Filed
Pursuant to Rule 424(b)(5)
Registration No. 333-249870
PROSPECTUS
SUPPLEMENT
(to the Prospectus dated November 23, 2020)

19,896,000 Shares
of Common Stock
We
are selling 19,896,000 shares of our common stock. Our common stock is listed on the Nasdaq Stock
Market under the symbol “TFFP.” On August 11, 2023, the last reported sales price of our common stock on the Nasdaq
Stock Market was $0.42 per share.
As
of August 14, 2023, the aggregate market value of our outstanding common stock held by non-affiliates, or public float, was approximately
$17.2 million, based on 36,253,174 shares of our common stock, of which approximately 1,072,340 shares were held by affiliates, and a
price of $0.49 per share, which was the price at which our common stock was last sold on The Nasdaq Stock Market on June 22, 2023. We
have offered and sold $26,054.11 in value of shares of our common stock pursuant to General Instruction I.B.6 of Form S-3 during the
prior 12-calendar-month period that ends on and includes the date of this prospectus. Pursuant to General Instruction I.B.6 of Form S-3,
in no event will we sell securities registered on this registration statement in a public primary offering with a value exceeding more
than one-third of our public float in any 12-month period so long as our public float remains below $75 million.
Investing
in our securities involves a high degree of risk. Before making an investment decision, you should carefully review and consider all
of the information set forth in this prospectus supplement, the accompanying base prospectus and the documents incorporated by reference
herein and therein, including the risks and uncertainties described under “Risk Factors” beginning on page S-4 of
this prospectus supplement and the risk factors incorporated by reference into this prospectus supplement and the accompanying base prospectus.
Neither
the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus supplement or the accompanying base prospectus is truthful or complete. Any representation to the contrary is a criminal
offense.
| | |
Per Share | | |
Total | |
| Public offering price | |
$ | 0.2500 | | |
$ | 4,974,000 | |
| Underwriting discounts and commissions(1) | |
$ | 0.0175 | | |
$ | 348,180 | |
| Proceeds to us, before expenses | |
$ | 0.2325 | | |
$ | 4,625,820 | |
| (1) | Does
not include the reimbursement of certain expenses of the underwriter we have agreed to pay. Please see “Underwriting” beginning
on page S-9 for additional information regarding the total compensation to be received by the underwriter. |
We have granted the underwriter
a 30-day option to purchase up to an additional 2,984,400 shares of common stock from us at the public offering price less the underwriting
discount. If the underwriter exercises this option in full, the total underwriting discounts and commissions payable will be $400,407
and the total proceeds to us, before expenses, will be $5,319,693.
The underwriter expects to
deliver the shares on or about August 17, 2023.
The
Benchmark Company
The date of this prospectus supplement is August
14, 2023.
TABLE
OF CONTENTS
Prospectus Supplement
Base Prospectus
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
prospectus supplement and the accompanying base prospectus are part of a registration statement that we filed with the Securities and
Exchange Commission, or the SEC, utilizing a “shelf” registration process. From time to time, we may conduct an offering
to sell securities under the accompanying base prospectus and a related prospectus supplement that will contain specific information
about the terms of that offering, including the price, the amount of securities being offered and the plan of distribution. This prospectus
supplement describes the specific details regarding this offering and may add, update or change information contained in the accompanying
base prospectus. The base prospectus, dated November 23, 2020, including the documents incorporated by reference therein, provides general
information about us and our securities, some of which, such as the section entitled “Plan of Distribution,” may not apply
to this offering. This prospectus supplement and the accompanying base prospectus are an offer to sell only the securities offered hereby,
but only under circumstances and in jurisdictions where it is lawful to do so. Neither we nor the underwriter are making offers to sell
or solicitations to buy our securities in any jurisdiction in which an offer or solicitation is not authorized or in which the person
making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation.
If
information in this prospectus supplement is inconsistent with the accompanying base prospectus or the information incorporated by reference
with an earlier date, you should rely on this prospectus supplement. This prospectus supplement, together with the accompanying base
prospectus, the documents incorporated by reference into this prospectus supplement and the accompanying base prospectus and any free
writing prospectus we have provided for use in connection with this offering, include all material information relating to this offering.
Neither we nor the underwriter have authorized anyone to provide you with different or additional information, and you must not rely
on any unauthorized information or representations. You should assume that the information appearing in this prospectus supplement, the
accompanying base prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying base prospectus
and any free writing prospectus we have provided for use in connection with this offering is accurate only as of the respective dates
of those documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You
should carefully read this prospectus supplement, the accompanying base prospectus and the information and documents incorporated herein
by reference herein and therein, as well as any free writing prospectus we have provided for use in connection with this offering, before
making an investment decision. See “Incorporation of Certain Documents by Reference” and “Where You Can Find More Information”
in this prospectus supplement and in the accompanying base prospectus.
This
prospectus supplement and the accompanying base prospectus contain summaries of certain provisions contained in some of the documents
described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their
entirety by the full text of the actual documents, some of which have been filed or will be filed and incorporated by reference herein.
See “Where You Can Find More Information” in this prospectus supplement. We further note that the representations, warranties
and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference into this prospectus
supplement or the accompanying base prospectus were made solely for the benefit of the parties to such agreement, including, in some
cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty
or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly,
such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
This
prospectus supplement and the accompanying base prospectus contain and incorporate by reference certain market data and industry statistics
and forecasts that are based on independent industry publications and other publicly available information. Although we believe these
sources are reliable, estimates as they relate to projections involve numerous assumptions, are subject to risks and uncertainties, and
are subject to change based on various factors, including those discussed under “Risk Factors” in this prospectus supplement
and the accompanying base prospectus and under similar headings in the documents incorporated by reference herein and therein. Accordingly,
investors should not place undue reliance on this information.
PROSPECTUS
SUPPLEMENT SUMMARY
This
prospectus summary highlights information contained elsewhere in this prospectus supplement, the accompanying base prospectus and the
documents incorporated by reference herein and therein. This summary does not contain all of the information that you should consider
before deciding to invest in our securities. You should read this entire prospectus supplement and the accompanying base prospectus carefully,
including the section entitled “Risk Factors” in this prospectus supplement and our consolidated financial statements and
the related notes and the other information incorporated by reference into this prospectus supplement and the accompanying base prospectus,
before making an investment decision.
Our
Company
We
are a clinical stage biopharmaceutical company focused on developing and commercializing innovative drug products based on our patented
Thin Film Freezing, or TFF, technology platform. Based on our internal and sponsored testing and studies, we believe that our TFF platform
can significantly improve the solubility of poorly water-soluble drugs, which make up approximately 40% of marketed pharmaceuticals worldwide,
thereby improving the bioavailability and pharmacokinetics of those drugs. We believe that in the case of some new drugs that cannot
be developed due to poor water solubility, our TFF platform has the potential to increase the pharmacokinetic effect of the drug to a
level allowing for its development and commercialization. When administered as an inhaled dry powder for treatment of lung disorders,
we believe the TFF platform formulations can be used to increase efficacy and minimize systemic toxicities and drug-drug interactions.
As
of the date of this prospectus supplement, we have two product candidates in clinical trials, TFF Voriconazole Inhalation Powder, or
TFF VORI, and TFF Tacrolimus Inhalation Powder, or TFF TAC. To date, we have completed one Phase 1 study in healthy volunteers and
one Phase 1b study in patients with asthma exploring the safety, tolerability and pharmacokinetics of TFF VORI. As of the date of this
prospectus supplement, a Phase 2 clinical trial of TFF VORI in patients with invasive pulmonary aspergillosis has been initiated. We
have also completed one Phase 1 study in healthy volunteers examining the safety, tolerability and pharmacokinetics of TFF TAC. As of
the date of this prospectus supplement, a Phase 2 clinical trial of TFF TAC in lung transplant patients has been initiated.
We
are also actively engaged in the analysis and testing of dry powder formulations of several drugs and vaccines through parenteral, topical,
ocular, pulmonary and nasal applications through feasibility studies and material transfer agreements with U.S. and international pharmaceutical
companies and certain government agencies. We intend to initially focus on the development of inhaled dry powder drugs for the treatment
of pulmonary diseases and conditions. While the TFF platform was designed to improve solubility of poorly water-soluble drugs generally,
the researchers at University of Texas at Austin, or UT, found that the technology was particularly useful in generating dry powder particles
with properties which allow for superior inhalation delivery, especially to the deep lung, which is an area of extreme interest in respiratory
medicine. We believe that our TFF platform can significantly increase the number of pulmonary drug products that can be delivered directly
to the lung. We intend to design our dry powder drug products for use with dry powder inhalers, which are generally considered to be
the most effective and patient-friendly of all breath-actuated inhalers. We plan to focus on developing inhaled dry powder formulations
of existing off-patent drugs suited for lung diseases and conditions, which we believe includes dozens of potential drug candidates,
many of which have a potential market of over $1 billion.
We
intend to directly pursue the development of dry powder formulations of off-patent drugs through the U.S. Food and Drug Administration’s,
or FDA’s, 505(b)(2) regulatory pathway and in corresponding regulatory paths in other foreign jurisdictions. The 505(b)(2) pathway
contains full reports of investigations of safety and effectiveness but at least some of the information required for approval comes
from studies not conducted by or for the NDA applicant. 505(b)(2) products have the potential advantage of significantly lower development
costs and shorter development timelines versus traditional new molecular entities. The clinical requirements for a 505(b)(2) drug candidate
can vary widely from product to product depending primarily on whether the product candidate claims a new indication, provides for a
different route of administration or claims improved safety compared to the existing approved product, and may include bioequivalence
trials, limited safety and efficacy trials, or full Phase I through III trials. Unless the FDA releases a guidance document, the clinical
requirement for a 505(b)(2) product candidate is typically not known until the drug sponsor has a pre-IND and an end of Phase 2 meeting
with the FDA. For example, based on our meetings to date with the FDA, we believe we may need to conduct additional clinical trials beyond
the current Phase 2 trials for TFF VORI and TFF TAC prior to filing for marketing approval for either product.
In
June 2020, TFF TAC was awarded orphan drug status. We also believe that in some cases our other dry powder drug products may qualify
for the FDA’s orphan drug status.
We
intend to commercialize our TFF platform and internally developed product candidates through the following means:
| |
● |
We
may out-license our internally developed product candidates, such as TFF VORI and TFF TAC, or agree to jointly develop such products
with a third-party pharmaceutical company; |
| |
● |
Upon
and subject to receipt of the requisite approvals, we may directly commercialize our internally developed product candidates through
a combination of our internal direct sales and third-party marketing and distribution partnerships; and |
| |
● |
We
may pursue the licensing of our TFF platform or a joint development arrangement for a particular field of use with a third-party
pharmaceutical company. |
Company
Information
We
were incorporated under the laws of the state of Delaware on January 24, 2018. Our principal executive offices are located at 1751 River
Run, Suite 400, Fort Worth, Texas 76107, and our telephone number is (817) 438-6168. Our website address is www.tffpharma.com.
The information contained in, or accessible through, our website is not incorporated by reference into this prospectus supplement, and
you should not consider any information contained in, or that can be accessed through, our website as part of this prospectus supplement
or in deciding whether to purchase our common stock.
We
own unregistered trademarks, including our company name. All other trademarks or trade names referred to in this prospectus supplement
are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus supplement are
referred to without the symbols ® and ™, but such references should not be construed as any indication that their respective
owners will not assert, to the fullest extent under applicable law, their rights thereto.
Additional
Information
For
additional information related to our business and operations, please refer to the reports incorporated herein by reference, including
our Annual Report on Form 10-K for the year ended December 31, 2022 as filed with the SEC on March 31 2023, as described in the section
entitled “Incorporation of Certain Documents by Reference” in this prospectus supplement.
The
Offering
The
following is a brief summary of some of the terms of the offering and is qualified in its entirety by reference to the more detailed
information appearing elsewhere in this prospectus supplement and the accompanying base prospectus. For a more complete description of
the terms of our common stock, see “The Securities We May Offer—Common Stock” in the accompanying base prospectus.
| Common stock offered by us |
|
19,896,000 shares of our common stock (22,880,400 shares if the underwriter
exercises its over-allotment option in full). |
| |
|
|
| Offering price |
|
$0.25
per share of common stock. |
| |
|
|
| Common
stock to be outstanding after this offering |
|
56,089,085 shares (59,073,485 shares if the underwriter exercises its
over-allotment option in full). |
| |
|
|
| Use of proceeds |
|
We estimate that our net proceeds from this offering will be approximately
$4,360,820 (or approximately $5,054,694 if the underwriter exercises its over-allotment option in full), after deducting the underwriting
discounts and commissions and the estimated offering expenses payable by us. We expect to use the net proceeds from this offering for
working capital and general corporate purposes. See “Use of Proceeds” for additional information. |
| |
|
|
| Risk factors |
|
Investing in our common stock involves a high degree of risk. You should carefully consider the information under “Risk Factors” in this prospectus supplement and the other risks identified in the documents included or incorporated by reference in this prospectus supplement and the accompanying base prospectus before deciding to invest in our common stock. |
| |
|
|
| Lock-ups |
|
Pursuant to certain “lock-up” agreements our officers and directors have agreed, and pursuant to the Underwriting Agreement we have agreed that the company and our subsidiaries, from the date hereof until the earlier of (i) December 31, 2023, or (ii) the company making a public release of (a) meaningful Phase 2 initial readout data and/or compassionate use data for TFF-VORI, and (b) meaningful Phase 2 initial readout data for TFF-TAC, subject to certain exceptions (including tax covering sales and sales under Rule 10b5-1 plans by our officers and directors and our issuing shares under our existing at-the-market offering program and our equity incentive plans) each will not sell, transfer or dispose of, directly or indirectly, any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of capital stock. See “Underwriting” for more information. |
| |
|
|
| NASDAQ
Stock Market symbol |
|
“TFFP” |
The
number of shares of our common stock expected to be outstanding after this offering is based on 36,193,085 shares of common stock outstanding
as of June 30, 2023, and excludes the following:
| |
● |
5,283,289
shares of our common stock issuable upon exercise of outstanding options as of June 30, 2023, which have a weighted average exercise
price of $3.39 per share; |
| |
|
|
| |
● |
5,747,792
shares of our common stock issuable upon exercise of outstanding warrants as of June 30, 2023, which have a weighted average exercise
price of $1.70 per share; |
| |
● |
1,621,740
shares of common stock reserved for issuance and available for future grant under our 2018 Stock Incentive Plan and 2021 Stock Incentive
Plan as of June 30, 2023; |
| |
|
|
| |
● |
up to 2,984,400 shares of common stock issuable pursuant to the underwriter’s
over-allotment option; and |
| |
|
|
| |
● |
397,920 shares of common stock issuable upon exercise of the underwriter’s
warrant (457,608 shares if the underwriter exercises its over-allotment option in full). |
Unless otherwise stated or the context requires otherwise, all information
in this prospectus supplement assumes that the option to purchase up to 2,984,400 additional shares of common stock that we have granted
to the underwriter is not exercised.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. Before investing in our securities, you should carefully consider the risks, uncertainties
and assumptions contained in this prospectus supplement and discussed under the heading “Risk Factors” included in our Quarterly
Report on Form 10-Q for the quarter ended June 30, 2023 filed with the SEC on August 14, 2023, or as revised or supplemented by subsequent
filings, which are on file with the SEC and are incorporated herein by reference, and which may be amended, supplemented or superseded
from time to time by other reports we file with the SEC in the future. Our business, financial condition, results of operations and future
growth prospects could be materially and adversely affected by any of these risks. In these circumstances, the market price of our common
stock could decline, and you may lose all or part of your investment.
Risks
Related to this Offering and Our Common Stock
As
an investor, you may lose all of your investment.
Investing
in our common stock involves a high degree of risk. As an investor, you may never recoup all, or even part, of your investment and you
may never realize any return on your investment. You must be prepared to lose all of your investment.
Because
we will have broad discretion and flexibility in how the net proceeds from this offering are used, we may use the net proceeds in ways
in which you disagree.
We
intend to use the net proceeds from this offering for working capital and general corporate purposes. See “Use of Proceeds”
for additional information. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds
of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not
have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible
that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management
to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
We
will need additional financing following this offering to execute our business plan and fund operations, which additional financing may
not be available on reasonable terms, or at all.
As
of June 30, 2023, we had total assets of approximately $13.6 million and working capital of approximately $8.2 million. As of June 30,
2023, our working capital included approximately $7.7 million of cash and cash equivalents. We believe that the net proceeds of this
offering, plus our cash on-hand as of the date of this prospectus supplement, is sufficient to fund our proposed operating plan through
the first quarter of 2024, including initial clinical data readouts for TFF VORI and TFF TAC which are expected by the end of 2023. However,
we will need additional capital by the first half of 2024 to fund our continued operations, including through to the marketing approval
for TFF VORI and TFF TAC, assuming such approval can be obtained at all, and to engage in the substantial development of any other of
our drug candidates, such as formulation, early stage animal testing and formal toxicology studies. We intend to seek additional funds
through various financing sources, including the sale of our equity and debt securities, licensing fees for our technology and co-development
and joint ventures with industry partners, with a preference towards licensing fees for our technology and co-development and joint ventures
with industry partners. In addition, we will consider alternatives to our current business plan that may enable to us to achieve revenue
producing operations and meaningful commercial success with a smaller amount of capital. However, there can be no guarantees that such
funds will be available on commercially reasonable terms, if at all. If such financing is not available on satisfactory terms, we may
be unable to further pursue our business plan and we may be unable to continue operations, in which case you may lose your entire investment.
Obtaining additional financing contains risks, including:
| ● | additional
equity financing may not be available to us on satisfactory terms and any equity we are able
to issue could lead to dilution for current stockholders; |
| | | |
| ● | loans
or other debt instruments may have terms and/or conditions, such as interest rate, restrictive
covenants and control or revocation provisions; |
| | | |
| ● | the
current environment in capital markets combined with our capital constraints may prevent
us from being able to obtain adequate debt financing; and |
| | | |
| ● | if
we fail to obtain required additional financing to grow our business we may need to seek
bankruptcy protection in the near term. |
The
report of our independent registered public accounting firm for the year ended December 31, 2022 states that due to our lack of revenue
from commercial operations, significant losses and need for additional capital there is substantial doubt about our ability to continue
as a going concern.
There
can be no assurance that we will be able to produce meaningful clinical data for TFF VORI or TFF TAC by year end, or at all, or that
any such clinical data will warrant the further clinical development of either product candidate.
As
of the date of this prospectus supplement, we have underway a Phase 2 clinical trial of TFF VORI in patients with invasive pulmonary
aspergillosis and a Phase 2 clinical trial of TFF TAC in lung transplant patients. Based on our current site activations, patient enrollment
and efforts to accelerate patient pre-screening and screening activities, we expect to report meaningful initial clinical data readouts
for TFF VORI and TFF TAC by the end of 2023. However, clinical testing is difficult to design and implement and delays due to unforeseen
events are not uncommon. There can be no assurance that our Phase 2 clinical trials for TFF VORI and TFF TAC will produce meaningful
initial clinical data readouts by the end of 2023 or that any such data will be positive and warrant the further clinical development
of TFF VORI or TFF TAC. The absence of meaningful initial clinical data readouts for TFF VORI and TFF TAC may also adversely impact our
ability to raise capital for our operations.
The
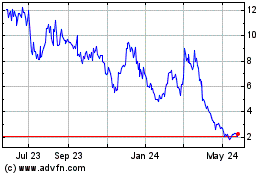
market price of our shares may be subject to fluctuation and volatility.
You
could lose all or part of your investment. The market price of our common stock is subject to wide fluctuations in response to various
factors, some of which are beyond our control. Since shares of our common stock were sold in our initial public offering in October 2019
at a price of $5.00 per share, the reported high and low sales prices of our common stock have ranged from $21.14 to $0.32 through August
11, 2023. The market price of our shares on the Nasdaq Stock Market may fluctuate as a result of a number of factors, some of which are
beyond our control, including, but not limited to:
| |
● |
actual
or anticipated variations in our and our competitors’ results of operations and financial condition; |
| |
● |
market
acceptance of our product candidates; |
| |
● |
changes
in earnings estimates or recommendations by securities analysts, if our shares are covered by analysts; |
| |
● |
development
of technological innovations or new competitive products by others; |
| |
|
|
| |
● |
announcements
of technological innovations or new products by us; |
| |
● |
whether
we can publish and when we are able to publish the results of preclinical or clinical trials for our product candidates; |
| |
● |
failure
by us to achieve a publicly announced milestone; |
| |
● |
delays
between our expenditures to develop and market new or enhanced products and the generation of sales from those products; |
| |
● |
developments
concerning intellectual property rights, including our involvement in litigation brought by or against us; |
| |
|
|
| |
● |
regulatory
developments and the decisions of regulatory authorities as to the approval or rejection of new or modified products; |
| |
|
|
| |
● |
changes
in the amounts that we spend to develop, acquire or license new products, technologies or businesses; |
| |
|
|
| |
● |
changes
in our expenditures to promote our product candidates; |
| |
|
|
| |
● |
our
sale or proposed sale, or the sale by our significant stockholders, of our shares or other securities in the future; |
| |
|
|
| |
● |
changes
in key personnel; |
| |
|
|
| |
● |
success
or failure of our research and development projects or those of our competitors; |
| |
|
|
| |
● |
the
trading volume of our shares; and |
| |
|
|
| |
● |
general
economic and market conditions and other factors, including factors unrelated to our operating performance. |
We
have received a notice of delisting or failure to satisfy a continued listing rule from the Nasdaq.
On
March 2, 2023, we received a notice of delisting from the Nasdaq Stock Market, LLC. The notice stated that we had fallen below compliance
with respect to the continued listing standard set forth in Rule 5450(a)(1) of the Nasdaq Listing Rules because the closing bid price
of our common stock over the previous 30 consecutive trading-day period had fallen below $1.00 per share.
Pursuant
to the notice and Rule 5810(c)(3)(A) of the Nasdaq Listing Rules, we have 180 days from the date of the notice, or until August 29, 2023,
to regain compliance with the minimum bid price requirement in Rule 5450(a)(1) by achieving a closing bid price for our common stock
of at least $1.00 per share over a minimum of 10 consecutive business days. If we do not regain compliance with Rule 5450(a)(1) during
the initial 180-day period, we may be eligible for additional time to regain compliance, subject to our transfer to the Nasdaq Capital
Market and compliance with the Nasdaq’s continued listing requirement for market value of publicly held shares and all other initial
listing standards for The Nasdaq Capital Market, with the exception of the bid price requirement, and our provision of certain undertakings
to the Nasdaq. As of the date of this prospectus supplement, we intend to submit to the Nasdaq Stock Market our request for an additional
180 days to regain compliance with the minimum bid price requirement along with our application to transfer from the Nasdaq Global Market
to the Nasdaq Capital Market. However, there can be no assurance that we will be afforded additional time to regain compliance with the
minimum bid price requirement following the initial 180-day period. If we are unable to regain compliance with Nasdaq Listing Rule 5450(a)(2)
in a timely manner, the Nasdaq will commence suspension and delisting procedures.
These
factors and any corresponding price fluctuations may materially and adversely affect the market price of our shares and result in substantial
losses being incurred by our investors. In the past, following periods of market volatility, public company stockholders have often instituted
securities class action litigation. If we were involved in securities litigation, it could impose a substantial cost upon us and divert
the resources and attention of our management from our business.
FORWARD-LOOKING
STATEMENTS
This
prospectus supplement, the accompanying base prospectus and the reports incorporated by reference herein and therein contain forward-looking
statements. The words “believe,” “may,” “will,” “potentially,” “estimate,”
“continue,” “anticipate,” “intend,” “could,” “would,” “project,”
“plan,” “expect” and similar expressions that convey uncertainty of future events or outcomes are intended to
identify forward-looking statements. These forward-looking statements include, but are not limited to, statements concerning the following:
| |
● |
our
future financial and operating results; |
| |
● |
our
intentions, expectations and beliefs regarding anticipated growth, market penetration and trends in our business; |
| |
● |
the
timing and success of our plan of commercialization; |
| |
● |
our
ability to successfully develop and clinically test our product candidates; |
| |
● |
our
ability to file for FDA approval of our product candidates through the 505(b)(2) regulatory pathway; |
| |
● |
our
ability to obtain FDA approval for any of our product candidates; |
| |
● |
our
ability to comply with all U.S. and foreign regulations concerning the development, manufacture and sale of our product candidates; |
| |
● |
our
ability to raise additional capital as and when needed; |
| |
● |
the
effects of market conditions on our stock price and operating results; |
| |
● |
our
ability to maintain, protect and enhance our intellectual property; |
| |
● |
the
effects of increased competition in our market and our ability to compete effectively; |
| |
● |
costs
associated with initiating and defending intellectual property infringement and other claims; |
| |
● |
the
attraction and retention of qualified employees and key personnel; |
| |
● |
future
acquisitions of or investments in complementary companies or technologies; and |
| |
● |
our
ability to comply with evolving legal standards and regulations, particularly concerning requirements for being a public company. |
These
forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading
“Risk Factors” and elsewhere in this prospectus supplement, the accompanying base prospectus and the reports incorporated
by reference herein and therein. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from
time to time. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent
to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking
statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed
in this prospectus supplement, the accompanying base prospectus and the reports incorporated by reference herein and therein may not
occur and actual results could differ materially and adversely from those anticipated or implied in our forward-looking statements.
You
should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected
in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events
and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes
responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any
forward-looking statements for any reason after the date of this prospectus supplement to conform these statements to actual results
or to changes in our expectations, except as required by law.
You
should read this prospectus supplement, the accompanying base prospectus and the reports incorporated by reference herein and therein
with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially
different from what we expect.
USE
OF PROCEEDS
We estimate that our net
proceeds from this offering will be approximately $4,360,820 (or approximately $5,054,694 if the underwriter exercises its
over-allotment option in full), after deducting the underwriting discounts and commissions and estimated offering expenses payable
by us.
We
expect to use the net proceeds from this offering for working capital and general corporate purposes. This represents our best estimate
of the manner in which we will use the net proceeds we receive from this offering based upon the current status of our business, but
we have not reserved or allocated amounts for specific purposes and we cannot specify with certainty how or when we will use any of the
net proceeds. The amounts and timing of our actual use of the net proceeds from this offering will vary depending on numerous factors,
including the factors described under “Risk Factors” located elsewhere in this prospectus supplement, the accompanying base
prospectus or in the information incorporated by reference herein or therein. As a result, our management will have broad discretion
in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds from
this offering.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
Common
Stock
We
are offering shares of our common stock in this offering. See “The Securities We May Offer—Common Stock” in the accompanying
base prospectus for more information regarding our shares of common stock.
UNDERWRITING
We entered into an underwriting
agreement, dated August 14, 2023, with The Benchmark Company, LLC, or the underwriter, with respect to the shares of our common stock
subject to this offering. Subject to the terms and conditions in the underwriting agreement, we have agreed to sell to the underwriter,
and the underwriter has agreed to purchase from us, 19,896,000 shares of our common stock.
The
underwriting agreement provides that the obligation of the underwriter to purchase all of the shares of our common stock being offered
to the public is subject to approval of legal matters by counsel and the satisfaction of other conditions. For example, conditions include,
among others, the continued accuracy of representations and warranties made by us in the underwriting agreement, delivery of legal opinions
and the absence of any material changes in our assets, business or prospects after the date of this prospectus supplement. The underwriter
is obligated to purchase all of the shares in this offering, if they purchase any of our shares.
The underwriter has advised
us that it proposes to offer the shares of our common stock directly to the public at the public offering price listed on the cover page
of this prospectus supplement and to selected dealers at the public offering price less a selling concession of up to $0.0175 per share
for the common stock. The underwriter may offer the shares through one or more of its affiliates or selling agents. Upon execution of
the underwriting agreement, the underwriter will be obligated to purchase the shares at the price and upon the terms stated therein. If
all of the shares are not sold at the initial public offering price, the underwriter may change the offering price and the other selling
terms.
Pursuant
to the underwriting agreement, we have agreed to indemnify the underwriter against certain liabilities, including liabilities under the
Securities Act, or to contribute to payments which the underwriter or other indemnified parties may be required to make in respect of
any such liabilities.
Underwriting
Discount and Expenses
The underwriting discount
is equal to the initial public offering price per share, less the amount paid by the underwriter to us per share. The underwriting discount
was determined through an arms’ length negotiation between us and the underwriter. We have agreed to sell the shares of our common
stock to the underwriter at the price of $0.2325 per share, which represents the initial public offering price of our shares set forth
on the cover page of this prospectus supplement less a 7% underwriting discount.
The
following table provides information regarding the amount of the underwriting discounts to be paid to the underwriter by us. The amounts
shown assume both no exercise and full exercise of the underwriter’s option to purchase additional shares.
| | |
| | |
Total | |
| | |
Per
Share | | |
Without
Option to
Purchase
Additional
Shares | | |
With
Option to
Purchase
Additional
Shares | |
| Public offering price | |
$ | 0.2500 | | |
$ | 4,974,000 | | |
$ | 5,720,100 | |
| Underwriting discounts and commissions paid by us | |
$ | 0.0175 | | |
$ | 348,180 | | |
$ | 400,407 | |
| Proceeds to us, before expenses | |
$ | 0.2325 | | |
$ | 4,625,820 | | |
$ | 5,319,693 | |
We estimate that the total
expenses, but excluding underwriting discounts and commissions, will be approximately $240,000, all of which are payable by us. This figure
includes expense reimbursements we have agreed to pay the underwriter for reimbursement of its expenses related to the offering up to
a maximum aggregate expense allowance of $140,000, which amount includes a $15,000 non-accountable expense allowance.
Option
to Purchase Additional Shares
We have granted to the underwriter
an option, exercisable for 30 days from the date of this prospectus supplement, to purchase, from time to time, in whole or in part, up
to an aggregate of 2,984,400 shares from us at the public offering price set forth on the cover page of this prospectus supplement, less
underwriting discounts and commissions. This option may be exercised only if the underwriter sells more shares than the total number set
forth on the cover page of this prospectus supplement.
Underwriters
Warrant
We have agreed to issue to
The Benchmark Company, LLC and its designees, a warrant to purchase shares of our common stock (up to 2% of the shares of common stock
sold in this offering). This warrant is exercisable at $0.3125 per share (125% of the price of the common stock sold in this offering),
commencing on the 180th day following the effective date of this offering and expiring five years from the effective date of this offering.
The warrant and the shares of common stock underlying the warrant are deemed compensation by FINRA and are therefore subject to a six-month
lock-up pursuant to FINRA Rule 5110(e)(1), except as permitted under FINRA Rule 5110(e)(2). The Benchmark Company, LLC and its designees
(or permitted assignees under the FINRA Rules) will not sell, transfer, assign, pledge, or hypothecate this warrant or the securities
underlying this warrant, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the
effective economic disposition of this warrant or the underlying securities for a period of six months from the effective date of the
offering.
Lock-Up
Agreements
Pursuant
to certain “lock-up” agreements our officers and directors have agreed and pursuant to the Underwriting Agreement the company
has agreed for itself and its subsidiaries, from the date hereof until the earlier of (i) December 31, 2023, or (ii) the company making
a public release of (a) meaningful Phase 2 initial readout data and/or compassionate use data for TFF-VORI, and (b) meaningful Phase
2 initial readout data for TFF-TAC, subject to certain exceptions (including tax covering sales and sales under Rule 10b5-1 plans by
our officers and directors and our issuing shares under our existing at-the-market offering program and our equity incentive plans) each
will not sell, transfer or dispose of, directly or indirectly, any shares of our capital stock or any securities convertible into or
exercisable or exchangeable for shares of capital stock.
Stock
Exchange
As
of the date of this prospectus supplement, our common stock is listed on the Nasdaq Global Market under the symbol “TFFP,”
however in our effort to regain compliance with the Nasdaq Stock Market’s minimum bid price requirement we intend to submit our
application to transfer from the Nasdaq Global Market to the Nasdaq Capital Market. See, “Risk Factors - We have received a
notice of delisting or failure to satisfy a continued listing rule from the Nasdaq.”
Stabilization
In
connection with this offering, the underwriter may engage in activities that stabilize, maintain or otherwise affect the price of the
shares of our common stock during and after this offering, including:
| ● | stabilizing
transactions; |
| |
● |
purchases
to cover positions created by short sales. |
Stabilizing
transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of the shares
of our common stock while this offering is in progress. Stabilization transactions permit bids to purchase our common stock so long as
the stabilizing bids do not exceed a specified maximum. These transactions may also include making short sales of the shares of our common
stock, which involve the sale by the underwriter of a greater number of shares of our common stock than it is required to purchase in
this offering and purchasing shares of our common stock on the open market to cover short positions created by short sales.
The
underwriter may close out any covered short position by purchasing shares in the open market. In making this determination, the underwriter
will consider, among other things, the price of shares available for purchase in the open market. A short position is more likely to
be created if the underwriter is concerned that there may be downward pressure on the price of the shares of our common stock in the
open market that could adversely affect investors who purchased in this offering.
These
stabilizing transactions, short sales and purchases to cover positions created by short sales may have the effect of raising or maintaining
the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result of these
activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. The underwriter
may carry out these transactions on the Nasdaq Stock Market, in the over-the-counter market, or otherwise. Neither we nor the underwriter
make any representation or prediction as to the effect that the transactions described above may have on the price of the shares. Neither
we nor the underwriter make any representation that the underwriter will engage in these stabilization transactions or that any transaction,
once commenced, will not be discontinued without notice.
Affiliations
The
underwriter and its affiliates are full service financial institutions engaged in various activities, which may include securities trading,
commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing
and brokerage activities. The underwriter and its affiliates may from time to time in the future engage with us and perform services
for us or in the ordinary course of their business for which they will receive customary fees and expenses. In the ordinary course of
their various business activities, the underwriter and its affiliates may make or hold a broad array of investments and actively trade
debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account
and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of us.
The underwriter and its affiliates may also make investment recommendations and/or publish or express independent research views in respect
of these securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in
these securities and instruments.
Electronic
Offer, Sale and Distribution of Securities
A
prospectus supplement and accompanying prospectus in electronic format may be made available on the websites maintained by one or more
of the underwriter or selling group members. The underwriter may agree to allocate a number of shares to selling group members for sale
to their online brokerage account holders. Internet distributions will be allocated by the underwriter and selling group members that
will make internet distributions on the same basis as other allocations. Other than the prospectus supplement and accompanying prospectus
in electronic format, the information on these websites is neither part of, nor incorporated by reference into, this prospectus supplement
and accompanying prospectus or the registration statement of which this prospectus supplement and accompanying prospectus forms a part,
has not been approved or endorsed by us, and should not be relied upon by investors.
Offer
Restrictions Outside the United States
Other
than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered
by this prospectus supplement in any jurisdiction where action for that purpose is required. The securities offered by this prospectus
supplement may not be offered or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements
in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances
that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus
supplement comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution
of this prospectus supplement. This prospectus supplement does not constitute an offer to sell or a solicitation of an offer to buy any
securities offered by this prospectus supplement in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice
to Prospective Investors in Canada
Shares
of our common stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are “accredited investors,”
as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are
permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations.
Any resale of the shares of our common stock must be made in accordance with an exemption from, or in a transaction not subject to, the
prospectus requirements of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus
supplement and accompanying prospectus (including any amendment hereto or thereto) contains a misrepresentation, provided that the remedies
for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s
province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s
province or territory for particulars of these rights or consult with a legal advisor.
Pursuant
to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section
3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply
with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice
to Prospective Investors in the European Economic Area and the United Kingdom
In
relation to the Member States of the European Economic Area and the United Kingdom (each, a Relevant State), no offer of shares of our
common stock which are the subject of the offering contemplated by this prospectus supplement and accompanying prospectus to the public
may be made in that Relevant State other than:
| |
● |
to
any legal entity that is a “qualified investor” as defined in the Prospectus Regulation; |
| |
● |
to
fewer than 150 natural or legal persons (other than “qualified investors” as defined in the Prospectus Regulation),
subject to obtaining the prior consent of the relevant representative or representatives nominated by us for any such offer; or |
| |
● |
in
any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided
that no such offer of shares of our common stock described in this prospectus supplement and accompanying prospectus shall result in
a requirement for the publication of a prospectus, by us or the underwriter, pursuant to Article 3 of the Prospectus Regulation.
Each
purchaser of shares of our common stock described in this prospectus supplement and accompanying prospectus located within a Relevant
State will be deemed to have represented, acknowledged and agreed that (1) it is a “qualified investor” within the meaning
of the Prospectus Regulation; and (2) in the case of any shares of our common stock acquired by it as a financial intermediary as
that term is used in Article 5(1) of the Prospectus Regulation, each such financial intermediary will be deemed to have represented,
acknowledged and agreed that the shares of our common stock acquired by it in the offer have not been acquired on a non-discretionary basis
on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an
offer to the public other than their offer or resale in a Relevant State to qualified investors, as that term is defined in the Prospectus
Regulation, or in circumstances in which the prior consent of the underwriter has been given to the offer or resale; or where shares
of our common stock have been acquired by it on behalf of persons in any Relevant State other than qualified investors, the offer of
those shares of our common stock to it is not treated under the Prospectus Regulation as having been made to such persons.
For
purposes of this provision, the expression an “offer to the public” in relation to the shares of our common stock in any
Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares
of our common stock to be offered so as to enable an investor to decide to purchase or subscribe to the shares and the expression “Prospectus
Regulation” means Regulation (EU) 2017/1129.
We
and the underwriter have not authorized and do not authorize the making of any offer of shares of our common stock through any financial
intermediary on their behalf, other than offers made by the underwriter with a view to the final placement of the shares as contemplated
in this prospectus supplement and accompanying prospectus. Accordingly, no purchaser of the shares of our common stock, other than the
underwriter, is authorized to make any further offer of the shares on behalf of us or the underwriter.
References
to the Prospectus Regulation includes, in relation to the UK, the Prospectus Regulation as it forms part of UK domestic law by virtue
of the European Union (Withdrawal) Act 2018.
The
above selling restriction is in addition to any other selling restrictions set out below.
Additional
Notice to Prospective Investors in the United Kingdom
The
communication of this prospectus supplement and accompanying prospectus and any other document or materials relating to the issue of
the shares of our common stock offered hereby is not being made, and such documents and/or materials have not been approved, by an authorized
person for the purposes of section 21 of the United Kingdom’s Financial Services and Markets Act 2000, as amended (the FSMA). Accordingly,
such documents and/or materials are not being distributed to, and must not be passed on to, the general public in the United Kingdom.
The communication of such documents and/or materials as a financial promotion is only being made to those persons in the United Kingdom
who have professional experience in matters relating to investments and who fall within the definition of investment professionals (as
defined in Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Financial
Promotion Order), or who fall within Article 49(2)(a) to (d) of the Financial Promotion Order, or who are any other persons to whom
it may otherwise lawfully be made under the Financial Promotion Order (all such persons together being referred to as “relevant
persons”). In the United Kingdom, the shares of our common stock offered hereby are only available to, and any investment or investment
activity to which this prospectus supplement relates will be engaged in only with, relevant persons. Any person in the United Kingdom
that is not a relevant person should not act or rely on this prospectus supplement or any of its contents.
Any
invitation or inducement to engage in investment activity (within the meaning of Section 21 of the FSMA) in connection with the
issue or sale of the shares of our common stock may only be communicated or caused to be communicated in circumstances in which Section 21(1)
of the FSMA does not apply to us.
All
applicable provisions of the FSMA must be complied with in respect to anything done by any person in relation to the shares of our common
stock in, from or otherwise involving the United Kingdom.
Notice
to Prospective Investors in Hong Kong
Shares
of our common stock may not be offered or sold by means of any document other than (i) in circumstances which do not constitute
an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), (ii) to “professional investors”
within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder or (iii) in
other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance
(Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to shares of our common stock may be issued or may
be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or
the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong
Kong) other than with respect to shares of our common stock which are or are intended to be disposed of only to persons outside Hong
Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong)
and any rules made thereunder.
Notice
to Prospective Investors in Japan
No
registration pursuant to Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended)
(the FIEL) has been made or will be made with respect to the solicitation of the application for the acquisition of the shares of our
common stock.
Accordingly,
the shares of our common stock have not been, directly or indirectly, offered or sold and will not be, directly or indirectly, offered
or sold in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including
any corporation or other entity organized under the laws of Japan) or to others for re-offering or re-sale, directly
or indirectly, in Japan or to, or for the benefit of, any resident of Japan except pursuant to an exemption from the registration requirements,
and otherwise in compliance with, the FIEL and the other applicable laws and regulations of Japan.
For
Qualified Institutional Investors (QII)
Please
note that the solicitation for newly-issued or secondary securities (each as described in Paragraph 2, Article 4 of the FIEL) in relation
to the shares of our common stock constitutes either a “QII only private placement” or a “QII only secondary distribution”
(each as described in Paragraph 1, Article 23-13 of the FIEL). Disclosure regarding any such solicitation, as is otherwise
prescribed in Paragraph 1, Article 4 of the FIEL, has not been made in relation to the shares of our common stock. The shares of our
common stock may only be transferred to QIIs.
For Non-QII Investors
Please
note that the solicitation for newly-issued or secondary securities (each as described in Paragraph 2, Article 4 of the FIEL) in relation
to the shares of our common stock constitutes either a “small number private placement” or a “small number private
secondary distribution” (each as is described in Paragraph 4, Article 23-13 of the FIEL). Disclosure regarding any such
solicitation, as is otherwise prescribed in Paragraph 1, Article 4 of the FIEL, has not been made in relation to the shares of our common
stock. The shares of our common stock may only be transferred en bloc without subdivision to a single investor.
Notice
to Prospective Investors in Singapore
This
prospectus supplement and accompanying prospectus have not been registered as a prospectus with the Monetary Authority of Singapore.
Accordingly, this prospectus supplement and accompanying prospectus and any other document or material in connection with the offer or
sale, or invitation for subscription or purchase, of shares of our common stock may not be circulated or distributed, nor may the shares
of our common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly,
to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter
289 of Singapore (the SFA), (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the
conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any
other applicable provision of the SFA.
Where
shares of our common stock are subscribed or purchased under Section 275 by a relevant person which is: (i) a corporation (which
is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one
or more individuals, each of whom is an accredited investor; or (ii) a trust (where the trustee is not an accredited investor) whose
sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures
of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation
or that trust has acquired shares of our common stock under Section 275 except: (a) to an institutional investor under Section 274
of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified
in Section 275 of the SFA; (b) where no consideration is given for the transfer; or (c) by operation of law.
LEGAL
MATTERS
The
validity of the securities offered by this prospectus supplement will be passed upon for us by Greenberg Traurig, LLP, Irvine, California.
Golenbock Eiseman Assor Bell & Peskoe LLP, New York, New York, is acting as counsel for the underwriter in connection with this
offering.
EXPERTS
The
consolidated financial statements as of and for the fiscal year ended December 31, 2022, incorporated by reference into this
prospectus supplement from the company’s Annual Report on Form 10-K for the year ended December 31, 2022, have been so
incorporated in reliance on the report of Marcum, LLP, an independent registered public accounting firm (the report on the financial
statements contains an explanatory paragraph regarding the Company's ability to continue as a going concern), as stated in their
report which is incorporated by reference herein, and has been so incorporated in reliance upon such report and upon the authority
of such firm as experts in accounting and auditing.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
The
SEC permits us to “incorporate by reference” the information and reports we file with it. This means that we can disclose
important information to you by referring to another document. The information that we incorporate by reference is considered to be part
of this prospectus supplement, and later information that we file with the SEC automatically updates and supersedes this information.
We incorporate by reference the documents listed below, except to the extent information in those documents is different from the information
contained in this prospectus supplement, and all future documents filed with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the
Exchange Act (other than the portions thereof deemed to be furnished to the SEC pursuant to Item 9 or Item 12) until we terminate the
offering of these securities:
| |
● |
our
Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which was filed on March 31, 2023; |
| |
● |
our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, which was filed on May 11, 2023; |
| |
● |
our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, which was filed on August 14, 2023; |
| |
● |
our Current Reports on Form 8-K, which was filed on January
11, 2023, February
9, 2023, February
17, 2023, April 6,
2023, June 23, 2023 and August 15, 2023; |
| |
● |
the
description of our common stock in our Form 8-A12B, which was filed on October 22, 2019, and any amendments or reports filed for
the purpose of updating this description; and |
| |
● |
all
documents we file with the SEC under Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus and
prior to the termination of this offering made by way of this prospectus. |
To
the extent that any statement in this prospectus supplement is inconsistent with any statement that is incorporated by reference and
that was made on or before the date of this prospectus supplement, the statement in this prospectus supplement shall supersede such incorporated
statement. The incorporated statement shall not be deemed, except as modified or superseded, to constitute a part of this prospectus
supplement or the registration statement. Statements contained in this prospectus supplement as to the contents of any contract or other
document are not necessarily complete and, in each instance, we refer you to the copy of each contract or document filed as an exhibit
to our various filings made with the SEC.
You
may request a copy of these filings, at no cost, by writing or telephoning us at the following address or telephone number:
TFF
Pharmaceuticals, Inc.
1751
River Run, Suite 400
Fort
Worth, Texas 76107
Attention:
Corporate Secretary
Telephone:
(817) 438-6168
Email:
investorinfo@tffpharma.com
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement under the Securities Act (SEC File No. 333-249870) that registers the securities offered
hereby. The registration statement, including the exhibits and schedules attached thereto and the information incorporated by reference
therein, contains additional relevant information about the securities and our company, which we are allowed to omit from this prospectus
supplement pursuant to the rules and regulations of the SEC. In addition, we file annual, quarterly and current reports and proxy statements
and other information with the SEC. Our SEC filings are available on the SEC’s website at www.sec.gov. Copies of certain information
filed by us with the SEC are also available free of charge on our website at www.tffpharma.com. We have not incorporated by reference
into this prospectus supplement the information on our website and it is not a part of this document.
PROSPECTUS
$100,000,000
TFF Pharmaceuticals, Inc.
Common Stock
Debt Securities
Warrants
Subscription Rights
Units
4,000,000 Shares of
Common Stock
Offered by a Selling
Stockholder
We may issue securities from
time to time in one or more offerings of up to $100,000,000 in aggregate offering price. This prospectus describes the general terms of
these securities and the general manner in which these securities will be offered. We will provide the specific terms of these securities
in supplements to this prospectus. The prospectus supplements will also describe the specific manner in which these securities will be
offered and may also supplement, update or amend information contained in this document. You should read this prospectus and any applicable
prospectus supplement before you invest.
We may offer these securities
in amounts, at prices and on terms determined at the time of offering. The securities may be sold directly to you, through agents, or
through underwriters and dealers. If agents, underwriters or dealers are used to sell the securities, we will name them and describe their
compensation in a prospectus supplement.
In
addition, the selling stockholder identified in this prospectus or any of its pledges, donees, transferees or other successors-in-interests
may offer to sell, from time to time, in amounts at prices and on terms determined at the time of the offering, up to 4,000,000 shares
of our common stock under this prospectus. These sales may occur through ordinary brokerage transactions, directly to market makers of
our shares or through any other means described in the section of this prospectus entitled “Plan of Distribution” beginning
on page 16 or by any applicable prospectus supplement. We will not receive any proceeds from the sale of common stock by the
selling stockholder, but we will incur expenses in connection with the sale of those shares. We and the selling stockholder may offer
securities at the same time or in separate transactions.
Our common stock is listed
on The NASDAQ Capital Market under the symbol “TFFP”. On November 3, 2020, the last reported sale price of our common
stock on The NASDAQ Capital Market was $14.06 per share.
Investing in these securities
involves significant risks. See “Risk Factors” included in any accompanying prospectus supplement and in the documents incorporated
by reference in this prospectus for a discussion of the factors you should carefully consider before deciding to purchase these securities.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy
or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is November 23, 2020
TABLE OF CONTENTS
ABOUT
THIS PROSPECTUS
This prospectus is part of
a registration statement that we filed with the Securities and Exchange Commission, which we refer to as the “SEC,” utilizing
a “shelf” registration process. Under this shelf registration process, we may from time to time sell any combination of the
securities described in this prospectus in one or more offerings for an aggregate initial offering price of up to $100,000,000.
This prospectus provides you
with a general description of the securities we may offer. From time to time, we may provide one or more prospectus supplements that will
contain specific information about the terms of the offering. The prospectus supplement may also add, update or change information contained
in this prospectus. You should read both this prospectus and any accompanying prospectus supplement together with the additional information
described under the heading “Where You Can Find More Information” beginning on page 18 of this prospectus.
The
selling stockholder also may use the shelf registration statement to sell an aggregate amount of 4,000,000 shares of our common stock
from time to time in the public market. We will not receive any proceeds from the sale of common stock by the selling stockholder. The
selling stockholder will deliver a supplement with this prospectus, if required, to update the information contained in this prospectus.
The selling stockholder may sell its shares of common stock through any means described in the section entitled “Plan of Distribution”
or in an accompanying prospectus supplement. As used herein, the term “selling stockholder” includes the selling stockholder
and its pledges, donees, transferees or other successors-in-interest.
We and the selling stockholder
have not authorized anyone to provide you with information different from that contained in or incorporated by reference in this prospectus,
any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC. We do not take any responsibility
for, and cannot provide any assurance as to the reliability of, any information other than the information contained or incorporated by
reference in this prospectus, any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC.
Neither this prospectus nor any accompanying prospectus supplement constitutes an offer to sell or the solicitation of an offer to buy
any securities other than the securities described in the accompanying prospectus supplement or an offer to sell or the solicitation of
an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume that the information
appearing in this prospectus, any prospectus supplement, the documents incorporated by reference and any related free writing prospectus
is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed
materially since those dates.
Unless the context otherwise
indicates, references in this prospectus to “we,” “our” and “us” refer, collectively, to TFF Pharmaceuticals,
Inc., a Delaware corporation, and its subsidiaries.
ABOUT
TFF Pharmaceuticals, INC.
TFF Pharmaceuticals, Inc.
(NASDAQ: TFFP) is a clinical-stage biopharmaceutical company focused on developing and commercializing innovative drug products based
on our patented Thin Film Freezing, or TFF, technology platform. We believe, and early testing confirms, that our TFF platform can significantly
improve the solubility of poorly water-soluble drugs, a class of drugs that makes up approximately 33% of the major pharmaceuticals worldwide,
thereby improving the pharmacokinetic effect of those drugs. We believe that in the case of some new drugs that cannot be developed due
to poor water-solubility, our TFF platform has the potential to improve the pharmacokinetic effect of the drug to a level allowing for
its development and commercialization. In November 2019, we initiated Phase I human clinical trials of our lead product, TFF Vori, and
in June 2020 we commenced Phase I human clinical trials of our TFF Tac-Lac product in Melbourne, Victoria, Australia, but in July 2020,
the Phase I trials of our TFF Tac-Lac product were delayed due to a resurgence of COVID-19 in the Melbourne area. A second clinical trial
site in Brisbane, Queensland, Australia was opened and dosing in the Phase 1 clinical trial resumed in Australia during the third
quarter 2020. We expect that dosing in this trial will be completed in the fourth quarter 2020. As of the date of this prospectus, we
have not progressed the development of any other of our drug candidates to human clinical trials and our efforts have focused on the formulation,
early stage animal testing and formal toxicology studies of our initial drug candidates in preparation for our first clinical trials.
We intend to initially focus
on the development of inhaled dry powder drugs for the treatment of pulmonary diseases and conditions. While our TFF platform was designed
to improve solubility of poorly water-soluble drugs generally, we have found that the technology is particularly useful in generating
dry powder particles with properties that allow for superior inhalation delivery, especially to the deep lung, which is an area of extreme
interest in respiratory medicine. We believe that our TFF platform can significantly increase the number of pulmonary drug products that
can be delivered by way of breath-actuated inhalers, which are generally considered to be the most effective and patient-friendly means
of delivering medication directly to the lungs. Our dry powder drug products will be designed for use with dry powder inhalers, which
are generally considered to be the most effective of all breath-actuated inhalers. We plan to focus on developing inhaled dry powder formulations
of existing off-patent drugs intended for lung diseases and conditions, which we believe includes dozens of potential drug candidates,
many of which have a potential market ranging from $100 million to over $500 million.
Our principal executive offices
are located at 2600 Via Fortuna, Suite 360, Austin, Texas 78746, and our telephone number is (737) 802-1973.
THE OFFERING
We
may offer and sell, from time to time, in one or more offerings, any combination of debt and equity securities that we describe in this
prospectus having a total initial offering price not exceeding $100,000,000 at prices and on terms to be determined by market conditions
at the time of any offering. This prospectus provides you with a general description of the securities we may offer. Each time we offer
a type or series of securities under this prospectus, we will provide a prospectus supplement that will describe the specific amounts,
prices and other important terms of the securities.
In
addition, the selling stockholder, Lung Therapeutics, Inc., or LTI, or its donees, pledges, transferees or other successors-in-interests
may offer to sell an aggregate of 4,000,000 shares of our common stock from time to time in the public market under this prospectus. We
will not receive any proceeds from the sale of shares of common stock by the selling stockholder. The selling stockholder will deliver
a supplement with this prospectus, if required, to update the information contained in this prospectus. The selling stockholder may sell
its shares of common stock through any means described in the section entitled “Plan of Distribution” or in an accompanying
prospectus supplement. See “Selling Stockholder” on page 6 for more information on the selling stockholder.
The
prospectus supplement also may add, update or change information contained in this prospectus or in documents we have incorporated by
reference into this prospectus. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus
or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
RISK
FACTORS
Investing in our securities
involves significant risks. You should carefully consider the risks and uncertainties described in this prospectus and any accompanying
prospectus supplement, including the risk factors in our most recent Annual Report on Form 10-K, any subsequently filed Quarterly Report
on Form 10-Q or Current Report on Form 8-K, together with all of the other information appearing
in or incorporated by reference into this prospectus and any applicable prospectus supplement, before making an investment decision
pursuant to this prospectus and any accompanying prospectus supplement relating to a specific offering.
Our business, financial condition
and results of operations could be materially and adversely affected by any or all of these risks or by additional risks and uncertainties
not presently known to us or that we currently deem immaterial that may adversely affect us in the future.
NOTE
REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains,
and any accompanying prospectus supplement will contain, forward-looking statements within the meaning of Section 27A of the Securities
Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act,
and the Private Securities Litigation Reform Act of 1993. Also, documents that we incorporate by reference into this prospectus, including
documents that we subsequently file with the SEC, will contain forward-looking statements. Forward-looking statements are those that predict
or describe future events or trends and that do not relate solely to historical matters. You can generally identify forward-looking statements
as statements containing the words “may,” “will,” “could,” “should,” “expect,”
“anticipate,” “intend,” “estimate,” “believe,” “project,” “plan,”
“assume” or other similar expressions, or negatives of those expressions, although not all forward-looking statements contain
these identifying words. All statements contained or incorporated by reference in this prospectus and any prospectus supplement regarding
our business strategy, future operations, projected financial position, potential strategic transactions, proposed licensing arrangements,
projected sales growth, estimated future revenues, cash flows and profitability, projected costs, potential outcome of litigation, potential
sources of additional capital, future prospects, future economic conditions, the future of our industry and results that might be obtained
by pursuing management’s current plans and objectives are forward-looking statements.
You should not place undue
reliance on our forward-looking statements because the matters they describe are subject to certain risks, uncertainties and assumptions
that are difficult to predict. Our forward-looking statements are based on the information currently available to us and speak only as
of the date on the cover of this prospectus, the date of any prospectus supplement, or, in the case of forward-looking statements incorporated
by reference, the date of the filing that includes the statement. Over time, our actual results, performance or achievements may differ
from those expressed or implied by our forward-looking statements, and such difference might be significant and materially adverse to
our security holders. Except as required by law, we undertake no obligation to update publicly any forward-looking statements, whether
as a result of new information, future events or otherwise.
We have identified some of
the important factors that could cause future events to differ from our current expectations and they are described in this prospectus
and supplements to this prospectus under the caption “Risk Factors,” as well as in our most recent Annual Report on Form 10-K,
including under the captions “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and
Results of Operations,” and in other documents that we may file with the SEC, all of which you should review carefully. Please consider
our forward-looking statements in light of those risks as you read this prospectus and any prospectus supplement.
USE OF PROCEEDS
Unless
otherwise specified in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities described
in this prospectus for general corporate and operations purposes and to fund our anticipated growth. The applicable prospectus supplement
will provide more details on the use of proceeds of any specific offering. We will not receive any proceeds from the sale of common stock
by the selling stockholder.
SELLING STOCKHOLDER
All
of the 4,000,000 shares of common stock registered for sale by the selling stockholder pursuant to this prospectus are owned by the Lung
Therapeutics, Inc., or LTI, a Texas corporation. We are registering such shares to permit the selling stockholder and its pledges, donees,
transferees or other successors-in-interest that receive their shares after the date of this prospectus to resell the shares in the manner
contemplated under the “Plan of Distribution”.
We
were incorporated under the laws of the state of Delaware on January 24, 2018 by LTI. In March 2018, we completed a Series A preferred
stock financing with third-party investors, at which time we acquired certain of LTI’s non-core intellectual property rights
and other assets, all of which relate to our Thin Film Freezing technology, in exchange for 4,000,000 shares of our common stock.
LTI is an early stage biotechnology company focused on the development of certain technologies in the pulmonary field. We are no longer
a subsidiary of LTI; however, LTI currently provides us with office space and certain administrative services and equipment for no charge,
from time to time on an as-needed basis, and three of our directors, Aaron Fletcher, Robert Mills and Brian Windsor, are also members
of the board of directors of LTI and Mr. Windsor is the Chief Executive Officer of LTI.
The
following table sets forth information with respect to the selling stockholder and the shares of common stock beneficially owned by the
selling stockholder, including shares that may be offered under this prospectus. The information is based on information provided by or
on behalf of the selling stockholder to us as of the date of this prospectus. For purposes of the table below, we have assumed that after
termination of this offering none of the shares covered by this prospectus that are currently owned by the selling stockholder will be
held by the selling stockholder.
Name of Selling Stockholder | |
Shares of
Common Stock
Beneficially
Owned Prior
to Offering | | |
Percent
of Shares
Owned
Before
Offering | | |
Maximum
Number of
Shares That
May Be Sold
Pursuant to
this Prospectus | | |
Shares of
Common Stock
Beneficially
Owned After
Offering | | |
Percent
of Shares
Owned
After
Offering | |
| Lung Therapeutics, Inc. | |
| 4,000,000 | | |
| 17.9 | % | |
| 4,000,000 | | |
| — | | |
| — | |
THE
SECURITIES WE MAY OFFER
We may offer and sell, from
time to time in one or more offerings, any combination of common stock, debt securities, warrants, subscription rights and units having
an aggregate initial offering price not exceeding $100,000,000. In this prospectus, we refer to the common stock, debt securities, warrants,
subscription rights and units that we may offer collectively as “securities.”
Common Stock
We are authorized to issue
45,000,000 shares of $0.001 par value common stock. Holders of shares of common stock are entitled to one vote per share on all matters
to be voted upon by the stockholders generally. Stockholders are entitled to receive such dividends as may be declared from time to time
by the board of directors out of funds legally available therefor, and in the event of liquidation, dissolution or winding up of the company
to share ratably in all assets remaining after payment of liabilities. The holders of shares of common stock have no preemptive, conversion,
subscription or cumulative voting rights.
This prospectus provides a
general description of the securities we may offer other than our common stock. Each time we sell any of our securities under this prospectus,
we will, to the extent required by law, provide a prospectus supplement that will contain specific information about the terms of the
offering. The prospectus supplement may also add, update or change information in this prospectus. For more information, see “About
this Prospectus.”
Description of Debt Securities
We may offer debt securities
which may be senior or subordinated. We refer to the senior debt securities and the subordinated debt securities collectively as debt
securities. The following description summarizes the general terms and provisions of the debt securities. We will describe the specific
terms of the debt securities and the extent, if any, to which the general provisions summarized below apply to any series of debt securities
in the prospectus supplement relating to the series and any applicable free writing prospectus that we authorize to be delivered.
We may issue senior debt securities
from time to time, in one or more series, which may be issued under a senior indenture to be entered into between us and a senior trustee
to be named in a prospectus supplement, which we refer to as the senior trustee. We may issue subordinated debt securities from time to
time, in one or more series, which may be issued under a subordinated indenture to be entered into between us and a subordinated trustee
to be named in a prospectus supplement, which we refer to as the subordinated trustee. While it is highly likely that any debt securities
we issue will be issued under an indenture, we reserve the right to issue debt securities other than under an indenture pursuant to an
exemption from the indenture requirement under the Trust Indenture Act of 1939. Any debt securities issued by us other than pursuant to
an indenture will subject the purchasers of such debt securities to certain unique risks arising from the lack of a trustee charged with
the responsibility of monitoring the debt securities and enforcing the rights of the holders of such debt securities, which will be set
forth in a prospectus supplement filed with regard to such unindentured debt securities.
The forms of senior indenture
and subordinated indenture are filed as exhibits to the registration statement of which this prospectus forms a part. Together, the senior
indenture and the subordinated indenture are referred to as the indentures and, together, the senior trustee and the subordinated trustee
are referred to as the trustees. This prospectus briefly outlines some of the provisions of the indentures. The following summary of the
material provisions of the indentures is qualified in its entirety by the provisions of the indentures, including definitions of certain
terms used in the indentures. Wherever we refer to particular sections or defined terms of the indentures, those sections or defined terms
are incorporated by reference in this prospectus or the applicable prospectus supplement. You should review any indentures that are filed
as exhibits to the registration statement of which this prospectus forms a part for additional information.
If we issue debt securities
other than under an indenture, we will likely be limited to issuing a maximum of $50 million of such debt securities and it is also likely
that such debt securities will be unsecured and subordinated. Any indenture regarding debt securities issued by us will not limit the
amount of debt securities that we may issue. The debt securities or applicable indenture, if any, will provide that debt securities may
be issued up to an aggregate principal amount authorized from time to time by us and may be payable in any currency or currency unit designated
by us or in amounts determined by reference to an index.
General
The following is a summary
of the general terms of the debt securities we may issue under an indenture or otherwise, except as otherwise described in a prospectus
supplement.
The senior debt securities
will constitute our unsubordinated general obligations and will rank pari passu with our other unsubordinated obligations. The subordinated
debt securities will constitute our subordinated general obligations and will be junior in right of payment to our senior indebtedness
(including senior debt securities).
The debt securities will be
our unsecured obligations unless otherwise specified in the applicable prospectus supplement. Any secured debt or other secured obligations
will be effectively senior to the debt securities to the extent of the value of the assets securing such debt or other obligations.
The applicable prospectus
supplement and any free writing prospectus will include any additional or different terms of the debt securities or any series being offered,
including the following terms:
| |
● |
the title and type of the debt securities; |
| |
● |
whether the debt securities will be issued under an indenture; |
| |
● |
whether the debt securities will be senior or subordinated debt securities, and, with respect to subordinated debt securities, the terms on which they are subordinated; |
| |
● |
the aggregate principal amount of the debt securities; |
| |
● |
the price or prices at which we will sell the debt securities; |
| |
● |
the maturity date or dates of the debt securities and the right, if any, to extend such date or dates; |
| |
● |
the rate or rates, if any, per year, at which the debt securities will bear interest, or the method of determining such rate or rates; |
| |
● |
the date or dates from which such interest will accrue, the interest payment dates on which such interest will be payable or the manner of determination of such interest payment dates and the related record dates; |
| |
● |
the right, if any, to extend the interest payment periods and the duration of that extension; |
| |
● |
the manner of paying principal and interest and the place or places where principal and interest will be payable; |
| |
● |
provisions for a sinking fund, purchase fund or other analogous fund, if any; |
| |
● |
any redemption dates, prices, obligations and restrictions on the debt securities; |
| |
● |
the currency, currencies or currency units in which the debt securities will be denominated and the currency, currencies or currency units in which principal and interest, if any, on the debt securities may be payable; |
| |
● |
any conversion or exchange features of the debt securities; |
| |
● |
whether and upon what terms the debt securities may be defeased; |
| |
● |
any events of default or covenants in addition to or in lieu of those set forth in any indenture; |
| |
● |
whether the debt securities will be issued in definitive or global form or in definitive form only upon satisfaction of certain conditions; |
| |
● |
whether the debt securities will be guaranteed as to payment or performance; |
| |
● |
if the debt securities of the series will be secured by any collateral and, if so, a general description of the collateral and the terms and provisions of such collateral security, pledge or other agreements; and |
| |
● |
any other material terms of the debt securities. |
The applicable prospectus
supplement will also describe any applicable material U.S. federal income tax consequences. When we refer to “principal” in
this section with reference to the debt securities, we are also referring to “premium, if any.”
We may from time to time,
without notice to or the consent of the holders of any series of debt securities, create and issue further debt securities of any such
series ranking equally with the debt securities of such series in all respects (or in all respects other than (1) the payment of interest
accruing prior to the issue date of such further debt securities or (2) the first payment of interest following the issue date of such
further debt securities). Such further debt securities may be consolidated and form a single series with the debt securities of such series
and have the same terms as to status, redemption or otherwise as the debt securities of such series.
You may present debt securities
for exchange and you may present debt securities for transfer in the manner, at the places and subject to the restrictions set forth in
the debt securities and the applicable prospectus supplement. We will provide you those services without charge, although you may have
to pay any tax or other governmental charge payable in connection with any exchange or transfer, as set forth in the debt securities or
any indenture.
Debt securities may bear interest
at a fixed rate or a floating rate. Debt securities bearing no interest or interest at a rate that at the time of issuance is below the
prevailing market rate (original issue discount securities) may be sold at a discount below their stated principal amount.
We may issue debt securities
with the principal amount payable on any principal payment date, or the amount of interest payable on any interest payment date, to be
determined by reference to one or more currency exchange rates, securities or baskets of securities, commodity prices or indices. You
may receive a payment of principal on any principal payment date, or a payment of interest on any interest payment date, that is greater
than or less than the amount of principal or interest otherwise payable on such dates, depending on the value on such dates of the applicable
currency, security or basket of securities, commodity or index. Information as to the methods for determining the amount of principal
or interest payable on any date, the currencies, securities or baskets of securities, commodities or indices to which the amount payable
on such date will be set forth in the applicable prospectus supplement.
Certain Terms of the Senior
Debt Securities
The following is a summary
of the general terms of the senior debt securities we may issue under a senior indenture, except as otherwise described in a prospectus
supplement.
Covenants. Unless we
indicate otherwise in a prospectus supplement, the senior debt securities will not contain any financial or restrictive covenants, including
covenants restricting either us or any of our subsidiaries from incurring, issuing, assuming or guaranteeing any indebtedness secured
by a lien on any of our or our subsidiaries’ property or capital stock, or restricting either us or any of our subsidiaries from
entering into sale and leaseback transactions.
Consolidation, Merger and
Sale of Assets. Unless we indicate otherwise in a prospectus supplement, we may not consolidate with or merge into any other person,
in a transaction in which we are not the surviving corporation, or convey, transfer or lease our properties and assets substantially as
an entirety to any person, in either case, unless:
| |
● |
the successor entity, if any, is a U.S. corporation, limited liability company, partnership or trust (subject to certain exceptions provided for in the senior indenture); |
| |
● |
the successor entity assumes our obligations on the senior debt securities and under the senior indenture; |
| |
● |
immediately after giving effect to the transaction, no default or event of default shall have occurred and be continuing; and |
| |
● |
certain other conditions are met. |
No Protection in the Event
of a Change in Control. Unless we indicate otherwise in a prospectus supplement with respect to a particular series of senior debt
securities, the senior debt securities will not contain any provisions that may afford holders of the senior debt securities protection
in the event we have a change in control or in the event of a highly leveraged transaction (whether or not such transaction results in
a change in control).
Events of Default.
Unless we indicate otherwise in a prospectus supplement with respect to a particular series of senior debt securities, the following are
events of default under the senior indenture for any series of senior debt securities:
| |
● |
failure to pay interest on any senior debt securities of such series when due and payable, if that default continues for a period of 90 days (or such other period as may be specified for such series); |
| |
● |
failure to pay principal on the senior debt securities of such series when due and payable whether at maturity, upon redemption, by declaration or otherwise (and, if specified for such series, the continuance of such failure for a specified period); |
| |
● |
default in the performance of or breach of any of our covenants or agreements in the senior indenture applicable to senior debt securities of such series, other than a covenant breach which is specifically dealt with elsewhere in the senior indenture, and that default or breach continues for a period of 90 days after we receive written notice from the trustee or from the holders of 25% or more in aggregate principal amount of the senior debt securities of such series; |
| |
● |
certain events of bankruptcy or insolvency, whether or not voluntary; and |
| |
● |
any other event of default provided for in such series of senior debt securities as may be specified in the applicable prospectus supplement. |
Unless we indicate otherwise
in a prospectus supplement, the default by us under any other debt, including any other series of debt securities, is not a default under
the senior indenture.
If an event of default other
than an event of default specified in the fourth bullet point above occurs with respect to a series of senior debt securities and is continuing
under the senior indenture, then, and in each such case, either the trustee or the holders of not less than 25% in aggregate principal
amount of such series then outstanding under the senior indenture (each such series voting as a separate class) by written notice to us
and to the trustee, if such notice is given by the holders, may, and the trustee at the request of such holders shall, declare the principal
amount of and accrued interest on such series of senior debt securities to be immediately due and payable, and upon this declaration,
the same shall become immediately due and payable.
If an event of default specified
in the fourth bullet point above occurs with respect to us and is continuing, the entire principal amount of and accrued interest, if
any, on each series of senior debt securities then outstanding shall become immediately due and payable.
Unless otherwise specified
in the prospectus supplement relating to a series of senior debt securities originally issued at a discount, the amount due upon acceleration
shall include only the original issue price of the senior debt securities, the amount of original issue discount accrued to the date of
acceleration and accrued interest, if any.
Upon certain conditions, declarations
of acceleration may be rescinded and annulled and past defaults may be waived by the holders of a majority in aggregate principal amount
of all the senior debt securities of such series affected by the default, each series voting as a separate class. Furthermore, prior to
a declaration of acceleration and subject to various provisions in the senior indenture, the holders of a majority in aggregate principal
amount of a series of senior debt securities, by notice to the trustee, may waive an existing default or event of default with respect
to such senior debt securities and its consequences, except a default in the payment of principal of or interest on such senior debt securities
or in respect of a covenant or provision of the senior indenture which cannot be modified or amended without the consent of the holders
of each such senior debt security. Upon any such waiver, such default shall cease to exist, and any event of default with respect to such
senior debt securities shall be deemed to have been cured, for every purpose of the senior indenture; but no such waiver shall extend
to any subsequent or other default or event of default or impair any right consequent thereto. For information as to the waiver of defaults,
see “—Modification and Waiver.”
The holders of a majority
in aggregate principal amount of a series of senior debt securities may direct the time, method and place of conducting any proceeding
for any remedy available to the trustee or exercising any trust or power conferred on the trustee with respect to such senior debt securities.
However, the trustee may refuse to follow any direction that conflicts with law or the senior indenture, that may involve the trustee
in personal liability or that the trustee determines in good faith may be unduly prejudicial to the rights of holders of such series of
senior debt securities not joining in the giving of such direction and may take any other action it deems proper that is not inconsistent
with any such direction received from holders of such series of senior debt securities. A holder may not pursue any remedy with respect
to the senior indenture or any series of senior debt securities unless:
| |
● |
the holder gives the trustee written notice of a continuing event of default; |
| |
● |
the holders of at least 25% in aggregate principal amount of such series of senior debt securities make a written request to the trustee to pursue the remedy in respect of such event of default; |
| |
● |
the requesting holder or holders offer the trustee indemnity satisfactory to the trustee against any costs, liability or expense; |
| |
● |
the trustee does not comply with the request within 60 days after receipt of the request and the offer of indemnity; and |
| |
● |
during such 60-day period, the holders of a majority in aggregate principal amount of such series of senior debt securities do not give the trustee a direction that is inconsistent with the request. |
These limitations, however,
do not apply to the right of any holder of a senior debt security to receive payment of the principal of and interest, if any, on such
senior debt security in accordance with the terms of such debt security, or to bring suit for the enforcement of any such payment in accordance
with the terms of such debt security, on or after the due date for the senior debt securities, which right shall not be impaired or affected
without the consent of the holder.
The senior indenture requires
certain of our officers to certify, on or before a fixed date in each year in which any senior debt security is outstanding, as to their
knowledge of our compliance with all covenants, agreements and conditions under the senior indenture.
Satisfaction and Discharge.
We can satisfy and discharge our obligations to holders of any series of senior debt securities if:
| |
● |
we pay or cause to be paid, as and when due and payable, the principal of and any interest on all senior debt securities of such series outstanding under the senior indenture; or |
| |
● |
all senior debt securities of such series have become due and payable or will become due and payable within one year (or are to be called for redemption within one year) and we deposit in trust a combination of cash and U.S. government or U.S. government agency obligations that will generate enough cash to make interest, principal and any other payments on the debt securities of that series on their various due dates. |
Under current U.S. federal
income tax law, the deposit and our legal release from the senior debt securities would be treated as a taxable event, and beneficial
owners of such debt securities would generally recognize any gain or loss on such senior debt securities. Purchasers of the senior debt
securities should consult their own advisers with respect to the tax consequences to them of such deposit and discharge, including the
applicability and effect of tax laws other than the U.S. federal income tax law.
Defeasance. Unless
the applicable prospectus supplement provides otherwise, the following discussion of legal defeasance and discharge and covenant defeasance
will apply to any senior series of senior debt securities issued under the indentures.
Legal Defeasance. We
can legally release ourselves from any payment or other obligations on the senior debt securities of any series (called “legal defeasance”)
if certain conditions are met, including the following:
| |
● |
We deposit in trust for your benefit and the benefit of all other direct holders of the senior debt securities of the same series a combination of cash and U.S. government or U.S. government agency obligations that will generate enough cash to make interest, principal and any other payments on the senior debt securities of that series on their various due dates. |
| |
● |
There is a change in current U.S. federal income tax law or an IRS ruling that lets us make the above deposit without causing you to be taxed on the senior debt securities any differently than if we did not make the deposit and instead repaid the senior debt securities ourselves when due. |
| |
● |
We deliver to the trustee a legal opinion of our counsel confirming the tax law change or ruling described above. |
If we ever did accomplish
legal defeasance, as described above, you would have to rely solely on the trust deposit for repayment of the debt securities. You could
not look to us for repayment in the event of any shortfall.
Covenant Defeasance.
Without any change of current U.S. federal tax law, we can make the same type of deposit described above and be released from some of
the covenants in the senior debt securities (called “covenant defeasance”). In that event, you would lose the protection of
those covenants but would gain the protection of having money and securities set aside in trust to repay the senior debt securities. In
order to achieve covenant defeasance, we must do the following (among other things):
| |
● |
We must deposit in trust for your benefit and the benefit of all other direct holders of the senior debt securities of the same series a combination of cash and U.S. government or U.S. government agency obligations that will generate enough cash to make interest, principal and any other payments on the senior debt securities of that series on their various due dates. |
| |
● |
We must deliver to the trustee a legal opinion of our counsel confirming that under current U.S. federal income tax law we may make the above deposit without causing you to be taxed on the senior debt securities any differently than if we did not make the deposit and instead repaid the senior debt securities ourselves when due. |
If we accomplish covenant
defeasance, you can still look to us for repayment of the senior debt securities if there were a shortfall in the trust deposit. In fact,
if one of the events of default occurred (such as our bankruptcy) and the debt securities become immediately due and payable, there may
be such a shortfall. Depending on the events causing the default, you may not be able to obtain payment of the shortfall.
Modification and Waiver.
We and the trustee may amend or supplement the senior indenture or the senior debt securities without the consent of any holder:
| |
● |
to comply with the requirements of the SEC in order to effect or maintain the qualification of the indenture under the Trust Indenture Act of 1939, as amended, or the Trust Indenture Act; |
| |
● |
to convey, transfer, assign, mortgage or pledge any assets as security for the senior debt securities of one or more series; |
| |
● |
to evidence the succession of a corporation, limited liability company, partnership or trust to us, and the assumption by such successor of our covenants, agreements and obligations under the senior indenture; |
| |
● |
to add to our covenants such new covenants, restrictions, conditions or provisions for the protection of the holders, and to make the occurrence, or the occurrence and continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default; |
| |
● |
to cure any ambiguity, defect or inconsistency in the senior indenture or in any supplemental indenture or to conform the senior indenture or the senior debt securities to the description of senior debt securities of such series set forth in this prospectus or any applicable prospectus supplement; |
| |
● |
to provide for or add guarantors with respect to the senior debt securities of any series; |
| |
● |
to establish the form or forms or terms of the senior debt securities as permitted by the senior indenture; |
| |
● |
to evidence and provide for the acceptance of appointment under the senior indenture by a successor trustee, or to make such changes as shall be necessary to provide for or facilitate the administration of the trusts in the senior indenture by more than one trustee; |
| |
● |
to add to, delete from or revise the conditions, limitations and restrictions on the authorized amount, terms, purposes of issue, authentication and delivery of any series of senior debt securities; |
| |
● |
to make any change to the senior debt securities of any series so long as no senior debt securities of such series are outstanding; or |
| |
● |
to make any change that does not adversely affect the rights of any holder in any material respect. |
Other amendments and modifications
of the senior indenture or the senior debt securities issued may be made, and our compliance with any provision of the senior indenture
with respect to any series of senior debt securities may be waived, with the consent of the holders of a majority of the aggregate principal
amount of the outstanding senior debt securities of all series affected by the amendment or modification (voting together as a single
class); provided, however, that each affected holder must consent to any modification, amendment or waiver that:
| |
● |
extends the final maturity of any senior debt securities of such series; |
| |
● |
reduces the principal amount of any senior debt securities of such series; |
| |
● |
reduces the rate or extends the time of payment of interest on any senior debt securities of such series; |
| |
● |
reduces the amount payable upon the redemption of any senior debt securities of such series; |
| |
● |
changes the currency of payment of principal of or interest on any senior debt securities of such series; |
| |
● |
reduces the principal amount of original issue discount securities payable upon acceleration of maturity or the amount provable in bankruptcy; |
| |
● |
waives a default in the payment of principal of or interest on the senior debt securities; |
| |
● |
changes the provisions relating to the waiver of past defaults or changes or impairs the right of holders to receive payment or to institute suit for the enforcement of any payment or conversion of any senior debt securities of such series on or after the due date therefor; |
| |
● |
modifies any of the provisions of these restrictions on amendments and modifications, except to increase any required percentage or to provide that certain other provisions cannot be modified or waived without the consent of the holder of each senior debt security of such series affected by the modification; or |
| |
● |
reduces the above-stated percentage of outstanding senior debt securities of such series whose holders must consent to a supplemental indenture or to modify or amend or to waive certain provisions of or defaults under the senior indenture. |
It shall not be necessary
for the holders to approve the particular form of any proposed amendment, supplement or waiver, but it shall be sufficient if the holders’
consent approves the substance thereof. After an amendment, supplement or waiver of the senior indenture in accordance with the provisions
described in this section becomes effective, the trustee must give to the holders affected thereby certain notice briefly describing the
amendment, supplement or waiver. Any failure by the trustee to give such notice, or any defect therein, shall not, however, in any way
impair or affect the validity of any such amendment, supplemental indenture or waiver.
No Personal Liability of
Incorporators, Stockholders, Officers, Directors. The senior indenture provides that no recourse shall be had under any obligation,
covenant or agreement of ours in the senior indenture or any supplemental indenture, or in any of the senior debt securities or because
of the creation of any indebtedness represented thereby, against any of our incorporators, stockholders, officers or directors, past,
present or future, or of any predecessor or successor entity thereof under any law, statute or constitutional provision or by the enforcement
of any assessment or by any legal or equitable proceeding or otherwise. Each holder, by accepting the senior debt securities, waives and
releases all such liability.
Concerning the Trustee.
The senior indenture provides that, except during the continuance of an event of default, the trustee will not be liable except for
the performance of such duties as are specifically set forth in the senior indenture. If an event of default has occurred and is continuing,
the trustee will exercise such rights and powers vested in it under the senior indenture and will use the same degree of care and skill
in its exercise as a prudent person would exercise under the circumstances in the conduct of such person’s own affairs.
The senior indenture and the
provisions of the Trust Indenture Act incorporated by reference therein contain limitations on the rights of the trustee thereunder, should
it become a creditor of ours or any of our subsidiaries, to obtain payment of claims in certain cases or to realize on certain property
received by it in respect of any such claims, as security or otherwise. The trustee is permitted to engage in other transactions, provided
that if it acquires any conflicting interest (as defined in the Trust Indenture Act), it must eliminate such conflict or resign.
We may have normal banking
relationships with the senior trustee in the ordinary course of business.
Unclaimed Funds. All
funds deposited with the trustee or any paying agent for the payment of principal, premium, interest or additional amounts in respect
of the senior debt securities that remain unclaimed for two years after the date upon which such principal, premium or interest became
due and payable will be repaid to us. Thereafter, any right of any holder of senior debt securities to such funds shall be enforceable
only against us, and the trustee and paying agents will have no liability therefor.
Governing Law. The
senior indenture and the senior debt securities will be governed by, and construed in accordance with, the internal laws of the State
of New York.
Certain Terms of the Subordinated
Debt Securities
The following is a summary
of the general terms of the subordinated debt securities we may issue under a subordinated indenture, except as otherwise described in
a prospectus supplement.
Other than the terms of the
subordinated indenture and subordinated debt securities relating to subordination or otherwise as described in the prospectus supplement
relating to a particular series of subordinated debt securities, the terms of the subordinated indenture and subordinated debt securities
are identical in all material respects to the terms of the senior indenture and senior debt securities.
Additional or different subordination
terms may be specified in the prospectus supplement applicable to a particular series.
Subordination. The
indebtedness evidenced by the subordinated debt securities is subordinate to the prior payment in full of all of our senior indebtedness,
as defined in the subordinated indenture. During the continuance beyond any applicable grace period of any default in the payment of principal,
premium, interest or any other payment due on any of our senior indebtedness, we may not make any payment of principal of or interest
on the subordinated debt securities (except for certain sinking fund payments). In addition, upon any payment or distribution of our assets
upon any dissolution, winding-up, liquidation or reorganization, the payment of the principal of and interest on the subordinated debt
securities will be subordinated to the extent provided in the subordinated indenture in right of payment to the prior payment in full
of all our senior indebtedness. Because of this subordination, if we dissolve or otherwise liquidate, holders of our subordinated debt
securities may receive less, ratably, than holders of our senior indebtedness. The subordination provisions do not prevent the occurrence
of an event of default under the subordinated indenture.
The term “senior indebtedness”
of a person means with respect to such person the principal of, premium, if any, interest on, and any other payment due pursuant to any
of the following, whether outstanding on the date of the subordinated indenture or incurred by that person in the future:
| |
● |
all of the indebtedness of that person for money borrowed; |
| |
● |
all of the indebtedness of that person evidenced by notes, debentures, bonds or other securities sold by that person for money; |
| |
● |
all of the lease obligations that are capitalized on the books of that person in accordance with generally accepted accounting principles; |
| |
● |
all indebtedness of others of the kinds described in the first two bullet points above and all lease obligations of others of the kind described in the third bullet point above that the person, in any manner, assumes or guarantees or that the person in effect guarantees through an agreement to purchase, whether that agreement is contingent or otherwise; and |
| |
● |
all renewals, extensions or refundings of indebtedness of the kinds described in the first, second or fourth bullet point above and all renewals or extensions of leases of the kinds described in the third or fourth bullet point above; |
unless, in the case of any particular indebtedness,
renewal, extension or refunding, the instrument creating or evidencing it or the assumption or guarantee relating to it expressly provides
that such indebtedness, renewal, extension or refunding is not superior in right of payment to the subordinated debt securities. Our senior
debt securities constitute senior indebtedness for purposes of the subordinated debt indenture.
Description of Warrants
We may issue warrants for
the purchase of shares of common stock, debt securities, and/or units from time to time. We may issue warrants independently or together
with common stock and/or debt securities, and the warrants may be attached to or separate from those securities. If we issue
warrants, they will be evidenced by warrant agreements or warrant certificates issued under one or more warrant agreements, which will
be contracts between us and the holders of the warrants or an agent for the holders of the warrants. We encourage you to read the prospectus
supplement that relates to any warrants we may offer, as well as the complete warrant agreement or warrant certificate that contain the
terms of the warrants. If we issue warrants, the forms of warrant agreements and warrant certificates, as applicable, relating
to the warrants will be filed as exhibits to the registration statement that includes this prospectus, or as an exhibit to a filing with
the SEC that is incorporated by reference into this prospectus.
Description of Subscription Rights
We
may issue rights to purchase our securities. The rights may or may not be transferable by the persons purchasing or receiving the rights.
In connection with any rights offering, we may enter into a standby underwriting, standby purchase or other arrangement with one or more
underwriters or other persons pursuant to which such underwriters or other persons would purchase any offered securities remaining unsubscribed
for after such rights offering. In connection with a rights offering to holders of our capital stock a prospectus supplement will be distributed
to such holders on or after the record date for receiving rights in the rights offering set by us.
We
will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a current
report on Form 8-K that we file with the SEC, forms of the subscription rights, standby underwriting agreement or other agreements, if
any. The prospectus supplement relating to any rights that we offer will include specific terms relating to the offering, including, among
other matters:
| |
● |
the date of determining the security holders entitled to the rights distribution; |
| |
● |
the aggregate number of rights issued and the aggregate amount of securities purchasable upon exercise of the rights; |
| |
● |
the conditions to completion of the rights offering; |
| |
● |
the date on which the right to exercise the rights will commence and the date on which the rights will expire; and |
| |
● |
any applicable federal income tax considerations. |
Each
right would entitle the holder of the rights to purchase the principal amount of securities at the exercise price set forth in the applicable
prospectus supplement. Rights may be exercised at any time up to the close of business on the expiration date for the rights provided
in the applicable prospectus supplement. After the close of business on the expiration date, all unexercised rights will become void.
Holders
may exercise rights as described in the applicable prospectus supplement. Upon receipt of payment and the rights certificate properly
completed and duly executed at the corporate trust office of the rights agent, if any, or any other office indicated in the prospectus
supplement, we will, as soon as practicable, forward the securities purchasable upon exercise of the rights. If less than all of the rights
issued in any rights offering are exercised, we may offer any unsubscribed securities directly to persons other than stockholders, to
or through agents, underwriters or dealers or through a combination of such methods, including pursuant to standby underwriting or purchase
arrangements, as described in the applicable prospectus supplement.
Description of Units
We may issue units comprised
of one or more of the other securities described in this prospectus in any combination from time to time. Each unit will be issued so
that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights
and obligations of a holder of each included security. If we issue units, they will be evidenced by unit agreements or unit certificates
issued under one or more unit agreements, which will be contracts between us and the holders of the units or an agent for the holders
of the units. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or
transferred separately, at any time or at any time before a specified date. We encourage you to read the prospectus supplement that relates
to any units we may offer, as well as the complete unit agreement or unit certificate that contain the terms of the units. If
we issue units, the forms of unit agreements and unit certificates, as applicable, relating to the units will be filed as exhibits to
the registration statement that includes this prospectus, or as an exhibit to a filing with the SEC that is incorporated by reference
into this prospectus.
PLAN
OF DISTRIBUTION
We and the selling stockholder
may sell our securities from time to time in any manner permitted by the Securities Act, including any one or more of the following ways:
| |
● |
to or through underwriters; |
| |
● |
to or through broker-dealers (acting as agent or principal); |
| |
● |
in “at the market” offerings, within the meaning of Rule 415(a)(4) of the Securities Act, to or through a market maker or into an existing trading market, on an exchange or otherwise; and/or |
| |
● |
directly to purchasers, through a specific bidding or auction process or otherwise. |
The securities may be sold
at a fixed price or prices, which may be changed, at market prices prevailing at the time of sale, at prices relating to the prevailing
market prices or at negotiated prices.
Offers to purchase offered
securities may be solicited by agents designated by us from time to time. Any agent involved in the offer or sale of the offered securities
in respect of which this prospectus is delivered will be named, and any commissions payable by us will be set forth, in the applicable
prospectus supplement. Unless otherwise set forth in the applicable prospectus supplement, any agent will be acting on a reasonable best
efforts basis for the period of its appointment. Any agent may be deemed to be an underwriter, as that term is defined in the Securities
Act, of the offered securities so offered and sold.
We and the selling stockholder
will set forth in a prospectus supplement the terms of the offering of our securities, including:
| |
● |
the name or names of any agents, underwriters or dealers; |
| |
● |
the purchase price of our securities being offered and the proceeds we will receive from the sale; |
| |
● |
any over-allotment options under which underwriters may purchase additional securities from us; |
| |
● |
any agency fees or underwriting discounts and commissions and other items constituting agents’ or underwriters’ compensation; |
| |
● |
the public offering price; |
| |
● |
any discounts or concessions allowed or reallowed or paid to dealers; and |
| |
● |
any securities exchanges on which such securities may be listed. |
If we or the selling stockholder
offer securities to be sold to the public by means of an underwritten offering, either through underwriting syndicates represented by
managing underwriters or directly by the managing underwriters, we or the selling stockholder will execute an underwriting agreement with
an underwriter or underwriters, and the names of the specific managing underwriter or underwriters, as well as any other underwriters,
will be set forth in the applicable prospectus supplement. In addition, the terms of the transaction, including commissions, discounts
and any other compensation of the underwriters and dealers, if any, will be set forth in the applicable prospectus supplement, which prospectus
supplement will be used by the underwriters to make resales of the offered securities. If underwriters are utilized in the sale of the
offered securities, the offered securities will be acquired by the underwriters for their own account and may be resold from time to time
in one or more transactions, including:
| |
● |
transactions on The NASDAQ Capital Market or any other organized market where the securities may be traded; |
| |
● |
in the over-the-counter market; |
| |
● |
in negotiated transactions; or |
| |
● |
under delayed delivery contracts or other contractual commitments. |
We or the selling stockholder
may grant to the underwriters options to purchase additional offered securities to cover over-allotments, if any, at the public offering
price with additional underwriting discounts or commissions, as may be set forth in the applicable prospectus supplement. If we or the
selling stockholder grant any over-allotment option, the terms of the over-allotment option will be set forth in the applicable prospectus
supplement.
We or the selling stockholder
may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the
public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery
on a specified date in the future. The conditions to these contracts and the commissions to be paid for solicitation of these contracts
will be described in the prospectus supplement.
We or the selling stockholder
may indemnify agents, underwriters and dealers against specified liabilities, including liabilities incurred under the Securities Act,
or to contribution by us to payments they may be required to make in respect of such liabilities. Agents, underwriters or dealers, or
their respective affiliates, may be customers of, engage in transactions with or perform services for us, the selling stockholder or our
respective affiliates, in the ordinary course of business.
Unless otherwise specified
in the applicable prospectus supplement, each class or series of securities will be a new issue with no established trading market, other
than our common stock, which is traded on The NASDAQ Capital Market. We may elect to list any other class or series of securities on any
exchange and, in the case of our common stock, on any additional exchange. However, unless otherwise specified in the applicable prospectus
supplement, we will not be obligated to do so. It is possible that one or more underwriters may make a market in a class or series of
securities, but the underwriters will not be obligated to do so and may discontinue any market making at any time without notice. We cannot
give any assurance as to the liquidity of the trading market for any of the offered securities.
Any underwriter may engage
in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange
Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids
to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other
short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in the open market
after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from
a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions.
Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue
any of the activities at any time.
We
have advised the selling stockholder that the anti-manipulation rules of Regulation M may apply to sales of shares in the market and to
the activities of the selling stockholder its affiliates. This regulation may limit the timing of purchases and sales of any of the shares
of common stock offered in this prospectus by the selling stockholder. The anti-manipulation rules under the Exchange Act may apply to
sales of shares in the market and to the activities of the selling stockholder and its affiliates.
To comply with the securities
laws of certain states, if applicable, the securities offered by this prospectus will be offered and sold in those states only through
registered or licensed brokers or dealers.
LEGAL MATTERS
The validity of the issuance
of the securities offered by this prospectus has been passed upon for us by Greenberg Traurig, LLP, Irvine, California.
EXPERTS
The consolidated financial
statements as of and for the fiscal years ended December 31, 2019 and 2018, incorporated by reference into this prospectus supplement
from the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 have been so incorporated in reliance on the
report of Marcum, LLP, an independent registered public accounting firm, as stated in their report which is incorporated by reference
herein, and has been so incorporated in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC
a registration statement on Form S-3 under the Securities Act that registers the securities to be sold in this offering. In addition,
we file annual, quarterly and current reports and proxy statements and other information with the SEC. Our SEC filings are and will become
available to the public over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we
file with the SEC at its public reference facilities at 100 F Street N.E., Washington, D.C. 20549. You can also obtain copies of
the documents upon the payment of a duplicating fee to the SEC. Please call the SEC at 1-800-SEC-0330 for further information on
the operation of the public reference facilities. Copies of certain information filed by us with the SEC are also available on our website
at https://ir.tffpharma.com/financial-information/sec-filings. We have not incorporated by reference into this prospectus the information
on our website and it is not a part of this document.
This prospectus does not contain
all of the information set forth in the registration statement and the exhibits and schedules thereto. Some items are omitted in
accordance with the rules and regulations of the SEC. You should review the information and exhibits included in the registration
statement for further information about us and the securities we are offering. Statements in this prospectus concerning any document
we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and
are qualified by reference to these filings. You should review the complete document to evaluate these statements.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to incorporate
by reference the information we file with it, which means that we can disclose important information to you by referring you to another
document that we have filed separately with the SEC. You should read the information incorporated by reference because it is an important
part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior
to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the information
in this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part
the information or documents listed below that we have filed with the SEC (Commission File No. 001-39102):
| |
● |
Our Annual Report on Form 10-K for the fiscal
year ended December 31, 2019 filed with the SEC on March 27, 2020; |
| |
● |
Our Annual Report on Form 10-K/A for the fiscal
year ended December 31, 2019 filed with the SEC on April 29, 2020; |
| |
● |
Our Quarterly Report on Form 10-Q for the
quarter ended March 31, 2020 filed with the SEC on May 14, 2020; |
| |
● |
Our Quarterly Report on Form 10-Q for the
quarter ended June 30, 2020 filed with the SEC on August 13, 2020; |
| |
● |
Our Quarterly Report on Form 10-Q for the
quarter ended September 30, 2020 filed with the SEC on November 5, 2020; |
| |
● |
Our definitive Proxy Statement on Schedule 14A filed with the SEC on August 28, 2020; and |
| |
● |
The description of our common stock set forth in our registration statement on Form 8-A12B filed with the SEC on October 22, 2019. |
We also incorporate by reference
any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that
are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act made after the effective date of this registration statement of which this prospectus is a part and until
we terminate this offering. Information in such future filings updates and supplements the information provided in this prospectus. Any
statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously
filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed
document modify or replace such earlier statements.
We will furnish without charge
to each person, including any beneficial owner, to whom a prospectus is delivered, upon written or oral request, a copy of any or all
of the reports or documents incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits that
are specifically incorporated by reference into such documents. You can access the reports and documents incorporated by reference into
this prospectus at https://ir.tffpharma.com/financial-information/sec-filings. You may also direct any requests for reports or documents
to:
TFF Pharmaceuticals, Inc.
2600 Via Fortuna, Suite 360
Austin, Texas 78746
Attention: Corporate Secretary
Telephone: (737) 802-1973
Email: investorinfo@tffpharma.com
You should rely only on information
contained in, or incorporated by reference into, this prospectus. We have not authorized anyone to provide you with information different
from that contained in this prospectus or incorporated by reference into this prospectus. We are not making offers to sell the securities
in any jurisdiction in which such an offer or solicitation is not authorized or in which the person making such offer or solicitation
is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
The Delaware General Corporation
Law provides that corporations may include a provision in their certificate of incorporation relieving directors of monetary liability
for breach of their fiduciary duty as directors, provided that such provision shall not eliminate or limit the liability of a director
(i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good
faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payment of a dividend or unlawful stock
purchase or redemption, or (iv) for any transaction from which the director derived an improper personal benefit. Our amended and restated
certificate of incorporation provides that directors are not liable to us or our stockholders for monetary damages for breach of their
fiduciary duty as directors to the fullest extent permitted by Delaware law. In addition to the foregoing, our amended and restated certificate
of incorporation provides that we shall indemnify directors and officers to the fullest extent permitted by law and we have entered into
indemnification agreements with each of our directors and executive officers.
The above provisions in our
amended and restated certificate of incorporation may have the effect of reducing the likelihood of derivative litigation against directors
and may discourage or deter stockholders or management from bringing a lawsuit against directors for breach of their fiduciary duty, even
though such an action, if successful, might otherwise have benefited us and our stockholders. However, we believe that the foregoing provisions
are necessary to attract and retain qualified persons as directors.
Insofar as indemnification
for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant
to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public
policy as expressed in the Securities Act and is, therefore, unenforceable.

19,896,000 Shares of Common
Stock
The Benchmark
Company
August 14, 2023
TFF Pharmaceuticals (NASDAQ:TFFP)
Historical Stock Chart
From Apr 2024 to May 2024
TFF Pharmaceuticals (NASDAQ:TFFP)
Historical Stock Chart
From May 2023 to May 2024