UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of February 2024
Commission File No. 001-40997
BRIGHT MINDS BIOSCIENCES INC.
(Translation of registrant's name into English)
19 Vestry Street,
New York, NY 10013
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F
Form 20-F [ X ] Form 40-F [ ]
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1) [ ]
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7) [ ]
SUBMITTED HEREWITH
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
BRIGHT MINDS BIOSCIENCES INC.
/s/ Ryan Cheung
__________________________
Ryan Cheung
Chief Financial Officer
Date: February 12, 2024
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Financial Statements
For the three months ended December 31, 2023 and 2022
(Expressed in Canadian Dollars)
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Financial Position
(Expressed in Canadian dollars)
| |
|
|
|
December 31, |
|
|
September 30, |
|
| As at |
Notes |
|
|
2023
(unaudited)
|
|
|
2023
(audited) |
|
| |
|
|
|
$ |
|
|
$ |
|
| ASSETS |
|
|
|
|
|
|
|
|
| Current Assets |
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
9 |
|
|
6,761,647 |
|
|
6,747,986 |
|
| Sales tax receivable |
|
|
|
45,040 |
|
|
36,981 |
|
| Prepaids |
|
|
|
32,071 |
|
|
27,692 |
|
| |
|
|
|
6,838,758 |
|
|
6,812,659 |
|
| Non-Current Assets |
|
|
|
|
|
|
|
|
| Right-of-use asset |
11 |
|
|
48,300 |
|
|
66,413 |
|
| TOTAL ASSETS |
|
|
|
6,887,058 |
|
|
6,879,072 |
|
| |
|
|
|
|
|
|
|
|
| LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
| Current Liabilities |
|
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
5,7 |
|
|
799,597 |
|
|
207,307 |
|
| Lease liability - current portion |
11 |
|
|
54,563 |
|
|
73,549 |
|
| TOTAL LIABILITIES |
|
|
|
854,160 |
|
|
280,856 |
|
| |
|
|
|
|
|
|
|
|
| Shareholders' equity |
|
|
|
|
|
|
|
|
| Share capital |
6 |
|
|
35,046,808 |
|
|
33,914,308 |
|
| Pre-funded warrants |
6 |
|
|
831,834 |
|
|
831,834 |
|
| Reserves |
6 |
|
|
3,385,546 |
|
|
3,399,097 |
|
| Deficit |
|
|
|
(33,231,290 |
) |
|
(31,547,023 |
) |
| TOTAL SHAREHOLDERS' EQUITY |
|
|
|
6,032,898 |
|
|
6,598,216 |
|
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
6,887,058 |
|
|
6,879,072 |
|
Nature and continuance of operations (Note 1)
|
Approved on behalf of the Board of Directors:
|
|
|
|
|
|
|
"Ian McDonald"
|
|
"Nils Bottler"
|
|
Director
|
|
Director
|
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Comprehensive Loss
(Expressed in Canadian dollars - Unaudited)
| For the three months ended |
Notes |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
|
$ |
|
|
$ |
|
| EXPENSES |
|
|
|
|
|
|
|
| Consulting fees |
6,7 |
|
29,942 |
|
|
31,352 |
|
| Directors' compensation |
6,7 |
|
113,230 |
|
|
523,964 |
|
| Foreign exchange |
|
|
(14,731 |
) |
|
37,134 |
|
| Marketing, advertising, and investor relations |
|
|
36,600 |
|
|
41,070 |
|
| Office and administrative |
11 |
|
69,236 |
|
|
62,690 |
|
| Professional fees |
7 |
|
152,173 |
|
|
138,616 |
|
| Regulatory and filing |
|
|
72,293 |
|
|
40,924 |
|
| Research and development |
6,7,10 |
|
1,225,524 |
|
|
1,459,678 |
|
| Net and comprehensive loss |
|
|
(1,684,267 |
) |
|
(2,335,428 |
) |
| |
|
|
|
|
|
|
|
| Basic and diluted loss per share |
|
|
(0.44 |
) |
|
(0.65 |
) |
| |
|
|
|
|
|
|
|
| Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
| -basic and diluted |
|
|
3,842,679 |
|
|
3,587,091 |
|
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Changes in Shareholders’ Equity
(Expressed in Canadian Dollars - Unaudited)
| |
|
Share Capital |
|
|
|
|
|
|
|
|
|
| |
|
Number of
shares * |
|
|
Share capital |
|
|
Pre-funded
warrants |
|
|
Reserves |
|
|
Deficit |
|
|
Total |
|
| |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
| Balance as at September 30, 2022 |
|
3,518,472 |
|
|
32,237,844 |
|
|
- |
|
|
2,479,466 |
|
|
(24,174,798 |
) |
|
10,542,512 |
|
| Private placement - common shares |
|
194,800 |
|
|
1,217,500 |
|
|
- |
|
|
- |
|
|
- |
|
|
1,217,500 |
|
| Private placement - pre-funded warrants |
|
- |
|
|
- |
|
|
831,834 |
|
|
- |
|
|
- |
|
|
831,834 |
|
| Share issue costs |
|
- |
|
|
(26,976 |
) |
|
- |
|
|
- |
|
|
- |
|
|
(26,976 |
) |
| Warrants exercised |
|
28,800 |
|
|
253,440 |
|
|
- |
|
|
- |
|
|
- |
|
|
253,440 |
|
| Share-based compensation (Note 6) |
|
- |
|
|
- |
|
|
- |
|
|
484,143 |
|
|
- |
|
|
484,143 |
|
| Net loss |
|
- |
|
|
- |
|
|
- |
|
|
- |
|
|
(2,335,428 |
) |
|
(2,335,428 |
) |
| Balance as at December 31, 2022 |
|
3,742,072 |
|
|
33,681,808 |
|
|
831,834 |
|
|
2,963,609 |
|
|
(26,510,226 |
) |
|
10,967,025 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance as at September 30, 2023 |
|
3,772,072 |
|
|
33,914,308 |
|
|
831,834 |
|
|
3,399,097 |
|
|
(31,547,023 |
) |
|
6,598,216 |
|
| Private placement - common shares |
|
661,765 |
|
|
900,000 |
|
|
- |
|
|
- |
|
|
- |
|
|
900,000 |
|
| RSUs exercised |
|
30,000 |
|
|
232,500 |
|
|
- |
|
|
(232,500 |
) |
|
- |
|
|
- |
|
| Share-based compensation (Note 6) |
|
- |
|
|
- |
|
|
- |
|
|
218,949 |
|
|
- |
|
|
218,949 |
|
| Net loss |
|
- |
|
|
- |
|
|
- |
|
|
- |
|
|
(1,684,267 |
) |
|
(1,684,267 |
) |
| Balance as at December 31, 2023 |
|
4,463,837 |
|
|
35,046,808 |
|
|
831,834 |
|
|
3,385,546 |
|
|
(33,231,290 |
) |
|
6,032,898 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* On July 14, 2023, the Company completed a share consolidation on the basis of 1 new common share to 5 old common shares (Note 6). For accounting purposes, recognition of the share consolidation has been made retroactively such that all share and per share numbers have been adjusted to reflect the share consolidation.
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
Bright Minds Biosciences Inc.
Condensed Interim Consolidated Statements of Cash Flows
(Expressed in Canadian Dollars - Unaudited)
For the three months ended |
Notes |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
|
$ |
|
|
$ |
|
| Operating activities |
|
|
|
|
|
|
|
| Net loss for the period |
|
|
(1,684,267 |
) |
|
(2,335,428 |
) |
| Non-cash items: |
|
|
|
|
|
|
|
| Depreciation - right-of-use asset |
11 |
|
18,113 |
|
|
18,112 |
|
| Foreign exchange |
|
|
42,295 |
|
|
7,049 |
|
| Share-based compensation |
6 |
|
218,949 |
|
|
484,143 |
|
| Interest on lease liability |
11 |
|
2,971 |
|
|
6,055 |
|
Changes in non-cash operating working capital items: |
|
|
|
|
|
|
|
| Sales tax receivable |
|
|
(8,059 |
) |
|
55,243 |
|
| Other receivables |
|
|
- |
|
|
41,261 |
|
| Prepaids |
|
|
(4,379 |
) |
|
6,272 |
|
| Accounts payable and accrued liabilities |
|
|
592,290 |
|
|
(946,379 |
) |
| Net cash used in operating activities |
|
|
(822,087 |
) |
|
(2,663,672 |
) |
| |
|
|
|
|
|
|
|
| Financing activities |
|
|
|
|
|
|
|
| Private placement proceeds |
6 |
|
900,000 |
|
|
1,217,500 |
|
| Share issue costs |
|
|
- |
|
|
(26,976 |
) |
| Pre-funded warrant proceeds |
|
|
- |
|
|
831,834 |
|
| Warrant exercise proceeds |
|
|
- |
|
|
253,440 |
|
| Principal portion of lease liability |
11 |
|
(21,957 |
) |
|
(21,488 |
) |
| Net cash from financing activities |
|
|
878,043 |
|
|
2,254,310 |
|
| |
|
|
|
|
|
|
|
| Change in cash and cash equivalents |
|
|
55,956 |
|
|
(409,362 |
) |
| Effect of foreign exchange on cash |
|
|
(42,295 |
) |
|
(7,049 |
) |
| Cash and cash equivalents, beginning of period |
|
|
6,747,986 |
|
|
11,627,913 |
|
| |
|
|
|
|
|
|
|
| Cash and cash equivalents, end of period |
|
|
6,761,647 |
|
|
11,211,502 |
|
| |
|
|
|
|
|
|
|
| SUPPLEMENTARY INFORMATION |
|
|
|
|
|
|
|
| Fair value of RSUs exercised |
|
|
232,500 |
|
|
- |
|
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
1. NATURE AND CONTINUANCE OF OPERATIONS
Bright Minds Biosciences Inc. (the "Company") was incorporated under the Business Corporations Act of British Columbia on May 31, 2019. The Company's objective is to generate income and achieve long term profitable growth through the development of therapeutics to improve the lives of patients with certain severe and life-altering diseases. On February 8, 2021, the Company started trading on the Canadian Stock Exchange ("CSE") under the symbol DRUG. On May 17, 2021, the Company started trading on the OTCQB under the symbol BMBIF. On November 8, 2021, the Company started trading on the NASDAQ under the symbol DRUG. The registered address of the Company is located at 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7, Canada. The head office address of the Company is located at 19 Vestry Street, New York, NY 10013, USA.
These condensed interim consolidated financial statements have been prepared on a going concern basis which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future. As at December 31, 2023, the Company is not able to finance day to day activities through operations and has incurred a loss of $1,684,267 for the three months ended December 31, 2023. The Company has a deficit of $33,231,290 since inception and negative operating cash flows. As at December 31, 2023, the Company has working capital of $5,984,598 (September 30, 2023 - $6,531,803). The continuing operations of the Company are dependent upon its ability to attain profitable operations and generate funds therefrom. Management intends to finance operating costs with equity financings, loans from directors and companies controlled by directors and/or private placement of common shares.
2. STATEMENT OF COMPLIANCE AND BASIS OF PREPARATION
Statement of compliance
The Company applies International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"). These unaudited condensed interim consolidated financial statements have been prepared in accordance with International Accounting Standard 34 - Interim Financial Reporting. Accordingly, they do not include all of the information required for full annual financial statements required by IFRS as issued by the IASB. The policies applied in these unaudited condensed interim consolidated financial statements are based on IFRSs issued and outstanding as of February 12, 2024, the date the Board of Directors approved the statements. The same accounting policies and methods of computation are followed in these unaudited condensed interim consolidated financial statements as compared with the most recent annual financial statements as at and for the year ended September 30, 2023 except as noted below. Any subsequent changes to IFRS that are given effect in the Company's annual financial statements for the year ending September 30, 2024 could result in restatement of these unaudited condensed interim consolidated financial statements.
Basis of preparation
Depending on the applicable IFRS requirements, the measurement basis used in the preparation of these condensed interim consolidated financial statements is cost, net realizable value, fair value or recoverable amount. These condensed interim consolidated financial statements, except for the statement of cash flows, are based on the accrual basis.
3. SIGNIFICANT ACCOUNTING POLICIES
Basis of consolidation
These condensed interim consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries Bright Minds Biosciences LLC, a Delaware limited liability company, and Bright Minds Bioscience Pty Ltd., a proprietary company registered under the Corporations Act of Australia on June 24, 2021. On June 10, 2021, the CEO (the "Chief Executive Officer") of the Company transferred, assigned and conveyed all of his membership interests in Bright Minds Biosciences LLC to the Company.
3. SIGNIFICANT ACCOUNTING POLICIES (continued)
A subsidiary is an entity that the Company controls, either directly or indirectly, where control is defined as the power to govern the financial and operating policies of an entity so as to obtain benefits from its activities. The financial results of the Company's subsidiaries are included in the condensed interim consolidated financial statements from the date that control commences until the date that control ceases. The accounting policies of the Company's subsidiaries have been aligned with the policies adopted by the Company. When the Company ceases to control a subsidiary, the financial statements of that subsidiary are de-consolidated.
Inter-company balances and transactions, and any income and expenses arising from inter-company transactions, have been eliminated in these condensed interim consolidated financial statements.
Foreign currency translation
The functional currency of the Company, Bright Minds Biosciences LLC and Bright Minds Bioscience Pty Ltd. is the Canadian dollar and the presentation currency of the Company is the Canadian dollar. Transactions in currencies other than the functional currency are recorded at the rates of exchange prevailing on the transaction date. Monetary assets and liabilities that are denominated in foreign currencies are translated at the rates prevailing at each reporting date. Non-monetary assets and liabilities denominated in foreign currencies that are measured at fair value are retranslated to the functional currency at the exchange rate at the date the fair value was determined. Non-monetary items that are measured in terms of historical cost in a foreign currency are not retranslated. Foreign currency translation differences are recognized in profit or loss.
Please refer to Note 3 of the audited consolidated financial statements of the Company for the year ended September 30, 2023 for full disclosure of the significant accounting policies.
4. INTANGIBLE ASSETS
Psilocybinlabs Ltd. ("PL") was incorporated under the laws of the province of British Columbia on April 25, 2019, with the incorporator share being held by a company controlled by the CEO of the Company. On May 17, 2019, this share was transferred to the Company. On April 25, 2019, PL entered into a confirmatory assignment and waiver (the "CAW") with an individual, which was amended and restated on May 17, 2019. Pursuant to the amended and restated CAW, this individual assigned all of the right, title and interest, including all other intellectual property rights (the Rights, as described) to PL. As compensation for the assignment of the Rights, PL issued 100,000 common shares valued at $2,000 to this individual. On August 7, 2019, the Company then purchased the 100,000 common shares of PL by issuing 20,000 common shares of the Company valued at $2,000, with the reacquisition being recorded as an asset acquisition. On September 29, 2022, the directors of the Company agreed to wind-up and dissolve PL and the carrying value of the intangible asset was impaired during the year ended September 30, 2022.
5. ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
| |
|
December 31,
2023 |
|
|
September 30,
2023 |
|
| |
|
$ |
|
|
$ |
|
| Accounts payable |
|
799,597 |
|
|
182,307 |
|
| Accrued liabilities |
|
- |
|
|
25,000 |
|
| Total accounts payable and accrued liabilities |
|
799,597 |
|
|
207,307 |
|
6. SHARE CAPITAL
Authorized share capital
Unlimited number of common shares without par value.
On July 14, 2023, the Directors of the Company approved the consolidation of the Company's issued and outstanding common shares on a 5:1 basis. All common shares, stock options, restricted share units and warrant references in these condensed interim consolidated financial statements reflect the effect of the share consolidation.
Issued share capital for the three months ended December 31, 2023
On December 22, 2023, the Company issued 661,765 Units of the Company at a price per unit of $1.36 for aggregate gross proceeds of $900,000. Each Unit is comprised of one common share and one common share purchase warrant of the Company. Each Warrant is exercisable to acquire one common share of the Company at an exercise price of $1.70 per share until December 22, 2028.
On December 13, 2023, 30,000 RSUs were exercised and $232,500 was reclassified from reserves to share capital upon the exercise.
Issued share capital for the year ended September 30, 2023
On December 2, 2022, the Company issued 133,200 pre-funded warrants ("PFWs") of the Company at a price per PFW of $6.245 and 194,800 Units of the Company at a price per Unit of $6.25 for aggregate gross proceeds of $2,049,334. Each PFW is exercisable into one Unit at an exercise price of $0.005 per Unit on the date that is the earlier of (a) the date the holder thereof elects to exercise the PFWs and pays the exercise price, and (b) December 2, 2024. Each Unit is comprised of one common share and one common share purchase warrant ("Warrant") of the Company. Each Warrant is exercisable to acquire one common share of the Company at an exercise price of $6.75 per share until December 2, 2024.
The PFWs are classified as a component of permanent shareholders' equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the Units with which they were issued, are immediately exercisable, do not embody an obligation for the Company to repurchase its shares, and permit the holders to receive a fixed number of common shares upon exercise. In addition, such PFWs do not provide any guarantee of value or return. The Company valued the PFWs at issuance, concluding that their sales price approximated their fair value, and a total of $831,834 is recorded to the PFWs.
On March 10, 2023, 30,000 RSUs were exercised and $232,500 was reclassified from reserves to share capital upon the exercise.
During the year ended September 30, 2023, an aggregate of 28,800 warrants were exercised for gross proceeds of $253,440.
Issued share capital for the year ended September 30, 2022
On April 11, 2022, the Company entered into a scientific advisory board agreement with Karl Deisseroth ("Deisseroth") pursuant to which the Company will pay Deisseroth a monthly fee of US$4,166.66 and issued an aggregate 5,000 common shares (the "Payment Shares") in the capital of the Company at a fair market value of $5.45 per share (total fair market value of $27,250). The Payment Shares will be issued in escrow and released to Deisseroth over a period of four years commencing on March 8, 2023 (see Note 8).
6. SHARE CAPITAL (continued)
On August 30, 2022, the Company issued 571,600 Units of the Company at a price per unit of $7.00 for aggregate gross proceeds of $4,001,200. Each Unit is comprised of one common share and one common share purchase warrant of the Company. Each Warrant is exercisable to acquire one common share of the Company at an exercise price of $8.80 per share until August 30, 2024. The agent was paid a cash finder's fee $280,084 and expenses of $176,065 and received compensation warrants entitling them to purchase an aggregate of 26,808 Units of the Company at a per unit price of $7.00 for a period of twenty-four months following closing, with the Units having the same terms as the Units sold pursuant to the Offering. An advisor was additionally paid a cash finder's fee of $259,245 and received compensation warrants entitling them to purchase an aggregate of 18,232 Units of the Company at a per unit price of $7.00 for a period of twenty-four months following closing, with the Units having the same terms as the Units sold pursuant to the Offering. The Company incurred additional share issue costs of $84,585 in connection with the offering.
In September 2022, 45,040 compensation warrants were exercised for gross proceeds of $315,277. Upon exercise, $531,000 was reclassified from reserves to share capital.
During the year ended September 30, 2022, 529,960 warrants priced at $0.25, $8.80, and $47.30 per unit were exercised for gross proceeds of $1,653,170.
Escrowed securities
On January 28, 2021, the Company entered into an escrow agreement under National Policy 46-201 Escrow for Initial Public Offerings (the "Policy") in connection with the listing of common shares of the Company on the CSE, whereby 570,560 common shares of the Company and 389,600 share purchase warrants (exercised on April 23, 2021), being an aggregate of 960,160 securities, were deposited to be held in escrow. As the Company is defined as an emerging issuer under the Policy, the escrowed securities will be released as follows:
- 96,016 - on the date that the Company's shares are listed on the CSE (February 8, 2021); and
- 144,024 - 6, 12, 18, 24, 30 and 36 months after the listing date.
As at December 31, 2023, 144,024 common shares remain in escrow which were released subsequently.
Stock options
The Company's stock option plan provides for stock options to be issued to directors, officers, employees and consultants of the Company, its subsidiaries and any personal holding company of such individuals so that they may participate in the growth and development of the Company. Subject to the specific provisions of the stock option plan, eligibility, vesting period, terms of the options and the number of options granted are to be determined by the Board of Directors at the time of grant. The stock option plan allows the Board of Directors to issue up to 10% of the Company's outstanding common shares as stock options.
Options granted during the three months ended December 31, 2023
No options were granted during the three-month period ended December 31, 2023.
Options granted during the year ended September 30, 2023
On December 1, 2022, the Company granted 60,000 options to the Chief Medical Officer of the Company. The options have an exercise price of $8.25 per share, expire on December 1, 2027 and vest as follows: 25% on the first anniversary of the grant date, 25% on the second anniversary of the grant date, 25% on the third anniversary of the grant date, and 25% on the fourth anniversary of the grant date. The fair value of these stock options was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $8.25; ii) share price: $7.75; iii) term: 5 years; iv) volatility: 141.61%; v) discount rate: 3.05%; and dividends: nil.
On December 1, 2022, the Company and a consultant mutually agreed to cancel 16,000 options that were previously granted on April 28, 2021.
6. SHARE CAPITAL (continued)
On February 16, 2023, the Company granted 47,000 options to the consultants and a director of the Company. The options have an exercise price of $5.25 per share, expire on February 16, 2028 and vest as follows: 25% on the first anniversary of the grant date, 25% on the second anniversary of the grant date, 25% on the third anniversary of the grant date, and 25% on the fourth anniversary of the grant date. The fair value of these stock options was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $5.25; ii) share price: $4.85; iii) term: 5 years; iv) volatility: 135.92%; v) discount rate: 3.45%; and dividends: nil.
The following table summarizes the movements in the Company's outstanding stock options for the three-month period ended December 31, 2023 and the year ended September 30, 2023:
| |
|
Number of options |
|
|
Weighted average
exercise price |
|
| |
|
|
|
|
|
|
| Balance at September 30, 2022 |
|
183,161 |
|
$ |
18.20 |
|
| Granted |
|
107,000 |
|
$ |
6.93 |
|
| Cancelled(1), (2) |
|
(78,000 |
) |
$ |
20.52 |
|
| Balance at September 30, 2023 and December 31, 2023 |
|
212,161 |
|
$ |
11.65 |
|
(1) 30,000 and 16,000 options were forfeited 90 days after the termination of the services of a former Chief Medical Officer and a director of the Company.
As at December 31, 2023, the options have a weighted average remaining life of 3.02 years (September 30, 2023 - 3.28).
The following table summarizes the stock options issued and outstanding:
| |
|
|
|
|
Options Outstanding and Exercisable |
|
|
|
|
Expiry Date |
|
Number of
options |
|
|
Exercisable |
|
|
Exercise price |
|
|
Remaining life
(Years) |
|
| September 21, 2024 |
|
1,761 |
|
|
1,761 |
|
$ |
38.20 |
|
|
0.73 |
|
| November 17, 2025 |
|
71,400 |
|
|
71,400 |
|
$ |
6.25 |
|
|
1.88 |
|
| April 28, 2026 (2) |
|
16,000 |
|
|
8,000 |
|
$ |
38.00 |
|
|
2.33 |
|
| June 15, 2026 |
|
16,000 |
|
|
8,000 |
|
$ |
38.00 |
|
|
2.46 |
|
| December 1, 2027 |
|
60,000 |
|
|
15,000 |
|
$ |
8.25 |
|
|
3.92 |
|
| February 16, 2028 |
|
47,000 |
|
|
- |
|
$ |
5.25 |
|
|
4.13 |
|
(2) On December 1, 2022, the Company and a consultant mutually agreed to cancel 16,000 options, and an additional 16,000 options were cancelled on the retirement of a consultant.
Restricted share unit plan
The Company's restricted share unit ("RSU") plan provides RSUs to be issued to directors, officers, employees and consultants of the Company, its subsidiaries and any personal holding company of such individuals so that they may participate in the growth and development of the Company. Subject to the specific provisions of the RSU plan, eligibility, vesting period, terms of the RSUs and the number of RSUs granted are to be determined by the Board of Directors at the time of the grant. The RSU plan allows the Board of Directors to issue common shares of the company as equity settled RSUs, provided that, when combined, the maximum number of common shares reserved for issuance under all share-based compensation arrangements of the Company does not exceed 10% of the Company's outstanding common shares.
On December 1, 2022, the Company issued 220,000 RSUs to the directors of the Company. These RSUs vest on an annual basis over a period of four years commencing on December 1, 2022 and expiring on December 1, 2027. The estimated fair value of these RSUs is $1,705,000 and will be recognized as an expense over the vesting period of the RSUs.
6. SHARE CAPITAL (continued)
On April 27, 2022, the Company issued 20,000 RSUs to a director of the Company and these RSU's vest as follows: 25% on the date of grant and 25% each on April 27, 2024, 2025 and 2026. The estimated fair value of these RSUs is $127,000 and will be recognized as an expense over the vesting period of the RSUs.
On February 4, 2022 and February 11, 2022, the Company issued 5,000 RSUs and 7,000 RSUs, respectively. These RSUs vest on an annual basis over a period of four years commencing on February 1, 2023. The estimated fair value of these RSUs is $181,250 and will be recognized as an expense over the vesting period of the RSUs.
The following table summarizes the movements in the Company's outstanding RSUs for the three-month period ended December 31, 2023 and the year ended September 30, 2023:
| |
|
Equity settled |
|
|
Cash settled |
|
|
Total |
|
|
Weighted average
exercise price |
|
| Balance at September 30, 2022 |
|
108,000 |
|
|
- |
|
|
108,000 |
|
$ |
13.15 |
|
| Granted |
|
220,000 |
|
|
- |
|
|
220,000 |
|
$ |
7.75 |
|
| Exercised |
|
(30,000 |
) |
|
- |
|
|
(30,000 |
) |
$ |
7.75 |
|
| Forfeited* |
|
(76,000 |
) |
|
- |
|
|
(76,000 |
) |
$ |
6.25 |
|
| Balance at September 30, 2023 |
|
222,000 |
|
|
- |
|
|
222,000 |
|
$ |
10.89 |
|
| Exercised |
|
(30,000 |
) |
|
- |
|
|
(30,000 |
) |
$ |
7.75 |
|
| Balance at December 31, 2023 |
|
192,000 |
|
|
- |
|
|
192,000 |
|
$ |
11.38 |
|
* On November 23, 2022, 76,000 RSUs were forfeited on the termination of the services of former Chief Medical Officer of the Company.
As at December 31, 2023, the RSUs have a weighted average remaining life of 3.81 years (September 30, 2023 - 4.07 years).
The following table summarizes the RSUs issued and outstanding:
| |
|
|
|
|
RSUs Outstanding and Exercisable |
|
|
|
|
Expiry Date |
|
Number of RSUs |
|
|
Exercisable |
|
|
Exercise price |
|
|
Remaining life
(Years) |
|
| February 1, 2027 |
|
5,000 |
|
|
1,250 |
|
$ |
15.25 |
|
|
3.09 |
|
| February 1, 2027 |
|
7,000 |
|
|
1,750 |
|
$ |
15.00 |
|
|
3.09 |
|
| April 27, 2027 |
|
20,000 |
|
|
5,000 |
|
$ |
38.20 |
|
|
3.32 |
|
| December 1, 2027 |
|
160,000 |
|
|
50,000 |
|
$ |
7.75 |
|
|
3.92 |
|
Share-based compensation expense recognized in the consolidated statements of comprehensive loss is comprised of the following:
| |
|
For the three months ended: |
|
| |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
$ |
|
|
$ |
|
| Stock options |
|
108,401 |
|
|
104,375 |
|
| Restricted share units - equity settled grants |
|
110,548 |
|
|
379,768 |
|
| Total equity settled share-based compensation expense |
|
218,949 |
|
|
484,143 |
|
| Restricted share units - cash settled grants |
|
- |
|
|
- |
|
| Total share-based compensation expense |
|
218,949 |
|
|
484,143 |
|
6. SHARE CAPITAL (continued)
Share-based compensation expense is included in the consolidated statements of comprehensive loss as follows:
| |
|
For the three months ended |
|
| |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
$ |
|
|
$ |
|
| Consulting fees |
|
17,306 |
|
|
716 |
|
| Directors' compensation |
|
113,230 |
|
|
523,964 |
|
| Research and development |
|
88,413 |
|
|
(40,537 |
) |
| Total share-based compensation expense |
|
218,949 |
|
|
484,143 |
|
Warrants
The following table summarizes the movements in the Company's outstanding warrants for the three-month period ended December 31, 2023 and the year ended September 30, 2023:
| |
|
Number of warrants |
|
|
Weighted average
exercise price |
|
| Balance at September 30, 2022 |
|
881,520 |
|
$ |
23.89 |
|
| Issued |
|
194,800 |
|
|
6.75 |
|
| Exercised |
|
(28,800 |
) |
|
8.80 |
|
| Balance at September 30, 2023 |
|
1,047,520 |
|
$ |
21.12 |
|
| Issued |
|
661,765 |
|
|
1.70 |
|
| Balance at December 31, 2023 |
|
1,709,285 |
|
$ |
13.60 |
|
On March 17, 2021, the Company issued 26,533 compensation warrants to underwriters. The fair value of these share purchase warrants of $521,000 was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $37.85; ii) share price: $33.25; iii) term: 3 years; iv) volatility: 100%; v) discount rate: 0.35%; and dividends: nil. The fair value of these broker warrants was recorded as a reduction against share capital.
On August 30, 2022, the Company granted 45,040 compensation warrants at an exercise price of $7.00 per compensation warrant expiring on August 30, 2024. Each compensation warrant comprises the one Unit under the same terms of the offering which closed on August 30, 2022. The fair value of these compensation warrants of $315,000 was measured using the Black Scholes option pricing model using the following inputs: i) exercise price: $7.00; ii) share price: $14.65; iii) term: 2 years; iv) volatility: 147.31%; v) discount rate:3.63%; and dividends: nil.
As at December 31, 2023, the warrants have a weighted average remaining life of 2.27 (September 30, 2023 - 0.80) years.
6. SHARE CAPITAL (continued)
The following table summarizes the warrants issued and outstanding:
| |
|
|
|
Warrants Outstanding |
|
| Expiry Date |
|
Number of
warrants |
|
|
Exercise price |
|
|
Remaining life
(Years) |
|
| July 30, 2024 (1) |
|
62,960 |
|
$ |
0.25 |
|
|
0.58 |
|
| March 17, 2024 |
|
339,488 |
|
$ |
47.30 |
|
|
0.21 |
|
| March 17, 2024 |
|
26,533 |
|
$ |
37.85 |
|
|
0.21 |
|
| August 30, 2024 |
|
378,700 |
|
$ |
8.80 |
|
|
0.67 |
|
| August 30, 2024 |
|
26,808 |
|
$ |
8.80 |
|
|
0.67 |
|
| August 30, 2024 |
|
18,231 |
|
$ |
8.80 |
|
|
0.67 |
|
| December 2, 2024 |
|
194,800 |
|
$ |
6.75 |
|
|
0.92 |
|
| December 22, 2028 |
|
661,765 |
|
$ |
1.70 |
|
|
4.98 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
(1) On June 15, 2021, the Company entered into warrant exercise agreements with the two warrant holders, whereby the warrant holders authorized the Company to issue only such number of common shares (or other class of voting securities of the Company, if applicable) as will result in the warrant holders and any other person (as defined) holding less than the threshold number of 4.99% (as defined) of any class of voting securities of the Company as of the date of exercise or conversion of the warrants.
7. RELATED PARTY TRANSACTIONS
Related party transactions were recorded at the exchange value, which is the consideration determined and agreed to by the related parties. The Company's related parties include directors, key management and companies controlled by directors and key management.
Included in accounts payable and accrued liabilities as at December 31, 2023 was $57,187 (September 30, 2023 - $51,480) owing to the directors of the Company and the companies controlled by key management personnel.
Compensation of Key Management Personnel
Key management personnel are those persons that have authority and responsibility for planning, directing and controlling the activities of the Company, directly and indirectly, and by definition include the directors of the Company.
The following table summarizes expenses related to key management personnel:
| |
|
For the three months ended: |
|
| |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
$ |
|
|
$ |
|
| Professional fees |
|
30,000 |
|
|
30,000 |
|
| Research and development |
|
132,377 |
|
|
190,200 |
|
| Share-based compensation included in directors' compensation |
|
113,230 |
|
|
523,964 |
|
| Share-based compensation included in research and development |
|
45,832 |
|
|
(127,256 |
) |
| |
|
321,439 |
|
|
616,908 |
|
See Note 8 for related party contractual obligations.
8. CONTRACTUAL OBLIGATIONS
License agreement
On April 23, 2021, the Company entered into an exclusive license agreement with equity (the "LA") with the Board of Trustees of the UIC (the "University"), whereby the University granted to the Company, in all fields of use and worldwide, an exclusive, non-transferable license with the right to sublicense under the University's rights in and to the Patent Rights (as defined) and a non-exclusive, non-transferable license with the right to sublicense under the University's rights in and to the Technical Information (as defined) to make, have made, construct, have constructed, use, import, sell, and offer for sale royalty-bearing Product (as defined). As consideration for the grant of license, the Company will pay the following amounts (in US$) to the University:
- Signing Fee - a signing fee of $100,000 less $15,000 in option fees was paid (CDN$105,502) and 12,600 common shares of the Company were issued to the University (see Note 6);
- Net Sales - royalties on Net Sales (as defined) ranging from 3% (under $1 billion) to 4.5% (over $2 billion), with such royalty payments being credited toward the annual minimum for the license year in which the royalty payment accrues;
- Sublicensee Revenues - royalties (as for net sales above) on Sublicensee Revenue (as defined), with such royalty payments being credited toward the annual minimum for the license year in which the royalty payment accrues and 12% on all non-royalty revenue until the Company has raised $7.5 million and then 10% thereafter.
- Annual Minimums - if the total royalties paid to the University for any license year are less than the following annual minimums, the Company must pay the University the amount equal to the shortfall:
- Years 1 and 2 - $nil;
- Year 3 - $5,000;
- Year 4 - $15,000;
- Year 5 - $35,000;
- Year 6 and thereafter - $50,000; and
- After first commercial sale - $250,000 or net sales royalty, whichever is higher.
- Milestone Payments - milestone payments after the occurrence of the following milestone events:
Prior to any sublicensing agreements, joint ventures or change of control:
- $10,000 upon dosing the first patient in a Phase I trial;
- $50,000 upon dosing the first patient in the first Phase II trial;
- $250,000 upon dosing the first patient in a Phase III trial in the first clinical indication; and
- $2 million upon the first commercial sale of each clinical indication.
After any sublicensing agreements, joint ventures or change of control:
- As above;
- $250,000 upon dosing the first patient in each Phase II trial;
- $500,000 upon dosing the first patient in each Phase III trial; and
- $2 million upon the first commercial sale of each clinical indication.
Unless otherwise agreed to in writing by the University, the Company will reimburse the University for all documented costs and expenses in connection with the Patent Rights, including the preparation, filing, prosecution, maintenance and defense thereof. From time to time, the anticipated costs and expenses may be significant and, upon request, the Company will pay the estimated costs and expenses in advance of such costs and expenses being incurred by the University.
8. CONTRACTUAL OBLIGATIONS (continued)
The term of the LA ends on the later of the last to expire of the Patent Rights, expiration of regulatory exclusivity for Product or when the Company provides notice that use of Technical Information has ceased. The University has the right to terminate the LA if the Company fails to make any required payments or is in breach of any provision of the LA. The Company may terminate the LA at any time upon providing at least 90 days written notice to the University.
Related party contracts
On June 5, 2020, the Company entered into an independent consultant agreement (the "ICA") whereby the consultant, a private corporation incorporated in the State of California, USA, was engaged and the consultant's representative will serve as the Company's Chief Medical Officer, with the services being provided in California. As compensation for performing these services, the consultant or the consultant's representative will participate in the Company's equity incentive plans and will be eligible for cash payments in respect of fees at such time as the Company begins to compensate other C-level personnel in cash and in similar proportion to total compensation (the "fees"). The non-cash portion of the consultant's fees was in the form of a grant of 30,000 vested stock options and 76,000 RSUs. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by either party giving the other 30 days written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless either party gives the other 30 days written notice. In March 2021, the Board of Directors authorized a monthly fee of US$15,000 and increased it to US$25,000 in August 2021. The Chief Medical Officer's engagement was terminated effective November 23, 2022 and the RSUs and options were cancelled (see Note 6).
On October 29, 2020, the Company entered into an independent contractor agreement (the "ICA") whereby the contractor was engaged to serve as the Company's Chief Science Officer on an as-needed basis. The contractor will be compensated for these services as determined by the Board of Directors of the Company. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by the Company providing five working days written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless the Company provides the contractor with 30 days written notice. In March 2021, the Board of Directors authorized a monthly fee of US$15,000 and increased it to US$25,000 in August 2021. Services provided by the Chief Science Officer ceased at the end of March 2022 and no further payments were made or due by the Company from April 2022 onwards. The Chief Science Officer later resigned at the end of June 2022.
The Company entered into several director indemnity agreements (the "DIAs") with the directors of the Company. Pursuant to the DIAs and subject to all applicable laws, including the applicable limitations and restrictions set forth in the Business Corporations Act (British Columbia), the Company will:
- Indemnify and save harmless the Directors against and from:
- any and all charges or claims by reason of them being or having been a director of the Company or another corporation, at a time when the other corporation is or was an affiliate of the Company, or at the request of the Company;
- any and all costs, damages, expenses, fines, liabilities, losses and penalties (the "Consequences") which they may sustain, incur or be liable for in consequence of their acting as a director of the Company, whether sustained or incurred by reason of their negligence, default, breach of duty or trust, failure to exercise due diligence or otherwise in relation to the Company or any of its affairs; and
- in particular, and without in any way limiting the generality of the foregoing, any and all Consequences which they may sustain, incur or be liable for as a result of or in connection with the release or presence in the environment of substances, contaminants, litter, waste, effluent, refuse, pollutants or deleterious materials and that arise out of or are in any way connected with the management, operation, activities or existence of the Company or by virtue of them holding any other directorship with any other entity at the Company's request.
- gross up any indemnity payment made pursuant to the DIAs by the amount of any income tax payable by the Directors in respect of that payment; and
8. CONTRACTUAL OBLIGATIONS (continued)
- indemnify the Directors for the amount of all costs they incur in obtaining any Court approval required to enable or require the Company to make a payment to them under the DIAs, or enforce the DIAs against the Company, including without limitation legal fees and disbursements on a full indemnity basis.
Notwithstanding the above-noted, the Company will have no obligation to indemnify or save harmless the Directors in respect of any liability for which they are entitled to indemnity pursuant to any valid and collectible policy of insurance obtained and maintained by the Company, to the extent of the amounts actually collected by the Directors under the insurance policy.
On April 11, 2022, the Company entered into a scientific advisory board agreement with Karl Deisseroth ("Deisseroth") pursuant to which the Company will pay Deisseroth a monthly fee of US$4,167 and issued an aggregate 5,000 common shares (the "Payment Shares") in the capital of the Company (see Note 6).
On November 13, 2022, the Company entered into an ICA whereby the contractor was engaged to serve as the Chief Medical Officer of the Company effective December 1, 2022. The Company agreed to pay a signing bonus of US$35,000 upon the execution of the ICA and a fee of US$205,000 annually, payable in monthly installments. The Company also agreed to reimburse for reasonable and approved expenses arising in connection with the performance of the services. The services will continue for an initial term of one year unless sooner terminated. The ICA can be terminated by the Company providing one month written notice, the contractor providing three months' written notice or by mutual written agreement. At the end of the initial term, the ICA will automatically be extended for additional one-year period(s) unless the Company provides the contractor with 30 days written notice. In connection with the ICA, the Company granted 60,000 options with an exercise price of $8.25 per share (see Note 6).
Scientific advisory board agreements
The Company entered into numerous scientific advisory board agreements (the "SABAs") whereby the advisors were retained to serve as members of the Company's scientific advisory board and as consultants to the Company and senior management in the areas of scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisors hourly rates of $150 and $160 per hour. The Company also granted 26,000 stock options to the advisors as part of the Company's November 17, 2020 and April 28, 2021 grant of options of which 4,000 options were cancelled on January 21, 2021. In addition, the Company granted 12,000 RSU's to the advisors of the Company on February 4, 2022 and February 11, 2022 (see Note 6). The advisors have the same hour requirements and restrictions as noted below. The services will continue for initial terms of one year unless sooner terminated. At the end of the initial terms, the SABAs will automatically be extended for an additional one-year period(s) unless either party gives the other 30 days written notice.
Consulting agreements
The Company has entered into numerous consulting agreements (the "CAs") whereby the consultants were retained to serve as advisors to the Company and senior management in the areas of public relations and content creation and scientific, technical and business advice. As compensation for performing these services, the Company will pay the advisors hourly rates between US$30 to US$600. The Company also granted 60,400 stock options to six advisors as part of the Company's November 17, 2020 and April 28, 2021 grant of options of which 32,000 options were cancelled on December 1, 2022 and January 30, 2023 (see Note 6). The advisors being paid $400 and $600 per hour will reserve at least six full days of services to the Company and such additional days as requested by the Company each annual period, but not to exceed 36 full days of service per year unless otherwise agreed and up to a maximum of 288 hours total per year, unless otherwise agreed. The services will continue for initial terms of one year unless sooner terminated. At the end of the initial terms, the CAs will automatically be extended for an additional one-year period(s) unless either party gives the other 30 days written notice.
9. FINANCIAL INSTRUMENTS AND CAPITAL MANAGEMENT
The following table summarizes the carrying value of financial assets and liabilities:
| |
|
|
December 31,
2023 |
|
|
September 30,
2023 |
|
| FVTPL |
|
|
$ |
|
|
$ |
|
| Cash |
|
|
6,675,397 |
|
|
6,661,736 |
|
| Guaranteed investment certificate |
|
|
86,250 |
|
|
86,250 |
|
| Cash and cash equivalents |
|
|
6,761,647 |
|
|
6,747,986 |
|
| Amortized cost |
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
799,597 |
|
|
207,307 |
|
Fair value measurement
Financial assets and liabilities that are recognized on the consolidated statement of financial position at fair value can be classified in a hierarchy that is based on the significance of the inputs used in making the measurements.
The levels in the hierarchy are:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 - inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices); and
Level 3 - inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs).
The Company's cash and cash equivalents is classified as Level 1, whereas accounts payable and accrued liabilities are classified as Level 2. As at December 31, 2023, the Company believes that the carrying values of cash and cash equivalents and accounts payable and accrued liabilities approximate their fair values because of their nature and relatively short maturity dates or durations.
Financial risk management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes. The type of risk exposure and the way in which such exposure is managed is provided as follows:
Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company's primary exposure to credit risk is on its cash and cash equivalents balance. As at December 31, 2023, the Company had cash and cash equivalents of $6,761,647 which was held with major banks in Canada, United States and Australia. Because deposits are with three banks, there is a concentration of credit risk. This risk is managed by using major banks that are high credit quality financial institutions as determined by rating agencies. The maximum exposure to credit risk is the carrying amount of the Company's financial instruments. The credit risk is assessed as low.
Foreign exchange risk
Foreign currency risk is the risk that the fair values of future cash flows of a financial instrument will fluctuate because they are denominated in currencies that differ from the respective functional currency. As at December 31, 2023, the Company had the following foreign currency balances - cash (US$573,703 and AU$602,203), receivables (AU$6,381), prepaids (US$11,145 and AU$95) and accounts payable and accrued liabilities (US$61,046, €525, and AU$666,959). A 10% fluctuation in the US$ and AU$ against the Canadian dollar would have an impact of approximately $64,000 on comprehensive loss.
9. FINANCIAL INSTRUMENTS AND CAPITAL MANAGEMENT (continued)
Liquidity risk
Liquidity risk arises through the excess of financial obligations over available financial assets due at any point in time. The Company's objective in managing liquidity risk is to maintain sufficient readily available reserves in order to meet its liquidity requirements at any point in time. The Company's main source of funding has been the issuance of equity securities for cash, primarily through private placements. The Company's access to financing is always uncertain. There can be no assurance of continued access to significant equity funding. As at December 31, 2023, the Company had cash and cash equivalents of $6,761,647 to cover current liabilities of $799,597.
Capital management
Management's objective is to manage its capital to ensure that there are adequate capital resources to safeguard the Company's ability to continue as a going concern through the optimization of its capital structure. The capital structure consists of share capital and working capital. In order to achieve this objective, management makes adjustments to it in light of changes in economic conditions and risk characteristics of the underlying assets. To maintain or adjust the capital structure, management may invest its excess cash in interest bearing accounts of Canadian chartered banks and/or raise additional funds externally as needed. The Company is not subject to externally imposed capital requirements. The Company's management of capital did not change during the period ended December 31, 2023.
10. RESEARCH AND DEVELOPMENT
Research and development expense recognized in the consolidated statements of comprehensive loss is comprised of the following:
| |
|
For the three months ended: |
|
| |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
$ |
|
|
$ |
|
| Laboratory costs |
|
3,650 |
|
|
9,220 |
|
| Novel drug development |
|
849,403 |
|
|
1,062,951 |
|
| Patents and related payments |
|
1,315 |
|
|
1,150 |
|
| Salary and subcontractors |
|
282,743 |
|
|
426,893 |
|
| Share-based compensation (see Note 6) |
|
88,413 |
|
|
(40,536 |
) |
| |
|
1,225,524 |
|
|
1,459,678 |
|
11. PREMISES LEASES
Commencing September 1, 2021, the Company entered into an apartment lease in New York, New York USA for a term of one year at a monthly base rent of US$5,300. Commencing September 1, 2022, the Company extended the lease for a term of two years at a monthly base rent of US$5,510 for the first year and US$5,630 for the second year of the lease.
(a) Right-of-Use Assets
As at December 31, 2023, $48,300 of right-of-use assets are recorded as follows:
| |
|
$ |
|
| |
|
|
|
| As at September 30, 2022 |
|
138,863 |
|
| Depreciation |
|
(72,450 |
) |
| As at September 30, 2023 |
|
66,413 |
|
| Depreciation |
|
(18,113 |
) |
| As at December 31, 2023 |
|
48,300 |
|
11. PREMISES LEASES (continued)
(b) Lease Liabilities
Minimum lease payments in respect of lease liabilities and the effect of discounting are as follows:
| |
|
Three months ended
December 31,
2023 |
|
|
Year ended
September 30,
2023 |
|
| |
|
|
|
|
|
|
| Undiscounted minimum lease payments: |
|
|
|
|
|
|
| Less than one year |
$ |
58,396 |
|
$ |
80,509 |
|
| Two to three years |
|
- |
|
|
- |
|
| |
|
58,396 |
|
|
80,509 |
|
| Effect of discounting |
|
(3,833 |
) |
|
(6,960 |
) |
| Present value of minimum lease payments |
|
54,563 |
|
|
73,549 |
|
| Less current portion |
|
(54,563 |
) |
|
(73,549 |
) |
| Long-term portion |
$ |
- |
|
$ |
- |
|
(c) Lease Liability Continuity
The lease liability continuity is as follows:
| |
|
$ |
|
| |
|
|
|
| As at September 30, 2022 |
|
139,911 |
|
| Cash flows: |
|
|
|
| Principal payments |
|
(66,362 |
) |
| As at September 30, 2023 |
|
73,549 |
|
| Principal payments |
|
(18,986 |
) |
| As at December 31, 2023 |
|
54,563 |
|
During the three months December 31, 2023, interest of $2,971 and depreciation of $18,113 is included in the office and administrative expense on the consolidated statements of comprehensive loss.
This Management Discussion and Analysis ("MD&A") provides a detailed analysis of the business of Bright Minds Biosciences Inc. (the "Company") and describes the Company's financial results for the first quarter ended December 31, 2023. This MD&A should be read in conjunction with the condensed interim consolidated financial statements of the Company and related notes for the first quarter ended December 31, 2023, and the Company's audited consolidated financial statements for the year ended September 30, 2023, and the relates notes. The Company's reporting currency is the Canadian dollar and all amounts in this MD&A are expressed in the Canadian dollars.
Management's Responsibility
The Company's management ("Management") is responsible for the preparation and presentation of the financial statements and this MD&A. The financial statements have been prepared in accordance with International Financial Accounting Standards ("IFRS") as issued by the International Accounting Standards Board. This MD&A is dated as of February 12, 2024 and has been prepared in accordance with the requirements of securities regulators, including National Instrument 51-102 of the Canadian Securities Administrators.
Forward-Looking Statements
This MD&A may include forward-looking statements including opinions, assumptions, estimates, the Company's assessment of future plans and operations, and, more particularly, statements concerning: the Company's milestone projections, including the timing, and costs; the effects of COVID-19 on the Company and its operations; the performance of the science team and related research and development subcontractors, Management and the Board of Directors ("Board") of the Company; current and future strategic partnerships; and the business plan of the Company, generally, including the eventual monetization of the portfolio of patented, selective serotonin (5-HT2C and 5-HT2A-receptor subtypes) agonists described later below. When used in this document, the words "will," "anticipate," "believe," "estimate," "expect," "intent," "may," "project," "should," and similar expressions are intended to be among the statements that identify forward-looking statements. The forward-looking statements are founded on the basis of expectations and assumptions made by the Company which include, but are not limited to: the financial strength of the Company; the eventual market for Company's products; the ability of the Company to obtain and retain applicable licences; and the successful development and implementation of a commercialization strategy, generally. Forward-looking statements are subject to a wide range of risks and uncertainties, and although the Company believes that the expectations represented by such forward-looking statements are reasonable, there can be no assurance that such expectations will be realized. Any number of important factors could cause actual results to differ materially from those in the forward-looking statements including, but not limited to, risks associated with the pharmaceutical industry in general, infringement on intellectual property, failure to benefit from current and future partnerships or successfully integrate acquisitions, actions and initiatives of federal and provincial governments and changes to government policies and the execution and impact of these actions, initiatives and policies, competition from other industry participants, adverse U.S., Canadian and global economic conditions, failure to comply with certain regulations, departure of key management personnel or inability to attract and retain talent regulatory and other factors more fully described from time to time in the reports and filings made by the Company with securities regulatory authorities. Except as required by applicable laws, the Company does not undertake any obligation to publicly update or revise any forward-looking statements.
Any financial outlook and future-oriented financial information contained in this document regarding prospective financial performance, financial position or cash flows is based on assumptions about future events, including economic conditions and proposed courses of action based on management's assessment of the relevant information that is currently available. Projected operational information contains forward-looking information and is based on a number of material assumptions and factors, as are set out above.
These projections may also be considered to contain future-oriented financial information or a financial outlook. The actual results of the Company's operations for any period will likely vary from the amounts set forth in these projections and such variations may be material. Actual results will vary from projected results. Readers are cautioned that any such financial outlook and future-oriented financial information contained herein should not be used for purposes other than those for which it is disclosed herein. The Company has no policy for updating forward looking information beyond the procedures required under applicable securities laws.
BACKGROUND
The Company was incorporated under the Business Corporations Act of British Columbia, Canada, on May 31, 2019. The Company's objective is to generate income and achieve long term profitable growth through the development of therapeutics to improve the lives of patients with certain severe and life-altering diseases. On February 8, 2021, the Company commenced trading on the Canadian Stock Exchange ("CSE") under the symbol DRUG. In addition, the Company began trading on the NASDAQ on November 8, 2021 under the same symbol. The Company's corporate headquarters is 19 Vestry St, New York, NY 10013, USA, and it's registered Canadian address is 1500 - 1055 West Georgia Street, Vancouver, British Columbia, V6E 4N7, Canada.
QUARTERLY HIGHLIGHTS
● Continued research and development ("R&D") of its pipeline programs according to plan, as discussed below.
OVERALL PERFORMANCE
The Company incurred a net loss of $1,684,267 for the three months ended December 31, 2023, compared to a net loss of $2,335,428 for the comparable period. The Company expects to continue to raise additional capital through dilutive equity financings and seek additional investment opportunities to further the development of therapeutics to improve the lives of patients with certain severe and life-altering diseases. The company may also pursue strategic partnerships and licensing opportunities with collaborators, which may or may not generate non-dilutive funds.
GENERAL BUSINESS OVERVIEW
Overview
The Company is a biotechnology company dedicated to developing the next-generation therapeutics to improve the lives of patients with severe and life-altering diseases. The Company is focused on new chemical entities (NCEs) for a variety of central nervous system disorders, including but not limited to pediatric epilepsies, as well as other neuro-psychiatric disorders, including but not limited to depression. The Company's R&D efforts focus on medical indications based on its expertise in 5-HT (serotonin) mediated diseases.
The Company does not advocate for the legalization of psychedelic substances for recreational use or otherwise, and its business is oriented to the discovery of novel, FDA/EMA-approved and regulated serotonergic therapeutics rather than the use of substances such as psilocybin or other psychedelics in new therapies. The Company does not have any direct or indirect involvement with illegal selling, production or distribution of substances in jurisdictions in which it operates.
Targeted Next Generation CNS and Neuro-Psychiatric Therapies
Serotonin (5-HT) is the most prominent neurotransmitter in the brain and modulates many biological functions. Dysfunction of serotonin receptors, transporters, and associated neurocircuits is fundamental to many diseases including epilepsies and neuro-psychiatric disorders such as depression. The class of medications known as selective serotonin reuptake inhibitors ("SSRIs"), such as Prozac®, Zoloft®, and Lexapro®, are widely used in the treatment of depression with a market of US$14.3 Billion.1 Similarly, other serotonergic drugs are widely used in the treatment of pain (Triptans in migraine),2 Alzheimer's and Parkinson's disease related psychosis (Pimavanserin),3 and seizures (Fintepla).4 The off-label use of psilocybin extracts in depression and cluster headache, as well as encouraging clinical trial data with psilocybin and MDMA in depression and PTSD illustrate the potential for advancing serotonergic therapies in neuropsychiatry, pain and substance use disorders (SUD). The full potential of serotonin-based therapeutics has not been achieved due to the lack of medications that are selective and specific to certain serotonin receptor subtypes that are fundamental to disease pathology, without non-specific effects, or other off-target effects on other serotonin receptors in the body that are associated with cardiac toxicities and have resulted in previous drugs being withdrawn from the market.
1 Research and Markets, "Global Antidepressants Market (2020 to 2030) - COVID-19 Implications and Growth" (21 April 2020), online during : Intrado GlobeNewswire <https://www.globenewswire.com/news-release/2020/04/21/2019282/0/en/Global-Antidepressants-Market-2020-to-2030-COVID-19-Implications-and-Growth.html>.
2 Samar Nicolas & Diala Nicolas, "Triptans" (26 May 2020), online: National Center for Biotechnology Information <https://www.ncbi.nlm.nih.gov/books/NBK554507/>.
3 Cerner Multum, "Pimavanserin" (5 February 2020), online: Drugs.com <https://www.drugs.com/mtm/pimavanserin.html>.
4 "Fintepla FDA Approval History", online: Drugs.com <https://www.drugs.com/history/fintepla.html>.
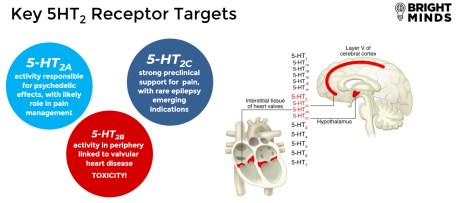
The Company has a portfolio of patented, selective serotonin (5-HT2C, 5-HT2A and 5-HT2C/A-receptor subtypes) agonists that were identified by using high-throughput screening methods in combination with advanced molecular modeling techniques to interrogate the interaction between the drug and its targeted receptors to increase downstream signaling while avoiding off-target effects.

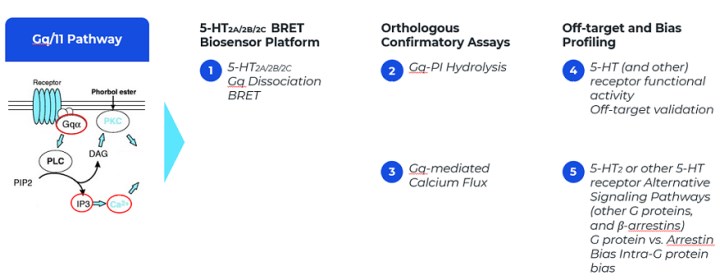
Drug Development Process
The current objective of the Company is to advance the investigational drugs to clinical trials, or to achieve strategic partnerships and/or license agreements with earlier, pre-clinical R&D programs/assets. To achieve this the Company is focused on achieving the following milestones:
1. Lead and back-up compounds synthesis and selection.
2. In-vitro and in-vivo pharmacology screening for lead and back-up compounds as well as studies demonstrating efficacy and safety in relevant animal models.
3. Investigational new drug (IND) package. The package includes pharmacology, drug metabolism, pharmacokinetics and toxicology studies. This package is completed in GLP (good laboratory practice) lab conditions. IND application should be approved by FDA.
4. First in human studies in Australia: Single Dose/Multiple Ascending Dose escalation studies (SAD and MAD) and phase 1b efficacy studies. Studies have been completed in 2023.
5. Phase 2 clinical studies to evaluate efficacy. Initiation of Phase 2 is planned in 2024.
Completion and ongoing work of major R&D Projects:
5-HT2C: BMB-101 for the treatment of rare pediatric epilepsies and other neurological and neuro-psychiatric disorders:
|
|
|
Studies Completed & Other Milestones Accomplished:
|
|
|
|
As of Period Ended,
January 2024
|
Ongoing
|
|
Chemistry, Manufacturing and Controls ("CMC"):
|
|
|
|
● Completion of GLP lot of BMB-101 drug substance for use in toxicology studies
● Manufacturing of GMP batch for human studies
● Formulation development work
● Production of drug product for clinical trial
|
● Stability work for drug substance and drug product
● Improved formulation development planned
|
|
Pharmacology & Toxicology:
|
|
|
Pharmacodynamics
|
● BRET assays, 5-HT receptors profiling; PD in animal models of Dravet:
● Scn1aLab zebrafish model of Dravet syndrome
● Mouse 6-Hz psychomotor seizure model
● Hyperthermia-induced seizures in Scn1a+/- mice
● SmartCube assay
|
● Completed
|
|
|
Safety Pharmacology
|
● In Vitro Safety Pharmacology Profiling at Eurofins
|
● Completed
|
|
|
ADME/PK
|
● In vitro/in vivo and cross-species ADME/PK, human PK prediction
|
● Completed
|
|
|
Toxicology
|
● 28-day toxicology, 90-day toxicology
|
● Completed
|
|
|
|
● Chronic toxicology initiated
|
● Initiated December 2023
|
|
In vivo & In vitro Efficacy; Other MoA-Related Studies
|
|
|
● Fentanyl self-administration rat model of Opioid Use
● Binge eating disorder rat model (backup compound)
● Testing of BMB-101 for behavioral changes in APP+ps1 mice
|
|
|
● Clinical Studies
|
|
|
| First in human clinical studies initiated in Australia 09.22 |
● Placebo controlled phase 1 SAD, MAD and food effects studies.
● Biomarkers analysis from clinical studies
|
● Study successfully completed October 2023
|
| Phase II clinical study |
Open label phase IIa study in photosensitive epilepsies
|
Study planning ongoing
|
5-HT2A: Novel compounds for the treatment of Depression and other neuro-psychiatric disorders
Three separate 5-HT2A-agonist programs are ongoing in-house:
1. Lead program BMB-202 is a fast-on-fast-off (FOFO) compound exhibiting high Cmax and short plasma half-life
2. Mixed 5-HT2A/2C compounds, exhibiting longer plasma half-life, suitable for once daily administration.
3. Non-hallucinogenic 5-HT2A - agonists, an investigational program attempting to identify the mechanism triggering the dissociative effect of classical psychedelics such as psilocybin.
|
|
|
Studies Completed & Other Milestones Accomplished:
|
|
|
|
As of Period Ended,
January 2024
|
Ongoing
|
|
Chemistry, Manufacturing and Controls ("CMC"):
|
|
|
|
● SAR work on 5-HT2A/C agonists
|
● Production of novel Ibogaine analogs for SAR work Production of gram-level of selected compounds for dose range finding toxicology studies
|
|
|
|
● Production of BMB-202 material for GLP-tox Backup program for 5HT2A selective lead BMB-202 ongoing
|
● Completed
|
|
|
|
● Pre-formulation and salt screen studies - BMB-202
|
●
|
|
|
|
● Investigational Non-hallucinogenic 5-HT2A program
|
●
|
|
Pharmacology & Toxicology:
|
|
|
Pharmacodynamics
|
● BRET assays, 5-HT receptors profiling
● Head Twitch Assays
● SmartCube assay
● Identification of potential preclinical drug candidate In vivo studies in rat models of depression
|
● BRET assays, profiling in 5-HTrodent receptors profiling
● Head Twitch Assays
● Forced Swim Test in rat studies
● Locomotor activity in rat studies
● 2A/2C drug candidates with improved profiles.
● IN delivery PD optimization of selected compounds
|
|
|
Safety Pharmacology
|
● In Vitro Safety Pharmacology Profiling at Eurofins for the lead and backup compounds
● In-vitro Safety Pharmacology Profiling at Eurofins - other candidates and follow-up screens
|
● Completed
|
|
|
ADME/PK
|
● In vitro ADME assays ADMEPK package for the lead and backup compounds
● In-vitro/in-vivo and cross-species ADMEPK, human PK prediction for selected compounds
● Modelling of ADMEPK properties in silico
● Profiling ongoing for the backup compounds In-vitro/in-vivo and cross-species ADMEPK, human PK prediction for selected compounds; IN delivery PK
|
● Completed
|
|
|
Toxicology
|
● N/ADRF studies - BMB-202 (rats and dogs)
|
● DRF studies completed, 28-day toxicology studies planned Formulation /Dosage level planned
|
|
In vivo & In vitro Efficacy; Other MoA-Related Studies
|
|
|
● In vivo studies in rat models of depression Animal model strategy refined
● Analysis of Head Twitch data (time-course)
● Neuroplasticity in vitro and in-vivo studies
|
● Other animal models of depression assessments
● Pilot study to assess neuroplasticity effects of BMB compounds
● Assessment of lead candidate in pain models ongoing
|
LIQUIDITY AND CAPITAL RESOURCES
To date, the Company's R&D activities and other operations have been financed through the issuance of equity securities. The Company reviews its working capital position and expected position to manage its liquidity, ensuring that the Company has sufficient cash to meet operational needs.
The Company will require additional capital to fund R&D activities and any significant expansion of operations. Potential sources of capital could include dilutive equity financing, non-dilutive government funding opportunities, new strategic partnership/licensing agreements to fund some or all costs of development, and or debt issuances. There can be no assurance that the Company will be able to obtain the capital sufficient to meet any or all of the Company's needs. The availability of equity or debt financing will be affected by, among other things, the results of our R&D, our ability to obtain regulatory approvals, the market acceptance of our development milestones, the state of the capital markets generally, strategic alliance agreements and other relevant commercial considerations. In addition, if the Company raises additional funds by issuing equity securities, the existing security holders will likely experience dilution, and any incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict the Company's operations. Any failure on the Company's part to raise additional funds on terms favorable or at all may require the Company to significantly change or curtail the current or planned operations in order to conserve cash until such time, if ever, that sufficient proceeds from operations are generated, and could result in the Company not taking advantage of business opportunities, in the termination or delay of clinical trials for our products or in curtailment of the product development programs designed.
At December 31, 2023, the Company had working capital of $5,984,598, including cash of $6,761,647. On December 22, 2023, the Company issued 661,765 Units of the Company at a price per unit of $1.36 for aggregate gross proceeds of $900,000. Each Unit is comprised of one common share and one common share purchase warrant of the Company. Each Warrant is exercisable to acquire one common share of the Company at an exercise price of $1.70 per share until December 22, 2028.
On July 14, 2023, the Directors of the Company approved the consolidation of the Company's issued and outstanding common shares on a 5:1 basis. All common shares, stock options and warrant references in these consolidated financial statements reflect the effect of the share consolidation.
On December 13, 2023, 30,000 RSUs were exercised and $232,500 was reclassified from reserves to share capital upon the exercise.
The Company's current and expected cash resources are sufficient to satisfy working capital requirements of running the operations for the following twelve months; however, the Company has not realized a source of revenue therefore, Management will continue to seek new sources of capital to maintain its operations.
The financial statements of the Company have been prepared in accordance with IFRS applicable to a going concern, which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future.
Management believes that its expected cash resources will be sufficient to fund operations for the next twelve months of research and development while maintaining adequate working capital. The Company continually reassesses the adequacy of its cash resources, evaluating existing research projects and/or potential collaboration opportunities, to determine when and how much additional funding is required.
PREVIOUS FINANCINGS - USE OF PROCEEDS VARIATIONS
|
Date of Issuance/Sale
|
Security Type
|
Number of Securities
|
Issue/Sale Price
|
|
September 30, 2020
|
Common Shares
|
124,788(1)
|
$6.25
|
|
November 2, 2020
|
Common Shares
|
325,828(1)
|
$6.25
|
|
February 3, 2021
|
Common Shares
|
3,200(1)
|
$6.25
|
|
March 17, 2021
|
Common Shares
|
683,977(2)
|
$37.85
|
|
August 30, 2022
|
Common shares
|
571,600(3)
|
$7.00
|
|
December 2, 2022
|
Common Shares
|
194,800(4)
|
$6.25
|
|
December 2, 2022
|
Pre-funded warrants
|
133,200(4)
|
$6.245
|
|
December 22, 2023
|
Common Shares
|
661,765(5)
|
$1.36
|
____________________
Notes:
(1) The use of these financing proceeds as described in the November 18, 2020 Preliminary Prospectus were for research and development activities, as well as working capital and general corporate purposes; there were no variances from this disclosure.
(2) The use of these financing proceeds as described in the February 23, 2021 news release were for research and development activities, as well as working capital and general corporate purposes; there were no variances from this disclosure.
(3) The use of these financing proceeds as described in the August 22, 2022 news release were for working capital and general corporate purposes; there were no variances from this disclosure.
(4) The use of these financing proceeds as described in the November 28, 2022 news release were for research and development activities, as well as working capital and general corporate purposes; there were no variances from this disclosure.
(5) The use of these financing proceeds as described in the December 6, 2023 news release were for research and development activities, as well as working capital and general corporate purposes; there were no variances from this disclosure.
OUTSTANDING SHARE DATA
The Company's share capital as of date of this MD&A is:
|
|
Balance
|
|
|
|
|
Shares issued and outstanding
|
4,463,837
|
|
Share purchase warrants
|
1,709,285
|
|
Pre-funded warrants
|
133,200
|
|
Restricted share units
|
192,000
|
|
Stock options
|
212,161
|
|
|
|
RESULTS OF OPERATIONS AND FIRST QUARTER DISCUSSION
For the Three Months Ended December 31, 2023
Overall Analysis
The Company incurred a net loss of $1,684,267 for the three months ended December 31, 2023, respectively, compared to a net loss of $2,335,428 for the comparable period. The Company decreased its overall research and development activity for the period due to timing of various program starts and completions.
Research and Development Expenditure Analysis
The following table summarizes the material components of research and development expenditure across its drug portfolio:
|
|
For the three months ended
|
|
Drug Portfolio
|
December 31,
2023
|
December 31,
2022
|
|
|
$
|
$
|
|
5-HT2A
|
401,924
|
301,852
|
|
5-HT2C
|
440,114
|
879,847
|
|
5-HT2C/A
|
383,486
|
277,979
|
|
TOTAL
|
1,225,524
|
1,459,678
|
During the three months ended December 31, 2023, the Company decreased its expenditures across the three drugs in its portfolio due to timing differences in program starts.
SELECTED QUARTERLY INFORMATION FOR MOST RECENT COMPLETED QUARTERS
|
|
December 31,
2023
|
September 30,
2023
|
June 30,
2023
|
March 31,
2023
|
|
|
$
|
$
|
$
|
$
|
|
|
|
|
|
|
|
Net profit (loss)
|
(1,684,267)
|
(1,433,018)
|
(1,458,301)
|
(2,050,499)
|
|
Basic profit (loss) per share
|
(0.44)
|
(0.38)
|
(0.39)
|
(0.55)
|
|
Diluted profit (loss) per share
|
(0.44)
|
(0.38)
|
(0.39)
|
(0.55)
|
|
|
December 31,
2022
|
September 30,
2022
|
June 30,
2022
|
March 31,
2022
|
|
|
$
|
$
|
$
|
$
|
|
|
|
|
|
|
|
Net profit (loss)
|
(2,335,428)
|
(2,961,015)
|
(2,658,378)
|
(4,487,503)
|
|
Basic profit (loss) per share
|
(0.65)
|
(1.05)
|
(1.10)
|
(1.90)
|
|
Diluted profit (loss) per share
|
(0.65)
|
(1.05)
|
(1.10)
|
(1.90)
|
For the first quarter of the 2024 fiscal year, the Company reduced overall net loss through reduced research and development costs. For the four quarters in the 2023 fiscal year, the Company reduced overall expenditures primarily driven by a decrease in research and developmental spending. Between the four reporting quarters comprising the 2022 fiscal year end, the Company ramped up its research and development activity with supporting overhead costs utilizing financing proceeds as planned. The trending increase in net loss driven by increased research and development activity and overhead costs should be expected as long as the Company has adequate working capital resources.
FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
The following table summarizes the carrying value of financial assets and liabilities:
| |
|
December 31,
2023 |
|
|
September 30,
2023 |
|
| FVTPL |
|
$ |
|
|
$ |
|
| Cash |
|
6,675,397 |
|
|
6,661,736 |
|
| Guaranteed investment certificate |
|
86,250 |
|
|
86,250 |
|
| Cash and cash equivalents |
|
6,761,647 |
|
|
6,747,986 |
|
| Amortized cost |
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
|
799,597 |
|
|
207,307 |
|
Fair value measurement
Financial assets and liabilities that are recognized on the statement of financial position at fair value can be classified in a hierarchy that is based on the significance of the inputs used in making the measurements.
The levels in the hierarchy are:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 - inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices); and
Level 3 - inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs).
The Company's cash and cash equivalents is classified as Level 1, whereas accounts payable and accrued liabilities are classified as Level 2. As at December 31, 2023, the Company believes that the carrying values of cash and cash equivalents and accounts payable and accrued liabilities approximate their fair values because of their nature and relatively short maturity dates or durations.
Financial risk management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors approves and monitors the risk management processes. The type of risk exposure and the way in which such exposure is managed is provided as follows:
Credit risk
Credit risk is the risk that one party to a financial instrument will fail to discharge an obligation and cause the other party to incur a financial loss. The Company's primary exposure to credit risk is on its cash and cash equivalents balance. As at December 31, 2023, the Company had cash and cash equivalents of $6,761,647 which was held with major banks in Canada, United States and Australia. Because deposits are with three banks, there is a concentration of credit risk. This risk is managed by using major banks that are high credit quality financial institutions as determined by rating agencies. The maximum exposure to credit risk is the carrying amount of the Company's financial instruments. The credit risk is assessed as low.
Foreign exchange risk
Foreign currency risk is the risk that the fair values of future cash flows of a financial instrument will fluctuate because they are denominated in currencies that differ from the respective functional currency. As at December 31, 2023, the Company had the following foreign currency balances - cash (US$573,703 and AU$602,203), receivables (AU$6,381), prepaids (US$11,145 and AU$95) and accounts payable and accrued liabilities (US$61,046, €525, and AU$666,959). A 10% fluctuation in the US$ and AU$ against the Canadian dollar would have an impact of approximately $64,000 on comprehensive loss.
Liquidity risk
Liquidity risk arises through the excess of financial obligations over available financial assets due at any point in time. The Company's objective in managing liquidity risk is to maintain sufficient readily available reserves in order to meet its liquidity requirements at any point in time. The Company's main source of funding has been the issuance of equity securities for cash, primarily through private placements. The Company's access to financing is always uncertain. There can be no assurance of continued access to significant equity funding. As at December 31, 2023, the Company had cash and cash equivalents of $6,761,647 to cover current liabilities of $799,597.
Capital management
Management's objective is to manage its capital to ensure that there are adequate capital resources to safeguard the Company's ability to continue as a going concern through the optimization of its capital structure. The capital structure consists of share capital and working capital. In order to achieve this objective, management makes adjustments to it in light of changes in economic conditions and risk characteristics of the underlying assets. To maintain or adjust the capital structure, management may invest its excess cash in interest bearing accounts of Canadian chartered banks and/or raise additional funds externally as needed. The Company is not subject to externally imposed capital requirements. The Company's management of capital did not change during the period ended December 31, 2023.
RELATED PARTY TRANSACTIONS
Compensation of Key Management Personnel
Key management personnel are those persons that have authority and responsibility for planning, directing and controlling the activities of the Company, directly and indirectly, and by definition include the directors of the Company. All compensation is measured at fair market value.
Included in accounts payable and accrued liabilities as at December 31, 2023 was $57,187 (September 30, 2023 - $51,480) owing to the directors of the Company and the companies controlled by key management personnel.
The following table summarizes expenses related to key management personnel:
| |
|
For the three months ended: |
|
| |
|
December 31,
2023 |
|
|
December 31,
2022 |
|
| |
|
$ |
|
|
$ |
|
| Professional fees |
|
30,000 |
|
|
30,000 |
|
| Research and development |
|
132,377 |
|
|
190,200 |
|
| Share-based compensation included in directors' compensation |
|
113,230 |
|
|
523,964 |
|
| Share-based compensation included in research and development |
|
45,832 |
|
|
(127,256 |
) |
| |
|
321,439 |
|
|
616,908 |
|
Professional fees include amounts paid or accrued to a private Company owned by Ryan Cheung, the Chief Financial Officer of the Company. Research and development comprise fees paid or accrued to Dr. Revati Shreeniwas and Dr. Alan Kozikowski (former directors and December 31, 2022 only), as well as Dr. Mark Smith and Jan Torlief Pedersen both of which are director and/or officers of the Company. Share-based compensation includes the portion stock-based compensation attributed to various directors and officers of the Company as at the date of the option grant.
CRITICAL ACCOUNTING ESTIMATES
Critical accounting estimates
The preparation of the financial statements in conformity with IFRS requires management to make estimates, judgments and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Certain of the Company's accounting policies and disclosures require key assumptions concerning the future and other estimates that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities or disclosures within the next fiscal year. Where applicable, further information about the assumptions made is disclosed in the notes specific to that asset or liability. The critical accounting estimates and judgments set out below have been applied consistently to all periods presented in these financial statements.
CHANGES IN ACCOUNTING POLICIES
New standards and interpretations not yet adopted
A number of new standards, amendments to standards and interpretations are not yet effective for the period June 30, 2023 and have not been applied in preparing these financial statements. The following new standards have not been adopted which may impact the Company in future:
IAS 1 - Presentation of Financial Statements
An amendment to IAS 1 clarifies the criterion for classifying a liability as non-current relating to the right to defer settlement of a liability for at least 12 months after the reporting period.
IAS 1 has amended the definition of material to "information is material if omitting, misstating or obscuring it could reasonably be expected to influence decisions that the primary users of general-purpose financial statements make on the basis of those financial statements, which provide financial information about a specific reporting entity." The previous definition of material from IAS1 was "omissions or misstatements of items are material if they could, individually or collectively, influence the economic decisions that users make on the basis of the financial statements. Materiality depends on the size and nature of the omission or misstatement judged in the surrounding circumstances. The size or nature of the item, or a combination of both, could be the determining factor."
IAS 8 - Accounting Policies, Changes in Accounting Estimates and Errors
IAS 8 amended the definition of material reflect the changes outlined above under IAS 1.
IAS 12 and IFRIC 23 - Income Taxes
IAS 12 currently provides guidance on current and deferred tax assets and liabilities however uncertainty may exist on how tax law applies to certain transactions. IFRIC 23 provides guidance on how to address uncertainty related to tax treatments.
RISK AND UNCERTAINTIES
Limited Operating History
The Company has a very limited history of operations and is considered a start-up company. As such, the Company is subject to many risks common to such enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial and other resources and lack of revenues. There is no assurance that the Company will be successful in achieving a return on shareholders' investment and the likelihood of the Company's success must be considered in light of its early stage of operations.
The Company's actual financial position and results of operations may differ materially from the expectations of the Company's management.
The Company's actual financial position and results of operations may differ materially from management's expectations. The Company has experienced some changes in its operating plans and certain delays in its plans. As a result, the Company's revenue, net income and cash flow may differ materially from the Company's projected revenue, net income and cash flow. The process for estimating the Company's revenue, net income and cash flow requires the use of judgment in determining the appropriate assumptions and estimates. These estimates and assumptions may be revised as additional information becomes available and as additional analyses are performed. In addition, the assumptions used in planning may not prove to be accurate, and other factors may affect the Company's financial condition or results of operations.
The Company may not be successful in its efforts to identify, license or discover additional product candidates.
Although a substantial amount of the Company's effort will focus on the continued research and pre-clinical testing, potential approval and commercialization of its existing product candidates, the success of its business also depends in part upon its ability to identify, license or discover additional product candidates. The Company's research programs or licensing efforts may fail to yield additional product candidates for clinical development for a number of reasons, including but not limited to the following:
• the Company's research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates;
• the Company may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates;
• the Company's product candidates may not succeed in pre-clinical or clinical testing;
• the Company's product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval;
• competitors may develop alternatives that render the Company's product candidates obsolete or less attractive;
• product candidates the Company develops may be covered by third parties' patents or other exclusive rights;
• the market for a product candidate may change during the Company's program so that such a product may become unreasonable to continue to develop;
• a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
• a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors.
If any of these events occurs, the Company may be forced to abandon its development efforts to identify, license or discover additional product candidates, which would have a material adverse effect on its business and could potentially cause the Company to cease operations. Research programs to identify new product candidates require substantial technical, financial and human resources. The Company may focus its efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
There is no assurance that the Company will turn a profit or generate immediate revenues
There is no assurance as to whether the Company will be profitable, earn revenues, or pay dividends. The Company has incurred and anticipates that it will continue to incur substantial expenses relating to the development and initial operations of its business. The payment and amount of any future dividends will depend upon, among other things, the Company's results of operations, cash flow, financial condition, and operating and capital requirements. There is no assurance that future dividends will be paid, and, if dividends are paid, there is no assurance with respect to the amount of any such dividends.
The Company as a going concern
The continued operation of the Company as a going concern is dependent upon the Company's ability to generate positive cash flows and/or obtain additional financing sufficient to fund continuing activities and acquisitions. While the Company continues to review its operations in order to identify strategies and tactics to increase revenue streams and financing opportunities, there is no assurance that the Company will be successful in such efforts; if the Company is not successful, it may be required to significantly reduce or limit operations, or no longer operate as a going concern. It is also possible that operating expenses could increase in order to grow the business. If the Company does not significantly increase its revenue to meet these increased operating expenses and/or obtain financing until its revenue meets these operating expenses, its business, financial condition and operating results could be materially adversely affected. The Company cannot be sure when or if it will ever achieve profitability and, if it does, it may not be able to sustain or increase that profitability.
The Company's intellectual property and licences thereto
The Company's success will depend in part on its ability to protect and maintain its intellectual property rights and its licenses. No assurance can be given that the license or rights used by the Company will not be challenged, invalidated, infringed or circumvented, nor that the rights granted thereunder will provide competitive advantages to the Company. It is not clear whether the pending patent applications will result in the issuance of patents. There is no assurance that the Company will be able to enter into licensing arrangements, develop or obtain alternative technology in respect of patents issued to third parties that incidentally cover its production processes. Moreover, the Company could potentially incur substantial legal costs in defending legal actions which allege patent infringement or by instituting patent infringement suits against others. The Company's commercial success also depends on the Company not infringing patents or proprietary rights of others and not breaching the exclusive license granted to the Company. There can be no assurance that the Company will be able to maintain such licenses that it may require to conduct its business or that such licences have been obtained at a reasonable cost. Furthermore, there can be no assurance that the Company will be able to remain in compliance with its licenses. Consequently, there may be a risk that such licenses may be withdrawn with no compensation or penalties to the Company.
The Company not achieving timelines for project development set out in this Prospectus
The Company's business is dependent on a number of key inputs and their related costs including raw materials and supplies related to its operations, as well as electricity, water and other utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition operating results, and timelines for project development of the Company. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on the business, financial condition, operating results, and timelines for project development of the Company.
The Company faces product liability exposure, which, if not covered by insurance, could result in significant financial liability.
The risk of product liability is inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Product candidates and products that we may commercially market in the future may cause, or may appear to have caused, injury or dangerous drug reactions, and expose the Company to product liability claims. These claims might be made by patients who use the product, healthcare providers, pharmaceutical companies, corporate collaborators or others selling such products. If the Company's product candidates during clinical trials were to cause adverse side effects, the Company may be exposed to substantial liabilities. Regardless of the merits or eventual outcome, product liability claims or other claims related to the Company's product candidates may result in:
• decreased demand for our products due to negative public perception;
• injury to our reputation;
• withdrawal of clinical trial participants or difficulties in recruiting new trial participants;
• initiation of investigations by regulators;
• costs to defend or settle related litigation;
• a diversion of management's time and resources;
• substantial monetary awards to trial participants or patients;
• product recalls, withdrawals or labeling, marketing or promotional restrictions;
• loss of revenues from product sales; and
• the inability to commercialize any of product candidates, if approved.
The Company intends to obtain clinical trial insurance once a clinical trial is initiated. However, the insurance coverage may not be sufficient to reimburse the Company for any expenses or losses it may suffer. Insurance coverage is becoming increasingly expensive, and, in the future, the Company, or any of its collaborators, may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts or at all to protect against losses due to liability. Even if the Company's agreements with any future collaborators entitle it to indemnification against product liability losses, such indemnification may not be available or adequate should any claim arise. The Company's inability to obtain sufficient product liability insurance at an acceptable cost to protect against product liability claims could prevent or inhibit the commercialization of its product candidates. If a successful product liability claim or series of claims is brought against the Company for uninsured liabilities or in excess of insured liabilities, its assets may not be sufficient to cover such claims and its business operations could be impaired.
Should any of the events described above occur, this could have a material adverse effect on the Company's business, financial condition and results of operations.
The Company has international operations, which subject us to risks inherent with operations outside of Canada.
The Company has international operations and may seek to obtain market approvals in foreign markets that it deems could generate significant opportunities. However, even with the cooperation of a commercialization partner, conducting drug development in foreign countries involves inherent risks, including, but not limited to: difficulties in staffing, funding and managing foreign operations; different and unexpected changes in regulatory requirements; export restrictions; tariffs and other trade barriers; different reimbursement systems; economic weaknesses or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labour laws for employees living or travelling abroad; supply chain and raw materials management; difficulties in protecting, acquiring, enforcing and litigating intellectual property rights; fluctuations in currency exchange rates; and potentially adverse tax consequences.
If the Company were to experience any of the difficulties listed above, or any other difficulties, its international development activities and its overall financial condition may suffer and cause it to reduce or discontinue our international development and market approval efforts.
Exchange rate fluctuations between the U.S. dollar and the Canadian dollar may negatively affect the Company's earnings and cash flows.
The Company's functional currency is the Canadian dollar. The Company may incur expenses Canadian Dollars and U.S. dollars. As a result, we are exposed to the risks that the Canadian dollar may devalue relative to the U.S. Dollar, or, if the Canadian dollar appreciates relative to the U.S. Dollar, that the inflation rate in Canada may exceed such rate of devaluation of the Canadian dollar, or that the timing of such devaluation may lag behind inflation in Canada. The Company cannot predict any future trends in the rate of inflation in Canada or the rate of devaluation, if any, of the Canadian dollar against the U.S. Dollar.
If patent laws or the interpretation of patent laws change, the Company's competitors may be able to develop and commercialize our discoveries.
Important legal issues remain to be resolved as to the extent and scope of available patent protection for biopharmaceutical products and processes in Canada and other important markets outside Canada, such as Europe or the United States. As such, litigation or administrative proceedings may be necessary to determine the validity, scope and ownership of certain of our and others' proprietary rights. Any such litigation or proceeding may result in a significant commitment of resources in the future and could force the Company to do one or more of the following: cease selling or using any of its future products that incorporate a challenged intellectual property, which would adversely affect its revenue; obtain a license or other rights from the holder of the intellectual property right alleged to have been infringed or otherwise violated, which license may not be available on reasonable terms, if at all; and redesign its future products to avoid infringing or violating the intellectual property rights of third parties, which may be time-consuming or impossible to do. In addition, changes in patent laws in Canada and other countries may result in allowing others to use its discoveries or develop and commercialize our products. The Company cannot provide assurance that the patents it obtains will afford it significant commercial protection.
The Company may not be able to enforce its intellectual property rights throughout the world. This risk is exacerbated because it expects that one or more of its product candidates will be manufactured and used in a number of foreign countries.
The laws of foreign countries may not protect intellectual property rights to the same extent as the laws of Canada. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This risk is exacerbated for the Company because it expects that future product candidates could be manufactured, and used in a number of foreign countries.
The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to life sciences. This could make it difficult to stop the infringement or other misappropriation of the Company's intellectual property rights. For example, several foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, some countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents and trade secrets may provide limited or no benefit.
Most jurisdictions in which the Company intends to apply for patents have patent protection laws similar to those of Canada, but some of them do not. For example, the Company may do business in the future in countries that may not provide the same or similar protection as that provided in Canada. Additionally, due to uncertainty in patent protection law, the Company has not filed applications in many countries where significant markets exist.
Proceedings to enforce patent rights in foreign jurisdictions could result in substantial costs and divert the Company's efforts and attention from other aspects of its business. Accordingly, efforts to protect intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in Canada, the U.S., and foreign countries may affect our ability to obtain adequate protection for the Company's technology and the enforcement of its intellectual property.
The lack of product for commercialization
If the Company cannot successfully develop, manufacture and distribute its products, or if the Company experiences difficulties in the development process, such as capacity constraints, quality control problems or other disruptions, the Company may not be able to develop market-ready commercial products at acceptable costs, which would adversely affect the Company's ability to effectively enter the market. A failure by the Company to achieve a low-cost structure through economies of scale or improvements in cultivation and manufacturing processes would have a material adverse effect on the Company's commercialization plans and the Company's business, prospects, results of operations and financial condition.
The lack of experience of the Company/Management in marketing, selling, and distribution products
Our management's lack of experience in marketing, selling, and distributing our products could lead to poor decision-making, which could result in cost-overruns and/or the inability to produce the desired products. Although management of the Company intends to hire experienced and qualified staff, this inexperience could also result in the company's inability to consummate revenue contracts or any contracts at all. Any combination of the aforementioned may result in the failure of the Company and a loss of your investment.
The size of the Company's target market is difficult to quantify, and investors will be reliant on their own estimates on the accuracy of market data.
Because the industry in which the Company operates is in a nascent stage with uncertain boundaries, there is a lack of information about comparable companies available for potential investors to review in deciding whether to invest in the Company and, few, if any, established companies whose business model the Company can follow or upon whose success the Company can build. Accordingly, investors will have to rely on their own estimates in deciding about whether to invest in the Company. There can be no assurance that the Company's estimates are accurate or that the market size is sufficiently large for its business to grow as projected, which may negatively impact its financial results.
The Company continues to sell shares for cash to fund operations, capital expansion, mergers and acquisitions that will dilute the current shareholders.
There is no guarantee that the Company will be able to achieve its business objectives. The continued development of the Company will require additional financing. The failure to raise such capital could result in the delay or indefinite postponement of current business objectives or the Company going out of business. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, the terms of such financing will be favourable to the Company.
If additional funds are raised through issuances of equity or convertible debt securities, existing shareholders could suffer significant dilution, and any new equity securities issued could have rights, preferences and privileges superior to those of holders of Common Shares. The Company's articles permit the issuance of an unlimited number of Common Shares, and shareholders will have no pre-emptive rights in connection with such further issuance. The directors of the Company have discretion to determine the price and the terms of issue of further issuances. In addition, from time to time, the Company may enter into transactions to acquire assets or the shares of other companies. These transactions may be financed wholly or partially with debt, which may temporarily increase the Company's debt levels above industry standards. Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for the Company to obtain additional capital and to pursue business opportunities, including potential acquisitions. The Company may require additional financing to fund its operations to the point where it is generating positive cash flows. Negative cash flow may restrict the Company's ability to pursue its business objectives.
The Company's officers and directors may be engaged in a range of business activities resulting in conflicts of interest.
The Company may be subject to various potential conflicts of interest because some of its officers and directors may be engaged in a range of business activities. In addition, the Company's executive officers and directors may devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to the Company. In some cases, the Company's executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to the Company's business and affairs and that could adversely affect the Company's operations. These business interests could require significant time and attention of the Company's executive officers and directors.
In addition, the Company may become involved in other transactions which conflict with the interests of its directors and officers who may from time-to-time deal with persons, firms, institutions or companies with which the Company may be dealing, or which may be seeking investments similar to those desired by it. The interests of these persons could conflict with those of the Company. In addition, from time to time, these persons may be competing with the Company for available investment opportunities. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws. In particular, if such a conflict of interest arises at a meeting of the Company's directors, a director who has such a conflict will abstain from voting for or against the approval of such participation or such terms. In accordance with applicable laws, the directors of the Company are required to act honestly, in good faith and in the best interests of the Company.
In certain circumstances, the Company's reputation could be damaged.
Damage to the Company's reputation can be the result of the actual or perceived occurrence of any number of events, and could include any negative publicity, whether true or not. The increased usage of social media and other web-based tools used to generate, publish and discuss user-generated content and to connect with other users has made it increasingly easier for individuals and groups to communicate and share opinions and views regarding the Company and its activities, whether true or not. Although the Company believes that it operates in a manner that is respectful to all stakeholders and that it takes care in protecting its image and reputation, the Company does not ultimately have direct control over how it is perceived by others. Reputation loss may result in decreased investor confidence, increased challenges in developing and maintaining community relations and an impediment to the Company's overall ability to advance its projects, thereby having a material adverse impact on financial performance, financial condition, cash flows and growth prospects.
Negative Operating Cash Flow
The Company's business has incurred losses since its inception. Although the Company expects to become profitable, there is no guarantee that will happen, and the Company may never become profitable. The Company currently has a negative operating cash flow and may continue to have a negative operating cash flow for the foreseeable future. To date, the Company has not generated any revenues and a large portion of the Company's expenses are fixed, including expenses related to facilities, equipment, contractual commitments and personnel. As a result, the Company expects for its net losses from operations to improve. The Company's ability to generate additional revenues and potential to become profitable will depend largely on its ability to manufacture and market its products and services. There can be no assurance that any such events will occur or that the Company will ever become profitable. Even if the Company does achieve profitability, the Company cannot predict the level of such profitability. If the Company sustains losses over an extended period of time, the Company may be unable to continue its business.
Need for additional financing
The Company believes that it will have sufficient capital to operate its business for at least 12 months following Listing. However, it is possible that costs associated with the operation of the Company's business will exceed its projections depending on the timing of future operating and capital expenses. Assuming the Company's existing funds sustain its operations for this period, the Company believes that it may thereafter require additional capital for additional product development, sales and marketing operations, other operating expenses and for general corporate purposes to fund growth in the Company's markets. The Company does not know how much additional funding it may require. The Company may therefore be required to seek other sources of financing in the future, which sources (assuming it is able to locate such alternative sources of financing) may be on terms less favorable to the Company than those in the Special Warrant Offering. Any additional equity financing may be dilutive to shareholders, and debt financing, if available, may involve restrictive covenants. If additional funds are raised through the issuance of equity securities, the percentage ownership of the shareholders of the Company will be reduced, shareholders may experience additional dilution in net book value per share, or such equity securities may have rights, preferences or privileges senior to those of the holders of the Common Shares. If adequate funds are not available on acceptable terms, the Company may be unable to develop or enhance its products and services, take advantage of future opportunities or respond to competitive pressures, any of which could have a material adverse effect on its business, financial condition and operating results, or the Company may be forced to cease operations.
Uncertainty of Use of Proceeds
Although the Company has set out its intended use of proceeds from this Offering, these intended uses are estimates only and may be subject to change. While management does not contemplate any material variation, management does retain broad discretion in the application of such proceeds. The failure by the Company to apply these funds effectively could have a material adverse effect on the Company's business, including the Company's ability to achieve its stated business objectives.
If the Company has a material weakness in its internal controls over financial reporting, investors could lose confidence in the reliability of its financial statements, which could result in a decrease in the value of its
securities.
One or more material weaknesses in the Company's internal controls over financial reporting could occur or be identified in the future. In addition, because of inherent limitations, the Company's internal controls over financial reporting may not prevent or detect misstatements, and any projections of any evaluation of effectiveness of internal controls to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the Company's policies or procedures may deteriorate. If the Company fails to maintain the adequacy of its internal controls, including any failure or difficulty in implementing required new or improved controls, its business and results of operations could be harmed, the Company may not be able to provide reasonable assurance as to its financial results or meet its reporting obligations and there could be a material adverse effect on the price of its securities.
Difficulties with Forecasts
The Company must rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the pharmaceutical industry in Canada. A failure in the demand for its products and services to materialize as a result of competition, technological change or other factors could have a material adverse effect on the business, results of operations and financial condition of the Company.
COVID-19 may materially and adversely affect the Company's business and financial results.
The Company's business could be materially and adversely affected by health epidemics in regions where the Company conducts research and development activities.
In December 2019, a novel strain of COVID-19 was reported in China. Since then, COVID-19 has spread globally. On March 11, 2020, the World Health Organization (WHO) declared the outbreak of COVID-19 as a "pandemic", or a worldwide spread of a new disease. Many countries around the world, including Canada, the United States and most countries in Europe, have imposed quarantines and restrictions on travel and mass gatherings to slow the spread of the virus, and have closed non-essential businesses.
The COVID-19 pandemic and any other health epidemics have the potential to cause significant disruption in the operations of the laboratories upon whom the Company relies, including laboratories situated in various parts of the United States and Europe. The Company is reliant on the continued operations of such laboratories. The regulations imposed by governments in response to the COVID-19 pandemic may cause laboratories to operate at limited occupancy rates, which may slow the rate at which research and development activities can be conducted. The Company may not have control over the protocols adopted in response to the COVID-19 pandemic by such laboratories in response to the regulations imposed by the governments in the regions in which they operate. The effects of such protocols and/or regulations may negatively impact productivity, disrupt our business and delay our research and development timelines, as well as potentially impact our financial condition and result of operations. The magnitude of these potential effects is uncertain and will depend, in part, on the length and severity of the COVID-19 pandemic and the restrictions imposed by governments in response.
MANAGEMENT'S RESPONSIBILITY FOR THE FINANCIAL STATEMENTS
The information provided in this report is the responsibility of management. In the preparation of these statements, estimates are sometimes necessary to make a determination of future values for certain assets or liabilities. Management believes such estimates have been based on careful judgments and have been properly reflected in the accompanying financial statements.
ADDITIONAL INFORMATION
Additional information relating to the Company, is available on the Canadian System for Electronic Document Analysis and Retrieval ("SEDAR") website at www.sedar.com.
Form 52-109F2
Certification of Interim Filings
Full Certificate
I, Ian McDonald, Chief Executive Officer of Bright Minds Biosciences Inc., certify the following:
1. Review: I have reviewed the interim financial report and interim MD&A (together, the "interim filings") of Bright Minds Biosciences Inc. (the "issuer") for the interim period ended December 31, 2023.
2. No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings.
3. Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings.
4. Responsibility: The issuer's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as those terms are defined in National Instrument 52-109 Certification of Disclosure in Issuers' Annual and Interim Filings, for the issuer.
5. Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer's other certifying officer(s) and I have, as at the end of the period covered by the interim filings
(a) designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that
(i) material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings are being prepared; and
(ii) information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation; and
(b) designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the issuer's GAAP.
5.1 Control framework: The control framework the issuer's other certifying officer(s) and I used to design the issuer's ICFR is Internal Control - Integrated Framework Issued by the Committee of Sponsoring Organization of the Treadway Commission in 2013.
5.2 ICFR - material weakness relating to design: N/A
5.3 Limitation on scope of design: N/A
1
6. Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer's ICFR that occurred during the period beginning on October 1, 2023 and ended on December 31, 2023 that has materially affected, or is reasonably likely to materially affect, the issuer's ICFR.
Date: February 12, 2024
/s/ Ian McDonald
_______________________
Chief Executive Officer
2
Form 52-109F2
Certification of Interim Filings
Full Certificate
I, Ryan Cheung, Chief Financial Officer of Bright Minds Biosciences Inc., certify the following:
1. Review: I have reviewed the interim financial report and interim MD&A (together, the "interim filings") of Bright Minds Biosciences Inc. (the "issuer") for the interim period ended December 31, 2023.
2. No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings.
3. Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim filings.
4. Responsibility: The issuer's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (DC&P) and internal control over financial reporting (ICFR), as those terms are defined in National Instrument 52-109 Certification of Disclosure in Issuers' Annual and Interim Filings, for the issuer.
5. Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer's other certifying officer(s) and I have, as at the end of the period covered by the interim filings
(a) designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that
(i) material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings are being prepared; and
(ii) information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation; and
(b) designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the issuer's GAAP.
5.1 Control framework: The control framework the issuer's other certifying officer(s) and I used to design the issuer's ICFR is Internal Control - Integrated Framework Issued by the Committee of Sponsoring Organization of the Treadway Commission in 2013.
5.2 ICFR - material weakness relating to design: N/A
5.3 Limitation on scope of design: N/A
1
6. Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer's ICFR that occurred during the period beginning on October 1, 2023 and ended on December 31, 2023 that has materially affected, or is reasonably likely to materially affect, the issuer's ICFR.
Date: February 12, 2024
/s/ Ryan Cheung
_______________________
Chief Financial Officer
2
Bright Minds Biosciences (NASDAQ:DRUG)
Historical Stock Chart
From Mar 2024 to Apr 2024
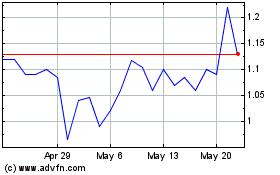
Bright Minds Biosciences (NASDAQ:DRUG)
Historical Stock Chart
From Apr 2023 to Apr 2024