Moderna Gets Vaccine Booster Jab Approval from Health Canada
November 15 2021 - 10:18AM
Dow Jones News
By Adriano Marchese
Canada's health regulator has approved Moderna Inc.'s Covid-19
vaccine as a booster dose in individuals over the age of 18, the
biotechnology company said.
On Monday, Moderna said Health Canada has authorized the use of
a third jab to adults who have completed the primary series at
least six months prior.
"Health Canada based this authorization on the totality of
scientific evidence shared by the company, including a data
analysis from the Phase 2 clinical study of mRNA-1273, which was
amended to offer a booster dose of mRNA-1273 at the 50 ug dose
level to interested participants 6-8 months following their second
dose," the company said.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
November 15, 2021 10:03 ET (15:03 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
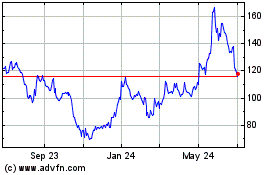
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
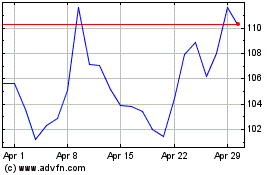
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Apr 2023 to Apr 2024