false
0000728387
0000728387
2023-11-27
2023-11-27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT PURSUANT TO
SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
Date of report (Date of earliest event reported): November 27, 2023
|
| |
|
Perspective Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
| |
|
Delaware
(State or Other Jurisdiction
of Incorporation)
|
001-33407
(Commission
File Number)
|
41-1458152
(IRS Employer
Identification No.)
|
2401 Elliott Avenue, Suite 320, Seattle, Washington 98121
(Address of Principal Executive Offices) (Zip Code)
(509) 375-1202
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
☐
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
| |
|
|
☐
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
| |
|
|
☐
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
| |
|
|
☐
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, $0.001 par value
|
CATX
|
NYSE American
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On November 27, 2023, Perspective Therapeutics, Inc. (the “Company”) issued a press release announcing the publication of four preclinical studies in support of the Company's discovery pipeline. A copy of the press release is filed as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference into this Item 8.01.
On November 28, 2023, the Company issued a press release announcing the publication of the first human SPECT images utilizing the alpha-emitting isotope of 212Pb, which was labeled to the Company's proprietary theranostic VMT-α-NET product. A copy of the press release is filed as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated by reference into this Item 8.01.
On November 28, 2023, the Company posted an updated corporate presentation on its website at www.perspectivetherapeutics.com. A copy of the presentation is filed as Exhibit 99.3 to this Current Report on Form 8-K and is incorporated by reference into this Item 8.01.
|
Item 9.01
|
Financial Statements and Exhibits.
|
| |
104
|
Cover Page Interactive Data File (embedded within the Inline XBRL document).
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Dated: November 28, 2023
Perspective Therapeutics, Inc., a Delaware corporation
By: /s/ Johan (Thijs) Spoor
Johan (Thijs) Spoor, Chief Executive Officer
Exhibit 99.1

Perspective Therapeutics Announces Publication of Four Preclinical Studies in Peer-Reviewed Journals
SEATTLE – November 27, 2023 – Perspective, Therapeutics, Inc. (NYSE AMERICAN: CATX), today announced the publication of four preclinical studies in support of the Company’s discovery pipeline. The studies were published in the European Journal of Nuclear Medicine and Molecular Imaging, Journal of Nuclear Medicine, and Pharmaceuticals.
“The results from these preclinical studies are compelling and continue to support our ongoing clinical efforts to advance our novel lead-based targeted alpha-particle therapies. Overall, these data demonstrate a dose-dependent therapeutic benefit of our treatment candidate [212Pb]VMT-α-NET in mouse models, and also demonstrate the high radiolabeling yields and high radiochemical purity and stability that are achievable using our radiopharmaceutical production process,” commented Michael Schultz, PhD, Chief Science Officer of Perspective Therapeutics. “Additionally, our collaborative investigations with our academic research partners are enhancing our understanding of biomarkers (e.g., NGal) that can be used in optimizing the therapeutic window for not only our alpha-particle therapies, but all tumor targeted radiopharmaceutical therapies. The use of these biomarkers has the potential to improve our ability to monitor patient safety and optimize the effectiveness of treatment. We are grateful to all our collaborators and look forward to generating and contributing additional data to our community in 2024.”
Publication One
Title: Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake
Summary: This study in Pharmaceuticals (November 2023, 16(11), 1605) notes that the molar activity (AM) can play a crucial role in tumor uptake, especially in receptor-mediated uptake, such as in neuroendocrine tumors. In this work, the influence of the AM-to-cell uptake of 203/212Pb-PSC-TOC was investigated in multiple different cell lines to develop a more detailed understanding of the tracer in preparation for clinical use. This study provides independent confirmatory evidence of the stability of the 212Bi daughter radionuclide in formulation upon the decay of 212Pb, generating >95% radiochemical purity in their study.
Key Highlights:
|
●
|
The quality control showed a radiochemical yield greater than 95% in most cases for 203/212Pb-PSC-TOC, confirming stability of the 212Bi in the Perspective chelator.
|
|
●
|
Higher AM, correlated positively with cell uptake. A moderate AM of 15–40 MBq/nmol showed the highest cell uptake.
|
|
●
|
No uptake limitation was found in the first 24 to 48 hours. However, authors suggest that further escalation experiments with higher AM should be performed.
|
Author Affiliations: University Hospital Carl Gustav Carus, Technical University Dresden, Dresden, Germany; The University of Iowa, Iowa City, Iowa, USA.
Publication Two
Title: Structural Modifications Toward Improved Lead-203/Lead-212 Peptide-Based Image-Guided Alpha-Particle Radiopharmaceutical Therapies for Neuroendocrine Tumors
Summary: This study in the European Journal of Nuclear Medicine and Molecular Imaging (in press as of November 2023) aims to improve the performance of somatostatin receptor subtype 2 (SSTR2)-targeted radionuclide imaging and therapy through structural modifications to Tyr3-octreotide (TOC)-based radiopharmaceuticals. The findings suggest that PSC-PEG2-TOC (i.e., VMT-a-NET) is a promising candidate for Pb-based targeted therapy for SSTR2 positive tumors.
Key Highlights:
|
●
|
The data demonstrated that the modified radiopeptide drug conjugate (RPDC), [203Pb]PSC-PEG2-TOC, significantly improved tumor-targeting properties, including receptor binding, tumor accumulation and retention, and exhibited faster renal clearance as compared to [203Pb]-DOTATOC.
|
|
●
|
Treatment resulted in a dose-dependent therapeutic effect with minimal signs of toxicity in a tumor xenograft mouse model.
|
|
●
|
Fractionated administrations of 3.7 MBq [212Pb]PSC-PEG2-TOC over three doses further improved antitumor effectiveness, resulting in 80% survival (70% complete response) over 120 days in the mouse model.
|
Author Affiliations: Korea Military Academy, Seoul, Republic of Korea; Perspective Therapeutics, Coralville, Iowa, USA; The University of Iowa, Iowa City, Iowa, USA.
Publication Three
Title: Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets
Summary: This study published in the Journal of Nuclear Medicine (November 2023, 64 (11) 1791-1797) aims to optimize the production and separation of high specific activity 203Pb using electroplated Thallium targets suitable for cyclotron irradiation. This work contributes to the broader body of knowledge to satisfy the growing interest in clinical applications for theranostic match pair (203Pb/212Pb).
Key Highlights:
|
●
|
The data confirmed the development of an efficient electroplating method for routine 203Pb production.
|
|
●
|
The data demonstrated that 203Pb production was fast and could be achieved in under 1.5 hours, the method employed a simple separation process that resulted in high recovery yields of approximately 95%, and high purity.
|
|
●
|
The study showed that the novel electroplating method is compatible with a 24 MeV incident beam and high current without using a degrader.
|
|
●
|
Results corroborated evidence published in a recent article by our colleagues at the University of Alberta (Nelson et al., Nuclear Medicine and Biology 116–117 (2023) 108314) that the production of 203Pb is relatively straightforward and scalable.
|
Author Affiliations: University of Alabama at Birmingham, Alabama, USA; Perspective Therapeutics, Coralville, Iowa, USA; The University of Iowa, Iowa City, Iowa, USA.
Publication Four
Title: Pre-clinical Evaluation of Biomarkers for Early Detection of Nephrotoxicity Following Alpha-Particle Radioligand Therapy
Summary:
This publication in the European Journal of Nuclear Medicine and Molecular Imaging 2023 (in press as of November 2023) highlights findings from a pre-clinical study evaluating biomarkers for early detection of nephrotoxicity following alpha-particle radioligand therapy (α-RLT). The results suggest that increased biomarker urinal neutrophil gelatinase-associated lipocalin (NGAL) secretion could be an additional diagnostic tool to improve our understanding of the potential for subclinical acute kidney injury (AKI) arising from radionuclide therapies and have the potential to guide more effective therapies with a lower risk of chronic kidney disease (CKD).
Key Highlights:
|
●
|
Early secretion of urinary biomarker NGAL may be a valuable tool for early detection of kidney injury in response to radioligand therapies and has the potential to assist in optimizing therapies.
|
|
●
|
Identification and feasibility of the use of urinary epidermal growth factor as a biomarker response readout that may provide evidence of early tubular damage in response to radioligand therapies.
|
Author Affiliations: Nationwide Children's Hospital, Columbus, Ohio, USA; The University of Iowa, Iowa City, Iowa, USA; Korea Military Academy, Seoul, Republic of Korea; and The Ohio State University College of Veterinary Medicine, Columbus, Ohio, USA.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope Lead-212 to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions in the United States. The Company has also developed a proprietary Lead-212 generator to secure key isotopes for clinical trial and commercial operations.
In addition to its targeted alpha therapy programs, Perspective is the sole producer of Cesium-131 brachytherapy seeds which are commercially available in the United States for the treatment of prostate cancer and other solid tumors.
For more information, please visit the Company's website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things: the Company’s belief that use of certain biomarkers has the potential to improve the ability to monitor patient safety and optimize the effectiveness of treatment; the Company’s expectation that early secretion of urinary biomarker NGAL may be a valuable tool for early detection of kidney injury in response to radioligand therapies and has the potential to assist in optimizing therapies; the Company’s expectation that identification and feasibility of the use of urinary epidermal growth factor as a biomarker response readout may provide evidence of early tubular damage in response to radioligand therapies; the Company’s prediction that complementary imaging diagnostics that incorporate certain targeting peptides provide the opportunity to personalize treatment and optimize patient outcomes; the Company’s expectation that its "theranostic" approach enables the ability to see specific tumors and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments; the Company's clinical development plans and the expected timing thereof; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the Company’s ability to continue as a going concern; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; whether there is sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, FDA fast track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Transition Report on Form 10-KT and the Company's most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports to be filed with the SEC and available at www.sec.gov.
Forward-looking statements contained in this press release are made as of this date, and the Company undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Exhibit 99.2

Perspective Therapeutics Announces First Ever Human SPECT Imaging of Pb-212 with VMT-α-NET by Clinical Collaborators in Germany
| |
-
|
Imaging was conducted as part of a clinical study at Technical University of Dresden in Germany
|
| |
-
|
Image was selected as “Image of the Month” in the European Journal of Nuclear Medicine and Molecular Imaging, November 2023
|
| |
-
|
Radiolabeling was performed on-site using a Company-provided generator
|
SEATTLE – November 28, 2023 – Perspective Therapeutics, Inc. (“Perspective” or “the Company”) (NYSE AMERICAN: CATX), today announced the publication of the first human SPECT images utilizing the alpha-emitting isotope of 212Pb, which was labeled to the Company’s proprietary theranostic VMT-α-NET product. The imaging was conducted as part of a series of four neuroendocrine tumor (NET) patients who were administered VMT-α-NET at a clinical study site in Germany. The Company is developing VMT-α-NET for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2)-expressing neuroendocrine tumors.
VMT-α-NET is being administered under the supervision of Prof. Dr. Jörg Kotzerke, Director of the Department of Nuclear Medicine at the Technical University of Dresden in Germany. The patient received 90 MBq (2.4mCi) of [212Pb]VMT-α-NET intravenously, and whole-body scintigraphy and SPECT/CT images were acquired 2 hours, 5 hours, and 19 hours after injection. Images were collected on a Symbia Intevo T6 (Siemens Healthineers) using a high-energy collimator. The SPECT/CT images showed high accumulation of [212Pb]VMT-α-NET in liver metastases and were consistent with the previously acquired [68Ga]DOTATATE PET/CT. High tumor retention can be observed in the planar and SPECT/CT images over time. As expected, due to the short half-life of 212Pb (10.6 hours), the images acquired after 19 hours showed a high level of noise due to the low count statistics. The patient showed no early or acute side effects.
“We are pleased to be able to provide our patients with Perspective’s novel targeted alpha-particle therapy. This imaging study represents a world-first and shows that [212Pb]VMT-α-NET can be imaged after administration,” commented Dr. Kotzerke. “Particularly encouraging is the uptake and the retention in the tumors, and the concordance of this data with existing standard of care imaging. NET patients are in desperate need for new treatment options that are safe and effective.”
Under certain expanded access circumstances, VMT-α-NET may be made available to qualified doctors in some countries. Perspective Therapeutics supplies a generator, drug precursors and isotopes for the local production of its proprietary radiotherapeutic, VMT-α-NET.
"Expanding our collaboration with Prof. Dr. Kotzerke and his colleagues at the Technical University of Dresden is a key milestone as we work to improve access to VMT-α-NET, our potentially life-changing treatment, to patients with burdensome NETs,” said Thijs Spoor, Chief Executive Officer at Perspective Therapeutics. “The use of post-therapy SPECT imaging to confirm tumor uptake and clearance from healthy organs is of particular interest moving forward, as it means that post-therapy dosimetry can be used in next-cycle treatment planning by physicians using our products. We trust the healthcare systems that value dosimetry and patient safety will work with us to be able to have this covered as a routine part of patient care.”
Dr. Kotzerke and his team will continue to monitor patients’ progress over the coming months. The publication is entitled “First-in-human SPECT/CT imaging of [212Pb]Pb-VMT-α-NET in a patient with metastatic neuroendocrine tumor” and is available in the European Journal of Nuclear Medicine and Molecular Imaging at https://link.springer.com/article/10.1007/s00259-023-06529-1. It follows a similar publication in Clinical Nuclear Medicine from earlier this year showing successful SPECT imaging of Perspective’s surrogate diagnostic isotope 203Pb which was also linked to VMT-α-NET.
Recruitment is ongoing for the use of VMT-α-NET in imaging and therapeutic trials at leading U.S. institutions. VMT-α-NET is categorized as an investigational new drug by the U.S. Food and Drug Administration. The use of VMT-α-NET in qualifying expanded access situations is entirely separate from and outside the scope of the Company's Phase 1 trial.
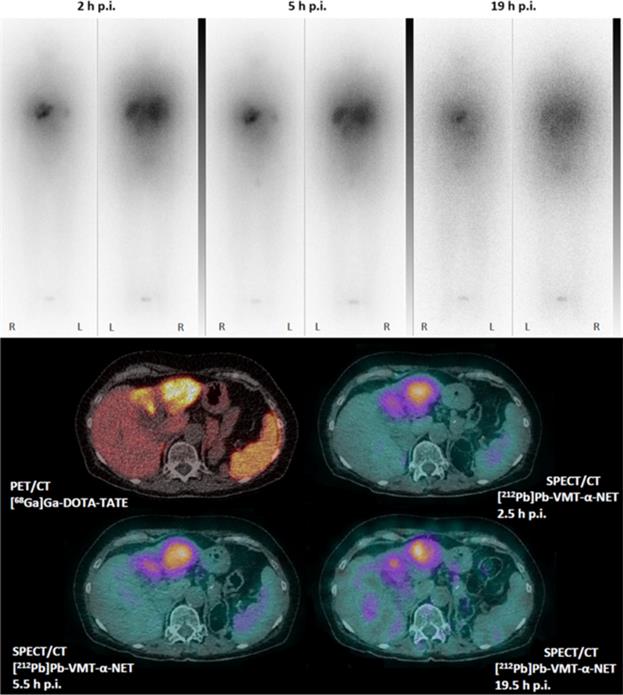
Michler, E., Kästner, D., Brogsitter, C. et al. First-in-human SPECT/CT imaging of [212Pb]Pb-VMT-α-NET in a patient with metastatic neuroendocrine tumor. Eur J Nucl Med Mol Imaging (2023). https://doi.org/10.1007/s00259-023-06529-1
About Neuroendocrine Tumors
Neuroendocrine tumors form in cells that interact with the nervous system or in glands that produce hormones. They can originate in various parts of the body, most often in the gut or the lungs and can be benign or malignant. Neuroendocrine tumors are typically classified as pancreatic neuroendocrine tumors or non-pancreatic neuroendocrine tumors. According to cancer.net, it is estimated that more than 12,000 people in the United States are diagnosed with a NET each year. Importantly, neuroendocrine tumors are associated with a relatively long duration of survival compared to other tumors and as a result, there are approximately 175,000 people living with this diagnosis.
About VMT-α-NET
VMT-α-NET is a clinical stage targeted alpha particle therapy (TAT) radiopharmaceutical being developed for the treatment and diagnosis of somatostatin receptor subtype 2 (SSTR2) expressing neuroendocrine tumors, which are a rare and difficult-to-treat type of cancer. VMT-α-NET incorporates Perspective Therapeutics' proprietary lead-specific chelator (PSC) to bind Pb-203 for SPECT imaging, and Pb-212 for alpha particle therapy.
About Perspective Therapeutics, Inc.
Perspective Therapeutics, Inc., is a diversified medical technology and radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body. The Company has a proprietary technology that utilizes the alpha emitting isotope Lead-212 to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides which provide the opportunity to personalize treatment and optimize patient outcomes. This “theranostic” approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments.
The Company’s melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions. The Company has also developed a proprietary Lead-212 generator to secure key isotopes for clinical trial and commercial operations.
Perspective is the sole producer of Cesium-131 brachytherapy seeds which are commercially available in the United States for the treatment of prostate cancer and other solid tumors.
For more information, please visit the Company’s website at www.perspectivetherapeutics.com.
Safe Harbor Statement
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things: the Company’s expectation that VMT-α-NET may be made available to qualified doctors in some countries under compassionate use circumstances; the Company’s belief that VMT-α-NET is a potentially life-changing treatment; the Company’s expectation that post-therapy dosimetry should open up a range of patient care options for the physicians who will be using the Company’s products; the Company’s prediction that the healthcare systems that value dosimetry and patient safety will work with the Company to be able to have post-therapy SPECT imaging covered as a routine part of patient care; the Company’s expectation that Dr. Kotzerke and his team will continue to monitor patients’ progress over the coming months; the Company’s prediction that complementary imaging diagnostics that incorporate certain targeting peptides provide the opportunity to personalize treatment and optimize patient outcomes; the Company’s expectation that its "theranostic" approach enables the ability to see specific tumors and then treat it to potentially improve efficacy and minimize toxicity associated with many other types of cancer treatments; the Company's clinical development plans and the expected timing thereof; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; the Company’s ability to continue as a going concern; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; whether there is sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, FDA fast track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Transition Report on Form 10-KT and the Company's most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports to be filed with the SEC and available at www.sec.gov.
Forward-looking statements contained in this press release are made as of this date, and the Company undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law.
Investor Relations Contact:
LifeSci Advisors
Chuck Padala
E: chuck@lifesciadvisors.com
Exhibit 99.3
v3.23.3
Document And Entity Information
|
Nov. 27, 2023 |
| Document Information [Line Items] |
|
| Entity, Registrant Name |
Perspective Therapeutics, Inc.
|
| Document, Type |
8-K
|
| Document, Period End Date |
Nov. 27, 2023
|
| Entity, Incorporation, State or Country Code |
DE
|
| Entity, File Number |
001-33407
|
| Entity, Tax Identification Number |
41-1458152
|
| Entity, Address, Address Line One |
2401 Elliott Avenue, Suite 320
|
| Entity, Address, City or Town |
Seattle
|
| Entity, Address, State or Province |
WA
|
| Entity, Address, Postal Zip Code |
98121
|
| City Area Code |
509
|
| Local Phone Number |
375-1202
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock
|
| Trading Symbol |
CATX
|
| Security Exchange Name |
NYSEAMER
|
| Entity, Emerging Growth Company |
false
|
| Amendment Flag |
false
|
| Entity, Central Index Key |
0000728387
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
IsoRay (AMEX:ISR)
Historical Stock Chart
From Mar 2024 to Apr 2024
IsoRay (AMEX:ISR)
Historical Stock Chart
From Apr 2023 to Apr 2024