UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
|
|
|
|
|
þ
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the quarterly period ended September 30, 2010
OR
|
|
|
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
Commission File Number 000-51512
Cardiac Science Corporation
(Exact name of registrant as specified in its charter)
|
|
|
|
Delaware
(State or Other Jurisdiction of
Incorporation or Organization)
|
|
94-3300396
(I.R.S. Employer
Identification No.)
|
|
|
|
|
3303 Monte Villa Parkway, Bothell, WA
(Address of Principal Executive Offices)
|
|
98021
(Zip Code)
|
(425) 402-2000
(Registrant’s Telephone Number, Including Area Code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed
by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days.
þ
Yes
o
No
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant
to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for
such shorter period that the registrant was required to submit and post such files).
o
Yes
o
No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer or a smaller reporting company. See definition of “large
accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act.
|
Large accelerated filer
o
|
Accelerated filer
þ
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
o
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of
the Exchange Act). Yes
o
No
þ
The number of shares of the registrant’s Common Stock outstanding at November 2, 2010 was
23,867,815.
TABLE OF CONTENTS
Page 2 of 41
PART I — FINANCIAL INFORMATION
Item 1. Financial Statements
CARDIAC SCIENCE CORPORATION AND SUBSIDIARIES
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
|
|
|
December 31,
|
|
|
(in thousands, except share amounts)
|
|
2010
|
|
|
2009
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
6,690
|
|
|
$
|
26,866
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $807 and
$1,017, respectively
|
|
|
20,781
|
|
|
|
24,228
|
|
|
Inventories
|
|
|
23,902
|
|
|
|
23,581
|
|
|
Prepaid expenses and other current assets
|
|
|
2,775
|
|
|
|
3,702
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
54,148
|
|
|
|
78,377
|
|
|
Other assets
|
|
|
687
|
|
|
|
872
|
|
|
Machinery and equipment, net of accumulated depreciation and amortization
of $19,432 and $17,490, respectively
|
|
|
8,019
|
|
|
|
8,406
|
|
|
Deferred income taxes
|
|
|
31
|
|
|
|
31
|
|
|
Intangible assets, net of accumulated amortization of $20,671 and $17,876,
respectively
|
|
|
25,183
|
|
|
|
27,988
|
|
|
Investments in unconsolidated entities
|
|
|
308
|
|
|
|
386
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
88,376
|
|
|
$
|
116,060
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND EQUITY
|
|
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
10,568
|
|
|
$
|
11,030
|
|
|
Accrued liabilities
|
|
|
13,323
|
|
|
|
12,216
|
|
|
Warranty liability
|
|
|
3,933
|
|
|
|
4,028
|
|
|
Deferred revenue
|
|
|
7,744
|
|
|
|
7,919
|
|
|
Corrective action liabilities
|
|
|
18,757
|
|
|
|
15,249
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
54,325
|
|
|
|
50,442
|
|
|
Deferred income taxes
|
|
|
5,389
|
|
|
|
5,389
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
59,714
|
|
|
|
55,831
|
|
|
Equity:
|
|
|
|
|
|
|
|
|
|
Cardiac Science Corporation shareholders’ equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock (10,000,000 shares authorized), $0.001 par value, no shares
issued or outstanding as of September 30, 2010 and December 31, 2009,
respectively
|
|
|
—
|
|
|
|
—
|
|
|
Common stock (65,000,000 shares authorized), $0.001 par value, 23,867,815
and 23,566,320 shares issued and outstanding at
September 30, 2010 and December 31, 2009, respectively
|
|
|
233,094
|
|
|
|
231,159
|
|
|
Accumulated other comprehensive income
|
|
|
117
|
|
|
|
304
|
|
|
Accumulated deficit
|
|
|
(206,000
|
)
|
|
|
(172,527
|
)
|
|
|
|
|
|
|
|
|
|
Total Cardiac Science Corporation shareholders’ equity
|
|
|
27,211
|
|
|
|
58,936
|
|
|
Noncontrolling interests
|
|
|
1,451
|
|
|
|
1,293
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
28,662
|
|
|
|
60,229
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
88,376
|
|
|
$
|
116,060
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Page 3 of 41
CARDIAC SCIENCE CORPORATION AND SUBSIDIARIES
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
Nine Months Ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
(In thousands, except share and per share data)
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Products
|
|
$
|
29,985
|
|
|
$
|
34,646
|
|
|
$
|
90,424
|
|
|
$
|
101,564
|
|
|
Service
|
|
|
4,495
|
|
|
|
4,238
|
|
|
|
13,266
|
|
|
|
13,098
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
34,480
|
|
|
|
38,884
|
|
|
|
103,690
|
|
|
|
114,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Products
|
|
|
15,244
|
|
|
|
17,194
|
|
|
|
46,319
|
|
|
|
49,261
|
|
|
Corrective action costs
|
|
|
—
|
|
|
|
18,500
|
|
|
|
11,000
|
|
|
|
18,500
|
|
|
Service
|
|
|
3,149
|
|
|
|
3,095
|
|
|
|
9,499
|
|
|
|
9,376
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of revenues
|
|
|
18,393
|
|
|
|
38,789
|
|
|
|
66,818
|
|
|
|
77,137
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
16,087
|
|
|
|
95
|
|
|
|
36,872
|
|
|
|
37,525
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
4,266
|
|
|
|
4,270
|
|
|
|
13,100
|
|
|
|
11,358
|
|
|
Sales and marketing
|
|
|
11,215
|
|
|
|
11,923
|
|
|
|
36,429
|
|
|
|
34,392
|
|
|
General and administrative
|
|
|
7,058
|
|
|
|
6,571
|
|
|
|
20,652
|
|
|
|
18,536
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
22,539
|
|
|
|
22,764
|
|
|
|
70,181
|
|
|
|
64,286
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(6,452
|
)
|
|
|
(22,669
|
)
|
|
|
(33,309
|
)
|
|
|
(26,761
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
7
|
|
|
|
23
|
|
|
|
25
|
|
|
|
55
|
|
|
Other income
|
|
|
229
|
|
|
|
158
|
|
|
|
323
|
|
|
|
555
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income
|
|
|
236
|
|
|
|
181
|
|
|
|
348
|
|
|
|
610
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before income tax expense
|
|
|
(6,216
|
)
|
|
|
(22,488
|
)
|
|
|
(32,961
|
)
|
|
|
(26,151
|
)
|
|
Income tax expense
|
|
|
(117
|
)
|
|
|
(43,923
|
)
|
|
|
(308
|
)
|
|
|
(42,563
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(6,333
|
)
|
|
|
(66,411
|
)
|
|
|
(33,269
|
)
|
|
|
(68,714
|
)
|
|
Less: Net income attributable to noncontrolling
interests
|
|
|
(43
|
)
|
|
|
(135
|
)
|
|
|
(204
|
)
|
|
|
(476
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to Cardiac Science Corporation
|
|
$
|
(6,376
|
)
|
|
$
|
(66,546
|
)
|
|
$
|
(33,473
|
)
|
|
$
|
(69,190
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share attributable to Cardiac
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Science Corporation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
(0.27
|
)
|
|
$
|
(2.85
|
)
|
|
$
|
(1.41
|
)
|
|
$
|
(2.98
|
)
|
|
Diluted
|
|
$
|
(0.27
|
)
|
|
$
|
(2.85
|
)
|
|
$
|
(1.41
|
)
|
|
$
|
(2.98
|
)
|
|
Weighted average shares outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
23,831,807
|
|
|
|
23,368,778
|
|
|
|
23,717,287
|
|
|
|
23,209,181
|
|
|
Diluted
|
|
|
23,831,807
|
|
|
|
23,368,778
|
|
|
|
23,717,287
|
|
|
|
23,209,181
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial
statements.
Page 4 of 41
CARDIAC SCIENCE CORPORATION AND SUBSIDIARIES
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
Nine Months Ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
(In thousands)
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(6,333
|
)
|
|
$
|
(66,411
|
)
|
|
$
|
(33,269
|
)
|
|
$
|
(68,714
|
)
|
|
Adjustments to reconcile net loss to net cash used in
operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation
|
|
|
577
|
|
|
|
674
|
|
|
|
1,797
|
|
|
|
1,887
|
|
|
Depreciation and amortization
|
|
|
1,516
|
|
|
|
1,595
|
|
|
|
4,724
|
|
|
|
4,662
|
|
|
Amortization of debt issuance costs
|
|
|
45
|
|
|
|
—
|
|
|
|
45
|
|
|
|
—
|
|
|
Deferred income taxes
|
|
|
—
|
|
|
|
43,996
|
|
|
|
—
|
|
|
|
42,231
|
|
|
Changes in operating assets and liabilities, net of
businesses acquired:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable, net
|
|
|
3,237
|
|
|
|
(5,552
|
)
|
|
|
3,219
|
|
|
|
3,165
|
|
|
Inventories
|
|
|
634
|
|
|
|
9
|
|
|
|
(363
|
)
|
|
|
(1,321
|
)
|
|
Prepaid expenses and other assets
|
|
|
553
|
|
|
|
509
|
|
|
|
1,213
|
|
|
|
545
|
|
|
Accounts payable
|
|
|
(1,976
|
)
|
|
|
1,672
|
|
|
|
(250
|
)
|
|
|
(785
|
)
|
|
Accrued liabilities
|
|
|
593
|
|
|
|
(672
|
)
|
|
|
1,172
|
|
|
|
(963
|
)
|
|
Warranty liability
|
|
|
(48
|
)
|
|
|
56
|
|
|
|
(63
|
)
|
|
|
65
|
|
|
Corrective action liabilities
|
|
|
(2,695
|
)
|
|
|
18,500
|
|
|
|
3,508
|
|
|
|
18,500
|
|
|
Deferred revenue
|
|
|
(55
|
)
|
|
|
539
|
|
|
|
(175
|
)
|
|
|
(131
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(3,952
|
)
|
|
|
(5,085
|
)
|
|
|
(18,442
|
)
|
|
|
(859
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of machinery and equipment
|
|
|
(289
|
)
|
|
|
(1,034
|
)
|
|
|
(1,634
|
)
|
|
|
(2,688
|
)
|
|
Purchase of intangible assets
|
|
|
—
|
|
|
|
(370
|
)
|
|
|
—
|
|
|
|
(370
|
)
|
|
Proceeds from repayment of note
|
|
|
80
|
|
|
|
—
|
|
|
|
82
|
|
|
|
110
|
|
|
Cash paid for acquisitions
|
|
|
—
|
|
|
|
27
|
|
|
|
—
|
|
|
|
(54
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(209
|
)
|
|
|
(1,377
|
)
|
|
|
(1,552
|
)
|
|
|
(3,002
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options and issuance of
shares under employee stock purchase plan
|
|
|
73
|
|
|
|
161
|
|
|
|
225
|
|
|
|
897
|
|
|
Minimum tax withholding on restricted stock awards
|
|
|
(12
|
)
|
|
|
(13
|
)
|
|
|
(106
|
)
|
|
|
(110
|
)
|
|
Debt issuance costs
|
|
|
(181
|
)
|
|
|
—
|
|
|
|
(181
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
|
(120
|
)
|
|
|
148
|
|
|
|
(62
|
)
|
|
|
787
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
86
|
|
|
|
74
|
|
|
|
(120
|
)
|
|
|
47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash and cash equivalents
|
|
|
(4,195
|
)
|
|
|
(6,240
|
)
|
|
|
(20,176
|
)
|
|
|
(3,027
|
)
|
|
Cash and cash equivalents, beginning of period
|
|
|
10,885
|
|
|
|
37,868
|
|
|
|
26,866
|
|
|
|
34,655
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, end of period
|
|
$
|
6,690
|
|
|
$
|
31,628
|
|
|
$
|
6,690
|
|
|
$
|
31,628
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of noncash investing and financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase (decrease) in accrued machinery and equipment purchases
|
|
$
|
(8
|
)
|
|
$
|
126
|
|
|
$
|
(93
|
)
|
|
$
|
(117
|
)
|
|
Conversion of accounts receivable to notes receivable
|
|
$
|
—
|
|
|
$
|
228
|
|
|
$
|
—
|
|
|
$
|
228
|
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial
statements.
Page 5 of 41
CARDIAC SCIENCE CORPORATION AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
1. Summary of Significant Accounting Policies
Organization and Description of Business
Cardiac Science develops, manufactures, and markets a family of advanced diagnostic and
therapeutic cardiology devices and systems, including automated external defibrillators (AED),
electrocardiograph devices (ECG/EKG), cardiac stress treadmills and systems, diagnostic
workstations, Holter monitoring systems, hospital defibrillators, vital signs monitors, cardiac
rehabilitation telemetry systems and cardiology data management systems (Informatics) that connect
with hospital information (HIS), electronic medical record (EMR), and other information systems.
The Company sells a variety of related products and consumables and provides a portfolio of
training, maintenance, and support services. Cardiac Science, the successor to the cardiac
businesses that established the trusted Burdick
®
, HeartCentrix
®
,
Powerheart
®
and Quinton
®
brands, is headquartered in Bothell, Washington.
With customers in more than 100 countries worldwide, the Company has operations in North America,
Europe, and Asia.
The Company is the result of the combination of Quinton Cardiology Systems, Inc. (“Quinton”)
and Cardiac Science, Inc. (“CSI”) pursuant to a merger transaction (the “Merger”) that was
completed on September 1, 2005.
Basis of Presentation
The Company follows accounting standards set by the Financial Accounting Standards Board,
commonly referred to as the “FASB”. The FASB sets U.S. generally accepted accounting principles
(“U.S. GAAP”) that the Company follows to ensure it consistently reports its financial position,
results of operations, and cash flows. References to U.S. GAAP issued by the FASB in the Company’s
notes to its unaudited condensed consolidated financial statements are to the FASB Accounting
Standards Codification, sometimes referred to as the “Codification” or “ASC”.
The condensed consolidated balance sheet dated September 30, 2010, the condensed consolidated
statements of operations for the three and nine month periods ended September 30, 2010 and 2009,
and the condensed consolidated statements of cash flows for the three and nine month periods ended
September 30, 2010 and 2009 have been prepared by the Company and are unaudited. The condensed
consolidated balance sheet dated December 31, 2009 was derived from the Company’s audited financial
statements. Certain information and note disclosures normally included in annual financial
statements prepared in accordance with U.S. GAAP have been omitted pursuant to the rules and
regulations of the Securities and Exchange Commission (the “SEC”). The notes to the audited
consolidated financial statements included in the Company’s annual report on Form 10-K for the
fiscal year ended December 31, 2009 provide a summary of significant accounting policies and
additional financial information that should be read in conjunction with this report.
The accompanying unaudited condensed consolidated financial statements present
the Company on a consolidated basis, including the Company’s wholly owned subsidiaries and its
majority owned joint ventures. All intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of the unaudited condensed consolidated financial statements and related
disclosures in conformity with U.S. GAAP requires management to make estimates and assumptions that
affect the reported amounts of assets and liabilities, the disclosure of contingent assets and
liabilities at the date of the financial statements and revenues and expenses during the periods
reported. These estimates include the collectibility of accounts receivable, the recoverability of
inventory, the adequacy of warranty liabilities, the adequacy of our liabilities for corrective
actions, the valuation of stock awards, the fair value of patent rights, estimated worldwide
effective tax rates in U.S. and foreign jurisdictions, the realizability of investments, the
realizability of deferred tax assets and valuation and useful lives of tangible and intangible
assets, among others. The market for the Company’s products is characterized by intense
competition, rapid technological development and frequent new product introductions, all of which
could affect the future realizability of the Company’s assets. Estimates and assumptions are
reviewed periodically, and the effects of revisions are reflected in the unaudited condensed
consolidated financial statements in the period they are determined to be necessary.
Net Loss Per Share
Basic loss per share is computed by dividing net loss attributable to Cardiac Science
Corporation by the weighted average number of shares of common stock outstanding during the period.
Diluted loss per share is computed by dividing net loss attributable to Cardiac Science Corporation
by the weighted average number of common and dilutive common equivalent
Page 6 of 41
shares outstanding during the period. Common equivalent shares consist of shares issuable upon
the exercise of stock options, non-vested stock awards, warrants and issuance of shares under the
Company’s 2002 Employee Stock Purchase Plan (“ESPP”) using the treasury stock method. Common
equivalent shares are excluded from the calculation if their effect is antidilutive.
The following table sets forth the computation of basic and diluted net loss per share
attributable to Cardiac Science Corporation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
Nine Months Ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
(in thousands, except share data)
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
Numerator:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to Cardiac Science
Corporation
|
|
$
|
(6,376
|
)
|
|
$
|
(66,546
|
)
|
|
$
|
(33,473
|
)
|
|
$
|
(69,190
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares for basic calculation
|
|
|
23,831,807
|
|
|
|
23,368,778
|
|
|
|
23,717,287
|
|
|
|
23,209,181
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares for diluted calculation
|
|
|
23,831,807
|
|
|
|
23,368,778
|
|
|
|
23,717,287
|
|
|
|
23,209,181
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table sets forth the number of antidilutive shares issuable upon exercise of
stock options, non-vested stock awards, ESPP and warrants excluded from the computation of diluted
net loss attributable to Cardiac Science Corporation per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
Nine Months Ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
Antidilutive shares issuable upon exercise of stock options
|
|
|
2,749,718
|
|
|
|
3,117,503
|
|
|
|
2,749,718
|
|
|
|
3,117,503
|
|
|
Antidilutive shares issuable upon exercise of warrants
|
|
|
12,500
|
|
|
|
211,276
|
|
|
|
12,500
|
|
|
|
212,276
|
|
|
Antidilutive shares related to non-vested stock awards and
ESPP
|
|
|
1,450,508
|
|
|
|
915,377
|
|
|
|
1,450,508
|
|
|
|
915,377
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
4,212,726
|
|
|
|
4,244,156
|
|
|
|
4,212,726
|
|
|
|
4,245,156
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer and Vendor Concentrations
For the three and nine month periods ended September 30, 2010 and 2009, the Company did not
have any customers that accounted for more than 10% of our revenues.
For the three and nine month periods ended September 30, 2010, the Company had one vendor in
each period that accounted for more than 10% of purchases during these periods. For the three and
nine month periods ended September 30, 2009, the Company did not have any vendors which accounted
for 10% of purchases during these periods. Although components are available from other sources, a
key vendor’s inability or unwillingness to supply components in a timely manner or on terms
acceptable to the Company could adversely affect the Company’s ability to meet customers’ demands.
Recent Accounting Pronouncements
In January 2010, the FASB issued guidance to amend the disclosure requirements related to
recurring and nonrecurring fair value measurements. The guidance requires new disclosures on the
transfers of assets and liabilities between Level 1 (quoted prices in active market for identical
assets or liabilities) and Level 2 (significant other observable inputs) of the fair
value measurement hierarchy, including the reasons and the timing of the transfers.
Additionally, the guidance requires a roll forward of activities on purchases, sales, issuance, and
settlements of the assets and liabilities measured using significant unobservable inputs (Level 3
fair value measurements). The guidance became effective for the Company with the reporting period
beginning January 1, 2010, except for the disclosure on the roll forward activities for Level 3
fair value measurements, which will become effective for the Company with the reporting period
beginning January 1, 2011. Other than requiring additional disclosures, adoption of this new
guidance did not and will not have a material impact on the Company’s financial statements.
In September 2009, the Emerging Issues Task Force (“EITF”) reached a consensus related to
revenue arrangements with multiple deliverables, which the FASB then issued as an Accounting
Standards Update (“ASU”), 2009-13. The ASU amends Accounting Standards Codification (“ASC”)
Subtopic 605-25, “Revenue Recognition — Multiple Element Arrangements” and provides application
guidance on whether multiple deliverables exist in a revenue arrangement, how the deliverables
should be separated and how the consideration should be allocated to one or more units of
accounting. This ASU establishes a selling
Page 7 of 41
price hierarchy for determining the selling price of a
deliverable based on vendor-specific objective evidence, if available, third-party evidence, or
estimated selling price if neither vendor-specific nor third-party evidence is available. The ASU
can be applied on a prospective basis or in certain circumstances on a retrospective basis. The
Company plans to adopt this ASU on a prospective basis beginning January 1, 2011. The Company
believes the adoption of this ASU may have an impact on its financial position and results of
operations, however it is uncertain whether the impact will be material. The Company is continuing
to evaluate this ASU as of September 30, 2010.
In September 2009, the EITF reached a consensus related to revenue arrangements that include
software elements, which the FASB issued as ASU, 2009-14. The ASU amends ASC Subtopic 985-605,
“Software — Revenue Recognition.” Previously, companies that sold tangible products with “more
than incidental” software were required to apply software revenue recognition guidance. This
guidance often delayed revenue recognition for the delivery of the tangible product. Under the new
guidance, tangible products that have software components that are “essential to the functionality”
of the tangible product will be excluded from the software revenue recognition guidance. The new
guidance will include factors to help companies determine what is “essential to the functionality.”
Software-enabled products will now be subject to other revenue guidance and will likely follow the
guidance for multiple deliverable arrangements issued by the FASB in September 2009 in ASU 2009-13.
The new guidance is to be applied on a prospective basis for revenue arrangements entered into or
materially modified in fiscal years beginning on or after June 15, 2010, with earlier application
permitted. The Company plans to adopt this ASU on a prospective basis beginning January 1, 2011.
The Company believes the adoption of this ASU may have an impact on its financial position and
results of operations, however it is uncertain whether the impact will be material. The Company is
continuing to evaluate this ASU as of September 30, 2010.
2. Subsequent Events
Proposed Merger with Opto Circuits
On October 19, 2010, the Company entered into an Agreement and Plan of Merger (the “Merger
Agreement”) with Opto Circuits (India) Ltd., a public limited company incorporated under the law of
the nation of India (“Opto Circuits” or “Parent”), and Jolt Acquisition Company, a Delaware
corporation and wholly-owned subsidiary of Parent (“Merger Sub”) under which Opto Circuits has
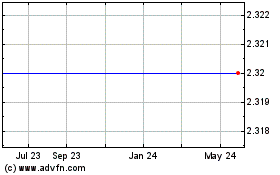
agreed to acquire all of the outstanding shares of the Company’s common stock for $2.30 per share
(the “Offer Price”). The aggregate value of the proposed transaction is approximately $58,000,000.
A copy of the Merger Agreement and a joint press release announcing the transaction were filed as
exhibits to a Current Report on Form 8-K filed by the Company with the SEC on October 19, 2010.
The boards of directors of the Company, Opto Circuits and Merger Sub have unanimously approved
the Merger Agreement and the transactions contemplated thereby, which will take the form of an
all-cash tender offer by Merger Sub to acquire up to 100% of the Company’s outstanding shares of
common stock at the Offer Price (the “Offer”), followed by a second-step merger of Merger Sub with
and into the Company. The Offer by Opto Circuits, which commenced on November 1, 2010, is subject
to various conditions, including (i) the valid tender of shares representing the greater of (x) at
least a majority of the fully diluted shares (which assumes conversion or exercise of all
outstanding options to acquire shares, or any other rights, options or warrants to acquire shares)
or (y) at least 60% of the outstanding shares, and (ii) other customary closing conditions. Unless
extended by Opto Circuits, the Offer will expire on November 30, 2010.
Pursuant to the Merger Agreement, following the consummation of the Offer, and subject to the
satisfaction or waiver of certain conditions set forth in the Merger Agreement, Merger Sub will
merge with and into the Company, with the Company as the surviving corporation in the merger (the
“
Merger
”). At the effective time of the Merger, all remaining outstanding shares not
tendered in the Offer (other than shares owned by Opto Circuits or Merger Sub or any other direct
or indirect wholly-owned subsidiary of Opto Circuits, shares owned by the Company as treasury stock
or shares held by stockholders who properly exercise their appraisal rights pursuant to and in
compliance with the Delaware General Corporation Law) will
be cancelled and converted into the right to receive the Offer Price in cash, without interest
and subject to applicable withholding.
As described below, several class action complaints have been filed in Washington State and
Delaware courts seeking, among other relief, injunctive relief enjoining the Transaction. See Note
12 (Commitments and Contingencies).
Upon completion of the Merger, the Company will become a wholly-owned subsidiary of Opto
Circuits. Opto Circuits intends to fund the purchase with its existing cash and existing credit
lines. There are no assurances that the proposed transaction will be consummated on the expected
timetable, or at all. If the Merger Agreement is terminated under certain circumstances, the
Company could be liable for termination fees of up to $1,300,000 which includes reimbursement of
legal and other fees incurred by Opto Circuits up to $300,000 plus a termination fee of $1,000,000.
Page 8 of 41
Pending Sale of Biotel, Inc. Investment
On November 8, 2010, Biotel, Inc. announced that it had signed a definitive merger agreement
with CardioNet, Inc. to be acquired in an all cash transaction valued at approximately $11,000,000,
or $3.84 per share of Biotel stock. At September 30, 2010, the Company held 180,269 shares of
Biotel stock valued at $1.26 per share. The Company accounts for these shares as available for
sale, and recorded the fair value of the shares of $228,000 at September 30, 2010 as investments in
unconsolidated entities in the accompanying unaudited condensed consolidated balance sheet.
The agreement is subject to Biotel, Inc. shareholder approval and is expected to close by
December 31, 2010. Should the agreement close as expected, the Company will receive approximately
$690,000 in cash based upon the shares held as of September 30, 2010. The Company would recognize a
realized gain of approximately $320,000 based on the historical cost of approximately $2.05 per
share, or approximately $370,000.
3. Liquidity
As of September 30, 2010, the Company’s cash and cash equivalents totaled $6,690,000. The
Company is currently in the process of addressing two separate voluntary corrective actions
relating to its AED products, the first of which was initially announced in November 2009 and the
second of which was initially announced in February 2010. See Note 4 (Corrective Action
Liabilities).
The Company has recorded charges totaling $32,000,000 related to these two voluntary
corrective actions, of which $21,000,000 was recorded during 2009 and an additional $11,000,000 was
recorded during the second quarter of 2010. This additional $11,000,000 charge was associated with
the Company’s plan, first announced in July 2010, to replace or repair approximately 24,000 AEDs
used by certain first responders and medical facilities in the United States and includes an
estimate for devices located outside of the United States which the Company believes that it is
probable that it would be required to repair or replace in the future. Through September 30, 2010,
the Company had used cash of $13,243,000 in carrying out these corrective actions, with remaining
accrued corrective action liabilities of $18,757,000 at September 30, 2010. See Note 4 (Corrective
Action Liabilities).
The costs recorded are estimates and actual costs that the Company incurs to complete the
corrective actions could be more or less than the remaining $18,757,000 accrual as of September 30,
2010. The Company expects that it will satisfy substantially all of the requirements under its
ongoing corrective actions during the remainder of 2010 and during 2011 and expects to spend a
significant amount of the remaining accrued corrective action liability costs during this same
period, which will negatively impact the Company’s cash position. In addition, the Company has
incurred significant legal and advisory fees in the fourth quarter in connection with the Company’s
pending merger with Opto Circuits. The Company expects to operate at a loss and to use cash in its
operations during the fourth quarter of 2010.
The Company believes it has addressed issues raised by the Food and Drug Administration
(“FDA”) in connection with the voluntary field corrective action first announced in November 2009.
See Note 4 (Corrective Action Liabilities). However, the Company is in ongoing discussions with the
FDA regarding the remaining issues raised by the FDA in its warning letter from February 2010 which
asserted inadequacies relating to the Company’s procedures associated with the evaluation,
investigation and follow up of complaints, procedures to verify the effectiveness of corrective and
preventative actions and procedures relating to certain design requirements. If the Company is
unable to satisfy the FDA’s concerns, the Company may be subject to regulatory action by the FDA,
including but not limited to seizure, injunction, halting shipment of the Company’s products and/or
civil monetary penalties. Any such actions could significantly disrupt the Company’s ongoing
business and operations and have a material adverse effect on its financial position and results of
operations. Additionally, quality issues and related corrective actions may adversely impact the
Company’s projected revenues in the near term.
Accordingly, the Company may be required to reduce costs, which could adversely impact the
Company’s forecasts for revenue growth in future periods.
On July 16, 2010, the Company entered into an amended and restated line of credit agreement
with Silicon Valley Bank which, among other things, increased the amount available for borrowing
from $5,000,000 to $15,000,000. The Company did not have any borrowings under its line of credit
agreement at September 30, 2010. However, as of November 9, 2010, the Company had borrowed
$3,000,000 under this line of credit and may have additional borrowings under the line of credit
during the remainder of the fourth quarter of 2010. The Company’s ability to borrow under this
agreement is primarily based on outstanding accounts receivable and, to a lesser extent, on its
inventory holdings. Additionally, the credit facility contains certain covenants related to minimum
liquidity levels and tangible net worth which must be maintained at all times under the agreement.
See Note 9 (Credit Facility). The Company was in compliance with its financial covenants under the
line of credit as of September 30, 2010, and through the date of this report. However, given the
ongoing risks associated with regulatory matters and the potential negative impact they could have
on the Company’s revenues and accounts receivable in future periods, as well as other potential
negative factors such as higher than anticipated operating losses in future periods, new or
Page 9 of 41
unexpected legal claims or other material adverse events, the Company can provide no assurance that
it will be able to continue to comply with the covenants under the line of credit agreement.
Failure to comply with the covenants under the line of credit could result in a default under the
line of credit agreement with Silicon Valley Bank. In such event, if the Company was unable to
obtain a waiver of the default, it would be unable to continue to borrow under this facility and
the default could result in an acceleration of any outstanding indebtedness, either of which would
have a material adverse impact on the Company’s financial condition and liquidity.
Given the above factors, the Company plans to closely monitor its actual and projected
operating results and cash balances during the remainder of 2010 and beyond. Additionally, the
Company is focused on executing its corrective action plans and reducing operating losses during
the fourth quarter of 2010. The Company intends to reduce operating losses through both revenue
growth and by reducing operating expenses during 2011. If the Company’s actual operating results
are not in line with its projected results, the Company intends to, and believes it has the ability
to, make such cost reductions should they become necessary. However, the Company believes its
existing cash and cash equivalents and ability to borrow under its available line of credit will be
sufficient to fund its anticipated operating losses for at least the next twelve months, as well as
to meet working capital requirements and fund anticipated capital expenditures and other
obligations, including the estimated remaining costs of the ongoing corrective actions.
Ultimately, the Company anticipates it will have sufficient operating profits, and/or cash
generated from operations in future periods, to repay any borrowings under its line of credit.
Further, the Company intends to renew its line of credit agreement with Silicon Valley Bank in July
2011. However, there can be no assurance that the Company will be successful in renewing its line
of credit agreement with Silicon Valley Bank in the future. To the extent the Company is not
successful in renewing its line of credit, or if other adverse events or circumstances arise during
the remainder of 2010 or 2011 which could require additional funding beyond that available under
its current line of credit, the Company may need to seek additional or alternative sources of
financing. There can be no assurance that the Company would be able to obtain financing from
alternative sources and, if such financing were to be available, it may be expensive and/or
dilutive to stockholders.
4. Corrective Action Liabilities
November 2009 Corrective Actions
At the end of the second quarter of 2009, the Company voluntarily ceased shipments of certain
of its AED products due to two instances the Company became aware of involving the failure of AEDs
to deliver therapy during a resuscitation attempt, apparently as a consequence of a malfunction of
one of the components used in the manufacture of the affected AED products. On August 10, 2009, the
Company resumed production and shipments of the AED products after implementing a more stringent
process to test for defects in the component at issue.
During the third quarter of 2009, the Company conducted a thorough review and analysis of the
potential for certain of its AED products shipped prior to August 10, 2009 to fail to perform a
rescue due to the component issue described above. The Company determined that the component at
issue has the unlikely potential to fail and that routine self-tests performed by the AED may not
detect a component that has malfunctioned. The Company also determined that approximately 300,000
AEDs shipped between June 2003 and June 2009 are potentially impacted by the component issue. In
November 2009 the Company announced it was initiating a voluntary field corrective action to
enhance the reliability of the affected AED units in the field through a software update related to
the AEDs’ routine self test functionality. The Company recorded a charge of $18,500,000 in the
third quarter of 2009 related to this corrective action. During the first quarter of 2010, the
Company completed development of the initial software update related to certain models of its AEDs
and made this update available to its customers. The Company introduced the remaining versions of
the software update for all remaining AED models in June 2010.
In February 2010, the Company received a warning letter from the FDA noting, among other
things, that the voluntary field corrective action it announced in November 2009 is inadequate
since the software update is intended to improve the products’ ability to detect the potential
component defect, but is not designed to prevent component failure. In April 2010, the FDA
published additional information regarding this corrective action, further clarifying the AED
models impacted by the potential component defect and providing further guidance to users of the
Company’s affected AEDs. Additionally, the FDA noted that the Company’s software update addresses
“some, but not all electrical component defects.”
Following discussions with the FDA, in July 2010, the Company announced it will replace
approximately 24,000 AEDs used by certain first responders and medical facilities in the United
States that are included in the customer population covered by the voluntary corrective action
first announced in November 2009. The population of first responders includes police, fire and
ambulance services and medical facilities include hospitals, medical clinics, dialysis centers and
assisted living facilities. The Company recorded an additional $11,000,000 charge during the second
quarter of 2010, representing the estimated costs of repair or replacement of these devices, as
well as an estimate of the cost to repair or replace certain devices
Page 10 of 41
located outside of the United
States that the Company believes that it is probable it would be required to be performed in the
future. The Company expects that it will satisfy substantially all of the requirements under this
corrective action during 2011.
Concurrent to the Company’s July 2010 announcement, the FDA published a communication
regarding the Company’s updated corrective action plan, citing the Company’s commitment to repair
or replace approximately 24,000 AEDs deployed in high risk and/or frequent use settings, such as
with first responders and medical facilities within the United States. The FDA recommended that all
other impacted customers follow the process, as outlined by the Company, for implementing the
software update which has been made available to customers through the Company’s website or through
a software kit shipped directly to the customer. The Company believes this updated recommendation
by the FDA addressed all outstanding issues with the Company’s previously communicated plan to
address potential component defects through a field software update as originally announced in
November 2009.
February 2010 Corrective Action
During the first quarter of 2010, the Company announced it was initiating a worldwide
voluntary medical device recall after determining that approximately 12,200 AEDs may not be able to
deliver therapy during a resuscitation attempt, which may lead to serious adverse events or death.
These AEDs were manufactured in a way that makes them potentially susceptible to failure under
certain conditions. The FDA was informed of this situation in February 2010. The Company recorded a
charge of $2,500,000 in the fourth quarter of 2009 related to this voluntary recall. As of
September 30, 2010, the Company has replaced substantially all of the devices included in this
corrective action and is currently expecting this matter to be complete by the end of the fourth
quarter of 2010.
Recorded Charges
As of September 30, 2010, the Company has recorded charges totaling $32,000,000 representing
estimated costs related to the two voluntary corrective actions described above. Charges totaling
$21,000,000 were included in cost of revenues on the consolidated statements of operations for the
year ended December 31, 2009 and charges of $11,000,000 were included in cost of revenues on the
unaudited condensed consolidated statement of operations for the nine month period ended September
30, 2010.
The Company’s corrective action liabilities are included in corrective action liabilities on
the unaudited condensed consolidated balance sheets and are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charged to
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning
|
|
product cost
|
|
|
|
|
|
|
|
|
|
End of
|
|
(in thousands)
|
|
of period
|
|
of revenues
|
|
Adjustments
|
|
Expenditures
|
|
period
|
|
Nine months ended
September 30, 2010
|
|
$
|
15,249
|
|
|
$
|
11,000
|
|
|
$
|
—
|
|
|
$
|
(7,492
|
)
|
|
$
|
18,757
|
|
The costs of these voluntary corrective actions are estimates. The actual costs incurred by
the Company to implement the voluntary corrective actions could vary significantly based on a
number of factors, including the outcome of discussions or negotiations with applicable regulatory
bodies in geographies outside the U.S., the number of impacted devices, the customer
and geographical segments related to the impacted devices, the logistical processes employed
by the Company to address the issues, the customer response rate in implementing the corrective
action plans, the level of required follow up with customers, the extent to which the Company
relies on third party assistance to carry out the corrective actions, the extent to which AED units
recovered from affected customers can be repaired and used as replacement units for other
customers, and the length of time and other resources required to complete the corrective actions,
among others. However, as of September 30, 2010, the Company believes its remaining accrued
corrective action liabilities on the unaudited condensed consolidated balance sheet as of September
30, 2010 will be sufficient to fund the remaining expected costs of these matters.
The Company expects that it will satisfy substantially all of the requirements under its
ongoing corrective actions during the remainder of 2010 and during 2011, which will continue to
have a negative impact on cash flows during this same period, and possibly later periods. Further,
as a result of these charges, the Company also considered the impact the corrective actions could
have on the carrying value of indefinite lived intangible assets or other long lived assets
including property and equipment and intangible assets subject to amortization, or the
realizability of its inventories as of September 30, 2010. Based on the Company’s analysis, it was
determined that no adjustments to the carrying value of these assets were required as of September
30, 2010.
The results of the Company’s evaluation of the component issues described herein and any
responsive actions taken by the Company are subject to significant uncertainty. The Company’s
policy is to assess the likelihood of any potential corrective actions, voluntary or not,
associated with its products as well as ranges of possible or probable costs associated
Page 11 of 41
with such activities, when appropriate. A determination of the amount of the liability required for this or
other contingencies is made after an analysis of each known issue. A liability is recorded and
charged to cost of revenues if and when the Company determines that a loss is probable and the
amount of the loss can be reasonably estimated. Additionally, the Company discloses contingencies
for which a material loss is reasonably possible, but not probable.
5. Equity and Comprehensive Income (Loss)
For the Company, components of other comprehensive income (loss) consist of unrealized gains
and losses on available-for-sale securities, net of related income tax effects, if applicable, and
translation gains and losses related to consolidation of financial statements from foreign
subsidiaries and joint ventures. Available for sale securities are classified as investments in
unconsolidated entities on the unaudited condensed consolidated balance sheets.
The Company reports its noncontrolling interests in consolidated joint ventures as a component
of equity in the Company’s unaudited condensed consolidated balance sheets separate from Cardiac
Science Corporation’s equity. Transactions that do not result in the deconsolidation of the
subsidiary are recorded as equity transactions, while those transactions that do result in a change
from noncontrolling to controlling ownership, or a deconsolidation of the subsidiary, are recorded
in net income (loss) with the gain or loss measured at fair value.
The following tables reflect the changes in equity attributable to both Cardiac Science
Corporation and the noncontrolling interests of the joint ventures in which the Company has a
majority, but not total, ownership interest for the three and nine month periods ended September
30, 2010 and 2009, respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable to
|
|
|
|
|
|
|
|
|
|
|
Cardiac Science
|
|
|
Noncontrolling
|
|
|
Total
|
|
|
(in thousands)
|
|
Corporation
|
|
|
Interests
|
|
|
Equity
|
|
|
Equity at June 30, 2010
|
|
$
|
32,884
|
|
|
$
|
1,276
|
|
|
$
|
34,160
|
|
|
Comprehensive loss:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(6,376
|
)
|
|
|
43
|
|
|
|
(6,333
|
)
|
|
Realized loss on available sale securities
|
|
|
(76
|
)
|
|
|
—
|
|
|
|
(76
|
)
|
|
Unrealized losses on available for sale securities
|
|
|
(8
|
)
|
|
|
|
|
|
|
(8
|
)
|
|
Foreign currency translation adjustments
|
|
|
148
|
|
|
|
132
|
|
|
|
280
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss)
|
|
|
(6,312
|
)
|
|
|
175
|
|
|
|
(6,137
|
)
|
|
Stock-based transactions and compensation expense under
employee benefit plans
|
|
|
639
|
|
|
|
—
|
|
|
|
639
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity at September 30, 2010
|
|
$
|
27,211
|
|
|
$
|
1,451
|
|
|
$
|
28,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity at June 30, 2009
|
|
$
|
131,274
|
|
|
$
|
869
|
|
|
$
|
132,143
|
|
|
Comprehensive income (loss):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(66,546
|
)
|
|
|
135
|
|
|
|
(66,411
|
)
|
|
Unrealized losses on available for sale securities,
net of related tax of $125
|
|
|
(213
|
)
|
|
|
—
|
|
|
|
(213
|
)
|
|
Foreign currency translation adjustments
|
|
|
75
|
|
|
|
31
|
|
|
|
106
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss)
|
|
|
(66,684
|
)
|
|
|
166
|
|
|
|
(66,518
|
)
|
|
Stock-based transactions and compensation expense under
employee benefit plans
|
|
|
832
|
|
|
|
—
|
|
|
|
832
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity at September 30, 2009
|
|
$
|
65,422
|
|
|
$
|
1,035
|
|
|
$
|
66,457
|
|
|
|
|
|
|
|
|
|
|
|
|
Page 12 of 41
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable to
|
|
|
|
|
|
|
|
|
|
|
Cardiac Science
|
|
|
Noncontrolling
|
|
|
Total
|
|
|
(in thousands)
|
|
Corporation
|
|
|
Interests
|
|
|
Equity
|
|
|
Equity at December 31, 2009
|
|
$
|
58,936
|
|
|
$
|
1,293
|
|
|
$
|
60,229
|
|
|
Comprehensive loss:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(33,473
|
)
|
|
|
204
|
|
|
|
(33,269
|
)
|
|
Realized loss on available for sale securities
|
|
|
(76
|
)
|
|
|
—
|
|
|
|
(76
|
)
|
|
Unrealized gains on available for sale securities
|
|
|
7
|
|
|
|
—
|
|
|
|
7
|
|
|
Foreign currency translation adjustments
|
|
|
(118
|
)
|
|
|
(46
|
)
|
|
|
(164
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss)
|
|
|
(33,660
|
)
|
|
|
158
|
|
|
|
(33,502
|
)
|
|
Stock-based transactions and compensation expense under
employee benefit plans
|
|
|
1,935
|
|
|
|
—
|
|
|
|
1,935
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity at September 30, 2010
|
|
$
|
27,211
|
|
|
$
|
1,451
|
|
|
$
|
28,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity at December 31, 2008
|
|
$
|
131,703
|
|
|
$
|
545
|
|
|
$
|
132,248
|
|
|
Comprehensive income (loss):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
(69,190
|
)
|
|
|
476
|
|
|
|
(68,714
|
)
|
|
Unrealized gains on available for sale securities,
net of related tax of ($44)
|
|
|
161
|
|
|
|
—
|
|
|
|
161
|
|
|
Foreign currency translation adjustments
|
|
|
48
|
|
|
|
14
|
|
|
|
62
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income (loss)
|
|
|
(68,981
|
)
|
|
|
490
|
|
|
|
(68,491
|
)
|
|
Stock-based transactions and compensation expense under
employee benefit plans
|
|
|
2,700
|
|
|
|
—
|
|
|
|
2,700
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity at September 30, 2009
|
|
$
|
65,422
|
|
|
$
|
1,035
|
|
|
$
|
66,457
|
|
|
|
|
|
|
|
|
|
|
|
|
6. Segment Reporting
The Company is required to disclose selected information about operating segments. The Company
also reports related disclosures about products and services, geographic areas and major customers.
An operating segment is defined as a component of an enterprise that engages in business activities
from which it may earn revenues and incur expenses whose separate financial information is
available and is evaluated regularly by the Company’s chief operating decision makers, or decision
making group, to perform resource allocations and performance assessments.
The Company’s chief operating decision makers are the Chief Executive Officer and other senior
executive officers of the Company. Based on evaluation of the Company’s financial information,
management believes that the Company operates in one reportable segment with its various cardiology
products and services.
The Company’s chief operating decision makers evaluate revenue performance of product lines,
both domestically and internationally. However, operating, strategic and resource allocation
decisions are based primarily on the Company’s overall performance in its operating segment.
The following table summarizes revenues by product line:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
Nine Months Ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
(in thousands)
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
Defibrillation products
|
|
$
|
18,149
|
|
|
$
|
21,646
|
|
|
$
|
53,062
|
|
|
$
|
61,413
|
|
|
Cardiac monitoring products
|
|
|
11,836
|
|
|
|
13,000
|
|
|
|
37,362
|
|
|
|
40,151
|
|
|
Service
|
|
|
4,495
|
|
|
|
4,238
|
|
|
|
13,266
|
|
|
|
13,098
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
34,480
|
|
|
$
|
38,884
|
|
|
$
|
103,690
|
|
|
$
|
114,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table summarizes revenues, which are attributed based on the geographic location
of the customers:
Page 13 of 41
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
Nine Months Ended
|
|
|
|
|
September 30,
|
|
|
September 30,
|
|
|
(in thousands)
|
|
2010
|
|
|
2009
|
|
|
2010
|
|
|
2009
|
|
|
Domestic
|
|
$
|
25,698
|
|
|
$
|
27,099
|
|
|
$
|
76,103
|
|
|
$
|
79,189
|
|
|
Foreign
|
|
|
8,782
|
|
|
|
11,785
|
|
|
|
27,587
|
|
|
|
35,473
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
34,480
|
|
|
$
|
38,884
|
|
|
$
|
103,690
|
|
|
$
|
114,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Substantially all intangible assets are domestic. Long-lived assets located outside of the
United States are not material.
7. Inventories
Inventory is valued at the lower of cost or market. Cost is determined using a standard cost
method, including material, labor and factory overhead. Inventories were comprised of the
following:
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
|
|
|
December 31,
|
|
|
(in thousands)
|
|
2010
|
|
|
2009
|
|
|
Raw materials
|
|
$
|
16,283
|
|
|
$
|
18,752
|
|
|
Finished goods
|
|
|
7,619
|
|
|
|
4,829
|
|
|
|
|
|
|
|
|
|
|
Total inventories
|
|
$
|
23,902
|
|
|
$
|
23,581
|
|
|
|
|
|
|
|
|
|
The Company performs a detailed analysis of its inventories on a quarterly basis, or more
frequently should circumstances arise. Such circumstances could include, but would not be limited
to, changes in a product bill of material due to design or quality improvements, product failures
related to faulty or defective materials, decisions regarding product life cycles, and the
Company’s ability to sell refurbished products, among others. This analysis is performed
to ensure inventory items are carried at the lower of cost, on a weighted average basis, or market,
and that the Company has adequately reserved any excess and/or obsolete items in inventory.
8. Intangible Assets
The following table sets forth the balances of intangible assets at September 30, 2010 (in
thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Useful life
|
|
Cost
|
|
|
Amortization
|
|
|
Net
|
|
|
Intangible assets not subject to amortization:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Burdick trade name
|
|
|
|
$
|
3,400
|
|
|
$
|
—
|
|
|
$
|
3,400
|
|
|
Cardiac Science trade name
|
|
|
|
|
11,380
|
|
|
|
—
|
|
|
|
11,380
|
|
|
Cardiac Science Deutschland trade name
|
|
|
|
|
182
|
|
|
|
—
|
|
|
|
182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets not subject to
amortization
|
|
|
|
|
14,962
|
|
|
|
—
|
|
|
|
14,962
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets subject to amortization:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cardiac Science customer relationships
|
|
5 years
|
|
|
8,650
|
|
|
|
(8,650
|
)
|
|
|
—
|
|
|
Cardiac Science developed technology
|
|
8 years
|
|
|
11,330
|
|
|
|
(7,199
|
)
|
|
|
4,131
|
|
|
Burdick distributor relationships
|
|
10 years
|
|
|
1,400
|
|
|
|
(1,085
|
)
|
|
|
315
|
|
|
Burdick developed technology
|
|
7 years
|
|
|
860
|
|
|
|
(860
|
)
|
|
|
—
|
|
|
Patents and patent applications
|
|
5-10 years
|
|
|
960
|
|
|
|
(940
|
)
|
|
|
20
|
|
|
Patent rights
|
|
6-13 years
|
|
|
7,692
|
|
|
|
(1,937
|
)
|
|
|
5,755
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets subject to amortization
|
|
|
|
|
30,892
|
|
|
|
(20,671
|
)
|
|
|
10,221
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
$
|
45,854
|
|
|
$
|
(20,671
|
)
|
|
$
|
25,183
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s estimated expense for the amortization of intangibles for each full year ended
December 31 is summarized as follows (in thousands):
Page 14 of 41
|
|
|
|
|
|
|
2010 (Q4 only)
|
|
$
|
548
|
|
|
2011
|
|
|
2,190
|
|
|
2012
|
|
|
2,187
|
|
|
2013
|
|
|
1,567
|
|
|
2014
|
|
|
623
|
|
|
Thereafter
|
|
|
3,106
|
|
9. Credit Facility
On July 16, 2010, the Company entered into an amended and restated line of credit agreement
with Silicon Valley Bank which, among other things, increased the amount available for borrowing in
revolving credit from $5,000,000 to $15,000,000. The Company’s ability to borrow under this
agreement is based largely on its accounts receivable outstanding at the time of borrowing and, to
a lesser extent, on its inventory holdings. Additionally, the credit facility contains certain
covenants related to minimum liquidity levels as well as certain levels of tangible net worth which
must be maintained at all times under the agreement. Interest on borrowings, if any, will be based
on the Wall Street Journal Prime rate plus 2.5%. Interest is payable monthly, on the last day of
the month, and all obligations are due on July 14, 2011. In addition, upon finalizing the agreement
in July 2010, the Company paid Silicon Valley Bank a non-refundable commitment fee of $175,000 and
any unused balances under this facility will bear monthly fees equal to 0.25% per annum on the
average unused portion of the revolving line. The Company granted Silicon Valley Bank a first
priority security interest in substantially all of its assets to secure its obligations under the
line of credit.
At September 30, 2010, the Company did not have any borrowings under this or any other line of
credit. However, as of November 9, 2010, the Company had borrowed $3,000,000 million under this
credit facility and may have additional borrowings under the line of credit during the remainder of
the fourth quarter of 2010.
10. Warranty Liability
Changes in the warranty liability for the nine months ended September 30, 2010 were as
follows:
|
|
|
|
|
|
|
|
|
Nine Months Ended
|
|
|
(in thousands)
|
|
September 30, 2010
|
|
|
Warranty liability, beginning of the period
|
|
$
|
4,028
|
|
|
Charged to product cost of revenues, net
|
|
|
1,316
|
|
|
Warranty expenditures
|
|
|
(1,411
|
)
|
|
|
|
|
|
|
Warranty liability, end of the period
|
|
$
|
3,933
|
|
|
|
|
|
|
Page 15 of 41
11. Stock-Based Compensation Plans
The Company maintains an ESPP, as well as several equity incentive plans under which it may
grant non-qualified stock options, incentive stock options and non-vested stock awards to
employees, non-employee directors and consultants.
The fair value of each option grant and ESPP purchase is estimated using the Black-Scholes
option-pricing model with the following weighted-average assumptions and the fair value of the
non-vested stock awards is calculated based on the market value of the shares awarded at date of
grant:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended
|
|
Nine months ended
|
|
|
|
September 30,
|
|
September 30,
|
|
|
|
2010
|
|
2009
|
|
2010
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock options plans:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Volatility
|
|
|
—
|
|
|
|
55.0
|
%
|
|
|
55.5
|
%
|
|
|
53.8
|
%
|
|
Expected term (years)
|
|
|
—
|
|
|
|
6.00
|
|
|
|
6.00
|
|
|
|
6.50
|
|
|
Risk-free interest rate
|
|
|
—
|
|
|
|
3.1
|
%
|
|
|
3.3
|
%
|
|
|
2.6
|
%
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Fair value of options granted
|
|
$
|
—
|
|
|
$
|
1.66
|
|
|
$
|
1.03
|
|
|
$
|
2.11
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee stock purchase plan:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Volatility
|
|
|
47.0
|
%
|
|
|
40.0
|
%
|
|
|
47.0
|
%
|
|
|
40.0
|
%
|
|
Expected term (years)
|
|
|
0.5
|
|
|
|
0.5
|
|
|
|
0.5
|
|
|
|
0.5
|
|
|
Risk-free interest rate
|
|
|
2.7
|
%
|
|
|
2.1
|
%
|
|
|
2.7
|
%
|
|
|
2.1
|
%
|
|
Expected dividend yield
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Fair value of employee stock purchase rights
|
|
$
|
0.79
|
|
|
$
|
1.73
|
|
|
$
|
0.79
|
|
|
$
|
1.73
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-vested stock:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of non-vested stock awards granted
|
|
$
|
—
|
|
|
$
|
3.04
|
|
|
$
|
2.32
|
|
|
$
|
4.05
|
|
In January 2010, the Company granted to certain of its executives 625,000 non-vested stock awards
as part of a long term performance based restricted stock unit award program. These awards will
vest at the end of a three-year performance period, with 50% based on the achievement of a
specified revenue compound annual growth rate with certain adjustments and 50% based on specified
combined operating cash flow (or suitable financing in place to fund operations beyond 2012) with
certain adjustments. The Company has granted an additional 78,500 non-vested stock awards during
2010. These non-vested stock awards vest ratably over a four-year period in annual increments
beginning one year from the date of grant. Non-vested stock awards require no payment from the
employee, with the exception of employee related federal taxes. As part of the Merger described in
Note 2 (Subsequent Events), all non-vested stock awards outstanding immediately prior to the
effective time of the Merger will be fully accelerated in vesting and will be cancelled at the
effective time of the Merger and the holder of the award will be entitled to receive, from Opto
Circuits, an amount in cash equal to the product of $2.30 multiplied by the number of cancelled
stock awards.
Page 16 of 41
The following table summarizes information about the non-vested stock award activity
during the three and nine month periods ended September 30, 2010:
|
|
|
|
|
|
|
|
|
Shares
|
|
Non-vested balance, December 31, 2009
|
|
|
1,045,460
|
|
|
Granted
|
|
|
675,000
|
|
|
Vested
|
|
|
(41,738
|
)
|
|
Forfeited
|
|
|
(89,194
|
)
|
|
|
|
|
|
|
Non-vested balance, March 31, 2010
|
|
|
1,589,528
|
|
|
Granted
|
|
|
28,500
|
|
|
Vested
|
|
|
(147,288
|
)
|
|
Forfeited
|
|
|
(19,637
|
)
|
|
|
|
|
|
|
Non-vested balance, June 30, 2010
|
|
|
1,451,103
|
|
|
Granted
|
|
|
—
|
|
|
Vested
|
|
|
(17,924
|
)
|
|
Forfeited
|
|
|
(24,465
|
)
|
|
|
|
|
|
|
Non-vested balance, September 30, 2010
|
|
|
1,408,714
|
|
|
|
|
|
|
In April 2010, the Company granted 7,500 shares of non-qualified stock options. Stock options
typically vest over a four-year period with 25% vesting after one year from the date of the grant
and then monthly thereafter (1/36
th
) for the next 3 years.
The Company issued 48,062 and 42,731 shares of common stock during the three month periods
ended September 30, 2010 and 2009, respectively, and 158,206 and 120,435 shares of common stock
during the nine month periods ended September 30, 2010 and 2009, respectively in connection with
the ESPP and received total proceeds of $73,000, $115,000, $225,000 and $372,000, respectively.
12. Commitments and Contingencies
Other Commitments
As of September 30, 2010, the Company had purchase obligations of approximately $39,596,000
consisting of outstanding purchase orders issued in the normal course of business.
Guarantees and Indemnities
During its normal course of business, the Company has made certain guarantees, indemnities and
commitments under which it may be required to make payments in relation to certain transactions.
These indemnities include intellectual property and other indemnities to the Company’s customers
and suppliers in connection with the sales of its products, and indemnities to directors and
officers of the Company to the maximum extent permitted under the laws of the State of Delaware.
Historically, the Company has not incurred any losses or recorded any liabilities related to
performance under these types of indemnities.
Legal Proceedings
On October 19, 2010, the Company announced that it had entered into a definitive merger
agreement with Opto Circuits, under which Opto Circuits has agreed to acquire all of the
outstanding shares of the Company’s common stock at $2.30 USD per share. Following that
announcement, certain Company stockholders filed similar lawsuits relating to this transaction
against the Company, Board members, and in some cases the Chief Executive Officer and Chief
Financial Officer. Those lawsuits include:
Lionel Patenaude v. Cardiac Science Corp., et
al.
, Court of Chancery of the State of Delaware, Civil Action No. 5923-VCP;
Mindy Creamer v. Cardiac Science Corp., et al.
Superior Court of
the State of Washington, Case No. 10-2-08782-4;
Robert Gluck v. Ruediger Naumann-Etienne, et
al.
, Superior Court of the State of Washington, Case No. 10-2-08912-6;
Mark Rapport v.
David Marver, et al.
, Superior Court of the State of Washington, Case No. 10-2-009005-1; and
Bagge v. Naumann-Etienne, et al.
, Superior Court of the State of Washington, Case No.
10-2-09045-1.
The plaintiffs in these cases
generally assert that the Company’s Directors and/or executives
breached fiduciary duties owed to stockholders, and that the Company either breached its fiduciary
duty to stockholders or in some manner participated with the individual defendants in their
respective alleged breaches, that the Company and Opto Circuits failed to provide adequate disclosures to stockholders
concerning the proposed transaction and that Opto Circuits and its Merger Sub also participated and are
also liable. The plaintiffs generally allege that the price announced for the tender offer is
inadequate, and that various
Page 17 of 41
individual defendants failed to take adequate steps to maximize stockholder value, and that the Company itself either failed to take adequate steps or aided and
abetted the individual defendants. The complaints seek to enjoin the defendants from proceeding
with, consummating, or closing the Merger, or to rescind and set aside the proposed transaction.
The Company believes that these complaints lack merit, and intends to defend these cases
vigorously.
The Company is subject to various other legal proceedings arising in the normal course of
business. In the opinion of management, the ultimate resolution of these proceedings is not
expected to have a material effect on the Company’s consolidated financial position, results of
operations or cash flows.
13. Income Taxes
The Company records its quarterly provision for income taxes based on the estimated worldwide
effective tax rates for the full year, which is determined based on estimates of pre-tax income or
losses for the full year, and applicable tax rates in jurisdictions in which income and losses are
expected to be generated. The Company estimates its worldwide effective tax rate for the year ended
December 31, 2010, excluding the U.S. jurisdictions, will be approximately 31%. This estimate
relates primarily to the Company’s foreign operations which generally are forecasted to be
marginally profitable during 2010.
Based on authoritative guidance, the Company has excluded the U.S. jurisdiction from its
worldwide effective tax rate analysis which was calculated separately and estimated to be zero as
of September 30, 2010 and for the full year of 2010. The Company excluded the U.S. jurisdiction
based on guidance that a jurisdiction should be excluded from the worldwide effective tax rate and
calculated separately to the extent such jurisdiction anticipates an ordinary loss for the year or
has generated ordinary losses on a year to date basis for which no tax benefit can be recognized.
The Company has maintained its valuation allowance on domestic deferred tax assets as the positive
and negative evidence which existed as of September 30, 2010 does not warrant a change from its
position as of December 31, 2009. As such, the Company believes it is not more likely than not that
it will be able to realize the benefits of the expected income tax losses generated during the
current year.
For the nine month period ended September 30, 2010, the Company recorded a worldwide effective
tax rate of 34% which includes the Company’s estimated worldwide effective tax rate, excluding the
U.S. jurisdictions, of 31% adjusted primarily for an insignificant discrete expense item in the
amount of $37,000 associated with an unfavorable resolution of a tax matter in a foreign
jurisdiction which was recorded in the three month period ended September 30, 2010, partially
offset by other favorable true-ups related to tax filings in foreign jurisdictions. Based on this,
the Company recorded income tax expense of $117,000 and $308,000 for the three and nine month
periods ended September 30, 2010, respectively.
The Company recorded a worldwide effective tax rate of 163% for the nine month period ended
September 30, 2009, resulting in income tax expense of $43,923,000 and $42,563,000 in the three and
nine month periods ended September 30, 2009, respectively. The prior year worldwide tax expense was
primarily comprised of the non-cash charge of $43,950,000 to increase the valuation allowance
against deferred tax assets and did not exclude the U.S. jurisdiction from the analysis as noted
above.
Based on management’s review of the Company’s tax positions, the Company had no significant
unrecognized tax positions as of September 30, 2010 and December 31, 2009. The Company’s continuing
practice is to recognize interest and/or penalties related to income tax matters in income tax
expense. At September 30, 2010 and December 31, 2009, the Company had no accrued interest related
to uncertain tax positions and no accrued penalties.
The Company is subject to U.S. federal income tax as well as income tax in multiple state and
foreign jurisdictions as well as federal, state and foreign filings related to Quinton and CSI. As
a result of the NOL carryforwards, substantially all tax years are open for U.S. federal and state
income tax matters. Foreign tax filings are open for years 2007 forward.
14. Derivatives
The Company is exposed to foreign currency exchange-rate fluctuations in the normal course of
its business, which the Company manages from time to time through the use of forward foreign
exchange contracts. Forward foreign exchange contracts are used to hedge the impact of fluctuations
of foreign exchange on certain assets or liabilities denominated in a currency other than the
functional currency of the Company or its subsidiaries. Currently, these forward foreign exchange
contracts do not qualify for hedge accounting. The Company uses forward foreign exchange contracts
to mitigate risk and does not intend to engage in speculative transactions. The forward foreign
exchange contracts are entered into by the Company and its subsidiaries primarily to hedge foreign
denominated accounts receivable and foreign denominated intercompany payables. These contracts do
not contain any credit-risk-related contingent features.
Page 18 of 41
The Company seeks to manage the counterparty risk associated with these forward foreign
exchange contracts by limiting transactions to counterparties with which the Company has an
established banking relationship. In addition, the contracts are limited to a time period of less
than one year, generally three months or less.
During each of the three and nine month periods ended September 30, 2010, these forward
foreign exchange contracts resulted in a net realized loss of $281,000 and a net realized gain of
$62,000, respectively. During each of the three and nine month periods ended September 30, 2009,
these forward foreign exchange contracts resulted in net realized losses of $89,000 and $81,000,
respectively. The realized gains and losses were partially offset by realized and unrealized gains
and losses on foreign denominated accounts receivable and foreign intercompany payables in the same
periods. Realized gains and losses related to forward foreign exchange contracts are recorded in
other income (loss), net and the assets and liabilities for these contracts are recorded in prepaid
and other assets and accrued liabilities. The Company had no outstanding forward foreign exchange
contracts as of September 30, 2010 or December 31, 2009.
15. Fair Value Measurement
Fair value measurements are determined under a three-level hierarchy that prioritizes the
inputs to valuation techniques used to measure fair value, distinguishing between market
participant assumptions developed based on market data obtained from sources independent of the
reporting entity (“observable inputs”) and the reporting entities own assumptions about market
participant assumptions developed based on the best information available in the circumstances
(“unobservable inputs”). Level 1 inputs consist of quoted prices (unadjusted) in active markets for
identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and
liabilities in active markets or inputs that are observable for the asset or liability, either
directly or indirectly through market corroboration, for substantially the full term of the
financial instrument. Level 3 inputs are unobservable inputs based on the Company’s own assumptions
used to measure assets and liabilities at fair value. Classification of a financial asset or
liability within the hierarchy is determined based on the lowest level input that is significant to
the fair value measurement. There were no changes to the valuation techniques during the three and
nine month periods ended September 30, 2010.
The Company’s investments in certain unconsolidated entities, which totaled $228,000 at
September 30, 2010, are comprised primarily of investments in equity securities of unconsolidated
entities that are carried at fair value and are included in investments in unconsolidated entities
on the Company’s unaudited condensed consolidated balance sheet, using quoted market prices in
active markets (level 1 inputs).
The Company has other financial instruments in addition to its investments in unconsolidated
entities noted above which consist primarily of cash, accounts receivable, notes receivable,
accounts payable and other accrued liabilities which are presented in the unaudited condensed
consolidated financial statements at their approximate fair values at September 30, 2010 due to the
short-term nature of their settlements.
16. Restructuring Costs
On January 14, 2009, the Company announced the implementation of a restructuring plan which
included a 12% reduction in work force, primarily impacting the Company’s product development,
manufacturing, and customer service organizations. The restructuring was implemented in order to
realign the Company’s cost structure and become more flexible and efficient in operations.
Additionally, as the Company has established a historical practice of providing similar termination
benefits, it was determined that the restructuring plan was an ongoing benefit arrangement, rather
than a one time termination benefit. The Company recorded severance charges of approximately
$1,203,000 in the fourth quarter of 2008 as management, having the appropriate authority,
determined the amount of benefits to be provided was probable and estimable as of December 31,
2008.
During the nine month period ended September 30, 2009, the Company made cash severance
payments of $888,000 related to this restructuring and all remaining costs associated with this
restructuring were paid prior to December 31, 2009. While there are periodic departures resulting in severance payments, there were no broad based
restructuring costs incurred during the three and nine month periods ended September 30, 2010 and
2009.
Page 19 of 41
|
|
|
|
|
Item 2.
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
This Quarterly Report on
Form 10-Q
contains forward-looking statements relating to Cardiac
Science Corporation (“Cardiac Science”), including forward-looking statements relating to the
proposed acquisition of Cardiac Science by Opto Circuits (India) Limited (“Opto Circuits”) and the
expected timetable for completing the transaction. Such statements are based on the current
assumptions and expectations of Cardiac Science’ and Opto Circuits’ management and are neither
promises nor guarantees. Except for historical information, the following discussion contains
forward-looking statements within the meaning of the Private Securities Litigation Reform Act of
1995. All statements other than statements of historical fact, including our expectations relating
to future results of operations, liquidity or financial position, completion of corrective action
plans; costs associated with corrective actions, cost reductions, resolution of pending regulatory
issues, closing of the proposed transaction with Opto Circuits; the effects of disruption from the
proposed transaction with Opto Circuits on the Company’s business and other matters, made in this
Quarterly Report on
Form 10-Q
are forward looking. Additionally, actual results of the proposed
transaction with Opto Circuits could vary materially as a result of a number of factors, including:
uncertainties as to whether the holders of sufficient shares of Cardiac Science common stock are
tendered in the Offer and whether the conditions to closing of the Offer are met; the possibility
that competing offers will be made; the risk that stockholder litigation in connection with the
Offer or the Merger may result in significant costs of defense, indemnification and liability and
the possibility that the Merger Agreement may be terminated under certain circumstances. The words
“believe,” “expect,” “intend,” “anticipate,” “will,” “may,” variations of such words, and similar
expressions identify forward-looking statements, but their absence does not mean that the statement
is not forward-looking. These forward-looking statements reflect management’s current expectations
and involve risks and uncertainties. Our actual results could differ materially from results that
may be anticipated by such forward-looking statements due to various uncertainties.
The principal factors that could cause or contribute to such differences include, but are not
limited to, the factors discussed in the section entitled “Risk Factors” in Part 1 — Item 1A of
our Annual Report on
Form 10-K
for the year ended December 31, 2009, those discussed in Part II —
Item 1A of our Quarterly Report on
Form 10-Q
for the quarters ended March 31, 2010 and June 30,
2010 and those discussed elsewhere in this report. Readers are cautioned not to place undue
reliance on these forward-looking statements, which speak only as of the date of this report. We
undertake no obligation to revise any forward-looking statements to reflect events or circumstances
that may subsequently arise. Readers are urged to review and consider carefully the various
disclosures made in this report and in our other filings made with the Securities and Exchange
Commission (“SEC”) that disclose and describe the risks and factors that may affect our business,
prospects and results of operations.
You should read the following discussion and analysis in conjunction with our unaudited
condensed consolidated financial statements and related notes included elsewhere in this report.
Operating results for the three and nine month periods ended September 30, 2010 are not necessarily
indicative of future results including the full fiscal year. You should also refer to our Annual
Consolidated Financial Statements, Notes thereto, and Management’s Discussion and Analysis of
Financial Condition and Results of Operations and “Risk Factors” contained in our Annual Report on
Form 10-K for the year ended December 31, 2009, filed with the SEC on March 15, 2010.
Business Overview
We develop, manufacture, and market a family of advanced diagnostic and therapeutic cardiology
devices and systems, including automated external defibrillators (AEDs), electrocardiograph devices
(ECG/EKG), cardiac stress testing treadmills and systems, diagnostic work stations, Holter
monitoring systems, hospital defibrillators, vital signs monitors, cardiac rehabilitation telemetry
systems, and cardiology data management systems (Informatics) that connect with hospital
information (HIS), electronic medical record (EMR), and other information systems. We sell a
variety of related products and consumables, and provide a portfolio of training, maintenance, and
support services. We are the successor to the cardiac businesses that established the trusted
Burdick®, HeartCentrix®, Quinton® and
Powerheart® brands and are headquartered in Bothell, Washington. With customers in more
than 100 countries worldwide, we have operations in North America, Europe, and Asia.
Page 20 of 41
Critical Accounting Estimates and Policies
To prepare financial statements that conform with U.S. generally accepted accounting
principles (“U.S. GAAP”), we must select and apply accounting policies and make estimates and
judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and
related disclosure of contingent assets and liabilities. We base our accounting estimates on
historical experience and on various other assumptions that we believe to be reasonable under the
circumstances, the results of which form our basis for making judgments about the carrying values
of assets and liabilities that are not readily apparent from other sources. Actual results may
differ from these estimates under different assumptions or conditions.
There are certain critical accounting estimates that we believe require significant judgment
in the preparation of our consolidated financial statements. We consider an accounting estimate to
be critical if it requires us to make assumptions because information was not available at the time
or it included matters that were highly uncertain at the time we were making the estimate, and
changes in the estimate or different estimates that we reasonably could have selected would have
had a material impact on our financial condition or results of operations.
Deferred Tax Assets and Income Taxes
. We account for income taxes under the asset and
liability method under which deferred income taxes are provided for the temporary differences
between the financial reporting basis and the tax basis of our assets and liabilities and operating
losses and tax credit carryforwards. This process involves calculating our current tax obligation
or refund and assessing the nature and measurements of temporary differences resulting from
differing treatment of items for tax and accounting purposes. These differences result in deferred
tax assets and liabilities. In each period, we assess the likelihood that our deferred tax assets
will be recovered from existing deferred tax liabilities or future taxable income. If required, we
will recognize a valuation allowance to reduce such deferred tax assets to amounts that are more
likely than not to be ultimately realized. To the extent that we establish a valuation allowance or
change this allowance in a period, we adjust our tax provision or tax benefit in the consolidated
statements of operations. We use our judgment to determine estimates associated with the
calculation of our provision or benefit for income taxes, and in our evaluation of the need for a
valuation allowance recorded against our net deferred tax assets.
During the third quarter of 2009, we evaluated the expected realization of our deferred tax
assets and determined an increase to our valuation allowance of $54.6 million was required of which
$42.2 million was included in the consolidated statement of operations as deferred tax expense.
Factors we considered in making such an assessment included, but were not limited to past
performance, including our recent history of operating results on a U.S. GAAP basis, our recent
history of generating taxable income, our history of recovering net operating loss carryforwards
for tax purposes and our expectation of future taxable income, both considering our past history in
predicting future results and considering current macroeconomic conditions and issues facing our
industry and customers.
Stock-Based Compensation.
We account for stock-based compensation based on fair value of the
options or restricted stock units at the date of grant. Share-based compensation cost is measured
at the grant date and is recognized as expense over the related vesting period. Determining the
fair value of share-based awards at the grant date requires judgment, including estimating future
volatility and expected term. If actual forfeiture results differ significantly from estimates,
stock-based compensation expense and our results of operations could be materially impacted.
Indefinite Lived Intangible Assets.
Our indefinite lived intangible assets are comprised
primarily of trade names, which were acquired in our acquisition of Spacelabs Burdick, Inc. in 2003
and the merger transaction between Quinton Cardiology Systems, Inc. and Cardiac Science, Inc. in
2005. We use our judgment to estimate the fair value of each of these indefinite lived intangible
assets. Our judgment about fair value is based on our expectation of future cash flows and an
appropriate discount rate.
We believe the Burdick and Cardiac Science trade names have indefinite lives and, accordingly,
we do not amortize the trade names. We evaluate this conclusion annually or more frequently if
events and circumstances indicate that the asset(s) might be impaired and make a judgment about
whether there are factors that would limit our ability to benefit from the trade name in the
future. If there were such factors, we would start amortizing the trade name over the expected
remaining period in which we believed it would continue to provide benefit.
Additionally, we periodically evaluate whether the carrying value of our intangible assets
might be impaired. For our trade names, this evaluation is performed annually or more frequently if
events occur that suggest there may be an impairment loss, and involves comparing the carrying
amount to our estimate of fair value.
Valuation of Long-Lived Assets.
We review long-lived assets, such as machinery and equipment,
and intangible assets subject to amortization, for impairment whenever events or changes in
circumstances indicate that the carrying amount of an asset group may not be recoverable.
Recoverability of asset groups to be held and used is measured by a comparison of the
Page 21 of 41
carrying amount of an asset group to estimated undiscounted future cash flows expected to be
generated by the asset group. If the carrying amount of an asset group exceeds its estimated future
cash flows, an impairment charge is recognized on our consolidated statements of operations and as
a reduction to value of the asset group on our condensed consolidated balance sheets. We use our
judgment to estimate the useful lives of our intangible assets subject to amortization and evaluate
the remaining useful lives annually.
Assets to be disposed of would be separately presented in the unaudited condensed consolidated
balance sheet and reported at the lower of the carrying amount or fair value less costs to sell,
and are no longer depreciated. The assets and liabilities of a disposal group classified as held
for sale would be presented separately in the appropriate asset and liability sections of the
unaudited condensed consolidated balance sheet.
Accounts Receivable.
Accounts receivable represent a significant portion of our assets. We
make estimates of the collectability of accounts receivable, including analyzing historical
write-offs, changes in our internal credit policies and customer concentrations when evaluating the
adequacy of our allowance for doubtful accounts. Different estimates regarding the collectability
of accounts receivable may have a material impact on the timing and amount of reported bad debt
expense and on the carrying value of accounts receivable.
Inventories.
Inventories represent a significant portion of our assets. We value inventories
at the lower of cost, on an average cost basis, or market. We regularly perform a detailed analysis
of our inventories to determine whether adjustments are necessary to reduce inventory values to
estimated net realizable value. We consider various factors in making this determination, including
the salability of individual items or classes of items, recent sales history and predicted trends,
industry market conditions and general economic conditions. Different estimates regarding the net
realizable value of inventories could have a material impact on our reported net inventory and cost
of revenues, and thus could have a material impact on the condensed consolidated financial
statements as a whole.
Warranty.
We provide warranty service covering many of the products and systems we sell. We
estimate and accrue for future costs of providing warranty service, which relate principally to the
hardware components of the systems, when the systems are sold. Our estimates are based, in part, on
our warranty claims history and our cost to perform warranty service. Differences could result in
the amount of the recorded warranty liability and cost of revenues if we made different judgments
or used different estimates.
Corrective Action Costs.
We provide for additional warranty costs associated with field
corrective actions and recalls when the likelihood of incurring costs associated with these matters
becomes probable and estimable. We record estimated warranty costs associated with field corrective
actions and recalls as part of cost of revenues on the unaudited condensed consolidated statements
of operations and within corrective action liabilities on the unaudited condensed consolidated
balance sheets. We evaluate the adequacy of our accruals for these initiatives on a regular basis
and will make adjustments to our estimates if facts and circumstances indicate that changes to our
initial estimates would be appropriate.
As of September 30, 2010, we have recorded charges totaling $32.0 million representing the
estimated costs related to the two voluntary corrective actions. We recorded a charge of $18.5
million in the third quarter of 2009, representing the estimated costs of implementing a software
update relating to the voluntary corrective action first announced in November 2009 covering nearly
300,000 AEDs shipped between June 2003 and June 2009, and a charge of $2.5 million in the fourth
quarter of 2009 representing the estimated costs of replacing approximately 12,200 AEDs that were
the subject of the corrective action announced in February 2010. As further described below, we
recorded an additional charge of $11.0 million during the second quarter of 2010 related to the
updated corrective action plan first announced in July 2010.
The costs of these voluntary corrective actions are estimates. Our actual costs incurred to
implement the voluntary corrective actions could vary significantly based on an number of factors,
including the outcome of discussions or negotiations with applicable regulatory bodies in
geographies outside the United States, the number of impacted devices, the customer and
geographical segments related to the impacted devices, the logistical processes we employ to
address the issues, the customer response rate in implementing the corrective action plans, the
level of required follow up with customers, the extent to which we rely on third party assistance
to carry out the corrective actions, the extent to which AED units recovered from affected
customers can be repaired and used as replacement units for other customers, and the length of time
and other resources required to complete the corrective actions, among others.
In February 2010, we received a warning letter from the Food & Drug Administration (“FDA”)
noting, among other things, that the voluntary field corrective action we announced in November
2009 is inadequate since it is intended to improve the products’ ability to detect the potential
component problem, but is not designed to prevent component failure. The FDA letter also asserted
other inadequacies, including our procedures relating to the evaluation, investigation and follow
up of complaints, procedures to verify the effectiveness of corrective and preventative actions and
procedures relating to certain design requirements. In April 2010, the FDA published additional
information regarding our corrective action announced in
Page 22 of 41
November 2009, further clarifying the AED models impacted by the potential component defect
and provided further guidance to users of our affected AEDs. Additionally, the FDA noted that our
software update addresses “some, but not all electrical component defects.”
Following discussions with the FDA, in July 2010, we announced that we will replace
approximately 24,000 AEDs used by certain first responders and medical facilities in the United
States that are included in the customer population covered by the voluntary corrective action
first announced in November 2009. The population of first responders includes police, fire and
ambulance services and medical facilities include hospitals, medical clinics, dialysis centers and
assisted living facilities. We recorded an additional $11.0 million charge during the second
quarter of 2010, representing the estimated costs for repair or replacement of these devices, as
well as an estimate of the cost of repair or replacement of certain devices located outside of the
United States that the Company believes that it is probable it would be required to be performed in
the future.
In July 2010, the FDA published a communication regarding our updated corrective action plan,
citing our plan to repair or replace approximately 24,000 AEDs used by certain first responders and
medical facilities within the United States. The FDA recommended that all other impacted customers
follow the process, as outlined by the Company, for implementing the software update which has been
made available to customers through our website or through a software kit shipped directly to the
customer. With this updated recommendation by the FDA, we believe we have addressed all outstanding
issues raised by the FDA with respect to our previously communicated plan to address potential
component defects through a field software update as originally announced in November 2009.
Software Revenue Recognition.
We account for the licensing of software and arrangements where
software is considered more than incidental to the product as software revenue arrangements. We use
judgment when determining the appropriate accounting for our software revenue arrangements,
including whether an arrangement includes multiple elements, and if so, whether vendor-specific
objective evidence (“VSOE”) of fair value exists for those elements. A portion of software revenue
is recorded as unearned due to undelivered elements including, in some cases, software
implementation and post-delivery support. The amount of revenue allocated to undelivered elements
is based on the sales price of each element when sold separately (VSOE) using the residual method.
Revenue from post-delivery support is recognized over the service period, which is typically a
year and revenue from software implementation services is recognized as the services are provided
(based on VSOE of fair value). When significant implementation activities are required, we
recognize revenue from software and services upon installation. Changes to the elements in a
software arrangement and the ability to identify VSOE of fair value for those elements could
materially impact the amount of earned and unearned revenue.
Revenue Recognition.
Revenue from sales of hardware products is generally recognized when
title transfers to the customer, typically upon shipment. Some of our customers are distributors
that sell goods to third party end users. Except for certain identified distributors where
collection may be contingent on distributor resale, we recognize revenue on sales of products made
to distributors when title transfers to the distributor and all significant obligations have been
satisfied. In making a determination of whether significant obligations have been met, we evaluate
any installation or integration obligations to determine whether those obligations are
inconsequential or perfunctory. In cases where the remaining installation or integration obligation
is not determined to be inconsequential or perfunctory, we defer the portion of revenue associated
with the fair value of the installation and integration obligation until these services have been
completed.
Distributors do not have price protection and generally do not have product return rights,
except if the product is defective upon shipment or shipped in error, and in some cases upon
termination of the distributor agreement. For certain identified distributors where collection may
be contingent on the distributor’s resale, revenue recognition is deferred and recognized on a
“sell through” or cash basis. The determination of whether sales to distributors are contingent on
resale is subjective because we must assess the financial wherewithal of the distributor to pay
regardless of resale. For sales to distributors, we consider several factors, including past
payment history, where available, trade references, bank account balances, Dun & Bradstreet reports
and any other financial information provided by the distributor, in assessing whether the
distributor has the financial wherewithal to pay regardless of, or prior to, resale of the product
and that collection of the receivable is not contingent on resale.
We offer limited volume price discounts and rebates to certain distributors. Volume price
discounts are on a per order basis based on the size of the order and are netted against the
revenue recorded at the time of shipment. We have some arrangements with key distributors that
provide for volume discounts based on meeting certain quarterly or annual purchase levels or based
on sales volumes of certain of our products during a defined period in which we offer a promotion.
Rebates are paid quarterly or annually and are accrued for as incurred.
We consider program management packages and training and other services as separate units of
accounting when sold with an AED based on the fact that the items have value to the customer on a
stand alone basis and could be acquired from
Page 23 of 41
another vendor. Fair value is determined to be the price at which they are sold to customers
on a stand alone basis. Training revenue is deferred and recognized at the time the training
occurs. AED program management services revenue, pursuant to annual or multi-year agreements that
exist with some customers, is deferred and amortized on a straight-line basis over the related
contract period.
We offer optional extended service contracts to customers. Fair value is determined to be the
price at which they are sold to customers on a stand alone basis. Service contract revenues are
recognized on a straight-line basis over the term of the extended service contracts, which
generally begin after the expiration of the original warranty period. For services performed, other
than pursuant to warranty and extended service contract obligations, revenue is recognized when the
service is performed and collection of the resulting receivable is reasonably assured.
Recent Accounting Pronouncements
In January 2010, the FASB issued guidance to amend the disclosure requirements related to
recurring and nonrecurring fair value measurements. The guidance requires new disclosures on the
transfers of assets and liabilities between Level 1 (quoted prices in active market for identical
assets or liabilities) and Level 2 (significant other observable inputs) of the fair value
measurement hierarchy, including the reasons and the timing of the transfers. Additionally, the
guidance requires a roll forward of activities on purchases, sales, issuance, and settlements of
the assets and liabilities measured using significant unobservable inputs (Level 3 fair value
measurements). The guidance became effective for us with the reporting period beginning January 1,
2010, except for the disclosure on the roll forward activities for Level 3 fair value measurements,
which will become effective for us with the reporting period beginning January 1, 2011. Other than
requiring additional disclosures, adoption of this new guidance did not and will not have a
material impact on our consolidated financial statements.
In September 2009, the Emerging Issues Task Force (EITF) reached a consensus related to
revenue arrangements with multiple deliverables, which the FASB then issued as an Accounting
Standards Update (“ASU”), 2009-13. The ASU amends Accounting Standards Codification (“ASC”)
Subtopic 605-25, “Revenue Recognition — Multiple Element Arrangements” and provides application
guidance on whether multiple deliverables exist in a revenue arrangement, how the deliverables
should be separated and how the consideration should be allocated to one or more units of
accounting. This ASU establishes a selling price hierarchy for determining the selling price of a
deliverable based on vendor-specific objective evidence, if available, third-party evidence, or
estimated selling price if neither vendor-specific nor third-party evidence is available. The ASU
can be applied on a prospective basis or in certain circumstances on a retrospective basis. We plan
to adopt this ASU on a prospective basis beginning January 1, 2011. We believe the adoption of this
ASU may have an impact on our financial position and results of operations; however, we are
uncertain whether the impact will be material. We are continuing to evaluate this ASU as of
September 30, 2010.
In September 2009, the EITF reached a consensus related to revenue arrangements that include
software elements, which the FASB issued as ASU, 2009-14. The ASU amends ASC Subtopic 985-605,
“Software — Revenue Recognition.” Previously, companies that sold tangible products with “more
than incidental” software were required to apply software revenue recognition guidance. This
guidance often delayed revenue recognition for the delivery of the tangible product. Under the new
guidance, tangible products that have software components that are “essential to the functionality”
of the tangible product will be excluded from the software revenue recognition guidance. The new
guidance will include factors to help companies determine what is “essential to the functionality.”
Software-enabled products will now be subject to other revenue guidance and will likely follow the
guidance for multiple deliverable arrangements issued by the FASB in September 2009 in ASU 2009-13.
The new guidance is to be applied on a prospective basis for revenue arrangements entered into or
materially modified in fiscal years beginning on or after June 15, 2010, with earlier application
permitted. We plan to adopt this ASU on a prospective basis beginning January 1, 2011. We believe
the adoption of this ASU may have an impact on our financial position and results of operations;
however, we are uncertain whether the impact will be material. We are continuing to evaluate this
ASU as of September 30, 2010.
Pending Merger Transaction
As further described in Note 2 to the unaudited condensed consolidated financial statements,
On October 19, 2010, we entered into the Merger Agreement with Opto Circuits and Merger Sub under
which Opto Circuits agreed to acquire all of the outstanding shares of our common stock for $2.30
per share. The Offer commenced on November 1, 2010, and unless extended by Opto Circuits, will
expire on November 30, 2010. The aggregate value of the proposed transaction is approximately $58.0
million.
Under certain circumstances specified in the Merger Agreement, the Company may be required to
pay Opto Circuits a termination fee of $1.3 million if the Merger is not commenced. Those
circumstances include (i) termination by Opto Circuits if the Company’s board changes its
recommendation with respect to the transaction, approves or recommends any alternative acquisition
proposal, or permits the Company to enter into an agreement related to an alternative acquisition
proposal and
Page 24 of 41
(ii) termination by the Company if the Company’s board approves an acquisition proposal determined
to be a superior proposal provided the acquisition proposal was not received in violation of
restrictions imposed under the Merger Agreement on the solicitation of third-party proposals to
acquire the Company or its assets and certain procedural requirements were satisfied. If Opto
Circuits terminates the Merger Agreement because the Company breaches or fails to perform any of
its representations, warranties, covenants or other agreements contained in the Merger Agreement,
the Company may be required to pay Opto Circuits an amount equal to $0.3 million to reimburse Opto
Circuits for its expenses incurred in connection with the Merger Agreement. In addition, the Merger
Agreement provides that if the Company terminates the Merger Agreement because of a failure by
Merger Sub to (i) commence the Offer in violation of the Merger Agreement or (ii) accept for
payment and to purchase validly tendered shares pursuant to the Offer, the Company may elect to
require Opto Circuits to pay a termination fee equal to $1.3 million or pursue any other remedy
available at law or in equity.
If the closing conditions associated with the Merger Agreement are not satisfied the market
price of our common stock could be adversely impacted and we may not be able to achieve
pre-transaction levels of activity in various functions, including sales of our products, among
others. Under this scenario, we will continue to closely monitor our remaining obligations
associated with our ongoing field corrective actions and our operating expenses in light of our
actual and anticipated future revenues and gross profit. We may need to take additional steps to
reduce operating expenses in the future if our revenues and gross profit do not grow over time.
However, we expect to see operating expenses decrease during 2011 as we complete our planned
product launches and other ongoing initiatives near completion by the end of 2010. Additionally, we
expect lower operating expenses beyond 2010 in relation to legal and other advisory fees which will
decline upon consummation of the pending merger agreement with Opto Circuits. Further, we expect to
see modest revenue growth in the fourth quarter of 2010. As of September 30, 2010, we had $6.7
million of cash and cash equivalents on our unaudited condensed consolidated balance sheet and have
not borrowed under our amended line of credit agreement with Silicon Valley Bank. As such, we
expect our existing cash and cash equivalents as well as our availability under our $15.0 million
line of credit to be sufficient to allow us to meet working capital needs and fulfill our remaining
obligations under our field corrective actions for at least the next twelve months. See further
discussion under Liquidity and Capital Resources below.
Results of Operations
Summary of Results for the Three and Nine Months Ended September 30, 2010
|
|
|
•
|
|
We generated revenue of $34.5 million for the three month period ended September 30,
2010, representing a decrease of approximately 11% over the three month period ended
September 30, 2009. This decrease was due mostly to a reduction in AED sales in Japan
and, to a lesser extent, softness in the market for medical devices, among other
factors.
|
|
|
|
|
|
•
|
|
We generated revenue of $103.7 million for the nine month period ended September 30,
2010, representing a decrease of approximately 10% over the nine month period ended
September 30, 2009. This decrease was also due mostly to reductions in AED sales in
Japan and, to a lesser extent, softness in the market for medical devices.
|
|
|
|
|
|
•
|
|
We recorded a charge of $11.0 million during the nine month period ended September
30, 2010 related to an estimate of additional costs associated with our updated plan to
repair or replace approximately 24,000 AEDs used by first responders and medical
facilities in the United States and an estimate of the cost of repair or replacement of
certain devices located outside of the United States that the Company believes that it
is probable that it would be required to be performed in the future.
|
|
|
|
|
|
•
|
|
We incurred a net loss of $6.4 million and $33.5 million for the three and nine month
periods ended September 30, 2010, respectively, compared a net loss of $66.5 million and
$69.2 million for the three and nine month periods ended September 30, 2009,
respectively.
|
|
|
|
|
|
•
|
|
We used cash from operations of $4.0 million and $18.4 million for the three and nine
month periods ended September 30, 2010, respectively, compared to cash used in
operations of $5.1 million and $0.9 million for the three and nine month periods ended
September 30, 2009, respectively.
|
|
|
|
|
|
•
|
|
We successfully negotiated an amendment to our line of credit agreement with Silicon
Valley Bank which was finalized in July 2010, increasing the amount available for
borrowing to $15.0 million. This agreement expires in July 2011.
|
|
|
|
|
|
•
|
|
We introduced multiple cardiac monitoring products, including two new Cardiac Stress
Systems.
|
Page 25 of 41
Looking Forward
We expect revenue for the full year 2010 to be down slightly compared to the prior year, with
decreases in both defibrillation and cardiac monitor revenues; service revenue will likely be flat
or increase slightly compared to the prior year.
We expect our cardiac monitoring revenues to grow during the fourth quarter of 2010 as a
result of recently announced and planned new product introductions and as a result of improved
marketing and demand generation capabilities which were put into place during 2009. Additionally,
cardiac monitoring revenues have been weakened in 2010 due to general market weakness for many of
our products as well disruption in the marketplace related to rumors of a potential transaction
involving the sale of all or part of our Company. With the announcement of our pending transaction
with Opto Circuits, we expect the distractions in the marketplace to subside and our cardiac
monitoring revenues will grow in the fourth quarter of 2010 as we continue to develop and introduce
new products and as we experience increased sales related to new products previously introduced in
2010. If we are unable to successfully execute on our new product development plans or if the
market is not receptive to our new products, our cardiac monitoring revenues could be adversely
affected in future periods.
We expect our defibrillation revenue to grow moderately during the fourth quarter of 2010
relative to the first three quarters of 2010, although we expect our full year defibrillation
revenue to decline relative to the prior year. We are continuing to face challenges in the market
place related to regulatory and quality issues associated with our AEDs as well as increased
competition due to the re-entry of a significant competitor into the market earlier in 2010.
Additionally, we continue to face challenges in the global economy, as well as declines in our
defibrillation revenue in Japan, due to the termination of our OEM Supply and Purchase Agreement
(the “OEM Agreement”) with Nihon Kohden Corporation (“Nihon Kohden”), our former exclusive
distributor of AEDs in Japan, in June 2010. Although, we have entered into an agreement with a new
distribution partner in Japan, we do not expect to launch a co-branded AED with this distribution
partner in Japan until the second half of 2011. With this agreement in place, we expect to begin
re-establishing our presence in the Japanese market in the latter half of 2011. If we are not
successful in launching a co-branded AED with our new distribution partner or if our new partner is
not successful in recapturing market share in Japan, our international defibrillation revenue
growth could be negatively impacted in future periods.
We expect the global market for AEDs to grow over time, as awareness of the benefits of AEDs
continues to increase and as the prevalence of mandates requiring deployment of AEDs in public
venues continues to rise. As the market continues to grow and as we overcome what we believe to be
temporary challenges associated with our regulatory and quality issues, we believe that we will be
able to grow our defibrillation revenues over time, both domestically and overseas. We believe our
direct presence in several countries in Europe will result in continued growth in sales in these
markets over time, though again we may experience softness in the near term due to the quality and
regulatory issues, the return of a competitor to the market and continued general economic factors.
We are in the process of working with the FDA on our recent regulatory and quality matters
which were raised in the FDA’s warning letter we received in February 2010. As part of this
process, in July 2010 we announced our plans to repair or replace approximately 24,000 AEDs
deployed in high risk and/or frequent use settings, such as with first responders and medical
facilities in the United States. This represents a subset of the customers covered by the voluntary
corrective action we originally announced in November 2009, which affected nearly 300,000 AED
devices shipped between June 2003 and June 2009. The FDA also provided an updated public
communication regarding these plans in July 2010 that recommended that all other customers impacted
by our voluntary corrective action announced in November 2009, who are not first responders or
medical facilities, follow our instructions for installing the software update on their AEDs which
is available on our website or by ordering a software kit from us. With this updated recommendation
by the FDA, we believe we have addressed all outstanding issues with our previously communicated
plan to address potential component defects through a field software update as originally announced
in November 2009.
We are still addressing the remaining matters noted by the FDA in its February 2010 warning
letter. These include assertions by the FDA that our procedures relating to the evaluation,
investigation and follow up of complaints, procedures to verify the effectiveness of corrective and
preventative actions, and procedures relating to certain design requirements are inadequate. To the
extent we are unable to address these matters to the satisfaction of the FDA, we could be
required to temporarily cease shipments of our products, which could ultimately impact
our ability to fulfill customer orders and maintain market share. Further, to the extent we are not
able to satisfy the FDA, we may be subject to further regulatory action by the FDA, including
seizure, injunction and/or civil monetary penalties. Any such regulatory
actions could significantly disrupt our ongoing business and operations and have a material
adverse effect on our financial position and results of operations. While we are
optimistic that we will be successful in addressing our ongoing regulatory matters with the FDA,
our AED revenues may continue to be adversely affected by these circumstances during 2010, and
potentially beyond.
Page 26 of 41
Our future gross profits and gross margins will be dependent upon our overall revenue volume,
by product mix, market pricing and our costs. Increasing volumes provide economies of scale and
tend to enhance our gross margin. AEDs generally have a higher gross margin than our monitoring
products. Accordingly, if our product mix shifts toward a relatively higher proportion of AEDs, our
overall gross margins will increase and if the mix shifts toward a relatively higher proportion of
monitoring products, our overall gross margins are likely to decline. Market pricing for our
products has declined slightly in recent years. As we face challenges in retaining our AED market
share in the face of circumstances discussed elsewhere in this report, we may experience additional
pricing pressure, which may, in turn, result in a reduction of our gross margins. Finally, any
increases in costs, including any unexpected additional costs associated with the ongoing or new
corrective actions or recalls relating to our AEDs, or any other quality issues related to our
products that may arise in the future, would also negatively affect our gross margins. Our actual
gross profit and margins will be dependent on the aggregation of these and other factors.
We expect our operating expenses to decrease during 2011, although operating expenses will
likely be slightly higher for the full year 2010 as compared to the prior year. Our operating
expenses have increased in 2010 due largely to increased costs associated with regulatory affairs
and quality assurance as we continue to upgrade our internal capabilities in these areas. We have
also incurred higher research and development and marketing expenses associated with new product
development initiatives and planned product launches during the second half of 2010. We expect to
see our operating expenses associated with product development and marketing activities to decrease
in 2011 as we complete our planned product launches and other ongoing initiatives near completion
during 2010. However, we have incurred significant legal and advisory fees during the third quarter
of 2010 related to our pending transaction with Opto Circuits as well as other strategic
initiatives. We expect our legal and advisory fees related to these matters, as well as legal costs
needed to address pending litigation matters discussed elsewhere in this report, to continue during
the fourth quarter of 2010 and potentially beyond 2010.
We expect to realize an operating loss in 2010 due to softness in our revenue, our ongoing
investments in new product development and product launches and as we make improvements to our
quality and information systems. In addition, the accrual of $11.0 million related to our updated
corrective action announced in July 2010, will add significantly to our operating loss for the
year.
Revenues
We derive our revenues primarily from the sale of our non-invasive cardiology products and
related consumables, and to a lesser extent, from services related to these products, including
training. We categorize our revenues as (1) defibrillation products, which include our AEDs,
hospital defibrillators and related accessories; (2) cardiac monitoring products, which include
capital equipment, software products and related accessories and supplies; and (3) service, which
includes service contracts, CPR/AED training services, AED program management services, equipment
maintenance and repair, replacement part sales and other services. We derive a portion of our
service revenue from sales of separate extended maintenance arrangements. We defer and recognize
these revenues over the applicable maintenance period.
Revenues for the three and nine month periods ended September 30, 2010 and 2009 were as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
% Change
|
|
|
Ended
|
|
|
Nine Months Ended
|
|
|
% Change
|
|
|
Nine Months Ended
|
|
|
(dollars in thousands)
|
|
September 30, 2010
|
|
|
2009 to 2010
|
|
|
Septemebr 30, 2009
|
|
|
September 30, 2010
|
|
|
2009 to 2010
|
|
|
September 30, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Defibrillation products
|
|
$
|
18,149
|
|
|
|
(16.2
|
%)
|
|
$
|
21,646
|
|
|
$
|
53,062
|
|
|
|
(13.6
|
%)
|
|
$
|
61,413
|
|
|
% of revenue
|
|
|
52.7
|
%
|
|
|
|
|
|
|
55.7
|
%
|
|
|
51.2
|
%
|
|
|
|
|
|
|
53.6
|
%
|
|
Cardiac monitoring products
|
|
|
11,836
|
|
|
|
(9.0
|
%)
|
|
|
13,000
|
|
|
|
37,362
|
|
|
|
(6.9
|
%)
|
|
|
40,151
|
|
|
% of revenue
|
|
|
34.3
|
%
|
|
|
|
|
|
|
33.4
|
%
|
|
|
36.0
|
%
|
|
|
|
|
|
|
35.0
|
%
|
|
Service
|
|
|
4,495
|
|
|
|
6.1
|
%
|
|
|
4,238
|
|
|
|
13,266
|
|
|
|
1.3
|
%
|
|
|
13,098
|
|
|
% of revenue
|
|
|
13.0
|
%
|
|
|
|
|
|
|
10.9
|
%
|
|
|
12.8
|
%
|
|
|
|
|
|
|
11.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
$
|
34,480
|
|
|
|
(11.3
|
%)
|
|
$
|
38,884
|
|
|
$
|
103,690
|
|
|
|
(9.6
|
%)
|
|
$
|
114,662
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Defibrillation products revenue decreased 16% for the three month period ended September
30, 2010 from the comparable period in 2009 due primarily to declines in international sales of
30%, or $3.1 million, including a decrease of $2.9 million due to a significant decline in AED shipments to our former exclusive distributor
in Japan, Nihon Kohden, as compared to the three month period ended September 30, 2009.
Defibrillation products revenue decreased 14% for the nine month period ended September 30,
2010 from the comparable period in 2009 due primarily to declines in international sales of 26%,
including a decrease of approximately
Page 27 of 41
23%, or $7.5 million, due to a significant decline in AED shipments to Nihon Kohden during the first nine months of 2010 as compared to the same period in
2009. The remainder of the decrease in defibrillation products revenue was due primarily to the
adverse impact and publicity associated with the FDA warning letter we received in February 2010 as
well as continued weakness in the economy which provides challenges for our AED business both
domestically and internationally.
Cardiac monitoring products revenue decreased by 9% and 7% for the three and nine month
periods ended September 30, 2010, respectively, from the comparable periods in 2009 due primarily
to general weakness in the market as our physician customers defer purchases of capital equipment
in the face of continuing uncertainty about reimbursement levels for procedures used in providing
patient care and, to a lesser extent to distractions in the market place with both customers and
our sales force related to rumors regarding the possible sale of all or part of our Company. In
addition, we were not able to ship approximately $1.0 million in recently introduced cardiac
monitoring products that were ordered for delivery during the third quarter due to initial delays
in availability of components in quantities sufficient to meet demand. We expect to ship the
backordered products during the fourth quarter of 2010.
Service revenue increased 6% and 1% for the three month and nine month periods ended September
30, 2010, respectively, from the comparable periods in 2009 due primarily to implementation of a
new third party sales strategy which is successfully generating new and renewal service contracts
and other training programs with our customers.
Gross Profit
Gross profit is revenues less the cost of revenues. Cost of revenues consists primarily of the
costs associated with manufacturing, assembling and testing our products, amortization of certain
intangibles, overhead costs, compensation, including stock-based compensation and other costs
related to manufacturing support and logistics. Cost of revenues also includes costs associated
with corrective actions and recalls associated with our products. We rely on third parties to
manufacture certain of our product components. Accordingly, a significant portion of our cost of
revenues consists of payments to these manufacturers. Cost of service revenue consists of customer
support costs, training and professional service expenses, parts and compensation. Our hardware
products include a warranty period that includes factory repair services or replacement parts. We
accrue estimated expenses for warranty obligations at the time products are shipped.
Gross profit for the three and nine month periods ended September 30, 2010 and 2009 was as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
% Change
|
|
|
Ended
|
|
|
Nine Months Ended
|
|
|
% Change
|
|
|
Nine Months Ended
|
|
|
(dollars in thousands)
|
|
September 30, 2010
|
|
|
2009 to 2010
|
|
|
September 30, 2009
|
|
|
September 30, 2010
|
|
|
2009 to 2010
|
|
|
September 30, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Products
|
|
$
|
14,741
|
|
|
|
(15.5
|
%)
|
|
$
|
17,452
|
|
|
$
|
44,104
|
|
|
|
(15.7
|
%)
|
|
$
|
52,303
|
|
|
% of products revenue
|
|
|
49.2
|
%
|
|
|
|
|
|
|
50.4
|
%
|
|
|
48.8
|
%
|
|
|
|
|
|
|
51.5
|
%
|
|
Corrective action costs
|
|
|
—
|
|
|
|
n/m
|
|
|
|
(18,500
|
)
|
|
|
(11,000
|
)
|
|
|
40.5
|
%
|
|
|
(18,500
|
)
|
|
Service
|
|
|
1,346
|
|
|
|
17.8
|
%
|
|
|
1,143
|
|
|
|
3,768
|
|
|
|
1.2
|
%
|
|
|
3,722
|
|
|
% of service revenue
|
|
|
29.9
|
%
|
|
|
|
|
|
|
27.0
|
%
|
|
|
28.4
|
%
|
|
|
|
|
|
|
28.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total gross profit
|
|
$
|
16,087
|
|
|
|
16833.7
|
%
|
|
$
|
95
|
|
|
$
|
36,872
|
|
|
|
(1.7
|
%)
|
|
$
|
37,525
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of total revenue
|
|
|
46.7
|
%
|
|
|
|
|
|
|
0.2
|
%
|
|
|
35.6
|
%
|
|
|
|
|
|
|
32.7
|
%
|
Gross profit from products decreased for the three and nine month periods ended September
30, 2010 from the comparable periods in 2009 due in part to significant declines in both revenue
and gross profit related to lower AED sales to our former exclusive distributor in Japan, Nihon
Kohden. Additionally, gross margins from products decreased during the three and nine month periods
ended September 30, 2010 from the comparable periods in 2009 due primarily to changes in product
mix, cost increases in certain product components, and inefficiencies in our factory due to our
recent recall activities.
Charges associated with corrective action costs of $11.0 million incurred in the nine month
period ended September 30, 2010 represent estimated additional costs associated with our voluntary
corrective action related to our AEDs which we previously announced in November 2009. During the
second quarter of 2010, we recorded an additional charge of $11.0 million related to our estimated
costs associated with repairing or replacing approximately 24,000 AEDs used by certain first
responders and medical facilities in the United States, and also includes an estimate of costs to
repair or replace certain devices located outside of the United States which we believe that it is
probable that we would be required to perform in the future. This represents a subset of the
customer based affected by the November 2009 corrective action. The estimated costs associated with
repairing or replacing these devices consist primarily of materials, shipping and other logistical
costs required to manage the corrective actions and replace the units with our customers. There was
a similar charge during the three and
Page 28 of 41
nine month periods ended September 30, 2009 of $18.5 million. See the “Looking Forward” section above for additional information regarding the impact of possible
corrective actions on gross profit in future periods.
Gross profit from service remained flat for the nine months ended September 30, 2010 from the
comparable period in 2009. Gross profit from service increased for the three month period ended
September 30, 2010 from the comparable period in 2009 due primarily to increased program management
services associated with our AEDs.
Operating Expenses
Operating expenses include expenses related to research and development, sales, marketing and
other general and administrative expenses required to run our business, including stock-based
compensation.
Research and development expenses consist primarily of salaries and related expenses for
development and engineering personnel, fees paid to consultants, and prototype costs related to the
design, development, testing and enhancement of products. Several components of our research and
development activities require significant funding, the timing of which can cause significant
quarterly variability in our expenses.
Sales and marketing expenses consist primarily of salaries, commissions and related expenses
for personnel engaged in sales, marketing and sales support functions as well as costs associated
with corporate and product branding, promotional and other marketing activities.
General and administrative expenses consist primarily of employee salaries and related
expenses for executive, finance, accounting, information technology, regulatory affairs, quality
assurance and human resources personnel as well as bad debt expense, professional fees, legal fees,
and other corporate expenses.
Operating expenses for the three and nine month periods ended September 30, 2010 and 2009 were
as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% Change 2010 to
|
|
|
|
|
|
|
|
|
|
|
% Change
|
|
|
|
|
|
|
|
Three Months
|
|
|
2009
|
|
|
Three Months
|
|
|
Nine Months
|
|
|
2010 to 2009
|
|
|
Nine Months
|
|
|
|
|
Ended
|
|
|
(Increase) /
|
|
|
Ended
|
|
|
Ended
|
|
|
(Increase) /
|
|
|
Ended
|
|
|
(dollars in thousands)
|
|
September 30, 2010
|
|
|
Decrease
|
|
|
September 30, 2009
|
|
|
September 30, 2010
|
|
|
Decrease
|
|
|
September 30, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
4,266
|
|
|
|
0.1
|
%
|
|
$
|
4,270
|
|
|
$
|
13,100
|
|
|
|
(15.3
|
%)
|
|
$
|
11,358
|
|
|
% of total revenue
|
|
|
12.4
|
%
|
|
|
|
|
|
|
11.0
|
%
|
|
|
12.6
|
%
|
|
|
|
|
|
|
9.9
|
%
|
|
Sales and marketing
|
|
|
11,215
|
|
|
|
5.9
|
%
|
|
|
11,923
|
|
|
|
36,429
|
|
|
|
(5.9
|
%)
|
|
|
34,392
|
|
|
% of total revenue
|
|
|
32.5
|
%
|
|
|
|
|
|
|
30.7
|
%
|
|
|
35.1
|
%
|
|
|
|
|
|
|
30.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative
|
|
|
7,058
|
|
|
|
(7.4
|
%)
|
|
|
6,571
|
|
|
|
20,652
|
|
|
|
(11.4
|
%)
|
|
|
18,536
|
|
|
% of total revenue
|
|
|
20.5
|
%
|
|
|
|
|
|
|
16.9
|
%
|
|
|
19.9
|
%
|
|
|
|
|
|
|
16.2
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
$
|
22,539
|
|
|
|
1.0
|
%
|
|
$
|
22,764
|
|
|
$
|
70,181
|
|
|
|
(9.2
|
%)
|
|
$
|
64,286
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of total revenue
|
|
|
65.4
|
%
|
|
|
|
|
|
|
58.5
|
%
|
|
|
67.7
|
%
|
|
|
|
|
|
|
56.1
|
%
|
Research and development costs remained relatively flat for the three month period ended
September 30, 2010 as compared to the same period in the prior year. The increase in research and
development expenses for the nine month periods ended September 30, 2010 from the comparable period
in 2009 was due primarily to outside design costs and other professional services relating to new
cardiac monitoring product introductions during the third quarter of 2010 and planned product
introductions during the fourth quarter of 2010.
The decrease in sales and marketing expenses for the three month period ended September 30,
2010 from the comparable period in 2009 was due primarily to decreased commissions due to lower
sales during the period as well as reduced travel and related expenses associated with cost control
measures implemented in 2010.
The increase in sales and marketing expenses for the nine month period ended September 30,
2010 from the comparable period in 2009 was due primarily to increased consulting and business
development fees for lead generation activities and promotions for product launches during the
second half of 2010, as well as increased salaries and benefits associated with general
compensation increases and changes in staffing mix. Additionally, sales and marketing expenses for
the nine month period ended September 30, 2010 included labor and severance costs associated with
our former Senior Vice President of Sales and Marketing for which no similar charges existed in the
prior year.
Page 29 of 41
The increase in general and administrative expenses for the three and nine month periods
ended September 30, 2010 from the comparable periods in 2009 was due primarily to professional fees
and other costs associated with our efforts to sell all or part of our company, which culminated in
the entry into the Merger Agreement with Opto Circuits as well as increased salaries and benefits
resulting from general compensation increases and higher costs associated with certain employee
retention plans. Additionally, general and administrative expenses increased for the nine month
period ended September 30, 2010 from the comparable period in 2009 due to increased headcount and
consulting costs associated with expanding our internal capabilities in our regulatory affairs and
quality assurance functions as well as increased costs associated with enhancing our information
technology infrastructure and capacity.
Other Income and Expense
Other income (loss), net was income of $0.2 million for both the three month periods ended
September 30, 2010 and 2009. Other income, net for the three month period ended September 30, 2010
consisted primarily of $0.2 million of income from royalty agreements. Other income, net for the
three month period ended September 30, 2009 consisted primarily of net foreign currency exchange
gains of $0.1 million and income from royalty agreements of $0.1 million.
Other income (loss), net was income of $0.3 million for the nine month period ended September
30, 2010 as compared to income of $0.6 million during the same period in 2009. Other income, net
for the nine month period ended September 30, 2010 consisted primarily of income from royalty
agreements of $0.4 million and other miscellaneous income items totaling $0.1 million, partially
offset by net foreign currency exchange losses of ($0.2) million. Other income, net for the nine
month period ended September 30, 2009 consisted primarily of net foreign currency exchange gains of
$0.3 million and income from royalty agreements of $0.3 million.
Income Taxes
During the three and nine month periods ended September 30, 2010, we recorded income tax
expense of $0.1 million and $0.3 million, respectively, compared to income tax expense of $43.9
million and $42.6 million in the three and nine month periods ended September 30, 2009,
respectively. Income tax expense for the three and nine month periods ended September 30, 2009
included a non-cash charge of $44.0 million to increase the valuation allowance associated with our
deferred tax assets. Our worldwide effective tax rate, excluding the U.S. jurisdictions, for the
nine month period ended September 30, 2010 was 34% as compared to a worldwide effective tax rate of
163% for the same period in the prior year.
The difference in our estimated worldwide effective tax rate for the nine month period ended
September 30, 2010 as compared to the same period in the prior year is due largely to the non-cash
charge in the prior year to increase the valuation allowance against our deferred tax assets. In
addition, we have excluded the U.S. jurisdiction from our estimate of the worldwide effective tax
rate for the full year of 2010 and for the year to date worldwide effective tax rate for the nine
month period ended September 30, 2010. We excluded the U.S. jurisdiction based on authoritative
guidance that a jurisdiction should be excluded from the worldwide effective tax rate and
calculated separately to the extent such jurisdiction anticipates an ordinary loss for the year or
has ordinary losses for the year to date for which no tax benefit can be recognized. The U.S
jurisdiction was not excluded from our estimated worldwide effective tax rate for the same period
in the prior year, and our estimated worldwide effective tax rate was based on estimates of pre-tax
income or losses for the full year, applicable tax rates in jurisdictions in which income and
losses were expected to be generated, including the U.S., and expected U.S. federal and state tax
credits.
The increase of our worldwide effective tax rate from 29% to 34% between the six month period
ended June 30, 2010 and the nine month period ended September 30, 2010 is due primarily to an
insignificant discrete expense item in the amount of $0.04 million in the current period associated
with an unfavorable resolution of a tax matter in a foreign jurisdiction, which is partially offset
by favorable true-ups related to tax filings in foreign jurisdictions.
We have maintained the valuation allowance on our domestic deferred tax assets as the evidence
available at September 30, 2010 does not warrant a change and as such, we believe it is not more
likely than not that we will be able to realize the benefits of the losses anticipated in the
current year. If in future periods we generate taxable income, the valuation allowance may be
released in part, or in total, when it becomes more likely than not that the deferred tax assets
will be realized. Until this occurs, we will continue to record a deferred tax benefit for any
domestic losses that might be incurred in the future and we will record deferred tax expense to
increase the recorded valuation allowance for any such additional losses. The tax expense recorded
in the quarter ended September 30, 2010 relates primarily to our foreign operations, which
generally are forecasted to be marginally profitable during 2010.
Page 30 of 41
Liquidity and Capital Resources
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months
|
|
|
|
|
|
|
Three Months
|
|
|
Nine Months
|
|
|
|
|
|
|
Nine Months
|
|
|
|
|
Ended
|
|
|
% Change
|
|
|
Ended
|
|
|
Ended
|
|
|
% Change
|
|
|
Ended
|
|
|
(dollars in thousands)
|
|
September 30, 2010
|
|
|
2009 to 2010
|
|
|
September 30, 2009
|
|
|
September 30, 2010
|
|
|
2009 to 2010
|
|
|
September 30, 2009
|
|
|
|
|
Cash used in operating activities
|
|
$
|
(3,952
|
)
|
|
|
22.3
|
%
|
|
$
|
(5,085
|
)
|
|
$
|
(18,442
|
)
|
|
|
(2046.9
|
%)
|
|
$
|
(859
|
)
|
|
Cash used in investing activities
|
|
|
(209
|
)
|
|
|
84.8
|
%
|
|
|
(1,377
|
)
|
|
|
(1,552
|
)
|
|
|
48.3
|
%
|
|
|
(3,002
|
)
|
|
Cash provided by (used in) financing
activities
|
|
|
(120
|
)
|
|
|
(181.1
|
%)
|
|
|
148
|
|
|
|
(62
|
)
|
|
|
(107.9
|
%)
|
|
|
787
|
|
|
Effect of exchange rates on cash and
cash equivalents
|
|
|
86
|
|
|
|
16.2
|
%
|
|
|
74
|
|
|
|
(120
|
)
|
|
|
(355.3
|
%)
|
|
|
47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash and cash equivalents
|
|
$
|
(4,195
|
)
|
|
|
(32.8
|
%)
|
|
$
|
(6,240
|
)
|
|
$
|
(20,176
|
)
|
|
|
(566.5
|
%)
|
|
$
|
(3,027
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash used in operating activities of $4.0 million for the three month period ended
September 30, 2010 resulted from our net loss of $6.3 million plus adjustments of $2.1 million for
net non-cash items included in our net loss and a net decrease in working capital of $0.2 million.
The net non-cash items included in our net loss related primarily to depreciation and amortization
and stock-based compensation. The net decrease in working capital of $0.2 million was due primarily
to a decrease in accounts receivable of $3.2 million, a decrease in inventory of $0.6 million, an
increase in accrued liabilities of $0.6 million and a decrease in prepaid expenses of $0.5 million,
substantially offset by cash used to satisfy our ongoing corrective action liabilities of $2.7
million and a decrease in accounts payable of $2.0 million. Cash used in operating activities of
$5.1 million for the three month period ended September 30, 2009 resulted from our net loss of
$66.4 million and an increase in accounts receivable of $5.6 million, substantially offset by
non-cash items included in net income of $46.2 million consisting primarily of the increase in our
valuation allowance on our deferred tax assets of $44.0 million. Additionally, cash used in
operations for the three month period ended September 30, 2009 included the unfavorable impact of
$18.5 million associated with our cost estimate for our AED field corrective action announced in
November 2009.
Cash used in operating activities of $18.4 million for the nine month period ended September
30, 2010 resulted from our net loss of $33.3 million plus adjustments of $6.6 million for net
non-cash items included in our net loss and a net decrease in working capital of $8.3 million. The
net non-cash items included in our net loss related primarily to depreciation and amortization and
stock-based compensation. The net decrease in working capital of $8.3 million was due primarily to
an increase in our corrective action liabilities associated with the additional $11.0 million
estimate for costs to repair or replace approximately 24,000 AEDs in the United States and
potentially outside of the United States which was accrued during the second quarter of 2010.
Additionally, the net decrease in working capital was due to a decrease in accounts receivable of
$3.2 million, an increase of accrued liabilities of $1.2 million and an increase in prepaid
expenses of $1.2 million, substantially offset by cash used in the amount of $7.5 million used to
satisfy our ongoing corrective action liabilities. Cash used in operating activities of $0.9
million for the nine month period ended September 30, 2009 resulted from our net loss of $68.7
million substantially offset non-cash items included in net income, excluding cash, of $48.8
million and increases in current liabilities, most notably the $18.5 million accrued liability for
our corrective action liabilities, and other working capital fluctuations. The net non-cash items
included in net income related primarily to the increase in our valuation allowance on deferred tax
assets and to a lesser extent to depreciation and amortization and stock-based compensation.
We anticipate that we will continue to use cash in operations during the fourth quarter of
2010 due in part to our anticipation of operating losses for the quarter and in connection with
significant legal and advisory fees relating to our pending merger with Opto Circuits.
Additionally, we will continue to use cash to satisfy our obligations associated with our ongoing
corrective action liabilities associated with our AEDs described elsewhere in this report. See Note
4 (Corrective Action Liabilities) to the unaudited condensed consolidated financial statements. Our
use of cash to fund operations may be further impacted by other general business factors such as
our ability to successfully sell our products and deliver our services, collect our accounts
receivables, optimize lead times and inventory levels, and manage our expenses.
Net cash used in investing activities for the three and nine month periods ended September 30,
2010 and September 30, 2009 consisted of payments for capital expenditures related primarily to
investments in information technology and new manufacturing equipment, including tooling for
products under development.
Net cash provided by (used in) financing activities for the three and nine month periods ended
September 30, 2010 and 2009 consisted of proceeds from exercises of stock options and sales of
common stock under our Employee Stock Purchase Plan, less required minimum tax withholdings on
restricted stock awards remitted to taxing authorities. Additionally, net cash
used in financing activities for the three and nine months ended September 30, 2010 included
$0.2 million of debt issuance costs associated with our amended line of credit agreement with
Silicon Valley Bank. There were no similar charges during the three and nine month periods ended
September 30, 2009.
Page 31 of 41
We may be affected by economic, financial, competitive, legislative, regulatory, business and
other factors beyond our control. As discussed in Note 4 (Corrective Action Liabilities) to the
unaudited condensed consolidated financial statements and elsewhere in this report, in February
2010, we received a warning letter from the FDA noting, among other things, that the voluntary
field corrective action undertaken in November 2009 is inadequate. In April 2010, the FDA published
additional information regarding this corrective action, further clarifying the AED models impacted
by the potential component defect and provided further guidance to users of our affected AEDs.
Additionally, the FDA noted that our software update addresses “some, but not all electrical
component defects.” In July 2010, concurrent with our announcement relating to our updated
corrective action plan, the FDA published a communication regarding our updated corrective action
plan, citing our commitment to repair or replace approximately 24,000 high risk and/or frequently
used AEDs with first responders and medical facilities within the United States. The FDA
recommended that all other impacted customers follow the process, as we outlined, for implementing
the software update which has been made available to customers through our website or through a
software kit shipped directly to the customer. With this updated recommendation by the FDA, we
believe we have addressed all outstanding issues with our previously communicated plan to address
potential component defects through a field software update as originally announced in November
2009.
We are in ongoing discussions with the FDA regarding the remaining matters it raised in its
warning letter. These include assertions by the FDA that our procedures relating to the evaluation,
investigation and follow up of complaints, procedures to verify the effectiveness of corrective and
preventative actions, and procedures relating to certain design requirements are inadequate. It is
possible that we may need to take additional actions beyond those incurred to date depending on the
outcome of those discussions. If we are unable to satisfy the FDA’s remaining concerns, we may be
subject to regulatory action by the FDA, including but not limited to seizure, injunction, halting
shipment of our products and/or civil monetary penalties. Any such actions could significantly
disrupt our ongoing business and operations and have a material adverse effect on our financial
position and results of operations. Additionally, quality issues and related corrective actions, as
well as other matters such as the recent re entry into the market of the former AED market leader,
may adversely impact our projected revenues in the near term. Accordingly, we may be required to
reduce costs, which could adversely impact our forecasts for revenue growth in future periods.
As of September 30, 2010, we had cash and cash equivalents of $6.7 million. We expect to incur
operating losses during the fourth quarter of 2010 and to use cash in operations. Additionally, we
will continue to use cash to satisfy our obligations associated with our ongoing corrective action
liabilities for which we have accrued $32.0 million in the aggregate and have $18.8 million in
estimated remaining costs as of September 30, 2010. The costs recorded are estimates and actual
costs that we will incur to complete the corrective actions could be more or less than the $18.8
million that is remaining in our accrued corrective action liabilities on the unaudited condensed
consolidated balance sheet as of September 30, 2010. We expect to satisfy a significant portion of
the requirements under the ongoing corrective actions during the remainder of 2010 and during 2011
and we expect to spend a significant amount of the remaining accrued corrective action liability
costs during this period, which will negatively impact our cash position for the remainder of 2010
and during 2011.
On July 16, 2010, we amended our existing line of credit agreement with Silicon Valley Bank to
increase the amount available for borrowing on a revolving credit basis from $5.0 million to $15.0
million. This new agreement expires in July 14, 2011 (the “maturity date”). Our ability to borrow
under this agreement is based largely on our accounts receivable outstanding at the time of
borrowing and, to a lesser extent, on our inventory holdings. Interest on borrowings, if any, will
be based on the Wall Street Journal Prime rate plus 2.5%. Interest is payable monthly, on the last
day of the month, and all obligations are due on the maturity date. In addition, upon finalizing
our agreement in July 2010, we paid Silicon Valley Bank a non-refundable commitment fee of $175,000
and any unused balances under this facility will bear monthly fees equal to 0.25% per annum on the
average unused portion of the revolving line. We granted Silicon Valley Bank a first priority
security interest in substantially all of our assets to secure our obligations under the above line
of credit. At September 30, 2010, we did not have any borrowings under this, or any other, line of
credit. However, as of November 9, 2010, we had borrowed $3.0 million under our line of credit and
may have additional borrowings under our line of credit during the remainder of the fourth quarter
of 2010.
We believe our existing cash and cash equivalents, together with available borrowings under
our amended line of credit with Silicon Valley Bank discussed above, will be sufficient to fund our
anticipated operating losses, meet working capital requirements and fund anticipated capital
expenditures and other obligations, including the estimated remaining costs of the ongoing
corrective actions, for at least the next twelve months. However, our ability to continue borrowing
under our line of credit depends in part on our continued compliance with certain minimum liquidity
and tangible net worth covenants under the line of credit agreement, as well as other covenants.
We were in compliance with our financial covenants under the line of credit as of September 30,
2010, and through the date of this report. However, given the ongoing risks associated with
regulatory matters and the potential negative impact they could have on our operating
performance and financial condition in future periods, as well as other potential negative factors
such as higher than anticipated operating losses in future periods, new or unexpected legal claims
or other material adverse events, we can provide no assurance that we will be able to continue to
comply with the covenants under the line of credit agreement. Failure to comply with the covenants under the line of credit
Page 32 of 41
could result in a default under the line of credit agreement. In such
event, if we were unable to obtain a waiver of the default, we would be unable to continue to
borrow under this facility and the default could result in an acceleration of any outstanding
indebtedness, either of which would have a material adverse impact on our financial condition and
liquidity.
Given the above factors, including those risks associated with the pending merger agreement
with Opto Circuits discussed in the Pending Merger Transaction section of this report, we plan to
closely monitor our projected operating results and cash balances during the remainder of 2010, and
beyond. To the extent our actual operating results are not in line with our projected results, we
intend to reduce costs, to the extent necessary, to enable us to continue operations for at least
the next twelve months. While such cost reductions might negatively impact future growth
opportunities, we believe we have the ability to make such reductions, should they become
necessary. Absent the consummation of the pending transaction with Opto Circuits, our ability to
grow the business through possible acquisitions funded by cash or other borrowing has been reduced
substantially for the foreseeable future, as we will be required to use our existing cash and cash
equivalents, as well as borrowings under our line of credit, to fund our ongoing corrective actions
and operating losses. Further, we intend to renew our line of credit agreement with Silicon Valley
Bank in July 2011. However, there can be no assurance that we will be successful in renewing our
line of credit agreement with Silicon Valley Bank in the future. To the extent we are not
successful in renewing our line of credit, or if other adverse events or circumstances arise during
the remainder of 2010 or 2011 which could require additional funding beyond that available under
our current line of credit, we may need to seek additional or alternative sources of financing.
There can be no assurance that we would be able to obtain financing from alternative sources and,
if such financing were to be available, it may be expensive and/or dilutive to stockholders.
For more information on the factors that may impact our financial results, please see the Risk
Factors included in our Annual Report on Form 10-K filed with the Securities and Exchange
Commission on March 15, 2010, our Quarterly Reports on Form 10-Q for the quarters ended March 31,
2010 and June 30, 2010 and elsewhere in this report.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
We develop products in the U.S. and sell them worldwide. As a result, our financial results
could be affected by factors such as changes in foreign currency exchange rates or weak economic
conditions in foreign markets. Since the majority of our revenues are currently priced in U.S.
dollars and are translated to local currency amounts, a strengthening of the dollar could make our
products less competitive in foreign markets.
Item 4. Controls and Procedures
The Company maintains disclosure controls and procedures (as defined under Rules 13a-15(e) and
15d-15(e) of the Securities Exchange Act of 1934, as amended). Management, under the supervision
and with the participation of our chief executive officer and our chief financial officer,
evaluated the effectiveness and design of the Company’s disclosure controls and procedures pursuant
to Exchange Act Rule 13a-15(b) as of September 30, 2010. Based on that evaluation, our chief
executive officer and our chief financial officer concluded that the Company’s disclosure controls
and procedures were effective as of September 30, 2010.
There were no changes in our internal control over financial reporting that occurred during
our fiscal quarter ended September 30, 2010 that have materially affected, or are reasonably likely
to materially affect, our internal control over financial reporting.
Page 33 of 41
PART II — OTHER INFORMATION
Item 1. Legal Proceedings
On October 19, 2010, we announced that we entered into a definitive merger agreement with Opto
Circuits (India) Ltd. (“Opto Circuits”), under which Opto Circuits has agreed to acquire all of the
outstanding shares of the Company’s common stock at $2.30 USD per share. Following that
announcement, certain of our stockholders filed similar lawsuits relating to this transaction
against us, our Board members, and in some cases our Chief Executive Officer and Chief Financial
Officer. Those lawsuits include:
Lionel Patenaude v. Cardiac Science Corp., et al.
, Court
of Chancery of the State of Delaware, Civil Action No. 5923-VCP;
Mindy Creamer v. Cardiac
Science Corp., et al.
Superior Court of the State of Washington, Case No. 10-2-08782-4;
Robert Gluck v. Ruediger Naumann-Etienne, et al.
, Superior Court of the State of
Washington, Case No. 10-2-08912-6;
Mark Rapport v. David Marver, et al.
, Superior Court of
the State of Washington, Case No. 10-2-009005-1; and
Bagge v. Naumann-Etienne, et al.
,
Superior Court of the State of Washington, Case No. 10-2-09045-1.
The plaintiffs in these cases generally assert that the our Directors and/or executives
breached fiduciary duties owed to stockholders, and that we either breached our fiduciary duty to
stockholders or in some manner participated with the individual defendants in their respective
alleged breaches, that we and Opto Circuits failed to provide adequate disclosures to stockholders concerning the
proposed transaction and that Opto Circuits and its Merger Sub also participated and are also liable.
The plaintiffs generally allege that the price announced for the tender offer is inadequate, and
that various individual defendants failed to take adequate steps to maximize stockholder value, and
that we either failed to take adequate steps or aided and abetted the individual defendants. The
complaints seek to enjoin the defendants from proceeding with, consummating, or closing the Merger,
or to rescind and set aside the proposed transaction. We believe that these complaints lack merit,
and intend to defend these cases vigorously.
We are subject to various other legal proceedings arising in the normal course of business. In
the opinion of management, the ultimate resolution of these proceedings is not expected to have a
material effect on our consolidated financial position, results of operations or cash flows.
Item 1A. Risk Factors
Failure to complete the proposed merger with Opto Circuits could negatively impact our stock price
and adversely affect our future financial condition, operations and prospects.
The proposed merger with Opto Circuits may not be completed for numerous reasons, including
the following:
|
|
•
|
|
uncertainties as to whether sufficient shares may be tendered by the Company’s
stockholders needed to satisfy the minimum tender condition to the closing of the
tender offer
|
|
|
|
|
•
|
|
the existence of a “Company Material Adverse Effect” as defined in the merger
agreement
|
|
|
|
|
•
|
|
the risk that competing offers or acquisition proposals may be made
|
|
|
|
|
•
|
|
the possibility that various conditions to the consummation of the tender offer or
the merger may not be satisfied or waiver, including that a governmental entity may
prohibit, delay or refuse to grant approval for the consummation of the offer or the
merger
|
|
|
|
|
•
|
|
the risk that stockholder litigation in connection with the tender offer or the
merger may result in significant costs of defense, indemnification and liability or
result in a delay in closing the transaction.
|
If the merger is not completed, it could negatively impact our stock price and adversely
affect our future financial condition, operations and prospects. In addition, our current and
prospective employees may experience uncertainty about their future role with us until Opto
Circuits’ strategies with regard to us are announced or executed. This may adversely affect our
ability to attract and retain key management, research and development, manufacturing, sales and
marketing and other personnel. As a result, there may be delays before we can achieve
pre-transaction levels of activity in various functions, including sales of our products and
research and development, which may have a material adverse effect on our future financial
performance. If the tender offer or other transactions contemplated by the merger agreement do not
occur, we will nonetheless remain liable for significant legal and other expenses that we have
incurred related to the transaction.
If the merger agreement is terminated and our board of directors determines to seek another
merger or business combination, it may not be able to find a partner willing to pay an equivalent
or more attractive price than that which would have been paid in the merger with Opto Circuits.
Our cash and cash equivalents may not be sufficient to fund future operations and we may
not be able to secure additional sources of financing or obtain financing on acceptable terms.
Our cash and cash equivalents as of September 30, 2010, totaled $6.7 million. We expect to
incur operating losses during the fourth quarter of 2010 and to use cash in operations.
Additionally, we have two ongoing voluntary corrective actions
Page 34 of 41
relating to our AED products, the costs of which have been estimated at $32.0 million in the
aggregate, of which $18.8 million remains as an accrued liability as of September 30, 2010. We
expect to spend a significant portion of the remaining accrued costs associated with these
corrective actions by the end of 2011 which will negatively impact our cash position for the rest
of 2010 and during 2011.
We cannot be certain our cash and cash equivalents, together with borrowings under our line of
credit described below, will be sufficient to meet our operating needs for at least the next twelve
months as we may be affected by economic, financial, competitive, legislative, regulatory, business
and other factors beyond our control.
On July 16, 2010, we amended our existing line of credit agreement with Silicon Valley Bank
which now provides up to $15.0 million of revolving credit. Our ability to borrow under this
agreement is based largely on our accounts receivable outstanding at the time of borrowing and, to
a lesser extent, on our inventory holdings. Interest on borrowings, if any, will be based on the
Wall Street Journal Prime rate plus 2.5%. This new agreement expires in July 2011 and all amounts
outstanding under this facility will be due and payable at that time. We may be unable to extend
this facility when it expires or refinance amounts outstanding at maturity, which could have a
material adverse effect on our liquidity.
Accordingly, we may be required to reduce costs, which could adversely impact our forecasts
for revenue growth in future periods which in turn could also adversely impact our forecasts for
future cash flows. In addition, we may be forced to borrow from our available line of credit or may
need to seek additional or alternative sources of financing. There can be no assurance that we
would be able to obtain financing from alternative sources and, if such financing were to be
available, it may be expensive and/or dilutive to stockholders.
We may be unable to comply with our financial covenants.
As of September 30, 2010 we had no borrowings under our $15.0 million line of credit facility
with Silicon Valley Bank. However, as of November 9, 2010, we borrowed $3.0 million under this
credit facility and we may have additional borrowings during the remainder of the fourth quarter of
2010. Our ability to borrow is based largely on our existing accounts receivable and to a lesser
extent on our inventory levels. Additionally, the credit facility contains certain covenants
related to minimum liquidity levels as well as certain levels of tangible net worth which must be
maintained at all times under the agreement. We were in compliance with these covenants as of
September 30, 2010 and through the date of this report. Given our ongoing risks associated with
regulatory matters and the potential negative impact they could have on our revenues and accounts
receivable in future periods, as well as other potential negative factors such as higher than
anticipated operating losses in future periods, new or unexpected legal claims or other material
adverse events, we can provide no assurance we will be able to continue to comply with the
covenants under the line of credit agreement. Failure to comply with these covenants could result
in a default under the line of credit agreement with Silicon Valley Bank. In such event if we were
unable to obtain a waiver of the default, we would be unable to continue to borrow under this
facility and the default could result in the acceleration of any outstanding indebtedness, either
of which could have a material adverse impact on results of operations and our financial condition.
With the exception of the risk factors above, there have been no new risk factors or updates
to risk factors that we are aware of from the risk factors set forth in Part I, Item 1A in our
Annual Report on Form 10-K for the year ended December 31, 2009 filed with the Securities and
Exchange Commission (“SEC”) on March 15, 2010, in Part II, Item 1A in our Quarterly Report on Form
10-Q for the quarter ended March 31, 2010 filed with the SEC on May 7, 2010 and in Part II, Item 1A
in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 filed with the SEC on
August 6, 2010.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Removed and Reserved
Item 5. Other Information
None.
Item 6. Exhibits
Page 35 of 41
|
|
|
|
|
No.
|
|
Description
|
|
2.1
|
|
Agreement and Plan of Merger, dated as of October 19, 2010, by and among Cardiac Science Corporation,
Opto Circuits (India) Ltd. and Jolt Acquisition Company. (1)
|
|
|
|
|
|
2.2
|
|
Amendment No. 1 to Agreement and Plan of Merger, dated as of October 29, 2010, by and among Cardiac
Science Corporation, Opto Circuits (India) Ltd. and Jolt Acquisition Company. (2)
|
|
|
|
|
|
31.1
|
|
Certification of Chief Executive Officer pursuant to Section 302(a) of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
31.2
|
|
Certification of Chief Financial Officer pursuant to Section 302(a) of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
32.1
|
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
32.2
|
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
(1)
|
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K (File No
000-51512) filed on October 19, 2010.
|
|
|
|
(2)
|
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K (File No 000-51512)
filed on October 29, 2010.
|
Page 36 of 41
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly
caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
CARDIAC SCIENCE CORPORATION
|
|
|
|
By:
|
/s/ Michael K. Matysik
|
|
|
|
|
Michael K. Matysik
|
|
|
|
|
Senior Vice President and Chief Financial Officer
(Principal Financial Officer)
|
|
|
|
|
|
|
Date: November 9, 2010
|
|
Page 37 of 41
Cardiac Science Corp (MM) (NASDAQ:CSCX)
Historical Stock Chart
From Apr 2024 to May 2024
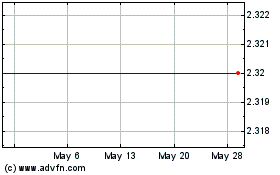
Cardiac Science Corp (MM) (NASDAQ:CSCX)
Historical Stock Chart
From May 2023 to May 2024