Kala Pharmaceuticals Stock Jumps 79% as FDA Accepts Drug Application For Eye Condition
December 27 2022 - 4:49PM
Dow Jones News
By Sabela Ojea
Shares of Kala Pharmaceuticals Inc. on Tuesday jumped about 79%
in after-hours trading after the company said the U.S. Food and
Drug Administration accepted its investigational new drug
application for the treatment of an eye rare condition through its
KPI-012 product candidate.
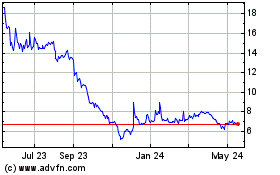
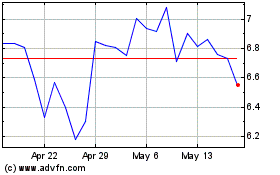
Shares were trading about 79% higher at $7.01. For the year,
shares are down about 94%.
The pharmaceutical company said KPI-012 is aimed at treating
persistent corneal epithelial defects, which can result after a
corneal injury.
"We are working closely with investigators to initiate our Phase
2b clinical trial of KPI-012 for PCED in the first quarter of
2023," Chief Medical Officer Kim Brazzell said.
Write to Sabela Ojea at sabela.ojea@wsj.com; @sabelaojeaguix
(END) Dow Jones Newswires
December 27, 2022 16:34 ET (21:34 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
KALA BIO (NASDAQ:KALA)
Historical Stock Chart
From May 2024 to Jun 2024
KALA BIO (NASDAQ:KALA)
Historical Stock Chart
From Jun 2023 to Jun 2024