Registration No. 333-
WASHINGTON, D.C. 20549
Biocept, Inc.
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Michael W. Nall
Biocept, Inc.
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Frederick T. Muto
Charles J. Bair
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box.
☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box.
☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box.
☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
☐
SUBJECT TO COMPLETION, DATED AUGUST 18, 2017
PROSPECTUS

2,901,306 Shares of Common Stock
This prospectus relates to the disposition from time to time of up to 2,901,306 shares of our common stock by the selling stockholders named in this prospectus. We are not selling any common stock under this prospectus and will not receive any of the proceeds from the sale of shares by the selling stockholders.
The selling stockholders identified in this prospectus, or their permitted transferees or other successors-in-interest that may be identified in a supplement to this prospectus or, if required, a post-effective amendment to the registration statement of which this prospectus is a part, may offer the shares from time to time through public or private transactions at fixed prices, at prevailing market prices, at varying prices determined at the time of sale, or at privately negotiated prices. We provide more information about how the selling stockholders may sell their shares of common stock in the section entitled “Plan of Distribution” beginning on page 5 of this prospectus. We will not be paying any underwriting discounts or commissions in connection with any offering of common stock under this prospectus.
Our common stock is listed on The NASDAQ Capital Market under the symbol “BIOC.” On August 17, 2017, the last reported sale price of our common stock on The NASDAQ Capital Market was $1.21.
__________________________________________
Investing in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties included herein under the heading “Risk Factors” on page 3 of this prospectus, and under similar headings in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, which has been filed with the Securities and Exchange Commission and is incorporated by reference in this prospectus and in the other documents that are filed after the date hereof and incorporated by reference into this prospectus.
__________________________________________
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
__________________________________________
The date of this prospectus is , 2017.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, using the “shelf” registration process. Under this process, the selling stockholders may from time to time, in one or more offerings, sell the common stock described in this prospectus.
You should rely only on the information contained in or incorporated by reference into this prospectus (as supplemented and amended). We have not authorized anyone to provide you with different information. This document may only be used where it is legal to sell these securities. The information contained in this prospectus (and in any supplement or amendment to this prospectus) is accurate only as of the date on the front of the document, and any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since those dates.
We urge you to read carefully this prospectus (as supplemented and amended), together with the information incorporated herein by reference as described under the heading “Incorporation of Certain Information by Reference” before deciding whether to invest in any of the common stock being offered.
This prospectus incorporates by reference market data, industry statistics and other data that have been obtained from, or compiled from, information made available by third parties. We have not independently verified their data. This prospectus and the information incorporated herein by reference includes trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus are the property of their respective owners.
i
PROSPECTUS SUMM
ARY
This summary highlights selected information appearing elsewhere or incorporated by reference into this prospectus and may not contain all of the information that is important to you. You should read this prospectus and any free writing prospectus that we have authorized for use in connection with this offering carefully, including the risks and uncertainties included herein under the heading “Risk Factors” beginning on page 3 in this prospectus and incorporated by reference from our most recent Annual Report on Form 10-K, before making an investment decision.
Company Overview
We are an early stage molecular oncology diagnostics company that develops and commercializes proprietary circulating tumor cell, or CTC, and circulating tumor DNA, or ctDNA, assays utilizing a standard blood sample, or “liquid biopsy.” Our current and planned assays are intended to provide information to aid healthcare providers to identify specific oncogenic alterations that may qualify a subset of cancer patients for targeted therapy at diagnosis, progression or for monitoring in order to identify specific resistance mechanisms. Often, traditional methodologies such as tissue biopsies are insufficient or unavailable to provide the molecular subtype information necessary for clinical decisions. Our assays have the potential to provide more contemporaneous information on the characteristics of a patient’s disease compared with traditional methodologies such as tissue biopsy and radiographic imaging. Additionally, our proprietary blood collection tubes, which allow for the intact transport of liquid biopsy samples for research use only from regions around the world, are anticipated to be sold to laboratory supply distributor(s) commencing in the six months ended December 31, 2017.
Our current assays and our planned future assays focus on key solid tumor indications utilizing our Target-Selector
TM
liquid biopsy technology platform for the biomarker analysis of CTCs and ctDNA from a standard blood sample. Our patented Target-Selector CTC offering is based on an internally developed microfluidics-based cell capture and analysis platform, with enabling features that change how CTC testing is used by clinicians. Our patent pending Target-Selector ctDNA technology enables mutation detection with enhanced sensitivity and specificity, and is applicable to nucleic acid from ctDNA or other sample types, such as CTCs, bone marrow, or cerebrospinal fluid. Our Target-Selector CTC and ctDNA platforms provide both biomarker detection as well as monitoring capabilities, and require only a patient blood sample. We believe that our Target-Selector platform technology has the potential to be developed and commercialized as in vitro diagnostic (IVD) test kits, and we are currently pursuing this option.
At our corporate headquarters facility located in San Diego, California, we operate a clinical laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and accredited by the College of American Pathologists, or CAP. We also performed research and development that led to our current assays, and we also intend to perform research and development for planned assays, at this facility. In addition, we manufacture our microfluidic channels, related equipment and certain reagents. The assays we offer and intend to offer are classified as laboratory developed tests (LDTs) under CLIA regulations. CLIA certification is required before any clinical laboratory, including ours, may perform testing on human specimens for the purpose of obtaining information for the diagnosis, prevention, or treatment of disease or the assessment of health. In addition, we participate in and have received CAP accreditation, which includes rigorous bi-annual laboratory inspections and adherence to specific quality standards.
Corporate Information
We maintain our principal executive offices at 5810 Nancy Ridge Drive, San Diego, California 92121. Our telephone number is (858) 320-8200 and our website address is
www.biocept.com
. The information contained in, or that can be accessed through, our website is not incorporated into and is not part of this prospectus. We were incorporated in California on May 12, 1997 and reincorporated as a Delaware corporation on July 30, 2013.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an emerging growth company until the earlier of (a) December 31, 2019, (b) the last day of the fiscal year in which we have total annual gross revenue of at least $1.0 billion, (c) the last day of the fiscal year in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30
th
and (d) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
The Offering
The
selling stockholders named in this prospectus may offer and sell up to
2,901,306
shares of our common stock. Our common stock is currently listed on The NASDAQ Capital Market under the symbol “BIOC.” Shares of common stock that may be offered under this prospectus will be fully paid and non-assessable. We will not receive any of the proceeds of sales by the selling
1
stockholders of any of t
he common stock covered by this prospectus. Throughout this prospectus, when we refer to the shares of our common stock being registered on behalf of the selling stockholders for offer and sale, we are referring to the shares of common stock sold to the se
lling stockholders, as well as the shares of common stock issuable upon exercise of the warrants sold to the selling stockholders, each as described below under the section entitled “Selling Stockholders”. When we refer to the selling stockholders in this
prospectus, we are referring to the selling stockholders identified in this prospectus and, as applicable, their permitted transferees or other successors-in-interest that may be identified in a supplement to this prospectus or, if required, a post-effecti
ve amendment to the registration statement of which this prospectus is a part
.
2
RISK FACTORS
An investment in our common stock involves a high degree of risk. Before deciding whether to invest in our common stock, you should consider carefully the risks and uncertainties discussed under the heading “Risk Factors” contained in our in our Annual Report on Form 10-K for the year ended December 31, 2016, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2017, which are incorporated by reference in this prospectus, as the same may be amended, supplemented or superseded by the risks and uncertainties described under similar headings in the other documents that are filed by us after the date hereof and incorporated by reference into this prospectus. Additional risks not currently known to us or that we currently believe are immaterial may also significantly impair our business operations. Please also read carefully the section below entitled “Special Note Regarding Forward-Looking Statements.”
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, each prospectus supplement, the documents incorporated by reference herein and any free writing prospectus that we have authorized for use in connection with this offering contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which are subject to the “safe harbor” created by those sections. We may, in some cases, use words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. Readers are cautioned not to place undue reliance on these forward-looking statements, which are based on the information available to management at this time and which speak only as of this date. Examples of our forward-looking statements include:
|
|
•
|
our ability to increase sales of our assays and services;
|
|
|
•
|
our ability to continually develop new cancer diagnostic assays and enhance our current assays and future assays;
|
|
|
•
|
our ability to effectively compete with other diagnostic assays, methods and services that now exist or may hereafter be developed;
|
|
|
•
|
our ability to expand our business internationally;
|
|
|
•
|
our ability to obtain coverage and adequate reimbursement from governmental and other third-party payors for assays and services;
|
|
|
•
|
our expectations regarding the use of our existing cash and the expected net proceeds of this offering;
|
|
|
•
|
our ability to enter into agreements with commercialization partners for the sales, marketing and commercialization of our current assays and our planned future assays;
|
|
|
•
|
our ability to satisfy any applicable United States and international regulatory requirements with respect to assays and services; and
|
|
|
•
|
our ability to obtain or maintain patents or other appropriate protection for the intellectual property utilized in our current and planned assays and services.
|
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. The forward-looking statements contained in this prospectus are excluded from the safe harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act.
This prospectus also incorporates by reference estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
3
USE OF PROCEEDS
The selling stockholders will receive all of the net proceeds from sales of the common stock sold pursuant to this prospectus. We will receive no proceeds from sales of the common stock sold pursuant to this prospectus.
SELLING STOCKHOLDERS
Private Placement Transaction
On August 9, 2017, we entered into a common stock and warrant purchase agreement with the selling stockholders pursuant to which we issued and sold in a private placement an aggregate of 1,466,667 shares of common stock, together with a warrant to purchase an additional 1,434,639 shares of common stock, for an aggregate purchase price of approximately $2.2 million. The warrant has an exercise price per share equal to $1.50, is immediately exercisable and will expire on the fifth anniversary of the original issuance date.
Pursuant to the common stock and warrant purchase agreement, we agreed to file the registration statement of which this prospectus is a part to cover the resale of the shares of common stock issued to the selling stockholders, as well as the shares of common stock issuable upon exercise of the warrant issued to the selling stockholders (collectively, the “Registrable Securities”), and to keep such registration statement effective until the date on which all of the 2,901,306 shares registered for resale under this registration statement have been sold or can be sold publicly without condition or restriction under Rule 144 under the Securities Act.
We are registering the resale of the Registrable Securities to permit each of the selling stockholders identified below, or their permitted transferees or other successors-in-interest that may be identified in a supplement to this prospectus or, if required, a post-effective amendment to the registration statement of which this prospectus is a part, to resell or otherwise dispose of the Registrable Securities in the manner contemplated under “Plan of Distribution” in this prospectus (as may be supplemented and amended). Throughout this prospectus, when we refer to the shares of our common stock being registered on behalf of the selling stockholders, we are referring to the Registrable Securities, and when we refer to the selling stockholders in this prospectus, we are referring to the purchasers under the common stock and warrant purchase agreement and, as applicable, their permitted transferees or other successors-in-interest that may be identified in a supplement to this prospectus or, if required, a post-effective amendment to the registration statement of which this prospectus is a part.
The selling stockholders may sell some, all or none of their Registrable Securities. We do not know how long the selling stockholders will hold the Registrable Securities before selling them, and we currently have no agreements, arrangements or understandings with the selling stockholders regarding the sale or other disposition of any of the Registrable Securities. The Registrable Securities covered hereby may be offered from time to time by the selling stockholders.
The following table sets forth the name of each selling stockholder, the number and percentage of our outstanding shares of common stock beneficially owned by the selling stockholders as of August 10, 2017, the number of Registrable Securities that may be offered under this prospectus, and the number and percentage of our outstanding shares of common stock beneficially owned by the selling stockholders assuming all of the Registrable Securities covered hereby are sold. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to our common stock. Generally, a person “beneficially owns” shares of our common stock if the person has or shares with others the right to vote those shares or to dispose of them, or if the person has the right to acquire voting or disposition rights within 60 days. The number of shares in the column “Number of Shares Offered” represents all of the Registrable Securities that a selling stockholder may offer and sell from time to time under this prospectus.
All information contained in the table below and the footnotes thereto is based upon information provided to us by the selling stockholders. The percentage of shares owned prior to and after the offering is based on 30,253,743 shares of common stock outstanding as of August 10, 2017.
|
|
Before Offering
|
|
After Offering
|
|
Name and Address
|
Number of
Shares
Beneficially
Owned
|
Percentage of Shares Beneficially Owned
|
Number of
Shares
Offered
|
Number of
Shares
Beneficially
Owned
|
Percentage of
Shares
Beneficially
Owned
|
|
Ally Bridge LB Healthcare Master Fund Limited
(1)
|
4,582,306
(2)
|
14.5%
|
2,901,306
|
1,681,000
|
5.3%
|
|
|
(1)
|
Ally Bridge LB Management Limited owns the sole voting share in Ally Bridge LB Healthcare Master Fund Limited. Mr. Fan Yu and Mr. Bin Li are the shareholders and directors of Ally Bridge LB Management Limited. Ally Bridge LB
|
4
|
|
|
Management Limited, by virtue o
f it being the holder of sole voting share of Ally Bridge LB Healthcare Master Fund Limited, and each of Mr. Yu and Mr. Li, by virtue of being a shareholder and director of Ally Bridge LB Management Limited, may be deemed to have voting control and investm
ent discretion over the securities held by Ally Bridge LB Healthcare Master Fund Limited. The address for Ally Bridge LB Healthcare Master Fund Limited is
Unit 1602, 16/F, Wheelock House, 20 Pedder Street, Central, Hong Kong
.
|
|
|
(2)
|
Includes a warrant to purchase 1,434,639 shares of common stock that is exercisable by Ally Bridge LB Healthcare Master Fund Limited.
|
Other Relationships with the Selling Stockholders
On October 14, 2016, Ally Bridge LB Healthcare Master Fund Limited acquired, through a registered public offering, an aggregate of 1,681,000 shares of our common stock and warrants to purchase an additional 1,681,000 shares of our common stock. Each warrant has an exercise price of $1.10 per share, is immediately exercisable and will expire on the fifth anniversary of the original issuance date. The price per share and related warrant was $1.10. Ally Bridge LB Healthcare Master Fund Limited paid an aggregate purchase price of approximately $1,849,100. The purchase was funded from the working capital of Ally Bridge LB Healthcare Master Fund Limited.
On January 17, 2017, January 18, 2017 and January 19, 2017, Ally Bridge LB Healthcare Master Fund Limited disposed of, respectively, 876,399, 549,708 and 254,893 shares of our common stock. Subsequently, Ally Bridge LB Healthcare Master Fund Limited exercised its warrants to purchase an additional 1,681,000 shares of our common stock.
Except with respect to the foregoing, none of the selling stockholders has, or within the past three years has had, any position, office or other material relationship with us.
PLAN OF DISTRIBUTION
We are registering the shares of common stock issued to the selling stockholders to permit the resale of these shares of common stock by the selling stockholders from time to time from after the date of this prospectus. We will not receive any of the proceeds from the sale by the selling stockholders of the shares of common stock. We will bear the fees and expenses incident to our obligation to register the shares of common stock, however the selling stockholders will bear legal and advisor fees, commissions and discounts, if any, attributable to their respective sales of shares.
Each selling stockholder may, from time to time, sell any or all of its shares of common stock covered hereby on The NASDAQ Capital Market or any other stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or privately negotiated prices. A selling stockholder may use any one or more of the following methods when selling shares:
|
|
•
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
•
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
•
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
•
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
•
|
privately negotiated transactions;
|
|
|
•
|
underwritten transactions;
|
|
|
•
|
settlement of short sales, to the extent permitted by law;
|
|
|
•
|
in transactions through broker-dealers that agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share;
|
|
|
•
|
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
|
•
|
through the distribution of the common stock by any selling stockholder to its partners, members or stockholders;
|
|
|
•
|
a combination of any such methods of sale; or
|
|
|
•
|
any other method permitted pursuant to applicable law.
|
5
The selling stockholders may also sell the shares of common stock under Rule 144 under the Securities Act, if ava
ilable, rather than under this prospectus.
If underwriters are used in the sale, the shares of common stock will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. In connection with any such underwritten sale of shares of common stock, underwriters may receive compensation from the selling stockholders, for whom they may act as agents, in the form of discounts, concessions or commissions. If the selling stockholders use an underwriter or underwriters to effectuate the sale of shares of common stock, we and/or they will execute an underwriting agreement with those underwriters at the time of sale of those shares of common stock. To the extent required by law, the names of the underwriters will be set forth in a prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes the prospectus supplement and the accompanying prospectus used by the underwriters to sell those securities. The obligations of the underwriters to purchase those shares of common stock will be subject to certain conditions precedent, and unless otherwise specified in a prospectus supplement, the underwriters will be obligated to purchase all the shares of common stock offered by such prospectus supplement if any of such shares of common stock are purchased. Any public offering price and any discounts or concessions allowed or re-allowed or paid to dealers may be changed from time to time.
Broker-dealers engaged by the selling stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440-1.
In connection with the sale of the shares of common stock or interests therein, the selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the shares of common stock in the course of hedging the positions they assume. The selling stockholders may also sell the shares of common stock short and deliver these securities to close out their short positions or to return borrowed shares in connection with such short sales, or loan or pledge the shares of common stock to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares of common stock offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholders and any broker-dealers or agents that are involved in selling the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such selling stockholders, broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Selling stockholders who are “underwriters” within the meaning of Section 2(11) of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act and may be subject to certain statutory liabilities of, including but not limited to, Sections 11, 12 and 17 of the Securities Act and Rule 10b-5 under the Exchange Act. Each selling stockholder has informed us that it is not a registered broker-dealer or an affiliate of a registered broker-dealer.
We are required to pay certain fees and expenses incurred by us incident to the registration of the shares of common stock of the selling stockholders. We have agreed to indemnify the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act. We may be indemnified by the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act that may arise from any written information furnished to us by the selling stockholders specifically for use in this prospectus.
The selling stockholders will be subject to the prospectus delivery requirements of the Securities Act, including Rule 172 thereunder, unless an exemption therefrom is available.
We agreed to cause the registration statement of which this prospectus is a part to remain effective until the date on which all of the shares registered for resale under the registration statement have been sold or can be sold publicly without condition or restriction under Rule 144 under the Securities Act. The shares of common stock will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the shares of common stock covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the shares of common stock may not simultaneously engage in market making activities with respect to the shares of common stock for the applicable
6
restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the selling stockholders will be subject
to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of common stock by the selling stockholders or any other person. We will make copies
of this prospectus available to the selling stockholders and have informed them of the need to deliver a copy of this prospectus at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
The selling stockholders may decide not to sell any or all of the shares of common stock we registered on behalf of the selling stockholders pursuant to the registration statement of which this prospectus forms a part.
Once sold under the registration statement of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
VALIDITY OF COMMON STOCK
The validity of the common stock being offered hereby has been passed upon for us by Cooley LLP, San Diego, California.
EXPERTS
Mayer Hoffman McCann P.C., our independent registered public accounting firm, has audited our balance sheets as of December 31, 2015 and 2016, and the related statements of operations and comprehensive loss, changes in shareholders’ equity/(deficit) and cash flows for each of the two years in the period ended December 31, 2016, as set forth in their report. We have incorporated by reference our financial statements in this prospectus and in this registration statement in reliance on the report of Mayer Hoffman McCann P.C. given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the resale of the common stock the selling stockholders are offering under this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the common stock offered by the selling stockholders under this prospectus, we refer you to the registration statement and the exhibits filed as a part of the registration statement. You may read and copy the registration statement, as well as our reports, proxy statements and other information, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference room. The SEC also maintains an Internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC, including Biocept. The SEC’s Internet site can be found at www.sec.gov. We maintain a website at www.biocept.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus, and you should not consider it part of this prospectus.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference the information and reports we file with it, which means that we can disclose important information to you by referring you to these documents. The information incorporated by reference is an important part of this prospectus. We incorporate by reference, as of their respective dates of filing, the documents listed below that we have filed with the SEC and any documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the termination of the offering of securities under this prospectus (except in each case the information contained in such documents to the extent “furnished” and not “filed”):
|
|
•
|
our Annual Report on Form 10-K for the fiscal year ended December 31, 2016;
|
|
|
•
|
our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2017 and June 30, 2017;
|
|
|
•
|
the information specifically incorporated by reference into our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 from our definitive proxy statement on Schedule 14A (other than information furnished rather than filed) filed with the SEC on March 30, 2017;
|
|
|
•
|
our Current Reports on Form 8-K filed with the SEC on January 25, 2017, February 9, 2017, March 23, 2017, March 30, 2017, May 5, 2017 and August 10, 2017 (in each case, except for information contained therein which is furnished rather than filed); and
|
7
|
|
•
|
the description of our common stock, which is registered under Section 12 of the Exchange Act, in our registration statement on Form 8-A, filed with the SEC on January 28, 2014, including any amendments or reports filed for the
purpose of updating such description.
|
Upon request, we will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered a copy of the documents incorporated by reference into this prospectus. You may request a copy of these filings, and any exhibits we have specifically incorporated by reference as an exhibit in this prospectus, at no cost by writing or telephoning us at the following address:
Biocept, Inc.
5810 Nancy Ridge Drive
San Diego, California 92121
Telephone: (858) 320-8200
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR
SECURITIES ACT LIABILITY
As permitted by the Delaware General Corporation Law, we have entered into indemnity agreements with each of our directors and executive officers, that require us to indemnify such persons against any and all expenses (including attorneys’ fees), witness fees, damages, judgments, fines, settlements and other amounts incurred in connection with any action, suit or proceeding, whether actual or threatened, to which any such person may be made a party by reason of the fact that such person is or was a director, an officer or an employee of ours or any of our affiliated enterprises, provided that such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to our best interests and, with respect to any criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification thereunder.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in such Act and is therefore unenforceable.
8
PART
II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution
The
following table sets forth the estimated costs and expenses payable by the registrant in connection with the common stock being registered. The selling stockholders will not bear any portion of such expenses. All the amounts shown are estimates, except for the SEC registration fee
.
|
SEC registration fee
|
|
$
|
407
|
|
|
Accounting fees and expenses
|
|
|
5,000
|
|
|
Legal fees and expenses
|
|
|
30,000
|
|
|
Printing and miscellaneous fees and expenses
|
|
|
4,593
|
|
|
Total
|
|
$
|
40,000
|
|
Item 15. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act.
The registrant’s amended certificate of incorporation provides for indemnification of its directors and executive officers to the maximum extent permitted by the Delaware General Corporation Law, and the registrant’s amended and restated bylaws provide for indemnification of its directors and executive officers to the maximum extent permitted by the Delaware General Corporation Law.
In addition, the registrant has entered into indemnification agreements with each of its current directors and executive officers. These agreements will require the registrant to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to the registrant and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. The registrant also intends to enter into indemnification agreements with its future directors and executive officers.
The common stock and warrant purchase agreement that the registrant entered into with the selling stockholders identified in the prospectus included in this registration statement provides for cross-indemnification in connection with registration of the registrant’s common stock on behalf of such selling stockholders, including for some liabilities arising under the Securities Act.
Item 16. Exhibits
The list of exhibits is set forth under the heading entitled “Index to Exhibits” at the end of this registration statement and is incorporated herein by reference.
Item 17. Undertakings
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
|
(i)
|
to include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
|
|
(ii)
|
to
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement
; and
|
|
|
(iii)
|
to
include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement
;
|
II-1
provided, however,
that paragraphs (1)(i), (1)(ii) and (1)(iii)
do not apply if the information required to be included in a post-effective amendment by those paragraphs
is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prosp
ectus filed pursuant to Rule 424(b) that is part of the registration statement
.
(2) That
, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide
offering thereof
.
(3) To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering
.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i)
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement
; and
(ii)
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial
bona fide
offering thereof.
Provided, however,
that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date
.
(5)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.
Provided, however
, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use
.
(6)
That, for the purpose of determining liability of the registrant under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide
offering thereof
.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue
.
II-2
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in San Diego, California, on the 18
th
day of August, 2017.
|
|
BIOCEPT, INC.
|
|
|
|
|
|
|
By:
|
/s/ Michael W. Nall
|
|
|
|
Michael W. Nall
|
|
|
|
Chief Executive Officer and President
|
POWER OF ATTORNEY
Know All Persons By These Presents
,
that each person whose signature appears below constitutes and appoints
Michael W. Nall and Timothy C. Kennedy
, and each of them, as his true and lawful attorneys-in-fact and agents, each with the full power of substitution, for him and in his name, place or stead, in any and all capacities, to sign any and all amendments to this Registration Statement (including post-effective amendments), and to sign any registration statement for the same offering covered by this Registration Statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act, and all post-effective amendments thereto, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their, his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof
.
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ Michael W. Nall
|
|
Chief Executive Officer and President
|
|
August 18, 2017
|
|
Michael W. Nall
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/ Timothy C. Kennedy
|
|
Chief Financial Officer, Senior VP of Operations and Secretary
|
|
August 18, 2017
|
|
Timothy C. Kennedy
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
|
|
|
|
/s/ David F. Hale
|
|
Chairman and Director
|
|
August 18, 2017
|
|
David F. Hale
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Marsha A. Chandler
|
|
Director
|
|
August 18, 2017
|
|
Marsha A. Chandler
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Bruce E. Gerhardt
|
|
Director
|
|
August 18, 2017
|
|
Bruce E. Gerhardt
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Bruce A. Huebner
|
|
Director
|
|
August 18, 2017
|
|
Bruce A. Huebner
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Edward Neff
|
|
Director
|
|
August 18, 2017
|
|
Edward Neff
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Ivor Royston
|
|
Director
|
|
August 18, 2017
|
|
Ivor Royston
|
|
|
|
|
|
|
|
|
|
|
|
/s/ M. Faye Wilson
|
|
Director
|
|
August 18, 2017
|
|
M. Faye Wilson
|
|
|
|
|
II-3
INDEX TO EXHIBITS
|
Exhibit Number
|
|
Description of Exhibit
|
|
|
|
|
|
3.1
|
|
Certificate of Amendment of Certificate of Incorporation (incorporated by reference to Exhibit 3.1.4 of the Registrant’s Current Report on Form 8-K, filed with the SEC on February 14, 2014).
|
|
3.2
|
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2.1 of the Registrant’s Current Report on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
3.3
|
|
Certificate of Amendment of Certificate of Incorporation (incorporated by reference to Exhibit 3.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on September 29, 2016).
|
|
4.1
|
|
Reference is made to Exhibits 3.1, 3.2 and 3.3.
|
|
4.2
|
|
Amended and Restated Investor Rights Agreement, dated as of October 31, 2011, among the Registrant and certain investors named therein (incorporated by reference to Exhibit 10.12 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.3
|
|
Specimen Common Stock certificate of Biocept, Inc. (incorporated by reference to Exhibit 4.3 of the Registrant’s Annual Report on Form 10-K, filed with the SEC on March 28, 2017).
|
|
4.4
|
|
Form of Representative’s Warrant, dated February 10, 2014 (incorporated by reference to Exhibit 4.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), as amended, filed with the SEC on November 20, 2013).
|
|
4.5
|
|
Form of Warrant issued to the lenders under the Loan and Security Agreement, dated as of April 30, 2014, by and among Biocept, Inc., Oxford Finance LLC, as collateral agent, and the lenders party thereto from time to time, including Oxford Finance LLC (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on May 6, 2014).
|
|
4.6
|
|
Form of Warrant to Purchase Common Stock (incorporated by reference to Exhibit 4.5 of the Registrant’s Registration Statement on Form S-1 (File No. 333-201437), filed with the SEC on February 6, 2015).
|
|
4.7
|
|
Warrant to Purchase Preferred Stock, dated September 10, 2012, issued by the Registrant in favor of ARE-SD Region No. 18, LLC (incorporated by reference to Exhibit 10.11.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.8
|
|
Warrant to Purchase Common Stock, dated September 10, 2013, issued by the Registrant in favor of ARE-SD Region No. 18, LLC (incorporated by reference to Exhibit 10.11.6 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.9
|
|
Warrant to Purchase Preferred Stock dated as of January 21, 2009, issued by the Registrant in favor of Goodman Co. Ltd. (incorporated by reference to Exhibit 10.17.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.10
|
|
Warrant to Purchase Common Stock dated as of July 31, 2013, issued by the Registrant in favor of Goodman Co. Ltd. (incorporated by reference to Exhibit 10.17.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.11
|
|
Form of Warrant to Purchase Preferred Stock, issued by the Registrant in favor of various investors under the Note and Warrant Purchase Agreement dated as of January 13, 2012 (incorporated by reference to Exhibit 10.19.3 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.12
|
|
Form of Amendment of Warrant to Purchase Preferred Stock, dated as of September 13, 2013 (incorporated by reference to Exhibit 10.19.4 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.13
|
|
Form of Warrant to Purchase Common Stock, issued by the Registrant in favor of various investors under the Note and Warrant Purchase Agreement dated as of June 28, 2013 (incorporated by reference to Exhibit 10.20.2 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.14
|
|
Form of Warrant to Purchase Common Stock, issued by the Registrant in favor of various guarantors under the Reimbursement Agreement dated as of July 11, 2013 (incorporated by reference to Exhibit 10.21.1 of the Registrant’s Registration Statement on Form S-1 (File No. 333-191323), filed with the SEC on September 23, 2013).
|
|
4.15
|
|
Form of Common Stock Purchase Warrant issued to the investors under the Securities Purchase Agreement, dated April 29, 2016, by and among Biocept, Inc. and the purchasers signatory thereto (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on April 29, 2016).
|
|
4.16
|
|
Form of Warrant to Purchase Common Stock (incorporated by reference to Exhibit 4.16 of the Registrant’s Post-Effective Amendment to Registration Statement on Form S-1 (File No. 333-213111), filed with the SEC on October 14, 2016).
|
|
4.17
|
|
Form of Common Stock Purchase Warrant issued to the investors under the Securities Purchase Agreement, dated March 28, 2017, by and among Biocept, Inc. and the purchasers signatory thereto (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on March 30, 2017).
|
|
Exhibit Number
|
|
Description of Exhibit
|
|
4.18
|
|
Common Stock Purchase Warrant issued by the Registrant in favor of Ally Bridge LB Healthcare Master Fund Limited under the Common Stock and Warrant Purchase Agreement dated August 9, 2017 (incorporated by reference to Exhibit 4.1 of the Registrant’s Current Report on Form 8-K, filed with the SEC on August 10, 2017).
|
|
5.1
|
|
Opinion of Cooley LLP.
|
|
23.1
|
|
Consent of Mayer Hoffman McCann P.C.
|
|
23.2
|
|
Consent of Cooley LLP (included in Exhibit 5.1).
|
|
24.1
|
|
Power of Attorney (included in the signature pages to this registration statement).
|
Biocept (NASDAQ:BIOC)
Historical Stock Chart
From Mar 2024 to Apr 2024
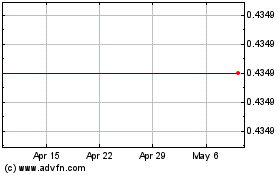
Biocept (NASDAQ:BIOC)
Historical Stock Chart
From Apr 2023 to Apr 2024