Boston Scientific Says FDA Clears Exalt Model D Single-Use Duodenoscope
December 13 2019 - 2:14PM
Dow Jones News
By Michael Dabaie
Boston Scientific Corp. (BSX) said the U.S. Food and Drug
Administration gave 510(k) clearance of the Exalt Model D
Single-Use Duodenoscope for use in endoscopic retrograde
cholangiopancreatography procedures.
The EXALT Model D Duodenoscope is a disposable duodenoscope and
was granted Breakthrough Device Designation from the FDA, Boston
Scientific said.
The device was been developed as an alternative to reusable
duodenoscopes, eliminating the need for duodenoscope reprocessing
and repairs, and allowing physicians to use a new, sterile device
for every procedure.
The company plans to commence a limited market release of the
device in the U.S. during the first quarter of 2020.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
December 13, 2019 13:59 ET (18:59 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
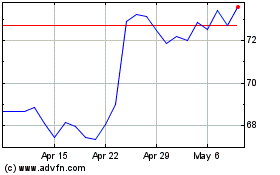
Boston Scientific (NYSE:BSX)
Historical Stock Chart
From Mar 2024 to Apr 2024
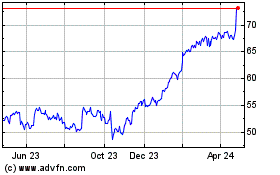
Boston Scientific (NYSE:BSX)
Historical Stock Chart
From Apr 2023 to Apr 2024