0001643918
false
0001643918
2023-10-06
2023-10-06
0001643918
dei:BusinessContactMember
2023-10-06
2023-10-06
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
AS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION
ON OCTOBER 6, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Biodexa Pharmaceuticals PLC
(Exact name of registrant as specified in its
charter)
| England and Wales |
2834 |
Not Applicable |
|
(State or Other Jurisdiction of
Incorporation or Organization) |
(Primary Standard Industrial
Classification Code Number) |
(IRS Employer
Identification No.) |
1 Caspian Point
Caspian Way
Cardiff, CF10 4DQ, United Kingdom
Tel: +44 29 20480 180
(Address, including zip code, and telephone
number, including area code, of registrant’s principal executive offices)
Donald J. Puglisi
Puglisi & Associates
850 Library Ave., Suite 204
Newark, Delaware 19711
Tel: (302) 738-6680
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
Copies of communications to:
| Jason S. McCaffrey |
Michael F. Nertney |
| Mintz, Levin, Cohn, Ferris, Glovsky & Popeo, P.C. |
Ellenoff Grossman & Schole LLP |
| One Financial Center |
1345 Avenue of the Americas |
| Boston, Massachusetts 02111 |
New York, New York 10105 |
| Telephone: (617) 542-6000 |
Telephone: (212) 370-1300 |
| Facsimile: (617) 542-2241 |
Facsimile: (212) 370-7889 |
Approximate date of commencement of proposed
sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this
Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities
for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed
pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ¨
If an emerging growth company that prepares its
financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition
period for complying with any new or revised financial accounting standard provided pursuant to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant hereby amends this Registration Statement on such
date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states
that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until
the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary
prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities
and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting an
offer to buy these securities in any state or jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS—SUBJECT
TO COMPLETION, DATED OCTOBER 6, 2023

Class A Units consisting of American Depositary
Shares Representing Ordinary Shares and Series E Warrants and
Class B Units consisting of Pre-Funded Warrants
to purchase American Depositary Shares and Series E Warrants (and American Depositary Shares Representing Ordinary Shares Underlying Pre-Funded
Warrants and Series E Warrants)
___________________
This preliminary prospectus,
or prospectus, relates to the offering of Class A Units, or Class A Units, of Biodexa Pharmaceuticals PLC, a public limited
company organized under the laws of England and Wales, or the Company, at an assumed public offering price of $ per Class A Unit,
the last reported sales price of our American Depositary Shares, or Depositary Shares, on the NASDAQ Capital Market on , 2023. Each Class A
Unit consists of one Depositary Share (representing 400 of our ordinary shares, nominal value £0.001 per share, or Ordinary Shares)
and one Series E warrant to purchase one Depositary Share at an exercise price of $ per share,
which will expire on the five-year anniversary of the initial exercise date, or a Series E Warrant.
We are also offering to those
purchasers, if any, whose purchase of Class A Units in this offering would otherwise result in such purchaser, together with its
affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding
Ordinary Shares immediately following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses,
Class B Units, in lieu of Class A Units that would otherwise result in such purchaser’s beneficial ownership exceeding
4.99% (or, at the election of the purchaser, 9.99%) of our outstanding Ordinary Shares. Each Class B Unit consists of one pre-funded
warrant, or Pre-Funded Warrants, exercisable for one Depositary Share and a Series E Warrant (together with the Depositary Shares
representing Ordinary Shares underlying such Pre-Funded Warrants and Series E Warrants, the Class B Units, and, together with the
Class A Units, the units) at an assumed public offering price of $ per Class B Unit, the closing price of our Depositary Shares
on , 2023, minus $0.0001, and the exercise price of each Pre-Funded Warrant included in the Class B Units will be $0.0001 per share. The
Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are exercised
in full. For each Class B Unit we sell, the number of Class A Units we are offering will be decreased on a one-for-one basis.
The Class A Units and
the Class B Units have no stand-alone rights and will not be issued or certificated as stand-alone securities. The Depositary Shares,
Pre-Funded Warrants and Series E Warrants comprising such units are immediately separable and will be issued separately in this offering.
The Depositary Shares or Pre-Funded Warrants, as the case may be, and the Series E Warrants included in the Class A Units and
the Class B Units can only be purchased together in this offering, but the securities contained in the Class A Units or Class B
Units will be immediately separable upon issuance and will be issued separately. Because we will issue one Series E Warrant for each Class
A Unit and for each Class B Unit sold in this offering, the number of Series E Warrants sold in this offering will not change as a result
of a change in the number of Class A Units and Class B Units sold. The Depositary Shares issuable from time to time upon exercise of the
Pre-Funded Warrants and Series E Warrants are also being offered by this prospectus.
Our Depositary Shares are
listed on the NASDAQ Capital Market under the symbol “BDRX”. We have assumed a public offering price of $ per Class A Unit,
which represents the last reported sale price of our Depositary Shares as reported on the NASDAQ Capital Market on , 2023. The final public
offering price will be determined through negotiation between us and the underwriters in the offering and may be at a discount to the
current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final
public offering price.
There is no established trading
market for the Pre-Funded Warrants or Series E Warrants being offered, and we do not expect a market to develop. In addition, we do not
intend to apply for the listing of the Pre-Funded Warrants or the Series E Warrants on any national securities exchange or other trading
market. Without an active trading market, the liquidity of the warrants will be limited.
We are also a “foreign
private issuer,” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act, and are exempt from certain
rules under the Exchange Act that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section
14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing”
profit recovery provisions under Section 16 of the Exchange Act. Moreover, we are not required to file periodic reports and financial
statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
Investing in our
securities involves risks. See “Risk Factors” beginning on page 11 of this prospectus for a discussion of the factors
you should carefully consider before deciding to purchase these securities.
___________________
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
| |
|
Per
Class A
Unit(1) |
|
|
Per Class B
Unit(2) |
|
|
Total |
|
| Public offering price |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Underwriter discounts and commissions(3) |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
| Proceeds, before expenses, to us |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
________
| (1) | The public offering price and underwriting discount corresponds, in respect of the Class A Units,
to (i) a public offering price per Depositary Share of $ ($
net of the underwriting discount) and (ii) a public offering price per Series E Warrant of $ ($
net of the underwriting discount). |
| (2) | The public offering price and underwriting discount in respect of the Class B Units corresponds to
(i) a public offering price per Pre-Funded Warrant of $ ($
net of the underwriting discount) and (ii) a public offering price per Series E Warrant of $ ($
net of the underwriting discount). |
| (3) | We have agreed to pay certain expenses of the underwriters in this offering. We refer you to “Underwriting”
on page 100 for additional information regarding underwritten compensation. |
The offering is being
underwritten on a firm commitment basis. We have granted a 45-day option to the underwriters to purchase up to an additional
Depositary Shares and/or Series E Warrants to purchase an additional Depositary Shares, each at its respective public offering
price, less the underwriting discounts payable by us, to cover over-allotments, if any. The option may be used to purchase
Depositary Shares and/or Series E Warrants, or any combination thereof, as determined by the underwriters.
The underwriters expect to
deliver the securities to investors on or about , 2023.
Sole Book Running Manager
Ladenburg Thalmann
The date of this prospectus is
, 2023
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of
a registration statement that we filed with the Securities and Exchange Commission, or SEC. As permitted by the rules and regulations
of the SEC, the registration statement filed by us includes additional information not contained in this prospectus. You may read the
registration statement and the other reports we file with the SEC at the SEC’s website or its offices described below under the
headings “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference.”
This prospectus does not constitute
an offer to sell or the solicitation of an offer to buy any securities other than the securities described in this prospectus or an offer
to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You
should assume that the information appearing in this prospectus, the documents incorporated by reference and any related free writing
prospectus is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have
changed materially since those dates.
Neither we nor the underwriters
have authorized anyone to provide you with any information or to make any representations other than that contained in this prospectus
or in any free writing prospectus we may authorize to be delivered or made available to you. We take no responsibility for, and can provide
no assurance as to the reliability of, any other information that others may give you. Neither we nor the underwriters are making an offer
to sell securities in any jurisdiction in which the offer or sale is not permitted. The information in this prospectus is accurate only
as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our securities and the information
in any free writing prospectus that we may provide to you in connection with this offering is accurate only as of the date of that free
writing prospectus. Our business, financial condition, results of operations and prospects may have changed since those dates.
To the extent there is a conflict
between the information contained in this prospectus, on the one hand, and the information contained in any document incorporated by reference
in this prospectus, on the other hand, you should rely on the information in this prospectus, provided that if any statement in one of
these documents is inconsistent with a statement in another document having a later date — for example, a document
incorporated by reference in this prospectus — the statement in the document having the later date modifies or supersedes
the earlier statement.
We further note that the representations,
warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein
were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among
the parties to such agreement, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should
not be relied on as accurately representing the current state of our affairs.
Unless the context specifically
indicates otherwise, references in this prospectus to “Biodexa Pharmaceuticals plc,” “Biodexa,” “the Company,”
“we,” “our,” “ours,” “us,” “the Group,” or similar terms refer to Biodexa
Pharmaceuticals plc and its consolidated subsidiaries.
For investors outside the
United States: We have not, and the underwriters have not, taken any action to permit this offering, or to permit the possession or distribution
of this prospectus, in any jurisdiction where action for that purpose is required, other than the United States. Persons outside the United
States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to the offering
of the Depositary Shares and the distribution of this prospectus outside of the United States.
PRESENTATION OF FINANCIAL AND OTHER INFORMATION
Our
financial statements are prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting
Standards Board and adopted by the European Union. We have made rounding adjustments to some of the figures included in this prospectus.
Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
We
prepare our consolidated financial statements in British pounds sterling. Except as otherwise stated, all monetary amounts in this prospectus
are presented in British pounds sterling.
In this prospectus, unless
otherwise specified or the context otherwise requires:
| · | “$” and “U.S. dollar” each refer to the United States dollar (or units thereof);
and |
| · | “£,” “pence” and “p” each refer to the British pound sterling
(or units thereof). |
On March 27, 2023, following
shareholder approval, we effected a one-for-20 reverse split of our ordinary shares, nominal value £0.02 per share, or Ordinary
Shares, and our Ordinary Shares began trading on AIM, a market operated by the London Stock Exchange plc, or AIM, on a split-adjusted
basis as of such date. No fractional shares were issued in connection with the reverse stock split. On March 24, 2023, our shareholders
approved the cancellation of admission of our Ordinary Shares on AIM and this cancellation became effective on April 26, 2023.
Concurrently with the reverse
split, and in order to continue meeting The NASDAQ Stock Market LLC’s, or NASDAQ, minimum 500,000 publicly held shares requirement
pursuant to Rule 5550(a)(4), on March 27, 2023 we effected a ratio change in the number of Ordinary Shares represented by the Depositary
Shares from 25 Ordinary Shares per Depositary Share to five Ordinary Shares per Depositary Share.
On July 5, 2023, and in an
effort to bring our Depositary Share price into compliance with NASDAQ’s minimum bid price per share requirement, we effected a
ratio change in the number of Ordinary Shares represented by our Depositary Shares from five Ordinary Shares per Depositary Share to 400
Ordinary Shares per Depositary Share. No fractional Depositary Shares were issued.
The change in the number of
Ordinary Shares resulting from the reverse stock split and change in the number of Depositary Shares (and the underlying Ordinary Shares)
resulting from the change in ratio, including any changes resulting from fractional Depositary Shares not being issued to holders in connection
with the Depositary Share ratio change, has been applied retroactively to all share and per share amounts presented in this prospectus,
to the extent applicable. As a result of retroactively applying changes resulting from fractional Depositary Shares not being issued to
holders in connection with the Depositary Share ratio change, the amount of Ordinary Shares issued in prior transactions may not equal
the amount of Depositary Shares such Depositary Shares are currently exercisable for.
MARKET AND INDUSTRY DATA
Market data and certain industry
data and forecasts used throughout this prospectus were obtained from sources we believe to be reliable, including market research databases,
publicly available information, reports of governmental agencies, and industry publications and surveys. We have relied on certain data
from third-party sources, including internal surveys, industry forecasts, and market research, which we believe to be reliable based on
our management's knowledge of the industry. While we are not aware of any misstatements regarding the industry data presented in this
prospectus, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed
under the heading “Risk Factors” and elsewhere in this prospectus.
Solely
for convenience, the trademarks and trade names in this prospectus may be referred to without the ® and ™ symbols, but such
references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable
law, their rights thereto. The trademarks, trade names and service marks in this prospectus are the property of their respective owners.
PROSPECTUS SUMMARY
This summary highlights
information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your
investment decision. Before deciding to invest in our securities, you should read this entire prospectus and the documents incorporated
by reference herein and therein carefully, including our financial statements and related notes, the information in the section “Risk
Factors,” “Where You Can Find More Information” and “Incorporation of Certain Documents by Reference.”
Company Overview
We
are a clinical stage biopharmaceutical company developing a pipeline of products aimed at primary and metastatic cancers of the brain.
Our lead candidate, MTX110, is being studied in aggressive rare/orphan brain cancer indications including recurrent glioblastoma and diffuse
midline glioma.
MTX110
is a liquid formulation of the histone deacetylase, or HDAC, inhibitor, panobinostat. Our proprietary formulation enables delivery
of the product via convection-enhanced delivery, or CED, at potentially chemotherapeutic doses directly to the site of the tumor, by-passing
the blood-brain barrier and avoiding systemic toxicity.
Our
clinical assets are supported by three proprietary drug delivery technologies focused on improving bio-delivery and bio-distribution of
drugs through either sustained delivery (Q-SpheraTM), direct delivery (MidaSolveTM), or targeted delivery (MidaCoreTM):
| |
· |
Our Q-Sphera platform: Our disruptive polymer microsphere microtechnology is used for sustained delivery to prolong and control the release of therapeutics over an extended period of time, from weeks to months. |
| |
· |
Our MidaSolve platform: Our innovative oligosaccharide nanotechnology is used to solubilize drugs so that they can be administered in liquid form directly and locally into tumors. |
| |
· |
MidaCore platform: Our gold nanoparticle nanotechnology is used for targeting sites of disease by using either chemotherapeutic agents or immunotherapeutic agents. |
Our Strategy
In
early 2023, we decided to re-position the Company as a therapeutics (as opposed to drug delivery) company. As a result, the delivery of
proof-of-concept clinical data is the primary focus of our business model going forward.
We
aim to deploy our proprietary technologies to develop proof of concept formulations and then enter into licensing agreements with third
party pharmaceutical companies.
Development
Our
intention is to build a balanced portfolio of clinical-stage development assets, ideally focused on oncology and on rare or orphan indications.
Our only current clinical-stage asset, MTX110, is in Phase I development for three rare or orphan brain cancers.
Our
research and development programs may, like MTX110, be based on one or more of our enabling technologies.
Our
aim is to enter into research and development collaborations with third parties to develop proof-of-concept formulations of their proprietary
compounds using our proprietary drug delivery technologies. We do not intend to expand our internal pipeline of drug delivery based programs.
Manufacturing
To
establish proof-of-concept in pre-clinical studies for potential licensees, we are able to manufacture non-good manufacturing practice,
or GMP, Q-Sphera products at pilot scale at our Cardiff, Wales facility. Our intention is to technology transfer the manufacturing of
clinical trial supplies and, ultimately, full GMP commercial manufacture to a third party contract manufacturing organizations. We would
expect a licensee to assume the cost of manufacturing GMP product and commercial scale-up pursuant to a technology transfer agreement.
MTX110
is currently being manufactured to GMP standards at a contract manufacturing organization.
Commercialization
Once
proof-of-concept has been established, we intend to license our products to a partner who would complete the development of, and subsequently
market and sale, the product in an agreed upon licensed territory. In addition to reimbursement of development costs, the partner would
be expected to make milestone payments based on sales targets and royalty payments.
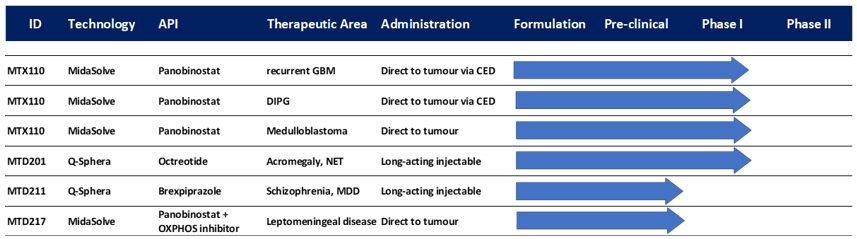
Our Pipeline and Platform Technologies
We
are actively pursuing the development of MTX110 in three Phase I studies. Our other product candidates, MTD201 (Q-octreotide) and MTD211
(Q-brexpiprazole) are not being actively developed but are available for licensing. MTD217 is an internal preclinical research program
recently added to our portfolio.
Our
development pipeline includes six projects, as follows:
Recent Developments
MTX110 Developments
On June 1, 2022, we announced
that upon submitting an application to the United States Food and Drug Administration, or the FDA, our development program of MTX110 for
the treatment of recurrent glioblastoma had been granted Fast Track designation by the agency. Fast Track is a process designed to facilitate
the development and expedite the review of treatments for serious conditions and that potentially address unmet medical needs. Drugs that
are granted this designation are given the opportunity for more frequent interactions with the FDA, as well as potential pathways for
expedited approval.
On January 12, 2023, we announced
that, following completion of one-month treatment with MTX110 in our first patient, our Phase I study of MTX110 in recurrent glioblastoma
(also known as the MAGIC-G1 study) would continue with a planned dose escalation following positive recommendation from the study’s
Data Safety Monitoring Board, or DSMB. MAGIC-G1 is an open-label, dose escalation study designed to assess the feasibility and safety
of intermittent infusions of MTX110 administered by CED via implanted refillable pump and catheter. The study aims to recruit two cohorts
(cohort A and cohort B), each with a minimum of four patients; the first cohort will receive MTX110 following implantation of the CED
system and the second cohort will also receive MTX110 but with the option of the treating investigator to re-position the catheter into
an area of new lesion upon progression, with the objective of increasing tumor coverage and survival.
The first patient in cohort
A was dosed at 60uM of MTX110 via direct-to-tumor delivery and has received 13 48-hour infusions over a period of 19 weeks. No treatment-associated
adverse events were noted in the patient during this period. Following successful completion of the first month of treatment, the DSMB
reviewed the available data and recommended dose escalation in the study to 90uM, which we believe is the optimal dose. To date, there
have been no dose-limiting toxicities.
On October 3, 2023, we announced
the completion of recruitment into cohort A with the minimum number of four patients. Enrollment in cohort B is expected to commence upon
the approval of the study by the DMSB, which is expected towards the end of October 2023.
On July 10, 2023, we announced
the completion of enrollment and the treatment of nine pediatric patients with diffuse midline glioma, or DMG, in the ongoing Phase I
study of MTX110 at Columbia University Irving Medical Center. All of the patients, aged four to 17 years old, received radiation therapy
as per the institutions’ standard of care. Each patient subsequently underwent surgery with implantation of an intratumoral catheter
and a programmable subcutaneous pump and eight out of nine received two infusions of MTX110 via CED separated by a period of one week.
Concentrations of 30, 60 or 90 µM were delivered with no intra-patient dose escalation. To date, no dose limiting toxicities related
to the study drug have been reported. We expect full results to be announced in the first quarter of 2024.
Change
in Ratio of Depositary Shares
On
July 5, 2023, in an effort to bring our Depositary Share price into compliance with NASDAQ’s minimum bid price per share requirement,
we effected a ratio change in the number of Ordinary Shares represented by our Depositary Shares from five Ordinary Shares per Depositary
Share to 400 Ordinary Shares per Depositary Share. This had the effect of a one-for-80 reverse split of the Depositary Shares. No
fractional Depositary Shares were issued in connection with the ratio change.
Regained Compliance with NASDAQ Continued
Listing Requirements
Our Depositary Shares are
currently listed on the NASDAQ Capital Market. We are required to meet certain qualitative and financial tests to maintain the listing
of our Depositary Shares on NASDAQ. On January 31, 2023, we received a letter from NASDAQ stating that, for the previous 30 consecutive
business days, the bid price for our Depositary Shares had closed below the minimum $1.00 bid price per share requirement for continued
listing on the NASDAQ Capital Market under NASDAQ Listing Rule 5550(a)(2). On July 20, 2023, we received written notice from NASDAQ notifying
us that for the preceding 10 consecutive business days the closing bid price for our Depositary Shares had been $1.00 or greater. Accordingly,
NASDAQ determined that we had regained compliance with NASDAQ Listing Rule 5550(a)(2).
NASDAQ further notified us
that we are subject to a mandatory panel monitor for a period of one year from July 20, 2023. If, within that one-year monitoring period,
the NASDAQ Listing Qualifications Staff finds us out of compliance with the rules that was subject to the previous exception, the Listing
Qualifications Staff will issue a delisting determination letter and we will have the opportunity to request a hearing with the Nasdaq
Hearings Panel.
Having regained compliance
with these rules, we are now in compliance with all applicable requirements for continued listing on the NASDAQ Capital Market. We cannot
assure you that we will remain in compliance with all applicable requirements for continued listing on the NASDAQ Capital Market.
June
AGM and June GM
On
June 14, 2023, we held our annual general meeting of shareholders, or June AGM, and our shareholders passed resolutions, among other procedural
items, to approve the allotment of, and disapplication of pre-emption rights in respect of, up to 7.0 billion Ordinary Shares, or Shareholder
Approval. On June 14, 2023, we also held a general meeting of shareholders, or June GM, and our shareholders passed resolutions to (x)(i)
re-designate our deferred shares into A Deferred Shares, or the Re-Designation, and (ii) subdivide our Ordinary Shares of £0.02
nominal value each into one ordinary share of £0.001 nominal value and 19 B Deferred Shares of £0.001 nominal value each,
each the Subdivision, which became effective on June 15, 2023 and (y) adopt new articles of association, or the Articles of Association,
which make consequential amendments to the existing articles of association of the Company to reflect the Re-Designation and the Subdivision,
together with certain other changes to reflect that the Ordinary Shares are no longer admitted to trading on AIM. As is standard for deferred
shares, each B Deferred Share has very limited rights and is effectively valueless. The B Deferred Shares have the rights and restrictions
as set out in the Articles of Association and do not entitle the holder thereof to receive notice of or attend and vote at any general
meeting of the Company or to receive a dividend or other distribution.
May 2023
Registered Direct Offering
On
May 26, 2023, we completed the closing of a registered direct offering, or the May 2023 Registered Direct Offering, with institutional
investors, or the Investors, for the sale of 110,679,610 Ordinary Shares represented by 279,699 Depositary Shares at a price per Depositary
Share of $12.00, for aggregate gross proceeds of $3.32 million.
Pursuant
to the terms of that certain securities purchase agreement entered into in connection with the May 2023 Offering, as defined below, or
Purchase Agreement, following Shareholder Approval on June 14, 2023, on June 20, 2023, we issued to the Investors (i) Series C Warrants
exercisable for an aggregate of 415,043 Depositary Shares representing 166,017,300 Ordinary Shares and (ii) Series D Warrants exercisable
for an aggregate of 279,699 Depositary Shares representing 110,679,610 Ordinary Shares, or the Warrant Private Placement, and together
with the May 2023 Registered Direct Offering, the May 2023 Offering. The warrants were exercisable at an exercise price of $16.00 per
Depositary Share, subject to adjustments for certain dilutive equity issuances. The warrants were exercisable upon receipt of Shareholder
Approval. The Series C Warrants will expire one year from the initial exercise date and may be exercised on a cashless basis, subject
to the satisfaction of payment of not less than the nominal value of the ordinary shares under the provisions of the United Kingdom Companies
Act of 2006, or the Companies Act. The Series D Warrants will expire five years from the initial exercise date. A holder of the warrants
may not exercise the warrants if the holder, together with its affiliates, would beneficially own more than 4.99% or 9.99% (such amount
to be determined at the option of the holder) of the number of Ordinary Shares outstanding immediately after giving effect to such exercise.
The Purchase Agreement contains customary representations, warranties and covenants of the Company and each Investor, and customary indemnification
provisions for a transaction of this type, including taking reasonable steps to maintain the Depositary Shares listed on NASDAQ.
Pursuant
to the terms and conditions set forth in that certain Placement Agency Agreement, dated as of May 23, 2023 and entered into in connection
with the May 2023 Offering, or the Placement Agent Agreement, by and between the Company and Ladenburg Thalmann & Co. Inc., or the
Placement Agent, we agreed to pay the Placement Agent a cash fee in an amount equal to 8.0% of the aggregate gross proceeds of the May
2023 Offering, and to issue to the Placement Agent, warrants to purchase Depositary Shares equal to 4.0% of the total Depositary Shares
(or Depositary Share equivalents) issued in the connection with the May 2023 Offering, or the May 2023 Placement Agent Warrants. Following
Shareholder Approval on June 14, 2023, we issued to the Placement Agent May 2023 Placement Agent Warrants having substantially the same
terms as the Series D Warrants, except that the exercise price of the May 2023 Placement Agent Warrant is 125% of the offering price of
the Depositary Shares issued in the May 2023 Registered Direct Offering and the term of the May 2023 Placement Agent Warrants terminates
on the three-year anniversary of the initial exercise date as defined in the May 2023 Placement Agent Warrant. We also agreed to pay the
Placement Agent a management fee equal to 1.0% of the gross proceeds raised in the May 2023 Offering and an expense allowance of up to
$85,000 for legal fees and other out-of-pocket expenses.
Additionally,
pursuant to the terms of the Purchase Agreement, we agreed to not issue any securities that are subject to a price reset based on the
trading prices of our Ordinary Shares or upon a specified or contingent event in the future or enter into any agreement to issue securities
at a future determined price for a period of one year following the closing date of the Warrant Private Placement, subject to an exception.
Reverse Stock Split,
Change of Name and AIM Cancellation
On
March 24, 2023, we held a general meeting where our shareholders approved, among other things, (i) a change of our name to “Biodexa
Pharmaceuticals PLC,” (ii) the allotment of up to 100% of our fully diluted share capital for future share issuances through our
annual general meeting in 2025, (iii) the cancellation of admission of our Ordinary Shares from trading on AIM, and (iv) a one-for-20
reverse stock split of our Ordinary Shares. The reverse stock split of our Ordinary Shares was effective as of March 27, 2023.
Concurrently with the reverse
split, and in order to continue meeting NASDAQ’s minimum 500,000 publicly held shares requirement pursuant to Rule 5550(a)(4), on
March 27, 2023, we changed the ratio of Depositary Shares from one Depositary Share representing 25 Ordinary Shares to a new ratio of
one Depositary Share representing five Ordinary Shares. This had the effect of a one-for-four reverse split of our Depositary Shares.
No fractional Depositary Shares were issued.
Additionally,
as noted above, our shareholders approved the cancellation of admission of our Ordinary Shares on AIM. This cancellation became effective
on April 26, 2023. The Depositary Shares trade exclusively on the NASDAQ Capital Market under the symbol “BDRX.” There is
currently no market for our Ordinary Shares.
February 2023 Private
Placement
On
February 15, 2023, we completed a private placement, or the February Private Placement, with certain institutional investors, for the
sale of (i) 3,250,200 Ordinary Shares represented by 8,125 Depositary Shares, (ii) 12,931,200 Ordinary Shares represented by 32,327 Depositary
Shares, issuable upon the exercise of Series A Warrants issued in the February Private Placement, (iii) 19,396,400 Ordinary Shares represented
by 48,491 Depositary Shares, issuable upon the exercise of Series B Warrants issued in the February Private Placement, and (iv) up to
71,749,600 Ordinary Shares represented by 179,374 Depositary Shares, issuable upon the exercise of pre-funded warrants issued in the February
Private Placement, subject to certain reset provisions set forth in the pre-funded warrants, at an initial purchase price of $185.60 per
Depositary Share, for aggregate gross proceeds of approximately $6.0 million.
In
addition, in connection with the February Private Placement, on February 9, 2023 we entered into a Waiver to the Securities Purchase Agreement,
dated as of December 13, 2022, or the Waiver, by and between the Company and Armistice Capital, LLC, or Armistice, as amended on December
16, 2022, or the December SPA, providing for a permanent waiver of certain equity issuance prohibitions and participation rights under
the December SPA. In connection therewith, we agreed to, subject to receipt of stockholder approval, issue to Armistice Series A Warrants
exercisable for 625,000 Ordinary Shares represented by 1,562 Depositary Shares. Further, we issued to the Placement Agent, in connection
with their engagement as placement agent in the February Private Placement, placement agent warrants to purchase 536,800 Ordinary Shares
represented by 1,342 Depositary Shares, or the February Placement Agent Warrants.
On
March 24, 2023, we held a general meeting where our shareholders approved, among other things, the allotment of, and disapplication of
preemption rights with respect to, the Ordinary Shares to be issued under the Series A Warrants, the Series B Warrants, certain of the
pre-funded warrants, and the February Placement Agent Warrants. We subsequently issued the warrants to the investors and the Placement
Agent.
Our Corporate Information
Our
principal executive offices are located at 1 Caspian Point, Caspian Way, Cardiff, CF10 4DQ, United Kingdom. The telephone number at our
principal executive office is +44 29 20480 180. Our corporate website is located at www.biodexapharma.com. Information contained on our
website is not part of, or incorporated in, this prospectus. Our authorized representative in the United States is Donald J. Puglisi of
Puglisi and Associates. Our agent for service in the United States is Donald J. Puglisi of Puglisi and Associates, located at 850 Library
Avenue, Suite 204, Newark, Delaware 19711. Our Depositary Shares are traded on the NASDAQ Capital Market under the symbol “BDRX.”
Implications of Being a Foreign Private Issuer
We are incorporated as a public
limited company in England and Wales, are we are deemed to be a “foreign private issuer” for the purposes of the reporting
rules under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In our capacity as a foreign private issuer, we are
exempt from certain rules under the Exchange Act that would otherwise apply if we were a company incorporated in the United States, including:
| · | the requirement to file periodic reports and financial statements with the SEC as frequently or as promptly
as United States companies with securities registered under the Exchange Act; |
| · | the requirement to file financial statements in accordance with accounting principles generally accepted
in the United States, or U.S. GAAP; |
| · | the proxy rules, which impose certain disclosure and procedural requirements for proxy solicitations;
and |
| · | the requirement to comply with Regulation FD, which imposes certain restrictions on the selective disclosure
of material information. |
In addition, our officers,
directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section
16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our Ordinary Shares. Accordingly,
an investor may receive less information about us that it would receive about a public company incorporated in the United States.
Additional Information
For additional information
related to our business and operations, please refer to the reports incorporated herein by reference, including our Annual Report on Form
20-F for the year ended December 31, 2022, as filed with the SEC on April 28, 2023, as amended by Form 20-F/A on May 5, 2023, and our
Reports on Form 6-K, as filed with the SEC, as described in the section titled “Incorporation of Certain Information by Reference.”
| The Offering |
| |
| Class A Units offered by us |
|
We are offering Class A Units, each Class A Unit consisting of one Depositary Share and one Series E Warrant to purchase one Depositary Share. |
| |
|
|
| Public Offering Price Per Class A Unit |
|
$ per each Class A Unit, based upon an assumed public offering price of $ , the closing price of our Depositary Shares on the NASDAQ Capital Market on , 2023. |
| |
|
|
| Class B Units offered by us |
|
We are also offering to those purchasers, if any, whose purchase of Class A Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding Ordinary Shares immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, Class B Units, in lieu of Class A Units that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding Ordinary Shares. Each Class B Unit consists of one Pre-Funded Warrant to purchase one Depositary Share and one Series E Warrant to purchase one Depositary Share. This prospectus also relates to the offering of the Depositary Shares issuable upon exercise of the Pre-Funded Warrants. |
| |
|
|
| Public Offering Price Per Class B Unit |
|
$ per each Class B Unit, based upon an assumed public offering price of $ , the closing price of our Depositary Shares on the NASDAQ Capital Market on , 2023, minus $0.0001, and the exercise price of each Pre-Funded Warrant included in the Class B Units will be $0.0001 per share. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Class B Unit we sell, the number of Class A Units we are offering will be decreased on a one-for-one basis. |
| |
|
|
| Series E Warrants offered by us |
|
Each unit includes one Series E Warrant.
Each Series E Warrant will have an exercise price of $ per share, will be exercisable following
the initial exercise date, and will expire on the fifth anniversary of the initial exercise date. This prospectus also relates to the
offering of the Depositary Shares issuable upon exercise of the Series E Warrants.
|
| |
|
|
Total Ordinary Shares outstanding immediately prior to this
offering |
|
Ordinary Shares (including those represented by Depositary Shares). |
| |
|
|
Total Ordinary Shares to be outstanding immediately after this
offering |
|
Ordinary Shares (including those represented by Depositary Shares), or Ordinary Shares (including those represented by Depositary Shares) if the underwriters exercise their option in full, in each case assuming the sale of all units covered by this prospectus, and no exercise of the Pre-Funded Warrants and Series E Warrants issued in this offering. |
| |
|
|
Underwriters’ option to purchase additional Depositary Shares
and/or warrants |
|
We have granted the underwriters an option, exercisable for forty-five (45) days after the date of this prospectus, to purchase up to an additional Depositary Shares and/or Series E Warrants at the public offering price, less the underwriting discounts payable by us, which may be purchased in any combination of Depositary Shares and/or Series E Warrants. |
| Depositary Shares |
|
Each Depositary Share represents 400 Ordinary
Shares.
The depositary (through its custodian) will hold
the Ordinary Shares underlying your Depositary Shares. You will have rights as provided in the deposit agreement among us, The Bank of
New York Mellon, as depositary, and all owners and holders from time to time of Depositary Shares issued thereunder. You may, among other
things, cancel your Depositary Shares and withdraw the underlying ordinary shares against a fee paid to the depositary (which may be reimbursable
by the Company). In certain limited instances described in the deposit agreement, we may amend or terminate the deposit agreement without
your consent. If you continue to hold your Depositary Shares, you agree to be bound by the terms of the deposit agreement then in effect.
To better understand the terms of the Depositary
Shares and the deposit agreement, including applicable fees and charges, you should carefully read “Description of American Depositary
Shares” in this prospectus. You should also read the deposit agreement, which is an exhibit to the registration statement that
includes this prospectus. |
| |
|
|
| Depositary |
|
The Bank of New York Mellon |
| |
|
|
| Use of proceeds |
|
We intend to use the net proceeds from this offering for funding our product candidates, general corporate purposes and working capital. |
| |
|
|
| Risk factors |
|
You should read the “Risk Factors” section starting on page 11 of this prospectus, together with all of the other information included and incorporated by reference in this prospectus, before deciding to invest in our securities. |
| |
|
|
| Market and trading symbol |
|
Our Depositary Shares are listed on the NASDAQ Capital Market under the symbol “BDRX.” We do not intend to list the Pre-Funded Warrants or Series E Warrants on any securities exchange or national recognized trading system. Without a trading market, the liquidity of the Pre-Funded Warrants and Series E Warrants will be extremely limited. |
Assumptions Used Throughout This Prospectus
The total number of Ordinary Shares outstanding
as of the date of this prospectus and after this offering is based on 365,799,722 shares outstanding as of August 31, 2023, assumes
the sale of units based on an assumed public offering price of $ , the last reported sales
price of a Depositary Share on the NASDAQ Capital Market on , 2023,
and excludes the following other securities as of August 31, 2023:
| · | 121,340 Ordinary Shares issuable upon the exercise of stock options outstanding under our equity incentive plans at a weighted-average
exercise price of £5.05 per share; |
| · | 134 Ordinary Shares issuable upon the exercise of stock options assumed in connection with the acquisition of DARA BioSciences, Inc.,
or DARA, at a weighted average exercise price of $1,903.40 per share; |
| · | warrants, issued to Armistice in an October 2019 private placement, exercisable for 375 Depositary Shares (representing 150,000 Ordinary
Shares) at an exercise price of $320.00 per Depositary Share; |
| · | warrants, issued to the placement agent in connection with an October 2019 private placement, exercisable for 17 Depositary Shares
(representing 6,800 Ordinary Shares) at an exercise price of $10,000.00 per Depositary Share; |
| · | warrants, issued to Armistice in a May 2020 private placement, exercisable for 406 Depositary Shares (representing 162,400 Ordinary
Shares) at an exercise price of $320.00 per Depositary Share; |
| · | warrants, issued to various investors in a May 2020 private placement, exercisable for 415 Depositary Shares (representing 166,000
Ordinary Shares) at an exercise price of $3,280.00 per Depositary Share; |
| · | warrants, issued to the placement agent in connection with the May 2020 private placement, exercisable for 17 Depositary Shares (representing
6,800 Ordinary Shares) at an exercise price of $3,300.00 per Depositary Share; |
| · | warrants, issued in a May 2020 placing in the United Kingdom, exercisable for 349,600 Ordinary Shares, at an exercise price of £6.80
per share; |
| · | warrants, issued in August 2022, exercisable for 16,400 Ordinary Shares, at an exercise price of £2.70 per Ordinary Share; |
| · | warrants, issued in February 2023 to the Placement Agent in connection with the December 2022 registered direct offering, exercisable
for 49 Depositary Shares (representing 19,600 Ordinary Shares) at an exercise price of $400.00 per Depositary Share; |
| · | warrants, issued in February 2023 to the Placement Agent in connection with the February Private Placement, exercisable for 1,293
Depositary Shares (representing 517,200 Ordinary Shares) at an exercise price of $232.00 per Depositary Share; |
| · | Series C Warrants, issued in June 2023, exercisable for 256,324 Depositary Shares (representing 102,529,600 Ordinary Shares), at an
exercise price of $16.00 per Depositary Share (with a cashless exercise option); |
| · | Series D Warrants, issued in June 2023, exercisable for 279,699 Depositary Shares (representing 110,679,610 Ordinary Shares), at an
exercise price of $16.00 per Depositary Share; |
| · | warrants, issued in June 2023, to the Placement Agent in connection with the May 2023 Registered Direct Offering, exercisable for
11,067 Depositary Shares (representing 4,426,800 Ordinary Shares), at an exercise price of $15.00 per Depositary Share; and |
| · | warrants issuable in connection with this offering, including to the representative of the underwriters, or the Representative. |
Except as otherwise noted, all information in this
prospectus reflects and assumes (i) no sale of Class B Units, which, if sold, for each Depositary Share underlying a Pre-Funded
Warrant, the number of Depositary Shares that we are offering will be decreased on a one-for-one basis, (ii) no exercise of outstanding
options issued under our equity incentive plans, (iii) no exercise of any warrants issued in this offering and (iv) no exercise
of the underwriters’ option to purchase additional Depositary Shares and/or Series E Warrants, or any combination thereof.
RISK FACTORS
Our business has significant
risks. You should consider carefully the risks set forth below and other information in this prospectus,
including the information contained under the heading “Risk Factors” in our Annual Report on Form 20-F for the year ended
December 31, 2022 and incorporated herein by reference, before you decide to purchase our securities. These risks and uncertainties
are not the only risks and uncertainties we may face. Additional risks and uncertainties not presently known to us, or that we currently
consider immaterial could also negatively affect our business, financial condition, results of operations, prospects, profits and stock
prices. If any of the risks described below actually occur, our business, financial condition, results of operations, prospects, profits
and stock prices could be materially adversely affected. See also the information contained under
the heading “Cautionary Statement Regarding Forward-Looking Statements” herein.
Summary of Risk Factors
The
occurrence of one or more of the events or circumstances described in this section titled “Risk Factors,” alone or
in combination with other events or circumstances, may materially adversely affect our business, financial condition and operating results.
In that event, the trading price of our securities could decline, and you could lose all or part of your investment. Such risks include,
but are not limited to:
| • | In 2020, our license agreement related to panobinostat, the active pharmaceutical ingredient in our MTX110
product, was terminated by Secura Bio, Inc. Because of this, we believe that the relevant Secura Bio, Inc. patents may delay a launch
of MTX110, which could have a material adverse effect on our business, financial condition and results of operations. |
| • | We have incurred significant losses since our inception and anticipate that we will continue to incur
losses in the future; |
| • | Our requirement for additional financing in the short-term represents a material uncertainty that raises
substantial doubt about our ability to continue as a going concern. |
| • | If we require or seek to raise additional capital to fund our operations and we fail to obtain necessary
financing, we may be unable to complete the development of our product candidates. |
| • | Our operations are in early-stage development with no sources of recurring revenue and there is no assurance
that we will successfully develop and license our product candidates or ever become profitable. |
| • | We are exposed to political, regulatory, social and economic risk relating to the United Kingdom’s
exit from the European Union. |
| • | We have undertaken in the past, and may in the future undertake, strategic acquisitions. Failure to integrate
acquisitions could adversely affect our value. |
| • | Our future success is dependent on product development and the ability to successfully license our product
candidates to partners who can seek regulatory approval and commercialization of our product candidates. |
| • | Our development efforts are in the early stages. All of our product candidates are in clinical development
or preclinical development phases. If we are unable to advance our product candidates through clinical development, obtain regulatory
approval and ultimately commercialize our product candidates, or experience significant delays in doing so, our business will be materially
harmed. |
| • | The results of preclinical studies and early clinical trials are not always predictive of future results.
Any product candidate that we advance in clinical trials may not achieve favorable results in later clinical trials, if any, or receive
marketing approval. |
| • | The regulatory approval processes in the United States and Europe are lengthy, time consuming and inherently
unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business may be substantially
harmed. |
| • | We seek to establish agreements with potential licensing partners and collaborators and, if we are not
able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans. |
| • | If we enter into agreements with a licensing or collaboration partner for the development and commercialization
of our product candidates, our prospects with respect to those product candidates will depend in significant part on the success of those
collaborations. |
| • | The commercial success of any of our product candidates is not guaranteed. |
| • | The pharmaceutical and biotechnology industries are highly competitive. |
| • | Changes in health care policies, laws and regulations, including legislative measures aimed at reducing
health care costs, may impact our ability to obtain approval for or commercialize any of our future product candidates, if approved. |
| • | Coverage and adequate reimbursement may not be available for our
current or any future product candidates, which could make it difficult for us to sell profitably, if approved. |
| • | Our business may be adversely affected by economic conditions and current economic weakness. |
| • | Our business may be impacted by political events, war, terrorism, business interruptions and other geopolitical
events and uncertainties beyond our control. |
| • | We may in the future be unable to retain and recruit qualified scientists, key executives, key employees or key consultants, may delay
our development efforts or otherwise harm our business. |
| • | Public health crises, such as the COVID-19 pandemic, have had, and could in the future have, a negative
effect on our business. |
| • | Our success depends in part on our ability to protect rights in our intellectual property, which cannot
be assured. |
| • | We rely on third parties to conduct our preclinical and clinical trials. If these third parties do not
successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize
our product candidates and our business could be substantially harmed. |
| • | We rely on third parties to manufacture our product candidates, and we expect to continue to rely on third
parties for the clinical as well as any future commercial supply of our product candidates and other future product candidates. The development
of our current and future product candidates, and the commercialization of any approved products, could be stopped, delayed or made less
profitable if any such third party fails to provide us with sufficient clinical or commercial quantities of such product candidates or
products, fails to do so at acceptable quality levels or prices or fails to achieve or maintain satisfactory regulatory compliance. |
| • | We are dependent on third party suppliers, and if we experience problems with any of these third parties,
the manufacturing of our product candidates could be delayed, which could harm our results of operations. |
| • | If we cannot meet NASDAQ’s continued listing requirements, NASDAQ may delist our Depositary Shares,
which could have an adverse impact on the liquidity and market price of our Depositary Shares. |
| • | The price of our Depositary Shares may be volatile. |
| • | The liquidity of our Depositary Shares may have an adverse effect on share price. |
| • | Shareholder ownership interests in the Company may be diluted as a result of, among other things, future
financings and/or additional acquisitions, and may have a material negative effect on the market price of our securities. |
| • | Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud. |
| • | As a foreign private issuer, we are not required to comply with many of the corporate governance standards
of NASDAQ applicable to companies incorporated in the United States. |
| • | Your right to participate in any future rights offerings may be limited, which may cause dilution to your holdings. |
| • | Protections found in provisions under the United Kingdom City Code on Takeovers and Mergers may delay
or discourage a takeover attempt, including attempts that may be beneficial to holders of our Ordinary Shares and Depositary Shares. |
| • | You will incur immediate and substantial dilution as a result of this offering. |
| • | Because our management will have broad discretion and flexibility in how the net proceeds from this offering
are used, our management may use the net proceeds in ways with which you disagree or which may not prove effective. |
| • | There is no public market for the warrants being offered in this offering. |
| • | The terms of the warrants could impede our ability to enter into certain transactions or obtain additional
financing. |
| | | |
| • | Holders of warrants purchased in this offering will have no rights as Depositary Share holders until such holders exercise their warrants
and acquire our Depositary Shares, except as set forth in the warrants. |
| • | The Series E Warrants are speculative in nature. |
Risks Related to Our Financial Operations and Capital Needs
We have incurred significant losses since
our inception and anticipate that we will continue to incur losses in the future.
We are an early-stage biopharmaceutical
company. Investment in biopharmaceutical product development is highly speculative because we entail substantial upfront capital expenditures
and significant risk that a product candidate will fail in development, will fail to gain regulatory approval or otherwise fail to become
commercially viable. We continue to incur significant development and other expenses related to our ongoing operations. As a result, we
are not profitable and have incurred substantial losses since our inception. For the six months ended June 30, 2023, we had a net loss
of £3.57 million and an accumulated deficit of £138.97 million. For the years ended December 31, 2022, 2021 and 2020 we had
a net loss of £7.66 million, £5.46 million and £22.19 million, respectively.
We expect to continue to incur
losses for the foreseeable future, and do not expect these losses to reduce as we continue our development of, and work with any licensing
partners to seek regulatory approvals for, our product candidates.
We may encounter unforeseen
expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future
net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If we fail to find
licensing partners, if we abandon any development programs, or if any of our licensed product candidates fail in clinical trials or do
not gain regulatory approval, or if approved, fail to achieve market acceptance, we may never become profitable. Even if we achieve profitability
in the future, we may not be able to sustain profitability in subsequent periods. Our prior losses and expected future losses have had
and will continue to have an adverse effect on our shareholders’ equity and working capital.
Our requirement for additional financing
in the short-term represents a material uncertainty that raises substantial doubt about our ability to continue as a going concern.
We have experienced net losses
and significant cash outflows from cash used in operating activities over the past years as we develop our portfolio.
Our future viability is dependent
on our ability to raise cash from financing activities to finance our development plans until commercialization, generate cash from operating
activities and to successfully obtain regulatory approval to allow marketing of our development products. Our failure to raise capital
as and when needed could have a negative impact on our financial condition and ability to pursue our business strategies.
Our consolidated
financial statements have been presented on a going concern basis, which contemplates the realization of assets and the satisfaction
of liabilities in the normal course of business. As at December 31, 2022 and June 30, 2023, we had cash and cash equivalents of
£2.84 million and £5.23 million, respectively. If we do not raise funds in this offering, we believe we currently have
enough cash to fund our planned operations into the second quarter of 2024.
We have prepared cash flow
forecasts and considered the cash flow requirement for the next three years, including the period 12 months from the date of approval
of the financial statements. Following registered direct offerings in December 2022 and May 2023 and a private placement in February 2023,
which raised aggregate proceeds of approximately $9.7 million, we updated our forecasts. The updated forecasts show that further financing
will be required before the second quarter of 2024. Failure to secure additional funding before the second quarter of 2024 could result
in the Company being placed into administration.
We believe the environment
for financing of small and micro-cap biotech companies is as challenging as it has been since the financial crisis of 2008-2010. While
this may present acquisition and/or merger opportunities with other companies with limited or no access to financing, any attendant financing
is likely to be dilutive. We and our advisors continue to evaluate financing options, including those connected to acquisitions
and/or mergers, potentially available to us, including fundraising and the partnering of assets and technologies of the Company. The alternatives
being considered are all at an early stage and are contingent upon the agreement of counterparties and accordingly, there can be no assurance
that any of the alternative courses of action to finance the Company will be successful. This requirement for additional financing in
the short term represents a material uncertainty that may cast doubt upon our ability to continue as a going concern. Should it become
evident in the future that there are no realistic financing options available to the Company which are actionable before our cash resources
run out, then we will no longer be a going concern. In such circumstances, we would no longer be able to prepare financial statements
under paragraph 25 of IAS 1. Instead, the financial statements would be prepared on a liquidation basis and asses would be stated at net
realizable value and all liabilities would be accelerated to current liabilities.
We believe there are adequate
options and time available to secure additional financing for the Company and after considering the uncertainties, we considered it appropriate
to continue to adopt the going concern basis in preparing the financial information.
Our ability to continue as
a going concern is dependent upon our ability to obtain additional capital and/or dispose of assets, for which there can be no assurance
we will be able to do on a timely basis, on favorable terms or at all.
Our operations are in early-stage development with no sources
of recurring revenue and there is no assurance that we will successfully develop and license our product candidates or ever become profitable.
We are at a relatively early
stage of our commercial development. To date, we have generated a minimal amount of revenue from our product candidates. Our ability to
generate revenue and become and remain profitable depends, in part, on our ability to successfully find a licensing partner for our product
candidates, or other product candidates we may in-license or acquire, and have such candidates successfully commercialized. Our current
strategy is, once proof-of-concept of our product candidates has been established, to generate revenue via a partner, thereby earning
royalty and/or milestone income; however, this is not expected to materialize in the foreseeable future, and there can be no guarantee
we will be able to find a licensing partner for our product candidates. Even if our product candidates were to successfully achieve regulatory
approval, we do not know when any of the product candidates will generate revenue, if at all. Our ability to generate revenue from our
product candidates also depends on a number of additional factors, including our ability, and the ability of any licensing partners, to:
| • | successfully complete development activities; |
| • | complete and submit new drug applications to the European Medicines Agency, or the EMA, the Medicines
and Healthcare Products Regulatory Agency in the United Kingdom, or the MHRA, the FDA, and any other foreign regulatory authorities, and
obtain regulatory approval for products for which there is a commercial market; |
| • | set a commercially viable price; |
| • | obtain commercial qualities of the products at acceptable cost levels; |
| • | develop and maintain a commercial organization capable of sales, marketing and distribution in the markets
where the product is to be sold; and |
| • | obtain adequate reimbursement from third-parties, including government, departments and healthcare payors. |
In addition, because of the
numerous risks and uncertainties associated with product development, including that our product candidates may not advance through development
or achieve the endpoints of applicable clinical trials, we are unable to predict the timing or amount of increased expenses, or when or
if we will be able to achieve or maintain profitability. Even if we are able to complete the process described above, we anticipate incurring
significant costs.
Even if we are able to generate
royalty and/or milestone revenues from the sale of product candidates, we may not become profitable and may need to obtain additional
funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we
may be unable to continue our operations at planned levels and may be forced to cease or reduce our operations.
There can be no assurance
that we will operate profitably, produce a reasonable return, if any, on investment, or remain solvent. If our strategy proves unsuccessful,
stockholders could lose all or part of their investment.
If we require or seek to raise additional
capital to fund our operations and we fail to obtain necessary financing, we may be unable to complete the development of our product
candidates.
We expect to continue to spend
substantial amounts of our cash resources going forward in order to advance the development of our product candidates. We believe that the net proceeds of this offering, together with cash on hand, will be sufficient to fund our operations through , assuming
we sell all of the securities being offered hereby at the assumed public offering price, and we believe that we will need to raise additional
capital to fund our operations thereafter.
Until such time as we can
generate a sufficient amount of revenue from the product candidates we license, if ever, we expect that we may finance future cash needs
through, among other things, public or private equity or debt offerings. Such offerings may take place in the United Kingdom, the United
States or other foreign countries. However, if we are unable to raise capital when needed, or on terms acceptable to us, our business
could be significantly harmed. If we raise additional funds through the issuance of debt or additional equity securities, such issuance
could result in dilution to our existing shareholders and/or increased fixed payment obligations. Furthermore, these securities may have
rights senior to those of our Ordinary Shares and could contain covenants that would restrict our operations and potentially impair our
competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual
property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events
could significantly harm our business, financial condition and prospects.
Our forecast of the
period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and
involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed
elsewhere in this “Risk Factors” section. We have based this estimate on assumptions that may prove to be wrong,
and we could utilize our available capital resources sooner than we currently expect. Our cash forecasts are based on assumptions
that may prove to be wrong, and we could use our available capital resources sooner than we currently expect. Changing circumstances
could cause us to consume capital significantly faster than we currently anticipate, and we may need to spend more than currently
expected because of circumstances beyond our control. Our future funding requirements, both near and long-term, will depend on many
factors, including, but not limited to:
| • | any acquisitions and the commercialization of other assets, including licensed assets; |
| • | the initiation, progress, timing, costs and results of clinical trials for any product candidates we advance
to clinical trials; |
| • | the attainment of milestones and the need to make any royalty payments on any of our product candidates
or any other future product candidates; |
| • | the number and characteristics of product candidates we in-license or acquire and develop; |
| • | the outcome, timing and cost of regulatory approvals by the EMA, the MHRA, the FDA and any other comparable
foreign regulatory authorities, including the potential for such regulatory authorities to require that we perform more studies, or more
costly studies, than those we currently expect; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims or other intellectual property
rights; and |
| • | the effect of competing technological and market developments. |
Further, our forecast also
does not reflect the possibility that we may not be able to access a portion of our existing cash, cash equivalents and investments due
to market conditions. For example, on March 10, 2023, the United States Federal Deposit Insurance Corporation took control and was appointed
receiver of Silicon Valley Bank. If other banks and financial institutions enter receivership or become insolvent in the future in response
to financial conditions affecting the banking system and financial markets, our ability to access our existing cash, cash equivalents
and investments may be threatened and could have a material adverse effect on our business and financial condition.
If a lack of available capital
means that we are unable to expand our operations or otherwise capitalize on our business opportunities, our business, financial condition
and results of operations could be materially adversely affected.
In previous years, we and our independent
registered public accounting firm have identified material weaknesses in our internal control over financial reporting. Any failure by
us to maintain an effective system of internal controls or provide reliable financial and other information in the future, may cause investors
to lose confidence in our financial statements and SEC filings and the market price of our securities may be materially and adversely
affected.
The Sarbanes-Oxley Act of
2002, or the Sarbanes-Oxley Act, requires, among other things, that we maintain effective internal controls for financial reporting and
disclosure controls and procedures. We are required, under Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on,
among other things, the effectiveness of our internal control over financial reporting. This assessment includes disclosures of any material
weaknesses identified by management in its internal control over financial reporting.
A material weakness is a control
deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable
possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on
the effectiveness of our internal control over financial reporting. However, for as long as we remain a non-accelerated filer, we are
not required to comply with the independent registered public accounting firm attestation requirement.
In previous years, we and
our independent registered public accounting firm have identified material weaknesses in our internal controls over financial reporting.
Although we have instituted remedial measures to address the material weaknesses identified and to continually review and evaluate our
internal control systems to allow management to report on the sufficiency of our internal control over financial reporting, we cannot
assure you that we will not discover additional weaknesses in our internal control over financial reporting. Any such additional weaknesses
or failure to adequately remediate any existing weakness could materially and adversely affect our financial condition and results of
operations, as well as our ability to accurately report our financial condition and results of operations in a timely and reliable
manner.
Additionally, the material
weaknesses previously identified, or other material weaknesses or significant deficiencies we may become aware of in the future, could
result in our determining that our controls and procedures are not effective in future periods or could result in a material misstatement
of the consolidated financial statements that would not be prevented or detected.
Any failure to maintain effective
internal controls over financial reporting could severely inhibit our ability to accurately report our financial condition, results of
operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent
registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial
reporting once that firm begin its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial
statements and reports, the market price of our Ordinary Shares and/or Depositary Shares could decline, and we could be subject to sanctions
or investigations by NASDAQ, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control
over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict
our future access to the capital markets.
Risks Related to Our Business, Strategy and
Industry
In 2020, our license agreement related to panobinostat, the active pharmaceutical ingredient in our MTX110 product, was terminated by
Secura Bio. Because of this, we believe that the relevant Secura Bio patents may delay a launch of MTX110, which could have a material
adverse effect on our business, financial condition and results of operations.
We entered into a License Agreement, executed on or about June 6, 2017, or the License Agreement, by and between Biodexa Limited (formerly
known as Midatech Limited) and Novartis AG, or Novartis, which Novartis subsequently transferred to Secura Bio, or the Secura License
Agreement. Pursuant to the Secura License Agreement, Biodexa Limited was granted a worldwide, sublicensable license to certain patents
of panobinostat, the active pharmaceutical ingredient of our development product MTX110. Biodexa Limited’s rights are limited to
the treatment of brain cancer in humans, administered by convection-enhanced delivery. We received a letter dated June 1, 2020, sent on
behalf of Secura Bio purporting to terminate the Secura License Agreement “effective immediately,” the reason specified being
that we were proposing to liquidate the Company. Despite our assurances to the contrary, and despite our repeated requests that Secura
Bio withdraw its termination, Secura Bio reaffirmed the termination and reasons therefor and the agreement was thus terminated. We received
a further letter sent on behalf of Secura Bio dated May 21, 2021 purporting to terminate the Secura License Agreement a second time for
alleged material breaches of the agreement, and demanding a non-exclusive, fully paid-up, royalty-free, perpetual license to our MTX110
intellectual property. This demand was refused based upon, among other things, Secura Bio’s previous termination of the License
Agreement in 2020.
We view MTX110 as an important asset and currently have three ongoing clinical trials for MTX110 and may commence further clinical trials
as part of our MTX110 clinical program. While we continue to enjoy freedom to use panobinostat for research purposes and we plan to continue
to pursue development of MTX110, we believe that the relevant Secura Bio patents may delay a launch of MTX110 for use in patients with
DMG. We do not, however, anticipate it would have any impact on launching MTX110 for use in patients with glioblastoma multiforme. If
we are unable to launch a product candidate until the patent expires, there could be a material adverse effect on our business, financial
condition and results of operations.
Further, should Secura Bio continue to interfere with our ongoing business by, among other things, challenging the legality of the termination
of the Secura License Agreement, the uncertainty and diversion of time and resources associated could have a material adverse effect on
our business, financial condition and prospects, and we cannot assure you that we would be successful in resolving such dispute.
We are exposed to political, regulatory,
social and economic risk relating to the United Kingdom’s exit from the European Union.
Following the result of a
referendum in 2016, the United Kingdom left the European Union on January 31, 2020, commonly referred to as Brexit. Pursuant to the formal
withdrawal arrangements agreed between the United Kingdom and the European Union, the United Kingdom was subject to a transition period
until December 31, 2020, during which European Union rules continued to apply. The Trade and Cooperation Agreement between the United
Kingdom and the European Union, which outlines the future trading relationship between the United Kingdom and the European Union, was
agreed in December 2020. The impact of the new trade agreement on the general and economic conditions in the United Kingdom remains uncertain.
There may be, for example, additional costs in materials and equipment sourced from the European Union and/or delays that could have a
material adverse effect on our business, financial condition and results of operations.
From a regulatory perspective,
the United Kingdom’s withdrawal from the European Union could bear significant complexity and risks. A basic requirement of
European Union law relating to the grant of a marketing authorization for a medicinal product in the European Union is that the applicant
is established in the European Union. Following the withdrawal of the United Kingdom from the European Union, marketing authorizations
previously granted to applicants established in the United Kingdom may no longer be valid. Moreover, the scope of a marketing authorization
for a medicinal product granted by the European Commission pursuant to the centralized procedure might not, in the future, include the
United Kingdom. In these circumstances, an authorization granted by competent United Kingdom authorities would be required to place medicinal
products on the United Kingdom market.
Any of these factors could
significantly increase the complexity of our activities in the European Union and in the United Kingdom, could depress our economic activity
and restrict our access to capital, which could have a material adverse effect on our business, financial condition and results of operations
and reduce the price of our Ordinary Shares and Depositary Shares.
We have undertaken in the past, and may in the future undertake,
strategic acquisitions. Failure to integrate acquisitions could adversely affect our value.
One of the ways we have
grown our pipeline and business in the past is through strategic acquisitions of other businesses, products, and technologies. We may,
from time to time, evaluate additional acquisition opportunities, and may, in the future, strategically make further acquisitions of,
and investments in, businesses, products and technologies when we believe the opportunity is advantageous to our prospects. There can
be no assurance that in the future we will be able to find appropriate acquisitions or investments. In connection with these acquisitions
or investments, we may:
| • | issue stock that would dilute our shareholders’ percentage of ownership; |
| • | be obligated to make milestone or other contingent or non-contingent payments; |
| • | incur debt and assume liabilities; and/ or |
| • | incur amortization expenses related to intangible assets or incur large and immediate write-offs. |
We also may be unable to find
suitable acquisition candidates and may not be able to complete acquisitions on favorable terms, if at all, or obtain adequate financing
for such acquisitions. If we do complete an acquisition, this may not ultimately strengthen our competitive position or ensure that we
will not be viewed negatively by customers, financial markets or investors. Further, acquisitions could also pose numerous additional
risks to our operations, including:
| • | problems integrating the purchased business, products or technologies without substantial costs, delays
or other problems; |
| • | increases to our expenses; |
| • | the failure to have discovered undisclosed liabilities of the acquired asset or company for which we may
not be adequately indemnified; |
| • | diversion of management’s attention from their day-to-day responsibilities and our core business; |
| • | inability to enforce indemnification and non-compete agreements; |
| • | the failure to successfully incorporate acquired products or technologies into our business; |
| • | the failure of the acquired business, products or technologies to perform as well as anticipated; |
| • | the failure to realize expected synergies and cost savings; |
| • | unexpected safety issues and/or clinical trial failure of the acquisition’s products; |
| • | harm to our operating results or financial condition, particularly during the first several reporting
periods after the acquisition is completed; |
| • | entrance into markets in which we have limited or no prior experience; and |
| • | potential loss of key employees or customers, particularly those of the acquired entity. |
We may not be able to complete
one or more acquisitions or effectively integrate the operations, products or personnel gained through any such acquisition without a
material adverse effect on our business, financial condition and results of operations.
Our future success is dependent on product development and the
ability to successfully license our product candidates to partners who can seek regulatory approval and commercialization of our product
candidates.
We continue to conduct research
and development for our product candidates and, to a lesser extent, clinical trials for certain of our product candidates; however there
can be no assurance that any of our targeted developments will be successful. We must develop functional products that address specific
market needs. We must therefore engage in new development activities, which may not produce innovative, commercially viable results in
a timely manner or at all. In addition, we may not be able to develop new technologies or identify specific market needs that are addressable
by our technologies, or technologies available to us. We may encounter delays and incur additional development and production costs and
expenses, over and above those expected, in order to develop technologies and products suitable for licensing. If any of our development
programs are curtailed, this may have a material adverse effect on our business and financial conditions.
Our business is dependent
on our ability to complete the development of product candidates, and license our product candidates to partners who will seek to obtain
regulatory approval for and commercialize our product candidates in a timely manner. Any licensing partner cannot commercialize a product
without first obtaining regulatory approval from the appropriate regulatory authorities in a country. Before obtaining regulatory approvals
for the commercial sale of any product candidate for a target indication, it must be demonstrated with substantial evidence gathered in
preclinical and well-controlled clinical studies that the product candidate is safe and effective for use for that target indication and
that the manufacturing facilities, processes and controls are adequate. The process of developing, obtaining regulatory approval for and
commercializing product candidates is long, complex and costly. Even if a product candidate were to successfully obtain approval from
the EMA, the MHRA, the FDA and/or comparable foreign regulatory authorities, any approval might contain significant limitations related
to use restrictions for certain age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval
study or risk management requirements. If our product candidates are unable to obtain regulatory approval in one or more jurisdictions,
or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue
the development of any other product candidate. Furthermore, even if a product candidate obtains approval from the regulatory authorities,
it is likely that, in order to obtain royalty and/or milestone revenue from any of our licensing partners, our licensing partners may
need to expand their commercial operations, establish commercially viable pricing and obtain approval for adequate reimbursement from
third parties and government departments and healthcare payors for such products. If our product candidates are unable to successfully
be commercialized, we may not be able to earn sufficient revenues to continue our business.
Our development efforts are in the early
stages. All of our product candidates are in clinical development or preclinical development phases. If we are unable to advance our product
candidates through clinical development, obtain regulatory approval and ultimately commercialize our product candidates, or experience
significant delays in doing so, our business will be materially harmed.
Clinical testing is expensive
and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial
process. The results of any preclinical studies and early clinical trials of our product candidates may not be predictive of the results
of later-stage clinical trials, even after seeing promising results in earlier clinical trials. Product candidates in later stages of
clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial
clinical trials. A number of companies in the biopharmaceutical industry, including many with greater resources and experience than us,
have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising
results in earlier trials.
In 2018, we embarked on first-in-human
clinical trial program for our MTD201 and MTX110 products, with MTD201 completing our Phase I study in the third quarter of 2018, with
an additional Phase I study completed in the third quarter of 2019. The MTX110 Phase I clinical study completed in the fourth quarter
of 2020. In 2020, Phase I clinical trials of MTX110 were initiated by the University of Texas in medulloblastoma, and by Columbia University
in DMG. In November 2022, a Phase I clinical trial in recurrent glioblastoma was initiated by Duke University. In connection with the
termination of our MTD201 program, we have determined not to conduct additional clinical trials in humans, other than pilot trials to
establish proof of concept in indications other than those for which the drug is approved. We expect our licensing partners will be responsible
for future clinical trials. We and any of our current or potential licensing partners may experience delays in ongoing or future clinical
trials and we do not know whether planned clinical trials will begin or enroll subjects on time, need to be redesigned or be completed
on schedule, if at all.
There is no assurance that
clinical trials of MTX110 or any other future clinical trials our other product candidates, will be successful or will generate positive
clinical data and we may not receive marketing approval from the FDA, European Commission, or other regulatory authorities for any of
our product candidates. We have limited experience submitting new drug applications, or NDAs, biologics license applications, or BLAs,
and investigational new drug applications, or INDs, to the FDA, as well as clinical trial applications, or CTAs, or marketing authorization
applications, or MAAs, to the EMA. MTX110 is in initial clinical development, currently being studied in an ongoing investigator-initiated
study. There can be no assurance that the FDA will permit any of our future NDAs, BLAs, or INDs, including the NDA for MTX110 or any future
INDs for our other product candidates, to go into effect in a timely manner or at all. Without an IND or CTA for a product candidate,
we will not be permitted to conduct clinical trials in the United States or the European Union, respectively, of such product candidate.
Drug
or biological product development is a difficult, long, time-consuming, expensive and uncertain process, and delay or failure can occur
at any stage of any of our clinical trials. Failure to obtain regulatory approval for our product candidates will prevent us from commercializing
and marketing them. Clinical trials may be delayed, suspended or prematurely terminated for a variety of reasons, such as:
| • | delay or failure to complete preclinical studies; |
| • | insufficient financial and other resources to complete the necessary preclinical studies and clinical
trials; |
| • | delay or failure in reaching agreement with the applicable regulatory authorities on a trial design; |
| • | delay or failure in obtaining authorization to commence a trial or inability to comply with conditions
imposed by a regulatory authority regarding the scope or design of a clinical study; |
| • | delay or failure in reaching agreement on acceptable terms with prospective contract research organizations,
or CROs, and clinical trial providers and sites, the terms of which can be subject to extensive negotiation and may vary significantly
among different CROs and trial sites; |
| • | delay or failure in obtaining institutional review board, or IRB, or the approval of other reviewing entities,
including foreign regulatory authorities, to conduct a clinical trial at each site; |
| • | failure to recruit, or subsequent withdrawal of, clinical trial sites from clinical trials as a result
of changing standards of care or the ineligibility of a site to participate in our clinical trials; |
| • | delay or failure in recruiting and enrolling suitable subjects to participate in a trial; |
| • | delay or failure in having subjects complete a trial or return for post-treatment follow-up; |
| • | clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance
with regulatory requirements, or dropping out of a trial; |
| • | inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged
in other clinical trial programs, including some that may be for the same indication; |
| • | failure of third party clinical trial managers or clinical sites to satisfy contractual duties or meet
expected deadlines; |
| • | failure to receive the recommendation of health technology assessment bodies such as the U.S. Agency for
Healthcare Research and Quality, and other relevant international bodies or agencies responsible for pricing and utilization determinations; |
| • | delay or failure in adding new clinical trial sites; |
| • | ambiguous or negative interim results, or results that are inconsistent with earlier results; |
| • | from the EMA, the MHRA, the FDA, the IRB, data safety monitoring boards, or other regulatory authority,
or results from earlier stage or concurrent preclinical and clinical studies, which might require modification to the protocol for a given
study; |
| • | decisions by the EMA, the MHRA, the FDA, the IRB, other regulatory authorities, or us, or recommendation
by a data safety monitoring board or other regulatory authority, to suspend or terminate a clinical trial at any time for safety issues
or for any other reason; |
| • | unacceptable risk-benefit profile or unforeseen safety issues or adverse side effects; |
| • | failure to demonstrate a benefit from using a drug over existing marketed products; |
| • | manufacturing issues, including problems with manufacturing or obtaining from third parties sufficient
quantities of raw materials, active pharmaceutical ingredients, or API, or product candidates for use in clinical trials; and |
| • | changes in governmental regulations or administrative actions or lack of adequate funding to continue
the clinical trial. |
Many of these factors are
beyond our control. If we experience delays in the completion of, or termination of, any ongoing or future clinical trial of our product
candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of
these product candidates will be delayed. In addition, any delays in completing clinical trials may slow down our product candidate development
and approval process and jeopardize the ability to commence product sales and generate revenues. Any of these occurrences may harm our
business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement
or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates. It is possible
that none of our product candidates will ever complete successfully the clinical development process and obtain regulatory approval, even
if we expend substantial time and resources seeking such approval.
Negative results in the development
of our lead product candidates may also prevent or delay our ability to continue or conduct clinical programs or receive regulatory approvals
for our other product candidates. For example, although we believe our preclinical studies and animal testing of MTX110 demonstrate indications
of acceptable safety and effectiveness profiles, future clinical trials may fail to demonstrate adequate levels of safety or effectiveness.
Moreover, anti-tumor activity may be different in each tumor type that we plan to evaluate in the clinical trial. Therefore, even though
we plan to pursue clinical development for multiple tumor types, the tumor response may be low in patients with some cancers compared
to others. As a result, we may be required to discontinue development of MTX110 for patients with those tumor types and/or mutations due
to insufficient clinical benefit, while continuing development for a more limited population of patients. Consequently, in order to obtain
regulatory approval, we may have to reach agreement with the FDA on defining the optimal patient population, study design, and size, any
of which may require significant additional resources and delay our clinical trials and ultimately the approval, if any, of any of our
product candidates.
We may experience setbacks
that could delay or prevent regulatory approval of, or our ability to commercialize, our product candidates, including:
| • | negative or inconclusive results from our preclinical studies or clinical trials or positive results from the clinical trials of others
for product candidates similar to ours leading to their approval, and evolving to a decision or requirement to conduct additional preclinical
testing or clinical trials or abandon a program; |
| • | product-related side effects experienced by patients or subjects in our clinical trials or by individuals using drugs or therapeutics
that we, the FDA, other regulators or others view as relevant to the development of to our product candidates; |
| • | delays in submitting INDs or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators
to commence a clinical trial, or a suspension or termination of a clinical trial once commenced; |
| • | conditions imposed by the FDA or comparable foreign authorities regarding the scope or design of our clinical trials, including our
clinical endpoints; |
| • | inability to maintain compliance with regulatory requirements, including current good manufacturing practices, or cGMP, and complying
effectively with other procedures; |
| • | inadequate supply or quality of product candidates or other materials necessary for the conduct of our clinical trials; |
| • | greater than anticipated clinical trial costs; |
| • | inability to compete with other therapies; |
| • | poor efficacy of our product candidates during clinical trials; |
| • | trial results taking longer than anticipated; |
| • | trials being subjected to fraud or data capture failure or other technical mishaps leading to the invalidation of our trials; |
| • | the results of our trials not supporting application for conditional approval in the EU; |
| • | unfavorable FDA or other regulatory agency inspection and review of a clinical trial site; |
| • | failure of our third-party contractors or investigators to comply with regulatory requirements or otherwise meet their contractual
obligations in a timely manner, or at all; |
| • | delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight
around clinical development generally or with respect to our technology in particular; or |
| • | varying interpretations of data by the FDA and similar foreign regulatory agencies. |
In addition, because we have
limited financial and personnel resources and are focusing primarily on developing our lead product candidates, we may forgo or delay
pursuit of other future product candidates that may prove to have greater commercial potential and may fail to capitalize on viable commercial
products or profitable market opportunities. If we do not accurately evaluate the commercial potential or target market for a future product
candidate, we may relinquish valuable rights to those future product candidates through collaboration, licensing, or other royalty arrangements
in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such future product
candidates.
The results of preclinical studies and early clinical trials
are not always predictive of future results. Any product candidate that we advance in clinical trials may not achieve favorable results
in later clinical trials, if any, or receive marketing approval.
The research and development
of drugs and biological products is expensive and extremely risky. Only a small percentage of product candidates that enter the development
process ever receive marketing approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates,
we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates in humans. The outcome of clinical
testing is uncertain. We may face unforeseen challenges in our product candidate development strategy, and we can provide no assurances
that any of our clinical trials will be conducted as planned or completed on schedule, or at all, that we will ultimately be successful
in our current and future clinical trials, or that our product candidates will be able to receive regulatory approval. A failure of one
or more clinical trials can occur at any stage of testing, which may result from a multitude of factors, including, among other things,
flaws in study design, dose selection issues, placebo effects, patient enrollment criteria and failure to demonstrate favorable safety
or efficacy. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials,
and preliminary or interim results of a clinical trial do not necessarily predict final results. For example, it is not uncommon for product
candidates to exhibit unforeseen safety or efficacy issues when tested in humans despite promising results in preclinical animal models.
Accordingly, we cannot assure you that any clinical trials that we may conduct will demonstrate consistent or adequate efficacy and safety
to support marketing approval. In general, the FDA and regulatory authorities outside the United States require two adequate and well-controlled
clinical trials demonstrating safety and effectiveness, including a Phase III clinical trial, before granting marketing approval of a
drug product.
Many companies in the pharmaceutical
industry have suffered significant setbacks in late-stage clinical trials even after achieving promising results in preclinical testing
and earlier-stage clinical trials, and we cannot be certain that we will not face similar setbacks. Moreover, preclinical and clinical
data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed
satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products. Furthermore,
the failure of MTX110 or any other product candidates that we develop to demonstrate safety and efficacy in any clinical trial could negatively
impact the perception of other product candidates that we develop or cause regulatory authorities to require additional testing before
approving any of our product candidates.
If we are required to conduct
additional clinical trials or other testing of MTX110 or our other product candidates, if we are unable to successfully complete clinical
trials of MTX110 or our other product candidates or other testing, if the results of these trials or tests are not positive or are only
modestly positive, if there are safety concerns or if we determine that the observed safety or efficacy profile would not be competitive
in the marketplace, we may:
| • | be delayed in obtaining marketing approval for MTX110 or other product candidates we develop; |
| • | not obtain marketing approval at all; |
| • | obtain marketing approval in some countries and not in others; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| • | be subject to additional post-marketing testing requirements; or |
| • | have the product removed from the market after obtaining marketing approval. |
Our product development costs
will also increase if we experience delays in clinical trials or in obtaining marketing approvals. We do not know whether any of our clinical
trials will continue or begin as planned, will need to be restructured or will be completed on schedule, or at all. We may also decide
to change the design or protocol of one or more of our clinical trials, including to add additional patients or arms, which could result
in increased costs and expenses or delays. Significant clinical trial delays also could shorten any periods during which we may have the
exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our
ability to successfully commercialize our product candidates and may harm our business and results of operations.
We or our collaborators may experience delays
or difficulties in the enrollment and/or retention of patients in clinical trials, which could delay or prevent our receipt of necessary
regulatory approvals.
Successful and timely completion
of clinical trials will require that we or our collaborators sponsoring trials for our product candidates enroll a sufficient number of
patients. Patient enrollment, which is an important factor in the timing of clinical trials, is affected by many factors, including the
size and nature of the patient population and competition for patients eligible for our clinical trials with competitors which may have
ongoing clinical trials for product candidates that are under development to treat the same indications as one or more of our product
candidates, or approved products for the conditions for which we are developing our product candidates.
Trials may be subject to
delays as a result of patient enrollment taking longer than anticipated or patient withdrawal. We may not be able to initiate or continue
clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate
in these trials as required by the FDA, EMA, or comparable foreign regulatory authorities. We cannot predict how successful we or our
collaborators will be at enrolling subjects in future clinical trials. Subject enrollment is affected by other factors including:
| • | the severity and difficulty of diagnosing the disease under investigation; |
| • | the eligibility and exclusion criteria for the trial in question; |
| • | the size of the patient population and process for identifying patients; |
| • | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| • | the design of the trial protocol; |
| • | the perceived risks and benefits of the product candidate in the trial in relation to other available
therapies, including any new products that may be approved for the indications we are investigating; |
| • | the availability of competing commercially available therapies and other competing therapeutic candidates’
clinical trials for the disease or condition under investigation, including for melanoma and other cancers in a first-line setting; |
| • | the willingness of patients to be enrolled in our clinical trials; |
| • | the risk that subjects enrolled in clinical trials will drop out of our trials before completion; |
| • | our ability to obtain and maintain clinical trial subject informed consents |
| • | the efforts to facilitate timely enrollment in clinical trials; |
| • | the patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; and |
| • | the proximity and availability of clinical trial sites for prospective patients. |
Inability to enroll a sufficient
number of patients for clinical trials would result in significant delays and could require us to abandon one or more clinical trials
altogether. Enrollment delays in these clinical trials may result in increased development costs for our product candidates, which would
cause the value of our company to decline and limit our ability to obtain additional financing. Furthermore, we expect to rely on contract
research organizations (CROs) and clinical trial sites to ensure the proper and timely conduct of our clinical trials and we will have
limited influence over their performance.
The regulatory approval processes in the United States and Europe
are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product
candidates, our business may be substantially harmed.
The time required to obtain
approval for a product candidate by the EMA, the MHRA, the FDA and other comparable foreign regulatory authorities is unpredictable, and
it typically takes many years following the commencement of preclinical studies and clinical trials, if approval is obtained at all, and
depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations,
or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical
development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate, and it is possible that
we may never obtain regulatory approval for any product candidate we may seek to develop in the future. Neither we nor any current or
future collaborator is permitted to market any drug product candidates in the United States until we receive regulatory approval of a
NDA from the FDA, and we cannot market it in the European Union or the United Kingdom until we receive a marketing authorization approval
from the EMA or the MHRA, respectively, or in any other country until we obtain regulatory authorization as required under the laws of
such country.
Our product candidates could
fail to receive regulatory approval from the EMA, the MHRA, the FDA and other comparable foreign regulatory authorities for many reasons,
including:
| • | disagreement with the design or implementation of the clinical trials; |
| • | failure to demonstrate that a product candidate is safe and effective for its proposed indication; |
| • | failure of clinical trial results to meet the level of statistical significance required for approval; |
| • | failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety
risks; |
| • | disagreement with our interpretation of data from preclinical studies or clinical trials; |
| • | the insufficiency of data collected from clinical trials of our product candidates to support the submission
and filing of a NDA, BLA, MAA or other submission or to obtain regulatory approval; |
| • | regulatory authorities may find deficiencies in good clinical practice, or GCP, compliance or may find
our record keeping, or the record keeping of our clinical trial sites, to be inadequate; |
| • | disapproval of the manufacturing processes or facilities of third party manufacturers with whom we or
any licensing partner contracts with for clinical and commercial supplies; or |
| • | changes in approval policies or regulations that render the preclinical and clinical data insufficient
for approval. |
Of the large number of products
in development, only a small percentage successfully complete the FDA, EMA, MHRA, or other comparable regulatory approval processes and
are commercialized. The lengthy approval and marketing authorization process as well as the unpredictability of future clinical trial
results may result in our failing to obtain regulatory approval and marketing authorization to market our product candidates, which would
significantly harm our business, financial condition, results of operations and prospects.
In addition, the EMA, the
MHRA, the FDA and other comparable foreign regulatory authorities may require more information, including additional preclinical or clinical
data to support approval, which may delay or prevent approval and any commercialization plans, or we or any licensing partner may decide
to abandon the development program. If approval were to be obtained, regulatory authorities may approve any of our product candidates
for fewer or more limited indications than is requested, may grant approval contingent on the performance of costly post-marketing clinical
trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful
commercialization of that product candidate. In addition, if our product candidate produces undesirable side effects or safety issues,
the regulatory authorities (the FDA, MHRA, EMA or a comparable foreign regulatory authority) may require the establishment of Risk Evaluation
and Mitigation Strategy, or REMS, which may, for instance, restrict distribution of the products and impose burdensome implementation
requirements on us or any licensing partner. Any of the foregoing scenarios could materially harm the commercial prospects for our product
candidates.
Our product candidates may cause undesirable
side effects or have other properties that could delay or prevent their regulatory approval and limit the commercial profile of an approved
label, and such side effects or other properties could result in significant negative consequences following any marketing approval of
any of our product candidates.
Undesirable side effects caused
by any of our product candidates could cause us, our licensing partners, if any, or regulatory authorities to interrupt, delay or halt
clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the EMA, the MHRA, the FDA
or other comparable foreign regulatory authority. Results of the clinical trials could reveal a high and unacceptable severity and prevalence
of side effects or risks associated with a product candidate’s use. In such an event, our trials could be suspended or terminated
and the regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all
targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the
trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects
significantly.
Additionally, if undesirable
side effects of our products are identified following marketing approval, a number of potentially significant negative consequences could
result, including:
| • | marketing of such product may be suspended; |
| • | a product recall or product withdrawal; |
| • | regulatory authorities may withdraw approvals of such product or may require additional warnings on the
label; |
| • | the requirement to develop a REMS for each product or, if a strategy is already in place, to incorporate
additional requirements under the REMS, or to develop a similar strategy as required by a comparable foreign regulatory authority; |
| • | the requirement to conduct additional post-market studies; and |
| • | being sued and held liable for harm caused to subjects or patients. |
Consequently, our reputation and business operations
may suffer.
Any of these events could prevent the achievement
or maintaining of market acceptance of the particular product or product candidate, if approved, and could significantly harm our business,
results of operations and prospects.
Even if we receive regulatory approval of any product candidates,
we will be subject to ongoing regulatory oversight and continued regulatory review, which may result in significant additional expense
and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with any of
our product candidates.
Our product candidates, if
they receive regulatory approval, will be subject to the ongoing requirements of the EMA, the MHRA, the FDA and other regulatory agencies
governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import,
export, advertising, promotion, recordkeeping and reporting of safety and other post-market information. These requirements include submissions
of safety and other post-marketing information and reports, establishment registration and listing, as well as continued compliance with
cGMP and GCP requirements for any clinical trials that we conduct post-approval. The safety profile of any product is closely monitored
by the EMA, the MHRA, the FDA and other regulatory authorities after approval. If the EMA, the MHRA, the FDA or other regulatory authorities
become aware of new safety information after approval of any of our products or product candidates, regulatory authorities may require
labeling changes or establishment of a risk mitigation strategy or similar strategy, impose significant restrictions on a product’s
indicated uses or marketing, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance.
In addition, manufacturers of drug and biological
products and their facilities are subject to continual review and periodic inspections by the EMA, the MHRA, the FDA and other governmental
regulatory authorities for compliance with cGMP regulations. If a previously unknown problem with a product, such as adverse events of
unanticipated severity or frequency, or a problem with the facility where the product is manufactured is discovered, a regulatory agency
may impose restrictions on that product, the manufacturing facility or the party commercializing the product, including requiring recall
or withdrawal of the product from the market or suspension of manufacturing. If our product candidates or the manufacturing facilities
for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
| • | issue warning letters or untitled letters; |
| • | mandate modifications to, or the withdrawal of, marketing and promotional materials or require corrective
information to be provided to healthcare practitioners; |
| • | require the violating party to enter into a consent decree, which can include the imposition of various
fines, reimbursements of inspection costs, required due dates for specific actions and penalties for noncompliance; |
| • | seek an injunction or impose civil or criminal penalties or monetary fines; |
| • | require revisions to the labeling, including limitations on approved uses or the addition of additional
warnings, contraindications or other safety information, including boxed warnings; |
| • | suspend, vary or withdraw regulatory approval; |
| • | require additional post-market clinical trials to assess the safety of the product; |
| • | suspend any ongoing clinical studies; |
| • | refuse to approve pending applications or supplements to applications filed by us or any licensing partner; |
| • | suspend or impose restrictions on operations, the products, manufacturing or ourselves; |
| • | require a change to the product labeling; or |
| • | seize or detain products, refuse to permit the import or export of products or require a product recall. |
In the EU, the EMA may require an equivalent risk
management plan (RMP). Non-compliance with European Union requirements regarding safety monitoring or pharmacovigilance can also result
in significant financial penalties. Similarly, failure to comply with the European Union’s requirements regarding the protection
of personal information can also lead to significant penalties and sanctions.
The occurrence of any of these
events or penalties described above may inhibit our ability to generate revenue from product candidates that are commercialized by any
of our licensing partners.
The FDA’s, EMA’s, MHRA’s, and
other comparable foreign regulatory authorities’ policies may change and additional government regulations may be enacted that could
prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements
or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval
that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
Obtaining and maintaining regulatory approval
of MTX110 or any of our other product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory
approval of MTX110 or our other product candidates in other jurisdictions.
Obtaining and maintaining
regulatory approval of MTX110 or any of our other product candidates in one jurisdiction does not guarantee that we will be able to obtain
or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction
may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product
candidate, similar foreign regulatory authorities must also approve the manufacturing, marketing and promotion of the product candidate
in those countries. Approval and licensure procedures vary among jurisdictions and can involve requirements and administrative review
periods different from, and greater than, those in the United States, including additional studies or clinical trials as clinical trials
conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the
United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some
cases, the price that we intend to charge for our products is also subject to approval.
We may also submit marketing
applications in other countries. Regulatory authorities in jurisdictions outside of the United States have requirements for approval of
product candidates with which we must comply prior to marketing in those jurisdictions. Obtaining similar foreign regulatory approvals
and compliance with similar foreign regulatory requirements could result in significant delays, difficulties and costs for us and could
delay or prevent the introduction of our products in certain countries. We do not have any product candidates approved for sale in any
jurisdiction, including international markets, and we do not have experience in obtaining regulatory approval in international markets.
If we fail to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, our target
market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
We seek to establish agreements with potential licensing partners
and collaborators and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and
commercialization plans.
Our current development and
commercialization strategy is to deploy our proprietary drug delivery technologies to formulate a compelling portfolio of novel first-in-class
sustained release formulations of products with significant commercial potential for licensing to pharmaceutical company partners at proof-of-concept
stage, which would potentially result in revenue generation from product royalty and/or milestone deals. We seek to work with licensing
or collaboration partners for the development and commercialization of one or more of our product candidates. For example, in January
2019, we entered into that certain Licensing, Collaboration and Distribution Agreement, or the CMS License Agreement, with China Medical
System Holdings Limited, or CMS, as guarantor, and two of its wholly owned subsidiaries, CMS Bridging Limited, or CMS Bridging, and CMS
Medical Hong Kong Limited, or CMS Medical HK, each a CMS Party, pursuant to which, among other things, we agreed to license certain of
our products to the CMS Parties in exchange for, among other things, royalty revenue. In June 2020, we announced a research and development
collaboration with Dr. Reddy’s Laboratories Ltd., or Dr. Reddy’s, under which we evaluated the feasibility of applying Q-Sphera
technology to molecules nominated by Dr. Reddy’s. The collaboration was subsequently terminated by mutual agreement. In July
2020, we announced a similar collaboration with Janssen Pharmaceutical NV, a subsidiary of Johnson & Johnson, or Janssen. The collaboration
with Janssen concluded in September 2023. Future collaborators may include large and mid-size pharmaceutical companies, regional
and national pharmaceutical companies and biotechnology companies.
We face significant competition
in seeking appropriate licensing or collaboration partners. Whether we reach a definitive agreement will depend, among other things, upon
our assessment of the partner’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed
partner’s evaluation of a number of factors. Those factors may include the potential differentiation of our product candidate from
competing product candidates, design or results of clinical trials, the likelihood of approval by the FDA or comparable foreign regulatory
authorities and the regulatory pathway for any such approval, the potential market for the product candidate, the costs and complexities
of manufacturing and delivering the product to patients and the potential of competing products. The partner may also consider alternative
product candidates or technologies for similar indications that may be available for collaboration and whether such collaboration could
be more attractive than the one with us for our product candidate.
These agreements are complex
and time consuming to negotiate and document. Further, there have been a significant number of recent business combinations among large
pharmaceutical companies that have resulted in a reduced number of potential future licensing and collaboration partners.
We may not be able to negotiate agreements with
these potential partners on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development
of the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other
development programs.
If we enter into agreements with a licensing
or collaboration partner for the development and commercialization of our product candidates, our prospects with respect to those product
candidates will depend in significant part on the success of those collaborations.
Some of our revenues are currently
derived from licensing or collaboration agreements with other biopharmaceutical companies, research institutes and universities, and we
expect a material amount of our revenue in the future will be derived from these and similar agreement. We may enter into additional agreements
with a licensing or collaboration partner for the development and commercialization of certain of our product candidates. If we enter
into such agreements, we will have limited control over the amount and timing of resources that our partners will dedicate to the development
or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on any future licensing
partners’ ability to successfully perform the functions assigned to them in these arrangements. In addition, any future licensing
or collaboration partner may have the right to abandon research or development projects and terminate applicable agreements, including
funding obligations, prior to or upon the expiration of the agreed upon terms.
Agreements involving our product
candidates pose a number of risks, including:
| • | partners have significant discretion in determining the efforts and resources that they will apply to
these matters; |
| • | partners may not perform their obligations as expected; |
| • | partners may not pursue development and commercialization of our product candidates or may elect not to
continue or renew development or commercialization programs, based on clinical trial results, changes in their strategic focus or available
funding or external factors, such as an acquisition, that divert resources or create competing priorities; |
| • | partners may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical
trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical
testing; |
| • | a partner with marketing and distribution rights to one or more products may not commit sufficient resources
to the marketing and distribution of such product or products; |
| • | disagreements with partners, including disagreements over proprietary rights, contract interpretation
or the preferred course of development, might cause delays or termination of the research, development or commercialization of product
candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration,
any of which would be time-consuming and expensive; |
| • | partners may not properly maintain or defend our intellectual property rights or may use our proprietary
information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information
or expose us to potential litigation; |
| • | partners may infringe the intellectual property rights of third parties, which may expose us to litigation
and potential liability; and |
| • | agreements may be terminated and, if terminated, may result in a need for additional capital to pursue
further development or commercialization of the applicable product candidates. |
Agreements may not lead to
development or commercialization of product candidates in the most efficient manner, or at all. If any future partners of ours is involved
in a business combination, it could decide to delay, diminish or terminate the development or commercialization of any product candidate
licensed to it by us.
The commercial success of any of our product
candidates is not guaranteed.
There can be no assurance
that any of our product candidates currently in development will be successfully developed into any commercially viable product or products
and/or be manufactured in commercial quantities at an acceptable cost or be marketed successfully and profitably. If we, or our partners,
encounter delays at any stage, and fail successfully to address such delays, it may have a material adverse effect on our business, financial
condition and prospects. In addition, our success will depend on the market’s acceptance of these products and there can be no guarantee
that this acceptance will be forthcoming or that our technologies will succeed as an alternative to competing products. If a market fails
to develop or develops more slowly than anticipated, we may be unable to recover the costs we may have incurred in the development of
particular products and may never achieve profitable royalty or licensing revenues from that product.
The pharmaceutical and biotechnology industries
are highly competitive.
The development and commercialization
of new drug products is highly competitive. Our business faces competition from a range of major and specialty pharmaceutical and biotechnology
companies worldwide with respect to our product candidates, and will face competition in the future with respect to any product candidates
that we may seek to develop or commercialize.
There are a number of pharmaceutical
and biotechnology companies that currently market and sell products or are pursuing development of products similar to our technology
and product candidates. With respect to our product candidates, from a technology perspective, we believe other companies in the sustained
release space include Medincell S.A., InnoCore Technologies BV, GP Pharm, S.A., Peptron, Inc., and Nanomi B.V. In addition, other companies
using gold nanoparticle technologies include CytImmune Sciences, Inc., and Nanospectra Biosciences, Inc. There are also a number of companies
developing therapies for glioblastoma including CNS Pharmaceuticals, Inc., Plus Therapeutics Inc., and TME Pharma N.V.
Some of these competitive
products and therapies are based on scientific approaches that are the same or similar to our approach, and others are based on entirely
different approaches. Potential competitors also include academic institutions, government agencies and other public and private research
organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing
and commercialization.
Our competitors in the biotechnology
and pharmaceutical industries may have superior research and development capabilities, products, manufacturing capability or sales and
marketing expertise. Many of our competitors may have significantly greater financial and human resources and may have more experience
in research and development.
As a result of these factors,
our competitors may obtain regulatory approval of their products more rapidly than we are able to or may obtain patent protection of other
intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop
products that are more effective, more widely used and less costly than our own product candidates, and may be more successful in commercializing
their products.
We anticipate that we will
face increased competition in the future as new companies enter our markets and alternative products and technologies become available.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among
a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly
through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining
qualified scientific, management and commercial personnel, as well as in acquiring technologies complementary to, or necessary for, our
programs.
Changes in health care policies, laws and regulations,
including legislative measures aimed at reducing health care costs, may impact our ability to obtain approval for or commercialize any
of our future product candidates, if approved.
All aspects of
our business, including research and development, manufacturing, marketing, pricing, sales, litigation, and intellectual property rights,
are subject to extensive legislation and regulation. Changes in applicable U.S. federal and state laws and agency regulation, as well
as foreign laws and regulations, could have a materially negative impact on our business. In the United States and in some other jurisdictions,
there have been a number of legislative and regulatory changes and proposed changes regarding the health care system that could prevent
or delay marketing approval of our product candidates or any potential future product candidates of ours, restrict or regulate post-approval
activities, or affect our ability to profitably sell any product candidates for which we obtain marketing approval. Increased scrutiny
by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us
to more stringent product labeling and post-marketing testing and other requirements. Congress also must reauthorize the FDA’s user
fee programs every five years and often makes changes to those programs in addition to policy or procedural changes that may be negotiated
between the FDA and industry stakeholders as part of this periodic reauthorization process. Congress most recently reauthorized the user
fee programs in September 2022 without any substantive policy changes.
Among policy
makers and payors in the United States and elsewhere, there is significant interest in promoting changes in health care systems with the
stated goals of containing health care costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry
has been a focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, Congress passed
the ACA, which substantially changed the way health care is financed by both the government and private insurers, and significantly impacts
the U.S. pharmaceutical industry.
There remain
judicial and Congressional challenges to certain aspects of the ACA, and as a result, certain sections of the ACA have not been fully
implemented or effectively repealed. However, following several years of litigation in the federal courts, in June 2021, the U.S. Supreme
Court upheld the ACA when it dismissed a legal challenge to the law’s constitutionality. Further legislative and regulatory changes
under the ACA remain possible, although the new federal administration under President Biden has signaled that it plans to build on the
ACA and expand the number of people who are eligible for health insurance subsidies under it. It is unknown what form any such changes
or any law would take, and how or whether it may affect the pharmaceutical industry as a whole or our business in the future. We expect
that changes or additions to the ACA, the Medicare and Medicaid programs, and changes stemming from other health care reform measures,
especially with regard to health care access, financing or other legislation in individual states, could have a material adverse effect
on the health care industry in the United States.
The uncertainty
around the future of the ACA, and in particular the impact to reimbursement levels, may lead to uncertainty or delay in the purchasing
decisions of our customers, which may in turn negatively impact our product sales. If there are not adequate reimbursement levels, our
business and results of operations could be adversely affected.
In addition,
other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare
payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and was extended by
the Consolidated Appropriations Act for 2023, and will remain in effect through 2032 unless additional Congressional action is taken.
In addition, the
Drug Supply Chain Security Act, or DSCSA, enacted in 2013 imposed obligations on manufacturers of pharmaceutical products related to product
tracking and tracing, and in February 2022, FDA released proposed regulations to amend the national standards for licensing of wholesale
drug distributors by the states; establish new minimum standards for state licensing third-party logistics providers; and create a federal
system for licensure for use in the absence of a State program, each of which is mandated by the DSCSA. Other legislative and regulatory
proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products.
We are unsure whether additional legislative changes will be enacted, or whether the current regulations, guidance or interpretations
will be changed, or whether such changes will have any impact on our business.
Additionally,
there has been heightened governmental scrutiny in the United States of biopharmaceutical pricing practices considering the rising cost
of prescription drugs and biologics. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal
and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing
and manufacturer patient programs, and reform government program reimbursement methodologies for products. For example, state legislatures
are increasingly passing legislation and implementing regulations designed to control pharmaceutical pricing, including price or patient
reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures,
and, in some cases, designed to encourage importation from other countries and bulk purchasing. In December 2020, the U.S. Supreme Court
held unanimously that federal law does not preempt the states’ ability to regulate pharmaceutical benefit managers and other members
of the health care and pharmaceutical supply chain, an important decision that may lead to further and more aggressive efforts by states
in this area.
Most recently,
in August 2022, President Biden signed into the law the Inflation Reduction Act of 2022, or the IRA. Among other things, the IRA has multiple
provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States.
Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government
if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis
and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by
Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single
source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs
starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such
drug will decrease. Any additional federal or state health care reform measures could limit the amounts that third-party payers will
pay for future health care products and services, and, in turn, could significantly reduce the projected value of certain development
projects and reduce our profitability.
Outside of the United States,
particularly in the European Union, the coverage status and pricing of prescription pharmaceuticals and biologics is subject to governmental
control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing
approval for a product. Furthermore, the requirements may differ across the E.U. Member States. To obtain coverage and reimbursement or
pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product
candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is
set at unsatisfactory levels, our business could be harmed. Also, at national level, actions have been taken to enact transparency and
anti-gift laws (similar to the US Physician Payments Sunshine Act) regarding payments between pharmaceutical companies and healthcare
professionals.
Coverage
and adequate reimbursement may not be available for our current or any future product candidates, which could make it difficult for us
to sell profitably, if approved.
Market
acceptance and sales of any product candidates that we commercialize, if approved, will depend in part on the extent to which reimbursement
for these products and related treatments will be available from third-party payors, including government health administration authorities,
managed care organizations and private health insurers. Significant uncertainty exists as to the coverage and reimbursement status of
any products for which we may obtain regulatory approval. Third-party payors decide which therapies they will pay for and establish reimbursement
levels. Third-party payors in the United States often rely upon Medicare coverage policy and payment limitations in setting their own
coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for
any product candidates that we develop will be made on a payor-by-payor basis. One payor’s determination to
provide coverage for a drug does not assure that other payors will also provide coverage for the drug. Additionally,
a third-party payor’s decision to provide coverage for a therapy does not imply that an adequate reimbursement rate will be approved.
Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical
products, therapies and services, in addition to questioning their safety and efficacy. We may incur significant costs to conduct expensive
pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of our product candidates, in addition
to the costs required to obtain FDA approvals. Our product candidates may not be considered medically necessary or cost-effective.
Each
payor determines whether or not it will provide coverage for a therapy, what amount it will pay the manufacturer for the therapy, and
on what tier of its list of covered drugs, or formulary, it will be placed. The position on a payor’s formulary, generally determines
the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy
by patients and physicians. Patients who are prescribed treatments for their conditions and providers prescribing such services generally
rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products, and
providers are unlikely to prescribe our products, unless coverage is provided and reimbursement is adequate to cover a significant portion
of the cost of our products and their administration. Therefore, coverage and adequate reimbursement is critical to new medical product
acceptance.
In the United States, no uniform
policy of coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for our products
can differ significantly from payor to payor. As a result, obtaining coverage and reimbursement approval of a product from a government
or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific,
clinical and cost-effectiveness data for the use of our products on a payor-by-payor basis, with no assurance that coverage and adequate
reimbursement will be obtained. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be
adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Additionally, third-party
payors may not cover, or provide adequate reimbursement for, long-term follow-up evaluations required following the use of product candidates,
once approved.
A
primary trend in the U.S. healthcare industry and elsewhere is cost containment. Third-party payors have attempted to control costs by
limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be
available for any product that we may commercialize and, if reimbursement is available, what the level of reimbursement will be. Even
if favorable coverage and reimbursement status is attained for one or more product candidates for which we receive regulatory approval,
less favorable coverage policies and reimbursement rates may be implemented in the future. Inadequate coverage and reimbursement may impact
the demand for, or the price of, any drug for which we obtain marketing approval. If coverage and adequate reimbursement are not available,
or are available only to limited levels, we may not be able to successfully commercialize our current and any future product candidates
that we develop.
We are subject to environmental laws and regulations that govern
the use, storage, handling and disposal of hazardous materials and other waste products.
We are subject to environmental
laws and regulations governing the use, storage, handling and disposal of hazardous materials and other waste products. We have health
and safety policies and procedures in place to assess the risks associated with use of hazardous materials, and the assessment includes
information for employees on how the substances should be used to avoid contamination of the environment and inadvertent exposure to themselves
and their colleagues. Despite our precautions for handling and disposing of these materials, we cannot eliminate the risk of accidental
contamination or injury. In the event of a hazardous waste spill or other accident, we could be liable for damages, penalties or other
forms of censure. If we fail to comply with any laws or regulations, or if an accident occurs, we may have to pay significant penalties
and may be held liable for any damages that result. This liability could exceed our financial resources and could harm our reputation.
We may also have to incur significant additional costs to comply with current or future environmental laws and regulations. Our failure
to comply with any government regulation applicable to our laboratory and the materials used in our laboratory may adversely affect our
ability to develop, produce, market or partner any products we may commercialize or develop.
Our employees, principal investigators,
consultants and commercial partners may engage in misconduct or other improper activities, including noncompliance with regulatory standards
and requirements, which could have a material adverse effect on our business.
We are exposed to the risk
of employee fraud or other misconduct by our employees, principal investigators, consultants and commercial partners. Misconduct by such
parties could include intentional failures to comply with applicable regulations, provide accurate information to regulatory authorities,
comply with manufacturing standards, comply with healthcare fraud and abuse laws and regulations, report financial information or data
accurately, or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry
are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws
and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive
programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of
clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Business Conduct
and Ethics, but it is not always possible to identify and deter employee misconduct, and the precautions we have taken to detect and prevent
this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations
or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted
against us and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on
our business and results of operations, including the imposition of significant fines or other sanctions.
Unexpected facility shutdowns or system failures may occur and
our disaster recovery plans may not be sufficient.
We depend on the performance,
reliability and availability of our properties, machinery, and laboratory equipment and information technology systems. We may not be
able to access our facilities as a result of events beyond our control, such as extreme weather conditions, quarantines, flood, fire,
theft, terrorism and acts of God.
Further, any damage to or
failure of our equipment and/or systems could also result in disruptions to our operations. A complete or partial failure of our information
technology systems, or those of our CROs and other third parties on which we rely, or corruption of data could result in our inability
to access information that we need in order to meet our obligations to our customers or a breach of confidentiality with respect to our
or our customers’ proprietary information. If such an event were to occur and cause interruptions in our operations, it could result
in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed or ongoing clinical
trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data.
Our disaster recovery plans may not adequately address every potential event and our insurance policies may not cover any loss in full
or in part (including losses resulting from business interruptions) or damage that we suffer fully or at all. The occurrence of one or
more of these events could have a material adverse effect on our business, financial position, reputation or prospects, and might lead
to a claim for damages.
Our business may be adversely affected by economic conditions
and current economic weakness.
Any economic downturn either
globally, regionally or locally in any country in which we operate may have an adverse effect on the demand for any products derived from
our product candidates. A more prolonged economic downturn may lead to an overall decline in our sales, limiting our ability to generate
a profit and positive cash flow. The markets in which we expect the products to be offered are directly affected by many national and
international factors that are beyond our control, such as political, economic, currency, social and other factors.
Our business may be impacted by political
events, war, terrorism, business interruptions and other geopolitical events and uncertainties beyond our control.
War, terrorism, geopolitical
uncertainties and other business interruptions could cause damage to disrupt or cancel the conduct of our planned clinical trials on a
global or regional basis, which could have a material adverse effect on our business, clinical sites or vendors with which we do business.
Such events could also decrease patient demand to enroll in our clinical trials or make it difficult or impossible for us to deliver products
and services to our clinical investigational sites. In addition, territorial invasions can lead to cybersecurity attacks on companies,
such as ours, located far outside of the conflict zone. In the event of prolonged business interruptions due to geopolitical events, we
could incur significant losses, require substantial recovery time and experience significant expenditures in order to resume our business
or clinical operations. We have no operations in Russia or Ukraine, but we do not and cannot know if the current uncertainties in these
geopolitical areas, which are unfolding in real-time, may escalate and result in broad economic and security conditions or rationing of
medical supplies, which could limit our ability to conduct clinical trials or result in material implications for our business.
We are exposed to the risks of doing business internationally.
We have in the past, and may
in the future, operate outside of the United Kingdom. These international operations are subject to a number of risks inherent in operating
in different countries. These include, but are not limited to, risks regarding:
| • | currency exchange rate fluctuations; |
| • | restrictions on repatriation of earnings; |
| • | difficulty of effective enforcement of contractual provisions in local jurisdictions;’ |
| • | inadequate intellectual property (including confidentiality) protection in foreign countries; |
| • | public health epidemics or outbreaks, such as COVID-19; |
| • | trade-protection measures, import or export licensing requirements and fines, penalties or suspension or
revocation of export privileges; and |
| • | changes in a specific country’s or a region’s political or economic conditions, including
the implications of the United Kingdom’s withdrawal from the European Union. |
The occurrence of any of these events or conditions
could adversely affect our ability to increase or maintain our operations in various countries.
We are exposed to risks related to currency
exchange rates.
We currently conduct a portion
of our operations outside of the United Kingdom. Because we use the British pound sterling as our financial statement reporting currency,
changes in currency exchange rates have had and could have a significant effect on our operating results when our operating results are
translated from the local currency into the British pound sterling. Exchange rate fluctuations between local currencies and the British
pound sterling create risk in several ways, including the following: weakening of the British pound sterling, as seen, for example, following
the results of the Brexit referendum, may increase the British pound sterling cost of overseas research and development expenses and the
cost of sourced product components outside the United Kingdom; strengthening of the British pound sterling may decrease the value of our
revenues denominated in other currencies; the exchange rates on non-sterling transactions and cash deposits can distort our financial
results; and commercial pricing and profit margins are affected by currency fluctuations. Future changes in currency exchange rates could
have a material adverse effect on our financial results.
We are subject to cybersecurity risks and other cyber incidents,
including the misappropriation of our information and other breaches of information security that may result in disruption and the incurrence
of costs in an effort to minimize those risks.
In the normal course of conducting
our business, we collect and store sensitive data on our networks, including intellectual property, personal information of our employees,
and our proprietary business information and that of our customers, vendors and business partners. Despite the security measures
we have in place and any additional measures we may implement in the future to safeguard our systems and to mitigate potential security
risks, our facilities and systems, and those of our third-party service providers, could be vulnerable to security breaches, computer
viruses, lost or misplaced data, programming errors, human errors, acts of vandalism or other events. Any steps we take to deter and mitigate
these risks may not be successful and may cause us to incur increasing costs. Any disruption of our systems or security breach or event
resulting in the misappropriation, loss or other unauthorized disclosure of confidential information, whether by us directly or by our
third-party service providers, could damage our reputation, result in the incurrence of costs, expose us to the risks of litigation and
liability, result in regulatory penalties under laws that protect privacy of personal information, disrupt our business or otherwise affect
our results of operations.
We may incur substantial costs in our efforts
to comply with evolving global data protection laws and regulations, and any failure or perceived failure by us to comply with such laws
and regulations may harm our business and operations.
We
maintain a large quantity of sensitive information, including confidential business and personal information in connection with the conduct
of our clinical trials and related to our employees, and we are subject to laws and regulations governing the privacy and security of
such information. In the United States, there are numerous federal and state privacy and data security laws and regulations governing
the collection, use, disclosure and protection of personal information, including federal and state health information privacy laws, federal
and state security breach notification laws, and federal and state consumer protection laws. The legislative and regulatory landscape
for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues, including
with respect to regulatory enforcement and private litigation, which may affect our business and is expected to increase our compliance
costs and exposure to liability. In the United States, numerous federal and state laws and regulations could apply to our operations or
the operations of our partners, including state data breach notification laws, state health information privacy laws, and federal and
state consumer protection laws and regulations, that govern the collection, use, disclosure, and protection of health-related and other
personal information. In addition, we may obtain health information from third parties (including research institutions from which we
obtain clinical trial data) that are subject to privacy and security requirements under HIPAA, as amended by HITECH and regulations promulgated
thereunder. Depending on the facts and circumstances, we could be subject to significant penalties if we obtain, use, or disclose, or
are subject to an actual or alleged data breach regarding, individually identifiable health information in a manner that is not authorized
or permitted by HIPAA.
In
the European Union, the General Data Protection Regulation (EU) 2016/679, or GDPR, lays down the legal framework for data protection and
privacy. The GDPR applies directly in all European Union member states (until December 31, 2020, this included the United Kingdom) and
applies to companies with an establishment in the European Economic Area, or EEA, and to certain other companies not in the EEA that offer
or provide goods or services to individuals located in the EEA or monitor the behavior of individuals located in the EEA. In the United
Kingdom, the GDPR has been converted into United Kingdom domestic law, pursuant to the Data Protection, Privacy and Electronic Communications
(Amendments etc.) (EU Exit) Regulations 2019 (as amended), which makes some minor technical amendments to ensure the GDPR is operable
in the United Kingdom, or the UK GDPR. The UK GDPR is also supplemented by the Data Protection Act 2018. United Kingdom and European Union
data protection law is therefore aligned. The GDPR and UK GDPR implement stringent operational requirements for controllers of personal
data, including, for example, expanded disclosures about how personal information is to be used, limitations on retention of information,
increased requirements pertaining to health data and pseudonymized (i.e., key-coded) data, increased cyber security requirements, mandatory
data breach notification requirements and higher standards for controllers to demonstrate that they have obtained a valid legal basis
for certain data processing activities. The GDPR provides that European Union member states may make their own further laws and regulations
in relation to the processing of genetic, biometric or health data, which could result in differences between member states, limit our
ability to use and share personal data or could cause our costs to increase, and harm our business and financial condition.
Failure
to comply with European Union laws, including failure under the GDPR and UK GDPR, Data Protection Act 2018, ePrivacy Directive and other
laws relating to the security of personal data may result in fines up to €20 million (or £17.5 million under the UK GDPR) or
up to 4% of the total worldwide annual turnover of the preceding financial year, if greater, and other administrative penalties including
criminal liability, which may be onerous and adversely affect our business, financial condition, results of operations and prospects.
The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory
authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. In addition, the GDPR
includes restrictions on cross-border data transfers. Failure to comply with the GDPR and related
laws may lead to increased risk of private actions from data subjects and consumer not-for-profit organizations, including a new form
of class action that is available under the GDPR. While we do not routinely handle or process personal data, we do maintain a database
of employee information; however, since the GDPR and UK GDPR only came into effect recently, the potential risks associated with non-compliance
therewith are uniquely difficult to predict.
We may in the future be unable to retain
and recruit qualified scientists, key executives and directors, key employees or key consultants,
may delay our development efforts or otherwise harm our business.
Our future development and
prospects depend to a large degree on the experience, performance and continued service of our senior management team, including members
of our Board of Directors. We have invested in our management team at all levels. We have entered into contractual arrangements with our
directors and senior management team with the aim of securing the services of each of them. However, retention of these services or the
identification of suitable replacements cannot be guaranteed. There can be no guarantee that the services of the current directors and
senior management team will be retained, or that suitably skilled and qualified individuals can be identified and employed, which may
adversely impact our ability to develop our technologies and/or provide our services at the time requested by our customers or our ability
to market our services and technologies, and otherwise to grow our business, could be impaired. The loss of the services of any of the
directors or other members of the senior management team and the costs of recruiting replacements may have a material adverse effect on
us and our commercial and financial performance.
The ability to continue to
attract and retain employees with the appropriate expertise and skills also cannot be guaranteed. Finding and hiring any additional personnel
and replacements could be costly and might require us to grant significant equity awards or other incentive compensation, which could
adversely impact our financial results, and there can be no assurance that we will have sufficient financial resources to do so. Effective
product development and innovation, upon which our success is dependent, is in turn dependent upon attracting and retaining talented technical
and scientific personnel, who represent a significant asset and serve as the source of our technological and product innovations. If we
are unable to hire, train and retain such personnel in a timely manner, the development and introduction of our products could be delayed
and our ability to sell our products and otherwise to grow our business will be impaired and the delay and inability may have a detrimental
effect upon our performance.
Risks related to global public health concerns
Public health crises, such as the COVID-19
pandemic, have had, and could in the future have, a negative effect on our business.
Pandemics
or disease outbreaks, such as the COVID-19 pandemic, have created and may continue to create significant volatility, uncertainty and economic
disruption in the markets we operate in and may negatively impact business and healthcare activity globally. In response to the COVID-19
pandemic, governments around the world imposed measures designed to reduce the transmission of COVID-19 and individuals continue to respond
to the fear of contracting COVID-19. It is not possible to accurately predict the extent of the adverse effects of the pandemic on
our business. However, we have experienced certain impacts and may experience others which, if they continue for an extended period of
time, could have material adverse effects on our operations and the execution of our business plans. For example, we experienced some
delays in our clinical trials, in particular our Phase I trials of MTX110 in DMG at Columbia University and in medulloblastoma at the
University of Texas. Individuals defer seeking treatment, physicians have fewer in-person meetings to recruit and enroll patients, and
recruited patients are hindered by restrictions in traveling to and accessing clinical sites. In addition, resources at hospitals have
been diverted to dealing with the pandemic, causing delays in scheduling screening evaluations, implant procedures, and follow-up monitoring
visits. As a result of the foregoing factors, the expected timeline for data readouts of our clinical trials may be negatively impacted,
which would adversely affect our business.
The
extent to which fear of exposure to or actual effects of COVID-19, new variants, disease outbreak, epidemic or a similar widespread health
concern impacts our business will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such
as the speed and extent of geographic spread of the disease, the duration of the outbreak, travel restrictions, the efficacy of vaccination
and treatment; impact on the United States, United Kingdom and international healthcare systems, the United States, United Kingdom economy
and worldwide economy; the timing, scope and effectiveness of United States, United Kingdom and international governmental response; and
the impact on the health, well-being and productivity of our employees. To the extent the COVID-19 pandemic adversely affects our business
and financial results, it may also have the effect of heightening many of the other risks described in this “Risk Factors”
section.
Risks related to our intellectual property
Our success depends in part on our ability
to protect rights in our intellectual property, which cannot be assured.
Our success and ability to
compete effectively are in large part dependent upon exploitation of proprietary technologies and products that we have developed internally
or have acquired or in-licensed. To date, we have relied on copyright, trademark and trade secret laws, as well as confidentiality procedures,
non-compete and/or work for hire invention assignment agreements and licensing arrangements with our employees, consultants, contractors,
customers and vendors, to establish and protect our rights to our technology and, to the best extent possible, control the access to and
distribution of our technology, software, documentation and other proprietary information, all of which offer only limited protection.
Where we have the right to do so under our agreements, we seek to protect our proprietary position by filing patent applications in the
United States, the United Kingdom and worldwide related to our novel technologies and products that are important to our business. The
patent positions of biotechnology and pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions
and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial
value of our patents, including those patent rights licensed to us by third parties, are highly uncertain. There can be no assurance that:
| • | the scope of our patents provides and will provide us with exclusivity with respect to any or all of our
product candidates and technologies, as well as any other technologies and/or products that address the same problems as our technologies
and product candidates by a different means, whether in the same manner as us or not; |
| • | pending or future patent applications will be issued as patents; |
| • | our patents, and/or those patents to which we are licensed, are and will remain valid and enforceable
and will not be subject to invalidity or revocation proceedings and that such proceedings will not result in a complete or partial loss
of rights; |
| • | our entitlement to exploit patents from time to time (including patents registered solely in our name
or our affiliates’ name or in the joint names of us or an affiliate and a third party or patents which are licensed to us) is and
will be sufficient to protect our core intellectual property rights against third parties, our commercial activities from competition
or to support comprehensively our ability to develop and market our proposed products either now or in the future; |
| • | the lack of any particular patents or rights to exploit any particular patents, and the scope of our patents,
will not have a material adverse effect on our ability to develop and market our proposed product candidates, either now or in the future; |
| • | we have or will have the resources to pursue any infringer of: (i) patents registered in our name (whether
solely or jointly with a third party) from time to time; or (ii) patents licensed to us where we or an affiliate have the financial responsibility
to bring such infringement actions pursuant to the relevant license agreement; |
| • | we will develop technologies or product candidates which are patentable, either alone or in conjunction
with third parties; |
| • | the ownership, scope or validity of any patents registered in our name (either solely or jointly) from
time to time will not be challenged by third parties, including parties with whom we, or any affiliate, have entered into collaboration
projects or co-ownership arrangements and that any such challenge will not be successful; |
| • | any patent or patent application owned solely or jointly by us will not be challenged on grounds that
we failed to identify the correct inventors or that we failed to comply with our duty of disclosure to the United States Patent and Trademark
Office or any equivalent office in a foreign jurisdiction having a disclosure requirement; |
| • | any issued patent in our sole or joint name from time to time will not be challenged in one or more post-grant
proceedings, including but not limited to inter partes review, derivation proceedings, interferences, and that like; and that any
such challenge will not result in a complete or partial loss of rights to such issued patent or patents; |
| • | any patent applications in our sole or joint name from time to time will not be opposed by any third party,
including parties to collaboration, co-existence and any other contractual relationship with us or any of its members; |
| • | the license agreements between us and third parties are and will be valid and subsisting in the future
or until their expiry dates, and that we have complied with our contractual obligations under the license agreements; |
| • | all intellectual property capable of being commercialized that is or has been generated pursuant to collaboration
agreements between us and third parties will be or has been identified; |
| • | all intellectual property generated pursuant to collaboration agreements and to which we have a contractual
entitlement or generated by employees has been lawfully assigned into our sole name (or to one of our subsidiaries); |
| • | in respect of all intellectual property generated pursuant to a collaboration agreement between us and
a third party to which we and that third party have a joint contractual entitlement, that such intellectual property has been lawfully
assigned into joint names and the rights between us and that third party are properly regulated by a co-ownership agreement; and |
| • | beyond contractual warranties, the licensors of intellectual property to us or our affiliates own the
relevant patents and that those patents have not and will not be the subject of, or subject to, infringement, invalidity or revocation
actions. |
The steps we have taken
to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our
intellectual property rights, both inside and outside of the United Kingdom and United States. The rights already granted under any of
our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection
or competitive advantages we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or
if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products
similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights,
we do not know whether any of the pending patent applications for any of our licensed compounds will result in the issuance of patents
that protect our technology or products, or which will effectively prevent others from commercializing competitive technologies and products.
Although we have a number of issued patents covering our technology, our pending applications cannot be enforced against third parties
practicing the technology claimed in such applications unless and until a patent issues from such applications. Further, the examination
process may require us to narrow the claims, which may limit the scope of patent protection that may be obtained. Because the issuance
of a patent is not conclusive as to its inventorship, scope, validity or enforceability, issued patents that we own or have licensed from
third parties may be challenged in the courts or patent offices in the European Union, United Kingdom, the United States and other foreign
jurisdictions. Overall, such challenges may result in the loss of patent protection, the narrowing of claims in such patents, or the invalidity
or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology
and products, or limit the duration of the patent protection for our technology and products. Protecting against the unauthorized use
of our patented technology, trademarks and other intellectual property rights is expensive, difficult and may in some cases not be possible.
In some cases, it may be difficult or impossible to detect third party infringement or misappropriation of our intellectual property rights,
even in relation to issued patent claims, and proving any such infringement may be even more difficult.
The patent prosecution process
is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable
cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of inventions made in the course of our
development and commercialization activities before it is too late to obtain patent protection on them. Further, given the amount of time
required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire
before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms where they are available in any
countries where we are prosecuting patents. However, the applicable authorities, including the FDA in the United States, and any equivalent
regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to
grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to
take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their
product earlier than might otherwise be the case. Changes in either the patent laws or interpretation of the patent laws in the European
Union, the United Kingdom, the United States and other countries may diminish the value of our patents or narrow the scope of our patent
protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United Kingdom or the United
States, and these foreign laws may also be subject to change. Publication of discoveries in the scientific literature often lag behind
the actual discoveries, and patent applications typically are not published until 18 months after filing or, in some cases, not at all.
Therefore, we cannot be certain that we were the first to make the inventions claimed in our owned or licensed patents or pending patent
applications, or that we were the first to file for patent protection of such inventions.
Previously, in the United
States, assuming the other requirements for patentability are met, the first to make the claimed invention was entitled to the patent.
Outside the United States, the first to file a patent application is entitled to the patent. In March 2013, the United States transitioned
to a “first to file” system in which the first inventor to file a patent application will be entitled to the patent. Under
either the previous or current system, third parties will be allowed to submit prior art prior to the issuance of a patent by the United
States Patent and Trademark Office, and may become involved in opposition, derivation, reexamination, inter-partes review or interference
proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding
or litigation could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position with
respect to third parties.
Our commercial success depends, in part, upon our not infringing
intellectual property rights owned by others.
Although we believe that we
have proprietary platforms for our technologies and product candidates, we cannot determine with certainty whether any existing third
party patents or the issuance of any third party patents in the future would require us to alter our technology, obtain licenses or cease
certain activities. We may become subject to claims by third parties that our technology infringes their intellectual property rights,
in which case we will have no option other than to defend the allegation, which may be possible to resolve through negotiation or which
might result in court proceedings. An adverse outcome in any of these circumstances is that we might be subject to significant liabilities,
be required to cease using a technology or to pay license fees (both prospectively and retrospectively); and may be subject to the payment
of significant damages. We could incur substantial costs in any litigation or other proceedings relating to patent rights, even if it
is resolved in our favor. If the proceedings occur in the United States, it is likely that we will be responsible for our own legal costs,
no matter the outcome of the litigation. In contrast, in the United Kingdom, the losing party typically is ordered to pay the winning
party’s costs, although it is rare to have a complete recovery of all costs from the losing side. Some of our competitors may be
able to sustain the costs of complex litigation more effectively or for a longer time than we can because of their substantially greater
resources. In addition, uncertainties or threatened or actual disputes relating to any patent, patent application or other intellectual
property right (including confidential information) could have a material adverse effect on our ability to market a product, enter into
collaborations in respect of the affected products, or raise additional funds.
The policing of unauthorized
use of our patented technologies and product candidates is difficult and expensive. There can be no assurance that the steps we take will
prevent misappropriation of, or prevent an unauthorized third party from obtaining or using, the technologies, know-how and products we
rely on. In addition, effective protection may be unavailable or limited in some jurisdictions. Any misappropriation of our proprietary
technology, product candidates and intellectual property could have a negative impact on our business and our operating results. Litigation
may be necessary in the future to enforce or protect our rights or to determine the validity or scope of the proprietary rights of others.
Litigation could cause us to incur substantial costs and divert resources and management attention away from our daily business and there
can be no guarantees as to the outcome of any such litigation. In addition, a defendant in any such litigation may counterclaim against
us, resulting in additional time and expense to defend against such a counterclaim, which defense may not be successful.
We may become involved in lawsuits to protect
or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe on
our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation
may be necessary in the future to enforce or defend our intellectual property rights, to protect our trade secrets or to determine the
validity and scope of our own intellectual property rights or the proprietary rights of others. This can be expensive and time consuming.
Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend our intellectual
property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating
our intellectual property. Litigation could result in substantial costs and diversion of management resources, which could harm our business
and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned by or licensed to us is invalid
or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover
the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated,
held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual
property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of
litigation.
Third parties may initiate legal proceedings
alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material
adverse effect on the success of our business.
Our commercial success depends
upon our ability and the ability of our collaborators and licensing partners to develop, manufacture, market and sell our product candidates,
and to use our proprietary technologies without infringing the proprietary rights of third parties. We may become party to, or threatened
with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology.
Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we
are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party
to continue developing and commercializing our products and technology. However, we may not be able to obtain any required license on
commercially reasonable terms or at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors
access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing
technology or product. In addition, in any such proceeding or litigation, we could be found liable for monetary damages. A finding of
infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which
could materially harm our business. Any claims by third parties that we have misappropriated our confidential information or trade secrets
could have a similar negative impact on our business.
We may be subject to claims that our employees have wrongfully
used or disclosed alleged trade secrets of their former employers.
Many of our employees, including
our senior management, were previously employed at other biotechnology or pharmaceutical companies. Some of these employees, including
members of our senior management, executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous
employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for
us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other
proprietary information, of any such employee’s former employer. We are not aware of any threatened or pending claims related to
these matters or concerning the agreements with our senior management, but in the future litigation may be necessary to defend against
such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property
rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a
potential distraction to management.
If we are unable to protect the confidentiality of our trade
secrets, our business and competitive position could be harmed.
In addition to seeking patents
for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other
proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure
and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific
collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention
or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements
and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches.
In addition, a court may determine that we failed to take adequate steps to protect our trade secrets, in which case it may not be possible
to enforce our trade secret rights. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult,
expensive and time-consuming, and the outcome is unpredictable. In addition, some may be less willing or unwilling to protect trade secrets.
If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent
such competitor from using that technology or information to compete with us, which could harm our competitive position.
We may face potential product liability,
and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms
patients or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be
revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims.
In carrying out our activities,
we may potentially face contractual and statutory claims, or other types of claims from customers, suppliers and/or investors. In addition,
we are exposed to potential product liability risks that are inherent in the research, development, production and supply of products.
Subjects enrolled in our clinical trials, consumers, healthcare providers or other persons administering or selling products based on
our and our collaborators’ technology may be able to bring claims against us based on the use of such products. If we cannot successfully
defend ourselves against claims that any product candidates commercialized caused injuries, we could incur substantial costs and liabilities.
Irrespective of their merits or actual outcome, liability claims may result in:
| • | decreased demand for any product candidates that we may develop; |
| • | withdrawal of clinical trial participants; |
| • | termination of clinical trials; |
| • | significant negative media attention and injury to our reputation; |
| • | significant costs to defend the related litigation; |
| • | substantial monetary awards to trial subjects or patients; |
| • | diversion of management and scientific resources from our business operations; and |
| • | the inability to commercialize any products that we may develop. |
While we have obtained product
liability coverage, our insurance coverage may not be sufficient to cover our entire product liability related expenses or losses and
may not cover us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the
future, we may not be able to maintain insurance coverage at a reasonable cost, in sufficient amounts or upon adequate terms to protect
us against losses due to product liability. If we determine that it is prudent to increase our product liability, we may be unable to
obtain this increased product liability insurance on commercially reasonable terms or at all. Large judgments have been awarded in class
action or individual lawsuits based on drugs that had unanticipated side effects, including side effects that may be less severe than
those of our products. A successful product liability claim or series of claims brought against us could cause the price of the Ordinary
Shares and/or Depositary Shares to decline and, if judgments exceed our insurance coverage, could decrease our cash and have a material
adverse effect our business, results of operations, financial condition and prospects.
Risks Related to our Relationships with Third
Parties
We rely on third parties to conduct our
preclinical and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines,
we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We are, and may continue to
be, reliant on other parties for the successful development and commercialization of many of our product candidates. We rely upon CROs
for the conduct of our clinical studies. We rely on these parties for execution of our preclinical and clinical trials, and control only
certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance
with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs or collaboration partners does
not relieve us of our regulatory responsibilities. We also rely on third parties to assist in conducting our preclinical studies in accordance
with good laboratory practices, or GLP, and requirements with respect to animal welfare. We and our CROs or collaboration or licensing
partners are required to comply with GCP, which are regulations and guidelines enforced by the MHRA, the FDA, the EMA and comparable foreign
regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCP through periodic inspections
of trial sponsors, principal investigators and trial sites. If we or any of our CROs or partners fail to comply with applicable GCP, the
clinical data generated in our clinical trials may be deemed unreliable and the EMA, the MHPA, the FDA or comparable foreign regulatory
authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot be assured that
upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with
GCP requirements. In addition, our clinical trials must be conducted with product produced under cGMP requirements. Failure to comply
with these regulations may require us to repeat preclinical and clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees,
and except for remedies available to us under such agreements with such CROs, we cannot control whether or not they devote sufficient
time and resources to our on-going clinical, nonclinical and preclinical programs. If CROs do not successfully carry out their contractual
duties or obligations or meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to
the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, then our clinical trials may be extended,
delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As
a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase
and our ability to generate revenues could be delayed.
If any of our relationships
with these third parties terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable
terms, or at all. Entering into arrangements with alternative CROs, clinical trial investigators or other third parties involves additional
cost and requires management focus and time, in addition to requiring a transition period when a new CRO, clinical trial investigator
or other third party begins work. If third parties do not successfully carry out their contractual duties or obligations or meet expected
deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain are compromised due to the failure
to adhere to our clinical protocols, regulatory requirements or for other reasons, any clinical trials such third parties are associated
with may be extended, delayed or terminated, and we may not be able to obtain marketing approval for or successfully commercialize our
product candidates. As a result, we believe that our financial results and the commercial prospects for our product candidates in the
subject indication would be harmed, our costs could increase and our ability to generate revenue could be delayed.
Because we have relied on
third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties
may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third
party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this
information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available
to identify and monitor our third party providers. To the extent we are unable to identify and successfully manage the performance of
third party service providers in the future, our business may be adversely affected. Though we carefully manage our relationships with
our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges
will not have a material adverse impact on our business, financial condition and prospects.
We rely on third parties to manufacture
our product candidates, and we expect to continue to rely on third parties for the clinical as well as any future commercial supply of
our product candidates and other future product candidates. The development of our current and future product candidates, and the commercialization
of any approved products, could be stopped, delayed or made less profitable if any such third party fails to provide us with sufficient
clinical or commercial quantities of such product candidates or products, fails to do so at acceptable quality levels or prices or fails
to achieve or maintain satisfactory regulatory compliance.
We do not currently have,
and we do not plan to build, the infrastructure or capability internally to manufacture current product candidates or any future product
candidates for use in the conduct of our clinical trials or, if approved, for commercial supply. We rely on, and expect to continue to
rely on, contract manufacturing organizations (CMOs). Reliance on third-party contractors may expose us to more risk than if we were to
manufacture our product candidates ourselves. We do not control the manufacturing processes of the CMOs we contract with and are dependent
on those third parties for the production of our product candidates in accordance with relevant applicable regulations, such as cGMP,
which include, among other things, quality control, quality assurance and the maintenance of records and documentation.
In complying with the manufacturing
regulations of the FDA and other comparable foreign regulatory authorities, we and our third-party manufacturers must spend significant
time, money and effort in the areas of design and development, testing, production, record-keeping and quality control to assure that
the product candidates meet applicable specifications and other regulatory requirements. If either we or our CMOs fail to comply with
these requirements, we may be subject to regulatory enforcement action, including the seizure of product candidates and shutting down
of production.
Even if we are able to establish
and maintain agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| • | reliance on the third party for regulatory, compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third party; |
| • | the possible misappropriation of our proprietary information, including our trade secrets and know-how;
and |
| • | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or
inconvenient for us. |
We or our third-party manufacturers
may encounter shortages in the raw materials or APIs necessary to produce our product candidates in the quantities needed for our clinical
trials or, if our product candidates are approved, in sufficient quantities for commercialization or to meet an increase in demand, as
a result of capacity constraints or delays or disruptions in the market for the raw materials or active pharmaceutical ingredients, including
shortages caused by the purchase of such raw materials or APIs by our competitors or others. The failure by us or our third-party manufacturers
to obtain the raw materials or APIs necessary to manufacture sufficient quantities of our product candidates, may have a material adverse
effect on our business.
Our third-party manufacturers
are subject to inspection and approval by regulatory authorities before we can commence the manufacture and sale of any of our product
candidates, and thereafter are subject to ongoing inspection from time to time. Our third-party manufacturers may not be able to comply
with cGMP regulations or similar regulatory requirements outside of the United States. Our failure, or the failure of our third-party
manufacturers, to comply with applicable regulations could result in regulatory actions, such as the issuance of notices of inspectional
observations, warning letters or sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays,
suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or drugs, operating restrictions
and criminal prosecutions, any of which could significantly and adversely affect supplies of our products. If any of our third-party suppliers
fails to comply with cGMP or other applicable manufacturing regulations, our ability to develop and commercialize our product candidates
could suffer significant interruptions.
Any disruption, such as a
fire, natural hazards or vandalism at our CMOs, or any impacts on our CMOs due to health pandemics, could significantly interrupt our
manufacturing capability. We currently do not have alternative production plans in place or disaster-recovery facilities available. In
case of a disruption, we will have to establish alternative manufacturing sources. This would require substantial capital on our part,
which we may not be able to obtain on commercially acceptable terms or at all. Additionally, we would likely experience months of manufacturing
delays as we build facilities or locate alternative suppliers and seek and obtain necessary regulatory approvals. If this occurs, we will
be unable to satisfy manufacturing needs on a timely basis, if at all. If changes to CMOs occur, then there also may be changes to manufacturing
processes inherent in the setup of new operations for our product candidates and any products that may obtain approval in the future.
Any such changes could require the conduct of bridging studies before we can use any materials produced at new facilities or under new
processes in clinical trials or, for any products reaching approval, in our commercial supply. Further, business interruption insurance
may not adequately compensate us for any losses that may occur and we would have to bear the additional cost of any disruption. For these
reasons, a significant disruptive event of any CMOs could have drastic consequences, including placing our financial stability at risk.
Our product candidates and
any drugs that we may develop may compete with other product candidates and drugs for access to manufacturing facilities. There are no
assurances we would be able to enter into similar arrangements with other manufacturers that operate under cGMP regulations and that might
be capable of manufacturing for us. Any performance failure on the part of our existing or future manufacturers could delay clinical development
or marketing approval.
If we were to experience an
unexpected loss of supply of or if any supplier were unable to meet our clinical or commercial demand for any of our product candidates,
we could experience delays in our planned clinical studies or commercialization. We could be unable to find alternative suppliers of acceptable
quality and experience that can produce and supply appropriate volumes at an acceptable cost or on favorable terms. Moreover, our suppliers
are often subject to strict manufacturing requirements and rigorous testing requirements, which could limit or delay production. The long
transition periods necessary to switch manufacturers and suppliers, if necessary, would significantly delay our clinical trials and, for
any product candidates that reach approval, the commercialization of our products, which would materially adversely affect our business,
financial condition and results of operation.
We are dependent on third party suppliers,
and if we experience problems with any of these third parties, the manufacturing of our product candidates could be delayed, which could
harm our results of operations.
We are dependent upon certain
qualified suppliers, of which there are a limited number, for the supply of raw materials, components, devices and manufacturing equipment,
some of which are manufactured or supplied by small companies with limited resources and experience to support commercial pharmaceutical
and biologics production. Additionally, these suppliers may also have upstream suppliers who supply materials, components, devices and
manufacturing equipment, which may indirectly impact our business operations. Thus, the success of our business may be adversely affected
by the underperformance of third parties, exploitation by third parties of our commercial dependence and by unforeseen interruptions to
third parties’ businesses. Although the existence of several alternative suppliers for each function mitigates the risks associated
with this dependence, as does the availability of commercial insurance in respect of the impact of accidental events, the failure of a
third party to properly to carry out their contractual duties or regulatory obligations could be highly disruptive to our business. Supply
chain failures can result in significant clinical or commercial supply interruptions which could materially hamper our ability to conduct
clinical trials or to supply adequate commercial supplies, and efforts to qualify new suppliers can be costly and time consuming. Further,
any action taken by a third party that is detrimental to our reputation could have a negative impact on our ability to register our trademarks
and/or market and sell our products.
For some of these raw materials,
components, devices and manufacturing equipment, we rely and may in the future rely on sole source vendors or a limited number of vendors.
The supply of the reagents and other specialty materials and equipment that are necessary to produce our product candidates could be reduced
or interrupted at any time. In such case, identifying and engaging an alternative supplier or manufacturer could result in delay, and
we may not be able to find other acceptable suppliers or manufacturers on acceptable terms, or at all. Switching suppliers or manufacturers
may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines. If we change suppliers
or manufacturers for commercial production, applicable regulatory agencies may require us to conduct additional studies or trials. If
key suppliers or manufacturers are lost, or if the supply of the materials is diminished or discontinued, we may not be able to develop,
manufacture and market our product candidates in a timely and competitive manner, or at all. An inability to continue to source product
from any of these suppliers, which could be due to a number of issues, including regulatory actions or requirements affecting the supplier,
adverse financial or other strategic developments experienced by a supplier, labor disputes or shortages, unexpected demands or quality
issues, could adversely affect our ability to satisfy demand for our product candidates, which could adversely and materially affect our
product sales and operating results or our ability to conduct clinical trials, either of which could significantly harm our business.
As we continue to develop
our product candidates and manufacturing processes, we expect that we will need to obtain rights to and supplies of certain materials
and equipment to be used as part of that process. We may not be able to obtain rights to such materials on commercially reasonable terms,
or at all, and if we are unable to alter our process in a commercially viable manner to avoid the use of such materials or find a suitable
substitute, it would have a material adverse effect on our business. Even if we are able to alter our process so as to use other materials
or equipment, such a change may lead to a delay in our clinical development and/or commercialization plans. If such a change occurs for
product candidate that is already in clinical testing, the change may require us to perform both in vitro or in vivo comparability studies
and to collect additional data from patients prior to undertaking more advanced clinical trials. These factors could cause the delay of
studies or trials, regulatory submissions, required approvals or commercialization of product candidates that we develop, cause us to
incur higher costs and prevent us from commercializing our product candidates successfully.
Our counterparties may become insolvent.
There is a risk that parties
with whom we trade or have other business relationships with (including partners, joint venturers, customers, suppliers, subcontractors
and other parties) may become insolvent. This may be due to general economic conditions or factors specific to that company. In the event
that a party with whom we trade becomes insolvent, this could have an adverse impact on our revenues and profitability.
Our relationships with customers, healthcare
providers, physicians, prescribers, purchasers, third party payors, charitable organizations and patients are subject to applicable anti-kickback,
fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages,
reputational harm and diminished profits and future earnings.
Although we do not currently
have any products on the market, upon commercialization of any of our product candidates, if approved, we will be subject to additional
healthcare statutory and regulatory requirements and oversight by federal and state governments in the United States as well as foreign
governments in the jurisdictions in which we conduct our business. Healthcare providers, physicians and third-party payors in the United
States and elsewhere play a primary role in the recommendation and prescription of drug and biological products. Arrangements with third-party
payors and customers can expose pharmaceutical manufacturers to broadly applicable fraud and abuse and other healthcare laws and regulations,
including, without limitation, the federal Anti-Kickback Statute, or AKS, and the False Claims Act, or FCA, which may constrain the business
or financial arrangements and relationships through which such companies sell, market and distribute pharmaceutical products. In particular,
the research of any of our product candidates, as well as the promotion, sales and marketing of healthcare items and services, as well
as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing
and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion,
structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these
laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials.
The healthcare laws that may
affect us include: the federal fraud and abuse laws, including the AKS; false claims and civil monetary penalties laws, including the
FCA and Civil Monetary Penalties Law; federal data privacy and security laws, including HIPAA, as amended by HITECH; and the federal Physician
Payments Sunshine Act related to ownership and investment interests and payments and/or other transfers of value made to or held by physicians
(including doctors, dentists, optometrists, podiatrists, and chiropractors) and teaching hospitals and, beginning in 2022, information
regarding payments and transfers of value provided to other healthcare professionals, such as physician assistants and nurse practitioners
among others, during the previous year. In addition, many states have similar laws and regulations that may differ from each other and
federal law in significant ways, thus complicating compliance efforts. Moreover, several states require pharmaceutical companies to comply
with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal
government and may require manufacturers to report information related to payments and other transfers of value to physicians and other
healthcare providers or marketing expenditures. Additionally, some state and local laws require the registration of pharmaceutical sales
representatives in the jurisdiction.
The scope and enforcement
of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of
the lack of applicable precedent and regulations. Ensuring business arrangements comply with applicable healthcare laws, as well as responding
to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from
other aspects of its business.
It is possible that governmental
and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case
law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and
we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including
the imposition of significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, reputational harm,
possible exclusion from participation in federal and state funded healthcare programs, contractual damages and the curtailment or restricting
of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or
other agreement to resolve allegations of non-compliance with these laws. Further, if any of the physicians or other healthcare providers
or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to significant
criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Any action for violation
of these laws, even if successfully defended, could cause a pharmaceutical manufacturer to incur significant legal expenses and divert
management’s attention from the operation of the business. Therefore, even if we are successful in defending against any such actions
that may be brought against us, our business may be impaired. Prohibitions or restrictions on sales or withdrawal of future marketed products
could materially affect business in an adverse way.
Risks Related to Ownership of Our Securities and Our Status as a
U.S. Listed Company
If we cannot meet NASDAQ’s continued
listing requirements, NASDAQ may delist our Depositary Shares, which could have an adverse impact on the liquidity and market price of
our Depositary Shares.
Our Depositary Shares are
currently listed on the NASDAQ Capital Market. We are required to meet certain qualitative and financial tests to maintain the listing
of our Depositary Shares on NASDAQ. On January 31, 2023, we received a letter from NASDAQ stating that, for the previous 30 consecutive
business days, the bid price for our Depositary Shares had closed below the minimum $1.00 bid price per share requirement for continued
listing on the NASDAQ Capital Market under NASDAQ Listing Rule 5550(a)(2). On July 20, 2023, we received written notice from NASDAQ notifying
us that for the preceding 10 consecutive business days the closing bid price for our Depositary Shares had been $1.00 or greater. Accordingly,
NASDAQ determined that we had regained compliance with NASDAQ Listing Rule 5550(a)(2).
NASDAQ further notified us
that we are subject to a mandatory panel monitor for a period of one year from July 20, 2023. If, within that one-year monitoring period,
the NASDAQ Listing Qualifications Staff finds us out of compliance with the rules that was subject to the previous exception, the Listing
Qualifications Staff will issue a delisting determination letter and we will have the opportunity to request a hearing with the Nasdaq
Hearings Panel.
While we have regained compliance
with all applicable requirements for continued listing on the NASDAQ Capital Market, we cannot assure you that we will remain in compliance
with all applicable requirements for continued listing on the NASDAQ Capital Market. If, in the future, we fail to sustain compliance
with all applicable requirements for continued listing on NASDAQ, including during the one-year monitoring period, our Depositary Shares
may be subject to delisting by NASDAQ. This could inhibit the ability of our holders of Depositary Shares to trade their shares in the
open market, thereby severely limiting the liquidity of such shares. Although holders may be able to trade their shares of Depositary
Shares on the over-the-counter market, there can be no assurance that this would occur. Further, the over-the-counter market provides
significantly less liquidity than NASDAQ and other national securities exchanges, is thinly traded and highly volatile, has fewer market
makers and is not followed by analysts. As a result, your ability to trade or obtain quotations for these securities may be more limited
than if they were quoted on NASDAQ or other national securities exchanges.
The price of our Depositary Shares may be
volatile.
The trading price of our Depositary
Shares in the United States has fluctuated, and is likely to continue to fluctuate, substantially in response to various factors, some
of which are beyond our control, including limited trading volume. The stock market in general, and the market for pharmaceutical and
biotechnology stocks in particular, have experienced extreme volatility that has often been unrelated to the operating performance of
these companies. As a result of this volatility, investors may not be able to sell their Depositary Shares at or above the price paid
for the Depositary Shares. In addition, there is no current market for our Ordinary Shares and we do not expect to list our Ordinary Shares
on any exchange or have them quoted for trading on any over-the-counter trading system in the foreseeable future.
In addition to the factors
discussed in this “Risk Factors” section, the factors that could cause volatility in the market price of each Depositary
Share include:
| • | the success of competitive products or technologies; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | announcements by us or our competitors of new products, significant acquisitions, strategic partnerships,
joint ventures, collaborations or capital commitments; |
| • | the progress of preclinical development, laboratory testing and clinical trials of our product candidates
or those of our competitors; |
| • | the results from our clinical programs and any future trials we may conduct; |
| • | developments in the clinical trials of potentially similar competitive products; |
| • | EMA, MHRA, FDA or international regulatory or legal developments; |
| • | failure of any of our product candidates, if approved, to achieve commercial success; |
| • | developments or disputes concerning patent applications, issued patents or other proprietary intellectual
property rights; |
| • | the recruitment or departure of key personnel; |
| • | the level of expenses related to any of our product candidates or clinical development programs; |
| • | litigation or public concern about the safety of our products; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations
by securities analysts; |
| • | actual and anticipated fluctuations in our operating results; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | announcements or expectations of additional financing efforts; |
| • | rumors relating to us or our competitors; |
| • | sales of our Depositary Shares by us, our insiders or our other shareholders; |
| • | changes in the structure of healthcare payment systems; |
| • | market conditions in the pharmaceutical and biotechnology sectors, or general volatility in the market
due to other factors; |
| • | third party reimbursement policies; |
| • | Brexit and any resulting economic or currency volatility; |
| • | developments concerning current or future collaborations, strategic alliances, joint ventures or similar
relationships; and |
| • | reviews of long-term values of our assets, which could lead to impairment charges that could reduce our
earnings. |
These and other market and
industry factors may cause the market price and demand for our Depositary Shares to fluctuate substantially, regardless of our actual
operating performance, which may limit or prevent investors from selling their Depositary Shares at or above the price paid for the Depositary
Shares, and may otherwise negatively affect the liquidity of our Ordinary Shares and Depositary Shares. The realization of
any of the above risks or any of a broad range of other risks, including those described in these “Risk Factors,” could
have a dramatic and material adverse impact on the market price of our Depositary Shares.
The liquidity of our Depositary Shares may
have an adverse effect on share price.
In July 2023, we changed the
ratio of our Ordinary Shares to Depositary Shares, which had the effect of a one-for-eighty reverse split of Depositary Shares and reduced
the amount of Depositary Shares publicly traded. There is a risk that there may not be sufficient liquidity in the market to accommodate
significant increases in selling activity or the sale of a large block of our securities. Our Depositary Shares have historically had
limited trading volume, which may also result in volatility.
We may be subject to securities litigation, which is expensive
and could divert management attention.
The market price of our Depositary
Shares may be volatile, and in the past, some companies that have experienced volatility in the market price of their stock have been
subject to securities class action litigation. Any lawsuit to which we are a party, with or without merit, may result in an unfavorable
judgment. We also may decide to settle lawsuits on unfavorable terms.
Any such negative outcome
could result in payments of substantial damages or fines, damage to our reputation or adverse changes to our business practices. Defending
against litigation is costly and time-consuming, and could divert our management’s attention and our resources. Furthermore, during
the course of litigation, there could be negative public announcements of the results of hearings, motions or other interim proceedings
or developments, which could have a negative effect on the market price of our Depositary Shares.
Shareholder ownership interests in the Company may be diluted
as a result of, among other things, future financings and/or additional acquisitions, and may have a material negative effect on the market
price of our securities.
We may seek to raise additional
funds from time to time in public or private issuances of equity and such financings may take place in the near future or over the longer
term. Sales of our securities offered through future equity offerings may result in substantial dilution to the interests of our current
shareholders. The sale of a substantial number of securities to investors, or anticipation of such sales, could make it more difficult
for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales.
We may also issue Ordinary
Shares (and Depositary Shares underlying such Ordinary Shares) or other securities convertible into Ordinary Shares from time to time
for future acquisitions. The issuance of the securities underlying these instruments, or perception that issuance may occur, will have
a dilutive impact on other shareholders and could have a material negative effect on the market price of our Depositary Shares.
If equity research analysts do not publish research or publish
inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our
securities will depend in part on the research and reports that equity research analysts publish about us or our business. If
no or few equity research analysts cover our Company, the trading price for our securities would
be negatively impacted. We do not have any control over the analysts or the content and opinions included in their reports. The price
of our securities could decline if one or more equity research analysts downgrade our securities
or issue other unfavorable commentary or research about us. If one or more equity research analysts
ceases coverage of us or fails to publish reports on us regularly, demand for our securities could
decrease, which in turn could cause the trading price or trading volume of our securities to
decline.
The rights of holders of Depositary Shares
are not the same as the rights of holders of Ordinary Shares.
We are a public limited company
incorporated under the laws of England and Wales. The Depositary Shares represent a beneficial ownership interest in our Ordinary Shares.
The rights of holders of Depositary Shares are governed by English law, our constitutional documents, and the deposit agreement pursuant
to which the Depositary Shares are issued. The rights and terms of the Depositary Shares are designed to replicate, to the extent reasonably
practicable, the rights attendant to the Ordinary Shares, for which there is currently no active trading market in the United States.
However, because of aspects of United Kingdom law, our constitutional documents and the terms of the deposit agreement, the rights of
holders of Depositary Shares will not be identical to and, in some respects, may be less favorable than, the rights of holders of Ordinary
Shares.
You may not have the same voting rights
as the holders of our Ordinary Shares and may not receive voting materials in time to be able to exercise your right to vote.
Holders of our Depositary
Shares do not have the same rights as shareholders who hold our Ordinary Shares directly and may only exercise their voting rights with
respect to the underlying Ordinary Shares in accordance with the provisions of the deposit agreement. Holders of the Depositary Shares
will appoint the depositary or its nominee as their representative to exercise the voting rights attaching to the Ordinary Shares represented
by the Depositary Shares. When a general meeting is convened, if you hold Depositary Shares, you may not receive sufficient notice of
a shareholders’ meeting to permit you to withdraw the Ordinary Shares underlying your Depositary Shares to allow you to vote with
respect to any specific matter. Further, we cannot assure purchasers of Depositary Shares that they will receive voting materials in time
to instruct the depositary to vote, and it is possible that they, or persons who hold their Depositary Shares through brokers, dealers
or other third parties, will not have the opportunity to exercise a right to vote. Furthermore, the depositary will not be liable for
any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result,
purchasers of Depositary Shares may not be able to exercise their right to vote and they may lack recourse if their Depositary Shares
are not voted as they request.
You may not receive distributions on Ordinary Shares represented
by Depositary Shares or any value for them if it is illegal or impractical to make them available to holders of Depositary Shares.
The depositary of the Depositary
Shares has agreed to pay to you distributions with respect to cash or other distributions it or the custodian receives on Ordinary Shares
or other deposited securities after deducting its agreed fees and expenses. You will receive these distributions in proportion to the
number of Ordinary Shares your Depositary Shares represent. However, the depositary is not responsible if it decides that it is unlawful
or impractical to make a distribution available to any holders of Depositary Shares. We have no obligation to take any other action to
permit the distribution of our Depositary Shares, Ordinary Shares, rights or anything else to holders of our Depositary Shares. As a result,
you may not receive the distributions made on Ordinary Shares or any value from them if it is illegal or impractical for us to make them
available to you. These restrictions may have a material adverse effect on the value of your Depositary Shares.
You may be subject to limitations on transfer of your Depositary
Shares.
Your Depositary Shares are
transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems
expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer or register transfers of your
Depositary Shares generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it
advisable to do so because of any requirement of law or government or governmental body, or under any provision of the deposit agreement,
or for any other reason.
We have no present intention to pay dividends
on our Ordinary Shares in the foreseeable future and, consequently, your only opportunity to achieve a return on your investment during
that time may be if the price of Depositary Shares appreciates.
We have no present intention
to pay dividends on our Ordinary Shares in the foreseeable future. Any determination by our Board of Directors to pay dividends will depend
on many factors, including our financial condition, results of operations, legal requirements and other factors. Accordingly, if the price
of the Depositary Shares falls in the foreseeable future and you sell your Depositary Shares, you will lose money on your investment,
without the likelihood that this loss will be offset in part or at all by cash dividends.
We are a non-accelerated filer, and the reduced reporting obligations
applicable to non-accelerated filers may make our securities less attractive to investors.
We are a “non-accelerated
filer” under the rules of the SEC. For as long as we remain a “non-accelerated filer,” our independent registered public
accounting firm is not required to deliver an annual attestation report on the effectiveness of our internal control over financial reporting.
We will cease to be a non-accelerated filer if (a) the aggregate market value of our outstanding Ordinary Shares held by non-affiliates
as of the last business day of our most recently completed second fiscal quarter is $75 million or more and we reported annual net revenues
of greater than $100 million for our most recently completed fiscal year or (b) the aggregate market value of our outstanding
Ordinary Shares held by non-affiliates as of the last business day of our most recently completed second fiscal quarter is $700 million
or more, regardless of annual net revenues. If we cease to be a non-accelerated filer, we would be subject to the requirement
for an annual attestation report by our independent registered public accounting firm on the effectiveness of our internal control over
financial reporting. We cannot predict whether investors will find our securities less attractive if we rely on this exemption. If some
investors find our securities less attractive as a result, there may be a less active trading market for our securities and our stock
price may be more volatile and may decline.
Our disclosure controls and procedures may
not prevent or detect all errors or acts of fraud.
We are subject to certain
periodic reporting requirements of the Exchange Act. We design our disclosure controls and procedures to reasonably assure that information
we are required to disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded,
processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure
controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not
absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments
in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented
by the individual acts of some persons, by collusion of two or more people, or by an unauthorized override of the controls. Accordingly,
because of the inherent limitations in our control system, misstatements or insufficient disclosure due to error or fraud may occur and
we may not detect them.
Any failure to maintain effective
internal controls and procedures over financial reporting could severely inhibit our ability to accurately report our financial condition,
results of operations or cash flows.
We are incurring increased costs as a result
of operating as a public company, and management will be required to devote substantial time to new compliance initiatives.
We are currently a public
company in the United Kingdom and United States, and as such we are incurring significant legal, accounting and other expenses that we
did not incur as a private company. We are currently subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act,
the Dodd-Frank Wall Street Reform and Protection Act, as well as rules adopted, and to be adopted, by the SEC and the NASDAQ. Our management
and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, we expect these rules
and regulations to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and
costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and
officer liability insurance and we may be required to incur substantial costs to maintain the sufficient coverage. We cannot predict or
estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could
also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our board committees or
as members of our senior management.
Risks Related to Investing in a Foreign Private Issuer or United
Kingdom Company
We are a “foreign private issuer”
under the rules and regulations of the SEC and, as a result, are exempt from a number of rules under the Exchange Act and are permitted
to file less information with the SEC than a company incorporated in the United States.
We are incorporated as a public
limited company in England and Wales and are deemed to be a “foreign private issuer” under the rules and regulations of the
SEC. As a foreign private issuer, we are exempt from certain rules under the Exchange Act that would otherwise apply if we were a company
incorporated in the United States, including:
| • | the requirement to file periodic reports and financial statements with the SEC as frequently or as promptly
as United States companies with securities registered under the Exchange Act; |
| • | the requirement to file financial statements prepared in accordance with U.S. GAAP; |
| • | the proxy rules, which impose certain disclosure and procedural requirements for proxy or consent solicitations;
and |
| • | the requirement to comply with Regulation FD, which imposes certain restrictions on the selective disclosure
of material information. |
In addition, our officers,
directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section
16 of the Exchange Act and the related rules with respect to their purchases and sales of Ordinary Shares and Depositary Shares. Accordingly,
you may receive less information about us than you would receive about a public company incorporated in the United States and may be afforded
less protection under the United States federal securities laws than you would be if we were incorporated in the United States.
Additional reporting requirements may apply if we lose our status
as a foreign private issuer.
As a foreign private issuer,
we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to
U.S. domestic issuers. In order to maintain our current status as a foreign private issuer, either (1) a majority of our voting
securities must be either directly or indirectly owned of record by non-residents of the United States or (2)(a) a majority of our
executive officers or directors cannot be U.S. citizens or residents, (b) more than 50% of our assets must be located outside the
United States and (c) our business must be administered principally outside the United States.
If we lose our status as a
foreign private issuer, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic
issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes
in our corporate governance practices in accordance with various SEC and NASDAQ rules. The regulatory and compliance costs to us under
U.S. securities laws if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be significantly
higher than the cost we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would
increase our legal and financial compliance costs and would make some activities highly time consuming and costly. We also expect that
if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and
expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially
higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified
members of our Board of Directors.
As a foreign private issuer, we are not
required to comply with many of the corporate governance standards of NASDAQ applicable to companies incorporated in the United States.
Our Board of Directors is
required to maintain an audit committee comprised solely of three or more directors satisfying the independence standards of NASDAQ applicable
to audit committee members. As a foreign private issuer, however, we are not required to comply with most of the other corporate governance
rules of NASDAQ, including the requirement to maintain a majority of independent directors, and nominating and compensation committees
of our Board of Directors comprised solely of independent directors. Although United Kingdom corporate governance rules which we abide
by have comparable requirements, holders of Depositary Shares may not be afforded the benefits of the corporate governance standards of
NASDAQ to the same extent applicable to companies incorporated in the United States.
Your right to participate in any future rights offerings may
be limited, which may cause dilution to your holdings.
Under English law, shareholders
usually have preemptive rights to subscribe on a pro rata basis in the issuance of new shares for cash. The exercise of preemptive rights
by certain shareholders not resident in the United Kingdom may be restricted by applicable law or practice in the United Kingdom and overseas
jurisdictions. We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we
cannot make rights available to shareholders in the United States unless we register the rights and the securities to which the rights
relate under the Securities Act or an exemption from the registration requirements is available. Also, under the deposit agreement, the
depositary bank will not make rights available to Depositary Share holders unless either both the rights and any related securities are
registered under the Securities Act, or the distribution of them to Depositary Share holders is exempted from registration under the Securities
Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause
such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under
the Securities Act. If the depositary does not distribute the rights, it may, under the deposit agreement, either sell them, if possible,
or allow them to lapse. Accordingly, Depositary Share holders may be unable to participate in our rights offerings and may experience
dilution in their holdings. We are also permitted under English law to disapply preemptive rights (subject to the approval of our shareholders
by special resolution or the inclusion in our articles of association of a power to disapply such rights) and thereby exclude certain
shareholders, such as overseas shareholders, from participating in a rights offering (usually to avoid a breach of local securities laws).
It may be difficult for you to bring any
action or enforce any judgment obtained in the United States against us or members of our Board of Directors, which may limit the remedies
otherwise available to you.
We are incorporated as a public
limited company in England and Wales and all of our assets are located outside the United States. In addition, all of the members of our
Board of Directors are nationals and residents of countries, including the United Kingdom, outside of the United States. Most or all of
the assets of these individuals are located outside the United States. As a result, it may not be possible for investors to effect service
of process within the United States upon such persons or to enforce judgments obtained in U.S. courts against them or us, including judgments
predicated upon the civil liability provisions of the U.S. federal securities laws.
The United States and the
United Kingdom do not currently have a treaty providing for the reciprocal recognition and enforcement of judgments (other than arbitration
awards) in civil and commercial matters. Consequently, a final judgment for payment given by a court in the United States, whether or
not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in England and Wales. In addition,
uncertainty exists as to whether the English and Welsh courts would entertain original actions brought in England and Wales against us
or our directors or executive officers predicated upon the securities laws of the United States or any state in the United States. Any
final and conclusive monetary judgment for a definite sum obtained against us in U.S. courts would be treated by the courts of England
and Wales as a cause of action in itself and sued upon as a debt so that no retrial of the issues would be necessary, provided that certain
requirements are met consistent with English law and public policy. Whether these requirements are met in respect of a judgment based
upon the civil liability provisions of the U.S. securities laws is an issue for the English court making such decision. If an English
court gives judgment for the sum payable under a U.S. judgment, the English judgment will be enforceable by methods generally available
for this purpose.
As a result, U.S. investors
may not be able to enforce against us or our executive officers, board of directors or certain experts named herein who are residents
of the United Kingdom or countries other than the United States any judgments obtained in U.S. courts in civil and commercial matters,
including judgments under the U.S. federal securities laws.
We intend to operate so as to be treated
exclusively as a resident of the United Kingdom for tax purposes, but the relevant tax authorities may treat us as also being a resident
of another jurisdiction for tax purposes.
Under current English law,
the decisions of the English courts and the published practice of His Majesty’s Revenue and Customs suggest that we are likely to
be regarded as being a United Kingdom resident and should remain so if, as we intend that, (i) all major meetings of our Board of Directors
and most routine meetings are held in the United Kingdom with a majority of directors present in the United Kingdom for those meetings;
(ii) at those meetings there are full discussions of, and decisions are made regarding, the key strategic issues affecting us and our
subsidiaries; (iii) those meetings are properly minuted; (iv) at least some of our directors, together with supporting staff, are based
in the United Kingdom; and (v) we have permanent staffed office premises in the United Kingdom sufficient to discharge our functions.
Even if we are considered
by His Majesty’s Revenue and Customs as resident in the United Kingdom for United Kingdom tax purposes, as expected, we would nevertheless
not be treated as resident in the United Kingdom if (a) we were concurrently resident in another jurisdiction (applying the tax residence
rules of that jurisdiction) that has a double tax treaty with the United Kingdom and (b) there is a tiebreaker provision in that tax treaty
which allocates exclusive residence to that other jurisdiction. Because this analysis is highly factual and may depend on future changes
in our management and organizational structure, there can be no assurance regarding the final determination of our tax residence. Should
we be treated as resident for tax purposes in another jurisdiction other than the United Kingdom, we would be subject to taxation in such
jurisdiction in accordance with such jurisdiction’s laws, which could result in additional costs and expenses.
The rights of our shareholders may differ from the rights typically
offered to shareholders of a U.S. corporation.
We are incorporated under
English law. The rights of holders of Ordinary Shares and, therefore, certain of the rights of holders of our Depositary Shares, are governed
by English law, including the provisions of the Companies Act, and by our Articles of Association. These rights differ in certain respects
from the rights of shareholders in typical U.S. corporations. See “Memorandum and Articles of Association—Differences in
corporate law” for a description of the principal differences between the provisions of the Companies Act applicable to us and,
for example, the Delaware General Corporation Law relating to shareholders’ rights and protections.
Protections found in provisions under the
United Kingdom City Code on Takeovers and Mergers may delay or discourage a takeover attempt, including attempts that may be beneficial
to holders of our Ordinary Shares and Depositary Shares.
The United Kingdom City Code
on Takeovers and Mergers, or the City Code, applies, among other things, to an offer for a public limited company whose registered office
is in the United Kingdom and has its place of central management and control in United Kingdom, as determined by United Kingdom Panel
on Takeovers and Mergers, or the Panel. The Panel has confirmed we are currently subject to the City Code.
The City Code provides a framework
within which takeovers of certain companies organized in the United Kingdom are regulated and conducted. The following is a brief summary
of some of the most important rules of the City Code:
| • | In connection with a potential offer, if following an approach by or on behalf of a potential bidder,
the company is “the subject of rumor or speculation” or there is an “untoward movement” in the company’s
share price, there is a requirement for the potential bidder to make a public announcement about a potential offer for the company, or
for the company to make a public announcement about its review of a potential offer. |
| • | When interests in shares carrying 10% or more of the voting rights of a class have been acquired by an
offeror (i.e., a bidder) in the offer period (i.e., before the shares subject to the offer have been acquired) or within the previous
12 months, the offer must be in cash or be accompanied by a cash alternative for all shareholders of that class at the highest price paid
by the offeror or any person acting in concert with them in that period. Further, if an offeror or any person acting in concert with them
acquires any interest in shares during the offer period, the offer for the shares must be in cash or accompanied by a cash alternative
at a price at least equal to the price paid for such shares during the offer period. |
| • | If after an announcement is made, the offeror or any person acting in concert with them acquires an interest
in shares in an offeree company (i.e., a target) at a price higher than the value of the offer, the offer must be increased accordingly. |
| • | The board of directors of the offeree company must appoint a competent independent adviser whose advice
on the financial terms of the offer must be made known to all the shareholders, together with the opinion of the board of directors of
the offeree company. |
| • | Favorable deals for selected shareholders are not permitted, except in certain circumstances where independent
shareholder approval is given and the arrangements are regarded as fair and reasonable in the opinion of the financial adviser to the
offeree. |
| • | All shareholders must be given the same information. |
| • | Those issuing documents in connection with a takeover must include statements taking responsibility for
the contents thereof. |
| • | Profit forecasts, quantified financial benefits statements and asset valuations must be made to specified
standards and must be reported on by professional advisers. |
| • | Misleading, inaccurate or unsubstantiated statements made in documents or to the media must be publicly
corrected immediately. |
| • | Actions during the course of an offer by the offeree company, which might frustrate the offer are generally
prohibited unless shareholders approve these plans. Frustrating actions would include, for example, lengthening the notice period for
directors under their service contract or agreeing to sell off material parts of the target group. |
| • | Stringent requirements are laid down for the disclosure of dealings in relevant securities during an offer,
including the prompt disclosure of positions and dealing in relevant securities by the parties to an offer and any person who is interested
(directly or indirectly) in 1% or more of any class of relevant securities. |
| • | Employees of both the offeror and the offeree company and the trustees of the offeree company’s
pension scheme must be informed about an offer. In addition, the offeree company’s employee representatives and pension scheme trustees
have the right to have a separate opinion on the effects of the offer on employment appended to the offeree board of directors’
circular or published on a website. |
If we are deemed or become a passive foreign investment company,
or PFIC, for U.S. federal income tax purposes in 2023 or in any prior or subsequent year, this may result in adverse U.S. federal income
tax consequences for U.S. taxpayers that are holders of our securities.
We will be treated as a PFIC
for U.S. federal income tax purposes in any taxable year in which either (1) at least 75% of our gross income is “passive income”
or (2) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive
income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities
and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes
amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a
non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly,
at least a 25% interest (by value) is taken into account.
We do not believe we were
a PFIC for 2022, but there can be no assurance that we will not be a PFIC in 2023 or for any other taxable year, as our operating results
for any such years may cause us to be a PFIC. If we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable
year during which a U.S. shareholder owns our securities, and such U.S. shareholder does not make an election to treat us as a “qualified
electing fund,” or a QEF, or make a “mark-to-market” election, then “excess distributions” to a U.S. shareholder,
and any gain realized on the sale or other disposition of our securities will be subject to special rules. Under these rules: (1) the
excess distribution or gain would be allocated ratably over the U.S. shareholder’s holding period for the securities; (2) the amount
allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be
taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate
of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed
with respect to the resulting tax attributable to each such other taxable year. In addition, if the United States Internal Revenue Service,
or the IRS, determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late
for a U.S. shareholder to make a timely QEF or mark-to-market election. U.S. shareholders who hold or have held our securities during
a period when we were or are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject
to exceptions for U.S. shareholders who made a timely QEF or mark-to-market election. However, because we do not intend to prepare or
provide the information that would permit the making of a valid QEF election, such an election will not be available to United States
holders.
Changes to U.S. and non-U.S. tax laws could materially adversely
affect our Company and holders of our Ordinary Shares and the Depositary Shares.
The Tax Cuts and Jobs Act,
which was legislation bringing about broad changes in the existing U.S. corporate tax system, was enacted in the United States in December
2017. The Tax Cuts and Jobs Act made significant changes to the U.S. federal income tax laws. Certain provisions of the Tax Cuts and Jobs
Act could have an adverse effect on the Company or holders of our Ordinary Shares or Depositary Shares. The U.S. Treasury Department and
the IRS continue to interpret and issue guidance on how provisions of the Tax Cuts and Jobs Act will be applied and administered. The
interpretations of many provisions of the Tax Cuts and Jobs Act are still unclear. We cannot predict when or to what extent any additional
U.S. federal tax laws, regulations, interpretations, or rulings clarifying the Tax Cuts and Jobs Act will be issued or the impact of any
such guidance on investors or the Company. Holders of Ordinary Shares and Depositary Shares are urged to consult their own tax advisors
regarding the effect of the Tax Cuts and Jobs Act and other potential changes to the U.S. federal tax laws.
We are unable to predict what
tax changes may be enacted in the future or what effect such changes would have on our business, but such changes could affect our effective
tax rates in countries where we have operations and could have an adverse effect on our overall tax position in the future, along with
increasing the complexity, burden and cost of tax compliance. In addition, such changes could impact the holders of Ordinary Shares or
Depositary Shares.
Risks Related to This Offering
You will incur immediate and substantial dilution as a result
of this offering.
After giving effect to the
sale by us of Depositary Shares (or Pre-Funded Warrants exercisable for Depositary Shares) and
accompanying warrants in this offering at an assumed combined public offering price of $ per
Depositary Share (or $ per Pre-Funded Warrant) and accompanying warrants, after deducting underwriter
fees and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $ per Depositary Share.
For a further description of the dilution that investors in this offering may experience, see “Dilution.”
In the past, we have issued
Depositary Shares, Ordinary Shares and warrants in public offerings and private placements of our securities. Our issuance of Depositary
Shares or Ordinary Shares in the future, and the exercise of outstanding stock options, warrants or stock options or warrants that we
may issue in the future, may result in additional dilution to investors in this offering.
Because our management will have broad discretion and flexibility
in how the net proceeds from this offering are used, our management may use the net proceeds in ways with which you disagree or which
may not prove effective.
We currently intend to use the net proceeds from
this offering as discussed under “Use of Proceeds” in this prospectus. We have not allocated specific amounts of the
net proceeds from this offering for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility
in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net
proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used
appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us.
The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition,
operating results and cash flow.
The liquidity and trading volume of our Depositary Shares could
be low.
The liquidity and trading
volume of our Depositary Shares has at times been low in the past and could again be low in the future. If the liquidity and trading volume
of our Depositary Shares is low, this could adversely impact the trading price of our shares, our ability to issue stock and our stockholders’
ability to obtain liquidity in their shares.
There is no public market for the warrants being offered in this
offering.
The public offering price
for the securities will be determined by negotiations between us, the underwriters and prospective investors, and may not be indicative
of prices that will prevail in the trading market. We do not intend to apply to list the warrants on NASDAQ or any nationally recognized
trading system, and accordingly, there will be no trading market for such warrants. In the absence of an active public trading market:
| · | you may not be able to resell your securities at or above the public offering price; |
| · | the market price of our Depositary Shares may experience more price volatility; and |
| · | there may be less efficiency in carrying out your purchase and sale orders. |
The terms of the warrants could impede our ability to enter into
certain transactions or obtain additional financing.
The terms of the warrants
require us, upon the consummation of any “fundamental transaction” (as defined in the securities), to, among other obligations,
cause any successor entity resulting from the fundamental transaction to assume all of our obligations under the warrants and the associated
transaction documents. In addition, holders of warrants are entitled to participate in any fundamental transaction on an as-converted
or as-exercised basis, which could result in the holders of our Depositary Shares receiving a lesser portion of the consideration from
a fundamental transaction. The terms of the warrants could also impede our ability to enter into certain transactions or obtain additional
financing in the future.
We may be required to repurchase certain
of our warrants.
Under the terms of the Series
E Warrants, in the event of certain “Fundamental Transactions” (as defined in the related warrant
agreement, which generally includes any merger with another entity, the sale, transfer or other disposition of all or substantially all
of our assets to another entity, or the acquisition by a person of more than 50% of our Ordinary Shares), each warrant holder will have
the right at any time prior to the consummation of the Fundamental Transaction to require us to repurchase the warrant for a purchase
price in cash equal to the Black Scholes value (as calculated under the warrant agreement) of the then remaining unexercised portion of
such warrant on the date of such Fundamental Transaction, which may materially adversely affect our financial condition and/or results
of operations and may prevent or deter a third party from acquiring us.
Holders of warrants purchased in this offering will have no rights
as Depositary Share holders until such holders exercise their warrants and acquire our Depositary Shares, except as set forth in the warrants.
Except as set forth in the warrants, until holders
of warrants acquire our Depositary Shares upon exercise of the warrants, holders of the warrants have no rights with respect to our Depositary
Shares underlying such warrants, the holders will be entitled to exercise the rights of a holder of Depositary Shares only as to matters
for which the record date occurs after the exercise date.
The Series E Warrants are speculative in nature.
The Series E Warrants offered hereby do not confer
any rights of Depositary Share ownership on their holders, such as voting rights or the right to receive dividends, but rather merely
represent the right to acquire Depositary Shares at a fixed price. Specifically, holders of the Series E warrants may acquire the Depositary
Shares issuable upon exercise of such warrants at an exercise price of $ per Depositary Share.
Moreover, following this offering, the market value of the warrants is uncertain and there can be no assurance that the market value
of the warrants will equal or exceed their respective public offering prices.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus contains forward-looking
statements that are based on our management’s beliefs and assumptions and on information currently available. This section should
be read in conjunction with our financial statements and related notes incorporated by reference into this prospectus. The statements
contained in this prospectus that are not historical facts are forward-looking statements within the meaning of Section 27A of the
Securities Act and Section 21E of the Exchange Act.
Forward-looking statements
can be identified by words such as “believe,” “anticipate,” “may,” “might,” “can,”
“could,” “continue,” “depends,” “expect,” “expand,” “forecast,”
“intend,” “predict,” “plan,” “rely,” “should,” “will,” “may,”
“seek,” or the negative of these terms and other similar expressions, although not all forward-looking statements contain
these words. You should read these statements carefully because they discuss future expectations, contain projections of future results
of operations or financial condition, or state other “forward-looking” information. These statements relate to our future
plans, objectives, expectations, intentions and financial performance and the assumptions that underlie these statements.
These forward-looking statements
are subject to a number of risks, uncertainties, and assumptions, including, but not limited to, those described in “Risk Factors.”
These forward-looking statements reflect our beliefs and views with respect to future events and are based on estimates and assumptions
as of the date of this prospectus and are subject to risks and uncertainties. We discuss many of these risks in greater detail in the
section titled “Risk Factors” and elsewhere in this prospectus. Given these uncertainties, you should not place undue
reliance on these forward-looking statements. We qualify all of the forward-looking statements in this prospectus by these cautionary
statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons
actual results could differ materially from those anticipated in any forward-looking statements, whether as a result of new information,
future events or otherwise.
In addition, statements that
“we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon
information available to us as of the date of this prospectus, and although we believe such information forms a reasonable basis for such
statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a
thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors
are cautioned not to unduly rely upon these statements.
This prospectus also contains
estimates, projections and other information concerning our industry, our business, and our target markets for certain diseases, including
data regarding the estimated size of those markets. Information that is based on estimates, forecasts, projections, market research or
similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and
circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other
data from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical
and general publications, government data, and similar sources.
USE OF PROCEEDS
Assuming we sell all units
offered pursuant to this prospectus, we estimate the net proceeds from this offering will be approximately $ million (or approximately
$ million if the underwriters exercise their over-allotment option in full), based on an assumed public offering price of $ per Class
A Unit (the last reported sale price of our Depositary Shares on the NASDAQ Capital Market on , 2023), after deducting underwriting discounts
and commissions and estimated offering expenses payable by us as described in “Underwriting,” assuming we only sell
Class A Units and excluding the proceeds, if any, from the cash exercise of the Pre-Funded Warrants and Series E Warrants sold in this
offering.
We intend to use the net proceeds
from this offering for funding our product candidates, general corporate purposes and working capital.
The allocation of the net
proceeds of the offering represents our estimates based upon our current plans and assumptions regarding industry and general economic
conditions, our future revenues and expenditures.
The amounts and timing of
our actual use of net proceeds will vary depending on numerous factors, including the relative success and cost of our research and development
programs and our ability to gain access to additional financing. As a result, our management will have broad discretion in the application
of the net proceeds, and investors will be relying on our management’s judgment regarding the application of the net proceeds of
this offering. In addition, we might decide to postpone or not pursue certain development activities if the net proceeds from the offering
and any other sources of cash are less than expected.
Pending the application of
the net proceeds as described above, we will hold the net proceeds from this offering in short-term, interest-bearing, securities.
We believe that the net proceeds
of this offering, together with cash on hand, will be sufficient to fund our operations through , assuming we sell all of the securities
being offered hereby at the assumed public offering price, and we believe that we will need to raise additional capital to fund our operations
thereafter. Additional capital may not be available on terms favorable to us, or at all. If we raise additional funds by issuing equity
securities, our stockholders may experience dilution. Debt financing, if available, may involve restrictive covenants or additional security
interests in our assets. Any additional debt or equity financing that we complete may contain terms that are not favorable to us or our
stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to
relinquish some rights to our technologies or products or grant licenses on terms that are not favorable to us. If we are unable to raise
adequate funds, we may have to delay, reduce the scope of, or eliminate some or all of, our development programs or liquidate some or
all of our assets.
DIVIDEND POLICY
Since
inception, we have never declared or paid any cash dividends on our Ordinary Shares and do not anticipate paying any cash dividends on
our Ordinary Shares or the Depositary Shares in the foreseeable future. We intend to retain all available funds and any future earnings
to fund the development and expansion of our business. As a result, investors in the Ordinary Shares and Depositary Shares will benefit
in the foreseeable future only if the Ordinary Shares and Depositary Shares appreciate in value.
Any
determination to pay dividends in the future would be at the discretion of our Board of Directors and will depend upon our results of
operations, cash requirements, financial condition, contractual restrictions, and any future debt agreements and is subject to compliance
with applicable laws, including the Companies Act, which requires English companies to have profits available for distribution equal to
or greater than the amount of the proposed dividend.
CAPITALIZATION
The following table sets forth
our capitalization as of June 30, 2023:
| · | on a pro forma as-adjusted basis to give effect to the sale by us of Class A Units (each Class A Unit
consisting of one Depositary Share and one Series E Warrant to purchase one Depositary Share) in this offering at an assumed public offering
price of $ per Class A Unit, which is the last reported sale price of our Depositary Shares on the NASDAQ Capital Market on , 2023, after
deducting the estimated underwriting discounts and commissions and estimated offering expenses, and assuming no sale of Class B Units
(each Class B Unit consisting of one Pre-Funded Warrant and one Series E Warrant to purchase one Depositary Share) in this offering and
no exercise of any warrants included in the units, after deducting the estimated underwriting discounts and commissions and estimated
offering expenses in this offering. |
The adjusted amounts shown
below are unaudited and represent management’s estimate. The pro forma information set forth in the table below is illustrative
only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. The information
in this table should be read in conjunction with and is qualified by reference to the financial statements and notes thereto and other
financial information contained in this prospectus.
| (£ in thousands) | |
As of June 30, 2023 | |
| | |
Actual | | |
Pro Forma As-Adjusted (unaudited) (1) | |
| | |
| | |
| |
| Cash and cash equivalents | |
| 5,227 | | |
| | |
| Long-term debt | |
| 380 | | |
| | |
| Total equity | |
| 5,299 | | |
| | |
| Total capitalization | |
| 5,679 | | |
| | |
_____________
| (1) | A $1.00 increase or decrease in the assumed public offering price of $ per Class A Unit, which is the
last reported sale price of our Depositary Shares on the NASDAQ Capital Market on , 2023, would increase or decrease, as appropriate,
our pro-forma as adjusted cash and cash equivalents, total equity and total capitalization by approximately £ million,
assuming the number of units offered by us as set forth on the cover page of this prospectus remains the same, and after deducting
the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
Similarly, an increase or
decrease of 10,000 in the number of units offered by us, based on the assumed public offering price of $ per
Class A Unit, would increase or decrease our pro-forma as adjusted cash and cash equivalents, total equity and total capitalization by
approximately £ million, after deducting underwriting discounts and commissions and estimated
offering expenses payable by us. The information discussed above is illustrative only and will adjust based on the actual public offering
price, the actual number of units we offer in this offering, and other terms of this offering determined at pricing.
The total number of Ordinary
Shares outstanding as of the date of this prospectus and after this offering is based on 277,971,722 shares outstanding as of June 30,
2023, assumes the sale of units based on an assumed public offering price of $ , the last
reported sales price of a Depositary Share on the NASDAQ Capital Market on ,
2023, and excludes the following other securities as of June 30, 2023:
| · | 121,340 Ordinary Shares issuable upon the exercise of stock options
outstanding under our equity incentive plans at a weighted-average exercise price of £5.05 per share; |
| · | 134 Ordinary Shares issuable upon the exercise of stock options assumed in connection with the acquisition
of DARA, at a weighted average exercise price of $1,903.40 per share; |
| · | warrants, issued to Armistice in an October 2019 private placement, exercisable for 375 Depositary Shares
(representing 150,000 Ordinary Shares) at an exercise price of $320.00 per Depositary Share; |
| · | warrants, issued to the placement agent in connection with an October 2019 private placement, exercisable
for 17 Depositary Shares (representing 6,800 Ordinary Shares) at an exercise price of $10,000.00 per Depositary Share; |
| · | warrants, issued to Armistice in a May 2020 private placement, exercisable for 406 Depositary Shares (representing
162,400 Ordinary Shares) at an exercise price of $320.00 per Depositary Share; |
| · | warrants, issued to various investors in a May 2020 private placement, exercisable for 415 Depositary
Shares (representing 166,000 Ordinary Shares) at an exercise price of $3,280.00 per Depositary Share; |
| · | warrants, issued to the placement agent in connection with the May 2020 private placement, exercisable
for 17 Depositary Shares (representing 6,800 Ordinary Shares) at an exercise price of $3,300.00 per Depositary Share; |
| · | warrants, issued in a May 2020 placing in the United Kingdom, exercisable for 349,600 Ordinary Shares,
at an exercise price of £6.80 per share; |
| · | warrants, issued in August 2022, exercisable for 16,400 Ordinary Shares, at an exercise price of £2.70
per Ordinary Share; |
| · | warrants, issued in February 2023 to the Placement Agent in connection with the December 2022 registered
direct offering, exercisable for 49 Depositary Shares (representing 19,600 Ordinary Shares) at an exercise price of $400.00 per Depositary
Share; |
| · | warrants, issued in February 2023 to the Placement Agent in connection with the February Private Placement,
exercisable for 1,293 Depositary Shares (representing 517,200 Ordinary Shares) at an exercise price of $232.00 per Depositary Share; |
| · | Series C Warrants, issued in June 2023, exercisable for 256,324 Depositary Shares (representing 102,529,600
Ordinary Shares), at an exercise price of $16.00 per Depositary Share (with a cashless exercise option); |
| · | Series D Warrants, issued in June 2023, exercisable for 279,699 Depositary Shares (representing 110,679,610
Ordinary Shares), at an exercise price of $16.00 per Depositary Share; |
| · | warrants, issued in June 2023, to the Placement Agent in connection with the May 2023 Registered Direct
Offering, exercisable for 11,067 Depositary Shares (representing 4,426,800 Ordinary Shares), at an exercise price of $15.00 per Depositary
Share; and |
| · | warrants issuable in connection with this offering, including to the Representative. |
Except as otherwise noted,
all information in this prospectus reflects and assumes (i) no sale of Class B Units, which, if sold, for each Depositary Share
underlying a Pre-Funded Warrant, the number of Depositary Shares that we are offering will be decreased on a one-for-one basis, (ii) no
exercise of outstanding options issued under our equity incentive plans, (iii) no exercise of any warrants issued in this offering
and (iv) no exercise of the underwriters’ option to purchase additional Depositary Shares and/or Series E Warrants, or any
combination thereof.
DILUTION
If you invest in our securities,
your ownership interest may be diluted to the extent of the difference between the amount per unit paid by purchasers, assuming that only
Class A Units are issued and no value is attributed to the warrants, in this public offering and the as adjusted net tangible book
value per share of our Depositary Shares immediately after the closing of this offering. Such calculation does not reflect any potential dilution
associated with the sale and exercise of warrants, which would cause the actual dilution to you to be higher.
Our
net tangible book value as of June 30, 2023 was approximately £5.29 million, or $9.65 per Depositary Share. Net tangible book value
per Depositary Share is determined by dividing our total tangible assets, less total liabilities, by the number of our Ordinary Shares
outstanding as of June 30, 2023, and multiplying such amount by 400 (one Depositary Share represents 400 Ordinary Shares).
After
giving effect to the sale of Class A Units by us in this offering
at an assumed public offering price of $ per Class A Unit and after deducting estimated underwriting discounts and
commissions, and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2023 would have
been approximately $ million, or $ per Depositary Share.
This represents an immediate decrease in net tangible book value of $ per Depositary Share to
our existing shareholders and an immediate dilution of $ per Depositary Share to investors purchasing units
in this offering. The final public offering price will be determined through negotiation between us and the underwriters in the offering
and may be at a discount to the current market price. Therefore, the assumed public offering price used throughout this prospectus may
not be indicative of the final public offering price.
The
following table illustrates this dilution on a per Depositary Share basis:
| | |
| As at June 30, 2023 | |
| | |
| Per American Depositary Shares | |
| Assumed public offering price per Depositary Share | |
$ | | |
| Historical net tangible book value as of June 30, 2023 | |
$ | 9.65 | |
| Increase to net tangible book value per Depositary Share attributable to investors purchasing our Depositary Shares in this offering | |
$ | | |
| As Adjusted net tangible book value per Depositary Share as of June 30, 2023, after giving effect to this offering | |
$ | | |
| Dilution per Depositary Share to the investors in this offering | |
$ | | |
To
the extent that outstanding options or other securities are exercised or converted into Ordinary Shares or Depositary Shares, the investors
purchasing our Depositary Shares in this offering will experience significant further dilution. In addition, we may choose to raise additional
capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating
plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these
securities could result in further dilution to our shareholders.
The total number of Ordinary
Shares outstanding as of the date of this prospectus and after this offering is based on 277,971,722 shares outstanding as of June 30,
2023, assumes the sale of Class A Units based on an assumed public offering price of $ ,
the last reported sales price of a Depositary Share on the NASDAQ Capital Market on
, 2023, and excludes the following other securities as of June 30, 2023:
| · | 121,340 Ordinary Shares issuable upon the exercise of stock options
outstanding under our equity incentive plans at a weighted-average exercise price of £5.05 per share; |
| · | 134 Ordinary Shares issuable upon the exercise of stock options assumed in connection with the acquisition
of DARA BioSciences, Inc., or DARA, at a weighted average exercise price of $1,903.40 per share; |
| · | warrants, issued to Armistice in an October 2019 private placement, exercisable for 375 Depositary Shares
(representing 150,000 Ordinary Shares) at an exercise price of $320.00 per Depositary Share; |
| · | warrants, issued to the placement agent in connection with an October 2019 private placement, exercisable
for 17 Depositary Shares (representing 6,800 Ordinary Shares) at an exercise price of $10,000.00 per Depositary Share; |
| · | warrants, issued to Armistice in a May 2020 private placement, exercisable for 406 Depositary Shares (representing
162,400 Ordinary Shares) at an exercise price of $320.00 per Depositary Share; |
| · | warrants, issued to various investors in a May 2020 private placement, exercisable for 415 Depositary
Shares (representing 166,000 Ordinary Shares) at an exercise price of $3,280.00 per Depositary Share; |
| · | warrants, issued to the placement agent in connection with the May 2020 private placement, exercisable
for 17 Depositary Shares (representing 6,800 Ordinary Shares) at an exercise price of $3,300.00 per Depositary Share; |
| · | warrants, issued in a May 2020 placing in the United Kingdom, exercisable for 349,600 Ordinary Shares,
at an exercise price of £6.80 per share; |
| · | warrants, issued in August 2022, exercisable for 16,400 Ordinary Shares, at an exercise price of £2.70
per Ordinary Share; |
| · | warrants, issued in February 2023 to the Placement Agent in connection with the December 2022 registered
direct offering, exercisable for 49 Depositary Shares (representing 19,600 Ordinary Shares) at an exercise price of $400.00 per Depositary
Share; |
| · | warrants, issued in February 2023 to the Placement Agent in connection with the February Private Placement,
exercisable for 1,293 Depositary Shares (representing 517,200 Ordinary Shares) at an exercise price of $232.00 per Depositary Share; |
| · | Series C Warrants, issued in June 2023, exercisable for 256,324 Depositary Shares (representing 102,529,600
Ordinary Shares), at an exercise price of $16.00 per Depositary Share (with a cashless exercise option); |
| · | Series D Warrants, issued in June 2023, exercisable for 279,699 Depositary Shares (representing 110,679,610
Ordinary Shares), at an exercise price of $16.00 per Depositary Share; and |
| · | warrants, issued in June 2023, to the Placement Agent in connection with the May 2023 Registered Direct
Offering, exercisable for 11,067 Depositary Shares (representing 4,426,800 Ordinary Shares), at an exercise price of $15.00 per Depositary
Share. |
Except as otherwise noted, all information in this
prospectus reflects and assumes (i) no sale of Class B Units, which, if sold, for each Depositary Share underlying a Pre-Funded
Warrant, the number of Depositary Shares that we are offering will be decreased on a one-for-one basis, (ii) no exercise of outstanding
options issued under our equity incentive plans, (iii) no exercise of any warrants issued in this offering and (iv) no exercise
of the underwriters’ option to purchase additional Depositary Shares and/or Series E Warrants, or any combination thereof.
BUSINESS
Overview
We
are a clinical stage biopharmaceutical company developing a pipeline of products aimed at primary and metastatic cancers of the brain.
Our lead candidate, MTX110, is being studied in aggressive rare/orphan brain cancer indications including recurrent glioblastoma and diffuse
midline glioma, or DMG.
MTX110
is a liquid formulation of the histone deacetylase, or HDAC inhibitor, panobinostat. Our proprietary formulation enables delivery
of the product via convection-enhanced delivery, or CED, at potentially chemotherapeutic doses directly to the site of the tumor, by-passing
the blood-brain barrier and avoiding systemic toxicity.
Our
clinical assets are supported by three proprietary drug delivery technologies focused on improving bio-delivery and bio-distribution of
drugs through either sustained delivery (Q-SpheraTM), direct delivery (MidaSolveTM), or targeted delivery (MidaCoreTM):
| · | Our Q-Sphera platform: Our disruptive polymer microsphere microtechnology is used for sustained
delivery to prolong and control the release of therapeutics over an extended period of time, from weeks to months. |
| · | Our MidaSolve platform: Our innovative oligosaccharide nanotechnology is used to solubilize
drugs so that they can be administered in liquid form directly and locally into tumors. |
| · | MidaCore platform: Our gold nanoparticle nanotechnology is used
for targeting sites of disease by using either chemotherapeutic agents or immunotherapeutic agents. |
Additional
information regarding the general development of our business is set forth in our Annual Report on Form 20-F for the year ended December
31, 2022, as amended.
Recent Developments
MTX110 Developments
On June 1, 2022, we announced
that upon submitting an application to the FDA, our development program of MTX110 for the treatment of recurrent glioblastoma had been
granted Fast Track designation by the agency. Fast Track is a process designed to facilitate the development and expedite the review of
treatments for serious conditions and that potentially address unmet medical needs. Drugs that are granted this designation are given
the opportunity for more frequent interactions with the FDA, as well as potential pathways for expedited approval.
On January 12, 2023, we announced
that, following completion of one-month treatment with MTX110 in our first patient, our Phase I study of MTX110 in recurrent glioblastoma
(also known as the MAGIC-G1 study) would continue with a planned dose escalation following positive recommendation from the study’s
DSMB. MAGIC-G1 is an open-label, dose escalation study designed to assess the feasibility and safety of intermittent infusions of MTX110
administered by CED via implanted refillable pump and catheter. The study aims to recruit two cohorts (cohort A and cohort B), each with
a minimum of four patients; the first cohort will receive MTX110 following implantation of the CED system and the second cohort will also
receive MTX110 but with the option of the treating investigator to re-position the catheter into an area of new lesion upon progression,
with the objective of increasing tumor coverage and survival.
The first patient in cohort
A was dosed at 60uM of MTX110 via direct-to-tumor delivery and has received 13 48-hour infusions over a period of 19 weeks. No treatment-associated
adverse events were noted in the patient during this period. Following successful completion of the first month of treatment, the DSMB
reviewed the available data and recommended dose escalation in the study to 90uM, which we believe is the optimal dose. To date, there
have been no dose-limiting toxicities.
On October 3, 2023, we announced
the completion of recruitment into cohort A with the minimum number of four patients. Enrollment in cohort B is expected to commence upon
the approval of the study by the DMSB, which is expected towards the end of October 2023.
On July 10, 2023, we announced
the completion of enrollment and the treatment of nine pediatric patients with DMG in the ongoing Phase I study of MTX110 at Columbia
University Irving Medical Center. All of the patients, aged four to 17 years old, received radiation therapy as per the institutions’
standard of care. Each patient subsequently underwent surgery with implantation of an intratumoral catheter and a programmable subcutaneous
pump and eight out of nine received two infusions of MTX110 via CED separated by a period of one week. Concentrations of 30, 60 or 90
µM were delivered with no intra-patient dose escalation. To date, no dose limiting toxicities related to the study drug have been
reported. We expect full results to be announced in the first quarter of 2024.
Change
in Ratio of Depositary Shares
On
July 5, 2023, in an effort to bring our Depositary Share price into compliance with NASDAQ’s minimum bid price per share requirement,
we effected a ratio change in the number of Ordinary Shares represented by our Depositary Shares from five Ordinary Shares per Depositary
Share to 400 Ordinary Shares per Depositary Share. This had the effect of a one-for-80 reverse split of the Depositary Shares. No
fractional Depositary Shares were issued.
Regained Compliance with NASDAQ Continued
Listing Requirements
Our Depositary Shares are
currently listed on the NASDAQ Capital Market. We are required to meet certain qualitative and financial tests to maintain the listing
of our Depositary Shares on NASDAQ. On January 31, 2023, we received a letter from NASDAQ stating that, for the previous 30 consecutive
business days, the bid price for our Depositary Shares had closed below the minimum $1.00 bid price per share requirement for continued
listing on the NASDAQ Capital Market under NASDAQ Listing Rule 5550(a)(2). On July 20, 2023, we received written notice from NASDAQ notifying
us that for the preceding 10 consecutive business days the closing bid price for our Depositary Shares had been $1.00 or greater. Accordingly,
NASDAQ determined that we had regained compliance with NASDAQ Listing Rule 5550(a)(2).
NASDAQ further notified us
that we are subject to a mandatory panel monitor for a period of one year from July 20, 2023. If, within that one-year monitoring period,
the NASDAQ Listing Qualifications Staff finds us out of compliance with the rules that was subject to the previous exception, the Listing
Qualifications Staff will issue a delisting determination letter and we will have the opportunity to request a hearing with the Nasdaq
Hearings Panel.
Having regained compliance
with these rules, we are now in compliance with all applicable requirements for continued listing on the NASDAQ Capital Market. We cannot
assure you that we will remain in compliance with all applicable requirements for continued listing on the NASDAQ Capital Market.
June
AGM and June GM
On
June 14, 2023, we held the June AGM and our shareholders passed resolutions, among other procedural items, to approve the allotment of,
and disapplication of pre-emption rights in respect of, up to 7.0 billion Ordinary Shares. On June 14, 2023, we also held the June GM,
and our shareholders passed resolutions to approve the (x)(i) Re-Designation, and (ii) the Subdivision, which became effective on June
15, 2023 and (y) adopt new Articles of Association, which make consequential amendments to the existing articles of association of the
Company to reflect the Re-Designation and the Subdivision, together with certain other changes to reflect that the Ordinary Shares are
no longer admitted to trading on AIM. As is standard for deferred shares, each B Deferred Share has very limited rights and is effectively
valueless. The B Deferred Shares have the rights and restrictions as set out in the Articles of Association and do not entitle the holder
thereof to receive notice of or attend and vote at any general meeting of the Company or to receive a dividend or other distribution.
May 2023
Registered Direct Offering
On
May 26, 2023, we completed the closing of the May 2023 Registered Direct Offering, with the Investors for the sale of 110,679,610 Ordinary
Shares represented by 279,699 Depositary Shares at a price per Depositary Share of $12.00, for aggregate gross proceeds of $3.32 million.
Pursuant
to the terms of Purchase Agreement entered into in connection with the May 2023 Offering, following Shareholder Approval on June 14, 2023,
on June 20, 2023, we issued to the Investors (i) Series C Warrants exercisable for an aggregate of 415,043 Depositary Shares representing
166,017,300 Ordinary Shares and (ii) Series D Warrants exercisable for an aggregate of 279,699 Depositary Shares representing 110,679,610
Ordinary Shares. The warrants were exercisable at an exercise price of $16.00 per Depositary Share, subject to adjustments for certain
dilutive equity issuances. The warrants were exercisable upon receipt of Shareholder Approval. The Series C Warrants will expire one year
from the initial exercise date and may be exercised on a cashless basis, subject to the satisfaction of payment of not less than the nominal
value of the ordinary shares under the provisions of the Companies Act. The Series D Warrants will expire five years from the initial
exercise date. A holder of the warrants may not exercise the warrants if the holder, together with its affiliates, would beneficially
own more than 4.99% or 9.99% (such amount to be determined at the option of the holder) of the number of Ordinary Shares outstanding immediately
after giving effect to such exercise. The Purchase Agreement contains customary representations, warranties and covenants of the Company
and each Investor, and customary indemnification provisions for a transaction of this type, including taking reasonable steps to maintain
the Depositary Shares listed on NASDAQ.
Pursuant
to the terms and conditions set forth in the Placement Agency Agreement, we agreed to pay the Placement Agent a cash fee in an amount
equal to 8.0% of the aggregate gross proceeds of the Offering, and to issue to the Placement Agent, the May 2023 Placement Agent Warrants.
Following Shareholder Approval on June 14, 2023, we issued to the Placement Agent May 2023 Placement Agent Warrants having substantially
the same terms as the Series D Warrants, except that the exercise price of the May 2023 Placement Agent Warrant is 125% of the offering
price of the Depositary Shares issued in the May 2023 Registered Direct Offering and the term of the May 2023 Placement Agent Warrants
terminates on the three-year anniversary of the initial exercise date as defined in the May 2023 Placement Agent Warrant. We also agreed
to pay the Placement Agent a management fee equal to 1.0% of the gross proceeds raised in the May 2023 Offering and an expense allowance
of up to $85,000 for legal fees and other out-of-pocket expenses.
Additionally,
pursuant to the terms of the Purchase Agreement, we agreed to not issue any securities that are subject to a price reset based on the
trading prices of our Ordinary Shares or upon a specified or contingent event in the future or enter into any agreement to issue securities
at a future determined price for a period of one year following the closing date of the Warrant Private Placement, subject to an exception.
Reverse Stock Split,
Change of Name and AIM Cancellation
On
March 24, 2023, we held a general meeting where our shareholders approved, among other things, (i) a change of our name to “Biodexa
Pharmaceuticals PLC,” (ii) the allotment of up to 100% of our fully diluted share capital for future share issuances through our
annual general meeting in 2025, (iii) the cancellation of admission of our Ordinary Shares from trading on AIM, and (iv) a one-for-20
reverse stock split of our Ordinary Shares. The reverse stock split of our Ordinary Shares was effective as of March 27, 2023.
Concurrently
with the reverse split, and in order to continue meeting NASDAQ’s minimum 500,000 publicly held shares requirement pursuant to Rule
5550(a)(4), on March 27, 2023, we changed the ratio of Depositary Shares from one Depositary Share representing 25 Ordinary Shares to
a new ratio of one Depositary Share representing five Ordinary Shares. This had the effect of a one-for-four reverse split of our Depositary
Shares. No fractional Depositary Shares were issued.
Additionally,
as noted above, our shareholders approved the cancellation of admission of our Ordinary Shares on AIM. This cancellation became effective
on April 26, 2023. The Depositary Shares trade exclusively on the NASDAQ Capital Market under the symbol “BDRX.” There is
currently no market for our Ordinary Shares.
February 2023 Private
Placement
On
February 15, 2023, we completed the February Private Placement with certain institutional investors, for the sale of (i) 3,250,000 Ordinary
Shares represented by 8,125 Depositary Shares, (ii) 12,931,200 Ordinary Shares represented by 32,327 Depositary Shares, issuable upon
the exercise of Series A Warrants issued in the February Private Placement, (iii) 19,396,400 Ordinary Shares represented by 48,491 Depositary
Shares, issuable upon the exercise of Series B Warrants issued in the February Private Placement, and (iv) up to 71,749,600 Ordinary Shares
represented by 179,374 Depositary Shares, issuable upon the exercise of pre-funded warrants issued in the February Private Placement,
subject to certain reset provisions set forth in the pre-funded warrants, at an initial purchase price of $185.60 per Depositary Share,
for aggregate gross proceeds of approximately $6.0 million.
In
addition, in connection with the February Private Placement, on February 9, 2023 we entered into the Waiver providing for a permanent
waiver of certain equity issuance prohibitions and participation rights under the December SPA. In connection therewith, we agreed to,
subject to receipt of stockholder approval, issue to Armistice Series A Warrants exercisable for 625,000 Ordinary Shares represented by
1,562 Depositary Shares. Further, we issued to the Placement Agent, in connection with their engagement as placement agent in the February
Private Placement, the February Placement Agent Warrants.
On
March 24, 2023, we held a general meeting where our shareholders approved, among other things, the allotment of, and disapplication of
preemption rights with respect to, the Ordinary Shares to be issued under the Series A Warrants, the Series B Warrants, certain of the
pre-funded warrants, and the February Placement Agent Warrants. We subsequently issued the warrants to the investors and the Placement
Agent.
Our Strategy
In
early 2023, we decided to re-position the Company as a therapeutics (as opposed to drug delivery) company. As a result, the delivery of
proof-of-concept clinical data is the primary focus of our business model going forward.
We
aim to deploy our proprietary technologies to develop proof of concept formulations and then enter into licensing agreements with third
party pharmaceutical companies.
Development
Our
intention is to build a balanced portfolio of clinical-stage development assets, ideally focused on oncology and on rare or orphan indications.
Our only current clinical-stage asset, MTX110, is in Phase I development for three rare or orphan brain cancers.
Our
research and development programs may, like MTX110, be based on one or more of our enabling technologies.
Our
aim is to enter into research and development collaborations with third parties to develop proof-of-concept formulations of their proprietary
compounds using our proprietary drug delivery technologies. We do not intend to expand our internal pipeline of drug delivery based programs.
Manufacturing
To
establish proof-of-concept in pre-clinical studies for potential licensees, we are able to manufacture non-GMP Q-Sphera products at pilot
scale at our Cardiff, Wales facility. Our intention is to technology transfer the manufacturing of clinical trial supplies and, ultimately,
full GMP commercial manufacture to a third party CMO. We would expect a licensee to assume the cost of manufacturing GMP product and commercial
scale-up pursuant to a technology transfer agreement.
MTX110 is currently being
manufactured to GMP standards at a CMO.
Commercialization
Once
proof-of-concept has been established, we intend to seek to license our products to a partner who would complete the development, and
subsequently market and sale, of the product in an agreed upon licensed territory. In addition to reimbursement of development costs,
the partner would be expected to make milestone payments based on sales targets and royalty payments.
Our Pipeline and Platform
Technologies
We
are actively pursuing the development of MTX110 in three Phase I studies. Our other product candidates, MTD201, Q-octreotide and MTD211,
Q-brexpiprazole are not being actively developed but are available for licensing. MTD217 is an internal preclinical research program recently
added to our portfolio. Our development pipeline included six projects as follows:

Clinical Stage Assets
MTX110. Using
our MidaSolve technology in combination with panobinostat, an otherwise insoluble drug, MTX110 is designed for direct-to-tumor treatment
of intractable brain cancers. Panobinostat is currently marketed under the brand Farydak® which is used orally in combination therapy
for the treatment of multiple myeloma. We are currently researching the utility of MTX110 to proof of concept stage in three indications:
Glioblastoma
Multiforme (GBM): GBM is the most common and aggressive form of brain cancer in adults, usually occurring in the white matter
of the cerebrum. Treatments include radiation, surgical resection and chemotherapy although, in almost all cases, tumors recur. Based
on available date from the American Association of Neurosurgeons, there are approximately 2-3/100,000 population diagnoses of GBM per
annum. Survival with standard of care treatment ranges from approximately 13 months in patient with an unmethylated MGMT gene promotor
to approximately 30 months in patients with a highly methylated MGMT gene promotor.
Following
IND approval in December 2021, we are in the process of recruiting patients in a Phase I study to assess the utility of MTX110 in recurrent
GBM. The Phase I study is an open-label, dose escalation study designed to assess the feasibility and safety of intermittent infusions
of MTX110 administered by CED via implanted refillable pump and catheter. The study aims to recruit two cohorts, each with a minimum of
four patients; the first cohort will receive MTX110 only and the second cohort will receive MTX110 in combination with lomustine.
Diffuse
Midline Glioma (DMG): DMG, formerly known as diffuse intrinsic pontine glioma (DIPG), tumors are located in the pons (middle)
of the brain stem and are diffusely infiltrating. Occurring mostly in children, approximately 1,000 patients worldwide are diagnosed with
DMG per annum and median survival is approximately 10 months. There is no effective treatment since surgical resection is not possible.
The standard of care is radiotherapy, which transiently improves symptoms and survival. Chemotherapy does not improve survival and one
likely reason is that many anti-cancer drugs cannot cross the blood-brain barrier to access the tumor.
In
October 2020, we reported the first-in-human study by the University of California, San Francisco of MTX110 in DMG using a CED system.
The Phase I study established a recommended dose range for Phase II, a good safety and tolerability profile but also encouraging survival
data in the seven patients treated.
Medulloblastoma: Medulloblastomas
are malignant embryonal tumors that start in the cerebellum. They are invasive and, unlike most brain tumors, spread through the cerebrospinal
fluid, or CSF, and frequently metastasize to different locations in the brain and spinal cord. Treatments include resection, radiation
and chemotherapy. Approximately 350 patients are diagnosed with medulloblastoma per annum and 3,800 people are living with the disease
in the United States. The cumulative survival rate is approximately 60%, 52%, and 47% at 5 years, 10 years, and 20 years, respectively;
however, recurrence is nearly always fatal with no established standard of care.
The
University of Texas is undertaking a Phase I exploratory study in recurrent medulloblastoma patients using direct administration of MTX110
into the fourth ventricle, enabling it to circulate throughout the CSF.
In
2020, our non-exclusive worldwide, sublicensable license to certain patents of panobinostat was terminated by Secura Bio. We view MTX110
as an important asset and currently have two ongoing clinical trials for MTX110 and intend to commence two further clinical trials as
part of our MTX110 clinical program. We continue to enjoy freedom to use panobinostat for research purposes and we plan to continue to
pursue development of MTX110. We believe that the relevant Secura Bio patents may delay a launch of MTX110 for use in patients with DMG,
however we do not anticipate it would have any impact on launching MTX110 for use in patients with GBM. If we are unable to launch a product
candidate until the patent expires, there could be a material adverse effect on our business, financial condition and results of operations.
For additional information
regarding MTX110, see “—Recent Developments—MTX110 Developments.
Technologies
In
2020, following a strategic review, we pivoted from a largely singular focus on the clinical development and manufacturing scale up of
MTD201 to a strategy based on a broader, but earlier stage, pipeline designed to optimize opportunities for partnering success. In early
2023 we refined our strategy to focus on our clinical stage assets and ceased further development of our internal pipeline of drug delivery
programs.
Q-Sphera
Our
Q-Sphera technology employs 3-D printing techniques to encapsulate medicines in polymer-based bioresorbable microspheres. Q-Sphera is
a precise, scalable, efficient, and environmentally friendly microparticle manufacturing platform. From a clinical perspective, Q-Sphera
ensures monodispersed microparticles that release active drug compounds into the body in a tightly controlled, highly predictable and
linear manner over an extended period of time.
Q-Sphera
is the next generation polymer microsphere technology which simplifies manufacturing, facilitates the sustained release of products and
delivers formulations with significant patient, healthcare professional and payor benefits. Our polymer microsphere platform has been
developed to enable sustained release delivery solutions for peptide and small-molecule therapeutics through precise definition of the
properties of polymer microparticles into which active compounds can be incorporated. Microspheres are small, spherical particles that
can be utilized as a time release drug capsule. This technology contributes to our oncology franchise as well as potential applications
in endocrinology and other disease areas.
Current
reactor-based emulsion manufacturing technology has been in use for over 20 years and, despite several issues, it continues to be used
by the vast majority of the market as there are limited alternatives. Reactor-based emulsion processes, used by most existing products,
require large infrastructure, are energy intensive, inefficient and wasteful, producing large quantities of unusable particles, and utilize
large volumes of toxic organic solvents that are damaging to the environment.
The
microspheres may be injected to form depots in the body, which release drugs over predictable, sustained periods from one week up to several
months.
MTD211.
We have developed a long-acting formulation of brexpiprazole using Q-Sphera technology. Marketed under the brand name Rexulti® by
Otsuka Pharmaceutical Co., Ltd, brexpiprazole is indicated for the treatment of schizophrenia and adjunctive treatment of major depressive
disorder and is currently only available as an immediate release oral tablet. The market for anti-psychotic drugs is shifting towards
long-acting formulations for reasons of improved patient compliance and lowering of payor costs associated with patient hospitalization
events. MTD211 is available for licensing.
MTD201.
Following the announcement of a strategic review, we ceased further in-house development of MTD201, our Q-Sphera formulation of octreotide
for acromegaly and neuroendocrine tumors. We completed two clinical trials for MTD201, a 2018 first-in-human Phase I study compared MTD201
with Novartis’ Sandostatin LAR Depot®, and second Phase I study of MTD201 completed in January 2020 which compared subcutaneous
administration with intramuscular administration in healthy volunteers and showed similar pharmacokinetics and bioavailability in healthy
volunteers. Together, the two Phase I studies of MTD201 serve as clinical validation of the Q-Sphera technology, confirming many of Q-Sphera’s
features and benefits for patients, payors and licensees. MTD201 is also available for licensing.
MidaSolve
Our
MidaSolve nano inclusion technology is utilized for potent, small molecule chemotherapeutics that have minimal solubility in water at
biological pH, which means they cannot normally be injected and limits them to oral administration in solid form. When reformulated with
MidaSolve technology, the complexed molecules solubilize such that the molecule can be administered in liquid form into the body. This
enables local infusion directly into the tumor, thus extending the available routes of administration for drugs that otherwise would be
limited to oral forms only.
The
complexed molecules comprise a hydrophobic (‘water-fearing’) inner surface and a hydrophilic (‘water-loving’)
outer surface, and as a result are capable of forming host-guest complexes with normally water-insoluble molecules. A hydrophobic, poorly
water-soluble drug can associate with the inner, more hydrophobic surface of the MidaSolve host, while the hydrophilic outer surface allows
the complex to dissolve at biological pH.
MTX110.
Using our MidaSolve technology in combination with panobinostat, an otherwise insoluble drug, MTX110 is designed for direct-to-tumor treatment
of intractable brain cancers. The resulting complex is readily soluble in water at therapeutic concentrations, thus enabling liquid administration
routes directly into the tumor that otherwise would not be possible. Given in its natural oral form, panobinostat does not cross the blood-brain
barrier, and thus does not reach brain tumors. It is also a highly toxic substance and, when given orally, suffers from significant dose-limiting
side effects. MidaSolve allows an alternate means of delivery in liquid form, and MTX110, our soluble form of panobinostat, is infused
directly into the tumor. Direct delivery of MTX110 bypasses the blood-brain barrier and ensures adequate drug exposure to tumor cells
without exposing the rest of the body to potentially toxic concentrations. MTX110’s intratumoral delivery thus provides a significant
potential for treatment of DMG and, potentially, other forms of brain cancer.
MTD217.
Our MTD217 program explores simultaneous inhibition of key metabolic pathways in cancer, including glycolysis and oxidative phosphorylation
, or OXPHOS. The research is centered around a water-soluble drug formulation that can be infused or injected directly into the cancer
microenvironment, disrupting metabolic functions in a highly localized manner, thus limiting off-target toxicity. We have been able to
demonstrate up to a six-fold synergistic effect of administering MTX110 with an OXPHOS inhibitor in vitro with three patient-derived
cells lines. Our initial target is treatment of leptomeningeal disease, a lethal complication in which metastatic cancer cells invade
the cerebrospinal fluid and central nervous system.
MidaCore
MidaCore
is a leading innovation in ‘ultra-small’ nanomedicine and is designed for targeted delivery to enable improved delivery of
therapeutics to tumor cells and the immune system. In oncology treatments MidaCore provides a nano complex (less than 5nm in size, or
approximately 80,000 times smaller than the width of a hair) that carries conventional small molecule chemo-therapeutic payloads and delivers
these to the tumor site in high concentrations. In immunotherapy, treatments, MidaCore acts as a nanocarrier complex for synthetic immuno-peptides
that stimulate the immune system to seek out and destroy cancer cells via immune mediated vaccine processes. These small complexes can
enter immune processing cells to induce T-cell mediated immune responses specifically against tumor cells, viral infected host cells or
autoimmune disease.
The
MidaCore technology platform is based upon ‘ultra-small’ nanoparticle drug conjugates, which at 2-4nm in size are among the
smallest particles in biomedical use. They are composed of a core of gold atoms decorated with a permutation of therapeutic and/or targeting
molecules. The small size and multi-functional arrangement around the gold core underpin the ability to improve biodistribution, and target
tumor and/or immune sites providing a new generation of oncology drugs.
Commercial Agreements, Strategic Partnerships and Collaborations
We are currently collaborating
with a number of biopharmaceutical companies, research institutes and universities on several of our development programs involving our
core technologies.
On July 21, 2020, we announced
a collaboration with the European affiliate of a global pharmaceutical company to deploy our in-house expertise and proprietary drug delivery
platforms towards product candidates nominated by the collaborating company. On January 17, 2022, we announced the extension of this collaboration
and disclosed the collaborator as Janssen, an affiliate of Johnson & Johnson. On March 9, 2022, we announced we had extended this
collaboration to include another large molecule. The final deliverables were provided to Janssen and the collaboration concluded in September
2023.
CMS License Agreement.
On January 29, 2019, we entered into the CMS License Agreement with CMS, as guarantor, and the Licensees. The CMS License Agreement
was effective as of February 26, 2019. Pursuant to the terms of the CMS License Agreement, we agreed to license to the Licensees the exclusive
right to use our technology and our intellectual property rights and information and data related to certain of our clinical and pre-clinical
products (i.e. MTD201, MTX110, MTX102, MTR103 and MTD119), together with any other pipeline products or line extensions which are in or
which enter pre-clinical or clinical development in the first three years following the effective time of the CMS License Agreement, together
the Products, to develop and commercialize the Products in China, including Macau, Hong Kong and Taiwan, with the same rights in certain
countries in south east Asia in respect of which the Licensees notifies us that such licensee wants a license after the grant of a regulatory
approval of any of the Products by the FDA, EMA or by the regulatory authorities in the United Kingdom, France, Germany or Switzerland,
collectively the Territory, such activities to be conducted by the Licensee(s) and affiliates of CMS and local partners as permitted sub-licensees.
The Licensees have the exclusive right to import, obtain market approvals and register, market, distribute, promote and sell the Products
in the Territory at the Licensees’ sole discretion, and in the event we choose not to or fail to meet the Licensees’ binding
orders for the Products under certain circumstances, will be granted the right to manufacture the Products itself. The Licensees will
be restricted from supplying the Products to any customers outside of the Territory, while we will be restricted from supplying the Products
into the Territory, except through the Licensees.
In addition, we agreed to
assist the Licensees (and/or any affiliate of CMS) with their applications for marketing approvals for the Products in the Territory,
which approvals, if granted, will be exercised by CMS Bridging or CMS Medical HK, unless it is being transferred to us when we are entitled
to terminate the CMS License Agreement for material breach by CMS Bridging or CMS Medical HK. We will manufacture the Products for the
Licensees and their sub-licensees, which Products will be subject to exclusive purchase and supply arrangements with the Licensees for
the Territory.
Further, we agreed to permit the Licensees to identify
their own product and line extension targets in respect of which, if we agree, we will carry out initial development and then will, for
a technology transfer fee, the amount of which will be dependent on the circumstances, transfer the specific program know-how and data
to enable the Licensees to continue to develop using our platform technologies and then to commercialize in the Territory. We will receive
a low single digit royalty on the Net Sales (as such term is defined in the CMS License Agreement) in the Territory. The Licensees will
own any intellectual property rights it creates and any data they collect during the development process and will license such rights
and data to us for the purposes of manufacturing the products in question and also to commercialize the products outside the Territory,
for which we will pay the Licensees a low double digit royalty.
The Licensees shall pay us
lump sum payments on a Product-by-Product basis (in U.S. dollars) upon the achievement of certain regulatory approvals (in six, or potentially
seven, figure amounts) and sales performance milestones (in seven, or potentially, eight figure amounts), as well as royalties upon Net
Sales (as a low double digit percentage for the Products other than MTX110, for which the royalty will be a single digit percentage) in
the Territory.
The CMS License Agreement
may be terminated by either party for specific material breaches or insolvency. In particular, our rights to terminate are limited to
breaches of certain non-compete restrictions, failure to pay milestones or royalties, insolvency, or a failure to develop and/or commercialize
particular Products in particular countries after the grant of an FDA or EMA regulatory approval. In addition, we have the right to terminate
the agreement if the Licensee directly or indirectly infringes upon our intellectual property rights or challenges their validity or,
in relation to a particular Product and a particular Territory at any time, because the Licensee has made a determination that it no longer
wishes to develop and/or commercialize the Product in that country in the Territory. The CMS License Agreement also includes customary
indemnification for a transaction of this type.
Additional Information
Additional information regarding the Company, including
our intellectual property, sales and marketing, research and development capabilities, competitors and regulatory environment, is set
forth in our Annual Report on Form 20-F for the year ended December 31, 2022, as amended.
PRINCIPAL SHAREHOLDERS
The following table sets forth information, to
our knowledge, as of August 31, 2023, regarding the beneficial ownership of Ordinary Shares (including those represented by Depositary
Shares), including:
| · | each person, or group of affiliated persons, that is known by us to be a beneficial owner of 3% or more
of Ordinary Shares (based on information in our share register and information provided by such persons), if any; |
| · | each member of our Board of Directors; |
| · | each member of our senior management; and |
| · | all members of our Board of Directors and our senior management, taken as a group. |
Beneficial ownership of shares
is determined under rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment
power. Except as noted by footnote, and subject to community property laws where applicable, we believe, based upon the information provided
to us, that the persons and entities named in the table below have sole voting and investment power with respect to all Ordinary Shares
shown as beneficially owned by them. The percentage of beneficial ownership is based upon 365,799,722 Ordinary Shares outstanding as of
August 31, 2023. Ordinary Shares subject to options or warrants currently exercisable or exercisable within 60 days of August 31, 2023
are deemed to be outstanding and beneficially owned by the person holding the options or warrants for the purposes of computing the percentage
of beneficial ownership of that person and any group of which that person is a member, but are not deemed outstanding for the purpose
of computing the percentage of beneficial ownership for any other person. Unless otherwise indicated, the address for each holder listed
below is Biodexa Pharmaceuticals plc, 1 Caspian Point, Caspian Way, Cardiff, CF10 4DQ, United Kingdom. All holders of Ordinary Shares,
including those shareholders listed below, have the same voting rights with respect to such shares.
| Name of Beneficial Owner | |
Amount and Nature
Of Ownership | |
Percent
of Class |
| | |
| |
|
| Directors and Senior Management: | |
| |
|
| Stephen Parker | |
-- | |
* |
| Simon Turton | |
2,766 | |
* |
| Stephen Stamp (1) | |
30,250 | |
* |
| Sijmen de Vries | |
1,312 | |
* |
| Dmitry Zamoryakhin (2) | |
7,031 | |
* |
| Directors and senior management as a group (5 persons) (3) | |
41,359 | |
* |
__________________
| * | Represents beneficial ownership of less than one percent. |
| (1) | Shares owned by Mr. Stamp include 27,750 Ordinary Shares subject to outstanding stock options which are
exercisable at August 31, 2023 or will become exercisable within 60 days after such date. |
| (2) | Shares owned by Dr. Zamoryakhin are 7,031 Ordinary Shares subject to outstanding stock options which are
exercisable at August 31, 2023 or will become exercisable within 60 days after such date. |
| (3) | Shares owed by all directors and senior management as a group include 34,781 Ordinary Shares subject to
outstanding stock options, each of which are exercisable at August 31, 2023, or will become exercisable within 60 days after such date. |
As of August 31, 2023, approximately
0.2% of our outstanding Ordinary Shares was held by registered shareholders with addresses in the United Kingdom (excluding shares
held by the custodian under our depositary agreement with The Bank of New York Mellon), and we had 190 holders of record in the United
Kingdom. As of August 31, 2023, The Bank of New York Mellon, as depositary for the Depositary Shares, held 364,067,307 Ordinary Shares,
representing 99.5% of the issued share capital held at that date. As of August 31, 2023, we had two holders of record with an address
in the United States. The number of holders of record or registered holders in the United States or United Kingdom is not representative
of the number of beneficial holders or of the residence of beneficial holders.
Based on our share register,
we believe that we are not directly or indirectly controlled by another corporation or government, or by any other natural or legal persons.
There are no arrangement that may result in a change of control. Our major shareholders do not have different voting rights than other
holders of our Ordinary Shares.
To our knowledge, other than
due to the expiration of unexercised warrants held by our shareholders or as otherwise disclosed or incorporated by reference elsewhere
in this prospectus, there has been no significant change in the percentage ownership of our Ordinary Shares held by the principal shareholders
listed above in the last three years.
DESCRIPTION OF SECURITIES WE ARE OFFERING
The following description
summarizes certain terms of the Pre-Funded Warrants and Series E Warrants included in this offering. The material terms and provisions
of our Ordinary Shares are described under the caption “Description of Share Capital”. This summary does not purport
to be complete and is qualified in its entirety by the provisions of our Articles of Association and the warrants offered hereby, copies
of which are filed with the SEC as exhibits to the Registration Statement on Form F-1 of which this prospectus forms a part, and to applicable
law.
We are offering (i) Class A
Units, each Class A Unit consisting of one Depositary Share and one Series E Warrant to purchase one Depositary Share, and (ii) Class B
Units, each Class B Unit consisting of one Pre-Funded Warrant to purchase one Depositary Share and one Series E Warrant to purchase one
Depositary Share. For each Class B Unit we sell, the number of Class A Units we are offering will be decreased on a one-for-one basis.
Each Depositary Share and
Pre-Funded Warrant and accompanying Series E Warrant included in each unit will be immediately separable upon issuance and will be
issued separately will be immediately separable upon issuance and will be issued separately. The units will not be issued or certificated.
We are also registering the Depositary Shares included in the Class A Units and the Depositary Shares issuable upon exercise of the
Pre-Funded Warrants and Series E Warrants included in the units offered hereby.
Pre-Funded Warrants
The following description of the Pre-Funded
Warrants we are offering is a summary and is qualified in its entirety by reference to the provisions of the Pre-Funded Warrants, the
form of which is filed as an exhibit to the Registration Statement on Form F-1 of which this prospectus forms a part.
Duration and Exercise Price
Each Pre-Funded Warrant offered hereby will have
an initial exercise price per share equal to $0.0001. The Pre-Funded Warrants are exercisable at
any time after the initial exercise date until exercised in full and they do not expire. Pursuant to a warrant agency agreement between
us and , as warrant agent, the Pre-Funded Warrants will be issued in book-entry form and shall initially be represented by one or more
global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company, or DTC, and registered in the
name of Cede & Co., a nominee of DTC, or otherwise as directed by DTC.
Exercisability
The Pre-Funded
Warrants will be exercisable, at the option of the holder, in whole or in part, by delivering to us a duly executed exercise notice
accompanied by payment in full for the number of Depositary Shares purchased upon such exercise (except in the case of a cashless exercise,
as discussed below). A holder (together with its affiliates) may not exercise any portion of the Pre-Funded
Warrants to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding
Ordinary Shares immediately after exercise. However, upon notice from the holder to us, the holder may decrease or increase the holder’s
beneficial ownership limitation, which may not exceed 9.99% of the number of outstanding Ordinary Shares immediately after giving effect
to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded
Warrants, provided that any increase in the beneficial ownership limitation will not take effect until 61 days following notice
to us. Purchasers in this offering may also elect, prior to the issuance of the Pre-Funded Warrants,
to have the initial exercise limitation set at 9.99% of our outstanding Ordinary Shares. No fractional Depositary Shares will be issued
in connection with the exercise of a Pre-Funded Warrants. In lieu of fractional Depositary
Shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to
the next whole share.
Cashless Exercise
At the time a holder exercises
its Pre-Funded Warrants, in lieu of making the cash payment otherwise contemplated to be
made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either
in whole or in part) the net number of Depositary Shares determined according to a formula set forth in the Pre-Funded
Warrants.
Transferability
Subject to applicable laws, a Pre-Funded
Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate
instruments of transfer.
Exchange Listing
There is no trading market available for the Pre-Funded
Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Pre-Funded
Warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder
Except as otherwise provided in the Pre-Funded
Warrants, the holders of the Pre-Funded Warrants do not have any voting rights, dividends
or other rights as a holder of our capital stock until they exercise the warrants.
Fundamental Transaction
In the event of a fundamental
transaction, as described in the Pre-Funded Warrants and generally including any reorganization,
recapitalization or reclassification of our Ordinary Shares, the sale, transfer or other disposition of all or substantially all of our
properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Ordinary
Shares, or any person or group becoming the beneficial owner of more than 50% of the voting power represented by our outstanding Ordinary
Shares, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise
of the Pre-Funded Warrants the kind and amount of securities, cash or other property that
the holders would have received had they exercised the Pre-Funded Warrants immediately prior
to such fundamental transaction.
Series E Warrants
The following description of the Series E
Warrants we are offering is a summary and is qualified in its entirety by reference to the provisions of the Series E Warrant, the
form of which is filed as an exhibit to the Registration Statement on Form F-1 of which this prospectus forms a part.
Duration and Exercise Price
Each Series E Warrant
offered hereby will have an initial exercise price per share equal to $ . The Series E Warrants
will be immediately exercisable upon issuance and will expire on the fifth anniversary of their initial exercise date. The exercise price
and number of Depositary Shares issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits,
reorganizations or similar events affecting our Depositary Shares and/or Ordinary Shares and the exercise price. Pursuant to a warrant
agency agreement between us and , as warrant agent, the Series E Warrants will be issued in book-entry form and shall initially be represented
only by one or more global warrants deposited with the warrant agent, as custodian on behalf of DTC, and registered in the name of Cede
& Co., a nominee of DTC, or as otherwise directed by DTC.
Exercisability
The Series E Warrants
will be exercisable, at the option of the holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied
by payment in full for the number of Depositary Shares purchased upon such exercise (except in the case of a cashless exercise, as discussed
below). A holder (together with its affiliates) may not exercise any portion of the Series E Warrant to the extent that the holder
would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding Ordinary Shares immediately after exercise. However,
upon notice from the holder to us, the holder may decrease or increase the holder’s beneficial ownership limitation, which may not
exceed 9.99% of the number of outstanding Ordinary Shares immediately after giving effect to the exercise, as such percentage ownership
is determined in accordance with the terms of the Series E Warrants, provided that any increase in the beneficial ownership limitation
will not take effect until 61 days following notice to us. Purchasers in this offering may also elect, prior to the issuance of the
Series E Warrants, to have the initial exercise limitation set at 9.99% of our outstanding Ordinary Shares. No fractional Depositary
Shares will be issued in connection with the exercise of a Series E Warrant. In lieu of fractional Depositary Shares, we will either
pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless Exercise
If, at the time a holder exercises
its Series E Warrants, a registration statement registering the issuance of the securities underlying the Series E Warrants
under the Securities Act is not then effective or available and an exemption from registration under the Securities Act is not available
for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in
payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net
number of Depositary Shares determined according to a formula set forth in the Series E Warrants.
Transferability
Subject to applicable laws,
a Series E Warrant may be transferred at the option of the holder upon surrender of the Series E Warrant to us together with
the appropriate instruments of transfer.
Exchange Listing
There is no trading market
available for the Series E Warrants on any securities exchange or nationally recognized trading system. We do not intend to list
the Series E Warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder
Except as otherwise provided
in the Series E Warrants, the holders of the Series E Warrants do not have any voting rights, dividends or other rights as a
holder of our capital stock until they exercise the warrants.
Fundamental Transaction
In the event of a fundamental
transaction, as described in the Series E Warrants and generally including any reorganization, recapitalization or reclassification
of our Ordinary Shares, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation
or merger with or into another person, the acquisition of more than 50% of our outstanding Ordinary Shares, or any person or group becoming
the beneficial owner of more than 50% of the voting power represented by our outstanding Ordinary Shares, the holders of the Series E
Warrants will be entitled to receive upon exercise of the Series E Warrants the kind and amount of securities, cash or other property
that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. Additionally,
as more fully described in the Series E Warrant, in the event of certain fundamental transactions, the holders of the Series E
Warrants will be entitled to receive consideration in an amount equal to the Black Scholes value of the Series E Warrants on the
date of consummation of the transaction.
Representative Warrants
The following description
of the Representative Warrants (as defined below) we are offering is a summary and is qualified in its entirety by reference to the provisions
of the Representative Warrant, the form of which is filed as an exhibit to the Registration Statement on Form F-1 of which this prospectus
forms a part.
We have agreed to issue warrants
to the Representative, or Representative Warrants, upon the closing of this offering, which entitle the Representative to purchase up
to Depositary Shares (or Depositary Shares upon the exercise of the over-allotment option in full). The Representative Warrants will have
an exercise price equal to $ per Depositary Share. The Representative Warrants will be exercisable immediately upon issuance, at any time
and from time to time, in whole or in part, during the five-year period commencing from the commencement of sales of this offering, and
otherwise on substantially similar terms to the Series E Warrants issued to the investors as part of this offering. The Representative
Warrants and the Depositary Shares underlying the Representative Warrants are being registered on a registration statement of which this
prospectus is a part.
DESCRIPTION OF SHARE CAPITAL
The following describes
our issued share capital, summarizes the material provisions of our Articles of Association and highlights certain differences in corporate
law in the United Kingdom and the United States. This description of our share capital and summary of our Articles of Association is not
complete, and is qualified by reference to our Articles of Association. You should read our Articles of Association, which are filed as
an exhibit to the registration statement of which this prospectus forms a part, for the provisions that are important to you.
The following describes our issued share capital,
summarizes the material provisions of our articles of association and highlights certain differences in corporate law in the United Kingdom
and the United States. Please note that this summary is not intended to be exhaustive. For further information please refer to the full
version of our Articles of Association, which is included as an exhibit to the registration statement of which this prospectus is part.
General
We are a public limited company
organized under the laws of England and Wales under registered number 09216368. Our registered office is 1 Caspian Point, Caspian Way,
Cardiff, CF10 4DQ, United Kingdom. The principal legislation under which we operate and our shares are issued is the Companies Act.
Issued Share Capital
Our issued share capital as
of August 31, 2023 was 365,799,722. Each Ordinary Share has a nominal value £0.001 per share. Each issued Ordinary Share is fully
paid. We currently have 1,000,001 A Deferred Shares, 4,063,321,420 B Deferred Shares and no preference shares in our issued share capital.
There is no limit to the number
of Ordinary Shares or preference shares that we are authorized to issue, as the concept of authorized capital is no longer applicable
under the provisions of the Companies Act. There are no conversion rights, redemption provisions or sinking fund provisions relating to
any Ordinary Shares.
We are not permitted under
English law to hold our own Ordinary Shares unless they are repurchased by us and held in treasury. We do not currently hold any of our
own Ordinary Shares.
History of Share Capital
On September 30, 2020, we
issued 1,250 Ordinary Shares to be purchased under the plan set up in 2017 with our employees and directors to acquire Ordinary Shares
via salary sacrifice arrangement, or the Share Incentive Plan, at £0.001 per share, to the trust of the Share Incentive Plan.
On February 19, 2021, we issued
to certain affiliates of the placement agent in our May 2020 offering, 15,200 Ordinary Shares represented by 38 Depositary Shares upon
the exercise of warrants issued in May 2020 at an exercise price of $3,300.00 per share.
On July 6, 2021, we issued
1,754,386 Ordinary Shares at £5.70 per share to certain non-U.S. investors in a placing in the United Kingdom for aggregate gross
proceeds of £10.0 million.
On March 22, 2022, we issued
one Ordinary Share upon the exercise of one warrant issued in February 2019 to a certain institutional investor at an exercise price of
£200 per share.
On
May 3, 2022, we issued 1,250 Ordinary Shares to be purchased under the Share Incentive Plan at £0.02 per share to the trust of the
Share Incentive Plan.
On
August 3, 2022, we issued warrants to purchase 16,666 Ordinary Shares to a certain institutional investor at an exercise price of £2.70
per share.
On
September 26, 2022, we effected a ratio change to the Depositary Shares, pursuant to which the ratio of Ordinary Shares to Depositary
Shares was changed such that one Depositary Share represented 25 Ordinary Shares. Our Ordinary Shares were not affected by this change
and no fractional Depositary Shares were issued.
On
December 16, 2022, we sold to an institutional investor 492,400 Ordinary Shares represented by 1,231 Depositary Shares in a registered
direct offering at $320.00 per Depositary Share, resulting in gross proceeds of approximately $0.4 million.
On
February 15, 2023, we completed the closing of the February Private Placement pursuant to which we sold to certain institutional investors
(1) 3,250,000 Ordinary Shares represented by 8,125 Depositary Shares at $185.60 per Depositary Share,
(2) 12,931,200 Ordinary Shares represented by 32,327 Depositary Shares, issuable upon the exercise of Series A Warrants issued in the
February Private Placement at an exercise price of $214.40 per warrant, (3) 19,396,400
Ordinary Shares represented by 48,491 Depositary Shares, issuable upon the exercise of Series B Warrants issued in the February Private
Placement at an exercise price of $214.40 per warrant, and (4) up to 71,749,600 Ordinary
Shares represented by 179,374 Depositary Shares, issuable upon the exercise of pre-funded warrants issued in the February Private Placement at
an exercise price of $0.032 per warrant, for aggregate gross proceeds of approximately $6.0 million.
We also issued unregistered February Placement Agent Warrants to purchase a total of 536,800 Ordinary Shares represented by 1,342 Depositary
Shares to the Placement Agent at an exercise price of $400.00 per warrant for 49 warrants and an exercise price of $232.00 per
warrant for 1,293 warrants, and Series A Warrants to purchase 625,000 Ordinary Shares represented
by 1,562 Depositary Shares at an exercise price of $214.40 per warrant to an investor
pursuant to the Waiver.
On
March 27, 2023, following shareholder approval, we effected a one-for-20 reverse split of our Ordinary Shares, and our Ordinary Shares
began trading on AIM on a split-adjusted basis as of such date. No fractional shares were issued in connection with the reverse stock
split.
Concurrently
with the reverse split, and in an effort to bring the Depositary Shares price into compliance with NASDAQ’s minimum requirement
for 500,000 listed Depositary Shares, on March 27, 2023, we effected a ratio change in the number of our Ordinary Shares represented by
the Depositary Shares from 25 Ordinary Shares per Depositary Share to five Ordinary Shares per Depositary Share. No fractional Depositary
Shares were issued.
Between
March 27, 2023, and the date hereof, we have issued 95,137,075 Ordinary Shares upon the exercise of 237,841 pre-funded warrants, Series
A Warrants and Series B Warrants issued in the February Private Placement.
On
May 26, 2023, we completed the closing of the May 2023 Registered Direct Offering, with institutional investors of (1) 166,017,300 Ordinary
Shares represented by 415,043 Depositary Shares, issuable upon the exercise of the Series C Warrants at an exercise price of $16.00 per
warrant, (2) 110,679,610 Ordinary Shares represented by 279,699 Depositary Shares issuable upon the exercise of the Series D Warrants
at an exercise price of $16.00 per warrant and, (3) 4,426,800 Ordinary Shares represented by 11,067 Depositary Shares issuable upon the
exercise of the May 2023 Placement Agent Warrants at an exercise price of $15.00 per warrant.
On
June 20, 2023 we issued the Series C Warrants, Series D Warrants and May 2023 Placement Agent Warrants to the investors and the Placement
Agent after receiving required shareholder approval of the allotment of, and disapplication of pre-emption rights with respect to the
Ordinary Shares to be issued under the Series C Warrants, Series D Warrants and May 2023 Placement Agent Warrants at our June GM.
Between
June 20, 2023, and the date hereof, we have issued 151,315,700 Ordinary Shares upon the exercise of 378,289 Series C Warrants and Series
D Warrants issued in the May 2023 Registered Direct Offering.
On
July 5, 2023, we effected a ratio change to the Depositary Shares, pursuant to which the ratio of Ordinary Shares to Depositary Shares
was changed such that one Depositary Share represented 400 Ordinary Shares. Our Ordinary Shares were not affected by this change and no
fractional Depositary Shares were issued.
Options
We have established the Biodexa
Pharmaceuticals PLC 2014 Enterprise Management Incentive Scheme pursuant to which we have issued options to purchase Ordinary Shares to
employees and directors. As of June 30, 2023, there were options to purchase 121,340 Ordinary Shares. The options lapse after ten years
from the date of the grant. As of June 30, 2023, the weighted average remaining life of the options was 7.9 years.
In connection with our acquisition of DARA in December
2015, we assumed all of DARA’s outstanding options, or DARA Options. As of June 30, 2023 there were outstanding DARA Options to
purchase 134 Ordinary Shares with a weighted average remaining life of 2.0 years.
Warrants
October 2019 and May 2020 Warrants
In
October 2019, we completed a private placement with certain institutional investors, or the October Private Placement, where we issued
warrants to certain investors, or the October Private Placement Warrants, and the placement agent, Wainwright, or the Wainwright October
Warrants. In May 2020, we completed a private placement with certain institutional investors, or the May Private Placement, where we issued
warrants to certain investors, or the May Private Placement Warrants, the placement agent, Wainwright, or the Wainwright May Warrants
and Armistice, or the May Armistice Warrants. The following is a brief summary of the October Private Placement Warrants, Wainwright October
Warrants, May Private Placement Warrants, Wainwright May Warrants and the May Armistice Warrants issued in connection with the October
Private Placement and May Private Placement, as applicable, and is subject in all respects to the provisions contained in the applicable
warrants, which, with respect to the October Private Placement Warrants and Wainwright October Warrants, are filed as exhibits to our
Report on Form 6-K dated October 24, 2019, and for the May Private Placement Warrants, May Armistice Warrants and Wainwright May Warrants,
are filed as exhibits to our Report on Form 6-K dated May 20, 2020. In December 13, 2022, the exercise price of the October Private Placement
Warrants granted to Armistice and the May Private Placement Warrants was reduced to $320.00. Unless otherwise stated, references to warrants
in this section include the October Private Placement Warrants, May Private Placement Warrants, Wainwright October Warrants and Wainwright
May Warrants.
Exercisability.
The October Private Placement Warrants and Wainwright October Warrants became exercisable on December 23, 2019. The May Private Placement
Warrants, May Armistice Warrants and Wainwright May Warrants became exercisable upon issuance. The October Private Placement Warrants,
May Private Placement Warrants and May Armistice Warrants will expire five and one-half years from the initial exercise date, and the
Wainwright October Warrants and Wainwright May Warrants will expire on October 22, 2024 and May 18, 2025, respectively. The holder shall
deliver the aggregate exercise price for the Depositary Shares specified in the exercise notice within two trading days following the
date of exercise (subject to the ‘cashless exercise’ arrangements described below).
Cashless
Exercise. With respect to the October Private Placement Warrants and Wainwright October Warrants, if, more than six months after
the date of issuance of such warrants, there is no effective registration statement registering, or no current prospectus available for,
the resale of the Depositary Shares underlying such warrants, the holder may exercise the warrant, in whole or in part, on a cashless
basis. With respect to the May Private Placement Warrants and Wainwright May Warrants, if there is no effective registration statement
registering, or no current prospectus available for, the resale of the Depositary Shares underlying such warrants, the holder may exercise
the warrant, in whole or in part, on a cashless basis.
Exercise
Price. The exercise price of (i) each October Private Placement Warrant and Wainwright October Warrant is $320.00 and $10,000.00
per Depositary Share, respectively and (ii) each May Private Placement Warrant, May Armistice Warrant and Wainwright May Warrant is $3,280.00,
$320.00 and $3,300.00 per Depositary Share, respectively, each subject to the ‘cashless exercise’ arrangements described above
and to adjustment as described below.
Beneficial
Ownership Limitation. A holder shall have no right to exercise any portion of a warrant, to the extent that, after giving effect
to such exercise, such holder, together with such holder’s affiliates, and any persons acting as a group together with such holder
or any such affiliate, would beneficially own in excess of, at the initial option of the holder thereof, 4.99% or 9.99%, as applicable,
of the number of Ordinary Shares outstanding immediately after giving effect to the issuance of the Ordinary Shares underlying the Depositary
Shares upon such exercise. The holder of the warrant, upon notice to us, may increase or decrease the beneficial ownership limitation
to a percentage not to exceed 9.99%, provided that any increase in the beneficial ownership limitation shall not be effective until 61
days following notice to us. Beneficial ownership of the holder and its affiliates will be determined in accordance with Section 13(d)
of the Exchange Act, and the rules and regulations promulgated thereunder.
Stock
dividends and stock splits. If we pay a stock dividend or otherwise make a distribution payable in Depositary Shares or Ordinary
Shares, or any other equity or equivalent securities, subdivide or combine outstanding Depositary Shares or Ordinary Shares, or reclassify
Depositary Shares, Ordinary Shares or any shares of our capital stock, the exercise price of each warrant will be adjusted by multiplying
the then exercise price by a fraction, the numerator of which shall be the number of Depositary Shares (excluding treasury shares, if
any) outstanding immediately before such event, and the denominator of which shall be the number of Depositary Shares outstanding immediately
after such event.
Rights
Offerings; pro rata distributions. If we issue Ordinary Share equivalents or rights to purchase shares, warrants, securities or other
property pro rata to holders of Depositary Shares, a holder of a warrant will be entitled to acquire, subject to the beneficial ownership
limitation described above, such securities or property that such holder could have acquired if such holder had held the number of Depositary
Shares issuable upon complete exercise of the warrant immediately prior to the date a record is taken for such issuance. If we declare
or make any dividend or other distribution of assets or rights to acquire assets to holders of Depositary Shares or Ordinary Shares, a
holder of a warrant will be entitled to participate, subject to the beneficial ownership limitation, in such distribution to the same
extent that the holder would have participated therein if the holder had held the number of Depositary Shares issuable upon full exercise
of the warrant.
Fundamental
Transaction. If we effect a fundamental transaction, including, among other things, a merger, sale of substantially all of our assets,
tender offer, exchange offer and other business combination transactions, then upon any subsequent exercise of a warrant, the holder thereof
shall have the right to receive, for each Ordinary Share represented by the Depositary Shares that would have been issuable upon such
exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of the successor’s or acquiring
corporation’s securities, if it is the surviving corporation, and any additional consideration receivable as a result of such fundamental
transaction by a holder of the number of Ordinary Shares represented by the Depositary Shares for which the warrant is exercisable immediately
prior to such fundamental transaction.
Transferability. Each
warrant and all rights thereunder are transferable, in whole or in part, upon surrender of the warrant, together with a written assignment
of the warrant subject to applicable securities laws; provided, however, that the Wainwright October Warrants and Wainwright May Warrants
are subject to certain FINRA transfer restrictions. We do not intend to apply for listing of the warrants on any securities exchange or
other trading system.
No
Rights as Shareholder Until Exercise. Except as set forth in the warrants, the holders of the warrants do not have any voting
rights, dividends or other rights as a holder of our capital stock until they exercise the warrants.
May 2020 United Kingdom Placing Warrants
On May 22, 2020, we issued
333,333 units, with each unit comprising one new Ordinary Share and one UK Warrant. The exercise price of the UK Warrants is £6.80
per share and it expires five years and six months from the issuance date. We also issued UK Warrants to purchase a total of 16,400 Ordinary
Shares to Turner Pope, the placing agent, in connection with the closing of such offering, on the same terms and conditions as the other
investors in the offering.
August 2022 Warrants
On August 3, 2022, we issued
warrants to purchase 16,400 Ordinary Shares to Strand Hanson Limited, in payment for services rendered. The exercise price of such warrants
is £2.70 per share and they expire three years from the issuance date.
Placement Agent February Warrants
The
following is a brief summary of the February Placement Agent Warrants issued in connection with the February Private Placement, and is
subject in all respects to the provisions contained in the warrant, which, is filed as an exhibit to our Report on Form 6-K dated February
9, 2023. All pre-funded warrants, Series A Warrants and Series B Warrants issued in connection with the February Private Placement have
been exercised.
Exercisability.
The February Placement Agent Warrants are exercisable and expire three years from the initial exercise date. The holder shall deliver
the aggregate exercise price for the Depositary Shares specified in the exercise notice within two trading days following the date of
exercise (subject to the ‘cashless exercise’ arrangements described below).
Cashless
Exercise. The February Private Placement Agent Warrants may only be exercised on a cashless basis if, at the time of exercise,
there is no effective registration statement registering with a current prospectus available for resale of the Depositary Shares underlying
the February Private Placement Agent Warrants.
Exercise
Price. The exercise price of a portion of the February Placement Agent Warrants are $400.00 per Depositary Share and another
portion is $232.00 per Depositary Share, respectively, each subject to the ‘cashless exercise’ arrangements described above
and to adjustment as described herein.
Beneficial
Ownership Limitation. A holder shall have no right to exercise any portion of a February Placement Agent Warrant, to the extent
that, after giving effect to such exercise, such holder, together with such holder’s affiliates, and any persons acting as a group
together with such holder or any such affiliate, would beneficially own in excess of 4.99% of the number of Ordinary Shares outstanding
immediately after giving effect to the issuance of the Ordinary Shares underlying the Depositary Shares upon such exercise. The holder
of such warrant, upon notice to us, may increase or decrease the beneficial ownership limitation to a percentage not to exceed 9.99%,
provided that any increase in the beneficial ownership limitation shall not be effective until 61 days following notice to us. Beneficial
ownership of the holder and its affiliates will be determined in accordance with Section 13(d) of the Exchange Act, and the rules and
regulations promulgated thereunder.
Stock
dividends and stock splits. If we pay a stock dividend or otherwise make a distribution payable in Depositary Shares or Ordinary
Shares, or any other equity or equivalent securities, subdivide or combine outstanding Depositary Shares or Ordinary Shares, or reclassify
Depositary Shares, Ordinary Shares or any shares of our capital stock, the exercise price of each warrant will be adjusted by multiplying
the then exercise price by a fraction, the numerator of which shall be the number of Depositary Shares (excluding treasury shares, if
any) outstanding immediately before such event, and the denominator of which shall be the number of Depositary Shares outstanding immediately
after such event.
Rights
Offerings; pro rata distributions. If we issue Ordinary Share equivalents or rights to purchase shares, warrants, securities or other
property pro rata to holders of Depositary Shares, a holder of a warrant will be entitled to acquire, subject to the beneficial ownership
limitation described above, such securities or property that such holder could have acquired if such holder had held the number of Depositary
Shares issuable upon complete exercise of the warrant immediately prior to the date a record is taken for such issuance. If we declare
or make any dividend or other distribution of assets or rights to acquire assets to holders of Depositary Shares or Ordinary Shares, a
holder of a warrant will be entitled to participate, subject to the beneficial ownership limitation, in such distribution to the same
extent that the holder would have participated therein if the holder had held the number of Depositary Shares issuable upon full exercise
of the warrant.
Fundamental
Transaction. If we effect a fundamental transaction, including, among other things, a merger, sale of substantially all of our assets,
tender offer, exchange offer and other business combination transactions, then upon any subsequent exercise of a warrant, the holder thereof
shall have the right to receive, for each Ordinary Share represented by the Depositary Shares that would have been issuable upon such
exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of the successor’s or acquiring
corporation’s securities, if it is the surviving corporation, and any additional consideration receivable as a result of such fundamental
transaction by a holder of the number of Ordinary Shares represented by the Depositary Shares for which the warrant is exercisable immediately
prior to such fundamental transaction. In addition, in the event of a fundamental transaction that is (i) an all cash or substantially
all cash transaction, (ii) a “Rule 13e-3 transaction” as defined in Rule 13e-3 under the Exchange Act, or (iii) with certain
limited exceptions, a fundamental transaction involving a person or entity not traded on a national securities exchange or other established
trading market, including, but not limited to, the London Stock Exchange, AIM, The New York Stock Exchange, Inc., The NYSE MKT, The NASDAQ
Global Select Market, The NASDAQ Global Market, The NASDAQ Capital Market, the OTC QX, the OTC QB or the Over-the-Counter Bulletin Board,
then the Company or any successor entity will pay at the holder’s option, exercisable at any time concurrently with or within 30
days after the consummation of the fundamental transaction, an amount of cash equal to the value of the warrant as determined in accordance
with the Black Scholes option pricing model.
Transferability. Each
warrant and all rights thereunder are transferable, in whole or in part, upon surrender of the warrant, together with a written assignment
of the warrant subject to applicable securities laws; provided, however, that the February Placement Agent Warrants are subject to certain
FINRA transfer restrictions. We do not intend to apply for listing of the warrants on any securities exchange or other trading system.
No
Rights as Shareholder Until Exercise. Except as set forth in the warrants, the holders of the warrants do not have any voting
rights, dividends or other rights as a holder of our capital stock until they exercise the warrants.
Series C Warrants, Series D Warrants
and Placement Agent Warrants
The
following is a brief summary of the Series C Warrants, Series D Warrants and the May 2023 Placement Agent Warrants issued in connection
with the Warrant Private Placement, and is subject in all respects to the provisions contained in the applicable warrants, which, are
filed as exhibits to our Report on Form 6-K dated May 24, 2023. Unless otherwise stated, references to warrants in this subsection include
the Series C Warrants, Series D Warrants and the May 2023 Placement Agent Warrants.
Exercisability.
The warrants became exercisable on June 14, 2023. The Series C Warrants, Series D Warrants and May 2023 Placement Agent Warrants expire
one year, five years and three years, respectively from the initial exercise date. The holder shall deliver the aggregate exercise price
for the Depositary Shares specified in the exercise notice within two trading days following the date of exercise (subject to the ‘cashless
exercise’ arrangements described below).
Cashless
Exercise. The Series C Warrants and Series D Warrants may be exercised on a cashless basis. With respect to the Series C Warrants,
the aggregate number of Depositary Shares issuable in such cashless exercise pursuant to any given notice of exercise electing to effect
an alternative cashless exercise shall equal the product of (x) the aggregate number of Depositary Shares that would be issuable upon
exercise of the Series C warrant in accordance with the terms of thereof if such exercise were by means of a cash exercise rather than
a cashless exercise and (y) 1.00. The Series D Warrants and May 2023 Placement Agent Warrants may be exercised on a cashless basis,
if and only if, we have not filed a registration statement registering the Depositary Shares underlying such warrants within six months
of the initial exercise date.
Exercise
Price. The exercise price of each Series C Warrant and Series D Warrant is $16.00 per Depositary Share and the exercise price
of each May 2023 Placement Agent Warrant is $15.00 per Depositary Share.
Beneficial
Ownership Limitation. A holder shall have no right to exercise any portion of a warrant, to the extent that, after giving effect
to such exercise, such holder, together with such holder’s affiliates, and any persons acting as a group together with such holder
or any such affiliate, would beneficially own in excess of 9.99% (or in the case of the May 2023 Placement Agent Warrants, 4.99%), of
the number of Ordinary Shares outstanding immediately after giving effect to the issuance of the Ordinary Shares underlying the Depositary
Shares upon such exercise. The holder of the warrant, upon notice to us, may increase or decrease the beneficial ownership limitation
to a percentage not to exceed 9.99%, provided that any increase in the beneficial ownership limitation shall not be effective until 61
days following notice to us. Beneficial ownership of the holder and its affiliates will be determined in accordance with Section 13(d)
of the Exchange Act, and the rules and regulations promulgated thereunder.
Stock
dividends and stock splits. If we pay a stock dividend or otherwise make a distribution payable in Depositary Shares or Ordinary
Shares, or any other equity or equivalent securities, subdivide or combine outstanding Depositary Shares or Ordinary Shares, or reclassify
Depositary Shares, Ordinary Shares or any shares of our capital stock, the exercise price of each warrant will be adjusted by multiplying
the then exercise price by a fraction, the numerator of which shall be the number of Depositary Shares (excluding treasury shares, if
any) outstanding immediately before such event, and the denominator of which shall be the number of Depositary Shares outstanding immediately
after such event.
Rights
Offerings; pro rata distributions. If we issue Ordinary Share equivalents or rights to purchase shares, warrants, securities or other
property pro rata to holders of Depositary Shares, a holder of a warrant will be entitled to acquire, subject to the beneficial ownership
limitation described above, such securities or property that such holder could have acquired if such holder had held the number of Depositary
Shares issuable upon complete exercise of the warrant immediately prior to the date a record is taken for such issuance. If we declare
or make any dividend or other distribution of assets or rights to acquire assets to holders of Depositary Shares or Ordinary Shares, a
holder of a warrant will be entitled to participate, subject to the beneficial ownership limitation, in such distribution to the same
extent that the holder would have participated therein if the holder had held the number of Depositary Shares issuable upon full exercise
of the warrant.
Fundamental
Transaction. If we effect a fundamental transaction, including, among other things, a merger, sale of substantially all of our assets,
tender offer, exchange offer and other business combination transactions, then upon any subsequent exercise of a warrant, the holder thereof
shall have the right to receive, for each Ordinary Share represented by the Depositary Shares that would have been issuable upon such
exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of the successor’s or acquiring
corporation’s securities, if it is the surviving corporation, and any additional consideration receivable as a result of such fundamental
transaction by a holder of the number of Ordinary Shares represented by the Depositary Shares for which the warrant is exercisable immediately
prior to such fundamental transaction. In addition, with respect to the Series C Warrants, Series D Warrants and the May 2023 Placement
Agent Warrants, in the event of a fundamental transaction that is (i) an all cash or substantially all cash transaction, (ii) a “Rule
13e-3 transaction” as defined in Rule 13e-3 under the Exchange Act, or (iii) with certain limited exceptions, a fundamental transaction
involving a person or entity not traded on a national securities exchange or other established trading market, including, but not limited
to, the London Stock Exchange, AIM, The New York Stock Exchange, Inc., The NYSE MKT, The NASDAQ Global Select Market, The NASDAQ Global
Market, The NASDAQ Capital Market, the OTC QX, the OTC QB or the Over-the-Counter Bulletin Board, then the Company or any successor entity
will pay at the holder’s option, exercisable at any time concurrently with or within 30 days after the consummation of the fundamental
transaction, an amount of cash equal to the value of the warrant as determined in accordance with the Black Scholes option pricing model.
Transferability. Each
warrant and all rights thereunder are transferable, in whole or in part, upon surrender of the warrant, together with a written assignment
of the warrant subject to applicable securities laws; provided, however, that the May 2023 Placement Agent Warrants are subject to certain
FINRA transfer restrictions. We do not intend to apply for listing of the warrants on any securities exchange or other trading system.
No
Rights as Shareholder Until Exercise. Except as set forth in the warrants, the holders of the warrants do not have any voting
rights, dividends or other rights as a holder of our capital stock until they exercise the warrants.
Articles of Association
Shares and Rights
Attaching to Them
Objects
The objects of our Company
are unrestricted.
Share Rights
Subject to any special rights
attaching to shares already in issue, our shares may be issued with or have attached to them any preferred, deferred or other special
rights or privileges or be subject to such restrictions as we may resolve by ordinary resolution of the shareholders or decision of our
board.
Voting Rights
Without prejudice to any rights
or restrictions as to voting rights attached to any shares forming part of our share capital from time to time, the voting rights attaching
to shares are as follows:
| · | on a show of hands every shareholder who is present in person and each duly authorized representative present in person of a shareholder
that is a corporation shall have one vote; |
| · | on a show of hands, each proxy present in person has one vote for and one vote against a resolution if the proxy has been duly appointed
by more than one shareholder and the proxy has been instructed by one or more of those shareholders to vote for the resolution and by
one or more other of those shareholders to vote against it; |
| · | on a show of hands, each proxy present in person has one vote for and one vote against a resolution if the proxy has been duly appointed
by more than one shareholder entitled to vote on the resolution and either: (1) the proxy has been instructed by one or more of those
shareholders to vote for the resolution and has been given any discretion by one or more other of those shareholders to vote and the proxy
exercises that discretion to vote against it; or (2) the proxy has been instructed by one or more of those shareholders to vote against
the resolution and has been given any discretion by one or more other of those shareholders to vote and the proxy exercises that discretion
to vote for it; and |
| · | on a poll every shareholder who is present in person or by proxy shall have one vote for each share of which he is the holder. |
At any general meeting a resolution
put to the vote of the meeting shall be decided on a show of hands unless a poll is demanded. Subject to the provisions of the Companies
Act, a poll may be demanded by:
| · | the chairman of the meeting; |
| · | at least five shareholders present in person or by proxy and entitled to vote; |
| · | any shareholder(s) present in person or by proxy and representing in the aggregate not less than 10% of the total voting rights of
all shareholders having the right to vote on the resolution; or |
| · | any shareholder(s) present in person or by proxy and holding shares conferring a right to vote on the resolution on which there have
been paid up sums in the aggregate equal to not less than 10% of the total sums paid up on all shares conferring that right. |
Restrictions on Voting
No shareholder shall be entitled
to vote at any general meeting or at any separate class meeting in respect of any share held by him unless all calls or other sums payable
by him in respect of that share have been paid.
The Board of Directors may
from time to time make calls upon the shareholders in respect of any money unpaid on their shares and each shareholder shall (subject
to at least 14 days’ notice specifying the time or times and place of payment) pay at the time or times so specified the amount
called on his shares. If a call remains unpaid after it has become due and payable, and the fourteen days’ notice provided by the
Board of Directors has not been complied with, any share in respect of which such notice was given may be forfeited by a resolution of
the Board of Directors.
A shareholder’s right
to attend general or class meetings of the Company or to vote in respect of his shares may be suspended by the Board of Directors in accordance
with our Articles of Association if he fails to comply with a proper request for the disclosure of interests regarding the shares. See
“—Other United Kingdom Law Considerations—Disclosure of Interests in Shares” in this prospectus.
Dividends
We may, by ordinary resolution,
declare a dividend to be paid to the share owners according to their respective rights and interests in profits, and may fix the time
for payment of such dividend. No dividend may be declared in excess of the amount recommended by the directors. The Board of Directors
may from time to time declare and pay to our share owners such interim dividends as appear to the directors to be justified by our profits
available for distribution. There are no fixed dates on which entitlement to dividends arises on our ordinary shares.
The share owners may pass,
on the recommendation of the directors, an ordinary resolution to direct that all or any part of a dividend to be paid by distributing
specific assets, in particular paid up shares or debentures of any other body corporate. Our articles of association also permit, with
the prior authority of an ordinary resolution of shareholders, a scrip dividend scheme under which share owners may be given the opportunity
to elect to receive fully paid Ordinary Shares instead of cash, or a combination of shares and cash, with respect to future dividends.
By the way of the exercise
of a lien, if a share owner owes us any money relating in any way to shares, the Board of Directors may deduct any of this money from
any dividend on any shares held by the share owner, or from other money payable by us in respect of the shares. Money deducted in this
way may be used to pay the amount owed to us.
Unclaimed dividends and other
money payable in respect of a share can be invested or otherwise used by directors for our benefit until they are claimed. A dividend
or other money remaining unclaimed 12 years after it first became due for payment will be forfeited and shall revert to the Company.
A shareholder’s right
to receive dividends on his shares may, if they represent more than 0.25% of the issued shares of that class, be suspended by the directors
if he fails to comply with a proper request for the disclosure of interests regarding the shares. See “—Other United Kingdom
Law Considerations—Disclosure of Interests in Shares” in this prospectus.
Change of Control
There is no specific provision
in our Articles of Association that would have the effect of delaying, deferring or preventing a change of control. We are, however, subject
to the provisions of the City Code, which contains detailed provisions regulating the timing and manner of any takeover offer for those
of the Company’s shares which confer voting rights. See “—Other United Kingdom Law Considerations—City Code
on Takeovers and Mergers” in this prospectus.
Variation of Rights
Whenever our share capital
is divided into different classes of shares, all or any of the rights attached to any class may be varied or abrogated in such manner
(if any) as may be provided by those rights or (in the absence of any such provision) either with the consent in writing of the holders
of at least 75% of the issued shares of that class or with the authority of a special resolution passed at a separate general meeting
of the holders of the shares of that class.
Alteration of Share Capital and Repurchases
Subject to the provisions
of the Companies Act, and without prejudice to any relevant special rights attached to any class of shares, we may, from time to time:
| · | increase our share capital by allotting and issuing new shares in accordance with our Articles of Association and any relevant shareholder
resolution; |
| · | consolidate all or any of our share capital into shares of a larger nominal amount (i.e., par value) than the existing shares; |
| · | subdivide any of our shares into shares of a smaller nominal amount (i.e., par value) than our existing shares; or |
| · | redenominate our share capital or any class of share capital. |
Preemptive Rights and New Issuance of Shares
Under the Companies Act, the
issuance of equity securities (except shares held under an employees’ share scheme) that are to be paid for wholly in cash must
be offered first to the existing holders of equity securities in proportion to the respective nominal amounts (i.e., par values) of their
holdings on the same or more favorable terms, unless a special resolution to the contrary has been passed or the articles of association
otherwise provide an exclusion from this requirement (which exclusion can be for a maximum of five years after which our shareholders’
approval would be required to renew the exclusion). In this context, “equity securities” means ordinary shares (and would
exclude shares that, with respect to dividends or capital, carry a right to participate only up to a specified amount in a distribution),
and any and all rights to subscribe for or convert securities into such ordinary shares. This differs from U.S. law, under which shareholders
generally do not have pre-emptive rights unless specifically granted in the certificate of incorporation or otherwise.
The
Board seeks general authority to allot shares on a non-pre-emptive basis at each annual general meeting. By way of resolutions passed
at our annual general meeting held on June 14, 2023, authorities were given to the directors to generally allot shares in the Company,
or to grant rights to subscribe for or to convert or exchange any security into shares in the Company, up to an aggregate nominal amount
of £140,000,000, for a period up to the conclusion of our annual general meeting to be held in 2026. Pre-emptive rights under the
Companies Act will not apply in respect of allotment of shares for cash made pursuant to such authority.
Transfer of Shares
Any certificated shareholder
may transfer all or any of his shares by an instrument of transfer in the usual common form or in any other manner which is permitted
by the Companies Act and approved by the Board of Directors. Any written instrument of transfer shall be signed by or on behalf of the
transferor and (in the case of a partly paid share) the transferee.
All transfers of uncertificated
shares shall be made in accordance with and subject to the provisions of the Uncertificated Securities Regulations 2001 and the facilities
and requirements of its relevant system. The Uncertificated Securities Regulations 2001 permit shares to be issued and held in uncertificated
form and transferred by means of a computer-based system.
The Board of Directors may
decline to register any transfer of any share unless it is:
| · | a share on which the Company has no lien; |
| · | in respect of only one class of shares; |
| · | in favor of a single transferee or not more than four transferees; |
| · | duly stamped or duly certificated or otherwise shown the satisfaction of the Board of Directors to be
exempt from any required stamp duty; or |
| · | delivered for registration at the Company’s registered office or such other place as the Board of Directors may decide, accompanied
by the certificate for the shares to which it relates (other than uncertificated shares) and any other evidence the Board of Directors
may reasonably require to provide the title to such share of the transferor. |
If the Board of Directors
declines to register a transfer it shall, as soon as practicable and in any event within two months after the date on which the transfer
is lodged, send to the transferee notice of the refusal, together with reasons for the refusal.
CREST
CREST is a computerized paperless
share transfer and settlement system which allows securities to be transferred by electronic means, without the need for a written instrument
of transfer. The Articles of Association are consistent with CREST membership and, among other things, allow for the holding and transfer
of shares in uncertificated form.
Shareholder Meetings
Annual General Meetings
In accordance with the Companies
Act, we are required in each year to hold an annual general meeting in addition to any other general meetings in that year and to specify
the meeting as such in the notice convening it. The annual general meeting shall be convened whenever and wherever the board sees fit,
subject to the requirements of the Companies Act, as described in “—Differences in Corporate Law—Annual General Meeting”
and “—Differences in Corporate Law—Notice of General Meetings” in this prospectus.
Notice of General Meetings
The arrangements for the calling
of general meetings are described in “—Differences in Corporate Law—Notice of General Meetings” in this
prospectus.
Subject to certain conditions,
holders of Depositary Shares are entitled to receive notices under the terms of the deposit agreement relating to the Depositary Shares.
See “Description of American Depositary Shares—Voting Rights” in this prospectus.
Quorum of General Meetings
No business shall be transacted
at any general meeting unless a quorum is present, but the absence of a quorum shall not preclude the appointment, choice or election
of a chairman which shall not be treated as part of the business of the meeting. At least two shareholders present in person or by proxy
and entitled to vote shall be a quorum for all purposes.
Class Meetings
The provisions in the Articles
of Association relating to general meetings apply to every separate general meeting of the holders of a class of shares except that:
| · | no member, other than a member of the Board of Directors, shall be entitled to notice of it or attend
such meeting unless he is a holder of shares of that class; |
| · | the quorum for such class meeting shall be two holders in person or by proxy representing not less than
one-third in nominal value of the issued shares of the class; |
| · | at the class meeting, a holder of shares of the class present in person or by proxy may demand a poll
and shall on a poll be entitled to one vote for every shares of the class held by him; and |
| · | if at any adjourned meeting of such holders a quorum is not present at the meeting, one holder of shares
of the class present in person or by proxy at an adjourned meeting constitutes a quorum. |
Directors
Number of Directors
We may not have less than
two directors on our Board of Directors. We have no maximum number of directors, though we may fix a maximum number by ordinary resolution
of the shareholders. We may, by ordinary resolution of the shareholders, vary the minimum and any maximum number of directors from time
to time.
Appointment of Directors
Subject to the provisions
of the Articles of Association, we may, by ordinary resolution of the shareholders, elect any person to be a director, either to fill
a casual vacancy or as an addition to the existing board.
Without prejudice to the power
to appoint any person to be a director by shareholder resolution, the Board of Directors has the power to appoint any person to be a director,
either to fill a casual vacancy or as an addition to the existing Board of Directors. Any director appointed by the Board of Directors
will hold office only until the earlier to occur of the close of the next following annual general meeting and someone being appointed
in his stead at that meeting. Such a director is eligible for re-election at that meeting but shall not be taken into account in determining
the directors or the number of directors who are to retire by rotation at such meeting.
Rotation of Directors
At every annual general meeting,
one-third of the directors or, if their number is not a multiple of three, then the number nearest to and not exceeding one-third, shall
retire from office and each director must retire from office at least once every three years. If there are fewer than three directors,
one director shall make himself or herself available for re-election
The directors to retire on
each occasion shall be those subject to retirement by rotation who have been longest in office since their last election, but as between
persons who became or were re-elected directors on the same day those to retire shall (unless they otherwise agree amongst themselves)
be determined by lot.
A director who retires at
the annual general meeting shall be eligible for re-election.
The shareholders may, at the
meeting at which a director retires, fill the vacated office by electing a person and in default the retiring director shall, if willing
to continue to act, be deemed to have been re-elected, unless at such meeting it is expressly resolved not to fill such vacated office
or unless a resolution for the re-election of such director shall have been put to the meeting and lost or such director has given notice
in writing to us that he is unwilling to be re-elected or such director has attained the retirement age applicable to him as director
pursuant to the Companies Act.
Director’s Interests
The Board of Directors may
authorize, to the fullest extent permitted by law, any matter proposed to them which would otherwise result in a director infringing his
duty to avoid a situation in which he has, or can have, a direct or indirect interest that conflicts, or possibly may conflict, with our
interests and which may reasonably be regarded as likely to give rise to a conflict of interest. A director shall not, save as otherwise
agreed by him, be accountable to us for any benefit which he (or a person connected with him) derives from any matter authorized by the
directors and any contract, transaction or arrangement relating thereto shall not be liable to be avoided on the grounds of any such benefit.
Subject to the requirements
under Sections 175, 177 and 182 of the Companies Act (which require a director to avoid a situation in which he has, or can have, a direct
or indirect interest that conflicts, or possibly conflicts, with our interests, and to declare any interest that he has, whether directly
or indirectly, in a proposed or existing transaction or arrangement with us), and provided that he has disclosed to the Board of Directors
the nature and extent of any interest of his in accordance with the Companies Act and the Articles of Association, a director notwithstanding
his office:
| · | may be a party to, or otherwise interested in, any transaction or arrangement with us or in which we are
otherwise interested; |
| · | may be a director or other officer of, or employed by, or a party to any transaction or arrangement with,
or otherwise interested in, any body corporate promoted by us or in which we are otherwise interested; and |
| · | shall not, by reason of his office, be accountable to us for any benefit which he derives from any such office or employment or from
any such transaction or arrangement or from any interest in any such body corporate and no such transaction or arrangement shall be liable
to be avoided on the ground of any such interest or benefit. |
In the case of interests arising
where a director is in any way, directly or indirectly, interested in (a) a proposed transaction or arrangement with us or (b) a transaction
or arrangement that has been entered into by us and save as otherwise provided by the Articles of Association, such director shall not
vote at a meeting of the Board of Directors or of a committee of the Board of Directors on any resolution concerning such matter in which
he has a material interest (otherwise than by virtue of his interest in shares, debentures or other securities of, or otherwise in or
through, us) unless his interest or duty arises only because the case falls within one or more of the following paragraphs:
| · | the resolution relates to the giving to him or a person connected with him of a guarantee, security or indemnity in respect of money
lent to, or an obligation incurred by him or such a person at the request of or for the benefit of, us or any of our subsidiaries; |
| · | the resolution relates to the giving of a guarantee, security or indemnity in respect of a debt or obligation of ours or any of our
subsidiaries for which the director or a person connected with him has assumed responsibility in whole or part under a guarantee or indemnity
or by the giving of security; |
| · | the resolution relates in any way to any other company in which he is interested, directly or indirectly
and whether as an officer or shareholder or otherwise howsoever, provided that he and any persons connected with him do not to his knowledge
hold an interest in shares representing one per cent or more of any class of the equity share capital of such company or of the voting
rights available to shareholder of such company; |
| · | the resolution relates in any way to an arrangement for the benefit of our employees or any employees
of our subsidiaries which does not award him as such any privilege or benefit not generally awarded to the employees to whom such arrangement
relates; |
| · | the resolution relates in any way to the purchase or maintenance for the directors of insurance; or |
| · | the resolution is in respect of any matter in which the interest of the director cannot reasonably be regarded as conflicting. |
A director shall not be counted
in the quorum present at a meeting in relation to a resolution on which he is not entitled to vote.
If a question arises at a
meeting of the Board of Directors or of a committee of the Board of Directors as to the right of a director to vote or be counted in the
quorum, and such question is not resolved by his voluntarily agreeing to abstain from voting or not to be counted in the quorum, the question
may, before the conclusion of the meeting, be referred to the chairman of the meeting and his ruling in relation to any director other
than himself shall be final and conclusive except in a case where the nature or extent of the interest of the director concerned has not
been fairly disclosed.
An interest of a person connected
with a director shall be treated as an interest of the director and Section 252 of the Companies Act shall determine whether a person
is connected with a director.
Directors’ Fees and Remuneration
Each of the directors shall
be paid a fee at such rate as may from time to time be determined by the Board of Directors (or for the avoidance of doubt any duly authorized
committee of the Board of Directors) provided that the aggregate of all such fees so paid to directors shall not exceed £600,000
per annum, or such higher amount as may from time to time be determined by ordinary resolution of shareholders.
Each director may be paid
his reasonable traveling, hotel and other expenses of attending and returning from meetings of the Board of Directors or committees thereof
of or general meetings or separate meetings of the holders class of shares or of debentures and shall be paid all expenses properly and
reasonably incurred by him in the conduct of the Company’s business or in the discharge of his duties as a director. Any director
who, by request, goes or resides abroad for any purposes required by us or who performs services which in the opinion of the Board of
Directors go beyond the ordinary duties of a director may be paid such extra remuneration as the Board of Directors may determine.
An executive director shall
receive such remuneration as the Board of Directors may determine, and either in addition to or in lieu of his remuneration as a director
as detailed above.
Age Limitations and Share Ownership
We do not have any age limitations
for our directors, nor do we have mandatory retirement as a result of reaching a certain age. Our directors are not required to hold any
shares in the Company.
Borrowing Power
Our directors may exercise
all the powers of the Company to borrow or raise money and mortgage or charge all or any part of our undertaking, property and assets
(present and future), and uncalled capital. Subject to the Companies Act, the directors may also create and issue debentures, other loan
stock and other securities, whether outright or as collateral security for any debt, liability or obligation of the Company or of any
third party. Our directors are required to restrict the borrowings of the Company to ensure that the aggregate principal amount of borrowings
at any one time outstanding and all of its subsidiary undertakings (other than intra-Group borrowing) shall not at any time, without the
previous sanction of an ordinary resolution of the Company, exceed two times the gross asset value of the Company and our subsidiaries.
Liability of Biodexa and its Directors and
Officers
Subject to the provisions
on indemnities set out in Companies Act, every director, alternate director or former director (and of any associated company) shall be
entitled to be indemnified out of our assets against all costs and liabilities incurred by him or her in relation to any proceedings or
any regulatory investigation or action which relate to anything done or omitted or alleged to have been done or omitted by him or her
as a director so long as the indemnities do not cover liability for breach of duty to the Company or cover any fine, costs or related
expense in connection with any proceedings for default on the part of the director. Lawful indemnities extend to the provision of funds
to him or her by the Company to meet expenditure incurred or to be incurred by him in defending himself in any proceedings (whether civil
or criminal) or in connection with an application for statutory relief or in an investigation by a regulatory authority which must however
be repaid where such proceedings, application, investigation or action are in connection with any alleged negligence, default, breach
of duty or breach of trust by him or her in relation to the Company (or any associated company of ours) and he or she is convicted or
found in default thereof. Under English law, any provision that purports to exempt a director of a company (to any extent) from any liability
that would otherwise attach to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company
is void.
Under a deed poll declared
by us on August 5, 2015, or the Deed of Indemnity, our Board of Directors and our Company Secretary are indemnified against costs and
liabilities incurred in connection with their office, other than any liability owed by such person to the Company itself (or any of our
associated entities) and other than indemnification for liabilities in certain circumstances, which are prohibited by virtue of the Companies
Act. The Deed of Indemnity provides that a director may also be lent sums to finance any relevant defense costs, provided that, in the
event such proceedings involve criminal or civil matters in which the person is convicted or has a judgment made against him or her, then
such loan must be repaid. Our total aggregate liability under the Deed of Indemnity is £5 million.
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to a charter
provision, by-law, contract, arrangements, statute or otherwise, we acknowledge that in the opinion of the SEC such indemnification is
against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Other United Kingdom Law Considerations
Mandatory Purchases and Acquisitions
Pursuant to Sections 979 to
991 of the Companies Act, where a takeover offer has been made for us and the offeror has acquired or unconditionally contracted to acquire
not less than 90% in value of the shares to which the offer relates and not less than 90% of the voting rights carried by those shares,
the offeror may give notice to the holder of any shares to which the offer relates which the offeror has not acquired or unconditionally
contracted to acquire that he wishes to acquire, and is entitled to so acquire, those shares on the same terms as the general offer. The
offeror would do so by sending a notice to the outstanding minority shareholders telling them that it will compulsorily acquire their
shares. Such notice must be sent within three months of the last day on which the offer can be accepted in the prescribed manner. The
squeeze-out of the minority shareholders can be completed at the end of six weeks from the date the notice has been given, following which
the offeror can execute a transfer of the outstanding shares in its favor and pay the consideration to us, and we would hold the consideration
on trust for the outstanding minority shareholders. The consideration offered to the outstanding minority shareholders whose shares are
compulsorily acquired under the Companies Act must, in general, be the same as the consideration that was available under the takeover
offer.
Sell Out
The Companies Act also gives
our minority shareholders a right to be bought out in certain circumstances by an offeror who has made a takeover offer for all of our
shares. The holder of shares to which the offer relates, and who has not otherwise accepted the offer, may require the offeror to acquire
his shares if, prior to the expiry of the acceptance period for such offer, (i) the offeror has acquired or agreed to acquire not less
than 90% in value of the voting shares, and (ii) not less than 90% of the voting rights carried by those shares. The offeror may impose
a time limit on the rights of minority shareholders to be bought out that is not less than three months after the end of the acceptance
period. If a shareholder exercises his rights to be bought out, the offeror is required to acquire those shares on the terms of this offer
or on such other terms as may be agreed.
Disclosure of Interests in Shares
Pursuant to Part 22 of the
Companies Act, we are empowered by notice in writing to any person whom we know or have reasonable cause to believe to be interested in
our shares, or at any time during the three years immediately preceding the date on which the notice is issued has been so interested,
requiring such person within a reasonable time to disclose to us particulars of that person’s interest and (so far as is within
his knowledge) particulars of any other interest that subsists or subsisted in those shares. The Articles of Association specify that
a response is required from such person within 14 days after service of any such notice.
Under the Articles of Association,
if a person defaults in supplying us with the required particulars in relation to the shares in question, or Default Shares, the directors
may by notice direct that:
| · | in respect of the Default Shares, the relevant member shall not be entitled to attend or vote (either
in person or by proxy) at any general meeting or of a general meeting of the holders of a class of shares or upon any poll or to exercise
any right conferred by the Default Shares; and/or |
| · | where the Default Shares represent at least 0.25% of their class, (a) any dividend (or any part of a dividend) payable in respect
of the Default Shares shall be retained by us without liability to pay interest, (b) the shareholder may not be entitled to elect to receive
shares instead of a dividend, and (c) no transfers by the relevant member of any Default Shares may be registered (unless the member himself
is not in default and the transfer does not relate to Default Shares, the transfer is exempt or that the transfer is permitted under the
U.K. Uncertificated Securities Regulations 2001). |
Purchase of Own Shares
Under English law, a limited
company may only purchase or redeem its own shares out of the distributable profits of the company or the proceeds of a fresh issue of
shares made for the purpose of financing the purchase, provided that they are not restricted from doing so by their articles. A limited
company may not purchase or redeem its own shares if, as a result of the purchase, there would no longer be any issued shares of the company
other than redeemable shares or shares held as treasury shares. Shares must be fully paid in order to be repurchased.
Subject to the above, we may
purchase our own shares in the manner prescribed below. We may make a market purchase of our own fully paid shares pursuant to an ordinary
resolution of shareholders. The resolution authorizing the purchase must:
| · | specify the maximum number of shares authorized to be acquired; |
| · | determine the maximum and minimum prices that may be paid for the shares; and |
| · | specify a date, not being later than five years after the passing of the resolution, on which the authority to purchase is to expire. |
We may purchase our own fully
paid shares otherwise than on a recognized investment exchange pursuant to a purchase contract authorized by resolution of shareholders
before the purchase takes place. Any authority will not be effective if any shareholder from whom we propose to purchase shares votes
on the resolution and the resolution would not have been passed if he had not done so. The resolution authorizing the purchase must specify
a date, not being later than five years after the passing of the resolution, on which the authority to purchase is to expire.
Distributions and Dividends
Under the Companies Act, before
a company can lawfully make a distribution or dividend, it must ensure that it has sufficient distributable reserves (on a non-consolidated
basis). The basic rule is that a company’s profits available for the purpose of making a distribution are its accumulated, realized
profits, so far as not previously utilized by distribution or capitalization, less its accumulated, realized losses, so far as not previously
written off in a reduction or reorganization of capital duly made. The requirement to have sufficient distributable reserves before a
distribution or dividend can be paid applies to us and to each of our subsidiaries that has been incorporated under English law.
It is not sufficient that
we, as a public company, have made a distributable profit for the purpose of making a distribution. An additional capital maintenance
requirement is imposed on us to ensure that the net worth of the company is at least equal to the amount of its capital. A public company
can only make a distribution:
| · | if, at the time that the distribution is made, the amount of its net assets (that is, the total excess
of assets over liabilities) is not less than the total of its called up share capital and undistributable reserves; and |
| · | if, and to the extent that, the distribution itself, at the time that it is made, does not reduce the amount of the net assets to
less than that total. |
City Code on Takeovers and Mergers
The
Company is a public limited company incorporated in, and with its registered office in, the United Kingdom but its securities are not
admitted to trading on a regulated market or multilateral trading facility in the United Kingdom (or a stock exchange in the Channel Islands
or the Isle of Man). The City Code shall only apply to the Company if it is considered by the Panel to have its place of central management
and control in the United Kingdom (or the Channel Islands or the Isle of Man). This is known as the “residency test”. The
way in which the test for central management and control is applied for the purposes of the City Code may be different from the way in
which it is applied by the United Kingdom tax authorities, HMRC. Under the City Code, the Panel typically considers where the majority
of the directors of the Company are resident, amongst other factors, for the purposes of determining where the Company has its place of
central management and control. Three of the four directors of the Company are currently resident in the United Kingdom and the place
of central management and control of the Company is intended, for the time being, to remain in the United Kingdom meaning that the Company
and its shareholders will have the benefit of the protections that the City Code affords, including, but not limited to, under Rule 9
of the City Code as set out below.
The
City Code is issued and administered by the Panel. The City Code provides a framework within which takeovers of companies subject to it
are conducted. In particular, the City Code contains certain rules in respect of mandatory offers. Under Rule 9 of the City Code, if a
person:
| · | acquires an interest in our shares which, when taken together with shares in which he or persons acting
in concert with him are interested, carries 30% or more of the voting rights of our shares; or |
| · | who, together with persons acting in concert with him, is interested in shares that in the aggregate carry
not less than 30% and not more than 50% of the voting rights in us, acquires additional interests in shares that increase the percentage
of shares carrying voting rights in which that person is interested, |
the acquirer, and depending
on the circumstances, its concert parties would be required (except with the consent of the Panel) to make a cash offer for our outstanding
shares at a price not less than the highest price paid for any interests in the shares by the acquirer or its concert parties during the
previous 12 months.
Notwithstanding
the above, the Company may cease to be subject to the City Code in the future if there are any changes that lead to the Company being
deemed to no longer have its place of central management and control in the United Kingdom, Channel Islands or the Isle of Man.
Exchange Controls
There are no governmental
laws, decrees, regulations or other legislation in the United Kingdom that may affect the import or export of capital, including the availability
of cash and cash equivalents for use by us, or that may affect the remittance of dividends, interest, or other payments by us to non-resident
holders of our Ordinary Shares or Depositary Shares, other than withholding tax requirements. There is no limitation imposed by English
law or in the Articles of Association on the right of non-residents to hold or vote shares.
Differences in Corporate
Law
The
applicable provisions of the Companies Act differ from laws applicable to U.S. corporations and their shareholders. Set forth below is
a summary of certain differences between the provisions of the Companies Act applicable to us and the Delaware General Corporation
Law relating to shareholders’ rights and protections. This summary is not intended to be a complete discussion of the respective
rights and it is qualified in its entirety by reference to English law and Delaware law.
| |
|
England and Wales |
|
Delaware |
| Number of Directors |
|
Under the Companies Act, a public limited company must have at least two directors and the number of directors may be fixed by or in the manner provided in a company’s articles of association. |
|
Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws. |
| |
|
|
|
|
| Removal of Directors |
|
Under the Companies Act, shareholders may remove a director without cause by an ordinary resolution (which is passed by a simple majority of those voting in person or by proxy at a general meeting) irrespective of any provisions of any service contract the director has with the company, provided 28 clear days’ notice of the resolution has been given to the company and its shareholders. On receipt of notice of an intended resolution to remove a director, the company must forthwith send a copy of the notice to the director concerned. Certain other procedural requirements under the Companies Act must also be followed such as allowing the director to make representations against his or her removal either at the meeting or in writing. |
|
Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (a) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, shareholders may effect such removal only for cause, or (b) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he is a part. |
| |
|
|
|
|
Vacancies on Board of
Directors |
|
Under English law, the procedure by which directors, other than a company’s initial directors, are appointed is generally set out in a company’s articles of association, provided that where two or more persons are appointed as directors of a public limited company by resolution of the shareholders, resolutions appointing each director must be voted on individually. |
|
Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (a) otherwise provided in the certificate of incorporation or by-laws of the corporation or (b) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. |
| |
|
|
|
|
| Annual General Meeting |
|
Under the Companies Act, a public limited company must hold an annual general meeting in each six-month period following the company’s annual accounting reference date. |
|
Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. |
| |
|
England and Wales |
|
Delaware |
|
General Meeting
|
|
Under the Companies Act, a general meeting of
the shareholders of a public limited company may be called by the directors.
Shareholders holding at least 5% of the paid-up
capital of the company carrying voting rights at general meetings can require the directors to call a general meeting and, if the directors
fail to do so within a certain period, may themselves convene a general meeting. |
|
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. |
| |
|
|
|
|
Notice of General
Meetings |
|
Under the Companies Act, 21 clear days’ notice must be given for an annual general meeting and any resolutions to be proposed at the meeting. Subject to a company’s articles of association providing for a longer period, at least 14 clear days’ notice is required for any other general meeting. In addition, certain matters, such as the removal of directors or auditors, require special notice, which is 28 clear days’ notice. The shareholders of a company may in all cases consent to a shorter notice period, the proportion of shareholders’ consent required being 100% of those entitled to attend and vote in the case of an annual general meeting and, in the case of any other general meeting, a majority in number of the members having a right to attend and vote at the meeting, being a majority who together hold not less than 95% in nominal value of the shares giving a right to attend and vote at the meeting. |
|
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour, and purpose or purposes of the meeting. |
| |
|
|
|
|
| Proxy |
|
Under the Companies Act, at any meeting of shareholders, a shareholder may designate another person to attend, speak and vote at the meeting on their behalf by proxy. |
|
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director. |
| |
|
|
|
|
| Pre-emptive Rights |
|
Under the Companies Act, “equity securities,” being (i) shares in the company other than shares that, with respect to dividends and capital, carry a right to participate only up to a specified amount in a distribution (“ordinary shares”) or (ii) rights to subscribe for, or to convert securities into, ordinary shares, proposed to be allotted for cash must be offered first to the existing equity shareholders in the company in proportion to the respective nominal value of their holdings, unless an exception applies or a special resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise in each case in accordance with the provisions of the Companies Act. |
|
Under Delaware law, shareholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation. |
| |
|
England and Wales |
|
Delaware |
| Authority to Allot |
|
Under the Companies Act, the directors of a company must not allot shares or grant of rights to subscribe for or to convert any security into shares unless an exception applies or an ordinary resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise in each case in accordance with the provisions of the Companies Act. |
|
Under Delaware law, if the corporation’s charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. It may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. |
| |
|
|
|
|
Liability of Officers and
Directors |
|
Under the Companies Act, any provision, whether
contained in a company’s articles of association or any contract or otherwise, that purports to exempt a director of a company,
to any extent, from any liability that would otherwise attach to him in connection with any negligence, default, breach of duty or breach
of trust in relation to the company is void.
Any provision by which a company directly or indirectly
provides an indemnity, to any extent, for a director of the company or of an associated company against any liability attaching to him
in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he is a director is
also void except as permitted by the Companies Act, which provides exceptions for the company to (a) purchase and maintain insurance against
such liability; (b) provide a “qualifying third party indemnity” (being an indemnity against liability incurred by the director
to a person other than the company or an associated company or criminal proceedings in which he is not convicted); and (c) provide a “qualifying
pension scheme indemnity” (being an indemnity against liability incurred in connection with the company’s activities as trustee
of an occupational pension plan). |
|
Under Delaware law, a corporation’s certificate
of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its stockholders
for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
· any
breach of the director’s duty of loyalty to the corporation or its stockholders;
· acts
or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
· intentional
or negligent payment of unlawful dividends or stock purchases or redemptions; or
· any
transaction from which the director derives an improper personal benefit. |
| |
|
England and Wales |
|
Delaware |
| Voting Rights |
|
Under English law, unless a poll is demanded by
the shareholders of a company or is required by the chairman of the meeting or the company’s articles of association, shareholders
shall vote on all resolutions on a show of hands. Under the Companies Act, a poll may be demanded by (a) not fewer than five shareholders
having the right to vote on the resolution; (b) any shareholder(s) representing not less than 10% of the total voting rights of all the
shareholders having the right to vote on the resolution; or (c) any shareholder(s) holding shares in the company conferring a right to
vote on the resolution being shares on which an aggregate sum has been paid up equal to not less than 10% of the total sum paid up on
all the shares conferring that right. A company’s articles of association may provide more extensive rights for shareholders to
call a poll, and in our case the number in clause (a) above is reduced from five to three.
Under English law, an ordinary resolution is passed
on a show of hands if it is approved by a simple majority (more than 50%) of the votes cast by shareholders present (in person or by proxy)
and entitled to vote. If a poll is demanded, an ordinary resolution is passed if it is approved by holders representing a simple majority
of the total voting rights of shareholders present, in person or by proxy, who, being entitled to vote, vote on the resolution. Special
resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present, in person or by proxy, at the
meeting. |
|
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder. |
| |
|
|
|
|
Shareholder Vote on
Certain
Transactions |
|
The Companies Act provides for schemes of arrangement,
which are arrangements or compromises between a company and any class of shareholders or creditors and used in certain types of reconstructions,
amalgamations, capital reorganizations or takeovers. These arrangements require:
· the
approval at a shareholders’ or creditors’ meeting convened by order of the court, of a majority in number of shareholders
or creditors representing 75% in value of the capital held by, or debt owed to, the class of shareholders or creditors, or class thereof
present and voting, either in person or by proxy; and
· the
approval of the court. |
|
Generally, under Delaware law, unless the certificate of incorporation
provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially
all of a corporation’s assets or dissolution requires:
· the
approval of the board of directors; and
· approval
by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than
one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. |
| |
|
England and Wales |
|
Delaware |
Standard of Conduct
for Directors |
|
Under English law, a director owes various statutory
and fiduciary duties to the company, including:
· to
act in the way he considers, in good faith, would be most likely to promote the success of the company for the benefit of its members
as a whole;
· to
avoid a situation in which he has, or can have, a direct or indirect interest that conflicts, or possibly conflicts, with the interests
of the company;
· to
act in accordance with the company’s constitution and only exercise his powers for the purposes for which they are conferred;
· to
exercise independent judgment;
· to
exercise reasonable care, skill and diligence;
· not
to accept benefits from a third party conferred by reason of his being a director or doing, or not doing, anything as a director; and
· to
declare any interest that he has, whether directly or indirectly, in a proposed or existing transaction or arrangement with the company. |
|
Delaware law does not contain specific provisions
setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts
of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they
reasonably believe to be in the best interest of the stockholders.
Directors of a Delaware corporation owe fiduciary
duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good
faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform
himself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director
act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal
gain or advantage. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed
basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption
may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct
upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation.
In addition, under Delaware law, when the board
of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances,
have a duty to obtain the highest value reasonably available to the shareholders. |
| |
|
|
|
|
| Stockholder Suits |
|
Under English law, generally, the company, rather than its shareholders, is the proper claimant in an action in respect of a wrong done to the company or where there is an irregularity in the company’s internal management. Notwithstanding this general position, the Companies Act provides that (i) a court may allow a shareholder to bring a derivative claim (that is, an action in respect of and on behalf of the company) in respect of a cause of action arising from a director’s negligence, default, breach of duty or breach of trust and (ii) a shareholder may bring a claim for a court order where the company’s affairs have been or are being conducted in a manner that is unfairly prejudicial to some of its shareholders. |
|
Under Delaware law, a stockholder may initiate
a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
· state
that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter
devolved on the plaintiff by operation of law; and
· allege
with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for
the plaintiff’s failure to obtain the action; or
· state
the reasons for not making the effort.
Additionally, the plaintiff must remain a stockholder
through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court
of Chancery. |
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
General
The
Depositary Shares representing our Ordinary Shares are listed on the NASDAQ Capital Market under the symbol “BDRX.” The Bank
of New York Mellon is the depositary for the Depositary Shares. Each Depositary Share represents ownership of 400 Ordinary Shares deposited
with the Bank of New York Mellon London Branch, as custodian for the depositary. Each Depositary Share also represents ownership of any
other securities, cash or other property which may be held by the depositary. The depositary’s principal office at which the Depositary
Shares will be administered and its principal executive office are located at 240 Greenwich Street, New York, NY 10286.
You
may hold Depositary Shares either (1) directly (a) by having an American Depositary Receipt, or ADR, which is a certificate evidencing
a specific number of Depositary Shares, registered in your name, or (b) by having Depositary Shares registered in your name in the Direct
Registration System, or (2) indirectly by holding a security entitlement in Depositary Shares through your broker or other financial institution.
If you hold Depositary Shares directly, you are a registered Depositary Share holder. This description assumes you are a Depositary Share
holder. If you hold the Depositary Shares indirectly, you must rely on the procedures of your broker or other financial institution to
assert the rights of Depositary Share holders described in this section. You should consult with your broker or financial institution
to find out what those procedures are.
The
Direct Registration System, or DRS, is a system administered by The Depository Trust Company, or DTC, pursuant to which the depositary
may register the ownership of uncertificated Depositary Shares, which ownership shall be evidenced by periodic statements issued by the
depositary to the Depositary Share holders entitled thereto.
We
will not treat Depositary Share holders as our shareholders and accordingly, Depositary Shares holders will not have shareholder rights.
English law governs shareholder rights. The depositary will be the holder of the Ordinary Shares underlying the Depositary Shares. Holders
of Depositary Shares will have the rights of Depositary Share holders. A Deposit Agreement sets out Depositary Share holder rights as
well as the rights and obligations of the depositary. The laws of the State of New York govern the Deposit Agreement and the Depositary
Shares.
The
following is a summary of the material provisions of the Deposit Agreement. For more complete information, you should read the entire
Deposit Agreement and the form of American Depositary Receipt, which are filed as exhibits to this prospectus. See “Where You
Can Find More Information” beginning on page 103 of this prospectus.
Holding the Depositary Shares
How will you hold your Depositary Shares?
You may hold Depositary Shares
either (i) directly (a) by having an ADR, which is a certificate evidencing a specific number of Depositary Shares, registered
in your name, or (b) by holding Depositary Shares in DRS, or (ii) indirectly through your broker or other financial institution.
If you hold Depositary Shares directly, you are a Depositary Share holder. This description assumes you hold your Depositary Shares directly.
Depositary Shares will be issued through DRS unless you specifically request certificated ADRs. If you hold the Depositary Shares indirectly,
you must rely on the procedures of your broker or other financial institution to assert the rights of Depositary Share holders described
in this section. You should consult with your broker or financial institution to find out what those procedures are.
Dividends and Other
Distributions
How will you receive
dividends and other distributions on the Ordinary Shares?
The
depositary has agreed to pay to you the cash dividends or other distributions it or the custodian receives on Ordinary Shares or other
deposited securities, after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary
shares your Depositary Shares represent as of the record date (which will be as close as practicable to the record date for Ordinary Shares)
set by the depositary with respect to the Depositary Shares.
Cash. The
depositary will convert any cash dividend or other cash distribution we pay on the Ordinary Shares or any net proceeds from the sale of
any Ordinary Shares, rights, securities, or other entitlements into U.S. dollars if it can do so on a practicable basis, and can
transfer the U.S. dollars to the United States. If that is not practical or lawful or if any government approval is needed and
cannot be obtained, the deposit agreement allows the depositary either to distribute the foreign currency to the Depositary Share holders,
or to hold the foreign currency for the account of the Depositary Share holders, in which case it will not invest the foreign currency
and it will not be liable for any interest.
Before
making a distribution, any taxes or other governmental charges, together with fees and expenses of the depositary, that must be paid,
will be deducted. It will distribute only whole U.S. dollars and cents and will round fractional cents down to the nearest whole
cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some
or all of the value of the distribution.
Shares. The
depositary may distribute additional Depositary Shares representing any Ordinary Shares it distributes as a dividend or free distribution
to the extent reasonably practicable and permissible under law. The depositary will only distribute whole Depositary Shares. It will sell
Ordinary Shares which would require it to deliver a fractional Depositary Share and distribute the net proceeds in the same way as it
does with cash. If the depositary does not distribute additional Depositary Shares, the outstanding Depositary Shares will, to the extent
permissible by law, also represent the new Ordinary Shares. The depositary may also sell all or a portion of the Ordinary Shares that
is has not distributed, and distribute the net proceeds in the same way as it does with cash. Additionally, the depositary may sell a
portion of the distributed Ordinary Shares sufficient to pay its fees and expenses, and any taxes and governmental charges, in connection
with that distribution.
Elective
Distributions in Cash or Shares. If we offer holders of our Ordinary Shares the option to receive dividends in either cash or
Ordinary Shares, the depositary, after consultation with us and having received timely notice as described in the deposit agreement of
such elective distribution by us, has discretion to determine to what extent such elective distribution will be made available to you
as a holder of the Depositary Shares. We must first instruct the depositary to make such elective distribution available to you and furnish
it with satisfactory evidence that it is legal to do so. The depositary could decide it is not legal or reasonably practical to make such
elective distribution available to you. In such case, the depositary shall, on the basis of the same determination as is made in respect
of the ordinary shares for which no election is made, distribute either cash in the same way as it does in a cash distribution, or additional
Depositary Shares representing Ordinary Shares in the same way as it does in a share distribution. The depositary is not obligated to
make available to you a method to receive the elective dividend in Ordinary Shares rather than in Depositary Shares. There can be no assurance
that you will be given the opportunity to receive elective distributions on the same terms and conditions as the holders of Ordinary Shares.
Rights
to Purchase Additional Shares. If we offer holders of our Ordinary Shares any rights to subscribe for additional Ordinary Shares
or any other rights, the depositary may after consultation with us and having received timely notice as described in the deposit agreement
of such distribution by us, make these rights available to you. We must first instruct the depositary to make such rights available to
you and furnish the depositary with satisfactory evidence that it is legal to do so. If the depositary decides it is not legal and reasonably
practicable to make the rights available, or if rights have been made available but have not been exercised and appear to be about to
lapse, the depositary may, if it determines it is lawful and reasonably practicable to do so, endeavor to sell the rights and distribute
the net proceeds in the same way as it does with cash. The depositary will allow rights that are not distributed or sold to lapse. In
that case, you will receive no value for them.
If
the depositary makes rights available to you, it will establish procedures to distribute such rights and enable you to exercise the rights
upon your payment of applicable fees, charges and expenses incurred by the depositary and taxes and/or other governmental charges. The
depositary shall not be obliged to make available to you a method to exercise such rights to subscribe for Ordinary Shares (rather than
Depositary Shares).
The
depositary may sell a portion of the distributed rights sufficient to pay its fees and expenses, and any taxes and governmental charges,
in connection with that distribution.
There
can be no assurance that you will be given the opportunity to exercise rights on the same terms and conditions as the holders of Ordinary
Shares, or be able to exercise such rights.
U.S. securities
laws may restrict transfers and cancellation of the Depositary Shares represented by shares purchased upon exercise of rights. For example,
you may not be able to trade these Depositary Shares freely in the United States. In this case, the depositary may deliver restricted
depositary shares that have the same terms as the Depositary Shares described in this section, except for changes needed to put the necessary
restrictions in place.
Other
Distributions. Subject to receipt of timely notice, as described in the Deposit Agreement, from us with the request to make any
such distribution available to you, and provided the depositary has determined such distribution is lawful and reasonably practicable
and in accordance with the terms of the deposit agreement, the depositary will send to you anything else we distribute on deposited securities
by any means it thinks is practicable, upon your payment of applicable fees, charges and expenses incurred by the depositary and taxes
and/or other governmental charges. If the depositary cannot make a distribution in this way, it may endeavor to sell what we distributed
and distribute the net proceeds in the same way as it does with cash. If the depositary is unable to sell what we distributed, it may
dispose of such property in any way it deems reasonably practicable under the circumstances for nominal or no consideration. The depositary
may sell a portion of the distributed securities or property sufficient to pay its fees and expenses and any taxes and governmental charges
in connection with that distribution.
The depositary
is not responsible if it decides that it is unlawful or impractical to make a distribution available to any Depositary Share holders.
We have no obligation to register Depositary Shares, ordinary shares, rights or other securities under the Securities Act. We also have
no obligation to take any other action to permit the distribution of Depositary Shares, ordinary shares, rights or anything else to Depositary
Share holders. This means that you may not receive the distributions we make on our ordinary shares or any value for them if it is illegal
or impractical for us or the depositary to make them available to you.
Deposit, Withdrawal and Cancellation
How are Depositary
Shares issued?
The
depositary will deliver Depositary Shares if you or your broker deposit Ordinary Shares or evidence of rights to receive Ordinary Shares
with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or
fees, the depositary will register the appropriate number of Depositary Shares in the names you request and will deliver the Depositary
Shares to or upon the order of the person or persons entitled thereto.
How do Depositary
Share holders cancel an American Depositary Share?
You
may turn in your Depositary Shares at the depositary’s principal office or by providing appropriate instructions to your broker.
Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary
will deliver the Ordinary Shares and any other deposited securities underlying the Depositary Shares to you or a person you designate
at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its principal
office, to the extent permitted by law.
How do Depositary
Share holders interchange between Certificated Depositary Shares and Uncertificated Depositary Shares?
You
may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated Depositary Shares. The depositary will
cancel that ADR and will send you a statement confirming that you are the owner of uncertificated Depositary Shares. Alternatively, upon
receipt by the depositary of a proper instruction from a holder of uncertificated Depositary Shares requesting the exchange of uncertificated
Depositary Shares for certificated Depositary Shares, the depositary will execute and deliver to you an ADR evidencing those Depositary
Shares.
Voting Rights
How do you vote?
You
may instruct the depositary to vote the Ordinary Shares or other deposited securities underlying your Depositary Shares. Otherwise,
you could exercise your right to vote directly if you withdraw the Ordinary Shares. However, you may not know about the meeting sufficiently
enough in advance to withdraw the Ordinary Shares.
If
we ask for your instructions and upon timely notice from us as described in the deposit agreement, the depositary will notify you of the
upcoming vote and arrange to deliver our voting materials to you by regular, ordinary mail delivery, or by electronic transmission. The
materials will (i) describe the matters to be voted on and (ii) explain how you may instruct the depositary to vote the Ordinary
Shares or other deposited securities underlying your Depositary Shares as you direct. For your voting instructions to be valid, the depositary
must receive them in writing on or before the date specified. The depositary will try, as far as practical, subject to applicable law
and the provisions of the deposit agreement, the deposited securities and our articles of association, to vote or to have its agents vote
the ordinary shares or other deposited securities as you instruct. The depositary will only vote or attempt to vote as you instruct.
We
cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote the Ordinary
Shares underlying your Depositary Shares. In addition, the depositary and its agents are not responsible for failing to carry out voting
instructions or for the manner in which any vote is cast. This means that you may not be able to exercise your right to vote and
you may have no recourse if the Ordinary Shares underlying your Depositary Shares are not voted as you requested.
In
order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited securities,
if we request the depositary to act, we will give the depositary notice of any such meeting and details concerning the matters to be voted
at least 30 days in advance of the meeting date.
Fees and Expenses
What fees and expenses
am I responsible for paying?
As
a Depositary Share holder, you will be required to pay the following service fees to the depositary bank and certain taxes and governmental
charges (in addition to any applicable fees, expenses, taxes and other governmental charges payable on the deposited securities represented
by any of your Depositary Shares):
Persons depositing or withdrawing our ordinary
shares or Depositary Share holders must pay: |
|
For: |
| 5.00 USD (or less) per 100 Depositary Share (or portion of 100 Depositary Shares) |
|
Issue of Depositary Shares, including issues resulting from a distribution of our ordinary shares or rights or other property |
| |
|
|
| |
|
Cancellation of Depositary Shares for the purpose of withdrawal, including if the deposit agreement terminates |
| |
|
|
| 0.05 USD (or less) per Depositary Share |
|
Any cash distribution to Depositary Share holders |
| A fee equivalent to the fee that would be payable if securities distributed to Depositary Share holders had been our Ordinary Shares and the Depositary Shares had been deposited for issuance of Depositary Shares |
|
Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to Depositary Share holders |
| |
|
|
| 0.05 USD (or less) per Depositary Share per calendar year |
|
Depositary services |
| |
|
|
| Registration or transfer fees |
|
Transfer and registration of shares of our Ordinary Shares on our share register to or from the name of the depositary or its agent when persons deposit or withdraw our ordinary shares |
| |
|
|
| Expenses of the Depositary |
|
Cable and facsimile transmissions (when expressly provided in the deposit agreement) |
| |
|
|
| |
|
Converting foreign currency to U.S. dollars |
| |
|
|
| Taxes and other governmental charges the depositary or the custodian has to pay on any Depositary Share or our Ordinary Shares underlying Depositary Shares, such as stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
| |
|
|
| Any charges incurred by the depositary or its agents for servicing the deposited securities |
|
As necessary |
The depositary collects its
fees for delivery and surrender of Depositary Shares directly from investors depositing our ordinary shares or surrendering Depositary
Shares for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions
to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The
depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or
by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from
any cash distribution payable (or by selling a portion of securities or other property distributable) to Depositary Share holders
that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those
services are paid.
In
performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service
providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The
depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and
not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction
spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate
assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying
or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any
currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method
by which that rate will be determined will be the most favorable to Depositary Share holders, subject to the depositary’s obligations
under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
The
depositary has agreed to reimburse us for a portion of certain expenses it incurs that are related to establishment and maintenance of
the ADR program. There are limits on the amount of expenses for which the depositary will reimburse us, but the amount of reimbursement
available to us is not related to the amounts of fees the depositary collects from investors. Further, the depositary has agreed to reimburse
us certain fees payable to the depositary by holders of Depositary Shares. Neither we nor the depositary can determine the exact amount
to be made available to us because (i) the number of Depositary Shares that will be issued and outstanding, (ii) the level of service
fees to be charged to holders of Depositary Shares and (iii) its reimbursable expenses related to the program are not known at this time.
Payment of Taxes
You
will be responsible for any taxes or other governmental charges payable, or which become payable, on your Depositary Shares or on the
deposited securities represented by any of your Depositary Shares. The depositary may refuse to register or transfer your Depositary Shares
or allow you to withdraw the deposited securities represented by your Depositary Shares until such taxes or other charges are paid. It
may apply payments owed to you or sell deposited securities represented by your Depositary Shares to pay any taxes owed and you will remain
liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of Depositary Shares
to reflect the sale and pay to you any net proceeds, or send to you any property, remaining after it has paid the taxes. You agree to
indemnify us, the depositary, the custodian and each of their respective agents, directors, employees and affiliates for, and hold each
of them harmless from, any claims with respect to taxes and additions to tax (including applicable interest and penalties thereon) arising
from any refund of taxes, reduced rate of withholding at source or other tax benefit obtained for or by you. Your obligations under this
paragraph shall survive any transfer of ADRs, any surrender of ADRs and withdrawal of deposited securities or the termination of the deposit
agreement.
Reclassifications,
Recapitalizations and Mergers, etc.
| If we: |
|
Then: |
| ·Change the nominal or par value of our ordinary shares |
|
The cash, ordinary shares or other securities received by the depositary will become deposited securities. |
| |
|
|
| ·Reclassify, split up or consolidate any of the deposited securities |
|
Each Depositary Share will automatically represent its equal share of new deposited securities. |
| |
|
|
| ·Distribute securities on the ordinary shares that are not distributed to you |
|
The depositary may also deliver new Depositary Shares or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities. The depositary may also sell the new deposited securities and distribute the net proceeds if we are unable to assure the depositary that the distribution (a) does not require registration under the Securities Act or (b) is exempt from registration under the Securities Act. |
| |
|
|
| ·Recapitalize, reorganize, merge, liquidate, sell all or substantially all of our assets, or take any similar action |
|
Any replacement securities received by the depositary shall be treated as newly deposited securities and either the existing Depositary Shares or, if necessary, replacement Depositary Shares distributed by the depositary will represent the replacement securities. The depositary may also sell the replacement securities and distribute the net proceeds if the replacement securities may not be lawfully distributed to all Depositary Share holders. |
Upon
any change in our par value, split-up, subdivision cancellation, consolidation or any other reclassification of our Ordinary Shares, or
upon any recapitalization, reorganization, merger, amalgamation or consolidation or sale of assets affecting us or to which we otherwise
are a party, any securities which shall be received by the depositary or a custodian in exchange for, or in conversion of or replacement
or otherwise in respect of, our ordinary shares shall, to the extent permitted by law, be treated as new deposited securities under the
deposit agreement, and the ADRs shall, subject to the provisions of the deposit agreement and applicable law, evidence Depositary Shares
representing the right to receive such additional securities. Alternatively, the depositary may, with our approval, and shall, if we request,
subject to the terms of the deposit agreement and receipt of an opinion of our counsel satisfactory to the depositary that such distributions
are not in violation of any applicable laws or regulations, execute and deliver additional ADRs as in the case of a stock dividend on
our ordinary shares, or call for the surrender of outstanding ADRs to be exchanged for new ADRs, in either case, as well as in the event
of newly deposited ordinary shares, with necessary modifications to the form of ADR specifically describing such new deposited securities
and/or corporate change. Notwithstanding the foregoing, in the event that any security so received may not be lawfully distributed to
some or all Depositary Share holders, the depositary may, with our approval, and shall if we request, subject to receipt of an opinion
from our counsel satisfactory to the depositary that such distributions are not in violation of any applicable laws or regulations, sell
such securities at public or private sale, at such place or places and upon such terms as it may deem proper and may allocate the net
proceeds of such sales (net of fees and charges of, and expenses incurred by, the depositary and taxes and governmental charges) for the
account of the Depositary Share holders otherwise entitled to such securities upon an averaged or other practicable basis without regard
to any distinctions among such Depositary Share holders and distribute the net proceeds so allocated to the extent practicable as in the
case of a distribution received in cash pursuant to the deposit agreement. In accordance with the provisions of the deposit agreement,
the depositary is not responsible for (i) any failure to determine that it may be lawful or feasible to make such securities available
to Depositary Share holders in general or any Depositary Share holder in particular, (ii) any foreign exchange exposure or loss incurred
in connection with such sale, or (iii) any liability to the purchaser of such securities.
Amendment and Termination
How may the deposit agreement be amended?
Upon
any change in our par value, split-up, subdivision cancellation, consolidation or any other reclassification of our Ordinary Shares, or
upon any recapitalization, reorganization, merger, amalgamation or consolidation or sale of assets affecting us or to which we otherwise
are a party, any securities which shall be received by the depositary or a custodian in exchange for, or in conversion of or replacement
or otherwise in respect of, our ordinary shares shall, to the extent permitted by law, be treated as new deposited securities under the
deposit agreement, and the ADRs shall, subject to the provisions of the deposit agreement and applicable law, evidence Depositary Shares
representing the right to receive such additional securities. Alternatively, the depositary may, with our approval, and shall, if we request,
subject to the terms of the deposit agreement and receipt of an opinion of our counsel satisfactory to the depositary that such distributions
are not in violation of any applicable laws or regulations, execute and deliver additional ADRs as in the case of a stock dividend on
our ordinary shares, or call for the surrender of outstanding ADRs to be exchanged for new ADRs, in either case, as well as in the event
of newly deposited ordinary shares, with necessary modifications to the form of ADR specifically describing such new deposited securities
and/or corporate change. Notwithstanding the foregoing, in the event that any security so received may not be lawfully distributed to
some or all Depositary Share holders, the depositary may, with our approval, and shall if we request, subject to receipt of an opinion
from our counsel satisfactory to the depositary that such distributions are not in violation of any applicable laws or regulations, sell
such securities at public or private sale, at such place or places and upon such terms as it may deem proper and may allocate the net
proceeds of such sales (net of fees and charges of, and expenses incurred by, the depositary and taxes and governmental charges) for the
account of the Depositary Share holders otherwise entitled to such securities upon an averaged or other practicable basis without regard
to any distinctions among such Depositary Share holders and distribute the net proceeds so allocated to the extent practicable as in the
case of a distribution received in cash pursuant to the deposit agreement. In accordance with the provisions of the deposit agreement,
the depositary is not responsible for (i) any failure to determine that it may be lawful or feasible to make such securities available
to Depositary Share holders in general or any Depositary Share holder in particular, (ii) any foreign exchange exposure or loss incurred
in connection with such sale, or (iii) any liability to the purchaser of such securities.
How may the deposit agreement be terminated?
The
depositary will terminate the deposit agreement if we ask it to do so, in which case the depositary will give notice to you at least 90 days
prior to termination. The depositary may also terminate the deposit agreement if the depositary has told us that it would like to resign,
or if we have removed the depositary, and in either case we have not appointed a new depositary within 90 days. In either such case,
the depositary must notify you at least 30 days before termination.
After
termination, the depositary and its agents will do the following under the deposit agreement but nothing else: collect distributions on
the deposited securities, sell rights and other property and deliver ordinary shares and other deposited securities upon cancellation
of Depositary Shares after payment of any fees, charges, taxes or other governmental charges. Six months or more after termination, the
depositary may sell any remaining deposited securities by public or private sale. After that, the depositary will hold the money it received
on the sale, as well as any other cash it is holding under the deposit agreement, for the pro rata benefit of the Depositary
Share holders that have not surrendered their Depositary Shares. It will not invest the money and has no liability for interest. The depositary’s
only obligations will be to account for the money and other cash. After termination, our only obligations under the deposit agreement
will be to indemnify the depositary and to pay fees and expenses of the depositary that we agreed to pay.
Books of Depositary
The
depositary will maintain Depositary Share holder records at its depositary office. You may inspect such records at such office during
regular business hours but solely for the purpose of communicating with other holders in the interest of our business or matters relating
to the Depositary Shares or the deposit agreement.
The
depositary will maintain facilities in New York to record and process the issuance, cancellation, combination, split-up and transfer
of ADRs.
These
facilities may be closed from time to time, to the extent not prohibited by law or if any such action is deemed necessary or advisable
by the depositary, in good faith, at any time or from time to time because of any requirement of law, any government or governmental body
or commission or any securities exchange on which the ADRs or Depositary Shares are listed, or under any provision of the deposit agreement
or provisions of, or governing, the deposited securities, or any meeting of our shareholders or for any other reason.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations
of the Depositary; Limits on Liability to Holders of Depositary Shares
The deposit agreement expressly
limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary and
its directors, officers, affiliates, employees and agents.
We and the depositary, and
each of our and their respective directors, officers, affiliates, employees and agents:
| · | are only obligated to take the actions specifically set forth in the deposit agreement without negligence
or bad faith; |
| · | are not liable to Depositary Share holders, beneficial owners or any third parties if prevented or forbidden
from, or subjected to any civil or criminal penalty or restraint on account of, or delayed in, doing or performing any act or thing required
by the terms of the deposit agreement or the ADRs, by reason of any provision of any present or future law or regulation of the United
States or any state thereof, the United Kingdom or any other country, or of any other governmental authority or regulatory authority or
stock exchange, or on account of the possible criminal or civil penalties or restraints or by reason of any provision, present or future
of our constituent documents or any provision of or governing any deposited securities, or by reason of any act of God or war or other
circumstances beyond their control, (including, without limitation, nationalization, expropriation, currency restrictions, work stoppage,
strikes, civil unrest, revolutions, rebellions, explosions and computer failure beyond such party’s control); |
| · | are not liable to Depositary Share holders, beneficial owners or
any third parties by reason of any exercise of, or failure to exercise, any discretion provided for in the deposit agreement or in our
constituent documents or provisions of or governing deposited securities; |
| · | are not liable to Depositary Share holders, beneficial owners or
any third parties for the inability of any holder of Depositary Shares to benefit from any distribution, offering, right or other benefit
made available to holders of deposited securities that is not made available to holders of Depositary Shares under the terms of the deposit
agreement; |
| · | have no obligation to appear in, prosecute or defend any action,
suit or other proceeding in respect of any deposited securities, the ADRs, or the deposit agreement, which in its opinion may involve
it in expense or liability, unless indemnity satisfactory to it against all expenses (including fees and disbursements of counsel) and
liabilities be furnished as often as may be required (and no custodian shall be under any obligation whatsoever with respect to such proceedings,
the responsibility of the custodian being solely to the depositary); |
| · | may rely and shall be protected in acting upon any written notice,
request, opinion or other document believed by it to be genuine and to have been signed or presented by the proper party or parties; |
| · | disclaim any liability for any action/inaction in reliance on the advice or information of legal counsel,
accountants, any person presenting ordinary shares for deposit, holders and beneficial owners (or authorized representatives) of Depositary
Shares, or any other person believed in good faith to be competent to give such advice or information; and |
| · | disclaim any liability to Depositary Share holders, beneficial owners or any third parties for any indirect, special, punitive or
consequential damages for any breach of the terms of the deposit agreement or otherwise. |
Neither
the depositary nor the custodian shall be liable under the deposit agreement or otherwise for the failure by any Depositary Share holder
or beneficial owner to obtain the benefits of credits on the basis of non-U.S. tax paid against such Depositary Share holder’s or
beneficial owner’s income tax liability. The depositary and any of its agents also disclaim any liability for any failure to carry
out any instructions to vote, the manner in which any vote is cast or the effect of any vote or failure to determine that any distribution
or action may be lawful or reasonably practicable or for allowing any rights to lapse in accordance with the provisions of the deposit
agreement, the failure or timeliness of any notice from us, the content of any information submitted to it by us for distribution to you
or for any inaccuracy of any translation thereof, any investment risk associated with the acquisition of an interest in the deposited
securities, the validity or worth of the deposited securities, the credit-worthiness of any third party, or for any tax consequences that
may result from ownership of Depositary Shares, Ordinary Shares or deposited securities. The depositary and its agents shall not be liable
for any acts or omissions made by a successor depositary.
In
connection with the sale of securities, including, without limitation, deposited securities, the deposit agreement provides that the depositary
shall not have any liability for the price received in connection with any such sale, the timing thereof or any delay in action or omission
to act nor shall it be responsible for any error or delay in action, omission to act, default or negligence on the part of the party so
retained in connection with any such sale or proposed sale.
The
depositary will have the right, in its sole discretion, to refer any claim or dispute arising directly or indirectly from the relationship
created by the deposit agreement to arbitration in accordance with the Commercial Arbitration Rules of the American Arbitration Association.
The arbitration proceeding shall be conducted by three arbitrators, one nominated by the Depositary, one nominated by the Company, and
one nominated by the two party-appointed arbitrators. Any judgment, rendered by the arbitrators may be enforced in any court having jurisdiction
thereof. The arbitration shall occur in New York, New York, and the procedural law of such arbitration shall be New York law. The arbitration
provisions of the deposit agreement do not preclude you from pursuing claims under the Securities Act or the Exchange Act in federal courts.
In addition, the deposit agreement provides that each party to the deposit agreement (including each holder, beneficial owner and holder
of interests in the ADRs) irrevocably waives, to the fullest extent permitted by applicable law, any right it may have to a trial by jury
in any lawsuit or proceeding against the depositary or us related to our ordinary shares, the Depositary Shares or the deposit agreement.
In
the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will
issue, deliver or register a transfer of a Depositary Share, split up, subdivide or combine Depositary Shares, make a distribution on
a Depositary Share, or permit withdrawal of ordinary shares, the depositary may require:
| · | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees
charged by third parties for the transfer of any Ordinary Shares or other deposited securities and payment of applicable fees, expenses
and charges of the depositary; |
| · | satisfactory proof of the identity and genuineness of any signature or any other matters contemplated
in the deposit agreement; and |
| · | compliance with applicable laws and governmental regulations, and such reasonable regulations and procedures as the depositary may
establish, from time to time, consistent with the deposit agreement and applicable law, including presentation of transfer documents. |
The depositary may refuse
to issue and deliver Depositary Shares or register transfers of Depositary Shares when the transfer books of the depositary or our transfer
books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Ordinary Shares Underlying
your Depositary Shares
Depositary Share holders have
the right to cancel their Depositary Shares and withdraw the underlying Ordinary Shares at any time except:
| · | when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed
our transfer books; (ii) the transfer of Ordinary Shares is blocked to permit voting at a shareholders’ meeting; or (iii) we are
paying a dividend on our Ordinary Shares; |
| · | when you owe money to pay fees, taxes and similar charges; |
| · | when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations
that apply to Depositary Shares or to the withdrawal of Ordinary Shares or other deposited securities; |
| · | other circumstances specifically contemplated by Section I.A.(1) of the General Instructions to Form F-6
(as such General Instructions may be amended from time to time); or |
| · | for any other reason if the depositary determines, in good faith, that it is necessary or advisable to
prohibit withdrawals. |
This right of withdrawal may
not be limited by any other provision of the deposit agreement.
Pre-release of Depositary Shares
The
deposit agreement permits the depositary to deliver Depositary Shares before deposit of the underlying ordinary shares. This is called
a pre-release of the Depositary Shares. The depositary may also deliver Ordinary Shares upon cancellation of pre-released Depositary Shares
(even if the Depositary Shares are cancelled before the pre-release transaction has been closed out). A pre-release is closed out as soon
as the underlying Ordinary Shares are delivered to the depositary. The depositary may receive Depositary Shares instead of Ordinary Shares
to close out a pre-release. The depositary may pre-release Depositary Shares only under the following conditions: (i) before or at
the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer
(a) beneficially owns the Ordinary Shares or Depositary Shares to be deposited, (b) agrees to indicate the depositary as owner
of such Ordinary Shares or Depositary Shares in its records and to hold such Ordinary Shares or Depositary Shares in trust for the depositary
until such ordinary shares or Depositary Shares are delivered to the depositary or the custodian, (c) unconditionally guarantees
to deliver such Ordinary Shares or Depositary Shares to the depositary or the custodian, as the case may be, and (d) agrees to any
additional restrictions or requirements that the depositary deems appropriate; (ii) the pre-release is fully collateralized with
cash, United States government securities or other collateral that the depositary considers appropriate; and (iii) the depositary
must be able to close out the pre-release on not more than five business days’ notice. Each pre-release is subject to further indemnities
and credit regulations as the depositary considers appropriate. In addition, the depositary will normally limit the number of Depositary
Shares that may be outstanding at any time as a result of pre-release to 30% of the aggregate number of Depositary Shares then outstanding,
although the depositary may disregard the limit from time to time, if it thinks it is appropriate to do so.
Direct Registration System
The
deposit agreement provides that, to the extent available by the depositary, Depositary Shares shall be evidenced by ADRs issued through
DRS/Profile unless certificated ADRs are specifically requested by the Depositary Share holder. DRS is the system administered by DTC
pursuant to which the depositary may register the ownership of uncertificated Depositary Shares, which ownership shall be evidenced by
periodic statements issued by the depositary to the Depositary Share holders entitled thereto. The Profile Modification System, or Profile,
is a required feature of DRS which allows a DTC participant, claiming to act on behalf of a Depositary Share holder, to direct the depositary
to register a transfer of those Depositary Shares to DTC or its nominee and to deliver those Depositary Shares to the DTC account of that
DTC participant without receipt by the depositary of prior authorization from the Depositary Share holder to register such transfer.
In connection with and in
accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit
agreement understand that the depositary will not verify, determine or otherwise ascertain that the DTC participant which is claiming
to be acting on behalf of a Depositary Share holder in requesting registration of transfer and delivery described in the paragraph above
has the actual authority to act on behalf of the Depositary Share holder (notwithstanding any requirements under the Uniform Commercial
Code)
TAXATION
The
following summary contains a description of the material U.S. federal income tax and United Kingdom tax consequences of the acquisition,
ownership and disposition of Ordinary Shares and Depositary Shares, but it does not purport to be a comprehensive description of all the
tax considerations that may be relevant to a decision to purchase Ordinary Shares or Depositary Shares. The summary is based upon the
on the tax laws of the United States and regulations thereunder and the tax laws of the United Kingdom and regulations thereunder as of
the date hereof, which are subject to change.
Certain United Kingdom
Tax Considerations
The following is a general
summary of certain United Kingdom tax considerations relating to the ownership and disposal of our Ordinary Shares or Depositary Shares
and does not address all possible tax consequences relating to an investment in our Ordinary Shares or Depositary Shares. It is based
on United Kingdom tax law and generally published His Majesty’s Revenue & Customs, or HMRC, practice as of the date of
this prospectus, both of which are subject to change, possibly with retrospective effect. A United Kingdom tax year runs from April 6th
in any year to April 5th in the following year.
Save as provided otherwise,
this summary applies only to a person who is the absolute beneficial owner of our Ordinary Shares or Depositary Shares and who is resident
(and, in the case of an individual, domiciled) in the United Kingdom for tax purposes and who is not resident for tax purposes in any
other jurisdiction and does not have a permanent establishment or fixed base in any other jurisdiction with which the holding of our Ordinary
Shares or Depositary Shares is connected, or a U.K. Holder. A person who is not a U.K. Holder, including a person (a) who is not
resident (or, if resident, is not domiciled) in the United Kingdom for tax purposes, including an individual and company who trades
in the United Kingdom through a branch, agency or permanent establishment in the United Kingdom to which an Ordinary Share or Depositary
Share is attributable, or (b) who is resident or otherwise subject to tax in a jurisdiction outside the United Kingdom, is recommended
to seek the advice of professional advisors in relation to their taxation obligations.
This summary is for general
information only and is not intended to be, nor should it be considered to be, legal or tax advice to any particular investor. It does
not address all of the tax considerations that may be relevant to specific investors in light of their particular circumstances or to
investors subject to special treatment under United Kingdom tax law. In particular this summary:
| · | only applies to an absolute beneficial owner of Ordinary Shares or Depositary Shares and any dividend paid in respect of that Ordinary
Share where the dividend is regarded for United Kingdom tax purposes as that person’s own income (and not the income of some other
person); and |
| · | (a) only addresses the principal United Kingdom tax consequences for an investor who holds Ordinary
Shares or Depositary Shares as a capital asset, (b) does not address the tax consequences that may be relevant to certain special
classes of investor such as a dealer, broker or trader in shares or securities and any other person who holds Ordinary Shares or Depositary
Shares otherwise than as an investment, (c) does not address the tax consequences for a holder that is a financial institution, insurance
company, collective investment scheme, pension scheme, charity or tax-exempt organization, (d) assumes that a holder is not an officer
or employee of the company (nor of any related company) and has not (and is not deemed to have) acquired the Ordinary Shares or Depositary
Shares by virtue of an office or employment, and (e) assumes that a holder does not control or hold (and is not deemed to control
or hold), either alone or together with one or more associated or connected persons, directly or indirectly (including through the holding
of Depositary Shares), an interest of 10% or more in the issued share capital (or in any class thereof), voting power, rights to profits
or capital of the company, and is not otherwise connected with the company. |
This summary further assumes
that a holder of Depositary Shares is the beneficial owner of the underlying Ordinary Shares for United Kingdom direct tax purposes.
POTENTIAL INVESTORS IN
THE DEPOSITARY SHARES SHOULD SATISFY THEMSELVES PRIOR TO INVESTING AS TO THE OVERALL TAX CONSEQUENCES, INCLUDING, SPECIFICALLY, THE CONSEQUENCES
UNDER UNITED KINGDOM TAX LAW AND HMRC PRACTICE OF THE ACQUISITION, OWNERSHIP AND DISPOSAL OF THE ORDINARY SHARES OR DEPOSITARY SHARES,
IN THEIR OWN PARTICULAR CIRCUMSTANCES BY CONSULTING THEIR OWN TAX ADVISERS.
Taxation of Dividends
Withholding Tax. An
individual holder of Ordinary Shares or Depositary Shares who is not a U.K. Holder will not be chargeable to United Kingdom income tax
on a dividend paid by the Company, unless such holder carries on (whether solely or in partnership) a trade, profession or vocation in
the United Kingdom through a branch or agency in the United Kingdom to which the Ordinary Shares or Depositary Shares are attributable.
In these circumstances, such holder may, depending on his or her individual circumstances, be chargeable to United Kingdom income tax
on a dividend received from the Company.
A dividend received by individual U.K. Holders
will be subject to United Kingdom income tax. The rate of United Kingdom income tax that is chargeable on dividends received in either
the tax year 2022/2023 or the tax year 2023/2024 by an individual U.K. Holder who is (i) an additional rate taxpayer is 39.35%, (ii) a
higher rate taxpayer is 33.75%, and (iii) a basic rate taxpayer is 8.75%. An individual U.K. Holder may be entitled to a tax-free
dividend allowance (in addition to their personal allowance) of £2,000 for the tax year 2022/2023 and £1,000 for the tax year
2023/2024, being the amount of dividend income that the relevant individual can receive before United Kingdom income tax is payable. Dividends
within the dividend allowance will still count towards the relevant individual's basic, higher or additional rate bands, however. An individual’s
dividend income is treated as the top slice of their total income that is chargeable to United Kingdom income tax. Dividends which are
covered by an individual’s personal income tax allowance do not count towards and are ignored for the dividend allowance.
Corporation Tax. A U.K. Holder within
the charge to United Kingdom corporation tax may be entitled to exemption from United Kingdom corporation tax in respect of dividend payments
in respect of an Ordinary Share. If the conditions for the exemption are not satisfied or such U.K. Holder elects for an otherwise exempt
dividend to be taxable, United Kingdom corporation tax will be chargeable on the dividend. From April 1, 2023, the main rate of corporation
tax of 25% will apply to companies with profits in excess of £250,000. A lower rate of corporation tax of 19% will apply to companies
with profits of up to £50,000, and a marginal scaled rate between 19% and 25% will apply to companies with profits between £50,000
and £250,000. If potential investors are in any doubt as to their position, they should consult their own professional advisers.
A corporate holder of Ordinary
Shares or Depositary Shares that is not a U.K. Holder will not be subject to United Kingdom corporation tax on a dividend received from
the company, unless it carries on a trade in the United Kingdom through a permanent establishment to which the Ordinary Shares or Depositary
Shares are attributable. In these circumstances, such holder may, depending on its individual circumstances and if the exemption from
United Kingdom corporation tax discussed above does not apply, be chargeable to United Kingdom corporation tax on dividends received from
the Company.
Taxation of Disposals
U.K.
Holders. A disposal or deemed disposal of Ordinary Shares or Depositary Shares by an individual U.K. Holder may, depending on his
or her individual circumstances, give rise to a chargeable gain or to an allowable loss for the purpose of United Kingdom capital gains
tax. The principal factors that will determine the capital gains tax position on a disposal of Ordinary Shares or Depositary Shares are
the extent to which the holder realizes any other capital gains in the tax year in which the disposal is made, the extent to which the
holder has incurred capital losses in that or any earlier tax year and the level at which the annual exempt amount for United Kingdom
capital gains tax (the “annual exempt amount”) is set by the United Kingdom government for that tax year. The annual exempt
amount for the 2022/2023 tax year is £12,300 and for the 2023/2024 tax year is £6,000. If, after all allowable deductions,
an individual U.K. Holder’s total taxable income for the relevant tax year exceeds the basic rate income tax limit, a taxable capital
gain accruing on a disposal of an Ordinary Share or a Depositary Shares is taxed at the rate of 20%. In other cases, a taxable capital
gain accruing on a disposal of our Ordinary Shares or Depositary Shares may be taxed at the rate of 10% or the rate of 20% or at a combination
of both rates.
An
individual U.K. Holder who ceases to be resident in the United Kingdom (or who fails to be regarded as resident in a territory outside
the United Kingdom for the purposes of double taxation relief) for a period of less than five calendar years and who disposes of Ordinary
Shares or Depositary Shares during that period of temporary non-United Kingdom residence may be liable to United Kingdom capital gains
tax on a chargeable gain accruing on such disposal on his or her return to the United Kingdom (or upon ceasing to be regarded as resident
outside the United Kingdom for the purposes of double taxation relief) (subject to available exemptions or reliefs).
A
disposal (or deemed disposal) of Ordinary Shares or Depositary Shares by a corporate U.K. Holder may give rise to a chargeable gain or
an allowable loss for such holder for the purpose of United Kingdom corporation tax.
Any
gain or loss in respect of currency fluctuations over the period of holding Ordinary Shares or Depositary Shares is also brought into
account on a disposal.
Non-U.K.
Holders. An individual holder who is not a U.K. Holder will not be liable to United Kingdom capital gains tax on capital gains realized
on the disposal of Ordinary Shares or Depositary Shares unless such holder carries on (whether solely or in partnership) a trade, profession
or vocation in the U.K. through a branch or agency in the United Kingdom to which the Ordinary Shares or Depositary Shares are attributable.
In these circumstances, such holder may, depending on his or her individual circumstances, be chargeable to United Kingdom capital gains
tax on chargeable gains arising from a disposal of his or her Ordinary Shares or Depositary Shares.
A
corporate holder of Ordinary Shares or Depositary Shares that is not a U.K. Holder will not be liable for United Kingdom corporation tax
on chargeable gains realized on the disposal of Ordinary Shares or Depositary Shares unless it carries on a trade in the United Kingdom
through a permanent establishment to which the Ordinary Shares or Depositary Shares are attributable. In these circumstances, a disposal
(or deemed disposal) of Ordinary Shares or Depositary Shares by such holder may give rise to a chargeable gain or an allowable loss for
the purposes of United Kingdom corporation tax.
Inheritance Tax
If
for the purposes of the Double Taxation Relief (Taxes on Estates of Deceased Persons and on Gifts) Treaty United States of America Order
1979 (SI 1979/1454) between the United States and the United Kingdom an individual holder is at the time of their death or a transfer
made during their lifetime, domiciled in the United States and is not a national of the United Kingdom, any Ordinary Shares or Depositary
Shares beneficially owned by that holder should not generally be subject to United Kingdom inheritance tax, provided that any applicable
United States federal gift or estate tax liability is paid, except where (i) the Ordinary Shares or Depositary Shares are part of
the business property of a United Kingdom permanent establishment or pertains to a United Kingdom fixed base used for the performance
of independent personal services; or (ii) the Ordinary Shares or Depositary Shares are comprised in a settlement unless, at the time
the settlement was made, the settlor was domiciled in the United States and not a national of the United Kingdom (in which case no charge
to United Kingdom inheritance tax should apply).
Stamp Duty and Stamp Duty Reserve Tax
The United Kingdom stamp duty,
or stamp duty, and United Kingdom stamp duty reserve tax, or SDRT, treatment of the issue and transfer of, and the agreement to transfer,
an ordinary share outside a depositary receipt system or a clearance service is discussed in the paragraphs under “General”
below. The stamp duty and SDRT treatment of such transactions in relation to such systems is discussed in the paragraphs under “Depositary
Receipt Systems and Clearance Services” below.
General
An
agreement to transfer an ordinary share will normally give rise to a charge to SDRT at the rate of 0.5% of the amount or value of the
consideration payable for the transfer. SDRT is, in general, payable by the purchaser.
The
transfer of an Ordinary Share would be subject to stamp duty at the rate of 0.5% of the consideration given for the transfer (rounded
up to the next £5). The purchaser is liable to HMRC for the payment of the stamp duty (if any). Under current HMRC guidance, no
stamp duty should be payable on a written instrument transferring a Depositary Share or on a written agreement to transfer a Depositary
Share, on the basis that the Depositary Share is not regarded as either “stock” or a “marketable security” for
United Kingdom stamp duty purposes.
If
a duly stamped transfer completing an agreement to transfer is produced within six years of the date on which the agreement is made (or,
if the agreement is conditional, the date on which the agreement becomes unconditional) any SDRT already paid is generally repayable,
normally with interest, and any SDRT charge yet to be paid is canceled to avoid a double charge as the stamp duty has been paid.
No
SDRT or stamp duty is chargeable in respect of shares that are admitted to trading on a “recognized growth market” and not
listed on any “recognized stock exchange,” or the AIM Exemption. For so long as the Ordinary Shares were admitted to trading
on AIM (which qualifies as a “recognized growth market”) and not listed on a market that would qualify as a “recognized
stock exchange,” the AIM Exemption would apply and the transfer of Ordinary Shares or agreement to transfer Ordinary Shares would
be exempt from the charge to stamp duty and/or SDRT (as applicable) under the AIM Exemption. Following the cancellation of admission of
the Ordinary Shares on AIM, the AIM Exemption no longer applies.
Depositary Receipt Systems and Clearance
Services
The
Court of Justice of the European Union in C-569/07 HSBC Holdings Plc, Vidacos Nominees Limited v The Commissioners of Her Majesty’s
Revenue & Customs and the First-tier Tax Tribunal decision in HSBC Holdings Plc and the Bank of New York Mellon
Corporation v The Commissioners of Her Majesty’s Revenue & Customs, have considered the provisions of the European
Union Council Directive 69/335/EEC, which was subsequently substituted by the European Union Council Directive 2008/7/EEC, or the E.U.
Directives. Following these decisions HMRC has publicly confirmed that issues or transfers of shares of United Kingdom incorporated companies,
such as us, to a clearance service (such as, in our understanding, DTC) or a depositary receipt system will not be charged to United Kingdom
SDRT at 1.5% where that issue or transfer is an integral part of a raising of new capital.
It
was announced as part of the United Kingdom Budget 2017 by the United Kingdom government that the 1.5% stamp duty and SDRT charge will
not be enforced on the issue of shares by United Kingdom incorporated companies (and transfers of such shares where the transfer is integral
to new capital raising) into clearance services and depositary receipt systems following Brexit. However, the United Kingdom government
could potentially introduce new United Kingdom legislation with the effect that a future issue or transfer of our Ordinary Shares into
a clearance service or depositary receipt system (even where such an issue or transfer is an integral part of the raising of new capital
by the company) may potentially become chargeable to 1.5% stamp duty or SDRT.
Where
an ordinary share is transferred (i) to, or to a nominee for, a person whose business is or includes the provision of clearance services
or (ii) to, or to a nominee for a person whose business is or includes issuing depositary receipts and that transfer is not integral
to the raising of new capital by the company, stamp duty or SDRT would generally be chargeable at the rate of 1.5% of the amount or value
of the consideration given or, in certain circumstances, the value of the shares. Such a stamp duty or SDRT charge will arise if the transfer
takes place at a time when the Ordinary Shares are admitted to trading on AIM such that the AIM Exemption would apply. Following the cancellation
of the admission of the Ordinary Shares on AIM, however, the AIM Exemption no longer is available.
There
is an exception from the 1.5% charge on the transfer to, or to a nominee , a clearance service where the clearance service has made and
maintained an election under section 97A(1) of the Finance Act 1986, which has been approved by HMRC. If such an election were made by
a clearance service, SDRT at the rate of 0.5% of the amount or value of the consideration payable for the transfer would arise on any
transfer of an ordinary share into such a clearance service and on subsequent agreements to transfer such share within such clearance
service. It is our understanding that DTC has not to date made an election under section 97A(1) of the Finance Act of 1986.
Any
liability for stamp duty or SDRT in respect of a transfer into a clearance service or depositary receipt system, or in respect of a transfer
within such a service, which does arise, will strictly be accountable to HMRC by the clearance service or depositary receipt system operator
or their nominee, as the case may be, but will, in practice, be payable by the participants in the clearance service or depositary receipt
system.
Certain United States Taxation Matters
The following is a summary
of material United States federal income tax consequences of the ownership and disposition of Depositary Shares by United States holders
(as defined below). This summary is for general information only and is not tax advice. Each investor should consult its tax advisor with
respect to the tax consequences of the ownership and disposition of our securities.
This summary is based on
provisions of the Internal Revenue Code of 1986, as amended, or the Code, United States Treasury regulations promulgated thereunder
(whether final, temporary, or proposed), administrative rulings, and judicial interpretations thereof, and the Convention Between
the Government of the United Kingdom of Great Britain and Northern Ireland and the Government of the United States of America for
the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and on Capital Gains of 2001,
as amended (referred to below as the “United States-U.K. Treaty”), all as in effect on the date hereof, and all of which are subject to change, possibly with retroactive effect.
For purposes of this discussion,
the term “United States holder” means a holder of our Ordinary Shares or Depositary Shares that is, for United States federal
income tax purposes:
| · | an individual who is a citizen or resident of the United States; |
| · | a corporation or other entity taxable as a corporation that is created or organized in the United States
or under the laws of the United States or any state thereof or the District of Columbia; |
| · | an estate the income of which is subject to United States federal income taxation regardless of its source;
or |
| · | any trust if (a) a court within the United States is able to exercise primary supervision over the administration
of the trust and one or more United States persons have the authority to control all substantial decisions of the trust, or (b) such trust
has a valid election in effect under applicable United States Treasury regulations to be treated as a United States person. |
This summary addresses only
the United States federal income tax considerations for United States holders that acquire and hold the Depositary Shares as capital assets
within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all aspects
of United States federal income taxation that may be relevant to a holder in light of its particular circumstances, or that may apply
to holders that are subject to special treatment under the United States federal income tax laws (including, for example, banks, financial
institutions, underwriters, insurance companies, dealers in securities or foreign currencies, traders in securities who elect the mark-to-market
method of accounting for their securities, persons subject to the alternative minimum tax, persons that have a functional currency other
than the United States dollar, tax-exempt organizations (including private foundations), mutual funds, subchapter S corporations, partnerships
or other pass-through entities for United States federal income tax purposes, certain expatriates, corporations that accumulate earnings
to avoid United States federal income tax, persons who hold Depositary Shares as part of a hedge, straddle, constructive sale, conversion
or other integrated transaction, persons who acquire Depositary Shares through the exercise of options or other compensation arrangements,
persons who own (or are treated as owning) 10% or more of our outstanding voting stock, or persons who are not United States holders).
In addition, this discussion does not address any aspect of state, local, foreign, estate, gift or other tax law that may apply to holders
of Depositary Shares.
The United States federal
income tax treatment of a partner in a partnership (including any entity or arrangement treated as a partnership for United States federal
income tax purposes) generally will depend on the status of the partner and the activities of the partnership. A partner in such a partnership
should consult its tax advisor regarding the associated tax consequences.
Consequences Relating to Ownership and Disposition
of Depositary Shares
Ownership of Depositary
Shares. For United States federal income tax purposes, a holder of Depositary Shares will generally be treated as if such holder directly
owned the ordinary shares represented by such Depositary Shares.
Distributions on Depositary
Shares. Subject to the discussion below under “—Passive Foreign Investment Company Rules,” the gross
amount of any distribution on Depositary Shares (including withheld taxes, if any) made out of our current or accumulated earnings and
profits (as determined for United States federal income tax purposes) will generally be taxable to a United States holder as dividend
income on the date such distribution is actually or constructively received. Any such dividends paid to corporate United States holders
generally will not qualify for the dividends received deduction that may otherwise be allowed under the Code. Distributions in excess
of our current and accumulated earnings and profits would generally be treated first as a non-taxable return of capital to the extent
of the United States holder’s basis in the Depositary Shares, and thereafter as capital gain. However, since we do not calculate
our earnings and profits under United States federal income tax principles, it is expected that any distribution on Depositary Shares
will be reported as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain
under the rules described above.
Dividends paid in currencies
other than the United States dollar, if any, will generally be taxable to a United States holder as ordinary dividend income in an amount
equal to the United States dollar value of the currency received on the date such distribution is actually or constructively received.
Such United States dollar value must be determined using the spot rate of exchange on such date, regardless of whether the non-United
States currency is actually converted into United States dollars on such date. The United States holder may realize exchange gain or loss
if the currency received is converted into United States dollars after the date on which it is actually or constructively received. In
general, any such gain or loss will be ordinary and will be treated as from sources within the United States for United States foreign
tax credit purposes.
Subject to the discussion
below under “—3.8% Medicare Tax on Net Investment Income,” dividends received by certain non-corporate
United States holders (including individuals) from a “qualified foreign corporation” may be eligible for reduced rates of
taxation, currently at a maximum rate of 20%, provided that certain holding period requirements and other conditions are satisfied. For
these purposes, a foreign corporation will generally be treated as a qualified foreign corporation with respect to dividends paid by that
corporation on shares that are readily tradable on an established securities market in the United States. United States Treasury Department
guidance indicates that the Depositary Shares, which are listed on the NASDAQ Capital Market, would be considered readily tradable on
an established securities market in the United States. However, there can be no assurance that the Depositary Shares will be considered
readily tradable on an established securities market in future years. A foreign corporation is also treated as a qualified foreign corporation
if it is eligible for the benefits of a comprehensive income tax treaty with the United States which is determined by the United States
Treasury Department to be satisfactory for purposes of these rules and which includes an exchange of information provision. The United
States Treasury Department has determined that the United States-U.K. Treaty meets these requirements. We would not constitute a qualified
foreign corporation for purposes of these rules if we are a passive foreign investment company for the taxable year in which we pay a
dividend or for the preceding taxable year, as discussed below under “—Passive Foreign Investment Company Rules.”
Subject to certain conditions
and limitations, non-United States taxes, if any, withheld on dividends paid by the Company may be treated as foreign taxes eligible for
a credit against a United States holder’s United States federal income tax liability under the United States foreign tax credit
rules. The rules governing the United States foreign tax credit are complex, and United States holders should consult their tax advisors
regarding the availability of the United States foreign tax credit under their particular circumstances.
Sale of Depositary Shares
A United States holder will
generally recognize gain or loss on any sale, exchange, redemption, or other taxable disposition of Depositary Shares in an amount equal
to the difference between the amount realized on the disposition and such holder’s tax basis in such securities. Subject to the
discussion below under “—Passive Foreign Investment Company Rules,” any gain or loss recognized by a United States
holder on a taxable disposition of Depositary Shares will generally be capital gain or loss and will be long-term capital gain or loss
if the holder’s holding period in such share exceeds one year at the time of the disposition. The deductibility of capital losses
is subject to limitations.
For a cash basis taxpayer,
units of foreign currency received will generally be translated into United States dollars at the spot rate on the settlement date of
the sale. In that case, no foreign currency exchange gain or loss will result from currency fluctuations between the trade date and the
settlement date of such sale. An accrual basis taxpayer may elect to apply the same rules applicable to cash basis taxpayers with respect
to the sale of ADRs that are traded on an established securities market, provided that the election must be applied consistently from
year to year and cannot be changed without the consent of the IRS. For an accrual method taxpayer who does not make such an election,
units of foreign currency received will generally be translated into United States dollars at the spot rate on the trade date of the sale.
Such an accrual basis taxpayer may recognize foreign currency exchange gain or loss based on currency fluctuations between the trade date
and the settlement date of such sale. In general, any such gain or loss will be ordinary and will be treated as from sources within the
United States for United States foreign tax credit purposes.
Passive Foreign Investment Company Rules
A foreign corporation is a
PFIC if either (1) 75% or more of its gross income for the taxable year is passive income or (2) the average percentage of assets held
by such corporation during the taxable year that produce passive income or that are held for the production of passive income is at least
50%. For purposes of applying the tests in the preceding sentence, the foreign corporation is deemed to own its proportionate share of
the assets, and to receive directly its proportionate share of the income, of any other corporation of which the foreign corporation owns,
directly or indirectly, at least 25% by value of the stock.
Based
upon estimates with respect to its income, assets, and operations, it is expected that we will not be a PFIC for the current taxable year.
However, because the determination of PFIC status must be made on an annual basis after the end of the taxable year and will depend on
the composition of the income and assets, as well as the nature of the activities, of our activities and those of our subsidiaries from
time to time, there can be no assurance that we will not be considered a PFIC for any taxable year.
If
we were to be classified as a PFIC for any taxable year in which a United States holder held the Depositary Shares, various adverse United
States tax consequences could result to such United States holders, including taxation of gain on a sale or other disposition of the shares
of the corporation, Depositary Shares at ordinary income rates and imposition of an interest charge on gain or on distributions with respect
to the shares, Depositary Shares. Unless a United States holder of PFIC shares elects, in either case if eligible, to be taxed annually
on a mark-to-market basis or makes a QEF election and certain other requirements are met, gain realized on the sale or other disposition
of PFIC shares would generally not be treated as capital gain. Instead, the United States holder would be treated as if the United States
holder had realized such gain ratably over the holder’s holding period for such securities. The amounts allocated to the taxable
year of sale or other disposition and to any year before the foreign corporation became a PFIC would be taxed as ordinary income. The
amount allocated to each other taxable year would be subject to tax at the highest rate in effect for such year, together with an interest
charge in respect of the tax attributable to each such year. Similar rules apply to the extent any distribution in respect of PFIC shares
exceeds 125% of the average annual distribution on such PFIC securities received by the shareholder during the preceding three years or
holding period, whichever is shorter. With certain exceptions, a foreign corporation is treated as a PFIC with respect to a shareholder
(or warrant holder, as applicable) if the corporation was a PFIC with respect to such holder at any time during the holder’s holding
period of the foreign corporation’s stock or warrants. Dividends paid to with respect to shares of a PFIC are not eligible for the
special tax rates applicable to qualified dividend income of certain non-corporate holders. Instead, such dividend income is taxable at
rates applicable to ordinary income.
If
we were to be treated as a PFIC, the tax consequences described above could be avoided by a “mark-to-market” election with
respect to the Depositary Shares. A United States holder making a “mark-to-market” election (assuming the requirements for
such an election are satisfied) generally would (i) be required to include as ordinary income the excess of the fair market value of the
Depositary Shares on the last day of the United States holder’s taxable year over the United States holder’s adjusted tax
basis in such Depositary Shares and (ii) be allowed a deduction in an amount equal to the lesser of (A) the excess, if any, of the United
States holder’s adjusted tax basis in the Depositary Shares over the fair market value of such Depositary Shares on the last day
of the United States holder’s taxable year or (B) the excess, if any, of the amount included in income because of the election for
prior taxable years over the amount allowed as a deduction because of the election for prior taxable years. In addition, upon a sale or
other taxable disposition of Depositary Shares, a United States holder would recognize ordinary income or loss (which loss could not be
in excess of the amount included in income because of the election for prior taxable years over the amount allowed as a deduction because
of the election for prior taxable years). If we were to be treated as a PFIC, different rules would apply to a United States holder making
a QEF election with respect to Depositary Shares. However, we do not intend to prepare or provide the information necessary for United
States shareholders to make a QEF election.
United
States holders are urged to consult their own tax advisors about the PFIC rules, including the availability of the “mark-to-market”
election.
3.8% Medicare Tax on “Net Investment
Income”
A 3.8% tax, or “Medicare
Tax,” is imposed on all or a portion of “net investment income,” which may include any gain realized or amounts received
with respect to Depositary Shares received by (i) United States holders that are individuals with modified adjusted gross income in excess
of certain thresholds, and (ii) certain estates and trusts. United States holders should consult their own tax advisors with respect to
the applicability of the Medicare Tax resulting from ownership or disposition of Depositary Shares.
Information Reporting and Backup Withholding
United States holders may
be subject to information reporting requirements and may be subject to backup withholding with respect to dividends on Depositary Shares
and on the proceeds from the sale, exchange, or disposition of Depositary Shares, currently at a rate of 24%, unless the United States holder provides an accurate
taxpayer identification number and complies with certain certification procedures or otherwise establishes an exemption from backup withholding.
Backup withholding is not an additional tax and amounts withheld may be allowed as a credit against the United States holder’s United
States federal income tax liability and may entitle the United States holder to a refund, provided that certain required information is
timely furnished to the IRS.
Foreign Asset Reporting
United States holders who
are individuals and who own “specified foreign financial assets” with an aggregate value in excess of $50,000 are generally
required to file an information statement along with their tax returns, currently on IRS Form 8938, with respect to such assets. “Specified
foreign financial assets” include securities issued by a non-United States issuer (which would include an investment in our securities)
that are not held in accounts maintained by financial institutions. Higher reporting thresholds apply to certain individuals living abroad
and to certain married individuals. Individuals who fail to report the required information could be subject to substantial penalties,
and such individuals should consult their own tax advisors concerning the application of these rules to their investment in Depositary
Shares.
U.S. Federal Income
Tax Treatment of the Series E Warrants
Neither
we nor a United States holder of a Series E Warrant will recognize gain or loss as a result of the United States holder’s receipt
of our Depositary Shares upon exercise of a Series E Warrant. A United States holder’s adjusted tax basis in the Depositary Shares
received will be an amount equal to the sum of (i) the United States holder’s adjusted tax basis in the Series E Warrant exercised;
and (ii) the amount of the exercise price for the Series E Warrant. If the Series E Warrants lapse without being exercised, the United
States holder will recognize capital loss in the amount equal to the United States holder’s adjusted tax basis in the Series E Warrants.
A United States holder’s holding period for Depositary Shares received upon exercise of a Series E Warrant will commence on the
date the Series E Warrant is exercised.
The
exercise price of a Series E Warrant is subject to adjustment under certain circumstances. If an adjustment increases a proportionate
interest of the holder of a Series E Warrant in the fully diluted Ordinary Shares without proportionate adjustments to the holders of
our Depositary Shares, a United States holder of Series E Warrants may be treated as having received a constructive distribution, which
may be taxable to the United States holder as a dividend.
U.S. Federal Income
Tax Treatment of the Pre-Funded Warrants
We
believe that our Pre-Funded Warrants should be treated as our Depositary Shares for U.S. federal income tax purposes, rather than as warrants.
Assuming this position is upheld, upon the exercise of a Pre-Funded Warrant, the holding period of the Pre-Funded Warrant should carry
over to the Depositary Shares received. Similarly, no gain or loss should be recognized upon the exercise of a Pre-Funded Warrant and
the tax basis of a Pre-Funded Warrant should carry over to the Depositary Shares received upon exercise, increased by the exercise price
of $0.0001 per share.
In
the event that the exercise price or conversion ratio of Pre-Funded Warrants is adjusted as a result of an action affecting the Depositary
Shares, such as a dividend being paid on the Depositary Shares, a United States holder may be treated as receiving a distribution from
the Company. Such deemed distributions may be treated as dividends and may be eligible for preferential tax rates, as described above.
However,
our position is not binding on the IRS and the IRS may treat the Pre-Funded Warrants as warrants to acquire our Depositary Shares. You
should consult your tax advisor regarding the U.S. federal tax consequences of an investment in the Pre-Funded Warrants.
THE
DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PARTICULAR INVESTOR. EACH PROSPECTIVE
INVESTOR IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISER ABOUT THE TAX CONSEQUENCES TO IT OF OWNING AND DISPOSING OF OUR SECURITIES
IN LIGHT OF SUCH PROSPECTIVE INVESTOR’S OWN CIRCUMSTANCES.
UNDERWRITING
We are offering the Class
A Units and Class B Units described in this prospectus through the underwriters named below. Ladenburg Thalmann & Co. Inc. is acting
as the representative of the underwriters in this offering, or the Representative. Subject to the terms and conditions of the underwriting
agreement, dated as of , 2023, the underwriters have agreed to purchase the
number of our securities set forth opposite its respective name below.
| Underwriters |
|
Number of Class
A Units |
|
Number of Class
B Units |
| Ladenburg Thalmann & Co. Inc. |
|
|
|
|
| Total |
|
|
|
|
A copy of the underwriting
agreement will be filed as an exhibit to the registration statement of which this prospectus is part.
We have
been advised by the underwriters that they propose to offer the Class A Units and Class B Units, if any, directly to the public
at the public offering price set forth on the cover page of this prospectus. Any securities sold by the underwriters to securities dealers
will be sold at the public offering price less a selling concession not in excess of $
per share and $ per Series E Warrant.
The underwriting agreement
provides that the underwriters’ obligation to purchase the securities we are offering is subject to conditions contained in the
underwriting agreement.
No action has been taken by
us or the underwriters that would permit a public offering of the Class A Units or Class B Units, or the Depositary Shares, Pre-Funded
Warrants and Series E Warrants included in the Class A Units or Class B Units in any jurisdiction outside the United States where action
for that purpose is required. None of our securities included in this offering may be offered or sold, directly or indirectly, nor may
this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the securities offering
hereby be distributed or published in any jurisdiction except under circumstances that will result in compliance with the applicable rules
and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions
relating to this offering of securities and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation
of any offer to buy the securities in any jurisdiction where that would not be permitted or legal.
The underwriters have advised
us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
Underwriting Discount and Expenses
The following table summarizes
the underwriting discount and commission to be paid to the underwriters by us.
| | |
Per Class A
Unit(1) | | |
Per Class B
Unit(2) | | |
Total
Without
Over-
Allotment | | |
Total With
Full Over-
Allotment | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Underwriting discounts and commissions to be paid to underwriters by us(3)(4) | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Proceeds, before expenses, to us | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
______________
| (1) | The public offering price and underwriting discount corresponds to, in respect of the Class A Units, |
(i) a public offering price per Depositary
Share of $ ($
net of the underwriting discount), and (ii) a public offering price per Series E Warrant of $
($ net of the underwriting discount).
| (2) | The public offering price and underwriting discount in respect of the Class B Units corresponds to (i) a public offering price per
Pre-Funded Warrant of $ ($ net
of the underwriting discount) and (ii) a public offering price per Series E Warrant of $
($ net of the underwriting discount). |
| (3) | We have also agreed to pay the Representative a management fee of 1.0% of the gross proceeds from the offering and to reimburse the
accountable expenses of the Representative, including a pre-closing expense allowance of up to a maximum of $25,000 and an additional
closing expense allowance up to a maximum of $150,000. |
| (4) | We have granted a 45-day option to the underwriters to purchase up to
additional Depositary Shares and/or Series E Warrants to purchase an additional
Depositary Shares at its respective public offering price set forth above less the underwriting discounts and commissions solely to cover
over-allotments, if any. |
We estimate the total expenses
payable by us for this offering to be approximately $ ,
which amount includes (i) the underwriting discount of $ ,
(ii) reimbursement of the accountable expenses of the underwriters, including the legal fees of the Representative, in an amount not to
exceed $25,000 for pre-closing expenses plus $150,000 for closing expenses, (iii) a management fee of approximately $
which represents 1.0% of the total gross proceeds payable to the Representative, and (iv) other estimated company expenses of approximately
$ , which includes legal, accounting, printing costs, and various fees associated with the registration and listing of our shares.
Over-allotment Option
We have granted to the underwriters
an option exercisable not later than 45 days after the date of this prospectus to purchase up to an additional Depositary Shares and/or
Series E Warrants to purchase up to an additional Depositary Shares at the public offering price per Depositary Share and the public offering
price per Series E Warrant set forth on the cover page hereto less the underwriting discounts and commissions. The underwriters may exercise
the option solely to cover overallotments, if any, made in connection with this offering. If any additional Depositary Shares and/or Series
E Warrants are purchased, the underwriters will offer these Depositary Shares and/or Series E Warrants on the same terms as those on which
the other securities are being offered.
Representative Warrants
We have agreed to issue Representative
Warrants to the Representative, upon the closing of this offering, which entitle it to purchase up to Depositary Shares (or Depositary
Shares assuming the exercise of the over-allotment option in full). The Representative Warrants will have an exercise price equal to $
per Depositary Share. The Representative Warrants will be exercisable immediately upon issuance, at any time and from time to time, in
whole or in part, during the five-year period commencing from the commencement of sales of this offering, and otherwise on substantially
similar terms to the Series E Warrants issued to investors as part of the offering. The Representative Warrants and the Depositary Shares
underlying the Representative Warrants are being registered on the registration statement of which this prospectus is a part.
Determination of Offering Price
Our Depositary Shares are
listed on the NASDAQ Capital Market under the symbol “BDRX”. We have assumed a public offering price of $ per Class A Unit,
which represents the last reported sale price of our Depositary Shares as reported on the NASDAQ Capital Market on , 2023.
The public offering price
of the securities offered by this prospectus will be determined by negotiation between us and the underwriters. Among the factors that
will be considered in determining the final public offering price of the securities:
| ● | our history and our prospects; |
| ● | the industry in which we operate; |
| ● | our past and present operating results; and |
| ● | the general condition of the securities markets at this time of this offering. |
The public offering price
stated on the cover page of this prospectus should not be considered an indication of the actual value of the securities sold in this
offering. That price is subject to change as a result of market conditions and other factors and we cannot assure you that the Depositary
Shares sold in this offering can be resold at or above the public offering price. There is no established
public trading market for the Pre-Funded Warrants or the Series E Warrants, and we do not expect such a market to develop. In addition,
we do not intend to apply for a listing of the Pre-Funded Warrants or the Series E Warrants on any national securities exchange or
other nationally recognized trading system.
Right of First Refusal
We have granted the Representative
the right of first refusal for a period of twelve months following the closing of this offering
and expiration of its term to act as sole bookrunner, exclusive placement agent or exclusive sales agent in connection with any financing
of the Company.
Listing
Our Depositary Shares are
listed on the NASDAQ Capital Market under the symbol “BDRX”. The closing price of our Depositary Shares on , 2023, as reported
by the NASDAQ Stock Market LLC, was $ per share.
There is no established public
trading market for the Series E Warrants or the Pre-Funded Warrants, and we do not expect such a market to develop. In addition, we do
not intend to apply for listing of the Series E Warrants or the Pre-Funded Warrants on any securities exchange or other trading system.
Lock-up Agreements
Our officers, directors and
each of their respective affiliates and associated partners, and certain other shareholders have agreed with the underwriters to be
subject to a lock-up period of following the closing date of this offering. This means that, during the applicable lock-up period, such
persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate
or otherwise dispose of, directly or indirectly, any Depositary Shares or Ordinary Shares or any securities convertible into, or exercisable
or exchangeable for, Depositary Shares or Ordinary Shares. Certain limited transfers are permitted during the lock-up period if the transferee
agrees to these lock-up restrictions. We have also agreed, in the underwriting agreement, to similar lock-up restrictions on the issuance
and sale of our securities for a period of following the closing date of this offering, although we will be permitted to issue stock options
or stock awards to directors, officers and employees under our existing plans. The Representative may, in its sole discretion and without
notice, waive the terms of any of these lock-up agreements.
Other Relationships
From time to time, certain
of the underwriters and their affiliates may provide in the future, various advisory, investment and commercial banking and other services
to us in the ordinary course of business, for which they will receive customary fees and commissions. The Representative acted as placement
agent in connection with our registered direct offerings and private placement consummated in December 2022, February 2023 and May 2023,
for which it received compensation.
Transfer Agent, Warrant Agent and Registrar
The transfer agent and registrar
for our Depositary Shares is The Bank of New York Mellon. The warrant agent for the Pre-Funded Warrants and Series E Warrants is .
Stabilization, Short Positions and Penalty Bids
The underwriters may engage
in syndicate covering transactions stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining
the price of our Depositary Shares;
| ● | Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order
to cover syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short
position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities
in the open market after pricing that could adversely affect investors who purchase in the offering. |
| ● | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific
maximum. |
| ● | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by
the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These syndicate covering transactions,
stabilizing transactions, and penalty bids may have the effect of raising or maintaining the market prices of our securities or preventing
or retarding a decline in the market prices of our securities. As a result the price of our Depositary Shares may be higher than the price
that might otherwise exist in the open market. Neither we nor the underwriters make any representation or prediction as to the effect
that the transactions described above may have on the price of our Depositary Shares. These transactions may be effected on the NASDAQ
Capital Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time. In connection
with this offering, the underwriters also may engage in passive market making transactions in our Depositary Shares in accordance with
Regulation M during a period before the commencement of offers or sales of Depositary Shares in this offering and extending through the
completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent
bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered
when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that
which might otherwise prevail in the open market and, if commenced, may be discontinued at any time. Neither we, nor the underwriters
make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on
the prices of our securities. In addition, neither we nor the underwriters make any representation that the underwriters will engage in
these transactions or that any transactions, once commenced will not be discontinued without notice.
Indemnification
We have agreed to indemnify
the underwriters against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments
that the underwriters may be required to make for these liabilities.
Electronic Distribution
A prospectus in electronic
format may be made available on the websites maintained by the underwriters, if any, participating in this offering and the underwriters
may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part
of this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the
underwriters, and should not be relied upon by investors.
LEGAL MATTERS
Certain legal matters in connection
with the securities offered hereby will passed upon for us by Mintz, Levin, Cohn, Ferris, Glovsky & Popeo, P.C., Boston, Massachusetts,
and Brown Rudnick LLP, London, United Kingdom. The Representative is being represented by Ellenoff, Grossman & Schole, LLP, New York,
New York.
EXPERTS
The financial statements as
of December 31, 2022, and for the each of the three years in the period then ended, incorporated by reference into this prospectus have
been so incorporated in reliance on a report of Mazars LLP, an independent registered accounting firm, given on authority of said firm
as experts in auditing and accounting. The report on the financial statements for the year ended December 31, 2022 contains an explanatory
paragraph regarding our ability to continue as a going concern.
Mazars LLP, London, United
Kingdom, is a member of the Institute of Chartered Accountants in England and Wales.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to periodic
reporting and other informational requirements of the Exchange Act, as applicable to foreign private issuers. Accordingly, we are required
to file reports, including annual reports on Form 20-F, and other information with the SEC. As a foreign private issuer, we are exempt
from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders under the federal proxy
rules contained in Sections 14(a), (b) and (c) of the Exchange Act, and our “insiders” are exempt from the reporting
and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. The SEC maintains an Internet site that contains
reports, proxy, information statements and other information regarding issuers at http://www.sec.gov. Copies of certain information filed
by us with the SEC are also available on our website at http://www.biodexapharma.com. Our website is not a part of this prospectus and
is not incorporated by reference in this prospectus.
This prospectus is part of
a registration statement we filed with the SEC. This prospectus omits some information contained in the registration statement in accordance
with SEC rules and regulations. You should review the information and exhibits in the registration statement for further information
on us and our consolidated subsidiaries and the securities we are offering. Statements in this prospectus concerning any document we filed
as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified
by reference to these filings. You should review the complete document to evaluate these statements. You can obtain a copy of the registration
statement from the SEC at the address listed above or from the SEC’s website.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate
by reference” the information we file with the SEC, which means that we can disclose important information to you by referring you
to another document filed separately with the SEC. The information incorporated by reference is an important part of this prospectus.
We incorporate by reference, as of their respective dates of filing, the documents listed below that we have filed with the SEC and any
documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus
and prior to the termination of the offering of securities under this prospectus (except in each case the information contained in such
documents to the extent “furnished” and not “filed”):
| · | our Annual Report on Form 20-F for the fiscal year ended December 31, 2022, filed with the SEC on April
28, 2023, as amended on May 5, 2023; |
| · | our Reports on Form 6-K and any amendments thereto furnished to the SEC on January 6, 2023, January 11,
2023, January 12, 2023, January 23, 2023, January 26, 2023, February 2, 2023, February 9, 2023, February 15, 2023,
March 8, 2023, March 10, 2023, March 24, 2023, March 27, 2023, March 29, 2023 (both filings), March 30, 2023, April 4,
2023 (both filings), April 5, 2023, April 7, 2023, April 12, 2023, April 19, 2023, April 28, 2023, May 18, 2023, May
22, 2023, May 24, 2023, May 25, 2023, May 26, 2023 (both filings), June 1, 2023, June 14, 2023, June 15, 2023, June 20, 2023, June 22,
2023, July 6, 2023 (both filings), July 10, 2023, July 24, 2023, September 29, 2023, and October 3, 2023, that we incorporate by reference
into this prospectus; and |
| · | the description of our Ordinary Shares and Depositary Shares contained in our registration statement on
Form 8-A, originally filed with the SEC on December 2, 2015, as amended on April 30, 2021, including any amendments or reports filed for
the purposes of updating such description. |
We are also incorporating
by reference all subsequent Annual Reports on Form 20-F that we file with the SEC and certain reports on Form 6-K that we furnish to the
SEC after the date of this prospectus (if they state that they are incorporated by reference into this prospectus) prior to the termination
of this offering. In all cases, you should rely on the later information over different information included in this prospectus or any
accompanying prospectus supplement.
Unless
expressly incorporated by reference, nothing in this prospectus shall be deemed to incorporate by reference information furnished to,
but not filed with, the SEC. Copies of all documents incorporated by reference in this prospectus, other than exhibits to those documents
unless such exhibits are specifically incorporated by reference in this prospectus, will be provided at no cost to each person, including
any beneficial owner, who receives a copy of this prospectus on the written or oral request of that person made to:
Biodexa Pharmaceuticals
PLC
1 Caspian Point
Caspian Way
Cardiff, CF10 4DQ, United
Kingdom
+44 29 2048 0180
You
may also access these documents on our website, www.biodexapharma.com. The information contained on, or that can be accessed
through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual
reference.
You
should rely only on information contained in, or incorporated by reference into, this prospectus. We have not authorized anyone to provide
you with information different from that contained in this prospectus or incorporated by reference in this prospectus. We are not making
offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized or in which the person making
such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
SERVICE OF PROCESS AND ENFORCEMENT OF CIVIL
LIABILITIES
We are incorporated under
the laws of England and Wales. All of our directors and officers of are residents of jurisdictions outside the United States. Our corporate
headquarters is located in the United Kingdom and all or a substantial portion of our assets, and all or a substantial portion of the
assets of our directors and officers, are located outside of the United States. As a result, it may be difficult for you to serve legal
process on us or our directors or have any of them appear in a U.S. court.
We
have appointed Donald J. Puglisi of Puglisi & Associates as our authorized agent upon whom process may be served in any action instituted
in any U.S. federal or state court having subject matter jurisdiction arising out of or based upon the securities offered by this prospectus.
We
understand that in England it may not be possible to bring proceedings or enforce a judgment of a U.S. court in respect of civil liabilities
based solely on the federal securities laws of the United States. In addition, awards of punitive damages in actions brought in the United
States or elsewhere may be unenforceable in England. An award of damages is usually considered to be punitive if it does not seek to compensate
the claimant for loss or damage suffered and is instead intended to punish the defendant. In addition to public policy aspects of enforcement,
such as the aforementioned, the enforceability of any judgment in England will depend on the particular facts of the case and the relevant
circumstances, for example (and expressly without limitation), whether there are any relevant insolvency proceedings which may affect
the ability to enforce a judgment. In addition, the United States and the United Kingdom have not currently entered into a treaty (or
convention) providing for the reciprocal recognition and enforcement of judgments (although both are contracting states to the New York
Convention on the Recognition and Enforcement of Foreign Arbitral Awards).
EXPENSES OF THE OFFERING
The following table sets
forth the expenses payable by us in connection with the sale and distribution of the securities being registered hereby. All amounts shown,
other than the SEC registration fee and FINRA filing fee, are estimates:
| SEC registration fee |
$ |
|
| FINRA filing fee |
|
|
| Printing and engraving |
|
|
| Accounting services |
|
|
| Legal fees and expenses |
|
|
| Depositary fees |
|
|
| Miscellaneous |
|
|
| Total |
$ |
|
______________
*To be completed by amendment

Biodexa Pharmaceuticals plc
Class A Units consisting of American Depositary
Shares Representing Ordinary Shares and Series E Warrants
and
Class B Units consisting
of Pre-Funded Warrants to purchase American Depositary Shares and Series E Warrants (and American Depositary Shares Representing Ordinary
Shares Underlying Pre-Funded Warrants and Series E Warrants)
PRELIMINARY PROSPECTUS
, 2023
Sole Book Running Manager
Ladenburg Thalmann
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 6. | Indemnification of Directors and Officers. |
The
Registrant’s articles of association provide that, subject to the Companies Act, every person who is or was at any time a director,
alternate director, or former director of the Registrant or of any of its subsidiaries may be indemnified out of the assets of the Registrant
against all costs, charges, expenses, losses, damages and liabilities incurred by him or her in performing his duties or the exercise
of his or her powers or otherwise in relation to such company. Generally, under the Companies Act, a company may not indemnify its directors
against personal liability covering: liability to the company in cases where the company sues the director (i.e., only liability to third
parties can be the subject of an indemnity); liability for fines for criminal conduct or fines imposed by a regulator; or other liabilities,
such as legal costs, in criminal cases where the director is convicted, or in civil cases brought by the company where the final judgment
goes against the director.
The
Registrant has entered into a deed of indemnity with each of its directors and officers. Except as prohibited by applicable law, these
deeds of indemnity may require the Registrant, among other things, to indemnify its directors and officers for certain expenses, including
attorneys’ fees, judgments, fines and settlement amounts incurred by such directors and officers in any action or proceeding arising
out of their service as a director or officer of the Registrant, or one of its subsidiaries, or arising out of the services provided to
another company or enterprise at the Registrant’s request.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers or persons
controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
| Item 7. | Recent Sales of Unregistered Securities |
The following information
is furnished with regard to all securities issued by the registrant within the last three years that were not registered under the Securities
Act of 1933, as amended. Unless otherwise indicated below, the issuance of such shares was deemed exempt from registration requirements
of the Securities Act, of 1933, as amended, as such securities were offered and sold outside of the United States to persons who were
neither citizens nor residents of the United States, pursuant to Regulation S, or such sales were exempt from registration under Section
4(2) of Securities Act of 1933, as amended and Rule 506 of Regulation D promulgated thereunder.
On September 30, 2020, we
issued 1,250 Ordinary Shares to be purchased under the plan set up in 2017 with our employees and directors to acquire Ordinary Shares
via salary sacrifice arrangement, or the Share Incentive Plan, at £0.02 per share, to the trust of the Share Incentive Plan.
On February 19, 2021, we issued
to certain Wainwright affiliates 15,200 Ordinary Shares represented by 38 Depositary Shares upon the exercise of warrants issued in May
2020 at an exercise price of $3,300.00 per share.
On July 6, 2021, we issued
1,754,386 Ordinary Shares at £5.70 per share to certain non-U.S. investors in a placing in the United Kingdom for aggregate gross
proceeds of £10.0 million.
On March 22, 2022, we issued
one Ordinary Share upon the exercise of one warrant issued in February 2019 to a certain institutional investor at an exercise price of
£200 per share.
On
May 3, 2022, we issued 1,250 Ordinary Shares to be purchased under the Share Incentive Plan at £0.02 per share to the trust of the
Share Incentive Plan.
On
August 3, 2022, we issued warrants to purchase 16,666 Ordinary Shares to a certain institutional investor at an exercise price of £2.70
per share.
On
December 16, 2022, we sold to an institutional investor 492,400 Ordinary Shares represented by 1,231 Depositary Shares in a registered
direct offering at $320.00 per Depositary Share, resulting in gross proceeds of approximately $0.4 million.
On
February 15, 2023, we completed the closing of the February Private Placement pursuant to which we sold to certain institutional investors
(1) 3,250,000 Ordinary Shares represented by 8,125 Depositary Shares at $185.60 per Depositary Share,
(2) 12,931,200 Ordinary Shares represented by 32,327 Depositary Shares, issuable upon the exercise of Series A Warrants issued in the
February Private Placement at an exercise price of $214.40 per warrant, (3) 19,396,400
Ordinary Shares represented by 48,491 Depositary Shares, issuable upon the exercise of Series B Warrants issued in the February Private
Placement at an exercise price of $214.40 per warrant, and (4) up to 71,749,600 Ordinary
Shares represented by 179,374 Depositary Shares, issuable upon the exercise of pre-funded warrants issued in the February Private Placement at
an exercise price of $0.032 per warrant, for aggregate gross proceeds of approximately $6.0 million.
We also issued unregistered placement agent warrants to purchase a total of 536,800 Ordinary Shares represented by 1,342 Depositary Shares
to the Placement Agent in the February Private Placement at an exercise price of $400.00 per warrant for 49 warrants and an
exercise price of $232.00 per warrant for 1,293 warrants, and Series A Warrants to purchase 625,000
Ordinary Shares represented by 1,562 Depositary Shares at an exercise price of $214.40 per warrant to
an investor pursuant to the Waiver.
Between
March 27, 2023, and the date hereof, we have issued 95,137,075 Ordinary Shares upon the exercise of 237,841 pre-funded warrants, Series
A Warrants and Series B Warrants issued in the February Private Placement.
On
May 26, 2023, we completed the closing of the May 2023 Registered Direct Offering, with institutional investors of (1) 166,017,300 Ordinary
Shares represented by 415,043 Depositary Shares, issuable upon the exercise of the Series C Warrants at an exercise price of $16.00 per
warrant, (2) 110,679,610 Ordinary Shares represented by 279,699 Depositary Shares issuable upon the exercise of the Series D Warrants
at an exercise price of $16.00 per warrant and, (3) 4,426,800 Ordinary Shares represented by 11,067 Depositary Shares issuable upon the
exercise of the May 2023 Placement Agent Warrants at an exercise price of $15.00 per warrant.
On
June 20, 2023 we issued the Series C Warrants, Series D Warrants and May 2023 Placement Agent Warrants to the investors and the Placement
Agent after receiving required shareholder approval of the allotment of, and disapplication of pre-emption rights with respect to the
Ordinary Shares to be issued under the Series C Warrants, Series D Warrants and May 2023 Placement Agent Warrants at our general meeting
held on June 14, 2023.
Between
June 20, 2023, and the date hereof, we have issued 151,315,700 Ordinary Shares upon the exercise of 378,289 Series C Warrants and Series
D Warrants issued in the May 2023 Registered Direct Offering.
| Item 8. | Exhibits and Financial Statement Schedules |
| (a) | The Exhibit Index is incorporated herein by reference. |
See the Exhibit Index attached
to this registration statement, which is incorporated herein by reference.
| (b) | Financial Statement Schedules. |
Schedules not listed above
have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or
the notes thereto.
The undersigned Registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this
registration statement: |
| (i) | to include any prospectus required by section 10(a)(3) of the Securities Act; |
| (ii) | to reflect in the prospectus any facts or events arising after the effective date of the registration
statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change
in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities
offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or
high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule
424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price
set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | to include any material information with respect to the plan of distribution not previously disclosed
in the registration statement or any material change to such information in the registration statement; |
| (2) | That, for the purpose of determining any liability under the Securities Act, each such post-effective
amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered
which remain unsold at the termination of the offering. |
| (4) | To file a post-effective amendment to the registration statement to include any financial statements required
by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information
otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the registrant includes in the
prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information
necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. |
| (5) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| (i) | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration
statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| (ii) | Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration
statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing
the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration
statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of
sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any
person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating
to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such
effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration
statement or made in any such document immediately prior to such effective date. |
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant
to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a
claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director,
officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director,
officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel
the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification
by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from
the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed
by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration
statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment
that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and
the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the
requirements for filing on Form F-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto
duly authorized, in London, United Kingdom, on this 6th day of October, 2023.
| |
|
BIODEXA PHARMACEUTICALS PLC |
| |
|
|
|
| |
|
By: |
/s/ Stephen Stamp |
| |
|
|
Stephen Stamp |
| |
|
|
Chief Executive Officer |
We,
the undersigned, hereby severally constitute and appoint each of Stephen Stamp and Stephen Parker, each in their individual capacity,
our true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and his name, place and stead,
in any and all capacities, to execute any and all amendments (including post-effective amendments) to this registration statement, to
sign any registration statement filed pursuant to Rule 462(b) of the Securities Act of 1933, as amended, and to cause the same to be filed
with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said
attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and desirable to be done
in and about the premises as fully and to all intents and purposes as we might or could do in person, hereby ratifying and confirming
all facts and things that said attorney-in-fact and agent, or his substitute may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities
Act of 1933, as amended, this Registration Statement on Form F-1 has been signed by the following persons in the capacities and on the
date indicated.
| Name and Signature |
|
Title(s) |
|
Date |
| |
|
|
|
|
| /s/ Stephen Stamp |
|
Chief Executive Officer, Director, |
|
October 6, 2023 |
| Stephen Stamp |
|
Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer |
|
|
| |
|
|
|
|
| /s/ Stephen Parker |
|
Non-Executive Chairman of the Board |
|
October 6, 2023 |
| Stephen Parker |
|
|
|
|
| |
|
|
|
|
| /s/ Simon Turton, Ph.D. |
|
Senior Independent Non-Executive Director |
|
October 6, 2023 |
| Simon Turton, Ph.D. |
|
|
|
|
| |
|
|
|
|
| /s/ Sijmen de Vries, M.D. |
|
Non-Executive Director |
|
October 6, 2023 |
| Sijmen de Vries, M.D. |
|
|
|
|
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of the Securities
Act of 1933, as amended, this Registration Statement on Form F-1 has been signed by the undersigned on this 6th day of October, 2023.
| By: |
/s/ Donald J. Puglisi |
|
| Name: |
Donald J. Puglisi |
|
| Title: |
Authorized Representative in the United States |
|
Exhibit Index
|
Exhibit
Number |
Title |
| |
|
| 1.1** |
Form of Underwriting Agreement between Biodexa Pharmaceuticals PLC and Ladenburg Thalmann & Co. Inc. |
| |
|
| 3.1 |
Articles of Association of Biodexa Pharmaceuticals PLC, adopted on June 14, 2023 (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form F-1 (File No. 333-272693), filed with the SEC on June 16, 2023). |
| |
|
| 4.1 |
Description of Securities Registered Under Section 12 of the Exchange Act (incorporated by reference to Exhibit 4.1 to the Company’s Registration Statement on Form F-1 (File No. 333-272693), filed with the SEC on June 16, 2023). |
| |
|
| 4.2 |
Specimen certificate representing ordinary shares of Biodexa Pharmaceuticals Plc (incorporated by reference to Exhibit 4.2 to the Company’s Registration Statement on Form F-1 (File No. 333-272693), filed with the SEC on June 16, 2023). |
| |
|
| 4.3 |
Form of Amended and Restated Deposit Agreement by and among Midatech Pharma PLC, The Bank of New York Mellon, as depositary, and all owners and holders from time to time of American Depositary Shares thereunder (incorporated by reference to Exhibit 1 to the Company’s Registration Statement on Form F-6 (File No. 333-252507), filed with the SEC on January 28, 2021). |
| |
|
| 4.4 |
Form of American Depositary Receipt (included in Exhibit 4.3 as Exhibit A thereto). |
| |
|
| 4.5 |
Form of Warrant issued on October 25, 2019 (incorporated by reference to Exhibit 4.1 of the Company’s Report on Form 6-K, filed with the SEC on October 24, 2019). |
| |
|
| 4.6 |
Form of Placement Agent Warrant issued on October 25, 2019 (incorporated by reference to Exhibit 4.2 of the Company’s Report on Form 6-K, filed with the SEC on October 24, 2019). |
| |
|
| 4.7 |
Form of Warrant issued on May 20, 2020 (incorporated by reference to Exhibit 4.1 of the Company’s Report on Form 6-K, filed with the SEC on May 20, 2020). |
| |
|
| 4.8 |
Form of Placement Agent Warrant issued on May 20, 2020 (incorporated by reference to Exhibit 4.2 of the Company’s Report on Form 6-K, filed with the SEC on May 20, 2020). |
| |
|
| 4.9 |
Form of Warrant Instrument issued on May 22, 2020 (incorporated by reference to Exhibit 4.3 of the Company’s Report on Form 6-K, filed with the SEC on May 20, 2020). |
| |
|
| 4.10 |
Form of Series A Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Report on Form 6-K, filed with the SEC on February 9, 2023). |
| |
|
| 4.11 |
Form of Placement Agent Warrant (incorporated by reference to Exhibit 4.4 of the Company’s Report on Form 6-K, filed with the SEC on February 9, 2023). |
| |
|
| 4.12 |
Form of Series C Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| |
|
| 4.13 |
Form of Series D Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| |
|
| 4.14 |
Form of Placement Agent Warrant (incorporated by reference to Exhibit 4.3 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| |
|
| 4.15** |
Form of Series E Warrant. |
| |
|
| 4.16** |
Form of Pre-Funded Warrant. |
| |
|
| 4.17** |
Form of Warrant Agency Agreement. |
| |
|
| 4.18** |
Form of Representative Warrant. |
| |
|
| 5.1** |
Opinion of Brown Rudnick LLP. |
| |
|
| 5.2** |
Opinion of Mintz, Levin, Cohn, Ferris, Glovsky & Popeo, P.C. |
| 10.1# |
Midatech Pharma PLC 2014 Enterprise Management Incentive Scheme (incorporated by reference to Exhibit 10.3 to the Company’s Registration Statement on Form F-4 (File No. 333-206305), originally filed with the SEC on August 11, 2015, as amended). |
| |
|
| 10.2# |
Form of Option Agreement (included in Exhibit 10.1). |
| |
|
| 10.3# |
Consultancy Agreement, dated as of April 15, 2014, by and between Midatech Limited and Chesyl Pharma Limited (incorporated by reference to Exhibit 10.17 to the Company’s Registration Statement on Form F-4 (File No. 333-206305), originally filed with the SEC on August 11, 2015, as amended). |
| |
|
| 10.4# |
Form of Appointment Letter between Midatech Pharma PLC and certain directors of Midatech Pharma PLC (incorporated by reference to Exhibit 10.22 to the Company’s Registration Statement on Form F-4 (File No. 333-206305), originally filed with the SEC on August 11, 2015, as amended). |
| |
|
| 10.5# |
Deed of Indemnity dated August 5, 2015 (incorporated by reference to Exhibit 10.23 to the Company’s Registration Statement on Form F-4 (File No. 333-206305), originally filed with the SEC on August 11, 2015, as amended). |
| |
|
| 10.6† |
License, Collaboration and Distribution Agreement, dated as of January 29, 2019, by and between Midatech Pharma PLC, CMS Bridging Limited, CMS Medical Hong Kong Limited and China Medical System Holdings Limited (incorporated by reference to Exhibit 4.17 of the Company’s Annual Report on Form 20-F for the year ended December 31, 2018, as amended, filed with the SEC on May 28, 2019). |
| |
|
| 10.7# |
The Share Incentive Plan (incorporated by reference to Exhibit 4.27 of the Company’s Annual Report on Form 20-F for the year ended December 31, 2018, filed with the SEC on April 24, 2018). |
| |
|
| 10.8†# |
Service Agreement dated as of September 9, 2019, by and between Midatech Pharma PLC and Stephen Stamp (incorporated by reference to Exhibit 10.1 of the Company’s Report on Form 6-K, filed with the SEC on September 19, 2019). |
| |
|
| 10.9 |
Form of Securities Purchase Agreement, dated as of December 13, 2022, by and between Midatech Pharma PLC and the purchaser identified on the signature page thereto (incorporated by reference to Exhibit 10.5 of the Company’s Report on Form 6-K, filed with the SEC on December 13, 2022). |
| |
|
| 10.12 |
Form of First Amendment to the Securities Purchase Agreement, dated as of December 16, 2022, by and between Midatech Pharma PLC and the purchaser identified on the signature page thereto (incorporated by reference to Exhibit 10.1 of the Company’s Report on Form 6-K, filed with the SEC on December 19, 2022). |
| |
|
| 10.10 |
Form of Securities Purchase Agreement, dated as of February 9, 2023, by and between Midatech Pharma PLC and the purchasers identified on the signature page thereto (incorporated by reference to Exhibit 10.1 to the Company’s Report on Form 6-K, filed with the SEC on February 9, 2023). |
| |
|
| 10.11# |
Service Agreement, dated as of July 12, 2021, by and between Midatech Pharma PLC and Dmitry Zamoryakhin (incorporated by reference to Exhibit 4.15 of the Company’s Annual Report on Form 20-F for the year ended December 31, 2021, filed with the SEC on April 26, 2022). |
| |
|
| 10.12# |
Terms of Appointment as Director, dated June 20, 2022, by and between Midatech Pharma PLC and Stephen Barry Parker (incorporated by reference to Exhibit 10.1 of the Company’s Report on Form 6-K, filed with the SEC on June 21, 2022). |
| |
|
| 10.13 |
Form of Registration Rights Agreement, dated as of February 9, 2023, by and between Midatech Pharma PLC and the investors identified on the signature pages thereto (incorporated by reference to Exhibit 10.2 of the Company’s Report on Form 6-K, filed with the SEC on February 9, 2023). |
| |
|
| 10.14 |
Form of Waiver, dated as of February 9, 2023, by and between Midatech Pharma PLC and a certain institutional investor (incorporated by reference to Exhibit 10.4 of the Company’s Report on Form 6-K, filed with the SEC on February 9, 2023). |
| |
|
| 10.15 |
Form of Securities Purchase Agreement, dated as of May 23, 2023, by and between Biodexa Pharmaceuticals PLC and the investors identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| |
|
| 10.16 |
Form of Registration Rights Agreement, dated as of May 23, 2023, by and between Biodexa Pharmaceuticals PLC and the investors identified on the signature pages thereto (incorporated by reference to Exhibit 10.2 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| 10.17 |
Placement Agency Agreement, dated as of May 23, 2023, by and between Biodexa Pharmaceuticals PLC and Ladenburg Thalmann & Co. Inc. (incorporated by reference to Exhibit 10.3 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| |
|
| 10.18 |
Form of Lock-up Agreement, dated as of May 23, 2023, by and between Biodexa Pharmaceuticals PLC and named directors and officers of Biodexa and other individuals and entities listed in the Schedule I to the Purchase Agreement (incorporated by reference to Exhibit 10.4 of the Company’s Report on Form 6-K, filed with the SEC on May 24, 2023). |
| |
|
| 10.19 |
Form of Amended and Restated Securities Purchase Agreement, dated as of May 25, 2023, by and between Biodexa Pharmaceuticals PLC and the investors identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 of the Company’s Report on Form 6-K/A, filed with the SEC on May 26, 2023). |
| |
|
| 21.1 |
Subsidiaries of Biodexa Pharmaceuticals PLC (incorporated by reference to Exhibit 8.1 to the Company’s Annual Report on Form 20-F for the year ended December 31, 2022, filed with the SEC on April 28, 2023). |
| |
|
| 23.1* |
Consent of Mazars LLP, independent registered public accounting firm. |
| |
|
| 23.2** |
Consent of Brown Rudnick LLP (included in Exhibit 5.1). |
| |
|
| 23.3** |
Consent of Mintz, Levin, Cohn, Ferris, Glovsky & Popeo, P.C. (included in Exhibit 5.2). |
| |
|
| 24.1* |
Powers of Attorney (included on signature page). |
| |
|
| 107* |
Filing Fee Table. |
___________
| ** | To be filed by amendment. |
| # | Management contract or compensatory plan or arrangement. |
| † | Certain portions of this exhibit (indicated by asterisks) have been omitted because they are not material and would likely cause competitive
harm to Biodexa Pharmaceuticals PLC if publicly disclosed. |
II-8
Exhibit 23.1
 |
30 Old Bailey
London
EC4M 7AU
United Kingdom
Tel: +44 (0)20 7063 4000
www.mazars.co.uk |
Consent of Independent Registered Public Accounting
Firm
The Board of Directors
Biodexa Pharmaceuticals PLC
1 Caspian Point
Caspian Way
Cardiff
CF10 4DQ
United Kingdom
The Board of Directors of Biodexa Pharmaceuticals PLC
We consent to the inclusion of our audit report in
the 20-F dated April 28, 2023 and amended on May 5, 2023, which is incorporated by reference in the Registration Statement to Form F-1
dated 06 October 2023, on the consolidated statement of financial position of Biodexa Pharmaceuticals PLC (the “Company”)
and its subsidiaries as of December 31, 2022, 2021 and 2020 and the related consolidated statements of comprehensive income, cash flow,
changes in equity for each of the three years in the period ended December 31, 2022, and the related notes. Our report contains a material
uncertainty paragraph regarding the Company’s ability to continue as a going concern.
We also consent to the reference to us under the caption
“Experts” in this Registration Statement.
/s/ Mazars LLP
Mazars LLP
London, United Kingdom
05 October 2023
Mazars LLP
Mazars LLP is the UK firm of Mazars, an integrated
international advisory and accountancy organisation. Mazars LLP is a limited liability partnership registered in England and Wales with
registered number OC308299 and with its registered office at 30 Old Bailey, London, EC4M 7AU. Registered to carry on audit work in the
UK by the Institute of Chartered Accountants in England and Wales. Details about our audit registration can be viewed at www.auditregister.org.uk
under reference number C001139861. VAT number: GB 839 8356 73
Exhibit 107
Calculation of Filing Fee Tables
FORM F-1
…………...
(Form Type)
Biodexa Pharmaceuticals PLC
………………………………………………………..
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward
Securities
| | |
Security
Type | |
Security Class Title(1) | |
Fee
Calculation
or Carry
Forward
Rule |
|
Amount
Registered |
|
Proposed
Maximum
Offering
Price Per
Unit | |
Maximum
Aggregate
Offering
Price(2)(3)(4)
| |
Fee Rate | |
Amount of
Registration
Fee |
|
| Newly Registered Securities |
|
| Fees to Be Paid | |
Equity | |
Class A Units(5), consisting of (i) Ordinary Shares, nominal
value £0.001 per share, represented by American Depositary Shares and (ii) Series E Warrants to purchase American Depositary Shares | |
Rule 457(o) |
|
|
|
| |
$ |
4,000,000.00 | |
$ |
0.00014760 | |
$ | 590.40 |
|
| Fees to Be Paid | |
Equity | |
Ordinary Shares, £0.001 per share, represented by American Depositary
Shares | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Series E Warrants to purchase American Depositary
Shares included in the Class A Units(4)(6) | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Ordinary Shares underlying the American Depositary Shares issuable
upon exercise of the Series E Warrants | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Class B Units(5), consisting of (i) Pre-Funded Warrants
to purchase American Depositary Shares and (ii) Series E Warrants to purchase American Depositary Shares | |
Rule 457(o) |
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Pre-Funded Warrants to purchase American Depositary Shares | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Ordinary Shares underlying the American Depositary Shares issuable
upon exercise of the Pre-Funded Warrants(7) | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Series E Warrants to purchase American Depositary
Shares included in the Class B Units(5) | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Ordinary Shares underlying the American Depositary Shares issuable
upon exercise of the Series E Warrants included in the Class B Units | |
|
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Representative’s Warrants to purchase American Depositary Shares(6) | |
Rule 457(g) |
|
|
|
| |
|
| |
|
| |
| |
|
| Fees to Be Paid | |
Equity | |
Ordinary shares underlying the American Depositary Shares issuable
upon exercise of the Representative’s Warrants(1)(8) | |
Rule 457(o) |
|
|
|
| |
$ |
200,000.00 | |
$ |
0.00014760 | |
$ | 29.52 |
|
| | |
| |
| |
|
|
|
|
| |
|
| |
|
| |
| |
|
| | |
| |
Total Offering Amounts | |
|
|
|
|
| |
$ |
4,200,000.00 | |
|
| |
$ | 619.92 |
|
| | |
| |
Total Fees Previously Paid | |
|
|
|
|
| |
|
| |
|
| |
$ | 0.00 |
|
| | |
| |
Total Fee Offsets | |
|
|
|
|
| |
|
| |
|
| |
$ | |
|
| | |
| |
Net Fee Due | |
|
|
|
|
| |
|
| |
|
| |
$ | 619.92 |
|
| (1) | American Depositary Shares issuable upon the deposit of the ordinary shares, nominal value £0.001
per share, or Ordinary Shares, registered hereby have been registered under a separate Registration Statement on Form F-6 (File No. 333-207186),
as amended. Each American Depositary Share represents 400 Ordinary Shares. |
| (2) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with
Rule 457(o) under the Securities Act of 1933 (the “Securities Act”). |
| (3) | Includes American Depositary Shares and/or Series E warrants that may be purchased by the underwriters pursuant to their option
to purchase additional American Depositary Shares and/or Series E warrants to cover over-allotments. |
| (4) | Pursuant to Rule 416 under the Securities Act, the securities registered hereby also include an indeterminate
number of additional securities as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations,
or other similar transactions. |
| (5) | The proposed maximum aggregate offering price of the Class A Units proposed to be sold in the offering
will be reduced on a dollar-for-dollar basis based on the aggregate offering price of any Class B Units offered and sold in the offering.
Accordingly, the proposed maximum aggregate offering price of the Class A Units and the Class B Units, if any, is $4,000,000. |
| (6) | No registration fee required pursuant to Rule 457(g). |
| (7) | No registration fee required pursuant to Rule 457(i). |
| | (8) | Estimated solely for the purpose of calculating the registration fee
pursuant to Rule 457(g) under the Securities Act. The Representative’s Warrants are exercisable at a per American Depositary Share
exercise price equal to 125% of the public offering price. As estimated solely for the purpose of calculating the registration fee pursuant
to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the Representative’s Warrants is equal
to $200,000 (which is equal to 4.0% of the proposed maximum aggregate offering price for the Class A Units and the Class B Units of $4,000,000). |
v3.23.3
Cover
|
Oct. 06, 2023 |
| Entity Addresses [Line Items] |
|
| Document Type |
F-1
|
| Amendment Flag |
false
|
| Entity Registrant Name |
Biodexa Pharmaceuticals PLC
|
| Entity Central Index Key |
0001643918
|
| Entity Incorporation, State or Country Code |
X0
|
| Entity Address, Address Line One |
1 Caspian Point
|
| Entity Address, Address Line Two |
Caspian Way
|
| Entity Address, City or Town |
Cardiff
|
| Entity Address, Country |
GB
|
| Entity Address, Postal Zip Code |
CF10 4DQ
|
| Country Region |
+44
|
| City Area Code |
29
|
| Local Phone Number |
20480 180
|
| Entity Emerging Growth Company |
false
|
| Business Contact [Member] |
|
| Entity Addresses [Line Items] |
|
| Entity Address, Address Line One |
850 Library Ave.
|
| Entity Address, Address Line Two |
Suite 204
|
| Entity Address, City or Town |
Newark
|
| Entity Address, State or Province |
DE
|
| Entity Address, Postal Zip Code |
19711
|
| City Area Code |
(302)
|
| Local Phone Number |
738-6680
|
| Contact Personnel Name |
Donald J. Puglisi
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Midatech Pharma (NASDAQ:MTP)
Historical Stock Chart
From Apr 2024 to May 2024
Midatech Pharma (NASDAQ:MTP)
Historical Stock Chart
From May 2023 to May 2024