false 0001770121 0001770121 2024-01-09 2024-01-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 9, 2024
SANA BIOTECHNOLOGY, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-39941 |
|
83-1381173 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification Number) |
188 East Blaine Street, Suite 400
Seattle, Washington 98102
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (206) 701-7914
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| |
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.0001 par value per share |
|
SANA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
Sana Biotechnology, Inc. (the “Company”) intends to discuss an updated corporate presentation (the “Corporate Presentation”) at the 42nd Annual J.P. Morgan Healthcare Conference on January 9, 2024. A copy of the Corporate Presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K (this “Current Report”) and is incorporated by reference herein.
By furnishing the information in this Item 7.01 of this Current Report, including Exhibit 99.1, the Company makes no admission as to the materiality of such information. The information contained herein is intended to be considered in the context of the Company’s filings with the U.S. Securities and Exchange Commission (the “SEC”) and other public announcements that the Company makes, by press release or otherwise, from time to time. The Company undertakes no duty or obligation to publicly update or revise the information contained in the Corporate Presentation, although it may do so from time to time as its management believes is appropriate. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through other public disclosure.
In accordance with General Instruction B.2 of Form 8-K, the information furnished with this Current Report, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (“Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
See the Exhibit Index below, which is incorporated by reference herein.
EXHIBIT INDEX
1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Sana Biotechnology, Inc. |
|
|
|
|
| Date: January 9, 2024 |
|
|
|
By: |
|
/s/ Bernard J. Cassidy |
| |
|
|
|
|
|
Bernard J. Cassidy |
| |
|
|
|
|
|
Executive Vice President and General Counsel |
2

Corporate Presentation January 2024
Exhibit 99.1

This presentation contains
forward-looking statements about Sana Biotechnology, Inc. (the “Company,” “we,” “us,” or “our”) within the meaning of the federal securities laws. All statements other than statements of historical
facts contained in this presentation, including, among others, statements regarding the Company’s strategy, expectations, cash runway and future financial condition, future operations, and prospects, are forward-looking statements. In some
cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,”
“design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,”
“predict,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of
these terms or other comparable terminology. The Company has based these forward-looking statements largely on its current expectations, estimates, forecasts and projections about future events and financial trends that it believes may affect its
financial condition, results of operations, business strategy and financial needs. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events.
These statements are subject to risks and uncertainties that could cause the actual results to vary materially, including, among others, the risks inherent in drug development such as those associated with the initiation, cost, timing, progress and
results of the Company’s current and future research and development programs, preclinical studies, and clinical trials. For a detailed discussion of the risk factors that could affect the Company’s actual results, please refer to the
risk factors identified in the Company’s SEC reports, including its Quarterly Report on Form 10-Q dated November 8, 2023. Except as required by law, the Company undertakes no obligation to update publicly any forward-looking statements for any
reason. Cautionary Note Regarding Forward-Looking Statements

Sana’s hypoimmune technology
goal is to overcome allogeneic rejection HIP technology provides foundation for potential multiple drugs across many therapeutic areas Begin 2024 with four clinical programs treating seven diseases SC291 oncology – NHL and CLL SC291 B-cell
mediated autoimmune – lupus nephritis, extrarenal lupus, and ANCA-associated vasculitis SC262 oncology – r/r NHL, initially in CD19 CAR T failures HIP primary islet cells in patients with type 1 diabetes Pipeline positioned to deliver
additional clinical data over time Regenerative medicine: SC379 (CNS disorders) and SC451 (type 1 diabetes) Hypoimmune allogeneic CAR T cells: SC255 (BCMA) and beyond Balance sheet allows potential for multiple data readouts Changing the
Possible for Patients Sana Biotechnology
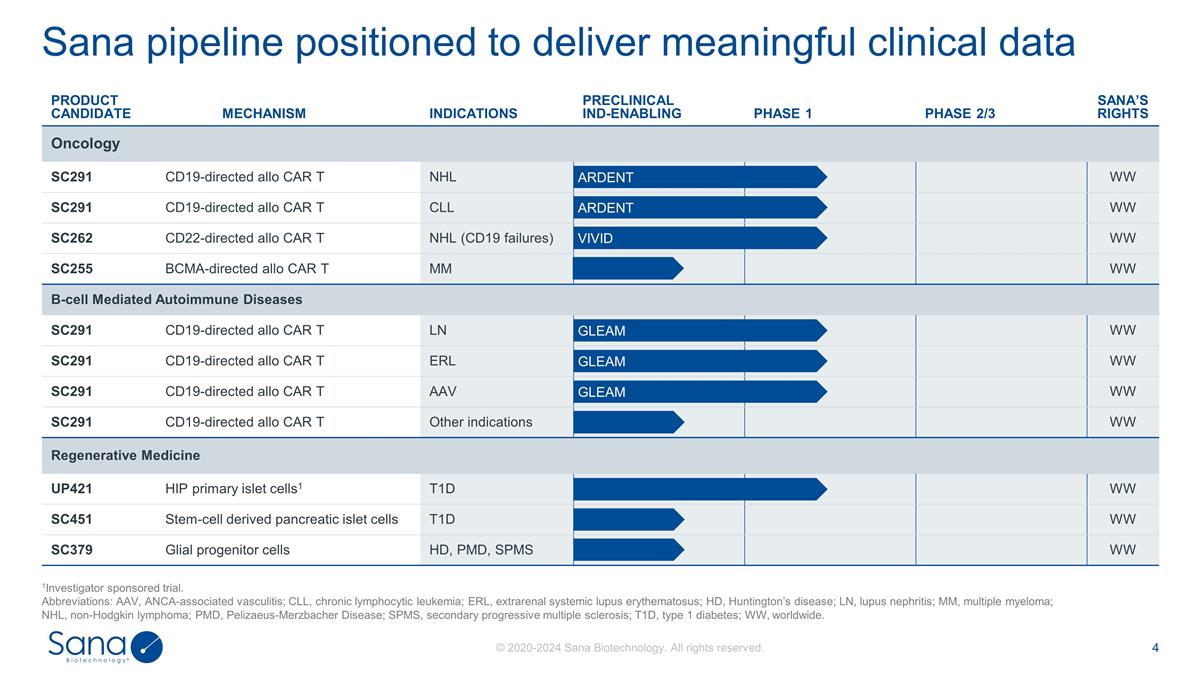
Sana pipeline positioned to deliver
meaningful clinical data 1Investigator sponsored trial. Abbreviations: AAV, ANCA-associated vasculitis; CLL, chronic lymphocytic leukemia; ERL, extrarenal systemic lupus erythematosus; HD, Huntington’s disease; LN, lupus nephritis; MM,
multiple myeloma; NHL, non-Hodgkin lymphoma; PMD, Pelizaeus-Merzbacher Disease; SPMS, secondary progressive multiple sclerosis; T1D, type 1 diabetes; WW, worldwide. PRODUCT CANDIDATE MECHANISM INDICATIONS PRECLINICAL IND-ENABLING PHASE 1 PHASE 2/3
SANA’S RIGHTS Oncology SC291 CD19-directed allo CAR T CD19-targeted allo CAR T NHL WW SC291 CD19-directed allo CAR T CD19-targeted allo CAR T CLL WW SC262 CD22-directed allo CAR T CD22-targeted allo CAR T NHL (CD19 failures) WW SC255
BCMA-directed allo CAR T BCMA-targeted allo CAR T MM WW B-cell Mediated Autoimmune Diseases SC291 CD19-directed allo CAR T CD19-targeted allo CAR T LN WW SC291 CD19-directed allo CAR T CD19-targeted allo CAR T ERL WW SC291 CD19-directed allo CAR T
CD19-targeted allo CAR T AAV WW SC291 CD19-directed allo CAR T CD19-targeted allo CAR T Other indications WW Regenerative Medicine UP421 HIP primary islet cells1 HIP Primary Islet Cells1 T1D WW SC451 Stem-cell derived pancreatic islet cells
Stem-cell derived pancreatic islet cells T1D WW SC379 Glial progenitor cells Glial progenitor cells HD, PMD, SPMS WW ARDENT GLEAM VIVID ARDENT GLEAM GLEAM
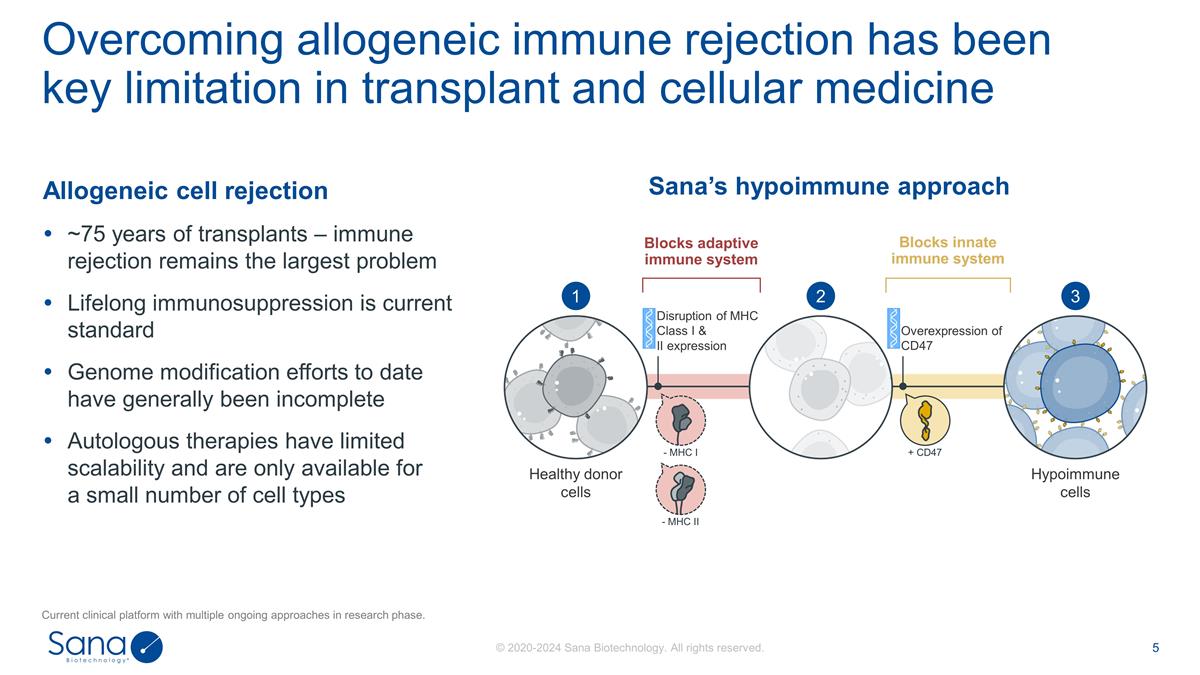
Allogeneic cell rejection ~75 years of
transplants – immune rejection remains the largest problem Lifelong immunosuppression is current standard Genome modification efforts to date have generally been incomplete Autologous therapies have limited scalability and are only available
for a small number of cell types Overcoming allogeneic immune rejection has been key limitation in transplant and cellular medicine Sana’s hypoimmune approach + CD47 - MHC I - MHC II Healthy donor cells Hypoimmune cells Disruption of MHC Class
I & II expression Overexpression of CD47 1 2 3 Blocks adaptive immune system Blocks innate immune system Current clinical platform with multiple ongoing approaches in research phase.
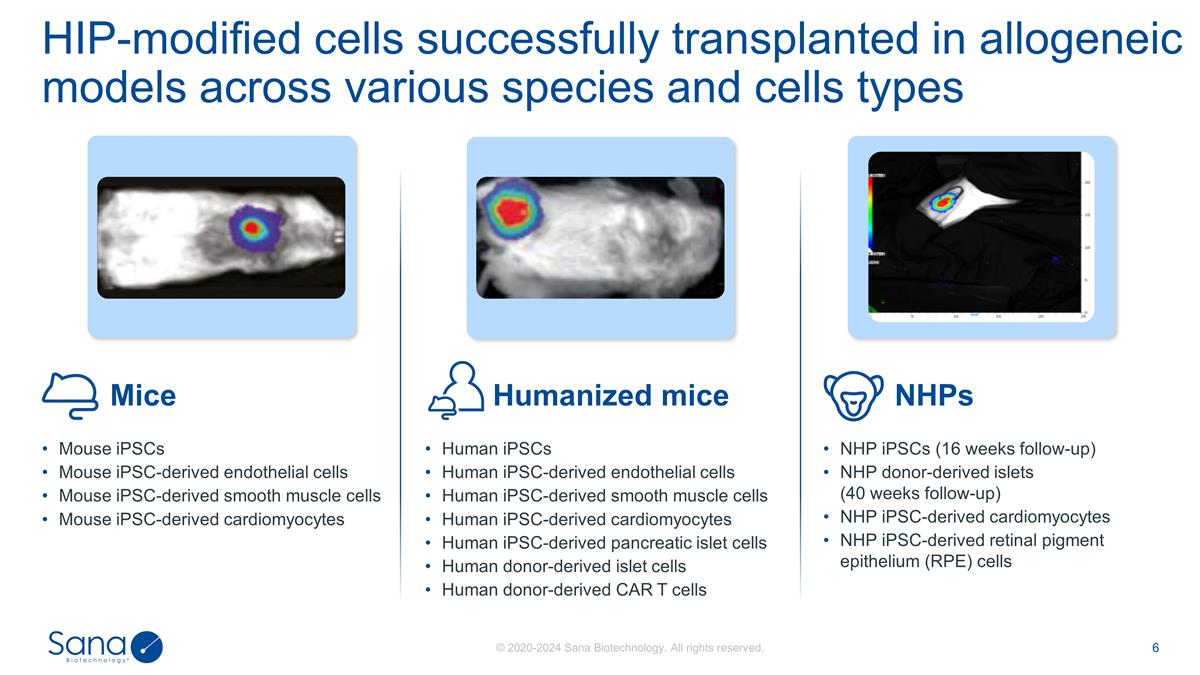
HIP-modified cells successfully
transplanted in allogeneic models across various species and cells types Mouse iPSCs Mouse iPSC-derived endothelial cells Mouse iPSC-derived smooth muscle cells Mouse iPSC-derived cardiomyocytes NHP iPSCs (16 weeks follow-up) NHP donor-derived
islets (40 weeks follow-up) NHP iPSC-derived cardiomyocytes NHP iPSC-derived retinal pigment epithelium (RPE) cells Human iPSCs Human iPSC-derived endothelial cells Human iPSC-derived smooth muscle cells Human iPSC-derived cardiomyocytes Human
iPSC-derived pancreatic islet cells Human donor-derived islet cells Human donor-derived CAR T cells Mice NHPs Humanized mice
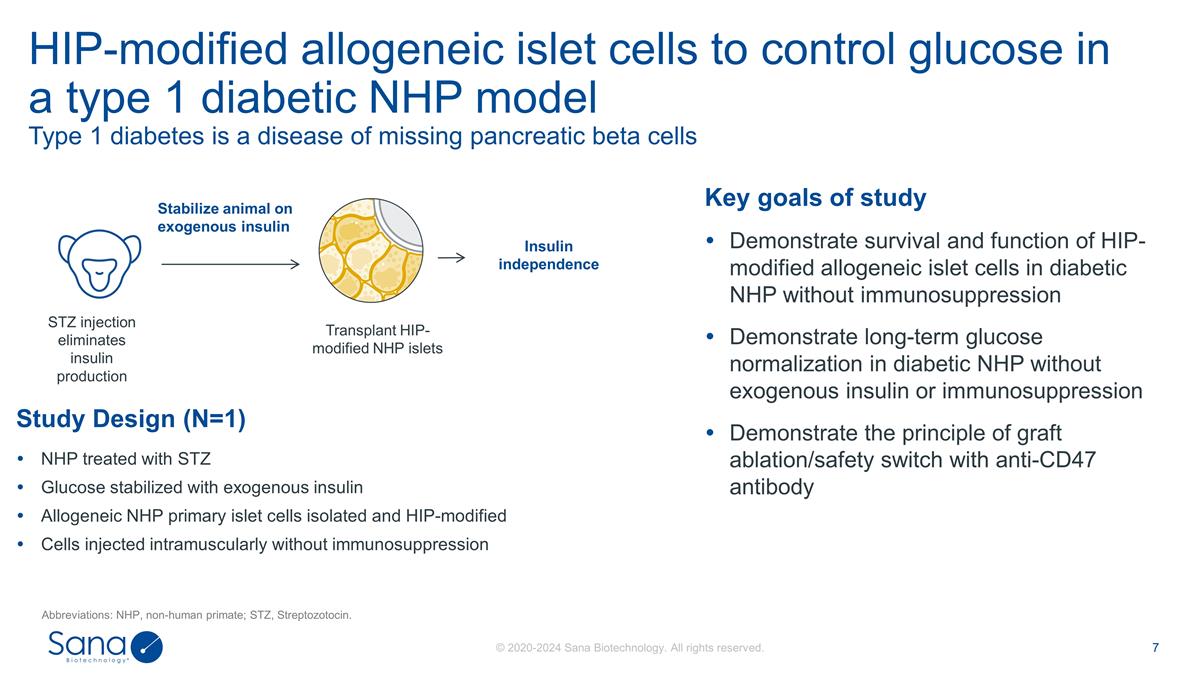
HIP-modified allogeneic islet cells to
control glucose in a type 1 diabetic NHP model Type 1 diabetes is a disease of missing pancreatic beta cells Abbreviations: NHP, non-human primate; STZ, Streptozotocin. Study Design (N=1) NHP treated with STZ Glucose stabilized with exogenous
insulin Allogeneic NHP primary islet cells isolated and HIP-modified Cells injected intramuscularly without immunosuppression STZ injection eliminates insulin production Transplant HIP-modified NHP islets Stabilize animal on exogenous insulin
Insulin independence Key goals of study Demonstrate survival and function of HIP-modified allogeneic islet cells in diabetic NHP without immunosuppression Demonstrate long-term glucose normalization in diabetic NHP without exogenous insulin or
immunosuppression Demonstrate the principle of graft ablation/safety switch with anti-CD47 antibody
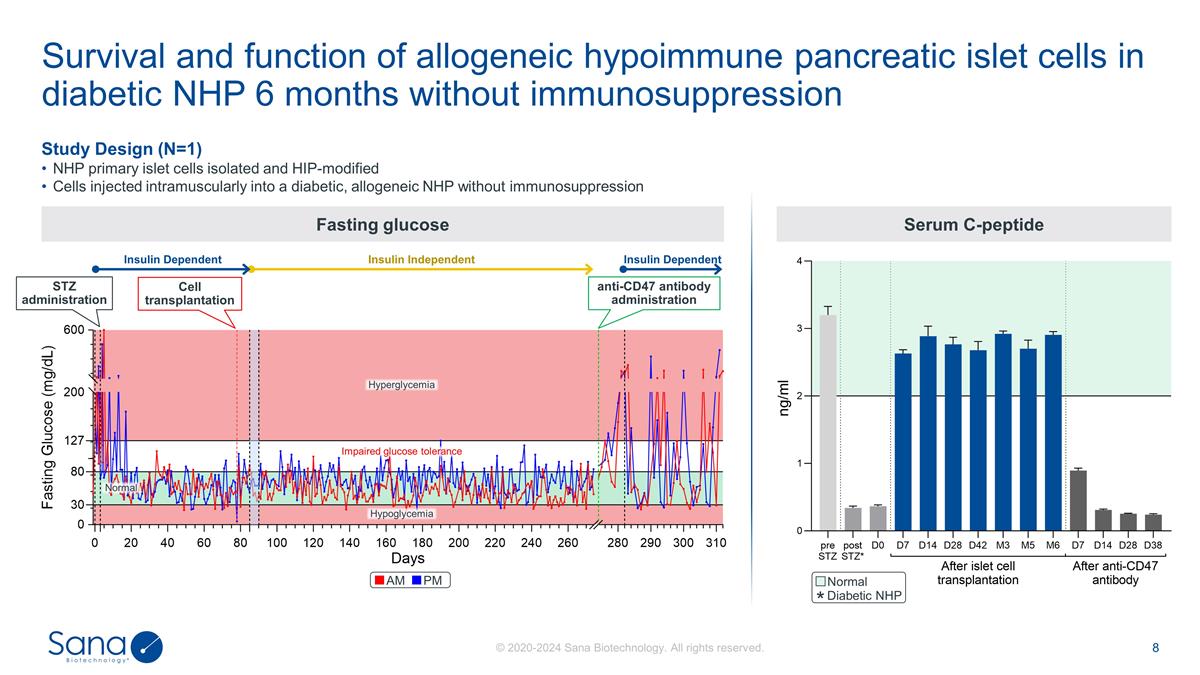
Study Design (N=1) NHP primary
islet cells isolated and HIP-modified Cells injected intramuscularly into a diabetic, allogeneic NHP without immunosuppression Fasting glucose Survival and function of allogeneic hypoimmune pancreatic islet cells in diabetic NHP 6 months without
immunosuppression STZ administration Cell transplantation Normal Hypoglycemia Impaired glucose tolerance Hyperglycemia anti-CD47 antibody administration Insulin Dependent Insulin Independent Insulin Dependent AM PM Serum C-peptide Diabetic NHP
Normal *

1Avezbakiyev et al. Blood. 2022 2Durie
et al. The Oncologist. 2020 3NIH Autoimmune Diseases Coordinating Committee: Autoimmune Diseases Research Plan, October 2017, U.S. 4t1dindex.org Near-term opportunities to apply HIP modifications to validated mechanisms with unmet need Blood
cancers: >100,000 patients/year1,2 Type 1 diabetes: >8 million patients4 B-cell mediated autoimmune diseases: >5 million patients3
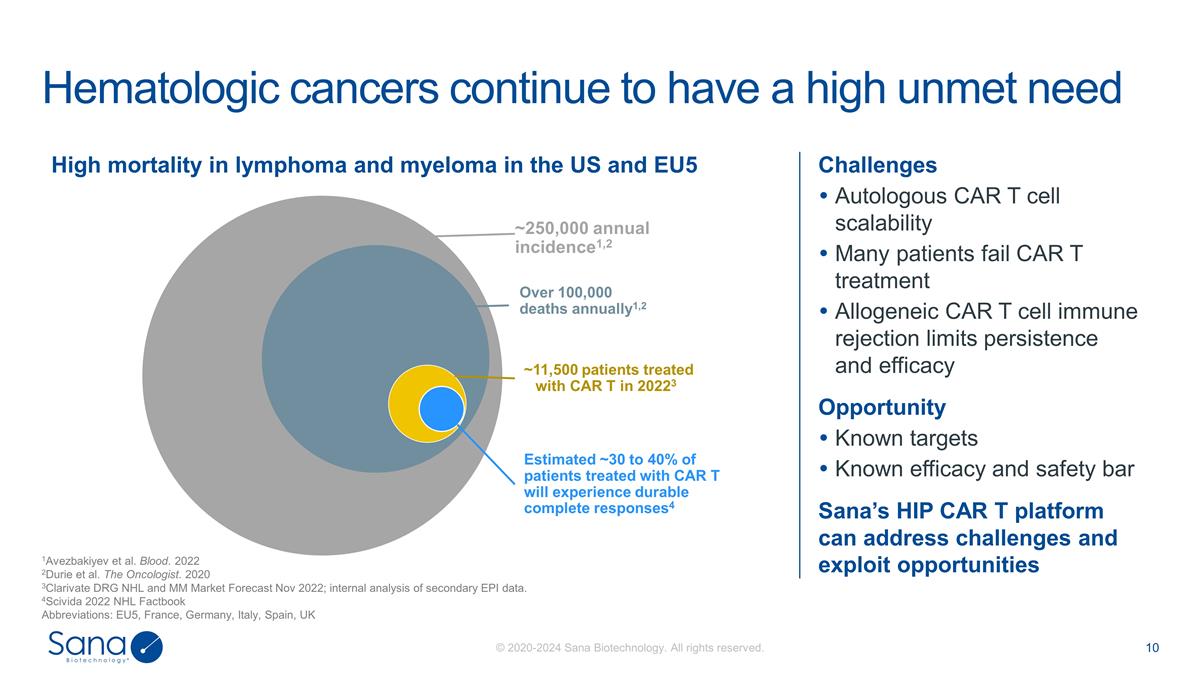
Challenges Autologous CAR T cell
scalability Many patients fail CAR T treatment Allogeneic CAR T cell immune rejection limits persistence and efficacy Opportunity Known targets Known efficacy and safety bar Sana’s HIP CAR T platform can address challenges and exploit
opportunities Hematologic cancers continue to have a high unmet need 1Avezbakiyev et al. Blood. 2022 2Durie et al. The Oncologist. 2020 3Clarivate DRG NHL and MM Market Forecast Nov 2022; internal analysis of secondary EPI data. 4Scivida 2022 NHL
Factbook Abbreviations: EU5, France, Germany, Italy, Spain, UK High mortality in lymphoma and myeloma in the US and EU5 ~250,000 annual incidence1,2 Over 100,000 deaths annually1,2 ~11,500 patients treated with CAR T in 20223 Estimated ~30 to 40% of
patients treated with CAR T will experience durable complete responses4
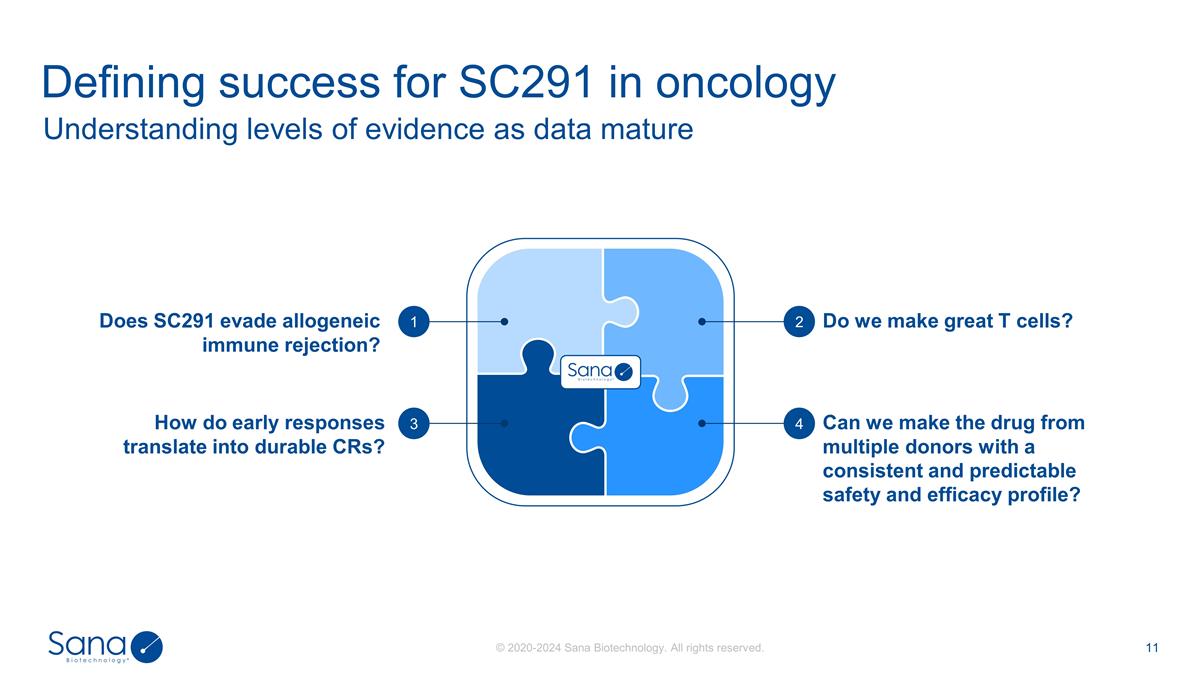
Understanding levels of evidence as
data mature Defining success for SC291 in oncology Does SC291 evade allogeneic immune rejection? Do we make great T cells? How do early responses translate into durable CRs? Can we make the drug from multiple donors with a consistent and predictable
safety and efficacy profile? 4 3 2 1
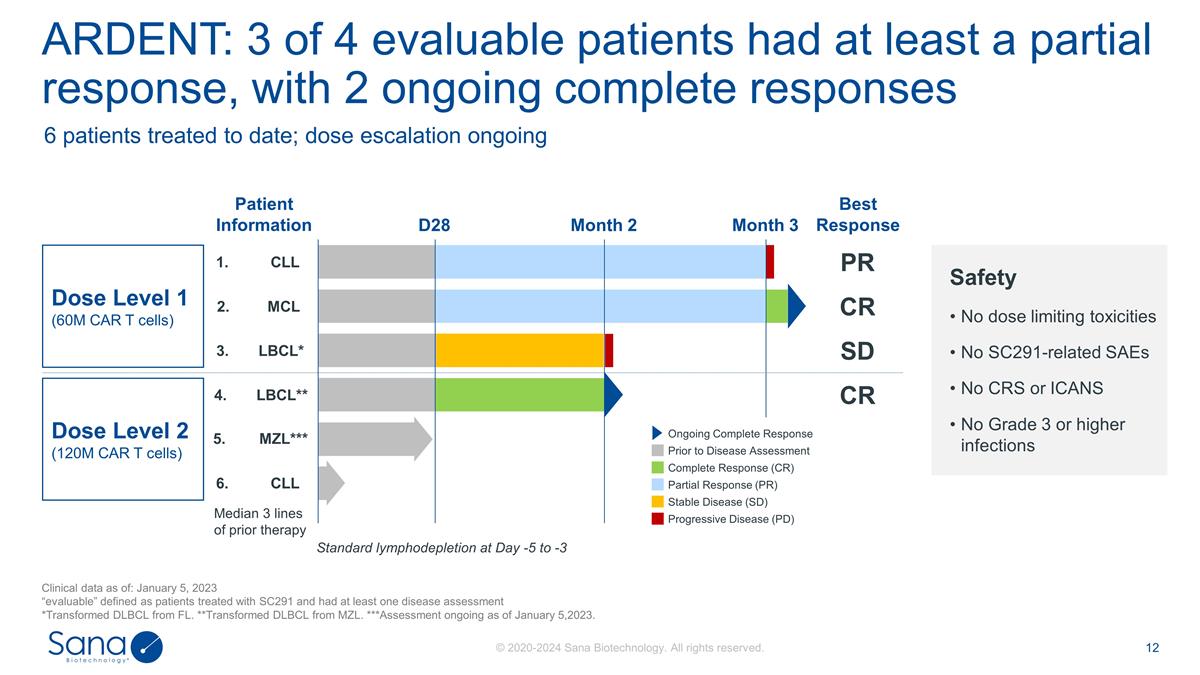
3. LBCL* 4. LBCL** 5. MZL*** 6. CLL
1. CLL 2. MCL ARDENT: 3 of 4 evaluable patients had at least a partial response, with 2 ongoing complete responses Clinical data as of: January 5, 2023 “evaluable” defined as patients treated with SC291 and had at least one disease
assessment *Transformed DLBCL from FL. **Transformed DLBCL from MZL. ***Assessment ongoing as of January 5,2023. Dose Level 1 (60M CAR T cells) Dose Level 2 (120M CAR T cells) Patient Information Safety No dose limiting toxicities No SC291-related
SAEs No CRS or ICANS No Grade 3 or higher infections Median 3 lines of prior therapy Standard lymphodepletion at Day -5 to -3 Progressive Disease (PD) Stable Disease (SD) Partial Response (PR) Complete Response (CR) Prior to Disease Assessment
Ongoing Complete Response D28 Month 2 Month 3 6 patients treated to date; dose escalation ongoing Best Response PR CR SD CR
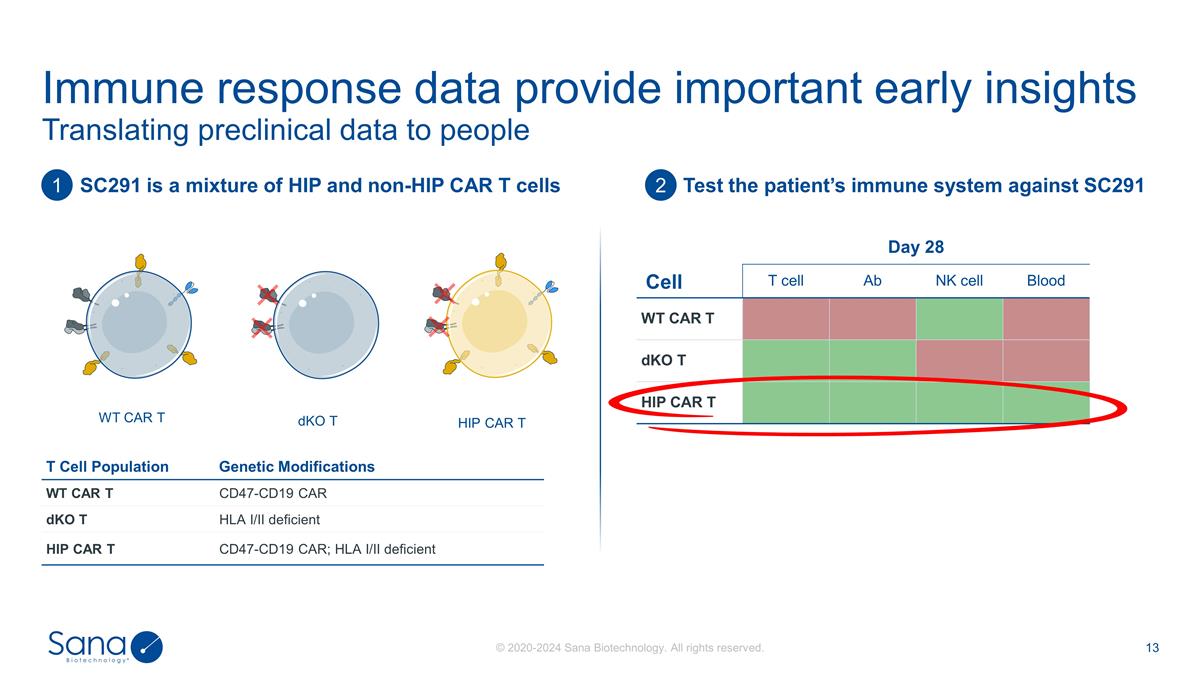
Translating preclinical data to
people Immune response data provide important early insights T Cell Population Genetic Modifications WT CAR T CD47-CD19 CAR dKO T HLA I/II deficient HIP CAR T CD47-CD19 CAR; HLA I/II deficient SC291 is a mixture of HIP and non-HIP CAR T cells Test
the patient’s immune system against SC291 Cell Day 28 Ab NK cell Blood T cell Ab NK cell Blood WT CAR T dKO T HIP CAR T WT CAR T HIP CAR T 1 2 dKO T
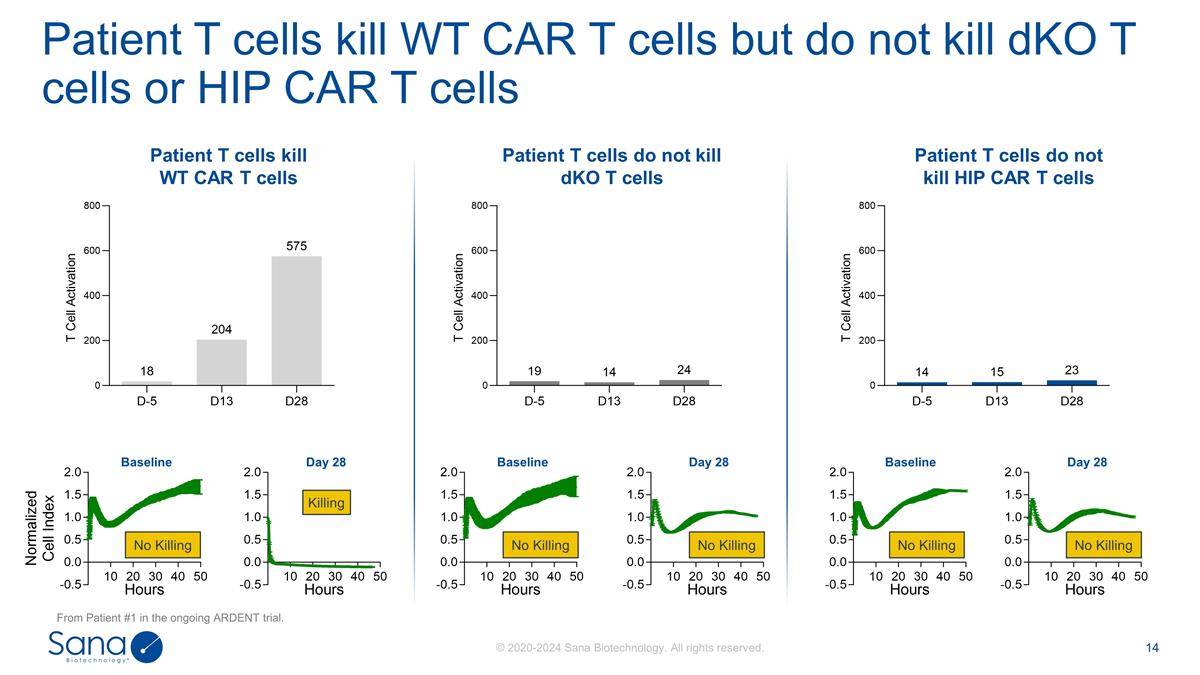
Patient T cells kill WT CAR T cells
but do not kill dKO T cells or HIP CAR T cells Patient T cells kill WT CAR T cells Patient T cells do not kill dKO T cells Patient T cells do not kill HIP CAR T cells No Killing Killing No Killing Day 28 Baseline Day 28 Baseline Day 28 Baseline No
Killing No Killing No Killing From Patient #1 in the ongoing ARDENT trial.
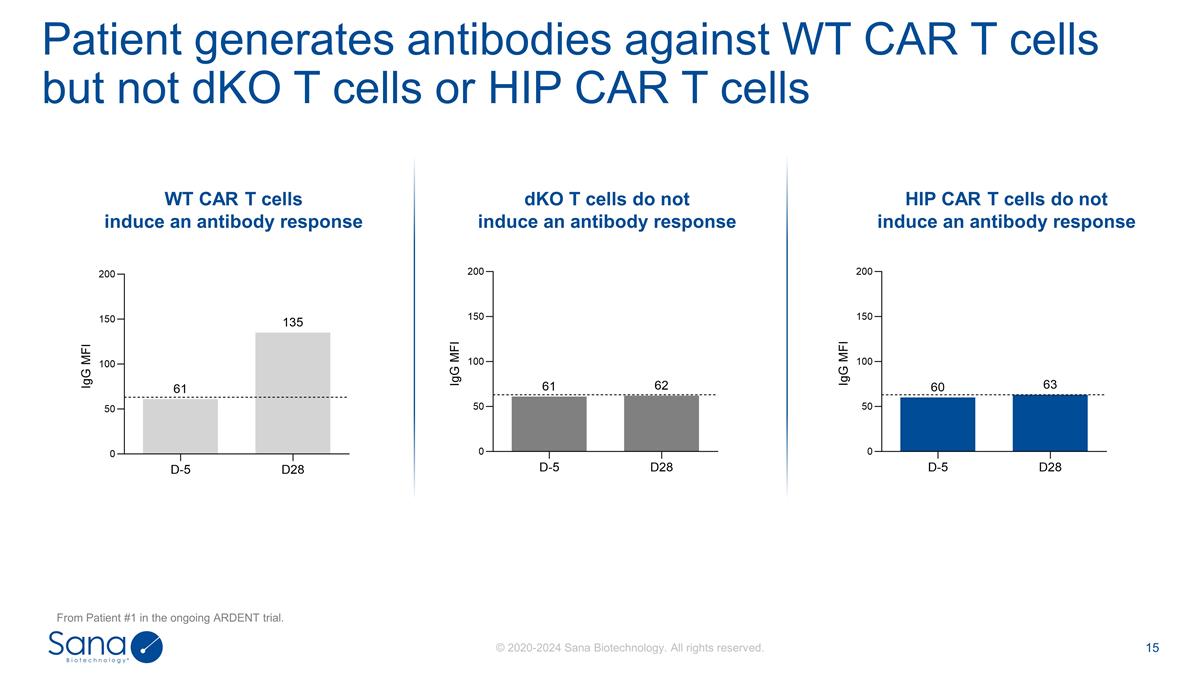
WT CAR T cells induce an antibody
response HIP CAR T cells do not induce an antibody response dKO T cells do not induce an antibody response Patient generates antibodies against WT CAR T cells but not dKO T cells or HIP CAR T cells From Patient #1 in the ongoing ARDENT
trial.
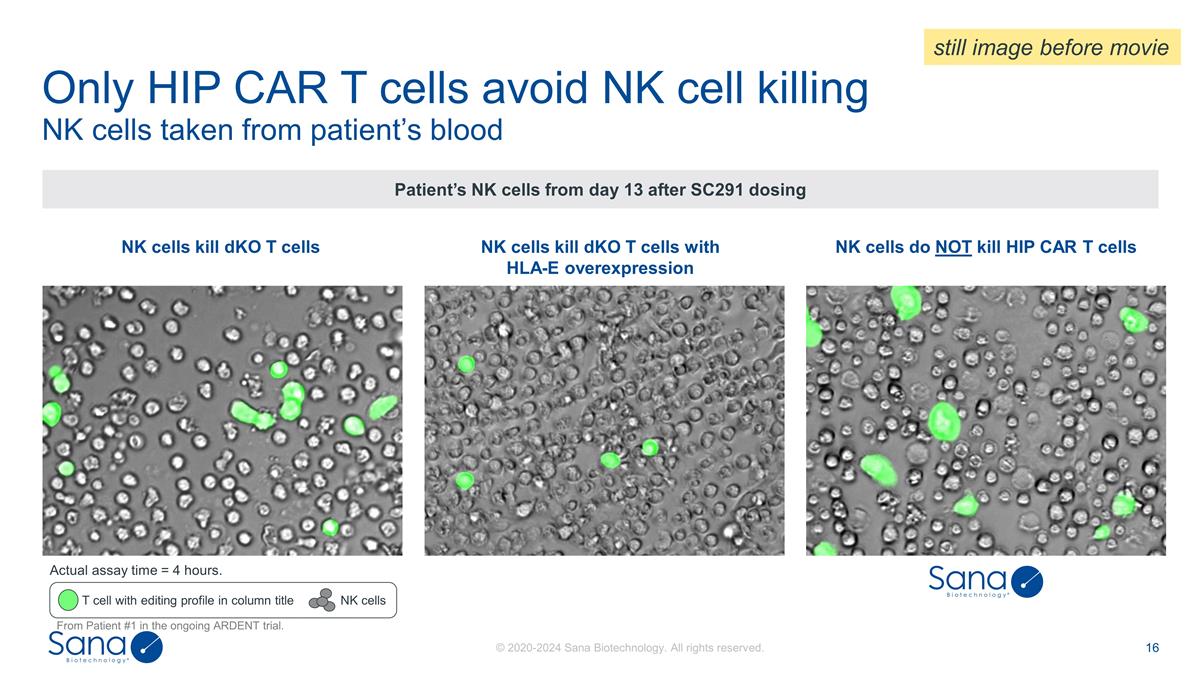
NK cells kill dKO T cells with
HLA-E overexpression NK cells kill dKO T cells NK cells taken from patient’s blood Only HIP CAR T cells avoid NK cell killing Patient’s NK cells from day 13 after SC291 dosing T cell with editing profile in column title NK cells NK cells
do NOT kill HIP CAR T cells Actual assay time = 4 hours. From Patient #1 in the ongoing ARDENT trial. still image before movie
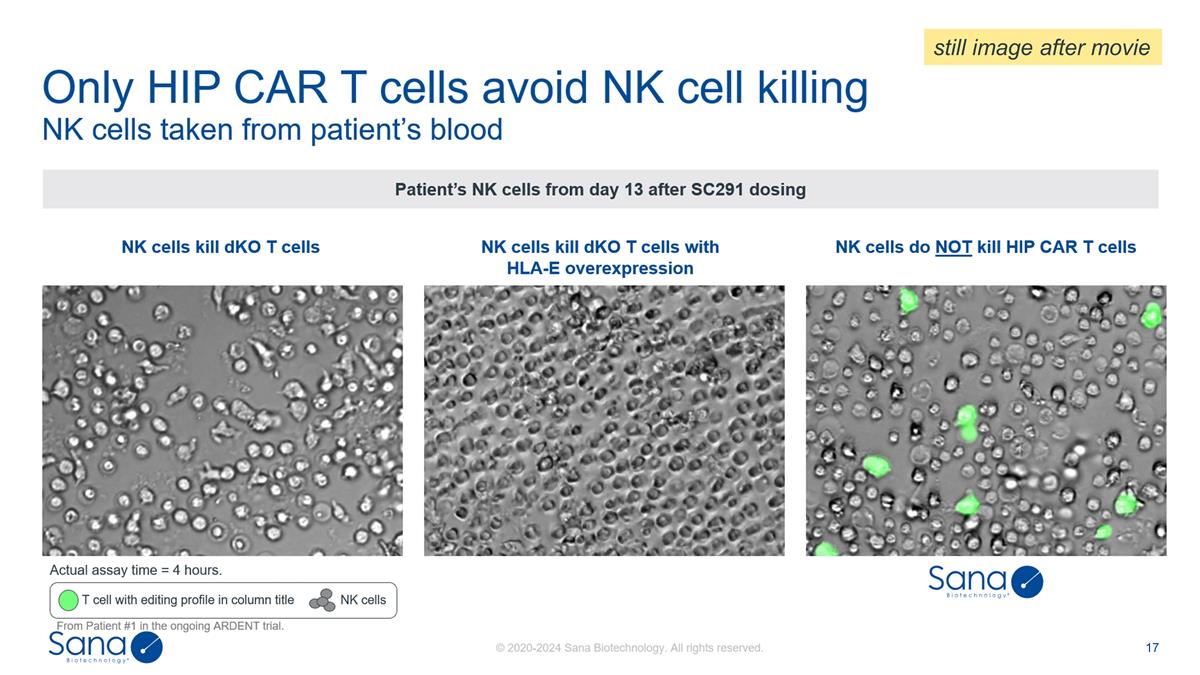
still image after movie
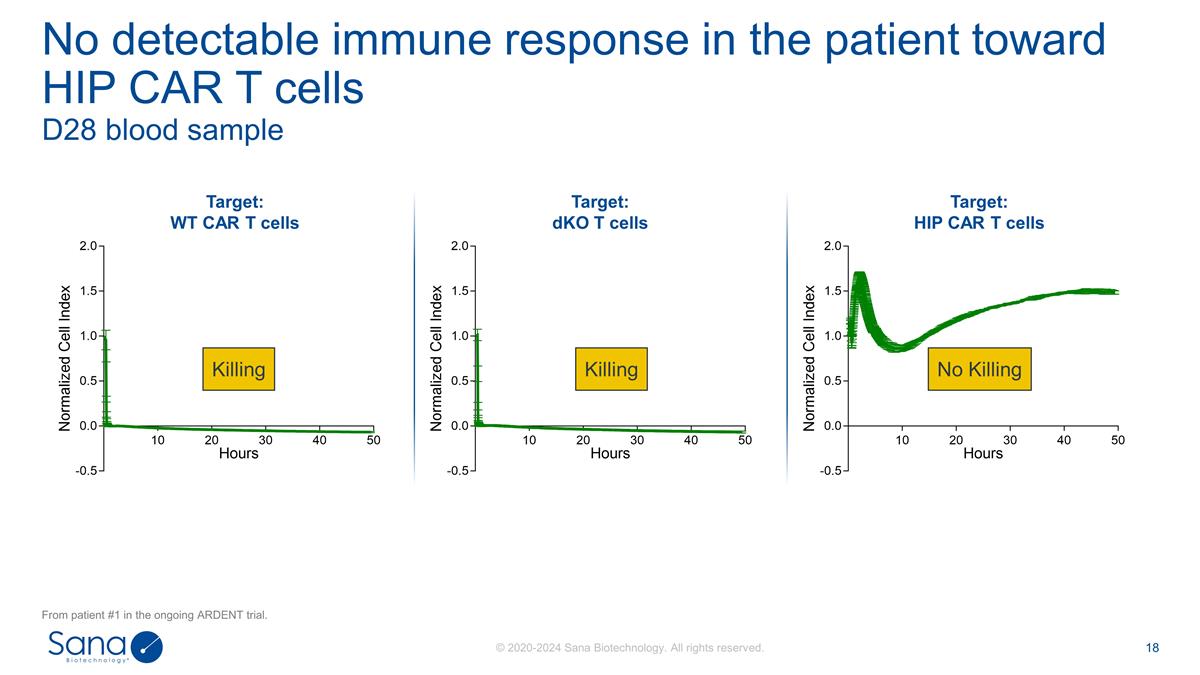
D28 blood sample No detectable
immune response in the patient toward HIP CAR T cells From patient #1 in the ongoing ARDENT trial. Target: WT CAR T cells Target: dKO T cells Target: HIP CAR T cells Killing No Killing Killing
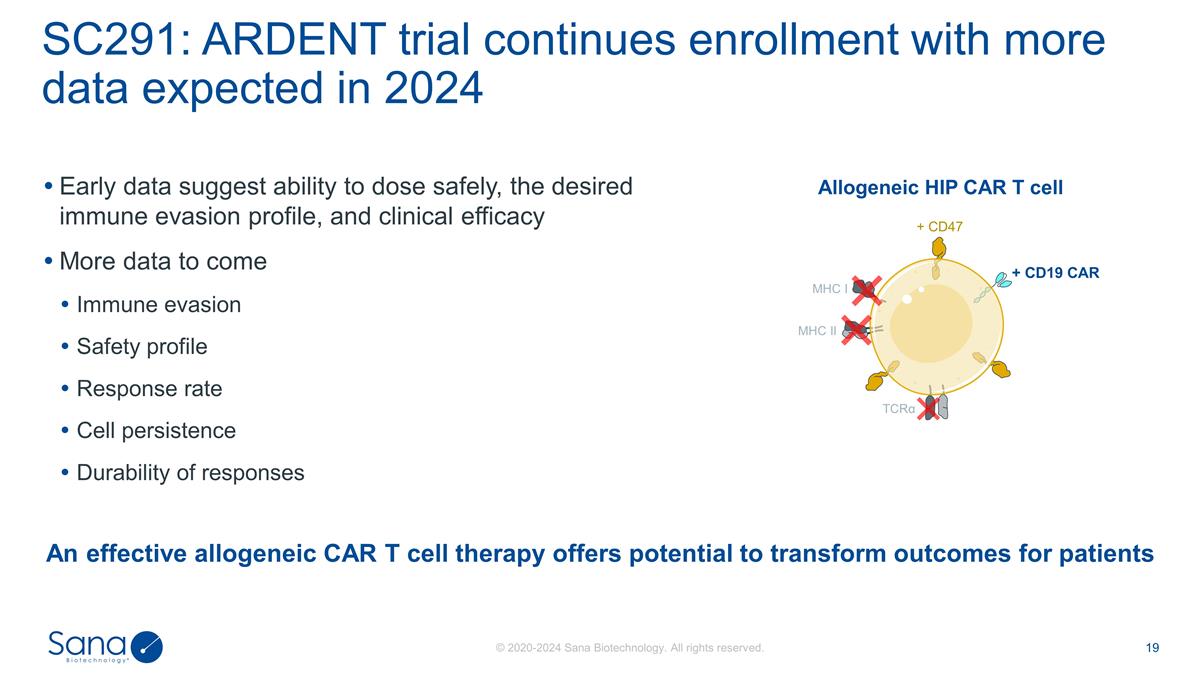
Early data suggest ability to dose
safely, the desired immune evasion profile, and clinical efficacy More data to come Immune evasion Safety profile Response rate Cell persistence Durability of responses SC291: ARDENT trial continues enrollment with more data expected in 2024 An
effective allogeneic CAR T cell therapy offers potential to transform outcomes for patients Allogeneic HIP CAR T cell MHC I MHC II + CD47 + CD19 CAR TCRα
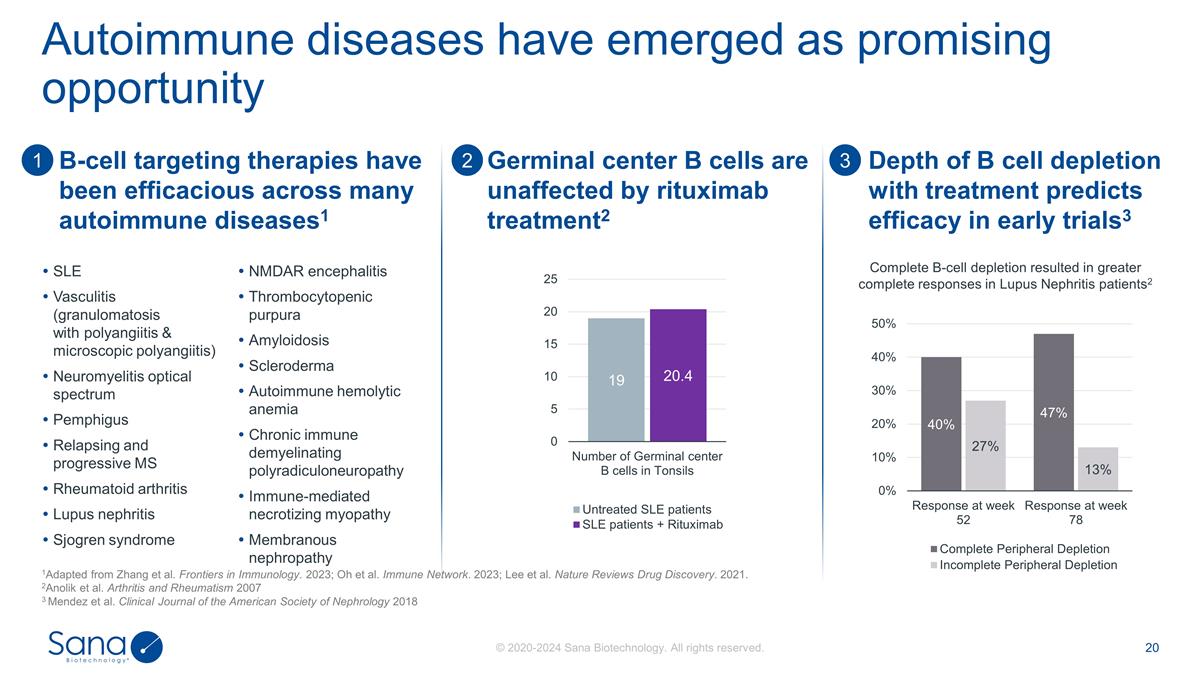
Autoimmune diseases have emerged as
promising opportunity 1Adapted from Zhang et al. Frontiers in Immunology. 2023; Oh et al. Immune Network. 2023; Lee et al. Nature Reviews Drug Discovery. 2021. 2Anolik et al. Arthritis and Rheumatism 2007 3 Mendez et al. Clinical Journal of the
American Society of Nephrology 2018 SLE Vasculitis (granulomatosis with polyangiitis & microscopic polyangiitis) Neuromyelitis optical spectrum Pemphigus Relapsing and progressive MS Rheumatoid arthritis Lupus nephritis Sjogren syndrome
NMDAR encephalitis Thrombocytopenic purpura Amyloidosis Scleroderma Autoimmune hemolytic anemia Chronic immune demyelinating polyradiculoneuropathy Immune-mediated necrotizing myopathy Membranous nephropathy B-cell targeting therapies have been
efficacious across many autoimmune diseases1 Germinal center B cells are unaffected by rituximab treatment2 1 3 2 Depth of B cell depletion with treatment predicts efficacy in early trials3 Complete B-cell depletion resulted in greater complete
responses in Lupus Nephritis patients2
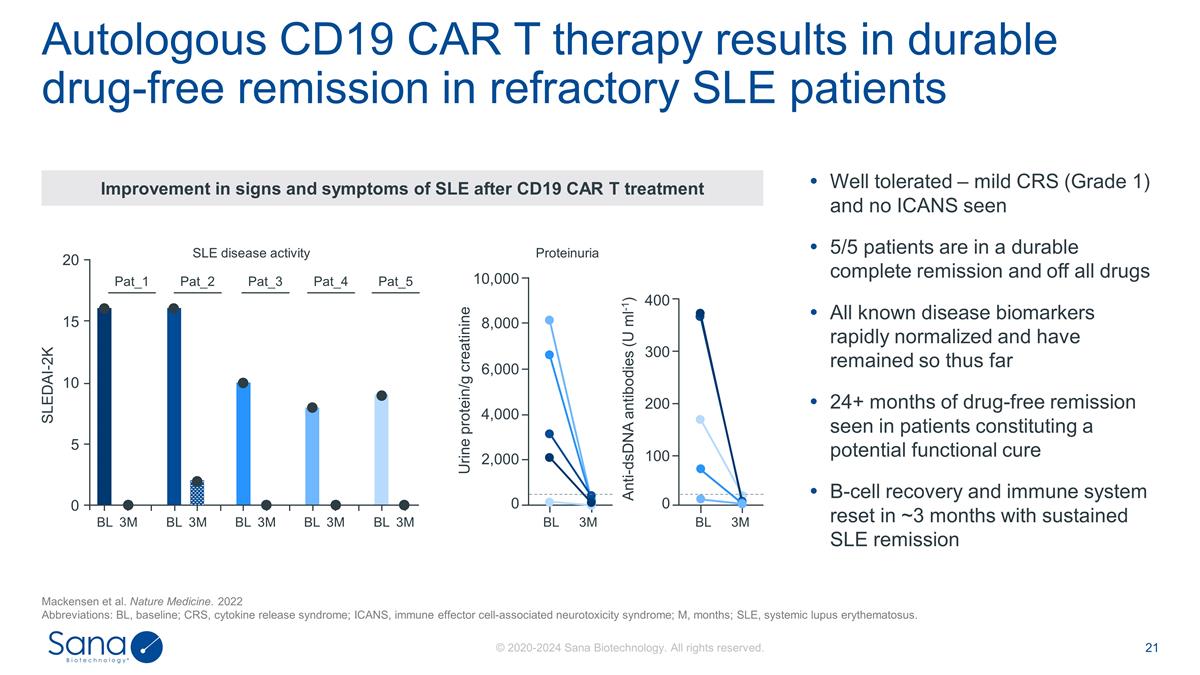
Autologous CD19 CAR T therapy
results in durable drug-free remission in refractory SLE patients Mackensen et al. Nature Medicine. 2022 Abbreviations: BL, baseline; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; M, months; SLE,
systemic lupus erythematosus. Well tolerated – mild CRS (Grade 1) and no ICANS seen 5/5 patients are in a durable complete remission and off all drugs All known disease biomarkers rapidly normalized and have remained so thus far 24+ months of
drug-free remission seen in patients constituting a potential functional cure B-cell recovery and immune system reset in ~3 months with sustained SLE remission 20 15 10 5 0 SLEDAI-2K BL 3M BL 3M BL 3M BL 3M BL 3M SLE disease activity Pat_1 Pat_2
Pat_3 Pat_4 Pat_5 Proteinuria BL 3M Urine protein/g creatinine 10,000 8,000 6,000 4,000 2,000 0 BL 3M 0 Anti-dsDNA antibodies (U ml-1) 100 200 300 400 Improvement in signs and symptoms of SLE after CD19 CAR T treatment
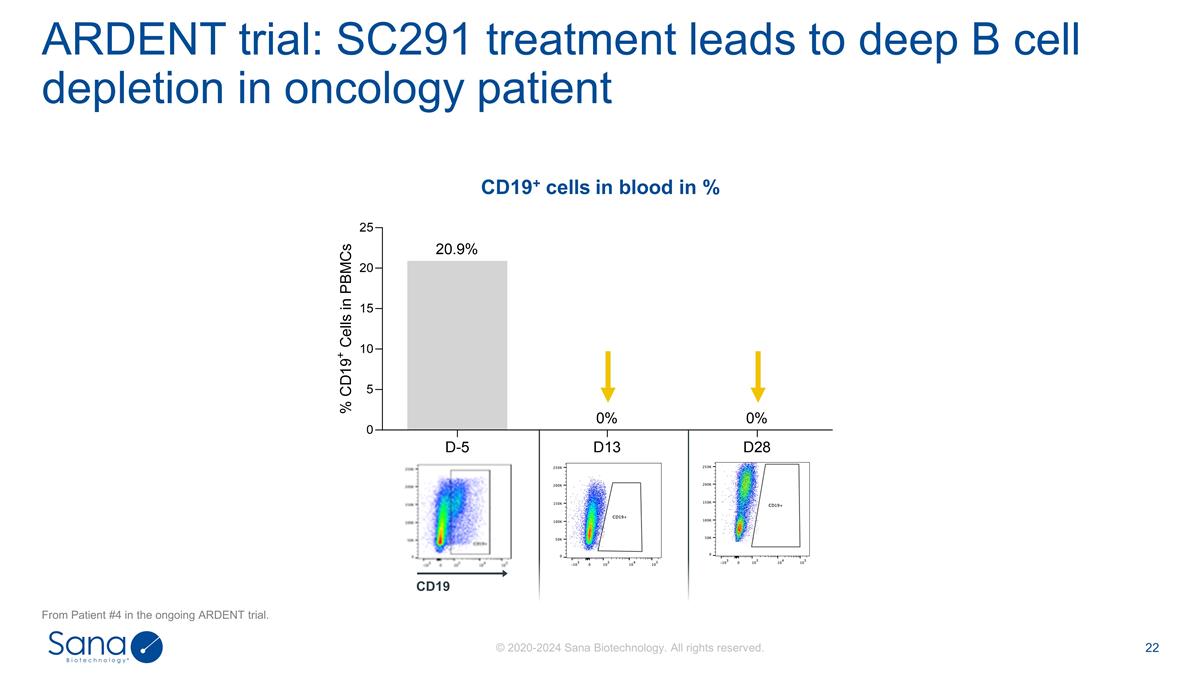
ARDENT trial: SC291 treatment leads
to deep B cell depletion in oncology patient From Patient #4 in the ongoing ARDENT trial. CD19 CD19+ cells in blood in %
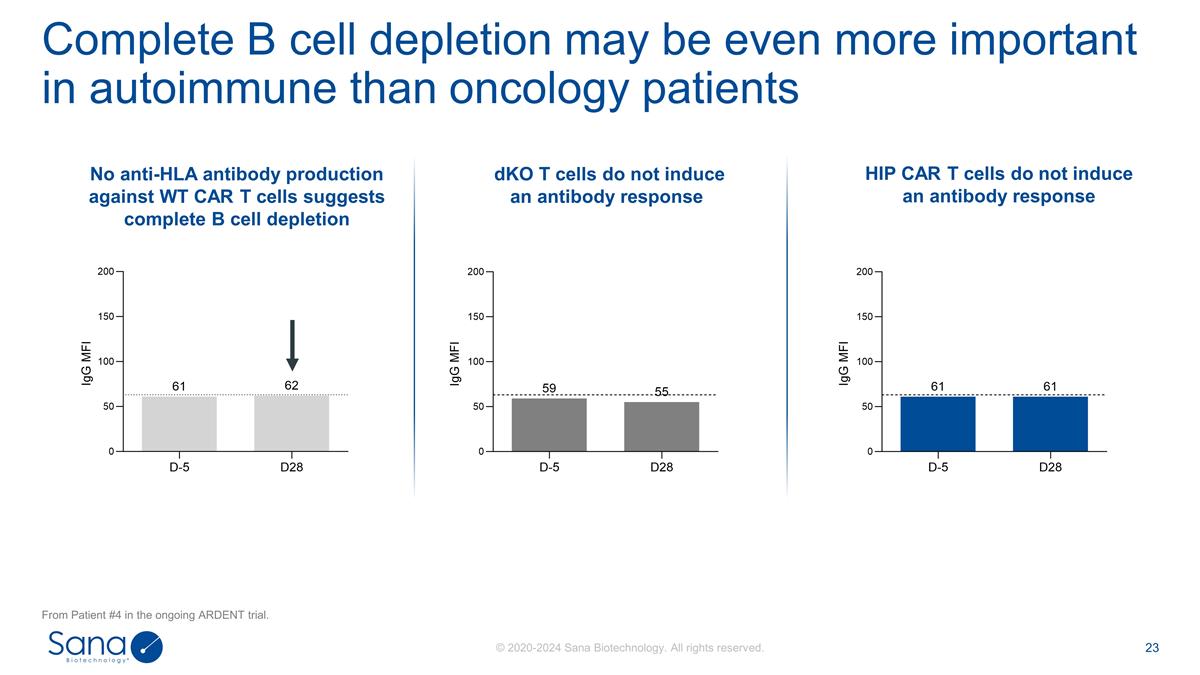
No anti-HLA antibody production
against WT CAR T cells suggests complete B cell depletion HIP CAR T cells do not induce an antibody response dKO T cells do not induce an antibody response Complete B cell depletion may be even more important in autoimmune than oncology
patients From Patient #4 in the ongoing ARDENT trial.
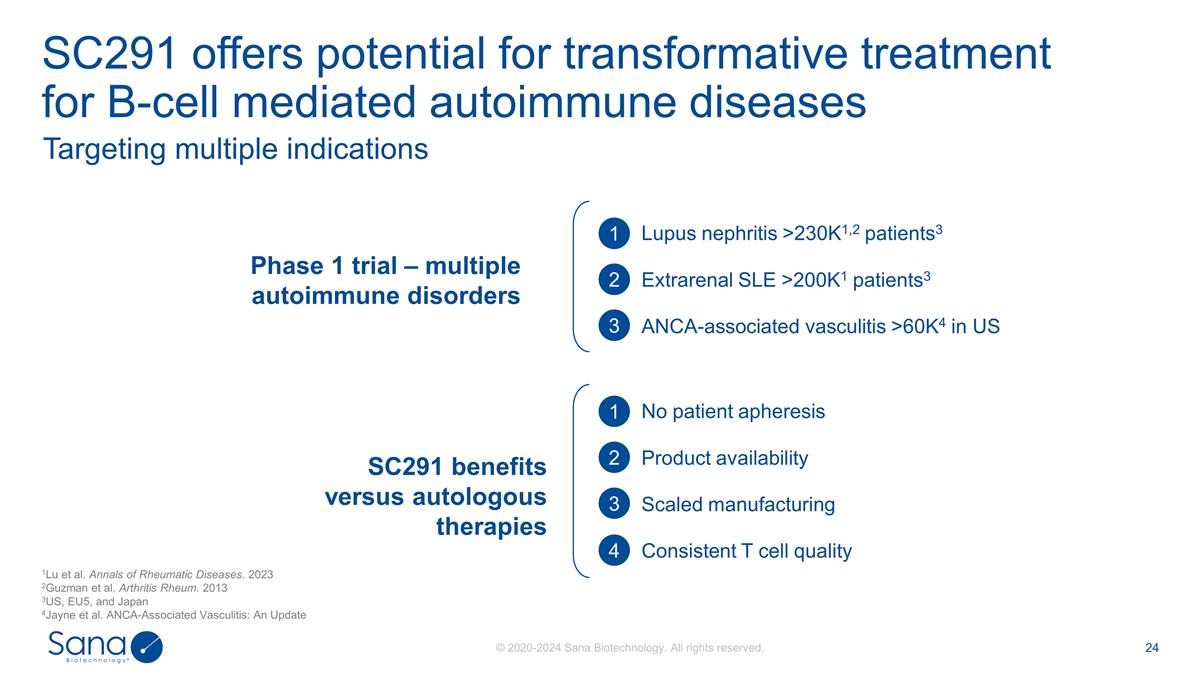
SC291 offers potential for
transformative treatment for B-cell mediated autoimmune diseases Targeting multiple indications Lupus nephritis >230K1,2 patients3 Extrarenal SLE >200K1 patients3 ANCA-associated vasculitis >60K4 in US 1 2 3 1 2 3 4 No patient apheresis
Product availability Scaled manufacturing Consistent T cell quality SC291 benefits versus autologous therapies Phase 1 trial – multiple autoimmune disorders 1Lu et al. Annals of Rheumatic Diseases. 2023 2Guzman et al. Arthritis Rheum. 2013
3US, EU5, and Japan 4Jayne et al. ANCA-Associated Vasculitis: An Update
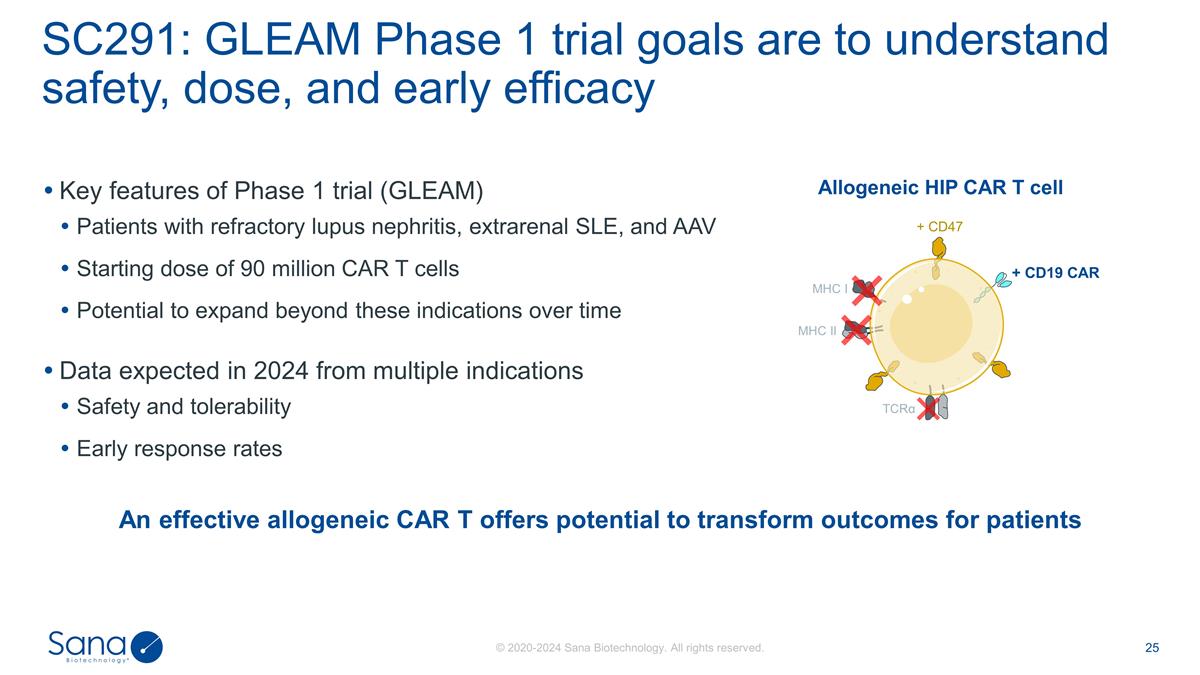
Key features of Phase 1 trial
(GLEAM) Patients with refractory lupus nephritis, extrarenal SLE, and AAV Starting dose of 90 million CAR T cells Potential to expand beyond these indications over time Data expected in 2024 from multiple indications Safety and tolerability Early
response rates SC291: GLEAM Phase 1 trial goals are to understand safety, dose, and early efficacy An effective allogeneic CAR T offers potential to transform outcomes for patients Allogeneic HIP CAR T cell MHC I MHC II + CD47 + CD19 CAR
TCRα
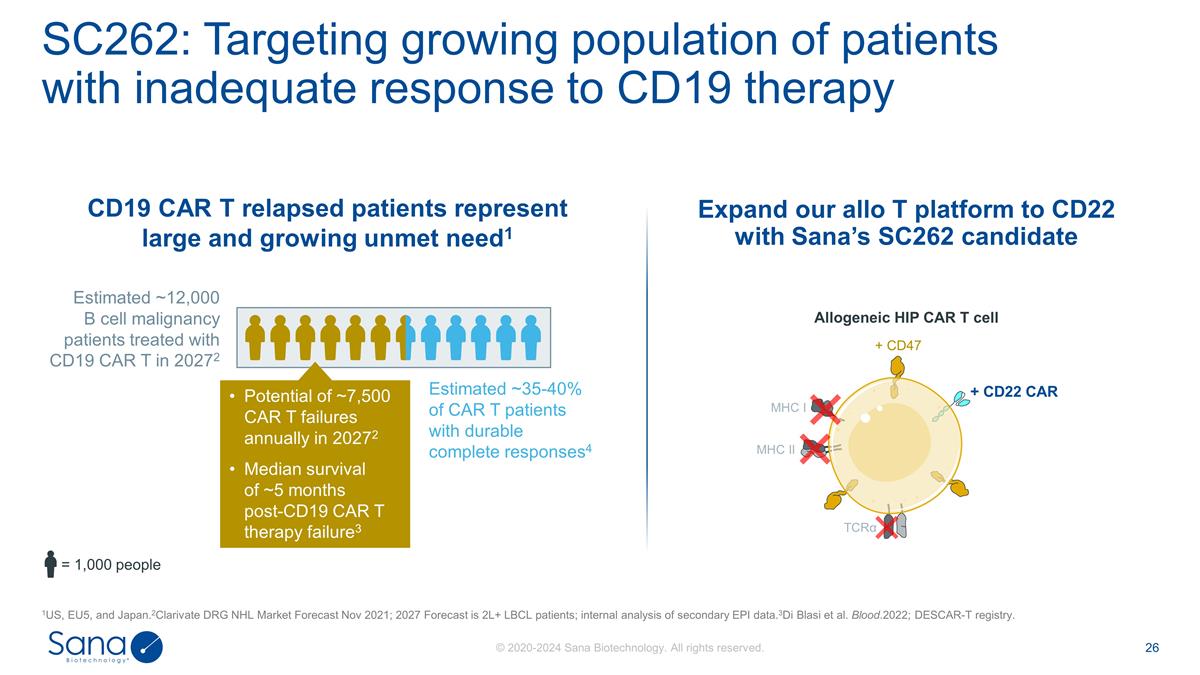
SC262: Targeting growing population
of patients with inadequate response to CD19 therapy 1US, EU5, and Japan.2Clarivate DRG NHL Market Forecast Nov 2021; 2027 Forecast is 2L+ LBCL patients; internal analysis of secondary EPI data.3Di Blasi et al. Blood.2022; DESCAR-T registry.
Estimated ~12,000 B cell malignancy patients treated with CD19 CAR T in 20272 CD19 CAR T relapsed patients represent large and growing unmet need1 = 1,000 people Estimated ~35-40% of CAR T patients with durable complete responses4 Potential of
~7,500 CAR T failures annually in 20272 Median survival of ~5 months post-CD19 CAR T therapy failure3 Expand our allo T platform to CD22 with Sana’s SC262 candidate Allogeneic HIP CAR T cell MHC I MHC II + CD47 + CD22 CAR TCRα
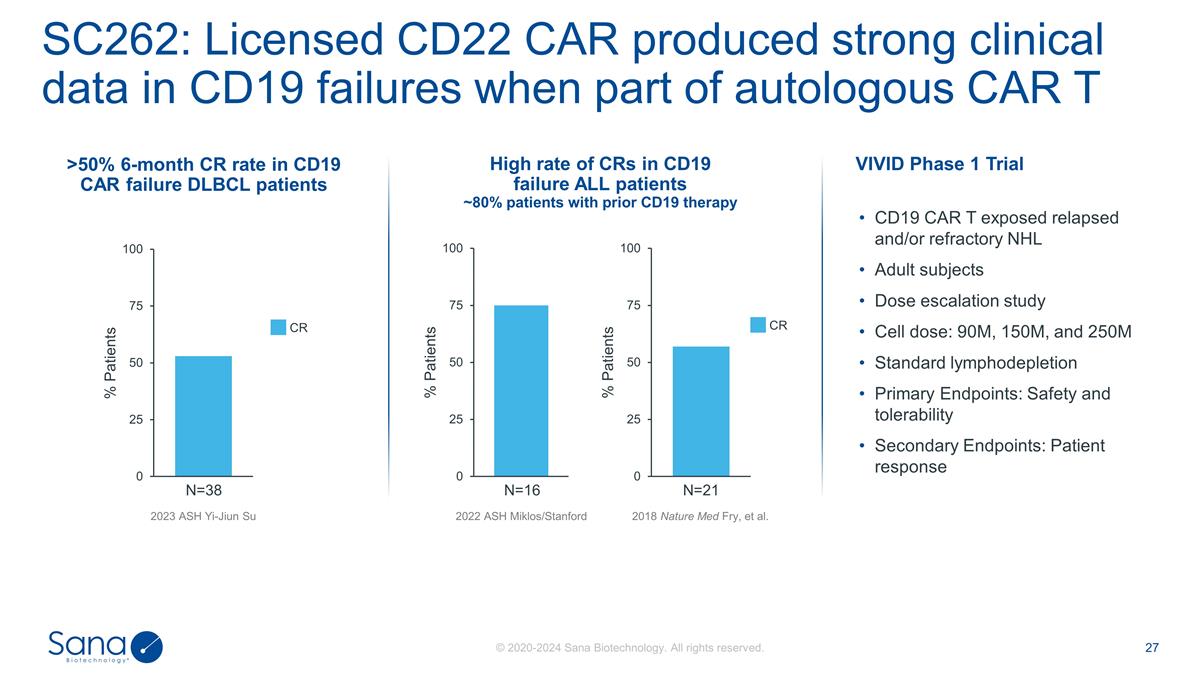
2023 ASH Yi-Jiun Su N=38 SC262:
Licensed CD22 CAR produced strong clinical data in CD19 failures when part of autologous CAR T N=16 N=21 >50% 6-month CR rate in CD19 CAR failure DLBCL patients High rate of CRs in CD19 failure ALL patients ~80% patients with prior CD19 therapy
2022 ASH Miklos/Stanford 2018 Nature Med Fry, et al. CR CR VIVID Phase 1 Trial CD19 CAR T exposed relapsed and/or refractory NHL Adult subjects Dose escalation study Cell dose: 90M, 150M, and 250M Standard lymphodepletion Primary Endpoints: Safety
and tolerability Secondary Endpoints: Patient response

Disease caused by autoimmune
destruction of insulin-producing pancreatic beta cells, resulting in no insulin production Type 1 diabetes is a large unmet need with >8M WW2 Short-term complications result from hypo- and hyperglycemia Long-term complications result from micro-
and macrovascular disease and end-organ damage: including heart attack, stroke, blindness, and kidney failure SC451 goal is euglycemia without any immunosuppression or exogenous insulin Type 1 diabetes represents a large unmet need with a loss of
~15 years of life1 1Rawshani et al. Lancet. 2018 2t1dindex.org DIGICOMPHOTO/SCIENCE PHOTO LIBRARY
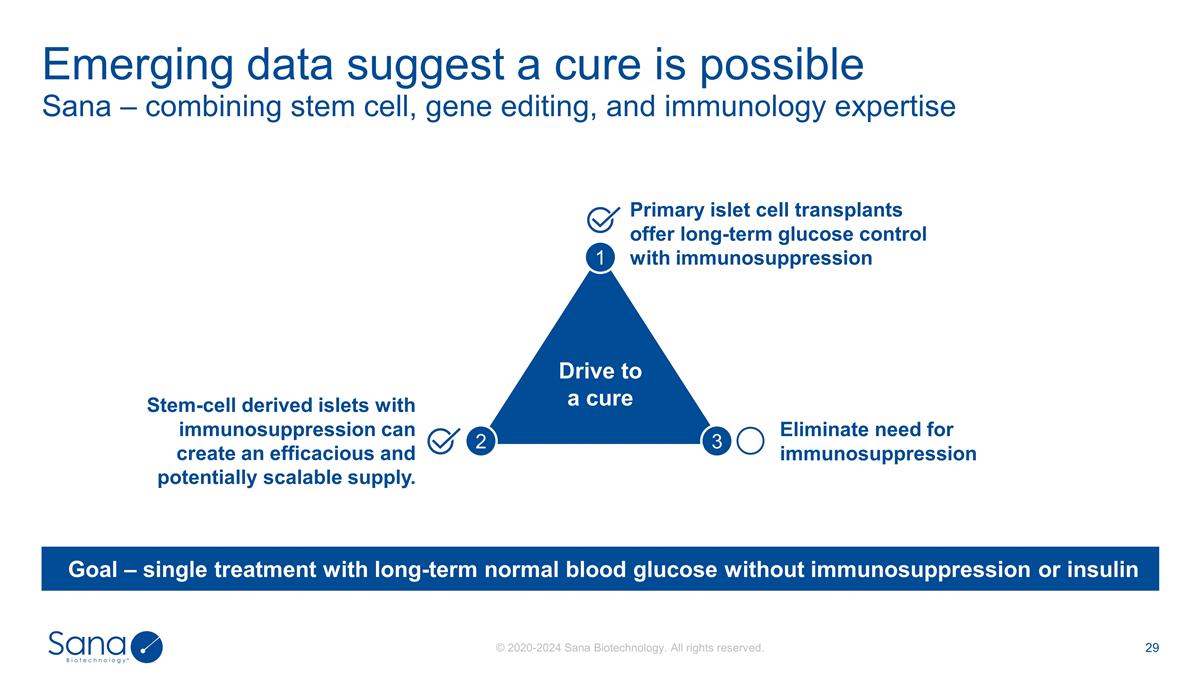
Sana – combining stem cell,
gene editing, and immunology expertise Emerging data suggest a cure is possible Eliminate need for immunosuppression Primary islet cell transplants offer long-term glucose control with immunosuppression Stem-cell derived islets with
immunosuppression can create an efficacious and potentially scalable supply. Goal – single treatment with long-term normal blood glucose without immunosuppression or insulin Drive to a cure 1 2 3
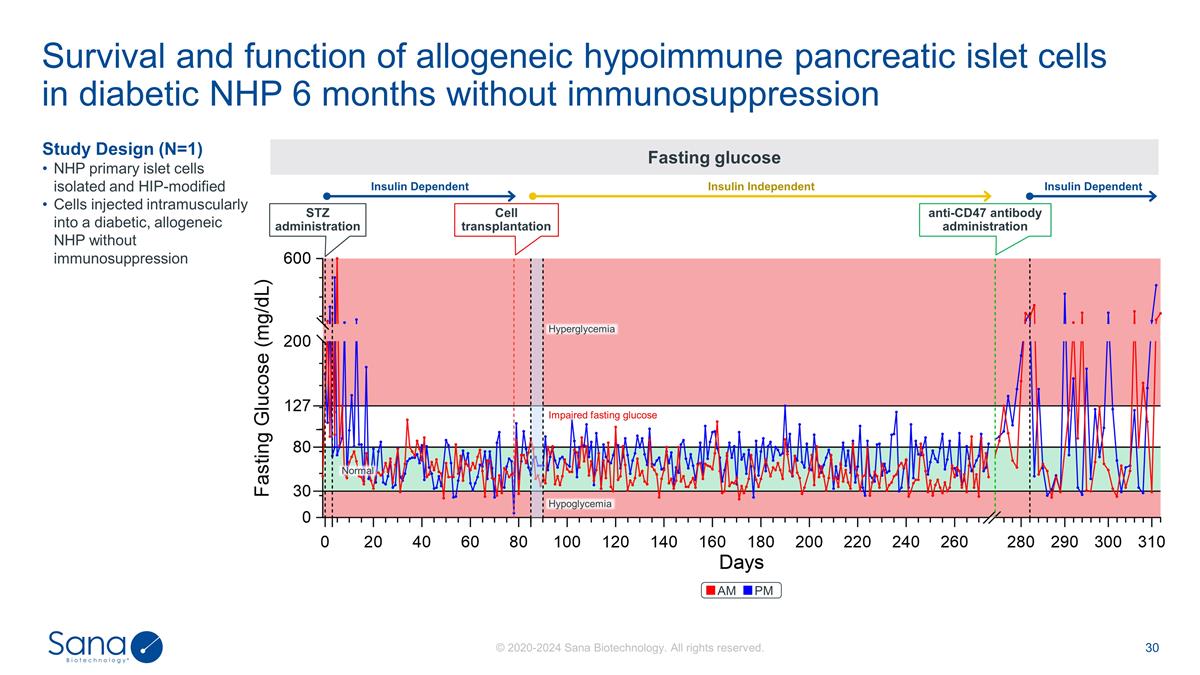
Survival and function of allogeneic
hypoimmune pancreatic islet cells in diabetic NHP 6 months without immunosuppression Study Design (N=1) NHP primary islet cells isolated and HIP-modified Cells injected intramuscularly into a diabetic, allogeneic NHP without immunosuppression
Fasting glucose STZ administration Cell transplantation Normal Hypoglycemia Impaired fasting glucose Hyperglycemia anti-CD47 antibody administration Insulin Dependent Insulin Independent Insulin Dependent AM PM
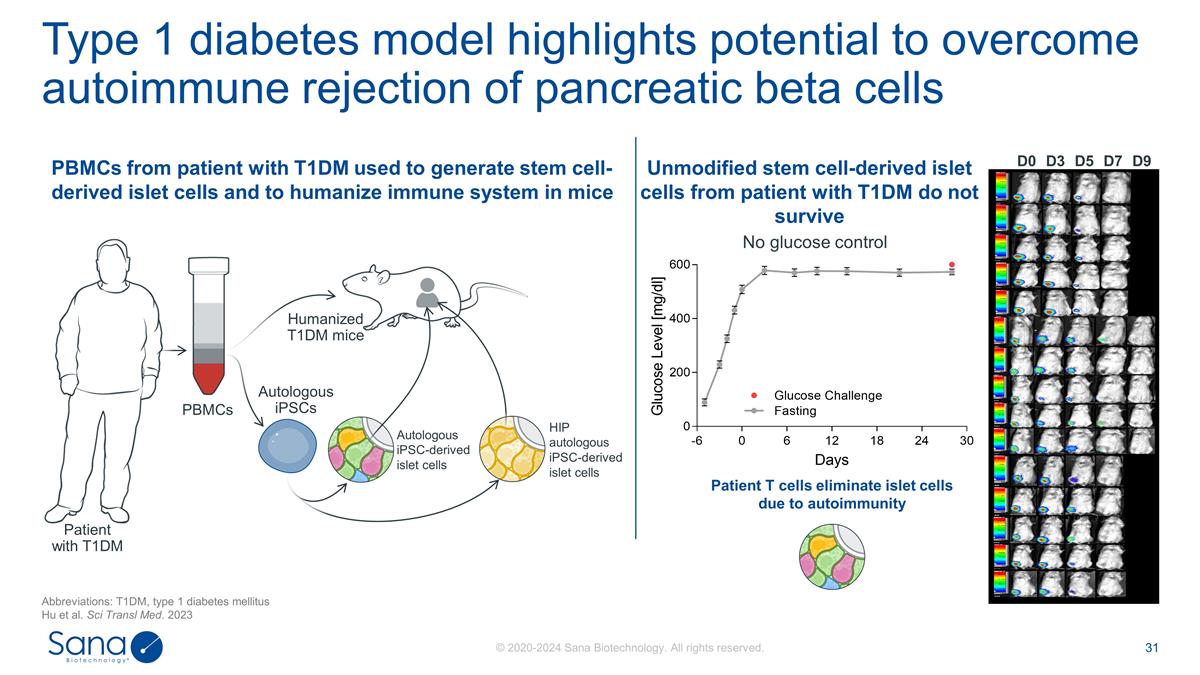
D0 D3 D7 D5 D9 No glucose control
Patient T cells eliminate islet cells due to autoimmunity Type 1 diabetes model highlights potential to overcome autoimmune rejection of pancreatic beta cells Abbreviations: T1DM, type 1 diabetes mellitus Hu et al. Sci Transl Med. 2023 Patient with
T1DM PBMCs Autologous iPSC-derived islet cells Humanized T1DM mice Autologous iPSCs HIP autologous iPSC-derived islet cells Unmodified stem cell-derived islet cells from patient with T1DM do not survive PBMCs from patient with T1DM used to generate
stem cell-derived islet cells and to humanize immune system in mice
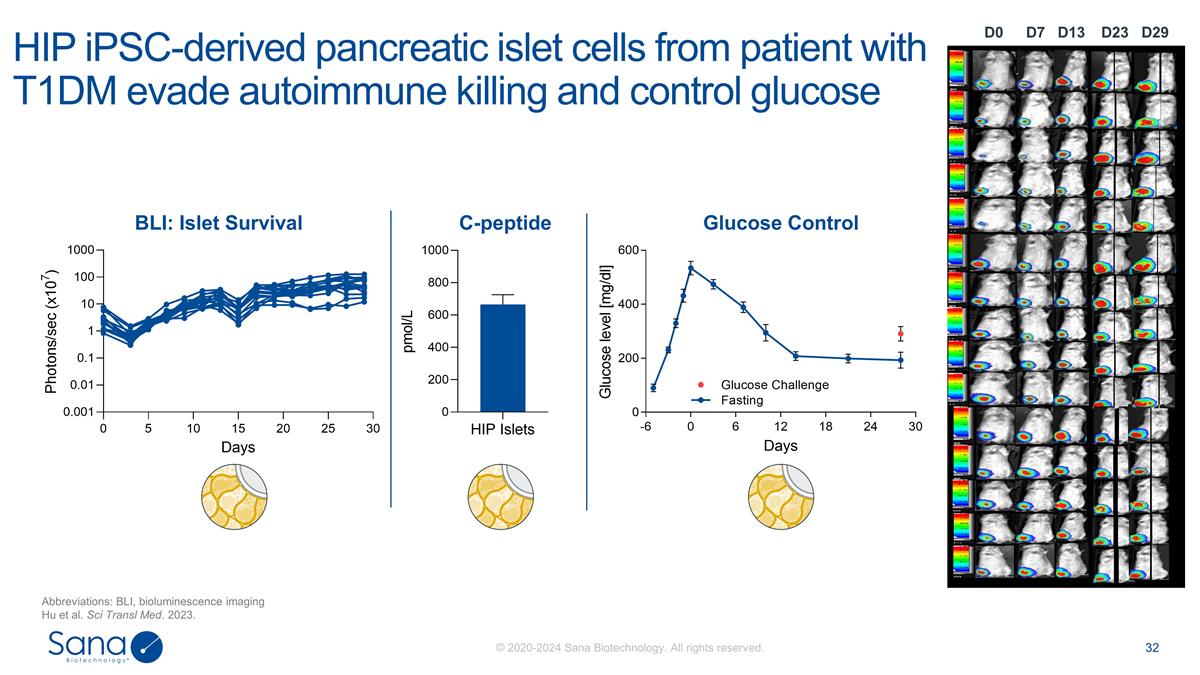
D7 D0 D23 D29 D13 HIP iPSC-derived
pancreatic islet cells from patient with T1DM evade autoimmune killing and control glucose Abbreviations: BLI, bioluminescence imaging Hu et al. Sci Transl Med. 2023. BLI: Islet Survival C-peptide Glucose Control
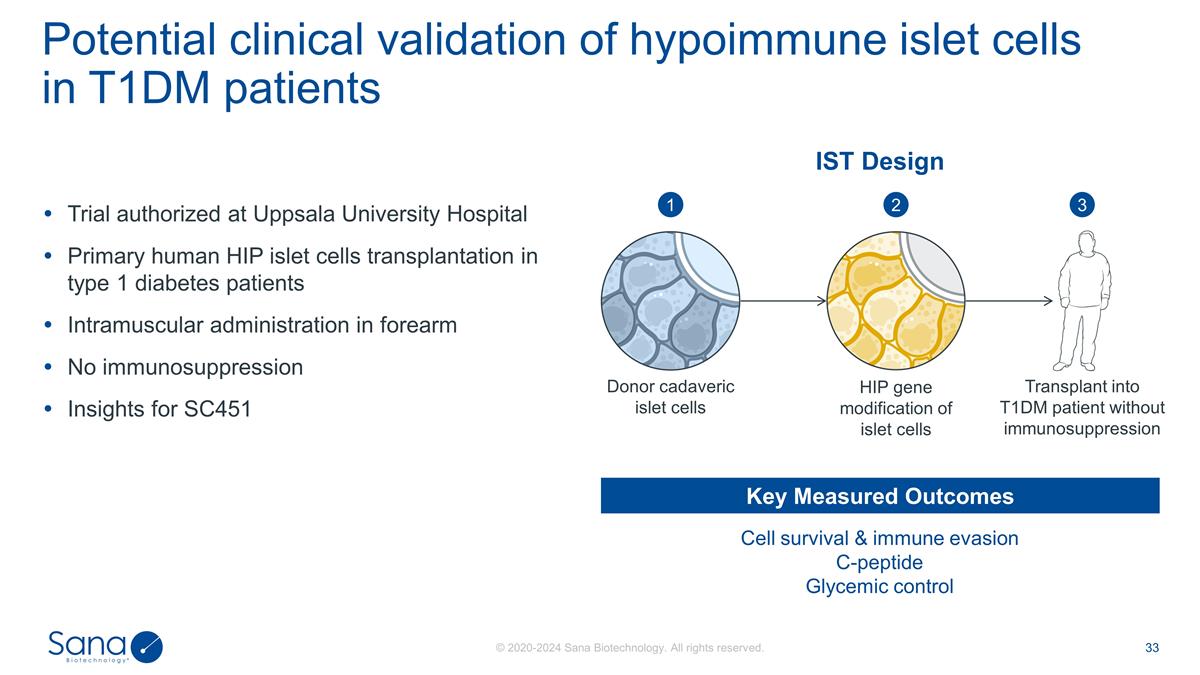
Trial authorized at Uppsala
University Hospital Primary human HIP islet cells transplantation in type 1 diabetes patients Intramuscular administration in forearm No immunosuppression Insights for SC451 Potential clinical validation of hypoimmune islet cells in T1DM patients
Cell survival & immune evasion C-peptide Glycemic control Key Measured Outcomes IST Design Transplant into T1DM patient without immunosuppression HIP gene modification of islet cells Donor cadaveric islet cells 1 2 3
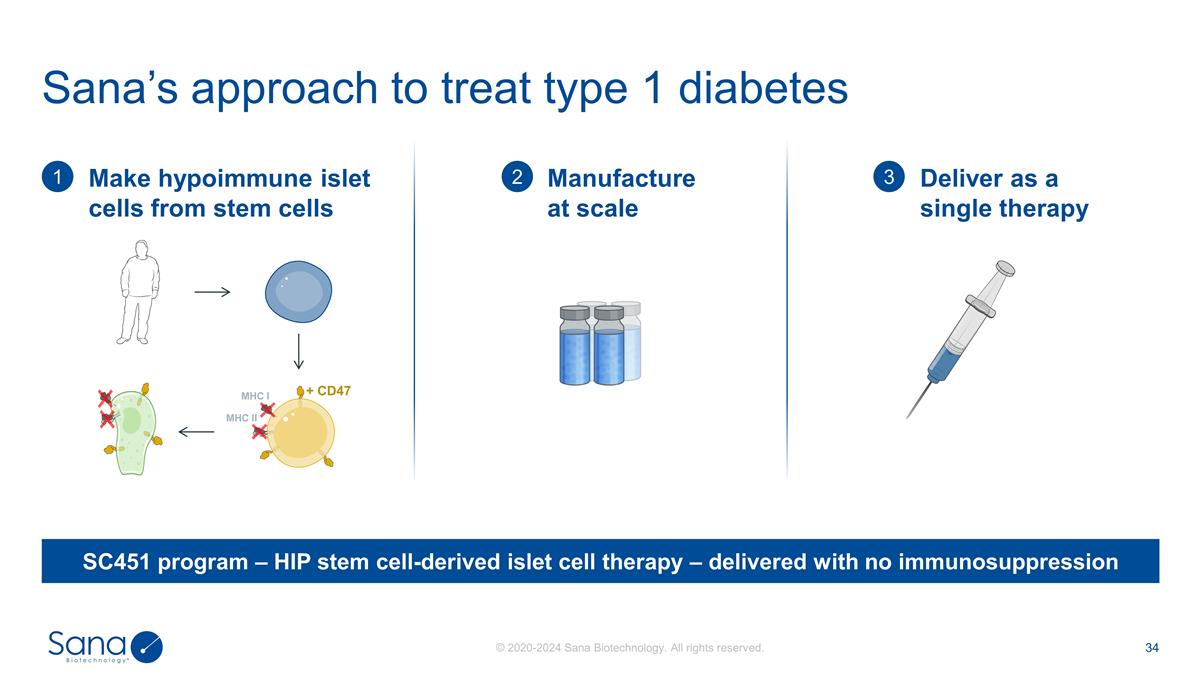
Sana’s approach to treat type
1 diabetes Make hypoimmune islet cells from stem cells 1 + CD47 MHC I MHC II Manufacture at scale 2 Deliver as a single therapy 3 SC451 program – HIP stem cell-derived islet cell therapy – delivered with no immunosuppression
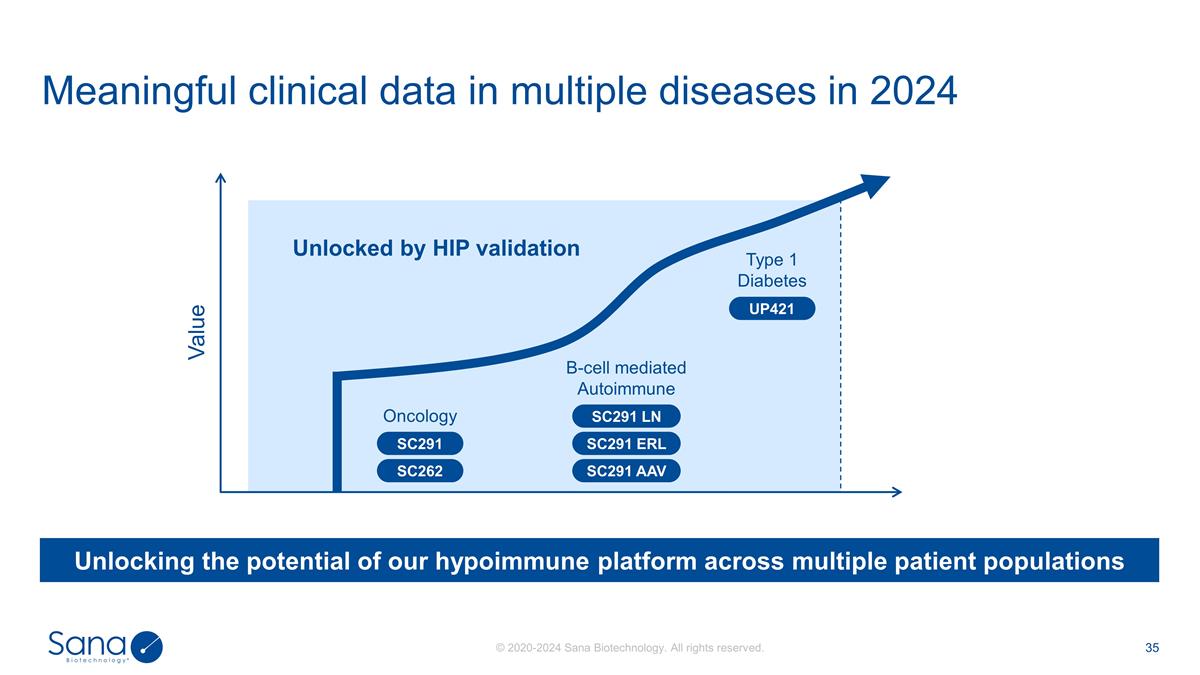
Meaningful clinical data in
multiple diseases in 2024 Unlocking the potential of our hypoimmune platform across multiple patient populations Value B-cell mediated Autoimmune Unlocked by HIP validation Oncology Type 1 Diabetes SC291 SC262 SC291 LN SC291 ERL SC291 AAV
UP421

v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Sana Biotechnology (NASDAQ:SANA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Sana Biotechnology (NASDAQ:SANA)
Historical Stock Chart
From Apr 2023 to Apr 2024