ARS Pharmaceuticals Soar 68% in Premarket Trading as FDA Panel Backs Neffy
May 12 2023 - 8:55AM
Dow Jones News
By Colin Kellaher
ARS Pharmaceuticals shares surged in premarket trading Friday
after the biopharmaceutical company won the backing of a U.S. Food
and Drug Administration advisory committee for its proposed
epinephrine nasal spray for the treatment of severe allergic
reaction, including anaphylaxis.
Shares of the San Diego company, which closed Wednesday at
$4.52, were recently up 68% premarket to $7.60. Trading in the
stock was halted during Thursday's session.
ARS late Thursday said the FDA panel voted that available data
support a favorable benefit-risk assessment for the spray, which
the company plans to market as "neffy," for adults and children who
weigh more than 66 pounds.
ARS said neffy, if approved by the FDA, would be the first
needle-free epinephrine product for severe allergic reactions.
The FDA, which usually follows the advice of its advisory
committees but it isn't bound by the recommendations, has set a
mid-2023 target action date for neffy.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
May 12, 2023 08:40 ET (12:40 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
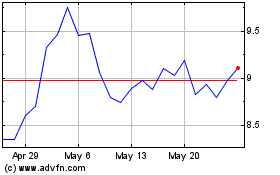
ARS Pharmaceuticals (NASDAQ:SPRY)
Historical Stock Chart
From Apr 2024 to May 2024
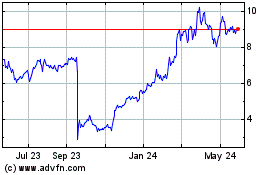
ARS Pharmaceuticals (NASDAQ:SPRY)
Historical Stock Chart
From May 2023 to May 2024