0001347242
false
0001347242
2023-09-11
2023-09-11
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): September 11, 2023
Lipella
Pharmaceuticals Inc.
(Exact name of registrant as specified in its charter)
| Delaware |
|
005-93847 |
|
20-2388040 |
(State or other jurisdiction
of incorporation) |
|
(Commission File
Number) |
|
(IRS Employer
Identification No.) |
7800
Susquehanna St., Suite 505
Pittsburgh, PA |
|
15208 |
| (Address
of registrant’s principal executive office) |
|
(Zip
code) |
Registrant’s
telephone number, including area code: (412) 901-0315
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant
under any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written communications
pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material
pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which
registered |
| Common
Stock, par value $0.0001 per share |
|
LIPO |
|
The
Nasdaq Stock Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☒
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 |
Regulation FD
Disclosure. |
On
September 11, 2023, Lipella Pharmaceuticals Inc. (the “Company”) presented at the H.C. Wainwright 25th
Annual Global Investment Conference in New York City, New York to prospective investors, analysts, industry participants and other
attendees in order to provide an update on its product pipeline and discuss recent business developments, including its Phase
2a clinical trial results for its drug candidate, LP-10. The presentation materials furnished herewith as Exhibit 99.1
(the “Presentation Materials”) were used in connection with such presentation, are incorporated into this Item 7.01
by reference and will be posted on the Company’s website at https://lipella.com. Information contained on the Company’s
website is not incorporated by reference into and should not be considered to be part of this Current Report on Form 8-K (this
“Form 8-K”).
The
information contained in this Form 8-K under Item 7.01, including Exhibit 99.1 attached hereto, is deemed to be ”furnished”
and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended
(the “Exchange Act”), and shall not be deemed incorporated by reference in any filing under the Securities Act of
1933, as amended (the “Securities Act”), or the Exchange Act, whether made before or after the date hereof, except
as shall be expressly set forth by specific reference to this Form 8-K in such filing. The information set forth in this Item
7.01 of this Form 8-K and Exhibit 99.1 attached hereto shall not be deemed an admission as to the materiality of any information
in this Form 8-K that is required to be disclosed solely to satisfy the requirements of Regulation FD.
Forward-Looking
Statements
Exhibit
99.1 attached hereto contains, and may indicate, forward-looking statements within the meaning of Section 27A of the Securities
Act, Section 21E of the Exchange Act and as defined in the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking
statements include, but are not limited to,
statements that express the Company’s intentions, beliefs, expectations, strategies, predictions or any other statements
related to the Company’s future activities, or future events or conditions, including without limitation, those statements
relating to the Company’s product pipeline, anticipated timing and clinical trial results for LP-10 in the Presentation
Materials, and the Company’s clinical trials and/or trial results for its other products now or in the future, which can
be identified by terminology such as “may,” “will,” “expects,” “anticipates,”
“aims,” “potential,” “future,” “intends,”
“plans,” believes,” “estimates,” “continue,” “likely to,” and other similar
expressions intended to identify forward-looking statements, although not all forward-looking statements contain these identifying
words. These statements are not historical facts and are based on current expectations, estimates and projections about the Company’s
business based, in part, on assumptions made by its management. These statements are not guarantees of future performance and
involve risks, uncertainties and assumptions that are difficult to predict, many of which are beyond the Company’s control.
Any forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation to update
any forward-looking statement to reflect events or circumstances after the date of this Form 8-K, except as required by applicable
law.
| Item 9.01 |
Financial Statements and Exhibits. |
(d)
Exhibits
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf
by the undersigned hereunto duly authorized.
| Date: September 11, 2023 |
Lipella Pharmaceuticals Inc. |
| |
|
|
| |
By: |
/s/ Jonathan Kaufman |
| |
|
Name: Jonathan Kaufman
Title: Chief Executive Officer |
Exhibit 99.1

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO lipellapharmaceuticals.com

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO Disclaimers Theinformation inthispresentation isbeing provided soyoucanfamiliarize yourself with LipellaPharmaceuticals Inc.(“Lipella,”the“Company,” “we,” “us,” or“our”) during thisinformational meeting.We request thatyoukeepanyinformation weprovide atthismeeting confidential andthatyoudonotdisclose anyoftheinformation toanyotherparties without theCompany’s priorexpresswritten permission. Although theCompany believes theinformation contained hereinisaccurate inallmaterialrespects, theCompany doesnotmakeanyrepresentation orwarranty, eitherexpressorimplied, astotheaccuracy, completeness orreliability oftheinformation contained inthispresentation . 2 Forward-LookingStatements Thepresentation includes certain “forward -looking statements .”Allstatements, other thanstatements of historical fact, included inthispresentation regarding, among other things, ourstrategy, future operations, financial position, anticipated dividends, projected costs, prospects, pipeline and opportunities, sources of growth, successful implementation of our proprietary technology, plans and objectives areforward-looking statements .Forward-looking statements canbeidentified bywords suchas“may,” “will,” “could,” “continue,” “would,” “should,” “potential,” “target,” “goal,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “predicts,” “expects,” “projects” and similar references to future periods.Forward-looking statements arebased on our current expectations and assumptions regarding future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short- and long-term business operations and objectives, andfinancialneeds.Because forward-looking statements relatetothefuture, theyaresubject toinherentuncertainties, risksandchanges incircumstances thataredifficult topredict .Ouractual results may differ materially from those contemplated by the forward-looking statements .We caution you, therefore, against relying on any of these forward-looking statements .Theyare neither statements ofhistorical fact norguarantees orassurancesoffuture performance .Therearerisks,uncertainties andother factors, both knownandunknown,thatcould causeactualresultstodiffer materially from those intheforward-looking statements whichinclude, butarenotlimited to,regional, nationalorglobal political, economic, business,competitive, market andregulatory conditions, andother factors. Anyforward-looking statement made byusisbased upon thereasonable judgment ofourmanagement atthetime suchstatement ismade andspeaks onlyasofthedate onwhich itismade.Factors or events that could causeouractual resultstodiffer mayemerge from time totime, anditisnot possible forustopredict allofthem.Weundertake noobligation toupdate anyforward-looking statement, whether asaresultofnewinformation, future developments orotherwise, except asmayberequired byapplicable law. Nothing contained hereinis,orshallberelied upon as,apromise orrepresentation asto thepast orfuture.TheCompany expressly disclaims anyandallliability relating to orresulting from theuseof this presentation .Inaddition, theinformation contained inthispresentation isasofthedate hereof, andtheCompany hasnoobligation toupdate suchinformation, including intheevent that suchinformation becomes inaccurate.Youshould not construe thecontents of thispresentation orother information weprovide atthismeeting aslegal,tax,accounting orinvestment advice orarecommendation .You shouldconsult yourowncounsel andtaxandfinancialadvisorsastolegalandrelated matters concerning thematters described herein.

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO Mission Lipella is committed to providing supportive care to cancer survivors. Our mission is to develop and commercialize treatments for serious diseases resulting from cancer treatments including radiation and chemotherapy. 3

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need Business Growth Strategy Focused on rare and orphan drug indications and leveraging the 505(b)(2) pathway. Lipella anticipates this strategy will lower cost and allow for a faster approval process Why rare and orphan drugs? • Lipella can be first to market by pursuing conditions for which there are no current FDA approved treatments. • Requires smaller and less costly drug trials with greater flexibility from the FDA. • Orphandrugmarket exclusivity limits additional market entrants. • 7 years in the US, 10 years in the EU and Japan. 4 Why leverage the 505(b)(2) pathway? • Bypasses Phase 1 trials by utilizing drugs and mechanisms of action where safety and efficacy have already been established. • Canimmediately initiate Phase 2 clinical trials. • Potentially mitigates risk from a CMC, safety and clinical development standpoint. • Greater flexibility from the FDA.
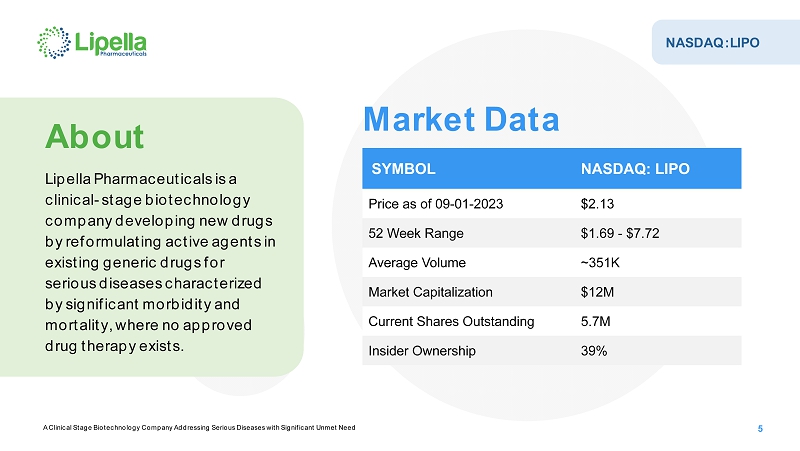
A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO About Lipella Pharmaceuticals is a clinical-stage biotechnology company developing new drugs by reformulating active agents in existing generic drugs for serious diseases characterized by significant morbidity and mortality, where no approved drug therapy exists. SYMBOL NASDAQ: LIPO Price as of 09-01-2023 $2.13 52 Week Range $1.69 -$7.72 Average Volume ~351K Market Capitalization $12M Current Shares Outstanding 5.7M Insider Ownership 39% Market Data 5

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need Overview of Opportunity Drug Development 6 Drug Delivery Proprietary drug delivery technology with potential applications in bladder, urethra, oral cavity, esophageal and colonic. Designed for use with other lipophilic drugs; potential for technology partnerships, commercial licensing agreements. Lead product candidate, LP -10, a 505(b)(2) drug candidate for hemorrhagic cystitis. LP-10 granted FDA Orphan Drug Designation; potential candidate for multiple accelerated pathways. Successfully completed Phase 2a clinical trial; FDA Type B meeting anticipated in 4Q 2023. Peak annualrevenue estimates in hemorrhagic cystitis exceed $1 billion. Addressable market of approximately 60,000 potential new patients each year.

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need 7 Executive Summary Emerging, clinical -stage, proprietary 505(b)(2) opportunity Drug-Delivery Focused Biotech Impressive Revenue Potential Patented liposomal local drug delivery 505(b)(2) Optimized for epithelial tissues Lower systemic drug exposure Pilot manufacturing Transferrable CMC Approved phase -2a IND Potential for accelerated approval pathways NDAefficient development strategy (4) American Cancer Society Cancer Treatment and Survivorship Fact and Figures 2019 -2021, (5) based on the Company’s 30% estimate (6) 8% estimate, (7) based on the Company’s estimate, (8) $20,000 average revenue per each of an estimated 60,000 patients treated per year. $20,000 annual revenue per patient 7 $1.2 billion annual revenue potential 8 Prostate and other pelvic malignancies Over 3,000,000 survivors in US 4 Pelvic radiation theraphy Over 900,000 survivors in US 5 Radiation- hemorrhagic cystitis Over 60,000 patients per year 6

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO Prostate Cancer Cancer Survivorship and Potential Addressable Market Expected annual revenue per patient is $20,000 Market penetration of 60,000 patients (50%) yields $1.2 billionannual revenue in US Pelvic Radiation Survivors 700,000 1 Severe HC Survivors 42,000 2 (1) American Cancer Society Cancer Treatment and Survivorship Fact and Figures 2019 -2021. (2) Based on the Company’s estimate. 8 Uterine Corpus Cancers Pelvic Radiation Survivors 200,000 1 Severe HC Survivors 12,000 2 Rectal & Other Pelvic Pelvic Radiation Survivors 300,000 1 Severe HC Survivors 18,000 2 Breast Cancer x-DNA Chemotherapy Survivors 360,000 1 Severe HC Survivors 36,000 2 Leukemia & Lymphoma x-DNA Chemotherapy Survivors 180,000 1 Severe HC Survivors 18,000 2 Other Tumors x-DNA Chemotherapy Survivors 60,000 1 Severe HC Survivors 6,000 2

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need Asset Pipeline LP-10: Liposomal tacrolimus for hemorrhagic cystitis Proprietary liposomal formulation being evaluated as a treatment for hemorrhagic cystitis. A growing pipeline with strong patent protection 9 Product Candidate Target Indication Preclinical Phase 1 Phase 2 Phase 3 Marketing Approval Next Anticipated Milestone LP-10 Hemorrhagic Cystitis FDA Type-B mtg anticipated 4Q2023 LP-310 Oral Lichen Planus Phase-2a IND approval anticipated 4Q 2023 LP-310:Liposomal tacrolimus for oral lichen planus Proprietary liposomal oral rinse formulation of LP -10 as a potential treatment for oral lichen planus.

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO Millions of patients are diagnosed each yearwith pelvic malignancies including prostate cancer, and cancers of the uterine corpus, requiring treatment. 20% of these patients receive pelvic radiation therapy during the treatment of their cancer, in addition to surgery and chemotherapy. Hemorrhagic cystitis is a potentially chronic, highly morbid condition with no approved drug therapy, and a four percent mortality rate. Years after receiving treatment, many patients acquire hemorrhagic cystitis, uncontrolled bladder blood loss. An increasing US population is surviving cancer therapies, including pelvic radiation, and chemotherapies that damage DNA. 10 Hemorrhagic Cystitis, Uncontrolled Urinary Blood Loss Hemorrhagic cystitis is a serious, life -threatening bladder damage from pelvic radiation therapy and/or bladder -toxic chemotherapy.

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need LP-10 for Hemorrhagic Cystitis Liposomal tacrolimus treatment for hemorrhagic cystitis Potent vasoconstrictor; reduces capillary blood flow to the bladder lumen Potent anti-inflammatory; inhibits cytokine cascade and reduces injury to the bladder tissue Well known pharmacologic mechanisms increase the probability of efficacy Sterile powder Easy to deliver Low COGS In-house CMC 11

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO • Well Known mechanism of action • Pre-clinical efficacy • 505(b)(2) pathway • Potential $1.2 billion revenue • US FDA IND approval • Accelerated path -way candidate • Patent protection secured for LP-10 through 2034 12 LP-10: an Outstanding Drug Candidate Multiple reasons increase LP -10 probability of success Pre-clinical efficacy FDA Orphan Designation US FDA IND approval Accelerated path- way candidate Well known mechanism of action 505(b)(2) pathway Ongoing clinical evidence Potential $1.2 billion revenue

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need • Multi-center, dose -escalation study • 13 subjects with moderate to severe refractory hemorrhagic cystitis • Subjects treated with up to two courses of LP-10 intravesical bladder instillations. 13 LP-10: Phase 2a Trial A Phase 2a clinical trial (NCT01393223): • All subjects tolerated LP -10 instillations with no serious adverse events (SAE) reported • LP-10 demonstrated short duration of systemic uptake • Decreased cystoscopic bleeding hematuria and improved urinary incontinence • 58% of patients achieved complete or near complete resolution of bleeding per cystoscopy Top line results • Type-B meeting with the FDA anticipated Q4, 2023 • Breakthrough Therapy Designation Request expected Q4, 2023 • Phase-2b IND submission Q1, 2024 Next Steps

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need Mean Comment Age, years 67 Range 25-89 Race • White 9 • Non-White 4 Radiation induced HC 11 Chemotherapy induced HC 2 Cancer • Prostate cancer 9 • Bladder cancer 2 • Lymphoma 2 Duration of HC years 4 Range 1 -14 years Prior HC treatment 13 medication, HBO, catheters, surgical procedures 14 LP-10 Phase 2a Trial Patient Demographics

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need Number Comment Product related significant adverse events 0 Subject discontinuations 0 Number of subjects with adverse events 6/13 12 AEs reported in 6 subjects 2 mg: 4 AEs in 3 subjects 4 mg: 2 AEs in 1 subject 8 mg: 6 AEs in 2 subjects Bladder spasm, urinary urgency, pain and dysuria 4 Artificial urinary sphincter malfunction 1 Weakness and dizziness 1 Low blood pressure 1 Flank pain 1 Urinary retention due to bladder blood clot 1 Headache 1 Arthralgia 1 Urinary tract infection 1 15 Adverse events (AE) LP-10 Safety

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO 16 Bleeding: Cystoscopy & Microscopy

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO 17 Hematuria & Incontinence

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO 18 Dose Response: Bleeding by Cystoscopy

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO 19 Dose Response: Urinary Urge Incontinence

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO 20 LP-10 Phase 2b Trial Design 40 HC subjects randomized 50:50 LP-10 therapy two courses Delayed- treatment Cystoscopy at baseline Cystoscopy at week 4 Cystoscopy at week 4 LP-10 therapy two courses Cystoscopy at baseline Expedited treatment group Delayed treatment group Cystoscopy at week 8 40 HC subjects randomized 50:50 LP-10 therapy two courses Delayed- treatment Cystoscopy at baseline Cystoscopy at week 4 Cystoscopy at week 4 LP-10 therapy two courses Cystoscopy at baseline Expedited treatment group Delayed treatment group Cystoscopy at week 8 Number of bleeding sites as measured by cystoscopy at 4 weeks after instillation): achievement of a clinically relevant (at least 1 category) decrease in number of bleeding sites. This end point is suggested as a direct measurement for hemorrhagic cystitis and potentially serves as a surrogate endpoint for the indication of refractory moderate to severe HC . Primary Efficacy Endpoint: Key criteria: Prospective, 40 -subject, multi -center clinical trial, randomized 1:1: expedited treatment group, delayed treatment group Subjects randomized into the delayed treatment group will alternatively receive four weeks standard of care HC treatment instead of LP -10 All study subjects will be evaluated for urinary bleeding as measured by cystoscopy at four weeks (after cystoscopic evaluation, subjects in the delayed treatment group will be treated with LP -10).

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO • Painful and comes with the risk of complications including infections, scarring, stress and depression. • Has malignant potential. • Most currently available therapies are palliative rather than curative. Oral Lichen Planus Mouth Rinse 21 LP-310 for Oral Lichen Planus Oral Lichen Planus (OLP)is a chronic inflammatory, T-cell-mediated autoimmune oral mucosal disease • Oral rinse formulation of LP -10 • Increased local concentration in oral cavity while minimalizing systemic toxicity • 505(b)(2) pathway, platform technology expansion • Low COGS and fast development plan LP-310 • Large market size opportunity (6 million US)with no current approved therapy • Phase 2a IND approval anticipated Q4, 2023 LP-310

NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need • Lipellamaintains a sterile manufacturing facility in Pittsburgh, PA for the production of clinical supplies and research products. • LP-10 has been produced at high quantities in this facility for Lipella'sclinical trials. • The facility is also used for development of liposomal formulations intended for intravesical delivery. • Lipella is currently collaborating with Cook Myocyte (a subsidiary of Cook Medical) regarding commercial grade manufacturing. 22 Manufacturing Capabilities

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO Experienced Management Team Lipella is led by an experienced team with complementary skillset and years of experience working together. Jonathan Kaufman, PhD Chief Executive Officer • 23+ Years Experience • Co-founded Lipella in 2005 • Co-founder of Knopp Biosciences. CFO of SemprusBiosciences. CSO LaunchCyte LLC. • PhD Penn. MBA Wharton. 23 Michael Chancellor, MD Chief Medical Officer • 30+ Years Experience • Lipella co-founder. Joined Lipella in 2008 • Co-founder of Cook -Myosite; Professor of Urology, William Beaumont Medical Center. • MD University of Michigan. Michele Gruber Director of Operations • 12+ Years Experience • Joined Lipella in 2009 • CMC development, facilities, R&D management. • BS Carnegie Mellon Janet Okonski Director Clinical Operations • 20+ Years Experience • Joined Lipella in 2021 • Clinical trial management, safety monitoring • BS Indiana University of Pennsylvania
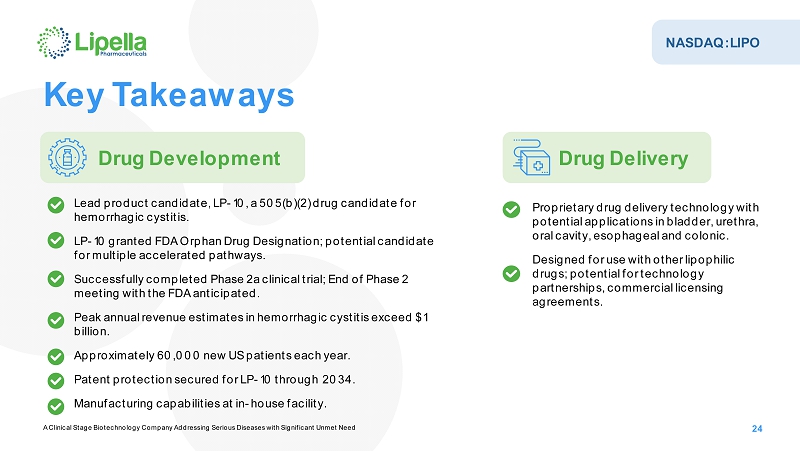
NASDAQ:LIPO A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need 24 Key Takeaways Drug Development Drug Delivery Proprietary drug delivery technology with potential applications in bladder, urethra, oral cavity, esophageal and colonic. Designed for use with other lipophilic drugs; potential for technology partnerships, commercial licensing agreements. Lead product candidate, LP -10, a 505(b)(2) drug candidate for hemorrhagic cystitis. LP-10 granted FDA Orphan Drug Designation; potential candidate formultiple accelerated pathways. Successfully completed Phase 2a clinical trial; End of Phase 2 meeting with the FDA anticipated. Peak annualrevenue estimates in hemorrhagic cystitis exceed $1 billion. Approximately 60,000 new US patients each year. Patent protection secured for LP -10 through 2034. Manufacturing capabilities at in -house facility.

A Clinical Stage Biotechnology Company Addressing Serious Diseases with Significant Unmet Need NASDAQ:LIPO lipellapharmaceuticals.com LipellaPharmaceuticals Inc. 7800 Susquehanna Street, Suite 505 Pittsburgh, PA 15208 412-894-1853
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
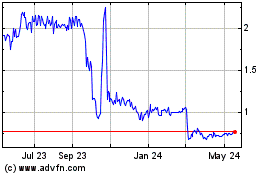
Lipella Pharmaceuticals (NASDAQ:LIPO)
Historical Stock Chart
From Apr 2024 to May 2024
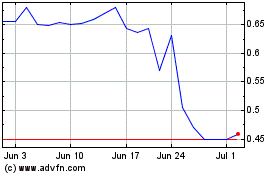
Lipella Pharmaceuticals (NASDAQ:LIPO)
Historical Stock Chart
From May 2023 to May 2024