As filed with the Securities and Exchange Commission on December , 2023.
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_______________
Form F-3
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
_______________
Virax Biolabs Group Limited
(Exact name of Registrant as specified in its charter)
_______________
Not Applicable
(Translation of Registrant’s name into English)
_______________
|
|
|
|
|
|
|
|
|
|
Cayman Islands |
|
2835 |
|
Not Applicable |
(State or other jurisdiction of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
20 North Audley Street
London, W1K 6LX
United Kingdom
Telephone: +44 020 7788 7414
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
_______________
Virax Biolabs USA Management, Inc.
23501 Cinco Ranch Blvd. Ste H120-289
Katy, TX 77494
+44 020 7788 7414
(Name, address, including zip code, and telephone number, including area code, of agent for service)
_______________
Copies of all communications, including communications sent to agent for service, should be sent to:
|
Lawrence S. Venick, Esq.
Loeb & Loeb LLP
2206-19 Jardine House
1 Connaught Place, Central
Hong Kong SAR
Telephone: +852-3923-1111
Fax: +852-3923-1100 |
_______________
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement becomes effective.
|
|
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. |
☒ |
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. |
☐ |
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. |
☐ |
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. |
☐ |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act: Emerging growth company. |
☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 7(a)(2)(B) of the Securities Act. |
☐ |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to such Section 8(a), may determine.
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
EXPLANATORY NOTE
This registration statement contains two prospectuses:
•a base prospectus which covers the offering, issuance and sale by us of up to US$30,000,000 of our ordinary shares, preferred stock, debt securities, warrants and/or units; and
•a secondary offering prospectus which covers the offer and sale by the selling stockholders described therein of up to 15,195,292 ordinary issuable upon the exercise of the New Warrants (defined below) issued to Armistice Capital Master Fund Ltd. and Placement Agent Warrants (defined below) issued to certain affiliates of H.C. Wainwright & Co., LLC.
The base prospectus immediately follows this explanatory note. The secondary offering prospectus immediately follows the base prospectus. The 15,195,292 ordinary shares that may be offered and sold under the secondary offering prospectus are not included in the US$30,000,000 of securities that may be offered, issued and sold by us under the base prospectus.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated December 5, 2023
PROSPECTUS

US$30,000,000
Ordinary Shares
Preferred Shares
Debt Securities
Warrants
Units
Rights
From time to time, we may offer, issue and sell up to US$30,000,000 of any combination of the securities described in this prospectus in one or more offerings. We may also offer securities as may be issuable upon conversion, redemption, repurchase, exchange or exercise of any securities registered hereunder, including any applicable antidilution provisions.
This prospectus provides a general description of the securities we may offer. Each time we offer securities, we will provide specific terms of the securities offered in a supplement to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before you invest in any of the securities being offered.
This prospectus may not be used to sell our securities unless accompanied by a prospectus supplement. The prospectus supplement or any related free writing prospectus may also add to, update, supplement or clarify information contained in this prospectus.
Pursuant to General Instruction I.B.5. of Form F-3, in no event will we sell the securities covered hereby in a public primary offering with a value exceeding more than one-third of the aggregate market value of our Ordinary Shares in any 12-month period so long as the aggregate market value of our outstanding Ordinary Shares held by non-affiliates remains below $75,000,000. The aggregate market value of our outstanding voting and non-voting common equity held by non-affiliates is approximately $4,827,696 based on the closing price of $0.24 per ordinary share on November 27, 2023 and 14,840,381 ordinary shares held by non-affiliates. During the 12 calendar months prior to and including the date of this prospectus, we have not offered or sold any securities pursuant to General Instruction I.B.5 of Form F-3.
Our Ordinary Shares are listed on the Nasdaq Capital Market under the symbol “VRAX.” The applicable prospectus supplement will contain information, where applicable, as to other listings, if any, on the Nasdaq Capital Market or other securities exchange of the securities covered by the prospectus supplement.
Investing in our securities involves a high degree of risk. See “Risk Factors” on page 18 of this prospectus and in the documents incorporated by reference in this prospectus, as updated in the applicable prospectus supplement, any related free writing prospectus and other future filings we make with the Securities and Exchange Commission that are incorporated by reference into this prospectus, for a discussion of the factors you should consider carefully before deciding to purchase our securities.
We may sell these securities directly to investors, through agents designated from time to time or to or through underwriters or dealers. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. If any underwriters are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such underwriters and any applicable commissions or discounts will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth in a prospectus supplement.
Investing in our securities is highly speculative and involves a significant degree of risk. Virax Biolabs Group Limited, which we refer to as "Virax Cayman", is a holding company incorporated as an exempted company under the laws of the Cayman Islands. As a holding company with no material operations of our own, Virax Cayman conduct a substantial majority of our sales and trading activities through our operating entity established in Singapore, Virax Biolabs Pte. Limited, which we refer to as SingaporeCo. Currently, Virax Cayman indirectly owns 95.65% of the equity interests in SingaporeCo. However, some of Virax Cayman’s operations are currently conducted through our operating entities established in the British Virgin Islands, Hong Kong and Shanghai, primarily, Logico Bioproducts Corp., Virax Immune T-Cell Medical Device Company Limited and Shanghai Xitu Consulting Co., Limited, which we refer to as Logico BVI, Virax Immune T-Cell and Shanghai Xitu, respectively. Our Ordinary Shares offered in this prospectus are shares of our Cayman Islands holding company.
Recent statements by the Chinese government have indicated an intent to exert more oversight and control over offerings that are conducted overseas and/or foreign investments in China based issuers. Any future action by the Chinese government expanding the categories of industries and companies whose foreign securities offerings are subject to government review could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly decline or be worthless.
Recently, the PRC government initiated a series of regulatory actions and made a number of public statements on the regulation of business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas using a variable interest entity structure, adopting new measures to extend the scope of cybersecurity reviews, and expanding efforts in anti-monopoly enforcement. We are not subject to these regulatory actions or statements, as we do not have a variable interest entity structure and our business does not involve the collection of user data, implicate cybersecurity, or involve any other type of restricted industry. Because these statements and regulatory actions are new, however, it is highly uncertain how soon legislative or administrative regulation making bodies in China will respond to them, or what existing or new laws or regulations will be modified or promulgated, if any, or the potential impact such modified or new laws and regulations will have on our daily business operations or our ability to accept foreign investments and list on an U.S. exchange.
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. In accordance with the HFCA Act, trading in securities of any registrant on a national securities exchange or in the over-the-counter trading market in the United States may be prohibited if the Public Company Accounting Oversight Board (the “PCAOB”) determines that it cannot inspect or fully investigate the registrant’s auditor for three consecutive years beginning in 2021, and, as a result, an exchange may determine to delist the securities of such registrant. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, which, if enacted, would amend the HFCA Act and require the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period before our securities may be prohibited from trading or delisted if our auditor is unable to meet the PCAOB inspection requirement. Pursuant to the HFCA Act, the PCAOB issued a Determination Report on December 16, 2021 which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. In addition, the PCAOB’s report
identified the specific registered public accounting firms which are subject to these determinations. On August 26, 2022, the PCAOB entered into a Statement of Protocol with the China Securities Regulatory Commission and the Ministry of Finance of the PRC and, as summarized in the “Statement on Agreement Governing Inspections and Investigations of Audit Firms Based in China and Hong Kong” published on the U.S. Securities and Exchange Commission’s official website, the parties agreed to the following: (i) in accordance with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation; (ii) the PCAOB shall have direct access to interview or take testimony from all personnel of the audit firms whose issuer engagements are being inspected or investigated; (iii) the PCAOB shall have the unfettered ability to transfer information to the SEC, in accordance with the Sarbanes-Oxley Act; and (iv) the PCAOB inspectors shall have access to complete audit work papers without any redactions, with view-only procedures for certain targeted pieces of information such as personally identifiable information. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, whether the PCAOB will continue to be able to satisfactorily conduct inspections of PCAOB-registered public accounting firms headquartered in mainland China and Hong Kong is subject to uncertainty and depends on a number of factors out of our, and our auditor’s, control. The PCAOB is continuing to demand complete access in mainland China and Hong Kong moving forward and is already making plans to resume regular inspections in early 2023 and beyond, as well as to continue pursuing ongoing investigations and initiate new investigations as needed. In the future, if there is any regulatory change or step taken by PRC regulators that does not permit our auditor to provide audit documentations located in China or Hong Kong to the PCAOB for inspection or investigation, or the PCAOB expands the scope of the Determination so that we are subject to the HFCA Act, as the same may be amended, you may be deprived of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities, including trading on the national exchange and trading on “over-the-counter” markets, may be prohibited under the HFCA Act. On December 29, 2022, a legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”), was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two. Our registered public accounting firm, Reliant CPA PC , is not headquartered in mainland China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determination. Notwithstanding the foregoing, if the PCAOB is not able to fully conduct inspections of our auditor’s work papers in China, you may be deprived of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities may be prohibited under the HFCA Act.
Within the organization, investor cash inflows have all been received by Virax Cayman. Cash to fund Virax Cayman’s operations is transferred from Virax Cayman down through our Singapore, Hong Kong, BVI entities and then into our Chinese entity through capital contributions and loans. However, the PRC government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China, and investment in Chinese companies, which are governed by the Foreign Investment Law and Company Law, and the dividends and distributions from Shanghai Xitu is subject to relevant regulations and restrictions on dividends and payment to parties outside of China. Transfers among our Singapore and Hong Kong entities are not restricted under Singapore and Hong Kong Laws. No dividends or distribution have been made by our subsidiaries or by Virax Cayman to date and we intend to reinvest all cash into our subsidiaries for the foreseeable future. For the years ended March 31, 2023 and 2022, there was no transfer between Virax Cayman and its subsidiaries.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is ________, 2023.
TABLE OF CONTENTS
|
|
|
Page |
About this Prospectus |
9 |
|
|
Commonly Used Defined Terms |
10 |
|
|
Note Regarding Forward-Looking Statements |
11 |
|
|
Company Overview |
12 |
|
|
Risk Factors |
18 |
|
|
Use of Proceeds |
19 |
|
|
Dilution |
19 |
|
|
Description of Share Capital |
20 |
|
|
Description of Our Debt Securities |
27 |
|
|
Description of Our Warrants |
28 |
|
|
Description of Our Units |
29 |
|
|
Plan of Distribution |
30 |
|
|
Legal Matters |
32 |
|
|
Experts |
32 |
|
|
Where You Can Find More Information |
32 |
|
|
Information Incorporated by Reference |
33 |
|
|
Enforceability of Civil Liabilities |
35 |
|
|
Indemnification for Securities Act Liabilities |
37 |
We are responsible for the information contained and incorporated by reference in this prospectus, in any accompanying prospectus supplement, and in any related free writing prospectus we prepare or authorize. We have not authorized anyone to give you any other information, and we take no responsibility for any other information that others may give you. If you are in a jurisdiction where offers to sell, or solicitations of offers to purchase, the securities offered by this documentation are unlawful, or if you are a person to whom it is unlawful to direct these types of activities, then the offer presented in this document does not extend to you. The information contained in this document speaks only as of the date of this document, unless the information specifically indicates that another date applies. Neither the delivery of this prospectus or any accompanying prospectus supplement, nor any sale of securities made under these documents, will, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus, any accompanying prospectus supplement or any free writing prospectus we may provide you in connection with an offering or that the information contained or incorporated by reference is correct as of any time subsequent to the date of such information. You should assume that the information in this prospectus or any accompanying prospectus supplement, as well as the information incorporated by reference in this prospectus or any accompanying prospectus supplement, is accurate only as of the date of the documents containing the information, unless the information specifically indicates that another date applies. Our business, financial condition, results of operations and prospects may have changed since those dates.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, under the Securities Act of 1933, as amended, or the Securities Act, using a “shelf” registration process. Under this shelf registration process, we may from time to time sell Ordinary Shares, preferred shares, warrants to purchase Ordinary Shares or preferred shares, debt securities or any combination of the foregoing, either individually or as units comprised of one or more of the other securities, in one or more offerings up to a total dollar amount of US$30,000,000. We have provided you in this prospectus a general description of the securities we may offer. Each time we sell securities under this shelf registration, we will, to the extent required by law, provide a prospectus supplement that will contain specific information about the terms of that offering. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus or in any documents that we have incorporated by reference into this prospectus. To the extent there is a conflict between the information contained in this prospectus and the prospectus supplement or any related free writing prospectus, you should rely on the information in the prospectus supplement or the related free writing prospectus; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date – for example, a document filed after the date of this prospectus and incorporated by reference into this prospectus or any prospectus supplement or any related free writing prospectus – the statement in the document having the later date modifies or supersedes the earlier statement.
We have not authorized any dealer, agent or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus and any accompanying prospectus supplement, or any related free writing prospectus that we may authorize to be provided to you. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or an accompanying prospectus supplement, or any related free writing prospectus that we may authorize to be provided to you. This prospectus and the accompanying prospectus supplement, if any, do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and the accompanying prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference (as our business, financial condition, results of operations and prospects may have changed since that date), even though this prospectus, any applicable prospectus supplement or any related free writing prospectus is delivered or securities are sold on a later date.
As permitted by SEC rules and regulations, the registration statement of which this prospectus forms a part includes additional information not contained in this prospectus. You may read the registration statement and the other reports we file with the SEC at its website or at its offices described below under “Where You Can Find More Information.”
Unless the context otherwise requires, all references in this prospectus to “VRAX,” “we,” “us,” “our,” “the Company,” “the “Registrant” or similar words refer to Virax Biolabs Group Limited, together with our subsidiaries.
COMMONLY USED DEFINED TERMS
Unless we explicitly state otherwise or the context otherwise indicates clearly, all references in this proxy statement to “we,” “us,” “our,” or “our Group” refer to Virax Biolabs Group Limited and its subsidiaries, namely, Virax Biolabs (UK) Limited, Virax Biolabs Group Holdings Limited, Virax Biolabs FZ LLC, Virax Biolabs Trading B.V., Virax Biolabs USA Management, Inc., Virax Biolabs Limited, Virax Immune T-Cell Medical Device Company Limited, Virax Biolabs Pte. Limited, Logico Bioproducts Corp., and Shanghai Xitu Consulting Co., Limited.
•The “Company” or “Virax Cayman” refers to Virax Biolabs Group Limited.
•“GBP” or “GB£” refers to the legal currency of the United Kingdom.
•“HKD” or “HK$” refers to the legal currency of Hong Kong.
•“RMB” or “Renminbi” refers to the legal currency of China.
•“IVD” refers to in-vitro diagnostics.
•“PRC” or “China” refers to the People’s Republic of China, including Hong Kong and Macau.
•“Prospectus” refers to the public offering prospectus unless we explicitly state otherwise, or the context otherwise indicates clearly.
•“SGD” or “S$” refers to the legal currency of Singapore.
•“United Kingdom” or “UK” refers to the England, Scotland, Wales and Northern Ireland for the purpose of this prospectus.
•“$” or “U.S. dollars” or “USD” refers to the legal currency of the United States.
We have made rounding adjustments to some of the figures included in this prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
Our business is primarily conducted in Europe, and the financial records of our subsidiaries in Asia are maintained in USD, and our functional currency is USD. Our consolidated financial statements are presented in U.S. dollars. We use U.S. dollars as the reporting currency in our consolidated financial statements and in this prospectus.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and our SEC filings that are incorporated by reference into this prospectus contain or incorporate by reference forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. All statements other than statements of historical fact are “forward-looking statements,” including any projections of earnings, revenue or other financial items, any statements of the plans, strategies and objectives of management for future operations, any statements concerning proposed new projects or other developments, any statements regarding future economic conditions or performance, any statements of management’s beliefs, goals, strategies, intentions and objectives, and any statements of assumptions underlying any of the foregoing. The words “believe,” “anticipate,” “estimate,” “plan,” “expect,” “intend,” “may,” “could,” “should,” “potential,” “likely,” “projects,” “continue,” “will,” and “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements reflect our current views with respect to future events, are based on assumptions and are subject to risks and uncertainties. We cannot guarantee that we actually will achieve the plans, intentions or expectations expressed in our forward-looking statements and you should not place undue reliance on these statements. There are a number of important factors that could cause our actual results to differ materially from those indicated or implied by forward-looking statements. These important factors include those discussed under the heading “Risk Factors” contained or incorporated by reference in this prospectus and in the applicable prospectus supplement and any free writing prospectus we may authorize for use in connection with a specific offering. These factors and the other cautionary statements made in this prospectus should be read as being applicable to all related forward-looking statements whenever they appear in this prospectus. Except as required by law, we undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
COMPANY OVERVIEW
Overview
Virax Cayman is a holding company incorporated as an exempted company under the laws of the Cayman Islands. As a holding company with no material operations of our own, Virax Cayman conducts our operations through its subsidiaries in the United Kingdom, the United States, Singapore, Hong Kong, China and British Virgin Islands and has been operating since 2013.
Virax Biolabs Group Limited and its subsidiaries is a global innovative biotechnology company focused on the prevention, detection, diagnosis, and risk management of viral diseases with a particular interest in the field of T-Cell in Vitro Diagnostics. The Company is in the process of developing and manufacturing tests that can predict adaptive immunity to viral diseases as well as identify individuals suffering from T-cell exhaustion linked to post viral syndromes. The Company's mission is to protect people from viral diseases and help with the early diagnosis of post viral syndromes associated with T-cell exhaustion and chronic fatigue through the provision of diagnostic tests, tests for adaptive immunity and education through a wellness mobile application which could allow people to make informed decisions regarding their viral risks.
Diagnostics test kits are distributed through our ViraxClear and ViraxVet brands. Currently, we do not manufacture or develop any product that we sell in our ViraxClear and ViraxVet product portfolios, and we act as a distributor of third-party suppliers’ products. Our Company also seeks to maximize consumers’ access to our products and services through competitive pricing and regular evaluations of our pricing arrangements and contracts with our distributors.
We also expect to launch an upcoming brand, ViraxImmune, with the intention of providing an immunology profiling platform that assesses each individual’s immune risk profile against major global viral diseases as well as helping with the early diagnosis of post viral syndromes associated with T-cell exhaustion and chronic fatigue. We are in the process of developing a T-Cell Test under the ViraxImmune brand and will apply for regulatory agency approval. We believe that the T-Cell Tests and immunology platform we are developing under the ViraxImmune brand will be particularly useful in assisting in the threat analysis of the major viruses faced globally as well as helping with the early diagnosis of indications associated with T-cell exhaustion and chronic fatigue. Initially, we will be focusing in diseases associated with post viral syndromes including but not limited to COVID-19, Human Papillomavirus (better known as HPV), Malaria, Hepatitis B, and Herpes (better known as HSV-1). The results and education for specific viruses will be delivered through our mobile-based immunology application.
Recent Developments
Initial Public Offering
On July 20, 2022, Virax entered into an underwriting agreement with Boustead Securities, LLC, as representatives of the several underwriters, in connection with its initial public offering (“IPO”) of 1,350,000 Ordinary Shares, at a price of $5.00 per share, before deducting underwriting discounts, commissions, and other related expenses. The shares began trading on the Nasdaq Capital Market on July 21, 2022. The Company issued Representative’s Warrant to purchase up to 108,675 ordinary shares at $6.00 per share, dated July 20, 2022, to Boustead Securities, LLC. On July 25, 2022, the Company consummated its IPO generating gross proceeds to the Company of $7,762,500, before deducting underwriting discounts and other related expenses.
2022 PIPE Financing
On November 3, 2022, Virax entered into a Securities Purchase Agreement (the “November SPA”) with an accredited investor for a private placement offering (“2022 Private Placement”), pursuant to which the Company received gross proceeds of approximately $3,844,500, before deducting placement agent fees and other offering expenses, in consideration of (i) 1,165,000 Ordinary Shares; (ii) pre-funded warrants to purchase 1,165,000 Ordinary Shares, and (iii) warrants to purchase 3,495,000 Ordinary Shares at a combined purchase price of $1.65 per Ordinary Share and one and a half Ordinary Warrant, or approximately $1.65 per Pre-Funded Warrant and one and a half
Ordinary Warrant if purchasing the Pre-Funded Warrants. The warrants have an exercise price of $1.73 per share. The November SPA contains customary representations and warranties and agreements of the Company and the purchaser and customary indemnification rights and obligations of the parties. The 2021 Private Placement closed on November 8, 2022. Concurrently with the signing of the November SPA, we entered into a registration rights agreement to file with the Securities and Exchange Commission a registration statement covering the resale of all of the registrable securities under the registration rights agreement.
2023 PIPE Financing
On March 8, 2023, Virax entered into a Securities Purchase Agreement (the “PIPE Securities Purchase Agreement”) with an accredited investor for a private placement offering (“2023 Private Placement”), pursuant to which the Company received gross proceeds of approximately $4,000,000, before deducting placement agent fees and other offering expenses, in consideration of (i) 1,500,000 Ordinary Shares; (b) Pre-Funded Warrants to purchase 2,343,309 Ordinary Shares, (iii) Series A Options to purchase 3,497,412 Ordinary Shares, and (iv) Series B Options to purchase 3,843,309 Ordinary Shares at a purchase price of $1.04077 per Ordinary Share and associated Preferred Options and a purchase price of $1.04067 per Pre-Funded Warrant and associated Preferred Options (the “PIPE Offering”). The Preferred Options have an exercise price of $0.80202 per share. In addition, the Company issued warrants to purchase up to 269,032 Ordinary Shares at $1.3010 per share to certain designees of H.C. Wainwright & Co., placement agent of the Offering. The PIPE Securities Purchase Agreement contains customary representations and warranties and agreements of the Company and the purchaser and customary indemnification rights and obligations of the parties. The 2023 Private Placement closed on March 10, 2023. Concurrently with the signing of the PIPE Securities Purchase Agreement, we entered into a registration rights agreement to file with the Securities and Exchange Commission a registration statement covering the resale of all of the registrable securities under the registration rights agreement.
2023 Warrant Inducement
On October 11, 2023, we entered into an inducement offer letter agreement (the “Inducement Letter”) with a certain holder (the “Holder”) of existing Series A and B Options (the “Existing Warrants”) to purchase ordinary shares of the Company. The Existing Warrants were issued on March 10, 2023 and each has an exercise price of $0.80202 per share.
Pursuant to the Inducement Letter, the Holder agreed to exercise for cash its Existing Warrants to purchase an aggregate of 7,340,721 ordinary shares of the Company at a reduced exercise price of $0.2934 per share in consideration for the Company’s agreement to issue new warrants to purchase ordinary share (the “New Warrants”), as described below, to purchase up to 14,681,442 of the Company’s ordinary share of par value US$0.0001 each (the “New Warrant Shares”). The Company received an aggregate gross proceeds of approximately $2.15 million from the exercise of the Existing Warrants by the Holder, before deducting placement agent fees and other offering expenses payable by the Company. Each New Warrant will have an exercise price equal to $0.2934 per share. The New Warrants will be immediately exercisable from the date of issuance until the five-year anniversary of the initial exercise date. The exercise price and number of Ordinary Shares issuable upon exercise is subject to appropriate adjustment in the event of share dividends, share splits, subsequent rights offerings, pro rate distributions, reorganizations, or similar events affecting the Company’s Ordinary Shares and the exercise price.
In addition, on October 16, 2023, the Company issued warrants to purchase up to 513,850 Ordinary Shares of US$0.0001 par value each at $0.36675 per share to certain designees of H.C. Wainwright & Co., placement agent of the offering. The Inducement Letter contains customary representations, warranties and covenants by the Company which were made only for the purposes of such agreements and as of specific dates, were solely for the benefit of the parties to such agreements and may be subject to limitations agreed upon by the contracting parties. Concurrently with the signing of the Inducement Letter Agreement, we agreed to file with the Securities and Exchange Commission a registration statement covering the resale of all of the registrable securities under the registration rights agreement.
Corporate History and Structure
Structural Overview
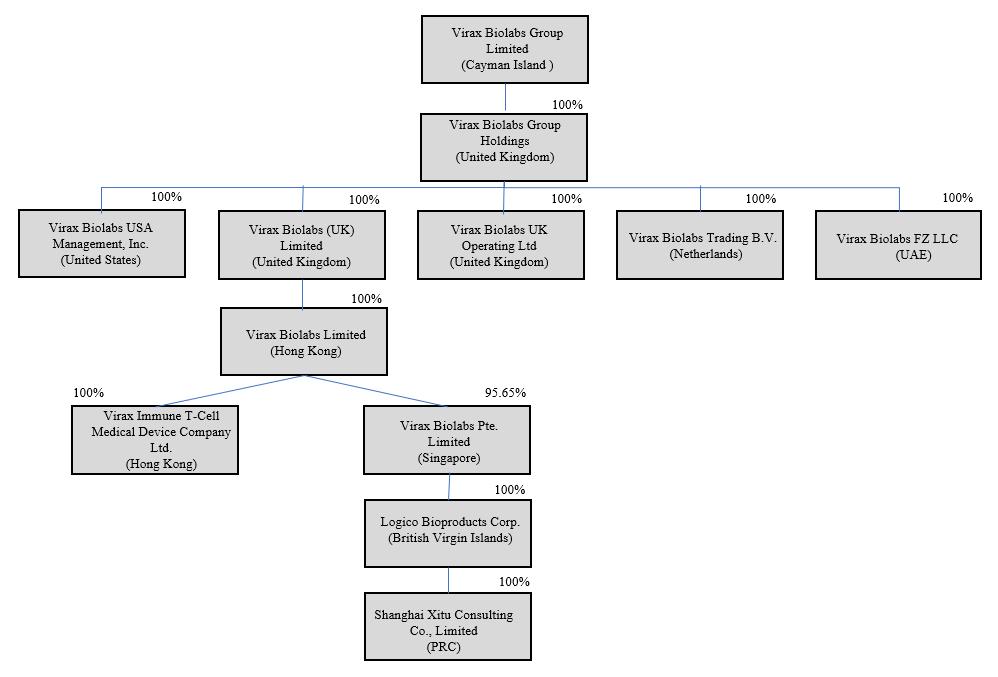
Virax Cayman is a holding company incorporated as an exempted company under the laws of the Cayman Islands that owns all of the outstanding capital stock of Virax Biolabs (UK) Limited and Virax Biolabs USA Management, Inc., our wholly-owned subsidiaries. Virax Biolabs (UK) Limited, in turn, owns all of the outstanding capital stock of Virax Biolabs Limited, our wholly-owned Hong Kong subsidiary. Virax Biolabs Limited owns all of the outstanding capital stock of Virax Immune T-Cell Medical Device Company Limited, our wholly-owned Hong Kong subsidiary, and 95.65% of the outstanding capital stock of Virax Biolabs Pte. Limited, our operating subsidiary incorporated in Singapore. Virax Biolabs Pte. Limited owns all of the outstanding capital stock of Logico Bioproducts Corp., a wholly-owned British Virgin Islands and a subsidiary of Virax Biolabs Pte. Limited. Logico Bioproducts Corp., in turn, owns all of the outstanding capital stock of Shanghai Xitu, a wholly-owned subsidiary of Logico Bioproducts Corp. and a wholly foreign owned enterprise based in China.
We completed a reorganization and share exchange of our company in September 2021 (the “Reorganization”). Pursuant to the Reorganization, all shareholders of Virax Biolabs Limited (HK) transferred their shares, 102,478,548 ordinary shares in total, to Virax Biolabs (UK) Limited, in exchange for an aggregate of (i) 2,549,028 newly issued Class A Ordinary Shares and (ii) 7,034,306 newly issued Class B Ordinary Shares of Virax Biolabs Group Limited. On June 19, 2022, Virax Cayman underwent a shareholding restructuring whereby the Company’s authorized share capital became a single class of shares of Ordinary Shares and all of the then issued shares were re-designated as Ordinary Shares.
Organization Structure and Purpose
Virax Biolabs Group Limited (“Virax Cayman”) — Virax Biolabs Group Limited is a Cayman Islands exempted company incorporated on September 2, 2021, previously named as “Virax Biolabs (Cayman) Limited” and effected a name change to “Virax Biolabs Group Limited” on January 19, 2022. Structured as a holding company with no material operations, Virax Cayman conducts our operations through its operating subsidiaries in the Hong Kong, Singapore, British Virgin Islands and China.
Virax Biolabs (UK) Limited — Virax Biolabs (UK) Limited was incorporated on August 19, 2021 under the laws of the United Kingdom, a wholly-owned subsidiary of Virax Cayman and structured as a holding company with no material operations.
Virax Biolabs USA Management, Inc. — Virax Biolabs USA Management, Inc. was incorporated on August 1, 2022 under the laws of the United States, a wholly-owned subsidiary of Virax Cayman and structured as a management company for operations within the United States.
Virax Biolabs Limited (“HKco”) — Virax Biolabs Limited, incorporated on April 14, 2020 under the laws of Hong Kong, was previously named as “Shanghai Biotechnology Devices Limited” and effected a name change to “Virax Biolabs Limited” on July 12, 2021. Virax Biolabs Limited, our wholly-owned Hong Kong subsidiary, serves as a holding company.
Virax Immune T-Cell Medical Device Company Limited (“Virax Immune T-Cell”) — Virax Immune T-Cell Medical Device Company Limited, a wholly-owned subsidiary of HKco, incorporated on January 16, 2017 under the laws of Hong Kong, was previously named as “Stork Nutrition Asia Limited” and effected a name change to “Virax Immune T-Cell Medical Device Company Limited” on September 10, 2021. It is primarily engaged in the research and development of T-Cell blood analysis.
Virax Biolabs Pte. Limited (“SingaporeCo”) — Virax Biolabs Pte. Limited, incorporated on May 4, 2013 under the laws of Singapore, was previously named as “Natural Source Group Pte. Limited” and effected a name change to “Virax Biolabs Pte. Limited” on July 2, 2021. 95.65% of its capital stock is owned by Virax Biolabs Limited and the remaining 4.35% is owned by independent third party shareholders. It is our operating company, primarily engaged in the trading and sales of our products and running primarily day to day operations.
Logico Bioproducts Corp. (“Logico BVI”) — Logico Bioproducts Corp., a wholly-owned subsidiary of SingaporeCo, is a limited liability company incorporated in the British Virgin Islands on January 21, 2011, and is primarily engaged in the trading and sales of our products.
Shanghai Xitu Consulting Co., Limited (“Shanghai Xitu”) — Shanghai Xitu, a wholly-owned subsidiary of Logico BVI and a wholly foreign owned enterprise, is a limited liability company incorporated on October 27, 2017 in China. Shanghai Xitu is primarily engaged in procurement.
Virax Biolabs Group Holdings Ltd (“Virax UK HoldCo”) — Virax Biolabs Group Holdings Limited was incorporated on February 22, 2023 under the laws of the United Kingdom, a wholly-owned subsidiary of the Company and structured as a holding company.
Virax Biolabs FZ-LLC (“Virax Dubai”) — Virax Biolabs FZ-LLC was incorporated on April 18, 2023 under the laws of the United Kingdom, a wholly-owned subsidiary of the Company and is primarily engaged as a regional distribution company.
Virax Biolabs Trading B.V. (“Virax Netherlands”) — Virax Biolabs Trading B.V. was incorporated on August 4, 2023 under the laws of the Netherlands, a wholly-owned subsidiary of the Company and is primarily engaged as a regional distribution company.
Virax Biolabs UK Operating LLC (“Virax UK Operating”) — Virax Biolabs UK Operating was incorporated on August 4, 2023 under the laws of the United Kingdom, a wholly-owned subsidiary of the Company and is primarily engaged as a regional operating company.
The following diagram illustrates our corporate structure:
Implications of Being an Emerging Growth Company and a Foreign Private Issuer
Emerging Growth Company
As a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012, and may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
•being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in our filings with the SEC;
•not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
•reduced disclosure obligations regarding executive compensation in periodic reports, proxy statements and registration statements; and
•exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our Ordinary Shares pursuant to this offering. However, if certain events occur before the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.235 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company before the end of such five-year period.
In addition, Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and acknowledge such election is irrevocable pursuant to Section 107 of the JOBS Act.
Foreign Private Issuer
We are a “foreign private issuer,” as defined by the SEC. As a result, in accordance with the rules and regulations of The Nasdaq Stock Market LLC, or Nasdaq, we may comply with home country governance requirements and certain exemptions thereunder rather than complying with Nasdaq corporate governance standards. We may choose to take advantage of the following exemptions afforded to foreign private issuers:
•Exemption from filing quarterly reports on Form 10-Q or provide current reports on Form 8-K disclosing significant events within four (4) days of their occurrence.
•Exemption from Section 16 rules regarding sales of Ordinary Shares by insiders, which will provide less data in this regard than shareholders of U.S. companies that are subject to the Exchange Act.
•Exemption from the Nasdaq rules applicable to domestic issuers requiring disclosure within four (4) business days of any determination to grant a waiver of the code of business conduct and ethics to directors and officers. Although we will require board approval of any such waiver, we may choose not to disclose the waiver in the manner set forth in the Nasdaq rules, as permitted by the foreign private issuer exemption.
•Exemption from the requirement that our board of directors have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities.
•Exemption from the requirements that director nominees are selected, or recommended for selection by our board of directors, either by (i) independent directors constituting a majority of our board of directors’ independent directors in a vote in which only independent directors participate, or (ii) a committee comprised solely of independent directors, and that a formal written charter or board resolution, as applicable, addressing the nominations process is adopted.
Furthermore, Nasdaq Rule 5615(a)(3) provides that a foreign private issuer, such as us, may rely on our home country corporate governance practices in lieu of certain of the rules in the Nasdaq Rule 5600 Series and Rule 5250(d),
provided that we nevertheless comply with Nasdaq’s Notification of Noncompliance requirement (Rule 5625), the Voting Rights requirement (Rule 5640) and that we have an audit committee that satisfies Rule 5605(c)(3), consisting of committee members that meet the independence requirements of Rule 5605(c)(2)(A)(ii). If we rely on our home country corporate governance practices in lieu of certain of the rules of Nasdaq, our shareholders may not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq. If we choose to do so, we may utilize these exemptions for as long as we continue to qualify as a foreign private issuer.
Although we are permitted to follow certain corporate governance rules that conform to Cayman Islands requirements in lieu of many of the Nasdaq corporate governance rules, we intend to comply with the Nasdaq corporate governance rules applicable to foreign private issuers.
Corporate Information
Our principal executive office is located at 20 North Audley Street London, W1K 6LX, United Kingdom. Our telephone number is +44 020 7788 7414. Our registered office in the Cayman Islands is located at the office of Ogier Global (Cayman) Limited, 89 Nexus Way, Camana Bay, Grand Cayman, KY1-9009, Cayman Islands.
Our agent for service of process in the United States is Virax Biolabs USA Management, Inc., located at 23501 Cinco Ranch Blvd. Ste H120-289, Katy, TX 77494. Our principal website is located at https://viraxbiolabs.com/. Information contained on, or that can be accessed through, our website is not a part of, and shall not be incorporated by reference into, this prospectus.
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider the risk factors set forth under “Risk Factors” described in our most recent annual report on Form 20-F, filed on June 14, 2023, as supplemented and updated by subsequent current reports on Form 6-K that we have filed with the SEC, together with all other information contained or incorporated by reference in this prospectus and any applicable prospectus supplement and in any related free writing prospectus in connection with a specific offering, before making an investment decision. Each of the risk factors could materially and adversely affect our business, operating results, financial condition and prospects, as well as the value of an investment in our securities, and the occurrence of any of these risks might cause you to lose all or part of your investment.
USE OF PROCEEDS
Except as described in any prospectus supplement and any free writing prospectus in connection with a specific offering, we currently intend to use the net proceeds from the sale of the securities offered under this prospectus to fund the development and commercialization of our projects and the growth of our business, primarily working capital, and for general corporate purposes. We may also use a portion of the net proceeds to acquire or invest in technologies, products and/or businesses that we believe will enhance the value of our Company, although we have no current commitments or agreements with respect to any such transactions as of the date of this prospectus. We have not determined the amount of net proceeds to be used specifically for the foregoing purposes. As a result, our management will have broad discretion in the allocation of the net proceeds and investors will be relying on the judgment of our management regarding the application of the proceeds of any sale of the securities. If a material part of the net proceeds is to be used to repay indebtedness, we will set forth the interest rate and maturity of such indebtedness in a prospectus supplement. Pending use of the net proceeds will be deposited in interest bearing bank accounts.
DILUTION
If required, we will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests of investors purchasing securities in an offering under this prospectus:
•the net tangible book value per share of our equity securities before and after the offering;
•the amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering; and
•the amount of the immediate dilution from the public offering price which will be absorbed by such purchasers.
DESCRIPTION OF SHARE CAPITAL
The following description of our capital stock (which includes a description of securities we may offer pursuant to the registration statement of which this prospectus, as the same may be supplemented, forms a part) does not purport to be complete and is subject to and qualified in its entirety by our Amended and Restated Memorandum and Articles of Association (“M&A”) and by the applicable provisions of Cayman Islands law.
We are an exempted company incorporated with limited liability under the laws of the Cayman Islands and our affairs are governed by:
•Memorandum and Articles of Association;
•The Companies Act (Revised) of the Caymans Islands, which is referred to as the Companies Act below; and
•Common law of the Cayman Islands.
•Our authorized share capital is US$50,000 divided into 500,000,000 Ordinary Shares of $0.0001 par value each.
Ordinary Shares
All of our outstanding Ordinary Shares are fully paid and non-assessable. Certificates representing the Ordinary Shares are issued in registered form. Our shareholders, whether or not they are non-residents of the Cayman Islands, may freely hold and transfer their Ordinary Shares in accordance with our Memorandum and Articles.
Dividends
The holders of our Ordinary Shares are entitled to such dividends as may be declared by our board of directors. Our Articles provide that our board of directors may declare and pay dividends if justified by our financial position and permitted by law. Our articles of association also provides that, subject to the Companies Act, the Company may by also by ordinary resolution declare dividends in accordance with the respective rights of the shareholders but no dividend shall exceed the amount recommended by the directors.
Voting Rights
Holders of our Ordinary Shares vote on all matters submitted to a vote of our shareholders, except as may otherwise be required by law. In respect of matters requiring shareholders’ vote, each ordinary share is entitled to one vote. At any general meeting a resolution put to the vote of the meeting shall be decided on a show of hands unless voting by poll is duly demanded by the chairman of the meeting, by at least two shareholders having the right to vote on the resolutions, or by shareholder(s) together holding at least 10% of the total voting rights of all our shareholders having the right to vote at such general meeting. A quorum required for a meeting of shareholders consists of one or more shareholders who holds at least one-third of our issued voting shares. Shareholders’ meetings may be held annually. Each general meeting, other than an annual general meeting, shall be an extraordinary general meeting. Extraordinary general meetings may be called by a majority of our board of directors or upon a requisition of any one or more shareholders holding at the deposit of the requisition at least 10% of the aggregate share capital of our company that carries the right to vote at a general meeting, in which case on advance notice of at least 7 clear days is required for the convening of our annual general meeting and other general meetings by requisition of our shareholders.
Any ordinary resolution to be made by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the Ordinary Shares cast in a meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes attaching to the Ordinary Shares cast in a meeting.
A special resolution will be required for important matters such as amending our memorandum and articles of association or changing the name of the Company.
There are no limitations on non-residents or foreign shareholders in the memorandum and articles of association to hold or exercise voting rights on the Ordinary Shares imposed by foreign law or by the charter or other constituent document of our company. However, no person will be entitled to vote at any general meeting or at any separate meeting of the holders of the Ordinary Shares unless the person is registered as of the record date for such meeting and unless all calls or other sums presently payable by the person in respect of Ordinary Shares in the Company have been paid.
Winding Up; Liquidation
Subject to any special rights, privileges or restrictions as to the distribution of available surplus assets on liquidation applicable to any class or classes of shares (1) if we are wound up and the assets available for distribution among our shareholders are more than sufficient to repay the whole of the capital paid up at the commencement of the winding up, the excess shall be distributed pari passu among our shareholders in proportion to the amount paid up at the commencement of the winding up on the shares held by them, respectively, and (2) if we are wound up and the assets available for distribution among our shareholders as such are insufficient to repay the whole of the paid-up capital, those assets shall be distributed so that, as nearly as may be, the losses shall be borne by our shareholders in proportion to the capital paid up, or which ought to have been paid up, at the commencement of the winding up on the shares held by them, respectively.
Calls on Ordinary Shares and Forfeiture of Ordinary Shares
Our directors may from time to time make calls on our shareholders in respect of any moneys unpaid on their shares including any premium in a notice served to such shareholders at least 14 clear days prior to the specified time of payment. Any Ordinary Shares that have been called upon and remain unpaid are subject to forfeiture.
Redemption of Ordinary Shares
The Companies Act and our Memorandum and Articles permit us to purchase our own shares. In accordance with our Articles, provided the necessary shareholders or board approval have been obtained and requirements under the Companies Act have been satisfied, we may issue shares on terms that are subject to redemption at our option on such terms and in such manner as may be determined by our board of directors.
Inspection of Books and Records
Holders of our Ordinary Shares have no general right under our Articles to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements.
Issuance of Additional Shares
Our Memorandum and Articles authorize our board of directors to issue additional Ordinary Shares from time to time as our board of directors shall determine, to the extent of available authorized but unissued shares. Issuance of these shares may dilute the voting power of holders of Ordinary Shares.
Anti-Takeover Provisions
Some provisions of our Memorandum and Articles may discourage, delay or prevent a change of control of our company or management that shareholders may consider favorable. Our authorized, but unissued Ordinary Shares are available for future issuance without shareholders’ approval and could be utilized for a variety of corporate purposes, including future offerings to raise addition capital, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved Ordinary Shares could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Exempted Company
We are an exempted company with limited liability under the Companies Act. The Companies Act distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except that an exempted company:
does not have to file an annual return of its shareholders with the Registrar of Companies;
is not required to open its register of members for inspection;
does not have to hold an annual general meeting;
may not issue negotiable or bearer shares, but may issue shares with no par value;
may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance);
may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands;
may register as a limited duration company; and
may register as a segregated portfolio company.
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of the company.
Preferred Shares
As all the current authorized share capital is designated as Ordinary Shares, shareholders’ special resolution will be needed to amend the Company’s M&A to alter its authorized share capital if the Company decides to issue preferred shares. After such resolution and amendment, the Board is empowered to allot and/or issue (with or without rights of renunciation), grant options over, offer or otherwise deal with or dispose of any unissued shares of the Company (whether forming part of the original or any increased share capital), either at a premium or at par, with or without preferred, deferred or other special rights or restrictions, whether in regard to dividend, voting, return of capital or otherwise and to such persons, on such terms and conditions, and at such times as the Board may decide and they may allot or otherwise dispose of them to such persons (including any director of the Board) on such terms and conditions and at such time as the Board may determine.
You should refer to the prospectus supplement relating to the series of preferred shares being offered for the specific terms of that series, including:
title of the series and the number of shares in the series;
the price at which the preferred shares will be offered;
the dividend rate or rates or method of calculating the rates, the dates on which the dividends will be payable, whether or not dividends will be cumulative or noncumulative and, if cumulative, the dates from which dividends on the preferred shares being offered will cumulate;
the voting rights, if any, of the holders of preferred shares being offered;
the provisions for a sinking fund, if any, and the provisions for redemption, if applicable, of the preferred shares being offered, including any restrictions on the foregoing as a result of arrearage in the payment of dividends or sinking fund installments;
the liquidation preference per share;
the terms and conditions, if applicable, upon which the preferred shares being offered will be convertible into our Ordinary Shares, including the conversion price, or the manner of calculating the conversion price, and the conversion period;
the terms and conditions, if applicable, upon which the preferred shares being offered will be exchangeable for debt securities, including the exchange price, or the manner of calculating the exchange price, and the exchange period;
any listing of the preferred shares being offered on any securities exchange;
a discussion of any material federal income tax considerations applicable to the preferred shares being offered;
the relative ranking and preferences of the preferred shares being offered as to dividend rights and rights upon liquidation, dissolution or the winding up of our affairs;
any limitations on the issuance of any class or series of preferred shares ranking senior or equal to the series of preferred shares being offered as to dividend rights and rights upon liquidation, dissolution or the winding up of our affairs; and
any additional rights, preferences, qualifications, limitations and restrictions of the series.
Upon issuance, the preferred shares will be fully paid and nonassessable, which means that its holders will have paid their purchase price in full and we may not require them to pay additional funds.
Any preferred share terms selected by the Board could decrease the amount of earnings and assets available for distribution to holders of our Ordinary Shares or adversely affect the rights and power, including voting rights, of the holders of our Ordinary Shares without any further vote or action by the stockholders. The rights of holders of our Ordinary Shares will be subject to, and may be adversely affected by, the rights of the holders of any preferred shares that may be issued by us in the future. The issuance of preferred shares could also have the effect of delaying or preventing a change in control of our company or make removal of management more difficult.
Anti-money Laundering—Cayman Islands
In order to comply with legislation or regulations aimed at the prevention of money laundering, we are required to adopt and maintain anti-money laundering procedures and may require subscribers to provide evidence to verify their identity and source of funds. Where permitted, and subject to certain conditions, we may also delegate the maintenance of our anti-money laundering procedures (including the acquisition of due diligence information) to a suitable person.
We reserve the right to request such information as is necessary to verify the identity of a subscriber. In some cases the directors may be satisfied that no further information is required since an exemption applies under the Anti-Money Laundering Regulations (Revised) of the Cayman Islands, as amended and revised from time to time (the “Regulations”). Depending on the circumstances of each application, a detailed verification of identity might not be required where:
•the subscriber makes the payment for their investment from an account held in the subscriber’s name at a recognized financial institution; or
•the subscriber is regulated by a recognized regulatory authority and is based or incorporated in, or formed under the law of, a recognized jurisdiction; or
•the application is made through an intermediary which is regulated by a recognized regulatory authority and is based in or incorporated in, or formed under the law of a recognized jurisdiction and an assurance is provided in relation to the procedures undertaken on the underlying investors.
For the purposes of these exceptions, recognition of a financial institution, regulatory authority, or jurisdiction will be determined in accordance with the Regulations by reference to those jurisdictions recognized by the Cayman Islands Monetary Authority as having equivalent anti-money laundering regulations.
In the event of delay or failure on the part of the subscriber in producing any information required for verification purposes, we may refuse to accept the application, in which case any funds received will be returned without interest to the account from which they were originally debited.
We also reserve the right to refuse to make any redemption payment to a shareholder if our directors or officers suspect or are advised that the payment of redemption proceeds to such shareholder might result in a breach of applicable anti-money laundering or other laws or regulations by any person in any relevant jurisdiction, or if such refusal is considered necessary or appropriate to ensure our compliance with any such laws or regulations in any applicable jurisdiction.
If any person resident in the Cayman Islands knows or suspects or has reason for knowing or suspecting that another person is engaged in criminal conduct or is involved with terrorism or terrorist property and the information for that knowledge or suspicion came to their attention in the course of their business in the regulated sector, or other trade, profession, business or employment, the person will be required to report such knowledge or suspicion to (i) a nominated officer (appointed in accordance with the Proceeds of Crime Act (Revised) of the Cayman Islands) or the Financial Reporting Authority of the Cayman Islands, pursuant to the Proceeds of Crime Act (Revised), if the disclosure relates to criminal conduct or money laundering or (ii) to a police constable or a nominated officer (pursuant
to the Terrorism Act (Revised) of the Cayman Islands) or the Financial Reporting Authority, pursuant to the Terrorism Act (Revised), if the disclosure relates to involvement with terrorism or terrorist financing and terrorist property. Such a report shall not be treated as a breach of confidence or of any restriction upon the disclosure of information imposed by any enactment or otherwise.
Data Protection in the Cayman Islands – Privacy Notice
This privacy notice explains the manner in which we collect, process, and maintain personal data about investors of the Company pursuant to the Data Protection Act (Revised)1 of the Cayman Islands, as amended from time to time and any regulations, codes of practice, or orders promulgated pursuant thereto (the “DPA”).
We are committed to processing personal data in accordance with the DPA. In our use of personal data, we will be characterized under the DPA as a “data controller,” whilst certain of our service providers, affiliates, and delegates may act as “data processors” under the DPA. These service providers may process personal information for their own lawful purposes in connection with services provided to us.
By virtue of your investment in the Company, we and certain of our service providers may collect, record, store, transfer, and otherwise process personal data by which individuals may be directly or indirectly identified.
Your personal data will be processed fairly and for lawful purposes, including (a) where the processing is necessary for us to perform a contract to which you are a party or for taking pre-contractual steps at your request, (b) where the processing is necessary for compliance with any legal, tax, or regulatory obligation to which we are subject, or (c) where the processing is for the purposes of legitimate interests pursued by us or by a service provider to whom the data are disclosed. As a data controller, we will only use your personal data for the purposes for which we collected it. If we need to use your personal data for an unrelated purpose, we will contact you.
We anticipate that we will share your personal data with our service providers for the purposes set out in this privacy notice. We may also share relevant personal data where it is lawful to do so and necessary to comply with our contractual obligations or your instructions or where it is necessary or desirable to do so in connection with any regulatory reporting obligations. In exceptional circumstances, we will share your personal data with regulatory, prosecuting, and other governmental agencies or departments, and parties to litigation (whether pending or threatened), in any country or territory including to any other person where we have a public or legal duty to do so (e.g. to assist with detecting and preventing fraud, tax evasion, and financial crime or compliance with a court order).
Your personal data shall not be held by the Company for longer than necessary with regard to the purposes of the data processing.
We will not sell your personal data. Any transfer of personal data outside of the Cayman Islands shall be in accordance with the requirements of the DPA. Where necessary, we will ensure that separate and appropriate legal agreements are put in place with the recipient of that data.
We will only transfer personal data in accordance with the requirements of the DPA and will apply appropriate technical and organizational information security measures designed to protect against unauthorized or unlawful processing of the personal data and against the accidental loss, destruction, or damage to the personal data.
If you are a natural person, this will affect you directly. If you are a corporate investor (including, for these purposes, legal arrangements such as trusts or exempted limited partnerships) that provides us with personal data on individuals connected to you for any reason in relation to your investment into the Company, this will be relevant for those individuals and you should inform such individuals of the content.
You have certain rights under the DPA, including (a) the right to be informed as to how we collect and use your personal data (and this privacy notice fulfils our obligation in this respect), (b) the right to obtain a copy of your personal data, (c) the right to require us to stop direct marketing, (d) the right to have inaccurate or incomplete personal data corrected, (e) the right to withdraw your consent and require us to stop processing or restrict the processing, or not begin the processing of your personal data, (f) the right to be notified of a data breach (unless the breach is unlikely to be prejudicial), (g) the right to obtain information as to any countries or territories outside the Cayman Islands to
which we, whether directly or indirectly, transfer, intend to transfer, or wish to transfer your personal data, general measures we take to ensure the security of personal data, and any information available to us as to the source of your personal data, (h) the right to complain to the Office of the Ombudsman of the Cayman Islands, and (i) the right to require us to delete your personal data in some limited circumstances.
If you consider that your personal data has not been handled correctly, or you are not satisfied with our responses to any requests you have made regarding the use of your personal data, you have the right to complain to the Cayman Islands’ Ombudsman. The Ombudsman can be contacted by calling +1 (345) 946-6283 or by email at info@ombudsman.ky.
DESCRIPTION OF OUR DEBT SECURITIES
We may issue debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. Such convertible debt may be exchangeable for and/or convertible into shares of ordinary shares or any of the other securities that may be sold under this prospectus. The debt securities will be issued under one or more separate indentures between us and a designated trustee. We will include in a prospectus supplement the specific terms of each series of senior or subordinated debt securities being offered, including the terms, if any, on which a series of senior or subordinated debt securities may be convertible into or exchangeable for other securities. In addition, the material terms of any indenture, which will govern the rights of the holders of our senior or subordinated debt securities will be set forth in the applicable prospectus supplement.
We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the debt securities that we may offer under this prospectus, as well as the complete indenture that contains the terms of the debt securities.
DESCRIPTION OF OUR WARRANTS
We may issue warrants to purchase our debt or equity securities or securities of third parties or other rights, including rights to receive payment in cash or securities based on the value, rate or price of one or more specified commodities, currencies, securities or indices, or any combination of the foregoing. Warrants may be issued independently or together with any other securities and may be attached to, or separate from, such securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and a warrant agent. The terms of any warrants to be issued and a description of the material provisions of the applicable warrant agreement will be set forth in the applicable prospectus supplement.
We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the warrants that we may offer under this prospectus, as well as the complete warrant agreements and warrant certificates that contain the terms of the warrants.
DESCRIPTION OF OUR UNITS
We may issue units consisting of any combination of the other types of securities offered under this prospectus in one or more series. We may evidence each series of units by unit certificates that we will issue under a separate agreement. We may enter into unit agreements with a unit agent. Each unit agent will be a bank or trust company that we select. We will indicate the name and address of the unit agent in the applicable prospectus supplement relating to a particular series of units.
We urge you to read the applicable prospectus supplement and any related free writing prospectus, as well as the complete unit certificate that contains the terms of the units.
Transfer Agent and Registrar
The transfer agent and registrar for our Ordinary Shares is Continental Stock Transfer & Trust Company. The transfer agent and registrar’s address is 1 State Street, 30th Floor, New York, NY 10004.
NASDAQ Capital Market Listing
Our Ordinary Shares are listed on the Nasdaq Capital Market under the symbol “VRAX.”
PLAN OF DISTRIBUTION
We may sell the securities offered through this prospectus (i) to or through underwriters or dealers, (ii) directly to purchasers, including our affiliates, (iii) through agents, or (iv) through a combination of any these methods. The securities may be distributed at a fixed price or prices, which may be changed, market prices prevailing at the time of sale, prices related to the prevailing market prices, or negotiated prices. The prospectus supplement will include the following information:
•the terms of the offering;
•the names of any underwriters or agents;
•the name or names of any managing underwriter or underwriters;
•the purchase price of the securities;
•any over-allotment options under which underwriters may purchase additional securities from us;
•the net proceeds from the sale of the securities;
•any delayed delivery arrangements;
•any underwriting discounts, commissions and other items constituting underwriters’ compensation;
•any initial public offering price;
•any discounts or concessions allowed or reallowed or paid to dealers;
•any commissions paid to agents; and
•any securities exchange or market on which the securities may be listed.
Sale Through Underwriters or Dealers
Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement. If underwriters are used in the sale, the underwriters will acquire the securities for their own account, including through underwriting, purchase, security lending or repurchase agreements with us. The underwriters may resell the securities from time to time in one or more transactions, including negotiated transactions. Underwriters may sell the securities in order to facilitate transactions in any of our other securities (described in this prospectus or otherwise), including other public or private transactions and short sales. Underwriters may offer securities to the public either through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters. Unless otherwise indicated in the prospectus supplement, the obligations of the underwriters to purchase the securities will be subject to certain conditions, and the underwriters will be obligated to purchase all the offered securities if they purchase any of them. The underwriters may change from time to time any public offering price and any discounts or concessions allowed or reallowed or paid to dealers.
If dealers are used in the sale of securities offered through this prospectus, we will sell the securities to them as principals. They may then resell those securities to the public at varying prices determined by the dealers at the time of resale. The prospectus supplement will include the names of the dealers and the terms of the transaction.
We will provide in the applicable prospectus supplement any compensation we will pay to underwriters, dealers or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers.
Direct Sales and Sales Through Agents
We may sell the securities offered through this prospectus directly. In this case, no underwriters or agents would be involved. Such securities may also be sold through agents designated from time to time. The prospectus supplement will name any agent involved in the offer or sale of the offered securities and will describe any commissions payable to the agent. Unless otherwise indicated in the prospectus supplement, any agent will agree to use its reasonable best efforts to solicit purchases for the period of its appointment.
We may sell the securities directly to institutional investors or others who may be deemed to be underwriters within the meaning of the Securities Act with respect to any sale of those securities. The terms of any such sales will be described in the prospectus supplement.
Delayed Delivery Contracts
If the prospectus supplement indicates, we may authorize agents, underwriters or dealers to solicit offers from certain types of institutions to purchase securities at the public offering price under delayed delivery contracts. These contracts would provide for payment and delivery on a specified date in the future. The contracts would be subject only to those conditions described in the prospectus supplement. The applicable prospectus supplement will describe the commission payable for solicitation of those contracts.
Market Making, Stabilization and Other Transactions
Unless the applicable prospectus supplement states otherwise, other than our Ordinary Shares, all securities we offer under this prospectus will be a new issue and will have no established trading market. We may elect to list offered securities on an exchange or in the over-the-counter market. Any underwriters that we use in the sale of offered securities may make a market in such securities, but may discontinue such market making at any time without notice. Therefore, we cannot assure you that the securities will have a liquid trading market.
Any underwriter may also engage in stabilizing transactions, syndicate covering transactions and penalty bids in accordance with Rule 104 under the Securities Exchange Act. Stabilizing transactions involve bids to purchase the underlying security in the open market for the purpose of pegging, fixing or maintaining the price of the securities. Syndicate covering transactions involve purchases of the securities in the open market after the distribution has been completed in order to cover syndicate short positions.
Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a syndicate covering transaction to cover syndicate short positions. Stabilizing transactions, syndicate covering transactions and penalty bids may cause the price of the securities to be higher than it would be in the absence of the transactions. The underwriters may, if they commence these transactions, discontinue them at any time.
General Information
Agents, underwriters, and dealers may be entitled, under agreements entered into with us, to indemnification by us against certain liabilities, including liabilities under the Securities Act. Our agents, underwriters, and dealers, or their affiliates, may be customers of, engage in transactions with or perform services for us, in the ordinary course of business.
LEGAL MATTERS
Except as otherwise set forth in the applicable prospectus supplement, certain legal matters in connection with the securities offered pursuant to this prospectus will be passed upon for us by Loeb & Loeb LLP to the extent governed by the laws of the State of New York, and by Ogier to the extent governed by the laws of the Cayman Islands. If legal matters in connection with offerings made pursuant to this prospectus are passed upon by counsel to underwriters, dealers or agents, such counsel will be named in the applicable prospectus supplement relating to any such offering.
EXPERTS
The consolidated financial statements of Virax Biolabs Group Limited as of March 31, 2023 and for the year then ended, have been incorporated by reference herein in reliance upon the report of Reliant CPA PC, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The registered business address of Reliant CPA PC is 895 Dove Street, Suite 300, Newport Beach, CA 92660, United States.
The consolidated financial statements of Virax Biolabs Group Limited as of March 31, 2022 and for the year then ended, have been incorporated by reference herein in reliance upon the report of BF Borgers CPA PC, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The registered business address of BF Borgers CPA PC is 5400 W Cedar Ave, Lakewood, CO 80226, United States.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
This prospectus is part of a registration statement we filed with the SEC. This prospectus, which constitutes part of the registration statement, does not contain all of the information set forth in the registration statement and the exhibits and schedules thereto. For further information with respect to the Company and its securities, reference is made to the registration statement and the exhibits and any schedules filed therewith. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov.
We are subject to the information reporting requirements of the Exchange Act and we are required to file reports, proxy statements and other information with the SEC. These reports, proxy statements, and other information are available for inspection and copying at the SEC’s website referred to above. We also maintain a website at www.viraxbiolabs.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with the SEC. This means that we can disclose important information to you by referring you to those documents. Any statement contained in a document incorporated by reference in this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein, or in any subsequently filed document, which also is incorporated by reference herein, modifies or supersedes such earlier statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We hereby incorporate by reference into this prospectus the following documents that we have filed with the SEC under the Exchange Act:
•our Annual Report on Form 20-F for the year ended March 31, 2023, filed with the SEC on June 14, 2023;
•our Current Reports on Form 6–K filed with the SEC on April 19, 2023, April 21, 2023, May 4, 2023, June 14, 2023, July 18, 2023, August 3, 2023, September 1, 2023, September 12, 2023, October 4, 2023, October 12, 2023, October 31, 2023, and November 8, 2023 (in each case, except for information contained therein which is furnished rather than filed); and
•the description of our Class A common stock in our registration statement on Form 8–A filed with the SEC on June 30, 2022, including any amendments thereto or reports filed for the purpose of updating such description.
•All documents filed by Virax under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, that are filed (excluding, however, information we furnish to the SEC) (i) by us after the date of the initial registration statement and prior to its effectiveness and (ii) by us after the date of this prospectus and prior to the termination of any offering under this registration statement.
Any statement contained in this prospectus, or in a document all or a portion of which is incorporated by reference, shall be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, any applicable prospectus supplement and any related free writing prospectus or any document incorporated by reference modifies or supersedes such statement. Any such statement so modified or superseded shall not, except as so modified or superseded, constitute a part of this prospectus.
Upon request, we will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered a copy of the documents incorporated by reference into this prospectus. You may request a copy of these filings, and any exhibits we have specifically incorporated by reference as an exhibit in this prospectus, at no cost by writing or telephoning us at the following:
Virax Biolabs Group Limited
20 North Audley Street
London, W1K 6LX
United Kingdom
Telephone: +44 020 7788 7414
You may also access these documents, free of charge on the SEC’s website at www.sec.gov or on the “Investors” page of our website at https://viraxbiolabs.com/. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information on, or that can be accessed from, our website as part of this prospectus or any accompanying prospectus supplement.
This prospectus is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration statement. You should read the exhibits carefully for provisions that may be important to you.
We have not authorized anyone to provide you with information other than what is incorporated by reference or provided in this prospectus or any prospectus supplement. We are not making an offer of these securities in any state where such offer is not permitted. You should not assume that the information in this prospectus or in the documents incorporated by reference is accurate as of any date other than the date on the front of this prospectus or those documents.
ENFORCEABILITY OF CIVIL LIABILITIES
We are an exempted company with limited liability incorporated under the laws of the Cayman Islands and our affairs are governed by our second amended and restated memorandum and articles of association and the Companies Act, and the common law of the Cayman Islands. We are incorporated in the Cayman Islands because of certain benefits associated with being a Cayman Islands company, such as political and economic stability, an effective judicial system, a favorable tax system, the absence of foreign exchange control or currency restrictions and the availability of professional and support services. However, certain disadvantages accompany incorporation in the Cayman Islands. These disadvantages include, but are not limited to, the following: (i) the Cayman Islands has a less developed body of securities laws as compared to the United States and provides less protection for investors; and (ii) Cayman Islands companies may not have standing to sue before the federal courts of the United States.
Our constitutional documents do not contain provisions requiring that disputes, including those arising under the securities laws of the United States, among us, our officers, directors and shareholders, be arbitrated.
Substantially all of our assets are located outside the United States. In addition, most of our directors and executive officers are nationals or residents of jurisdictions other than the United States and substantially all of their assets are located outside the United States. As a result, it may be difficult or impossible for you to effect service of process within the United States upon us or these persons, or to enforce judgments obtained in U.S. courts against us or them, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States. It may also be difficult for you to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our executive officers and directors.
We have appointed Virax Biolabs USA Management, Inc. as our agent to receive service of process with respect to any action brought against us in the United States in connection with this offering under the federal securities laws of the United States or of any State in the United States.
Cayman Islands
We have been advised by Ogier, our counsel as to Cayman Islands law, there is uncertainty as to whether the courts of the Cayman Islands would:
recognize or enforce judgments of U.S. courts obtained against us or our directors or officers predicated upon the civil liability provisions of securities laws of the United States or any state in the United States; or
entertain original actions brought in each respective jurisdiction against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States
Ogier has further advised us that although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign judgement, without any re-examination or re-litigation of matters adjudicated upon, provided such judgment:
(a)is given by a foreign court of competent jurisdiction;
(b)imposes on the judgment debtor a liability to pay a liquidated sum for which the judgment has been given;
(d)is not in respect of taxes, a fine or a penalty;
(e)was not obtained by fraud; and
(f)is not of a kind the enforcement of which is contrary to natural justice or the public policy of the Cayman Islands.
Subject to the above limitations, in appropriate circumstances, a Cayman Islands court may give effect in the Cayman Islands to other kinds of final foreign judgments such as declaratory orders, orders for performance of contracts and injunctions.
As a result of all of the above, public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a U.S. company.
Singapore
Singapore has no arrangement for the reciprocal enforcement of judgments with the United States. It is possible that the Singapore courts may not (i) recognize and enforce judgments of courts in the United States, based upon the civil liability provisions of the securities laws of the United States or any state or territory of the United States, or (ii) enter judgments in original actions brought in the Singapore courts based solely on the civil liability provisions of these securities laws. An in personam final and conclusive judgment in the federal or state courts of the United States under which a fixed or ascertainable sum of money is payable may generally be enforced as a debt in the Singapore courts under the common law as long as it is established that the Singapore courts have jurisdiction over the judgment debtor, subject to the applicable substantive and procedural laws of Singapore. Additionally, the court where the judgment was obtained must have had international jurisdiction over the party sought to be bound in the local proceedings. However, the Singapore courts are unlikely to enforce a foreign judgment if (a) the foreign judgment is inconsistent with a prior local judgment that is binding on the same parties; (b) the enforcement of the foreign judgment would contravene the public policy of Singapore; (c) the proceedings in which the foreign judgment was obtained were contrary to principles of natural justice; (d) the foreign judgment was obtained by fraud; or (e) the enforcement of the foreign judgment amounts to the direct or indirect enforcement of a foreign, penal, revenue or other public laws.
In particular, the Singapore courts may potentially not allow the enforcement of any foreign judgment for a sum payable in respect of taxes, fines, penalties or other similar charges, including the judgments of courts in the United States based upon the civil liability provisions of the securities laws of the United States or any state or territory of the United States. In respect of civil liability provisions of the United States federal and state securities law which permit punitive damages against us and our Directors or Executive Officers, we are unaware of any decision by the Singapore courts which has considered the specific issue of whether a judgment of a United States court based on such civil liability provisions of the securities laws of the United States or any state or territory of the United States is enforceable in Singapore.
Hong Kong
There is uncertainty as to whether the courts of Hong Kong would (i) recognize or enforce judgments of United States courts obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States or (ii) entertain original actions brought in Hong Kong against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States.
A judgment of a court in the United States predicated upon U.S. federal or state securities laws may be enforced in Hong Kong at common law by bringing an action in a Hong Kong court on that judgment for the amount due thereunder, and then seeking summary judgment on the strength of the foreign judgment, provided that the foreign judgment, among other things, is (1) for a debt or a definite sum of money (not being taxes or similar charges to a foreign government taxing authority or a fine or other penalty) and (2) final and conclusive on the merits of the claim, but not otherwise. Such a judgment may not, in any event, be so enforced in Hong Kong if (a) it was obtained by fraud; (b) the proceedings in which the judgment was obtained were opposed to natural justice; (c) its enforcement or recognition would be contrary to the public policy of Hong Kong; (d) the court of the United States was not jurisdictionally competent; or (e) the judgment was in conflict with a prior Hong Kong judgment.
Hong Kong has no arrangement for the reciprocal enforcement of judgments with the United States. As a result, there is uncertainty as to the enforceability in Hong Kong, in original actions or in actions for enforcement, of judgments of United States courts of civil liabilities predicated solely upon the federal securities laws of the United States or the securities laws of any State or territory within the United States.
China
There is uncertainty as to whether the courts of China would (1) recognize or enforce judgments of United States courts obtained against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or (2) be competent to hear original actions brought in each respective jurisdiction, against us or such persons predicated upon the securities laws of the United States or any state thereof.
The recognition and enforcement of foreign judgments are mainly provided for under the Chinese Civil Procedure Law. Chinese courts may recognize and enforce foreign judgments in accordance with the requirements of the Chinese Civil Procedure Law and other applicable laws and regulations based either on treaties between China and the country where the judgment is made or in reciprocity between jurisdictions. Accordingly, there is uncertainty whether China courts will recognize or enforce judgments of United States or Cayman Islands Courts because China does not have any treaties or other agreements with the Cayman Islands or the United States that provide for the reciprocal recognition and enforcement of foreign judgments as of the date of this prospectus. Further, under Chinese Civil Procedure Law, Chinese courts will not enforce a foreign judgment against us or our officers and directors if the court decides that such judgment violates the basic principles of PRC law or national sovereignty, security or social public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States or in the Cayman Islands.
Under the PRC Civil Procedure Law, foreign shareholders may originate actions based on PRC law against a company in China for disputes if they can establish sufficient nexus to the PRC for a PRC court to have jurisdiction, and meet other procedural requirements, including, among others, the plaintiff must have a direct interest in the case, and there must be a concrete claim, a factual basis and a cause for the suit. However, it will be difficult for U.S. shareholders to originate actions against us in the PRC in accordance with PRC laws because we are incorporated under the laws of the Cayman Islands and it will be difficult for U.S. shareholders, by virtue only of holding our ordinary shares, to establish a connection to the PRC for a PRC court to have jurisdiction as required under the PRC Civil Procedure Law.
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
|
|
|
|
|
PRELIMINARY PROSPECTUS (SUBJECT TO COMPLETION) |
|
DATED December , 2023 |
UP TO 15,195,292 Ordinary Shares

Virax Biolabs Group Limited
This prospectus relates to the offer and resale of purchase warrants (the “New Warrants”) to purchase up to 14,681,442 ordinary shares, par value $0.0001 per share (the “Ordinary Shares”), which were issued by us pursuant to a letter agreement dated October 11, 2023 (the “Letter Agreement”) and placement agent warrants (the “Placement Agent Warrants” and together with the New Warrants, the “Warrants”) to purchase up to an aggregate of 513,850 Ordinary Shares, which were issued by us pursuant to an engagement agreement, dated September 22, 2023 (the “Engagement Agreement”). The holders of the Ordinary Shares are each referred to herein as a “Selling Shareholder”.
The exercise price of the New Warrants is $0.2934 per share. The exercise price of the Placement Agent Warrants is $0.36675 per share. See “Use of Proceeds”. The Selling Shareholders, or its respective transferees, pledgees, donees or other successors-in-interest, may sell the Ordinary Shares through public or private transactions at prevailing market prices, at prices related to prevailing market prices or at privately negotiated prices. The Selling Shareholders may sell any, all or none of the securities offered by this prospectus, and we do not know when or in what amount the Selling Shareholders may sell their Ordinary Shares hereunder following the effective date of this registration statement. We provide more information about how the Selling Shareholders may sell its Ordinary Shares in the section titled “Plan of Distribution” on page 30.
We are registering the Ordinary Shares on behalf of the Selling Shareholders to be offered and sold by them from time to time. While we will not receive any proceeds from the sale of the Ordinary Shares by any selling shareholder, we will receive proceeds from the exercise of any Warrants for cash. We have agreed to bear all of the expenses incurred in connection with the registration of the Ordinary Shares. The Selling Shareholders will pay or assume discounts, commissions, fees of underwriters, selling brokers or dealer managers and similar expenses, if any, incurred for the sale of the Ordinary Shares.
Our Ordinary Shares is traded on The Nasdaq Capital Market under the symbol “VRAX.” On November 27, 2023, the reported sales price of our Ordinary Shares on The Nasdaq Capital Market was $0.24 per share.
Investment in our Ordinary Shares involves a high degree of risk. See “Risk Factors” beginning on page 18, in our periodic reports filed from time to time with the Securities and Exchange Commission, which are incorporated by reference in this prospectus and in any applicable prospectus supplement. You should carefully read this prospectus and the accompanying prospectus supplement, together with the documents we incorporate by reference, before you invest in our ordinary shares.
We are both an “emerging growth company” and a “foreign private issuer” as defined under the U.S. federal securities laws and, as such, may elect to comply with certain reduced public company reporting requirements for this and future filings.
Investing in our Ordinary Shares is highly speculative and involves a significant degree of risk. Virax Biolabs Group Limited, which we refer to as "Virax Cayman", is a holding company incorporated as an exempted company
under the laws of the Cayman Islands. As a holding company with no material operations of our own, Virax Cayman conduct a substantial majority of our sales and trading activities through our operating entity established in Singapore, Virax Biolabs Pte. Limited, which we refer to as SingaporeCo. Currently, Virax Cayman indirectly owns 95.65% of the equity interests in SingaporeCo. However, some of Virax Cayman’s operations are currently conducted through our operating entities established in the British Virgin Islands, Hong Kong and Shanghai, primarily, Logico Bioproducts Corp., Virax Immune T-Cell Medical Device Company Limited and Shanghai Xitu Consulting Co., Limited, which we refer to as Logico BVI, Virax Immune T-Cell and Shanghai Xitu, respectively. Our Ordinary Shares offered in this prospectus are shares of our Cayman Islands holding company.
Recent statements by the Chinese government have indicated an intent to exert more oversight and control over offerings that are conducted overseas and/or foreign investments in China based issuers. Any future action by the Chinese government expanding the categories of industries and companies whose foreign securities offerings are subject to government review could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly decline or be worthless.
Recently, the PRC government initiated a series of regulatory actions and made a number of public statements on the regulation of business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas using a variable interest entity structure, adopting new measures to extend the scope of cybersecurity reviews, and expanding efforts in anti-monopoly enforcement. We are not subject to these regulatory actions or statements, as we do not have a variable interest entity structure and our business does not involve the collection of user data, implicate cybersecurity, or involve any other type of restricted industry. Because these statements and regulatory actions are new, however, it is highly uncertain how soon legislative or administrative regulation making bodies in China will respond to them, or what existing or new laws or regulations will be modified or promulgated, if any, or the potential impact such modified or new laws and regulations will have on our daily business operations or our ability to accept foreign investments and list on an U.S. exchange.
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. In accordance with the HFCA Act, trading in securities of any registrant on a national securities exchange or in the over-the-counter trading market in the United States may be prohibited if the Public Company Accounting Oversight Board (the “PCAOB”) determines that it cannot inspect or fully investigate the registrant’s auditor for three consecutive years beginning in 2021, and, as a result, an exchange may determine to delist the securities of such registrant. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, which, if enacted, would amend the HFCA Act and require the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections for two consecutive years instead of three, thus reducing the time period before our securities may be prohibited from trading or delisted if our auditor is unable to meet the PCAOB inspection requirement. Pursuant to the HFCA Act, the PCAOB issued a Determination Report on December 16, 2021 which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. In addition, the PCAOB’s report identified the specific registered public accounting firms which are subject to these determinations. On August 26, 2022, the PCAOB entered into a Statement of Protocol with the China Securities Regulatory Commission and the Ministry of Finance of the PRC and, as summarized in the “Statement on Agreement Governing Inspections and Investigations of Audit Firms Based in China and Hong Kong” published on the U.S. Securities and Exchange Commission’s official website, the parties agreed to the following: (i) in accordance with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation; (ii) the PCAOB shall have direct access to interview or take testimony from all personnel of the audit firms whose issuer engagements are being inspected or investigated; (iii) the PCAOB shall have the unfettered ability to transfer information to the SEC, in accordance with the Sarbanes-Oxley Act; and (iv) the PCAOB inspectors shall have access to complete audit work papers without any redactions, with view-only procedures for certain targeted pieces of information such as personally identifiable information. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, whether the PCAOB will continue to be able to satisfactorily conduct inspections of PCAOB-registered public accounting firms headquartered in mainland China and Hong Kong is subject to uncertainty and
depends on a number of factors out of our, and our auditor’s, control. The PCAOB is continuing to demand complete access in mainland China and Hong Kong moving forward and is already making plans to resume regular inspections in early 2023 and beyond, as well as to continue pursuing ongoing investigations and initiate new investigations as needed. In the future, if there is any regulatory change or step taken by PRC regulators that does not permit our auditor to provide audit documentations located in China or Hong Kong to the PCAOB for inspection or investigation, or the PCAOB expands the scope of the Determination so that we are subject to the HFCA Act, as the same may be amended, you may be deprived of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities, including trading on the national exchange and trading on “over-the-counter” markets, may be prohibited under the HFCA Act. On December 29, 2022, a legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”), was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two. Our registered public accounting firm, Reliant CPA PC, is not headquartered in mainland China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determination. Notwithstanding the foregoing, if the PCAOB is not able to fully conduct inspections of our auditor’s work papers in China, you may be deprived of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities may be prohibited under the HFCA Act.
Within the organization, investor cash inflows have all been received by Virax Cayman. Cash to fund Virax Cayman’s operations is transferred from Virax Cayman down through our Singapore, Hong Kong, BVI entities and then into our Chinese entity through capital contributions and loans. However, the PRC government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China, and investment in Chinese companies, which are governed by the Foreign Investment Law and Company Law, and the dividends and distributions from Shanghai Xitu is subject to relevant regulations and restrictions on dividends and payment to parties outside of China. Transfers among our Singapore and Hong Kong entities are not restricted under Singapore and Hong Kong Laws. No dividends or distribution have been made by our subsidiaries or by Virax Cayman to date and we intend to reinvest all cash into our subsidiaries for the foreseeable future. For the years ended March 31, 2023 and 2022.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or the accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2023.
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
Page |
About this Prospectus |
|
42 |
Special Note Regarding Forward-Looking Statements |
|
44 |
Company Overview |
|
45 |
The Offering |
|
51 |
Risk Factor |
|
52 |
Use of Proceeds |
|
57 |
Description of Share Capital and Governing Documents |
|
58 |
Selling Shareholder |
|
61 |
Plan of Distribution |
|
63 |
Enforcement of Civil Liabilities |
|
65 |
Legal Matters |
|
68 |
Experts |
|
68 |
Incorporation of Certain Information by Reference |
|
69 |
Where You Can Find Additional Information |
|
71 |
i
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form F–3 that we filed with the Securities and Exchange Commission (the “SEC”) using the “shelf” registration process. Under this shelf registration process, the selling stockholders may, from time to time, sell the securities offered by them described in this prospectus. We will not receive any proceeds from the sale by such selling stockholders of the securities offered by them described in this prospectus.
Neither we nor the selling shareholders have authorized anyone to provide you with any information or to make any representations other than those contained in this prospectus or any applicable prospectus supplement or any free writing prospectuses prepared by or on behalf of us or to which we have referred you. Neither we nor the selling stockholders take responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Neither we nor the selling stockholders will make an offer to sell these securities in any jurisdiction where the offer or sale is not permitted.
We may also provide a prospectus supplement to add information to, or update or change information contained in, this prospectus. Any statement contained in this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in such prospectus supplement modifies or supersedes such statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus. You should read both this prospectus and any applicable prospectus supplement together with the additional information to which we refer you in the sections of this prospectus entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference.”
Unless the context indicates otherwise, references in this prospectus to the “Company,” “Virax,” “we,” “us,” “our” and similar terms refer to Virax Biolabs Group Limited and its consolidated subsidiaries.
As permitted by SEC rules and regulations, the registration statement of which this prospectus forms a part includes additional information not contained in this prospectus. You may read the registration statement and the other reports we file with the SEC at its website or at its offices described below under “Where You Can Find Additional Information.”
Unless we explicitly state otherwise or the context otherwise indicates clearly, all references in this proxy statement to “we,” “us,” “our,” or “our Group” refer to Virax Biolabs Group Limited and its subsidiaries, namely, Virax Biolabs (UK) Limited, Virax Biolabs USA Management, Inc., Virax Biolabs Limited, Virax Immune T-Cell Medical Device Company Limited, Virax Biolabs Pte. Limited, Logico Bioproducts Corp., and Shanghai Xitu Consulting Co., Limited.
The “Company” or “Virax Cayman” refers to Virax Biolabs Group Limited.
“GBP” or “GB£” refers to the legal currency of the United Kingdom.
“HKD” or “HK$” refers to the legal currency of Hong Kong.
“RMB” or “Renminbi” refers to the legal currency of China.
“IVD” refers to in-vitro diagnostics.
“PRC” or “China” refers to the People’s Republic of China, including Hong Kong and Macau.
“Prospectus” refers to the public offering prospectus unless we explicitly state otherwise or the context otherwise indicates clearly.
“SGD” or “S$” refers to the legal currency of Singapore.
“United Kingdom” or “UK” refers to the England, Scotland, Wales and Northern Ireland for the purpose of this prospectus.
“$” or “U.S. dollars” or “USD” refers to the legal currency of the United States.
We have made rounding adjustments to some of the figures included in this prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
Our business is primarily conducted in Europe, and the financial records of our subsidiaries in Asia are maintained in USD, and our functional currency is USD. Our consolidated financial statements are presented in U.S. dollars. We use U.S. dollars as the reporting currency in our consolidated financial statements and in this prospectus.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “goal,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements and opinions contained in this prospectus are based upon information available to us as of the date of this prospectus and, while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. Forward-looking statements include statements about:
timing of the development of future business;
capabilities of our business operations;
expected future economic performance;
competition in our market;
continued market acceptance of our services and products;
protection of our intellectual property rights;
changes in the laws that affect our operations;
inflation and fluctuations in foreign currency exchange rates;
our ability to obtain and maintain all necessary government certifications, approvals, and/or licenses to conduct our business;
continued development of a public trading market for our securities;
the cost of complying with current and future governmental regulations and the impact of any changes in the regulations on our operations;
managing our growth effectively;
projections of revenue, earnings, capital structure and other financial items;
fluctuations in operating results;
dependence on our senior management and key employees; and
other factors set forth under “Risk Factors.”
You should refer to the section titled “Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus forms a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
COMPANY OVERVIEW
Overview
Virax Cayman is a holding company incorporated as an exempted company under the laws of the Cayman Islands. As a holding company with no material operations of our own, Virax Cayman conducts our operations through its subsidiaries in the United Kingdom, the United States, Singapore, Hong Kong, China and British Virgin Islands and has been operating since 2013.
Virax Biolabs Group Limited and its subsidiaries is a global innovative biotechnology company focused on the prevention, detection, diagnosis, and risk management of viral diseases with a particular interest in the field of T-Cell in Vitro Diagnostics. The Company is in the process of developing and manufacturing tests that can predict adaptive immunity to viral diseases as well as identify individuals suffering from T-cell exhaustion linked to post viral syndromes. The Company's mission is to protect people from viral diseases and help with the early diagnosis of post viral syndromes associated with T-cell exhaustion and chronic fatigue through the provision of diagnostic tests, tests for adaptive immunity and education through a wellness mobile application which could allow people to make informed decisions regarding their viral risks.
Diagnostics test kits are distributed through our ViraxClear and ViraxVet brands. Currently, we do not manufacture or develop any product that we sell in our ViraxClear and ViraxVet product portfolios, and we act as a distributor of third-party suppliers’ products. Our Company also seeks to maximize consumers’ access to our products and services through competitive pricing and regular evaluations of our pricing arrangements and contracts with our distributors.
We also expect to launch an upcoming brand, ViraxImmune, with the intention of providing an immunology profiling platform that assesses each individual’s immune risk profile against major global viral diseases as well as helping with the early diagnosis of post viral syndromes associated with T-cell exhaustion and chronic fatigue. We are in the process of developing a T-Cell Test under the ViraxImmune brand and will apply for regulatory agency approval. We believe that the T-Cell Tests and immunology platform we are developing under the ViraxImmune brand will be particularly useful in assisting in the threat analysis of the major viruses faced globally as well as helping with the early diagnosis of indications associated with T-cell exhaustion and chronic fatigue. Initially, we will be focusing in diseases associated with post viral syndromes including but not limited to COVID-19, Human Papillomavirus (better known as HPV), Malaria, Hepatitis B, and Herpes (better known as HSV-1). The results and education for specific viruses will be delivered through our mobile-based immunology application.
Recent Developments
Initial Public Offering
On July 20, 2022, Virax entered into an underwriting agreement with Boustead Securities, LLC, as representatives of the several underwriters, in connection with its initial public offering (“IPO”) of 1,350,000 Ordinary Shares, at a price of $5.00 per share, before deducting underwriting discounts, commissions, and other related expenses. The shares began trading on the Nasdaq Capital Market on July 21, 2022. The Company issued Representative’s Warrant to purchase up to 108,675 ordinary shares at $6.00 per share, dated July 20, 2022, to Boustead Securities, LLC. On July 25, 2022, the Company consummated its IPO generating gross proceeds to the Company of $7,762,500, before deducting underwriting discounts and other related expenses.
2022 PIPE Financing
On November 3, 2022, Virax entered into a Securities Purchase Agreement (the “November SPA”) with an accredited investor for a private placement offering (“2022 Private Placement”), pursuant to which the Company received gross proceeds of approximately $3,844,500, before deducting placement agent fees and other offering expenses, in consideration of (i) 1,165,000 Ordinary Shares; (ii) pre-funded warrants to purchase 1,165,000 Ordinary Shares, and (iii) warrants to purchase 3,495,000 Ordinary Shares at a combined purchase price of $1.65 per Ordinary Share and one and a half Ordinary Warrant, or approximately $1.65 per Pre-Funded Warrant and one and a half Ordinary Warrant if purchasing the Pre-Funded Warrants. The warrants have an exercise price of $1.73 per share. The
November SPA contains customary representations and warranties and agreements of the Company and the purchaser and customary indemnification rights and obligations of the parties. The 2021 Private Placement closed on November 8, 2022. Concurrently with the signing of the November SPA, we entered into a registration rights agreement to file with the Securities and Exchange Commission a registration statement covering the resale of all of the registrable securities under the registration rights agreement.
2023 PIPE Financing
On March 8, 2023, Virax entered into a Securities Purchase Agreement (the “PIPE Securities Purchase Agreement”) with an accredited investor for a private placement offering (“2023 Private Placement”), pursuant to which the Company received gross proceeds of approximately $4,00,000, before deducting placement agent fees and other offering expenses, in consideration of (i) 1,500,000 Ordinary Shares; (b) Pre-Funded Warrants to purchase 2,343,309 Ordinary Shares, (iii) Series A Options to purchase 3,497,412 Ordinary Shares, and (iv) Series B Options to purchase 3,843,309 Ordinary Shares at a purchase price of $1.04077 per Ordinary Share and associated Preferred Options and a purchase price of $1.04067 per Pre-Funded Warrant and associated Preferred Options (the “PIPE Offering”). The Preferred Options have an exercise price of $0.80202 per share. In addition, the Company issued warrants to purchase up to 269,032 Ordinary Shares at $1.3010 per share to certain designees of H.C. Wainwright & Co., placement agent of the Offering. The PIPE Securities Purchase Agreement contains customary representations and warranties and agreements of the Company and the purchaser and customary indemnification rights and obligations of the parties. The 2023 Private Placement closed on March 10, 2023. Concurrently with the signing of the PIPE Securities Purchase Agreement, we entered into a registration rights agreement to file with the Securities and Exchange Commission a registration statement covering the resale of all of the registrable securities under the registration rights agreement.
2023 Warrant Inducement
On October 11, 2023, we entered into an inducement offer letter agreement (the “Inducement Letter”) with a certain holder (the “Holder”) of existing Series A and B Options (the “Existing Warrants”) to purchase ordinary shares of the Company. The Existing Warrants were issued on March 10, 2023 and each has an exercise price of $0.80202 per share.
Pursuant to the Inducement Letter, the Holder agreed to exercise for cash its Existing Warrants to purchase an aggregate of 7,340,721 ordinary shares of the Company at a reduced exercise price of $0.2934 per share in consideration for the Company’s agreement to issue new warrants to purchase ordinary share (the “New Warrants”), as described below, to purchase up to 14,681,442 of the Company’s ordinary share of par value US$0.0001 eaach (the “New Warrant Shares”). The Company received an aggregate gross proceeds of approximately $2.15 million from the exercise of the Existing Warrants by the Holder, before deducting placement agent fees and other offering expenses payable by the Company. Each New Warrant will have an exercise price equal to $0.2934 per share. The New Warrants will be immediately exercisable from the date of issuance until the five year anniversary of the initial exercise date. The exercise price and number of Ordinary Shares issuable upon exercise is subject to appropriate adjustment in the event of share dividends, share splits, subsequent rights offerings, pro rate distributions, reorganizations, or similar events affecting the Company’s Ordinary Shares and the exercise price.
In addition, on October 16, 2023, the Company issued warrants to purchase up to 513,850 Ordinary Shares of par value US$0.0001 each at $0.36675 per share to certain designees of H.C. Wainwright & Co., placement agent of the offering. The Inducement Letter contains customary representations, warranties and covenants by the Company which were made only for the purposes of such agreements and as of specific dates, were solely for the benefit of the parties to such agreements and may be subject to limitations agreed upon by the contracting parties. Concurrently with the signing of the Inducement Letter Agreement, we agreed to file with the Securities and Exchange Commission a registration statement covering the resale of all of the registrable securities under the registration rights agreement.
Corporate History and Structure
Structural Overview
Virax Cayman is a holding company incorporated as an exempted company under the laws of the Cayman Islands that owns all of the outstanding capital stock of Virax Biolabs (UK) Limited and Virax Biolabs USA Management, Inc., our wholly-owned subsidiaries. Virax Biolabs (UK) Limited, in turn, owns all of the outstanding capital stock of Virax Biolabs Limited, our wholly-owned Hong Kong subsidiary. Virax Biolabs Limited owns all of the outstanding capital stock of Virax Immune T-Cell Medical Device Company Limited, our wholly-owned Hong Kong subsidiary, and 95.65% of the outstanding capital stock of Virax Biolabs Pte. Limited, our operating subsidiary incorporated in Singapore. Virax Biolabs Pte. Limited owns all of the outstanding capital stock of Logico Bioproducts Corp., a wholly-owned British Virgin Islands and a subsidiary of Virax Biolabs Pte. Limited. Logico Bioproducts Corp., in turn, owns all of the outstanding capital stock of Shanghai Xitu, a wholly-owned subsidiary of Logico Bioproducts Corp. and a wholly foreign owned enterprise based in China.
We completed a reorganization and share exchange of our company in September 2021 (the “Reorganization”). Pursuant to the Reorganization, all shareholders of Virax Biolabs Limited (HK) transferred their shares, 102,478,548 ordinary shares in total, to Virax Biolabs (UK) Limited, in exchange for an aggregate of (i) 2,549,028 newly issued Class A Ordinary Shares and (ii) 7,034,306 newly issued Class B Ordinary Shares of Virax Biolabs Group Limited. On June 19, 2022, Virax Cayman underwent a shareholding restructuring whereby the Company’s authorized share capital became a single class of shares of Ordinary Shares and all of the then issued shares were re-designated as Ordinary Shares.
Organization Structure and Purpose
Virax Biolabs Group Limited (“Virax Cayman”) — Virax Biolabs Group Limited is a Cayman Islands exempted company incorporated on September 2, 2021, previously named as “Virax Biolabs (Cayman) Limited” and effected a name change to “Virax Biolabs Group Limited” on January 19, 2022. Structured as a holding company with no material operations, Virax Cayman conducts our operations through its operating subsidiaries in the Hong Kong, Singapore, British Virgin Islands and China.
Virax Biolabs (UK) Limited — Virax Biolabs (UK) Limited was incorporated on August 19, 2021 under the laws of the United Kingdom, a wholly-owned subsidiary of Virax Cayman and structured as a holding company with no material operations.
Virax Biolabs USA Management, Inc. — Virax Biolabs USA Management, Inc. was incorporated on August 1, 2022 under the laws of the United States, a wholly-owned subsidiary of Virax Cayman and structured as a management company for operations within the United States.
Virax Biolabs Limited (“HKco”) — Virax Biolabs Limited, incorporated on April 14, 2020 under the laws of Hong Kong, was previously named as “Shanghai Biotechnology Devices Limited” and effected a name change to “Virax Biolabs Limited” on July 12, 2021. Virax Biolabs Limited, our wholly-owned Hong Kong subsidiary, serves as a holding company.
Virax Immune T-Cell Medical Device Company Limited (“Virax Immune T-Cell”) — Virax Immune T-Cell Medical Device Company Limited, a wholly-owned subsidiary of HKco, incorporated on January 16, 2017 under the laws of Hong Kong, was previously named as “Stork Nutrition Asia Limited” and effected a name change to “Virax Immune T-Cell Medical Device Company Limited” on September 10, 2021. It is primarily engaged in the research and development of T-Cell blood analysis.
Virax Biolabs Pte. Limited (“SingaporeCo”) — Virax Biolabs Pte. Limited, incorporated on May 4, 2013 under the laws of Singapore, was previously named as “Natural Source Group Pte. Limited” and effected a name change to “Virax Biolabs Pte. Limited” on July 2, 2021. 95.65% of its capital stock is owned by Virax Biolabs Limited and the remaining 4.35% is owned by independent third party shareholders. It is our operating company, primarily engaged in the trading and sales of our products and running primarily day to day operations.
Logico Bioproducts Corp. (“Logico BVI”) — Logico Bioproducts Corp., a wholly-owned subsidiary of SingaporeCo, is a limited liability company incorporated in the British Virgin Islands on January 21, 2011, and is primarily engaged in the trading and sales of our products.
Shanghai Xitu Consulting Co., Limited (“Shanghai Xitu”) — Shanghai Xitu, a wholly-owned subsidiary of Logico BVI and a wholly foreign owned enterprise, is a limited liability company incorporated on October 27, 2017 in China. Shanghai Xitu is primarily engaged in procurement.
Virax Biolabs Group Holdings Ltd (“Virax UK HoldCo”) — Virax Biolabs Group Holdings Limited was incorporated on February 22, 2023 under the laws of the United Kingdom, a wholly-owned subsidiary of the Company and structured as a holding company.
Virax Biolabs FZ-LLC (“Virax Dubai”) — Virax Biolabs FZ-LLC was incorporated on April 18, 2023 under the laws of the United Kingdom, a wholly-owned subsidiary of the Company and is primarily engaged as a regional distribution company.
Virax Biolabs Trading B.V. (“Virax Netherlands”) — Virax Biolabs Trading B.V. was incorporated on August 4, 2023 under the laws of the Netherlands, a wholly-owned subsidiary of the Company and is primarily engaged as a regional distribution company.
Virax Biolabs UK Operating LLC (“Virax UK Operating”) — Virax Biolabs UK Operating was incorporated on August 4, 2023 under the laws of the United Kingdom, a wholly-owned subsidiary of the Company and is primarily engaged as a regional operating company.
The following diagram illustrates our corporate structure:
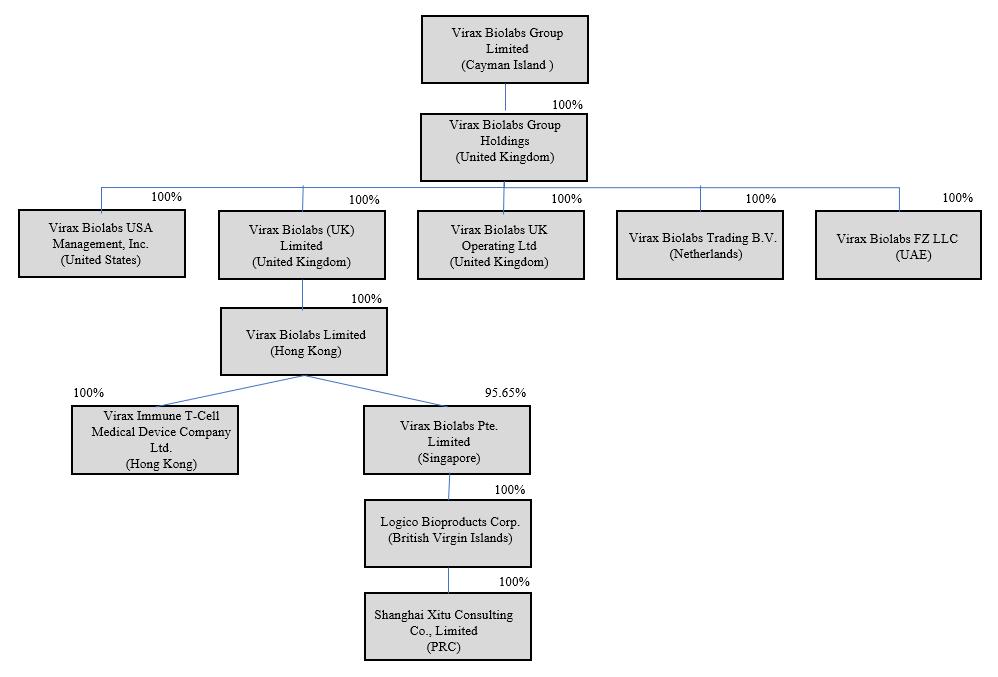
Implications of Being an Emerging Growth Company and a Foreign Private Issuer
Emerging Growth Company
As a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012, and may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in our filings with the SEC;
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
reduced disclosure obligations regarding executive compensation in periodic reports, proxy statements and registration statements; and
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our Ordinary Shares pursuant to this offering. However, if certain events occur before the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.235 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company before the end of such five-year period.
In addition, Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and acknowledge such election is irrevocable pursuant to Section 107 of the JOBS Act.
Foreign Private Issuer
We are a “foreign private issuer,” as defined by the SEC. As a result, in accordance with the rules and regulations of The Nasdaq Stock Market LLC, or Nasdaq, we may comply with home country governance requirements and certain exemptions thereunder rather than complying with Nasdaq corporate governance standards. We may choose to take advantage of the following exemptions afforded to foreign private issuers:
•Exemption from filing quarterly reports on Form 10-Q or provide current reports on Form 8-K disclosing significant events within four (4) days of their occurrence.
•Exemption from Section 16 rules regarding sales of Ordinary Shares by insiders, which will provide less data in this regard than shareholders of U.S. companies that are subject to the Exchange Act.
•Exemption from the Nasdaq rules applicable to domestic issuers requiring disclosure within four (4) business days of any determination to grant a waiver of the code of business conduct and ethics to directors and officers. Although we will require board approval of any such waiver, we may choose not to disclose the waiver in the manner set forth in the Nasdaq rules, as permitted by the foreign private issuer exemption.
•Exemption from the requirement that our board of directors have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities.
•Exemption from the requirements that director nominees are selected, or recommended for selection by our board of directors, either by (i) independent directors constituting a majority of our board of directors’ independent directors in a vote in which only independent directors participate, or (ii) a committee comprised
solely of independent directors, and that a formal written charter or board resolution, as applicable, addressing the nominations process is adopted.
Furthermore, Nasdaq Rule 5615(a)(3) provides that a foreign private issuer, such as us, may rely on our home country corporate governance practices in lieu of certain of the rules in the Nasdaq Rule 5600 Series and Rule 5250(d), provided that we nevertheless comply with Nasdaq’s Notification of Noncompliance requirement (Rule 5625), the Voting Rights requirement (Rule 5640) and that we have an audit committee that satisfies Rule 5605(c)(3), consisting of committee members that meet the independence requirements of Rule 5605(c)(2)(A)(ii). If we rely on our home country corporate governance practices in lieu of certain of the rules of Nasdaq, our shareholders may not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq. If we choose to do so, we may utilize these exemptions for as long as we continue to qualify as a foreign private issuer.
Although we are permitted to follow certain corporate governance rules that conform to Cayman Islands requirements in lieu of many of the Nasdaq corporate governance rules, we intend to comply with the Nasdaq corporate governance rules applicable to foreign private issuers.
Corporate Information
Our principal executive office is located at 20 North Audley Street London, W1K 6LX, United Kingdom. Our telephone number is +44 020 7788 7414. Our registered office in the Cayman Islands is located at the office of Ogier Global (Cayman) Limited, 89 Nexus Way, Camana Bay, Grand Cayman, KY1-9009, Cayman Islands.
Our agent for service of process in the United States is Virax Biolabs USA Management, Inc., located at Virax Biolabs USA Management, Inc. Our principal website is located at https://viraxbiolabs.com/. Information contained on, or that can be accessed through, our website is not a part of, and shall not be incorporated by reference into, this prospectus.
The Offering
|
|
|
Ordinary Shares to be Offered by the Selling Shareholders |
|
Up to 15,195,292 of our Ordinary Shares. These 15,195,292 Ordinary Shares consist of (i) 14,681,442 Ordinary Shares (the “New Warrant Shares”) issued or issuable upon the exercise of new warrants (the “New Warrant”) pursuant to the inducement offer letter agreement, dated as of October 11, 2023, entered into between the Company and Armistice Capital Master Fund Ltd. (the “Inducement Offer Letter Agreement”); and (ii) 513,850 Ordinary Shares issued or issuable upon the exercise of placement agent warrants (the “Placement Warrants”) that were issued to certain designees of H.C. Wainwright & Co., placement agent of 2023 Warrant Inducement. |
|
|
|
Warrants |
|
Each New Warrant will be immediately exercisable, will expire five (5) years from the date of issuance, or October 16, 2028 and has an exercise price of $0.2934 per share, subject to adjustment as set forth therein. Each Placement Agent Warrant will be immediately exercisable, will expire five (5) years from the date of issuance, or October 16, 2028 and have an exercise price of $0.36675 per share, subject to adjustment as set forth therein. |
|
|
|
Ordinary shares outstanding before this offering |
|
18,115,398 Ordinary Shares. |
|
|
|
Warrants outstanding before this offering |
|
Warrants to purchase 7,609,753 Ordinary Shares. |
|
|
|
Use of Proceeds |
|
We will not receive any of the proceeds from the sale of Ordinary Shares by the Selling Shareholders pursuant to this prospectus. The Selling Shareholders will pay any agent’s commissions and expenses they incur for brokerage, accounting, tax or legal services or any other expenses that they incur in disposing of the Ordinary Shares. We will bear all other costs, fees and expenses incurred in effecting the registration of the Ordinary Shares covered by this prospectus and any prospectus supplement. |
|
|
|
Nasdaq Global Market Symbol |
|
Our ordinary shares are listed on The Nasdaq Capital Market under the symbol “VRAX.” |
|
|
|
Risk Factors |
|
See “Risk Factors” and other information included in this prospectus for a discussion of factors you should consider before investing in our securities. |
RISK FACTOR
Our business is subject to a number of risks and uncertainties, including those risks discussed at length in Item 3D (“Risk Factors”) in our 2023 Form 20-F incorporated into this prospectus by reference. These risks include among others those summarized below. Investing in our company and its securities involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information in this prospectus, including the information incorporated by reference to our 2023 Form 20-F, before investing in our company and our securities. If any of these risks materialize, our business, financial condition, operating results and prospects could be materially and adversely affected. In that event, the price or value of our Ordinary Shares in the public market could decline, and you could lose part or all of your investment.
The following is a summary of some of the principal risks we face. The list below is not exhaustive, and investors should read the risks described under the heading “Risk Factors” in our 2023 Form 20-F incorporated by reference herein, as well as the additional risks set forth in this section, in full.
We have limited operating history, have incurred operating losses for the years ended March 31, 2023 and 2022 and expect to incur significant losses for the foreseeable future. We may not generate sufficient revenue or become profitable or, if we achieve profitability, we may not be able to sustain it.
If we are not successful in leveraging the ViraxImmune platform to discover, develop and commercialize additional products and services, our ability to expand our business and achieve our strategic objectives would be impaired.
Our efforts to develop a T-Cell In-Vitro Diagnostic Test may not be successful, and it may not yield the insights we expect at all or on a timetable that allows us to develop or commercialize any new diagnostic products.
If we are not successful in obtaining regulatory approvals for our ViraxImmune products, we may not be able to commercialize our products in the expected timeframe or at all, and our ability to expand our business and achieve our strategic objectives would be impaired.
We will face significant challenges in successfully commercializing our products, particularly in new markets.
Our business, financial condition and results of operations will depend on the market acceptance and increased demand of our products by CROs, hospitals, governments and public health departments, as well as physicians others in the medical community, and the growing proportion of the population who are interested in taking personal charge over their health and wellbeing.
The success of some of our products partially depends on the continued demand for diagnostic and products linked to COVID-19 and other major viral diseases.
The success of our proprietary technology T-Cell testing requires us to proceed through clinical and validation studies successfully, which is not guaranteed.
New market opportunities may not develop as quickly as we expect, limiting our ability to market and sell our products successfully.
We have supply contracts with four of our key suppliers, and any disruptions from such key suppliers could adversely affect our business and results of operations.
We rely on a limited number of suppliers or, in many cases, single suppliers, for laboratory equipment and materials and may not be able to find replacements or immediately transition to alternative suppliers.
Our suppliers may experience development or manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
We have a significant customer concentration, with a limited number of customers accounting for a large portion or all of our revenues.
Our efforts to discover and develop products and services related to COVID-19 and major viral threats, namely ViraxImmune products, may not be successful from either a platform extension or commercialization perspective.
We may be liable for improper collection, use or appropriation of personal information provided by our customers.
The in-vitro diagnostics industry is subject to rapid change, which could make our diagnostics platform and related products and services that we develop obsolete.
Our business could suffer if we lose the services of, or are unable to attract and retain, key members of our senior management, key advisors or other personnel.
We depend on our information technology systems and any failure of these systems could harm our business.
We face risks related to natural disasters, health epidemics and other outbreaks, specifically the coronavirus, which could significantly disrupt our operations.
If we are not able to adequately protect our proprietary intellectual property and information, and protect against third party claims that we are infringing on their intellectual property rights, our results of operations could be adversely affected.
If we are unable to adequately protect our intellectual property rights, or if we are accused of infringing on the intellectual property rights of others, our competitive position could be harmed or we could be required to incur significant expenses to enforce or defend our rights.
We intend to apply for patents in the United States, subject to approval from the relevant regulatory bodies. If we do not obtain protection under the Hatch-Waxman Amendments and similar non-U.S. legislation for extending the term of patents covering each of our product candidates, our business may be materially harmed.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage and changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products.
We enjoy only limited geographical protection with respect to certain patents and may face difficulties in certain jurisdictions, which may diminish the value of intellectual property rights in those jurisdictions.
Litigation or other proceedings or third-party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling our products or affect our stock price.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
Third parties may assert ownership or commercial rights to inventions we develop, which could have a material adverse effect on our business.
The regulatory environment for IVD could change, resulting a new procedure for achieving approvals for various global marketplaces which might adversely affect Virax’s ability to enter various markets.
If we fail to comply with extensive regulations of domestic and international regulatory authorities, sales of our products in new markets and the development and commercialization of any new product candidates could be delayed or prevented.
If we or our suppliers fail to comply with ongoing regulatory requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
We may potentially be subject to product liability claims.
Risks Related to this Offering
The price of the Ordinary Shares has been, and is likely to continue to be, highly volatile, which could result in substantial losses for purchases of Ordinary Shares in this offering.
The price of the Ordinary Shares has been, and is likely to continue to be, highly volatile. The stock market in general and the market for smaller pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, purchasers of securities sold pursuant to this registration statement may not be able to sell their Ordinary Shares at or above the price paid by such purchasers and, as such, they may lose some or all of their investment. Additionally, in the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us in light of the significant stock price volatility we and other pharmaceutical companies have experienced in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
If we fail to meet applicable listing requirements, Nasdaq may delist our Ordinary Shares from trading, in which case the liquidity and market price of our Ordinary Shares could decline.
We cannot assure you that we will be able to meet the continued listing standards of Nasdaq in the future. If we fail to comply with the applicable listing standards and Nasdaq delists our Ordinary Shares, we and our shareholders could face significant material adverse consequences, including:
•a limited availability of market quotations for our Ordinary Shares;
•reduced liquidity for our Ordinary Shares;
•a determination that our Ordinary Shares are “penny stock”, which would require brokers trading in our Ordinary Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our Ordinary Shares;
•a limited amount of news about us and analyst coverage of us; and
•a decreased ability for us to issue additional equity securities or obtain additional equity or debt financing in the future.
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer listed on Nasdaq, our securities would not be covered securities and we would be subject to regulations in each state in which we offer our securities.
The exercise of outstanding purchase warrants and share options will have a dilutive effect on the percentage ownership of our capital stock by existing shareholders.
As of the date of this F-3, we had outstanding warrants to acquire 15,464,324 Ordinary Shares. A number of warrants have exercise prices above our Ordinary Shares’ recent trading prices, but the holders have the right, in certain circumstances, to effect a cashless exercise of such warrants. If a significant number of such warrants and share options are exercised by the holders, the percentage of our Ordinary Shares owned by our existing shareholders will be diluted.
We are an emerging growth company within the meaning of the Securities Act and may take advantage of certain reduced reporting requirements.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from requirements applicable to other public companies that are not emerging growth companies, including, most significantly, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 for so long as we remain an emerging growth company. As a result, if we elect not to comply with such auditor attestation requirements, our investors may not have access to certain information they may deem important.
The JOBS Act also provides that an emerging growth company does not need to comply with any new or revised financial accounting standards until such date that a private company is otherwise required to comply with such new or revised accounting standards. We do not plan to “opt out” of such exemptions afforded to an emerging growth company. As a result of this election, our financial statements may not be comparable to those of companies that comply with public company effective dates.
We qualify as a foreign private issuer and, as a result, we will not be subject to U.S. proxy rules and will be subject to Exchange Act reporting obligations that permit less detailed and less frequent reporting than that of a U.S. domestic public company.
Upon the closing of the Company's IPO, we have begun reporting under the Exchange Act as a non-U.S. company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K upon the occurrence of specified significant events. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules thereunder. Therefore, our shareholders may not know on a timely basis when our officers, directors and principal shareholders purchase or sell our Ordinary Shares. In addition, foreign private issuers are not required to file their annual report on Form 20-F until one hundred twenty (120) days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within seventy-five (75) days after the end of each fiscal year. Foreign private issuers also are exempt from Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from Nasdaq corporate governance listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.
As a foreign private issuer, we are permitted to take advantage of certain provisions in the Nasdaq rules that allow us to follow our home country law for certain governance matters. Certain corporate governance practices in our home country, the Cayman Islands, may differ significantly from corporate governance listing standards. We have adopted
Cayman Islands practices in lieu of certain requirements of Rule 5635 of the Nasdaq Stock Market LLC Rules which, among others, means we do not have to obtain shareholders' approval for certain dilutive events, such as (i) certain acquisition of stock or assets of another company; (ii) an issuance of shares that will result in a change of control of the company; (iii) the establishment or amendment of certain equity based compensation plans and arrangements; and (iv) certain transactions (other than a public offering) involving issuances of a 20% or more interest or voting power in the company at a price that is less than the minimum price defined therein. As such, our shareholders may be afforded less protection than they would otherwise enjoy under the Nasdaq corporate governance listing standards applicable to U.S. domestic issuers.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our Ordinary Shares are directly or indirectly held by residents of the United States and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq rules. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting and other expenses that we will not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange.
USE OF PROCEEDS
All of the securities offered by the selling securityholders pursuant to this prospectus will be sold by the selling securityholders for their respective accounts. We will not receive any of the proceeds from these sales.
The selling securityholders will pay any underwriting discounts and commissions and expenses incurred by the selling stockholders for brokerage, accounting, tax or legal services or any other expenses incurred by the selling stockholders in disposing of the securities. We will bear the costs, fees and expenses incurred in effecting the registration of the securities covered by this prospectus, including all registration and filing fees, Nasdaq listing fees and fees and expenses of our counsel and our independent registered public accounting firm.
DESCRIPTION OF SHARE CAPITAL AND GOVERNING DOCUMENTS
The following description of our capital stock (which includes a description of securities we may offer pursuant to the registration statement of which this prospectus, as the same may be supplemented, forms a part) does not purport to be complete and is subject to and qualified in its entirety by our Amended and Restated Memorandum and Articles of Association (“M&A”) and by the applicable provisions of Cayman Islands law.
We are an exempted company incorporated with limited liability under the laws of the Cayman Islands and our affairs are governed by:
Memorandum and Articles of Association;
The Companies Act (Revised) of the Caymans Islands, which is referred to as the Companies Act below; and
Common law of the Cayman Islands.
Our authorized share capital is US$50,000 divided into 500,000,000 Ordinary Shares of $0.0001 par value each.
Ordinary Shares
All of our outstanding Ordinary Shares are fully paid and non-assessable. Certificates representing the Ordinary Shares are issued in registered form. Our shareholders, whether or not they are non-residents of the Cayman Islands, may freely hold and transfer their Ordinary Shares in accordance with our Memorandum and Articles.
Dividends
The holders of our Ordinary Shares are entitled to such dividends as may be declared by our board of directors. Our Articles provide that our board of directors may declare and pay dividends if justified by our financial position and permitted by law. Our articles of association also provides that, subject to the Companies Act, the Company may by also by ordinary resolution declare dividends in accordance with the respective rights of the shareholders but no dividend shall exceed the amount recommended by the directors.
Voting Rights
Holders of our Ordinary Shares vote on all matters submitted to a vote of our shareholders, except as may otherwise be required by law. In respect of matters requiring shareholders’ vote, each ordinary share is entitled to one vote. At any general meeting a resolution put to the vote of the meeting shall be decided on a show of hands unless voting by poll is duly demanded by the chairman of the meeting, by at least two shareholders having the right to vote on the resolutions, or by shareholder(s) together holding at least 10% of the total voting rights of all our shareholders having the right to vote at such general meeting. A quorum required for a meeting of shareholders consists of one or more shareholders who holds at least one-third of our issued voting shares. Shareholders’ meetings may be held annually. Each general meeting, other than an annual general meeting, shall be an extraordinary general meeting. Extraordinary general meetings may be called by a majority of our board of directors or upon a requisition of any one or more shareholders holding at the deposit of the requisition at least 10% of the aggregate share capital of our company that carries the right to vote at a general meeting, in which case on advance notice of at least 7 clear days is required for the convening of our annual general meeting and other general meetings by requisition of our shareholders.
Any ordinary resolution to be made by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the Ordinary Shares cast in a meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes attaching to the Ordinary Shares cast in a meeting.
A special resolution will be required for important matters such as amending our memorandum and articles of association or changing the name of the Company.
There are no limitations on non-residents or foreign shareholders in the memorandum and articles of association to hold or exercise voting rights on the Ordinary Shares imposed by foreign law or by the charter or other constituent document of our company. However, no person will be entitled to vote at any general meeting or at any separate meeting of the holders of the Ordinary Shares unless the person is registered as of the record date for such meeting and unless all calls or other sums presently payable by the person in respect of Ordinary Shares in the Company have been paid.
Winding Up; Liquidation
Subject to any special rights, privileges or restrictions as to the distribution of available surplus assets on liquidation applicable to any class or classes of shares (1) if we are wound up and the assets available for distribution among our shareholders are more than sufficient to repay the whole of the capital paid up at the commencement of the winding up, the excess shall be distributed pari passu among our shareholders in proportion to the amount paid up at the commencement of the winding up on the shares held by them, respectively, and (2) if we are wound up and the assets available for distribution among our shareholders as such are insufficient to repay the whole of the paid-up capital, those assets shall be distributed so that, as nearly as may be, the losses shall be borne by our shareholders in proportion to the capital paid up, or which ought to have been paid up, at the commencement of the winding up on the shares held by them, respectively.
Calls on Ordinary Shares and Forfeiture of Ordinary Shares
Our directors may from time to time make calls on our shareholders in respect of any moneys unpaid on their shares including any premium in a notice served to such shareholders at least 14 clear days prior to the specified time of payment. Any Ordinary Shares that have been called upon and remain unpaid are subject to forfeiture.
Redemption of Ordinary Shares
The Companies Act and our Memorandum and Articles permit us to purchase our own shares. In accordance with our Articles, provided the necessary shareholders or board approval have been obtained and requirements under the Companies Act have been satisfied, we may issue shares on terms that are subject to redemption at our option on such terms and in such manner as may be determined by our board of directors.
Inspection of Books and Records
Holders of our Ordinary Shares have no general right under our Articles to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements.
Issuance of Additional Shares
Our Memorandum and Articles authorize our board of directors to issue additional Ordinary Shares from time to time as our board of directors shall determine, to the extent of available authorized but unissued shares. Issuance of these shares may dilute the voting power of holders of Ordinary Shares.
Anti-Takeover Provisions
Some provisions of our Memorandum and Articles may discourage, delay or prevent a change of control of our company or management that shareholders may consider favorable. Our authorized, but unissued Ordinary Shares are available for future issuance without shareholders’ approval and could be utilized for a variety of corporate purposes, including future offerings to raise addition capital, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved Ordinary Shares could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Exempted Company
We are an exempted company with limited liability under the Companies Act. The Companies Act distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except that an exempted company:
does not have to file an annual return of its shareholders with the Registrar of Companies;
is not required to open its register of members for inspection;
does not have to hold an annual general meeting;
may not issue negotiable or bearer shares, but may issue shares with no par value;
may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance);
may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands
may register as a limited duration company; and
may register as a segregated portfolio company.
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of the company.
Listing
Our Ordinary Shares are listing on the Nasdaq Capital Market under the symbol “VRAX”.
Transfer Agent and Registrar of Shares
The transfer agent and registrar for our Ordinary Shares is Continental Stock Transfer & Trust Company. The transfer agent and registrar’s address is 1 State Street, 30th Floor, New York, NY 10004.
SELLING SHAREHOLDERS
The Ordinary Shares being offered by the selling shareholders are those issuable to the selling shareholders upon exercise of the New Warrants and Placement Warrants (collectively, the “Warrants”). For additional information regarding the issuances of those securities, see “Recent Developments – 2023 Warrant Inducement” above. We are registering the Ordinary Shares in order to permit the Selling Shareholders to resell the Ordinary Shares issuable upon exercise of the Warrants for resale from time to time. Except with respect to the Placement Agent, which acted as a placement agent and/or underwriter in connection with our 2022 and 2023 PIPE Financing, the selling shareholders have not had any material relationship with us within the past three years.
The table below lists the selling shareholders and other information regarding the beneficial ownership of the Ordinary Shares by the selling shareholders. Beneficial ownership is determined in accordance with rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under the rules of the SEC, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or “investment power,” which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of a security if that person has the right to acquire beneficial ownership of such security within 60 days.
The second column lists the number of Ordinary Shares beneficially owned by the selling shareholders, based on its ownership of the Ordinary Shares and warrants, as the date of this prospectus, assuming exercise of the Warrants held by the selling shareholders on that date, without regard to any limitations on exercises.
The third column lists the Ordinary Shares being offered by this prospectus by the Selling Shareholders.
In accordance with the terms of a warrant inducement agreement with the selling shareholder, this prospectus generally covers the resale of the number of Ordinary Shares issuable upon exercise of the Warrants issued to the selling shareholders in the “Recent Developments – 2023 Warrant Inducement” described above, determined as if the outstanding Warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC. The fourth column assumes the sale of all of the Ordinary Shares offered by the selling shareholder pursuant to this prospectus.
Under the terms of the Warrants, a selling shareholder may not exercise any such warrants to the extent such exercise would cause such selling shareholder, together with its affiliates and attribution parties, to beneficially own a number of Ordinary Shares which would exceed 4.99% of our then outstanding Ordinary Shares following such exercise, excluding for purposes of such determination Ordinary Shares issuable upon exercise of such warrants which have not been exercised. The number of Ordinary Shares in the second and fourth columns do not reflect this limitation. The selling shareholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name of Selling Shareholder |
|
Number of ordinary shares owned prior to offering |
|
Maximum number of ordinary shares to be sold pursuant to this Prospectus |
|
Number of ordinary shares owned after offering |
Armistice Capital Master Fund Ltd. (1) |
|
1,809,728 |
|
14,681,442 |
|
0 |
Michael Vasinkevich (2) |
|
329,506 |
|
329,506 |
|
0 |
Noam Rubinstein (2) |
|
161,863 |
|
161,863 |
|
0 |
Craig Schwabe (2) |
|
17,342 |
|
17,342 |
|
0 |
Charles Worthman (2) |
|
5,139 |
|
5,139 |
|
0 |
|
|
(1) |
Includes (i) 14,681,442 Ordinary Shares issuable upon exercise of Warrants and (ii) 7,108,000 Ordinary Shares that are held in abeyance. The securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The warrants are subject to a beneficial ownership limitation of 4.99%, which such limitation restricts the Selling Stockholder from exercising that portion of the warrants that would result in the Selling Stockholder and its affiliates owning, after exercise, a number of shares of common stock in excess of the beneficial ownership limitation. The address of Armistice Capital Master Fund Ltd. is c/o Armistice Capital, LLC, 510 Madison Avenue, 7th Floor, New York, NY 10022. |
|
|
(2) |
The selling shareholder is affiliated with the Placement Agent. The Placement Agent is a registered broker-dealer, has a registered address of c/o H.C. Wainwright & Co., LLC, 430 Park Ave, 3rd Floor, New York, NY 10022, and has sole voting and dispositive power over the securities held. The number of shares being registered hereby for resale consist of shares of ordinary shares issuable upon exercise of placement agent warrants, which were received as compensation in connection with the 2023 Warrant Inducement. The selling shareholder acquired the placement agent warrants in the ordinary course of business and, at the time of acquisition of the securities that are registered for resale, the selling shareholder had no agreements or understanding, directly or indirectly, with any person to distribute such securities. |
PLAN OF DISTRIBUTION
Each Selling Shareholder (for the purposes of this section, the “Selling Shareholders”) of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the Nasdaq Capital Market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Shareholder may use any one or more of the following methods when selling securities:
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction;
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
an exchange distribution in accordance with the rules of the applicable exchange;
privately negotiated transactions;
settlement of short sales;
in transactions through broker-dealers that agree with the Selling Shareholders to sell a specified number of such securities at a stipulated price per security;
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
a combination of any such methods of sale; or
any other method permitted pursuant to applicable law.
The Selling Shareholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Shareholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Shareholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, the Selling Shareholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Shareholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Shareholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Shareholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Shareholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the Selling Shareholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Shareholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the ordinary shares for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the ordinary shares by the Selling Shareholders or any other person. We will make copies of this prospectus available to the Selling Shareholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
ENFORCEMENT OF CIVIL LIABILITIES
We are an exempted company with limited liability incorporated under the laws of the Cayman Islands and our affairs are governed by our second amended and restated memorandum and articles of association and the Companies Act, and the common law of the Cayman Islands. We are incorporated in the Cayman Islands because of certain benefits associated with being a Cayman Islands company, such as political and economic stability, an effective judicial system, a favorable tax system, the absence of foreign exchange control or currency restrictions and the availability of professional and support services. However, certain disadvantages accompany incorporation in the Cayman Islands. These disadvantages include, but are not limited to, the following: (i) the Cayman Islands has a less developed body of securities laws as compared to the United States and provides less protection for investors; and (ii) Cayman Islands companies may not have standing to sue before the federal courts of the United States.
Our constitutional documents do not contain provisions requiring that disputes, including those arising under the securities laws of the United States, among us, our officers, directors and shareholders, be arbitrated.
Substantially all of our assets are located outside the United States. In addition, most of our directors and executive officers are nationals or residents of jurisdictions other than the United States and substantially all of their assets are located outside the United States. As a result, it may be difficult or impossible for you to effect service of process within the United States upon us or these persons, or to enforce judgments obtained in U.S. courts against us or them, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States. It may also be difficult for you to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our executive officers and directors.
We have appointed Virax Biolabs USA Management, Inc. as our agent to receive service of process with respect to any action brought against us in the United States in connection with this offering under the federal securities laws of the United States or of any State in the United States.
Cayman Islands
We have been advised by Ogier, our counsel as to Cayman Islands law, there is uncertainty as to whether the courts of the Cayman Islands would:
recognize or enforce judgments of U.S. courts obtained against us or our directors or officers predicated upon the civil liability provisions of securities laws of the United States or any state in the United States; or
entertain original actions brought in each respective jurisdiction against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States
Ogier has further advised us that although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign judgment, without any re-examination or re-litigation of matters adjudicated upon, provided such judgment:
•is given by a foreign court of competent jurisdiction;
•imposes on the judgment debtor a liability to pay a liquidated sum for which the judgment has been given;
•is not in respect of taxes, a fine or a penalty;
•was not obtained by fraud; and
•is not of a kind the enforcement of which is contrary to natural justice or the public policy of the Cayman Islands.
Subject to the above limitations, in appropriate circumstances, a Cayman Islands court may give effect in the Cayman Islands to other kinds of final foreign judgments such as declaratory orders, orders for performance of contracts and injunctions.
As a result of all of the above, public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board of directors or controlling shareholders than they would as public shareholders of a U.S. company.
Singapore
Singapore has no arrangement for the reciprocal enforcement of judgments with the United States. It is possible that the Singapore courts may not (i) recognize and enforce judgments of courts in the United States, based upon the civil liability provisions of the securities laws of the United States or any state or territory of the United States, or (ii) enter judgments in original actions brought in the Singapore courts based solely on the civil liability provisions of these securities laws. An in personam final and conclusive judgment in the federal or state courts of the United States under which a fixed or ascertainable sum of money is payable may generally be enforced as a debt in the Singapore courts under the common law as long as it is established that the Singapore courts have jurisdiction over the judgment debtor, subject to the applicable substantive and procedural laws of Singapore. Additionally, the court where the judgment was obtained must have had international jurisdiction over the party sought to be bound in the local proceedings. However, the Singapore courts are unlikely to enforce a foreign judgment if (a) the foreign judgment is inconsistent with a prior local judgment that is binding on the same parties; (b) the enforcement of the foreign judgment would contravene the public policy of Singapore; (c) the proceedings in which the foreign judgment was obtained were contrary to principles of natural justice; (d) the foreign judgment was obtained by fraud; or (e) the enforcement of the foreign judgment amounts to the direct or indirect enforcement of a foreign, penal, revenue or other public laws.
In particular, the Singapore courts may potentially not allow the enforcement of any foreign judgment for a sum payable in respect of taxes, fines, penalties or other similar charges, including the judgments of courts in the United States based upon the civil liability provisions of the securities laws of the United States or any state or territory of the United States. In respect of civil liability provisions of the United States federal and state securities law which permit punitive damages against us and our Directors or Executive Officers, we are unaware of any decision by the Singapore courts which has considered the specific issue of whether a judgment of a United States court based on such civil liability provisions of the securities laws of the United States or any state or territory of the United States is enforceable in Singapore.
Hong Kong
There is uncertainty as to whether the courts of Hong Kong would (i) recognize or enforce judgments of United States courts obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States or (ii) entertain original actions brought in Hong Kong against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States.
A judgment of a court in the United States predicated upon U.S. federal or state securities laws may be enforced in Hong Kong at common law by bringing an action in a Hong Kong court on that judgment for the amount due thereunder, and then seeking summary judgment on the strength of the foreign judgment, provided that the foreign judgment, among other things, is (1) for a debt or a definite sum of money (not being taxes or similar charges to a foreign government taxing authority or a fine or other penalty) and (2) final and conclusive on the merits of the claim, but not otherwise. Such a judgment may not, in any event, be so enforced in Hong Kong if (a) it was obtained by fraud; (b) the proceedings in which the judgment was obtained were opposed to natural justice; (c) its enforcement or recognition would be contrary to the public policy of Hong Kong; (d) the court of the United States was not jurisdictionally competent; or (e) the judgment was in conflict with a prior Hong Kong judgment.
Hong Kong has no arrangement for the reciprocal enforcement of judgments with the United States. As a result, there is uncertainty as to the enforceability in Hong Kong, in original actions or in actions for enforcement, of judgments of United States courts of civil liabilities predicated solely upon the federal securities laws of the United States or the securities laws of any State or territory within the United States.
China
There is uncertainty as to whether the courts of China would (1) recognize or enforce judgments of United States courts obtained against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or (2) be competent to hear original actions brought in each respective jurisdiction, against us or such persons predicated upon the securities laws of the United States or any state thereof.
The recognition and enforcement of foreign judgments are mainly provided for under the Chinese Civil Procedure Law. Chinese courts may recognize and enforce foreign judgments in accordance with the requirements of the Chinese Civil Procedure Law and other applicable laws and regulations based either on treaties between China and the country where the judgment is made or in reciprocity between jurisdictions. Accordingly, there is uncertainty whether China courts will recognize or enforce judgments of United States or Cayman Islands Courts because China does not have any treaties or other agreements with the Cayman Islands or the United States that provide for the reciprocal recognition and enforcement of foreign judgments as of the date of this prospectus. Further, under Chinese Civil Procedure Law, Chinese courts will not enforce a foreign judgment against us or our officers and directors if the court decides that such judgment violates the basic principles of PRC law or national sovereignty, security or social public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States or in the Cayman Islands.
Under the PRC Civil Procedure Law, foreign shareholders may originate actions based on PRC law against a company in China for disputes if they can establish sufficient nexus to the PRC for a PRC court to have jurisdiction, and meet other procedural requirements, including, among others, the plaintiff must have a direct interest in the case, and there must be a concrete claim, a factual basis and a cause for the suit. However, it will be difficult for U.S. shareholders to originate actions against us in the PRC in accordance with PRC laws because we are incorporated under the laws of the Cayman Islands and it will be difficult for U.S. shareholders, by virtue only of holding our ordinary shares, to establish a connection to the PRC for a PRC court to have jurisdiction as required under the PRC Civil Procedure Law.
LEGAL MATTERS
The validity of the securities offered by this prospectus and other legal matters concerning this offering relating to Cayman Islands law will be passed upon for us by Ogier. Certain legal matters relating to U.S. law will be passed upon for us by Loeb & Loeb LLP.
EXPERTS
The consolidated financial statements of Virax Biolabs Group Limited as of March 31, 2023 and for the year then ended, have been incorporated by reference herein in reliance upon the report of Reliant CPA PC, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The registered business address of Reliant CPA PC is 895 Dove Street, Suite 300, Newport Beach, CA 92660, United States.
The consolidated financial statements of Virax Biolabs Group Limited as of March 31, 2022 and for the year then ended, have been incorporated by reference herein in reliance upon the report of BF Borgers CPA PC, independent registered public accounting firm, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
The registered business address of BF Borgers CPA PC is 5400 W Cedar Ave, Lakewood, CO 80226, United States.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with the SEC. This means that we can disclose important information to you by referring you to those documents. Any statement contained in a document incorporated by reference in this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein, or in any subsequently filed document, which also is incorporated by reference herein, modifies or supersedes such earlier statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We hereby incorporate by reference into this prospectus the following documents that we have filed with the SEC under the Exchange Act:
•our Annual Report on Form 20-F for the year ended March 31, 2023, filed with the SEC on June 14, 2023;
•our Current Reports on Form 6–K filed with the SEC on April 19, 2023, April 21, 2023, May 4, 2023, June 14, 2023, July 18, 2023, August 3, 2023, September 1, 2023, September 12, 2023, October 4, 2023, October 12, 2023, October 31, 2023, and November 8, 2023 (in each case, except for information contained therein which is furnished rather than filed); and
•the description of our Class A common stock in our registration statement on Form 8–A filed with the SEC on June 30, 2022, including any amendments thereto or reports filed for the purpose of updating such description.
•All documents filed by Virax under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, that are filed (excluding, however, information we furnish to the SEC) (i) by us after the date of the initial registration statement and prior to its effectiveness and (ii) by us after the date of this prospectus and prior to the termination of any offering under this registration statement.
Any statement contained in this prospectus, or in a document all or a portion of which is incorporated by reference, shall be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, any applicable prospectus supplement and any related free writing prospectus or any document incorporated by reference modifies or supersedes such statement. Any such statement so modified or superseded shall not, except as so modified or superseded, constitute a part of this prospectus.
Upon request, we will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered a copy of the documents incorporated by reference into this prospectus. You may request a copy of these filings, and any exhibits we have specifically incorporated by reference as an exhibit in this prospectus, at no cost by writing or telephoning us at the following:
Virax Biolabs Group Limited
20 North Audley Street
London, W1K 6LX
United Kingdom
Telephone: +44 020 7788 7414
You may also access these documents, free of charge on the SEC’s website at www.sec.gov or on the “Investors” page of our website at https://viraxbiolabs.com/. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information on, or that can be accessed from, our website as part of this prospectus or any accompanying prospectus supplement.
This prospectus is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration statement. You should read the exhibits carefully for provisions that may be important to you.
We have not authorized anyone to provide you with information other than what is incorporated by reference or provided in this prospectus or any prospectus supplement. We are not making an offer of these securities in any state where such offer is not permitted. You should not assume that the information in this prospectus or in the documents incorporated by reference is accurate as of any date other than the date on the front of this prospectus or those documents.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-3 under the Securities Act relating to this offering. This prospectus does not contain all of the information contained in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself for a complete description of its terms.
You may read and copy the registration statement, including the related exhibits and schedules, and any document we file with the SEC without charge at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
We are subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those requirements we file reports with the SEC. Those other reports or other information may be inspected without charge at the locations described above. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm within four months after the fiscal year end, or such applicable time as required by the SEC.
We maintain a principal website at https://viraxbiolabs.com/. Information contained on, or that can be accessed through, our website is not a part of, and shall not be incorporated by reference into, this prospectus.

Virax Biolabs Group Limited
Up to 15,195,292
Ordinary Shares
_________________________
PROSPECTUS
, 2023
_________________________
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 8. Indemnification of Directors and Officers
Cayman Islands law does not limit the extent to which a company’s M&A may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy. Subject to the provisions of the Companies Act the Company may indemnify each existing or former director (including alternate director), secretary and other officer of the Company (including an investment adviser or an administrator or liquidator) and their personal representatives against:
a)all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by the existing or former director (including alternate director), secretary or officer in or about the conduct of the Company's business or affairs or in the execution or discharge of the existing or former director's (including alternate director's), secretary’s or officer’s duties, powers, authorities or discretions; and
b)without limitation to paragraph (a), all costs, expenses, losses or liabilities incurred by the existing or former director (including alternate director), secretary or officer in defending (whether successfully or otherwise) any civil, criminal, administrative or investigative proceedings (whether threatened, pending or completed) concerning the Company or its affairs in any court or tribunal, whether in the Cayman Islands or elsewhere.
No such existing or former director (including alternate director), secretary or officer, however, shall be indemnified in respect of any matter arising out of his own dishonesty.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 9. Exhibits
* Filed herein.
(a)The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii)To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b).
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i)Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii)Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time
of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii)Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii)The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv)Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b)That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c)Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in London, United Kingdom, on December 5, 2023.
|
|
|
|
|
|
|
|
|
|
|
|
VIRAX BIOLABS GROUP LIMITED |
|
|
By: |
|
/s/ James Foster |
|
|
|
|
Name: James Foster |
|
|
|
|
Title: Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS that each person whose signature appears below hereby constitutes and appoints James Foster and Jason Davis, and each of them, his or her true and lawful attorneys-in-fact and agents, with full power to act separately and full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement and all additional registration statements pursuant to Rule 462(b) of the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the SEC, granting unto each said attorney-in-fact and agent full power and authority to do and perform each and every act in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or either of them or his or her or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
|
|
|
|
|
|
|
|
|
Name |
|
Position |
|
Date |
/s/ James Foster |
|
Chief Executive Officer |
|
December 5, 2023 |
James Foster |
|
(Principal executive officer) and Director |
|
|
|
|
|
|
|
/s/ Jason Davis |
|
Chief Financial Officer |
|
December 5, 2023 |
Jason Davis |
|
(Principal financial and accounting officer) |
|
|
|
|
|
|
|
/s/ Nigel McCracken |
|
Chief Operating Officer |
|
December 5, 2023 |
Nigel McCracken |
|
|
|
|
|
|
|
|
|
/s/ Mark Ternouth |
|
Director and Chief Technical Officer |
|
December 5, 2023 |
Mark Ternouth |
|
|
|
|
|
|
|
|
|
/s/ Yair Erez |
|
Independent Director |
|
December 5, 2023 |
Yair Erez |
|
|
|
|
|
|
|
|
|
/s/ Evan Norton |
|
Independent Director |
|
December 5, 2023 |
|
|
|
|
|
Evan Norton |
|
|
|
|
|
|
|
|
|
/s/ Nelson Haight |
|
Independent Director |
|
December 5, 2023 |
Nelson Haight |
|
|
|
|
AUTHORIZED REPRESENTATIVE
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Virax Biolabs Group Limited has signed this registration statement on December 5, 2023.
|
|
|
|
|
|
|
Virax Biolabs Group Limited |
|
|
|
|
By: |
/s/ Nelson Haight |
|
|
Name: Nelson Haight |
|
|
Title: Director |
Exhibit 5.1













Exhibit 23.1
Report of Independent Registered Public Accounting Firm
To the shareholders and the board of directors of Virax Biolabs Group Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of Virax Biolabs Group Limited (the "Company") as of March 31, 2022, the related statements of profit and loss and comprehensive loss, changes in equity (deficit), and cash flows for the year then ended, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2022, and the results of its operations and its cash flows for the year then ended, in conformity with the International Financial Reporting Standards as issued by the International Accounting Standards Board.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s significant operating losses raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ BF Borgers CPA PC
BF Borgers CPA PC (PCAOB ID 5041)
Served as Auditor from 2021 to 2023
Lakewood, CO
August 12, 2022
Exhibit 23.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of
Virax Biolabs Group Limited
We consent to the incorporation by reference in the Form F-3 Registration Statement of Virax Biolabs Group Limited (“the Company”) of our report dated June 14, 2023 with respect to our audit of the consolidated statement of financial position of March 31, 2023, and consolidated statements of profit and loss and other comprehensive loss, changes in equity and cash flows for the year then ended appearing in the Annual Report on Form 20-F of the Company for the year ended March 31, 2023.
We also consent to the reference to us under the caption “Experts” in the Registration Statement.
/s/ Reliant CPA PC
Reliant CPA PC
Certified Public Accountants
Newport Beach, CA
December 5, 2023
Exhibit 107.1
Calculation of Filing Fee Tables
Form F-3
(Form Type)
Virax Biolabs Group Limited
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Security
Type |
|
Security
Class
Title |
|
Fee
Calculation
or Carry
Forward Rule |
|
|
Amount
Registered (1)(2)(3) |
|
|
Proposed
Maximum
Offering
Price Per
Unit (4) |
|
|
Maximum
Aggregate
Offering
Price |
|
|
Fee
Rate |
|
|
Amount of
Registration
Fee |
|
Fees to be paid |
|
Equity |
|
Ordinary Shares |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Fees to be paid |
|
Equity |
|
Preferred Shares |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Fees to be paid |
|
Debt |
|
Debt Securities |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Fees to be paid |
|
Other |
|
Warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Fees to be paid |
|
Other |
|
Units |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Fees to be paid |
|
Other |
|
Rights |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Fees to be paid |
|
Unallocated (Universal) Shelf |
|
- |
|
|
457(o) |
|
|
|
(1) |
|
|
|
(5) |
|
|
$30,000,000 |
|
|
|
|
|
|
|
|
$4,428 |
|
Fees
to be paid |
|
Equity |
|
Ordinary Shares, par value $0.0001 per share |
|
|
457 |
(c) |
|
|
15,195,292 |
|
|
$ |
0.22 |
|
|
$3,342,964.24 |
|
|
|
$ |
0.00014760 |
|
|
$ |
493.43 |
|
|
|
Total Offering Amounts |
|
|
|
|
|
|
$ 33,342,964.24 |
|
|
|
|
0.00014760 |
|
|
$ |
4,921.43 |
|
|
|
Total Fees Previously Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
Total Fee Offsets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
Net Fee Due |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
4,921.43 |
|
|
|
(1) |
The registrant is registering an indeterminate number of securities for offer and sale from time to time at indeterminate prices, which shall have an aggregate offering price not to exceed $30,000,000. In addition, pursuant to Rule 416(a) under the Securities Act of 1933, as amended, this registration statement shall be deemed to cover any additional number of securities that may be issued from time to time to prevent dilution as a result of a distribution, split, combination, or similar transaction. Securities registered hereunder may be sold separately, or together with other securities registered hereunder. Includes consideration to be received by the registrant, if applicable, for registered securities that are issuable upon exercise, conversion, or exchange of other registered securities. |
(2) |
Represents the maximum number of ordinary shares offered by the Selling Shareholders named in this Registration Statement. |
|
|
(3) |
This Registration Statement includes an indeterminate number of additional ordinary shares issuable for no additional consideration pursuant to any stock dividend, stock split, recapitalization or other similar transaction effected without the receipt of consideration, which results in an increase in the number of outstanding ordinary shares. In the event of a stock split, stock dividend or similar transaction involving our ordinary shares, in order to prevent dilution, the number of shares registered shall be automatically increased to cover the additional shares in accordance with Rule 416(a) under the Securities Act of 1933, as amended (the “Securities Act”). |
(4) |
Estimated in accordance with Rule 457(c) of the Securities Act solely for the purpose of computing the amount of the registration fee. The maximum price per share and the maximum aggregate offering price are based on the average of the $0.23 (high) and $0.21 (low) sale price of the ordinary shares as reported on the Nasdaq Capital Market on November 29, 2023. |
(5) |
The proposed maximum aggregate offering price per class of security will be determined from time to time by the registrant in connection with the issuance by the registrant of the securities registered hereunder and is not specified as to each class of security pursuant to Instructions to the Calculation of Filing Fee Tables and Related Disclosure (2)(A)(iii)(b) of Form F-3 under the Securities Act. |
Virax Biolabs (NASDAQ:VRAX)
Historical Stock Chart
From Apr 2024 to May 2024
Virax Biolabs (NASDAQ:VRAX)
Historical Stock Chart
From May 2023 to May 2024