Bluebird Bio Shares Slide 41% on Black-Box Label, Higher Price for Sickle-Cell Treatment
December 08 2023 - 4:11PM
Dow Jones News
By Ben Glickman
Shares of Bluebird bio fell after the company's sickle-cell
disease treatment received a black-box warning and was announced at
a higher price point than a competitor's treatment.
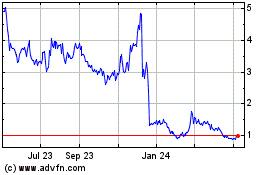
The stock was down 41% to $2.84 on Friday. Shares are down 60%
this year.
The company's Lyfgenia was approved by the Food and Drug
Administration for the treatment of sickle-cell disease. The FDA
also approved Casgevy, a sickle-cell treatment developed by Vertex
Pharmaceuticals and CRISPR Therapeutics using Crispr
gene-modification technology.
The FDA said that Lyfgenia would include a black-box warning
related to certain cases of blood cancer in patients treated with
the drug.
Bluebird said the treatment would come with a $3.1 million price
tag, compared with the $2.2 million price for Casgevy.
Evercore analysts said in a research note that the announcements
gave Casgevy an advantage because of its lack of black-box label
and lower price.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
December 08, 2023 15:56 ET (20:56 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
bluebird bio (NASDAQ:BLUE)
Historical Stock Chart
From Apr 2024 to May 2024
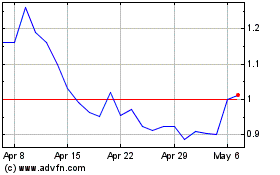
bluebird bio (NASDAQ:BLUE)
Historical Stock Chart
From May 2023 to May 2024