Autolus Seeks FDA OK of Obe-Cel in Adult B-Cell Acute Lymphoblastic Leukemia
November 27 2023 - 7:54AM
Dow Jones News
By Colin Kellaher
Autolus Therapeutics has filed for U.S. Food and Drug
Administration approval of obecabtagene autoleucel, its lead
investigational chimeric antigen receptor T-cell, or CAR-T,
therapy, for adult relapsed/refractory B-cell acute lymphoblastic
leukemia.
The London-based clinical-stage biopharmaceutical company on
Monday said it is also on track to submit a marketing authorization
application for the therapy, also known as oba-cel, to the European
Medicines Agency in the first half of 2024.
Autolus said its FDA filing is based on data from a pivotal
Phase 2 study of oba-cel in patients with the cancer that affects
the blood and bone marrow.
Autolus in June said the study had met its primary endpoint,
with 76% of dosed patients achieving a complete response or
complete response with incomplete haematological recovery.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
November 27, 2023 07:39 ET (12:39 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
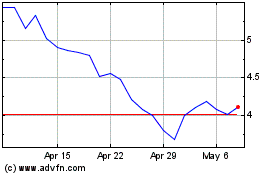
Autolus Therapeutics (NASDAQ:AUTL)
Historical Stock Chart
From Apr 2024 to May 2024
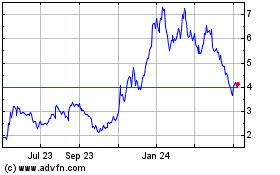
Autolus Therapeutics (NASDAQ:AUTL)
Historical Stock Chart
From May 2023 to May 2024