Ionis Pharmaceuticals, AstraZeneca Get FDA Approval for Rare Genetic Disease Treatment
December 21 2023 - 7:38PM
Dow Jones News
By Ben Glickman
Ionis Pharmaceuticals and AstraZeneca's Wainua has been approved
by the U.S. Food and Drug Administration for treating a symptom of
a rare genetic disease.
The FDA approved Wainua, which has generic name eplontersen, for
the treatment of the polyneuropathy of hereditary
transthyretin-mediated amyloidosis in adults.
Ionis said Wainua would be available in the U.S. in January.
Regulatory reviews elsewhere in the world are currently
underway.
The disease, also known as hAATR, can cause polyneuropathy, a
form of nerve damage. The companies said Wainua is the only
approved medicine for treating polyneuropathy from hATTR.
The approval was based on a 35-week interim analysis from a
Phase 3 study.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
December 21, 2023 19:23 ET (00:23 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
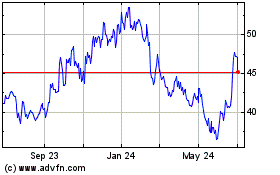
Ionis Pharmaceuticals (NASDAQ:IONS)
Historical Stock Chart
From May 2024 to Jun 2024
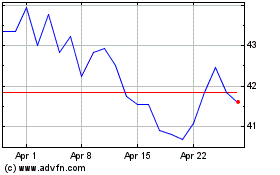
Ionis Pharmaceuticals (NASDAQ:IONS)
Historical Stock Chart
From Jun 2023 to Jun 2024