NASDAQ false 0000872912 0000872912 2024-01-05 2024-01-05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 5, 2024
DELCATH SYSTEMS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-16133 |
|
06-1245881 |
(State or other jurisdiction
of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 1633 Broadway, Suite 22C New York, NY 10019 |
|
10019 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (212) 489-2100
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| |
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol |
|
Name of each exchange on which registered |
| Common Stock, $.01 par value |
|
DCTH |
|
The NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 5, 2024, Delcath Systems, Inc. (the “Company”) made available an updated corporate presentation that may be used in connection with presentations at conferences and investor meetings, which can be found on the Company’s website (the “Corporate Presentation”). The Corporate Presentation is furnished as Exhibit 99.1 and incorporated by reference in this Item 7.01.
The information furnished under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference into any of the Company’s filings with the Securities and Exchange Commission under the Exchange Act or the Securities Act of 1933, as amended, whether made before or after the date hereof, regardless of any general incorporation language in such a filing, except as otherwise expressly stated in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
DELCATH SYSTEMS, INC. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Gerard Michel |
|
|
|
|
Name: |
|
Gerard Michel |
|
|
|
|
Title: |
|
Chief Executive Officer |

Exhibit 99.1 Corporate Presentation NASDAQ: DCTH January 2024

Table of Contents Forward-Looking Statement 3 Investment Summary 4 Unmet
Need: Liver-Dominant Cancers 5 HEPZATO KIT™ 8 Metastatic Uveal Melanoma (mUM) 11 FOCUS Trial 16 HEPZATO KIT: Commercialization 20 Reimbursement & Pricing 25 Next Steps: Future Indications 30 References 39 2

Forward-Looking Statement The Private Securities Litigation Reform Act
of 1995 provides a safe harbor for forward- ability to successfully enter into any necessary purchase and sale agreements with looking statements made by the Company or on its behalf. This presentation contains users of the HEPZATO KIT; the timing
and results of the Company’s clinical trials; our forward-looking statements, which are subject to certain risks and uncertainties that determination whether to continue a clinical trial program or to focus on other can cause actual results to
differ materially from those described. The words alternative indications; the impact of the COVID-19 pandemic or other pandemics on “anticipate,” “believe,” “continue,” “could,”
“estimate,” “expect,” “intend,” “may,” the completion of our clinical trials; the impact of the presentations at major medical “plan,” “potential,” “predict,”
“project,” “should,” “target,” “will,” “would” and similar conferences and future clinical results consistent with the data presented; expressions are intended to identify forward-looking
statements, although not all uncertainties relating to the timing and results of research and development projects; forward-looking statements contain these identifying words. and uncertainties regarding the Company’s ability to obtain
financial and other resources for any research, development, clinical trials and commercialization Factors that may cause such differences include, but are not limited to, uncertainties activities. These factors, and others, are discussed from time
to time in our filings relating to: the Company’s ability to successfully commercialize the HEPZATO KIT; the with the Securities and Exchange Commission. Company's successful management of the HEPZATO KIT supply chain, including You should not
place undue reliance on these forward-looking statements, which securing adequate supply of critical components necessary to manufacture and speak only as of the date they are made. We undertake no obligation to publicly assemble the HEPZATO KIT;
successful FDA inspections of the facilities of Delcath and update or revise these forward-looking statements to reflect events or circumstances third-party suppliers/manufacturers; the Company's successful implementation and after the date they are
made. management of the HEPZATO KIT Risk Evaluation and Mitigation Strategy; the potential of the HEPZATO KIT as a treatment for patients with primary and metastatic disease in the liver; our ability to obtain reimbursement for commercialized
product; the Company’s 3

Delcath Investment Summary HEPZATO KIT Commercial Opportunity High
Penetration • FDA approval for • Ultra orphan pricing • Included in NCCN Guidelines mUM* 8/14/23 • Focused call points • First and only FDA approved • 1Q 2024 US Launch whole-liver directed therapy • US mUM
TAM ~$600M Experienced Significant upside beyond Multiple 2024 Catalysts Management Team mUM • Launch • Expertise in commercializing • Strong efficacy signals in • Revenue build high value, specialty multiple other tumor
types • CHOPIN enrollment completion products • Unique interventional • Initiate trials in other indications • TheraSphere (BSX) veterans oncology asset * metastatic Uveal Melanoma (mUM) 4

HIGH UNMET NEED: Liver-Dominant Cancers 5

Liver-Dominant Cancers: High Incidence with High Unmet Medical Need * US
Incidence of Liver-Dominant Cancers Up to (partial set shown) of patients with liver metastases are not amenable to surgical resection % 50,000 80 1 largely due to extensive tumor burden Delcath Opportunity 40,000 • Limited Overall Survival -
Unresectable Liver Cancer 30,000 • Liver: Common Site of Metastases 20,000 o Often the life-limiting organ • Limited Effective Systemic Treatments 10,000 o Systemic Therapies: low efficacy o Immuno-oncology agents become less effective
in the 0 presence of metastases mUM ICC mBC mNET mPC mCRC HCC *Metastatic Uveal Melanoma (mUM) 1 Reddy S, et al. Isolated hepatic perfusion for patients with liver metastases, Ther Adv Med Oncol. 2014 Jul; 6(4): 180-194. 6

Current Liver-Directed Therapies MAJORITY OF TREATMENT 3 SIRT (Y90)
Trans Arterial Chemo 2 Embolization (TACE) • Beads obstruct blood flow to tumor and elute chemo • Radioactive beads delivered into a portion of the liver • 50-60k treatments and rising per year • 10-15k treatments and rising
per year Limitations Many tumors not Tumors recur and Diffuse disease cannot be Neither approved for the imageable and micro- retreatment options limited treated with a tumor-by- treatment of mUM and metastases are common, due to damage to tumor
modality (TACE) and lacking substantial high neither TACE or Y90 can vasculature (TACE) and bilobar treatment is quality data set to support treat the entire liver hepatotoxicity (Y90) hepatotoxic (Y90) usage 2 Xu L, T, Funchain P, F, Bena J, F, Li
M, Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332. doi: 10.1159/000495113. 3 Lane AM, Kim IK, Gragoudas ES. Survival Rates in Patients After Treatment for
Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018 Sep 1;136(9):981- 986. 7

8

Percutaneous Hepatic Perfusion (PHP) Effective, Safe & Repeatable
Liver-focused Disease Control 1. Isolation Hepatic venous flow is isolated, enabling >6X greater local Isolation concentration of chemo Filtration 2. Saturation Melphalan (chemo) treats micro and macro lesions simultaneously regardless of
location in the liver Saturation 3. Filtration Proprietary filters remove greater than 4 85% of chemo from the body 4 Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J
Immunotherap Cancer. 2019 Nov 13;7(1):299. 9

Indication Statement HEPZATO KIT (melphalan) for Injection/Hepatic
Delivery System HEPZATO KIT is indicated as a liver-directed treatment for adult patients with uveal melanoma with unresectable hepatic metastases affecting less than 50% of the liver and no extrahepatic disease, or extrahepatic disease limited to
the bone, lymph nodes, subcutaneous tissues, or lung that is amenable to resection or radiation. • Indicated Patient Population Includes: o No HLA genotype restrictions o Treatment naïve and previously treated patients 10

Metastatic Uveal Melanoma (mUM) 11

mUM: Beachhead Market Opportunity • ~2,000 newly diagnosed cases
of primary uveal 2 melanoma per year in the US • 50% metastasize with the liver involved in >90% of 5,6 2,3 cases of metastatic disease (1,000 mUM patients) ~50% metastasize • 1-year OS rate of patients with metastatic disease 1,2 to
the liver in the liver is 13% 2,7 o median survival ranging from 4 to 15 months 1 Reddy S, et al. Isolated hepatic perfusion for patients with liver metastases, Ther Adv Med Oncol. 2014 Jul; 6(4): 180-194. 2 Xu L, T, Funchain P, F, Bena J, F, Li M,
Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332. doi: 10.1159/000495113. 3 Lane AM, Kim IK, Gragoudas ES. Survival Rates in Patients After Treatment for
Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018 Sep 1;136(9):981- 986. 5 Krantz BA, et al. Uveal Melanoma: Epidemiology, Etiology, and Treatment of Primary Disease. Clin Ophthalmol. 2017;11:279-289. 6 Eschelman DJ et al. Transhepatic Therapies
for Metastatic Uveal Melanoma. Semin Intervent Radiol. 2013;30(1):39-48. 7 Carvajal RD, et al. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br J Ophthalmol. 2017;101(1):38-44. 12
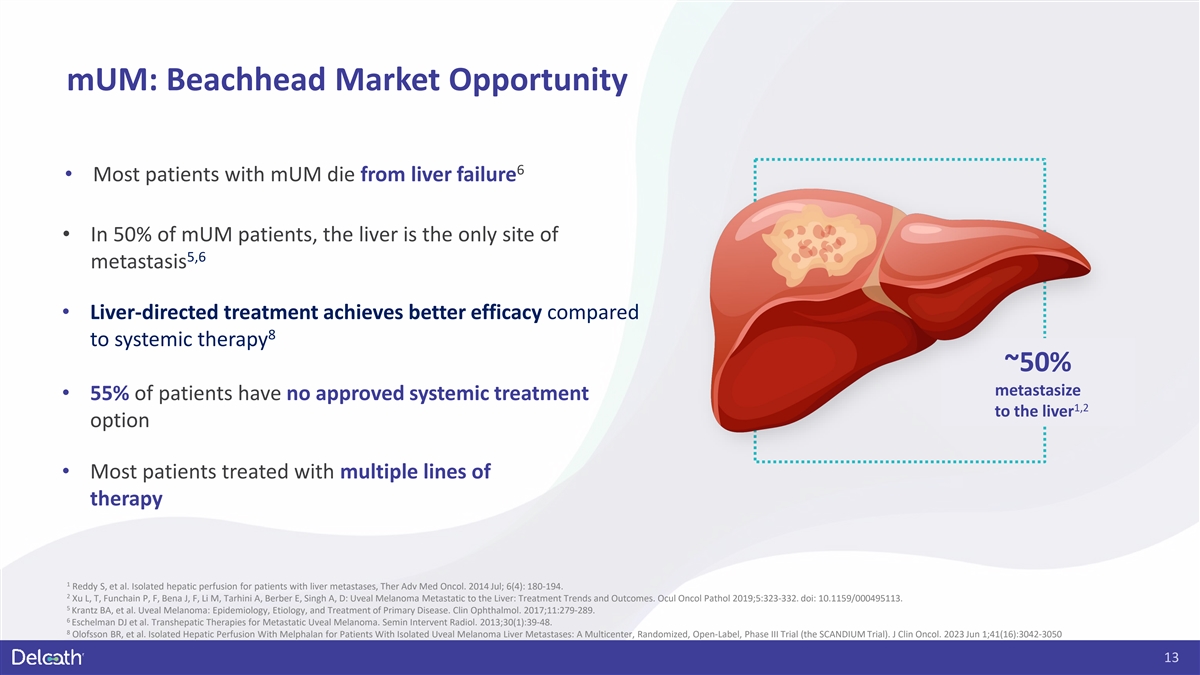
mUM: Beachhead Market Opportunity 6 • Most patients with mUM die
from liver failure • In 50% of mUM patients, the liver is the only site of 5,6 metastasis • Liver-directed treatment achieves better efficacy compared 8 to systemic therapy ~50% metastasize • 55% of patients have no approved
systemic treatment 1,2 to the liver option • Most patients treated with multiple lines of therapy 1 Reddy S, et al. Isolated hepatic perfusion for patients with liver metastases, Ther Adv Med Oncol. 2014 Jul; 6(4): 180-194. 2 Xu L, T, Funchain
P, F, Bena J, F, Li M, Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332. doi: 10.1159/000495113. 5 Krantz BA, et al. Uveal Melanoma: Epidemiology, Etiology, and
Treatment of Primary Disease. Clin Ophthalmol. 2017;11:279-289. 6 Eschelman DJ et al. Transhepatic Therapies for Metastatic Uveal Melanoma. Semin Intervent Radiol. 2013;30(1):39-48. 8 Olofsson BR, et al. Isolated Hepatic Perfusion With Melphalan for
Patients With Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial). J Clin Oncol. 2023 Jun 1;41(16):3042-3050 13

Diffuse/Miliary Metastatic Pattern in mUM Diffuse Disease is Difficult
to Treat with Other Liver Directed Options • Solitary liver lesions are often treated with surgery or ablation. • True nature of the disease may only be seen upon visual confirmation. • Radiographically, metastatic Uveal Melanoma
can initially present only as focal lesions. • Traditional liver-directed therapy mechanism of action is not optimal if a whole liver treatment is needed. • Whole organ therapy delivers medication to a specific organ then filters out the
medication to minimize systemic exposure. Actual patient sent for a liver resection based upon radiographic diagnosis* * Data on File . 14

Patient Journey MILESTONE MILESTONE Surveillance Decision Metastases
Detected Initial Screening • Ophthalmologist/ • Surgical Oncologist Ocular Oncologist Screening frequency Surgical Resection Initial diagnosis & treatment Treatment Decision Surveillance dependent on risk level • Enucleation
• Radiation • Higher risk patients (50%) • Photodynamic therapy are screened at a high • Interventional frequency • Medical Oncologist Most patients receive Radiologist Surveillance at Academic both systemic and •
Lower risk patients (50%) Liver-directed therapy Center o Ophthalmology liver-directed are screened at a lower o Optometrists therapies frequency o Eye Specialist • Medical Oncologist • Medical Oncologist Gene expression profiling
Surveillance at Systemic Therapy may occur Nonacademic Center ~2,000 patients ~1,000 patients ~800 patients eligible • Ophthalmologist • Ocular Oncologist Pre-Metastatic | 3-5 years Post-Metastatic 15

U.S. Registration Trial for the Treatment of Patients with mUM
16

FOCUS TRIAL 9 Summary of Efficacy Results Endpoints HEPZATO KIT (N=91)
ORR, n 33 (36.3%) DOR, Median in months 14.0 DCR, n 67 (73.6%) PFS, Median in months 9.0 OS, Median in months 20.53 • Full analysis with final data cut pending publication • HEPZATO Tx every 6-8 weeks up to a maximum of 6 cycles •
Prescribing Information includes ORR, DOR and response categories • Trial powered to show an ORR advantage over a meta-analysis of Best Alternative Care o Checkpoint inhibitors, chemotherapy, other liver-directed therapy • Lower bound of
FOCUS ORR (26.4%) is significantly higher than the upper bound of the meta-analysis (8.3%) 9 DOI: 10.1200/JCO.2022.40.16_suppl.9510 Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 9510-9510. 17

FOCUS TRIAL * Published mUM Prospective and Retrospective Studies
Median OS Median PFS Clinical Study/Publication Study Type Treatment N 1 year OS (months) (months) AL FOCUS Single-Arm HEPZATO 91 20.53 80% 9.03 systemic and liver- 10 Khoja et al 2019 Meta-Analysis 912 10.2 NA 3.3 directed therapies systemic and
liver- 11 Rantala et al 2019 Meta-Analysis 2,494 12.84 NA NA directed therapies 12 TN Piulats et al 2021 Single-Arm ipi plus nivo 52 12.7 NA 3.0 13 AL Heppt et al 2019 Single-Arm ipi plus (pembro or nivo) 64 16.1 NA 3.0 TN tebentafusp 252 21.7 73%
3.3 14 Nathan et al 2021 Randomized TN control 126 16 59% 2.9 TN = Treatment Naïve, AL = Any Line Ipi = ipilimUMab, nivo = nivolumab, pembro = pemUMab *Studies from 2019 or later with >50 patients 10 Khoja L, et al. Meta-analysis in
metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019 Aug 1, 30(8): 1370-1380. 11 Ranjala, E, et al. Overall survival after
treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019 Dec; 29(6): 561–568 12 Piulats, J, et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label,
Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). Journal of Clinical Oncology 39, no. 6 (February 20, 2021) 586-598. 13 Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a
retrospective, multi-center study. J Immunotherapy Cancer. 2019 Nov 13;7(1):299. 14 Nathan, P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med 2021; 385:1196-1206 18

FOCUS TRIAL Adverse Events Adverse Reactions (N=95) >10% ALL GRADES
(%) GRADES 3 OR 4 (%) Hypotension 13 3 Dyspnea 23 2 Abdominal Pain 39 1 Diarrhea 17 1 Musculoskeletal Pain 46 1 Hemorrhage 15 1 Nausea 57 0 Vomiting 35 0 Fatigue 65 0 Pyrexia 16 0 Groin Pain 11 0 • Most hematological side effects result Cough
15 0 from melphalan Headache 19 0 Lethargy 12 0 • Side effect profile similar to standard melphalan use Dizziness 11 0 Contusion 17 0 Decreased appetite 16 0 Adverse reactions are described further in the HEPZATO KIT PI. 19

FOCUS TRIAL Adverse Reactions Related to Study Treatment Occurring in
≥10% of Patients (N=95) ALL GRADES (%) GRADES 3 OR 4 (%) Adverse Events Thrombocytopenia* 64 55 Leukopenia* 44 34 Anemia* 61 33 Neutropenia* 35 29 International normalized ratio increased 29 8 Activated partial thromboplastin time prolonged 26
8 Aspartate aminotransferase increased 27 3 Hypocalcemia 12 3 Blood bilirubin increased 11 3 Alanine aminotransferase increased 31 2 Blood alkaline phosphatase increased 25 2 Troponin I increased 12 2 Abdominal pain upper 18 1 Dyspnea 11 1 Nausea 47
0 Fatigue 43 0 Vomiting 27 0 Contusion 16 0 • Most hematological side effects result Asthenia 13 0 Back pain 13 0 from melphalan Decreased appetite 13 0 Abdominal pain 12 0 • Side effect profile similar to standard Lethargy 12 0
melphalan use Groin pain 11 0 Headache 11 0 * Anemia includes anemia, febrile bone marrow aplasia, hemoglobin decreased, normochromic normocytic anemia, red blood cell count decreased. Leukopenia includes leukopenia, lymphocyte count decreased,
lymphopenia, and white Adverse reactions are described further in the HEPZATO KIT PI. blood cell count decreased. Neutropenia includes neutropenia and neutrophil count decreased. Thrombocytopenia includes thrombocytopenia and platelet count
decrease. 20

HEPZATO KIT: Commercialization 21

Delivering an Innovative Treatment with a Well-Trained Team Treatment
with HEPZATO KIT involves training and a team approach. The team members below complete a preceptorship and proctorship as well as a risk evaluation and mitigation strategy (REMS) training. Interventional radiologist leads and performs the vascular
interventional procedure Perfusionist establishes, monitors, and controls the extracorporeal pump and veno- venous bypass circuit Anesthesiologist manages sedation, analgesia, and respiratory and cardiovascular support All REMS materials are
available at www.HEPZATOKITREMS.com or by calling the REMS Coordinating Center at 1-833-632-0457. 22

Referring Centers HEPZATO Medical Medical Specialized, Targeted Sales
Treating Center Oncologist Oncologist Teams Trained Treating Team Three Complementary Teams of Representatives: Perfusionist Interventional Radiologist Clinical Specialists Anesthesiologist Liver-Directed Therapy Representatives Medical Medical
Oncologist Medical Ocular Oncologist Oncologist Oncologist Oncology Managers Medical Medical Oncologist Oncologist Medical Oncologist 23

CENTER CITY STATE Treatment Sites FULLY TRAINED Duke University Durham
NC Interest from 20+ sites Moffitt Cancer Center Tampa Bay FL Ohio State University Columbus OH • Focused on a subset of sites to ensure we University of Tennessee Memphis TN achieve our planned activation targets throughout the year PENDING
PROCTORSHIP Mayo Clinic Jacksonville Jacksonville FL Stanford University Stanford CA Thomas Jefferson University Philadelphia PA University of California, Los Angeles Los Angeles CA PENDING PRECEPTORSHIP St. John (Cedars) Los Angeles CA University
of Wisconsin Madison WI STRONG INTEREST/COMMITMENT Cleveland Clinic Cleveland OH Emory University Atlanta GA Honor Health Scottsdale AZ Massachusetts General Hospital Boston MA Memorial Sloan Kettering New York NY Piedmont Healthcare Atlanta GA
University of Miami Miami FL Fully Trained Pending Preceptorship University of Washington Fred Hutchinson Cancer Center Seattle WA Pending Proctorship Strong Interest/Commitment Status as of January 2024 24

Demonstrated Demand for FDA Approved Treatment in mUM KIMMTRAK •
$42.1 million in Q3 2023 US sales ($168M annualized revenue) • Captured an estimated 40% share of eligible patients within 12 months • Approximately 360* patients are eligible for KIMMTRAK KIMMTRAK U.S. Quarterly Sales (USD) • Only
45%* of mUM patients are eligible for treatment due to HLA restriction HEPZATO KIT: FDA Approved August 14, 2023 to Treat Patients with Liver-Dominant mUM • Approximately 800 patients potentially eligible for treatment • HEPZATO has no
HLA genotype restrictions KIMMTRAK • Patients often receive both systemic and liver-directed treatment • Only FDA approved drug for 100% of all mUM patients, with appropriate liver involvement *Due to HLA genotype restrictions
25

HEPZATO KIT: Reimbursement & Pricing 26

Reimbursement Medicare Patients Private Payer Patients • Follow
Medicare guidelines • C-Code and J-Code under review and expected 2024 H1 o For rare disease o Patients to be treated as outpatients • Majority of patients will be outpatient • Medical Prior-Authorization of patients likely
required o Drug directly covered by o Delcath has engaged a hub service to assist with benefit Medicare as pass through verification and navigation • Centers of Excellence (Prospective Payment System (PPS) exempt and NCI designated
Cancer Centers) have the leverage to negotiate favorable rates and reimbursement terms o ~50% of target sites are PPS exempt or NCI Cancer Centers 27
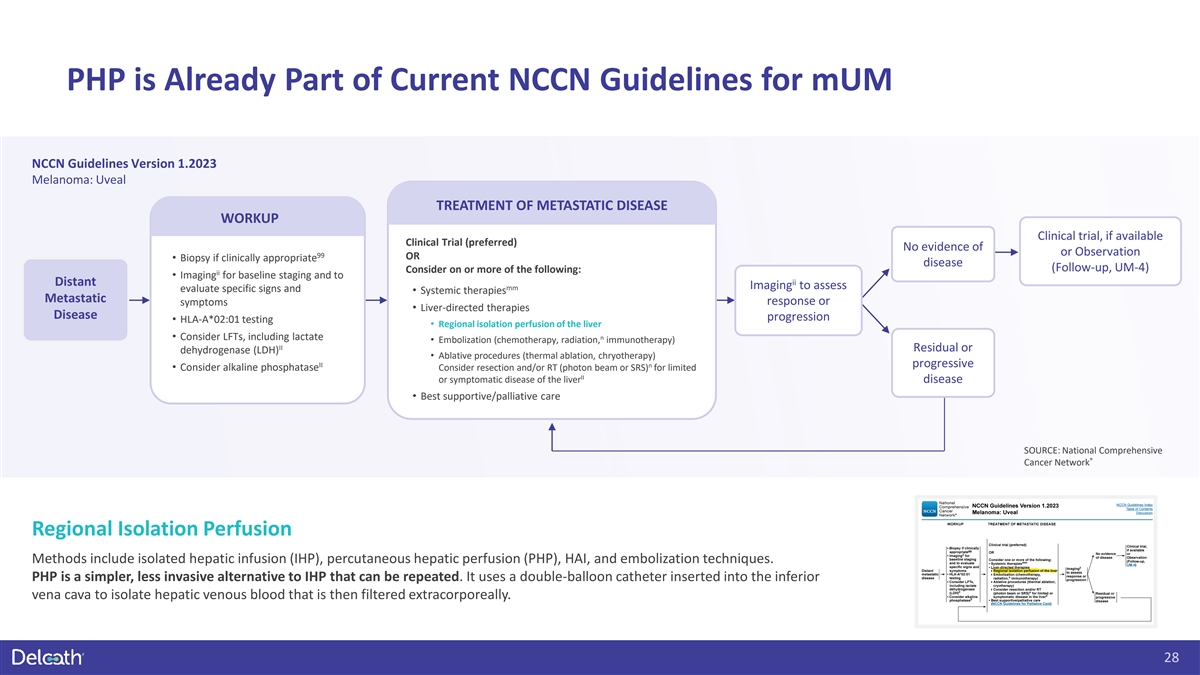
PHP is Already Part of Current NCCN Guidelines for mUM NCCN Guidelines
Version 1.2023 Melanoma: Uveal TREATMENT OF METASTATIC DISEASE WORKUP Clinical trial, if available Clinical Trial (preferred) No evidence of or Observation 99 OR • Biopsy if clinically appropriate disease (Follow-up, UM-4) Consider on or more
of the following: ii • Imaging for baseline staging and to ii Distant Imaging to assess mm evaluate specific signs and • Systemic therapies Metastatic response or symptoms • Liver-directed therapies Disease progression •
HLA-A*02:01 testing • Regional isolation perfusion of the liver • Consider LFTs, including lactate n • Embolization (chemotherapy, radiation, immunotherapy) II Residual or dehydrogenase (LDH) • Ablative procedures (thermal
ablation, chryotherapy) II n progressive • Consider alkaline phosphatase Consider resection and/or RT (photon beam or SRS) for limited II or symptomatic disease of the liver disease • Best supportive/palliative care SOURCE: National
Comprehensive ® Cancer Network Regional Isolation Perfusion Methods include isolated hepatic infusion (IHP), percutaneous hepatic perfusion (PHP), HAI, and embolization techniques. PHP is a simpler, less invasive alternative to IHP that can be
repeated. It uses a double-balloon catheter inserted into the inferior vena cava to isolate hepatic venous blood that is then filtered extracorporeally. 28

Components of Hospital Reimbursement Assuming Outpatient Pass Through
Status with C-Code DRUG HEALTHCARE FACILITY FEE “PHYSICIAN” PAYMENT • MDs primarily on salary but physician payments • ASP+6% (CMS) • The existing CPT codes should capture all and associated RVUs are still relevant
steps of the procedure • Likely similar for commercial • The existing CPT codes should capture all steps • Believe the existing codes will provide payers of the procedure payment competitive with other • Believe the existing
codes will provide payment interventional procedures competitive with other interventional procedures CPT Code mapping complete No meaningful impact on treatment decisions 29

HEPZATO KIT Pricing Consistent with Only Other Approved mUM Therapy At
First Assessment (first time to discontinue treatment because of progression) DRUG DOSE COST* TREATMENTS #** TOTAL COST KIMMTRAK $19,289 24 $462,936 HEPZATO $182,500 2 $365,000 Mean HEPZATO treatment vs. mean treatment duration of KIMMTRAK (per
pivotal trials) DRUG DOSE COST* MEAN TREATMENTS #*** TOTAL COST KIMMTRAK $19,289 41 $790,849 HEPZATO $182,500 4.1 $748,250 *Dose Cost ASP calculated using 7/2023 CMS payment allowance limit ** Minimum treatments prior to determining progression
based on trial protocols *** Mean from published phase 3 trials 30

NEXT STEPS: Future Indications 31

Clinical Rationale for Broad Development Effort Melphalan has
demonstrated clinical activity in multiple tumor types Promising ORR, DCR and PFS signals seen across multiple tumor types with CHEMOSAT in Europe and in earlier studies with IHP In many solid tumor patients, liver metastases are often life limiting
HEPZATO is the only liver-directed treatment that can repeatedly treat the whole liver Potential for significant improvement in survival Converting unresectable liver metastases into resectable metastases and adjuvant usage to prevent recurrence
Potential for sequential usage with Immune-Oncology (I/O) agents Liver metastases reduce I/O therapy efficacy due to the tumor microenvironment inducing immune tolerance, HEPZATO may reduce this effect 32

Strong Correlation of IHP and PHP Efficacy in mUM Patients IHP activity
in CRC and NET 15 IHP / Melphalan in mCRC Meta-analysis of 8 mUM clinical studies N=154 ORR 50% 16 Endpoint IHP (%) PHP (%) Van Iersel mPFS 7.4 months mOS 24.8 months mOS 17.1 17.3 N=120 ORR 61% 17 Alexander mOS 17.4 months mPFS 7.2 9.6 2-year
survival 34% hPFS 10 9.5 IHP in mNET Complications 39.1 23.8 ORR 50% DOR 15 months 18 Grover mhPFS 7 months Mortality 5.5 1.8 mOS 48 months IHP, or Intrahepatic Perfusion, is an invasive surgical technique for delivering high doses of chemotherapy
to the liver; procedure related mortality and morbidity prevented common usage. PHP is a minimally invasive, safer procedure which accomplishes the same goals as IHP and can be performed up to 6 times. 15 Bethlehem MS et al. Meta-Analysis of
Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers (Basel). 2021 Sep 21;13(18):4726. 16 Van Iersel LB, Gelderblom H, Vahrmeijer AL, et al. Isolated hepatic melphalan perfusion of
colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol. 2008;19:1127–34Grover A et al. Isolated Hepatic Perfusion with 200 mg Melphalan for Advanced Noncolorectal Liver Metastases. Surgery. (2005). 136. 1176-82.
17 Alexander HR Jr, Bartlett DL, Libutti SK, et al. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol. 2009;16:1852–9 18
Grover AC, Libutti SK, Pingpank JF, Helsabeck C, Beresnev T, Alexander HR. Isolated hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery.
2004;136(6):1176- 1182. doi:https://doi.org/10.1016/j.surg.2004.06.044 33

Rationale for Combining HEPZATO with IO Therapy Liver Metastases
Suppress IO Therapy Efficacy 34

Encouraging Signal of Efficacy for PHP and I/O Drug Combination From
Phase 1b Part of the Chopin Trial % Change of Target Lesions from Baseline by Response Category 19 • N=7 in Phase 1b portion of the trial • RP2D: IPI 1mg/kg and NIVO 3mg/kg. Well tolerated, no DLTs or deaths. • 1CR, 6 PR and 1 PD
(85.7% ORR, 100% DCR) – meta-analysis of prior IO trials has shown ORR<<10% • As of 11/15/22 the median follow-up was 29.1 months, the median PFS was 29.1 months, and the median duration of response was 27.1 months. All patients
are still alive. • 3 of 4 patients who subsequently experienced PD continued with treatment in the form of repeated Melphalan Chemosat treatments • Ongoing randomized Phase 2 (control is Chemosat) has recruited 50% of N=76 patients and
will provide an interim analysis at N=40 patients Ipilimumab + melphalan liver Ipilimumab + Ipilimumab + melphalan liver Ipilimumab + Inclusion nivolumab* chemo-saturation nivolumab* nivolumab* chemo-saturation nivolumab* *c1: 1+1mg/kg, c2: 1+3mg/kg
19 Tong TML et al. Combining Melphalan Percutaneous Hepatic Perfusion with Ipilimumab Plus Nivolumab in Advanced Uveal Melanoma: First Safety and Efficacy Data from the Phase Ib Part of the Chopin Trial. Cardiovasc Intervent Radiol. 2023
Mar;46(3):350- 359. 35

Market Expansion U.S. TAM Significant Upside >$1B per year 50,000 *
U.S. Incidence of Liver-Dominant Cancers 40,000 30,000 20,000 10,000 0 mUM ICC mBC mNET mPC mCRC HCC *Metastatic Uveal Melanoma (mUM) 36

HEPZATO KIT: Financials and Team 37

Capital Structure and Share Information DCTH (NASDAQ) Share Listing -
Current a. As of September 30, 2023; includes 19.7M of Common plus; 1.1M Preferred E & E-1, 1.5M of Preferred F-2; 4.8M of Preferred F-3; Conversion of convertible notes .5M; & a 28.6M Shares Outstanding 1.0M Pre-funded Warrants as
converted. Does not include Tranche B Outstanding Warrants. b $40.5M b. As of September 30, 2023; (10-Q filing on November 13, Cash and Cash Equivalents 2023) which includes $35M received from Tranche A warrants exercised 21 days after receipt of
FDA approval c for HEPZATO; 7.8M Warrants Outstanding c. As of September 30, 2023; 3.6M warrants at a $10 exercise price and 4.17M Tranche B warrants for an 4.1M Stock Options Granted aggregate exercise price $25 million exercisable until the
earlier of 3/31/2026 or 21 days following recording at least $10 million in quarterly U.S. revenue. d $9.7M 2023 Q3 Cash Burn d. Q3 Net cash used in operating activities e. Includes $5.0M of notes convertible at $11.98 per common e share equivalent,
$9.8M Debt f. Used NASDAQ closing price information starting on December 20, 2022 – December 20, 2023 f $2.25 - $7.99 52 week Low – High g. 30-day average calculated between on December 4, 2023 – January 4, 2024 g 351,474 30d
Average Daily Volume 38

Multi-Disciplinary, Experienced Leadership Team Gerard Michel John
Purpura BOARD OF DIRECTORS CHIEF EXECUTIVE OFFICER CHIEF OPERATING OFFICER John R. Sylvester Chairman • Past VP and Exec Director roles of Reg. • 30+ yrs. pharma/medtech experience Affairs for Bracco Diagnostics Dr. Roger G. Stoll, Ph.D.
Director • C-suite roles at Vericel Corp, Biodel, • Senior roles Sanofi-Aventis, Bolar & NPS Elizabeth Czerepak Director Pharma, Luitpold Pharma & Eon Labs • M.S. Microbiology, B.S. Biology & • M.S. Mgmt.. &
Policy and B.S. Chemistry Geology from the Univ. of Rochester Steven Salamon Director and Biology at the State University of NY School of Medicine at Stony Brook Dr. Gil Aharon, Ph.D. Director • M.B.A. Simon School of Business & Leadership
Gerard Michel CEO Kevin Muir Sandra Pennell Vojislav Vukovic, MD PhD GENERAL MANAGER, INTERVENTIONAL ONCOLOGY SVP, FINANCE CHIEF MEDICAL OFFICER • 20+ yrs. medtech/bioTx sales & • 20+ years' biotech financial oversight •
Oncology dev. exec, global clinical marketing experience experience expertise • Senior leadership roles at BTG, • Manages global financial affairs, U.S. • Former CMO at Aileron, Taiho, Synta ClearFlow, Aragon Surgical, Kensey GAAP
compliance • MD, Univ. of Sarajevo | MSc, PhD, Univ. Nash Corporation, and Kyphon • Led finance at Invivyd of Toronto • Field Artillery officer, U.S. Army • VP at Vericel Corp • Published, AACR, ASCO, ASH, ESMO •
B.S. in Management Systems member • MSc, Accountancy, Univ. of Illinois Engineering, U.S. Military Academy at West Point 39

Thank You 40

References 1. Reddy S, et al. Isolated hepatic perfusion for patients
with liver metastases, Ther Adv Med Oncol. 2014 Jul; 6(4): 180-194. 2. Xu L, T, Funchain P, F, Bena J, F, Li M, Tarhini A, Berber E, Singh A, D: Uveal Melanoma Metastatic to the Liver: Treatment Trends and Outcomes. Ocul Oncol Pathol 2019;5:323-332.
doi: 10.1159/000495113. 3. Lane AM, Kim IK, Gragoudas ES. Survival Rates in Patients After Treatment for Metastasis From Uveal Melanoma. JAMA Ophthalmol. 2018 Sep 1;136(9):981- 986. 4. Heppt, M, et al. Combined immune checkpoint blockade for
metastatic uveal melanoma: a retrospective, multi-center study. J Immunotherap Cancer. 2019 Nov 13;7(1):299. 5. Krantz BA, et al. Uveal Melanoma: Epidemiology, Etiology, and Treatment of Primary Disease. Clin Ophthalmol. 2017;11:279-289 6. Eschelman
DJ et al. Transhepatic Therapies for Metastatic Uveal Melanoma. Semin Intervent Radiol. 2013;30(1):39-48. 7. Carvajal RD, et al. Metastatic Disease from Uveal Melanoma: Treatment Options and Future Prospects. Br J Ophthalmol. 2017;101(1):38-44. 8.
Olofsson BR, et al. Isolated Hepatic Perfusion With Melphalan for Patients With Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial). J Clin Oncol. 2023 Jun 1;41(16):3042-3050. 9. DOI:
10.1200/JCO.2022.40.16_suppl.9510 Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 9510-9510. 10. Khoja L, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international
rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019 Aug 1, 30(8): 1370- 1380. 11. Ranjala, E, et al. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019 Dec;
29(6): 561–568 12. Piulats, J, et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). Journal of Clinical
Oncology 39, no. 6 (February 20, 2021) 586-598. 13. Heppt, M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunotherapy Cancer. 2019 Nov 13;7(1):299. 14. Nathan, P, et al. Overall
Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med 2021; 385:1196-1206 15. Bethlehem MS et al. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver
Metastases. Cancers (Basel). 2021 Sep 21;13(18):4726. 16. Van Iersel LB, Gelderblom H, Vahrmeijer AL, et al. Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol.
2008;19:1127–34Grover A et al. Isolated Hepatic Perfusion with 200 mg Melphalan for Advanced Noncolorectal Liver Metastases. Surgery. (2005). 136. 1176-82. 17. Alexander HR Jr, Bartlett DL, Libutti SK, et al. Analysis of factors associated
with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol. 2009;16:1852–9. 18. Grover AC, Libutti SK, Pingpank JF, Helsabeck C, Beresnev T, Alexander HR. Isolated
hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery. 2004;136(6):1176-1182. doi:https://doi.org/10.1016/j.surg.2004.06.044 19. Tong TML et al.
Combining Melphalan Percutaneous Hepatic Perfusion with Ipilimumab Plus Nivolumab in Advanced Uveal Melanoma: First Safety and Efficacy Data from the Phase Ib Part of the Chopin Trial. Cardiovasc Intervent Radiol. 2023 Mar;46(3):350-359.
41
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Delcath Systems (NASDAQ:DCTH)
Historical Stock Chart
From Apr 2024 to May 2024
Delcath Systems (NASDAQ:DCTH)
Historical Stock Chart
From May 2023 to May 2024