| PROSPECTUS |
Filed pursuant to Rule 424(b)(3) |
| |
Registration
No. 333-267591
|

3,336,730 Shares
of Common Stock
Issuable
upon Exercise of Outstanding Warrants
7,642,038
Shares of Common Stock
Issuable
upon Conversion of Senior Subordinated Convertible Notes
This
prospectus relates to the resale from time to time, by the Selling Securityholders identified in this prospectus under the caption “Selling
Securityholders” of up to 3,336,730 shares of our common stock which are issuable upon the exercise of outstanding warrants
(the “Warrants”), and 7,642,038 shares of our common stock which are issuable upon the conversion of the outstanding
Senior Subordinated Convertible Notes (the “Notes”). We previously issued the Notes and the Warrants to the Selling Securityholders
in multiple private placements, pursuant to several Securities Purchase Agreements entered in December 2022, February 2023, March 2023,
April 2023 and July 2023.
The
Selling Securityholders may, from time to time, sell, transfer or otherwise dispose of any or all of their common stock or interests
in their common stock on any stock exchange, market or trading facility on which the common stock is traded or in private transactions.
These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market
price, at varying prices determined at the time of sale, or at negotiated prices. See “Plan of Distribution” in this prospectus
for more information. We will not receive any proceeds from the resale or other disposition of the common stock by the Selling Securityholders.
However, Evofem will receive the proceeds of any cash exercise of the Warrants. See “Use of Proceeds” beginning on page 59
and “Plan of Distribution” beginning on page 130 of this prospectus for more information.
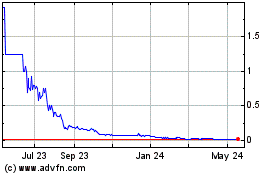
Our
common stock is traded on the OTCQB under the symbol “EVFM.” On August 1, 2023, the last reported sale
price of our common stock as reported on the OTCQB was $0.4400 per share.
You
should read this prospectus, together with additional information described under the headings “Where You Can Find More Information,”
carefully before you invest in any of our securities.
The
registrant is currently in default on certain financial obligations. The Company has considered and continues to consider filing for
bankruptcy protection. In the event that the Company files for bankruptcy, holders of our common stock, including stock reserved for
the Notes and the Warrants being registered for resale in this prospectus, will likely receive no recovery.
An
investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should
consider carefully the risks and uncertainties described in the section captioned “Risk
Factors” on page 8 herein and in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed
with the Securities and Exchange Commission (the SEC) on April 27, 2023.
Neither
the SEC nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
The
date of this Prospectus is August 14, 2023
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
You
should rely only on the information we have included or in this prospectus. Neither we nor the Selling Securityholders have authorized
any dealer, salesman or other person to give any information or to make any representation other than those contained in this prospectus.
This prospectus does not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered
securities to which they relate, nor does this prospectus constitute an offer to sell or the solicitation of an offer to buy securities
in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You
should not assume the information contained in this prospectus is accurate on any date subsequent to the date set forth on the front
of the document, even though this prospectus is delivered, or securities are sold, on a later date.
This
prospectus contains or incorporates by reference summaries of provisions contained in certain documents and agreements described herein.
All the summaries are qualified in their entirety by the actual documents. Copies of some of the documents and/or agreements referred
to herein have been filed as exhibits to the registration statement of which this prospectus forms a part, and you may obtain copies
of those documents and/or agreements as described in this prospectus under the heading “Where You Can Find More Information.”
PROSPECTUS
SUMMARY
This
summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all the
information you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be
read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read all such documents carefully,
especially the risk factors included herein and our audited consolidated financial statements and the related notes included herein,
before deciding to buy shares of our common stock. Unless the context requires otherwise, references in this prospectus to “Evofem,”
“Company,” “we,” “us” and “our” refer to Evofem Biosciences, Inc. and our subsidiaries.
Company
Overview
We
are a San Diego-based commercial-stage biopharmaceutical company committed to developing and commercializing innovative products to address
unmet needs in women’s sexual and reproductive health. Our first commercial product, Phexxi, was approved by the FDA on May 22,
2020 and is the first and only FDA-approved, hormone-free, woman-controlled, on-demand prescription contraceptive gel for women. We commercially
launched Phexxi in September 2020 in the United States. We intend to commercialize Phexxi in global markets through partnerships or licensing
agreements.
While
we have increased annual net sales of Phexxi year over year since launch, we have not yet reached cash flow breakeven; we continue
to incur operating losses and negative cash flows from operating activities, as we have done since inception. As of August 3, 2023,
we had $0.3 million in restricted and unrestricted cash. If we are unable to raise additional capital finance our operations
when needed or on acceptable terms, we may be forced to delay, reduce and/or eliminate one or more of our business initiatives.
Additionally, we may be required to utilize proceeds of subsequent financings to redeem outstanding debt, in which case we would not
be able to use such funds to support our ongoing operations. As noted in the discussion of risk factors later in this document, we
received a Notice of Default on the Baker Bros. Purchase Agreement. As of June 30, 2023, we owe $95.7 million, in aggregate, under
this agreement.
Additionally,
we are currently over 90 days past due on a significant amount of vendor
obligations. Our current indebtedness and accounts payable to these vendors is approximately $15.7 million, in aggregate, as of June 30,
2023. If we are unable to refinance, extend or repay our substantial indebtedness owed to our secured and unsecured lenders, this would
have a material adverse effect on our financial condition and ability to continue as a going concern and could cause investors to lose
their entire investment in the Company.
Given
our current financial condition, we have considered and continue to consider filing for bankruptcy protection. While we have not initiated
bankruptcy proceedings, we caution that trading in our securities is highly speculative and poses substantial risks relating to the potential
of bankruptcy proceedings. Trading prices for our securities may bear little or no relationship to the actual recovery, if any, by holders
of our securities in Bankruptcy proceedings, if any.
Phexxi
as a Contraceptive; Commercial Strategies
Our
sales force promotes Phexxi directly to obstetrician/gynecologists and their affiliated health professionals, who collectively write
the majority of prescriptions for contraceptive products. Our sales force consists of 16 sales representatives, three business managers
and a VP of sales, supported by a self-guided virtual health care provider (HCP) learning platform. Additionally, we offer women direct
access to Phexxi via our telehealth platform. Using the platform, women can directly meet with an HCP to determine their eligibility
for a Phexxi prescription and, if eligible, have the prescription written by the HCP, filled, and mailed directly to them by a third-party
pharmacy.
Our
commercial strategy for Phexxi includes marketing and product awareness campaigns targeting women of reproductive potential in the U.S.,
including the approximately 23 million women who are not using hormonal contraception and the approximately 18.8 million women who are
using a prescription contraceptive, some of whom, particularly pill users, may be ready to move to an FDA-approved, non-invasive hormone-free
contraceptive, as well as certain identified target HCP segments. In addition to marketing and product awareness campaigns, our commercial
strategy includes payer outreach and execution of our consumer digital and media strategy.
According
to our market research since Phexxi’s commercial launch, HCPs indicate they would recommend Phexxi to approximately:
| |
● |
47%
of patients experiencing side effects from current contraception; |
| |
● |
37%
of patients using non-hormonal prescription contraception; |
| |
● |
36%
of patients seeking pregnancy prevention; and |
| |
● |
19%
of patients using hormonal prescription contraception. |
Additional
research into the demographics of more than 5,000 women who are using Phexxi revealed that 79% of Phexxi users are between 18 to 34 years
of age. Among the subset of Phexxi users for whom prior contraceptive data is available (n=2,512), 80% of women who had recently started
Phexxi were not on any method of prescription contraception. Another 20% switched to Phexxi from either oral contraceptives, hormonal
rings or patches.
Market
acceptance and sales of Phexxi will depend in part on the extent to which reimbursement is available from third-party payers, which include
government health administration authorities, managed care organizations, private health insurers and PBMs. Third-party payers decide
which therapies they will pay for and establish reimbursement levels. Decisions regarding the extent of coverage and amount of reimbursement
to be provided for any product are made on a payer-by-payer basis. One payer’s determination to provide coverage for a drug does
not assure that other payers will also provide coverage and adequate reimbursement for that drug.
Managed
care organizations and other private insurers frequently adopt their own payment or reimbursement reductions. The continued integration
between commercial health plans and PBMs has increased the negotiating power of these entities. Third-party payers increasingly employ
formularies, which may not include all the products approved for a particular indication, to control costs by negotiating discounted
prices in exchange for formulary inclusion. We continue to work with health plans and PBMs to secure additional formulary positioning
for Phexxi.
Coverage
for and access to Phexxi is expected to further increase as additional insurers and PBMs comply with the current Health Resources and
Services Administration (HRSA) Women’s Preventive Services Guidelines, which took effect on January 1, 2023 for calendar year plans,
and adhere to the updated guidance related to contraceptive access that was issued by the U.S. Departments of Labor, Health and Human
Services (HHS), and the Treasury (the “Departments”) in January 2022. Collectively, these agencies specify that most insurers
and PBMs must provide coverage, with no out-of-pocket costs to women, for FDA-approved contraceptive products, like Phexxi, prescribed
by healthcare providers.
To
comply with these Guidelines, payers are increasingly covering Phexxi by:
| |
● |
adding
Phexxi to formulary (commercial insurers) or preferred drug list (Medicaid); |
| |
● |
removing
the requirement for a Prior Authorization letter from the HCP (commercial insurers); and/or |
| |
● |
moving
Phexxi to $0 copay (commercial insurers). |
We
continue working to increase the number of lives covered and to gain a preferred formulary position for Phexxi. We gained coverage for
32.5 million lives in 2022 and have added 22.1 million lives since December 31, 2022. We currently have 65% coverage within our Commercial
and Medicaid books of business, including 19.3 million lives covered at no out-of-pocket cost as of May 9, 2023 and approximately 13.7
million lives covered under our December 2020 contract award from the U.S. Department of Veterans Affairs. As of March 2023, the Phexxi
approved claims rate increased to 81%.
Phexxi
is classified in the databases and pricing compendia of Medi-Span and First Databank, two major drug information databases that payers
can consult for pricing and product information, as the first and only “vaginal pH modulator.”
In
2022, Evofem developed and introduced a new educational chart for patients and HCPs that details high-level information about birth control
methods currently available to women in the U.S., including the vaginal pH modulator. This
new educational tool has been extremely well received and has had a positive impact with HCPs and patients alike.
We
have not paid our Fiscal Year 2023 PDUFA Invoice for Phexxi to the FDA. The balance due continues to incur interest and penalties. As
a result, any drug application or supplement we submit will be considered incomplete and will not be accepted for consideration for filing
until all fees, interest and penalties are paid. We are unable to determine the full impact of this non-payment on the Company.
Research
and Development
EVO100
for the Prevention of Chlamydia and Gonorrhea
Based
on positive and statistically significant top-line results of our Phase 2B/3 AMPREVENCE trial, in October 2020 we initiated the
Phase 3 EVOGUARD clinical trial to evaluate EVO100 for the prevention of urogenital chlamydia and gonorrhea infections in women.
This randomized, placebo-controlled clinical trial enrolled 1,903 women with a prior chlamydia or gonorrhea infection who were at risk
for future infection.
On
October 11, 2022, we reported that EVOGUARD did not meet its primary efficacy endpoint. The Company believes COVID-19 related
changes in clinical site operations, subject behavior and actions including deviations from following the clinical study protocol requirements
related to STI acquisition, detection, and prevention contributed to this outcome. The product safety profile was consistent with what
has been observed in prior clinical trials, and only two women (0.1%) in the study discontinued due to adverse events. Due to financial
constraints, we discontinued investment in this clinical program.
EVO200 for Recurrent Bacterial Vaginosis
Our investigational candidate
for the reduction of recurrent bacterial vaginosis (BV), EVO200 vaginal gel, uses the same proprietary vaginal pH modulator platform
as Phexxi. In a Phase 1 dose-finding trial for this indication, the highest dose formulation of the study drug demonstrated reduced vaginal
pH for up to seven days following a single administration. The FDA has designed EVO200 as a Qualified Infectious Disease Product (QIDP)
for this indication. Due to financial constraints, all further development of EVO200 is on hold.
Multipurpose
Prevention Technology Vaginal Gel for HIV Prevention
In
December 2021, we launched a collaboration with Orion Biotechnology Canada Ltd. (Orion) to evaluate the compatibility and stability of
Orion’s novel CCR5 antagonist, OB-002, in Phexxi with the goal of developing a Multipurpose Prevention Technology (MPT) product
candidate for indications including the prevention of HIV in women. Assuming positive preclinical results, Evofem and Orion will seek
government and philanthropic funding for subsequent clinical trials of any resulting MPT vaginal gel product candidate.
Thin
Film Project
In
February 2020, we contracted with the University of South Australia to explore the development of a vaginally applied thin film
as a second-generation vaginal pH modulator product. The lead thin film candidates have been selected, and stability data has been generated
with positive results. The project is currently on hold due to financial constraints.
Senior
Subordinated Convertible Notes
On
December 20, 2022, the Company entered into a Securities Purchase Agreement (the December 2022 SPAs), with certain investors (the December
2022 Notes Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 8.0% Senior Subordinated
Notes due December 21, 2025 with an aggregate issue price of $2.3 million (the December 2022 Notes), which included an original issue
discount of $0.8 million (ii) warrants (the December 2022 Warrants) to purchase up to 369,230 shares of the Company’s common stock,
$0.0001 par value per share, and (iii) an aggregate 70 shares of Series D Preferred Stock (the Preferred Shares) (collectively, the Offering).
The Offering closed on December 21, 2022, with net proceeds to the Company from the Offering, after deducting offering expenses, of $1.25
million. The December 2022 Notes are convertible at $6.25, and the December 2022 Warrants have a strike price of $6.25.
In
February and March 2023, the Company entered into securities purchase agreements with certain investors providing for the sale and issuance
of senior subordinate convertible notes (collectively, the February and March 2023 SPAs). The February and March 2023 SPAs included (i)
convertible promissory notes with aggregate original principal amounts of approximately $1.4 million, $0.6 million, and $0.5 million,
respectively (the February and March 2023 Notes), and (ii) warrants to purchase an aggregate 553,846, 240,000, and 215,384 shares of
common stock, respectively (the February and March 2023 Warrants and collectively, the February and March 2023 Offerings). The 2023 Offerings
closed on February 17, 2023 (the February 2023 Closing), and March 13, 2023, March 20, 2023 (the March 2023 Closing), respectively, with
gross proceeds to the Company, before deducting offering expenses, of approximately $0.9 million, $0.4 million, and $0.3 million, respectively.
The February and March 2023 SPAs also included a Registration Rights Agreement that us to register the common stock underlying the February
and March 2023 Notes and Warrants within the timeframes specified therein. In addition, the Company issued warrants to purchase an aggregate
99,692 and 43,200 shares of common stock in February and March 2023 Closing to the placement agent.
In
April 2023, the Company entered into a securities purchase agreement with certain investors providing for the sale and issuance of senior
subordinate convertible notes (the April 2023 SPA). The April 2023 SPA included (i) convertible promissory notes with aggregate original
principal amounts of approximately $0.8 million (the April 2023 Notes), and (ii) warrants to purchase 615,384 shares of common stock
(the April 2023 Warrants and collectively, the April 2023 Offering). The April 2023 Offering closed on April 5, 2023 (the April 2023
Closing), with net proceeds to the Company, after deducting offering expenses, of approximately $0.5 million. The April 2023 SPA also
included a Registration Rights Agreement that requires the Company to register the common stock underlying the April 2023 Notes and April
2023 Warrants within the timeframes specified therein.
This
prospectus covers the resale or other disposition by the Selling Securityholders of the shares of common stock issuable upon the exercise
Warrants and conversion of the Notes.
Summary
of Risk Factors
The
risk factors described below are a summary of the principal risk factors associated with an investment in Evofem. These are not the only
risks we face. You should carefully consider the following risk factors, together with all of the other information included in this
prospectus, including the financial statements and related notes, when deciding to invest in us. You should be aware that the occurrence
of any of the events described in this Risk Factors section and elsewhere in this prospectus could have a material adverse effect on
our business, financial position, results of operations and cash flows and the trading price of our securities could decline and you
could lose all or part of your investment.
Risks
Related to Our Financial Condition and Capital Requirements
| |
○ |
We
received a Notice of Default on the Baker Bros. Purchase Agreement. |
| |
|
|
| |
○ |
We
are currently over 90 days past due on a significant amount of vendor obligations, including pursuant to previous lease agreements.
We may not be able to refinance, extend or repay our substantial indebtedness owed to our secured and unsecured lenders, which would
have a material adverse effect on our financial condition and ability to continue as a going concern. |
| |
○ |
We
have incurred significant losses and negative cash flows since our inception and anticipate we will continue to incur significant
losses and negative cash flow for the foreseeable future. |
| |
|
|
| |
○ |
We
must raise significant additional funds to finance our operations and to remain a going concern. If we are unable to raise additional
capital when needed or on acceptable terms, we may be forced to delay, reduce and/or eliminate one or more of our business initiatives. |
| |
|
|
| |
○ |
We
have a limited number of shares of common stock available for future issuance which could adversely affect our ability to raise capital
or consummate strategic transactions. |
Risks
Related to Potential Bankruptcy
| |
○ |
We
are subject to risks and uncertainties associated with potential bankruptcy proceedings including a long and protracted restructuring. |
| |
|
|
| |
○ |
Our
financial results may be volatile and may not reflect historical trends. |
Risks
Related to Commercialization of Phexxi
| |
○ |
Failure
to successfully commercialize Phexxi for prevention of pregnancy would likely cause our business to fail. |
| |
|
|
| |
○ |
We
face competition from other medical device, biotechnology and biopharmaceutical companies and our operating results will suffer if
we are unable to compete effectively. |
| |
|
|
| |
○ |
Phexxi
may not gain sufficient market acceptance among physicians, patients or the medical community, thereby limiting our potential to
generate revenue, which will undermine our future growth prospects. |
| |
|
|
| |
○ |
The
commercial success of Phexxi or any future approved products will depend in significant measure on the label claims that the FDA
or other regulatory authorities approve for those products. |
| |
|
|
| |
○ |
We
will need to obtain FDA approval of any proposed product names, and any failure or delay associated with such approval may adversely
affect our business. |
| |
|
|
| |
○ |
The
proportion of the contraceptive market that is made up of generic products continues to increase, making introduction of a branded
contraceptive difficult and expensive. |
Risks
Related to the Development of Our Product Candidates
| |
○ |
Our
inability to develop our vaginal pH modulator for additional indications could have an adverse effect on our business and our ability
to successfully market Phexxi for the prevention of pregnancy. |
| |
|
|
| |
○ |
Indemnity
claims from lawsuits or damages against our clinical trial sites could cause us to incur substantial liabilities and to limit commercialization
of Phexxi and any other product candidates we may develop. |
| |
|
|
| |
○ |
Clinical
trials are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of
applicable regulatory authorities. |
Risks
Related to Regulatory Approval of Our Product Candidates
| |
○ |
If
our clinical trials fail to satisfactorily demonstrate the safety and efficacy of our product candidates to the FDA and other comparable
foreign regulators, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development
and commercialization of our product candidates. |
| |
|
|
| |
○ |
Even
though we have received approval from the FDA in the United States to market Phexxi for the prevention of pregnancy, we may fail
to receive similar approval outside the United States. |
| |
|
|
| |
○ |
We
have not paid our Fiscal Year 2023 PDUFA Invoice for Phexxi to the FDA and the balance due continues to incur interest, penalties
and may apply retroactively. We cannot submit any new applications or supplements until paid. |
Risks
Related to Our Post-Marketing Legal and Regulatory Compliance
| |
○ |
Developments
after a product reaches the market may adversely affect sales of the product. |
| |
|
|
| |
○ |
Product
liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of Phexxi. If we are
unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits
of, our insurance coverage, a material liability claim could adversely affect our financial condition. |
| |
|
|
| |
○ |
If
we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur
costs that could have a material adverse effect on our business, financial condition or results of operations. |
Risks
Related to Our Intellectual Property
| |
○ |
Our
rights to develop and commercialize Phexxi are subject, in part, to the terms and conditions of licenses granted to us by third parties.
The patent protection and patent prosecution of Phexxi is dependent on third parties. |
| |
|
|
| |
○ |
If
we are unable to obtain and maintain patent protection for Phexxi or other proprietary technologies we may develop, or if the scope
of the patent protection we have or will obtain is not sufficiently broad, our competitors could develop and commercialize products
and technology similar or identical to our products and technology, and our ability to successfully commercialize Phexxi, our product
candidates and other proprietary technologies we may develop may be adversely affected. |
| |
|
|
| |
○ |
We
may not be able to protect our intellectual property and proprietary rights throughout the world. |
| |
|
|
| |
○ |
Issued
patents covering Phexxi and other proprietary technologies we may develop could be found invalid or unenforceable if challenged in
court or before administrative bodies in the United States or abroad. |
| |
|
|
| |
○ |
If
we do not obtain patent term extensions (PTE) for our products or product candidates, our business may be materially harmed. |
| |
|
|
| |
○ |
The
patent protection and patent prosecution for our product candidates are dependent on third parties, including Rush University. |
| |
|
|
| |
○ |
If
an event of default occurs under our issued and outstanding secured convertible notes issued pursuant to the Baker Bros. Purchase
Agreement, the note holders could take possession of all assets owned by us, including any directly owned intellectual property. |
| |
|
|
| |
○ |
If
we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed. |
| |
|
|
| |
○ |
We
may be subject to claims that our employees have wrongfully used or disclosed or wrongfully use alleged trade secrets of their former
employers. |
| |
|
|
| |
○ |
We
may not be successful in obtaining necessary rights to any product candidate we may develop through acquisitions and in-licenses. |
| |
|
|
| |
○ |
Some
intellectual property that we have in-licensed may have been discovered through government funded programs and thus may be subject
to federal regulations such as “march-in” rights, certain reporting requirements and a preference for U.S.-based companies.
Compliance with such regulations may limit our exclusive rights and limit our ability to contract with non-U.S. manufacturers. |
| |
|
|
| |
○ |
In April 2023, the Company received a Paragraph IV certification
notice letter (the “Padagis Notice Letter”) regarding an Abbreviated New Drug Application (“ANDA”) submitted
to the FDA by Padagis Israel Pharmaceuticals Inc. (“Padagis”). The ANDA seeks approval from the FDA to commercially manufacture,
use, or sell a generic version of Phexxi® under 21 U.S.C. § 355(j) prior to the expiration of United States Patent
Nos. 10,568,855; 11,337,989; and 11,439,610 listed in the FDA’s Orange Book: Approved Drug Products with Therapeutic Equivalence
Evaluations (collectively the “Phexxi Patents”). In the Padagis Notice Letter, Padagis claims that the Phexxi Patents
are invalid under various grounds. |
| |
|
|
| |
|
On June 1, 2023, the Company filed a complaint for patent
infringement in Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals, et al., in the United States District
Court for the District of New Jersey. The case was assigned number 2:23-cv-03003. The complaint alleges that Padagis’ proposed
generic version of Phexxi infringes the Phexxi Patents. The relief sought by the Company is a declaration of infringement and an
injunction of FDA approval of Padagis’ proposed generic version of Phexxi until expiration of the Phexxi Patents in 2033. Until
the earlier of final judgment or the passage of 30 months from the receipt of the Padagis Notice Letter, the FDA is prohibited from
approving Padagis’ ANDA to market its proposed generic version of Phexxi. The Company also subsequently filed a substantively
identical action in the United States District Court for the District of Delaware, Evofem Biosciences, Inc. et al. v. Padagis Israel
Pharmaceuticals, et al., which was assigned number 1:23-cv-00606-UNA. The Company is not aware of any answer or counterclaim filed
by Padagis in either action against the Company at this time. |
Risks
Related to Our Reliance on Third Parties
| |
○ |
Our
success relies on third-party suppliers and one contract manufacturer. Any failure by these third parties, including their inability
to successfully perform and comply with regulatory requirements, could negatively impact our business and our ability to market Phexxi
and develop and market our product candidates, and our business could be substantially harmed. |
| |
|
|
| |
○ |
We
have no significant internal distribution capabilities. We intend to engage third-party distributors for distribution of products
outside the United States, if approved, and have engaged additional third-party wholesale distributors for the distribution of Phexxi
in the United States. Our inability to identify, or enter into an agreement with, any such third-party distributor would likely have
a material adverse effect on our business and operations. |
Risks
Related to Our Commercialization of Health Care Products
| |
○ |
Changes
in health care laws and regulations may eliminate current requirements for health insurance plans to cover and reimburse FDA-cleared
or FDA-approved contraceptive products without cost sharing, which could reduce demand for products such as Phexxi. |
| |
|
|
| |
○ |
Despite
FDA-approval for Phexxi, and even if we are successful in obtaining regulatory approval to market other product candidates in the
United States, revenues may be adversely affected if Phexxi or any other approved product does not obtain coverage and adequate reimbursement
from third-party payers in the United States. |
| |
|
|
| |
○ |
Health
care legislative reform measures may have a negative impact on our business and results of operations. |
| |
|
|
| |
○ |
Our
business may be adversely affected by unfavorable macroeconomic conditions, including the COVID-19 pandemic. |
Risks
Related to Our Business Operations
| |
○ |
We
will need to expand the size of our organization, and we may experience difficulties in managing this growth or be unable to successfully
commercialize Phexxi, develop any product candidates or otherwise implement our business plan. |
Risks
Related to Our Common and Preferred Stock
| |
○ |
Our
management has identified material weaknesses in our internal controls and procedures. |
| |
|
|
| |
○ |
Our
shares of common stock have been delisted from the Nasdaq Capital Market which has and could result in, among other things, a decline
in the price of our common stock and less liquidity for holders of shares of our common stock. |
| |
|
|
| |
○ |
Our
stock price is and may continue to be volatile. |
| |
|
|
| |
○ |
There
may not be an active, liquid trading market for our equity securities. |
| |
|
|
| |
○ |
Because
our Common Stock is subject to the “penny stock” rules, brokers cannot generally solicit the purchase of our Common Stock,
which adversely affects its liquidity and market price. |
| |
|
|
| |
○ |
Our
common stock could be further diluted as the result of the issuance of additional shares of common stock, convertible securities,
warrants or options. |
| |
|
|
| |
○ |
We
are and may continue to be subject to short-selling strategies. |
| |
|
|
| |
○ |
Our
business could be negatively affected as a result of the actions of activist stockholders. |
| |
|
|
| |
○ |
We
may become a defendant in one or more stockholder derivative or class-action litigation(s), and any such future lawsuit(s) may adversely
affect our business, financial condition, results of operations and cash flows. |
Our
Corporate Information
Our
principal executive offices are located at 7770 Regents Rd. Suite 113-618, San Diego, California 92122 and our telephone number is (858)
550-1900. Our website is located at www.evofem.com. Information contained in, or that can be accessed through, our website is not incorporated
by reference into this prospectus, and you should not consider information on our website to be part of this prospectus.
Smaller
Reporting Company
We
are a “smaller reporting company” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange
Act, and have elected to take advantage of certain of the scaled disclosure available for smaller reporting companies.
The
Offering
| Common
stock offered by the Selling Securityholders |
|
Up
to 3,336,730 shares of common stock issuable upon exercise of the Warrants, and 7,642,038 shares upon conversion of
the Notes. |
| |
|
|
| Common
stock outstanding after this offering |
|
14,738,977
shares, assuming the exercise in full of the
Notes and the Warrants and assuming all common shares are sold by the Selling Securityholders. |
| |
|
|
| Use
of proceeds |
|
We
will not receive any proceeds from the sale of shares of common stock offered by the Selling Securityholders under this prospectus.
However, Evofem will receive the proceeds of any cash exercise of the Warrants. If we receive proceeds, we currently intend to use
the proceeds for the continuation of commercialization activities related to Phexxi, other general corporate purposes and other capital
expenditures. |
| |
|
|
| Offering
Price |
|
The
Selling Securityholders may sell all or a portion of their shares through public or private transactions at prevailing market prices
or privately negotiated prices. |
| |
|
|
| Listing
Information |
|
Our
common stock is traded on the OTCQB Venture Market under the symbol “EVFM.” |
| |
|
|
| Risk
Factors |
|
An
investment in our securities involves a high degree of risk. See the section entitled “Risk Factors” of this prospectus. |
The
number of shares of our common stock to be outstanding after this offering is based on 1,727,690 shares of common stock outstanding as
of March 31, 2023 and excludes as of such date:
| |
● |
4,843
shares of common stock issuable upon the exercise of stock options outstanding as of March 31, 2023, at a weighted-average exercise
price of $7,900 per share; 1,058 shares of stock options were forfeited after March 31, 2023, at a weighted-average exercise
price of $14,390 per share; |
| |
● |
3,180,282
shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2023 at a weighted-average exercise price
of $35.70 per share; 122,741 shares of common stock issued in connection with the exercise of warrants after March 31, 2023; |
| |
● |
3,353,845
shares of common stock issuable upon the exercise
of warrants granted after March 31, 2023 with a weighted-average exercise price of $1.25 per share; |
| |
● |
76,407,245
shares of common stock issuable upon conversion of principal and accrued interest underlying issued and outstanding convertible notes
assuming a weighted-average conversion price of $1.64 per share, assuming a conversion date of March 31, 2023; |
| |
● |
14,238,827
shares of common stock issuable upon the exercise of rights to receive common stock outstanding as of March 31, 2023; 1,243,152
shares of common stock issued in connection with the exercise of rights after March 31, 2023; |
| |
● |
15,218,227
shares of common stock issuable upon the exercise of rights increased after March 31, 2023 with a weighted-average exercise price
of $0.8125 per share; |
| |
● |
4,769
shares of common stock reserved for future awards under the Amended and Restated Evofem Biosciences, Inc. 2014 Equity Incentive Plan; |
| |
● |
575
shares of common stock reserved for future awards under the Amended 2018 Inducement Equity Incentive Plan; and |
| |
● |
509
shares of common stock reserved for future awards under the 2019 Employee Stock Purchase Plan. |
RISK
FACTORS
An
investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider
carefully the risks and uncertainties described below and in the section captioned “Risk Factors” contained in our Annual
Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the SEC on April 27, 2023, and other filings we make with
the SEC from time to time. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow
could suffer materially. In such event, the trading price of our common stock could decline, and you might lose all or part of your investment.
Risks
Related to Our Financial Condition and Capital Requirements
We
are currently in Default of the Securities Purchase and Security Agreement with Baker Brothers.
On
March 7, 2023, Baker Bros. Advisors, LP (the Designated Agent) provided a Notice of Event of Default and Reservation of Rights (the Notice
of Default) relating to the Securities Purchase and Security Agreement dated April 23, 2020, and subsequently amended (SPA), by and amount
the Company, Designated Agent, the Guarantors and Baker Purchasers. The Notice of Default claims that the Company has failed to maintain
the “Required Reserve Amount” as required by Section 2.7 of the Third Amendment to the Securities Purchase Agreement and
Section 8.1(e) of the SPA. The Designated Agent claims such failure constitutes an immediate Event of Default pursuant to Section 9.1(e)
of the SPA. The Designated Agent, at the direction of the Baker Purchasers, has accelerated repayment of the outstanding balance payable
and elected its remedies pursuant to Section 5.07(b) of the Securities Purchase Agreement. As a result, approximately $92.7 million
representing two times the sum of the outstanding balance and all accrued and unpaid interest thereon and all other amounts due under
the SPA and other documents is due and payable within three business days of receipt of the Notice of Default. As of the date of the
filing of this prospectus, the Company has sufficient required reserve number of shares upon the effectuation of the Reverse Stock Split.
The failure to cure the default or otherwise settle or resolve, could have a significant negative financial impact on the Company,
could result in litigation, and could result in the assets of the company being seized, attached or otherwise utilized to satisfy the
debt.
We
are currently over 90 days past due on a significant amount of vendor obligations. We may not be able to refinance, extend or repay our
substantial indebtedness owed to our secured and unsecured lenders, which would have a material adverse effect on our financial condition
and ability to continue as a going concern.
As
of June 30, 2023, we have approximately $15.7 million in accounts payable with approximately $15.3 million that
is over 90 days past due (not including the Baker Notes described herein). If we are unable to repay these amounts, as well as our existing
debt obligations at maturity, and we are otherwise unable to extend the maturity dates or refinance these obligations, we would be in
default. We cannot provide any assurances that we will be able to raise the necessary amount of capital to repay these obligations or
that we will be able to extend the maturity dates or otherwise refinance these obligations. Upon a default, our secured lenders would
have the right to exercise their rights and remedies to collect, which would include foreclosing on our assets. Accordingly, a default
would have a material adverse effect on our business, and we would likely be forced to seek bankruptcy protection.
Our
audited financial statements included a statement that there is a substantial doubt about our ability to continue as a going concern
and a continuation of negative financial trends could result in our inability to continue as a going concern.
Our
management has determined that there is a substantial doubt about our ability to continue as a going concern over the next 12 months
from the Form 10-K filing date of April 27, 2023. Our independent auditors have included a “going concern”
explanatory paragraph in their report on our financial statements as of and for the year ended December 31, 2022 as filed in this Registration
Statement. The reaction of investors to the inclusion of a going concern statement by our independent auditors, and our
potential inability to continue as a going concern, could materially adversely affect the price of our common stock.
Additionally,
if our operating results fail to improve we could violate additional debt covenants, our liquidity could be further adversely impacted
and we may need to seek additional sources of funding. There is no assurance that we will be able to raise additional capital to fund
our operations or that debt or equity financing will be available in sufficient amounts or on acceptable terms. If our operating results
fail to improve, then our financial condition could render us unable to continue as a going concern.
We
have incurred significant losses and negative cash flows since our inception and anticipate we will continue to incur significant losses
and negative cash flow for the foreseeable future.
We
have incurred yearly losses and negative cash flows since our inception, including net losses of $2.4 million and $32.0 million
for the three months ended March 31, 2023 and 2022, and $76.7 million and $205.2 million for the years ended December 31, 2022 and 2021,
respectively. As of March 31, 2023 and December 31, 2022, we had an accumulated deficit of $941.0 million and $938.7 million, respectively.
Negative cash flows from our operations are expected to continue for the foreseeable future. To date, we have devoted substantially all
our financial resources to the development and commercialization of Phexxi for hormone-free contraception and to the development of EVO100
for the prevention of chlamydia and gonorrhea and our other product candidates, as well as providing general and administrative support
for our operations. Our utilization of cash has historically been highly dependent on these development programs and the commercialization
of Phexxi in the United States. In October 2022, we discontinued development of EVO100 for the prevention of chlamydia and gonorrhea
and have no plans to advance clinical development of this program or to significantly invest in other clinical programs or product candidates
for the foreseeable future. We plan to allocate capital to fund our continued commercialization efforts. Our cash expenses will also
continue to be dependent on the terms and conditions of our contracts with service providers and any license partners.
To
date, we have financed our operations primarily through the sale of equity securities, notes, warrants, convertible notes, convertible
preferred stock and through other debt arrangements. The amount of our future net losses will depend, in large part, on our ability to
generate revenue from the sale of Phexxi, the rate of our future expenditures and our ability to obtain funding through equity or debt
financings, strategic collaborations or grants which may be particularly challenging or impossible in light of market conditions, especially
in light of the ongoing COVID-19 pandemic. The commercialization and development of biopharmaceutical products involves a substantial
degree of risk.
We
expect to continue to incur significant operating expenses and to continue to incur significant losses for the foreseeable future as
we:
| |
● |
incur
sales, marketing, and distribution costs to commercialize Phexxi, including media and digital promotional campaigns; |
| |
● |
incur
costs associated with the commercial manufacturing of Phexxi; |
| |
● |
implement
post-approval changes and process improvements to manufacturing; |
| |
● |
seek
regulatory and marketing approvals for Phexxi outside the United States and reimbursement for Phexxi or any product candidates we
may choose to develop in the future; |
| |
● |
continue
our efforts to identify, assess, acquire, and/or develop other product candidates; |
| |
● |
make
milestone, royalty or other payments under third-party license agreements; |
| |
● |
seek
to maintain, protect, and expand our intellectual property portfolio; and |
| |
● |
seek
to attract and retain skilled personnel. |
Due
in part to circumstances related to the COVID-19 pandemic, we delayed the commercial launch of Phexxi from June 2020 until September
2020. The COVID-19 pandemic led to slower than forecasted uptake of Phexxi due to reduced access to medical offices and HCPs as well
as changes in sexual behavior among consumers, particularly during periods of lockdown and the emergence of variant strains of the virus.
Should we experience any further delays or encounter issues with the commercialization, some of which may result in part due to the ongoing
COVID-19 pandemic, we may incur significant additional expenses.
The
net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of
our results of operations may not be a good indication of our future performance. Due to the recurring losses, negative cash flows from
operating activities since inception, and net working capital at December 31, 2022, the report of our independent registered public accountant
on our financial statements as of and for the years ended December 31, 2022 and 2021 filed with this Registration Statement includes
explanatory language describing the existence of substantial doubt about our ability to continue as a going concern. In addition, our
management has further determined that there is a substantial doubt about our ability to continue as a going concern over the next 12
months from the Form 10-K filing date of April 27, 2023.
Although
we have generated revenue from product sales, we may never be profitable. Our operating results may differ from any guidance we may announce.
Our
current business is substantially dependent on the commercial success of Phexxi. The commercial launch of Phexxi took place on September
8, 2020, and although we have generated revenue from sales of Phexxi, we may never achieve or sustain profitability. Our ability to generate
revenue and achieve and sustain profitability depends on our ability, alone or with strategic collaborators, to successfully commercialize
Phexxi and, to a lesser extent, any future products we may license or develop and commercialize. Our ability to generate future revenue
from product sales depends heavily on our success in many areas, including, but not limited to:
| |
● |
the
rate and degree of market acceptance for Phexxi and any other product candidates that may be approved in the future; |
| |
● |
the
effectiveness of our commercialization strategy for Phexxi and any other product candidates that may be approved in the future, either
directly or with one or more distribution partners, including the effectiveness of our sales force, the Phexxi telehealth platform,
media and digital campaigns, and contracted tele-sales vendor; |
| |
● |
reimbursement
and pricing for Phexxi and any other approved product candidates in amounts that support profitability; |
| |
● |
successfully
competing against other contraceptive products; |
| |
● |
manufacturing
Phexxi and establishing and maintaining supply and manufacturing relationships with third parties that are commercially feasible,
as well as complying with applicable regulatory requirements and meeting our supply needs in sufficient quantities to meet market
demand for Phexxi; |
| |
● |
our
ability to adapt in a dynamic and challenging pandemic environment; |
| |
● |
obtaining
regulatory approval of Phexxi in territories outside of the United States; |
| |
● |
manufacturing
any investigational product(s), should we choose to advance their clinical development, funding and successfully completing clinical
development, and obtaining regulatory approval; |
| |
● |
protecting,
maintaining and enforcing our intellectual property rights, including patents, trade secrets and know-how; |
| |
● |
negotiating
favorable terms in any collaboration, licensing or other arrangements into which we may enter; and |
| |
● |
attracting,
hiring and retaining qualified personnel. |
From
time to time, we may provide guidance as to our anticipated future performance and certain unit shipment information, prescription and
prescriber statistics, website and search statistics and other metrics. We may fail to achieve the performance described in any such
guidance, and any information or metrics we may provide may be not be indicative of future results. In addition, we provide co-pay assistance
to commercially insured patients with an approved Phexxi prescription and utilize a sample program to promote demand for Phexxi. The
co-pay program reduces the amount of profit we realize per unit sold, however it is a value program to patients that we aim to continue
in 2023. Because of the expense to run the program, we will look to modify the business rules surrounding the co-pay program in the future,
particularly as payers increasingly cover Phexxi at $0 co-pay to comply with HRSA guidelines; compliance is mandated beginning January
1, 2023 and enforcement action is anticipated. If we are not able to generate sufficient revenue from product sales of Phexxi, the revenue
from product sales of Phexxi is not sufficiently profitable, we fail to meet our guidance, or our information or metrics is not indicative
of our future results of operations, this could materially and adversely affect our business results of operations, the price of our
common stock, our financial condition and our ability to raise additional capital.
We
will need to raise significant additional funds to finance our operations, including the commercialization of Phexxi, and to remain a
going concern. If we are unable to raise additional capital when needed or on acceptable terms, we may be forced to delay, reduce and/or
eliminate one or more of our business initiatives or to cease our operations entirely.
We
have incurred significant losses and negative cash flows since our inception. Our ability to raise additional funds will depend, in part,
on our ability to successfully commercialize Phexxi in the United States. If, for whatever reason, we are unsuccessful in these efforts,
it may make any necessary debt, equity or alternative financing more difficult, more costly and more dilutive. Attempting to secure additional
financing will divert our management from our day-to-day activities, which may adversely affect our ability to commercialize Phexxi.
In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all.
Furthermore, the global credit and financial markets have experienced extreme volatility and disruptions in recent history, particularly
for life science companies. If the equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult,
more costly and more dilutive. If we are unable to raise additional funds when needed or on acceptable terms, we may be unable to continue
commercializing Phexxi as a contraceptive. In addition, we may be required to delay, scale back or eliminate some or all of our business
initiatives or be forced to cease operations entirely. To the extent we raise additional capital through the sale of equity, convertible
debt or other securities convertible into equity, the ownership interest of our stockholders will be diluted, and the terms of these
new securities may include liquidation or other preferences that adversely affect the rights of our stockholders. Future debt financings,
if available at all, would likely involve agreements with additional covenants limiting or restricting our ability to take specific actions,
such as incurring additional debt, making capital expenditures, making additional product acquisitions or declaring dividends. If we
raise additional funds through strategic collaborations, alternative non-dilutive financing, such as royalty-based financing, or licensing
arrangements with third parties, we may have to relinquish valuable rights to our product candidates or future revenue streams or grant
licenses on terms that are not favorable to us.
Moreover,
if we are unable to continue as a going concern, we may be forced to liquidate our assets and the values we receive for our assets in
liquidation or dissolution could be significantly lower than the values reflected in our financial statements. Given the amounts currently
owed pursuant to the Adjuvant Notes, the Baker Notes and other debt arrangements, holders of our common stock may not receive value for
their shares in the event of a liquidation.
We
have certain obligations pursuant to our issued and outstanding promissory notes, convertible notes and related note purchase agreements,
and our failure to comply with these obligations could have a material adverse effect on our business, financial condition or results
of operations.
In
April 2020, we entered into a Securities Purchase and Securities Agreement (the “Baker Bros. Purchase Agreement”) with certain
institutional investors and their designated agent pursuant to which we issued and sold secured convertible promissory notes in an aggregate
principal amount of $25.0 million and warrants to purchase shares of our common stock. In November 2021, we entered into the first amendment
to the Baker Bros. Purchase Agreement which extends the affirmative covenant to achieve $100.0 million in cumulative net sales of Phexxi
by June 30, 2022 to June 30, 2023. On March 7, 2023, Baker Bros. Advisors, LP (the Designated Agent)
provided a Notice of Event of Default and Reservation of Rights (the Notice of Default) relating to the Baker Bros. Purchase Agreement.
In
October 2020, we entered into a Securities Purchase Agreement (the Adjuvant Purchase Agreement) pursuant to which we issued
and sold to certain institutional investors unsecured convertible promissory notes in an aggregate principal amount of $25.0 million.
On April 4, 2022, we entered into the first amendment to the Adjuvant Purchase Agreement (the First
Adjuvant Amendment). The First Adjuvant Amendment extended, effective as of the date on which we achieved the Qualified Financing
Threshold upon the closing of the May 2022 Public Offering, the affirmative covenant to achieve $100.0 million in cumulative net sales
of Phexxi by June 30, 2022 to June 30, 2023. The First Adjuvant Amendment also provided for an adjustment to the conversion price of
the Adjuvant Notes such that the conversion price for these Notes, effective as of the reverse stock split the conversion price will
now be the lesser of (i) $5.4279 and (ii) 100% of the lowest price per share of common stock (or with respect to securities convertible
into common stock, 100% of the applicable conversion price) sold in any equity financing until we have met the Qualified Financing Threshold.
In
January 2022, we entered into a Securities Purchase Agreement (the 2022 Purchase Agreement) with certain institutional
investors pursuant to which we issued warrants and unsecured subordinate promissory notes with an original principal amount of $5.9
million (the January 2022 Notes). In March 2022, we entered into a Securities Purchase Agreement (the March
2022 Purchase Agreement) with certain institutional investors pursuant to which we issued warrants and unsecured subordinate promissory
notes with an original principal amount of $7.5 million (the March 2022 Notes; collectively with the Baker Bros.
Notes, the Adjuvant Notes and the January 2022 Notes, the Notes).
These
debt arrangements limit our ability to incur debt, merge, or declare dividends and, in certain circumstances, and with respect to the
January 2022 Notes and March 2022 Notes, the holders may require us to redeem outstanding amounts out of gross proceeds raised in certain
subsequent offerings which could mean money raised in these offerings would not ultimately be able to be used to fund our ongoing operations.
The Baker Notes are secured by substantially all of our assets, and we are currently in default. Our failure to make payments as due
under any of the Notes could be an event of default under all of the Notes. Events of default under these arrangements could also include,
but are not limited to, a material breach of representations, our failure to comply with our obligation to convert convertible notes,
our failure to perform or observe, and in certain instances, cure, certain covenants, including, but not limited to, covenants requiring
us to maintain the listing of shares of our common stock on the OTCQB and, assuming no further amendment of current terms, to achieve
cumulative net sales of Phexxi of at least $100.0 million by June 30, 2023. In the event of a default and depending on the terms of each
Note, a holder of the Notes may be entitled to redemption premiums, treble amounts and other remedies described in their respective agreements.
Any default could materially and adversely impact our business, results of operations and financial condition, as well as increase our
need to raise additional capital, cause us to cease our operations entirely and may result in the holders of our common stock not receiving
any value for their investment.
On
December 20, 2022, we entered into a securities purchase agreement (SPA), with certain investors (the Investors) providing
for the sale and issuance of senior secured convertible notes due in the aggregate original principal amount of $2,307,692 (the Notes),
warrants to purchase an aggregate 369,230 shares of common stock (Warrants) and an aggregate 70 shares of Series D Preferred Stock (the
Preferred Shares) (collectively, the Offering). The Offering closed on December 21, 2022 (the Closing Date) and as a result, we issued
an aggregate $2,307,692 in aggregate principal amount of Notes and the Warrants to purchase 369,230 shares of common stock. Each Investor paid approximately $650 for each $1,000 of principal amount of Notes, Preferred Shares and Warrants. Our net proceeds from the
Offering, after deducting offering expenses were approximately $1,250,000.
On
December 19, 2022, we entered into the First Amendment to Forbearance Agreement (the Amendment) effective as of December 15, 2022 (the
Amendment Effective Date) to amend certain provisions of the of the Secured Creditor Forbearance Agreement dated September 15, 2022.
The Amendment revises the Secured Creditor Forbearance Agreement to (i) amend the Fifth Recital Clause to clarify that the Purchasers
consent to any additional indebtedness pari passu, but nor senior to that of the Purchases, in an amount not to exceed $5,000,000,
and (ii) strike and entirely replace Section 4 to clarify the terms of the Purchasers’ consent to Interim Financing (as defined
therein). No other revisions were made to the Secured Creditor Forbearance Agreement, including the requirement under Section 5. Agreement
to Forbear where the Forbearance Termination Event means, among other things, the first date after December 31, 2022 on which our total
cash falls below $1,000,000.
In
February, March and April 2023, the Company entered into securities purchase agreements with certain investors providing for the sale
and issuance of senior secured convertible notes (collectively, the 2023 SPAs). The 2023 SPAs included (i) convertible promissory notes
with aggregate original principal amounts of approximately $1.4 million, $0.6 million, $0.5 million and $0.8 million, respectively (the
2023 Notes), and (ii) warrants to purchase an aggregate 553,846, 240,000, 215,384 and 615,384 shares of common stock, respectively (the
2023 Warrants and collectively, the 2023 Offerings). The 2023 Offerings closed on February 17, 2023 (the February 2023 Closing), March
13, 2023, March 20, 2023 (the March 2023 Closing) and April 5, 2023 (the April 2023 Closing), respectively, with net proceeds to the
Company, after deducting offering expenses, of approximately $0.7 million, $0.3 million, $0.3 million, and $0.5 million, respectively.
The 2023 SPAs also included a Registration Rights Agreement that us to register the common stock underlying the 2023 Notes and 2023 Warrants
within the timeframes specified therein. In addition, the Company issued warrants to purchase an aggregate 99,692 and 43,200 shares of
common stock in February and March 2023 Closing to the placement agent.
In July 2023, we entered
into an SPA with certain investors providing for the sale and issuance of senior secured convertible notes due in the aggregate
original principal amount of $1.5 million (the Notes), and warrants to purchase an aggregate 1,200,000 shares of common stock
(Warrants) (collectively, the July 2023 Offering). The July 2023 Offering closed on July 3, 2023 with net proceeds to the Company,
after deducting offering expenses, of approximately $1.0 million.
A
failure to comply with these obligations, triggering additional events of default, or other breach could have a material adverse effect
on our business, financial condition or results of our operations.
We
have a limited number of shares of common stock available for future issuance which could adversely affect our ability to raise capital
or consummate strategic transactions.
We
are currently authorized to issue 500,000,000 shares of common stock under our amended and restated certificate of incorporation. As
of July 3, 2023, we have issued 2,524,239 shares of common stock and approximately 189.1 million shares of common stock
were committed for issuance giving effect to the assumed exercise of all outstanding warrants, options, purchase rights and the assumed
conversion of all issued and outstanding convertible notes. The conversion prices of the Adjuvant Convertible Notes (as amended) and
Baker Convertible Notes may also be subject to adjustment depending on the price of issuances in future financings as described above.
These adjustments would further increase the numbers of shares of common stock to be reserved as a result of these adjustments. Due to
the limited number of authorized shares common stock available for future issuance, we may not able to raise additional equity capital
or complete a merger or other business combination unless we increase the number of shares we are authorized to issue. We would need
to seek stockholder approval to increase the number of our authorized shares of Common Stock, and we can provide no assurance that we
would succeed in amending our amended and restated certificate of incorporation to increase the number of shares of Common Stock we are
authorized to issue which could negatively impact our business, prospects and results of operations.
Use
of net operating loss carryforwards may be limited and U.S. federal income tax reform could adversely affect us.
Our
ability to utilize our net operating loss (NOL) carryforwards and other tax attributes to offset future taxable income or tax liabilities
may be limited as a result of ownership changes. Corresponding rules may apply under state tax laws. Even if there is no limitation on
utilization of our NOL carryforwards as the result of an ownership change, the utilization of NOL carryforwards may be limited by other
applicable laws. Pursuant to the TCJA passed in December 2017, carryforwards originating from a loss incurred in a year after 2017 are
limited and may reduce taxable income in any post-2020 year by no more than 80% of the pre-NOL taxable income in such year. The Coronavirus
Aid, Relief and Economic Security Act (the CARES Act) temporarily suspended this 80% taxable income limitation, allowing an NOL carryforward
to fully offset taxable income in tax years beginning before January 1, 2021. Additional legislation or regulation which could affect
our tax burden could be enacted by any governmental authority. We cannot predict the timing or extent of such tax-related developments
which could have a negative impact on our financial results, including a potential increase in federal corporate tax rates generally.
We cannot estimate how the changes in tax law from this legislation will affect our tax liability in future years, but we have recorded
a valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future
benefits from those assets. We have established a full valuation allowance for our deferred tax assets due to uncertainties as to their
utilization. While we use our best judgment in attempting to quantify and reserve for our tax obligations. A challenge by a taxing authority,
our ability to utilize tax benefits such as carryforwards or tax credits, or a deviation from other tax-related assumptions may cause
actual results to deviate from previous estimates.
Risks
Related to Potential Bankruptcy
Given
our current financial condition, we have considered and continue to consider filing for bankruptcy protection. While we have not initiated
bankruptcy proceedings, we caution that trading in our securities is highly speculative and poses substantial risks relating to the potential
of bankruptcy proceedings. Trading prices for our securities may bear little or no relationship to the actual recovery, if any, by holders
of our securities in Bankruptcy proceedings, if any.
We
are subject to risks and uncertainties associated with potential bankruptcy proceedings.
Our
operations and ability to develop and execute our business plan, our financial condition, our liquidity and our continuation as a going
concern, are subject to risks and uncertainties associated potential or actual bankruptcy. These risks include the following:
| |
● |
our
ability to prosecute, confirm and consummate a plan of reorganization with respect to the Chapter 11 proceedings; |
| |
|
|
| |
● |
the
high costs of bankruptcy proceedings and related fees; |
| |
|
|
| |
● |
our
ability to obtain sufficient financing to allow us to emerge from bankruptcy and execute our business plan post emergence; |
| |
|
|
| |
● |
our
ability to maintain our current relationships with or attract new suppliers, service providers, customers, employees, and other third
parties; |
| |
|
|
| |
● |
our
ability to maintain contracts that are critical to our operations; |
| |
|
|
| |
● |
our
ability to execute our business plan in the current depressed commodity price environment; |
| |
|
|
| |
● |
our
ability to attract, motivate and retain key employees; |
| |
|
|
| |
● |
the
ability of third parties to seek and obtain court approval to terminate contracts and other agreements with us or make other third-party
motions in the proceedings; |
| |
|
|
| |
● |
the
ability of third parties to seek and obtain court approval to convert Chapter 11 proceedings to Chapter 7 proceedings, if applicable;
and |
| |
|
|
| |
● |
the
actions and decisions of our creditors and other third parties who have interests in proceedings that may be inconsistent with our
plans. |
Delays
in filing for or moving forward with the proceedings increase the risks of our being unable to reorganize our business and emerge from
bankruptcy and increase our costs associated with the bankruptcy process.
These
risks and uncertainties could affect our business and operations in various ways. For example, negative events associated with either
Chapter 11 or Chapter 7 proceedings could adversely affect our relationships with our suppliers, service providers, customers, employees,
and other third parties, which in turn could adversely affect our operations and financial condition. Also, we need the prior approval
of the Bankruptcy Court for transactions outside the ordinary course of business, which may limit our ability to respond timely to certain
events, take advantage of certain opportunities or pursue our business strategies. Because of the risks and uncertainties associated
with potential proceedings, we cannot accurately predict or quantify the ultimate impact that events that may occur during the proceedings
will have on our business, financial condition and results of operations.
Our
businesses could suffer from a long and protracted restructuring.
Our
future results could be dependent upon the successful confirmation and implementation of a bankruptcy plan or other alternative restructuring
transaction, including a sale of all or substantially all of our assets. A long period of operations under Bankruptcy Court protection
could have a material adverse effect on our business, financial condition, results of operations and liquidity. Failure to obtain confirmation
of a Chapter 11 plan or approval and consummation of an alternative restructuring transaction in a timely manner may harm our ability
to obtain financing to fund our operations, and there is a significant risk that the value of our securities and assets would be substantially
eroded to the detriment of all stakeholders. If a Chapter 11 plan that complies with the applicable provisions of the Bankruptcy Code
cannot be agreed upon, it is possible that we would have to liquidate our assets, in which case it is likely that holders of claims would
receive substantially less favorable treatment than they would receive if we were to emerge as a viable, reorganized entity.
If
filed, for as long as bankruptcy proceedings continue, we will be required to incur substantial costs for professional fees and other
expenses associated with the administration of the Chapter 11 or Chapter 7 proceedings. Chapter 11 proceedings may also require us to
seek debtor-in-possession financing to fund operations. If we are unable to obtain such financing on favorable terms or at all, our chances
of successfully reorganizing our business may be seriously jeopardized, the likelihood that we instead will be required to liquidate
our assets may be enhanced, and, as a result, any securities in us could become further devalued or become worthless.
In
the event we decide to initiate bankruptcy proceedings, there can be no assurance that we will successfully reorganize and emerge from
Chapter 11 proceedings or, if we do successfully reorganize, as to when we would emerge from the Chapter 11 proceedings. Even after a
Chapter 11 plan is confirmed and implemented, our operating results may be adversely affected by the possible reluctance of prospective
lenders, suppliers and other counterparties to do business with a company that recently emerged from bankruptcy proceedings.
In
certain instances, a Chapter 11 case may be converted to a case under Chapter 7 of the Bankruptcy Code.
We
have not yet filed for bankruptcy and therefore have not yet decided upon Chapter 11 or Chapter 7. However, should we choose to pursue
Chapter 11, upon a showing of cause, the Bankruptcy Court may convert our Chapter 11 case to a case under Chapter 7 of the Bankruptcy
Code. In such event, a Chapter 7 trustee would be appointed or elected to liquidate our assets for distribution in accordance with the
priorities established by the Bankruptcy Code. We believe that liquidation under Chapter 7 would result in significantly smaller distributions
being made to our creditors because of (i) the likelihood that the assets would have to be sold or otherwise disposed of in a distressed
fashion over a short period of time rather than in a controlled manner and as a going concern, (ii) additional administrative expenses
involved in the appointment of a Chapter 7 trustee, and (iii) additional expenses and claims, some of which would be entitled to priority,
that would be generated during the liquidation and from the rejection of leases and other executory contracts in connection with a cessation
of operations.
If
we choose to file under Chapter 11, we may be subject to claims that will not be discharged in the bankruptcy proceedings, which could
have a material adverse effect on our financial condition and results of operations.
The
Bankruptcy Code provides that the confirmation of a plan of reorganization discharges a debtor from substantially all debts arising prior
to confirmation. With few exceptions, all claims that arose prior to confirmation of the plan of reorganization (i) would be subject
to compromise and/or treatment under the plan of reorganization and/or (ii) would be discharged in accordance with the Bankruptcy Code
and the terms of the plan of reorganization. Any claims not ultimately discharged through a plan of reorganization could be asserted
against the reorganized entities and may have an adverse effect on our financial condition and results of operations on a post-reorganization
basis.
Our
financial results may be volatile and may not reflect historical trends.
During
bankruptcy proceedings, we expect our financial results to continue to be volatile as asset impairments, asset dispositions, restructuring
activities and expenses, contract terminations and rejections, and claims assessments occur, which may significantly impact our consolidated
financial statements. As a result, our historical financial performance is likely not indicative of our financial performance after the
date of the bankruptcy filing.
In
addition, if we emerge from Chapter 11, the amounts reported in subsequent consolidated financial statements may materially change relative
to historical consolidated financial statements, including as a result of revisions to our operating plans pursuant to a plan of reorganization.
We also may be required to adopt fresh start accounting, in which case our assets and liabilities will be recorded at fair value as of
the fresh start reporting date, which may differ materially from the recorded values of assets and liabilities on our consolidated balance
sheets. Our financial results after the application of fresh start accounting also may be different from historical trends.
Risks
Related to Commercialization of Phexxi and Any Other Approved Product Candidates
Our
success will depend heavily on whether we can successfully commercialize our only commercially available product, Phexxi, for prevention
of pregnancy. Failure to successfully commercialize Phexxi for the prevention of pregnancy would likely cause our business to fail.
Our
overall success will rely heavily on the commercial success of Phexxi vaginal gel for prevention of pregnancy. Failure to successfully
commercialize Phexxi for the prevention of pregnancy would likely cause our business to fail. There are numerous examples of failures
to meet high expectations of market potential for new product launches in the health care space, including by pharmaceutical companies
with more experience and resources than us. If the commercialization of Phexxi is unsuccessful or perceived as disappointing, our stock
price could decline significantly.
If
we are unable to establish and maintain effective internal sales and marketing capabilities, or enter into agreements with third parties
to market and sell Phexxi, our ability to generate revenue would be adversely affected.
Although
our employees may have previously marketed, commercialized and sold other pharmaceutical products, including contraceptives, while employed
at other companies, we have limited experience selling and marketing Phexxi. We may face difficulties recruiting and hiring sales representatives
and otherwise obtaining these marketing capabilities. Any failure or delay in the timely development of our internal commercialization
capabilities could adversely impact the potential for commercial success of Phexxi.
If
we are unable to effectively train and equip our sales force, our ability to successfully commercialize Phexxi will be harmed.
We
may not be able to maintain the requisite sales force to market Phexxi. Even if we are able to maintain the requisite sales force, Phexxi
is a newly marketed drug with a new mechanism of action as a vaginal pH modulator and, therefore, none of the members of our sales force
has extensive experience promoting Phexxi. We expect to continue to expend significant time and resources to train our sales consultants
in marketing Phexxi. In addition, we must train our sales force to ensure that an appropriate and compliant message about Phexxi is being
delivered. If we are unable to effectively train our sales force and equip them with compliant and effective materials, including medical
and sales literature to help them appropriately inform and educate physicians regarding the potential benefits of Phexxi, our efforts
to successfully commercialize Phexxi could be put in jeopardy, which would negatively impact our ability to generate product revenues.
Our
use of social media platforms to market and promote a prescription product, e.g. Phexxi, presents risks and operational challenges.
We
believe that our customer base and potential patient populations for Phexxi are active on social media, and we have engaged and intend
to continue to engage through those platforms to elevate our national marketing presence in direct-to-consumer marketing. Social media
practices in the pharmaceutical, biotechnology and medical device industries are evolving, which creates uncertainty and risk of noncompliance
with regulations applicable to our business. For example, patients may use social media platforms to comment on the effectiveness of,
or adverse experiences with, our product, which could result in regulatory reporting obligations or the need for us to conduct an investigation.
The use of influencers and patient ambassadors to promote Phexxi also may be subject to federal truth-in-advertising laws enforced by
the Federal Trade Commission (FTC), as well as comparable state consumer protection laws, and we are responsible for training those influencers
on the compliant messages they can deliver to consumers about Phexxi. Any actual or perceived non-compliance by our influencers and patient
ambassadors with those requirements could lead to an investigation by the FTC or a comparable state agency or could lead to allegations
of misleading advertising by private plaintiffs. In addition, there is a risk of inappropriate disclosure of sensitive information or
negative or inaccurate posts or comments about us or our product on any social networking website. If any of these events were to occur
or we otherwise fail to comply with any applicable regulations, we could incur liability, face restrictive regulatory actions or incur
other harm to our business such as reputational damage.
We
face competition from other medical device, biotechnology and biopharmaceutical companies and our operating results will suffer if we
are unable to compete effectively.
The
medical device, biotechnology and biopharmaceutical industries, and the women’s health sector, are intensely competitive. Significant
competition among various contraceptive products already exists. Existing products have name recognition, are marketed by companies with
established commercial infrastructures, and are marketed with greater financial, technical and personnel resources than we have. To compete
and gain market share, any new product must demonstrate advantages in efficacy, convenience, tolerability or safety, among other things.
In addition, new products developed by others could emerge as competitors to Phexxi. These products could potentially offer an alternative
form of non-hormonal contraception that is more convenient, is more effective and/or provides protection over longer periods of time
as compared to Phexxi. We also compete with these organizations to recruit management, scientists, and sales and marketing and clinical
development personnel. Any failure to attract and retain such personnel could negatively affect our level of expertise and our ability
to execute our business plan. We also face competition in connection with identifying and engaging in strategic transactions and, should
we choose to advance the clinical development of our product candidates, in establishing clinical trial sites and enrolling subjects
for clinical trials and funding those trials. If we are not able to compete effectively against our current and future competitors, our
business will not grow and our financial condition and operations will suffer.
Our
potential competitors include large, well-established pharmaceutical companies and specialty pharmaceutical companies who have significantly
more resources than Evofem. These companies include Merck & Co., Inc., Allergan PLC, Pfizer Inc., Bayer AG, Johnson & Johnson,
CooperSurgical Inc. and Mylan Inc. Additionally, several generic manufacturers currently market and continue to introduce new generic
contraceptives.
Phexxi
and any other approved products may not gain sufficient market acceptance among physicians, patients or the medical community, thereby
limiting our potential to generate revenue, which will undermine our future growth prospects.
Even
though Phexxi has been approved by the FDA for commercial sale for the prevention of pregnancy, and even if any of our other product
candidates are approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any new product
by physicians, patients and the medical community will depend on a number of factors, including:
| |
● |
demonstrated
evidence of efficacy and safety and potential advantages compared to competing products; |
| |
● |
perceptions
by the medical community, physicians, and patients, regarding the safety and effectiveness of the product and the willingness of
the target patient population to try it and of physicians to prescribe it; |
| |
● |
relative
convenience and ease of administration compared to other products approved for the same indication; |
| |
● |
the
regulatory label requirements for the product, including any potential restrictions on use or precautionary statements; |
| |
● |
sufficient
third-party insurance coverage and adequate reimbursement; |
| |
● |
the
willingness of wholesalers and pharmacies to stock the products; |
| |
● |
the
prevalence and severity of any adverse side effects; |
| |
● |
the
ability to sufficiently educate physicians with respect to the product’s safety and efficacy; and |
| |
● |
availability
of alternative products and the cost-effectiveness of our product relative to competing products. |
If
any approved product that we may license, develop or sell, including Phexxi, does not provide a benefit over currently available options,
that product is unlikely to achieve market acceptance, and we will not generate sufficient revenues to achieve profitability.
The
telehealth market is immature and unpredictable, and if it does not develop, if it develops more slowly than we expect, if it encounters
negative publicity over privacy issues, if it fails to engage sufficient numbers of providers, or if limitations on reimbursement or
new state law regulatory requirements impede our ability to implement our telehealth platform, the growth of our business will be harmed.
We
operate a telehealth platform where women can directly meet with HCPs to determine their eligibility for Phexxi and potentially have
prescriptions written. The telehealth market is relatively new and unproven, and it is uncertain whether it will achieve and sustain
high levels of demand, consumer acceptance and market adoption. Our success will depend to a substantial extent on the willingness of
women to use our telehealth platform. Negative publicity concerning our telehealth solution or the telehealth industry as a whole could
limit market acceptance of the Phexxi telehealth platform. Additionally, telehealth laws are rapidly changing, especially in light of
the COVID-19 pandemic and attendant public health emergency. Many states loosened telehealth restrictions to facilitate remote care,
but these changes are typically by executive order and are intended to be temporary for the duration of the public health emergency.
There is no guarantee that telehealth will be permitted in the same way in the future. Changes by state professional licensing boards
to the standards of care or other requirements governing the practice of telehealth, including imposition of new requirements for prescriptions
from state and federal regulatory bodies, could impact the success of our telehealth solution. Similarly, individual and health care
industry concerns or negative publicity regarding patient confidentiality and privacy in the context of telehealth could limit market
acceptance of our platform. If any of these events occurs, it could have a material adverse effect on our business, financial condition
or results of operations.
The
success of Phexxi will depend on the availability of competitive products and women’s preferences, in addition to the market’s
acceptance of our new form contraception.
The
commercial success of Phexxi will depend upon the contraceptive market as well as market acceptance of Phexxi as a new form of prevention
of pregnancy, a vaginal pH modulator. Risks related to market acceptance include, among other things:
| |
● |
minimum
acceptable contraceptive efficacy rates and the related regulatory label requirements, including any potential restrictions on use
or precautionary statements; |
| |
● |
perceived
safety differences of hormonal and/or non-hormonal contraceptive options; |
| |
● |
changing
women’s preferences; |
| |
● |
the
effect of the Affordable Care Act (ACA) on pharmaceutical coverage, reimbursement and pricing, and the coverage of preventable services
(including contraception under certain conditions); and |
| |
● |
new
generic contraceptive options including the possibility of a future potential generic version of Phexxi. |
For
example, the pregnancy rate for typical use of Phexxi in the FDA-approved label is higher than many other forms of contraceptives, and
we cannot be certain that the associated risk of unintended pregnancy will not deter adoption of Phexxi as a method of pregnancy prevention.
In addition, Phexxi’s label contains a warning related to use by women with a history of recurrent urinary tract infections, which
could limit the willingness of HCPs to prescribe or certain women to use Phexxi. These risks could reduce the market potential for Phexxi
or any future contraceptive product we may seek to develop, and place pressure on our business, financial condition, results of operations
and prospects.
The
commercial success of Phexxi and/or any future approved products will depend in significant measure on the label claims that the FDA
or other regulatory authorities approve for those products.
The
commercial success of Phexxi vaginal gel and/or future approved products, if any, will depend in significant measure upon the prescribing
information and the patient-directed labeling describing the product’s features, benefits and risks.
We
are required to submit all revisions to approved product labeling for Phexxi as part of a supplemental NDA to the FDA for review and
approval. In addition, the FDA must review and approve proposed labeling for any of our product candidates as part of the NDA pre-market
review process. Failure to achieve approval from the FDA or other regulatory authorities of product labeling containing certain types
of information on features or benefits will prevent or substantially limit our advertising and promotion of such features in order to
differentiate our product candidates from those products already existing in the market. This failure would have a material adverse impact
on our business, financial condition, results of operations and prospects.
The
FDA and other regulatory agencies actively enforce laws and regulations prohibiting the promotion of off-label uses for prescription
drugs and medical devices. If we are found or alleged to have improperly promoted our commercial product for off-label uses, we may become
subject to significant liability.
The
FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products such as Phexxi.
In particular, a product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected
in the product’s approved labeling. Promotional labeling for Phexxi, and for any other of our products that may receive marketing
approval, must be submitted to FDA at the time of first use. The agency actively solicits reports from health care professionals about
improper drug manufacturer promotional claims or activities. If we are found to have promoted Phexxi for any off-label use, we may become
subject to significant liability and potentially reputational harm. The federal government has levied large civil and criminal fines
against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has
also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed
or curtailed. If we cannot successfully manage the promotion of Phexxi or any of our product candidates, if approved in the future, to
ensure compliance with these legal and regulatory requirements, we could become subject to significant liability, which would materially
adversely affect our business and financial condition.
We
will need to obtain FDA approval of any proposed product names, and any failure or delay associated with such approval may adversely
affect our business.
Any
name we intend to use for our current or future product candidates will require approval from the FDA regardless of whether we have secured
a formal trademark registration from the USPTO. The FDA typically conducts a review of proposed product names, including an evaluation
of the potential for confusion with other product names. The FDA may also object to a product name if it believes the name inappropriately
implies medical claims or contributes to an overstatement of efficacy. If the FDA objects to any of our proposed product names, we may
be required to adopt alternative names for our product candidates. If we adopt alternative names, we would lose any goodwill or brand
recognition developed for previously used names and marks, as well as the benefit of our existing trademark applications for such product
candidate and may be required to expend significant additional resources in an effort to identify a suitable product name that would
qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. We may be unable
to build a successful brand identity for a new trademark in a timely manner or at all, which would limit our ability to commercialize
the product.
If
we suffer negative publicity concerning the safety or efficacy of Phexxi or our product candidates in development, our reputation and
the commercialization of Phexxi could be harmed and we may be forced to cease development of such product candidates.
If
concerns should arise about the actual or anticipated clinical outcomes regarding the safety or efficacy of any of our current or future
product candidates, such concerns could adversely affect the market’s perception of these candidates. Such concerns could lead
to a decline in investors’ expectations, adverse effects on our results of operations and a decline in the price of our common
stock.
We
rely, and expect to continue to rely, on market research conducted internally and on our behalf to evaluate the potential commercial
acceptance of Phexxi for the prevention of pregnancy, and any other future product candidates.
We
have contracted with and expect to continue to perform market research and to contract with third parties to perform research on our
behalf. These research findings may not be indicative or predictive of actual or overall market acceptance and any future market research
may not be indicative of the acceptance for Phexxi for contraception or any future product candidates we may develop. Moreover, our internal
and external research that have informed our views with respect to our sales and marketing strategy, payer coverage, pricing and reimbursement
with respect to Phexxi may prove to be incorrect. For example, we believe that women that are most likely to use Phexxi as their primary
method of preventing pregnancy are those who are unwilling to use hormone-based contraceptives and are unsatisfied with other commercially
available non-hormonal alternatives. If our market research has overestimated the size of this population or the willingness of these
women to try Phexxi, the commercialization of Phexxi may be less successful than we or others expect.
There
can be no assurance on the accuracy or completeness of certain facts, forecasts and other statistics obtained from various government
publications, market data providers and other independent third-party sources, including industry expert reports, contained in this Registration
Statement or other statements we may make from time to time.
Certain
facts, forecasts and other statistics contained herein and that we may discuss from time to time have been derived from various government
publications, market data providers and other third-party sources. While we have no reason to believe that this information is false
or misleading or that any fact has been omitted that would render this information false or misleading, we cannot guarantee the accuracy
and completeness of this information. While we have taken reasonable care to ensure that these facts, forecasts and other statistics
have been accurately reproduced from their respective sources, these facts, forecasts and other statistics have not been independently
verified by us, our directors, advisers or any other parties and none of us make any representation as to the accuracy or completeness
of such information. Due to possibly flawed or ineffective collection methods or discrepancies between published information and market
practice and other problems, the facts, forecasts and statistics contained herein may be inaccurate or may not be comparable to information
produced by other parties. Therefore, you should give consideration as to how much weight or importance you should attach to or place
on these facts, forecasts or statistics and in all cases, but particularly with respect to market size, this information should not be
unduly relied upon.
The
proportion of the contraceptive market that is made up of generic products continues to increase, making the introduction of a branded
contraceptive difficult and expensive.
The
proportion of the U.S. market that is made up of generic products has been increasing over time. This trend is occurring in the women’s
health segment as well, where many of the most popular oral contraceptive pills brands have experienced genericization. Assuming this
trend continues, it may be more challenging to introduce Phexxi, or any future approved contraceptive product candidate we may develop
as a branded contraceptive, at a price that will maximize our revenue and profits. Also, there may be additional marketing costs to introduce
Phexxi in order to overcome the trend towards generics and to gain access to reimbursement by payers. If we are unable to introduce any
future approved product candidate at a price that is commensurate with that of current branded products, or if we are unable to gain
reimbursement from payers for Phexxi, or if patients are unwilling to pay any price differential between Phexxi and a generic contraceptive
product, our revenues will be limited. We are currently covering the cost of Phexxi for the first month for women with commercial insurance
whose health plans do not reimburse for Phexxi or whose health plans require a co-pay for Phexxi, and we are covering the cost of subsequent
refills of Phexxi at a $25 co-pay for these women if their co-pay is above that amount with a cap of $650 annual benefit to each patient.
However, we cannot be certain that these initiatives will be successful in overcoming general inclinations of physicians and their patients
to avoid branded contraceptives and these initiatives may become prohibitively expensive. If we choose to curtail our co-pay programs,
demand for Phexxi may decrease. In addition, if health care plans do not add Phexxi to their covered formularies within the timelines
we expect or impose more restrictive co-pay than we expect, our costs of providing these incentive programs will increase beyond our
expectations and reduce our product margins and net revenues from sales of Phexxi.
Our
business has been adversely affected and could continue to be materially and adversely affected in the future by the ongoing COVID-19
pandemic.
Any
outbreak or pandemic of a contagious disease, such as COVID-19 and its variants, or other adverse public health developments, could have
a material and adverse effect on our operations, results of operations and financial condition. The COVID-19 pandemic led to the implementation
of various responses, including government-imposed quarantines, travel restrictions and other public health safety measures, as well
as adverse impacts on health care resources, facilities and providers, in California, across the United States and in other countries.
A number of health care systems have had to restructure operations to prioritize caring for COVID-19 patients and limit or cease other
activities. The severe burden on health care systems caused by this pandemic has impaired the ability of physicians to diagnose and treat
patients with non-COVID-19 related conditions, including routine women’s health visits, and impaired the ability of many clinical
research sites to continue existing studies, start new studies, enroll new patients and monitor patients in clinical trials. The COVID-19
pandemic and government measures taken in response have had a significant impact, both direct and indirect, on businesses, commerce and
commercial spending, as significant reductions in business related activities have occurred, unemployment has risen, supply chains have
been disrupted, and certain manufacturing and clinical development activities have been curtailed or suspended. The continued impact
of COVID-19 on our operations or those of our third-party partners and suppliers will depend on future developments, which are highly
uncertain and cannot be predicted with confidence, including the ultimate duration of the pandemic, additional or modified government
actions, the success of ongoing vaccination efforts, the emergence, prevalence and strength of variant strains, actions taken to contain
or treat the disease as well as the continued impact on local, regional, national and international markets, among others.
Our
business has been adversely affected by the COVID-19 pandemic. In response to the pandemic and in accordance with direction from state
and local government authorities, we took precautionary measures in 2020 and 2021 intended to help minimize the risk of the virus to
our employees, including temporarily requiring most employees to work remotely (which in turn increases the threat to our cyber security
and data accessibility, and communication matters) and suspending all non-essential travel worldwide for our employees. We are heavily
reliant on our employees to perform the day to day operation of our business, and to the extent multiple employees are unavailable at
the same time due to an outbreak or to personal illness, our ability to complete these day to day operations may be impaired. Further,
the COVID-19 pandemic has already affected and will likely continue to affect our commercialization activities for Phexxi. For example,
in light of the COVID-19 pandemic, particularly the restrictions on physician interactions, we made the strategic decision to delay the
commercial launch of Phexxi from June 2020 to September 2020. In light of the COVID-19 pandemic, we also made the decision to reduce
our target initial internal sales force and rely more on telehealth for marketing, including the Phexxi telehealth platform. Nevertheless,
the restrictions on in person contact have limited the ability of our sales representatives to meet with HCPs in person and have also
significantly reduced the number of visits by patients to physician offices. These factors may continue to slow the rate of adoption
of Phexxi. The public health response to the COVID-19 pandemic included universal recommendations for social distancing, individual and
household quarantines, and clinic visits for health emergencies only. With respect to our clinical development efforts, the completion
of enrollment in our Phase 3 EVOGUARD clinical trial evaluating EVO100 for the prevention of chlamydia and gonorrhea in women
was delayed due in part due to challenges related to COVID-19 and the Omicron variant. We also believe changes in clinical site operations,
subject behavior and actions including deviations from following the clinical study protocol requirements related to STI acquisition,
detection, and prevention contributed to the outcome of EVOGUARD, which did not achieve its endpoints. As and if COVID-19 and
its variants continue to affect individuals, businesses and industries, economies and markets around the globe, we and our third party
partners and suppliers may experience further effects on our business and results of operations stemming directly or indirectly from
the pandemic, some of which could severely impact our business, results of operations and financial condition.
Risks
Related to the Development of Our Product Candidates
Our
inability to develop our vaginal pH modulator for additional indications could have an adverse effect on our business and our ability
to successfully market Phexxi for the prevention of pregnancy.
We
believe our vaginal pH modulator gel may be useful in certain indications outside of the prevention of pregnancy. In August 2019, we
completed the Phase 2B/3 AMPREVENCE clinical trial to evaluate EVO100 for the prevention of urogenital chlamydia in women and
for the prevention of urogenital gonorrhea in women. AMPREVENCE results demonstrated that the trial met both its primary and secondary
endpoints, with women receiving EVO100 experiencing a relative risk reduction for chlamydia and gonorrhea infection of 50% and 78%, respectively,
compared to women receiving placebo.
On
October 11, 2022 we announced that the completed Phase 3 EVOGUARD clinical trial evaluating EVO100 for the prevention of chlamydia
and gonorrhea did not achieve its endpoints. As a result of this outcome, together with limited financial resources, we discontinued
further investment this clinical program. This trial failure could impede our ability to market Phexxi for the prevention of pregnancy.
Lastly, if we do not obtain regulatory approvals for additional indications for Phexxi, there will likely be a material adverse effect
on our business, results of operations or our financial condition.
Indemnity
claims from lawsuits or damages against our clinical trial sites could cause us to incur substantial liabilities and to limit commercialization
of Phexxi and any other product candidates we may develop.
In
connection with our clinical trials, our third-party investigators and clinical trial sites face inherent risk of liability exposure
from patients enrolled in our clinical trials. We have entered into indemnification agreements with each of our clinical trial sites
obligating us to defend the sites against third-party claims or reimburse the sites should they incur certain costs or liability in connection
with our clinical trials.
We
currently carry product liability insurance with policy limits we believe are customary for similarly situated companies and adequate
to provide us with coverage for foreseeable risks. Although we maintain such insurance, any claim that may be brought against us could
result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or is in excess of the
limits of our insurance coverage.
If
we or our clinical trial sites cannot successfully defend against product liability or other health related claims, we may incur substantial
liabilities. Regardless of merit or eventual outcome, liability claims and/or litigation may result in decreased demand for Phexxi and
any other product candidates we may develop, injury to our reputation, negative media attention and the diversion of our management’s
time and attention from our product development and commercialization efforts to address claim related matters.
The
success of our business is also expected to depend in part upon our ability to identify, license, discover, develop or commercialize
additional product candidates. Failure to identify additional product candidates would have a negative impact on our business and operations.
Although
a substantial amount of our effort will focus on the commercialization of Phexxi for the prevention of pregnancy, the success of our
business is also expected to depend in part upon our ability to identify, license, discover, develop or commercialize additional product
candidates. We are seeking to license, or otherwise obtain, product and technology rights to a variety of products and product candidates
in the field of women’s health, but there can be no assurance we will be able to do so, or do so on favorable terms. There are
risks, uncertainties and costs associated with identifying, licensing and advancing product candidates through successful clinical development.
We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Our research
programs or licensing efforts may fail to yield additional product candidates for clinical development and commercialization for a number
of reasons, including but not limited to the following:
| |
● |
our
research or business development methodology or search criteria and process may be unsuccessful in identifying potential product
candidates; |
| |
● |
we
may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates; |
| |
● |
our
product candidates may not succeed in preclinical or clinical testing; |
| |
● |
our
potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products
unmarketable or unlikely to receive marketing approval; |
| |
● |
competitors
may develop alternatives that render our product candidates obsolete or less attractive; |
| |
● |
product
candidates we develop may be covered by third parties’ patents or other exclusive rights; |
| |
● |
the
market for a product candidate may change during our clinical development program such that a product may become unreasonable to
continue to develop; |
| |
● |
research
and development programs are quite costly, and we may be unable to obtain the financing and resources to initiate, conduct or complete
them; |
| |
● |
a
product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and, |
| |
● |
a
product candidate may not be accepted as safe and effective by patients, the medical community or third-party payers. |
If
any of these events occur, we may be forced to abandon our development efforts for a program or programs, or we may not be able to identify,
license, partner, discover, develop or commercialize additional product candidates, which could have a material adverse effect on our
business, financial condition or results of operations. Moreover, even if we were able to obtain the rights to additional product candidates,
there can be no assurance these candidates will ever be advanced successfully through clinical development.
Clinical
trials are costly, time consuming and inherently risky, and may fail to demonstrate safety and efficacy to the satisfaction of applicable
regulatory authorities.
Clinical
development is expensive, time consuming and involves significant risk. We cannot guarantee any clinical trials will be conducted as
planned or completed on schedule, if at all. In addition, certain of our product candidates have been targeted toward the prevention
of STIs. It may be especially difficult to recruit patients to participate in our clinical trials when doing so may require patients
to refrain from using other methods of infection prevention. A failure of one or more clinical trials can occur at any stage of development.
Events that may prevent successful or timely completion of clinical development include, but are not limited to:
| |
● |
inability
to obtain the funding necessary to initiate or complete any clinical trial; |
| |
● |
inability
to generate satisfactory preclinical, toxicology or other in vivo or in vitro data or to develop diagnostics capable
of supporting the initiation or continuation of clinical trials; |
| |
● |
delays
in reaching agreement on acceptable terms with clinical research organizations (CROs) and clinical trial sites and principal investigators,
the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
| |
● |
delays
or failure in obtaining required institutional review board (IRB) approval at each clinical trial site; |
| |
● |
failure
to obtain or delays in obtaining authorization from regulatory authorities to conduct or begin a clinical trial; |
| |
● |
delays
in recruiting or failure to recruit sufficient eligible patients in our clinical trials; |
| |
● |
failure
to manufacture clinical trial scale quantities of our product candidate; |
| |
● |
failure
by clinical sites, CROs or other third parties to adhere to clinical trial requirements or protocols; |
| |
● |
failure
by clinical sites, CROs or other third parties to perform in accordance with the good clinical practices requirements of the FDA,
applicable laws or applicable foreign regulatory requirements; |
| |
● |
patients
withdrawing from our clinical trials; |
| |
● |
adverse
events or other issues of concern significant enough for an IRB to suspend or terminate a clinical trial or for the FDA, or comparable
foreign regulatory authority, to put an IND or comparable foreign clinical trial application on clinical hold; |
| |
● |
occurrence
of adverse events associated with our product candidates that may make it more difficult to recruit subjects or cause other material
delays in the clinical programs; |
| |
● |
changes
in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
| |
● |
the
cost of clinical trials of our product candidates; |
| |
● |
negative
or inconclusive results from our clinical trials that may result in our deciding, or regulators requiring us, to conduct additional
clinical trials or abandon development programs in other ongoing or planned indications for a product candidate; and |
| |
● |
delays
in reaching agreement on acceptable terms with third-party manufacturers and the time for manufacture of sufficient quantities of
our product candidates for use in clinical trials. |
In
addition to the possible events described above, our clinical trials may also be impacted by matters beyond our control. For example,
conditions and circumstances surrounding the ongoing COVID-19 pandemic delayed enrollment in our Phase 3 EVOGUARD trial and may,
in future, again make it difficult for us and our third-party service providers to recruit, enroll, retain and monitor patients in these
trials, disrupt the necessary logistic and manufacturing activities related to our clinical trials, require us to adjust our trial protocols,
lead to a failure to collect in a timely manner key data necessary to support trial endpoints or otherwise compromise our ability to
collect reliable data, result in delays in related communications and activities with the FDA or other comparable regulatory organizations
and may affect our clinical trials in ways we may not presently predict.
Any
inability to successfully complete clinical development and obtain regulatory approval for one or more of our product candidates could
result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes
to our product candidates, we may need to conduct additional non-clinical studies and/or clinical trials to show the results obtained
from such new formulation or manufacturing process are consistent with previous results obtained. Clinical trial delays could also shorten
any periods during which our products have patent protection and may allow competitors to develop and bring products to market before
we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
Due
in part to our limited financial resources, we may fail to select or capitalize on the most scientifically, clinically or commercially
promising or profitable indications or therapeutic areas for our product candidates and we may be unable to pursue and complete the clinical
trials we would like to pursue and complete.
We
have limited financial and technical resources to determine the indications on which we should focus the development efforts for our
product candidates and any future candidates we may choose to develop. Due to our limited available financial resources, we may be required
to curtail clinical development programs and activities that might otherwise have led to more rapid progress of our product candidates,
or product candidates we may in the future choose to develop, through the regulatory and development processes. We may make incorrect
determinations regarding the indications and clinical trials on which to focus our available resources. The decisions to allocate our
research, management and financial resources towards particular indications may not lead to the development of viable commercial products
and may divert resources from better opportunities. Similarly, our decisions to delay or terminate development programs may also cause
us to miss valuable opportunities.
Risks
Related to Regulatory Approval of Our Product Candidates
We
are required to obtain regulatory approval prior to marketing or commercializing any of our product candidates and we also must obtain
regulatory approval from international authorities should we elect to commercialize Phexxi outside of the United States. To obtain regulatory
approval, we must complete our preclinical studies and clinical trials in compliance with the regulatory approval requirements of the
FDA and any applicable and comparable foreign regulators. If our clinical trials fail to satisfactorily demonstrate the safety and efficacy
of our product candidates to the FDA and other comparable foreign regulators, we may incur additional costs or experience delays in completing,
or ultimately be unable to complete, the development and commercialization of our product candidates.
With
the exception of Phexxi vaginal gel for the prevention of pregnancy, which has been approved by the FDA for U.S. marketing and patient
use, we are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining marketing
approval from the FDA. Comparable foreign regulatory authorities impose similar restrictions, and we do not have marketing approval for
Phexxi in any country outside of the United States except Nigeria, where it is approved and may potentially be launched as Femidence.
We may never receive such approvals, and we may need to complete extensive preclinical development and clinical trials to demonstrate
the safety and efficacy of our product candidates in other populations before we may be able to obtain these approvals.
Any
inability to complete preclinical and clinical development successfully could result in additional costs to us and impair our ability
to generate revenues. Moreover, if (i) we are required to conduct additional clinical trials or other nonclinical testing of our product
candidates beyond the trials and testing we currently contemplate, (ii) we are unable to successfully complete clinical trials of our
product candidates or other testing, (iii) the results of these clinical trials or tests are unfavorable, uncertain or are only modestly
favorable or (iv) there are unacceptable safety concerns associated with our product candidates, we may:
| |
● |
be
delayed in obtaining marketing approval for our product candidates; |
| |
● |
not
obtain marketing approval at all; |
| |
● |
obtain
approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed
warnings; |
| |
● |
be
subject to additional post-marketing testing or other requirements; or |
| |
● |
be
required to remove the product from the market after obtaining marketing approval. |
Even
if we complete the necessary clinical trials for our product candidates, the marketing approval process is expensive, time consuming
and uncertain and may prevent us from obtaining approvals for the commercialization of our product candidates. If we are not able to
obtain, or if there are delays in obtaining, required marketing approvals, we will not be able to commercialize our product candidates,
and our ability to generate revenue will be materially impaired.
To
date, we have not received approval from the FDA or regulatory authorities in other jurisdictions to market any of our product candidates,
with the exception of Phexxi vaginal gel, which is approved by FDA for the prevention of pregnancy and, as Femidence, by the National
Agency for Food and Drug Administration and Control of Nigeria. Despite the experience of our management team in completing successful
regulatory filings for other companies, we have only submitted one NDA to date for Phexxi as a contraceptive product and four regulatory
submissions to foreign regulatory authorities, so we have limited experience in filing and supporting the applications necessary to obtain
marketing approvals for our product candidates. Securing marketing approval requires the submission of extensive preclinical and clinical
data and supporting information to regulatory authorities for each therapeutic indication in the relevant patient population to establish
the product candidate’s safety and effectiveness for that indication. Securing marketing approval also requires the submission
of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities.
Regulatory authorities may determine that our unapproved product candidates or any potential future product candidate is not effective,
is only moderately effective or has undesirable or unintended side effects, toxicities, safety profiles or other characteristics that
preclude us from obtaining marketing approval for the product or that limit or restrict its commercial use.
The
process of obtaining marketing approvals is expensive, may take many years, if approval is obtained at all, and can vary substantially
based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in marketing
approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory
review for each submitted product application, may cause delays in the approval or rejection of an application. Regulatory authorities
have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient
for approval and require additional preclinical studies, clinical trials or other trials. In addition, varying interpretations of the
data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing
approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product
not commercially viable. If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the
commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
Currently,
we are listed on the FDA’s list of companies in arrears for non-payment of its annual fees under PDUFA and as such, any drug application
or supplement submitted by us will be considered incomplete and will not be accepted for consideration until all fees due and payable
are paid.
From
time to time, we may report top-line data from our clinical trials. These top-line data may differ from complete trial results once additional
data are received and evaluated by the FDA or comparable foreign regulatory authorities.
Top-line
data are based on a preliminary analysis of currently available efficacy and safety data, and therefore these results are subject to
change, either by us or the FDA (or comparable foreign regulatory authorities), following a comprehensive review of the more extensive
data we expect to receive when the full data set becomes available. Top-line data are based on important assumptions, estimations, calculations
and information currently available to us. As a result, the top-line results may differ from the full data, or different conclusions
or considerations may qualify these top-line results, once the complete data have been received and fully evaluated. If these initial
data analyses differ from the results of the full data analyses, in a manner not favorable to the development of our product candidates,
our business, financial condition, results of operations, prospects and, ultimately, the value of our common stock could be adversely
affected.
Even
though we have received approval from the FDA in the United States to market Phexxi for the prevention of pregnancy, and, as Femidence,
by the National Agency for Food and Drug Administration and Control of Nigeria, we may fail to receive similar approval in other territories
outside the United States.
To
market a new product outside the United States, we must obtain separate marketing approvals in each jurisdiction and comply with numerous
and varying regulatory requirements of other countries, including clinical trials, commercial sales, pricing manufacture distribution
and safety requirements. The time required to obtain approval in other countries might differ from, and be longer than, that required
to obtain FDA approval. The marketing approval process in other countries may include all the risks associated with obtaining FDA approval
in the United States, as well as other risks. In addition, in many countries outside the United States, a new product must receive pricing
and reimbursement approval prior to commercialization. This can result in substantial delays in these countries. Additionally, the product
labeling requirements outside the United States are different and may be inconsistent with the United States labeling requirements, negatively
affecting our ability to market our products in countries outside the United States.
In
addition, if we are unable to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal
of marketing approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. In such an event, our
ability to market to our full target market will be reduced and our ability to realize the full market potential of Phexxi will be harmed,
which could have a materially adverse effect on our business, financial condition, results of operations and prospects.
The
discontinuation of further investment in the development of Phexxi as a preventative of chlamydia and gonorrhea infection in women could
have a material adverse effect on our ability to generate additional revenues.
The
ability to market the additional use of Phexxi as a preventative measure for chlamydia and gonorrhea infection in women would have given
us the ability to increase our revenues and thus profits from sales for that purpose. After the Phase 3 EVOGUARD clinical trial
did not achieve its endpoints, and due to a lack of financial resources, we discontinued further investment in this program. This change
may have a negative effect on our stock price and our financial condition overall.
If
we are unable to take full advantage of regulatory programs designed to expedite drug development or provide other incentives, our development
programs may be adversely impacted.
There
are a number of incentive programs administered by the FDA and other regulatory bodies to facilitate development of drugs in areas of
unmet medical need. Phexxi may not qualify for or maintain designations under these or other incentive programs under any of the FDA’s
existing or future programs to expedite drug development in areas of unmet medical need. Our inability to fully take advantage of these
incentive programs may require us to run larger trials, incur delays, lose opportunities that may not otherwise be available to us, lose
marketing exclusivity for which we would otherwise be eligible and incur greater expense in the development of our product candidates.
We
have not paid our Fiscal Year 2023 PDUFA Invoice to the FDA and cannot submit any application or supplements to the FDA, and the amount
payable continues to accrue interest and penalties.
We
have not paid our Fiscal Year 2023 PDUFA Invoice for Phexxi to the FDA in the amount of $0.4 million with an original due date of November
7, 2022, and grace period through December 6, 2022. Beyond this date, interest and penalties have begun to be applied retroactively to
the original due date of October 7, 2022. On May 27, 2023, we received a letter from the FDA’s collection agency, and states
that if payment-in-full or an agreement is not established, the FDA will have no other alternative than to refer our account to the Department
of Justice for enforced collection and or other federal agencies for administrative offset. As a result, any drug application or
supplement we submit will be considered incomplete and will not be accepted for consideration for filing until all fees, interest and
penalties are paid.
Risks
Related to Our Post-Marketing Legal and Regulatory Compliance
Even
though we have obtained FDA approval for Phexxi for prevention of pregnancy, we will remain subject to ongoing regulatory requirements.
Even
though Phexxi vaginal gel has been approved by the FDA for the prevention of pregnancy we are and will be subject to ongoing regulatory
requirements with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of
post-marketing clinical trials and submission of safety, efficacy and other post-approval information, including both federal and state
requirements in the United States and requirements of comparable foreign regulatory authorities.
In
addition, manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory
authority requirements, including ensuring quality control and manufacturing procedures conform to cGMP regulations and corresponding
foreign regulatory manufacturing requirements. Accordingly, we and our contract manufacturers will be subject to continual review and
inspections to assess compliance with cGMP and adherence to commitments made in any NDA submission to the FDA or any other type of domestic
or foreign MAA.
Any
regulatory approvals we receive for Phexxi, or for any other product candidates we may seek to develop, may be subject to limitations
on the approved indicated uses for which the product candidate may be marketed or to the conditions of approval, or contain requirements
for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy
of the product candidate. We will be required to report adverse reactions and production problems, if any, to the FDA and comparable
foreign regulatory authorities. Any new legislation addressing drug safety issues could result in delays in product development or commercialization,
or increased costs to assure compliance.
If
a regulatory agency discovers previously unknown problems with Phexxi or a future product, such as adverse events of unanticipated severity
or frequency, or problems with the facility where the product is manufactured, or it disagrees with the promotion, marketing or labeling
of a product, the regulatory agency may impose restrictions on that product or on us, including requiring withdrawal of the product from
the market. If we are unable to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among
other things:
| |
● |
issue
warning letters; |
| |
● |
impose
civil or criminal penalties; |
| |
● |
suspend
or withdraw regulatory approval; |
| |
● |
suspend
any of our ongoing clinical trials; |
| |
● |
refuse
to approve pending applications or supplements to approved applications submitted by us; |
| |
● |
impose
restrictions on our operations, including closing our contract manufacturers’ facilities; or |
| |
● |
require
a product recall. |
Any
government investigation of alleged violations of law would require us to expend significant time and resources in response and could
generate adverse publicity. Any inability to comply with ongoing regulatory requirements may significantly and adversely affect our ability
to develop and commercialize our products and the value of our business and our operating results would be adversely affected.
Developments
after a product reaches the market may adversely affect sales of the product.
Even
though Phexxi has been approved in the United States for the prevention of pregnancy and even assuming any of our other product candidates
were to be approved, certain developments may decrease market demand for our products, including the following:
| |
● |
the
re-review of products that are already marketed; |
| |
● |
new
scientific information and evolution of scientific theories; |
| |
● |
the
recall or loss of marketing approval of products that are already marketed; |
| |
● |
changing
government standards or public expectations regarding safety, efficacy or labeling changes; and |
| |
● |
greater
examination of advertising and promotion. |
In
the past, clinical trials and post-marketing surveillance of certain marketed drugs have raised concerns that have led to recalls, withdrawals
or addition of restrictive labeling of marketed products. If previously unknown side effects are discovered with one of the active ingredients
in, or if there is an increase in negative publicity regarding known side effects related to Phexxi or any of our product candidates
following marketing approval, this could significantly reduce demand for the product or require us to take actions that could negatively
affect sales, including removing the product from the market, restricting its distribution or applying for labeling changes.
Product
liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of Phexxi for the prevention
of pregnancy. If we are unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from,
or beyond the limits of, our insurance coverage, a material liability claim could adversely affect our financial condition.
We
face an inherent risk of product liability exposure in commercializing Phexxi for the prevention of pregnancy and other product candidates
we may seek to develop or commercialize. If serious adverse events or undesirable side effects occur during or following the commercialization
of Phexxi, or during the clinical investigation or post marketing of Phexxi or our other product candidates, the following events could
occur which would materially and adversely affect our business:
| |
● |
regulatory
authorities may require the addition of specific warnings or contraindications to product labeling or the issuance of alerts to physicians,
pharmacies and the general public; |
| |
● |
we
may be required to change the way Phexxi or our other product candidates are administered or to revise the labeling of Phexxi or
our other product candidates; |
| |
● |
we
may be subject to promotional and marketing limitations on Phexxi and our product candidates; |
| |
● |
sales
of Phexxi and our other approved products, if any, may decrease significantly; |
| |
● |
regulatory
authorities may require us to take Phexxi or, should any of our other product candidates be approved, our other approved products
off the market; |
| |
● |
IRBs
may suspend or terminate our clinical trials; |
| |
● |
regulatory
authorities may impose a clinical hold, which could result in substantial delays and adversely impact our ability to continue development
of our product candidates; |
| |
● |
we
may be required to conduct additional clinical trials with more patients or over longer periods of time than anticipated; |
| |
● |
we
may be required to implement risk evaluation and mitigation strategies (REMS), which could result in substantial cost increases and
have a negative impact on our ability to commercialize Phexxi or our other approved products, if any; |
| |
● |
we
may be required to limit the patients who can receive Phexxi or our product candidates; |
| |
● |
we
may be subject to litigation or product liability claims; and |
| |
● |
our
reputation may suffer. |
Any
of these events could prevent us from achieving or maintaining market acceptance of Phexxi or our other product candidates, or could
substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenues
from Phexxi or our other product candidates. Serious adverse events or side effects could require Phexxi to be taken off the market,
may require them to be packaged with safety warnings or may otherwise limit our sales.
Further,
if we cannot successfully defend ourselves against these product liability claims, we may incur substantial liabilities. Regardless of
merit or eventual outcome, liability claims may result in decreased demand for Phexxi or other product candidates we may seek to develop,
injury to our reputation, negative media attention and the diversion of our management’s time and attention from our product development
and commercialization efforts to address claim related matters.
We
will need to maintain liability insurance coverage as we continue to commercialize Phexxi and conduct clinical trials for our product
candidates. This insurance may become increasingly expensive and difficult to procure. In the future, this insurance may not be available
to us at all or may only be available at a very high cost and, if available, may not be adequate to cover all liabilities we may incur.
In addition, while we have increased our liability insurance coverage in connection with the commercialization of Phexxi, we cannot be
certain our coverage limits will be sufficient to cover liability claims we may face. We will also need to increase liability coverage
if any other product candidate we may seek to develop is approved. If we are not able to obtain and maintain insurance coverage at a
reasonable cost or in an amount adequate to satisfy any liability that may arise, our business could be harmed, possibly materially.
If
we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur
costs that could have a material adverse effect on our business, financial condition or results of operations.
Our
research and development activities and our third-party manufacturer’s and suppliers’ activities may involve the controlled
storage, use, and disposal of hazardous materials. We and our manufacturer and supplier, and our potential future manufacturers and suppliers,
are and will be subject to laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials.
In some cases, these hazardous materials and various wastes resulting from their use may be stored at our and our current and potential
future manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause
an interruption of our commercialization efforts, research and development efforts and business operations; environmental damage resulting
in costly clean-up; and liabilities under applicable laws and regulations governing the use, storage, handling, and disposal of these
materials and specified waste products. Although we believe the safety procedures utilized by us and our current third-party manufacturers
for handling and disposing of materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee
this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable
for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail
our use of specified materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex,
change frequently, and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our
future compliance. We do not currently carry biological or hazardous waste insurance coverage.
Risks
Related to Our Intellectual Property
Our
rights to develop and commercialize Phexxi are subject, in part, to the terms and conditions of licenses granted to us by third parties.
The patent protection and patent prosecution of Phexxi is dependent on third parties.
We
are reliant upon licenses to certain patent rights and proprietary technology from third parties that are important or necessary to the
commercialization of Phexxi. For example, the Rush License Agreement includes intellectual property rights to Phexxi. This agreement
requires us, as a condition to the maintenance of our license and other rights, to make milestone and royalty payments and satisfy certain
performance obligations. As of March 31, 2023, there are approximately $0.6 million accrued expenses in the condensed consolidated
balance sheets pursuant to the Rush License Agreement, we have obtained a waiver of any potential claim of breach based on any provisions
requiring us to timely exploit the licensed patent or make minimum royalty payments.
In
addition, with respect to Phexxi, Rush University has the right, in certain instances, to control the defense against any infringement
litigation arising from the manufacture or development (but not the sale) of Phexxi. While the Rush License Agreement requires Rush University
to indemnify us for certain losses arising from these claims, this indemnification may not be sufficient to adequately compensate us
for any related losses or the potential loss of our ability to manufacture and develop Phexxi. In general, the agreements under which
we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may
be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what
we believe to be the scope of our rights to the relevant intellectual property or technology or increase what we believe to be our financial
or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition,
results of operations, and prospects. Moreover, if disputes over intellectual property we have licensed prevent or impair our ability
to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize
the affected product candidate, which could have a material adverse effect on our business, financial conditions, results of operations,
and prospects.
Our
licensors may have relied on third-party consultants or collaborators or on funds from third parties such that our licensors are not
the sole and exclusive owners of the patents we in-license. If other third parties have ownership rights to our in-licensed patents,
they may be able to license such patents to our competitors, and our competitors could market competing products and technology. This
could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
If
we are unable to obtain and maintain patent protection for Phexxi for the prevention of pregnancy, or other proprietary technologies
we may develop, or if the scope of the patent protection we have or will obtain is not sufficiently broad, our competitors could develop
and commercialize products and technology similar or identical to our products and technology, and our ability to successfully commercialize
our product candidates, and other proprietary technologies we may develop may be adversely affected.
Our
success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect
to our products, product candidates and other proprietary technologies we may develop. We seek to protect our proprietary position by
in-licensing intellectual property and filing patent applications in the United States and abroad relating to Phexxi and other proprietary
technologies we may develop. If we or our licensors are unable to obtain or maintain patent protection with respect to Phexxi and other
proprietary technologies we may develop, our business, financial condition, results of operations, and prospects could be materially
harmed.
Changes
in either the patent laws or their interpretation in the United States and other countries may diminish our ability to protect our inventions,
obtain, maintain, and enforce our intellectual property rights and, more generally, could affect the value of our intellectual property
or narrow the scope of our owned and licensed patents. With respect to both in-licensed and owned intellectual property, we cannot predict
whether the patent applications we and our licensors are currently pursuing will issue as patents in any particular jurisdiction or whether
the claims of any issued patents will provide sufficient protection from competitors or other third parties. Our pending and issued patent
claims for Phexxi are not broad, and it is possible that a competitor may seek to make modifications to their product in an effort to
design around our patent claims and avoid infringement. Furthermore, if any such competitor or third party is able to demonstrate bioequivalence
without infringing our patents, then such a competitor or third party would then be able to introduce a competitive generic product onto
the market once any available regulatory exclusivity has expired. The FDA has broad discretion in determining whether a potential competitive
product demonstrates bioequivalence; we are not able to predict the extent to which a competitor or third party might be able to demonstrate
bioequivalence without infringing our patents.
The
patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or
license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible we will be unsuccessful
in our efforts to identify patentable aspects of our research and development output in time to obtain patent protection. Although we
enter into non-disclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of our research
and development output, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers,
consultants, advisors, and other third parties, any of these parties may breach the agreements and disclose such output before a patent
application is filed, thereby jeopardizing our ability to seek patent protection. In addition, our ability to obtain and maintain valid
and enforceable patents depends on whether the differences between our inventions and the prior art allow our inventions to be patentable
over the prior art. Furthermore, publications of discoveries in the scientific literature often lag the actual discoveries, and patent
applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases
not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in any of our owned
or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such
inventions.
The
patent position of biotechnology and biopharmaceutical companies generally is highly uncertain, involves complex legal and factual questions,
and has been the subject of much litigation in recent years. As a result, the issuance, scope, validity, enforceability, and commercial
value of our patent rights are highly uncertain. Our owned or in-licensed pending and future patent applications may not result in patents
being issued which protect Phexxi and other product candidates or proprietary technologies that we may seek to develop or which effectively
prevent others from commercializing competitive technologies and product candidates.
Moreover,
the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted
after issuance. Even if patent applications we license or own currently or in the future issue as patents, they may not issue in a form
that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise
provide us with any competitive advantage. Any patents we own or in-license may be challenged, narrowed, circumvented, or invalidated
by third parties. Consequently, we do not know whether Phexxi and other proprietary technology will be protectable or remain protected
by valid and enforceable patents. Our competitors or other third parties may be able to circumvent our patents by developing similar
or alternative technologies or products in a non-infringing manner which could materially adversely affect our business, financial condition,
results of operations and prospects.
The
issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and our patents may be challenged
in the courts or patent offices in the United States and abroad. We or our licensors may be subject to a third-party pre-issuance submission
of prior art to the USPTO, or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes
review, or interference proceedings or other similar proceedings challenging our owned or licensed patent rights. An adverse determination
in any such submission, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, our owned or in-licensed
patent rights, allow third parties to commercialize generic versions of our products, product candidates and other proprietary technologies
we may develop and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products
without infringing third-party patent rights. Moreover, we, or one of our licensors, may have to participate in interference proceedings
declared by the USPTO to determine priority of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent
office, that challenge our or our licensor’s priority of invention or other features of patentability with respect to our owned
or in-licensed patents and patent applications. Such challenges may result in loss of patent rights, loss of exclusivity, or in patent
claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing
similar or identical technology and products, or limit the duration of the patent protection of our product candidates and other proprietary
technologies we may develop. Such proceedings also may result in substantial cost and require significant time from our scientists and
management, even if the eventual outcome is favorable to us.
In
addition, given the amount of time required for the commercialization, development, testing, and regulatory review of our products and
product candidates, patents protecting such products and product candidates might expire before or shortly after such products or product
candidates are fully commercialized. For the U.S. patent that we licensed from Rush University, three Orders Granting Interim Extension
(OGIEs) were received from the USPTO, extending the expiration of this patent to September 2023. Moving forward, we will be
relying on our directly owned patent formulas and patent application families for patent protection for Phexxi. As a result, our
intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical
to ours. Moreover, some of our owned and in-licensed patents and patent applications may in the future be co-owned with third parties.
If we are unable to obtain an exclusive license to any such third-party co-owners’ interest in such patents or patent applications,
such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market
competing products and technology. In addition, we may need the cooperation of any such co-owners of our patents in order to enforce
such patents against third parties, and such cooperation may not be provided to us. Furthermore, our owned and in-licensed patents may
be subject to a reservation of rights by one or more third parties. Any of the foregoing could have a material adverse effect on our
competitive position, business, financial conditions, results of operations, and prospects.
We
may not be able to protect our intellectual property and proprietary rights throughout the world.
Filing,
prosecuting, and defending patents on our products, product candidates and other proprietary technologies we may develop in all countries
throughout the world would be prohibitively expensive, and the laws of foreign countries may not protect our rights to the same extent
as the laws of the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries
outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions.
Competitors may use our technology in jurisdictions where we have not obtained patent protection to develop their own products and, further,
may export otherwise infringing products to territories where we have patent protection but enforcement is not as strong as that in the
United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective
or sufficient to prevent them from competing.
Many
companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The
legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets,
and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it difficult
for us to stop the infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary
rights generally. In addition, some jurisdictions, such as Europe, Japan, and China, may have a higher standard for patentability than
in the United States, including for example the requirement of claims having literal support in the original patent filing and the limitation
on using supporting data that is not in the original patent filing. Under those heightened patentability requirements, we may not be
able to obtain sufficient patent protection in certain jurisdictions even though the same or similar patent protection can be secured
in United States and other jurisdictions.
Proceedings
to enforce our intellectual property and proprietary rights in foreign jurisdictions could result in substantial costs and divert our
efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly,
could put our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail
in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly,
our efforts to enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial
advantage from the intellectual property we develop or license.
Many
countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition,
many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent
owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors are forced to
grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and
our business, financial condition, results of operations, and prospects may be adversely affected.
Obtaining
and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements
imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic
maintenance fees, renewal fees, annuity fees, and various other government fees on patents and applications will be due to be paid to
the USPTO and various government patent agencies outside of the United States over the lifetime of our owned or licensed patents and
applications. In certain circumstances, we rely on our licensing partners to pay these fees due to U.S. and non-U.S. patent agencies.
The USPTO and various non-United States government agencies require compliance with several procedural, documentary, fee payment, and
other similar provisions during the patent application process. We are also dependent on our licensors to take the necessary action to
comply with these requirements with respect to our licensed intellectual property. In some cases, an inadvertent lapse can be cured by
payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which non-compliance
can result in abandonment or lapse of the patent or patent application, resulting in a partial or complete loss of patent rights in the
relevant jurisdiction. In such an event, potential competitors might be able to enter the market with similar or identical products or
technology, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Changes
in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
Changes
in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding
the prosecution of patent applications and the enforcement or defense of issued patents. Assuming other requirements for patentability
are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside
the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America
Invents Act (the America Invents Act) enacted in September 2011, the United States transitioned to a first inventor to file system in
which, assuming other requirements for patentability are met, the first inventor to file a patent application will be entitled to the
patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a
patent application in the USPTO after March 2013, but before we do could therefore be awarded a patent covering an invention of ours
even if we had made the invention before it was made by such third party. This will require us to be cognizant going forward of the time
from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential
for a period after filing or until issuance, we cannot be certain that we or our licensors were the first to either (i) file any patent
application related to Phexxi and other proprietary technologies we may develop or (ii) invent any of the inventions claimed in our or
our licensor’s patents or patent applications.
The
America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also
may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional
procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes
review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard
in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding
sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first
presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims
that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the
America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed
patent applications and the enforcement or defense of our owned or in-licensed issued patents, all which could have a material adverse
effect on our business, financial condition, results of operations, and prospects.
In
addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly
uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened
the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and
enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws
and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent
portfolio and our ability to protect and enforce our intellectual property in the future.
Issued
patents covering Phexxi and other proprietary technologies we may develop could be found invalid or unenforceable if challenged in court
or before administrative bodies in the United States or abroad.
If
we or one of our licensors initiated legal proceedings against a third party to enforce a patent covering Phexxi or other proprietary
technologies we may develop, the defendant could counterclaim that such patent is invalid or unenforceable. In patent litigation in the
United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could
be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds
for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information
from the USPTO, or made a misleading statement, during prosecution. Third parties may raise claims challenging the validity or enforceability
of our owned or in-licensed patents before administrative bodies in the United States or abroad, even outside the context of litigation.
Such mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings,
and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of,
cancellation of, or amendment to our patents in such a way that they no longer cover Phexxi and other proprietary technologies we may
develop. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question,
for example, we cannot be certain that there is no invalidating prior art, of which we or our licensing partners and the patent examiner
were unaware during prosecution. If a third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose
at least part, and perhaps all, of the patent protection on our product candidates and other proprietary technologies we may develop.
Such a loss of patent protection would have a material adverse impact on our business, financial condition, results of operations, and
prospects.
In April 2023, the Company
received a Paragraph IV certification notice letter (the Padagis Notice Letter) regarding an Abbreviated New Drug Application (ANDA)
submitted to the FDA by Padagis Israel Pharmaceuticals Inc. (Padagis). The ANDA seeks approval from the FDA to commercially manufacture,
use, or sell a generic version of Phexxi® under 21 U.S.C. § 355(j) prior to the expiration of United States Patent
Nos. 10,568,855; 11,337,989; and 11,439,610 listed in the FDA’s Orange Book: Approved Drug Products with Therapeutic Equivalence
Evaluations (collectively the Phexxi Patents). In the Padagis Notice Letter, Padagis claims that the Phexxi Patents are invalid under
various grounds.
On June 1, 2023, the Company
filed a complaint for patent infringement in Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals, et al.,
in the United States District Court for the District of New Jersey. The case was assigned number 2:23-cv-03003. The complaint alleges
that Padagis’ proposed generic version of Phexxi infringes the Phexxi ® Patents. The relief sought by the Company is a declaration
of infringement and an injunction of FDA approval of Padagis’ proposed generic version of Phexxi until expiration of the Phexxi
Patents in 2033. Until the earlier of final judgment or the passage of 30 months from the receipt of the Padagis Notice Letter, the FDA
is prohibited from approving Padagis’ ANDA to market its proposed generic version of Phexxi. The Company also subsequently filed
a substantively identical action in the United States District Court for the District of Delaware, Evofem Biosciences, Inc. et al.
v. Padagis Israel Pharmaceuticals, et al., which was assigned number 1:23-cv-00606-UNA. The Company is not aware of any answer or
counterclaim filed by Padagis in either action against the Company at this time.
If
we do not obtain a Patent Term Extension (PTE) for our products or product candidates, our business may be materially harmed.
One
or more of our owned or in-licensed U.S. patents covers Phexxi for the prevention of pregnancy, and depending upon the timing, duration
and specifics of any FDA marketing approval of any other product candidate we may develop, our patents may be eligible for limited PTE
under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a PTE of up to 5 years as compensation for patent term lost during
the FDA regulatory review process. A PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product
approval, only one patent may be extended, and only those claims covering the approved drug, a method for using it, or a method for manufacturing
it may be extended. Similar patent term restoration provisions to compensate for commercialization delay caused by regulatory review
are also available in certain foreign jurisdictions, such as in Europe under Supplemental Protection Certificate (SPC). Further, if the
FDA determines that the Phexxi does not represent the first permitted commercial marketing or use of the product, or the active ingredients,
we may fail to satisfy applicable requirements which could materially harm us and our operations.
For
the U.S. patent that we licensed from Rush University, three
Orders Granting Interim Extension (OGIEs) were received from the USPTO, extending the expiration of this patent to September 2023.
A third request for interim patent term extension was filed on December 7, 2022. If granted, the expiration of this patent would be extended
to 2024. However, we may not be granted a full five-year PTE for this patent or any similar extension outside the United States, such
as SPC for the European patents, because of, for example, our inability to exercise due diligence during the testing phase or regulatory
review process, our inability to apply within applicable deadlines, our inability to apply prior to expiration of relevant patents, or
if we are otherwise unable to satisfy applicable requirements. Moreover, the applicable time or the scope of patent protection afforded
could be less than our or Rush University’s request. If we or Rush University are unable to obtain PTE, or the term of any such
extension is shorter than what we request, our competitors may obtain approval of competing products following our patent expiration,
and our business, financial condition, results of operations, and prospects could be materially harmed.
The
patent protection and patent prosecution for our product candidates are dependent on third parties, including Rush University.
While
we normally seek to obtain the right to control prosecution, maintenance and enforcement of the patents relating to our products and
product candidates, there may be times, such as with respect to our agreement with Rush University, when the filing and prosecution activities
for patents relating to our products or product candidates are controlled by our licensors or collaboration partners. If any of our current
or future licensing or collaboration partners fail to prosecute, maintain and enforce such patents and patent applications in a manner
consistent with the best interests of our business, including by payment of all applicable fees for patents covering our products or
product candidates, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, our ability
to develop and commercialize Phexxi for the prevention of pregnancy may be adversely affected and we may not be able to prevent competitors
from making, using and selling competing products. In addition, even where we have the right to control patent prosecution of patents
and patent applications we have licensed to and from third parties, we may still be adversely affected or prejudiced by actions or inactions
of our licensees, our licensors and their counsel that took place prior to the date upon which we assumed control over patent prosecution.
If
an event of default continues and remains uncured under our issued and outstanding secured convertible notes issued pursuant to the Baker
Bros. Purchase Agreement, the note holders could take possession of all assets owned by us, including any directly owned intellectual
property.
On
March 7, 2023, Baker Bros. Advisors, LP (the Designated Agent) provided a Notice of Event of Default and Reservation of Rights (the Notice
of Default) relating to the Securities Purchase and Security Agreement dated April 23, 2020, and subsequently amended (SPA), by and among
Evofem, Designated Agent, the Guarantors and Baker Purchasers. The Notice of Default claims that the Company has failed to maintain the
“Required Reserve Amount” as required by Section 2.7 of the Third Amendment to the Securities Purchase Agreement and Section
8.1(e) of the SPA. The Designated Agent claims such failure constitutes an immediate Event of Default pursuant to Section 9.1(e) of the
SPA. The Designated Agent, at the direction of the Baker Purchasers, has accelerated repayment of the outstanding balance payable and
elected its remedies pursuant to Section 5.07(b) of the Securities Purchase Agreement. As a result, approximately $92.7 million,
representing two times the sum of the outstanding balance and all accrued and unpaid interest thereon and all other amounts due under
the SPA and other documents, is due and payable within three business days of receipt of the Notice of Default. We disagree with the
Designated Agent’s claims and have invited the Designated Agent to reconsider and rescinded its Notice of Default and request for
payment, for which no formal request for payment has yet been made. Given our current inability to pay any amounts due under the Baker
Bros. Purchase Agreement or under the convertible notes, the Designated Agent of these note holders has the right to take possession
of all of our assets and/or pursue any available legal remedies against
us. As of the date of this Registration Statement, the Baker Notes remain outstanding, and the Company has sufficient required reserve number
of shares upon the effectuation of the 2023 Reverse Stock Split.
We
may be subject to claims challenging the inventorship of our patents and other intellectual property.
We
or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or
in-licensed patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we or our licensors may
have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our products or
product candidates and other proprietary technologies we may develop. Litigation may be necessary to defend against these and other claims
challenging inventorship or our or our licensor’s ownership of our owned or in-licensed patents, trade secrets or other intellectual
property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual
property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our products, product candidates
and other proprietary technologies we may develop. Even if we are successful in defending against such claims, litigation could result
in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect
on our business, financial condition, results of operations and prospects.
If
we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In
addition to seeking and maintaining patents for Phexxi and other proprietary technologies we may develop, we also rely on trade secrets
and confidentiality agreements to protect our unpatented know-how, technology, and other proprietary information and to maintain our
competitive position. With respect to Phexxi, we consider trade secrets and know-how to be one of our important sources of intellectual
property. Trade secrets and know-how can be difficult to protect. In particular, our trade secrets and know-how in connection with Phexxi
and other proprietary technology we may develop over time may be disseminated within the industry through independent development, the
publication of journal articles describing the methodology, and the movement of personnel with scientific positions in academic and industry.
We
seek to protect these trade secrets and other proprietary technology, in part, by entering into non-disclosure and confidentiality agreements
with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract
manufacturers, consultants, advisors, and other third parties. We also enter into confidentiality and invention or patent assignment
agreements with our employees and consultants. We cannot guarantee we have entered into such agreements with each party that may have
or have had access to our trade secrets or proprietary technology and processes. Despite these efforts, any of these parties may breach
the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies
for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and
time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling
to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other
third party, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade
secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position would be materially
and adversely harmed.
We
may be subject to claims that third parties have an ownership interest in our trade secrets. For example, we may have disputes arise
from conflicting obligations of our employees, consultants or others who are involved in developing our products and product candidates.
Litigation may be necessary to defend against these and other claims challenging ownership of our trade secrets. If we fail in defending
any such claims, in addition to paying monetary damages, it may lose valuable trade secret rights, such as exclusive ownership of, or
right to use, trade secrets that are important to a product candidate and other proprietary technologies we may develop. Such an outcome
could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result
in substantial costs and be a distraction to management and other employees.
We
may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As
is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at other biotechnology
or pharmaceutical companies, including our competitors or potential competitors. Although we have no knowledge of any claims against
us, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary
information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending
against these claims, litigation could result in substantial costs and be a distraction to management. To date, none of our employees
have been subject to such claim.
We
may be at risk that our former employees may wrongfully use or disclose our trade secrets.
In
addition to patent protection, we rely heavily upon know-how and trade secret protection, as well as non-disclosure agreements and invention
assignment agreements with our employees, consultants, and third parties, to protect our confidential and proprietary information, especially
where we do not believe patent protection is appropriate or obtainable. In addition to contractual measures, we try to protect the confidential
nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the
case of misappropriation of a trade secret by an employee, former employee, consultant, former consultant or third party with authorized
access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant
from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide
an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret
can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed
by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade
secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive
position could be harmed.
We
may not be successful in obtaining necessary rights to any product candidate we may develop through acquisitions and in-licenses.
We
currently have rights to intellectual property covering Phexxi. Other pharmaceutical companies and academic institutions may also have
filed or are planning to file patent applications potentially relevant to our business. To avoid infringing these third-party patents,
we may find it necessary or prudent to obtain licenses to such patents from such third-party intellectual property holders. However,
we may be unable to secure such licenses or otherwise acquire or in-license any compositions, methods of use, processes, or other intellectual
property rights from third parties that we identify as necessary for Phexxi and other proprietary technologies we may develop. The licensing
or acquisition of third-party intellectual property rights is a competitive area, and several more established companies may pursue strategies
to license or acquire third-party intellectual property rights we may consider attractive or necessary. These established companies may
have a competitive advantage over us due to their size, capital resources and greater clinical development and commercialization capabilities.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable
to license or acquire third-party intellectual property rights on terms that would allow it to make an appropriate return on our investment
or at all. If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing
intellectual property rights we have, we may have to abandon development or commercialization of the relevant program, product or product
candidate, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
We
may be subject to claims that our employees, consultants, or advisors have wrongfully used or disclosed alleged trade secrets of their
current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Many
of our employees, consultants and advisors are currently or were previously employed at universities or other biotechnology or biopharmaceutical
companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and advisors
do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these individuals
have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s
current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition
to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against
such claims, litigation could result in substantial costs and be a distraction to our management.
In
addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual
property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with
each party who, in fact, conceives or develops intellectual property that it regards as its own. The assignment of intellectual property
rights may not be self-executing, or the assignment agreements may be breached, and we may be forced to bring claims against third parties,
or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. Such claims
could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Third-party
claims of intellectual property infringement, induced intellectual property infringement, misappropriation or other violation against
us or our collaborators may prevent or delay the development and commercialization of our products, product candidates and other proprietary
technologies we may develop.
The
contraceptive market is competitive and dynamic. Due to the significant research and development activities that are taking place by
several companies in this field, including us and our competitors, the intellectual property landscape is in flux, and it may remain
uncertain in the future. There may be significant intellectual property related litigation and proceedings relating to our owned and
in-licensed and other third-party intellectual property and proprietary rights in the future.
Our
commercial success depends in part on our and our collaborators’ ability to avoid infringing, inducing infringement, misappropriating
and otherwise violating the patents and other intellectual property rights of third parties. There is a substantial amount of complex
litigation involving patents and other intellectual property rights in the biotechnology and biopharmaceutical industries, as well as
administrative proceedings for challenging patents, including interference, derivation and reexamination proceedings before the USPTO
or oppositions and other comparable proceedings in foreign jurisdictions. As discussed above, recently, due to changes in U.S. law referred
to as patent reform, new procedures including inter partes review and post-grant review have been implemented. As stated above,
this reform adds uncertainty to the possibility of challenge to our patents in the future.
Numerous
U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which we intend to commercialize
Phexxi and in which we are developing other proprietary technologies. As the biotechnology and biopharmaceutical industries expand and
more patents are issued, the risk increases that our product candidate may give rise to claims of infringement of the patent rights of
others. We cannot assure you that Phexxi and other proprietary technologies we may develop will not infringe existing or future patents
owned by third parties. We may not be aware of patents that have already been issued and that a third party, for example, a competitor
in the fields in which we are commercializing or developing our products or product candidates, might assert are infringed by our current
or future product candidates, including claims to compositions, formulations, methods of manufacture or methods of use or treatment that
cover our product candidates. It is also possible that patents owned by third parties of which we are aware, but which we do not believe
are relevant to our products, product candidates and other proprietary technologies we may develop, could be found to be infringed by
our products or product candidate. In addition, because patent applications can take many years to issue, there may be currently pending
patent applications that may later result in issued patents that our products or product candidate may infringe.
Third
parties may currently have patents or obtain patents in the future and may claim that use of our technology or the manufacture, use or
sale of our product candidates infringes upon these patents. In the event a third party claims we infringed their patents or that we
are otherwise employing their proprietary technology without authorization and initiates litigation against us, even if we believe such
claims are without merit, a court of competent jurisdiction could hold that such patents are valid, enforceable and infringed by our
technology, products or product candidates. In this case, the holders of such patents may be able to block our ability to commercialize
the applicable product candidate or technology unless we obtain a license under the applicable patents, or until such patents expire
or are finally determined to be held invalid or unenforceable. Such a license may not be available on commercially reasonable terms or
at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the
rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. If
we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, we may be unable to commercialize
our products, product candidates or technology or such commercialization efforts may be significantly delayed, which could in turn significantly
harm our business.
Defense
of infringement claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion
of management and other employee resources from our business, and may impact our reputation. In the event of a successful claim of infringement
against us, we may be enjoined from further developing or commercializing our infringing products or technology. In addition, we may
have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses
from third parties, pay royalties and/or redesign our infringing products or technology, which may be impossible or require substantial
time and monetary expenditure. In that event, we would be unable to further develop and commercialize our product, product candidates
or technology, which could harm our business significantly. Further, we cannot predict whether any required license would be available
at all or whether we would be available on commercially reasonable terms. In the event we could not obtain a license, we may be unable
to further develop our product, product candidates and commercialize our product and product candidates, if approved, which could harm
our business significantly. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties
or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual
property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations
such as the commercialization of Phexxi, if, as a result of actual or threatened patent infringement claims, we are unable to enter licenses
on acceptable terms.
Engaging
in litigation defending us against third parties alleging infringement of patent and other intellectual property rights is very expensive,
particularly for a company of our size, and time-consuming. Some of our competitors may be able to sustain the costs of litigation or
administrative proceedings more effectively than we can because of greater financial resources. Patent litigation and other proceedings
may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other
proceedings could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could have a material adverse
effect on our business, financial condition or results of operations.
In
the ordinary course, we have been and again may become involved in lawsuits to protect or enforce our patents and other intellectual
property rights, which could be expensive, time consuming, and unsuccessful.
Competitors
or other third parties may infringe our patents or the patents of our licensing partners. We have and may again be required to defend
against claims of infringement or otherwise engage in legal action to protect our intellectual property. Any commercial success we may
achieve with Phexxi for the prevention of pregnancy may incentivize third parties to challenge or infringe our intellectual property.
In addition, our patents or the patents of our licensing partners also may become involved in inventorship, priority or validity disputes.
To counter or defend against these claims is expensive and time consuming. In an infringement proceeding, a court may decide a patent
owned or in-licensed by us is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the
grounds our owned and in-licensed patents do not cover the technology in question. An adverse result in any litigation proceeding could
put one or more of our owned or in-licensed patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the
substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential
information could be compromised by disclosure during this type of litigation.
Even
if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant
expenses and could distract our personnel from their normal responsibilities. In addition, there could be public announcements of the
results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results
to be negative, it could have a substantial adverse effect on the price of our common stock. These litigation or proceedings could substantially
increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution
activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our
competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater
financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation
of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
Some
intellectual property that we have in-licensed may have been discovered through government funded programs and thus may be subject to
federal regulations such as “march-in” rights, certain reporting requirements and a preference for U.S.-based companies.
Compliance with such regulations may limit our exclusive rights, and limit our ability to contract with non-U.S. manufacturers.
Intellectual
property rights we have licensed or may in the future license are generated through the use of U.S. government funding and are therefore
subject to certain federal regulations. As a result, the U.S. government may have certain rights to intellectual property embodied in
our current product or our current or future product candidates pursuant to the Bayh-Dole Act of 1980 (Bayh-Dole Act) and implementing
regulations. These U.S. government rights in certain inventions developed under a government-funded program include a non-exclusive,
non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has
the right to require us or our licensors to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions
to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action
is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under
federal regulations (also referred to as “march-in rights”). The U.S. government also has the right to take title to these
inventions if we, or the applicable licensor, fail to disclose the invention to the government and fail to file an application to register
the intellectual property within specified time limits. These time limits have recently been changed by regulation, and may change in
the future. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance
with which may require us or the applicable licensor to expend substantial resources. In addition, the U.S. government requires that
any products embodying the subject invention or produced through the use of the subject invention be manufactured substantially in the
United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable
but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture
substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference
for U.S. manufacturers may limit our ability to contract with non-U.S. product manufacturers for products covered by such intellectual
property. To the extent any of our current or future intellectual property is generated through the use of U.S. government funding, the
provisions of the Bayh-Dole Act may similarly apply.
If
our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest
and our business may be adversely affected.
Our
registered or unregistered trademarks or trade names have in the ordinary course of our business been challenged and may again be challenged
by third parties. These trademarks and trade names may also be infringed, circumvented or may not be registered with the USPTO or determined
to be infringing on other marks. During trademark registration proceedings, we may receive rejections of our applications by the USPTO
or in other foreign jurisdictions. Although we are given an opportunity to respond to those rejections, we may be unable to overcome
such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity
to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed
against our trademarks, and our trademarks may not survive such proceedings. Moreover, any name we have proposed to use with our product
or product candidates in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register
it, as a trademark. Similar requirements exist in Europe. The FDA typically conducts a review of proposed product names, including an
evaluation of potential for confusion with other product names. Furthermore, in many countries, owning and maintaining a trademark registration
may not provide an adequate defense against a subsequent infringement claim asserted by the owner of a senior trademark.
We
may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners
or customers in our markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to
ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, we may be subject to
potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate
variations of our registered or unregistered trademarks or trade names or that allege we have infringed on their trademarks and trade
names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be
able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights or to
defend ourselves in suits related to our trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective
and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results
of operations and prospects.
Intellectual
property rights do not necessarily address all potential threats.
The
degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations
and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
| |
● |
others may
be able to make products that are similar to our products or product candidates or utilize similar technology but that are not covered
by the claims of the patents that we license or may own; |
| |
● |
we, or our current or future
licensors or collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application
that we license or may own in the future; |
| |
● |
we, or our current or future
licensors or collaborators, might not have been the first to file patent applications covering certain of our or their inventions; |
| |
● |
others may independently
develop similar or alternative technologies or duplicate any of our technology without infringing our owned or licensed intellectual
property rights; |
| |
● |
it is possible
that our current or future pending owned or licensed patent applications will not lead to issued patents; |
| |
● |
issued patents that we
hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors or other third
parties; |
| |
● |
our competitors or other
third parties might conduct research and development activities in countries where we do not have patent rights and then use the
information learned from such activities to develop competitive products for sale in our major commercial markets; |
| |
● |
we may not develop additional
proprietary technologies that are patentable; |
| |
● |
the patents of others may
harm our business; and |
| |
● |
we may choose not to file
a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual
property. |
Should
any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations, and
prospects.
Risks
Related to Our Reliance on Third Parties
Our
success relies on third-party suppliers and one contract manufacturer. Any failure by these third parties, including their inability
to successfully perform and comply with regulatory requirements, could negatively impact our business and our ability to develop and
market our products or product candidates, and our business could be substantially harmed.
We
have a small number of employees and no internal manufacturing capability. Our management does not expect to manufacture any products
and expects to rely solely on third parties to manufacture our products, including our FDA-approved commercial product Phexxi, and as
such we will be subject to inherent uncertainties related to product safety, availability and security. We currently have only one contract
manufacturer for drug product, DPT Laboratories, Ltd. (DPT), with whom we entered into a supply and manufacturing agreement in November
2019 (the “Manufacturing Agreement”). Pursuant to the Manufacturing Agreement, subject only to a supply failure, we are obligated
to purchase all of our requirements with respect to Phexxi from DPT. We expect to rely on DPT to increase the manufacturing of Phexxi
in amounts needed to support commercialization. If DPT does not perform as agreed or is unable to increase manufacturing of Phexxi as
needed to support commercialization, including as a result of being adversely affected by COVID-19, or terminates our agreement, we will
be required to replace them as our manufacturer, and we may be unable to do so on a timely basis, on similar terms or at all. Furthermore,
we have only a single source of supply for some of the key raw materials and components of Phexxi, and while we believe we would be able
to obtain supplies through alternative sources if needed, alternate sources of supply may not be readily available and alternate sources
of supply may also be affected by COVID-19.
Moreover,
we do not control the manufacturing processes for the production of Phexxi, which must be made in accordance with relevant regulations
including, among other things, quality control, quality assurance, compliance with cGMP and the maintenance of records and documentation.
In the future, it is possible that our suppliers or manufacturers may fail to comply with FDA regulations, the requirements of other
regulatory bodies or our own requirements, any of which would result in suspension or prevention of commercialization and/or manufacturing
of our products or product candidates, including Phexxi; suspension of ongoing research; disqualification of data or other enforcement
actions such as product recall, injunctions, civil penalties or criminal prosecutions against us. Furthermore, we may be unable to replace
any supplier or manufacturer with an alternate supplier or manufacturer on a commercially reasonable or timely basis, or at all.
If
we were to experience an unexpected loss of supply of, or if any supplier or manufacturer were unable to meet our demand for Phexxi or
our product candidates, we could experience delays in research, planned clinical trials and/or commercialization. We might be unable
to find alternative suppliers or manufacturers with FDA approval, of acceptable quality, and that are able to supply products/ingredients
in the appropriate volumes and at an acceptable cost. The long transition periods necessary to switch manufacturers and suppliers would
significantly delay our timelines, including our commercialization timeline, which would materially adversely affect our business, financial
conditions, results of operations and prospects.
In
addition, our reliance on DPT, and potential future third-party manufacturers, exposes us to the following additional risks:
| |
● |
we may be unable
to identify other manufacturers on acceptable terms or at all; |
| |
● |
our third-party manufacturers
might be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical
and commercial needs, if any; |
| |
● |
DPT and potential future
third-party manufacturers may not be able to execute our manufacturing procedures appropriately; |
| |
● |
our future
third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required
to supply our clinical trials or to successfully produce, store and distribute our products; |
| |
● |
manufacturers are subject
to ongoing periodic unannounced inspections by the FDA and corresponding state agencies to ensure strict compliance with cGMPs and
other government regulations and corresponding foreign standards, and we do not have control over third-party manufacturers’
compliance with these regulations and standards; |
| |
● |
we may not own, or may
have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process
for our product or product candidates; and, |
| |
● |
our third-party manufacturers
could breach or terminate their agreements with us. |
Each
of these risks could impact the continued availability of Phexxi or could result in higher costs or deprive us of potential product revenue
and, should we resume research and development activities, could delay our clinical trials, the approval of our product candidates by
the FDA or the commercialization of our product candidates. In addition, we rely on third parties to perform release testing on our products
and product candidates prior to delivery to patients. If these tests are not appropriately conducted and test data are not reliable,
patients could be put at risk of serious harm, which could result in product liability suits.
The
manufacture of medical products is complex and requires significant expertise and capital investment, including the development of advanced
manufacturing techniques and process controls. Manufacturers of medical products often encounter difficulties in production, particularly
in scaling up and validating initial production and absence of contamination. These problems include difficulties with production costs
and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel,
timely availability of raw materials, lot consistency, as well as compliance with strictly enforced federal, state and foreign regulations.
Furthermore, if contaminants are discovered in our supply of our product or product candidates or in the manufacturing facilities, such
manufacturing facilities may need to be closed for an extended period to investigate and remedy the contamination. We cannot be assured
that any stability or other issues relating to the manufacture of our products or product candidates will not occur in the future. Additionally,
our manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable
political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual
obligations, our ability to provide our product candidates to patients in clinical trials would be jeopardized and our ability to distribute
any approved products would be harmed. Any delay or interruption in the supply of clinical trial supplies, could delay the completion
of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require
us to commence new clinical trials at additional expense or terminate clinical trials completely. There is no assurance that our manufacturer
will be successful in establishing a larger-scale commercial manufacturing process for Phexxi or other product candidates that achieves
our objectives for manufacturing capacity and cost of goods. There is no assurance that our manufacturers will be able to manufacture
or continue to manufacture any approved products, including Phexxi, to specifications acceptable to the FDA or other regulatory authorities,
to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential future demand.
Any delay or failure in the production of any approved products would impair our ability to commercialize and obtain revenue from these
products. These circumstances would materially harm our business, results of operations, financial conditions and prospects.
We
have no significant internal distribution capabilities. We intend to engage third-party distributors for distribution of products outside
the United States, if approved, and have engaged additional third-party wholesale distributors for the distribution of Phexxi in the
United States. Our inability to identify, or enter into an agreement with, any such third-party distributor, would likely have a material
adverse effect on our business and operations.
If
we are unable to engage additional wholesale distributors and/or maintain our relationship with our wholesale distributors within the
United States for Phexxi, our domestic commercialization activities may be disrupted. If we are able to identify and enter into a strategic
relationship with one or more third party collaborators for the development of Phexxi outside of the United States, we intend to work
with that third party or third parties to obtain marketing approval for Phexxi in each relevant jurisdiction and to enter into distribution
agreements with such third party or parties for distribution of Phexxi in each relevant jurisdiction outside the United States. We cannot
guarantee that we will be able to enter into any such additional wholesale distribution agreements on commercially reasonable terms,
or at all, or that we will be able to identify any third party collaborators for the development and commercialization of Phexxi outside
the United States or that we will be able to enter into any such distribution agreement with any such third party for the distribution
of Phexxi outside the United States. For our current distribution agreements and for any future distribution agreements we may enter
into, we would be subject to uncertainties related to such distribution services, including the quality of such distribution services.
For example, distributors may not have the capacity to supply sufficient product if demand increases rapidly. Further, we would be dependent
on the distributors to ensure that the distribution process accords with applicable foreign and U.S. regulations, which include, among
other things, compliance with current good documentation practices, the maintenance of certain records, and compliance with other regulations,
including, without limitation, the Foreign Corrupt Practices Act (FCPA) and the Drug Supply Chain Security Act (DSCSA) in the United
States. Failure to comply with these requirements could result in significant remedial action, including enforcement action requiring
distributors to implement physical changes or improvements to their facilities, suspension of distribution or recall product. Additionally,
any failure by us to forecast demand for finished product, including Phexxi, and failure by us to ensure our distributors have appropriate
capacity to distribute such quantities of finished product, could result in an interruption in the supply of certain products and a decline
in sales of that product. If we grant any such third-party distributor the right to manufacture any applicable product, we would also
be subject to the risk factors set forth above with respect to third-party manufacturing of our product as well as the requirement to
have any such additional manufacturer pre-approved by FDA or other relevant regulatory authorities. Further, third-party distributors
may not perform as agreed or may terminate their agreements with us. Any significant problem or disruption that our distributors experience,
including any disruption resulting from the COVID-19 pandemic, could delay or interrupt our sale of products in the applicable jurisdiction
until the applicable distributor cures the problem or until we identify and negotiate an acceptable agreement with an alternative distributor,
if one is available. Due to the global nature of the COVID-19 pandemic, we may be unable to find any alternative distributor. Any failure
or delay in distributing products would likely have a negative impact on our business and operations.
We
rely and intend to rely on third parties for the execution of our development programs for our product candidates and for the delivery
of telehealth services through the Phexxi telehealth platform. Failure of these third parties to provide services of a suitable quality,
in accordance with applicable regulations and within acceptable time frames may cause the delay or failure of our development programs.
We
employ a business model that relies on the outsourcing of certain functions, tests and services to CROs, medical institutions and other
specialist providers, including, without limitation, the conduct, management and monitoring of our ongoing and planned clinical trials.
As a result, we rely on these third parties for, among other things, quality assurance, clinical monitoring, clinical data management
and regulatory expertise. We also intend to engage a CRO for all future clinical trial requirements needed to file for regulatory approvals.
There is no assurance that such organizations or individuals will be able to provide the functions, tests or services as agreed upon,
or to the requisite quality. We will rely on the efforts of these organizations and individuals and could suffer significant delays in
the development of our product or processes should they fail to perform as expected.
There
is also no assurance that these third parties will not make errors in, or simply fail to be effective in, the design, management or retention
of our data or data systems. Any failures by such third parties could lead to a loss of data or data integrity, which in turn could lead
to delays in clinical development and obtaining regulatory approval. Third parties may not pass FDA or other regulatory audits, which
could delay or prohibit regulatory approval. In addition, the cost of such services could significantly increase over time. If these
third parties do not successfully carry out their contractual duties or meet expected deadlines, regulatory approval of our current or
any future product candidates may be delayed, prevented or cost significantly more than expected, all which would have a material adverse
effect on our business, financial conditions, results of operations and prospects.
The
Phexxi telehealth platform is designed to provide physicians with on-demand educational support, and to remove certain barriers to women’s
access to Phexxi by removing the need for an in-office visit. With the Phexxi telehealth platform, women can directly meet with an HCP
to determine their eligibility for a Phexxi prescription and potentially have it written by the HCP, filled, and mailed directly to them
by a third-party pharmacy. These telehealth platform services are not core to our business of developing and commercializing innovative
products to address unmet needs in women’s sexual and reproductive health. These services are also subject to complex federal and
state laws and regulations and professional practice standards, and we do not have the resources to provide these telehealth services
internally. Any pharmacy that fills Phexxi prescriptions will be fully independent from us. We do not control or own or possess any ownership
stake in any pharmacy that we expect may fill prescriptions for Phexxi or in any telehealth service provider. All prescriptions will
be routed through our independent third-party telehealth service providers. If our telehealth service providers fail to perform or fail
to perform in compliance with applicable laws, regulations and standards of care, our business, financial condition, commercial launch
of Phexxi and results of operation would be adversely affected.
If
we are unable to enter into or maintain strategic relationships or collaborations with respect to Phexxi for the prevention of pregnancy
or for our future product candidates, or if we are unable to realize the potential benefits from such collaborations, our business, financial
condition, commercialization prospects and results of operation may be materially adversely affected.
We
do not presently expect to commercialize Phexxi, assuming international marketing approval is obtained, outside of the United States
unless we enter into a strategic relationship or collaboration with a third party. If we are successful in identifying and in-licensing
the rights to additional product candidates, our expected strategy with respect to the development of any such future product candidates
is to supplement internal efforts with third-party collaborations. We face significant competition in seeking appropriate collaborators.
Collaborations are complex and time-consuming arrangements to negotiate and document.
Our
success in entering into a definitive agreement for any collaboration will depend upon, among other things, our assessment of the collaborator’s
resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a
number of factors. Those factors may include the design and outcomes of the clinical trials, the collaborator’s history of regulatory
compliance, the likelihood of approval by regulatory authorities, the potential market for the product, the costs and complexities of
manufacturing and delivering such products to customers, the potential of competing products, the strength of the intellectual property
and industry and market conditions generally. The collaborator may also consider alternative products or technologies for similar indications
that may be available to collaborate on with one of our competitors and whether such collaboration could be more attractive than the
one with us for our products or product candidates.
Any
potential collaboration agreement into which we might enter may call for licensing or cross-licensing of potentially blocking patents,
know-how or other intellectual property. Due to the potential overlap of data, know-how and intellectual property rights, there can be
no assurance that one of our collaborators will not dispute our right to use, license or distribute such data, know-how or other intellectual
property rights, and this may potentially lead to disputes, liability or termination of the collaboration.
We
may also be restricted under existing and future collaboration agreements from entering into agreements on certain terms with other potential
collaborators and may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If that were to occur,
we may have to curtail the development of a particular product, reduce or delay our development program, delay commercialization, reduce
the scope of sales or marketing activities, or increase expenditures and undertake development or commercialization activities at our
own expense. If we elect to fund development or commercialization activities on our own, we will need to obtain additional capital, which
may not be available to us on acceptable terms or at all. Absent sufficient funds, we may not be able to commercialize a product candidate.
If we enter into a collaboration agreement regarding a product or product candidate, we could be subject to, among other things, the
following risks, each of which may materially harm our business, commercialization prospects and financial condition:
| |
● |
we may not
be able to control the amount and timing of resources that the collaborator devotes to the product development program; |
| |
● |
we may experience financial
difficulties and thus not commit sufficient financial resources to the product development program; |
| |
● |
we may be required to relinquish
important rights to the collaborator such as marketing, distribution and intellectual property rights; |
| |
● |
a collaborator could move
forward with a competing product developed either independently or in collaboration with third parties, including our competitors; |
| |
● |
a collaborator could terminate
the agreement either for convenience, if permitted, or for our breach; or |
| |
● |
business combinations or
significant changes in a collaborator’s business strategy may adversely affect our willingness to complete our obligations
under any arrangement. |
As
a result, a collaboration may not result in the successful development or commercialization of our product or product candidates. In
addition, actions taken by a collaborator within its licensed territory, many of which we may not be able to control, could negatively
impact our development or commercialization of the product or product candidate in the United States.
We
enter into various contracts in the normal course of our business in which we indemnify the other party to the contract. In the event
we must perform under these indemnification provisions, it could have a material adverse effect on our business, financial condition
and results of operations.
In
the normal course of business, we periodically enter into or will enter into manufacturing, distribution, wholesale, academic, commercial,
service, collaboration, licensing, consulting and other agreements that contain indemnification provisions. With respect to our academic
and other research agreements, including the Rush License Agreement, we typically indemnify the institution and related parties from
losses arising from claims relating to the products, processes or services made, used, sold or performed pursuant to the agreements for
which we have secured licenses, and from claims arising from our or our sublicensees’ exercise of rights under the agreement. With
respect to collaboration agreements, we may have to indemnify our collaborators from any third-party product liability claims that could
result from the production, use or consumption of the product, as well as for alleged infringements of any patent or other intellectual
property right owned by a third party. With respect to consultants, we indemnify them from claims arising from performance of their services
in accordance with legal and contractual requirements.
If
our obligations under an indemnification provision exceed applicable insurance coverage or if we were denied insurance coverage, our
business, financial condition and results of operations could be adversely affected. Similarly, if we are relying on a collaborator to
indemnify us and the collaborator is denied insurance coverage or the indemnification obligation exceeds the applicable insurance coverage,
and if the collaborator does not have other assets available to indemnify us, our business, financial condition and results of operations
could be adversely affected.
Risks
Related to Our Commercialization of Health Care Products
Phexxi
and any other approved product may face follow-on competition sooner than anticipated.
Although
Phexxi vaginal gel is FDA-approved for commercialization in the United States, it and any of our product candidates that may achieve
regulatory approval in the future may face competition from generic products earlier or more aggressively than anticipated, depending
upon how well such approved products perform in the United States prescription drug market. In addition to creating the 505(b)(2) NDA
pathway, the Hatch-Waxman Amendments to the Federal Food, Drug, and Cosmetic Act (FDCA) authorized the FDA to approve generic drugs that
are the same as drugs previously approved for marketing under the NDA provisions of the statute pursuant to an Abbreviated New Drug Application
(ANDA). An ANDA relies on the preclinical and clinical testing conducted for a previously approved reference listed drug (RLD) and must
demonstrate to the FDA that the generic drug product is identical to the RLD with respect to the active ingredients, the route of administration,
the dosage form, and the strength of the drug and also that it is “bioequivalent” to the RLD. The FDA is prohibited by statute
from approving an ANDA when certain marketing or data exclusivity protections apply to the RLD. If any such competitor or third party
is able to demonstrate bioequivalence without infringing our patents, then this competitor or third party may then be able to introduce
a competing generic product onto the market.
Phexxi
is indicated for the prevention of pregnancy and was granted three (3) years of data exclusivity that expired on May 22,
2023, and it has been designated as an RLD by the FDA. As such, the three-year exclusivity period should block FDA from approving either
a subsequent ANDA or 505(b)(2) NDA that relies in whole or in part on our protected clinical data. We cannot predict the interest of
potential follow-on competitors in the future Phexxi market, whether someone will attempt to invalidate our period of exclusivity or
otherwise force the FDA to take other actions, or how quickly others may seek to come to market with competing products after the three-year
data exclusivity period ends. Future product candidates may also receive marketing exclusivity under the FDCA after approval that may
similarly be subject to challenge or uncertainty.
If
the FDA approves generic versions of our products, it could negatively impact our future revenue, profitability and cash flows and substantially
limit our ability to obtain a return on our investments in those product candidates.
Changes
in health care laws and regulations may eliminate current requirements for health insurance plans to cover and reimburse FDA-cleared
or FDA-approved contraceptive products without cost sharing, which could reduce demand for products such as Phexxi. Our management expects
our success will be dependent on the willingness or ability of patients to pay out-of-pocket for Phexxi should they not be able to obtain
third-party reimbursement or should such reimbursement be limited.
We
cannot be certain that third-party reimbursement will remain available for Phexxi vaginal gel for the prevention of pregnancy, or if
reimbursement is available, that the amount of any such reimbursement would not change. We provide a financial assistance program for
Phexxi patients to offset any co-pay or patient out of pocket costs, but we do not know if this program will be successful in increasing
market acceptance or that such program will not prove to be prohibitively costly. Demand for Phexxi may decrease if we elect to discontinue
our co-pay programs. The ACA and subsequent regulations enacted by the U.S. Department of Health and Human Services (DHHS) require, under
certain conditions, health plans to provide coverage for women’s preventive care, including all forms of FDA-cleared or FDA-approved
contraception, without imposing any cost sharing on the plan beneficiary. These regulations ensure that women who wish to use an approved
form of contraception may request it from their doctors and their health insurance plan must cover all costs associated with such products,
under certain conditions. In January 2022, the DHHS, Department of Labor, and Treasury Department jointly issued guidance on implementation
of this ACA mandate, among other things. The recently issued federal guidance makes clear that all FDA-approved or cleared contraceptive
products that are determined by an individual’s medical provider to be medically appropriate for such individual must be covered
without-cost sharing, regardless of whether the product is specifically identified in the FDA’s Birth Control Guide.
However,
certain members of Congress and other stakeholders may attempt to repeal or repeal and replace the ACA and corresponding regulations,
as more fully described below, which could eliminate the requirement for health plans to cover women’s preventive care without
cost sharing. Even if the ACA is not repealed, the DHHS regulations to specifically enforce the preventive health coverage mandate could
be repealed or modified; for example, the Trump administration in 2017 altered the mandate to allow certain employers and insurers to
opt-out of birth control coverage for religious or moral reasons, which was partially upheld by the Supreme Court in July 2020. The DHHS,
Department of Labor, and Treasury Department are expected to initiate rulemaking in 2022 that would amend existing regulations to account
for recent litigation. We cannot predict the timing or impact of any future rulemaking or changes in the law. Any repeal or elimination
of the preventive care coverage rules would mean that women seeking to use prescribed forms of contraceptives may have to pay some portion
of the cost for such products out-of-pocket, which could deter some women from using prescription contraceptive products, such as Phexxi,
at all. We expect that health care reform measures that may be adopted in the future may result in more rigorous coverage criteria and
lower reimbursement, and in additional downward pressure on the price that may be charged for Phexxi or any of our product candidates,
if approved. Even if we obtain coverage for any approved products, the resulting reimbursement payment rates might not be adequate or
may require a co-pay that patients find unacceptably high. Patients are unlikely to use any products we may market unless coverage is
provided and reimbursement is adequate to cover a significant portion of the cost of those products. As a result, we expect that our
success, to some degree, will be dependent on the willingness of patients to pay out-of-pocket for Phexxi in the event that their third-party
payer either does not cover and reimburse Phexxi or requires payment of a portion of Phexxi by the patient, thus increasing the patient’s
overall cost to use Phexxi. This could reduce market demand for Phexxi or any future product candidates we may seek to develop, if and
when they receive FDA approval, which would have a material adverse effect on our business, financial conditions, and prospects.
We
may also experience pressure from payers as well as state and federal government authorities concerning certain promotional approaches
that we may implement such as our co-pay programs. Certain state and federal enforcement authorities and members of Congress have initiated
inquiries about co-pay programs. Some state legislatures have been considering proposals that would restrict or ban co-pay coupons. For
example, legislation was recently signed into law in California that would limit the use of co-pay coupons in cases where a lower cost
generic drug is available and if individual ingredients in combination therapies are available over the counter at a lower cost. It is
possible that similar legislation could be proposed and enacted in additional states. If we are unsuccessful with or discontinue our
co-pay programs, or we are unable to secure adequate coverage from third-party payers, we may experience financial pressure which would
have a material adverse effect on our business and make it difficult to commercialize successfully.
Despite
FDA-approval for Phexxi and even if we are successful in obtaining regulatory approval to market other product candidates in the United
States, revenues may be adversely affected if Phexxi or any other the product does not obtain coverage and adequate reimbursement from
third-party payers in the United States.
Market
acceptance and sales of Phexxi vaginal gel or any other product candidates that we may seek to commercialize will depend in part on the
extent to which reimbursement for these products will be available from third-party payers, including government health administration
authorities, managed care organizations and private health insurers. Third-party payers decide which therapies they will pay for and
establish reimbursement levels. Third-party payers in the United States often rely upon Medicare coverage policy and payment limitations
in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement
to be provided for any product or product candidates that we develop will be made on a payer-by-payer basis. One payer’s determination
to provide coverage for a drug does not assure that other payers will also provide coverage and adequate reimbursement for the drug.
Additionally, a third-party payer’s decision to provide coverage for a therapy does not imply that an adequate reimbursement rate
will be approved.
Third-party
payers are increasingly challenging the prices charged for pharmaceutical and medical device products, including Phexxi. The U.S. government
and other third-party payers are increasingly limiting both coverage and the level of reimbursement for new drugs and medical devices,
in addition to questioning their safety and efficacy. Coverage decisions can depend upon clinical and economic standards that disfavor
new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available.
We may incur significant costs to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness
of our future products, in addition to the costs required to obtain the necessary FDA marketing approvals. Third-party payer coverage
may not be available to patients for Phexxi or any future product we may seek to commercialize. If third-party payers do not provide
coverage and adequate reimbursement for Phexxi or our other product candidates, if approved, HCPs may not prescribe them or patients
may ask their HCPs to prescribe competing products with more favorable reimbursement.
Managed
care organizations and other private insurers frequently adopt their own payment or reimbursement reductions. Consolidation among managed
care organizations has increased the negotiating power of these entities. Third-party payers increasingly employ formularies to control
costs by negotiating discounted prices in exchange for formulary inclusion. Failure to obtain timely or adequate pricing or formulary
placement for Phexxi or any future product we may seek to commercialize, or obtaining such pricing or placement at unfavorable pricing
levels, could materially adversely affect our business, financial conditions, results of operations and prospects.
The
pharmaceutical and medical device industries are highly regulated and subject to various fraud and abuse, data privacy, transparency,
and other health care laws, including, without limitation, the U.S. Federal Anti-Kickback Statute, the U.S. Federal False Claims Act
and the FCPA.
HCPs
and third-party payers play a primary role in the recommendation and prescription of drug products and medical devices that are granted
marketing approval. Our current and future arrangements with health care professionals, principal investigators, consultants, third-party
payers, customers and other organizations may expose us to broadly applicable fraud and abuse and other health care laws and regulations
in the United States. These regulations are complex, and even minor irregularities can potentially give rise to claims that a statute
or prohibition has been violated. The laws that may affect our ability to operate include, among others:
| |
● |
the federal
Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering
or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service
reimbursable under a federal health care program, such as the Medicare and Medicaid programs; |
| |
|
|
| |
● |
federal civil and criminal
false claims laws, including the False Claims Act, which can be enforced by private individuals through civil whistleblower or qui
tams actions, and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting,
or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payers that are false or fraudulent; |
| |
|
|
| |
● |
the Health Insurance Portability
and Accountability Act (HIPAA) which, among other things, created new federal criminal statutes that prohibit executing a scheme
to defraud any health care benefit program and making false statements relating to health care matters; |
| |
|
|
| |
● |
HIPAA, as amended by the
Health Information Technology for Economic and Clinical Health Act (HITECH), and its implementing regulations, which imposes certain
requirements on certain covered HCPs, health plans, and health care clearinghouses as well as their respective business associates
that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to
the privacy, security, and transmission of individually identifiable health information; |
| |
|
|
| |
● |
the Physician Payments
Sunshine Act, enacted as part of the ACA, which requires manufacturers of drugs, devices, biologics, and medical supplies to report
annually to the Centers for Medicare & Medicaid Services (CMS) information related to payments and other transfers of value to
physicians, as defined by such law, teaching hospitals, and certain advanced non-physician health care practitioners and ownership
and investment interests held by physicians and their immediate family members; and, |
| |
|
|
| |
● |
foreign and state law equivalents
of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by
any third-party payer, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical
industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or
otherwise restrict payments that may be made to HCPs and other potential referral sources; state laws that require product manufacturers
to report information related to payments and other transfers of value to physicians and other HCPs or marketing expenditures; state
and local laws that require the registration of pharmaceutical sales representatives; and state and foreign laws governing the privacy
and security of health information in certain circumstances, many of which differ from each other in significant ways and which may
conflict, thus complicating compliance efforts. |
The
scope and enforcement of these laws and regulations is uncertain and subject to rapid change. Notably, in November 2020, DHHS finalized
significant changes to the regulations implementing the Anti-Kickback Statute, as well as the civil monetary penalty rules regarding
beneficiary inducements, with the goal of offering the health care industry more flexibility and reducing the regulatory burden associated
with those fraud and abuse laws, particularly with respect to value-based arrangements among industry participants. Regulatory authorities
might challenge our current or future activities under these laws. Any such challenge could have a material adverse effect on our reputation,
business, results of operations and financial condition. These risks may be increased where there are evolving interpretations of applicable
regulatory requirements, such as those applicable to manufacturer co-pay programs. Pharmaceutical manufacturer co-pay programs, including
pharmaceutical manufacturer donations to patient assistance programs offered by charitable foundations, are the subject of ongoing litigation,
enforcement actions and settlements (involving other manufacturers and to which we are not a party) and evolving interpretations of applicable
regulatory requirements and certain state laws, and any change in the regulatory or enforcement environment regarding such programs could
impact our ability to offer such programs. In addition, efforts to ensure that our business arrangements with third parties will comply
with these laws will involve substantial costs. Any investigation of us or the third parties with whom we contract, regardless of the
outcome, would be costly and time consuming. If our operations are found to be in violation of any of these laws or any other governmental
regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation,
damages, monetary fines, imprisonment, disgorgement of profits, possible exclusion and debarment from participation in Medicare, Medicaid
and other federal health care programs, debarment under the FDCA, additional reporting or oversight obligations if we become subject
to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law, contractual damages, reputational
harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
Health
care legislative reform measures may have a negative impact on our business and results of operations.
In
the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and
proposed changes regarding the health care system that could prevent or delay marketing approval of product candidates, restrict or regulate
post-approval activities, and affect our ability to profitably sell any product or product candidates for which we obtain marketing approval.
Among
policy makers and payers in the United States and elsewhere, there is significant interest in promoting changes in health care systems
with the stated goals of containing health care costs, improving quality and/or expanding access. In the United States, the pharmaceutical
industry has been a focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, Congress
passed the ACA, which substantially changed the way health care is financed by both the government and private insurers, and significantly
impacts the United States pharmaceutical industry. As another example, the 2021 Consolidated Appropriations Act signed into law on December
27, 2020 incorporated extensive health care provisions and amendments to existing laws, including a requirement that all manufacturers
of drug products covered under Medicare Part B report the product’s Average Sales Price (ASP) to DHHS beginning on January 1, 2022,
subject to enforcement via civil money penalties.
There
remain judicial and Congressional challenges to certain aspects of the ACA, and as a result certain sections of the ACA have not been
fully implemented or effectively repealed. However, following several years of litigation in the federal courts, in June 2021, the U.S.
Supreme Court upheld the ACA when it dismissed a legal challenge to the ACA’s constitutionality. Further legislative and regulatory
changes under the ACA remain possible, although the new federal administration under President Biden has signaled that it plans to build
on the ACA and expand the number of people who are eligible for health insurance subsidies under it. It is unknown what form any such
changes or any law would take, and how or whether it may affect the biopharmaceutical industry as a whole or our business in the future.
We expect that changes or additions to the ACA, the Medicare and Medicaid programs, such as changes allowing the federal government to
directly negotiate drug prices, and changes stemming from other health care reform measures, especially with regard to health care access,
financing or other legislation in individual states, could have a material adverse effect on the health care industry in the U.S.
Additionally,
the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost
employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminated the health insurer tax. Further,
the Bipartisan Budget Act of 2018, among other things, amended the ACA, effective January 1, 2019, to close the coverage gap in most
Medicare drug plans, commonly referred to as the “donut hole”. In addition, CMS published a final rule that would give states
greater flexibility, effective January 1, 2020, in setting benchmarks for insurers in the individual and small group marketplaces, which
may have the effect of relaxing the essential health benefits required under the ACA for plans sold through such marketplaces.
The
uncertainty around the future of the ACA, and in particular the impact to reimbursement levels, may lead to uncertainty or delay in the
purchasing decisions of our customers, which may in turn negatively impact our product sales. If there are not adequate reimbursement
levels, our business and results of operations could be adversely affected.
In
addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions
to Medicare payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and will
remain in effect through 2030 unless additional Congressional action is taken. However, the Medicare sequester reductions under the Budget
Control Act of 2011 will be suspended from May 1, 2020 through December 31, 2020 due to the COVID-19 pandemic, pursuant to provisions
of the CARES Act which also extended the sequester by one year, through 2030, in order to offset the added expense of the 2020 cancellation.
The suspension was subsequently extended through March 31, 2022, with a reduction of the suspension to 1% sequester through June 30,
2022.
In
addition, in 2013, the Drug Supply Chain Security Act (DSCSA) enacted imposed obligations on manufacturers of pharmaceutical products
related to product tracking and tracing. On December 20, 2019, President Trump signed the Further Consolidated Appropriations Act for
2020 into law (P.L. 116-94) that includes a piece of bipartisan legislation called the CREATES Act. The CREATES Act aims to address the
concern articulated by both the FDA and others in the industry that some brand manufacturers have improperly restricted the distribution
of their products, including by invoking the existence of a REMS for certain products, to deny generic and biosimilar product developers
access to samples of brand products. The CREATES Act establishes a private cause of action that permits a generic or biosimilar product
developer to sue the brand manufacturer to compel it to furnish the necessary samples on “commercially reasonable, market-based
terms.” Whether and how generic and biosimilar product developments will use this new pathway, as well as the likely outcome of
any legal challenges to provisions of the CREATES Act, remain highly uncertain and its potential effects on our future commercial products
are unknown. Other legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional
activities for pharmaceutical products. We are unsure whether additional legislative changes will be enacted, or whether the current
regulations, guidance or interpretations will be changed, or whether such changes will have any impact on our business.
Additionally,
there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices considering the rising cost
of prescription drugs and biologics. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal
and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing
and manufacturer patient programs, and reform government program reimbursement methodologies for products. For example, state legislatures
are increasingly passing legislation and implementing regulations designed to control pharmaceutical pricing, including price or patient
reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures,
and, in some cases, designed to encourage importation from other countries and bulk purchasing. In December 2020, the U.S. Supreme Court
unanimously held that federal law does not preempt the states’ ability to regulate PBMs or other members of the health care and
pharmaceutical supply chain, an important decision that may lead to further and more aggressive efforts by states in this area.
At
the federal level, DHHS has solicited feedback on various measures intended to lower drug prices and reduce the out of pocket costs of
drugs and has implemented others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage
plans the option to use step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that
was effective January 1, 2019. In addition, in 2020, the FDA finalized a rulemaking to establish a system whereby state governmental
entities could lawfully import and distribute prescription drugs sourced from Canada. The Biden Administration, which assumed control
of the Executive Branch on January 20, 2021, has also indicated that lowering prescription drug prices is a priority. For example, in
July 2021, President Biden issued a sweeping executive order on promoting competition in the American economy that includes several mandates
pertaining to the pharmaceutical and health care insurance industries. Among other things, the executive order directs the FDA to work
towards implementing a system for importing drugs from Canada (following on the Trump administration notice-and-comment rulemaking on
Canadian drug importation finalized in October 2020). The Biden order also called on DHHS to release a comprehensive plan to combat high
prescription drug prices, and it includes several directives regarding the Federal Trade Commission’s oversight of potentially
anticompetitive practices within the pharmaceutical industry. The drug pricing plan released by DHHS in September 2021 in response to
the executive order makes clear that the Biden Administration supports aggressive action to address rising drug prices, including allowing
DHHS to negotiate the cost of Medicare Part B and D drugs, but such significant changes will require either new legislation to be passed
by Congress or time-consuming administrative actions. The implementation of cost containment measures or other health care reforms may
prevent us from being able to generate revenue, attain profitability, or commercialize our products.
Current
and future health care legislation could have a significant impact on our business. There is uncertainty with respect to the impact these
changes, if any, may have, and any changes likely will take time to unfold. In addition, it is possible that additional governmental
action is taken to address the COVID-19 pandemic. Any additional federal or state health care reform measures could limit the amounts
that third-party payers will pay for health care products and services, and, in turn, could significantly reduce the projected value
of certain development projects and reduce our profitability.
We
may be subject to numerous and varying privacy and security laws, and our failure to comply could result in penalties and reputational
damage.
We
and our third-party service providers are subject to laws and regulations covering data privacy and the protection of personal information
including health information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there
has been an increasing focus on privacy and data protection issues which may affect our business. In the United States, we and our third-party
service providers may be subject to state security breach notification laws, state health information privacy laws and federal and state
consumer protections laws which impose requirements for the collection, use, disclosure and transmission of personal information. These
laws overlap and often conflict and each of these laws are subject to varying interpretations by courts and government agencies, creating
complex compliance issues for us and our third-party service providers. In particular, our Phexxi telehealth platform and our online,
digital and media marketing strategies are required to comply with these laws and regulations. If we fail to comply with applicable laws
and regulations, we could be subject to penalties or sanctions, including criminal penalties if we knowingly obtain information that
is protected by HIPAA (protected health information) from a covered entity or business associate in a manner that is not authorized or
permitted by HIPAA or for aiding and abetting a violation of HIPAA.
The
regulatory environment surrounding information security, data collection, and privacy is increasingly demanding. We are subject to numerous
U.S. federal and state laws and regulations governing the protection of health, personal information, and financial information of our
customers, clinical subjects, clinical investigators, employees, and vendors/business contacts. For example, California has implemented
the California Confidentiality of Medical Information Act that imposes restrictive requirements regulating the use and disclosure of
health information and other personally identifiable information, and California has recently adopted the CCPA, which went into effect
in January of 2020. The CCPA mirrors a number of the key provisions of the EU General Data Protection Regulation (GDPR) described below.
The CCPA establishes a new privacy framework for covered businesses by creating an expanded definition of personal information, establishing
new data privacy rights for consumers in the State of California, imposing special rules on the collection of consumer data from minors,
and creating a new and potentially severe statutory damages framework for violations of the CCPA and for businesses that fail to implement
reasonable security procedures and practices to prevent data breaches. Additionally, a new privacy law, the California Privacy Rights
Act (CPRA), was a ballot measure approved by California voters in the election on November 3, 2020, and certain provisions are effective
as of January 1, 2022 with full effectiveness as of January 1, 2023. The CPRA modifies and expands the CCPA significantly, and among
other things, creates the California Privacy Protection Agency with full administrative power, authority and jurisdiction to implement
and enforce CCPA. CPRA transferred rulemaking authority from the California attorney General to the California Privacy Protection Agency
effective July 1, 2021 with final CPRA regulations due by July 1, 2022. CPRA enforcement will begin July 1, 2023. The CCPA creates the
potential for further uncertainty, additional costs and expenses in our efforts to comply with California privacy requirements and additional
potential for harm and liability for failure to comply. Virginia and Colorado enacted similar data protection laws in 2021, and other
U.S. states have proposals under consideration, increasing the regulatory compliance risk.
Numerous
other countries have, or are developing, laws governing the collection, use and transmission of personal information as well. EU member
states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations.
On
May 25, 2018, the GDPR went into effect, implementing a broad data protection framework that expanded the scope of EU data protection
law, including to non-EU entities that process, or control the processing of, personal data relating to individuals located in the EU,
including clinical trial data. The GDPR sets out a number of requirements that must be complied with when handling the personal data
of EU based data subjects, including: providing expanded disclosures about how their personal data will be used; higher standards for
organizations to demonstrate that they have obtained valid consent or have another legal basis in place to justify their data processing
activities; the obligation to appoint data protection officers in certain circumstances; new rights for individuals to be “forgotten”
and rights to data portability, as well as enhanced current rights (e.g. access requests); the principal of accountability and demonstrating
compliance through policies, procedures, training and audit; and a new mandatory data breach regime. In particular, medical or health
data, genetic data and biometric data where the latter is used to uniquely identify an individual are all classified as “special
category” data under the GDPR and afford greater protection and require additional compliance obligations. Further, EU member states
have a broad right to impose additional conditions—including restrictions—on these data categories. This is because the GDPR
allows EU member states to derogate from the requirements of the GDPR mainly in regard to specific processing situations (including special
category data and processing for scientific or statistical purposes). As the EU states continue to reframe their national legislation
to harmonize with the GDPR, we will need to monitor compliance with all relevant EU member states’ laws and regulations, including
where permitted derogation from the GDPR are introduced.
We
will also be subject to evolving EU laws on data export if we transfer data outside the EU to ourselves or third parties. The GDPR only
permits exports of data outside the EU where there is a suitable data transfer solution in place to safeguard personal data (e.g. the
EU Commission approved Standard Contractual Clauses). On July 16, 2020, the Court of Justice of the EU (CJEU) issued a landmark opinion
in the case Maximilian Schrems vs. Facebook (Case C-311/18) (Schrems II). This decision calls into question certain data transfer mechanisms
as between the EU member states and the US. The CJEU is the highest court in Europe and the Schrems II decision heightens the burden
on data importers to assess U.S. national security laws on their business future actions of EU data protection authorities are difficult
to predict at the early date. Consequently, there is some risk of any data transfers from the EU being halted. If we have to rely on
third parties to carry out services for us, including processing personal data on our behalf, we are required under GDPR to enter into
contractual arrangements to help ensure that these third parties only process such data according to our instructions and have sufficient
security measures in place. Any security breach or non-compliance with our contractual terms or breach of applicable law by such third
parties could result in enforcement actions, litigation, fines and penalties or adverse publicity and could cause customers to lose trust
in us, which would have an adverse impact on our reputation and business. Any contractual arrangements requiring the processing of personal
data from the EU to us in the United States will require greater scrutiny and assessments as required under Schrems II and may have an
adverse impact on cross-border transfers of personal data or increase costs of compliance. The GDPR provides an enforcement authority
to impose large penalties for noncompliance, including the potential for fines of up to €20 million or 4% of the annual global revenues
of the noncompliant company, whichever is greater. We will be subject to GDPR when we have a EU presence or “establishment”
(e.g. EU based subsidiary or operations), when conducting clinical trials with EU based data subjects, whether the trials are conducted
directly by us or through a vendor or partner, or offering approved products or services to EU based data subjects, regardless of whether
involving a EU based subsidiary or operations.
Applicable
data privacy and data protection laws may conflict with each other, and by complying with the laws or regulations of one jurisdiction,
we may find that we are violating the laws or regulations of another jurisdiction. Despite our efforts, we may not have fully complied
in the past and may not in the future. If we become liable under laws or regulations applicable to us, we may be required to pay significant
fines and penalties, our reputation may be harmed, and we may be forced to change the way we operate. That could require us to incur
significant expenses, which could significantly affect our business.
Our
business may be adversely affected by unfavorable macroeconomic conditions, including the COVID-19 pandemic.
Various
macroeconomic factors could adversely affect our business, our results of operations and our financial condition, including changes in
inflation, interest rates and foreign currency exchange rates and overall economic conditions and uncertainties, including those resulting
from political instability (including workforce uncertainty), trade disputes between nations and the current and future conditions in
the global financial markets. For example, if inflation or other factors were to significantly increase our business costs, we may be
unable to pass through price increases to patients. The cost of importing similar products from foreign markets may affect our sales
in any domestic market.
In
addition, U.S. and global financial markets have experienced disruption due to various macroeconomic and geopolitical events. These include,
but are not limited to, rising inflation, rising interest rates, the risk of a recession and other ongoing global conflicts. For example,
on March 10, 2023, Silicon Valley Bank (SVB) was closed by the California Department of Financial Protection and Innovation, which appointed
the FDIC as receiver. At the time of the closure we held assets in an account with SVB, however we were able to retrieve such funds and
move them to other institutions, and as of the date of this Registration Statement, we do not have funds in an SVB account. On
March 12, 2023, the FDIC announced that Signature Bank was closed and that the FDIC was appointed as receiver. On March 13, 2023, the
FDIC announced that all of SVB’s deposits and substantially all of its assets had been transferred to a newly created, full-service
FDIC-operated bridge bank, SVBB. SVBB assumed all loans that were previously held by SVB. On March 27, 2023, First-Citizens Bank &
Trust Company assumed all of SVBB’s customer deposits and certain other liabilities and acquired substantially all of SVBB’s
loans and certain other assets from the FDIC. While we have had full access to the assets and were able to successfully protect them
since March 13, 2023, we may be impacted by other disruptions to the U.S. banking system caused by the recent developments involving
SVB, including potential delays in our ability to transfer funds and potential delays in making payments to vendors while new banking
relationships are established. We cannot predict at this time to what extent our or our collaborators, employees, suppliers, contract
manufacturers and/or vendors could be negatively impacted by these and other macroeconomic and geopolitical events.
Interest
rates and the ability to access credit markets could also adversely affect the ability of patients, payers and distributors to purchase,
pay for and effectively distribute our product if, and when approved. Similarly, these macroeconomic factors could affect the ability
of our current or potential future third-party manufacturers, sole source or single source suppliers, licensors or licensees to remain
in business, or otherwise manufacture or supply our product candidate. Failure by any of them to remain in business could affect our
ability to manufacture Phexxi or any of our future product candidates.
The
COVID-19 pandemic may continue to affect the macroeconomic factors and the credit markets in a manner that is detrimental to our business.
Moreover, some physician offices appear to be negatively impacted by restrictions on elective procedures and office visits related to
the pandemic. To the extent physician offices are again closed or visits are again reduced, patients could be less likely to be prescribed
Phexxi. Even with our planned telehealth efforts through efforts such as the Phexxi telehealth platform, we may not be able to effectively
commercialize Phexxi for the prevention of pregnancy as a result of our reduced sales force, any reduction in physician office visits
and other circumstances related to the COVID-19 pandemic. The pandemic may continue to adversely affect us and our business in manner
we may be unable to reliably predict or quantify.
Also,
as a result of the current geopolitical tensions and conflict between Russia and Ukraine, and the recent invasion by Russia of Ukraine,
the governments of the United States, European Union, Japan and other jurisdictions have recently announced the imposition of sanctions
on certain industry sectors and parties in Russia and the regions of Donetsk and Luhansk, as well as enhanced export controls on certain
products and industries. These and any additional sanctions and export controls, as well as any counter responses by the governments
of Russia or other jurisdictions, could adversely affect, directly or indirectly, the global supply chain, with negative implications
on the availability and prices of raw materials, energy prices, and our customers, as well as the global financial markets and financial
services industry.
Risks
Related to Our Business Operations
As
we mature and expand our sales and marketing infrastructure, we will need to expand the size of our organization. If we experience difficulties
in managing this growth or are unable to attract and retain management and other key personnel, we may be unable to successfully commercialize
our products, develop any product candidates or otherwise implement our business plan.
As
of July 3, 2023, we had a total of 37 full-time employees and one part-time employee. In addition, we use third-party consultants
to assist with finance, including regulatory filings, sales, marketing and market access research and programs, as well as general and
administrative activities. As our development and commercialization plans and strategies continue to develop, we expect that we will
expand the size of our employee base for managerial, operational, sales, marketing, financial, regulatory affairs and other resources.
Future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain,
motivate and integrate additional employees. In addition, management may have to divert a disproportionate amount of its attention away
from day-to-day activities and devote a substantial amount of time to managing these growth activities, which would lead to disruptions
in our operations. We cannot provide assurance that we will be able to retain adequate staffing levels to run our operations and/or to
accomplish all the objectives that we otherwise would seek to accomplish, or that our staffing levels may turn out to be too robust for
our actual business activity.
Our
ability to compete in the highly competitive pharmaceutical industry depends upon our ability to attract and retain highly qualified
managerial and key personnel. We are highly dependent on our senior management, and the loss of the services of any members of our senior
management team could impede, delay or prevent the development and commercialization of our product or product candidates, hurt our ability
to raise additional funds and negatively impact our ability to implement our business plan. If we lose the services of any of these individuals,
we might not be able to find suitable replacements on a timely basis or at all, and our business could be harmed as a result. We do not
maintain “key man” insurance policies on the lives of these individuals.
We
might not be able to attract or retain qualified management and other key personnel in the future due to the intense competition for
qualified personnel among biotechnology, medical device, biopharmaceutical and other businesses, particularly in the San Diego area where
we are headquartered. As a result, we may be required to expend significant financial resources in our employee recruitment and retention
efforts, including the grant of significant equity incentive awards which would be dilutive to stockholders. Many of the other companies
within the contraceptive industry with whom we compete for qualified personnel have greater financial and other resources, different
risk profiles and longer histories in the industry than we do. They also may provide more diverse opportunities and better chances for
career advancement. If we are not able to attract and retain the necessary personnel to accomplish our business objectives or if we are
not able to effectively manage any future growth, we may experience constraints that will harm our ability to implement our business
strategy and achieve our business objectives.
Our
current or future employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper
activities, including non-compliance with legal requirements or regulatory standards.
We
may become exposed to the risk of employees, independent contractors, principal investigators, consultants, suppliers, commercial partners
or vendors engaging in fraud or other misconduct. Misconduct by employees, independent contractors, principal investigators, consultants,
suppliers, commercial partners and vendors could include intentional conduct such as failures: (i) to comply with FDA or other regulators’
regulations; (ii) to provide accurate information to such regulators; or (iii) to comply with manufacturing standards established by
us and/or required by law. In particular, sales, marketing and business arrangements in the health care industry are subject to extensive
laws, regulations and industry guidance intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These
laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer
incentive programs and other business arrangements. Misconduct by current or future employees, independent contractors, principal investigators,
consultants, suppliers, commercial partners and vendors could also involve the improper use of information obtained in the course of
clinical trials, which could result in regulatory or civil sanctions and serious harm to our reputation. It is not always possible to
identify and deter misconduct by employees, independent contractors, principal investigators, consultants, suppliers, commercial partners
and vendors, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged
risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance
with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending or asserting our
rights, those actions could have a significant adverse impact on our business and we may be subject to significant civil, criminal and
administrative penalties, including, without limitation, damages, monetary fines, individual imprisonment, disgorgement of profits, possible
exclusion from participation in Medicare, Medicaid and other federal health care programs, additional reporting or oversight obligations
if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law, contractual
damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
We
may be vulnerable to disruption, damage and financial obligations as a result of information technology system failures, cybersecurity
breaches, loss of data or other disruptions that could compromise our proprietary information or other sensitive information.
Despite
the implementation of security measures and internal policies and controls, any of the internal computer systems belonging to us or our
third-party service providers are vulnerable to damage from computer viruses, unauthorized access, natural disasters, malicious attack,
human error, and telecommunication and electrical failure. Cybersecurity risks continue to increase for our industry, including for our
third party vendors, who may hold some of our data, and the proliferation of new technologies and the increased sophistication and activities
of the actors behind such attacks present risks for compromised or lost data, which could result in substantial costs and harm to our
reputation. Any system failure, accident, security breach or data breach that causes interruptions in our own or in third-party service
vendors’ operations could result in a material disruption of our commercialization or product development programs. For example,
the loss of clinical study data from future clinical trials could result in liability, delays in our or our partners’ regulatory
approval efforts and significantly increase our costs to recover or reproduce the lost data. Further, our information technology and
other internal infrastructure systems, including firewalls, servers, leased lines and connection to the Internet, face the risk of systemic
failure, which could disrupt our operations. In addition, our commercialization of Phexxi is partially reliant on the use of the Phexxi
telehealth platform and our other digital or media marketing strategies. We are in turn reliant on third parties and limited internal
resources to ensure the Phexxi telehealth platform and these other digital and marketing resources function appropriately. Our commercialization
of Phexxi may be adversely affected to the extent the Phexxi telehealth platform and our other online marketing resources do not work
properly or are disrupted. To the extent any disruption or security breach results in a loss or damage to our data or applications, sensitive
information or inappropriate disclosure of confidential or proprietary information, we may incur resulting liability and reputation damage,
our product development programs and competitive position may be adversely affected and the further commercialization or development
of our products may be delayed. Furthermore, we may incur additional costs to remedy the damage caused by these disruptions or security
breaches and these costs could be significant.
The
United States federal and various state and foreign governments have adopted or proposed requirements regarding the collection, distribution,
use, security, and storage of personally identifiable information and other data relating to individuals, and federal and state consumer
protection laws are being applied to enforce regulations related to the collection, use, and dissemination of data. Some of these federal,
state and foreign government requirements include obligations of companies to notify individuals and others of security breaches involving
health information or particular personally identifiable information, which could result from breaches experienced by us or by our vendors,
contractors, or organizations with which we have formed strategic relationships. Even though we may have contractual protections with
such vendors, contractors, or other organizations, notifications and follow-up actions related to a security breach could impact our
reputation, cause us to incur significant costs, including legal expenses, harm customer confidence, hurt our expansion into new markets,
cause us to incur remediation costs, or cause us to lose existing customers.
The
techniques used by criminal elements to attack computer systems are sophisticated, change frequently and may originate from less regulated
or remote areas of the world. For example, there may be an increased risk of cybersecurity attacks by state actors due to the current
conflict between Russia and Ukraine. Recently, Russian ransomware gangs have threatened to increase hacking activity against critical
infrastructure of any nation or organization that retaliates against Moscow for its invasion of Ukraine. Any such increase in such attacks
on our third-party provider or other systems could adversely affect our network systems or other operations. We may not be able to address
these techniques proactively or implement adequate preventative measures. There can be no assurance that we will promptly detect any
such disruption or security breach, if at all. If our computer systems are compromised, we could be subject to fines, damages, reputational
harm, litigation and enforcement actions, and we could lose trade secrets, the occurrence of which could harm our business, in addition
to possibly requiring substantial expenditures of resources to remedy. For example, any such event that leads to unauthorized access,
use or disclosure of personal information, including personal information regarding our patients or employees, could harm our reputation,
require us to comply with federal and/or state breach notification laws and foreign law equivalents, and otherwise subject us to liability
under laws and regulations that protect the privacy and security of personal information. In addition, the loss of data from clinical
trials for our drug or biologic candidates could result in delays in our regulatory approval efforts and significantly increase our costs
to recover or reproduce data and a cybersecurity breach could adversely affect our reputation and could result in other negative consequences,
including disruption of our internal operations, increased cyber security protection costs, lost revenues or litigation. Despite precautionary
measures to prevent unanticipated problems that could affect our IT systems, sustained or repeated system failures that interrupt our
ability to generate and maintain data could adversely affect our ability to operate our business.
Any
such security breach may compromise information stored on our networks and may result in significant data losses or theft of our intellectual
property or proprietary business information, it may also subject us to significant fines, penalties or liabilities for any noncompliance
with certain privacy and security laws. In addition, our liability insurance may not be sufficient in type or amount to cover us against
claims related to security breaches, cyber-attacks and other related breaches. A cybersecurity breach could adversely affect our reputation
and could result in other negative consequences, including disruption of our internal operations, increased cybersecurity protection
costs, lost revenue or litigation.
We
expect to continue to incur increased costs as a result of operating as a public company and our management will be required to devote
substantial time to compliance initiatives and corporate governance practices.
As
a public company, we incur and expect to continue to incur additional significant legal, accounting and other expenses in relation to
our status as a public reporting company. Now that we are no longer an emerging growth company, we expect these expenses will further
increase. We may need to hire additional accounting, finance and other personnel in connection with our continuing efforts to comply
with the requirements of being a public company, and our management and other personnel will need to continue to devote a substantial
amount of time towards maintaining compliance with these requirements. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently
implemented by the SEC and the OTC Markets have imposed various requirements on public companies, including establishment and maintenance
of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote
a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial
compliance costs and will make some activities more time-consuming and costly.
While
we remain a smaller reporting company and have revenues of less than $100 million per year, we will not be required to include an attestation
report on internal control over financial reporting issued by our independent registered public accounting firm. If and when we are required
to achieve compliance with regulatory auditor attestation report requirements within the prescribed period, we will be engaged in a process
to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will
need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and
document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate
through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal
control over financial reporting. As described herein, we have identified one or more material weaknesses. This could result in an adverse
reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Loss
of 39 employees during the 2022 RIF and 11 employees during the March 2023 RIF and the inability to attract and retain qualified key
management personnel would impair our ability to implement our business plan.
Our
success largely depends on the continued service of key management, advisors and other specialized personnel, including Saundra Pelletier
our Chief Executive Officer, who is employed at-will and for whom we do not have “key man” insurance coverage. On October
10, 2022 Alex Fitzpatrick, our General Counsel and Secretary, tendered his resignation effective October 14, 2022 and his position as
General Counsel has not been filled, but rather we have hired outside counsel to perform those duties. On March, 3, 2023 Justin J. File,
our Chief Financial Officer tendered his resignation effective April 3, 2023. On March 6, 2023, our Board of Directors appointed Albert
Altro as Interim Chief Financial Officer and on April 13, 2023, our Board of Directors appointed Ivy Zhang as Chief Financial Officer
and Secretary which corresponded with Albert Altro’s departure
As
a result of the RIF in the fourth quarter of 2022, we reduced our workforce by 39 employees. As a result of the RIF in the first quarter
of 2023, we further reduced our workforce by 11 employees. The loss of one or more members of our management team or other key employees
or advisors could delay our commercialization efforts and could also have a material and adverse effect on our business, financial condition,
results of operations and prospects. Our future success will depend in large part on our continued ability to attract and retain other
highly qualified management personnel, as well as personnel with expertise in women’s health care, drug development, governmental
regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions,
government entities and other organizations (many of whom have substantially greater financial resources than us), and we might not be
able to attract or retain these key employees on conditions that are economically acceptable. Our inability to attract and retain these
key employees could prevent us from achieving our objectives and implementing our business strategy, which could have a material adverse
effect on our business and prospects.
In
connection with the two RIFs, and/or the departure of key personnel, we may be subject to certain separation payments, legal actions
or other claims.
As
a result of the RIFs in the fourth quarter of 2022 and first quarter of 2023, we reduced our workforce by 39 and 11 employees, respectively.
Also, on March 3, 2023, Justin J. File, our Chief Financial Officer, tendered his resignation effective April 3, 2023. We are and may
continue to be responsible for the payment of all earned and unpaid wages, vacation, bonuses and other forms of compensation due to certain
employees. Our failure to pay such may result in claims being filed against us and us being subject to further penalties for any violations.
The failure to successfully remediate any such disputes or pay any amounts payable could negatively impact our business, financial conditions,
results of operations and prospects.
We
are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering
laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets.
We can face criminal liability and other serious consequences for violations which can harm our business.
We
are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations,
various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls,
the FCPA, the U.S. domestic bribery statute contained in 18 United States Code (U.S.C.) § 201, the U.S. Travel Act, the USA PATRIOT
Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption
laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other partners from authorizing, promising,
offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector.
We may engage third parties for clinical trials outside of the United States, to sell our products abroad once we enter a commercialization
phase, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect
interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations.
We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other partners, even if
we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above
may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax
reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
We
or the third parties upon whom we depend may be adversely affected by earthquakes, medical epidemics or pandemics, or other natural disasters.
These natural disasters may be exacerbated by the effects of climate change.
Our
principal offices are located in our facilities in San Diego, California. Any unplanned event, such as flood, fire, explosion, earthquake,
extreme weather condition, medical epidemics or pandemics, power shortage, telecommunication failure or other natural or man-made accidents
or incidents, including the COVID-19 pandemic, that results in us being unable to fully utilize our facilities, effects the ability of
our employees working remotely to communicate with us and our systems, or that affects the operations of our third party manufacturers,
distributors, service providers or consultants may have a material and adverse effect on our ability to operate our business and have
significant negative consequences on our financial and operating conditions. These natural events may become worse over time due to the
ongoing effects of climate change. Any business interruption may have a material and adverse effect on our business, financial condition,
results of operations and prospects.
Risks
Related to Our Common Stock
Our
shares of common stock have been delisted from the Nasdaq Capital Market which have and could result in, among other things, a decline
in the price of our common stock and less liquidity for holders of shares of our common stock.
Our
common stock was listed on the Nasdaq Capital Market, but as a result of our failure to maintain a minimum $1.00 per share bid price
requirement for continued inclusion on the Nasdaq Capital Market pursuant to the Bid Price Requirement, on October 27, 2022, we were
delisted. Since July 12, 2021, the closing bid price for our common stock has been below $1.00 per share. On August 23, 2021, we received
a deficiency letter from the Staff of Nasdaq notifying us, that, for the preceding 30 consecutive trading days, the closing bid price
for shares of our common stock was below the minimum $1.00 per share requirement and that we had failed to comply with the Bid Price
Requirement. In accordance with Nasdaq rules, we were provided until the Compliance Date to regain compliance with the Bid Price Requirement.
We did not evidence compliance with the Bid Price Requirement by the Compliance Date and, as a result, the Staff of Nasdaq notified us
on February 22, 2022 that shares of our common stock were subject to delisting unless we timely requested a hearing before the Nasdaq
Hearings Panel. On October 27, 2022, the Nasdaq Stock Market, LLC filed the Notification of Removal From Listing and Registration Under
12(b) of the Securities Exchange Act of 1934 with the SEC.
Delisting
from the Nasdaq Capital Market has made trading our common stock more difficult for investors, potentially leading to declines in our
share price and liquidity. Other possible consequences could include: a default under our Notes, an adverse effecting on our ability
to obtain equity financing at acceptable terms or at all, a negative effect on the common stock trading volume, price, and an increase
in the stock volatility, and a possible loss of confidence by shareholders, employees, and business partners. As noted above, in the
event of a default, or continued default under our Notes, holders of our common stock may not receive the value of their investment.
Our
stock price is and may continue to be volatile.
Our
Common Stock is currently quoted for public trading on the OTCQB under the symbol “EVFM”. The market price for our common
stock is volatile and may continue to fluctuate significantly in response to a number of factors, many of which we cannot control, such
as quarterly fluctuations in financial results, the timing and our ability to advance the development of our product candidates or changes
in securities analysts’ recommendations, any of which could cause the price of our common stock to fluctuate substantially. Each
of these factors, among others, could harm your investment in our securities and could result in your being unable to resell any of our
securities that you purchase at a price equal to or above the price you paid.
In
addition, the stock market in general and the market for biopharmaceutical companies in particular, have experienced extreme volatility
that has often been unrelated to companies operating performance. The market price for our common stock may be influenced by many factors,
including:
| |
● |
the delisting
of our common stock from Nasdaq; |
| |
● |
failure to file all future required filings in a timely fashion; |
| |
● |
the loss of key personnel; |
| |
● |
the results of our efforts
to commercialize Phexxi or any other approved products; |
| |
● |
failure or discontinuation
of any of our research programs; |
| |
● |
the results of our efforts
to discover, develop, acquire or in-license product candidates or products, if any; actual or anticipated results from, and any delays
in, any future clinical trials, as well as results of regulatory reviews relating to the approval of any product candidates we may
choose to develop; |
| |
● |
the level of expenses related
to any product candidates that we may choose to develop or clinical development programs we may choose to pursue; |
| |
● |
commencement or termination
of any collaboration or licensing arrangement; |
| |
● |
disputes or other developments
relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technology; |
| |
● |
announcements by us or
our competitors of significant acquisitions, strategic partnerships, joint ventures and capital commitments; |
| |
● |
additions or departures
of key scientific or management personnel; |
| |
● |
variations in our financial
results or those of companies that are perceived to be similar to us; |
| |
● |
new products, product candidates
or new uses for existing products introduced or announced by our competitors, and the timing of these introductions or announcements; |
| |
● |
results of clinical trials
of product candidates of our competitors; |
| |
● |
general economic and market
conditions and other factors that may be unrelated to our operating performance or the operating performance of our competitors,
including changes in market valuations of similar companies, wars, terrorism and political unrest, outbreak of disease (e.g., the
COVID-19 pandemic), boycotts and other business restrictions; |
| |
● |
regulatory or legal developments
in the United States and other countries; |
| |
● |
changes in the structure
of health care payment systems; |
| |
● |
conditions or trends in
the biotechnology and biopharmaceutical industries; |
| |
● |
actual or anticipated changes
in earnings estimates, development timelines or recommendations by securities analysts; |
| |
● |
announcement or expectation
of additional financing efforts and related debt and equity issuances; |
| |
● |
sales of common stock by
us or our stockholders in the future, as well as the overall trading volume of our common stock; |
| |
● |
stockholder activism; |
| |
● |
any stockholder derivative
actions; and |
| |
● |
other factors described
in this “Risk Factors” section. |
Between
January 1, 2021 and December 31, 2021, the closing sales price of our common stock reported on the Nasdaq Capital Market ranged between
$46.25 and $610 per share. Upon being listed on the OTCQB Marketplace on October 10, 2022 the closing sales price started at $21.25,
was $8.00 as of December 30, 2022, and was $1.00 as of May 26, 2023. These broad market fluctuations may adversely affect the trading
price or liquidity of our common stock. In the past, following periods of volatility in companies’ stock prices, securities class-action
litigation has often been instituted against such companies. Such litigation, if instituted against us, could result in substantial costs
and diversion of management’s attention and resources, which could materially and adversely affect our business and financial condition.
There
may not be an active, liquid trading market for our equity securities.
Our
common stock trades exclusively on the OTCQB Marketplace. Trading volumes on the OTCQB Marketplace can fluctuate significantly, which
could make it difficult for investors to execute transactions in our securities and could cause declines or volatility in the prices
of our equity securities.
Because
our Common Stock is subject to the “penny stock” rules, brokers cannot generally solicit the purchase of our Common Stock,
which adversely affects its liquidity and market price.
The
SEC has adopted regulations which generally define “penny stock” to be an equity security that has a market price of less
than $5.00 per share, subject to specific exemptions. The market price of our Common Stock on the OTCQB Marketplace is presently less
than $5.00 per share and therefore we are considered a “penny stock” company according to SEC rules. Further, we do not expect
our stock price to rise above $5.00 in the foreseeable future. The “penny stock” designation requires any broker-dealer selling
our securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine
that the purchaser is reasonably suitable to purchase the securities. These rules limit the ability of broker-dealers to solicit purchases
of our Common Stock and therefore reduce the liquidity of the public market for our shares.
Moreover,
as a result of apparent regulatory pressure from the SEC and the Financial Industry Regulatory Authority (FINRA), a growing number of
broker-dealers decline to permit investors to purchase and sell or otherwise make it difficult to sell shares of penny stocks. The “penny
stock” designation may have a depressive effect upon our Common Stock price.
Because
we do not have sufficient authorized capital on a fully diluted basis, the excess outstanding
capital exposes us to liability, and we will need to increase our authorized capital, effectuate a reverse split or obtain effective
waivers from derivative securityholders.
As
of December 31, 2022, and May 31, 2023, our authorized capital consists of 500,000,000 shares of common stock and 5,000,000 shares of
Preferred Stock. As of December 31, 2022, of the authorized common stock, 984,786 shares were issued and outstanding and 24,658,948
shares were reserved for issuance under pending conversions of convertible notes, purchase rights, warrants and all other derivatives.
As of May 31, 2023, of the authorized common stock, 1,727,690 shares were issued and outstanding and approximately 189.1 million shares
were reserved for issuance under pending conversions of convertible notes, rights, warrants and all other derivatives. As such, our fully
diluted capital structure is presently well above the amount of common stock we are authorized to issue. Therefore, until we either increase
our authorized common stock, effectuate a reverse split, or obtain waivers from the holders of the outstanding derivative securities
both and with respect to their rights to an adequate reserve from which to receive the shares of common stock which underlie their respective
securities, we are exposed to the risk of liability arising from the excess fully diluted capitalization. In addition to the dilutive
effect any exercises of the derivative securities would have, in the event we are unable to obtain the requisite approvals or waivers,
or we are delayed in those efforts, the Company and your investment in us would be at risk.
We
may not obtain requisite approval to approve an increase in the authorized, reverse split or other corporate action relating to the common
stock.
Given the current ownership structure
of our common stock, it is possible that any future proposals made to the shareholders of our common stock may not pass or be approved.
Since no one person or group owns a majority of the currently issued and outstanding shares of common stock, we cannot guarantee
that we could obtain requisite shareholder approval to effectuate a reverse split, an increase in the authorized number of common shares,
or other corporate action that could benefit us and your investment. Additionally, we cannot guarantee approval by FINRA, SEC or any
other governmental or regulatory agency needed to effectuate a corporate action.
Our
common stock could be further diluted as the result of the issuance of additional shares of common stock, convertible securities, warrants
or options.
In
the past, we have issued common stock, convertible securities (such as convertible notes) and warrants in order to raise capital. We
have also issued common stock as compensation for services and incentive compensation for our employees, directors and certain vendors.
We have shares of common stock reserved for issuance upon the exercise of certain of these securities and may increase the shares reserved
for these purposes in the future. Our issuance of additional common stock, convertible securities, options and warrants could affect
the rights of our stockholders, could reduce the market price of our common stock or could result in adjustments to exercise prices of
outstanding warrants (resulting in these securities becoming exercisable for, as the case may be, a greater number of shares of our common
stock), or could obligate us to issue additional shares of common stock to certain of our stockholders.
A
significant portion of our total outstanding shares of common stock may be sold into the public market at any point, which could cause
the market price of our common stock to drop significantly, even if our business is doing well.
Sales
of a substantial number of shares of our common stock in the public market could occur. These sales, or the perception in the market
that holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Future issuances of
our securities may cause additional reduction in the percentage interests of our current stockholders in the voting power, liquidation
value, our book and market value, and in any future earnings. As of May 31, 2023, there were approximately 4,391 shares of our
common stock subject to outstanding options which have been registered on registration statements on Form S-8. Furthermore, as of May
31, 2023, there were an aggregate of approximately 189.1 million shares subject to outstanding warrants to purchase our common stock,
reserved for issuance upon conversion of our issued and outstanding convertible notes, and outstanding purchase rights. We have granted
(or are required to grant) certain of our security holders registration rights pursuant to our agreements with these holders, including
agreements requiring us to register for resale the shares of our common stock issued upon the conversion or exercise of our convertible
notes and related warrants.
The
issuance or resale of our common stock issued to our security holders upon conversion of convertible notes or upon exercise of our warrants
or options could cause the market price of our common stock to decline. In addition, the increase in the number of issued shares of our
common stock issuable upon conversion of our convertible notes or upon exercise of our warrants may have an incidental anti-takeover
effect in that these additional shares could be used to dilute the stock ownership of parties seeking to obtain control of us. The resulting
increased number of issued shares could discourage the possibility of, or render more difficult, certain mergers, tender offers, proxy
contests or other change of control or ownership transactions.
We
are and may continue to be subject to short selling strategies.
Short
sellers of our stock may be manipulative and may attempt to drive down the market price of shares of our Common Stock. Short selling
is the practice of selling securities that the seller does not own but rather has, borrowed from a third party with the intention of
buying identical securities back at a later date to return to the lender. The short seller hopes to profit from a decline in the value
of the securities between the sale of the borrowed securities and the purchase of the replacement shares, as the short seller expects
to pay less in that purchase than it received in the sale. As it is therefore in the short seller’s best interests for the price
of the stock to decline, many short sellers (sometime known as “disclosed shorts”) publish, or arrange for the publication
of, negative opinions regarding the relevant issuer and its business prospects to create negative market momentum and generate profits
for themselves after selling a stock short. Although traditionally these disclosed shorts were limited in their ability to access mainstream
business media or to otherwise create negative market rumors, the rise of the Internet and technological advancements regarding document
creation, videotaping and publication by weblog (blogging) have allowed many disclosed shorts to publicly attack a company’s credibility,
strategy and veracity by means of so-called “research reports” that mimic the type of investment analysis performed by large
Wall Street firms and independent research analysts. These short attacks have, in the past, led to selling of shares in the market, on
occasion in large scale and broad base. Issuers who have limited trading volumes and are susceptible to higher volatility levels than
large-cap stocks, can be particularly vulnerable to such short seller attacks. These short seller publications are not regulated by any
governmental, self-regulatory organization or other official authority in the United States, are not subject to certification requirements
imposed by the SEC and, accordingly, the opinions they express may be based on distortions or omissions of actual facts or, in some cases,
fabrications of facts. In light of the limited risks involved in publishing such information, and the enormous profit that can be made
from running a successful short attack, unless the short sellers become subject to significant penalties, it is more likely than not
that disclosed short sellers will continue to issue such reports.
Significant
short selling of a company’s stock creates an incentive for market participants to reduce the value of that company’s common
stock. Short selling may lead to the placement of sell orders by short sellers without commensurate buy orders because the shares borrowed
by short sellers do not have to be returned by any fixed period of time. If a significant market for short selling our common stock develops,
the market price of our common stock could be significantly depressed.
Continued
failure to remediate current material weaknesses and establish and maintain effective internal controls in accordance with Section 404
of the Sarbanes-Oxley Act could have a material adverse effect on our business and stock price.
As
a publicly traded company, we are required to comply with the SEC’s rules implementing Sections 302 and 404 of the Sarbanes-Oxley
Act, which requires management to certify financial and other information in our quarterly and annual reports and provide an annual management
report on the effectiveness of controls over financial reporting. As discussed below, we have identified internal control weaknesses,
and need to undertake various actions, such as implementing new internal controls, new systems and procedures and hiring additional accounting
or internal audit staff, which could increase our operating expenses. In addition, we may identify additional deficiencies in our internal
control over financial reporting as part of that process.
In
addition, if we are unable to resolve internal control deficiencies in a timely manner, investors could lose confidence in the accuracy
and completeness of our financial reports and the market price of our common stock could be negatively affected.
We
identified material weaknesses in our internal control over financial reporting as of December 31, 2022 and March 31, 2023 and
these or other material weaknesses could continue to materially impair our ability to report accurate financial information in a timely
manner.
Item
9A of our Annual Report on Form 10-K, as filed with the Securities and Exchange Commission (SEC) on April 27, 2023,
and Item 4 of our Quarterly Report on Form 10-Q, as filed with the SEC on June 16, 2023, our management, with the participation
of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and
procedures as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act as of December 31, 2022 and March 31, 2023 and
identified material weaknesses described below.
A
material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that a reasonable
possibility exists that a material misstatement of our annual or interim financial statements would not be prevented or detected on a
timely basis.
Our
management identified material weaknesses in the Company’s internal control over financial reporting primarily related to limited
finance and accounting staffing levels that are not commensurate with the Company’s complexity and its financial accounting and
reporting requirements. The Company has had several organization changes, including the resignation of the some of its named executives,
including the principal financial officer. Turnover of these key management positions of the Company led the financial reporting staff
to rely increasingly on outsourced service providers and specialists, without adequate resources to monitor and operate internal controls
of financial reporting.
Based
on the above, the Company did not fully implement components of the COSO framework, including elements of the control environment, risk
assessment, control activities, information and communication, and monitoring activities components.
Remediation
Activities:
Management
is continuing to evaluate the material weaknesses discussed above and is in the process of implementing its remediation plan, which includes
the hiring of additional resources. However, we cannot provide assurance as to when our remediation efforts will be complete and the
material weaknesses cannot be considered remediated until the applicable controls have operated for a sufficient period of time and management
has concluded, through testing, that these controls are operating effectively. We cannot assure you that the measures we have taken to
date, and are continuing to implement, will be sufficient to remediate the material weaknesses we have identified or avoid potential
future material weaknesses.
As
a result, we may not be able to remediate on a timely basis such that our financial statements may not be available on a timely
basis or we may discover additional material weaknesses related to control environments, risk assessment, control activities, monitoring
activities and/or information and communication or other matters, any of which could cause investors to lose confidence in us and lead
to, among other things, unanticipated legal, accounting and other expenses, delays in filing required financial disclosures, enforcement
actions by government authorities, fines, penalties, the delisting of our securities, a decline in the prices of our securities, liabilities
arising from stockholder litigation and defaults under our various debt obligations.
We
are a “smaller reporting company”, and the reduced disclosure requirements applicable to smaller reporting companies may
make our common stock less attractive to investors.
We
are a “smaller reporting company” under SEC regulations. For so long as we remain a smaller reporting company, we will be
permitted to and intend to rely on exemptions from certain disclosure requirements applicable to other public companies that are not
smaller reporting companies. These exemptions include:
| |
● |
for
so long as we remain a smaller reporting company with annual revenues of less than $100 million per year and a public float value
as of our most recently completed second fiscal quarter of less than $700 million, not being required to comply with the auditor
attestation requirements in the assessment of our internal control over financial reporting; and |
| |
● |
reduced
disclosure obligations regarding executive compensation. |
We
cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our
common stock less attractive as a result, there may be a less active trading market for our common stock and the price of our common
stock price may be more volatile.
We
do not anticipate paying any cash dividends on our capital stock in the foreseeable future; capital appreciation, if any, will be your
sole source of gain as a holder of our common stock.
We
have never declared or paid cash dividends on shares of our common stock. As noted above, we are also restricted from paying dividends
pursuant to our debt arrangements. Except as may be required to redeem our issued and outstanding promissory notes or shares of Series
B-2 Convertible Preferred Stock, we currently plan to retain all our future earnings, if any, and any cash received through future financings
to finance the growth and development of our business. Accordingly, capital appreciation, if any, of our common stock will be the sole
source of gain for our common stockholders for the foreseeable future.
Provisions
in our amended and restated certificate of incorporation, our bylaws or Delaware law might discourage, delay or prevent a change in control
of the Company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions
in our amended and restated certificate of incorporation, our bylaws or Delaware law may discourage, delay or prevent a merger, acquisition
or other change in control stockholders may consider favorable, including transactions in which our stockholders might otherwise receive
a premium for their shares. These provisions could also limit the price investors might be willing to pay in the future for shares of
our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible
for appointing the members of our management team, these provisions might frustrate or prevent any attempts by our stockholders to replace
or remove the current management by making it more difficult for our stockholders to replace members of our board of directors. These
provisions include the following:
| ● | a
classified board of directors with three-year staggered terms, which may delay the ability
of stockholders to change the membership of a majority of our board of directors; |
| ● | prohibiting
our stockholders from calling a special meeting of stockholders or acting by written consent
other than unanimous written consent; |
| ● | permitting
our board of directors to issue additional shares of our preferred stock, with such rights,
preferences and privileges as they may designate, including the right to approve an acquisition
or other changes in control; |
| ● | establishing
an advance notice procedure for stockholder proposals to be brought before an annual meeting,
including proposed nominations of persons for election to our board of directors; |
| ● | providing
that our directors may be removed only for cause; |
| ● | providing
that vacancies on our board of directors may be filled only by a majority of directors then
in office, even though less than a quorum; and |
| ● | requiring
the approval of our board of directors or the holders of a supermajority of our outstanding
shares of capital stock to amend our bylaws and certain provisions of our certificate of
incorporation. |
Claims
for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us
and may reduce the amount of money available to us.
Our
amended and restated certificate of incorporation and amended and restated bylaws provides that we will indemnify our directors and officers,
in each case to the fullest extent permitted by Delaware law. In addition, as permitted by Section 145 of the DGCL, our amended and restated
bylaws and our indemnification agreements that we have entered with our directors and officers provide that:
| ● | We
will indemnify our directors and officers for serving us in those capacities, or for serving
other business enterprises at our request, to the fullest extent permitted by Delaware law.
Delaware law provides that a corporation may indemnify such person if such person acted in
good faith and in a manner such person reasonably believed to be in or not opposed to the
best interests of the registrant and, with respect to any criminal proceeding, had no reasonable
cause to believe such person’s conduct was unlawful. |
| ● | We
may, in our discretion, indemnify employees and agents in those circumstances where indemnification
is permitted by applicable law. |
| ● | We
are required to advance expenses, as incurred, to our directors and officers in connection
with defending a proceeding, except that such directors or officers shall undertake to repay
such advances if it is ultimately determined that such person is not entitled to indemnification. |
| ● | We
will not be obligated pursuant to our amended and restated bylaws to indemnify a person with
respect to proceedings initiated by that person against us or our other indemnities, except
with respect to proceedings authorized by our board of directors or brought to enforce a
right to indemnification. |
| ● | The
rights conferred in our amended and restated bylaws are not exclusive, and we are authorized
to enter into indemnification agreements with our directors, officers, employees and agents
and to obtain insurance to indemnify such persons. |
| ● | We
may not retroactively amend our bylaw provisions to reduce our indemnification obligations
to directors, officers, employees and agents. |
If
securities analysts cease publishing research or reports about our business, or if they publish negative evaluations of our common stock,
the price of our common stock could decline.
The
trading market for our common stock relies in part on the research and reports industry or financial analysts publish about us or our
business. We do not have any control over these analysts. If one or more of the analysts covering our business downgrade their evaluations
of our common stock, the price of our common stock could decline. In addition, if one or more of these analysts cease coverage or cease
regularly publishing reports on our business, we could lose visibility in the financial markets, which in turn could cause our common
stock price or trading volume to decline.
Our
business could be negatively affected as a result of the actions of activist stockholders.
It
is possible that one or more of our stockholders may publicly voice opposition to our financing strategy and/or certain aspects of our
corporate governance and strategy, or undertake a proxy contest to reconstitute our board. Proxy contests have been waged against many
companies in the biopharmaceutical industry over the last several years. If faced with a proxy contest or other type of stockholder activism,
we may not be able to respond successfully to the contest or other type of activism which would be disruptive to our business. Even if
we are successful, our reputation and/or business could be adversely affected by a proxy contest or other form of stockholder activism
because:
| ● | responding
to proxy contests and other actions by activist stockholders can be costly and time-consuming,
disrupting operations and diverting the attention of management and employees; |
| ● | perceived
uncertainties as to our company and future strategic direction may result in the loss of
potential financing, acquisitions, collaboration, in-licensing or other business opportunities,
and may make it more difficult to attract and retain qualified personnel and business partners;
and |
| ● | if
individuals are elected to our board of directors with a specific agenda, it may adversely
affect our ability to effectively and timely implement our strategic plan and create additional
value for our stockholders. |
Any
or all of these activities could cause our stock price to decline or experience periods of volatility, and could be particularly problematic
as our company seeks to transition to a commercial enterprise in a challenging environment.
We
may become a defendant in one or more stockholder derivative or class-action litigations, and any such future lawsuit may adversely affect
our business, financial condition, results of operations and cash flows.
We
and certain of our officers and directors may become defendants in one or more future stockholder derivative actions or other class-action
lawsuits. These lawsuits would divert our management’s attention and resources from our ordinary business operations, and we would
likely incur significant expenses associated with their defense (including, without limitation, substantial attorneys’ fees and
other fees of professional advisors and potential obligations to indemnify current and former officers and directors who are or may become
parties to such actions). If these lawsuits do arise, we may be required to pay material damages, consent to injunctions on future conduct
and/or suffer other penalties, remedies or sanctions. In addition, any such future stockholder lawsuits could adversely impact our reputation
and/or to launch and commercialize Phexxi, thereby harming our ability to generate revenue. Accordingly, the ultimate resolution of these
matters could have a material adverse effect on our business, financial condition, results of operation and cash flow and, consequently,
could negatively impact the trading price of our common stock.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking
statements that involve substantial risks and uncertainties. The forward-looking statements are contained principally in the section
entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” All statements, other
than statements of historical facts, contained in this prospectus, including statements regarding our strategy, future operations, future
financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. Words such as, but
not limited to, “anticipate,” “aim,” “believe,” “contemplate,” “continue,”
“could,” “design,” “estimate,” “expect,” “intend,” “may,” “might,”
“plan,” “possible,” “potential,” “predict,” “project,” “seek,”
“should,” “suggest,” “strategy,” “target,” “will,” “would,” and
similar expressions or phrases, or the negative of those expressions or phrases, are intended to identify forward-looking statements,
although not all forward-looking statements contain these identifying words.
These forward-looking statements
include, among other things, statements about:
| |
● |
our ability to continue as a going concern; |
| |
● |
our ability to remediate the material weaknesses in our internal
controls and procedures identified by management; |
| |
● |
potential bankruptcy proceedings and the effect of those proceedings
on our ongoing and future operations; |
| |
● |
the effect of the Notice of Default received from Baker Bros. Advisors,
LP and our ability to resolve the same; |
| |
● |
our ability to obtain necessary approvals of a reverse split proposal
or any other corporate action needing stockholder, FINRA, or other approvals; |
| |
● |
our ability to file Annual and Quarterly Reports on a timely basis; |
| |
● |
our ability to raise additional capital to fund our operations; |
| |
● |
our ability to achieve and sustain profitability; |
| |
● |
our estimates regarding our future performance including, without
limitation, any estimates of potential future revenues; |
| |
● |
estimates regarding market size; |
| |
● |
our estimates regarding expenses, revenues, financial performance
and capital requirements, including the length of time our capital resources will sustain our operations; |
| |
● |
our ability to maintain the listing of our shares on the OTCQB®
Venture Market; |
| |
● |
our ability to comply with the provisions and requirements of our
debt arrangements, to manage the current defaults pursuant to our debt arrangements and to pay amounts owed, including any amounts
that may be accelerated, pursuant to our debt arrangements; |
| |
● |
estimates regarding health care providers’ (HCPs) recommendations
of Phexxi® (lactic acid, citric acid, and potassium bitartrate) vaginal gel (Phexxi) to patients; |
| |
● |
the rate and degree of market acceptance of Phexxi; |
| |
● |
our ability to successfully commercialize Phexxi and continue to
develop our sales and marketing capabilities; |
| |
● |
our estimates regarding the effectiveness of our marketing campaigns; |
| |
● |
our strategic plans for our business, including the commercialization
of Phexxi; |
| |
● |
the impacts of the ongoing COVID-19 pandemic including, without
limitation, its impact on our business and the commercialization of Phexxi; |
| |
● |
the potential for changes to current regulatory mandates requiring
health insurance plans to cover U.S. Food and Drug Administration (FDA)-cleared or -approved contraceptive products without cost
sharing; |
| |
● |
our ability to obtain or maintain third-party payer coverage and
adequate reimbursement, and our reliance on the willingness of patients to pay out-of-pocket for Phexxi absent full or partial third-party
payer reimbursement; |
| |
● |
our ability to obtain the necessary regulatory approvals to market
and commercialize any product candidate we may seek to develop; |
| |
● |
the success, cost and timing of our potential future clinical trials,
if any; |
| |
● |
our ability to protect and defend our intellectual property position
and our reliance on third party licensors; |
| |
● |
our ability to obtain additional patent protection for our product
and product candidates; |
| |
● |
our dependence on third parties for the manufacture of Phexxi and
in the conduct of potential future clinical trials, if any; |
| |
● |
our ability to expand our organization to accommodate potential
growth; and |
| |
● |
our ability to retain and attract key personnel. |
Although we believe that we
have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based
on our projections of the future that are subject to known and unknown risks and uncertainties and other factors that may cause our actual
results, level of activity, performance or achievements expressed or implied by these forward-looking statements, to differ. We may not
actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance
on our forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement. Forward-looking
statements should be regarded solely as our current plans, estimates and beliefs. You should read this prospectus and the documents that
we have filed as exhibits to this prospectus and incorporated by reference herein completely and with the understanding that our actual
results may be materially different from the plans, intentions and expectations disclosed in the forward-looking statements we make.
Moreover, we operate in a very competitive and rapidly changing environment and new risks emerge from time to time. It is not possible
for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor,
or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may
make. The forward-looking statements contained in this prospectus are made as of the date of this prospectus, and we do not assume any
obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required
by applicable law.
USE
OF PROCEEDS
We
are not selling any securities under this prospectus and will not receive any proceeds from the sale of the common stock offered by this
prospectus by the Selling Stockholders. However, we may receive proceeds from the cash exercise of the Warrants, which, if exercised
in cash at the current exercise price with respect to all Warrants, would result in gross proceeds to us of approximately $4.2 million.
The proceeds from such Warrant exercises, if any, will be used for working capital and general corporate purposes. We cannot predict
when or whether the Warrants will be exercised, and it is possible that some or all of the Warrants may expire unexercised. For information
about the Selling Securityholders, see “Selling Securityholders.”
The
Selling Securityholders will pay any underwriting discounts and commissions and expenses incurred by the Selling Securityholders for
brokerage or legal services or any other expenses incurred by the Selling Stockholders in disposing of the shares of common stock offered
hereby. We will bear all other costs, fees and expenses incurred in effecting the registration of the shares of common stock covered
by this prospectus, including all registration and filing fees and fees and expenses of our counsel and accountants.
MARKET
PRICE AND DIVIDEND INFORMATION
Market
Information
Our
common stock is listed on the OTCQB Venture Market under the symbol “EVFM.” The last reported sale price of our common stock
on August 1, 2023 was $0.4400.
Holders
As
of August 1, 2023, there were 13 stockholders of record of our common stock.
Dividends
We
have never declared or paid any cash dividends on our common stock. We intend to retain future earnings, if any, to finance the expansion
of our business. As a result, the Company does not anticipate paying any cash dividends in the foreseeable future.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The
terms “we,” “us,” “our,” “Evofem” or the “Company” refer collectively to
Evofem Biosciences, Inc. and its wholly-owned subsidiaries, unless otherwise stated. All information presented in this prospectus is
based on our fiscal year. Unless otherwise stated, references to particular years, quarters, months or periods refer to our fiscal years
ending December 31 and the associated quarters, months and periods of those fiscal years.
You
should read the following discussion and analysis of our financial condition and results of operations together with our condensed consolidated
financial statements and related notes appearing elsewhere in this prospectus. For additional context with which to understand our financial
condition and results of operations, see the audited consolidated financial statements and accompanying notes contained therein as of
December 31, 2022 and 2021 (2022 Audited Annual Financial Statements) in the Company’s Annual Report on Form 10-K as filed
with the SEC on April 27, 2023 (2022 Annual Report), and the condensed financial statements and accompanying notes contained
therein as of March 31, 2023 and 2022 (2023 Q1 Interim Financial Statements) in the Company’s Quarterly Report on Form 10-Q as
filed with the SEC on June 16, 2023. This discussion and analysis contains forward-looking statements that involve risks, uncertainties
and assumptions. The actual results may differ materially from those anticipated in these forward-looking statements as a result of certain
factors. Unless otherwise defined in this section, the defined terms in this section have the meanings set forth in the 2022 Audited
Financial Statements.
Recent
Developments
In
April 2023, the Company entered into a securities purchase agreement with certain investors providing for the sale and issuance of senior
subordinate convertible notes (the April 2023 SPA). The April 2023 SPA included (i) convertible promissory notes with aggregate original
principal amounts of approximately $0.8 million (the April 2023 Notes), and (ii) warrants to purchase 615,384 shares of common stock
(the April 2023 Warrants and collectively, the April 2023 Offering). The April 2023 Offering closed on April 5, 2023 (the April 2023
Closing), with net proceeds to the Company, after deducting offering expenses, of approximately $0.5 million. The April 2023 SPA also
included a Registration Rights Agreement that requires the Company to register the common stock underlying the April 2023 Notes and April
2023 Warrants within the timeframes specified therein.
Upon
the April 2023 Closing, the conversion and strike prices, as applicable, of the Baker Notes, Baker Warrants, the May 2022 Common Warrants,
the June 2022 Baker Warrants, the Adjuvant Notes, the December 2022 Notes and Warrants, and the Notes and Warrants in the February and
March 2023 Closing reset to $0.8125 per share, accordingly. Additionally, the Company’s outstanding Purchase Rights increased by
approximately 15,218,227 shares since March 31, 2023.
On
April 24, 2023, Gillian Greer, PhD., resigned as member of the Company’s board of directors. Dr. Greer’s resignation was
not the result of any dispute or disagreement with the Company on any matter relating to the Company’s operations, policies or
practices.
Ivy
Zhang was appointed Chief Financial Officer and Secretary of Evofem, effective April 13, 2023. Ms. Zhang’s employment is “at-will”,
meaning that either she or the Company are entitled to terminate her employment at any time and for any reason, with or without cause.
As consideration for her employment, Ms. Zhang is entitled to receive an annualized base salary of $410,000.00 (subject to review and
adjustment in accordance with the Company’s normal performance review practices); will also be eligible to receive, at the sole
discretion of the Company’s Board, an annual end-of-year incentive bonus in an amount up to 40% of her base salary; and is eligible
to receive other employment benefits on the same terms as other employees of the Company.
In
April 2023, the Company received a Paragraph IV certification notice letter (the “Padagis Notice Letter”) regarding an Abbreviated
New Drug Application (“ANDA”) submitted to the FDA by Padagis Israel Pharmaceuticals Inc. (“Padagis”). The ANDA seeks
approval from the FDA to commercially manufacture, use, or sell a generic version of Phexxi®
under 21 U.S.C. § 355(j) prior to the expiration of United States Patent Nos.
10,568,855; 11,337,989; and 11,439,610 listed in the FDA’s Orange Book: Approved Drug
Products with Therapeutic Equivalence Evaluations (collectively the “Phexxi ® Patents”).
In the Padagis Notice Letter, Padagis claims that the Phexxi ® Patents are
invalid under various grounds.
On
June 1, 2023, the Company filed a complaint for patent infringement in Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals,
et al., in the United States District Court for the District of New Jersey. The case was assigned number 2:23-cv-03003.
The complaint alleges that Padagis’ proposed generic version of Phexxi ®
infringes the Phexxi ® Patents. The relief sought by the Company is a declaration
of infringement and an injunction of FDA approval of Padagis’ proposed generic version of Phexxi
® until expiration of the Phexxi ® Patents in 2033.
Until the earlier of final judgment or the passage of 30 months from the receipt of the Padagis Notice Letter, the FDA is prohibited
from approving Padagis’ ANDA to market its proposed generic version of PHEXXI®. The Company also subsequently filed
a substantively identical action in the United States District Court for the District of Delaware, Evofem Biosciences, Inc. et al.
v. Padagis Israel Pharmaceuticals, et al., which was assigned number 1:23-cv-00606-UNA. The Company is not aware of any answer
or counterclaim filed by Padagis in either action against the Company at this time.
The
2023 Reverse Stock Split became effective on May 18, 2023 upon the opening of trading on the OTCQB (the Effective Time). At the Effective Time, every 125 shares of the Company’s issued and outstanding common stock were
automatically converted into one share of common stock, without any change to the par value per share. In addition, proportionate
adjustments were made to the per share exercise price and the number of shares issuable upon the exercise of all outstanding stock
options and warrants to purchase shares of common stock, the number of shares issuable upon the vesting of all RSAs, and the number
of shares of common stock reserved for issuance pursuant to the Company’s equity incentive compensation plans, convertible
notes and convertible preferred stock. Any stockholder who would otherwise be entitled to a fractional share of the Company’s
common stock created as a result of the Reverse Stock Split is entitled to receive a cash payment equal to the product of such
resulting fractional interest in one share of the Company’s common stock multiplied by the closing trading price of the
Company’s common stock on the trading day immediately preceding the Effective Time. These interim condensed consolidated
financial statements are retrospectively adjusted for this 2023 Reverse Stock Split.
In July 2023, we entered
into an SPA with certain investors providing for the sale and issuance of senior secured convertible notes due in the aggregate
original principal amount of $1.5 million (the Notes), and warrants to purchase an aggregate 1,200,000 shares of common stock
(Warrants) (collectively, the July 2023 Offering). The July 2023 Offering closed on July 3, 2023 with net proceeds to the Company,
after deducting offering expenses, of approximately $1.0 million.
In August 2023,
the Company entered into SPAs with certain investors providing for the sale and issuance of senior subordinate convertible notes due
in the aggregate original principal amount of $1.0 million (the August Notes) and warrants to purchase an aggregate of 799,999 shares
of common stock (the August Warrants and collectively, the August Offering). The August Offering closed on August 4, 2023, with net proceeds
to the Company, after deducting offering expenses, of approximately $0.5 million.
In August 2023, certain investors party to the December 2022 Notes and the February 2023 Notes exchanged $1.8 million total outstanding notes
including accrued interest for 1,800 shares of Series E-1 Convertible Preferred Stock, par value $0.0001 per share. The holders of shares of Series E-1 Preferred Stock shall entitle
the holder thereof to vote together with the common shareholders as a single class and to cast that number of votes per share as is equal
to the number of shares of common stock into which it is then convertible. The Series E-1 Preferred Stock is convertible into shares of
common stock at a rate of $0.40 per share subject to adjustment as provided in the Certificate of Designation (the “Conversion
Rate”). Each holder of Series E-1 Preferred Stock is entitled to receive dividends paid exclusively in the form of common stock
(the “Dividends”) payable to the holders of the Series E-1 Preferred Stock on a monthly basis.
Overview
We
are a San Diego-based commercial-stage biopharmaceutical company committed to developing and commercializing innovative products to address
unmet needs in women’s sexual and reproductive health. Our first commercial product, Phexxi, was approved by the FDA on May 22,
2020 and is the first and only FDA-approved, hormone-free, woman-controlled, on-demand prescription contraceptive gel for women. We commercially
launched Phexxi in September 2020 in the United States. We intend to commercialize Phexxi in global markets through partnerships or licensing
agreements.
Phexxi
as a Contraceptive; Commercial Strategies
Our
sales force promotes Phexxi directly to obstetrician/gynecologists and their affiliated health professionals, who collectively write
the majority of prescriptions for contraceptive products. Our sales force consists of 16 sales representatives, three business managers
and a VP of sales, supported by a self-guided virtual health care provider (HCP) learning platform. Additionally, we offer women direct
access to Phexxi via our telehealth platform. Using the platform, women can directly meet with an HCP to determine their eligibility
for a Phexxi prescription and, if eligible, have the prescription written by the HCP, filled, and mailed directly to them by a third-party
pharmacy.
Our
commercial strategy for Phexxi includes marketing and product awareness campaigns targeting women of reproductive potential in the U.S.,
including the approximately 23 million women who are not using hormonal contraception and the approximately 18.8 million women who are
using a prescription contraceptive, some of whom, particularly pill users, may be ready to move to an FDA-approved, non-invasive hormone-free
contraceptive, as well as certain identified target HCP segments. In addition to marketing and product awareness campaigns, our commercial
strategy includes payer outreach and execution of our consumer digital and media strategy.
According
to our market research since Phexxi’s commercial launch, HCPs indicate they would recommend Phexxi to approximately:
| ● | 47%
of patients experiencing side effects from current contraception; |
| ● | 37%
of patients using non-hormonal prescription contraception; |
| ● | 36%
of patients seeking pregnancy prevention; and |
| ● | 19%
of patients using hormonal prescription contraception. |
Additional
research into the demographics of more than 5,000 women who are using Phexxi revealed that 79% of Phexxi users are between 18 to 34 years
of age. Among the subset of Phexxi users for whom prior contraceptive data is available (n=2,512), 80% of women who had recently started
Phexxi were not on any method of prescription contraception. Another 20% switched to Phexxi from either oral contraceptives, hormonal
rings or patches.
In
February 2021, we launched a direct-to-consumer advertising campaign, known as “Get Phexxi,” designed to increase awareness
and educate women on the benefits of Phexxi. The campaign highlighted some of the struggles women face when choosing among the many available
methods of contraception, including the lack of control with condoms, daily use of the pill, and abstinence required for cycle tracking.
In
September 2021, we launched a national brand ambassador campaign featuring Emmy Award-winning celebrity Annie Murphy, designed to broaden
awareness and drive uptake of Phexxi. This award-winning campaign, known as “House Rules,” has significantly raised our target
audience awareness of Phexxi. To date, the House Rules campaign has grown brand awareness by 45% and garnered 42 million video views
and over 33,000 telehealth exits. More importantly, it has also helped drive significant increases in new HCPs recommending and prescribing
Phexxi.
Over
the course of 2021, ex-factory units grew quarter over quarter, with the most significant growth in the fourth quarter following “House
Rules;” Phexxi units shipped increased 73% as compared to the prior quarter, propelled by a 56% increase in new patients starting
Phexxi and a 111% increase in refills as compared to the prior quarter.
The
first quarter of 2022 reflected anticipated softness in Phexxi prescription and dispensed unit growth due to the annual reset of patient
healthcare deductibles, which impacted most contraceptive brands, as well as from adjustments to Evofem’s patient support programs
in January 2022 intended to increase the profit margin on Phexxi units dispensed and support continued net product sales growth. As forecasted,
Phexxi total prescriptions and dispensed units rebounded in March 2022 and continued to grow in the second and third quarters of 2022.
Coverage
for and access to Phexxi is expected to further increase as additional insurers and PBMs comply with the current Health Resources and
Services Administration (HRSA) Women’s Preventive Services Guidelines, which took effect on January 1, 2023 for calendar year plans
and adhere to the updated guidance related to contraceptive access that was issued by the U.S. Departments of Labor, Health and Human
Services (HHS), and the Treasury (the “Departments”) in January 2022. Collectively, these agencies specify that most insurers
and PBMs must provide coverage, with no out-of-pocket costs to women, for FDA-approved contraceptive products, like Phexxi, prescribed
by healthcare providers.
To
comply with these Guidelines, payers are increasingly covering Phexxi by adding Phexxi to formulary (commercial insurers) or preferred
drug list (Medicaid); removing the requirement for a Prior Authorization letter from the HCP (commercial insurers); and/or moving Phexxi
to $0 copay (commercial insurers).
We
continue working to increase the number of lives covered and to gain a preferred formulary position for Phexxi. We gained coverage for
32.5 million lives in 2022 and have added 22.1 million lives since December 31, 2022. We currently have 65% coverage within our Commercial
and Medicaid books of business, including 19.3 million lives covered at no out-of-pocket cost as of May 9, 2023 and approximately 13.7
million lives covered under our December 2020 contract award from the U.S. Department of Veterans Affairs. As of March 2023, the Phexxi
approved claims rate increased to 81%.
On
January 1, 2021, as a result of our participation in the Medicaid National Drug Rebate Program, the U.S. Medicaid population gained access
to Phexxi. Medicaid provides health coverage to approximately 68 million members, including approximately 16.8 million women between
19 to 49 years of age.
Phexxi
is classified in the databases and pricing compendia of Medi-Span and First Databank, two major drug information databases that payers
can consult for pricing and product information, as the first and only “vaginal pH modulator.”
In
2022, Evofem developed and introduced a new educational chart for patients and HCPs that details high-level information about birth control
methods currently available to women in the U.S., including the vaginal pH modulator. This new educational tool has been extremely well
received and has had a positive impact with HCPs and patients alike.
EVO100
for the Prevention of Chlamydia and Gonorrhea
Based
on positive and statistically significant top-line results of our Phase 2B/3 AMPREVENCE trial, in October 2020 we initiated the
Phase 3 EVOGUARD clinical trial to evaluate EVO100 for the prevention of urogenital chlamydia and gonorrhea infections in women.
This randomized, placebo-controlled clinical trial enrolled 1,903 women with a prior chlamydia or gonorrhea infection who were at risk
for future infection.
On
October 11, 2022, we reported that EVOGUARD did not meet its primary efficacy endpoint. The Company believes COVID-19 related
changes in clinical site operations, subject behavior and actions including deviations from following the clinical study protocol requirements
related to STI acquisition, detection, and prevention contributed to this outcome. The product safety profile was consistent with what
has been observed in prior clinical trials, and only two women (0.1%) in the study discontinued due to adverse events. Due to financial
constraints, we discontinued investment in this clinical program.
Multipurpose
Prevention Technology Vaginal Gel for HIV Prevention
In
December 2021, we launched a collaboration with Orion Biotechnology Canada Ltd. (Orion) to evaluate the compatibility and stability of
Orion’s novel CCR5 antagonist, OB-002, in Phexxi with the goal of developing a Multipurpose Prevention Technology (MPT) product
candidate for indications including the prevention of HIV in women. Assuming positive preclinical results, Evofem and Orion will seek
government and philanthropic funding for subsequent clinical trials of any resulting MPT vaginal gel product candidate.
Financial
Operations Overview
Net
Product Sales
Our
revenue recognition is based on unit shipments from our third-party logistics warehouse to our customers, which consist of wholesale
distributors, retail pharmacies, and a mail-order specialty pharmacy. We have recognized net product sales in the United States since
the commercial launch of Phexxi in September 2020.
We
intend to out-license commercialization rights for Phexxi to one or more pharmaceutical companies or other qualified potential partners
for countries or regions outside of the United States. We are currently in discussion with potential partners for various geographies.
We cannot forecast when or if these arrangements will be secured, the structure or potential amount of revenues from these arrangements,
whether upfront, milestone-related or related to future Phexxi sales (assuming approval of Phexxi for commercial sale outside of the
United States), or to what degree these arrangements would affect our development plans, future revenues and overall capital requirements.
In
October 2021, we submitted the registration for our hormone-free contraceptive vaginal gel to the Mexican Regulatory Agency Comisión
Federal para la Protección contra Riesgos Sanitarios. In addition to submitting for registration in Mexico, we have also submitted
marketing applications for Phexxi under the trademark Femidence™ in Nigeria, Ethiopia, and Ghana. These were the first of several
strategic regulatory submissions planned under Evofem’s 2020 Global Health Agreement with Adjuvant Capital. In October 2022, Phexxi
was approved in Nigeria, where the product will be potentially marketed under the brand name Femidence™. This is the first regulatory
approval for the contraceptive vaginal gel outside the U.S.
Operating
Expenses
Cost
of Goods Sold
Inventory
costs include all purchased materials, direct labor and manufacturing overhead.
We
are obligated to pay quarterly royalty payments, pursuant to our license agreement with Rush University, in amounts equal to a single-digit
percentage of the gross amounts we receive on a quarterly basis less certain deductions incurred in the quarter based on a sliding scale.
We are also obligated to pay a minimum annual royalty amount of $100,000 to the extent these earned royalties do not equal or exceed
$100,000 in any given year. A minimum annual royalty amount of $100,000 was first required for the annual period commencing on January
1, 2021. These royalty costs were immaterial and $0.3 million for the three months ended March 31, 2023 and 2022, respectively, and were
included in the costs of goods sold in the condensed consolidated financial statements. Such royalty costs were $1.1 million and $0.2
million for the years ended December 31, 2022 and 2021, respectively, and was included in the costs of goods sold in the consolidated
financial statements.
Research
and Development Expenses
Research
and development expenses decreased significantly in the three months ended March 31, 2023 compared to the prior year quarter primarily
due to the completion of EVOGUARD clinical trial in the fourth quarter of 2022. As previously noted, we have discontinued this
program and placed additional programs on hold, and therefore expect a significant reduction in clinical trial expense in 2023 versus
2022 levels.
Our
research and development expenses primarily consist of costs associated with the continuous improvements related to Phexxi commercialization
efforts. These expenses include:
| |
● |
continuous
improvements of manufacturing and analytical efficiency; |
| |
● |
on-going
product characterization and process optimization; |
| |
● |
evaluation
of back-up contract manufacturing organizations to support future commercial forecast and reduce cost of goods sold; |
| |
● |
alternative
raw material evaluation to secure an uninterrupted supply chain and reduce cost of goods sold; |
| |
● |
employee-related
expenses, including salaries, benefits, travel and noncash stock-based compensation expense; and |
| |
● |
facilities,
depreciation and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, depreciation
of leasehold improvements and equipment, and research and other supplies. |
In
prior years, research and development expenses also included costs associated with the now-complete EVOGUARD trial, including:
| |
● |
costs
related to compliance with drug development regulatory requirements; |
| |
● |
external
development expenses incurred under arrangements with third parties, such as fees paid to clinical research organizations (CROs)
relating to our clinical trials; |
| |
● |
costs
of acquiring and evaluating clinical trial data such as investigator grants, patient screening fees, laboratory work and statistical
compilation and analysis, and fees paid to consultants; and, |
| |
● |
costs
to acquire, develop and manufacture clinical trial materials, including fees paid to contract manufacturers. |
We
expense internal and third-party research and development expenses as incurred. The following table summarizes research and development
expenses by product candidate (in thousands):
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Allocated
third-party development expenses: | |
| | | |
| | |
| Phexxi
for prevention of chlamydia/gonorrhea- Phase 3 (EVOGUARD) | |
$ | 93 | | |
$ | 8,287 | |
| Total
allocated third-party development expenses | |
| 93 | | |
| 8,287 | |
| | |
| | | |
| | |
| Unallocated
internal research and development expenses: | |
| | | |
| | |
| Noncash
stock-based compensation expenses | |
| 40 | | |
| 175 | |
| Payroll
and related expenses | |
| 313 | | |
| 1,366 | |
| Outside
services costs | |
| 30 | | |
| 245 | |
| Other | |
| 64 | | |
| 318 | |
| Total
unallocated internal research and development expenses | |
| 447 | | |
| 2,104 | |
| Total
research and development expenses | |
$ | 540 | | |
| 10,391 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Allocated
third-party development expenses: | |
| | | |
| | |
| Phexxi
for prevention of chlamydia/gonorrhea- Phase 3 (EVOGUARD) | |
$ | 17,374 | | |
$ | 23,779 | |
| Unallocated
internal research and development expenses: | |
| | | |
| | |
| Noncash
stock-based compensation expenses | |
| 553 | | |
| 1,357 | |
| Payroll
and related expenses | |
| 3,820 | | |
| 4,967 | |
| Outside
services costs | |
| 1,240 | | |
| 1,696 | |
| Other | |
| 2,045 | | |
| 1,330 | |
| Total
unallocated internal research and development expenses | |
| 7,658 | | |
| 9,350 | |
| Total
research and development expenses | |
$ | 25,032 | | |
$ | 33,129 | |
Selling
and Marketing Expenses
In
connection with our overall cost reduction strategy, our selling and marketing expenses decreased significantly in the three months ended
March 31, 2023 compared to the prior year quarter due to reductions in media and marketing activities related to ongoing Phexxi promotional
strategies, including direct to consumer (DTC) and HCP advertising, and the Phexxi telehealth platform.
Our
selling and marketing expenses consist primarily of Phexxi commercialization costs, the Phexxi telehealth platform, our sample program,
training, salaries, benefits, travel, noncash stock-based compensation expense, and other related costs for our employees and consultants.
General
and Administrative Expenses
Our
general and administrative expenses decreased significantly in the three months ended March 31, 2023 compared to the prior year quarter
primarily due to decreased general legal expenses and recruiting and financing related fees.
Our
general and administrative expenses consist primarily of salaries, benefits, travel, business development expenses, investor relations
expenses, noncash stock-based compensation, and other related costs for our employees and consultants performing executive, administrative,
finance, legal and human resource functions. Other general and administrative expenses include facility-related costs not otherwise included
in research and development or selling and marketing, and professional fees for accounting, auditing, tax and legal fees, and other costs
associated with obtaining and maintaining our patent portfolio.
Other
Income (Expense)
Other
income (expense) consists primarily of interest expense and the change in fair value of financial instruments issued in various capital
raise transactions. The change in fair value of financial instruments was recognized as a result of mark-to-market adjustments for those
financial instruments.
Results
of Operations
Three
Months Ended March 31, 2023 Compared to Three Months Ended March 31, 2022 (in thousands):
Net
Product Sales
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| Product
sales, net | |
$ | 5,809 | | |
$ | 4,251 | | |
$ | 1,558 | | |
| 37 | % |
The
increase in product sales, net, was primarily due to the continued growth in Phexxi ex-factory unit sales from the impact of Phexxi promotional
strategies.
Cost
of Goods Sold
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| Cost
of goods sold | |
$ | 1,376 | | |
$ | 1,066 | | |
$ | 310 | | |
| 29 | % |
The
increase in cost of goods sold was primarily due to the increase in sales in the current period versus the same period in the prior year.
Research
and Development Expenses
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| Research
and development | |
$ | 540 | | |
$ | 10,391 | | |
$ | (9,851 | ) | |
| (95 | )% |
The
decrease in research and development expenses was primarily due to an $8.0 million decrease in clinical trial costs associated with EVOGUARD,
which was completed in the fourth quarter of 2022, and a $1.2 million decrease in personnel costs due to reduced headcount and lower
noncash stock-based compensation.
Selling
and Marketing Expenses
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| Selling
and marketing | |
$ | 3,854 | | |
$ | 12,705 | | |
$ | (8,851 | ) | |
| (70 | )% |
The
decrease in selling and marketing expenses was primarily due to a $5.8 million decrease in marketing and DTC promotion costs, including
media agency fees, a $2.0 million decrease in a personnel costs due to reduced headcount and lower noncash stock-based compensation,
a $0.4 million decrease in costs for outside services associated with medical affairs and marketing activities, and a $0.4 million decrease
in facilities costs.
General
and Administrative Expenses
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| General
and administrative | |
$ | 3,618 | | |
$ | 9,018 | | |
$ | (5,400 | ) | |
| (60 | )% |
The
decrease in general and administrative expenses was primarily due to a $2.6 million decrease in a personnel costs due to reduced headcount
and lower noncash stock-based compensation, a $2.0 million decrease in professional services fees related to legal and finance, and a
$0.5 million decrease in costs for outside services.
Total
other income (expense), net
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| Total
other income (expense), net | |
$ | 1,228 | | |
$ | (2,956 | ) | |
$ | 4,184 | | |
| (142 | )% |
Total
other income (expense), net, for the three months ended March 31, 2023 primarily included a $1.6 million gain from the change in fair
value of financial instruments, $0.2 million gain from the disposal of our fleet cards, offset by $0.6 million interest expense related
to the Adjuvant Note.
Total
other income (expense), net, for the three months ended March 31, 2022, primarily included a $1.6 million recorded loss from the change
in fair value of the Baker Notes, January and March 2022 notes, and derivative liabilities, as described in Note 4- Debt to the
2023 Q1 Interim Financial Statements, as a result of mark-to-market adjustments during the quarter, a $0.9 million loss
on issuance of the January and March 2022 notes and warrants, and $0.5 million in interest expense related to convertible notes.
Year
Ended December 31, 2022 Compared to Year Ended December 31, 2021 (in thousands):
Net
Product Sales
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| Product
sales, net | |
$ | 16,837 | | |
| 8,244 | | |
$ | 8,593 | | |
| 104 | % |
The
increase in net product sales was primarily due to continued growth in ex-factory Phexxi unit sales and an increase in net sales from
the impact of Phexxi promotional strategies and gross-to-net initiatives implemented in 2022.
Cost
of Goods Sold
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| Cost
of goods sold | |
$ | 4,415 | | |
| 4,055 | | |
$ | 360 | | |
| 9 | % |
The
increase in cost of goods sold was primarily due to increase in royalty costs associated with the growth in Phexxi net sales, partially
offset by the reversal of excess & obsolete inventory reserve recorded in 2021.
Research
and Development Expenses
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| Research
and development | |
$ | 25,032 | | |
| 33,129 | | |
$ | (8,097 | ) | |
| (24 | )% |
The
decrease in research and development expenses was primarily due to a $7.2 million decrease in clinical trial costs associated with EVOGUARD,
a $1.1 million decrease in payroll and related expenses due to reduced headcount, and a $0.8 million decrease in noncash stock-based
compensation. These decreases were partially offset by a $1.1 million increase in facilities and other research and development related
activities.
Selling
and Marketing Expenses
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| Selling
and marketing | |
$ | 43,951 | | |
| 113,152 | | |
| (69,201 | ) | |
| (61 | )% |
The
decrease in selling and marketing expenses was primarily due to a $61.0 million decrease in media and marketing costs related to ongoing
promotional strategies, a $6.3 million decrease in payroll and related expenses due to reduced headcount, $1.6 million in the Phexxi
sample program, and a $1.3 million decrease in facilities costs. These aggregated decreases were partially offset by a $2.0 million increase
in noncash stock-based compensation.
General
and Administrative Expenses
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| General
and administrative | |
$ | 27,563 | | |
| 24,709 | | |
$ | 2,854 | | |
| 12 | % |
The
increase in general and administrative expenses was primarily due to a $7.7 million increase in legal, corporate, and financing related
expenses. This increase was partially offset by a decrease of $3.4 million in noncash stock-based compensation expense and a $1.4 million
decrease in payroll related expenses due to reduced headcount.
Total
Other Expense, Net
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| Total
other expense, net | |
$ | 7,470 | | |
$ | (38,374 | ) | |
$ | 45,844 | | |
| (119 | )% |
Total
other expense, net, for the year ended December 31, 2022, primarily due to gains of $92.2 million from the change in fair value of the
liability classified warrants issued in 2022, and $2.5 million from the partial extinguishment of the Adjuvant Notes. These gains were
partially offset by losses of: $73.0 million recorded upon issuance of financial instruments, primarily from the June 2022 Baker Warrants,
$10.3 million from the change in the fair value of the May Notes as a result of mark-to-market adjustments and $2.0 million from the
change in fair value of the Baker Notes as a result of mark-to-market adjustments unrelated to changes in credit risk, and $2.2 million
in interest expense related to the Adjuvant Notes.
Total
other expense, net, for the year ended December 31, 2021 primarily included $4.7 million in interest expense related to the Baker Notes
and the Adjuvant Notes and a $33.7 million recorded loss as a result of mark-to-market adjustments including the recorded loss from the
change in fair value of the Baker Notes and the recorded gain from the change in fair value of the derivative liability
Liquidity
and Capital Resources
Overview
As
of March 31, 2023, we had a working capital deficit of $63.3 million and an accumulated deficit of $941.0 million. We have financed our
operations to date primarily through the issuance of preferred stock, common stock, warrants and convertible and term notes; cash received
from private placement transactions; and, to a lesser extent, product sales. As of March 31, 2023, we had approximately $0.6 million
in cash and cash equivalents, and $0.9 million in restricted cash available for use from the Adjuvant Notes. Our cash and cash equivalents
include amounts held in checking accounts. According to management estimates, liquidity resources as of March 31, 2023 are
not sufficient to maintain the Company’s cash flow needs for the twelve months from the date of issuance of these condensed consolidated
financial statements.
We
have incurred losses and negative cash flows from operating activities since inception. During the three months ended March 31, 2023,
we received proceeds of approximately $1.6 million, in aggregate, before debt issuance costs, from the sale of senior subordinate convertible
notes and warrants. During the year ended December 31, 2022, we received gross proceeds of $11.5 million from the sale of notes and warrants
in three registered direct offerings, net proceeds of $7.4 million from the sale and issuance of common stock pursuant to the Stock Purchase
Agreement, net proceeds of $18.1 million upon the sale and issuance of common stock and warrants from the May 2022 Public Offering, and
$25.2 million from the exercise of common warrants.
We
aim to reach operational earnings before interest, taxes, depreciation, amortization (EBITDA) break even on a normalized basis by year-end
2023 and anticipate that we will continue to restructure our trade payables with extended terms and to attempt to cure existing defaults.
We have implemented measures, including headcount reductions in November 2022 and March 2023, to right size our cost structure with projected
revenues. For 2023, we expect research and development expenses to decrease significantly primarily due to the completion of EVOGUARD
and discontinuation of this clinical program in October 2022; selling and marketing expenses to decrease significantly due to reductions
in media and marketing activities related to ongoing Phexxi promotional strategies; and general and administrative expenses to decrease
slightly due to reductions in headcount partially offset by increased professional and consulting expenses.
Despite
the letter of default, our senior lenders have not yet taken additional actions in accordance with their contractual rights. If we can
cure existing defaults, we currently expect our liquidity resources as of March 31, 2023, together with the net proceeds from the 2023
Offerings, defined below, cost reductions, restructuring of outstanding account payable and liquidity tactics to be sufficient to fund
our planned operations into the third quarter of 2023. As of March 31, 2023, our significant commitments include the Baker Notes, as
described in Note 4- Debt, our office lease, fleet leases, and our supply and manufacturing agreement with our Phexxi manufacturer,
as described in Note 7- Commitments and Contingencies to the 2023 Q1 Interim Financial Statements. The purpose of
these commitments is to further the commercialization of Phexxi. We expect to fund these commitments through debt and equity issuances
and product sales.
Our
management is currently evaluating different strategies to obtain the required funding for our operations. These strategies may include,
but are not limited to: public and private placements of equity and/or debt, licensing and/or collaboration arrangements and strategic
alternatives with third parties, corporate restructuring, or other potential funding from third parties. Our ability to secure funding
is subject to numerous risks and uncertainties, including the impact of geopolitical turmoil related to the ongoing
hostilities in Ukraine and economic uncertainty related to rising inflation and disruptions in the global supply chain. As a result,
there can be no assurance that these funding efforts will be successful. Our ability to raise additional funds, and the terms on which
those funds may be raised, will be dependent, in part, on how successful the commercialization of Phexxi is, the success of our cost
reduction and gross-to-net improvement efforts, the accuracy of our estimates regarding cash needed to fund our operations, our ability
to comply with the terms of our debt arrangements, and whether we are able to gain revenue traction prior to raising additional funds.
If
we are not able to obtain required additional funding when and as needed, through equity financings or other means, or if we are unable
to obtain funding on terms favorable to us, the shortfall in funds raised, or such unfavorable terms, will likely have a material adverse
effect on our operations and strategic plan for future growth. If we cannot successfully raise the funding necessary to implement our
current and ongoing liquidity tactics or as necessary to comply with obligations pursuant to our debt arrangements (including any acceleration
of those obligations), we may be forced to make further reductions in spending, expand on our extended payment terms with suppliers,
liquidate assets where possible, suspend or curtail planned programs, and/or cease operations entirely. Any of these developments would
materially and adversely affect our financial condition and business prospects and could even cause us to be unable to continue as a
going concern. If we are unable to continue as a going concern, we may have to liquidate our assets and, in doing so, we may receive
less than the value at which those assets are carried on our financial statements. Any of these developments would materially and adversely
affect the price of our stock and the value of an investment in our stock. As a result, our financial statements include explanatory
disclosures expressing substantial doubt about our ability to continue as a going concern.
The
opinion of our independent registered public accounting firm on our audited financial statements as of and for the years ended December
31, 2022 and 2021 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Future
reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern.
Our audited consolidated financial statements as of and for the years ended December 31, 2022 and 2021 and our unaudited condensed consolidated
financial statements as of March 31, 2023 and for the three months ended March 31, 2023 and 2022 included in this prospectus do not include
any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might be necessary
should we be unable to continue our operations.
2023
Debt Financings
In
February, March and April 2023, we entered into securities purchase agreements with certain investors providing for the sale and issuance
of senior secured convertible notes (collectively, the 2023 SPAs). The 2023 SPAs included (i) convertible promissory notes with aggregate
original principal amounts of approximately $1.4 million, $0.6 million, $0.5 million and $0.8 million, respectively (the 2023 Notes),
and (ii) warrants to purchase an aggregate 553,846, 240,000, 215,384, and 615,384 shares of common stock, respectively (the 2023 Warrants
and collectively, the 2023 Offerings). The 2023 Offerings closed on February 17, 2023 (the February 2023 Closing), March 13, 2023 and
March 20, 2023 (the March 2023 Closings) and April 5, 2023 (the April 2023 Closing), respectively, with gross proceeds to the
Company, before deducting offering expenses, of approximately $0.9 million, $0.4 million, $0.3 million, and $0.5
million, respectively. The 2023 SPAs also included a Registration Rights Agreement requiring us to register the common stock underlying
the 2023 Notes and 2023 Warrants within the time frames specified therein. In addition, the Company issued warrants to purchase an aggregate
99,692 and 43,200 shares of common stock in February and March 2023 Closing to the placement agent.
In July 2023,
we entered into an SPA with certain investors providing for the sale and issuance of senior secured convertible notes due in the aggregate
original principal amount of $1.5 million (the Notes), and warrants to purchase an aggregate 1,200,000 shares of common stock (Warrants)
(collectively, the July 2023 Offering). The July 2023 Offering closed on July 3, 2023 with net proceeds to the Company, after deducting
offering expenses, of approximately $1.0 million.
Upon
the April 2023 Closing, the conversion and strike prices, as applicable, of the Baker Notes, Baker Warrants, the May 2022 Common Warrants,
the June 2022 Baker Warrants, the Adjuvant Notes, the December 2022 Notes and Warrants, and the Notes and Warrants in the February and
March 2023 Closing reset to $0.8125 per share, accordingly. Additionally, the Company’s outstanding Purchase Rights increased by
approximately 15,218,227 since March 31, 2023.
2022
Debt and Equity Financings
We
received net proceeds of $10.0 million, before issuance
costs, from the sale of notes and warrants in two registered direct offerings in the first quarter of 2022. These notes were then exchanged
for the May 2022 Notes during the May 2022 Exchange transaction, which were subsequently exchanged for Purchase Rights during the debt
restructuring in September 2022 with a total outstanding balance of $21.8 million immediately prior to the restructuring.
We received
net proceeds of $18.1 million upon the sale and issuance of common stock and warrants from an underwritten public offering in May
2022, net proceeds of $7.4 million from the sale and issuance of common stock pursuant to the Stock Purchase Agreement, and $25.2
million from the exercise of common warrants.
We
received gross proceeds of $2.3 million, before
issuance costs, from the sale of notes, warrants and non-convertible Series D preferred stock in December 2022.
2021
Equity Financings
We
received proceeds of approximately $28.0 million,
net of underwriting discounts, from a public offering in March 2021, upon the issuance of 9,142 shares of our common stock, and approximately
$4.2 million, net of underwriting discounts, from the issuance of 1,371 shares of common stock upon exercise of the underwriters’
overallotment option in April 2021.
We
received proceeds of approximately $46.8 million,
net of underwriting discounts and fees, from a public offering in May 2021, upon the issuance of 26,666 shares of common stock and common
warrants to purchase 26,666 shares of common stock. We received approximately $2.4 million and $0.1 million, both net of underwriting
discounts, from the issuance of 1,358 shares of common stock and 4,000 common warrants, respectively, upon exercise of the underwriter’s
overallotment option in May 2021.
We
received proceeds of approximately $9.6 million,
net of offering expenses, from a registered direct offering in October 2021, upon the issuance of 5,000 shares of Series B-1 Convertible
Preferred Stock and 5,000 shares of Series B-2 Convertible Preferred Stock.
Summary
Statements of Cash Flows
The
following table sets forth a summary of the net cash flow activity for the three months ended March 31, 2023 and 2022 (in thousands):
| | |
Three
Months Ended March 31, | | |
2023
vs. 2022 | |
| | |
2023 | | |
2022 | | |
$
Change | | |
%
Change | |
| Net
cash, cash equivalents and restricted cash used in operating activities | |
$ | (4,940 | ) | |
$ | (20,919 | ) | |
$ | 15,979 | | |
| (76 | )% |
| Net
cash, cash equivalents and restricted cash used in investing activities | |
| (3 | ) | |
| (66 | ) | |
| 63 | | |
| (95 | )% |
| Net
cash, cash equivalents and restricted cash provided by financing activities | |
| 1,701 | | |
| 15,129 | | |
| (13,428 | ) | |
| (89 | )% |
| Net
decrease in cash, cash equivalents and restricted cash | |
$ | (3,242 | ) | |
$ | (5,856 | ) | |
$ | 2,614 | | |
| (45 | )% |
Cash
Flows from Operating Activities. During the three months ended March 31, 2023 and 2022, the primary use of cash, cash equivalents
and restricted cash was to fund the commercialization of Phexxi and to support general and administrative operations and, in 2022, to
fund the Phase 3 clinical trial to evaluate EVO100 for the prevention of chlamydia and gonorrhea.
Cash
Flows from Financing Activities. During the three months ended March 31, 2023, the primary source of cash, cash equivalents and restricted
cash was the sale of senior subordinate convertible notes and warrants for proceeds of approximately $1.6 million, in aggregate, before
the debt issuance costs. During the three months ended March 31, 2022, the primary source of cash, cash equivalents and restricted cash
was the sale of 8,647 shares of common stock for net proceeds of approximately $5.7 million (including $5.4 million in cash and cash
equivalents and $0.3 million in other receivables) and gross proceeds of $10.0 million from the sale of term notes and warrants.
The
following table sets forth a summary of the net cash flow activity for the years ended December 31, 2022 and 2021 (in thousands):
| | |
Year
Ended December 31, | | |
2022
vs. 2021 | |
| | |
2022 | | |
2021 | | |
$
Change | | |
%
Change | |
| Net
cash, cash equivalents and restricted cash used in operating activities | |
$ | (70,410 | ) | |
$ | (146,667 | ) | |
$ | 76,257 | | |
| (52 | )% |
| Net
cash, cash equivalents and restricted cash used in investing activities | |
| (341 | ) | |
| (2,689 | ) | |
| 2,348 | | |
| (87 | )% |
| Net
cash, cash equivalents and restricted cash provided by financing activities | |
| 61,939 | | |
| 90,693 | | |
| (28,754 | ) | |
| (32 | )% |
| Net
(decrease) increase in cash, cash equivalents and restricted cash | |
$ | (8,812 | ) | |
$ | (58,663 | ) | |
$ | 49,851 | | |
| (85 | )% |
Cash
Flows from Operating Activities. During the years ended December 31, 2022 and 2021, the primary use of cash, cash equivalents and
restricted cash was to fund commercialization of our lead product Phexxi, to fund the Phase 3 clinical trial to evaluate EVO100 for the
prevention of chlamydia and gonorrhea, and to support selling and marketing and general and administrative operations.
Cash
Flows from Investing Activities. During the year ended December 31, 2022, the change in net cash, cash equivalents and restricted
cash used in investing activities was primarily due to $0.3 million in purchases of property and equipment. During the year ended December
31, 2021, the change in net cash, cash equivalents and restricted cash used in investing activities was primarily due to $2.9 million
in purchases of property and equipment, offset by a $0.3 million cash inflow from the sale of Softcup line of business.
Cash
Flows from Financing Activities. During the year ended December 31, 2022, the primary source of cash, cash equivalents and restricted
cash was provided from the issuance of 181,320 shares of common stock, warrants to purchase 568,000 shares of common stock and pre-funded
warrants to purchase 102,680 shares of common stock for net proceeds of $24.9 million; the issuance of 282,518 shares of our common
stock for net proceeds of $25.2 million from the exercise of common warrants; and the issuance of 15,714 shares of common stock for net
proceeds of $7.4 million and net proceeds of $11.5 million from the sale of term notes and warrants, net of original issue discount when
applicable.
During
the year ended December 31, 2021, the primary source of cash, cash equivalents and restricted cash was provided from the issuance of
38,539 shares of common stock and 4,000 shares of common warrants for proceeds of approximately $81.5 million, net of underwriting discounts,
the issuance of 245 shares of our common stock under the 2019 Employee Stock Purchase Plan (ESPP) with proceeds of approximately $0.3
million, the issuance of 84 shares of common stock from the exercise of common warrants for proceeds of approximately $0.2 million, the
issuance of 5,000 shares of Series B-1 Convertible Preferred Stock and 5,000 shares of Series B-2 Convertible Preferred Stock for proceeds
of approximately $9.6 million, net of offering expenses, offset by $0.3 million in payments of tax withholdings related to vesting of
restricted stock awards and $1.0 million in payments for financing issuance costs.
Operating
and Capital Expenditure Requirements
Our
specific future operating and capital expense requirements are difficult to forecast. However, we can anticipate the general types of
expenses and areas in which they might occur in 2023 as follows: we expect all our operating expenses to decrease significantly due to
the reasons stated under “Operating Expenses” above.
Contractual
Obligations and Commitments
Operating
Leases
Operating
lease right-of-use assets and lease liabilities were $3.9 and $4.9 million on March 31, 2023, respectively, and were $4.4
and $5.4 million on December 31, 2022, respectively. On December 31, 2022, operating lease ROU assets and lease liabilities were $4.4
million and $5.4 million, respectively, and were $5.4 million and $6.8 million, respectively, on December 31, 2021. See Note 7-
Commitments and Contingencies to the 2023 Q1 Interim Financial Statements for more detailed discussions
on leases and financial statements information under ASC 842, Leases.
Other
Contractual Commitments
As
described in Note 7- Commitments and Contingencies to the 2023 Q1 Interim Financial Statements, in November
2019, the Company entered into a supply and manufacturing agreement with a third-party to manufacture Phexxi, with potential to manufacture
other product candidates in accordance with all applicable current good manufacturing practice regulations, pursuant to which the Company
has certain minimum purchase commitments based on the forecasted product sales. The amounts purchased under the supply and manufacturing
agreement were none for the months ended March 31, 2023 and 2022, and $1.0 million and $3.0 million for the years ended December 31,
2022 and 2021, respectively.
Intellectual
Property Rights
As
described in Note 7- Commitments and Contingencies to the 2023 Q1 Interim Financial Statements, royalty costs owed
to Rush University pursuant to the Rush License Agreement were an immaterial amount and $0.3 million for the three months ended March
31, 2023 and 2022, respectively, and $1.1 million and $0.2 million for the years ended December 31, 2022 and 2021, respectively. As of
December 31, 2022 and 2021, approximately $0.6 million and an immaterial amount were included in accrued expenses in the consolidated
balance sheets.
Other
Matters
Recently
Adopted Accounting Pronouncements
In
August 2020, the FASB issued ASU No. 2020-06, Debt (ASU No. 2020-06), removing, modifying, and adding certain disclosure
requirements of ASC 470, Debt with Conversion and Other Options, and ASC 815, Derivatives and Hedging - Contracts in Entity’s
Own Equity (ASC 815). ASU No. 2020-06 will be effective for the Company beginning January 1, 2024 and early adoption is allowed.
The adoption of ASU No. 2020-06 on January 1, 2022 did not have a material impact on the Company’s 2023 Q1 Interim Financial
Statements and 2022 Audited Annual Financial Statements.
In
March 2022, the FASB issued ASU No. 2022-02, Financial Instruments - Credit Losses (ASU No. 2022-02). This is an amendment to ASU 2016-13,
where it eliminates the accounting guidance for troubled debt restructuring by creditors in Subtopic 310-40, Receivables—Troubled
Debt Restructurings by Creditors, while enhancing disclosure requirements for certain loan refinancings and restructurings by creditors
when a borrower is experiencing financial difficulty. ASU No. 2022-02
was
effective for the Company beginning January 1, 2023 since the Company adopted ASU 2016-13 on January 1, 2020. The adoption of this new
standard did not have a material impact on the Company’s 2023 Q1 Interim Financial Statements and 2022 Audited Annual Financial
Statements consolidated financial statements.
Recently
Issued Accounting Pronouncements — Not Yet Adopted
From
time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standards setting bodies
that are adopted as of the specified effective date. The Company believes the impact of recently issued standards and any issued but
not yet effective standards will not have a material impact on its consolidated financial statements upon adoption.
Critical
Accounting Policies
Our
consolidated financial statements have been prepared in accordance with generally accepted accounting principles (GAAP) in the
United States. The preparation of consolidated financial statements requires us to make use of estimates, assumptions and judgments
that affect the reported amounts of assets, expenses, and liabilities, as well as the disclosure of contingent liabilities on the
date of the consolidated financial statements. Management bases its estimates, assumptions, and judgments on historical experience
and on various other factors it believes to be reasonable under the circumstances. Different estimates, assumptions and judgments
may change the estimate used in the preparation of our consolidated financial statements, which, in turn, could materially change
our results from those reported. Management evaluates its use of estimates, assumptions, and judgments on an ongoing basis. However,
if our assumptions change, we may need to revise our estimates, or take other corrective actions, either of which may have a
material adverse effect on our consolidated statements of operations, liquidity, and financial condition. We believe the following
critical accounting policies involve significant areas where management applies estimates, assumptions, and judgments in the
preparation of our consolidated financial statements. See Note 2- Summary of Significant Accounting Policies to the 2022 Audited
Annual Financial Statements.
DESCRIPTION
OF BUSINESS
Item
1. Business.
Overview
Company
Overview
We
are a San Diego-based commercial-stage biopharmaceutical company committed to developing and commercializing innovative products to address
unmet needs in women’s sexual and reproductive health. Our first commercial product, Phexxi, was approved by the FDA on May 22,
2020 and is the first and only FDA-approved, hormone-free, woman-controlled, on-demand prescription contraceptive gel for women. We commercially
launched Phexxi in September 2020 in the United States. We intend to commercialize Phexxi in global markets through partnerships or licensing
agreements.
Phexxi
as a Contraceptive; Commercial Strategies
Our
sales force promotes Phexxi directly to obstetrician/gynecologists and their affiliated health professionals, who collectively write
the majority of prescriptions for contraceptive products. As of September 30, 2022, our sales force consisted of 50 sales representatives
and 6 business managers, supported by a self-guided virtual health care provider (HCP) learning platform. After the RIF, as discussed
below, our sales force consists of 20 sales representatives and 3 business managers. Additionally, we offer women direct access to Phexxi
via our telehealth platform. Using the platform, women can directly meet with an HCP to determine their eligibility for a Phexxi prescription
and, if eligible, have the prescription written by the HCP, filled, and mailed directly to them by a third-party pharmacy.
Our
comprehensive commercial strategy for Phexxi includes marketing and product awareness campaigns targeting women of reproductive potential
in the United States as well as certain identified target HCP segments. Our target audience includes the approximately 23 million women
who are not using hormonal contraception and the approximately 18.8 million women who are using a prescription contraceptive, some of
whom, particularly pill users, may be ready to move to an FDA-approved, non-invasive hormone-free contraceptive. In addition to marketing
and product awareness campaigns, our commercial strategy includes payer outreach and execution of our consumer digital and media strategy.
According
to our market research since Phexxi’s commercial launch, HCPs indicate they would recommend Phexxi to approximately:
| ● | 47%
of patients experiencing side effects from current contraception; |
| ● | 37%
of patients using non-hormonal prescription contraception; |
| ● | 36%
of patients seeking pregnancy prevention; and |
| ● | 19%
of patients using hormonal prescription contraception. |
Additional
research into the demographics of more than 5,000 women who are using Phexxi revealed that 79% of Phexxi users are between 18 to 34 years
of age. Among the subset of Phexxi users for whom prior contraceptive data is available (n=2,512), 80% of women who had recently started
Phexxi were not on any method of prescription contraception. Another 20% switched to Phexxi from either oral contraceptives, hormonal
rings or patches.
In
February 2021, we launched a direct-to-consumer advertising campaign, known as “Get Phexxi,” designed to increase awareness
and educate women on the benefits of Phexxi. The campaign highlighted some of the struggles women face when choosing among the many available
methods of contraception, including the lack of control with condoms, daily use of the pill, and abstinence required for cycle tracking.
In
September 2021, we launched a national brand ambassador campaign featuring Emmy Award-winning celebrity Annie Murphy, designed to broaden
awareness and drive uptake of Phexxi. This award-winning campaign, known as “House Rules,” has significantly raised our target
audience awareness of Phexxi. To date, the House Rules campaign has grown brand awareness by 45% and garnered 42 million video views
and over 33,000 telehealth exits. More importantly, it has also helped drive significant increases in new HCPs recommending and prescribing
Phexxi.
Over
the course of 2021, ex-factory units grew quarter over quarter, with the most significant growth in the fourth quarter following “House
Rules;” Phexxi units shipped increased 73% as compared to the prior quarter, propelled by a 56% increase in new patients starting
Phexxi and a 111% increase in refills as compared to the prior quarter.
The
first quarter of 2022 reflected anticipated softness in Phexxi prescription and dispensed unit growth due to the annual reset of patient
healthcare deductibles, which impacted most contraceptive brands, as well as from adjustments to Evofem’s patient support programs
in January 2022 intended to increase the profit margin on Phexxi units dispensed and support continued net product sales growth.
Approved
claims for Phexxi have increased throughout 2022, and currently 71% of Phexxi claims are being approved, up from a low of 55% at launch
in September 2020.
In
the second quarter of 2022, we successfully negotiated an agreement with one of the nation’s largest pharmacy benefit managers
(PBMs) to ensure most women covered by this plan can fill their Phexxi prescription. The agreement took effect on July 1, 2022 and is
representative of approximately 48 million lives.
We
continue working to increase the number of lives covered and to gain a preferred formulary position for Phexxi. Currently Phexxi has
coverage for approximately 60% of U.S. commercial lives. This includes:
| ● | U.S.
Department of Veterans Affairs: Our December 2020 contract award from the U.S. Department
of Veterans Affairs covers approximately 13.7 million commercial lives. |
| ● | The
U.S. Medicaid population: Medicaid provides health coverage to approximately 68 million
members, including approximately 16.8 million women 19 to 49 years of age gaining access
to Phexxi on January 1, 2021 through our participation in the Medicaid National Drug Rebate
Program. |
| ● | Pharmacy
Benefits Manager: As mentioned above, we successfully negotiated a contract with one
of the largest PBMs in the nation, which added Phexxi to its formulary with no restrictions
for most women covered by the plan. The agreement took effect July 1, 2022. |
Approximately
18 million commercial lives have access to Phexxi at no out-of-pocket cost. This is due in part to one of the largest plans in California
covering Phexxi with $0 copay effective August 1, 2022.
Coverage
for and access to, Phexxi is expected to further increase as additional insurers and PBMs comply with the January 2022 guidance regarding
access to contraception in the U.S. from the Health Resources and Services Administration (HRSA) and the U.S. Department of Labor.
The new guidance specifies that most insurers and PBMs must provide coverage, with no out-of-pocket costs to women, for FDA-approved
contraceptive products, like Phexxi, prescribed by healthcare providers. Compliance with the January 2022 guidance is expected on or
before January 1, 2023.
Phexxi
is classified in the databases and pricing compendia of Medi-Span and First Databank, two major drug information databases that payers
can consult for pricing and product information, as the first and only “vaginal pH modulator.”
As
of January 1, 2023, most insurers and pharmacy benefit managers (PBMs) must provide coverage, with no out-of-pocket costs (e.g. $0
copay) to the subscriber or dependent, for FDA-approved contraceptive products, like Phexxi, prescribed by healthcare providers.
As
a result, to comply with these Guidelines, payers are increasingly covering Phexxi by:
–
Adding Phexxi to formulary (commercial insurers) or preferred drug list (Medicaid)
–
Removing the requirement for a Prior Authorization letter from the HCP (commercial insurers)
–
Moving Phexxi to $0 copay (commercial insurers)
In
2022, Evofem developed and introduced a new educational chart for patients and HCPs that details high-level information about birth control
methods currently available to women in the U.S., including the vaginal pH modulator. This new educational tool has been extremely well
received and has had a positive impact with HCPs and patients alike.
EVO100
for the Prevention of Chlamydia and Gonorrhea
Based
on positive and statistically significant top-line results of our Phase 2B/3 AMPREVENCE trial, in October 2020 we initiated the
Phase 3 EVOGUARD clinical trial to evaluate EVO100 for the prevention of urogenital chlamydia and gonorrhea infections in women.
This randomized, placebo-controlled clinical trial enrolled 1,903 women with a prior chlamydia or gonorrhea infection who were at risk
for future infection.
On
October 11, 2022, we reported that EVOGUARD did not meet its primary efficacy endpoint. The Company believes COVID-19 related
changes in clinical site operations, subject behavior and actions including deviations from following the clinical study protocol requirements
related to STI acquisition, detection, and prevention contributed to this outcome. The product safety profile was consistent with what
has been observed in prior clinical trials, and only two women (0.1%) in the study discontinued due to adverse events. Due to financial
constraints, we discontinued investment in this clinical program.
Multipurpose
Prevention Technology Vaginal Gel for HIV Prevention
In
December 2021, we launched a collaboration with Orion Biotechnology Canada Ltd. (Orion) to evaluate the compatibility and stability of
Orion’s novel CCR5 antagonist, OB-002, in Phexxi with the goal of developing a Multipurpose Prevention Technology (MPT) product
candidate for indications including the prevention of HIV in women. Assuming positive preclinical results, Evofem and Orion will seek
government and philanthropic funding for subsequent clinical trials of any resulting MPT vaginal gel product candidate.
Our
Strategy
Key
elements of our strategy include:
●
Successfully commercialize Phexxi. Our primary focus is the successful commercialization of Phexxi in the United States.
Outside the United States, we intend to commercialize Phexxi through strategic partnerships or license agreements in several key target
regions, including the Greater European Union plus the United Kingdom (EU), Asia Pacific (APAC), and Latin America (LATAM). We believe
this approach will allow us to effectively deploy our capital to maximize the inherent value of Phexxi for the benefit of all stakeholders.
●
Leverage our vaginal pH modulator platform to develop and commercialize novel, first-in-class products for women. Following
the successful development and FDA approval of Phexxi for the prevention of pregnancy, we are continuing the clinical development of
our vaginal pH modulator platform to develop additional product candidates.
●
Expand our intellectual property position by pursuing opportunities to extend the exclusivity of our highly differentiated and
proprietary product candidates. We intend to aggressively pursue additional and new patent applications to broaden our intellectual
property portfolio. We continue to seek domestic and international patent protection and endeavor to proactively file patent applications
for new commercially valuable inventions.
●
Build our product portfolio and leverage our U.S. sales force through business development. We intend to opportunistically
acquire or in-license additional products and/or product candidates to enhance our offerings and complement our core competencies in
women’s health.
Contraceptive
Market Overview
United
States Contraceptive Market
The
total U.S. contraceptive market was valued at $8.3 billion in 2022 and is expected to reach approximately $12 billion by 2030 with a
compound annual growth rate of 4.7% (source: April 2022 Research and Markets U.S. Contraceptive Market Report).
In
the U.S., current contraceptive options include:
| | ● | devices
designed to prevent pregnancy through physical means, such as condoms and diaphragms; |
| ● | hormone-based
pharmaceutical products, including oral contraceptives (OCs), vaginal rings, transdermal
patches, intramuscular injections, subcutaneous implants and intrauterine devices (IUDs).
These can be associated with undesirable side effects such as weight gain, loss of libido
and mood changes that may lead women to discontinue their use and seek alternative contraceptive
methods. Further, a peer-reviewed analysis published in the journal PLOS Medicine
in March 2023 found that the use of all kinds of hormonal birth control is associated with
a slight increase in the risk of breast cancer; |
| | ● | a
hormone-free copper IUD; and |
| | ● | Phexxi,
a prescription vaginal pH modulator that was introduced to the market in September 2020. |
The
only non-hormonal option within the top five sales-generating segments in 2019 was the male condom, which is an over-the-counter (OTC)
product. Besides condoms, the only currently available OTC products in the United States are nonoxynol-9 containing (N-9) spermicides.
These surfactant-based products can potentially cause genital irritation and inflammation that may increase the risk of contracting human
immunodeficiency virus (HIV) or other STIs from an infected partner. The FDA requires specific warnings to appear on all N-9 products
that state: “this product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected
partner” as well as: “Do not use if you or your sex partner has HIV/AIDS. If you do not know if you or your sex partner is
infected, choose another form of birth control method.”
As
shown in the chart below, in the United States, 13.0 million women use no method of birth control, putting them at risk of unintended
pregnancy. An additional 10.3 million women in the United States rely on condoms or some other form of non-hormonal OTC birth control
(e.g. rhythm, withdrawal). Another 20.0 million women in the United States use prescription birth control methods, which are predominantly
hormone-based with the sole exception of the copper IUD.
U.S.
Contraceptive Market

Source:
Daniels-K-and-Abma-J.-Current-Contraceptive-Status-Among-Women-Aged-15-49_NCHS-Data-Brief-Number-388-October-2020.pdf (evofem.com)
Market
Opportunity: Contraception
Hundreds
of millions of women worldwide seek sexual and reproductive health products that provide them with their self-defined control of their
individual needs during their (on average) 30+ years of fertility. However, an estimated 257 million women who want to avoid pregnancy
are not using safe, modern methods of contraception and nearly half of all pregnancies - 121 million each year - are unintended according
to the United Nations 2022 State of World Population 2022 report.
Innovation
and new product introductions in the women’s reproductive and sexual health care arena have been limited when compared to other
therapeutic categories. While several new contraceptive category entrants have been introduced in recent years, we believe Phexxi is
the first innovative contraceptive method introduced in the United States since NuvaRing was approved by the FDA in 2001.
According
to the CDC, reducing the percentage of all unintended pregnancies has been one of the National Health Promotion Objectives since its
establishment in 1980. Despite efforts to reduce their incidence, over two million unintended pregnancies occur in the United States
annually. Following decades of minimal change or increase, the percentage of unintended pregnancies in the United States decreased slightly
from 2008 to 2011. Despite this decrease, 45%, or 2.8 million of the 6.1 million total pregnancies in the United States, were unintended
in 2011 (Finer et al., NEJM, 2016).
Our
Commercial Product
Phexxi
as a Contraceptive
Phexxi
vaginal gel is the only FDA-approved, hormone-free, on-demand, woman-controlled prescription contraceptive drug product available in
the United States. We believe Phexxi’s attributes address significant gaps and underserved and unmet needs in the contraceptive
market and make it an attractive contraceptive choice for women.
Phexxi
key benefits:
| ● | Hormone-free:
Phexxi is an innovative gel that works to prevent pregnancy without the use of hormones.
Because Phexxi is completely hormone-free, women do not have to worry about the hormone related
side effects like weight gain, mood swings, or blood clots which are associated with hormonal
birth control methods. |
| ● | Only
when you need it: With Phexxi, women no longer need to have birth control in their bodies
24/7. Phexxi is used in the moment, right before each and every act of sex, so no daily commitment
is required. This also makes Phexxi easily reversible, providing women with a flexible option
for family planning. |
| ● | First
in class: Phexxi is the first and only hormone-free prescription birth control gel that
women control. Phexxi works to prevent pregnancy by maintaining the vaginal pH, which reduces
sperm motility, and lowers the chance of sperm reaching the egg. This revolutionary mechanism
of action is unique to Phexxi, meaning there are no other products like it in the market. |
| ● | Woman-controlled:
Phexxi puts women in control of their bodies and their pregnancy prevention. With Phexxi,
there is no need to rely on a partner to bring a condom and no need to head into the doctor’s
office for an injection or procedure to prevent pregnancy. The quick and easy pre-sex application
is designed with spontaneity and convenience in mind. |
We
believe Phexxi is a disruptive entry to the U.S. contraceptive landscape. Phexxi is designed to address underserved and unmet needs in
the birth control market, as seen in the table below. Women are becoming highly aware of the hormones that they put in their bodies,
with ~75% of women having some concerns or completely opposing hormonal birth control. These women are a part of the approximately 23
million women who are currently not using hormonal birth control methods, and who we are seeing as a large subset of early adopters of
Phexxi.
Our
sales data further support the uptick of early adopters with almost half of prescriptions coming from women who were not using a method
of contraception in the previous year. This data indicates that the Phexxi reach goes beyond those women who have fallen out of the birth
control funnel, and extends to a robust amount of women who are switching from other prescription birth control methods to Phexxi, further
highlighting that the key attributes of Phexxi are appealing to a wide range of women. Additionally, we are seeing that the majority
of women (~80%) starting Phexxi are under the age of 40, which is promising for long term adoption of the brand.
Prescription
Contraceptive Products and Associated Benefits
Phexxi
is designed to address underserved and unmet needs in the birth control market, as seen in the table below.
| Product
Class | |
Non-Hormonal | | |
No
Systemic Side
Effects | | |
Non-invasive | | |
Convenient | |
| Vaginal
pH Modulator (i.e. Phexxi) | |
| ✔ | | |
| ✔ | | |
| ✔ | | |
| ✔ | |
| 28
Day OCs | |
| | | |
| | | |
| ✔ | | |
| | |
| Extended
Regimen OCs | |
| | | |
| | | |
| ✔ | | |
| | |
| Hormone
Releasing IUDs | |
| | | |
| | | |
| | | |
| ✔ | |
| Copper
IUD | |
| ✔ | | |
| ✔ | | |
| | | |
| ✔ | |
| Implant | |
| | | |
| | | |
| | | |
| ✔ | |
| Vaginal
Ring | |
| | | |
| | | |
| ✔ | | |
| ✔ | |
| Transdermal
Patch | |
| | | |
| | | |
| ✔ | | |
| | |
Vaginal
pH Modulator Mechanism of Action
A
normal vaginal pH of 3.5 to 4.5 is important for maintaining good vaginal health. At this optimal pH level, the vagina contains a balance
of necessary healthy bacteria. Additionally, a vaginal pH in this range is inhospitable to sperm as well as certain viral and bacterial
pathogens.
Phexxi
was developed to have acid-buffering (pH 3.5), lubricating and viscosity-retaining properties to provide effective acidification of the
male ejaculate in the vagina. Typically, the introduction of semen (pH = 7.2-8.0) into the vagina causes a rise in pH above 6.0 due to
the alkalinity of the ejaculate, which neutralizes the normally acidic vaginal environment and allows for the survival of sperm. The
active ingredients in Phexxi produce a normal vaginal pH (3.5-4.5) even in the presence of semen, creating an inhospitable environment
for sperm. The maintenance of the acidic vaginal pH reduces the availability of calcium ions which are needed to drive sperm tail movement.
In vitro studies show immediate sperm motility reduction. Phexxi prevents pregnancy by reducing sperm motility, inhibiting sperm
from reaching the ovum to form a zygote. Other properties contributing to Phexxi’s mechanism of action are its capacity to maintain
sufficient viscosity even upon
The
diagram below shows the respective pH levels of the vagina and semen.
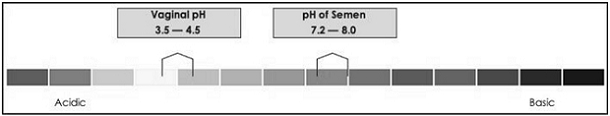
Commercialization
Strategy
Our
strategy is to commercialize Phexxi and to leverage our vaginal pH modulator platform to develop and commercialize novel, first-in-class
products for women. We have deployed a dedicated sales team and developed a telehealth platform and media strategy focused on maximizing
the commercial return from Phexxi in the United States.
Outside
of the United States, we are focused on three key regions – the European Union, Asia Pacific (APAC), and Latin America (LATAM).
Our intent is to establish regional and/or global partnerships in these regions by either sublicensing the commercialization rights or
entering into distribution agreements with one or more third parties for the commercialization of Phexxi and our product candidate. We
expect these third parties to be involved in the regulatory process in their respective markets as well as any clinical trials to support
regulatory submissions, if required.
Commercialization
of Phexxi in the United States
We
believe the United States market is the largest commercial opportunity for Phexxi. Our sales force promotes Phexxi directly to obstetrician/gynecologists
and their affiliated health professionals, who collectively write the majority of prescriptions for contraceptive products. As of April
7, 2023, our sales force consisted of 16 sales representatives and three business managers, supported by a self-guided virtual health
care provider (HCP) learning platform. Additionally, we offer women direct access to Phexxi via our telehealth platform. Using the platform,
women can directly meet with an HCP to determine their eligibility for a Phexxi prescription and, if eligible, have the prescription
written by the HCP, filled, and mailed directly to them by a third-party pharmacy.
Our
commercial strategy for Phexxi includes targeting women of reproductive potential in the United States, including the approximately 23
million women who are not using hormonal contraception and the approximately 18.8 million women who are using a prescription contraceptive,
some of whom, particularly pill users, may be ready to move to an FDA-approved, non-invasive, hormone-free contraceptive, as well as
certain identified target HCP segments.
According
to our market research since Phexxi’s commercial launch, HCPs indicate they would recommend Phexxi to approximately:
| ● | 47%
of patients experiencing side effects from current contraception; |
| ● | 37%
of patients using non-hormonal prescription contraception; |
| ● | 36%
of patients seeking pregnancy prevention; and |
| ● | 19%
of patients using hormonal prescription contraception. |
Additional
research into the demographics of more than 5,000 women who are using Phexxi revealed that 79% of Phexxi users are between 18 to 34 years
of age. Among the subset of Phexxi users for whom prior contraceptive data is available (n=2,512), 80% of women who had recently started
Phexxi were not on any method of prescription contraception. Another 20% switched to Phexxi from either oral contraceptives, hormonal
rings or patches.
In
February 2021, we launched a direct-to-consumer advertising campaign, known as “Get Phexxi,” designed to increase awareness
and educate women on the benefits of Phexxi. The campaign highlighted some of the struggles women face when choosing among the many available
methods of contraception, including the lack of control with condoms, daily use of the pill, and abstinence required for cycle tracking.
In
September 2021, we launched a national brand ambassador campaign featuring Emmy Award-winning celebrity Annie Murphy designed to broaden
awareness and drive uptake of Phexxi. This award-winning campaign, known as “House Rules,” significantly raised our brand
awareness among our target audience and helped drive significant increases in new HCPs recommending and prescribing Phexxi.
In
January 2022 we adjusted our patient support programs to increase the profit margin on Phexxi units dispensed. These adjustments, coupled
with growth in prescriptions and dispensed units, enabled us to achieve record Phexxi net product sales in 2022.
Our
experienced team of key account directors and medical affairs team also focus on educating key payer accounts, pharmacy benefit managers
(PBMs), key opinion leaders and medical associations about the importance of offering a wider set of options to women seeking non-hormonal,
woman-controlled contraceptive methods. These educational activities have been supported by presentation of clinical data at key national
congresses (such as the annual meetings of the American College of Obstetricians and Gynecologists, the Society of Family Planning, the
American Society for Reproductive Medicine, and Nurse Practitioners in Women’s Health), clinical publications, and additional market
development activities.
Pricing
Strategy
Our
pricing strategy for Phexxi was informed by extensive payer research including discussions with decision makers at major health plans
and PBMs across the United States who control nearly 83 million commercial lives. Based on this gathered intelligence, we initially priced
Phexxi at $267.50 per box of 12 applicators. As of October 1, 2022, Phexxi is priced at $338.10 per box of 12 applicators, which when
annualized is comparable to all other commercially available branded contraceptives.
Phexxi
is classified in the databases and pricing compendia of Medi-Span and First Databank, two major drug information databases that payers
consult for pricing and product information, as the first and only “Vaginal pH Modulator.”
Third-party
Payers
Market
acceptance and sales of Phexxi will depend in part on the extent to which reimbursement is available from third-party payers, which include
government health administration authorities, managed care organizations, private health insurers and PBMs. Third-party payers decide
which therapies they will pay for and establish reimbursement levels. Decisions regarding the extent of coverage and amount of reimbursement
to be provided for any product are made on a payer-by-payer basis. One payer’s determination to provide coverage for a drug does
not assure that other payers will also provide coverage and adequate reimbursement for that drug.
Managed
care organizations and other private insurers frequently adopt their own payment or reimbursement reductions. The continued integration
between commercial health plans and PBMs has increased the negotiating power of these entities. Third-party payers increasingly employ
formularies, which may not include all the products approved for a particular indication, to control costs by negotiating discounted
prices in exchange for formulary inclusion.
In
the second quarter of 2022, we successfully negotiated a contract with one of the largest PBMs in the nation, which added Phexxi to its
formulary with no restrictions for most women covered by the plan. The agreement took effect July 1, 2022 and is representative of approximately
48 million lives.
We
participate in government programs that include the 340B and the Medicaid Drug Rebate Program, which took effect January 1, 2021, and
affords access to Phexxi for the U.S. Medicaid population, comprising approximately 68 million members, including approximately 16.8
million women 19-49 years of age.
We
continue working to increase the number of lives covered and to gain a preferred formulary position for Phexxi. We gained coverage for
32.5 million lives in 2022 and have added 22.1 million lives since December 31, 2022. We currently have 65% coverage within our Commercial
and Medicaid books of business, including 19.3 million lives covered at no out-of-pocket cost as of May 9, 2023 and approximately 13.7
million lives covered under our December 2020 contract award from the U.S. Department of Veterans Affairs. As of March 2023, the Phexxi
approved claims rate increased to 81%.
Affordable
Care Act
The
Affordable Care Act (ACA) guarantees coverage of women’s preventive services, including free birth control and contraceptive counseling,
for all individuals and covered dependents with reproductive capacity. This includes all contraceptives approved, granted, or cleared
by the FDA.
History
Under
section 2713 of the Public Health Service (PHS) Act, group health plans and health insurers are required to cover preventive care and
screenings under guidelines issued by the Health Resources and Services Administration (HRSA). PHS Act section 2713 took effect when
added by the Affordable Care Act (ACA) in 2010.
HRSA
guidelines issued in 2019 required broad coverage of contraceptive care and services for women. HRSA issued updated guidelines in late
2021, under which:
| a. | The
full range of FDA- approved, -granted, or -cleared contraceptives, effective family planning
practices, and sterilization procedures should be available as part of contraceptive care. |
| b. | The
full range of contraceptives includes those currently listed in the FDA’s Birth Control
Guide and any additional contraceptives approved, granted, or cleared by the FDA. |
The
current HRSA Women’s Preventive Services Guidelines took effect on January 1, 2023, for calendar year plans.
Separately,
on January 10, 2022, the U.S. Department of Health and Human Services (HHS), alongside the Departments of Labor and of the Treasury (the
“Departments”) issued updated guidance related to contraceptive access.
Under
the Departments’ FAQ Update:
| a. | Plans
are required to cover an FDA- approved, cleared, or granted contraceptive, if a provider
deems it medically necessary, at $0 cost share, whether or not it is specifically identified
in the current FDA Birth Control Guide. |
| b. | Plans
may not require patients to try and fail multiple options within a method, or force trying
and failing other methods, if a provider deems a product medically necessary. |
The
Departments also established clear communications channels for consumers with concerns about their plan’s compliance with HSRA
requirements.
Collectively,
this new guidance specifies that most insurers and pharmacy benefit managers (PBMs) must provide coverage, with no out-of-pocket costs
to women, for FDA-approved contraceptive products, like Phexxi® (lactic acid, citric acid and potassium bitartrate), prescribed
by healthcare providers.
In
July 2022 after the fall of Roe v. Wade and in the wake of action in many states to restrict access to emergency contraception, the Departments
released further guidance regarding birth control coverage. Key points of this guidance include:
| – | Most
private health plans and health insurance issuers must cover contraceptives at no additional
cost to individuals under the Affordable Care Act no matter where they live or work. |
| – | Violators
of the preventive care coverage requirements may be subject to the $100 per person per day
excise tax under section 4980D of the Internal Revenue Code or a civil monetary penalty under
PHS Act section 2723. |
| – | The
Departments “will take enforcement action as warranted.” |
As
of January 1, 2023, most insurers and pharmacy benefit managers (PBMs) must provide coverage, with no out-of-pocket costs (e.g. $0
copay) to the subscriber or dependent, for FDA-approved contraceptive products, like Phexxi, prescribed by healthcare providers.
As
a result, to comply with these Guidelines, payers are increasingly covering Phexxi by:
| – | Adding
Phexxi to formulary (commercial insurers) or preferred drug list (Medicaid) |
| | | |
| – | Removing
the requirement for a Prior Authorization letter from the HCP (commercial insurers) |
| | | |
| – | Moving
Phexxi to $0 copay (commercial insurers) |
Birth
Control Guide
In
2022 Evofem developed and introduced a new educational chart for patients and HCPs that details high-level information about birth control
methods currently available to women in the U.S., including the vaginal pH modulator. This new educational tool has been extremely well
received and has had a positive impact with HCPs and patients alike.
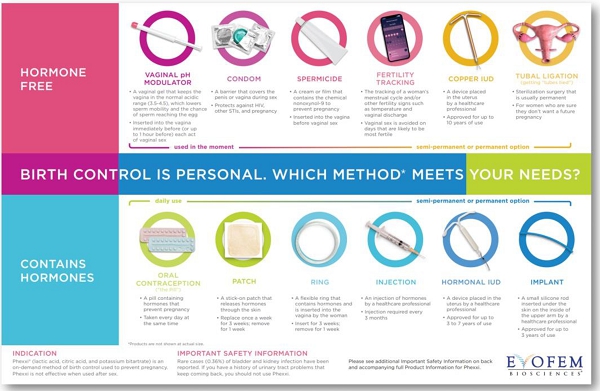
Contraceptive
Market Landscape
The
modern contraception market was established in 1960 with the introduction of “the pill,” the first oral contraceptive widely
available to women in the United States. As shown in the timeline below, there was no notable innovation providing additional options
in women’s reproductive health until almost 30 years after the introduction of “the pill,” when pharmaceutical companies
introduced the non-hormonal copper IUD and synthetic hormonal products with different hormonal delivery systems, including the hormonal
IUD, implants, the patch, and vaginal ring.
We
expect that Phexxi vaginal gel will grow the prescription birth control user market when considering the 28.3 million women who are currently
at risk for pregnancy and do not use hormone-based contraceptives as their primary form of contraception. Additionally, as women’s
expectations change throughout their contraceptive journey, we expect Phexxi to compete for market share in at least three categories:
| |
1. |
Hormonal
short acting reversible contraceptives consisting of oral contraceptive pills, patches, and rings; |
| |
|
|
| |
2. |
Long-Acting
Reversible Contraception, comprising IUDs, implants, and injectables; and |
| |
|
|
| |
3. |
OTC
methods, dominated primarily by the male condom. |
U.S.
Market
Unless
otherwise noted, the source for all data in this section is Daniels K and Abma J, Current Contraceptive Status Among Women Aged 15–49:
United States, 2017–2019, which was published in NCHS Data Brief No. 388 in October 2020.
Prescription
Contraception
In
the United States, an estimated 18.8 million women use prescription contraception.
Oral
contraceptives (OCs), also known as the pill, are the most commonly used form of birth control in the United States today. There are
two main kinds of hormonal OCs: combination birth control pills, which contain both estrogen and progestin, and the progestin only pill.
Use of either kind is associated with a slight increase in the risk of breast cancer. OCs typically must be taken at the same time every
day to be the most effective.
LARC
Long-acting
reversible contraception (LARC) is not dependent on user adherence, which appeals to those who benefit from a passive form of birth control
with no daily requirement to take a pill. LARC methods include the Intrauterine Device (IUD) and the Contraceptive Implant.
IUDs
The
copper IUD was introduced to the market in 1988 and provides protection by disrupting sperm motility and damaging sperm so that they
are prevented from joining with an ovum. Today, the copper IUD is principally marketed by Cooper Surgical, Inc. as Paragard.
The
hormonal IUD is principally offered under the brand names, Kyleena, Skyla and Mirena, a family of products from Bayer Pharmaceuticals.
All IUDs must be inserted and removed by a physician.
Many
women have opted against the IUD for 1) fear of a bad insertion experience; a peer-reviewed study published in 2015 found that “all
women had a high expectation of pain prior to IUD insertion.” (source: Brima et al. A comparison of the expected and actual
pain experienced by women during insertion of an intrauterine contraceptive device. Open Access J Contracept. 2015 Feb 16;6:21-26. doi:
10.2147/OAJC.S74624.) and 2) concern over having something in them (i.e. a “foreign body effect”), which has been frequently
demonstrated in medical literature. (source: Ferguson et al. Patient Opinions About Foreign Body Contraceptives. Womens Health Rep
(New Rochelle). 2020 Oct 8;1(1):451-458. doi: 10.1089/whr.2020.0048.). Among women who opt in to the insertion procedure, many decide
to remove their IUD due to the hormonal and other side effects that they experience.
Implants
The
contraception implant must be implanted under the skin and removed by a qualified HCP, requiring a medical procedure. It provides contraception
by releasing hormones over a three-year period. The implant is marketed in the United States as Nexplanon by Organon.
Contraceptive
Patch
The
weekly contraceptive patch was introduced in 2000 by Johnson & Johnson’s Janssen division; however, deaths resulting from venous
thromboembolism due to hormonal exposure had a significant negative impact on the patch and led to label changes restricting utilization.
Following the loss of exclusivity, Johnson & Johnson’s Janssen division exited women’s health care and contraception
as a promotional category. A new branded patch was launched in late 2020 under the brand name Twirla (Agile Therapeutics) and is competing
against a generic entrant Xulane (Mylan).
Vaginal
Ring
The
hormonal vaginal ring was introduced to the market in 2001 by Merck & Co.; generic versions are now available. The ring is used for
three weeks and then removed for a week during menses and a new hormonal vaginal ring is inserted. The efficacy of the vaginal ring is
similar to hormonal oral contraception. A meta-analysis of 18 studies found that users of the vaginal ring reported more vaginal irritation
and discharge than combination pill users, but less nausea, acne, irritability, depression, and emotional changes (source: Lopez et
al. Skin patch and vaginal ring versus combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013 Apr 30;2013(4):CD003552.
doi: 10.1002/14651858.CD003552.pub4).
An
annual hormonal vaginal ring was launched in the United States in 2020 under the brand name Annovera (Mayne Pharma).
Injectables
The
primary injectable hormonal contraceptive on the market is Depo-Provera offered by Pfizer Inc. Each injection provides protection for
up to 12 to 14 weeks, but patients must receive injections once every 12 weeks to get optimal contraceptive protection. Depo-Provera
was introduced to the market in 1992.
Non-prescription
OTC Products
In
the United States, an estimated 10.3 million women rely on OTC products for their contraceptive needs.
Condoms
are the dominant product offering in OTC sales. Approximately six million women depend on condom use as their only method of birth control.
The predominant brands are Trojan (Church & Dwight) and Durex (Reckitt Benckiser).
Additional
OTC products include spermicides, which are available in sponges, jelly/creams and foams. Spermicides rely on Nonoxynol-9 (N-9), a detergent,
and have very limited utilization. The FDA requires specific warnings to appear on all N-9 products that state: “this product does
not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected partner” as well as: “Do
not use if you or your sex partner has HIV/AIDS. If you do not know if you or your sex partner is infected, choose another form of birth
control method.
Vaginal
pH Modulator
New
adopters of Phexxi are expected to come equally from each category discussed, as interest in Phexxi falls into three distinct segments:
(1) those women who are not currently using hormone-based contraceptives; (2) those women seeking an alternative to hormonal contraception;
and (3) those women who are expected to utilize Phexxi as added protection to their current form of birth control. Our market research
has indicated that the hormone-free, on-demand, woman-controlled aspect of Phexxi makes it an attractive option across the entire competitive
set.
Ex-United
States Markets
In
markets outside of the United States, we intend to establish regional and/or global partnerships by either sublicensing the commercialization
rights or entering into distribution agreements with one or more third parties for the commercialization of Phexxi and/or the applicable
product candidate in that market.
In
October 2021, we submitted the registration for our hormone-free contraceptive vaginal gel to the Mexican Regulatory Agency COFEPRIS
(Comisión Federal para la Protección contra Riesgos Sanitarios) (COFEPRIS). We have also submitted marketing applications
for Phexxi under the trademark Femidence™ in Nigeria, Ethiopia, and Ghana. These were the first of several strategic regulatory
submissions planned under Evofem’s 2020 Global Health Agreement with Adjuvant Capital.
In
October 2022, Phexxi was approved in Nigeria, where the product will be potentially marketed under the brand name Femidence™. This
is the first regulatory approval for the contraceptive vaginal gel outside the U.S.
Manufacturing
We
outsource the manufacturing of Phexxi (and our investigational product candidates) to a third party. We are currently contracted with
a gel manufacturer to manufacture Phexxi in accordance with all applicable current good manufacturing practices (cGMP) regulations, as
well as in compliance with all applicable laws and other relevant regulatory agency requirements for manufacture of pharmaceutical drug
products and combination drug-device products. As of December 31, 2022, we estimated that we had manufactured inventory on hand to support
approximately nine months of anticipated demand for Phexxi. An additional six Phexxi batches, included in other non-current assets in
the consolidated balance sheets, had been manufactured and awaited release as of that date and are therefore not include in this estimated
timing.
Our
Pipeline
Phase
3: EVO100 for STI Prevention
Until
October 2022, we were evaluating EVO100 for the prevention of urogenital chlamydia and gonorrhea in women. Chlamydia and gonorrhea are
among the many bacterial and viral pathogens that require a higher pH environment to thrive. in 2018, the CDC reported that infections
with these two sexually transmitted pathogens cost the U.S. healthcare system $1 billion, in aggregate direct and indirect costs. There
are no FDA-approved drugs to prevent these sexually transmitted diseases (STIs), and we believe there is a clear need for new prophylactics
given the rising incidence and increasing antibiotic resistance of gonorrhea.
The
FDA has granted Fast Track designation and Qualified Infectious Disease Product (QIDP) designation to EVO100 for the prevention of both
chlamydia and gonorrhea in women.
Our
Phase 2B/3 trial (AMPREVENCE) achieved its primary and secondary endpoints, demonstrating statistically significant reductions
in chlamydia and gonorrhea infections of 50% and 78%, respectively, in women receiving EVO100 vs. placebo. Based on these highly positive
clinical outcomes we initiated a Phase 3 clinical trial (EVOGUARD) to evaluate EVO100 for these potential indications in 2020.
This randomized, placebo-controlled clinical trial enrolled 1,903 women with a prior chlamydia or gonorrhea infection who were at risk
for future infection.
On
October 11, 2022, we reported that EVOGUARD did not meet its primary efficacy endpoint. We believe COVID-19 related changes in
clinical site operations, subject behavior and actions including deviations from following the clinical study protocol requirements related
to STI acquisition, detection, and prevention contributed to this outcome. The product safety profile was consistent with what has been
observed in prior clinical trials, and only two women (0.1%) in the study discontinued due to adverse events.
We
believe there is a path forward for EVO100 and may in the future conduct a new Phase 3 clinical trial of EVO100 for these potential indications.
However, due to financial constraints, we discontinued investment in this clinical program in October 2022.
Phase
2- Ready: EVO200 Vaginal Gel for Recurrent Bacterial Vaginosis
Our
investigational candidate for the reduction of recurrent bacterial vaginosis (BV), EVO200 vaginal gel, uses the same proprietary vaginal
pH modulator platform as Phexxi. In a Phase 1 dose-finding trial for this indication, the highest dose formulation of the study drug
demonstrated reduced vaginal pH for up to seven days following a single administration. The FDA has designed EVO200 as a Qualified Infectious
Disease Product (QIDP) for this indication. Due to financial constraints, all further development of EVO200 is on hold.
Pre-clinical:
MPT Vaginal Gel for HIV Prevention
In
December 2021, we launched a collaboration with Orion Biotechnology Canada Ltd. (Orion) to evaluate the compatibility and stability of
Orion’s novel CCR5 antagonist, OB-002, in Phexxi with the goal of developing a Multipurpose Prevention Technology (MPT) product
candidate for indications including the prevention of HIV in women. Assuming positive preclinical results, Evofem and Orion will seek
government and/or philanthropic funding for subsequent clinical trials of any resulting MPT vaginal gel product candidate.
Thin
Film Project
In
February 2020, we contracted with the University of South Australia to explore the development of a vaginally applied thin film as a
second-generation vaginal pH modulator product. The lead thin film candidates have been selected, and stability data has been generated
with positive results. The project is currently on hold due to financial constraints.
Rush
License Agreement
In
2014, we entered into an amended and restated license agreement with Rush University (the Rush License Agreement) pursuant to which Rush
University granted us an exclusive, worldwide license of certain patents and know-how related to our multipurpose vaginal pH modulator
technology (the Rush License IP) authorizing us to make, distribute and commercialize products and processes for any and all therapeutic,
prophylactic and/or diagnostic uses, including, without limitation, use for female vaginal health and/or birth control. Pursuant to the
Rush License Agreement, we are obligated to pay quarterly royalty payments in amounts equal to a single-digit percentage of the gross
amounts we receive on a quarterly basis less certain deductions incurred in the quarter based on a sliding scale. We are also obligated
to pay a minimum annual royalty amount of $100,000 to the extent these earned royalties do not equal or exceed $100,000 in a given year.
A minimum annual royalty amount of $100,000 was first required for the annual period commencing on January 1, 2021. The royalty costs
for the year ended December 31, 2022 were $1.1 million and immaterial for the first quarter of 2023.
We
also have the right to sub-license our rights to affiliates (without the prior approval of Rush University) and to third parties (with
the prior written approval of Rush University). To the extent Rush University approves of a third-party sub-license, in lieu of any royalty
payment obligation under the Rush License Agreement, we would then be under an obligation to pay Rush University a sub-license fee equal
to a percentage of any sublicensing revenue received from any third-party sub-licensee. Rush University retained a royalty free, non-exclusive
license from us for the Rush License IP for non-commercial research purposes.
The
Rush License Agreement contains additional customary representations and warranties, covenants, indemnification and insurance and confidentiality
provisions for agreements of its type. The Rush License Agreement may be terminated upon mutual written consent of both parties or by
a non-breaching party if the other party commits a breach or default of any covenant in the agreement and fails to cure this breach within
30 days after receiving written notice of the breach or default.
Unless
terminated in accordance with its terms, the Rush License Agreement continues until the expiration, revocation or invalidation of the
last of the patents or the abandonment of the last patent application included within the licensed patents and technology, including
any patent claiming an improvement made during the term of the Rush License Agreement in the course of research supported or developed
by Rush University utilizing the technology.
Intellectual
Property
We
strive to protect the proprietary vaginal pH modulator gel technology both internationally and domestically. We seek and maintain patents
intended to cover our product candidates, and their methods of use, as well as any other inventions that are commercially important to
the development of our business. We endeavor to properly file patent applications for new inventions we believe may have commercial value.
We also may rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate
for, patent protection.
Our
success will depend on our ability, in part: to obtain and maintain patent and other proprietary protection for commercially important
technology, inventions and know-how related to our business; to defend and enforce our patents and other intellectual property rights;
and to preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and other proprietary
rights of third parties. We will also rely on continuing technological innovation and in-licensing opportunities to develop and maintain
our proprietary position.
As
of February 10, 2023, we owned or had exclusive license to approximately 49 issued patents and allowed applications in the United States
and other countries and jurisdictions, and had approximately 22 patent applications pending in the United States and other countries
and jurisdictions. This includes four U.S. patents which cover Phexxi and its labeled indication that are listed in the U.S. FDA publication
Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book):
| ● | U.S.
Patent No. 11,337,989: Method of use patent covering contraception using the L-Lactic Acid
Phexxi formulation; |
| ● | U.S.
Patent No. 11,439,610: Composition of matter patent covering compositions containing L-Lactic
Acid, including the Phexxi formulation; |
| ● | U.S.
Patent No. 10,568,855: Method of use patent covering contraception using the L-Lactic Acid
Phexxi formulation; and, |
| ● | U.S.
Patent No. 6,706,276: Composition of matter patent covering Phexxi. |
We
solely own several patent application families relating to the composition and therapeutic use of our vaginal pH modulator gel, which,
upon issue, would expire at the earliest in 2033. We also have the Rush License IP, which provides general protection for our vaginal
pH modulator platform. Our vaginal pH modulator platform could be eligible for regulatory extensions to at least 2026 in the United States
and in certain European jurisdictions, if granted by those regulatory bodies. For the U.S. patent that we licensed from Rush University,
three Orders Granting Interim Extension (OGIEs) were received from the USPTO, extending the expiration of this patent to September 2023.
We believe that our licensed and solely owned non-hormonal birth control gel patents and pending patent applications, combined with our
substantial know-how in this field, will continue to provide opportunities for us to establish a significant barrier to competitor entry
into the market.
In
addition to patents, we rely, and expect to rely, on trade secrets and know-how to develop and maintain our competitive positions. For
example, certain aspects of the composition, manufacturing, and use of Phexxi are protected by unpatented trade secrets and know-how.
Although trade secrets and know-how can be difficult to protect, we seek to protect our proprietary technology and processes, in part,
through confidentiality agreements with our employees, consultants, scientific advisors, collaborators, and contractors. We also seek
to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical
and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems,
agreements or security measures may be breached and we may not have adequate remedies for these incidents. In addition, our trade secrets
and know-how may otherwise become known or may be independently discovered by competitors. To the extent our consultants, contractors
or collaborators use intellectual property owned by third parties in their work for us, disputes may arise as to the rights in related
or resulting intellectual property, including trade secret, know-how and inventions.
Trademark
Basics and Strategy
We
own or have rights to various trademarks, copyrights and trade names used in our business, including Evofem, Phexxi and Femidence. All
of our logos and trademarks appearing in this Registration Statement are the property of Evofem Biosciences, Inc. All other third-party
trademarks appearing in this Registration Statement are the property of their respective holders. Our use or display of other
parties’ trademarks, trade dress, or products in this Registration Statement is not intended to, and does not, imply a relationship
with, or endorsement or sponsorship of us, by the trademark, trade dress, or product owner.
Government
Regulation and Product Approval
The
research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of our
products are subject to extensive regulation by governmental authorities in the United States and other countries. The processes for
obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with
applicable statutes and regulations and other regulatory requirements, require the expenditure of substantial time and financial resources.
In
the United States, the FDA regulates drugs and other medical products under the Federal Food, Drug, and Cosmetic Act (FDCA) and its implementing
regulations. Failure to comply with the applicable United States requirements may subject us to administrative or judicial sanctions,
such as FDA refusal to approve pending New Drug Applications (NDAs), warning letters, product recalls, product seizures, total or partial
suspension of production or distribution, injunctions and/or criminal prosecution.
Drug
Development and FDA Review and Approval Process
Our
product candidates may not be marketed in the United States until the product has received FDA approval. The steps to be completed before
a drug may be marketed in the United States include:
| a. | completion
of preclinical laboratory tests, animal studies, and formulation studies, performed in accordance
with the FDA’s Good Laboratory Practice (GLP) regulations; |
| b. | submission
to the FDA of an Investigational New Drug (IND) application to permit human clinical testing
of the therapeutic candidate; |
| c. | approval
by an independent institutional review board (IRB) or ethics committee at each clinical trial
site before each clinical trial may be initiated; |
| d. | completion
of adequate and well-controlled human clinical trials in accordance with applicable IND regulations,
current good clinical practices (cGCPs), and other clinical-trial related regulations to
establish the safety and efficacy of the investigational drug for each proposed indication; |
| e. | submission
to the FDA of an NDA for marketing approval, including payment of application user fees; |
| f. | satisfactory
completion of an FDA inspection of the manufacturing facility or facilities at which the
drug is produced to assess compliance with current Good Manufacturing Practice (cGMP) regulations; |
| g. | satisfactory
completion of FDA bioresearch monitoring inspections of selected investigational sites at
which the drug product was subject to clinical trials to assess compliance with cGCP regulations;
and |
| h. | FDA
review and approval of the NDA, including satisfactory completion of an FDA advisory committee
review of the product candidate, where appropriate or if applicable, prior to any commercial
marketing or sale of the product in the United States. |
Before
testing any drug product candidate, including our product candidates, in humans, the product candidate must undergo rigorous preclinical
testing. The preclinical developmental stage generally involves laboratory evaluations of drug chemistry, formulation and stability,
as well as studies to evaluate toxicity in animals, which support subsequent clinical testing. The sponsor must submit the results of
the preclinical studies, together with manufacturing information, analytical data, any available clinical data or literature and a proposed
clinical protocol, to the FDA as part of the IND. An IND is a request for authorization from the FDA to administer an investigational
product to humans, and must become effective before human clinical trials may begin.
Preclinical
tests include laboratory evaluation of product chemistry, toxicity and formulation, as well as in vitro and animal studies to assess
the potential for adverse events and in some cases to establish a rationale for therapeutic use. The conduct of preclinical studies is
subject to federal regulations and requirements, including GLP regulations for safety/toxicology studies. Some long-term preclinical
testing, such as animal tests of reproductive adverse events and carcinogenicity, may continue after an IND for an investigational drug
candidate is submitted to the FDA and human clinical trials have been initiated.
The
results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an
IND, which must become effective before human clinical trials in the United States may begin and is required to be updated annually.
An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions
about issues such as the conduct of the trials as outlined in the IND and imposes a clinical hold. In such a case, the IND sponsor and
the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. Clinical holds also may be imposed
by the FDA at any time before or during studies due to safety concerns or non-compliance. We currently have two active INDs on file with
the FDA: one for EVO100 for the prevention of urogenital chlamydia and urogenital gonorrhea, and one for EVO200.
Clinical
trials involve the administration of the investigational drug to human subjects under the supervision of qualified investigators. Clinical
trials are conducted under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness
criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND. The trial protocol and informed consent information
for trial subjects in clinical trials must also be approved by an IRB for each institution where the trials will be conducted, and each
IRB must monitor the trial until completion; an IRB may halt a trial under its jurisdiction for safety reasons. Trial subjects must sign
an informed consent form before participating in a clinical trial. Clinical testing also must satisfy extensive good clinical practice
regulations and regulations for informed consent and privacy of individually identifiable information.
Clinical
trials necessary for product approval are typically conducted in three sequential phases, although the phases may overlap.
| a. | Phase
1: The product candidate is initially introduced into healthy human subjects and
tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion.
In the case of some products for severe or life-threatening diseases, such as cancer, especially
when the product may be too inherently toxic to ethically administer to healthy volunteers,
the initial human testing is often conducted in patients. |
| | | |
| b. | Phase
2: This phase involves studies in a limited patient population to identify possible
adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for
specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| | | |
| c. | Phase
3: Larger clinical trials are undertaken to further evaluate dosage, clinical efficacy
and safety in an expanded patient population, often at geographically dispersed clinical
study sites. These studies are intended to establish the overall risk-benefit ratio of the
product candidate and provide, if appropriate, an adequate basis for product labeling. These
trials may include comparisons with placebo and/or other comparator treatments. The duration
of treatment is often extended to mimic the actual use of a product during marketing. |
Post-approval
trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to
gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow
up. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
In
addition, information about certain clinical trials, including details of the protocol and study results, must be submitted within specific
timeframes to the National Institutes of Health for public dissemination on the ClinicalTrials.gov data registry. Information related
to the product, patient population, phase of investigation, study sites and investigators and other aspects of the clinical trial is
made public as part of the registration of the clinical trial. Sponsors are also obligated to disclose the results of their clinical
trials after completion. Disclosure of the results of these trials can be delayed in some cases for up to two years after the date of
completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development
programs.
Assuming
successful completion of the required clinical testing, the results of the preclinical studies and of the clinical trials, together with
detailed information relating to the product’s chemistry, manufacturing, and controls and proposed labeling, are submitted to the
FDA in the form of an NDA requesting approval to market the product for one or more indications. An NDA must be accompanied by payment
of a significant user fee to the FDA (for example, for the fiscal year ending December 31, 2023, this application fee exceeds $3.2 million).
Annual program fees are also assessed on each sponsor of an approved NDA after a drug’s approval. Section 505(b)(1) and Section
505(b)(2) of the FDCA are the provisions governing the type of NDAs that may be submitted under the FDCA. Section 505(b)(1) is the traditional
pathway for new chemical entities when no other new drug containing the same active pharmaceutical ingredient or active moiety, which
is the molecule or ion responsible for the action of the drug substance, has been approved by the FDA. As an alternate pathway to FDA
approval for new or improved formulations of previously approved products, a company may file a Section 505(b)(2) NDA. Section 505(b)(2)
permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or
for the applicant and for which the applicant has not obtained a right of reference.
During
the 60 days after submission, the FDA reviews any NDA submitted to ensure that it is sufficiently complete for substantive review before
the FDA accepts the NDA for filing. The FDA may request additional information rather than accept the NDA for filing. Once the submission
is accepted for filing, the FDA begins an in-depth review. Under the goals and policies agreed to by the FDA under the Prescription Drug
User Fee Act, or PDUFA, the FDA has agreed to certain performance goals in the review of NDAs. For most applications involving first-in-kind
molecular entities, FDA has ten months from the filing date in which to complete its initial review of a standard application and respond
to the applicant, and six months from the filing date for an application with priority review. Priority review can be applied to drugs
intended to treat a serious condition and that the FDA determines offer major advances in treatment by providing a significant improvement
in safety or effectiveness, or that provide a treatment where no adequate therapy exists. Even if the NDA is filed by the FDA, companies
cannot be sure that any approval will be granted on a timely basis, if at all. Moreover, the FDA does not always meet its PDUFA goal
dates, and the review process for both standard and priority new drug applications may be extended by FDA for three additional months
to consider certain late-submitted information, or information intended to clarify information already provided in the submission. The
FDA may also refer the application to an appropriate advisory committee, typically a panel of independent clinicians and other experts,
for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendations
of the advisory committee, but it typically considers such recommendations when making final decisions on marketing approval. The FDA
also may require submission of a risk evaluation and mitigation strategy or “REMS” plan if it determines that a REMS is necessary
to ensure that the benefits of the drug outweigh its risks and to assure the safe use of the drug or biological product. The REMS plan
could include medication guides, physician communication plans, assessment plans and/or elements to assure safe use, such as restricted
distribution methods, patient registries or other risk minimization tools. The FDA determines the requirement for a REMS, as well as
the specific REMS provisions, on a case-by-case basis. If the FDA concludes a REMS plan is needed, the sponsor of the NDA must submit
a proposed REMS. The FDA will not approve an NDA without a REMS, if required.
Before
approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with cGCPs. Additionally, the FDA will
inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with
cGMP requirements is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in
the indication studied.
The
approval process is lengthy and often difficult, and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not
satisfied or may require additional clinical or other data and information. On the basis of the FDA’s evaluation of the NDA and
information, including the results of the inspection of the manufacturing facilities, it issues either an approval letter or a Complete
Response Letter, or CRL. A CRL generally outlines the deficiencies in the submission and may require substantial additional testing,
or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s
satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions
in two or six months depending on the type of information included. Even with the submission of additional information responding to
the deficiencies identified in a prior CRL, however, the FDA ultimately may decide that a new drug application does not satisfy the regulatory
criteria for approval.
When
issued, an NDA approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications
as described in the application. Further, depending on the specific risk(s) to be addressed, the FDA may require that certain contraindications,
warnings or precautions be included in the product labeling, require that post-approval trials, including Phase 4 clinical trials, be
conducted to further assess a product’s safety after approval, require testing and surveillance programs to monitor the product
after commercialization, or impose other conditions, including distribution and use restrictions or other risk management mechanisms
under a REMS, which can materially affect the potential market and profitability of the drug. Moreover, the FDA may prevent or limit
further marketing of a product based on the results of post-marketing trials or surveillance programs. Once granted, product approvals
may be withdrawn if compliance with regulatory requirements is not maintained or problems are identified following initial marketing
or any time thereafter, and certain types of changes to the approved product, such as adding new indications, manufacturing changes and
additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval
Requirements in the United States
Following
approval of a new product or indication, the manufacturer and the approved product are subject to continuing regulation by the FDA, including,
among other things, monitoring and record-keeping activities, reporting of adverse experiences, and complying with promotion and advertising
requirements, which include restrictions on promoting approved drugs for unapproved uses or patient populations (known as “off-label
use”). Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such
uses. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company
that is found to have improperly promoted off-label uses may be subject to significant liability, including adverse publicity, enforcement
action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA. Prescription
drug promotional materials also must be submitted to the FDA in conjunction with their first use. Further, if there are any modifications
to the approved drug, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required
to submit and obtain FDA approval of a new NDA or NDA supplement, which may require the applicant to develop additional data or conduct
additional preclinical studies or clinical trials.
Any
limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Product
approvals may be withdrawn for non-compliance with regulatory standards or if problems occur while the product is on the market.
FDA
regulations require that products be manufactured in specific approved facilities and in accordance with cGMPs. The cGMP regulations
include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product
containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls,
records and reports and returned or salvaged products. The manufacturing facilities for our product and product candidates must meet
cGMP requirements and satisfy the FDA or comparable foreign regulatory authorities’ satisfaction before any product candidate is
approved and our commercial products can be manufactured. Evofem relies, and expects to continue to rely, on third parties for the production
of clinical and commercial quantities of its products and product candidates in accordance with cGMPs. These manufacturers must also
comply with cGMPs that require, among other things, quality control and quality assurance, the maintenance of records and documentation,
and the obligation to investigate and correct any deviations from cGMP. Manufacturers and other entities involved in the manufacture
and distribution of approved drugs or combination products are required to register their establishments with the FDA and certain state
agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements
and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control
to maintain cGMP compliance. The discovery of violative conditions, including failure to conform to cGMPs, could result in enforcement
actions, and the discovery of problems with a product after approval may result in restrictions on a product, manufacturer or holder
of an approved NDA, including recall.
After
approval of a drug is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained
or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse
events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may
result in mandatory revisions to the approved labeling to add new safety information, or imposition of additional post-market surveillance
or clinical trials to assess new safety risks. Other potential consequences include, among other things:
| ● | restrictions
on the marketing or manufacturing of the product, complete withdrawal of the product from
the market or product recalls; |
| | | |
| ● | fines,
warning letters or other enforcement-related letters or clinical holds on investigational
or post-approval clinical trials; |
| | | |
| ● | refusal
of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation
of product approvals; |
| | | |
| ● | product
seizure or detention, or refusal to permit the import or export of products; |
| | | |
| ● | injunctions
or the imposition of civil or criminal penalties; and |
| | | |
| ● | consent
decrees, corporate integrity agreements, debarment, or exclusion from federal health care
programs; or mandated modification of promotional materials and labeling and the issuance
of corrective information. |
In
addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act (PDMA), which regulates
the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug
distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose
requirements to ensure accountability in distribution. More recently, the Drug Supply Chain Security Act (DSCSA), was enacted with the
aim of building an electronic system to identify and trace certain prescription drugs distributed in the United States, including most
biological products. The DSCSA mandates phased-in resource-intensive obligations for pharmaceutical manufacturers, wholesale distributors,
and dispensers over a 10-year period that is expected to culminate in November 2023. On February 4, 2022, FDA announced the availability
of the proposed rule “National Standards for the Licensure of Wholesale Drug Distributors and Third-Party Logistics Providers”
(Docket No. FDA-2020-N-1663) as required by the Drug Supply Chain Security Act (DSCSA). The proposed rule, when finalized, would provide
greater assurance that supply chain participants are sufficiently vetted and qualified to distribute prescription drugs, further strengthening
the supply chain. On May 24, 2022, FDA extended the comment period for the proposed Rule to September 6, 2022, to allow interested stakeholders
additional time to submit comments. As of the date of this Registration Statement, the FDA had not provided a subsequent update
on the proposed rule.
From
time to time, new legislation and regulations may be implemented that could significantly change the statutory provisions governing the
approval, manufacturing and marketing of products regulated by the FDA. It is impossible to predict whether further legislative or regulatory
changes will be enacted, or FDA regulations, guidance or interpretations will be changed or what the impact of such changes, if any,
may be.
Hatch-Waxman
Act and Marketing Exclusivity
Under
the Drug Price Competition and Patent Term Restoration Act of 1984 (the Hatch-Waxman Amendments) to the Federal Food, Drug, and Cosmetic
Act (FDCA), Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the
NDA provisions of the statute and also enacted Section 505(b)(2) of the FDCA. To obtain approval of a generic drug, an applicant must
submit an abbreviated new drug application (ANDA), to the agency. In support of such applications, a generic manufacturer may rely on
the preclinical and clinical testing conducted for a drug product previously approved under an NDA, known as the reference listed drug
(RLD). Specifically, in order for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect
to the active ingredients, the route of administration, the dosage form, and the strength of the drug. In contrast, Section 505(b)(2)
permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for
the applicant and for which the applicant has not obtained a right of reference. A Section 505(b)(2) applicant may eliminate the need
to conduct certain preclinical or clinical studies, if it can establish that reliance on studies conducted for a previously-approved
product is scientifically appropriate. Unlike the ANDA pathway used by developers of bioequivalent versions of innovator drugs, which
does not allow applicants to submit new clinical data other than bioavailability or bioequivalence data, the 505(b)(2) regulatory pathway
does not preclude the possibility that a follow-on applicant would need to conduct additional clinical trials or nonclinical studies;
for example, they may be seeking approval to market a previously approved drug for new indications or for a new patient population that
would require new clinical data to demonstrate safety or effectiveness. The FDA may then approve the new product for all or some of the
label indications for which the RLD has been approved, or for any new indication sought by the Section 505(b)(2) applicant, as applicable.
Upon
approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s
product or an approved method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. The
Orange Book listing for the Phexxi vaginal gel NDA includes two patents covering the product’s composition of matter and its method
of use in prevention of pregnancy. Except for patents covering methods of use for which the follow-on applicant is not seeking approval,
the applicant is required to certify to the FDA concerning any patents listed in the Orange Book for the RLD, when an ANDA applicant
submits its application to the FDA. To the extent the Section 505(b)(2) applicant is relying on studies conducted for an already approved
product, such an applicant is also required to certify to the FDA concerning any patents listed for the approved product in the Orange
Book to the same extent that an ANDA applicant would.
Specifically,
an ANDA or 505(b)(2) applicant for a follow-on drug product with respect to each patent must certify that: (i) the required patent information
has not been filed by the original applicant; (ii) the listed patent already has expired; (iii) the listed patent has not expired, but
will expire on a specified date and approval is sought after patent expiration; or (iv) the listed patent is invalid, unenforceable or
will not be infringed by the manufacture, use or sale of the new product.
If
a Paragraph I or II certification is filed, the FDA may make approval of the application effective immediately upon completion of its
review. If a Paragraph III certification is filed, the approval may be made effective on the patent expiration date specified in the
application, although a tentative approval may be issued before that time. If an application contains a Paragraph IV certification, a
series of events will be triggered, the outcome of which will determine the effective date of approval of the ANDA or 505(b)(2) application.
A
certification that the new product will not infringe the RLD’s listed patents or that such patents are invalid is called a Paragraph
IV certification. If the follow-on applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice
of the Paragraph IV certification to the NDA and patent holders for the RLD once the applicant’s NDA has been accepted for filing
by the FDA. The NDA and patent holders may then initiate a legal challenge to the Paragraph IV certification. The filing of a patent
infringement lawsuit within 45 days of their receipt of a Paragraph IV certification automatically prevents the FDA from approving the
ANDA or 505(b)(2) NDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent or a decision
in the infringement case that is favorable to the ANDA or 505(b)(2) applicant. Alternatively, if the listed patent holder does not file
a patent infringement lawsuit within the required 45-day period, the follow-on applicant’s ANDA or 505(b)(2) NDA will not be subject
to the 30-month stay.
In
addition, under the Hatch-Waxman Amendments, the FDA may not approve an ANDA or 505(b)(2) NDA until any applicable period of non-patent
exclusivity for the referenced RLD has expired. These market exclusivity provisions under the FDCA also can delay the submission or the
approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to
the first applicant to gain approval of an NDA for a drug containing a new chemical entity. A drug is a new chemical entity if the FDA
has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action
of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another
company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required
for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement.
The
FDCA also provides three years of marketing exclusivity for a NDA, 505(b)(2) NDA, or supplement to an existing NDA if new clinical investigations,
other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval
of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the
conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving follow-on applications
for drugs containing the original active agent. Five-year and three-year exclusivity also will not delay the submission or approval of
a traditional NDA filed under Section 505(b)(1) of the FDCA. However, an applicant submitting a traditional NDA would be required to
either conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary
to demonstrate safety and effectiveness. The three-year new product exclusivity for the Phexxi NDA expired on May 22, 2023. The product’s
intellectual property also includes four U.S. patents which cover Phexxi and its labeled indication that are listed in the U.S. FDA publication
Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book); these patents are expected to protect Phexxi
into 2033.
On
May 5, 2023, the Company disclosed receipt of a Paragraph IV Certification Notice Letter (“Notice Letter”) from Padagis Israel
Pharmaceuticals Ltd. advising that Padagis has submitted an Abbreviated New Drug Application (ANDA) to the FDA seeking authorization
to manufacture, use or sell a generic version of Phexxi in the U.S. In the Notice Letter, Padagis states that it intends to market a
generic version of Phexxi before the expiration of the three Orange Book listed patents held by the Company providing Phexxi market exclusivity
into 2033.
On
June 6, 2023, within 45 days of receipt of the Notice Letter, the Company filed a patent infringement suit against Padagis in a federal
district court, triggering an automatic 30-month stay which would prevent the FDA from issuing final approval of Padagis’ ANDA
until the expiration of the stay. The Company intends to vigorously defend its intellectual property rights relating to Phexxi.
Designation
of and Exclusivity for Qualified Infectious Disease Products
In
2012 as part of the Food Drug Administration Safety and Innovation Act, Congress passed legislation known as the Generating Antibiotic
Incentives Now Act (GAIN Act), which amended the FDCA to encourage the development of antibacterial and antifungal drug products that
treat pathogens that cause serious and life-threatening infections. The law grants an additional five years of marketing exclusivity
upon the approval of an NDA for a drug product previously designated by FDA as a QIDP. As a result, if applicable to a designated QIDP,
upon approval the periods of five-year new chemical entity exclusivity and three-year new clinical investigation exclusivity would become
ten years and eight years, respectively.
A
QIDP is defined in the GAIN Act to mean “an antibacterial or antifungal drug for human use intended to treat serious or life-threatening
infections, including those caused by: (1) an antibacterial or antifungal resistant pathogen, including novel or emerging infectious
pathogens;” or (2) certain “qualifying pathogens.” A “qualifying pathogen” is a pathogen that has the potential
to pose a serious threat to public health (e.g., resistant gram positive pathogens, multi-drug resistant gram negative bacteria, multi-drug
resistant tuberculosis and Clostridium difficile) and that is included in a list established and maintained by FDA. A drug sponsor
may request FDA to designate its product as a QIDP any time before the submission of an NDA for that indication. FDA must make a QIDP
determination within 60 days of the designation request. A product designated as a QIDP may be granted priority review by FDA upon submission
and can also qualify for “Fast Track” status, described further below. We have received two QIDP designations from the FDA
for EVO100 for the prevention of urogenital infection in women with both chlamydia and gonorrhea and one for EVO200 for BV.
Fast
Track and Priority Review Designations
The
FDA is authorized to designate certain products for expedited development or review if they are intended to address an unmet medical
need in the treatment of a serious or life-threatening disease or condition. These programs include Fast Track designation and priority
review designation.
To
be eligible for a Fast Track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat
a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need by providing a therapy
where none exists or a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. Fast Track designation
provides opportunities for more frequent interactions with the FDA review team to expedite development and review of the product. The
FDA may also review sections of the NDA for a Fast Track product on a rolling basis before the complete application is submitted, if
the sponsor and the FDA agree on a schedule for the submission of the application sections, and the sponsor pays any required user fees
upon submission of the first section of the NDA. Fast Track designation may be withdrawn by the sponsor or rescinded by the FDA if the
designation is no longer supported by data emerging in the clinical trial process. A product candidate designated as a QIDP is eligible
for Fast Track designation under the provisions of the GAIN Act, but the NDA sponsor must specifically request Fast Track designation
from the agency as with non-infectious disease product candidates. Fast Track designation may be requested concurrent with or at any
time after the QIDP designation. In addition, although QIDP designation may be requested prior to submission of an Investigational New
Drug Application (IND), a request for Fast Track designation may only be made concurrently with, or any time after, submission of an
IND.
The
FDA also may designate a product for priority review if it is a drug or biologic that treats a serious condition and, if approved, would
provide a significant improvement in safety or effectiveness. The FDA determines at the time that the marketing application is submitted,
on a case- by-case basis, whether the proposed drug represents a significant improvement in treatment, prevention or diagnosis of disease
when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the
treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient
compliance that may lead to improvement in serious outcomes, or evidence of safety and effectiveness in a new subpopulation. A priority
review designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s
goal for taking action on a marketing application from ten months to six months for an original new molecular entity NDA from the date
of filing. Although the FDA automatically gives priority review designation to the first application submitted for a specific drug product
and indication for which a QIDP designation was granted, a subsequent application from the same sponsor for the same product and indication
will receive priority review designation only if it otherwise meets the criteria for priority review.
Finally,
even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions
for qualification or decide that the time period for FDA review or approval will not be shortened. Furthermore, Fast Track designation
and priority review do not change the standards for approval and may not ultimately expedite the development or approval process.
We
have received two Fast Track designations from the FDA for EVO100 for the prevention of urogenital chlamydia and gonorrhea infection
in women.
Patent
Term Restoration in the United States
Depending
upon the timing, duration and specifics of FDA approval of our drug candidates, some of our U.S. patents may be eligible for limited
PTE under other provisions of the Hatch-Waxman Amendments. These PTEs permit a patent restoration term of up to five years as compensation
for any patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend
the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period
is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission
date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension, and
the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office (USPTO) in consultation
with the FDA, reviews and approves the application for any PTE or restoration.
Other
United States Governmental Regulations and Environmental Matters
If
we establish international operations, we will be subject to compliance with the United States Foreign Corrupt Practices Act of 1977,
as amended (the FCPA), which prohibits corporations and individuals from paying, offering to pay, or authorizing the payment of anything
of value to any foreign government official, government staff member, political party, or political candidate to obtain or retain business
or to otherwise influence a person working in an official capacity. We also may be implicated under the FCPA for activities by our partners,
collaborators, contract research organizations, vendors or other agents.
Importantly,
United States authorities that enforce the FCPA, including the Department of Justice, deem most health care professionals and other employees
of foreign hospitals, clinics, research facilities and medical schools in countries with public health care or public education systems
to be “foreign officials” under the FCPA. If and when we interact with foreign health care professionals and researchers
in testing and marketing our products abroad, we must have policies and procedures in place sufficient to prevent us and agents acting
on our behalf from providing any bribe, gift or gratuity, including excessive or lavish meals, travel or entertainment in connection
with marketing our products and services or securing required permits and approvals such as those needed to initiate clinical trials
in foreign jurisdictions. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting
provisions requiring the maintenance of books and records that accurately and fairly reflect all transactions of the corporation, including
international subsidiaries, and the development and maintenance of an adequate system of internal accounting controls for international
operations.
Our
present and future business has been and will continue to be subject to various other laws and regulations. Various laws, regulations
and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage,
movement, import and export and use and disposal of hazardous or potentially hazardous substances used in connection with our research
work are or may be applicable to our activities. Certain agreements involving exclusive license rights, if any, or acquisitions, if any,
may be subject to national or supranational antitrust regulatory control, the effect of which cannot be predicted. The extent of government
regulation, which might result from future legislation or administrative action, cannot accurately be predicted.
Review
and Approval of Drug Products in the European Union
In
addition to regulations in the United States, we are and will be subject, either directly or through our distribution partners, to a
variety of regulations in other jurisdictions governing, among other things, clinical trials and future commercial sales and distribution
of our products, if approved in those markets.
We
must obtain the requisite approvals from regulatory authorities in non-U.S. countries prior to the commencement of clinical trials or
marketing of a product in those countries. Moreover, the time required to obtain approval in other countries and jurisdictions might
differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure
regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively
impact the regulatory process in others. As of January 31, 2020, the United Kingdom (UK) is no longer a member state of the European
Union (EU), and therefore a separate marketing authorization application (MAA) and approval will be required to market a medicinal product
in the UK.
We
are currently assessing the optimal regulatory legal basis for the Phexxi MAA in the EU and the UK. As in the United States, medicinal
products can be marketed in the EU only if a marketing authorization from the competent regulatory agencies has been obtained. Similar
to the United States, the various phases of preclinical and clinical research in the EU are subject to significant regulatory controls.
Pursuant
to the European Clinical Trials Directive, a system for the approval of clinical trials in the EU has been implemented through national
legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of an EU
member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent
ethics committee has issued a favorable opinion. Clinical trial applications must be accompanied by an investigational medicinal product
dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member
states and further detailed in applicable guidance documents. In April 2014, the new Clinical Trials Regulation, Regulation EU No 536/2014
(Clinical Trials Regulation) was adopted and it came into application on January 31, 2022. The Clinical Trials Regulation will be directly
applicable in all the EU member states, repealing the current Clinical Trials Directive 2001/20/EC. Conduct of all clinical trials performed
in the EU will continue to be bound by currently applicable provisions until the new Clinical Trials Regulation becomes applicable. The
extent to which ongoing clinical trials will be governed by the Clinical Trials Regulation will depend on when the Clinical Trials Regulation
becomes applicable and on the duration of the individual clinical trial. If a clinical trial continues for more than three years from
the day on which the Clinical Trials Regulation becomes applicable, the Clinical Trials Regulation will at that time begin to apply to
the clinical trial.
The
new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the EU. The main characteristics of
the regulation include: a streamlined application procedure via a single entry point; a single set of documents to be prepared and submitted
for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment
of applications for clinical trials, which is divided in two parts. Part I is assessed by the competent authorities of all EU member
states in which an application for authorization of a clinical trial has been submitted. Part II is assessed separately by each EU member
state concerned. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics
committees in the assessment procedure will continue to be governed by the national law of the concerned EU member state. However, overall
related timelines will be defined by the Clinical Trials Regulation.
To
obtain marketing approval of a drug in the EU, an applicant must submit an MAA either under a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all
EU member states, Iceland, Lichtenstein and Norway. The centralized procedure is compulsory for specific products, including for medicines
produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products (such as
gene-therapy, somatic cell-therapy or tissue-engineered medicines) and products with a new active substance indicated for the treatment
of certain diseases. For products with a new active substance indicated for the treatment of certain diseases and products that are highly
innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional. Under the centralized
procedure the maximum timeframe for the evaluation of an MAA by the EMA is 210 days, excluding clock stops, when additional written or
oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human
Use (CHMP). Accelerated assessment might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a
major public health interest, particularly from the point of view of therapeutic innovation. The timeframe for the evaluation of an MAA
under the accelerated assessment procedure is of 150 days, excluding stop-clocks.
The
decentralized procedure is available to applicants who wish to market a product in specific EU member states where such product has not
received marketing approval in any EU member states before. The decentralized procedure provides for an applicant to apply to one-member
state to assess the application (the reference member state) and specifically list other member states in which it wishes to obtain approval
(concerned member states). Under this procedure, an applicant submits an application based on identical dossiers and related materials,
including a draft summary of product characteristics, and draft labelling and package leaflet, to the reference member state and each
concerned member state. The reference member state prepares a draft assessment report and drafts of the related materials within 210
days after receipt of a valid application which is then reviewed and approved commented on by the concerned member states. Within 90
days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide
whether to approve the assessment report and related materials.
In
the EU, only products for which marketing authorizations have been granted may be promoted. A marketing authorization is valid for five
years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit
balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder must
provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including
all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases
to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent
authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization
which is not followed by the actual placing of the drug on the EU market (in case of centralized procedure) or on the market of the authorizing
member state within three years after authorization ceases to be valid (the so-called sunset clause). Even if authorized to be marketed
in the EU, prescription-only medicines may only be promoted to health care professionals, not the general public. All promotion should
be in accordance with the particulars listed in the summary of product characteristics. Promotional materials must also comply with various
laws, and codes of conduct developed by pharmaceutical industry bodies in the EU which govern (among other things) the training of sales
staff, promotional claims and their justification, comparative advertising, misleading advertising, endorsements, and (where permitted)
advertising to the general public. Failure to comply with these requirements could lead to the imposition of penalties by the competent
authorities of the EU member states. The penalties could include warnings, orders to discontinue the promotion of the drug product, seizure
of promotional materials, fines and possible imprisonment.
EU
Regulatory Exclusivity
In
the EU, new products authorized for marketing (i.e., reference products) qualify for eight years of data exclusivity and an additional
two years of market exclusivity upon marketing authorization. The data exclusivity period prevents generic applicants from relying on
the pre-clinical and clinical trial data contained in the dossier of the reference product when applying for a generic marketing authorization
in the EU during a period of eight years from the date on which the reference product was first authorized in the EU. The market exclusivity
period prevents a successful generic applicant from commercializing its product in the EU until ten years have elapsed from the initial
authorization of the reference product in the EU. The ten-year market exclusivity period can be extended to a maximum of eleven years
if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new
therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical
benefit in comparison with existing therapies.
Rest
of the World Regulation
For
other countries outside of the EU and the United States, such as countries in Eastern Europe, Latin America, Asia, or Africa, the requirements
governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from jurisdiction to jurisdiction. Additionally,
the clinical trials must be conducted in accordance with cGCP requirements and the applicable regulatory requirements and the ethical
principles that have their origin in the Declaration of Helsinki.
Other
U.S. Health Care Laws and Regulations
We
must comply with various U.S. federal and state laws, rules and regulations pertaining to health care fraud and abuse, including anti-kickback
laws. HCPs and third-party payers play a primary role in the recommendation and prescription of drug products and medical devices. Our
current and future arrangements with health care professionals, principal investigators, consultants, third-party payers and customers
may expose us to broadly applicable fraud and abuse and other health care laws and regulations. Such restrictions under applicable federal
and state health care laws and regulations, include but are not limited to the following:
Anti-Kickback
Statute – the Federal Anti-Kickback Statute, among other things, prohibits persons from knowingly and willfully soliciting,
offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of
an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federally funded
health care programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific
intent to violate the statute in order to have committed a violation. In addition, the government may assert that a claim that includes
items or services resulting from a violation of the Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes
of the False Claims Act.
Civil
and Criminal False Claims Laws – the federal civil and criminal false claims laws, including the federal False Claims Act,
which can be enforced by private citizens through civil whistleblower or qui tam actions, prohibit, among other things, individuals or
entities from knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent
or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government.
Health
Insurance Portability and Accountability Act of 1996 – the federal Health Insurance Portability and Accountability Act
of 1996 (HIPAA) prohibits, among other things, individuals or entities from executing a scheme to defraud any health care benefit program
or making any false statements relating to health care matters; as in the case of the Federal Anti-Kickback Statute, a person or entity
does not need to have actual knowledge of the statute or specific intent to violate the statute in order to have committed a violation.
Additionally, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH), and its implementing
regulations impose certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and
transmission of individually identifiable health information without appropriate authorization, on entities subject to the law, such
as certain HCPs, health plans, and health care clearinghouses and their respective business associates that perform services for them
that involve the creation, use, maintenance or disclosure of, individually identifiable health information.
False
Statements Statute – the federal False Statements Statutes prohibits knowingly and willfully falsifying, concealing or
covering up a material fact or making any materially false statement to the federal government, including executive or administrative
agencies.
Sunshine
Act – the federal transparency or “sunshine” requirements of the ACA requires certain manufacturers of drugs,
devices, biologics and medical supplies to annually report to the Department of Health and Human Services (the DHHS) information related
to payments and other transfers of value made to physicians, teaching hospitals and certain advanced non-physician health care practitioners,
as well as ownership and investment interests held by physicians and their immediate family members.
State
Transparency Laws – some U.S. state laws require pharmaceutical companies to comply with the pharmaceutical industry’s
voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug
manufacturers to report information related to payments to HCPs and other HCPs or marketing expenditures; some state laws require pharmaceutical
companies to implement compliance programs and to track and report gifts, compensation and other remuneration provided to physicians,
in addition to requiring drug manufacturers to report information related to payments to physicians and other HCPs or marketing expenditures
and pricing information; and some state and local laws require the registration of pharmaceutical sales representatives.
State
and Foreign Regulatory Concerns – there are analogous State and foreign laws and regulations, such as State Anti-Kickback
and False Claims laws, which may apply to sales or marketing arrangements and claims involving health care items or services reimbursed
by non-governmental third-party payers, including private insurers. State and foreign laws also govern the privacy and security of health
and personal information. These laws differ from each other in significant ways and may conflict, while applying simultaneously with
HIPAA, thus complicating compliance efforts.
The
scope and enforcement of these laws is uncertain and subject to rapid change. Notably, in November 2020, DHHS finalized significant changes
to the regulations implementing the Anti-Kickback Statute, as well as the civil monetary penalty rules regarding beneficiary inducements,
with the goal of offering the health care industry more flexibility and reducing the regulatory burden associated with those fraud and
abuse laws, particularly with respect to value-based arrangements among industry participants. Regulatory authorities might challenge
our current or future activities under these laws, regulations, and safe harbors. Any such challenge could have a material adverse effect
on our reputation, business, results of operations and financial condition. In addition, efforts to ensure that our business arrangements
with third parties will comply with these laws will involve substantial costs. Any investigation of us or the third parties with whom
we contract, regardless of the outcome, would be costly and time consuming. If our operations are found to be in violation of any of
these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative
penalties, including, without limitation, damages, monetary fines, imprisonment, disgorgement of profits, possible exclusion from participation
in Medicare, Medicaid and other federal health care programs, debarment under the FDCA, additional reporting or oversight obligations
if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law, contractual
damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
Health
Care Reform and Potential Changes to Laws and Regulations
In
the United States and some foreign jurisdictions, there have been, and continue to be, legislative and regulatory changes both enacted
and proposed related to the health care system, which could prevent or delay marketing approval of our product candidates, restrict or
regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In particular, the FDA’s and other regulatory authorities’ policies may change and additional government regulations may
be enacted. For example, in December 2016, the 21st Century Cures Act (Cures Act), was passed by Congress and signed into law. The Cures
Act, among other things, is intended to modernize the regulation of drugs and devices and to spur innovation, but its ultimate implementation
is uncertain. In addition, in August 2017, the FDA Reauthorization Act was signed into law, which reauthorized the FDA’s user fee
programs and included additional drug and device provisions that build on the Cures Act. A subsequent FDA reauthorization package was
finalized by Congress on September 30, 2022, and several other FDA-related changes are being proposed in Congress, including several
within a “Cures 2.0” bill that is likely to have bipartisan support. If we are slow or unable to adapt to changes in existing
requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any
marketing approval that we otherwise may have obtained and we may not achieve or sustain profitability, which would adversely affect
our business, prospects, financial condition and results of operations.
Among
policy makers and payers in the United States and elsewhere, there is significant interest in promoting changes in health care systems
with the stated goals of containing health care costs, improving quality and/or expanding access. In the United States, the pharmaceutical
industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. For example,
in March 2010, the ACA was enacted, which, among other things, increased the minimum Medicaid rebates owed by most manufacturers under
the Medicaid Drug Rebate Program; introduced a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate
Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; extended the Medicaid Drug Rebate Program
to utilization of prescriptions of individuals enrolled in Medicaid managed care plans; imposed mandatory discounts for certain Medicare
Part D beneficiaries as a condition for manufacturers’ outpatient drugs coverage under Medicare Part D; and established a Center
for Medicare Innovation at the U.S. Centers for Medicare and Medicaid Services (CMS) to test innovative payment and service delivery
models to lower Medicare and Medicaid spending. As another example, the 2021 Consolidated Appropriations Act, signed into law on December
27, 2020, incorporated extensive health care provisions and amendments to existing laws, including a requirement that all manufacturers
of drug products covered under Medicare Part B report the product’s average sales price (ASP) to DHHS beginning on January 1, 2022,
subject to enforcement via civil money penalties.
Since
its enactment, there have been judicial and congressional challenges to certain aspects of the ACA, and as a result certain sections
of the ACA have not been fully implemented or effectively repealed. However, following several years of litigation in the federal courts,
in June 2021, the U.S. Supreme Court upheld the ACA when it dismissed a legal challenge to the ACA’s constitutionality. Further
legislative and regulatory changes under the ACA remain possible, although the new federal administration under President Biden has signaled
that it plans to build on the ACA and expand the number of people who are eligible for health insurance subsidies under it. It is unknown
what form any such changes or any law would take, and how or whether it may affect the pharmaceutical industry as a whole or our business
in the future. We expect that changes or additions to the ACA, the Medicare and Medicaid programs, such as changes allowing the federal
government to directly negotiate drug prices, and changes stemming from other health care reform measures, especially with regard to
health care access, financing or other legislation in individual states, could have a material adverse effect on the health care industry
in the United States.
Other
legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare
payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and will remain in
effect through 2030 unless additional congressional action is taken. The Coronavirus Aid, Relief, and Economic Security Act (the CARES
Act), which was signed into law on March 27, 2020, and was designed to provide financial support and resources to individuals and businesses
affected by the COVID-19 pandemic, suspended the 2% Medicare sequester from May 1, 2020 through December 31, 2020, and extended the sequester
by one year, through 2030, in order to offset the added expense of the 2020 cancellation. The suspension was subsequently extended through
March 31, 2022, with a reduction of the suspension to 1% sequester through June 30, 2022. On July 1, 2022 the Medicare sequester increased
to 2%.
As
another example, on December 20, 2019, President Trump signed the Further Consolidated Appropriations Act for 2020 into law (P.L. 116-94)
that includes a piece of bipartisan legislation called the Creating and Restoring Equal Access to Equivalent Samples Act of 2019 (the
CREATES Act). The CREATES Act aims to address the concern articulated by both the FDA and others in the industry that some brand manufacturers
have improperly restricted the distribution of their products to deny generic product developers access to samples of brand products.
Because generic product developers need samples to conduct certain comparative testing required by the FDA, some have attributed the
inability to timely obtain samples as a cause of delay in the entry of generic products. To remedy this concern, the CREATES Act establishes
a private cause of action that permits a generic product developer to sue the brand manufacturer to compel it to furnish the necessary
samples on “commercially reasonable, market-based terms.” Whether and how generic product developers will use this new pathway,
as well as the likely outcome of any legal challenges to provisions of the CREATES Act, remain highly uncertain and its potential effects
on our future commercial products are unknown. Other new laws may result in additional reductions in Medicare and other health care funding,
which could have an adverse effect on customers for our approved product and, accordingly, our financial operations.
Additionally,
there has been heightened governmental scrutiny in the United States of manufacturers’ pharmaceutical pricing practices in light
of the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several recent congressional inquiries and proposed
and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship
between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. DHHS has solicited
feedback on various measures intended to lower drug prices and reduce the out-of-pocket costs of drugs and has implemented others under
its existing authority. For example, in 2020, the FDA finalized a rulemaking to establish a system whereby state governmental entities
could lawfully import and distribute prescription drugs sourced from Canada. More recently, in July 2021, President Biden issued a sweeping
executive order on promoting competition in the American economy that includes several mandates pertaining to the pharmaceutical and
health care insurance industries. Among other things, the executive order directs the FDA to work towards implementing a system for importing
drugs from Canada (following on the Trump administration notice-and-comment rulemaking on Canadian drug importation that was finalized
in October 2020). The Biden order also called on DHHS to release a comprehensive plan to combat high prescription drug prices, and it
includes several directives regarding the Federal Trade Commission’s oversight of potentially anticompetitive practices within
the pharmaceutical industry. The drug pricing plan released by DHHS in September 2021 in response to the executive order makes clear
that the Biden Administration supports aggressive action to address rising drug prices, including allowing DHHS to negotiate the cost
of Medicare Part B and D drugs, but such significant changes will require either new legislation to be passed by Congress or time-consuming
administrative actions.
Coverage,
Pricing, and Reimbursement
Sales
of Evofem’s products approved for marketing by the FDA and foreign regulatory authorities depend, in part, on the extent to which
such products will be covered by third-party payers, such as government health programs, commercial insurance and managed care organizations.
In the United States, no uniform policy of coverage and reimbursement for drug or biological products exists. Accordingly, decisions
regarding the extent of coverage and amount of reimbursement to be provided for any of Evofem’s FDA-approved products will be made
on a payer-by-payer basis. Prescriptions generated through the Phexxi telehealth platform may be subject to additional payer requirements.
As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific
and clinical support for the use of our approved products to each payer separately, with no assurance that coverage and adequate reimbursement
will be obtained.
The
U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs
to limit the growth of government-paid health care costs, including price-controls, restrictions on reimbursement and requirements for
substitution of generic products for branded prescription drugs. For example, the ACA contains provisions that may reduce the profitability
of drug products through increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed
care plans, and mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’
share of sales to federal health care programs. Adoption of general controls and measures, coupled with the tightening of restrictive
policies in jurisdictions with existing controls and measures, could limit payments for pharmaceutical drugs. The Medicaid Drug Rebate
Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the
DHHS as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid
patients. The ACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’
rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs from 15.1% of average manufacturer price
(AMP), to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended
release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying
the statutory definition of AMP. The ACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical
manufacturers to pay rebates on Medicaid managed care utilization and by enlarging the population potentially eligible for Medicaid drug
benefits. Congress has expressed its intention to repeal or repeal and replace the ACA. If that is done, many if not all of the provisions
of the ACA may no longer apply to prescription drugs.
The
marketability of any products for which Evofem has or will receive regulatory approval for commercial sale may suffer if the government
and third-party payers fail to provide adequate coverage and reimbursement. An increasing emphasis on cost containment measures in the
United States has increased, and Evofem expects will continue to increase, the pressure on pharmaceutical pricing. Coverage policies
and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or
more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in
the future.
In
addition, in most foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements
governing drug pricing and reimbursement vary widely from country to country. Some countries provide that drug products may be marketed
only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness
of our product candidate to currently available therapies (so called health technology assessment) in order to obtain reimbursement or
pricing approval. For example, the EU provides options for its member states to restrict the range of medicinal products for which their
national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state
may approve a specific price for the medicinal product, or it may instead adopt a system of direct or indirect controls on the profitability
of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement
limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of Evofem’s approved
drug products. Historically, products launched in the EU do not follow price structures of the United States and generally prices tend
to be significantly lower.
Corporate
Information
Our
corporate headquarters are located at 7770 Regents Rd, Suite 113-618, San Diego, CA 92122-1967, and our telephone number is (858) 550-1900.
Our website is located at www.evofem.com. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports
on Form 8-K, and amendments to reports filed pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended
(the Exchange Act) will be made available free of charge on our website as soon as reasonably practicable after we electronically file
these materials with, or furnish them to, the Securities and Exchange Commission (SEC) on their website located at www.sec.gov. The contents
of our website are not incorporated into this Registration Statement, and our reference to the URL for our website is intended to be
an inactive textual reference only. The information contained on, or that can be accessed through, our website is not a part of this
Registration Statement.
Employees
As
of August 1, 2023, we had a total of 38 full-time employees and one part-time employee. We also engage
consultants and contract workers on an as-needed basis. We believe that relations with our employees and consultants are
good.
MANAGEMENT
Executive
Officers and Directors
Executive
Officers
The
following table sets forth certain information regarding our executive officers and their respective ages as of April 30, 2023. All executive
officers are at-will employees.
| |
Saundra
Pelletier |
| |
|
|
| |
 |
Chief
Executive Officer, Evofem Biosciences, Inc.
Age:
53 |
| |
|
|
| |
Background
●
Since joining the Company in 2015, Ms. Pelletier has been responsible for Evofem’s rapid growth and evolution, including the
Company’s transition to the public market in January 2018 and multiple equity financing rounds that have raised in excess of
$500 million.
●
Under her leadership, the Company launched its first commercial product in September 2020. Phexxi is the first and only hormone-free,
on-demand, prescription vaginal gel approved in the United States for the prevention of pregnancy.
●
Ms. Pelletier brings more than two decades of broad executive leadership experience to Evofem, including a strong track record driving
multiple billion-dollar product launches, expanding commercial capabilities in ex-U.S. markets and advocating for women’s health.
Throughout her career, she has had oversight and accountability for Sales, Marketing, Operations, Medical Affairs, Regulatory Affairs,
Manufacturing, Customer Service, Business Development, and Strategic Partnerships.
●
Ms. Pelletier was previously the founding CEO of Woman Care Global (WCG), an international nonprofit organization focused on creating
sustainable supply chains that delivered products to women in more than 100 developing countries. Under her leadership, WCG secured
approximately $68M in committed funding from major foundations and USAID.
●
Earlier in her career, Ms. Pelletier served as Corporate Vice President and Global Franchise Leader for G.D. Searle, where she managed
a $250 million business unit focused on women’s healthcare. She later moved to Women First Healthcare, where she served as
Vice President of Pharmaceuticals and raised $40 million in capital.
●
She is a Director of TRACON Pharmaceuticals, Inc., a clinical stage biopharmaceutical company focused on novel targeted therapeutics
for cancer, where she serves as the chair of the Governance/Nomination Committee and is a member of the Audit Committee.
●
Ms. Pelletier is a published author, skilled moderator and coveted keynote speaker. She has appeared at TEDx San Diego, Harvard School
of Public Health, Davos World Economic Forum, the Clinton Global Initiative, MAKERS Conference, Women Deliver, University of Virginia’s
Darden School of Business, University of Oregon’s Lundquist School of Business and University of California, San Diego. She
was named as a New Champion for Reproductive Health by the United Nations Foundation, awarded the Athena San Diego’s Pinnacle
Award for Life Sciences, named 2019 Businesswoman of the Year by the San Diego Business Journal, and named to Inc. Magazine’s
2020 Female Founders 100 List. |
| |
Ivy
Zhang |
| |
|
|
| |
 |
Chief
Financial Officer
Age:
45 |
| |
|
|
| |
Background
● Ivy
Zhang is a trusted leader who is dedicated to advancing Evofem Biosciences’ mission of addressing the unmet sexual and reproductive
health needs of women. She has more than 14 years of financial and accounting experience spanning diverse industries, including pharmaceuticals
and medical devices, and leads the Company’s finance organization and financial activities including financial planning and
analysis, accounting, external audit, tax, controllership, and treasury functions.
● Ms.
Zhang re- joined Evofem as Chief Financial Officer and Secretary in April 2023 from HUYABIO International, where she served as Vice
President Controller.
● Previously
Ms. Zhang held increasingly senior finance roles at Evofem from March 2018 until November 2022. She served as Director of SEC Reporting
and SOX Compliance until her promotion to Controller in April 2020.
● Earlier
in her career, she served in finance positions for approximately seven years at Ernst & Young LLP, and for more than two and
a half years at SeaSpine Holdings Corporation (a public medical and therapeutic technology and device company).
● Ms.
Zhang holds a Master’s in Assurance from Virginia Tech and a Master’s in Economics from the University of Victoria, Canada.
She is a certified public accountant (CPA) in the state of California. |
The
Board of Directors
Our
Board currently consists of eight seats with three vacancies (two for Class II and one for Class III). Vacancies on the Board may be
filled by potential candidates nominated by the Nominating and Corporate Governance Committee of the Board, who may seek out potential
candidates that meet the criteria for selection as a Board nominee and have the specific qualities or skills being sought, and one or
more of such candidates may be appointed as directors as appropriate and in accordance with the Company’s organizational documents.
The vacancies, if filled, will be filled until the end of the class term. Our Board is divided into three classes as set forth below,
each serving staggered three-year terms until their respective successors are duly elected and qualified:
| ● | Our
Class I directors are Kim Kamdar, Ph.D., Colin Rutherford, and Lisa Rarick, M.D. and their
terms expire at the Annual Meeting of Stockholders in 2024; |
| ● | Our
Class II director is Tony O’Brien and his term expires at the Annual Meeting of Stockholders
in 2025; and |
| ● | Our
Class III director is Saundra Pelletier and her term expires at the Annual Meeting of Stockholders
in 2023. |
The
following table lists the names, ages as of April 30, 2023, and positions of the individuals who serve as our directors:
| |
|
Name
and Principal Occupation |
|
Age |
|
Director
Since |
|
Board
Committees |
|
Other
Current Public Directorships |
| |
|
|
|
|
|
|
|
|
|
|
| Class
I Directors |
| |
|
|
|
|
|
|
|
|
|
|
 |
|
Colin
Rutherford | Independent
Current
member of the board of Biopharma Hifas da Terra SA |
|
64 |
|
2015 |
|
A |
|
Mitchells
& Butlers Plc Renaissance Services SAOG Brookgate Limited |
| |
|
|
|
|
|
|
|
|
|
|
 |
|
Kim
P. Kamdar, Ph.D. | Independent
Managing
Partner, Domain Associates, LLC |
|
55 |
|
2011 |
|
A,
C, N |
|
Seraphina
Therapeutics, Inc. Truvian Sciences |
| |
|
|
|
|
|
|
|
|
|
|
 |
|
Lisa
Rarick, M.D. | Independent
Board-certified
Obstetrician/ Gynecologist and Regulatory Affairs Expert |
|
63 |
|
2020 |
|
N |
|
|
| |
|
|
|
|
|
|
|
|
|
|
| Class
II Directors |
| |
|
|
|
|
|
|
|
|
|
|
 |
|
Tony
O’Brien | Independent
Former
Director General of Ireland’s Health Service Executive |
|
60 |
|
2018 |
|
A,
C |
|
Global
Leadership and Governance Solutions Limited |
| |
|
|
|
|
|
|
|
|
|
|
| Class
III Directors |
| |
|
|
|
|
|
|
|
|
|
|
 |
|
Saundra
Pelletier | Interim Chair of the Board of Directors
President
and Chief Executive Officer, Evofem Biosciences, Inc. |
|
53 |
|
2013 |
|
|
|
TRACON
Pharmaceuticals |
| A |
Audit
Committee |
| C |
Compensation
Committee |
| N |
Nominating
and Corporate Governance Committee |
 |
Committee
Chair |
Non-Employee
Directors
 |
|
|
|
|
|
| |
|
|
KEY
EXPERIENCE AND QUALIFICATIONS
We
believe Dr. Kamdar is qualified to serve on our Board based on her extensive experience working and serving on the boards of directors
of life sciences companies and her experience working in the venture capital industry. |
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
CAREER
HIGHLIGHTS
● Managing
Partner of Domain Associates, LLC, a life sciences venture capital firm (since 2005)
● Chair
of the board of directors of Seraphina Therapeutics, Inc. and Truvian Sciences
● Member
of the board of directors of several private companies including Alume, Epic Sciences, Epitel and Pleno Inc.
● Member
of the board of directors of several public companies including NASDAQ: SERA and NASDAQ: OMIC
● Past
investments include Ariosa (acquired by Roche), Corthera (acquired by Novartis), BiPar Sciences (acquired by Sanofi-Aventis) and
Omniome (acquired by NASDAQ: PACB)
● Kauffman
Fellow with MPM Capital (MPM) (2003 through 2004)
● Research
director at Novartis, where she built and led a research team that focused on the biology, genetics and genomics of model organisms
(1995 to 2003)
● Author
of ten papers as well as the inventor of seven patents
● Advisory
board member of Dr. Eric Topol’s NIH supported Clinical and Translational Science Award for Scripps Medicine
EDUCATION
● B.A.
from Northwestern University
● Ph.D.
in Biochemistry and Genetics from Emory University |
|
| |
|
|
| |
|
|
|
Kim
Kamdar, Ph.D., 55
Independent
Director
Since: April 2011 (Private Evofem);
January
2018 (Evofem Biosciences)
Committees:
●
Audit
●
Compensation Committee
●
Nominating and Corporate Governance (Chair) |
|
|
|
 |
|
|
|
|
|
| |
|
|
KEY
EXPERIENCE AND QUALIFICATIONS
We
believe Mr. O’Brien’s extensive experience as an executive and member of the boards of directors for health
care and life sciences companies qualifies him to be a member of our Board. |
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
CAREER
HIGHLIGHTS
● Director
General of Ireland’s Health Service Executive (HSE), an organization responsible for the provision of health and personal social
services for the residents of Ireland (2012 to 2018)
● Chief
Operating Officer of the Department of Health’s Special Delivery Unit and a member of the Department’s Management
Board (2011 to 2014)
● Director
of Clinical Strategy and Programs in the HSE (2011 to 2012)
● Chief
Executive Officer of the National Treatment Purchase Fund (2011 to 2013)
● Chief
Advisor to the HSE on the implementation of the National Cancer Control Strategy (2006 to 2010)
● Project
Director for the National Plan for Radiation Oncology (2005 to 2008)
● Chairman
of the National Cancer Registry Board (2009 to 2012)
● Founding
Chief Executive Officer of the National Cancer Screening Service (2007 to 2011)
● Director
of BreastCheck, CervicalCheck (2002 to 2010)
● Associate
and Interim Director of the National Cancer Control Programme (2007 to 2011)
● Chief
Executive of the Irish Family Planning Association (1991 to 2002)
● Chief
Executive of the UK Family Planning Association (1995 to 1996)
● Chartered
Director of the Institute of Directors in Ireland
● Adjunct
Assistant Professor in Health Strategy and Management at Trinity College Dublin
OTHER
PROFESSIONAL EXPERIENCE AND COMMUNITY INVOLVEMENT
● Director
and owner of Global Leadership And Governance Solutions Limited, a private limited company organized in the Republic of Ireland
EDUCATION
● M.Sc.
in Management Practice from Trinity College, University of Dublin |
|
| |
|
|
| |
|
|
|
Tony
O’Brien, 60
Independent
Director
Since: January 2018
Committees:
●
Audit
●
Compensation (Chair) |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
 |
|
|
|
|
|
| |
|
|
KEY
EXPERIENCE AND QUALIFICATIONS
Ms.
Pelletier’s service as Evofem’s CEO and extensive experience in women’s healthcare brings Evofem’s Board
of Directors invaluable guidance and insight. With more than twenty-five years providing broad executive leadership, including successes
in driving multiple, billion-dollar product launches, expanding commercial capabilities in global markets and advocating for women’s
health, Ms. Pelletier continues to lead the Board of Directors with a clear focus on continuing Evofem’s successes.
Since
joining Evofem Ms. Pelletier has led the Company’s rapid growth and evolution, including its transition to the public market
in January 2018. Ms. Pelletier has also led the Evofem team in multiple successful equity financing rounds, raising more than $500
million. Under her leadership, Evofem launched its first commercial product in September 2020. Phexxi is the first and only hormone-free,
on-demand, prescription vaginal gel approved in the United States for the prevention of pregnancy. |
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
| |
|
|
|
|
Saundra
Pelletier, 53
Chief
Executive Officer
Director
Since: February 2013 (Private Evofem); January 2018 (Evofem Biosciences) |
|
|
|
|
| |
|
|
|
|
| |
|
CAREER
HIGHLIGHTS
● President
and Chief Executive Officer of Evofem Biosciences, Inc. (since January 2018)
● Member
of the Board of Directors, President and Chief Executive Officer of Private Evofem (2013 to 2018)
● Founding
CEO of WomanCare Global (WCG Cares), an international nonprofit organization focused on creating sustainable supply chains that delivered
products to women in more than 100 developing countries
● Corporate
Vice President and Global Franchise Leader for G.D. Searle, where she managed women’s health care business and teams
● Presenter
and speaker at the Harvard T. H. Chan School of Public Health, the Davos World Economic Forum, the Clinton Global Initiative, the
International Conference on Climate Change, the MAKERS Conference, Women Deliver, the International Conference on Family Planning,
Reproductive Health Supplies Coalition, the University of Virginia’s Darden School of Business, the National Community Oncology
Dispensing Association, Fearless in Pharma, the Women’s Health Innovation Summit, the University of Oregon’s Lundquist
School of Business, Husson University and the Rady School of Management at the University of California, San Diego
● Member,
Board of Directors of TRACON Pharmaceuticals, Inc., a clinical stage biopharmaceutical company focused on the development and commercialization
of novel targeted therapeutics for cancer, where she serves as the Chair of the Governance/Nomination Committee and is a member of
the Audit Committee
AWARDS
AND RECOGNITION
● Awarded
San Diego Magazine’s Woman of the Year (2021)
● Director
of the Year Honoree from the San Diego Corporate Directors Forum (2021)
● Received
the Lifetime Legacy Award from the National Women of Influence (2021)
● Girls
Inc. San Diego SHE LEADS Trailblazer Award (2021)
● Recognized
as a Women of Influence in Life Sciences by the San Diego Business Journal (2021)
● MM+M
Hall of Femme Honoree (2021)
● PharmaVoice
100 Most Inspiring People (2021, 2020)
● Business
Intelligence Group (BIG) Innovation Award (2021)
● Enterprising
Women of the Year Honoree (2021)
● Recognized
as One of the 500 Most Influential People in San Diego by the San Diego Business Journal (2020, 2021)
● Inc.
Magazine’s Female Founders 100 List (2020)
● Awarded
an Honorary Doctor of Business Administration from Husson University (2020)
● San
Diego Business Journal’s Business Woman of the Year (2019)
● Awarded
the Athena San Diego Pinnacle Award for Life Sciences
● Named
a “New Champion for Reproductive Health” by the United Nations Foundation
● Profiled
by The New York Times, Inc. Magazine, NPR, Bangor Daily News, CNN, San Diego Business Journal, The San Diego Union-Tribune, Fierce
Pharma, Pharma Voice, Life Science Leader, Cosmopolitan, Bustle, Glamour, Marie Claire and Vogue
EDUCATION
● Bachelor
of Science in Business Administration – Husson University
● Bachelor
of Science in Communications – New England School of Communications |
|
 |
|
|
|
|
|
| |
|
|
KEY
EXPERIENCE AND QUALIFICATIONS
We
believe that Dr. Rarick is qualified to serve as a member of our Board because of her extensive experience in health care/women’s
health matters as well as her vast prior experience with regulatory matters and the life sciences industry. |
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
CAREER
HIGHLIGHTS
● Board-certified
obstetrician/gynecologist and regulatory affairs expert with 35 years’ experience in women’s health and 15
years’ experience leading several offices within the U.S. Food and Drug Administration (FDA)
● Began
her career at the FDA as a Medical Officer, responsible for the management of products indicated for a variety of reproductive health
conditions, including oral, transdermal and vaginal contraceptives (1988)
● Director
for the Division of Reproductive and Urologic Products (DRUP) at the FDA (1996)
● Held
several management roles in the Center for Drug Evaluation and Research (CDER), including Deputy Director of the Office of Drug
Evaluation 2 and Associate Director in the Office of the Center Director
● Focused
on HIV prevention, pregnancy prevention, pre- and post-pregnancy care and menopausal therapy in her final year at the FDA in
the Office of Women’s Health
● Reproductive
health and regulatory affairs consultant, helping numerous companies navigate the development of their products from early-stage
development through FDA approval
● Member
of the Scientific Advisory Committee for the National Institute of Child Health and Human Development (since 2004)
● Member
of the board of directors for Alliance Partners 360 from (2017 to 2019)
● Family
Planning clinical care provider (2020 to present)
EDUCATION
● B.S.
and M.D. from the Loma Linda University School of Medicine
● Completed
residency training in Obstetrics and Gynecology at Georgetown University |
|
| |
|
|
| |
|
|
| |
|
|
|
Lisa
Rarick, M.D., F.A.C.O.G., 63
Independent
Director
Since: 2020
Committees:
●
Nominating and Corporate Governance
|
|
|
|
 |
|
|
|
|
|
| |
|
|
KEY
EXPERIENCE AND QUALIFICATIONS
We
believe that Mr. Rutherford is qualified to serve as a member of our Board because of his prior experience as a member
of Private Evofem’s board of directors and his many years of finance and operations leadership experience in the
health care and life sciences industries. |
|
| |
|
|
|
|
| |
|
|
|
|
| |
|
CAREER
HIGHLIGHTS
● Former
Chairman and CEO of LSE quoted European finance specialist Euro-Sales Plc (with 18 offices across Europe), sold to
Royal Bank of Scotland Plc (2000 to 2002)
● Former
Chairman of SGI Funds, a Guernsey-, Cayman- and Hong Kong-based diversified fund management group (2004 to 2009)
● Former
Chairman and CEO of the LSE quoted UK fund management group, MAM Funds Plc (2008 to 2011)
● Former
Member of the board and Audit Committee Chairman of Mitchells & Butlers Plc, the LSE’s largest quoted hospitality
group (2013 to 2021)
● Former
Member of the board and Audit Committee Chairman of the MSE quoted Oil & Gas shipping logistics business, Renaissance Services
SAOG, based in Muscat and Dubai (2007 to 2019)
● Former
Chairman of European Health Care Group before its acquisition by two U.S.-based hedge funds (2012 to 2014)
● Current
Member of the Board of Meallmore Health Care Group (2014 to Present)
● Current
Member of the Board of Spanish based Biopharma Hifas da Terra SA, a leader in the field of mycotherapy-related oncology
products (2018 to Present)
● Current
Chairman of Brookgate Limited, a UK property development business backed by Goldman Sachs and Sixth Street (2010 to Present)
● Former
visiting Professor at Edinburgh University’s Business School
EDUCATION
●
A member of the Scottish Institute of Chartered Accountants, he graduated in Accountancy and Finance from Heriot Watt University
in 1980 and qualified with Deloitte (formerly Touche Ross) in 1984.
●
Harvard Business School Alumni, having attended over a 10 year period and subsequently Chairing the HBS/YPO Presidents leadership
seminar for 5 years. |
| |
|
| |
|
|
Colin
Rutherford, 64
Independent
Director
Since: November 2015 (Private Evofem); January 2018 (Evofem Biosciences)
Committees:
●
Audit (Chair)
|
|
|
| |
|
|
| |
|
|
| |
|
|
Audit
Committee and Financial Expert
| Audit
Committee |
 |
|
Chair:
Colin
Rutherford
|
Members:
Kim Kamdar, Ph.D.
Tony O’Brien
|
Meetings
in 2022: 4 |
| |
|
|
|
| |
Our
Audit Committee’s role and responsibilities are set forth in the Audit Committee’s
written charter.
Principal
Responsibilities:
● Reviews
annual financial statements;
● Considers
matters relating to accounting policy and internal controls;
● Reviews
the scope of annual audits;
● Assists
the Board in its oversight of Evofem’s financial statements, including internal control over financial reporting;
● Reviews
and discusses with senior management the guidelines and policies by which Evofem assesses and manages risk;
● Assists
the Board in its oversight of the qualifications, independence, and performance of Evofem’s independent registered public accounting
firm, including responsibility for the appointment, compensation, retention, and oversight of the work of the firm;
● Assists
the Board in its oversight of the performance of Evofem’s internal audit function, including responsibility for the appointment,
replacement, reassignment, or dismissal of, and being involved in the performance reviews of, Evofem’s internal auditor; and
● Assists
the Board in its oversight of Evofem’s compliance with legal and regulatory requirements, including reviewing periodically
with management any significant legal, compliance, and regulatory matters that have arisen or that may have a material impact on
Evofem’s business, financial statements, or compliance policies, Evofem’s relations with regulators and governmental
agencies, and any material reports or inquiries from regulators and government agencies.
All
members of the Audit Committee satisfy the current independence standards promulgated by the SEC, OTCQB and Nasdaq, as such standards
apply specifically to members of audit committees. The Board has determined that Mr. Rutherford is an “audit committee financial
expert,” as the SEC has defined that term in Item 407 of Regulation S-K. Please also see the report of the Audit Committee
set forth elsewhere in this proxy statement.
A
copy of the Audit Committee’s written charter is publicly available on our website at www.evofem.com. |
| |
|
Code
of Conduct and Ethics
We
have adopted a Code of Business Conduct and Ethics that applies to our officers, directors and employees, which is available on our website
at www.evofem.com and will be made available to stockholders without charge, upon request, in writing to our Corporate Secretary, Evofem
Biosciences, Inc., 7770 Regents Rd. Suite 113-618, San Diego, California 92122. The Code of Business Conduct and Ethics contains general
guidelines for conducting the business of our company consistent with the highest standards of business ethics and is intended to qualify
as a “code of ethics” within the meaning of Section 406 of the Sarbanes-Oxley Act of 2002 and Item 406 of Regulation S-K.
In addition, disclosure regarding any amendments to, or waivers from, provisions of our Code of Business Conduct and Ethics that apply
specifically to our directors, principal executive officer and principal financial officer will be included in a Current Report on Form
8-K within four business days following the date of the amendment or waiver, unless website posting or the issuance of a press release
of such amendments or waivers is then permitted by the rules of the OTCQB.
Family
Relationships
Our
executive officers are appointed by, and serve at the discretion of, our board of directors. There are no family relationships among
any of our directors or executive officers.
EXECUTIVE
COMPENSATION
Summary
Compensation Table
The
following table summarizes information concerning the compensation awarded to, earned by, or paid for services rendered in all capacities
by our named executive officers during the years ended December 31, 2022 and 2021:
Name
and
Principal
Position | |
Year
Ended December
31, | | |
Salary
($) | | |
Bonus
($)(1) | | |
Restricted
Stock Awards(2) ($) | | |
Option
Awards(2) ($) | | |
All
Other Compensation(3) ($) | | |
Total ($) | |
| Saundra
Pelletier Chief Executive Officer | |
| 2022 | | |
| 812,083 | | |
| 203,021
| (4) | |
| — | (5) | |
| 293,850
| (6) | |
| $15,716
| (7) | |
| 1,324,670 | |
| | |
| 2021 | | |
| 812,083 | | |
| — | (8) | |
| 171,400
(9) | | |
| 1,787,940
| (10) | |
| $20,521 | (11) | |
| 2,791,944 | |
| Justin
J. File Chief Financial Officer | |
| 2022 | | |
| 589,240 | | |
| — | (12) | |
| — | (13) | |
| 176,310
| (14) | |
| 1,903 | | |
| 767,453 | |
| | |
| 2021 | | |
| 589,240 | | |
| — | (15) | |
| 68,560
| (16) | |
| 383,130
| (17) | |
| 1,242 | | |
| 1,042,172 | |
| Russ
Barrans(18) Chief Commercial Officer | |
| 2022 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| | |
| 2021 | | |
| 471,960
| (19) | |
| — | (20) | |
| 117,330
| (21) | |
| 383,130
| (22) | |
| 67,030
| (23) | |
| 1,039,450 | |
| Alexander
A. Fitzpatrick(24) General Counsel | |
| 2022 | | |
| 409,865
| (25) | |
| — | | |
| — | | |
| 117,540
| (26) | |
| 10,233
| (27) | |
| 537,638 | |
| | |
| 2021 | | |
| 469,310 | | |
| — | (28) | |
| 68,560
| (29) | |
| 383,130
| (30) | |
| 8,740
| (31) | |
| 929,740 | |
| Katherine
Atkinson(32) Chief Commercial Officer | |
| 2022 | | |
| 450,000 | | |
| — | | |
| — | (33) | |
| 39,180
| (34) | |
| 3,558 | | |
| 492,738 | |
| | |
| 2021 | | |
| 0 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| (1) |
Consists
of an earned cash incentive bonus as approved by the Compensation Committee in respect of the named executive officer’s performance
and the Company’s performance during each respective fiscal year. |
| (2) |
Amounts
listed in this column represent the aggregate fair value on the date of vesting of the Company’s equity awards granted to the
named executive officers determined in accordance with Financial Accounting Standards Board (FASB) ASC Topic 718, Compensation-Stock
Compensation (FASB ASC Topic 718). See Note 11 to our 2022 Audited Annual Financial Statements included in our Annual Report
for details as to the assumptions used to determine the fair value of these awards. |
| (3) |
All
Other Compensation primarily includes premiums paid for group term life insurance, except for Ms. Pelletier, Mr. Barrans, and Mr.
Fitzpatrick as discussed in notes (7) and (11), (23), and (27) and (31), respectively, below. |
| (4) |
Due
to the Company’s financial constraints, Ms. Pelletier’s cash incentive bonus for fiscal 2022 has yet to be determined
and approved by the Compensation Committee. Based on the achievement of corporate goals for fiscal 2022, it will be a maximum of
25% of Ms. Pelletier’s annual salary and thus no greater than $203,021. |
| (5) |
On
February 18, 2022, the Company granted Ms. Pelletier 400 shares of common stock issued as Restricted Stock Awards (RSAs),
which are subject to vesting upon the verification by our Compensation Committee of the achievement of certain to the Company’s
achievement of certain performance milestones in 2022. On December 31, 2022, these RSAs were cancelled due to forfeiture under these
vesting provisions. |
| (6) |
On
February 18, 2022, the Company granted Ms. Pelletier 400 stock options which vest in a series of forty-eight (48) successive
equal monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement
date of February 18, 2022. |
| (7) |
In
2022, All Other Compensation for Ms. Pelletier includes (i) a $1,903 premium paid for group term life insurance and (ii) $13,812
in fringe benefits paid on behalf of Ms. Pelletier. |
| (8) |
Ms.
Pelletier’s accrued cash incentive bonus for fiscal 2021 was cancelled by the Compensation Committee due to the Company’s
financial constraints. |
| (9) |
On
February 3, 2021, the Company granted Ms. Pelletier 266 shares of common stock issued as Restricted Stock Awards (RSAs), of
which 106 vested in connection with the Company’s achievement of certain performance milestones in 2021. Of these RSAs,
the Company withheld 56 shares of common stock to satisfy statutory tax withholding requirements upon vesting of such RSAs
during 2021. |
| (10) |
On
February 3, 2021, the Company granted Ms. Pelletier 373 stock options, which vest in a series of forty-eight (48) successive
equal monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement
date of February 3, 2021. |
| (11) |
In
2021, All Other Compensation for Ms. Pelletier includes (i) a $1,242 premium paid for group term life insurance and (ii) $19,279
in fringe benefits paid on behalf of Ms. Pelletier. |
| (12) |
Mr.
File resigned from his position as Chief Financial Officer effective April 3, 2023, as of which date the Compensation Committee had
yet to determine and approve any cash incentive bonus for fiscal 2022. |
| (13) |
On
February 18, 2022, the Company granted Mr. File 240 shares of common stock issued as RSAs, which are subject to vesting upon
the verification by our Compensation Committee of the achievement of certain to the Company’s achievement of certain performance
milestones in 2022. On December 31, 2022, these RSAs were cancelled due to forfeiture under these vesting provisions. |
| (14) |
On
February 18, 2022, the Company granted Mr. File 240 stock options, which vest in a series of forty-eight (48) successive equal
monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement date
of February 18, 2022. |
| (15) |
Mr.
File’s prorated accrued cash incentive bonus for fiscal 2021 was cancelled by the Compensation Committee due to the Company’s
financial constraints. |
| (16) |
On
February 3, 2021, the Company granted Mr. File 106 shares of common stock issued as RSAs, of which 42 vested in connection
with the Company’s achievement of certain performance milestones in 2021. Of these RSAs, the Company withheld 22 shares
of common stock to satisfy statutory tax withholding requirements upon vesting of such RSAs during 2021. |
| (17) |
On
February 3, 2021, the Company granted Mr. File 80 stock options, which vest in a series of forty-eight (48) successive equal
monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement date
of February 3, 2021. |
| (18) |
Russ
Barrans retired from his position as Chief Commercial Officer in November 2021. |
| (19) |
Consists
of (i) $447,379 paid to Mr. Barrans pursuant to Mr. Barrans’ employment agreement with the Company and (ii) $24,581 paid to
Mr. Barrans for vacation payout. |
| (20) |
Mr.
Barrans’ prorated accrued cash incentive bonus for fiscal 2021 was cancelled by the Compensation Committee due to the Company’s
financial constraints. |
| (21) |
On
February 3, 2021, the Company granted Mr. Barrans 106 shares of common stock issued as RSAs, of which 42 vested in
connection with the Company’s achievement of certain performance milestones in 2021. Of these RSAs, the Company withheld 16
shares of common stock to satisfy statutory tax withholding requirements upon vesting of the RSAs during 2021. The Company also
withheld 10 shares of common stock to satisfy statutory tax withholding requirements upon vesting of the third tranche of
RSAs during 2021 that were granted in July 2019. |
| (22) |
On
February 3, 2021, the Company granted Mr. Barrans 80 stock options, which vest in a series of forty-eight (48) successive
equal monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement
date of February 3, 2021. |
| (23) |
2021
All Other Compensation for Mr. Barrans includes (i) a $3,119 premium paid for group term life insurance and (ii) $63,911 in severance
pay. |
| (24) |
Alex
Fitzpatrick became a named executive officer as of December 31, 2021 as a result of Mr. Barrans’ retirement. He resigned from
his position as General Counsel in October 2022. |
| (25) |
Consists
of (i) $386,399 paid to Mr. Fitzpatrick pursuant to Mr. Fitzpatrick’s employment agreement with the Company and (ii) $23,466
paid to Mr. Fitzpatrick for vacation payout. |
| (26) |
Mr.
Fitzpatrick’s prorated accrued cash incentive bonus for fiscal 2021 was cancelled by the Compensation Committee due to the
Company’s financial constraints. |
| (27) |
On
February 18, 2022, the Company granted Mr. Fitzpatrick 160 shares of common stock issued as RSAs, which are subject to vesting
upon the verification by our Compensation Committee of the achievement of certain to the Company’s achievement of certain performance
milestones in 2022. On December 31, 2022, these RSAs were cancelled due to forfeiture under their vesting provisions. |
| (28) |
On
February 18, 2022, the Company granted Mr. Fitzpatrick 160 stock options, which vest in a series of forty-eight (48) successive
equal monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement
date of February 18, 2022. |
| (29) |
On
February 3, 2021, the Company granted Mr. Fitzpatrick 106 shares of common stock issued as RSAs, of which 42 vested
in connection with the Company’s achievement of certain performance milestones in 2021. Of these RSAs, the Company withheld
22 shares of common stock to satisfy statutory tax withholding requirements upon vesting of such RSAs during 2021. |
| (30) |
On
February 3, 2021, the Company granted Mr. Fitzpatrick 80 stock options which vest in a series of forty-eight (48) successive
equal monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement
date of February 3, 2021. |
| (31) |
2021
All Other Compensation for Mr. Fitzpatrick includes (i) a $2,322 premium paid for group term life insurance and (ii) $6,418 in fringe
benefits paid on behalf of Mr. Fitzpatrick. |
| (32) |
Katherine
Atkinson became a named executive officer on May 31, 2022, when she was appointed Chief Commercial Officer. Her position was terminated
effective March 17, 2023, as of which date the Compensation Committee had yet to determine and approve any cash incentive bonus for
fiscal 2022. |
| (33) |
On
February 18, 2022, the Company granted Ms. Atkinson 133 shares of common stock issued as RSAs, which are subject to vesting
upon the verification by our Compensation Committee of the achievement of certain to the Company’s achievement of certain performance
milestones in 2022. On December 31, 2022, these RSAs were cancelled due to forfeiture under their vesting provisions. |
| (34) |
On
February 18, 2022, the Company granted Ms. Atkinson 53 stock options, which vest in a series of forty-eight (48) successive
equal monthly installments upon completion of each additional month of service for the Company measured from the vesting commencement
date of February 18, 2022. |
Employment,
Severance and Separation Agreements
Current
Executive Officers
Our
current principal executive officer (Ms. Pelletier) was appointed to her office in January 2018 in connection with a reverse merger
between private Evofem and Neothetics, Inc. (the Merger). The amounts reported for her in the Summary Compensation Table above include
compensation paid to or earned by her pursuant to offer letters for her services provided as our principal executive officer pursuant
to her offer letter and subsequent employment agreement described below.
Our
current Chief Financial Officer (Ms. Zhang) was appointed to her office in April 2023.
Current
Employment Agreements
On
July 2, 2018, we entered into an employment agreement with Ms. Pelletier. Pursuant to the terms of this agreement, Ms. Pelletier is eligible
to receive an annual base salary of $812,083 and a target bonus as a base salary up to 100%, payable in the discretion of our Board.
The
employment agreement also entitles Ms. Pelletier to (i) participate in benefit/welfare plans and fringe benefits provided generally to
our senior executives, (ii) receive reimbursement for ordinary and reasonably incurred business expenses and (iii) receive paid vacation
and holiday time in accordance with policies generally applicable to our senior executives. Ms. Pelletier may terminate his or her employment
for good reason after giving us thirty days to correct or “cure” the circumstances giving rise to a termination for good
reason, and she may terminate her employment upon at least thirty days’ prior written notice to us for any reason other than for
good reason. We may terminate the employment Ms. Pelletier without prior written notice for cause or in the event of her disability.
We may also terminate her employment without cause on thirty days’ prior written notice. The employment agreement will be automatically
terminated upon the death of the Ms. Pelletier. If her employment is terminated by us for cause, by reason of her death or disability,
or as a result of the applicable executive officer’s resignation without good reason, we agreed to pay the terminated executive
officer the amount of our accrued obligations as of the date of such termination. If an executive officer’s employment is terminated
without cause or the applicable executive officer resigns for good reason, then we have agreed to make the payments set forth below.
On April 13, 2023, we entered into
an employment agreement with Ms. Zhang. Pursuant to the terms of this agreement, Ms. Zhang is eligible to receive an annual base salary
of $410,000 and a target bonus as a base salary up to 40%, payable at the discretion of our Board.
The employment agreement also entitles
Ms. Zhang to (i) participate in benefit/welfare plans and fringe benefits provided generally to our senior executives and (ii) receive
paid vacation and holiday time in accordance with policies generally applicable to our senior executives. Ms. Zhang is an “at will”
employee and may terminate her employment at any time and for any reason without notice.
Severance
Obligations
Saundra
Pelletier
If
Ms. Pelletier is terminated by us other than for cause or Ms. Pelletier resigns for good reason, then pursuant to her employment agreement,
we have agreed to pay and provide to Ms. Pelletier: (i) all accrued obligations as of the date of termination, (ii) any accrued but unpaid
bonus for the prior fiscal year, (iii) a pro-rated bonus for the year in which the termination occurs as of her termination date, (iv)
an amount equal to eighteen months of her then-current base salary in a lump sum and (v) eighteen months of continuing health benefits
coverage, each subject to the conditions outlined in the agreement. In addition, fifty percent (50%) of any unvested and outstanding
equity interests Ms. Pelletier may have shall immediately vest and become exercisable, in each case subject to the conditions outlined
in her equity agreements. If Ms. Pelletier’s employment is terminated without cause or if Ms. Pelletier resigns for good reason,
in each case within three months prior to or twelve months following a change of control, then we have agreed to pay and provide to Ms.
Pelletier: (i) all accrued obligations as of the date of termination, (ii) an amount equal to twenty-four months of her then-current
base salary in a lump sum, (iii) any accrued but unpaid bonus for the prior fiscal year, (iv) her target annual bonus for the year in
which the termination occurs at the rate in effect immediately prior to such termination multiplied by a factor of 2.0 and (v) twenty-four
months of continuing health benefits coverage, each subject to the conditions outlined in the agreement. In addition, any unvested and
outstanding equity interests Ms. Pelletier may have shall fully vest and become exercisable, in each case subject to the conditions outlined
in her equity agreements.
Justin
J. File
Justin
J. File resigned as the Company’s Chief Financial Officer effective April 4, 2023. Mr. File’s resignation was voluntary and
not for good reason, and there was no severance obligation.
Severance
Tax Matters
All
payments made and benefits available to each executive officer in connection with his or her employment agreement will or were intended
to comply with Section 409A of the Internal Revenue Code of 1986, as amended, (the Code) in accordance with the terms of his or her employment
agreement. In the event the benefit provided to an employee (i) constitutes “parachute payments” within the meaning of Section
280G of the Code, and (ii) would otherwise be subject to the excise tax imposed by Section 4999 of the Code, then such “Payments”
will be reduced. The reduced amount will be either (x) the largest portion of the Payment that would result in no portion of the Payment
being subject to the excise tax or (y) the largest portion, up to and including the total, of the Payment, whichever amount results in
the executive officer’s receipt, on an after-tax basis, of the greater amount of the Payment notwithstanding that all or some portion
of such benefits may be taxable under Section 4999 of the Code. If a reduction in payments or benefits constituting “parachute
payments” is necessary to limit or avoid a certain employee’s excise tax, the reduction shall occur at the election of such
employee (provided, however, that such election shall be subject to our approval if made on or after the effective date of the event
that triggers the Payment) and may reduce cash payments, cancel accelerated vesting of stock award, and/or reduce employee benefits in
any order or combination that maximizes the amount of such reduced amount. In the event that acceleration of vesting of stock award compensation
is to be reduced, such acceleration of vesting shall be cancelled in the reverse order of the date of grant of such executive officer’s
stock awards unless the executive officer elects a different order for cancellation.
Outstanding
Equity Awards at December 31, 2022
The
following table shows the outstanding equity awards held by our named executive officers as of December 31, 2022.
| |
|
Option
Awards |
| Name |
|
Number
of Securities Underlying Unexercised Options Exercisable |
|
|
Number
of Securities Underlying Unexercised Options Unexercisable |
|
|
Option
Exercise
Price
($) |
|
|
Option
Grant
Date |
|
Option
Expiration
Date |
| Saundra
Pelletier |
|
|
3 |
(1) |
|
|
— |
|
|
|
149,756.25 |
|
|
6/3/2013 |
|
6/3/2023 |
| |
|
|
22 |
(2) |
|
|
— |
|
|
|
86,925.00 |
|
|
9/28/2016 |
|
9/28/2026 |
| |
|
|
440 |
|
|
|
— |
|
|
|
13,668.75 |
|
|
3/12/2018 |
|
3/12/2028 |
| |
|
|
167 |
|
|
|
— |
|
|
|
3,937.50 |
|
|
7/31/2018 |
|
7/31/2028 |
| |
|
|
151 |
|
|
|
— |
|
|
|
6,468.75 |
|
|
11/28/2018 |
|
11/28/2028 |
| |
|
|
151 |
|
|
|
8 |
|
|
|
9,131.25 |
|
|
2/5/2020 |
|
2/5/2030 |
| |
|
|
171 |
|
|
|
202 |
|
|
|
6,093.75 |
|
|
2/3/2021 |
|
2/3/2031 |
| |
|
|
83 |
|
|
|
316 |
|
|
|
917.50 |
|
|
2/18/2022 |
|
2/18/2032 |
| Justin
J. File(4) |
|
|
12 |
(3) |
|
|
— |
|
|
|
86,925.00 |
|
|
9/28/2016 |
|
9/28/2026 |
| |
|
|
160 |
|
|
|
— |
|
|
|
13,668.75 |
|
|
3/12/2018 |
|
3/12/2028 |
| |
|
|
60 |
|
|
|
— |
|
|
|
3,937.50 |
|
|
7/31/2018 |
|
7/31/2028 |
| |
|
|
55 |
|
|
|
— |
|
|
|
6,468.75 |
|
|
11/28/2018 |
|
11/28/2028 |
| |
|
|
50 |
|
|
|
2 |
|
|
|
9,131.25 |
|
|
2/5/2020 |
|
2/5/2030 |
| |
|
|
36 |
|
|
|
43 |
|
|
|
6,093.75 |
|
|
2/3/2021 |
|
2/3/2031 |
| |
|
|
50 |
|
|
|
190 |
|
|
|
917.50 |
|
|
2/18/2022 |
|
2/18/2032 |
| Katherine
Atkinson(5) |
|
|
8 |
|
|
|
23 |
|
|
|
783.75 |
|
|
12/1/2021 |
|
12/1/2031 |
| |
|
|
11 |
|
|
|
42 |
|
|
|
917.50 |
|
|
2/18/2022 |
|
2/18/2032 |
| Alexander
A. Fitzpatrick(6) |
|
|
146 |
|
|
|
— |
|
|
|
13,668.75 |
|
|
3/12/2018 |
|
3/12/2028 |
| |
|
|
55 |
|
|
|
— |
|
|
|
3,937.50 |
|
|
7/31/2018 |
|
7/31/2028 |
| |
|
|
53 |
|
|
|
— |
|
|
|
6,468.75 |
|
|
11/28/2018 |
|
11/28/2028 |
| |
|
|
47 |
|
|
|
— |
|
|
|
9,131.25 |
|
|
2/5/2020 |
|
2/5/2030 |
| |
|
|
33 |
|
|
|
— |
|
|
|
6,093.75 |
|
|
2/3/2021 |
|
2/3/2031 |
| |
|
|
23 |
|
|
|
— |
|
|
|
917.50 |
|
|
2/18/2022 |
|
2/18/2032 |
| (1) |
The
share numbers and exercise prices reflected are those of options issued to the executive upon completion of the Merger in January
2018. These options were issued upon completion of the Merger in exchange for options to purchase 139 shares of Private Evofem
common stock, which were fully vested upon grant, at an exercise price of $3,843.75 per share awarded to the executive
by Evofem Operations in 2013 (See more detail described in Note 3 to our Consolidated Financial Statements included in our Annual
Report on Form 10-K for the year ended December 31, 2018). |
| (2) |
The
share numbers and exercise prices reflected are those of options issued to the executive upon completion of the Merger in January
2018. These options were issued upon completion of the Merger in exchange for options to purchase an aggregate of 874 shares
of Private Evofem common stock at an exercise price of $2,231.25 per share awarded to the executive by Private Evofem in 2016. |
| (3) |
The
share numbers and exercise prices reflected are those of options issued to the executive upon completion of the Merger in January
2018. These options were issued upon completion of the Merger in exchange for options to purchase an aggregate of 480 shares
of Private Evofem common stock at an exercise price of $2,231.25 per share awarded to the executive by Private Evofem in 2016. |
| (4) |
Justin
File resigned as the Company’s Chief Financial Officer effective April 3, 2023. |
| (5) |
The
employment of Katherine Atkinson as the Company’s Chief Commercial Officer was terminated effective March 17, 2023. |
| (6) |
Alexander
Fitzpatrick resigned as the Company’s General Counsel effective October 14, 2022; he had 90 days thereafter to exercise his
vested options, after which date they were cancelled. |
Employee
Benefit and Equity Incentive Plans
Stock
Compensation Plans
Summary
of the Amended and Restated 2014 Plan
The
Company initially adopted the 2007 Stock Plan (the 2007 Plan) in March 2007, under which 113 shares of common stock were reserved
for issuance to employees, non-employee directors, and consultants of the Company. The Company ceased granting any additional awards
under our 2007 Plan, and presently grants equity awards under the Amended and Restated 2014 Plan.
On
September 15, 2014, our Board adopted, and our stockholders approved, the 2014 Equity Incentive Plan. The 2014 Equity Incentive Plan,
as amended and restated, provides incentives that will assist us to attract, retain, and motivate employees, including officers, consultants,
and directors. We may provide these incentives through the grant of stock options, stock appreciation rights, restricted stock, restricted
stock units, performance shares, and units and other cash-based or share-based awards. In addition, the Amended and Restated 2014 Plan
contains a mechanism through which we may adopt a deferred compensation arrangement in the future.
A
total of 88 shares of our common stock was initially authorized and reserved for issuance under the Amended and Restated 2014 Plan.
As of August 1, 2023, a total of 5,756 shares of our common stock were reserved and available for issuance under the
Amended and Restated 2014 Plan. Per the terms of the Amended and Restated 2014 Plan, this reserve will automatically increase on
each January 1 through 2024, by an amount equal to the smaller of:
| ● | 4%
of the number of shares of common stock issued and outstanding on the immediately preceding
December 31; or |
| ● | an
amount determined by our Board. |
Appropriate
adjustments will be made in the number of authorized shares and other numerical limits in the Amended and Restated 2014 Plan and in outstanding
awards to prevent dilution or enlargement of participants’ rights in the event of a stock split or other change in our capital
structure. Shares subject to awards which expire or are cancelled or forfeited will again become available for issuance under the Amended
and Restated 2014 Plan.
The
Amended and Restated 2014 Plan is administered by the Compensation Committee of our Board. Pursuant to the provisions of the Amended
and Restated 2014 Plan, the Compensation Committee determines, in its discretion, the persons to whom and the times at which awards are
granted, the sizes of such awards and all of their terms and conditions. The Compensation Committee has the authority to construe and
interpret the terms of the Amended and Restated 2014 Plan and awards granted under it. The Amended and Restated 2014 Plan provides, subject
to certain limitations, for indemnification by us of any director, officer, or employee against all reasonable expenses, including attorneys’
fees, incurred in connection with any legal action arising from such person’s action or failure to act in administering the Amended
and Restated 2014 Plan.
In
the event of a change in control as described in the Amended and Restated 2014 Plan, the acquiring or successor entity may assume or
continue all or any awards outstanding under the Amended and Restated 2014 Plan or substitute substantially equivalent awards. The Compensation
Committee may provide for the acceleration of vesting of any or all outstanding awards upon such terms and to such extent as it determines,
except that the vesting of all awards held by members of the Board who are not employees will automatically be accelerated in full upon
a change in control. Any award held by a participant whose service has not terminated prior to a change in control that is not assumed,
continued, or substituted for in connection with a change in control or are not exercised or settled prior to the change in control will
terminate effective as of the time of the change in control. Notwithstanding the foregoing, except as otherwise provided in an award
agreement governing any award, in the discretion of the Compensation Committee, any award that is not assumed, continued, or substituted
for in connection with a change in control shall, subject to the provisions of applicable law, become fully vested and exercisable and/or
settleable as of a date prior to, but conditioned upon, the consummation of the change in control. The Amended and Restated 2014 Plan
also authorizes the Compensation Committee, in its discretion and without the consent of any participant, to cancel each or any outstanding
award denominated in shares upon a change in control in exchange for a payment to the participant with respect to each vested share subject
to the cancelled award (and each unvested share, if so determined by the Compensation Committee) of an amount equal to the excess of
the fair market value of the consideration to be paid per share of common stock in the change in control transaction over the exercise
price per share, if any, under the award. The vesting schedules of all outstanding options of the Company, excluding any shares issuable
pursuant to the assumed equity incentive plan of Private Evofem, were fully accelerated in connection with the Merger and termination
of employment or service arrangement with the Company.
The
Amended and Restated 2014 Plan will continue in effect until it is terminated, provided, however, that all awards will be granted, if
at all, within ten years of its effective date. The Compensation Committee may amend, suspend or terminate the Amended and Restated 2014
Plan at any time, provided that without stockholder approval, the Amended and Restated 2014 Plan cannot be amended by the Compensation
Committee without stockholder approval, except as described above, to increase the number of shares authorized, change the class of persons
eligible to receive incentive stock options, or effect any other change that would require stockholder approval under any applicable
law or listing rule.
Summary
of the 2018 Inducement Equity Incentive Plan
On
July 24, 2018, upon the recommendation of our Compensation Committee, the Board approved our 2018 Inducement Equity Incentive Plan and
reserved 133 shares of our common stock to be used exclusively for grants of awards to individuals that were not previously employees
or directors of the company, as an inducement to the individual’s entry into employment with the company within the meaning of
Rule 5635(c)(4) of the Nasdaq Listing Rules. On February 25, 2020, the Board approved an increase to the number of shares of our common
stock reserved and available for issuance under the 2018 Inducement Equity Incentive Plan to 666 shares. The 2018 Inducement Equity
Incentive Plan was adopted without stockholder approval pursuant to Rule 5635(c)(4). The 2018 Inducement Equity Incentive Plan provides
for the grant of equity-based awards, including options, restricted and unrestricted stock awards, and other stock- based awards, and
its terms are substantially similar to the Amended and Restated 2014 Plan, but with such other terms and conditions intended to comply
with the Nasdaq inducement award exception. As of May 31, 2023, there were 64 shares of options outstanding and 594
shares available for grant under the 2018 Inducement Equity Incentive Plan.
2019
Employee Stock Purchase Plan
On
May 7, 2019, the Board approved the 2019 ESPP, which was approved by stockholders at the 2019 annual meeting held on June 5, 2019 and
which authorizes the issuance of up to 266 shares of common stock pursuant to purchase rights granted to employees. This authorized
number of shares may be increased annually on the first day of each of the Company’s fiscal years beginning in 2020 and ending
on the first day of 2029, in an amount equal to the lesser of (i) 533 shares, (ii) two percent (2%) of the shares of common stock
outstanding on the last day of the immediately preceding fiscal year, or (iii) such lesser number of shares as is determined by the Board.
The 2019 ESPP enables eligible full-time and part-time employees to purchase shares of the Company’s common stock through payroll
deductions of between 1% and 15% of eligible compensation during an offering period. A new offering period begins approximately every
June 15 and December 15. At the last business day of each offering period, the accumulated contributions made during the offering period
will be used to purchase shares. The purchase price is 85% of the lesser of the fair market value of the common stock on the first or
the last business day of an offering period. The maximum number of shares of common stock that may be purchased by any participant during
an offering period will be equal to $25,000 divided by the fair market value of the common stock on the first business day of an offering
period.
In
October 2022, the Board terminated the offering period ending December 15, 2022, refunded all employee contributions, and suspended future
offering periods. Additionally, the authorized number of shares available for issuance under the 2019 ESPP was not increased on January
1, 2023. The Board may in future reinstitute the ESPP if and when our common stock resumes trading on a national stock exchange.
As
of August 1, 2023, there were 937 shares of common stock purchased and 509 shares of our common stock reserved and available for
issuance under the 2019 ESPP.
Private
Evofem Equity Incentive Plan
The
Private Evofem Equity Incentive Plan was assumed by the Company in connection with the Merger and shares of Private Evofem common stock
issuable pursuant to options previously granted under the Private Evofem Equity Incentive Plan became options to purchase our common
stock upon completion of the Merger. No new awards may be granted under the Private Evofem Equity Incentive Plan. As of May 31, 2023,
a total of 66 shares of our common stock were reserved for issuance upon the exercise of outstanding options under the Private Evofem
Equity Incentive Plan.
Perquisites,
Health, Welfare and Retirement Benefits
Our
executive officers are eligible to participate in all of our employee benefit plans, including our medical, dental, vision, group life
and disability insurance plans, in each case on the same basis as other employees.
Director
Compensation
The
following table sets forth the compensation (cash and equity) received by our non-employee directors during the year ended December 31,
2022.
| Name | |
Fees
Earned or
Paid
in Cash ($) | | |
Option
Awards(1)
($) | | |
Totals
($) | |
| Kim
Kamdar, Ph.D. | |
| 75,981 | | |
| 11,849 | | |
| 87,830 | |
| Tony
O’Brien | |
| 75,000 | | |
| 11,849 | | |
| 86,849 | |
| Lisa
Rarick, M.D. | |
| 55,000 | | |
| 11,849 | | |
| 66,849 | |
| Colin
Rutherford | |
| 70,000 | | |
| 11,849 | | |
| 81,849 | |
| Gillian
Greer, Ph.D.(2) | |
| 59,437 | | |
| 11,849 | | |
| 71,286 | |
| Jenny
Yip(3) | |
| — | | |
| — | | |
| — | |
| (1) | Amounts
listed in this column represent the aggregate fair value of the option awards computed as
of the grant date of each option award in accordance with FASB ASC Topic 718, rather than
amounts paid to or realized by the named individual. There can be no assurance that options
will be exercised (in which case no value will be realized by the individual) or that the
value on exercise will approximate the fair value as computed in accordance with FASB ASC
Topic 718. The assumptions used in the valuation of these awards are set forth in Note 11-
Stock-based Compensation to our Consolidated Financial Statements on our Annual Report. |
| (2) | Dr.
Greer resigned from the Board effective April 24, 2023. |
| (3) | Ms.
Yip elected to forego any compensation as director. She resigned from the Board effective
February 10, 2023. |
The
following table shows the outstanding equity awards held by our non-employee directors as of December 31, 2022.
| |
|
Option
Awards |
| Name |
|
Number
of Securities Underlying Unexercised Options Exercisable |
|
|
Number
of Securities Underlying Unexercised Options Unexercisable |
|
|
Option
Exercise Price ($) |
|
|
Option
Grant
Date |
|
Option
Expiration
Date |
| Kim
Kamdar, Ph.D. |
|
|
3 |
|
|
|
— |
|
|
$ |
6,793.20 |
|
|
6/16/2015 |
|
6/16/2025 |
| |
|
|
4 |
|
|
|
— |
|
|
$ |
1,220.40 |
|
|
6/21/2016 |
|
6/21/2026 |
| |
|
|
5 |
|
|
|
— |
|
|
$ |
2,322.00 |
|
|
5/11/2017 |
|
5/11/2027 |
| |
|
|
1 |
|
|
|
— |
|
|
$ |
2,365.20 |
|
|
6/20/2017 |
|
6/20/2027 |
| |
|
|
6 |
|
|
|
— |
|
|
$ |
1,258.20 |
|
|
5/8/2018 |
|
5/8/2028 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
1,089.00 |
|
|
6/5/2019 |
|
6/5/2029 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
910.80 |
|
|
5/12/2020 |
|
5/12/2030 |
| |
|
|
48 |
|
|
|
— |
|
|
$ |
225.00 |
|
|
5/12/2021 |
|
5/12/2031 |
| |
|
|
— |
|
|
|
48 |
|
|
$ |
29.64 |
|
|
5/4/2022 |
|
5/4/2032 |
| Tony
O’Brien |
|
|
13 |
|
|
|
— |
|
|
$ |
1,312.20 |
|
|
3/12/2018 |
|
3/12/2028 |
| |
|
|
6 |
|
|
|
— |
|
|
$ |
415.80 |
|
|
7/24/2018 |
|
7/24/2028 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
1,089.00 |
|
|
6/5/2019 |
|
6/5/2029 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
910.80 |
|
|
5/12/2020 |
|
5/12/2030 |
| |
|
|
48 |
|
|
|
— |
|
|
$ |
225.00 |
|
|
5/12/2021 |
|
5/12/2031 |
| |
|
|
— |
|
|
|
48 |
|
|
$ |
29.64 |
|
|
5/4/2022 |
|
5/4/2032 |
| Lisa
Rarick, M.D. |
|
|
37 |
|
|
|
2 |
|
|
$ |
1,053.00
|
|
|
2/25/2020 |
|
2/25/2030 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
910.80
|
|
|
5/12/2020 |
|
5/12/2030 |
| |
|
|
48 |
|
|
|
— |
|
|
$ |
225.00
|
|
|
5/12/2021 |
|
5/12/2031 |
| |
|
|
— |
|
|
|
48 |
|
|
$ |
29.64
|
|
|
5/4/2022 |
|
5/4/2032 |
| Colin
Rutherford |
|
|
— |
|
|
|
— |
|
|
$ |
7,855.20
|
|
|
3/8/2017 |
|
3/8/2027 |
| |
|
|
21 |
|
|
|
— |
|
|
$ |
1,312.20
|
|
|
3/12/2018 |
|
3/12/2028 |
| |
|
|
6 |
|
|
|
— |
|
|
$ |
1,258.20
|
|
|
5/8/2018 |
|
5/8/2028 |
| |
|
|
2 |
|
|
|
— |
|
|
$ |
378.00
|
|
|
7/31/2018 |
|
7/31/2028 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
1,089.00
|
|
|
6/5/2019 |
|
6/5/2029 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
910.80
|
|
|
5/12/2020 |
|
5/12/2030 |
| |
|
|
48 |
|
|
|
— |
|
|
$ |
225.00
|
|
|
5/12/2021 |
|
5/12/2031 |
| |
|
|
— |
|
|
|
48 |
|
|
$ |
29.64
|
|
|
5/4/2022 |
|
5/4/2032 |
| Gillian
Greer, Ph.D.(1) |
|
|
13 |
|
|
|
— |
|
|
$ |
1,312.20
|
|
|
3/12/2018 |
|
3/12/2028 |
| |
|
|
6 |
|
|
|
— |
|
|
$ |
1,258.20
|
|
|
5/8/2018 |
|
5/8/2028 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
1,089.00
|
|
|
6/5/2019 |
|
6/5/2029 |
| |
|
|
26 |
|
|
|
— |
|
|
$ |
910.80
|
|
|
5/12/2020 |
|
5/12/2030 |
| |
|
|
48 |
|
|
|
— |
|
|
$ |
225.00
|
|
|
5/12/2021 |
|
5/12/2031 |
| |
|
|
— |
|
|
|
48 |
|
|
$ |
29.64
|
|
|
5/4/2022 |
|
5/4/2032 |
(1)
Dr. Greer resigned from the Board effective April 24, 2023.
Our
Non-Employee Director Compensation Policy
In
February 2022, our Compensation Committee amended the Non-Employee Director Compensation Policy as described below which took effect
April 1, 2022.
| |
● |
Each
non-employee director will receive an annual cash retainer in the amount of $40,000 per year. |
| |
● |
The
Chairperson of the Board will receive an additional annual cash retainer in the amount of $30,000 per year. |
| |
● |
The
Chairperson of the Audit Committee will receive additional annual cash compensation in the amount of $20,000 per year for such chairperson’s
service on the Audit Committee. Each non-chairperson member of the Audit Committee will receive additional annual cash compensation
in the amount of $10,000 per year for such member’s service on the Audit Committee. |
| |
● |
The
Chairperson of the Compensation Committee will receive additional annual cash compensation in the amount of $15,000 per year for
such chairperson’s service on the Compensation Committee. Each non-chairperson member of the Compensation Committee will receive
additional annual cash compensation in the amount of $7,500 per year for such member’s service on the Compensation Committee. |
| |
● |
The
Chairperson of the Nominating and Corporate Governance Committee will receive additional annual cash compensation in the amount of
$10,000 per year for such chairperson’s service on the Nominating and Corporate Governance Committee. Each non-chairperson
member of the Nominating and Corporate Governance Committee will receive additional annual cash compensation in the amount of $5,000
per year for such member’s service on the Nominating and Corporate Governance Committee. |
| |
● |
Each
non-employee director will receive a stock option grant with an initial grant equal to 48 shares of the Company’s common
stock upon a director’s initial appointment or election to the Board, vesting quarterly over a 3 year period and an annual
stock option grant equal 48 shares of the Company’s common stock on the date of each annual stockholder’s meeting
thereafter, fully vesting in one year from the date of grant. |
The
February 2022 amendment of the Non-Employee Director Compensation Policy, effective as of April 1, 2022, reduced the annual cash retainer
for each non-employee director from $50,000 per year to $40,000 per year, the annual cash retainer for the chairperson of the Board from
$40,000 to $30,000, and the annual cash compensation for the chairperson of the Nominating and Corporate Governance Committee from $11,250
per year to $10,000 per year.
PRINCIPAL
STOCKHOLDERS
The
following table sets forth certain information concerning the ownership of our common stock as of May 31, 2023, by (i) those persons
who are known to us to be the beneficial owner(s) of more than five percent of our common stock, (ii) each of our directors and named
executive officers and (iii) all of our directors and named executive officers as a group.
As
of May 31, 2023, 2,181,019 shares of common stock and 70 shares of Series D Non-Convertible Preferred Stock were issued and outstanding.
The holders of the Series D Non-Convertible Preferred Shares were entitled to vote at the Special Meeting of Stockholders on March 15,
2023, together with the holders of our common stock, only to approve a reverse split. The Series D Non-Convertible Preferred Shares carry
no other voting, dividend, distribution or other rights and are therefore excluded from the table below. We redeemed each share of
the Series D Non-Convertible Preferred Shares at $1.00 per share in July 2023.
The
number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules
of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. In the cases of holders
who are not directors, director nominees, and named executive officers, Schedules 13G or 13D filed with the SEC (and, consequently, ownership
reflected here) often reflect holdings as of a date prior to May 31, 2023. Under such rules, beneficial ownership generally includes
any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has
the right to acquire within 60 days after May 31, 2023, through the exercise of stock options, warrants or other rights. Unless otherwise
indicated in the footnotes to this table, we believe that each of the stockholders named in this table has sole voting and investment
power with respect to the shares indicated as beneficially owned. Unless otherwise noted, the address of the persons in the table below
is that of the Company.
| Name
and Address of Beneficial Owner | |
Shares Beneficially Owned | | |
Percent
of Class Beneficially Owned | |
| 5%
Stockholders | |
| | |
| |
| None | |
| | |
| |
| Directors
and Named Executive Officers | |
| | | |
| | |
| Kim
Kamdar, Ph.D.(1) | |
| 180 | | |
| * | |
| Tony
O’Brien(2) | |
| 173 | | |
| * | |
| Lisa
Rarick, M.D.(3) | |
| 167 | | |
| * | |
| Colin
Rutherford(4) | |
| 180 | | |
| * | |
| Saundra
Pelletier(5) | |
| 2,833 | | |
| 0.1 | % |
| Ivy
Zhang(6) | |
| 104 | | |
| * | |
| Directors
and executive officers as a group (6 Persons)(7) | |
| 3,637 | | |
| 0.1 | % |
*
Includes beneficial ownership of less than 1% of the outstanding shares of Evofem’s common stock.
| (1) |
Consists
of (i) 10 shares of common stock held by Dr. Kamdar, and (ii) 170 shares of common stock that may be acquired pursuant to the exercise
of stock options within 60 days of August 1, 2023. |
| (2) |
Consists
of (i) 4 shares of common stock held by Mr. O’Brien, and (ii) 169 shares of common stock that may be acquired pursuant to the
exercise of stock options within 60 days of August 1, 2023. |
| (3) |
Consists
of (i) 5 shares of common stock held by Dr. Rarick, and (ii) 162 shares of common stock that may be acquired pursuant to the exercise
of stock options within 60 days of August 1, 2023. |
| (4) |
Consists
of 180 shares of common stock that may be acquired by Mr. Rutherford pursuant to the exercise of stock options within 60 days of
August 1, 2023. |
| (5) |
Consists
of (i) 1,493 shares of common stock held by Ms. Pelletier, and (ii) 1,340 shares of common stock that may be acquired pursuant
to the exercise of stock options within 60 days of August 1, 2023. |
| (6) |
Consists
of (i) 104 shares of common stock held by Ms. Zhang as of August 1, 2023. |
| (7) |
Consists
of (i) 1,616 shares of common stock held by our current executive officers and directors, and (ii) 2,021 shares of common
stock that may be acquired by our current executive officers and directors pursuant to the exercise of stock options within 60 days
after August 1, 2023. |
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Related
Person Transactions
Company
Policy Regarding Related Party Transactions
Our
Audit Committee is responsible for reviewing and approving all transactions in which we are a participant and in which any parties related
to us, including our executive officers, directors, beneficial owners of more than 5% of our securities, immediate family members of
the foregoing persons, and any other persons whom our Board determines may be considered related parties, has or will have a direct or
indirect material interest. If advanced approval is not feasible, the Audit Committee has the authority to ratify a related party transaction
at the next Audit Committee meeting. For purposes of our Audit Committee charter, a material interest is deemed to be any consideration
received by such a party in excess of the lesser of $120,000 per year or 1% of the average of our total assets for the last two completed
fiscal years.
In
reviewing and approving such transactions, the Audit Committee shall obtain, or shall direct our management to obtain on its behalf,
all information that our committee believes to be relevant and important to a review of the transaction prior to its approval. Following
receipt of the necessary information, a discussion shall be held of the relevant factors if deemed to be necessary by our committee prior
to approval. If a discussion is not deemed to be necessary, approval may be given by written consent of our committee. This approval
authority may also be delegated to the Chairperson of the Audit Committee in respect of any transaction in which the expected amount
is less than $500,000.
The
Audit Committee or its chairperson, as the case may be, shall approve only those related party transactions that are determined to be
in, or not inconsistent with, the best interests of us and our stockholders, taking into account all available facts and circumstances
as our committee or the Chairperson determines in good faith to be necessary. These facts and circumstances will typically include, but
not be limited to, the material terms of the transaction, the nature of the related party’s interest in the transaction, the significance
of the transaction to the related party and the nature of our relationship with the related party, the significance of the transaction
to us, and whether the transaction would be likely to impair (or create an appearance of impairing) the judgment of a director or executive
officer to act in our best interest. No member of the Audit Committee may participate in any review, consideration, or approval of any
related party transaction with respect to which the member or any of his or her immediate family members is the related party, except
that such member of the Audit Committee will be required to provide all material information concerning the related party transaction
to the Audit Committee.
Except
as otherwise set forth below, during the years ended December 31, 2022 and 2021 and to date there were no transactions to which we will
be a party, nor are there any currently proposed transactions to which we will be a party, in which:
| ● | the
amounts involved exceeded or will exceed the lesser of $120,000 per year or 1% of the average
of our total assets for the last two completed fiscal years; and |
| ● | any
of our directors, nominees for director, executive officers or holders of more than 5% of
our outstanding capital stock, or any immediate family member of, or person sharing the household
with, any of these individuals or entities, had or will have a direct or indirect material
interest. |
Series
B and C Convertible Preferred Stock
On
October 12, 2021, the Company completed the initial closing of a registered direct offering with Keystone Capital Partners (Keystone
Capital) (the Initial October 2021 Registered Direct Offering), whereby the Company issued 5,000 shares of Series B-1 Convertible Preferred
Stock, par value $0.0001 per share, at a price of $1,000.00 per share. The Company received proceeds from the Initial October 2021 Registered
Direct Offering of approximately $4.6 million, net of offering expenses.
On
October 26, 2021, the Company completed the additional closing of the October 2021 Registered Direct Offering (the Additional October
2021 Registered Direct Offering), whereby the Company issued 5,000 shares of Series B-2 Convertible Preferred Stock, par value $0.0001
per share, at a price of $1,000.00 per share. The Company received proceeds from the Additional October 2021 Registered Direct Offering
of approximately $5.0 million, net of offering expenses.
The
Series B-1 and B-2 Convertible Preferred Stock were convertible into shares of common stock at any time at a conversion price per share
of the greater of $1,125 (Fixed Conversion Price), or the price computed as the product of 0.85 multiplied by the arithmetic average
of the closing sale prices of a share of the Company’s common stock during the five consecutive trading-day period immediately
preceding the conversion date (Variable Conversion Price).
On
October 12, 2021, Keystone Capital converted its 5,000 shares of B-1 Convertible Preferred Stock at a conversion price of $1,181.25 per
share into 4,232 shares of the Company’s common stock. Pursuant to the terms of the Series B-2 Convertible Preferred Stock, the
Fixed Conversion Price was adjusted during the first quarter of 2022 for certain dilutive issuances. The adjustment period ended on April
25, 2022 and the Fixed Conversion Price was fixed at $332.50 from the sale of common stock pursuant to the Seven Knots Purchase Agreement.
During March 2022 and April 2022, Keystone Capital converted their 1,200 shares of B-1 Convertible Preferred Stock at a conversion
price of $587.50 per share into 2,347 shares of the Company’s common stock. Pursuant to the terms of the Series B-2 Convertible
Preferred Stock, the Fixed Conversion Price was adjusted during the first quarter of 2022 for certain dilutive issuances.
On
March 24, 2022, the Company entered into an exchange agreement with the holder of its Series B-2 Convertible Preferred Stock, pursuant
to which the holder agreed to exchange 1,700 shares of the Series B-2 Convertible Preferred Stock in consideration for 1,700 shares of
the Company’s Series C Convertible Preferred Stock, par value $0.0001 per share, $1,000.00 per share stated value. The Series C
and Series B-2 Preferred Stock had substantially similar terms except with respect to voting provisions; the holders of Series C Preferred
Stock had the right to cast, at the 2022 Annual Meeting of Stockholders, 50,000 votes per share of Series C Preferred Stock on the proposal
that our Amended and Restated Certificate of Incorporation, as amended (the Certificate of Incorporation), be amended to effect a reverse
stock split of the issued and outstanding shares of common stock, provided, that such votes must be counted by the Company in the same
proportion as the aggregate shares of common stock voted on the proposal.
On
May 4, 2022, following the 2022 Annual Meeting of Stockholders and pursuant to the May 2022 Exchange, the remaining 2,100 shares of Series
B-2 Convertible Preferred Stock and 1,700 shares of Series C Convertible Preferred Stock were exchanged for Senior Subordinated Notes
with an aggregate principal amount of $4.8 million and warrants to purchase up to 6,666 shares of common stock.
Effective
December 15, 2021, the Company amended and restated its certificate of incorporation, under which the Company is currently authorized
to issue up to 5,000,000 shares of preferred stock, $0.0001 par value per share.
Series
D Non-Convertible Preferred Stock
On
December 16, 2022, the Company filed a Certificate of Designation of Series D Non-Convertible Preferred Stock, par value $0.0001 per
share (the Series D Preferred Shares). An aggregate of 70 shares has been authorized. They are not convertible into shares of common
stock, have limited voting rights equal to 1% of the total voting power of the then-outstanding shares of common stock entitled to vote
per share, are not entitled to dividends, and were required to be redeemed by us, once our shareholders approved a reverse split, as
described in the Certificate of Designation. All 70 shares of the Series D Preferred were subsequently issued in connection with the
December 2022 Securities Purchase Agreement as discussed in Note 4-Debt to the 2023 Q1 Interim Financial Statements. Since the Series
D Preferred Shares can only be settled in cash, they are recorded as a liability within accrued expenses in the consolidated balance
sheets. The amount related to the liability is de minimus. All shares of the Series D Preferred were redeemed in July 2023.
Series
E-1 Convertible Preferred Stock
On
August 7, 2023 the Company filed the Certificate of Designation creating the Series E-1 Preferred Stock. The Certificate of Designation,
which forms a part of the Company’s Amended and Restated Articles of Incorporation, specifies the terms of the Series E-1 Preferred
Stock.
The
Certificate of Designation authorizes a total of 2,300 shares of Series E-1 Preferred Stock. The holders of shares of Series E-1 Preferred
Stock shall entitle the holder thereof to vote together with the common shareholders as a single class and to cast that number of votes
per share as is equal to the number of shares of common stock into which it is then convertible. The Series E-1 Preferred Stock is convertible
into shares of common stock at a rate of $0.40 per share subject to adjustment as provided in the Certificate of Designation (the “Conversion
Rate”). Each holder of Series E-1 Preferred Stock is entitled to receive dividends paid exclusively in the form of common stock
(the “Dividends”) payable to the holders of the Series E-1 Preferred Stock on a monthly basis.
On
August 7, 2023, the Company entered into Exchange Agreements with certain investors providing for the exchange of senior secured
convertible notes due in the aggregate original principal amount of $1.8 million into an aggregate 1,800 shares of Series E-1 Preferred
Stock (the “Preferred Shares”). As a result, the Company issued an aggregate
1,800 Preferred Shares in exchange for the outstanding debt held by certain investors in aggregate value of $1.8 million.
Indemnification
Arrangements
We
entered into indemnification agreements with each of our officers and directors and purchased directors’ and officers’ liability
insurance. Our indemnification agreements and amended and restated bylaws require us to indemnify our directors and officers to the fullest
extent permitted under Delaware law.
Employment
Arrangements
We
entered into employment arrangements with certain of our named executive officers as is further described under “Current Employment
Agreements” above.
USE
OF PROCEEDS
We
will not receive any proceeds from the sale of shares of common stock offered by the Selling Securityholders under this prospectus. However,
Evofem will receive the proceeds of any cash exercise of the Warrants. If all of the Warrants were exercised for cash, we would receive
aggregate proceeds of approximately $4.2 million. If we receive proceeds, we currently intend to use the proceeds for the continuation
of commercialization activities related to Phexxi and other general corporate purposes and other capital expenditures.
SELLING
SECURITYHOLDERS
This
prospectus covers the resale or other disposition by the Selling Securityholders identified in the table below of up to 10,978,768
shares of common stock issuable upon the exercise Warrants and/or conversion of Notes.
We
are registering the shares of common stock in order to permit the Selling Securityholders to offer the shares of common stock for resale
from time to time. The registration of such common stock does not necessarily mean, however, that any of the shares of common stock will
be offered or sold by the Selling Securityholders. We will not receive any proceeds from the sale of the common stock by the Selling
Securityholders, and we have borne and will continue to bear the costs relating to the registration of these shares of common stock,
other than commissions and discounts of agents or broker-dealers and transfer taxes, if any.
The
Warrants and Notes held by the Selling Securityholders contain limitations which prevent the holder from exercising those Warrants or
converting the Notes, if such exercise or conversion would cause the Selling Securityholders, together with certain related parties,
to beneficially own a number of shares of common stock which would exceed 4.99% of our then outstanding common stock following such exercise
or conversion, excluding for purposes of such determination, common stock issuable upon exercise of the Warrants which have not been
exercised or common stock issuable upon conversion of the Notes which have not been exercised.
The
table below sets forth, as of August 1, 2023, the following information regarding the Selling Securityholders:
| |
a. |
the
name of the Selling Securityholders; |
| |
b. |
the
number of shares of common stock owned by the Selling Securityholders prior to this offering, without regard to any beneficial ownership
limitations contained in the Warrants; |
| |
c. |
the
number of shares of common stock to be offered by the Selling Securityholders in this offering; |
| |
d. |
the
number of shares of common stock to be owned by the Selling Securityholders assuming the sale of all of the shares of common stock
covered by this prospectus; and |
| |
e. |
the
percentage of our issued and outstanding shares of common stock to be owned by the Selling Securityholders assuming the sale of all
of the common stock covered by this prospectus based on the number of shares of common stock issued and outstanding as of August 1, 2023. |
Except
as described above, the number of shares of common stock beneficially owned by the Selling Securityholders have been determined in accordance
with Rule 13d-3 under the Exchange Act and includes, for such purpose, shares of common stock that the Selling Securityholders have the
right to acquire within 60 days after August 1, 2023.
Because
the Selling Securityholders identified in the table may sell some or all of the shares of common stock beneficially owned and covered
by this prospectus, and because there are currently no agreements, arrangements or understandings with respect to the sale of any of
the shares of common stock, no estimate can be given as to the number of shares of common stock available for resale hereby that will
be held by the Selling Securityholders upon termination of this offering. In addition, the Selling Securityholders may have sold, transferred
or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time and from time to time, the shares of common stock
they beneficially own in transactions exempt from the registration requirements of the Securities Act after the date on which they provided
the information set forth in the table below. We have, therefore, assumed for the purposes of the following table, that the Selling Securityholders
will sell all of the shares of common stock owned beneficially that are covered by this prospectus, but will not sell any other shares
of common stock that they presently own. The Selling Securityholders have not held any position or office, or otherwise had a material
relationship, with us or any of our subsidiaries within the past three years other than as a result of the ownership of our common stock
or other securities.
| Name of Selling Securityholder | |
Shares of Common Stock Beneficially Owned prior to the Offering | | |
Maximum Number of Shares of Common Stock to be Sold Pursuant to this Prospectus | | |
Shares of Common Stock Beneficially Owned after Offering(18) | | |
Percentage of Shares Beneficially Owned after Offering(1) | |
| Keystone Capital Partners, LLC | |
| — | | |
| 1,552,798 | (2) | |
| 0 | | |
| 0 | % |
| Cavalry Fund I LP | |
| — | | |
| 480,227 | (3) | |
| 0 | | |
| 0 | % |
| Mercer Street Global Opportunity Fund, LLC | |
| — | | |
| 1,117,320 | (4) | |
| 0 | | |
| 0 | % |
| Seven Knots, LLC | |
| — | | |
| 481,268 | (5) | |
| 0 | | |
| 0 | % |
| Pinz Capital Special Opportunities Fund, LP | |
| — | | |
| 172,723 | (6) | |
| 0 | | |
| 0 | % |
| Stratgyx, LLC | |
| — | | |
| 233,050 | (7) | |
| 0 | | |
| 0 | % |
| Jim Fallon | |
| — | | |
| 223,463 | (8) | |
| 0 | | |
| 0 | % |
| Walleye Opportunities Master Fund Ltd. | |
| — | | |
| 3,504,683 | (9) | |
| 0 | | |
| 0 | % |
| ISJ Capital, LLC | |
| — | | |
| 897,
618 | (10) | |
| 0 | | |
| 0 | % |
| Joseph Gunnar & Co., LLC | |
| — | | |
| 142,892 | (11) | |
| 0 | | |
| 0 | % |
| Peter D. Villari | |
| — | | |
| 62,339 | (12) | |
| 0 | | |
| 0 | % |
| Keith Ernst | |
| — | | |
| 249,356 | (13) | |
| 0 | | |
| 0 | % |
| Dennis Stuart | |
| — | | |
| 124,678 | (14) | |
| 0 | | |
| 0 | % |
| Jeffrey Woodward | |
| — | | |
| 62,339 | (15) | |
| 0 | | |
| 0 | % |
| Tim Ayer | |
| — | | |
| 249,356 | (16) | |
| 0 | | |
| 0 | % |
| Jason Kanner | |
| — | | |
| 498,713 | (17) | |
| 0 | | |
| 0 | % |
| Chris Diangelo | |
| — | | |
| 246,919 | (18) | |
| 0 | | |
| 0 | % |
| Robert & Lynn Demattei | |
| — | | |
| 61,729 | (19) | |
| 0 | | |
| 0 | % |
| Daniel Katcher | |
| — | | |
| 61,729 | (20) | |
| 0 | | |
| 0 | % |
| Jeff Colins | |
| — | | |
| 61,729 | (21) | |
| 0 | | |
| 0 | % |
| Proactive Capital Partners, LP | |
| — | | |
| 493,839 | (22) | |
| 0 | | |
| 0 | % |
| (1) |
Percentage is based on 3,760,209
shares of common stock outstanding as of August 1, 2023 assuming the resale of all of the shares of common stock covered
by this prospectus and giving effect to the 4.99% beneficial ownership blockers in the Warrants. |
| |
|
| (2) |
Consists of 1,189,721
shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 363,077 shares of common
stock underlying the Warrants. |
| |
|
| (3) |
Consists of 394,074
shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 86,153 shares of
common stock underlying the Warrants. |
| |
|
| (4) |
Consists of 994,244
shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 123,076 shares of common
stock underlying the Warrants. |
| |
|
| (5) |
Consists of 395,115
shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 86,153 shares of common
stock underlying the Warrants. |
| |
|
| (6) |
Consists of 148,108
shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 24,615 shares of
common stock underlying the Warrants. |
| (7) |
Consists
of 177,666 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 55,384
shares of common stock underlying the Warrants. |
| |
|
| (8) |
Consists
of 198,848 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 24,615
shares of common stock underlying the Warrants. |
| |
|
| (9) |
Consists
of 2,366,221 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 1,138,462
shares of common stock underlying the Warrants. |
| |
|
| (10) |
Consists
of 682,234 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 215,384
shares of common stock underlying the Warrants. |
| |
|
| (11) |
Consists
of 142,892 shares of common stock underlying the Warrants. |
| |
|
| (12) |
Consists
of 31,570 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 30,769
shares of common stock underlying the Warrants. |
| |
|
| (13) |
Consists
of 126,280 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 123,076
shares of common stock underlying the Warrants. |
| |
|
| (14) |
Consists
of 63,140 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 61,538
shares of common stock underlying the Warrants. |
| |
|
| (15) |
Consists
of 31,570 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 30,769
shares of common stock underlying the Warrants. |
| |
|
| (16) |
Consists
of 126,280 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 123,076
shares of common stock underlying the Warrants. |
| |
|
| (17) |
Consists
of 252,560 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 246,153
shares of common stock underlying the Warrants. |
| |
|
| (18)
|
Consists
of 123,842 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 123,077
shares of common stock underlying the Warrants. |
| |
|
| (19) |
Consists
of 30,960 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 30,769
shares of common stock underlying the Warrants. |
| |
|
| (20)
|
Consists
of 30,960 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 30,769
shares of common stock underlying the Warrants. |
| |
|
| (21)
|
Consists
of 30,960 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 30,769
shares of common stock underlying the Warrants. |
| |
|
| (22) |
Consists
of 247,685 shares of common stock underlying the Notes assuming the accrued interest as of August 1, 2023 and 246,154 shares
of common stock underlying the Warrants. |
| |
|
| (23) |
We
have assumed, for the purposes of the following table, that the Selling Securityholders will sell all of the shares of common stock
owned beneficially that are covered by this prospectus but will not sell any other shares of common stock that they presently own. |
DESCRIPTION
OF SECURITIES
Capital
Stock
The
following description of our common stock and preferred stock summarizes the material terms and provisions of our common stock and the
preferred stock. For the complete terms of our common stock and preferred stock, please refer to our amended and restated certificate
of incorporation and our amended and restated bylaws, each as amended to date. The terms of our capital stock may also be affected by
the Delaware General Corporation Law (the “DGCL”). The summary below is qualified in its entirety by reference to our amended
and restated certificate of incorporation and amended and restated bylaws, as in effect at the time of any offering of securities under
this prospectus.
General
Our
amended and restated certificate of incorporation authorizes us to issue up to 500,000,000 shares of common stock, $0.0001 par value
per share, and 5,000,000 shares of preferred stock, $0.0001 par value per share.
Common
Stock
Voting
Each
holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the
election of directors. Our amended and restated certificate of incorporation and amended and restated bylaws do not provide for cumulative
voting rights. Because of this absence of cumulative voting, the holders of a majority of the shares of common stock entitled to vote
in any election of directors can elect all of the directors standing for election, if they should so choose.
Dividends
Subject
to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive ratably
those dividends, if any, as may be declared from time to time by our board of directors (our “Board of Directors”) out of
legally available funds.
Liquidation
In
the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets
legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of
any liquidation preferences that may be granted to the holders of any then outstanding shares of preferred stock.
Rights
and Preferences
Holders
of common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable
to the common stock. The rights, preferences, and privileges of the holders of common stock are subject to, and may be adversely affected
by, the rights of the holders of shares of any series of preferred stock, which we may designate and issue in the future.
Fully-paid
All
of the outstanding shares of our common stock are, and the shares of common stock issued upon the conversion of any securities convertible
into our common stock will be, fully paid and non-assessable. The shares of common stock offered by this prospectus or upon the conversion
of any preferred stock or debt securities or exercise of any warrants offered pursuant to this prospectus, when issued and paid for,
will also be, fully paid and non-assessable.
Stock
Exchange Listing
Our
common stock is listed on OTCQB Venture Market under the symbol “EVFM.”
Preferred
Stock
Our
Board of Directors has the authority, without further action by the stockholders, to issue up to 5,000,000 shares of preferred stock
in one or more series and:
| |
1. |
to
establish from time to time the number of shares to be included in each such series; |
| |
2. |
to
fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions
thereon; and |
| |
3. |
to
increase or decrease the number of authorized shares of any such series (but not below the number of shares of such series then outstanding). |
Our
Board of Directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting
power or other rights of the holders of our common stock. In connection with the Company’s prior entry into a rights agreement
(the Rights Agreement) with Pacific Stock Transfer, as rights agent, the Board approved a certificate of designation setting
forth the rights, preferences and limitations of 1,000,000 shares of Series A Preferred Stock. This certificate was filed with the Secretary
of State of the State of Delaware on March 24, 2020. The Rights Agreement expired in accordance with its terms on March 24, 2021, and
no shares of Series A Preferred Stock were ever issued. The issuance of preferred stock, while providing flexibility in connection with
possible acquisitions and other corporate purposes, could, among other things, delay, defer or prevent a change of control of the Company
and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock.
If
we offer a specific series of preferred stock under this prospectus, we will describe the terms of the preferred stock in the prospectus
supplement for such offering and will file a copy of the restated certificate establishing the terms of the preferred stock with the
SEC. To the extent required, this description will include:
| |
● |
the
title and stated value; |
| |
● |
the
number of shares offered, the liquidation preference, if any, per share and the purchase price; |
| |
● |
the
dividend rate(s), period(s) and/or payment date(s), or method(s) of calculation for such dividends; |
| |
● |
whether
dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
| |
● |
the
procedures for any auction and remarketing, if any; |
| |
● |
the
provisions for a sinking fund, if any; |
| |
● |
the
provisions for redemption, if applicable; |
| |
● |
any
listing of the preferred stock on any securities exchange or market; |
| |
● |
whether
the preferred stock will be convertible into our common stock, and, if applicable, the conversion price (or how it will be calculated)
and conversion period; |
| |
● |
whether
the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price (or how it will be calculated)
and exchange period; |
| |
● |
voting
rights, if any, of the preferred stock; |
| |
● |
a
discussion of any material and/or special United States federal income tax considerations applicable to the preferred stock; |
| |
● |
the
relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding
up of the affairs of the Company; and |
| |
● |
any
material limitations on issuance of any class or series of preferred stock ranking pari passu with or senior to the series
of preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of the Company. |
Series
B Preferred Stock
In
connection with a registered direct offering completed in October 2021, the Company filed Certificates of Designation of Preferences,
Rights and Limitations (the “Certificates of Designation”) with the Secretary of State of the State of Delaware to designate
5,000 shares of Series B-1 Convertible Preferred Stock, par value $0.0001 per share (the “Series B-1 Preferred Stock”), and
5,000 shares of Series B-2 Convertible Preferred Stock, par value $0.0001 per share (the “Series B-2 Preferred Stock”; collectively
with the Series B-1 Preferred Stock, the “Series B Preferred Stock”). As of December 31, 2021, there were no shares of Series
B-1 Preferred Stock issued and outstanding, and 5,000 shares of Series B-2 Convertible Preferred Stock were issued and outstanding.
Each
share of Series B-2 Preferred Stock is convertible (subject to a customary 19.99% exchange cap limitation and a customary 4.99%/9.99%
beneficial ownership limitation), at the holder’s option, into shares of the Company’s common stock The Series B-2
Convertible Preferred Stock may be converted into shares of the Company’s common stock at any time at a Fixed Conversion
Price per share as defined. The Fixed Conversion Price was adjusted during the first quarter of 2022 for certain dilutive issuances.
The adjustment period ended on April 25, 2022 and the Fixed Conversion Price was fixed at $332.50 from the sale of common stock pursuant
to the Seven Knots Purchase Agreement.
Each
holder of shares of Series B-2 Preferred Stock is entitled to receive dividends on a pari passu basis, when and if declared, on an as-converted-basis
with the holders of shares of the Company’s common stock, and each share of Series B-2 Preferred Stock carries a liquidation preference
equal to $1,000.00 per share for any voluntary or involuntary liquidation or winding up of the Company as well as for certain deemed
liquidation events described in the Certificates of Designation.
The
holders of the Series B-2 Preferred Stock will not have the right to vote on any matter presented to the holders of the Company’s
common stock but, the Company may not take the following actions without the prior consent of the holders of at least a majority of the
shares of Series B-2 Preferred Stock then outstanding: (i) alter or change adversely the powers, preferences or rights given to the Series
B-1 Preferred Stock or Series B-2 Preferred Stock or alter or amend the Certificates of Designation, (ii) amend the Company’s certificate
of incorporation in any manner that adversely affects any rights of the holders of the Series B-1 Preferred Stock or Series B-2 Preferred
Stock, (iii) increase the number of authorized shares of Series B-1 Preferred Stock or Series B-2 Preferred Stock, or (iv) enter into
any agreement with respect to any of the foregoing.
Commencing
in October 2025 and unless prohibited by Delaware law, the Company will be required to redeem the issued and outstanding shares of Series
B-2 Preferred Stock within 60 days following the request of the holders of a majority of the then issued and outstanding shares of Series
B-2 Preferred Stock at a price of $1,000 per share. If Delaware law then prohibits the Company from completing a redemption of all the
then outstanding shares of Series B-2 Preferred Stock, the Company will be required to ratably redeem the maximum number of shares of
Series B-2 Preferred Stock on a pro rata basis as permitted by Delaware law.
There
is no established trading market for the Series B Preferred Stock, and the Company does not intend to list the Series B Preferred Stock
on any securities exchange or nationally recognized trading system. Without a trading market, the liquidity of the Series B Preferred
Stock may be extremely limited.
Series
D Non-Convertible Preferred Stock
On
December 16, 2022, the Company filed a certificate of designation to create the Series D Non-Convertible Preferred Stock (the “Certificate
of Designation”) with the Secretary of State, Division of Corporations in Delaware. The Certificate of Designation, which forms
a part of the Company’s Amended and Restated Articles of Incorporation, specifies the terms of the Series D Non-Convertible Preferred
Stock (the Series D Preferred Stock). The Certificate of Designation authorizes a total of 70 shares of Series D Preferred. The holders
of shares of Series D Preferred Stock will not be entitled to receive dividends and are not convertible into shares of Common Stock.
Each share of Series D Preferred Stock has voting rights equal to 1% of the total voting power of the then-outstanding shares of common
stock of the Company entitled to vote for the approval of a reverse split of the Company’s common stock. The Series D Preferred
Stock is not authorized to vote on any other matters. However, the voting power attributable to any share of Series D Preferred Stock
shall be automatically reduced, as necessary such that the aggregate voting power of the holders shall not exceed 9.99%. The Company
had the option to redeem the Shares of Series D Preferred Stock (the Optional Redemption), at any time and from time to time on
or after the Date of Issuance. Further, the Company was required to redeem the shares of Series D Preferred Stock (the Mandatory
Redemption) following the approval of a reverse split of the Company’s common stock by the Company’s stockholders.
On March 15, 2023, the Company held a Special Meeting of Stockholders at which its stockholders approved an amendment to its Certificate
of Incorporation to effectuate a reverse stock split of the outstanding shares of its common stock by a ratio of not less than 1-for-20
and not more than 1-for-125 at any time on or prior to March 15, 2024, with the exact ratio to be set at a whole number within such range
by the board of directors (the 2023 Reverse Stock Split). The Company redeemed the Series D Preferred Stock in July 2023.
The
2023 Reverse Stock Split became effective on May 18, 2023 upon the opening of trading on the OTCQB (the Effective Time). Trading of
the Company’s common stock on the OTCQB resumed, on a post-split adjusted basis, on May 18, 2023, under the trading symbol
“EVFMD.” At the Effective Time, every 125 shares of the Company’s issued and outstanding common stock were
automatically converted into one share of common stock, without any change to the par value per share. In addition, proportionate
adjustments were made to the per share exercise price and the number of shares issuable upon the exercise of all outstanding stock
options and warrants to purchase shares of common stock, the number of shares issuable upon the vesting of all RSAs, and the number
of shares of common stock reserved for issuance pursuant to the Company’s equity incentive compensation plans, convertible
notes and convertible preferred stock. Any stockholder who would otherwise be entitled to a fractional share of the Company’s
common stock created as a result of the 2023 Reverse Stock Split is entitled to receive a cash payment equal to the product of such
resulting fractional interest in one share of the Company’s common stock multiplied by the closing trading price of the
Company’s common stock on the trading day immediately preceding the Effective Time. These interim condensed consolidated
financial statements are retrospectively adjusted for this 2023 Reverse Stock Split.
Series E-1 Convertible Preferred Stock
On August 7, 2023 the Company
filed the Certificate of Designation creating the Series E-1 Preferred Stock. The Certificate of Designation, which forms a part of the
Company’s Amended and Restated Articles of Incorporation, specifies the terms of the Series E-1 Preferred Stock.
The Certificate of Designation
authorizes a total of 2,300 shares of Series E-1 Preferred Stock. The holders of shares of Series E-1 Preferred Stock shall entitle
the holder thereof to vote together with the common shareholders as a single class and to cast that number of votes per share as is equal
to the number of shares of common stock into which it is then convertible. The Series E-1 Preferred Stock is convertible into shares
of common stock at a rate of $0.40 per share subject to adjustment as provided in the Certificate of Designation (the “Conversion
Rate”). Each holder of Series E-1 Preferred Stock is entitled to receive dividends paid exclusively in the form of common stock
(the “Dividends”) payable to the holders of the Series E-1 Preferred Stock on a monthly basis.
Registration
Rights Agreements
On
January 17, 2018, in connection with the Merger, we entered into a registration rights agreement with certain of our stockholders, including
funds managed by Invesco Ltd., discretionary investment funds managed by Woodford Investment Management as discretionary investment manager,
and funds managed by Domain Partners VII, L.P. Pursuant to the registration rights agreement, we were required to file a registration
statement with respect to shares of our capital stock, (the “Registrable Securities”), held by the stockholders who are party
to this agreement. Subject to limited exceptions, we are required to maintain the effectiveness of this registration statement until
the Registrable Securities covered by this registration have been disposed of or are no longer Registrable Securities. In addition, the
rights holders have the right to demand we effect the registration of any or all the Registrable Securities and/or effectuate the distribution
of any or all their Registrable Securities subject to certain exceptions and limitations. The rights holders also have customary piggyback
registration rights, subject to the limitations set forth in the registration rights agreement. In connection with these obligations,
we filed a registration statement on Form S-3 (No. 333-223731) on March 16, 2018 and amended on March 27, 2018, which was declared effective
on April 3, 2018.
On
April 10, 2019, in connection with a securities purchase agreement and private placement (the 2019 Private Placement), we entered into
a registration rights agreement with PDL BioPharma, Inc., a Delaware corporation, funds discretionally managed by Invesco Asset Management
Ltd. and funds managed by Woodford Investment Management Limited. Pursuant to the registration rights agreement, we were required to
(i) file a registration statement with the SEC within 30 days following the first closing of the 2019 Private Placement (the “First
Closing”) registering for resale the shares of our common stock issued in the First Closing and the shares of our common stock
issuable upon exercise of the warrants issued in the First Closing (the First Closing Registration Statement), (ii) use our commercially
reasonable efforts to have the First Closing Registration Statement declared effective, (iii) file a registration statement with the
SEC within 30 days following the second closing of the 2019 Private Placement (the “Second Closing”) registering for resale
the shares of our common stock issued in the Second Closing and the shares of our common stock issuable upon exercise of the warrants
issued in the Second Closing (the “Second Closing Registration Statement”), (iv) use our commercially reasonable efforts
to have the Second Closing Registration Statement declared effective and (v) maintain the effectiveness of the First Closing Registration
Statement and Second Closing Registration Statement until all registrable securities have been sold or may be sold without volume or
manner-of-sale restrictions pursuant to Rule 144 under the Securities Act.
The
registration rights agreement contains customary terms and conditions for transactions of this type, and includes liquidated damages
penalties in the event that we fail to satisfy or maintain the specified filing and effectiveness time periods in the registration rights
agreement.
In
connection with these obligations, we filed a registration statement on Form S-3 (No. 333-231126) on April 30, 2019 which was declared
effective on May 7, 2019, and filed a registration statement on Form S-3 (No. 333-232303) on June 24, 2019 which was declared effective
on July 2, 2019.
On
April 23, 2020, we entered into a securities purchase and security agreement with certain institutional investors and their designated
agent, pursuant to which we issued and sold to these purchasers convertible senior secured promissory notes in an aggregate principal
amount of up to $25.0 million and warrants to purchase shares of our common stock. These purchasers may require us to enter into a registration
rights agreement, pursuant to which we would grant these purchasers certain demand resale registration rights with respect to the common
stock issuable upon conversion of their notes and warrants. The rights under the registration rights agreement will terminate upon the
earlier of the tenth anniversary of the date of the agreement or automatically once all applicable registrable securities (i) have been
sold pursuant to an effective registration statement, (ii) have been sold by these purchasers pursuant to Rule 144 under the Securities
Act or (iii) may be resold by these purchasers without limitations as to volume or manner or sale pursuant to Rule 144.
On
October 14, 2020, in connection with a securities purchase agreement and private placement of convertible promissory notes, we entered
into a registration rights agreement with Adjuvant Global Health Technology Fund, L.P., and Adjuvant Global Health Technology Fund DE,
L.P. Pursuant to the registration rights agreement, we are required to file a registration statement with the SEC within 30 days following
the conversion of notes purchased in the private placement with an outstanding balance of at least $5 million registering for resale
the shares of our common stock issued upon conversion of these notes. Subject to limited exceptions, we are required to use our commercially
reasonable efforts to have this registration statement declared effective, and to maintain the effectiveness of this registration statement
until all applicable registrable securities have been sold or may be sold without volume or manner-of-sale restrictions pursuant to Rule
144 under the Securities Act.
On
December 20, 2022, the Company entered into a securities purchase agreement (SPA) with certain investors providing for the sale and issuance
of senior secured convertible notes due in the aggregate original principal amount of $2.3 million (the December 2022 Notes), warrants
to purchase an aggregate 369,230 shares of common stock (the December 2022 Warrants) and an aggregate 70 shares of Series D Preferred
Stock (the Preferred Shares) (collectively, the Offering). The Offering closed on December 21, 2022 with net proceeds to the Company
from the Offering, after deducting offering expenses, of approximately $1.25 million. In connection with the Offering, the Company entered
into a Registration Rights Agreement that requires the Company to register the common shares underlying the Warrants and Notes, which
is included in the prospectus.
In
February, March and April 2023, we entered into SPAs with certain investors providing for the sale and issuance of senior secured convertible
notes (collectively, the 2023 SPAs). The 2023 SPAs included (i) convertible promissory notes with aggregate original principal amounts
of approximately $1.4 million, $0.6 million, $0.5 million and $0.8 million, respectively (the 2023 Notes), and (ii) warrants to purchase
an aggregate 553,846, 240,000, 215,384 and 615,384 shares of common stock, respectively (the 2023 Warrants and, collectively,
the 2023 Offerings). The 2023 Offerings closed on February 17, 2023 (the February 2023 Closing), March 13, 2023 and March 20, 2023 (the
March 2023 Closings) and April 5, 2023 (the April 2023 Closing), respectively, with gross proceeds to the Company, before deducting offering
expenses, of approximately $0.9 million, $0.3 million, $0.3 million, and $0.5 million, respectively. The 2023 SPAs also included a Registration
Rights Agreement requiring us to register the common stock underlying the 2023 Notes and 2023 Warrants within the timeframes specified
therein, which is included in the prospectus. In addition, the Company issued warrants to purchase an aggregate 99,692 and 43,200 shares
of common stock in the February and March 2023 Closings to the placement agent.
In
July 2023, we entered into SPAs with certain investors providing for the sale and
issuance of senior secured convertible notes due in the aggregate original principal amount of $1.5 million (the July Notes), and warrants
to purchase an aggregate 1,200,000 shares of common stock (the July 2023 Warrants) (collectively, the July 2023 Offering). The July 2023
Offering closed on July 3, 2023with net proceeds to the Company, after deducting offering expenses, of approximately $1.0 million.
Possible
Anti-Takeover Effects of Delaware Law and Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Provisions
of the DGCL and our amended and restated certificate of incorporation and amended and restated bylaws could make it more difficult to
acquire the Company by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These provisions,
summarized below, are expected to discourage certain types of coercive takeover practices and takeover bids that our Board of Directors
may consider inadequate and to encourage persons seeking to acquire control of the company to first negotiate with our Board of Directors.
We believe that the benefits of increased protection of our ability to negotiate with the proponent of an unfriendly or unsolicited proposal
to acquire or restructure the company outweigh the disadvantages of discouraging takeover or acquisition proposals because, among other
things, negotiation of these proposals could result in an improvement of their terms.
Classified
Board
Our
amended and restated certificate of incorporation and our amended and restated bylaws provide that our Board of Directors is divided
into three classes. The directors designated as Class I directors have terms that will expire at the annual meeting of stockholders in
2024. The directors designated as Class II directors will have terms expiring at the annual meeting of stockholders in 2025, and the
director designated as a Class III director will have terms expiring at the annual meeting of stockholders in 2023. Directors for each
class will be elected at the annual meeting of stockholders held in the year in which the term for that class expires and thereafter
will serve for a term of three years. At any meeting of stockholders for the election of directors at which a quorum is present, the
election will be determined by a plurality of the votes cast by the stockholders entitled to vote at the election. Under the classified
board provisions, it would take at least two elections of directors for any individual or group to gain control of our Board of Directors.
Accordingly, these provisions could discourage a third party from initiating a proxy contest, making a tender offer or otherwise attempting
to gain control of the Company.
Removal
of Directors
Our
amended and restated bylaws provide that our stockholders may only remove our directors with cause, as defined in the amended and restated
bylaws.
Amendment
Our
amended and restated certificate of incorporation and our amended and restated bylaws provide that the affirmative vote of the holders
of at least 80% of our voting stock then outstanding is required to amend certain provisions relating to the number, term, election and
removal of our directors, stockholder notice procedures, the calling of special meetings of stockholders and the indemnification of directors.
Size
of Board and Vacancies
Our
amended and restated bylaws provide that the number of directors on our Board of Directors is fixed exclusively by our Board of Directors.
Newly created directorships resulting from any increase in our authorized number of directors will be filled by a majority of the members
of our Board of Directors then in office, provided that a majority of the entire Board of Directors, or a quorum, is present and any
vacancies in our Board of Directors resulting from death, resignation, retirement, disqualification, removal from office or other cause
will be filled generally by the majority vote of our remaining directors in office, even if less than a quorum is present.
Special
Stockholder Meetings
Our
amended and restated certificate of incorporation provides that only the Chairman of our Board of Directors, our Chief Executive Officer,
or our Board of Directors pursuant to a resolution adopted by a majority of the total number of directors it would have if there were
no vacancies may call special meetings of our stockholders.
Stockholder
Action by Unanimous Written Consent
Our
amended and restated certificate of incorporation expressly eliminates the right of our stockholders to act by written consent other
than by unanimous written consent.
Requirements
for Advance Notification of Stockholder Nominations and Proposals
Our
amended and restated bylaws provide advance notice procedures with respect to stockholder proposals and nomination of candidates for
election as directors other than nominations made by or at the direction of our Board of Directors or a committee of our Board of Directors.
No
Cumulative Voting
The
DGCL provides that stockholders are denied the right to cumulate votes in the election of directors unless our certificate of incorporation
provides otherwise. Our amended and restated certificate of incorporation does not provide for cumulative voting.
Undesignated
Preferred Stock
The
authority that is possessed by our Board of Directors to issue preferred stock could potentially be used to discourage attempts by third
parties to obtain control of the company through a merger, tender offer, proxy contest, or otherwise by making it more difficult or more
costly to obtain control of the company. Our Board of Directors may issue additional shares of preferred stock with voting rights or
conversion rights that, if exercised, could adversely affect the voting power of the holders of common stock.
Authorized
but Unissued Shares
Our
authorized but unissued shares of common stock and preferred stock will be available for future issuance without stockholder approval.
We may use additional shares for a variety of purposes, including future public offerings to raise additional capital, to fund acquisitions
and as employee compensation. The existence of authorized but unissued shares of common stock and preferred stock could render more difficult
or discourage an attempt to obtain control of the Company by means of a proxy contest, tender offer, merger or otherwise.
The
above provisions may deter a hostile takeover or delay a change in control or management of the Company.
Transfer
Agent and Registrar
The
transfer agent and registrar for our capital stock is Pacific Stock Transfer, Inc. The transfer agent and the registrar’s address
is 6725 Via Austi Pkwy Suite 300, Las Vegas, Nevada 89119.
Common
Stock
We
are registering for resale the shares of common stock issuable upon exercise of the Warrants and conversion of the Notes.
PLAN
OF DISTRIBUTION
The
Selling Securityholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling shares of
common stock or interests in shares of common stock received after the date of this prospectus from the Selling Securityholders as a
gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of
their shares of common stock or interests in shares of common stock on any stock exchange, market or trading facility on which the shares
are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at
prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The
Selling Securityholders may use any one or more of the following methods when disposing of shares or interests therein:
| |
a. |
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
b. |
block
trades in which the broker-dealer will attempt to sell the securities as agent, but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
c. |
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
d. |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
e. |
privately
negotiated transactions; |
| |
f. |
short
sales effected after the date the registration statement of which this prospectus is a part is declared effective by the SEC; |
| |
g. |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
h. |
broker-dealers
may agree with the Selling Securityholders to sell a specified number of such shares at a stipulated price per share; |
| |
i. |
a
combination of any such methods of sale; and |
| |
j. |
any
other method permitted pursuant to applicable law. |
The
Selling Securityholders may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned
by it and, if it defaults in the performance of its secured obligations, the pledgees or secured parties may offer and sell the shares
of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable
provision of the Securities Act amending the list of Selling Securityholders to include the pledgee, transferee or other successors in
interest as Selling Securityholders under this prospectus. The Selling Securityholders also may transfer the shares of common stock in
other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for
purposes of this prospectus.
In
connection with the sale of our common stock or interests therein, the Selling Securityholders may enter into hedging transactions with
broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging
the positions they assume. The Selling Securityholders may also sell shares of our common stock short and deliver these securities to
close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The Selling
Securityholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or
more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this
prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or
amended to reflect such transaction).
The
aggregate proceeds to the Selling Securityholders from the sale of the common stock offered by them will be the purchase price of the
common stock less discounts or commissions, if any. The Selling Securityholders reserve the right to accept and, together with their
agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents.
We will not receive any of the proceeds from this offering. Upon any exercise of the warrants by payment of cash, however, we will receive
the exercise price of the warrants.
The
Selling Securityholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the
Securities Act, provided that it meets the criteria and conforms to the requirements of that rule.
The
Selling Securityholders and any underwriters, broker-dealers or agents that participate in the sale of the common stock or interests
therein may be “underwriters” within the meaning of Section 2(a)(11) of the Securities Act. Any discounts, commissions, concessions
or profit they earn on any resale of the shares may be underwriting discounts and commissions under the Securities Act. Any Selling Securityholder
who is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act will be subject to the prospectus delivery
requirements of the Securities Act.
To
the extent required, the shares of our common stock to be sold, the name of the Selling Securityholder, the respective purchase prices
and public offering prices, the names of any agents, dealer or underwriter, and any applicable commissions or discounts with respect
to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the
registration statement that includes this prospectus.
In
order to comply with the securities laws of certain states, if applicable, the common stock may be sold in these jurisdictions only through
registered or licensed brokers or dealers. In addition, in some states, the common stock may not be sold unless (i) it has been registered
or qualified for sale or (ii) an exemption from registration or qualification requirements is available and is complied with.
We
have advised the Selling Securityholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of
shares in the market and to the activities of the Selling Securityholders and their affiliates. In addition, to the extent applicable
we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the Selling Securityholders
for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Securityholders may indemnify any
broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising
under the Securities Act.
We
have agreed to indemnify the Selling Securityholders against liabilities, including liabilities under the Securities Act and state securities
laws, relating to the registration of the shares offered by this prospectus.
We
have agreed with the Selling Securityholders to keep the registration statement of which this prospectus constitutes a part effective
until the earlier of (1) such time as all of the shares covered by this prospectus have been disposed of pursuant to and in accordance
with such registration statement or (2) the date on which all of the shares may be sold without volume or manner-of-sale restrictions
pursuant to Rule 144 of the Securities Act and without the requirement for the Company to be in compliance with the current public information
requirement under Rule 144.
LEGAL
MATTERS
The
validity of the securities being offered by this prospectus supplement will be passed upon for us Procopio, Cory, Hargreaves & Savitch,
LLP, San Diego, California.
EXPERTS
The
financial statements of Evofem Biosciences, Inc. as of December 31, 2022 and 2021, and for each of the two years in the period ended
December 31, 2022, included in this prospectus have been audited by Deloitte & Touche LLP, an independent registered public
accounting firm, as stated in their report. Such financial statements are included in reliance upon the report of such firm given their
authority as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
The
SEC maintains a website at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers
that file electronically with the SEC. You may access the registration statement, of which this prospectus form is a part, at the SEC’s
website. Our reports on Forms 10-K, 10-Q and 8-K, and amendments to those reports filed pursuant to Section 13(a) or 15(d) of the Exchange
Act, are also available for download, free of charge, as soon as reasonably practicable after these reports are filed with the SEC, at
our website at www.evofem.com. The content contained in, or that can be accessed through, our website is not a part of this prospectus.
In addition, our common stock is listed for trading on The OTC Marketplace under the symbol “EVFM.”
This
prospectus is only part of a Registration Statement on Form S-1 that we have filed with the SEC under the Securities Act, and therefore
omits certain information contained in the Registration Statement. We have also filed exhibits and schedules with the Registration Statement
that are excluded from this prospectus, and you should refer to the applicable exhibit or schedule for a complete description of any
statement referring to any contract or other document. You may obtain a copy of these documents and contracts from the SEC’s web
site or our web site.
INDEX
TO FINANCIAL STATEMENTS
For
the Three Months Ended March 31, 2023
For
the Year Ended December 31, 2022
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONDENSED
CONSOLIDATED BALANCE SHEETS
(Unaudited)
(In
thousands, except par value and share data)
| | |
March
31, 2023 | | |
December
31, 2022 | |
| Assets | |
| | | |
| | |
| Current
assets: | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 639 | | |
$ | 2,769 | |
| Restricted
cash | |
| 895 | | |
| 1,207 | |
| Trade
accounts receivable, net | |
| 7,404 | | |
| 1,126 | |
| Inventories | |
| 5,478 | | |
| 5,379 | |
| Prepaid
and other current assets | |
| 2,098 | | |
| 2,218 | |
| Total
current assets | |
| 16,514 | | |
| 12,699 | |
| Property
and equipment, net | |
| 3,698 | | |
| 3,940 | |
| Operating
lease right-of-use assets | |
| 3,902 | | |
| 4,406 | |
| Other
noncurrent assets | |
| 1,920 | | |
| 4,118 | |
| Total
assets | |
$ | 26,034 | | |
$ | 25,163 | |
| Liabilities,
convertible and redeemable preferred stock and stockholders’ deficit | |
| | | |
| | |
| Current
liabilities: | |
| | | |
| | |
| Accounts
payable | |
$ | 18,797 | | |
$ | 14,984 | |
| Convertible
notes payable - carried at fair value (Note 4) | |
| 23,807 | | |
| 39,416 | |
| Convertible
notes payable - Adjuvant (Note 4) | |
| 26,833 | | |
| 26,268 | |
| Accrued
expenses | |
| 3,454 | | |
| 4,124 | |
| Accrued
compensation | |
| 1,375 | | |
| 2,175 | |
| Operating
lease liabilities – current | |
| 2,226 | | |
| 2,311 | |
| Derivative
liabilities | |
| 122 | | |
| 1,676 | |
| Other
current liabilities | |
| 3,194 | | |
| 2,876 | |
| Total
current liabilities | |
| 79,808 | | |
| 93,830 | |
| Operating
lease liabilities – noncurrent | |
| 2,627 | | |
| 3,133 | |
| Total
liabilities | |
| 82,435 | | |
| 96,963 | |
| Commitments
and contingencies (Note 7) | |
| - | | |
| - | |
| Convertible
and redeemable preferred stock, $0.0001
par value | |
| | | |
| | |
| Series
B-1, B-2 and C convertible preferred stock, 5,000,
5,000, and 1,700 shares
authorized, respectively, and no shares
issued and outstanding at March 31, 2023 and December 31, 2022 | |
| — | | |
| — | |
| Convertible
and redeemable preferred stock | |
| — | | |
| — | |
| Stockholders’
deficit: | |
| | | |
| | |
| Preferred
stock, $0.0001 par
value; 5,000,000 shares
authorized; no equity-classified
preferred stock issued and outstanding at March 31, 2023 and December 31, 2022 | |
| — | | |
| — | |
| Common
stock, $0.0001 par
value; 500,000,000 shares
authorized; 1,727,690 and
984,786 shares
issued and outstanding at March 31, 2023 and December 31, 2022, respectively | |
| — | | |
| — | |
| Additional
paid-in capital | |
| 819,660 | | |
| 817,367 | |
| Accumulated
other comprehensive income | |
| 64,987 | | |
| 49,527 | |
| Accumulated
deficit | |
| (941,048 | ) | |
| (938,694 | ) |
| Total
stockholders’ deficit | |
| (56,401 | ) | |
| (71,800 | ) |
| Total
liabilities, convertible and redeemable preferred stock and stockholders’ deficit | |
$ | 26,034 | | |
$ | 25,163 | |
See
accompanying notes to the condensed consolidated financial statements (unaudited).
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
(In
thousands, except share and per share data)
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Product
sales, net | |
$ | 5,809 | | |
$ | 4,251 | |
| | |
| | | |
| | |
| Operating
expenses: | |
| | | |
| | |
| Cost
of goods sold | |
| 1,376 | | |
| 1,066 | |
| Research
and development | |
| 540 | | |
| 10,391 | |
| Selling
and marketing | |
| 3,854 | | |
| 12,705 | |
| General
and administrative | |
| 3,618 | | |
| 9,018 | |
| Total
operating expenses | |
| 9,388 | | |
| 33,180 | |
| Loss
from operations | |
| (3,579 | ) | |
| (28,929 | ) |
| Other
income (expense): | |
| | | |
| | |
| Interest
income | |
| 18 | | |
| 1 | |
| Other
expense | |
| (318 | ) | |
| (471 | ) |
| Gain
on issuance of financial instruments, net | |
| - | | |
| — | |
| Loss
on issuance of financial instruments | |
| (84 | ) | |
| (852 | ) |
| Change
in fair value of financial instruments | |
| 1,612 | | |
| (1,634 | ) |
| Total
other income (expense), net | |
| 1,228 | | |
| (2,956 | ) |
| Loss
before income tax | |
| (2,351 | ) | |
| (31,885 | ) |
| Income
tax expense | |
| (3 | ) | |
| (3 | ) |
| Net
loss | |
| (2,354 | ) | |
| (31,888 | ) |
| Series
B-1 and B-2 convertible preferred stock deemed dividends | |
| - | | |
| - | |
| Convertible
preferred stock deemed dividends | |
| — | | |
| (81 | ) |
| Net
loss attributable to common stockholders | |
$ | (2,354 | ) | |
$ | (31,969 | ) |
| Net
loss per share attributable to common stockholders, basic and diluted | |
$ | (1.85 | ) | |
$ | (360.25 | ) |
| Weighted-average
shares used to compute net loss per share attributable to common stockholders, basic and diluted | |
| 1,271,524 | | |
| 88,741 | |
See
accompanying notes to condensed consolidated financial statements (unaudited).
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONDENSED
CONSOLIDATED STATEMENTS OF COMPREHENSIVE OPERATIONS
(Unaudited)
(In
thousands, except share and per share data)
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Net
loss | |
$ | (2,354 | ) | |
$ | (31,888 | ) |
| Other
comprehensive income: | |
| | | |
| | |
| Change
in fair value of financial instruments attributed to credit risk change | |
| 15,460 | | |
| 181 | |
| Comprehensive
income (loss) | |
$ | 13,106 | | |
$ | (31,707 | ) |
See
accompanying notes to condensed consolidated financial statements (unaudited).
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONDENSED
CONSOLIDATED STATEMENTS OF CONVERTIBLE AND REDEEMABLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
(Unaudited)
(In
thousands, except share data)
| | |
Shares | | |
Amount | | |
Capital | | |
Income | | |
Deficit | | |
Deficit | |
| | |
Stockholders’
Deficit | |
| | |
Common
Stock | | |
Additional
Paid-in | | |
Accumulated
Other Comprehensive | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Income | | |
Deficit | | |
Deficit | |
| Balance
at December 31, 2022 | |
| 984,786 | | |
$ | — | | |
$ | 817,367 | | |
$ | 49,527 | | |
$ | (938,694 | ) | |
$ | (71,800 | ) |
| Issuance
of common stock upon cash exercise of warrants | |
| 24,200 | | |
| — | | |
| 67 | | |
| — | | |
| — | | |
| 67 | |
| Issuance
of common stock upon noncash exercise of purchase rights | |
| 718,704 | | |
| — | | |
| 180 | | |
| — | | |
| — | | |
| 180 | |
| Issuance
of February and March 2023 Notes (see Note 4) | |
| — | | |
| — | | |
| 1,629 | | |
| — | | |
| — | | |
| 1,629 | |
| Change
in fair value of financial instruments attributed to credit risk change (see Note 4) | |
| — | | |
| — | | |
| — | | |
| 15,460 | | |
| — | | |
| 15,460 | |
| Stock-based
compensation | |
| — | | |
| — | | |
| 417 | | |
| — | | |
| — | | |
| 417 | |
| Net
loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| (2,354 | ) | |
| (2,354 | ) |
| Balance
at March 31, 2023 | |
| 1,727,690 | | |
$ | — | | |
$ | 819,660 | | |
$ | 64,987 | | |
$ | (941,048 | ) | |
$ | (56,401 | ) |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Income | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
Stockholders’
Deficit | |
| | |
Series
B Convertible and Redeemable Preferred Stock | | |
Series
C Convertible and Redeemable Preferred Stock | | |
Common
Stock | | |
Additional
Paid-in | | |
Accumulated
Other Comprehensive | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Income | | |
Deficit | | |
Deficit | |
| Balance
at December 31, 2021 | |
| 5,000 | | |
$ | 4,740 | | |
| — | | |
$ | — | | |
| 86,666 | | |
$ | — | | |
$ | 751,276 | | |
$ | 5,089 | | |
$ | (860,680 | ) | |
$ | (104,315 | ) |
| Balance,
value | |
| 5,000 | | |
$ | 4,740 | | |
| — | | |
$ | — | | |
| 86,666 | | |
$ | — | | |
$ | 751,276 | | |
$ | 5,089 | | |
$ | (860,680 | ) | |
$ | (104,315 | ) |
| Issuance
of common stock - Stock Purchase Agreement (see Note 8) | |
| — | | |
| — | | |
| — | | |
| — | | |
| 9,673 | | |
| — | | |
| 5,400 | | |
| — | | |
| — | | |
| 5,400 | |
| Conversion
of series B-2 convertible preferred stock | |
| (650 | ) | |
| (619 | ) | |
| — | | |
| — | | |
| 978 | | |
| — | | |
| 708 | | |
| — | | |
| — | | |
| 708 | |
| Exchange
of series B-2 convertible preferred stock (see Note 8) | |
| (1,700 | ) | |
| (1,616 | ) | |
| 1,700 | | |
| 1,616 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Convertible
preferred stock deemed dividends | |
| — | | |
| 16 | | |
| — | | |
| 1 | | |
| — | | |
| — | | |
| (81 | ) | |
| — | | |
| — | | |
| (81 | ) |
| Restricted
stock awards issued | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,259 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Change
in fair value of financial instruments attributed to credit risk change (see Note 4) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 181 | | |
| — | | |
| 181 | |
| Modification
of the Baker Warrants (see Note 8) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 828 | | |
| — | | |
| — | | |
| 828 | |
| Stock-based
compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,067 | | |
| — | | |
| — | | |
| 1,067 | |
| Net
loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (31,888 | ) | |
| (31,888 | ) |
| Balance
at March 31, 2022 | |
| 2,650 | | |
$ | 2,521 | | |
| 1,700 | | |
$ | 1,617 | | |
| 98,576 | | |
$ | — | | |
$ | 759,198 | | |
$ | 5,270 | | |
$ | (892,568 | ) | |
$ | (128,100 | ) |
| Balance,
value | |
| 2,650 | | |
$ | 2,521 | | |
| 1,700 | | |
$ | 1,617 | | |
| 98,576 | | |
$ | — | | |
$ | 759,198 | | |
$ | 5,270 | | |
$ | (892,568 | ) | |
$ | (128,100 | ) |
See
accompanying notes to condensed consolidated financial statements (unaudited).
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
(In
thousands)
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Cash
flows from operating activities: | |
| | | |
| | |
| Net
loss | |
$ | (2,354 | ) | |
| (31,888 | ) |
| Adjustments
to reconcile net loss to net cash, cash equivalents and restricted cash used in operating activities: | |
| | | |
| | |
| Loss
on issuance of financial instruments | |
| 84 | | |
| 852 | |
| Change
in fair value of financial instruments | |
| (1,612 | ) | |
| 1,634 | |
| Baker
Warrants modification | |
| — | | |
| 828 | |
| Stock-based
compensation | |
| 417 | | |
| 1,067 | |
| Depreciation | |
| 245 | | |
| 265 | |
| Noncash
lease expenses | |
| 504 | | |
| 395 | |
| Noncash
interest expenses | |
| 565 | | |
| 523 | |
| Noncash
inventory reserve | |
| - | | |
| - | |
| Noncash
instrument exchange expense | |
| - | | |
| — | |
| Loss
on disposal of property and equipment | |
| - | | |
| — | |
| Financial
instrument modification expense | |
| - | | |
| — | |
| Changes
in operating assets and liabilities: | |
| | | |
| | |
| Accounts
receivable | |
| (6,278 | ) | |
| 2,033 | |
| Inventories | |
| (99 | ) | |
| 755 | |
| Prepaid
and other assets | |
| 1,518 | | |
| (1,036 | ) |
| Accounts
payable | |
| 3,813 | | |
| 3,735 | |
| Accrued
expenses and other liabilities | |
| (352 | ) | |
| (85 | ) |
| Accrued
compensation | |
| (800 | ) | |
| 469 | |
| Operating
lease liabilities | |
| (591 | ) | |
| (466 | ) |
| Net
cash, cash equivalents and restricted cash used in operating activities | |
| (4,940 | ) | |
| (20,919 | ) |
| Cash
flows from investing activities: | |
| | | |
| | |
| Proceeds
from sale of Softcup line of business | |
| — | | |
| - | |
| Purchases
of property and equipment | |
| (3 | ) | |
| (66 | ) |
| Maturities
of short-term investments | |
| — | | |
| — | |
| Net
cash, cash equivalents and restricted cash used in investing activities | |
| (3 | ) | |
| (66 | ) |
| Cash
flows from financing activities: | |
| | | |
| | |
| Proceeds
from issuance of common stock and warrants, net of discounts and commissions - public offerings | |
| - | | |
| - | |
| Proceeds
from issuance of common stock - Stock Purchase Agreement | |
| — | | |
| 5,480 | |
| Proceeds
from issuance of common stock, exercise of warrants | |
| 61 | | |
| — | |
| Proceeds
from issuance of common stock, net of commissions - ATM transactions | |
| - | | |
| — | |
| Proceeds
from issuance of common stock - ESPP and exercise of stock options | |
| - | | |
| - | |
| Proceeds
from issuance of preferred stock - registered direct offering | |
| — | | |
| - | |
| Payments
under term notes | |
| | - | |
| — | |
| Borrowings
under term notes | |
| 1,640 | | |
| 10,000 | |
| Cash
repurchase of fractional common stock after the reverse stock split | |
| - | | |
| — | |
| Cash
paid for financing costs | |
| — | | |
| (351 | ) |
| Payments
of tax withholdings related to vesting of restricted stock awards | |
| — | | |
| - | |
| Net
cash, cash equivalents and restricted cash provided by financing activities | |
| 1,701 | | |
| 15,129 | |
| Net
change in cash, cash equivalents and restricted cash | |
| (3,242 | ) | |
| (5,856 | ) |
| Cash,
cash equivalents and restricted cash, beginning of period | |
| 4,776 | | |
| 13,588 | |
| Cash,
cash equivalents and restricted cash, end of period | |
$ | 1,534 | | |
$ | 7,732 | |
| Supplemental
disclosure of noncash investing and financing activities: | |
| | | |
| | |
| Financing
and debt issuance costs included in accounts payable and accrued expenses | |
$ | 125 | | |
$ | 61 | |
| Receivable
from issuance of common stock - Stock Purchase Agreement | |
$ | — | | |
$ | 331 | |
| Purchases
of property and equipment included in accounts payable and accrued expenses | |
$ | 140 | | |
$ | 57 | |
| Conversion
of series B-2 convertible preferred stock to common stock | |
$ | — | | |
$ | 644 | |
| Exchange
of series B-2 convertible preferred stock to series C convertible preferred stock | |
$ | — | | |
$ | 1,616 | |
| Issuance
of common stock upon exercise of purchase rights | |
$ | 180 | | |
$ | — | |
See
accompanying notes to condensed consolidated financial statements (unaudited).
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
NOTES
TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS |
1.
Description of Business and Basis of Presentation
Description
of Business
Evofem
is a San Diego-based, commercial-stage biopharmaceutical company committed to developing and commercializing innovative products to address
unmet needs in women’s sexual and reproductive health.
The
Company’s first commercial product, Phexxi® (lactic acid, citric acid, and potassium bitartrate) vaginal gel (Phexxi),
was approved by the U.S. Food and Drug Administration (FDA) on May 22, 2020 and is the first and only FDA-approved, hormone-free, woman-controlled,
on-demand prescription contraceptive gel for women. The Company commercially launched Phexxi in September 2020. Phexxi net product sales
were $16.8 million in 2022.
Basis
of Presentation and Principles of Consolidation
The
Company prepared the unaudited interim condensed consolidated financial statements included in this Registration Statement in
accordance with accounting principles generally accepted (GAAP) in the United States for interim financial information and the rules
and regulations of the Securities and Exchange Commission (SEC) related to quarterly reports on Form 10-Q.
The
Company’s financial statements are presented on a consolidated basis, which include the accounts of the Company and its wholly-owned
subsidiaries. Intercompany accounts and transactions have been eliminated in consolidation. The unaudited interim condensed consolidated
financial statements do not include all information and disclosures required by GAAP for annual audited financial statements and should
be read in conjunction with the Company’s consolidated financial statements and notes thereto for the year ended December 31, 2022
included in its Annual Report on Form 10-K as filed with the SEC on April 27, 2023 (the 2022 Audited Annual Financial Statements).
The
unaudited interim condensed consolidated financial statements included in this report have been prepared on the same basis as the Company’s
audited consolidated financial statements and include all adjustments (consisting only of normal recurring adjustments) necessary for
a fair statement of the financial position, results of operations, cash flows, and statements of convertible and redeemable preferred
stock and stockholders’ deficit for the periods presented. The results for the three months ended March 31, 2023 are not necessarily
indicative of the results expected for the full year. The condensed consolidated balance sheet as of December 31, 2022 was derived from
the 2022 Audited Financial Statements.
Reverse
Stock Split
On
March 15, 2023, the Company’s shareholders approved a
reverse stock split between 1-for-20 and not more than 1-for-125 at any time on or prior to March 15, 2024. The Company determined on
a ratio of 1-for-125 for the 2023 Reverse Stock Split. On May 18, 2023, the Reverse Stock Split became effective.
The interim condensed consolidated financial statements are retrospectively adjusted for this 2023 Reverse Stock Split. See Note
10- Subsequent Events for further discussions on the Reverse Stock Split and other subsequent events.
Risks,
Uncertainties and Going Concern
Any
disruptions in the commercialization of Phexxi and/or its supply chain could have a material adverse effect on the Company’s business,
results of operations and financial condition.
The
condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets
and settlement of liabilities, in the normal course of business, and does not include any adjustments to reflect the possible future
effects on the recoverability and classification of assets or amounts and classification of liabilities that may result from the outcome
of this uncertainty.
The
Company’s principal operations have been related to research and development, including the development of Phexxi, and to its commercially
related sales and marketing efforts. Additional activities have included raising capital, identifying alternative manufacturing to lower
the cost of goods sold (COGS), seeking ex-U.S. licensing partners to add non-dilutive capital to the balance sheet, and establishing
and maintaining a corporate infrastructure to support a commercial product. The Company has incurred operating losses and negative cash
flows from operating activities since inception. In the first quarter of 2023, as described in Note 4- Debt and Note 8- Stockholders’
Deficit, the Company received gross proceeds of approximately $1.6
million, in aggregate, from the sale and issuance
of senior subordinated convertible notes and warrants in three closings. As of March 31, 2023, the Company had cash and cash equivalents
of $0.6 million,
$0.9 million
in restricted cash from the Adjuvant Notes (as defined in Note 4- Debt) that is available for use, a working capital deficit of
$63.3 million
and an accumulated deficit of $941.0
million.
Effective
October 3, 2022, the Company’s common stock is listed on the OTC Venture Market (the OTCQB) of the OTC Markets Group, Inc., a centralized
electronic quotation service for over-the-counter securities, under the symbol “EVFM.” The OTCQB imposes, among other requirements,
a minimum $0.01 per
share bid price requirement (the Bid Price Requirement) for continued inclusion on the OTCQB. The closing bid price for the Company’s
common stock must remain at or above $0.01
per share to comply with the Bid Price Requirement
for continued listing.
In
October 2022, the Company reported that its Phase 3 clinical trial (EVOGUARD) did not achieve its efficacy endpoints. The Company
has discontinued investment in this development program.
In
March 2023, the Company received a Notice of Event of Default and Reservation of Rights (the Notice of Default) from Baker Bros
claiming that the Company has failed to maintain the required shares reserved amount per the Third Baker Amendment as defined in Note
4- Debt. In addition, the Notice of Default resulted in a cross default under all outstanding debt.
Management’s
plans to meet the Company’s cash flow needs in the next 12 months include generating recurring product revenue, restructuring its
current payables, curing the event of default under its debt arrangements, and obtaining additional funding such as through the issuance
of its capital stock, non-dilutive financings, or through collaborations or partnerships with other companies, including license agreements
for Phexxi in foreign markets.
While
the Company has increased net product sales in each consecutive fiscal year since the launch of Phexxi in September 2020, the Company
anticipates it will continue to incur net losses for the foreseeable future. According to management estimates, liquidity resources as
of March 31, 2023 are not sufficient to maintain the Company’s cash flow needs for the twelve months from the date of issuance
of these condensed consolidated financial statements.
If
the Company is not able to obtain the required funding, through a significant increase in revenue, equity or debt financings, license
agreements for Phexxi in foreign markets, or other means, or is unable to obtain funding on terms favorable to the Company, or if the
event of default under its existing debt arrangements is not cured or there is another event of default affecting the notes payable,
there will be a material adverse effect on commercialization and development operations and the Company’s ability to execute its
strategic development plan for future growth. If the Company cannot successfully raise additional funding and implement its strategic
development plan, the Company may be forced to make further reductions in spending, including spending in connection with its commercialization
activities, extend payment terms with suppliers, liquidate assets where possible at a potentially lower amount than as recorded in the
condensed consolidated financial statements, suspend or curtail planned operations or cease operations entirely. Any of these could materially
and adversely affect the Company’s liquidity, financial condition and business prospects, and the Company would not be able to
continue as a going concern. The Company has concluded that these circumstances and the uncertainties associated with the Company’s
ability to obtain additional equity or debt financing on terms that are favorable to the Company, or at all, and otherwise succeed in
its future operations raise substantial doubt about the Company’s ability to continue as a going concern.
2.
Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions
that affect the amounts reported in the condensed consolidated financial statements and the notes thereto.
Significant
estimates affecting amounts reported or disclosed in the condensed consolidated financial statements include, but are not limited to:
the assumptions used in measuring the revenue gross-to-net variable consideration items; the trade accounts receivable credit loss reserve
estimate; the discount rate used in estimating the fair value of the lease right-of-use (ROU) assets and lease liabilities;
the assumptions used in estimating the fair value of convertible notes, warrants and purchase rights issued; the useful lives of property
and equipment; the recoverability of long-lived assets; and clinical trial accruals; the assumptions used in estimating the fair value
of stock-based compensation expense. These assumptions are more fully described in Note 3- Revenue, Note 4- Debt, Note 6- Fair
Value of Financial Instruments, Note 7- Commitments and Contingencies, and Note 9- Stock-based Compensation. The Company bases its estimates on historical experience and other market-specific or other relevant
assumptions that it believes to be reasonable under the circumstances and adjusts when facts and circumstances dictate. The estimates
are the basis for making judgments about the carrying values of assets, liabilities and recorded expenses that are not readily apparent
from other sources. As future events and their effects cannot be determined with precision, actual results may materially differ from
those estimates or assumptions.
Segment
Reporting
Operating
segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation
by the chief operating decision-maker, the Chief Executive Officer of the Company, in making decisions regarding resource allocation
and assessing performance. The Company views its operations and manages its business in one
operating segment.
Concentrations
of Credit Risk
Financial
instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash, cash equivalents
and restricted cash. Deposits in the Company’s checking, time deposit and investment accounts are maintained in federally insured
financial institutions and are subject to federally insured limits or limits set by Securities Investor Protection Corporation. The Company
invests in funds through a major U.S. bank and is exposed to credit risk in the event of default to the extent of amounts recorded on
the condensed consolidated balance sheets.
The
Company has not experienced any losses in such accounts and believes it is not exposed to significant concentrations of credit risk on
its cash, cash equivalents and restricted cash balances on amounts in excess of federally insured limits due to the financial position
of the depository institutions in which these deposits are held. The Company’s deposits were primarily held in Silicon Valley Bank
prior to their closure by regulators, however, the Company was subsequently able to regain full access to all its deposits and moved
these to a different financial institution.
The
Company is also subject to credit risk related to its trade accounts receivable from product sales. Its customers are located in the
United States and consist of wholesale distributors, retail pharmacies, and a mail-order specialty pharmacy. The Company extends credit
to its customers in the normal course of business after evaluating their overall financial condition and evaluates the collectability
of its accounts receivable by periodically reviewing the age of the receivables, the financial condition of its customers, and its past
collection experience. Historically, the Company has not experienced any credit losses. As of March 31, 2023, based on the evaluation
of these factors, the Company did not record an allowance for doubtful accounts.
Phexxi
is distributed primarily through three major distributors and a mail-order pharmacy, who receive service fees calculated as a percentage
of the gross sales, and fee per units shipped, respectively. These entities are not obligated to purchase any set number of units and
distribute Phexxi on demand as orders are received. For the three months ended March 31, 2023 and 2022, the Company’s three largest
customers combined made up approximately 86%
and 70%
of its gross product sales, respectively. As of March 31, 2023 and March 31, 2022, the Company’s three largest customers combined
made up 94%
and 73%,
respectively, of its trade accounts receivable balance.
Significant
Accounting Policies
There
have been no changes to the significant accounting policies that were described in Note 2- Summary of Significant Accounting Policies
of the 2022 Audited Financial Statements in the Company’s Annual Report.
Cash,
Cash Equivalents and Restricted Cash
Cash
and cash equivalents consist of readily available cash in checking accounts and money market funds. Restricted cash consists of cash
held in monthly time deposit accounts and letters of credit, which are collateral for the Company’s credit cards, facility leases
and fleet leases, as described in Note 7- Commitments and Contingencies. During the quarter ended March 31, 2023, the letters
of credit of $0.3
million for its fleet leases have been released.
Additionally, the remaining $0.9
million of the $25.0
million received from the issuance of Adjuvant
Notes (as defined in Note 4- Debt) in the fourth quarter of 2020, is classified as restricted cash as the Company is contractually
obligated to use the funds for specific purposes. Upon receipt of a notice of default from its landlord on March 20, 2023, for failing
to pay March 2023 rent timely resulting in a breach under the office lease agreement, the Company’s letter of credit in the amount
of $0.8
million, in restricted cash, has been recovered
by the landlord.
The
following table provides a reconciliation of cash, cash equivalents and restricted cash, reported within the condensed consolidated statements
of cash flows (in thousands):
Schedule
of Reconciliation of Cash and Restricted Cash
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Cash
and cash equivalents | |
$ | 639 | | |
$ | 2,761 | |
| Restricted
cash | |
| 895 | | |
| 4,171 | |
| Restricted
cash included in other noncurrent assets | |
| — | | |
| 800 | |
| Total
cash, cash equivalents and restricted cash presented in the condensed consolidated statements of cash flows | |
$ | 1,534 | | |
$ | 7,732 | |
Net
Loss Per Share
Basic
net loss per common share is calculated by dividing the net loss by the weighted-average number of common shares outstanding during the
period, without consideration for potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss by
the weighted-average number of common shares and potentially dilutive securities outstanding for the period determined using the treasury-stock
and if-converted methods. For purposes of the diluted net loss per share calculation, potentially dilutive securities are excluded from
the calculation of diluted net loss per share because their effect would be anti-dilutive and, therefore, basic and diluted net loss
per share were the same for all periods presented. Potentially dilutive securities excluded from the calculation of diluted net loss
per share are summarized in the table below. Common shares were calculated for the convertible preferred stock and the convertible debt
using the if-converted method.
Schedule
of Potentially Dilutive Securities Excluded from Calculation of Diluted Net Loss Per Share
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Unvested
restricted common stock subject to repurchase | |
| — | | |
| 1,258 | |
| Common
stock to be purchased under the 2019 ESPP | |
| — | | |
| 288 | |
| Options
to purchase common stock | |
| 4,843 | | |
| 7,183 | |
| Warrants
to purchase common stock | |
| 3,180,282 | | |
| 52,448 | |
| Series
B-2 and C convertible preferred stock | |
| — | | |
| 4,444 | |
| Purchase
rights to purchase common stock | |
| 14,238,827 | | |
| — | |
| Convertible
debt | |
| 18,042,988 | | |
| 9,969 | |
| Total | |
| 35,466,940 | | |
| 75,590 | |
Recently
Adopted Accounting Pronouncements
In
March 2022, the FASB issued ASU No. 2022-02, Financial Instruments - Credit Losses (ASU No. 2022-02). This is an amendment to ASU 2016-13,
where it eliminates the accounting guidance for troubled debt restructuring by creditors in Subtopic 310-40, Receivables—Troubled
Debt Restructurings by Creditors, while enhancing disclosure requirements for certain loan refinancings and restructurings by creditors
when a borrower is experiencing financial difficulty. ASU No. 2022-02 was effective for the Company beginning January 1, 2023 since the
Company adopted ASU 2016-13 on January 1, 2020. The adoption of this new standard did not have a material impact on the Company’s
condensed consolidated financial statements.
3.
Revenue
The
Company recognizes revenue from the sale of Phexxi in accordance with ASC 606, Revenue from Contracts with Customers (ASC 606).
The provisions of ASC 606 require the following steps to determine revenue recognition: (1) identify the contract(s) with a customer;
(2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to
the performance obligations in the contract; and (5) recognize revenue when (or as) the entity satisfies a performance obligation.
In
accordance with ASC 606, the Company recognizes revenue when its performance obligation is satisfied by transferring control of the product
to a customer. In accordance with the Company’s contracts with customers, control of the product is transferred upon the conveyance
of title, which occurs when the product is sold to and received by a customer. The Company’s customers are located in the United
States and consist of wholesale distributors, retail pharmacies, and a mail-order specialty pharmacy. Payment terms typically range from
31
to 66
days, include prompt pay discounts, and vary
by customer. Trade accounts receivable due to the Company from contracts with its customers are stated separately in the balance sheet,
net of various allowances as described in the Trade Accounts Receivable policy in Note 2- Summary of Significant Accounting Policies
to the 2022 Audited Financial Statements.
The
amount of revenue recognized by the Company is equal to the amount of consideration that is expected to be received from the sale of
product to its customers. Revenue is only recognized when the performance obligation is satisfied. To determine whether a significant
reversal will occur in future periods, the Company assesses both the likelihood and magnitude of any such potential reversal of revenue.
Phexxi
is sold to customers at the wholesale acquisition cost (WAC), or in some cases at a discount to WAC. However, the Company records product
revenue, net of reserves for applicable variable consideration. These types of variable consideration reduce revenue and include the
following:
| ● | Distribution
services fees |
| ● | Prompt
pay and other discounts |
| ● | Product
returns |
| ● | Chargebacks |
| ● | Rebates |
| ● | Patient
support programs, including our co-pay programs |
An
estimate for variable consideration is made with each sale and is recorded in conjunction with the revenue being recognized. To calculate
the variable consideration, the Company uses the expected value method. If the estimated amount is payable to a customer, it is recorded
as a reduction to accounts receivable. If the estimated amount is payable to an entity other than a customer, it is recorded as a current
liability. An estimated amount of variable consideration may differ from the actual amount. At each balance sheet date, these provisions
are analyzed and adjustments are made if necessary. Any adjustments made to these provisions would also affect net product revenue and
earnings.
In
accordance with ASC 606, the Company must make significant judgments to determine the estimate for certain variable consideration. For
example, the Company must estimate the percentage of end-users that will obtain the product through public insurance such as Medicaid
or through private commercial insurance. To determine these estimates, the Company relies on historical sales data showing the amount
of various end-user consumer types, inventory reports from the wholesale distributors and mail-order specialty pharmacy, and other relevant
data reports. Because Phexxi was launched in September 2020, this historical data is limited. Due to limits on historical data, the Company
has also used trend analysis and professional judgment in developing these estimates.
The
specific considerations that the Company uses in estimating these amounts related to variable consideration are as follows:
Distribution
services fees – The Company pays distribution service fees to its wholesale distributors and mail-order specialty pharmacy.
These fees are a contractually fixed percentage of WAC and are calculated at the time of sale based on the purchase amount. The Company
considers these fees to be separate from the customer’s purchase of the product, therefore, they are recorded in other current
liabilities on the condensed consolidated balance sheet.
Prompt
pay and other discounts – The Company incentivizes its customers to pay their invoices on time through prompt pay discounts.
These discounts are an industry standard practice, and the Company offers a prompt pay discount to each wholesale distributor and retail
pharmacy customer. The specific prompt pay terms vary by customer and are contractually fixed. Prompt pay discounts are typically taken
by the Company’s customers, so an estimate of the discount is recorded at the time of sale based on the purchase amount. Prompt
pay discount estimates are recorded as contra trade accounts receivable on the condensed consolidated balance sheet.
The
Company may also give other discounts to its customers to incentivize purchases and promote customer loyalty. The terms of such discounts
may vary by customer. These discounts reduce gross product revenue at the time the revenue is recognized.
Chargebacks
– Certain government entities and covered entities (e.g. Veterans Administration, 340B covered entities) are able to purchase
the product at a price discounted below WAC. The difference between the government or covered entity purchase price and the wholesale
distributor purchase price of WAC will be charged back to the Company. The Company estimates the amount of each chargeback channel based
on the expected number of claims in each channel and related chargeback that is associated with the revenue being recognized for product
that remains in the distribution channel at the end of each reporting period. Estimated chargebacks are recorded as contra trade accounts
receivable on the condensed consolidated balance sheet.
Rebates
– The Company is subject to mandatory discount obligations under the Medicaid and Tricare programs. The rebate amounts for
these programs are determined by statutory requirements or contractual arrangements. Rebates are owed after the product has been dispensed
to an end user and the Company has been invoiced. Rebates for Medicaid and Tricare are typically invoiced in arrears. The Company estimates
the amount in rebates based on the expected number of claims and related cost that is associated with the revenue being recognized for
product that remains in the distribution channel at the end of each reporting period. Rebate estimates are recorded as other current
liabilities on the condensed consolidated balance sheet.
Patient
support programs – One type of patient support program the Company offers is a co-pay program to commercially insured patients
whose insurance requires a co-pay to be made when filling their prescription. This is a voluntary program that is intended to provide
financial assistance to patients meeting certain eligibility requirements. The Company estimates the amount of financial assistance for
these programs based on the expected number of claims and related cost that is associated with the revenue being recognized for product
that remains in the distribution channel at the end of each reporting period. Patient support programs estimates are recorded as other
current liabilities on the condensed consolidated balance sheet.
Product
returns – Customers have the right to return product that is within six months or less of the labeled expiration date or that
is past the expiration date by no more than six months. Phexxi was commercially launched in September 2020. The Company uses historical
sales and return data to estimate future product returns. Product return estimates are recorded as other current liabilities on the condensed
consolidated balance sheet.
As
of March 31, 2023 and December 31, 2022, the variable considerations discussed above were recorded in the condensed consolidated balance
sheet and consisted of $0.2
million and $0.1
million, respectively, in contra trade accounts
receivable and $3.1
million and $2.6
million, respectively, in other current liabilities.
4.
Debt
Convertible
Notes
Baker
Bros. Notes
On
April 23, 2020, the Company entered into a Securities Purchase and Security Agreement (the Baker Bros. Purchase Agreement) with certain
affiliates of Baker Bros. Advisors LP, as purchasers (the Baker Purchasers), and Baker Bros. Advisors LP, as designated agent, pursuant
to which the Company agreed to issue and sell to the Baker Purchasers (i) convertible senior secured promissory notes (the Baker Notes)
in an aggregate principal amount of up to $25.0
million and (ii) warrants to purchase shares
of common stock (the Baker Warrants) in a private placement.
At
the initial closing date of April 24, 2020 (the Baker Initial Closing), the Company issued and sold Baker Notes with an aggregate principal
amount of $15.0
million (the Baker First Closing Notes) and Baker
Warrants exercisable for 1,639
shares of common stock.
Following
the Baker Initial Closing, the Baker Purchasers had an option to purchase from the Company up to $10.0
million of Baker Notes (the Baker Purchase Rights)
at the Baker Purchasers’ discretion at any time prior to the Company receiving at least $100.0
million in aggregate gross proceeds from one
or more sales of equity securities.
On
June 5, 2020 (the Exercise Date), the Baker Purchasers exercised the Baker Purchase Rights. At the second closing date of June 9, 2020
(the Baker Second Closing), the Baker Purchasers acquired the remaining Baker Notes with an aggregate principal amount of $10.0
million and Baker Warrants exercisable for 1,092
shares of common stock. Upon the completion of
the underwritten public offering in June 2020, the exercise price of the Baker Warrants was $4,575
per share. The Baker Warrants have a five-year
term with a cashless exercise provision and are
immediately exercisable at any time from their respective issuance date.
The
Baker Notes have a five-year
term, with no pre-payment ability during the
first three
years. Interest on the unpaid principal balance
of the Baker Notes (the Baker Outstanding Balance) accrues at 10.0%
per annum with interest accrued during the first year from the two respective closing dates recognized as payment-in-kind. The effective
interest rate for the period was 10.0%.
Accrued interest beyond the first year of the respective closing dates is to be paid in arrears on a quarterly basis in cash or recognized
as payment-in-kind, at the direction of the Baker Purchasers. As discussed below, with the amendment to the Baker Bros. Purchase Agreement,
interest payments were paid-in kind. Interest pertaining to the Baker Notes for the three months ended March 31, 2023 and 2022 was approximately
$1.4
million and $0.7
million, respectively, which was added to the
outstanding principal balance. The Company accounts for the Baker Notes under the fair value method as described below and, therefore,
the interest associated with the Baker Notes is included in the fair value determination. As of March 31, 2023, the Baker Notes could
be converted into 57,426,766
shares of common stock.
The
Baker Notes are callable by the Company on 10
days’ written notice beginning on the third
anniversary of the initial closing date of April 24, 2020. The call price will equal 100%
of the Baker Outstanding Balance plus accrued and unpaid interest if the Company’s common stock as measured using a 30-day
volume weighted average price (VWAP) is greater than the benchmark price of $9,356.25
as stated in the Baker Bros. Purchase Agreement,
or 110%
of the Baker Outstanding Balance plus accrued and unpaid interest if the VWAP is less than such benchmark price. The Baker Purchasers
also have the option to require the Company to repurchase all or any portion of the Baker Notes in cash upon the occurrence of certain
events. In a repurchase event, as defined in the Baker Bros. Purchase Agreement, the repurchase price will equal 110%
of the Baker Outstanding Balance plus accrued and unpaid interest. In an event of default or the Company’s change of control, the
repurchase price will equal to the sum of (x) three times of the Baker Outstanding Balance plus (y) the aggregate value of future interest
that would have accrued. The Baker Notes were convertible at any time at the option of the Baker Purchasers at the conversion price of
$4,575
per share prior to the First and Second Baker
Amendments (as defined below).
On
November 20, 2021, the Company entered into the first amendment to the Baker Bros. Purchase Agreement (the First Baker Amendment), in
which each Baker Purchaser had the right to convert all or any portion of the Baker Notes into common stock at a conversion price equal
to the lesser of (a) 4,575
and (b) 115%
of the lowest price per share of common stock (or, as applicable with respect to any equity securities convertible into common stock,
115%
of the applicable conversion price) sold in one or more equity financings until the Company has met a qualified financing threshold defined
as one or more equity financings resulting in aggregate gross proceeds to the Company of at least $50
million (the Financing Threshold).
The
First Baker Amendment also extended, effective upon the Company’s achievement of the Financing Threshold, the affirmative covenant
to achieve $100.0
million in cumulative net sales of Phexxi by
June 30, 2022 to June 30, 2023. Additionally per the First Baker Amendment, if in any equity financing closing on or prior to the date
the Company has met the Financing Threshold, the Company was required to issue warrants to purchase capital stock of the Company (or
other similar consideration), the Company was also required to issue to the Baker Purchasers an equivalent coverage of warrants (or other
similar consideration) on the same terms as if the Baker Purchasers participated in the financing in an amount equal to the then outstanding
principal of Baker Notes held by the Baker Purchasers. In satisfaction of this requirement and in connection with the closing of the
May 2022 Public Offering, the Company issued warrants to purchase 582,886
shares of the Company’s common stock at
an exercise price of $93.75
per share (the June 2022 Baker Warrants). As
required by the terms of the First Baker Amendment, the June 2022 Baker Warrants have substantially the same terms as the warrants issued
in the May 2022 Public Offering. Refer to Note 8 - Stockholders’ Deficit for
further information. The exercise price of the initial Baker Warrants and the June 2022 Baker Warrants was reset to $1.625
per share with the February and March 2023 Notes
issuance, both as discussed below, and further reset to $0.8125
per share along with the April 2023 Notes issuance.
On
March 21, 2022, the Company entered into the second amendment to the Baker Bros. Purchase Agreement (the Second Baker Amendment), pursuant
to which each Baker Purchaser now has the right to convert all or any portion of the Baker Notes into Common Stock at a conversion price
equal to the lesser of (a) $725.81
or (b) 100%
of the lowest price per share of common stock (or as applicable with respect to any equity securities convertible into common stock,
100%
of the applicable conversion price) sold in any equity financing until the Company has (i) met the qualified financing threshold by June
30, 2022, defined as a single underwritten financing resulting in aggregate gross proceeds to the Company of at least $20
million (Qualified Financing Threshold) and (ii)
the publication of its top-line results from its EVOGUARD clinical trial (the Clinical Trial Milestone) by October 31, 2022. The
Second Baker Amendment also provides that the exercise price of the Baker Warrants will equal the conversion price of the Baker Notes.
The Company met the Qualified Financing Threshold upon the closing of the May 2022 Public Offering, and as of September 30, 2022, the
conversion price and exercise price of the Baker Warrants was reset to $93.75.
The Company achieved the Clinical Trial Milestone in October 2022. Also, with the achievement of the Qualified Financing Threshold and
the Clinical Trial Milestone, the affirmative covenant to achieve $100.0
million in cumulative net sales of Phexxi was
extended to June 30, 2023.
On
September 15, 2022, the Company entered into the third amendment to the Baker Bros. Purchase Agreement (the Third Baker Amendment), pursuant
to which the conversion was amended to equal to $26.25,
subject to adjustment for certain dilutive Company equity issuance adjustments for a two-year
period, removal of an interest make-whole payment
due in certain circumstances, and certain change of control and liquidation payment amounts were reduced from three times the outstanding
amounts of the Baker Notes to two times the outstanding amounts. In addition, the Third Baker Amendment provides that the Company may
make future interest payments to the Baker Purchasers in kind or in cash, at the Company’s option.
The
Baker Notes contain various customary affirmative and negative covenants agreed to by the Company, including timely payment, in cash,
of the quarterly interest payment and maintaining an active listing. On September 12, 2022, the Company received a default notice from
the Baker Purchasers due to its failure of making the required payments of accrued interest for the first and second quarters of 2022
in the aggregate amount of $1.4
million and being delisted from Nasdaq. As a
result of the cross-default provisions applicable to the Adjuvant Notes and the May 2022 Notes (both, as discussed below), the Company
was also in default of these Notes. On September 15, 2022, the Company entered into a (i) Forbearance Agreement (the Secured Creditor
Forbearance Agreement) with the Baker Purchasers, pursuant to which the Baker Purchasers agreed to forbear from exercising any of their
rights and remedies during the Forbearance Period (as defined), but solely with respect to the specified events of default (Forbearance
Termination Event) provided under the Secured Creditor Forbearance Agreement, which includes among other things, the first date after
December 31, 2022, on which the Company’s cash falls below $1.0
million. In exchange for the forbearance and
the Third Baker Amendment, the Company agreed to adjust the aggregate principal balance of the Baker Notes to $44.2
million, which includes the delinquent interest
payments of $1.4
million that the Baker Purchasers agreed to forego
in cash, as well as an immaterial amount of legal fees incurred by the Baker Purchasers’ counsel.
On
December 19, 2022, the Company entered into the First Amendment to Forbearance Agreement (the Amendment) effective as of December 15,
2022 (the Amendment Effective Date) to amend certain provisions of the of the Secured Creditor Forbearance Agreement dated September
15, 2022. The Amendment revises the Secured Creditor Forbearance Agreement to (i) amend the Fifth Recital Clause to clarify that the
Purchasers consent to any additional indebtedness pari passu, but not senior to that of the Purchasers, in an amount not to exceed
$5.0
million, and (ii) strike and entirely replace
Section 4 to clarify the terms of the Purchasers’ consent to Interim Financing (as defined therein). No other revisions were made
to the Secured Creditor Forbearance Agreements.
On
March 7, 2023, Baker Bros. Advisors, LP (the Designated Agent) provided a Notice of Event of Default and Reservation of Rights (the Notice
of Default) relating to the Baker Bros. Purchase Agreement. The Notice of Default claims that the Company has failed to maintain the
“Required Reserve Amount” as required by the Third Baker Amendment. The Designated Agent, at the direction of the Baker Purchasers,
has accelerated repayment of the outstanding balance payable. As a result, approximately $92.7
million representing two times the sum of the
outstanding balance and all accrued and unpaid interest thereon and all other amounts due under the Baker Bros. Purchase Agreement and
other documents was due and payable within three
business days of receipt of the Notice of Default.
In addition, the Notice of Default resulted in a cross default under all outstanding debt. As of the date of the filing of this Registration
Statement the Baker Notes remain outstanding, and the Company has sufficient required reserve number of shares upon the effectuation
of the 2023 Reverse Stock Split. The failure to cure the default or otherwise settle or resolve, could have a significant negative
financial impact on the Company, could result in litigation, and could result in the assets of the company being seized, attached or
otherwise utilized to satisfy the debt.
The
Company evaluated whether any of the Embedded Features required bifurcation as a separate component of equity. The Company elected the
fair value option (FVO) under ASC 825, Financial Instruments (ASC 825), as the Baker Notes are qualified financial instruments and are,
in whole, classified as liabilities. Under the FVO, the Company recognized the hybrid debt instrument at fair value, inclusive of the
Embedded Features with changes in fair value related to changes in the Company’s credit risk being recognized as a component of
accumulated other comprehensive income in the condensed consolidated balance sheets. All other changes in fair value were recognized
in the condensed consolidated statements of operations.
For
the quarter ended March 31, 2023, using the valuation methods discussed in Note 6- Fair Value Financial Instruments, the Company recorded a gain of $15.5 million due to changes in fair value of the Baker notes, and recorded
as a component of other comprehensive income due to changes in the underlying instrument-specific credit risk for the Baker Notes. The
fair value of the Baker Notes was determined by estimating the fair value of the Market Value of Invested Capital (“MVIC”)
of the Company. This was estimated using forms of the cost and market approaches. In the Cost approach, an adjusted net asset value method
was used to determine the net recoverable value of the Company, including an estimate of the fair of the Company’s intellectual
property. The estimated fair value of the Company’s intellectual property was valued using a relief from royalty method which required
management to make significant estimates and assumptions related to forecasts of future revenue, and the selection of the royalty and
discount rates. If the resulting fair value is not estimated as greater than the contractual payout, the fair value of the Baker Notes
then becomes the Company’s MVIC available for distribution.
As
of March 31, 2023, the Baker Notes are recorded at fair value in the condensed consolidated balance sheet as short-term convertible notes
payable with a total balance of $23.8
million, and the total outstanding balance including
principal and accrued interest is $93.3
million.
Adjuvant
Notes
On
October 14, 2020, the Company entered into a Securities Purchase Agreement (the Adjuvant Purchase Agreement) with Adjuvant Global Health
Technology Fund, L.P., and Adjuvant Global Health Technology Fund DE, L.P. (together, the Adjuvant Purchasers), pursuant to which the
Company sold unsecured convertible promissory notes (the Adjuvant Notes) in aggregate principal amount of $25.0
million.
The
Adjuvant Notes have a five-year
term, and in connection with certain Company
change of control transactions, the Adjuvant Notes may be prepaid at the option of the Company or will become payable on the date of
the consummation of a change of control transaction at the option of the Adjuvant Purchasers. The Adjuvant Notes have interest accruing
at 7.5%
per annum on a quarterly basis in arrears to the outstanding balance of the Adjuvant Notes and are recognized as payment-in-kind. The
effective interest rate for the period was 7.7%.
Interest
expense for the Adjuvant Notes for the three months ended March 31, 2023 and 2022 consisted of the following (in thousands):
Schedule
of interest expense
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Coupon
interest | |
$ | 497 | | |
$ | 513 | |
| Amortization
of issuance costs | |
| 68 | | |
| 10 | |
| Total | |
$ | 565 | | |
$ | 523 | |
The
Adjuvant Notes are convertible, subject to customary 4.99%
and 19.99%
beneficial ownership limitations, into shares of the Company’s common stock, par value $0.0001
per share, at any time at the option of the Adjuvant
Purchasers at a conversion price of $6,843.75
per share. In connection with certain Company
change of control transactions, the Adjuvant Notes may be prepaid at the option of the Company or will become payable at the option of
the Adjuvant Purchasers. To the extent not previously prepaid or converted, the Adjuvant Notes were originally automatically convertible
into shares of the Company’s common stock at a conversion price of $6,843.75
per share immediately following the earliest
of the time at which the (i) 30-day
value-weighted average price of the Company’s common stock was $18,750
per share, or (ii) the Company achieved cumulative
net sales from the sales of Phexxi of $100.0
million, provided such net sales are achieved
prior to July 1, 2022.
On
April 4, 2022, the Company entered into the first amendment to the Adjuvant Purchase Agreement (the Adjuvant Amendment). The Adjuvant
Amendment extended, effective as of the next date the Company achieved the Qualified Financing Threshold upon the closing of the May
2022 Public Offering, the affirmative covenant to achieve $100.0
million in cumulative net sales of Phexxi by
June 30, 2022 to June 30, 2023. The Adjuvant Amendment also provided for an adjustment to the conversion price of the Adjuvant Notes
such that the conversion price (the Conversion Price) for these Notes, effective as of the May 2022 reverse stock split the conversion
price will now be the lesser of (i) $678.49
and (ii) 100%
of the lowest price per share of common stock (or with respect to securities convertible into common stock, 100%
of the applicable conversion price) sold in any equity financing until the Company has met the Qualified Financing Threshold. Effective
as of the Company’s achievement of the Qualified Financing Threshold, the automatic conversion provisions in the Agreement were
further amended to provide that the Adjuvant Notes will automatically convert into shares of the Company’s common stock at the
Conversion Price immediately following the earliest of the time at which the (i) 30-day
value-weighted average price of the Company’s common stock is $18,750
per share, or (ii) the Company achieves cumulative
net sales from the sales of Phexxi of $100.0
million, provided such net sales are achieved
prior to July 1, 2023.
The
Adjuvant Notes contain various customary affirmative and negative covenants agreed to by the Company. On September 12, 2022, the Company
was in default of the Adjuvant Notes due to the default with the Baker Notes under the cross-default provision. On September 15, 2022,
the Company entered into a (i) Forbearance Agreement (the Adjuvant Forbearance Agreement) with the Adjuvant Purchasers, pursuant to which
the Adjuvant Purchasers agreed to forbear from exercising any of their rights and remedies during the Forbearance Period as defined in
therein, but solely with respect to the specified events of default provided under the Adjuvant Forbearance Agreement.
On
September 15, 2022, the Company also entered into the second amendment to the Adjuvant Purchase Agreement (the Second Adjuvant
Amendment), pursuant to which the conversion price per share was reduced to $26.25,
subject to adjustment for certain dilutive Company equity issuance adjustments for a two-year period.
In addition, the Company entered into an exchange agreement, pursuant to which the Adjuvant Purchasers agreed to exchange 10%
of the outstanding amount of the Adjuvant Notes as of September 15, 2022 (or $2.9 million)
for rights to receive 109,842 shares
of common stock (Adjuvant Purchase Rights). The number of shares for each Adjuvant Purchase Right is initially fixed, but is subject
to certain customary adjustments, and, until the second anniversary of issuance, adjustments for certain dilutive Company equity
issuances. Refer to Note 8- Stockholders’ Deficit for discussion
regarding additional issuances of Purchase Rights under this provision. The Adjuvant Purchase Rights expire on June 28, 2027 and do
not have an exercise price per share and, therefore, will not result in cash proceeds to the Company. As of March 31, 2023, all
Adjuvant Purchase Rights remain outstanding. The conversion price of the Adjuvant Notes were further reset to $1.625 per
share with the February 2023 Notes issuance, as discussed below. Subsequent to March 31, 2023, the conversion price adjusted to
$0.8125,
as discussed in Note 10– Subsequent Events.
The
Adjuvant Notes are accounted for in accordance with authoritative guidance for convertible debt instruments and are classified as current
liabilities in the condensed consolidated balance sheet. The aggregate proceeds of $25.0
million was initially classified as restricted
cash for financial reporting purposes due to contractual stipulations that specify the types of expenses the money can be spent on and
how it must be allocated. Its conversion feature is required to be bifurcated as an embedded derivative due to the fact that the Company
does not have sufficient number of shares reserved upon conversion as of March 31, 2023 and December 31, 2022; however, the fair value
of such feature is immaterial for both periods. As of March 31, 2023 and December 31, 2022, $0.9
million in proceeds remained, which are included
in restricted cash on the condensed consolidated balance sheets. See Note 7- Fair Value of Financial Instruments for a description of
the accounting treatment for the Adjuvant Purchase Rights.
Due
to the execution of the Adjuvant Forbearance and the Second Adjuvant Amendment, the Company reviewed the Adjuvant Notes in accordance
with Topics ASC 470-50 – Modifications and Extinguishments and ASC 470-60 – Troubled Debt Restructurings by Debtors.
The Company concluded that although changes in the structure of the debt met certain qualitative factors to qualify as a troubled
debt restructuring (TDR), the effective interest rate post changes was greater than the original effective interest rate and, therefore,
failed the quantitative test to be a TDR. The Adjuvant Notes were evaluated in accordance with ASC 470-50 and were determined to have
failed certain qualitative factors to qualify as a modification and, therefore, were accounted for as an extinguishment. The Company
removed the old debt from its books and recorded the new, revised debt and concurrently recognized a gain of approximately $2.5
million upon extinguishment, included in change
in fair value of financial instruments within the condensed consolidated statements of operations for the third quarter of 2022.
As
discussed above, on March 7, 2023, the Company received a Notice of Event of Default and Reservation of Rights (the Notice of Default)
from Baker Bros. resulting in a cross default under the all outstanding debt and as such, the Company was not in compliance with all
applicable covenants as of March 31, 2023. However, upon the 2023 Reverse Stock Split effectuated on May 18, 2023, the Company
now has sufficient required reserve number of shares.
As
of March 31, 2023, the Adjuvant Notes are recorded in the condensed consolidated balance sheet as short-term convertible notes payable
with a total balance of $26.8
million. The balance is comprised of $22.3
million in principal, net of unamortized debt
issuance costs, and $4.5
million in accrued interest.
As
of March 31, 2023 and assuming the current conversion price of $1.625
per share, the Adjuvant Notes could be converted
into 16,512,880
shares of common stock.
Term
Notes
January
and March 2022 Notes
On
January 13, 2022, the Company entered into a Securities Purchase Agreement (the January 2022 Purchase Agreement) with institutional investors
(the January 2022 Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 5.0%
Senior Subordinated Notes due 2025 with an aggregate issue price of $5.9
million (the January 2022 Notes), which included
an original issue discount of $0.9
million, and (ii) warrants (the January 2022
Warrants) to purchase up to 8,003
shares of the Company’s common stock, $0.0001
par value per share. The January 2022 Warrants
have an exercise price of $735.00
per share and were initially exercisable beginning
on July 15, 2022 with a five-year
term. Pursuant to the terms of the March 2022 Purchase Agreement
(as defined below), the January 2022 Warrants became exercisable on March 1, 2022, as described in more detail below.
On
March 1, 2022, the Company entered into a Securities Purchase Agreement (the March 2022 Purchase Agreement) with institutional investors
(the March 2022 Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 5.0%
Senior Subordinated Notes due 2025 with an aggregate issue price of $7.45
million (the March 2022 Notes), which included
an original issue discount of $2.45
million, and (ii) warrants (the March 2022 Warrants)
to purchase up to 8,303
shares of the Company’s common stock, $0.0001
par value per share. The March 2022 Warrants
have an exercise price of $897.56
per share and are immediately exercisable with
a five-year term.
The
January and March 2022 Notes carried an interest rate of 5%
per annum, which was subject to increase to 18%
upon an event of default. The January and March 2022 Notes were able to be prepaid, in whole or in part, at the Company’s option
together with all accrued and unpaid interest and fees as of the date of the repayment. The holders of the January and March 2022 Notes
were able to require the Company to redeem their respective notes upon the occurrence of an event of default with a redemption premium
of 25%.
The holders of the January and March 2022 Notes were also able to require the Company to redeem their respective notes upon the occurrence
of certain subsequent transactions.
Pursuant
to the terms of the January and March 2022 Purchase Agreements, the Company agreed to certain restrictions on effecting variable rate
transactions so long as the January and March 2022 Notes were outstanding. Also, pursuant to the terms of the January and March 2022
Purchase Agreements, the January and March 2022 Purchasers had certain rights to participate in subsequent issuances of the Company’s
securities, subject to certain exceptions.
The
Company evaluated the January and March 2022 Notes to determine if any embedded components qualified as a derivative requiring bifurcation
in accordance with ASC 815. The Company determined that the embedded put option and interest rate increase feature would both require
bifurcation and separate accounting. Therefore, the Company elected to use the fair value option under ASC 825, Financial Instruments
(ASC 825) for the January and March 2022 Notes inclusive of the embedded features.
The
Company evaluated the January and March 2022 Warrants and determined that in accordance with ASC 815 the warrants should be recorded
at fair value and classified as a derivative liability in the condensed consolidated balance sheet. Both the January and March 2022 Notes
and Warrants were marked-to-market at each reporting date.
Under
the valuation methods as described in Note 6- Fair Value of Financial Instruments
the Company recorded the following in the condensed consolidated financial statements related to the January and March 2022 Notes and
Warrants during the three months ended March 31, 2022: (i) $0.2
million in notes at issuance; (ii) $10.6
million in warrants at issuance as a derivative
liability; (iii) a $0.9
million loss on issuance; and (iv) a $2.8
million gain in fair value of financial instruments
as a result of the mark-to-market adjustment on the January and March 2022 Warrants. On May 4, 2022, the January and March 2022 Notes
were exchanged pursuant to the May 2022 Exchange, as defined below, and therefore no longer outstanding since May 2022.
Interest
pertaining to the January 2022 Notes and March 2022 Notes for the three months ended March 31, 2022 was approximately $0.1
million and immaterial, respectively. Since the
Company accounts for the January and March 2022 Notes under the fair value method, the interest was included in the determination of
the fair value, and the debt issuance costs were expensed.
May
2022 Notes
On
May 4, 2022, the Company entered into amendment and exchange agreements (the May 2022 Exchange) with the holder of issued and outstanding
Series B-2 and C Preferred Stock, Seven Knots, and the January and March 2022 Notes Purchasers (collectively, the May 2022 Notes Purchasers),
pursuant to which they agreed to exchange all of the January and March 2022 Notes, 2,100
shares of Series B-2 Convertible Preferred Stock,
1,700
shares of Series C Convertible Preferred Stock,
and 4,266 shares
of the Company’s Common Stock for (i) new 5.0%
Senior Subordinated Notes with an aggregate principal amount of $22.3
million (the May 2022 Notes), (ii) 1,666
new shares of Common Stock and (iii) new warrants
to purchase up to 6,666
shares of Common Stock (the May 2022 Warrants).
The May 2022 Warrants have an exercise price of $309.56
per share and were exercisable immediately with
a five-year
term. The 2,100
shares of Series B-2 Convertible Preferred Stock,
1,700
shares of Series C Convertible Preferred Stock,
and 4,266 shares
of the Company’s Common Stock that were exchanged in the May 2022 Exchange were retired by the Company. All exchange transactions
aforementioned were cashless.
The
May 2022 Notes are substantially similar to the January and March 2022 Notes, except that (i) the maturity date of the May 2022 Notes
was August 1, 2022 and (ii) the holders of the May 2022 Notes may require the Company to redeem or exchange up to 100%
of the May 2022 Notes upon the occurrence of certain subsequent transactions (each, a Subsequent Transaction Optional Redemption). Pursuant
to the terms of the May 2022 Notes and subject to certain conditions described in the May 2022 Notes, if the Company completed an underwritten
public offering of at least $20
million complying with certain conditions (a
Qualified Underwritten Offering) and the holder of the May 2022 Notes did not participate in the Qualified Underwritten Offering, then
the holder would have forfeited their right to Subsequent Transaction Optional Redemption solely with respect to that Qualified Underwritten
Offering and amounts that may have been due pursuant to the May 2022 Notes would not have been due and payable until the three-month
anniversary of the Qualified Underwritten Offering.
The
May 2022 Public Offering qualified as the Qualified Underwritten Offering and, in connection with the May 2022 Public Offering, the holders
of the May 2022 Notes waived certain of their preemptive and redemption rights and the Company redeemed $5.9
million of the May 2022 Notes. The holders of
the May 2022 Notes also waived the maturity date of the May 2022 Notes until October 31, 2022.
The
May 2022 Notes contain various customary affirmative and negative covenants agreed to by the Company. The May 2022 Notes also include
other customary events of default, which include the suspension of trading of shares of the Company’s common stock on the Nasdaq
Capital Market for a period of more than five
trading days. On September 12, 2022, the Company
was in default of the May Notes due to the default with the Baker Notes under the cross-default provision. As a result, the interest
rate was increased to 18%
for the duration of the default and the holders of the May 2022 Notes had the right to request redemption for 125%
of the amounts then owed pursuant to the May 2022 Notes.
On
September 15, 2022, the Company entered into exchange agreements with each of the May 2022 Notes Purchasers (the May 2022 Notes Exchange
Agreements), pursuant to which the May 2022 Notes Purchasers agreed to exchange all outstanding balance of the May Notes as of September
15, 2022 using the higher interest rate and redemption premium aforementioned for purchase rights (the May Note Purchase Rights) to receive
832,237
shares of common stock. As a result, the May
Notes are no longer outstanding as of December 31, 2022. The number of right shares for each May Note Purchase Right is initially fixed,
but is subject to certain customary adjustments, and, until the second anniversary of issuance, adjustments for certain dilutive Company
equity issuances, as further discussed in Note 8- Stockholders’ Deficit and
expire on June 28, 2027. The May 2022 Notes Purchasers also waived certain anti-dilution share adjustment provisions with respect to
shares underlying the May 2022 Warrants.
The
Company evaluated the May 2022 Notes and determined that in accordance with ASC 470 the notes should be accounted for as a modification
of the January and March 2022 Notes. The Company further evaluated the May 2022 Notes to determine if any embedded components qualified
as a derivative requiring bifurcation in accordance with ASC 815. The Company determined that the embedded put options and interest rate
increase feature would all require bifurcation and separate accounting. Therefore, the Company elected to use the fair value option under
ASC 825, Financial Instruments (ASC 825) for the May 2022 Notes inclusive of the embedded features.
The
Company evaluated the May 2022 Warrants and determined that, in accordance with ASC 815, the warrants should be recorded at fair value
and classified as a derivative liability in the condensed consolidated balance sheet. Both the May 2022 Notes and Warrants are marked-to-market
at each reporting date before the exchange as described above.
December
2022 Notes
On
December 20, 2022, the Company entered into a Securities Purchase Agreement (the December 2022 Purchase Agreement), with certain investors
(the December 2022 Notes Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 8.0%
Senior Subordinated Notes due December 21, 2025 with an aggregate issue price of $2.3
million (the December 2022 Notes), which included
an original issue discount of $0.8
million (ii) warrants (the December 2022 Warrants)
to purchase up to 369,230
shares of the Company’s common stock, $0.0001
par value per share, and (iii) an aggregate 70
shares of Series D Preferred Stock (the Preferred
Shares) (collectively, the Offering). The Offering closed on December 21, 2022, with net proceeds to the Company from the Offering, after
deducting offering expenses, of $1.25
million. The December 2022 Notes are convertible
at $6.25,
and the December 2022 Warrants have a strike price of $6.25.
The
December 2022 Notes interest rate is subject to increase to 12%
upon an event of default and have no Company right to prepayment prior to maturity, however, the Company can redeem the respective notes
at a redemption premium of 32.5%.
The December 2022 Notes Purchasers can also require the Company to redeem their notes at the respective premium rate tied to the occurrence
of certain subsequent transactions, as well as require the Company to redeem the December 2022 Notes in the event of subsequent placements
(as defined). Also, pursuant to the terms of the December 2022 Purchase Agreement, the December 2022 Notes Purchasers have certain rights
to participate in subsequent issuances of the Company’s securities, subject to certain exceptions. Additionally, the December 2022
Notes conversion rate and warrant strike price are subject to adjustment upon the issuance of other securities (as defined) less than
the stated conversion rate and strike price of $6.25.
Subsequent to December 31, 2022, the conversion and strike price adjusted to $1.625
as of March 31, 2023 and then to $0.8125
in April 2023, as discussed in Note 10–
Subsequent Events.
The
Company evaluated the December 2022 Notes and December 2022 Warrants, in accordance with ASC 480 – Distinguishing Liabilities
from Equity and determined both were liability instruments. The December 2022 Notes were then evaluated in accordance the requirements
of ASC 825, Financial Instruments (ASC 825) and concluded the Company was not precluded from electing the fair value option for
the December 2022 Notes; as such the December 2022 Notes are carried at fair value in the condensed consolidated balance sheets. Since
the December 2022 Warrants are also required to be recorded as liabilities in the Company’s condensed consolidated balance sheets,
they are also carried at fair value. Both the December 2022 Notes and Warrants are marked-to-market at each reporting date with changes
in fair value of the December 2022 Notes and Warrants are recorded recognized in the condensed consolidated statement of operations,
unless the change is concluded to be related to changes in the Company’s credit rating, in which case the change will be recognized
as a component of accumulated other comprehensive income in the condensed consolidated balance sheets.
February
and March 2023 Notes
On
February 17, March 13 and March 20, 2023, the Company entered into securities purchase agreements with certain investors providing for
the sale and issuance of senior subordinate convertible notes (collectively, the February and March 2023 SPAs). The February and March
2023 SPAs included (i) convertible promissory notes with aggregate original principal amounts of approximately $1.4
million, $0.6
million, and $0.5
million, respectively (the February and March
2023 Notes), and (ii) warrants to purchase an aggregate 553,846,
240,000,
and 215,384
shares of common stock, respectively (the February
and March 2023 Warrants and collectively, the February and March 2023 Offerings). The 2023 Offerings closed on February 17, 2023 (the
February 2023 Closing), and March 13, 2023, March 20, 2023 (the March 2023 Closing), respectively, with gross proceeds to the Company,
before deducting offering expenses, of approximately $0.9
million, $0.4
million, and $0.3
million, respectively. The February and March
2023 SPAs also included a Registration Rights Agreement that require us to register the common stock underlying the February and March
2023 Notes and Warrants within the timeframes specified therein. In addition, the Company issued warrants to purchase an aggregate 99,692
and 43,200
shares of common stock in February and March
2023 Closing to the placement agent.
Upon
the April 2023 Closing as discussed in Note 10– Subsequent Events, the conversion and strike prices, as applicable, of
the Baker Notes, Baker Warrants, the May 2022 Common Warrants, the June 2022 Baker Warrants, the Adjuvant Notes, the December 2022
Notes and Warrants, and the Notes and Warrants in the February and March 2023 Closing reset to $0.8125 per
share, accordingly. Additionally, the Company’s outstanding Purchase Rights increased by approximately 15,218,227 since
March 31, 2023.
5.
Balance Sheet Details
Inventories
Inventories
consist of the following (in thousands) for the period indicated:
Schedule
of Inventories
| | |
March
31, 2023 | | |
December
31, 2022 | |
| Raw
materials | |
$ | 740 | | |
$ | 758 | |
| Work
in process(1) | |
| 2,198 | | |
| 4,142 | |
| Finished
goods | |
| 2,540 | | |
| 1,748 | |
| Total(2) | |
$ | 5,478 | | |
$ | 6,648 | |
| (1) | The
work in process balance represents all production costs incurred for partially completed
goods. |
| (2)
| A
portion of the total inventory balance as of December 31, 2022 is included in other noncurrent assets. There was none
as of March 31, 2023. |
Prepaid
and Other Current Assets
Prepaid
and other current assets consist of the following (in thousands):
Schedule
of Prepaid and Other Current Assets
| | |
March
31, 2023 | | |
December
31, 2022 | |
| Insurance | |
$ | 789 | | |
$ | 1,387 | |
| Selling
and marketing related costs | |
| — | | |
| 44 | |
| Manufacturing
related costs | |
| 57 | | |
| 82 | |
| Other | |
| 1,252 | | |
| 705 | |
| Total | |
$ | 2,098 | | |
$ | 2,218 | |
Property
and Equipment, Net
Property
and equipment, net, consists of the following (in thousands):
Schedule
of Property and Equipment net
| | |
Useful
Life | |
March
31, 2023 | | |
December
31, 2022 | |
| Research
equipment | |
5
years | |
$ | 653 | | |
$ | 653 | |
| Computer
equipment and software | |
3
years | |
| 647 | | |
| 639 | |
| Office
furniture | |
5
years | |
| 881 | | |
| 881 | |
| Leasehold
improvements | |
5
years or less | |
| 3,388 | | |
| 3,388 | |
| Construction
in-process | |
— | |
| 1,563 | | |
| 1,568 | |
| Property
and equipment gross | |
| |
| 7,132 | | |
| 7,129 | |
| Less:
accumulated depreciation | |
| |
| (3,434 | ) | |
| (3,189 | ) |
| Total,
net | |
| |
$ | 3,698 | | |
$ | 3,940 | |
Depreciation
and amortization expense for property and equipment is disclosed in the condensed consolidated statements of cash flows.
Accrued
Expenses
Accrued
expenses consist of the following (in thousands):
Schedule
of Accrued Expenses
| | |
March
31, 2023 | | |
December
31, 2022 | |
| Clinical
trial related costs | |
$ | 2,498 | | |
$ | 2,574 | |
| Selling
and marketing related costs | |
| 520 | | |
| 674 | |
| Other | |
| 436 | | |
| 876 | |
| Total | |
$ | 3,454 | | |
$ | 4,124 | |
6.
Fair Value of Financial Instruments
Fair
Value of Financial Assets
The
fair values of the Company’s assets, including the money market funds, investments in marketable fixed income debt securities classified
as cash and cash equivalents, and restricted cash measured on a recurring basis as of March 31, 2023 and December 31, 2022, respectively,
are summarized in the following tables (in thousands):
Schedule
of Fair Value of Financial Assets
| | |
March
31, 2023 | | |
December
31, 2022 | |
| | |
Quoted
Prices in Active Markets for Identical Assets (Level
1) | | |
Significant
Other Observable Inputs (Level
2) | | |
Significant
Unobservable Inputs (Level
3) | | |
Total | | |
Quoted
Prices in Active Markets for Identical Assets (Level
1) | | |
Significant
Other Observable Inputs (Level
2) | | |
Significant
Unobservable Inputs (Level
3) | | |
Total | |
| Money
market funds (1) | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 2,612 | | |
$ | — | | |
$ | — | | |
$ | 2,612 | |
| Total
assets | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 2,612 | | |
$ | — | | |
$ | — | | |
$ | 2,612 | |
| (1) | Included
as a component of cash and cash equivalents and restricted cash on the accompanying condensed
consolidated balance sheet. |
Fair
Value of Financial Liabilities
The
following tables summarize the Company’s convertible debt instruments as of March 31, 2023 and December 31, 2022, respectively
(in thousands):
Schedule
of Fair Value of Financial Liabilities
| | |
| | |
| | |
| | |
| | |
Fair
Value | |
| As
of March 31, 2023 | |
Principal
Amount | | |
Unamortized
Issuance Costs | | |
Accrued
Interest | | |
Net
Carrying Amount | | |
Amount | | |
Leveling | |
| Baker
Notes(1)(2) | |
| 93,318 | | |
| — | | |
| — | | |
| 93,318 | | |
| 23,800 | | |
| Level
3 | |
| Adjuvant
Notes(3) | |
| 22,500 | | |
| (183 | ) | |
| 4,516 | | |
| 26,833 | | |
| — | | |
| N/A | |
| December
2022 Notes(1) | |
| 2,308 | | |
| — | | |
| — | | |
| 2,308 | | |
| 3 | | |
| Level
3 | |
| February
and March 2023 Notes (1) | |
| 2,523 | | |
| — | | |
| — | | |
| 2,523 | | |
| 4 | | |
| Level
3 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
Fair
Value | |
| As
of December 31, 2022 | |
Principal
Amount | | |
Unamortized
Issuance Costs | | |
Accrued
Interest | | |
Redemption
Amount | | |
Amount
Exchanged | | |
Net
Carrying Amount | | |
Amount | | |
Leveling | |
| Baker
Notes (1) (2) | |
$ | 45,528 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 45,528 | | |
$ | 39,260 | | |
| Level
3 | |
| Adjuvant
Notes (3) (4) | |
| 22,500 | | |
| (252 | ) | |
| 4,020 | | |
| — | | |
| — | | |
| 26,268 | | |
| — | | |
| N/A | |
| May
2022 Notes (1) | |
| 16,376 | | |
| — | | |
| 1,101 | | |
| 4,369 | | |
| (21,846 | ) | |
| — | | |
| — | | |
| N/A | |
| December
2022 Notes (1) | |
| 2,308 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 2,308 | | |
| 156 | | |
| Level
3 | |
| (1) | These
liabilities are/were carried at fair value in the condensed consolidated balance sheets.
As such, the principal and accrued interest was included in the determination of fair value.
The related debt issuance costs were expensed. |
| (2) | The
Baker Notes principal amount includes $7.1
million
and $5.6
million
of interest paid-in kind as of March 31, 2023, and December 31, 2022, respectively. |
| (3) | The
Adjuvant Notes are recorded in the condensed consolidated balance sheets at their net carrying
amount which includes principal and accrued interest, net of unamortized issuance costs. |
| (4) | The
principal amount and accrued interest of the Adjuvant Notes are net of the 10%
reduction in principal and interest of $2.5
million
and $0.4
million,
respectively, received in exchange for the issuance of Purchase Rights. |
The
following tables summarize the Company’s derivative liabilities as of March 31, 2023 and December 31, 2022 as discussed in Note
10- Stockholders’ Equity (Deficit) (in thousands):
Schedule
of Fair Value of Financial Liabilities
| | |
Fair
Value | |
| | |
March
31, 2023 (1) | | |
December
31, 2022 | | |
Leveling | |
| April
and June 2020 Baker Warrants | |
$ | — | | |
$ | 1 | | |
| Level
3 | |
| May
2022 Public Offering Warrant | |
| 6 | | |
| 303 | | |
| Level
3 | |
| June
2022 Baker Warrants | |
| 3 | | |
| 170 | | |
| Level
3 | |
| December
2022 Warrants | |
| 1 | | |
| 107 | | |
| Level
3 | |
| February
and March 2023 Warrants | |
| 6 | | |
| — | | |
| Level
3 | |
| Purchase
Rights | |
| 106 | | |
| 1,095 | | |
| Level
3 | |
| Total
Derivative Liabilities | |
$ | 122 | | |
$ | 1,676 | | |
| | |
| (1) |
As
of March 31, 2023, all warrants issued by the Company are subject to liability accounting due to potential settlement in cash, an insufficient
number of authorized shares and other adjustment mechanics. However, warrants with an exercise price greater than $2.50
per share were considered to be significantly
out of the money as of March 31, 2023 and therefore the value ascribed to those warrants was considered to be de minimus and
is therefore excluded from the above table. |
Change
in Fair Value of Level 3 Financial Liabilities
The
following table summarizes the changes in Level 3 financial liabilities related to Term Notes, Baker Notes and December 2022 Notes, and
February and March 2023 Notes measured at fair value on a recurring basis for the three months ended March 31, 2023 and 2022 (in thousands).
Schedule
of Change in Fair Value of Level 3 Financial Liabilities
| | |
Baker
First Closing Notes | | |
Baker
Second Closing Notes | | |
December
2022 Notes | | |
February
and March 2023 Notes | |
| Balance
at December 31, 2022 | |
$ | 23,556 | | |
$ | 15,704 | | |
$ | 156 | | |
$ | — | |
| Balance
at issuance | |
| — | | |
| — | | |
| — | | |
| 12 | |
| Exercises | |
| - | | |
| - | | |
| - | | |
| - | |
| Change
in fair value presented in the Condensed Consolidated Statements of Operations | |
| — | | |
| — | | |
| (153 | ) | |
| (8 | ) |
| Change
in fair value presented in the Condensed Consolidated Statements of Comprehensive Operations | |
| (9,276 | ) | |
| (6,184 | ) | |
| — | | |
| — | |
| Conversion
of series B-2 convertible preferred stock | |
| - | | |
| - | | |
| - | | |
| - | |
| Balance
at March 31, 2023 | |
$ | 14,280 | | |
$ | 9,520 | | |
$ | 3 | | |
$ | 4 | |
| | |
Term
Notes – January 2022 Notes | | |
Term
Notes – March 2022 Notes | | |
Term
Notes Total | | |
Baker
First Closing Notes | | |
Baker
Second Closing Notes | | |
Baker
Notes Total | |
| Balance
at December 31, 2021 | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 49,030 | | |
$ | 32,687 | | |
$ | 81,717 | |
| Balance
at issuance | |
| 116 | | |
| 149 | | |
| 265 | | |
| — | | |
| — | | |
| — | |
| Change
in fair value presented in the Condensed Consolidated Statements of Operations | |
| 2 | | |
| — | | |
| 2 | | |
| 2,732 | | |
| 1,821 | | |
| 4,553 | |
| Change
in fair value presented in the Condensed Consolidated Statements of Comprehensive Operations | |
| — | | |
| — | | |
| — | | |
| (109 | ) | |
| (72 | ) | |
| (181 | ) |
| Conversion
of series B-2 convertible preferred stock | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Balance
at March 31, 2022 | |
$ | 118 | | |
$ | 149 | | |
$ | 267 | | |
$ | 51,653 | | |
$ | 34,436 | | |
$ | 86,089 | |
| | |
Derivative
Liabilities Previously Classified as Equity Instruments | | |
May
2022 Public Offering Common Warrants | | |
June 2022
Baker Warrants | | |
December
2022 Warrants | | |
February
and March 2023 Warrants | | |
Purchase
Rights | | |
Derivative
Liabilities Total | |
| Balance
at December 31, 2022 | |
$ | 1 | | |
$ | 303 | | |
$ | 170 | | |
$ | 107 | | |
$ | — | | |
$ | 1,095 | | |
$ | 1,676 | |
| Balance
at issuance | |
| — | | |
| — | | |
| — | | |
| — | | |
| 6 | | |
| 77 | | |
| 83 | |
| Exercises | |
| — | | |
| (6 | ) | |
| — | | |
| — | | |
| — | | |
| (180 | ) | |
| (186 | ) |
| Change
in fair value presented in the condensed consolidated statements of operations | |
| (1 | ) | |
| (291 | ) | |
| (167 | ) | |
| (106 | ) | |
| — | | |
| (886 | ) | |
| (1,451 | ) |
| Change
in fair value presented in the Condensed Consolidated Statements of Comprehensive Operations | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| March
31, 2023 | |
$ | — | | |
$ | 6 | | |
$ | 3 | | |
$ | 1 | | |
$ | 6 | | |
$ | 106 | | |
$ | 122 | |
| | |
Derivative
Liability – Convertible Preferred Stock Conversion Feature | | |
Derivative
Liability – January 2022 Warrants | | |
Derivative
Liability – March 2022 Warrants | | |
Derivative
Liabilities Total | |
| Balance
at December 31, 2021 | |
$ | 202 | | |
$ | — | | |
$ | — | | |
$ | 202 | |
| Balance
at issuance | |
| — | | |
| 4,562 | | |
| 6,025 | | |
| 10,587 | |
| Change
in fair value presented in the Condensed Consolidated Statements of Operations | |
| (83 | ) | |
| (705 | ) | |
| (2,132 | ) | |
| (2,920 | ) |
| Change
in fair value presented in the Condensed Consolidated Statements of Comprehensive Operations | |
| — | | |
| — | | |
| — | | |
| — | |
| Conversion
of series B-2 convertible preferred stock | |
| (27 | ) | |
| — | | |
| — | | |
| (27 | ) |
| Balance
at March 31, 2022 | |
$ | 92 | | |
$ | 3,857 | | |
$ | 3,893 | | |
$ | 7,842 | |
Valuation
Methodology
Through
June 30, 2022, the fair value of the Baker Notes issued, and the change in fair value of the Baker Notes at the reporting date, were
determined using a Monte Carlo simulation-based model. The Monte Carlo simulation was used to take into account several embedded features
and factors, including the future value of our common stock, a potential change of control event, the probability of meeting certain
debt covenants, the maturity term of the Baker Notes, the probability of an event of voluntary conversion of the Baker Notes, the probability
of the failure to meet the affirmative covenant to achieve $100.0
million in cumulative net sales of Phexxi by
June 30, 2023, and the probability of exercise of the put right and the probability of exercise of our call right.
The
fair value of the Baker Notes are subject to uncertainty due to the assumptions that are used in the Monte Carlo simulation-based model.
These factors include but are not limited to the future value of the Company’s common stock, the probability and timing of a potential
change of control event, the probability of meeting certain debt covenants, the probability of an event of voluntary conversion of the
Baker Notes, exercise of the put right, and exercise of the Company’s call right. The fair value of the Baker Notes is sensitive
to these estimated inputs made by management that are used in the calculation.
Since
the third quarter of 2022, the fair value of the Baker Notes issued as described in Note 4- Debt, and subsequent changes in fair
value recorded at each reporting date, was determined by estimating the fair value of the Market Value of Invested Capital (“MVIC”)
of the Company. This was estimated using forms of the cost and market approaches. In the Cost approach, an adjusted net asset value method
was used to determine the net recoverable value of the Company, including an estimate of the fair of the Company’s intellectual
property. The estimated fair value of the Company’s intellectual property was valued using a relief from royalty method which required
management to make significant estimates and assumptions related to forecasts of future revenue, and the selection of the royalty (3.5%)
and discount (19.0%)
rates. The guideline public company method served as another valuation indicator. In this form of the Market approach, comparable market
revenue multiples were elected and applied to the Company’s forward revenue forecast to ultimately derive a MVIC indication. If
the resulting fair value from these approaches is not estimated as greater than the contractual payout, the fair value of the Baker Notes
then becomes only the Company MVIC available for distribution to this first lien note holder.
January
and March 2022 Notes
The
fair value of the January and March 2022 Notes issued as described in Note 4- Debt, and subsequent changes in fair value
recorded at each reporting date, was determined using a probability weighted expected return method (PWERM) model. PWERM was used to
take into account several factors, including the future value of the Company’s common stock, a potential change of control
event, the probability of meeting certain debt covenants, the maturity term of the January and March 2022 Notes, exercise of the put
right, and exercise of the Company’s call right.
May
2022 Notes
The
fair value of the May 2022 Notes issued as described in Note 4- Debt, and subsequent changes in fair value recorded at each reporting
date, was determined using a PWERM model. PWERM was used to take into account several factors, including the future value of the Company’s
common stock, a potential change of control event, the probability of meeting certain debt covenants, the maturity term of the January
and March 2022 Notes, exercise of the put right, and exercise of the Company’s call right.
December
2022 Notes and February and March 2023 Notes
The
fair value of the December 2022 Notes and February and March 2023 Notes issued as described in Note 4- Debt, were determined
using a Black-Scholes option pricing model using typical inputs such as underlying market price of the Company’s common stock,
the conversion/strike price, time to maturity of the December 2022 Notes and February and March 2023 Notes, guideline public
company volatilities and a risk-free interest rate.
Purchase
Rights
The
Adjuvant Purchase Rights and the May Note Purchase Rights (collectively Purchase Rights) contain certain provisions that are outside
the Company’s control under which the holders can force settlement in cash; as such, the Purchase Rights are recorded as derivative
liabilities in the condensed consolidated balance sheets. The Purchase Rights are valued using an option pricing model (OPM), like a
Black-Scholes Methodology with changes in the fair value being recorded in the condensed consolidated statements of operations. The assumptions
used in the OPM are considered level 3 assumptions and include, but are not limited to, the market value of invested capital, the cumulative
equity value of the Company as a proxy for the exercise price and the expected term the Purchase Rights will be held prior to exercise
and a risk-free interest rate.
Warrants
The
warrants contain certain provisions, which are outside the Company’s control, under which the holders can force settlement in cash,
as such, the warrants are recorded as derivative liabilities in the condensed consolidated balance sheets. In accordance with ASC 815
- Derivatives and Hedging, certain warrants previously classified as equity instruments were determined to be liability classified
(the Reclassified Warrants) due to the Company having an insufficient number of authorized shares as of March 31, 2023. The Company will
continue to re-evaluate the classification of its warrants at each balance sheet to determine the proper balance sheet classification
for them. The warrants are valued using an OPM based on the applicable assumptions, which include the exercise price of the warrants,
time to expiration, expected volatility of our peer group, risk-free interest rate, and expected dividends. The assumptions used in the
OPM are considered level 3 assumptions and include, but are not limited to, the market value of invested capital, the cumulative equity
value of the Company as a proxy for the exercise price, the expected term the warrants will be held prior to exercise and a risk-free
interest rate and probability of change of control event.
7.
Commitments and Contingencies
Operating
Leases
Fleet
Lease
In
December 2019, the Company and Enterprise FM Trust (the Lessor) entered into a Master Equity Lease Agreement whereby the Company leases
vehicles to be delivered by the Lessor from time to time with various monthly costs depending on whether the vehicles are delivered for
a term of 24
or 36
months, commencing on each corresponding delivery
date. The leased vehicles are for use by eligible employees of the Company’s commercial operations personnel. As of March 31, 2023,
there was a total of 25 leased
vehicles. The Company maintains a letter of credit as collateral in favor of the Lessor, which was included in restricted cash in the
condensed consolidated balance sheet. As December 31, 2022, this letter of credit was $0.3
million, which was released by the Lessor during
the quarter ended March 31, 2023. The Company determined that the leased vehicles are accounted for as operating leases under ASC 842.
In September 2022, the Company extended the lease term for an additional 12
months for the vehicles with a term of 24
months. The Company determined that such extension
is accounted for as a modification, for which the Company reassessed the lease classification and the incremental borrowing rate on the
modification date and accounted for accordingly.
2020
Lease and the First Amendment
On
October 3, 2019, the Company entered into an office lease for approximately 24,474
square feet (the High Bluff Premises) pursuant
to a non-cancelable lease agreement (the 2020 Lease). The 2020 Lease commenced on April 1, 2020 and will expire on September 30, 2025,
unless terminated earlier in accordance with its terms. The Company has a right to extend the term of the lease for an additional five
years, although at this time the Company does
not anticipate exercising such extension. The Company provided the landlord with a $750,000
security deposit in the form of a letter of credit
for the High Bluff Premises. On April 14, 2020, the Company entered into the first amendment to the 2020 Lease for an additional 8,816
rentable square feet of the same office location
(the Expansion Premises), which commenced on September 1, 2020 and will expire on September 30, 2025. The Company provided an additional
$50,000
in a letter of credit for the Expansion Premises.
As of December 31, 2022, restricted cash maintained as collateral for the Company’s security deposit was $0.8
million.
On
March 20 2023, the Company received a notice of default from its landlord for failing to timely pay March 2023 rent, resulting in a breach
under the agreement. As a result, the Company’s letter of credit in the amount of $0.8
million, in restricted cash, was recovered by
the landlord. As of the date of the filing of the Quarterly Report we are unable to estimate the amount of damages the
landlord may seek, if any, as a result of the breech. On March 31, 2023, the Company vacated the High Bluff Premises. Subsequent to March
31, 2023, the Company reached a settlement with the landlord to release the remaining future lease payment of $5.3
million.
2022
Sublease
On
May 27, 2022, the Company entered into a sublease agreement with AMN Healthcare, Inc. (AMN), pursuant to which the Company agreed to
sublease 16,637 rentable
square feet of the High Bluff Premises to AMN for a term commencing on June 15, 2022 and ending coterminous with the 2020 Lease on September
30, 2025, in exchange for the sum of approximately $87,000
per month, subject to an annual 3.5%
increase each year. Gross sublease income was
$0.3 million
for the three months ended March 31, 2023.
Supplemental
Financial Statement Information
Schedule
of Lease Cost
| | |
| |
Three
Months Ended March 31, | |
| Lease
Cost (in thousands) | |
Classification | |
2023 | | |
2022 | |
| Operating
lease expense | |
Research
and development | |
$ | 66 | | |
$ | 86 | |
| Operating
lease expense | |
Selling
and marketing | |
| 159 | | |
| 231 | |
| Operating
lease expense | |
General
and administrative | |
| 231 | | |
| 259 | |
| Total | |
| |
$ | 456 | | |
$ | 576 | |
Schedule
of Lease Term and Discount Rate
| Lease
Term and Discount Rate | |
March
31, 2023 | | |
December
31, 2022 | |
| Weighted
Average Remaining Lease Term (in years) | |
| 2.35 | | |
| 2.68 | |
| Weighted
Average Discount Rate | |
| 12 | % | |
| 12 | % |
Schedule
of Operating Lease Maturities
| Maturity
of Operating Lease Liabilities (in thousands) | |
March
31, 2023 | |
| Remainder
of 2023 (9 months) | |
$ | 1,774 | |
| Year
ending December 31, 2024 | |
| 2,312 | |
| Year
ending December 31, 2025 | |
| 1,511 | |
| Total
lease payments | |
| 5,597 | |
| Less:
imputed interest | |
| (744 | ) |
| Total | |
$ | 4,853 | |
Schedule
of Supplement Cash Outflows in Operating Leases
| | |
| | |
| |
| | |
Three
Months Ended March 31, | |
| Other
information (in thousands) | |
2023 | | |
2022 | |
| Cash
paid for amounts included in the measurement of lease liabilities: | |
| | | |
| | |
| Operating
cash outflows in operating leases | |
$ | 610 | | |
$ | 634 | |
Other
Contractual Commitments
In
November 2019, the Company entered into a supply and manufacturing agreement with a third party to manufacture Phexxi, with potential
to manufacture other product candidates in accordance with all applicable current good manufacturing practice regulations, pursuant to
which the Company has certain minimum purchase commitments based on the forecasted product sales. The amounts purchased under the supply
and manufacturing agreement were none
for both the three months ended March 31, 2023 and 2022.
Contingencies
From
time to time the Company may be involved in various lawsuits, legal proceedings, or claims that arise in the ordinary course of business.
As
of March 31, 2023, there were no other claims or actions pending against the Company which management believes has a probable, or reasonably
possible, probability of an unfavorable outcome. However, the Company may receive trade payable demand letters from its vendors that
could lead to potential litigation. As of March 31, 2023, approximately 65%
of our trade payables were greater than 90 days
past due.
In
April 2023, the Company received a Paragraph IV certification notice letter (the “Padagis Notice Letter”) regarding an Abbreviated
New Drug Application (“ANDA”) submitted to the FDA by Padagis Israel Pharmaceuticals Inc. (“Padagis”). The ANDA seeks
approval from the FDA to commercially manufacture, use, or sell a generic version of Phexxi®
under 21 U.S.C. § 355(j) prior to the expiration of United States Patent Nos.
10,568,855; 11,337,989; and 11,439,610 listed in the FDA’s Orange Book: Approved Drug
Products with Therapeutic Equivalence Evaluations (collectively the “Phexxi Patents”). In the Padagis Notice Letter,
Padagis claims that the Phexxi Patents are invalid under various grounds.
On
June 1, 2023, the Company filed a complaint for patent infringement in Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals,
et al., in the United States District Court for the District of New Jersey. The case was assigned number 2:23-cv-03003.
The complaint alleges that Padagis’ proposed generic version of Phexxi infringes the
Phexxi Patents. The relief sought by the Company is a declaration of infringement and an
injunction of FDA approval of Padagis’ proposed generic version of Phexxi until expiration
of the Phexxi Patents in 2033. Until the earlier of final judgment or the passage
of 30 months from the receipt of the Padagis Notice Letter, the FDA is prohibited from approving Padagis’ ANDA to market its proposed
generic version of Phexxi. The Company subsequently filed a substantively identical action in the United States District Court for the
District of Delaware, Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals, et al., which was assigned number 1:23-cv-00606-UNA. The
Company is not aware of any answer or counterclaim filed by Padagis in either action against the Company at this time.
Intellectual
Property Rights
In
2014, the Company entered into an amended and restated license agreement (the Rush License Agreement) with Rush University Medical Center
(Rush University) pursuant to which Rush University granted the Company an exclusive, worldwide license of certain patents and know-how
related to its multipurpose vaginal pH modulator technology. For the U.S. patent that we licensed from Rush University, three Orders
Granting Interim Extension (OGIEs) were received from the USPTO, extending the expiration of this patent to September 2023. Pursuant
to the Rush License Agreement, the Company is obligated to pay to Rush University an earned royalty based upon a percentage of net sales
in the range of mid-single digits. In September 2020, the Company entered into the first amendment to the Rush License Agreement, pursuant
to which the Company is also obligated to pay a minimum annual royalty amount of $100,000
to the extent the earned royalties do not equal
or exceed $100,000
commencing January 1, 2021. Such royalty costs,
included in cost of goods sold, were an immaterial amount and $0.3
million amount for the three months ended March
31, 2023 and 2022, respectively. As of March 31, 2023 and December 31, 2022, approximately $0.6
million were included in accrued expenses in
the condensed consolidated balance sheets.
8.
Stockholders’ Deficit
Warrants
In
April and June 2020, pursuant to the Baker Bros. Purchase Agreement, as discussed in Note 4 – Debt, the Company issued
warrants to purchase up to 2,732
shares of common stock in a private placement
at an exercise price of $4,575
per share. The Second Baker Amendment provides
that the exercise price of the Baker Warrants will equal the conversion price of the Baker Notes. As of March 31, 2023, the exercise
price of the Baker warrants was reset to $1.625
per share and then reset to $0.8125
per share subsequent to March 31, 2023 as discussed
in Note 10– Subsequent Events.
In
February and March 2023, pursuant to the 2023 Securities Purchase Agreement as discussed in Note 4- Debt, the Company issued
warrants to purchase up to 1,152,122 shares
of the Company’s common stock at an exercise price of $2.50 per
share. Subsequent to March 31, 2023, these warrants had their strike price reset to $0.8125.
As
of March 31, 2023, warrants to purchase up to 3,180,282
shares of the Company’s common stock remain
outstanding at a weighted average exercise price of $35.70
per share. All warrants issued by the Company
are subject to liability accounting due to potential settlement in cash, an insufficient number of authorized shares and other adjustment
mechanics. However, warrants with an exercise price greater than $2.50
per share were considered to be significantly
out of the money as of March 31, 2023 and therefore the value ascribed to those warrants was considered to be de minimus. In accordance
with ASC 815 - Derivatives and Hedging, certain warrants previously classified as equity instruments were determined to be liability
classified (the Reclassified Warrants) due to the Company having an insufficient number of authorized shares as of March 31, 2023. The
Company will continue to re-evaluate the classification of its warrants at each balance sheet to determine the proper balance sheet classification
for them. The fair value of the warrants is included in derivative liabilities in the condensed consolidated balance sheets. These warrants
are summarized below:
Schedule
of Warrants
| Type
of Warrants | |
Underlying
Common Stock to be Purchased | | |
Exercise
Price | | |
Issue
Date | |
Exercise
Period | |
| Common
Warrants | |
| 4 | | |
$ | 6,918.75 | | |
June
11, 2014 | |
| June
11, 2014 to June 11, 2024 | |
| Common
Warrants | |
| 452 | | |
$ | 14,062.50 | | |
May
24, 2018 | |
| May
24, 2018 to May 24 2025 | |
| Common
Warrants | |
| — | | |
$ | 14,062.50 | | |
June
26, 2018 | |
| June
26, 2018 to June 26, 2025 | |
| Common
Warrants | |
| 888 | | |
$ | 11,962.50 | | |
April
11, 2019 | |
| October
11, 2019 to April 11, 2026 | |
| Common
Warrants | |
| 1,480 | | |
$ | 11,962.50 | | |
June
10, 2019 | |
| December
10, 2019 to June 10, 2026 | |
| Common
Warrants | |
| 1,639 | | |
$ | 1.625 | | |
April
24, 2020 | |
| April
24, 2020 to April 24, 2025 | |
| Common
Warrants | |
| 1,092 | | |
$ | 1.625 | | |
June
9, 2020 | |
| June
9, 2020 to June 9, 2025 | |
| Common
Warrants | |
| 30,582 | | |
$ | 1,875.00 | | |
May
20, 2021 | |
| May
20, 2021 to May 22, 2023 | |
| Common
Warrants | |
| 8,003 | | |
$ | 735.00 | | |
January
13, 2022 | |
| March
1, 2022 to March 1, 2027 | |
| Common
Warrants | |
| 8,303 | | |
$ | 897.56 | | |
March
1, 2022 | |
| March
1, 2022 to March 1, 2027 | |
| Common
Warrants | |
| 6,666 | | |
$ | 309.56 | | |
May
4, 2022 | |
| May
4, 2022 to May 4, 2027 | |
| Common
Warrants | |
| 1,016,935 | | |
$ | 1.625 | | |
May
24, 2022 | |
| May
24, 2022 to May 24, 2027 | |
| Common
Warrants | |
| 582,886 | | |
$ | 1.625 | | |
June
28, 2022 | |
| May
24, 2022 to June 28, 2027 | |
| Common
Warrants | |
| 369,230 | | |
$ | 1.625 | | |
December
21, 2022 | |
| December
21, 2022 to December 21, 2027 | |
| Common
Warrants | |
| 653,538 | | |
$ | 2.50 | | |
February
17, 2023 | |
| February
17, 2023 to February 17, 2028 | |
| Common
Warrants | |
| 240,000 | | |
$ | 2.50 | | |
March
13, 2023 | |
| March
13, 2023 to March 13, 2028 | |
| Common
Warrants | |
| 258,584 | | |
$ | 2.50 | | |
March
20, 2023 | |
| March
20, 2023 to March 20, 2028 | |
| Total | |
| 3,180,282 | | |
| | | |
| |
| | |
Convertible
Preferred Stock
In
October 2021, the Company issued 5,000
shares of Series B-1 Convertible Preferred Stock,
par value $0.0001
per share, at a price of $1,000.00
per share, and 5,000
shares of Series B-2 Convertible Preferred Stock,
par value $0.0001
per share, at a price of $1,000.00
per share to Keystone Capital Partners (Keystone
Capital) through a registered direct offering.
The
Series B-1 and B-2 Convertible Preferred Stock were convertible into shares of common stock at any time at a conversion price per share
of the greater of Fixed Conversion Price or Variable Conversion Price as defined. All 5,000
shares of B-1 Convertible Preferred Stock were
converted in 2021. Pursuant to the terms of the Series B-2 Convertible Preferred Stock, the Fixed Conversion Price was adjusted during
the first quarter of 2022 for certain dilutive issuances. The adjustment period ended on April 25, 2022 and the Fixed Conversion Price
was fixed at $332.50
from the sale of common stock pursuant to the
Seven Knots Purchase Agreement. During March and April 2022, Keystone Capital converted their 1,200
shares of B-2 Convertible Preferred Stock at
a conversion price of $587.50
per share into 2,347
shares of the Company’s common stock.
On
March 24, 2022, the Company, entered into an exchange agreement with the holder of its Series B-2 Convertible Preferred Stock, pursuant
to which the holder agreed to exchange 1,700
shares of the Series B-2 Convertible Preferred
Stock in consideration for 1,700
shares of the Company’s Series C Convertible
Preferred Stock, par value $0.0001
per share, $1,000.00
per share stated value. Except with respect to
voting provisions, the Series C and Series B-2 Preferred Stock had substantially similar terms.
On
May 4, 2022, pursuant to the May 2022 Exchange, the remaining 2,100
shares of Series B-2 Convertible Preferred Stock
and 1,700
shares of Series C Convertible Preferred Stock
were exchanged for Senior Subordinated Notes with an aggregate principal amount of $4.8
million and warrants to purchase up to 6,666
shares of common stock.
Effective
December 15, 2021, the Company amended and restated its certificate of incorporation, under which the Company is currently authorized
to issue up to 5,000,000 shares
of preferred stock, par value $0.0001
per share.
Nonconvertible
Preferred Stock
On
December 16, 2022, the Company filed a Certificate of Designation of Series D Non-Convertible Preferred Stock, par value $0.0001
per share (the Series D Preferred Shares). An
aggregate of 70
shares was authorized, they were not convertible
into shares of common stock, had limited voting rights equal to 1%
of the total voting power of the then-outstanding
shares of common stock entitled to vote per shares, were not entitled to dividends, and are required to be redeemed by the Company, once
its shareholders have approved a reverse split, as described in the Certificate of Designation. All 70
shares of the Series D Preferred were subsequently
issued in connection with the December 2022 Securities Purchase Agreement as discussed in Note 5- Debt. As of the date of this Registration
Statement all Series D Preferred are still outstanding. Since the Series D Preferred Shares can only be settled in cash, they are
recorded as a liability within accrued expenses in the condensed consolidated balance sheets. The amount related to the liability is
de minimus.
Common
Stock
Effective
January 17, 2018, the Company amended and restated its certificate of incorporation, under which the Company was authorized to issue
up to 300,000,000 shares
of common stock, $0.0001 par
value per share. Effective December 15, 2021, the Company further amended its amended and restated certificate of incorporation to increase
the number of authorized shares of common stock to 500,000,000
shares.
Public
Offerings
In
May 2022, the Company completed an underwritten public offering (the May 2022 Public Offering), whereby the Company issued 181,320
shares of common stock and common warrants (the
May Common Stock Warrants) to purchase 362,640
shares of common stock at a price to the public
of $93.75.
The common warrants have an exercise price of $93.75
per share, a five-year
term, and were exercisable beginning on May 24,
2022. In the May 2022 Public Offering the Company also issued pre-funded warrants to purchase 102,680
shares of common stock and common warrants to
purchase 205,360
shares of common stock at a price to the public
of $93.63.
The pre-funded warrants had an exercise price of $0.125
per share, were exercisable beginning on May
24, 2022 were fully exercised after completion of this offering. The Company received proceeds from the May 2022 Public Offering of $18.1
million, net of $5.9
million debt repayment, underwriting discounts
and offering expenses. As discussed above in Warrants, the May Common Stock Warrants were impacted by dilution
adjustments and the strike price was reset to $1.625
during the first quarter of 2023, with a further
strike price reset to $0.8125
subsequent to March 31, 2023.
Common
Stock Purchase Agreement
On
February 15, 2022, the Company entered into a common stock purchase agreement (the Stock Purchase Agreement) with Seven Knots, LLC (Seven
Knots), pursuant to which Seven Knots agreed to purchase from the Company up to $50.0
million in shares of the Company’s common
stock. Sales made to Seven Knots were at the Company’s sole discretion, and the Company controlled the timing and amount of any
and all sales. The price per share was based on the market price of the Company’s common stock at the time of sale as computed
under the Stock Purchase Agreement. As consideration for Seven Knots’ commitment to purchase shares of common stock, the Company
issued 1,025
shares of common stock to Seven Knots as commitment
fee shares, and as of March 31, 2022, issued 8,648
shares of its common stock at a weighted average
purchase price of $675.00
per share. Effective May 18, 2022, the Company
and Seven Knots elected to terminate the Stock Purchase Agreement without any penalty or additional cost to the Company.
Sales
of common stock to Seven Knots are subject to customary 4.99%
and 19.99%
beneficial ownership limitations. The Stock Purchase
Agreement had a termination date of the earliest of March 1, 2024, or when Seven Knots has purchased from the Company $50.0
million in shares of the Company’s common
stock, or as otherwise determined by the Stock Purchase Agreement at the Company’s option.
Purchase
Rights
On
September 15, 2022, the Company entered into certain exchange agreements with the Adjuvant Purchasers and the May 2022 Notes Purchasers
to exchange, upon request, the Purchase Rights for an aggregate of 942,080
shares of the Company’s common stock. The
number of right shares for each Purchase Right is initially fixed at issuance, but is subject to certain customary adjustments, and,
until the second anniversary of issuance, adjustments for certain dilutive Company equity issuances and expire on June 28, 2027. Refer
to Note 6- Fair Value of Financial Instruments for the accounting treatment of the Purchase Rights. In connection with the February and
March 2023 Notes issuance during the first quarter of 2023, the Company increased the number of outstanding Purchase Rights by 10,467,332
due to the reset of its exercise price. During
the three months ended March 31, 2023, the Company issued 718,704
shares of common stock upon the exercises of
certain Purchase Rights. As of March 31, 2023, Purchase Rights of 14,238,827
shares of the Company’s common stock remained
outstanding. Subsequent to March 31, 2023, the Purchase Rights had an additional dilution adjustment.
Common
Stock Reserved for Future Issuance
Common
stock reserved for future issuance is as follows in common equivalent shares as of March 31, 2023:
Summary
of Common Stock Reserved for Future Issuance
| Common
stock issuable upon the exercise of stock options outstanding | |
| 4,843 | |
| Common
stock issuable upon the exercise of common stock warrants | |
| 3,180,282 | |
| Common
stock available for future issuance under the 2019 ESPP | |
| 509 | |
| Common
stock available for future issuance under the Amended and Restated 2014 Plan | |
| 4,769 | |
| Common
stock available for future issuance under the Amended Inducement Plan | |
| 575 | |
| Common
stock reserved for the exercise of purchase rights | |
| 14,238,827 | |
| Common
stock reserved for the conversion of convertible notes | |
| 76,407,245 | |
| Total
common stock reserved for future issuance | |
| 93,837,050 | |
9.
Stock-based Compensation
Equity
Incentive Plans
The
following table summarizes stock-based compensation expense related to stock options, restricted stock awards (RSAs) granted to employees,
non-employee directors and consultants, and the 2019 Employee Stock Purchase Plan (the 2019 ESPP) included in the condensed consolidated
statements of operations as follows (in thousands):
Schedule
of Stock-based Compensation Expense Related to Stock Options
| | |
2023 | | |
2022 | |
| | |
Three
Months Ended March 31, | |
| | |
2023 | | |
2022 | |
| Research
and development | |
$ | 40 | | |
$ | 175 | |
| Selling
and marketing | |
| 57 | | |
| 163 | |
| General
and administrative | |
| 320 | | |
| 729 | |
| Total | |
$ | 417 | | |
$ | 1,067 | |
Stock
Options
There
were zero
and 1,741
shares of stock options granted during the three
months ended March 31, 2023 and 2022, respectively. As of March 31, 2023, unrecognized stock-based compensation expense for employee
stock options was approximately $2.3
million, which the Company expects to recognize
over a weighted-average remaining period of 1.9
years, assuming all unvested options become fully
vested.
Restricted
Stock Awards
There
were zero
and 1,258
shares of performance-based RSAs granted during
the three months ended March 31, 2023 and 2022, respectively, to the Company’s executive management team. The vesting conditions
for the performance-based RSAs are connected to the Company’s achievement of certain performance milestones during the current
fiscal year.
For
the performance-based RSAs, (i) the fair value of the award is determined on the grant date; (ii) the Company assesses the probability
of achieving each individual milestone associated with the award using reasonable assumptions based on the Company’s operation
performance towards each milestone; (iii) the fair value of the shares subject to the milestone is expensed over the implicit service
period commencing once management believes the performance criteria is probable of being met; and (iv) the Company reassesses the probability
of achieving each individual milestone at each reporting date, and any change in estimate is accounted for through a cumulative adjustment
in the period when the change in estimate occurs. The non-performance based RSAs are valued at the fair value on the grant date and the
associated expenses will be recognized over the vesting period.
Employee
Stock Purchase Plan
The
purchase price under the 2019 ESPP is 85%
of the lesser of the fair market value of the
common stock on the first or the last business day of an offering period. The maximum number of shares of common stock that may be purchased
by any participant during an offering period is equal to $25,000
divided by the fair market value of the common
stock on the first business day of an offering period. In October 2022, the Board terminated the offering period ending December 15,
2022, refunded all employee contributions, and suspended future offering periods.
During
the three months ended March 31, 2023 and 2022, there were no
shares of common stock purchased under the 2019
ESPP.
The
fair market value of shares to be issued to employees under the 2019 ESPP is estimated using a Black-Scholes option-pricing model at
the grant date, which requires the use of subjective and complex assumptions, including (i) the expected stock price volatility, (ii)
the calculation of the expected term of the award, (iii) the risk-free interest rate and (iv) the expected dividend yield. No grant date
fair value calculation was performed during the three months ended March 31, 2023 and 2022.
10.
Subsequent Events
Subsequent
events were evaluated through the filing date of this Registration Statement.
Additional
Financings
In
April 2023, the Company entered into a securities purchase agreement with certain investors providing for the sale and issuance of senior
subordinate convertible notes (the April 2023 SPA). The April 2023 SPA included (i) convertible promissory notes with aggregate original
principal amounts of approximately $0.8
million (the April 2023 Notes), and (ii) warrants
to purchase 615,384
shares of common stock (the April 2023 Warrants
and collectively, the April 2023 Offering). The April 2023 Offering closed on April 5, 2023 (the April 2023 Closing), with net proceeds
to the Company, after deducting offering expenses, of approximately $0.5
million. The April 2023 SPA also included a Registration
Rights Agreement that requires the Company to register the common stock underlying the April 2023 Notes and April 2023 Warrants within
the timeframes specified therein.
Upon
the April 2023 Closing, the conversion and strike prices, as applicable, of the Baker Notes, Baker Warrants, the May 2022 Common Warrants,
the June 2022 Baker Warrants, the Adjuvant Notes, the December 2022 Notes and Warrants, and the Notes and Warrants in the February and
March 2023 Closing reset to $0.8125
per share, accordingly. Additionally, the Company’s
outstanding Purchase Rights increased by approximately 15,218,227
since March 31, 2023.
Sales
of Long-term Assets
In
late April 2023, the Company sold office furniture with net book value of $0.4
million for an immaterial amount, which resulted
in a loss of $0.3
million recorded in the second quarter of 2023.
Settlement
of Trade Payables
Subsequent
to March 31, 2023, the Company settled a portion of its trade payables with numerous vendors, which resulted in $1.2
million reduction in trade payable being recorded
as contra expense in the second quarter of 2023.
Receipt
of Paragraph IV Certification Notice Letter
In
April 2023, the Company received a Paragraph IV certification notice letter (the “Padagis Notice Letter”) regarding an Abbreviated
New Drug Application (“ANDA”) submitted to the FDA by Padagis Israel Pharmaceuticals Inc. (“Padagis”). The ANDA seeks
approval from the FDA to commercially manufacture, use, or sell a generic version of Phexxi®
under 21 U.S.C. § 355(j) prior to the expiration of United States Patent Nos.
10,568,855; 11,337,989; and 11,439,610 listed in the FDA’s Orange Book: Approved Drug
Products with Therapeutic Equivalence Evaluations (collectively the “Phexxi Patents”). In the Padagis Notice Letter,
Padagis claims that the Phexxi Patents are invalid under various grounds.
On
June 1, 2023, the Company filed a complaint for patent infringement in Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals,
et al., in the United States District Court for the District of New Jersey. The case was assigned number 2:23-cv-03003.
The complaint alleges that Padagis’ proposed generic version of Phexxi infringes the
Phexxi Patents. The relief sought by the Company is a declaration of infringement and an
injunction of FDA approval of Padagis’ proposed generic version of Phexxi until expiration
of the Phexxi Patents in 2033. Until the earlier of final judgment or the passage
of 30 months from the receipt of the Padagis Notice Letter, the FDA is prohibited from approving Padagis’ ANDA to market its proposed
generic version of Phexxi. The Company subsequently filed a substantively identical action in the United States District Court for the
District of Delaware, Evofem Biosciences, Inc. et al. v. Padagis Israel Pharmaceuticals, et al., which was assigned number 1:23-cv-00606-UNA. The
Company is not aware of any answer or counterclaim filed by Padagis in either action against the Company at this time.
Warrants
and Purchase Rights Exercises
Subsequent
to March 31, 2023, the Company received proceeds of approximately $0.1
million from the exercise of 122,741
common warrants. There are also noncash exercise
of 458,600
Purchase Rights for an equivalent number of shares
of common stock.
2023
Reverse Stock Split
On
March 15, 2023, the Company held a Special Meeting of its Stockholders in which the stockholders approved an amendment to the Company’s
Amended and Restated Certificate of Incorporation, as amended, to effectuate a one-time reverse stock split of the outstanding shares
of the Company’s common stock, par value $0.0001
per share, at a ratio of not less than 1-for-20
and not more than 1-for-125 (the 2023 Reverse Stock Split) at any time on or prior to March 15, 2024, with the exact ratio to
be set at a whole number within such range by the Company’s board of directors.
The
2023 Reverse Stock Split became effective on May 18, 2023 upon the opening of trading on the OTCQB (the Effective Time). Trading
of the Company’s common stock on the OTCQB resumed, on a post-split adjusted basis, on May 18, 2023, under the trading symbol “EVFMD.”
At
the Effective Time, every 125 shares of the Company’s issued and outstanding common stock were automatically converted into one
share of common stock, without any change to the par value per share. In
addition, proportionate adjustments were made to the per share exercise price and the number of shares issuable upon the exercise of
all outstanding stock options and warrants to purchase shares of common stock, the number of shares issuable upon the vesting of all
RSAs, and the number of shares of common stock reserved for issuance pursuant to the Company’s equity incentive compensation plans,
convertible notes and convertible preferred stock. Any stockholder who would otherwise be entitled to a fractional share of the Company’s
common stock created as a result of the 2023 Reverse Stock Split is entitled to receive a cash payment equal to the product of
such resulting fractional interest in one share of the Company’s common stock multiplied by the closing trading price of the Company’s
common stock on the trading day immediately preceding the Effective Time. These interim condensed consolidated financial statements are
retrospectively adjusted for this 2023 Reverse Stock Split.
In
July 2023, we entered into SPAs with certain investors providing for the sale and issuance of senior secured convertible notes
due in the aggregate original principal amount of $1.5
million (the July Notes), and warrants
to purchase an aggregate 1,200,000
shares of common stock (the July 2023 Warrants)
se (collectively, the July 2023 Offering). The July 2023 Offerings closed on July 3, 2023 with net proceeds to the Company,
after deducting offering expenses, of approximately $1.0
million.
Amendments
to Bylaws
On
July 11, 2023, our board of directors adopted and approved an amendment and restatement of our Amended and Restated Bylaws. Among the
changes, i) Board Consent is no longer required to reschedule the Company’s annual meeting and ii) the proportion of shares present
required to constitute a quorum at a meeting of stockholders changed from a majority to one-third of the outstanding shares of stock
entitled to vote, as permitted under § 216 of the Delaware General Corporation Law.
Additional Financings
In August 2023,
the Company entered into SPAs with certain investors providing for the sale and issuance of senior subordinate convertible notes due
in the aggregate original principal amount of $1.0 million (the August Notes) and warrants to purchase an aggregate of 799,999 shares
of common stock (the August Warrants and collectively, the August Offering). The August Offering closed on August 4, 2023, with net proceeds
to the Company, after deducting offering expenses, of approximately $0.5 million.
In August 2023, certain
investors party to the December 2022 Notes and the February 2023 Notes exchanged $1.8
million total outstanding notes including accrued interest for 1,800
shares of Series E-1 Convertible Preferred Stock, par value $0.0001
per share. The holders of shares of Series E-1 Preferred Stock shall entitle the holder thereof to vote together with the
common shareholders as a single class and to cast that number of votes per share as is equal to the number of shares of common stock
into which it is then convertible. The Series E-1 Preferred Stock is convertible into shares of common stock at a rate of $0.40
per share subject to adjustment as provided in the Certificate of Designation (the “Conversion Rate”). Each
holder of Series E-1 Preferred Stock is entitled to receive dividends paid exclusively in the form of common stock (the “Dividends”)
payable to the holders of the Series E-1 Preferred Stock on a monthly basis.
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the stockholders and the Board of Directors of Evofem Biosciences, Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of Evofem Biosciences, Inc. and subsidiaries (the “Company”) as
of December 31, 2022 and 2021, the related consolidated statements of operations, comprehensive operations, convertible and redeemable
preferred stock and stockholders’ deficit and cash flows, for each of the two years in the period ended December 31, 2022, and
the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present
fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations
and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally
accepted in the United States of America.
Going
Concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
1 to the financial statements, the Company has suffered recurring losses, negative cash flows from operations since inception and has
received a notice of default for its convertible notes, and does not have sufficient capital to repay such obligations, which are now
currently due. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans in
regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from
the outcome of this uncertainty.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities
laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits
provide a reasonable basis for our opinion.
Critical
Audit Matters
The
critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated
or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial
statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters
does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit
matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Debt
and Fair Value of Financial Instruments — Refer to Notes 5 and 7 to the financial statements
Critical
Audit Matter Description
In
April 2020, the Company entered into a Securities Purchase and Security Agreement with certain affiliates of Baker Bros. Advisors LP,
as purchasers, pursuant to which the Company agreed to issue and sell senior secured promissory notes (the “Baker Notes”)
in an aggregate principal amount of up to $25.0 million. The Baker Notes were issued and sold in two separate closings in April and June
2020 and remain outstanding at December 31, 2022. The Company elected the fair value option under ASC 825, Financial Instruments (“ASC
825”) and recognized the hybrid debt instrument at fair value inclusive of the embedded features. The fair value of the Baker Notes
was determined by estimating the fair value of the Market Value of Invested Capital of the Company. This was estimated using forms of
the cost and market approaches. In the Cost approach, an adjusted net asset value method was used to determine the net recoverable value
of the Company, including an estimate of the fair value of the Company’s intellectual property. The estimated fair value of the
Company’s intellectual property was valued using a relief from royalty method which required management to make significant estimates
and assumptions related to forecasts of future revenue, and the selection of the royalty and discount rates. As of December 31, 2022,
the Company recorded the fair value of the Baker Notes at $39.4 million.
We
identified the Company’s estimate of the fair value for the Baker Notes as a critical audit matter due to the significant estimates
and assumptions made by management related to forecasts of future revenue, and the selection of the royalty and discount rates to determine
the fair value of the Company’s intellectual property. This required a high degree of auditor judgment and an increased extent
of effort, including the need to involve our fair value specialists, when performing audit procedures to evaluate the reasonableness
of management’s forecasts of future revenue and the selection of the royalty and discount rates for the intellectual property.
How
the Critical Audit Matter Was Addressed in the Audit
Our
audit procedures related to the Company’s determination of the fair value of the Baker Notes included the following, among others:
-
We evaluated management’s ability to accurately forecast future revenue by comparing actual revenues to management’s historical
forecasts.
-
We evaluated the reasonableness of management’s forecasts of future revenue by comparing the forecasts to (1) historical results,
(2) internal communications to management and the Board of Directors, and (3) the overall estimated market size.
-
With the assistance of fair value specialists, we evaluated the reasonableness of the royalty rates, discount rates, and multiples by
(1) testing the underlying source information and mathematical accuracy of the calculations (2) developing a range of independent estimates
and comparing those to the discount rates selected by management and (3) understanding the facts and circumstances around the selected
royalty rate.
/s/
Deloitte & Touche LLP
San
Diego, CA
April
27, 2023 (July 7, 2023, as to the effects of the reverse stock split described in Note 14)
We
have served as the Company’s auditor since 2015. In 2023 we became the predecessor auditor.
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED
BALANCE SHEETS
(In
thousands, except par value and share data)
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Assets | |
| | | |
| | |
| Current
assets: | |
| | | |
| | |
| Cash
and cash equivalents | |
$ | 2,769 | | |
$ | 7,732 | |
| Restricted
cash | |
| 1,207 | | |
| 5,056 | |
| Trade
accounts receivable, net | |
| 1,126 | | |
| 6,449 | |
| Inventories | |
| 5,379 | | |
| 7,674 | |
| Prepaid
and other current assets | |
| 2,218 | | |
| 3,229 | |
| Total
current assets | |
| 12,699 | | |
| 30,140 | |
| Property
and equipment, net | |
| 3,940 | | |
| 5,774 | |
| Operating
lease right-of-use assets | |
| 4,406 | | |
| 5,395 | |
| Other
noncurrent assets | |
| 4,118 | | |
| 1,203 | |
| Total
assets | |
$ | 25,163 | | |
$ | 42,512 | |
| Liabilities,
convertible and redeemable preferred stock and stockholders’ deficit | |
| | | |
| | |
| Current
liabilities: | |
| | | |
| | |
| Accounts
payable | |
$ | 14,984 | | |
$ | 10,316 | |
| Convertible
notes payable - carried at fair value (Note 5) | |
| 39,416 | | |
| 81,717 | |
| Convertible
notes payable - Adjuvant (Note 5) | |
| 26,268 | | |
| 27,209 | |
| Accrued
expenses | |
| 4,124 | | |
| 8,370 | |
| Accrued
compensation | |
| 2,175 | | |
| 4,653 | |
| Operating
lease liabilities – current | |
| 2,311 | | |
| 2,332 | |
| Derivative
liabilities | |
| 1,676 | | |
| 202 | |
| Other
current liabilities | |
| 2,876 | | |
| 2,864 | |
| Total
current liabilities | |
| 93,830 | | |
| 137,663 | |
| Operating
lease liabilities – noncurrent | |
| 3,133 | | |
| 4,424 | |
| Total
liabilities | |
| 96,963 | | |
| 142,087 | |
| Commitments
and contingencies (Note 8) | |
| - | | |
| - | |
| Convertible
and redeemable preferred stock, $0.0001
par value | |
| | | |
| | |
| Series
B-1 convertible preferred stock, no
shares issued and outstanding as of December
31, 2022 and 2021 | |
| — | | |
| — | |
| Series
B-2 convertible preferred stock, no
shares and 5,000
shares issued and outstanding at December
31, 2022 and 2021, respectively | |
| — | | |
| 4,740 | |
| Convertible
and redeemable preferred stock | |
| — | | |
| 4,740 | |
| Stockholders’
deficit | |
| | | |
| | |
| Preferred
stock, $0.0001 par
value; 5,000,000 shares
authorized; no equity-classified
preferred stock issued and outstanding at December 31, 2022 and 2021 | |
| — | | |
| — | |
| Common
stock, $0.0001 par
value; 500,000,000 shares
authorized; 984,786 and
86,666 shares
issued and outstanding at December 31, 2022 and 2021, respectively | |
| — | | |
| — | |
| Additional
paid-in capital | |
| 817,367 | | |
| 751,276 | |
| Accumulated
other comprehensive income | |
| 49,527 | | |
| 5,089 | |
| Accumulated
deficit | |
| (938,694 | ) | |
| (860,680 | ) |
| Total
stockholders’ deficit | |
| (71,800 | ) | |
| (104,315 | ) |
| Total
liabilities, convertible and redeemable preferred stock and stockholders’ deficit | |
$ | 25,163 | | |
$ | 42,512 | |
See
accompanying notes to the consolidated financial statements.
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF OPERATIONS
(In
thousands, except share and per share data)
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Product
sales, net | |
$ | 16,837 | | |
$ | 8,244 | |
| | |
| | | |
| | |
| Operating
expenses: | |
| | | |
| | |
| Cost
of goods sold | |
| 4,415 | | |
| 4,055 | |
| Research
and development | |
| 25,032 | | |
| 33,129 | |
| Selling
and marketing | |
| 43,951 | | |
| 113,152 | |
| General
and administrative | |
| 27,563 | | |
| 24,709 | |
| Total
operating expenses | |
| 100,961 | | |
| 175,045 | |
| Loss
from operations | |
| (84,124 | ) | |
| (166,801 | ) |
| Other
income (expense): | |
| | | |
| | |
| Interest
income | |
| 85 | | |
| 15 | |
| Other
expense | |
| (2,087 | ) | |
| (4,732 | ) |
| Gain
on issuance of financial instruments, net | |
| (72,993 | ) | |
| — | |
| Change
in fair value of financial instruments | |
| 82,465 | | |
| (33,657 | ) |
| Total
other income (expense), net | |
| 7,470 | | |
| (38,374 | ) |
| Loss
before income tax | |
| (76,654 | ) | |
| (205,175 | ) |
| Income
tax expense | |
| (44 | ) | |
| (17 | ) |
| Net
loss | |
| (76,698 | ) | |
| (205,192 | ) |
| Series
B-1 and B-2 convertible preferred stock deemed dividends | |
| (1,316 | ) | |
| (1,047 | ) |
| Net
loss attributable to common stockholders | |
$ | (78,014 | ) | |
$ | (206,239 | ) |
| Net
loss per share attributable to common stockholders, basic and diluted | |
$ | (167.42 | ) | |
$ | (2,954.20 | ) |
| Weighted-average
shares used to compute net loss per share attributable to common stockholders, basic and diluted | |
| 465,967 | | |
| 69,812 | |
See
accompanying notes to the consolidated financial statements.
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF COMPREHENSIVE OPERATIONS
(In
thousands, except share and per share data)
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31 | |
| | |
2022 | | |
2021 | |
| Net
loss | |
$ | (76,698 | ) | |
$ | (205,192 | ) |
| Other
comprehensive income: | |
| | | |
| | |
| Change
in fair value of financial instruments attributed to credit risk change | |
| 44,438 | | |
| 5,089 | |
| Comprehensive
loss | |
$ | (32,260 | ) | |
$ | (200,103 | ) |
See
accompanying notes to consolidated financial statements.
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CONVERTIBLE AND REDEEMABLE PREFERRED STOCK
AND
STOCKHOLDERS’ EQUITY (DEFICIT)
(In
thousands, except share data)
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
|
Shares | | |
Amount | | |
Capital | | |
income | | |
Deficit | | |
(Deficit) | |
| | |
Series
B
| | |
Series
C
| | |
|
Stockholders’
Equity (Deficit) | |
| | |
Convertible
and
Redeemable
Preferred
Stock | | |
Convertible
and
Redeemable
Preferred
Stock | | |
|
Common
Stock | | |
Additional
Paid-in | | |
Accumulated
Other
Comprehensive | | |
Accumulated | | |
Total
Stockholders’
Equity | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
|
Shares | | |
Amount | | |
Capital | | |
income | | |
Deficit | | |
(Deficit) | |
| Balance
at December 31, 2020 | |
| — | | |
$ | — | | |
| — | | |
| — | | |
|
| 43,387 | | |
$ | — | | |
$ | 656,835 | | |
$ | — | | |
$ | (655,488 | ) | |
$ | 1,347 | |
| Issuance
of common stock in connection with the March 2021 and May 2021 Public Offering (see Note 10) | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 38,539 | | |
| — | | |
| 80,799 | | |
| — | | |
| — | | |
| 80,799 | |
| Issuance
of common stock - ESPP | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 245 | | |
| — | | |
| 297 | | |
| — | | |
| — | | |
| 297 | |
| Issuance
of common stock upon cash exercise of warrants | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 84 | | |
| — | | |
| 159 | | |
| — | | |
| — | | |
| 159 | |
| Issuance
of series B-1and B-2 convertible preferred stock deemed dividends | |
| 10,000 | | |
| 9,081 | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Conversion
of series B-1 convertible preferred stock | |
| (5,000 | ) | |
| (4,631 | ) | |
| — | | |
| — | | |
|
| 4,232 | | |
| — | | |
| 5,662 | | |
| — | | |
| — | | |
| 5,662 | |
| Deemed
dividends on series B-1 and B-2 convertible preferred stock | |
| — | | |
| 290 | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| (1,047 | ) | |
| — | | |
| — | | |
| (1,047 | ) |
| Restricted
stock awards issued | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 947 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Restricted
stock awards cancelled | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| (572 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Shares
withheld to cover taxes related to vesting of restricted stock awards | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| (196 | ) | |
| — | | |
| (327 | ) | |
| — | | |
| — | | |
| (327 | ) |
| Change
in fair value of financial instruments attributed to credit risk change | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| — | | |
| 5,089 | | |
| — | | |
| 5,089 | |
| Stock-based
compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| 8,898 | | |
| — | | |
| — | | |
| 8,898 | |
| Net
loss | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| — | | |
| — | | |
| (205,192 | ) | |
| (205,192 | ) |
| Balance
at December 31, 2021 | |
| 5,000 | | |
$ | 4,740 | | |
| — | | |
$ | — | | |
|
| 86,666 | | |
$ | — | | |
$ | 751,276 | | |
$ | 5,089 | | |
$ | (860,680 | ) | |
$ | (104,315 | ) |
| Beginning
balance, value | |
| 5,000 | | |
$ | 4,740 | | |
| — | | |
$ | — | | |
|
| 86,666 | | |
$ | - | | |
$ | 751,276 | | |
$ | 5,089 | | |
$ | (860,680 | ) | |
$ | (104,315 | ) |
| | |
Series
B | | |
Series
C | | |
|
Stockholders’
Equity (Deficit) | |
| | |
Convertible
and
Redeemable
Preferred
Stock | | |
Convertible
and
Redeemable
Preferred
Stock | | |
|
Common
Stock | | |
Additional
Paid-in | | |
Accumulated
Other
Comprehensive | | |
Accumulated | | |
Total
Stockholders’
Equity | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
|
Shares | | |
Amount | | |
Capital | | |
income | | |
Deficit | | |
(Deficit) | |
| Issuance
of common stock - Stock Purchase Agreement (Note 10) | |
| — | | |
$ | — | | |
| — | | |
$ | — | | |
|
| 16,739 | | |
$ | — | | |
| 7,953 | | |
$ | — | | |
$ | — | | |
| 7,953 | |
| Issuance
of common stock - May 2022 Public Offering (see Note 10) | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 181,320 | | |
| — | | |
| 1,239 | | |
| — | | |
| — | | |
| 1,239 | |
| Issuance
of common stock upon cash exercise of warrants and pre-funded warrants | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 385,198 | | |
| — | | |
| 41,932 | | |
| — | | |
| — | | |
| 41,932 | |
| Issuance
of common stock - ESPP | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 601 | | |
| — | | |
| 20 | | |
| — | | |
| — | | |
| 20 | |
| Issuance
of common stock - a360 Media | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 53,908 | | |
| — | | |
| 3,408 | | |
| — | | |
| — | | |
| 3,408 | |
| Issuance
of common stock upon noncash exercise of Purchase Rights | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 260,692 | | |
| — | | |
| 1,005 | | |
| — | | |
| — | | |
| 1,005 | |
| Conversion
of series B-2 convertible preferred stock (see Note 10) | |
| (1,200 | ) | |
| (1,143 | ) | |
| — | | |
| (72 | ) | |
|
| 2,347 | | |
| — | | |
| 1,251 | | |
| — | | |
| — | | |
| 1,251 | |
| Exchange
of series B-2 convertible preferred stock (see Note 10) | |
| (1,700 | ) | |
| (1,616 | ) | |
| 1,700 | | |
| 1,616 | | |
|
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Convertible
preferred stock deemed dividends | |
| — | | |
| 118 | | |
| — | | |
| 84 | | |
|
| — | | |
| — | | |
| (81 | ) | |
| — | | |
| — | | |
| (81 | ) |
| Restricted
stock awards issued | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| 1,258 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Restricted
stock awards cancelled | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| (1,258 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| May
2022 exchange transaction | |
| (2,100 | ) | |
| (2,099 | ) | |
| (1,700 | ) | |
| (1,628 | ) | |
|
| (2,600 | ) | |
| — | | |
| 3,655 | | |
| — | | |
| (1,316 | ) | |
| 2,339 | |
| Cash
repurchase of fractional common stock after the reverse stock split | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| (85 | ) | |
| — | | |
| (18 | ) | |
| — | | |
| — | | |
| (18 | ) |
| Issuance
of December 2022 Notes (see Note 5) | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| 1,344 | | |
| — | | |
| — | | |
| 1,344 | |
| Change
in fair value of financial instruments attributed to credit risk change | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| — | | |
| 44,438 | | |
| — | | |
| 44,438 | |
| Modification
of Baker Warrants (see Note 5) | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| 1,070 | | |
| — | | |
| — | | |
| 1,070 | |
| Stock-based
compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| 3,313 | | |
| — | | |
| — | | |
| 3,313 | |
| Net
loss | |
| — | | |
| — | | |
| — | | |
| — | | |
|
| — | | |
| — | | |
| — | | |
| — | | |
| (76,698 | ) | |
| (76,698 | ) |
| Balance
at December 31, 2022 | |
| — | | |
$ | — | | |
| — | | |
$ | — | | |
|
| 984,786 | | |
$ | — | | |
$ | 817,367 | | |
$ | 49,527 | | |
$ | (938,694 | ) | |
$ | (71,800 | ) |
| Ending
balance, value | |
| — | | |
$ | — | | |
| — | | |
$ | — | | |
|
| 984,786 | | |
$ | - | | |
$ | 817,367 | | |
$ | 49,527 | | |
$ | (938,694 | ) | |
$ | (71,800 | ) |
See
accompanying notes to the consolidated financial statements.
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CASH FLOWS
(In
thousands)
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Cash
flows from operating activities: | |
| | | |
| | |
| Net
loss | |
$ | (76,698 | ) | |
$ | (205,192 | ) |
| Adjustments
to reconcile net loss to net cash, cash equivalents and restricted cash used in operating activities: | |
| | | |
| | |
| Loss
on issuance of financial instruments | |
| 72,993 | | |
| — | |
| Change
in fair value of financial instruments | |
| (82,465 | ) | |
| 33,657 | |
| Stock-based
compensation | |
| 3,313 | | |
| 8,898 | |
| Depreciation | |
| 1,015 | | |
| 1,023 | |
| Noncash
lease expenses | |
| 1,031 | | |
| 1,404 | |
| Noncash
interest expenses | |
| 2,176 | | |
| 2,665 | |
| Noncash
inventory reserve | |
| (300 | ) | |
| 300 | |
| Noncash
instrument exchange expense | |
| 514 | | |
| — | |
| Loss
on disposal of property and equipment | |
| 926 | | |
| — | |
| Financial
instrument modification expense | |
| 1,067 | | |
| — | |
| Changes
in operating assets and liabilities: | |
| | | |
| | |
| Accounts
receivable | |
| 5,323 | | |
| (5,382 | ) |
| Inventories | |
| 1,566 | | |
| (21 | ) |
| Prepaid
and other assets | |
| 2,593 | | |
| 13,882 | |
| Accounts
payable | |
| 4,474 | | |
| (4 | ) |
| Accrued
expenses and other liabilities | |
| (4,106 | ) | |
| 5,471 | |
| Accrued
compensation | |
| (2,478 | ) | |
| (1,861 | ) |
| Operating
lease liabilities | |
| (1,354 | ) | |
| (1,507 | ) |
| Net
cash, cash equivalents and restricted cash used in operating activities | |
| (70,410 | ) | |
| (146,667 | ) |
| Cash
flows from investing activities: | |
| | | |
| | |
| Proceeds
from sale of Softcup line of business | |
| — | | |
| 250 | |
| Purchases
of property and equipment | |
| (341 | ) | |
| (2,939 | ) |
| Maturities
of short-term investments | |
| — | | |
| — | |
| Net
cash, cash equivalents and restricted cash (used in) provided by investing activities | |
| (341 | ) | |
| (2,689 | ) |
| Cash
flows from financing activities: | |
| | | |
| | |
| Proceeds
from issuance of common stock and warrants, net of discounts and commissions - public offerings | |
| 24,882 | | |
| 81,534 | |
| Proceeds
from issuance of common stock - exercise of warrants | |
| 25,211 | | |
| 159 | |
| Proceeds
from issuance of common stock, net of commissions - ATM transactions | |
| 7,438 | | |
| — | |
| Proceeds
from issuance of common stock - ESPP and exercise of stock options | |
| 20 | | |
| 297 | |
| Proceeds
from issuance of preferred stock - registered direct offering | |
| — | | |
| 10,000 | |
| Payments
under term notes | |
| (5,892 | ) | |
| — | |
| Borrowings
under convertible notes | |
| 11,500 | | |
| — | |
| Cash
repurchase of fractional common stock after the reverse stock split | |
| (18 | ) | |
| — | |
| Cash
paid for financing costs | |
| (1,202 | ) | |
| (970 | ) |
| Payments
of tax withholdings related to vesting of restricted stock awards | |
| — | | |
| (327 | ) |
| Net
cash, cash equivalents and restricted cash provided by financing activities | |
| 61,939 | | |
| 90,693 | |
| Net
change in cash, cash equivalents and restricted cash | |
| (8,812 | ) | |
| (58,663 | ) |
| Cash,
cash equivalents and restricted cash, beginning of period | |
| 13,588 | | |
| 72,251 | |
| Cash,
cash equivalents and restricted cash, end of period | |
$ | 4,776 | | |
$ | 13,588 | |
| Supplemental
cash flow information: | |
| | | |
| | |
| Cash
paid for interest | |
$ | 698 | | |
| 1,389 | |
| Cash
paid for taxes | |
$ | 26 | | |
$ | 11 | |
| Supplemental
disclosure of noncash investing and financing activities: | |
| | | |
| | |
| Right-of-use
assets obtained in exchange for operating lease liabilities | |
$ | 219 | | |
$ | — | |
| Purchases
of property and equipment included in accounts payable and accrued expenses | |
$ | 105 | | |
$ | 476 | |
| Conversion
of series B-2 and B-1, respectively, convertible preferred stock to common stock | |
$ | 1,187 | | |
$ | 1,032 | |
| Exchange
of series B-2 convertible preferred stock to series C convertible preferred stock | |
$ | 1,616 | | |
$ | — | |
| Issuance
of common stock for prepaid advertising | |
$ | 3,412 | | |
$ | — | |
| Exchange
of Adjuvant Notes for Purchase Rights | |
$ | 634 | | |
$ | — | |
| Exchange
of term notes for Purchase Rights | |
$ | 4,806 | | |
$ | — | |
| Issuance
of common stock from exercise of Purchase Rights | |
$ | 1,007 | | |
$ | — | |
See
accompanying notes to the consolidated financial statements.
EVOFEM
BIOSCIENCES, INC. AND SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
1.
Description of Business and Basis of Presentation
Description
of Business
Evofem
is a San Diego-based, commercial-stage biopharmaceutical company committed to developing and commercializing innovative products to address
unmet needs in women’s sexual and reproductive health.
The
Company’s first commercial product, Phexxi® (lactic acid, citric acid, and potassium bitartrate) vaginal gel (Phexxi),
was approved by the Food and Drug Administration (FDA) on May 22, 2020 and is the first and only FDA-approved, hormone-free, woman-controlled,
on-demand prescription contraceptive gel for women. The Company commercially launched Phexxi in September 2020.
Until
October 2022, the Company was developing EVO100 for the prevention of urogenital chlamydia and gonorrhea in women. Based on the positive,
statistically significant outcomes of a Phase 2B/3 trial (AMPREVENCE), the Company initiated a Phase 3 clinical trial (EVOGUARD) to evaluate
EVO100 for these potential indications in 2020. On October 11, 2022, the Company reported that EVOGUARD did not achieve its efficacy
endpoints. The Company has discontinued investment in this development program. We remain focused on continuing to meet the unmet contraceptive
need of millions of women with Phexxi.
Basis
of Presentation and Principles of Consolidation
The
Company prepared the consolidated financial statements in accordance with accounting principles generally accepted in the United States
(GAAP) and the rules and regulations of the Securities and Exchange Commission (SEC) related to annual reports on Form 10-K. The Company’s
financial statements are presented on a consolidated basis, which include the accounts of the Company and its wholly-owned subsidiaries.
Intercompany accounts and transactions have been eliminated in consolidation.
Risks,
Uncertainties and Going Concern
The
Company is susceptible to risks and uncertainties associated with the COVID-19 pandemic, which is affecting its employees, customers,
communities, and business operations, as well as the U.S. and global economies and financial markets.
Any
disruptions in the commercialization of Phexxi and/or its supply chain could have a material adverse effect on its business, results
of operations and financial condition. The full extent to which the ongoing COVID-19 pandemic will directly or indirectly impact the
Company’s business, results of operations and/or financial condition will depend on future developments that are highly uncertain,
including as a result of new information that may emerge concerning COVID-19, the success of ongoing COVID-19 vaccination efforts, the
emergence, prevalence and strength of variant strains, actions taken to contain or treat the disease, as well as the economic impact
on local, regional, national and international markets. The COVID-19 pandemic slowed the Company’s ability to generate product
sales of Phexxi due to reduced access to medical offices and HCPs.
The
consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and settlement
of liabilities, in the normal course of business, and does not include any adjustments to reflect the possible future effects on the
recoverability and classification of assets or amounts and classification of liabilities that may result from the outcome of this uncertainty.
The
Company’s principal operations have been related to research and development, including the development of Phexxi, and to its commercially
related sales and marketing efforts. Additional activities have included raising capital, recruiting personnel and establishing and maintaining
a corporate infrastructure to support a commercial product. The Company has incurred operating losses and negative cash flows from operating
activities since inception. As of December 31, 2022, the Company had cash and cash equivalents of $2.8
million and $0.9
million in restricted cash from the Adjuvant
Notes (as defined in Note 5- Debt) that is available for use, a working capital deficit of $81.1
million and an accumulated deficit of $938.7
million.
In
October 2022, the Company reported that EVOGUARD did not achieve its efficacy endpoints. The Company has discontinued investment in this
development program. In March 2023, the Company received a Notice of Event of Default and Reservation of Rights (the Notice of Default)
from Baker Bros claiming that the Company has failed to maintain the required shares reserved amount per the Third Baker Amendment as
defined in Note 5- Debt. In addition, the Notice of Default resulted in
a cross default under all outstanding debt.
Management’s
plans to meet its cash flow needs in the next 12 months include generating recurring product revenue, restructuring its current payables,
curing the event of default under its debt arrangements, and obtaining additional funding such as through the issuance of its capital
stock, non-dilutive financings, or through collaborations or partnerships with other companies, including license agreements for Phexxi
in foreign markets.
The
Company’s common stock began trading on the OTCQB® Venture Market (the OTCQB) of the OTC Markets Group, Inc., a centralized
electronic quotation service for over-the-counter securities, effective October 3, 2022 under the symbol “EVFM.” While the
Company’s common stock was previously listed on the Nasdaq Capital Market (Nasdaq) under the symbol “EVFM”, on August
11, 2022, it was suspended from trading on the Nasdaq due to noncompliance with the Nasdaq’s minimum bid price requirement. On
October 26, the Company’s common stock was formally delisted from Nasdaq. The delisting of the Company’s shares from Nasdaq
makes shares of the Company’s common stock less liquid and makes it more difficult for the Company to raise funds when and as needed
to fund its operations.
The
Company has recognized limited revenues since the launch of Phexxi in September 2020 and anticipates it will continue to incur net losses
for the foreseeable future. According to management estimates, liquidity resources as of December 31, 2022 are not sufficient to maintain
the Company’s cash flow needs for the twelve months from the date of issuance of these consolidated financial statements.
If
the Company is not able to obtain the required funding, through a significant increase in revenue, equity or debt financings, license
agreements for Phexxi in foreign markets, or other means, or is unable to obtain funding on terms favorable to the Company, or if the
event of default under its existing debt arrangements is not cured or there is another event of default affecting the notes payable,
there will be a material adverse effect on commercialization and development operations, seek bankruptcy protection, and the Company’s
ability to execute its strategic development plan for future growth. If the Company cannot successfully raise additional funding and
implement its strategic development plan, the Company may be forced to make further reductions in spending, including spending in connection
with its commercialization activities, extend payment terms with suppliers, liquidate assets where possible at a potentially lower amount
than as recorded in the consolidated financial statements, suspend or curtail planned operations or cease operations entirely. Any of
these could materially and adversely affect the Company’s liquidity, financial condition and business prospects, and the Company
would not be able to continue as a going concern. The Company has concluded that these circumstances and the uncertainties associated
with the Company’s ability to obtain additional equity or debt financing on terms that are favorable to the Company, or at all,
and otherwise succeed in its future operations raise substantial doubt about the Company’s ability to continue as a going concern.
2.
Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect
the amounts reported in the consolidated financial statements and the notes thereto.
Significant
estimates affecting amounts reported or disclosed in the consolidated financial statements include, but are not limited to: the assumptions
used in measuring the revenue gross-to-net variable consideration items; the trade accounts receivable credit loss reserve estimate;
the discount rate used in estimating the fair value of the lease right-of-use (ROU) assets and lease liabilities; the assumptions
used in estimating the fair value of convertible notes, warrants and purchase rights issued; the useful lives of property and equipment;
the recoverability of long-lived assets; and clinical trial accruals; the assumptions used in estimating the fair value of stock-based
compensation expense. These assumptions are more fully described in Note 3- Revenue, Note 5-
Debt, Note 7- Fair Value of Financial Instruments, Note 8- Commitments and Contingencies,
and Note 11- Stock-based Compensation. The Company bases
its estimates on historical experience and other market-specific or other relevant assumptions that it believes to be reasonable under
the circumstances and adjusts when facts and circumstances dictate. The estimates are the basis for making judgments about the carrying
values of assets, liabilities and recorded expenses that are not readily apparent from other sources. As future events and their effects
cannot be determined with precision, actual results may materially differ from those estimates or assumptions.
Segment
Reporting
Operating
segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation
by the chief operating decision-maker, the Chief Executive Officer of the Company, in making decisions regarding resource allocation
and assessing performance. The Company views its operations and manages its business in one
operating segment.
Concentrations
of Credit Risk
Financial
instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash, cash equivalents
and restricted cash. Deposits in the Company’s checking, time deposit and investment accounts are maintained in federally insured
financial institutions and are subject to federally insured limits or limits set by Securities Investor Protection Corporation. The Company
invests in funds through a major U.S. bank and is exposed to credit risk in the event of default to the extent of amounts recorded on
the consolidated balance sheets.
The
Company has not experienced any losses in such accounts and believes it is not exposed to significant concentrations of credit risk on
its cash, cash equivalents and restricted cash balances on amounts in excess of federally insured limits due to the financial position
of the depository institutions in which these deposits are held. The Company’s deposits were primarily held in Silicon Valley Bank
prior to their closure by regulators, however, the Company was subsequently able to regain full access to all its deposits and moved
these to a different financial institution.
The
Company is also subject to credit risk related to its trade accounts receivable from product sales. Its customers are located in the
United States and consist of wholesale distributors, retail pharmacies, and a mail-order specialty pharmacy. The Company extends credit
to its customers in the normal course of business after evaluating their overall financial condition and evaluates the collectability
of its accounts receivable by periodically reviewing the age of the receivables, the financial condition of its customers, and its past
collection experience. Historically, the Company has not experienced any credit losses. As of December 31, 2022, based on the evaluation
of these factors the Company did not record an allowance for doubtful accounts. Phexxi is distributed primarily through three major distributors
and a mail-order pharmacy, who receive service fees calculated as a percentage of the gross sales, and fee per units shipped, respectively.
These entities are not obligated to purchase any set number of units and distribute Phexxi on demand as orders are received. For the
years ended December 31, 2022, and 2021, the Company’s three largest customers combined made up approximately 77%
and 75%
of its gross product sales, respectively. As of December 31, 2022 and 2021, the Company’s four largest customers combined made
up 81%
and the Company’s three largest customers combined made up 75%,
respectively, of its trade accounts receivable balance.
Cash,
Cash Equivalents and Restricted Cash
Cash
and cash equivalents consist of readily available cash in checking accounts and money market funds. Restricted cash consists of cash
held in monthly time deposit accounts and letters of credit, which are collateral for the Company’s credit cards, facility leases
and fleet leases, as described in Note 8- Commitments and Contingencies. As of December 31, 2022, the Company
maintained letters of credit of $0.8
million and $0.3
million for its office lease and fleet leases,
respectively. Additionally, the remaining $0.9
million of the $25.0
million received from the issuance of Adjuvant
Notes (as defined below) in the fourth quarter of 2020 is classified as restricted cash due to the Company’s contractual obligation
to use the funds for specific purposes. Refer to Note 14 – Subsequent Events for forfeiture of the
$0.8
million letter of credit related to the office
lease.
The
following table provides a reconciliation of cash, cash equivalents and restricted cash, reported within the consolidated statements
of cash flows (in thousands):
Schedule
of Reconciliation of Cash and Restricted Cash
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Cash
and cash equivalents | |
$ | 2,769 | | |
$ | 7,732 | |
| Restricted
cash | |
| 1,207 | | |
| 5,056 | |
| Restricted
cash included in other noncurrent assets | |
| 800 | | |
| 800 | |
| Total
cash, cash equivalents and restricted cash presented in the consolidated statements of cash flows | |
$ | 4,776 | | |
$ | 13,588 | |
Trade
Accounts Receivable and Allowance
Trade
accounts receivable are amounts owed to the Company by its customers for product that has been delivered. The trade accounts receivable
are recorded at the invoice amount, less prompt pay and other discounts, chargebacks, and an allowance for credit losses, if any. The
allowance for credit losses is the Company’s estimate of losses over the life of the receivables. The Company determines the allowance
for credit losses based on its historical payment information by customer and the analysis of the trade accounts receivable balance by
customer segment. When the collectability of an invoice is no longer probable, the Company will create a reserve for that specific receivable.
If a receivable is determined to be uncollectible, it is charged against the general credit loss reserve or the reserve for the specific
receivable, if one exists.
Fair
Value of Financial Instruments
The
Company defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction
between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are
required to be recorded at fair value, the Company considers the principal or most advantageous market in which to transact and the market-based
risk. The Company applies fair value accounting for all assets and liabilities that are recognized or disclosed at fair value in the
consolidated financial statements on a recurring basis.
The
valuation of assets and liabilities are subject to fair value measurements using a three-tiered approach. Fair value measurement is classified
and disclosed by the Company in one of the following three categories:
| Level
1: |
|
Unadjusted
quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities; |
| |
|
|
| Level
2: |
|
Quoted
prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable,
either directly or indirectly, for substantially the full term of the asset or liability; |
| |
|
|
| Level
3: |
|
Prices
or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e. supported
by little or no market activity). |
The
carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, restricted cash, accounts payable, accrued
expenses and accrued compensation approximate their fair values due to their short-term nature.
The
Company believes that the Adjuvant Notes bear interest at a rate that approximates prevailing market rates for instruments with similar
characteristics and, accordingly, the carrying value of the Adjuvant Note, as defined below, approximates fair value. The Company estimates
the fair value of long-term debt utilizing an income approach. The Company uses a present value calculation to discount principal and
interest payments and the final maturity payment on these liabilities using a discounted cash flow model based on observable inputs.
The debt instrument is then discounted based on what the current market rates would be as of the reporting date. Based on the assumptions
used to value these liabilities at fair value, the debt instrument is categorized as Level 2 in the fair value hierarchy.
Inventories
Inventories,
consisting of purchased materials, direct labor and manufacturing overheads, are stated at the lower of cost, or net realizable value.
Cost is determined on a first-in, first-out basis. Net realizable value is the estimated selling price in the ordinary course of business,
less reasonably predictable costs of completion, disposal, and transportation. At each balance sheet date, the Company evaluates ending
inventories for excess quantities, obsolescence, or shelf-life expiration. The evaluation includes an analysis of the Company’s
current and future strategic plans, anticipated future sales, the price projections of future demand, and the remaining shelf life of
goods on hand. To the extent that management determines there are excess or obsolete inventory or quantities with a shelf life that is
too near its expiration for the Company to reasonably expect that it can sell those products prior to their expiration, the Company adjusts
the carrying value to estimated net realizable value in accordance with the first-in, first-out inventory costing method.
Property
and Equipment
Property
and equipment generally consist of research equipment, computer equipment and software and office furniture. Property and equipment are
recorded at cost and depreciated over the estimated useful lives of the assets (generally three
to five years) using the straight-line method. Leasehold
improvements are stated at cost and are amortized on a straight-line basis over the lesser of the remaining term of the related lease
or the estimated useful lives of the assets. Repairs and maintenance costs are charged to expense as incurred and improvements and betterments
are capitalized. When assets are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the consolidated
balance sheets and any resulting gain or loss is reflected in the consolidated statements of operations in the period realized.
Impairment
of Long-lived Assets
The
Company reviews property and equipment for impairment on an annual basis and whenever events or changes in circumstances indicate that
the carrying amount of such assets may not be recoverable. An impairment loss would be recognized when estimated future undiscounted
cash flows relating to the asset or asset group are less than its carrying amount. An impairment loss is measured as the amount by which
the carrying amount of an asset or asset group exceeds its fair value. The Company did not
recognize an impairment loss related to long-lived assets during the years ended December 31, 2022 and 2021.
Clinical
Trial Accruals
As
part of the process of preparing the financial statements, the Company is required to estimate expenses resulting from obligations under
contracts with vendors, clinical research organizations (CROs), consultants and under clinical site agreements relating to conducting
clinical trials. The financial terms of these contracts vary and may result in payment flows that do not match the periods over which
materials or services are provided under such contracts.
The
Company’s objective is to reflect the appropriate clinical trial expenses in our consolidated financial statements by recording
those expenses in the period in which services are performed and efforts are expended. The Company accounts for these expenses according
to the progress of the clinical trial as measured by patient progression and the timing of various aspects of the trial. Management determines
accrual estimates through financial models and discussions with applicable personnel and outside service providers as to the progress
of clinical trials.
During
a clinical trial, the Company adjusts the clinical expense recognition if actual results differ from its estimates. The Company makes
estimates of accrued expenses as of each balance sheet date based on the facts and circumstances known at that time. The Company’s
clinical trial accruals are partially dependent upon accurate reporting by CROs and other third-party vendors. The Company’s understanding
of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result
in reporting amounts that are too high or too low for any period.
Fair
Value of Warrants
Upon
the issuance of the warrants, they are initially measured at fair value and reviewed for the appropriate classification (liability or
equity). Warrants determined to require liability accounting are subsequently re-measured with changes in fair value being recognized
as a component of other income (expense), net in the consolidated statements of operations. Warrants are value using an option pricing
model based on the applicable assumptions, which include the exercise price of the warrants, time to expiration, expected volatility
of our peer group, risk-free interest rate, and expected dividends. The Company re-evaluates the classification of its warrants at each
balance sheet to determine the proper balance sheet classification for them. The assumptions used in the OPM are considered level 3 assumptions
and include, but are not limited to, the market value of invested capital, our cumulative equity value as a proxy for the exercise price,
the expected term the purchase rights will be held prior to exercise and a risk-free interest rate, and probability of change of control
events.
Leases
The
Company determines if an arrangement is a lease or implicitly contains a lease at inception based on the lease definition, and if the
lease is classified as an operating lease or finance lease in accordance with ASC 842, Leases (ASC 842). Operating leases are
included in operating lease ROU assets and operating lease liabilities in the Company’s consolidated balance sheets. ROU assets
represent the Company’s right to use an underlying asset for the lease term. Lease liabilities represent the Company’s obligation
to make lease payments arising from the lease. ROU assets and lease liabilities are recognized at commencement date or the Adoption Date
for existing leases based on the present value of lease payments over the lease term using an estimated discount rate.
For
leases which do not provide an implicit rate, the Company uses an incremental borrowing rate based on the information available at commencement
date or the Adoption Date in determining the present value of lease payments over a similar term. In determining the estimated incremental
borrowing rate, the Company considers a rate obtained from its primary banker for discussion purposes of a potential collateralized loan
with a term similar to the lease term; the Company’s historical borrowing capability in the market; and the Company’s costs
incurred for underwriting discounts and financing costs in its previous equity financings. For leases which have an implicit rate, the
Company uses the rate implicit in the lease to determine the present value of the lease payments. The ROU assets also include any lease
payments made and exclude lease incentives. For operating leases, lease expense is recognized on a straight-line basis over the lease
term. Lease and non-lease components within a contract are generally accounted for separately. Short-term leases of 12 months or less,
if any. are expensed as incurred which approximates the straight-line basis due to the short-term nature of the leases.
Operating
lease ROU assets and lease liabilities were $4.4 million
and $5.4 million
on December 31, 2022, respectively, and were $5.4 million
and $6.8 million
on December 31, 2021, respectively. See Note 8 - Commitments and Contingencies for more detailed
discussions on leases and financial statements information under ASC 842.
Revenue
The
Company recognizes revenue from the sale of Phexxi in accordance with ASC 606, Revenue from Contracts with Customers (ASC 606).
Revenue is recognized when the Company’s performance obligation is satisfied by transferring control of the product to a customer.
In accordance with the Company’s contracts with customers, control of the product is transferred upon the conveyance of title,
which occurs when the product is sold to and received by a customer. The amount of revenue recognized by the Company is equal to the
amount of consideration that is expected to be received from the sale of product to its customers.
An
estimate for variable consideration is made with each sale and is recorded in conjunction with the revenue being recognized. To calculate
the variable consideration, the Company uses the expected value method. If the estimated amount is payable to a customer, it is recorded
as a reduction to accounts receivable. If the estimated amount is payable to an entity other than a customer, it is recorded as a current
liability.
Research
and Development
Research
and development expenses include costs associated with the Company’s research and development activities, including, but not limited
to, payroll and personnel-related expenses, stock-based compensation expense, materials, laboratory supplies, clinical studies, and outside
services. Research and development costs are expensed as incurred, except when accounting for nonrefundable advance payments for goods
or services not yet received. These payments, if any, are capitalized at the time of payment and expensed as the related goods are delivered
or the services are performed.
Advertising
Costs
for producing advertising are expensed when incurred. Costs for communicating advertising, such as television commercial airtime and
print media space, are recorded as prepaid expenses and then expensed when the advertisement occurs.
Patent
Expenses
The
Company expenses all costs incurred relating to patent applications, including, but not limited to, direct application fees and the legal
and consulting expenses related to making such applications. Such costs are included in general and administrative expenses in the consolidated
statements of operations.
Stock-based
Compensation
Stock-based
compensation expense for stock options issued to employees, non-employee directors and consultants is measured based on estimating the
fair value of each stock option on the date of grant using the BSM option-pricing model.
The
following table summarizes the Company’s stock-based awards expensing policies for employees and non-employees:
Schedule
of Stock Option Expensing Policies
| |
|
Employees
and
Nonemployee
Consultants |
| Service
only condition |
|
Straight-line
based on the grant date fair value |
| |
|
|
| Performance
criterion is probable of being met: |
|
|
| |
|
|
| Service
criterion is complete |
|
Recognize
the grant date fair value of the award(s) once the performance criterion is considered probable of occurrence |
| |
|
|
| Service
criterion is not complete |
|
Expense
using an accelerated multiple-option approach(1) over
the remaining requisite service period |
| |
|
|
| Performance
criterion is not probable of being met and: |
|
No
expense is recognized until the performance criterion is considered probable at which point expense is recognized using an accelerated
multiple-option approach |
| (1) | The
accelerated multiple-option approach results in compensation expense being recognized for
each separately vesting tranche of the award as though the award was in substance multiple
awards and, therefore, results in accelerated expense recognition during the earlier vesting
periods. |
Fair
Value of Stock Options
The
fair value of stock options is determined using the BSM option-pricing model based on the applicable assumptions, which includes the
exercise price of warrants, time to expiration, expected volatility of our peer group, risk-free interest rate and expected dividend.
The Company records forfeitures when they occur.
Performance-based
Awards
For
performance-based RSAs (i) the fair value of the award is determined on the grant date, (ii) the Company assesses the probability of
the individual milestone under the award being achieved, and (iii) the fair value of the shares subject to the milestone is expensed
over the implicit service period commencing once management believes the performance criteria is probable of being met. If the performance-based
RSAs are modified, the Company applies the share-based payment modification accounting in accordance with ASC 718, Compensation-Stock
Compensation (ASC 718).
Income
Taxes
The
accounting guidance for uncertainty in income taxes prescribes a recognition threshold and measurement attribute criteria for the financial
statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized,
a tax position must be more likely than not to be sustained upon examination by taxing authorities based on the technical merits of the
position.
The
Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are
determined based on the difference between the financial reporting and the tax reporting basis of assets and liabilities and are measured
using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The Company provides
a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the
deferred tax assets will be realized. When the Company establishes or reduces the valuation allowance against its deferred tax assets,
its provision for income taxes will increase or decrease, respectively, in the period such determination is made.
Net
Loss per Share
Basic
net loss per common share is calculated by dividing the net loss by the weighted-average number of common shares outstanding during the
period, without consideration for potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss by
the weighted-average number of common shares and potentially dilutive securities outstanding for the period determined using the treasury-stock
and if-converted methods. For purposes of the diluted net loss per share calculation, potentially dilutive securities are excluded from
the calculation of diluted net loss per share because their effect would be anti-dilutive and therefore, basic and diluted net loss per
share were the same for all periods presented. Potentially dilutive securities excluded from the calculation of diluted net loss per
share are summarized in the table below. Common shares were calculated for the Series B-2 Convertible Preferred Stock and the convertible
debt using the if-converted method.
Schedule
of Potentially Dilutive Securities Excluded from Calculation of Diluted Net Loss Per Share
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Common
stock to be purchased under the 2019 ESPP | |
| — | | |
| 271 | |
| Options
to purchase common stock | |
| 5,672 | | |
| 5,666 | |
| Warrants
to purchase common stock | |
| 2,052,367 | | |
| 36,142 | |
| Series
B-2 convertible preferred stock | |
| — | | |
| 4,444 | |
| Purchase
rights to purchase common stock | |
| 4,490,202 | | |
| — | |
| Convertible
debt | |
| 18,105,684 | | |
| 9,537 | |
| Total | |
| 24,653,925 | | |
| 56,060 | |
| Potentially
dilutive securities (in shares) | |
| 24,653,925 | | |
| 56,060 | |
Recently
Adopted Accounting Pronouncements
In
August 2020, the FASB issued ASU No. 2020-06, Debt (ASU No. 2020-06), removing, modifying, and adding certain disclosure
requirements of ASC 470, Debt with Conversion and Other Options, and ASC 815, Derivatives and Hedging - Contracts in Entity’s
Own Equity (ASC 815). ASU No. 2020-06 will be effective for the Company beginning January 1, 2024 and early adoption is allowed.
The adoption of ASU No. 2020-06 did not have a material impact on the Company’s consolidated financial statements.
Recently
Issued Accounting Pronouncements — Not Yet Adopted
From
time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standards setting bodies
that are adopted as of the specified effective date. The Company believes the impact of recently issued standards and any issued but
not yet effective standards will not have a material impact on its consolidated financial statements upon adoption.
3.
Revenue
The
Company recognizes revenue from the sale of Phexxi in accordance with ASC 606, Revenue from Contracts with Customers (ASC 606).
The provisions of ASC 606 require the following steps to determine revenue recognition: (1) identify the contract(s) with a customer;
(2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to
the performance obligations in the contract; (5) recognize revenue when (or as) the entity satisfies a performance obligation.
In
accordance with ASC 606, the Company recognizes revenue when its performance obligation is satisfied by transferring control of the product
to a customer. In accordance with the Company’s contracts with customers, control of the product is transferred upon the conveyance
of title, which occurs when the product is sold to and received by a customer. The Company’s customers are located in the U.S.
and consist of wholesale distributors, retail pharmacies, and a mail-order specialty pharmacy. Payment terms vary by customer, but typically
range from 31
to 66
days and include prompt pay discounts. Trade
accounts receivable due to the Company from contracts with its customers are stated separately in the balance sheet, net of various allowances
as described in the Trade Accounts Receivable policy in Note 2- Summary of Significant Accounting Policies.
The
amount of revenue recognized by the Company is equal to the amount of consideration that is expected to be received from the sale of
product to its customers. Revenue is only recognized when the performance obligation is satisfied. To determine whether a significant
reversal will occur in future periods, the Company assesses both the likelihood and magnitude of any such potential reversal of revenue.
Phexxi
is sold to customers at the wholesale acquisition cost (WAC), or in some cases, at a discount to WAC. However, the Company records product
revenue, net of reserves for applicable variable consideration. These types of variable consideration reduce revenue and include the
following:
| |
● |
Distribution
services fees |
| |
● |
Prompt
pay and other discounts |
| |
● |
Product
returns |
| |
● |
Chargebacks
|
| |
● |
Rebates |
| |
● |
Patient
support programs, including our co-pay programs |
An
estimate for variable consideration is made with each sale and is recorded in conjunction with the revenue being recognized. To calculate
the variable consideration, the Company uses the expected value method. If the estimated amount is payable to a customer, it is recorded
as a reduction to accounts receivable. If the estimated amount is payable to an entity other than a customer, it is recorded as a current
liability. An estimated amount of variable consideration may differ from the actual amount. At each balance sheet date, these provisions
are analyzed and adjustments are made if necessary. Any adjustments made to these provisions would also affect net product revenue and
earnings.
In
accordance with ASC 606, the Company must make significant judgments to determine the estimate for certain variable consideration. For
example, the Company must estimate the percentage of end-users that will obtain the product through public insurance, such as Medicaid,
or through private commercial insurance. To determine these estimates, the Company relies on historical sales data showing the amount
of various end-user consumer types, inventory reports from the wholesale distributors and mail-order specialty pharmacy, and other relevant
data reports. Because Phexxi was launched in September 2020, this historical data is limited. Due to limits on historical data, the Company
has also used trend analysis, industry data, and professional judgment in developing these estimates.
The
specific considerations that the Company uses in estimating these amounts related to variable consideration are as follows:
Distribution
services fees – The Company pays distribution service fees to its wholesale distributors and mail-order specialty pharmacy.
These fees are a contractually fixed percentage of WAC and are calculated at the time of sale based on the purchase amount. The Company
considers these fees to be separate from the customer’s purchase of the product, therefore, they are recorded in other current
liabilities on the consolidated balance sheet.
Prompt
pay and other discounts – The Company incentivizes its customers to pay their invoices on time through prompt pay discounts.
These discounts are an industry standard practice, and the Company offers a prompt pay discount to each wholesale distributor and retail
pharmacy customer. The specific prompt pay terms vary by customer and are contractually fixed. Prompt pay discounts are typically taken
by the Company’s customers, so an estimate of the discount is recorded at the time of sale based on the purchase amount. Prompt
pay discount estimates are recorded as contra trade accounts receivable on the consolidated balance sheet.
The
Company may also give other discounts to its customers to incentivize purchases and promote customer loyalty. The terms of such discounts
may vary by customer. These discounts reduce gross product revenue at the time the revenue is recognized.
Chargebacks
– Certain government entities and covered entities (e.g. Veterans Administration, 340B covered entities) are able to purchase
the product at a price discounted below WAC. The difference between the government or covered entity purchase price and the wholesale
distributor purchase price of WAC will be charged back to the Company. The Company estimates the amount of each chargeback channel based
on the expected number of claims in each channel and related chargeback that is associated with the revenue being recognized for product
that remains in the distribution channel at the end of each reporting period. Estimated chargebacks are recorded as contra trade accounts
receivable on the consolidated balance sheet.
Rebates
– The Company is subject to mandatory discount obligations under the Medicaid and Tricare programs. The rebate amounts for
these programs are determined by statutory requirements or contractual arrangements. Rebates are owed after the product has been dispensed
to an end user and the Company has been invoiced. Rebates for Medicaid and Tricare are typically invoiced in arrears. The Company estimates
the amount in rebates based on the expected number of claims and related cost that is associated with the revenue being recognized for
product that remains in the distribution channel at the end of each reporting period. Rebate estimates are recorded as other current
liabilities on the consolidated balance sheet.
Patient
support programs – One type of patient support program the Company offers is a co-pay program to commercially insured patients
whose insurance requires a co-pay to be made when filling their prescription. This is a voluntary program that is intended to provide
financial assistance to patients meeting certain eligibility requirements. The Company estimates the amount of financial assistance for
these programs based on the expected number of claims and related cost that is associated with the revenue being recognized for product
that remains in the distribution channel at the end of each reporting period. Patient support programs estimates are recorded as other
current liabilities on the consolidated balance sheet.
Product
returns – Customers have the right to return product that is within six months or less of the labeled expiration date or that
is past the expiration date by no more than six months. Phexxi was commercially launched in September 2020 and there have been minimal
returns as of December 31, 2022. The Company uses historical sales and return data to estimate future product returns. Product return
estimates are recorded as other current liabilities on the consolidated balance sheet.
The
variable considerations discussed above were recorded in the consolidated balance sheet and consisted of $0.1
million in contra trade accounts receivable as
of both December 31, 2022 and 2021 and $2.6
million and $2.2
million in other current liabilities as of December
31, 2022 and 2021, respectively.
4.
Inventories
The
inventory costs include all purchased materials, direct labor and manufacturing overhead.
Inventories
consist of the following (in thousands) for the period indicated:
Schedule
of Inventories
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Raw
materials | |
$ | 758 | | |
$ | 574 | |
| Work
in process(1) | |
| 4,142 | | |
| 1,712 | |
| Finished
goods(2) | |
| 1,748 | | |
| 5,629 | |
| Total(3) | |
$ | 6,648 | | |
$ | 7,915 | |
| (1) | | The
work in process balance represents all production costs incurred for partially completed
goods, including inventory
designated for relabeling. |
| (2) | | The
finished goods balance as of December 31, 2021, includes $0.3
million
inventory reserve for estimated obsolescence and excess inventory based upon assumptions
about the future demand for Phexxi. |
| (3) | | A
portion of the total inventory balance is included in other noncurrent assets. |
5.
Debt
Convertible
Notes
Baker
Bros. Notes
On
April 23, 2020, the Company entered into a Securities Purchase and Security Agreement (the Baker Bros. Purchase Agreement) with certain
affiliates of Baker Bros. Advisors LP, as purchasers (the Baker Purchasers), and Baker Bros. Advisors LP, as designated agent, pursuant
to which the Company agreed to issue and sell to the Baker Purchasers (i) convertible senior secured promissory notes (the Baker Notes)
in an aggregate principal amount of up to $25.0
million and (ii) warrants to purchase shares
of common stock (the Baker Warrants) in a private placement.
At
the initial closing date of April 24, 2020 (the Baker Initial Closing), the Company issued and sold Baker Notes with an aggregate principal
amount of $15.0
million (the Baker First Closing Notes) and Baker
Warrants exercisable for 1,639
shares of common stock.
Following
the Baker Initial Closing, the Baker Purchasers had an option to purchase from the Company up to $10.0
million of Baker Notes (the Baker Purchase Rights)
at the Baker Purchasers’ discretion at any time prior to the Company receiving at least $100.0
million in aggregate gross proceeds from one
or more sales of equity securities.
On
June 5, 2020 (the Exercise Date), the Baker Purchasers exercised the Baker Purchase Rights. At the second closing date of June 9, 2020
(the Baker Second Closing), the Baker Purchasers acquired the remaining Baker Notes with an aggregate principal amount of $10.0
million and Baker Warrants exercisable for 1,092
shares of common stock. With the completion of
the underwritten public offering in June 2020 the exercise price of the Baker Warrants was $4,575.
The Baker Warrants have a five-year
term with a cashless exercise provision and are
immediately exercisable at any time from their respective issuance date.
The
Baker Notes have a five-year
term, with no pre-payment ability during the
first three
years. Interest on the unpaid principal balance
of the Baker Notes (the Baker Outstanding Balance) accrues at 10.0%
per annum with interest accrued during the first year from the two respective closing dates recognized as payment-in-kind. The effective
interest rate for the period was 10.0%.
Accrued interest beyond the first year of the respective closing dates are to be paid in arrears on a quarterly basis in cash or recognized
as payment-in-kind, at the direction of the Baker Purchasers. The Baker Purchasers elected to have the accrued interest for the first
quarter of 2021 paid-in-kind, and the accrued interest going forward to be paid in cash. Interest expense pertaining to the Baker Notes
for the years ended December 31, 2021 was approximately $2.8
million. As of December 31, 2021, the accrued
interest is recorded in the consolidated balance sheet in other current liabilities with a total balance of $0.7
million. As discussed below, with the amendment
to the Baker Bros. Purchase Agreement, interest payments were paid-in kind. The Company accounts for the Baker notes under the fair value
method as described below and, therefore, the interest associated with the Baker Notes is included in the fair value determination. As
of December 31, 2022, the Baker Notes could be converted into 11,207,734
shares of common stock.
The
Baker Notes are callable by the Company on 10
days’ written notice beginning on the third
anniversary of the Baker Initial Closing. The call price will equal 100%
of the Baker Outstanding Balance plus accrued and unpaid interest if the Company’s common stock as measured using a 30-day
volume weighted average price (VWAP) is greater than the benchmark price of $9,356.25
as stated in the Baker Bros. Purchase Agreement,
or 110%
of the Baker Outstanding Balance plus accrued and unpaid interest if the VWAP is less than such benchmark price. The Baker Purchasers
also have the option to require the Company to repurchase all or any portion of the Baker Notes in cash upon the occurrence of certain
events. In a repurchase event, as defined in the Baker Bros. Purchase Agreement, the repurchase price will equal 110%
of the Baker Outstanding Balance plus accrued and unpaid interest. In an event of default or the Company’s change of control, the
repurchase price will equal to the sum of (x) three times of the Baker Outstanding Balance plus (y) the aggregate value of future interest
that would have accrued (collectively, the Embedded Features of the Baker Notes), which was subsequently adjusted in an amendment to
the Baker Notes on September 15, 2022, as further described below. The Baker Notes were convertible at any time at the option of the
Baker Purchasers at the conversion price of $4,575
per share prior to the First Baker Amendment
as defined below.
On
November 20, 2021, the Company entered into the first amendment to the Baker Bros. Purchase Agreement (the First Baker Amendment), in
which each Baker Purchaser shall have the right to convert all or any portion of the Baker Notes into Common Stock at a conversion price
equal to the lesser of (a) $4,575
and (b) 115%
of the lowest price per share of common stock (or, as applicable with respect to any equity securities convertible into common stock,
115%
of the applicable conversion price) sold in one or more equity financings until the Company had met a qualified financing threshold,
defined as one or more equity financings resulting in aggregate gross proceeds to the Company of at least $50.0
million (Financing Threshold).
The
First Baker Amendment also extended, effective upon the Company’s achievement of the Financing Threshold, the affirmative covenant
to achieve $100.0
million in cumulative net sales of Phexxi by
June 30, 2022 to June 30, 2023. Additionally per the First Baker Amendment, if in any equity financing closing on or prior to the date
the Company has met the Financing Threshold, the Company was required to issue warrants to purchase capital stock of the Company (or
other similar consideration), the Company was also required to issue to the Baker Purchasers an equivalent coverage of warrants (or other
similar consideration) on the same terms as if the Baker Purchasers participated in the financing in an amount equal to the then outstanding
principal of the Baker Notes held by the Baker Purchasers. In satisfaction of this requirement and in connection with the closing of
the May 2022 Public Offering, the Company issued warrants to purchase 582,886
shares of the Company’s common stock at
an exercise price of $93.75
per share (the June 2022 Baker Warrants). As
required by the terms of the First Baker Amendment, the June 2022 Baker Warrants have substantially the same terms as the warrants issued
in the May 2022 Public Offering. Refer to Note 10 - Stockholders’ Equity (Deficit) for further information.
The exercise price of the initial Baker Warrants and the June 2022 Baker Warrants was reset to $26.25
per share along with the change of the conversion
price per the Third Baker Amendment and further reset to $4.0625
per share with the December 2022 Notes issuance,
both as discussed below.
On
March 21, 2022, the Company entered into the second amendment to the Baker Bros. Purchase Agreement (the Second Baker Amendment), pursuant
to which each Baker Purchaser now has the right to convert all or any portion of the Baker Notes into Common Stock at a conversion price
equal to the lesser of (a) $725.81
or (b) 100%
of the lowest price per share of common stock (or as applicable with respect to any equity securities convertible into common stock,
100%
of the applicable conversion price) sold in any equity financing until the Company has (i) met the qualified financing threshold by June
30, 2022, defined as a single underwritten financing resulting in aggregate gross proceeds to the Company of at least $20.0
million (Qualified Financing Threshold) and (ii)
the disclosure of its top-line results from its EVOGUARD clinical trial (the Clinical Trial Milestone) by October 31, 2022. The
Second Baker Amendment also provides that the exercise price of the Baker Warrants will equal the conversion price of the Baker Notes.
The Company met the Qualified Financing Threshold upon the closing of the May 2022 Public Offering, and as of September 30, 2022, the
conversion price and exercise price of the Baker Warrants was reset to $93.75.
The Company achieved the Clinical Trial Milestone in October 2022. Also, with the achievement of the Qualified Financing Threshold and
the Clinical Trial Milestone, the affirmative covenant to achieve $100.0
million in cumulative net sales of Phexxi was
extended to June 30, 2023. The Baker Warrants were reset to $26.25
per share per the Third Baker Amendment and further
reset to $4.0625
per share with the December 2022 Notes issuance,
both as discussed below. Subsequent to December 31, 2022, the conversion and strike price adjusted to $0.8125,
as discussed in Note 14 –
Subsequent Events.
On
September 15, 2022, the Company entered into the third amendment to the Baker Bros. Purchase Agreement (the Third Baker Amendment), pursuant
to which the conversion was amended to equal to $26.25,
subject to adjustment for certain dilutive Company equity issuance adjustments for a two-year
period, removal of an interest make-whole payment
due in certain circumstances, and certain change of control and liquidation payment amounts were reduced from three times the outstanding
amounts of the Baker Notes to two times the outstanding amounts. In addition, the Third Baker Amendment provides that the Company may
make future interest payments to the Baker Purchasers in kind or in cash, at the Company’s option. For the year ended December
31, 2022, the Company elected an in-kind interest payment and approximately $3.3
million was added to the outstanding principal
balance.
The
Company evaluated whether any of the Embedded Features required bifurcation as a separate component of equity. The Company elected the
fair value option (FVO) under ASC 825, Financial Instruments (ASC 825), as the Baker Notes are qualified financial instruments and are,
in whole, classified as liabilities. Under the FVO, the Company recognized the hybrid debt instrument at fair value, inclusive of the
Embedded Features with changes in fair value related to changes in the Company’s credit risk being recognized as a component of
accumulated other comprehensive income in the consolidated balance sheets. All other changes in fair value were recognized in the consolidated
statements of operations.
The
Baker Notes contain various customary affirmative and negative covenants agreed to by the Company, including timely payment, in cash,
of the quarterly interest payment and maintaining an active listing. On September 12, 2022, the Company received a default notice from
the Baker Purchasers due to its failure of making the required payments of accrued interest for the first and second quarters of 2022
in the aggregate amount of $1.4
million and being delisted from Nasdaq. As a
result of the cross-default provisions applicable to the Adjuvant Notes and the May 2022 Notes (both, as discussed below), the Company
was also in default of these Notes. On September 15, 2022, the Company entered into a (i) Forbearance Agreement (the Secured Creditor
Forbearance Agreement) with the Baker Purchasers, pursuant to which the Baker Purchasers agreed to forbear from exercising any of their
rights and remedies during the Forbearance Period (as defined), but solely with respect to the specified events of default (Forbearance
Termination Event) provided under the Secured Creditor Forbearance Agreement, which includes among other things, the first date after
December 31, 2022, on which the Company’s cash falls below $1.0
million. In exchange for the forbearance and
the Third Baker Amendment, the Company agreed to adjust the aggregate principal balance of the Baker Notes to $44.2
million, which includes the delinquent interest
payments of $1.4
million that the Baker Purchasers agreed to forego
in cash, as well as an immaterial amount of legal fees incurred by the Baker Purchasers’ counsel.
On
December 19, 2022, the Company entered into the First Amendment to Forbearance Agreement (the Amendment) effective as of December 15,
2022 (the Amendment Effective Date) to amend certain provisions of the of the Secured Creditor Forbearance Agreement dated September
15, 2022. The Amendment revises the Secured Creditor Forbearance Agreement to (i) amend the Fifth Recital Clause to clarify that the
Purchasers consent to any additional indebtedness pari passu, but not senior to that of the Purchasers, in an amount not to exceed
$5.0
million, and (ii) strike and entirely replace
Section 4 to clarify the terms of the Purchasers’ consent to Interim Financing (as defined therein). No other revisions were made
to the Secured Creditor Forbearance Agreements.
As
described more fully in Note 14 – Subsequent Events,
on March 7, 2023, the Company received a Notice of Event of Default and Reservation of Rights (the Notice of Default) from Baker Bros
claiming that the Company has failed to maintain the required shares reserved amount per the Third Baker Amendment. As a result of the
Notice of Default, approximately $92.7
million representing two times the sum of
the outstanding balance and all accrued and unpaid interest thereon and all other amounts due under
the SPA and other documents is due and payable within three
business days
of receipt of the Notice of Default. In addition, the Notice of Default resulted in a cross
default under all outstanding debt.
During
the year ended December 31, 2022, using the valuation methods discussed in Note 7- Fair Value of Financial Instruments,
the Company recorded a gain of $42.4
million due to changes in fair value of the Baker
Notes, of which $2.0
million is recorded in the consolidated statements
of operations as a result of mark-to-market adjustments unrelated to instrument-specific credit losses and $44.4
million, recorded as a component of other comprehensive
income due to changes in the underlying instrument-specific credit risk for the Baker Notes. Through June 30, 2023, the change in fair
value attributed to the change in the underlying instrument-specific credit risk was determined by taking the difference between the
fair value of the Baker Notes with and without the credit risk change and value of the collateral. For the second half of 2022, the fair
value of the Baker Notes was determined by estimating the fair value of the Market Value of Invested Capital (“MVIC”) of
the Company. This was estimated using forms of the cost and market approaches. In the Cost approach, an adjusted net asset value method
was used to determine the net recoverable value of the Company, including an estimate of the fair of the Company’s intellectual
property. The estimated fair value of the Company’s intellectual property was valued using a relief from royalty method which required
management to make significant estimates and assumptions related to forecasts of future revenue, and the selection of the royalty and
discount rates. If the resulting fair value is not estimated as greater than the contractual payout, the fair value of the Baker Notes
then becomes the Company’s MVIC available for distribution.
Adjuvant
Notes
On
October 14, 2020, the Company entered into a Securities Purchase Agreement (the Adjuvant Purchase Agreement) with Adjuvant Global Health
Technology Fund, L.P., and Adjuvant Global Health Technology Fund DE, L.P. (together, the Adjuvant Purchasers), pursuant to which the
Company sold unsecured convertible promissory notes (the Adjuvant Notes) in aggregate principal amount of $25.0
million.
The
Adjuvant Notes have a five-year
term, and in connection with certain Company
change of control transactions, the Adjuvant Notes may be prepaid at the option of the Company or will become payable on the date of
the consummation of a change of control transaction at the option of the Adjuvant Purchasers. The Adjuvant Notes have interest accruing
at 7.5%
per annum on a quarterly basis in arrears to the outstanding balance of the Adjuvant Notes and are recognized as payment-in-kind. The
effective interest rate for the period was 7.7%.
Interest
expense for the Adjuvant Notes consist of the following, and is included in short-term convertible notes payable on the consolidated
balance sheet as of December 31, 2022 (in thousands):
Schedule
of interest expense
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Coupon
interest | |
$ | 2,048 | | |
$ | 1,959 | |
| Amortization
of issuance costs | |
| 129 | | |
| 39 | |
| Total | |
$ | 2,177 | | |
$ | 1,998 | |
The
Adjuvant Notes are convertible, subject to customary 4.99%
and 19.99%
beneficial ownership limitations, into shares of the Company’s common stock, par value $0.0001
per share, at any time at the option of the Adjuvant
Purchasers at a conversion price of $6,843.75
per share. To the extent not previously prepaid
or converted, the Adjuvant Notes will automatically convert into shares of the Company’s common stock at a conversion price of
$6,843.75
per share. In connection with certain Company
change of control transactions, the Adjuvant Notes may be prepaid at the option of the Company or will become payable at the option of
the Adjuvant Purchasers. To the extent not previously prepaid or converted, the Adjuvant Notes were originally automatically convertible
into shares of the Company’s common stock at a conversion price of $6,843.75
per share immediately following the earliest
of the time at which the (i) 30-day
value-weighted average price of the Company’s common stock was $18,750
per share, or (ii) Company achieved cumulative
net sales from the sales of Phexxi of $100.0
million, provided such net sales are achieved
prior to July 1, 2022.
On
April 4, 2022, the Company entered into the first amendment to the Adjuvant Purchase Agreement (the Adjuvant Amendment). The Adjuvant
Amendment extended, effective as of the date the Company achieved the Qualified Financing Threshold upon the closing of the May 2022
Public Offering, the affirmative covenant to achieve $100.0
million in cumulative net sales of Phexxi by
June 30, 2022 to June 30, 2023. The Adjuvant Amendment also provided for an adjustment to the conversion price of the Adjuvant Notes
such that the conversion price (the Conversion Price) for these Notes, effective as of the reverse stock split the conversion price will
now be the lesser of (i) $678.49
and (ii) 100%
of the lowest price per share of common stock (or with respect to securities convertible into common stock, 100%
of the applicable conversion price) sold in any equity financing until the Company has met the Qualified Financing Threshold. In the
second quarter of 2022 and upon the closing of the May 2022 Public Offering, the conversion price was reset to $93.75.
Effective as of the Company’s achievement of the Qualified Financing Threshold, the automatic conversion provisions in the Agreement
were further amended to provide that the Adjuvant Notes will automatically convert into shares of the Company’s common stock at
the Conversion Price immediately following the earliest of the time at which the (i) 30-day
value-weighted average price of the Company’s common stock is $18,750
per share, or (ii) the Company achieves cumulative
net sales from the sales of Phexxi of $100.0
million, provided such net sales are achieved
prior to July 1, 2023.
The
Adjuvant Notes contain various customary affirmative and negative covenants agreed to by the Company. On September 12, 2022, the Company
was in default of the Adjuvant Notes due to the default with the Baker Notes under the cross-default provision. On September 15, 2022,
the Company entered into a (i) Forbearance Agreement (the Adjuvant Forbearance Agreement) with the Adjuvant Purchasers, pursuant to which,
the Adjuvant Purchasers agreed to forbear from exercising any of their rights and remedies during the Forbearance Period as defined in
therein, but solely with respect to the specified events of default provided under the Adjuvant Forbearance Agreement.
On
September 15, 2022, the Company also entered into the second amendment to the Adjuvant Purchase Agreement (the Second Adjuvant Amendment),
pursuant to which the conversion price per share was reduced to $26.25,
subject to adjustment for certain dilutive Company equity issuance adjustments for a two-year
period. In addition, the Company entered into
an exchange agreement, pursuant to which the Adjuvant Purchasers agreed to exchange 10%
of the outstanding amount of the Adjuvant Notes as of September 15, 2022 (or $2.9
million) for rights to receive 109,842
shares of common stock (Adjuvant Purchase Rights).
The number of shares for each Adjuvant Purchase Right is initially fixed, but is subject to certain customary adjustments, and, until
the second anniversary of issuance, adjustments for certain dilutive Company equity issuances. Refer to Note 10 -
Stockholders’ Equity (Deficit) for discussion regarding additional issuances of Purchase Rights under this provision. The Adjuvant
Purchase Rights expire on June 28, 2027 and do not have an exercise price per share and, therefore, will not result in cash proceeds
to the Company. As of December 31, 2022, all Adjuvant Purchase Rights remain outstanding. The conversion price of the Adjuvant Notes
were further reset to $4.0625
per share with the December 2022 Notes issuance,
as discussed below. Subsequent to December 31, 2022, the conversion price adjusted to $0.8125,
as discussed in Note 14 – Subsequent Events.
The
Adjuvant Notes are accounted for in accordance with authoritative guidance for convertible debt instruments and are classified as current
liabilities in the consolidated balance sheet. The aggregate proceeds of $25.0
million was initially classified as restricted
cash for financial reporting purposes due to contractual stipulations that specify the types of expenses the money can be spent on and
how it must be allocated. Its conversion feature is required to be bifurcated as an embedded derivative due to the fact that the Company
does not have sufficient number of shares reserved upon conversion. However, the fair value of such feature is immaterial as of December
31, 2022. As of December 31, 2022 and 2021, $0.9
million and $4.7
million in proceeds remained, which are included
in restricted cash on the consolidated balance sheets. See Note 7- Fair Value of Financial Instruments for a description of the accounting
treatment for the Adjuvant Purchase Rights.
Due
to the execution of the Adjuvant Forbearance and the Second Adjuvant Amendment, the Company reviewed the Adjuvant Notes in accordance
with Topics ASC 470-50 – Modifications and Extinguishments and ASC 470-60 – Troubled Debt Restructurings by Debtors.
The Company concluded that although changes in the structure of the debt met certain qualitative factors to qualify as a troubled
debt restructuring (TDR), the effective interest rate post changes was greater than the original effective interest rate and, therefore,
failed the quantitative test to be a TDR. The Adjuvant Notes were evaluated in accordance with ASC 470-50 and were determined to have
failed certain qualitative factors to qualify as a modification and, therefore, were accounted for as an extinguishment. The Company
removed the old debt from its books and recorded the new, revised debt and concurrently recognized a gain of approximately $2.5
million upon extinguishment, included in change
in fair value of financial instruments within the consolidated statements of operations.
As
discussed above and described more fully in Note 14 – Subsequent Events, on March 7, 2023, the Company
received a Notice of Event of Default and Reservation of Rights (the Notice of Default) from Baker Bros. resulting in a cross
default under the all outstanding debt and as such, the Company was not in compliance with all applicable covenants as of the filing
date of the Annual Report, including the cross-default provisions addressed by the Secured Creditor Forbearance Agreement
discussed above.
As
of December 31, 2022 and 2021, the Adjuvant Notes are recorded in the consolidated balance sheet as short-term convertible notes payable
with a total balance of $26.3
million and $27.2
million, respectively. As of December 31, 2022
and 2021, the balance is comprised of $22.3
million and $24.8
million, respectively, in principal, net of unamortized
debt issuance costs, and $4.0
million and $2.4
million, respectively, in accrued interest.
As
of December 31, 2022 and assuming the current conversion price of $4.0625
per share, the Adjuvant Notes could be converted
into 6,527,898
shares of common stock.
Term
Notes
January
and March 2022 Notes
On
January 13, 2022, the Company entered into a Securities Purchase Agreement (the January 2022 Purchase Agreement) with institutional investors
(the January 2022 Notes Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 5.0%
Senior Subordinated Notes due 2025 with an aggregate issue price of $5.9
million (the January 2022 Notes), which included
an original issue discount of $0.9
million, and (ii) warrants (the January 2022
Warrants) to purchase up to 8,003
shares of the Company’s common stock, $0.0001
par value per share. The January 2022 Warrants
have an exercise price of $735.00
per share and were initially exercisable beginning
on July 15, 2022 with a five-year
term. Pursuant to the terms of the March 2022 Purchase Agreement
(as defined below), the January 2022 Warrants became exercisable on March 1, 2022, as described in more detail below.
On
March 1, 2022, the Company entered into a Securities Purchase Agreement (the March 2022 Purchase Agreement) with institutional investors
(the March 2022 Notes Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 5.0%
Senior Subordinated Notes due 2025 with an aggregate issue price of approximately $7.5
million (the March 2022 Notes), which included
an original issue discount of approximately $2.5
million, and (ii) warrants (the March 2022 Warrants)
to purchase up to 8,303
shares of the Company’s common stock, $0.0001
par value per share. The March 2022 Warrants
have an exercise price of $897.56
per share and are immediately exercisable with
a five-year term.
The
January and March 2022 Notes carried an interest rate of 5%
per annum, which was subject to increase to 18%
upon an event of default. The January and March 2022 Notes were able to be prepaid, in whole or in part, at the Company’s option
together with all accrued and unpaid interest and fees as of the date of the repayment. The holders of the January and March 2022 Notes
were able to require the Company to redeem their respective notes upon the occurrence of an event of default with a redemption premium
of 25%.
The holders of the January and March 2022 Notes were also able to require the Company to redeem their respective notes upon the occurrence
of certain subsequent transactions.
Pursuant
to the terms of the January and March 2022 Purchase Agreements, the Company agreed to certain restrictions on effecting variable rate
transactions so long as the January and March 2022 Notes were outstanding. Also, pursuant to the terms of the January and March 2022
Purchase Agreements, the January and March 2022 Purchasers had certain rights to participate in subsequent issuances of the Company’s
securities, subject to certain exceptions.
The
Company evaluated the January and March 2022 Notes to determine if any embedded components qualified as a derivative requiring bifurcation
in accordance with ASC 815. The Company determined that the embedded put option and interest rate increase feature would both require
bifurcation and separate accounting. Therefore, the Company elected to use the fair value option under ASC 825, Financial Instruments
(ASC 825) for the January and March 2022 Notes inclusive of the embedded features.
The
Company evaluated the January and March 2022 Warrants and determined that in accordance with ASC 815 the warrants should be recorded
at fair value and classified as a derivative liability in the consolidated balance sheet. Both the January and March 2022 Notes and Warrants
were marked-to-market at each reporting date.
Under
the valuation methods as described in Note 7- Fair Value of Financial Instruments, the Company recorded the following in the consolidated financial statements related to the January and March 2022 Notes
and Warrants during the year ended December 31, 2022: (i) $0.2
million in notes at issuance; (ii) $10.6
million in warrants at issuance as a derivative
liability; and (iii) a $0.9
million loss on issuance. During the year ended
December 31, 2022, the Company recognized gains in fair value of financial instruments as a result of the mark-to-market adjustment on
the January and March 2022 Warrants of $10.6
million.
On
May 4, 2022, the January and March 2022 Notes were exchanged pursuant to the May 2022 Exchange, as defined below.
May
2022 Notes
On
May 4, 2022, the Company entered into amendment and exchange agreements (the May 2022 Exchange) with the holder of issued and outstanding
Series B-2 and C Preferred Stock, Seven Knots, and the January and March 2022 Notes Purchasers (collectively, the May 2022 Notes Purchasers),
pursuant to which they agreed to exchange all of the January and March 2022 Notes, 2,100
shares of Series B-2 Convertible Preferred Stock,
1,700
shares of Series C Convertible Preferred Stock,
and 4,266 shares
of the Company’s Common Stock for (i) new 5.0%
Senior Subordinated Notes with an aggregate principal amount of $22.3
million (the May 2022 Notes), (ii) 1,666
new shares of Common Stock and (iii) new warrants
to purchase up to 6,666
shares of Common Stock (the May 2022 Warrants).
The May 2022 Warrants have an exercise price of $309.56
per share and were exercisable immediately
with a five-year
term. The 2,100
shares of Series B-2 Convertible Preferred Stock,
1,700
shares of Series C Convertible Preferred Stock,
and 4,266 shares
of the Company’s Common Stock that were exchanged in the May 2022 Exchange were retired by the Company. All exchange transactions
aforementioned were cashless.
The
May 2022 Notes are substantially similar to the January and March 2022 Notes, except that (i) the maturity date of the May 2022 Notes
was August 1, 2022 and (ii) the holders of the May 2022 Notes may require the Company to redeem or exchange up to 100%
of the May 2022 Notes upon the occurrence of certain subsequent transactions (each, a Subsequent Transaction Optional Redemption). Pursuant
to the terms of the May 2022 Notes and subject to certain conditions described in the May 2022 Notes, if the Company completed an underwritten
public offering of at least $20
million complying with certain conditions (a
Qualified Underwritten Offering) and the holder of the May 2022 Notes did not participate in the Qualified Underwritten Offering, then
the holder would have forfeited their right to Subsequent Transaction Optional Redemption solely with respect to that Qualified Underwritten
Offering and amounts that may have been due pursuant to the May 2022 Notes would not have been due and payable until the three-month
anniversary of the Qualified Underwritten Offering.
The
May 2022 Public Offering qualified as the Qualified Underwritten Offering and, in connection with the May 2022 Public Offering, the holders
of the May 2022 Notes waived certain of their preemptive and redemption rights and the Company redeemed $5.9
million of the May 2022 Notes. The holders of
the May 2022 Notes also waived the maturity date of the May 2022 Notes until October 31, 2022.
The
May 2022 Notes contain various customary affirmative and negative covenants agreed to by the Company. The May 2022 Notes also include
other customary events of default, which include the suspension of trading of shares of the Company’s common stock on the Nasdaq
Capital Market for a period of more than five
trading days. On September 12, 2022, the Company
was in default of the May Notes due to the default with the Baker Notes under the cross-default provision. As a result, the interest
rate was increased to 18%
for the duration of the default and the holders of the May 2022 Notes had the right to request redemption for 125%
of the amounts then owed pursuant to the May 2022 Notes.
On
September 15, 2022, the Company entered into exchange agreements with each of the May 2022 Notes Purchasers (the May 2022 Notes
Exchange Agreements), pursuant to which the May 2022 Notes Purchasers agreed to exchange all outstanding balance of the May Notes as
of September 15, 2022 using the higher interest rate and redemption premium aforementioned for purchase rights (the May Note
Purchase Rights) to receive 832,237 shares
of common stock. As a result, the May Notes are no longer outstanding as of December 31, 2022. The number of right shares for each
May Note Purchase Right is initially fixed, but is subject to certain customary adjustments, and, until the second anniversary of
issuance, adjustments for certain dilutive Company equity issuances, as further discussed in Note 10 -
Stockholders’ Equity (Deficit) and expire on June 28, 2027. The May 2022 Notes Purchasers also waived certain
anti-dilution share adjustment provisions with respect to shares underlying the May 2022 Warrants.
The
Company evaluated the May 2022 Notes and determined that in accordance with ASC 470 the notes should be accounted for as a modification
of the January and March 2022 Notes. The Company further evaluated the May 2022 Notes to determine if any embedded components qualified
as a derivative requiring bifurcation in accordance with ASC 815. The Company determined that the embedded put options and interest rate
increase feature would all require bifurcation and separate accounting. Therefore, the Company elected to use the fair value option under
ASC 825, Financial Instruments (ASC 825) for the May 2022 Notes inclusive of the embedded features.
The
Company evaluated the May 2022 Warrants and determined that, in accordance with ASC 815, the warrants should be recorded at fair value
and classified as a derivative liability in the consolidated balance sheet. Both the May 2022 Notes and Warrants are marked-to-market
at each reporting date before the exchange as described above.
Under
the valuation methods as described in Note 7- Fair Value of Financial Instruments, the Company recorded the following in the consolidated financial statements related to the May 2022 Notes and Warrants
during the year ended December 31, 2022: (i) $22.3
million in notes at issuance; and (ii) $1.6
million in warrants at issuance as a derivative
liability. During the year ended December 31, 2022, the Company recognized losses in fair value of financial instruments as a result
of the mark-to-market adjustment on the May 2022 Notes of $10.3
million and gains in fair value of financial
instruments as a result of the mark-to-market adjustment on the May 2022 Warrants of $1.6
million.
December
2022 Notes
On
December 20, 2022, the Company entered into a Securities Purchase Agreement (the December 2022 Purchase Agreement), with certain investors
(the December 2022 Notes Purchasers) pursuant to which the Company agreed to sell in a registered direct offering (i) unsecured 8.0%
Senior Subordinated Notes due December 21, 2025 with an aggregate issue price of $2.3
million (the December 2022 Notes), which included
an original issue discount of $0.8
million (ii) warrants (the December 2022 Warrants)
to purchase up to 369,230
shares of the Company’s common stock, $0.0001
par value per share, and (iii) an aggregate 70
shares of Series D Preferred Stock (the Preferred
Shares) (collectively, the Offering). The Offering closed on December 21, 2022, with net proceeds to the Company from the Offering, after
deducting offering expenses, of $1.25
million. The December 2022 Notes are convertible
at $6.25,
and the December 2022 Warrants have a strike price of $6.25.
Upon the closing of the December 2022 Purchase Agreement, the conversion and strike price of the Baker Notes, the Baker Warrants, the
June 2022 Baker Warrants, the Adjuvant Notes and the May Common Stock Warrants, as discussed in Note 10 - Stockholders’
Equity (Deficit), reset to $4.0625
per share.
The
December 2022 Notes interest rate is subject to increase to 12%
upon an event of default and have no Company right to prepayment prior to maturity, however, the Company can redeem the respective notes
at a redemption premium of 32.5%.
The December 2022 Notes Purchasers can also require the Company to redeem their notes at the respective premium rate tied to the occurrence
of certain subsequent transactions, as well as require the Company to redeem the December 2022 Notes in the event of subsequent placements
(as defined). Also, pursuant to the terms of the December 2022 Purchase Agreement, the December 2022 Notes Purchasers have certain rights
to participate in subsequent issuances of the Company’s securities, subject to certain exceptions. Additionally, the December 2022
Notes conversion rate and warrant strike price are subject to adjustment upon the issuance of other securities (as defined) less than
the stated conversion rate and strike price of $6.25.
Subsequent to December 31, 2022, the conversion and strike price adjusted to $0.8125
as discussed in Note 14 –
Subsequent Events.
The
Company evaluated the December 2022 Notes and December 2022 Warrants, in accordance with ASC 480 – Distinguishing Liabilities
from Equity and determined both were liability instruments. The December 2022 Notes were then evaluated in accordance the requirements
of ASC 825, Financial Instruments (ASC 825) and concluded the Company was not precluded from electing the fair value option for
the December 2022 Notes; as such the December 2022 Notes are carried at fair value in the consolidated balance sheets. Since the December
2022 Warrants are also required to be recorded as liabilities in the Company’s consolidated balance sheets, they are also carried
at fair value. Both the December 2022 Notes and Warrants are marked-to-market at each reporting date with changes in fair value of the
December 2022 Notes and Warrants are recorded recognized in the consolidated statement of operations, unless the change is concluded
to be related to changes in the Company’s credit rating, in which case the change will be recognized as a component of accumulated
other comprehensive income in the consolidated balance sheets.
Under
the valuation methods as described in Note 7- Fair Value of Financial Instruments, the Company recorded the following in the consolidated financial statements related to the December 2022 Notes and
Warrants during the year ended December 31, 2022: (i) $156,000
in convertible notes payable carried at fair
value in the consolidated balance sheets, (ii) $143,000
in derivative liabilities for the warrants, and
(iii) $1.3
million additional paid-in capital upon the issuance
of financial instruments carried at fair value.
6.
Balance Sheet Details
Prepaid
and Other Current Assets
Prepaid
and other current assets consist of the following (in thousands):
Schedule
of Prepaid and Other Current Assets
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Insurance | |
$ | 1,387 | | |
$ | 1,144 | |
| Selling
and marketing related costs | |
| 44 | | |
| 1,134 | |
| Manufacturing
related costs | |
| 82 | | |
| 322 | |
| Other | |
| 705 | | |
| 629 | |
| Total | |
$ | 2,218 | | |
$ | 3,229 | |
| Prepaid
and other current assets | |
$ | 2,218 | | |
$ | 3,229 | |
Property
and Equipment, Net
Property
and equipment, net, consists of the following (in thousands):
Schedule
of Property and Equipment net
| | |
Useful
Life | |
2022 | | |
2021 | |
| | |
| |
December
31, | |
| | |
Useful
Life | |
2022 | | |
2021 | |
| Research
equipment | |
5
years | |
$ | 653 | | |
$ | 653 | |
| Computer
equipment and software | |
3
years | |
| 639 | | |
| 619 | |
| Office
furniture | |
5
years | |
| 881 | | |
| 881 | |
| Leasehold
improvements | |
5
years or less | |
| 3,388 | | |
| 3,388 | |
| Construction
in-process | |
— | |
| 1,568 | | |
| 2,407 | |
| Property
and equipment gross | |
| |
| 7,129 | | |
| 7,948 | |
| Less:
accumulated depreciation | |
| |
| (3,189 | ) | |
| (2,174 | ) |
| Total,
net | |
| |
$ | 3,940 | | |
$ | 5,774 | |
Depreciation
and amortization expense for property and equipment is disclosed in the consolidated statements of cash flows.
Other
Noncurrent Assets
Other
noncurrent assets consist of the following (in thousands):
Schedule
of Other Non Current Assets
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Restricted
cash included in noncurrent assets | |
$ | 800 | | |
$ | 800 | |
| Inventories,
long-term | |
| 1,270 | | |
| 241 | |
| Prepaid
directors & officers’ insurance | |
| 1,717 | | |
| 109 | |
| Other | |
| 331 | | |
| 53 | |
| Total | |
$ | 4,118 | | |
$ | 1,203 | |
Accrued
Expenses
Accrued
expenses consist of the following (in thousands):
Schedule
of Accrued Expenses
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Clinical
trial related costs | |
$ | 2,574 | | |
$ | 5,294 | |
| Selling
and marketing related costs | |
| 674 | | |
| 1,997 | |
| Legal
and other professional fees | |
| — | | |
| 550 | |
| Manufacturing
related costs | |
| — | | |
| 201 | |
| Other | |
| 876 | | |
| 328 | |
| Total | |
$ | 4,124 | | |
$ | 8,370 | |
| Accrued
expenses | |
$ | 4,124 | | |
$ | 8,370 | |
7.
Fair Value of Financial Instruments
Fair
Value of Financial Assets
The
fair values of the Company’s assets, including the money market funds, investments in marketable fixed income debt securities classified
as cash and cash equivalents, restricted cash, and Flex Note receivable, measured on a recurring basis are summarized in the following
tables, as applicable (in thousands):
Schedule
of Fair Value of Financial Assets
| | |
December
31, 2022 | | |
Quoted
Prices in Active Markets for Identical Assets (Level
1) | | |
Significant
Other Observable Inputs (Level
2) | | |
Significant
Unobservable Inputs (Level
3) | |
| Money
market funds (1) | |
$ | 2,612 | | |
$ | 2,612 | | |
$ | — | | |
$ | — | |
| Total
assets | |
$ | 2,612 | | |
$ | 2,612 | | |
$ | — | | |
$ | — | |
| | |
December
31, 2021 | | |
Quoted
Prices in Active Markets for Identical Assets (Level
1) | | |
Significant
Other Observable Inputs (Level
2) | | |
Significant
Unobservable Inputs (Level
3) | |
| Money
market funds (1) | |
$ | 11,176 | | |
$ | 11,176 | | |
$ | — | | |
$ | — | |
| Total
assets | |
$ | 11,176 | | |
$ | 11,176 | | |
$ | — | | |
$ | — | |
| (1) |
Included
as a component of cash and cash equivalents and restricted cash on the consolidated balance sheet. |
Fair
Value of Financial Liabilities
The
following table is a summary of the Company’s convertible debt instruments as of December 31, 2022 and 2021, respectively (in thousands).
Schedule
of Fair Value of Financial Liabilities
| | |
Principal | | |
Unamortized
Issuance |
| |
Accrued | | |
Redemption | | |
Amount | | |
Net
Carrying | | |
Fair
Value | |
| As
of December 31, 2022 | |
Amount | | |
Costs | | |
Interest | | |
Amount | | |
Exchanged | | |
Amount | | |
Amount | | |
Leveling | |
| Baker
Notes (1) (2) | |
$ | 45,528 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 45,528 | | |
$ | 39,260 | | |
| Level
3 | |
| Adjuvant
Notes (3) (4) | |
| 22,500 | | |
| (252 | ) | |
| 4,020 | | |
| — | | |
| — | | |
| 26,268 | | |
| — | | |
| N/A | |
| May
2022 Notes (1) | |
| 16,376 | | |
| — | | |
| 1,101 | | |
| 4,369 | | |
| (21,846 | ) | |
| — | | |
| — | | |
| N/A | |
| Dec
2022 Notes (1) | |
| 2,308 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 2,308 | | |
| 156 | | |
| Level
3 | |
| (1)
|
These
liabilities are/were carried at fair value in the consolidated balance sheets. As such, the principal and accrued interest was included
in the determination of fair value. The related debt issuance costs were expensed. |
| (2)
|
The
Baker Notes principal amount includes $5.6
million of interest paid-in
kind as of December 31, 2022. |
| (3)
|
The
Adjuvant Notes are recorded in the consolidated balance sheets at their net carrying amount which includes principal and accrued
interest, net of unamortized issuance costs. |
| (4)
|
The
principal amount and accrued interest of the Adjuvant Notes are net of the 10%
reduction in principal and interest of $2.5
million and $0.4
million, respectively,
received in exchange for the issuance of Purchase Rights. |
| | |
Principal | | |
Unamortized
Issuance | | |
Accrued | | |
Net
Carrying | | |
Fair
Value | |
| As
of December 31, 2021 | |
Amount | | |
Costs | | |
Interest | | |
Amount | | |
Amount | | |
Leveling | |
| Baker
Notes (1) (2) | |
$ | 27,323 | | |
$ | — | | |
$ | 698 | | |
$ | 28,021 | | |
$ | 81,717 | | |
| Level
3 | |
| Adjuvant
Notes (3) | |
| 25,000 | | |
| (146 | ) | |
| 2,355 | | |
| 27,209 | | |
| 27,209 | | |
| Level
3 | |
| (1)
|
These
liabilities are/were carried at fair value in the consolidated balance sheets. As such, the principal and accrued interest was included
in the determination of fair value. The related debt issuance costs were expensed. |
| (2)
|
The
Baker Notes principal amount includes $2.3
million of interest paid-in
kind as of December 31, 2021. |
| (3)
|
The
Adjuvant Notes are recorded in the consolidated balance sheets at their net carrying amount which includes principal and accrued
interest, net of unamortized issuance costs. |
The
following tables summarize the Company’s derivative liabilities as of December 31, 2022 and 2021 as discussed in Note
10- Stockholders’ Equity (Deficit) (in thousands):
Schedule
of Fair Value of Financial Liabilities
| | |
Fair
Value | |
| As
of December 31, 2022(1) | |
Amount | | |
Leveling | |
| April
and June 2020 Baker Warrants | |
$ | 1 | | |
| Level
3 | |
| May
2022 Public Offering Warrant | |
| 303 | | |
| Level
3 | |
| June
2022 Baker Warrants | |
| 170 | | |
| Level
3 | |
| December
2022 Warrants | |
| 107 | | |
| Level
3 | |
| Purchase
Rights | |
| 1,095 | | |
| Level
3 | |
| Total
Derivative Liabilities | |
$ | 1,676 | | |
| | |
| (1)
|
As
of December 31, 2022, all warrants issued by the Company are subject to liability accounting due to potential settlement in cash,
an insufficient number of authorized shares and other adjustment mechanics. However, warrants with an exercise price greater than
$6.250
per share were considered
to be significantly out of the money as of December 31, 2022 and therefore the value ascribed to those warrants was considered to
be de minimus and is therefore excluded from the above table. |
| | |
Fair
Value | |
| As
of December 31, 2021 | |
Amount | | |
Leveling | |
| Derivative
Liabilities - Convertible Preferred Stock | |
$ | 202 | | |
| Level
3 | |
Change
in Fair Value of Level 3 Financial Liabilities
The
Baker Warrants, as discussed in Note 5- Debt, were determined
to be classified as liabilities. Therefore, they were stated at fair value at issuance and subject to mark-to-market adjustments at each
reporting date until a subsequent event occurs that would change their classification. They were considered Level 3 instruments because
the fair value measurement was based, in part, on significant inputs not observed in the market.
The
following table summarizes the changes in Level 3 financial liabilities related to Term Notes, Baker Notes and December 2022 Notes measured
at fair value on a recurring basis for the years ended December 31, 2022 (in thousands).
Schedule
of Change in Fair Value of Level 3 Financial Liabilities
| | |
Term
Notes - January 2022 Notes | | |
Term
Notes - March 2022 Notes | | |
Term
Notes - May 2022 Notes | | |
Baker
First Closing Notes | | |
Baker
Second Closing Notes | | |
December
2022 Notes | | |
Total | |
| Balance
at December 31, 2021 | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 49,030 | | |
$ | 32,687 | | |
$ | — | | |
$ | 81,717 | |
| Balance
at issuance | |
| 116 | | |
| 149 | | |
| 447 | | |
| — | | |
| — | | |
| 156 | | |
| 868 | |
| Debt
repayment | |
| — | | |
| — | | |
| (5,892 | ) | |
| — | | |
| — | | |
| — | | |
| (5,892 | ) |
| Change
in fair value presented in the Condensed Consolidated Statements of Operations | |
| 4 | | |
| 2 | | |
| 10,251 | | |
| 1,189 | | |
| 792 | | |
| — | | |
| 12,238 | |
| Change
in fair value presented in the Statements of Comprehensive Operations | |
| | | |
| | | |
| | | |
| (26,663 | ) | |
| (17,775 | ) | |
| — | | |
| (44,438 | ) |
| Exchange
of notes (noncash) | |
| (120 | ) | |
| (151 | ) | |
| (4,806 | ) | |
| — | | |
| — | | |
| — | | |
| (5,077 | ) |
| Balance
at December 31, 2022 | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 23,556 | | |
$ | 15,704 | | |
$ | 156 | | |
$ | 39,416 | |
The
following table summarizes the changes in Level 3 financial liabilities related to Baker Notes measured at fair value on a recurring
basis for the years ended December 31, 2021 (in thousands).
| | |
Baker
First
Closing
Notes | | |
Baker
Second
Closing
Notes | | |
Total | |
| Balance
at December 31, 2020 | |
$ | 30,451 | | |
$ | 20,301 | | |
$ | 50,752 | |
| Initial
liability at issuance | |
| 21,632 | | |
| 14,422 | | |
| 36,054 | |
| Change
in fair value | |
| (3,053 | ) | |
| (2,036 | ) | |
| (5,089 | ) |
| Balance
at December 31, 2021 | |
$ | 49,030 | | |
$ | 32,687 | | |
$ | 81,717 | |
The
following table summarizes the changes in Level 3 financial liabilities related to derivative liabilities measured at fair value on a
recurring basis for the years ended December 31, 2022 (in thousands).
Schedule
of Change in Fair Value of Level 3 Financial Liabilities
| | |
Derivative
Liability - Convertible Preferred Stock Conversion Feature | | |
Derivative
Liabilities Previously Classified as Equity Instruments | | |
Derivative
Liability - January 2022 Warrants | | |
Derivative
Liability - March 2022 Warrants | | |
Derivative
Liability - May 2022 Warrants | | |
May
2022 Public Offering Common Warrants | | |
May
2022 Public Offering Pre-Funded Warrants | | |
June
2022
Baker Warrants | | |
December
2022 Warrants | | |
Purchase
Rights | | |
Derivative
Liabilities Total | |
| Balance
at December 31, 2021 | |
$ | 202 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 202 | |
| Balance
at issuance | |
| — | | |
| — | | |
| 4,562 | | |
| 6,025 | | |
| 1,613 | | |
| 18,074 | | |
| 4,633 | | |
| 70,238 | | |
| 107 | | |
| 6,284 | | |
| 111,536 | |
| Exercises | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (12,086 | ) | |
| (4,633 | ) | |
| — | | |
| — | | |
| (1,007 | ) | |
| (17,726 | ) |
| Change
in fair value presented in the consolidated statements of operations | |
| (83 | ) | |
| — | | |
| (4,562 | ) | |
| (6,025 | ) | |
| (1,613 | ) | |
| (5,685 | ) | |
| — | | |
| (70,068 | ) | |
| — | | |
| (4,182 | ) | |
| (92,218 | ) |
| Conversion
of series B-2 convertible preferred stock | |
| (46 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (46 | ) |
| Loss
on re-valuation of derivative liabilities presented in the consolidated statement of operations. | |
| — | | |
| 1 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1 | |
| May
2022 exchange transaction | |
| (73 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (73 | ) |
| Balance
at December 31, 2022 | |
$ | — | | |
$ | 1 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 303 | | |
$ | — | | |
$ | 170 | | |
$ | 107 | | |
$ | 1,095 | | |
$ | 1,676 | |
The
following table summarizes the changes in Level 3 financial liabilities related to derivative liabilities measured at fair value on a
recurring basis for the year ended December 31, 2021 (in thousands):
| | |
Derivative
Liabilities | |
| Beginning
balance | |
$ | — | |
| Initial
liability at issuance | |
| 550 | |
| Conversion
of series B-1 convertible preferred stock | |
| (275 | ) |
| Change
in fair value presented in the consolidated statements of operations | |
| (73 | ) |
| Ending
balance | |
$ | 202 | |
Valuation
Methodology
Baker
Notes
Through
June 30, 2022, the fair value of the Baker Notes issued, and the change in fair value of the Baker Notes at the reporting date, were
determined using a Monte Carlo simulation-based model. The Monte Carlo simulation was used to take into account several embedded features
and factors, including the future value of our common stock, a potential change of control event, the probability of meeting certain
debt covenants, the maturity term of the Baker Notes, the probability of an event of voluntary conversion of the Baker Notes, the probability
of the failure to meet the affirmative covenant to achieve $100.0
million in cumulative net sales of Phexxi by
June 30, 2023, and the probability of exercise of the put right and the probability of exercise of our call right.
The
fair value of the Baker Notes are subject to uncertainty due to the assumptions that are used in the Monte Carlo simulation-based model.
These factors include but are not limited to the future value of the Company’s common stock, the probability and timing of a potential
change of control event, the probability of meeting certain debt covenants, the probability of an event of voluntary conversion of the
Baker Notes, exercise of the put right, and exercise of the Company’s call right. The fair value of the Baker Notes is sensitive
to these estimated inputs made by management that are used in the calculation.
For
the second half of 2022, the fair value of the Baker Notes issued as described in Note 5- Debt, and subsequent changes in fair value recorded at each reporting date, was determined by estimating
the fair value of the Market Value of Invested Capital (“MVIC”) of the Company. This was estimated using forms of the cost
and market approaches. In the Cost approach, an adjusted net asset value method was used to determine the net recoverable value of the
Company, including an estimate of the fair of the Company’s intellectual property. The estimated fair value of the Company’s
intellectual property was valued using a relief from royalty method which required management to make significant estimates and assumptions
related to forecasts of future revenue, and the selection of the royalty (3.5%)
and discount (19.0%)
rates. The guideline public company method served as another valuation indicator. In this form of the Market approach, comparable market
revenue multiples were elected and applied to the Company’s forward revenue forecast to ultimately derive a MVIC indication. If
the resulting fair value from these approaches is not estimated as greater than the contractual payout, the fair value of the Baker Notes
then becomes only the Company MVIC available for distribution to this first lien note holder.
January
and March 2022 Notes
The
fair value of the January and March 2022 Notes issued as described in Note 5- Debt, and subsequent changes in
fair value recorded at each reporting date, were determined using a probability weighted expected return method (PWERM) model. PWERM
was used to take into account several factors, including the future value of the Company’s common stock, a potential change of
control event, the probability of meeting certain debt covenants, the maturity term of the January and March 2022 Notes, exercise of
the put right, and exercise of the Company’s call right.
May
2022 Notes
The
fair value of the May 2022 Notes issued as described in Note 5- Debt,
and subsequent changes in fair value recorded at each reporting date, were determined using a PWERM model. PWERM was used to take into
account several factors, including the future value of the Company’s common stock, a potential change of control event, the probability
of meeting certain debt covenants, the maturity term of the January and March 2022 Notes, exercise of the put right, and exercise of
the Company’s call right.
December
2022 Notes
The
fair value of the December 2022 Notes issued as described in Note 5- Debt, were determined using an Black-Scholes
option pricing model using typical inputs such as underlying market price of the Company’s common stock, the conversion/strike
price, time to maturity of the December 2022 Notes, guideline public company volatilities and a risk-free interest rate.
Purchase
Rights
The
Adjuvant Purchase Rights and the May Note Purchase Rights (collectively Purchase Rights) contain certain provisions that are outside
the Company’s control under which the holders can force settlement in cash; as such, the Purchase Rights are recorded as derivative
liabilities in the consolidated balance sheets. The Purchase Rights are valued using an option pricing model (OPM), like a Black-Scholes
Methodology with changes in the fair value being recorded in the consolidated statements of operations. The assumptions used in the OPM
are considered level 3 assumptions and include, but are not limited to, the market value of invested capital, the cumulative equity value
of the Company as a proxy for the exercise price and the expected term the Purchase Rights will be held prior to exercise and a risk-free
interest rate.
Warrants
The
warrants contain certain provisions, which are outside the Company’s control, under which the holders can force settlement in cash,
as such, the warrants are recorded as derivative liabilities in the consolidated balance sheets. In accordance with ASC 815 - Derivatives
and Hedging, certain warrants previously classified as equity instruments were determined to be liability classified (the Reclassified
Warrants) due to the Company having an insufficient number of authorized shares as of December 31, 2022. The Company will continue to
re-evaluate the classification of its warrants at each balance sheet to determine the proper balance sheet classification for them. The
warrants are valued using an OPM based on the applicable assumptions, which include the exercise price of the warrants, time to expiration,
expected volatility of our peer group, risk-free interest rate, and expected dividends. The assumptions used in the OPM are considered
level 3 assumptions and include, but are not limited to, the market value of invested capital, the cumulative equity value of the Company
as a proxy for the exercise price, the expected term the warrants will be held prior to exercise and a risk-free interest rate and probability
of change of control event.
8.
Commitments and Contingencies
Operating
Leases
Fleet
Lease
In
December 2019, the Company and Enterprise FM Trust (the Lessor) entered into a Master Equity Lease Agreement whereby the Company leases
vehicles to be delivered by the Lessor from time to time with various monthly costs depending on whether the vehicles are delivered for
a term of 24
or 36
months, commencing on each corresponding delivery
date. The leased vehicles are for use by eligible employees of the Company’s commercial operations personnel. The Company maintains
a letter of credit as collateral in favor of the Lessor, which was included in restricted cash in the consolidated balance sheet. As
of December 31, 2022 and 2021, this letter of credit was $0.3
million. The Company determined that the leased
vehicles are accounted for as operating leases under ASC 842. In September 2022, the Company extended the lease term for an additional
12
months for the vehicles with a term of 24
months. The Company determined that such extension
is accounted for as a modification, for which the Company reassessed the lease classification and the incremental borrowing rate on the
modification date and accounted for accordingly.
2020
Lease and the First Amendment
On
October 3, 2019, the Company entered into an office lease for approximately 24,474
square feet (the Existing Premises) pursuant
to a non-cancelable lease agreement (the 2020 Lease). The 2020 Lease commenced on April 1, 2020 and will expire on September 30, 2025,
unless terminated earlier in accordance with its terms. The Company has a right to extend the term of the lease for an additional five
years and does not anticipate exercising such
extension. The Company provided the landlord with a $750,000
security deposit in the form of a letter of credit
for the Existing Premises. On April 14, 2020, the Company entered into the first amendment to the 2020 Lease for an additional 8,816
rentable square feet of the same office location
(the Expansion Premises), which commenced on September 1, 2020 and will expire on September 30, 2025. The Company provided an additional
$50,000
in a letter of credit for the Expansion Premises.
As of December 31, 2022 and 2021, restricted cash maintained as collateral for the Company’s security deposit was $0.8
million. See default under lease agreement discussion
within Note 14 – Subsequent Events for information regarding breach of the 2020 lease subsequent
to December 31, 2022.
2022
Sublease
On
May 27, 2022, the Company entered into a sublease agreement with AMN Healthcare, Inc. (AMN), pursuant to which the Company agreed to
sublease 16,637 rentable
square feet of the Existing Premises to AMN for a term commencing on June 15, 2022 and ending coterminous with the 2020 Lease on September
30, 2025, in exchange for the sum of approximately $87,000
per month, subject to an annual 3.5%
increase each year. Gross sublease income was $0.6
million for the year ended December 31, 2022.
Sublease income expected to be received from AMN is $1.0
million, $1.1
million and $0.9
million in each of the years ended December 31,
2023, 2024 and 2025, respectively.
Schedule
of Lease Cost
| | |
| |
Year
Ended December 31, | |
| Lease
Cost (in thousands) | |
Classification | |
2022 | | |
2021 | |
| Operating
lease expense | |
Research
and development | |
$ | 210 | | |
$ | 499 | |
| Operating
lease expense | |
Selling
and marketing | |
| 886 | | |
| 1,012 | |
| Operating
lease expense | |
General
and administrative | |
| 597 | | |
| 827 | |
| Total | |
| |
$ | 1,693 | | |
$ | 2,338 | |
Schedule
of Lease Term and Discount Rate
| Lease
Term and Discount Rate | |
December
31, 2022 | | |
December
31, 2021 | |
| Weighted
Average Remaining Lease Term (in years) | |
| 2.68 | | |
| 3.58 | |
| Weighted
Average Discount Rate | |
| 12 | % | |
| 12 | % |
Schedule
of Operating Lease Maturities
| Maturity
of Operating Lease Liabilities (in thousands) | |
Year
Ended
December
31 | |
| 2023 | |
$ | 2,581 | |
| 2024 | |
| 2,360 | |
| 2025 | |
| 1,521 | |
| Total
lease payments | |
| 6,462 | |
| Less:
imputed interest | |
| (1,018 | ) |
| Total | |
$ | 5,444 | |
Schedule
of Supplement Cash Outflows in Operating Leases
| Other
information (in thousands) | |
Year
Ended December
31, 2022 | | |
Year
Ended December
31, 2021 | |
| Cash
paid for amounts included in the measurement of lease liabilities: | |
| | | |
| | |
| Operating
cash outflows in operating leases | |
$ | 2,639 | | |
$ | 2,426 | |
Other
Contractual Commitments
In
November 2019, the Company entered into a supply and manufacturing agreement with a third-party to manufacture Phexxi, with potential
to manufacture other product candidates in accordance with all applicable current good manufacturing practice regulations, pursuant to
which the Company has certain minimum purchase commitments based on the forecasted product sales. The amounts purchased under the supply
and manufacturing agreement were $1.0
million and $3.0
million for the years ended December 31, 2022
and 2021, respectively. As of December 31, 2022, the $1.0
million remains unpaid.
Contingencies
From
time to time the Company may be involved in various lawsuits, legal proceedings, or claims that arise in the ordinary course of business.
On December 14, 2020, a trademark dispute captioned TherapeuticsMD, Inc. v Evofem Biosciences, Inc., filed in the United States District
Court for the Southern District of Florida against the Company, alleging trademark infringement of certain trademarks owned by TherapeuticsMD
under federal and state law (Case No. 9:20-cv-82296). On July 17, 2022, the Company settled the lawsuit with TherapeuticsMD, pursuant
to which the Company agreed to rebrand its product by July 2024 to coincide with its marketing objectives.
As
of December 31, 2022, there were no other claims or actions pending against the Company, which management believe has a probable, or
reasonably possible, probability of an unfavorable outcome. However, the Company may receive trade payable demand letters from its vendors
that could lead to potential litigation. As of December 31, 2022, approximately 56.7%
of our trade payables were greater than 90 days past due.
Intellectual
Property Rights
In
2014, the Company entered into an amended and restated license agreement (the Rush License Agreement) with Rush University Medical Center
(Rush University) pursuant to which Rush University granted the Company an exclusive, worldwide license of certain patents and know-how
related to its multipurpose vaginal pH modulator technology. Pursuant to the Rush License Agreement, the Company is obligated to pay
to Rush University an earned royalty based upon a percentage of net sales in the range of mid-single digits. In September 2020, the Company
entered into the first amendment to the Rush License Agreement, pursuant to which the Company is also obligated to pay a minimum annual
royalty amount of $100,000
to the extent the earned royalties do not equal
or exceed $100,000
commencing January 1, 2021. Such royalty costs,
included in cost of goods sold, were $1.1
million and $0.2
million for the years ended December 31, 2022
and 2021, respectively. As of December 31, 2022 and 2021, approximately $0.6
million and immaterial
were included in accrued expenses in the consolidated
balance sheets.
9.
Reduction in Force
On
November 1, 2022, the Company’s Board of Directors approved a reduction in force (RIF) intended to conserve the Company’s
current cash resources. The Company reduced the current workforce by 39
employees, of which 8
were in research and development, 30
were in sales and marketing and one
was in general and administrative.
The
Company estimated its aggregate pre-tax charges of approximately $0.4
million (of which $0.3
million was recorded within research and development
expenses, $0.1
million, was recorded within sales and marketing
expense and approximately $14,000
within general and administrative expenses),
in connection with the reduction in force, primarily consisting of notice period and severance payments, employee benefits and related
costs. The Company effected the reduction in force by the end of November 2022. These one-time charges were incurred primarily in the
fourth quarter of 2022. As of December 31, 2022, any one-time charges not paid were considered to be de minimus. See Note
14 – Subsequent Events for additional RIF costs incurred subsequent
to December 31, 2022.
10.
Stockholders’ Equity
Warrants
In
April and June 2020, pursuant to the Baker Bros. Purchase Agreement, as discussed in Note 5- Debt, the Company issued in aggregate warrants to purchase up to 2,732
shares of the Company’s common stock in
a private placement at an exercise price of $4,575
per share. As discussed in Note
5- Debt, the Second Baker Amendment provides that the exercise price of the
Baker Warrants will equal the conversion price of the Baker Notes. As discussed in Note 5- Debt, as of December 31, 2022, the exercise price of the Baker warrants was reset to $4.0625
per share and subsequent to year end was reset
to $0.8125,
as further discussed in Note 14 – Subsequent Events.
In
January 2022, pursuant to the January 2022 Securities Purchase Agreement as discussed in Note 5- Debt, the Company issued warrants to purchase up to 8,003
shares of the Company’s common stock in
a registered direct offering at an exercise price of $735
per share. In March 2022, pursuant to the March
2022 Securities Purchase Agreement as discussed in Note 5- Debt,
the Company issued warrants to purchase up to 8,303
shares of common stock in a registered direct
offering at an exercise price of $897.50
per share.
In
May 2022, pursuant to the exchange agreement as described in Note 5- Debt, the Company issued common warrants to purchase up to 6,666
shares of common stock at an exercise price of
$309.56
per share. The warrants have a five-year
term and were exercisable beginning on May 4,
2022.
In
May 2022, pursuant to the May 2022 Public Offering as described below, the Company issued common warrants to purchase up to 568,000
shares of common stock at an exercise price of
$93.75
per share, and pre-funded warrants to purchase
up to 102,680
shares of common stock at an exercise price of
$0.125
per share. The warrants have a five-year
term and were exercisable beginning May 24, 2022.
The common warrants contain (and the pre-funded warrants contained) customary 4.99%
and 19.99%
limitations on exercise provisions. The exercise price and number of shares issuable upon exercise of the common warrants is subject
to adjustment for certain dilutive issuances, stock splits and similar recapitalization transactions. As part of the debt restructuring
in September 2022 as described Note 5- Debt, the exercise
price of the common warrants was reset to $26.25
per share, and an additional 619,350
warrants were issued to holders of remaining
unexercised warrants to reflect the dilutive adjustment resulting from the lower exercise price. Additionally, as part of the December
2022 Notes issuance as described in Note 5- Debt, the exercise
price of the common warrants was reset to $4.0625
per share, and an additional 136,304
warrants were issued to holders of remaining
unexercised warrants to reflect the dilutive adjustment resulting from the lower exercise price. No further adjustment to the holders
of remaining unexercised warrants exists after the adjustment related to the December 2022 Notes issuance. During the second quarter
of 2022, all pre-funded warrants were exercised for an immaterial amount of cash. During the year ended December 31, 2022, 282,518
shares of common warrants were exercised for
total proceeds of $25.2
million. Subsequent to December 31, 2022, these
warrants had their strike price reset to $0.8125,
as discussed in Note 14 – Subsequent Events.
In
June 2022, as required by the Second Baker Amendment, the Company issued the June 2022 Baker Warrants to purchase up to 582,886
shares of the Company’s common stock, $0.0001
par value per share. The June 2022 Baker Warrants
had an exercise price of $93.75
per share at issuance and a five-year
term and were exercisable beginning June 28,
2022. The June 2022 Baker Warrants also contain customary 4.99%
and 19.99%
limitations on exercise provisions. The exercise price and number of shares issuable upon exercise of the June 2022 Baker Warrants is
subject to adjustment for certain dilutive issuances, stock splits and similar recapitalization transactions. As part of the debt restructuring
in September 2022 as described Note 5- Debt, the exercise
price of the June 2022 Baker Warrants was reset to $26.25
per share and then was further reset to $4.0625
per share upon the December 2022 Notes issuance.
Subsequent to December 31, 2022, these warrants had their strike price reset to $0.8125,
as discussed in Note 14 – Subsequent Events.
In
December 2022, pursuant to the December 2022 Securities Purchase Agreement as discussed in Note 5- Debt, the Company issued warrants to purchase up to 369,230
shares of the Company’s common stock in
a registered direct offering at an exercise price of $6.25
per share. Subsequent to December 31, 2022, these
warrants had their strike price reset to $0.8125,
as discussed in Note 14 – Subsequent Events.
As
of December 31, 2022, warrants to purchase up to 2,052,361
shares of the Company’s common stock
remain outstanding at a weighted average exercise price of $56.25
per share. All warrants issued by the Company
are subject to liability accounting due to potential settlement in cash, an insufficient number of authorized shares and other adjustment
mechanics. However, warrants with an exercise price greater than $6.25
per share were considered to be significantly
out of the money as of December 31, 2022 and therefore the value ascribed to those warrants was considered to be de minimus. In
accordance with ASC 815 - Derivatives and Hedging, certain warrants previously classified as equity instruments were determined
to be liability classified (the Reclassified Warrants) due to the Company having an insufficient number of authorized shares as of December
31, 2022. The Company will continue to re-evaluate the classification of its warrants at each balance sheet to determine the proper balance
sheet classification for them. The fair value of the warrants is included in derivative liabilities in the consolidated balance sheets.
These warrants are summarized below:
Schedule
of Warrants
| Type
of Warrants | |
Underlying
Common Stock to be Purchased | | |
Exercise
Price | | |
Issue
Date | |
Exercise
Period |
| Common
Warrants | |
| 4 | | |
| 6,918.75 | | |
June
11, 2014 | |
June
11, 2014 to June 11, 2024 |
| Common
Warrants | |
| 452 | | |
$ | 14,062.50 | | |
May
24, 2018 | |
May
24, 2018 to May 24 2025 |
| Common
Warrants | |
| - | | |
$ | 14,062.50 | | |
June
26, 2018 | |
June
26, 2018 to June 26, 2025 |
| Common
Warrants | |
| 888 | | |
$ | 11,962.50 | | |
April
11, 2019 | |
October
11, 2019 to April 11, 2026 |
| Common
Warrants | |
| 1480 | | |
$ | 11,962.50 | | |
June
10, 2019 | |
December
10, 2019 to June 10, 2026 |
| Common
Warrants | |
| 1,639 | | |
$ | 4.0625 | | |
April
24, 2020 | |
April
24, 2020 to April 24, 2025 |
| Common
Warrants | |
| 1,092 | | |
$ | 4.0625 | | |
June
9, 2020 | |
June
9, 2020 to June 9, 2025 |
| Common
Warrants | |
| 30,582 | | |
$ | 1,875 | | |
May
20, 2021 | |
May
20, 2021 to May 22, 2023 |
| Common
Warrants | |
| 8,003 | | |
$ | 735.00 | | |
January
31, 2022 | |
January
31, 2022 to March 1, 2027 |
| Common
Warrants | |
| 8,303 | | |
| 897.56 | | |
March
1, 2022 | |
March
1, 2022 to March 1, 2027 |
| Common
Warrants | |
| 6,666 | | |
$ | 309.56 | | |
May
4, 2022 | |
May
4, 2022 to May 4, 2027 |
| Common
Warrants | |
| 1,041,136 | | |
$ | 4.0625 | | |
May
24, 2022 | |
May
24, 2022 to May 24, 2027 |
| Common
Warrants | |
| 582,886 | | |
| 4.0625 | | |
June
28, 2022 | |
May
24, 2022 to June 28, 2027 |
| Common
Warrants | |
| 369,230 | | |
$ | 6.25 | | |
December
21, 2022 | |
December
21, 2022 to December 21, 2027 |
| Total | |
| 2,052,361 | | |
| | | |
| |
|
Convertible
Preferred Stock
On
October 12, 2021, the Company completed the initial closing of a registered direct offering with Keystone Capital Partners (Keystone
Capital) (the Initial October 2021 Registered Direct Offering), whereby the Company issued 5,000
shares of Series B-1 Convertible Preferred Stock,
par value $0.0001
per share, at a price of $1,000.00
per share. The Company received proceeds from
the Initial October 2021 Registered Direct Offering of approximately $4.6
million, net of offering expenses. On October
26, 2021, the Company completed the additional closing of the October 2021 Registered Direct Offering (the Additional October 2021 Registered
Direct Offering), whereby the Company issued 5,000
shares of Series B-2 Convertible Preferred Stock,
par value $0.0001
per share, at a price of $1,000.00
per share. The Company received proceeds from
the Additional October 2021 Registered Direct Offering of approximately $5.0
million, net of offering expenses.
The
Series B-1 and B-2 Convertible Preferred Stock were convertible into shares of common stock at any time at a conversion price per share
of the greater of $1,125
(Fixed Conversion Price), or the price computed
as the product of 0.85
multiplied by the arithmetic average of the closing
sale prices of a share of the Company’s common stock during the five
consecutive trading-day period immediately preceding
the conversion date (Variable Conversion Price). On October 12, 2021, Keystone Capital converted their 5,000
shares of B-1 Convertible Preferred Stock at
a conversion price of $1,181.25
per share into 4,232
shares of the Company’s common stock. Pursuant
to the terms of the Series B-2 Convertible Preferred Stock, the Fixed Conversion Price was adjusted during the first quarter of 2022
for certain dilutive issuances. The adjustment period ended on April 25, 2022 and the Fixed Conversion Price was fixed at $332.50
from the sale of common stock pursuant to the
Seven Knots Purchase Agreement. During March and April 2022, Keystone Capital converted their 1,200
shares of B-1 Convertible Preferred Stock at
a conversion price of $587.50
per share into 2,347
shares of the Company’s common stock. Pursuant
to the terms of the Series B-2 Convertible Preferred Stock, the Fixed Conversion Price was adjusted during the first quarter of 2022
for certain dilutive issuances.
On
March 24, 2022, the Company entered into an exchange agreement with the holder of its Series B-2 Convertible Preferred Stock, pursuant
to which the holder agreed to exchange 1,700
shares of the Series B-2 Convertible Preferred
Stock in consideration for 1,700
shares of the Company’s Series C Convertible
Preferred Stock, par value $0.0001
per share, $1,000.00
per share stated value. Except with respect to
voting provisions, the Series C and Series B-2 Preferred Stock had substantially similar terms.
On
May 4, 2022, pursuant to the May 2022 Exchange, the remaining 2,100
shares of Series B-2 Convertible Preferred Stock
and 1,700
shares of Series C Convertible Preferred Stock
were exchanged for Senior Subordinated Notes with an aggregate principal amount of $4.8
million and warrants to purchase up to 6,666
shares of common stock.
The
Company evaluated its convertible preferred stock to determine if an embedded component qualified as a derivative requiring bifurcation
in accordance with ASC 815 Derivative and Hedging. The Company determined that the embedded conversion feature required bifurcation
and needed to be accounted for separately as a free standing financial instrument. As a result, the fair value of the conversion feature
is marked-to-market at each reporting date and is recorded on the consolidated balance sheet as a derivative liability. Changes in fair
value are recognized on the consolidated income statement.
The
Company also evaluated its convertible preferred stock and determined that it required mezzanine equity classification. The proceeds
from the offering were first allocated to the fair value of the derivative liability and the remaining balance to the convertible preferred
stock. The creation of the derivative liability resulted in a discount to the convertible preferred stock, at an amount equal to the
fair value of the derivative liability at issuance. The discount is accreted through a deemed dividend which is recorded on the consolidated
income statement. The entire discount to the Series B-1 Convertible Preferred Stock was accreted through a single deemed dividend when
it was converted into common stock immediately after the initial closing. The Company elected to accrete the discount to the Series B-2
Convertible Preferred Stock over the four-year
period from the issuance date to the date when
the preferred stock becomes redeemable, and such accretion was immaterial for the year ended December 31, 2021. A deemed dividend for
return of capital was also recorded as a result of the Series B-1 Convertible Preferred Stock conversion into common stock.
Under
the valuation methods as described in Note 7- Fair Value of Financial Instruments, the Company recorded the following in the consolidated financial statements related to the convertible preferred
stock issued in 2021: (i) an aggregate $9.6
million in convertible preferred stock, net of
offering expenses, at issuance; (ii) an aggregate $0.5
million discount to the convertible preferred
stock at issuance; (iii) an aggregate $0.5
million in derivative liabilities at issuance;
(iv) a $0.8
million deemed dividend for return of capital as a result of
the Series B-1 Convertible Preferred Stock conversion into common stock; (v) a $0.3
million deemed dividend for the accretion of
the discount to the Series B-1 Convertible Preferred Stock upon conversion into common stock; and (vi) a $0.1
million gain in fair value of financial instruments
as a result of the mark-to-market adjustment of the derivative liability at December 31, 2021. During the year ended December 31, 2022,
a loss of $0.1
million was recognized as a result of the mark-to-market
adjustment of the derivative liability.
Effective
December 15, 2021, the Company amended and restated its certificate of incorporation, under which the Company is currently authorized
to issue up to 5,000,000 shares
of preferred stock, $0.0001
par value per share.
Nonconvertible
Preferred Stock
On
December 16, 2022, the Company filed a Certificate of Designation of Series D Non-Convertible Preferred Stock, par value $0.0001
per share (the Series D Preferred Shares). An
aggregate of 70
shares has been authorized, they are not convertible
into shares of common stock, have limited voting rights equal to 1%
of the total voting power of the then-outstanding shares of common stock entitled to vote per shares, are not entitled to dividends,
and are required to be redeemed by us, once our shareholders have approved a reverse split, as described in the Certificate of Designation.
All 70
shares of the Series D Preferred were subsequently
issued in connection with the December 2022 Securities Purchase Agreement as discussed in in Note 5- Debt. Since the Series D Preferred Shares can only be settled in cash, they are recorded
as a liability within accrued expenses in the consolidated balance sheets. The amount related to the liability is de minimus.
Common
Stock
Effective
January 17, 2018, the Company amended and restated its certificate of incorporation, under which the Company was authorized to issue
up to 300,000,000 shares
of common stock, $0.0001 par
value per share. Effective December 15, 2021, the Company further amended its amended and restated certificate of incorporation to increase
the number of authorized shares of common stock to 500,000,000
shares.
Public
Offerings
In
March 2021, the Company completed an underwritten public offering (the March 2021 Public Offering), whereby the Company issued 9,142
shares of common stock at a price to the public
of $3,281.25
per share (the March 2021 Public Offering Price).
The Company received proceeds from the March 2021 Public Offering of approximately $28.0
million, net of underwriting discounts. In addition,
the Company granted the underwriters a 30
days overallotment option to purchase up to an
additional 1,371
shares of its common stock at the March 2021
Public Offering Price, less applicable underwriting discounts. On April 6, 2021, the underwriters exercised their overallotment option
in full and the Company received proceeds of approximately $4.2
million, net of underwriting discounts. The common
stock issued in the March 2021 Public Offering were registered pursuant to a shelf registration statement on Form S-3 filed with the
SEC on March 4, 2021 and declared effective on March 11, 2021.
In
May 2021, the Company completed an underwritten public offering (the May 2021 Public Offering), whereby the Company issued 26,666
shares of common stock at a price to the public
of $1,875
per share and common warrants to purchase 26,666
shares of common stock. The common warrants
have an exercise price of $1,875
per share and can be exercised any time through
May 22, 2023. The Company received proceeds from the May 2021 Public Offering of approximately $46.8
million, net of underwriting discounts and fees.
In addition, the Company granted the underwriters a 30-day
overallotment option to purchase up to an additional 4,000
shares of its common stock at $1,856.25
per share, less applicable underwriting discounts,
and/or common warrants to purchase 4,000
shares of common stock, at $18.75
per warrant, less applicable underwriting discounts.
On May 20, 2021, the underwriters exercised their overallotment option to purchase warrants in full and the Company received proceeds
of approximately $0.1
million, net of underwriting discounts. On May
24, 2021, the underwriters exercised their overallotment option to purchase common stock and the Company issued an additional 1,358
shares of common stock and received proceeds
of approximately $2.4
million, net of underwriting discounts. The common
stock issued in the May 2021 Public Offering were registered pursuant to a shelf registration statement on Form S-3 filed with the SEC
on March 4, 2021 and declared effective on March 11, 2021.
In
May 2022, the Company completed an underwritten public offering (the May 2022 Public Offering), whereby the Company issued 181,320
shares of common stock and common warrants (the
May Common Stock Warrants) to purchase 362,640
shares of common stock at a price to the public
of $93.75.
The common warrants have an exercise price of $93.75
per share, a five-year
term, and were exercisable beginning on May 24,
2022. In the May 2022 Public Offering the Company also issued pre-funded warrants to purchase 102,680
shares of common stock and common warrants to
purchase 205,360
shares of common stock at a price to the public
of $93.63.
The pre-funded warrants had an exercise price of $0.125
per share, were exercisable beginning on May
24, 2022 were fully exercised after completion of this offering. The Company received proceeds from the May 2022 Public Offering of $18.1
million, net of $5.9
million debt repayment, underwriting discounts
and offering expenses. As discussed above, in Warrants, the May Common Stock Warrants were impacted by dilution adjustments and the strike
price was reset to $4.0625
during the year ended December 31, 2022, with
a further strike price reset to $0.8125,
subsequent to December 31, 2022.
Common
Stock Purchase Agreement
On
February 15, 2022, the Company entered into a common stock purchase agreement (the Stock Purchase Agreement) with Seven Knots, LLC (Seven
Knots), pursuant to which Seven Knots agreed to purchase from the Company up to $50.0
million in shares of the Company’s common
stock. Sales made to Seven Knots were at the Company’s sole discretion, and the Company controlled the timing and amount of any
and all sales. The price per share was based on the market price of the Company’s common stock at the time of sale as computed
under the Stock Purchase Agreement. As consideration for Seven Knots’ commitment to purchase shares of common stock, the Company
issued 1,025
shares of common stock to Seven Knots as commitment
fee shares.
Sales
of common stock to Seven Knots were subject to customary 4.99%
and 19.99%
beneficial ownership limitations. The Stock Purchase Agreement had a termination date of the earliest of March 1, 2024, or when Seven
Knots has purchased from the Company $50.0
million in shares of the Company’s common
stock, or as otherwise determined by the Stock Purchase Agreement at the Company’s option.
Effective
May 18, 2022, the Company and Seven Knots elected to terminate the Stock Purchase Agreement without any penalty or additional cost to
the Company. Prior to termination, the Company issued a total of 15,714
shares of common stock under the Stock Purchase
Agreement for aggregate net proceeds of $7.4
million.
Unregistered
shares
On
June 8, 2022, the Company entered into an agreement for services with a360 Media, LLC (a360 Media), pursuant to which a360 Media will
provide professional media support and advertising services in exchange for, at a360 Media’s option, either (a) $860,119
in cash, or (b) 18,547
shares of the Company’s common stock at
a value of $46.38
per share. On July 18, 2022, the Company and
a360 Media entered into a similar agreement for professional media support and advertising services in exchange for, at a360 Media’s
option, either (a) $1,409,858
in cash, or (b) 12,802
shares of the Company’s common stock at
a value of $110.13
per share. On August 15, 2022, the Company and
a360 Media entered into a similar agreement for professional media support and advertising services in exchange for, at a360 Media’s
option, either (a) $1,142,048
in cash, or (b) 22,558
shares of the Company’s common stock at
a value of $50.63
per share. Pursuant to these three agreements,
the company issued an aggregate 53,908
unregistered shares of the Company’s common
stock to a360 Media.
The
Company evaluated the a360 Media agreement and determined that in accordance with ASC 480 Distinguishing Liabilities from Equity
(ASC 480) and ASC 718 Compensation-Stock Compensation (ASC 718), the common stock issued to a360 should be equity classified and
recorded as a prepaid asset in the consolidated balance sheet, which is then amortized to noncash stock-based compensation expense when
services are received. During the year ended December 31, 2022, the Company recorded $3.4
million in stock-based compensation expense,
which was recorded within sales and marketing expense in the consolidated statements of operations.
Purchase
Rights
On
September 15, 2022, the Company entered into certain exchange agreements with the Adjuvant Purchasers and the May 2022 Notes Purchasers
to exchange, upon request, the Purchase Rights for an aggregate of 942,080
shares of the Company’s common stock. The
number of right shares for each Purchase Right is initially fixed at issuance, but is subject to certain customary adjustments, and,
until the second anniversary of issuance, adjustments for certain dilutive Company equity issuances and expire on June 28, 2027. Refer
to Note 7- Fair Value of Financial Instruments for the
accounting treatment of the Purchase Rights. In connection with the December 2022 Notes issuance, the Company increased the number of
outstanding Purchase Rights by 3,808,814.
During the year ended December 31, 2022, the Company issued 260,692
shares of common stock upon the exercises of
certain Purchase Rights. As of December 31, 2022, Purchase Rights related to the Adjuvant Purchase Rights and May Note Purchase Rights
of 4,490,202
shares of the Company’s common stock remained
outstanding. Subsequent to December 31, 2022, the Purchase Rights had an additional dilution adjustment, as discussed in Note
14 – Subsequent Events.
Common
Stock Reserved for Future Issuance
Common
stock reserved for future issuance, on a one-for-one basis, is as follows in common equivalent shares as of December 31, 2022:
Summary
of Common Stock Reserved for Future Issuance
| Common
stock issuable upon the exercise of stock options outstanding | |
| 5,672 | |
| Common
stock issuable upon the exercise of common stock warrants | |
| 2,052,367 | |
| Common
stock issuance upon the exercise of purchase rights | |
| 4,490,202 | |
| Common
stock available for future issuance under the 2019 ESPP | |
| 509 | |
| Common
stock available for future issuance under the Amended and Restated 2014 Plan | |
| 3,989 | |
| Common
stock available for future issuance under the Amended Inducement Plan | |
| 525 | |
| Common
stock reserved for the conversion of convertible notes | |
| 18,105,684 | |
| Total
common stock reserved for future issuance | |
| 24,658,948 | |
11.
Stock-based Compensation
Equity
Incentive Plans
The
following table summarizes stock-based compensation expense related to stock options, restricted stock awards (RSAs) and RSUs granted
to employees, non-employee directors and consultants, and the 2019 ESPP (as defined below) included in the consolidated statements of
operations as follows (in thousands):
Schedule
of Stock-based Compensation Expense Related to Stock Options
| | |
2022 | | |
2021 | |
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Research
and development | |
$ | 553 | | |
$ | 1,357 | |
| Selling
and marketing | |
| 497 | | |
| 1,870 | |
| General
and administrative | |
| 2,263 | | |
| 5,671 | |
| Total | |
$ | 3,313 | | |
$ | 8,898 | |
The
2012 Equity Incentive Plan (the 2012 Plan) provides for the issuance of RSAs, RSUs, or non-qualified and incentive common stock options
to its employees, non-employee directors and consultants, from its authorized shares. In general, the options expire ten
years from the date of grant and generally vest
either (i) over a four-year
period, with 25%
exercisable at the end of one year from the employee’s hire date and the balance vesting ratably thereafter or (ii) over a three-year
period, with 25%
exercisable at the grant date and the balance vesting ratably thereafter. No further awards may be issued under the 2012 Plan.
On
September 15, 2014, the Company’s board of directors adopted, and stockholders approved, the 2014 Equity Incentive Plan (the 2014
Plan), which was amended and restated on each of May 2018 and February 26, 2019 (the Amended and Restated 2014 Plan). Per the terms of
the Amended and Restated 2014 Plan, the shares reserved will automatically increase on each January 1 through 2024, by an amount equal
to the smaller of (i) 4%
of the number of shares of common stock issued and outstanding on the immediately preceding December 31; or (ii) an amount determined
by our board of directors.
On
July 24, 2018, upon the recommendation by the Compensation Committee, the Company’s board of directors adopted the Evofem Biosciences,
Inc. 2018 Inducement Equity Incentive Plan (the Inducement Plan). Under the Inducement Plan, as amended, the number of authorized shares
total 666
shares. The only persons eligible to receive
awards under the Inducement Plan are individuals who satisfy the standards for inducement grant recipients under Nasdaq Marketplace Rule
5635(c)(4), generally, a person not previously an employee or director of the Company, or following a bona fide period of non-employment,
as an inducement material to the individual’s entering into employment with the Company.
Stock
Options
The
following table summarizes share option activity for the year ended December 31, 2022:
Schedule
of Stock Option Information
| | |
Options | | |
Weighted
Average Exercise Price | | |
Weighted
Average Remaining Contractual Term (in Years) | | |
Aggregate
Intrinsic Value (in thousands) | |
| Outstanding
as of December 31, 2021 | |
| 5,666 | | |
$ | 10,007.50 | | |
| 6.8 | | |
$ | — | |
| Granted | |
| 2,104 | | |
$ | 805.00 | | |
| | | |
| | |
| Exercised | |
| — | | |
$ | — | | |
| | | |
| | |
| Cancelled | |
| (2,098 | ) | |
$ | 6,851.25 | | |
| | | |
| | |
| Outstanding
as of December 31, 2022 | |
| 5,672 | | |
$ | 7,761.25 | | |
| 5.1 | | |
$ | — | |
| Options
expected to vest as of December 31, 2022 | |
| 5,672 | | |
$ | 7,761.25 | | |
| 5.1 | | |
$ | — | |
| Options
vested and exercisable as of December 31, 2022 | |
| 3,988 | | |
$ | 10,058.75 | | |
| 4.3 | | |
$ | — | |
The
following table summarizes certain information regarding stock options for the years ended December 31, 2022 and 2021 (in thousands,
except per share data):
| | |
2022 | | |
2021 | |
| Weighted
average grant date fair value per share of options granted during the period | |
$ | 645.00 | | |
$ | 272.50 | |
| Cash
received from options exercised during the period | |
$ | — | | |
$ | — | |
| Intrinsic
value of options exercised during the period | |
$ | — | | |
$ | — | |
As
of December 31, 2022, unrecognized stock-based compensation expense for employee stock options was approximately $2.9
million, which the Company expects to recognize
over a weighted-average remaining period of 2.2
years, assuming all unvested options become fully
vested.
Summary
of Assumptions
The
fair value of noncash stock-based compensation for stock options granted to employees and non-employees was estimated on the date of
grant using the Black-Scholes option pricing model based on the following weighted-average assumptions for options granted for the periods
indicated.
Schedule
of Weighted-Average Assumptions Rate
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Expected
volatility | |
| 102.5 | % | |
| 101.1 | % |
| Risk-free
interest rate | |
| 2.0 | % | |
| 0.7 | % |
| Expected
dividend yield | |
| — | % | |
| — | % |
| Expected
term (years) | |
| 6.0 | | |
| 5.9 | |
Expected
volatility. The expected volatility assumption is based on volatilities of a peer group of similar companies whose share prices are
publicly available. The peer group was developed based on companies in the biotechnology industry.
Risk-free
interest rate. The risk-free interest rate assumption is based on observed interest rates appropriate for the expected term of the
stock option grants.
Expected
dividend yield. The expected dividend yield assumption is based on the fact that the Company has never paid cash dividends and has
no present intention to pay cash dividends.
Expected
term. The expected term represents the period options are expected to be outstanding. Because the Company does not have historical
exercise behavior, it determines the expected term assumption using the practical expedient as provided for under ASC 718, Compensation-Stock
Compensation (ASC 718), which is the midpoint between the requisite service period and the contractual term of the option.
Restricted
Stock Awards
The
following table summarizes RSAs activity for the year ended December 31, 2022:
Schedule
of Restricted Stock Awards
| | |
Shares
(RSAs) | | |
Weighted
Average Fair Value per Share | |
| Unvested
as of December 31, 2021 | |
| — | | |
$ | — | |
| Granted | |
| 1,258 | | |
$ | 917.50 | |
| Forfeited | |
| (1,258 | ) | |
$ | 917.50 | |
| Released | |
| — | | |
$ | — | |
| Unvested
as of December 31, 2022 | |
| — | | |
$ | — | |
Of
the total RSAs granted during the years ended December 31, 2022 and 2021, zero
and 377
shares vested in accordance with the Company’s
achievement of the Performance-based RSAs milestones, respectively.
For
the performance-based RSAs, (i) the fair value of the award is determined on the grant date; (ii) the Company assesses the probability
of achieving each individual milestone associated with the award using reasonable assumptions based on the Company’s operation
performance towards each milestone; (iii) the fair value of the shares subject to the milestone is expensed over the implicit service
period commencing once management believes the performance criteria is probable of being met; and (iv) the Company reassesses the probability
of achieving each individual milestone at each reporting date, and any change in estimate is accounted for through a cumulative adjustment
in the period when the change in estimate occurs. The non-performance based RSAs and RSUs are valued at the fair value on the grant date
and the associated expenses will be recognized over the vesting period.
As
of December 31, 2022, there was no
unrecognized noncash stock-based compensation
expense related to unvested RSAs.
Employee
Stock Purchase Plan
On
May 7, 2019, the board of directors approved a 2019 Employee Stock Purchase Plan (the 2019 ESPP), which was approved by stockholders
at the 2019 annual meeting held on June 5, 2019. The 2019 ESPP initially authorized the issuance of 266
shares of common stock pursuant to purchase rights
granted to employees. In addition, the number of shares available for issuance under the 2019 ESPP will increase on January 1 of each
year until the first day of 2029, in an amount equal to the lesser of (i) 533
shares, (ii) 2%
of the shares of common stock outstanding on December 31, or (iii) such lesser number of shares as is determined by the board of directors.
This provision resulted in an additional 133
shares added to the total number of authorized
shares on January 1, 2022. The 2019 ESPP is intended to qualify as an employee stock purchase plan within the meaning of Section 423
of the Internal Revenue Code of 1986, as amended.
The
2019 ESPP enables eligible full-time and part-time employees to purchase shares of the Company’s common stock through payroll deductions
of between 1%
and 15%
of eligible compensation during an offering period. A new offering period begins around June 15 and December 15 of each year. At the
last business day of each offering period, the accumulated contributions made during the offering period will be used to purchase shares.
The purchase price is 85%
of the lesser of the fair market value of the common stock on the first or the last business day of an offering period. The maximum number
of shares of common stock that may be purchased by any participant during an offering period will be equal to $25,000
divided by the fair market value of the common
stock on the first business day of an offering period. During the years ended December 31, 2022 and 2021, there were 601
and 245
shares of common stock purchased under the 2019
ESPP, respectively. In October 2022, the Board terminated the current offering period ending December 15, 2022, refunded all employee
contributions, and suspended future offering periods.
The
Company recognized $0.1
million and $0.3
million in noncash stock-based compensation expense
related to the 2019 ESPP for the years ended December 31, 2022 and 2021, respectively. In October 2022, the Board terminated the current
offering period ending December 15, 2022, refunded all employee contributions, and suspended future offering periods. As of December
31, 2022, the Company had no unrecognized noncash stock-based compensation expense related to the 2019 ESPP.
The
fair value of shares to be issued to employees under the 2019 ESPP is estimated using a Black-Scholes option-pricing model at the grant
date, which requires the use of subjective and complex assumptions, including (i) the expected stock price volatility, (ii) the calculation
of the expected term of the award, (iii) the risk-free interest rate and (iv) the expected dividend yield. The following weighted average
assumptions were used in the calculation of fair value of shares under the 2019 ESPP at the grant dates for the period indicated.
Schedule
of Weighted-Average Assumptions Rate
| | |
Years
Ended December 31, | |
| | |
2022 | | |
2021 | |
| Expected
volatility | |
| 177.2 | % | |
| 83.9 | % |
| Risk-free
interest rate | |
| 2.3 | % | |
| 0.1 | % |
| Expected
dividend yield | |
| — | % | |
| — | % |
| Expected
term (years) | |
| 0.5 | | |
| 0.5 | |
12.
Employee Benefits
The
Company has a defined contribution 401(k) plan (401(k) Plan) for all qualifying employees. Employees are eligible to participate in the
plan beginning on the first day of the month following their three-month
anniversary of employment. Under the terms of
the 401(k) Plan, employees may make voluntary contributions as a percent of their compensation. The Company makes a safe-harbor contribution
of three percent (3.0%)
of each employee’s gross earnings, subject to Internal Revenue Service limitations. In the years ended December 31, 2022 and 2021,
the Company made safe-harbor contributions of approximately $0.6
million and $0.8
million, respectively.
13.
Income Taxes
The
Company is subject to taxation in the United States and various states jurisdictions. Tax years since inception remain open to examination
by the major taxing jurisdictions. The Company’s consolidated pretax loss for the years ended December 31, 2022 and 2021 were generated
by domestic as follows (in thousands). There are no consolidated pretax losses generated by foreign operations for the periods indicated.
Schedule
of Consolidated Pretax Loss from Continuing Operations
| | |
2022 | | |
2021 | |
| United
States | |
$ | (76,654 | ) | |
$ | (205,175 | ) |
| Total | |
$ | (76,654 | ) | |
$ | (205,175 | ) |
The
income tax provision for the years ended December 31, 2022 and 2021 consisted of the following (in thousands):
Schedule
of Income Tax Provision from Continuing Operations
| | |
2022 | | |
2021 | |
| United
States | |
$ | — | | |
$ | — | |
| State | |
| (44 | ) | |
| (17 | ) |
| Total
current tax provision | |
| (44 | ) | |
| (17 | ) |
| Total
deferred tax provision | |
| — | | |
| — | |
| Total | |
$ | (44 | ) | |
$ | (17 | ) |
The
reconciliation between the Company’s effective tax rate on loss before income tax and the statutory tax rate for the years ended
December 31, 2022 and 2021 was as follows:
Schedule
of Reconciliation on Effective Tax Rate on Loss Before Income Tax and Statutory Tax Rate
| | |
2022 | | |
2021 | |
| Statutory
rate | |
| 21.00 | % | |
| 21.00 | % |
| State
income tax, net of federal benefit | |
| 2.12 | % | |
| 1.17 | % |
| Nondeductible
expenses | |
| (0.41 | )% | |
| (0.48 | )% |
| Equity-based
expenses | |
| (1.82 | )% | |
| (0.70 | )% |
| Change
in fair value of purchase rights | |
| 22.60 | % | |
| — | % |
| Change
in fair value of financial instruments | |
| (20.00 | )% | |
| (3.44 | )% |
| Return
to provision | |
| (0.47 | )% | |
| (0.30 | )% |
| Tax
credits | |
| 1.41 | % | |
| 0.68 | % |
| Uncertain
tax positions | |
| (0.39 | )% | |
| (0.50 | )% |
| Change
in valuation allowance | |
| (24.11 | )% | |
| (17.43 | )% |
| Effective
tax rate | |
| (0.07 | )% | |
| — | % |
Deferred
income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial
reporting purposes and the amounts used for income tax purposes. The Company’s net deferred tax assets arising from its taxable
subsidiaries consisted of the following components as of December 31, 2022 and 2021 (in thousands):
Schedule
of Net Deferred Tax Assets Arising From Taxable Subsidiaries
| | |
2022 | | |
2021 | |
| Deferred
tax assets: | |
| | | |
| | |
| Net
loss carryforwards | |
$ | 126,056 | | |
$ | 112,891 | |
| Fixed
assets and intangibles | |
| 338 | | |
| 423 | |
| Research
and development capitalization | |
| 4,951 | | |
| — | |
| Research
and development credits | |
| 6,136 | | |
| 5,233 | |
| Stock-based
compensation | |
| 3,367 | | |
| 3,513 | |
| Other | |
| 2,247 | | |
| 2,726 | |
| Total
deferred tax assets | |
| 143,095 | | |
| 124,786 | |
| Deferred
tax liabilities | |
| | | |
| | |
| Lease
assets | |
| (1,011 | ) | |
| (1,218 | ) |
| Fixed
assets | |
| (113 | ) | |
| (101 | ) |
| Other | |
| (29 | ) | |
| — | |
| Less:
valuation allowance | |
| (141,942 | ) | |
| (123,467 | ) |
| Net
deferred tax assets | |
$ | — | | |
$ | — | |
In
assessing the ability to realize deferred tax assets, management considers whether it is more likely than not that some portion or all
the deferred tax assets will be realized. Generally, the ultimate realization of deferred tax assets is dependent upon the generation
of future taxable income during the periods in which those temporary differences become deductible. Based on historical performance and
future expectations, management has determined a valuation allowance is needed in respect to its ending deferred tax assets.
As
of December 31, 2022, the Company had net operating loss (NOL) carryforwards for federal income tax purposes of approximately $548.9
million, which will begin to expire in 2029 if
not utilized. As of December 31, 2022, the Company had NOL carryforwards in various states of approximately $212.8
million. The state carryforwards have varying
expiration dates beginning in 2029.
As
of December 31, 2022, the Company had federal and state research and development (R&D) tax credit carryforwards of approximately
$6.2
million and $2.5
million, respectively. As of December 31, 2021,
the Company had federal and state R&D tax credit carryforwards of approximately $5.1
million and $2.3
million, respectively. The federal R&D tax
credits begin to expire in 2031, unless utilized, and the state credits do not expire.
For
the tax years beginning on or after January 1, 2022, the Tax Cuts and Jobs Act of 2017 (“TCJA”) eliminates the option to
currently deduct research and development expenses and requires taxpayers to capitalize and amortize them over five years for research
activities performed in the United States and 15 years for research activities performed outside the United States pursuant to IRC Section
174. Although Congress is considering legislation that would repeal or defer this capitalization and amortization requirement, it is
not certain that this provision will be repealed or otherwise modified. If the requirement is not repealed or replaced, it will decrease
our tax deduction for research and development expense in future years.
The
following table summarized the activity related to the Company’s gross unrecognized tax benefits as of December 31, 2022 and 2021
(in thousands):
Schedule
of Activity Related to Gross Unrecognized Tax Benefits
| | |
2022 | | |
2021 | |
| Balance
at the beginning of the year | |
$ | 2,679 | | |
$ | 1,465 | |
| Adjustments
related to prior year tax positions | |
| 5 | | |
| 813 | |
| Increases
related to current year tax positions | |
| 304 | | |
| 401 | |
| Decreases
due to statute of limitation expiration | |
| — | | |
| — | |
| Balance
at end of year | |
$ | 2,988 | | |
$ | 2,679 | |
The
Company recognizes a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon
examination, including resolutions of any related appeals or litigation processes, based on the technical merits, and uncertain income
tax positions must meet a more likely than not recognition threshold to be recognized. The Company recognizes interest and penalties
related to unrecognized tax benefits within the income tax expense line in the consolidated statements of operations. There were no accrued
interest and penalties associated with unrecognized tax benefits as of December 31, 2022. The Company does not anticipate a significant
change in its uncertain tax benefits over the next 12 months.
Management
believes it is more likely than not that all significant tax positions taken to date would be sustained by the relevant taxing authorities.
Furthermore, the Company has not recognized any tax benefits to date because the Company has established a full valuation allowance for
its deferred tax assets due to uncertainties as to their ultimate realization.
Pursuant
to Internal Revenue Code (IRC) sections 382 and 383, use of the Company’s NOLs and R&D credit carryforwards may be limited
if a cumulative change in ownership of more than 50.0% (by value) occurs within a rolling three-year period. The Company completed a
formal Section 382 analysis through the period ending December 31, 2019, and determined they experienced ownership changes in 2010 and
2018. Accordingly, the Company has reduced its deferred tax asset for NOLs and R&D tax credits by the estimated impact of IRC sections
382 and 383 as of December 31, 2019. The Company has not completed a formal Section 382 analysis after the period ending December 31,
2019. Any future ownership changes could further impact the utilization of the NOLs and R&D tax credits, however given the full valuation
allowance this would not result in an impact to the Company’s tax expense.
14.
Subsequent Events
Subsequent
events were evaluated through the filing date of the Annual Report and were updated for the 2023 reverse stock split.
Additional
Financings
In
February, March and April 2023, the Company entered into securities purchase agreements with certain investors providing for the sale
and issuance of senior secured convertible notes (collectively, the 2023 SPAs). The 2023 SPAs included (i) convertible promissory notes
with aggregate original principal amounts of approximately $1.4
million, $0.6
million, $0.5
million and $0.8
million, respectively (the 2023 Notes), and (ii)
warrants to purchase an aggregate 553,846,
240,000,
215,384
and 615,384
shares of common stock, respectively (the 2023
Warrants and collectively, the 2023 Offerings). The 2023 Offerings closed on February 17, 2023 (the February 2023 Closing), March 13,
2023, March 20, 2023 (the March 2023 Closing) and April 5, 2023 (the April 2023 Closing), respectively, with net proceeds to the Company,
after deducting offering expenses, of approximately $0.7
million, $0.3
million, $0.3
million, and $0.5
million, respectively. The 2023 SPAs also included
a Registration Rights Agreement that us to register the common stock underlying the 2023 Notes and 2023 Warrants within the timeframes
specified therein. In addition, the Company issued warrants to purchase an aggregate 99,692
and 43,200
shares of common stock in February and March
2023 Closing to the placement agent.
Upon
the April 2023 Closing, the conversion and strike prices, as applicable, of the Baker Notes, Baker Warrants, the May 2022 Common Warrants,
the June 2022 Baker Warrants, the Adjuvant Notes, the December 2022 Notes and Warrants, and the Notes and Warrants in the February and
March 2023 Closing reset to $0.8125
per share, accordingly. Additionally, the Company’s
outstanding Purchase Rights increased by approximately 24.8
million since December 31, 2022.
Event
of Default
On
March 7, 2023, Baker Bros. Advisors, LP (the Designated Agent) provided a Notice of Event of Default and Reservation of Rights (the Notice
of Default) relating to the Securities Purchase and Security Agreement dated April 23, 2020, and subsequently amended (SPA), by and amount
the Company, Designated Agent, the Guarantors and Baker
Purchasers. The Notice of Default claims that the Company has failed to maintain the “Required
Reserve Amount” as required by Section 2.7 of the Third Amendment to the Securities Purchase Agreement and Section 8.1(e) of the
SPA. The Designated Agent claims such failure constitutes an immediate Event of Default pursuant to Section 9.1(e) of the SPA. The Designated
Agent, at the direction of the Baker Purchasers, has accelerated repayment of the outstanding balance payable and elected its remedies
pursuant to Section 5.07(b) of the Securities Purchase Agreement. As a result, approximately $92.7
million representing
two times the sum of the outstanding balance and all accrued and unpaid interest thereon and all other amounts due under the SPA and
other documents is due and payable within three
business days of receipt
of the Notice of Default. As of the date of the filing of the Annual Report, the Baker Notes remain outstanding.
The failure to cure the default or otherwise settle or resolve, could have a significant negative financial impact on the Company, could
result in litigation, and could result in the assets of the company being seized, attached or otherwise utilized to satisfy the debt.
2023
Reverse Stock Split
On
March 15, 2023, the Company held a Special Meeting of its Stockholders in which the stockholders approved an amendment to the Company’s
Certificate of Incorporation to
effectuate a reverse stock split of the outstanding shares of the Company’s common stock by a ratio of not less than 1-for-20 and
not more than 1-for-125 at any time on or prior to March 15, 2024,
with the exact ratio to be set at a whole number within such range by the Company’s board of directors.
The Reverse Stock Split
became effective on May 18, 2023 upon the opening of trading on the OTCQB (the Effective Time). Trading of the Company’s common
stock on the OTCQB continued, on a post-split adjusted basis, on May 18, 2023, under the ticker symbol “EVFMD.” At the Effective
Time, every 125 shares of the Company’s issued and outstanding common stock were automatically converted into one share of common
stock, without any change to the par value per share. In addition, proportionate adjustments were made to the per share exercise price
and the number of shares issuable upon the exercise of all outstanding stock options and warrants to purchase shares of common stock,
the number of shares issuable upon the vesting of all RSAs, and the number of shares of common stock reserved for issuance pursuant to
the Company’s equity incentive compensation plans, convertible notes and convertible preferred stock. Any stockholder who would
otherwise be entitled to a fractional share of the Company’s common stock created as a result of the Reverse Stock Split is entitled
to receive a cash payment equal to the product of such resulting fractional interest in one share of the Company’s common stock
multiplied by the closing trading price of the Company’s common stock on the trading day immediately preceding the Effective Time.
These consolidated financial statements are retrospectively adjusted for this Reverse Stock Split. Following the specified
20-day period, the Company’s ticker symbol changed back to “EVFM” on or around June 16, 2023.
Reduction
in Force
On
March 20, 2023 the Board of Directors of Evofem Biosciences, Inc. (the “Company”) approved a reduction in force (RIF) intended
to conserve the Company’s current cash resources and manage operating expenses.
The
Company estimates that it will incur aggregate pre-tax charges of approximately $0.1
million in connection with the reduction in force,
primarily consisting of notice period and severance payments, employee benefits and related costs. The Company expects that the reduction
in force will be complete by the end of the second quarter of 2023 and that these one-time charges will be incurred in the first quarter
of 2023.
Default
under Lease Agreement
On
March 20 2023, the Company received a notice of default from its landlord, for failing to pay March 2023 rent timely resulting in a breach
under the agreement. As a result, the Company’s letter of credit in the amount of $0.8
million, in restricted cash, has been recovered
by the landlord. As of the date of the filing of the Annual Report we are unable to estimate the amount of damages
the landlord may seek, if any, as a result of the breach.

3,336,730
Shares of Common Stock
Issuable
upon Exercise of Outstanding Warrants
7,642,038
Shares of Common Stock
Issuable
upon Conversion of Senior Subordinated Convertible Notes
PROSPECTUS
August
14, 2023
Evofem Biosciences (QB) (USOTC:EVFM)
Historical Stock Chart
From Feb 2025 to Mar 2025
Evofem Biosciences (QB) (USOTC:EVFM)
Historical Stock Chart
From Mar 2024 to Mar 2025