As filed with the Securities and Exchange Commission on May 23, 2024
Registration No. 333-[●]
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
Nexalin Technology, Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
|
3845 |
|
27-5566468 |
(State or other jurisdiction of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification No.) |
1776 Yorktown, Suite 550
Houston, TX 77056
(832) 260-0222
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Mark White
Chief Executive Officer
Nexalin Technology, Inc.
1776 Yorktown, Suite 550
Houston, TX 77056
(832) 260-0222
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Martin S. Siegel, Esq.
Warshaw Burstein, LLP
575 Lexington Avenue
New York, NY 10022
Telephone: (212) 984-7741 |
|
Andrew M. Tucker, Esq.
Nelson Mullins Riley & Scarborough LLP
101 Constitution Avenue NW, Suite 900
Washington, D.C. 20001
Telephone: (202) 689-2987 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| |
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☒
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is declared effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 23, 2024
Preliminary PROSPECTUS
NEXALIN TECHNOLOGY, INC.

Up to $7,000,000 of
Common Stock
We are offering on a reasonable best effort basis up to $7,000,000 of our common stock, $0.001 par value per share. We are offering each share of common stock at an assumed public offering price of $[●] per share.
The securities will be offered at a fixed price and are expected to be issued in a single closing. The offering will terminate on [●], 2024, unless completed sooner or unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We expect this offering to be completed not later than two business days following the commencement of sales in this offering (after the effective date of the registration statement of which this prospectus forms a part) and we will deliver all securities to be issued in connection with this offering delivery versus payment or receipt versus payment, as the case may be, upon receipt of investor funds received by us. Accordingly, neither we nor the Placement Agent (as defined below) have made any arrangements to place investor funds in an escrow account or trust account since the Placement Agent will not receive investor funds in connection with the sale of the securities offered hereunder.
We have engaged Maxim Group LLC (the “Placement Agent” or “Maxim”), to act as our exclusive placement agent in connection with this offering. The Placement Agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The Placement Agent is not purchasing or selling any of the securities we are offering and the Placement Agent is not required to arrange the purchase or sale of any specific number of securities or dollar amount. We have agreed to pay to the Placement Agent the Placement Agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus. Because we will deliver the securities to be issued in this offering upon our receipt of investor funds, there is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum offering requirement as a condition of closing of this offering. We may sell fewer than all of the shares of common stock offered hereby, which may significantly reduce the amount of proceeds received by us. Because there is no escrow account and no minimum number of securities or amount of proceeds, investors could be in a position where they have invested in us, but we have not raised sufficient proceeds in this offering to adequately fund the intended uses of the proceeds as described in this prospectus. See “Risk Factors” for more information regarding risks related to this offering. We will bear all costs associated with the offering. See “Plan of Distribution” for more information regarding these arrangements.
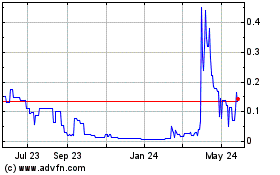
Our common stock is currently listed on the Nasdaq Capital Market under the symbol “NXL.” The last reported sale price of our common stock on the Nasdaq Capital Market on May 22, 2024, was $1.14 per share.
We are an “emerging growth company” under the federal securities laws and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. See the section titled “Prospectus Summary -- Implications of Being an Emerging Growth Company and a Smaller Reporting Company.”
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Investing in our securities involves a high degree of risk. Before buying any shares, you should carefully read the discussion of the material risks of investing in our securities under the heading “Risk Factors” beginning on page 17 of this prospectus.
Neither the Securities and Exchange Commission, any state securities commission, nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The following table sets forth the expected proceeds from this offering, assuming an offering price at the minimum of the proposed price range of the shares of our common stock:
| |
|
Proposed
Maximum
Offering
Price Per
Share |
|
|
Total |
|
| Public offering price |
|
$ |
|
|
|
$ |
|
|
| Placement agent fees(1) |
|
$ |
|
|
|
$ |
|
|
| Proceeds, before expenses, to us |
|
$ |
|
|
|
$ |
|
|
|
(1) |
See “Plan of Distribution” for a description of the compensation payable to the Placement Agent. |
Maxim Group LLC
The date of this prospectus is May 23, 2024
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment. Neither we, nor the Placement Agent, have authorized any other person to provide you with information that is different from, or adds to, that contained in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. Neither we nor the Placement Agent take responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of any securities in any jurisdiction in which such offer is unlawful.
No action is being taken in any jurisdiction outside the United States to permit a public offering of our securities or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this public offering and the distribution of this prospectus applicable to that jurisdiction.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-1 that we filed with the Securities and Exchange Commission (“SEC”). It omits some of the information contained in the registration statement and reference is made to the registration statement for further information with regard to us and the securities being offered hereby. You should review the information and exhibits in the registration statement for further information about us and the securities being offered hereby. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to the filings. You should review the complete document to evaluate these statements.
In this prospectus, unless the context requires otherwise, references to “we,” “us,” “our,” “Nexalin” or the “Company” refer to Nexalin Technology, Inc. and, where appropriate, its subsidiaries. Additionally, references to the “Board” refer to the board of directors of Nexalin Technology, Inc.
We have proprietary rights
to a number of trademarks and tradenames used in this prospectus which are important to our business, including Nexalin®
and the Nexalin logo. Solely for convenience, trademarks, service marks and tradenames referred to in this prospectus may appear without
the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the
fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks, service marks and tradenames.
This prospectus may also contain trademarks, service marks, tradenames and copyrights of other companies, which are the property of their
respective owners.
We have not, and the Placement Agent and its affiliates have not, authorized anyone to provide you with any information or to make any representation not contained or incorporated by reference in this prospectus or any related free writing prospectus. We do not, and the Placement Agent and its affiliates do not, take any responsibility for, and can provide no assurance as to the reliability of, any information that others may provide to you. This prospectus is not an offer to sell or an offer to buy securities in any jurisdiction where offers and sales are not permitted. The information in this prospectus is accurate only as of its date, regardless of the time of delivery of this prospectus or any sale of securities. You should also read and consider the information in the documents to which we have referred you under the caption “Where You Can Find More Information” in the prospectus.
PROSPECTUS SUMMARY
This summary
highlights information contained in greater detail elsewhere in this prospectus and does not contain all the information that you
should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire
prospectus including the “Risk Factors,” “Special Note Regarding Forward-Looking Statements and Industry
Data” included in this prospectus, “Management’s Discussion and Analysis of Financial Condition and Results of
Operations” in our Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the SEC on
March 27, 2024 (the “2023 Form 10-K”), as supplemented by our Quarterly Reports on Form 10-Q, and as may
be further amended, supplemented or superseded from time to time by our subsequent filings under the Exchange Act which are
incorporated herein by reference, and the financial statements and the notes to those financial statements.
Company Overview
Overview
We design and develop innovative neurostimulation products to uniquely and effectively help combat the ongoing global mental health epidemic. We developed an easy-to-administer medical device — referred to as “Generation 1” or “Gen-1” — that utilizes bioelectronic medical technology to treat anxiety and insomnia, without the need for drugs or psychotherapy. Our original Gen-1 devices are cranial electrotherapy stimulation (CES) devices that emit waveform at 4 milliamps during treatment and are presently classified by the U.S. Food and Drug Administration (the “FDA”) as a Class II device.
Medical professionals in the United States have utilized the Gen-1 device to administer to patients in clinical settings. While the Gen-1 device had been cleared by the FDA to treat depression, anxiety, and insomnia, three prevalent and serious diseases, because of the FDA’s December 2019 reclassification of CES devices, the Gen-1 device was reclassified as a Class II device for the treatment of anxiety and insomnia. We are required to file a new application under Section 510(k) of the Federal Food, Drug and Cosmetic Act (“510(k) Application”) to be approved by the FDA for the sales and marketing of our devices for the treatment of anxiety and insomnia. In the FDA’s December 2019 reclassification ruling, the treatment of depression with our device will require a Class III certification and require a new PMA (premarket approval) application to demonstrate safety and effectiveness.
While we continue providing services to medical professionals to support patients’ use of the Gen-1 devices which were in operation prior to December 2019, we are not making new sales or new marketing efforts of Gen-1 devices in the United States. We continue to derive revenue from devices which we sold or leased prior to the FDA’s December 2019 reclassification announcements. This revenue consists of monthly licensing fees and payments for the sale of electrodes and patient cables. We have suspended marketing efforts for new sales of devices related to the Gen-1 device for treatment of anxiety and insomnia in the United States until the Nexalin regulatory team decides on a new 510(k) application at 4 milliamps based on FDA comments expected to be received in late 2024. Our regulatory team continues to inform the FDA of the suspension of the marketing and sale of the Gen-1 products to new providers. We are currently analyzing whether to proceed with an amended application with the FDA for Gen-1 devices for the treatment of insomnia and anxiety.
The waveform that comprises the basis of Gen-2 and new Gen-3 headset devices is in pre-submission for review by the FDA for safety evaluation and eventual marketing in the United States. Determinations of the safety and efficacy of our devices in the United States are solely within the authority of the FDA. We plan to conduct decentralized clinical trials for the Gen-3 device in the U.S. and we continue to consult with the FDA as part of the pre-submission meetings.
Our Technology
We have designed and
developed a new advanced waveform technology to be emitted at 15 milliamps through new and improved medical devices referred to as
“Generation 2” or “Gen-2” and “Generation 3” or “Gen-3.” Gen-2 is a clinical use
device with a modern enclosure to emit the new 15 milliamp advanced waveform. Gen-3 is a new patient headset that will be prescribed
by licensed medical professionals in a virtual clinic setting similar to existing tele-health platforms. The Nexalin research team
believes that the new 15 milliamp Gen-2 and Gen-3 devices can penetrate deeper into the brain and stimulate associated structures of
mental illness, which we believe will generate enhanced patient response without any risk or unpleasant side effects. The Nexalin
regulatory team has made a strategic decision to develop strategies for pilot trials and/or pivotal trials in various mental health
disease states. In addition, a new PMA application in the United States is in strategic development for the treatment of depression
utilizing both Gen-2 and Gen-3. We plan to schedule additional pilot trials and/or pivotal trials for the new Gen-3 device for
anxiety and insomnia in the United States and China beginning in the late third quarter or early fourth quarter of 2024. Preliminary
data provided by The University of California, San Diego and recent published data from Asia supports the safety of utilizing our 15
milliamp waveform technology. However, the determination of safety and efficacy of medical devices in the United States is subject
to clearance by the FDA.
Currently, the waveform that comprises the basis of Gen-2 and new Gen-3 headset devices has been tested in research settings to develop safety data that has been submitted for review by the FDA for safety evaluation and eventual marketing in the United States and around the world. Determinations of the safety and efficacy of our devices in the United States are solely within the authority of the FDA.
A new pre-submission document in preparation of a new 510(k) and/or de novo application for our Gen-3 Halo headset at 15 milliamps was filed with the FDA in January of 2023. Formal comments to our pre-submission document filing were received in March of 2023. A formal meeting to address FDA comments took place on May 9, 2023.
A second FDA pre-submission document was submitted on February 13, 2024. FDA comments to this second pre-submission document were received on April 26, 2024. A formal teleconference was held with the FDA on April 30, 2024. The Nexalin regulatory team and the FDA came to a consensus on the Anxiety and Insomnia Clinical research protocols.
In part due to increasing incidence attributed to the devastating impacts of the COVID-19 pandemic, mental health and cognitive disorders are widespread across the globe and cause substantial health, social and economic losses, and hardships accordingly. Our focus is on the continued development of our innovative bioelectronic medical technologies and rapid regulatory approval. We intend to help reverse these losses, and hardships of these losses, by safely and effectively treating various mental health disorders associated with post Covid and long Covid mental disease states.
All our products are
non-invasive, undetectable to the human body and are designed to provide relief to those afflicted with mental health issues without
adverse side effects. We have a proprietary design that stabilizes currents, electromagnetic fields, and various frequencies —
referred to collectively as a waveform - particularly our proprietary, 15 milliamp patented waveform. Additionally, our devices
generate a proprietary high frequency carrier wave for deeper penetration into the brain. It is applied to the brain with an array
of electrodes on the forehead and behind each ear at the mastoid. The features of this proprietary waveform and the array of
electrodes allow the application of the waveform to the entire brain rather than a small, targeted area of the brain. To ensure
deeper penetration into the brain, we have created a waveform that is undetectable to the brain which allows the increase of the
power from < 4 mAmps to 15 mAmps, more than a 400% increase without incurring any patient discomfort, risk, or adverse side
effects. By increasing the power, our waveform can penetrate deeper into the brain and stimulate deep mid-brain structures
associated with mental illness. Our research data and clinical teams believe that a more powerful waveform will create a stronger
response in the brain. A stronger response creates a higher level of efficacy. This entire proprietary technique allows Nexalin to
provide a non-invasive and comfortable treatment, which we believe is more powerful than any FDA-cleared stimulation device in the
market. Current pilot study protocols and randomized clinical trials have been designed and submitted to the FDA to provide feedback
on final reports and data sets for the purpose of safety and efficacy evaluations in the future. Determinations of the safety and
efficacy of our devices are solely within the authority of the FDA.
We recognize that an additional barrier to treatment in today’s mental health treatment landscape — beyond the concerns about safety, efficacy and side-effects that have been associated with conventional mental health treatments such as ECT (shock therapy), drugs and psychotherapy is stigma. Industry reports and feedback indicate that many patients that struggle with mood disorders have the stigma of embarrassment associated with psychiatrists and psychotherapy (e.g., counselling with a therapist). Additional stigmas and other issues are associated with the side effects of medication prescribed by psychiatrists. When we researched the current pharmaceuticals model, public information highlighted the many side effects associated with such medications. Frequently, patients would stop taking the medication because of the uncomfortable side effects. Additional public information mentions dependency and withdrawal issues associated with medication for psychiatric disorders.
To address the embarrassment stigma, we are developing a new virtual clinic that will allow the physician to diagnose a mental health issue in the privacy of a tele-psychiatry virtual platform. After diagnosis, the physician will prescribe the Nexalin Gen-3 headset to the patient for treatment. Next, the Gen-3 device will be shipped to the patient’s home. After the patient receives the device, they will pair the headset device with an app in the patient’s smart phone. The app will communicate with the Nexalin cloud servers to authorize the device for treatment according to the protocol designed by the physician. The physician will monitor treatment compliance and other health related issues in a private physician dashboard that connects through the Nexalin app and cloud servers. We believe that to preserve product safety and integrity for home use, the headset device will require physician oversight that will include a prescription for use with a monthly authorization provided by the physician after a monthly virtual visit. All appointments will be in a virtual setting to provide privacy and convenience for the physician and patient. The Nexalin virtual clinic will be provided in a proprietary virtual platform currently in the design stage.
Our China Gen-2 15 milliamp device was approved in China by the NMPA for the treatment of insomnia and depression in China. This device and all other clinical devices will include a single use electrode for long term revenue streams. The USA Gen-2 device bears a fresh and modern appearance that meets the technology standards of the digital tech world of 2024. Early adopters of the Gen-1 device will be able to access additional firmware upgrades which are planned to enhance the previously purchased devices to the new symmetric15-milliamp waveform. Our Gen-2 device will be equipped with RFID technology that exchanges electrode usage data with a reader in the main device. The purpose of RFID is to track and maintain control of the proprietary single use electrode. Our electrode chip will be programmed to exchange data with the device and allow activation for a single treatment with a new electrode only. This ensures a recurring revenue stream on the device and protects against any generic knockoffs designed to avoid treatment costs. This upgrade in technology also ensures the proprietary nature of the electrodes that support treatment outcomes are sustained.
Overall, we believe that our advanced waveform, technological upgrades and the development of a modern headset monitored with our IT management platform will position us with the opportunity to disrupt the traditional mental health treatment model. Our mission is to remove the stigma of expensive psychotherapy or pharmaceuticals with the attendant side effects and dependency issues and replace such stigma with clinically proven and cost-effective technology that is easily accessible in the privacy of the patient’s home and monitored by licensed healthcare providers.
Formalized Joint Venture; China Related Activities
On May 31, 2023, the Company formalized an agreement related to the formation of a joint venture established to engage in the clinical development, marketing, sale and distribution of Nexalin’s second generation transcranial Alternating Current Stimulation (“tACS”) devices (“Gen-2 devices”) in China and other countries in the region. The Joint Venture is registered in Hong Kong.
As of the date of this prospectus”, (i) our operations are carried on outside of China; and (ii) the Joint Venture does not maintain any variable interest entity structure or operate any data center in China.
Under the Joint Venture Agreement, Wider Come Limited (“Wider”), a related party, is obligated to fund all operations for the initial 12-month period of the Joint Venture, after which Nexalin and Wider plan to jointly fund the Joint Venture’s operating expenses in accordance with their pro rata ownership.
The Joint Venture is controlled by a Board of Directors in which Wider is to have sole representation but neither the Company nor Wider has exclusive decision-making ability over day-to-day or significant operational decisions. Wider and Nexalin own 52% and 48% of the Joint Venture, respectively. In accordance with ASC 323 Investments - Equity Method and Joint Ventures (“ASC 323”) and ASC 810 - Consolidations (“ASC 810”), the Company recognized $5,783 of equity method investment income from the Joint Venture on a one-quarter reporting lag for the three months ended March 31, 2024, on the condensed consolidated statements of operations and comprehensive loss.
The investment in the Joint Venture is accounted for using the equity method of accounting. As of March 31, 2024 and December 31, 2023 the Company had an Equity Method Investment of $101,783 and $96,000, respectively, recorded on the condensed consolidated balance sheets. The Company invested $96,000 in the joint venture in September 2023 which is recorded on the consolidated balance sheet at December 31, 2023 as an Equity Method Investment. Wider invested $104,000. In accordance with ASC 323, the Company uses the equity method of accounting for its investment in the Joint Venture, an unconsolidated entity over which it does not have a controlling interest. The equity method of accounting requires the investment to be initially recorded at cost and subsequently adjusted for the Company’s share of equity in the unconsolidated entity’s earnings or losses. The Company evaluates the carrying amount of this investment in the Joint Venture for impairment in accordance with ASC 323. If the Company determines that a loss in the value of the investment is other than temporary, the Company writes down the investment to its estimated fair value. Any such losses are recorded to equity in income of unconsolidated entities in the Company’s consolidated statements of operations and comprehensive loss. The Company has made an election to classify distributions received from the Joint Venture using the nature of the distribution approach. Distributions received are classified as cash inflows from operating activities based on the nature of the activities of the unconsolidated entity.
Under the preceding terms of the collaborative arrangement between the Company and Wider, Wider served as an authorized distributor of the Company’s Gen-2 devices in Asia. As part of the consideration for Wider’s performance of its obligations to the Company prior to the formalization of the Joint Venture, the Company and certain designated Wider shareholders entered into stock issuance agreements for the issuance of 450,000 shares of the Company’s common stock, and simultaneously with the execution of this service agreement, Wider invested $200,000 to the Company. During the year ended December 31, 2020, the Company issued 150,000 shares to affiliates of Wider in satisfaction of the obligation. Under the terms of the collaborative agreement, designated shareholders of Wider are entitled to an additional 300,000 shares upon Wider’s achievement of certain milestones. The fair value of the 150,000 shares issued during the year ended December 31, 2020 (less the invested $200,000 in cash) resulted in a charge to stock-based compensation of $550,000 and was recorded in selling, general and administrative expenses on the consolidated statement of operations and comprehensive loss. During the twelve months ended December 31, 2023, the Company issued an additional 150,000 shares to affiliates of Wider in satisfaction of obligations pursuant to the collaborative agreement and also recognized its obligation to issue an additional 150,000 shares. The grant date fair value of the 300,000 shares issued and to be issued resulted in a charge to research and development of $1,500,000 and was recorded in selling, general and administrative expenses on the consolidated statement of operations and comprehensive loss.
In September of 2021, the NMPA approved the Gen-2 device for marketing and sale in China for the treatment of insomnia and depression. These treatment indications and clearances from the NMPA have allowed Wider to market and sell the Gen-2 device in China for the treatment of insomnia and depression.
Regulatory Background and Matters Related to our Business
United States
Medical devices commercially distributed in the United States require either FDA clearance of a 510(k) premarket notification submission, granting of a de novo request or Premarket Approval (PMA), unless an exemption exists. Under the FFDCA, as administered by the FDA, medical devices are classified into one of three classes — Class I, Class II or Class III — depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to ensure its safety and effectiveness. Regulatory control increases from Class I to Class III. Prior to December 20, 2019, in the United States, all cranial electrical stimulation (CES) technology was classified as a Class III medical device (high-risk).
Class II devices are moderate risk devices and are subject to the FDA’s general controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. Such special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. Most manufacturers of Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FFDCA requesting permission to commercially distribute the device.
Class III devices are deemed the highest risk devices by the FDA and generally include life-sustaining, life-supporting or some implantable devices or devices that have a new intended use or use advanced technology that is not substantially equivalent to that of a legally marketed device. Class III devices require a PMA. For a device that is Class III by default (because it is a novel device that was not previously classified and has no predicate), the manufacturer may request that the FDA reclassify the device into Class II or Class I via a de novo request.
To obtain 510(k) clearance, a premarket notification submission must be submitted to the FDA demonstrating that the proposed device is substantially equivalent to a predicate device. A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I (e.g., via the de novo classification process), or a device that was previously cleared through the 510(k) process. The FDA’s 510(k) review process usually takes from three to six months but can take longer.
After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness or that would constitute a major change or modification in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, a de novo request or PMA approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), de novo, or a PMA in the first instance. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or request the recall of the modified device until FDA has cleared or approved a 510(k), de novo or PMA for the modification.
The PMA process is more demanding than the 510(k) premarket notification process. In a PMA, the manufacturer must demonstrate that the device is safe and effective, and the PMA must be supported by extensive data, including data from preclinical studies and human clinical trials. The PMA must also contain, among other things, a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing and proposed labelling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review of a PMA, although in practice, the FDA’s review often takes significantly longer, and can take several years.
On December 20, 2019, the FDA issued new rulings related to CES devices for the treatment of anxiety, depression, and insomnia. As a result of these rulings, depression treatment with CES devices remained a Class III medical device and will require a full PMA that provides definitive clinical trial evidence of effectiveness and safety. A PMA is the most extensive application and process at the FDA. All CES manufacturers had one year to prepare and file intentions for the depression treatment with a PMA. CES devices that treat anxiety and insomnia were reclassified as Class II devices and required a new application in the form of a special control trial, a summary version of a PMA, requiring safety data and mild efficacy response. All CES manufacturers had one year to complete special control trials for anxiety and insomnia. We are presently analyzing our previous 510(k) Application for such treatment of anxiety and insomnia in accordance with the FDA reclassification ruling in December 2019. Our intent is to move forward with our new 15 milliamp waveform given its success in the China studies. We have also completed 2 prototypes of a Nexalin headset which can be used at home or in a clinical setting. The new headset will utilize the new 15 milliamp waveform. Final prototypes and design files for manufacturing have been completed.
We have made a strategic decision to file a new PMA for the treatment of depression with the Gen-2 and Gen-3 devices that administer the new advanced Nexalin waveform at 15 milliamps. The Gen-1 device was previously cleared by the FDA at 4 milliamps and the re-classification does not prevent us from servicing previously sold or leased devices. Providers may continue to use these devices for treatment purposes. Servicing consists of warranty coverage, electrode sales, and patient cable replacement. This servicing is included in the monthly lease payment. We continue to derive revenue from devices which we sold or leased prior to the FDA’s December 2019 reclassification announcements. This revenue consists of monthly license fees and payment for the sale of electrodes to clinical providers of our technology. As we are in the process of evaluating our new Gen-2 15 milliamp waveform for our technology, a strategic decision was made to not pursue a PMA for the treatment of depression on our existing Gen-1 device. Strategy development has begun for a full PMA for the treatment of depression for our next generation Gen-2 and Gen-3 devices.
China
The NMPA is the governmental authority principally responsible for the supervision and administration of medical devices in the Peoples Republic of China (the “PRC”). Medical devices in the PRC (including manufacturing, marketing, and sale) are subject to a mandatory filing/registration regime regulated by the NMPA. The exact filing pathways are mainly determined by the classification of such devices — like the United States, a three-class classification system, from Class I (lowest risk) to Class III (highest risk). Local testing and clinical trials are generally required for Class II and Class III devices. Some imported devices may need to be registered with a higher-level government authority than domestic devices.
As determined by the NMPA the three classes for devices are:
|
● |
Class I — Medical devices for which routine administration can ensure safety for users and the effectiveness of the device. |
|
● |
Class II — Medical devices that can only be safe and effective with further control in addition to routine administration. |
|
● |
Class III — Medical devices that are implanted into the patient’s body, pose a threat to the patient’s health, or provide sustenance or life support. |
All medical devices must be registered with the NMPA. An overseas device company must submit product samples to test with the NMPA. In addition, all included product information, packaging, and labels, and related material need to be translated into simplified Chinese. For a Class I device, simple product filing to NMPA is required. However, for Class II and Class III medical devices, the manufacturing company must meet all the requirements in the latest regulation, guidelines, and standards.
The NMPA approved the new Gen-2 15 milliamp device for the treatment of insomnia and depression. These treatment indications and clearances from the NMPA have allowed us to market and sell the Gen-2 device in China. Wider will be responsible for obtaining future NMPA registrations and approvals related to the marketing and sales of our devices in China.
Recent statements and regulatory actions by the Chinese government have targeted those companies whose operations involve cross-border data security or anti-monopoly concerns. Regarding data security, China has promulgated several important laws recently. Among them, on June 10, 2021, China promulgated the PRC Data Security Law (“DSL”), which became effective on September 1, 2021. The legislative intent for this law mainly includes regulating data processing activities, ensuring data security, promoting data development and utilization, protecting the data related legitimate rights and interests of individuals and organizations, and safeguarding national sovereignty, security and development interests. Article 36 provides that any Chinese entity that provides the data to foreign judicial or law enforcement agencies (regardless of whether directly or through a foreign entity) without approval from the Chinese authority would likely be deemed to be in violation of DSL. In addition, pursuant to Article 2 of Measures for Cybersecurity Reviews, the procurement of any network product or service by an operator of critical information infrastructure that affects or may affect national security shall be subjected to a cybersecurity review under the Measures. Pursuant to Article 35 of Cybersecurity Law of the People’s Republic of China, where “critical information infrastructure operators” purchase network products and services, which may influence national security, the operators are required to be subjected to a cybersecurity review. We do not operate any critical information infrastructure. As a result, we do not believe that these new legal requirements in China are applicable to us, including sales made to date by Wider as a distributor. However, the exact scope of the term “critical information infrastructure operator” remains unclear, so there can be no assurance that the Joint Venture will not be subjected to critical information infrastructure operator review in the future. Furthermore, in the event that the Joint Venture becomes an operator of critical information infrastructure in the future it may be subjected to the above-described regulation.
With regard to anti-monopoly concerns, Article 3 of Anti-Monopoly Law of the People’s Republic of China prohibits “monopolistic practices,” which include: a) the conclusion of monopoly agreements between operators; b) the abuse of dominant market position by operators; c) concentration of undertakings which has or may have the effect of eliminating or restricting market competition. Also, according to Article 19, the operator(s) will be assumed to have a dominant market position if it has following situation: a) an operator has 50% or higher market share in a relevant market; b) two operators have 66% or higher market share in a relevant market; c) three operators have 75% or higher market share in a relevant market. We believe that we have not conducted any monopolistic practices in China, and that recent statements and regulatory actions by the Chinese government do not impact our ability to conduct business, accept foreign investments, or list on a U.S. or other foreign stock exchange. However, there can be no assurance that regulators in China will not promulgate new laws and regulations or adopt new series of interpretations or regulatory actions which may require the Joint Venture to meet new requirements on the issues mentioned above.
Currently, these statements and regulatory actions of China authorities have had no impact on our daily business operation, including the sales and marketing efforts made to date of our Gen-2 devices in China through Wider. We do not believe that these statements and regulatory actions will have any impact on the Joint Venture. Further, we are a United States’ company with no physical presence in China, and we do not believe that the formation of the Joint Venture in Hong Kong and any resultant exposure to China regulatory actions will adversely impact our ability to accept foreign investments or list our securities on a United States or other foreign exchange. However, since these statements and regulatory actions from China authorities are relatively recent, it is highly uncertain how soon legislative or administrative regulation making bodies will respond and what existing or new laws or regulations or detailed implementations and interpretations will be modified or promulgated, if any, and the potential impact such modified or new laws and regulations will have on our daily business operation, the ability to accept foreign investments and list our securities on a United States or other foreign exchange. In the event any existing or new laws or regulations or detailed implementations and interpretations are modified or promulgated, we and the Joint Venture will take any and all actions to remain in compliance with any such laws or regulations or detailed implementations and interpretations thereof. See “Risk Factors — Risks Related to Doing Business in China.”
As a result of the formation of the Joint Venture, we are conducting our clinical research and implementing a business distribution plan for our devices in China and elsewhere through the Joint Venture, which we believe confers clinical, commercial, and regulatory advantages, but may subject us to significant regulatory, liquidity, and enforcement risks. Although we do not intend to have any physical presence in China, Hong Kong, Macau and Taiwan, the Joint Venture entity has physical presence in Hong Kong. Wider, as a China formed entity with its physical presence in China may be subject to regulatory actions and prohibitions from China regulatory entities and required to obtain certain approvals.
The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions under the civil law system may be cited for reference but have limited precedential value. Uncertainties in the interpretation and enforcement of Chinese laws and regulations could limit the legal protections available to us.”
Market and Industry Background
General
Historically, pharmaceutical solutions have been the first line of treatment for those who suffer from anxiety, insomnia, depression, and other mental health disorders. Beginning in 1950, for patients that were not responding to medication, ECT, also called “shock therapy,” became available. Over time, researchers began to look at alternative ways to inject electricity into the human brain. One such method was via implantable neurostimulators that required invasive surgery procedures associated with high cost and high risk. Implantable devices became the potential solution for those who would not take or could no longer take pharmaceuticals. The interest in electricity continued with the creation of small handheld devices powered by a direct current (DC) battery that the consumer could buy without any medical supervision. Clinical versions of DC stimulators, known as transcranial direct current stimulation (tDCS), were developed by researchers; many of these devices are still in research settings without industry support.
In 1992, a new neurostimulation technique emerged called trans-cranial magnetic stimulation (TMS). This technique evolved into repetitive trans-cranial magnetic stimulation (rTMS), which utilized repetitive magnetic pulse energy to stimulate the brain of patients struggling with depression. The American pharmaceutical industry embraced and funded this technology. The FDA cleared rTMS only for patients who had failed to respond to anti-depressants. Side effects, high cost and moderate efficacy continue to burden this technology sector.
Both insurance companies and healthcare providers are looking for alternative ways to decrease costs while still providing safe and effective treatments.
We believe that our new marketing and growth strategy in combination with our advanced 15 milliamp waveform, technological upgrades and the development of a modern headset monitored with our IT management platform, will position us for the opportunity to disrupt the traditional mental health treatment model. Our mission is to remove the stigma of expensive psychotherapy or pharmaceuticals with the attendant side effects and dependency issues and replace it with clinically proven and cost-effective technology that is easily accessible in the privacy of the patient’s home and monitored by licensed healthcare providers.
Anxiety Market
Anxiety disorders are considered the most prevalent of psychiatric disorders. Anxiety disorders include generalized anxiety disorder, social anxiety disorder, panic disorder, obsessive-compulsive disorder, post-traumatic stress disorder (PTSD) and phobias.
Insomnia Market
Insomnia is a common sleep disorder considered to be responsible for at least $63 billion in direct and indirect healthcare costs each year, according to the Harvard American Insomnia Study. A frightening number of insomnia cases are undiagnosed and untreated, even as the condition becomes a mounting financial burden on America’s employers and the healthcare system. Data surrounding sleep disorders demonstrate that insomnia is a growing problem that shows no signs of slowing down. Current market conditions present an opportunity to introduce technology that provides a safe, effective and drug-free alternative for those suffering from insomnia. We believe we have the ability to decrease the number of potentially addictive insomnia prescriptions needed by patients and offer physicians a non-pharmaceutical option to provide their patients. Additionally, we are developing a solution for home-based treatment for chronic insomnia and to improve sleep hygiene for its users.
Depression Market
Depression continues to be the leading cause of medical disability around the world. Poor efficacy, risk and adverse side effects of current anti-depressants are driving the preference for non-pharmacological therapies, which will limit growth for the pharmaceutical sector of the depression treatment market. This limitation will enhance the research and development of novel therapies that treat depression safely and effectively without adverse side effects. Historically, according to the CDC, only one-third of people with severe depression have taken anti-depressants.
Any decline in the depression medication market should indirectly accelerate the growth of the neurostimulator market. Management believes that, based on the market data and current trends, the depression market — like the anxiety and insomnia market — creates enormous potential for our products.
Prior to December 2019, our Gen-1 device was considered a Class III device. Treatment of depression in the United States is limited to Class III devices only. Prior to 2019, our existing Gen-1 4 milliamp medical device had been used to successfully treat depression in the U.S. The Gen-2 15 milliamp version of our device when introduced into the United States will be subject to approximately eighteen months of clinical study before our PMA application for depression will be accepted. Assuming we will be able to obtain successful classification from the FDA, we expect to market our device in the United States as a treatment for depression.
Substance Use Disorders (Opioid Addiction) Market
According to the National Institute on Drug Abuse (NIDA,) substance use, and substance use disorders cost the United States more than $740 billion a year in healthcare, crime and lost productivity costs; but dollars barely capture the devastating human cost of addiction to individuals, families and communities. According to the National Survey on Drug Use and Health, 19.7 million adults in the United States suffered from a substance use disorder in 2017.
The current success rate of the best drug and alcohol rehabilitation facilities is minimal. We believe that this represents a significant market opportunity for our company. The disease of addiction is brain-based in its nature. Currently brain-based treatments for the disease are only available to patients who can afford long-term expensive boutique treatment centers. We intend to demonstrate that a brain-based approach to addiction treatment will enhance a patient’s success at long-term recovery. Our hypothesis is that the current pilot study design at The University California, San Diego (see below) will provide a source of validation for this treatment modality in addiction treatment.
Chronic Pain Market
Originally, our waveform was designed as an electro-analgesic for pain. This refers to the ability to electrically interrupt the pain signaling process in the brain. By interrupting the pain signaling process in the brain, our products can reduce symptoms and discomfort associated with chronic pain. By reducing the symptoms and discomfort associated with chronic pain, physicians can reduce medications and avoid dependency issues related to opiate-based medications.
According to Research and Markets, the global chronic pain treatment market is predicted to progress at a CAGR of 5.8% from 2020 to 2030 and generate revenue of $151.7 billion in 2030.
Currently, we own an electrostimulation patent for a device that will apply electrodes to the brain, spine, and the place of injury. The placement of these electrodes in conjunction with our various waveforms creates an opportunity for us to treat chronic pain without medication. The Nexalin executive team is preparing strategies to develop a prototype of our existing patented design and introduce it into clinical trials for the treatment of chronic pain. In previous pilot studies, our existing Gen-1 product reduced pain in patients suffering from injuries originating in industrial accidents. However, we plan to use the new advanced waveform emitted at 15 milliamps into the new prototype pain device for new clinical trials for the treatment of chronic pain.
Alzheimer’s Disease and Dementia Market
Alzheimer’s disease is a degenerative brain disease and the most common form of dementia. Dementia is not a specific disease, but rather an overall term that describes a group of symptoms. According to the WHO, there are around 50 million people living with Alzheimer’s disease and other dementias worldwide.
According to Reports and Data, the global Alzheimer’s therapeutics market is projected to reach $13.7 billion by 2030 from $2.2 billion in 2020 with a substantial compound annual growth rate (CAGR) of 20% through the forecast period.
We believe our products could be leveraged to extend the quality of life for millions of people who are diagnosed with Alzheimer’s disease.
Marketing and Sales Efforts
We believe that our marketing and sales plan provides a long-term scalable business model. Our team will prepare the foundation and marketing assets necessary to launch the new virtual clinic model that will complement the traditional clinic model. Our sales model is to place more than 1,000 Gen-2 and Gen-3 devices on the global stage. The momentum and branding strategies of Nexalin providers will be leveraged to enhance the launch of a global sales plan. The Gen-2 device at 15 milliamps supported by the Gen-3 outpatient headset and our virtual digital management platform is intended to disrupt the current mental healthcare model. The Gen-2 and Gen-3 device at 15 milliamps will offer patients a cost effective and efficient treatment model for day-to-day mental health challenges. We believe those devices, with their advanced waveform, can treat existing mental health disorders associated with anxiety and insomnia. Additionally, new strategies are in research and development for FDA treatment indications of depression, substance use disorder, TBI (traumatic brain injury), PTSD, opioid addiction, alcoholism and chronic pain. Additional research and treatment efficacy are being investigated for the Alzheimer’s community for patient care and management.
Our plan is designed to triangulate and stimulate the physician, consumer, and manufacturer relationship. Trends in healthcare indicate consumers are involved in treatment decisions that concern their mental health. Because of the advancement in healthcare technologies, home-based care with medical supervision provides patients with a cost-effective and efficient treatment option. Home-based care also avoids the stigma associated with treatment for mental health disorders. In our current sales plan, we intend to launch with a physician provider in each state. These physicians will lead the Nexalin campaign in each state as that state’s primary provider. These preferred state providers will begin with the virtual clinic. Our digital marketing team will drive consumers with quality-of-life struggles related to mental health issues into the virtual clinic and then to the provider in the consumer’s state of residence. These initial state physicians providing mental health services in the virtual clinic, will also have the ability to offer treatment in their clinic. The in-clinic model will use the Gen-2 clinical device while the virtual clinic will use the Gen-3 headset. This initial launch plan with state providers will develop and support multiple marketing verticals to drive the Nexalin brand and treatment as an alternative to medications and psychotherapy. We will leverage this physician / patient community to establish a national network of physicians that offer mental health evaluations and the Nexalin treatment in either a clinical setting or in the privacy of the patient’s home with medical supervision through the future Nexalin app.
Most, if not all, patients treated in the Nexalin virtual clinic would be part of a digital community that supports brand awareness and the sharing of anonymous treatment outcomes in a social media setting. The Patient Activation Program will include a robust data gathering system on providers and patients (opt-in) that enhances our marketing strategies.
Insurance Reimbursement for Our Products
In January 2020, the Centers for Medicare & Medicaid Services (CMS) in conjunction with the Durable Medical Equipment for Medicare Administrative Contractors issued a code for Cranial Electrotherapy Stimulators (CES). CMS issues codes that are used by medical practitioners to obtain Medicare, Medicaid and private insurance reimbursement. The issuance of this code is the first time that a reimbursement code from CMS has been designated specifically for CES. The code does not guarantee reimbursement and is considered at this time, experimental. The Nexalin executive team plans to continue preparing clinical data and durability data to pursue long term clinical reimbursement.
Reimbursement strategies for this type of technology are complex and vary from one diagnosis to another. Nexalin plans to utilize an RFID system that will track treatments completed. This will simplify comparing our devices to pharmaceutical interventions. Beginning in 2024, a complete reimbursement assessment is being conducted and evaluated to develop a strategy to acquire reimbursement. We will employ a two-prong approach for eventual reimbursement. The first prong will evaluate the clinic-based product offered by physicians. The second prong will focus on tracking usage and response from the outpatient headset model that is tracked through the virtual platform. Frequently therapies that are used in the home are not classified as durable medical equipment and will fall into a reimbursement gap without coverage. We intend to work to successfully achieve a Level 2 code under the healthcare common procedure coding system. We will work to seek reimbursement for conditions in sequence with the home based and the clinic-based unit that will maximize value of treatment from a financial standpoint as well as monitoring the response by the patient community.
Research
Research is the fundamental core of any pharmaceutical or medical device company. Although small trials, with limited patients, can show promise for a treatment, they are generally not acceptable to the FDA for product approval. To commercialize a product for widespread use, multiple large-scale trials are required to demonstrate both efficacy and safety. In the past two decades, the cost of conducting such trials has more than doubled, with many small start-up companies unable to raise the necessary capital to complete these vital projects. The increase in cost reflects several variables which are required for successful clinical trial completion.
The various costs can include patient recruitment and retention expenses, physician, and nurse expenses, as well as the expenses of other healthcare providers. Various regulations, each more complex than the next, also have added significant cost to the process. Data collection, as well as data analysis, is also a significant portion of the study cost. Additionally, almost all studies are conducted through either a large university, with its underlying overhead for administrative costs and institutional review board approval, or through a contract research organization, which also adds significant overhead costs in addition to the hard cost of the study itself. Latest estimates for the cost per patient for an average trial is approximately $41,000.
In 2019, we began a research partnership with The University California, San Diego. Prior to the pandemic, two pilot clinical studies were undertaken with UCSD, however, these trials were paused due to the shutdown of college campuses in California. Beginning in the summer of 2022 and continuing to present, new discussions began to explore strategies for PTSD and Opiate dependency and mild traumatic brain injury (mTBI) with our new Gen-3 headset.
At UCSD the first clinical is studying the benefits of our Gen-2 device as a treatment for veterans suffering with opiate addiction. This pilot study was designed by UCSD and funded by the department of Veterans Affairs. For this opiate addiction study, the primary endpoint will be alleviating withdrawal symptoms. Secondary endpoints will include decreasing dosages of medication used in opiate addiction cessation, as well as Magnetoencephalography (MEG) gamma band improvement.
The other study at UCSD is focused on veterans suffering from mild traumatic brain injury (mTBI) and is funded by the United States Department of Defense. One of the primary symptoms associated with mTBI is PTSD (post-traumatic stress disorder). The primary endpoint associated with this study will be the assessment and reduction of post-concussion symptoms associated with PTSD. A secondary endpoint for the study will be improvement and MEG slow-wave abnormalities. Additional studies are planned for patients and veterans with suicidal thoughts related to mTBI.
In addition to UCSD, we are developing additional strategies to initiate further trials to address new FDA guidelines. These new strategies and pivotal trials will support new 510(k)s and/or de novo applications for anxiety and insomnia at 15 milliamps. These trials are in addition to the special control trials required by the FDA. Final trial designs are due to be executed after recommendations are reviewed from the April 30, 2024 meeting FDA pre-sub meetings. Other areas of research that will be designed and funded relate to the treatment of substance use disorders, TBI, PTSD, Alzheimer’s disease, and dementia.
Additional research in China is being performed with the goal of publishing the findings in a peer reviewed journal. All research will be controlled by our team, with all trial designs requiring written final approval by our Chief Medical Officer. Clinical updates will be required every 30 days. Frequent in-person WeChat meetings will also be performed to ensure the integrity of the research efforts.
In addition to clinical trials in China and current studies in the United States required by the FDA, an additional study is planned with the 15 milliamp Gen-2 and Gen-3 devices to evaluate a large cohort of patients with depression. This trial will include a double-blind study design with active and sham groups. Patient selection screening will evaluate 200-250 subjects to acquire the number of patients needed for a successful trial. Each patient, upon enrollment, will be evaluated extensively prior to initiation of therapy. Patients will be treated a minimum of 20 separate times, with pre- and post-test screening. Moreover, upon completion of therapy, post-test examination will be performed not only immediately thereafter but also over the course of one to three months to establish not only efficacy but durability of the treatment. The results of this study will provide the basis of the PMA with the FDA for the treatment of Depression.
At the start of 2021, an Alzheimer’s specific clinical trial was underway in China: “Transcranial alternating current stimulation for patients with mild Alzheimer’s disease.” Extensive cognitive pre- and post-evaluations are being performed at the beginning and conclusion of the study, with less rigorous evaluations before and after each therapy session. Because of issues related to Covid-19 in China, this trial was paused. Additionally, results of this trial will dictate additional testing strategies to determine specific treatment protocols for complex Alzheimer’s and dementia patients.
A final area of study includes the evaluation of chemical changes within the brain following transcranial stimulation. Chemicals, which are naturally formed in the brain, control many of our moods and thoughts, modulating feelings of pain, depression and generalized mood. These substances also drive cravings in substance use disorders. One of the specific areas of research is to validate changes of serotonin levels in the brain. Serotonin is a “feel good” chemical which has also been associated with learning. Other chemicals, such as dopamine, act in a reward center mechanism. Additionally, certain other neurons require specific chemicals to either fire or be inhibited from firing. These areas can be explored with specific radioactive markers in the brain for evaluation with PET MRI scans.
Clinical trials for the Gen-2 device were conducted in China during 2023. These clinical trials were funded by Wider, and its related companies, and was conducted at the Xuanwu Hospital, Capital Medical University in Beijing, China. The results were also published in General Psychiatry, an open-source, peer-reviewed scientific journal. The published results of the study concluded that repeated treatment with the Company’s neurostimulation device suggests an acute effect in reducing depressive symptoms in patients with treatment-resistant depression (“TRD”). In addition, no adverse events were observed during treatment. As part of the clinical study, 7 migraine patients were treated at the Xuanwu Hospital. Treatment was administered for 4 consecutive weeks via the forehead and both mastoid (twice per day, five days a week). Efficacy and adverse reactions were assessed at a two-week screening/baseline period followed by a four-week treatment phase. The study concluded that twice daily 15mA tACS, a unique form of non-invasive brain stimulation, offers an acute effective intervention for patients with TRD. The study showed that all patients with TRD had a significant reduction in depression symptoms after the four-week treatment, and all of them achieved a clinical response (defined as HAMD-17/ MADRS scores that decreased by 50% or more from the baseline). Additional Insomnia clinical trial results are expected in Q2 2024 with additional submissions for publication.
Virtual Clinic Digital Management Platform
We expect to capitalize on the post pandemic digital health model. Our team began researching IT digital development firms at the beginning of the pandemic. We have now completed our research and bidding process and have begun contract negotiations with a leading IT design team to begin work on an advanced, proprietary IT management platform that will eventually manage all aspects of the Nexalin virtual clinic model. The vision is to implement a virtual clinic model that will enable providers and clinics to integrate remote outpatients into an overall treatment process. Our IT platform goes well beyond telehealth and is designed to support all aspects of the treatment model in conjunction with various data sets to support marketing, data collection and patient monitoring. Our digital management platform will manage the entire clinical and outpatient headset business model. The proprietary IT platform will manage all aspects of a new virtual health center related to treatment for mental health. As the development of the new generations of our devices and the outpatient headset are developed, the digital platform will eventually manage and triangulate the relationship between the medical professional, the patient, and the manufacturer. The digital platform will handle logistics, data collection and user experience data for clinical evaluation. Additionally, there will be an app that the patients will install on their phones that will communicate with the outpatient headset. The app will upload user information that is HIPAA compliant to the IT management platform. Modules will be designed and implemented in the platform to collect biometric data. Biometric data will be utilized to evaluate patient response. A symptom exam for additional clinical validation will also be offered in the app. All data and user information will be stored in a secure, HIPAA compliant cloud computing center and access to the information will be managed through a secure and compliant dashboard management system. The medical professional will have access to all data to monitor outpatient experience, client response and general health and wellness information.
We will leverage our IT investment to create a lead management system for mental health physicians connecting prospective patients with providers. The medical professional will be able to engage in a telehealth virtual appointment with prospective patients to complete an evaluation and assess whether the patient is a candidate for the outpatient headset program. After the professional approves the device for the patient, we will automatically prepare shipment of the device directly to the outpatient consumer from the manufacturer. We will have an internal department to monitor shipment, and to answer questions through a help desk on how to set up and use the device. The medical professional can be reimbursed for the virtual appointment via the outpatient’s insurance for telehealth care which is becoming part of the new normal in the post-pandemic, digital-health world.
Additional design and implementation of modules related to social media marketing, bio-metric data collection and user experience will eventually complete the design of the IT management platform.
Manufacturing
In December 2021, we entered into a quality assurance agreement with Apical Instruments, an FDA-registered manufacturer, to ensure quality assurance of our products. We currently have enough design and manufacturing support to meet all projected company design and sales goals. Our regulatory team works closely with the Apical quality team to ensure all current compliance and testing standards are adhered to. All distribution channels will rely on a collaboration between the Apical and Nexalin teams.
Intellectual Property
Our commercial success depends in part on our ability to: obtain and maintain proprietary or intellectual property protection for our products, our core technologies and other know-how; operate without infringing on the proprietary rights of others; and prevent others from infringing on our proprietary or intellectual property rights. Our policy is to seek to protect our proprietary and intellectual property position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on the skills, knowledge, and experience of our scientific and technical personnel, as well as that of our advisors, consultants and other contractors. To help protect our proprietary know-how that is not patentable, we rely on trade secret protection and confidentiality agreements to protect our interests. As part of our hiring practices and as described in our Code of Ethics which is binding on all employees, our employees, consultants, and advisors are prohibited from disclosing confidential information and are required to assign to us the ideas, developments, discoveries and inventions important to our business.
We file patent applications directed to our key products to establish intellectual property positions. These patent applications are intended to protect these products as well as their uses in the treatment of diseases. We are the owner and inventor of three existing patents and four pending patents related to the electro-stimulation techniques related to our products and services. Our current patents cover a therapeutic electro-stimulation apparatus (the medical device) and the software used to create and administer the stimulation to the patient and our core technology utilized in the Gen-2 and Gen-3 system. We expect to file additional provisional and non-provisional patent applications and copyright protection pertaining to future Generation technology, proprietary software, and trademarks. The patent claims associated with the non-provisional patent applications will be defined and prepared in the filings. The intention is to continue building an intellectual property portfolio asset. Future research and development projects related to advancements in neurostimulation and neuromodulation technology will be identified and investigated for future patent filings.
Our trademark portfolio currently consists of registered trademark rights for the mark, NEXALIN TECHNOLOGY, in the United States. In connection with the ongoing development and advancement of our products and services in the United States and various international jurisdictions, we routinely seek to create protection for our marks and enhance their value by pursuing trademarks and service marks where available and when appropriate. In addition to patents and trademark protection, we rely upon unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position.
Competition
We plan to be the leader in brain-based health. We compete with traditional pharmaceutical therapies. All of these have side effects, such as drug dependency as well as adverse health risks.
We also compete with several neurostimulators at the high and low end of the market as well as implanted devices. All have either a high-risk profile or uncomfortable side effects with moderate efficacy. Our products were designed as a cost-effective option to all current reimbursed treatments available to the patient.
We believe that some existing neurostimulation products are either high risk, high cost and difficult to administer. In addition, they are invasive, frequently requiring surgery and multiple visits to a physician. Since many of the conditions require ongoing treatments, the difficulty and cost of administering them make them of limited utility for broad application.
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We qualify as an “emerging growth company,” as defined in the Jumpstart Our Business Start-ups Act of 2012, as amended, or the JOBS Act. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from some of the reporting requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
| |
● |
being permitted to present only two years of audited financial statements and only two years of related management’s discussion and analysis of financial condition and results of operations disclosures; |
| |
● |
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act; |
| |
● |
not being required to comply with any requirements that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
| |
● |
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| |
● |
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We may take advantage of these exemptions until the last day of our fiscal year following the fifth anniversary of the completion of our initial public offering which was completed on September 16, 2022. However, if any of the following events occur prior to the end of such five-year period, (i) our annual gross revenue exceeds $1.235 billion, (ii) we issue more than $1.0 billion of non-convertible debt in any three-year period or (iii) we become a “large accelerated filer,” (as defined in Rule 12b-2 under the Exchange Act), we will cease to be an emerging growth company prior to the end of such five-year period. We will be deemed to be a “large accelerated filer” at such time that we (a) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of our most recently completed second fiscal quarter, (b) have been required to file annual and quarterly reports under the Exchange Act, for a period of at least twelve months and (c) have filed at least one annual report pursuant to the Exchange Act. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are emerging growth companies. As a result, changes in rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
We are also a “smaller reporting company” as defined in the Exchange Act. We may take advantage of certain of the scaled disclosures available to smaller reporting companies until the fiscal year following the determination that our voting and non-voting common stock held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues are less than $100 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter.
THE OFFERING
| Number of shares of common stock being offered: |
|
[●] shares of common stock. |
| |
|
|
| Offering Price Per Share: |
|
$[●] per share. |
| |
|
|
| Common stock outstanding prior to this offering: |
|
7,436,562 shares. |
| |
|
|
| Common outstanding after this offering: |
|
[●] shares1. |
| |
|
|
| Use of proceeds: |
|
To be completed. See “Use of Proceeds” at page 54. |
| |
|
|
| Risk Factors: |
|
An investment in our securities involves a high degree of risk. You should read this prospectus carefully, including the section entitled “Risk Factors” beginning on page 17 of this prospectus and under similar headings in the other documents that are filed after the date hereof and incorporated by reference in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. |
| |
|
|
| Best Efforts Offering: |
|
We have agreed to offer and sell the securities offered hereby to the purchasers through the Placement Agent. The Placement Agent is not required to buy or sell any specific number or dollar amount of the securities offered hereby, but it will use its reasonable best efforts to solicit offers to purchase the securities offered by this prospectus. See “Plan of Distribution” beginning on page 61 of this prospectus. |
| |
|
|
| Transfer agent and registrar: |
|
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company. |
| |
|
|
| Nasdaq Capital Market Symbol: |
|
Our common stock is listed under the symbol “NXL.” |
|
1 |
The number of shares of our common stock that will be outstanding after this offering is based on 7,436,562 shares of common stock outstanding as of May 22, 2024 and excludes: |
|
● |
1,152,125 shares of our common stock issuable upon the exercise of outstanding and vested stock options under the Company’s 2023 Equity Incentive Plan (the “2023 Plan”); |
|
● |
347,875 shares of our common stock reserved for future issuance under our 2023 Plan; |
|
● |
3,443,314
shares of our common stock awarded as stock grants and/or options to certain executive officers, directors, employees and
consultants in lieu of cash compensation, some of which has not been earned, subject to stockholder approval of an amendment to the
2023 Plan to increase the number of shares available under such Plan; and |
|
● |
2,662,250 shares of common stock issuable upon exercise
of outstanding warrants issued in our initial public offering.
|
This prospectus reflects and assumes no exercise of outstanding options
or warrants and that all such options and warrants have been or will be earned.
RISK FACTORS
Investing in our common stock is speculative and involves a high degree of risk including the risk of a loss of your entire investment. Before you invest in our common stock, you should carefully consider the following risk factors, as well as the risks described in the “Risk Factors” contained in the 2023 Form 10-K. These risk factors contain, in addition to historical information, forward looking statements that involve risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward-looking statements. The occurrence of any of the adverse developments described in the following risk factors and in the documents incorporated herein by reference could materially and adversely harm our business, financial condition, results of operations or prospects. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock. In addition, the risks and uncertainties discussed below are not the only ones we face. Our business, financial condition, results of operations or prospects could also be harmed by risks and uncertainties not currently known to us or that we currently do not believe are material, and these risks and uncertainties could result in a complete loss of your investment. A summary of our risk factors is as follows:
Risks Related to Our Financial Position and Capital Needs
We have incurred significant losses since our inception. We expect to incur losses over the next several years and may never achieve or maintain profitability.
We are a Delaware corporation with a limited operating history. We have funded our operations to date primarily with proceeds from private investors and the sale of our stock, including the proceeds from our initial public offering completed in September 2022. We have had only limited sales of our products and services to date. For the year ended December 31, 2023 and the quarter ended March 31, 2024, we incurred a comprehensive loss in the amount of $4,685,427 and $1,040,997, respectively. Our accumulated deficit as of December 31, 2023 and at March 31, 2024, was $77,038,049 and $78,079,206, respectively.
We have devoted a substantial portion of our financial resources and efforts to research and development, including preclinical studies and clinical trials. We are still in the early stages of development of our products.
We expect to continue to incur significant expenses and operating losses over the next several years. Our net losses may fluctuate substantially from quarter to quarter and year to year. We anticipate that our expenses will increase significantly as we:
| |
● |
continue our ongoing and planned preclinical and clinical development of our existing and next Generation devices; |
| |
● |
initiate preclinical studies and clinical trials for any additional products that we may pursue in the future; |
| |
● |
seek to discover and develop additional treatment indications; |
| |
● |
seek regulatory approvals for any products that successfully complete clinical trials; |
| |
● |
ultimately establish sales, marketing and distribution infrastructure and scale up external manufacturing capabilities to commercialize any product for which we may obtain regulatory approval and intend to commercialize on our own; |
| |
● |
maintain, expand and protect our intellectual property portfolio; |
| |
● |
engage additional clinical, scientific, manufacturing and controls personnel; |
| |
● |
add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts; and |
| |
● |
incur additional legal, accounting and other expenses associated with operating as a public company. |
To become and remain profitable, we and our collaborators must succeed in developing and eventually commercializing future and existing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing preclinical studies and clinical trials of our products and preclinical program, obtaining regulatory approval, manufacturing, marketing and selling any products for which we may obtain regulatory approval, as well as discovering and developing additional products. Again, we are only in the preliminary stages of most of these activities. We may never succeed in these activities and, even if we do, may never generate revenues that are significant enough to achieve profitability.
Because of the numerous risks and uncertainties associated with product development, we are unable to accurately predict the timing or amount of expenses or when, or if, we will be able to achieve profitability. If regulatory authorities require us to perform studies in addition to those currently expected, or if there are any delays in the initiation and completion of our clinical trials or the development of any of our products, our expenses could increase.
Even if we achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the value of our common stock and could impair our ability to raise capital, expand our business, maintain our research and development efforts or continue our operations. A decline in the value of our common stock could also cause you to lose all or part of your investment.
We may not be able to continue as a going concern if we do not execute our business plan or obtain additional financing in the future if necessary.
Our independent accountant’s audit report included in the 2023 Form 10-K states that there is substantial doubt about our ability to continue as a going concern. We have incurred only losses since our inception, raising substantial doubt about our ability to continue as a going concern. Therefore, our ability to continue as a going concern is highly dependent upon us executing our business plan in the planned amount of time allotted and obtaining additional financing for our planned operations, if necessary. There can be no assurance that we will be able to raise any additional funds, or if we are able to raise additional funds, that such funds will be in the amounts required or on terms favorable to us.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We commenced active operations in 2010, and our operations to date have been largely focused on raising capital, identifying and developing our products and preclinical program, broadening our expertise in the development of our products and undertaking preclinical studies and conducting early-stage clinical trials. As a result of the FDA reclassification ruling in December 2019, we had to suspend marketing of our Gen-1 medical device for the treatment of anxiety and insomnia. We are presently evaluating whether to proceed with amending our prior application with the FDA for the treatment of insomnia and anxiety or filing new applications 510(k) for our next Generation devices.
Although we have developed second- and third-Generation versions of our medical device, these have not as yet been approved by the FDA for marketing or sales in the United States. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history.
We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our business objectives. We will need to transition at some point from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
We may require additional funding to meet our financial needs and to pursue our business objectives. If we are unable to raise capital when needed, we could be forced to delay, reduce or altogether cease our product development programs or commercialization efforts.
We are currently not cash flow positive and are not certain when and if we will be cash flow positive. We incurred a comprehensive loss in the amount of $4,685,427 for the year ended December 31, 2023 and $1,040,997 for the quarter ended March 31, 2024. We may need to obtain substantial additional funding in connection with our continuing operations and planned activities. Our future capital requirements will depend on many factors, including:
| |
● |
the timing, progress and results of our ongoing clinical trials of our products; |
| |
● |
the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials of other products that we may pursue; |
| |
● |
our ability to establish collaborations on favorable terms, if at all; |
| |
● |
the costs, timing and outcome of regulatory review of our products; |
| |
● |
the costs and timing of future commercialization activities, including product manufacturing, marketing, sales and distribution, for any of our products for which we receive marketing approval; |
| |
● |
the revenue, if any, received from commercial sales of our products for which we receive marketing approval; |
| |
● |
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and |
| |
● |
the costs of operating as a public company. |
Identifying potential products and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to continue our regulatory approvals and achieve product sales. In addition, our products, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that are cleared under FDA review. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or altogether cease our research and development programs or future commercialization efforts.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or products.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through equity offerings. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, future revenue streams, research programs or products or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to a third party to develop and market products that we would otherwise prefer to develop and market ourselves.
Risks Related to the Development of Our Products and Preclinical Programs
We depend on the success of our future products, some of which are in clinical development but have not completed advanced clinical trials. If we lose our existing or cannot obtain future regulatory approval for and successfully commercialize one or more of our products or if we experience significant delays in doing so, we may never become profitable.
The success of our products and preclinical program will depend on several additional factors, including:
| |
● |
successful completion of preclinical studies and requisite clinical trials; |
| |
● |
performing preclinical studies and clinical trials in compliance with the FDA or any comparable regulatory authority requirements; |
| |
● |
receipt of marketing approvals from applicable regulatory authorities; |
| |
● |
the ability of collaborators to manufacture sufficient quantity of product for development, clinical trials or potential commercialization; |
| |
● |
obtaining and maintaining patent, trademark and trade secret protection, and regulatory exclusivity for our products and preclinical program; |
| |
● |
making arrangements with third parties for manufacturing capabilities; |
| |
● |
launching commercial sales of products, if and when approved, whether alone or in collaboration with others; |
| |
● |
acceptance of the therapies, if and when approved, by healthcare providers, physicians, clinicians, patients and third-party payors; |
| |
● |
competing effectively with other therapies; |
| |
● |
obtaining and maintaining healthcare coverage and adequate reimbursement; and |
| |
● |
protecting our rights in our intellectual property portfolio. |
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our products, which would harm our bus
Our products and product candidates may be subject to reclassification by the FDA, and a change in the classification may have an adverse impact on our revenues or our abilities to obtain necessary regulatory approvals.
Originally, our technology was cleared for the treatment of anxiety, depression and insomnia. Each treatment indication with this technology was classified as Class III from a risk tolerance standpoint at the FDA. In December of 2019, the FDA passed a new ruling that separated anxiety and insomnia from the treatment of depression. CES devices that treat anxiety and insomnia were reclassified as Class II medical devices and require special control trials to be initiated, as well as the filing of a new 510(k) application for previously approved devices. The FDA continued to classify the treatment of depression for cranial stimulation as a Class III high risk device. In order to receive approval for treatment for depression, our devices will require a new pre-market application for this indication. We have decided not to pursue a depression indication for our Gen-1 device at such time.
Any further such reclassification by the FDA of an indication from a certain class of device to another during our development or post-commercialization for that indication could have a significant adverse impact due to the more rigorous and lengthy approval process required for a higher risk class medical device. Such a change in classification can significantly increase development costs and prolong the time for development and approval, thus delaying revenues. A reclassification of an indication after approval from a certain class of device to another could result in a change in classification for reimbursement, and there could be a significant negative impact on our revenues relatedly.
We plan to conduct decentralized clinical trials for the Gen-3 device in the U.S. and have consulted the FDA as part of the pre-submission meetings.
Success in preclinical studies or clinical trials may not be indicative of results in future clinical trials.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Our products may fail to show the desired safety and efficacy in all clinical trials.
If we experience delays or difficulties in the enrolment of patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate, continue or complete clinical trials of any product candidate that we develop if we and our collaborators are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or other comparable regulatory authority. We have limited experience enrolling patients in our clinical trials and cannot predict how successful we will be in enrolling patients in future clinical trials.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct the clinical trials for our products, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials or failing to comply with applicable regulatory requirements.
We rely on third parties, such as research institutions and Wider, which is based in China, to conduct some of our clinical trials. Our reliance upon research institutions, including hospitals, clinics and academics, provides us with less control over the timing and cost of clinical trials and the ability to recruit subjects. If we are unable to reach agreement with suitable research institutions on acceptable terms, or if any resulting agreement is terminated, we may be unable to quickly replace the research institution with another qualified institution on acceptable terms. Even if we do replace the institution, we may incur additional costs to conduct the trial at the new institution. We may not be able to secure and maintain suitable research institutions to conduct our clinical trials.
We rely on a collaboration with a third party for the quality assurance of our products, and we may seek additional collaborations in the future. If those collaborations are not successful, we may not be able to capitalize on the market potential of these products.
We are a party to a quality assurance agreement with a third party for the quality assurance of our products and may enter into additional collaborations in the future. We are dependent upon the success of our current and any future collaborators in performing their responsibilities in connection with the relevant collaboration. If we fail to maintain these collaborative relationships for any reason, we will need to perform the activities that we currently anticipate would be performed by our collaborators on our own at our sole expense. This could substantially increase our capital needs, and we may not have the capability or financial capacity to undertake these activities on our own, or we may not be able to find other collaborators on acceptable terms, or at all. This may limit the programs we are able to pursue and result in significant delays in the development, sale and manufacture of our product candidates and products, and may have a material adverse effect on our business, financial condition and results of operations.
Our dependence upon our current and potential future collaborations exposes us to a number of risks, including that our collaborators (i) may fail to cooperate or perform their contractual obligations, including financial obligations, (ii) may choose to undertake differing business strategies or pursue alternative technologies or (iii) may take an opposing view regarding ownership of clinical trial results or intellectual property.
Due to these factors and other possible events, we could suffer delays in the research, development or commercialization of our product candidates and future products or we may become involved in litigation or arbitration, which could be time consuming and expensive. We additionally may be compelled to split revenue with our collaborators, which could have a material adverse effect on our business, financial condition, and results of operations.
Risks Related to the Commercialization of Our Products
Even if any of our products receives marketing approval, it may fail to achieve the degree of market acceptance by healthcare providers, physicians, clinicians, patients, third-party payors and others in the medical community necessary for commercial success.
The degree of market acceptance of our products, if approved for commercial sale, will depend on a number of factors, including:
| |
● |
the efficacy and potential advantages compared to alternative treatments; |
| |
● |
the potential and perceived advantages and disadvantages of the products, including cost and clinical benefit relative to alternative treatments; |
| |
● |
the convenience and ease of administration compared to alternative treatments; |
| |
● |
the willingness of the target patient population to try new therapies and of healthcare providers, physicians, and clinicians to prescribe these therapies; |
| |
● |
acceptance by healthcare providers, physicians, clinicians, patients, operators of hospitals, including in-hospital formularies, and treatment facilities and parties responsible for coverage and reimbursement of the product; |
| |
● |
the availability of coverage and adequate reimbursement by third-party payors and government authorities; |
| |
● |
the ability to manufacture our product in sufficient quantities and yields; |
| |
● |
the strength and effectiveness of marketing and distribution support; |
| |
● |
the prevalence and severity of any side effects; |
| |
● |
limitations or warnings, including distribution or use restrictions, contained in the product’s approved labelling; |
| |
● |
the approval of other new products for the same indications; and |
| |
● |
the timing of market introduction of the approved product as well as competitive products. |
Any failure by any of our existing or future products that obtain regulatory approval to achieve market acceptance or commercial success would have a material adverse effect on our business prospects.
We may eventually compete for product sales with other companies, many of which will have greater resources or capabilities than we have or may succeed in developing better products or in developing products more quickly than we do, and we may not compete successfully with them.
Our industry is competitive and has been evolving rapidly with not only existing treatment options, but also the introduction of new technologies and products as well as the market activities of industry participants. We compete or may eventually compete with other companies and organizations that are marketing or developing therapies for our targeted disease indications, based on traditional pharmaceutical, medical device, or other neurostimulation therapy and technologies.
We also face competition in the neurostimulation field from academic institutions and governmental agencies. Many of our current and potential competitors have greater financial and human resources than we have, including more experience in research and development and more established sales, marketing and distribution capabilities.
We anticipate that competition in our industry will increase. In addition, the health care industry is characterized by rapid technological change, resulting in new product introductions and other technological advancements. Our competitors may develop and market products that render product candidates now or under development by us in the future, or any products manufactured or marketed by us, non-competitive or otherwise obsolete.
Coverage and adequate reimbursement may not be available for our current or any future products, which could make it difficult for us to sell profitably, if approved.
Market acceptance and sales of any products that we commercialize, if approved, will depend in part on the extent to which reimbursement for these products and related treatments will be available from third-party payors, including government health administration authorities, managed care organizations and other private health insurers. Third-party payors decide which therapies they will pay for and establish reimbursement levels. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for any products that we develop will be made on a payor-by-payor basis. One payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and adequate reimbursement for the product. Additionally, a third-party payor’s decision to provide coverage for a therapy does not imply that an adequate reimbursement rate will be approved. Each payor determines whether it will provide coverage for a therapy, what amount it will pay for the therapy and on what tier of its list of covered products, or formulary, it will be placed. The position on a payor’s formulary generally determines the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy by patients and physicians. Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products, and providers are unlikely to prescribe our products, unless coverage is provided, and reimbursement is adequate to cover a significant portion of the cost of our products and their administration.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Third-party payors have attempted to control costs by limiting coverage and limited reimbursement for medications and certain treatments utilizing digital technologies. We cannot be sure that coverage and reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. If coverage and adequate reimbursement are not available, or are available only to limited levels, we may not be able to successfully commercialize our current and any future products that we develop.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our products in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. If we cannot successfully defend ourselves against claims that our products or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| |
● |
reduced resources of our management to pursue our business strategy; |
| |
● |
decreased demand for any products or products that we may develop; |
| |
● |
injury to our reputation and significant negative media attention; |
| |
● |
withdrawal of clinical trial participants; |
| |
● |
initiation of investigations by regulators; |
| |
● |
product recalls, withdrawals or labelling, marketing or promotional restrictions; |
| |
● |
significant costs to defend the resulting litigation; |
| |
● |
substantial monetary awards paid to clinical trial participants or patients; |
| |
● |
the inability to commercialize any products that we may develop. |
We currently hold $1 million in product liability insurance coverage in the aggregate, with a per incident limit of $1 million, which may not be adequate to cover all liabilities that we may incur. We may need to increase our insurance coverage as we expand our clinical trials or if we commence commercialization of our products. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Our Business and Managing Our Growth
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
Recruiting and retaining qualified scientific and clinical personnel and, if we progress the development of any of our products, commercialization, manufacturing and sales and marketing personnel, will be critical to our success. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize our products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high-quality personnel, our ability to pursue our growth strategy will be limited.
We expect to expand our development and regulatory capabilities and potentially implement sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
As of May 22, 2024, we had 6 full-time employees, 1 part-time employee, and 7 consultants. As the clinical development of our products progresses, we also expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of research, product development and regulatory affairs, including a sales and marketing team for our existing products. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Significant disruptions of our information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
We are increasingly dependent on information technology systems and infrastructure, including mobile technologies, to operate our business. In the ordinary course of our business, we collect, store, process and transmit large amounts of sensitive information, including intellectual property, proprietary business information, personal information and other confidential information. It is critical that we do so in a secure manner to maintain the confidentiality, integrity and availability of such sensitive information. We have also outsourced elements of our operations, including elements of our information technology infrastructure, to third parties and, as a result, we manage a number of third-party vendors who may or could have access to our computer networks or our confidential information. In addition, many of those third parties in turn subcontract or outsource some of their responsibilities to other third parties. While all information technology operations are inherently vulnerable to inadvertent or intentional security breaches, incidents, attacks and exposures, the accessibility and distributed nature of our information technology systems, and the sensitive information stored on those systems, make such systems potentially vulnerable to unintentional or malicious, internal and external attacks on our technology environment. Potential vulnerabilities can be exploited from inadvertent or intentional actions of our employees, third-party vendors, or business partners or by malicious third parties. Attacks of this nature are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives (including industrial espionage) and expertise, including organized criminal groups, “hacktivists,” nation states and others. In addition to the extraction of sensitive information, such attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. In addition, the prevalent use of mobile devices increases the risk of data security incidents.
Significant disruptions of our third-party vendors’ information technology systems or other similar data security incidents could adversely affect our business operations and result in the loss, misappropriation and unauthorized access, use or disclosure of, or the prevention of access to, sensitive information, which could result in financial, legal, regulatory, business and reputational harm to us. In addition, information technology system disruptions, whether from attacks on our technology environment or from computer viruses, natural disasters, terrorism, war or telecommunication and electrical failures, could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data.
There is no way of knowing with certainty whether we have experienced any data security incidents that have not been discovered. While we have no reason to believe this to be the case, attackers have become very sophisticated in the way they conceal access to systems, and many companies that have been attacked are not aware that they have been attacked. Any event that leads to unauthorized access, use or disclosure of personal information, including personal information regarding our patients or employees, could disrupt our business, harm our reputation, compel us to comply with applicable federal and state breach notification laws and foreign law equivalents, subject us to time-consuming, distracting and expensive litigation, regulatory investigation and oversight or mandatory corrective action, require us to verify the correctness of database contents or otherwise subject us to liability under laws, regulations and contractual obligations, including those that protect the privacy and security of personal information. This could result in increased costs to us, and result in significant legal and financial exposure and reputational harm. In addition, any failure or perceived failure by us or our vendors or business partners to comply with our privacy, confidentiality or data security-related legal or other obligations to third parties, or any further security incidents or other inappropriate access events that result in the unauthorized access, release or transfer of sensitive information, which could include personally identifiable information, may result in governmental investigations, enforcement actions, regulatory fines, litigation or public statements against us by advocacy groups or others, and could cause third parties, including clinical sites, regulators or current and potential partners, to lose trust in us, or we could be subject to claims by third parties that we have breached our privacy- or confidentiality-related obligations. Moreover, data security incidents and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. While we have implemented security measures intended to protect our information technology systems and infrastructure, there can be no assurance that such measures will successfully prevent service interruptions or security incidents.
If we engage in future acquisitions or strategic collaborations, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
From time to time, we may evaluate various acquisitions and strategic collaborations, including licensing or acquiring intellectual property rights, technologies or businesses, as deemed appropriate to carry out our business plan. Any potential acquisition or strategic collaboration may entail numerous risks, including:
| |
● |
increased operating expenses and cash requirements; |
| |
● |
the assumption of additional indebtedness or contingent liabilities; |
| |
● |
assimilation of operations, intellectual property and products of an acquired company, including difficulties associated with integrating new personnel; |
| |
● |
the diversion of our management’s attention from our existing product programs and initiatives in pursuing such a strategic partnership, merger or acquisition; |
| |
● |
retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships; |
| |
● |
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or products and regulatory approvals; and |
| |
● |
our inability to generate revenue from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
We are subject to the risks of conducting business internationally.
The global tensions resulting from the Russia-Ukraine conflict and the conflict in Israel and the Gaza Strip have increased supply interruptions throughout the world and in the United States and may hinder our ability to find the materials we need to make our products. Although, to date, there has been minimal effect upon our business, supply disruptions could make it harder for us to find favorable pricing and reliable sources for the materials we need, putting upward pressure on our costs and increasing the risk that we may be unable to acquire the materials and services we need to continue to make certain products.
Risks Related to Doing Business in China
The medical industry in China is highly regulated and such regulations are subject to change which may affect approval and commercialization of our products.
A material portion of our research is expected to be conducted in China through the Joint Venture, which we believe confers clinical, commercial and regulatory advantages, but may subject the Joint Venture (and also potentially us) to significant regulatory, liquidity, and enforcement risks. The medical industry in China is subject to comprehensive government regulation and supervision, encompassing the approval, registration, manufacturing, packaging, licensing and marketing of new drugs. In recent years, the regulatory framework in China regarding the medical industry has undergone significant changes, and we expect that it will continue to undergo significant changes. Any such changes or amendments may result in increased compliance costs on our business or cause delays in or prevent the successful development or commercialization of our products in China and reduce the current benefits we believe are available to us from researching our products in China. The PRC authorities have become increasingly vigilant in enforcing laws in the medical industry and any failure by us or our partners to maintain compliance with applicable laws and regulations or obtain and maintain required licenses and permits may result in the suspension or termination of our business activities in China. We believe our strategy and approach are aligned with the PRC government’s regulatory policies, but we cannot ensure that our strategy and approach will continue to be aligned. In the event that there are changes, we and the Joint Venture will take any and all actions to remain in compliance with any such laws or regulations or detailed implementations and interpretations thereof.
There may be difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us based on foreign laws.
We are conducting our research in China through the Joint Venture. Also, the Joint Venture was formed under the laws of Hong Kong and is physically located in Hong Kong. Our joint venture partner, Wider, is located in China. As a result, it may be difficult to effect service of process upon the Joint Venture inside China. It may also be difficult to enforce in U.S. courts judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against the Joint Venture. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts against the Joint Venture predicated upon the civil liability provisions of the securities laws of the United States or any state.
It may be difficult for us to enforce our rights with respect to the Joint Venture. The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other forms of written arrangement with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment by us against Wider or the Joint Venture if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security, or the public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
It may be difficult for overseas regulators to conduct investigations or collect evidence within China.
It may be difficult for you or overseas regulators, such as the Securities and Exchange Commission (SEC), the Department of Justice (DOJ) and other authorities of the United States, to conduct investigations or collect evidence within China. For example, in China, there are significant legal and other obstacles to obtaining information, documents and materials needed for regulatory investigations or litigation outside China or otherwise with respect to foreign entities. Although the authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the United States may not be efficient in the absence of mutual and practical cooperation mechanism. Furthermore, according to Article 177 of the PRC Securities Law, which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC. Accordingly, without the consent of the competent PRC securities regulators and relevant authorities, no entity or individual may provide the documents and materials relating to securities business activities to overseas parties. While detailed interpretation of or implementing rules under Article 177 have yet to be promulgated, the inability for an overseas securities regulator to directly conduct investigation or evidence collection activities within China may further increase difficulties faced by you in protecting your interests.
The PRC’s economic, political and social conditions, as well as governmental policies, could affect the business environment and financial markets in China, and our ability to operate our business, maintain our liquidity and keep our access to capital.
We expect that a portion of our operations will be conducted in China through the Joint Venture. Accordingly, our business, results of operations, financial condition and prospects may be influenced to a significant degree by economic, political, legal and social conditions in China. China’s economy differs from the economies of developed countries in many respects, including with respect to the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. While the PRC economy has experienced significant growth over the past thirty years, growth has been uneven across different regions and among various economic sectors of China. The PRC government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures may benefit the overall PRC economy but may have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are currently applicable to us. In addition, in the past the PRC government implemented certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity in China, which may adversely affect our business and results of operation. More generally, if the business environment in China deteriorates from the perspective of domestic or international investment, our business in China may also be adversely affected.
Uncertainties with respect to the PRC legal system could adversely affect us.
The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions under the civil law system may be cited for reference but have limited precedential value.
In 1979, the PRC government began to promulgate a comprehensive system of laws and regulations governing economic matters in general. The overall effect of legislation over the past four decades has significantly enhanced the protection afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system, and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, the interpretation and enforcement of these laws and regulations involve uncertainties. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory provisions and contractual terms, it may be difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy. These uncertainties may affect our judgment on the relevance of legal requirements and our ability to enforce our contractual rights or tort claims. In addition, the regulatory uncertainties may be exploited through unmerited or frivolous legal actions or threats in attempts to extract payments or benefits from us.
In addition, any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention.
In May 2019, the Cyberspace Administration of China (“CAC”) issued strict guidelines for the collection and use of data by operators in China. At this time, Wider does not share any data from any hospital setting or research setting with Nexalin and Nexalin does not share any data from any hospital setting or research setting with Wider. All clinical data, patient data, provider data associated with China and the U.S. do not affect the design or statistical interpretation of preclinical or clinical studies in either country.
Uncertainties in the interpretation and enforcement of Chinese laws and regulations could limit the legal protections available to us.
The PRC legal system is based on written statutes and prior court decisions have limited value as precedents. Since these laws and regulations are relatively new and the PRC legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involves uncertainties.
From time to time, we may have to resort to administrative and court proceedings to enforce our legal rights, most notably our rights with respect to the Joint Venture. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. Furthermore, the PRC legal system is based in part on government policies and internal rules. As a result, we may not be able to keep ourselves updated with these policies and rules in time. Such uncertainties, including uncertainty over the scope and effect of our contractual property (including intellectual property) and procedural rights, could materially and adversely affect our business and impede our ability to continue our operations.
Restrictions on foreign currency may limit our ability to receive and use our revenue effectively.
The PRC government imposes controls on the conversion of the Renminbi into foreign currencies and, in certain cases, the remittance of foreign currency out of China. To date, the payments we have received from Wider have been in United States dollars, although in the future, payments from Wider or from the Joint Venture may be in Renminbi. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval of China’s State Administration of Foreign Exchange (SAFE), by complying with certain procedural requirements. However, approval from or registration with appropriate government authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans denominated in foreign currencies. As a result, we would need to obtain approval from SAFE to use cash generated from our operations to pay off any debt in a currency other than Renminbi owed to entities outside China, or to make other capital expenditure payments outside China in a currency other than Renminbi. The PRC government may restrict access to foreign currencies for current account transactions in the future. The foreign exchange control system could prevent us from obtaining sufficient foreign currencies to satisfy our foreign currency demands.
Fluctuation in exchange rates could have a negative effect on our results of operations and the value of an investment in the Company.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions in China and by China’s foreign exchange policies. Since June 2010, the Renminbi has fluctuated against the U.S. dollar, at times significantly and unpredictably. On November 30, 2015, the Executive Board of the International Monetary Fund, or IMF, completed the regular five-year review of the basket of currencies that make up the Special Drawing Right, or the SDR, and decided that with effect from October 1, 2016, the Renminbi is determined to be a freely usable currency and will be included in the SDR basket as a fifth currency, along with the U.S. dollar, the euro, the Japanese yen and the British pound. Since the fourth quarter of 2016, the Renminbi has depreciated significantly in the backdrop of a surging U.S. dollar and persistent capital outflows of China. With the development of the foreign exchange market and progress toward interest rate liberalization and Renminbi internationalization, the PRC government may in the future announce further changes to the exchange rate system, and we cannot assure you that the Renminbi will not appreciate or depreciate significantly in value against the U.S. dollar in the future. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar in the future.
Very limited hedging options are available in China to reduce our exposure to exchange rate fluctuations. As of the date of this prospectus, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may decide to enter into hedging transactions in the future, the availability and effectiveness of these hedges may be limited and we may not be able to adequately hedge our exposure or hedge our exposure at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert Renminbi into foreign currency or to convert foreign currency into Renminbi.
The approval of the CSRC, and other compliance procedures may be required in connection with any offering we may make and, if required, we cannot predict whether we will be able to obtain such approval.
We do not have any operations in China and will not have any operations other than the Joint Venture following its formation, of which there can be no assurance. As of the date of this prospectus, (i) our business operations are carried on outside of China; and (ii) we do not maintain any variable interest entity structure or operate any data center in China. We do not believe that sales of our devices to Wider to date constitute doing business in China. We may still be subject to PRC laws relating to, among others, data security and restrictions over foreign investments due to the complexity of the regulatory regime in China, and the recent statements and regulatory actions by the PRC government relating to data security may affect our business operations in China or even our ability to offer securities in the United States. Our securities are not being offered or sold directly or indirectly in China to or for the benefit of, legal or natural persons of the PRC. Therefore, we have not obtained the approval from either the China Securities Regulatory Commission (the “CSRC”) or the Cyberspace Administration of China (the “CAC”) for any offering we may make in the future, and we do not intend to obtain the approval from either the CSRC or the CAC in connection with any such future offering, since we do not believe that such approval is required under these circumstances. Under the PRC’s current legal system, Chinese citizens have the right to purchase securities publicly issued by overseas companies through legal channels and enjoy corresponding benefits of such ownership. Ownership of such securities does not require approval from the CSRC or the CAC.
On the website of the CSRC, the CSRC provides that in accordance with current laws and regulations, domestic Chinese residents can invest in overseas securities markets through legal channels such as purchasing qualified domestic institutional investor (QDII) fund product shares and participating in Shanghai Hong Kong stock transactions.
There can be no assurance however, that regulators in China will not take a contrary view or will not subsequently require us to undergo the approval procedures and subject us to penalties for non-compliance. The approval of the CSRC or the CAC, and other compliance procedures may be required in connection with any offering we may make and, if required, we cannot predict whether we will be able to obtain such approval.
Recent regulatory developments in China may subject the Joint Venture to additional regulatory review and disclosure requirement, expose the Joint Venture to government interference, or otherwise restrict our ability to offer securities and raise capital outside China, all of which could materially and adversely affect our business and the value of our securities.
In light of the recent statements by the Chinese government indicating its intention to exert more oversight and control over overseas offerings of China-based companies and the proposed CAC review for certain data processing operators in China, we may adjust our business operations in the future, to comply with PRC laws regulating our industry and our business operations through the Joint Venture. However, such efforts may not be completed in a liability-free manner or at all. We cannot that we will not be subject to PRC regulatory inspection and/or review relating to cybersecurity, especially when there remains significant uncertainty as to the scope and manner of the regulatory enforcement. If the Joint Venture is subject to regulatory inspection and/or review by the CAC or other PRC authorities or are required by them to take any specific actions, it could cause suspension or termination of the future offering of our securities, disruptions to our operations, result in negative publicity regarding our company, and divert our managerial and financial resources. The Joint Venture may also be subject to fines or other penalties, which could materially and adversely affect our business, financial condition, and results of operations.
We may be subject to PRC laws relating to, among others, data security and restrictions over foreign investments in value-added telecommunications services and other industry sectors set out in the Special Administrative Measures (Negative List) for the Access of Foreign Investment (2020 Edition). Specifically, we may be subject to PRC laws relating to the collection, use, sharing, retention, security, and transfer of confidential and private information, such as personal information and other data. These PRC laws apply not only to third-party transactions, but also to transfers of information between us and our wholly foreign-owned enterprises in China, and other parties with which we have commercial relations. These PRC laws and their interpretations and enforcement continue to develop and are subject to change, and the PRC government may adopt other rules and restrictions in the future. The recent regulatory developments in China, in particular with respect to restrictions on China-based companies raising capital offshore, and the government-led cybersecurity reviews of certain companies with VIE structure, may lead to additional regulatory review in China over our financing and capital raising activities in the United States. Pursuant to the PRC Cybersecurity Law, which was promulgated by the Standing Committee of the National People’s Congress on November 7, 2016 and took effect on June 1, 2017, personal information and important data collected and generated by a critical information infrastructure operator in the course of its operations in China must be stored in China, and if a critical information infrastructure operator purchases internet products and services that affect or may affect national security, it should be subject to cybersecurity review by the CAC.
The PRC Cybersecurity Law also establishes more stringent requirements applicable to operators of computer networks, especially to operators of networks which involve critical information infrastructure. The PRC Cybersecurity Law contains an overarching framework for regulating Internet security, protection of private and sensitive information, and safeguards for national cyberspace security and provisions for the continued government regulation of the Internet and content available in China. The PRC Cybersecurity Law emphasizes requirements for network products, services, operations and information security, as well as monitoring, early detection, emergency response and reporting. Due to the lack of further interpretations, the exact scope of “critical information infrastructure operator” remains unclear.
On July 10, 2021, the CAC publicly issued the Cybersecurity Review Measures (the “Draft Measures”) for public comments until July 25, 2021. According to the Draft Measures, the scope of cybersecurity reviews is extended to data processing operators engaging in data processing activities that affect or may affect national security. The Draft Measures further requires that any operator applying for listing on a foreign exchange must go through cybersecurity review if it possesses personal information of more than one million users. According to the Draft Measures, a cybersecurity review assesses potential national security risk that may be brought about by any procurement, data processing, or overseas listing. The review focuses on several factors, including, among others, (1) the risk of theft, leakage, corruption, illegal use or export of any core or important data, or a large amount of personal information, and (2) the risk of any critical information infrastructure, core or important data, or a large amount of personal information being affected, controlled or maliciously exploited by a foreign government after a company is listed overseas. While the Draft Measures have been released for consultation purposes, there is still uncertainty regarding the final content of the Draft Measures, its adoption timeline or effective date, its final interpretation and implementation, and other aspects. Furthermore, the Standing Committee of the National People’s Congress passed the Personal Information Protection Law of the PRC (“PIPL”), which became effective November 1, 2021, and requires general network operators to obtain a personal information protection certification issued by recognized institutions in accordance with the CAC regulation before such information can be transferred out of China.
Additionally, the Company does not currently believe any of the Company’s scientific data resulting from activities in China to be conducted by the Joint Venture would fall within the Measures for the Management of Scientific Data promulgated by the General Office of the PRC State Council. Therefore, we do not believe the PRC would prevent us from seeking foreign approval and commercialization of our product candidates. In the event the Joint Venture becomes subject to cybersecurity inspection and/or review by the CAC or other PRC authorities or are required by them to take any specific actions, we and the Joint Venture will take any and all actions to remain in compliance with any such laws or regulations or detailed implementations and interpretations thereof.
On July 30, 2021, in response to regulatory developments in China and actions adopted by the PRC government, the Chairman of the SEC issued a statement requesting additional disclosures from offshore issuers with China-based operating companies before their registration statements will be declared effective, including detailed disclosure related to variable interest entity structures and whether the variable interest entity and the issuer, when applicable, received or were denied permission from the PRC authorities to list on U.S. exchanges and the risks that such approval could be denied or rescinded.
On August 1, 2021, the CSRC stated that it had taken note of the new disclosure requirements announced by the SEC regarding the listings of Chinese companies and the recent regulatory development in China, and that the securities regulators in both countries should strengthen communications on regulating China-related issuers. In light of our business operations, we should not be required to undergo the CAC review for any offering that we may make. However, if the enacted version of the Draft Measures mandates clearance of cybersecurity review and other specific actions to be completed by companies aiming to offer securities outside China, we cannot assure you that the PRC regulatory authorities will not take a contrary view or will not subsequently require us to undergo the approval procedures and subject us to penalties for non-compliance, or that if we are required to obtain such clearance, such clearance can be timely obtained, or at all. If the Joint Venture becomes subject to cybersecurity inspection and/or review by the CAC or other PRC authorities or are required by them to take any specific actions, it could cause suspension or termination of the future offering of our securities, disruptions to our operations, result in negative publicity regarding our company, and divert our managerial and financial resources. We may also be subject to significant fines or other penalties, which could materially and adversely affect our business, financial condition and results of operations. In the event the Joint Venture becomes subject to cybersecurity inspection and/or review by the CAC or other PRC authorities or are required by them to take any specific actions, we and the Joint Venture will take any and all actions to remain in compliance with any such laws or regulations or detailed implementations and interpretations thereof.
The PRC government has significant influence by enforcing existing rules and regulation, adopting new ones, or changing relevant industrial policies in a manner that may materially increase our compliance cost, change relevant industry landscape or otherwise cause significant changes to our business operations in China, which could result in material and adverse changes in our operations and cause the value of our securities to significantly decline or be worthless.
The PRC government has significant influence by allocating resources, providing preferential treatment to particular industries or companies, or imposing industry-wide policies on certain industries. The PRC government may also amend or enforce existing rules and regulation, or adopt ones, which could materially increase our compliance costs of the Joint Venture, change the relevant industry landscape, or cause significant changes to the Joint Venture business operations in China. In addition, the PRC regulatory system is based in part on government policies and internal guidance, some of which are not published on a timely basis, or at all, and some of which may even have a retroactive effect. We may not be aware of all non-compliance incidents at all times, and we may face regulatory investigation, fines and other penalties as a consequence. As a result of the changes in the industrial policies of the PRC government, including the amendment to and/or enforcement of the related laws and regulations, companies with China-based operations, including us, and the industries in which we operate, face significant compliance and operational risks and uncertainties. For example, on July 24, 2021, Chinese state media, including Xinhua News Agency and China Central Television, announced a broad set of reforms targeting private education companies providing after-school tutoring services and prohibiting foreign investments in institutions providing such after-school tutoring services. As a result, the market value of certain U.S. listed companies with China-based operations in the affected sectors declined substantially. We are not aware of any similar regulations that may be adopted to significantly curtail our business operations. However, if such other adverse regulations or policies are adopted in China, the Joint Venture may be materially and adversely affected, which may significantly disrupt our operations and adversely affect our business. In the event any of the foregoing were to occur, we and the Joint Venture will take any and all actions to remain in compliance with any such regulations or policies.
We may be subject to anti-monopoly concerns as a result of our doing business in China.
Article 3 of Anti-Monopoly Law of the People’s Republic of China prohibits “monopolistic practices,” which include: a) the conclusion of monopoly agreements between operators; b) the abuse of dominant market position by operators; c) concentration of undertakings which has or may have the effect of eliminating or restricting market competition. Also, according to Article 19, the operator(s) will be assumed to have a dominant market position if it has following situation: a) an operator has 50% or higher market share in a relevant market; b) two operators have 66% or higher market share in a relevant market; c) three operators have 75% or higher market share in a relevant market. We believe we have not conducted any monopolistic practices in China, and that recent statements and regulatory actions by the Chinese government do not impact our ability to conduct business, accept foreign investments, create the Joint Venture with Wider or list on a U.S. or other foreign stock exchange. However, there can be no assurance that regulators in China will not promulgate new laws and regulations or adopt new series of regulatory actions which may require us or the Joint Venture to meet new requirements on the issues mentioned above.
We may be subject to regulatory and other risks if we were to operate Variable Interest Entities in China
In July 2021, the Chinese government provided new guidance on China-based companies raising capital outside of China, including through arrangements called variable interest entities (“VIEs”). In light of such developments, the SEC has imposed enhanced disclosure requirements on China-based companies seeking to register securities with the SEC. Although we do not have a VIE structure, due to our Joint Venture, any future Chinese, U.S. or other rules and regulations that place restrictions on capital raising or other activities may adversely affect our business and results of operations. If the business environment in China deteriorates from the perspective of domestic or international investment, or if relations between China and the United States or other governments deteriorate, the Chinese government may intervene with our operations and our business in China and United States, as well as the market price of our securities, may also be adversely affected.
Our business does not appear to be within the targeted areas of concern by the Chinese government. However, because of our Joint Venture, there is a risk that the Chinese government may in the future seek to affect operations of any company with any level of operations in Hong Kong or China, including its ability to offer securities to investors, list its securities on a U.S. or other foreign exchange, conduct its business or accept foreign investment. Substantial uncertainties and restrictions with respect to the political and economic policies of the PRC government and PRC laws and regulations could have a significant impact upon the business that we may be able to conduct in the PRC and accordingly on the results of our operations and financial condition. If any or all of the foregoing were to occur, it could, in turn, result in a material change in the Company’s operations and/or the value of its common stock and/or significantly limit or completely hinder its ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. Furthermore, in the event any of the foregoing were to occur or to be interpreted differently, we and the Joint Venture will take any and all actions to remain in compliance with any such laws or regulations or detailed implementations and interpretations thereof.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent protection for our technologies and products, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technologies and products similar or identical to ours, and our ability to successfully commercialize our technologies and products may be impaired.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our products. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our technologies and products. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage that we may have, which could harm our business and ability to achieve profitability. To protect our proprietary positions, we file patent applications in the United States and abroad related to our novel technologies and products that are important to our business. The patent application and prosecution processes are expensive and time-consuming. We and our current licensees, or any future licensors and licensees may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. We or our current licensees, or any future licensors or licensees may also fail to identify patentable aspects of our research and development before it is too late to obtain patent protection. Therefore, these and any of our patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, such as with respect to proper priority claims, inventorship, claim scope or patent term adjustments. If our current licensees, or any future licensors or licensees, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised and we might not be able to prevent third parties from making, using and selling competing products. If there are material defects in the form or preparation of our patents or patent applications, such patents or applications may be invalid and unenforceable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Any of these outcomes could impair our ability to prevent competition from third parties.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Furthermore, changes in patent laws in the United States, including the America Invents Act of 2011, may affect the scope, strength and enforceability of our patent rights or the nature of proceedings that may be brought by us related to our patent rights.
We may not be aware of all third-party intellectual property rights potentially relating to our current and future products. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until eighteen months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. Similarly, should we own any patents or patent applications in the future, we may not be certain that we were the first to file for patent protection for the inventions claimed in such patents or patent applications. As a result, the issuance, scope, validity and commercial value of our patent rights cannot be predicted with any certainty. Moreover, we may be subject to a third-party pre-issuance submission of prior art to the U.S. Patent and Trademark Office (the “USPTO”), or become involved in opposition, derivation, re-examination, inter partes review or interference proceedings, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
Our pending and future patent applications may not result in patents being issued that protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection against competing products or processes sufficient to achieve our business objectives, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. Alternatively, our competitors may seek approval to market their own products similar to or otherwise competitive with our products. In these circumstances, we may need to defend and/or assert our patents, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid and/or unenforceable.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technologies and products, or limit the duration of the patent protection of our technologies and products. In addition, given the amount of time required for the development, testing and regulatory review of new products, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our issued patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming and divert the time and attention of our management and scientific personnel. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, trademarks, copyrights or other intellectual property. In addition, in a patent infringement proceeding, there is a risk that a court will decide that a patent of ours is invalid or unenforceable, in whole or in part, and that we do not have the right to stop the other party from using the invention at issue. There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent’s claims narrowly or decide that we do not have the right to stop the other party from using the invention at issue on the grounds that our patents do not cover the invention. An adverse outcome in a litigation or proceeding involving our patents could limit our ability to assert our patents against those parties or other competitors and may curtail or preclude our ability to exclude third parties from making and selling similar or competitive products. Similarly, if we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
In any infringement litigation, any award of monetary damages we receive may not be commercially valuable. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings.
Accordingly, despite our efforts, we may not be able to prevent third parties from infringing, misappropriating or successfully challenging our intellectual property rights. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a negative impact on our ability to compete in the marketplace.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could significantly harm our business.
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell our products and use our proprietary technologies without infringing the intellectual property and other proprietary rights of third parties.
There is potential for a substantial amount of intellectual property litigation in our industry, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to our technology or products, including interference proceedings before the USPTO. Intellectual property disputes arise in a number of areas including with respect to patents, use of other proprietary rights and the contractual terms of license arrangements. Third parties may assert claims against us based on existing or future intellectual property rights. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance.
If we are found to infringe a third party’s intellectual property rights, we could be forced, including by court order, to cease developing, manufacturing or commercializing the infringing product candidate or product. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing product candidate.
However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our products or force us to cease some of our business operations. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative effect on our business.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent and trademark protection for our products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect our trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets. Monitoring unauthorized uses and disclosures of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be effective. In addition, we may not be able to obtain adequate remedies for any such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets.
Moreover, our competitors may independently develop knowledge, methods and know-how equivalent to our trade secrets. Competitors could purchase our products and replicate some or all of the competitive advantages we derive from our development efforts for technologies on which we do not have patent protection. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on products in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those in the United States. In some cases, we may not be able to obtain patent protection for certain licensed technology outside the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States, even in jurisdictions where we do pursue patent protection. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, even in jurisdictions where we do pursue patent protection or from selling or importing products made using our inventions in and into the United States or other jurisdictions.
Competitors may use our technologies in jurisdictions where we have not pursued and obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and preclinical programs and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents, if pursued and obtained, or marketing of competing products in violation of our proprietary rights generally.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us.
We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Regulatory Approval of Our Products and Other Legal Compliance Matters
Even if we complete the necessary preclinical studies and clinical trials, the regulatory approval process is expensive, time-consuming and uncertain and may prevent us or any future collaborators from obtaining approvals for the commercialization of some or all of our products. As a result, we cannot predict when or if, and in which territories, we, or any future collaborators, will obtain marketing approval to commercialize a product candidate.
Our products and the activities associated with their development and commercialization, including their design, research, testing, manufacture, safety, efficacy, quality control, recordkeeping, labelling, packaging, storage, approval, advertising, promotion, sale, distribution, import, export and reporting of safety and other post-market information, are subject to comprehensive regulation by the FDA and other foreign regulatory agencies including the NMPA. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. As a result of the FDA reclassification ruling in December 2019, which impacted the classification of our devices, we had to suspend marketing of our first-Generation medical device for the treatment of anxiety and insomnia. We are presently communicating with the FDA with regard to amending our previous 510(k) Application for the treatment of anxiety and insomnia with our Gen-1 device in accordance with the FDA ruling. Our Gen-2 and Gen-3 devices have completed development and are in the prototype stage of manufacturing and testing. Securing marketing approval from the FDA in the United States requires the submission of extensive testing and clinical data to regulatory authorities for each therapeutic indication to establish the candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. Our products may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
In addition, changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical, or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit, or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of our products, the commercial prospects for our products may be harmed and our ability to generate revenues will be impaired.
Failure to obtain marketing approval in foreign jurisdictions would prevent our products from being marketed in these territories. Any approval we are granted for our products in the United States would not assure approval of our products in foreign jurisdictions.
To market and sell our products in China and any other jurisdictions, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain approval from the FDA in the United States. The regulatory approval process outside the United States generally includes all the risks associated with obtaining approval from the FDA. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. However, failure to obtain approval in one jurisdiction may impact our ability to obtain approval elsewhere. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
The U.S. FDA, Chinese National Medical Products Administration and other comparable foreign regulatory authorities may not accept data from trials conducted in locations outside of their jurisdiction.
We have chosen, and may continue to choose, to conduct international clinical trials. The acceptance of study data by the FDA, NMPA or other comparable foreign regulatory authority from clinical trials conducted outside of their respective jurisdictions may be subject to certain conditions. In cases where data from foreign clinical trials are intended to serve as the basis for marketing approval in the United States, the FDA will generally not approve the application on the basis of foreign data alone unless (1) the data are applicable to the United States population and United States medical practice; (2) the trials are performed by clinical investigators of recognized competence and pursuant to Current Good Clinical Practice requirements; and (3) the FDA is able to validate the data through an on-site inspection or other appropriate means. The FDA may accept the use of some foreign data to support a marketing approval if the clinical trial meets certain requirements. Additionally, the FDA’s clinical trial requirements, including the adequacy of the subject population studied and statistical powering, must be met. Furthermore, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that the FDA, NMPA or any applicable foreign regulatory authority will accept data from trials conducted outside of its respective jurisdiction. If the FDA, NMPA or any applicable foreign regulatory authority does not accept such data, it would result in the need for additional trials, which would be costly and time-consuming and delay aspects of our business plan, and which may result in our product candidates not receiving approval for commercialization in the applicable jurisdiction.
Even if we obtain marketing approvals for our products, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products and compliance with such requirements may involve substantial resources, which could materially impair our ability to generate revenue.
Even if marketing approval of a product candidate is granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation, including the potential requirements to implement a risk evaluation and mitigation strategy or to conduct costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product. We must also comply with requirements concerning advertising and promotion for any of our products for which we obtain marketing approval. Promotional communications are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labelling. Thus, we will not be able to promote any products we develop for indications or uses for which they are not approved. In addition, manufacturers of approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements including ensuring quality control and manufacturing procedures, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We and our contract manufacturers could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance.
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other misconduct or failure to comply with applicable regulatory requirements. Misconduct by employees and independent contractors, such as principal investigators, consultants, commercial partners and vendors, could include failures to comply with regulations of the FDA and other comparable regulatory authorities, to provide accurate information to such regulators, to comply with manufacturing standards we have established, to comply with healthcare fraud and abuse laws, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and other business arrangements in the healthcare industry are subject to extensive laws intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of business activities, including, but not limited to, research, manufacturing, distribution, pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee and independent contractor misconduct could also involve the improper use of individually identifiable information, including, without limitation, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious
harm to our reputation. In addition, federal procurement laws impose substantial penalties for misconduct in connection with government contracts and require certain contractors to maintain a code of business ethics and conduct. It is not always possible to identify and deter employee and independent contractor misconduct, and any precautions we take to detect and prevent improper activities may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws. If any such actions are instituted against us, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate.
Our current and future relationships with healthcare professionals, principal investigators, consultants, customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, physician payment transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to penalties.
Healthcare providers, physicians, clinicians, and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors may expose us to broadly applicable fraud and abuse and other healthcare laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, that may constrain the business or financial arrangements and relationships through which we research, sell, market and distribute any products for which we obtain marketing approval. In addition, we may be subject to physician payment transparency laws and patient privacy and security regulation by the federal government and by the states and foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws that may affect our ability to operate include the following:
| |
● |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service, for which payment may be made, in whole or in part, under federal and state healthcare programs such as Medicare and Medicaid; |
| |
● |
federal civil and criminal false claims laws, including the federal False Claims Act, which impose criminal and civil penalties, including through civil whistle blower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| |
● |
the civil monetary penalties statute, which imposes penalties against any person or entity who, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent; |
| |
● |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of whether the payor is public or private, knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; |
| |
● |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on “covered entities,” including certain healthcare providers, health plans, and healthcare clearinghouses, as well as their respective “business associates” that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| |
● |
the federal Physician Payments Sunshine Act, created under Section 6002 of Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the “ACA,” and its implementing regulations, created annual reporting requirements for manufacturers of products, devices, biologicals and medical supplies for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and |
| |
● |
analogous state and foreign laws, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require companies to comply with voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or to adopt compliance programs as prescribed by state laws and regulations, or that otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts. |
Further, the ACA, among other things, amended the intent requirement of the federal Anti-Kickback Statute and certain criminal statutes governing healthcare fraud. A person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. In addition, the ACA provided that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
Efforts to ensure that our future business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and pursue our strategy. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including future collaborators, are found not to comply with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also affect our business.
Recently enacted and future legislation may increase the difficulty and cost for us and our collaborators to obtain marketing approval of and commercialize our products and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent, alter or delay marketing approval of our existing or future products, restrict or regulate post-approval activities and affect our ability to profitably sell any products for which we obtain marketing approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. For example, the ACA, which was enacted in the United States in March 2010, includes measures to change health care delivery, decrease the number of individuals without insurance, ensure access to certain basic health care services and contain the rising cost of care. The healthcare reform movement, including the enactment of the ACA, has significantly changed health care financing by both governmental and private insurers in the United States. With respect to pharmaceutical manufacturers, the ACA increased the number of individuals with access to health care coverage, but it simultaneously imposed, among other things, increased liability for rebates and discounts owed to certain entities and government health care programs, and new transparency reporting requirements under the Physician Payments Sunshine Act. For a detailed discussion of the ACA’s provisions of importance to the pharmaceutical industry, as well as a description of reform legislation passed subsequent to the ACA, see the section titled “Business — Government Regulation — Healthcare Reform Efforts” in the 2023 Form 10-K.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, as well as efforts to repeal or replace certain aspects of the ACA. We continue to evaluate the effect that the ACA and its possible repeal and replacement has on our business. It is uncertain the extent to which any such changes may impact our business or financial condition.
In addition to the ACA, other federal health reform measures have been proposed and adopted in the United States. For example, legislation has been enacted to reduce the level of reimbursement paid to providers under the Medicare program over time, as well as phase in alternative payment models for provider services under the Medicare program with the goal of incentivizing the attainment of pre-defined quality measures. As these measures are not fully in effect, and since the U.S. Congress could intervene to prevent their full implementation, at this time, it is unclear how payment reductions or the introduction of the quality payment program will impact overall physician reimbursement under the Medicare program. It is also unclear if changes in Medicare payments to providers would impact such providers’ willingness to prescribe and administer our existing or future products, if approved. Further, there has been heightened governmental scrutiny over the manner in which companies set prices for their marketed products. For example, there have been several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, review the relationship between pricing and patient programs, and reform government program reimbursement methodologies for products.
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our products, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labelling and post-marketing testing and other requirements.
Various new healthcare reform proposals are emerging at the federal and state level. It is also possible that additional governmental action will be taken in response to the COVID-19 pandemic. Any new federal and state healthcare initiatives that may be adopted could limit the amounts that federal and state governments will pay for healthcare products and services, and could harm our business, financial condition and results of operations.
Our business activities may be subject to the U.S. Foreign Corrupt Practices Act, or the FCPA, and similar anti-bribery and anti-corruption laws of other countries in which we operate, as well as U.S. and certain foreign export controls, trade sanctions and import laws and regulations. Compliance with these legal requirements could limit our ability to compete in foreign markets and subject us to liability if we violate them.
If we further expand our operations outside of the United States, we must dedicate additional resources to comply with numerous laws and regulations in each jurisdiction in which we plan to operate. Our business activities may be subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. The FCPA generally prohibits companies and their employees and third-party intermediaries from offering, promising, giving or authorizing the provision of anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Additionally, in many other countries, hospitals owned and operated by the government and doctors and other hospital employees would be considered foreign officials under the FCPA. Recently the SEC and DOJ have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty that all our employees, agents or contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, disgorgement and other sanctions and remedial measures and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international activities, our ability to attract and retain employees and our business, prospects, operating results and financial condition.
In addition, our products and technology may be subject to U.S. and foreign export controls, trade sanctions and import laws and regulations. Governmental regulation of the import or export of our products and technology, or our failure to obtain any required import or export authorization for our products, when applicable, could harm our international sales and adversely affect our revenue. Compliance with applicable regulatory requirements regarding the export of our products may create delays in the introduction of our products in international markets or, in some cases, prevent the export of our products to some countries altogether. Furthermore, U.S. export control laws and economic sanctions prohibit the shipment of certain products and services to countries, governments and persons targeted by U.S. sanctions. If we fail to comply with export and import regulations and such economic sanctions, penalties could be imposed, including fines and/or denial of certain export privileges. Moreover, any new export or import restrictions, new legislation or shifting approaches in the enforcement or scope of existing regulations, or in the countries, persons or products targeted by such regulations, could result in decreased use of our products by, or in our decreased ability to export our products to, existing or potential customers with international operations. Any decreased use of our products or limitation on our ability to export or sell access to our products would likely adversely affect our business.
Risks Related to this Offering
If you purchase shares in this offering, you will suffer immediate and substantial dilution of your investment.
The price per share of our common stock in this offering may exceed the net tangible book value per share of our common stock outstanding prior to this offering. Therefore, if you purchase shares in this offering, you may pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent shares are issued under outstanding options at exercise prices lower than the price of our common stock in this offering, you will incur further dilution. See the section entitled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this offering.
You may experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may at any time offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering.
This offering may cause the trading price of our common stock to decrease.
The number of shares of common stock underlying the securities we propose to issue and ultimately will issue if this offering is completed, may result in an immediate decrease in the market price of our common stock. This decrease may continue after the completion of this offering.
We have broad discretion in the use of our cash and cash equivalents, including the net proceeds we receive in this offering, and may not use them effectively.
Our management has broad discretion to use our cash and cash equivalents, including the net proceeds we receive in this offering, to fund our operations and could spend these funds in ways that do not improve our results of operations or enhance the value of our common stock, and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of product candidates. Pending their use to fund our operations, we may invest our cash and cash equivalents, including the net proceeds from this offering, in a manner that does not produce income or that loses value.
If we are not able to comply with the applicable continued listing requirements or standards of The Nasdaq Stock Market, Nasdaq could delist our common stock.
Our shares of our common stock are listed on the Capital Market tier of the Nasdaq Stock Market, or Nasdaq, under the symbol “NXL.” Nasdaq has rules for continued listing, including, without limitation, minimum market capitalization, minimum stockholders’ equity and other requirements. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including the Minimum Bid Price Rule (as discussed below) and those regarding director independence and independent committee requirements, minimum stockholders’ equity, and certain corporate governance requirements. There can be no assurances that we will be able to comply with the applicable listing standards.
We are required to maintain a minimum bid price of $1.00 per share. On May 10, 2023, the Company received written notice from Nasdaq notifying the Company that it was no longer in compliance with the minimum bid price requirement for continued listing on Nasdaq, as the closing bid price for the Company’s common stock was below $1.00 per share as set forth in the Nasdaq listing rules. The Company was afforded 180 calendar days, or until November 6, 2023, to regain compliance with the Nasdaq listing rules. The Company was unable to regain compliance with the bid price requirement by November 6, 2023.
On November 7, 2023, the Company submitted a letter to NASDAQ requesting a second 180-day period in order to regain compliance with NASDAQ Rule 5550(a)(2). The Company stated in that letter that it believed it will be able to cure the deficiency and increase its stock price to above $1.00 per share pursuant to its plan to do so. On November 7, 2023, the Company received written notice from the Nasdaq Listing Qualifications Department (the “Staff”) that the Company was not eligible for an additional 180 calendar day compliance period because the Company no longer complied with Nasdaq’s $5 million minimum stockholders’ equity initial listing requirement. On January 18, 2024, the Nasdaq Hearing Panel granted the Company a temporary exception to regain compliance with the Minimum Bid Price Rule until March 27, 2024. On March 6, 2024, the Nasdaq Hearing Panel granted the Company a temporary exception to regain compliance with the Minimum Bid Price Rule until April 25, 2024.
On March 7, 2024, The Company’s stockholders approved a proposed amendment to Nexalin’s Certificate of Incorporation (the “Amendment”), pursuant to which Nexalin’s Board of Directors is authorized, in its discretion, to proceed with a reverse stock split. The exact ratio of the reverse stock split would be within the 1-for-4 to 1-for-14 range, and, if enacted, will be determined by our Board and publicly announced by the Company prior to the effective time of the reverse stock split. The sole purpose for the proposed reverse stock split was to increase the per share market price of the Company’s Common Stock to meet the Nasdaq Minimum Bid Price Rule for continued listing on The Nasdaq Capital Market. The filing of the Amendment and the reverse stock split was only to be implemented if Nexalin’s Board determined they were necessary to regain and maintain compliance with the Nasdaq Minimum Bid Price Rule. The Company regained compliance with Nasdaq’s Minimum Bid Price Rule without the necessity of a reverse stock split and the Board did not exercise the authority given to it to file the proposed Amendment.
On April 23, 2024, the Company received notice from Nasdaq notifying the Company that it has regained compliance with Nasdaq’s minimum bid price requirement under Nasdaq Rule 5550(a)(2).
We
are also required to maintain a minimum stockholders’ equity of at least $2,500,000. On May 16, 2024, the Company
received written notice from Nasdaq notifying the Company that it was no longer in compliance with the minimum stockholders’
equity requirement for continued listing on Nasdaq, as the Company’s Quarterly Report on Form 10-Q for the quarter ended
March 31, 2024, reported stockholders’ equity of approximately $2,326,000. The Company is preparing a plan to regain
compliance with the minimum stockholders’ equity requirement, which, if accepted by Nasdaq, can result in a 180 day extension
to regain compliance, during which time the Company’s common stock will continue to be listed.
The trading price of our common stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. The stock market in general and the market for companies in our industry in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. In addition to the factors discussed in these “Risk Factors” sections, these factors include:
| |
● |
the commencement, enrollment or results of our planned and future clinical trials; |
| |
● |
the loss of any of our key scientific or management personnel; |
| |
● |
regulatory or legal developments in the United States, China and other countries; |
| |
● |
the success of competitive products or technologies; |
| |
● |
adverse actions taken by regulatory agencies with respect to our clinical trials or manufacturers; |
| |
● |
changes or developments in laws or regulations applicable to our products and preclinical program; |
| |
● |
changes to our relationships with collaborators, manufacturers or suppliers; |
| |
● |
the results of our testing and clinical trials; |
| |
● |
unanticipated safety concerns; |
| |
● |
announcements concerning our competitors or our industry in general; |
| |
● |
actual or anticipated fluctuations in our operating results; |
| |
● |
changes in financial estimates or recommendations by securities analysts; |
| |
● |
potential acquisitions; |
| |
● |
the results of our efforts to discover, develop, acquire or in-license additional products; |
| |
● |
the trading volume of our securities on Nasdaq; |
| |
● |
sales of our common stock by us, our executive officers and directors or our stockholders or the anticipation that such sales may occur in the future; |
| |
● |
general economic, political and market conditions and overall fluctuations in the financial markets in the United States or China; |
| |
● |
stock market price and volume fluctuations of comparable companies and, in particular, those that operate in our industry; and |
| |
● |
investors’ general perception of us and our business. |
These and other market and industry factors may cause the market price and demand for our common stock to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from selling their shares of our common stock at or above the price paid for the shares and may otherwise negatively affect the liquidity of our common stock. In addition, the stock market in general, and companies in our industry in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies.
Some companies that have experienced volatility in the trading price of their shares have been the subject of securities class action litigation. Any lawsuit to which we are a party, with or without merit, may result in an unfavorable judgment. We also may decide to settle lawsuits on unfavorable terms. Any such negative outcome could result in payments of substantial damages or fines, damage to our reputation or adverse changes to our business practices. Defending against litigation is costly and time-consuming and could divert our management’s attention and our resources. Furthermore, during litigation, there could be negative public announcements of the results of hearings, motions or other interim proceedings or developments, which could have a negative effect on the market price of our common stock.
If equity research analysts do not publish research or reports, or publish unfavorable research or reports about us, our business or our market, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that equity research analysts publish about us and our business. We do not currently have and may never obtain research coverage by equity research analysts. Equity research analysts may elect not to provide research coverage of our common stock, and such lack of research coverage may adversely affect the market price of our common stock. In the event we do have equity research analyst coverage, we will not have any control over the analysts, or the content and opinions included in their reports. The price of our shares could decline if one or more equity research analysts downgrade our shares or issue other unfavorable commentary or research about us. If one or more equity research analysts ceases coverage of us or fails to publish reports on us regularly, demand for our shares could decrease, which in turn could cause the trading price or trading volume of our common stock to decline.
We do not intend to pay cash dividends on our common stock in the foreseeable future.
We have never declared or paid any cash dividends on our common stock, and we currently do not anticipate paying any cash dividends in the foreseeable future. We intend to retain any earnings to finance the development and expansion of our products and business. Accordingly, our stockholders will not realize a return on their investments unless the trading price of our common stock appreciates.
Concentration of ownership of our common stock among our existing executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions and matters submitted to stockholders for approval.
Our executive officers, directors and current beneficial owners of 5% or more of our common stock and their respective affiliates, in the aggregate, beneficially own approximately 27.69% of our outstanding common stock, based on the number of shares of our common stock outstanding as of May [•], 2024. As a result, these persons, acting together, would be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors, any merger, consolidation or sale of all or substantially all of our assets or other significant corporate transactions. In addition, these persons, acting together, may have the ability to control the management and affairs of our company. Accordingly, this concentration of ownership may harm the market price of our common stock by:
| |
● |
delaying, deferring or preventing a change in control; |
| |
● |
entrenching our management and/or the board of directors; |
| |
● |
impeding a merger, consolidation, takeover or other business combination involving us; or |
| |
● |
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
In addition, some of these persons or entities may have interests different than yours. For example, because many of these stockholders purchased their shares at prices substantially below the price at which shares were sold in our public offering and have held their shares for a longer period, they may be more interested in selling our company to an acquirer than other investors, or they may want us to pursue strategies that deviate from the interests of other stockholders.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of our Company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws that became effective on December 1, 2021 (as amended August 11, 2022) may discourage, delay or prevent a merger, acquisition or other change in control of our company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
| |
● |
establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
| |
● |
require that stockholder actions must be affected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
| |
● |
limit who may call stockholder meetings; and |
| |
● |
require the approval of the holders of at least 66.66% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws or remove a director. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired more than 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. These provisions could discourage potential acquisition proposals and could delay or prevent a change in control transaction. They could also have the effect of discouraging others from making tender offers for our common stock, including transactions that may be in your best interests. These provisions may also prevent changes in our management or limit the price that investors are willing to pay for our stock.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware and the federal district courts of the United States of America are the exclusive forums for substantially all disputes between us and our stockholders, including claims under the Securities Act and the Exchange Act, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for:
| |
● |
any derivative action or proceeding brought on our behalf; |
| |
● |
any action asserting a breach of fiduciary duty; |
| |
● |
any action asserting a claim against us or any of our directors, officers, employees or agents arising under the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws; |
| |
|
|
| |
● |
any action or proceeding to interpret, apply, enforce or determine the validity of our amended and restated certificate of incorporation or our amended and restated bylaws; and |
| |
● |
any action asserting a claim against us or any of our directors, officers, employees or agents that is governed by the internal-affairs doctrine. |
Our amended and restated certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act and the Exchange Act.
These exclusive-forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage lawsuits against us and our directors, officers and other employees. If a court were to find either exclusive-forum provision in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving the dispute in other jurisdictions. We note that there is uncertainty as to whether a court would enforce such exclusive-forum provision and that provision may result in increased costs for investors to bring a claim. We also note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder, and that Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, we do not intend to take advantage of some of the exemptions from reporting requirements that are applicable to other public companies that are not emerging growth companies, including:
| |
● |
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act; |
| |
● |
not being required to comply with any requirements that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the consolidated financial statements; |
| |
● |
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| |
● |
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and the trading prices for our securities may be more volatile. We may take advantage of these exemptions until the last day of our fiscal year following the fifth anniversary of the completion of our initial public offering. However, if any of the following events occur prior to the end of such five-year period, (i) our annual gross revenue exceeds $1.235 billion, (ii) we issue more than $1.0 billion of non-convertible debt in any three-year period or (iii) we become a “large accelerated filer,” (as defined in Rule 12b-2 under the Exchange Act), we will cease to be an emerging growth company prior to the end of such five-year period. We will be deemed to be a “large accelerated filer” at such time that we (a) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of our most recently completed second fiscal quarter; (b) have been required to file annual and quarterly reports under the Exchange Act, for a period of at least twelve months; and (c) have filed at least one annual report pursuant to the Exchange Act.
Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements including reduced disclosure obligations regarding executive compensation in this Form 10-K and our other periodic reports and proxy statements.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are emerging growth companies. As a result, changes in rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
We have identified certain material weaknesses in our internal controls, which could impair our ability to produce accurate consolidated financial statements on a timely basis.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, the Sarbanes-Oxley Act and the rules and regulations of The Nasdaq Capital Market, or Nasdaq. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. As required by Section 404 of the Sarbanes-Oxley Act, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting in our Form 10-K filing for each fiscal year. This requires us to incur substantial additional professional fees and internal costs to expand our accounting and finance functions and we expend significant management efforts.
As of the fiscal year ended December 31, 2023, our management evaluated, with the participation of our chief executive officer and chief financial officer, the effectiveness of our disclosure controls and procedures, pursuant to Rule 13a-15(b) under the Exchange Act. Based upon that evaluation, our management concluded that our disclosure controls and procedures were not effective due to the following material weaknesses:
| |
● |
Lack of sufficient resources necessary to provide adequate segregation of duties related to the preparation and review of financial information used in financial reporting and review of controls over the financial reporting process, including the review of reconciliations and the accounting for the Company’s stock options that were granted in the current year; and |
| |
● |
Insufficient IT controls which are effectively designed and implemented, specifically related to user/superuser access to the Company’s financial reporting system. |
Our internal control over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements. If that were to happen, the market price of our common stock could decline and we could be subject to sanctions or investigations by Nasdaq, the SEC, or other regulatory authorities.
Legal Proceedings
From time-to-time, we may become involved in various legal proceedings that arise in the ordinary course of business or otherwise. Legal proceedings are subject to inherent uncertainties as to timing, outcomes, costs, expenses and time expenditures by our management and others on our behalf.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, future revenue, timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated products and prospects are forward-looking statements. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain. Forward-looking statements include, but are not limited to, statements about:
| |
● |
our plans to develop and commercialize our products; |
| |
|
|
| |
● |
our planned clinical trials for our products; |
| |
|
|
| |
● |
the timing of the availability of data from our clinical trials; |
| |
|
|
| |
● |
the timing of our selection of an initial clinical candidate from our program; |
| |
|
|
| |
● |
our ability to take advantage of benefits offered by current and pending legislation related to the development of products addressing antimicrobial resistance; |
| |
|
|
| |
● |
the timing of our planned regulatory filings; |
| |
|
|
| |
● |
the timing of and our ability to obtain and maintain regulatory approvals for our products; |
| |
|
|
| |
● |
the clinical utility of our products and their potential advantages compared to other treatments; |
| |
|
|
| |
● |
our commercialization, marketing and distribution capabilities and strategy; |
| |
|
|
| |
● |
our ability to establish and maintain arrangements for the manufacture of our products; |
| |
|
|
| |
● |
our ability to establish and maintain collaborations and to recognize the potential benefits of such collaborations; |
| |
|
|
| |
● |
our estimates regarding the market opportunities for our products; |
| |
|
|
| |
● |
our intellectual property position and the duration of our patent rights; |
| |
|
|
| |
● |
our estimates regarding future expenses, capital requirements and needs for additional financing; and |
| |
|
|
| |
● |
our expected use of proceeds from this offering. |
We caution you that the foregoing list may not contain all of the forward-looking statements made in this prospectus. We have based these forward-looking statements largely on our current expectations about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
USE OF PROCEEDS
We estimate that the
net proceeds from this offering will be approximately $[●] million, after deducting the Placement Agent’s compensation
and estimated offering expenses payable by us. However, because this is a best-efforts offering with no minimum number of securities
or amount of proceeds as a condition to closing, the actual offering amount, placement agent fees and net proceeds to us are not
presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus, and we
may not sell all or any of the securities we are offering. As a result, we may receive significantly less in net proceeds.
We currently intend to use the net proceeds of this offering for general corporate purposes, including working capital, operating expenses, and capital expenditures.
Circumstances that may give rise to a change in the use of proceeds and the alternate purposes for which the proceeds may be used include:
|
● |
The existence of other opportunities or the need to take advantage of changes in timing of our existing activities; |
|
● |
The need or desire on our part to accelerate, increase or eliminate existing initiatives due to, among other things, changing market conditions and competitive developments; and/or |
|
● |
If strategic opportunities present themselves (including acquisitions, joint ventures, licensing and other similar transactions). |
From time to time, we evaluate these and other factors and we anticipate continuing to make such evaluations to determine if the existing allocation of resources, including the proceeds of this offering, is being optimized.
DIVIDEND POLICY
We have never declared or paid a dividend, and we do not anticipate declaring or paying dividends in the foreseeable future. We currently intend to retain our future earnings, if any, to fund the development and growth of our business. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the board of directors deems relevant, and subject to the restrictions contained in any future financing instruments.
CAPITALIZATION
The following table sets forth our consolidated cash and capitalization, as of May [●], 2024. Such information is set forth on the following basis:
|
● |
on an actual basis; and |
|
● |
on a pro forma as adjusted basis to reflect the issuance of [●] shares of common stock in this offering based on an assumed public offering price of $[●] per share, after deducting estimated placement agent compensation and estimated offering expenses of $[●] payable by us and after the use of net proceeds therefrom. |
The information below is illustrative only. Our capitalization following the closing of this offering will change based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes included in the 2023 Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K.
| |
|
Actual |
|
|
Pro Forma
as adjusted |
|
| Cash, cash equivalents and investments |
|
$ |
|
|
|
$ |
|
|
| |
|
|
|
|
|
|
|
|
| Common stock, $0.001 par value; 100,000,000 shares authorized at May 22, 2024; 7,436,562 shares issued and outstanding at May 22, 2024; [●] shares issued and outstanding, pro forma |
|
$ |
|
|
|
$ |
|
|
| Additional paid-in capital |
|
$ |
|
|
|
$ |
|
|
| Accumulated deficit |
|
$ |
|
|
|
$ |
|
|
| Accumulated other comprehensive loss |
|
$ |
|
|
|
|
|
|
| Total stockholders’ equity |
|
$ |
|
|
|
$ |
|
|
| Total capitalization |
|
$ |
|
|
|
$ |
|
|
DILUTION
If you purchase securities in this offering, your ownership interest will be diluted to the extent of the difference between the public offering price per share of our Common Stock and the as adjusted net tangible book value per share of our Common Stock immediately after giving effect to this offering.
Our net tangible book value as of March 31, 2024, was approximately $[●] million, or approximately $[●] per share of our Common Stock. Our net tangible book value is the amount of our total tangible assets minus total liabilities. Net tangible book value per share as of March 31, 2024, is our net tangible book value divided by the number of shares of Common Stock outstanding as of March 31, 2024.
The information below is illustrative only. Our dilution following the closing of this offering will change based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes included in the 2023 Form 10-K and subsequent quarterly reports.
After giving effect to the sale of the maximum number of common stock offered hereby, or [●] number of shares in this offering at a public offering price of $[●] per share of common stock, and after deducting estimated placement agent compensation and estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31, 2024, would have been approximately $[●] million, or approximately $[●] per share of common stock. This amount represents an immediate increase in as adjusted net tangible book value of $[●] per share of common stock to our existing stockholders and an immediate dilution of $[●] per share of common stock to investors participating in this offering. We determine dilution per share of common stock to investors participating in this offering by subtracting as adjusted net tangible book value per share of common stock after giving effect to this offering from the public offering price per share of common stock paid by investors participating in this offering.
| Public offering price per share |
|
|
|
|
|
$ |
|
|
| Net tangible book value per share before this offering |
|
$ |
|
|
|
|
|
|
| Increase in net tangible book value per share attributable to this offering |
|
|
|
|
|
|
|
|
| As adjusted net tangible book value per share after giving effect to this offering |
|
|
|
|
|
|
|
|
| Dilution per share to new investors participating in this offering |
|
|
|
|
|
$ |
|
|
A $0.10 increase (decrease) in the assumed combined public offering price per share of $[●] would increase (decrease) the as adjusted net tangible book value by approximately $[●] ($[●]) per share and the dilution to new investors by $[●] ($[●]) per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated placement agent compensation and estimated offering expenses payable by us. An increase (decrease) of [●] in the number of shares offered by us would increase (decrease) the as adjusted net tangible book value by approximately $[●] ($[●]) per share and the dilution to new investors by $[●] ($[●]) per share, assuming no change in the public offering price per share of common stock, and after deducting estimated placement agent compensation and estimated offering expenses payable by us.
The number of shares of our Common Stock outstanding is based on an aggregate of 7,436,562 shares of our Common Stock outstanding as May 22, 2024, and excludes:
|
● |
1,152,125 shares of our common stock issuable upon the exercise of outstanding and vested stock options under the 2023 Plan with an average weighted exercise price of $[●]; |
|
● |
347,875 shares of our common stock reserved for future issuance under the 2023 Plan; |
|
● |
3,443,314
shares of our common stock awarded as stock grants and/or options to certain executive officers, directors, employees and
consultants in lieu of cash compensation, some of which has not been earned, subject to stockholder approval of an amendment to the
2023 Plan to increase the number of shares available under such Plan; and |
|
● |
2,662,250 shares of common stock issuable upon exercise
of outstanding warrants issued in our initial public offering.
|
This prospectus reflects and assumes no exercise of
outstanding options or warrants and that all such options and warrants have been or will be earned.
DESCRIPTION OF SECURITIES
The following description of our capital stock and certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries. You should also refer to the amended and restated certificate of incorporation and bylaws, which are filed as exhibits to the registration statement of which this prospectus is part.
General
Our Certificate of Incorporation, as amended to date, we are authorized to issue 100,000,000 shares of common stock, $0.001 par value per share.
Common Stock
Outstanding Shares
As of May 22, 2024, we had 7,436,562 shares of common stock outstanding, held of record by 867 stockholders.
Voting Rights
Each holder of common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders. The affirmative vote of holders of at least 66% of the voting power of all of the then-outstanding shares of capital stock, voting as a single class, will be required to amend certain provisions of our amended and restated certificate of incorporation, including provisions relating to amending our amended and restated bylaws, the classified board, the size of our board, removal of directors, director liability, vacancies on our board, special meetings, stockholder notices, actions by written consent and exclusive forum.
Dividends
Holders of our common stock are entitled to receive ratably any dividends that our board of directors may declare out of funds legally available for that purpose.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities.
Rights and Preferences
Holders of our common stock have no pre-emptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate in the future.
Fully Paid and Nonassessable
All outstanding shares of our common stock are fully paid and non-assessable, and the shares of common stock to be issued upon completion of this offering will be fully paid and non-assessable.
Anti-Takeover Provisions
Certificate of Incorporation and Bylaws to be in Effect Immediately Prior to Completion of this Offering
Our amended certificate of incorporation and amended and restated bylaws:
| |
● |
provide that the authorized number of directors may be changed only by resolution of our board of directors; |
| |
● |
provide that directors may only be removed for cause, which removal may be effected, subject to any limitation imposed by law, by the holders of at least 66% of the voting power of all of our then-outstanding shares of the capital stock entitled to vote generally at an election of directors; |
| |
● |
provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| |
● |
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent or electronic transmission; |
| |
● |
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice; |
| |
● |
provide that special meetings of our stockholders may be called only by the chairman of our board of directors, our chief executive officer or president or by our board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors; and |
| |
● |
not provide for cumulative voting rights, therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election if they should so choose. |
The amendment of any of these provisions would require approval by the holders of at least 66% of the voting power of all our then-outstanding common stock entitled to vote generally in the election of directors, voting together as a single class.
The combination of these provisions will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Because our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts. We believe that the benefits of these provisions, including increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company, outweigh the disadvantages of discouraging takeover proposals, because negotiation of takeover proposals could result in an improvement of their terms.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in a business combination with any interested stockholder for a period of three years following the date the person became an interested stockholder, with the following exceptions:
| |
● |
before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested holder; |
| |
● |
upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (a) by persons who are directors and also officers and (b) pursuant to employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; and |
| |
● |
on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 of the DGCL defines business combination to include the following:
| |
● |
any merger or consolidation involving the corporation and the interested stockholder; |
| |
● |
any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| |
● |
subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| |
● |
any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; and |
| |
● |
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 of the DGCL defines an “interested stockholder” as an entity or person who, together with the entity’s or person’s affiliates and associates, beneficially owns, or is an affiliate of the corporation and within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
A Delaware corporation may “opt out” of these provisions with an express provision in its certificate of incorporation. We have not opted out of these provisions, which may as a result, discourage or prevent mergers or other takeover or change of control attempts of us.
Choice of Forum
Our amended and restated certificate of incorporation will provide that the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us or any of our directors, officers, employees or agents arising under the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws; any action or proceeding to interpret, apply, enforce or determine the validity of our amended and restated certificate of incorporation or our amended and restated bylaws; and any action asserting a claim against us that is governed by the internal affairs doctrine. Our amended and restated certificate of incorporation will further provide that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with one or more actions or proceedings described above, a court could find the choice of forum provisions contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable.
Transfer Agent and Registrar
Our transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company.
Exchange Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “NXL.”
PLAN OF DISTRIBUTION
We are offering [●] shares of our common stock, for gross proceeds of approximately $7.0 million, before deduction of placement agent commissions and offering expenses, in a best-efforts offering.
Pursuant to a placement agency agreement, dated as of May [●], 2024, we have engaged Maxim Group LLC to act as our exclusive Placement Agent to solicit offers to purchase the securities offered by this prospectus. The Placement Agent is not purchasing or selling any securities, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of securities, other than to use its “reasonable best efforts” to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of securities being offered. Investors purchasing securities offered hereby will have the option to execute a securities purchase agreement with us. In addition to the rights and remedies available to all investors in this offering under federal and state securities laws, the investors which enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our securities in this offering. The Placement Agent may engage one or more subagents or selected dealers in connection with this offering.
The placement agency agreement provides that the Placement Agent’s obligations are subject to conditions contained in the placement agency agreement.
The shares will be offered at a fixed price and are expected to be issued in a single closing. There is no minimum number of shares to be sold or minimum aggregate offering proceeds for this offering to close.
We will deliver the securities being issued to the investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. We expect to deliver the securities being offered pursuant to this prospectus on or about [●], 2024.
Placement Agent Fees, Commissions and Expenses
Upon closing of this offering, we will pay the Placement Agent a cash transaction fee equal to 8.0% of the aggregate gross cash proceeds to use from the sale of the securities in the offering. In addition, we have agreed reimburse the Placement Agent for certain of its out-of-pocket expenses incurred in connection with this offering, including the Placement Agent’s legal fees, and actual travel and reasonable out-of-pocket expenses, in an amount not to exceed $110,000. If this offering is not completed, we have agreed to reimburse the Placement Agent for its actual expenses in an amount not to exceed $35,000.
The following table shows the public offering price, Placement Agent fees and proceeds, before expenses, to us, assuming the sale of all shares in this offering.
| |
|
Per Share |
|
|
Total |
|
| Public offering price |
|
$ |
|
|
|
$ |
|
|
| Placement agent fees (8.0%) |
|
$ |
|
|
|
$ |
|
|
| Proceeds to us (before expenses) |
|
$ |
|
|
|
$ |
|
|
We estimate that the total expenses of the offering, including registration and filing fees, printing fees and legal and accounting expenses, but excluding the Placement Agent fees, will be approximately $[●], all of which are payable by us. This figure includes, among other things, the Placement Agent’s expenses (including the legal fees, costs and expenses for the Placement Agent’s legal counsel) that we have agreed to reimburse.
Right of First Refusal
Pursuant to the terms of the placement agency agreement, subject to the closing of this offering, for a period of twelve (12) months after the commencement of sales of securities in this offering, the Placement Agent shall have a right of first refusal to act as lead managing underwriter and sole book runner, sole placement agent, or sole sales agent for any and all future public or private equity, equity-linked or debt (excluding commercial bank debt) offerings for which we retain the service of an underwriter, agent, advisor, finder or other person or entity in connection with such offering during such twelve (12) month period for us, or any successor to us or any of our subsidiaries. We shall not offer to retain any entity or person in connection with any such offering on terms more favorable than terms on which we offer to retain Maxim. Such offer shall be made in writing in order to be effective. Placement Agent shall notify us within ten (10) business days of its receipt of the written offer contemplated above as to whether or not it agrees to accept such retention. If the Placement Agent should decline such retention, we shall have no further obligations to the Placement Agent with respect to the offering for which we have offered to retain Placement Agent.
Other Compensation
We have also agreed to pay the Placement Agent a tail fee equal to 8% of the gross proceeds of any equity, equity-linked or debt or other capital raising activity, if any investor, who was contacted or introduced by the Placement Agent during the term of its engagement, provides us with capital in such a financing during the twelve-month period following the closing of the offering or expiration or termination of our engagement with the representative.
Lock-Up Agreements
We and each of our officers and directors have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any of our common stock or other securities convertible into or exercisable or exchangeable for our common stock for six months after this offering is completed without the prior written consent of the Placement Agent.
The Placement Agent may in its sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up period.
Indemnification
We have agreed to indemnify the Placement Agent against certain liabilities, including certain liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the Placement Agent may be required to make in respect of those liabilities.
Regulation M
The Placement Agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the placement agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the Placement Agent acting as principal. Under these rules and regulations, the Placement Agent (i) may not engage in any stabilization activity in connection with our securities and (ii) may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
Determination of Offering Price
The actual offering price of the securities we are offering was negotiated between us, the Placement Agent and the investors in the offering based on the trading of our shares of common stock prior to the offering, among other things. Other factors considered in determining the public offering price of the securities we are offering include our history and prospects, the market price of our common stock on Nasdaq, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, the general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Electronic Distribution
A prospectus in electronic
format may be made available on a website maintained by the Placement Agent. In connection with the offering, the Placement Agent may
distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe®
PDF will be used in connection with this offering.
Other than the prospectus in electronic format, the information on the Placement Agent’s website and any information contained in any other website maintained by the Placement Agent is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the Placement Agent in its capacity as Placement Agent and should not be relied upon by investors.
Other Relationships
The Placement Agent and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The Placement Agent and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses. However, except as disclosed in this prospectus, we have no present arrangements with the placement agent for any further services.
In the ordinary course of their various business activities, the Placement Agent and certain of its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the Placement Agent or its affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The Placement Agent and its affiliates may hedge such exposure by entering into transactions that consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the securities offered hereby. Any such short positions could adversely affect future trading prices of the securities offered hereby. The Placement Agent and certain of its affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
General
Other than in the United States, no action has been taken by us or the Placement Agent that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Canada
Shares of our common stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the Securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the Securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A-3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the Placement Agent are not required to comply with the disclosure requirements of NI 33-105 regarding Placement Agent’s conflicts of interest in connection with this offering.
United Kingdom
This document is only being distributed to and is only directed at persons who are “qualified investors” (as defined in the Prospectus Directive) who are (i) persons having professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order, (ii) high net worth entities, and (iii) other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. This document and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by any recipients to any other person in the United Kingdom. Any person who is not a relevant person should not act or rely on this document or any of its contents.
European Economic Area
In relation to each Member State of the European Economic Area that has implemented the Prospectus Directive (each, a “Relevant Member State”), an offer to the public of any securities described in this prospectus may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any ordinary shares may be made at any time under the following exemptions under the Prospectus Directive if they have been implemented in that Relevant Member State:
| |
(a) |
to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| |
(b) |
to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) per Relevant Member State, subject to obtaining the prior consent of the Placement Agent; or |
| |
(c) |
in any other circumstances falling within Article 3(2) of the Prospectus Directive; |
provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us or any Placement Agent of a prospectus pursuant to Article 3 of the Prospectus Directive or a supplemental prospectus pursuant to Article 16, of the Prospectus Directive or any measure implementing the Prospectus Directive in a Relevant Member State and each person who initially acquires any securities or to whom any offer is made on the basis of (a) above will be deemed to have represented, acknowledged and agreed that it is a “qualified investor” within the meaning of Article 2(1)(e) of this Prospectus Directive.
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Relevant Member State. The expression “Prospectus Directive” means Directive 2003/71/EC (as amended, including by Directive 2010/73/EU) and includes any relevant implementing measure in each Relevant Member State.
Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this prospectus nor any other offering or marketing material relating to the offering, the Company, or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or the CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
China
This prospectus does not constitute a public offer of the shares offered by this prospectus, whether by sale or subscription, in China. The shares are not being offered or sold directly or indirectly in China to or for the benefit of, legal or natural persons of the PRC.
Further, no legal or natural persons of China may directly or indirectly purchase any of the shares without obtaining all prior Chinese governmental approvals that are required, whether statutorily or otherwise. Persons who come into possession of this prospectus are required by the issuer and its representatives to observe these restrictions.
Hong Kong
The shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each Placement Agent has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
LEGAL MATTERS
The validity of the shares being offered by this prospectus will be passed upon for us by Warshaw Burstein, LLP. Certain legal matters related to this offering will be passed upon for the Placement Agent by Nelson Mullins Riley & Scarborough LLP.
EXPERTS
Marcum LLP, independent
registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K
for the year ended December 31, 2023, as set forth in their report (which report includes an explanatory paragraph referring to
the Company’s ability to continue as a going concern) which is incorporated by reference in this prospectus and elsewhere in the
registration statement. Our financial statements are incorporated by reference in reliance on Marcum LLP’s report, given on their
authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov.
Our web site address is http://www.nexalin.com. There we make available free of charge, on or through the investor relations section of our website, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with the SEC. The information on our web site, however, is not, and should not be deemed to be, a part of this prospectus. All website addresses in this prospectus are intended to be inactive textual references only.
This prospectus is part of a registration statement that we filed with the SEC and does not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC or us, as provided below. You may inspect a copy of the registration statement through the SEC’s website, as provided above.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. We incorporate by reference into this prospectus the documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (1) after the date of this prospectus and prior to the time that all of the securities offered by this prospectus are sold or the earlier termination of the offering, and (2) after the date of the initial registration statement of which this prospectus forms a part and prior to the effectiveness of the registration statement (except in each case in which the information contained in such documents is “furnished” and not “filed”).
This prospectus incorporates by reference the documents set forth below that have previously been filed with the SEC:
|
● |
our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed on March 27, 2024; |
|
● |
any future filings made with the SEC under Section 13(a), 13(c) or 15(d) of the Exchange Act.. |
Any statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded for purposes hereof to the extent that a statement contained in this prospectus or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may request a free copy of any of the documents incorporated by reference in this prospectus (other than exhibits, unless they are specifically incorporated by reference in the documents) by writing or telephoning us at the following address:
Nexalin Technology, Inc.
1776 Yorktown, Suite 550
Houston, TX 77056
(832) 260-0222
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than placement agent compensation, payable by us in connection with the sale of the shares being registered in the registration statement of which this prospectus forms a part. All amounts shown are estimates except for the SEC registration fee, the Financial Industry Regulatory Authority, or FINRA, filing fee and The Nasdaq Capital Market listing fee.
| |
|
Amount to be
Paid |
|
| SEC registration fee |
|
$ |
1,033 |
|
| FINRA filing fee |
|
|
1,550 |
|
| Printing and miscellaneous expenses |
|
|
10,000 |
|
| Legal fees and expenses |
|
|
80,000 |
|
| Placement Agent fees and expenses |
|
|
110,000 |
|
| Accounting fees and expenses |
|
|
30,000 |
|
| Transfer agent and registrar fees and expenses |
|
|
10,000 |
|
| Total |
|
$ |
242,583 |
|
|
(1) |
On April 15, 2024, the Registrant filed a registration statement on Form S-3 (File No. 333- 278702) (the “Prior Registration Statement”), and paid a registration fee of $833.42. The Prior Registration Statement was withdrawn by filing a Form RW on May 15, 2024. The Prior Registration Statement was not declared effective and no securities were sold under the Prior Registration Statement. In accordance with Rule 457(p) under the Securities Act, the Registrant is offsetting the registration fee for this registration statement against the fees previously paid in connection with the Prior Registration Statement. |
Item 14. Indemnification of Directors and Officers.
As permitted by Section 102 of the Delaware General Corporation Law, we have adopted provisions in our amended and restated certificate of incorporation and amended and restated bylaws that limit or eliminate the personal liability of our directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, directors exercise an informed business judgment based on all material information reasonably available to them. Consequently, a director will not be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
| |
● |
any breach of the director’s duty of loyalty to us or our stockholders; |
| |
● |
any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| |
● |
any act related to unlawful stock repurchases, redemptions or other distributions or payment of dividends; or |
| |
● |
any transaction from which the director derived an improper personal benefit. |
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission. Our amended and restated certificate of incorporation also authorizes us to indemnify our officers, directors and other agents to the fullest extent permitted under Delaware law.
As permitted by Section 145 of the Delaware General Corporation Law, our amended and restated bylaws provide that:
| |
● |
we may indemnify our directors, officers and employees to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions; |
| |
● |
we may advance expenses to our directors, officers and employees in connection with a legal proceeding to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions; and |
| |
● |
the rights provided in our bylaws are not exclusive. |
Our amended and restated certificate of incorporation, to be attached as Exhibit 3.2, and our amended and restated bylaws, to be attached as Exhibit 3.3, provide for the indemnification provisions described above and elsewhere herein. We have entered or will enter into, and intend to continue to enter, into separate indemnification agreements with our directors and officers that may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements generally require us, among other things, to indemnify our officers and directors against liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct. These indemnification agreements also generally require us to advance any expenses incurred by the directors or officers as a result of any proceeding against them as to which they could be indemnified. These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers and directors for liabilities, including reimbursement of expenses incurred, arising under the Securities Act of 1933, as amended, or the Securities Act.
We have purchased and currently intend to maintain insurance on behalf of each and every person who is or was a director or officer of our company against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
The form of Placement Agent Agreement, to be attached as Exhibit 1.1 hereto, provides for indemnification by the Placement Agent of us and our officers and directors who sign this Registration Statement for specified liabilities, including matters arising under the Securities Act.
Item 15. Recent Sales of Unregistered Securities.
None.
Item 16. Exhibits and Financial Statement Schedules.
| Exhibit No. |
|
Description of Document |
| 1.1** |
|
Underwriting Agreement dated as of September 15, 2022 between the Registrant and Maxim Group LLC |
| 3.1* |
|
Certificate of Incorporation, as amended and as currently in effect. |
| 3.2* |
|
Amended and Restated Bylaws. |
| 4.1* |
|
Form of Specimen stock certificate evidencing shares of common stock. |
| 4.2*** |
|
Form of Warrant Agent Agreement between the Company and Continental Stock Transfer & Trust Company |
| 4.3* |
|
Form of Warrant Certificate (filed as part of Exhibit 4.2) |
| 5.1 |
|
Opinion of Warshaw Burstein, LLP relating to the legality of the shares |
| 10.1***** |
|
Joint Venture Agreement between the Company and Wider Come Limited dated as of May 31, 2023. |
| 10.2***** |
|
Employment Agreement between the Company and Mark White dated as of July 1, 2023. |
| 10.3***** |
|
Services Agreement between the Company and David Owens, M.D. dated as of July 1, 2023. |
| 10.4* |
|
Quality Assurance Agreement between the Company and Apical Instruments dated December 31, 2020. |
| 10.5* |
|
Advisor Agreement with Leonard Osser dated as of December 22, 2021 |
| 10.6* |
|
Advisor Agreement with Tucker Anderson dated as of December 24, 2021 |
| 10.7* |
|
Advisor Agreement with Gian Domenico Trombetta dated December 24, 2021 |
| 10.8* |
|
Employment Agreement between the Company and Marilyn Elson dated as of January 11, 2022 |
| 10.9* |
|
Amendment and Deferral Agreement dated as of March 30, 2022 to Consulting Agreement between the Company and US Asian Consulting Group LLC |
| 10.10***** |
|
Employment Agreement between the Company and Michael Nketiah dated as of July 1, 2023. |
| 10.11* |
|
Form of Lock-Up Agreement. |
| 10.12* |
|
Consulting Agreement dated as of May 9, 2018 between the Company and US Asianc Consulting Group, LLC, as amended on January 2, 1019 and March 4, 2021 |
| 10.13* |
|
Amended and Restated Promissory Note in favor of Mark White dated as of January 1, 2023. |
| 10.14**** |
|
Distribution Authorization Agreement dated as of May 1, 2019 with Wider Come Limited. |
| 23.1 |
|
Consent of Marcum LLP, independent registered public accounting firm. |
| 23.2 |
|
Consent of Warshaw Burstein, LLP (included in Exhibit 5.1). |
| 99.1* |
|
Code of Ethics |
| 99.2* |
|
Audit Committee Charter |
| 99.3* |
|
Compensation Committee Charter |
| 99.4* |
|
Nominating and Corporate Governance Committee Charter |
| 107 |
|
Filing Fees |
| * |
Previously filed as an exhibit to Form S-1 as declared effective by the SEC on September 15, 2022 (SEC File Number 333-261989). |
| ** |
Previously filed as an exhibit to Form 8-K as filed with the SEC on September 20, 2022. |
| *** |
Previously filed as an exhibit to Form 8-K/A as filed with the SEC on September 20, 2022 |
| **** |
Previously filed as an exhibit to Form 10-Q as filed with the SEC on May 10, 2023 |
| ***** |
Previously filed as an exhibit to Form 10-Q as filed with the SEC on August 10, 2023 |
|
(b) |
Financial Statement Schedules. |
No financial statement schedules have been submitted because they are not required or are not applicable or because the information required is included in the consolidated financial statements or the notes thereto.
Item 17. Undertakings.
The undersigned Registrant hereby undertakes to provide to the Placement Agent at the closing specified in the Placement Agent agreement, certificates in such denominations and registered in such names as required by the Placement Agent to permit prompt delivery to each purchaser insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
| |
(1) |
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective. |
| |
(2) |
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
If we fail to develop and successfully commercialize other current and future products, our business and future prospects may be harmed and our business will be more vulnerable to any problems that we encounter in developing and commercializing our products.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized on May 23, 2024.
| |
NEXALIN TECHNOLOGY, INC. |
| |
|
| |
By: |
/s/
Mark White |
| |
|
Mark White |
| |
|
President
and Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, as amended, this pre effective amendment number 9 to the Registration Statement has been signed below by the following persons in the capacities and on the dates indicated.
| Name |
|
Position/Title |
|
Date |
| |
|
|
|
|
| /s/
Mark White |
|
Director, President and Chief
Executive Officer |
|
May 23, 2024 |
| Mark White |
|
(principal executive officer) |
|
|
| |
|
|
|
|
| /s/
David Owens |
|
Director, Chief Medical Officer |
|
May 23, 2024 |
| David Owens, M.D. |
|
|
|
|
| |
|
|
|
|
| /s/
Leslie Bernhard |
|
Chairman, Board of Directors |
|
May 23, 2024 |
| Leslie Bernhard |
|
|
|
|
| |
|
|
|
|
| /s/
Alan Kazden |
|
Director |
|
May 23, 2024 |
| Alan Kazden |
|
|
|
|
| |
|
|
|
|
| /s/
Ben Hu |
|
Director |
|
May 23, 2024 |
| Ben Hu, M.D |
|
|
|
|
Exhibit 5.1
|
WARSHAW BURSTEIN, LLP
575 Lexington Avenue
New York, NY 10022
Telephone: 212-984-7700
www.wbny.com |
 |
May 23, 2024
Nexalin Technology, Inc.
1776 Yorktown, Suite 550
Houston, Texas 77056
|
Re: |
Registration Statement on Form S-1 |
Ladies and Gentlemen:
We have acted as special counsel to Nexalin Technology, Inc., a Delaware corporation (the “Company”), in connection with its filing on the date hereof with the Securities and Exchange
Commission (the “Commission”) of a registration statement on Form S-1 (the “Registration Statement”) under the Securities Act of 1933, as amended (the “Act”), relating to the proposed public offering of shares of common stock of the Company,
par value $0.001 per share (the “Shares”), with a proposed maximum aggregate offering price of $7,000,000. The Shares will be sold by the Company pursuant to a placement agent agreement to be entered into by and between the Company and Maxim Group LLC (the “Agreement”).
This opinion is being furnished in connection with the requirements of Item 601(b)(5)
of Regulation S-K under the Act, and no opinion is expressed herein as to any matter
pertaining to the contents of the Registration Statement or related prospectus or
prospectus supplement (collectively, the “Prospectus”), other than as expressly stated herein with respect to the issuance of the Shares.
As such counsel, we have examined such matters of fact and questions of law as we
have considered appropriate for purposes of this letter. With your consent, we have
relied upon certificates and other assurances of officers of the Company and others
as to factual matters without having independently verified such factual matters.
We are opining herein as to the General Corporation Law of the State of Delaware (the
“DGCL”), and we express no opinion with respect to any other laws.
WARSHAW BURSTEIN, LLP
Nexalin Technology, Inc.
May 23, 2024
Page 2
Subject to the foregoing and the other matters set forth herein, it is our opinion
that when the Shares shall have been duly registered on the books of the transfer agent
and registrar therefor in the name or on behalf of the purchasers, and have been issued
by the Company against payment therefor (not less than par value) in the circumstances
contemplated by the Agreement, the issuance and sale of the Shares will have been
duly authorized by all necessary corporate action of the Company, and the Shares will
be validly issued, fully paid and nonassessable. In rendering the foregoing opinion, we have assumed that (i) the Company will comply
with all applicable notice requirements regarding uncertificated shares provided in
the DGCL and (ii) upon the issuance of any of the Shares, the total number of shares
of common stock issued and outstanding will not exceed the total number of shares of common stock that the Company is then authorized to issue under its Certificate of Incorporation.
This opinion is for your benefit in connection with the Registration Statement and
may be relied upon by you and by persons entitled to rely upon it pursuant to the
applicable provisions of the Act. We consent to your filing this opinion as an exhibit
to the Registration Statement and to the reference to our firm contained in the Prospectus
under the heading “Legal Matters.” In giving such consent, we do not thereby admit
that we are in the category of persons whose consent is required under Section 7 of the Act or the rules and regulations of the Commission thereunder.
| |
Very truly yours, |
| |
|
| |
/s/ Warshaw Burstein, LLP |
| |
|
| |
WARSHAW BURSTEIN. LLP |
Exhibit 23.1
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S
CONSENT
We consent to the incorporation
by reference in this Registration Statement of Nexalin Technology, Inc. (the “Company”) on Form S-1 of our report dated March
27, 2024, which includes an explanatory paragraph as to the Company’s ability to continue as a going concern, with respect to our
audits of the consolidated financial statements of the Company as of and for the years ended December 31, 2023 and 2022 appearing in the
Annual Report on Form 10-K of the Company for the year ended December 31, 2023. We also consent to the reference to our firm under the
heading “Experts” in the Prospectus, which is part of this Registration Statement.
/s/ Marcum LLP
Marcum LLP
Marlton,
New Jersey
May 23, 2024
Exhibit 107
Calculation of Filing Fee Tables
Form S-1
(Form Type)
Nexalin Technology, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered Securities and Carry
Forward Securities
| |
Security
Type |
Security
Class
Title |
Fee
Calculation
or Carry
Forward
Rule |
Amount
Registered |
Proposed
Maximum
Offering
Price Per
Share |
Proposed
Maximum
Aggregate
Offering
Price(1) |
Fee Rate |
Amount of
Registration
Fee |
Carry
Forward
Form
Type |
Carry
Forward
File
Number |
Carry
Forward
Initial
Effective
Date |
Filing Fee
Previously
Paid in
Connection
with
Unsold
Securities
to be
Carried
Forward |
| Newly Registered Securities |
| Fees to be Paid |
Equity |
Common Stock, par value $0.001 per share |
457(o) |
— |
— |
$7,000,000 |
$147.60 per
$1,000,000
(0.00014760) |
$1,033.20 |
— |
— |
— |
— |
| Fees Previously Paid |
Equity |
Common Stock, par value $0.001 per share |
457(c) |
— |
— |
$5,646,485 |
$147.60 per $1,000,000
(0.00014760) |
$833.42 |
S-3 |
333- 278702 |
— |
$833.42 |
| Carry Forward Securities |
| Carry Forward Securities |
— |
— |
— |
— |
|
— |
— |
— |
— |
— |
— |
— |
| Total Offering Amounts |
|
$7,000,000 |
|
|
|
|
|
|
| Total Fees Previously Paid |
|
|
|
$833.42 |
|
|
|
|
| Total Fee Offsets |
|
|
|
— |
|
|
|
|
| Net Fee Due |
|
|
|
$199.78 |
|
|
|
|
| (1) | Calculated pursuant to Rule 457(o) based on an estimate
of the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the registrant. |
Nexalin Technologies (NASDAQ:NXLIW)
Historical Stock Chart
From May 2024 to Jun 2024
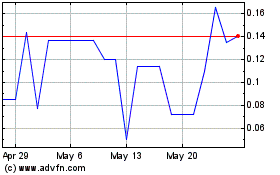
Nexalin Technologies (NASDAQ:NXLIW)
Historical Stock Chart
From Jun 2023 to Jun 2024