As filed
with the Securities and Exchange Commission on October 29, 2020
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM S-1
REGISTRATION
STATEMENT
UNDER
THE SECURITIES
ACT OF 1933
Hepion
Pharmaceuticals, Inc.
(Exact Name
of Registrant as Specified in its Charter)
Delaware
(State or other jurisdiction of
incorporation or organization)
|
2834
(Primary Standard Industrial
Classification Code Number)
|
46-2783806
(I.R.S. Employer
Identification Number)
|
399 Thornall
Street, First Floor
Edison,
NJ 08837
(732) 902-4000
(Address,
including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Robert
Foster
Chief
Executive Officer
Hepion
Pharmaceuticals, Inc.
399 Thornall
Street, First Floor
Edison,
NJ 08837
(732) 902-4000
(Name, address,
including zip code, and telephone number, including area code, of agent for service)
|
Copies
to:
|
Jeffrey
J. Fessler, Esq.
Sheppard, Mullin, Richter & Hampton LLP
30 Rockefeller Plaza
New York, NY 10112
Tel.: (212) 634-3067
|
Brad
L. Schiffman, Esq.
Melissa
Palat Murawsky, Esq.
Blank
Rome LLP
1271
Avenue of the Americas
New
York, NY 10020
Tel:
(212) 885-5442
|
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date hereof.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415
under the Securities Act of 1933, check the following box: ¨
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please
check the following box and list the Securities Act registration statement number of the earlier effective registration statement
for the same offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box
and list the Securities Act registration statement number of the earliest effective registration statement for the same offering.
¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box
and list the Securities Act registration statement number of the earliest effective registration statement for the same offering.
¨
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller
reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated
filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the
Exchange Act. (Check one):
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
Non-accelerated filer x
|
Smaller reporting company x
Emerging growth company x
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. x
CALCULATION
OF REGISTRATION FEE
Title
of Each Class of Securities
to Be Registered
|
|
Proposed
Maximum Aggregate
Offering Price(1)
|
|
|
Amount
of
Registration
Fee(2)
|
|
|
Common
Stock, $0.0001 par value per share (3)
|
|
$
|
34,500,000
|
|
|
$
|
3,764
|
|
(1)
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933,
as amended (the "Securities Act").
(2)
Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price.
(3)
Includes shares of common stock which may be issued on exercise of a 45-day option granted to the underwriters to cover over-allotments,
if any.
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until
the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter
become effective in accordance with Section 8(a) of the Securities Act of 1933, or until the Registration Statement shall
become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The
information contained in this preliminary prospectus is not complete and may be changed. These securities may not be sold until
the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not
an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale
is not permitted.
|
PRELIMINARY
PROSPECTUS
|
|
SUBJECT
TO COMPLETION
|
|
DATED
OCTOBER 29, 2020
|

Hepion
Pharmaceuticals, Inc.
We are offering
shares of our common stock, par value $0.0001 per share.
Our common
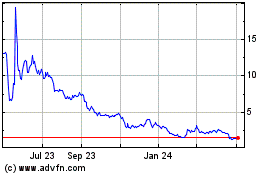
stock is listed on The Nasdaq Capital Market (“Nasdaq”), under the symbol “HEPA.” On October 28, 2020,
the last reported sale price of our common stock was $3.26 per share.
The final
public offering price of the shares of common stock in this offering will be determined through negotiation between us and the
underwriters in the offering and the recent market price used throughout this prospectus may not be indicative of the final offering
price.
We are an
“emerging growth company” under the federal securities laws and have elected to comply with certain reduced public
company reporting requirements.
Investing in our common stock
involves a high degree of risk. See “Risk Factors” beginning on page 6. Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
Per Share
|
|
|
Total
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting discounts and commissions(1)
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to us, before expenses
|
|
$
|
|
|
|
$
|
|
|
|
|
(1)
|
We
refer you to “Underwriting” beginning on page 14 for additional information regarding underwriters’
compensation.
|
We have
granted a 45-day option to the representative of the underwriters to purchase up to additional
shares of common stock solely to cover over-allotments, if any.
The underwriters
expect to deliver the shares of common stock to the purchasers on or about
, 2020.
ThinkEquity
a division of Fordham Financial Management, Inc.
The date
of this prospectus is , 2020


TABLE
OF CONTENTS
You
should rely only on the information contained or incorporated by reference in this prospectus
or in any free writing prospectus that we may authorize to be delivered or made available to you. We and the underwriters have
not authorized anyone to provide you with different information. We are offering to sell, and seeking offers to buy, our securities
only in jurisdictions where such offers and sales are permitted. The information in this prospectus is accurate only as of its
date, regardless of the time of its delivery or any sale of our securities.
Persons outside the United States
who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering
of our securities and the distribution of the prospectus outside the United States.
ABOUT
THIS PROSPECTUS
In this prospectus, “Hepion,”
“the Company,” “we,” “us,” and “our” refer to Hepion Pharmaceuticals, Inc.,
a Delaware corporation, and its subsidiaries, unless the context otherwise requires.
This
prospectus describes the specific information about the terms of this offering, the shares of common stock that we are selling
and the risks of investing in our common stock. You should read this prospectus, any free writing prospectus and the information
incorporated by reference in this prospectus before making your investment decision. See
“Where You Can Find More Information.” If any statement in one of these documents is inconsistent with a statement
in another document having a later date – for example, a document incorporated by reference in this prospectus – the
statement in the document having the later date modifies or supersedes the earlier statement.
Neither we, nor any of our officers,
directors, agents or representatives or underwriters, make any representation to you about the legality of an investment in our
common stock. You should not interpret the contents of this prospectus or any free writing prospectus to be legal, business, investment
or tax advice. You should consult with your own advisors for that type of advice and consult with them about the legal, tax, business,
financial and other issues that you should consider before investing in our securities.
PROSPECTUS
SUMMARY
This summary highlights certain
information appearing elsewhere in this prospectus and the documents incorporated by reference. This summary does not contain
all of the information you should consider before investing in our common shares. You should read this entire prospectus and the
documents incorporated by reference into this prospectus carefully before making an investment decision. References in this prospectus
to “we,” “us,” “our” and “Company” refer to Hepion Pharmaceuticals, Inc.
and its consolidated subsidiaries.
Business Overview
We are a biopharmaceutical company
headquartered in Edison, New Jersey, focused on the development of pleiotropic drug therapy for treatment of chronic liver disease.
This therapeutic approach targets fibrosis and hepatocellular carcinoma (“HCC”) associated with non-alcoholic steatohepatitis
(“NASH”), viral hepatitis, and other liver diseases. Our cyclophilin inhibitor, CRV431, is being developed to offer
benefits to address these multiple complex pathologies. CRV431 is a cyclophilin inhibitor that targets multiple biochemical pathways
involved in the progression of liver disease. Preclinical studies with CRV431 in NASH models demonstrated consistent reductions
in liver inflammation, fibrosis, and cancerous tumors. CRV431 additionally shows antiviral activity towards hepatitis B, C, and
D viruses which also trigger liver disease.
We have completed a Phase 1 program
with CRV431 demonstrating safety, tolerability, and pharmacokinetics (PK). Our program consisted of three different clinical trials
with CRV431, administered orally once daily, that included: 1) a Single Ascending Dose (SAD) study; 2) a Multiple Ascending Dose
(MAD) study; and 3) a Drug-Drug Interaction (DDI) study. The SAD, MAD, and DDI studies were comprised of 32, 25, and 18 healthy
subjects, respectively. Additionally, in the SAD study, 8 of the 32 subjects received placebo (24 received CRV431).
CRV431 appeared to be well-tolerated
in the Phase 1 program, and there were no serious adverse effects (SAEs). The few adverse effects (AEs) observed were mild to
moderate and mostly unrelated to study drug. The PK profile of each subject was characterized and CRV431 blood exposures were
similar to those needed to elicit efficacy in the preclinical studies.
We are currently conducting a
Phase 2a study in NASH patients with fibrosis scores of F2 and F3. The first dosing cohort of 75 mg CRV431 once daily orally is
underway.
NASH is the form of liver disease
that is triggered by what has come to be known as the “Western diet”, characterized especially by high-fat, high-sugar,
and processed foods. Among the effects of a prolonged Western diet is fat accumulation in liver cells (steatosis) which is described
as non-alcoholic fatty liver disease (“NAFLD”) and can predispose cells to injury. NAFLD may evolve
into NASH when the fatty liver begins to progress through stages of cell injury, inflammation, fibrosis, and carcinogenesis. People
who develop NASH often have additional predisposing conditions such as diabetes and hypertension, but the exact biochemical events
that trigger and maintain the progression are not well known. Many people in the early stages of disease do not have significant
symptoms and therefore do not know that they have it. NASH becomes evident and a major concern when the liver becomes fibrotic
and puts the individual at increased risk of developing cirrhosis and other complications. Individuals with advanced liver fibrosis
have significantly higher risk of developing liver cancer, although cancer may also arise in some patients before significant
hepatitis or fibrosis. NASH is increasing worldwide at an alarming rate due to the spread of the Western diet, obesity, and other
related conditions. Approximately 4-5% of the global population is estimated to have NASH, and that proportion is higher in the
USA. It is predicted that NASH will become the leading reason for individuals requiring a liver transplant in the USA as early
as 2020. Considering the serious outcomes linked to advancing NASH, the economic and social burden of the disease is enormous.
There are no simple blood tests to diagnose or track the progression of NASH, and no drugs are approved to specifically treat
the disease.
HCC is the major type of liver
cancer, accounting for 85-90% of all cases. NASH, hepatitis virus infection, and alcohol consumption all are major causes of HCC.
Globally, over 700,000 people die each year from liver cancer which is second only to lung cancer among all cancer-related
deaths. The high mortality is due to the fact that only around half of all people who develop HCC (in developed countries) receive
the diagnosis early enough to have an opportunity for therapeutic intervention. Additionally, recurrence rates are high, and current
treatment options remain limited.
HCC is a type of cancer in which
the tissue microenvironment plays a major role in its development. In most cases HCC is preceded by significant, long-term damage
to liver cells, inflammation and fibrosis. One-third of people with cirrhosis, a very advanced stage of liver disease, will eventually
progress to HCC. The chronic injury to the liver leads to many genetic mutations that eventually lead to transformation of cells
and formation of tumors. The noxious tissue microenvironment also promotes cancer by altering the function of immune cells and
endothelial cells which form tumor-supporting blood vessels. These various events underscore the importance of halting liver injury
and scarring as early and effectively as possible to prevent cancer development.
Viral hepatitis may be linked
to one or more viruses including hepatitis A, B, C, D, or E. Hepatitis B virus (“HBV”) is one of many hepatitis viruses
that selectively infect human liver cells and can establish persistent infections under certain conditions. Chronic infections,
especially by HBV, HCV, and HDV, cause progressive liver inflammation, fibrosis, cirrhosis, and cancer. Collectively, these infections
represent one of the 3 major triggers of progressive liver disease (NAFLD/NASH and alcohol being the others).
An HBV vaccine is available that,
if administered prior to HBV infection, assists the body in neutralizing the virus and blocking infection. However,
vaccination is not efficacious for people who are already infected with HBV, and the vaccine has not been historically available
to everyone. As a result, an estimated 240 million people worldwide have chronic HBV infection. Anti-HBV medications are used
widely by chronically infected individuals but usually are only effective in decreasing viral replication and viremia (virus in
the blood), and NOT in eradicating HBV from the liver. This is because HBV, unlike HCV, has evolved clever ways of persisting
in liver cells and evading the immune system. Thus, despite vaccines and anti-viral medications, chronic HBV infection remains
a huge global health problem. Chronic HBV infection is the leading cause of hepatocellular carcinoma, which kills around 350,000
people per year. A similar number of people die each year from cirrhosis and other complications arising from HBV.
We are developing CRV431 as our
lead molecule. CRV431 is a cyclophilin inhibitor that targets specific isomerases that play an important role in protein folding
in health and in disease. To date, in vitro and/or in vivo studies have demonstrated reductions
in HBV DNA, HBsAg, HBeAg, inhibition of virus uptake (NTCP transport inhibition), and stimulation of innate immunity. Importantly, in
vivo studies in a NASH model of fibrosis and HCC have repeatedly demonstrated CRV431 reduces fibrosis scores and overall
liver tumor burden. Hence, CRV431 is a pleiotropic molecule that may not only treat liver disease but may also serve to reduce
important risk factors (e.g., HBV) for developing the disease.
CRV431
CRV431 is a novel drug candidate
designed to target a class of proteins called cyclophilins, of which there are many isoforms. Cyclophilins play a role in health
and in the pathogenesis of certain diseases and are known as peptidyl prolyl isomerases. The isomerase activity plays an important
role in several biological processes including, for example, folding of proteins to confer certain 3-dimensional configurations.
Additionally, specific host cyclophilins (e.g., cyclophilin A, B, C, D) play a role in the pathogenesis of many diseases,
including liver disease and viral hepatitis.
Cyclophilins are pleiotropic
enzymes that play a role in injury and steatosis through mechanisms including cell death occurring through mitochondrial pore
permeability (cyclophilin D). Inhibition of cyclophilin D, therefore, may play an important role in protection from cell death.
Cyclophilin A binding to CD147 is known to play a role in inflammation, cyclophilin B plays a role in fibrosis through collagen
production, and cyclophilins also play a role in cirrhosis and cancer (e.g., cell proliferation and metastasis). Cyclophilin inhibition
with CRV431, therefore, may play an important role in reducing liver disease.
To date, we have completed eight
separate preclinical animal efficacy studies of CRV431 to assess antifibrotic activity. Each of these eight studies were conducted
by independent laboratory collaborations at The Scripps Research Institute (San Diego, CA), SMC Corporation (Tokyo, Japan), and
Physiogenex S.A.S. (France), Each of these studies demonstrated consistent and significant reductions in fibrosis in mice and
rats. CRV431 was also tested in Precision Cut Liver Slices and in Precision Cut Lung Slices in ex plants from human donors.
Again, CRV431 demonstrated an antifibrotic effect that was consistent with the animal study findings. These studies provide support
of advancing CRV431 into clinical trials for NASH, and potentially additional indications where fibrosis plays a role.
Important risk factors for development
of liver disease include viral hepatitis (HBV, HCV, HDV), alcohol, and non-alcoholic fatty liver disease and the more aggressive
form called non-alcoholic steatohepatitis. The life cycle of certain viruses, including for example, HBV, HIV, and hepatitis C
virus (“HCV”) infections are dependent on host proteins (cyclophilins) for the role they play in the virus life cycle
and propagation of the virus. CRV431 has been developed to inhibit the role of host cyclophilins and therefore interfere in viral
propagation. CRV431 does not directly target the virus and, as such, should be less susceptible to drug resistance, borne from
viral mutations.
Data in various cell lines
of either transfected or infected HBV demonstrates nanomolar efficacy (EC50 values) and micromolar toxicity (CC50 values).
The selective index (“SI”), therefore, is wide and suggests that CRV431 presents a viable clinical drug candidate
for the treatment of viral infections, including HBV. Additional testing in a transgenic mouse model of HBV indicated that
CRV431 reduced HBV DNA in the liver and HBsAg in serum. CRV431 is orally active and appears to be well tolerated.
On May 10,
2018, we submitted an Investigational New Drug Application (“IND”) to the U.S. Food and Drug Administration
(“FDA”) to support initiation of our CRV431 HBV clinical development program in the United States and received
approval in June 2018. We completed the first segment of our Phase 1 clinical activities for CRV431 in October 2018
wherein we reached a major clinical milestone of positive data from a Phase I trial of CRV431 in humans. This achievement
triggered the first milestone payment, as stated in the Merger Agreement for the acquisition of Ciclofilin
Pharmaceuticals, Inc. (“Ciclofilin”) and we paid a related milestone payment of approximately $346,000 to
Aurinia Pharmaceuticals, Inc. ("Aurinia") and $654,000 to the former Ciclofilin shareholders along with the issuance of 1,439
shares of our common stock with a fair value of $55,398, representing 2.5% of our issued and outstanding common stock as of
June, 2016, to the former Ciclofilin shareholders. Our CEO is a former Ciclofilin shareholder and received approximately
$274,000 and 603 shares of common stock and Petrus Wijngaard, a director of our company, received $2,805 and 6 shares of
common stock.
Additional
milestone payments could potentially be payable to the former Ciclofilin shareholders pursuant to the Ciclofilin Merger
Agreement as follows: (i) upon receipt of Phase II positive data from a proof of concept clinical trial of CRV431 in humans -
4,317 shares of common stock and $3,000,000, (ii) upon initiation of a Phase III trial of CRV431 - $5,000,000, and (iii) upon
acceptance by the FDA of a new drug application for CRV431 - $8,000,000 . In addition, on February 14, 2014, Ciclofilin had
entered into a Purchase and Sale Agreement to acquire Aurinia’s entire interest in CRV431. This agreement contains
future milestone payments payable by us based on clinical and marketing milestones of up to CAD $2.45 million. The milestone
payments payable to the former Ciclofilin shareholders will be subject to offset by certain of the clinical and marketing
milestone payments payable to Aurinia as follows: (a) the payments to the former Ciclofilin shareholders pursuant to (ii)
above would be offset by payment to Aurinia of CAD $450,000, and (b) the payments to the former Ciclofilin shareholders
pursuant to (iii) above would be subject to offset by payment to Aurinia of up to CAD $2,000,000. In addition to the above
clinical and milestone payments, the Aurinia Agreement provides for the following additional contingent payment obligations:
(x) a royalty of 2.5% on net sales of CRV431 which is uncapped, (y) a royalty of 5% on license revenue from CRV431 and (z) a
payment equal to 30% of the proceeds from a Liquidity Event (as defined in the Purchase and Sale Agreement) with respect to
Ciclofilin, of which approximately $150,000 plus interest will be paid to Aurinia upon the closing of this offering. The
maximum obligation under both (y) and (z) is CAD $5,000,000.
On June 17, 2019, we submitted an IND to the FDA to support
initiation of our CRV431 NASH clinical development program in the United States and received approval in July 2019. We completed
dosing of CRV431 in our MAD clinical trial in September, 2020.
ARTIFICAL INTELLIGENCE (AI)
We have created a proprietary AI called, “AI-POWRTM
to optimize the outcomes of our current clinical programs and to potentially identify novel indications for CRV431 and possibly
identify new targets and new drug molecules to broaden our pipeline.
AI-POWR™ is our acronym for Artificial Intelligence
- Precision Medicine; Omics that include genomics, proteomics, metabolomics, transcriptomics, and lipidomics;
World database access; and Response and clinical outcomes. AI-POWR™ allows for the selection
of novel drug targets, biomarkers, and appropriate patient populations. AI-POWR™ is used to identify responders from big
data sources using our multi-omics approach, while modelling inputs and scenarios to increase response rates. The components of
AI-POWR™ include access to publicly available databases, and in-house genomic and multi-omic big data, processed via machine
learning algorithms. AI outputs allow for improved response outcomes through enhanced patient selection, biomarker selection and
drug target selection. We believe AI outputs will help identify responders a priori and reduce the need for large sample
sizes through study design enrichment.
We intend to use AI-POWR™ to help identify which patients
will best respond to CRV431 for treatment of NASH patients, currently in a Phase 2a clinical trial. It is anticipated that applying
this proprietary platform to our drug development program will ultimately save time, resources and money. In so doing, we believe
that AI-POWR™ is a risk-mitigation strategy that should reap benefits all the way through from clinical trials to commercialization.
We believe that NASH is a very heterogenous disease and we need
to have a better understanding of interactions between changes to proteins, genes, lipids, and metabolites, to name a few, induced
by both drugs and disease. All of this is further complicated by variable drug concentrations, patient traits and temporal factors.
AI-POWR™ is designed to address many of these typical challenges, as we believe we can use our proprietary platform to shorten
development timelines and increase the delta between placebo and treatment groups. AI-POWR™ will be used to drive our ongoing
Phase 2a NASH program and identify additional potential indications for CRV431 to expand our footprint in the cyclophilin inhibition
therapeutic space.
Recent Developments
On July 30, 2020, our stockholders approved an amendment to our 2013 Equity Incentive Plan (the "Plan"), increasing the number of shares
of common stock issuable under the Plan to 2,500,000 from 40,535. As of September 30, 2020, 2,499,473 shares of our common stock are issuable
upon exercise of outstanding options under the Plan and 527 shares of common stock are reserved for future issuance under the Plan.
Risks Associated with our Business
Our business is subject to numerous risks and uncertainties,
including those highlighted and incorporated by reference in the section entitled “Risk Factors” immediately following
this prospectus summary. These risks include, but are not limited to, the following:
|
|
•
|
Our product candidate, CRV431, is in the early stages of clinical development and its commercial viability remains subject
to the successful outcome of current and future clinical trials, regulatory approvals and the risks generally inherent in the development
of pharmaceutical product candidates. If we are unable to successfully advance or develop or partner our product candidate, we
may be delayed or precluded further development or regulatory approval.
|
|
|
•
|
A pandemic, epidemic or outbreak of an infectious disease, such as COVID-19, may materially and adversely affect our business
and operations. The Company cannot estimate the length or gravity of the impact of the COVID-19 outbreak at this time, but if
the pandemic continues, it may have a material adverse effect on the Company’s results of future operations, financial position,
liquidity, and capital resources, and those of the third parties on which the Company relies in fiscal year 2020.
|
|
|
•
|
We have incurred losses since inception, anticipate that we will incur continued losses for the foreseeable future indicating
the possibility that we may not be able to operate in the future.
|
|
|
•
|
We will require substantial additional funding which may not be available to us on acceptable terms, or at all. If we fail
to raise the necessary additional capital, we may be unable to complete the development and commercialization of our product candidate,
or continue our development programs.
|
|
|
•
|
If we fail to comply with the continued listing requirements of The Nasdaq Capital Market, our common stock may be delisted
and the price of our common stock and our ability to access the capital markets could be negatively impacted.
|
|
|
•
|
Our product candidate and any future product candidates may exhibit undesirable side effects when used alone or in combination
with other approved pharmaceutical products or investigational new drugs, which may delay or preclude further development or regulatory
approval, or limit their use if approved.
|
|
|
•
|
If the results of preclinical studies or clinical trials for our product candidate, including those that are subject to existing
or future license or collaboration agreements, are unfavorable or delayed, we could be delayed or precluded from the further development
or commercialization of our product candidate, which could materially harm our business.
|
|
|
•
|
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials
may not be predictive of future trial results.
|
|
|
•
|
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently
unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidate, our business will be substantially
harmed.
|
|
|
•
|
We currently have no sales and marketing organization. If we are unable to establish a direct sales force in the United States
to promote our products, the commercial opportunity for our products may be diminished.
|
|
|
•
|
We may not be able to manufacture our product candidate in commercial quantities, which would prevent us from commercializing
our product candidate.
|
|
|
•
|
Our product candidate, if approved for sale, may not gain acceptance among physicians, patients and the medical community,
thereby limiting our potential to generate revenues.
|
|
|
•
|
You will experience immediate and substantial dilution in the book value per share of the common stock you purchase.
|
|
|
•
|
Management will have broad discretion as to the use of proceeds from this offering and might not use them effectively.
|
Corporate History and Information
We were incorporated in Delaware on May 15, 2013 for the
purpose of holding certain FV-100 assets of Synergy Pharmaceuticals Inc., or Synergy. We were a majority-owned subsidiary
of Synergy Pharmaceuticals Inc. (Synergy) until February 18, 2014, the date Synergy completed the spinout of our shares
of common stock. On July 18, 2019, we filed a certificate of amendment to our certificate of incorporation to change the Company’s
name from “ContraVir Pharmaceuticals, Inc.” to “Hepion Pharmaceuticals, Inc.” The name change became effective
as of July 18, 2019.
Our principal executive offices are located at 399 Thornall
Street, First Floor, Edison, New Jersey 08837. Our telephone number is (732) 902-4000 and our website address is www.hepionpharma.com.
The information on our website is not a part of, and should not be construed as being incorporated by reference into, this registration
statement or the accompanying prospectus.
THE OFFERING
|
Common stock offered
|
|
shares.
|
|
|
|
|
|
Over-allotment option
|
|
We have granted a 45-day option to the representative of the underwriters to purchase up to additional shares of common stock solely to cover over-allotments, if any, at the public offering price less underwriting discounts and commissions.
|
|
|
|
|
|
Common stock to be outstanding after this
offering (1)
|
|
shares of common stock (or shares of common stock if the underwriters exercise in full their option to purchase additional shares of common stock).
|
|
|
|
|
|
Use of proceeds
|
|
We
estimate that the net proceeds to us from this offering from the sale of the shares of our common stock will be approximately $
million, or approximately $ million if the underwriters exercise their option
to purchase additional shares in full, at the assumed public offering price of $ , the closing price of our common stock as reported
on The Nasdaq Capital Market on , 2020, and after deducting underwriting discounts and commissions and offering expenses payable
by us.
We
intend to use the net proceeds of this offering to fund our research and development activities and general
corporate purposes, including approximately $150,000 plus interest for a milestone payment, working capital, operating
expenses and capital expenditures. We may use the net proceeds from this offering to fund possible acquisitions of other
companies, products or technologies, though no such acquisitions are currently contemplated. See “Use of
Proceeds.”
|
|
Risk factors
|
|
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 6 of this prospectus and the risk factors included in our Form 10-K for the year ended December 31, 2019, which are incorporated by reference into this prospectus, for a discussion of factors to consider carefully before deciding to invest in shares of our common stock in this offering.
|
|
|
|
|
|
Nasdaq symbol
|
|
Our common stock is listed on The Nasdaq Capital Market under the symbol “HEPA.”
|
|
(1)
|
Based on 9,025,061 shares of common stock outstanding as of June 30, 2020 and excludes:
|
|
|
•
|
1,402,771 shares of our common stock issuable upon exercise of outstanding options at a weighted average price of $7.76 per
share;
|
|
|
•
|
2,536,566 shares of our common stock issuable upon exercise of outstanding warrants with a weighted-average exercise price
of $19.35 per share;
|
|
|
•
|
3,184 shares of our common stock issuable upon conversion of outstanding shares of Series A Convertible Preferred Stock;
|
|
|
•
|
16,839 shares of our common stock issuable upon conversion of outstanding shares of Series C Convertible Preferred Stock;
and
|
|
|
•
|
shares of our common stock issuable upon exercise of the warrant to be issued to the representative in connection with this
offering.
|
RISK FACTORS
An investment in our securities involves a high degree of
risk. You should carefully consider the risk factors contained in our periodic reports filed with the SEC, including our Annual Report on Form 10-K for the year ended December 31, 2019 and all of our quarterly reports on Form 10-Q,
which are incorporated by reference into this prospectus. Before deciding to invest in our securities, you should carefully consider
these risks, as well as other information we include or incorporate by reference in this prospectus.
If any of the events described in these risk factors actually
occurs, or if additional risks and uncertainties that are not presently known to us or that we currently deem immaterial later
materialize, then our business, prospects, results of operations and financial condition could be materially adversely affected.
In that event, the trading price of our securities could decline, and you may lose all or part of your investment in our securities.
The risks discussed below include forward-looking statements, and our actual results may differ substantially from those discussed
in these forward-looking statements. See “Cautionary Statement Regarding Forward-Looking Statements.”
Risks Related to our Business
The following items supplement
the risks related to our business previously reported in Part 1, “Item 1A. Risk Factors – Risks Related to Our Business”
of our Annual Report on Form 10-K for the year ended December 31, 2019:
Our
approach to the discovery and development of product candidates based on AI-POWR™ is
novel and unproven, and we do not know whether we will be able to develop any products of commercial value.
We
intend to leverage AI-POWR™ to potentially identify
novel indications for CRV431 and possibly identify new targets and new drug molecules to broaden our pipeline for
patients whose diseases have not been adequately addressed to date by other approaches and to design and conduct efficient clinical
trials with a higher likelihood of success. While we believe that applying AI-POWR™ to
create medicines for defined patient populations may potentially enable drug research and clinical development that is more efficient
than conventional drug research and development, our approach is both novel and unproven. Because our approach is both novel and
unproven, the cost and time needed to develop our product candidates is difficult to predict, and our efforts may not result in
the discovery and development of commercially viable medicines. We may also be incorrect about the effects of our product candidates
on the diseases of our defined patient populations, which may limit the utility of our approach or the perception of the utility
of our approach. Furthermore, our estimates of our defined patient populations available for study and treatment may be lower than
expected, which could adversely affect our ability to conduct clinical trials and may also adversely affect the size of any market
for medicines we may successfully commercialize. Our approach may not result in time savings, higher success rates or reduced costs
as we expect it to, and if not, we may not attract collaborators or develop new drugs as quickly or cost effectively as expected
and therefore we may not be able to commercialize our approach as originally expected.
AI-POWR™
may fail to help us discover and develop additional potential product candidates.
Any
drug discovery that we are conducting using AI-POWR™ may
not be successful in identifying compounds that have commercial value or therapeutic utility. AI-POWR™ may
initially show promise in identifying potential product candidates, yet fail to yield viable product candidates for clinical development
or commercialization for a number of reasons, including:
|
|
·
|
research programs to identify new product candidates will require
substantial technical, financial and human resources, and we may be unsuccessful in our efforts to identify new product candidates.
If we are unable to identify suitable additional compounds for preclinical and clinical development, our ability to develop product
candidates and obtain product revenues in future periods could be compromised, which could result in significant harm to our financial
position and adversely impact our stock price;
|
|
|
·
|
compounds found through AI-POWR™ may
not demonstrate efficacy, safety or tolerability;
|
|
|
·
|
potential product candidates may, on further study, be shown to have
harmful side effects or other characteristics that indicate that they are unlikely to receive marketing approval and achieve market
acceptance;
|
|
|
|
|
|
|
·
|
competitors may develop alternative therapies that render our potential
product candidates non-competitive or less attractive; or
|
|
|
·
|
a potential product candidate may not be capable of being produced
at an acceptable cost.
|
Risks Related to this Offering
Management will have broad discretion as to the use of
proceeds from this offering and might not use them effectively.
Our management will have broad discretion as to the application
of the net proceeds from this offering and our stockholders will not have the opportunity as part of their investment decisions
to assess whether the net proceeds are being used appropriately. You might not agree with our decisions, and our use of the proceeds
might not yield any return on your investment. Because of the number and variability of factors that will determine our use of
the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our failure to
apply the net proceeds of this offering effectively could compromise our ability to pursue our growth strategy and we might not
be able to yield a significant return, if any, in our investment of these net proceeds. You will not have the opportunity to influence
our decisions on how to use our net proceeds from this offering.
Investors in this offering will experience immediate and
substantial dilution and may experience further dilution in the future.
The
offering price per share of our common stock being offered will be higher than the net tangible book value per share of our common
stock. Therefore, if you purchase shares of common stock in this offering, you will incur immediate and substantial dilution in
the as adjusted net tangible book value per share of common stock from the price you pay for the common stock. For a further description
of the dilution that investors in this offering will experience, see “Dilution”.
Furthermore,
we expect that we will seek to raise additional capital from time to time in the future. Such financings may involve the
issuance of equity and/or securities convertible into or exercisable or exchangeable for our equity securities. In
addition, investors in this offering will be subject to increased dilution upon the exercise of outstanding stock options or
warrants or conversion of outstanding preferred stock. We also expect to continue to utilize equity-based compensation which
would further dilute investors. We have in the past issued, and could in the future issue, securities with anti-dilution
features which if triggered could
result in further dilution to our stockholders.
If we fail to comply with the continued listing requirements
of The Nasdaq Capital Market, our common stock may be delisted and the price of our common stock and our ability to access the
capital markets could be negatively impacted.
A delisting of our common stock from The Nasdaq Capital Market
could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our
common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable
to us, or at all, and may result in the potential loss of confidence by investors, employees and fewer business development opportunities.
A large number of shares issued in this offering may be
sold in the market following this offering, which may depress the market price of our common stock.
A large number of shares issued in this offering may be sold
in the market following this offering, which may depress the market price of our common stock. Sales of a substantial number of
shares of our common stock in the public market following this offering could cause the market price of our common stock to decline.
If there are more shares of common stock offered for sale than buyers are willing to purchase, then the market price of our common
stock may decline to a market price at which buyers are willing to purchase the offered shares of common stock and sellers remain
willing to sell the shares. All of the shares of common stock issued in the offering will be freely tradable without restriction
or further registration under the Securities Act.
We have not paid dividends in the past and have no immediate
plans to pay dividends.
We plan to reinvest all of our earnings, to the extent we have
earnings, in order to further develop our product candidate and to cover operating costs. We do not plan to pay any cash dividends
with respect to our securities in the foreseeable future. We cannot assure you that we would, at any time, generate sufficient
surplus cash that would be available for distribution to the holders of our common stock as a dividend. Therefore, you should not
expect to receive cash dividends on the common stock we are offering.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus and the documents we incorporate by reference
in this prospectus contain forward-looking statements within the meaning of Section 27A of the Securities Act, and Section
21E of the Exchange Act, and may involve material risks, assumptions and uncertainties. Statements that are not purely historical
should be considered forward-looking statements. Often they can be identified by the use of forward-looking words and phrases,
such as “may,” “will,” “believe,” “anticipate,” “expect,” “should,”
“optimistic” or “continue,” “estimate,” “intend,” “plan,” “would,”
“could,” “guidance,” “potential,” “opportunity,” “project,” “forecast,”
“confident,” “projections,” “schedule,” “designed,” “future” and the
like. These forward-looking statements reflect our current expectations and projections about future events and financial trends
that we believe may affect our business, financial condition and results of operations. These forward-looking statements are
subject to a number of risks, uncertainties and assumptions described under the section entitled “Risk Factors.”
These statements are not guarantees of future performance and
involve risks and uncertainties that are difficult to predict or are beyond our control. A number of important factors could cause
actual outcomes and results to differ materially from those expressed in these forward-looking statements. Consequently, readers
should not place undue reliance on such forward-looking statements. In addition, these forward-looking statements relate
to the date on which they are made.
The forward-looking statements reflect our current expectations
and are based on information currently available to us and on assumptions we believe to be reasonable. Forward-looking information
is subject to known and unknown risks, uncertainties and other factors that may cause our actual results, activities, performance
or achievements to be materially different from that expressed or implied by such forward-looking statements. Some of the risks, uncertainties, and other factors that could cause actual results to differ materially from estimates or projections
contained in the forward-looking
statements include, but are not limited to:
|
|
•
|
The impact of COVID-19 on our operations;
|
|
|
•
|
Our ability to compete with larger, better financed pharmaceutical companies;
|
|
|
•
|
Our uncertainty of developing marketable products;
|
|
|
•
|
Our ability to develop and commercialize our products;
|
|
|
•
|
Our ability to obtain regulatory approvals;
|
|
|
•
|
Our ability to maintain and protect intellectual property rights;
|
|
|
•
|
The inability to raise additional future financing and lack of financial and other resources;
|
|
|
•
|
Our ability to control product development costs;
|
|
|
•
|
We may not be able to attract and retain key employees;
|
|
|
•
|
We may not be able to compete effectively;
|
|
|
•
|
We may not be able enter into new strategic collaborations;
|
|
|
•
|
Changes in government regulation affecting product candidates could increase our development costs;
|
|
|
•
|
Our involvement in patent and other intellectual property litigation could be expensive and could divert management’s
attention;
|
|
|
•
|
The possibility that there will be no market acceptance for our products; and
|
|
|
•
|
Changes in third-party reimbursement policies could adversely affect potential future sales of any of our products that
are approved for marketing.
|
Although we have attempted to identify important factors that
could cause actual actions, events or results to differ materially from those described in forward-looking information, there
may be other factors that cause actions, events or results to differ from those anticipated, estimated or intended. Other than
as required by law, we do not assume any obligation to update any forward-looking information, whether as a result of new information,
future events or results or otherwise.
You should also read the matters described in “Risk Factors”
and the other cautionary statements made in this prospectus and the documents incorporated by reference into this prospectus. The
forward-looking statements in this prospectus and the documents incorporated by reference into this prospectus may not prove
to be accurate and therefore you are encouraged not to place undue reliance on forward-looking statements. You should read
this prospectus and the documents incorporated by reference into this prospectus completely.
This prospectus and the documents incorporated by reference
into this prospectus also include estimates and other statistical data made by independent parties and by us relating to market
size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned
not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the
future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
USE OF PROCEEDS
We estimate that the net proceeds to us
from this offering from the sale of the shares of our common stock will be approximately $ million, or approximately $ million
if the underwriters exercise their option to purchase additional shares in full, at the assumed public offering price of $ , the
closing price of our common stock as reported on the Nasdaq Capital Market on , 2020, and after deducting underwriting discounts
and commissions and offering expenses payable by us.
We
intend to use the net proceeds from this offering to fund our research and development activities and general corporate
purposes, including approximately $150,000 plus interest for a milestone payment, working capital, operating expenses and
capital expenditures. We may use the net proceeds from this offering to fund possible acquisitions of other companies,
products or technologies, though no such acquisitions are currently contemplated.This expected use of our net proceeds from
this offering represents our intentions based upon our current plans and business conditions, which could change in the
future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly
depending on numerous factors, including the progress of our drug candidate development, the status of and results from
clinical trials, as well as any collaborations that we may enter into with third parties for our drug candidate, and
any unforeseen cash needs.
As a result, our management will retain broad discretion over
the allocation of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding
the application of the net proceeds from this offering. The timing and amount of our actual expenditures will be based on many
factors, including cash flows from operations and the anticipated growth of our business.
DILUTION
If
you purchase securities in this offering, your interest will be diluted to the extent of the difference between the public offering
price and the net tangible book value per share of our common stock after this offering. Our net tangible book value as of June
30, 2020 was $13,231,154 million or $1.47 per share of common stock based on 9,025,061 shares of our common stock outstanding
as of that date. “Net tangible book value” is total assets minus the sum of liabilities and intangible assets. “Net
tangible book value per share” is net tangible book value divided by the total number of shares of common stock outstanding.
After giving effect to the sale by us in this offering of shares
of common stock at an assumed offering price of per share, which was the last reported sale price of our common stock on the Nasdaq
Capital Market on , 2020, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses
payable by us, our as adjusted net tangible book value as of June 30, 2020 would have been approximately $ million, or $
per share of common stock. This amount represents an immediate increase in net tangible book value of $ per share to existing stockholders
and an immediate dilution of $ per share to purchasers in this offering.
The following table illustrates the dilution:
|
Assumed public offering price per share
|
|
|
$
|
|
|
Net tangible book value per share as of June 30, 2020
|
$
|
1.47
|
|
|
|
Assumed increase in net tangible book value per share attributable to this offering
|
$
|
|
|
|
|
Assumed as adjusted net tangible book value per share as of June 30, 2020 after giving effect to this offering
|
|
|
$
|
|
|
Assumed dilution per share to new investors
|
|
|
$
|
|
The actual price at which shares are sold in this offering and
the actual amount of underwriting discounts and commissions and offering expenses payable by us may be lesser or greater than the
assumed amounts reflected in the table above.
The dilution information set forth in the table above is illustrative
only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
The above table is based on 9,025,061 shares of common stock
outstanding as of June 30, 2020, does not give effect to any exercise of the underwriters’ option to purchase additional
shares and excludes:
|
|
•
|
1,402,771 shares of our common stock issuable upon exercise of outstanding options at a weighted average price of $7.76 per
share;
|
|
|
•
|
2,536,566 shares of our common stock issuable upon exercise of outstanding warrants with a weighted-average exercise price
of $19.35 per share;
|
|
|
•
|
3,184 shares of our common stock issuable upon conversion of outstanding shares of Series A Convertible Preferred Stock;
|
|
|
•
|
16,839 shares of our common stock issuable upon conversion of outstanding shares of Series C Convertible Preferred Stock;
and
|
|
|
•
|
shares of our common stock issuable upon exercise of the warrant to be issued to the representative in connection with this
offering.
|
If we issue any additional shares in connection with outstanding
options or warrants or issue shares upon conversion of outstanding preferred stock there will be additional
dilution. In addition, we may choose to raise additional capital. To the extent that additional capital is raised through the sale
of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
CAPITALIZATION
The following table sets forth our cash and our capitalization
as of June 30, 2020 on:
|
|
•
|
an as adjusted basis to give effect to this offering (assuming no exercise of the underwriters’ option to purchase additional
shares) at an assumed offering price of $ per share, which was the last reported sale price of our common stock on the Nasdaq Capital
Market on , 2020, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable
by us.
|
The
following data is qualified in its entirety by, and should be read in conjunction with, our unaudited condensed consolidated financial
statements and notes thereto incorporated by reference in this prospectus. The as adjusted information set forth in the
table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering
determined at pricing.
|
|
|
As of June 30, 2020
|
|
|
|
|
Actual
|
|
|
As adjusted
|
|
|
Cash
|
|
$
|
17,561,813
|
|
|
|
|
|
|
Total long-term debt
|
|
$
|
176,585
|
|
|
|
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Preferred Stock, stated value $10.00 per share; 85,581 shares of Series A Convertible Preferred Stock issued and outstanding
|
|
|
855,808
|
|
|
|
|
|
|
Preferred Stock, stated value $1,000; 1,827 shares of Series C Convertible Preferred Stock issued and outstanding
|
|
|
861,033
|
|
|
|
|
|
|
Common Stock, par value $0.0001; 120,000,000 shares authorized; 9,025,061 shares issued and outstanding, actual; shares issued and outstanding, as adjusted
|
|
|
901
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
108,923,663
|
|
|
|
|
|
|
Accumulated deficit
|
|
|
(92,349,327
|
)
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
18,292,078
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
18,468,663
|
|
|
|
|
|
The
capitalization table above is based on the number of shares outstanding as of June 30, 2020, does not give effect to any exercise
of the underwriters’ option to purchase additional shares. The number of shares of our common stock that will
be outstanding after this offering is based on 9,025,061shares of common stock outstanding as of June 30, 2020 and excludes:
|
|
•
|
1,402,771 shares of our common stock issuable upon exercise of outstanding options at a weighted average price of $7.76 per
share;
|
|
|
•
|
2,536,566 shares of our common stock issuable upon exercise of outstanding warrants with a weighted-average exercise price
of $19.35 per share;
|
|
|
•
|
3,184 shares of our common stock issuable upon conversion of outstanding shares of Series A Convertible Preferred Stock;
|
|
|
•
|
16,839 shares of our common stock issuable upon conversion of outstanding shares of Series C Convertible Preferred Stock;
and
|
|
|
•
|
shares of our common stock issuable upon exercise of the warrant to be issued to the representative in connection with this
offering.
|
DESCRIPTION OF THE
SECURITIES WE ARE OFFERING
General
We are authorized to issue up to 120,000,000 shares of common
stock, $0.0001 par value per share, and 20,000,000 shares of preferred stock, $0.0001 par value per share.
As of June 30, 2020, there were 9,025,061 shares of our
common stock issued and outstanding, 85,581 shares of Series A convertible preferred stock outstanding, and 1,827 shares
of Series C convertible preferred stock outstanding. As of June 30, 2020, we had outstanding warrants to purchase an
aggregate of 2,536,566 shares of our common stock at an average weighted exercise price of $19.35 per share.
Common Stock
Holders of common stock are entitled to receive ratably dividends
out of funds legally available, if and when declared from time to time by our Board. We have never paid any cash dividends on our
common stock and our Board does not anticipate that we will pay cash dividends in the foreseeable future. The future payment of
dividends, if any, on our common stock is within the discretion of the Board and will depend upon earnings, capital requirements,
financial condition and other relevant factors. Holders of common stock are entitled to one vote for each share held on each matter
to be voted on by stockholders. There is no cumulative voting in the election of directors. In the event of liquidation, dissolution
or winding up of the affairs of us, holders of common stock are to share in all assets remaining after the payment of liabilities
and any preferential distributions payable to preferred stockholders, if any. The holders of common stock have no preemptive or
conversion rights and are not subject to further calls or assessments. There are no redemption or sinking fund provisions applicable
to the common stock. The rights of the holders of the common stock are subject to any rights that may be fixed for holders of preferred
stock, if any. All of the outstanding shares of common stock are fully paid and non-assessable. Our common stock is listed
on the Nasdaq Capital Market under the symbol “HEPA.”
Anti-Takeover
Effects of Certain Provisions of Hepion Certificate of Incorporation, Bylaws and the DGCL
Certain provisions
of our certificate of incorporation and bylaws, which are summarized in the following paragraphs, may have the effect of discouraging
potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder
might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management.
In particular, our certificate of incorporation and bylaws and Delaware law, as applicable, among other things:
• provide the Board
of Directors with the ability to alter the bylaws without stockholder approval; and
•
provide that vacancies on the Board of Directors may be filled by a majority of directors in office, although less than a quorum.
These provisions
are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking
to acquire control of Hepion to first negotiate with its board. These provisions may delay or prevent someone from acquiring or
merging with Hepion, which may cause the market price of Hepion common stock to decline.
Blank
Check Preferred. Our Board of Directors is authorized to create and issue from time to time,
without stockholder approval, up to an aggregate of 20,000,000 shares of preferred stock in one or more series and to establish
the number of shares of any series of preferred stock and to fix the designations, powers, preferences and rights of the shares
of each series and any qualifications, limitations or restrictions of the shares of each series.
The
authority to designate preferred stock may be used to issue series of preferred stock, or rights to acquire preferred stock, that
could dilute the interest of, or impair the voting power of, holders of the common stock or could also be used as a method of determining,
delaying or preventing a change of control.
Advance
Notice Bylaws. The Bylaws contain an advance notice procedure for stockholder proposals to
be brought before any meeting of stockholders, including proposed nominations of persons for election to our Board of
Directors. Stockholders at any meeting will only be able to consider proposals or nominations specified in the notice of
meeting or brought before the meeting by or at the direction of our Board of Directors or by a stockholder who was a
stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given Hepion's
corporate secretary timely written notice, in proper form, of the stockholder's intention to bring that business before the
meeting. Although the Bylaws do not give our Board of Directors the power to approve or disapprove stockholder nominations of
candidates or proposals regarding other business to be conducted at a special or annual meeting, the Bylaws may have the
effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage
or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise
attempting to obtain control of us.
UNDERWRITING
We have entered into an underwriting agreement,
dated , 2020, with ThinkEquity, a division of Fordham Financial Management, Inc., acting as the sole book-running manager (sometimes
referred to as the “representative”). Subject to the terms and conditions of the underwriting agreement, we have agreed
to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering
price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common stock listed
next to its name in the following table:
|
Underwriters
|
|
Number of Shares
|
|
ThinkEquity, a division of Fordham Financial Management, Inc.
|
|
|
|
|
Total
|
|
|
|
The underwriting agreement provides that
the obligations of the underwriters to pay for and accept delivery of the shares of common stock offered by this prospectus are
subject to various conditions and representations and warranties, including the approval of certain legal matters by their counsel
and other conditions specified in the underwriting agreement. The shares of common stock are offered by the underwriters, subject
to prior sale, when, as and if issued to and accepted by them. The underwriters reserve the right to withdraw, cancel or modify
the offer to the public and to reject orders in whole or in part. The underwriters are obligated to take and pay for all of the
shares of common stock offered by this prospectus if any such shares of common stock are taken, other than those shares of common
stock covered by the over-allotment option described below.
We have agreed to indemnify the underwriters
and certain of their affiliates and controlling persons (within the meaning of Section 15 of the Securities Act or Section 20 of
the Exchange Act), among others, against specified liabilities, including liabilities under the Securities Act, and to contribute
to payments the underwriters may be required to make in respect thereof.
Discounts, Commissions and Reimbursement
The underwriters propose to offer the shares
of common stock directly to the public at the public offering price set forth on the cover page of this prospectus. After the offering
to the public, the offering price and other selling terms may be changed by the underwriters without changing the proceeds we will
receive from the underwriters.
The following table summarizes the public
offering price, underwriting discounts and commissions and proceeds before expenses to us. The underwriting commissions are 7.0%
of the public offering price. The information assumes either no exercise or full exercise of the over-allotment option we granted
to the representative of the underwriters.
|
|
|
Per Share
|
|
|
Total Without
Over-allotment
Option
|
|
|
Total With
Over-allotment
Option
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
Underwriting discounts and commissions (7.0%)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
Proceeds,
before expenses, to us
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
We have paid an expense deposit of $25,000
to the representative, which will be applied against out-of-pocket accountable expenses that will be paid by us to the underwriters
in connection with this offering, and will be reimbursed to us to the extent not actually incurred in compliance with FINRA Rule
5110(g)(4)(A).
We have also agreed to pay certain of
the representative’s expenses relating to the offering, including (a) all filing fees and communication expenses
relating to the registration of the shares of common stock to be sold in the offering (including the share’s subject to
the underwriters’ over-allotment option) with the Commission; (b) all public filing system filing fees associated with
the review of the offering by FINRA; (c) all fees and expenses relating to the listing of such shares of common stock on The
Nasdaq Capital Market and such other stock exchanges as we and the representative together determine; (d) all fees, expenses
and disbursements relating to background checks of our officers and directors in an amount not to exceed $15,000 in the
aggregate; (e) all fees, expenses and disbursements relating to the registration or qualification of the common stock under
the “blue sky” securities laws of such states and other jurisdictions as the representative may reasonably
designate; (f) all fees, expenses and disbursements relating to the registration, qualification or exemption of the common
stock under the securities laws of such foreign jurisdictions as the representative may reasonably designate; (g) the costs
of all mailing and printing of the underwriting documents (including, without limitation, the underwriting agreement, any
blue sky surveys and, if appropriate, any agreement among underwriters, selected dealers’ agreement,
underwriters’ questionnaire and power of attorney), registration statements, prospectuses and all amendments,
supplements and exhibits thereto and as many preliminary and final prospectuses as the representative may reasonably deem
necessary; (h) the costs and expenses of a public relations firm; (i) the costs of preparing, printing and delivering
certificates representing the common stock; (j) fees and expenses of the transfer agent for the shares of common stock; (k)
stock transfer and/or stamp taxes, if any, payable upon the transfer of securities from us to the underwriters; (l) the costs
associated with post-closing advertising the offering in the national editions of the Wall Street Journal and New York Times;
(m) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and lucite
tombstones, each of which us or our designee shall provide within a reasonable time after the closing date in such quantities
as the representative may reasonably request, in an amount not to exceed $3,000; (n) the fees and expenses of our
accountants; (o) the fees and expenses of our legal counsel and other agents and representatives; (p) fees and expenses of
the representative’s legal counsel not to exceed $100,000; and (q) the $29,500 cost associated with the
underwriters’ use of Ipreo’s book-building, prospectus tracking and compliance software for the offering.
Our total estimated expenses of the offering,
including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts
and commissions, are approximately $ .
Over-allotment Option
We have granted a 45-day option to the
representative of the underwriters to purchase up to additional
shares of common stock (equal to 15% of the common stock sold in this offering) from us solely to cover over-allotments, if any,
at the public offering price less underwriting discounts and commissions.
Representative’s Warrants
Upon closing of this offering, we have
agreed to issue to the representative as compensation warrants to purchase a number of shares of common stock equal to 3% of the
aggregate number of shares of common stock sold in this offering, or the Representative’s Warrants. The Representative’s
Warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per share in this offering.
The Representative’s Warrants are exercisable at any time and from time to time, in whole or in part, during the four and
one half year period commencing 180 days from the effective date of the registration statement of which this prospectus is a part.
The Representative’s Warrants have
been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(e)(1) of FINRA. The representative
(or permitted assignees under Rule 5110(e)(2)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities
underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result
in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from the effective
date of the registration statement. In addition, the warrants provide for registration rights upon request, in certain cases. The
one-time demand registration right provided will not be greater than five years from the effective date of the registration statement
in compliance with FINRA Rule 5110(g)(8)(A). The unlimited piggyback registration right provided will not be greater than seven
years from the effective date of the registration statement in compliance with FINRA Rule 5110(g)(8)(D). We will bear all fees
and expenses attendant to registering the securities issuable on exercise of the warrants other than underwriting commissions incurred
and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain
circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However,
the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the
warrant exercise price.
Right of First Refusal
Until ,
2021 (six (6) months from the closing date), the representative will have an irrevocable right of first refusal to act as sole
investment banker, sole book-runner and/or sole placement agent, at its sole discretion, for each and every of our future public
equity and debt offerings, including all equity linked financings, for us, or any of our successors or subsidiaries, on terms customary
to the representative. The representative in conjunction with us, has the sole right to determine whether or not any other broker-dealer
shall have the right to participate in any such offering and the economic terms of any such participation.
Lock-Up Agreements
Pursuant to “lock-up” agreements,
we, our executive officers and directors, have agreed, without the prior written consent of the representative, not to, directly
or indirectly, offer pledge, sell, contract to sell, grant, lend or otherwise transfer or dispose of any of shares of (or enter
into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person
at any time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another,
in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise
any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of
any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any other securities
of ours or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of (i) six
months after the date of this prospectus in the case of directors and officers and (ii) three months after the date of this prospectus
in the case of the Company.
Determination of Offering Price
The public offering price of the securities
we are offering was negotiated between us and the underwriters based on the trading of our common stock prior to the offering,
among other things. Other factors considered in determining the public offering price of the shares include the history and prospects
of the Company, the stage of development of our business, our business plans for the future and the extent to which they have been
implemented, an assessment of our management, general conditions of the securities markets at the time of the offering and such
other factors as were deemed relevant.
Other Relationships
Certain of the underwriters and their affiliates
have, from time to time, provided and may in the future provide, various investment banking and other financial services for us
for which they may receive customary fees. In particular, ThinkEquity provided advisory services to us from July 2019 through February
2020 for which we paid ThinkEquity $17,000. In the course of their businesses, the underwriters and their affiliates may actively
trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their
affiliates may at any time hold long or short positions in such securities or loans.
In 2020, we engaged B. Riley FBR, Inc. (“B. Riley”)
to act as a placement agent. In connection with such engagement, we granted B. Riley a right for a tail fee equal to 7% of the
gross proceeds raised by us from certain investors in connection with certain financing transactions consummated by us within a
three-month period ending November 17, 2020.
Listing
Our common stock is listed on The Nasdaq
Capital Market under the symbol “HEPA”.
Price Stabilization, Short Positions
and Penalty Bids
In connection with this offering, the underwriters
may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. The underwriters may elect
to stabilize the price of our common stock by bidding for, and purchasing, common stock in the open market.
The underwriters may also impose a penalty
bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing a security in
this offering because the underwriter repurchases that security in stabilizing or short covering transactions.
Finally, the underwriters may bid for,
and purchase, shares of our common stock in market making transactions, including “passive” market making transactions
as described below.
These activities may stabilize or maintain
the market price of our common stock at a price that is higher than the price that might otherwise exist in the absence of these
activities. The underwriters are not required to engage in these activities, and may discontinue any of these activities at any
time without notice. These transactions may be effected on the Nasdaq Capital Market, in the over-the-counter market, or otherwise.
In connection with this offering, the underwriters
or their affiliates may engage in passive market making transactions in our common stock immediately prior to the commencement
of sales in this offering, in accordance with Rule 103 of Regulation M under the Exchange Act. Rule 103 generally provides that:
|
|
●
|
a passive market maker may not effect
transactions or display bids for our common stock in excess of the highest independent bid price by persons who are not
passive market makers;
|
|
|
●
|
net purchases by a passive market
maker on each day are generally limited to 30% of the passive market maker’s average daily trading volume in our common
stock during a specified two-month prior period or 200 shares of common stock, whichever is greater, and must be discontinued
when that limit is reached; and
|
|
|
●
|
passive market making bids must be identified as such.
|
Electronic Distribution
This prospectus in electronic format may
be made available on websites or through other online services maintained by one or more of the underwriters, or by their affiliates.
Other than this prospectus in electronic format, the information on any underwriter’s website and any information contained
in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus
forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter, and should not be
relied upon by investors.
Offer Restrictions Outside The United States
Other than in the United States, no action
has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any
jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly
or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of
any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance
with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised
to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This
prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus
in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document
under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission
and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations
Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the
securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section
708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth
in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree
represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations
Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its
transfer to the offeree under this prospectus.
China
The information in this document does not
constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding,
for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities
may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified
domestic institutional investors.”
European Economic Area — Belgium,
Germany, Luxembourg and Netherlands
The information in this document has been
prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC, or the
Prospectus Directive, as implemented in Member States of the European Economic Area, or a Relevant Member State, from the requirement
to produce a prospectus for offers of securities.
An offer to the public of securities has
not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus
Directive as implemented in that Relevant Member State:
• to legal entities that
are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose
is solely to invest in securities;
• to any legal entity that
has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than
€43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover
of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements);
• to fewer than 100 natural
or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to
obtaining the prior consent of our company or any underwriter for any such offer; or
• in any other circumstances
falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement
for the publication by us of a prospectus pursuant to Article 3 of the Prospectus Directive.
France
This document is not being distributed
in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning
of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of
the General Regulation of the French Autorité des marchés financiers, or AMF. The securities have not been offered
or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material
relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not
be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have
been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account,
as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French
Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle
restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2°
and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General
Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the
public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French
Monetary and Financial Code.
Ireland
The information in this document does not
constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish
regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within
the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005, or the Prospectus Regulations. The securities have
not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering,
except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or
legal persons who are not qualified investors.
Israel
The securities offered by this prospectus
have not been approved or disapproved by the Israeli Securities Authority, or the ISA, nor have such securities been registered
for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication
of a prospectus. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing the prospectus;
nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to
the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered
by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities
laws and regulations.
Italy
The offering of the securities in the
Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le
Societ-$$-Aga e la Borsa), or CONSOB, pursuant to the Italian securities legislation and, accordingly, no offering material
relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public
offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998, or Decree No. 58, other
than:
•
to Italian qualified investors, or Qualified Investors, as defined in Article 100 of Decree no.58 by reference to Article
34-ter of CONSOB Regulation no. 11971 of 14 May 1999, or Regulation no. 1197l, as amended; and
• in other circumstances
that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971
as amended.
Any offer, sale or delivery of
the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified
Investor solicits an offer from the issuer) under the paragraphs above must be:
• made by investment firms,
banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of
1 September 1993 (as amended), Decree No.58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and
• in compliance with all
relevant Italian securities, tax and exchange controls and any other applicable laws.
Any subsequent distribution of the securities
in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the
Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in
the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any
damages suffered by the investors.
Japan
The securities have not been and will not
be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended,
or the FIEL, pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified
Institutional Investors (as defined in and in accordance with Article2, paragraph 3 of the FIEL and the regulations promulgated
thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit
of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities
may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of
securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed
in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within
the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have
not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and
any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market
Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed
or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not
to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal
are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors
may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not
be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document
may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed
not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella
instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in
the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information
contained in it to any other person.
Switzerland
The securities may not be publicly offered
in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility
in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art.
652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the
SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document
nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in
Switzerland.
Neither this document nor any other offering
material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular,
this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory
Authority.
This document is personal to the recipient
only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities
have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental
authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab
Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab
Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating
to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within
the United Arab Emirates by us.
No offer or invitation to subscribe for
securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document
nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United
Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended, or FSMA)
has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis
to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may
not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in
circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be
distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person
in the United Kingdom.
Any invitation or inducement to engage
in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities
has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United
Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is
being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments
falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order
2005, or FPO, (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies,
unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant
persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to
purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this
document or any of its contents.
Canada
The securities may be sold in Canada
only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National
Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as
defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of
the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus
requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide
a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a
misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit
prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any
applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these
rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI
33-105), the underwriters are not required to comply with the disclosure requirements of NI33-105 regarding underwriter
conflicts of interest in connection with this offering.
LEGAL MATTERS
The validity of the shares of our common stock offered hereby
will be passed upon for us by Sheppard, Mullin, Richter & Hampton LLP, New York, New York. Blank Rome LLP, New York,
New York has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
EXPERTS
The consolidated financial statements as of
December 31, 2019 and December 31, 2018 and for the years ended December 31, 2019 and 2018, incorporated by
reference in this prospectus and in the registration statement have been so incorporated in reliance on the report of BDO
USA, LLP, an independent registered public accounting firm, incorporated by reference herein, given on the authority of
said firm as experts in auditing and accounting. The report on the consolidated financial statements contains an explanatory
paragraph regarding the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus, which constitutes a part of the registration
statement on Form S-1 that we have filed with the SEC under the Securities Act, does not contain all of the information
in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this
prospectus, you should refer to the registration statement and the exhibits filed as part of that document. Statements contained
in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each
instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of
these statements is qualified in all respects by this reference.
We are subject to the reporting requirements of the Exchange
Act and file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings,
including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. We also maintain
a website at http://www.hepionpharma.com, at which you may access these materials free of charge as soon as reasonably practicable
after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through,
our website is not part of this prospectus.
INCORPORATION OF CERTAIN DOCUMENTS BY
REFERENCE
The SEC allows us to “incorporate by reference”
information that we file with them. Incorporation by reference allows us to disclose important information to you by referring
you to those other documents. The information incorporated by reference is an important part of this prospectus, and information
that we file later with the SEC will automatically update and supersede this information. We filed a registration statement on
Form S-1 under the Securities Act with the SEC with respect to the securities being offered pursuant to this prospectus.
This prospectus omits certain information contained in the registration statement, as permitted by the SEC. You should refer to
the registration statement, including the exhibits, for further information about us and the securities being offered pursuant
to this prospectus. Statements in this prospectus regarding the provisions of certain documents filed with, or incorporated by
reference in, the registration statement are not necessarily complete and each statement is qualified in all respects by that reference.
Copies of all or any part of the registration statement, including the documents incorporated by reference or the exhibits, may
be obtained upon payment of the prescribed rates at the offices of the SEC listed above in “Where You Can Find More Information”.
We are incorporating by reference the documents listed below, which we have already filed with the SEC.
1. The Company’s Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on May 14, 2020;
2. Amendment Number 1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on June 15, 2020;
3. The
Company Quarterly Reports on Form 10-Q for (i) the quarter ended March 31, 2020, filed with the SEC on June 29, 2020
and (ii) the quarter ended June 30, 2020, filed with the SEC on August 12, 2020;
4. The Company’s Definitive Proxy Statement filed with the SEC on June 12, 2020;
5. The
Company’s Current Reports on Form 8-K filed with the SEC on January
6, 2020, January
9, 2020, January
28, 2020, February
12, 2020 (two
reports), February
20, 2020, March
12, 2020, March
27, 2020, March
30, 2020, April
17, 2020, May
14, 2020, May
19, 2020, May
20, 2020, June
10, 2020, June
22, 2020, June
29, 2020, July
7, 2020, July
30, 2020, August
5, 2020, August
12, 2020, August
27, 2020, September
1, 2020, September
17, 2020, September
29, 2020, October
5, 2020 and October 27, 2020; and
6. The description of the Company’s common stock contained in the registration statement on Form 8-A filed with the SEC on February 24, 2015 pursuant to Section 12 of the Exchange Act of 1934, as amended (the “Exchange Act”), including any amendment or report filed for the purpose of updating that description.
We also incorporate by reference all documents (other than Current
Reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related
to such items) that are subsequently filed by us with the Securities and Exchange Commission pursuant to Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act prior to the termination of the offering of the securities made by this prospectus (including documents
filed after the date of the initial Registration Statement of which this prospectus is a part and prior to the effectiveness of
the Registration Statement). These documents include periodic reports, such as Annual Reports on Form 10-K, Quarterly
Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy statements.
Any statement contained in this prospectus or in a document
incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded to the
extent that a statement contained in this prospectus or any subsequently filed document that is deemed to be incorporated by reference
into this prospectus modifies or supersedes the statement
You may request, and we will provide you with, a copy of these
filings, at no cost, by calling us at (732) 902-4000 or by writing to us at the following address:
Hepion Pharmaceuticals, Inc.
399 Thornall Street, First Floor
Edison, New Jersey, 08837
Attn.: Secretary
Shares
of Common Stock

Hepion Pharmaceuticals, Inc.
|
ThinkEquity
|
|
a division
of Fordham Financial Management, Inc.
|
,
2020
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table provides information
regarding the various actual and anticipated expenses (other than underwriting fees and expenses) payable by us in connection with
the issuance and distribution of the securities being registered hereby. All amounts shown are estimates except the SEC registration
fee.
|
Item
|
|
Amount
|
|
|
SEC registration fee
|
|
$
|
3,764
|
|
|
FINRA filing fee
|
|
|
*
|
|
|
Printing and engraving expenses
|
|
|
*
|
|
|
Legal fees and expenses
|
|
|
*
|
|
|
Accounting fees and expenses
|
|
|
*
|
|
|
Transfer agents’ fees and expenses
|
|
|
*
|
|
|
Miscellaneous costs
|
|
|
*
|
|
|
Total
|
|
|
*
|
|
|
*
|
To be provided by amendment
|
Item 14. Indemnification of Directors and Officers
The DGCL authorizes corporations to limit
or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’
fiduciary duties as directors and our amended and restated certificate of incorporation includes such an exculpation provision.
Our certificate of incorporation and by-laws include provisions that indemnify, to the fullest extent allowable under the DGCL,
the personal liability of directors or officers for monetary damages for actions taken as a director or officer of us, or for serving
at our request as a director or officer or another position at another corporation or enterprise, as the case may be. Our certificate
of incorporation and by-laws also provide that we must indemnify and advance reasonable expenses to our directors and officers,
subject to our receipt of an undertaking from the indemnified party as may be required under the DGCL. Our certificate of incorporation
expressly authorizes us to carry directors’ and officers’ insurance to protect us, our directors, officers and certain
employees for some liabilities. The limitation of liability and indemnification provisions in our amended and restated certificate
of incorporation and by-laws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary
duty. These provisions may also have the effect of reducing the likelihood of derivative litigation against our directors and officers,
even though such an action, if successful, might otherwise benefit us and our stockholders. However, these provisions do not limit
or eliminate our rights, or those of any stockholder, to seek non-monetary relief such as injunction or rescission in the event
of a breach of a director’s duty of care. The provisions do not alter the liability of directors under the federal securities
laws. In addition, your investment may be adversely affected to the extent that, in a class action or direct suit, we pay the costs
of settlement and damage awards against directors and officers pursuant to these indemnification provisions. There is currently
no pending material litigation or proceeding against any of our directors, officers or employees for which indemnification is sought.
Item 15. Recent Sales of Unregistered Securities
The Company has sold the securities described
below within the past three years which were not registered under the Securities Act. All of the sales listed below were made pursuant
to an exemption from registration afforded by Section 4(a)(2) of the Securities Act and Regulation D thereunder.
On March 13, 2019, the Company entered
into a securities purchase agreement (the “Securities Purchase Agreement”) with an accredited investor. Pursuant to
the Securities Purchase Agreement, the Company issued to the investor in a private placement (i) 47,429 shares of Company
common stock and (ii) an unsecured $1.25 million aggregate principal amount debenture.
On May 8, 2018, the Company
entered into a securities purchase agreement with Iliad Research and Trading, L.P. (“IRT”), pursuant to which the
Company issued to IRT a secured convertible promissory note in the aggregate principal amount of $3,325,000 for an aggregate
purchase price of $2,000,000 cash and $1,000,000 aggregate principal amount of investor notes payable to the Company in four
tranches of $250,000 upon request by the Company. Closing occurred on May 9, 2018.
Item 16. Exhibits and Financial Statement Schedules
|
|
(a)
|
Exhibits. See Exhibit Index set forth on page II-4 to this Registration Statement.
|
|
|
(b)
|
Financial Statements. Incorporated by reference into this registration statement.
|
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes
as follows:
(1) To file, during any period in which
offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by
Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts
or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof)
which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement.
Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering
range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes
in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the "Calculation
of Registration Fee" table in the effective registration statement; and
(iii) To include any material information
with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such
information in the registration statement.
provided, however, that
paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13
or Section 15(d) of the Exchange Act that are incorporated by reference in the Registration Statement
(2) That, for the purpose of determining
any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement
relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
(3) To remove from registration by means
of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining
any liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned
registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement,
regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such
purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will
be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus
of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating
to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing
prospectus relating to the offering containing material information about the undersigned registrant or our securities provided
by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an
offer in the offering made by the undersigned registrant to the purchaser.
(b)
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each
filing of the registrant's annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where
applicable, each filing of an employee benefit plan's annual report pursuant to Section 15(d) of the Exchange Act) that
is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the
securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof.
(c) Insofar as indemnification for
liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the
undersigned pursuant to the foregoing provisions, or otherwise, the undersigned has been advised that in the opinion of the
SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the
event that a claim for indemnification against such liabilities (other than the payment by the undersigned of expenses
incurred or paid by a director, officer or controlling person of the undersigned in the successful defense of any action,
suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being
registered, the undersigned will, unless in the opinion of our counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as
expressed in the Act and will be governed by the final adjudication of such issue.
(d) The
undersigned tegistrant hereby undertakes that:
(1) for
purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part
of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant
to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement
as of the time it was declared effective.
(2) for
the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof.
EXHIBIT INDEX
Exhibit
Number
|
|
Exhibit Description
|
|
1.1**
|
|
Form of Underwriting Agreement.
|
|
3.1(a)
|
|
Certificate of Incorporation of the Company (filed as Exhibit 3.1 to the Company’s registration statement on Form 10-12G filed with the Securities and Exchange Commission on August 8, 2013 and incorporated herein by reference).
|
|
3.1(b)
|
|
Certificate of Designation, Preferences and Rights of the Series A Convertible Preferred Stock of the Company filed with the Secretary of State of the State of Delaware on October 14, 2014 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 15, 2014 and incorporated herein by reference).
|
|
3.1(c)
|
|
Certificate of Designation, Preferences and Rights of the Series B Convertible Preferred Stock of the Company filed with the Secretary of State of the State of Delaware on December 18, 2014 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 18, 2014 and incorporated herein by reference).
|
|
3.1(d)
|
|
Certificate of Designation of Preferences, Rights and Limitations of Series C Convertible Preferred Stock filed with the Secretary of State of the State of Delaware on July 2, 2018 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on July 5, 2018 and incorporated herein by reference).
|
|
3.1(e)
|
|
Certificate of Designation of Preferences, Rights and Limitations of Series D Convertible Preferred Stock filed with the Secretary of State of the State of Delaware on April 26, 2019 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 8, 2019 and incorporated herein by reference).
|
|
3.1(f)
|
|
Certificate of Amendment of Certificate of Incorporation of Company dated May 25, 2018 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 29, 2018 and incorporated herein by reference);
|
|
3.1(g)
|
|
Certificate of Designation of Preference, Rights and Limitations of Series E Convertible Preferred Stock, filed with the Secretary of State of the State of Delaware on June 18, 2019 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 20, 2019 and incorporated herein by reference).
|
|
3.1(h)
|
|
Certificate of Amendment to the Certificate of Incorporation, filed with the Secretary of State of the State of Delaware on dated May 28, 2019 (incorporated by reference filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 31, 2019 and incorporated herein by reference)
|
|
3.1(i)
|
|
Certificate of Amendment to the Certificate of Incorporation, dated July 18, 2019 (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on July 23, 2019 and incorporated herein by reference).
|
|
3.2
|
|
By Laws of the Company (filed as Exhibit 3.2 to the Company’s registration statement on Form 10-12G filed with the Securities and Exchange Commission on August 8, 2013 and incorporated herein by reference).
|
|
4.1
|
|
Warrant Agency Agreement, dated as of July 2, 2018, by and between the Company and Philadelphia Stock Transfer, Inc. (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on July 5, 2018 and incorporated herein by reference).
|
|
4.2
|
|
Form of Warrant issued in April 2019 Offering (filed as Exhibit 4.1 to Form S-1 filed with the Securities and Exchange Commission on April 18, 2019 and incorporated herein by reference).
|
|
4.3
|
|
Form of Warrant issued in June 2019 Offering (filed as Exhibit 4.1 to Form S-1 filed with the Securities and Exchange Commission on June 5, 2019 and incorporated herein by reference).
|
|
4.4
|
|
Form of Warrant to be issued to the Investors (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K
filed with the Securities and Exchange Commission on March 30, 2016 and incorporated herein by reference).
|
|
4.5
|
|
Form of Warrant to be issued to the Investors (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K
filed with the Securities and Exchange Commission on April 25, 2017 and incorporated herein by reference).
|
|
4.6**
|
|
Form of Representative’s Warrant
|
Exhibit
Number
|
|
Exhibit Description
|
|
5.1**
|
|
Opinion of Sheppard, Mullin, Richter & Hampton LLP.
|
|
10.1
|
|
Executive Agreement, dated April 1, 2016, between the Company and John Cavan (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 31, 2016 and incorporated herein by reference).
|
|
10.2
|
|
2013 Equity Incentive Plan (filed as Exhibit 10.1 to the Company’s Form S-8 filed with the Securities and Exchange Commission on May 4, 2015 and incorporated herein by reference).
|
|
10.3
|
|
Executive Agreement, dated June 1, 2016, between the Company and Dr. Robert T. Foster (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 13, 2016 and incorporated herein by reference).
|
|
10.4*
|
|
Promissory Note dated April 13, 2020.
|
|
10.5*
|
|
Agreement and Plan of Merger by and among the Company,
Ciclofilin Acquisition Corp., Ciclofilin Pharmaceuticals, Inc. and Robert Foster, Pharm.D., PH.D., as Stockholder
Representative, dated as of May 26, 2016.
|
|
10.6
|
|
Amendment to 2013 Equity Incentive Plan (filed as Appendix A to the Company's Definitive Proxy Statement filed with the Securities and
Exchange Commission on June 12, 2020 and incorporated herein by reference).
|
|
21.1
|
|
List of Subsidiaries. (filed as Exhibit 21.1 to the Company’s Form 10-K filed with the Securities and Exchange Commission on May 14, 2020 and incorporated herein by reference).
|
|
23.1*
|
|
Consent of BDO USA, LLP, Independent Registered Public Accounting Firm.
|
|
23.2**
|
|
Consent of Sheppard, Mullin, Richter & Hampton, LLP (included as part of Exhibit 5.1).
|
|
24*
|
|
Power of Attorney (included on signature page hereto).
|
|
|
**
|
To be filed by amendment.
|
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, as amended, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements
for filing on Form S-1 and has duly caused this registration statement on Form S-1 to be signed on its behalf
by the undersigned, thereunto duly authorized, in Edison, New Jersey, on the 29th day of October 2020.
|
|
HEPION PHARMACEUTICALS, INC.
|
|
|
|
|
|
|
By:
|
/s/ Robert Foster
|
|
|
|
Robert Foster
|
|
|
|
President, Chief Executive Officer and Director
|
POWER OF ATTORNEY
KNOW
ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Robert Foster and John Cavan,
his true and lawful attorneys-in-fact and agent with full power of substitution and re-substitution, for him and in his name, place
and stead, in any and all capacities to sign any or all amendments (including, without limitation, post-effective amendments) to
this Registration Statement, any related Registration Statement filed pursuant to Rule 462(b) under the Securities Act of
1933 and any or all pre- or post-effective amendments thereto, and to file the same, with all exhibits thereto, and all other documents
in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agent, full power
and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully
for all intents and purposes as he might or could do in person, hereby ratifying and confirming that said attorneys-in-fact and
agent, or any substitute or substitutes for him, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities
Act of 1933, the following persons in the capacities and on the dates indicated have signed this Registration Statement below.
|
Signature
|
|
Title
|
Date
|
|
|
|
|
|
|
/s/ Robert Foster
|
|
President, Chief Executive Officer and Director
|
|
|
Robert Foster
|
|
(Principal Executive Officer)
|
October 29, 2020
|
|
|
|
|
|
|
/s/ John Cavan
|
|
Chief Financial Officer (Principal Financial
and
|
|
|
John Cavan
|
|
Accounting Officer)
|
October 29, 2020
|
|
|
|
|
|
|
/s/ Gary S. Jacob
|
|
Chairman of the Board
|
|
|
Gary S. Jacob
|
|
|
October 29, 2020
|
|
|
|
|
|
|
/s/ Timothy Block
|
|
Director
|
|
|
Timothy Block
|
|
|
October 29, 2020
|
|
|
|
|
|
|
/s/ Thomas H. Adams
|
|
Director
|
|
|
Thomas H. Adams
|
|
|
October 29, 2020
|
|
|
|
|
|
|
/s/ John Brancaccio
|
|
Director
|
|
|
John Brancaccio
|
|
|
October 29, 2020
|
|
|
|
|
|
|
/s/ Arnold Lippa
|
|
Director
|
|
|
Arnold Lippa
|
|
|
October 29, 2020
|
|
|
|
|
|
|
/s/ Petrus Wijngaard
|
|
Director
|
|
|
Petrus Wijngaard
|
|
|
October 29, 2020
|
Hepion Pharmaceuticals (NASDAQ:HEPA)
Historical Stock Chart
From Aug 2024 to Sep 2024
Hepion Pharmaceuticals (NASDAQ:HEPA)
Historical Stock Chart
From Sep 2023 to Sep 2024