false
0001178879
0001178879
2024-01-07
2024-01-07
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
8-K
CURRENT
REPORT PURSUANT TO
SECTION
13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of
earliest event reported): January 7, 2024
AMICUS THERAPEUTICS, INC.
(Exact
Name of Registrant as Specified in Its Charter)
| Delaware |
|
001-33497 |
|
71-0869350 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
47 Hulfish Street,
Princeton, New Jersey 08542
(Address of Principal
Executive Offices, and Zip Code)
609-662-2000
Registrant’s
Telephone Number, Including Area Code
(Former Name or Former Address, if Changed Since
Last Report.)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant
to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock Par Value $0.01 |
|
FOLD |
|
Nasdaq |
Indicate by check mark whether the registrant is an emerging growth
company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934
(17 CFR §240.12b-2). Emerging growth company ¨
If an emerging growth company, indicate
by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 2.02 – Results of Operations and Financial
Condition.
On January 7, 2024, Amicus Therapeutics, Inc.
(the “Company”) issued a press release announcing preliminary 2023 revenue and its 2024 strategic outlook, along with various
business updates. A copy of the press release is attached hereto as Exhibit 99.1. As previously announced, the Company will also
be presenting at the 42nd Annual J.P. Morgan Healthcare Conference on January 8th, 2024. A copy of the presentation
materials management will be using at the conference is also attached hereto as Exhibit 99.2. Both exhibits are incorporated herein
by reference.
The information furnished pursuant to this Item
2.02, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and
shall not be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly
set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits:
Signature Page
Pursuant to the requirements of the
Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly
authorized.
| |
AMICUS THERAPEUTICS, INC. |
| |
|
| Date: January 8, 2024 |
By: |
/s/ Ellen S. Rosenberg |
| |
Name: Ellen S. Rosenberg |
| |
Title: Chief Legal Officer and Corporate Secretary |
Exhibit 99.1

Amicus Therapeutics Reports Preliminary 2023
Revenue and Provides 2024 Strategic Outlook
2023 Total
Revenue of ~$399.4M, a 21% Increase Year-Over-Year
>2,400 People Living with Fabry Disease
on Galafold® Following a Year of Increased Demand
Expecting 2024 Galafold Revenue Growth of
11-16% at CER
Successful Launches of Pombiliti™
+ Opfolda™ Underway in the U.S., U.K., and Germany
PRINCETON, NJ,
January 7, 2024 – Amicus Therapeutics (Nasdaq: FOLD), a patient-dedicated global biotechnology company focused
on developing and commercializing novel medicines for rare diseases, today provided its preliminary and unaudited 2023 revenue, corporate
updates, and full-year 2024 outlook.
In 2023, Amicus met or exceeded its strategic
priorities, highlighted by:
| · | Sustaining
double-digit Galafold revenue growth |
| · | Securing
FDA, EMA, and MHRA approvals for Pombiliti + Opfolda |
| · | Initiating
successful global launches of Pombiliti + Opfolda |
| · | Advancing
next-generation pipeline programs |
| · | On-track
to achieving non-GAAP profitability in the fourth quarter of 2023 |
Preliminary and Unaudited 2023 Revenue:
| · | Total
revenue in 2023 reached ~$399.4 million, representing a year-over-year increase of
21%, reflecting strong operational growth measured at constant exchange rates (CER)1
of 20% and a favorable currency impact of approximately $2.7 million, or 1%. Fourth
quarter total revenue was ~$115.1 million. |
| | | |
| · | Galafold
(migalastat) net product sales in 2023 were ~$387.8 million, representing a year-over-year
increase of 18%, or 17% at CER. Fourth quarter Galafold net product sales were ~$106.6
million. |
| | | |
| · | Pombiliti
(cipaglucosidase alfa-atga) + Opfolda (miglustat) net product sales in 2023 were ~$11.6 million.
The commercial launch of Pombiliti + Opfolda is successfully underway in the three largest
markets with ~120 patients on treatment with commercial product or scheduled to be treated
as of the end of 2023. Fourth quarter Pombiliti + Opfolda net product sales were ~$8.5 Million. |
Bradley Campbell,
President and Chief Executive Officer of Amicus Therapeutics, Inc., stated, “Last year was an incredible year for Amicus highlighted
by the continued double-digit growth of Galafold sales and the global regulatory approvals of our second commercial therapy, which is
off to a fantastic launch. We expect 2024 to be a truly transformative year as we continue to drive significant revenue growth by treating
an increasing number of Fabry patients globally, executing on the global launches of Pombiliti and Opfolda, which we believe has the
potential to become the standard of care in a >$1B market today, and delivering our first full year of non-GAAP profitability. We
look forward to reporting on our progress throughout this year as we further our mission for people living with rare diseases.”
Amicus is focused on the following four key strategic
priorities in 2024:
| · | Delivering
double-digit Galafold revenue growth (11-16% at CER) |
| · | Ensuring
the successful global launches of Pombiliti + Opfolda |
| · | Advancing
ongoing studies to support medical and scientific leadership in Fabry and Pompe diseases |
| · | Achieving
full year non-GAAP profitability2 |
Mr. Campbell
will discuss the Amicus corporate objectives and key milestones in a presentation at the 42nd Annual J.P. Morgan Healthcare
Conference on Monday, January 8, 2024, at 2:15 p.m. PT. A live webcast of the presentation can be accessed through the Investors
section of the Amicus Therapeutics corporate website at http://ir.amicusrx.com/events.cfm,
and will be archived for 90 days.
1 In order to illustrate
underlying performance, Amicus discusses its results in terms of constant exchange rate (CER) growth. This represents growth calculated
as if the exchange rates had remained unchanged from those used in the comparative period. Full-year 2024 Galafold revenue guidance utilizes
actual exchange rate as of December 31, 2023.
2 Non-GAAP Net Income defined
as GAAP Net Income excluding the impact of share-based compensation expense, changes in fair value of contingent consideration, loss
on impairment of assets, depreciation and amortization, acquisition related income (expense), loss on extinguishment of debt, loss on
impairment of assets, restructuring charges, and income taxes.
About Galafold
Galafold® (migalastat)
123 mg capsules is an oral pharmacological chaperone of alpha-Galactosidase A (alpha-Gal A) for the treatment of Fabry disease in adults
who have amenable galactosidase alpha gene (GLA) variants. In these patients, Galafold works by stabilizing the body’s
own dysfunctional enzyme so that it can clear the accumulation of disease substrate. Globally, Amicus Therapeutics estimates that approximately
35 to 50 percent of people living with Fabry disease may have amenable GLA variants, though amenability rates within
this range vary by geography. Galafold is approved in more than 40 countries around the world, including the U.S., EU, U.K., and Japan.
U.S. INDICATIONS AND USAGE
Galafold is indicated
for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant
based on in vitro assay data.
This indication is approved
under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued
approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
U.S. IMPORTANT SAFETY INFORMATION
ADVERSE REACTIONS
The most common adverse
drug reactions reported with Galafold (≥10 %) are headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia.
DRUG INTERACTIONS
Avoid co-administration
of Galafold with caffeine at least 2 hours before and 2 hours after taking Galafold.
USE IN SPECIFIC POPULATIONS
There is insufficient
clinical data on Galafold use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. Advise women
of the potential risk to a fetus.
It is not known if Galafold
is present in human milk. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s
clinical need for Galafold and any potential adverse effects on the breastfed child from Galafold or from the underlying maternal condition.
Galafold is not recommended
for use in patients with severe renal impairment or end-stage renal disease requiring dialysis.
The safety and effectiveness
of Galafold have not been established in pediatric patients.
To
report Suspected Adverse Reactions, contact Amicus Therapeutics at 1-877-4AMICUS or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For additional
information about Galafold, including the full U.S. Prescribing Information, please visit https://www.amicusrx.com/pi/Galafold.pdf.
EU Therapeutic Indication
Galafold® (migalastat) is indicated for long-term treatment
of adults and adolescents aged 12 years and older with a confirmed diagnosis of Fabry disease (α-galactosidase A deficiency) and
who have an amenable mutation.
EU Important Safety Information
Treatment with Galafold should be initiated and
supervised by specialist physicians experienced in the diagnosis and treatment of Fabry disease. Galafold is not intended for concomitant
use with enzyme replacement therapy.
The safety and efficacy of Galafold in children
aged less than 12 years have not been established. No data are available.
Galafold is contraindicated in patients with
hypersensitivity to the active substance or to any of the excipients listed in the Summary of Product Characteristics (SmPC).
Galafold 123 mg capsules are not for children
(≥12 years) weighing less than 45 kg.
It is advised to periodically monitor renal function,
echocardiographic parameters and biochemical markers (every 6 months) in patients initiated on or switched to Galafold. In case of meaningful
clinical deterioration, further clinical evaluation or discontinuation of treatment with Galafold should be considered.
Galafold is not indicated for use in patients
with non-amenable mutations.
Galafold is not recommended for use in patients
with severe renal insufficiency, defined as estimated GRF less than 30 mL/min/1.73m2.
Food and caffeine should not be consumed at least
2 hours before and 2 hours after taking Galafold to give a minimum 4 hours fast.
Galafold is not recommended in women of childbearing
potential not using contraception. Galafold is not recommended during pregnancy. It is not known whether Galafold is secreted in human
milk.
The most common adverse reaction reported was
headache, which was experienced by approximately 10% of patients who received Galafold. For a complete list of adverse reactions, please
review the SmPC.
OVERDOSE: General medical care is recommended
in the case of Galafold overdose.
For complete information please see
the EU SmPC available at https://www.ema.europa.eu/en/medicines/human/EPAR/galafold
About Pombiliti + Opfolda
Pombiliti + Opfolda, is a two-component therapy
that consists of cipaglucosidase alfa-atga, a bis-M6P-enriched rhGAA that facilitates high-affinity uptake through the M6P receptor while
retaining its capacity for processing into the most active form of the enzyme, and the oral enzyme stabilizer, miglustat, that’s
designed to reduce loss of enzyme activity in the blood.
U.S. INDICATIONS AND USAGE
POMBILITI in combination with OPFOLDA is indicated
for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase [GAA] deficiency) weighing ≥40
kg and who are not improving on their current enzyme replacement therapy (ERT).
SAFETY INFORMATION
HYPERSENSITIVITY
REACTIONS INCLUDING ANAPHYLAXIS: Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily
available. If a severe hypersensitivity reaction occurs, POMBILITI should be discontinued immediately and appropriate medical treatment
should be initiated. INFUSION-ASSOCIATED REACTIONS (IARs): If severe IARs occur, immediately discontinue POMBILITI and initiate appropriate
medical treatment. RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS: Patients susceptible to fluid volume overload, or
those with acute underlying respiratory illness or compromised cardiac or respiratory function, may be at risk of serious exacerbation
of their cardiac or respiratory status during POMBILITI infusion. See PI for complete Boxed Warning. CONTRAINDICATION: POMBILITI
in combination with Opfolda is contraindicated in pregnancy. EMBRYO-FETAL TOXICITY: May cause embryo-fetal harm. Advise females
of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment and for at least 60 days
after the last dose. Adverse Reactions: Most common adverse reactions ≥ 5% are headache, diarrhea, fatigue, nausea, abdominal
pain, and pyrexia. Please see full PRESCRIBING INFORMATION, including BOXED WARNING, for POMBILITI (cipaglucosidase alfa-atga) LINK
and full PRESCRIBING INFORMATION for OPFOLDA (miglustat) LINK.
EU Important Safety Information
Pombiliti (cipaglucosidase alfa) Important
Safety Information
Posology
and Method of Administration: Pombiliti must be used in combination with miglustat 65 mg hard capsules. The recommended
dose of Pombiliti is 20 mg/kg of body weight every other week. The Pombiliti infusion should start 1 hour after taking miglustat capsules.
Paediatric population: The safety and efficacy of Pombiliti in combination with miglustat therapy in paediatric patients
less than 18 years old have not yet been established. No data are available. Contraindications: Life-threatening hypersensitivity
to the active substance, or to any of the excipients. Contraindication to miglustat. Anaphylaxis and infusion-associated reactions
(IARs): Serious anaphylaxis and IARs have occurred in some patients during infusion and following infusion with Pombiliti. Premedication
with oral antihistamine, antipyretics, and/or corticosteroids may be administered to assist with signs and symptoms related to IARs experienced
with prior enzyme replacement therapy (ERT) treatment. Reduction of the infusion rate, temporary interruption of the infusion, symptomatic
treatment with oral antihistamine, or antipyretics, and appropriate resuscitation measures should be considered to manage serious IARs.
If anaphylaxis or severe allergic reactions occur, infusion should be immediately paused, and appropriate medical treatment should be
initiated. The current medical standards for emergency treatment of anaphylactic reactions are to be observed and cardiopulmonary resuscitation
equipment should be readily available. The risks and benefits of re-administering Pombiliti following anaphylaxis or severe allergic
reaction should be carefully considered, and appropriate resuscitation measures made available. Risk of acute cardiorespiratory
failure in susceptible patients: Patients with acute underlying respiratory illness or compromised cardiac and/or respiratory
function may be at risk of serious exacerbation of their cardiac or respiratory compromise during infusions. Appropriate medical support
and monitoring measures should be readily available during Pombiliti infusion. Immune complex-related reactions: Immune
complex-related reactions have been reported with other ERTs in patients who had high IgG antibody titres, including severe cutaneous
reactions and nephrotic syndrome. If immune complex-related reactions occur, discontinuation of the administration of Pombiliti should
be considered and appropriate medical treatment should be initiated. The risks and benefits of re-administering Pombiliti following an
immune complex-related reaction should be reconsidered for each individual patient. Contraception in females: Reliable
contraceptive measures must be used by women of childbearing potential during treatment with Pombiliti in combination with miglustat,
and for 4 weeks after discontinuing treatment. Pregnancy: Pombiliti in combination with miglustat therapy is not recommended
during pregnancy. Breast feeding: It is not known if Pombiliti and miglustat are secreted in human breast milk. A decision
must be made whether to discontinue breast-feeding or to discontinue/abstain from Pombiliti in combination with miglustat therapy, taking
into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. Summary of the safety profile:
The most commonly reported adverse reactions only attributable to Pombiliti were chills (4.0%), dizziness (2.6%), flushing (2.0%),
somnolence (2.0%), chest discomfort (1.3%), cough, (1.3%), infusion site swelling (1.3%), and pain (1.3%). Reported serious adverse reactions
only attributable to Pombiliti were urticaria (2.0%), anaphylaxis (1.3%), pyrexia (0.7%), presyncope (0.7%), dyspnoea (0.7%), pharyngeal
oedema (0.7%), wheezing (0.7%), and hypotension (0.7%). Refer to SmPC for full list.
Opfolda (miglustat) 65 mg hard capsules Important
Safety Information
Posology and
Method of Administration: Opfolda must be used in combination with Pombiliti. The recommended dose is to be taken orally every
other week and is based on body weight. Opfolda should be taken approximately 1 hour but no more than 3 hours before the start of the
Pombiliti infusion. Paediatric population: The safety and efficacy of Opfolda in combination with Pombiliti therapy in paediatric
patients less than 18 years old have not yet been established. No data are available. Contraindications: Hypersensitivity to the
active substance or to any of the excipients. Contraindication to cipaglucosidase alfa. Food Interaction: Patients should fast
for 2 hours before and 2 hours after taking Opfolda. Contraception in females: Reliable contraceptive measures must be used by
women of childbearing potential during treatment with Opfolda in combination with Pombiliti, and for 4 weeks after discontinuing treatment.
Pregnancy: Opfolda crosses the placenta. Opfolda in combination with Pombiliti therapy is not recommended during pregnancy. Breast
feeding: It is not known if Opfolda and Pombiliti are secreted in human breast milk. A decision must be made whether to discontinue
breast-feeding or to discontinue/abstain from Opfolda in combination with Pombiliti therapy, taking into account the benefit of breastfeeding
for the child and the benefit of therapy for the woman. Summary of the safety profile: The most commonly reported adverse reaction
only attributable to Opfolda 65 mg was constipation (1.3%). Refer to SmPC for full list.
About Amicus Therapeutics
Amicus Therapeutics
(Nasdaq: FOLD) is a global, patient-dedicated biotechnology company focused on discovering, developing and delivering novel high-quality
medicines for people living with rare diseases. With extraordinary patient focus, Amicus Therapeutics is committed to advancing and expanding
a pipeline of cutting-edge, first- or best-in-class medicines for rare diseases. For more information please visit the company’s
website at www.amicusrx.com, and follow on X and LinkedIn.
Non-GAAP Financial Measures
In addition to financial information prepared
in accordance with U.S. GAAP, this press release also contains adjusted financial measures that we believe provide investors and management
with supplemental information relating to operating performance and trends that facilitate comparisons between periods and with respect
to projected information. These adjusted financial measures are non-GAAP measures and should be considered in addition to, but not as
a substitute for, the information prepared in accordance with U.S. GAAP. We typically exclude certain GAAP items that management does
not believe affect our basic operations and that do not meet the GAAP definition of unusual or non-recurring items. Other companies may
define these measures in different ways. When we provide our expectation for non-GAAP operating expenses on a forward-looking basis,
a reconciliation of the differences between the non-GAAP expectation and the corresponding GAAP measure generally is not available without
unreasonable effort due to potentially high variability, complexity and low visibility as to the items that would be excluded from the
GAAP measure in the relevant future period, such as unusual gains or losses. The variability of the excluded items may have a significant,
and potentially unpredictable, impact on our future GAAP results.
Forward Looking Statement
This press release contains "forward-looking
statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development
of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing
of the potential regulatory approval of our product candidates, commercialization plans, manufacturing and supply plans, financing plans,
and the projected revenues and cash position for the Company. The inclusion of forward-looking statements should not be regarded as a
representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this press release may turn
out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example,
with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities and pricing
and reimbursement authorities, are based on current information. Actual results may differ materially from those set forth in this release
due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or
preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll
patients in our clinical trials; the potential that regulatory authorities may not grant or may delay approval for our product candidates;
the potential that required regulatory inspections may be delayed or not be successful and delay or prevent product approval; the potential
that we may not be successful in negotiations with pricing and reimbursement authorities; the potential that we may not be successful
in commercializing Galafold and/or Pombiliti and Opfolda in Europe, the UK, the US and other geographies; the potential that preclinical
and clinical studies could be delayed because we identify serious side effects or other safety issues; the potential that we may not
be able to manufacture or supply sufficient clinical or commercial products; and the potential that we will need additional funding to
complete all of our studies, the manufacturing, and commercialization of our products. With respect to statements regarding corporate
financial guidance and financial goals and the expected attainment of such goals and projections of the Company's revenue, non-GAAP profitability
and cash position, actual results may differ based on market factors and the Company's ability to execute its operational and budget
plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the
year ended December 31, 2022, and on Form 10-Q for the quarter ended September 30, 2023. You are cautioned not to place
undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified
in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events
or circumstances after the date hereof.
CONTACT:
Investors:
Amicus Therapeutics
Andrew Faughnan
Vice President, Investor
Relations
afaughnan@amicusrx.com
(609) 662-3809
Media:
Amicus Therapeutics
Diana Moore
Head of Global Corporate Communications
dmoore@amicusrx.com
(609) 662-5079
FOLD-G
Exhibit 99.2
| AT THE FOREFRONT OF
THERAPIES FOR RARE DISEASES
42nd Annual J.P. Morgan
Healthcare Conference
January 8, 2024 |

| 2
Forward-Looking Statements
This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our
product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates,
commercialization plans, manufacturing and supply plans, financing plans, and the projected revenues and cash position for the Company. The inclusion of forward-looking statements
should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong and
can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress,
timing, and outcomes of discussions with regulatory authorities and pricing and reimbursement authorities, are based on current information. Actual results may differ materially from
those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate
that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities may not grant or
may delay approval for our product candidates; the potential that required regulatory inspections may be delayed or not be successful and delay or prevent product approval; the
potential that we may not be successful in negotiations with pricing and reimbursement authorities; the potential that we may not be successful in commercializing Galafold and/or
Pombiliti and Opfolda in Europe, the UK, the US and other geographies; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or
other safety issues; the potential that we may not be able to manufacture or supply sufficient clinical or commercial products; and the potential that we will need additional funding to
complete all of our studies, the manufacturing, and commercialization of our products. With respect to statements regarding corporate financial guidance and financial goals and the
expected attainment of such goals and projections of the Company's revenue, non-GAAP profitability and cash position, actual results may differ based on market factors and the
Company's ability to execute its operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the
year ended December 31, 2022, and on Form 10-Q for the quarter ended September 30, 2023. You are cautioned not to place undue reliance on these forward-looking statements,
which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update
this newsrelease to reflect events or circumstances after the date hereof.
Non-GAAP Financial Measures
In addition to financial information prepared in accordance with U.S. GAAP, this presentation also contains adjusted financial measures that we believe provide investors and
management with supplemental information relating to operating performance and trends that facilitate comparisons between periods and with respect to projected information. These
adjusted financial measures are non-GAAP measures and should be considered in addition to, but not as a substitute for, the information prepared in accordance with U.S. GAAP. We
typically exclude certain GAAP items that management does not believe affect our basic operations and that do not meet the GAAP definition of unusual or non-recurring items. Other
companies may define these measures in different ways. When we provide our expectation for non-GAAP operating expenses on a forward-looking basis, a reconciliation of the
differences between the non-GAAP expectation and the corresponding GAAP measure generally is not available without unreasonable effort due to potentially high variability,
complexity and low visibility as to the items that would be excluded from the GAAP measure in the relevant future period, such as unusual gains or losses. The variability of the excluded
items may have a significant, and potentially unpredictable, impact on our future GAAP results. |

| Definition:
əˈmēkəs (noun) Latin Friend
Our Passion
is for Patients
Our Mission:
We seek to deliver the highest
quality therapies for people living
with rare diseases
Our Vision:
Be a leader in rare disease drug
development and commercialization
leveraging our global capabilities
in bringing life-changing therapies
to patients
3 |
| 4
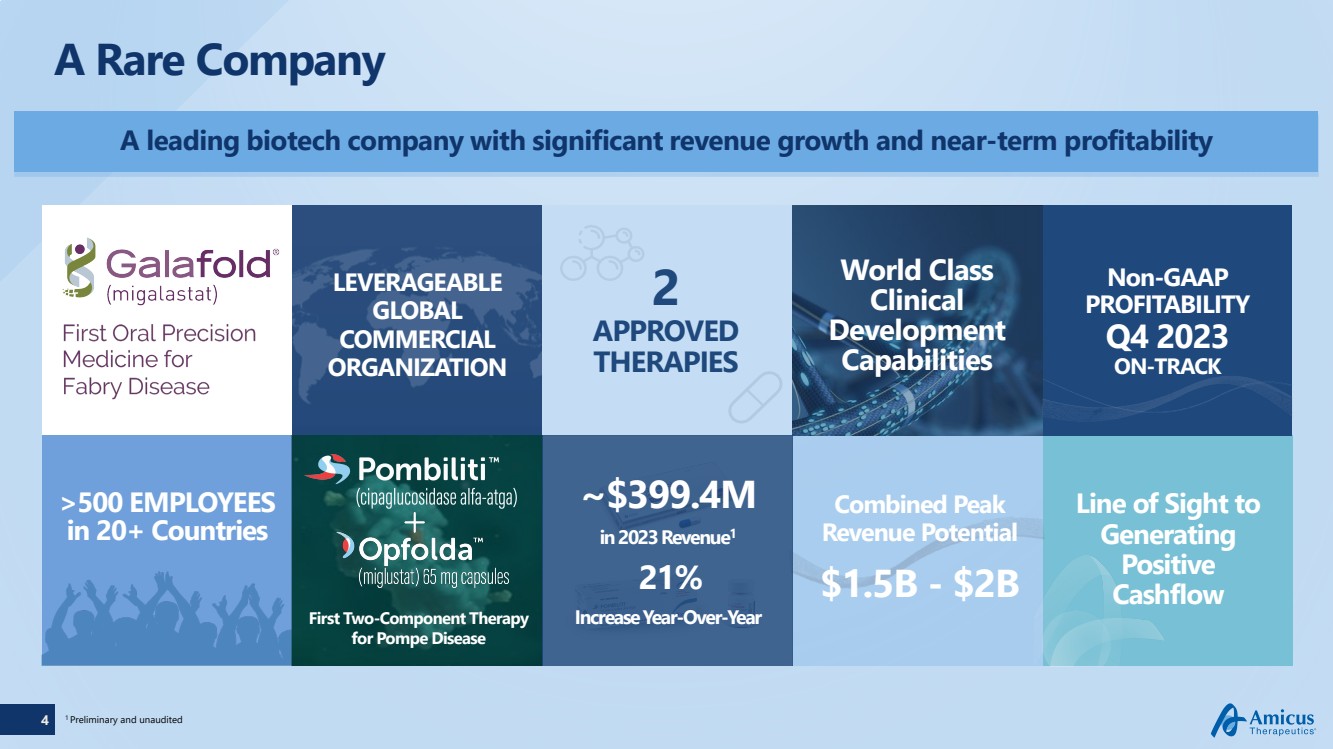
A leading biotech company with significant revenue growth and near-term profitability
A Rare Company
LEVERAGEABLE
GLOBAL
COMMERCIAL
ORGANIZATION
>500 EMPLOYEES
in 20+ Countries
First Two-Component Therapy
for Pompe Disease
2
APPROVED
THERAPIES
1 Preliminary and unaudited
Combined Peak
Revenue Potential
$1.5B - $2B
Non-GAAP
PROFITABILITY
Q4 2023
ON-TRACK
World Class
Clinical
Development
Capabilities
~$399.4M
in 2023 Revenue1
21%
Increase Year-Over-Year
Line of Sight to
Generating
Positive
Cashflow |
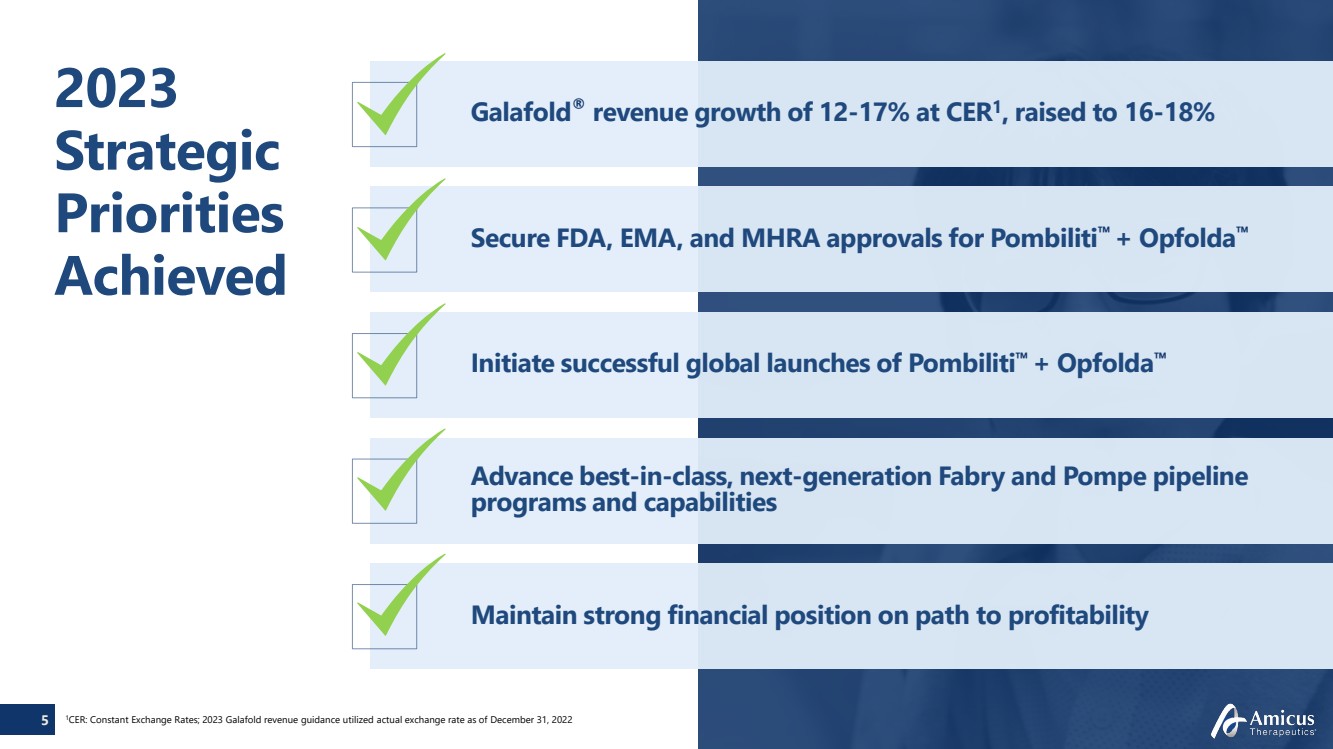
| Galafold® revenue growth of 12-17% at CER1, raised to 16-18%
Secure FDA, EMA, and MHRA approvals for Pombiliti™ + Opfolda™
Initiate successful global launches of Pombiliti™ + Opfolda™
Advance best-in-class, next-generation Fabry and Pompe pipeline
programs and capabilities
Maintain strong financial position on path to profitability
2023
Strategic
Priorities
Achieved
1 5 CER: Constant Exchange Rates; 2023 Galafold revenue guidance utilized actual exchange rate as of December 31, 2022 |

| 6
2023 Key Milestones
Successful Early Days of Pombiliti +
Opfolda Launch |
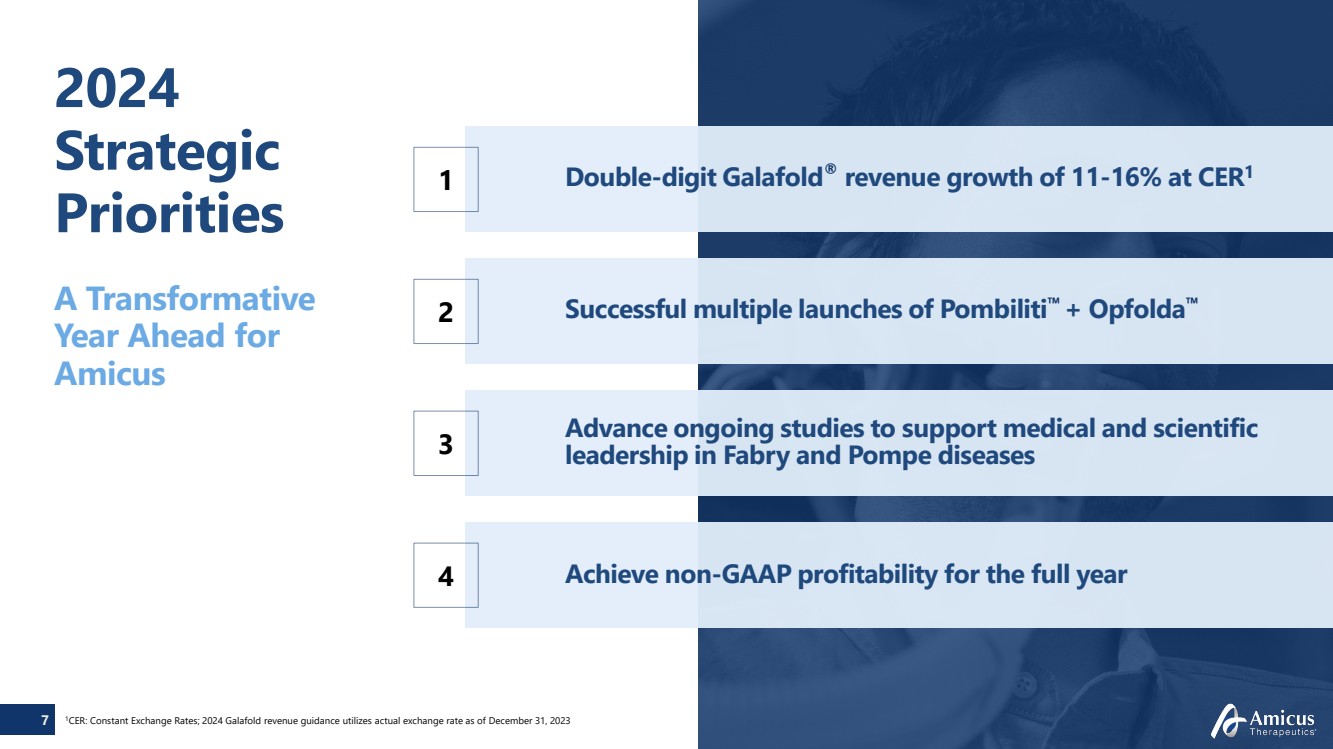
| Double-digit Galafold® revenue growth of 11-16% at CER1 1
Successful multiple launches of Pombiliti™ + Opfolda™
Advance ongoing studies to support medical and scientific
leadership in Fabry and Pompe diseases
Achieve non-GAAP profitability for the full year
2
3
4
A Transformative
Year Ahead for
Amicus
1CER: Constant Exchange Rates; 2024 Galafold revenue guidance utilizes actual exchange rate as of December 31, 2023
2024
Strategic
Priorities
7 |

| 8
Continued Growth of
Galafold® (migalastat)
Expanding leadership in the treatment of
Fabry disease |
| 9
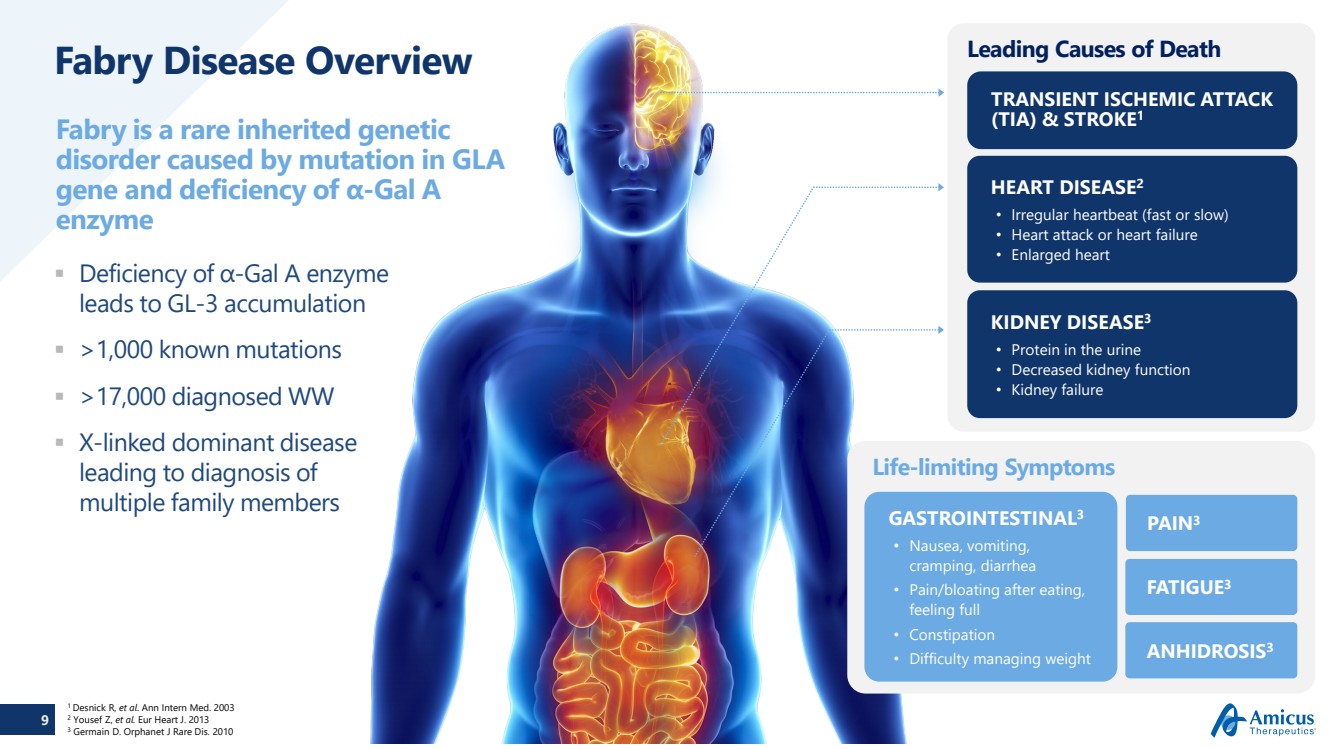
Fabry Disease Overview Deficiency of α-Gal A enzyme
leads to GL
-3 accumulation
>1,000 known mutations >17,000 diagnosed WW X-linked dominant disease
leading to diagnosis of
multiple family members
Fabry is a rare inherited genetic
disorder caused by mutation in GLA
gene and deficiency of α
-Gal A
enzyme
Leading Causes of Death
TRANSIENT ISCHEMIC ATTACK
(TIA) & STROKE
1
KIDNEY DISEASE
3
• Protein in the urine • Decreased kidney function • Kidney failure
HEART DISEASE
2
• Irregular heartbeat (fast or slow) • Heart attack or heart failure • Enlarged heart
Life
-limiting Symptoms
GASTROINTESTINAL
3
• Nausea, vomiting,
cramping, diarrhea
• Pain/bloating after eating,
feeling full
• Constipation • Difficulty managing weight
PAIN
3
FATIGUE
3
ANHIDROSIS
3
1 Desnick R, et al. Ann Intern Med. 2003 2 Yousef Z, et al. Eur Heart J. 2013 3 Germain D. Orphanet J Rare Dis. 2010 |
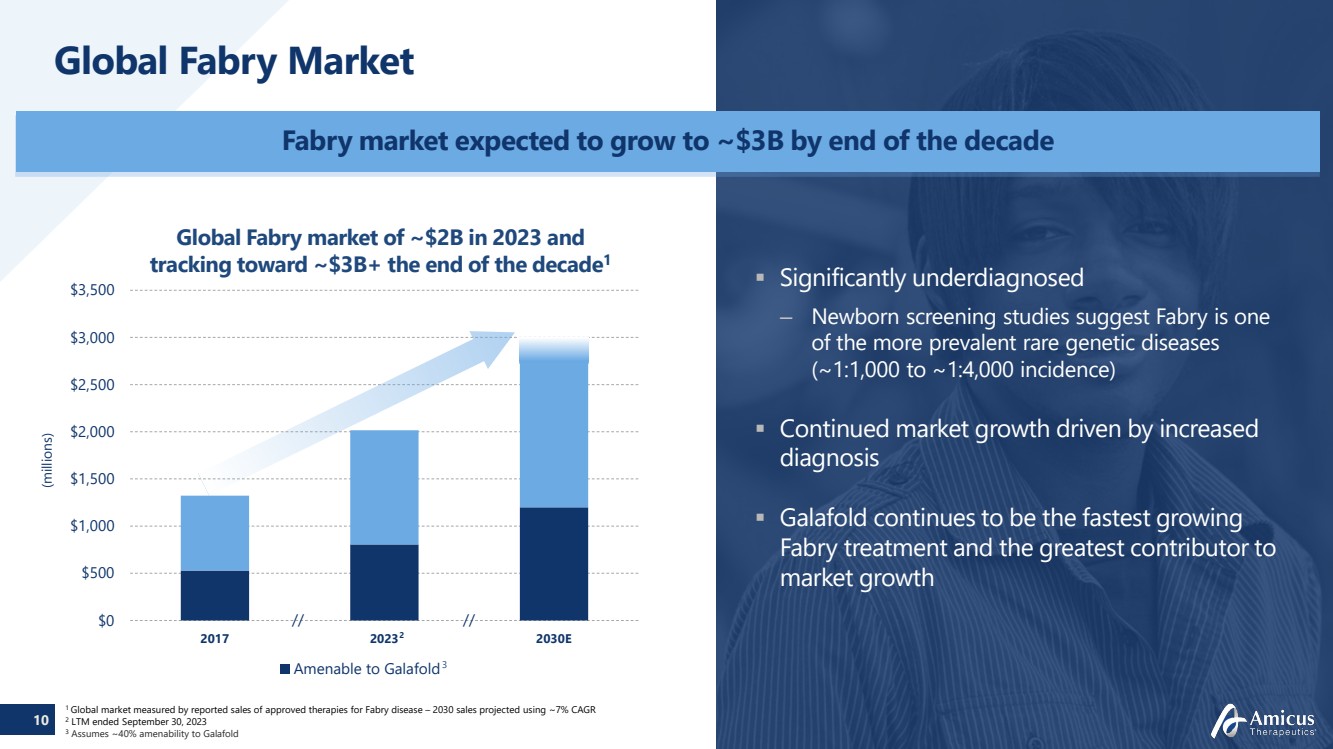
| 10
Global Fabry Market
(millions)
$0
$500
$1,000
$1,500
$2,000
$2,500
$3,000
$3,500
2017 2023 2030E
Amenable to Galafold
//
Global Fabry market of ~$2B in 2023 and
tracking toward ~$3B+ the end of the decade1
1 Global market measured by reported sales of approved therapies for Fabry disease – 2030 sales projected using ~7% CAGR 2 LTM ended September 30, 2023
3 Assumes ~40% amenability to Galafold
Fabry market expected to grow to ~$3B by end of the decade
Significantly underdiagnosed
– Newborn screening studies suggest Fabry is one
of the more prevalent rare genetic diseases
(~1:1,000 to ~1:4,000 incidence)
Continued market growth driven by increased
diagnosis
Galafold continues to be the fastest growing
Fabry treatment and the greatest contributor to
market growth
2
3
// |
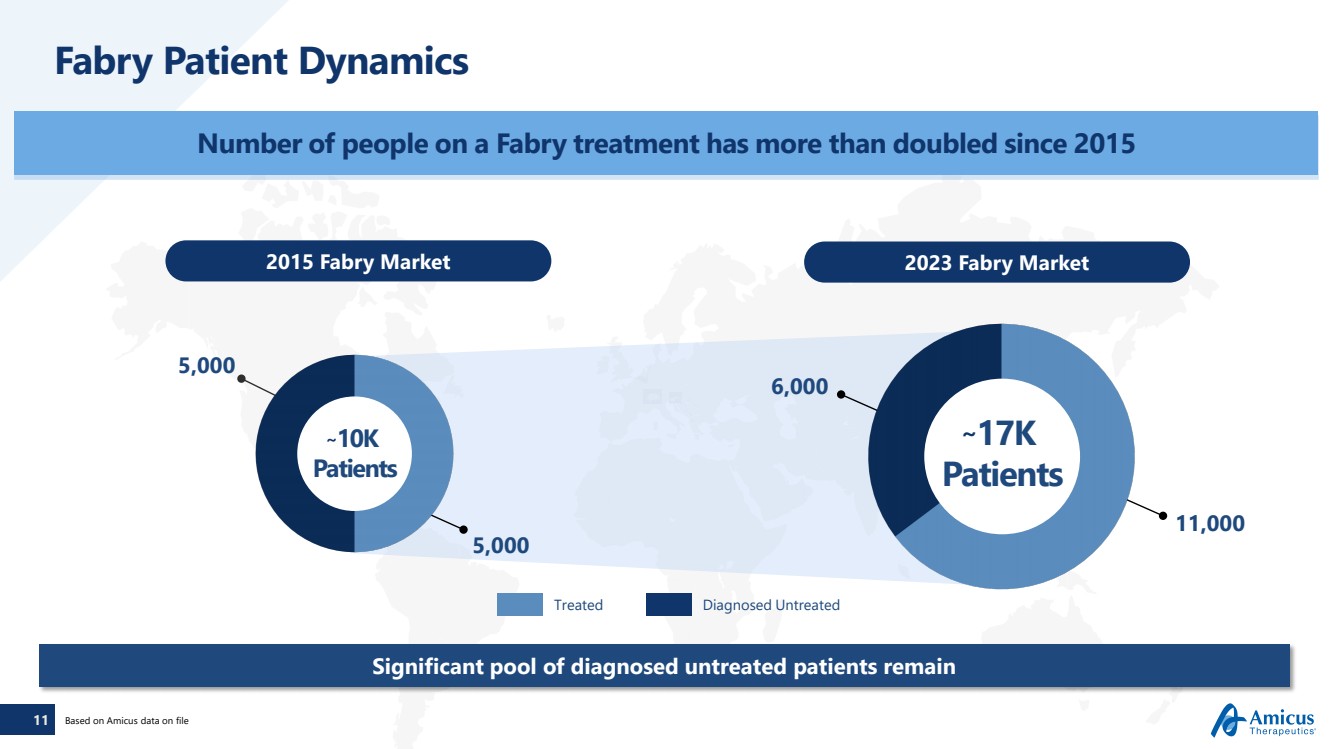
| 11
Fabry Patient Dynamics
Number of people on a Fabry treatment has more than doubled since 2015
2015 Fabry Market 2023 Fabry Market
~17K
Patients
Significant pool of diagnosed untreated patients remain
5,000
5,000
~10K
Patients
6,000
11,000
Based on Amicus data on file
Treated Diagnosed Untreated |
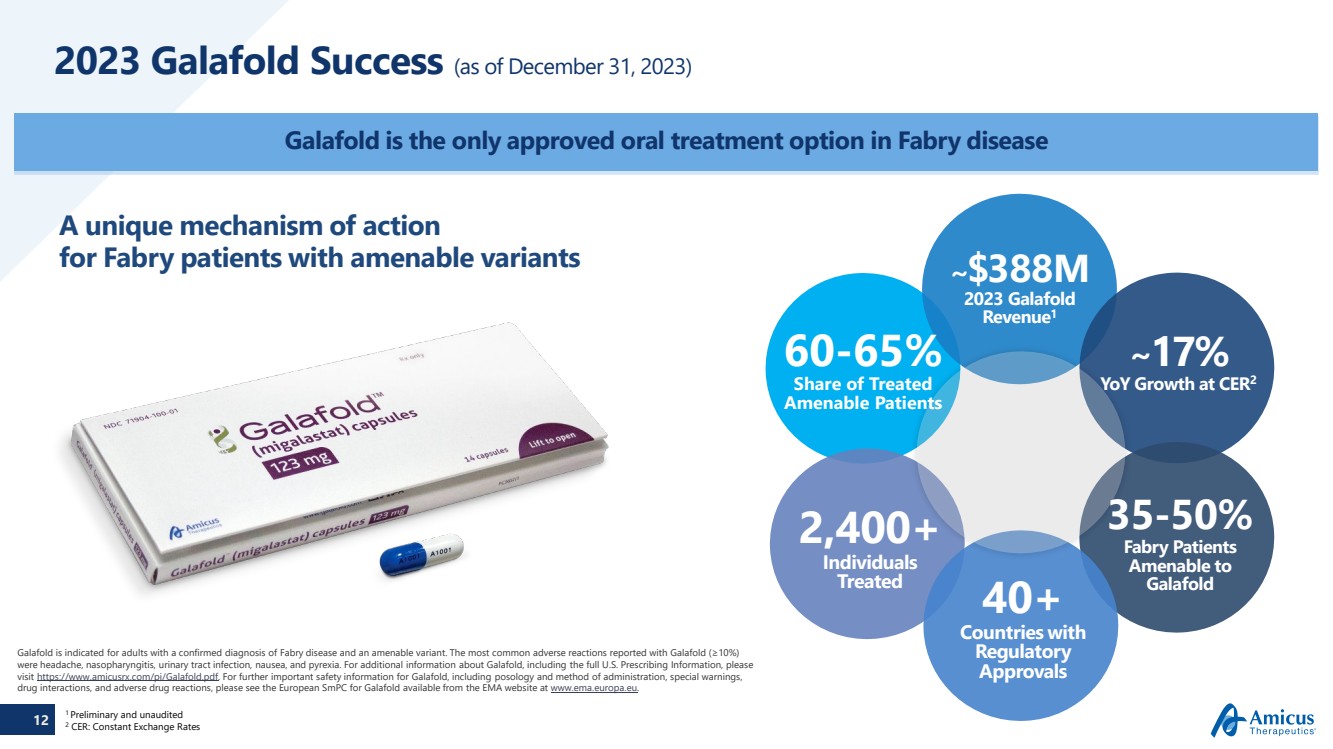
| 12
2023 Galafold Success (as of December 31, 2023)
Galafold is the only approved oral treatment option in Fabry disease
Galafold is indicated for adults with a confirmed diagnosis of Fabry disease and an amenable variant. The most common adverse reactions reported with Galafold (≥10%)
were headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia. For additional information about Galafold, including the full U.S. Prescribing Information, please
visit https://www.amicusrx.com/pi/Galafold.pdf. For further important safety information for Galafold, including posology and method of administration, special warnings,
drug interactions, and adverse drug reactions, please see the European SmPC for Galafold available from the EMA website at www.ema.europa.eu.
A unique mechanism of action
for Fabry patients with amenable variants
35-50%
Fabry Patients
Amenable to
Galafold 40+
Countries with
Regulatory
Approvals
2,400+
Individuals
Treated
~$388M
2023 Galafold
Revenue1
~17%
YoY Growth at CER2
60-65%
Share of Treated
Amenable Patients
1 Preliminary and unaudited
2 CER: Constant Exchange Rates |

| 13
Key Growth Drivers for 2024
Strong patient demand laying groundwork for continued double-digit Galafold growth
Improve diagnosis of patients through medical education,
screening, and testing
Drive market share of treated amenable patients
through excellent execution
Expand market through uptake in naïve population
as well as geographic and label expansion
Maintain >90% adherence and compliance through HCP
and patient education and support
13 |

| 14
Improving Diagnosis of Fabry Disease
Harnessing AI to improve diagnosis of people living with Fabry and predicting patient outcomes
Partnership with OM1 and leading healthcare
system in the U.S. to pilot algorithm
Finding undiagnosed patients and variation
in patient phenotype
Predicting health outcomes including serious
events
Applying AI tools to additional Fabry markets |

| 15
Launch Underway of
Pombiliti™ + Opfolda™
(cipaglucosidase alfa-atga) (miglustat)
Resetting expectations for people
living with late-onset Pompe disease |

| 16
Late-onset Pompe Disease is a Rare, Inherited Genetic Disorder Caused by
Mutation in GAA Gene and Deficiency of α-Glucosidase Enzyme
Significant unmet need
Deficiency of GAA leading to
lysosomal glycogen accumulation
and cellular dysfunction
Symptoms include systemic muscle weakness
that worsens over time
Respiratory failure is major
cause of mortality
~5,000-10,000 people diagnosed
globally
~$1.3B+ global Pompe ERT
sales1
Significantly underdiagnosed
1 Based on 12 months ended September 30, 2023. |
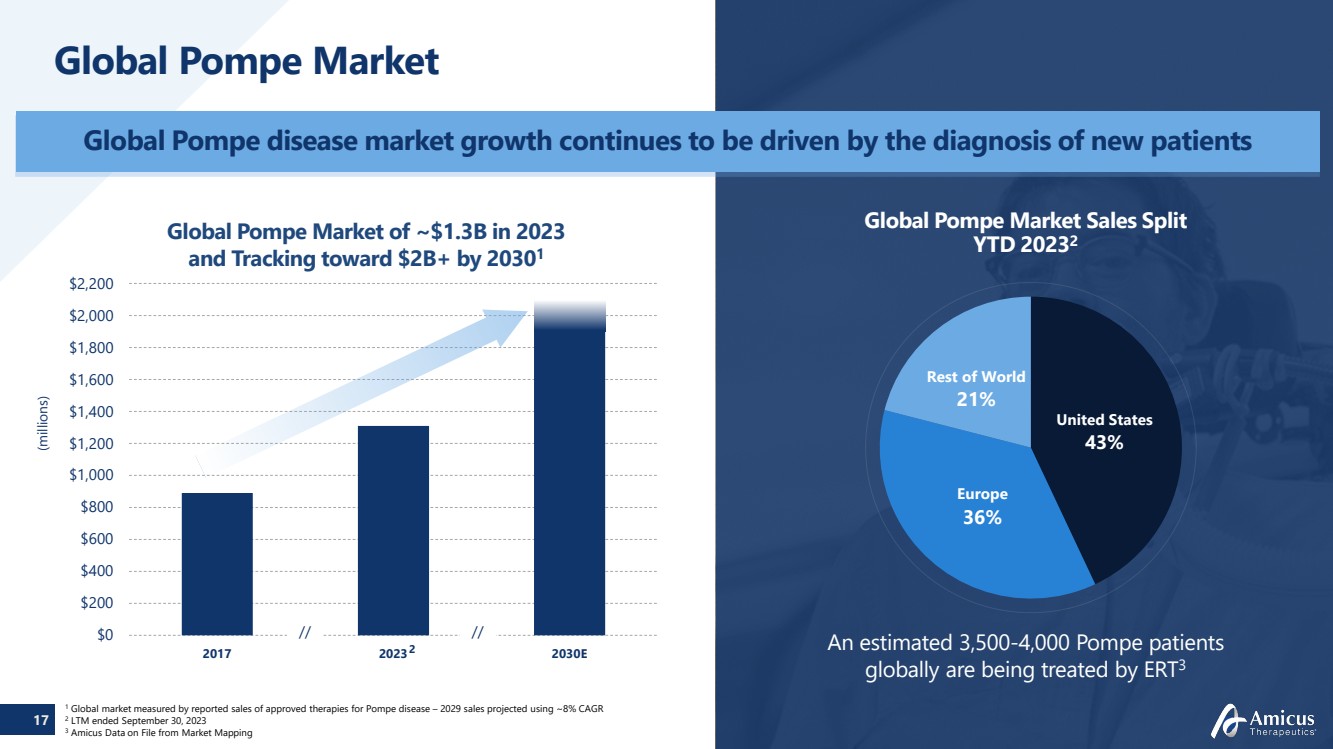
| 17
$0
$200
$400
$600
$800
$1,000
$1,200
$1,400
$1,600
$1,800
$2,000
$2,200
2017 2023 2030E
// //
Global Pompe Market
Global Pompe disease market growth continues to be driven by the diagnosis of new patients
An estimated 3,500-4,000 Pompe patients
globally are being treated by ERT3
Global Pompe Market Sales Split
YTD 20232
United States
43%
Europe
36%
Rest of World
21%
Global Pompe Market of ~$1.3B in 2023
and Tracking toward $2B+ by 20301
(millions)
1 Global market measured by reported sales of approved therapies for Pompe disease – 2029 sales projected using ~8% CAGR 2 LTM ended September 30, 2023
3 Amicus Data on File from Market Mapping
2 |
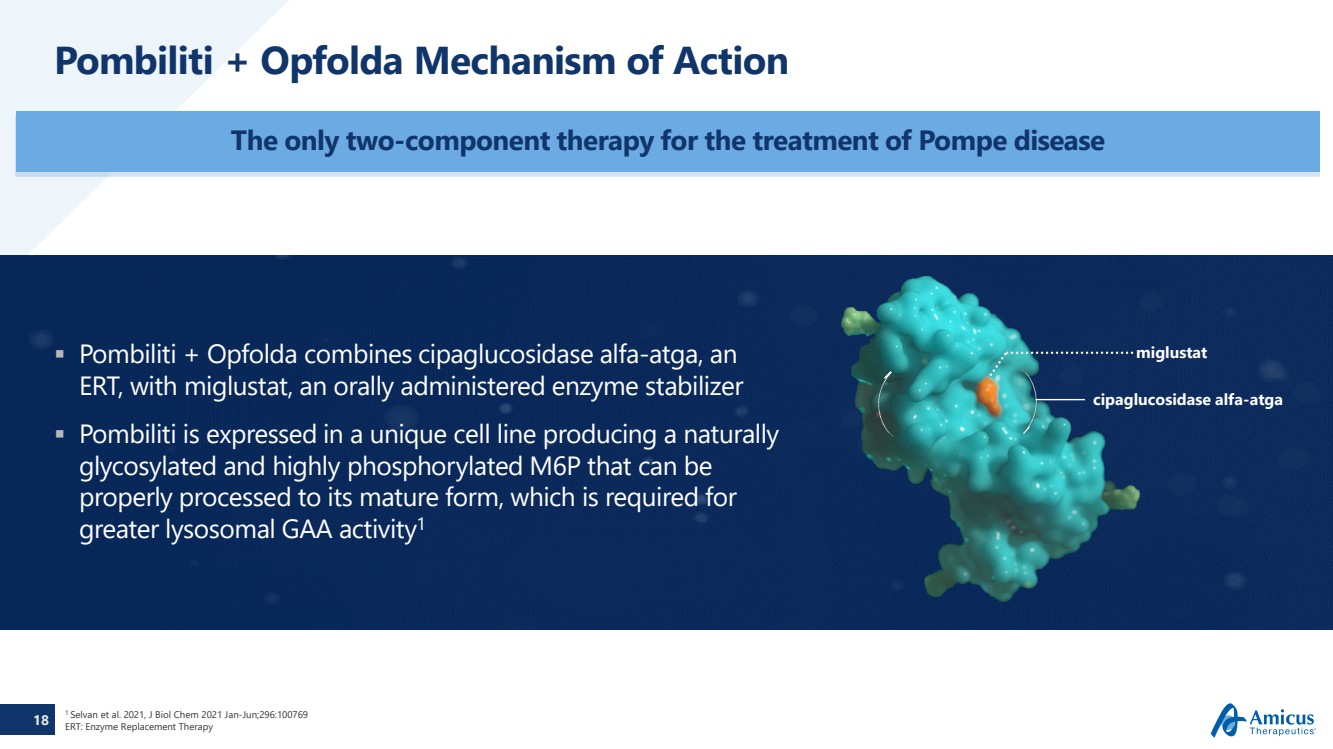
| 18
Pombiliti + Opfolda Mechanism of Action
The only two-component therapy for the treatment of Pompe disease
1 Selvan et al. 2021, J Biol Chem 2021 Jan-Jun;296:100769
ERT: Enzyme Replacement Therapy
Pombiliti + Opfolda combines cipaglucosidase alfa-atga, an
ERT, with miglustat, an orally administered enzyme stabilizer
Pombiliti is expressed in a unique cell line producing a naturally
glycosylated and highly phosphorylated M6P that can be
properly processed to its mature form, which is required for
greater lysosomal GAA activity1
miglustat
cipaglucosidase alfa-atga |
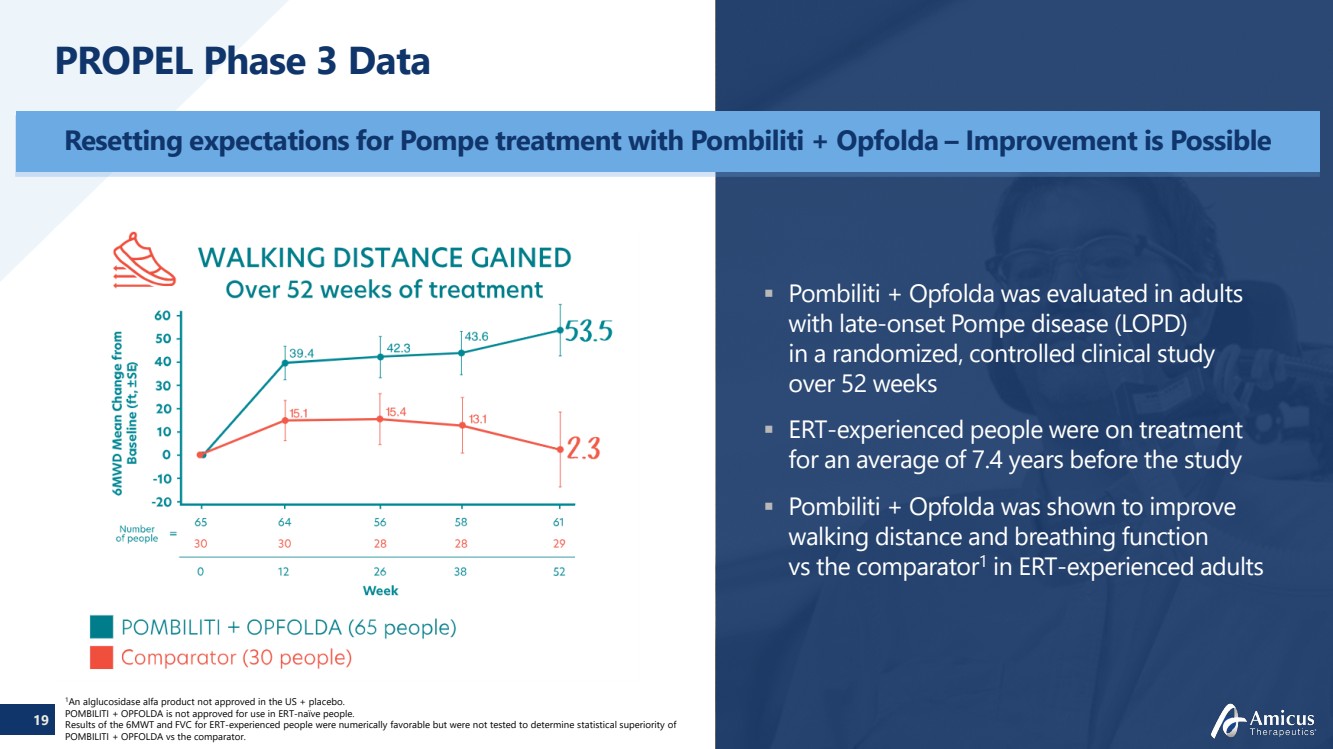
| 19
PROPEL Phase 3 Data
Resetting expectations for Pompe treatment with Pombiliti + Opfolda – Improvement is Possible
1An alglucosidase alfa product not approved in the US + placebo.
POMBILITI + OPFOLDA is not approved for use in ERT-naïve people.
Results of the 6MWT and FVC for ERT-experienced people were numerically favorable but were not tested to determine statistical superiority of
POMBILITI + OPFOLDA vs the comparator.
Pombiliti + Opfolda was evaluated in adults
with late-onset Pompe disease (LOPD)
in a randomized, controlled clinical study
over 52 weeks
ERT-experienced people were on treatment
for an average of 7.4 years before the study
Pombiliti + Opfolda was shown to improve
walking distance and breathing function
vs the comparator1 in ERT-experienced adults |
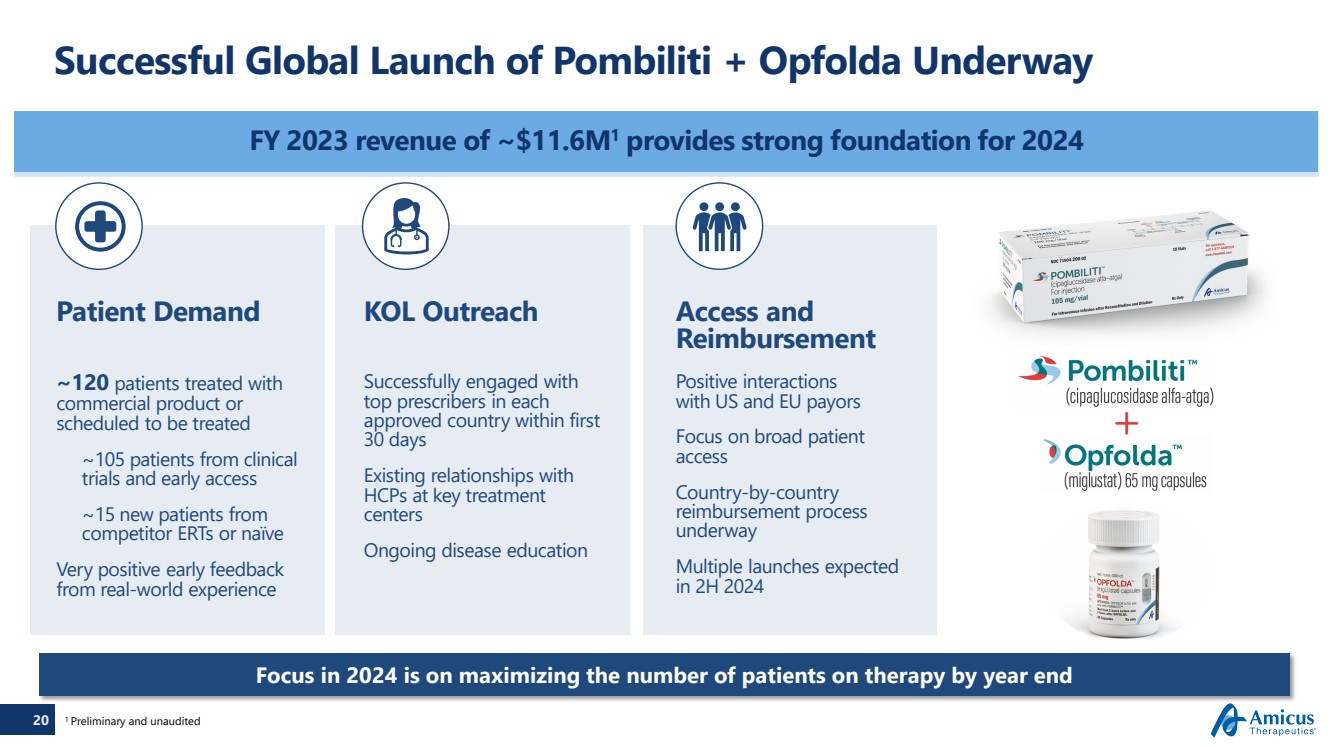
| 20
Successful Global Launch of Pombiliti + Opfolda Underway
FY 2023 revenue of ~$11.6M1 provides strong foundation for 2024
Access and
Reimbursement
Positive interactions
with US and EU payors
Focus on broad patient
access
Country-by-country
reimbursement process
underway
Multiple launches expected
in 2H 2024
~120 patients treated with
commercial product or
scheduled to be treated
~105 patients from clinical
trials and early access
~15 new patients from
competitor ERTs or naïve
Very positive early feedback
from real-world experience
KOL Outreach
Successfully engaged with
top prescribers in each
approved country within first
30 days
Existing relationships with
HCPs at key treatment
centers
Ongoing disease education
Patient Demand
20 1 Preliminary and unaudited
Focus in 2024 is on maximizing the number of patients on therapy by year end |

| 21
International: EU and U.K. Update and Market Opportunity
>1,300 patients are estimated to be treated in Europe1
>200 patients are estimated to be treated in the U.K.1
Broad experience from a wide set of KOLs through
clinical trials and early access programs
Leveraging EU label and regulatory outcome to extend
into other geographies
1 Amicus Data on File from Market Mapping
EU and U.K. Pompe markets collectively represent sizeable market opportunity
Strong indication statement:
Pombiliti™ (cipaglucosidase alfa) is a long-term enzyme
replacement therapy used in combination with the enzyme
stabiliser miglustat for the treatment of adults with late-onset Pompe disease (acid α glucosidase [GAA] deficiency)
All EAP and clinical trial patients transitioned
Multiple new patient starts from both switch and
naïve patients
Robust interest from physician community and
treatment centers
LAUNCH DYNAMICS |
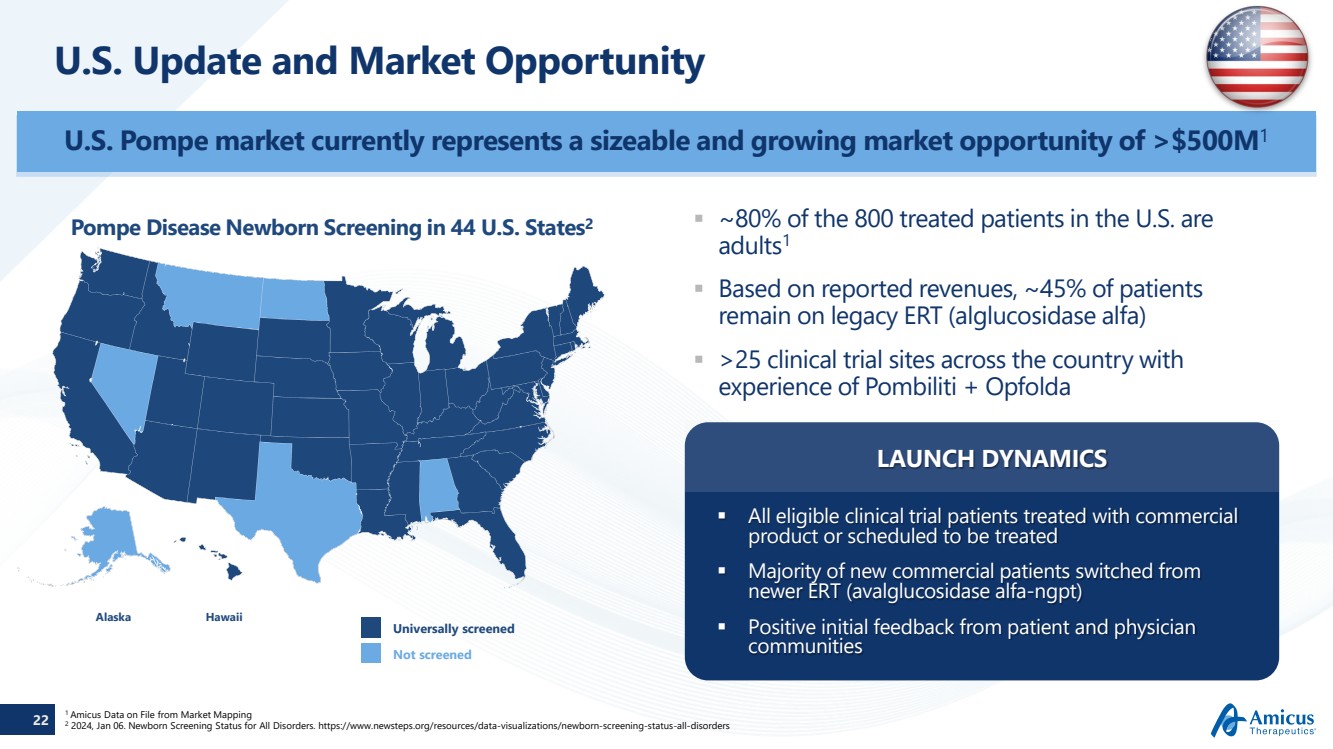
| 22
U.S. Update and Market Opportunity
Alaska Hawaii
Universally screened
Not screened
Pompe Disease Newborn Screening in 44 U.S. States2
1 Amicus Data on File from Market Mapping
2 2024, Jan 06. Newborn Screening Status for All Disorders. https://www.newsteps.org/resources/data-visualizations/newborn-screening-status-all-disorders
U.S. Pompe market currently represents a sizeable and growing market opportunity of >$500M1
~80% of the 800 treated patients in the U.S. are
adults1
Based on reported revenues, ~45% of patients
remain on legacy ERT (alglucosidase alfa)
>25 clinical trial sites across the country with
experience of Pombiliti + Opfolda
All eligible clinical trial patients treated with commercial
product or scheduled to be treated
Majority of new commercial patients switched from
newer ERT (avalglucosidase alfa-ngpt)
Positive initial feedback from patient and physician
communities
LAUNCH DYNAMICS
22 |

| 23
Regulatory and Clinical Updates
Building the body of evidence and expanding commercial access
>10 reimbursement dossiers submitted and multiple
regulatory submissions throughout 2024
Ongoing clinical studies in children with late-onset
Pompe disease and infantile-onset Pompe disease (IOPD)
Amicus registry for Pompe disease expected to continue
generating evidence on differentiated MOA and long-term effect |

| 24
Corporate Outlook
Delivering on our mission for patients
and shareholders |
| 25
Accelerating
total revenue
growth
Positioned for Significant Value Creation in 2024
Unlocking the value of two unique commercial therapies in sizeable and growing markets
1Non-GAAP Net Income defined as GAAP Net Income excluding the impact of stock-based compensation expense, changes in fair value of contingent consideration, loss on impairment of assets,
depreciation and amortization, acquisition related income (expense), loss on extinguishment of debt, restructuring charges and income taxes.
Clear line of
sight to
generating
positive
cashflow
Delivering
full-year
non-GAAP1
profitability |
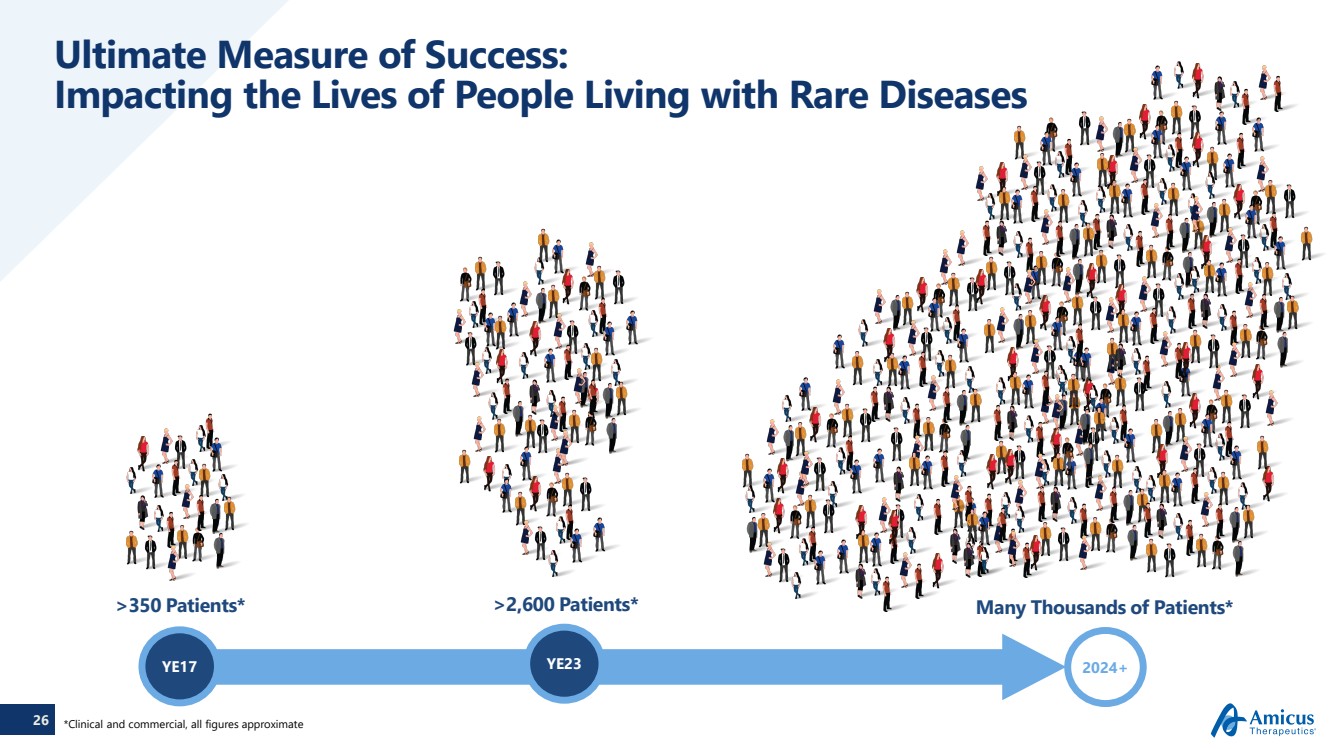
| 26
Ultimate Measure of Success:
Impacting the Lives of People Living with Rare Diseases
YE17 2024+
>2,600 Patients* Many Thousands of Patients*
YE23
>350 Patients*
*Clinical and commercial, all figures approximate |

| Thank You |

| Appendix |
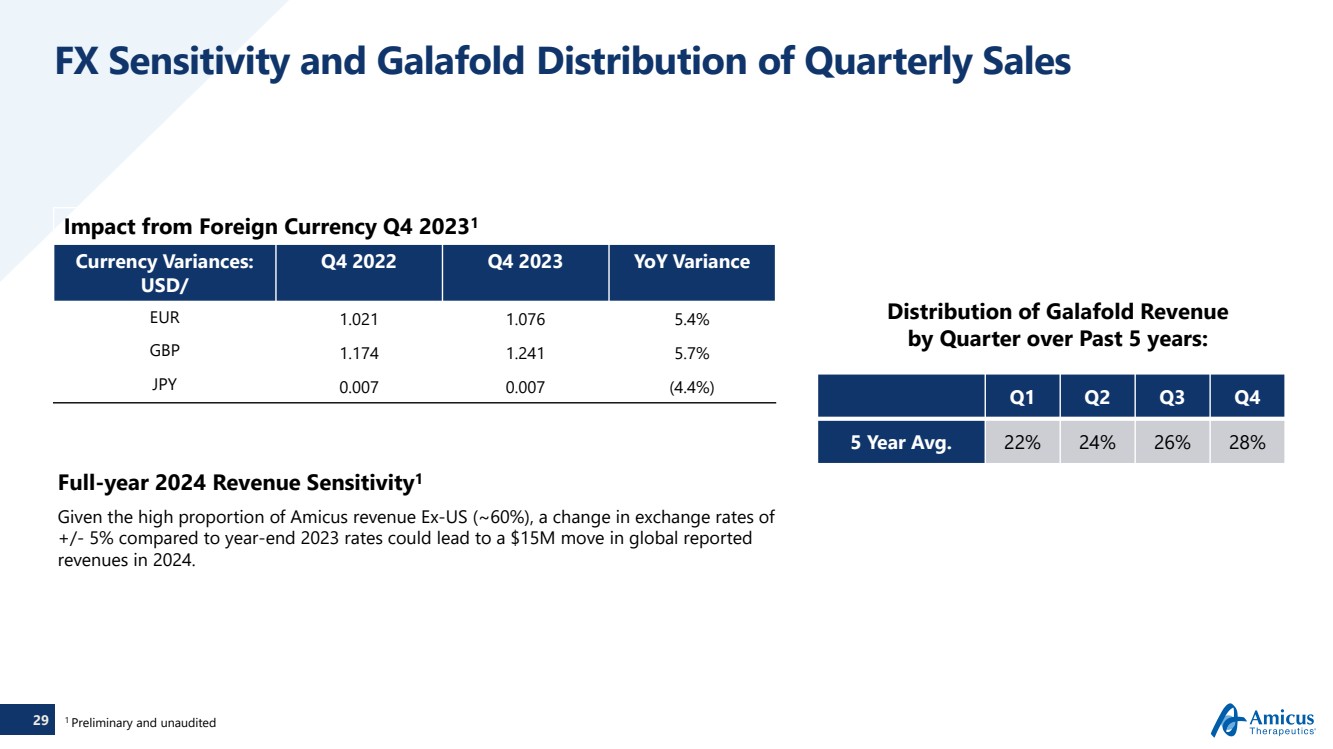
| 29
FX Sensitivity and Galafold Distribution of Quarterly Sales
Impact from Foreign Currency Q4 20231
Currency Variances:
USD/
Q4 2022 Q4 2023 YoY Variance
EUR 1.021 1.076 5.4%
GBP 1.174 1.241 5.7%
JPY 0.007 0.007 (4.4%)
Full-year 2024 Revenue Sensitivity1
Given the high proportion of Amicus revenue Ex-US (~60%), a change in exchange rates of
+/- 5% compared to year-end 2023 rates could lead to a $15M move in global reported
revenues in 2024.
Distribution of Galafold Revenue
by Quarter over Past 5 years:
Q1 Q2 Q3 Q4
5 Year Avg. 22% 24% 26% 28%
1 Preliminary and unaudited |
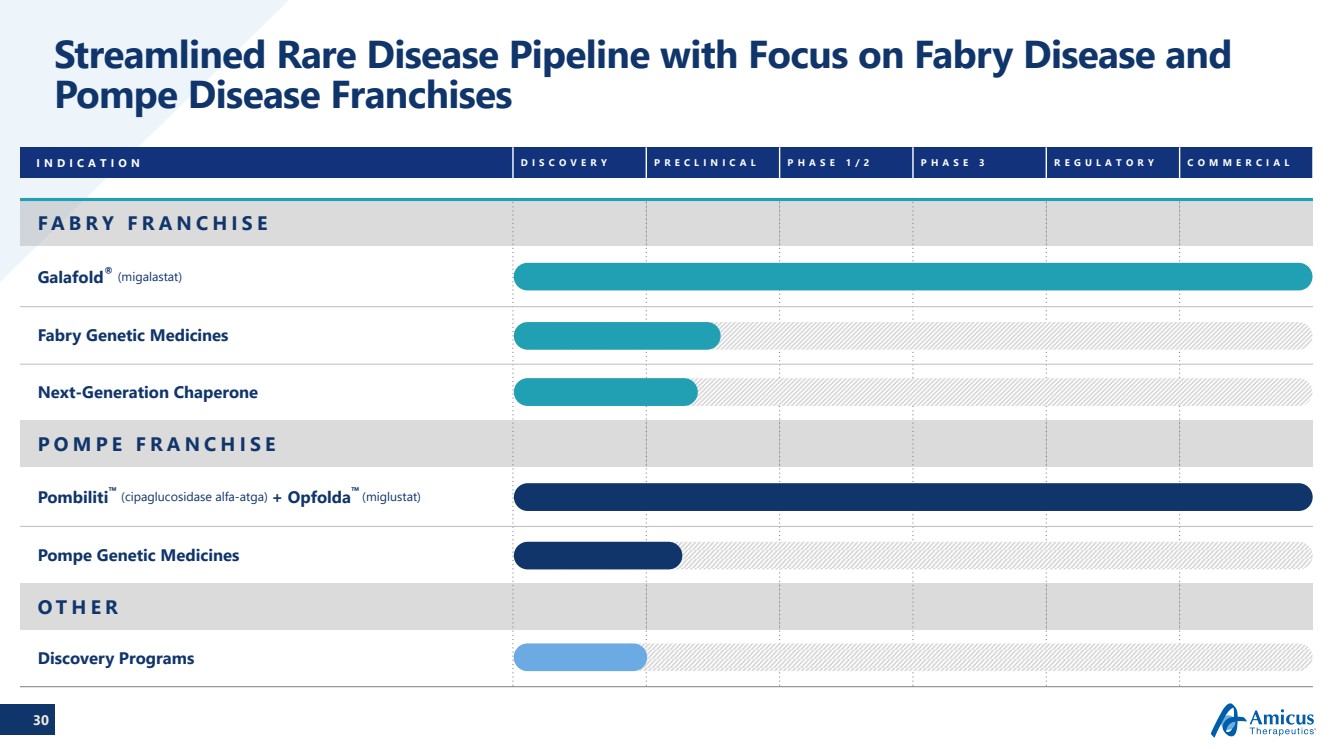
| 30
Streamlined Rare Disease Pipeline with Focus on Fabry Disease and
Pompe Disease Franchises
INDICATION DISCOVERY PRECLINICAL PHASE 1/2 PHASE 3 REGULATORY COMMERCIAL
FABRY FRANCHISE
Galafold® (migalastat)
Fabry Genetic Medicines
Next-Generation Chaperone
POMPE FRANCHISE
Pombiliti™ (cipaglucosidase alfa-atga) + Opfolda™ (miglustat)
Pompe Genetic Medicines
OTHER
Discovery Programs |
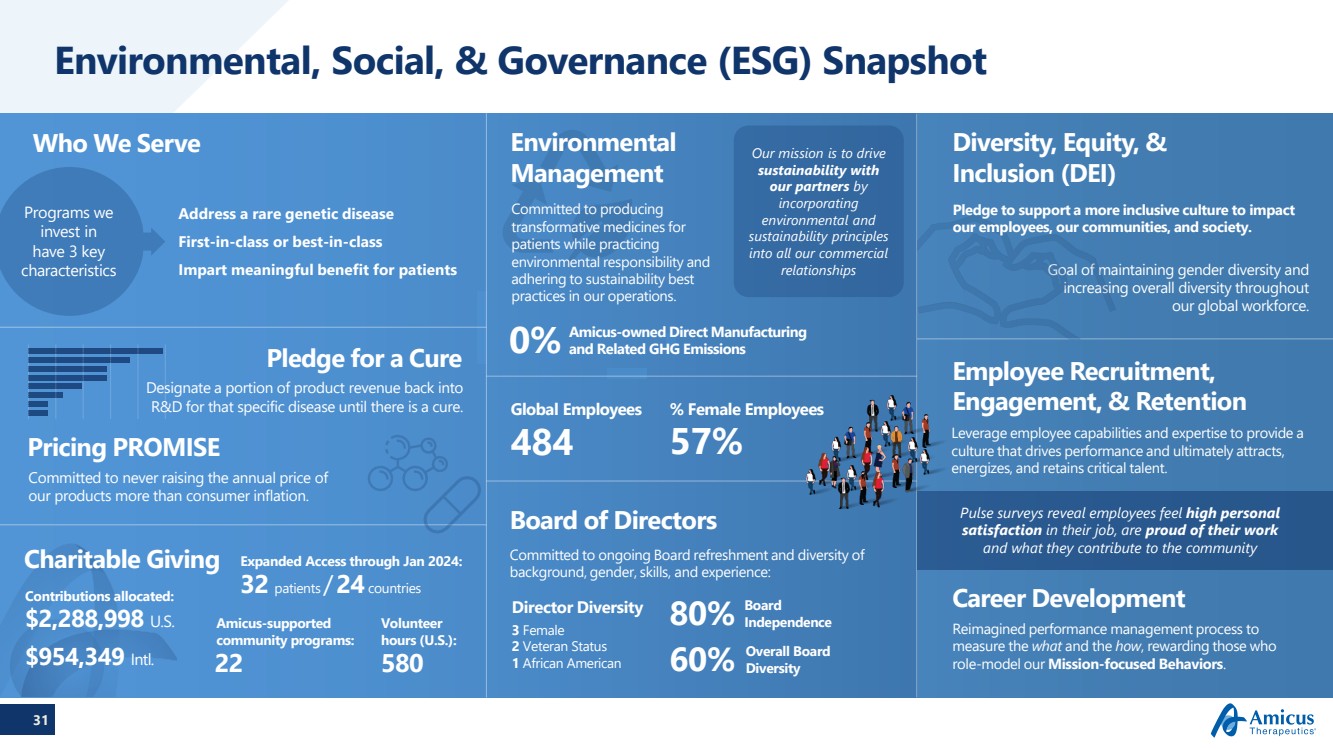
| 31
Environmental, Social, & Governance (ESG) Snapshot
Board of Directors
Committed to ongoing Board refreshment and diversity of
background, gender, skills, and experience:
80% Board
Independence
60% Overall Board
Diversity
Address a rare genetic disease
First-in-class or best-in-class
Impart meaningful benefit for patients
484
Global Employees
57%
% Female Employees
Who We Serve Our mission is to drive
sustainability with
our partners by
incorporating
environmental and
sustainability principles
into all our commercial
relationships
Pledge for a Cure
Designate a portion of product revenue back into
R&D for that specific disease until there is a cure.
Programs we
invest in
have 3 key
characteristics
3 Female
2 Veteran Status
1 African American
Director Diversity
Leverage employee capabilities and expertise to provide a
culture that drives performance and ultimately attracts,
energizes, and retains critical talent.
Employee Recruitment,
Engagement, & Retention
Pulse surveys reveal employees feel high personal
satisfaction in their job, are proud of their work
and what they contribute to the community
Career Development
Reimagined performance management process to
measure the what and the how, rewarding those who
role-model our Mission-focused Behaviors.
Committed to producing
transformative medicines for
patients while practicing
environmental responsibility and
adhering to sustainability best
practices in our operations.
Environmental
Management
0% Amicus-owned Direct Manufacturing
and Related GHG Emissions
Diversity, Equity, &
Inclusion (DEI)
Goal of maintaining gender diversity and
increasing overall diversity throughout
our global workforce.
580
Volunteer
hours (U.S.):
22
Amicus-supported
community programs:
32 patients /24countries
Expanded Access through Jan 2024:
Pricing PROMISE
Contributions allocated:
$2,288,998 U.S.
$954,349 Intl.
Charitable Giving
Committed to never raising the annual price of
our products more than consumer inflation.
Pledge to support a more inclusive culture to impact
our employees, our communities, and society. |
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Amicus Therapeutics (NASDAQ:FOLD)
Historical Stock Chart
From Mar 2024 to Apr 2024
Amicus Therapeutics (NASDAQ:FOLD)
Historical Stock Chart
From Apr 2023 to Apr 2024