UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 13E-3
RULE 13e-3 TRANSACTION STATEMENT
PURSUANT TO SECTION 13(e)
OF THE SECURITIES EXCHANGE ACT OF 1934
(Amendment No. 2)
Pardes
Biosciences, Inc.
(Name of the Issuer)
Pardes
Biosciences, Inc.
(Name of Person(s) Filing Statement)
Common Stock, par value $0.0001 per share
(Title of Class of Securities)
69945Q 105
(CUSIP Number
of Common Stock)
Thomas G. Wiggans
Chief Executive Officer
Pardes Biosciences, Inc.
2173 Salk Avenue, Suite 250
PMB#052
Carlsbad,
California 92008
(415) 649-8758
(Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications on Behalf of the Person(s) Filing Statement)
With copies to:
|
|
|
| Douglas N. Cogen, Esq.
Ethan A. Skerry, Esq. Ran
D. Ben-Tzur, Esq. Jennifer J. Hitchcock, Esq.
Michael S. Pilo, Esq.
Fenwick & West LLP
555 California Street, 12th Floor
San Francisco, California 94104
(415) 875-2300 |
|
Elizabeth H. Lacy
General Counsel and Corporate Secretary
Pardes Biosciences, Inc.
2173 Salk Avenue, Suite 250
PMB#052 Carlsbad,
California 92008 (415) 649-8758 |
This statement is filed in connection with (check the appropriate box):
|
|
|
|
|
| a. |
|
☐ |
|
The filing of solicitation materials or an information statement subject to Regulation 14A, Regulation 14C or Rule 13e-3(c) under the Securities Exchange Act of 1934. |
|
|
|
| b. |
|
☐ |
|
The filing of a registration statement under the Securities Act of 1933. |
|
|
|
| c. |
|
☒ |
|
A tender offer. |
|
|
|
| d. |
|
☐ |
|
None of the above. |
Check the following box if the soliciting materials or information statement referred to in checking box (a) are
preliminary copies: ☐
Check the following box if the filing is a final amendment reporting the results of the
transaction: ☐
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THIS
TRANSACTION, PASSED UPON THE MERITS OR FAIRNESS OF THIS TRANSACTION, OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS SCHEDULE 13E-3. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL
OFFENSE.
INTRODUCTION
This Amendment No. 2 (“Amendment No. 2”) amends and supplements
the Rule 13e-3 Transaction Statement on Schedule 13E-3 previously filed by Pardes Biosciences, Inc., a Delaware corporation
(“Pardes” or the “Company”), with the United States Securities and Exchange Commission (the “SEC”) on July 28, 2023 (as amended and restated on August 17, 2023, and as
may be further amended, restated or supplemented from time to time, the “Schedule 13E-3”). This Amendment No. 2 relates to the offer to purchase by MediPacific Sub, Inc., a
Delaware corporation (“Purchaser”), and wholly owned subsidiary of MediPacific, Inc. (“Parent”), all of the issued and outstanding shares (the “Shares”) of common stock, par
value $0.0001 per share (“Common Stock”), of Pardes that is the subject of the Rule 13e-3 transaction described below (other than (i) Shares held in the
treasury of Pardes immediately prior to the effective time of the Merger (as defined below), and (ii) Shares owned, directly or indirectly, by the Foresite Stockholders (as defined in that certain Agreement and Plan of Merger, dated as of
July 16, 2023, by and among Pardes, Purchaser and Parent (the “Merger Agreement”)), Parent, Purchaser or any other subsidiary of Parent at the commencement of the Offer and that are owned by Parent, Purchaser or any
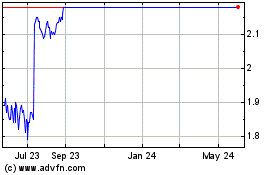
other subsidiary of Parent immediately prior to the effective time of the Merger), for a price of (i) $2.13 per Share and (ii) one non-transferable contingent value right per Share, upon the
terms and subject to the conditions set forth in the Offer to Purchase dated July 28, 2023 (as amended and restated on August 17, 2023, further amended on August 28, 2023, and as may be further amended or supplemented from time to
time, the “Offer to Purchase”) and in the related Letter of Transmittal, dated July 28, 2023, which, together with any further amendments or supplements thereto, collectively constitute the
“Offer.” The Offer is being made pursuant to the Merger Agreement. The Merger Agreement provides, among other things, for the terms and conditions of the Offer and the subsequent merger of Purchaser with and into Pardes (the
“Merger”) in accordance with Section 251(h) of the Delaware General Corporation Law.
The information contained in the Tender
Offer Statement filed under cover of Schedule TO by Parent with the SEC on July 28, 2023 (as amended and restated on August 17, 2023, further amended on August 28, 2023, and as may be further amended or supplemented from time to time,
the “Schedule TO”), including the Offer to Purchase, and the Solicitation/Recommendation Statement on Schedule 14D-9 filed by Pardes with the SEC on July 28,
2023 (as amended and restated on August 17, 2023, further amended on August 28, 2023, and as may be further amended or supplemented from time to time, the
“Schedule 14D-9”), is incorporated herein by reference.
All information contained in this Statement concerning Purchaser, Parent or their affiliates has been provided by such person and not by any other person.
Item 16 is hereby amended and supplemented by adding the following exhibits:
|
|
|
| Exhibit No. |
|
Description |
| (a)(1)(I) |
|
Supplement No. 1 to Amended and Restated Offer to Purchase, dated August 28, 2023 (incorporated herein by reference to Exhibit (a)(1)(H) to the Schedule TO). |
|
|
| (a)(1)(J) |
|
Solicitation/Recommendation Statement (Amendment No. 2) on Schedule 14D-9 (incorporated herein by reference to the Schedule 14D-9). |
|
|
| (a)(1)(K) |
|
Press Release issued by Purchaser on August 28, 2023 (incorporated herein by reference to Exhibit (a)(1)(I) to the Schedule TO). |
|
|
| (c)(10)+ |
|
Discussion Materials, dated April 13, 2023, from Leerink Partners LLC. |
|
|
| (c)(11)+ |
|
Discussion Materials, dated May 2023, from Leerink Partners LLC. |
|
|
| (c)(12)+ |
|
Discussion Materials, dated May 24, 2023, from Leerink Partners LLC. |
|
|
| (c)(13)+ |
|
Discussion Materials, dated May 25, 2023, from Leerink Partners LLC. |
|
|
| (c)(14)+ |
|
Discussion Materials, dated June 10, 2023, from Leerink Partners LLC. |
|
|
| (c)(15)+ |
|
Discussion Materials, dated June 11, 2023, from Leerink Partners LLC. |
| + |
Certain portions of this exhibit have been redacted and separately filed with the Securities and Exchange
Commission pursuant to a request for confidential treatment. |
SIGNATURES
After due inquiry and to the best of my knowledge and belief, I certify that the information set forth in this Statement is true, complete and correct.
|
|
|
|
|
|
|
| Dated: August 28, 2023 |
|
|
|
PARDES BIOSCIENCES, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Thomas G. Wiggans |
|
|
|
|
|
|
Name: Thomas G. Wiggans |
|
|
|
|
|
|
Title: Chief Executive Officer and |
|
|
|
|
|
|
Chair of the Board of Directors |

$1'5(3/$&(':,7+³>;<=@´68&+,'(17,),(',1)250$7,21
+$6%((1(;&/8'(')5207+,6(;+,%,7%(&$86(,7 , ,61270$7(5,$/$1' ,, ,67+(7<3(7+$77+(5(*,675$1775($76$635,9$7(25&21),'(17,$/ Exhibit (c)(10) &(57$,1&21),'(17,$/3257,2162)7+,6(;+,%,7+$9(%((120,77(' PROJECT PACIFIC STRATEGIC
ALTERNATIVES CONSIDERATIONS APRIL 13, 2023 Confidential

PROJECT PACIFIC SITUATION OVERVIEW • Pardes Biosciences is
evaluating its strategic options following the decision to suspend the clinical development of pomotrelvir, reduce headcount by 85% and conserve its current cash balance of ~$172 million. • Pardes’ attractive cash balance and the current
weakness in the capital markets provide Pardes with a range of strategic alternatives to optimize value for shareholders. – Merge with a private company – In-license or acquire clinical assets or new technologies – Merge with a
public company – Return capital to shareholders • Private company merger counterparties could have complementary assets and operating synergies or could be in different therapeutic areas that the board believes will create a compelling
story for new and existing investors. • In-licensing or acquiring assets should be explored but can be challenging given both the competitive intensity and funding requirements to acquire and develop high-quality, clinical-stage assets through
to value inflecting milestones. • Public-to-public mergers are rare for pre-commercial biopharma companies as public company targets with attractive assets often have financing options that are more efficient and feasible than a merger with a
cash-rich fallen angel. • The return of capital option may be more efficient than in the past given the emergence of parties willing to acquire cash-rich companies for up to 80-90% of their net cash balance plus CVRs via a tender offer that
can close in ~45 days vs the traditional dissolution process that can take up to three years to complete. • The typical private merger process can take approximately two to three months to announcement and can take up to another three to four
months to close in the event the transaction does not qualify for a sign-and-close. • Our experience as a leading strategic advisor, history of screening and sourcing select targets for a number of similarly situated clients, differentiated
knowledge resources and deep relationships with potential merger partners make us an ideal advisor for the board as it navigates options and executes potential strategic transactions. Confidential 1

PROJECT PACIFIC PUBLIC / PRIVATE MERGERS CAN WORK AS A GO PUBLIC
ALTERNATIVE FOR HIGH-QUALITY COMPANIES $ IN MILLIONS; SORTED BY ANNOUNCEMENT DATE (NEWEST TO OLDEST) RECENTLY Private Company Private Company Private Company Private Company Private Company ANNOUNCED Merger with Merger with Merger with Merger with
Merger with PUBLIC / PRIVATE MERGERS: March 2023 February 2023 December 2022 November 2022 November 2022 HIGH QUALITY COMPANIES CURRENTLY TRADING ON NASDAQ VIA A PUBLIC / PRIVATE MERGER: Private Company Private Company Private Company Private
Company Private Company Private Company Merger with Merger with Merger with Merger with Merger with Merger with Celtrix Pharmaceuticals May 2000 October 2020 October 2020 January 2018 November 2016 July 2016 • $98MM follow-on (Sep. 2021)
• $184MM follow-on (Nov. 2021) • $84MM follow-on (Jan. 2018) • $52MM follow-on (May 2017) • $144MM follow-on (Dec. 2017) • $403MM follow-on (Sep. 2017) • $450MM convertible debt • $270MM follow-on (Aug.2022)
• $121MM follow-on (May 2022) • $63MM follow-on (Nov. 2018) • $75MM follow-on (Jan. 2018) • $440MM follow-on (Jun. 2018) (Jan. 2018) • $91MM follow-on (Apr. 2019) • $46MM follow-on (Jan. 2020) • $159MM
proceeds under • $287MM follow-on (May 2019) $200M ATM (Dec. 2022) • $98MM follow-on (Dec. 2019) • $160MM follow-on (Sep. 2020) • $259MM follow-on (May 2020) • $288MM follow-on / $575MM • $299MM follow-on (Dec.
2020) • Acquisition by Ipsen (closed convertible debt (May 2021) March 2023) • Renovacor Acquisition • $275MM follow-on / $500MM (Dec. 2022) term loan/royalty (Oct. 2022) • $100MM follow-on (Oct. 2022) Acquired for Mkt. Cap:
$1.5B Mkt. Cap: $1.7B Mkt. Cap: $1.4B Mkt. Cap: $5.6B Mkt. Cap: $2.3B (1) $1.0B by Ipsen (1) Does not include $10/share CVRs. Note: Data as of 04/06/23. Recently announced transactions include private company mergers that have not yet closed.
Confidential 2 Source: FactSet, Dealogic, SEC filings and press releases.

PROJECT PACIFIC SELECTION METHODOLOGY FOR A PRIVATE COMPANY MERGER
TRANSACTION Leverage SVB Securities' relationships with high-quality private biopharma companies with stated interest in alternative go-public transactions MEDACorp knowledge resource enables diligence of potential opportunities Relationships with
100+ high quality private biopharma companies help expedite screening process Focused list of attractive target companies for Pardes to pursue Confidential 3

PROJECT PACIFIC CRITERIA FOR DETERMINING ATTRACTIVENESS OF A PRIVATE
COMPANY MERGER COUNTERPARTY ILLUSTRATIVE SCREENING CRITERIA USED BY PARDES BIOSCIENCES Attractiveness of target’s lead asset, pipeline, technology, and business case 1. 2. Near-term, value-driving catalysts within pro forma cash runway
Management, board and existing investor quality 3. 4. Readiness to be a U.S. publicly traded company Need for additional financing and commitment from private company investors to provide such 5. 6. Proposed valuation and ownership split including
premium assigned to private company over expected cash at close Fit and value assigned to public company’s programs 7. ILLUSTRATIVE SCREENING CRITERIA USED BY PRIVATE COMPANY 1. Sufficient cash on balance sheet to fund activities through key
value-driving catalysts 2. Quality of public company investor base – overlap with private company is often helpful Material litigation, liabilities or sustained operational commitments 3. Personnel with complementary domain expertise
(scientific, development, or commercial) 4. Confidential 4

PROJECT PACIFIC COMPONENTS OF VALUE IN A PRIVATE COMPANY MERGER PARDES
BIOSCIENCES PRIVATE TARGET Estimated net cash at close Base value is post-money valuation of last private financing • Primary value driver • Value may be adjusted based on market conditions • Ability to maximize cash preservation
via operational • Meaningful data generation or update since last streamlining financing could justify a value adjustment • Target valuation may be more negotiable in the absence of a high-quality institutional shareholder Valuation
supported by: Listing value Clinical programs / Intrinsic valuation Market based valuation other assets • Key analysis to inform • Crossover to IPO step- • Additional value driver • Potential for right inherent value of
future up, comparable partner to ascribe value cash flows • Dependent on equity companies analysis capital market • CVRs can be employed • Sensitive to changes in • Reference point to conditions to retain value for
assumptions inform value shareholders • Less reliable for earlier- • Dependent on market stage companies conditions Relative value, informed by the above metrics, will be determined via negotiation of the exchange ratio Confidential
5

CVRS CAN BE EMPLOYED TO RETAIN VALUE FOR PROJECT PACIFIC SHAREHOLDERS
Mechanism of Special dividend to Aduro shareholders of record prior Special dividend to OncoMed shareholders of record in to merger becoming effective March 2019 CVR Issuance Terminates at earlier of: • Payment by Mereo of each milestone
eligible to be 10 years Period attained • Expiration of period for each trigger event Non-tradable Non-tradable Tradability • Celgene exercises its option to license Oncomed’s etigilimab and pays associated $35mm milestone •
Disposition or license of Aduro’s non-renal assets before 12/31/19 Trigger • Revenue resulting from ownership of subsidiary Event(s) • Mereo enters partnership or other agreement established to hold any non-renal assets regarding
navicixizumab within 18 months of the merger closing • Additional Mereo ADRs based on exchange ratio calculated by dividing the milestone received by the • Any consideration paid to Aduro with respect to the Mereo’s 10-day VWAP
following announcement disposition or license of any non-renal assets that Celgene exercised its option (subject to issuance limitation of 40% of issued share capital) • Proceeds resulting from ownership in any Payment subsidiary established
by Aduro before the six- • 70% of net proceeds of milestone payments month anniversary of the closing date to hold any received by Mereo within 5 years of merger from non-renal assets future partnership or investment transactions in relation
to navicixizumab (subject to aggregate cap of $79.7mm) Confidential 6

PROJECT PACIFIC CASE STUDY: CONCENTRA’S ACQUISITION OF JOUNCE
POST REDX MERGER ANNOUNCEMENT TIMELINE • 02/23: Jounce announced an all-stock merger with Redx Pharma (37% / 63% split, respectively) • 03/14: Concentra offered to acquire Jounce for $1.80 cash per share + a CVR • 03/27: Jounce
announced acquisition by Concentra for $1.85 cash per share + CVRs TRANSACTION DETAILS • On 04/07, Concentra will commence a tender offer to acquire all outstanding shares of Jounce for $1.85 per share + CVRs ‒ Estimated / minimum Jounce
net cash at close: $115M / $110M ‒ Upfront cash of $1.85 per share represents 61% of current net cash per share, 85% of estimated net cash at close and 89% of minimum net cash ‒ Jounce’s liquidation analysis suggested distribution
would result in proceeds of 61% of current net cash and 89% of merger minimum cash • CVRs are for proceeds from (1) a transaction for Jounce’s programs and (2) certain specified cost savings (1) 80% of proceeds from a transaction for
Jounce’s programs within two years of merger close; 10-year tail on payments (2) 100% of the potential aggregate value of certain specified potential cost savings, including: o Any reduction in the amount of the expected lease obligation o Any
increase in net working capital • Concurrent with the merger, Jounce announced an 84% RIF (compared to a 57% RIF with the Redx merger) and restructuring costs of $6.5M • Jounce’s board also recommended not moving forward with the
Redx merger, causing Redx shares to decline 11% TRADING PERFORMANCE PERFORMANCE RELATIVE TO CASH PER SHARE Share TANGCAPITALMANAGEMENT Price 03/27: Announced $3.00 acquisition by Concentra Merger (2/23) + Offer (3/14): Merger (3/27): (1) Current Net
Cash Per Share: $3.01 57% RIF $1.80 + CVR $1.85 + CVRs $2.50 T-0 +1 Day T-0 +1 Day T-0 +1 Day (2) 03/14: Announced Est. Net Cash at Close Per Share: $2.16 Concentra’s offer to acquire $2.00 Jounce Share Price $0.99 $1.10 $1.06 $1.49 $1.51
$1.83 (3) Merger Min. Net Cash Per Share: $2.07 Change 11% 41% 21% $1.50 Share Price as a % of Net Cash Share Price as % of: $1.00 (1) Latest Net Cash 33% 37% 35% 50% 50% 61% (2) Est. Net Cash at Close 46% 51% 49% 69% 70% 85% $0.50 02/23: Announced
merger 03/06: Tang Capital acquired (3) with Redx a 9.7% stake in Jounce Merger Min. Net Cash 48% 53% 51% 72% 73% 88% $0.00 1/3 1/10 1/17 1/24 1/31 2/7 2/14 2/21 2/28 3/7 3/14 3/21 (1): Net cash per share of $3.01, assuming $160M last reported net
cash and 53.1 fully diluted shares outstanding using treasury stock methodology. (2): Net cash per share of $2.16, assuming $115M net cash at close and 53.1 fully diluted shares outstanding using treasury stock methodology. Confidential 7 (3): Net
cash per share of $2.07, assuming $110M net cash at close and 53.1 fully diluted shares outstanding using treasury stock methodology.

PROJECT PACIFIC ILLUSTRATIVE POTENTIAL MERGER CANDIDATES Oncology
Autoimmune & Inflammation (1) (1) (1) Vida Ventures (1) (1) (1) Portfolio Company (2) (1): Public company. (2): Highest stage of development not disclosed. Inbound Interest Source: Company websites. Confidential 8 Preclinical Phase 1 & 1/2
Phase 2 & 3

PROJECT PACIFIC ILLUSTRATIVE POTENTIAL MERGER CANDIDATES (CONT.)
Cardiology / X (1) (2) Cardio- CNS Anti-Viral Genetic Medicines Rare Diseases Others metabolic (4) (3) (4) (4) Project Nuage (5) (1): Excludes oncology focused companies. (2): Other therapeutics areas includes endocrinology, gastrointestinal
diseases, HCS therapies, hepatology, ophthalmology, pulmonology, xenotransplantation, companies with multiple therapeutic areas as well as a healthcare information technology platform. (3): Commercial-stage. (4): Public company. (5): Highest stage
of development not disclosed. Inbound Interest Source: Company websites. Confidential 9 Preclinical Phase 1 & 1/2 Phase 2 & 3 or Later

PROJECT PACIFIC ILLUSTRATIVE PRIVATE COMPANY MERGER PROCESS TIMELINE
THROUGH ANNOUNCEMENT MONTH APRIL MAY JUNE JULY WEEK OF 10 17 24 1 8 15 22 29 5 12 19 26 3 10 17 24 Weekly reoccurring meeting to track process Private company target selection Formally initiate outreach to selected parties Send process letter and
receive initial proposals Evaluate proposals and advance parties to further diligence Due diligence Management presentations Receipt of final proposals Negotiate final terms and definitive documentation If Nasdaq agrees no shareholder approval is
required, the merger can close shortly after announcement. Confidential 10

PROJECT PACIFIC Disclosures This information (including, but not
limited to, prices, quotes and statistics) has been obtained from sources that we believe reliable, but we do not represent that it is accurate or complete and it should not be relied upon as such. All information is subject to change without
notice. The information is intended for Institutional Use Only and is not an offer to sell or a solicitation to buy any product to which this information relates. SVB Securities LLC (“Firm”), its officers, directors, employees,
proprietary accounts and affiliates may have a position, long or short, in the securities referred to in this report, and/or other related securities, and from time to time may increase or decrease the position or express a view that is contrary to
that contained in this report. The Firm's research analysts, salespeople, traders and other professionals may provide oral or written market commentary or trading strategies that are contrary to opinions expressed in this report. The Firm's asset
management group and proprietary accounts may make investment decisions that are inconsistent with the opinions expressed in this document. The past performance of securities does not guarantee or predict future performance. Transaction strategies
described herein may not be suitable for all investors. This document may not be reproduced or circulated without SVB Securities’ written authority. Additional information is available upon request by contacting the Editorial Department, SVB
Securities LLC, 53 State Street, 40th Floor, Boston, MA 02109. Like all Firm employees, research analysts receive compensation that is impacted by, among other factors, overall firm profitability, which includes revenues from, among other business
units, Institutional Equities, Research, and Investment Banking. Research analysts, however, are not compensated for a specific investment banking services transaction. To the extent SVB Securities' research reports are referenced in this material,
they are either attached hereto or information about these companies, including prices, rating, market making status, price charts, compensation disclosures, Analyst Certifications, etc. is available on
https://svbsecurities.bluematrix.com/bluematrix/Disclosure2. SVB MEDACorp LLC (MEDACorp), an affiliate of SVB Securities LLC, is a global network of independent healthcare professionals (Key Opinion Leaders and consultants) providing industry and
market insights to SVB Securities and its clients. © 2023 SVB Securities LLC. All Rights Reserved. Member FINRA/SIPC. SVB Securities LLC is a member of SVB Financial Group. Confidential

Exhibit (c)(11) PROJECT PACIFIC PROPOSED HIGH PRIORITY PRIVATE COMPANY
TARGETS MAY 2023 Confidential

PROJECT PACIFIC ANHEART THERAPEUTICS INC. Business Description
Management • Clinical-stage global biopharmaceutical company developing Name Title novel precision oncology therapeutics Junyuan Jerry Wang, • Operating in the U.S. and China Chief Executive Officer & Co-Founder Ph.D. • Lead
program, taletrectinib, is ROS1 inhibitor currently in Bing Yan, M.D. Co-Founder & Chief Medical Officer Phase 2 trials for ROS1 fusion-positive NSCLC • Second program, AB-218, is a mIDH1 inhibitor in Phase 2 Lihua Zheng, Ph.D. Co-Founder
& Chief Strategy Officer Headquarters: New York, NY trials for multiple solid tumors with mIDH1 mutations Shuanglian Lian Li, SVP & Chief Medical Officer (US) • Pipeline also includes AB-329, an AXL inhibitor in Phase 1 M.D., Ph.D.
Date of Last Raise: 12/14/2021 combination studies for NSCLC or other solid tumors Edward Lang Jr. Chief Business Officer Amount Raised: $60.9M Company Highlights Taletrectinib Overview • Taletrectinib in-licensed from Daiichi Sankyo in
worldwide • Next-generation, CNS-active ROS1 tyrosine kinase inhibitor exclusive agreement in 2018 (TKI) with selectivity over TRKB • Co-development and co-commercialization agreement with • Ongoing global pivotal Phase 2 trial for
ROS1 TKI-naïve and Innovent in Greater China for $189 million upfront fees and TKI-pretreated patients with ROS1 fusion-positive NSCLC Select Key Investors milestones plus tiered royalties • TRUST-1 Phase 2 trial in China showed
proof-of-concept in • Development and commercialization agreement with NewG NSCLC patients with ~1.5-year follow-up time Lab in Korea for $7 million milestone payments and double- ‒ cORR of 92.5% (62/67) in ROS1 TKI-naïve patients
and digit royalties and 52.6% (20/38) in crizotinib-pretreated patients • Breakthrough Therapy Designation granted in both the U.S. ‒ Median PFS of 33.2 months in ROS1 TKI-naïve patients and China for ROS1+ NSCLC and 11.8 months in
crizotinib-pretreated patients ‒ Well-tolerable with low incidence of neurological AEs Fuzhou Investment Recent News Product Pipeline Early Late Clinical / • April 3, 2023: Appointed Edward Lang, Jr. as Chief Business Drug Indication
Preclinical Clinical Pivotal Trial Officer 1L ROS1 fusion- • March 31, 2023: Presented updated efficacy and safety data Taletrectinib / positive NSCLC AB-106 from taletrectinib Phase 2 study in patients with ROS1+ 2L ROS1 fusion- (ROS1 TKI)
NSCLC that continued to demonstrate clinically meaningful positive NSCLC efficacy outcomes 1L & 2L lower grade Safusidenib / glioma • February 2, 2023: Announced collaboration with Guardant AB-218 (mIDH1 Cholangiocarcinoma Health to
develop Guardant360 CDx and Guardant360 inhibitor) Other solid tumors TissueNext as companion diagnostics for taletrectinib in PD1 combo NSCLC advanced or metastatic ROS1+ NSCLC AB-329 Chemo combo for (AXL inhibitor) solid tumors Undisclosed
Undisclosed Assets Source: Company website, press releases, Pitchbook. Confidential 1

PROJECT PACIFIC ASHER BIOTHERAPEUTICS, INC. Business Description
Management • Biotechnology company developing cis-targeted Name Title immunotherapies for cancer, chronic viral infections, and Craig Gibbs, Ph.D. Chief Executive Officer autoimmune disorders • Cis-targeting platform improves selectivity
and overcomes Ivana Djuretic, Ph.D. Founder, Chief Scientific Officer pleiotropy seen in traditional targeted immunotherapies Kyle Elrod Chief Operating Officer • Cis-targeted candidates consist of an antibody connected to a Andy Yeung, Ph.D.
Founder, Chief Technology Officer Headquarters: S. San Francisco, CA modified immunomodulatory protein, such as a cytokine, Andrea Pirzkall, M.D. Chief Medical Officer designed to only activate specific immune cells with clinically Date of Last
Raise: 09/01/2021 validated function Don O’Sullivan, Ph.D Chief Business Officer Amount Raised: $108.0M – Engineered antibody directs binding with high specificity to immune cell of interest AB248 Overview – Attenuated
immunomodulatory protein avoids activation of off-target immune cells • Engineered IL-2 immunotherapy designed to specifically target CD8+ effector T cells, overcoming native IL-2’s side effects seen with clinical use Company Highlights
• Demonstrated superior selectivity and efficacy in multiple Select Key Investors • Lead program, AB248, has demonstrated preclinical PoC for preclinical models compared to other cytokine therapeutics cis-targeting platform • Dosed
first patient in Phase 1a/1b trial in January 2023 for the • Preclinical studies of AB248 murine surrogate show: treatment of locally advanced or metastatic solid tumors – Therapeutic profile differentiated from first- and second-
• Initial clinical data expected in 2H 2023 generation (non-alpha) IL-2-based therapies – Improved anti-tumor activity and tolerability Product Pipeline – Strong antitumor activity as mono- and combination- Candidate Indication
Discovery IND Enabling Phase 1 therapy AB248 (CD8+ T • Highly modular platform allows for efficient generation of new cell cis-targeted IL- Oncology 2 2) product candidates by reusing the already optimized AB821 (CD8+ T components of existing
candidates cell cis-targeted IL- Oncology 21) CAR-T (cis- Oncology targeted IL-2) Recent News CAR-T (cis- Oncology targeted IL-21) • January 17, 2023: Announced dosing of first patient in Phase Myeloid (targeted 1a/1b clinical trial of AB248,
a cis-targeted il-2 immunotherapy Oncology immune agonist) product candidate, for the treatment of locally advanced or AB359 (CD8+ T Chronic viral metastatic solid tumors cell cis-targeted IL- infections 2) • January 5, 2023: Appointed
independent director Elaine Sun Treg (cis-targeted Autoimmune to Board of Directors cytokine) disease Source: Company website, press releases, Pitchbook. Confidential 2

PROJECT PACIFIC BOUNDLESS BIO, INC. Business Description Management
• Next-generation precision oncology company advancing four Name Title first-in-class or best-in-class programs targeting Zachary Hornby President, Chief Executive Officer extrachromosomal DNA (ecDNA) in aggressive cancers Neil Abdollahian
Chief Business Officer • Company’s Spyglass platform combines proprietary ecDNA model systems with bespoke analytical tools to enable the Chris Hassig, Ph.D. Chief Scientific Officer interrogation of ecDNA cancer Klaus Wagner, M.D.,
Headquarters: San Diego, CA Chief Medical Officer • Developing proprietary software system, ecDNA Harboring Ph.D. Oncogenes (ECHO™), for detecting presence of ecDNA Date of Last Raise: 04/28/2021 Jessica Oien General Counsel oncogene,
which is the first portable precision medicine tool for Amount Raised: $105.0M identifying ecDNA oncogenes and built to be deployable at David Hinkle Vice President, Finance & Controller clinical trial sties or commercial labs • Initial
therapeutic programs will pursue specific solid tumor types where ecDNA are highly prevalent Spyglass Platform Overview • Consists of a comprehensive suite of proprietary ecDNA+/- Company Highlights models across tumor types and oncogene
amplifications Select Key Investors • Company is developing the first ever ecDNA-directed • Enables characterization of ecDNA in cancer cells and provides therapeutics (ecDTx) for ecDNA-driven cancers a synthetic lethality-based approach
to targeting ecDNA-bearing cancers • ecDNA are circles of DNA outside the chromosomes but still within the nucleus of a cell and can be rapidly replicated within • Preclinical results demonstrate ecDNA-enabled colorectal the cell,
causing high numbers of oncogene copies cancer cells have intrinsically elevated levels of replication stress and are hypersensitive to replication stress inducing - ecDNA frequently harbor oncogene amplifications and agents promote resistance by
enhancing genomic diversity, thereby enabling cancer cells to rapidly adapt under • Platform will be essential in understanding how ecDNA drives therapeutic pressure cancer progression and therapeutic resistance - More than half of all high
copy number amplifications in cancer occur on ecDNA Product Pipeline - Company developed directed-assembly techniques using short-read sequencing data to reconstruct ecDNA ecDNA Target Lead IND- Drug Discovery Phase 1/2 Validation Optimization
Enabling - Addressing 400k+ patients in the US with oncogene amplified cancers each year BBI-355 CHK1 BBI-825 Undisclosed Recent News ecDTx 3 Undisclosed • May 9, 2023: Announced first patient dosed in first-in-human ecDTx 4 Undisclosed Phase
1/2 clinical trial of BBI-355 in patients with solid tumors Diagnostic candidate ECHO Undisclosed harboring oncogene amplification • April 17, 2023: Presented data on the novel discovery of CHK1 as an ecDNA essential target in oncogene
amplified cancers at AACR 2023 Source: Company website, press releases, Pitchbook. Confidential 3

PROJECT PACIFIC DTX PHARMA, INC. Business Description Management
• Clinical-stage biotechnology company leveraging RNA-based Name Title therapeutics to treat genetic conditions Arthur T. Suckow, Ph.D. Chief Executive Officer • Initially applying FALCON platform technology to peripheral nervous system,
muscular and CNS disorders Bryan Laffitte, Ph.D. Chief Scientific Officer • R&D strategy is to select targets underlying the genetic cause Peter Condon Chief Business Officer Headquarters: San Diego, CA of the disease and engineer FALCON
siRNAs to repress the disease-causing gene Charles Allerson, Ph.D. SVP of Chemistry Date of Last Raise: 03/01/2021 • Lead program, DTX-1252, is a first-in-class FALCON siRNA Alan Joelson VP, Finance and Operations Amount Raised: $115.0M
therapeutic for Charcot-Marie-Tooth Disease Type 1A (CMT1A) Company Highlights DTX-1252 Overview • FALCON (Fatty Acid Ligand Conjugated OligoNucleotides) • First-in-class FALCON siRNA therapeutic platform conjugates combinations of fatty
acids to siRNAs to • In development for CMT1A, the most common inherited improve cellular uptake and biodistribution Select Key Investors neuromuscular disease affecting 150,000 patients in the US • Fatty acids improve biodistribution in
marketed diabetes drugs and EU such as insulin detemir, degludec, liraglutide and semaglutide • Reversed CMT1A in a mouse model by repressing PMP22 • Small size of fatty acids relative to siRNA keeps doses low • Induced
remyelination of axons to normal levels and increased relative to other delivery mechanisms muscle mass, grip strength, coordination and agility • FALCON siRNAs silence disease-causing genes in tissues • Progressing to clinical
development in 2023 beyond the liver to address disease throughout the body Product Pipeline Recent News • June 28, 2022: Appointed Peter Condon as Chief Business Drug Indication Discovery Preclinical Phase 1 Phase 2 Officer DTX-1252 CMT1A
• May 12, 2022: Announced appointment of Kathie M. Bishop to Muscle ND Board of Directors Disorder • March 1, 2021: Completed $100 million Series B financing to Rare CNS ND Disease advance its FALCON platform and pipeline of RNA
therapeutics ND Other Tissues Source: Company website, press releases, Pitchbook. Confidential 4

PROJECT PACIFIC HUMAN IMMUNOLOGY BIOSCIENCES, INC. Business
Description Management • Clinical-stage biopharmaceutical company leveraging human Name Title genetic and immunological insights to deliver targeted Travis Murdoch, M.D. Chief Executive Officer therapies to patients with severe immune-mediated
diseases Matthew Albert, M.D., • Programs aim to target, modulate or deplete cellular drivers of Chief Translational Officer Ph.D. immune-mediated diseases Carl Henrik Enell Chief Operating Officer • Potential to expand programs into
other indications sharing the Headquarters: S. San Francisco, CA same cellular drivers Uptal Patel, M.D. Chief Medical Officer Date of Last Raise: 11/01/2022 • Cellular approach includes plasma cells, neutrophils and mast cells Amount Raised:
$120.0M Felzartamab Overview Company Highlights • Human mAb directed against CD38 • Obtained exclusive worldwide rights, except for Greater China, • Phase 1b/2a POC trial (n=31) in primary membranous for felzartamab, and rights in
Greater China and South Korea nephropathy (PMN) showed for HIB210 (formerly MOR210) from MorphoSys in 2022 - Dose-dependent reduction in pathogenic antibody levels • MorphoSys received 15% equity in HIBio, $15 million upfront •
Proteinuria remission observed across patient groups, Select Key Investors cash payment for HIB210, and up to $1 billion in milestones including those starting with high aPLA2R titers or across both programs plus single- to low double-digit
royalties refractory to prior immunosuppressive therapies • Launched in November 2022 with $120 million financing • Median reductions of 45% in aPLA2R titers were • Incubated by ARCH Venture Partners and Monograph Capital observed
in most patients as early as one week, with deep responses (>50% reduction) in most patients at six months • Well tolerated with mild to moderate TEAEs Recent News • Currently being evaluated in Phase 2 trials in PMN, • April
18, 2023: Appointed Uptal Patel, M.D. as Chief Medical Immunoglobulin A Nephropathy and for antibody mediated Officer and Sunil Agarwal, M.D. as Board Member rejection (AMR) of renal allograft transplants • April 11, 2023: Announced positive
Phase 2 data on felzartamab for the treatment of primary membranous Product Pipeline nephropathy • November 1, 2022: Launched with $120 Million financing Drug Indication Preclinical Phase 1 Phase 2 Primary membranous • June 14, 2022:
Entered into equity participation and license nephropathy agreements with MorphoSys for felzartamab and MOR210 Felzartamab IgA Nephropathy (CD38) Antibody-mediated transplant rejection HIB210 Undisclosed (C5aR1) Mast cell Undisclosed program Source:
Company website, press releases, Pitchbook. Confidential 5

t PROJECT PACIFIC IMMPACT BIO, INC. Business Description Management
• Clinical-stage biotechnology company dedicated to the Name Title discovery of transformative chimeric antigen receptor (CAR) T- Sumant Ramachandra, M.D., Ph.D. President & CEO cell therapies for various cancers where other treatments
have been exhausted Vikram Lamba Chief Financial Officer • Next generation bispecific CAR T-cell technologies precisely Jonathan Benjamin, M.D, Ph.D. Chief Medical Officer distinguish cancer cells from normal cells, thereby eliminating
Headquarters: West Hills, CA the severe side effects endured by cancer patients Jim Johnston, Ph.D. Chief Scientific Officer Date of Last Raise: 01/20/2022 Venkat Yepuri Chief Operating Officer Amount Raised: $111.0M Sylvain Roy Chief Technical
Officer Company Highlights CD19-CD20 Bispecific CAR-T Overview • The company’s logic-gate-based CAR-T platforms address key • The company’s lead program is a CD19-CD20 bispecific ‘OR- biological challenges in treating
cancer and are specifically gate’ CAR-T designed to address antigen escape, a key designed to prevent antigen escape, prevent ‘on-target – off- challenge for current approved CD19 therapies Select Key Investors tumor’
toxicities, and overcome the immunosuppressive tumor • Updated data from the ongoing Phase 1 trial of patients with microenvironment relapsed or refractory B-cell non-Hodgkin’s lymphoma • OR-gated bispecific CAR-T is based on the
work of pioneering demonstrated a total of seven of eight patients achieved and oncology and immunology scientists from UCLA and the remain in complete remission (12 months median follow-up) MIGAL-Galilee Research Institute • Ongoing
investigational study (UCLA) demonstrated potential best-in-class safety and efficacy data from 10 patients who had received 3 prior lines of treatment - Demonstrated 90% ORR and 70% CR Recent News - Observed limited toxicity (no ICANS/neurotoxicity
and no CRS above grade 1) • February 8, 2023: Appointed Biren Amin to its Board • January 24, 2023: Announced FDA clearance of IND for novel bispecific CAR to treat aggressive B-cell lymphoma Product Pipeline • September 22, 2022:
Appointed Jonathan Benjamin, M.D., Ph.D. as Chief Medical Officer Lead IND Drug Indication Phase 1/1b • August 10, 2022: Appointed Vikram Lamba as Chief Financial Selection Enabling Officer and Head of Business Development CD19-CD20 NHL &
CLL Bispecific CAR-T • May 9, 2022: Appointed Sylvain Roy as Chief Technical Officer LOH Bispecific Lung Cancer CAR-T • April 19, 2022: Appointed Venkat Yepuri as Chief Operating Officer TGF-β x ND Glioblastoma CAR-T Bispecific
Source: Company website, press releases, Pitchbook. Confidential 6

PROJECT PACIFIC MBX BIOSCIENCES, INC. Business Description Management
• Biotechnology company focused on discovery, development, Name Title and commercialization of transformative peptide therapeutics Kent Hawryluk President & Chief Executive Officer • Founded by seasoned industry veterans with
expertise in endocrine drug development, including the development and Mary Jane Geiger, M.D., Chief Medical Officer commercialization of first-in-class endocrine therapeutics such Ph.D. as Forteo® and Humalog® Headquarters: Carmel, IN
Richard DiMarchi, Ph.D. Chief Scientific Officer • Advancing a pipeline of candidates with clinically validated targets, defined regulatory pathways and unmet medical needs Date of Last Raise: 11/14/2022 Richard Bartram Chief Financial Officer
in a variety of endocrine disorders Amount Raised: $115.0M Company Highlights MBX 2109 Overview • World-class technology platform of Precision Endocrine • MBX 2109 is an investigational parathyroid hormone (PTH) Peptides (PEPs),
innovative peptide hormone analogs peptide prodrug in development as a long-acting hormone engineered to have optimized pharmaceutical properties replacement therapy for hypoparathyroidism • Advancing a pipeline of PEPs that have been
selectively • Under physiological conditions, MBX 2109 (prodrug) is Select Key Investors modified using state-of-the-art and proprietary chemical designed to release the active PTH peptide, referred to as modifications to enhance the stability
and pharmacokinetics MBX 2099, at a precisely controlled rate compared to the native peptide hormone • Both MBX 2109 (prodrug) and MBX 2099 (active peptide) are • Aim to extend the duration of action of the PEP thereby designed to have
extended half-lives with the potential to necessitating few administrations enable once-weekly administration • Currently being evaluated in healthy subjects in a Phase 1 trial Product Pipeline Recent News • November 14, 2022: Announced
closing of $115 million Drug Indication Discovery Preclinical Phase 1 Series B financing led by Wellington Management • October 5, 2022: Announced advancements in phase 1 MBX 2109 Hypoparathyroidism clinical trial of MBX 2109 • July 26,
2022: Received Orphan Drug Designation for MBX Post-bariatric 2109 for the treatment of hypoparathyroidism MBX 1416 hypoglycemia (PBH) • April 11, 2022: Appointed Richard Bartram as Chief Financial Officer Additional Lead Undisclosed
Candidates Source: Company website, press releases, Pitchbook. Confidential 7

PROJECT PACIFIC PIPELINE THERAPEUTICS, INC. Business Description
Management • Clinical-stage biotechnology company focused on the Name Title development of small molecules designed to reactivate innate Carmine Stengone President and Chief Executive Officer repair pathways in order to restore function for
the treatment of neurodegenerative diseases. Daniel Lorrain, Ph.D. Chief Scientific Officer • Advancing a pipeline of programs to address CNS and neuro- otology conditions, including its lead clinical candidate, PIPE- Austin Chen, Ph.D. SVP,
Head of Research Headquarters: San Diego, CA 307, which is currently in development for the treatment of Peter Slover Chief Financial Officer multiple sclerosis (MS). Date of Last Raise: 4/17/23 Chief Medical Officer and SVP, Stephen Huhn, M.D.
Amount Raised: $50.0M Clinical Development Company Highlights • Currently no approved medicines that support myelin PIPE-307 Overview restoration, which represents a significant unmet medical need in the treatment of MS as it allows for the
repair of neuronal • Proprietary, oral, selective antagonist of muscarinic M1 damage. receptor in development for the treatment of MS. • In April 2023, announced global license and development • Completed multiple Phase 1 studies
in healthy volunteers: agreement with Janssen Pharmaceutica of Johnson & Select Key Investors – SAD/MAD study demonstrated PIPE-307 had PK data Johnson. consistent with preclinical modeling and was well tolerated - Janssen granted
exclusive license to research, develop across all dose cohorts and commercialize PIPE-307 in all indications – PET study to determine M1 receptor occupancy and - Pipeline retains ability to develop PIPE-307 for relapsing- clearance in the
brain after a single dose showed that remitting multiple sclerosis (RRMS) doses tested in the Phase 1 study achieved a level of - Pipeline received $50M upfront cash payment and $25M uptake in the human brain that have been associated with equity
investment from Johnson & Johnson Innovation remyelination observed in preclinical studies. - Pipeline eligible for ~$1B in clinical, regulatory and • Phase 2 trial in RRMS expected to initiate in 2H 2023 commercial milestones, as well as
tiered double-digit royalty payments - Additionally, Pipeline also received $25M equity Product Pipeline investment from Pipeline’s existing investors IND- • Also developing PIPE-791, a LPA1 receptor antagonist in IND- Drug Indication
Discovery Phase 1 Enabling enabling studies to evaluate its ability to promote myelin PIPE-307 restoration and treat neuroinflammation. (M1R Myelin Restoration antagonist) Recent News PIPE-791 Myelin Restoration, (LPA1R Neuroinflammation antagonist)
• April 17, 2023: Announced global license and development agreement for PIPE-307 with Janssen Discovery Axonal Repair • March 28, 2022: Announced receipt of FDA IND clearance to initiate a Phase 1b/2a study of PIPE-307 in patients with
relapsing-remitting MS Source: Company website, press releases, Pitchbook. Confidential 8

PROJECT PACIFIC Q32 BIO INC. Business Description Management •
Clinical-stage company pioneering a new approach to treating Name Title complement-mediated diseases Jodie Morrison Acting Chief Executive Officer • Lead candidate, ADX-097, is a tissue targeted CD3 antibody Shelia Violette, Ph.D. Chief
Scientific Officer fH1-5 fusion protein for the treatment of complement alternative pathway driven disease Jason Campagna, Chief Medical Officer M.D., Ph.D. • Most advanced program, ADX-914, is a potent IL-7Rα Headquarters: Waltham, MA
antagonist with the potential to be a best-in-class modulator of Adam Cutler Chief Financial Officer IL-7 signaling that is being developed in collaboration with Date of Last Raise: 10/29/2020 Horizon Therapeutics Amount Raised: $60.0M ADX-097
Overview Company Highlights • A bifunctional fusion protein containing two moieties of the first five consensus repeats of human factor H (fH1-5) linked to a • ADX-914 is currently being evaluated in a Phase 2 trial for humanized
anti-C3d antibody atopic dermatitis (AD) with an additional Phase 2 trial, in • Designed to target diseased tissue via binding to C3d, which is another indication, to be initiated in 2023 deposited at sites of complement activation, and
provide • Under the Horizon agreement, Horizon will fund development localized blockade Select Key Investors through the two Phase 2 trials of ADX-914 with Q32 being • ADX-097 may potently and durably block complement activity in
operationally responsible humans, while preserving the body’s ability to fight infection - Horizon has an option to acquire the program – exercisable effectively, thereby avoiding the potentially damaging effects of through a period
following the completion of the Phase 2 systemic complement inhibition trials • Preclinical models showed potent, durable, and efficacious local - Q32 received $55mm in upfront payment and, if the option alternative pathway complement blockade
at low doses that is exercised, is eligible to receipt up to an additional avoid systemic complement inhibition $645mm in closing and milestone payments plus tiered • Currently conducting a first-in-human, Phase 1, ascending dose royalties
(SAD/MAD) clinical study of ADX-097 for the treatment of complement disorders Recent News • October 27, 2022: Announced dosing of first patient in Phase 2 Product Pipeline trial of ADX-914 of atopic dermatitis with Horizon Therapeutics Drug
Indication Discovery Preclinical Phase 1 Phase 2 • September 20, 2022: Appointed Jodie Morrison, a Venture Complement Platform Partner at Atlas Venture, as Board of Director and acting Chief Executive Officer ADX-097 Complement • August
15, 2022: Entered into a collaboration and option mAb-CR1a disorders agreement with Horizon to develop ADX-914 for the treatment fAb-fH/CR1 of autoimmune diseases Partnered Program • May 26, 2022: Initiated first-in-human Phase 1 trial of
ADX-097 for the treatment of complement disorders ADX-914 AD Source: Company website, press releases, Pitchbook. Confidential 9

PROJECT PACIFIC Disclosures This information (including, but not
limited to, prices, quotes and statistics) has been obtained from sources that we believe reliable, but we do not represent that it is accurate or complete and it should not be relied upon as such. All information is subject to change without
notice. The information is intended for Institutional Use Only and is not an offer to sell or a solicitation to buy any product to which this information relates. SVB Securities LLC (“Firm”), its officers, directors, employees,
proprietary accounts and affiliates may have a position, long or short, in the securities referred to in this report, and/or other related securities, and from time to time may increase or decrease the position or express a view that is contrary to
that contained in this report. The Firm's research analysts, salespeople, traders and other professionals may provide oral or written market commentary or trading strategies that are contrary to opinions expressed in this report. The Firm's asset
management group and proprietary accounts may make investment decisions that are inconsistent with the opinions expressed in this document. The past performance of securities does not guarantee or predict future performance. Transaction strategies
described herein may not be suitable for all investors. This document may not be reproduced or circulated without SVB Securities’ written authority. Additional information is available upon request by contacting the Editorial Department, SVB
Securities LLC, 53 State Street, 40th Floor, Boston, MA 02109. Like all Firm employees, research analysts receive compensation that is impacted by, among other factors, overall firm profitability, which includes revenues from, among other business
units, Institutional Equities, Research, and Investment Banking. Research analysts, however, are not compensated for a specific investment banking services transaction. To the extent SVB Securities' research reports are referenced in this material,
they are either attached hereto or information about these companies, including prices, rating, market making status, price charts, compensation disclosures, Analyst Certifications, etc. is available on
https://svbsecurities.bluematrix.com/bluematrix/Disclosure2. SVB MEDACorp LLC (MEDACorp), an affiliate of SVB Securities LLC, is a global network of independent healthcare professionals (Key Opinion Leaders and consultants) providing industry and
market insights to SVB Securities and its clients. © 2023 SVB Securities LLC. All Rights Reserved. Member FINRA/SIPC. SVB Securities LLC is a member of SVB Financial Group. Confidential

Exhibit (c)(12) PROJECT PACIFIC STRATEGIC PROCESS UPDATE MAY 24, 2023
Confidential
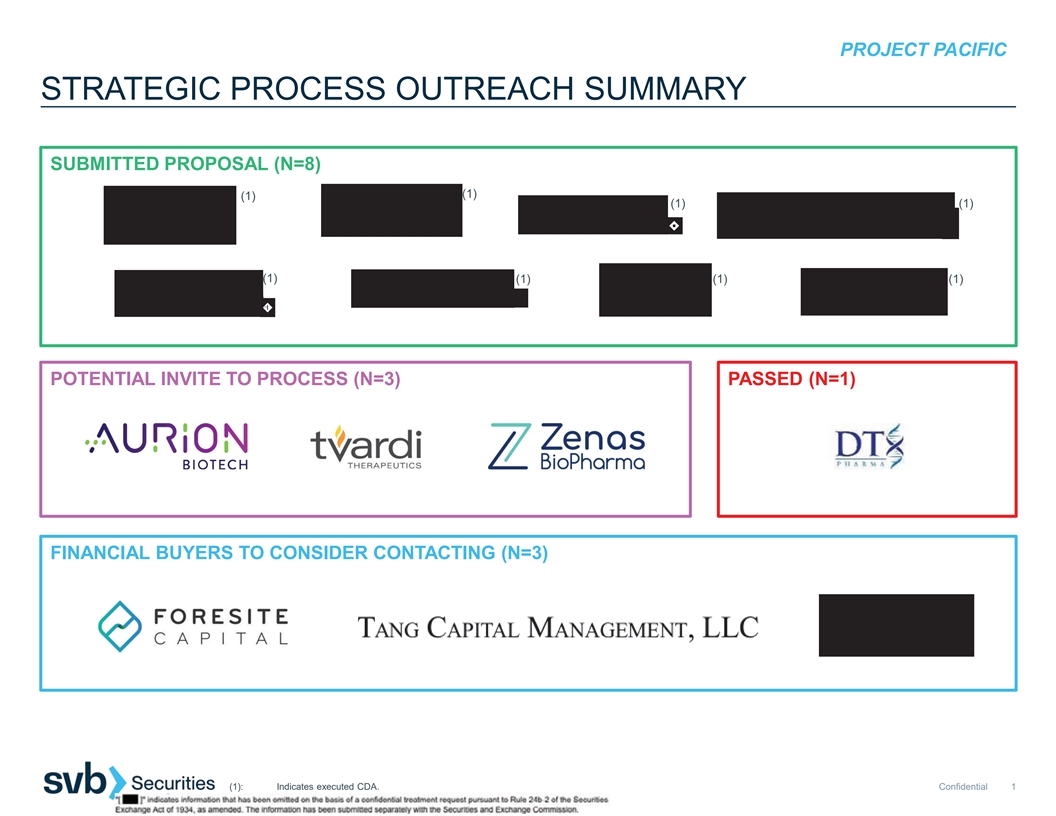
PROJECT PACIFIC STRATEGIC PROCESS OUTREACH SUMMARY SUBMITTED PROPOSAL
(N=8) (1) (1) (1) (1) (1) (1) (1) (1) POTENTIAL INVITE TO PROCESS (N=3) PASSED (N=1) FINANCIAL BUYERS TO CONSIDER CONTACTING (N=3) (1): Indicates executed CDA. Confidential 1

PROJECT PACIFIC HOW COUNTERPARTIES ARE VALUING PACIFIC AND THEMSELVES
$ IN USD MILLIONS. SORTED ALPHABETICALLY. PACIFIC VALUATION COUNTERPARTY PF OWNERSHIP ASSETS / PRO FORMA TARGET PIPE PARTY CASH LISTING TECHNOLOGY TOTAL VALUATION STEP-UP CASH RUNWAY (INSIDER COMMITMENT) PACIFIC PARTY PIPE (1) (2) $40 $5 - $45 $155
ND Through 2024 - 22.5% 77.5% - (3) (4) 140 10 - 150 250 1.25x Through 2024 $40 (100%) 34.1% 56.8% 9.1% Through 1H 140 5 - 145 357 1.10x - 28.9% 71.1% - 2025 140 - - 140 402-434 1.50-1.62x Into 2H 2026 - 24.4-25.8% 74.2-75.6% - 140 7 - 147 325 1.50x
Into 2026 75-125 (ND) 24.6-26.9% 54.4-59.4% 13.7-20.9% (5) (5) Through early 40 10 - 50 257 1.00x 110 (68%) 12.0% 61.6% 26.4% 2026 (6) 140 15 - 155 310 1.00x Through 2027 - 33.3% 66.7% - (7) 140 15 - 155 250 1.35x Into 2H 2026 - 38.3% 61.7% - (1):
PACIFIC net cash at close in excess of $40M to be returned to pre-transaction PACIFIC shareholders. (2): Pro forma runway includes ~$280M in non-dilutive funding from BARDA. (3): Runway through 2024 requires only $100M. (4): Concurrent PIPE not
required. Insiders and licensing partner have indicated $40M commitment to any potential PIPE. (5): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to PACIFIC stockholders
seeking to exit the transaction. (6): Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money for Series C and valuation for proposed transaction accordingly. (7): Existing Q32 shareholders have a signed a
letter committing at least $30 million for PIPE financing. This funding could be used to extend runway further or potentially return a portion of PACIFIC's cash to its shareholders. Confidential 2

PROJECT PACIFIC COUNTERPARTY CATALYSTS IN PRO FORMA CASH RUNWAY SORTED
ALPHABETICALLY. PF runway: Through 2024 PF runway: Through 2024 PF runway: Through 1H 2025 PF runway: Into 2H 2026 • BARDA contract (~$280M) • Taletrectinib/AB-106 in non-small cell lung • AB248 in solid tumors • BBI-355 in
oncogene amplified cancers – June 2023: Submit proposal cancer – 3Q23: Phase 1a monotherapy PD data – Mid-2024: Phase 1/2 clinical POC data – July-August 2023: Funding signal – 2H23: Global Phase 2 TRUST-II interim
– 2H23: Initiate Phase 1a/b anti-PD1 • BBI-825 in oncogene amplified cancers – September 2023: Contract data combination trial – 4Q23: IND filing • AER003 / AER004 / AER005 – 4Q23: Complete enrollment of global
– Late 2023 / early 2024: initiate Phase 1b – 2Q24: Initiate Phase 1/2 trial – 4Q23: Initiate Phase 1/2 trial Phase 2 TRUST-II monotherapy expansion cohorts – 2H25: Phase 1/2 clinical POC data – 1Q24: Initiate Phase 3
trial – 1H24: Initiate global Phase 3 TRUST-III – June 2024: Phase 1a/b preliminary • ecDTx 3 in oncogene amplified cancers – 4Q24: EUA /=and commercial launch confirmatory study monotherapy activity data – 4Q23: In
vivo POC – 2Q24: Pre-NDA type B meeting with FDA – 2H24 / 1Q25: Phase 1a/b anti-PD1 – 1H24: Development candidate selection (6-month follow up data) combination activity data – 2Q25: IND filing – 3Q24: Global Phase 2
TRUST-II final data • AB821 in solid tumors – 3Q25: Initiate Phase 1/2 trial – 4Q24: NDA submission in the US – 3Q23: GLP tox • ecDTx 4 in oncogene amplified cancers – 2Q25: US NDA approval – 3Q23: GMP
manufacturing and pilot – 1Q24: In vivo POC • Safusidenib/AB-218 in lower grade glioma, batch – 2H24: Development candidate selection cholangiocarcinoma – Q423: IND filing – 4Q25: IND filing – Ongoing: Enrollment
for Phase 2 global – 1Q24: Initiate Phase 1 trial – 1H26: Initiate Phase 1/2 trial studies – 2H24: Preliminary Phase 1 PD data • ECHO Platform – 2H23: IDE filing – 1Q24: CTA filing Note: Pro forma cash runway
includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 3

PROJECT PACIFIC COUNTERPARTY CATALYSTS IN PRO FORMA CASH RUNWAY
(CONT.) SORTED ALPHABETICALLY. PF runway: Into 2026 PF runway: Through early 2026 PF runway: Through 2027 PF runway: Into 2H 2026 • Felzartamab – 2Q23: reproductive tox data • IMPT-314 in ≥3L CAR-T naïve ABCL •
PIPE-307 in r/r MS • ADX-097 in complement-mediated renal • Felzartamab in PMN – 2H23: Phase 1 data – 2H23: Phase 2 POC initiation diseases – 2H23: Interim data from POC study in – 4Q24: Phase 2 pivotal data
– 2Q25: Phase 2 POC study completion – 2024 and 2025: Phase 2 AAV 12-week aCD20-refractory population – 3Q25: Phase 2 pivotal data update • PIPE-307 in other neurological disorders, safety/efficacy and topline data, –
1H24: Phase 3 initiation – 1Q26: BLA submission including MDD respectively • Felzartamab in IgAN – 2Q23: Clinical POC • IMPT-314 in CAR-T experienced ABCL – 2023 and 2024: initiate multiple Phase 2 – 2024 and
2025: Phase 2 renal basket trial • Felzartamab in LN – 1Q24: Phase 1 data POC studies 4-week / biomarker topline data and – 2H23: Phase 1 initiation – 2Q24: EOP1 meeting – 1Q25: Phase 2 POC studies completion
interim12-week data, respectively – 2H24: Clinical POC – 2H24: Phase 2 data update • PIPE-791 – 2H25-1H26: Phase 2/3 trial initiations in – 2024 and 2025: Phase 1b readouts – 1H25: BLA submission – 1H23: IND
filing complement-mediated renal diseases • Felzartamab in AMR • IMPT-314 in 2L HCT unintended ABCL – 1H24: Phase 1 HV study completion • ADX-096 – 1H25: IND submission – 1Q24: Clinical POC – 1Q25: Patient
data – 4Q24: Phase 1 PET study completion • Novel nanobodies – 2024: DC nomination – 1Q24: Phase 2 investigator sponsored trial – 1H26: BLA submission – 2Q25: Chronic tox studies • ADX-914 in atopic
dermatitis readout in AMR • IMPT-314 – 1Q26: patient data in 2L HCT – 3Q25: Biomarker studies completion – 2H23: Receipt of all additional • HIB210 in severe IMDs eligible ABCL – 3Q25: Phase 2a trial initiation
development funding from Horizon – 3Q23: Preclinical data supporting • IMPT-514 in r/r lupus nephritis • Calpain – 2H23: Phase 2 study completion superiority to avacopan – Mid 2023: IND clearance – 4Q25: IND
submission – 2H24: Phase 2 study completion in second – 1H23: Phase 1 trial initiation in healthy – 3Q24: Phase 1 first cohort data – 1Q26: Phase 1 study initiation (undisclosed) indication volunteers – 1Q25: EOP1
meeting – Late 2024 – early 2025: Horizon option – 1Q24: NHP chronic tox study readout – 3Q25: Phase 2 pivotal data exercise window (subject to a significant – 1H24: Subcutaneous formulation – 2Q26: BLA submission
option exercise fee) for the acquisition of developments insights • IMPT-514 in severe systemic lupus all of Q32’s assets related to ADX-914 – 1H24: Phase 2 study initiation in AAV erythematosus with extrarenal disease –
1H24: Phase 1 readout in healthy – 1Q24: IND amendment volunteers – 4Q24: Patient data • Advanced discovery programs – 2023: – 3Q26: BLA submission development candidate nomination • IMPT-514 in ANCA-associated
vasculitis – 2Q24: IND amendment – 1H25: Patient data – 4Q26: BLA submission • IMPT-514 in secondary progressive MS – 3Q24: IND amendment – Mid 2025: Patient data – 4Q26: BLA submission Note: Pro forma cash
runway includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 4

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: AERIUM | ANHEART
Transaction Structure • Reverse triangular merger • Reverse triangular merger (2) • Assumes closing 09/30/23 • Prepared to sign merger agreement and close transaction in 2H 2023 and Timing • AERIUM: $155M (includes $2M
current cash position and ongoing insider-led round which will fund • ANHEART: $250M (includes $5M of cash expected at 09/30/23 and $5M from ANHEART strategic AERIUM through September 2023) partnership) Implied Valuation (1) • PACIFIC:
$45M (based on $40M net cash at close + $5M for Nasdaq listing) • PACIFIC: $150M (based on $140M net cash at close + $10M for Nasdaq listing) Post-$ Last Round / • AERIUM’S post-money from last private round (Series A, 2022): ND
• ANHEART’S post-money from last private round (Series B, September 2021): $200M • Step-up: ND • Step-up: 1.25x Step-up • (Incl. optional $40M PIPE 100% covered by insiders and licensing partner) ANHEART: 56.8% /
Implied Ownership Split • (Excl. PIPE) AERIUM: 77.5% / PACIFIC: 22.5% PACIFIC: 34.1% / PIPE: 9.1% • (Excl. PIPE) ANHEART: 62.5% / PACIFIC: 37.5% • Concurrent PIPE not required • Concurrent PIPE not contemplated •
Insiders and licensing partner have indicated $40M commitment to any potential PIPE • AERIUM pursuing ~$280M BARDA contract to fund development to EUA and launch Concurrent Financing • ANHEART has also explored entering $30M investment
from SDIC at $250M pre-money (not included • AERIUM ongoing insider-led round to fund company through September 2023 in $40M of commitments from insiders and licensing partner) (2) • Pro forma cash (excl. ~$280M BARDA contract): $40M
(AERIUM: $0M expected at 09/30/23 ; (1) PACIFIC: $40M ) • Pro forma cash (incl. optional PIPE): $190M (ANHEART: $10M, including $5M expected at 09/30/23 Pro Forma Cash and (2) • Pro forma cash (incl. ~$280M BARDA contract): $320M
(AERIUM: $0M expected at 09/30/23 ; and $5M from licensing partner; PACIFIC: $140M; PIPE: $40M) Runway (1) PACIFIC: $40M ) • Runway: $100M funds through 2024 • Runway: through 2024 (with BARDA funding) • AER003 (preclinical)
– mAb antiviral treatment against SARS-CoV-2 occupying ACE2 footprint • Taletrectinib/AB-106 (Pivotal Phase 2) – ROS1 inhibitor for ROS1 fusion-positive NSCLC • AER004 (preclinical) – mAb antiviral treatment against
SARS-CoV-2 • Safusidenib/AB-218 (Phase 2) – mIDH1 inhibitor for lower grade glioma, cholangiocarcinoma and • AER005 (preclinical) – mAb antiviral treatment against SARS-CoV-2 binding outside Receptor Binding other tumors Key
Asset(s) Domain • AB-329 (Phase 1) – AXL inhibitor for combination with checkpoint inhibitors or chemotherapy in • Two mAbs planned to be developed in combination; third to be developed as contingency for viral NSCLC and other
solid tumors evolution • 5 undisclosed assets (Preclinical) • Taletrectinib/AB-106 in non-small cell lung cancer • BARDA contract (~$280M) – 2H23: Global Phase 2 TRUST-II interim data – June 2023: Submit proposal
– 4Q23: Complete enrollment of global Phase 2 TRUST-II – July-August 2023: Funding signal – 1H24: Initiate global Phase 3 TRUST-III confirmatory study Timing of Key – September 2023: Contract – 2Q24: Pre-NDA type B
meeting with FDA (6-month follow up data) • AER003 / AER004 / AER005 – 3Q24: Global Phase 2 TRUST-II final data Milestones – 4Q23: Initiate Phase 1/2 trial – 4Q24: NDA submission in the US – 1Q24: Initiate Phase 3 trial
– 2Q25: US NDA approval – 4Q24: EUA /=and commercial launch • Safusidenib/AB-218 in lower grade glioma, cholangiocarcinoma – Ongoing: Enrollment for Phase 2 global studies Plans for PACIFIC’s • Open to discussing
retaining PACIFIC employees for open positions, including CFO, GC and CBO, as • Open to discussions retention of certain personnel, particularly those in the finance and accounting well as PACIFIC’s public company infrastructure and
capabilities functions, during a transition period or full-time following a Transaction Employees • N/A • N/A Exclusivity • Financial: Piper Sandler Advisors • N/A • Legal: WilmerHale • Auditor: Deloitte •
Board Composition: 10 members; 6 designated by ANHEART and 4 designated by PACIFIC • ANHEART does not assume reliance on PACIFIC non-cash assets, facilities or business • Board Composition: 8 board members; 5 designated by AERIUM (one of
which could potentially be • Audit Status: Have completed audits in China for 2021 and 2022; in process of initiating conversion to independent director from PACIFIC board) and 3 designated by PACIFIC US GAAP standards with completion in mid
2023 • AERIUM would consider CVR for development or out licensing of PACIFIC assets Additional Information • Out-licensed Japanese commercial rights for taletrectinib to global pharmaceutical partner. Licensing • Audit Status: ND
will close concurrently with reverse merger; ANHEART to receive $15M in equity and non-dilutive (1) • Proposal allows most PACIFIC cash to be returned to pre-transaction shareholders upfront payments upon closing, $63M in potential milestone
payments, and high double-digit royalties on net sales • Octagon Investments, Decheng Capital, Laurion Capital, Sage Partners, Innovent Biologics and Select Investors • Omega Funds, F-Prime Capital Cenova (1): PACIFIC net cash at close
in excess of $40M to be returned to pre-transaction PACIFIC shareholders. (2): Based on assumption that ongoing insider-led round funds company through September 2023. Note: Bold indicates catalysts that could fall within the transaction window.
Confidential 5

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: ASHER BIO | BOUNDLESS
BIO Transaction Structure • Reverse triangular merger • Reverse triangular merger • Assumes closing of 07/31/23 • Entry into a definitive merger agreement within 30-45 days of being selected to move forward and Timing •
ASHER: $357M (includes $50M cash expected at 07/31/23) • BOUNDLESS: $402-434M (includes $142M of cash expected at 06/30/23) Implied Valuation • PACIFIC: $145M (based on $140M net cash at close + $5M for Nasdaq listing) • PACIFIC:
$140M (based on $140M net cash at close + $0M for Nasdaq listing) Post-$ Last Round / • ASHER’s post-money from last private round (Series B, September 2021): $324M • BOUNDLESS’ post-money from last private round (Series C,
May 2023): $268M • Step-up: 1.10x • Step-up: 1.50x-1.62x Step-up • (Excl. PIPE) ASHER: 71.1% / PACIFIC: 28.9% • (Excl. PIPE) BOUNDLESS: 74.2-75.6% / PACIFIC: 24.4-25.8% Implied Ownership Split Concurrent Financing • Not
required but open to exploring concurrent PIPE • No planned financing concurrent with the transaction • Pro forma cash: $190M (ASHER: $50M expected at 07/31/23; PACIFIC: $140M) Pro Forma Cash and • Pro forma cash: $282M (BOUNDLESS:
$142M expected at 06/30/23; PACIFIC: $140M) • Runway: through 1H 2025 • Runway: into 2H 2026 Runway • $60M of additional capital required to reach YE 2025 • AB248 (Phase 1a/b) – CD8-targeted IL2 for solid tumors •
BBI-355 (Phase 1/2) – oral CHK1 inhibitor for oncogene amplified cancers • AB821 (IND-enabling) – CD8-targeted IL21 for solid tumors • BBI-825 (IND-enabling) – extrachromosomal DNA directed therapies (exDTx) for
oncogene • AB359 (IND-enabling) – CD8-targeted IL2 for chronic viral infection amplified cancers Key Asset(s) • Cell therapy-targeted IL-2 / 21 (Discovery) – for cancer • ecDTx 3 (Discovery) – for oncogene
amplified cancers • Myeloid-targeted immune agonist (Discovery) – for cancer • ecDTx 4 (Discovery) – for oncogene amplified cancers • CD4+ T cell-targeted cytokine (Discovery) – for cancer • ECHO / ecDNA
Harboring Oncogenes (IND-enabling) – patient selection diagnostic assay • AB248 in solid tumors • BBI-355 in oncogene amplified cancers – 3Q23: Phase 1a monotherapy PD data – Mid-2024: Phase 1/2 clinical POC data
– 2H23: Initiate Phase 1a/b anti-PD1 combination trial • ecDTx 4 in oncogene amplified cancers • BBI-825 in oncogene amplified cancers – Late 2023 / early 2024: initiate Phase 1b monotherapy expansion cohorts – 1Q24: In
vivo POC – 4Q23: IND filing – June 2024: Phase 1a/b preliminary monotherapy activity data – 2H24: Development candidate selection – 2Q24: Initiate Phase 1/2 trial Timing of Key – 2H24 / 1Q25: Phase 1a/b anti-PD1
combination activity data – 4Q25: IND filing – 2H25: Phase 1/2 clinical POC data • AB821 in solid tumors – 1H26: Initiate Phase 1/2 trial Milestones • ecDTx 3 in oncogene amplified cancers – 3Q23: GLP tox •
ECHO Platform – 4Q23: In vivo POC – 3Q23: GMP manufacturing and pilot batch – 2H23: IDE filing – 1H24: Development candidate selection – Q423: IND filing – 1Q24: CTA filing – 2Q25: IND filing – 1Q24:
Initiate Phase 1 trial – 3Q25: Initiate Phase 1/2 trial – 2H24: Preliminary Phase 1 PD data Plans for PACIFIC’s • N/A • N/A Employees Exclusivity • N/A • N/A • Legal: Goodwin • Legal: Latham
& Watkins Advisors • Auditor: PWC • Auditor: KPMG • Board Composition: 8-9 board members; 7 designated by ASHER and 1-2 designated by PACIFIC to be mutually agreed upon • Audit Status: Completed 2020/2021 PCAOB audits;
2022 audit expected to be completed July • Board Composition: 10 members; 9 designated by BOUNDLESS and 1 designated by PACIFIC Additional Information 2023 • Audit Status: 5 years of audited financials, including 2022 • Open to
licensing or divestiture of PACIFIC assets to parties with appropriate expertise and capabilities • Leaps by Bayer, RA Capital, Sectoral, Piper Heartland, ARCH Venture, Nextech Invest, Fidelity, • Third Rock Ventures, Wellington, RA
Capital, Invus Public Equities, Boxer Capital, Mission Bay Wellington, Boxer, Redmile, Surveyor, PFM, Vertex Ventures, Logos, City Hill, GT Healthcare Select Investors Capital, Janus Henderson, Logos, Marshall Wace, Y-Combinator Capital, Alexandria
Venture Investments Note: Bold indicates catalysts that could fall within the transaction window. Confidential 6

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: HI-BIO | IMMPACT BIO
Transaction Structure • Reverse triangular merger • Reverse merger; proposing a simultaneous sign and close transaction • Sign definitive agreement and announce Transaction within 60 days of accepted proposal • Intends to
execute a definitive merger agreement within 45 days of signing term sheet and exclusivity agreement and Timing Financial: • HI-BIO: $325M (includes $22M cash at 03/31/23, excludes $30M from additional Series A • IMMPACT BIO: $257M
(includes $65M of cash expected at 06/30/23) closing in May 2023) Implied Valuation (1) • PACIFIC: $50M (based on $40M net cash at close + $10M for Nasdaq listing) • PACIFIC: $147M (based on $140M net cash at close + $7M for Nasdaq
listing) Post-$ Last Round / • HI-BIO’s post-money from last private round (Series A extension, November 2022): $217M • IMMPACT BIO’s post-money from last private round (Series B, January 2022): $257M • Step-up: 1.50x
• Step-up: 1.00x Step-up • (Excl. PIPE) HI-BIO: 68.9% / PACIFIC: 31.1% • (Excl. PIPE and share buyback) IMMPACT BIO: 63.1% / PACIFIC: 36.9% Implied Ownership Split • (Incl. PIPE) HI-BIO: 54.4-59.4% / PACIFIC: 24.6-26.9% /
PIPE: 13.7-20.9% • (Incl. PIPE and share buyback) IMMPACT BIO: 61.6% / PACIFIC: 12.0% / PIPE: 26.4% • $110M concurrent PIPE with support from existing investors for over $75M (term sheet signed with anchor investor); • $75-125
concurrent financing 30 additional investors in data room Concurrent Financing • Ongoing conversations with both new and existing investors regarding a potential financing • Concurrent share buyback of up to $100M • Pro forma cash
(incl. PIPE): $267-317M (HI-BIO: $52M, including ~$30M from the additional Pro Forma Cash and • Pro forma cash: $215M (IMMPACT: $65M; PACIFIC: $140M; PIPE: $110M; Share repurchase of $100M) Series A closing in May 2023; PACIFIC: $140M; PIPE:
$75-125M) • Runway: through early 2026 Runway • Runway: into 2026 • Felzartamab (Phase 2) – anti-CD38 antibody for primary membranous nephropathy (PMN), • IMPT-314 (Ph. 1) – CD19/CD20 bispecific CAR T cell therapy
for aggressive B-cell lymphoma (ABCL) immunoglobulin A nephropathy (IgAN), antibody-mediated rejection (AMR) and lupus • IMPT-514 (IND-enabling) – CD19/CD20 bispecific CAR T cell therapy for autoimmune diseases nephritis (LN) Key
Asset(s) • TGF-β platform (Discovery) – TGF-β in combination with a binder for IL13 receptor α2 (IL13Ra2) for glioblastoma • HIB210 (Phase 1) – anti-C5aR1 antibody for severe immune-mediated diseases (IMDs)
multiforme • Advanced discovery programs (Discovery) – directed against mast cells • IMPT-514 in r/r lupus nephritis – Mid 2023: IND clearance • IMPT-314 in ≥3L CAR-T naïve ABCL • Felzartamab –
2Q23: reproductive tox data • HIB210 in severe IMDs – 3Q24: Phase 1 first cohort data – 2H23: Phase 1 data • Felzartamab in PMN – 3Q23: Preclinical data supporting – 1Q25: EOP1 meeting – 4Q24: Phase 2
pivotal data – 2H23: Interim data from POC study in superiority to avacopan – 3Q25: Phase 2 pivotal data – 3Q25: Phase 2 pivotal data update aCD20-refractory population – 1H23: Phase 1 trial initiation in – 2Q26: BLA
submission – 1Q26: BLA submission – 1H24: Phase 3 initiation healthy volunteers • IMPT-514 in severe SLE with extrarenal disease • IMPT-314 in CAR-T experienced ABCL • Felzartamab in IgAN – 2Q23: Clinical POC
– 1Q24: NHP chronic tox study readout – 1Q24: IND amendment – 1Q24: Phase 1 data • Felzartamab in LN – 1H24: Subcutaneous formulation – 4Q24: Patient data – 2Q24: EOP1 meeting Timing of Key Milestones
– 2H23: Phase 1 initiation developments insights – 3Q26: BLA submission – 2H24: Phase 2 data update – 2H24: Clinical POC – 1H24: Phase 2 study initiation in • IMPT-514 in ANCA-associated vasculitis – 1H25:
BLA submission – 2024 and 2025: Phase 1b readouts ANCA-associated vasculitis (AAV) – 2Q24: IND amendment • IMPT-314 in 2L hematopoietic cell transplant (HCT) • Felzartamab in AMR – 1H24: Phase 1 readout in healthy
– 1H25: Patient data unintended ABCL – 1Q24: Clinical POC volunteers – 4Q26: BLA submission – 1Q25: Patient data – 1Q24: Phase 2 investigator sponsored • Advanced discovery programs – 2023: • IMPT-514
in secondary progressive MS – 1H26: BLA submission trial readout in AMR development candidate nomination – 3Q24: IND amendment • IMPT-314 – 1Q26: patient data in 2L HCT eligible ABCL – Mid 2025: Patient data –
4Q26: BLA submission Plans for PACIFIC’s • N/A • If specific PACIFIC employees fulfill open roles, they can be considered for combined company Employees • N/A • N/A Exclusivity • Financial: Jefferies •
Financial: Goldman Sachs • Legal: Cooley Advisors • Audit: Deloitte • Board Composition: Proportionate to the equity split and include combined company’s CEO (or as agreed upon in sign and close structure to not require vote
until post-close) • Board Composition: At least 1 director position designated by PACIFIC • Audit Status: 2 years of audited statements can be available within 3 months Additional Information • Audit Status: Completed AICPA audit
from inception to 12/31/22 • Proposes announcing PACIFIC share buyback upon announcement of transaction for up to $100M • May assign value to PACIFIC’s assets on case-by-case basis; open to PACIFIC monetizing assets prior to
transaction close • Bukwang Pharmaceutical, Decheng Capital, Foresite Capital Management, FutuRx, Hayan Health Networks, JJDC, • ARCH Venture Partners, Monograph Capital, Jeito Capital, MorphoSys JVC Investment Partners, Novartis Venture
Fund, OrbiMed, RM Global Partners, RMGLOBAL Healthcare Fund, Select Investors Surveyor Capital, Takeda Ventures, venBio (1): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to
PACIFIC stockholders seeking to exit the transaction. Note: Bold indicates catalysts that could fall within the transaction window. Confidential 7

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: PIPELINE THERAPEUTICS
| Q32 BIO Transaction Structure • Reverse triangular merger • Reverse triangular merger • Due diligence could be completed by both parties in 45 days and Timing • Q32: $250M (includes $31M cash at 03/31/23, excl. upcoming
payment of $22.5M from Horizon by the • PIPELINE: $310M (includes $130M cash expected at 06/30/23) Implied Valuation end of 3Q23 and $25M debt facility, of which $5M will be used to refinance debt with SVB) • PACIFIC: $155M (based on
$140M net cash at close + $15M for Nasdaq listing) • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) • Q32’s post-money from last private round (Convertible note, 2022; post-$ assumes conversion of the
Post-$ Last Round / • PIPELINE’s post-money from last private round (Series C, April 2023): $310M note at Series B price and 5% interest): $185M, including current option pool • Step-up: 1.00x Step-up • Step-up: 1.35x •
(Excl. PIPE) PIPELINE: 66.7% / PACIFIC: 33.3% • (Excl. PIPE) Q32: 61.7% / PACIFIC: 38.3% Implied Ownership Split • No planned financing concurrent with the transaction, however, PIPELINE is in discussions to extend • Not required
but open to exploring concurrent PIPE Series C by $25-50M, which would increase post-money for Series C and valuation for proposed • Existing shareholders have signed a letter committing at least $30M to a PIPE, which could be used to
Concurrent Financing transaction accordingly extend runway or return a portion of PACIFIC cash to shareholders • Pro forma cash: $213.5M (Q32: $73.5M, based on 1Q23 cash, $22.5M from Horizon and $20M of new Pro Forma Cash and • Pro forma
cash: $270M (PIPELINE: $130M; PACIFIC: $140M) debt; PACIFIC: $140M) • Runway: through 2027, not including potential milestones payments from Janssen related to PIPE-307 Runway • Runway: into 2H 2026 • ADX-097 (Phase 1) –
C3d-mAb fH complement inhibitor for multiple complement-mediated diseases 1-5 • PIPE-791 (IND-enabling) – LPA1 antagonist for multiple sclerosis (MS) and neuroinflammation of the kidney and IgG4 • PIPE-307 (Phase 1 complete)
– oral M1 antagonist for MS and major depressive disorder (MDD) • ADX-096 (Preclinical) – C3d-mAb CR1 complement inhibitor for complement-mediated diseases 1-10 – Entered into a global license and development agreement with
Janssen in April 2023 for $50M • Novel nanobodies (Discovery) – C3d-fab fH/CR1 complement inhibitors and next-gen targeted Key Asset(s) upfront plus $1B milestones and tiered double-digit royalties as well as $25M equity investment
complement inhibitors for neuromuscular and neurodegenerative diseases from JJDC • ADX-914 (Phase 2) – IL-7/TSLP receptor mAb for atopic dermatitis (collaboration and option deal with – PIPELINE retained ability to develop in r/r
MS Horizon in August 2022 for $55M upfront and up to $645M milestones and royalties) • ADX-097 in complement-mediated renal diseases – 2024 and 2025: Phase 2 AAV 12-week safety/efficacy and topline data, respectively • PIPE-791
– 2024 and 2025: Phase 2 renal basket trial 4-week / biomarker topline data and interim12-week • PIPE-307 in r/r MS – 1H23: IND filing data, respectively – 2H23: Phase 2 POC initiation – 1H24: Phase 1 HV study
completion – 2H25-1H26: Phase 2/3 trial initiations in complement-mediated renal diseases – 2Q25: Phase 2 POC study completion – 4Q24: Phase 1 PET study completion • ADX-096 – 1H25: IND submission Timing of Key •
PIPE-307 in other neurological disorders, – 2Q25: Chronic tox studies • Novel nanobodies – 2024: DC nomination including MDD – 3Q25: Biomarker studies completion Milestones • ADX-914 in atopic dermatitis – 2023
and 2024: initiate multiple Phase 2 – 3Q25: Phase 2a trial initiation – 2H23: Receipt of all additional development funding from Horizon POC studies • Calpain – 2H23: Phase 2 study completion – 1Q25: Phase 2 POC studies
completion – 4Q25: IND submission – 2H24: Phase 2 study completion in second (undisclosed) indication – 1Q26: Phase 1 study initiation – Late 2024 – early 2025: Horizon option exercise window (subject to a significant
option exercise fee) for the acquisition of all of Q32’s assets related to ADX-914 • Open to discussing the benefit of retaining members of PACIFIC in key functions. This could include Plans for PACIFIC’s • N/A members of
leadership and functional roles including key areas of clinical, regulatory, legal, and Employees finance. • 45 days • N/A Exclusivity • Legal: Gunderson Dettmer (corporate) & Mintz Levin (IP) • Legal: Goodwin Advisors
• Auditor: E&Y • Auditor: E&Y • Board Composition: Current Q32 board consists of 8 member, expect combined company board to include at least two board members from PACIFIC • Board Composition: 7-9 Board seats; up to 2
designated by PACIFIC • Audit Status: 2021 audit completed; 2022 audit underway • Audit Status: 2019-2021 audits completed; 2022 audit to be completed by 06/30/23 • Open to structures that would allow PACIFIC to monetize its
existing pipeline and IP (and retain the Additional Information • Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money resulting value for Series C and valuation for proposed transaction accordingly
• $30M committed by existing Q32 shareholders could be used to extend runway further or potentially return a portion of PACIFIC’s cash to its shareholders • Atlas Venture, OrbiMed, Acorn, Osage University Partners, Abingworth,
Sanofi Ventures, CU • JJDC, Casdin Capital, Franklin Templeton, Perceptive, Samsara, Suvretta, Cleva Pharma Capital Select Investors Healthcare Innovation Fund, Children’s Hospital Colorado Center for Innovation, Columbia, Harvard,
(Brace Pharma), Hadean Ventures, Sectoral, Versant, Baker Brothers, Hillhouse BMS Note: Bold indicates catalysts that could fall within the transaction window. Confidential 8

PROJECT PACIFIC ILLUSTRATIVE PROCESS TIMELINE MONTH M MA AY JUNE WEEK
OF 8 8 15 15 22 29 5 12 19 26 Weekly reoccurring meeting to track process Finalize private company targets 5 5/9 /9 Formally initiate outreach; send process letters to 5/10 - 5/10 - 5 5/12 interested parties Receive initial proposals from private
targets 5/23 Special committee meeting to evaluate proposals 5/24 Special committee meeting to confirm 2-3 private targets 5/26 for further diligence Send process letter for final bids 5/26 Management presentations 5/30 - 6/8 Due diligence 5/30
– 6/8 Receipt of final proposals 6/9 Special committee to determine interest in including 5/26 financial buyers in process Initiate outreach to financial buyers; send process letters 5/26 Receive proposals from financial buyers 6/9 Special
committee meeting to review private target and 6/12 financial buyer proposals Confidential 9 Financial Buyer Private Company Merger

APPENDIX

2 3333333 22 333333 PROJECT PACIFIC SUMMARY OF ADDITIONAL PROPOSED
PRIVATE COMPANY TARGETS Sorted alphabetically; $ in millions # of Other Clinical Total Last Pvt. Round Therapeutic Technology / Lead Lead Clinical Clinical Catalyst Raised Management Team (Title) Company Area Platform Lead Target Indication(s) Phase
POC? Programs in 18 Mos? to Date Date Post-$ Select Investors / Select Former Affiliations ADDITIONAL PROPOSED PRIVATE COMPANY TARGETS RECOMMENDED BY PACIFIC TEAM Greg Kunst (CEO) / Glaukos, Alcon, Kinetic Concept Corneal Phase 3 Michael Goldstein,
M.D. (CMO) / Ocular Therapeutix, AGTC, Novel, multi-step, proprietary Deerfield Management, Petrichor edema Complete Ophthal- and patented process to Allogeneic cornea Healthcare Capital, Flying L Partners, Eleven Biotherapeutics Aurion
Biotechnologies secondary to (Japan) / 0 $206 04/12/22 $215 mology culture healthy cells from cell therapy Falcon Vision, (KKR), Visionary Arnaud Lacoste, Ph.D. (CSO) / Novartis endothelial Preclinical donor cornea Ventures, Alcon David Rostov (CFO)
/ CorneaGen, Identity Digital, Lighthouse dysfunction (US) eDiscovery Slate Path Capital, 683 Capital, Imran Alibhai, Ph.D. (CEO) / PJ Solomon, Alexandria Venture Palkon Capital Management, Oncology, Investments, MPM Capital Liver and ArrowMark
Partners, ImpactAssets, Inflammatory Small molecule Dan Conn (CFO) / Morgan Stanley, PJ Solomon, DE Shaw & Co, Tvardi Therapeutics s s N/A breast Phase 2 0 84 06/23/21 200 Piedmont Capital Investment, and Fibrotic STAT3 inhibitor Brookfield
Asset Management cancer, IPF ShangBay Capital, Solas Diseases John Kauh, M.D. (CMO) / HUTCHMED, Glenmark BioVentures, Alexandria Venture Pharmaceuticals, Eli Lilly Investments Enavate Sciences, Longitude Hua Mu, Ph.D., M.D. (CEO) / Overland
Pharmaceuticals, Hillhouse Capital, Vivo Capital, Rock Springs Capital, Simcere Pharmaceutical Group, WuXi AppTec, Hutchison Capital, Perceptive Advisors, Agent MediPharma Autoimmune CD19xFcγRIIB IgG4-related Capital, Pivotal bioVenture
Partners, Zenas Biopharma N/A Phase 3 1 118 11/07/22 ND Joe Farmer (President & COO) / Xilio Therapeutics, TESARO, Diseases bifunctional mAb disease Superstring Capital, Fairmount, Cubist Pharmaceuticals Wellington Management, Tellus Simon
Lowry, M.D. (CMO) / Kinevant Science, Sun Pharma, BioVentures, Quan Venture Fund, Novartis, Pfizer Xencor OTHER PROPOSED PRIVATE COMPANIES REVIEWED Sanofi Ventures, Mass General Alex Martin (CEO) / Palladio Biosciences, Realm Therapeutics, Brigham
Ventures, RA Capital, Intercept Pharma, BioXel Autoimmune Inclusion Recruiting Hongsen Investment, Myeloma Jeffrey Wilkins, M.D. (CMO)/ Avalo Therapeutics, Lycera, Ceptaris Abcuro Diseases & N/A Anti-KLRG1 mAb body 0 $52 01/07/21 $70 Phase 2/3
Investment Fund, Pontifax, Samsara, Therapeutics, Cephalon, Ception Therapeutics Oncology myositis ShangBay Capital, ShangPharma Karen Tubridy (Chief Development Officer) / Verseau Tx, Akebia Innovation Therapeutics, Eleven Bio Bing Yao, Ph.D. (CEO)
/ Viela Bio, MedImmune, AstraZenca, Genentech NSCLC with Phase 3 Hillhouse Capital Group, Lilly Asia Robin LaChapelle (Chief Administrative Officer) / RLT Consulting, EGFR-mutant EGFR Exon Ready Ventures, OrbiMed, Octagon Capital Arrivent Oncology
N/A 0 260 12/16/22 335 MedImmune, AstraZeneca, Lockheed Martin selective inhibitor 20 Insertion (Approved Advisors, Boyu/Zoo Capital, Lyra Yang Wang, Ph.D. (CTO) / Viela Bio, MedImmune, Amgen, Pfizer Mutations in China) Capital, Catalio Capital
Dandan Dong, Ph.D. (CBO) / Vivo Capital, Visen Pharmaceutical, RareStone Group Norwest Venture Partners, F-Prime Lyn Baranowski (CEO) / Altavant Sciences, Pearl Therapeutics Capital Partners, Edmond de Idiopathic Mark Surber, Ph.D. (CSO) / Inhaled
Rothschild Investment Partners, Avalyn Pulmonology N/A pulmonary Phase 2 1 98 04/24/20 155 Marc Schneebaum (CFO) / Madrigal Pharmaceuticals, Synta pirfenidone Pivotal bioVenture Partners, Novo fibrosis Pharmaceuticals, Elevation Pharmaceuticals,
Aires Holdings A/S, RiverVest Venture Pharmaceuticals Partners, TPG Biotech Josep Bassaganya-Riera, Ph.D. (President & CEO) / Landos Biopharma Oral gut- Autoimmune Ulcerative Phase 3 Raquel Hontecillas, Ph.D. (CSO) / Landos Biopharma Nimmune Bio
N/A restricted 3 ND ND ND ND Diseases colitis ready Andrew Leber, Ph.D. (CDO) LANCL2 agonist Marek Ciszewski (CFO) / Landos Biopharma, Lantern Pharma, Epirus Biopharma, Verastem Oncology Diem Nguyen, Ph.D. (CEO) / Pfizer Naked plasmid PBM Capital,
Naitonal Institutes of Howard Rutman, M.D. (CMO) / Daiichi Sankyo, Pfizer Non-viral plasmid DNA Inflammatory designed to Osteo- Phase 2/3 Neurological Disorders and Stroke, Brandan O'Leary (COO) / Pfizer Xalud Therapeutics (pDNA)-delivered gene 2 64
08/24/21 303 Diseases express modified arthritis ready U.S. Dept. of Defense, U.S. Dept. of Kristin Murray (Chief Regulatory Officer) / Ultragenyx, Shire therapy platform version of IL-10 Health and Human Services Pharma, Pfizer, Wyeth Steve Vicik,
Ph.D. (CTO) / Wyeth, Pfizer Note: Bold indicates lead investors. Source: Company website, press releases, Pitchbook. Confidential 11

Exhibit (c)(13) PROJECT PACIFIC SUMMARY OF INITIAL INDICATIONS OF
INTEREST MAY 25, 2023 Confidential

PROJECT PACIFIC STRATEGIC PROCESS OUTREACH SUMMARY SUBMITTED PROPOSAL
(N=8) (1) (1) (1) (1) (1) (1) (1) (1) POTENTIAL INVITE TO PROCESS (N=3) PASSED (N=1) FINANCIAL BUYERS TO CONSIDER CONTACTING (N=3) (1): Indicates executed CDA. Confidential 1

PROJECT PACIFIC GUIDELINES FOR THE ASSESSMENT OF PRIVATE COMPANY
TARGETS’ PROPOSALS CATEGORY GUIDELINES Stage of Development • 4 = Phase 3 or pivotal; 3 = Phase 2; 2 = Phase 1; 1 = preclinical • Commercial potential of the lead program in lead indication Market Opportunity / Unmet • Size
of the lead indication’s addressable market Need • Limited competition from marketed drugs or candidates in development Quality of Data Generated to • Quality of clinical data published to date, especially relative to competitors
Date • Clinical proof-of-concept preferred Differentiation from • Differentiated mechanism of action or platform technology compared to peers Competitors Technical Advancement • Clinical validation or significant development
progress for underlying science or technology • Programs’ abilities to expand into other indications in the future Platform / Pipeline Potential • Novelty of underlying science • Probability of success for the platform or
pipeline in totality Number of Clinical Catalysts • Number of clinical data readouts occurring between 6-12 months following transaction close Between 6-12 Months Post-Close Pro Forma Cash Runway • 4 = 2H 2026 and beyond; 3 = to 1H 2026;
2 = to 2025; 1 = to 2024 • Existing investor syndicate Existing Investors / Ability to Bring • Concurrent financing requirement / insider and new investor commitments to concurrent New Investors into PIPE financing • Completeness
and experience of management team • Audited U.S. GAAP financial statements Public Company Readiness • Drafted or filed Form S-1 / other SEC disclosure document preparedness • Strong internal controls and progress toward becoming
public company-ready Valuation / Pro Forma Ownership • Low step-up to post-money of the target’s prior financing round Favorable to Pacific • Pacific pro forma ownership of combined company Potential for Partial Return of •
Proposed partial return of capital to pre-merger Pacific shareholders at transaction close Capital to Pacific Shareholders Confidential 2 BUSINESS ASSESSMENT SCIENTIFIC ASSESSMENT Lead Program

3 33 3333 33 3333 3 333 3 3 3 33 PROJECT PACIFIC SCIENTIFIC ASSESSMENT
OF PRIVATE COMPANY TARGETS SORTED BY SCIENTIFIC ASSESSMENT SCORE, THEN BY TOTAL COMPANY ASSESSMENT SCORE. Lead Program Subtotal for Platform / Market Quality of Data Scientific Stage of Differentiation Technical Pipeline Potential Opportunity /
Generated to Assessment Development from Competitors Advancement Unmet Need Date 15 33333333333333 14 33333333333333 12 3333333 10 333333 12 3333333 12 33333333333 12 33333333 12 3333333333 Confidential 3

3 3 3 3 33 3 33 333 PROJECT PACIFIC BUSINESS ASSESSMENT OF PRIVATE
COMPANY TARGETS SORTED BY SCIENTIFIC ASSESSMENT SCORE, THEN BY TOTAL COMPANY ASSESSMENT SCORE. Number of Existing Valuation / Pro Potential for Clinical Investors / Public Forma Partial Return Subtotal for Total for Catalysts Pro Forma Ability to
Bring Company Ownership of Capital to Business Company Between 6-12 Cash Runway New Investors Readiness Favorable to Pacific Assessment Assessment Months Post- into PIPE Pacific Shareholders Close (1) 15 30 33333333333333 ? 18 32 333333333333333333
17 29 3333333333333333 ? 17 27 3333333333333333 14 26 33333333333 ? 12 24 333333333333 ? (2) 921 333333 ? (3) 921 33 ?3333 (1): BOUNDLESS BIO’s proposed valuation of $402-434M is a 1.50-1.62x step up from the $268M post-money of its May 2023
Series C financing. (2): ANHEART expects interim data for its lead program’s, taletrectinib (AB-106), Phase 2 pivotal TRUST-II trial in ROS1 fusion-positive NSCLC in 2H 2023, which could fall within the potential transaction window. (3):
AERIUM currently holds ~$2M cash. The company expects ~$280M funding from a BARDA contract in September 2023, which could fall within the transaction window. AERIUM expects to submit a proposal for the contract in June 2023. The BARDA funding is
critical to fund AERIUM’s operations and for the proposal’s expected return of PACIFIC cash in excess of $40M to PACIFIC shareholders. Confidential 4

PROJECT PACIFIC HOW COUNTERPARTIES ARE VALUING PACIFIC AND THEMSELVES
$ IN USD MILLIONS. SORTED ALPHABETICALLY. PACIFIC VALUATION COUNTERPARTY PF OWNERSHIP ASSETS / PRO FORMA TARGET PIPE PARTY CASH LISTING TECHNOLOGY TOTAL VALUATION STEP-UP CASH RUNWAY (INSIDER COMMITMENT) PACIFIC PARTY PIPE (1) (2) $40 $5 - $45 $155
ND Through 2024 - 22.5% 77.5% - (3) (4) 140 10 - 150 250 1.25x Through 2024 $40 (100%) 34.1% 56.8% 9.1% Through 1H 140 5 - 145 357 1.10x - 28.9% 71.1% - 2025 140 - - 140 402-434 1.50-1.62x Into 2H 2026 - 24.4-25.8% 74.2-75.6% - 140 7 - 147 325 1.50x
Into 2026 75-125 (ND) 24.6-26.9% 54.4-59.4% 13.7-20.9% (5) (5) Through early 40 10 - 50 257 1.00x 110 (68%) 12.0% 61.6% 26.4% 2026 (6) 140 15 - 155 310 1.00x Through 2027 - 33.3% 66.7% - (7) 140 15 - 155 250 1.35x Into 2H 2026 - 38.3% 61.7% - (1):
PACIFIC net cash at close in excess of $40M to be returned to pre-transaction PACIFIC shareholders. (2): Pro forma runway includes ~$280M in non-dilutive funding from BARDA. (3): Runway through 2024 requires only $100M. (4): Concurrent PIPE not
required. Insiders and licensing partner have indicated $40M commitment to any potential PIPE. (5): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to PACIFIC stockholders
seeking to exit the transaction. (6): Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money for Series C and valuation for proposed transaction accordingly. (7): Existing Q32 shareholders have a signed a
letter committing at least $30 million for PIPE financing. This funding could be used to extend runway further or potentially return a portion of PACIFIC's cash to its shareholders. Confidential 5

PROJECT PACIFIC COUNTERPARTY CATALYSTS IN PRO FORMA CASH RUNWAY SORTED
ALPHABETICALLY. PF runway: Through 2024 PF runway: Through 2024 PF runway: Through 1H 2025 PF runway: Into 2H 2026 • BARDA contract (~$280M) • Taletrectinib/AB-106 in non-small cell lung • AB248 in solid tumors • BBI-355 in
oncogene amplified cancers – June 2023: Submit proposal cancer – 3Q23: Phase 1a monotherapy PD data – Mid-2024: Phase 1/2 clinical POC data – July-August 2023: Funding signal – 2H23: Global Phase 2 TRUST-II interim
– 2H23: Initiate Phase 1a/b anti-PD1 • BBI-825 in oncogene amplified cancers – September 2023: Contract data combination trial – 4Q23: IND filing • AER003 / AER004 / AER005 – 4Q23: Complete enrollment of global
– Late 2023 / early 2024: initiate Phase 1b – 2Q24: Initiate Phase 1/2 trial – 4Q23: Initiate Phase 1/2 trial Phase 2 TRUST-II monotherapy expansion cohorts – 2H25: Phase 1/2 clinical POC data – 1Q24: Initiate Phase 3
trial – 1H24: Initiate global Phase 3 TRUST-III – June 2024: Phase 1a/b preliminary • ecDTx 3 in oncogene amplified cancers – 4Q24: EUA and commercial launch confirmatory study monotherapy activity data – 4Q23: In vivo
POC – 2Q24: Pre-NDA type B meeting with FDA – 2H24 / 1Q25: Phase 1a/b anti-PD1 – 1H24: Development candidate selection (6-month follow up data) combination activity data – 2Q25: IND filing – 3Q24: Global Phase 2
TRUST-II final data • AB821 in solid tumors – 3Q25: Initiate Phase 1/2 trial – 4Q24: NDA submission in the US – 3Q23: GLP tox • ecDTx 4 in oncogene amplified cancers – 2Q25: US NDA approval – 3Q23: GMP
manufacturing and pilot – 1Q24: In vivo POC • Safusidenib/AB-218 in lower grade glioma, batch – 2H24: Development candidate selection cholangiocarcinoma – Q423: IND filing – 4Q25: IND filing – Ongoing: Enrollment
for Phase 2 global – 1Q24: Initiate Phase 1 trial – 1H26: Initiate Phase 1/2 trial studies – 2H24: Preliminary Phase 1 PD data • ECHO Platform – 2H23: IDE filing – 1Q24: CTA filing Note: Pro forma cash runway
includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 6

PROJECT PACIFIC COUNTERPARTY CATALYSTS IN PRO FORMA CASH RUNWAY
(CONT.) SORTED ALPHABETICALLY. PF runway: Into 2026 PF runway: Through early 2026 PF runway: Through 2027 PF runway: Into 2H 2026 • Felzartamab – 2Q23: reproductive tox data • IMPT-314 in ≥3L CAR-T naïve ABCL •
PIPE-307 in r/r MS • ADX-097 in complement-mediated renal • Felzartamab in PMN – 2H23: Phase 1 data – 2H23: Phase 2 POC initiation diseases – 2H23: Interim data from POC study in – 4Q24: Phase 2 pivotal data
– 2Q25: Phase 2 POC study completion – 2024 and 2025: Phase 2 AAV 12-week aCD20-refractory population – 3Q25: Phase 2 pivotal data update • PIPE-307 in other neurological disorders, safety/efficacy and topline data, –
1H24: Phase 3 initiation – 1Q26: BLA submission including MDD respectively • Felzartamab in IgAN – 2Q23: Clinical POC • IMPT-314 in CAR-T experienced ABCL – 2023 and 2024: initiate multiple Phase 2 – 2024 and
2025: Phase 2 renal basket trial • Felzartamab in LN – 1Q24: Phase 1 data POC studies 4-week / biomarker topline data and – 2H23: Phase 1 initiation – 2Q24: EOP1 meeting – 1Q25: Phase 2 POC studies completion
interim12-week data, respectively – 2H24: Clinical POC – 2H24: Phase 2 data update • PIPE-791 – 2H25-1H26: Phase 2/3 trial initiations in – 2024 and 2025: Phase 1b readouts – 1H25: BLA submission – 1H23: IND
filing complement-mediated renal diseases • Felzartamab in AMR • IMPT-314 in 2L HCT unintended ABCL – 1H24: Phase 1 HV study completion • ADX-096 – 1H25: IND submission – 1Q24: Clinical POC – 1Q25: Patient
data – 4Q24: Phase 1 PET study completion • Novel nanobodies – 2024: DC nomination – 1Q24: Phase 2 investigator sponsored trial – 1H26: BLA submission – 2Q25: Chronic tox studies • ADX-914 in atopic
dermatitis readout in AMR • IMPT-314 – 1Q26: patient data in 2L HCT – 3Q25: Biomarker studies completion – 2H23: Receipt of all additional • HIB210 in severe IMDs eligible ABCL – 3Q25: Phase 2a trial initiation
development funding from Horizon – 3Q23: Preclinical data supporting • IMPT-514 in r/r lupus nephritis • Calpain – 2H23: Phase 2 study completion superiority to avacopan – Mid 2023: IND clearance – 4Q25: IND
submission – 2H24: Phase 2 study completion in second – 1H23: Phase 1 trial initiation in healthy – 3Q24: Phase 1 first cohort data – 1Q26: Phase 1 study initiation (undisclosed) indication volunteers – 1Q25: EOP1
meeting – Late 2024 – early 2025: Horizon option – 1Q24: NHP chronic tox study readout – 3Q25: Phase 2 pivotal data exercise window (subject to a significant – 1H24: Subcutaneous formulation – 2Q26: BLA submission
option exercise fee) for the acquisition of developments insights • IMPT-514 in severe systemic lupus all of Q32’s assets related to ADX-914 – 1H24: Phase 2 study initiation in AAV erythematosus with extrarenal disease –
1H24: Phase 1 readout in healthy – 1Q24: IND amendment volunteers – 4Q24: Patient data • Advanced discovery programs – 2023: – 3Q26: BLA submission development candidate nomination • IMPT-514 in ANCA-associated
vasculitis – 2Q24: IND amendment – 1H25: Patient data – 4Q26: BLA submission • IMPT-514 in secondary progressive MS – 3Q24: IND amendment – Mid 2025: Patient data – 4Q26: BLA submission Note: Pro forma cash
runway includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 7

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: AERIUM | ANHEART
Transaction Structure • Reverse triangular merger • Reverse triangular merger (2) • Assumes closing 09/30/23 • Prepared to sign merger agreement and close transaction in 2H 2023 and Timing • AERIUM: $155M (includes $2M
current cash position and ongoing insider-led round which will fund • ANHEART: $250M (includes $5M of cash expected at 09/30/23 and $5M from ANHEART strategic AERIUM through September 2023) partnership) Implied Valuation (1) • PACIFIC:
$45M (based on $40M net cash at close + $5M for Nasdaq listing) • PACIFIC: $150M (based on $140M net cash at close + $10M for Nasdaq listing) Post-$ Last Round / • AERIUM’S post-money from last private round (Series A, 2022): ND
• ANHEART’S post-money from last private round (Series B, September 2021): $200M • Step-up: ND • Step-up: 1.25x Step-up • (Incl. optional $40M PIPE 100% covered by insiders and licensing partner) ANHEART: 56.8% /
Implied Ownership Split • (Excl. PIPE) AERIUM: 77.5% / PACIFIC: 22.5% PACIFIC: 34.1% / PIPE: 9.1% • (Excl. PIPE) ANHEART: 62.5% / PACIFIC: 37.5% • Concurrent PIPE not required • Concurrent PIPE not contemplated •
Insiders and licensing partner have indicated $40M commitment to any potential PIPE • AERIUM pursuing ~$280M BARDA contract to fund development to EUA and launch Concurrent Financing • ANHEART has also explored entering $30M investment
from SDIC at $250M pre-money (not included • AERIUM ongoing insider-led round to fund company through September 2023 in $40M of commitments from insiders and licensing partner) (2) • Pro forma cash (excl. ~$280M BARDA contract): $40M
(AERIUM: $0M expected at 09/30/23 ; (1) PACIFIC: $40M ) • Pro forma cash (incl. optional PIPE): $190M (ANHEART: $10M, including $5M expected at 09/30/23 Pro Forma Cash and (2) • Pro forma cash (incl. ~$280M BARDA contract): $320M
(AERIUM: $0M expected at 09/30/23 ; and $5M from licensing partner; PACIFIC: $140M; PIPE: $40M) Runway (1) PACIFIC: $40M ) • Runway: $100M funds through 2024 • Runway: through 2024 (with BARDA funding) • AER003 (preclinical)
– mAb antiviral treatment against SARS-CoV-2 occupying ACE2 footprint • Taletrectinib/AB-106 (Pivotal Phase 2) – ROS1 inhibitor for ROS1 fusion-positive NSCLC • AER004 (preclinical) – mAb antiviral treatment against
SARS-CoV-2 • Safusidenib/AB-218 (Phase 2) – mIDH1 inhibitor for lower grade glioma, cholangiocarcinoma and • AER005 (preclinical) – mAb antiviral treatment against SARS-CoV-2 binding outside Receptor Binding other tumors Key
Asset(s) Domain • AB-329 (Phase 1) – AXL inhibitor for combination with checkpoint inhibitors or chemotherapy in • Two mAbs planned to be developed in combination; third to be developed as contingency for viral NSCLC and other
solid tumors evolution • 5 undisclosed assets (Preclinical) • Taletrectinib/AB-106 in non-small cell lung cancer • BARDA contract (~$280M) – 2H23: Global Phase 2 TRUST-II interim data – June 2023: Submit proposal
– 4Q23: Complete enrollment of global Phase 2 TRUST-II – July-August 2023: Funding signal – 1H24: Initiate global Phase 3 TRUST-III confirmatory study Timing of Key – September 2023: Contract – 2Q24: Pre-NDA type B
meeting with FDA (6-month follow up data) • AER003 / AER004 / AER005 – 3Q24: Global Phase 2 TRUST-II final data Milestones – 4Q23: Initiate Phase 1/2 trial – 4Q24: NDA submission in the US – 1Q24: Initiate Phase 3 trial
– 2Q25: US NDA approval – 4Q24: EUA and commercial launch • Safusidenib/AB-218 in lower grade glioma, cholangiocarcinoma – Ongoing: Enrollment for Phase 2 global studies Plans for PACIFIC’s • Open to discussing
retaining PACIFIC employees for open positions, including CFO, GC and CBO, as • Open to discussions retention of certain personnel, particularly those in the finance and accounting well as PACIFIC’s public company infrastructure and
capabilities functions, during a transition period or full-time following a Transaction Employees • N/A • N/A Exclusivity • Financial: Piper Sandler Advisors • N/A • Legal: WilmerHale • Auditor: Deloitte •
Board Composition: 10 members; 6 designated by ANHEART and 4 designated by PACIFIC • ANHEART does not assume reliance on PACIFIC non-cash assets, facilities or business • Board Composition: 8 board members; 5 designated by AERIUM (one of
which could potentially be • Audit Status: Have completed audits in China for 2021 and 2022; in process of initiating conversion to independent director from PACIFIC board) and 3 designated by PACIFIC US GAAP standards with completion in mid
2023 • AERIUM would consider CVR for development or out licensing of PACIFIC assets Additional Information • Out-licensed Japanese commercial rights for taletrectinib to global pharmaceutical partner. Licensing • Audit Status: ND
will close concurrently with reverse merger; ANHEART to receive $15M in equity and non-dilutive (1) • Proposal allows most PACIFIC cash to be returned to pre-transaction shareholders upfront payments upon closing, $63M in potential milestone
payments, and high double-digit royalties on net sales • Octagon Investments, Decheng Capital, Laurion Capital, Sage Partners, Innovent Biologics and Select Investors • Omega Funds, F-Prime Capital Cenova (1): PACIFIC net cash at close
in excess of $40M to be returned to pre-transaction PACIFIC shareholders. (2): Based on assumption that ongoing insider-led round funds company through September 2023. Note: Bold indicates catalysts that could fall within the transaction window.
Confidential 8

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: ASHER BIO |
BOUNDLESS BIO Transaction Structure • Reverse triangular merger • Reverse triangular merger • Assumes closing of 07/31/23 • Entry into a definitive merger agreement within 30-45 days of being selected to move forward and
Timing • ASHER: $357M (includes $50M cash expected at 07/31/23) • BOUNDLESS: $402-434M (includes $142M of cash expected at 06/30/23) Implied Valuation • PACIFIC: $145M (based on $140M net cash at close + $5M for Nasdaq listing)
• PACIFIC: $140M (based on $140M net cash at close + $0M for Nasdaq listing) Post-$ Last Round / • ASHER’s post-money from last private round (Series B, September 2021): $324M • BOUNDLESS’ post-money from last private
round (Series C, May 2023): $268M • Step-up: 1.10x • Step-up: 1.50x-1.62x Step-up • (Excl. PIPE) ASHER: 71.1% / PACIFIC: 28.9% • (Excl. PIPE) BOUNDLESS: 74.2-75.6% / PACIFIC: 24.4-25.8% Implied Ownership Split Concurrent
Financing • Not required but open to exploring concurrent PIPE • No planned financing concurrent with the transaction • Pro forma cash: $190M (ASHER: $50M expected at 07/31/23; PACIFIC: $140M) Pro Forma Cash and • Pro forma
cash: $282M (BOUNDLESS: $142M expected at 06/30/23; PACIFIC: $140M) • Runway: through 1H 2025 • Runway: into 2H 2026 Runway • $60M of additional capital required to reach YE 2025 • AB248 (Phase 1a/b) – CD8-targeted IL2
for solid tumors • BBI-355 (Phase 1/2) – oral CHK1 inhibitor for oncogene amplified cancers • AB821 (IND-enabling) – CD8-targeted IL21 for solid tumors • BBI-825 (IND-enabling) – extrachromosomal DNA directed
therapies (exDTx) for oncogene • AB359 (IND-enabling) – CD8-targeted IL2 for chronic viral infection amplified cancers Key Asset(s) • Cell therapy-targeted IL-2 / 21 (Discovery) – for cancer • ecDTx 3 (Discovery)
– for oncogene amplified cancers • Myeloid-targeted immune agonist (Discovery) – for cancer • ecDTx 4 (Discovery) – for oncogene amplified cancers • CD4+ T cell-targeted cytokine (Discovery) – for cancer
• ECHO / ecDNA Harboring Oncogenes (IND-enabling) – patient selection diagnostic assay • AB248 in solid tumors • BBI-355 in oncogene amplified cancers – 3Q23: Phase 1a monotherapy PD data – Mid-2024: Phase 1/2
clinical POC data – 2H23: Initiate Phase 1a/b anti-PD1 combination trial • ecDTx 4 in oncogene amplified cancers • BBI-825 in oncogene amplified cancers – Late 2023 / early 2024: initiate Phase 1b monotherapy expansion
cohorts – 1Q24: In vivo POC – 4Q23: IND filing – June 2024: Phase 1a/b preliminary monotherapy activity data – 2H24: Development candidate selection – 2Q24: Initiate Phase 1/2 trial Timing of Key – 2H24 / 1Q25:
Phase 1a/b anti-PD1 combination activity data – 4Q25: IND filing – 2H25: Phase 1/2 clinical POC data • AB821 in solid tumors – 1H26: Initiate Phase 1/2 trial Milestones • ecDTx 3 in oncogene amplified cancers –
3Q23: GLP tox • ECHO Platform – 4Q23: In vivo POC – 3Q23: GMP manufacturing and pilot batch – 2H23: IDE filing – 1H24: Development candidate selection – Q423: IND filing – 1Q24: CTA filing – 2Q25: IND
filing – 1Q24: Initiate Phase 1 trial – 3Q25: Initiate Phase 1/2 trial – 2H24: Preliminary Phase 1 PD data Plans for PACIFIC’s • N/A • N/A Employees Exclusivity • N/A • N/A • Legal: Goodwin
• Legal: Latham & Watkins Advisors • Auditor: PWC • Auditor: KPMG • Board Composition: 8-9 board members; 7 designated by ASHER and 1-2 designated by PACIFIC to be mutually agreed upon • Audit Status: Completed
2020/2021 PCAOB audits; 2022 audit expected to be completed July • Board Composition: 10 members; 9 designated by BOUNDLESS and 1 designated by PACIFIC Additional Information 2023 • Audit Status: 5 years of audited financials, including
2022 • Open to licensing or divestiture of PACIFIC assets to parties with appropriate expertise and capabilities • Leaps by Bayer, RA Capital, Sectoral, Piper Heartland, ARCH Venture, Nextech Invest, Fidelity, • Third Rock
Ventures, Wellington, RA Capital, Invus Public Equities, Boxer Capital, Mission Bay Wellington, Boxer, Redmile, Surveyor, PFM, Vertex Ventures, Logos, City Hill, GT Healthcare Select Investors Capital, Janus Henderson, Logos, Marshall Wace,
Y-Combinator Capital, Alexandria Venture Investments Note: Bold indicates catalysts that could fall within the transaction window. Confidential 9

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: HI-BIO | IMMPACT BIO
Transaction Structure • Reverse triangular merger • Reverse merger; proposing a simultaneous sign and close transaction • Sign definitive agreement and announce Transaction within 60 days of accepted proposal • Intends to
execute a definitive merger agreement within 45 days of signing term sheet and exclusivity agreement and Timing Financial: • HI-BIO: $325M (includes $22M cash at 03/31/23, excludes $30M from additional Series A • IMMPACT BIO: $257M
(includes $65M of cash expected at 06/30/23) closing in May 2023) Implied Valuation (1) • PACIFIC: $50M (based on $40M net cash at close + $10M for Nasdaq listing) • PACIFIC: $147M (based on $140M net cash at close + $7M for Nasdaq
listing) Post-$ Last Round / • HI-BIO’s post-money from last private round (Series A extension, November 2022): $217M • IMMPACT BIO’s post-money from last private round (Series B, January 2022): $257M • Step-up: 1.50x
• Step-up: 1.00x Step-up • (Excl. PIPE) HI-BIO: 68.9% / PACIFIC: 31.1% • (Excl. PIPE and share buyback) IMMPACT BIO: 63.1% / PACIFIC: 36.9% Implied Ownership Split • (Incl. PIPE) HI-BIO: 54.4-59.4% / PACIFIC: 24.6-26.9% /
PIPE: 13.7-20.9% • (Incl. PIPE and share buyback) IMMPACT BIO: 61.6% / PACIFIC: 12.0% / PIPE: 26.4% • $110M concurrent PIPE with support from existing investors for over $75M (term sheet signed with anchor investor); • $75-125
concurrent financing 30 additional investors in data room Concurrent Financing • Ongoing conversations with both new and existing investors regarding a potential financing • Concurrent share buyback of up to $100M • Pro forma cash
(incl. PIPE): $267-317M (HI-BIO: $52M, including ~$30M from the additional Pro Forma Cash and • Pro forma cash: $215M (IMMPACT: $65M; PACIFIC: $140M; PIPE: $110M; Share repurchase of $100M) Series A closing in May 2023; PACIFIC: $140M; PIPE:
$75-125M) • Runway: through early 2026 Runway • Runway: into 2026 • Felzartamab (Phase 2) – anti-CD38 antibody for primary membranous nephropathy (PMN), • IMPT-314 (Ph. 1) – CD19/CD20 bispecific CAR T cell therapy
for aggressive B-cell lymphoma (ABCL) immunoglobulin A nephropathy (IgAN), antibody-mediated rejection (AMR) and lupus • IMPT-514 (IND-enabling) – CD19/CD20 bispecific CAR T cell therapy for autoimmune diseases nephritis (LN) Key
Asset(s) • TGF-β platform (Discovery) – TGF-β in combination with a binder for IL13 receptor α2 (IL13Ra2) for glioblastoma • HIB210 (Phase 1) – anti-C5aR1 antibody for severe immune-mediated diseases (IMDs)
multiforme • Advanced discovery programs (Discovery) – directed against mast cells • IMPT-514 in r/r lupus nephritis – Mid 2023: IND clearance • IMPT-314 in ≥3L CAR-T naïve ABCL • Felzartamab –
2Q23: reproductive tox data • HIB210 in severe IMDs – 3Q24: Phase 1 first cohort data – 2H23: Phase 1 data • Felzartamab in PMN – 3Q23: Preclinical data supporting – 1Q25: EOP1 meeting – 4Q24: Phase 2
pivotal data – 2H23: Interim data from POC study in superiority to avacopan – 3Q25: Phase 2 pivotal data – 3Q25: Phase 2 pivotal data update aCD20-refractory population – 1H23: Phase 1 trial initiation in – 2Q26: BLA
submission – 1Q26: BLA submission – 1H24: Phase 3 initiation healthy volunteers • IMPT-514 in severe SLE with extrarenal disease • IMPT-314 in CAR-T experienced ABCL • Felzartamab in IgAN – 2Q23: Clinical POC
– 1Q24: NHP chronic tox study readout – 1Q24: IND amendment – 1Q24: Phase 1 data • Felzartamab in LN – 1H24: Subcutaneous formulation – 4Q24: Patient data – 2Q24: EOP1 meeting Timing of Key Milestones
– 2H23: Phase 1 initiation developments insights – 3Q26: BLA submission – 2H24: Phase 2 data update – 2H24: Clinical POC – 1H24: Phase 2 study initiation in • IMPT-514 in ANCA-associated vasculitis – 1H25:
BLA submission – 2024 and 2025: Phase 1b readouts ANCA-associated vasculitis (AAV) – 2Q24: IND amendment • IMPT-314 in 2L hematopoietic cell transplant (HCT) • Felzartamab in AMR – 1H24: Phase 1 readout in healthy
– 1H25: Patient data unintended ABCL – 1Q24: Clinical POC volunteers – 4Q26: BLA submission – 1Q25: Patient data – 1Q24: Phase 2 investigator sponsored • Advanced discovery programs – 2023: • IMPT-514
in secondary progressive MS – 1H26: BLA submission trial readout in AMR development candidate nomination – 3Q24: IND amendment • IMPT-314 – 1Q26: patient data in 2L HCT eligible ABCL – Mid 2025: Patient data –
4Q26: BLA submission Plans for PACIFIC’s • N/A • If specific PACIFIC employees fulfill open roles, they can be considered for combined company Employees • N/A • N/A Exclusivity • Financial: Jefferies •
Financial: Goldman Sachs • Legal: Cooley Advisors • Audit: Deloitte • Board Composition: Proportionate to the equity split and include combined company’s CEO (or as agreed upon in sign and close structure to not require vote
until post-close) • Board Composition: At least 1 director position designated by PACIFIC • Audit Status: 2 years of audited statements can be available within 3 months Additional Information • Audit Status: Completed AICPA audit
from inception to 12/31/22 • Proposes announcing PACIFIC share buyback upon announcement of transaction for up to $100M • May assign value to PACIFIC’s assets on case-by-case basis; open to PACIFIC monetizing assets prior to
transaction close • Bukwang Pharmaceutical, Decheng Capital, Foresite Capital Management, FutuRx, Hayan Health Networks, JJDC, • ARCH Venture Partners, Monograph Capital, Jeito Capital, MorphoSys JVC Investment Partners, Novartis Venture
Fund, OrbiMed, RM Global Partners, RMGLOBAL Healthcare Fund, Select Investors Surveyor Capital, Takeda Ventures, venBio (1): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to
PACIFIC stockholders seeking to exit the transaction. Note: Bold indicates catalysts that could fall within the transaction window. Confidential 10

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST: PIPELINE
THERAPEUTICS | Q32 BIO Transaction Structure • Reverse triangular merger • Reverse triangular merger • Due diligence could be completed by both parties in 45 days and Timing • Q32: $250M (includes $31M cash at 03/31/23, excl.
upcoming payment of $22.5M from Horizon by the • PIPELINE: $310M (includes $130M cash expected at 06/30/23) Implied Valuation end of 3Q23 and $25M debt facility, of which $5M will be used to refinance debt with SVB) • PACIFIC: $155M
(based on $140M net cash at close + $15M for Nasdaq listing) • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) • Q32’s post-money from last private round (Convertible note, 2022; post-$ assumes
conversion of the Post-$ Last Round / • PIPELINE’s post-money from last private round (Series C, April 2023): $310M note at Series B price and 5% interest): $185M, including current option pool • Step-up: 1.00x Step-up •
Step-up: 1.35x • (Excl. PIPE) PIPELINE: 66.7% / PACIFIC: 33.3% • (Excl. PIPE) Q32: 61.7% / PACIFIC: 38.3% Implied Ownership Split • No planned financing concurrent with the transaction, however, PIPELINE is in discussions to extend
• Not required but open to exploring concurrent PIPE Series C by $25-50M, which would increase post-money for Series C and valuation for proposed • Existing shareholders have signed a letter committing at least $30M to a PIPE, which
could be used to Concurrent Financing transaction accordingly extend runway or return a portion of PACIFIC cash to shareholders • Pro forma cash: $213.5M (Q32: $73.5M, based on 1Q23 cash, $22.5M from Horizon and $20M of new Pro Forma Cash and
• Pro forma cash: $270M (PIPELINE: $130M; PACIFIC: $140M) debt; PACIFIC: $140M) • Runway: through 2027, not including potential milestones payments from Janssen related to PIPE-307 Runway • Runway: into 2H 2026 • ADX-097
(Phase 1) – C3d-mAb fH complement inhibitor for multiple complement-mediated diseases 1-5 • PIPE-791 (IND-enabling) – LPA1 antagonist for multiple sclerosis (MS) and neuroinflammation of the kidney and IgG4 • PIPE-307 (Phase
1 complete) – oral M1 antagonist for MS and major depressive disorder (MDD) • ADX-096 (Preclinical) – C3d-mAb CR1 complement inhibitor for complement-mediated diseases 1-10 – Entered into a global license and development
agreement with Janssen in April 2023 for $50M • Novel nanobodies (Discovery) – C3d-fab fH/CR1 complement inhibitors and next-gen targeted Key Asset(s) upfront plus $1B milestones and tiered double-digit royalties as well as $25M equity
investment complement inhibitors for neuromuscular and neurodegenerative diseases from JJDC • ADX-914 (Phase 2) – IL-7/TSLP receptor mAb for atopic dermatitis (collaboration and option deal with – PIPELINE retained ability to
develop in r/r MS Horizon in August 2022 for $55M upfront and up to $645M milestones and royalties) • ADX-097 in complement-mediated renal diseases – 2024 and 2025: Phase 2 AAV 12-week safety/efficacy and topline data, respectively
• PIPE-791 – 2024 and 2025: Phase 2 renal basket trial 4-week / biomarker topline data and interim12-week • PIPE-307 in r/r MS – 1H23: IND filing data, respectively – 2H23: Phase 2 POC initiation – 1H24: Phase 1
HV study completion – 2H25-1H26: Phase 2/3 trial initiations in complement-mediated renal diseases – 2Q25: Phase 2 POC study completion – 4Q24: Phase 1 PET study completion • ADX-096 – 1H25: IND submission Timing of Key
• PIPE-307 in other neurological disorders, – 2Q25: Chronic tox studies • Novel nanobodies – 2024: DC nomination including MDD – 3Q25: Biomarker studies completion Milestones • ADX-914 in atopic dermatitis –
2023 and 2024: initiate multiple Phase 2 – 3Q25: Phase 2a trial initiation – 2H23: Receipt of all additional development funding from Horizon POC studies • Calpain – 2H23: Phase 2 study completion – 1Q25: Phase 2 POC
studies completion – 4Q25: IND submission – 2H24: Phase 2 study completion in second (undisclosed) indication – 1Q26: Phase 1 study initiation – Late 2024 – early 2025: Horizon option exercise window (subject to a
significant option exercise fee) for the acquisition of all of Q32’s assets related to ADX-914 • Open to discussing the benefit of retaining members of PACIFIC in key functions. This could include Plans for PACIFIC’s • N/A
members of leadership and functional roles including key areas of clinical, regulatory, legal, and Employees finance. • 45 days • N/A Exclusivity • Legal: Gunderson Dettmer (corporate) & Mintz Levin (IP) • Legal: Goodwin
Advisors • Auditor: E&Y • Auditor: E&Y • Board Composition: Current Q32 board consists of 8 member, expect combined company board to include at least two board members from PACIFIC • Board Composition: 7-9 Board
seats; up to 2 designated by PACIFIC • Audit Status: 2021 audit completed; 2022 audit underway • Audit Status: 2019-2021 audits completed; 2022 audit to be completed by 06/30/23 • Open to structures that would allow PACIFIC to
monetize its existing pipeline and IP (and retain the Additional Information • Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money resulting value for Series C and valuation for proposed
transaction accordingly • $30M committed by existing Q32 shareholders could be used to extend runway further or potentially return a portion of PACIFIC’s cash to its shareholders • Atlas Venture, OrbiMed, Acorn, Osage University
Partners, Abingworth, Sanofi Ventures, CU • JJDC, Casdin Capital, Franklin Templeton, Perceptive, Samsara, Suvretta, Cleva Pharma Capital Select Investors Healthcare Innovation Fund, Children’s Hospital Colorado Center for Innovation,
Columbia, Harvard, (Brace Pharma), Hadean Ventures, Sectoral, Versant, Baker Brothers, Hillhouse BMS Note: Bold indicates catalysts that could fall within the transaction window. Confidential 11

PROJECT PACIFIC ILLUSTRATIVE PROCESS TIMELINE MONTH M MA AY JUNE WEEK
OF 8 8 15 15 22 29 5 12 19 26 Weekly reoccurring meeting to track process Finalize private company targets 5 5/9 /9 Formally initiate outreach; send process letters to 5/10 - 5/10 - 5 5/12 interested parties Receive initial proposals from private
targets 5/23 Special committee meeting to evaluate proposals 5/24 Special committee meeting to confirm 2-3 private targets 5/26 for further diligence Send process letter for final bids 5/26 Management presentations 5/30 - 6/8 Due diligence 5/30
– 6/8 Receipt of final proposals 6/9 Special committee to determine interest in including 5/26 financial buyers in process Initiate outreach to financial buyers; send process letters 5/26 Receive proposals from financial buyers 6/9 Special
committee meeting to review private target and 6/11 financial buyer proposals Confidential 12 Financial Buyer Private Company Merger

APPENDIX

PROJECT PACIFIC ILLUSTRATIVE FINANCIAL BUYER SCENARIO $ IN THOUSANDS,
EXCEPT PER SHARE VALUES. Illustrative Financial Buyer Scenario Estimated Net Cash Available to Financial Buyer $140,000 x Assumed Purchase Price as % of Net Cash 85-90% Estimated Financial Buyer Offer Value $119,000 - $126,000 ÷ Pacific Shares
Outstanding 61.7 Estimated Financial Buyer Offer Value per Share $1.93 - $2.04 Source: Pacific Management projections. Confidential 14

2 3333333 3 33233323 2 PROJECT PACIFIC SUMMARY OF ADDITIONAL PROPOSED
PRIVATE COMPANY TARGETS Sorted alphabetically; $ in millions # of Other Clinical Total Last Pvt. Round Therapeutic Technology / Lead Lead Clinical Clinical Catalyst Raised Management Team (Title) Company Area Platform Lead Target Indication(s) Phase
POC? Programs in 18 Mos? to Date Date Post-$ Select Investors / Select Former Affiliations ADDITIONAL PROPOSED PRIVATE COMPANY TARGETS RECOMMENDED BY PACIFIC TEAM Greg Kunst (CEO) / Glaukos, Alcon, Kinetic Concept Corneal Phase 3 Novel, multi-step,
proprietary edema Complete Deerfield Management, Petrichor Healthcare Michael Goldstein, M.D. (CMO) / Ocular Therapeutix, AGTC, Eleven Ophthal- and patented process to Allogeneic cornea Aurion Biotechnologies secondary to (Japan) / 0 $206 04/12/22
$215 Capital, Flying L Partners, Falcon Vision, (KKR), Biotherapeutics mology culture healthy cells from cell therapy endothelial Preclinical Visionary Ventures, Alcon Arnaud Lacoste, Ph.D. (CSO) / Novartis donor cornea dysfunction (US) David Rostov
(CFO) / CorneaGen, Identity Digital, Lighthouse eDiscovery John Hood, Ph.D. (CEO) / Impact Biomedicines,TargeGen Miguel de los Rios, Ph.D. (CSO) / Rift Biotherapeutics, Sevion Therapeutics, Ally Bridge Group, Avidity Partners, Perceptive Chimeros
Small molecule PTCH1 Oncology, Advisors, Piper Heartland Healthcare Capital, Endeavor BioMedicines N/A Hedgehog mutated solid Phase 2 0 163 2/7/2022 361 Raghu Saripalli (CFO) / Impact Bio, HemoLife Med, Wasserstein Perella, Fibrosis Revelation
Partners, Tekla Capital, T. Rowe Price, inhibitor tumors, IPF Deutsche Bank Omega Funds, Longitude Capital Srikanth Pendyala, M.D. (CMO) / BridgeBio, Theravance, Merck, Roche/Genentech Enavate Sciences, Longitude Capital, Vivo Hua Mu, Ph.D., M.D.
(CEO) / Overland Pharmaceuticals, Hillhouse Capital, Capital, Rock Springs Capital, Perceptive Simcere Pharmaceutical Group, WuXi AppTec, Hutchison MediPharma Autoimmune CD19xFcγRIIB IgG4-related Advisors, Agent Capital, Pivotal bioVenture
Zenas Biopharma N/A Phase 3 1 118 11/07/22 ND Joe Farmer (President & COO) / Xilio Therapeutics, TESARO, Cubist Diseases bifunctional mAb disease Partners, Superstring Capital, Fairmount, Pharmaceuticals Wellington Management, Tellus
BioVentures, Simon Lowry, M.D. (CMO) / Kinevant Science, Sun Pharma, Novartis, Pfizer Quan Venture Fund, Xencor OTHER PROPOSED PRIVATE COMPANIES REVIEWED Alex Martin (CEO) / Palladio Biosciences, Realm Therapeutics, Intercept Sanofi Ventures, Mass
General Brigham Pharma, BioXel Autoimmune Inclusion Recruiting Ventures, RA Capital, Hongsen Investment, Jeffrey Wilkins, M.D. (CMO)/ Avalo Therapeutics, Lycera, Ceptaris Abcuro Diseases & N/A Anti-KLRG1 mAb body 0 $52 01/07/21 $70 Phase 2/3
Myeloma Investment Fund, Pontifax, Samsara, Therapeutics, Cephalon, Ception Therapeutics Oncology myositis Karen Tubridy (Chief Development Officer) / Verseau Tx, Akebia ShangBay Capital, ShangPharma Innovation Therapeutics, Eleven Bio Bing Yao,
Ph.D. (CEO) / Viela Bio, MedImmune, AstraZenca, Genentech NSCLC with Phase 3 Robin LaChapelle (Chief Administrative Officer) / RLT Consulting, Hillhouse Capital Group, Lilly Asia Ventures, EGFR-mutant EGFR Exon Ready MedImmune, AstraZeneca, Lockheed
Martin Arrivent Oncology N/A 0 260 12/16/22 335 OrbiMed, Octagon Capital Advisors, Boyu/Zoo selective inhibitor 20 Insertion (Approved Yang Wang, Ph.D. (CTO) / Viela Bio, MedImmune, Amgen, Pfizer Capital, Lyra Capital, Catalio Capital Mutations in
China) Dandan Dong, Ph.D. (CBO) / Vivo Capital, Visen Pharmaceutical, RareStone Group Norwest Venture Partners, F-Prime Capital Lyn Baranowski (CEO) / Altavant Sciences, Pearl Therapeutics Idiopathic Partners, Edmond de Rothschild Investment Inhaled
Mark Surber, Ph.D. (CSO) / Avalyn Pulmonology N/A pulmonary Phase 2 1 98 04/24/20 155 Partners, Pivotal bioVenture Partners, Novo pirfenidone Marc Schneebaum (CFO) / Madrigal Pharmaceuticals, Synta fibrosis Holdings A/S, RiverVest Venture Partners,
TPG Pharmaceuticals, Elevation Pharmaceuticals, Aires Pharmaceuticals Biotech Josep Bassaganya-Riera, Ph.D. (President & CEO) / Landos Biopharma Oral gut- Raquel Hontecillas, Ph.D. (CSO) / Landos Biopharma Autoimmune Ulcerative Phase 3 Nimmune
Bio N/A restricted 3 ND ND ND ND Andrew Leber, Ph.D. (CDO) Diseases colitis ready LANCL2 agonist Marek Ciszewski (CFO) / Landos Biopharma, Lantern Pharma, Epirus Biopharma, Verastem Oncology Slate Path Capital, 683 Capital, Palkon Capital Imran
Alibhai, Ph.D. (CEO) / PJ Solomon, Alexandria Venture Investments, Oncology, Liver and MPM Capital Management, ArrowMark Partners, Inflammatory Small molecule Tvardi Therapeutics N/A breast Phase 2 0 84 06/23/21 200 ImpactAssets, Piedmont Capital
Investment, Dan Conn (CFO) / Morgan Stanley, PJ Solomon, DE Shaw & Co, Brookfield and Fibrotic STAT3 inhibitor cancer, IPF ShangBay Capital, Solas BioVentures, Alexandria Asset Management Diseases Venture Investments John Kauh, M.D. (CMO) /
HUTCHMED, Glenmark Pharmaceuticals, Eli Lilly Diem Nguyen, Ph.D. (CEO) / Pfizer Naked plasmid Howard Rutman, M.D. (CMO) / Daiichi Sankyo, Pfizer Non-viral plasmid DNA PBM Capital, Naitonal Institutes of Neurological Inflammatory designed to Osteo-
Phase 2/3 Brandan O'Leary (COO) / Pfizer Xalud Therapeutics (pDNA)-delivered gene 2 64 08/24/21 303 Disorders and Stroke, U.S. Dept. of Defense, Diseases express modified arthritis ready Kristin Murray (Chief Regulatory Officer) / Ultragenyx, Shire
Pharma, Pfizer, therapy platform U.S. Dept. of Health and Human Services version of IL-10 Wyeth Steve Vicik, Ph.D. (CTO) / Wyeth, Pfizer Note: Bold indicates lead investors. Source: Company website, press releases, Pitchbook. Confidential
15

Exhibit (c)(14) PROJECT PACIFIC PROCESS UPDATE JUNE 10, 2023
Confidential

PROJECT PACIFIC PROCESS SUMMARY STRATEGICS WHO HAVE CONDUCTED
MANAGEMENT PRESENTATIONS (N=3) (1) (1) (1)(2) FINANCIAL BUYERS CONTACTED (N=3) (1)(2)(4) (3) DE-PRIORITIZED STRATEGICS WHO HAVE SUBMITTED PROPOSALS STRATEGICS WHO PASSED (N=5) (N=1) (1) (1) (1) (1) (1) (1): Indicates executed CDA. (2): Due diligence
in process. Granted access to VDR. (3): FORESITE submitted a proposal on 06/05/23. (4): XOMA submitted a proposal on 05/30/23 and a revised proposal on 06/09/23. Confidential 1

PROJECT PACIFIC HOW PRIORITIZED COUNTERPARTIES ARE VALUING PACIFIC AND
THEMSELVES $ IN USD MILLIONS. SORTED ALPHABETICALLY. PACIFIC VALUATION COUNTERPARTY PF OWNERSHIP PROPOSED TRANSACTION RETURN OF CASH ASSETS / PRO FORMA TARGET PIPE PARTY STRUCTURE CAPITAL DELIVERED LISTING TECHNOLOGY CVR TOTAL VALUATION STEP-UP CASH
RUNWAY (INSIDER COMMITMENT) PACIFIC PARTY PIPE ACTIVE (1) (1) Sign-and-close $100 $40 $10 - None $50 $257 1.00x Until early 2026 $110 (68%) 12.0% 61.6% 26.4% (2) (2) S-4 110 30 15 - None 45 257 1.00x Until early 2026 120 (63%) 10.7% 60.9% 28.4% (3)
S-4 - 140 15 - None 155 310 1.00x Through 2027 - 33.3% 66.7% - (4) (5) (3) S-4 60 80 15 - None 95 310 1.00x [Through 2027] - 23.5% 76.5% - PASSED FOLLOWING SPECIAL COMMITTEE FEEDBACK PROVIDED ON 06/07/23 S-4 - $140 - - None $140 402-434 1.50-1.62x
Into 2H 2026 - 24.4-25.8% 74.2-75.6% - (6) (5) S-4 $40 100 - - None 100 402-434 1.50-1.62x [Into 2H 2026] - 18.7-19.9% 80.1-81.3% - (1): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction for up to $100M to provide
liquidity to PACIFIC stockholders seeking to exit the transaction. (2): IMMPACT BIO revised its proposed share buyback to return up to $110M to PACIFIC stockholders. (3): Ongoing discussions with investors to extend Series C by $25-50M, which would
increase post-money for Series C and valuation for proposed transaction accordingly. PIPELINE would not require a concurrent financing for the transaction. (4): PIPELINE would be open to returning $60M of the $140M available estimated net cash at
close to the shareholders of PACIFIC. (5): Pro forma runway per initial indication of interest. The company has since proposed a return of capital mechanism and has not given guidance on how that may impact the pro forma runway. (6): BOUNDLESS BIO
would consider a scenario that dividends any cash net of $100M from the PACIFIC balance sheet back to PACIFIC shareholders. Note: Green shading denotes revised proposal communicated following further feedback from PACIFIC’s special committee.
Blue shading denotes revised proposal communicated following initial feedback from PACIFIC’s special committee. Confidential 2

PROJECT PACIFIC FINANCIAL BUYERS’ OFFERS FOR PACIFIC Date of
Offer • May 30, 2023 • June 9, 2023 • June 5, 2023 • Cash tender offer • Cash tender offer for outstanding shares not Transaction Structure • “Back-end” merger to acquire any shares not tendered
currently owned by FORESITE Requested Response Date • N/A • June 8, 2023, 5pm ET (3 days from offer date) Timing • 3Q23 expected close • N/A Support Agreements • Certain of PACIFIC’s key shareholders • N/A
• Terminated as of closing of “back-end” merger Treatment of Stock Options • N/A • In-the-money options cashed out for intrinsic value • 30 days Exclusivity • 10-day extension if initial period lapses and
parties remain in negotiations unless party provides • N/A 10-day written notice of non-renewal Cash Consideration as $ per (1) (2) • $2.07 per share • $2.02 per share • $1.93 per share Share (4) • $125M cash (PACIFIC
net cash minus $14M, (5) assuming PACIFIC net cash at close is $139M) • $119M cash (assumes PACIFIC net cash at (3) • $127M cash (assumes PACIFIC net cash • Excess net cash at close greater than $139M will close is $141M) at close
is $141M) Aggregate Consideration be returned to existing PACIFIC shareholders • CVR for 80% of the net proceeds payable from • CVR for 80% of net proceeds from sale of • CVR for 80% of the net proceeds from the sale of any license
or disposition involving Pomotrelvir PACIFIC’s assets PACIFIC’s legacy assets, such as pomotrelvir, within one year of closing PBI-2158, the backup leads and the IP Cash Consideration as % of (6) (7) (8) • 90% • 90% •
84% PACIFIC Net Cash Cash Consideration as a Premium to Closing Price as • 11% • 8% • 3% (9) of 06/09/23 (1): Calculated assuming aggregate cash consideration of $127M, based on aggregate cash consideration equal to 90% of assumed
PACIFIC net cash at close per initial XOMA proposal received on 05/30/23, and ~61.7M PACIFIC common shares outstanding. Initial XOMA proposal of aggregate cash consideration equal to $135M, based on assumed PACIFIC net cash at close of $150M,
implied cash consideration of $2.19 per share. (2): Calculated based on aggregate cash consideration of $139M assumed PACIFIC net cash at close minus $14M and ~61.7M PACIFIC common shares outstanding per XOMA’s proposal received on 06/09/23.
(3): Assumed cash consideration equal to 90% of assumed PACIFIC net cash at close based on initial XOMA proposal received on 05/30/23. Initial proposed cash consideration of $135M was based on XOMA’s assumed PACIFIC cash at close of $150M.
(4): Cash consideration of $139M assumed PACIFIC net cash at close minus $14M, representing approximately 90% of the assumed net cash, per XOMA’s proposal received on 06/09/23. (5): Based on offer price of $1.93 per share and ~61.7M common
shares outstanding as of 05/01/23 per PACIFIC’s latest 10-Q filing, including ~16.8M shares (~27.2% of common shares outstanding) owned by FORESITE and its affiliates at the offer date. (6): Based aggregate consideration of $135M and assumed
PACIFIC net cash at close of $150M per XOMA’s proposal received on 05/30/23. (7): Based aggregate consideration of $125M and assumed PACIFIC net cash at close of $139M per XOMA’s proposal received on 06/09/23. (8): Based on $141M assumed
PACIFIC net cash at close per FORESITE proposal. Confidential 3 (9): Based on PACIFIC closing price of $1.87 per share as of 06/09/23.

2223 PROJECT PACIFIC DISCOUNTS TO NET CASH IMPLIED BY RECENT FINANCIAL
BUYERS’ PROPOSALS ($ in millions, except per share values) Terms of Proposal Cash Consideration Non-Contingent Offer Target As a % of Premium to Ann. Financial Cash per Equity Total per Aggregate Contingent Net Cash Net Cash Unaffected Offer
(1) Date Company Buyer Share per Share Share Value Value Rights at Close at Close Price Accepted 80% of the net proceeds payable from any (2) 05/30/23 $8.00 - $8.00 $53 license or disposition of the MEI's clinical $76 70% 11% assets 80% of the net
proceeds payable from any 05/22/23 5.75 - 5.75 480 570 84% 55% license or disposition of Atea’s programs Echo Lake (3) 03/21/23 1.60 - 1.60 58 None 87 67% 90% Capital 80% of the net proceeds for ten years post- closing from any license or
disposition of (4) 03/14/23 1.85 - 1.85 96 Jounce’s programs effected within two years 115 85% 75% of closing and 100% of the potential aggregate value of certain potential cost savings Mean: 76% 58% Median: 77% 65% 25th Percentile: 69% 44%
75th Percentile: 84% 78% (1): Estimated net cash at close per offer filing unless otherwise noted. (2): Expected net cash of ~$93M at 06/30/23 per the S-4 filing, net of ~$17M of total minimum operating lease payments (carrying value of $13M due to
present value discounting) as of 03/31/23. (3): $90M cash and cash equivalents net of $3M total liabilities as of 03/31/23. (4): Represents final offer accepted on 03/27/23, increased from the 03/14/23 offer of $1.80 per share + CVR. Note: Includes
unsolicited offers from financial buyers for biopharma companies trading below net cash. Excludes unsolicited offers for companies which total liabilities exceeded cash and cash equivalents at the time of the offer. Source: Company press releases
and filings. Confidential 4

APPENDIX

A. CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGICS THAT HAVE
SUBMITTED INDICATIONS OF INTEREST

PROJECT PACIFIC CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGICS
INVITED TO MANAGEMENT PRESENTATIONS SORTED ALPHABETICALLY. (1) PF runway: Until early 2026 PF runway: [Through 2027] • IMPT-314 in ≥3L CAR-T naïve ABCL • PIPE-307 in r/r MS – 2H23: Phase 1 data – 2H23: Phase 2 POC
initiation – 4Q24: Phase 2 pivotal data – 2Q25: Phase 2 POC study completion – 3Q25: Phase 2 pivotal data update • PIPE-307 in other neurological disorders, including MDD – 1Q26: BLA submission – 2023 and 2024:
initiate multiple Phase 2 POC studies • IMPT-314 in CAR-T experienced ABCL – 1Q25: Phase 2 POC studies completion – 1Q24: Phase 1 data • PIPE-791 – 2Q24: EOP1 meeting – 1H23: IND filing – 2H24: Phase 2 data
update – 1H24: Phase 1 HV study completion – 1H25: BLA submission – 4Q24: Phase 1 PET study completion • IMPT-314 in 2L HCT unintended ABCL – 2Q25: Chronic tox studies – 1Q25: Patient data – 3Q25: Biomarker
studies completion – 1H26: BLA submission – 3Q25: Phase 2a trial initiation • IMPT-314 – 1Q26: patient data in 2L HCT eligible ABCL • Calpain • IMPT-514 in r/r lupus nephritis – 4Q25: IND submission –
Mid 2023: IND clearance – 1Q26: Phase 1 study initiation – 3Q24: Phase 1 first cohort data – 1Q25: EOP1 meeting – 3Q25: Phase 2 pivotal data – 2Q26: BLA submission • IMPT-514 in severe systemic lupus erythematosus
with extrarenal disease – 1Q24: IND amendment – 4Q24: Patient data • IMPT-514 in ANCA-associated vasculitis – 2Q24: IND amendment – 1H25: Patient data • IMPT-514 in secondary progressive MS – 3Q24: IND
amendment – Mid 2025: Patient data (1): Pro forma runway per initial indication of interest. The company has since revised its proposal and has not given guidance on how that may impact the pro forma runway. Note: Pro forma cash runway
includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 7

PROJECT PACIFIC CATALYSTS IN PRO FORMA CASH RUNWAY OF DE-PRIORITIZED
STRATEGICS SORTED ALPHABETICALLY. PF runway: Through 2024 PF runway: Through 2024 PF runway: Through 1H 2025 • BARDA contract (~$280M) • Taletrectinib/AB-106 in non-small cell lung cancer • AB248 in solid tumors – June 2023:
Submit proposal – 2H23: Global Phase 2 TRUST-II interim data – 3Q23: Phase 1a monotherapy PD data – July-August 2023: Funding signal – 4Q23: Complete enrollment of global Phase 2 TRUST-II – 2H23: Initiate Phase 1a/b
anti-PD1 combination trial – September 2023: Contract – 1H24: Initiate global Phase 3 TRUST-III confirmatory study – Late 2023 / early 2024: initiate Phase 1b monotherapy • AER003 / AER004 / AER005 – 2Q24: Pre-NDA type
B meeting with FDA (6-month follow expansion cohorts – 4Q23: Initiate Phase 1/2 trial up data) – June 2024: Phase 1a/b preliminary monotherapy activity – 1Q24: Initiate Phase 3 trial – 3Q24: Global Phase 2 TRUST-II final data
data – 4Q24: EUA and commercial launch – 4Q24: NDA submission in the US – 2H24 / 1Q25: Phase 1a/b anti-PD1 combination activity – 2Q25: US NDA approval data • Safusidenib/AB-218 in lower grade glioma, cholangiocarcinoma
• AB821 in solid tumors – Ongoing: Enrollment for Phase 2 global studies – 3Q23: GLP tox – 3Q23: GMP manufacturing and pilot batch – Q423: IND filing – 1Q24: Initiate Phase 1 trial – 2H24: Preliminary Phase
1 PD data PF runway: Into 2026 PF runway: Into 2H 2026 • Felzartamab – 2Q23: reproductive tox data • ADX-097 in complement-mediated renal diseases • Felzartamab in PMN – 2024 and 2025: Phase 2 AAV 12-week
safety/efficacy and topline data, – 2H23: Interim data from POC study in aCD20-refractory population respectively – 1H24: Phase 3 initiation – 2024 and 2025: Phase 2 renal basket trial 4-week / biomarker topline data •
Felzartamab in IgAN – 2Q23: Clinical POC and interim12-week data, respectively • Felzartamab in LN – 2H25-1H26: Phase 2/3 trial initiations in complement-mediated renal diseases – 2H23: Phase 1 initiation • ADX-096
– 1H25: IND submission – 2H24: Clinical POC • Novel nanobodies – 2024: DC nomination – 2024 and 2025: Phase 1b readouts • ADX-914 in atopic dermatitis • Felzartamab in AMR – 2H23: Receipt of all
additional development funding from Horizon – 1Q24: Clinical POC – 2H23: Phase 2 study completion – 1Q24: Phase 2 investigator sponsored trial readout in AMR – 2H24: Phase 2 study completion in second (undisclosed) indication
• HIB210 in severe IMDs – Late 2024 – early 2025: Horizon option exercise window (subject to a – 3Q23: Preclinical data supporting superiority to avacopan significant option exercise fee) for the acquisition of all of
Q32’s assets related – 1H23: Phase 1 trial initiation in healthy volunteers to ADX-914 – 1Q24: NHP chronic tox study readout – 1H24: Subcutaneous formulation developments insights – 1H24: Phase 2 study initiation in AAV
– 1H24: Phase 1 readout in healthy volunteers • Advanced discovery programs – 2023: development candidate nomination Note: Pro forma cash runway includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that
could fall within the transaction window. Confidential 8

PROJECT PACIFIC CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGIC WHO
HAS PASSED (1) PF runway: [Into 2H 2026] • BBI-355 in oncogene amplified cancers – Mid-2024: Phase 1/2 clinical POC data • BBI-825 in oncogene amplified cancers – 4Q23: IND filing – 2Q24: Initiate Phase 1/2 trial
– 2H25: Phase 1/2 clinical POC data • ecDTx 3 in oncogene amplified cancers – 4Q23: In vivo POC – 1H24: Development candidate selection – 2Q25: IND filing – 3Q25: Initiate Phase 1/2 trial • ecDTx 4 in
oncogene amplified cancers – 1Q24: In vivo POC – 2H24: Development candidate selection – 4Q25: IND filing – 1H26: Initiate Phase 1/2 trial • ECHO Platform – 2H23: IDE filing – 1Q24: CTA filing (1): Pro forma
runway per initial indication of interest. The company has since proposed a return of capital mechanism and has not given guidance on how that may impact the pro forma runway. Note: Pro forma cash runway includes concurrent PIPE proceeds where
applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 9

B. INITIAL INDICATIONS OF INTEREST FROM STRATEGICS INVITED TO
MANAGEMENT PRESENTATIONS

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM STRATEGICS
INVITED TO MANAGEMENT PRESENTATIONS: IMMPACT | Q32 BIO (2) • Reverse merger; proposing a simultaneous sign and close transaction Transaction Structure • Reverse triangular merger • Intends to execute a definitive merger agreement
within 45 days of signing term sheet and exclusivity and Timing • Due diligence could be completed by both parties in 45 days agreement • IMMPACT BIO: $257M (includes $65M of cash expected at 06/30/23) • PIPELINE: $310M (includes
$130M cash expected at 06/30/23) Implied Valuation (1) • PACIFIC: $50M (based on $40M net cash at close + $10M for Nasdaq listing) • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) Post-$ Last Round / •
IMMPACT BIO’s post-money from last private round (Series B, January 2022): $257M • PIPELINE’s post-money from last private round (Series C, April 2023): $310M Step-up • Step-up: 1.00x • Step-up: 1.00x • (Excl.
PIPE and share buyback) IMMPACT BIO: 63.1% / PACIFIC: 36.9% Implied Ownership Split • (Excl. PIPE) PIPELINE: 66.7% / PACIFIC: 33.3% • (Incl. PIPE and share buyback) IMMPACT BIO: 61.6% / PACIFIC: 12.0% / PIPE: 26.4% • $110M
concurrent PIPE with support from existing investors for over $75M (term sheet signed with • No planned financing concurrent with the transaction, however, PIPELINE is in discussions to extend Concurrent Financing anchor investor); 30
additional investors in data room Series C by $25-50M, which would increase post-money for Series C and valuation for proposed • Concurrent share buyback of up to $100M transaction accordingly • Pro forma cash: $215M (IMMPACT: $65M;
PACIFIC: $140M; PIPE: $110M; Share repurchase of $100M) • Pro forma cash: $270M (PIPELINE: $130M; PACIFIC: $140M) Pro Forma Cash and Runway • Runway: Until early 2026 • Runway: through 2027, not including potential milestones
payments from Janssen related to PIPE-307 • PIPE-791 (IND-enabling) – LPA1 antagonist for multiple sclerosis (MS) and neuroinflammation • IMPT-314 (Ph. 1) – CD19/CD20 bispecific CAR T cell therapy for aggressive B-cell
lymphoma (ABCL) • PIPE-307 (Phase 1 complete) – oral M1 antagonist for MS and major depressive disorder (MDD) • IMPT-514 (IND-enabling) – CD19/CD20 bispecific CAR T cell therapy for autoimmune diseases Key Asset(s) •
Entered into a global license and development agreement with Janssen in April 2023 for $50M upfront • TGF-β platform (Discovery) – TGF-β in combination with a binder for IL13 receptor α2 (IL13Ra2) for plus $1B milestones
and tiered double-digit royalties as well as $25M equity investment from JJDC glioblastoma multiforme • PIPELINE retained ability to develop in r/r MS • IMPT-514 in r/r lupus nephritis • IMPT-314 in ≥3L CAR-T naïve ABCL
– Mid 2023: IND clearance – 2H23: Phase 1 data – 3Q24: Phase 1 first cohort data – 4Q24: Phase 2 pivotal data – 1Q25: EOP1 meeting – 3Q25: Phase 2 pivotal data update – 3Q25: Phase 2 pivotal data •
PIPE-791 – 1Q26: BLA submission – 2Q26: BLA submission • PIPE-307 in r/r MS – 1H23: IND filing • IMPT-314 in CAR-T experienced ABCL • IMPT-514 in severe SLE with extrarenal disease – 2H23: Phase 2 POC
initiation – 1H24: Phase 1 HV study completion – 1Q24: Phase 1 data – 1Q24: IND amendment – 2Q25: Phase 2 POC study completion – 4Q24: Phase 1 PET study completion – 2Q24: EOP1 meeting – 4Q24: Patient data
• PIPE-307 in other neurological disorders, – 2Q25: Chronic tox studies Timing of Key Milestones – 2H24: Phase 2 data update – 3Q26: BLA submission including MDD – 3Q25: Biomarker studies completion – 1H25: BLA
submission • IMPT-514 in ANCA-associated vasculitis – 2023 and 2024: initiate multiple Phase 2 – 3Q25: Phase 2a trial initiation • IMPT-314 in 2L hematopoietic cell transplant – 2Q24: IND amendment POC studies •
Calpain (HCT) unintended ABCL – 1H25: Patient data – 1Q25: Phase 2 POC studies completion – 4Q25: IND submission – 1Q25: Patient data – 4Q26: BLA submission – 1Q26: Phase 1 study initiation – 1H26: BLA
submission • IMPT-514 in secondary progressive MS • IMPT-314 – 1Q26: patient data in 2L HCT eligible – 3Q24: IND amendment ABCL – Mid 2025: Patient data – 4Q26: BLA submission Plans for PACIFIC’s • If
specific PACIFIC employees fulfill open roles, they can be considered for combined company • N/A Employees Exclusivity • N/A • 45 days • Financial: Jefferies • Legal: Gunderson Dettmer (corporate) & Mintz Levin (IP)
Advisors • Audit: Deloitte • Legal: Cooley • Auditor: E&Y • Board Composition: Proportionate to the equity split and include combined company’s CEO (or as agreed upon in sign and close structure to not require vote
until post-close) • Board Composition: 7-9 Board seats; up to 2 designated by PACIFIC • Audit Status: 2 years of audited statements can be available within 3 months • Audit Status: 2019-2021 audits completed; 2022 audit to be
completed by 06/30/23 Additional Information • Proposes announcing PACIFIC share buyback upon announcement of transaction for up to $100M • Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money
• May assign value to PACIFIC’s assets on case-by-case basis; open to PACIFIC monetizing assets prior for Series C and valuation for proposed transaction accordingly to transaction close • Bukwang Pharmaceutical, Decheng Capital,
Foresite Capital Management, FutuRx, Hayan Health • JJDC, Casdin Capital, Franklin Templeton, Perceptive, Samsara, Suvretta, Cleva Pharma Capital (Brace Select Investors Networks, JJDC, JVC Investment Partners, Novartis Venture Fund, OrbiMed,
RM Global Partners, Pharma), Hadean Ventures, Sectoral, Versant, Baker Brothers, Hillhouse RMGLOBAL Healthcare Fund, Surveyor Capital, Takeda Ventures, venBio (1): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction
for up to $110M to provide liquidity to PACIFIC stockholders seeking to exit the transaction per the Company’s revised proposal dated 06/09/23. The original proposal planned for a $100M share buyback. (2): PIPELINE would be open to returning
$60M of the $140M available estimated net cash at close to the shareholders of PACIFIC. The revised transaction structure was communicated following feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders
via dividend. Note: Bold indicates catalysts that could fall within the transaction window. Confidential 11

C. INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS: AERIUM | ANHEART Transaction Structure • Reverse triangular merger • Reverse triangular merger (2) • Assumes closing 09/30/23 • Prepared to sign merger agreement and close transaction in 2H 2023 and Timing •
AERIUM: $155M (includes $2M current cash position and ongoing insider-led round which will fund • ANHEART: $250M (includes $5M of cash expected at 09/30/23 and $5M from ANHEART strategic AERIUM through September 2023) partnership) Implied
Valuation (1) • PACIFIC: $45M (based on $40M net cash at close + $5M for Nasdaq listing) • PACIFIC: $150M (based on $140M net cash at close + $10M for Nasdaq listing) Post-$ Last Round / • AERIUM’S post-money from last
private round (Series A, 2022): ND • ANHEART’S post-money from last private round (Series B, September 2021): $200M • Step-up: ND • Step-up: 1.25x Step-up • (Incl. optional $40M PIPE 100% covered by insiders and
licensing partner) ANHEART: 56.8% / Implied Ownership Split • (Excl. PIPE) AERIUM: 77.5% / PACIFIC: 22.5% PACIFIC: 34.1% / PIPE: 9.1% • (Excl. PIPE) ANHEART: 62.5% / PACIFIC: 37.5% • Concurrent PIPE not required • Concurrent
PIPE not contemplated • Insiders and licensing partner have indicated $40M commitment to any potential PIPE • AERIUM pursuing ~$280M BARDA contract to fund development to EUA and launch Concurrent Financing • ANHEART has also
explored entering $30M investment from SDIC at $250M pre-money (not included • AERIUM ongoing insider-led round to fund company through September 2023 in $40M of commitments from insiders and licensing partner) (2) • Pro forma cash
(excl. ~$280M BARDA contract): $40M (AERIUM: $0M expected at 09/30/23 ; (1) PACIFIC: $40M ) • Pro forma cash (incl. optional PIPE): $190M (ANHEART: $10M, including $5M expected at 09/30/23 Pro Forma Cash and (2) • Pro forma cash (incl.
~$280M BARDA contract): $320M (AERIUM: $0M expected at 09/30/23 ; and $5M from licensing partner; PACIFIC: $140M; PIPE: $40M) Runway (1) PACIFIC: $40M ) • Runway: $100M funds through 2024 • Runway: through 2024 (with BARDA funding)
• AER003 (preclinical) – mAb antiviral treatment against SARS-CoV-2 occupying ACE2 footprint • Taletrectinib/AB-106 (Pivotal Phase 2) – ROS1 inhibitor for ROS1 fusion-positive NSCLC • AER004 (preclinical) – mAb
antiviral treatment against SARS-CoV-2 • Safusidenib/AB-218 (Phase 2) – mIDH1 inhibitor for lower grade glioma, cholangiocarcinoma and • AER005 (preclinical) – mAb antiviral treatment against SARS-CoV-2 binding outside
Receptor Binding other tumors Key Asset(s) Domain • AB-329 (Phase 1) – AXL inhibitor for combination with checkpoint inhibitors or chemotherapy in • Two mAbs planned to be developed in combination; third to be developed as
contingency for viral NSCLC and other solid tumors evolution • 5 undisclosed assets (Preclinical) • Taletrectinib/AB-106 in non-small cell lung cancer • BARDA contract (~$280M) – 2H23: Global Phase 2 TRUST-II interim data
– June 2023: Submit proposal – 4Q23: Complete enrollment of global Phase 2 TRUST-II – July-August 2023: Funding signal – 1H24: Initiate global Phase 3 TRUST-III confirmatory study Timing of Key – September 2023:
Contract – 2Q24: Pre-NDA type B meeting with FDA (6-month follow up data) • AER003 / AER004 / AER005 – 3Q24: Global Phase 2 TRUST-II final data Milestones – 4Q23: Initiate Phase 1/2 trial – 4Q24: NDA submission in the
US – 1Q24: Initiate Phase 3 trial – 2Q25: US NDA approval – 4Q24: EUA and commercial launch • Safusidenib/AB-218 in lower grade glioma, cholangiocarcinoma – Ongoing: Enrollment for Phase 2 global studies Plans for
PACIFIC’s • Open to discussing retaining PACIFIC employees for open positions, including CFO, GC and CBO, as • Open to discussions retention of certain personnel, particularly those in the finance and accounting well as
PACIFIC’s public company infrastructure and capabilities functions, during a transition period or full-time following a Transaction Employees • N/A • N/A Exclusivity • Financial: Piper Sandler Advisors • N/A •
Legal: WilmerHale • Auditor: Deloitte • Board Composition: 10 members; 6 designated by ANHEART and 4 designated by PACIFIC • ANHEART does not assume reliance on PACIFIC non-cash assets, facilities or business • Board
Composition: 8 board members; 5 designated by AERIUM (one of which could potentially be • Audit Status: Have completed audits in China for 2021 and 2022; in process of initiating conversion to independent director from PACIFIC board) and 3
designated by PACIFIC US GAAP standards with completion in mid 2023 • AERIUM would consider CVR for development or out licensing of PACIFIC assets Additional Information • Out-licensed Japanese commercial rights for taletrectinib to
global pharmaceutical partner. Licensing • Audit Status: ND will close concurrently with reverse merger; ANHEART to receive $15M in equity and non-dilutive (1) • Proposal allows most PACIFIC cash to be returned to pre-transaction
shareholders upfront payments upon closing, $63M in potential milestone payments, and high double-digit royalties on net sales • Octagon Investments, Decheng Capital, Laurion Capital, Sage Partners, Innovent Biologics and Select Investors
• Omega Funds, F-Prime Capital Cenova (1): PACIFIC net cash at close in excess of $40M to be returned to pre-transaction PACIFIC shareholders. (2): Based on assumption that ongoing insider-led round funds company through September 2023. Note:
Bold indicates catalysts that could fall within the transaction window. Confidential 13

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS: ASHER BIO | HI BIO Transaction Structure • Reverse triangular merger • Reverse triangular merger • Assumes closing of 07/31/23 • Sign definitive agreement and announce Transaction within 60 days of accepted
proposal and Timing • HI-BIO: $325M (includes $22M cash at 03/31/23, excludes $30M from additional Series A closing in • ASHER: $357M (includes $50M cash expected at 07/31/23) May 2023) Implied Valuation • PACIFIC: $145M (based on
$140M net cash at close + $5M for Nasdaq listing) • PACIFIC: $147M (based on $140M net cash at close + $7M for Nasdaq listing) Post-$ Last Round / • ASHER’s post-money from last private round (Series B, September 2021): $324M
• HI-BIO’s post-money from last private round (Series A extension, November 2022): $217M • Step-up: 1.10x • Step-up: 1.50x Step-up • (Excl. PIPE) HI-BIO: 68.9% / PACIFIC: 31.1% Implied Ownership Split • (Excl.
PIPE) ASHER: 71.1% / PACIFIC: 28.9% • (Incl. PIPE) HI-BIO: 54.4-59.4% / PACIFIC: 24.6-26.9% / PIPE: 13.7-20.9% • $75-125 concurrent financing • Not required but open to exploring concurrent PIPE Concurrent Financing • Ongoing
conversations with both new and existing investors regarding a potential financing • Pro forma cash: $190M (ASHER: $50M expected at 07/31/23; PACIFIC: $140M) • Pro forma cash (incl. PIPE): $267-317M (HI-BIO: $52M, including ~$30M from
the additional Series Pro Forma Cash and • Runway: through 1H 2025 A closing in May 2023; PACIFIC: $140M; PIPE: $75-125M) Runway • $60M of additional capital required to reach YE 2025 • Runway: into 2026 • AB248 (Phase 1a/b)
– CD8-targeted IL2 for solid tumors • AB821 (IND-enabling) – CD8-targeted IL21 for solid tumors • Felzartamab (Phase 2) – anti-CD38 antibody for primary membranous nephropathy (PMN), • AB359 (IND-enabling) –
CD8-targeted IL2 for chronic viral infection immunoglobulin A nephropathy (IgAN), antibody-mediated rejection (AMR) and lupus nephritis (LN) Key Asset(s) • HIB210 (Phase 1) – anti-C5aR1 antibody for severe immune-mediated diseases (IMDs)
• Cell therapy-targeted IL-2 / 21 (Discovery) – for cancer • Advanced discovery programs (Discovery) – directed against mast cells • Myeloid-targeted immune agonist (Discovery) – for cancer • CD4+ T
cell-targeted cytokine (Discovery) – for cancer • Felzartamab – 2Q23: reproductive tox data • AB248 in solid tumors • HIB210 in severe IMDs • Felzartamab in PMN – 3Q23: Preclinical data supporting –
3Q23: Phase 1a monotherapy PD data – 2H23: Interim data from POC study superiority to avacopan – 2H23: Initiate Phase 1a/b anti-PD1 combination trial in aCD20-refractory population – 1H23: Phase 1 trial initiation in healthy
– Late 2023 / early 2024: initiate Phase 1b monotherapy expansion cohorts – 1H24: Phase 3 initiation volunteers – June 2024: Phase 1a/b preliminary monotherapy activity data • Felzartamab in IgAN – 2Q23: Clinical POC
– 1Q24: NHP chronic tox study readout Timing of Key – 2H24 / 1Q25: Phase 1a/b anti-PD1 combination activity data • Felzartamab in LN – 1H24: Subcutaneous formulation – 2H23: Phase 1 initiation • AB821 in solid
tumors Milestones developments insights – 2H24: Clinical POC – 3Q23: GLP tox – 1H24: Phase 2 study initiation in ANCA- – 2024 and 2025: Phase 1b readouts – 3Q23: GMP manufacturing and pilot batch associated vasculitis
(AAV) • Felzartamab in AMR – Q423: IND filing – 1H24: Phase 1 readout in healthy volunteers – 1Q24: Clinical POC – 1Q24: Initiate Phase 1 trial • Advanced discovery programs – 2023: – 1Q24: Phase 2
investigator sponsored – 2H24: Preliminary Phase 1 PD data development candidate nomination trial readout in AMR Plans for PACIFIC’s • N/A • N/A Employees Exclusivity • N/A • N/A • Legal: Goodwin •
Financial: Goldman Sachs Advisors • Auditor: PWC • Board Composition: 8-9 board members; 7 designated by ASHER and 1-2 designated by PACIFIC to be mutually agreed upon • Board Composition: At least 1 director position designated by
PACIFIC Additional Information • Audit Status: Completed 2020/2021 PCAOB audits; 2022 audit expected to be completed July 2023 • Audit Status: Completed AICPA audit from inception to 12/31/22 • Open to licensing or divestiture of
PACIFIC assets to parties with appropriate expertise and capabilities • Third Rock Ventures, Wellington, RA Capital, Invus Public Equities, Boxer Capital, Mission Bay Select Investors • ARCH Venture Partners, Monograph Capital, Jeito
Capital, MorphoSys Capital, Janus Henderson, Logos, Marshall Wace, Y-Combinator Note: Bold indicates catalysts that could fall within the transaction window. Confidential 14

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS: Q32 BIO Transaction Structure • Reverse triangular merger and Timing • Q32: $250M (includes $31M cash at 03/31/23, excl. upcoming payment of $22.5M from Horizon by the end of 3Q23 and $25M debt facility, of which $5M will be
Implied Valuation used to refinance debt with SVB) • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) • Q32’s post-money from last private round (Convertible note, 2022; post-$ assumes conversion of the
note at Series B price and 5% interest): $185M, including Post-$ Last Round / current option pool Step-up • Step-up: 1.35x Implied Ownership Split • (Excl. PIPE) Q32: 61.7% / PACIFIC: 38.3% • Not required but open to exploring
concurrent PIPE Concurrent Financing • Existing shareholders have signed a letter committing at least $30M to a PIPE, which could be used to extend runway or return a portion of PACIFIC cash to shareholders • Pro forma cash: $213.5M
(Q32: $73.5M, based on 1Q23 cash, $22.5M from Horizon and $20M of new debt; PACIFIC: $140M) Pro Forma Cash and Runway • Runway: into 2H 2026 • ADX-097 (Phase 1) – C3d-mAb fH complement inhibitor for multiple complement-mediated
diseases of the kidney and IgG4 1-5 • ADX-096 (Preclinical) – C3d-mAb CR1 complement inhibitor for complement-mediated diseases 1-10 • Novel nanobodies (Discovery) – C3d-fab fH/CR1 complement inhibitors and next-gen targeted
complement inhibitors for neuromuscular and neurodegenerative Key Asset(s) diseases • ADX-914 (Phase 2) – IL-7/TSLP receptor mAb for atopic dermatitis (collaboration and option deal with Horizon in August 2022 for $55M upfront and up to
$645M milestones and royalties) • ADX-097 in complement-mediated renal diseases – 2024 and 2025: Phase 2 AAV 12-week safety/efficacy and topline data, respectively – 2024 and 2025: Phase 2 renal basket trial 4-week / biomarker
topline data and interim12-week data, respectively – 2H25-1H26: Phase 2/3 trial initiations in complement-mediated renal diseases • ADX-096 – 1H25: IND submission • Novel nanobodies – 2024: DC nomination Timing of Key
Milestones • ADX-914 in atopic dermatitis – 2H23: Receipt of all additional development funding from Horizon – 2H23: Phase 2 study completion – 2H24: Phase 2 study completion in second (undisclosed) indication – Late
2024 – early 2025: Horizon option exercise window (subject to a significant option exercise fee) for the acquisition of all of Q32’s assets related to ADX- 914 • Open to discussing the benefit of retaining members of PACIFIC in key
functions. This could include members of leadership and functional roles including key Plans for PACIFIC’s Employees areas of clinical, regulatory, legal, and finance. Exclusivity • N/A • Legal: Goodwin Advisors • Auditor:
E&Y • Board Composition: Current Q32 board consists of 8 member, expect combined company board to include at least two board members from PACIFIC • Audit Status: 2021 audit completed; 2022 audit underway Additional Information
• Open to structures that would allow PACIFIC to monetize its existing pipeline and IP (and retain the resulting value • $30M committed by existing Q32 shareholders could be used to extend runway further or potentially return a portion
of PACIFIC’s cash to its shareholders • Atlas, OrbiMed, Acorn, Osage University Partners, Abingworth, Sanofi Ventures, CU Healthcare Innovation Fund, Children’s Hospital Colorado Center for Select Investors Innovation, Columbia,
Harvard, BMS Note: Bold indicates catalysts that could fall within the transaction window. Confidential 15

D. INITIAL INDICATION OF INTEREST FROM STRATEGIC WHO HAS
PASSED

PROJECT PACIFIC INITIAL INDICATION OF INTEREST FROM STRATEGIC WHO HAS
PASSED: BOUNDLESS BIO (1) Transaction Structure • Reverse triangular merger and Timing • Entry into a definitive merger agreement within 30-45 days of being selected to move forward • BOUNDLESS: $402-434M (includes $142M of cash
expected at 06/30/23) Implied Valuation • PACIFIC: $140M (based on $140M net cash at close + $0M for Nasdaq listing) Post-$ Last Round / • BOUNDLESS’ post-money from last private round (Series C, May 2023): $268M Step-up •
Step-up: 1.50x-1.62x Implied Ownership Split • (Excl. PIPE) BOUNDLESS: 74.2-75.6% / PACIFIC: 24.4-25.8% Concurrent Financing • No planned financing concurrent with the transaction • Pro forma cash: $282M (BOUNDLESS: $142M expected
at 06/30/23; PACIFIC: $140M) Pro Forma Cash and Runway • Runway: into 2H 2026 • BBI-355 (Phase 1/2) – oral CHK1 inhibitor for oncogene amplified cancers • BBI-825 (IND-enabling) – extrachromosomal DNA directed therapies
(exDTx) for oncogene amplified cancers Key Asset(s) • ecDTx 3 (Discovery) – for oncogene amplified cancers • ecDTx 4 (Discovery) – for oncogene amplified cancers • ECHO / ecDNA Harboring Oncogenes (IND-enabling) –
patient selection diagnostic assay • BBI-355 in oncogene amplified cancers – Mid-2024: Phase 1/2 clinical POC data • ecDTx 4 in oncogene amplified cancers • BBI-825 in oncogene amplified cancers – 1Q24: In vivo POC
– 4Q23: IND filing – 2H24: Development candidate selection – 2Q24: Initiate Phase 1/2 trial – 4Q25: IND filing Timing of Key Milestones – 2H25: Phase 1/2 clinical POC data – 1H26: Initiate Phase 1/2 trial •
ecDTx 3 in oncogene amplified cancers • ECHO Platform – 4Q23: In vivo POC – 2H23: IDE filing – 1H24: Development candidate selection – 1Q24: CTA filing – 2Q25: IND filing – 3Q25: Initiate Phase 1/2 trial
Plans for PACIFIC’s Employees • N/A Exclusivity • N/A • Legal: Latham & Watkins Advisors • Auditor: KPMG • Board Composition: 10 members; 9 designated by BOUNDLESS and 1 designated by PACIFIC Additional
Information • Audit Status: 5 years of audited financials, including 2022 • Leaps by Bayer, RA Capital, Sectoral, Piper Heartland, ARCH Venture, Nextech Invest, Fidelity, Wellington, Boxer, Redmile, Surveyor, PFM, Select Investors Vertex
Ventures, Logos, City Hill, GT Healthcare Capital, Alexandria Venture Investments (1): BOUNDLESS BIO would consider a scenario that dividends any cash net of $100M from the PACIFIC balance sheet back to PACIFIC shareholders. The revised transaction
structure was communicated following feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders via dividend. Note: Bold indicates catalysts that could fall within the transaction window. Confidential
17

E. ILLUSTRATIVE PROCESS TIMELINE

PROJECT PACIFIC ILLUSTRATIVE PROCESS TIMELINE MONTH M MA AY Y JUNE
WEEK OF 8 8 15 15 2 22 2 2 29 9 5 5 12 19 26 Weekly reoccurring meeting to track process Finalize private company targets 5 5/9 /9 Formally initiate outreach; send process letters to 5/10 - 5/10 - 5 5/ /12 12 interested parties Receive initial
proposals from private targets 5/ 5/23 23 Special committee meeting to evaluate proposals 5 5/ /24 24 Special committee meeting to confirm 2-3 private targets 5/26 5/26 for further diligence Management presentations 6/ 6/1 1 –– 6/3 6/
Due diligence 6/5-6/16 Receipt of final proposals By 6/16 Special committee to determine interest in including 5/26 5/26 financial buyers in process Initiate outreach to financial buyers 5/ 5/26 26- -5/3 5/31 Receive proposals from financial buyers
6/9 Special committee meeting to review private target and 6/11 financial buyer proposals Confidential 19 Financial Buyer Private Company Merger

Exhibit (c)(15) PROJECT PACIFIC PROCESS UPDATE JUNE 11, 2023
Confidential

PROJECT PACIFIC PROCESS SUMMARY STRATEGICS WHO HAVE CONDUCTED
MANAGEMENT PRESENTATIONS (N=3) (1) (1) (1)(2) Withdrew from process on 06/08/23 Withdrew from process on 06/10/23 FINANCIAL BUYERS CONTACTED (N=3) (1)(2)(4) (3) DE-PRIORITIZED STRATEGICS WHO HAVE SUBMITTED PROPOSALS STRATEGIC WHO PASSED (N=5) (N=1)
(1) (1) (1) (1) (1) (1): Indicates executed CDA. (2): Due diligence in process. Granted access to VDR. (3): FORESITE submitted a proposal on 06/05/23 and a revised proposal on 06/10/23. (4): XOMA submitted a proposal on 05/30/23 and a revised
proposal on 06/09/23. Confidential 1

PROJECT PACIFIC HOW PRIORITIZED COUNTERPARTIES ARE VALUING PACIFIC AND
THEMSELVES $ IN USD MILLIONS. SORTED ALPHABETICALLY. PACIFIC VALUATION COUNTERPARTY PF OWNERSHIP PROPOSED TRANSACTION RETURN OF CASH ASSETS / PRO FORMA TARGET PIPE PARTY STRUCTURE CAPITAL DELIVERED LISTING TECHNOLOGY CVR TOTAL VALUATION STEP-UP CASH
RUNWAY (INSIDER COMMITMENT) PACIFIC PARTY PIPE ACTIVE (1) S-4 - $140 $15 - None $155 $310 1.00x Through 2027 - 33.3% 66.7% - (2) (3) (1) S-4 $60 80 15 - None 95 310 1.00x [Through 2027] - 23.5% 76.5% - PASSED FOLLOWING SPECIAL COMMITTEE FEEDBACK S-4
- $140 - - None $140 $402-434 1.50-1.62x Into 2H 2026 - 24.4-25.8% 74.2-75.6% - (4) (4) S-4 $40 100 - - None 100 402-434 1.50-1.62x [Into 2H 2026] - 18.7-19.9% 80.1-81.3% - (5) (5) Sign-and-close 100 40 $10 - None 50 257 1.00x Until early 2026 $110
(68%) 12.0% 61.6% 26.4% (6) (6) S-4 110 30 15 - None 45 257 1.00x Until early 2026 120 (63%) 10.7% 60.9% 28.4% (1): Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money for Series C and valuation for
proposed transaction accordingly. PIPELINE would not require a concurrent financing for the transaction. (2): PIPELINE would be open to returning $60M of the $140M available estimated net cash at close to the shareholders of PACIFIC. (3): Pro forma
runway per initial indication of interest. The company has since proposed a return of capital mechanism and has not given guidance on how that may impact the pro forma runway. (4): BOUNDLESS BIO would consider a scenario that dividends any cash net
of $100M from the PACIFIC balance sheet back to PACIFIC shareholders. (5): IMMPACT BIO plans to announce a share buyback upon announcement of the transaction for up to $100M to provide liquidity to PACIFIC stockholders seeking to exit the
transaction. (6): IMMPACT BIO revised its proposed share buyback to return up to $110M to PACIFIC stockholders. Note: Green shading denotes revised proposal communicated following further feedback from PACIFIC’s special committee. Blue shading
denotes revised proposal communicated following initial feedback from PACIFIC’s special committee. Confidential 2

PROJECT PACIFIC XOMA CORPORATION’S OFFERS FOR PACIFIC CURRENT
PRIOR Date of Offer • June 9, 2023 • May 30, 2023 • Cash tender offer Transaction Structure • “Back-end” merger to acquire any shares not tendered Requested Response Date • N/A Timing • 3Q23 expected
close Support Agreements • Certain of PACIFIC’s key shareholders • Terminated as of closing of “back-end” merger Treatment of Stock Options • In-the-money options cashed out for intrinsic value • 30 days
Exclusivity • 10-day extension if initial period lapses and parties remain in negotiations unless party provides 10-day written notice of non-renewal (1) (2) Cash Consideration as $ per Share • $2.06 per share • $2.07 per share (3)
• $127M cash (PACIFIC net cash minus $14M, assuming PACIFIC net cash at close is $141M) • Excess net cash at close greater than $139M will be (4) • $127M cash (assumes PACIFIC net cash at close is $141M) Aggregate Consideration
returned to existing PACIFIC shareholders • CVR for 80% of net proceeds from sale of PACIFIC’s assets • CVR for 80% of the net proceeds from the sale of PACIFIC’s legacy assets, such as pomotrelvir, PBI- 2158, the backup
leads and the IP Cash Consideration as % of PACIFIC (5) (6) • 90% • 90% Net Cash Cash Consideration as a Premium to • 10% • 11% (7) Closing Price as of 06/09/23 (1): Calculated based on aggregate consideration of $127M and
~61.7M common shares outstanding per PACIFIC’s latest 10-Q filing. (2): Calculated assuming aggregate cash consideration of $127M, based on aggregate cash consideration equal to 90% of assumed PACIFIC net cash at close per initial XOMA
proposal received on 05/30/23, and ~61.7M PACIFIC common shares outstanding. Initial XOMA proposal of aggregate cash consideration equal to $135M, based on assumed PACIFIC net cash at close of $150M, implied cash consideration of $2.19 per share.
(3): Cash consideration of $141M assumed PACIFIC net cash at close per management guidance minus $14M per XOMA’s proposal received on 06/09/23, representing approximately 90% of the assumed net cash. (4): Assumed cash consideration equal to
90% of assumed PACIFIC net cash at close based on initial XOMA proposal received on 05/30/23. Initial proposed cash consideration of $135M was based on XOMA’s assumed PACIFIC cash at close of $150M. (5): Based aggregate consideration of $127M
and assumed PACIFIC net cash at close of $141M per management guidance. (6): Based aggregate consideration of $135M and assumed PACIFIC net cash at close of $150M per XOMA’s proposal received on 05/30/23. Confidential 3 (7): Based on PACIFIC
closing price of $1.87 per share as of 06/09/23.

PROJECT PACIFIC FORESITE CAPITAL’S OFFERS FOR PACIFIC CURRENT
PRIOR Date of Offer • June 10, 2023 • June 5, 2023 Transaction Structure • Cash tender offer for outstanding shares not currently owned by FORESITE • June 12, 2023, 5pm ET (2 days from offer date) • June 8, 2023, 5pm ET
(3 days from offer date) Requested Response Date Timing • N/A Support Agreements • N/A Treatment of Stock Options • N/A Exclusivity • N/A Cash Consideration as $ per Share • $2.09 per share • $1.93 per share (1)
• $129M cash (PACIFIC net cash minus $12M, (2) assuming PACIFIC net cash at close is $141M) • $119M cash (assumes PACIFIC net cash at close is $141M) Aggregate Consideration • CVR for 90% of the net proceeds payable from any
• CVR for 80% of the net proceeds payable from any license or license or disposition involving Pomotrelvir within one disposition involving Pomotrelvir within one year of closing year of closing Cash Consideration as % of PACIFIC • 91%
• 84% (3) Net Cash Cash Consideration as a Premium to • 12% • 3% (4) Closing Price as of 06/09/23 (1): Based on assumed $141M PACIFIC net cash at close less $12M per FORESITE’s proposal dated 06/10/23. Cash tender to be equal
to aggregate consideration of $129M multiplied by the percentage of common shares not owned by FORESITE and its affiliates, ~16.8M shares (~27.2% of common shares outstanding). (2): Based on offer price of $1.93 per share and ~61.7M common shares
outstanding per PACIFIC’s latest 10-Q filing, including ~16.8M shares (~27.2% of common shares outstanding) owned by FORESITE and its affiliates at the offer date. (3): Based on $141M assumed PACIFIC net cash at close per FORESITE proposal.
Confidential 4 (4): Based on PACIFIC closing price of $1.87 per share as of 06/09/23.

2223 PROJECT PACIFIC DISCOUNTS TO NET CASH IMPLIED BY RECENT FINANCIAL
BUYERS’ PROPOSALS ($ in millions, except per share values) Terms of Proposal Cash Consideration Non-Contingent Offer Target As a % of Premium to Ann. Financial Cash per Equity Total per Aggregate Contingent Net Cash Net Cash Unaffected Offer
(1) Date Company Buyer Share per Share Share Value Value Rights at Close at Close Price Accepted 80% of the net proceeds payable from any (2) 05/30/23 $8.00 - $8.00 $53 license or disposition of the MEI's clinical $76 70% 11% assets 80% of the net
proceeds payable from any 05/22/23 5.75 - 5.75 480 570 84% 55% license or disposition of Atea’s programs Echo Lake (3) 03/21/23 1.60 - 1.60 58 None 87 67% 90% Capital 80% of the net proceeds for ten years post- closing from any license or
disposition of (4) 03/14/23 1.85 - 1.85 96 Jounce’s programs effected within two years 115 85% 75% of closing and 100% of the potential aggregate value of certain potential cost savings Mean: 76% 58% Median: 77% 65% 25th Percentile: 69% 44%
75th Percentile: 84% 78% (1): Estimated net cash at close per offer filing unless otherwise noted. (2): Expected net cash of ~$93M at 06/30/23 per the S-4 filing, net of ~$17M of total minimum operating lease payments (carrying value of $13M due to
present value discounting) as of 03/31/23. (3): $90M cash and cash equivalents net of $3M total liabilities as of 03/31/23. (4): Represents final offer accepted on 03/27/23, increased from the 03/14/23 offer of $1.80 per share + CVR. Note: Includes
unsolicited offers from financial buyers for biopharma companies trading below net cash. Excludes unsolicited offers for companies which total liabilities exceeded cash and cash equivalents at the time of the offer. Source: Company press releases
and filings. Confidential 5

APPENDIX

A. CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGICS THAT HAVE
SUBMITTED INDICATIONS OF INTEREST

PROJECT PACIFIC CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGIC WHO
HAS BEEN PRIORITIZED (1) PF runway: [Through 2027] • PIPE-307 in r/r MS – 2H23: Phase 2 POC initiation – 2Q25: Phase 2 POC study completion • PIPE-307 in other neurological disorders, including MDD – 2023 and 2024:
initiate multiple Phase 2 POC studies – 1Q25: Phase 2 POC studies completion • PIPE-791 – 1H23: IND filing – 1H24: Phase 1 HV study completion – 4Q24: Phase 1 PET study completion – 2Q25: Chronic tox studies
– 3Q25: Biomarker studies completion – 3Q25: Phase 2a trial initiation • Calpain – 4Q25: IND submission – 1Q26: Phase 1 study initiation (1): Pro forma runway per initial indication of interest. The company has since
revised its proposal and has not given guidance on how that may impact the pro forma runway. Note: Pro forma cash runway includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window.
Confidential 8

PROJECT PACIFIC CATALYSTS IN PRO FORMA CASH RUNWAY OF DE-PRIORITIZED
STRATEGICS SORTED ALPHABETICALLY. PF runway: Through 2024 PF runway: Through 2024 PF runway: Through 1H 2025 • BARDA contract (~$280M) • Taletrectinib/AB-106 in non-small cell lung cancer • AB248 in solid tumors – June 2023:
Submit proposal – 2H23: Global Phase 2 TRUST-II interim data – 3Q23: Phase 1a monotherapy PD data – July-August 2023: Funding signal – 4Q23: Complete enrollment of global Phase 2 TRUST-II – 2H23: Initiate Phase 1a/b
anti-PD1 combination trial – September 2023: Contract – 1H24: Initiate global Phase 3 TRUST-III confirmatory study – Late 2023 / early 2024: initiate Phase 1b monotherapy • AER003 / AER004 / AER005 – 2Q24: Pre-NDA type
B meeting with FDA (6-month follow expansion cohorts – 4Q23: Initiate Phase 1/2 trial up data) – June 2024: Phase 1a/b preliminary monotherapy activity – 1Q24: Initiate Phase 3 trial – 3Q24: Global Phase 2 TRUST-II final data
data – 4Q24: EUA and commercial launch – 4Q24: NDA submission in the US – 2H24 / 1Q25: Phase 1a/b anti-PD1 combination activity – 2Q25: US NDA approval data • Safusidenib/AB-218 in lower grade glioma, cholangiocarcinoma
• AB821 in solid tumors – Ongoing: Enrollment for Phase 2 global studies – 3Q23: GLP tox – 3Q23: GMP manufacturing and pilot batch – Q423: IND filing – 1Q24: Initiate Phase 1 trial – 2H24: Preliminary Phase
1 PD data PF runway: Into 2026 PF runway: Into 2H 2026 • Felzartamab – 2Q23: reproductive tox data • ADX-097 in complement-mediated renal diseases • Felzartamab in PMN – 2024 and 2025: Phase 2 AAV 12-week
safety/efficacy and topline data, – 2H23: Interim data from POC study in aCD20-refractory population respectively – 1H24: Phase 3 initiation – 2024 and 2025: Phase 2 renal basket trial 4-week / biomarker topline data •
Felzartamab in IgAN – 2Q23: Clinical POC and interim12-week data, respectively • Felzartamab in LN – 2H25-1H26: Phase 2/3 trial initiations in complement-mediated renal diseases – 2H23: Phase 1 initiation • ADX-096
– 1H25: IND submission – 2H24: Clinical POC • Novel nanobodies – 2024: DC nomination – 2024 and 2025: Phase 1b readouts • ADX-914 in atopic dermatitis • Felzartamab in AMR – 2H23: Receipt of all
additional development funding from Horizon – 1Q24: Clinical POC – 2H23: Phase 2 study completion – 1Q24: Phase 2 investigator sponsored trial readout in AMR – 2H24: Phase 2 study completion in second (undisclosed) indication
• HIB210 in severe IMDs – Late 2024 – early 2025: Horizon option exercise window (subject to a – 3Q23: Preclinical data supporting superiority to avacopan significant option exercise fee) for the acquisition of all of
Q32’s assets related – 1H23: Phase 1 trial initiation in healthy volunteers to ADX-914 – 1Q24: NHP chronic tox study readout – 1H24: Subcutaneous formulation developments insights – 1H24: Phase 2 study initiation in AAV
– 1H24: Phase 1 readout in healthy volunteers • Advanced discovery programs – 2023: development candidate nomination Note: Pro forma cash runway includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that
could fall within the transaction window. Confidential 9

PROJECT PACIFIC CATALYSTS IN PRO FORMA CASH RUNWAY OF STRATEGICS WHO
HAVE PASSED SORTED ALPHABETICALLY. (1) PF runway: [Into 2H 2026] PF runway: Until early 2026 • BBI-355 in oncogene amplified cancers • IMPT-314 in ≥3L CAR-T naïve ABCL – Mid-2024: Phase 1/2 clinical POC data –
2H23: Phase 1 data • BBI-825 in oncogene amplified cancers – 4Q24: Phase 2 pivotal data – 4Q23: IND filing – 3Q25: Phase 2 pivotal data update – 2Q24: Initiate Phase 1/2 trial – 1Q26: BLA submission – 2H25:
Phase 1/2 clinical POC data • ecDTx 3 in oncogene amplified cancers • IMPT-314 in CAR-T experienced ABCL – 4Q23: In vivo POC – 1Q24: Phase 1 data – 1H24: Development candidate selection – 2Q24: EOP1 meeting
– 2Q25: IND filing – 2H24: Phase 2 data update – 3Q25: Initiate Phase 1/2 trial – 1H25: BLA submission • ecDTx 4 in oncogene amplified cancers • IMPT-314 in 2L HCT unintended ABCL – 1Q24: In vivo POC –
1Q25: Patient data – 2H24: Development candidate selection – 1H26: BLA submission – 4Q25: IND filing – 1H26: Initiate Phase 1/2 trial • IMPT-314 – 1Q26: patient data in 2L HCT eligible ABCL • ECHO Platform
• IMPT-514 in r/r lupus nephritis – 2H23: IDE filing – Mid 2023: IND clearance – 1Q24: CTA filing – 3Q24: Phase 1 first cohort data – 1Q25: EOP1 meeting – 3Q25: Phase 2 pivotal data – 2Q26: BLA
submission • IMPT-514 in severe systemic lupus erythematosus with extrarenal disease – 1Q24: IND amendment – 4Q24: Patient data • IMPT-514 in ANCA-associated vasculitis – 2Q24: IND amendment – 1H25: Patient data
• IMPT-514 in secondary progressive MS – 3Q24: IND amendment – Mid 2025: Patient data (1): Pro forma runway per initial indication of interest. The company has since revised its proposal and has not given guidance on how that may
impact the pro forma runway. Note: Pro forma cash runway includes concurrent PIPE proceeds where applicable. Bold indicates catalysts that could fall within the transaction window. Confidential 10

B. INITIAL INDICATIONS OF INTEREST FROM STRATEGIC WHO HAS BEEN
PRIORITIZED

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM STRATEGIC WHO
HAS BEEN PRIORITIZED: PIPELINE (1) Transaction Structure • Reverse triangular merger and Timing • Due diligence could be completed by both parties in 45 days • PIPELINE: $310M (includes $130M cash expected at 06/30/23) Implied
Valuation • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) Post-$ Last Round / • PIPELINE’s post-money from last private round (Series C, April 2023): $310M Step-up • Step-up: 1.00x Implied
Ownership Split • (Excl. PIPE) PIPELINE: 66.7% / PACIFIC: 33.3% • No planned financing concurrent with the transaction, however, PIPELINE is in discussions to extend Series C by $25-50M, which would increase Concurrent Financing
post-money for Series C and valuation for proposed transaction accordingly • Pro forma cash: $270M (PIPELINE: $130M; PACIFIC: $140M) Pro Forma Cash and Runway • Runway: through 2027, not including potential milestones payments from
Janssen related to PIPE-307 • PIPE-791 (IND-enabling) – LPA1 antagonist for multiple sclerosis (MS) and neuroinflammation • PIPE-307 (Phase 1 complete) – oral M1 antagonist for MS and major depressive disorder (MDD) Key
Asset(s) • Entered into a global license and development agreement with Janssen in April 2023 for $50M upfront plus $1B milestones and tiered double- digit royalties as well as $25M equity investment from JJDC • PIPELINE retained ability
to develop in r/r MS • PIPE-791 – 1H23: IND filing • PIPE-307 in r/r MS – 1H24: Phase 1 HV study completion – 2H23: Phase 2 POC initiation – 4Q24: Phase 1 PET study completion – 2Q25: Phase 2 POC study
completion – 2Q25: Chronic tox studies Timing of Key Milestones • PIPE-307 in other neurological disorders, including MDD – 3Q25: Biomarker studies completion – 2023 and 2024: initiate multiple Phase 2 POC studies –
3Q25: Phase 2a trial initiation – 1Q25: Phase 2 POC studies completion • Calpain – 4Q25: IND submission – 1Q26: Phase 1 study initiation Plans for PACIFIC’s Employees • N/A Exclusivity • 45 days •
Legal: Gunderson Dettmer (corporate) & Mintz Levin (IP) Advisors • Auditor: E&Y • Board Composition: 7-9 Board seats; up to 2 designated by PACIFIC • Audit Status: 2019-2021 audits completed; 2022 audit to be completed by
06/30/23 Additional Information • Ongoing discussions with investors to extend Series C by $25-50M, which would increase post-money for Series C and valuation for proposed transaction accordingly • JJDC, Casdin Capital, Franklin
Templeton, Perceptive, Samsara, Suvretta, Cleva Pharma Capital (Brace Pharma), Hadean Ventures, Sectoral, Select Investors Versant, Baker Brothers, Hillhouse (1): PIPELINE would be open to returning $60M of the $140M available estimated net cash at
close to the shareholders of PACIFIC. The revised transaction structure was communicated following feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders via dividend. Note: Bold indicates catalysts that
could fall within the transaction window. Confidential 12

C. INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS: AERIUM | ANHEART Transaction Structure • Reverse triangular merger • Reverse triangular merger (2) • Assumes closing 09/30/23 • Prepared to sign merger agreement and close transaction in 2H 2023 and Timing •
AERIUM: $155M (includes $2M current cash position and ongoing insider-led round which will fund • ANHEART: $250M (includes $5M of cash expected at 09/30/23 and $5M from ANHEART strategic AERIUM through September 2023) partnership) Implied
Valuation (1) • PACIFIC: $45M (based on $40M net cash at close + $5M for Nasdaq listing) • PACIFIC: $150M (based on $140M net cash at close + $10M for Nasdaq listing) Post-$ Last Round / • AERIUM’S post-money from last
private round (Series A, 2022): ND • ANHEART’S post-money from last private round (Series B, September 2021): $200M • Step-up: ND • Step-up: 1.25x Step-up • (Incl. optional $40M PIPE 100% covered by insiders and
licensing partner) ANHEART: 56.8% / Implied Ownership Split • (Excl. PIPE) AERIUM: 77.5% / PACIFIC: 22.5% PACIFIC: 34.1% / PIPE: 9.1% • (Excl. PIPE) ANHEART: 62.5% / PACIFIC: 37.5% • Concurrent PIPE not required • Concurrent
PIPE not contemplated • Insiders and licensing partner have indicated $40M commitment to any potential PIPE • AERIUM pursuing ~$280M BARDA contract to fund development to EUA and launch Concurrent Financing • ANHEART has also
explored entering $30M investment from SDIC at $250M pre-money (not included • AERIUM ongoing insider-led round to fund company through September 2023 in $40M of commitments from insiders and licensing partner) (2) • Pro forma cash
(excl. ~$280M BARDA contract): $40M (AERIUM: $0M expected at 09/30/23 ; (1) PACIFIC: $40M ) • Pro forma cash (incl. optional PIPE): $190M (ANHEART: $10M, including $5M expected at 09/30/23 Pro Forma Cash and (2) • Pro forma cash (incl.
~$280M BARDA contract): $320M (AERIUM: $0M expected at 09/30/23 ; and $5M from licensing partner; PACIFIC: $140M; PIPE: $40M) Runway (1) PACIFIC: $40M ) • Runway: $100M funds through 2024 • Runway: through 2024 (with BARDA funding)
• AER003 (preclinical) – mAb antiviral treatment against SARS-CoV-2 occupying ACE2 footprint • Taletrectinib/AB-106 (Pivotal Phase 2) – ROS1 inhibitor for ROS1 fusion-positive NSCLC • AER004 (preclinical) – mAb
antiviral treatment against SARS-CoV-2 • Safusidenib/AB-218 (Phase 2) – mIDH1 inhibitor for lower grade glioma, cholangiocarcinoma and • AER005 (preclinical) – mAb antiviral treatment against SARS-CoV-2 binding outside
Receptor Binding other tumors Key Asset(s) Domain • AB-329 (Phase 1) – AXL inhibitor for combination with checkpoint inhibitors or chemotherapy in • Two mAbs planned to be developed in combination; third to be developed as
contingency for viral NSCLC and other solid tumors evolution • 5 undisclosed assets (Preclinical) • Taletrectinib/AB-106 in non-small cell lung cancer • BARDA contract (~$280M) – 2H23: Global Phase 2 TRUST-II interim data
– June 2023: Submit proposal – 4Q23: Complete enrollment of global Phase 2 TRUST-II – July-August 2023: Funding signal – 1H24: Initiate global Phase 3 TRUST-III confirmatory study Timing of Key – September 2023:
Contract – 2Q24: Pre-NDA type B meeting with FDA (6-month follow up data) • AER003 / AER004 / AER005 – 3Q24: Global Phase 2 TRUST-II final data Milestones – 4Q23: Initiate Phase 1/2 trial – 4Q24: NDA submission in the
US – 1Q24: Initiate Phase 3 trial – 2Q25: US NDA approval – 4Q24: EUA and commercial launch • Safusidenib/AB-218 in lower grade glioma, cholangiocarcinoma – Ongoing: Enrollment for Phase 2 global studies Plans for
PACIFIC’s • Open to discussing retaining PACIFIC employees for open positions, including CFO, GC and CBO, as • Open to discussions retention of certain personnel, particularly those in the finance and accounting well as
PACIFIC’s public company infrastructure and capabilities functions, during a transition period or full-time following a Transaction Employees • N/A • N/A Exclusivity • Financial: Piper Sandler Advisors • N/A •
Legal: WilmerHale • Auditor: Deloitte • Board Composition: 10 members; 6 designated by ANHEART and 4 designated by PACIFIC • ANHEART does not assume reliance on PACIFIC non-cash assets, facilities or business • Board
Composition: 8 board members; 5 designated by AERIUM (one of which could potentially be • Audit Status: Have completed audits in China for 2021 and 2022; in process of initiating conversion to independent director from PACIFIC board) and 3
designated by PACIFIC US GAAP standards with completion in mid 2023 • AERIUM would consider CVR for development or out licensing of PACIFIC assets Additional Information • Out-licensed Japanese commercial rights for taletrectinib to
global pharmaceutical partner. Licensing • Audit Status: ND will close concurrently with reverse merger; ANHEART to receive $15M in equity and non-dilutive (1) • Proposal allows most PACIFIC cash to be returned to pre-transaction
shareholders upfront payments upon closing, $63M in potential milestone payments, and high double-digit royalties on net sales • Octagon Investments, Decheng Capital, Laurion Capital, Sage Partners, Innovent Biologics and Select Investors
• Omega Funds, F-Prime Capital Cenova (1): PACIFIC net cash at close in excess of $40M to be returned to pre-transaction PACIFIC shareholders. (2): Based on assumption that ongoing insider-led round funds company through September 2023. Note:
Bold indicates catalysts that could fall within the transaction window. Confidential 14
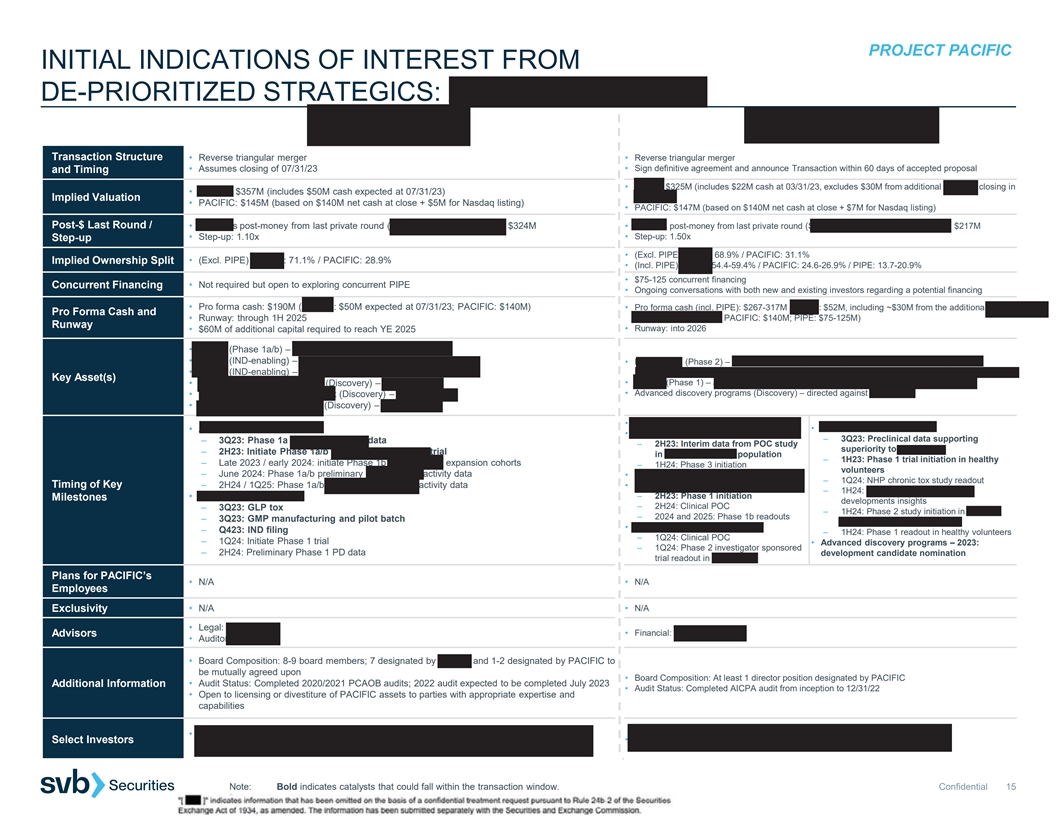
PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS: ASHER BIO | HI BIO Transaction Structure • Reverse triangular merger • Reverse triangular merger • Assumes closing of 07/31/23 • Sign definitive agreement and announce Transaction within 60 days of accepted
proposal and Timing • HI-BIO: $325M (includes $22M cash at 03/31/23, excludes $30M from additional Series A closing in • ASHER: $357M (includes $50M cash expected at 07/31/23) May 2023) Implied Valuation • PACIFIC: $145M (based on
$140M net cash at close + $5M for Nasdaq listing) • PACIFIC: $147M (based on $140M net cash at close + $7M for Nasdaq listing) Post-$ Last Round / • ASHER’s post-money from last private round (Series B, September 2021): $324M
• HI-BIO’s post-money from last private round (Series A extension, November 2022): $217M • Step-up: 1.10x • Step-up: 1.50x Step-up • (Excl. PIPE) HI-BIO: 68.9% / PACIFIC: 31.1% Implied Ownership Split • (Excl.
PIPE) ASHER: 71.1% / PACIFIC: 28.9% • (Incl. PIPE) HI-BIO: 54.4-59.4% / PACIFIC: 24.6-26.9% / PIPE: 13.7-20.9% • $75-125 concurrent financing • Not required but open to exploring concurrent PIPE Concurrent Financing • Ongoing
conversations with both new and existing investors regarding a potential financing • Pro forma cash: $190M (ASHER: $50M expected at 07/31/23; PACIFIC: $140M) • Pro forma cash (incl. PIPE): $267-317M (HI-BIO: $52M, including ~$30M from
the additional Series Pro Forma Cash and • Runway: through 1H 2025 A closing in May 2023; PACIFIC: $140M; PIPE: $75-125M) Runway • $60M of additional capital required to reach YE 2025 • Runway: into 2026 • AB248 (Phase 1a/b)
– CD8-targeted IL2 for solid tumors • AB821 (IND-enabling) – CD8-targeted IL21 for solid tumors • Felzartamab (Phase 2) – anti-CD38 antibody for primary membranous nephropathy (PMN), • AB359 (IND-enabling) –
CD8-targeted IL2 for chronic viral infection immunoglobulin A nephropathy (IgAN), antibody-mediated rejection (AMR) and lupus nephritis (LN) Key Asset(s) • HIB210 (Phase 1) – anti-C5aR1 antibody for severe immune-mediated diseases (IMDs)
• Cell therapy-targeted IL-2 / 21 (Discovery) – for cancer • Advanced discovery programs (Discovery) – directed against mast cells • Myeloid-targeted immune agonist (Discovery) – for cancer • CD4+ T
cell-targeted cytokine (Discovery) – for cancer • Felzartamab – 2Q23: reproductive tox data • AB248 in solid tumors • HIB210 in severe IMDs • Felzartamab in PMN – 3Q23: Preclinical data supporting –
3Q23: Phase 1a monotherapy PD data – 2H23: Interim data from POC study superiority to avacopan – 2H23: Initiate Phase 1a/b anti-PD1 combination trial in aCD20-refractory population – 1H23: Phase 1 trial initiation in healthy
– Late 2023 / early 2024: initiate Phase 1b monotherapy expansion cohorts – 1H24: Phase 3 initiation volunteers – June 2024: Phase 1a/b preliminary monotherapy activity data • Felzartamab in IgAN – 2Q23: Clinical POC
– 1Q24: NHP chronic tox study readout Timing of Key – 2H24 / 1Q25: Phase 1a/b anti-PD1 combination activity data • Felzartamab in LN – 1H24: Subcutaneous formulation – 2H23: Phase 1 initiation • AB821 in solid
tumors Milestones developments insights – 2H24: Clinical POC – 3Q23: GLP tox – 1H24: Phase 2 study initiation in ANCA- – 2024 and 2025: Phase 1b readouts – 3Q23: GMP manufacturing and pilot batch associated vasculitis
(AAV) • Felzartamab in AMR – Q423: IND filing – 1H24: Phase 1 readout in healthy volunteers – 1Q24: Clinical POC – 1Q24: Initiate Phase 1 trial • Advanced discovery programs – 2023: – 1Q24: Phase 2
investigator sponsored – 2H24: Preliminary Phase 1 PD data development candidate nomination trial readout in AMR Plans for PACIFIC’s • N/A • N/A Employees Exclusivity • N/A • N/A • Legal: Goodwin •
Financial: Goldman Sachs Advisors • Auditor: PWC • Board Composition: 8-9 board members; 7 designated by ASHER and 1-2 designated by PACIFIC to be mutually agreed upon • Board Composition: At least 1 director position designated by
PACIFIC Additional Information • Audit Status: Completed 2020/2021 PCAOB audits; 2022 audit expected to be completed July 2023 • Audit Status: Completed AICPA audit from inception to 12/31/22 • Open to licensing or divestiture of
PACIFIC assets to parties with appropriate expertise and capabilities • Third Rock Ventures, Wellington, RA Capital, Invus Public Equities, Boxer Capital, Mission Bay Select Investors • ARCH Venture Partners, Monograph Capital, Jeito
Capital, MorphoSys Capital, Janus Henderson, Logos, Marshall Wace, Y-Combinator Note: Bold indicates catalysts that could fall within the transaction window. Confidential 15

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM DE-PRIORITIZED
STRATEGICS: Q32 BIO Transaction Structure • Reverse triangular merger and Timing • Q32: $250M (includes $31M cash at 03/31/23, excl. upcoming payment of $22.5M from Horizon by the end of 3Q23 and $25M debt facility, of which $5M will be
Implied Valuation used to refinance debt with SVB) • PACIFIC: $155M (based on $140M net cash at close + $15M for Nasdaq listing) • Q32’s post-money from last private round (Convertible note, 2022; post-$ assumes conversion of the
note at Series B price and 5% interest): $185M, including Post-$ Last Round / current option pool Step-up • Step-up: 1.35x Implied Ownership Split • (Excl. PIPE) Q32: 61.7% / PACIFIC: 38.3% • Not required but open to exploring
concurrent PIPE Concurrent Financing • Existing shareholders have signed a letter committing at least $30M to a PIPE, which could be used to extend runway or return a portion of PACIFIC cash to shareholders • Pro forma cash: $213.5M
(Q32: $73.5M, based on 1Q23 cash, $22.5M from Horizon and $20M of new debt; PACIFIC: $140M) Pro Forma Cash and Runway • Runway: into 2H 2026 • ADX-097 (Phase 1) – C3d-mAb fH complement inhibitor for multiple complement-mediated
diseases of the kidney and IgG4 1-5 • ADX-096 (Preclinical) – C3d-mAb CR1 complement inhibitor for complement-mediated diseases 1-10 • Novel nanobodies (Discovery) – C3d-fab fH/CR1 complement inhibitors and next-gen targeted
complement inhibitors for neuromuscular and neurodegenerative Key Asset(s) diseases • ADX-914 (Phase 2) – IL-7/TSLP receptor mAb for atopic dermatitis (collaboration and option deal with Horizon in August 2022 for $55M upfront and up to
$645M milestones and royalties) • ADX-097 in complement-mediated renal diseases – 2024 and 2025: Phase 2 AAV 12-week safety/efficacy and topline data, respectively – 2024 and 2025: Phase 2 renal basket trial 4-week / biomarker
topline data and interim12-week data, respectively – 2H25-1H26: Phase 2/3 trial initiations in complement-mediated renal diseases • ADX-096 – 1H25: IND submission • Novel nanobodies – 2024: DC nomination Timing of Key
Milestones • ADX-914 in atopic dermatitis – 2H23: Receipt of all additional development funding from Horizon – 2H23: Phase 2 study completion – 2H24: Phase 2 study completion in second (undisclosed) indication – Late
2024 – early 2025: Horizon option exercise window (subject to a significant option exercise fee) for the acquisition of all of Q32’s assets related to ADX- 914 • Open to discussing the benefit of retaining members of PACIFIC in key
functions. This could include members of leadership and functional roles including key Plans for PACIFIC’s Employees areas of clinical, regulatory, legal, and finance. Exclusivity • N/A • Legal: Goodwin Advisors • Auditor:
E&Y • Board Composition: Current Q32 board consists of 8 member, expect combined company board to include at least two board members from PACIFIC • Audit Status: 2021 audit completed; 2022 audit underway Additional Information
• Open to structures that would allow PACIFIC to monetize its existing pipeline and IP (and retain the resulting value • $30M committed by existing Q32 shareholders could be used to extend runway further or potentially return a portion
of PACIFIC’s cash to its shareholders • Atlas, OrbiMed, Acorn, Osage University Partners, Abingworth, Sanofi Ventures, CU Healthcare Innovation Fund, Children’s Hospital Colorado Center for Select Investors Innovation, Columbia,
Harvard, BMS Note: Bold indicates catalysts that could fall within the transaction window. Confidential 16

D. INITIAL INDICATION OF INTEREST FROM STRATEGIC WHO HAS
PASSED

PROJECT PACIFIC INITIAL INDICATIONS OF INTEREST FROM STRATEGICS WHO
HAVE PASSED: BOUNDLESS BIO | IMMPACT (1) • Reverse merger; proposing a simultaneous sign and close transaction • Reverse merger; proposing a simultaneous sign and close transaction Transaction Structure • Intends to execute a
definitive merger agreement within 45 days of signing term sheet and exclusivity • Intends to execute a definitive merger agreement within 45 days of signing term sheet and exclusivity and Timing agreement agreement • IMMPACT BIO: $257M
(includes $65M of cash expected at 06/30/23) • IMMPACT BIO: $257M (includes $65M of cash expected at 06/30/23) Implied Valuation (1) (2) • PACIFIC: $50M (based on $40M net cash at close + $10M for Nasdaq listing) • PACIFIC: $50M
(based on $40M net cash at close + $10M for Nasdaq listing) Post-$ Last Round / • IMMPACT BIO’s post-money from last private round (Series B, January 2022): $257M • IMMPACT BIO’s post-money from last private round (Series B,
January 2022): $257M Step-up • Step-up: 1.00x • Step-up: 1.00x • (Excl. PIPE and share buyback) IMMPACT BIO: 63.1% / PACIFIC: 36.9% • (Excl. PIPE and share buyback) IMMPACT BIO: 63.1% / PACIFIC: 36.9% Implied Ownership Split
• (Incl. PIPE and share buyback) IMMPACT BIO: 61.6% / PACIFIC: 12.0% / PIPE: 26.4% • (Incl. PIPE and share buyback) IMMPACT BIO: 61.6% / PACIFIC: 12.0% / PIPE: 26.4% • $110M concurrent PIPE with support from existing investors for
over $75M (term sheet signed with • $110M concurrent PIPE with support from existing investors for over $75M (term sheet signed with Concurrent Financing anchor investor); 30 additional investors in data room anchor investor); 30 additional
investors in data room • Concurrent share buyback of up to $100M • Concurrent share buyback of up to $100M • Pro forma cash: $215M (IMMPACT: $65M; PACIFIC: $140M; PIPE: $110M; Share repurchase of $100M) • Pro forma cash:
$215M (IMMPACT: $65M; PACIFIC: $140M; PIPE: $110M; Share repurchase of $100M) Pro Forma Cash and Runway • Runway: Until early 2026 • Runway: Until early 2026 • IMPT-314 (Ph. 1) – CD19/CD20 bispecific CAR T cell therapy for
aggressive B-cell lymphoma (ABCL) • IMPT-314 (Ph. 1) – CD19/CD20 bispecific CAR T cell therapy for aggressive B-cell lymphoma (ABCL) • IMPT-514 (IND-enabling) – CD19/CD20 bispecific CAR T cell therapy for autoimmune diseases
• IMPT-514 (IND-enabling) – CD19/CD20 bispecific CAR T cell therapy for autoimmune diseases Key Asset(s) • TGF-β platform (Discovery) – TGF-β in combination with a binder for IL13 receptor α2 (IL13Ra2) for
• TGF-β platform (Discovery) – TGF-β in combination with a binder for IL13 receptor α2 (IL13Ra2) for glioblastoma multiforme glioblastoma multiforme • IMPT-514 in r/r lupus nephritis • IMPT-514 in r/r lupus
nephritis • IMPT-314 in ≥3L CAR-T naïve ABCL – Mid 2023: IND clearance • IMPT-314 in ≥3L CAR-T naïve ABCL – Mid 2023: IND clearance – 2H23: Phase 1 data – 3Q24: Phase 1 first cohort data
– 2H23: Phase 1 data – 3Q24: Phase 1 first cohort data – 4Q24: Phase 2 pivotal data – 1Q25: EOP1 meeting – 4Q24: Phase 2 pivotal data – 1Q25: EOP1 meeting – 3Q25: Phase 2 pivotal data update – 3Q25:
Phase 2 pivotal data – 3Q25: Phase 2 pivotal data update – 3Q25: Phase 2 pivotal data – 1Q26: BLA submission – 2Q26: BLA submission – 1Q26: BLA submission – 2Q26: BLA submission • IMPT-314 in CAR-T
experienced ABCL • IMPT-514 in severe SLE with extrarenal disease • IMPT-314 in CAR-T experienced ABCL • IMPT-514 in severe SLE with extrarenal disease – 1Q24: Phase 1 data – 1Q24: IND amendment – 1Q24: Phase 1
data – 1Q24: IND amendment – 2Q24: EOP1 meeting – 4Q24: Patient data – 2Q24: EOP1 meeting – 4Q24: Patient data Timing of Key Milestones – 2H24: Phase 2 data update – 3Q26: BLA submission – 2H24: Phase
2 data update – 3Q26: BLA submission – 1H25: BLA submission • IMPT-514 in ANCA-associated vasculitis – 1H25: BLA submission • IMPT-514 in ANCA-associated vasculitis • IMPT-314 in 2L hematopoietic cell transplant
– 2Q24: IND amendment • IMPT-314 in 2L hematopoietic cell transplant – 2Q24: IND amendment (HCT) unintended ABCL – 1H25: Patient data (HCT) unintended ABCL – 1H25: Patient data – 1Q25: Patient data – 4Q26:
BLA submission – 1Q25: Patient data – 4Q26: BLA submission – 1H26: BLA submission • IMPT-514 in secondary progressive MS – 1H26: BLA submission • IMPT-514 in secondary progressive MS • IMPT-314 – 1Q26:
patient data in 2L HCT eligible – 3Q24: IND amendment • IMPT-314 – 1Q26: patient data in 2L HCT eligible – 3Q24: IND amendment ABCL – Mid 2025: Patient data ABCL – Mid 2025: Patient data – 4Q26: BLA
submission – 4Q26: BLA submission Plans for PACIFIC’s • If specific PACIFIC employees fulfill open roles, they can be considered for combined company • If specific PACIFIC employees fulfill open roles, they can be considered
for combined company Employees Exclusivity • N/A • N/A • Financial: Jefferies • Financial: Jefferies Advisors • Audit: Deloitte • Audit: Deloitte • Legal: Cooley • Legal: Cooley • Board
Composition: Proportionate to the equity split and include combined company’s CEO (or as agreed • Board Composition: Proportionate to the equity split and include combined company’s CEO (or as agreed upon in sign and close
structure to not require vote until post-close) upon in sign and close structure to not require vote until post-close) • Audit Status: 2 years of audited statements can be available within 3 months • Audit Status: 2 years of audited
statements can be available within 3 months Additional Information • Proposes announcing PACIFIC share buyback upon announcement of transaction for up to $100M • Proposes announcing PACIFIC share buyback upon announcement of transaction
for up to $100M • May assign value to PACIFIC’s assets on case-by-case basis; open to PACIFIC monetizing assets prior • May assign value to PACIFIC’s assets on case-by-case basis; open to PACIFIC monetizing assets prior to
transaction close to transaction close • Bukwang Pharmaceutical, Decheng Capital, Foresite Capital Management, FutuRx, Hayan Health • Bukwang Pharmaceutical, Decheng Capital, Foresite Capital Management, FutuRx, Hayan Health Select
Investors Networks, JJDC, JVC Investment Partners, Novartis Venture Fund, OrbiMed, RM Global Partners, Networks, JJDC, JVC Investment Partners, Novartis Venture Fund, OrbiMed, RM Global Partners, RMGLOBAL Healthcare Fund, Surveyor Capital, Takeda
Ventures, venBio RMGLOBAL Healthcare Fund, Surveyor Capital, Takeda Ventures, venBio (1): BOUNDLESS BIO would consider a scenario that dividends any cash net of $100M from the PACIFIC balance sheet back to PACIFIC shareholders. The revised
transaction structure was communicated following feedback on the initial proposal regarding returning PACIFIC cash to pre-merger PACIFIC shareholders via dividend. (2): IMMPACT BIO plans to announce a share buyback upon announcement of the
transaction for up to $110M to provide liquidity to PACIFIC stockholders seeking to exit the transaction per the Company’s revised proposal dated 06/09/23. The original proposal planned for a $100M share buyback. Note: Bold indicates catalysts
that could fall within the transaction window. Confidential 18

E. ILLUSTRATIVE PROCESS TIMELINE

PROJECT PACIFIC ILLUSTRATIVE PROCESS TIMELINE MONTH M MA AY Y JUNE
WEEK OF 8 8 15 15 2 22 2 2 29 9 5 5 12 19 26 Weekly reoccurring meeting to track process Finalize private company targets 5 5/9 /9 Formally initiate outreach; send process letters to 5/10 - 5/10 - 5 5/ /12 12 interested parties Receive initial
proposals from private targets 5/ 5/23 23 Special committee meeting to evaluate proposals 5 5/ /24 24 Special committee meeting to confirm 2-3 private targets 5/26 5/26 for further diligence Management presentations 6/ 6/1 1 –– 6/3 6/
Due diligence 6/5-6/16 Receipt of final proposals By 6/16 Special committee to determine interest in including 5/26 5/26 financial buyers in process Initiate outreach to financial buyers 5/ 5/26 26- -5/3 5/31 Receive proposals from financial buyers
6/9 Special committee meeting to review private target and 6/11 financial buyer proposals Confidential 20 Financial Buyer Private Company Merger
Pardes Biosciences (NASDAQ:PRDS)
Historical Stock Chart
From Apr 2024 to May 2024
Pardes Biosciences (NASDAQ:PRDS)
Historical Stock Chart
From May 2023 to May 2024