UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
October 31, 2014
Date of Report (Date of earliest event reported)
MESA LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
Commission File Number: 0-11740
|
COLORADO
(State or other jurisdiction of
incorporation) |
84-0872291
(I.R.S. Employer
Identification No.) |
|
12100 WEST SIXTH AVENUE,
LAKEWOOD, COLORADO
(Address of principal executive offices) |
80228
(Zip Code) |
Registrant’s telephone number, including area code: (303) 987-8000
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|_| |
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|_| |
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|_| |
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|_| |
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
ITEM 8.01 OTHER EVENTS
On October 31, 2014, Amilabo SAS (“Amilabo”), a wholly owned subsidiary of Mesa Laboratories, Inc. (the “Company”), entered into and closed an asset purchase agreement (the “Agreement”) with ATI Atlas, Limited (“ATI”) whereby it acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of ATI. ATI is located near London and is a distributor of the Company’s biological indicator products.
ITEM 9.01 FINANCIAL STATEMENTS AND EXHIBITS
|
(d) |
Exhibits: |
| |
|
| |
99.1 Press release dated October 31, 2014. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
DATE: October 31, 2014 |
Mesa Laboratories, Inc.
(Registrant) |
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ John J. Sullivan, |
|
|
|
BY: |
John J. Sullivan, |
|
|
|
|
President and Chief Executive Officer |
|
Exhibit 99.1
Mesa Labs Acquires its UK and Irish Republic Distributor of Biological Indicators
Lakewood, Colorado, October 31, 2014 – Mesa Laboratories, Inc. (NASDAQ:MLAB) (we, us, our, “Mesa” or the “Company”) today announced the acquisition of substantially all of the assets and certain liabilities of ATI Atlas Limited, (“ATI”) by Amilabo SAS (“Amilabo”), the Company’s subsidiary located near Lyon, France. ATI, located near London, is a distributor of the Company’s biological indicator (“BI”) products in the UK.
Biological indicators are used to assess the effectiveness of sterilization processes, including steam, gas (such as Ethylene Oxide or Chlorine Dioxide), hydrogen peroxide and radiation, in the hospital, dental, medical device and pharmaceutical industries. ATI has been a distributor of Mesa’s BI products for nearly 16 years, selling primarily to the pharmaceutical and medical device manufacturing markets in the UK. After a short transition period, Amilabo will service the markets in the UK from their office near Lyon. Centralized supply of BI products from Lyon will improve the service to ATI’s customers by increasing product availability and reducing shipping time.
“This is the second acquisition of one of our distributors in Europe, following the purchase of Amilabo in April,” said John J. Sullivan, President and CEO of Mesa. “The acquisition of ATI will enable us to sell direct in the UK, an important market for our BI products. Selling direct in France, and now the UK, allows Mesa to capture additional margin and to better understand the needs of our customers, enabling us to grow our European BI business more effectively.”
“Mesa’s BI’s are well established with most major medical device manufacturers in the UK and Irish Republic, as the preferred choice for quality assurance. To meet market demands from a central location in Europe makes good sense for both Mesa and its customers,” said Nigel Chitty, Managing Director of ATI.
About Mesa Laboratories, Inc.
We pursue a strategy of focusing primarily on quality control products, which are sold into niche markets that are driven by regulatory requirements. We prefer markets that have limited competition where we can establish a commanding presence and achieve high gross margins. We are organized into three divisions across six physical locations. Our Instruments Division designs, manufactures and markets quality control instruments and disposable products utilized in connection with the healthcare, pharmaceutical, food and beverage, medical device, industrial hygiene, environmental air sampling and semiconductor industries. Our Biological Indicators Division manufactures and markets biological indicators and distributes chemical indicators used to assess the effectiveness of sterilization processes, including steam, gas, hydrogen peroxide, ethylene oxide and radiation, in the hospital, dental, medical device and pharmaceutical industries. Our Continuous Monitoring Division designs, develops and markets systems which are used to monitor various environmental parameters such as temperature, humidity and differential pressure to ensure that critical storage and processing conditions are maintained in hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies and a number of other laboratory and industrial environments.
Forward Looking Statements
This press release may contain information that constitutes "forward-looking statements." Generally, the words "believe," "expect," "intend," “anticipate,” "estimate," "project," "will" and similar expressions identify forward-looking statements, which generally are not historical in nature. However, the absence of these words or similar expressions does not mean that a statement is not forward-looking. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future — including statements relating to revenue growth and statements expressing general views about future operating results — are forward-looking statements. Management believes that these forward-looking statements are reasonable as and when made. However, caution should be taken not to place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our historical experience and present expectations or projections. These risks and uncertainties include, but are not limited to, those described in our Annual Report on Form 10-K for the year ended March 31, 2014, and those described from time to time in our subsequent reports filed with the Securities and Exchange Commission.
CONTACT: John J. Sullivan, Ph.D.; President and CEO, or John Sakys; CFO, both of Mesa Laboratories, Inc., +1-303-987-8000
For more information about the Company, please visit its website at www.mesalabs.com.
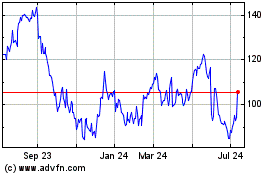
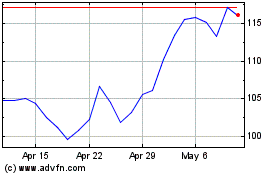
Mesa Laboratories (NASDAQ:MLAB)
Historical Stock Chart
From Aug 2024 to Sep 2024
Mesa Laboratories (NASDAQ:MLAB)
Historical Stock Chart
From Sep 2023 to Sep 2024