- Progenics is expecting a Food and Drug Administration (FDA) Advisory Committee meeting on June 11 – 12 to decide whether or not to recommend a Supplemental New Drug Application (sNDA).
- The Relistor sNDA is for a subcutaneous injection for opioid-induced constipation or OIC, in patients with chronic pain.
- If the sNDA is approved by the FDA, Progenics will receive an upfront payment of $50M along with up to $200M in milestone payments thereafter.
- Catalyst: Advisory Committee Reschedule Update
Progenics shares have not been high on investors’ radars in recent months because the FDA had rescheduled the set Advisory Committee (Adcom) meeting, which was due earlier this year. The FDA gave no guidance on when the meeting would be rescheduled, although many speculated it would be in the near term.
However, during first quarter financial results, Progenics announced that the FDA has rescheduled the meeting to June 11 and 12. This gives catalyst traders and investors a set date for which to trade and speculate on. These catalyst events tend to attract traders which in turn normally drive the stock price up under normal market conditions — especially an event with a specific date.
Salix and Progenics received a Complete Response Letter from the FDA in 2012. However, Salix appealed the FDA’s decision, believing that existing safety data from Relistor’s already approved indication was not properly considered. Since then, Salix has met with the organization to remedy the situation. In response, the FDA seeks input from an Adcom to better understand the situation.
We believe the FDA is “saving face” by asking for an Adcom after its decision to issue the CRL when the safety data was already sufficient. In this regard, an Adcom can “explain” the situation better to the FDA, and the organization can then approve with “a new and better understanding.”
Therefore, we believe this Adcom will go well and the sNDA will be approved by the FDA. It is always risky to hold through any type of binary event, but for the reasons given, we believe the companies have a better chance of success this time around.
- The Meaning of a Successful Adcom
A successful Adcom and approval of Relistor would be very meaningful for Progenics. The company is set to receive up to $50M upon U.S. marketing approval of an oral formulation of Relistor. Additionally, the company can receive up to $200M of commercialization milestone payments upon achievement of specified U.S. sales targets.
As of today, Progenics has a market cap of about $283M, so this income would immediately provide added value for shareholders. The company has $96.2M in cash as of May 9, so there should not be much additional risk due to capital raises. In addition to the cash on hand, these milestone payments would make Progenics significantly undervalued.
- Additional Value Drivers
Progenics does have proprietary products (products the company owns outright) in its pipeline. One such product is the company’s PSMA ADC technology, which has shown some success in clinical trials for prostate cancer.
A Phase II trial [of the PSMA technology] assessed the anti-tumor activity and tolerability of its antibody drug conjugate, PSMA ADC, in patients with metastatic castrate resistant prostate cancer. A total of 83 patients who had progressive disease despite treatment with at least one taxane containing chemotherapy received PSMA ADC. Enrollment of a chemotherapy naïve cohort is ongoing.
PSMA ADC technology is the company’s proprietary program which has a much better chance of longer term success if Progenics can receive these payments from Salix if Relistor receives the sNDA approval from the FDA. At that point, the company would be able to invest more into this and other programs.
- Chart
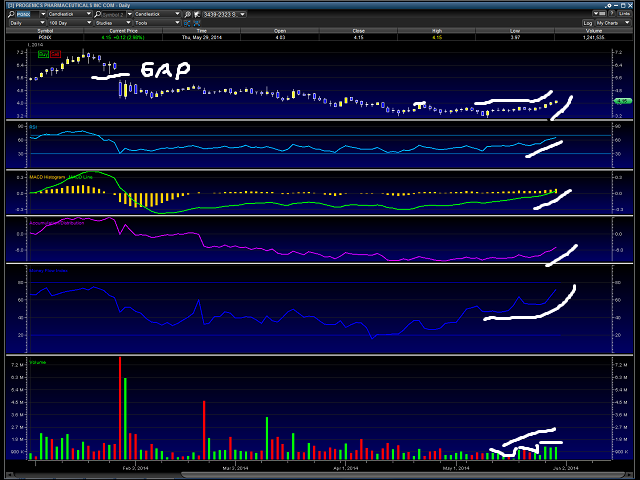
As I have marked above, we can see $PGNX is very close to breaking above an area which can cause a break out to over $4.50. Note MACD and volume trend.Traders have an old saying; “volume precedes price.” It certainty looks to me we are seeing this develop with $PGNX.
I think with EU dollar dropping against USD, that market continues a move up to 1925+ on S&P.
Target therefore is $4.50 ($4.40 to $4.61) for the short term, possibly at some point before June 11th filling the gap at $5.50 above.
- Conclusion
We think Progenics offers a good risk-to-reward opportunity at the current stock price levels for investors and traders alike. After the Biotech sell off over the last few weeks, we believe the company has become oversold and under-speculated. In the long term there will be competitors for Relistor and there is significant risk in the FDA approval process. However, success with Relistor would give the company ample cash from Salix to fund future trials of its propriety products without further dilution to shareholders.
For a small cap biotech, we feel it’s important to receive cash flow from a partnered product for use in funding potentially more valuable products, such as the company’s PSMA ADC technology. This technology ultimately will decide whether or not the company succeeds in the long term or not.







