false 0001770121 0001770121 2023-12-11 2023-12-11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 11, 2023
SANA BIOTECHNOLOGY, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-39941 |
|
83-1381173 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification Number) |
188 East Blaine Street, Suite 400
Seattle, Washington 98102
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (206) 701-7914
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.0001 par value per share |
|
SANA |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On December 11, 2023, the Company released an updated corporate presentation (the “Corporate Presentation”), a copy of which is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Current Report”) and is incorporated by reference herein.
In accordance with General Instruction B.2 of Form 8-K, the information furnished with this Current Report, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (“Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Forward-Looking Statements
This Current Report contains forward-looking statements, including regarding the timing or likelihood of the Company’s regulatory filings and the timing and availability of clinical data. These forward-looking statements reflect the Company’s views regarding current expectations and projections about future events and conditions and are based on currently available information. These forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and assumptions that are difficult to predict and the Risk Factors identified in the Company’s filings with the SEC, including the Company’s Annual Report on 10-K for the year ended December 31, 2022 and its Quarterly Report on Form 10-Q for the period ended September 30, 2023, and any subsequent Quarterly Reports on Form 10-Q; therefore, the Company’s actual results could differ materially from those expressed, implied or forecast in any such forward-looking statements. Expressions of future goals and expectations and similar expressions, including “may,” “will,” “should,” “could,” “aims,” “seeks,” “expects,” “plans,” “anticipates,” “intends,” “believes,” “estimates,” “predicts,” “potential,” “targets,” and “continue,” reflecting something other than historical fact are intended to identify forward-looking statements. Unless required by law, the Company undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events, or otherwise. However, readers should carefully review the reports and documents the Company files or furnishes from time to time with the SEC, particularly its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
See the Exhibit Index below, which is incorporated by reference herein.
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Sana Biotechnology, Inc. |
|
|
|
|
| Date: December 11, 2023 |
|
|
|
By: |
|
/s/ Bernard Cassidy |
|
|
|
|
|
|
Bernard Cassidy |
|
|
|
|
|
|
Executive Vice President and General Counsel |

Corporate Presentation December 2023
Exhibit 99.1

This presentation contains
forward-looking statements about Sana Biotechnology, Inc. (the “Company,” “we,” “us,” or “our”) within the meaning of the federal securities laws. All statements other than statements of historical
facts contained in this presentation, including, among others, statements regarding the Company’s strategy, expectations, cash runway and future financial condition, future operations, and prospects, are forward-looking statements. In some
cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,”
“design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,”
“predict,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of
these terms or other comparable terminology. The Company has based these forward-looking statements largely on its current expectations, estimates, forecasts and projections about future events and financial trends that it believes may affect its
financial condition, results of operations, business strategy and financial needs. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events.
These statements are subject to risks and uncertainties that could cause the actual results to vary materially, including, among others, the risks inherent in drug development such as those associated with the initiation, cost, timing, progress and
results of the Company’s current and future research and development programs, preclinical studies, and clinical trials. For a detailed discussion of the risk factors that could affect the Company’s actual results, please refer to the
risk factors identified in the Company’s SEC reports, including its Quarterly Report on Form 10-Q dated November 8, 2023. Except as required by law, the Company undertakes no obligation to update publicly any forward-looking statements for any
reason. Cautionary Note Regarding Forward-Looking Statements

Sana’s ambition is to repair or
replace any cell in the body. Technologies address fundamental barriers: Hypoimmune (HIP) technology: Overcoming immune rejection of allogeneic cells Fusogen technology: In vivo delivery of genomic modification reagents in a cell-specific
manner Overcoming immune rejection of allogeneic cells has potential to change cell therapy: Allogeneic CAR T cells that perform clinically like autologous CAR T cells can transform treatment of hematological malignancies Key to unlocking the
potential of stem cell-derived therapies such as pancreatic islet cells for the treatment of type 1 diabetes Three programs in the clinic... SC291 in oncology: Goal of understanding immune evasion and activity in multiple cancers SC291 in autoimmune
diseases: Goal of understanding activity in three indications HIP primary islets in patients with type 1 diabetes: Goal of understanding ability to overcome allogeneic and autoimmune destruction of cells ...and more to come. Pipeline poised to
deliver multiple clinical data readouts Hypoimmune allogeneic CAR T cells: SC262 (CD22), SC255 (BCMA), and beyond Regenerative medicine: SC451 (type 1 diabetes) and SC379 (CNS disorders) Balance sheet allows potential for multiple data
readouts Engineered Cells as Medicines Sana Biotechnology
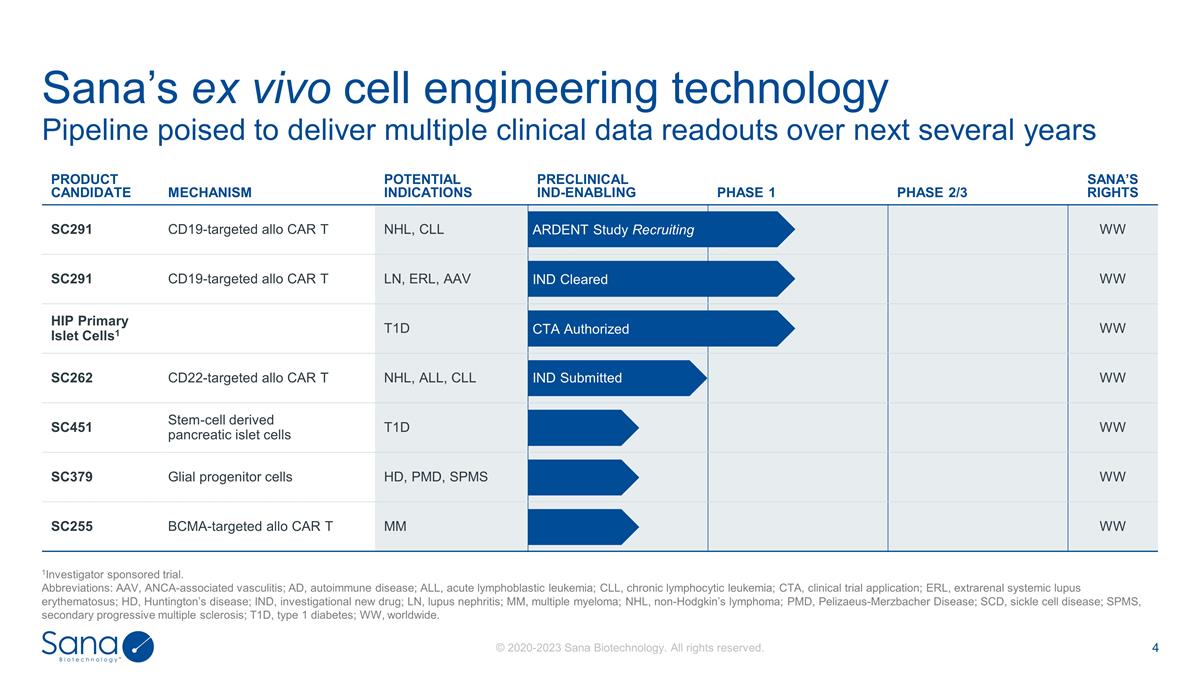
Pipeline poised to deliver multiple
clinical data readouts over next several years Sana’s ex vivo cell engineering technology 1Investigator sponsored trial. Abbreviations: AAV, ANCA-associated vasculitis; AD, autoimmune disease; ALL, acute lymphoblastic leukemia; CLL, chronic
lymphocytic leukemia; CTA, clinical trial application; ERL, extrarenal systemic lupus erythematosus; HD, Huntington’s disease; IND, investigational new drug; LN, lupus nephritis; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; PMD,
Pelizaeus-Merzbacher Disease; SCD, sickle cell disease; SPMS, secondary progressive multiple sclerosis; T1D, type 1 diabetes; WW, worldwide. PRODUCT CANDIDATE MECHANISM POTENTIAL INDICATIONS PRECLINICAL IND-ENABLING PHASE 1 PHASE 2/3 SANA’S
RIGHTS SC291 CD19-targeted allo CAR T NHL, CLL WW SC291 CD19-targeted allo CAR T LN, ERL, AAV WW HIP Primary Islet Cells1 T1D WW SC262 CD22-targeted allo CAR T NHL, ALL, CLL WW SC451 Stem-cell derived pancreatic islet cells T1D WW SC379 Glial
progenitor cells HD, PMD, SPMS WW SC255 BCMA-targeted allo CAR T MM WW ARDENT Study Recruiting CTA Authorized IND Submitted IND Cleared
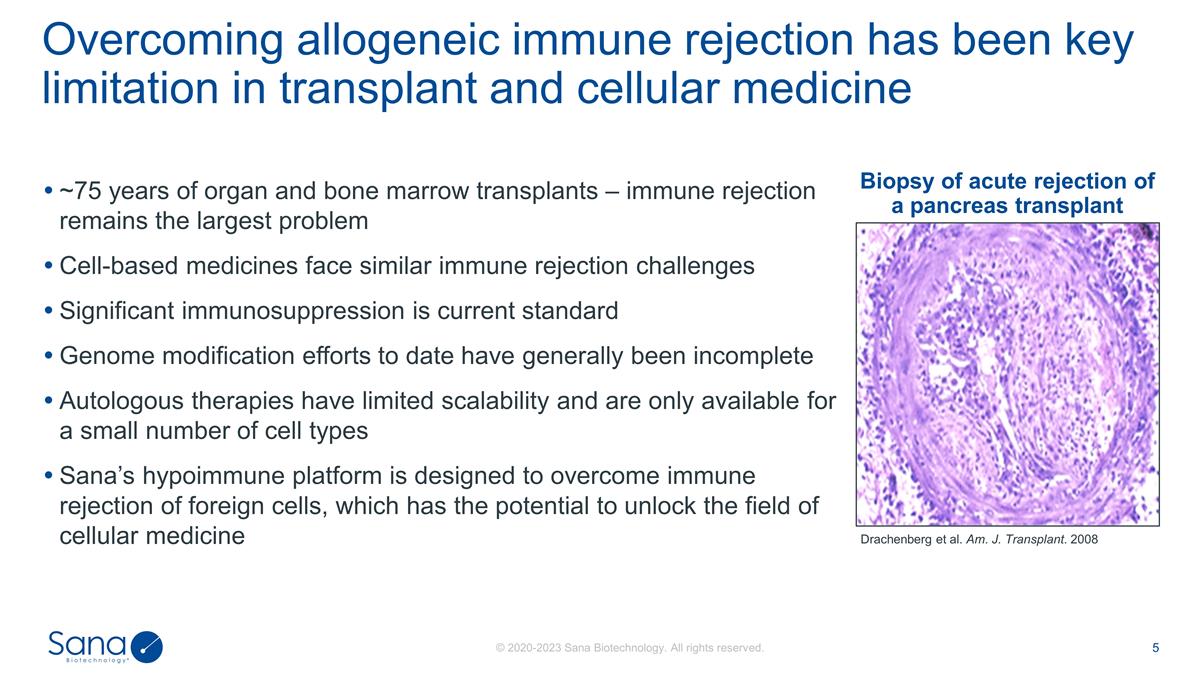
~75 years of organ and bone marrow
transplants – immune rejection remains the largest problem Cell-based medicines face similar immune rejection challenges Significant immunosuppression is current standard Genome modification efforts to date have generally been incomplete
Autologous therapies have limited scalability and are only available for a small number of cell types Sana’s hypoimmune platform is designed to overcome immune rejection of foreign cells, which has the potential to unlock the field of cellular
medicine Overcoming allogeneic immune rejection has been key limitation in transplant and cellular medicine Drachenberg et al. Am. J. Transplant. 2008 Biopsy of acute rejection of a pancreas transplant
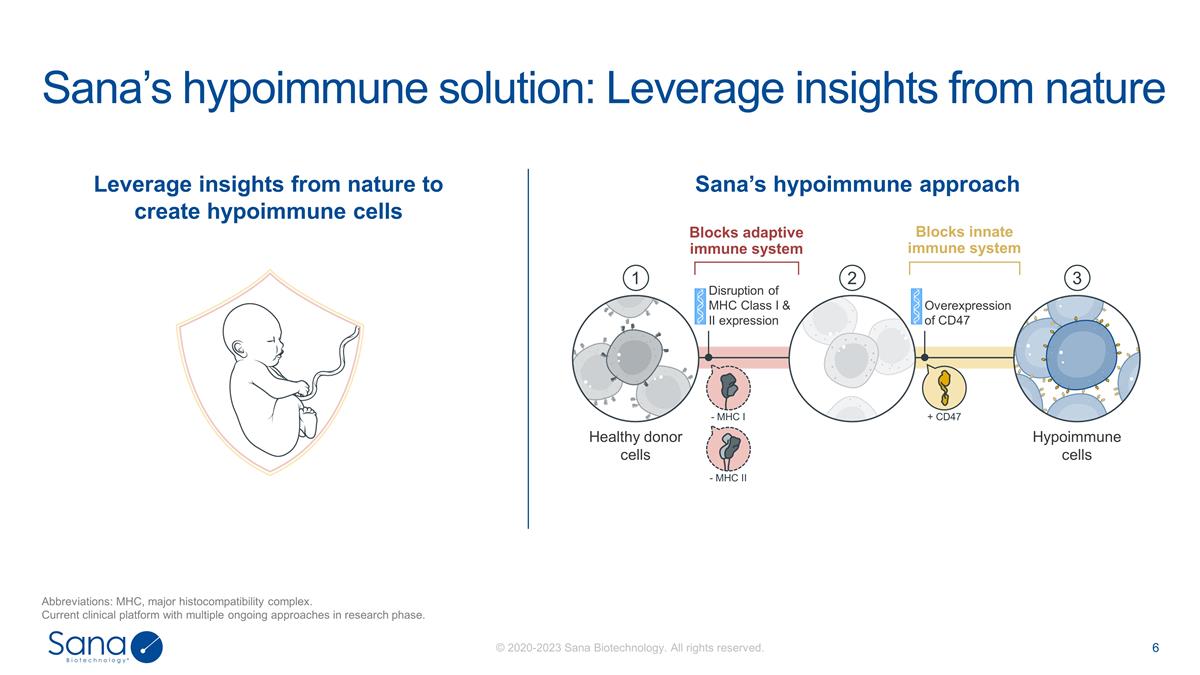
Sana’s hypoimmune solution:
Leverage insights from nature Abbreviations: MHC, major histocompatibility complex. Current clinical platform with multiple ongoing approaches in research phase. Leverage insights from nature to create hypoimmune cells Sana’s hypoimmune
approach + CD47 - MHC I - MHC II Healthy donor cells Hypoimmune cells Disruption of MHC Class I & II expression Overexpression of CD47 1 2 3 Blocks adaptive immune system Blocks innate immune system

Sana’s team has pioneered
hypoimmune technology
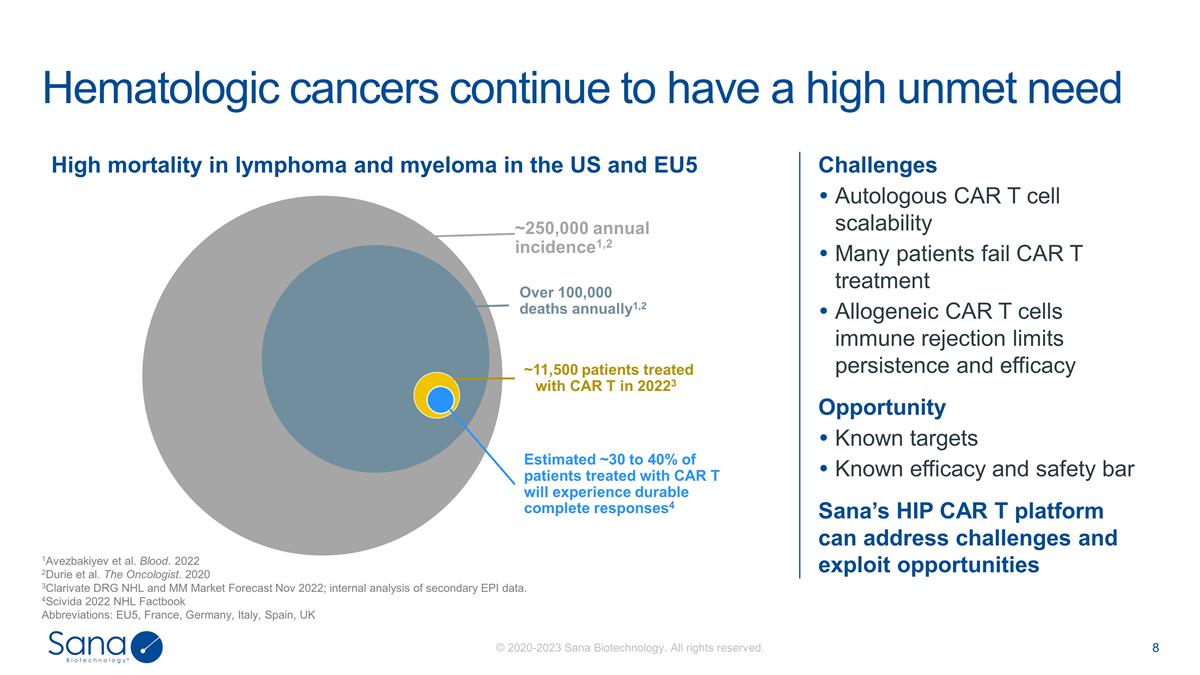
Challenges Autologous CAR T cell
scalability Many patients fail CAR T treatment Allogeneic CAR T cells immune rejection limits persistence and efficacy Opportunity Known targets Known efficacy and safety bar Sana’s HIP CAR T platform can address challenges and exploit
opportunities Hematologic cancers continue to have a high unmet need 1Avezbakiyev et al. Blood. 2022 2Durie et al. The Oncologist. 2020 3Clarivate DRG NHL and MM Market Forecast Nov 2022; internal analysis of secondary EPI data. 4Scivida 2022 NHL
Factbook Abbreviations: EU5, France, Germany, Italy, Spain, UK High mortality in lymphoma and myeloma in the US and EU5 ~250,000 annual incidence1,2 Over 100,000 deaths annually1,2 ~11,500 patients treated with CAR T in 20223 Estimated ~30 to 40% of
patients treated with CAR T will experience durable complete responses4
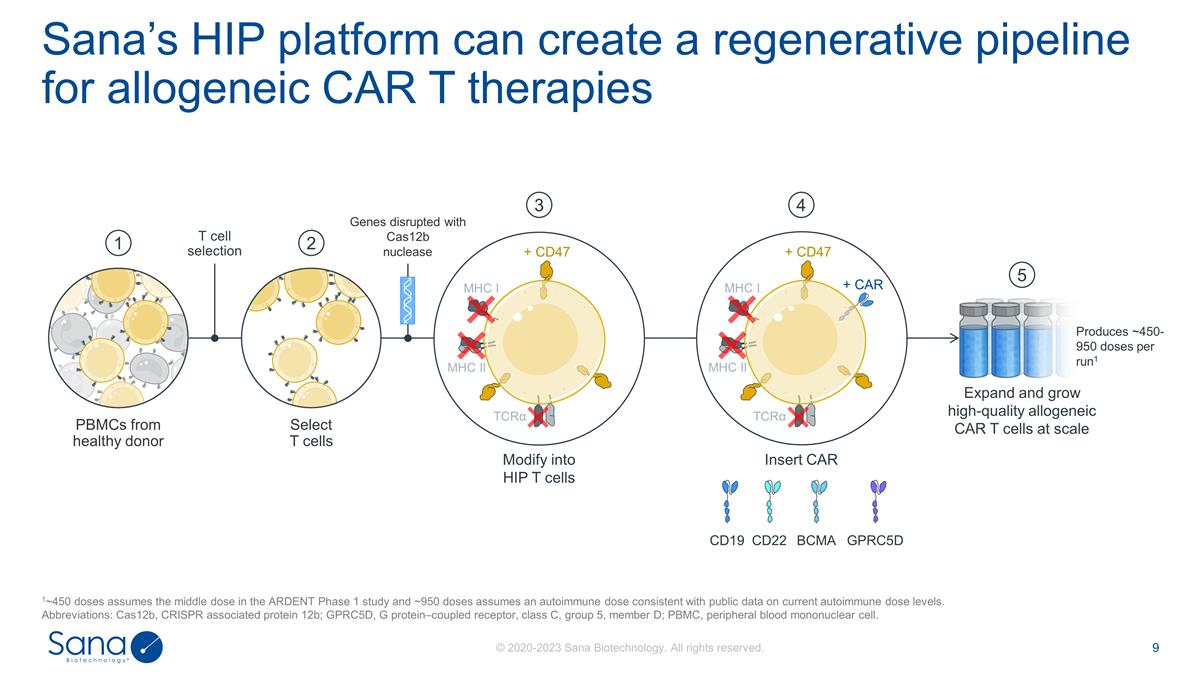
Sana’s HIP platform can create a
regenerative pipeline for allogeneic CAR T therapies 1~450 doses assumes the middle dose in the ARDENT Phase 1 study and ~950 doses assumes an autoimmune dose consistent with public data on current autoimmune dose levels. Abbreviations: Cas12b,
CRISPR associated protein 12b; GPRC5D, G protein–coupled receptor, class C, group 5, member D; PBMC, peripheral blood mononuclear cell. PBMCs from healthy donor 1 2 Select T cells T cell selection Expand and grow high-quality allogeneic CAR T
cells at scale 5 Modify into HIP T cells 3 Genes disrupted with Cas12b nuclease Insert CAR 4 CD22 CD19 BCMA GPRC5D Produces ~450-950 doses per run1 + CD47 MHC I MHC II TCRα MHC I MHC II TCRα + CAR + CD47
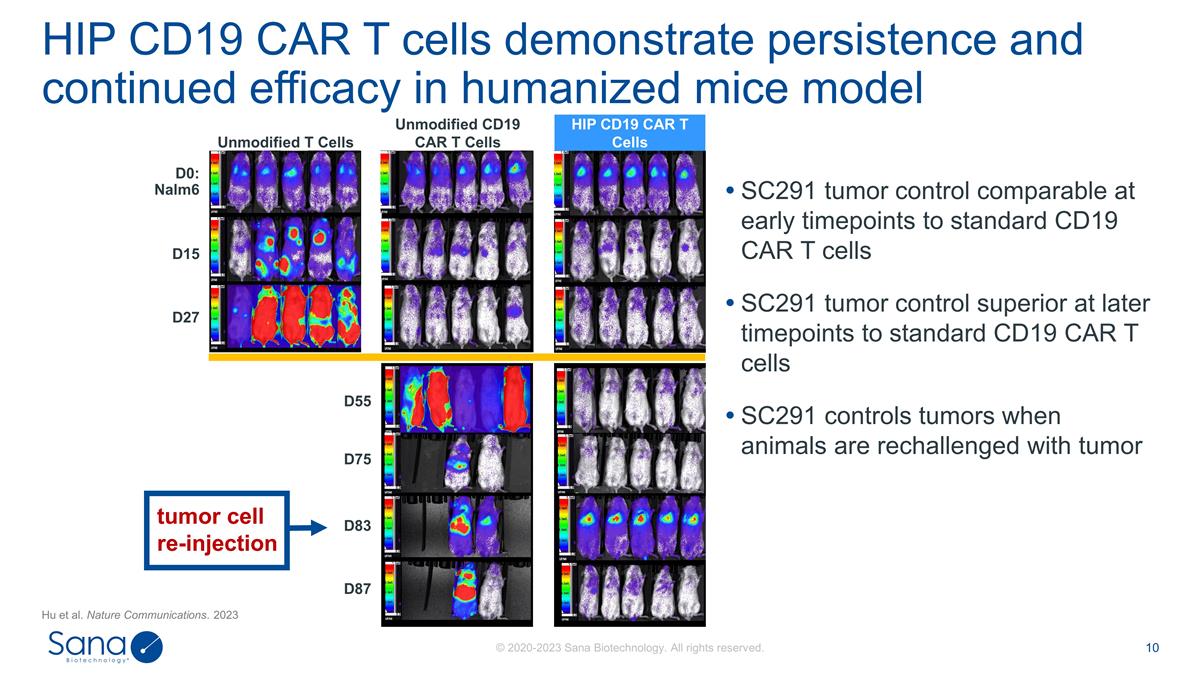
D55 D87 D75 D83 SC291 tumor control
comparable at early timepoints to standard CD19 CAR T cells SC291 tumor control superior at later timepoints to standard CD19 CAR T cells SC291 controls tumors when animals are rechallenged with tumor HIP CD19 CAR T cells demonstrate persistence and
continued efficacy in humanized mice model D0: Nalm6 D15 D27 Unmodified CD19 CAR T Cells Unmodified T Cells HIP CD19 CAR T Cells tumor cell re-injection Hu et al. Nature Communications. 2023
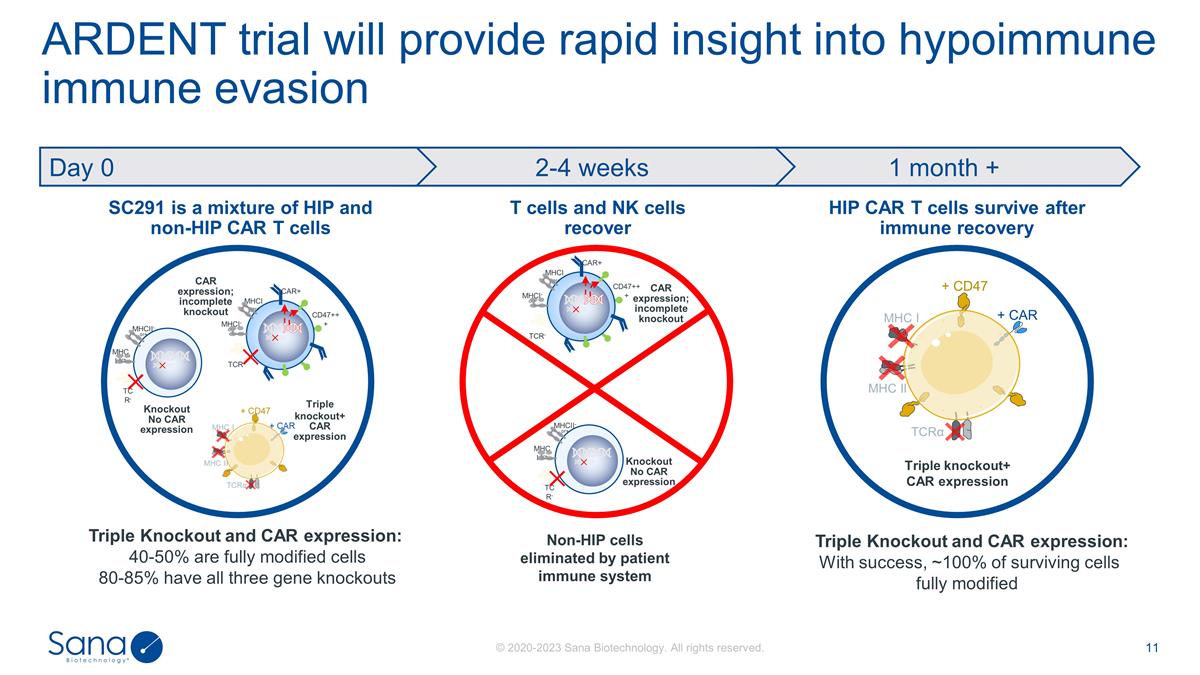
ARDENT trial will provide rapid
insight into hypoimmune immune evasion SC291 is a mixture of HIP and non-HIP CAR T cells HIP CAR T cells survive after immune recovery T cells and NK cells recover Triple Knockout and CAR expression: 40-50% are fully modified cells 80-85% have
all three gene knockouts Non-HIP cells eliminated by patient immune system Triple knockout+ CAR expression CAR expression; incomplete knockout MHCI-or+ TCR- CAR+ CD47+++ MHCII-or+ MHCI-or+ TCR- MHCII-or+ Knockout No CAR expression Triple knockout+
CAR expression MHC I MHC II TCRα + CAR + CD47 1 month + 2-4 weeks Day 0 CAR expression; incomplete knockout MHCI-or+ TCR- CAR+ CD47+++ MHCII-or+ MHCI-or+ TCR- MHCII-or+ Knockout No CAR expression Triple Knockout and CAR expression: With
success, ~100% of surviving cells fully modified
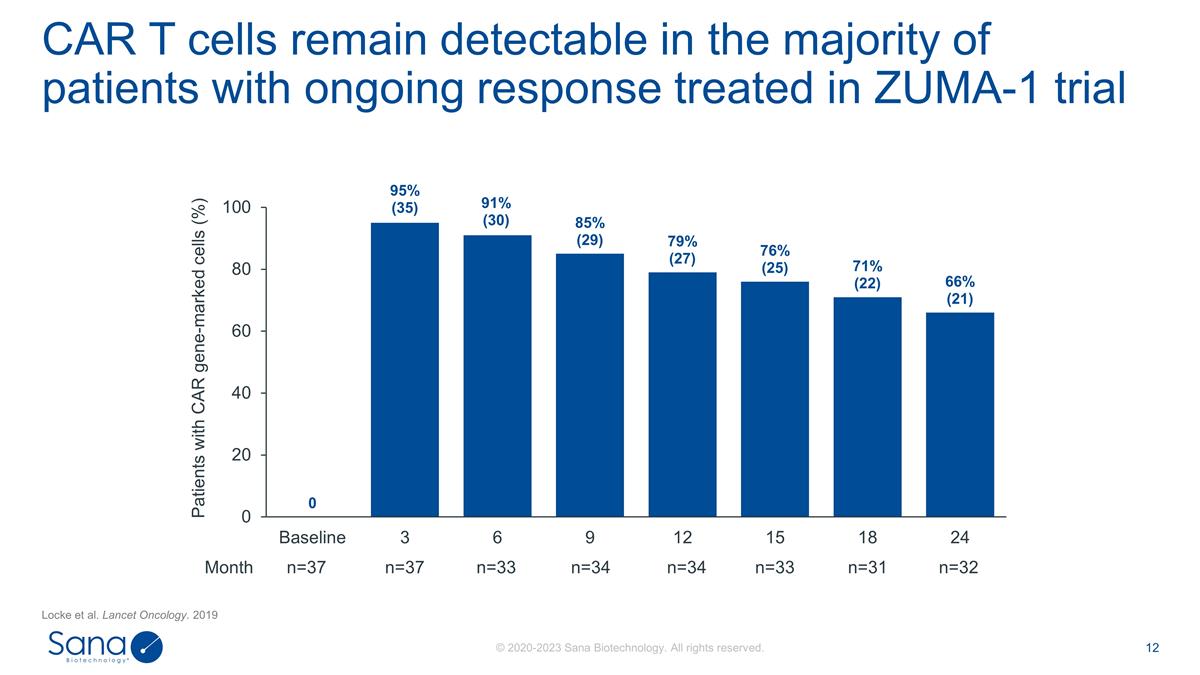
Locke et al. Lancet Oncology. 2019
CAR T cells remain detectable in the majority of patients with ongoing response treated in ZUMA-1 trial Patients with CAR gene-marked cells (%) Month n=37 n=37 n=33 n=34 n=34 n=33 n=31 n=32
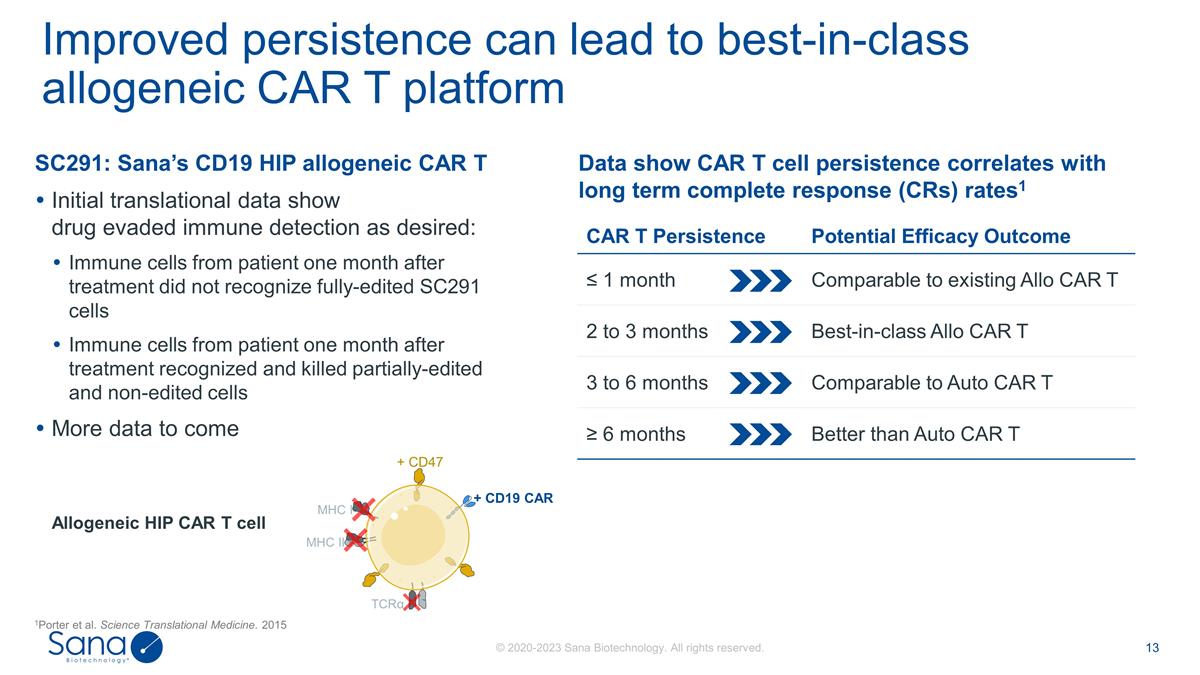
SC291: Sana’s CD19 HIP
allogeneic CAR T Initial translational data show drug evaded immune detection as desired: Immune cells from patient one month after treatment did not recognize fully-edited SC291 cells Immune cells from patient one month after
treatment recognized and killed partially-edited and non-edited cells More data to come Data show CAR T cell persistence correlates with long term complete response (CRs) rates1 Improved persistence can lead to best-in-class allogeneic CAR T
platform CAR T Persistence Potential Efficacy Outcome ≤ 1 month Comparable to existing Allo CAR T 2 to 3 months Best-in-class Allo CAR T 3 to 6 months Comparable to Auto CAR T ≥ 6 months Better than Auto CAR T Allogeneic HIP CAR T cell
MHC I MHC II TCRα + CD19 CAR + CD47 1Porter et al. Science Translational Medicine. 2015
CAR T cells have the potential
to transform autoimmune disorders like they have in blood cancers Depth of B-cell depletion correlates with clinical benefit CD19 CAR T cell therapy results in deep B-cell depletion Potential to deliver durable long-term remissions
SC291 has the scale and potential profile to change patient outcomes Drug product from oncology studies ready for use PoC studies across multiple diseases in near term B-cell targeting validated across multiple autoimmune diseases Adapted from Zhang
et al. Frontiers in Immunology. 2023; Oh et al. Immune Network. 2023; Lee et al. Nature Reviews Drug Discovery. 2021 Field has spent 25+ years identifying Systemic lupus erythematosus (SLE) Lupus Nephritis Vasculitis (Granulomatosis with
polyangiitis & Microscopic polyangiitis) Neuromyelitis optical spectrum Pemphigus Relapsing and progressive MS Rheumatoid Arthritis Sjogren syndrome NMDAR encephalitis Thrombocytopenic purpura Amyloidosis Scleroderma Autoimmune Hemolytic Anemia
Chronic immune demyelinating polyradiculoneuropathy Immune-mediated necrotizing myopathy Membranous nephropathy
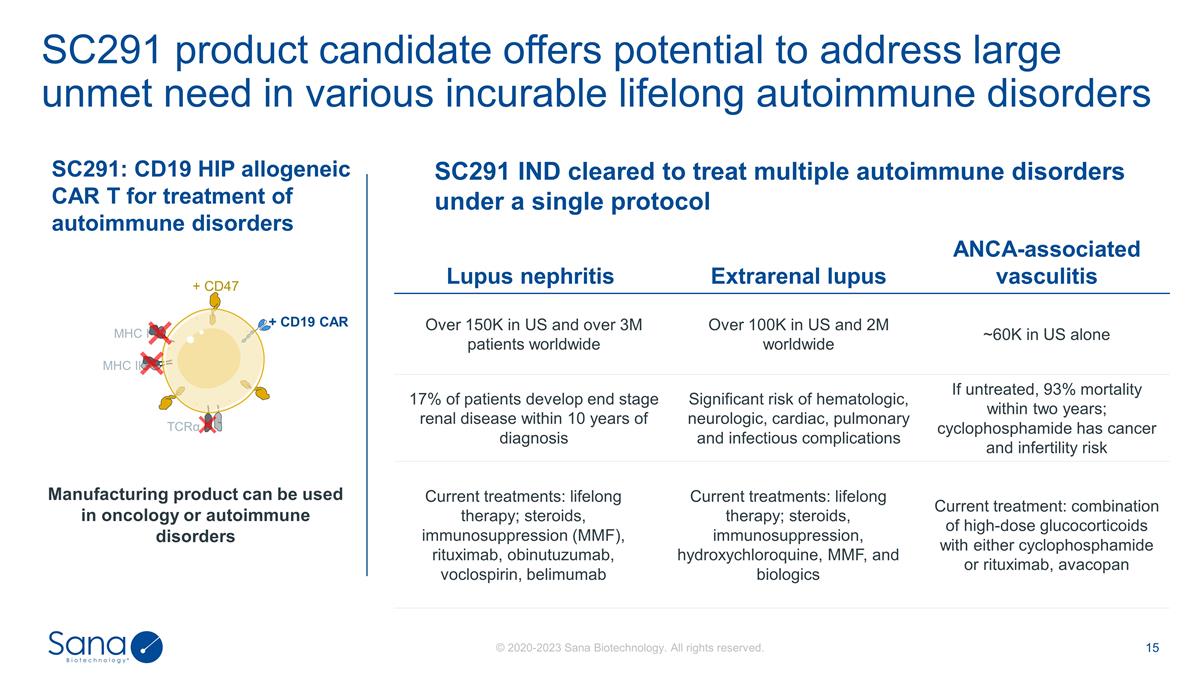
SC291 product candidate offers
potential to address large unmet need in various incurable lifelong autoimmune disorders SC291: CD19 HIP allogeneic CAR T for treatment of autoimmune disorders MHC I MHC II TCRα + CD19 CAR + CD47 SC291 IND cleared to treat multiple
autoimmune disorders under a single protocol Manufacturing product can be used in oncology or autoimmune disorders Lupus nephritis Extrarenal lupus ANCA-associated vasculitis Over 150K in US and over 3M patients worldwide Over 100K in US and
2M worldwide ~60K in US alone 17% of patients develop end stage renal disease within 10 years of diagnosis Significant risk of hematologic, neurologic, cardiac, pulmonary and infectious complications If untreated, 93% mortality within two
years; cyclophosphamide has cancer and infertility risk Current treatments: lifelong therapy; steroids, immunosuppression (MMF), rituximab, obinutuzumab, voclospirin, belimumab Current treatments: lifelong therapy; steroids, immunosuppression,
hydroxychloroquine, MMF, and biologics Current treatments: lifelong therapy, steroids, immunosuppression, and biologics Current treatment: combination of high-dose glucocorticoids with either cyclophosphamide or rituximab, avacopan
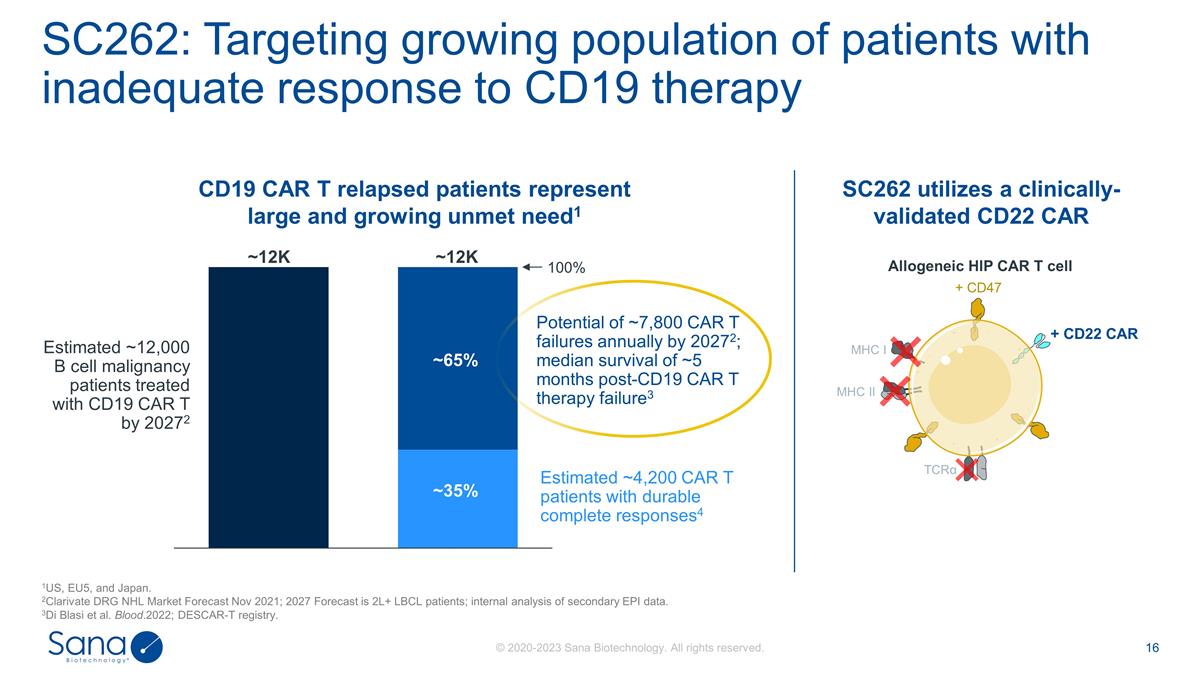
SC262: Targeting growing population
of patients with inadequate response to CD19 therapy 1US, EU5, and Japan. 2Clarivate DRG NHL Market Forecast Nov 2021; 2027 Forecast is 2L+ LBCL patients; internal analysis of secondary EPI data. 3Di Blasi et al. Blood.2022; DESCAR-T registry. ~65%
~35% ~12K ~12K Potential of ~7,800 CAR T failures annually by 20272; median survival of ~5 months post-CD19 CAR T therapy failure3 Estimated ~12,000 B cell malignancy patients treated with CD19 CAR T by 20272 Estimated ~4,200 CAR T patients with
durable complete responses4 Allogeneic HIP CAR T cell CD19 CAR T relapsed patients represent large and growing unmet need1 SC262 utilizes a clinically- validated CD22 CAR MHC I MHC II TCRα + CD47 + CD22 CAR
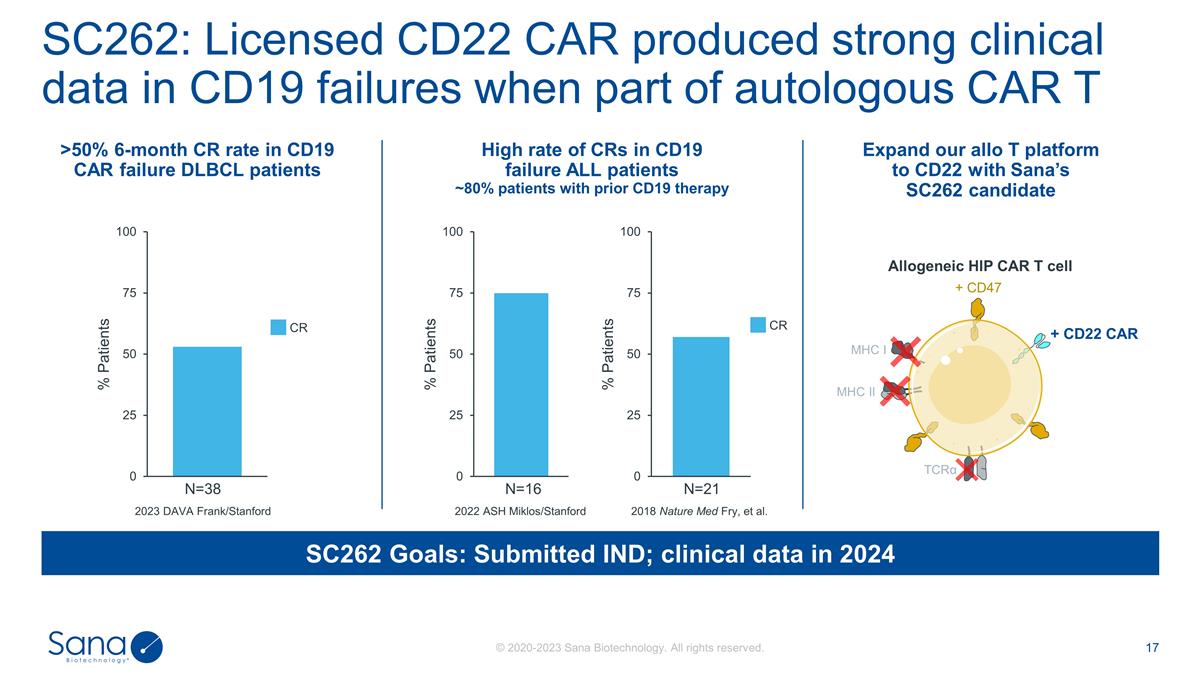
2023 DAVA Frank/Stanford SC262
Goals: Submitted IND; clinical data in 2024 N=38 SC262: Licensed CD22 CAR produced strong clinical data in CD19 failures when part of autologous CAR T N=16 N=21 >50% 6-month CR rate in CD19 CAR failure DLBCL patients High rate of CRs in CD19
failure ALL patients ~80% patients with prior CD19 therapy 2022 ASH Miklos/Stanford 2018 Nature Med Fry, et al. CR CR Expand our allo T platform to CD22 with Sana’s SC262 candidate Allogeneic HIP CAR T cell MHC I MHC II + CD47 + CD22 CAR
TCRα
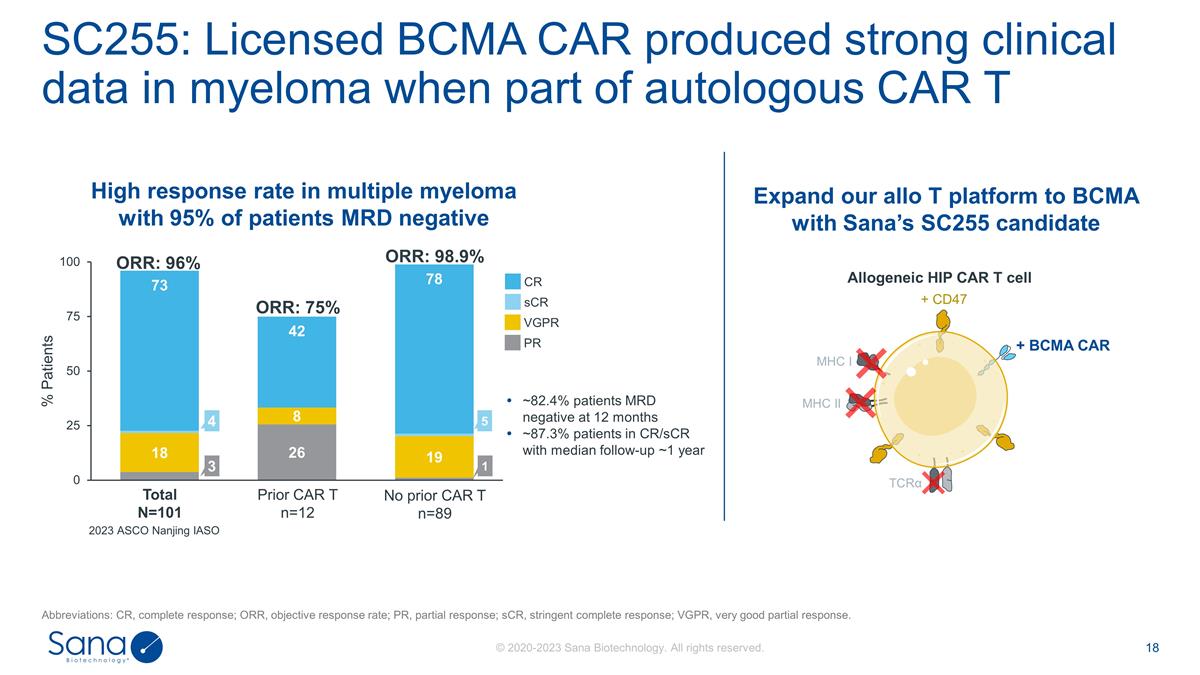
SC255: Licensed BCMA CAR produced
strong clinical data in myeloma when part of autologous CAR T ORR: 98.9% ORR: 96% ORR: % Total N=101 sCR CR PR VGPR Prior CAR T n=12 No prior CAR T n=89 1 ~82.4% patients MRD negative at 12 months ~87.3% patients in CR/sCR with median follow-up ~1
year 2023 ASCO Nanjing IASO Expand our allo T platform to BCMA with Sana’s SC255 candidate High response rate in multiple myeloma with 95% of patients MRD negative Allogeneic HIP CAR T cell Abbreviations: CR, complete response; ORR, objective
response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response. MHC I MHC II TCRα + BCMA CAR + CD47

Disease caused by autoimmune
destruction of insulin-producing beta cells in the pancreas; results in inability to control blood glucose Type 1 diabetes is a large unmet need with 1.9M patients in the U.S. and 2.4M in Europe2 Long-term complications: end-organ damage, including
heart attack, stroke, blindness, and kidney failure SC451 goal is euglycemia without exogenous insulin or immunosuppression Type 1 diabetes represents a large unmet need with a loss of ~15 years of life1 1Rawshani et al. Lancet. 2018 2Clarivate Type
1 Diabetes Landscape & Forecast, December 2022; internal analysis of secondary EPI data. DIGICOMPHOTO/SCIENCE PHOTO LIBRARY
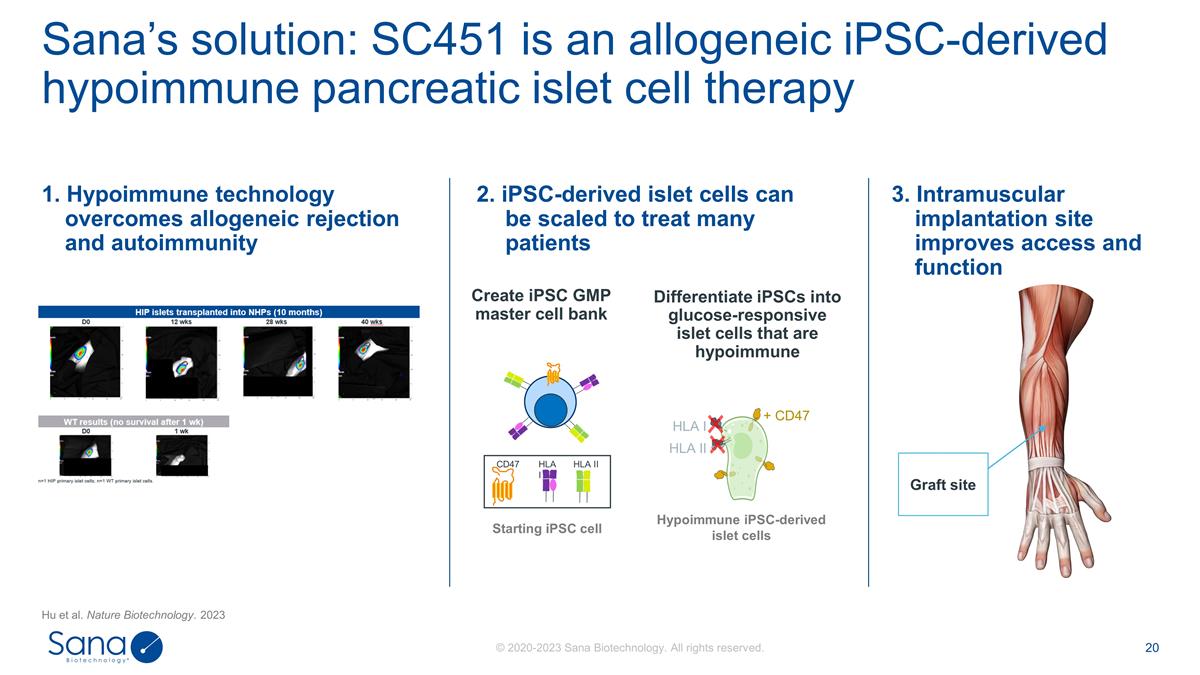
Sana’s solution: SC451 is an
allogeneic iPSC-derived hypoimmune pancreatic islet cell therapy Hu et al. Nature Biotechnology. 2023 Differentiate iPSCs into glucose-responsive islet cells that are hypoimmune + CD47 HLA I HLA II Hypoimmune iPSC-derived islet cells Create iPSC GMP
master cell bank Starting iPSC cell CD47 HLA I HLA II 1. Hypoimmune technology overcomes allogeneic rejection and autoimmunity 3. Intramuscular implantation site improves access and function 2. iPSC-derived islet cells can be scaled to treat many
patients Graft site
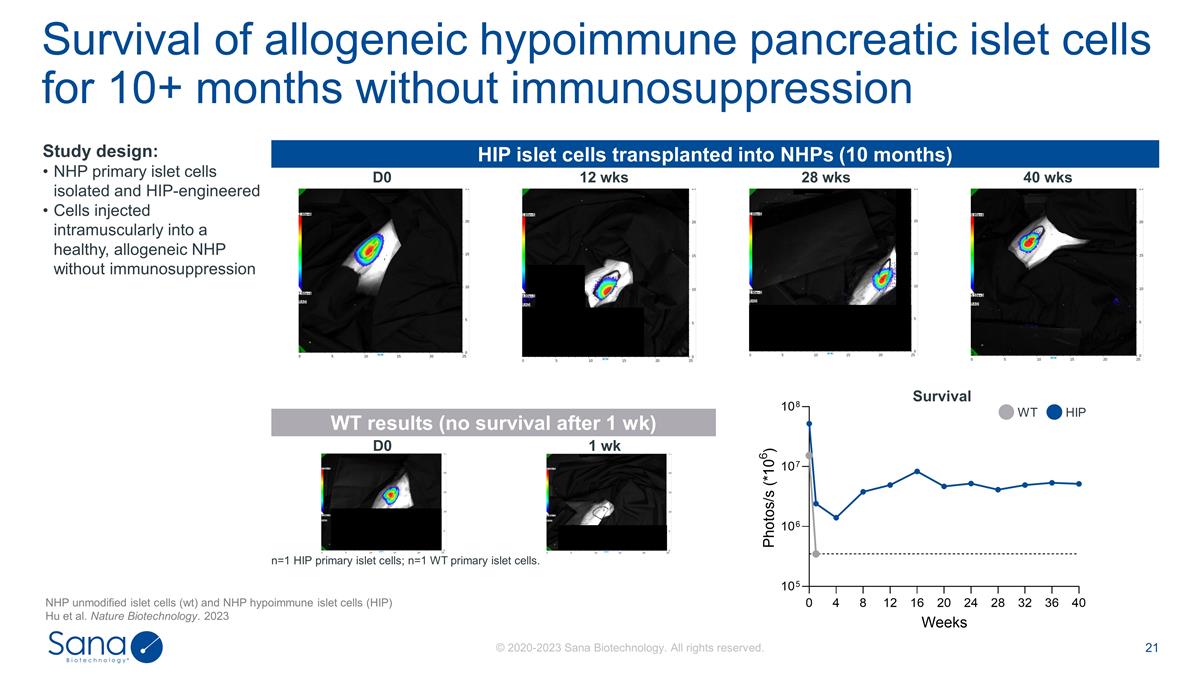
HIP islet cells transplanted into
NHPs (10 months) D0 12 wks 28 wks 40 wks WT results (no survival after 1 wk) D0 1 wk n=1 HIP primary islet cells; n=1 WT primary islet cells. Survival of allogeneic hypoimmune pancreatic islet cells for 10+ months without immunosuppression Study
design: NHP primary islet cells isolated and HIP-engineered Cells injected intramuscularly into a healthy, allogeneic NHP without immunosuppression WT HIP Survival NHP unmodified islet cells (wt) and NHP hypoimmune islet cells (HIP) Hu et al.
Nature Biotechnology. 2023
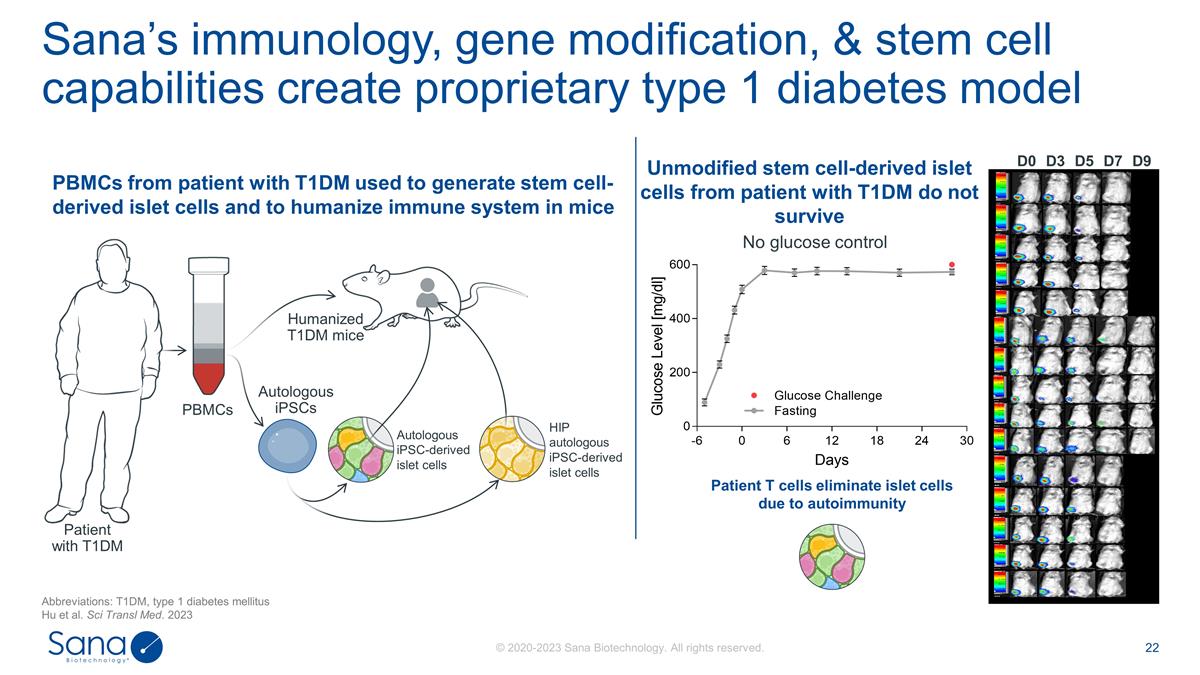
D0 D3 D7 D5 D9 No glucose control
Patient T cells eliminate islet cells due to autoimmunity Sana’s immunology, gene modification, & stem cell capabilities create proprietary type 1 diabetes model Abbreviations: T1DM, type 1 diabetes mellitus Hu et al. Sci Transl Med. 2023
Patient with T1DM PBMCs Autologous iPSC-derived islet cells Humanized T1DM mice Autologous iPSCs HIP autologous iPSC-derived islet cells Unmodified stem cell-derived islet cells from patient with T1DM do not survive PBMCs from patient with T1DM used
to generate stem cell-derived islet cells and to humanize immune system in mice
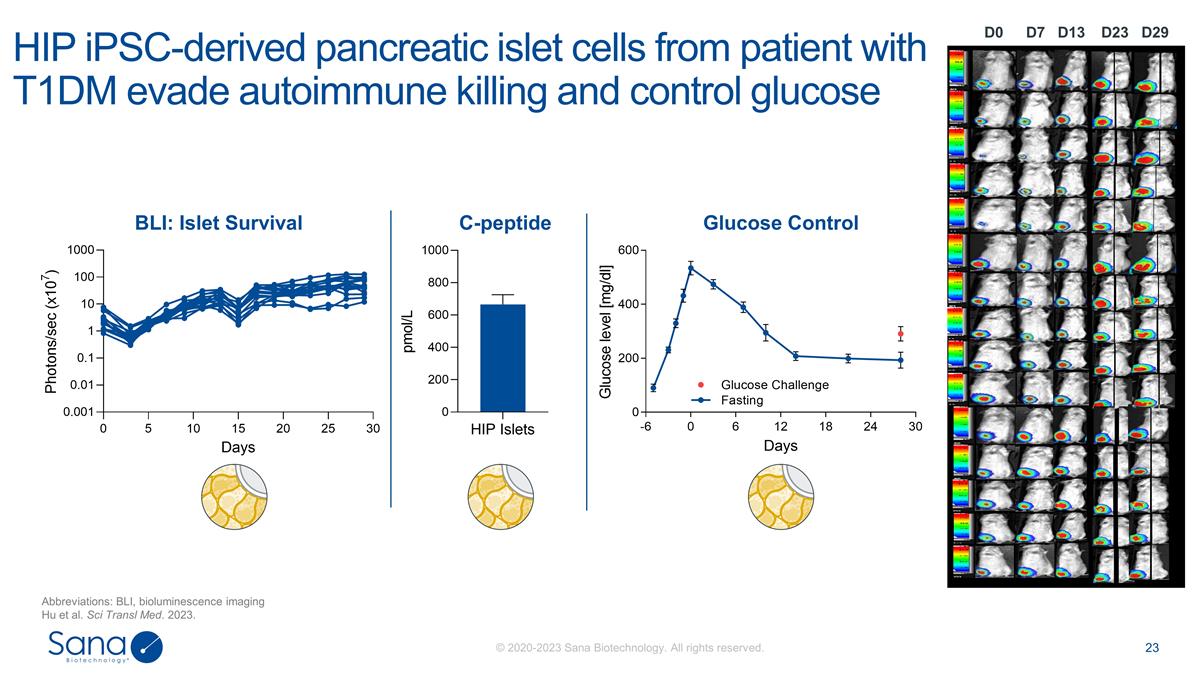
D7 D0 D23 D29 D13 HIP iPSC-derived
pancreatic islet cells from patient with T1DM evade autoimmune killing and control glucose Abbreviations: BLI, bioluminescence imaging Hu et al. Sci Transl Med. 2023. BLI: Islet Survival C-peptide Glucose Control
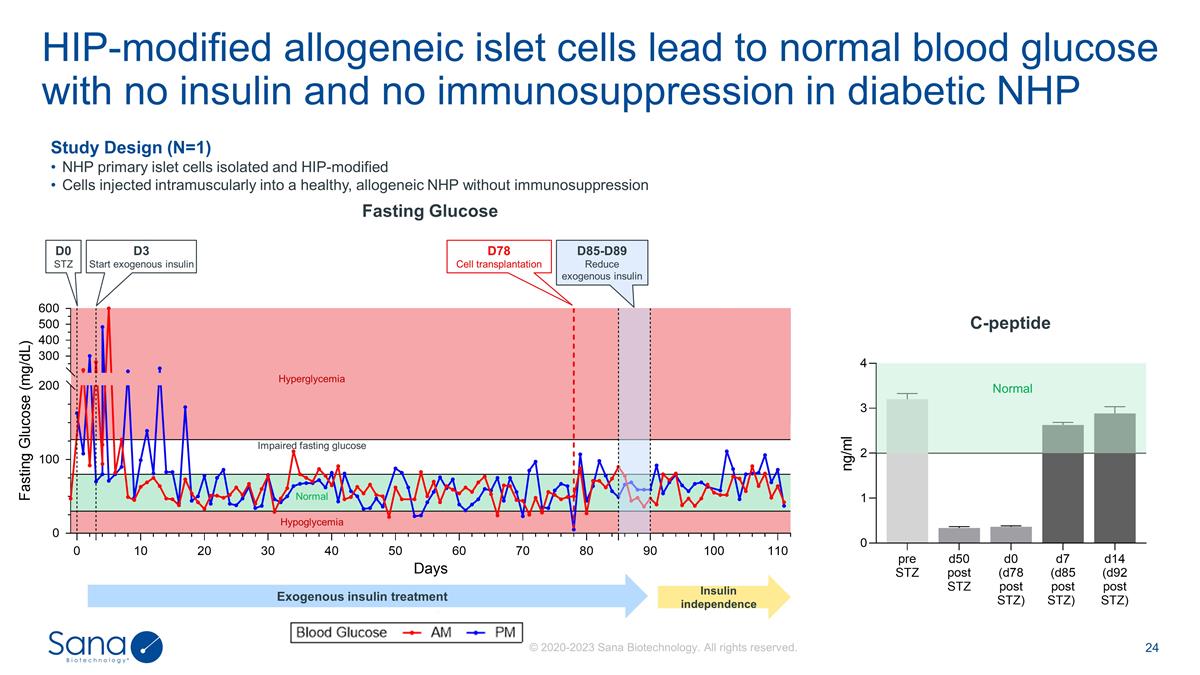
HIP-modified allogeneic islet cells
lead to normal blood glucose with no insulin and no immunosuppression in diabetic NHP Normal Hypoglycemia Impaired fasting glucose Hyperglycemia D78 Cell transplantation D85-D89 Reduce exogenous insulin D0 STZ D3 Start exogenous insulin Study Design
(N=1) NHP primary islet cells isolated and HIP-modified Cells injected intramuscularly into a healthy, allogeneic NHP without immunosuppression C-peptide Fasting Glucose Normal Exogenous insulin treatment Insulin independence © 2020-2023
Sana Biotechnology. All rights reserved.
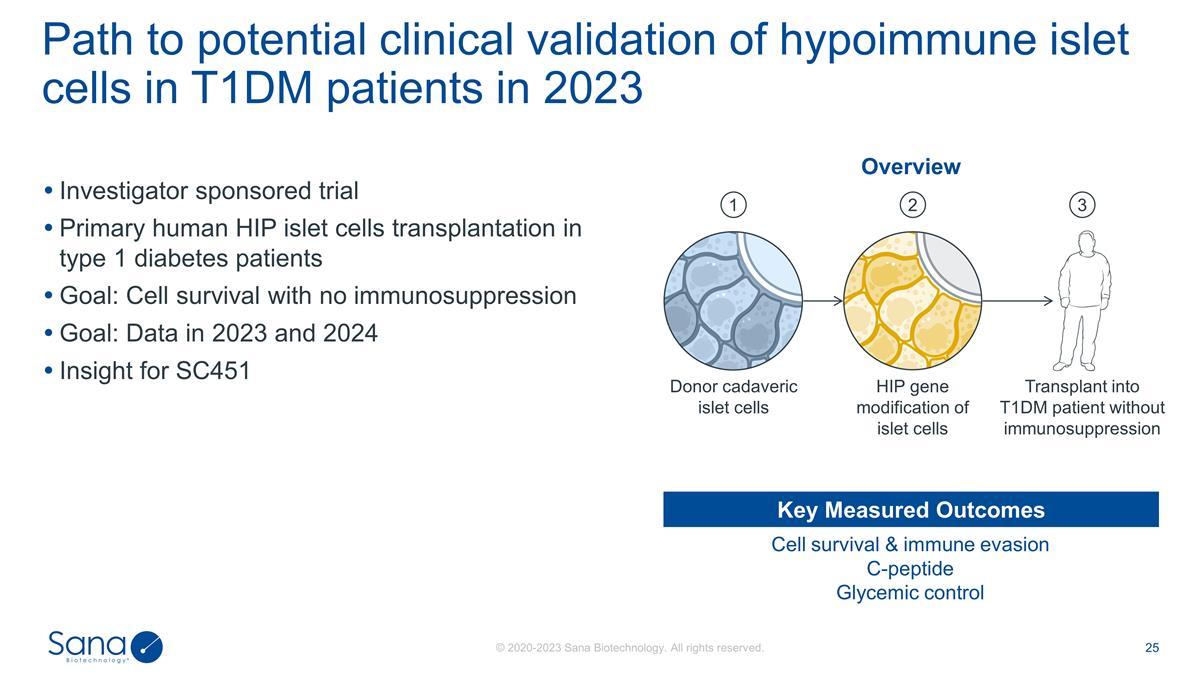
Investigator sponsored trial
Primary human HIP islet cells transplantation in type 1 diabetes patients Goal: Cell survival with no immunosuppression Goal: Data in 2023 and 2024 Insight for SC451 Path to potential clinical validation of hypoimmune islet cells in T1DM patients in
2023 Cell survival & immune evasion C-peptide Glycemic control Key Measured Outcomes Overview Transplant into T1DM patient without immunosuppression HIP gene modification of islet cells Donor cadaveric islet cells 1 2 3
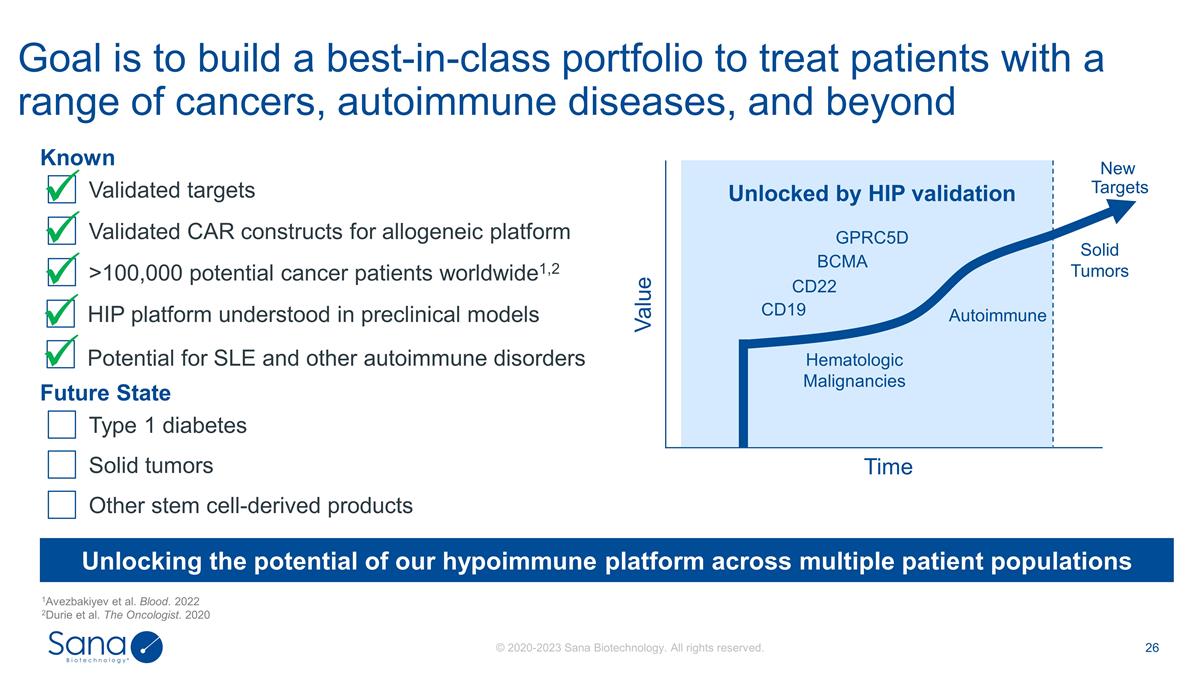
Goal is to build a best-in-class
portfolio to treat patients with a range of cancers, autoimmune diseases, and beyond 1Avezbakiyev et al. Blood. 2022 2Durie et al. The Oncologist. 2020 Unlocking the potential of our hypoimmune platform across multiple patient populations Value Time
CD19 CD22 BCMA Autoimmune Unlocked by HIP validation Solid Tumors New Targets Hematologic Malignancies GPRC5D Known ü >100,000 potential cancer patients worldwide1,2 Future State Validated CAR constructs for allogeneic platform ü
Validated targets ü Type 1 diabetes Solid tumors ü HIP platform understood in preclinical models Other stem cell-derived products Potential for SLE and other autoimmune disorders ü
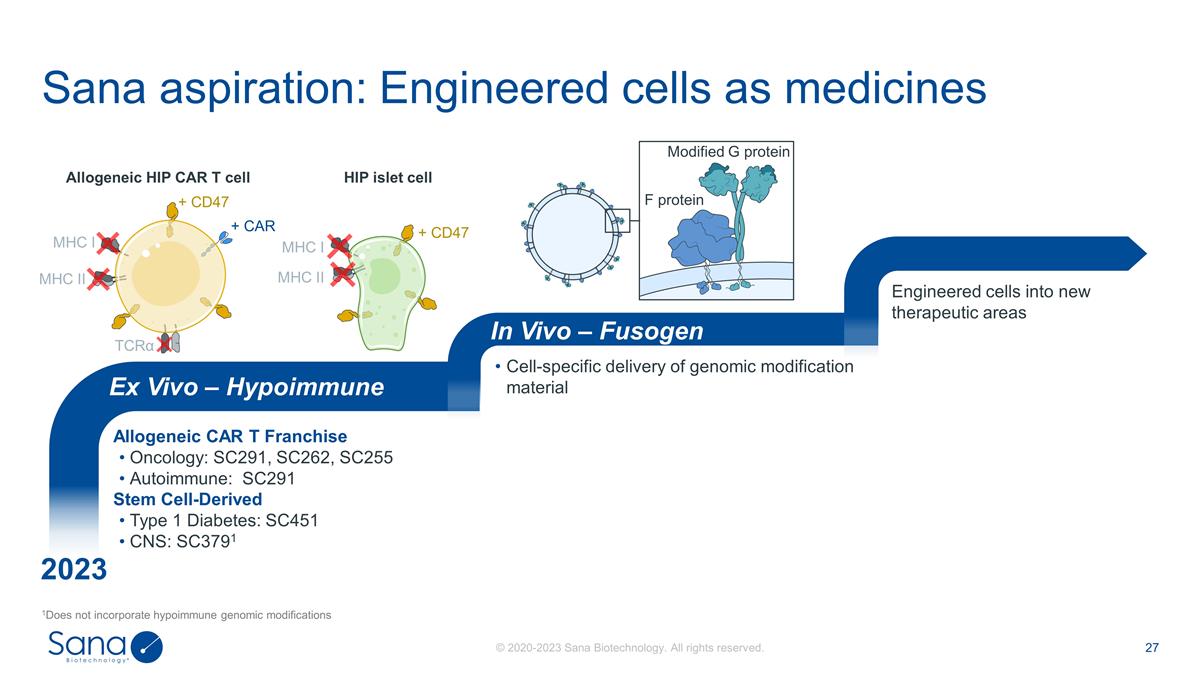
Sana aspiration: Engineered cells
as medicines 1Does not incorporate hypoimmune genomic modifications Allogeneic CAR T Franchise Oncology: SC291, SC262, SC255 Autoimmune: SC291 Stem Cell-Derived Type 1 Diabetes: SC451 CNS: SC3791 Cell-specific delivery of genomic modification
material Engineered cells into new therapeutic areas Ex Vivo – Hypoimmune In Vivo – Fusogen + CD47 MHC I MHC II Allogeneic HIP CAR T cell HIP islet cell Modified G protein F protein 2023 MHC I MHC II TCRα + CAR + CD47

v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Sana Biotechnology (NASDAQ:SANA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Sana Biotechnology (NASDAQ:SANA)
Historical Stock Chart
From Apr 2023 to Apr 2024