UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 13, 2016
INTREXON CORPORATION
(Exact Name of Registrant as Specified in Charter)
|
|
|
|
|
| Virginia |
|
001-36042 |
|
26-0084895 |
| (State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
20374 Seneca Meadows Parkway, Germantown, Maryland 20876
(Address of Principal Executive Offices) (Zip Code)
(301) 556-9900
(Registrant’s telephone number, including area code)
N/A
(Former name or
former address, if changed since last report)
Check the appropriate box below
if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01 |
Regulation FD Disclosure |
On January 13, 2016, Intrexon Corporation (the
“Company”) will deliver a presentation at the 2016 J.P. Morgan Healthcare Conference. The slides to be used during the presentation are furnished herewith as Exhibit 99.1 and are incorporated by reference into Item 7.01 of this
Current Report on Form 8-K.
As provided in General Instruction B.2 of Form 8-K, the information in this Item 7.01 and the exhibit
furnished hereunder will not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, nor will they be deemed to be incorporated by reference in any filing under the Securities Act of
1933, as amended, except as will be expressly set forth by specific reference in such a filing.
| Item 9.01. |
Financial Statements and Exhibits. |
See the Exhibit Index immediately following the signature page hereto, which is
incorporated herein by reference.
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
Date: January 13, 2016
|
|
|
| INTREXON CORPORATION |
|
|
| By: |
|
/s/ Donald P. Lehr |
|
|
Donald P. Lehr |
|
|
Chief Legal Officer |
3
EXHIBIT INDEX
|
|
|
| Exhibit
Number |
|
Description |
|
|
| 99.1 |
|
Intrexon Corporation Investor Presentation, dated January 13, 2016 |
4

Enabling Next-Generation Gene and Cell
Therapies with Better DNA® 34th Annual J.P. Morgan Healthcare Conference Samuel Broder, M.D. Senior Vice President, Health Sector Exhibit 99.1

Forward-Looking Statements Safe Harbor
Statement Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the Safe harbor Provisions of the Private Securities Litigation Reform Act of
1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s
business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be
materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s current and future ECCs and joint ventures; (ii) Intrexon’s
ability to successfully enter new markets or develop additional products, whether with its collaborators or independently; (iii) actual or anticipated variations in Intrexon’s operating results; (iv) actual or anticipated fluctuations in
Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (v) Intrexon’s cash position; (vi) market conditions in Intrexon’s industry; (vii) Intrexon’s ability,
and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (viii) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations
and policies; (ix) the rate and degree of market acceptance of any products developed by a collaborator under an ECC or through a joint venture; (x) Intrexon’s ability to retain and recruit key personnel; (xi) Intrexon’s expectations
related to the use of proceeds from its public offerings and other financing efforts; (xii) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xiii) Intrexon’s
expectations relating to its subsidiaries and other affiliates. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the
forward-looking statements, see the section entitled ”Risk Factors“ in Intrexon’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Intrexon’s subsequent
filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law. Non-GAAP Financial Measures This press
release presents Adjusted EBITDA and Adjusted EBITDA per share, which are non-GAAP financial measures within the meaning of applicable rules and regulations of the Securities and Exchange Commission (SEC). For a reconciliation of Adjusted EBITDA to
net loss attributable to Intrexon in accordance with generally accepted accounting principles and for a discussion of the reasons why the company believes that these non-GAAP financial measures provide information that is useful to investors see the
tables below under “Reconciliation of GAAP to Non-GAAP Measures.” Such information is provided as additional information, not as an alternative to Intrexon’s consolidated financial statements presented in accordance with GAAP, and
is intended to enhance an overall understanding of the Company’s current financial performance. © 2016 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such
materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such
securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as
to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise
under law upon distribution of any prospectus or similar offering document by Intrexon.

A Single Molecule at the Center of
Biology

Powering the Bioindustrial
Revolution™ Markets Human Plant Animal Bacteria Fungi HEALTH Gene and cellular therapies, API, biologics ENERGY Fuels, chemicals ENVIRONMENT Insect control, bioremediation FOOD Livestock, agriculture, aquaculture CONSUMER Personal care
products UltraVector® Platform Gene Expression Control and Optimization including RheoSwitch® gene switch DNA/RNA/Protein Engineering Broad Gene Delivery Expertise Multigenic Gene Systems Synthetic & Hybrid Modular Parts Bioinformatics
Genome Engineering including AttSite™ Recombinases Intrexon’s Better DNA® genetic technologies and expression host expertise target large markets and commercial opportunities Better DNA® Hosts Insect

Extensive Expertise in DNA Delivery
Electroporation Chemical Transduction Direct Injection Autologous Allogeneic Adeno- virus (Ad) Adeno-associated Virus (AAV) Retrovirus / Lentivirus AttSite™ Recombinases attR attL 5’ Cap Poly AAA mRNA Stem Cells Viral Delivery Utilize
many viruses and also generate a variety of serotype vectors for optimal delivery Non-Viral DNA/mRNA Delivery Provides for low immunogenicity and ability to accommodate large DNA constructs Cellular Delivery Engineer cells ex vivo to express
therapeutic genes and deliver to targeted sites including specific tissues Our broad expertise in DNA delivery includes a wide array of solutions to deliver payloads and drive optimal expression Sleeping Beauty

Intrexon’s Better DNA®
Engine Cellular Biofactories* Fibroblasts T Cells * Not complete list. Active health sector programs utilize cartilage cells, iPSCs, cardiomyocytes, EggPC™ cells, among others. Retinal L. lactis After Intrexon Bioengineering T cells engineered
with CARs or TCRs to better recognize cancer cells Retinal cells programmed to express certain proteins to treat blinding diseases Targeted expression of effectors in situ to treat GI/oral and other diseases A. B. Fibroblast cells re-wired to
produce or shutdown specific protein expression Reprogramming cells’ genetic code to drive new functionality with (A) single gene programs or (B) more complex multigene programs utilizing proprietary parts and engineering expertise Optimized
cell lines with significantly improved production of targeted APIs Yeast Engineer DNA constructs to correct gene defects in numerous CNS disorders Neuronal Re-tooling Cellular Engines for New Solutions in Health

Business Model Built to Capture Large
Market Opportunity Business model initiated in 2011 enabling broad application of our technology across many diverse end markets includes Exclusive Channel Collaborations (ECCs) and Research Collaborations Through our ECC model, Intrexon’s
cumulative TAF and milestone payments to date equal $240 million, cost recovery exceeds $102 million*, and our potential royalties for our collaborations range from single digit net sales to 50% net operating profits January 2011 1st ECC January
2016 30 ECCs and Research Collaborations * Cost recovery through Q3’2015 Exclusive Channel Collaborator (ECC) Exclusive Commercial License in a Field Strategic Access to Intrexon’s Technology Commercialize Valuable Products Intrexon
Technology Access Fees (TAF) Cost recovery / R&D Reimbursement Milestones Royalties

* Post split of all economics of deal
with biopharmaceutical business of Merck KGaA with ZIOPHARM Oncology including upfront and potential milestones. Including those economics would result in $245.5 million in TAF/milestones to date and >$1.1 Billion in potential milestones for the
health sector. $188 Million* in Technology Access Fees & Milestones Received to Date >$700 Million* in Potential Milestones in Current Pipeline R&D reimbursed and backend royalties across all programs Over 20 Programs and Collaborations
in our Health Sector Broad Engagement in Health Sector

Our Health Collaborations Across
Various Conditions Oncology: CAR-T, TCR, Gene/Cell Therapy Infectious Diseases: C. difficile, Pertussis Ocular Disease: Wet AMD Rare Skin Disorders: RDEB, Linear Scleroderma Rare Diseases: Friedreich’s Ataxia Cardiac Diseases: Heart Failure
Cartilage Repair: Off-The-Shelf Cells Metabolic and GI Disorders: Type 2 Diabetes, Oral mucositis, PKU Autoimmune: Allergies, GvHD Programs Utilizing Intrexon Technology Human Infertility: In Vitro Egg Maturation 2013 2012 2014 2015 Today
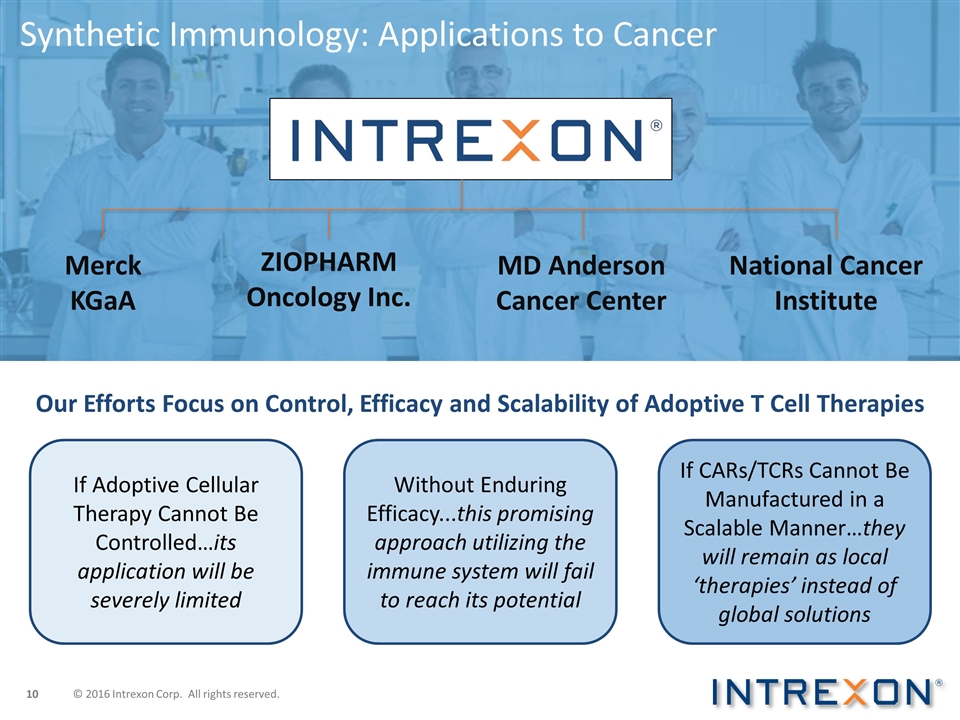
Synthetic Immunology: Applications
to Cancer Merck KGaA ZIOPHARM Oncology Inc. MD Anderson Cancer Center National Cancer Institute If Adoptive Cellular Therapy Cannot Be Controlled…its application will be severely limited Without Enduring Efficacy...this promising approach
utilizing the immune system will fail to reach its potential If CARs/TCRs Cannot Be Manufactured in a Scalable Manner…they will remain as local ‘therapies’ instead of global solutions Our Efforts Focus on Control, Efficacy and
Scalability of Adoptive T Cell Therapies

CAR-T: Intrexon / Merck KGaA,
Darmstadt, Germany *Note that timelines are event-driven and may change Potential differentiation and timelines: Potential to address limitations of 1st / 2nd generation gene and cell-based therapy May improve safety profile of CAR-Ts through
platform technologies including RheoSwitch® Intrexon engineering potential universal “off-the-shelf” treatment approach During Q2’2015 Merck KGaA selected first two novel CAR T targets of interest Furthered R&D efforts and
advanced these two targets in 2H of 2015 2016: Preclinical/clinical CAR-T cells targeting hematological tumors 2017: Test next-gen CAR-T cells for efficacy in solid tumors Intrexon / Biopharmaceutical business of Merck KGaA CAR T deal terms:
Intrexon received upfront payment of $115 million For first two CAR T targets of interest selected Intrexon will receive research funding and is eligible for up to $826 million in development and commercial milestones Intrexon also eligible for
certain technology development milestones Tiered royalties up to lower-double digits of net sales Intrexon shares economics of Merck KGaA collaboration equally with ZIOPHARM Oncology

Intrexon & ZIOPHARM: Delivering
Multimodal Gene Therapies Controlled Gene Therapy Adoptive Cell Therapies Viral & Non-Viral Gene Integration Autologous and Allogeneic Approach Sleeping Beauty Lentivirus & other viral delivery T Cells NK Cells (CAR T & TCR)

The Importance of Sleeping Beauty
“The Sleeping Beauty transposon-transposase system represents a unique non-viral system for introducing genes encoding T-cell receptors and chimeric antigen receptors into lymphocytes that can be of great value in the development of
personalized immunotherapies for patients with cancer.” Steven A. Rosenberg M.D., Ph.D. December 2015 Lowers cost of generating genetically modified T cells Conduit to Targeting Solid Tumors with TCRs Key to Unlock Personalized T Cell Therapy
Potential to Generate T cells with Minimal Ex Vivo Processing Advantages of Non-viral Sleeping Beauty Paradigm

In vivo model for myeloid
malignancies CAR-T target Lentiviral CAR+ T cells Platform Target CAR T cells for tumors with unmet needs T cells Lentivirus Intrexon and ZIOPHARM are utilizing lentiviral delivery for tumors with unmet needs outside of the crowded viral CD19+ CAR T
treatment landscape. We are rapidly advancing a CAR T target for myeloid malignancies. With encouraging pre-clinical data including CAR expression, cytotoxicity, and IFNγ production, a clinical trial is planned for 2016. Saline Untransduced T
cells Targeted CAR T Cells Non-targeted CAR T Cells Transduce T cells Expansion

Collaboration with MD Anderson
Cancer Center 2015 Development: Fully leveraging our clinical collaboration with MDACC to rapidly evaluate various strategies not limited to competitive CARs or TCRs Trial initiated in Q4’2015 with next-gen CD19 CAR design utilizing
Sleeping Beauty 2016 and Beyond: Exploring cutting edge immunology to harness the full capability of gene modified adoptive cell types, such as various immune-modulatory strategies driving T cell or NK cell anti-tumor activity: PD-1 and CTLA-4 knock
down Chimeric co-stimulatory receptors Transcription factors Cytokines (IL-2, IL-7, IL-15, IL-21 and IL-12) Chemokines

Next-gen RTS® Controlled Gene
Therapy Approach to Wet AMD Intrexon and Sun Pharmaceuticals formed joint venture to develop gene therapies for treatment of blinding ocular diseases 1st target is Wet Age-related Macular Degeneration (AMD); market est’d at $6B
Adeno-associated viral (AAV) delivery transduces target cells without systemic exposure and AAV ocular administration doesn’t require immuno-suppressants Next generation approach to Wet AMD utilizes RheoSwitch® platform for tunable
biologic therapy via eye drops or oral pills Anticipate filing IND in 2H 2016 Activator Ligand Delivered via Eye Drops or Pills Protein of Interest Gene of Interest

+AL -AL +AL Eyes harvested Day 0,
14, 24, 34 On-Off-On Expression with RheoSwitch® Platform AAV-RTS-EPO AAV2-RTS-IFNα In vivo pre-clinical data demonstrated ‘On-Off-On’ effect via RTS® platform and tight control with multiple proteins

RTS® Regulates Lead Therapeutic
Candidate Efficacy observed only with lead therapeutic candidate in presence of RTS® activator ligand veledimex AAV-RTS-XON AAV-RTS-XON AAV-CMV-FLuc No Treatment

Re-Engineering Fibroblasts into
Biotherapeutics Intrexon’s Gene Therapy Product Engine for FCX-007, FCX-013… Human Autologous Fibroblast Product (azficel-T BLA) Collection Culture Local Administration Vector Preparation Gene Packaging Gene Transduction FCX-007 –
Orphan drug candidate for Recessive Dystrophic Epidermolysis Bullosa (RDEB) , a debilitating disease with no approved treatment often lethal before the age of 30. Anticipate Phase I/II trial initiation in Q2’2016. FCX-013 – Drug
candidate for Linear Scleroderma which affects movement and skin development. Anticipate 2016 IND filing. New GM fibroblasts to treat chronic inflammatory and degenerative diseases of the joint
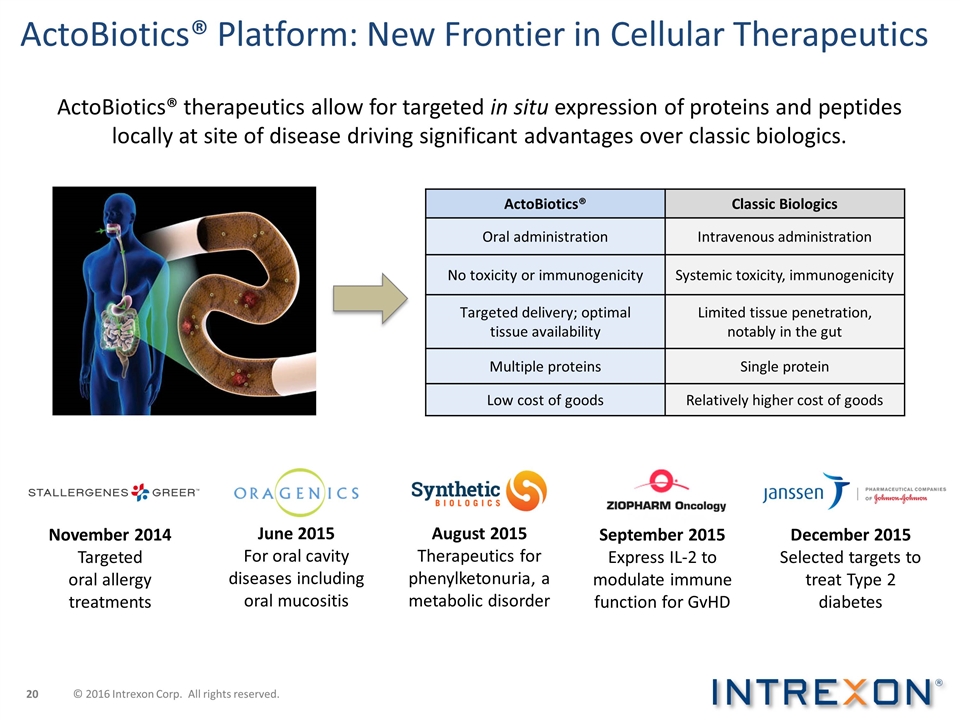
ActoBiotics® Classic Biologics
Oral administration Intravenous administration No toxicity or immunogenicity Systemic toxicity, immunogenicity Targeted delivery; optimal tissue availability Limited tissue penetration, notably in the gut Multiple proteins Single protein Low cost of
goods Relatively higher cost of goods November 2014 Targeted oral allergy treatments June 2015 For oral cavity diseases including oral mucositis August 2015 Therapeutics for phenylketonuria, a metabolic disorder September 2015 Express IL-2 to
modulate immune function for GvHD December 2015 Selected targets to treat Type 2 diabetes ActoBiotics® therapeutics allow for targeted in situ expression of proteins and peptides locally at site of disease driving significant advantages over
classic biologics. ActoBiotics® Platform: New Frontier in Cellular Therapeutics

Oral ActoBiotics® Stomach
Gastric emptying Pancreas Insulin secretion Glucagon secretion Liver Brain Appetite Glucose production Insulin sensitivity Muscle Adipose tissue Glucose uptake and storage Glucose uptake, Storage & utilization Adapted from
Gastroenterology 2007 132, 2131-2157 Intestinal Delivery Direct Effects Indirect Effects or Both Oral Tolerance Unique Opportunities in Diabetes with Oral ActoBiotics®

Advancement of Porcine to Human
Organ Xenotransplantation Organ Waiting List Transplant Kidney 108,238 14,879 Liver 15,275 5,651 Pancreas 1,040 187 Heart 4,198 2,331 Lung 1,552 1,694 Heart / Lung 49 12 Intrexon’s integrated technologies and proprietary platforms, including
our genetically diverse LoneStar™ Yucatan mini-swine families, offer end-to-end solutions for engineering of cells and organs for xenotransplantation products Global market for organ and tissue transplantation products and devices reached
$59.6 billion in 2014 and is estimated to reach $90 billion by 2020.* A critical shortage of suitable human organs for clinical transplantation driving increasing demand for synthetic and/or xenogenic organs. *BCC Research,
http://www.prweb.com/releases/2015/05/prweb12708017.htm

Attacking Global Health Epidemics at
their Source * http://www.idcostcalc.org/contents/dengue/index.html Mandacaru Itaberaba Panama 96% 92% 99% 94% 93% Pedra Branca Cayman Islands Aedes aegypti Oxitec’s lead health program centers on the primary vector for dengue, chikungunya,
yellow fever and Zika virus, the Aedes aegypti mosquito Efficacy field trials of Oxitec’s vector control solution in Brazil, Panama, and Cayman Islands show a 92-99% reduction of Aedes aegypti. Dengue virus is the fastest growing vector-borne
disease now present in +100 countries with up to 400 million people infected yearly according to CDC. Global cost of dengue is estimated to be $17 Billion including vector control, mortality and healthcare. Zika virus first two major outbreaks
occurred in 2007 and 2013 in islands in the Pacific Ocean. Now present in +10 countries in the Americas and source of new microcephaly epidemic in Brazil. Chikungunya outbreaks have occurred in parts of Africa, Europe, Southeast Asia, and islands in
the Indian/Pacific Oceans. In 2013, chikungunya was found for the first time in the Americas and between 2013-2014 over 1.1 million suspected cases were reported by PAHO.

New Zika Virus Epidemic Brazilian
cases of microcephaly (a birth defect that may be linked to Zika virus infection) in thousands December 1st, 2015 – World Health Organization (WHO) and Pan American Health Organization (PAHO) issued epidemiological alert given “increase
of congenital anomalies, Guillain-Barré syndrome, and other neurological and autoimmune syndromes in areas where Zika virus is circulating and their possible relation to the virus…In addition, Member States should continue efforts to
reduce the presence of mosquito vectors through an effective vector control strategy...”

Brazil: A Case Study Early 1970s No
Aedes aegypti No dengue Recent years 1.5 million cases of dengue in 2015, up 176% y-o-y with 811 dengue-related deaths First local transmission of chikungunya in 2014 and Zika virus entered in 2015 Over $1 billion spent on the dengue vector control
program annually Oxitec in Brazil – 2011 to Present Trials 2011–13 National Biosafety Approval granted 2014 Oxitec do Brasil established In April 2015, Piracicaba became world’s first municipality to release Oxitec mosquitoes 96%
of local Piracicaba residents support the Oxitec program Brazil press describe Oxitec solution as Aedes aegypti do bem: ‘the good mosquito’

Health Sector: 2016 Outlook &
XON/Collaborator Milestones Anticipate up to three INDs filed in oncology including lentiviral CAR T myeloid malignancies treatment, primary NK cells for AML, and gene therapy combining checkpoint inhibitor with Ad-RTS-IL-12 for GBM Anticipate IND
filing for next-gen RTS® controlled gene therapy for Wet AMD Anticipate Phase I/II trial initiation for rare skin disorder (RDEB) with FCX-007 Anticipate IND filing for rare skin disorder (Linear Scleroderma) with FCX-013 Anticipate IND filing
for C. difficile utilizing pioneering OG253 lantibiotic Potential Phase II clinical trial initiation for oral mucositis with ActoBiotics® Potential ANVISA approval for Oxitec’s solution in Brazil Launch of Oxitec solution in other regions
and expansion of pipeline to new vectors for other mosquito-borne diseases Expansion of API program targeting alkaloid production from microbes Continued progress on existing programs with potential data readouts on our ground-breaking multi-gene
cardiac therapy, EggPC℠ cell maturation in vitro for OvaTure℠ development, bacteriophage-based therapeutics, and treatments for infectious diseases Continued expansion of pipeline and collaborations across a number of new disease areas
including longevity, lysosomal storage diseases, Parkinson's disease, Huntington’s disease, Alzheimer's disease, amyotrophic lateral sclerosis, ischemia and spinal cord injury, epilepsy, diabetes, and xenotransplantation Strong cash position
of $352.6 million* *As of Q3’2015 Up to 8 INDs and clinical trial initiations Expansion of pipeline and collaborations

APPENDIX

Appendix: Diversified
XON/Collaborator Health Pipeline Field / Collaborators Indications Pre-Clinical Phase I Phase II Oncology (ZIOPHARM, Merck Serono, National Cancer Institute) Ad-RTS-IL-12: Breast cancer Malignant Glioma CAR/Cytokine Products: B-Cell Malignancies
Myeloid Malignancies RTS-Controlled T Cell Solid Tumors & Universal Donor Adoptive T Cell Immunotherapy: RTS-Controlled IL-12 in PBL GI & Oral Diseases (Intrexon NV) Inflammatory Bowel Disease – AG014 Autoimmune, Allergy &
Microbiome Oral Disease (Oragenics) Oral Mucositis – AG013 Allergic Diseases (STALLERGENES) ActoBiotics® Expressing Allergens Ocular Diseases (Sun Pharma) Age-related Macular Degeneration Orphan Ocular Diseases Rare Skin Disorders
(Fibrocell) Recessive Dystrophic Epidermolysis Bullosa Linear Scleroderma Auto-Immune (ZIOPHARM) Graft-versus-Host-Disease Rare Disease (Agilis) Friedreich’s Ataxia Infertility (OvaScience) Egg Cell Maturation Cartilage Repair (Histogenics)
Allogeneic Chondrocyte Cells Biotherapeutics (Synthetic Biologics) Pertussis Phenylketonuria Infectious Diseases (Oragenics) Lantibiotics for C. difficile

Appendix: Third Quarter and Nine
Month Results Fiscal Year 2015 Financials Third Quarter YTD Revenues $53.4 M $132.1 M Net loss attributable to Intrexon $38.2 M $51.8 M Basic EPS $(0.34) $(0.47) Adjusted EBITDA $3.8 M* $43.6 M* Basic adjusted EBITDA per share $0.03* $0.40* Total
Cash and Investments (excluding equity securities) $352.6 Million Total Equity Securities In Connection With ECCs $76.6 Million Basic weighted average shares outstanding 112.2 million YTD Cost Recovery revenues & Deal Money as % total cash opex
exclusive of operating expenses of consolidated subs 164%** YTD Cost Recovery revenues, Deal Money, and products and services revenues as % total cash opex 135% * Non-GAAP financial measure ** Excludes operating expenses of majority-owned
consolidated subsidiaries.

Appendix Intrexon Corporation and
Subsidiaries Reconciliation of GAAP to Non-GAAP Measures (Unaudited) Adjusted EBITDA and Adjusted EBITDA per share. To supplement Intrexon’s financial information presented in accordance with U.S. generally accepted accounting principles
(“GAAP”), Intrexon presents Adjusted EBITDA and Adjusted EBITDA per share. A reconciliation of Adjusted EBITDA to Intrexon’s net income or loss attributable to Intrexon under GAAP appears below. Adjusted EBITDA is a non-GAAP
financial measure that Intrexon calculates as net income or loss attributable to Intrexon adjusted for income tax expense or benefit, interest expense, depreciation and amortization, stock-based and executive incentive compensation, contribution of
services by shareholder, noncash research and development expenses related to the acquisition of Intrexon’s license agreement with the University of Texas MD Anderson Cancer Center, realized and unrealized appreciation or depreciation in the
fair value of equity securities, equity in net loss of affiliate and the change in deferred revenue related to upfront and milestone payments. Adjusted EBITDA and Adjusted EBITDA per share are key metrics for Intrexon’s management and Board of
Directors for evaluating the Company’s financial and operating performance, generating future operating plans and making strategic decisions about the allocation of capital. Management and the Board of Directors believe that Adjusted EBITDA
and Adjusted EBITDA per share are useful to understand the long-term performance of Intrexon’s core business and facilitate comparisons of the Company’s operating results over multiple reporting periods. Intrexon is providing this
information to investors and others to assist them in understanding and evaluating the Company’s operating results in the same manner as its management and board of directors. While Intrexon believes that these non-GAAP financial measures are
useful in evaluating its business, and may be of use to investors, this information should be considered as supplemental in nature and is not meant as a substitute for the related financial information prepared in accordance with GAAP. In addition,
these non-GAAP financial measures may not be the same as non-GAAP financial measures presented by other companies. Adjusted EBITDA and Adjusted EBITDA per share are not measures of financial performance under GAAP, and are not intended to represent
cash flows from operations nor earnings per share under GAAP and should not be used as an alternative to net income or loss as an indicator of operating performance or to represent cash flows from operating, investing or financing activities as a
measure of liquidity. Intrexon compensates for the limitations of Adjusted EBITDA and Adjusted EBITDA per share by using them only to supplement the Company’s GAAP results to provide a more complete understanding of the factors and trends
affecting the Company’s business. Adjusted EBITDA and Adjusted EBITDA per share have limitations as an analytical tool and you should not consider them in isolation or as a substitute for analysis of Intrexon’s results as reported under
GAAP. In addition to the reasons stated above, which are generally applicable to each of the items Intrexon excludes from its non-GAAP financial measure, Intrexon believes it is appropriate to exclude certain items from the definition of Adjusted
EBITDA for the following reasons: Interest expense may be subject to changes in interest rates which are beyond Intrexon’s control; Depreciation of Intrexon’s property and equipment and amortization of acquired identifiable intangibles
can be affected by the timing and magnitude of business combinations and capital asset purchases; Stock-based compensation expense is a noncash expense and may vary significantly based on the timing, size and nature of awards granted and also
because the value is determined using formulas which incorporate variables, such as market volatility. Executive incentive compensation expenses are determined by the compensation committee of the Board of Directors at the end of the year and may be
based on, among other items, the results of Adjusted EBITDA, exclusive of such expenses. Contribution of services by shareholder is a noncash expense which Intrexon excludes in evaluating its financial and operating performance; Unrealized and
realized appreciation or depreciation in the fair value of securities which Intrexon holds in its collaborators may be significantly impacted by market volatility and other factors which are outside of the Company’s control in the short term
and Intrexon intends to hold these securities over the long term except as provided above; Equity in net loss of affiliate reflects Intrexon’s proportionate share of the income or loss of entities over which the Company has significant
influence, but not control, and accounts for using the equity method of accounting. The Company’s acquisition of the license agreement with the University of Texas MD Anderson Cancer Center was a noncash expense Intrexon incurred to obtain
access to specific technologies, which are strategic to the Company. Intrexon believes excluding the impact of such losses or gains on these types of strategic investments from its operating results is important to facilitate comparisons between
periods; and GAAP requires Intrexon to account for its collaborations as multiple-element arrangements. As a result, the Company defers certain collaboration revenues because certain of its performance obligations cannot be separated and must be
accounted for as one unit of accounting. The collaboration revenues that Intrexon so defers arise from upfront and milestone payments received from the Company’s collaborators, which Intrexon recognizes over the future performance period even
though the Company’s right to such consideration is neither contingent on the results of Intrexon’s future performance nor refundable in the event of nonperformance. In order to evaluate Intrexon’s operating performance, its
management adjusts for the impact of the change in deferred revenue for these upfront and milestone payments in order to include them as a part of adjusted EBITDA when the transaction is initially recorded. The adjustment for the change in deferred
revenue removes the noncash revenue recognized during the period and includes the cash and stock received from collaborators for upfront and milestone payments during the period. Intrexon believes that adjusting for the impact of the change in
deferred revenue in this manner is important since it permits the Company to make quarterly and annual comparisons of the Company’s ability to consummate new collaborations or to achieve significant milestones with existing collaborators.
Further, Intrexon believes it is useful when evaluating its financial and operating performance, generating future operating plans and making strategic decisions about the allocation of capital.. Continued on next slide…

Appendix (continued) The following
table presents a reconciliation of net income attributable to Intrexon to EBITDA and also to Adjusted EBITDA, as well as the calculation of Adjusted EBITDA per share, for each of the periods indicated: Three months endedNine months endedSeptember
30,September 30,2015201420152014(In thousands)Net loss attributable to Intrexon$(38,213)$(52,725)$(51,779)$(100,653)Interest expense292210960258Income tax expense (benefit)(923)—80623Depreciation and
amortization4,8152,97012,0406,681EBITDA$(34,029)$(49,545)$(37,973)$(93,691)Stock-based and executive incentive compensation expense8,3634,21326,37414,770Contribution of services by shareholder—508—1,485Bad debt
expense578—1,562—Research and development license with MD Anderson Cancer Center paid in stock——59,579—Unrealized and realized (appreciation) depreciation in fair value of equity
securities30,45337,089(64,392)48,944Equity in net loss of affiliates2,4291,6196,5653,510Impact of change in deferred revenue related to upfront and milestone payments(4,025)7,71051,84133,736Adjusted EBITDA$3,769$1,594$43,556$8,754Weighted average
shares outstanding, basic 112,244,12999,888,203109,244,64198,711,564Weighted average shares outstanding, diluted 115,251,439101,487,136111,641,463100,497,766Adjusted EBITDA per share, basic $0.03$0.02$0.40$0.09Adjusted EBITDA per share, diluted
$0.03$0.02$0.39$0.09
Intrexon (NASDAQ:XON)
Historical Stock Chart
From Aug 2024 to Sep 2024
Intrexon (NASDAQ:XON)
Historical Stock Chart
From Sep 2023 to Sep 2024