false
0001682852
PRE 14A
0001682852
2023-01-01
2023-12-31
0001682852
2022-01-01
2022-12-31
0001682852
2021-01-01
2021-12-31
0001682852
2020-01-01
2020-12-31
0001682852
ecd:PeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2023-01-01
2023-12-31
0001682852
ecd:NonPeoNeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2023-01-01
2023-12-31
0001682852
ecd:PeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2022-01-01
2022-12-31
0001682852
ecd:NonPeoNeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2022-01-01
2022-12-31
0001682852
ecd:PeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2021-01-01
2021-12-31
0001682852
ecd:NonPeoNeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2021-01-01
2021-12-31
0001682852
ecd:PeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2020-01-01
2020-12-31
0001682852
ecd:NonPeoNeoMember
mrna:DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember
2020-01-01
2020-12-31
0001682852
ecd:PeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2023-01-01
2023-12-31
0001682852
ecd:NonPeoNeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2023-01-01
2023-12-31
0001682852
ecd:PeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2022-01-01
2022-12-31
0001682852
ecd:NonPeoNeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2022-01-01
2022-12-31
0001682852
ecd:PeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2021-01-01
2021-12-31
0001682852
ecd:NonPeoNeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2021-01-01
2021-12-31
0001682852
ecd:PeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2020-01-01
2020-12-31
0001682852
ecd:NonPeoNeoMember
mrna:IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember
2020-01-01
2020-12-31
0001682852
ecd:PeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2023-01-01
2023-12-31
0001682852
ecd:NonPeoNeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2023-01-01
2023-12-31
0001682852
ecd:PeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2022-01-01
2022-12-31
0001682852
ecd:NonPeoNeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2022-01-01
2022-12-31
0001682852
ecd:PeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2021-01-01
2021-12-31
0001682852
ecd:NonPeoNeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2021-01-01
2021-12-31
0001682852
ecd:PeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2020-01-01
2020-12-31
0001682852
ecd:NonPeoNeoMember
mrna:YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember
2020-01-01
2020-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2023-01-01
2023-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2023-01-01
2023-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2022-01-01
2022-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2022-01-01
2022-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2021-01-01
2021-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2021-01-01
2021-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2020-01-01
2020-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember
2020-01-01
2020-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2023-01-01
2023-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2023-01-01
2023-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2022-01-01
2022-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2022-01-01
2022-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2021-01-01
2021-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2021-01-01
2021-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2020-01-01
2020-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember
2020-01-01
2020-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2023-01-01
2023-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2023-01-01
2023-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2022-01-01
2022-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2022-01-01
2022-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2021-01-01
2021-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2021-01-01
2021-12-31
0001682852
ecd:PeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2020-01-01
2020-12-31
0001682852
ecd:NonPeoNeoMember
mrna:ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember
2020-01-01
2020-12-31
0001682852
1
2023-01-01
2023-12-31
0001682852
2
2023-01-01
2023-12-31
0001682852
3
2023-01-01
2023-12-31
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
SCHEDULE 14A
PROXY STATEMENT PURSUANT TO SECTION 14(a)
OF THE SECURITIES EXCHANGE ACT OF 1934
(Amendment No. )
 |
Filed by the Registrant |
 |
Filed by a Party other than the Registrant |
| Check the appropriate box: |
 |
Preliminary Proxy Statement |
 |
Confidential, for Use of the Commission Only (as permitted by Rule 14A-6(E)(2)) |
 |
Definitive Proxy Statement |
 |
Definitive Additional Materials |
 |
Soliciting Material under §240.14a-12 |
MODERNA, INC.

(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if
other than the Registrant)
| Payment of Filing Fee (Check all boxes that apply): |
 |
No fee required. |
 |
Fee paid previously with preliminary materials. |
 |
Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. |


Notice of Annual Meeting
of Shareholders
|
MONDAY, MAY 6, 2024
8:00 a.m., Eastern Time
www.virtualshareholdermeeting.com/MRNA2024
|
| |
|
HOW TO VOTE
Review your proxy statement and vote
in one of three ways:
|
| |
|
 |
Internet
www.proxyvote.com |
| |
|
 |
Telephone
1-800-690-6903 |
| |
|
 |
Mail
Complete, sign, date, and return your proxy card or voting instruction form |
YOUR VOTE IS IMPORTANT.
Even if you plan to participate in the Annual Meeting, we urge you to submit your proxy in advance to ensure your shares are represented.
This will not affect your right to participate in the meeting and to vote your shares at that time. For additional information on voting
and participating in the meeting, please see “Information About the 2024 Annual Meeting of Shareholders” beginning on page 83.
To the Shareholders of Moderna, Inc.:
You are cordially
invited to the Annual Meeting of Shareholders of Moderna, Inc., which will be held on Monday, May 6, 2024, beginning at 8:00 a.m., Eastern
Time (the Annual Meeting), for the following purposes:
| 1. |
To elect three Class III directors, each to serve for a three-year term expiring at the 2027 annual meeting of shareholders; |
| 2. |
To approve, on a non-binding, advisory basis, the compensation of our named executive officers; |
| 3. |
To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024; |
| 4. |
To approve a management proposal to amend our Certificate of Incorporation to provide shareholders the right to call a special meeting; |
| 5. |
To approve a management proposal to amend our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation; and |
| 6. |
To transact such other business as may be properly brought before the Annual Meeting or any adjournment or postponement thereof. |
The Annual Meeting
will be conducted virtually. You will be able to participate in the Annual Meeting online and submit your questions during the meeting
by visiting www.virtualshareholdermeeting.com/MRNA2024. You also will be able to vote your shares electronically during the Annual Meeting.
For more information about our virtual Annual Meeting, please see “Information About the 2024 Annual Meeting of Shareholders”
beginning on page 83.
Our
Board of Directors has fixed the close of business on March 7, 2024, as the Record Date for determining the shareholders that are entitled
to notice of and to vote at the Annual Meeting and any adjournment or postponement thereof.
This
proxy statement and our Annual Report on Form 10-K for the year ended December 31, 2023, are first being mailed on or about March [
], 2024, to all shareholders entitled to vote at the Annual Meeting. These materials also are available at www.proxyvote.com, using
the control number provided with your materials.
By order of the Board of Directors,

Stéphane Bancel
Chief Executive Officer and Director
Cambridge, Massachusetts
March [ ], 2024
 |
2024
Proxy statement |
1 |
Proxy Summary
This summary highlights certain
information from this Proxy Statement, but does not contain all the information that you should consider. Please read the entire Proxy
Statement before voting your shares. For more complete information regarding Moderna’s 2023 performance, please review our Annual
Report on Form 10-K for the year ended December 31, 2023, including the sections captioned "Special Note Regarding Forward-Looking Statements" and "Risk Factors", for a description of the substantial risks and uncertainties related to the forward-looking statements included herein. Expected product launches are subject to, among other risks, assumptions and uncertainties, clinical trial, regulatory and commercial success, and availability of supply.

When
|

Where
|

Record date
|
Monday,
May 6,
2024, at 8:00 a.m.,
Eastern time. |
The meeting
will be held virtually at
www.virtualshareholdermeeting.com/MRNA2024 |
March
7, 2024 |
Meeting Agenda
The matters we will act upon at
the Annual Meeting are:
| Proposal |
|
|
Board voting
recommendation |
|
Where to find
more information |
| Proposal 1: Elect three Class III directors, each for a three-year
term |
|
 |
FOR all nominees |
|
Page 8 |
| Proposal 2: Approve, on a non-binding, advisory basis the
compensation of our named executive officers |
|
 |
FOR |
|
Page 35 |
| Proposal 3: Ratify the appointment of Ernst & Young
LLP as our independent registered public accounting firm for 2024 |
|
 |
FOR |
|
Page 76 |
| Proposal 4: Amend our Certificate of Incorporation to provide
shareholders the right to call a special meeting |
|
 |
FOR |
|
Page 79 |
| Proposal 5: Amend our Certificate of Incorporation to reflect
new Delaware law provisions allowing for officer exculpation |
|
 |
FOR |
|
Page 81 |
 |
2024
Proxy statement |
2 |
Our Mission
| To deliver the greatest possible impact to people through mRNA medicines |
About Moderna
Moderna is a leader in the
creation of the field of mRNA medicines. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made
and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for
more than a decade, the Company has developed medicines at unprecedented speed and efficiency, including one of the earliest and
most effective COVID-19 vaccines.
Moderna’s mRNA platform has enabled the development of therapeutics and vaccines for
infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by
the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible
impact to people through mRNA medicines.
2023 Performance
| $6.7 billion |
|
48% U.S.
market share |
|
9 late-stage
programs |
| |
|
|
|
|
| in net product sales from COVID-19 vaccines in 2023. |
|
for COVID-19 vaccine sales to the retail market in the 2023 fall season, up from 37% in 2022 (Source: IQVIA). |
|
including Phase 3 programs for next-generation COVID, RSV, seasonal flu, seasonal flu + COVID, CMV, and INT (melanoma and non-small cell lung cancer) and late-stage programs for PA and MMA. |
| |
|
|
|
|
| $13.3 billion |
|
45 programs |
|
19 markets |
| |
|
|
|
|
| in cash, cash equivalents and investments as of December 31, 2023, available to fund future pipeline growth plans. |
|
in development, reflecting continued investment in the pipeline, laying the groundwork for future growth and profitability. |
|
with Moderna employees around the globe as of December 31, 2023, with a presence in key markets for our products. |
Our Strategic Priorities
Commercial
Execution: We are focused on commercial execution to drive sales growth and profitability. This
includes building on the current momentum from U.S. Spikevax sales and preparing for multiple product launches, including the expected
launch of our RSV vaccine candidate and other potential launches in 2025 and beyond.
Disciplined
Investment: We plan to continue to review our cost structure to find efficiencies, and to remain
disciplined around spending. We expect to leverage our existing manufacturing and commercial infrastructure as we launch new products
in our respiratory franchise in 2024 and 2025.
Executing
on Late-Stage Pipeline: We are focused on advancing our late-stage pipeline to drive organic sales
growth. Moderna expects to launch up to 15 products in the next five years, with up to four of those launches possibly occurring by 2025.
 |
2024
Proxy statement |
3 |
Proposal 1: Director Nominees
At the Annual Meeting, three Class
III directors will be elected for a three-year term. The Class III directors up for election are set forth below.
| Name |
Age |
Independent |
Principal occupation |
Committees* |
Other public boards |
Director
since |
| Class III directors nominated for re-election for
a three-year term |
Audit |
Comp |
Nom Gov |
Prod Dev |
Sci Tech |
|
|
| Robert Langer, Sc.D. |
75 |
 |
David H. Koch Institute Professor, MIT; Academic Co-Founder, Moderna |
|
|
 |
|
 |
2 |
2010 |
| Elizabeth Nabel, M.D. |
72 |
 |
Former President, Brigham Health |
|
 |
|
 |
 |
3 |
2015** |
| Elizabeth Tallett |
74 |
 |
Former Principal, Hunter Partners |
 |
 |
|
|
|
2 |
2020 |
| Continuing directors |
Noubar Afeyan,
Ph.D. Chairman |
61 |
 |
CEO, Flagship Pioneering; Co-founder and Chairman, Moderna |
|
|
 |
|
|
0 |
2010 Chairman since 2012 |
| Stéphane Bancel |
51 |
|
Chief Executive Officer, Moderna |
|
|
|
|
|
0 |
2011 |
| Stephen Berenson |
63 |
 |
Managing Partner, Flagship Pioneering |
 |
|
|
 |
|
1 |
2017 |
| Sandra Horning, M.D. |
75 |
 |
Former Chief Medical Officer and Global Head of Product Development, Roche |
|
|
 |
 |
 |
3 |
2020 |
| François Nader, M.D. |
67 |
 |
Former President, CEO and Executive Director, NPS Pharmaceuticals |
|
 |
|
 |
 |
1 |
2019 |
| Paul Sagan |
65 |
 |
Catalyst Advisor, General Catalyst |
 |
|
 |
|
|
0 |
2018 |

Chairman |

Member |
* Comp = Compensation and Talent
Nom Gov = Nominating and Corporate Governance
Prod Dev = Product Development
Sci Tech = Science and Technology
|
** Dr. Nabel was a member of our Board from
December 2015 to July 2020, and rejoined the Board in March 2021, following her retirement from Brigham Health. |
| |
|
|
|

|
The Board of Directors recommends a vote “FOR” the
election of each of the three nominees as a Class III director to serve for a three-year term. For more information on the nominees,
see page 8. |
 |
2024
Proxy statement |
4 |

Board Highlights
In 2023, our Board of Directors
contributed to Moderna’s strategic advancement through its oversight of management’s execution of our business plans and strategy.
Advice and guidance from the full Board, and relevant Committees where applicable, was instrumental in the following key accomplishments,
among others, in 2023.
| Commercial Execution |
|
2023 marked our first year of endemic commercial sales for our COVID-19 vaccine
in the U.S., and we achieved retail market share of 48%, up significantly from 37% U.S. retail market share in 2022 (Source: IQVIA).
Preparing for this launch entailed significant strategic investments and commercial execution decisions, which were guided by input
from our Board. The Board has similarly also been heavily engaged in preparations for the anticipated launch of RSV in 2024, our
second commercial product. |
| Pipeline Advancement |
|
The Board and Product Development Committee oversaw investments to advance our pipeline, such that
by year-end 2023, nine of our 45 programs were late-stage, including seven programs in Phase 3 and two rare disease programs. This
significant advancement of our programs lays the foundation for future growth. |
| Capital Allocation |
|
The Board continued to oversee significant capital allocation decisions in 2023. This included
investments in research & development to advance our pipeline, both internally and through external collaborations. It also
included investments in our internal manufacturing capabilities in Norwood and Marlborough, the UK, Australia, and Canada, and the
decision to right-size our manufacturing footprint and to restructure our relationships with contract manufacturers as we seek to
return our COVID franchise to profitability in 2024. |
| Board Succession |
|
The Board and Nominating and Corporate Governance
Committee are continuing to advance our Board succession planning and recruitment of new directors who will bring additional expertise
reflective of Moderna’s future strategic plan and increased scale. |
| Governance Enhancements |
|
In response to investor feedback, our Board approved several
enhancements to our governance practices, including (i) the adoption of majority voting for uncontested director elections, (ii)
the implementation of a proxy access bylaw, and (iii) requesting that shareholders approve the implementation of a right for shareholders
to call a special meeting (see Proposal No. 4). We have also enhanced our processes for assessment and mitigation of potential conflicts
of interest as we expand into new areas. |
| Human Capital and ESG Efforts |
|
The Board and its committees oversaw human capital and environmental,
social and governance (ESG) initiatives. This included efforts to refine our talent strategy as we shift into an endemic market,
focused on retention of key talent, development and performance management. ESG efforts included launching a double-materiality assessment
and climate risk analysis, as well as continuing to enhance transparency through our second ESG Report and ESG Day, and by publishing
Scope 1, 2 and 3 data on greenhouse gas emissions, as well as waste and water statistics. |
 |
2024
Proxy statement |
5 |
Proposal 2: Advisory Vote on Compensation of our Executive
Officers
Shareholders will be asked to
approve, on a non-binding, advisory basis, the compensation of Moderna’s named executive officers. This is commonly known
as a “say on pay” proposal.
Over the last several years,
we have evolved our executive compensation programs in order to:
| • |
Enhance alignment with shareholders through our pay-for-performance philosophy; |
| • |
Align with competitive market practices; and |
| • |
Reflect input received from shareholders. |
| Overall Performance |
|
• We
made significant progress in 2023 advancing our mRNA platform and our broad clinical pipeline.
• We
achieved 48% U.S. retail market share for the 2023 fall season, an 11 percentage point increase from 2022.
• Corporate
performance did not meet expectations for our financial goals, falling short on product sales and operating income objectives. |
| Base Salary |
|
• Modest increases in 2023, reflective of merit and market adjustments to better align our executives’ salaries to market, as well as cost-of-living adjustments across our broader employee base. |
| Bonus |
|
• 2023 corporate performance did not meet our expectations, resulting in our Executive Committee members, including our CEO, receiving below target annual bonuses, at 81% of target. |
| Long-Term Incentive |
|
• Increased the weighting to more performance-based restricted share units (PSUs), with the CEO PSU mix increasing to 50%, further tying executive compensation to the long-term achievement of our financial and pipeline expansion objectives, reinforcing our commitment to creating shareholder value. |
| Realizable Pay |
|
• Weighted
the vast majority of compensation for the CEO and our other named executive officers (NEOs) to “at-risk” compensation,
including bonus and equity awards (stock options, restricted stock units (RSUs) and PSUs) focusing on financial and operational
goals, stock price appreciation and pipeline development goals.
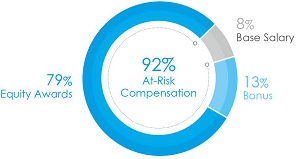
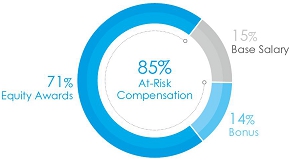
• 92%
at-risk target compensation for CEO, and 85% on average for other NEOs, see charts below.
• CEO
realizable pay demonstrates a strong correlation between stock price performance and pay; see page 41 for more details in the
“Pay for Performance” section of the Compensation Discussion & Analysis (CD&A). |
| Transparency |
|
• Augmented our CD&A to provide shareholders with visibility on the goals underlying our short-term and long-term incentive programs. |
| |
|
|
Our executive compensation program
is based on a pay-for-performance philosophy, which is reflected in both our annual and long-term incentive compensation programs.
We believe that a significant portion of each executive’s compensation should be variable and at-risk and tied to the achievement
of pre-established Company performance goals that drive value creation for our business and align our executives’ interests
with those of our shareholders. The largest component of our 2023 long-term incentive compensation program is delivered in the
form of stock options, which directly aligns payouts with outcomes for our shareholders.
The charts below set forth
the target total compensation mix for Mr. Bancel, our Chief Executive Officer, and our average NEO’s target
compensation for 2023.
| CEO Target Pay Mix |
|
Average NEO Target Pay Mix |
| |
|
|
 |
|
 |
 |
The
Board of Directors recommends a vote “FOR” approval,
on a non-binding, advisory basis, of the compensation of the Company’s Named Executive Officers. For more information,
see page 35. |
 |
2024
Proxy statement |
6 |
Proposal 3: Auditor Ratification
| • |
We are asking shareholders to ratify the appointment of Ernst & Young LLP as our independent auditor for the year ended December 31, 2024. |
| • |
The Audit Committee is committed to ensuring the independence of Ernst & Young, and has taken steps in recent years to ensure that we minimize spending on items with the firm other than the audit or audit-related matters. |
 |
The
Board of Directors recommends a vote “FOR” the
ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year
ending December 31, 2024. For more information, see page 76. |
Proposal 4: Amend Our Certificate of Incorporation
to Provide Shareholders the Right to Call a Special Meeting
| • |
In response to feedback from our investors, our Board of Directors is proposing to amend our Certificate of Incorporation to permit shareholders representing at least 20% of our outstanding shares to call a special meeting of shareholders. |
| • |
We believe that granting shareholders the right to call a special meeting, while setting a threshold of 20%, will grant investors with a sufficiently large economic and voting interest a valuable right, while also protecting against the potential disruption and possible loss to long-term shareholder value posed by setting a lower threshold. |
| • |
The special meeting right builds on other efforts earlier in 2024—including the adoption of a proxy access bylaw and the adoption of majority voting for uncontested director elections—to respond to investor feedback regarding Moderna’s governance practices. |
 |
The
Board of Directors recommends a vote “FOR” the
proposal to amend our Certificate of Incorporation to provide shareholders the right to call a special meeting. For more information,
see page 79. |
Proposal 5: Amend Our Certificate of Incorporation
to Reflect New Delaware Law Provisions Allowing for Officer Exculpation
| • |
In August 2022, Delaware law was amended to permit Delaware companies, like Moderna, to limit the personal liability of certain officers in limited circumstances, similar to protection that was already afforded to directors. |
| • |
The amended law permits a company to exculpate these officers for direct claims brought by shareholders for breach of an officer’s duty of care, but would not eliminate an officer’s monetary liability for breach of fiduciary duty claims brought by the company or in connection with derivative claims brought by shareholders on behalf of the company. It also would not allow officers to be exculpated for breaches of the duty of loyalty to the company, or acts or omissions that are not in good faith or that involve intentional misconduct, a knowing violation of law, or a situation in which an officer derived an improper personal benefit from a transaction. |
| • |
The Board of Directors is proposing to amend our Certificate of Incorporation to allow for the exculpation of officers of Moderna, consistent with the recent changes to Delaware law and to provide our executives similar protections to those already extended to our directors. |
| • |
The Board of Directors recognizes that our officers are called upon to make critical decisions, and that in an increasingly litigious environment our executive team can be exposed to substantial personal liability from litigants seeking to impose liability on the basis of hindsight and regardless of merit. |
| • |
The Board of Directors believes that providing the protection permitted under Delaware law will provide comfort to and empower our officers to best exercise their business judgment in the interests of Moderna and its shareholders, which will encourage agility and critical decision-making, while also enabling us to continue to attract and retain experienced and high-quality officers to the Company. |
 |
The
Board of Directors recommends a vote “FOR” the
proposal to amend our Certificate of Incorporation to allow for officer exculpation. For more information, see page 81. |
 |
2024
Proxy statement |
7 |
Proposal No. 1: Election of Directors
Our Board of Directors currently
has nine members, who are divided into three equal classes with staggered three-year terms. At the Annual Meeting, three Class
III directors will be elected for a three-year term. Each of these nominees is a Class III director whose current term is expiring.
Each director will continue in office until the election and qualification of a successor or until such director’s earlier
death, resignation, or removal.
Nominees
Our Nominating and Corporate
Governance Committee has recommended, and our Board of Directors has approved, Robert Langer, Elizabeth Nabel, M.D. and Elizabeth
Tallett as nominees for election as Class III directors at the Annual Meeting. Dr. Langer has served on the Board since 2010, Dr.
Nabel has served on the Board since 2015, and Ms. Tallett has served on the Board since 2020.
If you are a shareholder of record
and you sign your proxy card or vote over the Internet or by telephone but do not give instructions with respect to the voting
of directors, your shares will be voted FOR the election of Dr. Langer, Dr. Nabel and Ms. Tallett. We expect that the nominees
will serve if elected. However, if a director nominee is unable or declines to serve as a director at the time of the Annual Meeting,
proxies will be voted for any nominee who is designated by our Board of Directors to fill the resulting vacancy. If you own your
Moderna stock through a broker, bank, or other nominee and you do not give voting instructions, then your shares will not be voted
on this matter. For more information, please see “Information About the 2024 Annual Meeting of Shareholders—What if
I do not specify how my shares are to be voted?” on page 85.
Vote Required
The election of the Class III
directors requires a majority of the votes properly cast to be approved. If a director nominee does not receive a majority of the
votes cast in favor of his or her election to the Board, the director nominee will be required to tender his or her resignation
to the Board for consideration and action by the Board.
 |
The
Board of Directors recommends a vote “FOR” the
election of each of the three nominees as a Class III director to serve for a three-year term. |
 |
2024
Proxy statement |
8 |
Information About Moderna’s Directors
Nominees
|

Age: 75
Director since: 2010
Independent
Committees:
• Nominating
and Corporate Governance
• Science
and Technology
2023 Attendance: 86% |
Robert Langer, Sc.D. |
|
Why this director is valuable to Moderna
Dr. Langer is one of the world’s
most highly-cited researchers and decorated scientists. His pioneering academic work and extensive knowledge of medical, scientific
and intellectual property matters provide valuable strategic insights into the development of our products and advancement of our
pipeline into new areas. He is intimately familiar with Moderna as one of our founders and largest shareholders, and has decades
of experience applying scientific knowledge and insights to medical and commercial uses. His prior service to the U.S. Food and
Drug Administration (FDA) also provides him with insights into regulatory processes that are key to understanding how we might
obtain approval of our products.
Other Public Boards
• Seer,
Inc. (since 2020)
• Puretech
Health plc (since 2015)
• Abpro
Korea (2020-2024)
• Frequency
Therapeutics, Inc. (2016-2023)
• Rubius
Therapeutics, Inc. (2015-2019)
• Kala
Pharmaceuticals, Inc. (2009-2018)
Education
• B.S.
in chemical engineering from Cornell University
• Sc.D.
in chemical engineering from the Massachusetts Institute of Technology
Dr. Langer has been an Institute Professor at the Massachusetts Institute of Technology (MIT) since 2005, and has been a Professor at MIT since 1977. Dr. Langer served as a member of the Science Board to the U.S. Food and Drug Administration from 1995 to 2002, including as chairman for four years. He is an elected member of the National Academy of Sciences, the National Academy of Engineering, the National Academy of Medicine and the National Academy of Inventors. Dr. Langer has written over 1,550 articles and also has 1,450 issued or pending patents worldwide. Dr. Langer’s patents have been licensed or sublicensed to over 400 pharmaceutical, chemical, biotechnology and medical device companies.
|
|
|
|
|
| |
|
|
| |
|

Age: 72
Director since: 2015
Independent
Committees:
• Compensation
and Talent
• Science
and Technology (Chair)
• Product
Development
2023 Attendance: 100% |
Elizabeth Nabel, M.D. |
|
Why this director is valuable to Moderna
Dr. Nabel brings valuable strategic
insight as a medical doctor and professor of medicine who has spent decades in the healthcare industry. Dr. Nabel has served as
the chief executive of a large hospital organization, which informs her insights into the provision of care and how payors, including
government payors, approach the commercial market, as well as human capital management. Her experience working for governmental
organizations also helps guide our strategy related to the approval and regulation of our products. Her experience working in drug
development and serving as a director for other healthcare companies also provides valuable perspective on our industry.
Other Public Boards
• Medtronic
plc (since 2014)
• Lyell
Immunopharma, Inc. (since 2021)
• Accolade,
Inc. (since 2021)
Education
• B.A.
from St. Olaf College
• M.D.
from Cornell University Medical College
• Postgraduate
training in internal medicine and cardiovascular diseases at Brigham and Women’s Hospital and Harvard University
From 2010 to 2021, Dr. Nabel served as the President of Harvard University-affiliated Brigham Health, which includes Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, and the Brigham and Women’s Physician Organization. Dr. Nabel was also a Professor of Medicine at Harvard Medical School from 2010 to 2021. Following her retirement from Brigham Health, Dr. Nabel served as Executive Vice President for Strategy at ModeX Therapeutics, from 2021 to 2022, when the company was acquired by OPKO Health, Inc. Following the acquisition, Dr. Nabel also served as Chief Medical Officer for OPKO Health until August 2023. She now serves as the Chair of the Advisory Board of OPKO Health. Earlier in her career, Dr. Nabel held a variety of roles, including Director, at the National Heart, Lung and Blood Institute at the National Institutes of Health, a federal agency funding research, training and education programs to promote the prevention and treatment of heart, lung and blood diseases, from 1999 to 2009. She is an elected member of the National Academy of Medicine of the National Academy of Sciences.
|
|
|
|
|
| |
|
|
 |
2024
Proxy statement |
9 |
|

Age: 74
Director since: 2020
Independent
Committees:
Audit (Chair)
Compensation and Talent
2023 Attendance: 88% |
Elizabeth Tallett |
|
Why this director is valuable to Moderna
Ms. Tallett provides valuable
strategic insight based upon her extensive professional experience in the pharmaceutical industry, as an executive for organizations
at various stages of development, and as a public company director across different industries. Ms. Tallett’s experience is applied
in helping us identify the opportunities and challenges we face as a commercial-stage pharmaceutical company, as well as understanding
the dynamics impacting customers and payors. Ms. Tallett’s experience as a public company director, including as the Chair or lead
independent director for several public companies, also provides governance expertise, particularly in the areas of financial reporting,
human capital management, and risk management as we scale our organization globally and build out critical internal functions.
Other Public Boards
• Elevance
Health, Inc. (previously Anthem, Inc.) (since 2013), Chair since 2018
• Qiagen,
Inc. (since 2011)
• Principal
Financial Group (2001-2021)
• Meredith
Corp., Inc. (2008-2021)
Education
• Nottingham
University with a dual first class honours degree in mathematics and economics
Ms.
Tallett has spent more than 35 years in strategic leadership and operational roles in worldwide biopharmaceutical and consumer
products industries. From 2002 to 2015, she was a Principal of Hunter Partners, LLC, a management company for pharmaceutical,
biotechnology and medical device companies, and continues to consult with early-stage healthcare companies. She previously served
as President and Chief Executive Officer of Transcell Technologies Inc., President of Centocor Pharmaceuticals, a member of the
Parke-Davis Executive Committee, and Director of Worldwide Strategic Planning for Warner-Lambert Company. Ms. Tallett was a founding
member of the Biotechnology Council of New Jersey and chairs the board of trustees at Solebury School in Pennsylvania. She was
named a Financial Times Outstanding Director of the year in 2015 and recognized as one of the National Association of Corporate
Directors (NACD) Directorship 100 honorees in 2019.
|
|
|
|
|
| |
|
|
Continuing Directors
|

Age: 61
Director since: 2010
Chairman since: 2012
Term expires: 2025
Independent
Committees:
• Nominating
and Corporate Governance (Chair)
2023 Attendance: 92% |
Noubar Afeyan, Ph.D. |
|
Why this director is valuable to Moderna
Dr. Afeyan provides strategic
expertise and vision as one of our co-founders and as our Chairman since 2012. He has decades of experience co-founding, leading
and investing in numerous successful biotechnology companies, applying scientific insights to medical and commercial uses. This
experience helps guide our strategy as we continue to explore ways to expand our platform and as we reimagine the ways in which
we can impact patients. Dr. Afeyan’s experience leading several public and private company boards provides him with valuable leadership
experience as the Chairman of our Board.
Other Public Boards
• Omega
Therapeutics, Inc. (2016-2023), Chair
• Rubius
Therapeutics, Inc. (2013-2022)
• Seres
Therapeutics, Inc. (2012-2020)
• Evelo
Biosciences, Inc. (2014-2019)
• Kaleido
Biosciences, Inc. (2015-2019)
Education
• B.S.
in chemical engineering from McGill University
• Ph.D.
in biochemical engineering from the Massachusetts Institute of Technology
Dr.
Afeyan founded Flagship Pioneering, a company established in 1999 that creates bioplatform companies to transform human health
and sustainability, and serves as its Senior Managing Partner and Chief Executive Officer. He has served on the boards of numerous
privately and publicly held companies. Dr. Afeyan entered biotechnology during its emergence as an academic field and industry,
completing his doctoral work in biochemical engineering at MIT in 1987. He was a senior lecturer at MIT’s Sloan School of
Management where he taught courses on technology-entrepreneurship, innovation and leadership from 2000 to 2016, a lecturer of
business administration at Harvard Business School until 2020, and he currently serves as a member of the MIT Corporation. In
2022, Dr. Afeyan was elected to the National Academy of Engineering.
|
|
|
|
|
| |
|
|
 |
2024
Proxy statement |
10 |
|

Age: 51
Director since: 2011
Term expires: 2025
Committees: None
2023 Attendance: 100% |
Stéphane Bancel |
|
Why this director is valuable to Moderna
As our CEO for more than a decade,
Mr. Bancel plays a key role in setting the strategy for Moderna with the rest of the Board, and executing on that strategy with
our management team. Mr. Bancel is intimately familiar with our operations and is a key driver behind our culture and way of working.
Mr. Bancel’s experience prior to Moderna as an executive at other companies provides him with leadership experience and knowledge
of the pharmaceutical industry, including insight into opportunities for Moderna to reimagine how we can have the greatest impact
on patients.
Other Public Boards
• Qiagen
N.V. (2013-2021)
• Syros
Pharmaceuticals, Inc. (2013-2017)
Education
• Master
of Engineering degree from École Centrale Paris
• Master
of Science in chemical engineering from the University of Minnesota
• M.B.A.
from Harvard Business School
Mr. Bancel has served as our CEO since October 2011. Before joining Moderna, Mr. Bancel served for five years as CEO of the French diagnostics company bioMérieux SA. From July 2000 to March 2006, he served in various roles at Eli Lilly and Company, including as Managing Director, Belgium, and as Executive Director, Global Manufacturing Strategy and Supply Chain. Prior to Eli Lilly, Mr. Bancel served as Asia-Pacific Sales and Marketing Director for bioMérieux. He is currently a Venture Partner at Flagship Pioneering. In 2024, Mr. Bancel was elected to the National Academy of Engineering.
|
|
|
|
|
| |
|
|
|

Age: 63
Director since: 2017
Term expires: 2026
Independent
Committees:
• Audit
• Product
Development
2023 Attendance: 100% |
Stephen Berenson |
|
Why this director is valuable to Moderna
Mr. Berenson brings valuable
insight to the Board based upon decades of experience in the investment banking industry, working across different industries and
geographies. This experience has given Mr. Berenson a deep understanding of financial matters, including financial reporting, capital
allocation, and mergers & acquisitions, as well as an understanding of public company board governance, shareholder engagement,
regulation and risk management. His more recent experience at Flagship Pioneering provides Mr. Berenson with valuable insights
into the healthcare industry and the dynamics facing biotech companies at various stages of development.
Other Public Boards
• Seres
Therapeutics, Inc. (since 2019), Chair
Education
• S.B.
in mathematics from the Massachusetts Institute of Technology
Mr.
Berenson is a Managing Partner at Flagship Pioneering. He oversees Flagship’s capital formation and business development
activities, is deeply involved in firm-wide strategy and the firm’s talent agenda and is a member of the firm’s resource
allocation committee. Prior to joining Flagship, Mr. Berenson spent 33 years as an investment banker at J.P. Morgan. During his
last twelve years at J.P. Morgan, Mr. Berenson was Vice Chairman of Investment Banking and focused on providing high-touch strategic
advice to leading companies across all industries globally. He was co-founder of J.P. Morgan’s Global Strategic Advisory
Council and co-founder of the firm’s Board Initiative.
|
|
|
|
|
| |
|
|
 |
2024
Proxy statement |
11 |
|

Age: 75
Director since: 2020
Term expires: 2026
Independent
Committees:
• Product
Development (Chair)
• Nominating
and Corporate Governance
• Science
and Technology
2023 Attendance: 94% |
Sandra Horning, M.D. |
|
Why this director is valuable to Moderna
Dr. Horning brings decades of
experience in the healthcare industry to the Board as a practicing oncologist and investigator, professor of medicine, and an executive
leading pharmaceutical drug development with 15 new medicine approvals across multiple therapeutic areas. Dr. Horning provides
valuable insight to our research and development teams as they develop products across our portfolio. These insights inform how
we progress clinical development, engage with regulators and stakeholders, and prepare for commercialization of our product pipeline.
Dr. Horning’s experience as an executive and director at other healthcare companies also informs our understanding of healthcare
industry dynamics and our governance practices.
Other Public Boards
• Revolution
Medicines, Inc. (since 2023)
• Gilead
Sciences, Inc. (since 2020)
• Olema
Pharmaceuticals, Inc. (since 2020)
• EQRx,
Inc. (2021-2023)
Education
• M.D.
from the University of Iowa School of Medicine
• Completed
internal medicine training at the University of Rochester
• Post-graduate
fellowship in Oncology and Cancer Biology at Stanford University
Dr. Horning was the Chief Medical Officer and Global Head of Product Development of Roche, Inc., from 2014 until her retirement in 2019, and, prior to that, served as Global Head of Oncology Clinical Science at Roche from 2009 to 2013. Prior to Roche, Dr. Horning spent 25 years as a practicing oncologist, investigator and tenured Professor of Medicine at Stanford University School of Medicine, where she remains a Professor of Medicine Emerita. From 2005 to 2006, she served as President of the American Society of Clinical Oncology. From 2015 to 2018, Dr. Horning served on the Foundation Medicine Board of Directors.
|
|
|
|
|
| |
|
|
|

Age: 67
Director since: 2019
Term expires: 2026
Independent
Committees:
• Compensation
and Talent (Chair)
• Product
Development
• Science
and Technology
2023 Attendance: 100% |
François Nader, M.D. |
|
Why this director is valuable to Moderna
Dr. Nader brings decades of experience
in the healthcare industry to the Board, having served as an executive for organizations at various stages of development, including
as a public pharmaceutical company CEO. Dr. Nader helps guide our strategy through his insights into various stages of vaccine
development across our portfolio, including clinical development, engagement with regulators, and commercialization. His experience
on the boards of public and private healthcare companies from small cap to large cap across the globe help inform and guide our
growth as we scale our organization.
Other Public Boards
• BenevolentAI
(since 2021), Chair
• Talaris
Therapeutics, Inc. (2018-2023), Chair
• Acceleron
Pharma Inc. (2014-2021), Chair
• Alexion
Pharmaceuticals, Inc. (2017-2021)
• Prevail
Therapeutics Inc. (2018-2021), Chair
• Clementia
Pharmaceuticals Inc. (2014-2019)
• Advanced
Accelerator Applications S.A. (2016-2018)
• Baxalta
(2015-2016)
• NPS
Pharmaceuticals (2008-2015)
Education
• French
doctorate in medicine from St. Joseph University in Lebanon
• Physician
Executive M.B.A. from the University of Tennessee
Dr. Nader served as President, CEO and Executive Director of NPS Pharmaceuticals from 2008 until 2015, when the company was acquired. During his tenure as CEO, Dr. Nader transformed NPS Pharma into a leading global biotechnology company focused on delivering innovative therapies to patients with rare diseases. From September 2023 to January 2024, Dr. Nader served as the acting Chief Executive Officer of BenevolentAI while the company conducted a CEO search. Prior to NPS, Dr. Nader was a venture partner at Care Capital. He previously served on Aventis Pharma’s North America Leadership Team, holding a number of executive positions in integrated healthcare markets and medical and regulatory affairs. Dr. Nader previously led global commercial operations at the Pasteur Vaccines division of Rhone-Poulenc. He is a senior advisor for Blackstone Life Sciences. Dr. Nader is the former Chairman of BioNJ, New Jersey’s biotechnology trade organization, and previously served on the board of the Biotechnology Industry Organization.
|
|
|
|
|
| |
|
|
 |
2024
Proxy statement |
12 |
|

Age: 65
Director since: 2018
Term expires: 2026
Independent
Committees:
• Audit
• Nominating
and Corporate Governance
2023 Attendance: 100%
|
Paul Sagan |
|
Why this director is valuable to Moderna
Mr. Sagan brings valuable expertise
to our Board as a former public company CEO, and as an executive and advisor to companies across an array of industries, including
technology, media, and venture capital. Mr. Sagan has decades of experience guiding companies through early stage growth
to maturity and operating as public companies. He has particular insight into how digital technology can facilitate scaling and
growth, while also protecting against cybersecurity threats. His experience as an executive, director and advisor to both public
and private companies provides expertise, particularly in finance and accounting, human capital management and the application
of digital technologies, as we scale our organization globally and build out critical internal functions.
Other Public Boards
• VMware,
Inc. (2014-2023)
• Akamai
Technologies, Inc. (2005-2019)
• EMC
Corp. (2007-2016)
• iRobot,
Inc. (2010-2015)
Education
• B.S.
from the Medill School of Journalism at Northwestern University
Mr. Sagan is a Catalyst Advisor at General Catalyst, a venture capital firm, which he first joined in 2013 and became a Managing Director in 2018. From 2005 to 2013, Mr. Sagan served as CEO at Akamai Technologies, Inc. and was President from 1999 to 2010 and from October 2011 to 2013. Prior to joining Akamai, Mr. Sagan held senior management roles at Time Warner, where he helped to found RoadRunner, the world’s first consumer broadband service; Pathfinder, one of the first web portals that pioneered internet advertising; and NY1, the 24-hour cable news channel.
|
|
|
|
|
|
| |
|
|
 |
2024
Proxy statement |
13 |
Governance
Composition of our Board of Directors
Our Board currently consists of
nine members, but the Board has the authority to increase or decrease that size depending on an assessment of its needs and other
relevant circumstances at any given time.
Our Nominating and Corporate Governance
Committee (Nominating Committee) and our Board of Directors consider a broad range of factors when selecting nominees. We strive
to identify candidates who will further the interests of our shareholders. Among other things, we expect that all of our directors
will have the following experience and traits:
| • | substantial experience at a
strategic or policymaking level in a business, government, non-profit,
or academic organization of high standing, able to contribute
to Moderna’s strategic growth and able to offer advice
and guidance to Moderna’s senior management based on that
experience; |
| • | highly accomplished in their
respective fields; |
| • | the ability to contribute positively
to the Board’s collaborative culture; |
| • | knowledge of our business; |
| • | understanding of the competitive
landscape facing our business; and |
| • | expertise relevant to our growth
and business strategy. |
In addition, every nominee must
have sufficient time and availability to devote to Moderna’s affairs, a reputation for high ethical and moral standards,
an understanding of the fiduciary responsibilities assumed by public company directors, and the time and energy necessary to diligently
carry out those responsibilities, and role model our values and demonstrate a willingness to embrace the Moderna Mindsets, further
described in “ESG Topics—Human Capital Management” on page 31.
The Nominating Committee reviews
Board succession regularly, and in considering director candidates is focused on those individuals who can help guide Moderna
as it executes on its strategy over the next several years. This includes seeking out candidates with experience overseeing
global pharmaceutical companies, individuals with significant governmental or public policy experience, and individuals who can
help guide our growth as a digitally enabled and highly innovative company.
In building our Board, we also
believe that the following skills and experiences, while not exhaustive, are helpful in ensuring that our directors collectively
possess the skills and backgrounds necessary for us to execute on our strategic plans and to exercise the Board’s oversight
responsibilities on behalf of our shareholders. Skills and experiences shown below are generally reflective of the individual
having worked in the area, rather than experience obtained as a director in the relevant field.
| Skill/Experience |
Afeyan |
Bancel |
Berenson |
Horning |
Langer |
Nabel |
Nader |
Sagan |
Tallett |
| CEO Experience |
✓ |
✓ |
|
|
|
✓ |
✓ |
✓ |
✓ |
| Digital/Information
Security |
|
✓ |
✓ |
✓ |
|
✓ |
|
✓ |
✓ |
| Drug Development |
✓ |
✓ |
|
✓ |
✓ |
|
✓ |
|
✓ |
| Drug Commercialization |
✓ |
✓ |
|
|
|
|
✓ |
|
✓ |
| Finance/Accounting |
✓ |
✓ |
✓ |
✓ |
|
✓ |
✓ |
✓ |
✓ |
| Government/Regulatory |
✓ |
|
|
✓ |
✓ |
✓ |
✓ |
|
✓ |
| Healthcare
Industry |
✓ |
✓ |
|
✓ |
✓ |
✓ |
✓ |
|
✓ |
| Human Capital
Management |
✓ |
✓ |
✓ |
|
|
✓ |
✓ |
✓ |
✓ |
| International
Experience |
✓ |
✓ |
✓ |
|
|
✓ |
✓ |
✓ |
✓ |
| Investor
Experience |
✓ |
✓ |
✓ |
|
✓ |
|
✓ |
✓ |
✓ |
| Manufacturing/Supply
Chain |
✓ |
✓ |
|
|
|
|
✓ |
|
✓ |
| Science/Technology/R&D |
✓ |
✓ |
|
✓ |
✓ |
✓ |
✓ |
|
|
 |
2024
Proxy statement |
14 |
Our directors hold office until
their successors have been elected and qualified or until their earlier death, resignation, or removal. Directors may be removed
only for cause by the affirmative vote of the holders of at least two-thirds of the votes that all our shareholders would be entitled
to cast in an annual election of directors. Any vacancy on our Board of Directors, including a vacancy resulting from an enlargement
of the Board, may be filled only by vote of a majority of the directors then in office.
Board Diversity
The following Board Diversity Matrix
presents our Board diversity statistics in accordance with Nasdaq Rule 5606, as self-disclosed by our current directors. While
the Board satisfies the minimum objectives of Nasdaq Rule 5605(f)(3) by having at least one director who identifies as female
and at least one director who identifies as a member of an Underrepresented Minority (as defined by Nasdaq Rules), we note that
one of our directors also identifies as Middle Eastern. As we pursue future Board recruitment efforts, our Nominating Committee
will continue to seek out candidates who can contribute to the diversity of views and perspectives of the Board in accordance
with the committee’s Policies and Procedures for Director Candidates. This includes seeking out individuals of diverse ethnicities,
a balance in terms of gender, and individuals with diverse perspectives informed by other personal and professional experiences.
Board Diversity Matrix as of
March 7, 2024
| Part I: Gender
Identity |
Female |
Male |
Non-Binary |
Decline
to Disclose |
| Directors (9
total) |
3 |
5 |
- |
1 |
| Part
II: Demographic Background |
Female |
Male |
Non-Binary |
Decline
to Disclose |
| African American
or Black |
- |
- |
- |
- |
| Alaskan Native
or Native American |
- |
- |
- |
- |
| Asian |
- |
1 |
- |
- |
| Hispanic or
Latinx |
- |
- |
- |
- |
| Native Hawaiian
or Pacific Islander |
- |
- |
- |
- |
| White |
3 |
5 |
- |
- |
| Two or More
Races or Ethnicities |
- |
2 |
- |
- |
| LGBTQ+ |
|
|
- |
|
| Did Not Disclose
Demographic Background |
|
|
|
1 |
| Directors
who identify as Middle Eastern |
- |
1 |
- |
- |
Staggered Board
In consultation with our Nominating
Committee, our Board of Directors has determined that a staggered board structure, with directors divided into three classes with
staggered terms, remains appropriate for us at this time. At each annual meeting of shareholders, one class of directors will
be elected for a three-year term to succeed the directors of the same class whose terms are then expiring.
A staggered board provides for
stability, continuity and experience among our Board. This structure helps to resist pressure to focus on short-term results at
the expense of our long-term value and success, which is especially important in our industry given the multi-year time horizons
required for the successful development of pharmaceutical products and product candidates. Our Board also believes that this structure
helps to preserve institutional knowledge that can help us identify new opportunities, such as when our Board noted parallels
early in 2020 between our earlier efforts to develop a vaccine against Middle Eastern Respiratory Syndrome (MERS) and the disease
that came to be known as COVID-19. This led to the successful development and launch of our first product, our COVID-19 vaccine.
 |
2024
Proxy statement |
15 |
Board Highlights for 2023
During 2023, the Moderna Board
was focused on continuing to execute on the shift to an endemic, commercial market for the Company’s COVID-19 vaccine, while
also advancing the Company’s pipeline of products beyond COVID-19 and right-sizing the Company’s manufacturing footprint
to lay the foundation for future growth and profitability.
COVID-19
COVID-19 Vaccine Commercial
Launch. In 2023, the market for COVID-19 vaccines further evolved to reflect seasonal market dynamics, with sales
shifting significantly to the second half of the year and significantly lower demand than in 2021 and 2022. Additionally, 2023
marked the shift to an endemic, commercial market in the U.S., with most sales for COVID-19 vaccines occurring in the private
retail channel. The Board remained focused on strategic oversight of these evolving market dynamics and ensuring that Moderna
was ready for a significant product launch of the updated version of its COVID-19 vaccine, Spikevax, in the third quarter of 2023,
which targeted the XBB.1.5 variant of SARS-CoV-2. Launching in a commercial market significantly changed dynamics with respect
to marketing, contracting, distribution and accounting, all of which called on the Board’s expertise. In the U.S. market, the
Moderna team expanded its market share to 48% of the retail market for the 2023 fall vaccination season, up 11 percentage points
from 37% in 2022 (Source: IQVIA). The Company expects to leverage its experience in the commercial market for COVID-19 vaccines
in the anticipated launch of its RSV vaccine in 2024.
Right-Sizing Our Manufacturing
Footprint. Over the course of 2023, evolving market dynamics made clear that the manufacturing footprint that was
necessary for the production of our COVID-19 vaccines in a pandemic setting would no longer be necessary following the shift to
an endemic market. With the Board’s support and oversight, management acted quickly in the third quarter to right-size
the Company’s manufacturing footprint to reflect updated market forecasts. These actions are expected to help return
the COVID-19 vaccine franchise to profitability in 2024.
Pipeline Development
By the end of 2023, Moderna had
nine late-stage programs, including seven programs in Phase 3 development and two rare disease programs poised to enter pivotal
trials in 2024. The Board and its Product Development Committee played a key role in overseeing the advancement and strategic
investment in these programs, laying the groundwork to diversify our business beyond COVID-19 vaccines.
Respiratory Vaccine Franchise. Following
the announcement of positive efficacy data for the Company’s vaccine candidate against respiratory syncytial virus (RSV)
(mRNA-1345) in older adults in January 2023, the Company moved swiftly to apply for approval from regulators in key markets globally.
The Company is anticipating approval and is preparing for commercial launch of this product in 2024. During 2023,
the Company advanced Phase 3 studies for three additional respiratory vaccines programs beyond our original COVID-19 and RSV vaccines:
seasonal flu (mRNA-1010), next-generation COVID-19, which is designed to be refrigerator-stable (mRNA-1283), and our combination
vaccine against seasonal flu and COVID-19 (mRNA-1083). We believe that combination respiratory vaccines have the potential to
improve coverage while also reducing disease burden and producing savings for healthcare systems, both through lower cost of administration
and lower healthcare costs by preventing or reducing the need for care.
Individualized Neoantigen Therapy
(INT). The Board has also been actively engaged in overseeing the advancement of clinical trials and investments to prepare
for commercial readiness for our oncology program, INT (previously referred to as our personalized cancer vaccine) (mRNA-4157),
which we are advancing in collaboration with Merck. The Phase 3 trial of mRNA-4157 for melanoma launched in 2023 and is
enrolling globally. In December 2023, we launched a second Phase 3 trial of mRNA-4157 in non-small cell lung cancer, and
this study is also enrolling globally. Moderna broke ground on an additional manufacturing site in Marlborough, Massachusetts
in 2023, and this facility is expected to be dedicated to the production of INT.
Latent and Rare Diseases. The
Board and its committees continue to oversee our strategy to advance our broader pipeline, including investments in our development
programs and the talent to move them forward. This includes advancing our vaccines against latent and rare diseases and in other
areas. Our Phase 3 study for our vaccine candidate against cytomegalovirus (CMV) (mRNA-1647), the leading infectious cause of
birth defects in the U.S., is fully enrolled and could see an efficacy data readout in 2024. In rare diseases, the Paramount
study of our propionic acidemia (PA) program (mRNA-3927) and the Landmark study of our methylmalonic acidemia (MMA) program (mRNA-3705)
are both ongoing, and are expected to enter into pivotal studies in 2024.
 |
2024
Proxy statement |
16 |
Corporate Developments
Governance Updates. In
2023, the Board and Nominating Committee continued to assess feedback from Moderna’s investors regarding its governance
practices. As a result of this feedback, early in 2024, the Board approved updates to the Company’s bylaws to adopt majority
voting for uncontested director elections and to implement a proxy access bylaw on market terms, which will allow shareholders
with a significant, long-term ownership interest to
nominate director candidates for inclusion on the annual meeting ballot. The Board also is requesting approval from shareholders
to amend the Company’s Certificate of Incorporation to allow shareholders to call a special meeting (see “Proposal
4: Amend Our Certificate of Incorporation to Provide Shareholders the Right to Call a Special Meeting,” beginning on page 79). Additionally, during
2023, our Nominating Committee approved updates to our Corporate Governance Guidelines to require that directors obtain the approval
of the Chair of the Committee before taking on any new board or director roles, as described below under “Director Independence.”
Additionally, the Nominating Committee has implemented enhanced screening of potential conflicts as the scope of our business
has expanded.
Capital Allocation. During
2023, the Board played a significant role in steering the Company’s capital allocation strategy. Consistent with the Company’s
announced strategy, this included investments in the pipeline, as discussed above, as well as new manufacturing facilities in
the UK, Canada and Australia to support government partnerships, and investments to facilitate the commercialization of INT. The
Board and the Science and Technology Committee played a key role in overseeing collaborations with other companies, including
those that were announced with Immatics, CytomX and Life Edit Therapeutics. Lastly, the Board played a key role in overseeing
our share buyback program and the decision in the first half of the year to curtail buybacks to preserve capital for future investments.
Executive Compensation and Talent
Management. As discussed in greater detail elsewhere in this Proxy Statement, the Compensation and Talent Committee continues
to update our compensation programs to ensure ongoing alignment between our executives and investors, including tying our performance-based
programs to the Company’s strategy, evolving the weighting of different instruments in our equity programs, and evolving
our culture and ways of working as we seek to scale our operations and prepare for multiple product launches while maintaining
cost discipline. In addition, the Committee oversaw our talent management efforts, including performance, training and retention
programs and the results of our second global pay equity analysis.
ESG Initiatives. The
Board, acting primarily through the Nominating Committee, oversees Moderna’s efforts related to environmental, social and
governance (ESG) matters. In 2023, this included launching a climate-based risk assessment and a double-materiality assessment
to prepare Moderna for potential disclosure requirements related to these matters. In addition, the Committee oversaw efforts
to implement policies related to ESG, and increasing transparency on these matters, including reviewing our roadmap to achieving
publicly announced greenhouse gas reduction targets. For information, see “ESG Topics” below.
Director Independence
Our Corporate Governance Guidelines
provide that at least a majority of the members of the Board must meet the independence standards prescribed by rules of The Nasdaq
Stock Market. Our Board of Directors has determined that all current directors except Mr. Bancel, our Chief Executive Officer,
are independent, as defined by the Securities and Exchange Commission (SEC) and Nasdaq rules.
In making this determination, the
Board considered the relationships that each non-employee director has with Moderna and other relevant facts and circumstances.
There are no family relationships among any of our directors or executive officers.
Directors must notify the Chair
of the Nominating Committee and our Chief Legal Officer prior to accepting any new director roles or in connection with any significant
change in employment status so that the potential for conflicts or other factors that may compromise the director’s ability
to perform his or her duties may be fully assessed. Pre-clearance from the Chair of the Nominating Committee, acting on the advice
of the Chief Legal Officer and in consultation with the full Nominating Committee, where appropriate, is required before a director
may accept any such new roles. At least annually, the Board will evaluate all relationships between Moderna and each director
in light of relevant facts and circumstances for the purpose of determining whether a material relationship exists that might
signal a potential conflict of interest or otherwise interfere with such director’s ability to satisfy his or her responsibilities
as an independent director.
 |
2024
Proxy statement |
17 |
Board Leadership Structure
The role of Chairman of the Board
is separated from the role of Chief Executive Officer. Our Chief Executive Officer is responsible for recommending strategic decisions
and capital allocation to the Board and for ensuring the execution of the recommended plans. The Chairman is responsible for leading
the Board of Directors in its fundamental role of providing advice to and independent oversight of management. Our bylaws and
Corporate Governance Guidelines do not require that our Chairman and Chief Executive Officer positions be separate.
Limits on Board Service
Carrying out the duties and fulfilling
the responsibilities of a director requires a significant commitment of time and attention. The Board recognizes that excessive
time commitments can interfere with an individual’s ability to carry out and fulfill his or her duties effectively. In connection
with its assessment of director candidates for nomination, the Board will assess whether the performance of any director has been
or is likely to be adversely affected by excessive time commitments, including service on other boards.
Consistent with this belief, the
Board amended its Corporate Governance Guidelines in 2020 to adopt limits on the number of other boards on which directors may
serve. Under the revised Guidelines, directors who also serve as executives of public companies should not serve on more than
one board of a public company in addition to the Moderna Board, and other directors should not serve on more than three other
boards of public companies in addition to the Moderna Board, absent special circumstances, such as a period of transition.
Application to
Dr. Langer. Dr. Langer is in compliance with our policy related to board service
limits, and serves on the boards of two other public companies. The Nominating Committee notes that while Dr. Langer previously
served on four public company boards (in addition to ours) at the time our policy was adopted in 2020, in the last year he has
come off two of those boards.
Application to
Dr. Nabel. Dr. Nabel is also in compliance with our policy related to board service
limits and has been throughout her term. The Nominating Committee notes that for a temporary period, Dr. Nabel served as
the Chief Medical Officer, part-time, for OPKO Health, while also serving on two public company boards (in addition to ours).
In August 2023, Dr. Nabel ceased acting as Chief Medical Officer for OPKO Health and now serves on an Advisory Board for
that company.
Directors must notify the Chair
of the Nominating Committee and Chief Legal Officer in connection with accepting a seat on the board of directors of another business
and obtain approval before joining so we can fully assess the potential for conflicts or other factors that may compromise the
director’s ability to carry out his or her duties. See “Director Independence” above.
Age and Term Limits
The Board does not believe that
arbitrary limits on the number of consecutive terms a director may serve or on the directors’ ages are appropriate in light
of the substantial benefits resulting from a sustained focus on Moderna’s business, strategy, and industry over a significant
period of time.
Governance Documents
We have adopted a Code of Ethics
and Business Conduct that applies to our Board of Directors and all of our officers and employees. In addition, we have adopted
Corporate Governance Guidelines that formalize certain fundamental board policies and practices. Both of these documents are available
on the “Governance—Governance Documents” section of our Investor Relations website, https://investors.modernatx.com.
 |
2024
Proxy statement |
18 |
Board’s Role in Risk Oversight

 |
2024
Proxy statement |
19 |
Shareholder Engagement
We actively engage with our shareholders
throughout the year to solicit their views on our governance and compensation policies and practices, as well as current and emerging
ESG trends. As our investor base has grown, we have expanded our investor outreach program and practices to seek the views of
a broader range of investors, including those with a particular focus on socially responsible investing. Conversations throughout
the year led by our Investor Relations team are supplemented by outreach with members of our Corporate Governance team, senior
leadership and independent directors.
Following our 2023 Annual Meeting,
we initiated contact for governance engagements with our 25 largest investors that are unaffiliated with our officers and directors,
representing approximately 42% of our shares outstanding (as of September 2023), as well as several other investors who expressed
an interest in meeting on governance topics. Including affiliated investors, this outreach included shareholders representing
more than 55% of our shares outstanding. Governance outreach discussions focused on a number of topics that were voted on at the
2023 Annual Meeting, as well as other topics outlined below, including actions to adopt majority voting, implement proxy access,
provide shareholders the right to call a special meeting, implement officer exculpation, and our current board structure and executive
compensation programs.
Key Items Discussed with Shareholders in 2023-2024
| Board Composition
and Governance |
|
Executive
Compensation Programs |
|
ESG
Initiatives |
•
Director recruitment and Board composition
•
Board structure and independence
•
Majority voting
•
Proxy access
•
Special meeting right
•
Officer exculpation
|
|
•
Our 2023 say-on-pay vote
•
Program structure, including the weighting of components of equity awards
•
Transparency and disclosures on PSU goals
|
|
•
Access to medicines
•
Transparency reporting (e.g., ESG Report, SASB, EEO-1 Report)
•
Climate-related matters, including Scope 1, 2 and 3 emissions and reductions
•
Double-materiality analysis
|
Our discussions led to valuable
feedback from our investors, which was discussed among members of management and with our Board and Committees, as further discussed
below.
Board Composition and Governance.
Investor feedback informed our decision to adopt majority voting and to adopt a proxy access bylaw—both of which
were implemented in February 2024. Investor feedback also informed the proposal to amend our Certificate of Incorporation to grant
shareholders the right to call a special meeting, and the decision to set the threshold at 20% of outstanding shares. This investor
outreach also provided valuable feedback on the decision to pursue an amendment to our Certificate of Incorporation to allow for
officer exculpation, consistent with recent changes to Delaware law. Investor feedback has also informed our ongoing director
search efforts as we assess the ongoing evolution of our Board of Directors.
Executive Compensation Programs. Investor
feedback on our executive compensation programs was constructive, with support for our overall program design and emphasis on
tying the experience of investors to results for our executives through programs that are weighted toward equity. Our Compensation
and Talent Committee continues to incorporate this feedback into decisions around goal-setting and the structure of our compensation
programs. We have also undertaken to provide greater visibility on the goals for our PSU awards in this
Proxy Statement.
ESG initiatives. Investor
feedback on our ESG programs and transparency on these initiatives was quite positive. Most of our investors recognize Moderna’s
important role in promoting access to medicines, particularly through efforts like our mRNA Access program and diverse enrollment
targets in our clinical trials. Investors were also supportive of our efforts to provide greater transparency through our ESG
Report (which includes SASB and GRI disclosures) and ESG Day. Investors appreciated our efforts to disclose and obtain assurance
on our Scope 1, 2 and 3 greenhouse gas emissions, as well as our greenhouse gas reduction targets, and initiatives to assess climate
risk and to conduct a double-materiality analysis.
Going forward, we will continue
to engage with relevant stakeholders on these matters, continue to monitor the ESG reporting and disclosure landscape and assess
additional ESG reporting frameworks. For more information, please see “ESG Topics.” In addition to our one-on-one
discussions with investors, we host annual Investor Days throughout the year aimed at promoting understanding of our science and
development programs, including Vaccines Day and R&D Day.
 |
2024
Proxy statement |
20 |
Board Committees
As described below, our Board
of Directors has established five committees: Audit, Compensation and Talent, Nominating and Corporate Governance, Product Development
and Science and Technology. The Board of Directors may establish other committees from time to time. All members of the five Board
committees are independent directors.
The charter for each of the
committees is available on the “Governance—Governance Documents” section of our Investor Relations
website, https://investors.modernatx.com.
 |
Audit
Committee |
The Audit Committee’s responsibilities
include:
• appointing, approving
the compensation of, and assessing the independence of our independent registered public accounting firm;
• pre-approving
audit, audit-related and permissible non-audit services, and the terms of such services, to be provided by our independent
registered public accounting firm;
• reviewing
the overall audit plan with our independent registered public accounting firm and members of management responsible for
preparing our financial statements;
• reviewing
and discussing with management and our independent registered public accounting firm our annual and quarterly financial
statements and related disclosures, as well as the critical accounting policies and practices we use;
• coordinating
the oversight and reviewing the adequacy of our internal control over financial reporting;
• establishing
policies and procedures for the receipt and retention of accounting-related complaints and concerns;
• overseeing
the Company’s internal audit function, including internal audit plans, budgeting and staffing and reviewing any
findings resulting from audits;
• recommending,
based upon review and discussions with management and our independent registered public accounting firm, whether our audited
financial statements should be included in our Annual Report on Form 10-K;
• monitoring
the integrity of our financial statements and compliance with legal and regulatory requirements as they relate to our
financial statements and accounting;
• preparing
the Audit Committee Report required by SEC rules to be included in our annual proxy statement;
• reviewing
all related person transactions for potential conflicts of interest and approving all appropriate transactions;
• reviewing
quarterly earnings releases;
• discussing
the guidelines and policies that govern the process by which the Company’s exposure to risk is assessed and managed
by management; and
• exercising
general oversight over the Company’s information security and technology risks, including the Company’s information
security and related risk management programs.
|
Members:
• Ms. Tallett (Chair)
• Mr. Berenson
• Mr. Sagan
|
Meetings in 2023: 7
Independence:
Our Board of Directors has determined that each member of the Audit Committee meets the heightened
independence requirements for audit committee members prescribed by the SEC and Nasdaq, and that each has sufficient knowledge
in financial and auditing matters to serve on the Audit Committee.
Financial experts:
Our Board of Directors has determined that each of Ms. Tallett, Mr. Berenson and Mr. Sagan
is an “audit committee financial expert,” as defined in the applicable SEC rules.
|
Representative, recent discussion topics
| |
• | Financial
reporting and significant accounting items, including as a result of our shift to a commercial
market and in connection with the resizing of our manufacturing footprint |
| |
• | Ongoing
enhancements to our cybersecurity program, including onboarding of new personnel and
execution against our updated cybersecurity strategic roadmap |
| |
• | Ongoing
evolution of our internal audit, tax and compliance functions as we scale and grow as
a global organization |
| |
• | Capital
allocation strategy, including approach to investments, tax and oversight of our share
repurchase program |
 |
2024
Proxy statement |
21 |
 |
Compensation
and Talent Committee |
The Compensation and Talent Committee’s
responsibilities include:
• reviewing and establishing
our overall management compensation philosophy and policy;
• annually reviewing and recommending
to the Board corporate goals and objectives relevant to our CEO’s compensation, evaluating the CEO’s performance,
and making a recommendation to the Board for the CEO’s compensation;
• approving the cash and equity
compensation of our other executive officers;
• overseeing the operation
of all compensation plans, policies and programs in which executive officers participate, and other incentive, retirement,
health and welfare and equity plans for our broader employee population;
• reviewing the Company’s
talent initiatives and strategies to attract, develop and retain key employees, and reviewing succession planning for
key executive roles;
• overseeing the Company’s
human capital management strategies, policies, and practices, including those related to workforce belonging, inclusion
and diversity, employee engagement and internal pay equity;
• appointing and overseeing
compensation consultants and other advisors retained by the Committee;
• reviewing and recommending
to the Board of Directors the compensation of our directors; and
• preparing the Compensation
Committee Report required by SEC rules to be included in our annual proxy statement.
|
Members:
• Mr. Nader (Chair)
• Dr. Nabel
• Ms. Tallett
|
Meetings in 2023: 4
Independence:
Our Board of Directors has determined that each member of the Compensation and Talent Committee
meets the heightened independence requirements for compensation committee members prescribed by Nasdaq.
|
Representative, recent discussion topics
| |
• |
Ongoing evolution of our executive compensation
program, including the weighting of equity plans, goal-setting and the ties between our pay programs and strategy to ensure
alignment with investors |
| |
• |
Development of our future operating model as we seek to grow
sustainably and facilitate multiple product launches |
| |
• |
Maintaining Moderna’s culture and Mindsets |
| |
• |
Talent development efforts, including retention, development
and performance management |
| |
• |
Global pay practices and the results of our second annual pay
equity analysis |
| |
• |
Succession planning for key management roles |
 |
Nominating
and Corporate |
The Nominating and Corporate Governance
Committee’s responsibilities include:
• recommending to the Board
persons to be nominated for election as directors, and to serve on Committees and as Committee Chairs from among the directors;
• reviewing the composition
of the Board to ensure its members have the appropriate skills and expertise to oversee Moderna;
• reviewing and recommending
corporate governance practices to the Board;
• overseeing the evaluation
of our Board; and
• reviewing ESG matters pertaining
to the Company, including ESG policies and initiatives, such as political engagement, actions to mitigate risks related
to climate change, as well as philanthropic initiatives, such as the Moderna Charitable Foundation.
|
Governance Committee
Members:
• Dr. Afeyan (Chair)
• Dr. Horning
• Dr. Langer
• Mr. Sagan
|
| Meetings
in 2023: 4 |
Representative, recent discussion topics
| |
• |
Evolution of the Company’s
governance practices to implement majority voting, proxy access, a special meeting right, and officer exculpation, and reviewing
investor feedback on these topics |
| |
• |
Board succession planning and director
recruitment efforts to ensure appropriate expertise as we execute our Company strategy |
| |
• |
Rotation of committee assignments
and committee leadership roles |
| |
• |
Updates to our Corporate Governance
Guidelines and internal processes to ensure ongoing oversight of potential conflicts of interest as the Company expands into
new areas |
| |
• |
Expansion and evolution of ESG efforts,
including enhanced ESG reporting (Scope 1, 2 and 3 emissions), initiation of a climate risk assessment and double-materiality
analysis, and oversight of our sustainability strategy |
 |
2024
Proxy statement |
22 |
 |
Product
Development |
The Product Development Committee’s responsibilities
include:
• assessing
our product development strategy;
• reviewing product development plans for our pipeline and related investments;
• evaluating
management recommendations related to the further preclinical and clinical development of our programs, including the conduct of
pivotal trials;
• reviewing
R&D- and pipeline-related goals in performance-based compensation plans;
• providing
guidance and assisting in assessments of scientific talent; and
• advising
the Board on scientific and R&D aspects of licensing, strategic partnerships and acquisition or divestiture transactions.
|
Committee
Members:
• Dr.
Horning (Chair)
• Mr.
Berenson
• Dr. Nabel
• Dr.
Nader
|
| Meetings in 2023: 7 |
Representative, recent discussion topics
| |
• |
Advancement
of our late-stage pipeline, including design and launch of pivotal trials for seasonal flu, next-generation COVID, and combination
vaccine for COVID + seasonal flu |
| |
• |
Ongoing clinical
results from our Phase 2b trial for INT (melanoma), and the launch of Phase 3 trials of INT for melanoma and non-small cell
lung cancer |
| |
• |
R&D- and
pipeline-related goals included in 2024 performance-based compensation plans |
 |
Science and Technology |
The Science and Technology Committee’s
responsibilities include:
• reviewing,
evaluating and advising the Board regarding platform development, including advances in mRNA science, delivery science and manufacturing
process science and related investments;
• identifying
significant emerging science and technology issues and trends relevant to our mRNA platform;
• reviewing,
evaluating and advising the Board regarding potential new modalities and strategies to expand the utility of existing modalities;
and
• reviewing
and advising the Board regarding our intellectual property strategies.
|
Committee
Members:
• Dr.
Nabel (Chair)
• Dr.
Horning
• Dr.
Langer
• Dr.
Nader
|
| Meetings in 2023: 5 |
Representative, recent discussion topics
| |
• |
Oversight
of key external collaborations and investments with outside partners |
| |
• |
Platform research
strategy and budget |
| |
• |
Strategy related
to intellectual property and licensing |
Board and Committee Meetings
Each director is expected
to make reasonable efforts to attend all Board and applicable committee meetings. Attendance is taken into account by the Nominating
Committee and the Board when they assess directors for re-nomination to the Board. Each director is also expected to attend our
annual shareholder meetings, and all of our directors attended the 2023 Annual Meeting of Shareholders.
The full Board of Directors
met five times in formal meetings during 2023, in addition to holding frequent calls and informal sessions. Each director attended
(virtually or in person) at least 75% of the aggregate meetings of the Board (held while such person was a director) and meetings
held by all committees of the Board on which such person served.
The non-management directors
meet at regularly scheduled executive sessions without management participation. The Chair of the Board is an independent director
and presides at all Board meetings.
 |
2024
Proxy statement |
23 |
Board Self-Evaluation
Pursuant to our Corporate
Governance Guidelines, our Board has committed to conduct a self-evaluation at least annually to assess whether the Board and its
committees are functioning effectively. Our Board committees conduct self-evaluations periodically to assess whether they are functioning
effectively. Any such evaluation will consider the performance of the Board or the committee, as the case may be, as a unit (rather
than the performance of any individual director). The Nominating Committee oversees the Board’s annual self-evaluation process.
In 2023, the Board’s
self-evaluation utilized an independent third party who conducted a survey and individual interviews with each director regarding
the Board’s operations before reporting back to full Board of Directors. As a result of the feedback from this evaluation,
updates were made to the composition of the Board’s committees and leadership roles were rotated. Additional changes were implemented
to promote the Board’s effectiveness and to increase internal communications, among other changes. In the past, these self-evaluations
have similarly informed decisions regarding Board operations and Board composition, which have been acted upon by the Nominating
Committee.
Compensation Committee Interlocks and Insider
Participation
None of the members of our
Compensation and Talent Committee has at any time during the prior three years been one of our officers or employees. None of our
executive officers currently serves, or in the past fiscal year has served, as a member of the board of directors or compensation
committee of any entity that has one or more executive officers serving on our Board of Directors or Compensation and Talent Committee.
Director Onboarding
Moderna conducts an orientation
program for each new director to familiarize that individual with our business and strategic plans, key policies and practices,
principal officers and management structure, auditing and compliance processes, and Code of Business Conduct and Ethics.
How to Contact the Board
Any person who wishes to
contact the Board can do so:
| • |
By
e-mail to ir@modernatx.com; or |
| • |
By
reaching our Corporate Secretary as described on page 87 under “Information
about the 2024 Annual Meeting of Shareholders—How can I contact the Corporate Secretary?”. |
Any person who has a concern
about Moderna’s conduct, including with respect to accounting, internal controls, or auditing matters, may, in a confidential
or anonymous manner, communicate that concern to our Compliance Officer:
| • |
By
e-mail to ComplianceOfficer@modernatx.com (anonymity cannot be maintained); |
| • |
In
writing (which may be done anonymously), by mail to Moderna, Inc., Attention: Compliance Officer, 200 Technology Square, Cambridge,
Massachusetts 02139; |
| • |
Online at
https://moderna.whispli.com/compliancehotline (which
may be done anonymously); or |
| • |
By
calling the Compliance Hotline at 844-971-2551. |
Any person who wishes to
communicate directly with the Audit Committee may do so through the channels mentioned above, directing their communication to
the attention of the Chair of the Audit Committee.
 |
2024
Proxy statement |
24 |
Shareholder Recommendations for Director Candidates
The Nominating Committee
welcomes recommendations from shareholders for director candidates. Shareholder-recommended candidates will be evaluated in the
same manner as candidates that come to the committee’s attention from other sources.
To recommend a director candidate,
a shareholder should send the following information to our Corporate Secretary as described on page 87 under “Information about the 2024 Annual Meeting of Shareholders—How can I contact the Corporate
Secretary?”:
| • |
such
shareholder’s name and address of record; |
| • |
a
representation that such shareholder is a record holder of the Company’s securities, or if the shareholder is not a
record holder, evidence of ownership in accordance with Rule 14a-8(b)(2) of the Securities Exchange Act of 1934, as amended
(the Exchange Act); |
| • |
the
candidate’s name, age, business and residence address; |
| • |
the
educational background, current principal occupation or employment, and principal occupation or employment for the preceding
five full years of the candidate; |
| • |
a
description of the qualifications and background of the candidate, which address the minimum qualifications and other criteria
for Board membership approved by the Board; |
| • |
a
description of all arrangements or understandings between the shareholder and the candidate; and |
| • |
all
information relating to such candidate that is required to be disclosed in solicitations of proxies for election of directors
in an election contest pursuant to Regulation 14A under the Exchange Act (including such person’s written consent to
being named in the proxy statement as a nominee and to serving as a director if elected). |
The Nominating Committee
may seek further information from or about the shareholder making the recommendation and the recommended director candidate, including
information about all business and other relationships between the director candidate and the shareholder. Any shareholder recommendation
for a director candidate must be submitted to the Company not less than 120 calendar days prior to the date on which the Company’s
proxy statement was released to shareholders in connection with the previous year’s annual meeting.
 |
2024
Proxy statement |
25 |
Director Compensation
Annual Cash Retainers
Our non-employee directors
are eligible to receive the following cash retainers, which are pro-rated for partial-year service or time as Committee chairs,
and are paid quarterly:
| Annual
Retainer for service on the Board of Directors |
$ |
80,000 |
| Additional
Annual Retainer for service as: |
|
|
| Non-Executive
Chairman of the Board of Directors |
$ |
50,000 |
| Committee
Chair |
$ |
20,000 |
Equity Grants
Upon election to our Board
of Directors, each non-employee director is granted an equity award with an aggregate targeted grant date fair value of $400,000
(the Initial Grant). Beginning in April 2021, 75% of the value of each Initial Grant is delivered in stock options and 25% is delivered
in restricted stock units (RSUs). The stock option and RSU portions of the Initial Grant vest in full on the one-year anniversary
of the grant date if the recipient has continuously served as our director for that year.
On the date of each annual
meeting of shareholders, each continuing non-employee director is granted an equity award with an aggregate targeted grant date
fair value of $425,000 (the Annual Grant). Since April 2022, our directors have had the ability to choose to have the value of
each Annual Grant delivered solely as stock options or in the form of a mix of RSUs and stock options as chosen by each non-employee
director, provided that no more than 25% of such value may be delivered in the form of RSUs. The option and RSU portions of the
Annual Grant vest in full on the earlier of the one-year anniversary of the grant date and the next annual meeting of shareholders,
if the recipient has continuously served as a director through the applicable vesting date.
The stock option portion
of each director equity grant has an exercise price per share equal to the closing price of a share of Moderna’s common stock
on the date of grant, and an expiration period of ten years from the grant date. The number of stock options granted for any director
equity grant is determined by dividing the value attributable to the stock option award by the product of (i) the average closing
stock price over the preceding 20-trading days up to and including the last trading day immediately preceding the grant date and
(ii) the Black-Scholes ratio, which is calculated using the Black-Scholes value of a stock option on the grant date divided by
the closing stock price on the effective date of grant. The number of RSUs granted for any director equity grant is determined
by dividing the value attributable to the RSU award by the average closing stock price over the preceding 20-trading days up to
and including the last trading day immediately preceding the grant date. If a new non-employee director joins our Board of Directors
between annual meetings of shareholders, then such non-employee director will be granted a pro-rata portion of the Annual Grant
based on the time between such director’s appointment and our next annual meeting of shareholders. The director equity grants
are subject to full accelerated vesting upon a “sale event,” as defined in the Company’s 2018 Stock Option and
Incentive Plan, as amended from time to time (the 2018 Stock Plan).
Other Compensation Details
The aggregate amount of
cash and equity compensation paid to any non-employee director in a calendar year may not exceed $1,500,000 for the first year
of service and $1,000,000 for each year of service thereafter (or such other limits as may be set forth in the 2018 Stock Plan
or any similar provision of a successor plan).
Employee directors receive
no additional compensation for their service as a director. We reimburse all reasonable out-of-pocket expenses incurred by directors
for their attendance at meetings of our Board of Directors or any committee thereof. We have in the past provided, and may in the
future provide, security services to our directors.
 |
2024 Proxy statement |
26 |
Non-Employee Director Stock Ownership Policy
Our Stock Ownership Policy
provides that on or before December 31, 2024, or the fifth anniversary of an individual director’s appointment to the Board
(whichever is later), each non-employee director must own shares of Moderna’s common stock equal to at least six times the
amount of the annual cash retainer paid for regular service on the Board, exclusive of committee fees (i.e., $480,000 of Moderna
stock). For more information, see “Equity-Related Policies and Practices—Non-Employee Director and Executive Officer
Stock Ownership Policy,” below.
Non-Employee Director Compensation Table
The following table provides
information regarding the total compensation that was earned by or paid to each of our non-employee directors during the year ended
December 31, 2023. Mr. Bancel, our Chief Executive Officer, did not receive any additional compensation for his service as a director.
Mr. Bancel’s compensation is discussed in the Compensation Discussion and Analysis and compensation tables, which begin on
page 38. The amounts below for Option Awards and Stock Awards reflect the grant date fair value
of these awards, which, together, are lower than the target value due to the fact that the number of RSUs and stock options granted
was calculated based upon the 20-trading day trailing average closing price prior to grant, as described under “—Equity
Grants,” above.
| Name |
|
Fees Earned or
Paid in Cash |
|
Stock Awards(1)(2) |
|
Option Awards(1) |
|
All
Other
Compensation |
|
Total |
| Noubar
Afeyan, Ph.D.(3) |
|
$ |
150,000 |
|
$ |
— |
|
$ |
381,269 |
|
$ |
132,250(4) |
|
$ |
663,519 |
| Stephen
Berenson(5) |
|
|
90,000 |
|
|
95,262 |
|
|
285,983 |
|
|
— |
|
|
471,245 |
| Sandra
Horning, M.D.(6) |
|
|
100,000 |
|
|
— |
|
|
381,269 |
|
|
— |
|
|
481,269 |
| Robert
Langer, Sc.D.(7) |
|
|
80,000 |
|
|
— |
|
|
381,269 |
|
|
— |
|
|
461,269 |
| Elizabeth
Nabel, M.D.(8) |
|
|
95,000 |
|
|
95,262 |
|
|
285,983 |
|
|
— |
|
|
476,245 |
| François
Nader, M.D.(9) |
|
|
100,000 |
|
|
— |
|
|
381,269 |
|
|
— |
|
|
481,269 |
| Paul
Sagan(10) |
|
|
80,000 |
|
|
— |
|
|
381,269 |
|
|
— |
|
|
461,269 |
| Elizabeth Tallett(11) |
|
|
100,000 |
|
|
— |
|
|
381,269 |
|
|
— |
|
|
481,269 |
| (1) |
The amounts reported represent the aggregate grant
date fair value of the RSUs and stock options awarded to the non-employee directors in the year ended December 31, 2023, calculated
in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 718. Such grant
date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair
value of the equity awards reported in these columns are set forth in Note 12 to our Consolidated Financial Statements for
the year ended December 31, 2023, included in our Annual Report. The amounts reported in these columns reflect the grant date
fair value for each award, and, together, differ from the targeted amounts for the Annual Grant of $425,000, due to the fact
that a 20-trading day trailing average closing price convention is used for calculating the number of RSUs and stock options
granted. |
| (2) |
As of December 31, 2023, each non-employee director who selected
RSUs as part of their annual equity award held 732 unvested RSUs. |
| (3) |
As of December 31, 2023, Dr. Afeyan held outstanding options
to purchase a total of 171,068 shares of our common stock, 164,934 of which were vested. Dr. Afeyan is affiliated with Flagship
Pioneering, Inc. and prior to 2018, Flagship Pioneering, Inc. was granted equity for Dr. Afeyan’s service on our Board
of Directors. As of December 31, 2023, Flagship Pioneering, Inc. held options to purchase a total of 33,116 shares of our
common stock that were issued for such service. See “Security Ownership of Certain Beneficial Owners and Management”
for additional information regarding Flagship Pioneering’s and its affiliated entities’ beneficial ownership of
our common stock. |
| (4) |
The amount reported represents incremental costs borne by the
Company for the provision of security services to Dr. Afeyan in response to the heightened threat environment faced by individuals
associated with our Company. |
| (5) |
As of December 31, 2023, Mr. Berenson held options to purchase
a total of 168,181 shares of our common stock, 163,580 of which were vested. |
| (6) |
As of December 31, 2023, Dr. Horning held options to purchase
a total of 49,742 shares of our common stock, 43,607 of which were vested. |
| (7) |
As of December 31, 2023, Dr. Langer held options to purchase
a total of 299,636 shares of our common stock, 293,502 of which were vested. |
| (8) |
As of December 31, 2023, Dr. Nabel held options to purchase
a total of 13,882 shares of our common stock, 9,281 of which were vested. |
| (9) |
As of December 31, 2023, Dr. Nader held options to purchase
a total of 68,409 shares of our common stock, 62,275 of which were vested. |
| (10) |
As of December 31, 2023, Mr. Sagan held options to purchase
a total of 125,319 shares of our common stock, 119,185 of which were vested. |
| (11) |
As of December 31, 2023, Ms. Tallett held options to purchase
39,449 shares of our common stock, 33,315 of which were vested. |
 |
2024 Proxy statement |
27 |
Certain Relationships and Related Party Transactions
Other than the ordinary
course compensation agreements described under the sections entitled “Director Compensation” and “Compensation
Discussion and Analysis” and the transactions described below, since January 1, 2023, there has not been and there is not
currently proposed, any transaction or series of similar transactions to which we were, or will be, a party in which the amount
involved exceeded, or will exceed, $120,000 and in which any director, executive officer, holder of five percent or more of any
class of Moderna’s capital stock, or any member of the immediate family of, or entities affiliated with, any of the foregoing
persons, had, or will have, a direct or indirect material interest.
Agreements With Our Shareholders
Investor Rights Agreement
Prior to our IPO, we entered
into a second amended and restated Investor Rights Agreement with certain of our shareholders. The Investor Rights Agreement provided
the holders of approximately 44 million shares of our common stock rights with respect to the registration of those shares
under the Securities Act of 1933, as amended (the Securities Act), including demand registration rights, short-form registration
rights, and piggyback registration rights. Each of these rights granted under the Investor Rights Agreement terminated in December
2023.
Indemnification Agreements
We have entered into agreements
to indemnify our directors and executive officers. These agreements will, among other things, require us to indemnify these individuals,
to the maximum extent allowed under Delaware law, for certain expenses (including attorneys’ fees), judgments, fines, and
settlement amounts they reasonably incur in any action or proceeding, including any action by or in our right, on account of any
services undertaken by such person on behalf of Moderna or that person’s status as a member of our Board of Directors.
Policies for Approval of Related Party Transactions
We have adopted a written
policy providing that our Audit Committee is responsible for reviewing and overseeing related party transactions. For purposes
of this policy, a related person is defined as (i) any Moderna director or executive officer, (ii) any director nominee, (iii)
security holders known to us to beneficially own more than five percent of any class of Moderna’s voting securities, or (iv)
the immediate family members of any of such persons. In reviewing any related party transaction, the Audit Committee will take
into account, among other factors that it deems appropriate, whether the transaction is on terms no less favorable to Moderna than
terms generally available in a transaction with an unaffiliated third-party under the same or similar circumstances and the extent
of the related person’s interest in the transaction.
 |
2024 Proxy statement |
28 |
ESG Topics
At Moderna, our Environmental,
Social and Governance (ESG) strategy and corporate social responsibility program are built on a foundation of integrity, quality
and respect. These values provide a foundation for us to build and support long-term programs that demonstrate our commitment to
patients, employees, the environment and our local communities. Our Nominating and Corporate Governance Committee oversees ESG
matters and practices. The committee reports to the full Board on ESG matters and our progress on sustainability initiatives. Our
Chief Legal Officer, reporting to our CEO, leads our ESG strategy, with Executive Committee members overseeing additional elements
of particular ESG initiatives. Included below is a description of several topics that we view as key to promoting our long-term
value and impact. In June 2023, we published our second ESG Report, which provides further detail on these initiatives. Our ESG
Report can be found on our website at http://www.modernatx.com under the “Responsibility—Corporate policies”
section. Additionally, we hosted our second ESG Day in December 2023, where we discussed with investors our corporate mission and
key focus areas for our corporate responsibility strategy. During 2023, we also initiated a double-materiality analysis to inform
anticipated disclosure requirements and to validate our understanding of where we can create positive long-term value for our stakeholders.
Medicines for Patients
Moderna’s mission
is to deliver the greatest possible impact to people through mRNA medicines. Our priority as a relatively new commercial company
is to continue to accelerate the development of safe and effective mRNA medicines. To do so, we undertake sustained, long-term
investment in technology creation. We expect our ongoing investment in preclinical and clinical research and development to help
us continue to advance potentially life-changing therapeutics and vaccines where there are few or, in many cases, no treatment
options for patients. These areas include respiratory vaccines, latent and emerging vaccines, rare disease therapeutics, and oncology
therapeutics.
Our Global Public Health Strategy
In 2022, we first articulated
our global health strategy, which is centered on three key pillars: developing vaccines against priority pathogens, mRNA Access™
and regional manufacturing. We continue to focus on developing vaccines against priority pathogens identified in the World Health
Organization’s (WHO) R&D Blueprint, and those identified by the Coalition for Epidemic Preparedness Innovations (CEPI). Our
objective remains to create a portfolio of vaccines that are able to leverage our scale of manufacturing and the growing safety
database to more rapidly respond to outbreaks in the future. We have advanced clinical candidate vaccines against COVID-19, Zika,
chikungunya, HIV, Mpox, Nipah and pandemic influenza, many through public-private partnerships.
Our mRNA Access program
enables researchers around the world to utilize our mRNA technology platform to pursue research in their own labs on emerging and
neglected infectious diseases. During 2023, the number of institutions collaborating with us through mRNA Access nearly doubled,
to 16. In addition, we continue to advance regional manufacturing plants in Australia, Canada and the UK, in part to prepare for
future pandemics. Further detail on our global health strategy is included in our ESG Report.
Access to Medicines
Prior
to authorization of our first commercial product, our COVID-19 vaccine, we announced in December 2020 our commitment to access
to vaccines and therapeutics. This commitment is reflected in the following principles:
| • | We
are committed to developing a broad portfolio of vaccines and therapeutic solutions to
address epidemiological challenges worldwide. |
| | |
| • | We
will invest in R&D in areas of unmet need. |
| | |
| • | We
will work to include communities that have been under-represented in clinical research
in our development programs, as well as those who are disproportionately impacted by
the respective disease. |
| | |
| • | We aim to provide effective and affordable vaccines and
therapeutics to all populations. |
| | |
| • | We will price our products through differential pricing frameworks. |
| | |
| • | We are committed to participating in key public-private partnerships. |
| | |
| • | GAVI-eligible countries will get our lowest
prices, and we commit to an annual independent third-party audit of this commitment. |
 |
2024 Proxy statement |
29 |
In 2022, as part of our continued
support for achieving global health equity, we updated our COVID-19 patent pledge to provide that we will never enforce our patents
for COVID-19 vaccines against manufacturers in low- and middle-income countries in the Gavi COVAX Advance Market Commitment, provided
that the manufacturers produce vaccines solely for use in those countries. In non-AMC 92 countries, we remain willing to license
our technology for COVID-19 vaccines to manufacturers in those countries on commercially reasonable terms, which will enable us
to continue to invest in research to develop new vaccines, prepare for the next pandemic and meet other pressing areas of unmet
medical need.
Medicines for Rare Diseases
Our pipeline includes
potential therapeutics to address rare diseases, such as propionic acidemia (PA), methylmalonic acidemia (MMA), phenylketonuria,
glycogen storage disease type 1a (GSD1a), and autoimmune diseases that have not been addressed through traditional approaches.
During 2023, our most advanced programs for rare diseases, PA and MMA, demonstrated the potential of our platform to address rare
diseases. We expect to advance these two programs to pivotal trials in 2024. We have ongoing collaborations with Vertex Pharmaceuticals
to apply mRNA technology to addressing cystic fibrosis, with Chiesi Group to address pulmonary arterial hypertension, and with
the Institute for Life Changing Medicines (ILCM) to develop an mRNA therapeutic for Crigler-Najjar Syndrome Type 1 (CN-1), an ultra-rare
disease with only an estimated 70-100 known cases in the world.
Clinical Trial Diversity
We are committed to advancing
clinical trial health equity by identifying the barriers that currently impede inclusion, and implementing approaches to more efficiently
identify, engage, recruit and retain study participants from diverse backgrounds and vulnerable populations. When we recognized
racial-ethnic minority under-representation in the Phase 3 clinical trial of mRNA-1273, our original COVID-19 vaccine, we decided
to slow down the overall clinical trial enrollment to ensure representative diversity. At the conclusion of enrollment, the clinical
trial included more than 11,000 Participants of Color from diverse communities, representing 37% of the trial population. For the
Phase 3 clinical trial of our cytomegalovirus (CMV) vaccine candidate, we achieved our goal of enrolling 42% Participants of Color
in the US. Similarly, for the Phase 3 clinical trial of our RSV vaccine candidate, we again achieved our goal of enrolling at least
31% Participants of Color in the US.
Climate Change and the Environment
At Moderna, we are building
a company that seeks to drive change through what we make and how we make it. We believe that ensuring the health of our planet
is critical to positively impacting human health, and that it is our responsibility to grow Moderna in a way that protects the
planet and minimizes our impact on the environment. As an expanding company, we have an opportunity to grow in a way that puts
the protection of the environment as a key consideration in the design of new facilities, processes and products. As we continue
to expand our manufacturing capabilities, we are committed to protecting the planet, based on a three-pronged strategy: sustainability
by design, decarbonizing the value chain, and natural resource conservation.
We have set a goal of
achieving net-zero carbon emissions for our Scope 1 and 2 emissions by 2030. We are also committed to defining a Scope 3 target
that is in alignment with the Science-Based Targets Initiative.
Progress on climate and
environmental initiatives in 2023 included the following actions:
| • | Enhanced
our third-party assurance of energy and Scope 1 and Scope 2 emissions from Limited Assurance
to Reasonable Assurance; |
| | |
| • | Published metrics related
to Scope 3 emissions, water usage and waste management for our operations; |
| | |
| • | Incorporated sustainability
into the design and construction of new manufacturing plants in Marlborough, Massachusetts; Laval, Canada; Melbourne,
Australia; and Harwell, UK to reduce reliance on fossil fuels for thermal loads; |
| | |
| • | Completed a climate risk and
scenario analysis project to better inform our understanding of climate-related risks and opportunities to our
business; |
| | |
| • | Performed energy, water and
waste assessments at our manufacturing facility in Norwood, Massachusetts to identify opportunities to reduce fossil
fuel and water usage, improve operational efficiency and enhance our waste management program; and |
| | |
| • | Encouraged green transportation
among our employees by offering fully subsidized public transport, bike sharing and free electric vehicle charging
stations at our campuses. |
 |
2024 Proxy statement |
30 |
In February 2024,
we opened our new Moderna Science Center in Cambridge, Massachusetts, a purpose-built space to support our next chapter of
discovery. The high-performance building, which includes our principal executive offices, is targeting LEED Platinum Core
& Shell and LEED Zero Carbon certifications. The building includes geothermal heating and cooling, rooftop solar, an
exhaust air heat pump, and ultra-efficient building systems with acoustical and light pollution mitigation measures.
Further detail
on key performance indicators and our efforts to minimize our impact on the environment are included in our ESG Report and on our
website at modernatx.com under the “Responsibility—Corporate policies”
section.
Human Capital Management
We
recognize that our employees are key to our mission of delivering the greatest possible impact to people through mRNA medicines.
As an organization, we are bold, collaborative, curious and relentless. These values are underpinned by a core set of what we call
“basecamp” values—integrity, quality, respect. Additionally, with the continued rapid growth of our Company,
we articulated the Moderna Mindsets, which define how we behave, lead and make decisions. We believe our Mindsets are integral
to our future success, and we continue to integrate them into every facet of how we identify, onboard, grow and manage our highest
impact talent. New employees participate in our Moderna ONE onboarding program, which is an interactive, full-immersion learning
experience designed to provide the opportunity to engage with, better understand and learn how to apply the Mindsets in the workplace.

We operate in a highly
competitive environment for talent, particularly as we seek to attract and retain talent with experience in the biotechnology and
pharmaceutical sectors. Our workforce is highly educated, and as of December 31, 2023, 41% of our employees hold Ph.D., Doctorate,
M.D., J.D., or Master’s degrees. Among our employees, 49% are female. Among our leadership (which we define as employees
at the vice president level and above), as of December 31, 2023, approximately 39% are female. 45% of our U.S. employees identify
as racially or ethnically diverse as of December 31, 2023, an increase from 41% who identified as racially or ethnically diverse
the prior year. In 2023, for the second year in a row, an outside statistical pay equity analysis confirmed zero statistically
significant differences in pay across gender globally and across gender, race and ethnicity in the United States.
We had approximately 5,600
full-time employees as of December 31, 2023 in 19 markets around the world, with a presence in North America, Europe and the Asia-Pacific
region. We are committed to building a culture of inclusion and belonging for all. In 2023, we continued to act on our commitment
to belonging, inclusion and diversity by, among other things:
| • | continuing
our monitoring and reporting of Company-wide gender and ethnicity data; |
| | |
| • | including a belonging,
inclusion and diversity focus in every employee engagement survey; |
| | |
| • | continuing to invest in
our Employee Resource Groups (ERGs), which are voluntary, employee-led groups that harness
the power of belonging in service to our people, our Company and the community at large,
and adding a ninth ERG, mCare, which is dedicated to supporting and empowering employees
who are caregivers and parents; |
| | |
| • | gaining recognition from
Disability:IN Inclusion and the American Association of People with Disabilities as a
best place to work for disability inclusion; and |
| | |
| • | conducting diversity-related
events, celebrations and learning opportunities for employees throughout the year. |
To help promote alignment
between our employees and our shareholders, all employees participate in our equity programs through the receipt of equity awards,
and the percentage of equity as a component of overall pay mix increases with seniority. We believe that in addition to incentivizing
growth that leads to shareholder value, broad eligibility for our equity programs further embeds our “We Act Like Owners”
mindset and helps promote employee retention as these awards generally vest over a four-year period.
 |
2024 Proxy statement |
31 |
Our employees are highly
engaged, and our Company and team have been publicly recognized for our leadership, innovation and good corporate citizenship.
Science magazine has ranked us as a top employer for each of the last nine years. Additionally, in 2023, Biospace
ranked us the number one large employer in its 2024 Best Places to Work in Biopharma report for the third consecutive year. We
also received a perfect score from the Human Right Campaign’s Equality Index for 2023-2024. We measure employee engagement through
a vendor-supplied engagement software, using validated external benchmarks to track employee engagement factors.
Community
We launched the Moderna
Charitable Foundation in 2022 to support organizations and causes promoting public health and access to quality healthcare, advancing
scientific education and innovation, supporting local and global communities, and advocating for inclusion and diversity. We aspire
to make a lasting impact in these focus areas through grant-making activity, by supporting relief efforts during humanitarian crises,
and by supporting organizations that matter to Moderna employees through the Foundation’s matching gifts program. During 2023,
the Moderna Charitable Foundation made grants totaling approximately $7.2 million to local and global non-profit organizations
working on causes aligned with the Foundation’s mission, as well as $1.3 million in donations to match employee giving. In
2023, the Foundation continued to provide significant support to two organizations seeking to improve access to healthcare in Africa—Seed
Global Health and Amref. The Foundation has also committed to funding fellows focused on healthcare and advancing scientific education
in partnership with Ashoka, a leading organization for social entrepreneurship.
We encourage individual
employee volunteerism by providing our people with paid time off to volunteer at the organizations of their choice. During 2023,
70% of Moderna employees participated in tracked volunteering programs or employee giving, and volunteering hours increased 169%
from hours reported in 2022.
 |
2024 Proxy statement |
32 |
Management
Set forth below are the
biographies for our current Executive Committee members. These individuals are critical to Moderna’s success and are responsible
for leading our company to its next stage of development.
|

Age: 51
Joined Moderna and in current role since
October 2011 |
Stéphane Bancel,
Chief Executive Officer
As Chief Executive
Officer, Mr. Bancel chairs the Executive Committee and is responsible for executing on the strategy and operations for Moderna.
Following a restructuring of our commercial organization announced in December 2023, he also directly oversees commercial
operations for our approved products.
Before joining
Moderna in October 2011, Mr. Bancel served for five years as Chief Executive Officer of the French diagnostics company bioMérieux
SA. From July 2000 to March 2006, he served in various roles at Eli Lilly and Company, including as Managing Director, Belgium,
and as Executive Director, Global Manufacturing Strategy and Supply Chain. Prior to Eli Lilly, Mr. Bancel served as Asia-Pacific
Sales and Marketing Director for bioMérieux. He is currently a Venture Partner at Flagship Pioneering.
Education
• École
Centrale Paris, Master of Engineering
• University
of Minnesota, Master of Science in chemical engineering
• Harvard
Business School, M.B.A. |
|

Age: 58
Joined Moderna in October 2022 and in current
role since January 2023 |
Jerh Collins, Chief Technical
Operations and Quality Officer
Dr. Collins oversees technical
development, quality, and preclinical, clinical and commercial supply.
Before joining Moderna
in October 2022, Dr. Collins was employed by Novartis from 1993 to 2022, most recently as Chief Culture Officer. During his nearly
30 years at Novartis, Dr. Collins held roles of increasing responsibility focused on pharmaceutical production and manufacturing,
including roles serving as Head of Global Chemical Operations and Anti-Infectives and as Head of Global Chemical Operations.
Education
• University
College Cork, Bachelor of Science in chemistry
• University
College Cork, Ph.D. in organic chemistry |
|

Age: 58
Joined Moderna and in current role since
July 2021 |
Kate Cronin, Chief Brand Officer
Ms. Cronin oversees Moderna’s
communications, branding and marketing efforts.
Before joining Moderna
in July 2021, she was employed by Ogilvy Health from 2004 to 2021, in various roles, most recently as Global CEO. Prior to her
role as CEO, Ms. Cronin held numerous roles, including Global Managing Director, Managing Director of Ogilvy Public Relations’
New York office, and Co-President of Ogilvy Health in the United States. At Ogilvy, Ms. Cronin led integrated campaigns for the
firm’s largest long-term health clients including BMS, Boerhringer Ingelheim, Merck and Pfizer. Prior to Ogilvy, Ms. Cronin
was a partner at Porter Novelli.
Education
• Smith College, B.A. in biology |
|

Age: 44
Joined Moderna and in current role since
October 2019 |
Tracey Franklin, Chief Human Resources Officer
Ms. Franklin oversees
Moderna’s talent and organizational strategy.
Before joining Moderna
in October 2019, she was employed by Merck & Co. from 2004 to 2019 in positions of increasing responsibility, including most
recently Vice President, HR Chief Talent and Strategy Officer. Ms. Franklin’s previous leadership roles included responsibility
for HR for all divisions in the European region, head of HR for the UK and Ireland subsidiaries of Merck, and HR Operations leader
responsible for HR program implementation across Merck’s global footprint. She was based in Switzerland, the UK and the U.S.
Education
• Pennsylvania State University,
B.A. in communication arts and sciences
• Fairleigh Dickinson University,
Masters in industrial and organizational psychology |
 |
2024
Proxy statement |
33 |
|

Age: 48
Joined Moderna in January 2013 and in current
role since February 2015 |
Stephen Hoge, M.D., President
Dr. Hoge oversees Moderna’s
research & development efforts, from basic science through clinical development and regulatory approvals. He also oversees
early commercial efforts for many of our pipeline programs.
Before joining Moderna
in January 2013, he was employed by McKinsey & Company from 2005 to 2012, in roles of increasing responsibility, most recently
as a Partner and a leader in the firm’s healthcare practice. Dr. Hoge was a resident physician from 2004 to 2005 at New York
University/Bellevue Hospital.
Education
• Amherst
College, B.A. in neuroscience
• University
of California, San Francisco, M.D. |
|

Age: 52
Joined Moderna and in current role since
June 2021 |
Shannon Thyme Klinger,
Chief Legal Officer and Corporate Secretary
Ms. Klinger oversees legal
matters for Moderna, including corporate governance, intellectual property, litigation, compliance, privacy, as well as ESG strategy
and global security. She is also the President of the Moderna Charitable Foundation.
Before joining Moderna
in June 2021, she was employed by Novartis from 2008 to 2021, most recently as Chief Legal Officer and a member of the Novartis
Executive Committee. While at Novartis, Ms. Klinger also served as the Chief Ethics, Risk and Compliance Officer and Global Head
of Litigation, and as Global Head of Legal at Sandoz, a Novartis division. She began her in-house career at Barr Laboratories before
joining Solvay Pharmaceuticals as Senior Vice President and General Counsel. Ms. Klinger was previously a partner with Alston
& Bird.
Education
• University
of Notre Dame, B.A. in psychology
• University
of North Carolina at Chapel Hill, J.D. |
|

Age: 51
Joined Moderna and in current role since
January 2023 |
Brad Miller, Chief Information
Officer
Mr. Miller oversees Moderna’s
digital strategy and is responsible for building and operating critical enterprise services, including cybersecurity, and digital
experiences to enable the Company.
Before joining Moderna
in January 2023, Mr. Miller was employed by Capital One from 2021 to 2023 as Executive Vice President, Chief Technology Officer.
He was employed by Mastercard from 2017 to 2021 as Executive Vice President, Operations and Technology; by Citi from 2015 to 2017
as Managing Director, Head of Global Digital and Cloud Technology; and by Amazon and Microsoft in engineering roles earlier in
his career.
Education
• University
of Waterloo, B.S. in human factors
• University
of Nottingham, M.S. in human computer interaction in systems engineering |
|

Age: 47
Joined Moderna and in current role since
September 2022 |
James Mock, Chief Financial
Officer
Mr. Mock oversees Moderna’s
accounting, financial planning and analysis, business development, treasury, real estate, investor relations, internal audit and
tax functions.
Before joining Moderna
in September 2022, he was employed by PerkinElmer from 2018 to 2022 as Senior Vice President and Chief Financial Officer. Mr. Mock
joined PerkinElmer from General Electric, where he served in a number of positions between 1999 and 2018, most recently as Vice
President, Corporate Audit Staff.
Education
• St.
Lawrence University, B.A. in economics |
 |
2024
Proxy statement |
34 |
Proposal No. 2: Non-binding Advisory
Vote to Approve the Compensation of our Named Executive Officers
Our Board of Directors
is committed to excellence in governance. Consistent with good governance practices and the requirements of the Dodd-Frank Act,
shareholders have the opportunity to approve, on a non-binding, advisory basis, the compensation of Moderna’s named executive
officers. This is commonly known as a “say on pay” proposal.
The say on pay proposal
is not intended to address any specific item of compensation or the compensation of any particular officer, but rather the overall
compensation of our named executive officers and our compensation philosophy, policies, and practices discussed in this proxy
statement. The compensation of our named executive officers is disclosed in the Compensation Discussion and Analysis, the compensation
tables, and the related narrative disclosure contained in this proxy statement. As discussed in these disclosures, we believe
that our compensation policies and decisions are based on principles that reflect a “pay-for-performance” philosophy
and are strongly aligned with our shareholders’ interests and consistent with current market practices. Our executive compensation
program is designed to enable us to attract and retain talented and experienced executives to lead us successfully in a competitive
environment.
We are asking our shareholders
to vote for the following resolution:
“RESOLVED, that
the Company’s shareholders approve, on a non-binding, advisory basis, the compensation of the Company’s named executive
officers, as disclosed in this proxy statement, including the Compensation Discussion and Analysis, compensation tables and narrative
discussion.”
Before you vote, we recommend
that you read the Executive Compensation section that follows for complete information on our executive compensation programs
and philosophy.
This vote is advisory,
and therefore not binding on Moderna, the Board of Directors, or the Compensation and Talent Committee. However, our Board of
Directors and Compensation and Talent Committee value your opinion and intend to consider the outcome of the vote when making
compensation decisions in the future.
Vote Required
This non-binding, advisory
proposal will be approved if it receives an affirmative vote of a majority of the votes properly cast. If you ABSTAIN from voting
on Proposal No. 2, the abstention will have no effect on the results of this vote.
 |
The
Board of Directors recommends a vote “FOR” approval,
on a non-binding, advisory basis, of the compensation of the Company’s Named Executive Officers. |
 |
2024
Proxy statement |
35 |
Letter from the Compensation and Talent Committee
Dear Fellow Shareholders,
Fourteen years ago, Moderna’s
founders set out to change the world by making mRNA medicines a reality for humanity. Moderna’s platform is delivering on
the promise of mRNA as a result of significant investments since the Company’s founding. The COVID-19 pandemic challenged
the Moderna team to deliver at an unprecedented pace, and the mRNA platform successfully enabled Moderna to develop and deliver
one of the earliest and most effective vaccines against COVID-19. This success confirmed that our platform works, and we continue
to aggressively expand our R&D capabilities across multiple areas. Moderna currently has 45 development programs, nine of
which are in late-stage development, compared to 25 development programs at the end of 2020, when we only had one program in Phase 3.
2023 was a year of transition.
The Company fell short of its COVID-19 vaccine product sales and operating income goals as we adapted to an endemic market, but
the Company made significant progress in advancing its pipeline. The Moderna team pivoted to transform its COVID-19 business for
the endemic setting in 2023 by resizing its manufacturing footprint, positioning the COVID-19 franchise for profitability in 2024
and beyond. Moderna continues to enhance its commercial capability while building out systems and processes to ensure operational
excellence throughout the organization. With these operating improvements, and continued pipeline success, we hope to lay the
groundwork for future growth.
A key differentiator
at Moderna has always been its people and their willingness to innovate and drive solutions. Our executive compensation program
continues to be centered on at-risk, performance-based incentives, ensuring that Moderna’s executive compensation is linked
to both the Company’s financial results and execution of our long-term strategic objectives, which ultimately drive the creation
of long-term shareholder value. We have highlighted below key elements of the executive compensation framework and how they tie
to both our current and future expectations of performance for the Company and leadership team.
2023 Corporate Performance
— Executive Team Bonuses Paid at 81% of Target
| • |
Moderna fell short in
certain key areas for 2023 performance, as discussed in the following pages. As a result, the CEO and Executive Committee
bonuses were calculated by multiplying the 90% corporate funding by a 90% individual performance factor, which resulted in
a final 2023 bonus payout of 81% of target. This approach acknowledges that while each member of the Executive Committee made
meaningful contributions to our Company’s progress during 2023, the Company’s success in achieving its objectives requires
the Executive Committee to execute and deliver operationally as a team. Further details on corporate performance are included
in the “2023 Corporate Objectives” section on page 48. |
2021-2023 PSU Program
Update — 200% Performance Achievement
| • |
The
2021-2023 PSU program resulted in a maximum payout. 2021 was the first year we included PSUs in Moderna’s equity program,
and we set ambitious regulatory and clinical development goals that the Committee agreed would represent significant over-achievement
in terms of obtaining our first full product approvals in a number of major markets and significantly advancing and diversifying
our portfolio beyond our COVID-19 vaccine. |
| • |
While the vesting
percentage was 200% of target, the value that vested was 129% of the grant value as a result of the stock price decline over
the three-year performance period ($132.19 to $85.37). |
2023 Stock Price Impact
on Value of Equity Awards
| • |
Equity awards are the largest component
of our executive compensation program, representing no less than 70% of the target total compensation for each of our executives.
Therefore, stock price has a significant impact on the compensation realized by our executives, thus aligning our executive
and shareholder experiences. The CEO and average other NEO outstanding equity award values as of December 31, 2023 decrease
by 51% and 49%, respectively, when the awards are compared using the stock price from the previous year end ($179.62 vs. $99.45,
a 45% decrease). All stock options granted from 2021 through 2023 were underwater as of December 31, 2023, with exercise prices
for those options significantly above prevailing stock prices. |
In the fall of 2023,
we engaged with shareholders to discuss Moderna’s performance and compensation framework. Based on the valuable feedback
we have received from you, our shareholders, the Compensation and Talent Committee has taken deliberate actions regarding our
executive compensation program to reinforce our pay-for-performance philosophy and ensure alignment with our disciplined investment
strategy, as outlined below.
 |
2024
Proxy statement |
36 |
Increased PSU Weighting
for 2024 Long-Term Incentive Awards
| • |
Our CEO’s 2024 annual equity award is comprised of 50% of PSUs (increase from
25%) and 50% stock options (decrease from 75%). |
| • |
Other NEOs and Executive Committee members have an equal mix of PSUs, stock options
and RSUs for their annual equity awards, beginning in 2024 (PSU increase from 25% to 33 1/3%). |
| • |
This shift further ties executive compensation to the achievement of key pipeline advancement
and financial objectives, while achieving a balance in terms of maintaining stock options, which only deliver value with stock
price growth. |
Added Long-Term Financial
Metrics to our 2024 – 2026 PSU Program
| • |
Our
2024-2026 PSUs include long-term financial metrics for the first time, reflecting Moderna’s continued evolution as a
commercial company. |
| • |
The
financial metrics reflect the key goals that management has communicated and incentivizes achieving those goals by the end
of 2026. |
| • |
The program also
continues to remain focused on expanding and diversifying our portfolio into non-respiratory therapeutic areas to drive long-term
shareholder value. |
Enhanced CD&A
Disclosure
| • |
We have augmented our CD&A to provide shareholders
with a clearer understanding of our short-term and long-term incentive programs and the strong performance necessary to achieve
our financial and strategic objectives. |
| • |
These changes are part of our ongoing efforts to demonstrate the direct linkage between
compensation and company performance. |
In 2024, Moderna is focused
on executing its commercial strategy, investing with discipline, and advancing its late-stage pipeline to drive organic sales
growth. We have the utmost confidence in our Executive Committee to lead the organization, execute on the Company’s strategy,
and deliver on these ambitious goals.
We are grateful for your
support as we continue to navigate the exciting opportunities and challenges ahead. Your feedback is invaluable to us, and we
remain dedicated to open communication with our shareholders.
On behalf of the entire
Committee, we thank you for your partnership as we strive to bring revolutionary mRNA medicines to patients and create value for
all our shareholders.
Sincerely,
François Nader
Chair, Compensation and Talent Committee
 |
2024
Proxy statement |
37 |
Executive Compensation
Compensation Discussion and Analysis
This Compensation Discussion and
Analysis describes our executive compensation program and the 2023 compensation for our named executive officers (our NEOs). This Compensation
Discussion and Analysis should be read with the compensation tables and related disclosures for our NEOs.
Executive Summary
Moderna is advancing
messenger RNA (mRNA) therapeutics and vaccines to deliver the greatest possible impact to people through mRNA medicines. Our
mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing,
providing the Company the capability to pursue in parallel a robust pipeline of new development candidates. We are developing
therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases,
independently and with strategic collaborators.
2023 was a year of transition for
the Company, marked by a number of challenges in the COVID-19 vaccine market that caused us to fall short on our financial goals, but
with considerable progress as we advanced several other programs into late-stage development. Highlights included:
| • |
We achieved 48% retail U.S. market share for the 2023 COVID-19 vaccine fall season (compared to 37% in the 2022 fall season), demonstrating our ability to compete in a commercial market. |
| • |
We achieved significant progress advancing nine programs across our respiratory, latent, oncology and rare disease franchises into late-stage development. |
| • |
We pivoted to significantly resize our manufacturing infrastructure to reflect future anticipated market demand for our COVID-19 franchise. |
During 2023, we made significant
progress advancing our mRNA platform and our clinical pipeline, but from a financial perspective, our corporate performance did not meet
our expectations as we fell short of our objectives in terms of product sales and operating income. The corporate objectives for 2023
were:
| 1. |
Execute the operational and sales plan for COVID-19 vaccine. |
| 2. |
Build an unrivaled seasonal respiratory vaccine franchise. |
| 3. |
Execute on a bold campaign of cancer vaccine studies. |
| 4. |
Advance rare metabolic disease programs. |
| 5. |
Drive rapid advancement and growth in our latent vaccine portfolio. |
| 6. |
Deliver the next-generation pipeline and platform. |
| 7. |
Build a culture of learning, and strengthen our processes and digital systems. |
In designing our annual corporate
objectives, we focus on those shorter-term milestones that we believe will help us move closer to bringing new mRNA medicines to patients.
Our Executive Committee members are also granted performance-based restricted stock units (PSUs) with three-year performance periods that
maintain focus on our long-term strategy. Stock options help reinforce the long-term orientation of our executives as they have a four-year
vesting period and expire after ten years, and only convey value if our stock price appreciates. We believe this general design, which
focuses on long-term objectives, coupled with an ability to pivot quickly to adapt our compensation plans, has enabled us to deliver for
patients and our shareholders.
 |
2024
Proxy statement |
38 |
Corporate Performance Highlights
Our executive compensation program
seeks to incentivize and reward strong corporate performance. Highlights of our 2023 corporate performance are set forth below.
COVID-19 Vaccine Sales and Operating Income
| • |
The most heavily weighted factor in the assessment of our 2023 performance is product sales for our COVID-19 vaccine. While we achieved net product sales of $6.7 billion for the year, this performance fell short of our internal targets due to lower demand as we entered the endemic market. |
| • |
In terms of operating income, our operating loss of $4.2 billion for 2023 also fell significantly short of internal targets, with performance below threshold, resulting in no payout for this part of the scorecard. |
Advancing Our Pipeline and Science
By contrast, we made significant
strides in continuing to advance our pipeline, including:
| • |
Received two U.S. FDA supplemental Biologics License Application (BLA) approvals for our updated COVID-19 vaccines; |
| • |
Filed for the BLA for our RSV vaccine with the FDA; |
| • |
Initiated a Phase 3 trial of our combination vaccine against seasonal flu and COVID-19; |
| • |
Advanced our latent vaccine pipeline by fully enrolling participants in the cytomegalovirus (CMV) pivotal Phase 3 study to evaluate its efficacy, safety and immunogenicity; |
| • |
Identified a dose for expansion studies for our propionic acidemia therapeutic, and met our enrollment targets for our rare disease programs; and |
| • |
Demonstrated the clinical benefit of our INT program in melanoma, showing significant and clinically meaningful improvement in recurrence-free survival and reducing the risk of recurrence or death by 49% versus KEYTRUDA alone. |
Overview of Compensation for Our Named Executive Officers
Below is a summary of our NEOs for
2023 and a brief overview of the executive compensation actions for each of these NEOs. The individuals set forth below represent all
of our executive officers during 2023 for purposes of Rule 3b-7 of the Securities Exchange Act of 1934, as amended.
In response to the challenges Moderna
faced in meeting our corporate objectives for 2023, particularly with respect to meeting our financial goals, the Compensation Committee
decided to provide uniform bonuses to the CEO and other members of the Executive Committee (including all of our NEOs), establishing collective
accountability for the Company’s overall performance. Each member of the Executive Committee received an individual multiplier of
90%, which, when combined with the 90% corporate multiplier, resulted in bonus payments at 81% of target. This approach acknowledges that
while each member of the Executive Committee made meaningful contributions to our Company’s progress during 2023, the Company’s
success in achieving its objectives requires the Executive Committee to execute and deliver operationally as a team.
|

Chief Executive
Officer
Age: 51 |
|
Stéphane
Bancel |
|
| |
Compensation for Mr. Bancel for 2023 was as set forth below. |
| |
 |
Salary:
$1,575,000 (5% increase over 2022)
Bonus:
$1,913,625, based on target of 150% of salary, with 81% payout, reflecting 90%
individual assessment, as described above.
Equity Awards(1):
$15,000,000, delivered 75% in stock options and 25% in PSUs (based on 2022 performance)
|
| 1) | Amounts
shown for equity awards reflect target value, not grant date fair value. For grant date fair
value, see the Summary Compensation Table on page 59. |
 |
2024
Proxy statement |
39 |

Chief Financial
Officer
Age: 47
|
|
James
Mock |
|
| |
Compensation for Mr. Mock for 2023 was as set forth below. |
| |
 |
Salary:
$800,000 (6.7% increase over 2022)
Bonus:
$583,200, based on target of 90% of salary, with 81% payout, reflecting
90% individual assessment, as described above.
Equity Awards (1):
$3,500,000, delivered 50% in stock options, 25% in RSUs and 25% in PSUs
(based on 2022 performance)
|

President
Age: 48
|
|
Stephen
Hoge, M.D. |
|
| |
Compensation for Dr. Hoge for 2023 was as set forth below. |
| |
 |
Salary:
$1,050,000 (5% increase over 2022)
Bonus:
$850,500 based on target of 100% of salary, with
81% payout, reflecting 90% individual assessment, as described above.
Equity Awards (1):
$6,500,000, delivered 50% in stock options, 25%
in RSUs and 25% in PSUs (based on 2022 performance)
|
|

Former Chief
Commercial Officer
Age: 45
|
|
Arpa
Garay |
|
| |
Compensation
for Ms. Garay for 2023 was as set forth below. Ms. Garay served as our Chief Commercial Officer from May 2022 through December 2023.
In connection with her departure, Ms. Garay will be entitled to benefits under the Company’s Executive Severance Plan. Further
details are included under “Employment Arrangements with our NEOs” on page 55. |
| |
 |
Salary:
$850,000 (6.3% increase over 2022)
Bonus:
$619,650, based on target of 90% of salary, with 81%
payout, reflecting 90% individual assessment, as described above.
Equity Awards (1):
$3,500,000, delivered 50% in stock options, 25% in RSUs
and 25% in PSUs (based on 2022 performance)
|
|

Chief Legal Officer and Corporate Secretary
Age: 52
|
|
Shannon
Thyme Klinger |
|
| |
Compensation for Ms. Klinger for 2023 was as set forth below. |
| |
 |
Salary:
$800,000 (14.3% increase over 2022)
Bonus:
$583,200, based on target of 90% of salary, with 81%
payout, reflecting 90% individual assessment, as described above.
Equity Awards (1):
$3,500,000, delivered 50% in stock options, 25% in RSUs
and 25% in PSUs (based on 2022 performance)
|
| 1) | Amounts
shown for equity awards reflect target value, not grant date fair value. For grant date fair
value, see the Summary Compensation Table on page 59. |
 |
2024
Proxy statement |
40 |
Overview of Executive Compensation Program
Executive Compensation Philosophy
Our executive compensation program
is guided by our overarching philosophy of paying for demonstrable performance and impact. We are focused on our mission to “Deliver
the greatest possible impact to people through mRNA medicines.”
We believe that our compensation
philosophy helps align our team around executing on our mission, which ultimately leads to greater shareholder value. All full-time employees
at Moderna, regardless of their level, receive equity as part of their compensation, aligning them to investors and making them personally
invested in our mission. As we continue delivering on the promise of mRNA science, we recognize that our executive compensation programs
must also continue to attract and retain a talented team who can help us achieve this mission.
Consistent with this philosophy,
we have designed our executive compensation program to achieve the following primary goals:
| • |
Pay for performance by establishing competitive opportunities to incentivize
high performance and deliver greater rewards when corporate and individual performance exceed expectations and lower compensation
when corporate or individual performance falls short. Performance is measured by financial, operating and strategic performance,
return to shareholders and individual contributions. |
| • |
Attract, motivate and retain industry-leading talented individuals through
well-designed compensation programs that motivate our executives to achieve rigorous corporate objectives that are important to our
business and long-term success. |
| • |
Establish a competitive rewards program by evaluating the practices of our
peers and market data to validate that we are competitive with other companies who compete with us for talent. |
| • |
Align the interests of our executives and our shareholders to drive the
creation of sustainable long-term value. |
| • |
Balance a combination of compensation elements to achieve an appropriate
balance between cash and equity awards. The annual cash bonus is intended to motivate individuals to successfully execute on short-term
financial and strategic objectives. Equity awards are intended to focus executives on the long-term success of the organization. |
Pay for Performance
Pay for performance is a
foundational element within Moderna’s compensation program structure. We compare CEO total realizable pay (as defined below)
to stock price performance for Moderna and its peers. Our peer group represents companies of comparable size and business as
outlined by the criteria on page 45.
We looked at pay and performance
alignment over two periods as follows:
| • |
3-Year Analysis (2021-2023): The Company’s total shareholder
return (TSR) performance over the three-year period ending December 31, 2023 was at the 36th percentile among these peer companies
and the total realizable pay for our CEO over the same period was at the 4th percentile. |
| • |
5-Year Analysis (2019-2023): The Company’s TSR performance over the five-year period
ending December 31, 2023 was at the 100th percentile among these peer companies and the total realizable pay for our CEO over the
same period was at the 72nd percentile. |
The three-year analysis shows
that realizable pay and TSR performance were in the bottom left quadrant due to lagging share price performance. In comparison,
over five years, realizable pay and TSR performance were in the top right quadrant due to leading stock price performance. Both
analyses demonstrate our strong pay for performance alignment as the Company’s relative stock price performance ranked higher
than CEO relative pay.
| CEO
Realizable TDC Rank vs. 3-Year TSR Performance Rank |
CEO
Realizable TDC Rank vs. 5-Year TSR Performance Rank |
 |
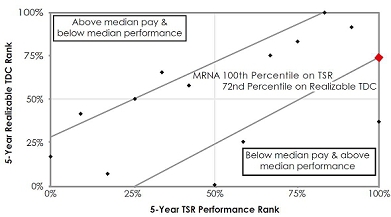 |
 |
2024
Proxy statement |
41 |
Realizable Total Direct Compensation
(Realizable TDC) is defined as the sum of the following components: actual base salaries, short-term incentive awards, and long-term incentive
awards paid over the preceding three- and five-year periods. The value of all in-the-money stock options granted during the preceding
three- and five-year period and RSUs and PSUs granted over the preceding three- and five-year periods (reflecting actual performance results
or estimated performance based on peer proxy disclosures) are valued as of December 31, 2023.
TSRs are calculated based on the
dividend adjusted closing price per share of common stock on the applicable exchange from January 1, 2021 through December 31, 2023 and
from January 1, 2019 through December 31, 2023 for the respective periods.
2023 Say on Pay Vote and Shareholder Engagement
In 2023, 88% of shareholders who voted at
our Annual Meeting on our “say on pay” proposal supported the pay actions we took in the prior year. We believe
this vote signals strong overall support for our pay programs and their general design. Based upon feedback from our investors since the
2023 Annual Meeting, our Compensation Committee has made the following enhancements:
| • |
increased the weighting in our long-term incentive program for performance-based restricted stock units (PSUs) for our executive team beginning in 2024, and |
| • |
continued
to provide greater visibility into those goals that will inform payouts on those PSU awards and for the goals in our corporate
scorecard. For additional detail, see “Letter from the Compensation and Talent Committee,” on page 36, and
“Governance—Shareholder Engagement” on page 20. |
Executive Compensation Program Design
Our executive compensation program
is designed to be competitive, and balance our goal of attracting, motivating, rewarding and retaining top-performing executives with
our goal of aligning their interests with those of our shareholders. Our Compensation Committee annually evaluates our executive compensation
program to ensure that it is consistent with our short-term and long-term goals and the dynamic nature of our business.
Our executive compensation program
consists of a mix of compensation elements that balance achievement of our short-term goals with our long-term performance. We provide
short-term incentive compensation opportunities in the form of annual cash bonuses, which focus on our achievement of annual corporate
goals. We also provide long-term incentive compensation opportunities in the form of equity awards, which, until recent years, consisted
primarily of stock options. Due to Moderna’s pre-commercial stage of development until 2020, our equity programs were primarily
focused on stock options to provide our team with a strong incentive to pursue growth that would result in stock price appreciation over
the long-term.
As the Company has matured, our
equity programs have evolved. In 2021, we introduced PSUs into our equity programs for our Executive Committee members. As further described
on page 53 below, these PSUs focused on achieving those goals that the Compensation Committee
recognized would be key to our long-term success, particularly once COVID-19 vaccine revenues peaked.
Early in 2021, Moderna had still
not received full approval for any product—just an emergency use authorization for its COVID-19 vaccine—and the COVID-19 vaccine
represented our only product to have reached a Phase 3 trial. Therefore, the goals for 2021-2023 reflected the importance of (i) obtaining
full approval for our COVID-19 vaccine in key markets, (ii) commencing Phase 3 or registrational studies for non-COVID programs, and (iii)
advancing our non-vaccine, therapeutic programs to positive Phase 1/2 early clinical readouts. Each of these goals for this first class
of PSUs was achieved above target. The goals for our other classes of PSUs are further described on page 54 below.
For the PSUs granted in 2021, 2022 and 2023, the long-term incentive mix was prescribed for each of our Executive Committee members as
described below. Beginning in 2024, PSUs will represent 50% of the weighting for equity awards to our CEO, and one-third of the weighting
for equity awards to our other Executive Committee members.
| |
2023 |
|
2024 |
| Equity Mix |
Stock Options |
PSUs |
RSUs |
|
Stock Options |
PSUs |
RSUs |
| CEO |
75% |
25% |
— |
|
50% |
50% |
— |
| Other Executive Committee Members |
50% |
25% |
25% |
|
33% |
33% |
33% |
 |
2024
Proxy statement |
42 |
Our executive compensation program
is also designed to incorporate sound practices for compensation governance as summarized below.
 |
Maintain
an Independent Compensation Committee. The Compensation Committee consists solely of independent directors. |
 |
Retain an Independent
Compensation Advisor. The Compensation Committee engages its own advisor to provide information, analysis and advice on
executive compensation decisions, independent of management. |
 |
Hold Annual Say-on-Pay
Vote. We put our executive compensation to an advisory vote of shareholders annually. |
 |
Deliver Significant
At-Risk Compensation. Our executive compensation program is designed so that a significant portion of our executive officers’
compensation is “at risk” based on our corporate and stock performance, to align the interests of our executives and
shareholders. |
 |
Use
a Pay-for-Performance Philosophy. The majority of our executive officers’ compensation is directly linked to corporate
performance and includes a significant long-term equity component, dependent upon our stock price and pipeline development goals. |
 |
Require 10b5-1
Plans. Require our executives to plan sales of Moderna stock in advance through the use of 10b5-1 plans. |
 |
Double-Trigger
Change of Control. Our Executive Severance Plan has double-trigger change of control provisions, requiring the termination
of employment or resignation for “good reason” within 12 months following a change of control for payments and accelerated
vesting of equity awards under the plan. |
 |
Maintain a Clawback
Policy. We have a clawback policy applicable to performance-based compensation for our Executive Committee, which would
apply in the event of a financial restatement or other improper conduct causing material financial, operational
or reputational harm. |
 |
Mitigate Undue
Risk-Taking. Employ the use of multiple performance goals and performing annual compensation risk assessments. |
| WHAT WE DON’T DO |
 |
No Special Health
and Welfare Benefits. Our executive officers participate in our health and welfare benefits programs on the same basis as
our other employees, other than eligibility for additional long-term disability insurance that we offer at the VP level and up. |
 |
No Executive
Retirement Plans. We do not offer pension or retirement plans to our executive officers that are different from or in addition
to those offered to our other employees. |
 |
No Post-Employment
Tax Payment Reimbursement. We do not provide any tax reimbursement payments (including “gross-ups”) on any change-in-control
or severance payments or benefits. |
 |
No Hedging or
Pledging Our Equity Securities. We prohibit our executive officers, the members of our Board and certain other employees
from hedging or pledging our securities. |
 |
No
Stock Option Re-Pricing under Current Stock Plan. Our 2018 Stock Plan does not permit stock options to be repriced to a
lower exercise or strike price without the approval of our shareholders. |
 |
2024
Proxy statement |
43 |
Governance of Executive
Compensation Program
Role of the Compensation
Committee and the Board
The Compensation Committee,
which is comprised entirely of independent directors, is responsible for discharging our Board of Directors’ responsibilities
relating to compensation of our directors and executives, overseeing our overall compensation structure, policies and programs,
and reviewing our processes and procedures for the consideration and determination of director and executive compensation. The
primary objective of the Compensation Committee is to develop and implement compensation policies and plans to attract and retain
key management personnel, motivate management to achieve our corporate goals and strategies, and align the interests of management
with the long-term interests of our shareholders. We have not adopted formal guidelines for allocating total compensation between
long-term and short-term compensation, cash compensation and non-cash compensation, or among different forms of non-cash compensation.
Our Compensation Committee
has engaged Pay Governance LLC, an independent executive compensation consultant, to provide guidance with respect to the development
and implementation of our compensation programs.
Pursuant to our Equity Award
Grant Policy, the Compensation Committee has delegated to our CEO and our Chief Human Resources Officer (CHRO) the authority to
approve grants of equity awards, subject to certain parameters, under the 2018 Stock Plan. See “Other Compensation Policies
and Practices—Equity Award Grant Policy.”
The Compensation Committee
reviews and approves the primary elements of compensation—base salary increases, annual cash bonuses, and annual equity awards—for
our NEOs (other than our CEO), as authorized by the Board of Directors pursuant to the Compensation Committee charter. Our Board
of Directors reviews and provides final approval for the primary elements of compensation awarded to our CEO after recommendation
by the Compensation Committee.
Compensation-Setting Factors
When reviewing and approving,
or recommending to the Board of Directors, as applicable, the amount of each compensation element and the target total compensation
opportunity for our executive officers, the Compensation Committee considers the following factors:
| • |
our performance during the year, based on business and corporate goals and priorities established by the CEO and the Board; |
| • |
each executive officer’s skills, experience and qualifications relative to other similarly situated executives at the companies in our compensation peer group; |
| • |
the scope of each executive officer’s role compared to other similarly situated executives at the companies in our peer group; |
| • |
the performance of each individual executive officer, based on an assessment of his or her contributions to our overall performance, ability to lead his or her department and work as part of a team, all of which reflect our values; |
| • |
compensation parity among our executive officers; |
| • |
our retention goals; |
| • |
the compensation practices of our peer group; and |
| • |
our CEO’s recommendations with respect to the compensation of our other executive officers. |
These factors provide the
framework for compensation decisions for each of our executive officers, including our NEOs. The Compensation Committee and the
Board of Directors, as applicable, do not assign relative weights or rankings to these factors, and do not consider any single
factor as determinative in the compensation of our executive officers.
Role of Management
In discharging its responsibilities,
the Compensation Committee works with management, including our CEO. Our management assists the Compensation Committee by providing
information on corporate and individual performance, market compensation data and management’s perspective on compensation
matters.
In addition, at the beginning
of each year, our CEO generally reviews the performance of our other executive officers, including our other NEOs, based on our
achievement of our corporate goals and each executive officer’s achievement of his or her departmental and individual goals
established for the prior year and his or her overall performance during that year. The Compensation Committee solicits and reviews
our CEO’s recommendations for base salary increases, annual cash bonuses, annual equity awards and any other compensation
opportunities for our other executive officers, including our other NEOs, and considers our CEO’s recommendations in determining
such compensation.
Role of Compensation Consultant
The Compensation Committee
engages an external compensation consultant to assist it by providing information, analysis and other advice relating to our executive
compensation program. For 2023, the Compensation Committee engaged Pay Governance as its compensation consultant to advise on executive
compensation matters including:
| • |
review and analysis of the compensation for our executive officers, including our NEOs, and our Board of Directors; |
| • |
assistance on incentive program design and discussion on executive compensation and governance trends; |
 |
2024
Proxy statement |
44 |
| • |
review and input on the Executive Compensation section of our Proxy Statement for our 2024 Annual Meeting of Shareholders; |
| • |
research, development and review of our compensation peer group; and |
| • |
support on other compensation matters as requested throughout the year. |
Pay Governance reports directly
to the Compensation Committee and to the Compensation Committee chairman. Pay Governance also coordinates with our management for
data collection and job matching for our executive officers. Our Compensation Committee charter requires that our compensation
consultant is independent of Company management. During 2023, Pay Governance did not provide services to us other than the services
to our Compensation Committee described herein. Our Compensation Committee performs an annual assessment of its compensation consultant’s
independence to determine whether the consultant is independent and in 2023 has determined that Pay Governance is independent pursuant
to the Nasdaq listing standards and SEC rules and has determined that no conflict of interest has arisen as a result
of the work performed.
Role of Market Data
For purposes of comparing
our executive compensation against the competitive market, the Compensation Committee reviews and considers the compensation levels
and practices of a group of peer companies. This compensation peer group consists of public biotechnology and pharmaceutical companies
against which we may compete for talent and that are similar to us across a number of factors, including market capitalization,
stage of development, geographical location and number of employees. The Compensation Committee reviews our compensation peer group
at least annually and makes adjustments to our peer group as necessary, taking into account changes in both our business and our
peer companies’ businesses. The Compensation Committee also uses market data from our compensation peer group and from the
Radford Global Life Sciences Compensation survey as one factor in evaluating whether the compensation for our executive officers
is competitive in the market. The Compensation Committee and the Board of Directors, as applicable, also rely on their own knowledge
and judgment in evaluating market data and making compensation decisions.
Since becoming a public company
in 2018, our Compensation Committee has used our peer group to assist in assessing annual base salary, target bonus and equity
awards for our NEOs and other senior level employees. To determine the composition of the peer group for 2023 the Compensation
Committee considered the following criteria:
| • |
commercial stage pharmaceutical and biotechnology companies with global revenues, double-digit pipelines and multiple late-stage candidates; |
| • |
market capitalization, generally selecting companies with a market capitalization around Moderna’s peer median; |
| • |
companies with significant commercial revenues (generally more than $2 billion annually); |
| • |
headcount factoring in Moderna’s growing headcount as company grows globally and commercializes; and |
| • |
R&D expenditure to provide context for scale of R&D organization and to balance revenue perspective as focus shifts to commercial revenue. |
 |
2024
Proxy statement |
45 |
Based on these factors, the
compensation peer group set forth below was adopted in October 2022 and was used in assessing compensation for our executives,
including those decisions that were made in February 2023, particularly related to salary and annual equity awards that are described
in this proxy statement.
2022-2023 Compensation
Peer Group
| AbbVie | |
BioMarin Pharmaceutical | |
Merck |
| Alnylam Pharmaceuticals | |
Bristol-Myers Squibb | |
Pfizer |
| Amgen | |
Eli Lilly | |
Regeneron Pharmaceuticals |
| BeiGene Ltd. | |
Gilead Sciences | |
Seagen (fka Seattle Genetics) |
| Biogen | |
Incyte Corporation | |
Vertex Pharmaceuticals |
In August 2023, based on
ongoing analysis from Pay Governance, the Compensation Committee made updates to the compensation peer group that informed decisions
made in February 2024 and that will inform decisions for the rest of the year. Updates were primarily aimed at removing and replacing
companies whose market capitalization and future anticipated revenues were more aligned to Moderna on these factors.
2023-2024 Compensation
Peer Group
| Alnylam Pharmaceuticals | |
Bristol-Myers Squibb | |
Pfizer |
| Amgen | |
Gilead Sciences | |
Regeneron Pharmaceuticals |
| BeiGene Ltd. | |
Incyte Corporation | |
Vertex Pharmaceuticals |
| Biogen | |
Jazz Pharmaceuticals + | |
|
| BioMarin Pharmaceutical | |
Merck | |
|
| + |
New addition to peer group for
2023-2024. |
| |
Removed AbbVie and Eli Lilly;
Seagen was acquired by Pfizer in December 2023 |
 |
2024
Proxy statement |
46 |
Primary Elements of Executive
Compensation Program
The primary elements of our
executive compensation program are:
| • |
base salary; |
| • |
short-term incentive compensation in the form of annual cash bonuses; and |
| • |
long-term incentive compensation in the form of annual equity awards. |
We do not have a specific
policy regarding the percentage allocation between short-term and long-term, or fixed and variable, compensation elements.
Our executive officers, including
our NEOs, are also eligible to participate in our standard employee benefit plans, such as our health and welfare benefits plans,
our employee stock purchase plan and our 401(k) Plan on the same basis as our other employees. In addition, as described below,
our executive officers, including our NEOs, are entitled to certain change-in-control severance payments and benefits pursuant
to our Executive Severance Plan, described herein.
Base Salary
We pay base salaries to our
executive officers, including our NEOs, as the fixed portion of their compensation to provide them with a reasonable degree of
personal income, and to attract and retain top-performing individuals. At the time of hire, base salaries are determined for our
executive officers, including our NEOs, based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting
Factors” above. Typically, at the beginning of each year, the Compensation Committee reviews base salaries for our executive
officers, including our NEOs, based on such factors to determine if an increase is appropriate. In addition, base salaries may
be adjusted in the event of a promotion or significant change in responsibilities.
Salary increases for each
of our NEOs were reviewed, approved and took effect in February 2023, and were reflective of merit and market adjustments to better
align each of these executive’s salaries to market, as well as cost-of-living adjustments applied across our broader employee
base. The actual salaries paid to our NEOs in 2023 are set forth in the “Summary Compensation Table.”
| |
|
2022 |
|
2023 |
|
Percent Change |
| Stéphane
Bancel |
|
$1,500,000 |
|
$1,575,000 |
|
5.0% |
| James
Mock |
|
750,000 |
|
800,000 |
|
6.7% |
| Stephen
Hoge, M.D. |
|
1,000,000 |
|
1,050,000 |
|
5.0% |
| Arpa
Garay |
|
800,000 |
|
850,000 |
|
6.3% |
| Shannon
Thyme Klinger |
|
700,000 |
|
800,000 |
|
14.3% |
Short-Term Incentive Compensation
Annual Cash Bonuses
We provide short-term incentive
compensation opportunities to our executive officers, including our NEOs, in the form of annual cash bonuses to drive our short-term
success. Our annual cash bonuses are tied to the achievement of annual corporate and individual performance goals pursuant to the
Company’s Senior Executive Cash Incentive Bonus Plan (the Bonus Plan). In addition, our Compensation Committee may grant
cash bonuses to the CEO or the other NEOs pursuant to the Bonus Plan based on individual performance during the year.
Corporate and Individual
Performance Goals
At the beginning of each
year, the Compensation Committee discusses with the CEO the annual corporate performance objectives that are intended to be the
most significant drivers of our short-term and long-term success.
In addition, at the beginning
of each year, our CEO, in consultation with each of the other executive officers, establishes individual performance goals for
each of the other executive officers, including our other NEOs. The individual performance goals are generally designed to align
the goals of our executive officers, including our NEOs, and his or her department with the corporate goals. The CEO discusses
with the Compensation Committee his overall goals for the year which are in line with the overall corporate objectives but also
include individual goals and action plans. The CEO’s goals and performance are ultimately evaluated, and his bonus is approved,
by the full Board, with input from the Compensation Committee.
At the beginning of the year
after the corporate performance objectives are established, the Compensation Committee, after reviewing management’s self-assessment,
evaluates specific achievements that are designed to advance the prior year’s corporate objectives, and our overall success
in the prior year, and determines the Company’s total percentage achievement level. Our
 |
2024
Proxy statement |
47 |
CEO evaluates the other executive
officers’, including the other NEOs’, achievement of their prior year’s individual performance goals, and makes
recommendations for total percentage achievement level. The Compensation Committee considers our CEO’s recommendations, and
independently reviews and approves the total percentage achievement level for each of the other executive officers, including our
other NEOs.
Target Annual Bonuses
At the time of hire, the
target annual bonus is determined for each of our executive officers, including our NEOs, and at the beginning of each year, the
Compensation Committee reviews and approves the target annual bonus for each such individual. The Compensation Committee considers
the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, with
an emphasis on market data from our compensation peer group for comparable positions while also factoring internal parity. Target
annual bonuses represent a specific percentage of annual base salary.
2023 Corporate Objectives
In early 2023, our Compensation
Committee set broad based corporate objectives that established the criteria for the funding of our annual bonus plan. These corporate
objectives were also designed to inform the more detailed goal setting by individual executive officers and their teams. These
goals and objectives were based upon our strategic plan, and focused on the following key objectives:
| (1) |
Continue to execute the operational and sales plan for COVID-19 vaccine. The metrics for this objective were designed to incentivize increasing vaccination rates globally to achieve significant market share. |
| (2) |
Build an unrivaled seasonal respiratory vaccine franchise. The metrics for this objective were designed to incentivize advancing our COVID-19, RSV and flu respiratory disease programs as well as our combination vaccine candidates to ensure alignment for commercial launch readiness. |
| (3) |
Execute on a bold campaign of cancer vaccine studies. The metrics for this objective were designed to incentivize executing our individualized neoantigen therapy (INT) through enrollment of patients in clinical studies and supporting the continued progress to enable its commercial launch. |
| (4) |
Advance rare metabolic disease programs. The metrics for this objective were designed to incentivize advancing our rare disease portfolio through enrollment and expansion of propionic acidemia to pivotal studies. |
| (5) |
Drive rapid advancement and growth in our latent vaccine portfolio. The metrics for this objective were designed to incentivize developing vaccines for viruses with unmet or underserved needs and progressing vaccine candidates through clinical trial phases. |
| (6) |
Deliver the next-generation pipeline and platform. The metrics for this objective were designed to incentivize delivering pipeline expansions and platform technology improvements. |
| (7) |
Build a culture of learning, and strengthen our processes and digital systems. The metrics for this objective were designed to incentivize increasing employee learning, and improving in priority processes and employee belonging. |
For each of these corporate
objectives, our Compensation Committee established criteria for assessing performance in terms of what achievements would be below
expectations, meet expectations or exceed expectations, with weighting assigned to each of these objectives as described on the
next page. For performance at target for each objective, 100% of the allocable portion of the bonus was payable. For performance
below target, but where threshold performance was met, 50% of the allocable portion of the bonus was payable. For maximum performance,
200% of the allocable portion of the bonus was payable.
In February 2024, the Compensation
Committee completed its assessment of management’s achievement of these corporate objectives for 2023, and concluded that
for these core corporate objectives, management performed at a level that merited funding the bonus pool at 90%.
In making its assessment,
the Compensation Committee noted that commercial performance fell short of where the Company expected to perform at the beginning
of 2023, albeit above threshold. In terms of operating income, the Company’s performance was below threshold performance,
in part due to expenses that were recognized during the year in connection with resizing the Company’s manufacturing footprint
and accounting-related tax expenses. No payout was made for operating income, and the Compensation Committee did not make any adjustments
for these metrics beyond the GAAP results. Together, these financial goals represented the most heavily weighted aspect of the
scorecard.
For non-financial metrics,
the Compensation Committee recognized that the Company continued to advance its pipeline and development programs with very strong
results, including preparing for a launch of its RSV vaccine in 2024; advancing next-generation and combination vaccines; enrolling
patients in clinical trials for the Company’s oncology programs; advancing rare disease therapeutic programs to prepare for
pivotal studies; completing enrollment in the Company’s CMV Phase 3 trial; and opening Investigational New Drug (IND)
applications for nine new products, including at least two in novel modalities or product concepts. Improvements to our platform
technology, improvements to priority processes and employee learning were all on target. However, employee belonging, as measured
in annual engagement surveys, fell short of expectations and no payout was made for this metric.
 |
2024
Proxy statement |
48 |
Set forth below is
a summary of the scorecard and the Compensation Committee’s assessment of performance. The maximum performance metric
for each goal is not disclosed, as these metrics are competitively sensitive, unless above target or maximum performance was
achieved, in which case performance is described below.
Corporate
Objective |
|
Goal
Weight |
|
Performance Metric Goals |
|
Actual Performance |
|
Committee
Assessment |
|
Payout |
| Maximize the impact of the COVID-19 vaccine |
|
|
|
|
|
|
|
|
|
|
•
Product Sales |
|
 |
|
•
Threshold:
$5.5B
•
Target:
$9.0B
|
|
$6.7B |
|
▼ |
|
10% |
• Operating Income |
|
 |
|
•
Threshold:
($3.2B)
•
Target:
($0.4B)
|
|
($4.2B) |
|
✖ |
|
0% |
| Build an unrivaled seasonal respiratory vaccine franchise |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
•
Expand portfolio of licensed respiratory vaccines |
|
 |
|
•
Threshold:
File two COVID-19 sBLAs (supplemental Biologics License Applications)
•
Target:
File three BLAs – Two COVID-19 sBLAs; RSV BLA
|
|
•
Filed
two COVID-19 sBLAs
•
Filed
RSV BLA
|
|
●
|
|
|
•
Prepare for commercial launch of RSV and flu vaccines |
|
 |
|
•
Threshold:
Align on demand and supply forecasts and secure capacity; create systems and processes to
accept sales by Q1 2024
•
Target:
Threshold goals; align on pricing and access strategies in each targeted country; and define
commercialization strategy
|
|
•
Threshold and target goals achieved |
|
● |
|
20% |
•
Advance clinical stage respiratory vaccines |
|
 |
|
•
Threshold:
Complete Phase 1/2 enrollment for next-gen COVID-19/ Flu combination vaccine;
enroll first patient in pediatric RSV combination vaccine
•
Target:
Complete interim analysis for next-gen COVID-19/ Flu combination vaccine
|
|
•
Initiated
Phase 3 studies for next-gen COVID-19 vaccine and COVID-19/ Flu combination vaccine
•
Initiated Phase 1 study for pediatric RSV + hMPV
|
|
◆ |
|
|
| Execute on a bold campaign of cancer vaccine studies |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
•
Demonstrate the potential clinical benefit of our Individualized Neoantigen Therapy (INT) program |
|
 |
|
•
Threshold:
Enroll ** patients in melanoma studies
•
Target:
Enroll ** patients in melanoma studies
|
|
•
Exceeded
maximum enrollment goal for melanoma studies
•
First
non-small cell lung cancer patient randomized January 5, 2024
|
|
▲ |
|
15% |
●
= At Target ▲=
Above Target ▼= Below Target ◆ = At Maximum ✖= Below Threshold
** Not disclosed; competitively sensitive
 |
2024
Proxy statement |
49 |
Corporate
Objective |
|
Goal
Weight |
|
Performance Metric Goals |
|
Actual Performance |
|
Committee
Assessment |
|
Payout |
| Advance rare metabolic disease programs |
|
 |
|
•
Threshold:
Enroll ** patients in Propionic acidemia (PA) study; dose first patient in cystic fibrosis (CFTR)
study
•
Target:
Identify recommended dose for PA expansion; initiate dosing a Phase 1/2 expansion or pivotal
study; and enroll ** new patients in rare disease studies (e.g., PA, CFTR, MMA, GSD1a, PKU)
|
|
•
PA
dose selected and expansion in progress
•
Met target enrollment goal for rare disease programs
|
|
● |
|
10% |
Drive rapid advancement
and growth in our latent or other vaccine portfolio |
|
 |
|
•
Threshold:
Enroll 80% of CMV P301
•
Target:
Enroll 80% of CMV P301; complete EBV infectious mononucleosis dose selection
|
|
•
CMV
P301 fully enrolled
•
Dosed
first patients in VZV, HSV, Norovirus, Lyme and EBV therapeutic vaccine studies
|
|
▲ |
|
15% |
| Deliver the next-generation pipeline and platform |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
•
Expand
and diversify our pipeline through use of our mRNA technology
•
Meaningfully
advance our mRNA technology to enhance our current modalities and invent novel modalities
|
|
 |
|
•
Threshold:
Open 6 IND/ CTAs
•
Target:
Open 8 IND/ CTAs with at least one novel product concept
|
|
•
9 INDs safe to proceed including two novel concepts |
|
● |
|
|
| |
|
•
Threshold:
Achieve 2 of 5 of platform objectives**
•
Target:
Achieve 3 of 5 of platform objectives**
|
|
•
Proof of biology or clinical demonstration for 3 of 5 platform objectives for current and novel modalities |
|
● |
|
10% |
Build a culture of learning and strengthen
our processes and digital systems |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
•
Demonstrate
improvement in priority processes
•
Invest in the development of our talent
•
Build
a culture of belonging and inclusion
|
|
 |
|
•
Threshold:
Achieve 1 of 3 key process improvement goals (e.g., ERM launch, CMC goals, CMO mgmt, workforce
planning)
•
Target:
Achieve 2 of 3 goals
|
|
•
Achieved 2 of 3 key
process improvement
goals |
|
● |
|
10% |
| |
|
•
Threshold:
Increase average learning hours per employee by 25%
•
Target:
Increase by 25% to 50%
|
|
•
67% average increase per employee |
|
◆ |
|
|
| |
|
•
Threshold:
Average engagement score of 72 to 73 based on surveys conducted during 2023
•
Target:
Average score of 74 to 75 (same or better than 2022)
|
|
•
Average engagement score of 71 |
|
✖ |
|
|
| Final Performance Assessment |
|
|
|
|
|
|
|
90% |
●
= At Target ▲=
Above Target ▼= Below Target ◆ = At Maximum ✖= Below Threshold
** Not disclosed; competitively sensitive
 |
2024
Proxy statement |
50 |
2023 Annual Bonus Determination
At the beginning of each
year, the Compensation Committee reviews the target annual bonuses of our executive officers, including our NEOs. The Compensation
Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors”
above, particularly market data from the companies in our compensation peer group. For 2023, our Board, acting on the recommendation
of the Compensation Committee, maintained the target bonus for our CEO at 150% of salary. The Compensation Committee similarly
maintained the target bonus for Dr. Hoge at 100% of salary, and for our other NEOs at 90% of salary. In maintaining these target
bonuses, the Board and Compensation Committee noted that targets were consistent with market trends for similarly situated executives,
and that the targets provide an enhanced incentive to achieve annual corporate objectives.
In February 2024, the Compensation
Committee and Board determined that due to the challenges Moderna faced in meeting our corporate objectives for 2023, particularly
with respect to meeting our financial goals, each member of the Executive Committee would receive a below-target individual multiplier
of 90%. When combined with the 90% corporate multiplier, this individual performance rating resulted in bonus payments at 81% of
target. This approach acknowledges that while each member of the Executive Committee made meaningful contributions to the Company’s
progress in 2023, the Company’s success in achieving its objectives requires the Executive Committee to deliver as a team.
The individual performance
factor for each NEO was determined by the Compensation Committee following discussion of the Company’s overall performance.
The Board and Compensation Committee agreed upon the decision to provide an individual performance multiplier of 90% to all of
our Executive Committee members, including our CEO. The individual performance factors, when multiplied by the target bonus reflecting
achievement of the corporate objective discussed above, resulted in individual bonus payouts below target, as described below.
These bonus payouts are reflected under the “Non-Equity Plan Incentive Compensation” column in the 2023 Summary Compensation
Table on page 59 below.
| Name | |
2023 Annual
Base Salary
($) | |
2023 Annual
Target Bonus
(% of salary) | |
2023 Annual
Target Bonus
($) | |
Corporate
Performance
Factor (%) | |
Individual
Performance
Factor (%) | |
Actual Cash
Bonus
($) |
| Stéphane Bancel | |
$1,575,000 | |
150% | |
$2,362,500 | |
90% | |
90% | |
$1,913,625 |
| James Mock | |
800,000 | |
90% | |
720,000 | |
90% | |
90% | |
583,200 |
| Stephen Hoge, M.D. | |
1,050,000 | |
100% | |
1,050,000 | |
90% | |
90% | |
850,500 |
| Arpa Garay | |
850,000 | |
90% | |
765,000 | |
90% | |
90% | |
619,650 |
| Shannon Thyme Klinger | |
800,000 | |
90% | |
720,000 | |
90% | |
90% | |
583,200 |
 |
2024
Proxy statement |
51 |
Long-Term Incentive Compensation
The Committee believes that LTI awards are a critical element of
compensation that provide a mechanism to align shareholders and executives, and to reinforce behaviors consistent with Moderna’s
strategic plan. The value of equity awards is directly related to stock price appreciation over time, which incentivizes our executive
officers to achieve long-term corporate goals and create long-term value for our shareholders. Equity awards also help us attract
and retain top-performing executive officers in a competitive market.
| Long-term incentive vehicle |
|
Standard vesting schedule |
|
Description |
| Stock Options |
|
• Ratably
over four years (25% vesting on first anniversary of grant date, 6.25% over the next 12 quarters) |
|
• Provide
strong incentives for our executives to pursue growth that would result in long-term stock price appreciation |
| Restricted Stock Units (RSUs) |
|
• Ratably
over four years (25% vesting on first anniversary of grant date, 6.25% over the next 12 quarters) |
|
• Reward
executives for growth in the price of our common stock as additional values are derived
from stock price appreciation
• Help
build actual stock ownership and are less dilutive to our shareholders than stock options
|
| Performance-based Restricted Stock Units (PSUs) |
|
• Cliff
vesting based on performance at the end of three-year performance period |
|
• Reward
executives for execution of long-term strategic and financial objectives, while providing a direct link to the creation of
sustainable shareholder value and execution of our strategic business plan |
2023 Equity Awards
The value of equity awards granted to our executive officers, including
our NEOs, varies based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting
Factors” above, particularly market data from the companies in our compensation peer group, as well as each individual’s
performance in the prior year. At the beginning of each year, the Compensation Committee reviews the equity awards for our executive
officers, including our NEOs, and determines the size of the annual equity awards it deems reasonable and appropriate based on
such factors.
At its February 2023 meeting, the Compensation Committee approved
the 2023 annual equity awards for our NEOs. The Board made this determination for our CEO upon the recommendation of the Compensation
Committee.
The target equity values below reflect the values approved by the
Compensation Committee. The values included in the Summary Compensation and Grants of Plan-Based Awards tables reflect the grant
date fair values calculated in accordance with FASB ASC Topic 718, which differ for these equity awards based on the fact that
the number of underlying shares granted is calculated based upon a 20-trading day trailing average closing price for our stock
immediately before the grant date. Therefore, those accounting values differ from the values included in this table. See “Equity-Related
Policies and Practices—Equity Award Grant Policy.”
The equity awards granted to our NEOs in 2023 are set forth in the
“Summary Compensation Table” and the “Grants of Plan-Based Awards for Fiscal Year 2023” table on page 61.
| |
Stock Options |
RSUs |
PSUs |
Total Target Value of
Equity Award |
| Stéphane Bancel(1) |
$ |
11,250,000 |
$ |
— |
$ |
3,750,000 |
$ |
15,000,000 |
| James Mock(2) |
|
1,750,000 |
|
875,000 |
|
875,000 |
|
3,500,000 |
| Stephen Hoge, M.D.(2) |
|
3,250,000 |
|
1,625,000 |
|
1,625,000 |
|
6,500,000 |
| Arpa Garay(2) |
|
1,750,000 |
|
875,000 |
|
875,000 |
|
3,500,000 |
| Shannon Thyme Klinger(2) |
|
1,750,000 |
|
875,000 |
|
875,000 |
|
3,500,000 |
| (1) |
The annual equity awards mix for the CEO was 75%
stock options and 25% PSUs. |
| (2) |
The annual equity awards mix for the NEOs other than
the CEO was 50% stock options, 25% RSUs and 25% PSUs. |
 |
2024
Proxy statement |
52 |
Determination of 2021 Earned PSUs
We introduced performance-based restricted stock units (PSUs) as
part of our compensation program for our Executive Committee in 2021. These PSUs have a three-year performance period and are
focused on advancing and diversifying our portfolio beyond COVID-19. At the time the performance goals were adopted for this class
of PSUs, we had just received emergency use authorization for our COVID-19 vaccine, but had not received full approval for any
product (including our COVID-19 vaccine). Our COVID-19 vaccine was also our only program to have reached a Phase 3 study. At the
time, the Compensation Committee and Board recognized that obtaining full approval in major markets for COVID-19 vaccines, and
advancing our portfolio beyond COVID-19 and infectious diseases would be key to laying the groundwork for long-term, sustainable
growth. The only current NEOs who were employed by us at the time this PSU was awarded and who are eligible to receive them are
Mr. Bancel and Dr. Hoge.
The Committee approved a vesting of 200% at its February 2024 meeting
for the 2021 PSUs. The table below summarizes the goals established for the three-year period along with the Committee’s
performance assessment of each goal. The maximum vesting reflects the significant momentum built cross our business and
our pipeline advancing our respiratory, latent, oncology and rare disease franchises.
| Performance Metric |
Metric
Weighting |
Threshold
(50% vesting) |
Target
(100% vesting) |
Maximum
(200% vesting) |
Actual |
Vesting Percentage |
Obtaining product approval under BLA from US FDA,
or equivalent approval for commercial distribution in another major market
|
25% |
1
(either in the US or the European Union)
|
2
(approval in both the US and the European Union)
|
4 or more
(all major market countries)
|
▲
|
COVID-19 approval in:
1. US
2. Canada
3. EU
4. UK
5. Japan
|
50% |
Initiation of Phase 3 or registrational study for any non-COVID
program
|
50% |
1 |
3 |
4+ |
▲ |
1. INT
Melanoma
2. Flu
3. RSV
4. Flu/COVID
5. CMV
|
100% |
Completing a non-vaccine Phase 1 or 2 trial producing positive
readouts, other than in infectious disease vaccines
|
25% |
1 |
2 |
3+ |
▲ |
1. INT
Phase 2b (melanoma)
2. PA Phase 1/2
3. MMA Phase 1/2
4. GSD1a Phase 1
|
50% |
| |
|
|
|
|
|
Total Vesting |
200% |
| ▼ Threshold ● Target ▲ Maximum |
Based on the Committee’s approval of the 200% vesting, the
following PSUs vested on February 14, 2024 for our NEOs that participated in the program:
| Name |
Number of
PSUs Granted
at Target |
|
Grant Date
Value(1) |
Vesting
Percentage |
Earned
Number of PSUs |
|
Value at
Vest(2) |
| Stéphane Bancel |
28,368 |
$ |
3,749,966 |
200% |
56,736 |
$ |
4,843,552 |
| Stephen Hoge, M.D. |
11,347 |
|
1,499,960 |
200% |
22,694 |
|
1,937,387 |
| (1) |
Values represent grant
date fair values calculated in accordance with FASB ASC Topic 718. |
| (2) |
The value realized upon the vesting
is calculated based on the closing market price of a share of our common stock on the date prior to the date of vesting. |
 |
2024
Proxy statement |
53 |
Outstanding PSU Awards
The table below summarizes the PSU awards that were granted in 2022,
2023 and 2024 that remain outstanding as of the date of this Proxy Statement, including an overview of the pre-established performance
metrics for each award.
| Grant Year |
|
2022 |
|
2023 |
|
2024 |
2025 |
2026 |
2027 |
| 2022 |
|
Key metrics:
• Advancing our combination
respiratory vaccine strategy
• Diversifying our pipeline
beyond respiratory vaccines
• Establishing manufacturing
plants
• Building out our digital
capabilities
|
|
|
|
| 2023 |
|
|
|
Key metrics:
• Obtaining
approvals for respiratory disease programs and growing our U.S. market share
• Advancing
our individualized neoantigen therapy (INT)
|
|
|
| 2024 |
|
|
|
|
|
Key Metrics:
• Delivering
on key externally communicated financial goals through 2026
• Executing
on our late-stage pipeline beyond respiratory
|
|
The inclusion of long-term financial metrics into our PSUs for the
first time in 2024 reflects our ongoing evolution and increased visibility into financial performance over a multi-year period
as a commercial company, particularly as we execute on the anticipated launch of additional respiratory products. We also remain
focused on expanding and diversifying our portfolio into non-respiratory therapeutic areas to drive long-term shareholder value.
2024 Long-Term Incentive Mix
At the February
2024 meeting, the Compensation Committee approved changes to the LTI mix for the CEO Compensation and other NEOs, shifting
additional weighting towards PSUs to further tie our executive compensation program to our long-term financial and pipeline
expansions objectives. We believe that this adjustment to the weighting of the LTI mix will continue to focus our executive
team on achieving those strategic objectives that will lead to the creation of sustainable shareholder value.
Increased
Weighting of PSUs for 2024 LTI Awards

 |
2024
Proxy statement |
54 |
Other Employee Benefits & Perquisites
Health and Welfare Benefits
Our executive officers, including our NEOs, are eligible to participate
in the same employee benefit plans that are generally available to all regular employees. These benefit plans include medical,
dental, and life and disability insurance plans, as well as well-being and time off programs. We sponsor a portion of the premiums
for health, life and disability insurance. Certain employees at the vice president level and above, including the NEOs, are eligible
for additional long-term disability insurance coverage.
2018 Employee Stock Purchase Plan
Our executive officers, including our NEOs (other than Mr. Bancel),
are eligible to participate in our 2018 Employee Stock Purchase Plan (the ESPP) on the same basis as our other full-time U.S.
employees. The ESPP is a broad-based stock ownership program that permits eligible employees to set aside a portion of their compensation
during a six-month offering period and use such contributions to purchase shares of our common stock at a purchase price equal
to 85% of the lower of the fair market value of the shares on the first business day of the offering period or the last business
day of the purchase period. During 2023, Ms. Garay was the only NEO that participated in the ESPP.
401(k) Savings Plan
We maintain a 401(k) plan that is tax-qualified under Section 401(a)
of the Internal Revenue Code of 1986, as amended (the Code), and is tax-exempt under Section 501(a) of the Code. Plan participants
can defer eligible compensation subject to applicable Code limits. We provide a matching contribution of up to 4.5% (100% of employee
contributions up to the first 3% of compensation, and then 50% of the next 3% of compensation). Once contributions are made, they
are fully vested. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are
not taxable to the employees until distributed from the 401(k) plan. Contributions can also be made on a Roth basis.
Limited Perquisites
We provided limited perquisites to our NEOs in 2023, consisting
primarily of security services and increased long-term disability insurance coverage, as well as reimbursing and paying related
taxes for relocation expenses. We believe that providing such perquisites was appropriate to assist our NEOs in the performance
of their duties, and for recruitment and retention purposes.
In response to the increased profile of our Company and our executives
as we pursued the development of a vaccine against COVID-19, beginning in 2020, the Company authorized the provision of personal
and home security services to certain of our executives, including some of the NEOs. These services continued into 2023 for certain
executives in response to the ongoing, heightened risk environment faced by these executives.
Employment Arrangements with our NEOs
Employment Offer Letters and Non-Compete, Non-Solicitation and Confidentiality Agreements
We generally enter into employment offer letters with new hires,
including each of our NEOs when they joined the Company. These offer letters set forth the basic terms and conditions of their
employment, including initial base salary, initial equity awards, eligibility to participate in our standard employee benefits
plans, the at-will employment relationship and, in certain cases, a one-time signing bonus and relocation benefits. These offer
letters also require that each NEO execute our standard employee confidentiality and assignment agreement. Each of our NEOs is
subject to our standard non-competition, non-solicitation, confidentiality, and assignment agreement, which provides for a perpetual
post-termination confidentiality covenant as well as post-termination non-competition and non-solicitation of customers, employees,
and consultants covenants for one year following termination.
Arpa Garay – Executive Separation Agreement and Release
In December 2023, we announced that following a restructuring of
the Company’s commercial organization and reporting lines, Arpa Garay, the Company’s Chief Commercial Officer, would
be departing the Company, and that effective as of December 8, 2023, Ms. Garay would no longer serve in that role or as an executive
officer of the Company. Ms. Garay has been asked to remain on as an employee of the Company through June 2024, and to serve in
an advisory capacity to Mr. Bancel and Dr. Hoge to assist with the transition. At the time of Ms. Garay’s departure, she
will be entitled to benefits under the Company’s Amended and Restated Executive Severance Plan, as amended on February 23,
2023 (Executive Severance Plan), subject to her execution of a separation agreement and release of claims in form and manner satisfactory
to the Company, as disclosed under “Executive Severance Plan” below. The benefits to which Ms. Garay will be entitled
under the Executive Severance Plan upon departure include: (i) twelve months of salary ($850,000), (ii) payment of her bonus for
the year of departure at target ($765,000); and (iii) COBRA coverage for 12 months. Ms. Garay will also be provided accelerated
vesting of 15% of any outstanding and unvested time-based equity awards, in accordance with the Company’s guidelines governing
involuntary terminations and up to 12 months from her final date of employment to exercise any vested but unexercised stock options.
Ms. Garay will continue to be eligible for her salary, benefits and vesting of time-based
 |
2024
Proxy statement |
55 |
equity awards while she remains employed by the Company, but she
will not be eligible for an equity award in 2024 or for a bonus for 2024 beyond the payments to which she is otherwise entitled
under the Executive Severance Plan.
Executive Severance Plan
We believe that the severance payments and benefits provided under
the Executive Severance Plans are appropriate in light of the post-employment compensation protections available to similarly
situated executive officers at companies in our compensation peer group and are an important component of each executive officer’s
overall compensation as they help us to attract and retain our key executives who could have other job alternatives that may appear
to them to be more attractive absent these protections.
In addition, we believe it is appropriate to provide enhanced severance
benefits in connection with certain employment terminations occurring in connection with a change in control in order to encourage
our executive officers to remain employed with us during an important time when their prospects for continued employment following
the transaction are often uncertain. The primary purpose of these arrangements is to keep our most senior executive officers focused
on pursuing potential corporate transactions that are in the best interests of our shareholders regardless of whether those transactions
may result in their own job loss. All of our NEOs are eligible for the Executive Severance Plan. Separate from the Executive Severance
Plan, our NEOs are also covered by the Company’s guidelines regarding involuntary terminations, which provide for accelerated
vesting of 15% of any outstanding and unvested time-based awards if termination occurs following the first anniversary of the
new hire grant date.
Termination not in connection with a change in control
The Executive Severance Plan provides that upon a termination of
employment by us other than for “Cause,” death, or “Disability,” or upon a resignation by an eligible
participant for “Good Reason” (in each case, as defined in the Executive Severance Plan), in either case outside of
the “change in control period” (i.e., the period beginning on the date of a “change in control” (as defined
in the Executive Severance Plan) and ending on the one-year anniversary of the change in control), the participant will be entitled
to receive, subject to the execution and delivery of a separation agreement and release containing, among other provisions, an
effective release of claims in favor of the Company and reaffirmation of the “restrictive covenants agreement” (as
defined in the Executive Severance Plan):
| (i) |
a severance amount equal to 12 months of the participant’s
annual base salary in effect immediately prior to such termination, payable over 12 months in the form of salary continuation, |
| (ii) |
an amount equal to the participant’s annual target bonus
in effect immediately prior to such termination, payable over 12 months, and |
| (iii) |
up to 12 monthly cash payments equal to the monthly employer
contribution that we would have made to provide health insurance for the applicable participant if he or she had remained
employed by us, based on the premiums as of the date of termination. |
Termination in connection with a change in control
The Executive Severance Plan also provides that upon a termination
of employment by us other than for Cause, death, or Disability or upon a resignation by an eligible participant for “Good
Reason,” in either case within the change in control period, the participant will be entitled to receive, in lieu of the
payments and benefits described above and subject to the execution and delivery of a separation agreement containing, among other
provisions, an effective release of claims in favor of the Company and reaffirmation of the restrictive covenants agreement:
| (i) |
a lump sum payment equal to 150% of the participant’s
annual base salary in effect immediately prior to such termination (or the participant’s annual base salary in effect
immediately prior to the change in control, if higher) paid in a cash lump sum as severance pay, |
| (ii) |
a lump sum payment equal to 150% of the participant’s annual
target bonus for the year such termination takes place (or the participant’s annual target bonus in effect immediately
prior to the change in control, if higher) (the Applicable Bonus), |
| (iii) |
a lump sum payment equal to (A) the participant’s Applicable
Bonus multiplied by (B) a fraction with a numerator equal to the number of full weeks elapsed in the then-current fiscal year
prior to the date of termination and with a denominator equal to 52, |
| (iv) |
a lump sum amount equal to the monthly employer contribution
that we would have made to provide health insurance for the participant if he or she had remained employed by us for 18 months
following the date of termination, based on the premiums as of the date of termination, |
| (v) |
for all outstanding and unvested equity awards of the Company
that are subject to time-based vesting held by the named executive officer, full accelerated vesting of such awards, and |
| (vi) |
for any performance-based equity awards, pro-rated acceleration
based on the better of target and actual performance against the performance metrics. |
 |
2024
Proxy statement |
56 |
The payments and benefits provided under the Executive Severance
Plan in connection with a change in control may not be eligible for a federal income tax deduction by us pursuant to Section 280G
of the Code. These payments and benefits may also subject an eligible participant, including the named executive officers, to
an excise tax under Section 4999 of the Code. If the payments or benefits payable to an eligible participant in connection with
a change in control would be subject to the excise tax imposed under Section 4999 of the Code, then those payments or benefits
will be reduced if such reduction would result in a greater net after-tax benefit to the applicable participant.
Equity-Related Policies and Practices
Equity Award Grant Policy
We have adopted an Equity Award Grant Policy that sets forth the
process and timing for us to follow when we grant equity awards for shares of our common stock to our employees, including our
executive officers, or advisors or consultants pursuant to any of our equity compensation plans. Pursuant to the policy, all grants
of equity awards must be approved in advance by our Board of Directors, the Compensation Committee or, subject to the delegation
requirements in the policy, our CEO or CHRO. The equity award granting authority delegated to our CEO and CHRO applies to employees
at the senior vice president level and below and to equity awards within the specific ranges set forth in the policy. All equity
awards for our Executive Committee members must be approved by the Compensation Committee or the full Board.
Generally, equity awards are granted on the following regularly
scheduled basis as set forth in the policy:
| • |
Equity awards granted in connection with the hiring
of a new employee are generally awarded on the fifth day of the month immediately following the month during which each new
employee is hired. |
| • |
Equity awards granted by our Board or the Compensation Committee
in connection with the promotion of an existing employee or the engagement of a new consultant are effective on the date of
approval by our Board or the Compensation Committee, as applicable, or such later date as specified in such approval. Our
Board and the Compensation Committee retain the discretion to grant equity awards at other times to the extent appropriate
in light of the circumstances of such awards. |
| • |
Equity awards granted to existing employees (other than in connection
with a promotion) will generally be granted, if at all, on an annual basis, including an annual award to all employees that
is generally granted at the end of the second trading day following the filing of our Annual Report on 10-K with the SEC;
additional grants for high-performing employees may be granted to certain employees at other times during the year. |
In addition, the Equity Award Grant Policy sets forth the way our
equity awards will be priced. If the grant of RSUs is denominated in dollars, the number of shares subject to each RSU award will
be determined by dividing the value of such award by the average closing market price on the Nasdaq Global Select Market of a
share of our common stock over the preceding 20 trading days, up to and including the last trading day immediately preceding the
effective date of grant (the 20-trading day trailing average price), and if the grant of an option is denominated in dollars,
the number of shares subject to such option will be determined by dividing the value of such award by the product of (i) the 20-trading
day trailing average price and (ii) the Black-Scholes ratio, which is calculated using the Black-Scholes value of an option on
the grant date divided by the closing market price on the Nasdaq Global Select Market of a share of our common stock on the effective
date of grant. The per share exercise price of all stock options will be at least equal to the closing market price on the Nasdaq
Global Select Market of a share of our common stock on the effective date of grant.
Non-Employee Director and Executive Officer Stock Ownership Policy
In 2019, the Compensation Committee adopted a Stock Ownership Policy,
which was subsequently amended in February 2021. As amended, the Stock Ownership Policy requires that by the fifth anniversary
of the original effectiveness date of the policy (i.e., December 31, 2024), or the fifth anniversary of an individual becoming
subject to the policy (whichever is later), that individual is required to hold a number of shares of Moderna stock equivalent
in value to a multiple of the individual’s salary or, in the case of directors, their annual cash retainer, as follows:
| • |
CEO: 7 times annual salary |
| • |
President: 6 times annual salary |
| • |
Other Executive Committee members: 3 times annual salary |
| • |
Directors: 6 times annual cash retainer |
In February 2021, the Stock Ownership Policy was revised by the
Compensation Committee to provide that only owned shares would count toward satisfaction of the ownership requirement, eliminating
credit previously granted for the value of vested but unexercised stock options. Until the requirements are met, covered individuals
who were subject to the policy as of December 31, 2020 are required to hold 100% of any stock underlying vested RSU awards until
the requirements are met, and individuals who are first subject to the policy on or after January 1, 2021 are required to hold
50% of any stock underlying vested RSU awards until the requirements are met.
 |
2024
Proxy statement |
57 |
As of December 31, 2023 each of Mr. Bancel and Dr. Hoge owned more
than the required amount of Moderna stock.
Clawback Policy
In February 2021, our Board of Directors adopted a clawback policy
applicable to all performance-based compensation granted to members of our Executive Committee, beginning in 2021. The policy
grants the Board or Compensation Committee discretion to recoup any performance-based compensation paid in excess of what otherwise
should have been delivered due to the Executive Committee member’s misconduct that resulted in a financial restatement.
In addition, the policy grants the Board or Compensation Committee discretion to recoup performance-based compensation in the
event that an Executive Committee member’s detrimental conduct causes material financial, operational or reputational harm
to the Company. In May 2023, our clawback policy was revised to reflect updated listing standard rules from Nasdaq and to require
that the Company seek a clawback of any excess performance-based compensation that was paid to our Executive Committee due to
an error or misstatement in our financial statements, regardless of fault or misconduct, other than in limited circumstances.
Policy Prohibiting Hedging and Pledging
Our Insider Trading Policy prohibits our executive officers, the
non-employee members of our Board of Directors and certain designated employees who in the course of the performance of their
duties have access to material, nonpublic information regarding the Company from engaging in the following transactions:
| • |
selling any of our securities that they do not own
at the time of the sale (a “short sale”); |
| • |
buying or selling puts, calls, other derivative securities of
the Company or any derivative securities that provide the economic equivalent of ownership of any of our securities or an
opportunity, direct or indirect, to profit from any change in the value of our securities or engaging in any other hedging
transaction with respect to our securities at any time; |
| • |
using our securities as collateral in a margin account; and |
| • |
pledging our securities as collateral for a loan (or modifying
an existing pledge). |
As of the date of this Proxy Statement, none of our NEOs had previously
sought or obtained approval from the Compensation Committee to engage in any hedging or pledging transaction involving our securities.
 |
2024
Proxy statement |
58 |
Executive Compensation Tables
2023 Summary Compensation Table
The following table provides
information regarding the total compensation awarded to, earned by, and paid to our NEOs for services rendered to us for the years
set forth below. Please note that in certain years these individuals were not NEOs and as such we are not including their compensation
for those years.
Name
and
Principal Position(1) | |
Year | |
Salary | |
Non-Equity
Plan
Incentive
Compensation | |
Bonus | |
Stock
Awards(2) | |
Option
Awards(2) | |
All
Other
Compensation | |
Total |
Stéphane
Bancel
Chief Executive Officer | |
2023 | |
$ | 1,563,462 | |
$ | 1,913,625 | |
$ | — | |
$ | 3,129,194 | |
$ | 9,387,713 | |
$ | 1,074,520 | |
$ | 17,068,514 |
| |
2022 | |
| 1,423,077 | |
| 2,700,000 | |
| — | |
| 3,597,152 | |
| 10,791,857 | |
| 851,562 | |
| 19,363,648 |
| |
2021 | |
| 990,385 | |
| 1,500,000 | |
| — | |
| 3,750,000 | |
| 11,250,000 | |
| 665,354 | |
| 18,155,739 |
James
Mock
Chief
Financial Officer | |
2023 | |
| 792,308 | |
| 583,200 | |
| — | |
| 1,460,282 | |
| 1,460,295 | |
| 21,100 | |
| 4,317,185 |
| |
2022 | |
| 227,885 | |
| 259,644 | |
| 1,000,000 | |
| 2,919,660 | |
| 2,919,680 | |
| 4,327 | |
| 7,331,196 |
Stephen
Hoge, M.D.
President | |
2023 | |
| 1,042,308 | |
| 850,500 | |
| — | |
| 2,711,792 | |
| 2,711,966 | |
| 22,600 | |
| 7,339,166 |
| |
2022 | |
| 953,846 | |
| 1,440,000 | |
| — | |
| 3,117,492 | |
| 3,117,579 | |
| 17,735 | |
| 8,646,652 |
| |
2021 | |
| 684,808 | |
| 819,000 | |
| — | |
| 3,000,000 | |
| 3,000,000 | |
| 299,624 | |
| 7,803,432 |
Arpa
Garay
Former Chief Commercial Officer | |
2023 | |
| 842,308 | |
| 619,650 | |
| — | |
| 1,460,282 | |
| 1,460,295 | |
| 63,413 | |
| 4,445,948 |
| |
2022 | |
| 458,462 | |
| 508,932 | |
| 1,500,000 | |
| 2,502,713 | |
| 2,502,742 | |
| 203,303 | |
| 7,676,152 |
Shannon
Thyme Klinger
Chief Legal Officer and Corporate Secretary | |
2023 | |
| 784,616 | |
| 583,200 | |
| — | |
| 1,460,282 | |
| 1,460,295 | |
| 24,103 | |
| 4,312,496 |
| |
2022 | |
| 692,590 | |
| 756,000 | |
| — | |
| 1,112,728 | |
| 1,112,705 | |
| 47,173 | |
| 3,721,196 |
| |
2021 | |
| 381,096 | |
| 344,000 | |
| 250,000 | |
| 6,000,000 | |
| 4,000,000 | |
| 514,051 | |
| 11,489,147 |
| (1) |
Mr. Mock and Ms.
Garay were hired on September 6, 2022 and May 31, 2022, respectively. Neither was an NEO for 2021; Ms. Klinger was not an
NEO for 2022. |
| (2) |
The amounts reported represent
the aggregate grant date fair value of the RSUs, PSUs and stock options, respectively, awarded to our NEOs in the year ended
December 31, 2023, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account
any estimated forfeitures. The assumptions used in calculating the grant date fair value of the equity awards reported in
these columns are set forth in Note 12 to our Consolidated Financial Statements for the year ended December 31, 2023 included
in our Annual Report. The amounts reported in these columns reflect the grant date fair value for each award, and, together,
differ from the targeted amounts for these grants, due to the fact that a 20-trading day trailing average closing price convention
is used for calculating the number of RSUs, PSUs and stock options granted. The grant date fair value of the PSUs are based
on the probable outcome of the applicable performance metrics. The amounts reported in this column reflect the accounting
cost for these awards and do not correspond to the actual economic value that may be received by the NEO upon the exercise
of the stock options or any sale of the underlying shares of common stock. |
Salary
Amounts represent the actual
amount of base salary paid for each NEO during the applicable year. NEOs and other employees are generally assessed for potential
salary increases at the beginning of each year. Percentage salary increases for each of our NEOs were approved and took effect
in February 2023 as follows: Mr. Bancel - 5%, to $1,575,000, Dr. Hoge - 5%, to $1,050,000, Mr. Mock - 6.7% to $800,000; Ms. Garay
- 6.3% to $850,000; and Ms. Klinger - 14.3% to $800,000. Salary increases were reflective of merit and market adjustments to better align each of these executive’s
salaries to market in the case of Mr. Mock, Ms. Garay and Ms. Klinger, as well as cost-of-living adjustments applied across our
broader employee base for each of these executives.
Non-Equity Plan Incentive Compensation
The amounts reported represent
annual bonuses earned by our NEOs for services during the applicable year, based on the achievement of Company and individual performance
objectives. Target bonuses for our NEOs are set as a percentage of annual salary, and for 2023 were maintained at 150% of salary
for our CEO, 100% of salary for our President, and 90% of salary for our other NEOs. For more information, see “Short-Term
Incentive Compensation” above.
 |
2024
Proxy statement |
59 |
Bonus
For Mr. Mock and Ms. Garay, in
2022, and for Ms. Klinger in 2021, the amount under “Bonus” represents amounts received as a signing bonus in connection
with each executive’s hiring. These signing bonuses are subject to a clawback if the executive voluntarily departs the Company
or is dismissed for cause within two years of the payment of the signing bonus.
Stock Awards
The amount reported represents
the aggregate grant date fair value of RSUs and PSUs awarded to the NEOs, calculated in accordance with FASB ASC Topic 718. Such
grant date fair value does not take into account any estimated forfeitures. The assumptions used in calculating the grant date
fair value of the RSUs and PSUs reported in this column are set forth in Note 12 to our Consolidated Financial Statements for the
year ended December 31, 2023 included in our Annual Report. The amount reported in this column reflects the accounting cost for
these equity awards and does not correspond to the actual economic value that may be received by the applicable NEO upon the vesting/settlement
of the RSUs and PSUs or any sale of the underlying shares of common stock. The grant date fair value of the 2023 PSUs, calculated
in accordance with FASB ASC Topic 718, assuming maximum achievement of the applicable performance metrics, are as follows: Mr.
Bancel - $6,258,388; Dr. Hoge - $2,711,792; Mr. Mock, Ms. Garay and Ms. Klinger - $1,460,282. Amounts for Mr. Mock and Ms. Garay
in 2022, and for Ms. Klinger in 2021, represent the value of new hire equity awards, which were set based upon market data for
executives of comparable caliber and experience, while also taking into account the value of equity awards that each of them would
forfeit by leaving their former employers to join Moderna.
Option Awards
The amounts reported represent
the aggregate grant date fair value of the stock options awarded to the NEOs during the applicable year, calculated in accordance
with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in
calculating the grant date fair value of the stock options reported in this column are set forth in Note 12 to our Consolidated
Financial Statements for the year ended December 31, 2023 included in our Annual Report. The amounts reported in this column reflect
the accounting cost for these stock options and do not correspond to the actual economic value that may be received by the NEOs
upon the exercise of the stock options or any sale of the underlying shares of common stock. Amounts for Mr. Mock and Ms.
Garay in 2022, and for Ms. Klinger in 2021, represent the value of new hire equity awards, which were set based upon market data
for executives of comparable caliber and experience, while also taking into account the value of equity awards that each of them
would forfeit by leaving their former employers to join Moderna.
All Other Compensation
The amounts set forth below provide
a detailed breakdown of the 2023 amounts reported above for All Other Compensation. These amounts consist of the following:
| • |
401(k) Match: Represents matching
contributions to the 401(k) account for the named executive officer. |
| • |
Relocation/Relocation Tax Expenses:
Represents benefits paid on behalf of Ms. Garay in 2023 in connection with her relocation to the Boston area to begin employment
with Moderna in 2022, and related tax gross-up payments for such expenses. |
| • |
Other Compensation: Represents incremental costs borne
by the Company for the provision of security services to the NEOs or members of their household in response to a heightened
threat environment, and the amount of premiums paid on behalf of the NEOs for supplemental long-term disability coverage,
which we offer to executives at the vice president level and above. For Mr. Bancel, the amount paid for security services
in 2023 was $1,053,767. |
| Name | 401(k)
Match | | Relocation | | Relocation
Tax
Expenses | | Other
Compensation | | Total | |
| Stéphane Bancel | |
$ | 14,850 | | |
$ | — | | |
$ | — | | |
$ | 1,059,670 | | |
$ | 1,074,520 | |
| James Mock | |
| 14,850 | | |
| — | | |
| — | | |
| 6,250 | | |
| 21,100 | |
| Stephen Hoge, M.D. | |
| 14,850 | | |
| — | | |
| — | | |
| 7,750 | | |
| 22,600 | |
| Arpa Garay | |
| 14,850 | | |
| 21,769 | | |
| 21,466 | | |
| 5,328 | | |
| 63,413 | |
| Shannon Thyme Klinger | |
| 14,850 | | |
| — | | |
| — | | |
| 9,253 | | |
| 24,103 | |
 |
2024
Proxy statement |
60 |
Grants of Plan-Based Awards
for Fiscal Year 2023
The following table – also
known as the Grants of Plan-Based Awards Table – sets forth the individual award, including stock options, RSUs and PSUs,
made to each of our NEOs during 2023. For a description of the types of awards indicated below, please see our “Compensation
Discussion and Analysis” above.
| Name | |
Grant Date(1) | |
Award Type | |
Estimated Future Payouts Under
Performance Share Units (#)(2) | |
Restricted
Stock Units (#)(3) | | |
Stock
Options
(#)(4) | | |
Stock Option
Exercise
Price(5) | |
Grant Date
Fair Value of
Awards(6) |
| |
|
|
Threshold | |
Target | |
Maximum | |
| |
|
|
|
| Stéphane Bancel | |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| | | |
| 128,830 | | |
$138.81 | |
$9,387,713 |
| |
February 28, 2023 | |
Annual Equity | |
11,271 | |
22,543 | |
45,086 | |
| | | |
| | | |
| |
3,129,194 |
| James Mock | |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| | | |
| 20,040 | | |
138.81 | |
1,460,295 |
| |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| 5,260 | | |
| | | |
| |
730,141 |
| |
February 28, 2023 | |
Annual Equity | |
2,630 | |
5,260 | |
10,520 | |
| | | |
| | | |
| |
730,141 |
| Stephen Hoge, M.D. | |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| | | |
| 37,217 | | |
138.81 | |
2,711,966 |
| |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| 9,768 | | |
| | | |
| |
1,355,896 |
| |
February 28, 2023 | |
Annual Equity | |
4,884 | |
9,768 | |
19,536 | |
| | | |
| | | |
| |
1,355,896 |
| Arpa Garay | |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| | | |
| 20,040 | | |
138.81 | |
1,460,295 |
| |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| 5,260 | | |
| | | |
| |
730,141 |
| |
February 28, 2023 | |
Annual Equity | |
2,630 | |
5,260 | |
10,520 | |
| | | |
| | | |
| |
730,141 |
| Shannon Thyme Klinger | |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| | | |
| 20,040 | | |
138.81 | |
1,460,295 |
| |
February 28, 2023 | |
Annual Equity | |
| |
| |
| |
| 5,260 | | |
| | | |
| |
730,141 |
| |
February 28, 2023 | |
Annual Equity | |
2,630 | |
5,260 | |
10,520 | |
| | | |
| | | |
| |
730,141 |
| (1) |
All annual equity
grants were approved by the Compensation Committee on February 14, 2023, with a grant date of February 28, 2023, for annual
stock option, RSU and PSU grants (the second trading day following the filing of our Annual Report 10-K with the SEC). |
| (2) |
Each PSU is subject to vesting
upon a determination by the Compensation Committee that the goals thereunder have been met. This determination is expected
to be made within two-and-a-half months of the conclusion of the performance period, which ends on December 31, 2025. |
| (3) |
Each RSU is subject to time-based
vesting, as described in the footnotes to the “Outstanding Equity Awards at 2023 Year-End” table below. |
| (4) |
Each stock option is subject
to time-based vesting, as described in the footnotes to the “Outstanding Equity Awards at 2023 Year-End” table
below. |
| (5) |
Based upon the closing price
of our common stock as reported on the Nasdaq Global Select Market on the date of grant. |
| (6) |
The amounts reported represent the aggregate grant
date fair value of the stock options, RSUs and PSUs, as applicable, awarded to the NEOs during 2023, calculated in accordance
with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used
in calculating the grant date fair value of the stock options, RSUs and PSUs, as applicable, reported in this column are set
forth in Note 12 to our Consolidated Financial Statements for the year ended December 31, 2023 included in our Annual Report.
The amounts reported in this column reflect the aggregate accounting cost for these equity awards, and do not correspond to
the actual economic value that may be received by the NEOs upon the exercise of the stock options, the vesting/settlement
of the RSUs or PSUs or any sale of the underlying shares of common stock. The grant date fair value of PSUs is based on probable
achievement of the performance metrics at target. |
 |
2024
Proxy statement |
61 |
Outstanding Equity Awards at
2023 Year-end
The table below sets forth information
regarding outstanding equity awards held by our NEOs as of December 31, 2023.
| Name | |
Grant Date(1) | |
Award
Type | |
First
Vesting Date | |
Number
Exercisable/
Vested | |
Number
Unexercisable/
Unvested | |
Option
Exercise
Price | | |
Option
Expiration Date | |
Market
Value(2) |
| Stéphane Bancel | |
2/23/2016 | |
Options | |
2/23/2017 | |
688,073(3) | |
— | |
$ | 10.90 | | |
2/23/2026 | |
$ | 60,928,864 |
| |
8/10/2016 | |
Options | |
8/10/2016 | |
558,394(3) | |
— | |
| 19.15 | | |
8/10/2026 | |
| 44,839,038 |
| |
8/10/2016 | |
Options | |
8/10/2016 | |
193,321(3) | |
— | |
| 19.15 | | |
8/10/2026 | |
| 15,523,676 |
| | |
2/23/2017 | |
Options | |
2/22/2018 | |
642,201(3) | |
— | |
| 12.21 | | |
2/23/2027 | |
| 56,025,615 |
| | |
2/28/2018 | |
Options | |
2/28/2019 | |
903,096(4) | |
14,335(4) | |
| 14.22 | | |
2/28/2028 | |
| 78,192,644 |
| | |
12/6/2018 | |
Options | |
6/13/2020 | |
4,587,155(3) | |
— | |
| 23.00 | | |
12/6/2028 | |
| 350,688,000 |
| | |
3/8/2019 | |
Options | |
3/8/2020 | |
593,592(3) | |
— | |
| 20.93 | | |
3/8/2029 | |
| 46,608,844 |
| | |
2/28/2020 | |
Options | |
2/28/2021 | |
604,200(5) | |
40,280(5) | |
| 25.93 | | |
2/28/2030 | |
| 47,382,170 |
| | |
2/9/2021 | |
Options | |
2/9/2022 | |
99,071(5) | |
45,034(5) | |
| 179.52 | | |
2/9/2031 | |
| — |
| | |
3/5/2021 | |
PSUs | |
| |
— | |
28,368(7) | |
| — | | |
| |
| 2,821,198 |
| | |
3/1/2022 | |
Options | |
3/1/2023 | |
64,034(5) | |
82,330(5) | |
| 149.52 | | |
3/1/2032 | |
| — |
| | |
3/1/2022 | |
PSUs | |
| |
— | |
24,058(7) | |
| — | | |
| |
| 2,392,568 |
| | |
2/28/2023 | |
Options | |
2/28/2024 | |
— | |
128,830(5) | |
| 138.81 | | |
2/28/2033 | |
| — |
| | |
2/28/2023 | |
PSUs | |
| |
— | |
22,543(7) | |
| — | | |
| |
| 2,241,901 |
| | |
| |
| |
| |
| |
| |
| | | |
| |
| 707,644,518 |
| James Mock | |
10/5/2022 | |
Options | |
10/5/2023 | |
10,969(5) | |
32,907(5) | |
| 125.62 | | |
10/5/2032 | |
| — |
| |
10/5/2022 | |
RSUs | |
10/5/2023 | |
— | |
17,432(5) | |
| — | | |
| |
| 1,733,612 |
| |
2/28/2023 | |
Options | |
2/28/2024 | |
— | |
20,040(5) | |
| 138.81 | | |
2/28/2033 | |
| — |
| | |
2/28/2023 | |
RSUs | |
2/28/2024 | |
— | |
5,260(5) | |
| — | | |
| |
| 523,107 |
| | |
2/28/2023 | |
PSUs | |
| |
— | |
5,260(7) | |
| — | | |
| |
| 523,107 |
| | |
| |
| |
| |
| |
| |
| | | |
| |
| 2,779,826 |
| Stephen Hoge, M.D. | |
8/10/2016 | |
Options | |
8/10/2016 | |
223,357(3) | |
— | |
| 19.15 | | |
8/10/2026 | |
| 17,935,567 |
| |
8/10/2016 | |
Options | |
8/10/2016 | |
96,660(3) | |
— | |
| 19.15 | | |
8/10/2026 | |
| 7,761,798 |
| |
2/23/2017 | |
Options | |
2/22/2018 | |
458,715(3) | |
— | |
| 12.21 | | |
2/23/2027 | |
| 40,018,297 |
| | |
10/3/2017 | |
Options | |
10/3/2018 | |
1,834,862(3) | |
— | |
| 12.21 | | |
10/3/2027 | |
| 160,073,361 |
| | |
2/28/2018 | |
Options | |
2/27/2019 | |
412,844(3) | |
— | |
| 14.22 | | |
2/28/2028 | |
| 35,186,694 |
| | |
3/8/2019 | |
Options | |
3/8/2020 | |
339,195(3) | |
— | |
| 20.93 | | |
3/8/2029 | |
| 26,633,591 |
| | |
2/28/2020 | |
Options | |
2/28/2021 | |
201,398(5) | |
13,428(5) | |
| 25.93 | | |
2/28/2030 | |
| 15,794,008 |
| | |
2/28/2020 | |
RSUs | |
2/28/2022 | |
— | |
2,411(6) | |
| — | | |
| |
| 239,774 |
| | |
2/9/2021 | |
Options | |
2/9/2022 | |
26,419(5) | |
12,009(5) | |
| 179.52 | | |
2/9/2031 | |
| — |
| | |
2/9/2021 | |
RSUs | |
2/9/2022 | |
— | |
2,612(5) | |
| — | | |
| |
| 259,763 |
| | |
3/5/2021 | |
PSUs | |
| |
— | |
11,347(7) | |
| — | | |
| |
| 1,128,459 |
| | |
3/1/2022 | |
Options | |
3/1/2023 | |
18,497(5) | |
23,785(5) | |
| 149.52 | | |
3/1/2032 | |
| — |
| | |
3/1/2022 | |
RSUs | |
3/1/2023 | |
— | |
5,865(5) | |
| — | | |
| |
| 583,274 |
| | |
3/1/2022 | |
PSUs | |
| |
— | |
10,425(7) | |
| — | | |
| |
| 1,036,766 |
| | |
2/28/2023 | |
Options | |
2/28/2024 | |
— | |
37,217(5) | |
| 138.81 | | |
2/28/2033 | |
| — |
| | |
2/28/2023 | |
RSUs | |
2/28/2024 | |
— | |
9,768(5) | |
| — | | |
| |
| 971,428 |
| | |
2/28/2023 | |
PSUs | |
| |
— | |
9,768(7) | |
| — | | |
| |
| 971,428 |
| | |
| |
| |
| |
| |
| |
| | | |
| |
| 308,594,208 |
 |
2024
Proxy statement |
62 |
| Name | |
Grant
Date(1) | |
Award
Type | |
First
Vesting Date | |
Number
Exercisable/
Vested | |
Number
Unexercisable/
Unvested | |
Option
Exercise
Price | | |
Option
Expiration Date | |
Market
Value(2) |
| Arpa Garay | |
6/5/2022 | |
Options | |
6/5/2023 | |
13,036(5) | |
21,730(5) | |
| 137.15 | | |
6/5/2032 | |
| — |
| |
6/5/2022 | |
RSUs | |
6/5/2023 | |
— | |
11,405(5) | |
| — | | |
| |
| 1,134,227 |
| | |
2/28/2023 | |
Options | |
2/28/2024 | |
— | |
20,040(5) | |
| 138.81 | | |
2/28/2033 | |
| — |
| | |
2/28/2023 | |
RSUs | |
2/28/2024 | |
— | |
5,260(5) | |
| — | | |
| |
| 523,107 |
| | |
2/28/2023 | |
PSUs | |
| |
— | |
5,260(7) | |
| — | | |
| |
| 523,107 |
| | |
| |
| |
| |
| |
| |
| | | |
| |
| 2,180,441 |
| Shannon Thyme Klinger | |
6/7/2021 | |
Options | |
6/7/2022 | |
26,355(5) | |
15,814(5) | |
| 219.57 | | |
6/7/2031 | |
| — |
| |
6/7/2021 | |
RSUs | |
6/7/2022 | |
— | |
6,832(5) | |
| — | | |
| |
| 679,442 |
| |
6/7/2021 | |
RSUs | |
6/7/2024 | |
— | |
9,108(8) | |
| — | | |
| |
| 905,791 |
| | |
3/1/2022 | |
Options | |
3/1/2023 | |
6,601(5) | |
8,490(5) | |
| 149.52 | | |
3/1/2032 | |
| — |
| | |
3/1/2022 | |
RSUs | |
3/1/2023 | |
— | |
2,094(5) | |
| — | | |
| |
| 208,248 |
| | |
3/1/2022 | |
PSUs | |
| |
— | |
3,721(7) | |
| — | | |
| |
| 370,053 |
| | |
2/28/2023 | |
Options | |
2/28/2024 | |
— | |
20,040(5) | |
| 138.81 | | |
2/28/2033 | |
| — |
| | |
2/28/2023 | |
RSUs | |
2/28/2024 | |
— | |
5,260(5) | |
| — | | |
| |
| 523,107 |
| | |
2/28/2023 | |
PSUs | |
| |
— | |
5,260(7) | |
| — | | |
| |
| 523,107 |
| | |
| |
| |
| |
| |
| |
| | | |
| |
| 3,209,748 |
| (1) |
Equity awards granted
prior to December 6, 2018 are subject to the terms of our 2016 Stock Option and Grant Plan, as amended from time to time (the
2016 Stock Plan), and equity awards granted on or after December 6, 2018 are subject to the terms of our 2018 Stock Plan. |
| (2) |
Market value reflects the value
of the applicable equity award, including both vested and unvested portions, based upon the closing price for the Company’s
common stock on December 29, 2023 of $99.45. |
| (3) |
The shares subject to the option
are fully vested. |
| (4) |
This option grant vests in three
tranches. The first tranche, consisting of 50% of the underlying shares, vests as follows: 25% of this tranche vested on the
first anniversary of the grant date, and the remainder vests in 12 equal quarterly installments thereafter. The second tranche,
consisting of 25% of the underlying shares, vests as follows: 25% of this tranche vested on the second anniversary of the
grant date, and the remainder vests in 12 equal quarterly installments thereafter. The third tranche, consisting of 25% of
the underlying shares, vests as follows: 25% of this tranche vests on the third anniversary of the grant date, and the remainder
will vest in 12 equal quarterly installments thereafter. Vesting is generally subject to Mr. Bancel’s continuous employment
with the Company through the applicable vesting date. |
| (5) |
25% of the shares subject to
the equity award vest on the first anniversary of the grant date and the remaining 75% vest in 12 equal quarterly installments
thereafter, generally subject to the NEO’s continuous service relationship with the Company through each applicable
vesting date. |
| (6) |
50% of the shares subject to
the equity award vest on the second anniversary of the grant date and the remaining 50% vest in 8 equal quarterly installments
thereafter, generally subject to the NEO’s continuous service relationship with the Company through each applicable
vesting date. |
| (7) |
Number of PSUs assumes performance
at target. The shares subject to the equity award are scheduled to vest within two and a half months of the conclusion
of the performance period, which ends on December 31st of the second calendar year after the grant date, and following a determination
by the Compensation Committee on whether the performance criteria have been satisfied; if an NEO remains employed for at least
one year of the performance period, he or she will be entitled to a pro rata award based upon time employed, subject to satisfaction
of the performance criteria. The number of shares subject to vesting reflect anticipated performance at target. |
| (8) |
The shares subject to the equity award vest in full
on the third anniversary of the grant date, subject to the NEO’s continuous service relationship with the Company through
the applicable vesting date. |
 |
2024
Proxy statement |
63 |
Option Exercises and Stock Vested
in Fiscal Year 2023
The following table sets forth
the number of shares acquired and the value realized upon exercises of stock options and vesting of RSUs during the fiscal year
ended December 31, 2023 by each of our NEOs.
| | |
Option
Awards | | |
Stock
Awards |
| | |
Number of
Shares
Acquired on
Exercise (#) | | |
Value
Realized on
Exercise ($)(1) | | |
Number of
Shares
Acquired on
Vesting (#) | | |
Value
Realized on
Vesting ($)(2) |
| Stéphane Bancel | |
| 2,027,155 | | |
$ | 300,714,563 | | |
| — | | |
$ | — |
| James Mock | |
| — | | |
| — | | |
| 5,810 | | |
| 605,751 |
| Stephen Hoge, M.D. | |
| — | | |
| — | | |
| 16,291 | | |
| 1,908,414 |
| Arpa Garay | |
| — | | |
| — | | |
| 6,843 | | |
| 814,837 |
| Shannon Thyme Klinger | |
| — | | |
| — | | |
| 6,181 | | |
| 726,519 |
| (1) |
The value realized
upon the exercise of option awards is calculated as the difference between the market price of the underlying security at
exercise and the exercise price of the options. |
| (2) |
The value realized upon the vesting of stock awards
is calculated based on the closing market price of a share of our common stock on the date prior to the date of vesting. |
All stock option exercises shown
in the table above were conducted pursuant to a 10b5-1 plan. For as long as they are executives, our NEOs are required to conduct
any sales of Moderna stock, including stock option exercises, pursuant to 10b5-1 plans. These plans require that the executive
commit to a trading plan in advance and at a time when the Company is in an open trading window under the Company’s Insider
Trading Policy.
The stock option exercises shown
above for Mr. Bancel were all exercises of a stock option award that was granted in August 2013 and that expired in August 2023.
In May 2022, Mr. Bancel announced his intention to fully exercise the award, exercising 40,000 options each Wednesday and Thursday
under a 10b5-1 plan until it was fully exercised. Mr. Bancel has committed to contributing the after-tax proceeds from the option
exercise to charitable causes. Mr. Bancel’s announcement regarding the exercise of the stock option and the contribution
of the proceeds to charitable causes can be found in the Current Report on Form 8-K filed by Moderna with the SEC on May 24, 2022.
 |
2024
Proxy statement |
64 |
Potential Payments on Termination or Change in Control
Our Executive Severance Plan, as described
above, provides for certain payments and benefits in the event of certain qualifying terminations of employment, including qualifying
terminations of employment in connection with a change in control of the Company.
The table below quantifies the potential
payments and benefits that would have become due to our NEOs, assuming that a qualifying termination occurred on December 31, 2023. In
the event of termination due to death or disability, each of our named executive officers would be eligible to have any outstanding but
unvested time-based equity accelerate on the same terms as other employees, up to a cap of $500 million, plus pro-rata vesting of any
PSUs. In the event of an involuntary termination, each of our NEOs would be eligible for accelerated vesting of 15% of any outstanding
and unvested time-based equity awards, in accordance with the Company’s severance guidelines.
The closing market price of a share
of our common stock on December 29, 2023 (the last trading day of 2023) was $99.45.
| |
Qualifying Termination Not
in Connection with a
Change in Control ($)(1) |
|
Qualifying Termination in
Connection with a Change
in Control ($)(2) |
|
|
Termination Due to
Death or Disability ($)
|
|
| Stéphane Bancel |
|
|
|
|
|
|
|
|
|
|
|
| Cash Severance Payment |
$ |
1,575,000 |
(3) |
|
$ |
2,362,500(4) |
|
|
$ |
— |
|
| Cash Incentive Bonus Payment |
|
2,362,500 |
(5) |
|
|
5,906,250(6) |
|
|
|
— |
|
| COBRA Premiums |
|
26,712 |
(7) |
|
|
40,069(8) |
|
|
|
— |
|
| Accelerated Equity Vesting |
|
627,452 |
(9) |
|
|
14,460,022(10) |
|
|
|
11,419,140(11) |
|
| James Mock |
|
|
|
|
|
|
|
|
|
|
|
| Cash Severance Payment |
|
800,000 |
(3) |
|
|
1,200,000(4) |
|
|
|
— |
|
| Cash Incentive Bonus Payment |
|
720,000 |
(5) |
|
|
1,800,000(6) |
|
|
|
— |
|
| COBRA Premiums |
|
26,712 |
(7) |
|
|
40,069(8) |
|
|
|
— |
|
| Accelerated Equity Vesting |
|
338,428 |
(9) |
|
|
2,779,826(10) |
|
|
|
2,256,719(11) |
|
| Stephen Hoge, M.D. |
|
|
|
|
|
|
|
|
|
|
|
| Cash Severance Payment |
|
1,050,000 |
(3) |
|
|
1,575,000(4) |
|
|
|
— |
|
| Cash Incentive Bonus Payment |
|
1,050,000 |
(5) |
|
|
2,625,000(6) |
|
|
|
— |
|
| COBRA Premiums |
|
26,712 |
(7) |
|
|
40,069(8) |
|
|
|
— |
|
| Accelerated Equity Vesting |
|
455,966 |
(9) |
|
|
7,306,578(10) |
|
|
|
5,988,865(11) |
|
| Arpa Garay |
|
|
|
|
|
|
|
|
|
|
|
| Cash Severance Payment |
|
850,000 |
(3) |
|
|
1,275,000(4) |
|
|
|
— |
|
| Cash Incentive Bonus Payment |
|
765,000 |
(5) |
|
|
1,912,500(6) |
|
|
|
— |
|
| COBRA Premiums |
|
26,342 |
(7) |
|
|
39,513(8) |
|
|
|
— |
|
| Accelerated Equity Vesting |
|
248,526 |
(9) |
|
|
2,180,441(10) |
|
|
|
1,657,334(11) |
|
| Shannon Thyme Klinger |
|
|
|
|
|
|
|
|
|
|
|
| Cash Severance Payment |
|
800,000 |
(3) |
|
|
1,200,000(4) |
|
|
|
— |
|
| Cash Incentive Bonus Payment |
|
720,000 |
(5) |
|
|
1,800,000(6) |
|
|
|
— |
|
| COBRA Premiums |
|
17,341 |
(7) |
|
|
26,012(8) |
|
|
|
— |
|
| Accelerated Equity Vesting |
|
347,379 |
(9) |
|
|
3,209,749(10) |
|
|
|
2,563,025(11) |
|
 |
2024
Proxy statement |
65 |
| (1) |
A “qualifying termination” means a termination other
than due to cause, death or disability or a resignation for good reason and “not in connection with a change in control”
means outside of the change in control period. |
| (2) |
A “qualifying termination” means a termination other than due to cause,
death or disability or a resignation for good reason and “in connection with a change in control” means within the change
in control period. |
| (3) |
Represents 12 months of the executive’s base salary. |
| (4) |
Represents 18 months of the executive’s base salary. |
| (5) |
Represents the NEO’s target annual bonus opportunity. |
| (6) |
Represents 150% of the NEO’s target annual bonus opportunity plus the NEO’s
target bonus opportunity, pro-rated for the number of full weeks elapsed in the then-current fiscal year prior to the date of termination. |
| (7) |
Represents 12 months of our contribution towards health insurance, based on our actual
costs to provide health insurance to the NEO as of the date of termination. |
| (8) |
Represents 18 months of our contribution towards health insurance, based on our actual
costs to provide health insurance to the NEO as of the date of termination. |
| (9) |
Represents the value of acceleration of 15% of the NEO’s unvested and outstanding time-based
equity awards. The NEO would remain eligible for the pro-rata portion of any outstanding PSUs based on actual performance, but such
PSUs would not be subject to accelerated vesting. |
| (10) |
Represents the value of acceleration of (i) vesting of 100% of the NEO’s unvested
and outstanding time-based equity awards and (ii) vesting of the NEO’s unvested and outstanding PSUs, based on the market price
of a share of our common stock on December 29, 2023, which was $99.45. |
| (11) |
Represents the value of acceleration of (i) vesting of 100% of the NEO’s unvested
and outstanding time-based equity awards and (ii) pro-rated vesting of the NEO’s unvested and outstanding PSUs, based on the
market price of a share of our common stock on December 29, 2023, which was $99.45. |
 |
2024
Proxy statement |
66 |
Equity Compensation Plan Information
The following table provides information
as of December 31, 2023 with respect to shares of our common stock that may be issued under our existing equity compensation plans.
| Plan Category | |
Number of Securities to
be Issued upon Exercise
of Outstanding Options
and Restricted Stock
Units (a) |
| |
Weighted-
Average
Exercise Price of
Outstanding Options (b) | |
Number of Securities
Remaining Available for
Future Issuance Under
Equity Compensation
Plans (Excluding
Securities Reflected in
Column (a)) |
|
| Equity Compensation Plans Approved by Shareholders(1) | |
| 30,684,628(2) | | |
$ | 56.14 | |
| 19,269,131(3)(4) | |
| Equity
Compensation Plans Not Approved by Shareholders | |
| — | | |
| N/A | |
| — | |
| TOTAL | |
| 30,684,628 | | |
$ | 56.14 | |
| 19,269,131 | |
| (1) |
Consists
of our 2018 Stock Plan, 2016 Stock Plan, and ESPP. Following our initial public offering, we have not and will not grant any awards
under our 2016 Stock Plan, but all outstanding awards under the 2016 Stock Plan will continue to be governed by their existing terms.
The shares of common stock underlying any awards granted under the 2016 Stock Plan or 2018 Stock Plan that are forfeited, canceled,
reacquired by us prior to vesting, satisfied without the issuance of stock, or otherwise terminated (other than by exercise) and
the shares of common stock that are withheld upon exercise of a stock option or settlement of such award to cover the exercise price
or tax withholding will be added to the shares of common stock available for issuance under the 2018 Stock Plan. |
| (2) |
Does not include purchase rights
accruing under the ESPP because the purchase right (and therefore the number of shares to be purchased) will not be determined until
the end of the purchase period. |
| (3) |
Consists of shares available for
future issuance under the ESPP and the 2018 Stock Plan. As of December 31, 2023, 3,172,648 shares of common stock were available
for issuance under the ESPP (which number includes shares subject to purchase during the current purchase period, which commenced
on December 1, 2023, and the exact number of which will not be known until the end of the purchase period on May 30, 2024), and 16,096,483
shares of common stock were available for issuance under the 2018 Stock Plan. Subject to the number of shares remaining in the share
reserve, under the ESPP, the maximum number of shares purchasable by any participant on any one purchase date for any purchase period,
including the current period, may not exceed 3,000 shares. |
| (4) |
The 2018 Stock Plan provides that
the number of shares reserved and available for issuance under the plan will automatically increase each January 1, beginning on
January 1, 2019, by 4% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such
lesser number of shares as determined by our Compensation Committee. The ESPP provides that the number of shares reserved and available
for issuance will automatically increase each January 1, beginning on January 1, 2020, by the least of 3,240,000 shares of our common
stock, 1% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such lesser number
of shares as determined by our Compensation Committee. In December 2023, the Compensation Committee authorized
an increase in the amount of shares reserved for future issuance under the 2018 Plan equivalent to 4% of our stock outstanding as
of December 31, 2023, with such increase taking effect on January 1, 2024. No such increase was authorized by the Compensation
Committee for the ESPP for 2024. The number does not include the increase to the 2018 Plan from January 1, 2024. |
 |
2024
Proxy statement |
67 |
CEO Pay Ratio
We present below the ratio of annual
total compensation of our median compensated employee to the annualized total compensation of Mr. Bancel, calculated in accordance with
Item 402(u) of Regulation S-K.
In identifying our median employee,
we reviewed the compensation of our entire employee population of approximately 5,600 global employees as of December 31, 2023. In performing
this analysis, we identified the median employee based on actual base pay during 2023, plus all cash bonuses, overtime pay and equity
awards received/granted during the year. After identifying the median employee, we then determined the cash bonus for 2023 (paid in 2024),
as well as other benefits such as 401(k) match, in the same method used to calculate and report Mr. Bancel’s compensation.
The 2023 total compensation for Mr.
Bancel, as reported above in the 2023 Summary Compensation Table on page 59 was $17,068,514,
which reflects all elements of his compensation as determined under Item 402 of Regulation S-K. The 2023 annual total compensation as
determined under Item 402 of Regulation S-K for our median employee, who is employed as a Finance Manager, was $209,627.
The ratio of Mr. Bancel’s total
compensation to our median employee’s total annual compensation for fiscal year 2023 is 81 to 1.
Pay Versus Performance
The following table reports the compensation
of our CEO (our Principal Executive Officer, or PEO) and the average compensation of the other Named Executive Officers (Other NEOs) as
reported in the Summary Compensation Table in our proxy statements for the past four years, as well as their “compensation actually
paid” (CAP), as calculated pursuant to SEC rules and certain performance measures required by the rules. The CAP values included
in the table below reflect a measure of compensation which is a combined realizable and realized pay measure predicated on fair value.
The grant date fair values included in the Summary Compensation Table (SCT) have been replaced with fair values reflecting the change
in value of equity awards during the fiscal year. The calculations do not reflect the actual sale of stock underlying equity awards or
the exercise of stock options by the executive.
| | |
| | |
| | |
| | |
Average
Compensation
Actually Paid
to Other
NEOs(2)
(e) | | |
Value of Initial
Fixed $100
Investment
Based on: | | |
| | |
Company
-Selected
Measure
[Product
Sales](4)(5)
(millions)
(i) | |
Year(1)
(a) | |
Summary
Compensation
Table Total
for PEO
(b) | | |
Compensation
Actually Paid
to PEO(2)
(c) | | |
Average SCT
Total for
Other NEOs
(d) | | |
| |
Moderna
TSR
(f) | | |
Peer
Group(3)
TSR
(g) | | |
Net Income
(millions)
(h) | | |
|
| 2023 | |
$ | 17,068,514 | | |
$ | (155,552,174) | | |
$ | 5,103,698 | | |
$ | (3,939,532) | | |
$ | 508 | | |
$ | 115 | | |
$ | (4,714) | | |
$ | 6,671 | |
| 2022 | |
| 19,363,648 | | |
| (306,219,630) | | |
| 5,822,260 | | |
| (8,967,479) | | |
| 918 | | |
| 111 | | |
| 8,362 | | |
| 18,435 | |
| 2021 | |
| 18,155,739 | | |
| 793,044,894 | | |
| 8,256,157 | | |
| 67,035,028 | | |
| 1,298 | | |
| 125 | | |
| 12,202 | | |
| 17,675 | |
| 2020 | |
| 12,855,275 | | |
| 469,967,605 | | |
| 5,286,409 | | |
| 54,985,594 | | |
| 534 | | |
| | |
| (747) | | |
| 200 | |
| (1) |
Mr. Bancel served as our PEO in each year shown.
The Other NEOs reflected in columns (d) and (e) above represent the following individuals for each of the years shown: |
| |
• |
2023 – Arpa Garay, Stephen Hoge, Shannon
Thyme Klinger and James Mock |
| |
• |
2022 – Juan Andres, Arpa Garay, Jorge Gomez,
Stephen Hoge, David Meline and James Mock |
| |
• |
2021 – Juan Andres, Stephen Hoge, Shannon Thyme
Klinger, David Meline and Corinne LeGoff |
| |
• |
2020 – Juan Andres, Stephen Hoge, Lorence Kim,
David Meline and Tal Zaks |
| |
|
|
| |
Biographical information for each of these individuals and their positions can be
found above, beginning on page 33, or in the proxy statement for the year upon which compensation was reported, under the heading
“Management.” |
| (2) |
The applicable Summary Compensation Table totals reported for the PEO and the
average of the Other NEOs for each year were subject to the following adjustments per Item 402(v)(2)(iii) of Regulation S-K to calculate
“compensation actually paid”: |
 |
2024
Proxy statement |
68 |
| | |
2023 | | |
2022 | | |
2021 | | |
2020 | |
| | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | |
| Summary Compensation Table | |
$ | 17,068,514 | | |
$ | 5,103,698 | | |
$ | 19,363,648 | | |
$ | 5,822,260 | | |
$ | 18,155,739 | | |
$ | 8,256,157 | | |
$ | 12,855,275 | | |
$ | 5,286,409 | |
| Adjustments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Deduction for amounts reported under the “Stock Awards”
and “Option Awards” columns of the Summary Compensation Table(a) | |
| (12,516,907) | | |
| (3,546,372) | | |
| (14,389,009) | | |
| (4,285,485) | | |
| (15,000,000) | | |
| (6,600,000) | | |
| (9,000,000) | | |
| (4,220,000) | |
| Increase/(decrease)
for the Inclusion of Rule 402(v) Equity Values(a) | |
| (160,103,781) | | |
| (5,496,858) | | |
| (311,194,269) | | |
| (10,504,254) | | |
| 789,889,155 | | |
| 65,378,871 | | |
| 466,112,330 | | |
| 53,919,185 | |
| Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year | |
| 7,294,658 | | |
| 2,224,773 | | |
| 18,930,378 | | |
| 5,957,933 | | |
| 25,410,484 | | |
| 7,893,516 | | |
| 53,681,961 | | |
| 14,619,557 | |
| Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards | |
| (15,002,894) | | |
| (4,007,912) | | |
| (216,035,770) | | |
| (5,734,897) | | |
| 577,920,879 | | |
| 29,752,324 | | |
| 347,020,376 | | |
| 30,590,579 | |
| Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year | |
| (152,395,545) | | |
| (3,713,719) | | |
| (114,088,877) | | |
| (10,254,023) | | |
| 186,557,792 | | |
| 27,733,031 | | |
| 65,409,993 | | |
| 10,481,461 | |
| Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year | |
| — | | |
| — | | |
| — | | |
| (473,267) | | |
| — | | |
| — | | |
| — | | |
| (1,772,412) | |
Compensation
Actually Paid | |
| (155,552,174) | | |
| (3,939,532) | | |
| (306,219,630) | | |
| (8,967,479) | | |
| 793,044,894 | | |
| 67,035,028 | | |
| 469,967,605 | | |
| 54,985,594 | |
| |
|
|
| (3) |
Peer group TSR reflects the Nasdaq Biotechnology Index for all four fiscal years disclosed, which aligns with the peer group used in our Annual Report on 10-K for each of these years. |
| (4) |
The Company has identified Product Sales from COVID-19 vaccines as the company-selected measure for the pay versus performance disclosure, as it represents the most important financial performance measure used to link compensation actually paid to the PEO and the Other NEOs in 2023 to the Company’s performance. |
| (5) |
Product Sales was chosen from the following three most important financial performance measures used by the Company to link compensation actually paid to the PEO and Other NEOs in 2023 to the Company’s performance: |
| |
Performance
Metrics |
| |
Product Sales from
COVID-19 Vaccines |
Refer to “Compensation
Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described on page 48. |
| |
Operating
Income |
Refer
to “Compensation Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described
on page 48. |
| |
Total
Shareholder Return (TSR) |
Stock
options are the most heavily weighted equity vehicle in our long-term incentive program (75% for the PEO and 50% for the Other NEOs).
The value of these equity awards is directly related to stock price appreciation over time, which incentivizes our executives to
achieve our long-term strategic and pipeline goals to create shareholder value. Total Shareholder Return on a $100 investment in
Moderna stock over a four-year period ending December 31, 2023 would have been $508.44. |
 |
2024
Proxy statement |
69 |
Supplemental Graphs & Narratives
| • |
Description of the relationship between Moderna’s
TSR and peer group TSR |
| • |
Descriptions of the relationships between Compensation Actually
Paid (CAP) and financial measures for the PEO and the average of the Other NEOs |
1. TSR: Company versus Peer Group

TSR for our peer group is based on the
Nasdaq Biotechnology Index, which reflects the Company’s industry sector and is also the peer group used in our Annual Report on
Form 10-K.
Moderna significantly outperformed peers
in 2021 and 2022, reflecting our rapid shift to a commercial company as we developed one of the earliest COVID-19 vaccines during the
pandemic. The COVID-19 vaccine was Moderna’s first commercial product when it was authorized for emergency use in the U.S. in December
2020, followed quickly by authorization or approval in markets globally.
Our stock price declined at the end
of 2023 compared to the prior year primarily driven by the lower demand for COVID-19 vaccines as we entered the endemic market. We anticipate
that the advancement of our pipeline, with as many as 15 products in the next five years, will build significant value for our investors,
which should be reflected in our stock price.
2. CAP versus Company TSR

Equity awards are the largest component
of our executive compensation program, representing no less than 70% of the target total compensation for each of our executives. Therefore,
TSR has a significant impact on our CAP. The impact for our CEO/PEO is more pronounced given the heavier weighting of stock options versus
the other NEOs (75% vs. 50%) and the larger size of his annual equity awards.
Prior to our development of the COVID-19
vaccine early in 2020, two members of our current executive team (Mr. Bancel and Dr. Hoge) were granted equity awards at then-prevailing
stock prices. From the time of our IPO in December 2018 through early February 2020—when we completed the first batch of our COVID-19
vaccine for testing—our stock price fluctuated between a low of $11.54 and a high of $29.79. Equity awards granted prior to the
development of our COVID-19 vaccine increased significantly in value during 2021, a year in which our closing stock price on December
31, 2021 was $253.98.
Our Other NEOs joined Moderna in 2021
and 2022 (Mr. Mock and Mses. Garay and Klinger) and were granted equity awards at higher stock prices and therefore, the impact of
stock price fluctuations on their CAP has been less pronounced. In addition, as of December 31, 2023, all of their previously granted
stock options are underwater, with grant prices significantly above prevailing stock prices. The decline in our stock price during 2022
and 2023 has resulted in negative CAP for our PEO and Other NEOs.
 |
2024
Proxy statement |
70 |
3. CAP versus Net Income
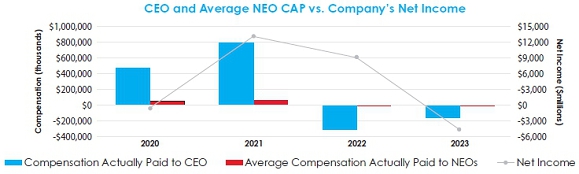
Our stock price appreciated significantly
during 2020 as we announced positive milestones in connection with the development of our COVID-19 vaccine and in anticipation of our
delivering on the promise of mRNA medicine. COVID-19 vaccines were the first medicine using mRNA technology, and prior to their launch
many questioned the ability to develop medicines using mRNA. While we recognized our initial sales to the U.S. and Canadian governments
in the last few weeks of 2020, we incurred significant costs during the year to produce the vaccine and to prepare for global distribution.
As a result, we experienced a loss in 2020, but experienced a significant growth in net income for 2021. 2022 also saw positive net income,
but a decline from the prior year. Moderna experienced a loss in 2023 driven by lower sales of our COVID-19 vaccine, as further described
in our Annual Report on Form 10-K.
4. CAP versus Company Selected Metric (CSM) – Product
Sales from COVID-19 Vaccines

The most heavily weighted factor in
Moderna’s corporate scorecard with respect to Moderna’s annual bonus plan is our product sales. Product sales from our COVID-19
vaccine are our primary source of revenue and are key to funding our operations and ongoing investments in the rest of our research and
development pipeline. Most of our other scorecard goals are tied to advancing our product pipeline.
Our product sales increased significantly
during 2021 with the sale of our first commercial product, the COVID vaccine, during the global pandemic. Our product sales increased
by an additional 4% between 2021 and 2022, but declined in 2023 as we moved into an endemic market and experienced lower than anticipated
vaccination rates. We believe that our ongoing investments in advancing our pipeline will lead to future revenues, and that this will
in turn lead to shareholder value creation that will be reflected in our stock price.
 |
2024
Proxy statement |
71 |
Other Compensation Policies and Practices
Tax and Accounting Considerations
Deductibility of Executive Compensation
Generally, Section 162(m) of the Code
(Section 162(m)) disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any
fiscal year to certain specified executive officers. Subject to certain transition rules which apply to remuneration provided pursuant
to written binding contracts which were in effect on November 2, 2017, and which are not subsequently modified in any material respect
and certain transition relief for newly public companies, for taxable years beginning after December 31, 2017, the exemption from the
deduction limit for “performance-based compensation” is no longer available. Consequently, for fiscal years beginning after
December 31, 2017, all remuneration in excess of $1 million paid to a specified executive will not be deductible unless it qualifies for
the transition relief described above.
In designing our executive compensation
program and determining the compensation of our executive officers, including our NEOs, the Compensation Committee considers a variety
of factors, including the potential impact of the Section 162(m) deduction limit. However, the Compensation Committee will not necessarily
limit executive compensation to that which is or may be deductible under Section 162(m). The deductibility of some types of compensation
depends upon the timing of an executive officer’s vesting or exercise of previously granted rights. Further, interpretations of
and changes in the tax laws, and other factors beyond the Compensation Committee’s control also affect the deductibility of compensation.
The Compensation Committee will consider various alternatives to preserving the deductibility of compensation payments and benefits to
the extent consistent with its compensation goals.
To maintain flexibility to compensate
our executive officers in a manner designed to promote our short-term and long-term corporate goals, the Compensation Committee has not
adopted a policy that all compensation must be deductible. The Compensation Committee believes that our shareholders’ interests are best
served if its discretion and flexibility in awarding compensation is not restricted in order to allow such compensation to be consistent
with the goals of our executive compensation program, even though some compensation awards may result in non-deductible compensation expense.
Accounting for Stock-Based Compensation
We follow the Financial Accounting Standard
Board’s Accounting Standards Codification Topic 718 (FASB ASC Topic 718) for our stock-based compensation awards. FASB ASC Topic
718 requires us to measure the compensation expense for all share-based payment awards made to our employees and non-employee members
of our Board of Directors, including stock options to purchase shares of our common stock and other stock awards, based on the grant date
“fair value” of these awards. This calculation is performed for accounting purposes and reported in the executive compensation
tables required by the federal securities laws, even though the recipient of the awards may never realize any value from their awards.
Taxation of “Parachute” Payments
Sections 280G and 4999 of the Code provide
that executive officers and directors who hold significant equity interests and certain other service providers may be subject to significant
additional taxes if they receive payments or benefits in connection with a change in control of the company that exceeds certain prescribed
limits, and that the company (or a successor) may forfeit a deduction on the amounts subject to this additional tax. We have not agreed
to provide any executive officer, including any NEO, with a “gross-up” or other reimbursement payment for any tax liability
that the executive officer might owe as a result of the application of Sections 280G or 4999 of the Code.
Section 409A of the Internal Revenue Code
Section 409A of the Code imposes additional
significant taxes in the event that an executive officer, director or service provider receives “deferred compensation” that
does not satisfy the requirements of Section 409A of the Code. Although we do not maintain a traditional nonqualified deferred compensation
plan, Section 409A of the Code does apply to certain severance arrangements, bonus arrangements and equity awards. We structure all our
severance arrangements, bonus arrangements and equity awards in a manner to either avoid the application of Section 409A or, to the extent
doing so is not possible, to comply with the applicable requirements of Section 409A of the Code.
 |
2024
Proxy statement |
72 |
Compensation and Talent Committee Report
The information contained in this Compensation
and Talent Committee Report shall not be deemed to be soliciting material or to be filed with the Securities and Exchange Commission,
subject to Regulations 14A or 14C of the Securities Exchange Act of 1934, as amended (the Exchange Act), or subject to the liabilities
of Section 18 of the Exchange Act. No portion of this Compensation and Talent Committee Report shall be deemed to be incorporated by reference
into any filing under the Securities Act of 1933, as amended (the Securities Act) or the Exchange Act, through any general statement incorporating
by reference in its entirety the proxy statement in which this report appears, except to the extent that Moderna specifically incorporates
this report or a portion of it by reference. In addition, this report shall not be deemed filed under either the Securities Act or the
Exchange Act.
The Compensation and Talent Committee
has reviewed and discussed the section captioned “Compensation Discussion and Analysis” with management. Based on such review
and discussions, the Compensation and Talent Committee recommended to the Board of Directors that this “Compensation Discussion
and Analysis” section be included in this proxy statement.
Respectfully submitted by the members
of the Compensation and Talent Committee of the Board of Directors:
François Nader (Chairperson)
Elizabeth Nabel
Elizabeth Tallett
 |
2024
Proxy statement |
73 |
Security Ownership of Certain Beneficial Owners
and Management
The following table sets
forth certain information known to us regarding beneficial ownership of our common stock as of March 7, 2024, or as of the date
otherwise set forth below, for:
| • |
each person or group of affiliated persons known by us to be the beneficial owner of more than five percent of our capital stock; |
| • |
each of our named executive officers; |
| • |
each of our directors; and |
| • |
all of our executive officers, directors, and director nominees as a group. |
Beneficial ownership is determined
in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Under those
rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment
power. Except as noted by footnote, and subject to community property laws where applicable, we believe, based on the information
provided to us, that the persons and entities named in the table below have sole voting and investment power with respect to all
common stock shown as beneficially owned by them.
The percentage of beneficial
ownership in the table below is based on 382,879,612 shares of common stock outstanding as of March 7, 2024. The table
shown below and the calculated percentage of beneficial ownership includes shares owned by each shareholder and all stock options
held by such shareholder that are either currently vested or will be vested within 60 days of March 7, 2024. Further details are
provided in the footnotes section below the table.
| | |
Shares Beneficially Owned |
| Name and Address
of Beneficial Owner(1) | |
Number | |
Percentage |
| Named Executive Officers, Directors and Director Nominees: | |
| |
|
| Stéphane
Bancel, Chief Executive Officer and Director(2) | |
30,113,590 | |
7.7% |
| Noubar
B. Afeyan, Ph.D., Chairman(3) | |
12,017,153 | |
3.1% |
| Robert
Langer, Sc.D., Director(4) | |
11,808,993 | |
3.1% |
| Stephen
Hoge, M.D., President(5) | |
5,312,014 | |
1.4% |
| Paul
Sagan, Director(6) | |
587,832 | |
* |
| Stephen
Berenson, Director(7) | |
190,384 | |
* |
| François
Nader, M.D., Director(8) | |
89,016 | |
* |
| Shannon
Thyme Klinger, Chief Legal Officer(9) | |
51,355 | |
* |
| Sandra
Horning, M.D., Director(10) | |
51,114 | |
* |
| Elizabeth
Tallett, Director(11) | |
40,152 | |
* |
| James
Mock, Chief Financial Officer(12) | |
27,216 | |
* |
| Arpa
Garay, Former Chief Commercial Officer(13) | |
25,150 | |
* |
| Elizabeth
Nabel, M.D., Director(14) | |
15,987 | |
* |
| All
executive officers, directors and director nominees as a group (13 persons) | |
60,329,956 | |
15.2% |
| Other 5% Shareholders: | |
| |
|
| Baillie Gifford & Co(15) | |
45,654,527 | |
11.9% |
| The Vanguard Group(16) | |
33,906,974 | |
8.9% |
| BlackRock, Inc.(17) | |
25,301,057 | |
6.6% |
| * |
Represents beneficial ownership
of less than one percent |
 |
2024 Proxy statement |
74 |
| (1) |
Unless otherwise indicated,
the address for each beneficial owner is c/o Moderna, Inc., 200 Technology Square, Cambridge, MA 02139. |
| (2) |
The shares reported herein consist of (a) 5,460,224 shares held directly by Stéphane Bancel, (b) 6,564,880 shares held
by OCHA LLC (OCHA), (c) 9,050,372 shares held by Boston Biotech Ventures, LLC (BBV), and (d) 9,038,114 shares of common stock
underlying outstanding stock options that are or will be immediately exercisable within 60 days of March 7, 2024. Mr. Bancel
is the controlling unit holder and sole managing member of each of OCHA and BBV. |
| (3) |
Consists of (a) 2,146,931 shares of common stock held by Noubar B. Afeyan, Ph.D., (b) 171,068 shares of common stock underlying
stock options held by Dr. Afeyan that are or will be immediately exercisable or vest within 60 days of March 7, 2024, (c)
8,100,794 shares of common stock held by Flagship Ventures Fund IV, L.P. (Flagship Fund IV), (d) 1,561,320 shares of common
stock held by Flagship Ventures Fund IV.Rx, L.P. (Flagship Fund IV.Rx, together with Flagship Fund IV collectively the Flagship
Funds), (e) 3,924 shares of common stock held by Flagship Pioneering, Inc. (Flagship Pioneering), and (f) 33,116 shares of
common stock underlying stock options held by Flagship Pioneering that are or will be immediately exercisable within 60 days
of March 7, 2024. Flagship Ventures Fund IV General Partner LLC (Flagship Fund IV GP) is the general partner of each of the
Flagship Fund IV and Flagship Fund IV.Rx. Dr. Afeyan is the sole manager of Flagship Fund IV GP and CEO and sole shareholder
of Flagship Pioneering and may be deemed to have voting and investment power with respect to all shares held by the Flagship
Funds and Flagship Pioneering. |
| (4) |
Consists of (a) 11,466,961 shares held by Robert Langer, (b) 14,132 shares held by Michael D. Langer Irrevocable Trust u/d/t
dated 12/14/95, (c) 14,132 shares held by Susan K. Langer Irrevocable Trust u/d/t dated 12/14/95, (d) 14,132 shares held by
Samuel A. Langer Irrevocable Trust u/d/t dated 12/14/95, and (e) 299,636 shares of common stock underlying outstanding stock
options that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (5) |
Consists of (a) 1,516,241 shares held by Stephen Hoge, (b) 151,933 shares held by a trust for the benefit of Dr. Hoge’s
spouse and children, of which his spouse is a trustee, (c) 4,116 shares held by Valhalla LLC, and (d) 3,639,724 shares of
common stock underlying outstanding stock options or unvested RSUs that are or will be immediately exercisable within 60 days
of March 7, 2024. Dr. Hoge disclaims beneficial ownership of the shares held in the trust. |
| (6) |
Consists of (a) 703 shares held, by Paul Sagan (b) 370,407 shares held by Paul Sagan Revocable Trust, (c) 76,452 shares held
by The Chatham Trust, (d) 14,951 shares held by Erwin Park LLC by, and (e) 125,319 shares of common stock underlying outstanding
stock options that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (7) |
Consists of (a) 21,471 shares held by Stephen Berenson and Louise Barzilay, Joint Tenants with Right of Survivorship, and
(b) 168,913 shares of common stock underlying outstanding stock options or unvested RSUs held by Mr. Berenson that are or
will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (8) |
Consists of (a) 20,607 shares held and (b) 68,409 shares of common stock underlying outstanding stock options held by François
Nader that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (9) |
Consists of (a) 8,673 shares held and (b) 42,682 shares of common stock underlying outstanding stock options or unvested RSUs
held by Shannon Thyme Klinger that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (10) |
Consists of (a) 1,373 shares held and (b) 49,741 shares of common stock underlying outstanding stock options or unvested RSUs
held by Sandra Horning that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (11) |
Consists of (a) 703 shares held and (b) 39,449 shares of common stock underlying outstanding stock options held by Elizabeth
Tallett that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (12) |
Consists of (a) 4,300 shares held and (b) 22,916 shares of common stock underlying outstanding stock options or unvested RSUs
held by James Mock that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (13) |
Consists of (a) 4,931 shares held and (b) 20,219 shares of common stock underlying outstanding stock options or unvested RSUs
held by Arpa Garay that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (14) |
Consists of (a) 1,373 shares held and (b) 14,614 shares of common stock underlying outstanding stock options or unvested RSUs
held by Elizabeth Nabel that are or will be immediately exercisable or vest within 60 days of March 7, 2024. |
| (15) |
Based solely on a Schedule 13G/A filed January 29, 2024 (the Baillie Gifford 13G/A). According to the Baillie Gifford 13G/A,
includes sole voting power with respect to 37,518,682 shares, shared voting power with respect to 0 shares, sole dispositive
power with respect to 45,654,527 shares and shared dispositive power with respect to 0 shares. The business address of Baillie
Gifford & Co is Calton Square, 1 Greenside Row, Edinburgh EH1 3AN, Scotland, UK. |
| (16) |
Based solely on a Schedule 13G/A filed February 13, 2024 (the Vanguard 13G/A). According to the Vanguard 13G/A, incudes sole
voting power with respect to 0 shares, shared voting power with respect to 416,058 shares, sole dispositive power with respect
to 32,506,908 shares, and shared dispositive power with respect to 1,400,066 shares. The business address of The Vanguard
Group is 100 Vanguard Blvd., Malvern, PA 19355. |
| (17) |
Based solely on a Schedule 13G/A filed January 29, 2024 (the BlackRock 13G/A). According to the BlackRock 13G/A, includes
sole voting power with respect to 23,024,008 shares, shared voting power with respect to 0 shares, sole dispositive power
with respect to 25,301,057 shares, and shared dispositive power with respect to 0 shares. The business address of BlackRock,
Inc. is 50 Hudson Yards, New York, NY 10001. |
 |
2024 Proxy statement |
75 |
Proposal No. 3: Ratification of Appointment of
Independent Registered Public Accounting Firm
Our Audit Committee has appointed
Ernst & Young LLP as our independent registered public accounting firm to audit our consolidated financial statements for the
year ending December 31, 2024. Ernst & Young has served as our independent registered public accounting firm since 2014. As
a matter of good corporate governance, we are asking shareholders to ratify this appointment. If this appointment is not ratified
at the Annual Meeting, the Audit Committee may reevaluate the selection of our auditors, but is not required to do so. Even if
shareholders ratify this appointment, the Audit Committee, in its sole discretion, may appoint another independent registered public
accounting firm at any time if the committee believes that such a change would be in the best interests of Moderna and its shareholders.
A representative of Ernst
& Young is expected to attend the Annual Meeting, will have an opportunity to make a statement if he or she wishes to do so,
and will be available to respond to appropriate questions from shareholders.
Fees Paid to the Independent Registered Public
Accounting Firm
During 2023, our organization
continued to grow geographically, and a number of our foreign subsidiaries were subject to new audit requirements as they matured.
Additionally, the shift to a commercial market in the U.S. significantly increased the number of our customers and this,
as well as other factors related to the growth of our business, as well as a reduced materiality threshold, increased the complexity
of our annual audit. As a result, we experienced an increase in our year-over-year audit fees from Ernst & Young.
Our Audit Committee remains
focused on ensuring the independence of Ernst & Young. In 2023, we significantly reduced spending on tax fees versus
2022 as we continued to hire internal talent and other service providers to perform certain tax-related services for which we had
previously engaged Ernst & Young. As we have disclosed in prior years, Ernst & Young was engaged to perform certain
tax-related services on a temporary basis during our rapid expansion into new jurisdictions during the pandemic, but over the last
two years we have been focused on limiting these engagements.
The following table presents
fees for professional audit services and other services rendered to us by Ernst & Young for the years ended December 31, 2023
and December 31, 2022:
| | |
2023 | | |
2022 |
| Audit fees(1) | |
$ | 5,271,731 | | |
$ | 3,733,808 |
| Audit-related fees(2) | |
| — | | |
| — |
| Tax fees(3) | |
| 115,872 | | |
| 1,198,000 |
| All other fees(4) | |
| 23,464 | | |
| 6,325 |
| Total Fees | |
$ | 5,411,067 | | |
$ | 4,938,133 |
| (1) |
Audit fees in
2023 and 2022 include fees for our annual audit, quarterly review procedures, statutory audits, comfort letters and consents,
and assistance with and review of documents filed with the SEC. |
| (2) |
There were no
audit-related fees incurred in 2023 or 2022. |
| (3) |
Tax fees include
tax advisory and transfer pricing services. As of 2023, Ernst & Young no longer provides tax return services to Moderna. |
| (4) |
All other fees in 2023 and 2022 consisted
of subscription fees for Ernst & Young’s online accounting research tool, and equal pay analysis for Moderna Switzerland. |
 |
2024 Proxy statement |
76 |
Audit Committee Pre-Approval of Audit and Permissible
Non-Audit Services of Independent Registered Public Accounting Firm
During 2023 and 2022, all
audit and non-audit services by our independent registered public accounting firm were pre-approved by our Audit Committee. The
Audit Committee may establish pre-approval policies and procedures, subject to SEC and Nasdaq rules and regulations, for such services.
The Audit Committee may delegate authority to pre-approve non-audit services to one or more members of the committee. Any decision
to pre-approve an activity must be reported to the full Audit Committee at its first meeting following such decision.
Auditor Independence
In 2023, Ernst & Young
did not provide any services to Moderna that would have caused our Audit Committee to reconsider that firm’s independence.
Vote Required
The ratification of the appointment
of Ernst & Young requires the affirmative vote of a majority of the votes properly cast. Abstentions will have no effect on
the results of this vote.
 |
The
Board of Directors recommends a vote “FOR” the
ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending
December 31, 2024. |
 |
2024 Proxy statement |
77 |
Audit Committee Report
The information contained
in the following Audit Committee Report shall not be deemed to be soliciting material or to be filed with the Securities and Exchange
Commission, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as
amended, or the Securities Exchange Act of 1934, as amended, except to the extent that Moderna specifically incorporates it by
reference in such filing.
The Audit Committee serves
as the representative of Moderna’s Board of Directors with respect to its oversight of:
| • |
Moderna’s accounting and financial reporting processes and the audit
of Moderna’s financial statements; |
| • |
the integrity of Moderna’s financial statements; |
| • |
Moderna’s compliance with legal and regulatory requirements; |
| • |
significant risks, including cybersecurity risks, reviewing Moderna’s policies for
risk assessment and risk management, and assessing the steps management has taken to control these risks; |
| • |
the performance and responsibilities of Moderna’s internal audit function; and |
| • |
the appointment, qualifications, and independence of the independent registered public accounting
firm. |
The Audit Committee also
reviews the performance of the independent registered public accounting firm in the annual audit of Moderna’s financial statements
and in assignments unrelated to the audit, and reviews the independent registered public accounting firm’s fees.
The Audit Committee is composed
of three non-employee directors. The Board of Directors has determined that each member of the Audit Committee is independent and
that Ms. Tallett, Mr. Berenson and Mr. Sagan each qualifies as an “audit committee financial expert” under SEC rules.
The Audit Committee provides
the Board of Directors such information and materials as it may deem necessary to apprise the Board of Directors of financial matters
requiring its attention. The Audit Committee reviews Moderna’s financial disclosures and meets privately, outside the presence
of management, with the independent registered public accounting firm and Moderna’s internal auditors. In fulfilling its
oversight responsibilities, the Audit Committee reviewed and discussed the audited financial statements in Moderna’s Annual
Report on Form 10-K for the year ended December 31, 2023, with management, Moderna’s internal auditors and Ernst & Young,
including a discussion of the quality and substance of the accounting principles, the reasonableness of significant judgments made
in connection with the audited financial statements, and the clarity of disclosures in the financial statements. The Audit Committee
reports on these meetings to the Board of Directors.
The Audit Committee has discussed
with Ernst & Young the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight
Board (PCAOB) and the SEC. In addition, the Audit Committee has received and reviewed the written disclosures and the letter from
Ernst & Young required by the applicable requirements of the PCAOB regarding that firm’s communications with the Audit
Committee concerning independence, and has discussed with Ernst & Young its independence from management and from Moderna,
and considered the compatibility of non-audit services with Ernst & Young’s independence. In addition, the Audit Committee
discussed Moderna’s internal controls over financial reporting and management’s assessment of the effectiveness of
those controls with management, Moderna’s internal auditors and Ernst & Young. The Audit Committee reviewed with both
Ernst & Young and Moderna’s internal auditors their audit plans, audit scope and identification of audit risks. Based
on these reviews and discussions, the Audit Committee recommended to the Board of Directors that Moderna’s audited consolidated
financial statements be included in Moderna’s Annual Report on Form 10-K for the year ended December 31, 2024, for filing
with the SEC.
The Audit Committee has selected
Ernst & Young as Moderna’s independent registered public accounting firm for the year ending December 31, 2024. The Board
of Directors recommends that shareholders ratify this selection at the Annual Meeting.
Respectfully submitted by
the members of the Audit Committee of the Board of Directors:
Elizabeth Tallett (Chairperson)
Stephen Berenson
Paul Sagan
 |
2024 Proxy statement |
78 |
Proposal No. 4: Amend Our Certificate of Incorporation
to Provide Shareholders the Right to Call a Special Meeting
Background and Proposed Amendment
This management proposal
seeks to provide to common shareholders owning at least 20% of Moderna’s outstanding stock the right to call a special meeting
of shareholders, in accordance with, and subject to, the provisions that would be set forth in our governing documents.
As part of our continuous
evaluation of our corporate governance practices, our Board regularly reviews our governing documents and considers feedback from
investors concerning possible updates. Currently, our Amended and Restated Certificate of Incorporation (the “Certificate
of Incorporation”) provides that only the Board may call a special meeting of shareholders.
In our engagement meetings
with shareholders, we have received consistent feedback that shareholders would appreciate an expansion of certain shareholder
rights. This feedback and engagement with shareholders informed the Board’s decision to amend our By-laws in February 2024 to implement
majority voting for uncontested director elections, as well as proxy access on market standard terms, both as described elsewhere
in this Proxy Statement. Additionally, the Board has decided that it is in the best interests of the Company and our shareholders
to permit shareholders that collectively hold a sufficiently large economic and voting interest in the Company to require that
the Company call a special meeting of shareholders, subject to specified procedures, provisions and requirements.
The Board, based on the recommendation
of the Nominating and Corporate Governance Committee, has unanimously recommended that the Company’s shareholders vote “FOR”
an amendment (the “Special Meeting Amendment”) to the Certificate of Incorporation to amend and revise Article V, Section
2 to read in its entirety as follows:
“2. Special Meetings.
Except as otherwise required by statute and subject to the rights, if any, of the holders of any series of Undesignated Preferred
Stock, special meetings of the stockholders of the Corporation may be called only by (i) the Board of Directors acting pursuant
to a resolution approved by the affirmative vote of a majority of the Directors then in office (whether or not there exist any
vacancies in previously authorized directorships at the time any such resolution is presented to the Board of Directors for adoption),
(ii) the chairman of the Board of Directors, or the chief executive officer or president (in the absence of a chief executive officer)
or (iii) the secretary of the Corporation following the secretary’s receipt of signed written requests to call a meeting
from the holders of at least 20% of the voting power of the capital stock of the Corporation issued and outstanding and entitled
to vote. Except as otherwise required by law, special meetings of stockholders of the Corporation may not be called by any other
person or persons. Advance notice of stockholder nominations and business to be brought before any special meeting of the stockholders
of the Corporation shall be given in the manner provided in the By-laws of the Corporation.”
If the shareholders approve
the Special Meeting Amendment at the Annual Meeting, the Company will file a Certificate of Amendment to our Certificate of Incorporation
in the form attached hereto as Appendix A. In accordance with Delaware law, however, our Board may elect to abandon the Special
Meeting Amendment without further action by the shareholders at any time prior to the effectiveness of the filing of the Special
Meeting Amendment with the Secretary of State of the State of Delaware, notwithstanding shareholder approval of the amendment.
Reasons for the Amendment
The Board recognizes that
providing a significant portion of the shareholders of a company the ability to call special meetings is viewed by many shareholders
as an important shareholder right. However, the Board also recognizes the need for appropriate parameters given that special meetings
of shareholders potentially can be disruptive to business operations and to long-term shareholder interests, can be misused and
can cause the Company to incur substantial expenses. Accordingly, the Board believes that the proposed 20% threshold for calling
special meetings of shareholders will balance these considerations, providing that special meetings can be called by shareholders
with a significant interest in the Company, while minimizing the risk of disruption to the Company and its operations that calling
a special meeting could entail. The Board’s decision to set this threshold at 20% was informed by feedback from investors
during outreach meetings over the last several months, and the Board believes this level of support strikes an appropriate balance
based upon the views expressed during those meetings.
 |
2024 Proxy statement |
79 |
The Board further believes
that the Special Meeting Amendment will further align the Company’s governance with prevailing market practices by granting the
right call a special meeting of shareholders to the Chairman of the Board, the Chief Executive Officer, or the President (in the
absence of a Chief Executive Officer).
Effect of the Amendment
As described above, the Special
Meeting Amendment would amend our Certificate of Incorporation to permit shareholders holding at least 20% of our outstanding stock
to request that the Secretary of the Company call a special meeting of shareholders. It would also grant the Chairman of
the Board, the Chief Executive Officer, or the President (in the absence of a Chief Executive Officer), the right to call a special
meeting of shareholders. Any business to be acted upon at a special meeting of shareholders would be subject to the applicable
provisions of our By-laws.
If the Special Meeting Amendment
is approved, the Board will adopt corresponding changes to the By-laws (which do not require shareholder approval) that will include
safeguards and requirements for calling special meetings, including the concepts outlined below and otherwise consistent with the
newly-effective shareholder rights provided in the Certificate of Incorporation:
| • |
To specify the information required to be set forth in a written request
to call a special meeting; |
| • |
To specify the requirements for proponents to update and supplement information submitted
in connection with a special meeting to ensure that information remains accurate in advance of the meeting; |
| • |
To grant the Board the discretion to determine whether to hold a special meeting if written
requests to hold a special meeting are revoked from holders and the relevant threshold is no longer met; |
| • |
To grant the Board the right to determine the place (if any), date and time of a special
meeting and to set the record date for the special meeting, as well as to determine any other business to be transacted at
the special meeting; and |
| • |
To specify that a shareholder written request to call a special meeting shall not be valid
if it (i) relates to an item of business that is not a proper subject for shareholder action under applicable law, (ii) relates
to an item that is the same or substantially similar to an item that was voted upon at a shareholder meeting within 120 days
prior to the receipt of shareholder requests, (iii) that is delivered during the period commencing 120 days prior to the anniversary
of the last annual meeting and ending on the date of the next annual meeting, (iv) violates Regulation 14A under the Exchange
Act, or (v) does not otherwise comply with the by-law related to special meetings. |
Other than the revisions
to Article V, Section 2 to reflect the text that appears above, the Special Meeting Amendment would not cause any changes to the
Certificate of Incorporation. If the Special Meeting Amendment is approved by shareholders, the Special Meeting Amendment would
become effective upon filing with the Delaware Secretary of State, which the Company would submit promptly following the Annual
Meeting of Shareholders.
 |
The
Board of Directors recommends a vote “FOR” this
proposal, for the reasons outlined above. |
 |
2024 Proxy statement |
80 |
Proposal No. 5: Amend Our Certificate of Incorporation
to Reflect New Delaware Law Provisions Allowing for Officer Exculpation
Background and Proposed Amendment
In August 2022, the State
of Delaware, which is the Company’s state of incorporation, enacted legislation that enables Delaware companies to limit
the liability of certain officers in limited circumstances. The amended Delaware law permits exculpation for direct claims brought
by shareholders for breach of an officer’s fiduciary duty of care, but would not permit companies to eliminate officers’
monetary liability for breach of fiduciary duty claims brought by the company itself or for derivative claims brought by shareholders
on behalf of the company. The new law also does not allow officers to be exculpated for breaches of the duty of loyalty, acts or
omissions not in good faith or that involve intentional misconduct or a knowing violation of law, or any transaction in which an
officer derived an improper personal benefit.
In light of this change in
law, the Board, based on the recommendation of the Nominating and Corporate Governance Committee, has unanimously recommended that
the Company’s shareholders vote “FOR” an amendment (the “Exculpation Amendment”) to the Company’s
Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) to add a new Article VIII, which
shall read in its entirety as follows:
“ARTICLE VIII. Limitation
of Liability for Officers. An Officer (as defined below) of the Corporation shall not be personally liable to the Corporation
or its stockholders for monetary damages for breach of his or her fiduciary duty as an officer of the Corporation, except for liability
(a) for any breach of the Officer’s duty of loyalty to the Corporation or its stockholders, (b) for acts or omissions not
in good faith or which involve intentional misconduct or a knowing violation of law, (c) for any transaction from which the Officer
derived an improper personal benefit, or (d) arising from any claim brought by or in the right of the Corporation. If the DGCL
is amended after the effective date of this Certificate to authorize corporate action further eliminating or limiting the personal
liability of Officers, then the liability of an Officer of the Corporation shall be eliminated or limited to the fullest extent
permitted by the DGCL, as so amended. For purposes of this Article VIII, “Officer” shall mean an individual who has
been duly appointed as an officer of the Corporation and who, at the time of an act or omission as to which liability is asserted,
is deemed to have consented to service by the delivery of process to the registered agent of the Corporation as contemplated by
10 Del. C. § 3114(b).
Any amendment, repeal
or modification of this Article VIII by either of (i) the stockholders of the Corporation or (ii) an amendment to the DGCL, shall
not adversely affect any right or protection existing at the time of such amendment, repeal or modification with respect to any
acts or omissions occurring before such amendment, repeal or modification of a person serving as a Director at the time of such
amendment, repeal or modification.”
The Exculpation Amendment
also includes a corresponding renumbering of the subsequent Articles in the Certificate of Incorporation and a technical amendment
in the (newly-renumbered) Article IX to clarify that the Board may adopt (in addition to amend or repeal) the Bylaws.
If the shareholders approve
this proposal at the Annual Meeting, the Company will file a Certificate of Amendment to our Certificate of Incorporation substantially
in the form attached hereto as Appendix B. In accordance with Delaware law, however, our Board may elect to abandon the Exculpation
Amendment without further action by the shareholders at any time prior to the effectiveness of the filing of the Exculpation Amendment
with the Secretary of State of the State of Delaware notwithstanding shareholder approval of the amendment.
Reasons for the Amendment
The Board believes it is
important to provide protection from certain liabilities and expenses that may discourage prospective or current officers from
serving the Company. The Board believes it is appropriate for public companies in states that allow exculpation of officers to
have exculpation clauses in their company charters. In the absence of such protection, qualified officers might be deterred from
serving as officers due to exposure to personal liability and the risk that substantial expense will be incurred. Our Board and
our shareholders expect our officers to effectively manage the Company’s challenges within a complex and ever-evolving environment.
The Board recognizes that our officers are called upon to make critical decisions, and that in an increasingly litigious environment
our executive team can be exposed to substantial personal liability from litigants seeking to impose liability on the basis of
hindsight and regardless of merit. The Board believes that providing the protection permitted under Delaware law will provide comfort
to and empower our officers to best exercise their business judgment in the interests of the Company and our shareholders, which
 |
2024 Proxy statement |
81 |
will encourage agility and
critical decision-making, while also enabling us to continue to attract and retain experienced and high-quality officers.
To the extent our peers adopt similar exculpation
clauses that limit the personal liability of officers, failing to adopt the Exculpation Amendment could negatively impact our recruitment
of exceptional officer candidates and retention of current officers.
Our Certificate of Incorporation
currently provides for the exculpation of directors but does not include a provision that allows for the exculpation of officers.
The Exculpation Amendment would more generally align the protections available to our directors under our governing documents and
Delaware law with those available to our officers, enabling them to exercise their business judgment in furtherance of the interests
of the shareholders without the potential for distraction posed by the risk of personal liability. The Exculpation Amendment would
also help to clarify the application of exculpation provisions to individuals serving as both a director and an officer. As is
currently the case with directors under our Certificate of Incorporation, this provision would not exculpate officers from liability
for breach of the duty of loyalty, acts or omissions not in good faith or that involve intentional misconduct or a knowing violation
of law, or any transaction in which the officer derived an improper personal benefit. Nor would this provision exculpate such officers
from liability for claims brought by or in the right of the Company, such as derivative claims.
The Exculpation Amendment
is aimed at striking a balance between shareholders’ interest in accountability and their interest in the Company being able
to attract and retain experienced and high-quality officers to work on its behalf. Accordingly, the Board believes that the Exculpation
Amendment is in the best interests of the Company and its shareholders.
Given the narrow class and
type of claims for which officers’ liability would be exculpated, the Board believes the Exculpation Amendment would not
negatively impact shareholder rights.
The Exculpation Amendment
may also reduce the Company’s future insurance needs and costs.
Effect of the Amendment
As described above, the Exculpation
Amendment would limit the liability of certain officers of the Company in limited circumstances. Other than the addition of a new
Article VIII with the text that appears above, and the renumbering of subsequent Articles of the Certificate of Incorporation,
the Exculpation Amendment would not cause any changes to the Company’s Certificate of Incorporation. If the Exculpation Amendment
is approved by shareholders, the Exculpation Amendment would become effective upon a filing with the Delaware Secretary of State,
which the Company would submit promptly following the Annual Meeting of Shareholders.
 |
The
Board of Directors recommends a vote “FOR” this
proposal, for the reasons outlined above. |
 |
2024 Proxy statement |
82 |
Information about the 2024 Annual Meeting of
Shareholders
To be held at 8:00 a.m. Eastern
Time on May 6, 2024.
Why am I receiving these materials?
Our Board of Directors is
providing these proxy materials to you in connection with a solicitation of proxies for use at the Annual Meeting. You are invited
to attend the Annual Meeting and we are asking you to vote on the proposals described in this proxy statement. All shareholders
as of the close of business on March 7, 2024, will receive the proxy materials and have the ability to access them online at www.proxyvote.com.
Who is entitled to vote at the Annual Meeting?
Holders of our common stock
at the close of business on March 7, 2024, the record date for the Annual Meeting (the Record Date), are entitled to notice of
and to vote at the Annual Meeting. Each shareholder is entitled to one vote for each share of our common stock held as of the Record
Date for each matter addressed at the meeting. As of the Record Date, there were 382,879,612 shares of common stock
outstanding and entitled to vote. You are entitled to vote shares that are held of record directly in your name and shares held
for you as the beneficial owner through a shareholder, bank, or other nominee. For more information, see “What is
the difference between holding shares as a shareholder of record and as a beneficial owner?”
What business will be conducted at the Annual
Meeting?
The following table shows
the proposals to be presented for a vote, the applicable voting requirements, and the Board’s recommendations.
| Proposal |
|
Vote
required to pass |
|
Voting
options |
|
Board’s
recommendation |
|
Effect
of abstentions and broker non-votes* |
| Elect
three Class III directors to hold office until the 2027 annual meeting of shareholders |
|
Each
director must receive an affirmative vote from a majority of the votes properly cast to be elected |
|
FOR,
AGAINST or ABSTAIN (for each director nominee) |
|
 |
FOR each
nominee |
|
No
effect |
| Approve,
on a non-binding, advisory basis, the compensation of our named executive officers |
|
A
majority of the votes properly cast |
|
FOR,
AGAINST or ABSTAIN |
|
 |
FOR |
|
No
effect |
| Ratify
the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December
31, 2024 |
|
A
majority of the votes properly cast |
|
FOR,
AGAINST or ABSTAIN |
|
 |
FOR |
|
No
effect |
| Proposal
to amend our Certificate of Incorporation to provide shareholders the right to call a special meeting |
|
A
majority of shares outstanding |
|
FOR,
AGAINST or ABSTAIN |
|
 |
FOR |
|
Treated
as a vote AGAINST the proposal |
| Proposal
to amend our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation |
|
A
majority of shares outstanding |
|
FOR,
AGAINST or ABSTAIN |
|
 |
FOR |
|
Treated
as a vote AGAINST the proposal |
| * |
See “What if I do not specify how my shares are to be
voted?” for an explanation of broker non-votes. |
The Board of Directors knows
of no other business to be brought before the Annual Meeting. Should any other matters be presented, the individuals named in the
proxy will have the authority to take action in regard to such matters as in their judgment seems advisable. If you hold shares
through a broker, bank, or other nominee, they will not be able to vote your shares on any other business that comes before the
Annual Meeting unless they receive instructions from you with respect to such matter.
 |
2024
Proxy statement |
83 |
What is the difference between holding shares
as a shareholder of record and as a beneficial owner?
Shareholder of record
If your shares are registered
directly in your name with Computershare Trust Company, N.A., our transfer agent, then you are considered the shareholder of record
with respect to those shares. As the shareholder of record, you have the right to grant your voting proxy directly to the individuals
listed on the proxy card or to vote online during the Annual Meeting.
Beneficial owner
If your shares were held
in a stock brokerage account or by a bank or other nominee on your behalf, then you are considered the beneficial owner of shares
held in “street name.” As the beneficial owner, you have the right to direct your broker, bank, or other nominee how
to vote your shares by following the voting instructions you receive. Your broker, bank, or other nominee has only limited authority
to vote your shares without your instructions. For more information, see “What if I do not specify how my shares
are to be voted?”
How can I attend the Annual Meeting?
Admission to the Annual Meeting
We will host the Annual Meeting
via the Internet. You will not be able to attend the meeting in person. Participation in the Annual Meeting as a shareholder, with
the right to vote and submit questions, is limited to shareholders as of the close of business on the Record Date. The meeting
can be accessed at: www.virtualshareholdermeeting.com/MRNA2024.
Other shareholders and members
of the public can also access the Annual Meeting at the URL above without a control number, but without the right to vote or submit
a question.
The webcast of the Annual
Meeting will begin at 8:00 a.m., Eastern Time, on May 6, 2024. Online access will begin at 7:45 a.m., Eastern Time, and we encourage
you to log into the Annual Meeting 5-15 minutes prior to the start time.
If you encounter difficulties
accessing the Annual Meeting, please call the technical support number that will be posted on the registration page for the meeting
website.
Shareholder of record
If you were a shareholder
of record at the close of business on the Record Date, you will need the 16-digit control number found on your Notice of Internet
Availability, your proxy card or the instructions that accompany your proxy materials. You do not need to do anything in advance
to access the Annual Meeting.
Beneficial owner
If you were a beneficial
owner at the close of business on the Record Date, you may attend the Annual Meeting. You will need the 16-digit control number
found on your Notice of Internet Availability, your proxy card or the instructions that accompany your proxy materials if you wish
to attend the Annual Meeting with the right to vote and submit a question. Even if you do not have your control number or were
not a shareholder as of the close of business on the Record Date, you can still access the meeting, but will not be able to vote
at the meeting or submit a question.
How do I vote?
Shareholder of record
If you are a shareholder
of record, you can vote in one of the following ways:
Internet. Go
to www.proxyvote.com to complete an electronic proxy card. You will need the control number from the proxy card you receive. You
will be responsible for any Internet access charges you incur. If you vote online, you do not need to return a proxy card by mail.
Votes by internet must be submitted by 11:59 p.m. on Sunday, May 5, 2024, Eastern time, to be counted.
By telephone. Dial
toll-free 1-800-690-6903 and follow the recorded instructions. You will need the control number from your proxy card. You will
be responsible for any telephone charges you incur. If you vote by telephone, you do not need to return a proxy card by mail. Votes
by telephone must be submitted by 11:59 p.m. on Sunday, May 5, 2024, Eastern time, to be counted.
By mail. Complete,
date, and sign your proxy card and return it promptly by mail in the envelope provided. The people named in the proxy card will
vote your shares in accordance with the instructions you provide. If you return the proxy card, but do not give voting instructions
on a particular matter, the people named in the proxy card will vote your shares in accordance with the recommendations of our
Board of Directors. Votes by mail must be received by the close of business on Friday, May 3, 2024, Eastern time, to be counted.
During the
meeting. If you plan to attend the virtual Annual Meeting, you may vote by following
the instructions provided online during the meeting. However, even if you plan to attend the virtual Annual Meeting, we encourage
you to submit your vote ahead of time by one of the methods listed above.
Beneficial owner
If your shares are held in
street name, follow the voting instructions you receive from your broker, bank, or other nominee.
 |
2024
Proxy statement |
84 |
Can I submit a question for the meeting?
Shareholders as of the Record
Date can submit questions in writing in advance of the meeting through www.proxyvote.com. Shareholders who attend the Annual Meeting
and log in as a shareholder using their control number (as described above) will also have an opportunity to submit questions in
writing during the meeting. We will try to answer as many submitted questions as time permits (whether submitted prior to or during
the portion of the meeting when questions may be submitted). If we receive substantially similar questions, we will group such
questions together and provide a single response to avoid repetition.
Can I change my vote or revoke my proxy?
Shareholder of record
If you are a shareholder
of record, you may revoke your proxy or change your proxy instructions at any time before your proxy is voted at the Annual Meeting
by:
| • |
entering a new vote by Internet or telephone; |
| • |
signing and returning a new proxy card with a later date; |
| • |
delivering a written revocation to our Corporate Secretary; or |
| • |
accessing the Annual Meeting and voting in person. |
Beneficial owner
If you are a beneficial owner,
you must contact the broker, bank, or other nominee holding your shares and follow their instructions to change your vote or revoke
your proxy.
What if I do not specify how my shares are to
be voted?
Shareholder of record
If you submit a proxy but
do not provide voting instructions, your shares will be voted:
| • |
FOR the election
of each of the nominees as Class III directors to serve for a three-year term (Proposal No. 1); |
| • |
FOR approval, on a non-binding, advisory
basis, of the compensation of Moderna’s named executive officers (Proposal No. 2); |
| • |
FOR the ratification of the appointment
of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024 (Proposal
No. 3); |
| • |
FOR the approval of an amendment
to our Certificate of Incorporation to provide shareholders the right to call a special meeting (Proposal No. 4); and |
| • |
FOR the approval of an amendment
to our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation (Proposal No. 5). |
| • |
In the discretion of the named proxy holders regarding any other matters properly presented
for a vote at the Annual Meeting. |
Beneficial owner
If you do not provide voting
instructions, then your broker, bank, or other nominee will only have authority to vote your shares with respect to Proposal No.
3 (ratification of the appointment of the independent registered public accounting firm). Your shares will not be voted with respect
to Proposals No. 1, 2, 4 and 5. This is known as a “broker non-vote.”
What is the quorum requirement for the Annual
Meeting?
A majority of the shares
of common stock outstanding and entitled to vote, in person or by proxy, constitutes a quorum for the transaction of business at
the Annual Meeting. As of the Record Date, there were a total of 382,879,612 shares of common stock outstanding, which
means that 191,439,807 shares of common stock must be represented in person or by proxy at the Annual Meeting
to have a quorum. If there is no quorum, a majority of the shares present at the Annual Meeting may adjourn the meeting to a later
date. Votes withheld, abstentions and broker non-votes will be counted for purposes of determining whether we have a quorum.
Who is soliciting my proxy?
Proxies are solicited by
and on behalf of our Board of Directors. The individuals named in the proxy have been designated as proxy holders by our Board
of Directors. When you return a proxy that is properly dated and executed, your shares represented by the proxy will be voted at
the Annual Meeting in accordance with your instructions. If you do not give specific instructions on your proxy card, your shares
will be voted in accordance with the recommendations of our Board of Directors. If any matters not described in this proxy statement
are properly presented at the Annual Meeting, the proxy holders will use their own judgment to determine how to vote your shares.
If the Annual Meeting is postponed or adjourned, the proxy holders can vote your shares on the new meeting date, unless you have
properly revoked your proxy, as described above.
 |
2024
Proxy statement |
85 |
How are proxies solicited and who is paying for
the solicitation?
Moderna will bear the entire
cost of this proxy solicitation, including the distribution of the proxy materials. Copies of solicitation materials will also
be made available to brokers, banks, and other nominees to forward to beneficial owners. The original solicitation of proxies may
be supplemented by solicitation by telephone, electronic communication, or other means by our directors, officers, employees, or
agents. Morrow Sodali, LLC has been retained to assist in soliciting proxies for a fee of $10,000 plus distribution costs and other
expenses.
Why did I receive more than one Notice of the
Annual Meeting?
If you receive more than
one proxy card or voting instruction form for the Annual Meeting, your shares may be registered in more than one name or in more
than one account. Please follow the voting instructions on each notice you received to ensure that all of your shares are voted.
I share an address with another shareholder,
and we received only one paper copy of the proxy materials. How may I obtain an additional copy?
Shareholders of Moderna common
stock who share a single address may receive only one copy of this proxy statement and Annual Report, unless we have received contrary
instructions from any shareholder at that address. This practice, known as “householding,” is designed to reduce our
printing and postage costs and the environmental impact of the Annual Meeting. Shareholders who participate in householding will
continue to receive separate proxy cards if they received a paper copy of proxy materials in the mail. If your household received
only a single set of our proxy materials and you would like a separate copy, or if your household received multiple copies of our
proxy materials and you want only a single copy next year, please notify our Corporate Secretary at the address provided below.
Shareholders who hold shares in street name may contact their brokerage firm, bank, or other nominee to request information about
householding.
How can I find out the results of the voting
at the Annual Meeting?
Preliminary voting results
will be announced at the Annual Meeting. In addition, final voting results will be published in a current report on Form 8-K that
we expect to file with the SEC within four business days after the Annual Meeting. If final voting results are not available to
us at that time, we intend to file a Form 8-K to publish preliminary results and, within four business days after we know the final
results, file an amendment to the Form 8-K to publish those results.
What is the deadline to propose actions for consideration
at next year’s annual meeting of shareholders or to nominate individuals to serve as directors?
Proposal to include in our proxy statement
Shareholders may present
proper proposals to be included in our proxy statement and considered at the next annual meeting of shareholders by submitting
their proposals in writing to our Corporate Secretary. For a shareholder proposal to be considered for inclusion in our proxy statement
for our 2025 annual meeting of shareholders (2025 Annual Meeting), our Corporate Secretary must receive the written proposal at
our principal executive offices no later than November [ ], 2024, unless the date of our 2025 Annual Meeting is held more
than 30 days before or after May 6, 2025, in which case the proposal must be received a reasonable time before we begin to print
and send proxy materials for the 2025 Annual Meeting. In addition, shareholder proposals must comply with the applicable requirements
of Rule 14a-8 under the Exchange Act.
Proposal that will not be included in our proxy
statement
Our bylaws contain an advance notice procedure for shareholders who wish to present a proposal before an annual meeting of
shareholders but do not intend for the proposal to be included in our proxy statement. These matters may only be brought before
the annual meeting by a shareholder of record entitled to vote at the annual meeting who has delivered timely written notice,
containing the information specified in our bylaws, to our Corporate Secretary. To be timely for our 2025 Annual Meeting,
our Corporate Secretary generally must receive the written notice at our principal executive offices between January 6, 2025,
and February 5, 2025. However, if we hold our 2025 Annual Meeting more than 30 days before or more than 60 days after May
6, 2025, then notice of a shareholder proposal that is not intended to be included in our proxy statement must be received
no later than the close of business on the later of (a) the 90th day before our 2025 Annual Meeting and (b) the 10th day following
the day on which public announcement of the date of our 2025 Annual Meeting is first made.
Director nominations
Our Nominating Committee
is responsible for selecting and recommending nominees for election as directors to the Board. For information on how you can propose
 |
2024
Proxy statement |
86 |
director candidates for consideration
by our Nominating Committee, see “Governance—Composition of Our Board of Directors—Shareholder Recommendations
for Director Candidates.”
In addition, a
shareholder, or a group of up to 20 shareholders, that has owned continuously for at least three years shares of our common
stock representing an aggregate of at least 3% of our outstanding shares, may nominate and include in our proxy materials the
greater of two director nominees or the number of nominees constituting 20% of our Board, or if such amount is not a whole
number, the closest whole number below 20%, provided that the shareholder(s) and nominee(s) satisfy the requirements in our
By-laws. Notice of director nominees pursuant to this proxy access by-law must be received between November
[ ], 2024 and December [ ], 2024. However, if we hold our 2025 Annual Meeting more than 30 days before or more than 60
days after May 6, 2025, then the proxy access notice must be received no earlier than the 120th day before our 2025 Annual
Meeting and no later than the close of business on the later of (a) the 90th day before our 2025 Annual Meeting and (b) the
10th day following the day on which public announcement of the date of our 2025 Annual Meeting is first made.
To directly nominate a director
for election at an annual meeting of shareholders, an eligible shareholder must provide the information required by our bylaws,
including, without limitation, the name and principal occupation or employment of the nominee, the number of shares of our capital
stock owned by the nominee, a description of arrangements or understandings between the nominee and the appointing shareholder
or shareholders concerning the nominee’s potential service on our Board of Directors and a completed questionnaire with respect
to the nominee’s background and qualifications. In addition, the appointing shareholder must give notice to our Corporate Secretary
in the time frame described above under “—Proposal that will not be included in our proxy statement.”
Furthermore, to
comply with universal proxy rules, shareholders who intend to solicit proxies in support of director nominees other than the
Company’s nominees must provide notice that sets forth the information required by Rule 14a-19 under the Exchange Act
no later than February 5, 2025.
How can I get a copy of Moderna’s Bylaws?
Our bylaws are part of our
public filings on the SEC’s website at www.sec.gov. You may also contact our Corporate Secretary as described immediately
below for a copy of our bylaws.
How can I contact the Corporate Secretary?
You can reach our Corporate
Secretary at the following address and phone number: Moderna, Inc., Attention: Corporate Secretary, 200 Technology Square, Cambridge,
Massachusetts 02139, (617) 714-6500, or via email at Corporate.Secretary@modernatx.com.
Where can I find more information about Moderna?
We file reports, proxy statements,
and other information with the SEC, which is all publicly available on the SEC’s website, http://www.sec.gov. You may also
find any document we file with the SEC (and more) on our website at http://www.modernatx.com under the “Investors”
heading. Information contained on, or that can be accessed through, our website is not intended to be incorporated by reference
into this proxy statement.
You should rely on the information
contained in this document to vote your shares at the Annual Meeting. Moderna has not authorized anyone to provide you with information
that is different from what is contained in this document. This document is dated March [ ], 2024. You should not assume
that the information contained in this document is accurate as of any later date, and the mailing of this document to shareholders
at any time after that date does not suggest otherwise. This proxy statement does not constitute a solicitation of a proxy in any
jurisdiction where, or to or from any person to whom, it is unlawful to make such proxy solicitations.
 |
2024
Proxy statement |
87 |
Appendix A
Certificate of Amendment - Special Meeting Right
Certificate of Amendment
of
Amended and Restated Certificate of Incorporation
of
Moderna, Inc.
Moderna, Inc. (the “Corporation”),
a corporation duly organized and existing under the laws of the State of Delaware pursuant to Section 242 of the General Corporation
Law of the State of Delaware (the “DGCL”), hereby certifies as follows:
| 1. |
The Amended and Restated Certificate of Incorporation of the Corporation,
as heretofore amended, is hereby amended by replacing Article V – Stockholder Action, Section 2, which shall read in
its entirety as follows: |
“ARTICLE V”
| |
2. |
Special Meetings. Except as otherwise
required by statute and subject to the rights, if any, of the holders of any series of Undesignated Preferred Stock, special
meetings of the stockholders of the Corporation may be called only by (i) the Board of Directors acting pursuant to a resolution
approved by the affirmative vote of a majority of the Directors then in office (whether or not there exist any vacancies in
previously authorized directorships at the time any such resolution is presented to the Board of Directors for adoption),
(ii) the chairman of the Board of Directors, or the chief executive officer or president (in the absence of a chief executive
officer) or (iii) the secretary of the Corporation following the secretary’s receipt of signed written requests to call
a meeting from the holders of at least 20% of the voting power of the capital stock of the Corporation issued and outstanding
and entitled to vote. Except as otherwise required by law, special meetings of stockholders of the Corporation may not be
called by any other person or persons. Advance notice of stockholder nominations and business to be brought before any special
meeting of the stockholders of the Corporation shall be given in the manner provided in the By-laws of the Corporation. |
| 2. |
The Board of Directors of the Corporation has adopted a resolution
approving and declaring advisable the foregoing amendment set forth in this Certificate of Amendment in accordance with the
provisions of Section 242 of the DGCL. |
| 3. |
The stockholders of the Corporation, at a meeting duly called and held pursuant to
Section 222 of the DGCL, duly adopted the amendments set forth in this Certificate of Amendment in accordance with the provisions
of Section 242 of the DGCL. |
| 4. |
The foregoing amendments were duly adopted in accordance with Section 242 of the DGCL. |
IN WITNESS WHEREOF, the undersigned, as a duly
authorized officer of the Corporation, has executed this Certificate of Amendment on ,
2024.
| |
Moderna,
Inc. |
|
| |
|
|
| |
Name:
Title: |
|
88
Appendix B
Certificate of Amendment - Officer Exculpation
Certificate of Amendment
of
Amended and Restated Certificate of Incorporation
of
Moderna, Inc.
Moderna, Inc. (the “Corporation”),
a corporation duly organized and existing under the laws of the State of Delaware pursuant to Section 242 of the General Corporation
Law of the State of Delaware (the “DGCL”), hereby certifies as follows:
| 1. |
The Amended and Restated Certificate of Incorporation of the Corporation,
as heretofore amended, is hereby amended by adding a new Article VIII – Limitation of Liability for Officers, which
shall read in its entirety as follows: |
“ARTICLE VIII
LIMITATION OF LIABILITY
FOR OFFICERS
An Officer (as defined below)
of the Corporation shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of his
or her fiduciary duty as an officer of the Corporation, except for liability (a) for any breach of the Officer’s duty of
loyalty to the Corporation or its stockholders, (b) for acts or omissions not in good faith or which involve intentional misconduct
or a knowing violation of law, (c) for any transaction from which the Officer derived an improper personal benefit, or (d) arising
from any claim brought by or in the right of the Corporation. If the DGCL is amended after the effective date of this Certificate
to authorize corporate action further eliminating or limiting the personal liability of Officers, then the liability of an Officer
of the Corporation shall be eliminated or limited to the fullest extent permitted by the DGCL, as so amended. For purposes of this
Article VIII, “Officer” shall mean an individual who has been duly appointed as an officer of the Corporation and who,
at the time of an act or omission as to which liability is asserted, is deemed to have consented to service by the delivery of
process to the registered agent of the Corporation as contemplated by 10 Del. C. § 3114(b).
Any amendment, repeal or
modification of this Article VIII by either of (i) the stockholders of the Corporation or (ii) an amendment to the DGCL, shall
not adversely affect any right or protection existing at the time of such amendment, repeal or modification with respect to any
acts or omissions occurring before such amendment, repeal or modification of a person serving as a Director at the time of such
amendment, repeal or modification.”
| 2. |
The Amended and Restated Certificate of Incorporation of the Corporation,
as heretofore amended, is hereby amended by retitling existing Articles VIII and IX as follows: |
“ARTICLE IX –
AMENDMENT OF BY-LAWS”
“ARTICLE X –
AMENDMENT OF CERTIFICATE OF INCORPORATION”
| 3. |
The Board of Directors of the Corporation has adopted a resolution
approving and declaring advisable the foregoing amendment set forth in this Certificate of Amendment in accordance with the
provisions of Section 242 of the DGCL. |
| 4. |
The stockholders of the Corporation, at a meeting duly called and held pursuant to
Section 222 of the DGCL, duly adopted the amendments set forth in this Certificate of Amendment in accordance with the provisions
of Section 242 of the DGCL. |
| 5. |
The foregoing amendments were duly adopted in accordance with Section 242 of the DGCL. |
IN WITNESS WHEREOF, the undersigned,
as a duly authorized officer of the Corporation, has executed this Certificate of Amendment on ,
2024.
| |
Moderna,
Inc. |
|
| |
|
|
| |
Name:
Title: |
89



v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Pay vs Performance Disclosure - USD ($)
|
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
Dec. 31, 2020 |
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Pay vs Performance [Table Text Block] |
|
Pay Versus Performance
The following table reports the compensation
of our CEO (our Principal Executive Officer, or PEO) and the average compensation of the other Named Executive Officers (Other NEOs) as
reported in the Summary Compensation Table in our proxy statements for the past four years, as well as their “compensation actually
paid” (CAP), as calculated pursuant to SEC rules and certain performance measures required by the rules. The CAP values included
in the table below reflect a measure of compensation which is a combined realizable and realized pay measure predicated on fair value.
The grant date fair values included in the Summary Compensation Table (SCT) have been replaced with fair values reflecting the change
in value of equity awards during the fiscal year. The calculations do not reflect the actual sale of stock underlying equity awards or
the exercise of stock options by the executive.
| | |
| | |
| | |
| | |
Average
Compensation
Actually Paid
to Other
NEOs(2)
(e) | | |
Value of Initial
Fixed $100
Investment
Based on: | | |
| | |
Company
-Selected
Measure
[Product
Sales](4)(5)
(millions)
(i) | |
Year(1)
(a) | |
Summary
Compensation
Table Total
for PEO
(b) | | |
Compensation
Actually Paid
to PEO(2)
(c) | | |
Average SCT
Total for
Other NEOs
(d) | | |
| |
Moderna
TSR
(f) | | |
Peer
Group(3)
TSR
(g) | | |
Net Income
(millions)
(h) | | |
|
| 2023 | |
$ | 17,068,514 | | |
$ | (155,552,174) | | |
$ | 5,103,698 | | |
$ | (3,939,532) | | |
$ | 508 | | |
$ | 115 | | |
$ | (4,714) | | |
$ | 6,671 | |
| 2022 | |
| 19,363,648 | | |
| (306,219,630) | | |
| 5,822,260 | | |
| (8,967,479) | | |
| 918 | | |
| 111 | | |
| 8,362 | | |
| 18,435 | |
| 2021 | |
| 18,155,739 | | |
| 793,044,894 | | |
| 8,256,157 | | |
| 67,035,028 | | |
| 1,298 | | |
| 125 | | |
| 12,202 | | |
| 17,675 | |
| 2020 | |
| 12,855,275 | | |
| 469,967,605 | | |
| 5,286,409 | | |
| 54,985,594 | | |
| 534 | | |
| | |
| (747) | | |
| 200 | |
| (1) |
Mr.
Bancel served as our PEO in each year shown. The Other NEOs reflected in columns (d) and (e) above represent the following
individuals for each of the years shown: 2023 – Arpa Garay, Stephen Hoge, Shannon
Thyme Klinger and James Mock 2022 – Juan Andres, Arpa Garay, Jorge Gomez,
Stephen Hoge, David Meline and James Mock 2021 – Juan Andres, Stephen Hoge, Shannon Thyme
Klinger, David Meline and Corinne LeGoff 2020 – Juan Andres, Stephen Hoge, Lorence Kim,
David Meline and Tal Zaks |
| |
 |
|
| |
 |
|
| |
 |
|
| |
 |
|
| (2) | | The applicable Summary Compensation Table totals reported for the PEO and the average of
the Other NEOs for each year were subject to the following adjustments per Item 402(v)(2)(iii) of Regulation S-K to calculate “compensation
actually paid”: |
| |
|
|
| (3) |
Peer group TSR reflects the Nasdaq Biotechnology Index for all four fiscal years disclosed, which aligns with the peer group used in our Annual Report on 10-K for each of these years. |
| (4) |
The Company has identified Product Sales from COVID-19 vaccines as the company-selected measure for the pay versus performance disclosure, as it represents the most important financial performance measure used to link compensation actually paid to the PEO and the Other NEOs in 2023 to the Company’s performance. |
| (5) |
Product Sales was chosen from the following three most important financial performance measures used by the Company to link compensation actually paid to the PEO and Other NEOs in 2023 to the Company’s performance: |
| (1) |
Mr. Bancel served as our PEO in each year shown.
The Other NEOs reflected in columns (d) and (e) above represent the following individuals for each of the years shown: |
| |
• |
2023 – Arpa Garay, Stephen Hoge, Shannon
Thyme Klinger and James Mock |
| |
• |
2022 – Juan Andres, Arpa Garay, Jorge Gomez,
Stephen Hoge, David Meline and James Mock |
| |
• |
2021 – Juan Andres, Stephen Hoge, Shannon Thyme
Klinger, David Meline and Corinne LeGoff |
| |
• |
2020 – Juan Andres, Stephen Hoge, Lorence Kim,
David Meline and Tal Zaks |
| |
|
|
| |
Biographical information for each of these individuals and their positions can be
found above, beginning on page 33, or in the proxy statement for the year upon which compensation was reported, under the heading
“Management.” |
| (2) |
The applicable Summary Compensation Table totals reported for the PEO and the
average of the Other NEOs for each year were subject to the following adjustments per Item 402(v)(2)(iii) of Regulation S-K to calculate
“compensation actually paid”: |
| | |
2023 | | |
2022 | | |
2021 | | |
2020 | |
| | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | |
| Summary Compensation Table | |
$ | 17,068,514 | | |
$ | 5,103,698 | | |
$ | 19,363,648 | | |
$ | 5,822,260 | | |
$ | 18,155,739 | | |
$ | 8,256,157 | | |
$ | 12,855,275 | | |
$ | 5,286,409 | |
| Adjustments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Deduction for amounts reported under the “Stock Awards”
and “Option Awards” columns of the Summary Compensation Table(a) | |
| (12,516,907) | | |
| (3,546,372) | | |
| (14,389,009) | | |
| (4,285,485) | | |
| (15,000,000) | | |
| (6,600,000) | | |
| (9,000,000) | | |
| (4,220,000) | |
| Increase/(decrease)
for the Inclusion of Rule 402(v) Equity Values(a) | |
| (160,103,781) | | |
| (5,496,858) | | |
| (311,194,269) | | |
| (10,504,254) | | |
| 789,889,155 | | |
| 65,378,871 | | |
| 466,112,330 | | |
| 53,919,185 | |
| Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year | |
| 7,294,658 | | |
| 2,224,773 | | |
| 18,930,378 | | |
| 5,957,933 | | |
| 25,410,484 | | |
| 7,893,516 | | |
| 53,681,961 | | |
| 14,619,557 | |
| Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards | |
| (15,002,894) | | |
| (4,007,912) | | |
| (216,035,770) | | |
| (5,734,897) | | |
| 577,920,879 | | |
| 29,752,324 | | |
| 347,020,376 | | |
| 30,590,579 | |
| Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year | |
| (152,395,545) | | |
| (3,713,719) | | |
| (114,088,877) | | |
| (10,254,023) | | |
| 186,557,792 | | |
| 27,733,031 | | |
| 65,409,993 | | |
| 10,481,461 | |
| Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year | |
| — | | |
| — | | |
| — | | |
| (473,267) | | |
| — | | |
| — | | |
| — | | |
| (1,772,412) | |
Compensation
Actually Paid | |
| (155,552,174) | | |
| (3,939,532) | | |
| (306,219,630) | | |
| (8,967,479) | | |
| 793,044,894 | | |
| 67,035,028 | | |
| 469,967,605 | | |
| 54,985,594 | |
| |
(a) |
Compensation Actually Paid excludes the Stock Awards and Option Awards columns from the relevant fiscal year’s Summary Compensation Table total. The Rule 402(v) Equity Values instead reflect the aggregate of the following components, as applicable: (i) the fair value as of the end of the listed fiscal year of unvested equity awards granted in that year; (ii) the change in fair value during the listed fiscal year of equity awards granted in prior years that remained outstanding and unvested at the end of the listed fiscal year; and (iii) the change in fair value during the listed fiscal year through the vesting date of equity awards granted in prior years that vested during the listed fiscal year, less the fair value at the end of the prior year of awards granted prior to the listed fiscal year that failed to meet applicable vesting conditions during the listed fiscal year. Equity values are calculated in accordance with FASB ASC Topic 718. |
| |
|
|
| (3) |
Peer group TSR reflects the Nasdaq Biotechnology Index for all four fiscal years disclosed, which aligns with the peer group used in our Annual Report on 10-K for each of these years. |
| (4) |
The Company has identified Product Sales from COVID-19 vaccines as the company-selected measure for the pay versus performance disclosure, as it represents the most important financial performance measure used to link compensation actually paid to the PEO and the Other NEOs in 2023 to the Company’s performance. |
| (5) |
Product Sales was chosen from the following three most important financial performance measures used by the Company to link compensation actually paid to the PEO and Other NEOs in 2023 to the Company’s performance: |
| |
Performance
Metrics |
| |
Product Sales from
COVID-19 Vaccines |
Refer to “Compensation
Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described on page 48. |
| |
Operating
Income |
Refer
to “Compensation Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described
on page 48. |
| |
Total
Shareholder Return (TSR) |
Stock
options are the most heavily weighted equity vehicle in our long-term incentive program (75% for the PEO and 50% for the Other NEOs).
The value of these equity awards is directly related to stock price appreciation over time, which incentivizes our executives to
achieve our long-term strategic and pipeline goals to create shareholder value. Total Shareholder Return on a $100 investment in
Moderna stock over a four-year period ending December 31, 2023 would have been $508.44. |
|
|
|
|
| Company Selected Measure Name |
|
Product Sales from COVID-19 vaccines
|
|
|
|
| Named Executive Officers, Footnote [Text Block] |
|
| (1) |
Mr. Bancel served as our PEO in each year shown.
The Other NEOs reflected in columns (d) and (e) above represent the following individuals for each of the years shown: |
| |
• |
2023 – Arpa Garay, Stephen Hoge, Shannon
Thyme Klinger and James Mock |
| |
• |
2022 – Juan Andres, Arpa Garay, Jorge Gomez,
Stephen Hoge, David Meline and James Mock |
| |
• |
2021 – Juan Andres, Stephen Hoge, Shannon Thyme
Klinger, David Meline and Corinne LeGoff |
| |
• |
2020 – Juan Andres, Stephen Hoge, Lorence Kim,
David Meline and Tal Zaks |
|
|
|
|
| PEO Total Compensation Amount |
[1] |
$ 17,068,514
|
$ 19,363,648
|
$ 18,155,739
|
$ 12,855,275
|
| PEO Actually Paid Compensation Amount |
[1],[2] |
$ (155,552,174)
|
(306,219,630)
|
793,044,894
|
469,967,605
|
| Adjustment To PEO Compensation, Footnote [Text Block] |
|
| | |
2023 | | |
2022 | | |
2021 | | |
2020 | |
| | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | |
| Summary Compensation Table | |
$ | 17,068,514 | | |
$ | 5,103,698 | | |
$ | 19,363,648 | | |
$ | 5,822,260 | | |
$ | 18,155,739 | | |
$ | 8,256,157 | | |
$ | 12,855,275 | | |
$ | 5,286,409 | |
| Adjustments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Deduction for amounts reported under the “Stock Awards”
and “Option Awards” columns of the Summary Compensation Table(a) | |
| (12,516,907) | | |
| (3,546,372) | | |
| (14,389,009) | | |
| (4,285,485) | | |
| (15,000,000) | | |
| (6,600,000) | | |
| (9,000,000) | | |
| (4,220,000) | |
| Increase/(decrease)
for the Inclusion of Rule 402(v) Equity Values(a) | |
| (160,103,781) | | |
| (5,496,858) | | |
| (311,194,269) | | |
| (10,504,254) | | |
| 789,889,155 | | |
| 65,378,871 | | |
| 466,112,330 | | |
| 53,919,185 | |
| Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year | |
| 7,294,658 | | |
| 2,224,773 | | |
| 18,930,378 | | |
| 5,957,933 | | |
| 25,410,484 | | |
| 7,893,516 | | |
| 53,681,961 | | |
| 14,619,557 | |
| Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards | |
| (15,002,894) | | |
| (4,007,912) | | |
| (216,035,770) | | |
| (5,734,897) | | |
| 577,920,879 | | |
| 29,752,324 | | |
| 347,020,376 | | |
| 30,590,579 | |
| Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year | |
| (152,395,545) | | |
| (3,713,719) | | |
| (114,088,877) | | |
| (10,254,023) | | |
| 186,557,792 | | |
| 27,733,031 | | |
| 65,409,993 | | |
| 10,481,461 | |
| Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year | |
| — | | |
| — | | |
| — | | |
| (473,267) | | |
| — | | |
| — | | |
| — | | |
| (1,772,412) | |
Compensation
Actually Paid | |
| (155,552,174) | | |
| (3,939,532) | | |
| (306,219,630) | | |
| (8,967,479) | | |
| 793,044,894 | | |
| 67,035,028 | | |
| 469,967,605 | | |
| 54,985,594 | |
| |
(a) |
Compensation Actually Paid excludes the Stock Awards and Option Awards columns from the relevant fiscal year’s Summary Compensation Table total. The Rule 402(v) Equity Values instead reflect the aggregate of the following components, as applicable: (i) the fair value as of the end of the listed fiscal year of unvested equity awards granted in that year; (ii) the change in fair value during the listed fiscal year of equity awards granted in prior years that remained outstanding and unvested at the end of the listed fiscal year; and (iii) the change in fair value during the listed fiscal year through the vesting date of equity awards granted in prior years that vested during the listed fiscal year, less the fair value at the end of the prior year of awards granted prior to the listed fiscal year that failed to meet applicable vesting conditions during the listed fiscal year. Equity values are calculated in accordance with FASB ASC Topic 718. |
|
|
|
|
| Non-PEO NEO Average Total Compensation Amount |
[1] |
$ 5,103,698
|
5,822,260
|
8,256,157
|
5,286,409
|
| Non-PEO NEO Average Compensation Actually Paid Amount |
[1],[2] |
$ (3,939,532)
|
(8,967,479)
|
67,035,028
|
54,985,594
|
| Adjustment to Non-PEO NEO Compensation Footnote [Text Block] |
|
| | |
2023 | | |
2022 | | |
2021 | | |
2020 | |
| | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | | |
PEO | | |
Average for
Other NEOs | |
| Summary Compensation Table | |
$ | 17,068,514 | | |
$ | 5,103,698 | | |
$ | 19,363,648 | | |
$ | 5,822,260 | | |
$ | 18,155,739 | | |
$ | 8,256,157 | | |
$ | 12,855,275 | | |
$ | 5,286,409 | |
| Adjustments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Deduction for amounts reported under the “Stock Awards”
and “Option Awards” columns of the Summary Compensation Table(a) | |
| (12,516,907) | | |
| (3,546,372) | | |
| (14,389,009) | | |
| (4,285,485) | | |
| (15,000,000) | | |
| (6,600,000) | | |
| (9,000,000) | | |
| (4,220,000) | |
| Increase/(decrease)
for the Inclusion of Rule 402(v) Equity Values(a) | |
| (160,103,781) | | |
| (5,496,858) | | |
| (311,194,269) | | |
| (10,504,254) | | |
| 789,889,155 | | |
| 65,378,871 | | |
| 466,112,330 | | |
| 53,919,185 | |
| Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year | |
| 7,294,658 | | |
| 2,224,773 | | |
| 18,930,378 | | |
| 5,957,933 | | |
| 25,410,484 | | |
| 7,893,516 | | |
| 53,681,961 | | |
| 14,619,557 | |
| Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards | |
| (15,002,894) | | |
| (4,007,912) | | |
| (216,035,770) | | |
| (5,734,897) | | |
| 577,920,879 | | |
| 29,752,324 | | |
| 347,020,376 | | |
| 30,590,579 | |
| Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year | |
| (152,395,545) | | |
| (3,713,719) | | |
| (114,088,877) | | |
| (10,254,023) | | |
| 186,557,792 | | |
| 27,733,031 | | |
| 65,409,993 | | |
| 10,481,461 | |
| Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year | |
| — | | |
| — | | |
| — | | |
| (473,267) | | |
| — | | |
| — | | |
| — | | |
| (1,772,412) | |
Compensation
Actually Paid | |
| (155,552,174) | | |
| (3,939,532) | | |
| (306,219,630) | | |
| (8,967,479) | | |
| 793,044,894 | | |
| 67,035,028 | | |
| 469,967,605 | | |
| 54,985,594 | |
| |
(a) |
Compensation Actually Paid excludes the Stock Awards and Option Awards columns from the relevant fiscal year’s Summary Compensation Table total. The Rule 402(v) Equity Values instead reflect the aggregate of the following components, as applicable: (i) the fair value as of the end of the listed fiscal year of unvested equity awards granted in that year; (ii) the change in fair value during the listed fiscal year of equity awards granted in prior years that remained outstanding and unvested at the end of the listed fiscal year; and (iii) the change in fair value during the listed fiscal year through the vesting date of equity awards granted in prior years that vested during the listed fiscal year, less the fair value at the end of the prior year of awards granted prior to the listed fiscal year that failed to meet applicable vesting conditions during the listed fiscal year. Equity values are calculated in accordance with FASB ASC Topic 718. |
|
|
|
|
| Compensation Actually Paid vs. Total Shareholder Return [Text Block] |
|
2. CAP versus Company TSR

|
|
|
|
| Compensation Actually Paid vs. Net Income [Text Block] |
|
3. CAP versus Net Income

|
|
|
|
| Compensation Actually Paid vs. Company Selected Measure [Text Block] |
|
4. CAP versus Company Selected Metric (CSM) – Product
Sales from COVID-19 Vaccines

|
|
|
|
| Total Shareholder Return Vs Peer Group [Text Block] |
|
1. TSR: Company versus Peer Group

|
|
|
|
| Tabular List [Table Text Block] |
|
| |
Performance
Metrics |
| |
Product Sales from
COVID-19 Vaccines |
Refer to “Compensation
Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described on page 48. |
| |
Operating
Income |
Refer
to “Compensation Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described
on page 48. |
| |
Total
Shareholder Return (TSR) |
Stock
options are the most heavily weighted equity vehicle in our long-term incentive program (75% for the PEO and 50% for the Other NEOs).
The value of these equity awards is directly related to stock price appreciation over time, which incentivizes our executives to
achieve our long-term strategic and pipeline goals to create shareholder value. Total Shareholder Return on a $100 investment in
Moderna stock over a four-year period ending December 31, 2023 would have been $508.44. |
|
|
|
|
| Total Shareholder Return Amount |
[1] |
$ 508
|
918
|
1,298
|
534
|
| Peer Group Total Shareholder Return Amount |
[1],[3] |
115
|
111
|
125
|
126
|
| Net Income (Loss) Attributable to Parent |
[1] |
$ (4,714,000,000)
|
$ 8,362,000,000
|
$ 12,202,000,000
|
$ (747,000,000)
|
| Company Selected Measure Amount |
[1],[4],[5] |
6,671,000,000
|
18,435,000,000
|
17,675,000,000
|
200,000,000
|
| PEO Name |
|
Mr. Bancel
|
Mr. Bancel
|
Mr. Bancel
|
Mr. Bancel
|
| Measure [Axis]: 1 |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Measure Name |
|
Product Sales from
COVID-19 Vaccines
|
|
|
|
| Measure [Axis]: 2 |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Measure Name |
|
Operating
Income
|
|
|
|
| Measure [Axis]: 3 |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Measure Name |
|
Total
Shareholder Return (TSR)
|
|
|
|
| PEO [Member] | Deduction for amounts reported under the “Stock Awards” and “Option Awards” columns of the Summary Compensation Table |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
[6] |
$ (12,516,907)
|
$ (14,389,009)
|
$ (15,000,000)
|
$ (9,000,000)
|
| PEO [Member] | Increase/(decrease) for the Inclusion of Rule 402(v) Equity Values |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
[6] |
(160,103,781)
|
(311,194,269)
|
789,889,155
|
466,112,330
|
| PEO [Member] | Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
7,294,658
|
18,930,378
|
25,410,484
|
53,681,961
|
| PEO [Member] | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
(15,002,894)
|
(216,035,770)
|
577,920,879
|
347,020,376
|
| PEO [Member] | Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
(152,395,545)
|
(114,088,877)
|
186,557,792
|
65,409,993
|
| PEO [Member] | Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
|
|
|
| Non-PEO NEO [Member] | Deduction for amounts reported under the “Stock Awards” and “Option Awards” columns of the Summary Compensation Table |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
[6] |
(3,546,372)
|
(4,285,485)
|
(6,600,000)
|
(4,220,000)
|
| Non-PEO NEO [Member] | Increase/(decrease) for the Inclusion of Rule 402(v) Equity Values |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
[6] |
(5,496,858)
|
(10,504,254)
|
65,378,871
|
53,919,185
|
| Non-PEO NEO [Member] | Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
2,224,773
|
5,957,933
|
7,893,516
|
14,619,557
|
| Non-PEO NEO [Member] | Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
(4,007,912)
|
(5,734,897)
|
29,752,324
|
30,590,579
|
| Non-PEO NEO [Member] | Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
(3,713,719)
|
(10,254,023)
|
27,733,031
|
10,481,461
|
| Non-PEO NEO [Member] | Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year |
|
|
|
|
|
| Pay vs Performance Disclosure [Table] |
|
|
|
|
|
| Adjustment to Compensation Amount |
|
|
$ (473,267)
|
|
$ (1,772,412)
|
|
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_AdjToCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_AdjToNonPeoNeoCompFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_AdjToPeoCompFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph vi
| Name: |
ecd_CoSelectedMeasureAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:decimalItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph vi
| Name: |
ecd_CoSelectedMeasureName |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph iii
| Name: |
ecd_CompActuallyPaidVsCoSelectedMeasureTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph ii
| Name: |
ecd_CompActuallyPaidVsNetIncomeTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph i
| Name: |
ecd_CompActuallyPaidVsTotalShareholderRtnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph vi
| Name: |
ecd_MeasureName |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_NamedExecutiveOfficersFnTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iii
| Name: |
ecd_NonPeoNeoAvgCompActuallyPaidAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph ii
| Name: |
ecd_NonPeoNeoAvgTotalCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iv
| Name: |
ecd_PeerGroupTotalShareholderRtnAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iii
| Name: |
ecd_PeoActuallyPaidCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 3
| Name: |
ecd_PeoName |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph ii
| Name: |
ecd_PeoTotalCompAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTableTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 6
| Name: |
ecd_TabularListTableTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 2
-Subparagraph iv
| Name: |
ecd_TotalShareholderRtnAmt |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 5
-Subparagraph iv
| Name: |
ecd_TotalShareholderRtnVsPeerGroupTextBlock |
| Namespace Prefix: |
ecd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ecd_MeasureAxis=1 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_MeasureAxis=2 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_MeasureAxis=3 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_ExecutiveCategoryAxis=ecd_PeoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=mrna_DeductionForAmountsReportedUnderTheStockAwardsAndOptionAwardsColumnsOfTheSummaryCompensationTableMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=mrna_IncreaseDecreaseForTheInclusionOfRule402vEquityValuesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=mrna_YearEndFairValueOfEquityAwardsGrantedDuringYearThatRemainedUnvestedAsOfLastDayOfYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=mrna_ChangeInFairValueFromLastDayOfPriorYearToLastDayOfYearOfUnvestedEquityAwardsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=mrna_ChangeInFairValueOfPriorYearsEquityAwardsThatVestedDuringTheYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_AdjToCompAxis=mrna_ChangeInFairValueOfPriorYearsEquityAwardsThatForfeitedDuringTheYearMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ecd_ExecutiveCategoryAxis=ecd_NonPeoNeoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Oct 2024 to Nov 2024
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Nov 2023 to Nov 2024