false000183186800018318682023-08-222023-08-220001831868us-gaap:SeriesAPreferredStockMember2023-08-222023-08-220001831868us-gaap:SeriesBPreferredStockMember2023-08-222023-08-22
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): August 22, 2023 |
SeaStar Medical Holding Corporation
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-39927 |
85-3681132 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
3513 Brighton Blvd, Suite 410 |
|
Denver, Colorado |
|
80216 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 844 427-8100 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock par value $0.0001 per share |
|
ICU |
|
The Nasdaq Stock Market LLC |
Warrants, each whole warrant exercisable for one share of Common Stock for $11.50 per share |
|
ICUCW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
SeaStar Medical Holding Corporation (the “Company”) is furnishing the following pursuant to Item 7.01, “Regulation FD Disclosure.”
On August 22, 2023, the Company posted a presentation to its website, www.seastarmedical.com, which was used by certain senior executives in a business update conference call held online on the same day. The presentation materials are attached hereto as Exhibit 99.1. The recorded webcast of the presentation may be accessed by visiting the Investors Events & Presentations section of the Company’s website at https://investors.seastarmedical.com/events-and-presentations/default.aspx.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
SeaStar Medical Holding Corporation |
|
|
By: |
/s/ Eric Schlorff |
Date: |
August 24, 2023 |
Name: |
Eric Schlorff |
|
|
Title: |
Chief Executive Officer |

EXHIBIT 9.1 Innovative Solutions for the Consequences of a Dysregulated Immune Response SeaStar Medical Business Update Call August 22, 2023

Forward-Looking Statements This presentation contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955. These forward-looking statements include, without limitation, SeaStar Medical’s expectations with respect to the timing of regulatory approval of its products, the expected timing on enrollment, generation of study results, submission of PMA and other corporate milestones, the ability of SCD to treat patients with AKI, and the potential benefits of SCD to treat other diseases. Words such as “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside SeaStar Medical’s control and are difficult to predict. Factors that may cause actual future events to differ materially from the expected results include, but are not limited to: (i) the risk that SeaStar may not be able to obtain regulatory approval of its SCD product candidates; (ii) the risk that SeaStar may not be able to raise sufficient capital to fund its operations, including clinical trials; (iii) the risk that SeaStar Medical and its current and future collaborators are unable to successfully develop and commercialize its products or services, or experience significant delays in doing so, including failure to achieve approval of its products by applicable federal and state regulators, (iv) the risk that SeaStar Medical may never achieve or sustain profitability; (v) the risk that SeaStar Medical may not be able to access funding under existing agreements, including the equity line of credit and forward purchase agreements; (vi) the risk that third-parties suppliers and manufacturers are not able to fully and timely meet their obligations, (vii) the risk of product liability or regulatory lawsuits or proceedings relating to SeaStar Medical’s products and services, (xiii) the risk that SeaStar Medical is unable to secure or protect its intellectual property, and (xi) other risks and uncertainties indicated from time to time in SeaStar Medical’s Annual Report on Form 10-K, including those under the “Risk Factors” section therein and in SeaStar Medical’s other filings with the SEC. (iii) The foregoing list of factors is not exhaustive. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and SeaStar Medical assume no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise.

Agenda Therapeutic Need & SCD Technology Update on HDE in Pediatric AKI Adult & Pediatric Program/Device Differentiation SCD Value Proposition NEUTRALIZE-AKI Study Update & Timelines Q&A AKI: acute kidney injury; HDE: Humanitarian Device Exemption; SCD: selective cytopheretic device
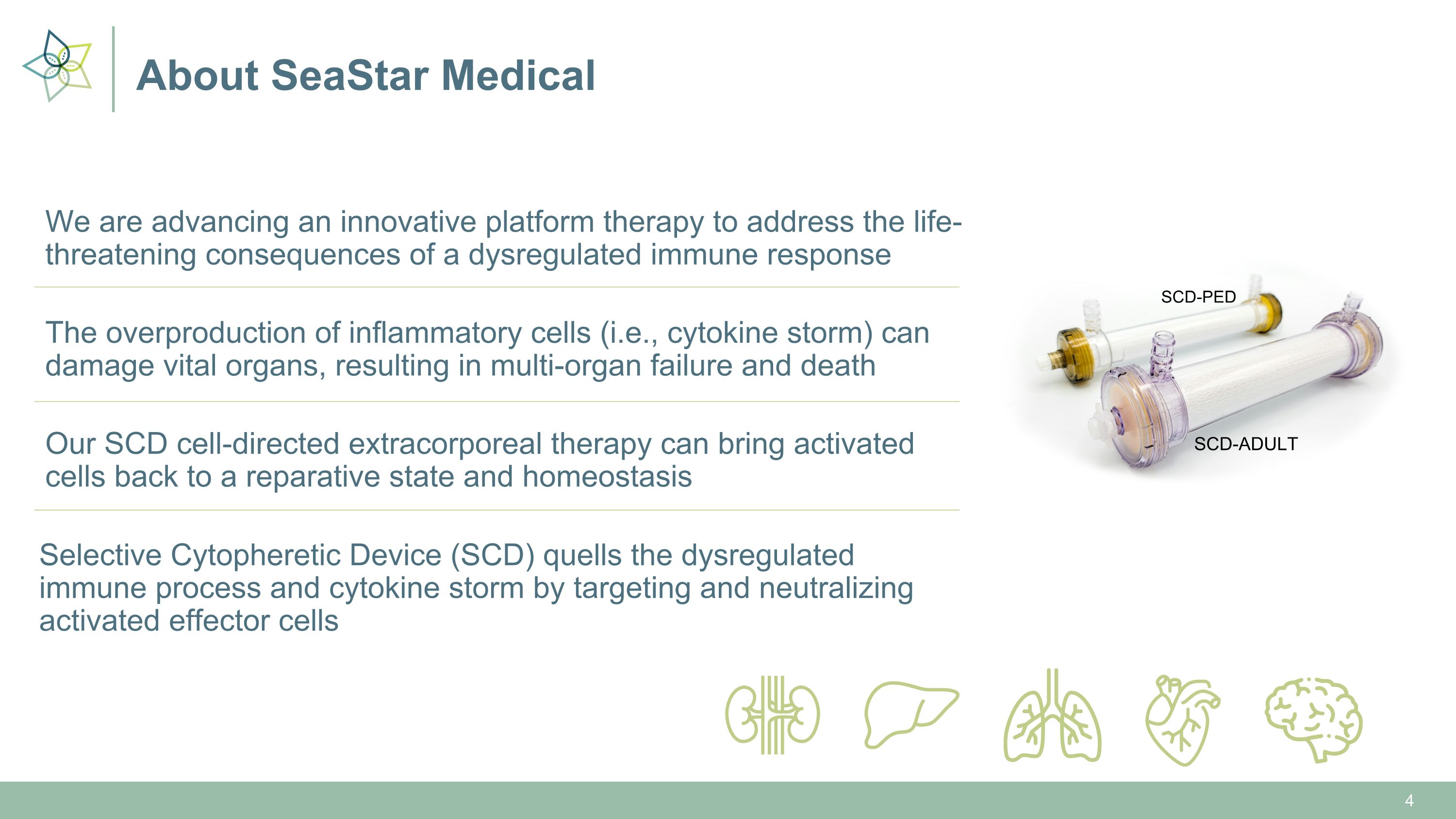
About SeaStar Medical We are advancing an innovative platform therapy to address the life-threatening consequences of a dysregulated immune response The overproduction of inflammatory cells (i.e., cytokine storm) can damage vital organs, resulting in multi-organ failure and death Our SCD cell-directed extracorporeal therapy can bring activated cells back to a reparative state and homeostasis Selective Cytopheretic Device (SCD) quells the dysregulated immune process and cytokine storm by targeting and neutralizing activated effector cells SCD-ADULT SCD-PED

Dysregulated Immune Response: Serious Condition Can Lead to �Permanent Organ Damage and Death Key Drivers Pulmonary infiltrates Lung injury Acute respiratory distress syndrome (ARDS) Cardiovascular shock Disseminated intravascular coagulant Renal failure (AKI) End-Organ Damage and Failure Permanent Organ Damage or Death Bacterial �Infection Viral �Infection Trauma Surgery Condition that Develops when Immune System �Responds too Aggressively to Injury or Infection Hyperinflammation / �Cytokine Storm Can Lead To
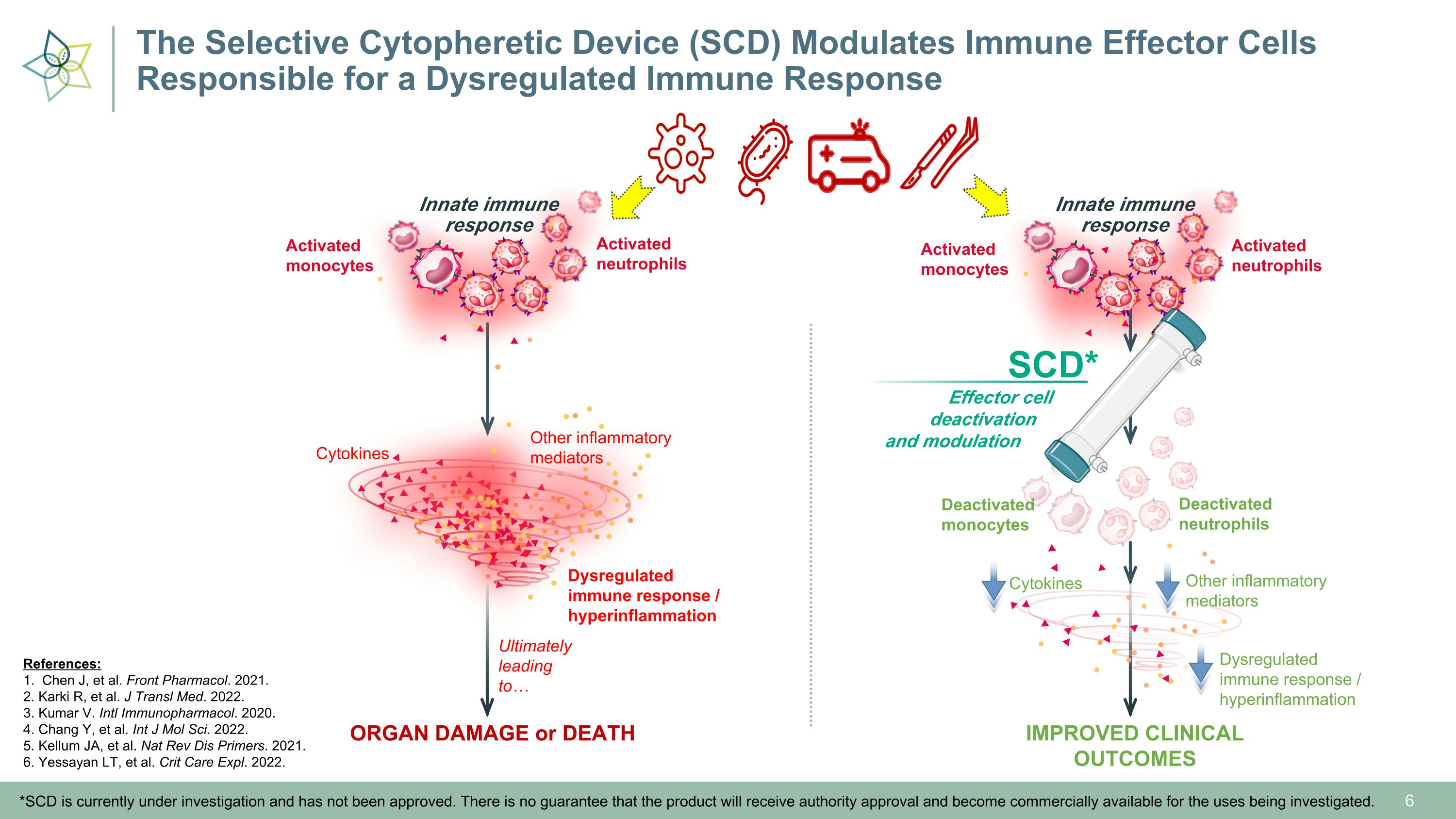
Deactivated�neutrophils Deactivated�monocytes Innate immune�response Activated�monocytes Activated�neutrophils IMPROVED CLINICAL OUTCOMES Other inflammatory�mediators Cytokines Dysregulated immune response / hyperinflammation Ultimately leading to… Effector cell � deactivation �and modulation SCD* *SCD is currently under investigation and has not been approved. There is no guarantee that the product will receive authority approval and become commercially available for the uses being investigated. Innate immune�response Activated�monocytes Activated�neutrophils ORGAN DAMAGE or DEATH Other inflammatory�mediators Cytokines Dysregulated immune response / hyperinflammation References: 1. Chen J, et al. Front Pharmacol. 2021. 2. Karki R, et al. J Transl Med. 2022. 3. Kumar V. Intl Immunopharmacol. 2020. 4. Chang Y, et al. Int J Mol Sci. 2022. 5. Kellum JA, et al. Nat Rev Dis Primers. 2021. 6. Yessayan LT, et al. Crit Care Expl. 2022. The Selective Cytopheretic Device (SCD) Modulates Immune Effector Cells �Responsible for a Dysregulated Immune Response
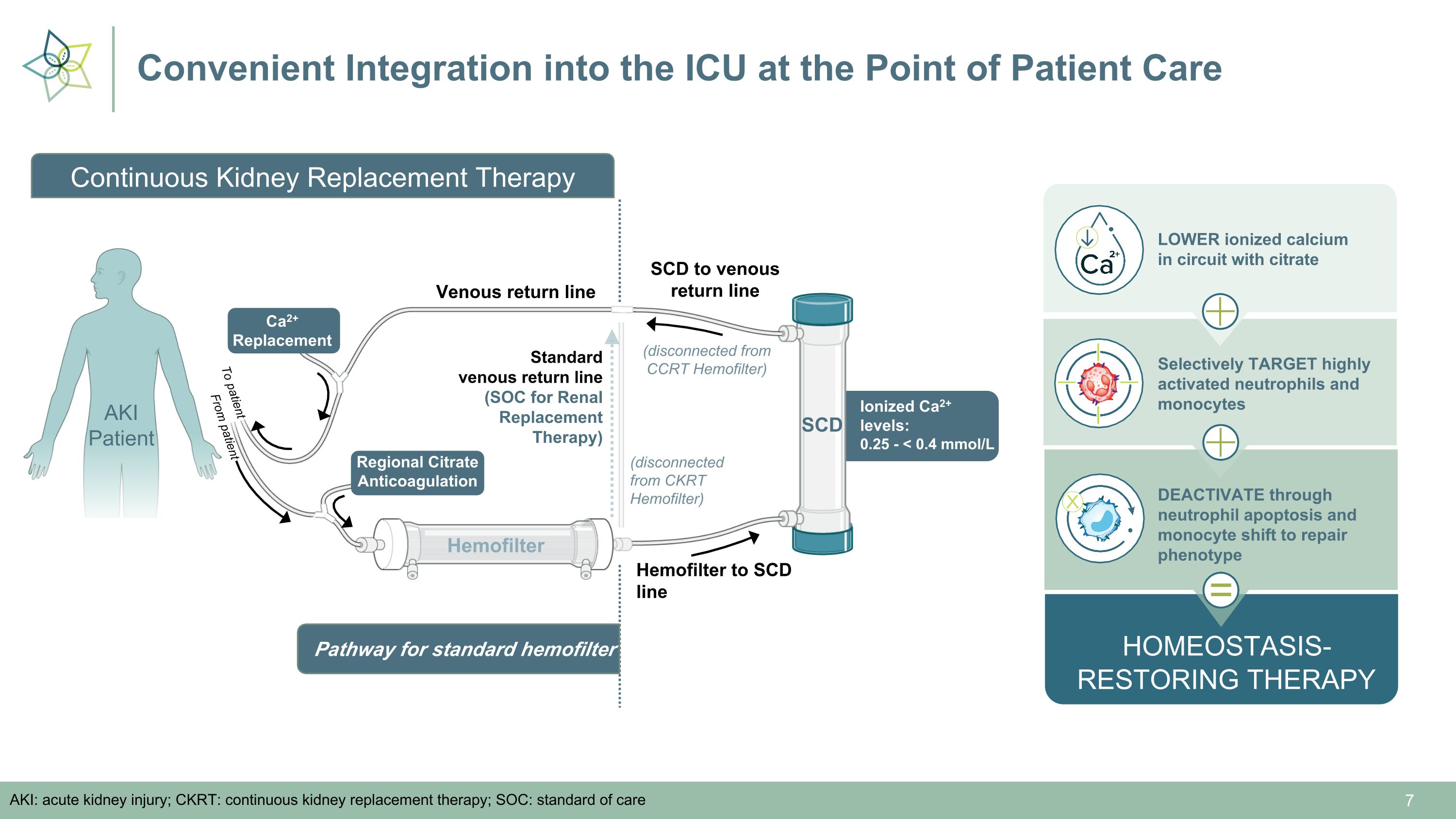
Convenient Integration into the ICU at the Point of Patient Care AKI: acute kidney injury; CKRT: continuous kidney replacement therapy; SOC: standard of care Standard �venous return line (SOC for Renal Replacement Therapy) Hemofilter Hemofilter to SCD line Ionized Ca2+ levels:�0.25 - < 0.4 mmol/L SCD Venous return line SCD to venous return line (disconnected from CKRT Hemofilter) Regional Citrate Anticoagulation Ca2+ �Replacement (disconnected from CCRT Hemofilter) HOMEOSTASIS- RESTORING THERAPY LOWER ionized calcium �in circuit with citrate Selectively TARGET highly activated neutrophils and monocytes DEACTIVATE through neutrophil apoptosis and monocyte shift to repair phenotype = From patient To patient Pathway for standard hemofilter AKI Patient Continuous Kidney Replacement Therapy
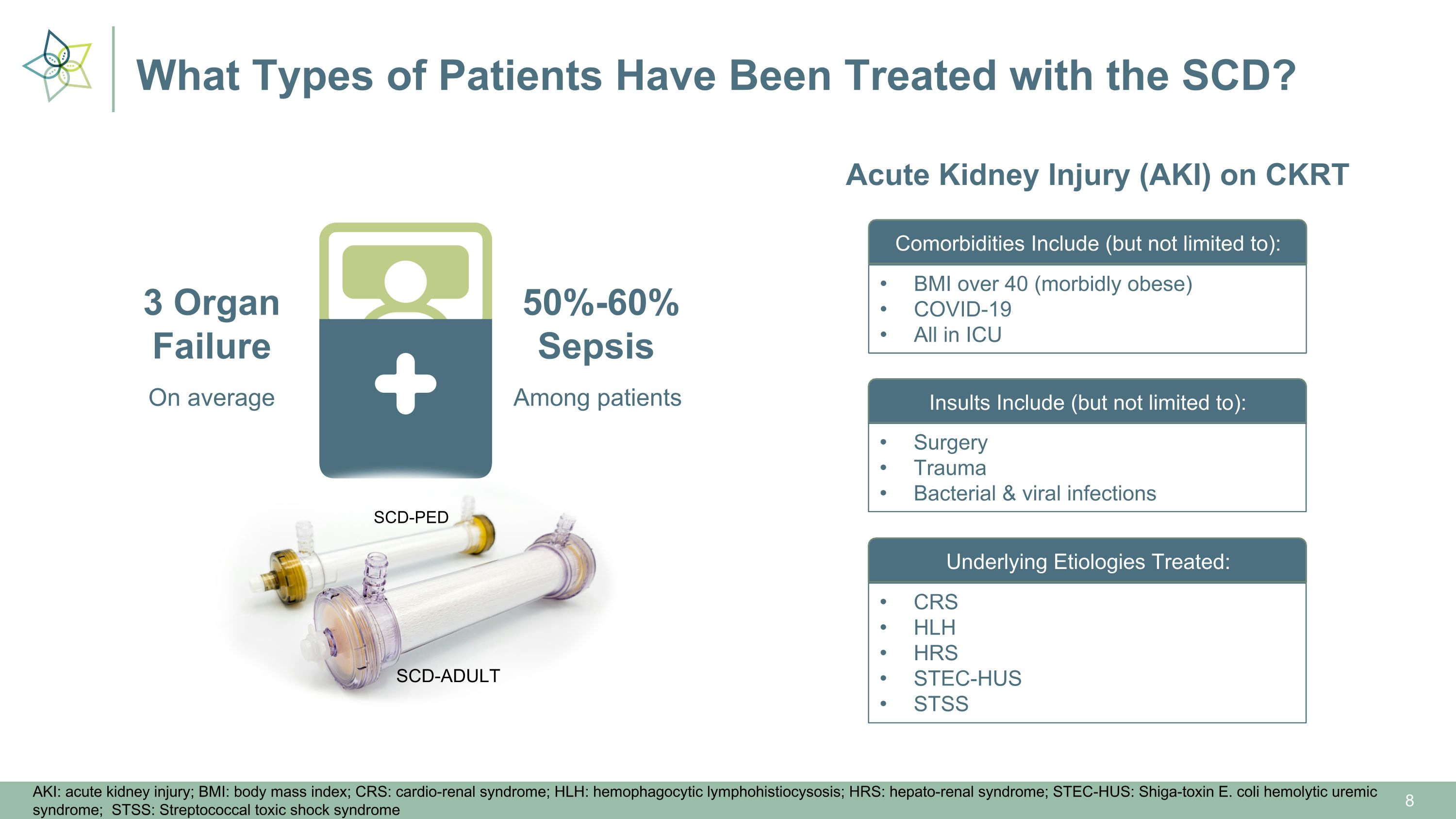
What Types of Patients Have Been Treated with the SCD? On average 3 Organ�Failure Among patients 50%-60%�Sepsis SCD-ADULT SCD-PED AKI: acute kidney injury; BMI: body mass index; CRS: cardio-renal syndrome; HLH: hemophagocytic lymphohistiocysosis; HRS: hepato-renal syndrome; STEC-HUS: Shiga-toxin E. coli hemolytic uremic syndrome; STSS: Streptococcal toxic shock syndrome Acute Kidney Injury (AKI) on CKRT Comorbidities Include (but not limited to): Insults Include (but not limited to): Underlying Etiologies Treated: BMI over 40 (morbidly obese) COVID-19 All in ICU Surgery Trauma Bacterial & viral infections CRS HLH HRS STEC-HUS STSS
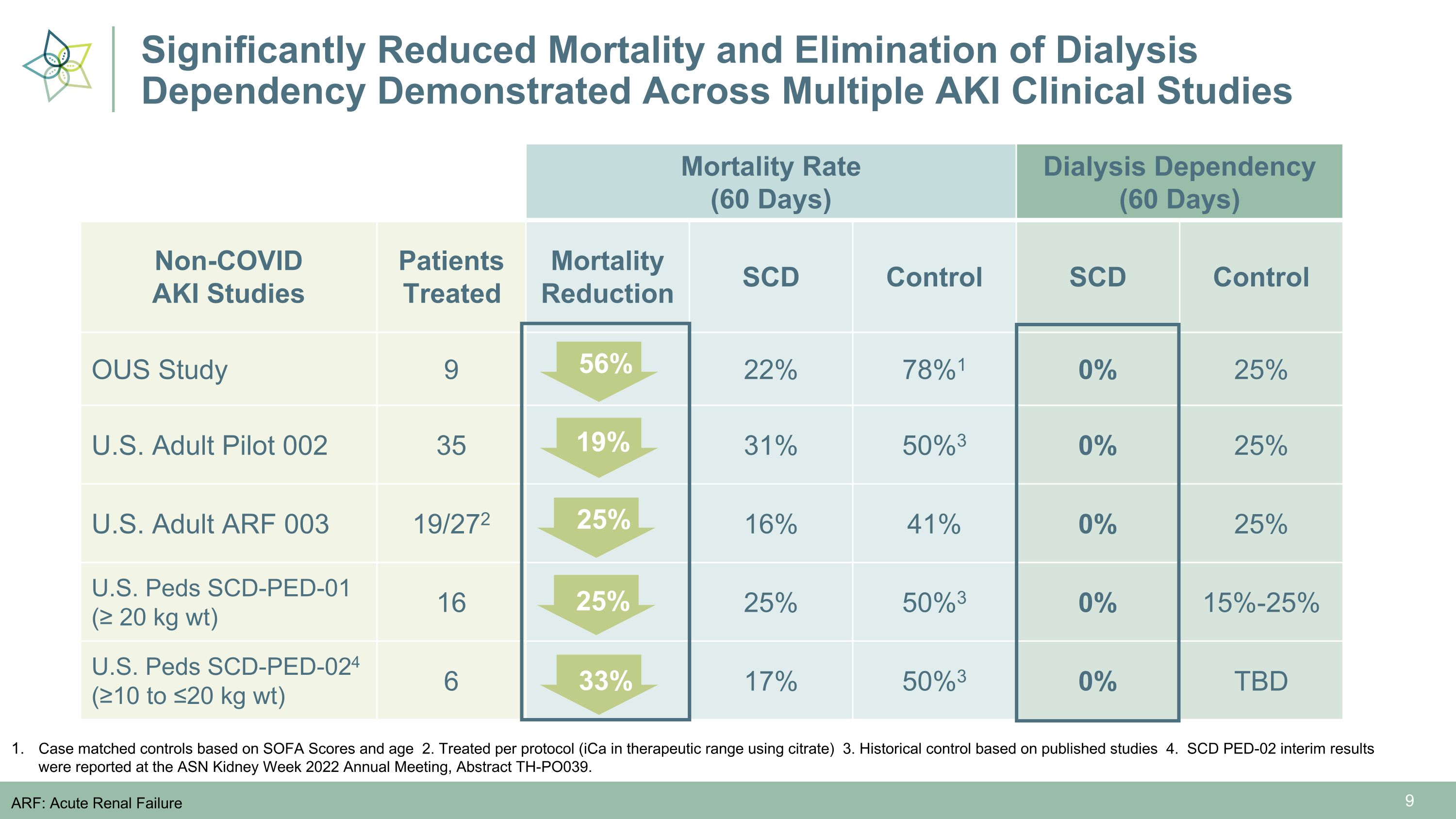
Significantly Reduced Mortality and Elimination of Dialysis Dependency Demonstrated Across Multiple AKI Clinical Studies Mortality Rate (60 Days) Control Dialysis Dependency (60 Days) Control Non-COVID �AKI Studies Patients Treated Mortality Reduction SCD Control SCD Control OUS Study 9 22% 78%1 0% 25% U.S. Adult Pilot 002 35 31% 50%3 0% 25% U.S. Adult ARF 003 19/272 16% 41% 0% 25% U.S. Peds SCD-PED-01 (≥ 20 kg wt) 16 25% 50%3 0% 15%-25% U.S. Peds SCD-PED-024 (≥10 to ≤20 kg wt) 6 17% 50%3 0% TBD 56% Case matched controls based on SOFA Scores and age 2. Treated per protocol (iCa in therapeutic range using citrate) 3. Historical control based on published studies 4. SCD PED-02 interim results were reported at the ASN Kidney Week 2022 Annual Meeting, Abstract TH-PO039. ARF: Acute Renal Failure 19% 25% 25% 33%

Safety Profile of SCD Across 5 Adult and Pediatric AKI Studies Study (Descriptor) # SCD-treated Patients # SCD Used per Patient (mean) Total # SCD Used Total SCD Exposure Time (hours) # AEs # SAEs # Device-related SAEs China Pilot 9 3.9 34 816 14 0 0 ARF-002 35 4.3 150 3,508 199 28 0 SCD-003 69 5.2 359 8,611.2 354 80 0 SCD-PED-01 16 5 80 1,936 47 12 0 SCD-005* 22 8.2 181 4,344 70 50 0 Totals 151 5.3 804 19,215.2 684 170 0 *All but 1 patient had AKI. Reference: Manuscript in review with a peer-reviewed journal. Pre-print available at https://www.authorea.com/doi/full/10.22541/au.169046178.83772599 151 critically ill ICU patients with AKI > 800 devices used and > 19,000 hours of exposure No device related infections or SAEs ~50% of these patients are septic
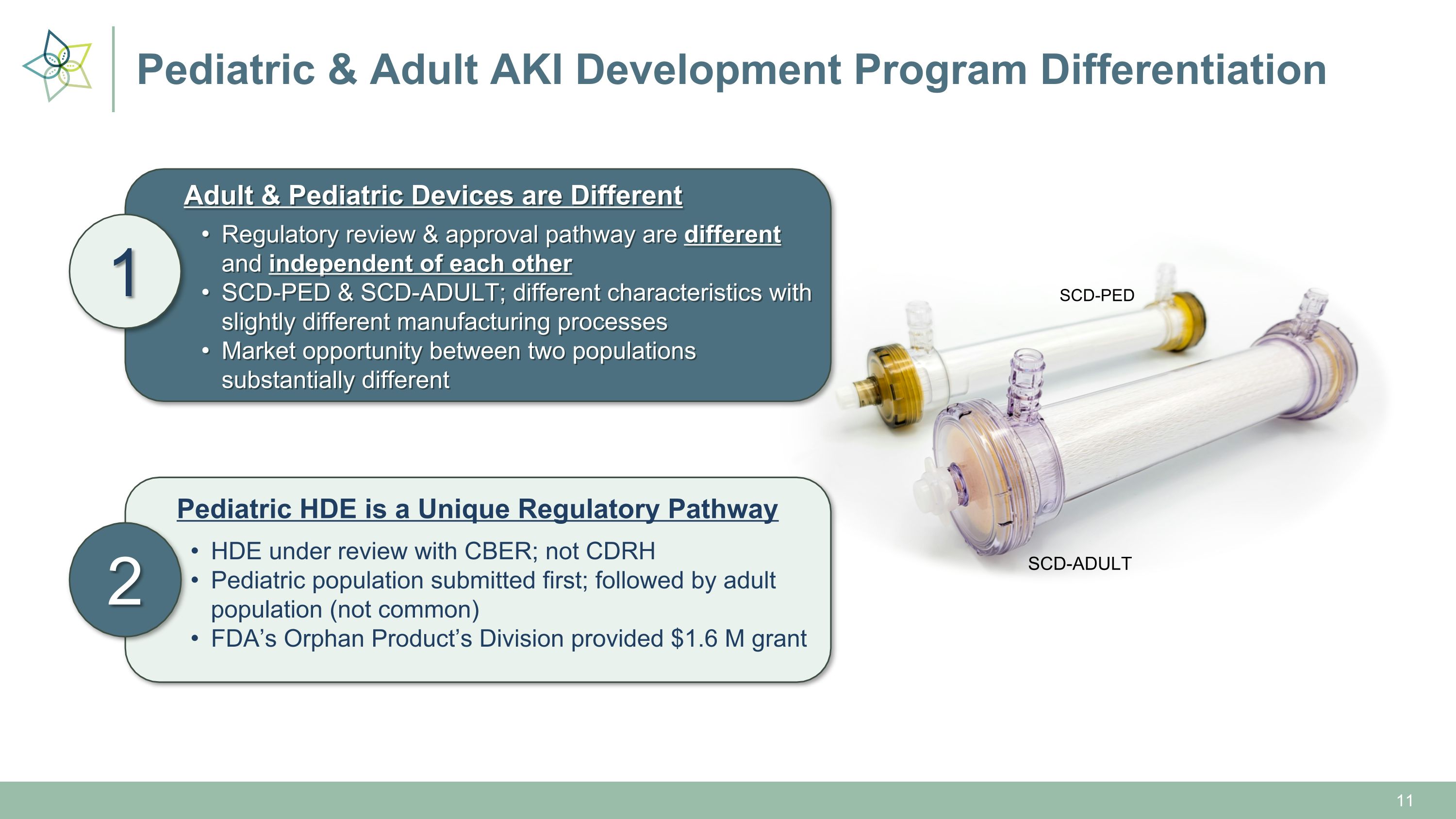
Pediatric & Adult AKI Development Program Differentiation 1 2 Adult & Pediatric Devices are Different Regulatory review & approval pathway are different and independent of each other SCD-PED & SCD-ADULT; different characteristics with slightly different manufacturing processes Market opportunity between two populations substantially different SCD-ADULT SCD-PED Pediatric HDE is a Unique Regulatory Pathway HDE under review with CBER; not CDRH Pediatric population submitted first; followed by adult population (not common) FDA’s Orphan Product’s Division provided $1.6 M grant

Pediatric SCD - Humanitarian Device Exemption (HDE) Update In May 2023, the Company received a letter from FDA indicating that the application is not approvable in its current form and outlining specific guidance as to how the application may be amended and resubmitted The company announced on May 9, 2023, that it would evaluate and pursue multiple pathways to find resolution Since that time, the Company has continued to work closely and collaboratively with FDA toward achieving this resolution to bring this therapy to this critically ill patient population Additionally, we have received & submitted to FDA written statements and testimonials from: Clinicians with experience using the device to improve clinical outcomes in pediatric patients Parents of children in the ICU who attest to the role of the SCD in their child’s recovery A national professional society of pediatric nephrologists expressing their concern with the ‘Not Approvable’ letter, urging FDA to ensure this device is rapidly made available to the pediatric community
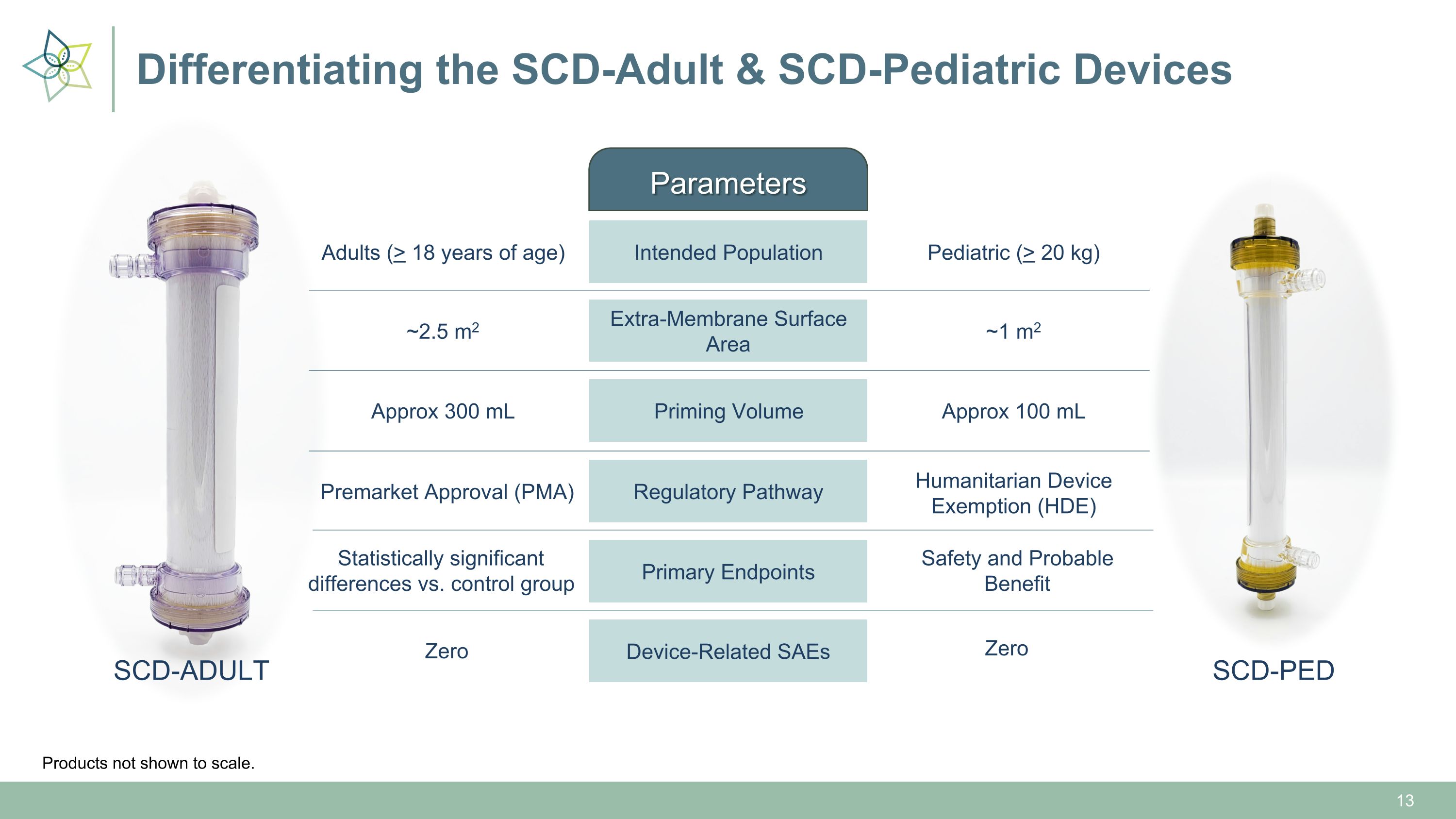
Differentiating the SCD-Adult & SCD-Pediatric Devices Parameters SCD-ADULT SCD-PED Intended Population Regulatory Pathway Extra-Membrane Surface Area Priming Volume Adults (> 18 years of age) Premarket Approval (PMA) ~2.5 m2 Approx 300 mL Pediatric (> 20 kg) Humanitarian Device Exemption (HDE) ~1 m2 Approx 100 mL Primary Endpoints Statistically significant differences vs. control group Safety and Probable Benefit Device-Related SAEs Zero Zero Products not shown to scale.
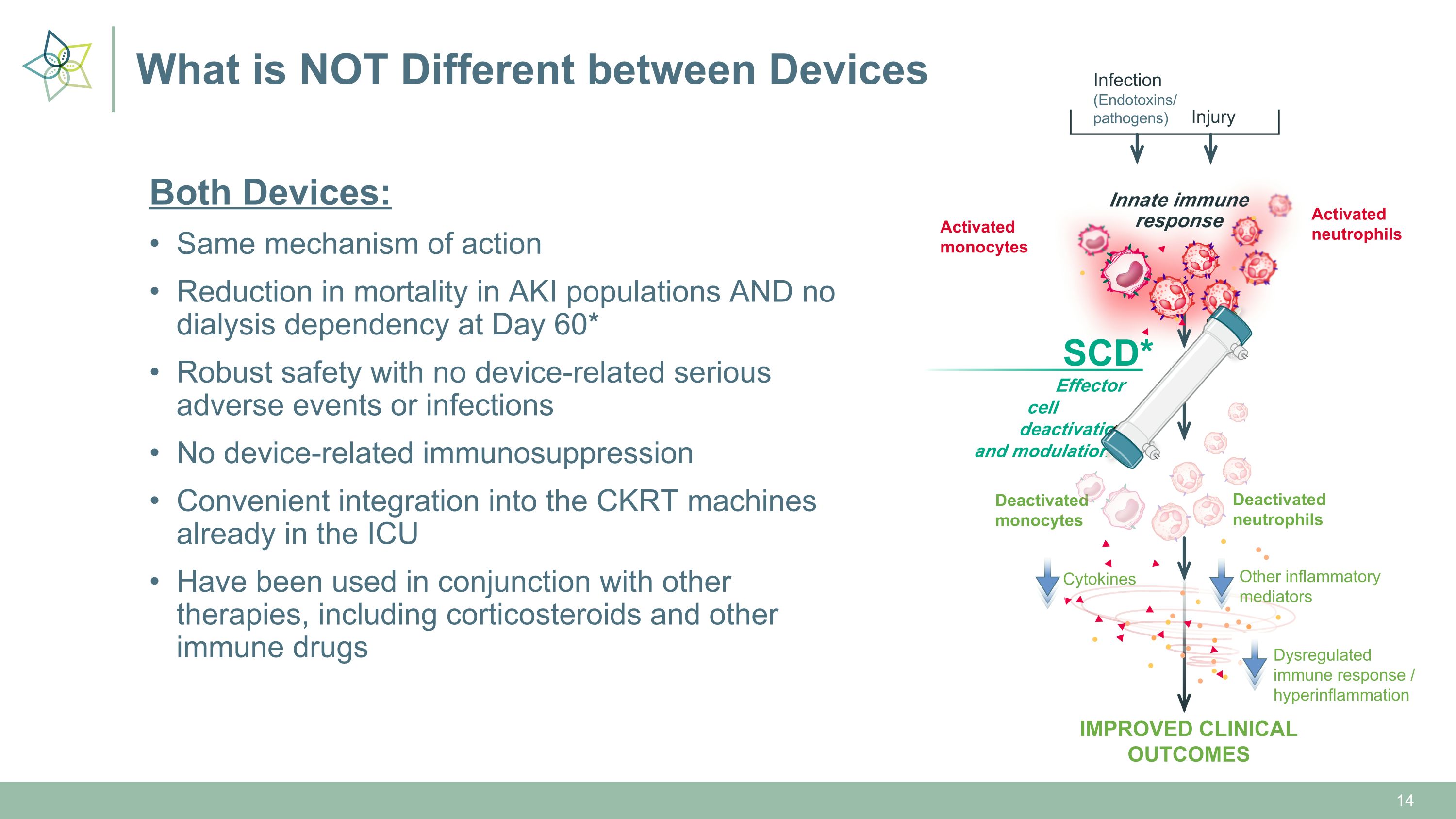
What is NOT Different between Devices Both Devices: Same mechanism of action Reduction in mortality in AKI populations AND no dialysis dependency at Day 60* Robust safety with no device-related serious adverse events or infections No device-related immunosuppression Convenient integration into the CKRT machines already in the ICU Have been used in conjunction with other therapies, including corticosteroids and other immune drugs Deactivated�neutrophils Deactivated�monocytes Infection�(Endotoxins/�pathogens) Injury Innate immune�response Activated�monocytes Activated�neutrophils IMPROVED CLINICAL OUTCOMES Other inflammatory�mediators Cytokines Dysregulated immune response / hyperinflammation Effector cell � deactivation �and modulation SCD*

Opportunity of Adult vs. Pediatric AKI 1 – Silver SA & Chertow GM. Nephron. 2017; 137(4):297-301. 4,000 US patients 210,000 US patients 50X Adult market is size of pediatric market = 1,000 patients

Financial Value Proposition for Adopting SCD Relevant Volumes 1,685 126 Procedures Unique Patients Current Cost $22,985 Average Inpatient Cost Per Episode $27,700 Average Post-Acute Care Cost Per Episode Clinical Utilization 7.1 21% 33% Avg. Length of Stay Avg. Readmission Rate PAC ER Visit Rate Single Center Example From Leading US Children’s Hospital PAC: Post-acute care $50,685 Total Source: Definitive Health Proprietary Database

SCD Value Proposition *WAC: wholesale acquisition cost; 1 – Coca SG, Singanamala S, Parikh CR. Kidney Int. 2012. 81(5):442-448. 2 – 2022 USRDS Annual Report; Chapter 9: End Stage Renal Disease; accessed 20-Aug-2023. CKD Chronic Kidney Disease ESRD End-Stage Renal Disease $~100,000: Cost of a single patient for 1 year of dialysis2 28X more likely to develop Severe AKI Acute Kidney Injury1 8X more likely to develop ~$10-20,000+: Total SCD cost for a full course (3-7 days)* (Disposable model; changed Q24h)
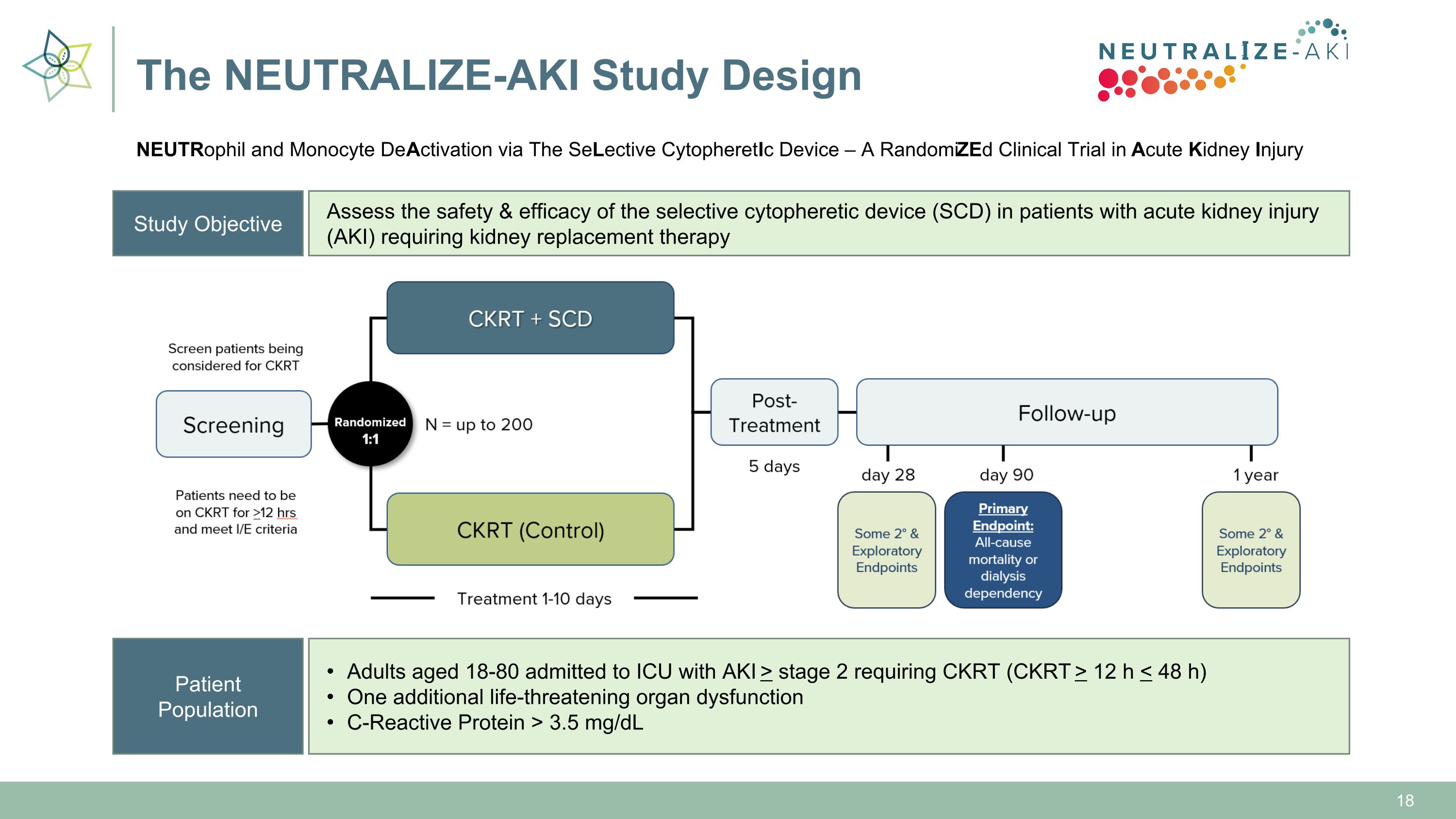
Study Objective Assess the safety & efficacy of the selective cytopheretic device (SCD) in patients with acute kidney injury (AKI) requiring kidney replacement therapy Patient Population Adults aged 18-80 admitted to ICU with AKI > stage 2 requiring CKRT (CKRT > 12 h < 48 h) One additional life-threatening organ dysfunction C-Reactive Protein > 3.5 mg/dL The NEUTRALIZE-AKI Study Design NEUTRophil and Monocyte DeActivation via The SeLective CytopheretIc Device – A RandomiZEd Clinical Trial in Acute Kidney Injury

Trial Update This week, we activated our 3rd clinical trial site as we progress toward our goal of 20-30 total trial sites Currently 2 patients enrolled in the study The Company will provide periodic updates when certain site activation and patient enrollment milestones are reached Target: Enroll 1 patient/site/month; progressing on track
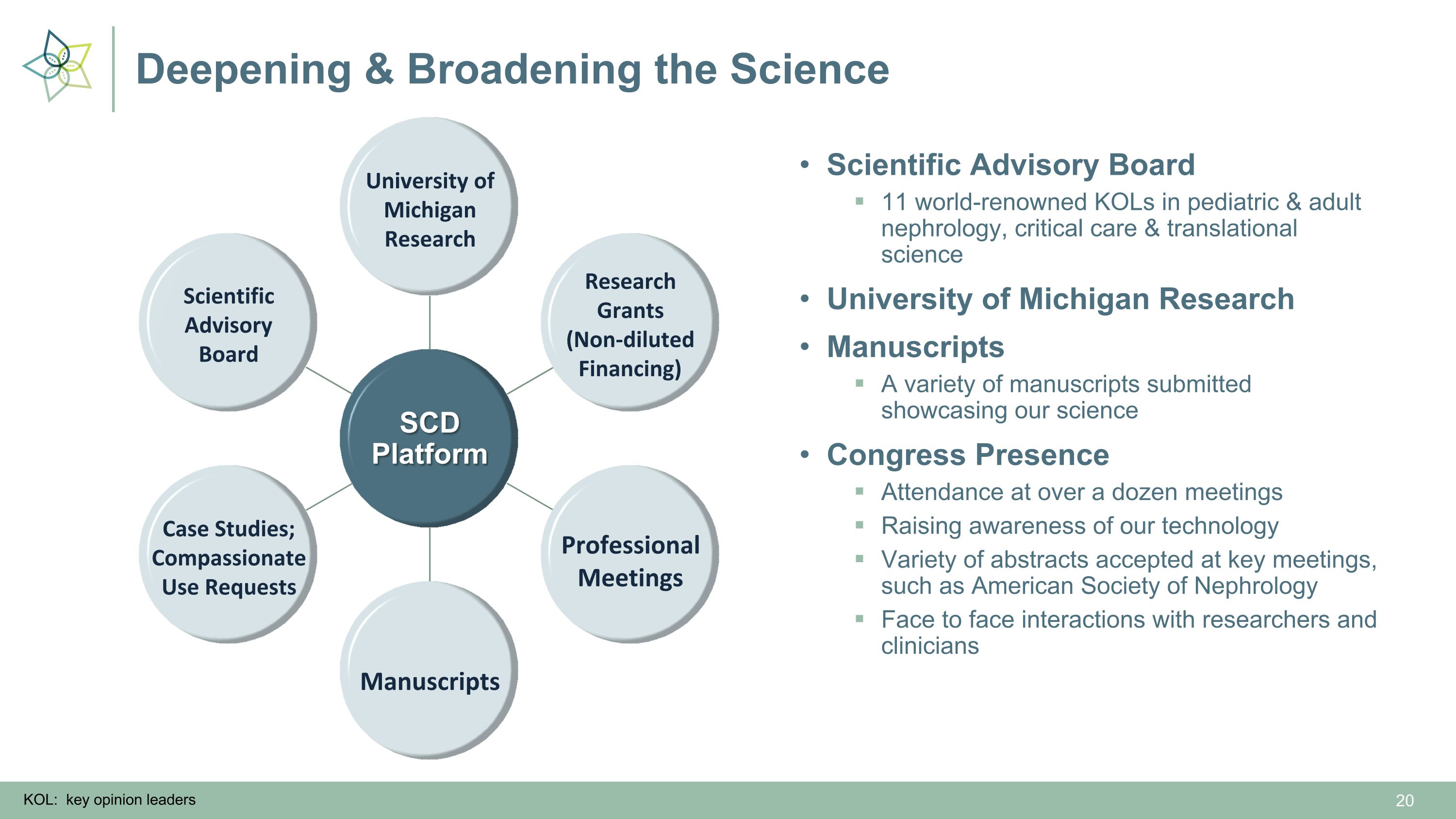
Deepening & Broadening the Science SCD Platform Professional Meetings Manuscripts Research Grants (Non-diluted Financing) University of Michigan Research Scientific Advisory Board Case Studies; Compassionate Use Requests Scientific Advisory Board 11 world-renowned KOLs in pediatric & adult nephrology, critical care & translational science University of Michigan Research Manuscripts A variety of manuscripts submitted showcasing our science Congress Presence Attendance at over a dozen meetings Raising awareness of our technology Variety of abstracts accepted at key meetings, such as American Society of Nephrology Face to face interactions with researchers and clinicians KOL: key opinion leaders

Platform Therapy Addressing Multiple High-Value Indications LVAD: Left Ventricular Assist Device American College of Physicians, ACP Hospitalist, Coding information from July 2019 Jamil, K et al The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records Journal of Medical Economics 2019;22(5):421-429 Agrawal, S et al Temporal Trends in Hospitalization for Acute Decompensated Heart Failure in the United States, 1998–2011, American Journal of Epidemiology 2016: 183(5) 462-470. (assumes only systolic patients) Expansion opportunities beyond AKI include: Acute Respiratory Distress Syndrome (TAM: 200,0001) Hepato-Renal Syndrome (TAM: 700,0002) Cardio Renal Syndrome (TAM: 400,0003) Pediatric Acute Kidney �Injury on CKRT Adult Acute Kidney� Injury on CKRT Cardiorenal Syndrome in Congestive Heart Failure (no LVAD) Cardiorenal Syndrome in Congestive Heart Failure (LVAD) Myocardial Stunning in End-Stage Renal Disease Hepatorenal Syndrome Exploratory clinical research underway to refine patient populations where SCD may be effective HDE Accepted for Review by FDA in July 2022 Potential FDA approval targeted by YE 2023, ~4,000 U.S. patients Initiated Pivotal Trial Activated first site in Q2 2023, ~210,000 U.S. patients
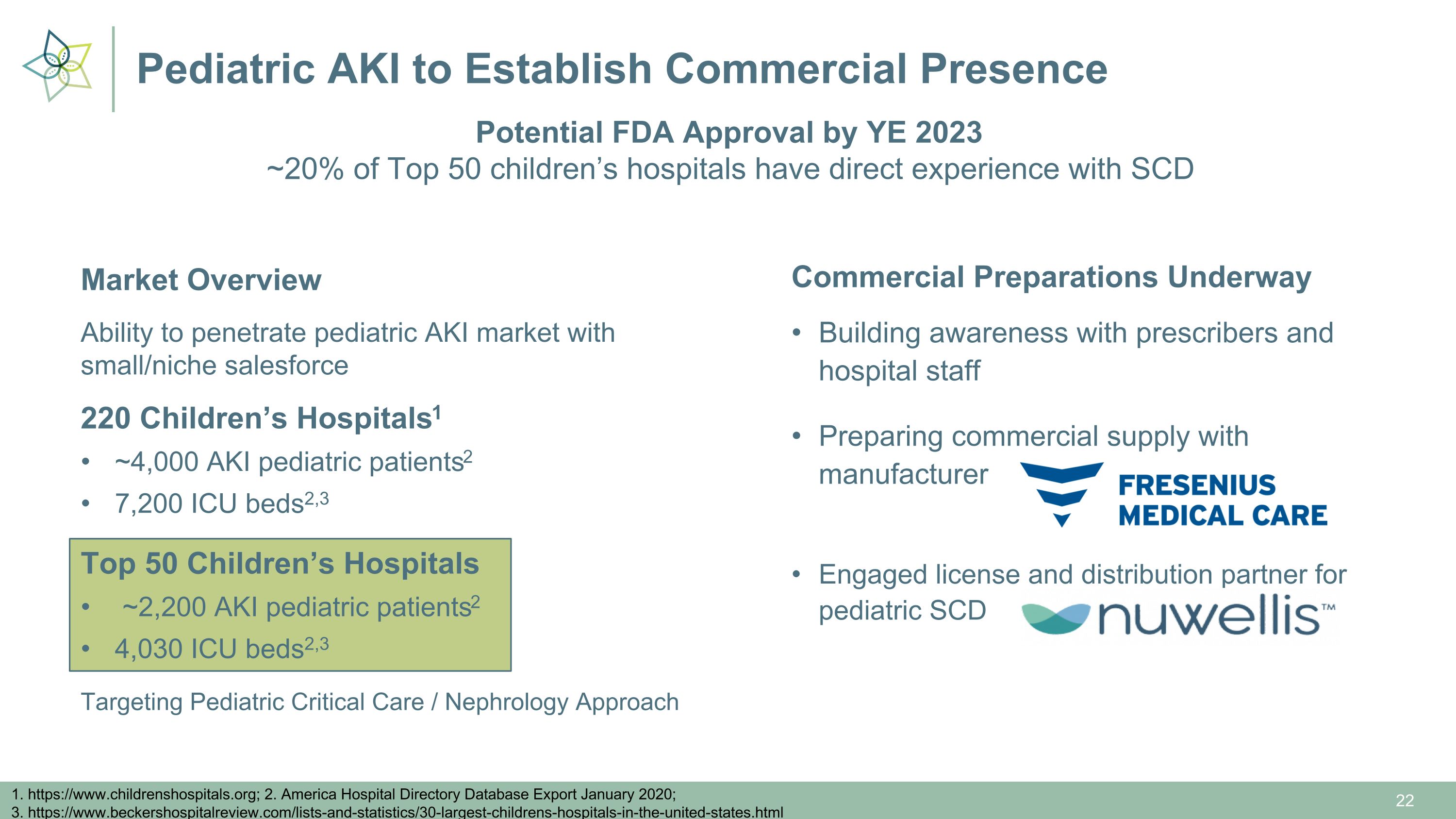
Pediatric AKI to Establish Commercial Presence 1. https://www.childrenshospitals.org; 2. America Hospital Directory Database Export January 2020; 3. https://www.beckershospitalreview.com/lists-and-statistics/30-largest-childrens-hospitals-in-the-united-states.html Market Overview Ability to penetrate pediatric AKI market with small/niche salesforce 220 Children’s Hospitals1 ~4,000 AKI pediatric patients2 7,200 ICU beds2,3 Targeting Pediatric Critical Care / Nephrology Approach Potential FDA Approval by YE 2023 ~20% of Top 50 children’s hospitals have direct experience with SCD Commercial Preparations Underway Building awareness with prescribers and hospital staff Preparing commercial supply with manufacturer Engaged license and distribution partner for pediatric SCD Top 50 Children’s Hospitals ~2,200 AKI pediatric patients2 4,030 ICU beds2,3
Summary Proprietary technology that has shown significant benefit against an unmet need Diligently working with FDA toward approval of HDE in children followed by commercial launch in pediatric AKI Activating sites & enrolling patients in the pivotal NEUTRALIZE-AKI trial in adult AKI Different regulatory pathway Different device than pediatrics Significantly larger patient population & market size Generating clinical data in demonstrating potential economic value of this technology Technology has potential applications in multiple acute & chronic high value indications using adult SCD with little to no modifications World class team focused on executing our business strategy & achieving our goal of improving patient outcomes and saving lives
v3.23.2
Document And Entity Information
|
Aug. 22, 2023 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Aug. 22, 2023
|
| Entity Registrant Name |
SeaStar Medical Holding Corporation
|
| Entity Central Index Key |
0001831868
|
| Entity Emerging Growth Company |
true
|
| Securities Act File Number |
001-39927
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
85-3681132
|
| Entity Address, Address Line One |
3513 Brighton Blvd,
|
| Entity Address, Address Line Two |
Suite 410
|
| Entity Address, City or Town |
Denver
|
| Entity Address, State or Province |
CO
|
| Entity Address, Postal Zip Code |
80216
|
| City Area Code |
844
|
| Local Phone Number |
427-8100
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
false
|
| Series A Preferred Stock [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common Stock par value $0.0001 per share
|
| Trading Symbol |
ICU
|
| Security Exchange Name |
NASDAQ
|
| Series B Preferred Stock [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Warrants, each whole warrant exercisable for one share of Common Stock for $11.50 per share
|
| Trading Symbol |
ICUCW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesAPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_SeriesBPreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
SeaStar Medical (NASDAQ:ICU)
Historical Stock Chart
From Apr 2024 to May 2024
SeaStar Medical (NASDAQ:ICU)
Historical Stock Chart
From May 2023 to May 2024