false
2023
FY
0001314052
0001314052
2022-10-01
2023-09-30
0001314052
2023-03-31
0001314052
2023-11-24
0001314052
2023-09-30
0001314052
2022-09-30
0001314052
2021-10-01
2022-09-30
0001314052
2020-10-01
2021-09-30
0001314052
2021-09-30
0001314052
2020-09-30
0001314052
us-gaap:CommonStockMember
2020-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2020-09-30
0001314052
us-gaap:RetainedEarningsMember
2020-09-30
0001314052
us-gaap:CommonStockMember
2021-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2021-09-30
0001314052
us-gaap:RetainedEarningsMember
2021-09-30
0001314052
us-gaap:CommonStockMember
2022-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2022-09-30
0001314052
us-gaap:RetainedEarningsMember
2022-09-30
0001314052
us-gaap:CommonStockMember
2020-10-01
2021-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2020-10-01
2021-09-30
0001314052
us-gaap:RetainedEarningsMember
2020-10-01
2021-09-30
0001314052
us-gaap:CommonStockMember
2021-10-01
2022-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2021-10-01
2022-09-30
0001314052
us-gaap:RetainedEarningsMember
2021-10-01
2022-09-30
0001314052
us-gaap:CommonStockMember
2022-10-01
2023-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2022-10-01
2023-09-30
0001314052
us-gaap:RetainedEarningsMember
2022-10-01
2023-09-30
0001314052
us-gaap:CommonStockMember
2023-09-30
0001314052
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001314052
us-gaap:RetainedEarningsMember
2023-09-30
0001314052
avxl:MichaelJFoxFoundationMember
2020-10-01
2021-09-30
0001314052
currency:AUD
2022-10-01
2023-09-30
0001314052
currency:AUD
2021-10-01
2022-09-30
0001314052
currency:AUD
2020-10-01
2021-09-30
0001314052
currency:AUD
2023-09-30
0001314052
currency:AUD
2022-09-30
0001314052
avxl:SecuritiesPurchaseAgreementMember
2021-06-21
2021-06-22
0001314052
avxl:SecuritiesPurchaseAgreementMember
2021-06-22
0001314052
avxl:EquityOfferingSalesAgreementMember
2022-10-01
2023-09-30
0001314052
avxl:EquityOfferingSalesAgreementMember
2021-10-01
2022-09-30
0001314052
avxl:EquityOfferingSalesAgreementMember
2020-10-01
2021-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
2023-02-02
2023-02-03
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommonStockIncludingCommitmentSharesMember
2022-10-01
2023-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommonStockIncludingCommitmentSharesMember
2021-10-01
2022-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommonStockExcludingCommitmentSharesMember
2022-10-01
2023-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommonStockExcludingCommitmentSharesMember
2021-10-01
2022-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
2022-10-01
2023-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
2021-10-01
2022-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommitmentSharesMember
2022-10-01
2023-09-30
0001314052
avxl:PurchaseAgreement2023Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommitmentSharesMember
2021-10-01
2022-09-30
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
2023-02-02
2023-02-03
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommonStockIncludingCommitmentSharesMember
2020-10-01
2021-09-30
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommonStockExcludingCommitmentSharesMember
2020-10-01
2021-09-30
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
us-gaap:CommonStockMember
2020-10-01
2021-09-30
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
avxl:CommitmentSharesMember
2020-10-01
2021-09-30
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
2022-10-01
2023-09-30
0001314052
avxl:PurchaseAgreement2019Member
avxl:LincolnParkCapitalFundLLCMember
2021-10-01
2022-09-30
0001314052
avxl:StockOptionPlan2015Member
2023-09-30
0001314052
avxl:StockOptionPlan2019Member
2022-10-01
2023-09-30
0001314052
avxl:StockOptionPlan2022Member
2021-10-01
2022-09-30
0001314052
avxl:StockOptionPlan2022Member
2022-03-24
2022-03-25
0001314052
avxl:StockOptionPlan2022Member
2023-09-30
0001314052
avxl:PurchaseWarrants1Member
2023-09-30
0001314052
avxl:PurchaseWarrants2Member
2023-09-30
0001314052
us-gaap:StockOptionMember
2021-09-30
0001314052
us-gaap:StockOptionMember
2021-10-01
2022-09-30
0001314052
us-gaap:StockOptionMember
2022-09-30
0001314052
us-gaap:StockOptionMember
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice1Member
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice1Member
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice2Member
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice2Member
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice3Member
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice3Member
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice4Member
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice4Member
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice5Member
2022-10-01
2023-09-30
0001314052
us-gaap:StockOptionMember
avxl:OptionPrice5Member
2023-09-30
0001314052
us-gaap:GeneralAndAdministrativeExpenseMember
2022-10-01
2023-09-30
0001314052
us-gaap:GeneralAndAdministrativeExpenseMember
2021-10-01
2022-09-30
0001314052
us-gaap:GeneralAndAdministrativeExpenseMember
2020-10-01
2021-09-30
0001314052
us-gaap:ResearchAndDevelopmentExpenseMember
2022-10-01
2023-09-30
0001314052
us-gaap:ResearchAndDevelopmentExpenseMember
2021-10-01
2022-09-30
0001314052
us-gaap:ResearchAndDevelopmentExpenseMember
2020-10-01
2021-09-30
0001314052
avxl:FederalMember
2023-09-30
0001314052
avxl:FederalMember
2022-09-30
0001314052
avxl:FederalMember
2022-10-01
2023-09-30
0001314052
us-gaap:StateAndLocalJurisdictionMember
2023-09-30
0001314052
us-gaap:StateAndLocalJurisdictionMember
2022-09-30
0001314052
us-gaap:ForeignCountryMember
2023-09-30
0001314052
us-gaap:ForeignCountryMember
currency:AUD
2023-09-30
0001314052
us-gaap:ForeignCountryMember
2022-09-30
0001314052
us-gaap:ForeignCountryMember
currency:AUD
2022-09-30
0001314052
avxl:FederalMember
2015-09-30
0001314052
avxl:FederalMember
2021-09-30
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
(Mark One)
☒ ANNUAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended September 30,
2023
☐ TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ______________
to________________
Commission file number: 001-37606
ANAVEX LIFE SCIENCES CORP.
(Exact name of registrant as specified in its
charter)
| Nevada |
|
98-0608404 |
| (State
or other jurisdiction of incorporation or organization) |
|
(I.R.S.
Employer Identification No.) |
| |
|
|
| 630 5th Avenue, 20th Floor, New York, NY USA |
|
10111 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
Registrant’s telephone number, including
area code 1-844-689-3939
Securities registered under Section 12(b) of
the Act:
| Common
Stock, $0.001 par value |
AVXL |
NASDAQ
Stock Market LLC |
| Title
of each class |
Trading
Symbol |
Name
of each exchange on which registered |
Securities registered pursuant to Section 12(g)
of the Act:
None
(Title of class)
| Indicate
by checkmark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. |
| |
Yes ☒
No ☐ |
| |
|
| Indicate
by checkmark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. |
| |
Yes ☐
No☒ |
| |
|
| Indicate
by checkmark whether the registrant has (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and
(2) has been subject to such filing requirements for the past 90 days. |
| |
Yes ☒
No ☐ |
| Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). |
| |
Yes ☒No ☐ |
| |
|
|
Indicate by checkmark whether the registrant is a large accelerated
filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions
of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging
growth company” in Rule 12b-2 of the Exchange Act.
|
| Large accelerated filer ☒ |
|
Accelerated
filer ☐ |
| Non-accelerated
filer ☐ |
|
Smaller
reporting company ☐ |
| |
|
Emerging
growth company☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use
the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act
☐ |
|
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal controls over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by
the registered public accounting firm that prepared or issued its audit report.
Yes ☒No ☐ |
| If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements |
| |
☐ |
| |
|
Indicate
by check mark whether any of those error corrections are restatements that required a recovery
analysis of incentive-based compensation received by any of the registrant’s executive
officers during the relevant recovery period pursuant to §240.10D-1(b). |
| |
☐ |
| |
| Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). |
| |
Yes ☐
No ☒ |
State the aggregate market value of the
voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last
sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently
completed second fiscal quarter: $667 million based on a price of $8.57 per share, being the closing price of the registrant’s
common stock on March 31, 2023.
Indicate the number of shares outstanding of
each of the registrant’s classes of common stock, as of the latest practicable date: 82,086,511 issued and outstanding as
of November 24, 2023.
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS
Forward Looking Statements.
This Annual Report on Form 10-K includes
forward-looking statements. All statements other than statements of historical facts contained in this Annual Report on Form 10-K,
including statements regarding our anticipated future clinical and regulatory milestone events, future financial position, business
strategy and plans and objectives of management for future operations, are forward-looking statements. The words “believe,”
“may,” “estimate,” “continue,” “anticipate,” “intend,” “expect,”
“should,” “forecast,” “potential,” “predict,” “could,” “would”,
“will”, “suggest,” “plan” and similar expressions, as they relate to us, are intended to identify
forward-looking statements. Such forward-looking statements include, without limitation, statements regarding:
| ● | volatility
in
our
stock
price
and
in
the
markets
in
general; |
| ● | our
ability
to
successfully
conduct
preclinical
studies
and
clinical
trials
for
our
product
candidates; |
| ● | our
ability
to
raise
additional
capital
on
favorable
terms
and
the
impact
of
such
activities
on
our
stockholders
and
stock
price; |
| ● | our
ability
to
generate
any
revenue
or
to
continue
as
a
going
concern; |
| ● | our
ability
to
execute
our
research
and
development
plan
on
time
and
on
budget; |
| ● | our
product
candidates’
ability
to
demonstrate
efficacy
or
an
acceptable
safety
profile; |
| ● | our
ability
to
obtain
the
support
of
qualified
scientific
collaborators; |
| ● | our
ability,
whether
alone
or
with
commercial
partners,
to
successfully
commercialize
any
of
our
product
candidates
that
may
be
approved
for
sale; |
| ● | our
ability
to
identify
and
obtain
additional
product
candidates; |
| ● | our
reliance
on
third
parties
in
non-clinical
studies
and
clinical
trials; |
| ● | our
ability
to
defend
against
product
liability
claims; |
| ● | our
ability
to
safeguard
against
security
breaches; |
| ● | our
ability
to
obtain
and
maintain
sufficient
intellectual
property
protection
for
our
product
candidates; |
| ● | our
ability
to
comply
with
our
intellectual
property
licensing
agreements; |
| ● | our
ability
to
defend
against
claims
of
intellectual
property
infringement; |
| ● | our
ability
to
comply
with
the
maintenance
requirements
of
the
government
patent
agencies; |
| ● | our
ability
to
protect
our
intellectual
property
rights
throughout
the
world; |
| ● | the
anticipated
start
dates,
durations
and
completion
dates
of
our
ongoing
and
future
clinical
trials; |
| ● | the
anticipated
designs
of
our
future
clinical
trials; |
| ● | our
ability
to
attract
and
retain
qualified
employees; |
| ● | the
impact
of
Fast
Track
designation
on
receipt
of
actual
FDA
approval; |
| ● | our
anticipated
future
regulatory
submissions
and
our
ability
to
receive
regulatory
approvals
to
develop
and
market
our
product
candidates,
including
any
orphan
drug
or
Fast
Track
designations;
and |
| ● | our
anticipated
future
cash
position
and
ability
to
obtain
funding
for
our
operations. |
We have based these forward-looking
statements largely on our current expectations and projections about future events, including the responses we expect from the
U.S. Food and Drug Administration, (“FDA”), and other regulatory authorities and financial trends that we believe may
affect our financial condition, results of operations, business strategy, preclinical studies and clinical trials, and financial
needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions including, without limitation,
the risks described in “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K. These risks are not exhaustive.
Other sections of this Annual Report on Form 10-K include additional factors which could adversely impact our business and financial
performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to
time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our
business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those
contained in any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events.
We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur and
actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable
laws including the securities laws of the United States, we assume no obligation to update or supplement forward-looking statements.
As used in this Annual Report on Form
10-K, the terms “we,” “us,” “our,”, “Company” and “Anavex” mean Anavex
Life Sciences Corp., unless the context clearly requires otherwise.
PART I
ITEM 1. BUSINESS
Overview and Strategy
Anavex Life Sciences Corp. is a clinical stage
biopharmaceutical company engaged in the development of differentiated therapeutics by applying precision medicine to central nervous
system (“CNS”) diseases with high unmet need. We analyze genomic data from clinical trials to identify biomarkers,
which we use in the analysis of our clinical trials.
Our lead product candidate, ANAVEX®2-73
(blarcamesine), is being developed to treat Alzheimer’s disease, Parkinson’s disease and potentially other central
nervous system diseases, including rare diseases, such as Rett syndrome, a rare severe neurological monogenic disorder caused by
mutations in the X-linked gene, methyl-CpG-binding protein 2 (“MECP2”).
We currently have two core programs and two
seed programs. Our core programs are at various stages of clinical and preclinical development, in neurodegenerative and neurodevelopmental
diseases.
The following table summarizes key information about our programs:
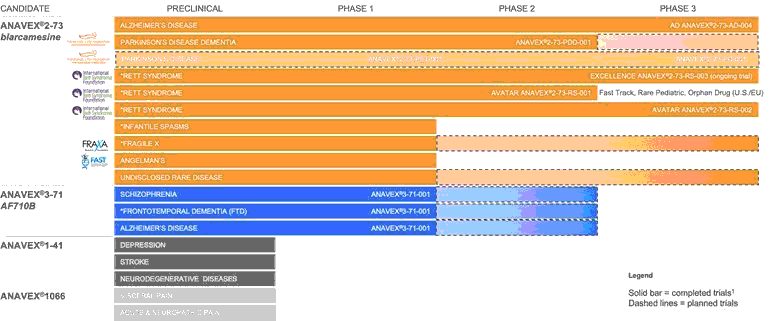
* = Orphan Drug Designation by the FDA;
1. EXCELLENCE: Ongoing trial
Anavex has a portfolio of compounds varying
in sigma-1 receptor (SIGMAR1) binding activities. The SIGMAR1 gene encodes the SIGMAR1 protein, which is an intracellular chaperone
protein with important roles in cellular communication. SIGMAR1 is also involved in transcriptional regulation at the nuclear envelope
and restores homeostasis and stimulates recovery of cell function when activated. In order to validate the ability of our compounds
to activate quantitatively the SIGMAR1, we performed, in collaboration with Stanford University, a quantitative Positron Emission
Tomography (PET) imaging scan in mice, which demonstrated a dose-dependent ANAVEX®2-73 (blarcamesine) target engagement
or receptor occupancy with SIGMAR1 in the brain.

Source: Reyes S et al., Sci Rep. 2021 Aug 25;11(1):17150
Cellular Homeostasis
Many diseases are possibly directly caused
by chronic homeostatic imbalances or cellular stress of brain cells. In pediatric diseases, such as Rett syndrome or infantile
spasms, the chronic cellular stress is possibly caused by the presence of a constant genetic mutation. In neurodegenerative diseases,
such as Alzheimer’s and Parkinson’s diseases, chronic cellular stress is possibly caused by age-correlated buildup
of cellular insult and hence chronic cellular stress. Specifically, defects in homeostasis of protein or ribonucleic acid (“RNA”)
lead to the death of neurons and dysfunction of the nervous system. The spreading of protein aggregates resulting in a proteinopathy,
a characteristic found in Alzheimer’s and Parkinson’s diseases that results from disorders of protein synthesis, trafficking,
folding, processing or degradation in cells. The clearance of macromolecules in the brain is particularly susceptible to imbalances
that result in aggregation and degeneration in nerve cells. For example, Alzheimer’s disease pathology is characterized by
the presence of amyloid plaques, and neurofibrillary tangles, which are aggregates of hyperphosphorylated Tau protein that are
a marker of other diseases known as tauopathies as well as inflammation of microglia. With the SIGMAR1 activation through SIGMAR1
agonists like ANAVEX®2-73 (blarcamesine), our approach is to restore cellular balance (i.e. homeostasis). Therapies
that correct defects in cellular homeostasis might have the potential to halt or delay neurodevelopmental and neurodegenerative
disease progression.
ANAVEX®2-73 (blarcamesine)-specific
Biomarkers
As part of some of our clinical trials, we
have incorporated a genomic analysis to better understand potential populations for whom our clinical programs might benefit. In
our clinical trials, a full genomic analysis of Alzheimer’s disease patients treated with ANAVEX®2-73 (blarcamesine)
has helped us identify actionable genetic variants. A significant impact of the genomic biomarkers SIGMAR1, the direct target of
ANAVEX®2-73 (blarcamesine) and COMT, a gene involved in memory function, on the drug response level was identified,
leading to an early ANAVEX®2-73 (blarcamesine)-specific biomarker hypothesis. We believe that excluding patients
with SIGMAR1 identified biomarker variant (approximately 10%-20% of the population) in prospective studies would identify approximately
80%-90% patients that would display clinically significant improved functional and cognitive scores. The consistency between the
identified DNA and RNA data related to ANAVEX®2-73 (blarcamesine), which are considered independent of Alzheimer’s
disease pathology, as well as multiple endpoints and time-points, provides support for the potential precision medicine clinical
development of ANAVEX®2-73 (blarcamesine) by using genetic biomarkers identified within the trial population itself
to target patients who are most likely to respond to ANAVEX®2-73 (blarcamesine) treatment. We may in the future
utilize such an approach in Alzheimer’s disease as well as indications like Parkinson’s disease dementia or Rett syndrome
in which ANAVEX®2-73 (blarcamesine) is currently being studied.
Clinical Trials Overview
Alzheimer’s Disease
In November 2016, we completed a Phase 2a clinical
trial, consisting of Part A and Part B, which lasted a total of 57 weeks, for ANAVEX®2-73 in mild-to-moderate Alzheimer’s
patients. This open-label randomized trial in Australia met both primary and secondary endpoints and was designed to assess the
safety and exploratory efficacy of ANAVEX®2-73 in 32 patients. ANAVEX®2-73 targets sigma-1 and muscarinic
receptors, which have been shown in preclinical studies to reduce stress levels in the brain believed to restore cellular homeostasis
and to reverse the pathological hallmarks observed in Alzheimer’s disease. In October 2017, we presented positive pharmacokinetic
(“PK”) and pharmacodynamic (“PD”) data from the Phase 2a clinical trial, which established a concentration-effect
relationship between ANAVEX®2-73 and trial measurements. These measures obtained from all patients who participated
in the entire 57 weeks include exploratory cognitive and functional scores as well as biomarker signals of brain activity. Additionally,
the clinical trial appeared to show that ANAVEX®2-73 activity was enhanced by its active metabolite (ANAVEX19-144),
which also targets the SIGMAR1 receptor and has a half-life approximately twice as long as the parent molecule.
Two consecutive trial extensions for the Phase
2a trial have allowed participants who completed the 52-week Part B of the trial to continue taking ANAVEX®2-73,
providing an opportunity to gather extended safety data for a cumulative time period of five years. In August 2020, patients completing
these Phase 2a trial extensions were granted continued access to treatment with ANAVEX®2-73 through the Australian
Government Department of Health – Therapeutic Goods Administration’s compassionate use Special Access Scheme.
A larger Phase 2b/3 double-blind, placebo-controlled
trial of ANAVEX®2-73 in Alzheimer’s disease commenced in August 2018. The Phase 2b/3 trial enrolled 509 patients,
which were treated with a convenient once-daily oral formulation of ANAVEX®2-73 for 48 weeks, randomized 1:1:1 to
two different ANAVEX®2-73 doses or placebo. The trial took place at 52 sites across North America, Europe and Australia.
Primary and secondary endpoints to assess safety and both cognitive and functional efficacy, were measured through the Alzheimer’s
Disease Assessment Scale – Cognitive Subscale test (“ADAS-Cog”), Alzheimer’s Disease Cooperative Study
– Activities of Daily Living (“ADCS-ADL”) and Clinical Dementia Rating – Sum of Boxes for cognition and
function (“CDR-SB”). In addition to the primary endpoints, the ANAVEX®2-73 Phase 2b/3 trial design incorporated
pre-specified statistical analyses related to potential genomic precision medicine biomarkers previously identified in the ANAVEX®2-73
Phase 2a clinical trial. The trial was completed in mid-2022 and, in December 2022, the Company presented positive topline results
from the Phase 2b/3 clinical trial.
ANAVEX®2-73 met the co-primary
endpoints ADAS-Cog and ADCS-ADL and key secondary endpoint CDR-SB. ANAVEX®2-73 treatment slowed decline of cognition
and function in patients with early Alzheimer’s disease over 48 weeks. Patients treated with ANAVEX®2-73 had
1.84 times higher odds, or likelihood, to improve cognitively compared to placebo, with a ADAS-Cog score threshold change of -0.5
points or better [Odds Ratio = 1.84 (p = 0.015)]. At clinically significant levels of improvement in function (ADCS-ADL score threshold
change of +3.5 points or better), patients treated with ANAVEX®2-73 had 2.67 times higher odds, or likelihood, to
improve function compared to placebo [Odds Ratio = 2.67 (p = 0.0255)]. Additionally, treatment with
ANAVEX®2-73 reduced cognitive decline at end of treatment, measured with the ADAS-Cog, as compared to placebo, by
45%, representing a treatment difference in mean score change of -1.85 points (p=0.033). Compared to placebo, ANAVEX®2-73
reduced clinical decline of cognition and function by 27% with mean score difference of -0.42 points (p=0.040) as measured by the
CDR-SB. ANAVEX®2-73 was generally safe and well tolerated. All statistical analyses were
performed by outside consultancy companies.
In September 2023, we provided additional
data demonstrating that the clinical effect was complemented by two independent biomarkers. A significant reduction in pathological
amyloid beta levels in plasma, as well as a significant slowing in the rate of pathological brain atrophy on MRI (Magnetic Resonance
Imaging) scans. Validated biomarkers of amyloid beta pathology, plasma Aβ42/40 ratio increased significantly (P = 0.048),
demonstrating strong anti-amyloid effects of ANAVEX®2-73 in Alzheimer’s disease patients, while MRI revealed
significant reduction in brain volume loss, including whole brain (P = 0.0005), comparing treatment to placebo.
Furthermore, all prespecified clinical
endpoints were further analyzed using a mixed model for repeated measures (MMRM). Under the multiplicity control rule, a trial
is successful in meeting the co-primary endpoints if the significance of each endpoint is P < 0.05, or if the significance of
only one co-primary endpoint is P < 0.025. If only one primary endpoint is significant at an α level of 0.025, then the
secondary endpoint will be evaluated at the same level of 0.025. The trial was successful, since the differences in the least-squares
mean (LSM) change from baseline to 48 weeks between the ANAVEX®2-73 and placebo groups were −1.783 [95% CI,
−3.314 to −0.251]; (P = 0.0226) for ADAS-Cog13, and −0.456 [95% CI, −0.831 to −0.080]; (P = 0.0175)
for CDR-SB in patients with early Alzheimer’s disease.
In the respective safety population,
common treatment-emergent adverse events included dizziness, which was transient and mostly mild to moderate in severity, and occurred
in 120 participants (35.8%) during titration and in 76 participants (25.2%) during maintenance with ANAVEX®2-73
and 10 (6.0%) during titration and 9 (5.6%) during maintenance with placebo.
A subsequent long-term open label extension
study of ANAVEX®2-73, entitled the ATTENTION-AD trial was initiated for patients who have completed the 48-week
Phase 2b/3 placebo-controlled trial referenced above. This trial extension for an additional 96 weeks is currently ongoing, and
provides an opportunity to evaluate longer term safety and efficacy of ANAVEX®2-73 in persons with Alzheimer’s
disease.
Rett Syndrome
In February 2016, we presented positive preclinical
data for ANAVEX®2-73 in Rett syndrome, a rare neurodevelopmental disease. The data demonstrated dose related and
significant improvements in an array of behavioral and gait paradigms in a mouse model with an MECP2-null mutation that causes
neurological symptoms that mimic Rett syndrome. The study was funded by the International Rett Syndrome Foundation (“Rettsyndrome.org”).
In January 2017, we were awarded a financial grant from Rettsyndrome.org of a minimum of $0.6 million to cover some of the costs
of a multicenter Phase 2 clinical trial of ANAVEX®2-73 for the treatment of Rett syndrome. This award was received
in quarterly instalments which commenced during fiscal 2018.
In March 2019, we commenced the first Phase
2 clinical trial in a planned Rett syndrome program of ANAVEX®2-73 for the treatment of Rett syndrome. The clinical
trials are being conducted in a range of patient age demographics and geographic regions, utilizing an oral liquid once-daily formulation
of ANAVEX®2-73.
The first Phase 2 trial, (ANAVEX®2-73-RS-001),
which took place in the United States, was completed in December 2020. This trial was a randomized double-blind, placebo-controlled
safety, tolerability, PK and efficacy trial of oral liquid ANAVEX®2-73 formulation in 25 adult female patients with
Rett syndrome over a 7-week treatment period including ANAVEX®2-73-specific genomic precision medicine biomarkers.
The primary endpoint of the trial was safety. The dosing of 5 mg ANAVEX®2-73 was well-tolerated and demonstrated
dose-proportional PK. All secondary efficacy endpoints of the trial showed statistically significant and clinically meaningful
response in the Rett Syndrome Behaviour Questionnaire (“RSBQ”) response, when compared to placebo, in the intent to
treat (“ITT”) cohort (all participants, p = 0.011). 66.7% of ANAVEX®2-73 treated subjects showed a statistically
significant improvement in RSBQ response as compared to 10% of the subjects on placebo in the ITT cohort (all participants, p =
0.011). ANAVEX®2-73 treatment resulted in a sustained improvement in Clinical Global Impression Improvement (CGI-I)
response throughout the 7-week clinical trial, when compared to placebo in the ITT cohort (all participants, p = 0.014). Consistent
with previous ANAVEX®2-73 clinical trials, patients carrying the common form of the SIGMAR1 gene treated with ANAVEX®2-73
experienced stronger improvements in the prespecified efficacy endpoints.
The
second, international trial of ANAVEX®2-73 for the treatment of Rett syndrome, called the AVATAR trial, commenced
in June 2019. This trial took place in Australia and the United Kingdom using a higher dose than the U.S. based Phase 2
trial for Rett syndrome. The trial was a Phase 3 randomized, double-blind, placebo-controlled trial to evaluate the safety and
efficacy of ANAVEX®2-73 in 33 adult patients over a 7-week treatment period including ANAVEX®2-73
specific precision medicine biomarkers. Based upon the input from the successful U.S. Phase 2 Rett syndrome trial (ANAVEX®2-73-RS-001),
we updated the endpoints for the AVATAR trial (ANAVEX®2-73-RS-002) to appropriately assess the clinically meaningful
outcome following International Conference on Harmonization (ICH) guidelines. These updates were approved by the respective regulatory
authorities in the U.K. and in Australia, respectively, where the AVATAR trial was conducted.
The data from the AVATAR trial was released
in February 2022. The clinical trial met all primary and secondary efficacy and safety endpoints, with consistent improvements
in primary efficacy endpoint, RSBQ response (p = 0.037), and secondary efficacy endpoints, ADAMS (p = 0.010) and CGI-I (p = 0.037)
response. Efficacy endpoints demonstrated statistically significant and clinically meaningful reductions in Rett syndrome symptoms.
Convenient once daily oral liquid doses of up to 30 mg of ANAVEX®2-73 were also well tolerated with good medication
compliance. All patients who participated in the trial were eligible to receive ANAVEX®2-73 under a voluntary open
label extension protocol.
In July 2020, we commenced the third trial
of ANAVEX®2-73 for the treatment of Rett syndrome, called the EXCELLENCE trial. This Phase 2/3 trial in pediatric
patients with Rett syndrome includes trial sites in Australia, the United Kingdom and Canada, and will evaluate the safety and
efficacy of ANAVEX®2-73 in approximately 84 pediatric patients, aged 5 to 18, over a 12-week treatment period incorporating
ANAVEX®2-73 specific precision medicine biomarkers. This trial completed enrollment in February 2023, exceeding
the original enrollment target. In June 2023, this trial completed dosing of all participants, and topline results are expected
in the second half of 2023. All patients who participate in the trial will be eligible to receive ANAVEX®2-73 under
a voluntary open label extension protocol, which is currently ongoing.
Parkinson’s Disease
In September 2016, we presented positive preclinical
data for ANAVEX®2-73 in an animal model of Parkinson’s disease, which demonstrated significant improvements
on behavioral, histopathological, and neuroinflammatory endpoints. The study was funded by the Michael J. Fox Foundation. Additional
data announced in October 2017 indicated that ANAVEX®2-73 induced robust neurorestoration in experimental Parkinsonism.
We believe the encouraging results we have gathered in this preclinical model, coupled with the favorable profile of this product
candidate in the Alzheimer’s disease trial, support the notion that ANAVEX®2-73 has the potential to treat
Parkinson’s disease dementia.
In October 2020, we completed a double-blind,
randomized, placebo-controlled proof-of-concept Phase 2 trial with ANAVEX®2-73 in Parkinson’s disease dementia
in Spain and Australia, to study the effect of the compound on both the cognitive and motor impairment of Parkinson’s disease.
The Phase 2 trial enrolled approximately 132 patients for 14 weeks, randomized 1:1:1 to two different ANAVEX®2-73
doses, 30 mg and 50 mg, or placebo. The ANAVEX®2-73 Phase 2 Parkinson’s disease dementia trial design incorporated
genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a Alzheimer’s disease trial.
The trial demonstrated that ANAVEX®2-73
was safe and well tolerated in oral doses up to 50 mg once daily. The results showed clinically meaningful, dose-dependent, and
statistically significant improvements in the Cognitive Drug Research (“CDR”) computerized assessment system analysis.
Treatment with ANAVEX®2-73 also resulted in clinically meaningful improvements as measured by the global composite
score of Parkinson’s disease symptom severity, MDS-Unified Parkinson’s Disease Rating Scale (“MDS-UPDRS”)
total score on top of standard of care including dopaminergic therapy, levodopa and other anti-PD medications after 14 weeks of
treatment, suggesting ANAVEX®2-73’s potential capability of slowing and reversing symptoms that progress in
Parkinson’s disease. In addition, the trial confirmed the precision medicine approach of targeting SIGMAR1 as a genetic biomarker
in response to ANAVEX®2-73 may result in improved clinical outcomes.
A 48-week Open Label Extension (“OLE”)
ANAVEX2-73-PDD-EP-001 Phase 2 trial was offered to participants after completion of the double-blind placebo-controlled ANAVEX2-73-PDD-001
Phase 2 trial discussed above. The OLE trial assessed safety, tolerability and efficacy, measuring among others, MDS-Unified Parkinson’s
Disease Rating Scale Parts I, II, III, REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Clinical Global Impression
– Improvement (CGI-I), as well as cognitive efficacy endpoint Montreal Cognitive Assessment (MoCA) over a 48-week period.
In March 2023, we reported the preliminary
ANAVEX2-73-PDD-EP-001 OLE trial data, which demonstrated longitudinal beneficial effects of ANAVEX®2-73 on the prespecified
primary and secondary objectives. Preliminary analysis reveals that ANAVEX®2-73 was found to be generally safe and
well tolerated; and safety findings in this trial were consistent with the known safety profile of ANAVEX®2-73.
In respect to efficacy, across all efficacy endpoints, patients performed better while on ANAVEX®2-73. While all
patients were on drug holiday due to COVID-19 between the DB EOT and the OLE Baseline, the respective efficacy endpoints, including
the MDS-UPDRS Part II + III and CGI-I, measured at the end of trial of the double-blind study (DB EOT) and the OLE Baseline, were
worsening, as expected in a progressive disease like Parkinson’s. However, when patients resumed daily oral ANAVEX®2-73
treatment, a consistent improvement was observed during the extension phase from OLE Baseline through OLE Week 24, and OLE Week
48, respectively. These results are consistent with the pattern observed for all efficacy measures in the extension phase. The
two endpoints, MDS-UPDRS Part II + III and CGI-I measured in this study are the planned primary and key secondary endpoints in
our forthcoming pivotal 6-month Parkinson’s disease study.
In January 2021, we were awarded a research
grant of $1.0 million from The Michael J. Fox Foundation for Parkinson’s Research to develop ANAVEX®2-73
for the treatment of Parkinson’s disease. The award will explore utilization of PET imaging biomarkers to enable measurement
of target engagement and pathway activation of the SIGMAR1 with clinically relevant doses, including in people with Parkinson’s
disease.
Schizophrenia, Frontotemporal Dementia and
Alzheimer’s disease
In July 2020, we commenced the First-in-Human
Phase 1 clinical trial of ANAVEX®3-71. ANAVEX®3-71 was previously granted orphan drug designation
for the treatment of Frontotemporal Dementia (“FTD”) by the FDA. ANAVEX®3-71 is an orally administered
small molecule targeting sigma-1 and M1 muscarinic receptors that is designed to be beneficial for neurodegenerative diseases.
In preclinical studies, ANAVEX®3-71 demonstrated disease-modifying activity
against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and
tau pathologies, as well as beneficial effects on mitochondrial dysfunction and neuroinflammation.
The Phase
1 clinical trial was a prospective double-blind, randomized, placebo-controlled trial in Australia. A total of 36 healthy male
and female subjects were included. Single escalating doses of ANAVEX®3-71 were administered in order to evaluate
the safety, tolerability, and PK of ANAVEX®3-71 and the effects of food and gender on its PK in healthy volunteers.
The trial
met its primary and secondary endpoints of safety, with no serious adverse events (“SAEs”) or dose-limiting toxicities
observed. ANAVEX®3-71 was well tolerated in all cohorts receiving ANAVEX®3-71 in single doses ranging
from 5 mg to 200 mg daily with no SAEs and no significant lab abnormalities in any subject. In the trial, ANAVEX®3-71
exhibited linear PK. Its pharmacokinetics was also dose proportional for doses up to 160 mg. Gender had no effect on the PK of
the drug and food had no effect on the bioavailability of ANAVEX®3-71. The trial also met the secondary objective
of characterizing the effect of ANAVEX®3-71 on electrocardiogram (“ECG”) parameters. There were no clinically
significant ECG parameters throughout the trial. Participant QTcF measures were normal across all dose groups with no difference
between ANAVEX®3-71 and placebo.
In October
2023 a peer-reviewed publication in the journal Neurobiology of Aging, titled “Early treatment with an M1 and sigma-1 receptor
agonist prevents cognitive decline in a transgenic rat model displaying Alzheimer-like amyloid pathology”, featured the orally
available small molecule ANAVEX®3-71 (AF710B). The preclinical study described the potential disease-modifying
properties of ANAVEX®3-71 on Alzheimer’s disease pathology as a possible drug candidate for a potential once
daily oral preventive strategy for Alzheimer’s disease.
Based
on these results, and ANAVEX®3-71’s pre-clinical profile, we intend to advance ANAVEX®3-71
into a biomarker-driven clinical development dementia program for the treatment of schizophrenia, FTD and Alzheimer’s disease,
evaluating longitudinal effect of treatment with ANAVEX®3-71. We believe the results of these clinical trials and
preclinical study could serve as a basis for advancing into respective registration trials in the U.S.
Our Pipeline
Our research and development pipeline includes
ANAVEX®2-73 currently in three different clinical trial indications, and several other compounds in different stages
of clinical and pre-clinical development.
Our proprietary SIGMACEPTOR™ Discovery
Platform produced small molecule drug candidates with unique modes of action, based on our understanding of sigma receptors. Sigma
receptors may be targets for therapeutics to combat many human diseases, both of neurodegenerative nature, including Alzheimer’s
disease, as well as of neurodevelopmental nature, like Rett syndrome. When bound by the appropriate ligands, sigma receptors influence
the functioning of multiple biochemical signals that are involved in the pathogenesis (origin or development) of disease. Multiple
viruses including SARS-CoV-2 (COVID-19) induce cellular stress by intrinsic mitochondrial apoptosis and other related cellular
processes, in order to ensure survival and replication. Hence, it is possible that SIGMAR1 could play a role in modulating the
cellular response to viral infection and ameliorate pathogenesis.
Compounds that have been subjects of our research
include the following:
ANAVEX®2-73 (blarcamesine)
We believe ANAVEX®2-73 may offer
a disease-modifying approach in neurodegenerative and neurodevelopmental diseases by activation of SIGMAR1. ANAVEX®2-73
is being developed in an oral liquid once-daily formulation for rare diseases such as Rett syndrome as well as an oral once-daily
capsule formulation for diseases such as Alzheimer’s disease.
In Rett syndrome, administration of ANAVEX®2-73
in liquid form resulted in both significant and dose related improvements in an array of behavioral paradigms in the MECP2 HET
Rett syndrome disease model. In addition, in a further experiment sponsored by Rettsyndrome.org, ANAVEX®2-73 was
evaluated in automatic visual response and respiration tests in 7-month old mice, an age at which advanced pathology is evident.
Vehicle-treated MECP2 mice demonstrated fewer automatic visual responses than wild-type mice. Treatment with ANAVEX®2-73
for four weeks significantly increased the automatic visual response in the MECP2 Rett syndrome disease mice. Additionally, chronic
oral dosing daily for 6.5 weeks of ANAVEX®2-73 starting at ~5.5 weeks of age was conducted in the MECP2 HET Rett
syndrome disease mouse model assessed the different aspects of muscular coordination, balance, motor learning and muscular strengths,
some of the core deficits observed in Rett syndrome. Administration of ANAVEX®2-73 resulted in both significant
and dose related improvements in an array of these behavioral paradigms in the MECP2 HET Rett syndrome disease model.
In May 2016 and June 2016, the FDA granted
Orphan Drug Designation to ANAVEX®2-73 for the treatment of Rett syndrome and infantile spasms, respectively. In
November 2019, the FDA granted to ANAVEX®2-73 the Rare Pediatric Disease (RPD) designation for the treatment of
Rett syndrome. The RPD designation is intended to encourage the development of treatments for rare pediatric diseases.
Further, in February 2020, the FDA granted
Fast Track designation for the ANAVEX®2-73 clinical development program for the treatment of Rett syndrome. The
FDA Fast Track program is designed to facilitate and expedite the development and review of new drugs to address unmet medical
needs in the treatment of serious and life-threatening conditions.
For Parkinson’s disease, data demonstrates
significant improvements and restoration of function in a disease-modifying animal model of Parkinson’s disease. Significant
improvements were seen on all measures tested: behavioral, histopathological, and neuroinflammatory endpoints. In October 2020,
we completed a double-blind, randomized, placebo-controlled proof-of-concept Phase 2 trial with ANAVEX®2-73 in Parkinson’s
disease dementia, to study the effect of the compound on both the cognitive and motor impairment of Parkinson’s disease.
The Phase 2 trial enrolled approximately 132 patients for 14 weeks, randomized 1:1:1 to two different ANAVEX®2-73
doses, 30mg and 50mg, or placebo. The ANAVEX®2-73 Phase 2 Parkinson’s disease dementia trial design incorporated
genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a Alzheimer’s disease trial.
The trial demonstrated that ANAVEX®2-73
was safe and well tolerated in oral doses up to 50mg once daily. The results showed clinically meaningful, dose-dependent, and
statistically significant improvements in the CDR computerized assessment system analysis. We anticipate conducting further clinical
trials of ANAVEX®2-73 in Parkinson’s disease dementia after submitting the results of the trial to the FDA
to obtain regulatory guidance.
In Alzheimer’s disease animal models,
ANAVEX®2-73 has shown pharmacological, histological and behavioral evidence as a potential neuroprotective, anti-amnesic,
anti-convulsive and anti-depressive therapeutic agent, due to its potent affinity to SIGMAR1 and moderate affinities to M1-4 type
muscarinic receptors. In addition, ANAVEX®2-73 has shown a potential dual mechanism which may impact amyloid, tau
pathology and inflammation. In a transgenic Alzheimer’s disease animal model Tg2576, ANAVEX®2-73 induced a
statistically significant neuroprotective effect against the development of oxidative stress in the mouse brain, as well as significantly
increased the expression of functional and synaptic plasticity markers that is apparently amyloid-beta independent. It also statistically
alleviated the learning and memory deficits developed over time in the animals, regardless of sex, both in terms of spatial working
memory and long-term spatial reference memory.
Based on the results of pre-clinical testing,
we initiated and completed a Phase 1 single ascending dose (SAD) clinical trial of ANAVEX®2-73. In this Phase 1
SAD trial, the maximum tolerated single dose was defined per protocol as 55-60 mg. This dose is above the equivalent dose shown
to have positive effects in mouse models of Alzheimer’s disease. There were no significant changes in laboratory or ECG parameters.
ANAVEX®2-73 was well tolerated below the 55-60 mg dose with only mild adverse events in some subjects. Observed
adverse events at doses above the maximum tolerated single dose included headache and dizziness, which were moderate in severity
and reversible. These side effects are often seen with drugs that target CNS conditions, including Alzheimer’s disease.
In November 2016, we completed a Phase 2a clinical
trial for ANAVEX®2-73, for the treatment of Alzheimer’s disease. The open-label randomized trial was designed
to assess the safety and exploratory efficacy of ANAVEX®2-73 in 32 patients with mild-to-moderate Alzheimer’s
disease. The Phase 2a trial met both primary and secondary objectives of the trial.
In July 2018, we presented
the results of a genomic DNA and RNA evaluation of the participants in the Phase 2a clinical trial. More than 33,000 genes were
analyzed using unbiased, data driven, machine learning, artificial intelligence (AI) system for analyzing DNA and RNA data in patients
treated with ANAVEX®2-73. The analysis identified genetic variants that impacted response to ANAVEX®2-73,
among them variants related to the SIGMAR1, the target for ANAVEX®2-73. Results showed that trial participants with
the common SIGMAR1 wild type gene variant, which is estimated to be about 80% of the population worldwide, demonstrated improved
cognitive (MMSE) and functional (ADCS-ADL) scores. The results from this evaluation supported the continued evaluation of genomic
information in subsequent clinical trials, since these signatures can now be applied to neurological indications tested in future
clinical trials with ANAVEX®2-73 including Alzheimer’s disease, Parkinson’s disease dementia and Rett
syndrome.
ANAVEX®2-73
data met prerequisite information in order to progress into a Phase 2b/3 placebo-controlled trial. On July 2, 2018, the Human Research
Ethics Committee in Australia approved the initiation of our Phase 2b/3, double-blind, randomized, placebo-controlled 48-week safety
and efficacy trial of ANAVEX®2-73 for the treatment of early Alzheimer’s disease. Clinical trial sites in
Canada, the United Kingdom, the Netherlands and Germany were also added. This Phase 2b/3 trial design incorporates inclusion of
genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a trial.
We believe preclinical data from our studies
also supports further research into the use of ANAVEX®2-73 as a potential platform drug for other neurodegenerative
diseases beyond Alzheimer’s disease, Parkinson’s disease or Rett syndrome, more specifically, epilepsy, infantile spasms,
Fragile X syndrome, Angelman syndrome, multiple sclerosis, and, more recently, tuberous sclerosis complex (TSC). ANAVEX®2-73
demonstrated significant improvements in all of these indications in the respective preclinical animal models.
In a preclinical study sponsored by the Foundation
for Angelman Syndrome, ANAVEX®2-73 was assessed in a mouse model for the development of audiogenic seizures. The
results indicated that ANAVEX®2-73 administration significantly reduced audiogenic-induced seizures in mice. In
a study sponsored by FRAXA Research Foundation regarding Fragile X syndrome, data demonstrated that ANAVEX®2-73
restored hippocampal brain-derived neurotrophic factor (BDNF) expression to normal levels. BDNF under-expression has been observed
in many neurodevelopmental and neurodegenerative pathologies. BDNF signaling promotes maturation of both excitatory and inhibitory
synapses. ANAVEX®2-73 normalization of BDNF expression could be a contributing factor for the positive preclinical
data observed in both neurodevelopmental and neurodegenerative disorders like Angelman and Fragile X syndromes.
In addition, preclinical data to-date also
indicates that ANAVEX®2-73 has the potential to demonstrate protective effects of mitochondrial enzyme complexes
during pathological conditions, which, if impaired, may play a role in the pathogenesis of neurodegenerative and neurodevelopmental
diseases.
In addition, preclinical data on ANAVEX®2-73
related to multiple sclerosis indicates that ANAVEX®2-73 may promote remyelination in multiple sclerosis disease.
Further, our data also demonstrates that ANAVEX®2-73 has the potential to provide protection for oligodendrocytes
(“OL’s”) and oligodendrocyte precursor cells (“OPC’s”), as well as central nervous system neurons
in addition to helping repair by increasing OPC proliferation and maturation in tissue culture.
In March 2018, we presented preclinical data
of ANAVEX®2-73 in a genetic mouse model of tuberous sclerosis complex (“TSC”). TSC is a rare genetic
disorder characterized by the growth of numerous benign tumors in many parts of the body with a high incidence of seizures. The
preclinical data demonstrated that treatment with ANAVEX®2-73 significantly increased survival and reduced seizures
in those mice.
ANAVEX®3-71
ANAVEX®3-71 is a clinical drug
candidate with a novel mechanism of action via SIGMAR1 activation and M1 muscarinic allosteric modulation, which has been shown
to enhance neuroprotection and cognition in Alzheimer’s disease models. ANAVEX®3-71 is a CNS-penetrable potential
disease-modifying treatment for cognitive impairments. We believe it is effective in very small doses against the major Alzheimer’s
hallmarks in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies, and also has beneficial effects
on inflammation and mitochondrial dysfunctions. ANAVEX®3-71 indicates extensive therapeutic advantages in Alzheimer’s
and other protein-aggregation-related diseases given its ability to enhance neuroprotection and cognition via SIGMAR1 activation
and M1 muscarinic allosteric modulation.
A preclinical study examined the response of
ANAVEX®3-71 in aged transgenic animal models and showed a significant reduction in the rate of cognitive deficit,
amyloid beta pathology and inflammation with the administration of ANAVEX®3-71. In April 2016, the FDA granted Orphan
Drug Designation to ANAVEX®3-71 for the treatment of FTD.
During pathological conditions ANAVEX®3-71
demonstrated the formation of new synapses between neurons (synaptogenesis) without causing an abnormal increase in the number
of astrocytes. In neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, synaptogenesis is believed
to be impaired. Additional preclinical data presented also indicates that in addition to reducing oxidative stress, ANAVEX®3-71
has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological conditions, which, if
impaired, are believed to play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
In July 2020, we commenced the first Phase
1 clinical trial of ANAVEX®3-71. The trial took place in Australia and was a double-blind, randomized, placebo-controlled,
Phase 1 trial to evaluate safety and tolerability, and PK of oral escalating doses of ANAVEX®3-71
including effects of food and gender in healthy volunteers. The trial met its primary and
secondary endpoints of safety, respectively, with no serious adverse events (SAEs) or dose-limiting toxicities observed, as
more fully described above under Clinical Trials Overview – Frontotemporal Dementia.
Based
on these results, and ANAVEX®3-71 pre-clinical profile, the Company intends to advance ANAVEX®3-71
into a biomarker-driven clinical development dementia program for the treatment of schizophrenia, FTD and Alzheimer’s disease,
evaluating longitudinal effect of treatment with ANAVEX®3-71. We believe the results of this clinical trial and
preclinical study could serve as a basis for advancing into respective registration trials in the U.S.
ANAVEX®1-41
ANAVEX®1-41 is a sigma-1 agonist.
Pre-clinical tests revealed significant neuroprotective benefits (i.e., protects nerve cells from degeneration or death) through
the modulation of endoplasmic reticulum, mitochondrial and oxidative stress, which damages and impairs cell viability. In addition,
in animal models, ANAVEX®1-41 prevented the expression of caspase-3, an enzyme that plays a key role in apoptosis
(programmed cell death) and loss of cells in the hippocampus, the part of the brain that regulates learning, emotion and memory.
These activities involve both muscarinic and SIGMAR1 systems through a novel mechanism of action.
Preclinical data presented also indicates that
ANAVEX®1-41 has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological
conditions, which, if impaired, are believed to play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
ANAVEX®1066
ANAVEX®1066, a mixed sigma-1/sigma-2
ligand, is designed for the potential treatment of neuropathic and visceral pain. ANAVEX®1066 was tested in two
preclinical models of neuropathic and visceral pain that have been extensively validated in rats. In the chronic constriction injury
model of neuropathic pain, a single oral administration of ANAVEX®1066 dose-dependently restored the nociceptive
threshold in the affected paw to normal levels while leaving the contralateral healthy paw unchanged. Efficacy was rapid and remained
significant for two hours. In a model of visceral pain, chronic colonic hypersensitivity was induced by injection of an inflammatory
agent directly into the colon and a single oral administration of ANAVEX®1066 returned the nociceptive threshold
to control levels in a dose-dependent manner. Companion studies in rats demonstrated the lack of any effects on normal gastrointestinal
transit with ANAVEX®1066 and a favorable safety profile in a battery of behavioral measures.
ANAVEX®1037
ANAVEX®1037 is designed for
the treatment of prostate and pancreatic cancer. It is a low molecular weight, synthetic compound exhibiting high affinity for
SIGMAR1 at nanomolar levels and moderate affinity for sigma-2 receptors and sodium channels at micromolar levels. In advanced pre-clinical
studies, this compound revealed antitumor potential. It has also been shown to selectively kill human cancer cells without affecting
normal/healthy cells and also to significantly suppress tumor growth in immune-deficient mice models. Scientific publications highlight
the possibility that these ligands may stop tumor growth and induce selective cell death in various tumor cell lines. Sigma receptors
are highly expressed in different tumor cell types. Binding by appropriate sigma-1 and/or sigma-2 ligands can induce selective
apoptosis. In addition, through tumor cell membrane reorganization and interactions with ion channels, we believe our drug candidates
may play an important role in inhibiting the processes of metastasis (spreading of cancer cells from the original site to other
parts of the body), angiogenesis (the formation of new blood vessels) and tumor cell proliferation.
ANAVEX®1037 is currently in
the pre-clinical and clinical testing stages of development, and there is no guarantee that the activity demonstrated in pre-clinical
models will be shown in human testing.
We continue to identify and initiate discussions
with potential strategic and commercial partners to most effectively advance our programs and increase stockholder value. Further,
we may acquire or develop new intellectual property and assign, license, or otherwise transfer our intellectual property to further
our goals.
Our Target Indications
We are developing compounds with potential
application to two broad categories and several specific indications, including:
Central Nervous System Diseases
| |
● |
Alzheimer’s
disease – In 2023, an estimated 6.7 million Americans aged 65 and older are suffering from Alzheimer’s disease.
The Alzheimer’s Association® estimates that the annual number of new cases of Alzheimer’s and other
dementias is projected to double by 2050. Medications on the market today treat only the symptoms of Alzheimer’s disease
and do not have the ability to stop its onset or its progression. We believe that there is an urgent and unmet need for both
a disease-modifying cure for Alzheimer’s disease as well as for better symptomatic treatments. |
| |
● |
Parkinson’s
disease – Parkinson’s disease is a progressive disease of the nervous system marked by tremors, muscular rigidity,
and slow, imprecise movement. It is associated with degeneration of the basal ganglia of the brain and a deficiency of the
neurotransmitter dopamine. Parkinson’s disease currently is estimated to afflict more than 10 million people worldwide,
typically middle-aged and elderly people. The Parkinson’s disease market is expected to reach $11.5 billion by 2029,
according to GlobalData. |
| |
● |
Rett
syndrome – Rett syndrome is a rare X-linked genetic neurological and developmental disorder that affects the way
the brain develops, including protein transcription, which is altered and as a result leads to severe disruptions in neuronal
homeostasis. It is considered a rare, progressive neurodevelopmental disorder and is caused by a single mutation in the
MECP2 gene. Because males have a different chromosome combination from females, boys who have the genetic MECP2 mutation
are affected in devastating ways. Most of them die before birth or in early infancy. For females who survive infancy,
Rett syndrome leads to severe impairments, affecting nearly every aspect of the child’s life; severe mental retardation,
their ability to speak, walk and eat, sleeping problems, seizures and even the ability to breathe easily. Rett syndrome
affects approximately 1 in every 10,000-15,000 females.
|
| ● | Fragile
X
–
Fragile
X
syndrome
(FXS)
is
the
most
prevalent
genetic
form
of
intellectual
disability
and
autism
spectrum
disorder,
primarily
affecting
boys.
As
with
most
neurodevelopmental
disorders,
FXS
is
considered
a
condition
of
synaptic
development
and
function.
The
disease
has
a
range
of
clinical
presentations
depending
on
the
specific
genetic
changes
associated
with
an
“expansion”
of
the
FMR1
gene.
The
disease
is
characterized
by
deficits
in
long-term
potentiation
and
homeostatic
plasticity.
FXS
has
been
detected
in
all
populations
and
ethnic
groups.
Researchers
do
not
know
the
exact
number
for
how
many
Americans
could
have
full
mutation
FXS.
Studies
estimate
that
the
disease
affects
approximately
1:4,000
males
and
1:6,000-8,000
females.
Worldwide,
more
than
1,400,000
people
could
be
affected
by
FXS. |
| |
|
|
| |
● |
Depression
– Depression is a major cause of morbidity worldwide according to the World Health Organization. The global antidepressant
drug market is projected to reach $21 Billion by 2030 according to Allied Market Research. Pharmaceutical treatment for depression
has been historically dominated by blockbuster brands. However, the dominance of the leading brands is waning, largely due
to an increase in the number of approvals for antidepressant drugs. |
| |
● |
Epilepsy
– Epilepsy is a common chronic neurological disorder characterized by recurrent unprovoked seizures. These seizures
are transient signs and/or symptoms of abnormal, excessive or synchronous neuronal activity in the brain. According to the
Centers for Disease Control and Prevention, in 2015 epilepsy affected 3.4 million Americans. Today, epilepsy is often controlled,
but not cured, with medication that is categorized as older traditional anti-epileptic drugs and second generation anti-epileptic
drugs. Because epilepsy afflicts sufferers in different ways, there is a need for drugs used in combination with both traditional
anti-epileptic drugs and second generation anti-epileptic drugs. |
| |
● |
Neuropathic
Pain – We define neuralgia, or neuropathic pain, as pain that is not related to activation of pain receptor cells in
any part of the body. Neuralgia is more difficult to treat than some other types of pain because it does not respond well
to normal pain medications. Special medications have become more specific to neuralgia and typically fall under the category
of membrane stabilizing drugs or antidepressants. |
Cancer
| |
● |
Malignant
Melanoma – Predominantly a skin cancer, malignant melanoma can also occur in melanocytes found in the bowel and the
eye. Malignant melanoma accounts for a large majority of skin cancer deaths. The treatment includes surgical removal of the
tumor, adjuvant treatment, chemo and immunotherapy, or radiation therapy. According to iHealthcareAnalyst, Inc. the worldwide
malignant melanoma market is expected to grow to $7.5 billion by 2029. |
| |
● |
Prostate
Cancer – Specific to men, prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive
system. Cancer cells may metastasize from the prostate to other parts of the body, particularly the bones and lymph nodes.
Drug therapeutics for prostate cancer are expected to increase to nearly $10.1 billion by the end of 2030 according to Market
Research Future. |
| |
● |
Pancreatic
Cancer – Pancreatic cancer is a malignant neoplasm of the pancreas. In the United States, approximately 62,000 new cases
of pancreatic cancer will be diagnosed this year and approximately 50,000 patients will die as a result of their cancer, according
to the American Cancer Society. Sales predictions by Market Data Forecast predict that the market for the global pharmaceutical
treatment of pancreatic cancer will increase to $3.7 billion by 2027. |
Competition
The drug discovery and development industry
is very competitive, characterized by rapid advancements in technology, where protection of proprietary advancements is essential.
Any product candidates that we may successfully develop and commercialize, may compete with existing therapies, or new therapies
that may become available in the future. Our commercial opportunities could be reduced or eliminated if our competitors develop
and commercialize products that are more effective, have fewer side effects, are more convenient or are less expensive than any
products that we may develop.
We believe our approach to the treatment
of Alzheimer’s disease and other CNS diseases differs from our competitors - our platform may offer a disease-modifying approach
in neurodegenerative and neurodevelopmental diseases by activation of SIGMAR1. In our preclinical studies, when activated by SIGMAR1
agonists, such as ANAVEX®2-73, SIGMAR1 demonstrated reduced cellular stress before and after RNA gene transcription.
Our studies confirm the potential existence of a predictive biomarker of response established through SIGMAR1 mRNA expression that
could be used in future clinical trials. Because of its role in maintaining neuronal homeostasis, we believe sigma receptors show
significant promise as viable targets for therapeutic molecules in an effort to treat Alzheimer’s disease and other CNS diseases
and disorders, including Parkinson’s disease and Rett syndrome, by restoring healthy gene expression.
At this time, our competitors are primarily
other biomedical development companies that are aiming to discover and develop compounds to be used in the treatment of Alzheimer’s
disease and other CNS diseases, and those companies already doing so. We also face competition from academic institutions and government
agencies, both in the United States and abroad.
Our competitors may have significantly
greater financial resources, an established presence in the market, expertise in research and development, manufacturing, preclinical
and clinical testing, may be in the process of obtaining regulatory approvals and marketing of approved products. These competitors
also compete with us in recruiting and retaining qualified scientific and technical personnel, establishing clinical trial sites
and patient registration for clinical trials, as well as in acquiring or developing technologies complementary to, or necessary
for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative
arrangements with large and established companies.
For additional discussion of the risks
related to competition, see Item 1A “Risk Factors.”
Patents, Trademarks and Intellectual
Property
We hold ownership or exclusive rights to twenty-two
U.S. patents, twenty-three U.S. patent applications, and various PCT or ex-U.S. patent applications relating to our drug candidates,
methods associated therewith, and to our research programs.
We own one issued U.S. patent entitled “ANAVEX®2-73
and certain anticholinesterase inhibitors composition and method for neuroprotection” claims a composition of matter of ANAVEX®2-73
directed to a novel and synergistic neuroprotective compound combined with donepezil and other cholinesterase inhibitors. This
patent is expected to expire in June 2034, absent any patent term extension for regulatory delays. We own one issued U.S. patent
entitled “A2-73 crystalline polymorph compositions of matter and methods of use thereof”. It claims crystals of A2-73
freebase or its fumarate salt, dosage forms and pharmaceutical formulations. This patent is expected to expire in July 2039, absent
any patent term extension for regulatory delays. We own four issued U.S. patents each with claims directed to crystalline forms
of ANAVEX®2-73. The first of these four patents claims crystalline forms of ANAVEX®2-73, dosage forms
and compositions containing crystalline ANAVEX®2-73, and methods of treatment for Alzheimer’s disease using
them. This patent is expected to expire in July 2036, absent any patent term extension for regulatory delays. The second of these
four patents claims pharmaceutical compositions containing a crystalline form of ANAVEX®2-73, and methods of treatment
for Alzheimer’s disease using the compositions. This patent is expected to expire in June 2036, absent any patent term extension
for
regulatory delays. The third of these four patents claims pharmaceutical compositions containing a crystalline form of ANAVEX®2-73,
and methods of treatment for Alzheimer’s disease using the compositions. This patent is expected to expire in June 2036,
absent any patent term extension for regulatory delays. The fourth of these four patents claims method of making certain crystalline
forms ANAVEX®2-73. This patent is expected to expire in October 2036, absent any patent term extension for regulatory
delays. We also own three issued U.S. patents for seizure treatment. The first of these three patents claims methods and dosage
forms for treating seizures, the dosage forms containing a low-dose anti-epilepsy drug combined with either: (i) ANAVEX®2-73
and its active metabolite ANAVEX®19-144; or (ii) ANAVEX®19-144. The second of these three patents
further claims a combination seizure treatment involving administration of an anti-epilepsy drug combined with (i) ANAVEX®19-144,
or (ii) ANAVEX 19-144® and ANAVEX 2-73®. The third of these three patents claims a dosage form for
seizure reduction, comprising (i) ANAVEX19-144®, (ii) ANAVEX 2-73®, or (iii) a combination of ANAVEX19-144®
and ANAVEX 2-73®; and optionally further comprising a low-dose anti-epilepsy drug. All three patents are
expected to expire in October 2035, absent any patent term extension for regulatory delays. We also own three issued U.S. patents
with claims directed to treating neurodevelopmental disorders. These patents claim methods for treating a neurodevelopmental disorder,
multiple sclerosis, or their related biochemical and functional abnormalities by administering ANAVEX®2-73, ANAVEX®19-144,
and/or ANAVEX®1-41 (another sigma receptor ligand similar to ANAVEX®2-73), or compositions thereof.
All three patents are expected to expire in January 2037, absent any patent term extension for regulatory delays. In addition,
we own one issued U.S. Patent with claims directed to methods of treating melanoma with a compound related to ANAVEX®2-73.
This patent is expected to expire in February 2030, absent any patent term extension for regulatory delays. We also own an issued
U.S. patent that claims crystalline forms of ANAVEX®19-144, dosage forms and compositions containing the crystalline
forms of ANAVEX®19-144, and methods of treatment for Alzheimer’s disease. This patent is expected to expire
in July 2036, absent any patent term extension for regulatory delays. Further, we own one issued U.S. Patent with claims directed
to methods of treating cardiac dysfunction with ANAVEX®2-73. This patent is expected to expire in July 2038, absent
any patent term extension for regulatory delays. Additionally, we own one issued U.S. Patent with claims directed to methods of
treating insomnia or anxiety with ANAVEX®2-73, ANAVEX®19-144, and/or ANAVEX®1-41.
This patent is expected to expire in September 2038, absent any patent term extension for regulatory delays. Further, we own one
issued U.S. Patent with claims directed to a method of treating systolic hypertension using ANAVEX®2-73. This patent
is expected to expire in July 2039, absent any patent term extension for regulatory delays. Additionally, we own one issued U.S.
Patent with claims directed to pharmaceutical dosage forms of (-) enantiomer of ANAVEX®2-73. This patent is expected
to expire in July 2036, absent any patent term extension for regulatory delays.
We also own two issued U.S. patents related
to ANAVEX®1066. The first of these two patents claims methods for treating or preventing pain using (+) ANAVEX®1066
isomer. The second patent claims methods for treating or preventing pain using (-) ANAVEX®1066 isomer. Both patents
are expected to expire in November 2036, absent any patent term extension for regulatory delays.
For ANAVEX®2-73, ANAVEX®19-144,
ANAVEX®1-41, and ANAVEX®1066, we also have granted or pending applications in Australia, Canada,
China, Europe, Japan, and Hong Kong, which are expected to expire after 2035.
With regard to ANAVEX®3-71,
we own exclusive rights to two issued U.S. patents with claims respectively directed to the ANAVEX®3-71 compound
and methods of treating various diseases including Alzheimer’s with the same. These patents are expected to expire in April
2030, and January 2030, respectively, absent any patent term extension for regulatory delays. We also own exclusive rights to related
patents or applications that are granted or pending in Australia, Canada, China, Europe, Japan, Korea, New Zealand, Russia, and
South Africa, which are expected to expire in January 2030.
We also own other patent applications directed
to enantiomers, crystals, formulations, uses, and patient selection methods that may provide additional protection for one or more
of our product candidates.
We regard patents and other intellectual property
rights as corporate assets. Accordingly, we attempt to optimize the value of intellectual property in developing our business strategy
including the selective development, protection, and exploitation of our intellectual property rights. In addition to filings made
with intellectual property authorities, we protect our intellectual property and confidential information by means of carefully
considered processes of communication and the sharing of information, and by the use of confidentiality and non-disclosure agreements
and provisions for the same in contractor’s agreements. While no agreement offers absolute protection, such agreements provide
some form of recourse in the event of disclosure, or anticipated disclosure.
Our
intellectual property position, like that of many biomedical companies, is uncertain and involves complex legal and technical questions
for which important legal principles are unresolved. For more information regarding challenges to our existing or future patents,
see “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K.
Government regulation
Government authorities in the United States,
at the federal, state and local levels, and other countries extensively regulate, among other things, the research, development,
testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution,
marketing and export and import of products such as those we are developing. A new drug must be approved by the FDA through the
NDA or ANDA process before it may be legally marketed in the United States. We are subject to various government regulations in
connection with the development of our pipeline.
U.S. Drug Development and Regulation
In the United States, the FDA regulates drugs
under the Federal Food, Drug, and Cosmetic Act and its implementing regulations (“FDCA”). The process of obtaining
regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations
require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at
any time during the product development process, approval process or post approval may subject an applicant to administrative or
judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval,
import refusal, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production
or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
Any agency or judicial enforcement action could have a material adverse effect on us.
Once a drug candidate is identified for development,
it enters the preclinical testing stage and an Investigational New Drug Application (“IND”) may be opened for the regulatory
development of the product. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as
well as other preclinical studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing
information and analytical data, to the FDA as part of the IND to conduct clinical trials. The sponsor must also include a protocol
detailing, among other things, the objectives of the clinical trials, the parameters to be used in monitoring the safety of the
trial, and the effectiveness criteria to be evaluated should the trial include an efficacy evaluation. Some preclinical testing
may continue even after the IND is filed. The IND automatically becomes effective thirty (30) days after receipt by the FDA, unless
the FDA, within the 30-day time period, places the clinical trial on a clinical hold. Clinical holds also may be imposed by the
FDA at any time before or during clinical trials due to safety concerns about ongoing or proposed clinical trials or non-compliance
with specific FDA requirements, and the trials may not begin or continue until the FDA notifies the sponsor that the hold has been
lifted.
All clinical trials must be conducted under
the supervision of one or more qualified investigators in accordance with FDA good clinical practice (“GCP”) requirements,
which include a requirement that all research subjects provide their informed consent in writing for their participation in any
clinical trial. Clinical trials must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject
selection inclusion and exclusion criteria and the safety and/or effectiveness criteria to be evaluated. Each protocol must be
submitted to the FDA as part of the IND, and timely safety reports must be submitted to the FDA and the investigators for serious
and unexpected adverse events. An Institutional Review Board (“IRB”) at each institution participating in the clinical
trial must review and approve each protocol before a clinical trial may commence at the institution and must also approve the information
regarding the trial as well as the informed consent form that must be provided to each trial participant or his or her legal representative,
monitor the study until completed and otherwise comply with all applicable IRB regulations.
Human clinical trials are typically conducted
in three sequential phases that may overlap or be combined in certain cases:
Phase 1: The compound is initially introduced
into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if
possible, to gain an early indication of its effectiveness. In most cases, initial Phase 1 clinical trials are conducted with healthy
volunteers. However, where the compound being evaluated is for the treatment of severe or life-threatening diseases, such as cancer,
and especially when the product may be too toxic to ethically administer to healthy volunteers, the initial human testing may be
conducted on patients with the target disease or condition. Sponsors sometimes subdivide their Phase 1 clinical trials into Phase
1a and Phase 1b clinical trials. Phase 1b clinical trials are typically aimed at confirming dosage, PK and safety in a larger number
of patients. Some Phase 1b clinical trials evaluate biomarkers or surrogate markers that may be associated with efficacy in patients
with specific types of diseases or conditions.
Phase 2: This phase involves clinical trials
in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of
the product for specific targeted diseases or conditions and to confirm dosage tolerance and appropriate dosage.
Phase 3: Phase 3 clinical trials are undertaken
to further evaluate dosage, clinical efficacy and safety in an expanded patient population, generally at geographically dispersed
clinical trial sites. These clinical trials, often referred to as “pivotal” or “confirmatory” clinical
trials, are intended to establish the overall risk-benefit ratio of the compound and provide, if appropriate, an adequate basis
for product approval and labeling.
The FDA or the sponsor may suspend a clinical
trial at any time on various grounds, including any finding that the research subjects or patients are being exposed to an unacceptable
health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is
not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected, serious
harm to study subjects. In addition, clinical trials may be overseen by an independent group of qualified experts organized by
the sponsor, known as a data safety monitoring board or committee. Depending on its charter, this board or committee may determine
whether a trial may move forward at designated check points based on access to certain data from the trial.
Phase 4: Phase 4 or post-approval trials may
also be conducted after a drug receives initial marketing approval. These trials, often referred to as “Phase 4” trials,
are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances,
the FDA may mandate the performance of such clinical trials as a condition of approval of continued marketing of the product.
During the development of a new drug, sponsors
are given several opportunities to meet with the FDA. These meetings can provide an opportunity for the sponsor to share information
about the progress of the application or clinical trials, for the FDA to provide advice, and for the sponsor and the FDA to reach
agreement on the next phase of development. These meetings may occur prior to the submission of an IND, at the end of Phase 2 clinical
trials, or before an NDA is ultimately submitted. Sponsors typically use the meetings at the end of the Phase 2 trials to discuss
Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trials that they believe will support approval of the
new drug. Meetings at other times may be made upon request and are subject to approval by the FDA.
Concurrent with clinical trials, companies
typically complete additional, animal or other non-clinical studies, develop additional information about the chemistry and physicochemical
characteristics of the drug, and finalize a process for manufacturing the product in commercial quantities in accordance with the
FDA’s current Good Manufacturing Practices (“cGMP”) requirements. The manufacturing process must consistently
produce quality batches of the drug and, among other things, the manufacturer must develop methods for testing the identity, strength,
quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must
be conducted to demonstrate the effectiveness of the packaging and that the compound does not undergo unacceptable deterioration
over its shelf life.
While the IND is active, progress reports summarizing
the results of ongoing clinical trials and nonclinical studies performed since the last progress report must be submitted on at
least an annual basis to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and
unexpected adverse events, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs,
findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important, increased incidence
of a serious adverse reaction compared to that listed in the protocol or investigator brochure.
There are also requirements governing the submission
of certain clinical trials and completed trial results to public registries. Sponsors of certain clinical trials of FDA-regulated
products are required to register and disclose specified clinical trial registration and results information, which is made publicly
available at www.clinicaltrials.gov. Failure to properly report clinical trial results can result in civil monetary penalties.
Disclosure of clinical trial results can often be delayed until the new product or new indication being studied has been approved.
U.S. review and approval process
The results of product development, preclinical
and other non-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted
on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of a New Drug Application
(“NDA”). The submission of an NDA is subject to the payment of substantial user fees; a waiver of which may be obtained
under certain limited circumstances.
The FDA reviews NDAs to determine, among other
things, whether the product is safe and effective for its intended use and whether it is manufactured in a cGMP-compliant manner,
which will assure and preserve the product’s identity, strength, quality and purity. Under the Prescription Drug User Fee
Act (“PDUFA”), the FDA has a goal of ten months from the date of “filing” of a standard, completed NDA
for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA
is submitted to the FDA because the FDA has approximately two months to make a “filing” decision after the application
is submitted. The FDA conducts a preliminary review of all NDAs within the first sixty days after submission, before accepting
them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional
information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The
resubmitted application also is subject to review before the FDA accepts it for filing.
The FDA may refer an application for a new
drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific
experts, that reviews, evaluates and provides a recommendation as to whether and under what conditions the application should be
approved. The FDA is not bound by the recommendations of such an advisory committee, but it considers advisory committee recommendations
carefully when making decisions.
Before approving an NDA, the FDA will also
inspect the facility where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing
processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product
within required specifications. Before approving an NDA, the FDA may also inspect one or more clinical trial sites to assure compliance
with GCP requirements and inspect the clinical trial records.
After the FDA evaluates an NDA, it will issue
an approval letter or a Complete Response Letter. A Complete Response Letter indicates that the review cycle of the application
is complete, and the application will not be approved in its present form. A Complete Response Letter usually describes the specific
deficiencies in the NDA identified by the FDA and may require additional clinical data, such as an additional pivotal Phase 3 trial
or other significant and time-consuming requirements related to clinical trials, nonclinical studies or product manufacturing.
If a Complete Response Letter is issued, the sponsor must resubmit the NDA, addressing all of the deficiencies identified in the
letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA does not
satisfy the criteria for approval. An approval letter authorizes commercial marketing of the drug with prescribing information
for specific indications.
The Pediatric Research Equity Act (“PREA”),
requires IND sponsors to conduct pediatric clinical trials for most drugs, for a new active ingredient, new indication, new dosage
form, new dosing regimen or new route of administration. Under PREA, original NDAs and supplements must contain a pediatric assessment
unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the
product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric
subpopulation for which the product is safe and effective. The sponsor or the FDA may request a deferral of pediatric clinical
trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that
the drug is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness
data needs to be collected before the pediatric clinical trials begin. The FDA must send a non-compliance letter to any sponsor
that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric
formulation.
If a drug receives FDA approval, the approval
may be limited to specific diseases and dosages, which could restrict the commercial value of the product. In addition, the FDA
may require testing and surveillance programs to monitor the safety of approved products which have been commercialized and may
require a sponsor to conduct post-marketing clinical trials (Phase 4 clinical trials), which are designed to further assess a drug’s
safety and effectiveness after NDA approval. The FDA may also place other conditions on approval, including a requirement for a
risk evaluation and mitigation strategy (“REMS”) to assure the safe use of the drug. If the FDA concludes a REMS is
needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required.
A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution
methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict
the commercial promotion, distribution, prescribing or dispensing of products. Marketing approval may be withdrawn for non-compliance
with REMS or other regulatory requirements, or if problems occur following initial marketing.
Post-approval requirements
Once an approval is granted, the FDA may withdraw
the approval if compliance with regulatory standards is not maintained or if problems occur after the drug reaches the market.
Later discovery of previously unknown problems with a drug may result in restrictions on the drug or even complete withdrawal of
the drug from the market. After approval, some types of changes to the approved drug, such as adding new indications, certain manufacturing
changes and additional labeling claims, are subject to further FDA review and approval. Manufacturers and other entities involved
in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state
agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP regulations
and other laws and regulations.
Any drug product manufactured or distributed
by us pursuant to FDA approval will be subject to continuing regulation by the FDA, including, among other things, record-keeping
requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug
sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with
FDA promotion and advertising requirements. FDA strictly regulates labeling, advertising, promotion and other types of information
regarding approved drugs that are placed on the market, and imposes requirements and restrictions on drug manufacturers, such as
those related to direct-to-consumer advertising, the prohibition on promoting products for uses or in patient populations that
are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific
and educational activities, and promotional activities involving the internet. Discovery of previously unknown problems or the
failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product for a certain
indication or withdrawal of the product from the market as well as possible civil or criminal sanctions. Failure to comply with
the applicable governmental requirements at any time during the product development process, approval process or after approval,
may subject an applicant or manufacturer to administrative or judicial civil or criminal sanctions and adverse publicity. The FDA
sanctions could include refusal to approve pending applications, withdrawal of an approval, clinical holds on post-marketing clinical
trials, enforcement letters, import refusals, product recalls, product seizures, total or partial suspension of production or distribution,
injunctions, fines, refusals of government contracts, mandated corrective advertising or communications with doctors, debarment,
restitution, disgorgement of profits, or civil or criminal penalties.
Expedited development and review programs
The FDA has a fast track designation program
that is intended to expedite or facilitate the process for reviewing new drug products that meet certain criteria. Specifically,
new drugs are eligible for fast track designation if they are intended to treat a serious or life-threatening disease or condition
and demonstrate the potential to address unmet medical needs for the disease or condition. With regard to a fast track product,
the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted, if the sponsor
provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines
that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
Any product submitted to the FDA for approval,
including a product with a fast track designation, may also be eligible for other types of FDA programs intended to expedite development
and review, such as priority review and accelerated approval.
A product is eligible for priority review if
it is intended to treat a serious condition, and if approved, would provide a significant improvement in safety or efficacy compared
to currently marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new
drug designated for priority review in an effort to facilitate the review. The FDA endeavors to review applications with priority
review designations within six months of the filing date, as compared to ten months for review of NDAs under its current PDUFA
review goals.
In addition, a product may be eligible for
accelerated approval. Drugs intended to treat serious or life-threatening diseases or conditions may be eligible for accelerated
approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical
benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely
to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity,
or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may
require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials.
Drugs receiving accelerated approval may be subject to expedited withdrawal procedures if the sponsor fails to conduct the required
post-marketing trials or if such trials fail to verify the predicted clinical benefit. In addition, the FDA currently requires
as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial
launch of the product.
The Food and Drug Administration Safety and
Innovation Act (“FDASIA”) established a category of drugs referred to as “breakthrough therapies” that
may be eligible to receive breakthrough therapy designation. A sponsor may seek FDA designation of a compound as a “breakthrough
therapy” if the product is intended, alone or in combination with one or more other products, to treat a serious or life-threatening
disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over
existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical
development. The designation includes all of the fast track program features, as well as more intensive FDA interaction and guidance.
The breakthrough therapy designation is a distinct status from both accelerated approval and priority review, which can also be
granted to the same drug if relevant criteria are met. If a product is designated as breakthrough therapy, the FDA will work to
expedite the development and review of such drug.
Fast track designation, priority review and
breakthrough therapy designation do not change the standards for approval but may expedite the development or approval process.
However, even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets
the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Orphan drug designation
Under the Orphan Drug Act, the FDA may grant
orphan designation to a drug intended to treat a rare disease or condition, which is a disease or condition that affects fewer
than 200,000 individuals in the United States or, if it affects more than 200,000 individuals in the United States, there is no
reasonable expectation that the cost of developing and making a drug product available in the United States for this type of disease
or condition will be recovered from sales of the product. Orphan designation must be requested before an NDA is submitted. After
the FDA grants orphan designation, the identity of the therapeutic agent and its potential orphan use are publicly disclosed by
the FDA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently
receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan
product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication
for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity
or inability to manufacture the product in sufficient quantities. The designation of such drug also entitles a party to financial
incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. However,
competitors may receive approval of different products for the indication for which the orphan product has exclusivity or obtain
approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan exclusivity also
could block the approval of one of our compounds for seven years if our compound is determined to be contained within the competitor’s
product for the same indication or disease, or if a competitor obtains approval of the same drug as defined by the FDA. In addition,
if an orphan designated product receives marketing approval for an indication broader than what is designated, it may not be entitled
to orphan exclusivity.
Abbreviated New Drug Applications, 505(b)(2)
Applications, and Marketing exclusivity
In seeking approval for a drug through an NDA,
applicants are required to list with the FDA each patent with claims that cover the applicant’s drug or an approved method
of use of the drug. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the
FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the “Orange Book.” Drugs
listed in the Orange Book can, in turn, be cited by competitors in support of approval of an Abbreviated New Drug Application,
or ANDA, or a 505(b)(2) application. In this case, the original NDA (the so-called pioneer drug) is known as the
“listed” drug or “reference-listed” drug. An ANDA provides for marketing of a drug that has the same active
ingredient and the same strength, route of administration and dosage form as the listed drug and has been shown through testing
to be bioequivalent to the listed drug or receives a waiver from bioequivalence testing. ANDA applicants are generally not required
to conduct or submit results of preclinical or clinical tests to prove the safety or effectiveness of their drug, other than the
requirement for bioequivalence testing. Drugs approved in this way are considered therapeutically equivalent, and are commonly
referred to as “generic equivalents” to the listed drug. These drugs then generally can be substituted by pharmacists
under prescriptions written for the original listed drug.
A 505(b)(2) application is a type of NDA that
relies, in part, upon data the applicant does not own and to which it does not have a right of reference. Such applications often
are submitted for changes to previously approved drug products, and rely on the FDA’s prior findings of safety and effectiveness
for a third party’s NDA to abbreviate the showings the sponsor of the 505(b)(2) application must make to establish that its
product is safe and effective.
The ANDA or 505(b)(2) applicant is required
to certify to FDA concerning any patents listed for the referenced approved drug in FDA’s Orange Book. Specifically, for
each listed patent, the applicant must certify that: (1) the required patent information has not been filed; (2) the
listed patent has expired; (3) the listed patent has not expired but will expire on a particular date and approval is sought
after patent expiration; or (4) the listed patent is invalid, unenforceable or will not be infringed by the new drug. A certification
that the new drug will not infringe the already approved drug’s listed patents or that such patents are invalid or unenforceable
is called a Paragraph IV certification. If the ANDA or 505(b)(2) applicant does not include a Paragraph IV certification,
the ANDA or 505(b)(2) application will not be approved until all of the listed patents claiming the referenced drug have expired,
except for any listed patents that only apply to uses of the drug not being sought by the ANDA or 505(b)(2) applicant.
If the ANDA or 505(b)(2) applicant has
made a Paragraph IV certification, the applicant must also send notice of a Paragraph IV Notice Letter to the NDA and patent holders
once the ANDA or 505(b)(2) application has been accepted for filing by FDA. The NDA and patent holders may then initiate a
patent infringement lawsuit in response to the notice of the Paragraph IV Notice Letter. The filing of a patent infringement lawsuit
within 45 days of the receipt of notice of a Paragraph IV Notice Letter automatically prevents FDA from approving the ANDA
until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, modification by a court or a decision
in the infringement case that is favorable to the ANDA or 505(b)(2) applicant. As discussed below, the ANDA or 505(b)(2) application
also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the reference-listed
drug has expired.
Market exclusivity provisions under the FDCA
can delay the submission or approval of certain marketing applications. The FDCA provides a five-year period of non-patent marketing
exclusivity within the United States to the first applicant to obtain approval of an NDA for a new chemical entity. A drug is a
new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule
or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not approve or even accept
for review an abbreviated new drug application (“ANDA”), or an NDA submitted under Section 505(b)(2) (a “505(b)(2)
NDA”), submitted by another company for another drug containing the same active moiety, regardless of whether the drug is
intended for the same indication as the original innovative drug or for another indication, where the applicant does not own or
have a legal right of reference to all the data required for approval. However, an application may be submitted after four years
if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator
NDA holder.
The FDCA alternatively provides three years
of marketing exclusivity for an NDA, or supplement to an existing NDA, if new clinical investigations, other than bioavailability
studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application,
for example new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the modification
for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving
ANDAs or 505(b)(2) NDAs for drugs containing the active ingredient for the original indication or condition of use. Five-year and
three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would
be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical
trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of marketing
exclusivity available in the United States. Pediatric exclusivity provides for an additional six months of marketing exclusivity
attached to another period of exclusivity if a sponsor conducts clinical trials in children in response to a written request from
the FDA. The issuance of a written request does not require the sponsor to undertake the described clinical trials. In addition,
orphan drug exclusivity, as described above, may offer a seven-year period of marketing exclusivity, except in certain circumstances.
United States Patent Term Restoration
Depending upon the timing, duration and specifics
of FDA approval of our future product candidates, some of our United States patents may be eligible for limited patent term extension
under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments.
The Hatch-Waxman Amendments permit restoration of the patent term of up to five years as compensation for patent term lost during
the FDA regulatory review process for a drug that has not been previously approved for commercial marketing. Patent-term restoration,
however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date and only
those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended. The patent-term
restoration period is generally one-half the time between the effective date of an IND and the submission date of an
NDA plus the time between the submission date of an NDA and the approval of that application, except that the review period is
reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug
is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The
U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension
or restoration. In the future, we may apply for restoration of patent term for our currently owned or licensed patents to add patent
life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in
the filing of the relevant NDA.
Foreign Sales
Sales outside the United States of potential
drug compounds we develop will also be subject to foreign regulatory requirements governing human clinical trials and marketing
for drugs. The requirements vary widely from country to country, but typically the registration and approval process takes several
years and requires significant resources. In most cases, if the FDA has not approved a potential drug compound for sale in the
United States, the potential drug compound may be exported for sale outside of the United States, only if it has been approved
in any one of the following: the European Union or a country in the European Economic Area (the countries in the European Union
and the European Free Trade Association) if the drug or device is marketed in that country or the drug or device is authorized
for general marketing in the European Economic Area, Canada, Australia, New Zealand, Japan, Israel, Switzerland and South Africa.
There are specific FDA regulations that govern this process.
U.S. coverage and reimbursement
Significant uncertainty exists as to the coverage
and reimbursement status of any compound for which we may seek regulatory approval. Sales in the United States will depend in part
on the availability of sufficient coverage and adequate reimbursement from third-party payors, which include government health
programs such as Medicare, Medicaid, CHIP, TRICARE and the Veterans Administration, as well as managed care organizations and private
health insurers. Prices at which we or our customers seek reimbursement for our therapeutic compounds can be subject to challenge,
reduction or denial by payors.
The process for determining whether a payor
will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will
pay for the product. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate
will be available. Additionally, in the United States there is no uniform policy among payors for coverage or reimbursement. Third-party
payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies,
but also have their own methods and approval processes. Therefore, coverage and reimbursement for products can differ significantly
from payor to payor. If coverage and adequate reimbursement are not available, or are available only at limited levels, successful
commercialization of, and obtaining a satisfactory financial return on, any product we develop may not be possible.
Third-party payors are increasingly challenging
the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety
and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for marketing, we may need to
conduct expensive studies in order to demonstrate the medical necessity and cost-effectiveness of any products, which would be
in addition to the costs expended to obtain regulatory approvals. Third-party payors may not consider our compounds to be medically
necessary or cost-effective compared to other available therapies, or the rebate percentages required to secure favorable coverage
may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient to realize an appropriate return
on our investment in drug development. Additionally, we or our collaborators may develop companion diagnostic
tests for use with our product candidates. Companion diagnostic tests require coverage and reimbursement separate and apart from
the coverage and reimbursement for their companion pharmaceutical or biological products. Similar challenges to obtaining coverage
and reimbursement, applicable to pharmaceutical or biological products, will apply to companion diagnostics.
Fraud and Abuse Laws
Federal and state health care laws and regulations
restrict business practices in the biopharmaceutical industry. In the biopharmaceutical industry, there are a number of federal
and state health care regulatory requirements that apply to entities, including, but not limited to, the federal and state fraud
and abuse laws. These laws include, but are not limited to, anti-kickback and self-referral law, civil false claims act law, criminal
false statement law, civil monetary penalty laws, exclusion law, and other civil, criminal, and administrative laws. Health care
laws, regulations, and guidance continuously evolve and are thereby subject to constant change.
The Federal Anti-Kickback Statute, 42 U.S.C.
§ 1320a-7b(b), among other things, prohibits the knowing and willful offer, payment, solicitation or receipt of any form of
remuneration, whether directly or indirectly and overtly or covertly in cash or in kind, in return for, or to induce the referral
of an individual for the:
| ● |
|
furnishing
or arranging for the furnishing of items or services reimbursable in whole or in part under Medicare, Medicaid or other federal
healthcare programs; or |
| ● |
|
purchase,
lease, or order of, or the arrangement or recommendation of the purchasing, leasing, or ordering of any item or service reimbursable
in whole or in part under Medicare, Medicaid or other federal healthcare programs. |
There are a number of narrow safe harbors to
the Federal Anti-Kickback Statute. Such safe harbors permit certain payments and business practices that, although they would otherwise
potentially implicate the Federal Anti-Kickback Statute, are not treated as an offense under the same if all of the requirements
of the specific applicable safe harbor are met. Actual knowledge of the statute or specific intent to violate it is not required
in order for a person or entity to have committed a violation.
The Federal Anti-Kickback Statute applies to
certain arrangements with healthcare providers, product end users and other parties, including marketing arrangements and discounts
and other financial incentives offered in connection with the sales of our products. Regulatory authorities may determine that
certain marketing, pricing, or other activities violate the Federal Anti-Kickback Statute or other applicable laws. Noncompliance
with the Federal Anti-Kickback Statute can result in civil, administrative and/or criminal penalties, restrictions on the ability
to operate in certain jurisdictions, and exclusion from participation in Medicare, Medicaid or other federal healthcare programs.
In addition, non-compliance can result in the need to curtail and/or restructure operations. Any penalties, damages, fines, exclusions,
curtailment or restructuring of operations could adversely affect the ability to operate a business, financial condition, and results
of operations. A violation of the Federal Anti-Kickback Statute can serve as a false or fraudulent claim for purposes of the civil
False Claims Act and the civil monetary penalties statute.
The federal Health Insurance Portability and
Accountability Act of 1996 (“HIPAA”) among other things, enacted numerous provisions that prohibit knowingly and willfully
executing, or attempting to execute, a scheme or artifice to defraud any healthcare benefit program or obtain, by means of false
or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of,
any healthcare benefit program, regardless of the payor (e.g., public or private), willfully obstructing a criminal investigation
of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact
or making any materially false, fictitious or fraudulent statements in connection with the delivery of, or payment for, healthcare
benefits, items or services relating to healthcare matters. These provisions include 18 U.S.C. §§ 286, 287, 669, 1035,
1347, and 1518, all as described further below.
The Ethics in Patient Referrals Act, commonly
known as the “Stark Law,” 42 U.S.C. § 1395nn, prohibits a physician from making referrals for certain “designated
health services” payable by Medicare to an entity in which the physician or an immediate family member of such physician
has an ownership or investment interest or with which the physician has entered into a compensation arrangement, unless a statutory
exception applies. There are a number of exceptions to the Stark Law. Such exceptions permit certain payments and arrangements
that, although they would otherwise potentially implicate the Stark Law, are not treated as a violation under the same if the requirements
of the specific exceptions are met. Violation of the Stark Law could result in denial of payment, disgorgement of reimbursements
received under a noncompliance arrangement, civil penalties, damages and exclusion from Medicare or other governmental programs.
These requirements are highly technical and there can be no guarantee that regulatory authorities will not determine or assert
that arrangements are in violation of the Stark Law and do not otherwise meet applicable Stark Law exceptions.
The federal false statements statute, 42 U.S.C.
§ 1320a-7b(a), prohibits knowingly and willfully falsifying, concealing, or omitting a material fact or making any materially
false statement in connection with the delivery of health care benefits, items, or services. Similarly, 18 U.S.C. § 1035 prohibits
a person or entity, in any matter involving a health care benefit program, from knowingly or willfully falsifying, concealing,
or covering up by any trick, scheme, or device a material fact; making any materially false, fictitious, or fraudulent statements
or representations; or making or using any materially false writing or document knowing the same to contain any materially false,
fictitious, or fraudulent statement or entry. In addition to criminal penalties, violation of these statutes may result in collateral
administrative sanctions, including exclusion from participation in Medicare, Medicaid and other federal health care programs.
18 U.S.C. § 669 prohibits knowingly and
willfully embezzling, stealing, or otherwise without authority converting to the use of any person or entity other than the rightful
owner, or intentionally misapplying any of the moneys, funds, securities, premiums, credits, property, or other assets of a health
care benefit program. In addition to criminal penalties, violation of this statute may result in collateral administrative sanctions,
including exclusion from participation in Medicare, Medicaid and other federal health care programs.
The criminal health care fraud statute, 18
U.S.C. § 1347, establishes criminal liability for whoever knowingly and willfully executes, or attempts to execute, a scheme
or artifice to defraud any health care benefit program, or to obtain, by means of false or fraudulent pretenses, representations,
or promises, any of the money or property owned by, or under the custody or control of, any health care benefit program, in connection
with the delivery of or payment for health care benefits, items, or services. In addition to criminal penalties, violation of this
statute may result in collateral administrative sanctions, including exclusion from participation in Medicare, Medicaid and other
federal health care programs. A person or entity need not have actual knowledge of this law or specific intent to commit a violation
of this law.
18 U.S.C. § 1518 establishes criminal
liability for whoever willfully prevents, obstructs, misleads, delays or attempts to prevent, obstruct, mislead, or delay the communication
of information or records relating to a violation of a Federal health care offense to a criminal investigator. In addition to criminal
penalties, violation of this statute may result in collateral administrative sanctions, including exclusion from participation
in Medicare, Medicaid and other federal health care programs.
18 U.S.C. § 286 establishes criminal liability
for whoever enters into any agreement, combination, or conspiracy to defraud the United States, or any department or agency thereof,
by obtaining or aiding to obtain the payment or allowance of any false, fictitious or fraudulent claim. In addition to criminal
penalties, violation of this statute may result in collateral administrative sanctions, including exclusion from participation
in Medicare, Medicaid and other federal health care programs.
18 U.S.C. § 287 establishes criminal liability
for whoever knowingly makes or presents a false, fictitious or fraudulent claim to the United States Government, including any
department or agency thereof. In addition to criminal penalties, violation of this statute may result in collateral administrative
sanctions, including exclusion from participation in Medicare, Medicaid and other federal health care programs.
The Federal False Claims Act, 31 U.S.C. §
3729, et seq., provides, in part, that the federal government—or a private party on behalf of the government—may bring
a lawsuit against any person whom it believes has knowingly presented, or caused to be presented, a false or fraudulent claim for
payment, or who has made a false statement or used a false record to get a claim paid or to avoid, decrease or conceal an obligation
to pay money to the federal government or who has knowingly retained an overpayment. Knowledge under the Federal False Claims Act
means actual knowledge, deliberate indifference, or reckless disregard. In addition, amendments in 1986 to the Federal False Claims
Act have made it easier for private parties to bring whistleblower lawsuits against companies.
The civil monetary penalties law, 42 U.S.C.
§ 1320a-7a, provides, in part, that the federal government may seek civil monetary penalties against any person who presents
or causes to be presented claims to a Federal health care program that the person knows or should know is for an item or services
that was not provided as claimed or is false or fraudulent, or the person has made a false statement or used a false record to
get a claim paid. The federal government may also seek civil monetary penalties for a wide variety of other conduct, including
offering remuneration to influence a Medicare or Medicaid beneficiary’s selection of providers and violations of the Federal
Anti-Kickback Statute.
Violations of the Federal False Claims Act
and/or the Civil Monetary Penalties Law can result in penalties ranging from $12,537 to $25,076 for each false claim violation
of the Federal False Claims Act and varying amounts based on the type of violation of the Civil Monetary Penalties Law, plus up
to three times the amount of damages that the federal government sustained. In addition, the federal government may also seek exclusion
from participation in all federal health care programs.
42 U.S.C. Section 1320a-7 provides that individuals
and entities can be mandatorily or permissively excluded from participation in federal health care programs. The grounds for mandatory
exclusion include, but are not limited to, conviction for a criminal offense related to the delivery of an item or service reimbursed
under a federal or state health care program, and a conviction related to health care fraud. The grounds for permissive exclusion
include, but are not limited to, criminal offenses relating to fraud inside and outside of health care, convictions related to
obstruction of an investigation or audit, and/or failure to disclose certain required information. Exclusion from federal health
care programs—whether mandatory or permissive—may mean that our customers may not be able to get reimbursed by federal
and/or state health care programs for use or dispensing of our products.
State Fraud and Abuse Provisions
Many states have also adopted some form of
anti-kickback and anti-referral laws and false claims acts and civil monetary penalties and other fraud and abuse provisions that
apply regardless of payer, in addition to items and services reimbursed under Medicaid and other state programs. A determination
of liability under such laws could result in fines, penalties, and exclusion, as well as restrictions on the ability to operate
in these jurisdictions.
Corporate liability can be present as a result
of the illegal activities of employees, representatives, contractors, collaborators, agents, subsidiaries, or affiliates, even
if they were not explicitly authorized. There can be no assurance that all employees, representatives, contractors, collaborators,
agents, subsidiaries or affiliates will comply with the foregoing laws at all times. Violation of the aforementioned and other
laws could result in whistleblower complaints, investigations, sanctions, settlements, prosecution, government oversight and reporting,
other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions
or other administrative remedies, suspension and/or debarment from contracting with certain governments or other persons, the loss
of privileges, reputational harm, contract damages, adverse media coverage and other collateral consequences. In addition, corporate
directors, officers, employees, and other representatives who engage in violations of these and other laws may face imprisonment,
fines, and penalties. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if a
company does not prevail in any possible civil or criminal litigation, business, financial condition, and results of operations
could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s
attention and resources and significant defense costs and other professional fees. Enforcement actions and sanctions could further
harm business, financial condition, and results of operations. Any of the consequences contained in this paragraph and section
could adversely affect the ability to operate the business, financial condition, and the results of operations.
Sunshine Act
The Sunshine Act requires manufacturers of
products reimbursed by Medicare, Medicaid or the Children’s Health Insurance Program (“CHIP”) to collect and
annually report detailed data to the Centers for Medicare and Medicaid Services (“CMS”) regarding payments or other
transfers of value to physicians, certain other health care providers (such as physicians assistants
and nurse practitioners), and teaching hospitals (“covered recipients”), as well as any ownership or investment
interest held by physicians and their immediate family members. The reporting data must be accompanied by an attestation as to
the accuracy of the data and failure to timely and accurately submit required information may result in civil monetary penalties.
Health Insurance Portability and Accountability
Act
Besides enacting the program integrity provisions
described above, HIPAA, also created a new set of privacy and security requirements. As amended by the Health Information Technology
for Economic and Clinical Health Act, and implementing regulations thereunder, HIPAA requires certain healthcare providers, health
plans and healthcare clearinghouses who conduct specified electronic healthcare transactions (“covered entities”),
as well as their independent contractors and agents who conduct certain activities involving protected health information on their
behalf (“business associates”) to comply with enumerated requirements relating to the privacy, security and transmission
of protected health information. Failure to comply with HIPAA can result in corrective action, as well as civil fines and penalties
and government oversight. Among other changes, HITECH made HIPAA security standards directly applicable to business associates,
increased the tiered civil and criminal fines and penalties that may be imposed against covered entities, business associates and
possibly other persons, and gave state attorneys general new authority to file actions to enforce HIPAA. Further, the breach notification
rule implemented under HITECH requires covered entities to notify affected individuals, the U.S. Department of Health and Human
Services Office of Civil Rights (“OCR”), the agency that enforces HIPAA, and for breaches affecting more than 500 individuals,
the media, of any breaches of unsecured protected health information. HIPAA does not create a private right of action for individuals,
though individuals may submit complaints related to HIPAA to OCR.
Legislative Activities Aimed at Controlling
Drug Costs
In the United States, there have been, and
continue to be proposed and enacted legislation at the federal and state levels designed to, among other things, bring more transparency
to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare,
and reform government program reimbursement methodologies for drugs. For example, in July 2021, the Biden administration released
an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription
drugs. In response to Biden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services (“HHS”)
released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety
of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance
these principles. In addition, the Inflation Reduction Act (“IRA”) passed on August 16, 2022. The IRA, among other
things, (1) directs HHS to negotiate the price of certain highly-utilized single-source drugs and biologics covered under Medicare
and (2) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions
will take effect progressively starting in fiscal year 2023, although they may be subject to legal challenges. It is currently
unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. Further, the
Biden administration released an additional executive order on October 14, 2022, directing HHS to submit a report within 90 days
on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models for lowering drug costs for
Medicare and Medicaid beneficiaries. We expect that additional U.S. federal healthcare reform measures will be adopted in the future,
any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could
result in reduced demand for our product candidates or additional pricing pressures.
Research and Development Expenses
Historically, a significant portion of our
operating expenses has related to research and development. See our Consolidated Financial Statements contained elsewhere in this
Annual Report for costs and expenses related to research and development, and other financial information for fiscal years 2023,
2022 and 2021.
Scientific Advisors
We are advised by
scientists and physicians with experience relevant to our Company and our product candidates. Our scientific advisors include
clinicians and scientists who are affiliated with a number of highly regarded medical institutions.
Employees
We currently have approximately forty full-time
employees, and we retain several independent contractors on a regular or as-needed basis. We believe that we have good relations
with our employees.
Available Information
Our internet website address is www.anavex.com.
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed
or furnished pursuant to section 13(a) or 15(d) of the Exchange Act are available there free of charge. We include our website
address in this report only as an inactive textual reference and do not intend it to be an active link to our website. The contents
of our website are not incorporated into this report.
ITEM 1A. RISK
FACTORS
Risk Factor Summary
The following is a summary of the risks and
uncertainties that could cause our business, financial condition or operating results to be harmed. We encourage you to carefully
review the full risk factors contained in this report in their entirety for additional information regarding these risks and uncertainties.
| ● | Our
history
of
losses
and
no
revenue
raises
a
risk
regarding
our
ability
to
continue
as
a
going
concern
in
the
future; |
| ● | We
have
a
very
limited
relevant
operating
history
upon
which
an
evaluation
of
our
performance
and
prospects
can
be
made.
We
may
never
be
able
to
successfully
develop
marketable
products
or
generate
any
revenue.
If
we
cannot
generate
sufficient
revenues,
we
may
suspend
or
cease
operations; |
| ● | Our
research
and
development
plans
will
require
substantial
additional
future
funding.
We
may
be
unable
to
raise
capital
when
needed,
which
would
force
us
to
delay,
reduce
or
eliminate
our
research
and
development
activities; |
| ● | Even
if
our
products
are
approved,
we
may
not
be
able
to
generate
significant
revenues
from
or
successfully
commercialize
them,
which
will
adversely
affect
our
financial
results
and
financial
condition
and
we
will
have
to
delay
or
terminate
some
or
all
of
our
research
and
development
plans
which
may
force
us
to
cease
operations; |
| ● | If
we
or
any
companion
diagnostic
collaborator
of
ours
are
unable
to
timely
develop
and
obtain
regulatory
approval
for
companion
diagnostic
tests
for
our
drug
candidates,
we
may
not
realize
the
commercial
potential
of
our
drug
candidates; |
| ● | Regulatory
authorities
may
not
accept
data
from
our
trials
conducted
outside
the
United
States; |
| ● | Fast
Track
designation
or
breakthrough
therapy
designation
that
we
have
received
or
may
seek
out
may
not
actually
lead
to
a
faster
FDA
review
and
approval
process; |
| ● | We
may
be
unable
to
maintain
any
benefits
associated
with
orphan
drug
designation,
including
market
exclusivity; |
| ● | If
we
fail
to
demonstrate
efficacy
in
our
non-clinical
studies
and
clinical
trials
our
future
business
prospects,
financial
condition
and
operating
results
will
be
materially
adversely
affected; |
| ● | If
we
do
not
obtain
the
support
of
qualified
scientific
collaborators,
our
revenue,
growth
and
profitability
will
likely
be
limited,
which
would
have
a
material
adverse
effect
on
our
business; |
| ● | We
may
not
be
able
to
develop,
market
or
generate
sales
of
our
products
to
the
extent
anticipated.
Our
business
may
fail
and
investors
could
lose
all
their
investment
in
our
Company; |
| ● | None
of
our
potential
drug
compounds
may
reach
the
commercial
market
and
our
business
may
fail; |
| ● | Our
technologies
and
future
products
may
be
rendered
undesirable
or
obsolete
if
our
competitors
succeed
in
developing
products
and
technologies
faster
or
that
are
more
effective
or
with
a
better
profile
than
our
own,
or
if
scientific
developments
change
our
understanding
of
the
potential
scope
and
utility
of
our
potential
products; |
| ● | We
have
advanced
our
research
and
development
efforts
on
the
treatment
of
neurodegenerative
and
central
nervous
system,
or
CNS,
disorders,
a
field
that
has
seen
very
limited
success
in
product
development; |
| ● | Our
reliance
on
third
parties
may
result
in
delays
in
completing,
or
a
failure
to
complete,
non-clinical
testing
or
clinical
trials
if
they
fail
to
perform
under
our
agreements
with
them
or
non-compliance
with
regulations; |
| ● | If
we
fail
to
compete
with
respect
to
partnering,
licensing,
mergers,
acquisitions,
joint
venture
and
other
collaboration
opportunities,
our
ability
to
research
and
develop
our
potential
drug
compounds
may
be
limited; |
| ● | The
use
of
any
of
our
products
in
clinical
trials
may
expose
us
to
liability
claims,
causing
our
business
to
suffer; |
| ● | If
we
are
unable
to
safeguard
against
security
breaches
with
respect
to
our
information
systems,
our
business
may
be
adversely
affected; |
| ● | Continuing
regulatory
obligations
and
ongoing
regulatory
review
may
result
in
additional
expense.
Our
compounds
could
be
subject
to
restrictions
on
marketing
or
withdrawal
from
the
market,
and
we
may
be
subject
to
penalties
when
and
if
any
of
them
are
approved; |
| ● | We
receive
Australian
government
research
and
development
income
tax
incentive
refunds.
Loss
of
access
to
such
incentives
could
have
a
negative
effect
on
our
future
cash
flows
and
the
funding
of
future
research
and
development
projects; |
| ● | Operating
our
business
internationally
carries
various
risks
which
could
materially
adversely
affect
our
business; |
| ● | Our
ability
to
use
our
net
operating
loss
carryforwards
and
tax
credit
carryforwards
may
be
subject
to
limitation; |
| ● | Healthcare
laws
and
regulations
could
expose
us
to
criminal
sanctions,
civil
and
administrative
penalties,
contractual
damages,
reputational
harm
and
diminished
profits
and
future
earnings,
among
other
penalties; |
| ● | We
expect
current
and
future
legislation
affecting
the
pharmaceutical
industry,
including
drug
pricing
reform,
to
impact
our
business
generally,
which
could
adversely
affect
our
business
operations; |
| ● | Failure
to
obtain
or
maintain
adequate
coverage
and
reimbursement
for
our
product
candidates,
if
approved,
could
limit
our
ability
to
market
those
products
and
decrease
our
ability
to
generate
product
revenue; |
| ● | Issuing
additional
shares
of
common
stock
will
result
in
the
dilution
of
our
existing
stockholders
and
may
cause
our
stock
price
to
fall.
A
decline
in
our
stock
price
could
affect
our
ability
to
raise
further
working
capital
and
adversely
affect
our
operations.
Raising
funds
at
lower
prices
would
severely
dilute
existing
or
future
investors; |
| ● | Our
stock
price
has
been
volatile
and
may
be
volatile
in
the
future
and
our
common
stock
may
become
the
target
of
a
“short
squeeze”; |
| ● | If
we
fail
to
maintain
an
effective
system
of
internal
control
over
financial
reporting,
we
may
not
be
able
to
accurately
report
our
financial
results
or
prevent
fraud.
Our
disclosure
controls
and
procedures
may
not
prevent
or
detect
all
errors
or
acts
of
fraud.
As
a
result,
stockholders
could
lose
confidence
in
our
financial
and
other
public
reporting,
which
would
harm
our
business
and
the
trading
price
of
our
common
stock; |
| ● | Patent
terms
may
be
inadequate
to
protect
our
competitive
position
on
our
product
candidates.
If
we
are
unable
to
obtain
and
maintain
sufficient
intellectual
property
protection
for
our
product
candidates,
our
competitors
could
develop
and
commercialize
product
candidates
similar
or
identical
to
ours,
and
our
ability
to
successfully
commercialize
our
product
candidates
may
be
impaired; |
| ● | If
we
fail
to
comply
with
our
obligations
in
intellectual
property
licensing
agreements
or
experience
disruptions
to
our
business
relationships
with
our
licensors,
we
could
lose
important
intellectual
property
rights.
If
we
are
unable
to
protect
the
confidentiality
of
our
trade
secrets,
our
business
would
be
harmed; |
| ● | Intellectual
property
infringement
claims
may
adversely
affect
our
development
and
commercialization
efforts; |
| ● | We
may
be
subject
to
claims
that
our
employees,
consultants
or
independent
contractors
have
wrongfully
used
or
disclosed
confidential
information
of
third
parties
or
that
our
employees
have
wrongfully
used
or
disclosed
alleged
trade
secrets
of
their
former
employers; |
| ● | Obtaining
and
maintaining
our
patent
protection
depends
on
compliance
with
various
requirements
imposed
by
governmental
patent
agencies.
Changes
in
patent
law
could
impair
our
ability
to
protect
our
product
candidates; |
| ● | We
may
become
involved
in
lawsuits
to
protect
or
enforce
our
patents
or
other
intellectual
property;
and |
| ● | We
may
fail
to
protect
our
intellectual
property
rights
or
the
confidentiality
of
our
trade
secrets. |
In addition to other information in this Annual
Report on Form 10-K, the following risk factors should be carefully considered in evaluating our business because such factors
may have a significant impact on our business, operating results, liquidity and financial condition. As a result of the risk factors
set forth below, actual results could differ materially from those projected in any forward-looking statements. Additional risks
and uncertainties not presently known to us, or that we currently consider to be immaterial, may also impact our business, operating
results, liquidity and financial condition. If any such risks occur, our business, operating results, liquidity and financial condition
could be materially affected in an adverse manner. Under such circumstances, the trading price of our securities could decline,
and you may lose all or part of your investment.
Risks Related to our Company
We have had a history of losses and no
revenue, which raises a risk regarding our ability to continue as a going concern in the future.
Since inception through September 30, 2023,
we have accumulated a deficit of approximately $293 million. We can offer no assurance that we will ever operate profitably or
that we will generate positive cash flow in the future. To date, we have not generated any revenues from our operations. Our history
of losses and no revenues creates a greater risk of our continued ability to continue as a going concern in the future. As a result,
our management expects the business to continue to experience negative cash flows for the foreseeable future and cannot predict
when, if ever, our business might become profitable. We will need to raise additional funds, and such funds may not be available
on commercially acceptable terms, if at all. If we are unable to raise funds on acceptable terms, we may not be able to execute
our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements. This
may seriously harm our business, financial condition and results of operations.
We are an early clinical stage pharmaceutical
research and development company and may never be able to successfully develop marketable products or generate any revenue. We
have a very limited relevant operating history upon which an evaluation of our performance and prospects can be made. There is
no assurance that our future operations will result in profits. If we cannot generate sufficient revenues, we may suspend or cease
operations.
We are an early clinical stage company and
have not generated any revenues to date and have no operating history. Moreover, we cannot be certain that our research and development
efforts will be successful or, if successful, that our potential drug compounds will ever be approved for sale to pharmaceutical
companies or generate commercial revenues. We have no relevant operating history upon which an evaluation of our performance and
prospects can be made. We are subject to all of the business risks associated with a new enterprise, including, but not limited
to, risks of unforeseen capital requirements, failure of potential drug compounds either in non-clinical testing or in clinical
trials, failure to establish business relationships and competitive disadvantages against larger and more established companies.
If we fail to become profitable, we may suspend or cease operations.
We will need additional funding and may
be unable to raise additional capital when needed, which would force us to delay, reduce or eliminate our research and development
activities.
To date, we have funded our operations primarily
through private placement of our equity securities, and grants or draws under our “at the market offering” in connection
with an Amended and Restated Sales Agreement, dated May 1, 2020, with Cantor Fitzgerald & Co. and SVB Leerink LLC (the “Sales
Agents”), pursuant to which we may offer and sell shares of common stock registered under an effective registration statement
from time to time through the Sales Agents or the through the Purchase Agreement with Lincoln Park Capital Fund, LLC (“Lincoln
Park”) pursuant to which the Company may direct Lincoln Park to purchase shares of common stock registered under an effective
registration statement. The Company currently does not have access to sell shares under the Sales Agreement. We will need to raise
additional funding and the current economic conditions may have a negative impact on our ability to raise additional needed capital
on terms that are favorable to our Company or at all. We may not be able to generate significant revenues for several years, if
at all. Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through equity or debt
financing. We cannot be certain that additional funding will be available on acceptable terms, or at all. If adequate funds are
not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research and development activities.
Risks Related to our Business
Even if we are able to develop our potential
drug compounds, we may not be able to receive regulatory approval, or if approved, we may not be able to generate significant revenues
or successfully commercialize our products, which will adversely affect our financial results and financial condition and we will
have to delay or terminate some or all of our research and development plans which may force us to cease operations.
All of our potential drug compounds are exclusively
focused on SIGMAR1 which has not previously been the subject of any approved drug products and will require extensive additional
research and development, including non-clinical testing and clinical trials, as well as regulatory approvals, before we can market
them. In particular, human therapeutic products are subject to rigorous non-clinical and clinical testing and other approval procedures
of the FDA and similar regulatory authorities in other countries. Various federal statutes and regulations also govern or influence
testing, manufacturing, safety, labeling, storage, and record-keeping related to such products and their marketing. We cannot predict
if or when any of the potential drug compounds we intend to develop will be approved for marketing. There are many reasons that
we may fail in our efforts to develop our potential drug compounds. These include:
| |
● |
the
possibility that non-clinical testing or clinical trials may show that our potential drug compounds are ineffective and/or
cause harmful side effects; |
| |
● |
regulators
may not authorize us to commence or continue a clinical trial or may impose a clinical hold or may limit the conduct of a
clinical trial through the imposition of a partial clinical hold; |
| |
● |
the
number of patients required for clinical trials for our drug candidates may be larger than we anticipate, enrollment in these
clinical trials may be slower than we anticipate, participants may drop out of these clinical trials at a higher rate than
we anticipate or the duration of these clinical trials may be longer than we anticipate; |
| |
● |
our
third-party contractors, including investigators, may fail to meet their contractual obligations to us in a timely manner,
or at all, or may fail to comply with regulatory requirements; |
| |
● |
our
potential drug compounds may prove to be too expensive to manufacture or administer to patients; |
| |
● |
our
potential drug compounds may fail to receive necessary regulatory approvals from the United States Food and Drug Administration
or foreign regulatory authorities in a timely manner, or at all; |
| |
● |
even
if our potential drug compounds are approved, we may not be able to produce them in commercial quantities or at reasonable
costs; |
| |
● |
even
if our potential drug compounds are approved, they may not achieve commercial acceptance; |
| |
● |
regulatory
or governmental authorities may apply restrictions to any of our potential drug compounds, which could adversely affect their
commercial success; and |
| |
● |
the
proprietary rights of other parties may prevent us or our potential collaborative partners from marketing our potential drug
compounds. |
If we fail to develop our potential drug compounds,
our financial results and financial condition will be adversely affected, we will have to delay or terminate some or all of our
research and development plans and may be forced to cease operations.
Our research and development plans will
require substantial additional future funding which could impact our operations and financial condition.
It will take several years before we can develop
potentially marketable products, if at all. Our research and development plans will require substantial additional capital, arising
from costs to:
| |
● |
conduct
research, non-clinical testing and human clinical trials; |
| |
● |
establish
pilot scale and commercial scale manufacturing processes and facilities; and |
| |
● |
establish
and develop quality control, regulatory, marketing, sales, finance and administrative capabilities to support these programs. |
Our future operating and capital needs will
depend on many factors, including:
| |
● |
the
pace of scientific progress in our research and development programs and the magnitude of these programs; |
| |
● |
the
scope and results of pre-clinical testing and human clinical trials; |
| |
● |
the
time and costs involved in obtaining regulatory approvals; |
| |
● |
the
time and costs involved in preparing, filing, prosecuting, securing, maintaining and enforcing patents; |
| |
● |
competing
technological and market developments; |
| |
● |
our
ability to establish additional collaborations; |
| |
● |
changes
in our existing collaborations; |
| |
● |
the
cost of manufacturing scale-up; and |
| |
● |
the
effectiveness of our commercialization activities. |
We base our outlook regarding the need for
funds on many uncertain variables. Such uncertainties include the success of our research initiatives, regulatory approvals, the
timing of events outside our direct control such as negotiations with potential strategic partners and other factors. Any of these
uncertain events can significantly change our cash requirements as they determine such one-time events as the receipt or payment
of major milestones and other payments.
Additional funds may be required to support
our operations and if we are unable to obtain them on favorable terms, we may be required to cease or reduce certain further research
and development programs of our drug product platform, sell some or all our intellectual property, merge with another entity or
scale back operations.
If we or any companion diagnostic collaborator
of ours are unable to successfully develop and obtain regulatory approval for companion diagnostic tests for our drug candidates,
or experience significant delays in doing so, we may not realize the commercial potential of our drug candidates.
We analyze genomic data from clinical trials
to identify biomarkers, which we use in the analysis of our clinical trials.
Identification of these patients will require
the use and development of companion diagnostics. According to the FDA’s 2014 guidance document on In Vitro Companion Diagnostic
Devices, for novel therapeutic products that depend on the use of a diagnostic test and where the diagnostic device could be essential
for the safe and effective use of the corresponding therapeutic product, the premarket application for the companion diagnostic
device should be developed and approved or cleared contemporaneously with the therapeutic.
We do not have experience or capabilities in
developing or commercializing diagnostics. It may be necessary to resolve issues such as selectivity/specificity, analytical validation,
reproducibility, or clinical validation of companion diagnostics during the development and regulatory approval processes. Moreover,
even if data from preclinical studies and early clinical trials appear to support development of a companion diagnostic for a drug
candidate, data generated in later clinical trials may fail to support the analytical and clinical validation of the companion
diagnostic. We and our future collaborators may encounter difficulties in developing, obtaining regulatory approval for, manufacturing
and commercializing companion diagnostics similar to those we face with respect to our drug candidates, including issues with achieving
regulatory clearance or approval, production of sufficient quantities at commercial scale and with appropriate quality standards,
and in gaining market acceptance. If we are unable to successfully develop companion diagnostics for our drug candidates, or experience
delays in doing so, the development of these drug candidates may be adversely affected, these drug candidates may not obtain marketing
approval, and we may not realize the full commercial potential of any of these therapeutics that have or may obtain marketing approval.
We may not be able to enter into arrangements with another diagnostic company to develop and obtain regulatory approval for of
an alternative diagnostic test for use in connection with the development and commercialization of our drug candidates or do so
on commercially reasonable terms, which could adversely affect and/or delay the development or commercialization of our therapeutic
candidates or therapeutics.
Companion diagnostics are subject to regulation
by the FDA and comparable foreign regulatory authorities as medical devices and will likely require separate regulatory approval
prior to commercialization. If we or third parties are unable to successfully develop companion diagnostics for our drug candidates,
or experience delays in doing so:
| |
● |
the
development of these drug candidates may be delayed because it may be difficult to identify patients for enrollment in our
clinical trials in a timely manner; |
| |
● |
these
drug candidates may not receive marketing approval if their safe and effective use depends on a companion diagnostic; and |
| |
● |
we
may not realize the full commercial potential of these drug candidates that receive marketing approval if, among other reasons,
we are unable to appropriately identify patients or types of tumors targeted by these drug candidates. |
Even if our drug candidates and any associated
companion diagnostics are approved for marketing, the need for companion diagnostics may slow or limit adoption of our drug candidates.
Our drug candidates may be perceived negatively compared to alternative treatments that do not require the use of companion diagnostics,
either due to the additional cost of the companion diagnostic or the need to complete additional prior to administering our drug
candidates.
If any of these events were to occur, our business
and growth prospects would be harmed materially.
All but one of our clinical
trials to date have been conducted outside the United States,
and the FDA and other foreign
regulatory authorities may not accept
data from such trials.
The acceptance
of study data from
clinical trials conducted
outside the United
States by the FDA may
be subject to certain conditions
or may not be accepted at all.
In cases where data from
foreign clinical trials
are intended to serve
as the sole basis
for regulatory approval in the
United States, the
FDA will generally
not approve the application
on the basis of foreign
data alone unless (i)
the data are
applicable to the United
States population and United
States medical practice;
(ii) the trials
were performed by clinical investigators
of recognized competence and pursuant
to good clinical practice regulations;
and (iii) the data
may be considered valid
without the need for
an on-site inspection by the
FDA, or if the
FDA considers such inspection to be
necessary, the FDA
is able to validate
the data through
an on-site inspection or other
appropriate means. Many foreign
regulatory bodies have similar
approval requirements. In addition,
such foreign trials would be subject
to the applicable local
laws of the foreign jurisdictions
where the trials are conducted.
There can be no assurance that
the FDA or any other
foreign regulatory authority
will accept data from
trials conducted outside
of the United States
or the applicable jurisdiction.
If the FDA
or any comparable foreign regulatory authority
does not accept such data, it
would result in the need for
additional trials, which would be costly
and time-consuming and delay aspects
of our business plan, and which
may result in our product candidates
not receiving approval or clearance
for commercialization in the
applicable jurisdiction.
We have received Fast Track designation
for one of our compounds and may seek such designation or breakthrough therapy and priority review for other compounds in the future.
Fast Track designation or breakthrough therapy designation may not actually lead to a faster FDA review and approval process.
For some of our compounds, including ANAVEX®2-73,
we hope to benefit from the FDA’s fast track and priority review programs. In February 2020, the FDA granted Fast Track designation
for the ANAVEX®2-73 clinical development program for the treatment of Rett syndrome. Programs with Fast Track designation
may benefit from early and frequent communications with the FDA, potential priority review and the ability to submit a rolling
application for regulatory review. Fast Track designation applies to both the product candidate and the specific indication for
which it is being studied. If any of our compounds receive Fast Track designation but do not continue to meet the criteria for
Fast Track designation, or if our clinical trials are delayed, suspended or terminated, or put on clinical hold due to unexpected
adverse events or issues with clinical supply, we will not receive the benefits associated with the Fast Track program. Furthermore,
Fast Track designation does not change the standards for approval. The receipt of Fast Track designation for a compound may not
result in a faster development or regulatory review or approval process compared to products considered for approval under conventional
FDA procedures and does not assure ultimate approval by the FDA. In addition, even if any product candidate qualifies for Fast
Track designation, the FDA may later decide that the product candidates no longer meet the conditions for qualification or decide
that the time period for FDA review or approval will not be shortened. Fast Track designation alone does not guarantee qualification
for the FDA’s priority review procedures.
Under FDA policies, a compound is eligible
for priority review, or review within a six-month time frame from the time a complete NDA is accepted for filing, if the compound
provides a significant improvement compared to marketed drugs in the treatment, diagnosis or prevention of a disease. The FDA determines
whether a drug qualifies for Priority Review after an NDA for such drug is submitted to the FDA. Therefore, until NDAs are submitted
for our compounds, we cannot be assured that they will be granted Priority Review. Additionally, even if Priority Review is granted
for one of our compounds, the FDA does not always meet its six-month PDUFA goal date for Priority Review and the review process
is often extended by FDA requests for additional information or clarification.
We may seek Breakthrough Therapy designation
for one or more of our current or future compounds. Designation as a Breakthrough Therapy is largely within the discretion of the
FDA. Accordingly, even if we believe that a compound meets the criteria for designation as a Breakthrough Therapy, the FDA may
disagree and instead determine not to make such designation. In any event, the receipt of a Breakthrough Therapy designation for
a product candidate may not result in a faster development process, review or approval compared to candidate products considered
for approval under non-expedited FDA review procedures and does not assure ultimate approval by the FDA. In addition,
even if one or more compounds qualify as breakthrough therapies, the FDA may later decide that the product no longer meets the
conditions for qualification and revoke the designation.
Fast track or breakthrough therapy designation
for our compounds may not actually lead to a faster review process, and a delay in the review process or in the approval of our
compounds will delay revenue from their potential sales and will increase the capital necessary to fund these compound development
programs.
We have received orphan drug designation for several of our
compounds, but we may be unable to maintain any benefits associated with orphan drug designation, including market exclusivity.
Under the Orphan Drug Act, the FDA may grant
orphan designation to a drug intended to treat a rare disease or condition or for which there is no reasonable expectation that
the cost of developing and making available in the United States a drug for a disease or condition will be recovered from sales
in the United States for that drug. If a product that has orphan drug designation subsequently receives the first FDA approval
for the indication for which it has such designation, the product is entitled to orphan product exclusivity, which means that the
FDA may not approve any other applications, including a full NDA, to market the same drug or biologic for the same indication for
seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity.
We have received orphan drug designation for
several of our compounds, but we may not be able to obtain or maintain orphan drug exclusivity in the United States for hose compounds.
We may not be the first to obtain marketing approval of any compound for which we have obtained orphan drug designation for the
orphan-designated indication due to the uncertainties associated with developing pharmaceutical products. In addition, exclusive
marketing rights in the United States may be limited if we seek FDA marketing approval for an indication broader than the orphan
designated indication. Additionally, any compound with orphan drug designation may lose such designation if the FDA later determines
that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the
product to meet the needs of patients with the rare disease or condition. Even after an orphan drug is approved, the FDA can subsequently
approve the same drug with the same active moiety for the same condition if the FDA concludes that the later drug is clinically
superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In addition, others
may obtain orphan drug exclusivity for products addressing the same diseases or conditions as products we are developing, thus
limiting our ability to compete in the markets addressing such diseases or conditions for a significant period of time. Orphan
drug designation neither shortens the development time or regulatory review time of a drug nor gives the product candidate any
advantage in the regulatory review or approval process or entitles the product candidate to priority review.
If we fail to demonstrate efficacy in
our non-clinical studies and clinical trials our future business prospects, financial condition and operating results will be materially
adversely affected.
The success of our research and development
efforts will be greatly dependent upon our ability to demonstrate potential drug compound efficacy in non-clinical studies, as
well as in clinical trials. Non-clinical studies involve testing potential drug compounds in appropriate non-human disease models
to demonstrate efficacy and safety. Regulatory agencies evaluate these data carefully before they will approve clinical testing
in humans. If certain non-clinical data reveals potential safety issues or the results are inconsistent with an expectation of
the potential drug compound’s efficacy in humans, the regulatory agencies may require additional more rigorous testing before
allowing human clinical trials. This additional testing will increase program expenses and extend timelines. We may decide to suspend
further testing on our potential drug compounds if, in the judgment of our management and advisors, the non-clinical test results
do not support further development.
Moreover, success in non-clinical testing and
early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the results of
later clinical trials will replicate the results of prior clinical trials and non-clinical testing. The clinical trial process
may fail to demonstrate that our potential drug compounds are safe for humans and effective for indicated uses. This failure would
cause us to abandon a drug candidate and may delay development of other potential drug compounds. Any delay in, or termination
of, our non-clinical testing or clinical trials will delay the filing of an IND and NDA with the FDA or the equivalent applications
with pharmaceutical regulatory authorities outside the United States and, ultimately, our ability to commercialize our potential
drug compounds and generate product revenues. In addition, we expect that our early clinical trials will involve small patient
populations. Because of the small sample size, the results of these early clinical trials may not be indicative of future results.
Also, the IND process may be extremely costly and may substantially delay the development of our potential drug compounds. Moreover,
positive results of non-clinical tests will not necessarily indicate positive results in subsequent clinical trials.
Following successful non-clinical testing,
potential drug compounds will need to be tested in a clinical development program to provide data on safety and efficacy prior
to becoming eligible for product approval and licensure by regulatory agencies. From the first human trial through to regulatory
approval can take many years and 10-12 years is not unusual for certain compounds.
If any of our future clinical development potential
drug compounds become the subject of problems, our ability to sustain our development programs will become critically compromised.
For example, efficacy or safety concerns may arise, whether or not justified, that could lead to the suspension or termination
of our clinical programs. Examples of problems that could arise include, among others:
| |
● |
efficacy
or safety concerns with the potential drug compounds, even if not justified; |
| |
● |
manufacturing
difficulties or concerns; |
| |
● |
regulatory
proceedings subjecting the potential drug compounds to potential recall; |
| |
● |
publicity
affecting doctor prescription or patient use of the potential drug compounds; |
| |
● |
pressure
from competitive products; or |
| |
● |
introduction
of more effective treatments. |
Each clinical phase is designed to test attributes
of the drug and problems that might result in the termination of the entire clinical plan can be revealed at any time throughout
the overall clinical program. The failure to demonstrate efficacy in our clinical trials would have a material adverse effect on
our future business prospects, financial condition and operating results.
If we do not obtain the support of qualified
scientific collaborators, our revenue, growth and profitability will likely be limited, which would have a material adverse effect
on our business.
We will need to establish relationships with
leading scientists and research institutions. We believe that such relationships are pivotal to establishing products using our
technologies as a standard of care for various indications. Additionally, although in discussion, there is no assurance that our
current research partners will continue to work with us or that we will be able to attract additional research partners. If we
are not able to establish scientific relationships to assist in our research and development, we may not be able to successfully
develop our potential drug compounds. If this happens, our business will be adversely affected.
We may not be able to develop, market
or generate sales of our products to the extent anticipated. Our business may fail and investors could lose all their investment
in our Company.
Assuming that we are successful in developing
our potential drug compounds and receiving regulatory clearances to market our products, our ability to successfully penetrate
the market and generate sales of those products may be limited by a number of factors, including the following:
| |
● |
If
our competitors receive regulatory approvals for and begin marketing similar products in the United States, the European Union,
Japan and other territories before we do, greater awareness of their products as compared to ours will cause our competitive
position to suffer; |
| |
● |
Information
from our competitors or the academic community indicating that current products or new products are more effective or offer
compelling other benefits than our future products could impede our market penetration or decrease our future market share;
and |
| |
● |
The
pricing and reimbursement environment for our future products, as well as pricing and reimbursement decisions by our competitors
and by payers, may have an effect on our revenues. |
If this happens, our business will be adversely
affected.
None of our potential drug compounds
may reach the commercial market for a number of reasons and our business may fail.
Successful research and development of pharmaceutical
products is high risk. Most products and development candidates fail to reach the market. Our success depends on the discovery
of new drug compounds that we can commercialize. It is possible that our products may never reach the market for a number of reasons.
They may be found ineffective or may cause harmful side-effects during non-clinical testing or clinical trials or fail to receive
necessary regulatory approvals. We may find that certain products cannot be manufactured at a commercial scale and, therefore,
they may not be economical to produce. Our potential products could also fail to achieve market acceptance or be precluded from
commercialization by proprietary rights of third parties. Our patents, patent applications, trademarks and other intellectual property
may be challenged, and this may delay or prohibit us from effectively commercializing our products. Furthermore, we do not expect
our potential drug compounds to be commercially available for a number of years, if at all. If none of our potential drug compounds
reach the commercial market, our business will likely fail and investors will lose all of their investment in our Company. If this
happens, our business will be adversely affected.
If our competitors succeed in developing
products and technologies faster or that are more effective or with a better profile than our own, or if scientific developments
change our understanding of the potential scope and utility of our potential products, then our technologies and future products
may be rendered undesirable or obsolete.
We face significant competition from industry
participants that are pursuing technologies in similar disease states to those that we are pursuing and are developing pharmaceutical
products that are competitive with our products. Nearly all of our industry competitors have greater capital resources, larger
overall research and development staffs and facilities, and a longer history in drug discovery and development, obtaining regulatory
approval and pharmaceutical product manufacturing and marketing than we do. With these additional resources, our competitors may
be able to respond to the rapid and significant technological changes in the biotechnology and pharmaceutical industries faster
than we can. Our future success will depend in large part on our ability to maintain a competitive position with respect to these
technologies. Rapid technological development, as well as new scientific developments, may result in our products becoming obsolete
before we can recover any of the expenses incurred to develop them. For example, changes in our understanding of the appropriate
population of patients who should be treated with a targeted therapy like we are developing may limit the drug’s market potential
if it is subsequently demonstrated that only certain subsets of patients should be treated with the targeted therapy.
We have advanced our research and development
efforts on the treatment of neurodegenerative and central nervous system, or CNS, disorders, a field that has seen very limited
success in product development.
We have advanced our
research and development efforts on addressing neurodegenerative, neurodevelopmental and CNS disorders. Collectively, efforts by
pharmaceutical companies in the field of neurodegenerative, neurodevelopmental and CNS disorders have seen very limited
successes in product development. The development of neurodegenerative and CNS therapies presents unique challenges, including
an imperfect understanding of the biology, the presence of the blood brain barrier, or BBB, that can restrict the flow of drugs
to the brain, a frequent lack of translatability of preclinical study results in subsequent clinical trials and dose selection,
and the product candidate having an effect that may be too small to be detected using the outcome measures selected in clinical
trials or if the outcomes measured do not reach statistical significance.
Our reliance on third parties, such as
university laboratories, contract manufacturing organizations and contract or clinical research organizations, may result in delays
in completing, or a failure to complete, non-clinical testing or clinical trials if they fail to perform under our agreements with
them or non-compliance with regulations.
In the course of product development, we may
engage university laboratories, other biotechnology companies or contract or clinical manufacturing organizations to manufacture
drug material for us to be used in non-clinical and clinical testing and contract research organizations to conduct and manage
non-clinical studies and clinical trials. If we engage these organizations to help us with our non-clinical and clinical programs,
many important aspects of this process have been and will be out of our direct control. If any of these organizations we may engage
in the future fail to perform their obligations under our agreements with them or fail to perform non-clinical testing and/or clinical
trials in a satisfactory manner, we may face delays in completing our clinical trials, as well as commercialization of any of our
potential drug compounds. Furthermore, any loss or delay in obtaining contracts with such entities may also delay the completion
of our clinical trials, regulatory filings and the potential market approval of our potential drug compounds.
In addition, any of these third parties may
engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements. Misconduct
by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that
violate the regulations of any regulatory authorities, including those laws requiring the reporting of true, complete and accurate
information to such authorities; healthcare fraud and abuse laws and regulations in the United States and abroad; or laws that
require the reporting of financial information or data accurately. It is not always possible to identify and deter misconduct by
employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling
unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming
from a failure to comply with these laws or regulations.
If we fail to compete successfully with
respect to partnering, licensing, mergers, acquisitions, joint venture and other collaboration opportunities, we may be limited
in our ability to research and develop our potential drug compounds.
Our competitors compete with us to attract
established biotechnology and pharmaceutical companies or organizations for partnering, licensing, mergers, acquisitions, joint
ventures or other collaborations. Collaborations include contracting with academic research institutions for the performance of
specific scientific testing. If our competitors successfully enter into partnering arrangements or license agreements with academic
research institutions, we will then be precluded from pursuing those specific opportunities. Since each of these opportunities
is unique, we may not be able to find a substitute. Other companies have already begun many drug development programs, which may
target diseases that we are also targeting, and have already entered into partnering and licensing arrangements with academic research
institutions, reducing the pool of available opportunities.
Universities and public and private research
institutions also compete with us. While these organizations primarily have educational or basic research objectives, they may
develop proprietary technology and acquire patent applications and patents that we may need for the development of our potential
drug compounds. In some instances, we will attempt to license this proprietary technology, if available. These licenses may not
be available to us on acceptable terms, if at all. If we are unable to compete successfully with respect to acquisitions, joint
venture and other collaboration opportunities, we may be limited in our ability to develop new products.
The use of any of our products in clinical
trials may expose us to liability claims, which may cost us significant amounts of money to defend against or pay out, causing
our business to suffer.
The nature of our business exposes us to potential
liability risks inherent in the testing, manufacturing and marketing of our products. We currently have one drug compound in clinical
trials, however, when any of our products enter clinical trials or become marketed products, they could potentially harm people
or allegedly harm people possibly subjecting us to costly and damaging product liability claims. Some of the patients who participate
in clinical trials are already ill when they enter a trial or may intentionally or unintentionally fail to meet the exclusion criteria.
The waivers we obtain may not be enforceable and may not protect us from liability or the costs of product liability litigation.
Although we intend to obtain product liability insurance, which we believe is adequate, we are subject to the risk that our insurance
will not be sufficient to cover claims. The insurance costs along with the defense or payment of liabilities above the amount of
coverage could cost us significant amounts of money and management distraction from other elements of the business, causing our
business to suffer.
If our information systems or data, or
those of third parties upon whom we rely, are or were compromised, our business may be adversely affected.
In the course of our business, we, or third
parties upon which we rely, may gather, collect, receive, use, transmit, store/retain or dispose of data and confidential information
(such as confidential employee information or health-related data), sensitive data, intellectual property and trade secrets.
Cyberattacks,
malicious internet-based activity, online and offline fraud and other similar activities threaten the confidentiality, integrity,
and availability of our sensitive information and information technology systems, and those of the third parties upon which we
rely. We, and the third parties upon which we may rely, may be subject to a variety of these evolving threats.
Although we
endeavor to protect confidential information through the implementation of security technologies, processes and procedures, it
is possible that an individual or group could defeat security measures and access sensitive information about our business and
employees. The existence of a remote workforce also poses increased risks to our information technology
systems and data, as more of our employees work from home, utilizing network connections outside our premises.
Any misappropriation, loss or other unauthorized
disclosure of confidential information gathered, stored or used by us or by third parties on our behalf, could have a material
impact on the operation of our business, including damaging our reputation with our employees, third parties and investors. We
could also incur significant costs implementing additional security measures and organizational changes, implementing additional
protection technologies, training employees or engaging consultants.
Our
contracts with third parties upon which we may rely, may not contain limitations of liability, and even where they do, there can
be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages, or claims
related to our data privacy and security obligations. In addition, we could incur increased litigation as a result of any
potential cyber-security breach and our insurance coverage may not be adequate or sufficient in type
or amount to protect us from or to mitigate liabilities arising out of our privacy and security practices.
We are not aware that we have experienced any
material misappropriation, loss or other unauthorized disclosure of confidential or personally identifiable information as a result
of a cyber-security breach or other act, however, a cyber-security breach or other act and/or disruption to our information technology
systems could have a material adverse effect on our business, prospects, financial condition or results of operations.
Even if we receive regulatory approval
for one or more compounds, we will be subject to continuing regulatory obligations and ongoing regulatory review, which may result
in significant additional expense. Additionally, our compounds, if approved, could be subject to labeling and other restrictions
on marketing or withdrawal from the market, and we may be subject to penalties, if we fail to comply with regulatory requirements
or if we experience unanticipated problems with our compounds, when and if any of them are approved.
Following potential approval of any our compounds,
the FDA may impose significant restrictions on a drug’s indicated uses or marketing or require potentially costly and time-consuming
post-approval studies, post-market surveillance or clinical trials to monitor the safety and efficacy of the drug. The FDA may
also require a Risk Evaluation and Mitigation Strategy (“REMS”) as a condition of approval of one or more of our compounds,
which could include requirements for a medication guide, physician communication plans or additional elements to ensure safe use
of the drug. Additional REMS elements may include restricted distribution methods, patient registries and other risk minimization
tools.
In addition, if the FDA or a comparable foreign
regulatory authority approves one or more of our compounds, the manufacturing processes, labeling, packaging, distribution, adverse
event reporting, storage, advertising, promotion, import, export and recordkeeping for the approved drug will be subject to additional
and potentially extensive ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing
information and reports, establishment registration, as well as continued compliance with cGMPs and GCP requirements for any clinical
trials that we conduct post-approval. Later discovery of previously unknown problems with our products, including adverse events
of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply
with regulatory requirements, may result in, among other things:
| |
● |
restrictions
on the marketing or manufacturing of our products, withdrawal of the product from the market or voluntary or mandatory product
recalls; |
| |
● |
fines,
restitutions, disgorgement of profits or revenues, warning letters, untitled letters or holds on clinical trials; |
| |
● |
restrictions
on product distribution or use, or requirements to conduct post-marketing studies or clinical trials; |
| |
● |
product
seizure or detention, or refusal to permit the import or export of our products; |
| |
● |
injunctions
or the imposition of civil or criminal penalties; and |
| |
● |
refusal
by the FDA to approve pending applications or supplements to approved applications filed by us or suspension or revocation
of approvals. |
The occurrence of any event or penalty described
above may limit our ability to commercialize our compounds and generate revenue, and could require us to expend significant time
and resources in response or generate negative publicity.
If any of our compounds are approved, our product
labeling, advertising and promotion will also be subject to regulatory requirements and ongoing regulatory review. The FDA strictly
regulates the promotional claims that may be made about drug products. In particular, a drug may not be promoted for uses that
are not approved by the FDA as reflected in the drug’s approved labeling. If we receive marketing approval for a compound,
physicians may nevertheless lawfully prescribe it to their patients in a manner that is inconsistent with the approved label. While
the FDA recently clarified that mere knowledge that a physician is prescribing an approved drug for off label use is not sufficient
to constitute unlawful off-label promotion, if we are found to have actively promoted such off label uses, we may become subject
to significant liability under the FDCA. The federal government has levied large civil and criminal fines against companies for
alleged improper promotion and has enjoined several companies from engaging in off-label promotion. Additionally, promotion for
off label uses could result in significant liability under the False Claims Act. The FDA has also requested that companies enter
into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
The FDA’s and other regulatory authorities’
policies are subject to change at any time, and additional government regulations may be enacted that could prevent, limit or delay
regulatory approval of our compounds. If we are unable to timely adapt to changes in existing requirements or the adoption of new
requirements or policies, or if we are not able to maintain regulatory compliance post-marketing, we may lose any marketing approval
that we may have obtained, and we may not achieve or sustain profitability.
Finally, we cannot predict the likelihood,
nature or extent of government regulation that may arise from future legislation or administrative or executive action, either
in the United States or abroad. It is difficult to predict how any such legislative, administrative or executive actions will be
implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority. If these legislative
or executive actions impose constraints on the FDA’s ability to engage in oversight and implementation activities in the
normal course, our business may be negatively impacted.
We receive Australian government
research and development income tax incentive refunds. If our research and development expenditures are not deemed to
be eligible for the refund, proposed modifications to the tax incentive program are enacted, or the tax incentive
program is discontinued by the Australian government, it could have a negative effect on our future cash flows and the
funding of future research and development projects.
Our subsidiary, Anavex Australia Pty Ltd.,
is incorporated in Australia where we are currently engaged in research and development activities for ANAVEX®2-73
and ANAVEX®3-71. Our subsidiary is eligible to participate in the Australian Federal Government’s
Research and Development Tax Incentive program, under which the government provides a cash refund for a portion of eligible
research and development expenditures (currently 43.5% to 48.5% depending on the entity’s corporate tax rate) by small Australian entities,
which are defined as Australian entities with less than $20 million (Australian) in revenue.
The Research and Development Tax Incentive
refund is offered by the Australian federal government for eligible research and development purposes based on the filing
of an annual application. As part of this program, our subsidiary applied for and received cash refunds from the Australian Taxation
Office, or the ATO, for a percentage of the research and development costs expended by our subsidiary in Australia. Since the fiscal
year ended September 30, 2015, we have been receiving Research and Development Tax Incentive refunds related to research
and development expenditures made.
Certain research and development expenses incurred
outside of Australia are also eligible for the Australian research and development tax incentive program, provided
we obtain an Advance Overseas Finding from AusIndustry, a division of the Australian Government’s Department of Industry,
Innovation and Science (“AusIndustry”). To receive an Advance Overseas Finding, the expenses must have been for
eligible research and development activities, as determined by AusIndustry, and the expenditures must have a scientific link to
the Australian activities, be unable to be conducted in Australia and the total actual and reasonably anticipated overseas
costs must be expected to be less than the total actual and reasonably anticipated expenditures for activities conducted within
Australia, as determined by AusIndustry at the time of application for an Advance Overseas Finding (“OSF”).
This OSF binds both
AusIndustry and the Commissioner of Taxation for three income years. However, for compliance purposes, specific issue guidance
jointly issued by AusIndustry and the ATO in 2014 provides that an OSF can apply for the duration of the overseas activity provided
the activities are not new or materially different then the activities described in the OSF. Currently, the Company is outside
of the binding three-year period with respect to OSF applicable to some of its programs being claimed in Australia.
To the extent that some or all of our research
and development expenditures are deemed to be “ineligible,” then our refunds may decrease or be eliminated. In addition,
the Australian government may in the future modify the requirements of, reduce the amounts of the refunds available under,
or discontinue the Research and Development Tax Incentive program. Any such change to our anticipated refunds or change
to the Research and Development Tax Incentive program would have a negative effect on our future cash flows.
A variety of risks are associated with
operating our business internationally which could materially adversely affect our business.
We are presently
conducting clinical development solely in Australia, United Kingdom, The Netherlands, Germany and Canada
and may choose to conduct additional international and U.S. clinical trials in the future. Additionally, while we have not taken
any steps to enter into any non-U.S. markets, we may do so in the future. Accordingly, we are subject to risks related to operating
in foreign countries, including:
| |
● |
different
standards of care in various countries that could complicate the evaluation of our product candidates; |
| |
● |
different
United States and foreign drug import and export rules; |
| |
● |
reduced
protection for intellectual property rights in certain countries; |
| |
● |
unexpected
changes in tariffs, trade barriers and regulatory requirements; |
| |
● |
economic
weakness, including inflation, or political instability in particular foreign economies and markets; |
| |
● |
compliance
with tax, employment, immigration, and labor laws for employees living or traveling abroad; |
| |
● |
compliance
with the FCPA and other anti-corruption and anti-bribery laws; |
| |
● |
foreign
taxes, including withholding of payroll taxes; |
| |
● |
foreign
currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident
to doing business in another country; |
| |
● |
workforce
uncertainty in countries where labor unrest is more common than in the United States; |
| |
● |
production
shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
| |
● |
different payor
reimbursement regimes, governmental payors or patient self-pay systems and price controls; |
| |
● |
potential liability
resulting from development work conducted by foreign partners; |
| |
● |
business interruptions
resulting from natural disasters, outbreaks of contagious diseases, such as COVID-19, or geopolitical actions, including war
and terrorism, or systems failure including cybersecurity breaches; and |
| |
● |
compliance with
evolving and expansive foreign regulatory requirements, including data privacy laws (such as the GDPR). |
Additionally, in connection with the ongoing
conflict between Russia and Ukraine, the U.S. government and European Union countries have imposed enhanced export controls on
certain products and sanctions on certain industry sectors and parties in Russia. The U.S. government has also indicated it will
consider imposing additional sanctions and other similar measures in the near future. Although we do not currently conduct any
clinical trials in Russia or Ukraine, further escalation of geopolitical tensions could have a broader impact that expands into
other markets where we do business or conduct certain research and development operations, which could adversely affect our business,
our supply chain for our product candidates, our collaborators or our ability to carry out our clinical trials.
Our
ability to use our net operating loss (“NOL”) carryforwards and certain tax credit carryforwards may be subject to
limitation.
As of September 30, 2023, we had approximately
$126.3 million of U.S. federal and $192.0 million of state and local NOL carryforwards. We had approximately $12.9 million of NOL
carryforwards in Australia as of the same period. Our NOL carryforwards are subject to review and possible adjustment by the U.S.
and state tax authorities. In addition, under Sections 382 and 383 of the Code and corresponding provisions of state law, if a
corporation undergoes an “ownership change,” which is generally defined as a greater than 50% change (by value) in
its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOL carryforwards and research
and development credits to offset its post-change income may be limited. This could limit the amount of NOLs or research and development
credit carryforwards that we can utilize annually to offset future taxable income or tax liabilities. Subsequent ownership changes
and changes to the U.S. tax rules in respect of the utilization of NOLs and research and development credits carried forward may
further affect the limitation in future years. In addition, at the state level, there may be periods during which the use of NOLs
is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
We conducted a Section 382 study during the
year ended September 30, 2021 and determined that, during the year ended September 30, 2015, there was a change in ownership which
resulted in $25.8 million of federal NOLs being subject to an annual limitation. During the year ended September 30, 2021, we reduced
our federal NOLs by $12.1 million and our research and development tax credit carryforwards by $0.8 million, which are the amount
of tax assets that will expire unutilized pursuant to the Section 382 study. This resulted in a reduction of $2.5 million of NOLs
and $0.8 million of research and development credits and a corresponding reduction in the valuation allowance of $3.3 million,
which was recorded in the 2021 fiscal year. Subsequent ownership changes in future years could trigger additional limitations of
our NOLs. During the year ended September 30, 2023 and 2022, we determined that there were no changes in ownership pursuant to
Section 382.
We are subject to healthcare laws and
regulations which may require substantial compliance efforts and could expose us to criminal sanctions, civil and administrative
penalties, contractual damages, reputational harm and diminished profits and future earnings, among other penalties.
Healthcare providers, physicians and others
will play a primary role in the recommendation and prescription of our products, if approved. Our arrangements with such persons
and third-party payors and our general business operations will expose us to broadly applicable fraud and abuse and other healthcare
laws and regulations that may constrain the business or financial arrangements and relationships through which we research, market,
sell and distribute our drugs, if we obtain marketing approval. Restrictions under applicable U.S. federal, state and foreign healthcare
laws and regulations include, but are not limited to, the following:
| |
● |
the U.S. federal
Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering,
receiving or providing remuneration, including any kickback, bribe or rebate, directly or indirectly, in cash or in kind,
to induce or reward, or in return for, either the referral of an individual for, or the purchase or lease, order or recommendation
of, any item, good, facility or service, for which payment may be made under federal healthcare programs such as Medicare
and Medicaid; |
| |
● |
U.S. federal civil
and criminal false claims laws and civil monetary penalties laws, including the civil False Claims Act, which impose criminal
and civil penalties, including those from civil whistleblower or qui tam actions, against individuals or entities for, among
other things, knowingly presenting, or causing to be presented, claims for payment that are false or fraudulent or making
a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government; |
| |
● |
The Stark Law prohibits
a physician from making referrals for certain “designated health services” payable by Medicare to an entity in
which the physician or an immediate family member of such physician has an ownership or investment interest or with which
the physician has entered into a compensation arrangement, unless a statutory exception applies. There are a number of exceptions
to the Stark Law. Such exceptions permit certain payments and arrangements that, although they would otherwise potentially
implicate the Stark Law, are not treated as a violation under the same if the requirements of the specific exceptions are
met. |
| |
● |
HIPAA, which among
other things, created additional federal criminal statutes that impose criminal and civil liability for, such actions as executing
or attempting to execute a scheme to defraud any healthcare benefit program or knowingly and willingly falsifying, concealing
or covering up a material fact or making false statements relating to healthcare matters; |
| |
● |
The privacy and
security provisions of HIPAA, which impose certain requirements on covered entities and their business associates, including
mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable
health information; |
| |
● |
U.S. federal transparency
requirements under the Physician Payments Sunshine Act, enacted as part of the Affordable Care Act that require applicable
manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid
or the Children’s Health Insurance Program, with specific exceptions, to track and annually report to CMS payments and
other transfers of value provided to physicians, certain other healthcare providers (such as physicians assistants and nurse
practitioners),and teaching hospitals, as well as ownership and investment interests held by physicians or their immediate
family members; and |
| |
● |
analogous state
or foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to items or services reimbursed
by any third-party payor, including commercial insurers, state marketing and/or transparency laws applicable to manufacturers
that may be broader in scope than the federal requirements, state laws that require biopharmaceutical companies to comply
with the biopharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated
by the federal government and state laws governing the privacy and security of health information in certain circumstances,
many of which differ from each other in significant ways and may not have the same effect as HIPAA, thus complicating compliance
efforts. |
Ensuring that our business arrangements with
third parties comply with applicable healthcare laws and regulations will likely be costly. It is possible that governmental authorities
will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable
fraud and abuse or other healthcare laws and regulations. If our operations were found to be in violation of any of these laws
or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative
penalties, damages, fines, disgorgement, imprisonment, possible exclusion from government funded healthcare programs, such as Medicare
and Medicaid, contractual damages, reputational harm, diminished profits and future earnings and curtailment of our operations,
any of which could substantially disrupt our operations. If the physicians or other providers or entities with whom we expect to
do business are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions,
including exclusions from government funded healthcare programs. We may incur significant costs achieving and maintaining compliance
with applicable federal and state privacy, security, and fraud laws. Any action against us for violation of these laws, even if
we successfully defend against it, could cause us to incur significant legal expenses and divert our attention from the operation
of our business.
We expect current and future legislation
affecting the pharmaceutical industry, including drug pricing reform, to impact our business generally, which could adversely affect
our business operations.
In the United States, there have been, and
continue to be proposed and enacted legislation at the federal and state levels designed to, among other things, bring more transparency
to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare,
and reform government program reimbursement methodologies for drugs. For example, in July 2021, the Biden administration released
an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription
drugs. In response to Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High
Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress
could pursue as well as potential administrative actions HHS can take to advance these principles. In addition, the IRA, among
other things, (1) directs HHS to negotiate the price of certain single-source drugs and biologics covered under Medicare and (2)
imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions
will take effect progressively starting in fiscal year 2023, although they may be subject to legal challenges. It is currently
unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. If any of our
products are subject to such negotiation, we may lose a significant amount of the revenues expected during the full life cycle
of these products. Further, the Biden administration released an additional executive order on October 14, 2022, directing HHS
to submit a report within 90 days on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models
for lowering drug costs for Medicare and Medicaid beneficiaries. We expect that additional U.S. federal healthcare reform
measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare
products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
The coverage and reimbursement status
of newly approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for our product candidates,
if approved, could limit our ability to market those products and decrease our ability to generate product revenue.
Significant uncertainty exists as to the coverage
and reimbursement status of any compound for which we may seek regulatory approval. Sales in the United States will depend in part
on the availability of sufficient coverage and adequate reimbursement from third-party payors, which include government health
programs such as Medicare, Medicaid, CHIP, TRICARE and the Veterans Administration, as well as managed care organizations and private
health insurers. Prices at which we or our customers seek reimbursement for our therapeutic compounds can be subject to challenge,
reduction or denial by payors.
The process for determining whether a payor
will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will
pay for the product. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate
will be available. Additionally, in the United States there is no uniform policy among payors for coverage or reimbursement. Third-party
payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies,
but also have their own methods and approval processes. Therefore, coverage and reimbursement for products can differ significantly
from payor to payor. If coverage and adequate reimbursement are not available, or are available only at limited levels, successful
commercialization of, and obtaining a satisfactory financial return on, any product we develop may not be possible.
Third-party payors
are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services,
in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved
for marketing, we may need to conduct expensive studies in order to demonstrate the medical necessity and cost-effectiveness of
any products, which would be in addition to the costs expended to obtain regulatory approvals. Third-party payors may not consider
our compounds to be medically necessary or cost-effective compared to other available therapies, or the rebate percentages required
to secure favorable coverage may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient
to realize an appropriate return on our investment in drug development. Additionally, we or our collaborators may develop
companion diagnostic tests for use with our product candidates. Companion diagnostic tests require coverage and reimbursement separate
and apart from the coverage and reimbursement for their companion pharmaceutical or biological products. Similar challenges to
obtaining coverage and reimbursement, applicable to pharmaceutical or biological products, will apply to companion diagnostics.
Our inability to promptly obtain coverage and adequate reimbursement from third-party payors for the
product candidates, and for us or our collaborators to obtain coverage and adequate reimbursement for related companion diagnostic
tests that may be developed, could have a material and adverse effect on our business, financial condition, results of operations
and prospects.
Risks Related to our Common Stock
A decline in the price of our common
stock could affect our ability to raise further working capital and adversely impact our operations and would severely dilute existing
or future investors if we were to raise funds at lower prices.
A prolonged decline in the price of our common
stock could result in a reduction in our ability to raise capital. Because our operations have been financed through the sale of
equity securities, a decline in the price of our common stock could be especially detrimental to our continued operations. Any
reduction in our ability to raise equity capital in the future would force us to reallocate funds from other planned uses and would
have a significant negative effect on our business plans and operations, including our ability to develop new products and continue
our current operations. If our stock price declines, there can be no assurance that we can raise additional capital or generate
funds from operations sufficient to meet our obligations. We believe the following factors could cause the market price of our
common stock to continue to fluctuate widely and could cause our common stock to trade at a price below the price at which you
purchase your shares of common stock:
| |
● |
actual or anticipated
variations in our quarterly operating results; |
| |
● |
announcements of
new services, products, acquisitions or strategic relationships by us or our competitors; |
| |
● |
changes in accounting
treatments or principles; |
| |
● |
changes in earnings
estimates by securities analysts and in analyst recommendations; and |
| |
● |
general political,
economic, regulatory and market conditions. |
The market price for our common stock may also
be affected by our ability to meet or exceed expectations of analysts or investors. Any failure to meet these expectations, even
if minor, could materially adversely affect the market price of our common stock.
If we issue additional shares of common
stock in the future, it will result in the dilution of our existing stockholders and may cause the share price of our common stock
to fall.
We have 200,000,000 shares of common stock
authorized for issuance and we also have 10,000,000 shares of preferred stock authorized. Our Board of Directors has the authority
to issue additional shares of preferred and common stock up to the authorized capital stated in the articles of incorporation.
Our Board of Directors may choose to issue some or all such shares of common stock to acquire one or more businesses or to provide
additional financing in the future. The issuance of any such shares of common stock will result in a reduction of the book value
or market price of the outstanding shares of our common stock. If we do issue any such additional shares of common stock, such
issuance also will cause a reduction in the proportionate ownership and voting power of all other stockholders. Further, any such
issuance may result in a change of control of our corporation. In the event we do issue or sell additional shares of common or
preferred stock, it may result in stockholder dilution and may cause our share price to fall.
Our stock price has been volatile and
may be volatile in the future.
Our stock price has been volatile at certain
times historically, and may be volatile in the future. We may incur rapid and substantial increases or decreases in our stock price
in the foreseeable future that are do not coincide in timing with the disclosure of news or developments by us. The stock market
in general, and the market for biotechnology and pharmaceutical companies in particular, has experienced extreme volatility that
has often been unrelated to the operating performance of particular companies. The market price for our common stock may be influenced
by many factors, including the following:
| |
● |
announcements of
new data, clinical trial results or those of companies that are perceived to be similar to us; |
| |
|
|
| |
● |
announcements related
to any delays in any preclinical or clinical trials related to our products; |
| |
|
|
| |
● |
announcements related
to our products’ ability to demonstrate efficacy or an acceptable safety profile of our product candidates or similar
announcements by companies that are perceived to be similar to us; |
| |
|
|
| |
● |
our ability to meet
or exceed expectations of analysts or investors; |
| |
|
|
| |
● |
news that the number
of patients required for clinical trials for our drug candidates may be larger than we anticipate, enrollment in these clinical
trials may be slower than we anticipate, participants may drop out of these clinical trials at a higher rate than we anticipate
or the duration of these clinical trials may be longer than we anticipate; |
| |
|
|
| |
● |
actions taken by
regulatory agencies with respect to our product candidates or the progress of our clinical trials, including with respect
to any fast track or orphan drug designations; |
| |
● |
announcements of
significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our strategic collaboration
partners or our competitors; |
| |
|
|
| |
● |
grants awarded to
us or companies that are perceived to be similar to us from outside entities; |
| |
|
|
| |
● |
variations in our
financial results or those of companies that are perceived to be similar to us; |
| |
|
|
| |
● |
trading volume of
our common stock; |
| |
|
|
| |
● |
developments concerning
our collaborations or partners; |
| |
|
|
| |
● |
the impact of the
COVID-19 outbreak and its effect on us; |
| |
|
|
| |
● |
the perception of
the biotechnology or pharmaceutical industries by the public, legislatures, regulators and the investment community; |
| |
|
|
| |
● |
developments or
disputes concerning intellectual property rights; |
| |
|
|
| |
● |
significant lawsuits,
including patent or stockholder litigation; |
| |
|
|
| |
● |
our ability or inability
to raise additional capital and the terms on which we raise it; |
| |
|
|
| |
● |
sales of our common
stock by us or our stockholders; |
| |
|
|
| |
● |
declines in the
market prices of stocks generally or of companies that are perceived to be similar to us; and |
| |
|
|
| |
● |
general economic,
industry and market conditions. |
In addition, companies trading in the stock
market in general, and The Nasdaq Capital Market in particular, have experienced extreme price and volume fluctuations that have
often been unrelated or disproportionate to the operating performance of these companies. These broad market and industry factors
may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods
of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if
instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could
materially and adversely affect our business, financial condition, results of operations and growth prospects. There can be no
guarantee that our stock price will remain at current prices.
Our common stock may become the target
of a “short squeeze.”
Securities of certain companies have experienced
significant and extreme volatility in stock price due to short sellers of shares of common stock, known as a “short squeeze.”
These short squeezes have caused extreme volatility in those companies and in the market and have led to the price per share of
those companies to trade at a significantly inflated rate that is disconnected from the underlying value of the company. Many investors
who have purchased shares in those companies at an inflated rate face the risk of losing a significant portion of their original
investment as the price per share has declined steadily as interest in those stocks have abated. There can be no assurance that
we will not, in the future be, a target of a short squeeze, and you may lose a significant portion or all of your investment if
you purchase our shares at a rate that is significantly disconnected from our underlying value.
If we fail to maintain an effective system
of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As
a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the
trading price of our common stock.
Pursuant to Section 404 of the Sarbanes-Oxley
Act, our management is required to report upon the effectiveness of our internal control over financial reporting. We cannot assure
you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in
the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately
report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over
financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness
or significant deficiency in our internal control over financial reporting, investors may lose confidence in the accuracy and completeness
of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations
by the Nasdaq Stock Market, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control
over financial reporting, or to implement or maintain other effective control systems required of public companies, could also
restrict our future access to the capital markets.
Our disclosure controls and procedures
may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements
of the Exchange Act. We designed our disclosure controls and procedures to reasonably assure that information we must disclose
in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized
and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures
or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance
that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making
can be faulty, and that breakdowns can occur because of simple error or mistake. For example, our directors or executive officers
could inadvertently fail to disclose a new relationship or arrangement causing us to fail to make a required related party transaction
disclosure. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people
or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements
due to error or fraud may occur and not be detected.
Risks Related to our Intellectual Property
If we are unable to obtain and maintain
sufficient intellectual property protection for our product candidates, or if the scope of the intellectual property protection
obtained is not sufficiently broad, our competitors could develop and commercialize product candidates similar or identical to
ours, and our ability to successfully commercialize our product candidates that we may pursue may be impaired.
Our success depends in large part on our ability
to obtain and maintain protection of our intellectual property, particularly patents, in the United States and other countries
with respect to our product candidates and technology. We seek to protect our proprietary position by filing patent applications
in the United States and abroad related to our product candidates or by in-licensing intellectual property. U.S. patents related
to ANAVEX®2-73 are directed to ANAVEX®2-73 in its various optical or crystal forms, its therapeutic indications,
and dosage forms comprising certain doses of ANAVEX®2-73 combined with another therapeutic agent. We may not be
able to obtain broader scope patent protection for ANAVEX®2-73 as a single drug or in other jurisdictions.
The patent position of biotechnology and pharmaceutical
companies generally is highly uncertain, involves complex legal, technological and factual questions and has in recent years been
the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the
laws of the United States, or vice versa. Further, we may not be aware of all third-party intellectual property rights potentially
relating to and/or interfering with our product candidates. Publications of discoveries in the scientific literature often lag
behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published
until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether we were the first
to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection
of such inventions. As a result, the issuance, scope, validity, enforceability and/or commercial value of our patent rights are
highly uncertain. Our pending and future patent applications may not result in patents being issued that protect our product candidates,
in whole or in part, or which could effectively prevent others from commercializing competitive product candidates. Even if our
patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent
competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent
our patents by developing similar or alternative product candidates in a non-infringing manner.
Moreover, we may be subject to a third-party
pre-issuance submission of prior art to the United States Patent and Trademark Office, or the USPTO, or become involved in opposition,
derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or
the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of,
or invalidate, our patent rights, allow third parties to commercialize our product candidates and compete directly with us, without
payment to us, or result in our inability to manufacture or commercialize drugs without infringing on third-party patent rights.
In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless
of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product
candidates.
In addition, the issuance of a patent is not
conclusive as to its inventorship, scope, validity and/or enforceability, and our patents may be challenged in the courts or patent
offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims
being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using
or commercializing similar or identical product candidates, or limit the duration of the patent protection of our product candidates.
Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting
such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may
not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
We hold ownership or exclusive rights to twenty-two
U.S. patents, twenty-three U.S. patent applications, and various PCT or ex-U.S. patent applications relating to our drug candidates,
methods associated therewith, and to our research programs. Neither patents nor patent applications ensure the protection of our
intellectual property for a number of reasons, including the following:
| |
1. |
Competitors may
interfere with our patenting process in a variety of ways. Competitors may claim that Anavex is not entitled to an issued
patent for a variety of legal reasons. Competitors may also claim that we are infringing their patents and restrict our freedom
to operate. If a court or, in some circumstances, a board of a national patent authority, agrees, we would lose some or all
of our patent protection. As a company, we have no meaningful experience with competitors interfering with our patents or
patent applications. |
| |
|
|
| |
2. |
Because of the time,
money and effort involved in obtaining and enforcing patents, our management may spend less time and resources on developing
potential drug compounds than they otherwise would, which could increase our operating expenses and delay product programs. |
| |
|
|
| |
3. |
Issuance of a patent
may not provide significant practical protection. If we receive a patent of narrow scope, then it may be possible for competitors
to design products that do not infringe our patent(s). |
| |
|
|
| |
4. |
Anavex is seeking
patent protection for a number of indications, combination products and drug regimens. The lack of patent protection in global
markets for a specific end product or indication may inhibit our ability to advance our compounds and may make Anavex less
attractive to potential partners. |
| |
5. |
Defending a patent
lawsuit takes significant time and can be very expensive. |
| |
|
|
| |
6. |
If a court decides
that an Anavex compound, its method of manufacture or use, infringes on a competitor’s patent, we may have to pay substantial
damages for infringement. |
| |
|
|
| |
7. |
A court may prohibit
us from making, selling or licensing the potential drug compound unless the patent holder grants a license. A patent holder
is not required to grant a license. If a license is available, we may have to pay substantial royalties or grant cross licenses
to our patents, and the license terms may be unacceptable. |
| |
|
|
| |
8. |
Redesigning our
potential drug compounds so that they do not infringe on other patents may not be possible or could require substantial funds
and time. |
It is also unclear whether our trade secrets
are adequately protected. While we use reasonable efforts to protect our trade secrets, our employees or consultants may unintentionally
or willfully disclose our information to competitors. Enforcing a claim that someone illegally obtained and is using our trade
secrets, like patent litigation, is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside
the United States are sometimes less willing to protect trade secrets. Our competitors may independently develop equivalent knowledge,
methods and know-how.
We may also support and collaborate in research
conducted by government organizations, hospitals, universities or other educational institutions. These research partners may be
unable or unwilling to grant us exclusive rights to technology or products derived from these collaborations.
If we do not obtain required intellectual property
licenses or rights, we could encounter delays in our product development efforts while we attempt to design around other patents
or even be prohibited from developing, manufacturing or selling potential drug compounds requiring these rights or licenses. There
is also a risk that legal disputes may arise as to the rights to technology or potential drug compounds developed in collaboration
with other parties, all with attendant risk, distraction, expense, and lack of predictability.
Patent terms may be inadequate to protect
our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United
States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S.
non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited.
Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from
competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory
review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing
products similar or identical to ours.
Depending upon the timing, duration and specifics
of FDA marketing approval of product candidates that we identify, one of the U.S. patents covering such product candidate or the
use thereof may be eligible for up to five years of patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act allows
a maximum of one patent to be extended per FDA approved product as compensation for the patent term lost during the FDA regulatory
review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of
product approval and only those claims covering such approved drug product, a method for using it or a method for manufacturing
it may be extended. Patent term extension also may be available in certain foreign countries upon regulatory approval of our product
candidates. Nevertheless, we may not be granted patent term extension either in the United States
or in any foreign country because of, for example, failing to exercise due diligence during the testing phase or regulatory review
process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing
to satisfy applicable requirements. Moreover, the term of extension, as well as the scope of patent protection during any such
extension, afforded by the governmental authority could be less than what we request. If we are unable to obtain patent term extension
or restoration, or the term of any such extension is less than we request, the period during which we will have the right to exclusively
market our product may be shortened and our competitors may obtain approval of competing products following our patent expiration
sooner, and our revenue could be reduced, possibly materially.
Also, there are detailed rules and requirements
regarding the patents that may be submitted to the FDA for listing in the Approved Drug Products with Therapeutic Equivalence Evaluations,
or the Orange Book. We may be unable to obtain patents covering our product candidates that contain one or more claims that satisfy
the requirements for listing in the Orange Book. Even if we submit a patent for listing in the Orange Book, the FDA may decline
to list the patent, or a manufacturer of generic drugs may challenge the listing. If one of our product candidates is approved
and a patent covering that product candidate is not listed in the Orange Book, a manufacturer of generic drugs would not have to
provide advance notice to us of any abbreviated new drug application filed with the FDA to obtain permission to sell a generic
version of such product candidate.
If we fail to comply with our obligations
in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to
our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We are party to an exclusive license agreement
with Life Science Research Israel Ltd., with respect to certain in-licensed intellectual property related to our ANAVEX®3-71
product candidate, and we may need to obtain additional licenses from others in the future. Our license agreement with Life Science
Research Israel Ltd. imposes, and we expect that future license agreements will impose, various development, diligence, commercialization,
and other obligations on us. In spite of our efforts, our licensors might conclude that we have materially breached our obligations
under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to
develop and commercialize products and technology covered by these license agreements. If these in-licenses are terminated, or
if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to
seek regulatory approval of, and to market, products identical to ours and we may be required to cease our development and commercialization
of ANAVEX®3-71 or other product candidates covered by any such future licenses. Any of the foregoing
could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Moreover, disputes may arise regarding intellectual
property subject to a licensing agreement, including:
| |
● |
the
scope of rights granted under the license agreement and other interpretation-related issues; |
| |
● |
the
extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is
not subject to the licensing agreement; |
| |
● |
the
sublicensing of patent and other rights under our collaborative development relationships; |
| |
● |
our
diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| |
● |
the
inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by
our licensors and us and our partners; and |
| |
● |
the
priority of invention of patented technology. |
In addition, the agreements under which we
currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements
may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could
narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe
to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on
our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that
we have licensed prevent or impair our ability to maintain our licensing arrangements on commercially acceptable terms, we may
be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect
on our business, financial conditions, results of operations, and prospects.
If we do not obtain required intellectual property
licenses or rights, we could encounter delays in our product development efforts while we attempt to design around other patents
or even be prohibited from developing, manufacturing or selling potential drug compounds requiring these rights or licenses. There
is also a risk that legal disputes may arise as to the rights to technology or potential drug compounds developed in collaboration
with other parties, all with attendant risk, distraction, expense, and lack of predictability.
Third-party claims of intellectual property
infringement may prevent or delay our development and commercialization efforts.
Our success will also depend in part on our
ability to commercialize our compounds without infringing the proprietary rights of others. However, our research, development
and commercialization activities may be subject to claims that we infringe or otherwise violate patents or other intellectual property
rights owned or controlled by third parties. We have not conducted extensive freedom of use patent searches and no assurance can
be given that patents do not exist or could be issued which would have an adverse effect on our ability to market our technology
or maintain our competitive position with respect to our technology. Because patent applications can take many years to issue,
there may be currently pending patent applications which may later result in issued patents that our product candidates may infringe.
In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents.
If our compounds or other subject matter are claimed under other United States patents or other international patents or are otherwise
protected by third party proprietary rights, we may be subject to infringement actions. In such event, we may challenge the validity
of such patents or other proprietary rights, or we may be required to obtain licenses from such companies in order to develop,
manufacture or market our technology. There can be no assurances that we would be successful in a challenge or be able to obtain
such licenses or that such licenses, if available, could be obtained on commercially reasonable terms. Furthermore, the failure
to succeed in a challenge, develop a commercially viable alternative or obtain needed licenses could be materially adverse. Adverse
consequences include delays in marketing some or all of our potential drug compounds based on our drug technology or the inability
to proceed with the development, manufacture or sale of potential drug compounds requiring such licenses. If we defend ourselves
against charges of patent infringement or to protect our proprietary rights against third parties, substantial costs will be incurred
regardless of whether we are successful. Such proceedings are typically protracted with no certainty of success. An adverse outcome
could subject us to significant liabilities to third parties and force us to curtail or cease the research and development of our
technology.
Parties making claims against us may obtain
injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize ANAVEX®2-73
or our other product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense
and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement
against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement,
pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require
substantial time and monetary expenditure. Additionally, parties making claims against us may be able to sustain the costs of complex
patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial
amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk
that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the
initiation and continuation of any litigation could have material adverse effect on our ability to raise additional funds or otherwise
have a material adverse effect on our business, results of operations, financial condition and prospects.
If we are unable to protect the confidentiality
of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed.
While we use reasonable efforts to protect
our trade secrets, our employees or consultants may unintentionally or willfully disclose our information to competitors. Enforcing
a claim that someone illegally obtained and is using our trade secrets, like patent litigation, is expensive and time consuming,
and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets.
Our competitors may independently develop equivalent knowledge, methods and know-how.
We seek to protect our confidential proprietary
information, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific
advisors, contractors and collaborators. These agreements are designed to protect our proprietary information. However, we cannot
be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our trade secrets
and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our
trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may
breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate
remedies for such breaches. We also seek to preserve the integrity and confidentiality of our confidential proprietary information
by maintaining physical security of our premises and physical and electronic security of our information technology systems, but
it is possible that these security measures could be breached. If any of our confidential proprietary information were to be lawfully
obtained or independently developed by a competitor, we would have no right to prevent such competitor from using that technology
or information to compete with us, which could harm our competitive position.
We may be subject to claims that our
employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or
that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical
industry, we employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies,
including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors
do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees,
consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade
secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may
be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we
may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful
in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may become involved in lawsuits to
protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful and could
harm our business.
Competitors may infringe our patents or other
intellectual property. Although we are not currently involved in any litigation, if we were to initiate legal proceedings against
a third party to enforce a patent covering ANAVEX®2-73 or our other product candidates, the defendant could counterclaim
that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant
counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged
failure to meet any of several statutory requirements, including lack of novelty, obviousness, written description or non-enablement.
Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld
relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions
of invalidity and unenforceability is unpredictable.
Interference or derivation proceedings provoked
by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect
to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt
to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license
on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same
technology. Our defense of litigation or interference or derivation proceedings may fail and, even if successful, may result in
substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could
have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research
programs, license necessary technology from third parties, or enter into development partnerships that would help us bring ANAVEX®2-73
or our other product candidates to market.
We may be subject to claims challenging
the inventorship of our patents and other intellectual property.
We or our licensors may be subject to claims
that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets,
or other intellectual property as an inventor or co-inventor. For example, we or our licensors may have inventorship disputes arise
from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. Litigation
may be necessary to defend against these and other claims challenging inventorship or our or our licensors’ ownership of
our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such
claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership
of, or right to use, intellectual property that is important to our product candidates. Even if we are successful in defending
against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of
the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
Obtaining and maintaining our patent
protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental
patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity
fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental
patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems
in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsel to pay these fees due to
non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural,
documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and
other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other
means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment
or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect
on our business.
We may not be able to protect our intellectual
property rights throughout the world.
Filing, prosecuting and defending patents on
our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights
in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some
foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States.
Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States,
or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors
may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also
export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United
States. These products may compete with our products and our patents or other intellectual property rights may not be effective
or sufficient to prevent them from competing.
Changes in patent law could diminish
the value of our patents and patent applications in general, thereby impairing our ability to protect our product candidates.
Changes in either the patent laws or interpretation
of the patent laws in the United States and other countries could increase the uncertainties and costs surrounding the prosecution
of patent applications and the enforcement or defense of issued patents. After March 2013, under the Leahy-Smith America Invents
Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in
which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled
to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party
that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention
of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant of the time
from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential
for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either (i)
file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our or our licensor’s
patents or patent applications. The America Invents Act also includes a number of significant changes that affect the way patent
applications will be prosecuted and also may affect patent litigation. Therefore, the America Invents Act and its implementation
could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the
enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business,
financial condition, results of operations, and prospects.
In addition, the patent positions of companies
in the development and commercialization of pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have
narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain
situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once
obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing
patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability
to protect and enforce our intellectual property in the future.
The risk factors disclosed in this Annual Report
on Form 10-K could materially and adversely affect our business, financial condition and results of operations. The risks described
herein are not the only risks we face. Our operations could also be affected by additional factors that are not presently known
to us or by factors that we currently consider immaterial to our business.
ITEM 1B. UNRESOLVED
STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We do not own any real property. We maintain
a corporate head office at 630 5th Avenue, 20th Floor, New York, NY, USA. Our lease costs for this office are approximately $10,000
per month. We believe our offices are suitable and adequate to operate our business currently, as they provide us with sufficient
space to conduct our operations.
ITEM 3. LEGAL
PROCEEDINGS
We know of no material
pending legal proceedings, other than ordinary routine litigation incidental to our business, to which our Company or our subsidiary
is a party or of which any of their property is subject. There are no proceedings in which any of our directors, officers or affiliates,
or any registered or beneficial stockholder holding more than 5% of our shares, is an adverse party or has a material interest
adverse to our or our subsidiary’s interest.
ITEM 4. MINE
SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market information
Our common stock is quoted on the Nasdaq Global
Select Stock Market (“Nasdaq”) under the symbol “AVXL.”
Holders of Common Stock
As of November 24,
2023, there were approximately 48 stockholders of record, and 82,086,511 shares of our common stock were issued and outstanding.
Most of our stockholders hold their shares in street name.
Dividends
We have not paid any cash dividends on our
common stock and have no intention of paying any dividends on the shares of our common stock. Our current policy is to retain earnings,
if any, for use in our operations and in the development of our business. Our future dividend policy will be determined from time
to time by our Board of Directors.
Recent Sales of Unregistered Securities
Since the beginning of our fiscal year ended
September 30, 2023, we have not sold any equity securities that were not registered under the Securities Act of 1933 that were
not previously reported in a quarterly report on Form 10-Q or in a current report on Form 8-K.
Repurchases of Equity Securities by Our
Company and Affiliated Purchasers
None.
ITEM 6 [Reserved]
ITEM 7 MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion should be read
in conjunction with our consolidated financial statements and related notes thereto included elsewhere in this report. Past operating
results are not necessarily indicative of results that may occur in future periods. This discussion contains forward-looking statements,
which involve a number of risks and uncertainties. See Forward Looking Statements included elsewhere in this report.
Financial Operations Overview
We
are in the development stage and have not earned any revenues since our inception in 2004. We do not anticipate earning any revenues
until we can establish an alliance with other companies to develop, co-develop, license, acquire or market our products.
Our
operating costs consist primarily of research and development activities including the cost of clinical studies and clinical supplies
as well as clinical drug manufacturing and formulation. Research and development expenses also include personnel related costs such as
salaries and wages, and third-party contract research organization (CRO) expenses in support of these clinical studies. Personnel costs
include salaries and wages, benefits, and non-cash stock-based compensation charges associated with options and other equity awards granted
to employees and consultants who are directly engaged in support of our research and development activities.
General and administrative expenses
consist of personnel costs, expenses for outside professional services and expenses associated with operating as a public company.
Personnel costs consist of salaries and wages, benefits and stock-based compensation for general and administrative personnel.
Outside professional services and public company expenses, include expenses related to compliance and reporting, additional insurance
expenses, audit and SOX compliance, expenses associated with patent research, applications and filings, investor and stockholder
relations activities and other administrative expenses and professional services.
Comparison of year ended September 30, 2023 to year
ended September 30, 2022
Operating Expenses
Our operating expenses for fiscal 2023 increased to
$55.8 million, from $51.0 million in fiscal 2022. The increase is attributable to an increase in research and development expenses
of $5.7 million in 2023 to $43.7 million.
The following table summarizes our research and development
expenses for the years ended September 30, 2023, and 2022 (in thousands):
| | |
2023 | |
2022 |
| Costs of external service
providers | |
$ | 22,542 | | |
$ | 18,102 | |
| Personnel costs | |
| 10,264 | | |
| 8,012 | |
| Stock-based compensation | |
| 10,812 | | |
| 11,250 | |
| License fees | |
| — | | |
| 500 | |
| Other common costs | |
| 99 | | |
| 52 | |
| Total research
and development costs | |
$ | 43,717 | | |
$ | 37,916 | |
External service providers cost by product
candidate was as follows (in thousands):
| | |
2023 | |
2022 |
| ANAVEX®2-73 | |
$ | 19,540 | | |
$ | 15,510 | |
| ANAVEX®3-71 | |
| 2,624 | | |
| 2,251 | |
| All other product candidates | |
| 6 | | |
| 298 | |
| Other external service
provider costs | |
| 372 | | |
| 43 | |
| Total external
service provider costs | |
$ | 22,542 | | |
$ | 18,102 | |
The
increase in external service provider costs from fiscal 2022 to fiscal 2023 is primarily due to (1) an increase in manufacturing
costs for both ANAVEX®2-73 and ANAVEX®3-71, in preparation for planned clinical trials or studies
and (2) an increase in clinical trial expenditures related to our Rett program in connection with the completed enrollment and
dosing of our Phase 2/3 Excellence pediatric clinical trial.
During
fiscal 2023, our personnel costs increased to $10.3 million from $8.0 million as a result of our expanded team. However,
this was offset by a decrease in stock-based compensation expense as a result of the vesting of previously awarded milestone-based
option awards.
General and administrative expenses
for fiscal 2023 decreased to $12.0 million, from $13.1 million in fiscal 2022, most significantly related to a decrease in non-cash
stock option compensation charges as a result of the vesting of previously awarded milestone-based option awards.
During
fiscal 2023, we utilized cash and cash equivalents of $27.8 million to fund our operations, compared to $24.2 million during fiscal
2022. Our cash position increased slightly to $151.0 million at September 30, 2023, an increase of $1.9 million over the prior
year. Cash for operations was generated through the issuance of
shares of common stock under the financing arrangements described below.
We
expect to continue to see an increase in our research and development expenditures as we advance our ANAVEX®2-73
clinical studies, including planned advancement of ANAVEX®2-73 for Parkinson’s
disease program, ongoing extension studies of our current clinical programs, continued advancement of our other pipeline compounds
such as ANAVEX®3-71, and as we continue to add additional staffing to manage and support these clinical initiatives.
Other income (net)
Net other income for the year ended
September 30, 2023 was $8.3 million as compared to $3.4 million for fiscal 2022. The primary reason for the increase in other
income was due to an increase in interest income earned on cash and cash equivalents, due to an increase in market wide interest
rates year over year.
During fiscal 2023, we recorded
$2.7 million in research and development incentive income, consisting of the Australian research and development incentive credit
administered through the Australian Tax Office, in connection with fiscal 2023 eligible expenditures. In comparison, research
and development incentive income for fiscal 2022 was $3.3 million in connection with fiscal 2022 eligible expenditures. We expect
to continue to receive support from the Australian government for various clinical trials being conducted within Australia.
Net loss
Net loss for fiscal 2023 was $47.5 million,
or $0.60 per share, compared to a net loss of approximately $48.0 million, or $0.62 per share for fiscal 2022.
Liquidity and Capital Resources
Working Capital (in thousands)
| |
|
2023 |
|
2022 |
| Current Assets |
|
$ |
154,386 |
|
|
$ |
152,705 |
|
| Current Liabilities |
|
|
12,534 |
|
|
|
10,214 |
|
| Working Capital |
|
$ |
141,852 |
|
|
$ |
142,491 |
|
At September 30, 2023, we had $151.0
million in cash and cash equivalents, an increase from $149.2 million at September 30, 2022.
We intend
to continue to use our capital resources to advance our clinical trials for ANAVEX®2-73 and ANAVEX®3-71,
and to perform work necessary to prepare for future development of our pipeline compounds.
Cash Flows
Following
is a summary of sources of cash flows for the years ended September 30, 2023 and 2022 (in thousands)
| |
|
2023 |
|
2022 |
| Cash flows used in operating activities |
|
$ |
(27,785 |
) |
|
$ |
(24,238 |
) |
| Cash flows provided by financing activities |
|
|
29,651 |
|
|
|
21,288 |
|
| Increase (decrease) in cash |
|
$ |
1,866 |
|
|
$ |
(2,950 |
) |
Cash flow used in operating activities
There was an increase in cash used in operating activities
of $1.9 million during fiscal 2023 primarily due to the collection of incentive and tax receivables in the comparable period.
Cash flow provided by financing activities
Cash provided by financing activities in
fiscal 2023 was $29.7 million, primarily attributable to cash received from the issuance of common shares at various market
prices under the 2023 Purchase Agreement (as defined below).
Cash provided by financing activities
in fiscal 2022 was $21.3 million, net of financing costs, primarily attributable to cash received
from the issuance of common shares at various market prices under the Sales Agreement.
Other Financings
2023 Purchase Agreement
On February 3, 2023, the Company entered into
a $150,000,000 purchase agreement (the “2023 Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“Lincoln
Park”), pursuant to which the Company has the right to sell and issue to Lincoln Park, and Lincoln Park is obligated to purchase,
up to $150.0 million in value of its shares of Common Stock from time to time over a three-year period until February 3, 2026.
On any business day and subject to certain
customary conditions, the Company may direct Lincoln Park to purchase up to 200,000 shares of Common Stock (such purchases, “Regular
Purchases”). The amount of a Regular Purchase may increase under certain circumstances based on the market price of the
Common Stock; provided, however, that Lincoln Park’s committed obligation under any Regular Purchase shall not exceed $4.0
million. The purchase price of shares of Common Stock will be based on the then prevailing market prices of such shares at the
time of sales as described in the Purchase Agreement. There are no limits on the price per share that Lincoln Park may pay to purchase
Common Stock under the Purchase Agreement. In addition, if the Company has directed Lincoln Park to purchase the full amount of
Common Stock available as a Regular Purchase on a given day, it may direct Lincoln Park to purchase additional amounts as “accelerated
purchases” and “additional accelerated purchases,” each as set forth in the Purchase Agreement.
The Purchase Agreement limits the Company’s
sale of shares of Common Stock to Lincoln Park to 15,606,426 shares of Common Stock, representing 19.99% of the shares of the Common
Stock outstanding on the date of the Purchase Agreement unless (i) stockholder approval is obtained to issue more than such
amount or (ii) the average price of all applicable sales of Common Stock to Lincoln Park under the Purchase Agreement equals
or exceeds the lower of (A) the closing price of the Common Stock on the Nasdaq Capital Market immediately preceding the Execution
Date or (B) the average of the closing price of the Common Stock on the Nasdaq Capital Market for the five Business Days immediately
preceding the Execution Date.
The Purchase Agreement also prohibits the Company
from directing Lincoln Park to purchase any shares of Common Stock if those shares, when aggregated with all other shares of Common
Stock then beneficially owned by Lincoln Park and its affiliates, would result in Lincoln Park and its affiliates having beneficial
ownership, at any single point in time, of more than 4.99% of the then total outstanding shares of Common Stock, as calculated
pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder.
In consideration for entering into the 2023
Purchase Agreement, the Company issued to Lincoln Park 75,000 shares of Common Stock as a commitment fee (the “initial commitment
shares”) during the year ended September 30, 2023 and agreed to issue up to 75,000 shares pro rata (collectively with the
initial commitment shares, the “commitment shares”), when and if, Lincoln Park purchased, at the Company’s discretion,
the $150.0 million aggregate commitment.
During the year ended September 30, 2023, the
Company issued to Lincoln Park an aggregate of 3,288,943 (2022: 0) shares of Common Stock under the 2023 Purchase Agreement, including
3,275,000 (2022: 0) shares of Common Stock for aggregate proceeds of $27.9 million (2022: $0) and 88,943 (2022: 0) commitment shares
(inclusive of the 75,000 initial commitment shares).
As of September 30, 2023, an amount of
$122.1 million in shares of our common stock remain available for purchase by Lincoln Park under the 2023 Purchase Agreement.
Controlled Equity Offering Sales
Agreement
On May 1, 2020, we entered into an Amended
and Restated Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and SVB Leerink LLC (the “Sales
Agents”), pursuant to which we may offer and sell shares of common stock registered under an effective registration statement
from time to time through the Sales Agents (the “At-the-Market Offering”).
Upon delivery of a placement notice based on
our instructions and subject to the terms and conditions of the Sales Agreement, the Sales Agents may sell shares of common stock
by methods deemed to be an “at the market offering”, in negotiated transactions at market prices prevailing at the
time of sale or at prices related to such prevailing market prices, or by any other method permitted by law, including negotiated
transactions, subject to our prior written consent. We are not obligated to make any sales of shares under the Sales Agreement.
We or the Sales Agents may suspend or terminate the At-the-Market Offering upon notice to the other party, subject to certain conditions.
The Sales Agents will act as agents on a commercially reasonable efforts basis consistent with their normal trading and sales practices,
applicable state and federal law, and rules and regulations and the rules of Nasdaq.
We have agreed to pay the Sales Agents’
commissions for their services of 3.0% of the gross proceeds from the sale of shares of Common Stock pursuant to the Sales Agreement.
We have also agreed to provide the Sales Agents with customary indemnification and contribution rights.
No shares were sold during the year ended September
30, 2023 under the Sales Agreement. The Company currently does not have access to sell shares of common stock with the Sales Agents.
During the year ended September 30, 2022, 1,623,813
shares were sold under the Sales Agreement for gross proceeds of $21.0 million (net proceeds of $20.3 million after deducting commissions
and offering expenses).
2019 Purchase Agreement
On June 7, 2019, we
entered into a Purchase Agreement (the “2019 Purchase Agreement”) with Lincoln Park, as amended on July 1, 2020, pursuant
to which Lincoln Park committed to purchase up to $50.0 million of our common stock. Concurrently with the execution of the 2019
Purchase Agreement in 2019, we issued 324,383 shares of our common stock to Lincoln Park as a fee for its commitment to purchase
shares of our common stock under the 2019 Purchase Agreement and became obligated to issue up to 162,191 shares pro rata, when
and if Lincoln Park purchased, at our discretion, the $50.0 million aggregate commitment.
During fiscal year 2021, the Company
issued to Lincoln Park an aggregate of 4,086,209 shares of common stock under the 2019 Purchase Agreement, including 4,007,996
shares of common stock for an aggregate purchase price of $24.1 million and 78,213 commitment shares. As of
September 30, 2023 and 2022, no shares of our common stock remained available for purchase by Lincoln Park under the 2019 Purchase
Agreement.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements
that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition,
revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our stockholders.
Application
of Critical Accounting Policies
Our
financial statements and accompanying notes are prepared in accordance with generally accepted accounting principles in the United
States. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts
of assets, liabilities, revenue and expenses. These estimates and assumptions are affected by management’s
application of accounting policies. We believe that understanding the basis and nature of the estimates and assumptions involved
with the following aspects of our financial statements is critical to an understanding of our financial statements.
We base our assumptions and estimates
on historical experience and other sources that we believe to be reasonable at the time. Actual results may vary from our estimates
due to changes in circumstances, politics, global economics, general business conditions and other factors. Our significant estimates
are related to the valuation of warrants and options.
There are accounting policies that
we believe are significant to the presentation of our financial statements. The most significant of these accounting policies
relates to the accounting for our research and development expenses and stock-based compensation expense.
Research and Development Expenses
Research
and development costs are expensed as incurred. These expenses are comprised of the costs of the Company’s
proprietary research and development efforts, including preclinical studies, clinical trials, manufacturing costs, employee salaries
and benefits and stock-based compensation expense, contract services including external research and development expenses incurred
under arrangements with third parties such as contract research organizations (“CROs”),
facilities costs, overhead costs and other related expenses. Milestone payments made by the Company to third parties are expensed
when the specific milestone has been achieved. Manufacturing costs are expensed as incurred in accordance with Accounting Standard
Codification (“ASC”)
730, Research and Development, as these materials have no alternative future use outside of their intended use.
Nonrefundable advance payments
for goods or services that will be used or rendered for future research and development activities are deferred and amortized
over the period that the goods are delivered, or the related services are performed, subject to an assessment of recoverability.
The Company makes estimates of costs incurred in relation to external CROs, and clinical site costs. The Company analyzes the
progress of clinical trials, including levels of patient enrollment, invoices received and contracted costs when evaluating the
adequacy of the amount expensed and the related prepaid asset and accrued liability. Significant judgments and estimates must
be made and used in determining the accrued balance and expense in any accounting period. The Company reviews and accrues CRO
expenses and clinical trial study expenses based on work performed and relies upon estimates of those costs applicable to the
stage of completion of a study. Accrued CRO costs are subject to revisions as such trials progress to completion. Revisions are
charged to expense in the period in which the facts that give rise to the revision become known. With respect to clinical site
costs, the financial terms of these agreements are subject to negotiation and vary from contract to contract. Payments under these
contracts may be uneven and depend on factors such as the achievement of certain events, the successful recruitment of patients,
the completion of portions of the clinical trial or similar conditions. The objective of our policy is to record expenses in our
financial statements based on actual services received and efforts expended. As such, expense accruals related to clinical site
costs are recognized based on our estimate of the degree of completion of the event or events specified in the specific clinical
trial contract.
In addition, we incur expenses
in respect of the acquisition of intellectual property relating to patents and trademarks. The probability of success and length
of time to develop commercial applications of the drugs subject to the acquired patents and trademarks is difficult to determine
and numerous risks and uncertainties exist with respect to the timely completion of the development projects. There is no assurance
the acquired patents and trademarks will ever be successfully commercialized. Due to these risks and uncertainties, we expense
the acquisition of patents and trademarks
Stock-based Compensation
We account for all stock-based payments and awards under
the fair value-based method.
The fair value of all share purchase
options and warrants are expensed over their contractual vesting period, or over the expected performance period for only the
portion of awards expected to vest, in the case of milestone-based vesting, with a corresponding increase to additional paid-in
capital.
Compensation costs for stock-based
payments with graded vesting are recognized on a straight-line basis. Stock-based compensation expense is adjusted for actual
forfeitures of unvested awards as they occur.
We have granted share purchase
option awards that vest upon achievement of certain performance criteria, or milestone-based awards. We estimate an implicit service
period for achieving performance criteria for each award and recognizes the resulting fair value as expense over the implicit
service period when we conclude that achieving the performance criteria is probable. We periodically review and update as appropriate
our estimates of implicit service periods and conclusions on achieving the performance criteria. Performance awards vest upon
achievement of the performance criteria.
We
use the Black-Scholes option valuation model to calculate the fair value of share purchase options and warrants at the date of
the grant. This model requires the input of subjective assumptions, including the expected price volatility, and expected life
of each award. These assumptions consist of estimates of future market conditions, which are inherently uncertain, and therefore,
are subject to management’s judgment. Changes in these assumptions
can materially affect the fair value estimates.
RECENT ACCOUNTING PRONOUNCEMENTS
For a discussion of recent accounting
pronouncements and their possible effect on our results, see Note 2 to our Consolidated Financial Statements found elsewhere in
this Annual Report.
ITEM 7A QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
We
invest our excess cash in investment-grade, interest-bearing securities. The
primary objective of our investment policy is to preserve principal and liquidity. To achieve this objective, our investment policy
allows for investments in domestic money market certificates, certificates of deposit, money market funds, bonds or commercial
papers, and establishes diversification and credit quality requirements and limits investments by maturity and issuer.
At September 30, 2023 and 2022, the majority of our excess cash was held
in a JP Morgan Chase Prime Money Market Fund. The average amount
invested at any given time throughout the year ended September 30, 2023 was $138.4 million (high: $145.4 million; low: $129.5
million) and the average rate of return was 4.69%. A hypothetical
100 basis point change in interest rates during any of the periods presented would not have a material impact on the fair market
value of our cash and cash equivalents as of September 30, 2023 and 2022 and would impact our net loss by approximately $1.4 million.
To date, we have not experienced a loss of principal on any of our investments and as of September 30, 2023 we did not have any
allowance for credit losses from our cash and cash equivalents.
Foreign Exchange Risk
We face foreign exchange risk as a result of entering
into transactions denominated in currencies other than U.S. dollars and as a result of the existence of sales and tax incentive
receivables denominated in other than U.S. dollars. Due to the uncertain timing of expected payments in foreign currencies, we
do not utilize any forward exchange contracts. All foreign transactions settle on the applicable spot exchange basis at the time
such payments are made. Volatile market conditions and supply chain shortages may result in significant changes in exchange rates,
and in particular a change in foreign currencies values relative to the U.S. dollar may affect our operating expenses as expressed
in U.S. dollars. An adverse movement in foreign exchange rates could have a material effect on payments made to foreign suppliers.
For the year ended September 30, 2023, a majority of
our expenses were denominated in U.S. dollars. A hypothetical 10% change in foreign exchange rates applied to foreign currency
transactions for the year ended September 30, 2023 would not have had a material impact on our consolidated financial statements.
At
September 30, 2023, we held net assets of $5.0 million (AUD $7.8 million) denominated in Australian dollars. A
hypothetical 10% change in foreign exchange rates at September 30, 2023 would result in a change in reported net assets of +/-
$0.5 million.
Inflation Risk
Inflation generally may affect us by
increasing our cost of labor and clinical trial costs. We do not believe that inflation has had a material impact on our results
of operations during the periods presented.
ITEM 8. FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA
ANAVEX
LIFE SCIENCES CORP.
CONSOLIDATED
FINANCIAL STATEMENTS
September
30, 2023
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board
of Directors and Shareholders
Anavex Life Sciences Corp.
Opinion on internal control over financial reporting
We have audited the internal control over financial reporting of Anavex
Life Sciences Corp. (a Nevada corporation) and subsidiaries (the “Company”) as of September 30, 2023, based on criteria established
in the 2013 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission
(“COSO”). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting
as of September 30, 2023, based on criteria established in the 2013 Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company
Accounting Oversight Board (United States) (“PCAOB”), the consolidated financial statements of the Company as of and for the
year ended September 30, 2023, and our report dated November 27, 2023 expressed an unqualified opinion on those financial statements.
Basis for opinion
The Company’s management is responsible for maintaining effective
internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included
in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an
opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered
with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and
the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those
standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial
reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting,
assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control
based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our
audit provides a reasonable basis for our opinion.
Definition and limitations of internal control over financial reporting
A company’s internal control over financial reporting is a process
designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements
for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting
includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded
as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts
and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
(3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s
assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting
may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk
that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures
may deteriorate.
/s/ GRANT THORNTON LLP
Melville, New York
November 27, 2023
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Anavex Life Sciences Corp.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Anavex
Life Sciences Corp. (a Nevada corporation) and subsidiaries (the “Company”) as of September 30, 2023 and 2022, the related
consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for each of the two
years in the period ended September 30, 2023, and the related notes (collectively referred to as the “financial statements”).
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company
as of September 30, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended
September 30, 2023, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company
Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of
September 30, 2023, based on criteria established in the 2013 Internal Control - Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission (“COSO”), and our report dated November 27, 2023 expressed an unqualified opinion.
Basis for opinion
These financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public
accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free
of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement
of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included
examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating
the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial
statements. We believe that our audits provide a reasonable basis for our opinion.
Critical audit matters
Critical audit matters are matters arising from the current period audit
of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts
or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments.
We determined that there are no critical audit matters.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2022.
Melville, New York
November 27, 2023
248
Report
of Independent Registered Public Accounting Firm
Shareholders
and Board of Directors
Anavex
Life Sciences Corp.
New
York, New York
Opinion
on the Consolidated Financial Statements
We
have audited the consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for the year
ended September 30, 2021, and the related notes (collectively referred to as the “consolidated financial statements”) of
Anavex Life Sciences Corp. (the “Company”). In our opinion, the consolidated financial statements present fairly, in all
material respects, the results of the Company’s operations and cash flows for the year ended September 30, 2021, in conformity
with accounting principles generally accepted in the United States of America.
Basis
for Opinion
These
consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion
on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the PCAOB
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted
our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable
assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audit included performing
procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and
performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts
and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that
our audit provides a reasonable basis for our opinion.
Critical
Audit Matters
Critical audit matters
are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated
to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and
(2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ BDO USA, P.C.
We served as the Company's
auditor from 2013 to 2022.
New York,
New York
November
24, 2021
Anavex
Life Sciences Corp.
Consolidated Balance Sheets
(in
thousands, except share and per share amounts)
| |
|
|
|
|
|
|
|
|
| |
|
September 30, |
| |
|
2023 |
|
2022 |
| |
|
|
|
|
| Assets |
|
|
|
|
|
|
|
|
| Current |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
151,024 |
|
|
$ |
149,158 |
|
| Incentive and tax receivables |
|
|
2,709 |
|
|
|
3,193 |
|
| Prepaid expenses and other current assets |
|
|
653 |
|
|
|
354 |
|
| Total Assets |
|
$ |
154,386 |
|
|
$ |
152,705 |
|
| |
|
|
|
|
|
|
|
|
| Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
| Current Liabilities |
|
|
|
|
|
|
|
|
| Accounts payable |
|
$ |
4,322 |
|
|
$ |
3,825 |
|
| Accrued liabilities - Note 3 |
|
|
7,295 |
|
|
|
5,945 |
|
| Deferred grant income - Note 4 |
|
|
917 |
|
|
|
444 |
|
| Total Liabilities |
|
|
12,534 |
|
|
|
10,214 |
|
| |
|
|
|
|
|
|
|
|
| Commitments and Contingencies - Note 6 |
|
|
— |
|
|
|
— |
|
| |
|
|
|
|
|
|
|
|
| Capital stock |
|
|
|
|
|
|
|
|
| Authorized: |
|
|
|
|
|
|
|
|
| 10,000,000 preferred stock, par value $0.001 per share |
|
|
— |
|
|
|
— |
|
| 200,000,000 common stock, par value $0.001 per share |
|
|
|
|
|
|
|
|
Issued and outstanding:
82,066,511 common shares (2022 - 77,942,815) |
|
|
82 |
|
|
|
78 |
|
| Additional paid-in capital |
|
|
434,839 |
|
|
|
387,977 |
|
| Accumulated deficit |
|
|
(293,069 |
) |
|
|
(245,564 |
) |
| Total Stockholders' Equity |
|
|
141,852 |
|
|
|
142,491 |
|
| Total Liabilities and Stockholders' Equity |
|
$ |
154,386 |
|
|
$ |
152,705 |
|
See
Accompanying Notes to Consolidated Financial Statements
Anavex
Life Sciences Corp.
Consolidated
Statements of Operations and Comprehensive Loss
(in
thousands, except share and per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years
Ended September 30, |
| |
|
2023 |
|
2022 |
|
2021 |
| Operating
expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| General
and administrative |
|
$ |
12,039 |
|
|
$ |
13,070 |
|
|
$ |
9,018 |
|
| Research
and development |
|
|
43,717 |
|
|
|
37,916 |
|
|
|
32,984 |
|
| Total
operating expenses |
|
|
55,756 |
|
|
|
50,986 |
|
|
|
42,002 |
|
| Operating
loss |
|
|
(55,756 |
) |
|
|
(50,986 |
) |
|
|
(42,002 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Other
income (expenses) |
|
|
|
|
|
|
|
|
|
|
|
|
| Grant
income |
|
|
25 |
|
|
|
— |
|
|
|
54 |
|
| Research
and development incentive income |
|
|
2,718 |
|
|
|
3,323 |
|
|
|
4,547 |
|
| Interest
income, net |
|
|
6,519 |
|
|
|
947 |
|
|
|
26 |
|
| Other
financing expense |
|
|
(964 |
) |
|
|
— |
|
|
|
— |
|
| Foreign
exchange loss |
|
|
(40 |
) |
|
|
(904 |
) |
|
|
(266 |
) |
| Total
other income, net |
|
|
8,258 |
|
|
|
3,366 |
|
|
|
4,361 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Income
tax expense, current |
|
|
(7 |
) |
|
|
(358 |
) |
|
|
(268 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Net
loss and comprehensive loss |
|
$ |
(47,505 |
) |
|
$ |
(47,978 |
) |
|
$ |
(37,909 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Net
Loss per share
Basic and diluted |
|
$ |
(0.60 |
) |
|
$ |
(0.62 |
) |
|
$ |
(0.54 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
average number of shares outstanding
Basic and diluted |
|
|
79,787,596 |
|
|
|
76,909,993 |
|
|
|
69,802,960 |
|
See
Accompanying Notes to Consolidated Financial Statements
Anavex
Life Sciences Corp.
For
the years ended September 30, 2023, 2022 and 2021
(in
thousands, except share and per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years ended September 30, |
| |
|
2023 |
|
2022 |
|
2021 |
| |
|
|
|
|
|
|
| Cash
Flows used in Operating Activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Net
loss |
|
$ |
(47,505 |
) |
|
$ |
(47,978 |
) |
|
$ |
(37,909 |
) |
| Adjustments
to reconcile net loss to net cash used in operations: |
|
|
|
|
|
|
|
|
|
|
|
|
| Non-cash
financing related charges |
|
|
845 |
|
|
|
— |
|
|
|
— |
|
| Stock-based
compensation |
|
|
16,370 |
|
|
|
18,379 |
|
|
|
8,231 |
|
| Changes
in working capital balances related to operations: |
|
|
|
|
|
|
|
|
|
|
|
|
| Incentive
and tax receivables |
|
|
484 |
|
|
|
5,944 |
|
|
|
(4,287 |
) |
| Prepaid
expenses and deposits |
|
|
(299 |
) |
|
|
1 |
|
|
|
88 |
|
| Accounts
payable |
|
|
497 |
|
|
|
(914 |
) |
|
|
751 |
|
| Accrued
liabilities |
|
|
1,350 |
|
|
|
330 |
|
|
|
2,298 |
|
| Deferred
grant income |
|
|
473 |
|
|
|
— |
|
|
|
444 |
|
| Net
cash used in operating activities |
|
|
(27,785 |
) |
|
|
(24,238 |
) |
|
|
(30,384 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash
Flows provided by Financing Activities |
|
|
|
|
|
|
|
|
|
|
|
|
| Issuance
of common shares |
|
|
27,875 |
|
|
|
20,985 |
|
|
|
153,219 |
|
| Share
issue costs |
|
|
— |
|
|
|
(707 |
) |
|
|
(5,551 |
) |
| Proceeds
from exercise of warrants |
|
|
— |
|
|
|
— |
|
|
|
1,467 |
|
| Proceeds
from exercise of stock options |
|
|
1,776 |
|
|
|
1,010 |
|
|
|
4,108 |
|
| Net
cash provided by financing activities |
|
|
29,651 |
|
|
|
21,288 |
|
|
|
153,243 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Increase
in cash and cash equivalents during the period |
|
|
1,866 |
|
|
|
(2,950 |
) |
|
|
122,859 |
|
| Cash
and cash equivalents, beginning of period |
|
|
149,158 |
|
|
|
152,108 |
|
|
|
29,249 |
|
| Cash
and cash equivalents, end of period |
|
$ |
151,024 |
|
|
$ |
149,158 |
|
|
$ |
152,108 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental
Cash Flow Information |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash
paid for state and local minimum income taxes |
|
$ |
136 |
|
|
$ |
327 |
|
|
$ |
140 |
|
See
Accompanying Notes to Consolidated Financial Statements
Anavex
Life Sciences Corp.
Consolidated
Statements of Changes in Stockholders' Equity
(in thousands, except share and per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Common
Stock |
|
|
|
Additional
Paid- |
|
Accumulated |
|
|
| |
|
Shares |
|
Par
Value |
|
in
Capital |
|
Deficit |
|
Total |
| |
|
|
|
|
|
|
|
|
|
|
| Balance,
October 1, 2020 |
|
|
62,045,198 |
|
|
$ |
62 |
|
|
$ |
186,852 |
|
|
$ |
(159,677 |
) |
|
$ |
27,237 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares
issued under 2019 purchase agreement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Purchase
shares |
|
|
4,007,996 |
|
|
|
4 |
|
|
|
24,107 |
|
|
|
— |
|
|
|
— |
24,111 |
|
| Commitment
shares |
|
|
78,213 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Shares
issued under Sales Agreement |
|
|
5,634,576 |
|
|
|
6 |
|
|
|
79,102 |
|
|
|
— |
|
|
|
79,108 |
|
| Less:
share issue costs |
|
|
— |
|
|
|
— |
|
|
|
(2,438 |
) |
|
|
— |
|
|
|
(2,438 |
) |
| Shares
issued pursuant to registered direct offering |
|
|
2,380,953 |
|
|
|
2 |
|
|
|
49,998 |
|
|
|
— |
|
|
|
50,000 |
|
| Less:
share issue costs |
|
|
— |
|
|
|
— |
|
|
|
(3,097 |
) |
|
|
— |
|
|
|
(3,097 |
) |
| Shares
issued pursuant to exercise of stock options |
|
|
1,421,529 |
|
|
|
1 |
|
|
|
4,107 |
|
|
|
— |
|
|
|
4,108 |
|
| Shares
issued pursuant to exercise of warrants |
|
|
350,000 |
|
|
|
— |
|
|
|
1,466 |
|
|
|
— |
|
|
|
1,466 |
|
| Share
based compensation |
|
|
— |
|
|
|
— |
|
|
|
8,231 |
|
|
|
— |
|
|
|
8,231 |
|
| Net
loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(37,909 |
) |
|
|
(37,909 |
) |
| Balance,
September 30, 2021 |
|
|
75,918,465 |
|
|
|
75 |
|
|
|
348,328 |
|
|
|
(197,586 |
) |
|
|
150,817 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares
issued under Sales Agreement |
|
|
1,623,813 |
|
|
|
2 |
|
|
|
20,983 |
|
|
|
— |
|
|
|
20,985 |
|
| Less:
share issue costs |
|
|
— |
|
|
|
— |
|
|
|
(723 |
) |
|
|
— |
|
|
|
(723 |
) |
| Shares
issued pursuant to exercise of stock options |
|
|
400,537 |
|
|
|
1 |
|
|
|
1,010 |
|
|
|
— |
|
|
|
1,011 |
|
| Share
based compensation |
|
|
— |
|
|
|
— |
|
|
|
18,379 |
|
|
|
— |
|
|
|
18,379 |
|
| Net
loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(47,978 |
) |
|
|
(47,978 |
) |
| Balance,
September 30, 2022 |
|
|
77,942,815 |
|
|
|
78 |
|
|
|
387,977 |
|
|
|
(245,564 |
) |
|
|
142,491 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares
issued under 2023 purchase agreement
Initial Commitment shares |
|
|
75,000 |
|
|
|
— |
|
|
|
845 |
|
|
|
— |
|
|
|
845 |
|
| Purchase
shares |
|
|
3,275,000 |
|
|
|
3 |
|
|
|
27,872 |
|
|
|
— |
|
|
|
27,875 |
|
| Commitment
shares |
|
|
13,943 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Shares
issued pursuant to exercise of stock options |
|
|
759,753 |
|
|
|
1 |
|
|
|
1,775 |
|
|
|
— |
|
|
|
1,776 |
|
| Share
based compensation |
|
|
— |
|
|
|
— |
|
|
|
16,370 |
|
|
|
— |
|
|
|
16,370 |
|
| Net
loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(47,505 |
) |
|
|
(47,505 |
) |
| Balance,
September 30, 2023 |
|
|
82,066,511 |
|
|
$ |
82 |
|
|
$ |
434,839 |
|
|
$ |
(293,069 |
) |
|
$ |
141,852 |
|
See
Accompanying Notes to Consolidated Financial Statements
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 9
Note
1 Business Description and Basis of Presentation
Business
Anavex
Life Sciences Corp. (“Anavex” or the “Company”) is a clinical stage biopharmaceutical company engaged in the
development of differentiated therapeutics by applying precision medicine to central nervous system (“CNS”) diseases with
high unmet need. Anavex analyzes genomic data from clinical trials to identify biomarkers, which are used in the analysis of its clinical
trials for the treatment of neurodegenerative and neurodevelopmental diseases.
The
Company’s lead compound ANAVEX®2-73 (blarcamesine) is being developed to treat Alzheimer’s disease, Parkinson’s
disease and potentially other central nervous system diseases, including rare diseases, such as Rett syndrome, a rare severe neurological
monogenic disorder caused by mutations in the X-linked gene, methyl-CpG-binding protein 2 (“MECP2”).
Basis
of Presentation
These
consolidated financial statements have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission
(“SEC”) and the instructions to Form 10-K and have been prepared under the accounting principles generally accepted in the
United States of America (“U.S. GAAP”).
Liquidity
All
of the Company’s potential drug compounds are in the clinical development stage and the Company cannot be certain that its research
and development efforts will be successful or, if successful, that its potential drug compounds will ever be approved for sales to pharmaceutical
companies or generate commercial revenues. To date, we have not generated any revenues from our operations. The Company expects the business
to continue to experience negative cash flows from operations for the foreseeable future and cannot predict when, if ever, our business
might become profitable.
Management
believes that the current working capital position will be sufficient to meet the Company’s working capital requirements beyond
the next 12 months after the date that these consolidated financial statements are issued. The process of drug development can be costly,
and the timing and outcomes of clinical trials are uncertain. The assumptions upon which the Company has based its estimates are
routinely evaluated and may be subject to change. The actual amount of the Company’s expenditures will vary depending upon
a number of factors including but not limited to the design, timing and duration of future clinical trials, the progress of the Company’s
research and development programs and the level of financial resources available. The Company has the ability to adjust its operating
plan spending levels based on the timing of future clinical trials.
Other
than our rights related to the Purchase Agreement (as defined below in Note 5), there can be no assurance that additional financing will
be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If the Company is not able
to obtain the additional financing on a timely basis, if and when it is needed, it will be forced to delay or scale down some or all
of its research and development activities.
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the
reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses in
the reporting period. The Company regularly evaluates estimates and assumptions related to accounting for research and development costs,
incentive income receivable, valuation and recoverability of deferred tax assets, stock based compensation, and loss contingencies. The
Company bases its estimates and assumptions on current facts, historical experience, and various other factors that it believes to be
reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and
liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by
the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between
the estimates and the actual results, future results of operations will be affected.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 10
Principles
of Consolidation
These
consolidated financial statements include the accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia
Pty Limited (“Anavex Australia”), a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated
under the laws of Germany, and Anavex Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company
transactions and balances have been eliminated.
Cash
and equivalents
The
Company considers only those investments which are highly liquid, readily convertible to cash and that mature within three months from
the date of purchase to be cash equivalents.
Highly
liquid investments that are considered cash equivalents include money market accounts, money market funds and certificates
of deposit. The carrying value of cash equivalents approximates fair value due to the short-term maturity of these securities. The Company’s
investment policy allows for investments in domestic money market certificates, certificates of deposit, money market funds, bonds or
commercial papers, and establishes diversification and credit quality requirements and limits investments by maturity and issuer. The
Company currently maintains its investments at one large well known financial institution.
The
Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. Accounts are guaranteed by
the Federal Deposit Insurance Corporation (FDIC) up to $250,000 under current regulations. At September 30, 2023 and 2022, substantially
all of the Company’s cash balances were in excess of these federally insured limits. The Company mitigates this risk by maintaining
the majority of its cash balances in a large well-known financial institution. The Company has not experienced any losses in such accounts.
Research
and Development Expenses
Research
and development costs are expensed as incurred. These expenses are comprised of the costs of the Company’s proprietary research
and development efforts, including preclinical studies, clinical trials, manufacturing costs, employee salaries and benefits and stock-based
compensation expense, contract services including external research and development expenses incurred under arrangements with third parties
such as contract research organizations (“CROs”), facilities costs, overhead costs and other related expenses. Milestone
payments made by the Company to third parties are expensed when the specific milestone has been achieved. Manufacturing costs are expensed
as incurred in accordance with Accounting Standard Codification (“ASC”) 730, Research and Development, as these materials
have no alternative future use outside of their intended use.
Nonrefundable
advance payments for goods or services that will be used or rendered for future research and development activities are deferred and
amortized over the period that the goods are delivered, or the related services are performed, subject to an assessment of recoverability.
The Company makes estimates of costs incurred in relation to external CROs, and clinical site costs. When evaluating the adequacy of
the accrued liabilities, the Company analyzes progress of the trials and studies including the phase or completion of events, invoices
received and contracted costs. Judgments and estimates are made in determining the accrued balances at the end of any reporting period.
Actual results could differ from the Company’s estimates. The Company’s historical accrual estimates have not been materially
different from actual costs.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 11
In
addition, the Company incurs expenses in respect of intellectual property costs relating to patents and trademarks. The probability of
success and length of time to develop commercial applications of the drugs subject to the underlying patent and trademark costs is difficult
to determine and numerous risks and uncertainties exist with respect to the timely completion of the development projects. There is no
assurance the drugs subject to the underlying patents and trademarks will ever be successfully commercialized.
Due
to these risks and uncertainties, the patent and trademark costs do not meet the definition of an asset and thus are expensed as incurred
within general and administrative expenses.
Research
and Development Incentive Income
The
Company is eligible to obtain certain research and development tax credits, including, through its wholly owned subsidiary Anavex Australia,
the Australian research and development tax incentive credit (the “Australia R&D credit”) through a program administered
through the Australian Tax Office (the “ATO”) and AusIndustry, a division of the Australian Government’s Department
of Industry, Innovation and Science (“AusIndustry”). The Australia R&D credit program provides for a cash refund based
on a percentage of eligible research and development activities undertaken in Australia by Anavex Australia. Anavex Australia is also
eligible under the Australia R&D credit program to receive the cash refund for certain research and development expenses incurred
by Anavex Australia outside of Australia, to the extent such expenses are pre-approved by AusIndustry pursuant to an advanced overseas
finding application.
The
Australia R&D credit program is available to eligible companies with an annual aggregate revenue of less than $20.0 million Australian
during the reimbursable period at a rate of 18.5% above the claimant’s company tax rate in Australia.
The
tax incentives are available on the basis of specific criteria with which the Company must comply. Although the tax incentive may be
administered through the local tax authority, the Company has accounted for the incentives outside of the scope of ASC Topic 740, Income
Taxes (“ASC 740”), since the incentives are not linked to the Company’s taxable income and can be realized regardless
of whether the Company has generated taxable income in the respective jurisdictions.
With
respect to the Australia R&D credit, as there is no authoritative guidance under GAAP for accounting for grants to for-profit business
entities, the Company accounts for the grant by analogy to IAS20 Accounting for Government Grants and Disclosure of Government Assistance
(“IAS 20”). The Company recognizes the Research and Development Incentive income as it incurs costs eligible for reimbursement
under the Australia R&D credit program when it is reasonably assured that the cash incentive will be received, as evidenced through
enrollment in the program and when the applicable conditions under the program have been met. The Company accrues for the amount of cash
refund it expects to receive in relation to research and development expenses outside of Australia only to the extent it has received
advanced approval from AusIndustry, pursuant to an approved advanced overseas finding application.
In
addition, Anavex Australia and Anavex Canada incur Goods and Services Tax (GST) on certain services provided by local vendors. As a domestic
entity in those jurisdictions, Anavex Australia and Anavex Canada are entitled to a refund of the GST paid. Similarly, Anavex Germany
incurs Value Added Tax (VAT) on certain services provided by local vendors, to which it is entitled to a refund of such VAT paid. The
Company’s estimate of the amount of cash refund it expects to receive related to GST and VAT incurred is included in Incentive
and tax receivables in the accompanying consolidated balance sheets.
License
Fees
The
Company expenses amounts paid to acquire licenses associated with products under development when the ultimate recovery of the amounts
paid is uncertain and the technology has no alternative future use when acquired. Acquisitions of technology licenses are charged to
expense or capitalized based on management’s assessment regarding the ultimate recoverability of the amounts paid and the potential
for alternative future use. The Company has determined that the technological feasibility for its product candidates is reached when
the requisite regulatory approvals are obtained to make the product available for sale.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 12
Basic
and Diluted Loss per Share
Basic
income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the weighted average number
of common shares outstanding during the period. Diluted income/(loss) per common share is computed by dividing net income/(loss) available
to common stockholders by the sum of (1) the weighted-average number of common shares outstanding during the period, (2) the dilutive
effect of the assumed exercise of options and warrants using the treasury stock method and (3) the dilutive effect of other potentially
dilutive securities. For purposes of the diluted net loss per share calculation, options and warrants are potentially dilutive securities
and are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive.
As
of September 30, 2023, diluted loss per share excludes 14,271,780 potentially dilutive common shares (2022 –13,329,616; 2021 –
11,540,903) related to outstanding options and warrants, as their effect was anti-dilutive.
Financial
Instruments
The
book value of the Company’s financial instruments, consisting of cash and equivalents, incentive and tax receivables, accounts
payable and accrued liabilities approximate their fair value due to the short-term maturity of such instruments. Unless otherwise noted,
it is management’s opinion that the Company is not exposed to significant interest, currency or credit risks arising from these
financial instruments.
Foreign
Currency Translation
The
functional currency of the Company is the US dollar. Monetary items denominated in a foreign currency are translated into US dollars
at exchange rates prevailing at the balance sheet date and non-monetary items are translated at exchange rates prevailing when the assets
were acquired, or obligations incurred. Foreign currency denominated expense items are translated at exchange rates prevailing on the
transaction date. Unrealized gains or losses arising from the translations are credited or charged to income in the period in which they
occur.
The
Company has determined that the functional currency of Anavex Australia Pty Limited, Anavex Germany GmbH, and Anavex Canada Ltd. is also
the US dollar.
Segment
and Geographic Reporting
Operating
segments are defined as components of an enterprise for which separate discrete information is available for evaluation by the chief
operating decision maker or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views
its operations and manages its business as one operating segment, which is the business of developing novel therapies for the management
of CNS diseases.
Grant
Income
Grant
income is recognized at the fair value of the grant when it is received, and all substantive conditions have been satisfied. Grants received
from government and other agencies in advance of the specific research and development costs to which they relate are deferred and recognized
in the consolidated statements of operations in the period they are earned, typically when the related research and development costs
are incurred.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 13
Income
Taxes
The
Company follows the provisions of ASC 740, which requires the asset and liability method of accounting for income taxes. Under the asset
and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences
between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets
and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences
are expected to be recovered or settled.
The
Company follows the provisions of ASC 740 regarding accounting for uncertainty in income taxes. The Company initially recognizes tax
positions in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities.
Such tax positions are initially and subsequently measured as the largest amount of tax benefit that is greater than 50% likely of being
realized upon ultimate settlement with the tax authority assuming full knowledge of the position and all relevant facts. Application
requires numerous estimates based on available information. The Company considers many factors when evaluating and estimating its tax
positions and tax benefits, and its recognized tax positions and tax benefits may not accurately anticipate actual outcomes. As additional
information is obtained, there may be a need to periodically adjust the recognized tax positions and tax benefits. These periodic adjustments
may have a material impact on the consolidated statements of operations.
The
Company recognizes interest and penalties related to current income tax expense on the interest income, net line, in the accompanying
consolidated statements of operations. Accrued interest and penalties, if any, are included in accrued liabilities on the consolidated
balance sheets.
Stock-based
Compensation
The
Company accounts for all stock-based payments and awards under the fair value method.
The
fair value of all share purchase options and warrants are expensed over their contractual vesting period, or over the expected performance
period for only the portion of awards expected to vest, in the case of milestone-based vesting, with a corresponding increase to additional
paid-in capital.
Compensation
costs for stock-based payments with graded vesting are recognized on a straight-line basis. Stock based compensation expense is adjusted
for actual forfeitures of unvested awards as they occur.
The
Company has granted share purchase option awards that vest upon achievement of certain performance criteria, or milestone-based awards.
The Company estimates an implicit service period for achieving performance criteria for each award and recognizes the resulting fair
value as expense over the implicit service period when it concludes that achieving the performance criteria is probable. The Company
periodically reviews and updates as appropriate its estimates of implicit service periods and conclusions on achieving the performance
criteria. Performance awards vest upon achievement of the performance criteria.
The
Company uses the Black-Scholes option valuation model to calculate the fair value of share purchase options and warrants at the date
of the grant. This model requires the input of subjective assumptions, including the expected price volatility and expected life of each award.
The Company uses the U.S. Treasury daily treasury yield curve rates for the expected term of the option as the risk-free rate. The expected
term represents the period that options granted are expected to be outstanding using the simplified method. The Company’s historical
share option exercise experience does not provide sufficient basis for estimating the expected term. Expected volatility is based on
the average of the daily share price changes over the expected term. The Company does not estimate forfeitures and elects to record actual
forfeitures as they occur. The Company has not paid any dividends on its common stock historically, therefore no assumption of dividend
payments is made in the model. These assumptions consist of estimates of future market conditions, which are inherently uncertain, and
therefore, are subject to management’s judgment. Changes in these assumptions can materially affect the fair value estimates.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 14
The
purchase price of options or warrants may be paid in cash or, if approved by the Company’s compensation committee (or in the case
of warrants by the Board of Directors) in advance, “net settled” in shares of the Company’s common stock. In a net
settlement of an option or warrant, the Company does not receive payment of the exercise price from the holder but reduces the number
of shares of common stock issued upon the exercise of the stock option or warrant by the smallest number of whole shares that have an
aggregate fair market value equal to or over the aggregate exercise price for the option shares covered by the option or warrant exercised.
Shares issued pursuant to the exercise of options and warrants are issued from the Company’s treasury.
Fair
Value Measurements
Fair
value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date.
Assets and liabilities that are measured at fair value are reported using a three-level fair value hierarchy that prioritizes the inputs
used to measure fair value. This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three
levels of inputs used to measure fair value are as follows:
Level
1 - quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at
the measurement date;
Level
2 - observable inputs other than Level 1, quoted prices for similar assets or liabilities in active markets, quoted prices for
identical or similar assets and liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose
significant value drivers are observable; and
Level
3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant
to the fair value of the assets or liabilities.
At
September 30, 2023 and 2022, the Company did not have any Level 2 or Level 3 assets or liabilities.
Recently
Adopted Accounting Pronouncements
Effective
October 1, 2021, the Company adopted Accounting Standards Update (“ASU”) 2019-12, “Simplifying the Accounting for Income
Taxes (ASC 740)”, which is intended to simplify various aspects related to accounting for income taxes by removing certain exceptions
to the general principles in Topic 740 and clarifying and amending existing guidance to improve consistent application. There was no
material impact on the Company’s operations, financial condition, or cash flows.
On
October 1, 2022, the Company adopted ASU 2021-10, Annual Disclosure Requirements for Business Entities Receiving Government Assistance
(Topic 832) – Disclosures by Business Entities about Government Assistance, which requires business entities to disclose information
about transactions with a government that are accounted for by applying a grant or contribution model by analogy. For transactions within
scope, the new standard requires the disclosure of information about the nature of the transaction, including significant terms and conditions,
as well as the amounts and specific financial statement line items affected by the transaction. The disclosure of the Company’s
research and development tax incentive income and receivable is detailed in Note 4.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 15
Note
3 Accrued Liabilities
The
principal components of accrued liabilities consist of (in thousands):
| Schedule of principal components of accrued liabilities |
|
|
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
| Accrued
clinical site and patient visits costs |
|
$ |
2,006 |
|
|
$ |
2,031 |
|
| Accrued
compensation and benefits |
|
|
1,360 |
|
|
|
1,298 |
|
| Fixed
contract accruals |
|
|
38 |
|
|
|
417 |
|
| Milestone
based contract accruals |
|
|
1,267 |
|
|
|
137 |
|
| All
other accrued liabilities |
|
|
2,624 |
|
|
|
2,062 |
|
| Total
accrued liabilities |
|
$ |
7,295 |
|
|
$ |
5,945 |
|
Note
4 Other Income
Grant
income
During
the year ended September 30, 2023, the Company received $0.5 million (2022: $0 2021: $0.5 million) of a $1.0 million research grant awarded
during the year ended September 30, 2021, by the Michael J. Fox Foundation for Parkinson’s Research. The grant will be used to
fund a clinical trial of the Company’s lead compound, ANAVEX®2-73 (blarcamesine) related to Parkinson’s disease.
The
grant income was deferred when received and amortized to other income as the related research and development expenditures are incurred.
During the year ended September 30, 2023, the Company recognized $25,000 (2022: $0; 2021: $54,100) of this grant on its statements of
operations within grant income. At September 30, 2023 an amount of $0.9 million (2022: $0.4 million) of this grant is recorded as deferred
grant income, representing the amount of this grant which has not yet been amortized to other income. The Company will recognize this
income on its statements of operations as the related expenditures are incurred to offset the income.
Research
and development incentive income
Research
and development incentive income represents the income earned by Anavex Australia of the Australia R&D credit. This cash incentive
is received by Anavex Australia, upon filing of a claim in connection with Anavex Australia’s annual income tax return.
During
the year ended September 30, 2023, the Company recorded research and development incentive income of $2.7 million (AUD 4.1 million) (2022:
$3.3 million (AUD 4.5 million); 2021: $4.5 million (AUD 6.1 million)) in respect of the Australia R&D credit for eligible research
and development expenses incurred during the year. This amount is included within Other income (expense) on the consolidated statements
of operations.
At
September 30, 2023, Incentive and tax receivables includes $2.5 million (AUD 3.9 million) (2022: $2.9 million (AUD 4.5 million) relating
to Australia R&D credits earned during the year that are expected to be reimbursed upon filing of the Company’s annual claim
under this program.
The
Australia R&D credit program is a self-assess program whereby the Company must assess its eligibility each year to determine (i)
if the entity is eligible (ii) if the specific R&D activities are eligible and (iii) if the individual R&D expenditures have
nexus to such R&D activities. The Company evaluates its eligibility under the tax incentive program as of each balance sheet date
based on the most current and relevant data available. Anavex Australia is able to continue to claim the R&D tax incentive for as
long as it remains eligible and continues to incur eligible research and development expenditures.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 16
Although
the Company believes that it has complied with all the relevant conditions of eligibility under the program for all periods claimed,
the ATO has the right to review the Company’s qualifying programs and related expenditures for a period of four years. If such
a review were to occur, the ATO may have different interpretations of certain eligibility requirements. If the ATO disagreed with the
Company’s assessments and any related subsequent appeals, it could require adjustment to and repayment of current or previous years’
claims already received. Additionally, if the Company was unable to demonstrate a reasonably arguable position taken on such claims,
the ATO could also assess penalties and interest on such adjustment.
Currently,
the Company’s tax incentive claims from 2019 to 2022 are open to potential review by the ATO. Additionally, the period open for
review is indefinite if the ATO suspects fraud. The Company has not provided any allowance for any such potential adjustments, should
they occur in the future.
Note
5 Equity Offerings
Common
Stock
Common
shares are voting and are entitled to dividends as declared at the discretion of the Board of Directors.
Preferred
Stock
The
Company’s Board of Directors (the “Board”) has the authority to issue preferred stock in one or more series
and to fix the rights, preferences, privileges, restrictions and the number of shares constituting any series or the designation of the
series.
Registered
Direct Offering
On
June 22, 2021, the Company and Deep Track Capital entered into a securities purchase agreement pursuant to which the Company sold to
Deep Track Capital an aggregate of 2,380,953 shares of common stock at $21 per share in a registered direct offering, for gross proceeds
of $50 million. Net proceeds of the offering were $46,902,981 after deducting offering fees and expenses.
Sales
Agreement
The
Company entered into a Controlled Equity Offering Sales Agreement on July 6, 2018, which was amended and restated on May 1, 2020 (the
“Sales Agreement”) with Cantor Fitzgerald & Co. and SVB Leerink LLC (together the “Sales Agents”), pursuant
to which the Company may offer and sell shares of common stock registered under an effective registration statement from time to time
through the Sales Agents (the “Offering”).
Upon
delivery of a placement notice based on the Company’s instructions and subject to the terms and conditions of the Sales Agreement,
the Sales Agents may sell the Shares by methods deemed to be an “at the market offering” offering, in negotiated transactions
at market prices prevailing at the time of sale or at prices related to such prevailing market prices, or by any other method permitted
by law, including negotiated transactions, subject to the prior written consent of the Company. The Company is not obligated to make
any sales of Shares under the Sales Agreement. The Company or Sales Agents may suspend or terminate the offering of Shares upon notice
to the other party, subject to certain conditions. The Sales Agents will act as agent on a commercially reasonable efforts basis consistent
with their normal trading and sales practices and applicable state and federal law, rules and regulations and the rules of
Nasdaq.
The
Company has agreed to pay the Sales Agents commissions for their services of up to 3.0% of the gross proceeds from the sale of the Shares
pursuant to the Sales Agreement. The Company also agreed to provide the Sales Agents with customary indemnification and contribution
rights. During the year ended September 30, 2023, no shares were sold pursuant to the Offering. During the year ended September 30, 2022,
1,623,813 shares were sold pursuant to the Offering for gross proceeds of $21.0 million (net proceeds of $20.3 million after deducting
offering expenses) (2021: 5,634,576 shares were sold for gross proceeds of $79.1 million, net proceeds of $76.7 million). At September
30, 2023, an amount of $142.4 million (2022: $142.4 million) was registered pursuant to an effective registration statement. The Company
currently does not have access to sell shares of common stock under the Sales Agreement.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 17
2023
Purchase Agreement
On
February 3, 2023, the Company entered into a $150.0 million purchase agreement (the “2023 Purchase Agreement”) with Lincoln
Park Capital Fund, LLC (“Lincoln Park”), pursuant to which the Company has the right to sell and issue to Lincoln Park, and
Lincoln Park is obligated to purchase, up to $150.0 million in value of its shares of common stock from time to time over a three-year
period until February 3, 2026.
In
consideration for entering into the 2023 Purchase Agreement, the Company issued to Lincoln Park 75,000 shares of common stock as a commitment
fee (the “initial commitment shares”) and agreed to issue up to an additional 75,000 shares pro rata, when and if, Lincoln
Park purchased, at the Company’s discretion, the $150.0 million aggregate commitment. The Company determined the fair value of
the initial commitment shares was $0.8 million with reference to the closing price of the Company’s shares on the Purchase Agreement
date. In addition, the Company incurred third party expenses of $0.1 million in connection with entering into the Purchase Agreement.
These amounts were expensed to other financing expense on the statements of operations during the year ended September 30, 2023.
During
the year ended September 30, 2023, the Company issued to Lincoln Park an aggregate of 3,288,943 (2022: 0) shares of common stock under
the 2023 Purchase Agreement, including 3,275,000 (2022: 0) shares of common stock for aggregate proceeds of $27.9 million (2022: $0)
and 13,943 (2022: 0) commitment shares.
At
September 30, 2023, an amount of $122.1 million remained available under the 2023 Purchase Agreement.
2019
Purchase Agreement
On
June 7, 2019, the Company entered into a $50.0 million purchase agreement (the “2019 Purchase Agreement”) with Lincoln Park,
as amended on July 1, 2020, pursuant to which the Company had the right to sell and issue to Lincoln Park, and Lincoln Park was obligated
to purchase, up to $50.0 million in value of its shares of common stock from time to time over a three-year period until July 1, 2022.
During
the year ended September 30, 2023 and 2022, the Company did not issue any shares to Lincoln Park under the 2019 Purchase Agreement.
During
the year ended September 30, 2021, the Company issued to Lincoln Park an aggregate of 4,086,209 shares of common stock under the 2019
Purchase Agreement, including 4,007,996 shares of common stock for an aggregate purchase price of $24.1 million and 78,213 commitment
shares.
At
September 30, 2023 and 2022, no shares remained available for issuance under the 2019 Purchase Agreement.
Note
6 Commitments and Contingencies
Lease
The
Company leases office space under an operating lease with an initial term of 12 months or less. Under the terms of the office lease,
the Company is required to pay its proportionate share of operating costs.
The
operating lease costs were as follows (in thousands):
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 18
| Schedule of operating lease costs |
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
| Operating lease costs |
|
$ |
118 |
|
|
$ |
73 |
|
|
$ |
131 |
|
Employee
401(k) Benefit Plan
The
Company has a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code. The plan covers all United States
based employees. United States based employees eligible to participate in the plan may contribute up to the current statutory limits
under the Internal Revenue Service regulations. The 401(k) plan permits the Company to make additional matching contributions on behalf
of contributing employees.
During
the year ended September 30, 2023, the Company made matching contributions under the 401(k) plan as follows (in thousands):
| Schedule of contributions under the plan |
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
Contributions
to 401(k) plan |
|
$ |
232 |
|
|
$ |
158 |
|
|
$ |
129 |
|
Litigation
The
Company is subject to claims and legal proceedings that arise in the ordinary course of business. Such matters are inherently uncertain,
and there can be no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of
any such matter will not have a material adverse effect upon the Company’s consolidated financial statements. The Company does
not believe that any of such pending claims and legal proceedings will have a material adverse effect on its consolidated financial statements.
Share
Purchase Warrants
At
September 30, 2023 and 2022, the Company had 160,000 warrants outstanding at a weighted average exercise price of $3.72 as follows:
| Schedule of share purchase warrants outstanding |
|
|
|
|
| Number |
|
Exercise Price |
|
Expiry Date |
| |
150,000 |
|
|
$ |
3.17 |
|
|
|
May 6, 2024 |
|
| |
10,000 |
|
|
$ |
12.00 |
|
|
|
April 21, 2026 |
|
| |
160,000 |
|
|
|
|
|
|
|
|
|
Stock–based
Compensation Plan
2015
Stock Option Plan
On
September 18, 2015, the Company’s board of directors approved a 2015 Omnibus Incentive Plan (the “2015 Plan”), which
provided for the grant of stock options and restricted stock awards to directors, officers, employees and consultants of the Company.
The
maximum number of our common shares reserved for issue under the plan was 6,050,553 shares, subject to adjustment in the event of a change
of the Company’s capitalization.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 19
2019
Stock Option Plan
On
January 15, 2019, the Board approved the 2019 Omnibus Incentive Plan (the “2019 Plan”), which provides for the grant of stock
options and restricted stock awards to directors, officers, employees, consultants and advisors of the Company.
The
maximum number of our common shares reserved for issue under the plan was 6,000,000 shares, subject to adjustment in the event of a change
of the Company’s capitalization.
During
the year ended September 30, 2022, 406,453 options previously available under the 2019 Plan and the 2015 Plan became available under
the 2022 Plan (as defined below).
2022
Stock Option Plan
On
March 25, 2022, the Board approved the 2022 Omnibus Incentive Plan (the “2022 Plan”). The 2022 Plan was approved by stockholders
on May 24, 2022. Under the terms of the 2022 Plan, 10,000,000 additional shares of Common Stock will be available for issuance under
the plan, in addition to the shares available under the 2019 Plan and the 2015 Plan. Any awards outstanding under a previous stock option
plan will remain subject to and be paid under such plan, and any shares subject to outstanding awards under a previous plan that subsequently
cease to be subject to such awards (other than by reason of settlement of the awards in shares) will automatically become available for
issuance under the 2022 Plan.
The
2022 Plan provides that it may be administered by the Board, or the Board may delegate such responsibility to a committee. The exercise
price will be determined by the Board at the time of grant shall be at least equal to the fair market value on such date. If the grantee
is a 10% stockholder on the grant date, then the exercise price shall not be less than 110% of fair market value of the Company’s
shares of common stock on the grant date. Stock options may be granted under the 2022 Plan for an exercise period of up to ten years
from the date of grant of the option or such lesser periods as may be determined by the Board, subject to earlier termination in accordance
with the terms of the 2022 Plan. At September 30, 2023, 3,849,917 options had been issued under the 2022 Plan and 6,661,535 options were
available for issue under the 2022 Plan, subject to stockholder approval.
The
following summarizes information about stock option activity during the years ended September 30, 2023 and 2022:
| Schedule of stock option activity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
|
|
| |
|
|
|
Weighted Average |
|
Average Grant |
|
Aggregate intrinsic |
| |
|
Number of |
|
Exercise Price |
|
Date Fair Value |
|
value |
| |
|
Options |
|
($) |
|
($) |
|
($) |
| Outstanding,
September 30, 2021 |
|
|
11,330,903 |
|
|
|
5.74 |
|
|
|
— |
|
|
|
140,132,451 |
|
| Granted |
|
|
2,358,000 |
|
|
|
10.13 |
|
|
|
7.07 |
|
|
|
— |
|
| Forfeited |
|
|
(118,750 |
) |
|
|
6.86 |
|
|
|
5.23 |
|
|
|
— |
|
| Exercised |
|
|
(400,537 |
) |
|
|
2.52 |
|
|
|
1.88 |
|
|
|
4,201,015 |
|
| Outstanding,
September 30, 2022 |
|
|
13,169,616 |
|
|
|
6.61 |
|
|
|
4.96 |
|
|
|
62,267,309 |
|
| Granted |
|
|
1,959,000 |
|
|
|
9.30 |
|
|
|
6.60 |
|
|
|
— |
|
| Expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Exercised |
|
|
(759,753 |
) |
|
|
2.34 |
|
|
|
0.95 |
|
|
|
4,629,026 |
|
| Forfeited |
|
|
(257,083 |
) |
|
|
12.00 |
|
|
|
6.74 |
|
|
|
— |
|
| Cancelled |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Outstanding,
September 30, 2023 |
|
|
14,111,780 |
|
|
|
7.12 |
|
|
|
5.27 |
|
|
|
22,290,069 |
|
| Exercisable,
September 30, 2023 |
|
|
9,604,614 |
|
|
|
5.19 |
|
|
|
3.94 |
|
|
|
22,290,069 |
|
The
following summarizes information about stock options at September 30, 2023 by a range of exercise prices:
| Schedule of summarizes information about stock options |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
|
|
|
|
|
|
| |
|
|
|
|
|
average |
|
Weighted |
|
|
|
|
| |
|
|
|
Number of |
|
remaining |
|
average |
|
|
|
Weighted |
| Range of exercises
prices |
|
outstanding |
|
contractual |
|
exercise |
|
Number of |
|
average |
| From |
|
To |
|
options |
|
life |
|
price |
|
vested options |
|
exercise price |
| $ |
0.92 |
|
|
|
3.00 |
|
|
|
3,280,309 |
|
|
|
4.74 |
|
|
|
2.39 |
|
|
|
3,280,309 |
|
|
$ |
2.39 |
|
| $ |
3.01 |
|
|
|
5.00 |
|
|
|
2,017,500 |
|
|
|
4.32 |
|
|
|
3.28 |
|
|
|
2,017,500 |
|
|
$ |
3.28 |
|
| $ |
5.01 |
|
|
|
9.00 |
|
|
|
5,260,054 |
|
|
|
6.21 |
|
|
|
6.90 |
|
|
|
3,304,137 |
|
|
$ |
6.10 |
|
| $ |
9.01 |
|
|
|
13.00 |
|
|
|
1,981,917 |
|
|
|
8.37 |
|
|
|
10.55 |
|
|
|
480,584 |
|
|
$ |
11.45 |
|
| $ |
13.01 |
|
|
|
25.00 |
|
|
|
1,572,000 |
|
|
|
7.49 |
|
|
|
18.29 |
|
|
|
522,084 |
|
|
$ |
18.57 |
|
| |
|
|
|
|
|
|
|
|
14,111,780 |
|
|
|
6.04 |
|
|
|
7.12 |
|
|
|
9,604,614 |
|
|
$ |
5.19 |
|
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 20
The
weighted average per share fair value of options vested during the year ended September 30, 2023 was $3.94 (2022: $4.69, 2021: $2.54).
At September 30, 2023, the weighted average contractual life of options outstanding was 6.0 years (2022: 6.4 years) and for options exercisable
was 4.75 years (2022: 5.1 years).
The
aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the quoted market price
of the Company’s stock for the options that were in-the-money at September 30, 2023.
The
Company recognized stock-based compensation expense of $16.4 million during the year ended September 30, 2023 (2022: $18.4 million; 2021:
$8.2 million) in connection with the issuance and vesting of stock options in exchange for services. These amounts have been included
in general and administrative expenses and research and development expenses on the Company’s consolidated statements of operations
as follows (in thousands):
| Schedule of general and administrative expenses and research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
| General
and administrative |
|
$ |
5,558 |
|
|
$ |
7,129 |
|
|
|
3,571 |
|
| Research
and development |
|
|
10,812 |
|
|
|
11,250 |
|
|
|
4,660 |
|
| Total
stock-based compensation |
|
$ |
16,370 |
|
|
$ |
18,379 |
|
|
|
8,231 |
|
An
amount of approximately $14.1 million in stock-based compensation is expected to be recorded over the remaining term of such options
and warrants through fiscal 2026.
The
fair value of each option and warrant award is estimated on the date of grant using the Black Scholes option pricing model based on the
following weighted average assumptions:
| Schedule of weighted average assumptions for fair value of each option award |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Risk-free
interest rate |
|
|
3.70 |
% |
|
|
3.11 |
% |
|
|
0.73 |
% |
| Expected
life of options (years) |
|
|
5.64 |
|
|
|
5.57 |
|
|
|
5.74 |
|
| Annualized
volatility |
|
|
85.13 |
% |
|
|
84.17 |
% |
|
|
93.43 |
% |
| Dividend
rate |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
The
fair value of stock compensation charges recognized during the years ended September 30, 2023, 2022 and 2021 was determined with reference
to the quoted market price of the Company’s shares on the grant date.
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 21
Note
7 Income Taxes
The
Company’s U.S. and foreign loss before income taxes are set forth below (in thousands):
| Schedule of loss before
income taxes |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| United
States |
|
$ |
(41,198 |
) |
|
$ |
(40,002 |
) |
|
$ |
(28,851 |
) |
| Foreign |
|
|
(6,300 |
) |
|
|
(7,618 |
) |
|
|
(8,790 |
) |
The
components of net deferred income tax assets as of September 30, 2023, 2022 and 2021 are as follows (in thousands):
| Schedule of components of
net deferred income tax assets |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Net
operating loss carryforwards |
|
$ |
46,462 |
|
|
$ |
46,208 |
|
|
$ |
34,982 |
|
| Research
and development tax credit carryforwards |
|
|
2,713 |
|
|
|
2,182 |
|
|
|
1,577 |
|
| Stock-based
compensation |
|
|
18,593 |
|
|
|
13,373 |
|
|
|
10,453 |
|
| Research
and development capitalization |
|
|
7,219 |
|
|
|
— |
|
|
|
— |
|
| Unpaid
charges |
|
|
1,559 |
|
|
|
894 |
|
|
|
89 |
|
| Intangible
asset costs |
|
|
593 |
|
|
|
388 |
|
|
|
323 |
|
| Foreign
exchange and other |
|
|
49 |
|
|
|
44 |
|
|
|
62 |
|
| Valuation
allowance of deferred tax assets |
|
|
(77,188 |
) |
|
|
(63,089 |
) |
|
|
(47,486 |
) |
| Net
deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
A
reconciliation of income tax expense at the statutory federal income tax rate and income taxes as reflected in the consolidated financial
statements for the years ended September 30, 2023, 2022 and 2021 is as follows (in thousands):
| Schedule
of reconciliation of income tax expense |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Income
tax benefit at statutory federal rate |
|
$ |
(9,975 |
) |
|
$ |
(10,000 |
) |
|
$ |
(7,934 |
) |
| Foreign
income taxed at other rates |
|
|
— |
|
|
|
(170 |
) |
|
|
(353 |
) |
| Permanent
differences relating to stock based compensation |
|
|
(601 |
) |
|
|
(714 |
) |
|
|
(4,379 |
) |
| Permanent
differences relating to GILTI inclusion |
|
|
165 |
|
|
|
— |
|
|
|
— |
|
| Permanent
differences relating to Section 162(m) |
|
|
— |
|
|
|
— |
|
|
|
816 |
|
| Other
permanent differences |
|
|
273 |
|
|
|
— |
|
|
|
741 |
|
| Adjustment
to tax assets based on Section 382 |
|
|
— |
|
|
|
— |
|
|
|
3,330 |
|
| Research
and development credits, net |
|
|
(37 |
) |
|
|
232 |
|
|
|
1,042 |
|
| State
and local taxes |
|
|
(4,122 |
) |
|
|
(4,975 |
) |
|
|
(7,022 |
) |
| Adjustment
to true up to prior years' tax provision |
|
|
206 |
|
|
|
24 |
|
|
|
48 |
|
| Effect
of change in statutory tax rates |
|
|
— |
|
|
|
— |
|
|
|
216 |
|
| State
minimum and excise taxes |
|
|
— |
|
|
|
358 |
|
|
|
268 |
|
| Change
in valuation allowances |
|
|
14,098 |
|
|
|
15,603 |
|
|
|
13,495 |
|
| Income
tax expense |
|
$ |
7 |
|
|
$ |
358 |
|
|
$ |
268 |
|
Anavex
Life Sciences Corp.
Notes
to the Consolidated Financial Statements
September
30, 2023 – Page 22
As
of September 30, 2023, the Company had U.S. federal net operating loss carryforwards of approximately $126.3 million (2022: $123.4 million)
of which $37.7 million will begin to expire in 2025 and $88.6 million can be carried forward indefinitely, state and local net operating
loss carryforwards of approximately $ 16.6 million (2022: $17.2 million) which will begin to expire in 2036, and Research and Development
tax credits of approximately $2.7 million (2022: $2.2 million) which will begin to expire in 2029. The Company had approximately $12.9
million (approximately AU$20.1 million) (2022: $10.6 million (approximately AU$ 14.9 million)) of net operating loss carryforwards in
Australia, which have an indefinite life, available to offset future taxable income in those jurisdictions.
The
Company evaluates its valuation allowance requirements based on available evidence. When circumstances change, and this causes a change
in management’s judgment about the recoverability of deferred tax assets, the impact of the change on the valuation allowance is
reflected in current income. Because management of the Company does not currently believe that it is more likely than not that the Company
will receive the benefit of these assets, a valuation allowance has been established at September 30, 2023 and 2022.
The
Tax Cuts and Jobs Act of 2017 (TCJA) has modified the IRC 174 expenses related to research and development for tax years beginning after
December 31, 2021. Under the TCJA, the Company must now capitalize the expenditures related to research and development activities and
amortize over five years for U.S. activities and 15 years for non-U.S. activities using a mid-year convention. Therefore, the capitalization
of research and development costs in accordance with IRC 174 resulted in a gross deferred tax asset of $7.2 million.
Uncertain
Tax Positions
The
Company files income tax returns in the U.S. federal jurisdiction and various state and local and foreign jurisdictions. The Company’s
tax returns are subject to tax examinations by U.S. federal and state tax authorities, or examinations by foreign tax authorities until
the respective statutes of limitation expire. The Company is subject to tax examinations by tax authorities for all taxation years commencing
on or after 2005.
Under
the provisions of the Internal Revenue Code, the net operating loss (“NOL”) carryforwards are subject to review and possible
adjustment by the Internal Revenue Service and state tax authorities. Under Section 382 of the Internal Revenue Code, NOL and tax credit
carryforwards may become subject to an annual limitation in the event of an over 50% cumulative change in the ownership interest of significant
stockholders over a three-year period, as well as similar state tax provisions.
The
Company conducted a Section 382 study during the year ended September 30, 2021 and determined that, during the year ended September 30,
2015, there was a change in ownership which resulted in $25.8 million of federal NOLs being subject to an annual limitation. During the
year ended September 30, 2021, the Company reduced its federal NOLs by $12.1 million and its Research and Development tax credit carryforwards
by $0.8 million, which are the amount of tax assets that will expire unutilized pursuant to the Section 382 study. This resulted in a
reduction of $2.5 million of NOLs and $0.8 million of research and development credits and a corresponding reduction in the valuation
allowance of $3.3 million, which was recorded in the 2021 fiscal year. Subsequent ownership changes in future years could trigger additional
limitations of the Company’s NOLs. During the year ended September 30, 2023 and 2022 the
Company determined that there were no changes in ownership pursuant to Section 382.
As
of September 30, 2023, the Company did not provide any foreign withholding taxes related to its foreign subsidiaries’ undistributed
earnings, as such earnings have been retained and are intended to be indefinitely reinvested to fund ongoing operations of the foreign
subsidiaries. It is not practicable to estimate the amount of taxes that would be payable upon remittance of these earnings, because
such tax, if any, is dependent upon circumstances existing if and when remittance occur.
Note
8 Subsequent Events
The
Company evaluates subsequent events occurring between the most recent balance sheet date and the date the financial statements are available
to be issued in order to determine whether the subsequent events are to be recorded and/or disclosed in the Company’s financial
statements and footnotes. The financial statements are considered to be available to be issued at the time they are filed with the Securities
and Exchange Commission (SEC).
There
were no subsequent events or transactions that required recognition or disclosure in the consolidated financial statements.
ITEM 9. CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL MATTERS
Not Applicable
ITEM 9A. CONTROLS
AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures
that are designed to provide reasonable assurance that material information required to be disclosed in our periodic reports filed
under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules
and forms and to provide reasonable assurance that such information is accumulated and communicated to our management, our chief
executive officer and our principal financial officer, to allow timely decisions regarding required disclosure.
We carried out an evaluation, under the supervision
and with the participation of our management, including our principal executive and principal financial officer, of the effectiveness
of the design and operation of our disclosure controls and procedures, as defined in Rule 13(a)-15(e) under the Exchange
Act. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls
and procedures were effective as of September 30, 2023.
Management’s Annual Report on Internal
Control over Financial Reporting
Our management is responsible for establishing
and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange
Act. Under the supervision and with the participation of our management, including our Principal Executive Officer and Principal
Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on criteria
established in the framework in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring
Organizations of the Treadway Commission (“COSO”).
Because of its inherent limitations,
internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness
to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
Based on this evaluation, our management
concluded that our internal controls over financial reporting were effective as of September 30, 2023.
The effectiveness of our internal control over
financial reporting as of September 30, 2023, has been audited by Grant Thornton LLP, an independent registered public accounting
firm, as stated in their report which appears in this Annual Report on Form 10-K.
Changes in Internal Control over Financial
Reporting
During the quarter ended September 30,
2023, there were no material changes in our internal control over financial reporting identified in management’s evaluation
pursuant to Rules 13a 15(d) or 15d 15(d) of the Exchange Act that materially affected, or are reasonably likely to materially affect,
our internal controls over financial reporting.
ITEM 9B OTHER
INFORMATION
None.
ITEM 9C DISCLOSURE
REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10 DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
Our directors are to be elected at our
annual meeting and each director elected is to hold office until his or her successor is elected and qualified. Our Board of Directors
may remove our officers at any time.
Our directors and executive officers,
their ages, positions held, and duration of such, are as follows:
| Name |
|
Position |
Age |
|
Date first appointed |
| Christopher Missling, PhD |
|
Director, President, Chief |
58 |
|
July 5, 2013 |
| |
|
Executive Officer, Secretary |
|
|
|
| Jiong Ma, PhD |
|
Director, Chair |
59 |
|
May 25, 2021 |
| Athanasios Skarpelos |
|
Director |
56 |
|
January 9, 2013 |
| Claus van der Velden, PhD |
|
Director |
51 |
|
March 2, 2018 |
| Steffen Thomas, PhD |
|
Director |
57 |
|
June 15, 2015 |
| Peter Donhauser, D.O. |
|
Director |
58 |
|
February 8, 2017 |
| Sandra Boenisch, CPA, CGA |
|
Principal Financial Officer, |
42 |
|
October 1, 2015 |
| |
|
Treasurer |
|
|
|
Board Leadership Structure
The Board of Directors is composed of
a majority of independent directors and the Chief Executive Officer of the Company.
Our Board of Directors has appointed
an independent Board Chair, Dr. Jiong Ma. As Board Chair, Dr. Ma has the authority, among other things, to call and preside over
meetings of our Board, to set meeting agendas, and to determine materials to be distributed to the Board. The Company believes
separation of the positions of Board Chair and Chief Executive Officer reinforces the independence of our Board in its oversight
of the business and affairs of the Company. In addition, the Company believes that having an independent Board Chair creates an
environment that is more conducive to objective evaluation and oversight of management’s performance, increasing management
accountability and improving the ability of our Board to monitor whether management’s actions are in the best interests of
the Company and its stockholders.
The Audit Committee, Compensation Committee,
and Nominating and Corporate Governance Committee each have oversight over specific areas of responsibility, as discussed further
below.
Board of Directors’ Role in Risk
Oversight
The Board of Directors is responsible
for oversight of the Company’s risk management process. The Board administers this oversight function directly through the
Board of Directors as a whole, as well as through the committees of the board. Areas of focus include economic risk, operational
risk, financial risk (accounting, investment or liquidity, and tax), competitive risk, legal and regulatory risk, cybersecurity
risk and compliance and reputational risks. The Board of Directors is supported by regular reporting by management, which is designed
to give the Board of Directors visibility over the Company’s operations and activities to adequately identify key risks and
understand management’s risk mitigation strategies.
Business Experience
The following is a brief account of
the education and business experience of directors and executive officers during at least the past five years, indicating their
principal occupations during the period, and the names and principal businesses of the organizations by which they were employed.
Christopher Missling, PhD. Christopher
Missling has over twenty years of healthcare industry experience in big pharmaceutical, biotech and investment banking. Most recently,
from March 2007 until his appointment by our Company, Dr. Missling served as the head of healthcare investment banking at Brimberg
& Co. in New York, New York. In addition, Dr. Missling served as the Chief Financial Officer of Curis, Inc. (NASDAQ:CRIS) and
ImmunoGen, Inc. (NASDAQ:IMGN). Dr. Missling earned his MS and PhD from the University of Munich and an MBA from Northwestern University
Kellogg School of Management and WHU Otto Beisheim School of Management.
Jiong Ma, PhD. Jiong Ma has over 25
years of experience in the investing, building and scaling of companies with a focus on innovative product launches in digital
health, technology and the new energy transition. Dr. Ma currently serves on the board of SES AI Corporation (NYSE: SES), and LinkinVax,
a biotech company, spin-off from the Vaccine Research Institute in France. Dr. Ma served as senior partner and a member of the
investment committee at Braemar Energy Ventures (“Braemar”). While at Braemar, Dr. Ma led investments in more than
15 companies involved in either resource efficiency, e-mobility, industrial digitalization, renewable energy, or deep tech, and
has achieved multiple successful exits through M&A and IPO. Dr. Ma has significant knowledge and expertise in the technology
industry, including information security. Prior to Braemar Energy Ventures, she was with the Venture Capital Group at 3i Group,
a global private equity firm, where she led investments across multiple stages in Digital Health, TMT and Cleantech. Preceding
the Venture Capital Group at 3i, Dr. Ma held several senior positions at Lucent Technologies and Bell Labs. Her responsibilities
included lead roles in product portfolio strategy, new product launches for Optical and Data Networking, and research and product
development. Dr. Ma was also a founding team member of Onetta Inc., a fiber networks company. She has a PhD in Electrical and Computer
Engineering from the University Colorado at Boulder and an MS in Electrical Engineering from Worcester Polytechnic Institute. Dr.
Ma is a Kauffman Fellow.
Athanasios Skarpelos. Athanasios
(Tom) Skarpelos is a self-employed investor with 20 years of experience working with private and public companies with a focus
on biotechnology companies involved in drug discovery and drug development projects. His experience has led to relationships with
researchers at academic institutes in Europe and North America. Mr. Skarpelos is a founder of Anavex.
Claus van der Velden, PhD. Claus
van der Velden, PhD, brings significant expertise in management, accounting, internal controls, information security and risk management.
Since May 2021, he has served as Managing Director (Chief Financial Officer) of NetCologne
GmbH, a regional telecommunication provider in Germany. From July 2011 to May 2021, he served as corporate head of Management
Accounting, Internal Audit and Risk Management at Stroeer SE & Co KGaA, a publicly listed German digital media company. Previously,
Dr. van der Velden served as the Director of Corporate Business Controlling for the Nutrition & Health business unit at Cognis,
a worldwide supplier of global nutritional ingredients and specialty chemicals. In this position, he was also a compliance representative
and a member of the global leadership team. After the acquisition of Cognis by BASF, he was responsible for the management accounting
processes of the BASF Nutrition & Health division, developing and producing mostly natural-source ingredients for the food
and healthcare industries. Dr. van der Velden started his career as a strategy consultant at an international marketing and strategy
consultancy firm. He studied in Kiel and Stockholm and received a degree in economics from the University of Kiel and later obtained
his doctorate in business management from the WHU-Otto Beisheim School of Management where he also previously taught economics.
Steffen Thomas, PhD. Steffen
Thomas, has over 15 years of experience as a European patent attorney and is currently practicing at Epping Hermann Fischer, a
major intellectual property law firm in Europe. Previously, he worked for Japan-based Takeda Pharmaceutical Company, the largest
pharmaceutical company in Asia and a top firm worldwide, as an in-house patent attorney. Prior to that, he worked for Nycomed Pharma,
acquired by Takeda in 2011 for approximately USD $10 billion. Dr. Thomas’ legal practice covers drafting of patent applications,
prosecuting patent applications before national and international patent offices, defending and challenging patents in opposition,
appeal, and nullity proceedings, enforcing patents before the infringement courts, and preparing opinions on patentability and
infringement in the technical field of chemistry. Dr. Thomas has particular expertise in small molecule pharmaceuticals. He holds
MS and PhD degrees in Chemistry from the University of Munich.
Peter Donhauser, D.O. Peter Donhauser,
had more than 20 years of expertise in clinical research prior to practicing osteopathic medicine with an integrated medical approach
in private practice beginning in 2000. He worked at the University Hospital of Munich in the fields of geriatrics and neuromusculoskeletal
diseases. During this time, he was a clinical trial investigator in multiple Phase 3 studies, including studies sponsored by Merck
Sharp & Dohme, Merck, Boehringer Mannheim, Roche, Servier and Sanofi. He received his human medicine degree at the University
of Munich and Doctor of Osteopathic Medicine (D.O.) from the German-American Academy for Osteopathy, or DAAO, a member of the European
Register for Osteopathic Physicians, or EROP, at the Philadelphia College of Osteopathic Medicine.
Sandra Boenisch, CPA, CGA. Ms.
Boenisch is a Chartered Professional Accountant (CPA, CGA) with approximately 20 years of accounting, audit and financial reporting
experience in a variety of industries, both in the United States and Canada. Ms. Boenisch was an independent consultant, providing
financial reporting services to a range of public companies in the United States and Canada since January 2012. From 2008 until
2012, Ms. Boenisch was employed at BDO Canada LLP (Vancouver, BC) where she was hired as a Senior Accountant and was later promoted
to Manager, Audit Assurance. Ms. Boenisch specialized in managing assurance engagements for public companies in the United States
and Canada. Prior to that, Ms. Boenisch worked for another public accounting firm from 2001 to 2008. As an independent consultant,
Ms. Boenisch has acquired considerable experience in finance, governance, and regulatory compliance. She holds a BComm from Laurentian
University.
Family Relationships
There are no family relationships between
any director or executive officer.
Involvement in Certain Legal Proceedings
There are no material proceedings to
which any director or executive officer or any associate of any such director or officer is a party adverse to our Company or has
a material interest adverse to our Company.
Delinquent Section 16(a) Reports
None.
Business Code of Conduct & Ethics
We have adopted a code of conduct and
ethics that applies to our directors, principal executive officer, principal financial officer, principal accounting officer or
controller, or persons performing similar functions, and to all employees. We have posted our policy on our website at www.anavex.com/code-of-conduct.
Insider Trading Policy
Our Insider Trading Policy prohibits
our directors, officers, employees and consultants from engaging in short sales, transactions in publicly traded options such as
put, calls or other derivative securities, hedging transactions and other inherently speculative transactions with respect to our
stock at any time. Our policy further prohibits such persons from engaging in transactions involving any loan, pledge or other
transfer of beneficial ownership of the Company’s securities without obtaining advance clearance of the proposed transaction
from our Insider Trading Compliance Officer.
Information Regarding Committees of the
Board of Directors
The Board has an Audit Committee, a
Compensation Committee and a Nominating and Corporate Governance Committee. The following table provides membership and meeting
information for 2023:
| Name | |
Audit
Committee | |
Compensation
Committee | |
Nominating
and Corporate Governance Committee |
| Christopher
Missling, PhD | |
| | | |
| | | |
| | |
| Athanasios
Skarpelos | |
| X | | |
| | | |
| | |
| Claus
van der Velden, PhD | |
| X* | | |
| X* | | |
| X* | |
| Steffen
Thomas, PhD | |
| X | | |
| X | | |
| X | |
| Peter
Donhauser, D.O. | |
| | | |
| X | | |
| X | |
| Jiong
Ma, PhD | |
| X | | |
| | | |
| | |
| Meetings
in 2023 | |
| 4 | | |
| 1 | | |
| — | |
*Committee Chair
Audit Committee and Audit Committee Financial
Experts
The Audit Committee is composed of four
directors, each of whom is independent. The Audit Committee operates under a charter that was adopted by our Board of Directors.
The Audit Committee oversees and reports to our Board of Directors on various auditing and accounting-related matters, including,
among other things, the maintenance of the integrity of our financial statements, reporting process and internal controls; the
selection, evaluation, compensation and retention of our independent registered public accounting firm; legal and regulatory compliance,
including our disclosure controls and procedures; and oversight over our risk management policies and procedures.
Our Board of Directors has determined
that Claus van der Velden is an “audit committee financial expert” as defined by applicable SEC and NASDAQ rules.
The Audit Committee has reviewed and
discussed the audited consolidated financial statements with management. The Audit
Committee has discussed with the Company’s independent registered public accounting firm the matters required to be discussed
by the applicable requirements of the Public Company Accounting Oversight Board (“PCAOB”) and the Securities and Exchange
Commission (the “SEC”). In addition, the Audit Committee has received the written disclosures and the letter from the
independent registered public accounting firm required by applicable requirements of the PCAOB regarding the firm’s communications
with the Audit Committee concerning independence and has discussed with the independent registered accounting firm its independence
from the Company and management. Based on the reviews and discussions referred to above, the Audit Committee recommended to the
Board that the audited consolidated financial statements for the Company for the fiscal year ended September 30, 2023 be included
in this Annual Report on Form 10-K for the year ended September 30, 2023.
The foregoing report has been furnished
by the Audit Committee.
Claus van der Velden (Chairman)
Athanasios Skarpelos
Steffen Thomas
Jiong Ma
Compensation Committee
The Compensation Committee is composed
of three directors, each of whom is independent. The Compensation Committee operates under a charter that was adopted by our Board
of Directors. The Compensation Committee assists our Board of Directors in discharging its responsibilities relating to compensation
of our directors and executive officers. Its responsibilities include, among other things: reviewing, approving and recommending
compensation programs and arrangements applicable to our officers; determining the objectives of our executive officer compensation
programs; overseeing the evaluation of our senior executives; administering our incentive compensation plans and equity-based plans,
including reviewing and granting equity awards to our executive officers; and reviewing and approving director compensation and
benefits. The Compensation Committee can delegate to other members of our Board of Directors, or an officer or officers of the
Company, the authority to review and grant stock-based compensation for employees who are not executive officers.
The Compensation Committee has the responsibilities
and authority designated by NASDAQ rules and the Compensation Committee Charter. Specifically, the Compensation Committee has the
sole discretion to select and receive advice from a compensation consultant, legal counsel or other adviser and is directly responsible
for oversight of their work. The Compensation Committee must also determine reasonable compensation to be paid to such advisors
by us.
The Compensation Committee met one time
during fiscal 2023 and acted by written consent as required.
Nominating and Corporate Governance
Committee
The Nominating and Corporate Governance
Committee (the “NCG Committee”) is appointed by the board to oversee and evaluate the Board’s performance and
the Company’s compliance with corporate governance regulations, guidelines and principles, to identify individuals qualified
to become Board members, to recommend to the Board proposed nominees for board membership, and to recommend to the Board directors
to serve on each standing committee. The NCG Committee seeks to assemble a Board that possesses the appropriate balance of professional
and industry knowledge, financial expertise and management experience that is necessary to oversee the Company’s business.
The NCG also recognizes the importance of diversity in board composition, including diversity of experience, gender and ethnicity
and seeks to continually strive towards optimal diversity. The NCG Committee operates under a charter that was adopted by our Board
of Directors.
The NCG Committee did not meet during
fiscal 2023 but acted by written consent as required.
ITEM 11. EXECUTIVE
COMPENSATION
The Company’s compensation objectives
are to offer our executive officers’ compensation and benefits that are competitive and meet our goals of attracting, retaining
and motivating highly skilled, talented management, which is necessary for the Company to achieve its financial and strategic objectives
and create long-term value for our stockholders.
A significant portion of the Company’s
executive compensation opportunity is related to factors that directly and indirectly influence shareholder value, including long-term
stock performance and operational performance. We believe the levels of compensation we provide should be competitive, reasonable
and appropriate for our business needs and circumstances.
Our Executive Compensation Program
and Philosophy
The intent of the Company’s compensation
program is to attract and retain talent, to create incentives for and to reward excellent performance. We seek to compensate our
executives in a manner that is competitive, rewards performance that creates shareholder value, recognizes individual contributions,
and encourages long-term value creation.
The Compensation Committee meets at
least once per year to review and evaluate executive compensation and each executive officer’s performance. The Compensation
Committee utilizes quantitative and qualitative factors, including the accomplishment of initiatives, attitude, and leadership
and applies overall judgment to assess performance, taking into account the financial condition of the Company. In setting compensation,
the Compensation Committee considers the outcome of the most recent say-on-pay vote, as well as stockholder feedback throughout
the year, when making compensation decisions for our executive officers. In our most recent say-on-pay vote, conducted at our 2021
annual meeting of stockholders, held on May 25, 2021, our stockholders approved the compensation of our named executive officers
on an advisory basis, with 88% of the votes cast in favor of the fiscal 2021 compensation of our named executive officers. Ultimately,
the Compensation Committee seeks to evaluate, based on the achievement of financial and nonfinancial objectives, the variable compensation,
including special awards, of executive officers of the Company and decide on the base salary and target discretionary bonus for
such persons taking into account relevant benchmark data.
The Compensation Committee believes
that a significant portion of each executive’s compensation opportunity should be tied to variable compensation and value
creation for shareholders. The Compensation Committee believes this mix provides an appropriate balance between the financial security
required to attract and retain qualified individuals, and the Compensation Committee’s goal of ensuring that executive compensation
rewards performance that benefits shareholders over the long term.
Compensation Risk Oversight
In administering our compensation program,
the Compensation Committee strives to achieve a balance among the elements of compensation to accomplish the objectives of the
program. The Compensation Committee reviews the Company’s overall compensation program in the context of the risks that may
be presented by the structure of our compensation program and the metrics used to determine compensation under that program. Based
upon this review, the Compensation Committee believes that our compensation program does not create a reasonable likelihood of
a material adverse effect on the Company.
Compensation Consultants
The Compensation Committee makes recommendations
to the Board for all compensation for executives, including the structure and design of the compensation programs. The Compensation
Committee is responsible for retaining and terminating compensation consultants and determining the terms and conditions of their
engagement. During fiscal 2023, the Compensation Committee did not engage any compensation consultants.
Elements of Executive Compensation
We focus our executive compensation
program on three related but distinct elements: base salary, cash bonuses and stock-related compensation.
Base Salary
Base salaries take into consideration
a number of factors, including the executive’s job performance, our corporate performance, and compensation practices observed
in the market.
Annual Discretionary Cash Bonuses
The Company has an annual discretionary
cash bonus program. We provide such bonuses to motivate executive officers to perform on behalf of general corporate goals and
to perform in their areas of responsibility. The Compensation Committee, or Board of Directors, works with the Chief Executive
Officer to evaluate the Company’s financial performance and overall financial condition to determine if discretionary bonuses
are to be paid.
Equity Compensation
Equity compensation includes stock option
grants within the terms of our 2022 Omnibus Incentive Plan. Each executive officer is eligible for stock option grants under the
Omnibus Incentive Plans which may vest over a required service period, “Time Based Awards”, or that vest upon achievement
of certain performance criteria, “Performance Awards”. Generally, our Compensation Committee grants Time Based Awards
at or near the date of hire. Such grants are intended to link executive compensation with stockholder value over time. Subsequent
awards are granted as Performance Awards, which are intended to align executive compensation with the company’s short-term
and long-term objectives. Our compensation committee selects performance goals that reflect the company’s short-term and
longer-term objectives to ensure executives, as well as all employees, are rewarded for the successful and timely accomplishment
of these objectives. Generally, these performance criteria include milestones in connection with the successful execution, enrollment
and completion of the Company’s clinical trials. Only our Board of Directors, acting in its sole discretion, or the Compensation
Committee grants options or Performance Awards to our executive officers.
We view stock options as one of the
more important components of our long-term, performance-based compensation philosophy.
We provide options through initial grants
at or near the date of hire and subsequent periodic/annual grants. Generally, initial option grants vest over a three-year period
and have an exercise price equal to the fair market value of our stock at the time of grant. Initial grant amounts are based on
ranges that take into consideration an executive’s job responsibilities and competitive market data.
We grant periodic additional stock options
to reflect the individual’s ongoing contributions to the long-term success and growth of the Company, to incentivize individuals
to remain with the Company and to provide a long-term incentive to achieve or exceed our corporate goals. We do not have a program,
plan or practice to time stock option grants to our executives in coordination with the release of material nonpublic information.
We have not re-priced any of our options and do not intend to re-price or otherwise adjust outstanding options at any time in the
future.
Other Compensation
Other components of compensation include
employee medical benefit plans and 401(k) benefit plan contributions.
Employee Medical Benefit Plans.
Our employee medical and welfare benefit plans include medical, dental, life, disability and accidental death and dismemberment
insurance.
401(k) Plan. We have a defined-contribution
savings plan under Section 401(k) of the Internal Revenue Code. The plan covers all United States based employees. United States
based employees eligible to participate in the plan may contribute up to the current statutory limits under the Internal Revenue
Service regulations. The 401(k) plan permits the Company to make additional matching contributions on behalf of contributing employees.
Compensation Committee Interlocks
and Insider Participation
No
member of the Compensation Committee has ever been an officer or employee of the Company. None of the executive officers currently
serves or has served on the Compensation Committee or board of directors of any other entity that has one or more executive officers
serving as a member of the Board of Directors or Compensation Committee of the Company.
Managing Compensation-Related Risks
Although a portion of the compensation
provided to our executive officers and other employees is performance-based, the executive compensation program does not encourage
excessive or unnecessary risk taking. This is primarily due to the fact that the compensation program is designed to encourage
executive officers and other employees to remain focused on both short-term and long-term strategic goals within the context of
our pay-for-performance compensation philosophy.
Clawback Policy
In November
2023, the Board of Directors adopted an executive officer compensation clawback policy that may be applied in the event of a material
financial restatement. The clawback policy covers all of the named executive officers and includes all incentive-based compensation.
Specifically, in the event of an accounting restatement, the Company must recover, reasonably promptly, erroneously awarded compensation
in amounts determined pursuant to the policy. Compensation that may be recoverable under the policy includes cash or equity-based
compensation for which the grant, payment or vesting (or any portion thereof) is or was predicated upon the achievement of specified
financial results that are impacted by the material financial restatement, and the amount of compensation that may be impacted
by the clawback policy is the difference between the amount paid or granted, and the amount that should have been paid or granted,
if calculated on the updated financials. Recovery under the policy with respect to an executive officer will not require the finding
of any misconduct by such executive officer or such executive officer being found responsible for the accounting error leading
to an accounting restatement.
Compensation Committee Report
The Compensation Committee
has reviewed and discussed with management the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K.
Based on this review and discussion, the Compensation Committee has recommended to the Board of Directors that the Compensation
Discussion and Analysis be included in this Annual Report on Form 10-K for the fiscal year ended September 30, 2023.
The foregoing report has been
furnished by the Compensation Committee.
Claus van der Velden, Committee
Chair
Steffen Thomas
Peter Donhauser
Summary Compensation
The particulars of compensation paid
to our named executive officers for the last two completed fiscal years:
| Name
and Principal Position |
|
Year |
|
Salary
($) |
|
Bonus
($) |
|
Option Awards
($) |
|
All
other Compensation
($)(1) |
|
Total
($) |
| Christopher Missling,
PhD | |
| 2023 | | |
| 700,000 | | |
| 124,753 | | |
| 3,066,200 | | |
| 3,500 | | |
| 3,894,453 | |
| President, Chief Executive
Officer, | |
| 2022 | | |
| 586,400 | | |
| 110,000 | | |
| 6,045,043 | | |
| 12,200 | | |
| 6,753,643 | |
| and Director | |
| 2021 | | |
| 550,000 | | |
| 110,000 | | |
| 8,745,457 | | |
| 11,600 | | |
| 9,417,057 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Sandra Boenisch(2) | |
| 2023 | | |
| 186,883 | | |
| — | | |
| 306,700 | | |
| 7,475 | | |
| 501,058 | |
| Principal Financial Officer
and | |
| 2022 | | |
| 174,900 | | |
| — | | |
| 277,603 | | |
| — | | |
| 452,503 | |
| Treasurer | |
| 2021 | | |
| 158,300 | | |
| — | | |
| 724,314 | | |
| — | | |
| 882,614 | |
| |
(1) |
Includes employer matching of defined contribution savings plans. |
| (2) | Compensation to Ms. Boenisch denominated in Canadian Dollars and has been translated to US dollars at an exchange rate of 0.7416
during the year ended September 30, 2023 (2022: 0.7831; 2021: 0.7915). |
Employment Agreements
Christopher Missling
We and Dr. Missling entered into an
employment agreement dated July 5, 2013, as amended and extended most recently by the third amendment effective April 7, 2022 (the
“CEO Employment Agreement”), whereby we currently pay Dr. Missling an annual base salary of $700,000. In addition,
Dr. Missling is eligible to earn an annual cash bonus for each whole or partial calendar year of up to twenty percent of his base
salary, and to participate in our employee benefit plans. We have agreed to indemnify Dr. Missling in connection with his provision
of services to us.
Sandra Boenisch
We and Ms. Boenisch entered into an
amended and restated employment agreement dated October 4, 2017, as amended and extended, whereby we currently pay Ms. Boenisch
an annual base salary of $264,000 Canadian dollars. Ms. Boenisch is eligible for discretionary salary increases.
Grants of Plan Based Awards
The following table sets forth the awards
granted for each named executive officer during the year ended September 30, 2023 under our 2022 Omnibus Incentive Plan:
| | |
| |
All
other option awards |
| Name | |
Grant
Date | |
Number
of securities underlying options (#)(1) | |
Exercise
or base price of option award ($/sh) | |
Grant
date fair value of option awards ($) (2) |
Christopher
Missling, PhD | |
March
31, 2023 | |
| 500,000 | | |
$ | 8.57 | | |
$ | 3,066,200 | |
Sandra
Boenisch
| |
March 31, 2023 | |
| 50,000 | | |
$ | 8.57 | | |
$ | 306,700 | |
(1) Represents shares of our common stock underlying options
awarded, each of which vest over time.
(2) Represents the fair value of each equity award on the date of grant, as computed in accordance with FASB ASC 718.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth for each
named executive officer and director certain information concerning the outstanding equity awards as of September 30, 2023.
| | |
| |
Option
Awards | |
| |
|
| Name | |
Number
of Securities Underlying Exercisable Options (#) | |
Number
of Securities Underlying Unexercisable Options (#) | |
Equity Incentive Plan
Awards: Number
of Securities Underlying Unexercised Unearned Options (#) | |
Option Exercise Price ($) | |
Option Expiration Date |
| Christopher | |
| 73,380 | | |
| — | | |
| — | | |
| 1.32 | | |
May 8, 2024 |
| Missling | |
| 500,000 | | |
| — | | |
| — | | |
| 0.92 | | |
April 2, 2025 |
| | |
| 187,500 | | |
| — | | |
| — | | |
| 5.04 | | |
Sept 18, 2025 |
| | |
| 379,625 | | |
| — | | |
| — | | |
| 6.26 | | |
July 5, 2026 |
| | |
| 861,429 | | |
| — | | |
| — | | |
| 7.06 | | |
July 18, 2026 |
| | |
| 500,000 | | |
| — | | |
| — | | |
| 3.28 | | |
Sept 22, 2026 |
| | |
| 450,000 | | |
| — | | |
| — | | |
| 5.92 | | |
May 12, 2027 |
| | |
| 400,000 | | |
| — | | |
| — | | |
| 3.30 | | |
Dec 13, 2027 |
| | |
| 450,000 | | |
| — | | |
| — | | |
| 2.30 | | |
May 15, 2028 |
| | |
| 409,500 | | |
| — | | |
| — | | |
| 2.58 | | |
Oct. 1, 2028 |
| | |
| 750,000 | | |
| — | | |
| — | | |
| 3.15 | | |
May 3, 2029 |
| | |
| 550,000 | | |
| — | | |
| — | | |
| 2.96 | | |
January 6, 2030 |
| | |
| 550,000 | | |
| — | | |
| — | | |
| 5.49 | | |
December 30, 2030 |
| | |
| — | | |
| 500,000 | | |
| — | | |
| 18.11 | | |
August 2, 2031 |
| | |
| — | | |
| 500,000 | | |
| — | | |
| 7.54 | | |
June 14, 2032 |
| | |
| — | | |
| 500,000 | | |
| — | | |
| 10.09 | | |
June 27, 2032 |
| | |
| — | | |
| 500,000 | | |
| | | |
| 8.57 | | |
March 31, 2032 |
| Sandra Boenisch | |
| 30,000 | | |
| — | | |
| — | | |
| 3.30 | | |
Dec 13, 2027 |
| | |
| 30,000 | | |
| — | | |
| — | | |
| 2.30 | | |
May 15, 2028 |
| | |
| 27,300 | | |
| — | | |
| — | | |
| 2.58 | | |
Oct. 1, 2028 |
| | |
| 35,000 | | |
| — | | |
| — | | |
| 2.93 | | |
June 4, 2029 |
| | |
| 70,000 | | |
| — | | |
| — | | |
| 2.96 | | |
January 6, 2030 |
| | |
| 50,000 | | |
| — | | |
| — | | |
| 5.49 | | |
December 30, 2030 |
| | |
| — | | |
| 40,000 | | |
| — | | |
| 18.11 | | |
August 2, 2031 |
| | |
| — | | |
| 40,000 | | |
| — | | |
| 10.09 | | |
June 27, 2032 |
| | |
| — | | |
| 50,000 | | |
| — | | |
| 8.57 | | |
March 31, 2032 |
Option Exercises and Stock Vested
The following table sets forth for each
named executive officer awards that were exercised during the year ended September 30, 2023:
| | |
Option
Awards |
| Name | |
Number
of shares acquired on exercise (#) | |
Value
realized on exercise ($) |
| Christopher
Missling | |
| 500,000 | | |
| 3,203,340 | |
| Sandra Boenisch | |
| — | | |
| — | |
Nonqualified Defined Contribution and
Other Nonqualified Deferred Compensation Plans
There was no nonqualified deferred compensation
for our named executive officers in fiscal 2023.
CEO Pay Ratio Disclosure
We are providing the following information
about the relationship of the annual total compensation of our employees and the annual total compensation of our CEO. Based on
the information for fiscal year 2023, we reasonably estimate that the ratio of our CEO’s annual total compensation to the
annual total compensation of our median employee was 7.7:1. Our pay ratio estimate has been calculated in a manner consistent with
Item 402(u) of Regulation S-K using the data and assumptions summarized below.
We identified the median employee by examining
the 2023 annual base salary compensation for all individuals, excluding our CEO. We excluded independent
contractors retained on an as needed basis, whose compensation is determined by an unaffiliated third party, and who are therefore
are not considered our employees for purposes of the pay ratio calculation.
We included all employees who were
employed by us as of September 30, 2023. We selected the determination date and measurement period because they are recent
periods for which employee census and compensation information are readily available. Salaries and wages were annualized for those
employees who were not employed for the full year of fiscal 2023. We selected annual base salary as our compensation measure because
it is readily available in our existing payroll systems, it is consistently calculated for each employee, and because it is a reasonable
proxy for total compensation for purposes of determining the median employee. We did not apply any cost-of-living adjustments to
the compensation of employees in jurisdictions other than the jurisdiction in which the CEO resides.
Once we identified our median employee, we
calculated such employee’s annual total compensation for 2023 in accordance with the requirements of Item 402(c)(2)(x) of
Regulation S-K, resulting in that employee’s annual total compensation of $512,245. The median employee’s annual total
compensation includes annualized base salary, annualized bonus, annualized 401(k) matching contributions, and the fair value of
awards granted during the fiscal year ended September 30, 2023 under our 2022 Omnibus Incentive Plan.
With respect to the CEO, we used the amount
reported as total compensation in the Summary Compensation Table included in this annual report on Form 10-K. Any estimates and
assumptions used to calculate total annual compensation are described in footnotes to the Summary Compensation Table.
Compensation of Directors
The table below shows the compensation
of our directors who were not our named executive officers for the fiscal year ended September 30, 2023:
| Name | |
Fees
Earned or Paid in Cash
($) | |
Stock
Awards
($) | |
Option
Awards
($) (1) | |
Non-Equity
Incentive Plan Compensation ($) | |
Nonqualified
Deferred Compensation Earnings ($) | |
All
Other Compensation ($) | |
Total
($) |
| Jiong
Ma | |
| 33,000 | | |
| — | | |
| 316,540 | | |
| — | | |
| — | | |
| — | | |
| 349,540 | |
| Claus van der Velden | |
| 41,000 | | |
| — | | |
| 316,540 | | |
| — | | |
| — | | |
| — | | |
| 357,540 | |
| Athanasios Skarpelos | |
| 25,000 | | |
| — | | |
| 316,540 | | |
| — | | |
| — | | |
| — | | |
| 341,540 | |
| Steffen Thomas | |
| 25,000 | | |
| — | | |
| 316,540 | | |
| — | | |
| — | | |
| — | | |
| 341,540 | |
| Peter Donhauser | |
| 25,000 | | |
| — | | |
| 316,540 | | |
| — | | |
| — | | |
| — | | |
| 341,540 | |
(1) Includes stock option awards valued
based on the aggregate grant date fair value of the award computed in accordance with FASB ASC Topic 718. The amounts shown in
the table above do not necessarily reflect the actual value that may be realized by the non-employee director upon vesting.
On September 30, 2023, the aggregate number of outstanding vested and unvested stock option awards held by each director was as
follows: Dr. Ma possessed options to purchase 160,000 shares, Dr. van der Velden possessed options to purchase 305,500 shares,
Mr. Skarpelos possessed options to purchase 355,500 shares, Dr. Thomas possessed options to purchase 405,500 shares and Dr. Donhauser
possessed options to purchase 305,500 shares.
We currently compensate non-employee
directors $25,000 per year, paid quarterly. We compensate Dr. Ma an additional $4,000 per quarter for acting as Chair. We compensate
Claus van der Velden an additional $4,000 per quarter for performing the functions of Chairman of the Audit Committee, Compensation
Committee and Nominating and Corporate Governance Committee.
We regularly grant members of the Board
of Directors awards of options. Each board member is granted options initially when they join the Board of Directors, which typically
vest over a three-year period. Additionally, we grant awards on an annual basis. Annual awards of options typically vest in full
on the first anniversary of grant date. In 2023, the annual grant was 50,000 options to each director.
In addition, directors are entitled
to reimbursement for reasonable travel and other out-of-pocket expenses incurred in connection with attendance at meetings of our
Board of Directors. Our Board of Directors may award further special remuneration to any director undertaking any special services
on our behalf other than services ordinarily required of a director.
Retirement or Similar Benefit Plans
There are no arrangements or plans in
which we provide retirement or similar benefits for our directors or executive officers.
Resignation, Retirement, Other Termination,
or Change in Control Arrangements
Potential Payments Upon Termination
The Company is a party to employment
contracts with our Chief Executive Officer and Principal Financial Officer that contain provisions for payment of severance upon
termination by either the Company without cause or by the employee for good reason. General terms of these arrangements are described
below.
Our CEO Employment Agreement with Dr.
Missling contains provisions regarding our obligations upon his termination and upon a Change in Control. Any capitalized term
not defined herein is used as defined in the CEO Employment Agreement. If Dr. Missling’s employment is terminated by us without
Cause, he is entitled to receive payments by us consisting of (i) reimbursement of any unpaid business expenses to which he is
entitled to reimbursement that were incurred prior to the effective date of his termination, (ii) all vested compensation and benefits
to which he is entitled as of the Termination Date, (iii) a severance payment consisting of the three times the sum of (a) his
annual salary in effect at the time of termination and (b) the average of the annual Bonuses payable to him for the last three
completed calendar years prior to the Termination Date, (iv) all outstanding and unvested stock options and all options previously
vested will become and remain exercisable for no less than three years from the Termination Date; (v) all of his unvested and outstanding
restricted stock, restricted stock units or other equity awards that are unvested and outstanding as of the Termination Date shall
vest and be settled within ten business days after the Termination Date, (vi) life insurance coverage until the end of the term
of the CEO Employment Agreement; and (vii) continued participation in all medical, dental and hospitalization benefits plans or
programs for Dr. Missling and his eligible dependents for 36 months or until he receives similar benefits at a new employer, at
his sole cost. If Dr. Missling’s employment is terminated by him for Good Reason, he is entitled to receive the same as the
above, however the severance payment will consist of three times his annual salary in effect at the time of termination and two
times the average annual Bonuses payable to him for the last three completed calendar years prior to the Termination Date.
If Dr. Missling was terminated by the
Company without cause on September 30, 2023, he would have been entitled to a severance payment of $2,444,750. If Dr. Missling
terminated his employment for Good Reason on September 30, 2023, he would have been entitled to a severance payment of $2,329,800.
Our CFO Employment Agreement with Ms.
Boenisch contains provisions regarding our obligations upon her termination. Any capitalized term not defined herein is used as
defined in the CFO Employment Agreement. Under the CFO Employment Agreement, if Ms. Boenisch is terminated without Cause, the Company
shall pay Ms. Boenisch severance compensation equal to six months base salary payable by the Company for the six-month period following
the Termination. In addition, the Company must provide Ms. Boenisch thirty (30) day notice of her Termination. If the Company opts
to have Ms. Boenisch cease providing services to the Company prior to the expiration of the thirty (30) day notice-period (the
“Notice Period”), Ms. Boenisch shall receive the Compensation and Benefits for the full length of the Notice Period
as if such period was not waived. In addition, any unvested stock options or stock awards vesting in the contract year of Termination
held by Ms. Boenisch as of the Date of Termination shall immediately vest.
If Ms. Boenisch was terminated by the
Company without cause on September 30, 2023, she would have been entitled to continued salary payments equal to $97,890 in total.
The following table presents accelerated
vesting for certain equity awards outstanding at the time of the executive’s termination for each Named Executive Officer,
if employment were terminated by either the Company without cause or by Dr. Missling for good reason on September 30, 2023:
| |
|
Vesting
Upon Termination |
| Named
Executive Officer |
|
Unvested
Stock Options (#) |
|
Stock
Option Awards Estimated Benefit ($)(1) |
| Dr. Christopher Missling |
|
|
2,550,000 |
|
|
|
583,000 |
|
| Sandra Boenisch |
|
|
— |
(2) |
|
|
— |
|
(1) Estimated benefit based on the closing
stock price of $6.55 at September 30, 2023.
(2) Ms. Boenisch’s unvested stock
options at September 30, 2023 all contained performance based vesting conditions, therefore such options would not automatically
vest upon Termination.
Potential Payments Upon Change in
Control
If the Company is subject to a Change
in Control, then the CEO Employment Agreement and the CFO Employment Agreement provide that all previously granted but unvested
stock options held by Dr. Missling and Ms. Boenisch shall vest.
The following table presents accelerated
vesting for certain equity awards outstanding to the Named Executive Officer, if a change in control had occurred at September
30, 2023:
| |
|
Vesting
Due to Change in Control |
| Named
Executive Officer |
|
Unvested
Stock Options (#) |
|
Stock
Option Awards Estimated Benefit ($)(1) |
| Dr. Christopher Missling |
|
|
2,550,000 |
|
|
|
583,000 |
|
| Sandra Boenisch |
|
|
180,000 |
|
|
|
53,000 |
|
(1) Estimated benefit based on the closing
stock price of $6.55 at September 30, 2023.
For a complete description of these
terms and conditions please refer to the CEO Employment Agreement and CFO Employment Agreement (and their amendments) filed as
exhibits to this Annual Report on Form 10-K.
ITEM 12. SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth, as of
November 24, 2023, certain information with respect to the beneficial ownership of our common stock by each stockholder known by
us to be the beneficial owner of more than 5% of our common stock and by each of our current directors and our named executive
officers and by our current directors and executive officers as a group. We have determined the number and percentage of shares
beneficially owned by such person in accordance with Rule 13d-3 under the Securities Exchange Act of 1934. This information does
not necessarily indicate beneficial ownership for any other purpose.
| | |
Name and address of | |
Amount and nature
of | |
Percent of |
| Title of class | |
beneficial owner | |
beneficial ownership | |
class (1) |
| Directors and Named Executive Officers | |
| | | |
| | |
| Common | |
Christopher Missling | |
| 7,311,644 | (2) | |
| 8.3 | % |
| Stock | |
(CEO/Director) | |
| | | |
| | |
| Common | |
Jiong Ma (Director, | |
| 65,000 | (3) | |
| * | |
| Stock | |
Chair) | |
| | | |
| | |
| Common | |
Claus van der Velden | |
| 222,167 | (4) | |
| * | |
| Stock | |
(Director) | |
| | | |
| | |
| Common | |
Athanasios Skarpelos | |
| 1,578,625 | (5) | |
| 1.9 | % |
| Stock | |
(Director) | |
| | | |
| | |
| Common | |
Steffen Thomas | |
| 322,167 | (6) | |
| * | |
| Stock | |
(Director) | |
| | | |
| | |
| Common | |
Peter Donhauser | |
| 224,332 | (7) | |
| * | |
| Stock | |
(Director) | |
| | | |
| | |
| Common | |
Sandra Boenisch | |
| 265,263 | (8) | |
| * | |
| Stock | |
(Principal Financial | |
| | | |
| | |
| | |
Officer) | |
| | | |
| | |
| Common | |
Directors
& Executive | |
| 9,989,198 | | |
| 11.2 | % |
| Stock | |
Officers
as a group (7 | |
| | | |
| | |
| | |
persons) | |
| | | |
| | |
| 5%
Holders | |
| |
| | | |
| | |
| Common | |
The Vanguard Group | |
| 4,144,500 | (9) | |
| 5.0 | % |
| Stock | |
100 Vanguard Blvd | |
| | | |
| | |
| | |
Malvern, PA 19355 | |
| | | |
| | |
| Common | |
State Street Corporation | |
| 5,812,605 | (9) | |
| 7.1 | % |
| Stock | |
1 Lincoln Street | |
| | | |
| | |
| | |
Boston, MA 02111 | |
| | | |
| | |
| Common | |
BlackRock, Inc. | |
| 5,578,159 | (10) | |
| 6.8 | % |
| Stock | |
55 East 52nd Street | |
| | | |
| | |
| | |
New York, NY 10055 | |
| | | |
| | |
| (1) | Percentage
of
ownership
is
based
on
82,086,511
of
our
common
stock
issued
and
outstanding
as
of
November
24,
2023.
Except
as
otherwise
indicated,
we
believe
that
the
beneficial
owners
of
the
common
stock
listed
above,
based
on
information
furnished
by
such
owners,
have
sole
investment
and
voting
power
with
respect
to
such
shares,
subject
to
community
property
laws
where
applicable.
Beneficial
ownership
is
determined
in
accordance
with
the
rules
of
the
Commission
and
generally
includes
voting
or
investment
power
with
respect
to
securities.
Shares
of
common
stock
subject
to
options
or
warrants
currently
exercisable
or
exercisable
within
60
days,
are
deemed
outstanding
for
purposes
of
computing
the
percentage
ownership
of
the
person
holding
such
option
or
warrants
but
are
not
deemed
outstanding
for
purposes
of
computing
the
percentage
ownership
of
any
other
person. |
| (2) | Includes
options
to
purchase
73,380
shares
of
our
common
stock
at
$1.32
per
share,
options
to
purchase
500,000
shares
of
our
common
stock
at
$0.92
per
share,
options
to
purchase
187,500
shares
of
our
common
stock
at
$5.04
per
share,
options
to
purchase
379,625
shares
of
our
common
stock
at
$6.26
per
share,
options
to
purchase
861,429
shares
of
our
common
stock
at
$7.06
per
share,
options
to
purchase
500,000
shares
of
our
common
stock
at
$3.28
per
share,
options
to
purchase
450,000
shares
of
our
common
stock
at
$5.92
per
share,
options
to
purchase
400,000
shares
of
our
common
stock
at
$3.30
per
share,
options
to
purchase
450,000
shares
of
our
common
stock
at
$2.30
per
share,
options
to
purchase
409,500
shares
of
our
common
stock
at
$2.58
per
share,
options
to
purchase
750,000
shares
of
our
common
stock
at
$3.15
per
share,
options
to
purchase
550,000
shares
of
our
common
stock
at
$2.96
per
share
and
options
to
purchase
550,000
shares
of
our
common
stock
at
$5.49
per
share
that
are
vested
or
are
vesting
within
60
days.
Excludes
options
to
purchase
500,000
shares
of
our
common
stock
at
$18.11
per
share,
options
to
purchase
500,000
shares
of
our
common
stock
at
$7.54
per
share,
options
to
purchase
500,000
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
500,000
shares
at
$8.57
per
share
that
do
not
vest
within
60
days. |
| (3) | Includes
options
to
purchase
23,333
shares
of
our
common
stock
at
$13.01
per
share,
options
to
purchase
25,000
shares
of
our
common
stock
at
$18.11
per
share
and
options
to
purchase
16,667
shares
of
our
common
stock
at
$10.09
per
share
that
have
vested
or
are
vesting
within
60
days.
Excludes
options
to
purchase
11,667
shares
of
common
stock
at
$13.01
per
share,
options
to
purchase
33,333
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$8.57
per
share
that
do
not
vest
within
60
days. |
| (4) | Includes
options
to
purchase
50,000
shares
of
our
common
stock
at
$2.60
per
share,
options
to
purchase
45,500
shares
of
our
common
stock
at
$2.58
per
share,
options
to
purchase
50,000
shares
of
our
common
stock
at
$2.96
per
share,
options
to
purchase
35,000
shares
of
our
common
stock
at
$5.49
per
share,
options
to
purchase
25,000
shares
of
our
common
stock
at
$18.11
per
share
and
options
to
purchase
16,667
shares
of
our
common
stock
at
$10.09
per
share
that
have
vested
or
are
vesting
within
60
days.
Excludes options
to
purchase
33,333
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$8.57
per
share
that
are
not
vesting
within
60
days. |
| (5) | Includes
options
to
purchase
100,000
shares
of
our
common
stock
at
$3.28
per
share,
options
to
purchase
45,500
shares
of
our
common
stock
at
$2.58
per
share,
options
to
purchase
50,000
shares
of
our
common
stock
at
$2.96
per
share,
options
to
purchase
35,000
shares
of
our
common
stock
at
$5.49
per
share,
options
to
purchase
25,000
shares
of
our
common
stock
at
$18.11
per
share
and
options
to
purchase
16,667
shares
of
our
common
stock
at
$10.09
per
share
that
have
vested
or
are
vesting
within
60
days.
Excludes
options
to
purchase
33,333
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$8.57
per
share
that
do
not
vest
within
60
days. |
| (6) | Includes
options
to
purchase
50,000
shares
of
our
common
stock
at
$1.76
per
share,
options
to
purchase
100,000
shares
of
our
common
stock
at
$3.28
per
share,
options
to
purchase
45,500
shares
of
our
common
stock
at
$2.58
per
share,
options
to
purchase
50,000
shares
of
our
common
stock
at
$2.96
per
share,
options
to
purchase
35,000
shares
of
our
common
stock
at
$5.49
per
share,
options
to
purchase
25,000
shares
of
our
common
stock
at
$18.11
per
share
and
options
to
purchase
16,667
shares
of
our
common
stock
at
$10.09
per
share
that
have
vested
or
are
vesting
within
60
days.
Excludes
options
to
purchase
33,333
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$8.57
per
share
that
are
not
vesting
within
60
days. |
| (7) | Includes
options
to
purchase
50,000
shares
of
our
common
stock
at
$5.39
per
share,
options
to
purchase
45,500
shares
of
our
common
stock
at
$2.58
per
share,
options
to
purchase
50,000
shares
of
our
common
stock
at
$2.96
per
share,
options
to
purchase
35,000
shares
of
our
common
stock
at
$5.49
per
share,
options
to
purchase
25,000
shares
of
our
common
stock
at
$18.11
per
share
and
options
to
purchase
16,667
shares
of
our
common
stock
at
$10.09
per
share
that
have
vested
or
are
vesting
within
60
days.
Excludes
options
to
purchase
33,333
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$8.57
per
share
that
are
not
vesting
within
60
days. |
| (8) | Includes
options
to
purchase
30,000
shares
of
our
common
stock
at
$3.30
per
share,
options
to
purchase
30,000
shares
of
our
common
stock
at
$2.30
per
share,
options
to
purchase
27,300
shares
of
our
common
stock
at
$2.58
per
share,
options
to
purchase
35,000
shares
of
our
common
stock
at
$2.93
per
share,
options
to
purchase
70,000
shares
of
our
common
stock
at
$2.96
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$5.49
per
share
that
have
vested
or
are
vesting
within
60
days.
Excludes
options
to
purchase
40,000
shares
of
our
common
stock
at
$18.11
per
share
options
to
purchase
40,000
shares
of
our
common
stock
at
$10.09
per
share
and
options
to
purchase
50,000
shares
of
our
common
stock
at
$8.57
per
share
that
do
not
vest
within
60
days. |
| (9) | Based
on
Schedule
13G
as
filed
with
the
SEC
and
dated
on
February
9,
2023 |
| (10) | Based
on
Schedule
13G
as
filed
with
the
SEC
and
dated
on
January
31,
2023 |
Change in Control
We are unaware of any contract or other arrangement, the
operation of which may at a subsequent date result in a change of control of our Company.
Securities Authorized for Issuance under Equity Compensation
Plans or Individual Compensation Arrangements
The following table summarizes certain information regarding
our equity compensation plan or individual compensation arrangements at September 30, 2023:
| Equity
Compensation Plan Information |
| Plan
Category |
|
Number
of securities to be issued upon exercise of outstanding options, warrants and rights
(a) |
|
Weighted-average
exercise price of outstanding options, warrants and rights
(b) |
|
Number
of securities remaining available for future issuances under equity compensation plans (excluding securities reflected in column (a))
(c) |
| Equity compensation plans approved by security holders |
|
|
22,050,553 |
|
|
|
7.40 |
|
|
|
6,661,535 |
|
| Equity compensation plans not approved by security holders |
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Total |
|
|
22,050,553 |
|
|
|
7.40 |
|
|
|
6,661,535 |
|
2022 Stock Option Plan
On March 25, 2022, the Board approved
the 2022 Omnibus Incentive Plan (the “2022 Plan”). The 2022 Plan was approved by stockholders on May 24, 2022. Under
the terms of the 2022 Plan, 10,000,000 shares of Common Stock were made available for issuance under the 2022 Plan, in addition
to the shares available under the 2019 Omnibus Incentive Plan (the “2019 Plan”) and the 2015 Omnibus Incentive Plan
(the “2015 Plan”). Any awards outstanding under a previous stock option plan will remain subject to and be paid under
such plan, and any shares subject to outstanding awards under a previous plan that subsequently cease to be subject to such awards
(other than by reason of settlement of the awards in shares) will automatically become available for issuance under the 2022 Plan.
The 2022 Plan provides that it may be
administered by the Board, or the Board may delegate such responsibility to a committee. The exercise price will be determined
by the Board at the time of grant shall be at least equal to the fair market value on such date. If the grantee is a 10% stockholder
on the grant date, then the exercise price shall not be less than 110% of fair market value of the Company’s shares of common
stock on the grant date. Stock options may be granted under the 2022 Plan for an exercise period of up to ten years from the date
of grant of the option or such lesser periods as may be determined by the Board, subject to earlier termination in accordance with
the terms of the 2022 Plan.
The purpose of the 2022 Plan is to retain
the services of valued key employees and consultants of our Company and such other persons, and to encourage such persons to acquire
a greater proprietary interest in our Company, thereby strengthening their incentive to achieve the objectives of the shareholders
of our Company. The purpose is also to serve as an aid and inducement in the hiring of new employees and to provide an equity incentive
to consultants.
ITEM 13. CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with related persons
There have been no transactions, since
October 1, 2022, or currently proposed transactions, in which we were or are to be a participant and the amount involved exceeds
the lesser of $120,000 or one percent of the average of the smaller reporting company’s total assets at year end for the
last two completed fiscal years, and in which any of the following persons had or will have a direct or indirect material interest.
| i. | any
director
or
executive
officer
of
our
Company; |
| ii. | any
beneficial
owner
of
shares
carrying
more
than
5%
of
the
voting
rights
attached
to
our
outstanding
shares
of
common
stock;
and |
| iii. | any
member
of
the
immediate
family
(including
spouse,
parents,
children,
siblings
and
in-laws)
of
any
of
the
foregoing
persons. |
Compensation of Named Executive
Officers and Directors
For information regarding compensation
of named executive officers and directors, please see “Item 11. Executive Compensation.”
Director Independence
Under the NASDAQ Stock Market Rules,
the Board has a responsibility to make an affirmative determination that those members of its Board that serve as independent directors
do not have any relationships with the Company and its businesses that would impair their independence. The Board has determined
that that Christopher Missling, PhD is not independent, as that term is defined by NASDAQ 5605(a)(2), because Mr. Missling serves
as our President, Chief Executive Officer, and Secretary.
The Board has determined that that Claus
van der Velden, Athanasios Skarpelos, Steffen Thomas, Peter Donhauser and Jiong Ma are independent, as that term is defined by
NASDAQ 5605(a)(2) and the applicable rules of the Commission.
Board Meetings
During fiscal 2023, each member of the
Board of Directors attended at least 75% of the aggregate of all meetings of the Board of Directors and all meetings of committees
of the Board of Directors on which such director served that were held during the period in which such director served.
ITEM 14. PRINCIPAL
ACCOUNTING FEES AND SERVICES
Fees Paid to Our Independent Registered
Public Accounting Firm
The following table sets forth the aggregate
fees billed or expected to be billed to our Company for professional services rendered by our independent registered public accounting
firm, Grant Thornton, LLP for the fiscal years ended September 30, 2023 and 2022:
| |
|
2023 |
|
2022 |
| Audit Fees |
|
$ |
444,098 |
|
|
$ |
398,900 |
|
| Audit Related Fees |
|
|
— |
|
|
|
— |
|
| Tax Fees |
|
|
— |
|
|
|
— |
|
| All Other Fees |
|
|
— |
|
|
|
— |
|
| Total Fees |
|
$ |
444,098 |
|
|
$ |
398,900 |
|
Audit Fees. Consist of
fees billed for professional services rendered for the audits of our financial statements, reviews of our interim financial statements
included in quarterly reports, services performed in connection with regular filings with the Commission for the fiscal years ended
September 30, 2023 and 2022 in connection with statutory and regulatory filings or engagements.
Policy on Pre-Approval by Audit Committee
of Services Performed by Independent Registered Public Accounting Firm
Our Audit Committee pre-approves all
services provided by our independent registered public accounting firm. All of the above services and fees were reviewed and approved
by our Audit Committee.
Our Audit Committee has considered the
nature and amount of fees billed or expected to be billed by Grant Thornton LLP and believes that the provision of services for
activities unrelated to the audit was compatible with maintaining Grant Thornton LLP’s independence.
PART IV
ITEM 15. EXHIBITS,
FINANCIAL STATEMENT SCHEDULES
| Exhibit |
|
| Number |
Description |
| (3) |
Articles of Incorporation and Bylaws |
| 3.1 |
Articles of Incorporation, as amended (incorporated by reference to our Annual Report on Form 10-K for the year ended September 30, 2021 filed on November 24, 2021) |
| 3.3 |
Bylaws (incorporated by reference to our Current Report on Form 8-K filed on September 28, 2007) |
| (4) |
Instruments Defining the Rights of Security Holders |
| 4.1 |
Description of Registrant’s Securities (incorporated by reference to our Annual Report on Form 10-K filed on November 28, 2022) |
| 4.2 |
Registration Rights Agreement, dated February 3, 2023, by and between the Company and Lincoln Park Capital Fund, LLC (incorporated by reference to our Quarterly Report on Form 10-Q filed on February 7, 2023) |
| (10) |
Material Contracts |
| 10.1^ |
2015 Omnibus Incentive Plan (incorporated by reference to our Annual Report on Form 10-K filed on December 29, 2015) |
| 10.2^ |
2019 Omnibus Incentive Plan (incorporated by reference to our Proxy Statement, dated February 11, 2019, as filed on February 11, 2019) |
| 10.3^ |
2022 Omnibus Incentive Plan (incorporated by reference to our Registration Statement on Form S-8, as filed on June 10, 2022) |
| 10.4^ |
Employment Agreement, dated as of July 5, 2013, by and between the Company and Christopher Missling, PhD (incorporated by reference to our Quarterly Report on Form 10-Q filed on August 13, 2014) |
| 10.5^ |
First Amendment to Employment Agreement, dated as of July 5, 2016, by and between the Company and Christopher Missling, PhD (incorporated by reference to our Current Report on Form 8-K filed on July 7, 2016) |
| 10.6^ |
Amended and Restated First Amendment to Employment Agreement, dated as of July 18, 2016, by and between the Company and Christopher Missling, PhD (incorporated by reference to our Current Report on Form 8-K filed on July 22, 2016) |
| 10.7^ |
Second Amendment to Employment Agreement, dated as of May 3, 2019 by and between the Company and Christopher Missling, PhD (incorporated by reference to our Quarterly Report on Form 10-Q filed on May 9, 2019) |
| 10.8^ |
Third Amendment to Employment Agreement, dated April 7, 2022 by and between the Company and Christopher Missling, PhD (incorporated by reference to our Current Report on Form 8-K filed on April 8, 2022) |
| 10.9^ |
Amended and Restated Employment Agreement by and between the Company and with Sandra Boenisch (incorporated by reference to our Annual Report on Form 10-K filed on December 11, 2017) |
| 10.10^ |
Amendment No. 1 to Amended and Restated Employment Agreement between the Company and Sandra Boenisch, dated February 4, 2020 (incorporated by reference to our Quarterly Report on Form 10-Q filed on February 6, 2020) |
| 10.11^ |
Amendment No. 2 to Amended and Restated Employment Agreement between the Company and Sandra Boenisch, dated February 28, 2022 (incorporated by reference to our Current Report on Form 8-K filed on March 4, 2022) |
| 10.12 |
Amended and Restated Sales Agreement, dated May 1, 2020, by and among the Company, Cantor Fitzgerald & Co. and SVB Leerink LLC (incorporated by reference to our Current Report on Form 8-K filed on May 1, 2020) |
* Filed herewith.
** The certification attached as Exhibit 32.1 that accompanies
this Form 10-K is not deemed filed with the SEC and is not to be incorporated by reference into any filing of the Registrant under
the Securities Act or the Exchange Act, whether made before or after the date of this Form 10-K, irrespective of any general incorporation
language contained in such filing.
^ Denotes a management contract or compensatory plan
or arrangement.
ITEM 16. FORM
10-K SUMMARY
Not Applicable.
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report
to be signed on its behalf by the undersigned thereunto duly authorized.
| Date: November
27, 2023 |
|
ANAVEX
LIFE SCIENCES CORP. |
| |
|
|
| |
|
By: |
|
/s/
Christopher Missling, PhD |
| |
|
Name: |
|
Christopher
Missling, PhD |
| |
|
Title: |
|
Chief
Executive Officer (Principal Executive Officer) |
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf
of the registrant and in the capacities and on the dates indicated.
| Signatures |
|
Title(s) |
|
Date |
| |
|
|
|
|
| /s/ Christopher
Missling, PhD |
|
|
|
November 27,
2023 |
| Christopher
Missling, PhD |
|
Chief
Executive Officer (Principal Executive Officer) and Director |
|
|
| |
|
|
|
|
| /s/
Sandra Boenisch |
|
|
|
November 27,
2023 |
| Sandra
Boenisch, CPA, CGA |
|
Principal
Financial Officer and Treasurer (Principal Accounting Officer) |
|
|
| |
|
|
|
|
| /s/
Jiong Ma, PhD |
|
|
|
November 27, 2023 |
| Jiong
Ma, PhD |
|
Director,
Chair |
|
|
| |
|
|
|
|
| /s/
Athanasios Skarpelos |
|
|
|
November 27, 2023 |
| Athanasios
Skarpelos |
|
Director |
|
|
| |
|
|
|
|
| /s/
Claus van der Velden, PhD |
|
|
|
November 27, 2023 |
| Claus
van der Velden, PhD |
|
Director |
|
|
| |
|
|
|
|
| /s/
Steffen Thomas, PhD |
|
|
|
November 27, 2023 |
| Steffen
Thomas, PhD |
|
Director |
|
|
| |
|
|
|
|
| /s/
Peter Donhauser, D.O. |
|
|
|
November 27, 2023 |
| Peter
Donhauser, D.O. |
|
Director |
|
|
90
EXHIBIT 14.1
BUSINESS
CODE OF CONDUCT & ETHICS
As a business engaged in
the discovery, development and commercialization of medicines for the treatment of various diseases, AnavexTM Life Sciences
Corp. (the “Anavex” or the “Company”) must always act in a way that reflects the highest
standards of corporate behavior and conduct itself in a legal and ethical manner. Each of us must always exercise good judgment
and common sense in making the necessary choices to advance the interests of the Company. The development and maintenance of relationships
of trust between each of us and government officials, health care professionals, patients, suppliers, customers, investors and
other employees is essential and expected. Obeying the law both in letter and in spirit is the foundation on which Anavex’s
ethical standards are built. In carrying out this policy, Anavex has adopted the following Business Code of Conduct and Ethics
(the “Code”). This Code is intended to cover Anavex’s and its affiliates’ directors, officers and
employees, as well agents and other parties acting on behalf, or for the benefit, of the Company and/or its affiliates (collectively,
“Covered Persons”).
Each Covered Person plays
an important role in the Company’s achievements. The Company is dedicated to its mission and preserving its reputation of
integrity in all the business it does. Anavex believes that building and maintaining a culture of compliance is integral to its
success and requires the cooperation and commitment of each Covered Person.
This Code is designed to
define the Company’s general expectations for legal and ethical business behavior for all Covered Persons. The Code is a
guide and may not address every legal or ethical issue nor is it an exhaustive description of all laws and policies that may apply
to our business. It is a statement of the Company’s expectations and obligations.
The Company views this Code
very seriously and expects all Covered Persons to abide by it. Any Covered Person who violates the standards set forth in the Code
may be subject to disciplinary action which, depending on the nature of the violation and the history of the Covered Person, may
range from a warning to a termination of employment and in appropriate cases, civil legal action or referral for regulatory or
criminal prosecution.
Understanding and daily
application of the principles of the Code provides a basis for making the right decisions for the Company; by embracing core values,
the Company creates a productive and respectful work environment poised to contribute to global healthcare. Each of us is responsible
for becoming familiar and following all the rules, regulations and policies that apply to our jobs and for seeking advice in any
situation where we are unsure of what to do.
Each Covered Person is expected
(i) to read and understand this Code which provides general principles to guide Covered Persons in making ethical decisions, and
its application to the performance of his or her business responsibilities and (ii) to conduct himself or herself in accordance
with this Code and to seek to avoid even the appearance of wrongdoing or improper behavior. Those who violate the standards in
this Code will be subject to disciplinary action, which may include suspension, termination and/or the reporting of violative conduct
to appropriate regulatory and criminal authorities. Any director, officer, or employee who observes or otherwise becomes aware
of conduct that violates, or could violate, the Code must take a prompt report of such violation to the Company. Any officer or
employee who fails to immediately report a Code violation, or perceived violation, or who violates any aspect of the Code may be
subject to disciplinary action, up to and including termination of employment.
If a law conflicts with
a policy in this Code, a Covered Person must comply with the law; however, if a local custom or policy conflicts with this Code,
a Covered Person must comply with this Code. If a Covered Person has any questions about these conflicts or this Code, he or she
should consult with the Chief Executive Officer (“CEO”).
The Company strongly
encourages dialogue among Covered Persons should awareness of situations that give rise to ethical questions transpire; Covered
Persons should discuss and articulate acceptable ways of handling those situations with the CEO. The Company shall provide a copy
of this Code to all new hires and, upon a change to the Code, to all current employees. Additionally, the Company shall also provide
its employees with periodic compliance training as a supplement this Code of Conduct.
The Company shall
take reasonable steps to monitor and audit compliance with the Code of Conduct, including the establishment of monitoring and auditing
systems that are reasonably designed to detect violations of the Code of Conduct. Additionally, the Company will periodically review
the Code of Conduct, and when necessary or desirable, make recommendations to ensure:
| · | its continued conformance to applicable law, |
| · | that it meets or exceeds industry standards, and |
| · | that any weaknesses revealed through monitoring, auditing and reporting systems are eliminated
or corrected. The Company may revise, change or amend the Code of Conduct, or any policies or procedures, at any time. |
After carefully reviewing
this Code, you must sign the acknowledgment attached hereto, indicating that you have received, read, understand and agree to comply
with this Code. The acknowledgment must be returned either electronically in a manner provided for by the Company within ten (10)
business days of your receipt of this Code and otherwise as required by the Company. All Covered Persons will each be required
to annually certify compliance with the Code. The failure to certify such compliance or any false certification, even if directed
by a supervisor, is grounds for disciplinary action by the Company, up to and including termination of employment.
| III. | HONEST AND ETHICAL CONDUCT |
Each Covered Person must
always conduct himself or herself in an honest and ethical manner. Each Covered Person must act with the highest standards of personal
and professional integrity and not tolerate others who attempt to deceive or evade responsibility for their actions. All actual
or potential conflicts of interest between personal and professional relationships must be handled honestly, ethically and in accordance
with the policies specified in this Code. In addition, all Covered Persons must be direct, honest and truthful in discussions with,
or requests for information from, the Board, regulatory agency officials and government officials, as well as in all dealings with
business partners and shareholders. Every Covered Person is accountable for adhering to the Company’s commitment to conduct
its business fairly, honestly and with integrity.
| IV. | COMPLIANCE WITH APPLICABLE GOVERNMENTAL LAWS, RULES AND REGULATIONS |
All Covered Persons must
respect and obey the laws, rules and regulations (including insider trading laws) of the jurisdictions in which we operate and
the rules and regulations applicable to the Company, its business and its affiliates (collectively, the “Company Group”),
including those of the NASDAQ and the Securities and Exchange Commission (the “SEC”). The Company shall hold
periodic training sessions to ensure all employees are aware of and comply with the relevant laws, rules and regulations associated
with their employment. Although not all Covered Persons are expected to know the details of the laws, rules and regulations to
which the Company Group is subject, it is important to understand enough to determine when it is necessary or appropriate to seek
advice from supervisors, managers or other persons, including the CEO and/or Board of Directors (“Board”), who
can provide guidance on such matters.
Disregard of the law will
not be tolerated. Violation of any applicable laws, rules and regulations may subject an individual, as well as one or more members
of the Company Group, to civil, administrative and/or criminal penalties and may harm their reputations. Covered Persons should
be aware that conduct and records, including e-mails, are subject to internal and external audits and to discovery by third parties
in the event of a government investigation or civil litigation. Consequently, it is in everyone’s best interest to understand
and comply with the laws, rules and regulations applicable to the Company Group.
Areas that require particular attention
to ensure proper compliance include:
| · | Antitrust and Competition Laws: It is the policy of the Company to comply with the antitrust and
competition laws of each country in which the Company does business. No director, officer or employee of the Company will engage
in anti-competitive conduct in violation of any antitrust or competition law. |
| · | Intellectual Property Laws: The Company requires that all scientific and technical information
generated, utilized and maintained by its employees and in strict compliance and conformity with applicable intellectual property
laws. |
| · | Workplace Safety Laws and Regulations: The Company requires full compliance with applicable workplace
safety and industrial hygiene standards required by law. Each of us has a responsibility for maintaining a safe and healthy working
environment for all persons at the Company, following safety and health rules and practices and reporting accidents, injuries and
unsafe equipment, practices or conditions. |
| · | Insider Trading Laws: All members of board of directors and of executive boards, and their auxiliary
staff in the Company Group, will be severely penalized if they make use of “hot information” (i.e. information that
is not available to the general public, facts which could substantially influence the price of a listed security) that is in their
possession to create a financial advantage for themselves or for some third party by purchasing (prior to the announcement of favorable
information) or selling (prior to the announcement of unfavorable information) stock that is listed on an exchange or other securities.
The insider trading provisions affect securities of Anavex Life Sciences Corp., and those of companies that are being acquired
or merged with Anavex or a Company with which Anavex is planning to enter into a strategic alliance, if the said securities are
listed on an exchange or traded in pre-market dealings. |
| · | Privacy and Data Protection Laws: The Company is committed to the protection of the individual
privacy of employees, persons who participate in the clinical trials of the Company’s drug candidates and products and individual
third parties who perform services on behalf of the Company. The Company must comply with applicable privacy laws and regulations
whenever the Company does business, including activities performed on behalf of the Company by third parties. Such laws and regulations
are complex and differ from country to country. Any employee who handles or oversees the handling of individually-identifiable
data and who has a concern or question about the proper handling of such data should contact the CEO and/or consult any applicable
Company policies. |
| · | Laws and Regulations relating to Records Retention: The Company must comply with all laws and regulations
mandating specified time periods for retaining various records of Company’s activities. It is the responsibility of each
direct report of the CEO working with his/her staff and the CEO, to establish and maintain a system for the retention and safekeeping
of all records that are required by law or Company Policy. |
| · | Laws Governing Pharmaceutical Products: The Company must comply with all laws and regulations governing
the manufacture, testing, review and approval, sales, marketing, shipment, storage, and destruction of pharmaceutical products
set forth by the Food and Drug Administration, the Drug Enforcement Administration, the local authorities and the European Union
and its member states. In conducting clinical trials of pharmaceutical products, the Company will comply with rules governing human
subject protection, animal welfare, and public disclosure of clinical trials for serious and life threatening diseases. The distribution
of pharmaceutical samples must comply with the Prescription Drug Marketing Act. These laws and regulations are complex, technical,
and comprehensive. Therefore, all employees shall take steps to become familiar with those areas directly relevant to their responsibilities
and shall consult with the CEO to obtain guidance on issues or areas of law or regulation that might arise during the normal course
of business. |
| · | Interacting with healthcare professionals: All employees, officers and directors must comply with
all applicable laws, regulations and professional ethical rules in their dealing with healthcare professionals. The purpose of
every interaction between the Company and a healthcare professional must be to benefit patients or enhance the practice of medicine.
You may inform healthcare professionals about Company products, provide scientific and medical information and promote medical
research. |
| · | Responsibility of Company Personnel: Every director, officer, or employee has the responsibility
to report a violation or suspected violation of this Policy. The Company promptly responds to all reports of suspected violations.
Any Director, officer, or employee who knows or believe that any other Director, officer, or employee of the Company, or anyone
else representing the Company, is violating or is suspected of violating this Policy should contact his supervisor or the CEO. |
All Covered Persons have
a duty to avoid business, financial or other direct or indirect relationships that conflict with the interests of the Company or
that divide his or her loyalty to the Company. A conflict of interest occurs when your personal interest interferes or appears
to interfere with the interests of the Company, or the Company Group as a whole. A conflict situation can arise, for example, when
a Covered Person takes actions or has interests that may make it difficult to perform his or her work for the Company honestly,
objectively and fairly. It is almost always a conflict for a Company officer or employee to work simultaneously for a competitor,
customer, or supplier or to work for a competitor as a consultant or board member. Conflicts of interest also arise when a Covered
Person, or any Family Member (as defined below) of such person, receives improper personal benefits as a result of his or her position
at the Corporation. Loans to, other than those made in the ordinary course of business, or guarantees of obligations of, employees
or their Family Members may also create a conflict of interest. Covered Persons may not participate in a joint venture, partnership
or other business arrangement with the Company, without the prior approval of a majority of the Board.
Any activity which even
appears to present a conflict must be avoided or terminated unless, after disclosure to the Company, it is determined that the
activity is not harmful to the Company or otherwise improper. It is our responsibility to disclose any material transaction or
relationship that could be expected to give rise to a conflict of interest to the CEO or Board of Directors. Managers may not authorize
conflict of interest matters or make determinations as to whether a problematic conflict of interest exists without first seeking
the approval of the CEO including a written description of the activity. Officers and directors may seek authorizations and determinations
from the Nominating and Corporate Governance Committee of the Board of Directors.
For purposes of this Code,
“Family Member” generally means a person’s spouse, parents, children and siblings, whether by blood, marriage
or adoption, or anyone residing in such person’s home.
| VI. | CORPORATE OPPORTUNITIES |
Covered Persons owe a duty
to the Company Group to advance their legitimate interests when the opportunity to do so arises. Covered Persons must offer to
the Company any business opportunities related to the Company Group’s target assets and business activities (as described
in the Company’s Registration Statement on Form S-1 relating to the Company’s initial public offering or any periodic
report filed by the Company from time to time with the SEC, together with any other assets that the Board determinates from time
to time will be a target asset or potential investment or business of the Company Group). Covered Persons are prohibited from:
(i) taking for themselves opportunities that are discovered through the use of Company Group property, information or position,
unless such opportunities are presented to the Board and the Board declines to pursue such opportunities; (ii) using Company Group
property, information or position for improper personal gain; or (iii) competing with any member of the Company Group. Any employee,
other than an officer, may only pursue a corporate opportunity if the CEO or Board waives in writing the Company’s right
to pursue the corporate opportunity. Corporate opportunities available to directors and officers may only be waived by the Board.
If the Company waives its right to pursue a corporate opportunity, Covered Persons may pursue such opportunities in a manner consistent
with this Code.
| VII. | COMPLIANCE PROCEDURES; REPORTING VIOLATIONS |
As part of its commitment
to ethical and legal conduct, Covered Persons must bring to attention information about suspected or known violations of law or
policy by any employee, contractor or agent of the Company to the CEO or an appropriate manager. Violations should be reported
regardless of the identity or position of the person suspected of engaging in improper conduct. The Company expects all Covered
Persons to work to ensure prompt and consistent action against violations of this Code. This Code covers a wide range of business
practices and procedures, but it does not address every applicable law or respond to every ethical question or concern that may
arise. Nonetheless, the general guidelines of this Code provide each Covered Person with the Company’s expectations regarding
business dealings. The Company understands that there may be some situations in which it is difficult to know right from wrong.
In determining the best course of action, each Covered Person should answer the following questions to help evaluate specific situations:
| 1. | Will my action comply with the intent and purpose of the Company’s policies and practices? |
| 2. | Will I compromise myself or the reputation of any member of the Company Group by this action if
it becomes known to my supervisor, colleagues, shareholders or friends? |
| 3. | Is this action honest in every respect? |
| 4. | Could this action appear inappropriate to others, even if it is ethical? |
If something you have seen,
heard or been asked to do (or not do) seems illegal, unethical or improper, it may very well be. Each Covered Person should use
his or her judgment before taking any action that could be deemed a violation of this Code or any law, rule or regulation or Company
policy. Furthermore, any Covered Person who becomes aware of (a) any existing or potential violation of this Code or any law, rule
or regulation or Company policy or (b) alleged irregularities of a general, operational or financial nature within the Company
(“Alleged Irregularities”), has an obligation to report his or her complaint or concern to his or her supervisor,
to the CEO, the Whistleblower Hotline or the Board. Alleged Irregularities concerning the functioning of the Board may be reported
directly to the CEO.
No Covered Person should
report any existing or potential violation of the Code or any law, rule or regulation, Company policy or any Alleged Irregularity
to any person who is involved in the matter giving rise to the existing or potential violation.
All concerns will be taken
seriously by the Company and, when appropriate, the Company will fully investigate each allegation. This may include talking to
any individuals directly involved, as well as to others who may possess information pertinent to the situation. Covered Persons
are expected to cooperate fully with internal investigations of wrongdoing or misconduct, and failure to cooperate fully with any
such investigations will lead to disciplinary action, up to and including termination.
The Company will not tolerate
any retaliation against any Covered Person for raising, in good faith, a possible violation of this Code or of a law, rule or regulation,
Company policy or any Alleged Irregularity. Retaliation for reporting a U.S. federal offense is illegal under U.S. federal law.
Any person who participates in retaliatory conduct will be subject to disciplinary action up to and including termination of employment
or office. Misusing this Code by knowingly or recklessly providing false information to the Company may also result in appropriate
disciplinary action. Any Covered Person who deliberately fails to report a matter of noncompliance will be subject to appropriate
disciplinary action.
Every director, officer,
manager and supervisor who receives a complaint or a report alleging or regarding an actual or potential violation of this Code
or of a law, rule or regulation, Company policy or regarding any Alleged Irregularity, has, without exception, the responsibility
to immediately communicate such complaint to the CEO.
Adherence to this Code is
a condition of working for the Company. If, after investigation, it is determined that a compliance violation has occurred, an
employee may be subject to discipline including, for example, training, referral to counseling, warning, reprimand, withholding
of a promotion or pay increase, demotion, reassignment, temporary suspension without pay, or termination of employment.
| VIII. | ACCOUNTING COMPLAINTS |
The Company’s policy
is to comply fully with all applicable financial reporting and accounting regulations. If any Covered Person has unresolved concerns
or complaints regarding questionable accounting, internal control or auditing matters concerning the Company, such person is encouraged
to submit such concerns or complaints in accordance with the Company’s Complaint Procedures.
We seek to outperform our
competition fairly and honestly. We seek competitive advantages through superior performance, never through unethical or illegal
business practices. Stealing proprietary information, possessing trade secret information that was obtained without the owner’s
consent, or inducing such disclosures by past or present employees or officers of other companies is prohibited. Each Covered Person
should endeavor to respect the rights of, and to deal fairly with the Company Group’s guests, suppliers, consultants, competitors,
employees, officers and other persons with whom the members of the Company Group transact business. No Covered Person should take
unfair advantage of anyone through manipulation, concealment, abuse of privileged information, misrepresentation of material facts,
or any other unfair dealing practice.
Directors, officers and
employees should maintain the confidentiality of information entrusted to them by the Company, except when disclosure is authorized
under the Company’s Disclosure Policy or as otherwise legally mandated. Confidential information includes all non-public
information that might be of use to competitors, or harmful to the Company, its suppliers or its customers, if disclosed. It also
includes information that suppliers, customers and individuals or institutions involved in clinical trials or other product development
activities have provided to the Company. Employees should take appropriate steps to limit distribution of such confidential information
to only those employees of the Company who have a need to know such information in order to carry out their job responsibilities.
It is the Policy of the Company that each of us must respect the proprietary information of other individuals or organizations
with which the Company does business. Information obtained from public sources can legitimately be used in the Company’s
business activities, but proprietary information obtained through improper means can never be used by any director, officer or
employee in carrying out his /her job responsibilities.
| XI. | NO ABUSE OF CORPORATE ASSETS |
Employees may not take personal
advantage of opportunities that are presented to them or discovered by them as a result of their position with the Company or through
their use of corporate property or information, unless authorized by the Board of Directors. Even opportunities that are acquired
privately by the employee may be questionable if they are related to the Company’s existing or proposed lines of business.
Significant participation in an investment or outside business opportunity that is directly related to our lines of business must
be pre-approved. Employees may not use their position at the Company or corporate property or information for improper personal
gain, nor should they compete with the Company in any way.
| XII. | MEDIA/PUBLIC DISCLOSURES |
It is our policy to disclose
material information concerning the Company and its work to the public only through specific limited channels to avoid inappropriate
or inaccurate publicity. Employees may not provide any information to the media about the Company and its work or customers, whether
off-the-record, for background only, confidentially or secretly. All inquiries or calls from the press should be referred to the
CEO.
| XIII. | PROTECTED INFORMATION |
The Company believes in
the importance of respecting the privacy of all of those with whom we do business. During normal business activities, the Company
may collect personal, sensitive or nonpublic information about various individuals, including our employees, customers, patients,
clinical trial participants and others with whom we conduct business. It is the Company’s policy to respect the privacy and
guard the confidentiality of personal, sensitive or nonpublic information, including protected health information. Accordingly,
employees must maintain and use such information in accordance with the law, and the Company’s even more stringent policies
and procedures. The Company will implement measures designed to protect the personal, sensitive or nonpublic information and to
appropriately maintain a system for protection of that information.
Patient personal data, including
Protected Health Information (PIH) and sensitive personal data may be subject to additional regulatory protections, such as a requirement
to have policy and security safeguards in place to protect it. We are responsible for ensuring that patient personal data is kept
secure and confidential and used solely for the purpose for which it was collected. Employees responsible for handling such patient
data are expected to become familiar with laws and regulations in the jurisdictions in which we conduct clinical trials.
The laws against insider
trading of the Company’s (or other) securities are specific and complex. Covered Persons may obtain, or come into regular
or occasional contact with, information that qualifies as “inside information” under applicable securities laws. Inside
information includes material information about the Company that is not available to the public. The unauthorized use by a Covered
Person of inside information is unethical and may also be unlawful. Covered Persons should never trade the shares or other securities
of the Company while in possession of inside information, nor should they disclose such information to any other person that might
engage in trading activities, unless in compliance with the Company’s Policy on Insider Information and Insider Trading.
In all cases, it is each Covered Person’s responsibility to ensure that he or she complies with all relevant securities laws
and regulations.
The Company recognizes the
popularity of social media as a business tool in appropriate circumstances and has implemented a policy outlining the use of social
media in its Employee Handbook. Business and personal use of social media relating to the Company, its business or its employees,
whether on or off working time or Company equipment, must comply with the Company’s Employee Handbook.
| XVI. | PROTECTION AND PROPER USE OF THE COMPANY GROUP’S ASSETS |
All Covered Persons should
protect the Company Group’s assets and ensure their efficient use. Theft, carelessness and waste have a direct impact on
the Company Group’s profitability. Any suspected incident of fraud or theft should be immediately reported to the Company’s
CEO or designee. All of the Company Group’s assets should be used for legitimate business purposes and should not be used
for non-company business, although incidental personal use may be permitted with the permission of your supervisor. The Company
has the ability, and reserves the right, to monitor all electronic and telephonic communication.
The Company has a responsibility
to ensure that it provides the investing public with information that accurately and fairly reflects the true value of its operations.
Accurate and fair records are essential to ensure the proper conduct of the Company’s business and its compliance with the
law.
The Company’s responsibilities
to its stakeholders and the investing public require that all of the Company’s and Company Group’s books, records,
accounts and financial statements be maintained in reasonable detail, appropriately reflect the Company’s transactions and
conform to applicable legal requirements, the Company’s system of internal controls and generally accepted accounting principles
(“GAAP”). The Company relies on the accuracy and completeness of its business records to (i) provide full, fair,
accurate, timely and understandable disclosure in the current reports, periodic reports and other information it files with or
submits to the SEC and in other public communications, such as press releases, earnings conference calls and industry conferences,
made by the Company or on the Company’s behalf, (ii) make management decisions and (iii) analyze its operations. The accuracy
of such records is essential for continued, long-term business success.
No false, misleading or
artificial entries may be made by any Covered Person in the books and records of the Company or the Company Group. All Covered
Persons with supervisory responsibility shall establish and implement appropriate internal accounting controls over all areas of
their responsibility to ensure the safeguarding of the Company’s assets and the accuracy of its financial records and reports.
The Company has adopted controls in accordance with internal needs and the requirements of applicable laws and regulations. These
established accounting practices and procedures must be followed to assure the complete and accurate recording of all transactions.
All Covered Persons, within their areas of responsibility, are expected to adhere to these procedures, as directed by the Chief
Financial Officer.
We all share the responsibility
of maintaining accurate records and reports. Even if you are not directly involved in financial reporting or accounting, you are
likely involved with financial records or reports of some kind—a contract, time sheet, invoice or expense report. In addition,
most employees have involvement with product or administrative activities or performance evaluations, which can affect our reported
financial condition or results. Therefore, regardless of whether you are otherwise required to be familiar with finance or accounting
matters, you are required to use all reasonable efforts to ensure that every business record or report with which you deal is accurate,
complete and reliable
Any accounting adjustments
that materially depart from GAAP must be approved by the Company’s Chief Financial Officer. In addition, all material off-balance-sheet
transactions, arrangements and obligations, contingent or otherwise, and other relationships of the Company with unconsolidated
entities or other persons that may have material current or future effects on the financial condition, changes in financial condition,
results of operations, liquidity, capital expenditures, capital resources or significant components of revenues or expenses must
be disclosed to the Company’s Chief Financial Officer.
The Audit Committee plays
an important role in ensuring the integrity of our public reports. If you believe that questionable accounting or auditing conduct
or practices have occurred or are occurring, you should notify the Audit Committee of the Board of Directors. In particular, our
CEO and senior financial officers should promptly notify the Audit Committee any such information of which he or she may become
aware including the accuracy of material disclosures in public filings, material weaknesses or significant deficiencies in internal
controls over financial reporting and any evidence of fraud that involves an employee with a significant role in our financial
reporting disclosures or internal controls or procedures.
No Covered Person may interfere
with or seek to improperly influence, directly or indirectly, the auditing of the Company’s financial records. Violation
of these provisions shall result in disciplinary action, up to and including termination of employment, and may also subject the
violator to substantial liability.
The Company has implemented
measures to ensure that its records will be appropriately safeguarded. The Company’s employees will retain records for as
long as they are required and in the manner required to meet its legal, regulatory, administrative and operational requirements,
after which they may be appropriately discarded. In many instances, there are legal requirements that certain records be retained
for specific periods of time and may be appropriately disposed of once that period of time has elapsed. Records necessary for business
reasons will be retained for a period of time that will reasonably assure the availability of those records when needed. Whenever
it becomes apparent that records of any type will be required in connection with a lawsuit or government investigation, all relevant
records should be preserved, and ordinary disposal or alteration of records pertaining to the subject of the litigation or investigation
should be suspended. If an employee is uncertain whether records under his or her control should be preserved because they might
relate to a lawsuit or investigation, he or she should contact the CEO.
| XIX. | DISCLOSURE OF COMPANY INFORMATION |
Information is a valuable
corporate asset. Open and effective dissemination of information is critical to the Company’s success. However, much of the
information to which Covered Persons have access at the Company is confidential and/or proprietary. Confidential information is
information that the Company considers private and that is not common knowledge outside the Company. Proprietary information is
information the Company owns, develops, pays to have developed, or to which it has an exclusive right. Because the disclosure of
such information could seriously damage the Company’s interests, safeguarding this information is the responsibility of all
employees of the Company and Covered Persons. Each employee and Covered Person must safeguard all confidential and proprietary
information. Covered Persons will not, without prior written approval of the Company, disclose information that has not already
been published or made publicly available.
Covered Persons may not
share proprietary information with anyone not entitled to know for a legitimate business reason including fellow employees, spouses,
other family members, and friends. Any access to confidential and proprietary information given to a third-party (outside of the
Company) for a legitimate business reason must be provided under a confidentiality agreement approved by the CEO with the advice
of legal counsel. Covered Persons will exercise due care to prevent the release or sharing of information beyond those people who
need to know such information to perform their job function. To avoid inadvertent disclosure of such information, you will avoid
discussions of Company information in public places such as elevators, public transportation, restaurants or on public telephones.
Only the CEO may respond to the media, the financial community or other outside inquiries about the Company. The obligation to
protect the Company’s Confidential and proprietary information continues even if employment with the Company ends.
Additionally, a substantial
part of the Company’s business involves use and licensing of patents, trademarks, copyrights and use of rights licensed from
others. The Company and its business partners invest extraordinary expertise and resources to make their products, and they expect
their intellectual property to be respected and protected. The confidentiality of the Company’s intellectual property is
critical to the success of our business and must be strictly maintained. Similarly, Company employees and Covered Persons should
never take or accept from others information or materials known or believed to contain the trade secrets of a competitor.
| XX. | PROPER USE OF COMPANY PROPERTY |
All Covered Persons are
expected to protect the Company’s assets and use them appropriately and efficiently. Theft, carelessness, and waste have
a direct impact on profitability. The Company property, such as office and lab supplies, computer equipment, and facilities are
expected to be used only for legitimate business purposes, although incidental personal use may be permitted. Employees may not,
however, use the Company’s name, any brand name or trademark owned or associated with the Company or letterhead stationery
for any personal purpose. Any misuse or suspected misuse of the Company’s assets must be immediately reported to the CEO.
Theft of any Company equipment,
property or funds, including without limitation, the submission of inappropriate or fraudulent requests for reimbursement of expenses,
will not be tolerated.
| XXI. | USE OF COMPANY EQUIPMENT, VOICE MAIL AND E-MAIL |
Technology is critical to
our success at the Company. Everyone who uses our technology in the course of their employment has the responsibility to use the
resources provided appropriately and securely. You will only use e-mail, voice mail systems, and other Company equipment (e.g.,
laptop computers, servers) for conducting business or for other purposes authorized by your supervisor. Reasonable, limited personal
use of these systems and equipment is permissible; however, you will not use these systems or equipment in connection with any
inappropriate or offensive material, including without limitation any pornographic or obscene material. The Company may monitor
and audit all usage of these systems and equipment to ensure integrity and to prevent misuse. There shall be no expectation of
privacy in your use of these systems or equipment.
| XXII. | QUALITY PRODUCTS AND PATIENT SAFETY |
The Company is committed
to carefully assessing the risks and benefits of our products before and after we bring them to market. We understand that it is
critical for patients and physicians to understand these risks and benefits before making treatment decisions, and the responsibility
of providing accurate product information is one the Company takes very seriously.
The Company conducts and
supports new medical research and recognizes that clinical trial and other research findings are an important means of adding to
the medical community’s body of knowledge. The Company strives to make all clinically significant scientific information
resulting from our clinical trials and other research publicly available and to ensure that any publications resulting from research
financially supported by the Company accurately disclose the Company’s support.
The Company collaborates
with appropriate regulatory authorities to determine the label information for each of our products in an effort to ensure that
accurate and complete information about the indications and safety of our products is provided.
The Company is committed
to developing and manufacturing the highest quality products in accordance with all relevant laws. Our dedication to quality is
the driving force of what we do for patients and for our Company. By focusing on quality in all internal and contracted operations
that support the development of our products, we will foster an environment of success and trust with physicians and patients.
We share the responsibility of maintaining
our high-quality standards by:
| · | Making patient safety a paramount focus of internal and contracted operations that support the
development of our products; |
| · | Following all applicable laws, regulations and Company policies and procedures; |
| · | Engaging only suppliers and other third parties who support the Company’s commitment to high
quality standards; |
| · | Never sacrificing quality to meet a deadline or target; and |
| · | Raising any quality questions or concerns through appropriate channels. |
Putting Patients First
The Company is committed
to conducting all its clinical trial activities in accordance with International Council for Harmonization - Good Clinical Practice
(ICH - GCP), the international ethical and scientific quality standard for designing, conducting, recording and reporting medical
trials that involve the participation of human subjects. Compliance with this standard provides public assurance that the rights,
safety and well-being of trial subjects are protected, and that the clinical trial data is credible. We protect patient rights
through appropriate informed consent procedures, compliance with ICH – GCP and documentation of our compliance with all applicable
privacy-related policies and regulations.
We only conduct or sponsor
trials under the direction of experienced investigators and in compliance with protocols that have received all necessary prior
approvals from institutional review boards (IRB), independent ethics committees (IEC) or other applicable authorized bodies or
agencies.
As part of our commitment
to improve the overall quality of life for people living with neurodegenerative and neurodevelopmental disorders, we recognize
the importance of fully understanding the needs and rights of the vulnerable populations that participate in our clinical trials.
The following principals must guide our clinical research:
Participants must not be
exposed to unnecessary risks.
Participants and
their caregivers must understand the nature and purpose of the clinical research via informed consent procedures.
Privacy and confidentiality
rules must be strictly adhered to.
Information gathered
must enable transparent and accurate reporting, interpretation and verification of study outcomes.
In working with patient
advocacy groups and organizations, always exercise respect and transparency, maintaining independence of these groups’ and
working cooperatively for the benefit of patients by being responsive to patient and physician requests and questions and respectful
of the physician-patient relationship. Employees are responsible for ensuring all product communications made with patient organizations
are factually accurate and consistent with legal and research requirements.
Working with Vendors
We work with various outside
vendors to perform certain research, development and commercial activities. These vendors must comply with all applicable international
and local healthcare program and regulatory requirements, including the FDA. Any employee who is responsible for such vendor’s
activities must ensure that the vendor understands that, in performing services on behalf of the Company, they must adhere to the
same high standards to which Anavex is held. Employees who are concerned that a vendor is not conducting its activities in compliance
with Anavex’s policies and procedures should report compliance concerns to the CEO.
Our vendor selection policy
is to select vendors based on the merits of their products, services and business practices and to purchase supplies based on need,
quality, service, price and other terms and conditions of sale. You may not establish a business relationship with a vendor if
you know or have reason to know that its business practices violate applicable laws or our Code of Conduct.
Environmental Compliance
It is our policy
to conduct our business in an environmentally responsible way that minimizes environmental impacts. We are committed to minimizing
and, if practicable, eliminating the use of any substance or material that may cause environmental damage, reducing waste generation
and disposing of all waste through safe and responsible methods, minimizing environmental risks by employing safe technologies
and operating procedures, and being prepared to respond appropriately to accidents and emergencies.
| XXIII. | RETENTION OF BUSINESS RECORDS |
Records retention policies
seek to establish consistent practices concerning how long records should be kept and when, in the normal course of business, they
should be destroyed. All Covered Persons must comply at all times with all laws, rules and regulations relating to records preservation,
all records retention policies and all document or record preservation notices. Records must be maintained for the duration of
the assigned retention periods. A record includes any information, regardless of physical format, which has been created or received
in the transaction of the Company’s business. Physical format of a record includes paper documents, CDs, DVDs, computer hard
disks, e-mail, floppy disks, microfiche, microfilm or all other media and data storage devices. The retention and proper disposal
of the Company Group’s records shall be in accordance with established Company policies and applicable legal and regulatory
requirements.
If the existence of any
pending or threatened legal action, subpoena or investigation is known or reported to you, promptly contact the CEO. You must retain
all records that may relate to any pending or threatened legal action, subpoena or investigation. If you have a question as to
whether a record pertains to a pending or threatened legal action, subpoena or investigation, contact the CEO before disposing
of the record in question.
| XXIV. | ANTI-BRIBERY AND ANTI-CORRUPTION POLICY |
This Anti-Bribery and Anti-Corruption
Policy of Anavex (the “Policy”) is designed to ensure compliance with the US Foreign Corrupt Practices Act of
1977 (the “FCPA”), as well as with the principles described in the Convention on Combating Bribery of Foreign
Public Officials in International Business Transactions and any local anti-bribery or anti-corruption laws existing where Anavex
and its affiliates operates (collectively, the “Anti-Corruption Laws”). This Policy applies to all Covered Persons.
Anavex takes a zero-tolerance
approach to bribery and corruption. All Company personnel are expected to conduct business legally and ethically, in accordance
with best practices. As a general principle, Anavex and its representatives shall not transfer or offer to transfer any type of
benefit for the purpose of influencing a public official to misuse his or her power or influence.
| A. | PURPOSE AND APPLICATION OF THIS POLICY |
The purpose of this Policy
is to establish the obligations of Anavex, and of those working on behalf of the Company, in observing and upholding its position
on bribery and corruption, and to provide guidance on how to recognize and deal with bribery and corruption issues. As the FCPA
has extraterritorial application, Anavex, along with all Covered Persons will be bound by these laws in relation to conduct in
all countries in which they operate. This Policy is not intended to supplant the Anti-Corruption Laws.
| B. | COMPLIANCE REQUIREMENTS |
Prohibited Activities
– No Covered Person shall, directly or indirectly give, offer or agree to give anything of value of any kind (including any
payment, loan, gift, reward, advantage, benefit or service) to a public official or to any person for the benefit of a public official:
(a) as consideration for an act or omission by the official in connection with the performance of his/her duties; or (b) to induce
the official to use his/her position to influence any acts or decisions of the government for which the official performs his/her
duties.
A “public official”
includes any person who holds a legislative, administrative or judicial position of a state; a person who performs public duties
or functions for a state, including a person employed by a board, commission, corporation state-owned company or other body or
authority that is established to perform a duty or function on behalf of a state, or is performing such a duty or function; and
an official or agent of a public international organization that is formed by two or more states or governments, or by two or more
such public international organizations. A “state” means any country, and includes any political subdivision of that
country (such as a province or territory); the government, and any department, or branch of that country or of a political subdivision
of that country; or any agency of that country or of a political sub-division of that country.
Bribes given through an
agent or received by a party other than a public official are also prohibited if the ultimate goal is to influence a public official
by conferring a benefit. Any question about whether someone is a public official should be directed to the CEO.
Covered Persons may not
make or authorize cash or cash equivalent (e.g. check) reimbursements or payments of any kind to individual public officials without
prior written authorization from the CEO.
No Facilitation
Payments – Anavex does not make “facilitation payments” or “kickbacks” of any kind, regardless
of whether such payments are permitted under applicable law. Facilitation payments are typically small, unofficial payments made
to secure or expedite a routine government action by a government official (such as the issuance of permits, licenses, provision
of mail pick-up and delivery etc.). Kickbacks are typically payments made in return for a business favor or advantage and can include
discounts or other types of cash incentives.
All Covered Persons must
avoid any activity that might lead to, or suggest, that a facilitation payment or kickback will be made by, on behalf of, or otherwise
in connection with the business of or for the benefit of the Company.
If asked to make a payment
on the Company’s behalf, always be mindful of what the payment is for and whether the amount requested is proportionate to
the goods or services provided. Always obtain a receipt which details the reason for the payment and evidences that the payment
went directly to the appropriate payee who provided the goods or services. Any suspicions, concerns or questions regarding a payment
should be raised with the CEO.
Lodging and Travel
Expenses – If any Covered Person proposes to reimburse the bona fide and reasonable travel and lodging expenses
of a public official, such Covered Person shall document such proposed reimbursement, lodging or services and shall consult with
the CEO to determine the propriety of any such proposed reimbursement and obtain the CEO’s prior written authorization before
making any offer to such Public Official. In any such case, the amount and purpose of such reimbursement, lodging or services must
be reasonable and must relate directly to either: (a) the promotion, demonstration, or explanation of the Company’s services
or operations with a government, government agency, or government-owned or government-controlled enterprise or the performance
of an official duty related to the Company; or (b) the execution or performance of a contract with a state or related organization.
Gifts and Hospitality
– This Policy does not prohibit normal, appropriate and modest hospitality to or from third parties. These customary courtesies
are designed to build goodwill among business partners.
The test to be applied is
whether in all the circumstances the gift or hospitality is reasonable and justifiable (both from the perspective of the provider
and recipient) rather than lavish and extraordinary; bearing in mind that what may normally be viewed as small or insignificant
in some countries can be of significant value in another. The intention behind the gift should always be considered and nothing
should be explicitly or implicitly expected or demanded in return.
The giving of gifts and
corporate hospitality or entertainment is not prohibited, if the following requirements are met:
| (a) | it is done in the normal course of the Company’s business and without the intention of, or
without a reasonable prospect of, influencing a public official to obtain or retain an improper business advantage, or to reward
the provision or retention of an improper business advantage, or in explicit or implicit exchange for favors or benefits; |
| (b) | it complies with applicable local law; |
| (c) | it does not include cash or a cash equivalent; |
| (d) | it must be properly recorded and disclosed, and not paid personally to avoid any approval or disclosure
requirements; |
| (e) | taking into account the reason for the gift or hospitality, it is of an appropriate type and value
in the applicable country/region and given at an appropriate time; |
| (f) | it is given openly and in the Company’s name, not secretly; and |
| (g) | it is not given or received frequently between the same individuals. |
Gifts or hospitality should
not be offered to Public Officials or government representatives, or politicians or political parties, without the prior approval
of the CEO.
Community Funding
– Government officials or their representatives may request or expect funding for consideration or approval of regulatory
matters involving the Company. The Company may be given opportunities to financially support development initiatives of communities
in proximity to its projects or make charitable contributions. Although it may be customary to do so, no Covered Person may make
or commit to such funding, contributions or payments to public officials on behalf of the Company without the prior written approval
of the Company’s CEO. In each of these scenarios, such payments or contributions may be prohibited under applicable laws
and, accordingly, in order to avoid involvement in improper conduct, it is critically important that the Company be diligent in
confirming details of the nature of the payment in question, including with respect to who the intended beneficiaries of the contribution
in question are, and how they will benefit.
Political Contributions
– No Covered Persons should make any contribution or provide any financial support to any political party or candidate on
behalf of Anavex, except as may be pre-approved by the Company’s CEO. No Political Contributions may be used as a subterfuge
for bribery.
In undertaking any political
activity that is not authorized by this policy or other policies of the Company, all Covered Persons will be deemed to be acting
in their personal capacity or that of their own corporate organization and not on behalf of the Anavex.
Record Keeping
– All accounts, invoices, memoranda and other documents and records relating to dealings with third parties must be prepared
and maintained with strict accuracy and completeness. Covered Persons must ensure that all expense reports relating to hospitality,
gifts or expenses incurred to third parties are submitted in accordance with the relevant Company policies and that the reasons
for the expenditures are specifically recorded. No accounts or transactions must be kept “off-book” for any reason,
including to facilitate or conceal improper payments. Recording of any payments in any way which would conceal their true nature
constitutes a violation of this Policy and applicable laws.
All document processing
payments, attachments to justify payment requests, classification of payments, authorizations, and certifications subject to this
Policy must be capable of being retrieved at least for five (5) years.
| C. | PENALTIES FOR NON-COMPLIANCE |
Individuals who fail to
comply with this Policy may face severe consequences which could include internal disciplinary action or termination of employment
or service arrangements without advance notice. If it appears that any Covered Person may have violated any applicable Anti-Corruption
Laws, then the Company may refer the matter to the appropriate regulatory authorities, which could lead to penalties, fines or
imprisonment for Anavex and/or the responsible person.
The Company may be held
liable where any of its employees, agents, contractors or other representatives has engaged in bribes or other forms of corruption
or misconduct, whether known or not by senior management. Companies charged under the Anti-Corruption Laws may be subject to significant
fines, negative effects on its share price and material damage to its reputation. A convicted company may also be ordered to forfeit
all proceeds obtained from an act of bribery.
| D. | POLICY IMPLEMENTATION AND OVERSIGHT |
The Company’s Board
has overall responsibility for ensuring this Policy complies with the Company’s legal and ethical obligations, and that all
those under the Company’s control comply with it.
The Company’s CEO
has primary responsibility for overseeing the implementation of this Policy, and for monitoring its suitability, adequacy and effectiveness.
Where appropriate the CEO may consult with other officers of the Company prior to making determinations in relation to this Policy.
Management at all levels is responsible for ensuring that those reporting to them are made aware of, understand and comply this
Policy.
The Company shall continue
to develop, implement, monitor and maintain a system of internal controls to facilitate and assess compliance with this Policy
by its employees, agents, contractors and consultants.
These systems shall include
the following elements:
- All
officers and directors, all corporate employees, all members of the executive committee and certain additional employees in positions
that require them to interact with public officials, approve payments or keep accounting records (the “Designated Employees”),
will receive relevant training on how to implement and adhere to this Policy. All Designated Employees will provide annual certification
of compliance with this Policy.
- Internal
audits will be conducted periodically to assess whether this Policy is effective in (i) increasing awareness among Covered Persons
of bribery and corruption issues, and the significance thereof; (ii) systematically reducing the risk of occurrence of bribery
and corruption related incidents involving the Company; (iii) establishing appropriate written records to evidence that reasonable
care and diligence have been taken to ensure compliance in these areas.
- Internal
audits will be conducted periodically in order to monitor instances of non-compliance by Covered Persons, with appropriate follow-up
action as warranted.
- A
report will be provided to the Board on an annual basis confirming that: (a) all annual certifications have been obtained, and
summarizing the results thereof; (b) an internal audit of the effectiveness of this Policy has been duly completed; and (c) material
issues that are identified are reported to the Board and appropriate corrective action is taken.
- The
Company shall conduct an anti-bribery risk review of projects or proposals involving business in new jurisdictions. The level of
due diligence conducted should be consistent with the level of risk to be managed, and must include an assessment of the prevalence
of bribery, corruption and other unacceptable behavior in such new market.
- Due
diligence on potential acquisition targets must include consideration of whether the target and its representatives have complied
with applicable bribery and corruption legislation. Employment, consultancy, agency and similar agreements entered into by the
Company, where the employees will be Designated Employees or consultants or agents are expected to have interaction with public
officials, shall require counterparties to acknowledge and agree that they understand, and shall comply with, this Policy.
| E. | COMMUNICATION OF THIS POLICY |
This Policy, along with
any updates hereto, will be posted on the Company’s website. A copy of the Policy will be provided to all Designated Employees
and to all agents, consultants and contractors of Anavex having interaction with public officials on behalf of Anavex, and they
will be advised that the Policy is available on the Anavex website for their review, and informed whenever significant changes
are made.
Education on this Policy
will form part of the orientation or induction process for all new Designated Employees. In addition, this Policy shall be communicated
to any agent, contractor or consultant having interaction with public officials on behalf of Anavex at the outset of the business
relationship, and as appropriate thereafter. For advice on these communications, please contact the Company’s CEO.
1. Reporting.
All Covered Persons must immediately report any known, suspected or suggested violations of this Policy to the Company’s
CEO. Alternatively, Covered Persons can report any known, suspected or suggested violations of this Policy through the Company’s
Whistleblower Hotline by one of the following methods:
a. Email.
By email on a confidential basis which is forwarded directly to a designated member of the Board of Directors (the “Board
Member”) to: anavexhotline@anavexcorp.com. This hotline email address is forwarded directly to the Board Member who will
maintain anonymity if so requested. However, employees may also use a non-identifiable or third party email address to submit anonymous
complaints to the hotline.
b. Audio
Message. Employees or others wishing to lodge a complaint or raise compliance concerns may contact 917-460-0668 to record
an audio message. An audio file will be created and will be forwarded to the Board Member.
Additionally, Covered Persons may contact the
Company’s CEO with a question or concern about the application of this Policy. Any questions or violation reports will be
addressed immediately and taken seriously, and can be made anonymously. The CEO, Board Member or his/her designee will investigate
any reported violations, and, if warranted, will determine an appropriate response, including corrective action and preventative
measures. All reports will be treated confidentially to every extent possible.
The Company will maintain a record of all reports
under this Policy, tracking their receipt, investigation and resolution.
The Company will promptly report any violations
of applicable law to appropriate regulatory authorities.
2. Protection.
The Company encourages openness and will support anyone who raises genuine concerns in good faith under this policy, even if they
turn out to be mistaken.
The Company is committed to ensuring no one
suffers any detrimental treatment as a result of refusing to take part in bribery or corruption, or because of reporting in good
faith their suspicion that an actual or potential bribery or other corruption offence has taken place, or may take place in the
future. Detrimental treatment includes dismissal, disciplinary action, threats or other unfavorable treatment connected with raising
a concern. If you believe that you have suffered any such treatment, you should inform the Company’s CEO immediately. If
the matter is not remedied, and you are an employee, you should raise it formally with the CEO.
The prevention, detection
and reporting of bribery offences and other forms of corruption are the responsibility of all those working for Anavex or on its
behalf. If you have any questions about this Policy, please do not hesitate to contact the Company’s CEO.
| XXV. | POLITICAL CONTRIBUTIONS |
The Company Group’s
funds or assets may not be contributed, directly or indirectly, to any political party, committee or candidate, or the holder of
any federal, state or local government office within the United States unless prior approval has been given by the Company’s
CEO. In countries other than the United States in which political contributions by companies are lawful, a political contribution
may be made only upon the prior specific written approval of the Company’s CEO. Covered Persons shall not be directed, pressured
or coerced in any manner by a director, officer or any individual acting in a managerial or supervisory capacity to make a contribution
to any political party or committee or to any candidate for or the holder of any government office.
| XXVI. | WORKPLACE COMPLIANCE |
It is the Company’s
policy to attract and retain ethically committed and competent employees and to provide them with the education, training and growth
opportunities that encourage professional development. The Company believes that it is important to establish key result areas
for individual performance and provide transparent and uniform assessment feedback that encourages continuous improvement. The
Company’s policies help foster an environment for employee development and investment into their success.
| A. | Equality and Harassment |
The Company is an equal
opportunity employer. The Company values a diverse workforce and proudly reflects a Company culture developed with a variety of
ethnic backgrounds, nationalities, races, ages, genders, political affiliates, sexual preferences, and religions. The Company is
committed to fostering a work environment in which all individuals are treated with respect and dignity and is committed to promoting
and maintaining an inclusive, high- performing culture. All team members embrace and leverage each other’s talents and backgrounds
and nourish innovative thinking to achieve their full potential and contribute to our shared success.
The Company does not unlawfully
discriminate, nor does it allow its employees to unlawfully discriminate. The Company expects that all relationships among persons
in the workplace will be business-like and free of unlawful discrimination, bias, prejudice and harassment. Prohibited harassment
includes conduct that is intended to or that has the effect of unreasonably interfering with a fellow employee’s work performance
or creating an environment that is intimidating, hostile, or offensive to the employee. Any hostile or demeaning behavior based
upon an employee’s race, religion, national origin, age, gender, disability, sexual preference, political affiliation, marital
status or veteran status will be construed as a violation of this policy and will be dealt with as such.
| B. | Workplace Safety and Health |
The Company conducts its
operations with the highest regard for the safety and health of all employees and is committed to maintaining a safe and healthy
workplace. The Company will endeavor to provide a safe and healthy workplace and to ensure that its operations do not cause any
adverse impact or injury to the environment or to the communities in and around its workspaces. Employees must comply with all
established safety rules and procedures, as well as all applicable federal, state and local health and safety laws including those
issued by the Occupational Safety and Health Administration. When an unsafe condition or practice is identified, appropriate action
must be taken promptly to correct the condition and to prevent it from happening again. Employees must report any violation of
a safety rule, procedure or law of which they are aware or any accident, workplace injury, or any situation presenting a danger
of injury.
It is our policy to maintain
a drug-free work environment. Use of illegal drugs, alcohol abuse and the misuse of legal drugs create serious health and safety
risks in the workplace. The sale, transfer, or possession of illegal drugs or being under the influence of such drugs is strictly
prohibited. Reporting to work or any Company business function under the influence of and/or impairment by any such substance or
alcohol is also strictly prohibited. It is important that cases of drug or alcohol abuse be brought to the CEO’s attention
immediately.
The Company is committed
to a safe working environment, free of threats, malicious behavior, intimidation and physical harm. All employees have a right
to work in a safe environment and share the responsibility for assuring each other’s safety. The Company prohibits violence-related
conduct including, but not limited to, physical assaults, fighting, threatening comments, intimidation and the intentional destruction
of any company property, employee property, or merchandise. Any comments or behavior that could be reasonably interpreted as intent
to do harm to employees, their family or their property will be considered a threat. Any employee who believes that he or she may
be the target of violence or threats of violence, or is aware of violent or threatening conduct by another individual, that could
result in injury to any employee or the destruction of property, has a responsibility to immediately report the situation to the
CEO.
The Company adheres to an
“Open Door Policy,” and encourages Company personnel to discuss with their manager or the CEO any compliance issues,
concerns, problems and/or suggestions without fear of retaliation and with the assurance that the matter will be kept as confidential
as possible.
The Company follows a strict
non-retaliation policy as part of its Code of Business Conduct. As a result, retaliation against any individual who reports discrimination,
harassment or violence or participates in an investigation of such reports is prohibited. Retaliation against an individual for
reporting discrimination, harassment or violence or for participating in an investigation of a claim of harassment or discrimination
is a serious violation of this Code and Company policies.
Unless otherwise agreed
in writing by the CEO, each Company employee is employed on an at-will basis. This means that there is no guarantee of continued
employment and the Company has the right to terminate an individual’s employment at any time with or without cause. Oral
representations cannot alter this relationship. Therefore, this Code of Conduct will not be construed or interpreted as creating
an implied contract with any employee that he or she may be discharged only for cause. Employment with the Company is voluntarily
entered into, and the employee is free to resign at will at any time, for any or no reason, with or without notice. Similarly,
the Company may terminate the employment relationship at will at any time, for any or no reason, with or without notice, so long
as there is no violation of applicable federal, state or local law.
| G. | Background Checks and Disclosure of Suspension/Debarment/Felony/Other Criminal Conviction |
The Company has determined
that for all positions within the Company, a criminal history records check is required for candidates, when information as to
a candidate’s criminal history is job-related to the position being sought. The determination has been made on the basis
of the particular requirements of the job, the employer’s business necessity, and applicable federal and state laws. In addition,
candidates may be required to submit to a post-offer drug screen. The Company is an equal opportunity employer and does not discriminate
on the basis of race, sex, age, national origin, religion, disability, genetic information, or any other characteristic protected
by federal, state or local laws.
In addition, the Company
requires the ongoing disclosure of all criminal convictions (including, among others, any conviction where an appeal is pending,
any plea of guilty or nolo contendere, and any participation in a first offender, deferred adjudication, or other similar arrangement
or program where judgment of conviction has been withheld), and may terminate the employment of any employee as a result, in the
discretion of management. To comply with these requirements, you must inform the CEO if you are convicted of a felony or misdemeanor
crime.
| XXVII. | WAIVERS OF OR CHANGES TO THE CODE OF BUSINESS CONDUCT AND ETHICS |
It may be appropriate for
a provision of this Code to be waived in a particular circumstance. Any waiver of, or changes to, this Code that apply to executive
officers or directors of the Company may be made only by the Nominating Committee or another committee of our Board comprised solely
of independent non-executive directors or a majority of our independent non-executive directors and must be promptly disclosed
to shareholders as required by law or regulation of the SEC and the rules of the Nasdaq. In particular, to the extent that such
committee determines to grant any waiver of this Code for an executive officer or director, the waiver shall be disclosed to shareholders
within four business days of such determination through a press release, providing website disclosure, or by filing a current report
on Form 8-K with the SEC. Any other Covered Person seeking a waiver should speak to his or her supervisor, who, in turn, should
obtain the written approval of the CEO regarding such matter.
For the avoidance of doubt,
no waiver shall be given from any provision of this Code that is not permitted under applicable law, rules or regulations.
The matters covered in this
Code are of the utmost importance to the Company, the Company Group as a whole, and their respective stakeholders and business
partners, and are essential to the Company Group’s ability to conduct its business in accordance with its stated values.
The Company expects all Covered Persons and persons with whom the Company Group transacts business to adhere to the standards set
forth in this Code in carrying out their duties to the Company Group. Individuals whose actions are deemed to be in violation of
this Code or other policies of the Company that may be adopted from time to time will be subject to disciplinary action, up to
and including suspension, termination and/or the reporting of violative conduct to the appropriate authorities and potential civil
liability and criminal prosecution.
| XXIX. | ADMINISTRATION AND IMPLEMENTATION |
The Board has overall responsibility
for administering and interpreting this Code. The CEO is responsible for the implementation of this Code.
This Code, as may be amended
from time to time, shall be posted on the Company’s website. The Company shall state in its annual proxy statement that this
Code is available on the Company’s website and provide the website address.
Approved: August 1, 2023
Exhibit 21.1
SUBSIDIARIES OF THE REGISTRANT
| Name of Subsidiary |
|
Jurisdiction of Incorporation or Organization |
| Anavex Australia Pty Limited |
|
Australia |
| Anavex Germany GmbH |
|
Germany |
| Anavex Canada Ltd. |
|
Ontario, Canada |
EXHIBIT 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
We have issued our reports dated November 27, 2023,
with respect to the consolidated financial statements and internal control over financial reporting included in the Annual Report of Anavex
Life Sciences Corp. on Form 10-K for the year ended September 30, 2023. We consent to the incorporation by reference of said reports in
the Registration Statements of Anavex Life Sciences Corp. on Forms S-3 (No. 333-218292, and No. 333-259788) and Forms S-8 (No. 333-219934,
No. 333-255166 and No. 333-265537).
/s/ GRANT THORNTON LLP
Melville, New York
November 27, 2023
EXHIBIT
23.2
Consent
of Independent Registered Public Accounting Firm
Anavex
Life Sciences Corp.
New York,
New York
We hereby
consent to the incorporation by reference in the Registration Statements on Form S-3 (No. 333-218292 and No. 333-259788) and Form S-8
(No. 333-219934, No. 333-255166 and No. 333-265537) of Anavex Life Sciences Corp. and subsidiaries of our report dated November 24, 2021,
relating to the consolidated financial statements of Anavex Life Sciences Corp. and subsidiaries, which appears in this Annual Report
on Form 10-K.
/s/ BDO USA, P.C.
New York,
New York
November
27, 2023
EXHIBIT
31.1
CERTIFICATION
I, Christopher Missling, certify that:
| 1. |
|
I have reviewed
this Annual Report on Form 10-K of Anavex Life Sciences Corp.; |
| |
|
|
| 2. |
|
Based on my knowledge,
this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements
made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this
report; |
| |
|
|
| 3. |
|
Based on my knowledge,
the financial statements, and other financial information included in this report, fairly present in all material respects the financial
condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| |
|
|
| 4. |
|
The registrant’s
other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in
Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f)
and 15d-15(f)) for the registrant and have: |
| |
|
|
| |
|
a) |
Designed such disclosure
controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material
information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities,
particularly during the period in which this report is being prepared; |
| |
|
|
|
| |
|
b) |
Designed such internal control
over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles; |
| |
|
|
|
| |
|
c) |
Evaluated the effectiveness
of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of
the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| |
|
|
|
| |
|
d) |
Disclosed in this report any
change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal
quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably
likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. |
|
The
registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation
of internal control over financial reporting, to the registrant’s auditors and the audit committee
of the registrant’s board of directors (or persons performing the equivalent functions):
|
| |
|
a) |
All significant deficiencies
and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely
affect the registrant’s ability to record, process, summarize and report financial information; and |
| |
|
|
|
| |
|
b) |
Any fraud, whether or not material,
that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
| Date: November 27, 2023 |
|
| |
|
| /s/ Christopher Missling |
|
| |
|
| Christopher
Missling, PhD |
|
Chief
Executive Officer, President, Secretary
(Principal Executive Officer) |
|
EXHIBIT 31.2
CERTIFICATION
I,
Sandra Boenisch, certify that:
| 1. |
|
I have reviewed
this Annual Report on Form 10-K of Anavex Life Sciences Corp.; |
| |
|
|
| 2. |
|
Based on my knowledge,
this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements
made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this
report; |
| |
|
|
| 3. |
|
Based on my knowledge,
the financial statements, and other financial information included in this report, fairly present in all material respects the financial
condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| |
|
|
| 4. |
|
The registrant’s
other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in
Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f)
and 15d-15(f)) for the registrant and have: |
| |
|
|
| |
|
a) |
Designed such disclosure
controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material
information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities,
particularly during the period in which this report is being prepared; |
| |
|
|
|
| |
|
b) |
Designed such internal control
over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles; |
| |
|
|
|
| |
|
c) |
Evaluated the effectiveness
of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of
the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| |
|
|
|
| |
|
d) |
Disclosed in this report any
change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal
quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably
likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. |
|
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| |
|
a) |
All
significant deficiencies and material weaknesses in the design or operation of internal control
over financial reporting which are reasonably likely to adversely affect the registrant’s
ability to record, process, summarize and report financial information; and |
| |
|
|
|
| |
|
b) |
Any
fraud, whether or not material, that involves management or other employees who have a significant
role in the registrant’s internal control over financial reporting. |
| Date: November 27, 2023 |
|
| |
|
| /s/ Sandra Boenisch |
|
| |
|
| Sandra
Boenisch, CPA, CGA |
|
Principal
Financial Officer, Treasurer
(Principal Financial and Accounting Officer) |
|
EXHIBIT 32.1
CERTIFICATION
PURSUANT TO
18
U.S.C. SECTION 1350
AS
ADOPTED PURSUANT TO
SECTION
906 OF THE SARBANES-OXLEY ACT OF 2002
In
connection with the Annual Report of Anavex Life Sciences Corp. (the “Company”) on Form 10-K for the fiscal year ending September
30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), each of the undersigned,
in the capacities and on the date indicated below, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section
906 of the Sarbanes-Oxley Act of 2002, that to the best of his or her knowledge:
| (1) |
The Report fully complies with the requirements
of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
| |
|
| (2) |
The information contained in the Report fairly presents,
in all material respects, the financial condition and results of operations of the Company. |
| Date: November 27, 2023
|
|
| |
|
| /s/
Christopher Missling |
|
| Christopher
Missling, PhD |
|
Chief Executive Officer, President, Secretary
(Principal Executive Officer) |
|
| /s/
Sandra Boenisch |
|
| Sandra
Boenisch, CPA, CGA |
|
| Principal
Financial Officer, Treasurer (Principal Financial and Accounting Officer) |
|
The
foregoing certification is being furnished solely to accompany the Report pursuant to 18 U.S.C. § 1350, and is not being filed for
purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing
of the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing. A signed
original of this written statement required by Section 906, or other document authenticating, acknowledging, or otherwise adopting the
signature that appears in typed form within the electronic version of this written statement required by Section 906, has been provided
to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
EXHIBIT 97.1
ANAVEX
LIFE SCIENCES CORP. NASDAQ RULE 5608
EXECUTIVE OFFICER COMPENSATION CLAWBACK Policy
Effective
NOVEMBER 8,
2023
1. Policy
Purpose. The purpose of this Anavex Life Sciences Corp. Nasdaq Rule 5608 Executive Officer Compensation Clawback Policy (this “Policy”)
is to enable Anavex Life Sciences Corp. and its subsidiaries and affiliates (the “Company”) to recover Erroneously
Awarded Compensation in the event that the Company is required to prepare an Accounting Restatement. This Policy is intended to comply
with the requirements set forth in Listing Rule 5608 of The Nasdaq Stock Market LLC and will be construed and interpreted in accordance
with such intent. Unless otherwise defined in this Policy, capitalized terms will have the meaning ascribed to such terms in Section
7.
2. Policy
Administration. This Policy will be administered by the Compensation Committee of the Board (the “Committee”)
unless the Board determines to administer this Policy itself. The Committee has full and final authority to make all determinations under
this Policy, in each case to the extent permitted under the Listing Rule and in compliance with (or pursuant to an exemption from the
application of) Section 409A of the Code. All determinations and decisions made by the Committee pursuant to the provisions of this Policy
will be final, conclusive and binding on all persons, including the Company, its affiliates, its stockholders and the Executive Officers.
Any action or inaction by the Committee with respect to an Executive Officer under this Policy in no way limits the Committee’s
actions or decisions not to act with respect to any other Executive Officer under this Policy or under any similar policy, agreement
or arrangement, nor will any such action or inaction serve as a waiver of any rights the Company may have against any Executive Officer
other than as set forth in this Policy.
3. Policy
Application. This Policy applies to all Incentive-Based Compensation received by a person (a) after beginning service as an Executive
Officer, (b) who served as an Executive Officer at any time during the performance period for such Incentive-Based Compensation, (c)
while the Company had a class of securities listed on a national securities exchange or a national securities association and (d) during
the three completed fiscal years immediately preceding the Accounting Restatement Date. In addition to such last three completed fiscal
years, the immediately preceding clause (d) includes any transition period that results from a change in the Company’s fiscal year
within or immediately following such three completed fiscal years, provided that a transition period between the last day of the Company’s
previous fiscal year end and the first day of its new fiscal year that comprises a period of nine to twelve months will be deemed a completed
fiscal year. For purposes of this Section 3, Incentive-Based Compensation is deemed received in the Company’s fiscal period during
which the Financial Reporting Measure specified in the Incentive-Based Compensation award is attained, even if the payment or grant of
the Incentive-Based Compensation occurs after the end of that period. For the avoidance of doubt, Incentive-Based Compensation that is
subject to both a Financial Reporting Measure vesting condition and a service-based vesting condition will be considered received when
the relevant Financial Reporting Measure is achieved, even if the Incentive-Based Compensation continues to be subject to the service-based
vesting condition.
4. Policy
Recovery Requirement. In the event of an Accounting Restatement, the Company must recover, reasonably promptly, Erroneously Awarded
Compensation, in amounts determined pursuant to this Policy. The Company’s obligation to recover Erroneously Awarded Compensation
is not dependent on if or when the Company files restated financial statements. Recovery under this Policy with respect to an Executive
Officer will not require the finding of any misconduct by such Executive Officer or such Executive Officer being found responsible for
the accounting error leading to an Accounting Restatement. In the event of an Accounting Restatement, the Company will satisfy the Company’s
obligations under this Policy to recover any amount owed from any applicable Executive Officer by exercising its sole and absolute discretion
in how to accomplish such recovery, to the extent permitted under the Listing Rule and in compliance with (or pursuant to an exemption
from the application of) Section 409A of the Code. The Company’s recovery obligation pursuant to this Section 4 will not apply
to the extent that the Committee, or in the absence of the Committee, a majority of the independent directors serving on the Board, determines
that such recovery would be impracticable and:
a. The
direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered. Before concluding that
it would be impracticable to recover any amount of Erroneously Awarded Compensation based on expense of enforcement, the Company must
make a reasonable attempt to recover such Erroneously Awarded Compensation, document such reasonable attempts to recover and provide
that documentation to the Stock Exchange;
b. Recovery
would violate home country law where that law was adopted prior to November 28, 2022. Before concluding that it would be impracticable
to recover any amount of Erroneously Awarded Compensation based on violation of home country law, the Company must obtain an opinion
of home country counsel, acceptable to the Stock Exchange, that recovery would result in such a violation, and must provide such opinion
to the Stock Exchange; or
c. Recovery
would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the registrant,
to fail to meet the requirements of Section 401(a)(13) or 411(a) of the Code.
5. Policy
Prohibition on Indemnification and Insurance Reimbursement. The Company is prohibited from indemnifying any current or former Executive
Officer against the loss of Erroneously Awarded Compensation. Further, the Company is prohibited from paying or reimbursing an Executive
Officer for purchasing insurance to cover any such loss.
6. Required
Policy-Related Filings. The Company will file all disclosures with respect to this Policy in accordance with the requirements of
the federal securities laws, including disclosures required by U.S. Securities and Exchange Commission filings.
7. Definitions.
a. “Accounting
Restatement” means an accounting restatement due to the material noncompliance of the Company with any financial reporting
requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial
statements that is material to the previously issued financial statements or that would result in a material misstatement if the error
were corrected in the current period or left uncorrected in the current period.
b. “Accounting
Restatement Date” means the earlier to occur of (i) the date the Board, a committee of the Board or the officers of the
Company authorized to take such action if the Board action is not required, concludes, or reasonably should have concluded, that the
Company is required to prepare an Accounting Restatement and (ii) the date a court, regulator or other legally authorized body directs
the Company to prepare an Accounting Restatement.
c. “Board”
means the board of directors of the Company.
d. “Code”
means the U.S. Internal Revenue Code of 1986, as amended. Any reference to a section of the Code or regulation thereunder includes such
section or regulation, any valid regulation or other official guidance promulgated under such section and any comparable provision of
any future legislation or regulation amending, supplementing, or superseding such section or regulation.
e. “Erroneously
Awarded Compensation” means, in the event of an Accounting Restatement, the amount of Incentive-Based Compensation previously
received that exceeds the amount of Incentive-Based Compensation that otherwise would have been received had it been determined based
on the restated amounts in such Accounting Restatement, and must be computed without regard to any taxes paid by the relevant Executive
Officer. Notwithstanding the foregoing, for Incentive-Based Compensation based on stock price or total stockholder return where the amount
of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement
(i) the amount of Erroneously Awarded Compensation must be based on a reasonable estimate of the effect of the Accounting Restatement
on the stock price or total stockholder return upon which the Incentive-Based Compensation was received and (ii) the Company must maintain
documentation of the determination of that reasonable estimate and provide such documentation to the Stock Exchange.
f. “Executive
Officer” means the Company’s president, principal financial officer, principal accounting officer (or if there is
no such accounting officer, the controller), any vice-president of the Company in charge of a principal business unit, division or function
(such as sales, administration or finance), any other officer who performs a policy-making function or any other person who performs
similar policy-making functions for the Company. An executive officer of the Company’s parent or subsidiary is deemed an “Executive
Officer” if the executive officer performs such policy making functions for the Company.
g. “Financial
Reporting Measure” means any measure that is determined and presented in accordance with the accounting principles used
in preparing the Company’s financial statements, and any measure that is derived wholly or in part from such measure, provided
that a Financial Reporting Measure is not required to be presented within the Company’s financial statements or included in a filing
with the U.S. Securities and Exchange Commission to qualify as a “Financial Reporting Measure.” For purposes of this Policy,
“Financial Reporting Measure” includes, but is not limited to, stock price and total stockholder return.
h. “Incentive-Based
Compensation” means any compensation that is granted, earned or vested based wholly or in part upon the attainment of a
Financial Reporting Measure.
i. “Stock
Exchange” means the national stock exchange on which the Company’s common stock is listed.
8. Acknowledgement.
Each Executive Officer will sign and return to the Company, within 30 calendar days following the later of (i) the effective date of
this Policy first set forth above or (ii) the date the individual becomes an Executive Officer, the Acknowledgement Form attached as
Exhibit A, pursuant to which the Executive Officer agrees to be bound by, and to comply with, the terms and conditions of this
Policy.
9. Severability.
The provisions in this Policy are intended to be applied to the fullest extent of the law. To the extent that any provision of this Policy
is found to be unenforceable or invalid under any applicable law, such provision will be applied to the maximum extent permitted, and
will automatically be deemed amended in a manner consistent with its objectives to the extent necessary to conform to any limitations
required under applicable law.
10. Amendment
and Termination. The Board may amend this Policy from time to time in its sole and absolute discretion and will amend this Policy
as it deems necessary to reflect the Listing Rule, to comply with (or maintain an exemption from the application of) Section 409A
of the Code. The Board may terminate this Policy at any time.
11. Other
Recovery Obligations and General Rights. To the extent that the application of this Policy would provide for recovery of Incentive-Based
Compensation that the Company recovers pursuant to Section 304 of the Sarbanes-Oxley Act or other recovery obligations, the amount the
relevant Executive Officer has already reimbursed the Company will be credited to the required recovery under this Policy. This Policy
will not limit the rights of the Company to take any other actions or pursue other remedies that the Company may deem appropriate under
the circumstances and under applicable law, in each case to the extent permitted under the Listing Rule and in compliance with (or pursuant
to an exemption from the application of) Section 409A of the Code. Nothing contained in this Policy will limit the Company’s ability
to seek recoupment, in appropriate circumstances (including circumstances beyond the scope of this Policy) and as permitted by other
applicable law, of any amounts from any individual, in each case to the extent permitted under the Listing Rule and in compliance with
(or pursuant to an exemption from the application of) Section 409A of the Code.
12. Successors.
This Policy is binding and enforceable against all Executive Officers and their beneficiaries, heirs, executors, administrators or other
legal representatives.
13. Governing
Law and Venue. This Policy and all rights and obligations hereunder are governed by and construed in accordance with the internal
laws of the State of Nevada, excluding any choice of law rules or principles that may direct the application of the laws of another jurisdiction.
EXHIBIT
A
ANAVEX
LIFE SCIENCES CORP. NASDAQ RULE 5608
EXECUTIVE OFFICER Compensation CLAWBACK Policy
Acknowledgement
Form
By
signing below, the undersigned acknowledges and confirms that the undersigned has received and reviewed a copy of the Anavex Life Sciences
Corp. Nasdaq Rule 5608 Executive Officer Compensation Clawback Policy (the “Policy”).
By
signing this Acknowledgement Form, the undersigned acknowledges and agrees that the undersigned is and will continue to be subject to
the Policy and that the Policy will apply both during and after the undersigned’s employment with Anavex Life Sciences Corp. and,
as applicable, its subsidiaries and affiliates (the “Company”). Further, by signing below, the undersigned
agrees to abide by the terms of the Policy, including, without limitation, by returning any Erroneously Awarded Compensation (as defined
in the Policy) to the Company to the extent required by, and in a manner consistent with, the Policy.
| |
EXECUTIVE
OFFICER |
| |
|
| |
Signature |
| |
|
| |
Print
Name |
| |
|
| |
Date |
v3.23.3
Cover - USD ($)
$ in Millions |
12 Months Ended |
|
|
Sep. 30, 2023 |
Nov. 24, 2023 |
Mar. 31, 2023 |
| Cover [Abstract] |
|
|
|
| Document Type |
10-K
|
|
|
| Amendment Flag |
false
|
|
|
| Document Annual Report |
true
|
|
|
| Document Transition Report |
false
|
|
|
| Document Period End Date |
Sep. 30, 2023
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
| Document Fiscal Year Focus |
2023
|
|
|
| Current Fiscal Year End Date |
--09-30
|
|
|
| Entity File Number |
001-37606
|
|
|
| Entity Registrant Name |
ANAVEX LIFE SCIENCES CORP.
|
|
|
| Entity Central Index Key |
0001314052
|
|
|
| Entity Tax Identification Number |
98-0608404
|
|
|
| Entity Incorporation, State or Country Code |
NV
|
|
|
| Entity Address, Address Line One |
630 5th Avenue
|
|
|
| Entity Address, Address Line Two |
20th Floor
|
|
|
| Entity Address, City or Town |
New York
|
|
|
| Entity Address, State or Province |
NY
|
|
|
| Entity Address, Country |
US
|
|
|
| Entity Address, Postal Zip Code |
10111
|
|
|
| City Area Code |
844
|
|
|
| Local Phone Number |
689-3939
|
|
|
| Title of 12(b) Security |
Common
Stock, $0.001 par value
|
|
|
| Trading Symbol |
AVXL
|
|
|
| Security Exchange Name |
NASDAQ
|
|
|
| Entity Well-known Seasoned Issuer |
Yes
|
|
|
| Entity Voluntary Filers |
No
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
| Entity Filer Category |
Large Accelerated Filer
|
|
|
| Entity Small Business |
false
|
|
|
| Entity Emerging Growth Company |
false
|
|
|
| Entity Shell Company |
false
|
|
|
| Entity Public Float |
|
|
$ 667
|
| Entity Common Stock, Shares Outstanding |
|
82,086,511
|
|
| ICFR Auditor Attestation Flag |
true
|
|
|
| Document Financial Statement Error Correction [Flag] |
false
|
|
|
| Auditor Name |
GRANT THORNTON LLP
|
|
|
| Auditor Location |
Melville, New York
|
|
|
| Auditor Firm ID |
248
|
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_IcfrAuditorAttestationFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Balance Sheets - USD ($)
$ in Thousands |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Current |
|
|
| Cash and cash equivalents |
$ 151,024
|
$ 149,158
|
| Incentive and tax receivables |
2,709
|
3,193
|
| Prepaid expenses and other current assets |
653
|
354
|
| Total Assets |
154,386
|
152,705
|
| Current Liabilities |
|
|
| Accounts payable |
4,322
|
3,825
|
| Accrued liabilities - Note 3 |
7,295
|
5,945
|
| Deferred grant income - Note 4 |
917
|
444
|
| Total Liabilities |
12,534
|
10,214
|
| Commitments and Contingencies - Note 6 |
|
|
| Capital stock Authorized:10,000,000 preferred stock, par value $0.001 per share |
|
|
| Capital stock Authorized: 200,000,000 common stock, par value $0.001 per Issued and outstanding: 82,066,511 common shares (2022 - 77,942,815) |
82
|
78
|
| Additional paid-in capital |
434,839
|
387,977
|
| Accumulated deficit |
(293,069)
|
(245,564)
|
| Total Stockholders' Equity |
141,852
|
142,491
|
| Total Liabilities and Stockholders' Equity |
$ 154,386
|
$ 152,705
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionValue received from shareholders in common stock-related transactions that are in excess of par value or stated value and amounts received from other stock-related transactions. Includes only common stock transactions (excludes preferred stock transactions). May be called contributed capital, capital in excess of par, capital surplus, or paid-in capital. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapitalCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of rental payment required by lease over rental income recognized. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 20
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481178/840-20-25-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 25
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479341/842-30-25-11
| Name: |
us-gaap_DeferredRentCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred stock, authorized |
10,000,000
|
10,000,000
|
| Preferred stock, par value |
$ 0.001
|
$ 0.001
|
| Common shares, authorized |
200,000,000
|
200,000,000
|
| Common shares, par value |
$ 0.001
|
$ 0.001
|
| Common shares, issued |
82,066,511
|
77,942,815
|
| Common shares, outstanding |
82,066,511
|
77,942,815
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Operations and Comprehensive Loss - USD ($)
$ in Thousands |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Operating expenses |
|
|
|
| General and administrative |
$ 12,039
|
$ 13,070
|
$ 9,018
|
| Research and development |
43,717
|
37,916
|
32,984
|
| Total operating expenses |
55,756
|
50,986
|
42,002
|
| Operating loss |
(55,756)
|
(50,986)
|
(42,002)
|
| Other income (expenses) |
|
|
|
| Grant income |
25
|
|
54
|
| Research and development incentive income |
2,718
|
3,323
|
4,547
|
| Interest income, net |
6,519
|
947
|
26
|
| Other financing expense |
(964)
|
|
|
| Foreign exchange loss |
(40)
|
(904)
|
(266)
|
| Total other income, net |
8,258
|
3,366
|
4,361
|
| Net loss before provision for income taxes |
(47,498)
|
(47,620)
|
(37,641)
|
| Income tax expense, current |
(7)
|
(358)
|
(268)
|
| Net loss and comprehensive loss |
$ (47,505)
|
$ (47,978)
|
$ (37,909)
|
| X |
- References
+ Details
| Name: |
avxl_NonOperatingIncomeFromGrants |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_OtherFinancingExpense |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ResearchAndDevelopmentIncentiveIncome |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net amount of operating interest income (expense). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.10)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_InterestIncomeExpenseNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Operations and Comprehensive Loss (Parenthetical) - $ / shares
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Income Statement [Abstract] |
|
|
|
| Net Loss per share, Basic |
$ (0.60)
|
$ (0.62)
|
$ (0.54)
|
| Net Loss per share, Diluted |
$ (0.60)
|
$ (0.62)
|
$ (0.54)
|
| Weighted average number of shares outstanding, Basic |
79,787,596
|
76,909,993
|
69,802,960
|
| Weighted average number of shares outstanding, Diluted |
79,787,596
|
76,909,993
|
69,802,960
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Cash Flows - USD ($)
$ in Thousands |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Cash Flows used in Operating Activities |
|
|
|
| Net loss |
$ (47,505)
|
$ (47,978)
|
$ (37,909)
|
| Adjustments to reconcile net loss to net cash used in operations: |
|
|
|
| Non-cash financing related charges |
845
|
|
|
| Stock-based compensation |
16,370
|
18,379
|
8,231
|
| Changes in working capital balances related to operations: |
|
|
|
| Incentive and tax receivables |
484
|
5,944
|
(4,287)
|
| Prepaid expenses and deposits |
(299)
|
1
|
88
|
| Accounts payable |
497
|
(914)
|
751
|
| Accrued liabilities |
1,350
|
330
|
2,298
|
| Deferred grant income |
473
|
|
444
|
| Net cash used in operating activities |
(27,785)
|
(24,238)
|
(30,384)
|
| Cash Flows provided by Financing Activities |
|
|
|
| Issuance of common shares |
27,875
|
20,985
|
153,219
|
| Share issue costs |
|
(707)
|
(5,551)
|
| Proceeds from exercise of warrants |
|
|
1,467
|
| Proceeds from exercise of stock options |
1,776
|
1,010
|
4,108
|
| Net cash provided by financing activities |
29,651
|
21,288
|
153,243
|
| Increase in cash and cash equivalents during the period |
1,866
|
(2,950)
|
122,859
|
| Cash and cash equivalents, beginning of period |
149,158
|
152,108
|
29,249
|
| Cash and cash equivalents, end of period |
151,024
|
149,158
|
152,108
|
| Supplemental Cash Flow Information |
|
|
|
| Cash paid for state and local minimum income taxes |
$ 136
|
$ 327
|
$ 140
|
| X |
- References
+ Details
| Name: |
avxl_DeferredGrantIncome |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsNoncashItemsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Changes in Stockholders' Equity - USD ($)
$ in Thousands |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
Total |
| Beginning balance, value at Sep. 30, 2020 |
$ 62
|
$ 186,852
|
$ (159,677)
|
$ 27,237
|
| Balance at beginning, shares at Sep. 30, 2020 |
62,045,198
|
|
|
|
| Purchase shares |
$ 4
|
24,107
|
|
|
| Purchase shares, shares |
4,007,996
|
|
|
|
| Commitment shares |
|
|
|
|
| Commitment shares, shares |
78,213
|
|
|
|
| Shares issued under Sales Agreement |
$ 6
|
79,102
|
|
79,108
|
| Shares issued under Sales Agreement, shares |
5,634,576
|
|
|
|
| Less: share issue costs |
|
(2,438)
|
|
(2,438)
|
| Shares issued pursuant to registered direct offering |
$ 2
|
49,998
|
|
50,000
|
| Shares issued pursuant to registered direct offering, shares |
2,380,953
|
|
|
|
| Less: share issue costs |
|
(3,097)
|
|
(3,097)
|
| Shares issued pursuant to exercise of stock options |
$ 1
|
4,107
|
|
4,108
|
| Shares issued pursuant to exercise of stock options, shares |
1,421,529
|
|
|
|
| Shares issued pursuant to exercise of warrants |
|
1,466
|
|
1,466
|
| Shares issued pusuant to exercise of warrants, shares |
350,000
|
|
|
|
| Share based compensation |
|
8,231
|
|
8,231
|
| Net loss |
|
|
(37,909)
|
(37,909)
|
| Ending balance, value at Sep. 30, 2021 |
$ 75
|
348,328
|
(197,586)
|
150,817
|
| Balance at ending, shares at Sep. 30, 2021 |
75,918,465
|
|
|
|
| Shares issued under Sales Agreement |
$ 2
|
20,983
|
|
20,985
|
| Shares issued under Sales Agreement, shares |
1,623,813
|
|
|
|
| Less: share issue costs |
|
(723)
|
|
(723)
|
| Shares issued pursuant to exercise of stock options |
$ 1
|
1,010
|
|
1,011
|
| Shares issued pursuant to exercise of stock options, shares |
400,537
|
|
|
|
| Share based compensation |
|
18,379
|
|
18,379
|
| Net loss |
|
|
(47,978)
|
(47,978)
|
| Ending balance, value at Sep. 30, 2022 |
$ 78
|
387,977
|
(245,564)
|
142,491
|
| Balance at ending, shares at Sep. 30, 2022 |
77,942,815
|
|
|
|
| Shares issued under 2023 purchase agreement Initial Commitment shares |
|
845
|
|
845
|
| Shares issued under 2023 purchase agreement Initial Commitment shares, shares |
75,000
|
|
|
|
| Purchase shares |
$ 3
|
27,872
|
|
27,875
|
| Purchase shares, shares |
3,275,000
|
|
|
|
| Commitment shares |
|
|
|
|
| Commitment shares, shares |
13,943
|
|
|
|
| Shares issued pursuant to exercise of stock options |
$ 1
|
1,775
|
|
1,776
|
| Shares issued pursuant to exercise of stock options, shares |
759,753
|
|
|
|
| Share based compensation |
|
16,370
|
|
16,370
|
| Net loss |
|
|
(47,505)
|
(47,505)
|
| Ending balance, value at Sep. 30, 2023 |
$ 82
|
$ 434,839
|
$ (293,069)
|
$ 141,852
|
| Balance at ending, shares at Sep. 30, 2023 |
82,066,511
|
|
|
|
| X |
- References
+ Details
| Name: |
avxl_CommitmentShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_CommitmentSharesShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_LessRegisteredDirectOfferingShareIssueCosts |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_LessSalesAgreementShareIssueCosts |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPursuantToExerciseOfStockOptions |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPursuantToExerciseOfStockOptionsShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPursuantToRegisteredDirectOffering |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPursuantToRegisteredDirectOfferingShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPusuantToExerciseOfWarrants |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedPusuantToExerciseOfWarrantsShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedUnder2023PurchaseAgreementInitialCommitmentShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedUnder2023PurchaseAgreementInitialCommitmentSharesShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedUnderSalesAgreement |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SharesIssuedUnderSalesAgreementShares |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of stock that has been repurchased during the period and has not been retired and is not held in treasury. Some state laws may mandate the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Business Description and Basis of Presentation
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Business Description and Basis of Presentation |
Note
1 Business Description and Basis of Presentation
Business
Anavex
Life Sciences Corp. (“Anavex” or the “Company”) is a clinical stage biopharmaceutical company engaged in the
development of differentiated therapeutics by applying precision medicine to central nervous system (“CNS”) diseases with
high unmet need. Anavex analyzes genomic data from clinical trials to identify biomarkers, which are used in the analysis of its clinical
trials for the treatment of neurodegenerative and neurodevelopmental diseases.
The
Company’s lead compound ANAVEX®2-73 (blarcamesine) is being developed to treat Alzheimer’s disease, Parkinson’s
disease and potentially other central nervous system diseases, including rare diseases, such as Rett syndrome, a rare severe neurological
monogenic disorder caused by mutations in the X-linked gene, methyl-CpG-binding protein 2 (“MECP2”).
Basis
of Presentation
These
consolidated financial statements have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission
(“SEC”) and the instructions to Form 10-K and have been prepared under the accounting principles generally accepted in the
United States of America (“U.S. GAAP”).
Liquidity
All
of the Company’s potential drug compounds are in the clinical development stage and the Company cannot be certain that its research
and development efforts will be successful or, if successful, that its potential drug compounds will ever be approved for sales to pharmaceutical
companies or generate commercial revenues. To date, we have not generated any revenues from our operations. The Company expects the business
to continue to experience negative cash flows from operations for the foreseeable future and cannot predict when, if ever, our business
might become profitable.
Management
believes that the current working capital position will be sufficient to meet the Company’s working capital requirements beyond
the next 12 months after the date that these consolidated financial statements are issued. The process of drug development can be costly,
and the timing and outcomes of clinical trials are uncertain. The assumptions upon which the Company has based its estimates are
routinely evaluated and may be subject to change. The actual amount of the Company’s expenditures will vary depending upon
a number of factors including but not limited to the design, timing and duration of future clinical trials, the progress of the Company’s
research and development programs and the level of financial resources available. The Company has the ability to adjust its operating
plan spending levels based on the timing of future clinical trials.
Other
than our rights related to the Purchase Agreement (as defined below in Note 5), there can be no assurance that additional financing will
be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If the Company is not able
to obtain the additional financing on a timely basis, if and when it is needed, it will be forced to delay or scale down some or all
of its research and development activities.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the
reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses in
the reporting period. The Company regularly evaluates estimates and assumptions related to accounting for research and development costs,
incentive income receivable, valuation and recoverability of deferred tax assets, stock based compensation, and loss contingencies. The
Company bases its estimates and assumptions on current facts, historical experience, and various other factors that it believes to be
reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and
liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by
the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between
the estimates and the actual results, future results of operations will be affected.
Principles
of Consolidation
These
consolidated financial statements include the accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia
Pty Limited (“Anavex Australia”), a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated
under the laws of Germany, and Anavex Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company
transactions and balances have been eliminated.
Cash
and equivalents
The
Company considers only those investments which are highly liquid, readily convertible to cash and that mature within three months from
the date of purchase to be cash equivalents.
Highly
liquid investments that are considered cash equivalents include money market accounts, money market funds and certificates
of deposit. The carrying value of cash equivalents approximates fair value due to the short-term maturity of these securities. The Company’s
investment policy allows for investments in domestic money market certificates, certificates of deposit, money market funds, bonds or
commercial papers, and establishes diversification and credit quality requirements and limits investments by maturity and issuer. The
Company currently maintains its investments at one large well known financial institution.
The
Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. Accounts are guaranteed by
the Federal Deposit Insurance Corporation (FDIC) up to $250,000 under current regulations. At September 30, 2023 and 2022, substantially
all of the Company’s cash balances were in excess of these federally insured limits. The Company mitigates this risk by maintaining
the majority of its cash balances in a large well-known financial institution. The Company has not experienced any losses in such accounts.
Research
and Development Expenses
Research
and development costs are expensed as incurred. These expenses are comprised of the costs of the Company’s proprietary research
and development efforts, including preclinical studies, clinical trials, manufacturing costs, employee salaries and benefits and stock-based
compensation expense, contract services including external research and development expenses incurred under arrangements with third parties
such as contract research organizations (“CROs”), facilities costs, overhead costs and other related expenses. Milestone
payments made by the Company to third parties are expensed when the specific milestone has been achieved. Manufacturing costs are expensed
as incurred in accordance with Accounting Standard Codification (“ASC”) 730, Research and Development, as these materials
have no alternative future use outside of their intended use.
Nonrefundable
advance payments for goods or services that will be used or rendered for future research and development activities are deferred and
amortized over the period that the goods are delivered, or the related services are performed, subject to an assessment of recoverability.
The Company makes estimates of costs incurred in relation to external CROs, and clinical site costs. When evaluating the adequacy of
the accrued liabilities, the Company analyzes progress of the trials and studies including the phase or completion of events, invoices
received and contracted costs. Judgments and estimates are made in determining the accrued balances at the end of any reporting period.
Actual results could differ from the Company’s estimates. The Company’s historical accrual estimates have not been materially
different from actual costs.
In
addition, the Company incurs expenses in respect of intellectual property costs relating to patents and trademarks. The probability of
success and length of time to develop commercial applications of the drugs subject to the underlying patent and trademark costs is difficult
to determine and numerous risks and uncertainties exist with respect to the timely completion of the development projects. There is no
assurance the drugs subject to the underlying patents and trademarks will ever be successfully commercialized.
Due
to these risks and uncertainties, the patent and trademark costs do not meet the definition of an asset and thus are expensed as incurred
within general and administrative expenses.
Research
and Development Incentive Income
The
Company is eligible to obtain certain research and development tax credits, including, through its wholly owned subsidiary Anavex Australia,
the Australian research and development tax incentive credit (the “Australia R&D credit”) through a program administered
through the Australian Tax Office (the “ATO”) and AusIndustry, a division of the Australian Government’s Department
of Industry, Innovation and Science (“AusIndustry”). The Australia R&D credit program provides for a cash refund based
on a percentage of eligible research and development activities undertaken in Australia by Anavex Australia. Anavex Australia is also
eligible under the Australia R&D credit program to receive the cash refund for certain research and development expenses incurred
by Anavex Australia outside of Australia, to the extent such expenses are pre-approved by AusIndustry pursuant to an advanced overseas
finding application.
The
Australia R&D credit program is available to eligible companies with an annual aggregate revenue of less than $20.0 million Australian
during the reimbursable period at a rate of 18.5% above the claimant’s company tax rate in Australia.
The
tax incentives are available on the basis of specific criteria with which the Company must comply. Although the tax incentive may be
administered through the local tax authority, the Company has accounted for the incentives outside of the scope of ASC Topic 740, Income
Taxes (“ASC 740”), since the incentives are not linked to the Company’s taxable income and can be realized regardless
of whether the Company has generated taxable income in the respective jurisdictions.
With
respect to the Australia R&D credit, as there is no authoritative guidance under GAAP for accounting for grants to for-profit business
entities, the Company accounts for the grant by analogy to IAS20 Accounting for Government Grants and Disclosure of Government Assistance
(“IAS 20”). The Company recognizes the Research and Development Incentive income as it incurs costs eligible for reimbursement
under the Australia R&D credit program when it is reasonably assured that the cash incentive will be received, as evidenced through
enrollment in the program and when the applicable conditions under the program have been met. The Company accrues for the amount of cash
refund it expects to receive in relation to research and development expenses outside of Australia only to the extent it has received
advanced approval from AusIndustry, pursuant to an approved advanced overseas finding application.
In
addition, Anavex Australia and Anavex Canada incur Goods and Services Tax (GST) on certain services provided by local vendors. As a domestic
entity in those jurisdictions, Anavex Australia and Anavex Canada are entitled to a refund of the GST paid. Similarly, Anavex Germany
incurs Value Added Tax (VAT) on certain services provided by local vendors, to which it is entitled to a refund of such VAT paid. The
Company’s estimate of the amount of cash refund it expects to receive related to GST and VAT incurred is included in Incentive
and tax receivables in the accompanying consolidated balance sheets.
License
Fees
The
Company expenses amounts paid to acquire licenses associated with products under development when the ultimate recovery of the amounts
paid is uncertain and the technology has no alternative future use when acquired. Acquisitions of technology licenses are charged to
expense or capitalized based on management’s assessment regarding the ultimate recoverability of the amounts paid and the potential
for alternative future use. The Company has determined that the technological feasibility for its product candidates is reached when
the requisite regulatory approvals are obtained to make the product available for sale.
Basic
and Diluted Loss per Share
Basic
income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the weighted average number
of common shares outstanding during the period. Diluted income/(loss) per common share is computed by dividing net income/(loss) available
to common stockholders by the sum of (1) the weighted-average number of common shares outstanding during the period, (2) the dilutive
effect of the assumed exercise of options and warrants using the treasury stock method and (3) the dilutive effect of other potentially
dilutive securities. For purposes of the diluted net loss per share calculation, options and warrants are potentially dilutive securities
and are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive.
As
of September 30, 2023, diluted loss per share excludes 14,271,780 potentially dilutive common shares (2022 –13,329,616; 2021 –
11,540,903) related to outstanding options and warrants, as their effect was anti-dilutive.
Financial
Instruments
The
book value of the Company’s financial instruments, consisting of cash and equivalents, incentive and tax receivables, accounts
payable and accrued liabilities approximate their fair value due to the short-term maturity of such instruments. Unless otherwise noted,
it is management’s opinion that the Company is not exposed to significant interest, currency or credit risks arising from these
financial instruments.
Foreign
Currency Translation
The
functional currency of the Company is the US dollar. Monetary items denominated in a foreign currency are translated into US dollars
at exchange rates prevailing at the balance sheet date and non-monetary items are translated at exchange rates prevailing when the assets
were acquired, or obligations incurred. Foreign currency denominated expense items are translated at exchange rates prevailing on the
transaction date. Unrealized gains or losses arising from the translations are credited or charged to income in the period in which they
occur.
The
Company has determined that the functional currency of Anavex Australia Pty Limited, Anavex Germany GmbH, and Anavex Canada Ltd. is also
the US dollar.
Segment
and Geographic Reporting
Operating
segments are defined as components of an enterprise for which separate discrete information is available for evaluation by the chief
operating decision maker or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views
its operations and manages its business as one operating segment, which is the business of developing novel therapies for the management
of CNS diseases.
Grant
Income
Grant
income is recognized at the fair value of the grant when it is received, and all substantive conditions have been satisfied. Grants received
from government and other agencies in advance of the specific research and development costs to which they relate are deferred and recognized
in the consolidated statements of operations in the period they are earned, typically when the related research and development costs
are incurred.
Income
Taxes
The
Company follows the provisions of ASC 740, which requires the asset and liability method of accounting for income taxes. Under the asset
and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences
between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets
and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences
are expected to be recovered or settled.
The
Company follows the provisions of ASC 740 regarding accounting for uncertainty in income taxes. The Company initially recognizes tax
positions in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities.
Such tax positions are initially and subsequently measured as the largest amount of tax benefit that is greater than 50% likely of being
realized upon ultimate settlement with the tax authority assuming full knowledge of the position and all relevant facts. Application
requires numerous estimates based on available information. The Company considers many factors when evaluating and estimating its tax
positions and tax benefits, and its recognized tax positions and tax benefits may not accurately anticipate actual outcomes. As additional
information is obtained, there may be a need to periodically adjust the recognized tax positions and tax benefits. These periodic adjustments
may have a material impact on the consolidated statements of operations.
The
Company recognizes interest and penalties related to current income tax expense on the interest income, net line, in the accompanying
consolidated statements of operations. Accrued interest and penalties, if any, are included in accrued liabilities on the consolidated
balance sheets.
Stock-based
Compensation
The
Company accounts for all stock-based payments and awards under the fair value method.
The
fair value of all share purchase options and warrants are expensed over their contractual vesting period, or over the expected performance
period for only the portion of awards expected to vest, in the case of milestone-based vesting, with a corresponding increase to additional
paid-in capital.
Compensation
costs for stock-based payments with graded vesting are recognized on a straight-line basis. Stock based compensation expense is adjusted
for actual forfeitures of unvested awards as they occur.
The
Company has granted share purchase option awards that vest upon achievement of certain performance criteria, or milestone-based awards.
The Company estimates an implicit service period for achieving performance criteria for each award and recognizes the resulting fair
value as expense over the implicit service period when it concludes that achieving the performance criteria is probable. The Company
periodically reviews and updates as appropriate its estimates of implicit service periods and conclusions on achieving the performance
criteria. Performance awards vest upon achievement of the performance criteria.
The
Company uses the Black-Scholes option valuation model to calculate the fair value of share purchase options and warrants at the date
of the grant. This model requires the input of subjective assumptions, including the expected price volatility and expected life of each award.
The Company uses the U.S. Treasury daily treasury yield curve rates for the expected term of the option as the risk-free rate. The expected
term represents the period that options granted are expected to be outstanding using the simplified method. The Company’s historical
share option exercise experience does not provide sufficient basis for estimating the expected term. Expected volatility is based on
the average of the daily share price changes over the expected term. The Company does not estimate forfeitures and elects to record actual
forfeitures as they occur. The Company has not paid any dividends on its common stock historically, therefore no assumption of dividend
payments is made in the model. These assumptions consist of estimates of future market conditions, which are inherently uncertain, and
therefore, are subject to management’s judgment. Changes in these assumptions can materially affect the fair value estimates.
The
purchase price of options or warrants may be paid in cash or, if approved by the Company’s compensation committee (or in the case
of warrants by the Board of Directors) in advance, “net settled” in shares of the Company’s common stock. In a net
settlement of an option or warrant, the Company does not receive payment of the exercise price from the holder but reduces the number
of shares of common stock issued upon the exercise of the stock option or warrant by the smallest number of whole shares that have an
aggregate fair market value equal to or over the aggregate exercise price for the option shares covered by the option or warrant exercised.
Shares issued pursuant to the exercise of options and warrants are issued from the Company’s treasury.
Fair
Value Measurements
Fair
value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date.
Assets and liabilities that are measured at fair value are reported using a three-level fair value hierarchy that prioritizes the inputs
used to measure fair value. This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three
levels of inputs used to measure fair value are as follows:
Level
1 - quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at
the measurement date;
Level
2 - observable inputs other than Level 1, quoted prices for similar assets or liabilities in active markets, quoted prices for
identical or similar assets and liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose
significant value drivers are observable; and
Level
3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant
to the fair value of the assets or liabilities.
At
September 30, 2023 and 2022, the Company did not have any Level 2 or Level 3 assets or liabilities.
Recently
Adopted Accounting Pronouncements
Effective
October 1, 2021, the Company adopted Accounting Standards Update (“ASU”) 2019-12, “Simplifying the Accounting for Income
Taxes (ASC 740)”, which is intended to simplify various aspects related to accounting for income taxes by removing certain exceptions
to the general principles in Topic 740 and clarifying and amending existing guidance to improve consistent application. There was no
material impact on the Company’s operations, financial condition, or cash flows.
On
October 1, 2022, the Company adopted ASU 2021-10, Annual Disclosure Requirements for Business Entities Receiving Government Assistance
(Topic 832) – Disclosures by Business Entities about Government Assistance, which requires business entities to disclose information
about transactions with a government that are accounted for by applying a grant or contribution model by analogy. For transactions within
scope, the new standard requires the disclosure of information about the nature of the transaction, including significant terms and conditions,
as well as the amounts and specific financial statement line items affected by the transaction. The disclosure of the Company’s
research and development tax incentive income and receivable is detailed in Note 4.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Accrued Liabilities
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Accrued Liabilities |
|
| Accrued Liabilities |
Note
3 Accrued Liabilities
The
principal components of accrued liabilities consist of (in thousands):
| Schedule of principal components of accrued liabilities |
|
|
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
| Accrued
clinical site and patient visits costs |
|
$ |
2,006 |
|
|
$ |
2,031 |
|
| Accrued
compensation and benefits |
|
|
1,360 |
|
|
|
1,298 |
|
| Fixed
contract accruals |
|
|
38 |
|
|
|
417 |
|
| Milestone
based contract accruals |
|
|
1,267 |
|
|
|
137 |
|
| All
other accrued liabilities |
|
|
2,624 |
|
|
|
2,062 |
|
| Total
accrued liabilities |
|
$ |
7,295 |
|
|
$ |
5,945 |
|
|
| X |
- References
+ Details
| Name: |
avxl_AccuredLiabilitiesTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_DisclosureAccruedLiabilitiesAbstract |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Other Income
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Other Income |
|
| Other Income |
Note
4 Other Income
Grant
income
During
the year ended September 30, 2023, the Company received $0.5 million (2022: $0 2021: $0.5 million) of a $1.0 million research grant awarded
during the year ended September 30, 2021, by the Michael J. Fox Foundation for Parkinson’s Research. The grant will be used to
fund a clinical trial of the Company’s lead compound, ANAVEX®2-73 (blarcamesine) related to Parkinson’s disease.
The
grant income was deferred when received and amortized to other income as the related research and development expenditures are incurred.
During the year ended September 30, 2023, the Company recognized $25,000 (2022: $0; 2021: $54,100) of this grant on its statements of
operations within grant income. At September 30, 2023 an amount of $0.9 million (2022: $0.4 million) of this grant is recorded as deferred
grant income, representing the amount of this grant which has not yet been amortized to other income. The Company will recognize this
income on its statements of operations as the related expenditures are incurred to offset the income.
Research
and development incentive income
Research
and development incentive income represents the income earned by Anavex Australia of the Australia R&D credit. This cash incentive
is received by Anavex Australia, upon filing of a claim in connection with Anavex Australia’s annual income tax return.
During
the year ended September 30, 2023, the Company recorded research and development incentive income of $2.7 million (AUD 4.1 million) (2022:
$3.3 million (AUD 4.5 million); 2021: $4.5 million (AUD 6.1 million)) in respect of the Australia R&D credit for eligible research
and development expenses incurred during the year. This amount is included within Other income (expense) on the consolidated statements
of operations.
At
September 30, 2023, Incentive and tax receivables includes $2.5 million (AUD 3.9 million) (2022: $2.9 million (AUD 4.5 million) relating
to Australia R&D credits earned during the year that are expected to be reimbursed upon filing of the Company’s annual claim
under this program.
The
Australia R&D credit program is a self-assess program whereby the Company must assess its eligibility each year to determine (i)
if the entity is eligible (ii) if the specific R&D activities are eligible and (iii) if the individual R&D expenditures have
nexus to such R&D activities. The Company evaluates its eligibility under the tax incentive program as of each balance sheet date
based on the most current and relevant data available. Anavex Australia is able to continue to claim the R&D tax incentive for as
long as it remains eligible and continues to incur eligible research and development expenditures.
Although
the Company believes that it has complied with all the relevant conditions of eligibility under the program for all periods claimed,
the ATO has the right to review the Company’s qualifying programs and related expenditures for a period of four years. If such
a review were to occur, the ATO may have different interpretations of certain eligibility requirements. If the ATO disagreed with the
Company’s assessments and any related subsequent appeals, it could require adjustment to and repayment of current or previous years’
claims already received. Additionally, if the Company was unable to demonstrate a reasonably arguable position taken on such claims,
the ATO could also assess penalties and interest on such adjustment.
Currently,
the Company’s tax incentive claims from 2019 to 2022 are open to potential review by the ATO. Additionally, the period open for
review is indefinite if the ATO suspects fraud. The Company has not provided any allowance for any such potential adjustments, should
they occur in the future.
|
| X |
- References
+ Details
| Name: |
avxl_DisclosureOtherIncomeAbstract |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_OtherIncomeDisclosureTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Equity Offerings
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Equity Offerings |
|
| Equity Offerings |
Note
5 Equity Offerings
Common
Stock
Common
shares are voting and are entitled to dividends as declared at the discretion of the Board of Directors.
Preferred
Stock
The
Company’s Board of Directors (the “Board”) has the authority to issue preferred stock in one or more series
and to fix the rights, preferences, privileges, restrictions and the number of shares constituting any series or the designation of the
series.
Registered
Direct Offering
On
June 22, 2021, the Company and Deep Track Capital entered into a securities purchase agreement pursuant to which the Company sold to
Deep Track Capital an aggregate of 2,380,953 shares of common stock at $21 per share in a registered direct offering, for gross proceeds
of $50 million. Net proceeds of the offering were $46,902,981 after deducting offering fees and expenses.
Sales
Agreement
The
Company entered into a Controlled Equity Offering Sales Agreement on July 6, 2018, which was amended and restated on May 1, 2020 (the
“Sales Agreement”) with Cantor Fitzgerald & Co. and SVB Leerink LLC (together the “Sales Agents”), pursuant
to which the Company may offer and sell shares of common stock registered under an effective registration statement from time to time
through the Sales Agents (the “Offering”).
Upon
delivery of a placement notice based on the Company’s instructions and subject to the terms and conditions of the Sales Agreement,
the Sales Agents may sell the Shares by methods deemed to be an “at the market offering” offering, in negotiated transactions
at market prices prevailing at the time of sale or at prices related to such prevailing market prices, or by any other method permitted
by law, including negotiated transactions, subject to the prior written consent of the Company. The Company is not obligated to make
any sales of Shares under the Sales Agreement. The Company or Sales Agents may suspend or terminate the offering of Shares upon notice
to the other party, subject to certain conditions. The Sales Agents will act as agent on a commercially reasonable efforts basis consistent
with their normal trading and sales practices and applicable state and federal law, rules and regulations and the rules of
Nasdaq.
The
Company has agreed to pay the Sales Agents commissions for their services of up to 3.0% of the gross proceeds from the sale of the Shares
pursuant to the Sales Agreement. The Company also agreed to provide the Sales Agents with customary indemnification and contribution
rights. During the year ended September 30, 2023, no shares were sold pursuant to the Offering. During the year ended September 30, 2022,
1,623,813 shares were sold pursuant to the Offering for gross proceeds of $21.0 million (net proceeds of $20.3 million after deducting
offering expenses) (2021: 5,634,576 shares were sold for gross proceeds of $79.1 million, net proceeds of $76.7 million). At September
30, 2023, an amount of $142.4 million (2022: $142.4 million) was registered pursuant to an effective registration statement. The Company
currently does not have access to sell shares of common stock under the Sales Agreement.
2023
Purchase Agreement
On
February 3, 2023, the Company entered into a $150.0 million purchase agreement (the “2023 Purchase Agreement”) with Lincoln
Park Capital Fund, LLC (“Lincoln Park”), pursuant to which the Company has the right to sell and issue to Lincoln Park, and
Lincoln Park is obligated to purchase, up to $150.0 million in value of its shares of common stock from time to time over a three-year
period until February 3, 2026.
In
consideration for entering into the 2023 Purchase Agreement, the Company issued to Lincoln Park 75,000 shares of common stock as a commitment
fee (the “initial commitment shares”) and agreed to issue up to an additional 75,000 shares pro rata, when and if, Lincoln
Park purchased, at the Company’s discretion, the $150.0 million aggregate commitment. The Company determined the fair value of
the initial commitment shares was $0.8 million with reference to the closing price of the Company’s shares on the Purchase Agreement
date. In addition, the Company incurred third party expenses of $0.1 million in connection with entering into the Purchase Agreement.
These amounts were expensed to other financing expense on the statements of operations during the year ended September 30, 2023.
During
the year ended September 30, 2023, the Company issued to Lincoln Park an aggregate of 3,288,943 (2022: 0) shares of common stock under
the 2023 Purchase Agreement, including 3,275,000 (2022: 0) shares of common stock for aggregate proceeds of $27.9 million (2022: $0)
and 13,943 (2022: 0) commitment shares.
At
September 30, 2023, an amount of $122.1 million remained available under the 2023 Purchase Agreement.
2019
Purchase Agreement
On
June 7, 2019, the Company entered into a $50.0 million purchase agreement (the “2019 Purchase Agreement”) with Lincoln Park,
as amended on July 1, 2020, pursuant to which the Company had the right to sell and issue to Lincoln Park, and Lincoln Park was obligated
to purchase, up to $50.0 million in value of its shares of common stock from time to time over a three-year period until July 1, 2022.
During
the year ended September 30, 2023 and 2022, the Company did not issue any shares to Lincoln Park under the 2019 Purchase Agreement.
During
the year ended September 30, 2021, the Company issued to Lincoln Park an aggregate of 4,086,209 shares of common stock under the 2019
Purchase Agreement, including 4,007,996 shares of common stock for an aggregate purchase price of $24.1 million and 78,213 commitment
shares.
At
September 30, 2023 and 2022, no shares remained available for issuance under the 2019 Purchase Agreement.
|
| X |
- References
+ Details
| Name: |
avxl_DisclosureEquityOfferingsAbstract |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_EquityOfferingTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments and Contingencies
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Note
6 Commitments and Contingencies
Lease
The
Company leases office space under an operating lease with an initial term of 12 months or less. Under the terms of the office lease,
the Company is required to pay its proportionate share of operating costs.
The
operating lease costs were as follows (in thousands):
| Schedule of operating lease costs |
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
| Operating lease costs |
|
$ |
118 |
|
|
$ |
73 |
|
|
$ |
131 |
|
Employee
401(k) Benefit Plan
The
Company has a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code. The plan covers all United States
based employees. United States based employees eligible to participate in the plan may contribute up to the current statutory limits
under the Internal Revenue Service regulations. The 401(k) plan permits the Company to make additional matching contributions on behalf
of contributing employees.
During
the year ended September 30, 2023, the Company made matching contributions under the 401(k) plan as follows (in thousands):
| Schedule of contributions under the plan |
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
Contributions
to 401(k) plan |
|
$ |
232 |
|
|
$ |
158 |
|
|
$ |
129 |
|
Litigation
The
Company is subject to claims and legal proceedings that arise in the ordinary course of business. Such matters are inherently uncertain,
and there can be no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of
any such matter will not have a material adverse effect upon the Company’s consolidated financial statements. The Company does
not believe that any of such pending claims and legal proceedings will have a material adverse effect on its consolidated financial statements.
Share
Purchase Warrants
At
September 30, 2023 and 2022, the Company had 160,000 warrants outstanding at a weighted average exercise price of $3.72 as follows:
| Schedule of share purchase warrants outstanding |
|
|
|
|
| Number |
|
Exercise Price |
|
Expiry Date |
| |
150,000 |
|
|
$ |
3.17 |
|
|
|
May 6, 2024 |
|
| |
10,000 |
|
|
$ |
12.00 |
|
|
|
April 21, 2026 |
|
| |
160,000 |
|
|
|
|
|
|
|
|
|
Stock–based
Compensation Plan
2015
Stock Option Plan
On
September 18, 2015, the Company’s board of directors approved a 2015 Omnibus Incentive Plan (the “2015 Plan”), which
provided for the grant of stock options and restricted stock awards to directors, officers, employees and consultants of the Company.
The
maximum number of our common shares reserved for issue under the plan was 6,050,553 shares, subject to adjustment in the event of a change
of the Company’s capitalization.
2019
Stock Option Plan
On
January 15, 2019, the Board approved the 2019 Omnibus Incentive Plan (the “2019 Plan”), which provides for the grant of stock
options and restricted stock awards to directors, officers, employees, consultants and advisors of the Company.
The
maximum number of our common shares reserved for issue under the plan was 6,000,000 shares, subject to adjustment in the event of a change
of the Company’s capitalization.
During
the year ended September 30, 2022, 406,453 options previously available under the 2019 Plan and the 2015 Plan became available under
the 2022 Plan (as defined below).
2022
Stock Option Plan
On
March 25, 2022, the Board approved the 2022 Omnibus Incentive Plan (the “2022 Plan”). The 2022 Plan was approved by stockholders
on May 24, 2022. Under the terms of the 2022 Plan, 10,000,000 additional shares of Common Stock will be available for issuance under
the plan, in addition to the shares available under the 2019 Plan and the 2015 Plan. Any awards outstanding under a previous stock option
plan will remain subject to and be paid under such plan, and any shares subject to outstanding awards under a previous plan that subsequently
cease to be subject to such awards (other than by reason of settlement of the awards in shares) will automatically become available for
issuance under the 2022 Plan.
The
2022 Plan provides that it may be administered by the Board, or the Board may delegate such responsibility to a committee. The exercise
price will be determined by the Board at the time of grant shall be at least equal to the fair market value on such date. If the grantee
is a 10% stockholder on the grant date, then the exercise price shall not be less than 110% of fair market value of the Company’s
shares of common stock on the grant date. Stock options may be granted under the 2022 Plan for an exercise period of up to ten years
from the date of grant of the option or such lesser periods as may be determined by the Board, subject to earlier termination in accordance
with the terms of the 2022 Plan. At September 30, 2023, 3,849,917 options had been issued under the 2022 Plan and 6,661,535 options were
available for issue under the 2022 Plan, subject to stockholder approval.
The
following summarizes information about stock option activity during the years ended September 30, 2023 and 2022:
| Schedule of stock option activity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
|
|
| |
|
|
|
Weighted Average |
|
Average Grant |
|
Aggregate intrinsic |
| |
|
Number of |
|
Exercise Price |
|
Date Fair Value |
|
value |
| |
|
Options |
|
($) |
|
($) |
|
($) |
| Outstanding,
September 30, 2021 |
|
|
11,330,903 |
|
|
|
5.74 |
|
|
|
— |
|
|
|
140,132,451 |
|
| Granted |
|
|
2,358,000 |
|
|
|
10.13 |
|
|
|
7.07 |
|
|
|
— |
|
| Forfeited |
|
|
(118,750 |
) |
|
|
6.86 |
|
|
|
5.23 |
|
|
|
— |
|
| Exercised |
|
|
(400,537 |
) |
|
|
2.52 |
|
|
|
1.88 |
|
|
|
4,201,015 |
|
| Outstanding,
September 30, 2022 |
|
|
13,169,616 |
|
|
|
6.61 |
|
|
|
4.96 |
|
|
|
62,267,309 |
|
| Granted |
|
|
1,959,000 |
|
|
|
9.30 |
|
|
|
6.60 |
|
|
|
— |
|
| Expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Exercised |
|
|
(759,753 |
) |
|
|
2.34 |
|
|
|
0.95 |
|
|
|
4,629,026 |
|
| Forfeited |
|
|
(257,083 |
) |
|
|
12.00 |
|
|
|
6.74 |
|
|
|
— |
|
| Cancelled |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Outstanding,
September 30, 2023 |
|
|
14,111,780 |
|
|
|
7.12 |
|
|
|
5.27 |
|
|
|
22,290,069 |
|
| Exercisable,
September 30, 2023 |
|
|
9,604,614 |
|
|
|
5.19 |
|
|
|
3.94 |
|
|
|
22,290,069 |
|
The
following summarizes information about stock options at September 30, 2023 by a range of exercise prices:
| Schedule of summarizes information about stock options |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
|
|
|
|
|
|
| |
|
|
|
|
|
average |
|
Weighted |
|
|
|
|
| |
|
|
|
Number of |
|
remaining |
|
average |
|
|
|
Weighted |
| Range of exercises
prices |
|
outstanding |
|
contractual |
|
exercise |
|
Number of |
|
average |
| From |
|
To |
|
options |
|
life |
|
price |
|
vested options |
|
exercise price |
| $ |
0.92 |
|
|
|
3.00 |
|
|
|
3,280,309 |
|
|
|
4.74 |
|
|
|
2.39 |
|
|
|
3,280,309 |
|
|
$ |
2.39 |
|
| $ |
3.01 |
|
|
|
5.00 |
|
|
|
2,017,500 |
|
|
|
4.32 |
|
|
|
3.28 |
|
|
|
2,017,500 |
|
|
$ |
3.28 |
|
| $ |
5.01 |
|
|
|
9.00 |
|
|
|
5,260,054 |
|
|
|
6.21 |
|
|
|
6.90 |
|
|
|
3,304,137 |
|
|
$ |
6.10 |
|
| $ |
9.01 |
|
|
|
13.00 |
|
|
|
1,981,917 |
|
|
|
8.37 |
|
|
|
10.55 |
|
|
|
480,584 |
|
|
$ |
11.45 |
|
| $ |
13.01 |
|
|
|
25.00 |
|
|
|
1,572,000 |
|
|
|
7.49 |
|
|
|
18.29 |
|
|
|
522,084 |
|
|
$ |
18.57 |
|
| |
|
|
|
|
|
|
|
|
14,111,780 |
|
|
|
6.04 |
|
|
|
7.12 |
|
|
|
9,604,614 |
|
|
$ |
5.19 |
|
The
weighted average per share fair value of options vested during the year ended September 30, 2023 was $3.94 (2022: $4.69, 2021: $2.54).
At September 30, 2023, the weighted average contractual life of options outstanding was 6.0 years (2022: 6.4 years) and for options exercisable
was 4.75 years (2022: 5.1 years).
The
aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the quoted market price
of the Company’s stock for the options that were in-the-money at September 30, 2023.
The
Company recognized stock-based compensation expense of $16.4 million during the year ended September 30, 2023 (2022: $18.4 million; 2021:
$8.2 million) in connection with the issuance and vesting of stock options in exchange for services. These amounts have been included
in general and administrative expenses and research and development expenses on the Company’s consolidated statements of operations
as follows (in thousands):
| Schedule of general and administrative expenses and research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
| General
and administrative |
|
$ |
5,558 |
|
|
$ |
7,129 |
|
|
|
3,571 |
|
| Research
and development |
|
|
10,812 |
|
|
|
11,250 |
|
|
|
4,660 |
|
| Total
stock-based compensation |
|
$ |
16,370 |
|
|
$ |
18,379 |
|
|
|
8,231 |
|
An
amount of approximately $14.1 million in stock-based compensation is expected to be recorded over the remaining term of such options
and warrants through fiscal 2026.
The
fair value of each option and warrant award is estimated on the date of grant using the Black Scholes option pricing model based on the
following weighted average assumptions:
| Schedule of weighted average assumptions for fair value of each option award |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Risk-free
interest rate |
|
|
3.70 |
% |
|
|
3.11 |
% |
|
|
0.73 |
% |
| Expected
life of options (years) |
|
|
5.64 |
|
|
|
5.57 |
|
|
|
5.74 |
|
| Annualized
volatility |
|
|
85.13 |
% |
|
|
84.17 |
% |
|
|
93.43 |
% |
| Dividend
rate |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
The
fair value of stock compensation charges recognized during the years ended September 30, 2023, 2022 and 2021 was determined with reference
to the quoted market price of the Company’s shares on the grant date.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant arrangements with third parties, which includes operating lease arrangements and arrangements in which the entity has agreed to expend funds to procure goods or services, or has agreed to commit resources to supply goods or services, and operating lease arrangements. Descriptions may include identification of the specific goods and services, period of time covered, minimum quantities and amounts, and cancellation rights. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Taxes
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Note
7 Income Taxes
The
Company’s U.S. and foreign loss before income taxes are set forth below (in thousands):
| Schedule of loss before
income taxes |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| United
States |
|
$ |
(41,198 |
) |
|
$ |
(40,002 |
) |
|
$ |
(28,851 |
) |
| Foreign |
|
|
(6,300 |
) |
|
|
(7,618 |
) |
|
|
(8,790 |
) |
The
components of net deferred income tax assets as of September 30, 2023, 2022 and 2021 are as follows (in thousands):
| Schedule of components of
net deferred income tax assets |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Net
operating loss carryforwards |
|
$ |
46,462 |
|
|
$ |
46,208 |
|
|
$ |
34,982 |
|
| Research
and development tax credit carryforwards |
|
|
2,713 |
|
|
|
2,182 |
|
|
|
1,577 |
|
| Stock-based
compensation |
|
|
18,593 |
|
|
|
13,373 |
|
|
|
10,453 |
|
| Research
and development capitalization |
|
|
7,219 |
|
|
|
— |
|
|
|
— |
|
| Unpaid
charges |
|
|
1,559 |
|
|
|
894 |
|
|
|
89 |
|
| Intangible
asset costs |
|
|
593 |
|
|
|
388 |
|
|
|
323 |
|
| Foreign
exchange and other |
|
|
49 |
|
|
|
44 |
|
|
|
62 |
|
| Valuation
allowance of deferred tax assets |
|
|
(77,188 |
) |
|
|
(63,089 |
) |
|
|
(47,486 |
) |
| Net
deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
A
reconciliation of income tax expense at the statutory federal income tax rate and income taxes as reflected in the consolidated financial
statements for the years ended September 30, 2023, 2022 and 2021 is as follows (in thousands):
| Schedule
of reconciliation of income tax expense |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Income
tax benefit at statutory federal rate |
|
$ |
(9,975 |
) |
|
$ |
(10,000 |
) |
|
$ |
(7,934 |
) |
| Foreign
income taxed at other rates |
|
|
— |
|
|
|
(170 |
) |
|
|
(353 |
) |
| Permanent
differences relating to stock based compensation |
|
|
(601 |
) |
|
|
(714 |
) |
|
|
(4,379 |
) |
| Permanent
differences relating to GILTI inclusion |
|
|
165 |
|
|
|
— |
|
|
|
— |
|
| Permanent
differences relating to Section 162(m) |
|
|
— |
|
|
|
— |
|
|
|
816 |
|
| Other
permanent differences |
|
|
273 |
|
|
|
— |
|
|
|
741 |
|
| Adjustment
to tax assets based on Section 382 |
|
|
— |
|
|
|
— |
|
|
|
3,330 |
|
| Research
and development credits, net |
|
|
(37 |
) |
|
|
232 |
|
|
|
1,042 |
|
| State
and local taxes |
|
|
(4,122 |
) |
|
|
(4,975 |
) |
|
|
(7,022 |
) |
| Adjustment
to true up to prior years' tax provision |
|
|
206 |
|
|
|
24 |
|
|
|
48 |
|
| Effect
of change in statutory tax rates |
|
|
— |
|
|
|
— |
|
|
|
216 |
|
| State
minimum and excise taxes |
|
|
— |
|
|
|
358 |
|
|
|
268 |
|
| Change
in valuation allowances |
|
|
14,098 |
|
|
|
15,603 |
|
|
|
13,495 |
|
| Income
tax expense |
|
$ |
7 |
|
|
$ |
358 |
|
|
$ |
268 |
|
As
of September 30, 2023, the Company had U.S. federal net operating loss carryforwards of approximately $126.3 million (2022: $123.4 million)
of which $37.7 million will begin to expire in 2025 and $88.6 million can be carried forward indefinitely, state and local net operating
loss carryforwards of approximately $ 16.6 million (2022: $17.2 million) which will begin to expire in 2036, and Research and Development
tax credits of approximately $2.7 million (2022: $2.2 million) which will begin to expire in 2029. The Company had approximately $12.9
million (approximately AU$20.1 million) (2022: $10.6 million (approximately AU$ 14.9 million)) of net operating loss carryforwards in
Australia, which have an indefinite life, available to offset future taxable income in those jurisdictions.
The
Company evaluates its valuation allowance requirements based on available evidence. When circumstances change, and this causes a change
in management’s judgment about the recoverability of deferred tax assets, the impact of the change on the valuation allowance is
reflected in current income. Because management of the Company does not currently believe that it is more likely than not that the Company
will receive the benefit of these assets, a valuation allowance has been established at September 30, 2023 and 2022.
The
Tax Cuts and Jobs Act of 2017 (TCJA) has modified the IRC 174 expenses related to research and development for tax years beginning after
December 31, 2021. Under the TCJA, the Company must now capitalize the expenditures related to research and development activities and
amortize over five years for U.S. activities and 15 years for non-U.S. activities using a mid-year convention. Therefore, the capitalization
of research and development costs in accordance with IRC 174 resulted in a gross deferred tax asset of $7.2 million.
Uncertain
Tax Positions
The
Company files income tax returns in the U.S. federal jurisdiction and various state and local and foreign jurisdictions. The Company’s
tax returns are subject to tax examinations by U.S. federal and state tax authorities, or examinations by foreign tax authorities until
the respective statutes of limitation expire. The Company is subject to tax examinations by tax authorities for all taxation years commencing
on or after 2005.
Under
the provisions of the Internal Revenue Code, the net operating loss (“NOL”) carryforwards are subject to review and possible
adjustment by the Internal Revenue Service and state tax authorities. Under Section 382 of the Internal Revenue Code, NOL and tax credit
carryforwards may become subject to an annual limitation in the event of an over 50% cumulative change in the ownership interest of significant
stockholders over a three-year period, as well as similar state tax provisions.
The
Company conducted a Section 382 study during the year ended September 30, 2021 and determined that, during the year ended September 30,
2015, there was a change in ownership which resulted in $25.8 million of federal NOLs being subject to an annual limitation. During the
year ended September 30, 2021, the Company reduced its federal NOLs by $12.1 million and its Research and Development tax credit carryforwards
by $0.8 million, which are the amount of tax assets that will expire unutilized pursuant to the Section 382 study. This resulted in a
reduction of $2.5 million of NOLs and $0.8 million of research and development credits and a corresponding reduction in the valuation
allowance of $3.3 million, which was recorded in the 2021 fiscal year. Subsequent ownership changes in future years could trigger additional
limitations of the Company’s NOLs. During the year ended September 30, 2023 and 2022 the
Company determined that there were no changes in ownership pursuant to Section 382.
As
of September 30, 2023, the Company did not provide any foreign withholding taxes related to its foreign subsidiaries’ undistributed
earnings, as such earnings have been retained and are intended to be indefinitely reinvested to fund ongoing operations of the foreign
subsidiaries. It is not practicable to estimate the amount of taxes that would be payable upon remittance of these earnings, because
such tax, if any, is dependent upon circumstances existing if and when remittance occur.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Subsequent Events
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
Note
8 Subsequent Events
The
Company evaluates subsequent events occurring between the most recent balance sheet date and the date the financial statements are available
to be issued in order to determine whether the subsequent events are to be recorded and/or disclosed in the Company’s financial
statements and footnotes. The financial statements are considered to be available to be issued at the time they are filed with the Securities
and Exchange Commission (SEC).
There
were no subsequent events or transactions that required recognition or disclosure in the consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Policies)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Use of Estimates |
Use
of Estimates
The
preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the
reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses in
the reporting period. The Company regularly evaluates estimates and assumptions related to accounting for research and development costs,
incentive income receivable, valuation and recoverability of deferred tax assets, stock based compensation, and loss contingencies. The
Company bases its estimates and assumptions on current facts, historical experience, and various other factors that it believes to be
reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and
liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by
the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between
the estimates and the actual results, future results of operations will be affected.
|
| Principles of Consolidation |
Principles
of Consolidation
These
consolidated financial statements include the accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia
Pty Limited (“Anavex Australia”), a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated
under the laws of Germany, and Anavex Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company
transactions and balances have been eliminated.
|
| Cash and equivalents |
Cash
and equivalents
The
Company considers only those investments which are highly liquid, readily convertible to cash and that mature within three months from
the date of purchase to be cash equivalents.
Highly
liquid investments that are considered cash equivalents include money market accounts, money market funds and certificates
of deposit. The carrying value of cash equivalents approximates fair value due to the short-term maturity of these securities. The Company’s
investment policy allows for investments in domestic money market certificates, certificates of deposit, money market funds, bonds or
commercial papers, and establishes diversification and credit quality requirements and limits investments by maturity and issuer. The
Company currently maintains its investments at one large well known financial institution.
The
Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. Accounts are guaranteed by
the Federal Deposit Insurance Corporation (FDIC) up to $250,000 under current regulations. At September 30, 2023 and 2022, substantially
all of the Company’s cash balances were in excess of these federally insured limits. The Company mitigates this risk by maintaining
the majority of its cash balances in a large well-known financial institution. The Company has not experienced any losses in such accounts.
|
| Research and Development Expenses |
Research
and Development Expenses
Research
and development costs are expensed as incurred. These expenses are comprised of the costs of the Company’s proprietary research
and development efforts, including preclinical studies, clinical trials, manufacturing costs, employee salaries and benefits and stock-based
compensation expense, contract services including external research and development expenses incurred under arrangements with third parties
such as contract research organizations (“CROs”), facilities costs, overhead costs and other related expenses. Milestone
payments made by the Company to third parties are expensed when the specific milestone has been achieved. Manufacturing costs are expensed
as incurred in accordance with Accounting Standard Codification (“ASC”) 730, Research and Development, as these materials
have no alternative future use outside of their intended use.
Nonrefundable
advance payments for goods or services that will be used or rendered for future research and development activities are deferred and
amortized over the period that the goods are delivered, or the related services are performed, subject to an assessment of recoverability.
The Company makes estimates of costs incurred in relation to external CROs, and clinical site costs. When evaluating the adequacy of
the accrued liabilities, the Company analyzes progress of the trials and studies including the phase or completion of events, invoices
received and contracted costs. Judgments and estimates are made in determining the accrued balances at the end of any reporting period.
Actual results could differ from the Company’s estimates. The Company’s historical accrual estimates have not been materially
different from actual costs.
In
addition, the Company incurs expenses in respect of intellectual property costs relating to patents and trademarks. The probability of
success and length of time to develop commercial applications of the drugs subject to the underlying patent and trademark costs is difficult
to determine and numerous risks and uncertainties exist with respect to the timely completion of the development projects. There is no
assurance the drugs subject to the underlying patents and trademarks will ever be successfully commercialized.
Due
to these risks and uncertainties, the patent and trademark costs do not meet the definition of an asset and thus are expensed as incurred
within general and administrative expenses.
|
| Research and Development Incentive Income |
Research
and Development Incentive Income
The
Company is eligible to obtain certain research and development tax credits, including, through its wholly owned subsidiary Anavex Australia,
the Australian research and development tax incentive credit (the “Australia R&D credit”) through a program administered
through the Australian Tax Office (the “ATO”) and AusIndustry, a division of the Australian Government’s Department
of Industry, Innovation and Science (“AusIndustry”). The Australia R&D credit program provides for a cash refund based
on a percentage of eligible research and development activities undertaken in Australia by Anavex Australia. Anavex Australia is also
eligible under the Australia R&D credit program to receive the cash refund for certain research and development expenses incurred
by Anavex Australia outside of Australia, to the extent such expenses are pre-approved by AusIndustry pursuant to an advanced overseas
finding application.
The
Australia R&D credit program is available to eligible companies with an annual aggregate revenue of less than $20.0 million Australian
during the reimbursable period at a rate of 18.5% above the claimant’s company tax rate in Australia.
The
tax incentives are available on the basis of specific criteria with which the Company must comply. Although the tax incentive may be
administered through the local tax authority, the Company has accounted for the incentives outside of the scope of ASC Topic 740, Income
Taxes (“ASC 740”), since the incentives are not linked to the Company’s taxable income and can be realized regardless
of whether the Company has generated taxable income in the respective jurisdictions.
With
respect to the Australia R&D credit, as there is no authoritative guidance under GAAP for accounting for grants to for-profit business
entities, the Company accounts for the grant by analogy to IAS20 Accounting for Government Grants and Disclosure of Government Assistance
(“IAS 20”). The Company recognizes the Research and Development Incentive income as it incurs costs eligible for reimbursement
under the Australia R&D credit program when it is reasonably assured that the cash incentive will be received, as evidenced through
enrollment in the program and when the applicable conditions under the program have been met. The Company accrues for the amount of cash
refund it expects to receive in relation to research and development expenses outside of Australia only to the extent it has received
advanced approval from AusIndustry, pursuant to an approved advanced overseas finding application.
In
addition, Anavex Australia and Anavex Canada incur Goods and Services Tax (GST) on certain services provided by local vendors. As a domestic
entity in those jurisdictions, Anavex Australia and Anavex Canada are entitled to a refund of the GST paid. Similarly, Anavex Germany
incurs Value Added Tax (VAT) on certain services provided by local vendors, to which it is entitled to a refund of such VAT paid. The
Company’s estimate of the amount of cash refund it expects to receive related to GST and VAT incurred is included in Incentive
and tax receivables in the accompanying consolidated balance sheets.
|
| License Fees |
License
Fees
The
Company expenses amounts paid to acquire licenses associated with products under development when the ultimate recovery of the amounts
paid is uncertain and the technology has no alternative future use when acquired. Acquisitions of technology licenses are charged to
expense or capitalized based on management’s assessment regarding the ultimate recoverability of the amounts paid and the potential
for alternative future use. The Company has determined that the technological feasibility for its product candidates is reached when
the requisite regulatory approvals are obtained to make the product available for sale.
|
| Basic and Diluted Loss per Share |
Basic
and Diluted Loss per Share
Basic
income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the weighted average number
of common shares outstanding during the period. Diluted income/(loss) per common share is computed by dividing net income/(loss) available
to common stockholders by the sum of (1) the weighted-average number of common shares outstanding during the period, (2) the dilutive
effect of the assumed exercise of options and warrants using the treasury stock method and (3) the dilutive effect of other potentially
dilutive securities. For purposes of the diluted net loss per share calculation, options and warrants are potentially dilutive securities
and are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive.
As
of September 30, 2023, diluted loss per share excludes 14,271,780 potentially dilutive common shares (2022 –13,329,616; 2021 –
11,540,903) related to outstanding options and warrants, as their effect was anti-dilutive.
|
| Financial Instruments |
Financial
Instruments
The
book value of the Company’s financial instruments, consisting of cash and equivalents, incentive and tax receivables, accounts
payable and accrued liabilities approximate their fair value due to the short-term maturity of such instruments. Unless otherwise noted,
it is management’s opinion that the Company is not exposed to significant interest, currency or credit risks arising from these
financial instruments.
|
| Foreign Currency Translation |
Foreign
Currency Translation
The
functional currency of the Company is the US dollar. Monetary items denominated in a foreign currency are translated into US dollars
at exchange rates prevailing at the balance sheet date and non-monetary items are translated at exchange rates prevailing when the assets
were acquired, or obligations incurred. Foreign currency denominated expense items are translated at exchange rates prevailing on the
transaction date. Unrealized gains or losses arising from the translations are credited or charged to income in the period in which they
occur.
The
Company has determined that the functional currency of Anavex Australia Pty Limited, Anavex Germany GmbH, and Anavex Canada Ltd. is also
the US dollar.
|
| Segment and Geographic Reporting |
Segment
and Geographic Reporting
Operating
segments are defined as components of an enterprise for which separate discrete information is available for evaluation by the chief
operating decision maker or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views
its operations and manages its business as one operating segment, which is the business of developing novel therapies for the management
of CNS diseases.
|
| Grant Income |
Grant
Income
Grant
income is recognized at the fair value of the grant when it is received, and all substantive conditions have been satisfied. Grants received
from government and other agencies in advance of the specific research and development costs to which they relate are deferred and recognized
in the consolidated statements of operations in the period they are earned, typically when the related research and development costs
are incurred.
|
| Income Taxes |
Income
Taxes
The
Company follows the provisions of ASC 740, which requires the asset and liability method of accounting for income taxes. Under the asset
and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences
between the financial statements carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets
and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences
are expected to be recovered or settled.
The
Company follows the provisions of ASC 740 regarding accounting for uncertainty in income taxes. The Company initially recognizes tax
positions in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities.
Such tax positions are initially and subsequently measured as the largest amount of tax benefit that is greater than 50% likely of being
realized upon ultimate settlement with the tax authority assuming full knowledge of the position and all relevant facts. Application
requires numerous estimates based on available information. The Company considers many factors when evaluating and estimating its tax
positions and tax benefits, and its recognized tax positions and tax benefits may not accurately anticipate actual outcomes. As additional
information is obtained, there may be a need to periodically adjust the recognized tax positions and tax benefits. These periodic adjustments
may have a material impact on the consolidated statements of operations.
The
Company recognizes interest and penalties related to current income tax expense on the interest income, net line, in the accompanying
consolidated statements of operations. Accrued interest and penalties, if any, are included in accrued liabilities on the consolidated
balance sheets.
|
| Stock-based Compensation |
Stock-based
Compensation
The
Company accounts for all stock-based payments and awards under the fair value method.
The
fair value of all share purchase options and warrants are expensed over their contractual vesting period, or over the expected performance
period for only the portion of awards expected to vest, in the case of milestone-based vesting, with a corresponding increase to additional
paid-in capital.
Compensation
costs for stock-based payments with graded vesting are recognized on a straight-line basis. Stock based compensation expense is adjusted
for actual forfeitures of unvested awards as they occur.
The
Company has granted share purchase option awards that vest upon achievement of certain performance criteria, or milestone-based awards.
The Company estimates an implicit service period for achieving performance criteria for each award and recognizes the resulting fair
value as expense over the implicit service period when it concludes that achieving the performance criteria is probable. The Company
periodically reviews and updates as appropriate its estimates of implicit service periods and conclusions on achieving the performance
criteria. Performance awards vest upon achievement of the performance criteria.
The
Company uses the Black-Scholes option valuation model to calculate the fair value of share purchase options and warrants at the date
of the grant. This model requires the input of subjective assumptions, including the expected price volatility and expected life of each award.
The Company uses the U.S. Treasury daily treasury yield curve rates for the expected term of the option as the risk-free rate. The expected
term represents the period that options granted are expected to be outstanding using the simplified method. The Company’s historical
share option exercise experience does not provide sufficient basis for estimating the expected term. Expected volatility is based on
the average of the daily share price changes over the expected term. The Company does not estimate forfeitures and elects to record actual
forfeitures as they occur. The Company has not paid any dividends on its common stock historically, therefore no assumption of dividend
payments is made in the model. These assumptions consist of estimates of future market conditions, which are inherently uncertain, and
therefore, are subject to management’s judgment. Changes in these assumptions can materially affect the fair value estimates.
The
purchase price of options or warrants may be paid in cash or, if approved by the Company’s compensation committee (or in the case
of warrants by the Board of Directors) in advance, “net settled” in shares of the Company’s common stock. In a net
settlement of an option or warrant, the Company does not receive payment of the exercise price from the holder but reduces the number
of shares of common stock issued upon the exercise of the stock option or warrant by the smallest number of whole shares that have an
aggregate fair market value equal to or over the aggregate exercise price for the option shares covered by the option or warrant exercised.
Shares issued pursuant to the exercise of options and warrants are issued from the Company’s treasury.
|
| Fair Value Measurements |
Fair
Value Measurements
Fair
value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal
or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date.
Assets and liabilities that are measured at fair value are reported using a three-level fair value hierarchy that prioritizes the inputs
used to measure fair value. This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three
levels of inputs used to measure fair value are as follows:
Level
1 - quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at
the measurement date;
Level
2 - observable inputs other than Level 1, quoted prices for similar assets or liabilities in active markets, quoted prices for
identical or similar assets and liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose
significant value drivers are observable; and
Level
3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant
to the fair value of the assets or liabilities.
At
September 30, 2023 and 2022, the Company did not have any Level 2 or Level 3 assets or liabilities.
|
| Recently Adopted Accounting Pronouncements |
Recently
Adopted Accounting Pronouncements
Effective
October 1, 2021, the Company adopted Accounting Standards Update (“ASU”) 2019-12, “Simplifying the Accounting for Income
Taxes (ASC 740)”, which is intended to simplify various aspects related to accounting for income taxes by removing certain exceptions
to the general principles in Topic 740 and clarifying and amending existing guidance to improve consistent application. There was no
material impact on the Company’s operations, financial condition, or cash flows.
On
October 1, 2022, the Company adopted ASU 2021-10, Annual Disclosure Requirements for Business Entities Receiving Government Assistance
(Topic 832) – Disclosures by Business Entities about Government Assistance, which requires business entities to disclose information
about transactions with a government that are accounted for by applying a grant or contribution model by analogy. For transactions within
scope, the new standard requires the disclosure of information about the nature of the transaction, including significant terms and conditions,
as well as the amounts and specific financial statement line items affected by the transaction. The disclosure of the Company’s
research and development tax incentive income and receivable is detailed in Note 4.
|
| X |
- References
+ Details
| Name: |
avxl_GrantIncomePolicyTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_LicenseFeesPolicyTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for sales incentives.
| Name: |
us-gaap_RevenueRecognitionIncentives |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482785/280-10-55-47
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy election for determining cost for share-based payment arrangement by either estimating forfeiture expected to occur or by recognizing effect of forfeiture upon occurrence. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (m)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationForfeituresPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Accrued Liabilities (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Accrued Liabilities |
|
| Schedule of principal components of accrued liabilities |
| Schedule of principal components of accrued liabilities |
|
|
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
| Accrued
clinical site and patient visits costs |
|
$ |
2,006 |
|
|
$ |
2,031 |
|
| Accrued
compensation and benefits |
|
|
1,360 |
|
|
|
1,298 |
|
| Fixed
contract accruals |
|
|
38 |
|
|
|
417 |
|
| Milestone
based contract accruals |
|
|
1,267 |
|
|
|
137 |
|
| All
other accrued liabilities |
|
|
2,624 |
|
|
|
2,062 |
|
| Total
accrued liabilities |
|
$ |
7,295 |
|
|
$ |
5,945 |
|
|
| X |
- References
+ Details
| Name: |
avxl_DisclosureAccruedLiabilitiesAbstract |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments and Contingencies (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Schedule of operating lease costs |
| Schedule of operating lease costs |
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
| Operating lease costs |
|
$ |
118 |
|
|
$ |
73 |
|
|
$ |
131 |
|
|
| Schedule of contributions under the plan |
| Schedule of contributions under the plan |
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
Contributions
to 401(k) plan |
|
$ |
232 |
|
|
$ |
158 |
|
|
$ |
129 |
|
|
| Schedule of share purchase warrants outstanding |
| Schedule of share purchase warrants outstanding |
|
|
|
|
| Number |
|
Exercise Price |
|
Expiry Date |
| |
150,000 |
|
|
$ |
3.17 |
|
|
|
May 6, 2024 |
|
| |
10,000 |
|
|
$ |
12.00 |
|
|
|
April 21, 2026 |
|
| |
160,000 |
|
|
|
|
|
|
|
|
|
|
| Schedule of stock option activity |
| Schedule of stock option activity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
|
|
| |
|
|
|
Weighted Average |
|
Average Grant |
|
Aggregate intrinsic |
| |
|
Number of |
|
Exercise Price |
|
Date Fair Value |
|
value |
| |
|
Options |
|
($) |
|
($) |
|
($) |
| Outstanding,
September 30, 2021 |
|
|
11,330,903 |
|
|
|
5.74 |
|
|
|
— |
|
|
|
140,132,451 |
|
| Granted |
|
|
2,358,000 |
|
|
|
10.13 |
|
|
|
7.07 |
|
|
|
— |
|
| Forfeited |
|
|
(118,750 |
) |
|
|
6.86 |
|
|
|
5.23 |
|
|
|
— |
|
| Exercised |
|
|
(400,537 |
) |
|
|
2.52 |
|
|
|
1.88 |
|
|
|
4,201,015 |
|
| Outstanding,
September 30, 2022 |
|
|
13,169,616 |
|
|
|
6.61 |
|
|
|
4.96 |
|
|
|
62,267,309 |
|
| Granted |
|
|
1,959,000 |
|
|
|
9.30 |
|
|
|
6.60 |
|
|
|
— |
|
| Expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Exercised |
|
|
(759,753 |
) |
|
|
2.34 |
|
|
|
0.95 |
|
|
|
4,629,026 |
|
| Forfeited |
|
|
(257,083 |
) |
|
|
12.00 |
|
|
|
6.74 |
|
|
|
— |
|
| Cancelled |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Outstanding,
September 30, 2023 |
|
|
14,111,780 |
|
|
|
7.12 |
|
|
|
5.27 |
|
|
|
22,290,069 |
|
| Exercisable,
September 30, 2023 |
|
|
9,604,614 |
|
|
|
5.19 |
|
|
|
3.94 |
|
|
|
22,290,069 |
|
|
| Schedule of summarizes information about stock options |
| Schedule of summarizes information about stock options |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted |
|
|
|
|
|
|
| |
|
|
|
|
|
average |
|
Weighted |
|
|
|
|
| |
|
|
|
Number of |
|
remaining |
|
average |
|
|
|
Weighted |
| Range of exercises
prices |
|
outstanding |
|
contractual |
|
exercise |
|
Number of |
|
average |
| From |
|
To |
|
options |
|
life |
|
price |
|
vested options |
|
exercise price |
| $ |
0.92 |
|
|
|
3.00 |
|
|
|
3,280,309 |
|
|
|
4.74 |
|
|
|
2.39 |
|
|
|
3,280,309 |
|
|
$ |
2.39 |
|
| $ |
3.01 |
|
|
|
5.00 |
|
|
|
2,017,500 |
|
|
|
4.32 |
|
|
|
3.28 |
|
|
|
2,017,500 |
|
|
$ |
3.28 |
|
| $ |
5.01 |
|
|
|
9.00 |
|
|
|
5,260,054 |
|
|
|
6.21 |
|
|
|
6.90 |
|
|
|
3,304,137 |
|
|
$ |
6.10 |
|
| $ |
9.01 |
|
|
|
13.00 |
|
|
|
1,981,917 |
|
|
|
8.37 |
|
|
|
10.55 |
|
|
|
480,584 |
|
|
$ |
11.45 |
|
| $ |
13.01 |
|
|
|
25.00 |
|
|
|
1,572,000 |
|
|
|
7.49 |
|
|
|
18.29 |
|
|
|
522,084 |
|
|
$ |
18.57 |
|
| |
|
|
|
|
|
|
|
|
14,111,780 |
|
|
|
6.04 |
|
|
|
7.12 |
|
|
|
9,604,614 |
|
|
$ |
5.19 |
|
|
| Schedule of general and administrative expenses and research and development expenses |
| Schedule of general and administrative expenses and research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
September
30, |
| |
|
2023 |
|
2022 |
|
2021 |
| General
and administrative |
|
$ |
5,558 |
|
|
$ |
7,129 |
|
|
|
3,571 |
|
| Research
and development |
|
|
10,812 |
|
|
|
11,250 |
|
|
|
4,660 |
|
| Total
stock-based compensation |
|
$ |
16,370 |
|
|
$ |
18,379 |
|
|
|
8,231 |
|
|
| Schedule of weighted average assumptions for fair value of each option award |
| Schedule of weighted average assumptions for fair value of each option award |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Risk-free
interest rate |
|
|
3.70 |
% |
|
|
3.11 |
% |
|
|
0.73 |
% |
| Expected
life of options (years) |
|
|
5.64 |
|
|
|
5.57 |
|
|
|
5.74 |
|
| Annualized
volatility |
|
|
85.13 |
% |
|
|
84.17 |
% |
|
|
93.43 |
% |
| Dividend
rate |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
| X |
- References
+ Details
| Name: |
avxl_ScheduleOfShareBasedPaymentWeightedAverageValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ScheduleOfSharePurchaseWarrantsOutstandingTableTextBlock |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of defined contribution pension plans or defined contribution other postretirement plans, separately for pension plans and other postretirement benefit plans. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 715
-SubTopic 70
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480794/715-70-50-1
| Name: |
us-gaap_DefinedContributionPlanDisclosuresTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the change in stock options.
| Name: |
us-gaap_ScheduleOfStockOptionsRollForwardTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Taxes (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Schedule of loss before income taxes |
| Schedule of loss before
income taxes |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| United
States |
|
$ |
(41,198 |
) |
|
$ |
(40,002 |
) |
|
$ |
(28,851 |
) |
| Foreign |
|
|
(6,300 |
) |
|
|
(7,618 |
) |
|
|
(8,790 |
) |
|
| Schedule of components of net deferred income tax assets |
| Schedule of components of
net deferred income tax assets |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Net
operating loss carryforwards |
|
$ |
46,462 |
|
|
$ |
46,208 |
|
|
$ |
34,982 |
|
| Research
and development tax credit carryforwards |
|
|
2,713 |
|
|
|
2,182 |
|
|
|
1,577 |
|
| Stock-based
compensation |
|
|
18,593 |
|
|
|
13,373 |
|
|
|
10,453 |
|
| Research
and development capitalization |
|
|
7,219 |
|
|
|
— |
|
|
|
— |
|
| Unpaid
charges |
|
|
1,559 |
|
|
|
894 |
|
|
|
89 |
|
| Intangible
asset costs |
|
|
593 |
|
|
|
388 |
|
|
|
323 |
|
| Foreign
exchange and other |
|
|
49 |
|
|
|
44 |
|
|
|
62 |
|
| Valuation
allowance of deferred tax assets |
|
|
(77,188 |
) |
|
|
(63,089 |
) |
|
|
(47,486 |
) |
| Net
deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
| Schedule of reconciliation of income tax expense |
| Schedule
of reconciliation of income tax expense |
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Income
tax benefit at statutory federal rate |
|
$ |
(9,975 |
) |
|
$ |
(10,000 |
) |
|
$ |
(7,934 |
) |
| Foreign
income taxed at other rates |
|
|
— |
|
|
|
(170 |
) |
|
|
(353 |
) |
| Permanent
differences relating to stock based compensation |
|
|
(601 |
) |
|
|
(714 |
) |
|
|
(4,379 |
) |
| Permanent
differences relating to GILTI inclusion |
|
|
165 |
|
|
|
— |
|
|
|
— |
|
| Permanent
differences relating to Section 162(m) |
|
|
— |
|
|
|
— |
|
|
|
816 |
|
| Other
permanent differences |
|
|
273 |
|
|
|
— |
|
|
|
741 |
|
| Adjustment
to tax assets based on Section 382 |
|
|
— |
|
|
|
— |
|
|
|
3,330 |
|
| Research
and development credits, net |
|
|
(37 |
) |
|
|
232 |
|
|
|
1,042 |
|
| State
and local taxes |
|
|
(4,122 |
) |
|
|
(4,975 |
) |
|
|
(7,022 |
) |
| Adjustment
to true up to prior years' tax provision |
|
|
206 |
|
|
|
24 |
|
|
|
48 |
|
| Effect
of change in statutory tax rates |
|
|
— |
|
|
|
— |
|
|
|
216 |
|
| State
minimum and excise taxes |
|
|
— |
|
|
|
358 |
|
|
|
268 |
|
| Change
in valuation allowances |
|
|
14,098 |
|
|
|
15,603 |
|
|
|
13,495 |
|
| Income
tax expense |
|
$ |
7 |
|
|
$ |
358 |
|
|
$ |
268 |
|
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of income before income tax between domestic and foreign jurisdictions. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_ScheduleOfIncomeBeforeIncomeTaxDomesticAndForeignTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Summary of Significant Accounting Policies (Details Narrative) - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Accounting Policies [Abstract] |
|
|
|
| Federal deposit insurance corporation |
$ 250,000
|
|
|
| Loss per share for potentially dilutive common shares |
14,271,780
|
13,329,616
|
11,540,903
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash deposited in financial institutions as of the balance sheet date that is insured by the Federal Deposit Insurance Corporation.
| Name: |
us-gaap_CashFDICInsuredAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.3
Accrued Liabilities (Details) - USD ($)
$ in Thousands |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Accrued Liabilities |
|
|
| Accrued clinical site and patient visits costs |
$ 2,006
|
$ 2,031
|
| Accrued compensation and benefits |
1,360
|
1,298
|
| Fixed contract accruals |
38
|
417
|
| Milestone based contract accruals |
1,267
|
137
|
| All other accrued liabilities |
2,624
|
2,062
|
| Total accrued liabilities |
$ 7,295
|
$ 5,945
|
| X |
- References
+ Details
| Name: |
avxl_AccruedClinicalSiteAndPatientVisitsCosts |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_DisclosureAccruedLiabilitiesAbstract |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_FixedContractAccruals |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_MilestoneBasedContractAccruals |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for incentive compensation awarded to employees and directors or earned by them based on the terms of one or more relevant arrangements. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedBonusesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Other Income (Details Narrative) - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
| Grant income |
$ 500,000
|
$ 0
|
$ 500,000
|
| Research and development incentive income |
2,700,000
|
3,300,000
|
4,500,000
|
| Non operating income from grant |
25,000
|
0
|
54,100
|
| Deferred grant income |
900,000
|
400,000
|
|
| Incentive and tax receivables |
2,500,000
|
2,900,000
|
|
| Australia, Dollars |
|
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
| Research and development incentive income |
4,100,000
|
4,500,000
|
6,100,000
|
| Incentive and tax receivables |
$ 3,900,000
|
$ 4,500,000
|
|
| Michael J Fox Foundation [Member] |
|
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
|
| Research and development incentive income |
|
|
$ 1,000,000
|
| X |
- References
+ Details
| Name: |
avxl_DeferredGrantIncome1 |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_GrantIncome |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_NonOperatingIncomeFromGrant |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_DefinedBenefitPlanDisclosureLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount due from parties in nontrade transactions, classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(5)(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_CurrencyAxis=currency_AUD |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Equity Offerings (Details Narrative) - USD ($)
|
|
|
12 Months Ended |
Feb. 03, 2023 |
Jun. 22, 2021 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Sales of agreement amount |
|
|
$ 142,400,000
|
$ 142,400,000
|
|
| Fair value of the initial commitment |
|
|
800,000
|
|
|
| Incurred expenses |
|
|
$ 100,000
|
|
|
| Securities Purchase Agreement [Member] |
|
|
|
|
|
| Number of common stock sold |
|
2,380,953
|
|
|
|
| Sale of stock, per share |
|
$ 21
|
|
|
|
| Gross proceeds from sale of stock |
|
$ 50,000,000
|
|
|
|
| Net proceed |
|
$ 46,902,981
|
|
|
|
| Equity Offering Sales Agreement [Member] |
|
|
|
|
|
| Number of common stock sold |
|
|
0
|
1,623,813
|
5,634,576
|
| Net proceed |
|
|
|
$ 20,300,000
|
$ 76,700,000
|
| Percentage of gross proceeds from sales |
|
|
3.00%
|
|
|
| Number of common stock sold, value |
|
|
|
21,000,000
|
|
| Sold for gross proceeds amount |
|
|
|
|
$ 79,100,000
|
| Purchase Agreement 2023 [Member] | Lincoln Park Capital Fund L L C [Member] |
|
|
|
|
|
| Value of shares obligated to purchase |
$ 150,000,000
|
|
|
|
|
| Number of shares issued, shares |
75,000
|
|
|
|
|
| Pro rata basic number of shares obligated to purchase |
75,000
|
|
|
|
|
| Proceeds from issuance or sale of equity |
$ 150,000,000
|
|
|
|
|
| Number of shares issued, value |
|
|
$ 27,900,000
|
$ 0
|
|
| Amount of shares remain available |
|
|
$ 122,100,000
|
|
|
| Purchase Agreement 2023 [Member] | Lincoln Park Capital Fund L L C [Member] | Common Stock Including Commitment Shares [Member] |
|
|
|
|
|
| Number of shares issued, shares |
|
|
3,288,943
|
0
|
|
| Purchase Agreement 2023 [Member] | Lincoln Park Capital Fund L L C [Member] | Common Stock Excluding Commitment Shares [Member] |
|
|
|
|
|
| Number of shares issued, shares |
|
|
3,275,000
|
0
|
|
| Purchase Agreement 2023 [Member] | Lincoln Park Capital Fund L L C [Member] | Commitment Shares [Member] |
|
|
|
|
|
| Number of shares issued, shares |
|
|
13,943
|
0
|
|
| Purchase Agreement 2019 [Member] | Lincoln Park Capital Fund L L C [Member] |
|
|
|
|
|
| Value of shares obligated to purchase |
$ 50,000,000
|
|
|
|
|
| Number of shares issued, shares |
|
|
0
|
0
|
|
| Purchase Agreement 2019 [Member] | Lincoln Park Capital Fund L L C [Member] | Common Stock Including Commitment Shares [Member] |
|
|
|
|
|
| Number of shares issued, shares |
|
|
|
|
4,086,209
|
| Purchase Agreement 2019 [Member] | Lincoln Park Capital Fund L L C [Member] | Common Stock Excluding Commitment Shares [Member] |
|
|
|
|
|
| Number of shares issued, shares |
|
|
|
|
4,007,996
|
| Purchase Agreement 2019 [Member] | Lincoln Park Capital Fund L L C [Member] | Commitment Shares [Member] |
|
|
|
|
|
| Number of shares issued, shares |
|
|
|
|
78,213
|
| Purchase Agreement 2019 [Member] | Lincoln Park Capital Fund L L C [Member] | Common Stock [Member] |
|
|
|
|
|
| Number of shares issued, value |
|
|
|
|
$ 24,100,000
|
| X |
- References
+ Details
| Name: |
avxl_FairValueOfInitialCommitment |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_IncurredExpenses |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_NumberOfSharesObligatedToPurchaseProrataBasic |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_PercentageOfGrossProceedsFromSales |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_SalesOfAgreementAmount |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_SoldForGrossProceedsAmount |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ValueOfSharesObligatedToPurchase |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCash received on stock transaction after deduction of issuance costs.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedOnTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of consideration received by subsidiary or equity investee in exchange for shares of stock issued or sold. Includes amount of cash received, fair value of noncash assets received, and fair value of liabilities assumed by the investor.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedPerTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodValueOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_SecuritiesPurchaseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_EquityOfferingSalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_PurchaseAgreement2023Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
dei_LegalEntityAxis=avxl_LincolnParkCapitalFundLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=avxl_CommonStockIncludingCommitmentSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=avxl_CommonStockExcludingCommitmentSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=avxl_CommitmentSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=avxl_PurchaseAgreement2019Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for pension benefit. Includes, but is not limited to, employer contribution to fund plan asset and payment to retiree. Excludes other postretirement benefit. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PensionContributions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionExpiration date of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=avxl_PurchaseWarrants1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=avxl_PurchaseWarrants2Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Commitments and Contingencies (Details 3) - Equity Option [Member] - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Offsetting Assets [Line Items] |
|
|
| Number of options, Outstanding beginning balance |
13,169,616
|
11,330,903
|
| Weighted average exercise price, Outstanding beginning balance |
$ 6.61
|
$ 5.74
|
| Weighted average grant date fair value, Outstanding beginning balance |
$ 4.96
|
|
| Aggregate intrinsic value, Outstanding beginning balance |
$ 62,267,309
|
$ 140,132,451
|
| Number of options, Granted |
1,959,000
|
2,358,000
|
| Weighted average exercise price, Granted |
$ 9.30
|
$ 10.13
|
| Weighted average grant date fair value, Granted |
$ 6.60
|
$ 7.07
|
| Number of options, Forfeited |
(257,083)
|
(118,750)
|
| Weighted average exercise price, Forfeited |
$ 12.00
|
$ 6.86
|
| Weighted average grant date fair value, Forfeited |
$ 6.74
|
$ 5.23
|
| Number of options, Exercised |
(759,753)
|
(400,537)
|
| Weighted average exercise price, Exercised |
$ 2.34
|
$ 2.52
|
| Weighted average grant date fair value, Exercised |
$ 0.95
|
$ 1.88
|
| Aggregate intrinsic value, Exercised |
$ 4,629,026
|
$ 4,201,015
|
| Number of options, Outstanding ending balance |
14,111,780
|
13,169,616
|
| Weighted average exercise price, Outstanding ending balance |
$ 7.12
|
$ 6.61
|
| Weighted average grant date fair value, Outstanding ending balance |
$ 5.27
|
$ 4.96
|
| Aggregate intrinsic value, Outstanding ending balance |
$ 22,290,069
|
$ 62,267,309
|
| Number of options, Exercisable |
9,604,614
|
|
| Weighted average exercise price, Exercisable |
$ 5.19
|
|
| Weighted average grant date fair value, Exercisable |
$ 3.94
|
|
| Aggregate intrinsic value, Exercisable |
$ 22,290,069
|
|
| X |
- References
+ Details
| Name: |
avxl_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisesPrice |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisesPrice |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated difference between fair value of underlying shares on dates of exercise and exercise price on options exercised (or share units converted) into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average grant-date fair value of non-vested options outstanding.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Commitments and Contingencies (Details 4) - $ / shares
|
12 Months Ended |
|
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Offsetting Assets [Line Items] |
|
|
|
| Weighted average remaining contractual life |
6 years
|
6 years 4 months 24 days
|
|
| Equity Option [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Number of outstanding options |
14,111,780
|
13,169,616
|
11,330,903
|
| Weighted average remaining contractual life |
6 years 14 days
|
|
|
| Weighted average exercise price |
$ 7.12
|
$ 6.61
|
$ 5.74
|
| Number of vested options |
9,604,614
|
|
|
| Weighted average exercise price options vested |
$ 5.19
|
|
|
| Equity Option [Member] | Option Price 1 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, Lower range limit |
0.92
|
|
|
| Range of exercise prices, Upper range limit |
$ 3.00
|
|
|
| Number of outstanding options |
3,280,309
|
|
|
| Weighted average remaining contractual life |
4 years 8 months 26 days
|
|
|
| Weighted average exercise price |
$ 2.39
|
|
|
| Number of vested options |
3,280,309
|
|
|
| Weighted average exercise price options vested |
$ 2.39
|
|
|
| Equity Option [Member] | Option Price 2 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, Lower range limit |
3.01
|
|
|
| Range of exercise prices, Upper range limit |
$ 5.00
|
|
|
| Number of outstanding options |
2,017,500
|
|
|
| Weighted average remaining contractual life |
4 years 3 months 25 days
|
|
|
| Weighted average exercise price |
$ 3.28
|
|
|
| Number of vested options |
2,017,500
|
|
|
| Weighted average exercise price options vested |
$ 3.28
|
|
|
| Equity Option [Member] | Option Price 3 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, Lower range limit |
5.01
|
|
|
| Range of exercise prices, Upper range limit |
$ 9.00
|
|
|
| Number of outstanding options |
5,260,054
|
|
|
| Weighted average remaining contractual life |
6 years 2 months 15 days
|
|
|
| Weighted average exercise price |
$ 6.90
|
|
|
| Number of vested options |
3,304,137
|
|
|
| Weighted average exercise price options vested |
$ 6.10
|
|
|
| Equity Option [Member] | Option Price 4 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, Lower range limit |
9.01
|
|
|
| Range of exercise prices, Upper range limit |
$ 13.00
|
|
|
| Number of outstanding options |
1,981,917
|
|
|
| Weighted average remaining contractual life |
8 years 4 months 13 days
|
|
|
| Weighted average exercise price |
$ 10.55
|
|
|
| Number of vested options |
480,584
|
|
|
| Weighted average exercise price options vested |
$ 11.45
|
|
|
| Equity Option [Member] | Option Price 5 [Member] |
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
| Range of exercise prices, Lower range limit |
13.01
|
|
|
| Range of exercise prices, Upper range limit |
$ 25.00
|
|
|
| Number of outstanding options |
1,572,000
|
|
|
| Weighted average remaining contractual life |
7 years 5 months 26 days
|
|
|
| Weighted average exercise price |
$ 18.29
|
|
|
| Number of vested options |
522,084
|
|
|
| Weighted average exercise price options vested |
$ 18.57
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest exercisable or convertible options. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe floor of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeLowerRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe ceiling of a customized range of exercise prices for purposes of disclosing shares potentially issuable under outstanding stock option awards on all stock option plans and other required information pertaining to awards in the customized range. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeUpperRangeLimit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options vested.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedNumberOfShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice2Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice3Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice4Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ShareBasedCompensationSharesAuthorizedUnderStockOptionPlansByExercisePriceRangeAxis=avxl_OptionPrice5Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Commitments and Contingencies (Details 5) - USD ($)
$ in Thousands |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Loss Contingencies [Line Items] |
|
|
|
| Total share based compensation |
$ 16,370
|
$ 18,379
|
$ 8,231
|
| General and Administrative Expense [Member] |
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
| Total share based compensation |
5,558
|
7,129
|
3,571
|
| Research and Development Expense [Member] |
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
| Total share based compensation |
$ 10,812
|
$ 11,250
|
$ 4,660
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482425/460-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-4
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-9
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483076/450-20-50-9
| Name: |
us-gaap_LossContingenciesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments and Contingencies (Details Narrative) - USD ($)
$ / shares in Units, $ in Millions |
|
12 Months Ended |
Mar. 25, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Operating lease term |
|
12 months
|
|
|
| Warrants outstanding |
|
160,000
|
160,000
|
|
| Warrants outstanding weighted average exercise price |
|
$ 3.72
|
$ 3.72
|
|
| Option issued |
|
3,849,917
|
|
|
| Weighted average grant date fair value of options vested |
|
$ 3.94
|
$ 4.69
|
$ 2.54
|
| Weighted average contractual life of options outstanding |
|
6 years
|
6 years 4 months 24 days
|
|
| Options exercisable |
|
4 years 9 months
|
5 years 1 month 6 days
|
|
| Share based compensation |
|
$ 16.4
|
$ 18.4
|
$ 8.2
|
| Remaining stock based compensation |
|
$ 14.1
|
|
|
| Stock Option Plan 2015 [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Maximum number of common shares reserved for future issuance |
|
6,050,553
|
|
|
| Stock Option Plan 2019 [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Additional shares of common stock available for issuance |
|
6,000,000
|
|
|
| Stock Option Plan 2022 [Member] |
|
|
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
|
| Additional shares of common stock available for issuance |
10,000,000
|
|
|
|
| Option granted |
|
|
406,453
|
|
| Option available issue |
|
6,661,535
|
|
|
| X |
- References
+ Details
| Name: |
avxl_OptionIssued |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_WarrantsOutstandingWeightedAverageExercise |
| Namespace Prefix: |
avxl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost to be recognized for option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of additional shares authorized for issuance under share-based payment arrangement.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term of exercisable stock options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationSharesAuthorizedUnderStockOptionPlansExercisePriceRangeExercisableOptionsWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=avxl_StockOptionPlan2015Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=avxl_StockOptionPlan2019Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=avxl_StockOptionPlan2022Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Income Taxes (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Income Tax Disclosure [Abstract] |
|
|
|
| United States |
$ (41,198)
|
$ (40,002)
|
$ (28,851)
|
| Foreign |
(6,300)
|
(7,618)
|
(8,790)
|
| Total |
$ (47,498)
|
$ (47,620)
|
$ (37,641)
|
v3.23.3
Income Taxes (Details 1) - USD ($)
$ in Thousands |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Income Tax Disclosure [Abstract] |
|
|
|
| Net operating loss carryforwards |
$ 46,462
|
$ 46,208
|
$ 34,982
|
| Research and development tax credit carryforwards |
2,713
|
2,182
|
1,577
|
| Stock-based compensation |
18,593
|
13,373
|
10,453
|
| Research and development capitalization |
7,219
|
|
|
| Unpaid charges |
1,559
|
894
|
89
|
| Intangible asset costs |
593
|
388
|
323
|
| Foreign exchange and other |
49
|
44
|
62
|
| Valuation allowance of deferred tax assets |
(77,188)
|
(63,089)
|
(47,486)
|
| Net deferred tax assets |
|
|
|
| X |
- References
+ Details
| Name: |
avxl_DeferredTaxAssetsResearchAndDevelopmentCapitalization |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
avxl_DeferredTaxAssetsUnpaidCharges |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from intangible assets including goodwill.
| Name: |
us-gaap_DeferredTaxAssetsGoodwillAndIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from in-process research and development costs expensed in connection with a business combination. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsInProcessResearchAndDevelopment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from unrealized losses on foreign currency transactions. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsUnrealizedCurrencyLosses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Income Taxes (Details 2) - USD ($)
$ in Thousands |
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Income Tax Disclosure [Abstract] |
|
|
|
| Income tax benefit at statutory federal rate |
$ (9,975)
|
$ (10,000)
|
$ (7,934)
|
| Foreign income taxed at other rates |
|
(170)
|
(353)
|
| Permanent differences relating to stock based compensation |
(601)
|
(714)
|
(4,379)
|
| Permanent differences relating to GILTI inclusion |
165
|
|
|
| Permanent differences relating to Section 162(m) |
|
|
816
|
| Other permanent differences |
273
|
|
741
|
| Adjustment to tax assets based on Section 382 |
|
|
3,330
|
| Research and development credits, net |
(37)
|
232
|
1,042
|
| State and local taxes |
(4,122)
|
(4,975)
|
(7,022)
|
| Adjustment to true up to prior years' tax provision |
206
|
24
|
48
|
| Effect of change in statutory tax rates |
|
|
216
|
| State minimum and excise taxes |
|
358
|
268
|
| Change in valuation allowances |
14,098
|
15,603
|
13,495
|
| Income tax expense |
$ 7
|
$ 358
|
$ 268
|
| X |
- References
+ Details
| Name: |
avxl_AdjustmentToTaxAssetsBasedOnSection382 |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_PermanentDifferencesRelatingToSection162m |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_PermanentDifferencesRelatingToStockBasedCompensation |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAll taxes not related to income of the entity or excise or sales taxes levied on the revenue of the entity that are not reported elsewhere. These taxes could include production, real estate, personal property, and pump tax. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_TaxesExcludingIncomeAndExciseTaxes |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
avxl_OperatingLossCarryForwardsExpirationYear |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_OperatingLossCarryforwardsDescription |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
avxl_ResearchAndDevelopmentTaxCreditsYear |
| Namespace Prefix: |
avxl_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible research tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwardsResearch |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OperatingLossCarryforwardsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in the valuation allowance for a specified deferred tax asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ValuationAllowanceDeferredTaxAssetChangeInAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CurrencyAxis=currency_AUD |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Anavex Life Sciences (NASDAQ:AVXL)
Historical Stock Chart
From Oct 2024 to Nov 2024
Anavex Life Sciences (NASDAQ:AVXL)
Historical Stock Chart
From Nov 2023 to Nov 2024