00018041762024FYfalseP3Y33.000http://fasb.org/us-gaap/2024#PrepaidExpenseAndOtherAssetsCurrenthttp://fasb.org/us-gaap/2024#PrepaidExpenseAndOtherAssetsCurrenthttp://fasb.org/us-gaap/2024#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2024#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2024#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2024#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2024#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2024#AccruedLiabilitiesAndOtherLiabilities66.7iso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:purebfly:segmentbfly:Votebfly:purchase_periodbfly:lease00018041762024-01-012024-12-310001804176us-gaap:CommonClassAMember2024-01-012024-12-310001804176bfly:WarrantsToPurchaseCommonStockMember2024-01-012024-12-3100018041762024-06-300001804176us-gaap:CommonClassAMember2025-02-130001804176us-gaap:CommonClassBMember2025-02-0900018041762024-10-012024-12-3100018041762024-12-3100018041762023-12-310001804176us-gaap:CommonClassAMember2023-12-310001804176us-gaap:CommonClassAMember2024-12-310001804176us-gaap:CommonClassBMember2023-12-310001804176us-gaap:CommonClassBMember2024-12-310001804176us-gaap:ProductMember2024-01-012024-12-310001804176us-gaap:ProductMember2023-01-012023-12-310001804176us-gaap:ProductMember2022-01-012022-12-310001804176us-gaap:ServiceOtherMember2024-01-012024-12-310001804176us-gaap:ServiceOtherMember2023-01-012023-12-310001804176us-gaap:ServiceOtherMember2022-01-012022-12-3100018041762023-01-012023-12-3100018041762022-01-012022-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2021-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassBMember2021-12-310001804176us-gaap:AdditionalPaidInCapitalMember2021-12-310001804176us-gaap:RetainedEarningsMember2021-12-3100018041762021-12-310001804176us-gaap:RetainedEarningsMember2022-01-012022-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2022-01-012022-12-310001804176us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2022-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassBMember2022-12-310001804176us-gaap:AdditionalPaidInCapitalMember2022-12-310001804176us-gaap:RetainedEarningsMember2022-12-3100018041762022-12-310001804176us-gaap:RetainedEarningsMember2023-01-012023-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2023-01-012023-12-310001804176us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2023-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassBMember2023-12-310001804176us-gaap:AdditionalPaidInCapitalMember2023-12-310001804176us-gaap:RetainedEarningsMember2023-12-310001804176us-gaap:RetainedEarningsMember2024-01-012024-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2024-01-012024-12-310001804176us-gaap:AdditionalPaidInCapitalMember2024-01-012024-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassAMember2024-12-310001804176us-gaap:CommonStockMemberus-gaap:CommonClassBMember2024-12-310001804176us-gaap:AdditionalPaidInCapitalMember2024-12-310001804176us-gaap:RetainedEarningsMember2024-12-310001804176bfly:OneCustomerMemberus-gaap:CustomerConcentrationRiskMemberbfly:AccountsReceivableBenchmarkMember2024-01-012024-12-310001804176bfly:NoCustomersMemberus-gaap:CustomerConcentrationRiskMemberbfly:AccountsReceivableBenchmarkMember2023-01-012023-12-310001804176bfly:OneCustomerMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2023-01-012023-12-310001804176bfly:OneCustomerMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2024-01-012024-12-310001804176bfly:OneCustomerMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2022-01-012022-12-310001804176bfly:ReportableSegmentMember2024-01-012024-12-310001804176bfly:ReportableSegmentMember2023-01-012023-12-310001804176bfly:ReportableSegmentMember2022-01-012022-12-310001804176srt:MinimumMember2024-01-012024-12-310001804176srt:MaximumMember2024-01-012024-12-310001804176us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2024-12-310001804176srt:MinimumMemberus-gaap:MachineryAndEquipmentMember2024-12-310001804176srt:MaximumMemberus-gaap:MachineryAndEquipmentMember2024-12-310001804176srt:MinimumMemberus-gaap:FurnitureAndFixturesMember2024-12-310001804176srt:MaximumMemberus-gaap:FurnitureAndFixturesMember2024-12-310001804176us-gaap:ComputerSoftwareIntangibleAssetMember2024-12-310001804176us-gaap:EmployeeSeveranceMember2024-07-010001804176bfly:AccruedLiabilitiesAndOtherLiabilitiesMemberus-gaap:EmployeeSeveranceMember2024-12-310001804176bfly:EmployeeStockPurchasePlanMember2024-01-012024-12-3100018041762020-05-262020-05-260001804176us-gaap:TransferredAtPointInTimeMemberus-gaap:ProductMember2024-01-012024-12-310001804176us-gaap:TransferredAtPointInTimeMemberus-gaap:ProductMember2023-01-012023-12-310001804176us-gaap:TransferredAtPointInTimeMemberus-gaap:ProductMember2022-01-012022-12-310001804176us-gaap:TransferredOverTimeMemberus-gaap:ServiceOtherMember2024-01-012024-12-310001804176us-gaap:TransferredOverTimeMemberus-gaap:ServiceOtherMember2023-01-012023-12-310001804176us-gaap:TransferredOverTimeMemberus-gaap:ServiceOtherMember2022-01-012022-12-310001804176country:US2024-01-012024-12-310001804176country:US2023-01-012023-12-310001804176country:US2022-01-012022-12-310001804176us-gaap:NonUsMember2024-01-012024-12-310001804176us-gaap:NonUsMember2023-01-012023-12-310001804176us-gaap:NonUsMember2022-01-012022-12-3100018041762025-01-012024-12-3100018041762026-01-012024-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueInputsLevel1Member2024-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueInputsLevel3Member2024-12-310001804176us-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176bfly:PublicWarrantsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueInputsLevel1Member2023-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176bfly:PrivateWarrantsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176us-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001804176us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2023-12-310001804176us-gaap:LeaseholdImprovementsMember2024-12-310001804176us-gaap:LeaseholdImprovementsMember2023-12-310001804176us-gaap:MachineryAndEquipmentMember2024-12-310001804176us-gaap:MachineryAndEquipmentMember2023-12-310001804176us-gaap:FurnitureAndFixturesMember2024-12-310001804176us-gaap:FurnitureAndFixturesMember2023-12-310001804176us-gaap:ConstructionInProgressMember2024-12-310001804176us-gaap:ConstructionInProgressMember2023-12-310001804176us-gaap:OtherCapitalizedPropertyPlantAndEquipmentMember2024-12-310001804176us-gaap:OtherCapitalizedPropertyPlantAndEquipmentMember2023-12-310001804176us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2024-01-012024-12-310001804176us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2023-01-012023-12-310001804176us-gaap:SoftwareAndSoftwareDevelopmentCostsMember2022-01-012022-12-310001804176us-gaap:TechnologyBasedIntangibleAssetsMember2024-12-310001804176us-gaap:TechnologyBasedIntangibleAssetsMember2023-12-310001804176us-gaap:OperatingIncomeLossMember2023-01-012023-12-310001804176us-gaap:CommonClassBMember2024-01-012024-12-310001804176bfly:A2012PlanMember2024-12-310001804176bfly:A2020PlanMember2024-12-310001804176us-gaap:EmployeeStockOptionMembersrt:MaximumMember2024-01-012024-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2022-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2023-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2024-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001804176bfly:PerformanceBasedRestrictedStockUnitsMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2022-01-012022-12-310001804176bfly:MarketBasedRestrictedStockUnitsMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2024-01-012024-12-310001804176bfly:MarketBasedRestrictedStockUnitsMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2023-01-012023-12-310001804176us-gaap:EmployeeStockOptionMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2024-01-012024-12-310001804176us-gaap:EmployeeStockOptionMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2023-01-012023-12-310001804176us-gaap:EmployeeStockOptionMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2022-01-012022-12-310001804176us-gaap:ShareBasedPaymentArrangementEmployeeMemberus-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-01-012022-12-310001804176us-gaap:ShareBasedPaymentArrangementEmployeeMemberus-gaap:EmployeeStockOptionMembersrt:MaximumMember2022-01-012022-12-310001804176us-gaap:ShareBasedPaymentArrangementNonemployeeMember2024-01-012024-12-310001804176us-gaap:ShareBasedPaymentArrangementNonemployeeMember2023-01-012023-12-310001804176us-gaap:ShareBasedPaymentArrangementNonemployeeMember2022-01-012022-12-310001804176us-gaap:EmployeeStockOptionMember2022-12-302022-12-300001804176bfly:PerformanceBasedRestrictedStockUnitsMember2022-12-302022-12-300001804176bfly:ServiceBasedRestrictedStockUnitsMember2022-12-302022-12-300001804176us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001804176bfly:EmployeeStockPurchasePlan2024Member2024-06-300001804176bfly:EmployeeStockPurchasePlan2024Member2024-01-012024-12-310001804176us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-12-310001804176us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-12-310001804176us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-12-310001804176us-gaap:SellingAndMarketingExpenseMember2024-01-012024-12-310001804176us-gaap:SellingAndMarketingExpenseMember2023-01-012023-12-310001804176us-gaap:SellingAndMarketingExpenseMember2022-01-012022-12-310001804176us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-12-310001804176us-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-12-310001804176us-gaap:GeneralAndAdministrativeExpenseMember2022-01-012022-12-310001804176us-gaap:CommonClassAMember2023-01-012023-12-310001804176us-gaap:CommonClassBMember2023-01-012023-12-310001804176us-gaap:CommonClassAMember2022-01-012022-12-310001804176us-gaap:CommonClassBMember2022-01-012022-12-310001804176us-gaap:EmployeeStockOptionMember2024-01-012024-12-310001804176us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001804176us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001804176us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001804176us-gaap:EmployeeStockMember2024-01-012024-12-310001804176us-gaap:EmployeeStockMember2023-01-012023-12-310001804176us-gaap:EmployeeStockMember2022-01-012022-12-310001804176us-gaap:WarrantMember2024-01-012024-12-310001804176us-gaap:WarrantMember2023-01-012023-12-310001804176us-gaap:WarrantMember2022-01-012022-12-310001804176us-gaap:DomesticCountryMember2024-12-310001804176us-gaap:DomesticCountryMember2023-12-310001804176us-gaap:StateAndLocalJurisdictionMember2024-12-310001804176us-gaap:StateAndLocalJurisdictionMember2023-12-310001804176us-gaap:DomesticCountryMember2024-01-012024-12-310001804176us-gaap:StateAndLocalJurisdictionMember2024-01-012024-12-310001804176us-gaap:OtherNonoperatingIncomeExpenseMember2022-01-012022-12-310001804176us-gaap:OtherNonoperatingIncomeExpenseMember2024-01-012024-12-310001804176bfly:PublicWarrantsMember2024-12-310001804176bfly:PublicWarrantsMemberus-gaap:CommonClassAMember2024-12-310001804176bfly:PublicWarrantsMemberbfly:WarrantRedemption0.01Member2024-12-310001804176bfly:PublicWarrantsMemberbfly:WarrantRedemption0.01Member2024-01-012024-12-310001804176bfly:PublicWarrantsMemberbfly:WarrantRedemption0.01Memberus-gaap:CommonClassAMember2024-01-012024-12-310001804176bfly:PublicWarrantsMemberbfly:WarrantRedemption0.10Member2024-12-310001804176bfly:PublicWarrantsMemberbfly:WarrantRedemption0.10Member2024-01-012024-12-310001804176bfly:PublicWarrantsMemberbfly:WarrantRedemption0.10Memberus-gaap:CommonClassAMember2024-01-012024-12-310001804176bfly:PrivateWarrantsMember2024-12-310001804176bfly:PrivateWarrantsMember2024-01-012024-12-310001804176bfly:PrivateWarrantsMemberbfly:WarrantRedemption0.01Member2024-12-310001804176bfly:PrivateWarrantsMemberbfly:WarrantRedemption0.10Member2024-12-3100018041762023-07-012023-09-300001804176us-gaap:InventoriesMembersrt:MinimumMember2024-01-012024-12-310001804176us-gaap:InventoriesMember2024-12-310001804176us-gaap:InventoriesMember2023-01-012023-12-310001804176us-gaap:CommonClassAMemberus-gaap:SubsequentEventMember2025-01-292025-01-290001804176us-gaap:CommonClassAMemberus-gaap:SubsequentEventMember2025-01-290001804176us-gaap:SubsequentEventMember2025-01-310001804176us-gaap:SubsequentEventMember2025-01-292025-01-29
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2024
or
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____ to _____
Commission file number: 001-39292
Butterfly Network, Inc.
(Exact name of registrant as specified in its charter)
| | | | | |
| Delaware | 84-4618156 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
1600 District Avenue
Burlington, Massachusetts 01803
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (781) 557-4800
Securities registered pursuant to Section 12(b) of the Exchange Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Class A common stock, $0.0001 par value per share | | BFLY | | The New York Stock Exchange |
Warrants to purchase one share of Class A common stock, each at an exercise price of $11.50 per share | | BFLY WS | | The New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | |
| Large accelerated filer | o | Accelerated filer | o |
| | | |
| Non-accelerated filer | x | Smaller reporting company | x |
| | | |
| | | Emerging growth company | o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No x
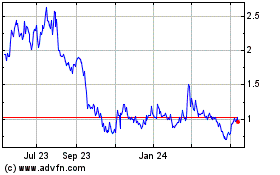
The aggregate market value of the registrant’s voting and non-voting equity held by non-affiliates of the registrant (without admitting that any person whose securities are not included in such calculation is an affiliate) computed by reference to the price at which the Class A common stock were last sold as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $133.7 million.
As of February 13, 2025, the registrant had 216,496,214 shares of Class A common stock outstanding and 26,426,937 shares of Class B common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement for its 2025 Annual Meeting of Stockholders (the “Proxy Statement”), to be filed within 120 days of the Registrant’s fiscal year ended December 31, 2024, are incorporated by reference in Part III of this Annual Report on Form 10-K. Except with respect to information specifically incorporated by reference in this Form 10-K, the Proxy Statement is not deemed to be filed as part of this Annual Report on Form 10-K.
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that relate to future events or our future financial performance regarding, among other things, our plans, strategies, and prospects, both business and financial. These statements are based on the beliefs and assumptions of our management team. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events, or results of operations, are forward-looking statements. Forward-looking statements contained in this Annual Report on Form 10-K include, but are not limited to, statements about:
•the success, cost, and timing of our product development activities, including the development of additional potential products;
•the potential attributes and benefits of our products and services;
•our ability to obtain and maintain regulatory authorization for our products, and any related restrictions and limitations on the use of any authorized product;
•our ability to identify, in-license, or acquire additional technology;
•our ability to maintain our existing license, manufacturing, and supply agreements;
•our ability to compete with other companies currently marketing or engaged in the development of ultrasound imaging devices, many of which have greater financial and marketing resources than us;
•the size and growth potential of the markets for our products and services, and the ability of each to serve those markets, either alone or in partnership with others;
•our estimates regarding expenses, revenue, capital requirements, and needs for additional financing; and
•our financial performance.
These statements may be preceded by, followed by, or include the words “believes,” “estimates,” “expects,” “projects,” “forecasts,” “may,” “will,” “should,” “seeks,” “plans,” “scheduled,” “anticipates,” or “intends” or similar expressions or phrases, or the negative of those expressions or phrases. The forward-looking statements are based on projections prepared by, and are the responsibility of, our management. Although we believe that our plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, we cannot assure you that we will achieve or realize these plans, intentions, or expectations. Forward-looking statements are inherently subject to risks, uncertainties, and assumptions relating to, among other things:
•our growth depends on our ability to attract and retain customers;
•our business could be harmed if we fail to manage our growth effectively;
•our current expectations and assumptions are subject to risks, assumptions, estimates, and uncertainties;
•our business is subject to a variety of U.S. and foreign laws, which are subject to change and could adversely affect our business;
•the pricing of our products and services and reimbursement for medical procedures conducted using our products and services;
•changes in applicable laws or regulations;
•our ability to protect or enforce our intellectual property rights;
•the ability to maintain the listing of our Class A common stock on the New York Stock Exchange; and
•economic downturns and political and market conditions beyond our control.
These and other factors that could cause actual results to differ from those implied by the forward-looking statements in this Annual Report on Form 10-K are more fully described in Item 1A under the heading “Risk Factors.” The risks described under the heading “Risk Factors” are not exhaustive. Other sections of this Annual Report on Form 10-K, such as the description of our Business set forth in Item 1 and our Management’s Discussion and Analysis of Financial Condition and Results of Operations set forth in Item 7, describe additional factors that could adversely affect our business, financial condition, or results of operations. New risk factors emerge from time to time, and it is not possible to predict all such risk factors, nor can we assess the impact of all such risk factors on our business or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements, which speak only as of the date hereof. All forward-looking statements attributable to the Company or persons acting on the Company’s behalf are expressly qualified in their entirety by the foregoing cautionary statements. We undertake no obligations to update or revise publicly any forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law.
SUMMARY OF RISK FACTORS
Our business is subject to numerous risks and uncertainties, including those further described below in Part I, Item 1A “Risk Factors” in this Annual Report on Form 10-K, that represent challenges that we face in connection with the successful implementation of our strategy and the growth of our business. In particular, the following considerations, among others, may offset our competitive strengths or have a negative effect on our business strategy, which could materially adversely affect our business, financial conditions, results of operations, future growth prospects or cause a decline in the price of our common stock:
•We have a limited operating history on which to assess the prospects for our business, we have generated limited revenue from sales of our products, and we have incurred losses since inception. We anticipate that we will continue to incur significant losses for at least the next several years as we continue to commercialize our existing products and services and seek to develop and commercialize new products and services.
•Our success depends upon market acceptance of our products and services, our ability to develop and commercialize existing and new products and services and generate revenues, and our ability to identify new markets for our technology.
•Medical device development is costly and involves continual technological change, which may render our current or future products obsolete.
•We will be dependent upon the success of our sales and customer acquisition and retention strategies.
•Our research and development efforts may not succeed in developing commercially successful products and technologies, which could adversely affect our business.
•We rely on limited or sole suppliers for some of the materials and components used in our products, and we may not be able to find replacements or immediately transition to alternative suppliers, which could have a material adverse effect on our business, financial condition, results of operations and reputation.
•If we are unable to continue the development of an adequate sales and marketing organization and/or if our direct sales organization is not successful, we may have difficulty achieving market awareness and selling our products in the future.
•We operate in highly competitive markets, competition may increase in the future, and our industry may be further disrupted.
•We are subject to extensive government regulation, which could restrict the development, marketing, sale and distribution of our products and could cause us to incur significant costs.
•We are subject to complex and evolving U.S. and foreign laws and regulations regarding privacy, data protection, and other matters. Many of these laws and regulations are subject to change and uncertain interpretation, and could result in claims, changes to our business practices, monetary penalties, increased cost of operations, or declines in customer growth or engagement, or otherwise harm our business.
•Increased cybersecurity requirements, vulnerabilities, threats, and more sophisticated and targeted computer crimes pose a risk to our systems, networks, products, solutions, services, and data, as well as our reputation, which could adversely affect our business
•If we are unable to protect our intellectual property, our ability to maintain any technological or competitive advantage over our competitors and potential competitors would be adversely impacted, and our business may be harmed.
•We may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our current or future products, and we cannot provide any assurances that we would be able to obtain such licenses.
•The Company’s outstanding warrants became exercisable for the Company’s Class A common stock on May 26, 2021. If the Company’s stock price reaches or exceeds $11.50, and outstanding warrants are exercised, the number of shares eligible for future resale in the public market will increase and result in dilution to our stockholders.
•If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, our ability to operate our business and investors’ views of us.
•We face the risk of product liability claims and may be subject to damages, fines, penalties and injunctions, among other things.
•We are currently subject to a securities class action lawsuit and stockholder derivative actions, the unfavorable outcome of which may have a material adverse effect on our financial condition, results of operations and cash flows.
These and other material risks we face are described more fully in Item 1A, Risk Factors, which investors should carefully review prior to making an investment decision with respect to the Company or its securities.
PART I
All brand names or trademarks appearing in this report are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this report is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners. Unless the context requires otherwise, references in this report to the “Company,” “we,” “us,” and “our” refer to Butterfly Network, Inc. and its wholly-owned subsidiaries.
Item 1. BUSINESS
Overview
We are an innovative digital health business transforming care through a unique combination of portable, semiconductor-based ultrasound technology, intuitive software, services and educational offerings that can make medical imaging more accessible than ever before. Due to its one-of-a-kind Ultrasound-on-Chip™ technology, Butterfly’s solution enables the practical application of ultrasound information into the clinical workflow through affordable hardware that fits in a healthcare professional’s pocket and is paired with cloud-connected software that is easily accessed through a mobile application.
While Butterfly is in the point-of-care ultrasound (“POCUS”) category, our technology is fundamentally different from incumbent POCUS devices, which we view as mere extensions of cart-based hospital workflows. In contrast, Butterfly is designed for true mobility, not just because it is a single, portable imaging device suitable for any doctor, nurse, and eventually, qualified chronic care patient, but also because it is powered by AI-driven tools, cloud connectivity, and seamless hospital integration. This combination of advanced, cloud-connected software and portable hardware is the key to mobility, allowing Butterfly to support large health systems while also functioning independently of them in remote or resource-limited settings. Wherever a doctor, nurse, or patient braves to go, Butterfly delivers imaging that adapts to their environment and remains securely connected.
With this proprietary, comprehensive portable ultrasound solution, that is protected by a robust intellectual property portfolio, we are on a mission to democratize healthcare by increasing access and use of ultrasound information wherever care is being delivered – whether a large healthcare system, a rural clinic, a global conflict zone or beyond. We are helping streamline and optimize deployment of ultrasound at scale across hospital systems with our Compass™ software that integrates into health system infrastructures, and connects across all departments and specialties. Furthermore, we envision a future where Butterfly’s imaging technology is fully integrated into hospital-at-home workflows, improving remote monitoring and management of patients’ health conditions from the comfort of their homes.
We market and sell the Butterfly solution to healthcare systems, as well as to physicians and healthcare providers through a direct sales force, distributors, and our eCommerce channel. We generated total revenue of $82.1 million and $65.9 million in the years ended December 31, 2024 and 2023, respectively. We also incurred net losses of $72.5 million and $133.7 million for the years ended December 31, 2024 and 2023, respectively.
We employ approximately 190 employees as of January 31, 2025 and sell our products in over 30 countries through our sales force and independent distributors and directly to physicians through our eCommerce channel. Outside of our core commercial geographies, Butterfly iQ+ is also being utilized in over 70 low resource settings around the world through global health partnerships. Butterfly iQ3 is currently FDA-cleared for sale in the United States and CE marked in Europe.
Corporate History and Information
The Company, formerly known as Longview Acquisition Corp. (“Longview”), was incorporated in Delaware in 2020 as a blank check company formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. Longview and Butterfly Network, Inc. (“Legacy Butterfly”), which was founded in 2011, completed a business combination (the “Business Combination”) on February 12, 2021, following which Longview was renamed Butterfly Network, Inc., and the business of Legacy Butterfly became our business.
Since our founding, Butterfly and our disruptive technology has been recognized by Prix Galien USA, Fierce50, TIME’s Best Inventions, Fast Company’s World Changing Ideas, CNBC Disruptor 50, and MedTech Breakthrough Awards, among other accolades.
We have wholly-owned subsidiaries organized in Australia, Germany, the Netherlands, the United Kingdom and Taiwan. Our principal executive offices are located at 1600 District Avenue, Burlington, Massachusetts 01803 and our telephone number is (781) 557-4800.
The Evolution of Ultrasound
Digital health is systematically changing the way healthcare practitioners deliver care by providing information that informs better decision-making, while increasing access and significantly reducing patient-care costs. Butterfly devices are designed for this new wave of medical care with an easy-to-use interface that displays ultrasound information on your smartphone or tablet in real-time.
Historically, the global ultrasound market has been dominated by traditional cart-based devices. These devices are accessible only to highly specialized, highly trained technicians and are located predominantly in hospitals, imaging centers, and physicians’ offices. Many healthcare institutions throughout the world lack the facilities and capital necessary to acquire and maintain expensive cart-based devices and cannot afford the highly trained individuals required to operate them.
Traditional cart-based equipment typically ranges from $30,000 to $120,000 or more per new device, plus specialized labor. More recently, we have seen the introduction of POCUS and handheld devices with an average price point of $10,000, based on $3,000 to $7,000 per probe, with some requiring three or more probes to cover a comparable range of cleared indications to the single Butterfly probe, and those devices often require an upfront software investment for access to advanced imaging modes (e.g. pulsed-wave Doppler) and workflow (e.g. cloud storage) that can reach upwards of $2,000. Further, these incumbent POCUS devices operate off the same 60-year-old, analog piezoelectric crystal technology as traditional cart-based ultrasound, which we believe limits the opportunity for future progress. Further, most piezoelectric crystals that are utilized in these incumbent ultrasound devices contain lead at levels deemed hazardous by regulators. In fact, the lead included in many traditional piezoelectric crystal ultrasound systems exceeds the allowable amount of lead under the EU’s Restrictions of Hazardous Substances Directive (“RoHS”), requiring many of our competitors to rely upon an exemption to RoHS specifically permitting lead in single crystal piezoelectric materials for ultrasound transducers in order to commercialize their products in the EU. In contrast, Butterfly’s Ultrasound-on-Chip™ technology allows us to offer much more versatile imaging capabilities on just one affordable probe without exceeding these limitations.
We believe the transition from traditional piezoelectric crystal sensors to semiconductor chip in ultrasound also gives us a competitive advantage because it allows our devices to benefit from Moore’s Law. Moore’s Law is a guiding principle of the semiconductor industry that states that the number of transistors on an integrated circuit will double every two years – meaning the computer processing power of a chip doubles every two years with minimal rise in cost. Much like the photography industry, when the digital camera’s image quality became equal to that of analog cameras, digital took over because it brought many other benefits – such as affordability, miniaturization, and advanced features for ease of use. Butterfly is on a path to driving that same transformation for the ultrasound industry toward more versatile, smaller, and consumer-friendly form factors.
Today, regulations still require a trained healthcare practitioner to operate our devices, but we are developing a technology roadmap to make it easier for users of all skill levels to use the device. We are focused on increasing the use of imaging during preventive care with the aim of providing the right information earlier in the care process for better, more rapid clinical decision making. As we continue to educate and empower ease-of-use, we believe that adoption of ultrasound information as a clinical assessment tool will grow and, in time, we will change the paradigm of care delivery. We believe that this information delivered through imaging with an intuitive user interface will further drive costs down and expand the use of imaging at clinical point-of-care.
Changing standards of care doesn't happen overnight, but our goal is to make this change happen much faster. We believe AI integration will be one key to accelerating the transition to handheld ultrasound, as it enables ease of use and automation, thereby removing barriers to adoption. To accelerate this process, we maintain an AI partnership ecosystem, Butterfly Garden™, that opens our software development kit (“SDK”) so third-party companies and developers can build and deploy new AI applications on our imaging platform, reaching our large installed base. For Butterfly, this model allows us to continue to broaden our base. As each of these companies develop on our platform, we believe we will have more of their customers buying Butterfly, while existing Butterfly customers will have access to even more capabilities.
We are also committed to accelerating adoption of POCUS through clinical education, not only by empowering the next generation of medical professionals with ultrasound skills and clinical integration knowledge via probe and software sale into medical schools for use in curriculum, but through our innovative collection of educational services and offerings.
Market Opportunity
We are on a journey to address a potential new market that we estimate exceeds $100 billion. We believe our solution addresses an unmet need across an addressable market of over 40 million healthcare practitioners, including approximately 10 million medical doctors, 30 million nurses and midwives, and 2 million veterinarians and veterinary technicians worldwide. Ultimately, our north star is to reach patients in alternative and home care settings, potentially with future, differentiated form factors intended to enable ease of use in the home, subject to receipt of required marketing authorizations.
In the near term, we are first driving adoption with healthcare practitioners, including doctors and nurses in healthcare systems and a focused group of initial customers in the veterinary market, comprised of companion animal, mixed animal, equine veterinarians, and veterinary academic institutions. Our newest Butterfly iQ3 device has demonstrated impressive uptake since its release in February 2024, driving further penetration into the market, with best-in-class image quality that has brought more ultrasound users over to Butterfly. As we look ahead, we will leverage the quality of Butterfly iQ3 and benefits of our Compass™ enterprise software to continue our focus on driving and expanding further into the hospital segment.
With our advanced digital capabilities made possible by our proprietary Ultrasound-on-Chip™ technology, we believe we can not only address this market, but move beyond the restrictions of the existing ultrasound market. Our affordable holistic solution provides valuable clinical information and workflow efficiency that is attractive to any healthcare systems that seek to improve care at lower cost. These attributes also may allow the use of our Butterfly devices beyond traditional health system environments to where health systems look to evolve, such as the home.
The advantages of our Ultrasound-on-Chip™ technology align with recent industry trends, including the shift to outside-the-hospital or in-home medical care, affordability, harnessing of AI and deep learning, collaboration through the cloud, disruptive medical innovation, and increasing access to care. In addition, by expanding the settings in which medical imaging can be done, the Butterfly device may provide opportunities for earlier detection and prevention of disease, while reducing cost. This aligns with the focus on consumer health empowerment, wellness, and acceleration of value-based care, all of which are important themes in the healthcare industry today.
Business Strategy
We believe that, with our current products and solutions, we have created a new standard for medical imaging, and we are focused on staying at the leading edge of technical innovation. We believe our current portfolio is only the first step in our development and we plan to continually improve it and expand our product and service offerings. We have a strong leadership team with disruptive healthcare and commercial expertise that energized and reoriented the organization in 2024. Under this leadership, we have established a focused path by:
•Nurturing and growing our core capabilities and clinical pathways.
•Capturing new and adjacent markets.
•Leveraging our Ultrasound-on-Chip™ technology into non-competitive markets through partnerships.
•Optimizing our resources.
As we execute on these strategies, Butterfly has become a more efficient business while simultaneously investing in long-term growth and innovation. Importantly, our expense reduction and cash preservation activities were paired with a continued commitment to increased productivity and efficiency. Because Butterfly devices are mobile and easy-to-use, healthcare practitioners can have access to ultrasound information outside of traditional settings. We continue to focus on the utility of our devices in global health contexts, including for building sustainable healthcare models in the developing world and for responding to natural and man-made humanitarian crises. For example, thousands of Butterfly devices have been distributed throughout the Ukraine, Gaza, and Israel via over 40 global health partnerships to support combat medics on the frontlines. We also remain committed to developing patient-focused delivery models, and we believe ultrasound imaging may find a market in home care settings with at-home medical personnel. These modalities have the potential to improve health outcomes, while avoiding expensive treatments, therefore generating economic value for both the patient
and payor, which is aligned with the healthcare mega-trend of value-based care. In March 2024, we introduced our Butterfly HomeCare Services Business, which strives to support caregivers with patient management outside the hospital for certain chronic conditions. In January 2025, Butterfly initiated its first pilot program for virtual chronic care management services with a major at-risk Medicare Advantage provider in the United States. The goal is to reduce readmissions of their congestive heart failure patient population through frequent AI-powered imaging. If successful, it will be our first foray into a services model.
Future phases of the HomeCare Services Business envision use of wearable devices and patient-performed scanning – all subject to appropriate regulatory approvals and authorizations. As our future devices one day reach new markets and enable more direct interaction with patients (e.g., remote patient monitoring), we believe this trend can accelerate, further improving outcomes and reducing costs. This reduction of costs has the potential to create economic value for the whole healthcare system across clinical applications and markets where ultrasound scanning is used. We will work diligently to validate target at-home applications through focused clinical trials and will seek additional regulatory authorizations, as needed.
Products
Our current product portfolio includes a combination of hardware and software, including Butterfly iQ3, Butterfly iQ+, Butterfly iQ+ Bladder, and Butterfly iQ+ Vet devices, software subscriptions, and professional services. We offer cloud-based software solutions to healthcare systems, teleguidance, in-app educational tutorials as well as our ScanLab™ education-only app, formal education programs through our Butterfly Academy™ software and Butterfly Certified™ courses, as well as professional services for large scale deployments.
Butterfly iQ+ and iQ3
In 2018, Legacy Butterfly commercially launched Butterfly iQ, the world’s first handheld, single-probe, whole-body ultrasound system using semiconductor technology. The company has continued to innovate, leveraging the benefits of Moore’s Law, to launch its second generation Butterfly iQ+ in 2020 and third generation iQ3 in 2024 – each with increased processing power and performance enhancements. We have over 145,000 unique Butterfly users to date.
Butterfly has two portable ultrasound devices on the market: our second-generation Butterfly iQ+ and third-generation Butterfly iQ3. Butterfly iQ+/iQ3 are both powered by our Ultrasound-on-Chip™ technology, allowing them to power whole-body imaging on a single handheld probe using digital semiconductor technology. Both of these small, handheld devices are priced competitively compared to incumbent, piezoelectric crystal-based ultrasound handhelds and carts. Butterfly iQ+ is our most affordable whole-body scanner offered, listed at approximately $2,700. Butterfly iQ3 – powered by our most advanced P4.3 chip which has double the processing power for best-in-class image quality, new advanced 3D imaging tools for easier use, and a smaller, more ergonomic design – is valued higher at approximately $3,900 per device, but still remains one of the lowest-cost handheld ultrasound devices on the market. Both of our devices are incredibly versatile, allowing providers to access over 20 anatomical presets, 6 imaging modes, and a suite of AI and other calculation tools via Butterfly’s simple mobile application interface, the availability of which are often dependent upon local marketing clearances and therefore availability may differ by country. Our software is designed to make the product easy to use and fully integrated with the clinical workflow, accessible on a user’s smartphone, tablet, and almost any hospital computer system connected to the Internet.
Our Butterfly iQ+/iQ3 devices connect directly to a compatible iPhone or Android smartphone or tablet to provide their imaging and software features for approximately two consecutive hours, according to average use as determined from field data analytics. Under normal conditions, the Butterfly iQ3 charges to full battery in two hours, and the Butterfly iQ+ in approximately five hours. The devices have over 20 ready-to-use anatomical presets generated in part with AI that are designed to optimize images obtained from scanning different areas of the body. Within the Butterfly application, users can utilize up to six imaging modes, including B-Mode, Color Doppler, M-Mode, Power Doppler, Pulsed-Wave Doppler, and Biplane Imaging™, as well as advanced tools iQ Slice™ and iQ Fan™.
In addition, advanced measuring tools can be used for a variety of specialties, including nursing and obstetrics. These features allow healthcare practitioners to perform surface area and volume measurements on the anatomical objects that are imaged and can use color Doppler to identify movement of fluid, similar to features provided by legacy products in the market.
•For obstetric clinicians, our devices’ tools can perform gestational age and amniotic fluid index calculations.
•The devices’ tools can provide automated bladder volume calculations with 3D visualizations, enable easier line placements using NeedleViz™ technology and Biplane Imaging™, or produce a B-line count (an indicator of wetness in the lungs) from just a six second ultrasound clip using the Auto B-line Counter.
•Using TeleGuidance™, healthcare practitioners can perform ultrasound remotely, providing real-time guidance by connecting with a novice user or peer directly from the Butterfly app. Through our TeleGuidance™ feature, healthcare practitioners can control the settings of the application while the device is in use and help the user identify the image.
•On the Butterfly iQ3, users can also access new automated image capture modes: iQ Slice™ and iQ Fan™. iQ Slice™ automatically steers the beam to scan an organ and capture up to 46 ultrasound slices at a time across a wide angle. iQ Fan™ is a dedicated lung tool that further builds on the core iQ Slice technology to allow providers to benefit from real-time, back-and-forth virtual fanning, making it easier to visualize A-lines and other lung conditions.
We believe these pre-set settings and intuitive operation features through smartphones will enable healthcare practitioners to adopt our devices, expanding our user base beyond the traditional ultrasound user base. This traditional base of ultrasound users has been limited because existing ultrasound devices often require unique environments and extensive training to operate, while the Butterfly devices were designed to be used by general and other healthcare practitioners across the healthcare industry.
Butterfly devices consist of both durable hardware and dynamic software solutions designed to make ultrasound imaging accessible to all healthcare practitioners, including nurses. We also sell accessories for our devices including cases, adaptors, and carts.
Software Subscriptions
We believe that the software and analytics capabilities of our solution, coupled with the Butterfly devices, empower smarter and expanded scanning, quality assurance, credentialing, documentation, and billing that can generate incremental revenue for both healthcare systems and independent practitioners but also reduce costs for payers from earlier detection and prevention of adverse downstream events due to suboptimal care decisions or treatment complications.
We currently offer different software membership plans, including Core Technology, our base software membership for individual users that is priced at approximately $300 per year, and Advanced Technology, our complete ultrasound solution for individual users that is priced at approximately $420 per year. In addition, we offer other membership plans that are specific to customer needs, including iQ+ Care for bladder scanner and vascular access application solutions, integrated software enterprise solutions to enable ultrasound deployments at scale, and medical education subscriptions for universities.
Through our software subscription options, users can upload scanned images to our HIPAA-compliant cloud, which has unlimited storage and links to electronic medical records (“EMRs”) on hospital and office systems, allowing for seamless transfer of images that can also be accessed from a desktop computer.
Butterfly iQ+ Bladder
In May 2024, we introduced our first specialty product, iQ+ Bladder, in the United States. With iQ+ Bladder, we expand outside of our core point-of-care ultrasound market to better serve the bladder scanning market with our proprietary Ultrasound-on-Chip™ technology. The bundled bladder solution includes an iQ+ Bladder probe, streamlined software, compact rolling cart, tablet, and power splitter, paired with premium assembly.
The rolling cart features storage space and a probe charging dock for enhanced convenience and readiness, and a large tablet is mounted to the cart with tilted brackets that rotate 360° and pan 180° for optimum viewing and positioning. The probe is paired with streamlined software, designed to address the specific needs of nurses and other providers who regularly conduct bladder scans. The application has a user-friendly interface, with no login or configurations necessary, allowing for immediate access to quick and intuitive scans. To empower confident, accurate measurement, the solution offers walkthrough guidance for proper probe placement, and it also leverages Butterfly’s advanced bladder visualization capabilities, including 3D rendering to confirm true bladder anatomy.
Butterfly for Enterprises
In 2022, we introduced an updated system-wide platform designed to support the scaled integration and deployment of our ultrasound hardware and Compass™ software across hospitals and health systems to empower image-informed clinical decisions from the bedside and encounter-based workflow. Leveraging Butterfly’s unique combination of a whole-body handheld ultrasound device, software, and services, our enterprise platform supports hospitals and health systems with a complete ultrasound solution. This system-wide offering promotes improved patient care via accessible imaging across multiple disciplines and care settings. Our device-agnostic software securely integrates into health systems’ clinical and administrative systems and workflows (e.g., Picture Archiving and Communication System (“PACS”) and EMR), including with non-Butterfly devices.
The Butterfly enterprise platform is built for organizations, including hospital systems, medical schools and residency programs. With this platform, institutions can rapidly and easily access ultrasound-enabled insights and oversight of their entire ultrasound program on one streamlined software solution. Benefits include, but are not limited to:
•Streamlined documentation & QA workflows,
•Increased billable exams,
•Centralized governance and program management,
•Cloud storage access,
•Ensured interoperability, and
•Education, proficiency, and credentialing management.
Educational Tools
In January 2024, ScanLab™ went live in the Apple App Store. Our AI-powered, educational ScanLab™ app provides written walkthroughs and reference imagery to guide real-time educational scanning. Enhancing the learning process are AI image quality indicators that provide real-time feedback for image adjustment and interactive AI labeling to help learners locate key anatomy.
Our platform features education tools to enable users to quickly gain proficiency in conducting exams, including hundreds of educational videos taught by experts. In 2023, we launched Butterfly Certified™, a complete set of virtual and in-person POCUS courses designed to provide practitioners with the skillsets necessary to meet local training or privileging requirements, delivered in collaboration with the Global Ultrasound Institute (“GUSI”). The hands-on training packages, which are expert-led and include tailored tracks across specialties that can be scaled for individual or department use, are available in the United States through direct Butterfly sales representatives. In 2021, we launched Butterfly Academy™, which provides embedded education and training to enable clinicians across care settings, to support long-term scaling of Butterfly throughout a healthcare system and for use in medical education applications.
Butterfly iQ+ Vet
In 2021, we launched Butterfly iQ+ Vet, a handheld ultrasound system that leverages the technology developed for our other iQ devices and is designed to bring value to veterinarians in a variety of care settings, helping to usher in a new standard for veterinary medicine.
As of December 31, 2024, iQ+ Vet is available in approximately 20 international markets, bringing first-of-its-kind innovation to veterinarians. The product includes a specially designed animal-specific probe for ease of use and maneuvering, Color-Doppler, and NeedleViz. We are changing the way that veterinarians deliver care, providing more information through imaging at the point-of-care, particularly since their patients do not speak.
When Butterfly launched its Auto B-Line Counter for human care in 2023, our Vet business saw an opportunity to use it in production cattle. There are one million cattle producers in the U.S. alone. They often prophylactically give their cattle expensive antibiotics to control disease spread and protect their herds. Our device has now been validated through publications affirming its utility in earlier detection of bovine respiratory disease to reduce antibiotic use – which could ultimately save time and money and has implications for supporting more sustainable upkeep of our cattle food supply.
Marketing and Sales
We market our products worldwide, in the U.S. through our targeted sales organization and internationally through both our direct sales force and our distributors. In the U.S., our sales organization is engaged in sales efforts and promotional activities primarily to healthcare institutions through direct sales and distributor partnerships. In the United States, Butterfly has been purchased by a clinician in most of the 100 largest healthcare systems. We use a variety of marketing tools to drive adoption, foster continued usage, and establish brand loyalty for our devices and software. We recognize the importance of the role of education in accelerating adoption of our products by those medical professionals without existing ultrasound skills.
We sell through three main channels:
•A targeted, regional, direct sales force focused on large healthcare system-wide implementations.
•An eCommerce website through which we sell our Butterfly devices to healthcare practitioners and veterinarians in these geographies, where allowed by local law.
•Distributor, veterinary, and affiliate relationships to unlock additional channels to supplement our direct and eCommerce sales.
Because healthcare institutions often make decisions to purchase on a system-wide level, we believe enterprise sales can generate economies of scale with larger volumes and larger numbers of users, while also increasing user retention. The health system channel also yields more comprehensive software subscriptions, which further increases our revenue from devices and subscriptions sold. We are working towards increasingly integrated solutions to maximize our value to large healthcare customers, as well as continuing to improve our sales and support infrastructure. Our ability to connect and integrate with traditional third-party ultrasound systems gives enterprise customers a solution to the governance and workflow challenges that may have previously limited the utilization and billing of point of care imaging devices. Health system customers deploying our solution can benefit from a streamlined clinical workflow that reduces the exam documentation burden typically associated with traditional ultrasound systems. By adopting Butterfly’s enterprise solution, customers can responsibly manage and optimize value from their fleets of point of care imaging devices.
Our international sales organization is focused on expanding access to innovative ultrasound technology through a strategic mix of direct sales, distribution partnerships, and e-commerce. We continue to refine our global go-to-market approach by optimizing our distribution network, investing in partner training, and ensuring regulatory compliance in key markets. Our international growth strategy prioritizes market expansion, healthcare provider education, and commercial execution, driving increased adoption across diverse healthcare ecosystems worldwide. We sell directly in Germany and the United Kingdom, and, through our distribution partnerships and e-commerce platform, our commercial footprint extends to approximately 40 countries.
We continue to develop our sales and marketing organization, which consists of a dedicated sales team, sales operations and sales support personnel that are complemented by a marketing team. As of January 31, 2025, we had approximately 60 people employed globally in sales, sales support, and marketing.
Geographic Areas
Butterfly is being used in over 100 countries. Outside of our core commercial geographies, Butterfly is also being utilized through collaborations with non-governmental organizations (“NGOs”) like the Gates Foundation to deliver our technology to underserved communities. Currently, we have placed our device with hundreds of NGOs, entities, and healthcare professionals that align with our mission to deliver care around the world and bring potentially lifesaving medical imaging to patients, often for the first time.
POCUS capabilities have been commercially available for decades, yet adoption in low-and-middle income countries is minimal. Butterfly’s global health program seeks to upend that paradigm by leveraging our technology to democratize medical imaging for marginalized and vulnerable populations around the world. In 2024, we completed the second phase of the world’s largest rapid POCUS deployment to sub-Saharan Africa, funded by a $5 million grant from the Gates Foundation. The initiative has brought 1,000 Butterfly iQ+ probes and ultrasound training to a region of the world that has
disproportionately high rates of maternal mortality. Preliminary findings from the first phase of deployment in Kenya demonstrate, among other findings, that:
•95% of the participant providers in Kenya are now using Butterfly to detect high-risk conditions and inform treatment decisions.
•80% of the participant providers have trained at least 2 other providers at their hospital to drive further wide scale use.
•80% of surveyed mothers reported experiencing happiness upon seeing their baby on screen.
We see our work in the areas of maternal and fetal health as the building blocks for continued impact toward better clinical assessment overall, and we believe our model is applicable to many more geographies and specialties. We anticipate leveraging our work in sub-Saharan Africa to continue improving access to imaging in other limited resource settings, and we aim to further expand our international customer base in the future.
In terms of geographic markets, for the fiscal year ended December 31, 2024, a substantial majority of our revenues were derived from sales to customers based in the United States. We believe our differentiated Butterfly handheld device and our growing user base of Butterfly practitioners, with sales to or agreements with most of the largest 100 U.S. healthcare systems and devices deployed across approximately 100 countries, position us well to compete in the existing ultrasound market and to potentially expand into emerging markets.
Research and Development
We plan to develop future applications, subject to appropriate marketing authorization, to leverage our unique hardware foundation and commitment to improving our software using AI. Simultaneously, we plan to enhance our software capabilities, pursuing regulatory authorizations as necessary, with new features to support clinical procedures. We also plan to further enhance workflow automations for our Compass™ software, in order to more deeply integrate our platform with healthcare systems, as we work with these customers to deploy Butterfly in their organizations.
In this way, we expect our solution will continue to innovate naturally, as well as through our enhancements to our proprietary technology. In order to pave the way for future phases of our HomeCare Services Business, we anticipate we will need to validate the at-home applications through focused clinical trials and also seek additional regulatory authorizations.
We believe these hardware developments, along with our software enhancements and user education initiatives, will bring ultrasound to even more healthcare systems and healthcare practitioners. We believe that with our differentiated and continually expanding solution, we have the potential to drive user adoption and change clinical behavior.
Beyond these hardware and software product roadmaps, we plan to develop new innovative products, services, and software applications in partnership with healthcare systems, leveraging our core technology and platform capabilities. Through this product development, we believe we will be positioned to remain on the forefront of medical imaging with a continued focus on both enabling access to more information at low cost and reduced effort and allowing us to enable healthcare practitioners to transform care with Butterfly through our education offerings, an intuitive interface, and AI that unlock the power of point-of-care information quickly and confidently.
Reimbursement
While we do not bill health plans directly, practitioners can leverage pre-existing, routine Current Procedural Terminology® codes (“CPT codes”) that enable them to obtain per-scan reimbursement in the specialties of anesthesiology, cardiology, critical care, emergency medicine, endocrinology, and ultrasound-guided procedures.
Competition
Several large companies currently constitute the bulk of ultrasound sales. High regulatory, distribution, manufacturing, and service-related long-term contractual costs represent significant barriers to entry for any new player. We expect that the existing market participants will remain strong active players in the future.
As a general matter, we view competition on two levels:
•Conventional ultrasound systems; and
•The development of other handheld ultrasound systems with the same or better attributes.
The primary competition comes from established market participants offering conventional ultrasound systems. While Butterfly's target is often non-traditional ultrasound users, we do compete with both traditional ultrasound manufacturers and other handheld ultrasound systems. However, Butterfly’s semiconductor technology differentiates us from our competitors in many ways, including the fact that our probes do not rely upon lead-based piezoelectric crystals that many of our competitors have relied upon since inception. Our semiconductor technology similarly enables a more versatile probe capable of performing whole-body scans, which is generally not possible with handheld ultrasound systems that rely upon piezoelectric crystals. Furthermore, Butterfly’s lack of reliance upon lead piezoelectric crystals allows us to remain RoHS-compliant in the EU without the use of the exemption from RoHS requirements for lead in single crystal piezoelectric materials in ultrasound transducers — an exemption that is not guaranteed to continue in perpetuity and that, as of October 2024, Butterfly has formally submitted a request to revoke to the European Commission.
Human Capital Resources
Our employees embody our mission to democratize healthcare and to make medical imaging accessible to everyone around the world by using our proprietary technology. We are committed to growing and cultivating an environment that values the diverse perspectives, backgrounds, experiences, and geographies of our employees and other stakeholders. We believe that our people are the reason for our success, and we have organized ourselves to maximize productivity and performance. We maintain a high bar for talent and actively work to build diversity within our workforce.
Demographics. As of January 31, 2025, we had approximately 190 employees. As of January 31, 2025, approximately 165 of our employees were located in the United States and approximately 25 of our employees were located outside the United States. None of our employees are represented by a labor union or are subject to a collective bargaining agreement. We supplement our employee population with independent contractors, contingent workers and temporary workforce support as needed. In July 2024, we entered into an agreement with a third-party global technology and business transformation partner to optimize and lower the cost of certain non-specialized technical functions. As part of the transition into this new partnership, a portion of the Company's workforce will be in lower-cost geographies.
Total Rewards. To attract qualified applicants to Butterfly and retain our employees, we offer a competitive total rewards package for all employees, consisting of market-competitive base salaries, annual target cash bonuses that recognize and reward company performance as well as individual results, long-term equity incentives that encourage our employees to focus on long-term value creation, and other comprehensive benefits, such as a 401(k) plan with employer matching and an Employee Stock Purchase Plan.
Employee Health. Aligned with our mission to make healthcare more accessible, we believe our employees should not have to worry about their health care costs. Butterfly offers employees medical, dental, and vision coverage that is covered at 100%, and we provide an employer-funded health savings account for out-of-pocket expenses. Our coverage encompasses mental, physical, and emotional well-being through our employee assistance program, which provides emotional support, work-life solutions, and other personal guidance resources. We are also focused on ensuring all of our employees, as well as temporary contractors and visitors to our sites, can work safely.
Manufacturing
Our Butterfly devices are built using both custom-made and off-the-shelf components supplied by vendors and contract manufacturers. The key custom-made component in the Butterfly probe is the ultrasound transducer module consisting of a custom micro-electro-mechanical systems ultrasound semiconductor chip and lens which are manufactured in Taiwan and then sent to Thailand for assembly.
We purchase some of our components and materials used in manufacturing, including the transducer module, from single sources. Although we believe that alternatives would be available, it would take time to identify and validate replacement components, which could negatively affect our ability to supply our products on a timely basis. We cannot give assurances that any alternative supplier would be able to recreate the manufacturing processes currently in use. To mitigate this risk, we typically carry a significant inventory of critical components.
Many of our Butterfly probes are manufactured, tested and shipped by Benchmark Electronics, Inc. (“Benchmark”) from its facilities in Thailand. We believe that this manufacturing strategy and supply chain is efficient and conserves capital.
However, in the event it becomes necessary to utilize a different contract manufacturer for our Butterfly products, we would experience additional costs and difficulties in doing so.
Key Agreements
Foundry Service Agreement with Taiwan Semiconductor Manufacturing Company Limited
We entered into a Foundry Service Agreement (the “FSA”) with Taiwan Semiconductor Manufacturing Company Limited (“TSMC”) in March 2019, as amended on October 1, 2020, under which TSMC agreed to manufacture integrated circuits used for the semiconductor chips in our probes. The FSA allows us to place purchase orders with TSMC, which are not binding until accepted by TSMC. The FSA also provides for TSMC to use commercially reasonable efforts to manufacture our products at TSMC and for us to meet monthly minimum purchase obligations. Under the FSA, we prepaid an amount to TSMC to be used against a portion of the purchase price for future purchases once the prepayment amount is reached. To the extent that we fail to fulfill our monthly wafer consumption requirement, TSMC has the right to deduct the shortfall from payments made by us to TSMC. In addition, we are required to buy back from TSMC unused raw wafers that TSMC purchases from its supplier.
The FSA also provides that TSMC will indemnify us for intellectual property infringement or misappropriation claims against us related to the wafer manufacturing process and that we will indemnify TSMC for any intellectual property infringement or misappropriation claims arising from TSMC’s compliance with our instructions, specifications, designs or requirements to manufacture, sell, or ship the wafers or arising from any harm caused by our medical device products.
The FSA’s current term expires on December 31, 2026, subject to automatic renewal for successive two-year terms unless terminated by either party upon three months’ notice prior to the end of the then-current term. The FSA may also be terminated by written notice at any time upon the bankruptcy or insolvency of or upon or after a material breach by the other party. Either party may terminate the FSA immediately, with or without cause, by giving the other party 12 months’ prior written notice of termination. In addition, TSMC may terminate the FSA if we do not place a purchase order for a period of 12 consecutive months or upon certain change of control transactions, including a merger, consolidation or other change of control or similar transactions to which we are party involving a semiconductor provider.
In connection with the FSA, we and TSMC developed a proprietary manufacturing process and continue to collaborate on manufacturing process improvements.
Manufacture and Supply Agreement with Benchmark Electronics, Inc.
In October 2015, we entered into a Manufacture and Supply Agreement (the “MSA”) with Benchmark, as amended on August 2019 and February 2021. Under the MSA, Benchmark agreed to manufacture our products pursuant to binding purchase orders, as well as non-binding forecasts. The parties have agreed to meet periodically regarding any minimum order quantities under the MSA.
Under the terms of the MSA, we granted Benchmark a non-exclusive, non-transferable, revocable, fully-paid, royalty-free license, without the right to sublicense, to use our technology solely to manufacture our products. The MSA provides that we will own any right, title and interest in any improvements or modifications to our technology made in the course of performance of Benchmark’s obligations under the MSA. We and Benchmark also agreed to indemnify each other against certain third-party claims.
The MSA’s current term expires on October 1, 2026, subject to automatic renewal for successive two-year terms unless either party gives 180 days’ prior written notice before the end of the then-current term to the other party electing not to renew the MSA. The MSA or any purchase order under the MSA may be terminated by either party for convenience upon 90 days’ prior written notice to the other party. The MSA may also be terminated by either party by written notice upon the occurrence of (i) a breach by the other party under the MSA which is not cured within 30 days after written notice by the terminating party, (ii) the other party becomes insolvent, dissolves, liquidates or ceases to conduct business or (iii) the occurrence of payment-related breaches. Benchmark may also terminate the MSA upon the filing of any petition against us under bankruptcy or similar laws, where such petition is not vacated within 10 days via court order.
Exclusive Distribution Agreement with Cardinal Health 105, Inc.
In July 2018, we entered into an Exclusive Distribution Agreement (the “Distribution Agreement”) with Cardinal Health 105, Inc. (“Cardinal Health”). Under the Distribution Agreement, Cardinal Health acts as the distribution agent and authorized distributor of record of our products to our customers, including, but not limited to, wholesalers, specialty distributors, physicians, clinics, hospitals, pharmacies and other healthcare providers, in the United States. Under the Distribution Agreement, we provide Cardinal Health with forecasts of the volume of our products to be handled and distributed by Cardinal Health. We make payments to Cardinal Health for its distribution services pursuant to a fee schedule. The Distribution Agreement’s current term expires on August 31, 2026. The Distribution Agreement is subject to automatic renewal for additional successive two-year terms unless terminated.
Intellectual Property
Protection of our intellectual property is a strategic priority for our business. We rely on a combination of patents, trademark, copyright, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies.
The patents owned and in-licensed by us are generally directed to the architecture of our ultrasonic imaging devices, our microfabricated ultrasonic transducers and machine learning for ultrasound applications. We have developed a portfolio of issued patents and pending patent applications directed to commercial products and technologies for potential development. We believe that our intellectual property is a core strength of our business, and our strategy includes the continued development of our patent portfolio.
As of February 13, 2025, we owned approximately 620 issued patents and pending patent applications in the United States and foreign jurisdictions, including the European Union and the United Kingdom. These issued patents and pending patent applications (if they were to be issued as patents) have expected expiration dates ranging between approximately 2030 and 2045.
In addition to patents, we also rely on trademarks, trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. We seek to protect our proprietary information and other intellectual property by generally requiring our employees, consultants, contractors, suppliers, outside scientific collaborators and other advisors to execute non-disclosure and assignment of invention agreements on commencement of their employment or engagement. Agreements with our employees also forbid them from using or incorporating the proprietary rights of third parties during their engagement with us. We also generally require confidentiality or material transfer agreements from third parties that receive our confidential data or materials.
License Agreements
We have entered into exclusive and non-exclusive licenses in the ordinary course of business relating to our technologies or other intellectual property rights or assets.
Exclusive (Equity) Agreement with Leland Stanford Junior University
In June 2013, we entered into an Exclusive (Equity) Agreement (the “Stanford Agreement”) with the Board of Trustees of the Leland Stanford Junior University (“Stanford”). Pursuant to the Stanford Agreement, Stanford granted us a co-exclusive, worldwide license to make, have made, use, import, offer to sell, and sell products covered by patent rights to Stanford’s wafer bonding technology. The rights licensed to us are for ultrasound applications using the wafer bonding technology excluding certain applications. As of December 23, 2023, the license became nonexclusive until the last licensed patent expires. The last licensed patent is currently expected to expire in 2030. The rights licensed to us are sublicensable, subject to Stanford’s prior approval. The Stanford Agreement outlines certain milestones to be met by us in connection with the development and sales of these products.
Under the terms of the Stanford Agreement, we paid a one-time, non-refundable upfront royalty fee. We are required to pay Stanford low single-digit royalties on all net sales of products that use the licensed technology, as well as a portion of any sublicensing revenues, during the term of the Stanford Agreement and if certain products using the licensed technology are made, used, imported, or offered for sale before the date the Stanford Agreement terminates, and those products are sold after the termination date, we will pay Stanford an earned royalty for our exercise of rights based on the net sales of those products. We are also obligated to pay Stanford annual license maintenance fees, which are fully creditable against any
royalty payments made by us for such year. We are also required to provide Stanford with periodic reports documenting our progress toward the development and commercialization of products using the licensed technology. Stanford is responsible under the agreement for preparing, filing and prosecuting patent claims and for maintaining the patents pertaining to the licensed technology.
Stanford may terminate the agreement in the event that we are materially delinquent on any payment, fail to diligently develop and commercialize a product incorporating the licensed technology, materially miss a milestone under the agreement, are in material breach of any substantive provision under the agreement, or knowingly provide any false report or are materially delinquent on any report, in each case which is not remedied within the applicable cure period. In addition, if we are not diligently developing and commercializing such a product incorporating the licensed technology, materially miss a milestone or knowingly provide a false report or are delinquent on any report, and we do not cure, the agreement shall not terminate, but it remains subject to termination by Stanford. We may terminate the agreement at any time upon at least 30 days’ prior written notice. Upon termination of the agreement, all rights to the licensed technology revert to Stanford. Our obligation to pay royalties accrued or accruable survives any termination or expiration of the agreement.
Government Regulation
The medical devices that we manufacture and distribute are subject to regulation by numerous regulatory bodies, including the FDA and comparable international regulatory agencies. These agencies require manufacturers of medical devices to comply with applicable laws and regulations governing the development, testing, manufacturing, packaging, labeling, marketing and distribution of medical devices. Devices are generally subject to varying levels of regulatory control, the most comprehensive of which requires that a clinical evaluation program be conducted before a device can be approved for marketing and commercial distribution. In addition, healthcare regulatory bodies in the United States and around the world impose a range of requirements related to paying for medical devices and the procedures in which they are used, including laws intended to prevent fraud, waste, and abuse of healthcare dollars.
U.S. Government and Regulatory Requirements
The medical devices that we manufacture and distribute are subject to regulation by numerous regulatory bodies, including the FDA and comparable international regulatory agencies. These agencies require manufacturers of medical devices to comply with applicable laws and regulations governing the development, testing, manufacturing, packaging, labeling, marketing and distribution of medical devices. Devices are generally subject to varying levels of regulatory control, the most comprehensive of which requires that a clinical evaluation program be conducted before a device can be approved for marketing and commercial distribution. In addition, healthcare regulatory bodies in the United States and around the world impose a range of requirements related to paying for medical devices and the procedures in which they are used, including laws intended to prevent fraud, waste, and abuse of healthcare dollars.
The Food, Drug, and Cosmetic Act (“FDCA”) classifies medical devices into three classes based on risk. Butterfly devices are considered Class II devices which are considered moderate risk. There are also Class I (lowest risk) and Class III (highest risk) devices, with more stringent regulatory requirements applicable to higher-risk devices. Commercial sales of Class II (except for Class II exempt devices) and Class III medical devices in the U.S. must be preceded by either a pre-market notification filing pursuant to Section 510(k) of the FDCA for Class II or the granting of a premarket approval for Class III. The development of a medical device typically requires extensive non-clinical testing and, for some devices, clinical testing involving human subjects.
After a device is placed on the market, regardless of its classification, numerous FDA regulatory requirements apply, including establishing registration and device listing, labeling, post-market record keeping and reporting, and the Quality System Regulation. These requirements are detailed, comprehensive, and require extensive investment and resources to comply with legal and regulatory requirements.
The FDA and the Federal Trade Commission (“FTC”) also regulate the advertising and promotion of our offerings to ensure that our claims are consistent with our regulatory clearances and approvals, that there is data to substantiate the claims, and that our materials are not false or misleading.
Pertaining to our veterinary devices, in the United States, the FDA does not require submission of a 510(k), PMA, or any premarket clearance or approval for devices used in veterinary medicine. Device manufacturers who exclusively manufacture or distribute veterinary devices are not required to register their establishments and list veterinary devices and
are exempt from post-marketing reporting. The FDA has regulatory oversight over veterinary devices and can take appropriate regulatory action if a veterinary device is misbranded or adulterated. It is the responsibility of the manufacturer and/or distributor of these articles to assure that these veterinary devices are safe, effective, and properly labeled.
The marketing, promotion, and sale of medical devices, drugs, and services are regulated by the U.S. Department of Health and Human Services (“HHS”) and comparable U.S. state and non-U.S. agencies responsible for reimbursement and regulation of the delivery of healthcare items and services, representing government’s interest in regulating the quality and cost of healthcare. Similar regulations are imposed in many global markets in which we do business.
While Butterfly does not submit claims for reimbursement, the U.S. federal healthcare laws apply when our customers submit claims for items or services that are reimbursed under Medicare, Medicaid, or other federally funded healthcare programs, including laws related to kickbacks, false claims, self-referrals, and healthcare fraud and abuse. These laws apply when claims are submitted for procedures that use our products. Similar state false claims, anti-kickback, anti-self-referral, and insurance laws also apply to state-funded Medicaid and other healthcare programs and private third-party payers. Any failure to comply with these laws and regulations could subject us or our officers and employees to criminal and civil financial penalties and expose us to civil liability and risk of further enforcement action under the U.S. Anti-Kickback Statute (“AKS”), the False Claims Act (“FCA”), or other healthcare fraud and abuse laws. In addition, as a manufacturer of U.S. FDA-cleared and -approved devices and drugs reimbursable by federal healthcare programs, we are subject to the U.S. Physician Payments Sunshine Act (the “Sunshine Act”), which requires us to annually track and report to the federal government certain payments and other transfers of value we make to U.S.-licensed physicians and other healthcare professionals or U.S. teaching hospitals, and to similar state equivalents.
The U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act of 2010, and similar anti-corruption and anti-bribery laws in other jurisdictions generally prohibit companies from making corrupt payments to or otherwise engaging in bribery of government officials. These laws apply to many of our customer interactions, as healthcare professionals in other countries are often considered government officials, and in some cases lay out requirements of how to operationalize compliance with the legal requirements. Failure to comply with these laws may expose us to criminal and civil enforcement actions, monetary fines and penalties, and reputational harm.
International Laws and Regulations
International marketing and distribution of medical devices are subject to regulation by foreign governments, and such regulations may vary substantially from country to country. The time required to obtain marketing authorization in a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ. There is a trend towards harmonization of quality system standards among the EU, United States, Canada and various other industrialized countries.
All new medical devices placed on the market or put into service in the EU must be compliant with and meet the general safety and performance requirements of the Medical Device Regulation (EU) No. 2017/745, which was implemented on May 26, 2021. Devices that conform to these requirements can be affixed with a CE marking and commercialized throughout the European Economic Area (“EEA”) and in Switzerland (subject to certain additional requirements). Prior to affixing a CE marking, manufacturers must demonstrate that their products comply with minimum standards of performance, safety, and quality, through a conformity assessment procedure that depends on the product’s classification. The classification of a medical device is determined by its intended purpose. Devices are classified from lowest to highest risk, as either Class I, IIa, IIb, or III. Classification is dependent on a variety of factors, including duration of use, whether the device is invasive or non-invasive, and whether the device is considered “active.” The competent authorities of the EU countries are responsible for regulating clinical investigations of medical devices and post-market surveillance of devices once they are placed on the market.
Outside of the EU, regulatory authorization needs to be sought on a country-by-country basis in order for us to market our products and each country may have its own processes and requirements for medical device licensing, approval/clearance, and regulation.
Data Privacy
Due to our global footprint and handling of personal data as both a data controller (on our own behalf) and data processor (on behalf of third parties, primarily customers), we are also subject to an extensive collection of global laws and
regulations protecting the privacy, security and integrity of the personal data, sensitive personal data, and patient health information that we create, receive, use, and maintain as a business.
Among the most relevant and material to our business, based on the volume and sensitivity of the data at issue, are: the U.S. Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information and Technology for Economic and Clinical Health Act (collectively “HIPAA”); the EU General Data Protection Regulation (Regulation (EU) 2016/679) (“GDPR”), similar U.K. legislation resulting from the European Union (Withdrawal) Act of 2018 (“U.K. GDPR”), and other EU country-level laws. In addition, there are also various U.S. state-level laws (e.g., the California Consumer Privacy Act), country regional laws, and proposed legislation that we monitor for applicability and impact to our business. These laws present a continuing challenge to businesses to structure their data collection, storage, use, and cross-border transmission in a compliant manner.
Many of these laws impose a significant compliance burden on organizations within their scope, and failure to comply can result in a variety of sanctions, including administrative fines for the most serious compliance failures up to 4-5% of a company’s total annual revenue of the preceding fiscal year (e.g., GDPR, U.K. GDPR, China PIPL). While there have been some recent enforcement actions by EU country-level data protection authorities resulting in substantial fines pursuant to GDPR, there remains uncertainty as to how data protection authorities throughout the rest of the globe will choose to interpret and enforce violations of applicable privacy and cybersecurity laws and regulations. Furthermore, these laws and regulations are continuously evolving, and further clarification in the form of implementing rules, guidelines, and related guidance from the data protection authorities is necessary to paint a full picture of the compliance obligations imposed on businesses within their scope.
Information Available on the Internet
Our internet address is www.butterflynetwork.com, to which we regularly post copies of our press releases as well as additional information about us. We recognize our website as a key channel of distribution to reach public investors and as a means of disclosing material non-public information to comply with our disclosure obligations under Securities and Exchange Commission (“SEC”) Regulation FD. Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, including exhibits, proxy, and information statements and all amendments to those reports, will be available to you free of charge through the Investors section of our website as soon as reasonably practicable after such materials have been electronically filed with, or furnished to, the SEC. The SEC maintains an internet site (www.sec.gov) that contains reports, proxy, and information statements and other information regarding issuers that file electronically with the SEC. We include our website address in this Annual Report on Form 10-K only as an inactive textual reference. Information contained in our website is not meant to be incorporated into, and does not constitute a part of, this Annual Report on Form 10-K or any of our other filings with the SEC.
Item 1A. RISK FACTORS
This Annual Report on Form 10-K contains forward-looking statements that involve risks and uncertainties. These statements include projections about our finances, plans and objectives for the future, future operating and economic performance, and other statements regarding future performance. These statements are not guarantees of future performance or events. Our actual results could differ materially from those discussed in this report. Factors that could cause or contribute to these differences include, but are not limited to, those discussed in the following section, as well as those discussed in Part II, Item 7 entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere throughout this report.
You should consider carefully the following risk factors, together with all of the other information included in this report. If any of the following risks, either alone or taken together, or other risks not presently known to us or that we currently believe to not be significant, develop into actual events, then our business, financial condition, results of operations, or prospects could be materially adversely affected. If that happens, the market price of our common stock could decline, and stockholders may lose all or part of their investment.
Unless the context otherwise requires, references in this section to “we,” “us,” “our,” and the “Company” refer to Butterfly Network, Inc. and its subsidiaries.
Risks Related to Our Financial Condition and Capital Requirements
We have a limited operating history on which to assess the prospects for our business, we have generated limited revenue from sales of our products, and we have incurred losses since inception. We anticipate that we will continue to incur significant losses for at least the next several years as we continue to commercialize our existing products and services and seek to develop and commercialize new products and services.
Since inception, we have devoted substantially all of our financial resources to develop our products and related services. We have financed our operations primarily through the issuance of equity and convertible debt securities. We have generated limited revenue from the sale of our products and services to date and have incurred significant losses. The amount of our future net losses will depend, in part, on sales and on-going development of our products and related services, the rate of our future expenditures, and our ability to obtain funding through the issuance of our securities, strategic collaborations, or grants. We expect to continue to incur significant losses for at least the next several years as we continue to commercialize our existing products and services and seek to develop and commercialize new products and services.
Our ability to generate future revenue from product and service sales depends heavily on our success in many areas, including, but not limited to:
•launching and commercializing current and future products and services, either directly or in conjunction with one or more collaborators or distributors;
•obtaining and maintaining marketing authorization with respect to each of our products and maintaining regulatory compliance throughout relevant jurisdictions;
•maintaining clinical and economical value for end-users and customers in changing environments;
•addressing any competing technological and market developments;
•negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter;
•establishing and maintaining distribution relationships with third-parties that can provide adequate (in amount and quality) infrastructure to support market demand for our products; and
•maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how.
We have incurred significant losses since inception. As such, you cannot rely upon our historical operating performance to make an investment decision about us.
Since our inception, we have engaged in R&D activities and launched our first product, Butterfly iQ, in 2018, our second product, Butterfly iQ+, in 2020, and our third product, Butterfly iQ3, in 2024. Since commercialization of the Butterfly iQ, we have also engaged in the continued development and sales of our enterprise software. We have financed our operations primarily through the issuance of equity securities and convertible debt. We have incurred net losses of $72.5 million, $133.7 million, and $168.7 million in the years ended December 31, 2024, 2023, and 2022, respectively. Our accumulated deficit as of December 31, 2024 was $802.1 million. We do not know whether or when we will become profitable. Our ability to generate revenue and achieve profitability depends upon our ability to accelerate the commercialization of our products and service offerings in line with the demand from current and future customers and our aggressive business strategy. We may be unable to achieve any or all of these goals.
Risks Related to Our Business and Operations
Our success depends upon market acceptance of our products and services, our ability to develop and commercialize existing and new products and services and generate revenues, and our ability to identify new markets for our technology.
We have developed, and we are engaged in the development of, ultrasound imaging solutions using our ultrasound-on-a-semiconductor-chip technology. We are commercializing Butterfly iQ+ and Butterfly iQ3 point-of-care ultrasound imaging devices. Our success will depend on the acceptance of our products and services in the U.S. and international healthcare markets. We are faced with the risk that the marketplace will not be receptive to our products and services over competing products, including traditional cart-based ultrasound devices used in hospitals, imaging centers, and physicians’ offices,
and that we will be unable to compete effectively. Factors that could affect our ability to successfully commercialize our current products and services and to commercialize any potential future products and services include:
•challenges of developing (or acquiring externally-developed) technology solutions that are adequate and competitive in meeting the requirements of next-generation design challenges; and
•dependence upon physicians’ and other healthcare practitioners’ acceptance of our products.
We cannot assure investors that our current products and services or any future products and services will gain broad market acceptance. If the market for our current products and services or any future products and services fails to develop or develops more slowly than expected, or if any of the services and standards supported by us do not achieve or sustain market acceptance, our business and operating results would be materially and adversely affected.
Medical device development is costly and involves continual technological change, which may render our current or future products obsolete.
The market for point-of-care medical devices is characterized by rapid technological change, medical advances, and evolving industry standards. Any one of these factors could reduce the demand for our devices or services or require substantial resources and expenditures for research, design and development to avoid technological or market obsolescence.
Our success will depend on our ability to enhance our current technology, services, and systems and develop or acquire and market new technologies to keep pace with technological developments and evolving industry standards, while responding to changes in customer needs. A failure to adequately develop or acquire device enhancements or new devices that will address changing technologies and customer requirements adequately, or to introduce such devices on a timely basis, may have a material adverse effect on our business, financial condition and results of operations.
We might have insufficient financial resources to improve existing devices, advance technologies and develop new devices at competitive prices. Technological advances by one or more competitors or future entrants into the field may result in our current devices becoming non-competitive or obsolete, which may decrease revenues and profits and adversely affect our business and results of operations.
We may encounter significant competition across our existing and future planned products and services and in each market in which we sell or plan to sell our products and services from various companies, many of which have greater financial and marketing resources than we do. Our primary competitors include the top five manufacturers of legacy cart-based incumbent ultrasound devices.
In addition, many of our competitors are well-established manufacturers with significant resources and may engage in aggressive marketing tactics. Competitors may also possess the ability to commercialize additional lines of products, bundle products, or offer higher discounts and incentives to customers in order to gain a competitive advantage. If the prices of competing products are lowered as a result, we may not be able to compete effectively.
We will be dependent upon the success of our sales and customer acquisition and retention strategies.
Our business is dependent upon the success of our sales and customer acquisition and retention strategies, and our marketing efforts are focused on developing a strong reputation with healthcare providers and increasing awareness of our products and services. If we fail to maintain a high quality of service or a high quality of device technology, we may fail to retain existing users or add new users. If we do not successfully continue our sales efforts and promotional activities, particularly to health systems and large institutions, or if existing users decrease their level of engagement, our revenue, financial results, and business may be significantly harmed. Our future success depends upon continued expansion of our commercial operations in the United States and internationally, as well as entering additional markets to commercialize our products and services. We believe that our growth will depend on the further development and commercialization of our current products and services, and marketing authorization of our future products and services. If we fail to expand the use of our products and services in a timely manner, we may not be able to expand our market share or to grow our revenue. Our financial performance will be substantially dictated by our success in adding, retaining, and engaging active users of our products. If customers do not perceive our products or services to be useful, reliable, and trustworthy, we may not be able to attract or retain customers or otherwise maintain or increase the frequency and duration of their engagement. As our business model is predicated on both hardware and software sales, there is risk that any decline in software renewal rates will adversely impact our business. To date, utilization of our software has varied across different medical specialties, but
usage does not directly correlate to renewal of subscriptions, as different medical specialties interact with the device in different ways depending on their clinical focus and routine. A decrease in customer retention, growth or engagement with our products and services may have a material and adverse impact on our revenue, business, financial condition, and results of operations.
Any number of factors could negatively affect customer retention, growth, and engagement, including:
•customers increasingly engaging with competing products;
•failure to introduce new and improved products and services;
•inability to continue to develop products for mobile devices that customers find engaging, that work with a variety of mobile operating systems and networks, and that achieve a high level of market acceptance;
•changes in customer sentiment about the quality or usefulness of our products and services or concerns related to privacy and data sharing, safety, security, or other factors;
•inability to manage and prioritize information to ensure customers are presented with content that is engaging, useful, and relevant to them;
•adverse changes in our products that are mandated by legislation or regulatory agencies, both in the United States and internationally; or
•technical or other problems preventing us from delivering products or services in a rapid and reliable manner or otherwise affecting the user experience.
Our research and development efforts may not succeed in developing commercially successful products and technologies, which could adversely affect our business.
Our technology on a microchip has the potential to allow us to monitor patients in various care settings due to its portability and cost. We expect our development path will be directed at accessing and optimizing our technology for use in various care settings, potentially including home scanning and or wearable patient technology, subject to appropriate regulatory authorization. We face risks associated with launching such new products. If we encounter development or manufacturing challenges or discover errors during our product development cycle, the product launch dates of new products may be delayed, which will cause delays in our ability to achieve our forecasted results. The expenses or losses associated with unsuccessful product development or launch activities or lack of market acceptance of our new products could adversely affect our business or financial condition.
We expect to generate an increasing portion of our revenue internationally in the future and may become subject to various additional risks relating to our international activities, which could adversely affect our business, operating results and financial condition.
During the years ended December 31, 2024, 2023, and 2022, approximately 23%, 21%, and 30%, respectively, of our total revenue was generated from customers located outside of the United States. We believe that a substantial percentage of our future revenue will come from international sources as we expand our sales and marketing opportunities internationally. We have limited experience operating internationally, and engaging in international business involves a number of difficulties and risks, including:
•required compliance with foreign regulatory requirements and laws, including regulations and laws relating to patient data and medical devices;
•trade relations among the United States and those foreign countries in which our current or future customers, distributors, manufacturers, and suppliers have operations, including protectionist measures such as tariffs and import or export licensing requirements, whether imposed by the United States or such foreign countries, in particular the strained trade relations between United States and China since 2018;
•difficulties protecting, procuring, or enforcing intellectual property rights internationally;
•required compliance with anti-bribery laws, such as the FCPA and the UK Bribery Act of 2010, data privacy requirements, labor laws, and anti-competition regulations;
•laws regulating the confidentiality of sensitive personal information and the circumstances under which such information may be released and/or collected;
•longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;
•political and economic instability and war or other military conflict, including the ongoing conflict occurring in Ukraine, which could have a material adverse impact on our sales in Europe and elsewhere; and
•potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements, and other trade barriers.
Furthermore, the new U.S. presidential administration has publicly expressed support for greater restrictions on free trade and the increase of tariffs on goods imported into the United States. If we dedicate significant resources to our international operations and are unable to manage these risks effectively, our business, operating results, and financial condition may be adversely affected.
If we are unable to attract, recruit, train, retain, motivate, and integrate key personnel, we may not achieve our goals.
There is substantial competition for key personnel, senior management, and qualified employees in the healthcare industry, and we may face increased competition for such a highly qualified scientific, technical, clinical, and management workforce in a highly competitive environment. While the increased availability of flexible, hybrid, or work-from-home arrangements has afforded us the ability to attract and retain talent from geographies remote from our physical offices, it has also expanded competition by allowing qualified employees within those same regions to pursue job opportunities throughout the country without the need to relocate. To help attract, retain, and motivate qualified employees in senior roles, we use equity-based awards and performance-based cash incentive awards. Sustained declines in our stock price, or lower stock price performance relative to competitors, can reduce the retention value of our equity-based awards, which can impact the competitiveness of our compensation. There can be no assurance that we will be successful in retaining existing personnel or recruiting new personnel.
From time to time, our efforts to attract, recruit, train, retain, integrate, and motivate key personnel may also subject us to litigation or other legal proceedings that may adversely affect our business. These legal proceedings may involve claims brought by current or former employees, government agencies, or others, through private actions, class actions, administrative proceedings, or other litigation. These legal proceedings may involve allegations of illegal, unfair, or inconsistent employment practices, including wage and hour, discrimination, harassment, wrongful termination, retaliation, violations of law, or other concerns. Even if the allegations against us are unfounded or we ultimately are not held liable, we may experience related negative publicity resulting in damage to our reputation. Further, the costs to defend ourselves may be significant and the litigation may subject us to substantial settlements, fines, penalties, or judgments against us and may consume management’s bandwidth and attention, some or all of which may negatively impact our financial condition and results of operations.
Having diverse representation and an inclusive workplace can also impact our ability to attract and retain talent and is an important driver of our ability to compete and innovate. As such, our ability to attract and retain diverse talent can impact our corporate reputation and have adverse consequences to our business.
The loss of one or more key employees, our inability to attract or develop additional qualified employees, and any delay in hiring key personnel could have a material adverse effect on our business results, cash flows, financial condition, or prospects.
We have chosen to engage a single supplier, TSMC, to supply and manufacture a key component of our products. If TSMC fails to fulfill its obligations under its existing contractual arrangements with us or does not perform satisfactorily, or if this relationship is terminated for other reasons, our ability to source our devices would be negatively and adversely affected. In addition, our obligation to purchase a minimum volume from TSMC may adversely affect our cash flows.
We have chosen to engage a single supplier, TSMC, a semiconductor manufacturer, to manufacture and supply all of the wafers used to create the semiconductor chips in our probes. See “Item 1. Business — Manufacturing — Key Agreements — Foundry Service Agreement with Taiwan Semiconductor Manufacturing Company Limited”. Since our contracts with TSMC are non-exclusive and do not commit TSMC to supply or manufacture quantities beyond the amounts included in our forecasts, TSMC may give other customers’ needs higher priority than ours, and we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms. If TSMC is unable to supply components or devices, our business would be harmed.
We entered into an FSA with TSMC, under which TSMC agreed to manufacture, and we committed to purchase, a minimum volume of the wafers used for the semiconductor chips in our probes. Our minimum purchase obligation could adversely affect our cash flows, such as in times when we have sufficient inventory and would otherwise be able to use our cash for other purposes. Pursuant to the FSA, we are required to buy back from TSMC any unused raw wafers. If we are required to buy back from TSMC any unused raw wafers pursuant to the FSA, our cash flows may be adversely impacted.
Geopolitical tensions between Taiwan and China have risen steadily in recent months. War or other military conflict in or near Taiwan, pandemics, and certain natural disasters, such as earthquakes which are commonplace in Taiwan, may result in the destruction or disruption of TSMC’s ability to supply wafers and have downstream implications for our Company.
If we were to lose TSMC as a component supplier, there can be no assurance that we will be able to identify or enter into agreements with alternative suppliers on a timely basis on acceptable terms, if at all. An interruption in our ability to sell and deliver our products or instruments to customers could occur if we encounter delays or difficulties in securing these components, if the quality of the components supplied do not meet our specifications, or if we cannot then obtain an acceptable substitute. If any of these events occur, our business and operating results could be harmed.
We rely on a single contract manufacturer, Benchmark, to test, assemble, and supply our finished products. If Benchmark fails to fulfill its obligations under its existing contractual arrangements with us or does not perform satisfactorily, our ability to source our devices could be negatively and adversely affected.
In October 2015, we entered into an MSA with Benchmark. Under the MSA, as amended effective in August 2019 and February 2021, Benchmark will manufacture our products pursuant to binding 90-day purchase orders, as well as non-binding 180-day “forecasts” estimating our product shipment requirements, submitted by us to Benchmark each month, which may become binding in certain cases. We also have certain inventory related obligations, including the obligation to purchase excess and obsolete components from Benchmark. See “Item 1. Business — Manufacturing — Key Agreements — Manufacture and Supply Agreement with Benchmark Electronics, Inc”.
In the event it becomes necessary to utilize a different contract manufacturer for our component products, we would experience additional costs, delays, and difficulties in obtaining such components as a result of identifying and entering into an agreement with a new contract manufacturer as well as preparing such new manufacturer to meet the logistical requirements associated with manufacturing our devices, and our business would suffer.
We have and may continue to experience pricing pressures from contract suppliers or manufacturers on which we rely.
Due to supply constraints, we have seen our costs increase in 2024, but we were largely able to offset these costs through manufacturing efficiencies and pricing actions. However, we expect there will continue to be supply constraints; our suppliers are continuing to raise prices and may continue to raise prices in the future, which we may not be able to offset through manufacturing efficiencies or pricing actions. Because we currently rely on TSMC to supply our custom components and on Benchmark to manufacture our finished products, such pricing pressures from either party could increase our costs and force us to increase the prices of our products if we are unable to enter into alternative arrangements with other suppliers or manufacturers, potentially leading to decreased customer demand.
We may experience manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
We may encounter unforeseen situations that would result in delays or shortfalls in our production as well as delays or shortfalls caused by our outsourced manufacturing suppliers and by other third-party suppliers who manufacture components for our products. The FDA (and comparable foreign regulatory authorities) has comprehensive and prescriptive guidelines for medical device component manufacturers, requiring these manufacturers to establish and maintain processes and procedures to adequately control environmental conditions that could adversely affect product quality and impact patient safety. Clean room standards are an example of these requirements. Failure of component manufacturers or other third-party suppliers to comply with applicable standards could delay the production of our products. If we are unable to keep up with demand for our products, our revenue could be impaired, market acceptance for our products could be adversely affected, and our customers might instead purchase our competitors’ products. Our inability to successfully manufacture our products would have a material adverse effect on our operating results.
We rely on limited or sole suppliers for some of the materials and components used in our products, and we may not be able to find replacements or immediately transition to alternative suppliers, which could have a material adverse effect on our business, financial condition, results of operations, and reputation.
We rely on limited or sole suppliers for certain materials and components that are used in our products. While we periodically forecast our needs for such materials and enter into standard purchase orders with them, we do not have long-term contracts with some of these suppliers. If we were to lose such suppliers, or if such suppliers were unable to fulfill our orders or to meet our manufacturing specifications, there can be no assurance that we will be able to identify or enter into agreements with alternative suppliers on a timely basis or on acceptable terms, if at all. If we are able to find a replacement supplier, such replacement supplier would need to be qualified and may require additional regulatory inspection or approval, which could result in further delay.
An interruption in our operations could occur if we encounter delays or difficulties in securing these materials and components, if the quality of the materials and components supplied do not meet our requirements, or if we cannot then obtain an acceptable substitute. The time and effort required to qualify a new supplier and ensure that the new materials and components provide the same or better quality results could result in significant additional costs. Any such interruption could significantly affect our business, financial condition, results of operations, and reputation. To mitigate this risk, we typically carry significant inventory of critical components. While we believe that our level of inventory is currently sufficient for us to continue the manufacturing of our products without a disruption to our business in the event that we must replace one of our suppliers, there can be no assurance that we can maintain this level of inventory in the future.
Acquisitions, joint ventures, or other strategic transactions could disrupt our business, cause dilution to our stockholders, and otherwise harm our business.
We may acquire other businesses, products, or technologies as well as pursue strategic alliances, joint ventures, technology licenses, investments in complementary businesses, or other strategic initiatives. For example, in November 2024, we announced our plan to form Octiv, LLC, a wholly-owned subsidiary dedicated to bringing our proprietary chip to new non-competitive markets. However, we may not be successful in achieving this objective. Other than the Business Combination, we have not made any acquisitions to date, and our ability to do so successfully is unproven. Any of these transactions could be material to our financial condition and operating results and expose us to many risks, including:
•disruption in our relationships with customers, distributors, manufacturers, or suppliers as a result of such a transaction;
•unanticipated liabilities related to acquired companies;
•difficulties integrating acquired personnel, technologies, and operations into our existing business;
•diversion of management’s time and focus away from operating our business to acquisition integration challenges;
•increases in our expenses and reductions in our cash available for operations and other uses; and
•possible write-offs or impairment charges relating to acquired businesses.
Foreign acquisitions involve unique risks in addition to those mentioned above, including those related to the integration of operations across different cultures and languages, currency risks, and the particular economic, political, and regulatory risks associated with specific countries.
In addition, the anticipated benefit of any acquisition or other strategic transaction may not materialize. Future acquisitions, dispositions, or other strategic initiatives could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities, amortization expenses, or write-offs of goodwill, any of which could harm our financial condition. We cannot predict the number, timing, or size of future joint ventures, acquisitions, or other strategic transactions, or the effect that any such transactions might have on our operating results.
If we are unable to continue the development of an adequate sales and marketing organization and/or if our direct sales organization is not successful, we may have difficulty achieving market awareness and selling our products in the future.
We must continue to develop and grow our sales and marketing organization and enter into partnerships or other arrangements to market and sell our products and/or collaborate with third parties, including distributors and others, to market and sell our products to maintain the commercial success of our devices and to achieve commercial success for any
of our future products. Developing and managing a direct sales organization is a difficult, expensive, and time-consuming process.
To continue to develop our sales and marketing organization to successfully achieve market awareness and sell our products, we must:
•continue to recruit and retain adequate numbers of effective and experienced sales and marketing personnel;
•effectively train our sales and marketing personnel in the benefits and risks of our products;
•establish and maintain successful sales, marketing, training, and education programs that educate health care professionals so they can appropriately inform their patients about our products;
•manage geographically dispersed sales and marketing operations; and
•effectively train our sales and marketing personnel on the applicable fraud and abuse laws that govern interactions with healthcare practitioners as well as current and prospective patients and maintain active oversight and auditing measures to ensure continued compliance.
We may not be able to successfully manage our sales force or increase our product sales at acceptable rates.
If we are unable to establish and maintain adequate sales and marketing capabilities, or enter into and maintain arrangements with third parties to sell and market our products, our business may be harmed.
We cannot guarantee that we will be able to maintain our current volume of sales in the future. A substantial reduction in sales could have a material adverse effect on our operating performance. To the extent that we enter into additional arrangements with third parties to perform sales or marketing services in the United States, Europe, or other countries, our product margins could be lower than if we directly marketed and sold our products. To the extent that we enter into co-promotion or other marketing and sales arrangements with other companies, any revenue received will depend on the skills and efforts of others, and we cannot predict whether these efforts will be successful. In addition, the growth of market acceptance of our products by healthcare practitioners outside of the United States will largely depend on our ability to continue to demonstrate the relative safety, effectiveness, reliability, cost-effectiveness, and ease of use of such products. If we are unable to do so, we may not be able to increase product revenue from our sales efforts in Europe or other countries. If we are unable to establish and maintain adequate sales, marketing, and distribution capabilities, independently or with others, our future revenue may be reduced and our business may be harmed.
We operate in highly competitive markets, competition may increase in the future, and our industry may be further disrupted.
Healthcare markets are characterized by rapidly evolving technology, frequent introduction of new products, intense competition, and pricing pressures. We face competition from international and domestic companies of all sizes. Competition is primarily focused on cost effectiveness, price, service, product performance, and technological innovation. Our ability to compete successfully may be adversely affected by factors such as:
•the introduction of new products or product enhancements by competitors;
•the development of new technology or the application of known or unknown technology;
•a failure to satisfy local market conditions and regulations, such as mandatory IP transfers, protectionist measures, and other government policies supporting increased local competition;
•the emergence of new market entrants;
•a failure to maintain or expand relationships with existing customers or attract new customers;
•cost of production or delivery, whether due to geographic location, currency fluctuations, taxes, duties, or otherwise, which may enable our competitors to offer greater discounts or lower prices;
•the perception of our brand and image in the market;
•a failure to successfully enter new geographic or adjacent product markets;
•changing regulatory standards, legal requirements, or enforcement rigor; or
•consolidation among customers, suppliers, channel partners, or competitors.
Our inability to obtain and maintain regulatory authorizations for, and supply commercial quantities of, our offerings as quickly and effectively as our competitors are able to could limit market acceptance. Furthermore, our markets are continually evolving and thus revenues and income are difficult to forecast. Any of these competitive factors could adversely affect our pricing, margins, and market share and have a material adverse effect on our business results, cash flows, financial condition, or prospects.
Unfavorable global economic conditions could adversely affect our business, financial condition, or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets, including changes in inflation, interest rates, and overall economic conditions and uncertainties. To the extent inflation or other factors increase our business costs, it may not be feasible to pass price increases on to our customers or offset higher costs through manufacturing efficiencies. Inflation could also adversely affect the ability of our customers to purchase our products. An economic downturn could result in a variety of risks to our business, including weakened demand for our products and our inability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also result in further constraints on our suppliers or cause future customers to delay making payments for our products. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
Changes in applicable tax laws and regulations could adversely affect our business.
We are subject to income and other non-income taxes (including sales, excise, and value-added) in the United States and foreign jurisdictions. Thus, the tax treatment of transactions we execute is subject to changes in tax laws or regulations, tax treaties, or positions by the relevant authority regarding the application, administration, or interpretation of these tax laws and regulations. These factors, together with the ambiguity of tax laws and regulations, the subjectivity of factual interpretations, and uncertainties regarding the geographic mix of earnings in any period, can affect our estimates of our effective tax rate and income tax assets and liabilities, result in changes in our estimates and accruals, and have a material adverse effect on our business results, cash flows, or financial condition. We are unable to predict what tax reforms may be proposed or enacted in the future or what effect such changes would have on our business; however, such changes could potentially result in higher tax expense and payments, along with increasing the complexity, burden, and cost of compliance.
Our ability to use net operating losses and certain other tax assets to offset future income may be subject to certain limitations.
As of December 31, 2024, we had federal net operating loss (“NOL”) carryforwards of approximately $634.8 million, of which approximately $73.7 million will begin to expire in 2031 if not utilized. Unused NOLs may be carried forward to offset future taxable income if we achieve profitability in the future, unless such NOLs expire under applicable tax laws. However, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOLs and other pre-change tax attributes (such as research tax credits) to offset post-change taxable income. State NOLs and other tax attributes may be similarly limited. Any such limitations may result in increased tax liabilities that could adversely affect our business, results of operations, financial position and cash flows.
We could be adversely affected by violations of the FCPA and other worldwide anti-bribery laws by us or our agents.
We are subject to the FCPA, which prohibits companies and their intermediaries from making payments in violation of law to non-U.S. government officials for the purpose of obtaining or retaining business or securing any other improper advantage. Our planned future reliance on independent distributors to sell our products internationally demands a high degree of vigilance in enforcing our policy against participation in corrupt activity, because these distributors could be deemed to be our agents, and we could be held responsible for their actions. Other U.S. companies in the medical device and pharmaceutical fields have faced criminal penalties under the FCPA for allowing their agents to deviate from appropriate practices in doing business with such non-U.S. government officials. We are also subject to similar anti-bribery laws in the jurisdictions in which we operate, including the UK Bribery Act of 2010, which also prohibits commercial bribery and makes it a crime for companies to fail to prevent bribery. We have limited experience in complying with these laws and in developing procedures to monitor compliance with these laws by our agents. These laws are complex and far-reaching in nature, and, as a result, we cannot assure investors that we would not be required in the future to alter one or more of our practices to be in compliance with these laws or any changes in these laws or the interpretation thereof.
Any violations of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction, involve significant costs and expenses, including legal fees, and could result in a material adverse effect on our business, prospects, financial condition, or results of operations. We could also incur severe penalties, including criminal and civil penalties, disgorgement, and other remedial measures.
Risks Related to Government Regulation and Other Legal Compliance Matters
We are subject to extensive government regulation, which could restrict the development, marketing, sale, and distribution of our products and could cause us to incur significant costs.
Our ultrasound imaging products and associated services are subject to extensive pre-market and post-market regulation by the FDA and various other federal, state, local, and foreign government authorities. For example, our operations are subject to regulations governing packaging and labeling requirements, adverse event reporting, quality system and manufacturing requirements, clinical testing, and recalls. For a discussion on the relevant regulatory regime, see , in Item 1, Business – Government Regulation.
We cannot assure that any new medical devices or new uses or modifications for our FDA-cleared or CE-marked devices will be cleared or approved in a timely or cost-effective manner, if cleared or approved at all. In the event we are unable to leverage existing or predicate devices for future products, we may experience delays and additional costs to obtain FDA approval or CE marking for such future products. Even if such clearances or approvals are received, they may not be for all indications for which we pursue. Because medical devices may only be marketed for cleared or approved indications, this could significantly limit the market for that product and may adversely affect our results of operations.
Our business is subject to unannounced inspections by the FDA to determine our compliance with FDA requirements. FDA inspections can result in inspectional observations on Form FDA 483s, warning letters, untitled letters, or other forms of more significant enforcement action. If the FDA concludes that we failed to comply with any regulatory requirements during an inspection, it could have a material adverse effect on our business and financial condition. We could incur substantial expense and harm to our reputation, and our ability to introduce new or enhanced products in a timely manner could be adversely affected.
Any interruption in the operations of our manufacturing facilities, or our suppliers’ or customers’ facilities, may impair our ability to deliver products or provide services.
We are dependent on our global production and operating network to develop, manufacture, assemble, supply, and service our offerings. A work stoppage, labor shortage, or other production limitation, including import or export restrictions and transportation issues, among others, could occur at our manufacturing facilities or at supplier or customer facilities, and negatively impact our reputation and market position. Such interruptions may occur for several reasons, including as a result of regulatory enforcement actions, tight credit markets or other financial distress, production constraints or difficulties, unscheduled downtimes, war, severe weather and natural disasters, fires and explosions, accidents, mechanical failures, pandemics, civil unrest, strikes, unpermitted releases of toxic or hazardous substances, other EH&S risks, sabotage, cybersecurity attacks, riots, or terrorist attacks.
Any significant event affecting one of our or our suppliers’ production or operating facilities may result in a disruption to our ability to supply customers, and standby capacity necessary for the reliable operation of the facility may not be sufficiently available. The impact of these risks is heightened if our production capacity is at or near full utilization (or if we lack alternative manufacturing sites) and could result in our inability to accept orders or deliver products in a timely manner. Additionally, significant capital investment to increase manufacturing capacity may be required to expand our business or meet increased demand for our products in the future. Any of these risks could have a material adverse effect on our business results, cash flows, financial condition, or prospects.
If we, our contract manufacturers or our component suppliers are unable to manufacture our products in sufficient quantities, on a timely basis, at acceptable costs, and in compliance with regulatory and quality requirements, the manufacturing and distribution of our devices could be interrupted, and our product sales and operating results could suffer.
We, our contract manufacturers, and our component suppliers are required to comply with the FDA’s Quality System Regulation (“QSR”), which is a complex regulatory framework that covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage, shipping, and servicing of
our devices. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic, sometimes unannounced, inspections by the FDA. We cannot assure investors that our facilities or our third-party manufacturers’ or suppliers’ facilities would pass any future quality system inspection. Failure of our or our third-party manufacturers and component suppliers to adhere to QSR requirements or take adequate and timely corrective action in response to an adverse quality system inspection finding could delay production of our products and lead to fines, difficulties in obtaining regulatory clearances, recalls, enforcement actions, including injunctive relief or consent decrees, or other consequences, which could have a material adverse effect on our financial condition or results of operations.
In addition, any of our products shipped internationally are also required to comply with the International Organization for Standardization (“ISO”) quality system standards as well as EU Regulations and norms in order to produce products for sale in the EU.
In addition, many countries such as Canada and Japan have very specific additional regulatory requirements for quality assurance and manufacturing. If we fail to continue to comply with current good manufacturing requirements, as well as ISO or other regulatory standards, we may be required to cease all or part of our operations until we comply with these regulations. Maintaining compliance with multiple regulators adds complexity and cost to our manufacturing and compliance processes.
Our current or future products may be subject to product recalls even after receiving FDA clearance or approval. A recall of our products, either voluntarily or at the direction of the FDA, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
The FDA and similar governmental bodies in other countries have the authority to require the recall of our products if we or our third-party manufacturers fail to comply with relevant regulations pertaining to, among other things, manufacturing practices, labeling, advertising, or promotional activities, or if new information is obtained concerning the safety or efficacy of these products. In February 2020, we initiated a voluntary recall of two software tools after being notified by the FDA that each of them required 510(k) clearance. The FDA evaluated the recall and subsequently terminated it in June 2020. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our reputation, results of operations, and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to product liability claims, be required to bear other costs, or be required to take other actions that may have a negative impact on our future sales and our ability to generate profits.
We may be subject to enforcement action if we engage in improper or off-label marketing or promotion of our products, including fines, penalties, and injunctions.
Our promotional materials and training methods must comply with the FDA and other applicable laws and regulations, including the prohibition of the promotion of unapproved, or off-label, uses. However, if the FDA determines that our promotional materials or training materials promote a 510(k)-cleared or approved medical device in a manner inconsistent with its labeling, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an Untitled Letter, a Warning Letter, injunction, seizure, civil fine, or criminal penalties. In addition to ensuring that the claims we make are consistent with our regulatory clearances or approvals, the FDA also ensures that promotional labeling for all regulated medical devices is neither false nor misleading.
It is also possible that other federal (such as the FTC), state, or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an off-label use, or to be false, unsubstantiated, or misleading, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged, and adoption of our products could be impaired, in addition to legal consequences, which may include fines, penalties, product liability claims, and other legal actions.
In some instances in our advertising and promotion, we may make claims regarding our product as compared to competing products, which may subject us to heightened regulatory scrutiny, enforcement risk, and litigation risks.
The FDA requires that promotional labeling be truthful and not misleading, including with respect to any comparative claims made about competing products or technologies. In addition to FDA implications, the use of comparative claims also presents risk of a lawsuit by the competitor under federal and state false advertising and unfair competition statutes (e.g., the Lanham Act) or unfair and deceptive trade practices law, and possibly also state libel law, or other similar foreign
laws. Such a suit may seek injunctive relief against further advertising, a court order directing corrective advertising, and compensatory and punitive damages where permitted by law. Further, notwithstanding the ultimate outcome of any Lanham Act or similar complaint, our reputation and relationship with certain customers or distribution partners may be harmed as a result of the allegations related to our products or our business practices more generally.
We are subject to federal, state, and foreign laws prohibiting “kickbacks” and false or fraudulent claims, and other fraud and abuse laws, transparency laws, and other health care laws and regulations, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
Our relationships with customers and third-party payers are subject to broadly applicable fraud and abuse and other health care laws and regulations that may constrain our sales, marketing, and other promotional activities by limiting the kinds of financial arrangements, including sales programs and certain customer and product support programs, we may have with hospitals, physicians, or other purchasers of medical devices. See Item 1, Business – Government Regulation.
Any failure to comply with these laws and regulations could subject us or our officers and employees to criminal and civil financial penalties. We are also subject to risks relating to changes in government and private medical reimbursement programs and policies, and changes in legal regulatory requirements in the U.S. and around the world. Implementation of further legislative or administrative reforms to these reimbursement systems, or adverse decisions relating to coverage of or reimbursement for our products by administrators of these systems, could have an impact on the acceptance of and demand for our products and the prices that our customers are willing to pay for them
We are subject to complex and evolving U.S. and foreign laws and regulations regarding privacy, data protection, and other matters. Many of these laws and regulations are subject to change and uncertain interpretation, and could result in claims, changes to our business practices, monetary penalties, increased cost of operations, or declines in customer growth or engagement, or otherwise harm our business.
Our products and systems receive, generate, and store significant volumes of personal and sensitive information, such as employee, customer, and patient data. Moreover, our digital ecosystem, which is intended to provide our customers with greater access to a broad array of personal and sensitive information to improve delivery of care to their patients, heightens our risks associated with the protection of such information. We have legal and contractual obligations regarding the protection of confidential and personal information and the appropriate collection, use, retention, protection, disclosure, transfer, and other processing of such data. Additionally, regulators within the United States and around the world are evaluating how best to regulate development and use of data as well as AI technologies. We are subject to various privacy law regimes in the different jurisdictions in which we operate, including comprehensive regulatory systems in Europe, Latin America, and sector-specific requirements in the United States. Certain international jurisdictions have enacted or are enacting data localization laws mandating that certain types of data collected in a particular jurisdiction be physically stored within that jurisdiction.
There are numerous U.S. federal and state laws and regulations related to the privacy and security of personal information. In particular, regulations promulgated pursuant to HIPAA establish privacy and security standards that limit the use and disclosure of protected health information (“PHI”), require the implementation of safeguards to protect the privacy and security of PHI and ensure the confidentiality, integrity, and availability of electronic PHI, and require the provision of notice in the event of a breach of PHI. If we are unable to properly protect the privacy and security of PHI, we could face liability for breach of our contracts with our customers. Further, if we fail to comply with applicable HIPAA privacy and security standards, we could face civil and criminal penalties. In addition, there are also various state-level laws (e.g., the California Consumer Privacy Act), both enacted and proposed, that we must monitor for applicability and impact to our business and for which we must implement necessary controls and other requirements (if applicable).
In addition, we are subject to the laws and regulations of foreign jurisdictions including, without limitation, the GDPR in the EU and the United Kingdom (“U.K.”) data protection legislation (including the GDPR, as it forms part of the law of the U.K. by virtue of the U.K. GDPR and the U.K. Data Protection Act 2018 (the “U.K. Data Protection Act”)). The GDPR is wide-ranging in scope and imposes numerous requirements on companies that process personal data, including requirements relating to having a legal basis for processing personal data, providing information to individuals regarding data processing activities, implementing safeguards to protect the security and confidentiality of personal data, providing notification of data breaches, and taking certain measures when engaging third-party processors. The GDPR permits data protection authorities to impose large penalties for violations of the GDPR, including potential fines of up to €20 million (£17.5 million for U.K.) or 4% of annual global revenues, whichever is greater. The GDPR also confers a private right of
action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. If we fail to comply with the GDPR, the U.K. GDPR, and the U.K. Data Protection Act, we could face fines, penalties, and harm to our reputation.
The GDPR also imposes strict rules on the transfer of personal data to countries outside the EU and the U.K., including the United States. The European Commission has issued standard contractual clauses for data transfers from controllers or processors in the EU (or otherwise subject to the GDPR) to controllers or processors established outside the EU. The standard contractual clauses require exporters to assess the risk of a data transfer on a case-by-case basis, including an analysis of the laws in the destination country. The U.K. is not subject to the European Commission’s standard contractual clauses but has published a U.K.-specific transfer mechanism, which enables transfers from the U.K. The U.K.-specific mechanism, the “International Data Transfer Agreement”, requires a similar risk assessment of the transfer as the standard contractual clauses. Further, the EU and United States have adopted its adequacy decision for the EU-U.S. Data Privacy Framework ("Framework"), which entered into force on July 11, 2023. This Framework provides that the protection of personal data transferred between the EU and the United States is comparable to that offered in the EU. This provides a further avenue to ensuring transfers to the United States are carried out in line with GDPR. There has been an extension to the Framework to cover U.K. transfers to the United States. The Framework could be challenged like its predecessor frameworks. This complexity and the additional contractual burden increases our overall risk exposure. There may be further divergence in the future, including with regard to administrative burdens.
Increased cybersecurity requirements, vulnerabilities, threats, and more sophisticated and targeted computer crimes pose a risk to our systems, networks, products, solutions, services, and data, as well as our reputation, which could adversely affect our business.
We manufacture and sell products that rely upon software and computer systems to operate properly and process and store confidential information. Our products often are connected to, and reside within, our customers’ information technology (“IT”) infrastructures. In some jurisdictions, we are expected to design our products to include appropriate cybersecurity protections, and regulatory authorities may review such protections when granting marketing authorizations. While we seek to protect our products and IT systems from unauthorized access, these measures may not be effective, particularly because techniques used to obtain unauthorized access or to sabotage systems change frequently, increase in sophistication, and often are not identified at the time that they are launched against a target. These risks apply to our installed base of products, products we currently sell, new products we will introduce in the future, and older technology that we no longer sell or service but remains in use by customers. Additionally, we offer software and cloud products that are developed by, controlled by, or are hosted by third-party providers. A cybersecurity breach of our systems or products, of our customers’ or service providers’ network security and systems, or of other third-party services could disrupt treatment being delivered to patients or interfere with our customers’ operations, and could lead to the loss of, damage to, or public disclosure of our employees’ and customers’ stored information, including personal data, such as PHI. Such an event could have serious negative consequences, including alleged customer or patient harm, obligations to notify enforcement authorities or users of our products, voluntary or forced recalls of or modifications to our products, regulatory actions, fines, penalties and damages, reduced demand for or use of our offerings by customers, harm to our reputation, and time-consuming and expensive litigation, any of which could have a material adverse effect on our business results, cash flows, financial condition, or prospects.
There are increasingly large volumes of information, including patient data, being generated that need to be securely processed and stored by healthcare organizations. There has been an increase in the frequency and sophistication of the cybersecurity threats we and our service providers face, and we expect these activities to continue to increase. Geopolitical tensions or conflicts, such as the conflict between Russia and Ukraine and conflict in the Middle East, and the increased adoption of AI technologies, may further heighten the risk of cyber-attacks. Additionally, leveraging AI capabilities to potentially improve internal functions and operations presents further risks and challenges, including the possibility of creating new attack methods for adversaries. The use of AI to support business operations carries inherent risks related to data privacy, IP, and security, such as intended, unintended, or inadvertent transmission of proprietary, confidential, or sensitive information, as well as challenges related to implementing and maintaining AI tools, such as developing and maintaining appropriate datasets for such support. If we fail to implement adequate safeguards, the use of AI may introduce additional operational vulnerabilities by producing inaccurate outcomes based on flaws in the underlying data or methodologies, or unintended results.
Furthermore, we may also be exposed to a more significant risk if such actions are taken by state or state-affiliated actors. The objectives of these cyber-attacks vary widely and may include, among other things, unauthorized access to personal, customer, or third-party information, disruptions in operations and the provision of services to customers, or theft of IP or
other sensitive assets or information belonging to us, our business partners, or customers. As such attacks become more effective, the risks in this area continue to grow. The back-up systems we have in place may not be adequate in the event of a failure or interruption. We may not have current capabilities to identify all vulnerabilities, which may allow others to exploit persistent potential exposures within our IT systems and products. We could suffer significant business disruption, including transaction errors, supply chain or manufacturing interruptions, processing inefficiencies, data loss, loss of customers, reputational damage, the loss of or damage to IP or other proprietary information, litigation, investigation, and possible liability to employees, customers, suppliers, patients, and regulatory authorities as a result of a successful cyber-attack. Further, our ability to effectively plan, forecast, and execute our business plan and comply with applicable laws and regulations may be impaired by such cyber-attacks. Any of the above could have a material adverse effect on our business results, cash flows, financial condition, or prospects, and on the timeliness of reporting our operating results.
We rely on software, SaaS, hardware, and other material components from a number of third parties to manufacture our products. If a material cyber incident impacting a supplier were to result in its prolonged inability to use, manufacture, and/or ship such components, this could impact our ability to manufacture and/or use our products. In addition, third-party sourced software components, malicious code, or a critical vulnerability emerging within such software could expose our customers to increased cyber risk. While we have undertaken efforts to mitigate cybersecurity risks, these efforts may not prevent all incidents.
If we were to experience a significant cybersecurity breach of our information systems or data, the costs associated with the investigation, remediation, and potential notification of the breach to customers, regulators, and counterparties, as well as any related litigation expenses, fines, penalties, or damages, could be material. In addition, our remediation efforts may not be successful. The data privacy and IT security insurance coverage we currently maintain may be inadequate. In addition, the market for such insurance continues to evolve and, in the future, our data privacy and IT security insurance coverage may be prohibitively expensive or not available on acceptable terms or in sufficient amounts, or at all.
Broad-based domestic and international government initiatives to reduce spending, particularly those related to healthcare costs, may reduce reimbursement rates for medical procedures, which will reduce the cost-effectiveness of our products and services.
Healthcare reforms, changes in healthcare policies, and changes to third-party coverage and reimbursements, including legislation enacted reforming the U.S. healthcare system and both domestic and foreign healthcare cost containment legislation, and any future changes to such legislation, may affect demand for our products and services and may have a material adverse effect on our financial condition and results of operations. The ongoing implementation of the Affordable Care Act in the United States, as well as state-level healthcare reform proposals, could reduce medical procedure volumes and impact the demand for medical device products or the prices at which we can sell products. The impact of this healthcare reform legislation, and practices including price regulation, competitive pricing, comparative effectiveness of therapies, technology assessments, and managed care arrangements are uncertain. There can be no assurance that current levels of reimbursement will not be decreased in the future, or that future legislation, regulation, or reimbursement policies of third parties will not adversely affect the demand for our products and services or our ability to sell products and provide services on a profitable basis. The adoption of significant changes to the healthcare system in the United States, the EEA, or other jurisdictions in which we may market our products and services, could limit the prices we are able to charge for our products and services or the amounts of reimbursement available for our products and services, could limit the acceptance and availability of our products and services, reduce medical procedure volumes, and increase operational and other costs.
Healthcare industry cost-containment measures could result in reduced sales of our products and services.
Most of our customers rely on third-party payers, including government programs and private health insurance plans, to reimburse some or all of the cost of the procedures in which our products are used. The continuing efforts of governmental authorities, insurance companies, and other payers of healthcare costs to contain or reduce these costs could lead to patients being unable to obtain approval for payment from these third-party payers. If third-party payer payment approval cannot be obtained by patients for procedures that use our products, sales of our products may decline significantly and our customers may reduce or eliminate purchases of our products. The cost-containment measures that healthcare providers are instituting, both in the U.S. and outside of the U.S., could harm our ability to operate profitably. For example, GPOs and IDNs have also concentrated purchasing decisions for some customers, which has led to downward pricing pressure for medical device companies.
Risks Related to Butterfly’s Intellectual Property
If we are unable to protect our intellectual property, our ability to maintain any technological or competitive advantage over our competitors and potential competitors would be adversely impacted, and our business may be harmed.
We rely on patent protection as well as trademark, copyright, trade secret, and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. As of February 13, 2025, we owned approximately 620 issued patents and pending patent applications in the United States and foreign jurisdictions, including the European Union and the United Kingdom. These issued patents and pending patent applications (if they were to be issued as patents) have expected expiration dates ranging between approximately 2030 and 2045. If we fail to protect our intellectual property, third parties may be able to compete more effectively against us, we may lose our technological or competitive advantage, or we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property.
We cannot assure investors that any of our currently pending or future patent applications will result in granted patents, and we cannot predict how long it will take for such patents to be granted or whether the scope of such patents, if granted, will adequately protect our products from competitors. It is possible that, for any of our patents that have been granted or that may be granted in the future, others will design alternatives that do not infringe upon our patented technologies. Further, we cannot assure investors that other parties will not challenge any patents granted to us, or that courts or regulatory agencies will hold our patents to be valid or enforceable. We cannot guarantee investors that we will be successful in defending challenges made against our patents and patent applications. Any successful third-party challenge to our patents could result in the unenforceability or invalidity of such patents, or to such patents being interpreted narrowly or otherwise in a manner adverse to our interests. Our ability to establish or maintain a technological or competitive advantage over our competitors may be diminished because of these uncertainties. For these and other reasons, our intellectual property may not provide us with any competitive advantage. For example:
•We or our licensors might not have been the first to make the inventions covered by each of our pending patent applications or granted patents;
•We or our licensors might not have been the first to file patent applications for our inventions. To determine the priority of these inventions, we may have to participate in interference proceedings or derivation proceedings declared by the U.S. Patent and Trademark Office (“USPTO”) that could result in substantial cost to us. No assurance can be given that our patent applications or granted patents (or those of our licensors) will have priority over any other patent or patent application involved in such a proceeding;
•Others may independently develop similar or alternative products and technologies or duplicate any of our products and technologies;
•It is possible that our owned or licensed pending patent applications will not result in granted patents, and even if such pending patent applications grant as patents, they may not provide a basis for intellectual property protection of commercially viable products, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties;
•We may not develop additional proprietary products and technologies that are patentable;
•The patents of others may have an adverse effect on our business; and
•While we apply for patents covering our products and technologies and uses thereof, as we deem appropriate, we may fail to apply for patents on important products and technologies in a timely fashion or at all, or we may fail to apply for patents in potentially relevant jurisdictions
Filing, prosecuting, and defending patents on current and future products in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, regardless of whether we are able to prevent third parties from practicing our inventions in the United States, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Even if we pursue and obtain issued patents in particular jurisdictions, our patent claims or other intellectual property rights may not be effective or sufficient to prevent third parties
from competing. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
If we fail to protect our intellectual property, third parties may be able to compete more effectively against us, we may lose our technological or competitive advantage, or we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property. For these and other reasons, our intellectual property may not provide us with any competitive advantage.
Our wafer bonding technology for ultrasound applications is licensed to us by Stanford. Any loss of our rights to this technology could prevent us from selling our products.
Our wafer bonding technology for use in ultrasound applications is licensed to us from Stanford on a non-exclusive basis. We also license on a non-exclusive basis 7 active patents from Stanford. We do not own the patents that underlie these licenses. Our rights to use the licensed technology and employ the inventions claimed in the licensed patents are subject to the continuation of and compliance with the terms of the license. Our principal obligations under the license agreements with Stanford include the following:
•royalty payments;
•meeting certain milestones pertaining to development, commercialization, and sales of products using the licensed technology;
•annual maintenance fees;
•using commercially reasonable efforts to develop and sell a product using the licensed technology and developing a market for such product; and
•providing certain reports.
If we breach any of these obligations, Stanford may have the right to terminate the licenses, which could result in us being unable to develop, manufacture, and sell products using the licensed technology. Termination of our license agreements with Stanford would have a material adverse effect on our business.
In addition, we are a party to a number of other agreements that include licenses to intellectual property, including non-exclusive licenses. We may need to enter into additional license agreements in the future. Our business could suffer, for example, if any current or future licenses terminate, if the licensors fail to abide by the terms of the license, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms.
We may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our current or future products, and we cannot provide any assurances that we would be able to obtain such licenses.
In addition to agreements pursuant to which we in-license intellectual property, we have in the past, and we will in the future, grant licenses under our intellectual property. For example, we licensed parts of our Ultrasound on a Chip™ and other components of our intellectual property portfolio to Forest Neurotech in 2023, subject to contractual restrictions. Through programs like our Powered by Butterfly™, we expect to continue strategically granting licenses to our intellectual property subject to customary contractual provisions. Like in-licenses, out-licenses are complex, and disputes may arise between us and our licensees. Moreover, our licensees may breach their obligations, or we may be exposed to liability due to our failure or alleged failure to satisfy our obligations. Any such occurrence could have an adverse effect on our business.
Licensing intellectual property involves complex legal, business, and scientific issues. Disputes may arise between us and our licensors regarding intellectual property subject to a license agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
•our right to sublicense patent and other rights to third parties under collaborative development relationships;
•our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our products, and what activities satisfy those diligence obligations; and
•the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners.
If disputes over licensed intellectual property prevent or impair our ability to maintain the licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product, or the dispute may have an adverse effect on our results of operations.
If we or any of our partners are sued for infringing the intellectual property rights of third parties, such litigation would be costly and time consuming, and an unfavorable outcome in any such litigation could have a material adverse effect on our business. We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming, and unsuccessful.
Our success also depends on our ability to develop, manufacture, market, and sell our products and perform our services without infringing upon the proprietary rights of third parties. Numerous U.S. and foreign-issued patents and pending patent applications owned by third parties exist in the fields in which we are developing products and services. As is common in the medical device industry, we also engage the services of specialized consultants and employees who are currently providing or previously provided services to our competitors, and we may become subject to claims that we, an employee, a consultant, or an independent contractor inadvertently or otherwise used or disclosed trade secrets, intellectual property, or other information proprietary to their former employers or their former or current clients. As part of a business strategy to impede our successful commercialization and entry into new markets, competitors may claim that our products and/or services infringe their intellectual property rights and may suggest that we enter into license agreements.
Even if such claims are without merit, we could incur substantial costs and the attention of our management and technical personnel could be diverted in defending us against claims of infringement made by third parties or settling such claims. Any adverse ruling by a court or administrative body, or perception of an adverse ruling, may have a material adverse impact on our ability to conduct our business and our finances. Moreover, third parties making claims against us may be able to obtain injunctive relief against us, which could block our ability to offer one or more products or services and could result in a substantial award of damages against us. In addition, since we sometimes indemnify customers, collaborators, or licensees, we may have additional liability in connection with any infringement or alleged infringement of third-party intellectual property.
There is a substantial amount of litigation involving patent and other intellectual property rights in the medical device space. As we face increasing competition and as our business grows, we will likely face more claims of infringement. If a third party claims that we or any of our licensors, customers, or collaboration partners infringe upon a third party’s intellectual property rights, we may have to:
•seek licenses that may not be available on commercially reasonable terms, if at all;
•abandon any infringing product or redesign our products or processes to avoid infringement;
•pay substantial damages including, in an exceptional case, treble damages and attorneys’ fees, which we may have to pay if a court decides that the product or proprietary technology at issue infringes upon or violates the third-party’s rights;
•pay substantial royalties or fees or grant cross-licenses to our technology; or
•defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources.
Competitors may infringe our patents or the patents that we license. In the event of infringement or unauthorized use, we may file one or more infringement lawsuits, which can also be expensive and time-consuming. An adverse result in any such litigation proceedings could put one or more of our patents at risk of being invalidated, being found to be unenforceable, or being interpreted narrowly and could put our patent applications at risk of not issuing. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Patent litigation can be very costly and time consuming. Many of our competitors are larger than we are and have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex patent litigation longer
than we could. In addition, the uncertainties associated with litigation, or an adverse outcome, could have a material adverse effect on our ability to raise any funds necessary to continue our operations, continue our internal research programs, in-license needed technology, expose us to significant liabilities, or enter into development partnerships that would help us bring our products to market.
Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products.
The America Invents Act (“AIA”) was signed into law on September 16, 2011, and many of the substantive changes under the AIA became effective on March 16, 2013. The AIA is the primary governing legislation in the United States, and many of the countries we operate within have similar governing legislation. Additionally, courts and administrative bodies often issue rulings on matters related to patent and intellectual property enforcement actions, which may either adversely or beneficially impact our ability to enforce our patent and intellectual property rights within the United States and elsewhere. The laws governing patent prosecution and enforcement are subject to change in unpredictable ways, and such changes may be influenced by rulings of courts and other administrative bodies. These changes may weaken our ability to obtain new patents and/or enforce the rights of our existing patents.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic, or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected.
We may use third-party open source software components in future products, and failure to comply with the terms of the underlying open source software licenses could restrict our ability to sell such products.
We have chosen, and we may choose in the future, to use open source software in our products, including our Software Development Kit which is meant to provide a governed ecosystem for third parties to create content and applications that will serve to enrich the overall software ecosystem and deliver additional clinical and product advancements for our users. Use and distribution of open source software may entail greater risks than use of third-party commercial software, as open source licensors generally do not provide warranties or other contractual protections regarding infringement claims or the quality of the code. Some open source licenses may contain unfavorable requirements that could allow our competitors to create similar products with less development effort and time and ultimately could result in a loss of product sales.
Although we intend to monitor any use of open source software to avoid subjecting our products to conditions we do not intend, the terms of many open source licenses have not been interpreted by U.S. courts, and there is a risk that any such licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to commercialize our products. Moreover, there is no assurance that our processes for controlling our use of open source software in our products will be effective. If we are held to have breached the terms of an open source software license, we could be required to seek licenses from third parties to continue offering our products on terms that are not economically feasible, to re-engineer our products, to discontinue the sale of our products if re-engineering could not be accomplished on a timely basis, or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, operating results, and financial condition.
We use third-party software that may cause errors or failures of our products that could lead to lost customers or harm to our reputation.
We use software licensed from third parties in our products. Any errors or defects in third-party software or other third-party software failures could result in errors, defects, or cause our products to fail, which could harm our business and be costly to correct. Many of these providers attempt to impose limitations on their liability for such errors, defects, or failures, and if enforceable, we may have additional liability to our customers or third-party providers that could harm our reputation and increase our operating costs.
We will need to maintain our relationships with third-party software providers and to obtain software from such providers that does not contain any errors or defects. Any failure to do so could adversely impact our ability to deliver reliable products to our customers and could harm our reputation and results of operations.
Risks Related to Our Securities and to Being a Public Company
The Company’s outstanding warrants became exercisable for the Company’s Class A common stock on May 26, 2021. If the Company’s stock price reaches or exceeds $11.50, and outstanding warrants are exercised, the number of shares eligible for future resale in the public market will increase and result in dilution to our stockholders.
As of February 13, 2025, there were 13,799,357 outstanding public warrants to purchase 13,799,357 shares of our Class A common stock at an exercise price of $11.50 per share. In addition, as of February 13, 2025, there were 6,853,333 private placement warrants outstanding exercisable for 6,853,333 shares of our Class A common stock at an exercise price of $11.50 per share. To the extent such warrants are exercised, additional shares of our Class A common stock will be issued, which will result in dilution to the holders of our Class A common stock and increase the number of shares eligible for resale in the public market.
The change in fair value of our warrants is the result of changes in stock price and warrants outstanding at each reporting period. The change in fair value of warrant liabilities represents the mark-to-market fair value adjustments to the outstanding warrants issued in connection with the initial public offering of Longview. Significant changes in our stock price or number of warrants outstanding may adversely affect our net loss in our consolidated statements of operations.
If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, our ability to operate our business, and investors’ views of us.
We are required to comply with Section 404 of the Sarbanes-Oxley Act. Section 404 of the Sarbanes-Oxley Act requires public companies to maintain effective internal control over financial reporting. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting. In addition, we may be required to have our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting.
If we fail to maintain the effectiveness of our internal controls, fail to comply in a timely manner with the requirements of the Sarbanes-Oxley Act, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, this could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our common stock and we could be subject to sanctions or investigations by the NYSE, the SEC, or other regulatory authorities, which would require additional financial and management resources. In addition, if our efforts to comply with new or changed laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Because we are a “controlled company” within the meaning of the NYSE rules, our stockholders may not have certain corporate governance protections that are available to stockholders of companies that are not controlled companies. The dual class structure of our common stock has the effect of concentrating voting power with our founder, which will limit an investor’s ability to influence the outcome of important transactions, including a change in control.
Shares of our Class B common stock have 20 votes per share, while shares of our Class A common stock have one vote per share. As of December 31, 2024, Dr. Rothberg holds all of the issued and outstanding shares of our Class B common stock and holds approximately 75% of the voting power of our capital stock and is able to control matters submitted to our stockholders for approval, including the election of directors, amendments of our organizational documents, and any merger, consolidation, or sale of all or substantially all of our assets or other major corporate transactions. Dr. Rothberg may have interests that differ from yours and may vote in a way with which you disagree and which may be adverse to your interests. This concentrated control may have the effect of delaying, preventing or deterring a change in control of the Company, could deprive our stockholders of an opportunity to receive a premium for their capital stock as part of a sale of the Company, and may affect the market price of shares of our Class A common stock.
Delaware law and provisions in our certificate of incorporation and bylaws could make a takeover proposal more difficult.
Our organizational documents are governed by Delaware law. Certain provisions of Delaware law and of our certificate of incorporation and bylaws could discourage, delay, defer, or prevent a merger, tender offer, proxy contest, or other change of control transaction that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares of our Class A common stock held by our stockholders. These anti-takeover provisions as well as certain provisions of Delaware law could make it more difficult for a third party to acquire the Company, even if the third party’s offer may be considered beneficial by many of our stockholders. As a result, our stockholders may be limited in their ability to obtain a premium for their shares. If prospective takeovers are not consummated for any reason, we may experience negative reactions from the financial markets, including negative impacts on the price of our common stock. These provisions could also discourage proxy contests and make it more difficult for our stockholders to elect directors of their choosing and to cause the Company to take other corporate actions that our stockholders desire.
Our certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings, and the federal district courts as the sole and exclusive forum for other types of actions and proceedings, in each case, that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain what such stockholders believe to be a favorable judicial forum for disputes with the Company or our directors, officers, or other employees.
Our certificate of incorporation provides that, unless we consent to the selection of an alternative forum, any (i) derivative action or proceeding brought on behalf of the Company; (ii) action asserting a claim of breach of a fiduciary duty owed by, or any other wrongdoing by, any current or former director, officer, or other employee or stockholder of the Company; (iii) action asserting a claim against the Company arising pursuant to any provision of the DGCL, our certificate of incorporation, or our bylaws; (iv) action to interpret, apply, enforce, or determine the validity of any provisions in the certificate of incorporation or bylaws; or (v) action asserting a claim against the Company or any director or officer of the Company governed by the internal affairs doctrine, shall, to the fullest extent permitted by law, be exclusively brought in the Court of Chancery of the State of Delaware or, if such court does not have subject matter jurisdiction thereof, the federal district court of the State of Delaware. Subject to the foregoing, the federal district courts of the United States are the exclusive forum for the resolution of any action, suit, or proceeding asserting a cause of action under the Securities Act. The exclusive forum provision does not apply to suits brought to enforce any liability or duty created by the Exchange Act. Any person or entity purchasing or otherwise acquiring an interest in any shares of our capital stock shall be deemed to have notice of, and to have consented to the forum provisions in, our certificate of incorporation. These choice-of-forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that he, she, or it believes to be favorable for disputes with the Company or our directors, officers, or other employees or stockholders, which may discourage such lawsuits. We note that there is uncertainty as to whether a court would enforce these provisions and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
Alternatively, if a court were to find these provisions of our certificate of incorporation inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could materially adversely affect our business, financial condition, and results of operations and result in a diversion of the time and resources of our management and board of directors.
Litigation Risks
We face the risk of product liability claims and may be subject to damages, fines, penalties, and injunctions, among other things.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing, and marketing of medical devices, including those which may arise from the misuse (including system hacking or other unauthorized access by third parties to our systems) or malfunction of, or design flaws in, our hardware and software products. This liability may vary based on the FDA classification associated with our devices and with the laws of the state or other applicable jurisdiction governing product liability standards applied to specification developers and/or manufacturers in a given negligence or strict liability lawsuit. We may be subject to product liability claims if our products cause, or merely appear to have caused, an injury. Claims may be made by patients, healthcare providers, or others selling our products. The
risk of product liability claims may also increase if our products are subject to a product recall, whether voluntary or mandatory, or government seizure. Product liability claims may be brought by individuals or by groups seeking to represent a class.
Although we have insurance at levels that we believe to be appropriate, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, the coverage may not be adequate to protect us against any future product liability claims. Further, if additional medical device products are approved or cleared for marketing, or if we launch additional 510(k)-exempt device products or products that are not FDA-regulated medical devices, we may seek additional insurance coverage. If we are unable to obtain insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect against potential product liability claims, we will be exposed to significant liabilities, which may harm our business. A product liability claim, recall, or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could result in significant costs and significant harm to our business.
We may be subject to claims against us even if the apparent injury is due to the actions of others or misuse of the device or a partner device. Healthcare providers may use our products in a manner that is inconsistent with the products’ labeling and that differs from the manner in which they were used in clinical studies and authorized for marketing by the FDA. Off-label use of products by healthcare providers is common, and any such off-label use of our products could subject us to additional liability, or require design changes to limit this potential off-label use once discovered. Defending a suit, regardless of merit, could be costly, could divert management attention, and might result in adverse publicity, which could result in the withdrawal of, or result in reduced acceptance of, our products in the market.
Additionally, we have entered into various agreements where we indemnify third parties for certain claims relating to our products. These indemnification obligations may require us to pay significant sums of money for claims that are covered by these indemnification obligations. We are not currently subject to any product liability claims; however, any future product liability claims against us, regardless of their merit, may result in negative publicity about us that could ultimately harm our reputation and could have a material adverse effect on our business, financial condition, and results of operations.
We are currently subject to a securities class action lawsuit and stockholder derivative actions, the unfavorable outcomes of which may have a material adverse effect on our financial condition, results of operations, and cash flows.
On February 16, 2022, a purported class action lawsuit was filed against us, certain of our executive officers and directors, and certain of Longview’s executive officers and directors prior to the Business Combination, that, as amended, alleges violations of the Securities Act, Exchange Act, and Rule 10b-5 and Rule 14a-9 promulgated thereunder. The alleged class consists of all persons or entities who purchased or otherwise acquired the Company’s stock between January 12, 2021 and November 15, 2021 and/or holders as of the record date for the special meeting of shareholders held on February 12, 2021 in connection with the approval of the Business Combination. The lawsuit is premised upon allegations that the defendants made false and misleading statements and/or omissions about its technology, customer pipeline, and post-Business Combination business and financial prospects, including the impact of the COVID-19 pandemic. Additionally, two stockholder derivative actions were filed against our board of directors and us as nominal defendant, alleging similar violations as the class action lawsuit.
While we intend to vigorously defend against these actions, there is no assurance that we will be successful in the defense or that insurance will be available or adequate to fund any settlement or judgment or the litigation and indemnification costs of the actions. These actions may divert management resources, we may incur substantial costs, and any unfavorable outcome may have a material adverse effect on our financial condition, results of operations, and cash flows.
General Risk Factors
The price of our common stock historically has been volatile, which may affect the price at which you could sell any shares of our common stock.
The market price for our common stock historically has been highly volatile and could continue to be subject to wide fluctuations in response to various factors. This volatility may affect the price at which you could sell the shares of our common stock, and the sale of substantial amounts of our common stock could adversely affect the price of our common
stock. Our stock price is likely to continue to be volatile and subject to significant price and volume fluctuations in response to market and other factors, including:
•the success of our or competing products or technologies;
•developments or disputes concerning issued patents, patent applications, or other intellectual property rights;
•regulatory or legal developments in the U.S. and other countries;
•the recruitment or departure of key personnel;
•the level of expenses related to our products;
•the results of our efforts to discover, develop, manufacture, acquire, or in-license our current and additional products;
•actual or anticipated changes in estimates as to financial results, timelines, or recommendations by securities analysts;
•variations in our financial results or the financial results of companies that are perceived to be similar to us;
•sales of a substantial number of shares of our common stock in the public market, or the perception in the market that the holders of a large number of shares intend to sell shares;
•changes in the structure of healthcare payment systems;
•general economic, industry, and market conditions; and
•the other factors summarized and described in this Risk Factors section.
Companies trading in the stock market in general have also experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business, financial condition, results of operations, and growth prospects.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
Item 1C. CYBERSECURITY
Butterfly Network, Inc. uses, stores, and processes data for and about our customers, employees, partners, and suppliers. We have implemented a cybersecurity risk management program that is designed to identify, assess, and mitigate risks from cybersecurity threats to this data and our systems.
Risk Management Strategy and Governance
Under the ultimate direction of our Chief Executive Officer, our Information Security Committee has primary responsibility for overseeing our management of cybersecurity risks. It is chaired by our Chief Information Security Officer ("CISO") who reports directly to our Chief Technology Officer. Other members of the committee include representation from information technology, quality, product, operations, sales, and compliance as well as advisory support from internal audit.
Our CISO, working with his team and the Information Security Committee, has primary responsibility for assessing and managing our cybersecurity threat management program. The current CISO has more than 20 years of experience in building and leading security, risk management, and compliance organizations across several industries, including med-tech, healthcare, and financial services, and many of these companies include highly-regulated Fortune 500 companies. The current CISO is an expert in NIST 800-53 (Rev-5), ISO27001, and other national and international security risk management disciplines.
The Information Security Committee meets periodically and as circumstances warrant to discuss and monitor prevention, detection, and remediation of risks from cybersecurity threats. When appropriate, cyber or information security incidents would be escalated by the CISO to our executive leadership team and/or our disclosure committee. On a regular basis, the CISO also updates the executive management team on developments within the cybersecurity sphere.
The Board of Directors has delegated oversight of the Company’s cybersecurity program to the Audit Committee of the Board of Directors. As provided in the Audit Committee Charter, the Audit Committee is responsible for reviewing reports on data management, security initiatives, significant existing and emerging cybersecurity risks, including cybersecurity incidents, the impact on the Company and its stakeholders of any significant cybersecurity incident, and any disclosure obligations arising from any such incidents.
Our CISO meets at least quarterly with the Audit Committee of the Board of Directors to discuss management’s ongoing cybersecurity risk management programs. He provides information about the sources and nature of risks the Company faces, how management assesses such risks – including in terms of likelihood and severity of impact, progress on vulnerability remediation, and current developments in the cybersecurity landscape. In turn, the Chair of the Audit Committee provides a readout to the full Board of Directors that includes a summary of the CISO’s presentation to enable discussion of cybersecurity risk management at the full board level.
Although risks from cybersecurity threats have to date not materially affected, and we do not believe they are reasonably likely to materially affect, us, our business strategy, results of operations, or financial condition, we could, from time to time, experience threats and security incidents relating to our and our third party vendors’ information systems. For more information, please see Part I, Item 1A “Risk Factors.”
Processes for the Identification of Cybersecurity Threats
Our Information Security team is responsible for monitoring our information systems for vulnerabilities and mitigating any issues. It works with other groups in the Company to understand the severity and the likelihood of the potential consequences of a cybersecurity incident and to make decisions about how to prioritize mitigation and other initiatives based on, among other things, materiality to the business. The Information Security team has processes designed to keep the Company apprised of the different threats in the cybersecurity landscape – this includes intelligence networks alerts, working with researchers, discussions with peers at other companies, monitoring social media, reviewing government alerts and other news items, and attending security conferences. The team also regularly monitors our internal network and our customer-facing network to identify security risks. In addition, the team has completed several assessments and threat modeling tabletop exercises, based on “what-if” scenarios.
We have a mandatory employee education program that is designed to raise awareness of cybersecurity threats to reduce our vulnerability as well as to encourage consideration of cybersecurity risks across functions. Security training is required upon hire for new employees, and on an annual basis for the rest of the workforce.
As part of the assessment of the protections we have in place to mitigate risks from cybersecurity threats, we engage third parties to conduct vendor risk assessments. To assess the effectiveness of our program, we also have engaged consultants to conduct penetration testing and other vulnerability assessment.
Before purchasing third-party technology or other solutions that involve exposure to the Company’s assets and electronic information, our Information Technology group requires those companies to complete a security review before being approved to work with the Company. We utilize an external tool to manage critical vendors. Vendors are assessed to determine inherent and residual risk.
Annually, the security team conducts a risk assessment that is informed by industry standards.
The Risk Assessment consists of:
•Listing the vulnerabilities company assets are exposed to;
•Identifying the threats that may exploit such vulnerabilities;
•Calculating inherent risk rating based on impact and exploitability; and
•Calculating residual risk by assessing mitigating controls .
Item 2. PROPERTIES
We currently maintain our executive offices in Burlington, Massachusetts under a lease for approximately 60,000 rentable square feet consisting of the entire building. In addition to serving as our corporate headquarters, the office supports our sales, marketing, R&D, and other general and administrative functions. The lease expires in 2033.
Additionally, we occupy other office space domestically in New York City. We also occupy office space internationally in Taiwan. We lease the office spaces under operating leases. We consider our current office space adequate for our current operations.
Item 3. LEGAL PROCEEDINGS
We are currently and may in the future be subject to legal proceedings, claims, and regulatory actions arising in the ordinary course of business. The outcome of any such matters, regardless of the merits, is inherently uncertain.
For more information about our legal proceedings and this item, see Note 17, “Commitments and Contingencies” in the Notes to the Consolidated Financial Statements in Part II, Item 8 “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K, which is incorporated herein by reference.
Item 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our Class A common stock and warrants to purchase Class A common stock are traded on the NYSE under the symbols “BFLY” and “BFLY WS” respectively.
Stockholders
As of February 13, 2025, the Company had 216,496,214 shares of Class A common stock issued and outstanding held of record by 54 holders, 26,426,937 shares of Class B common stock issued and outstanding held of record by six holders. As of February 13, 2025, the Company had 13,799,357 public warrants held of record by one holder and 6,853,333 private placement warrants issued in connection with Longview’s initial public offering held of record by six holders, with each warrant exercisable for one share of Class A common stock at a price of $11.50 per share. We believe the actual numbers of holders of our Class A common stock and public warrants are larger than the numbers of holders of record as many of our Class A common stock and public warrants are held by brokers and other institutions on behalf of an indeterminate number of beneficial owners. These numbers of holders of record also do not include holders whose shares or warrants may be held in trust by other entities.
Dividends
We have never declared or paid cash dividends on our capital stock. We intend to retain all of our future earnings to finance the growth and development of our business. We do not intend to pay cash dividends to our stockholders in the foreseeable future.
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Offerings
Not applicable.
Issuer Purchases of Equity Securities
Not applicable.
Securities Authorized for Issuance Under Equity Compensation Plans
Information about our equity compensation plans will be included in our definitive proxy statement to be filed with the SEC with respect to our 2025 Annual Meeting of Stockholders (the “Proxy Statement”) and is incorporated herein by reference to Item 12 of Part II of this Annual Report on Form 10-K.
Item 6. [RESERVED]
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the financial condition and results of operations should be read in conjunction with the financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements reflecting our current expectations, estimates and assumptions concerning events and financial trends that may affect our future operating results or financial position. Actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the sections entitled “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” appearing elsewhere in this Annual Report on Form 10-K.
Overview
We are an innovative digital health business transforming care through a unique combination of portable, semiconductor-based ultrasound technology, intuitive software, services, and educational offerings that can make medical imaging more accessible than ever before. Butterfly’s solution enables the practical application of ultrasound information into the clinical workflow through affordable hardware that fits in a healthcare professional’s pocket and is paired with cloud-connected software that is easily accessed through a mobile application.
Butterfly developed ultrasound devices that can perform whole-body imaging in a single handheld probe because it is powered by our proprietary semiconductor technology instead of piezoelectric crystals. Our Ultrasound-on-Chip™ makes ultrasound more accessible outside of large healthcare institutions, while our software is intended to make the product easy to use, fully integrated with the clinical workflow, and accessible on a user’s smartphone, tablet, and almost any hospital computer system connected to the Internet. We aim to enable the delivery of imaging information anywhere at point-of-care to drive earlier detection throughout the body and remote management of health conditions. We market and sell the Butterfly system, which includes probes, related accessories, and software subscriptions, to healthcare systems, physicians, and healthcare providers through a direct sales force, distributors, and our eCommerce channel.
Since 2022, we have taken significant actions to reduce our cost of operations and extend our cash runway and have reduced our annual cash requirements by approximately $180 million, to less than $50 million annually. As we look forward, we expect to continue to invest in our business in order to grow revenue. On January 31, 2025, we raised additional capital through the issuance and sale in a public offering of 27.6 million shares of our Class A common stock, generating proceeds of $86.9 million, before underwriting costs and expenses.
Key Performance Measures
We review the key performance measures discussed below to evaluate the business and measure performance, identify trends, formulate plans, and make strategic decisions. Our key performance measures may fluctuate over time as the adoption of our devices increases, which may shift the revenue mix more toward software and other services. The quarterly measures may be impacted by the timing of device sales.
Units fulfilled
We define units fulfilled as the number of devices whereby control is transferred to a customer. We do not adjust this measure for returns as our volume of returns has historically been low. We view units fulfilled as a key indicator of the growth of our business. We believe that this measure is useful to investors because it presents our core growth and performance of our business period over period.
Units fulfilled increased by 3,131, or 19.0%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. The increase was led by higher volume across all U.S. sales channels as we began selling our next-generation iQ3 probe alongside our existing iQ+ probe. We also saw increased probe sales in our international distributor channel after onboarding several new distribution territories in the current year.
Software and other services mix
We define software and other services mix as a percentage of our total revenue recognized in a reporting period that is based on software subscriptions and other related services, consisting primarily of our software as a service (“SaaS”) offering. We view software and other services mix as a key indicator of the profitability of our business, and thus we believe that this measure is useful to investors.
Software and other services mix decreased by 5.3 percentage points, to 33.9% for the year ended December 31, 2024 compared to the year ended December 31, 2023. Although our software and other services revenue increased in the current year, our software and other services mix decreased due to the even larger increase in product revenue realized in the current year.
Description of Certain Components of Financial Data
Revenue
Revenue consists of revenue from the sale of products, such as medical devices and accessories, and the sale of software and related services, classified as software and other services revenue on our consolidated statements of operations and comprehensive loss. Our software and related service offerings include SaaS subscriptions, product support and maintenance (“Support”), and software development kits ("SDKs") which may be perpetual or term-based. SaaS subscriptions include licenses for teams and individuals as well as enterprise-level subscriptions. For sales of products and perpetual SDKs, revenue is recognized at a point in time upon transfer of control to the customer. SaaS subscriptions, Support, and term-based SDKs are generally related to stand-ready obligations and are recognized ratably over time.
Over time, as adoption of our devices increases through further market penetration and as practitioners in the Butterfly network continue to use our devices, we expect our annual revenue mix to shift more toward software and other services. The quarterly revenue mix may be impacted by the timing of device sales. In 2024, due to the continued success of our next-generation iQ3 probe, our software and other services mix as a percentage of total revenue decreased.
To date, we have invested in building out our commercial footprint, with the ultimate goal of growing adoption at large-scale healthcare systems and driving awareness of the usability of ultrasound. As we expand our healthcare system software offerings and develop relationships with larger healthcare systems, we continue to expect a higher proportion of our sales in healthcare systems compared to eCommerce.
Cost of revenue
Cost of product revenue consists of product costs including manufacturing costs, personnel costs and benefits, inbound freight, packaging, warranty replacement costs, payment processing fees, and inventory obsolescence and write-offs. We expect our cost of product revenue to fluctuate over time due to the level of units fulfilled in any given period and fluctuate as a percentage of product revenue over time as our focus on operational efficiencies in our supply chain may be offset by increased prices of certain inventory components.
Cost of software and other services revenue consists of personnel costs, cloud hosting costs and payment processing fees. Because the costs and associated expenses to deliver our SaaS offerings are less than the costs and associated expenses of manufacturing and selling our devices, we anticipate an improvement in profitability and margin expansion over time as our revenue mix shifts increasingly towards software and other services. We plan to continue to invest additional resources to expand and further develop our SaaS and other service offerings which will be reflected in cost of revenue as amortization expense.
Research and development
Research and development expenses primarily consist of personnel costs and benefits, professional services, facilities-related expenses and depreciation, fabrication services, and software costs. Most of our research and development expenses are related to developing new products and services that have not reached the point of commercialization and improving our products and services that have been commercialized. Fabrication services include certain third-party engineering costs, product testing, and test boards. Research and development expenses are expensed as incurred. We expect to continue to make substantial investments in our product and software development, clinical, and regulatory capabilities.
Sales and marketing
Sales and marketing expenses primarily consist of personnel costs and benefits, advertising, conferences and events, facilities-related expenses, and software costs. We expect to increase our investments in our commercial capabilities.
General and administrative
General and administrative expenses primarily consist of personnel costs and benefits, insurance, patent fees, software costs, facilities-related expenses, and outside services. Outside services consist of professional services, legal fees and other professional fees.
Other
Operating expenses classified as other are expenses which we do not consider representative of our ongoing operations. These other expenses primarily consist of employee severance and benefits costs related to our reductions in force and business transformation initiative, litigation costs, and legal settlements.
Results of Operations
We operate as a single reportable segment to reflect the way our chief operating decision maker reviews and assesses the performance of the business. The accounting policies are described in Note 2 “Summary of Significant Accounting Policies” in our consolidated financial statements included in this Annual Report on Form 10-K.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, |
| | 2024 | | 2023 | | 2022 |
(in thousands) | | Dollars | | % of revenue | | Dollars | | % of revenue | | Dollars | | % of revenue |
| Revenue: | | | | | | | | | | | | |
| Product | | $ | 54,200 | | | 66.1 | % | | $ | 40,036 | | | 60.8 | % | | $ | 50,263 | | | 68.5 | % |
| Software and other services | | 27,856 | | | 33.9 | | | 25,864 | | | 39.2 | | | 23,127 | | | 31.5 | |
| Total revenue | | 82,056 | | | 100.0 | | | 65,900 | | | 100.0 | | | 73,390 | | | 100.0 | |
| Cost of revenue: | | | | | | | | | | | | |
| Product | | 24,380 | | | 29.7 | | | 40,655 | | | 61.7 | | | 26,804 | | | 36.5 | |
| Software and other services | | 8,845 | | | 10.8 | | | 8,389 | | | 12.7 | | | 7,126 | | | 9.7 | |
| | | | | | | | | | | | |
| Total cost of revenue | | 33,225 | | | 40.5 | | | 49,044 | | | 74.4 | | | 33,930 | | | 46.2 | |
| Gross profit | | 48,831 | | | 59.5 | | | 16,856 | | | 25.6 | | | 39,460 | | | 53.8 | |
| Operating expenses: | | | | | | | | | | | | |
| Research and development | | 37,800 | | | 46.1 | | | 55,616 | | | 84.4 | | | 88,044 | | | 120.0 | |
| Sales and marketing | | 41,567 | | | 50.7 | | | 39,073 | | | 59.3 | | | 59,494 | | | 81.1 | |
| General and administrative | | 39,810 | | | 48.5 | | | 49,613 | | | 75.3 | | | 77,596 | | | 105.7 | |
| Other | | 4,065 | | | 5.0 | | | 18,164 | | | 27.6 | | | 7,346 | | | 10.0 | |
| Total operating expenses | | 123,242 | | | 150.3 | | | 162,466 | | | 246.6 | | | 232,480 | | | 316.8 | |
| Loss from operations | | (74,411) | | | (90.8) | | | (145,610) | | | (221.0) | | | (193,020) | | | (263.0) | |
| Interest income | | 5,020 | | | 6.1 | | | 7,450 | | | 11.3 | | | 3,384 | | | 4.6 | |
| Interest expense | | (1,261) | | | (1.5) | | | — | | | — | | | (2) | | | — | |
| Change in fair value of warrant liabilities | | (1,859) | | | (2.3) | | | 4,544 | | | 6.9 | | | 20,859 | | | 28.4 | |
| Other income (expense), net | | (13) | | | — | | | (2) | | | — | | | 98 | | | 0.1 | |
| Loss before provision for income taxes | | (72,524) | | | (88.5) | | | (133,618) | | | (202.8) | | | (168,681) | | | (229.9) | |
| Provision (benefit) for income taxes | | (32) | | | — | | | 82 | | | 0.1 | | | 42 | | | 0.1 | |
| Net loss and comprehensive loss | | $ | (72,492) | | | (88.5) | | | $ | (133,700) | | | (202.9) | | | $ | (168,723) | | | (230.0) | |
Comparison of the Years Ended December 31, 2024 and 2023
Revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2024 | | 2023 | | Change | | % Change |
| Product | | $ | 54,200 | | | $ | 40,036 | | | $ | 14,164 | | | 35.4 | % |
| Software and other services | | 27,856 | | | 25,864 | | | 1,992 | | | 7.7 | |
| | $ | 82,056 | | | $ | 65,900 | | | $ | 16,156 | | | 24.5 | % |
Product revenue increased by $14.2 million, or 35.4%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This increase was primarily driven by higher product revenue across nearly all sales channels, from both volume and the impact of our iQ3 probe’s higher selling price. The increase in product revenue was negatively impacted by two large grant-based deployments to medical schools that occurred in the prior year and did not repeat in 2024. Excluding the prior-year large medical school deployments, product revenue increased 44.4% for the year ended December 31, 2024 compared to the year ended December 31, 2023.
Software and other services revenue increased by $2.0 million, or 7.7%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This increase was primarily driven by higher enterprise software revenue and increased licensing revenue from our Butterfly Garden and Powered by Butterfly partnerships, partially offset by lower renewals of individual subscriptions. Enterprise as a percentage of software revenue increased by approximately 5 percentage points year-over-year.
Cost of revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2024 | | 2023 | | Change | | % Change |
| Product | | $ | 24,380 | | | $ | 40,655 | | | $ | (16,275) | | | (40.0) | % |
| Software and other services | | 8,845 | | | 8,389 | | | 456 | | | 5.4 | |
| | $ | 33,225 | | | $ | 49,044 | | | $ | (15,819) | | | (32.3) | % |
| Percentage of revenue | | 40.5 | % | | 74.4 | % | | | | |
Cost of product revenue decreased by $16.3 million, or 40.0%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This decrease was primarily driven by the non-recurrence of a $21.1 million loss on excess inventory in 2023 related to inventory on-hand that was deemed excess. Excluding this loss, prior year cost of product revenue was $19.6 million, and cost of product revenue increased in 2024 due to higher probe sales volume in 2024 resulting in increased costs of $4.1 million. In addition, in 2024 we experienced higher warranty costs resulting from an increase in our standard warranty.
Cost of subscription revenue increased by $0.5 million, or 5.4%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This increase was primarily driven by higher software amortization expenses but was partially offset by lower cloud hosting costs.
Research and development
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2024 | | 2023 | | Change | | % Change |
| Research and development | | $ | 37,800 | | | $ | 55,616 | | | $ | (17,816) | | | (32.0) | % |
| Percentage of revenue | | 46.1 | % | | 84.4 | % | | | | |
Research and development expenses decreased by $17.8 million, or 32.0%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This decrease was primarily driven by reductions of $11.9 million in personnel costs resulting from our business transformation initiative in 2024 to optimize our non-specialized technical functions as well as the reductions in force carried out in 2023. Additional reductions of $3.2 million in facilities and software costs and $1.9 million in product development costs are largely attributable to increased efficiencies resulting from optimization efforts.
Sales and marketing
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2024 | | 2023 | | Change | | % Change |
| Sales and marketing | | $ | 41,567 | | | $ | 39,073 | | | $ | 2,494 | | | 6.4 | % |
| Percentage of revenue | | 50.7 | % | | 59.3 | % | | | | |
Sales and marketing expenses increased by $2.5 million, or 6.4%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This increase was primarily driven by $1.8 million of higher personnel costs and $1.4 million of higher marketing and event expenses, resulting from investments in our sales force and marketing functions in order to support the growth associated with the launch of the iQ3 probe. These increased costs were partially offset by a $0.6 million reduction in facilities and software costs.
General and administrative
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2024 | | 2023 | | Change | | % Change |
| General and administrative | | $ | 39,810 | | | $ | 49,613 | | | $ | (9,803) | | | (19.8) | % |
| Percentage of revenue | | 48.5 | % | | 75.3 | % | | | | |
General and administrative expenses decreased by $9.8 million, or 19.8%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. This decrease was primarily driven by reductions of $6.2 million in personnel costs resulting from our reductions in force carried out in 2023, $2.7 million in professional service fees for legal and other administrative services, and $1.5 million in insurance costs.
Other
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2024 | | 2023 | | Change | | % Change |
| Other | | $ | 4,065 | | | $ | 18,164 | | | $ | (14,099) | | | (77.6) | % |
| Percentage of revenue | | 5.0 | % | | 27.6 | % | | | | |
Other decreased by $14.1 million for the year ended December 31, 2024 compared to the year ended December 31, 2023. This decrease was primarily driven by reductions of $7.4 million of employee severance and benefits costs related to our reductions in force carried out in 2023 and $6.8 million in legal costs due to litigation and other legal matters. These costs are not representative of our ongoing operations.
Comparison of the Years Ended December 31, 2023 and 2022
Revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2023 | | 2022 | | Change | | % Change |
| Product | | $ | 40,036 | | | $ | 50,263 | | | $ | (10,227) | | | (20.3) | % |
| Software and other services | | 25,864 | | | 23,127 | | | 2,737 | | | 11.8 | |
| | $ | 65,900 | | | $ | 73,390 | | | $ | (7,490) | | | (10.2) | % |
Product revenue decreased by $10.2 million, or 20.3%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This decrease was primarily driven by a decline in probe sales in our distribution, global health, and eCommerce channels. Additionally, there were a number of large sales in these channels in 2022 that did not reoccur in 2023. Partially offsetting these declines were increases in overall prices.
Software and other services revenue increased by $2.7 million, or 11.8%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This increase was primarily driven by a higher volume of SaaS subscriptions sold in conjunction with new device sales, current year subscription renewals, and expanded service offerings. We saw an increase in enterprise software sales of $2.4 million while individual subscriptions were flat. Enterprise as percentage of software sales increased 6%.
Cost of revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2023 | | 2022 | | Change | | % Change |
| Product | | $ | 40,655 | | | $ | 26,804 | | | $ | 13,851 | | | 51.7 | % |
| Software and other services | | 8,389 | | | 7,126 | | | 1,263 | | | 17.7 | |
| | $ | 49,044 | | | $ | 33,930 | | | $ | 15,114 | | | 44.5 | % |
| Percentage of revenue | | 74.4 | % | | 46.2 | % | | | | |
Cost of product revenue increased by $13.9 million, or 51.7%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This increase was primarily driven by a $21 million loss on excess inventory related to inventory on-hand that was deemed excess due to a shift in our strategy and market conditions. This was partially offset by lower probe volume and operating efficiencies in manufacturing Butterfly iQ+.
Cost of subscription revenue increased by $1.3 million, or 17.7%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This increase was primarily driven by higher amortization expenses related to newly deployed internally developed software that supports our SaaS offerings.
Research and development
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2023 | | 2022 | | Change | | % Change |
| Research and development | | $ | 55,616 | | | $ | 88,044 | | | $ | (32,428) | | | (36.8) | % |
| Percentage of revenue | | 84.4 | % | | 120.0 | % | | | | |
Research and development expenses decreased by $32.4 million, or 36.8%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This decrease was primarily driven by reductions of $23.5 million in personnel costs resulting from our reductions in force over the past year, a decrease of $2.6 million in engineering and testing costs, and a decrease of $4.6 million in consulting fees as we developed our internal capabilities to perform previously outsourced functions.
Sales and marketing
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2023 | | 2022 | | Change | | % Change |
| Sales and marketing | | $ | 39,073 | | | $ | 59,494 | | | $ | (20,421) | | | (34.3) | % |
| Percentage of revenue | | 59.3 | % | | 81.1 | % | | | | |
Sales and marketing expenses decreased by $20.4 million, or 34.3%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This decrease was primarily driven by reductions of $13.6 million in personnel costs resulting from our reductions in force over the past year. Reductions of $3.3 million in marketing expenses and $2.3 million in travel expenses also contributed to the decrease. We have started to reinvest in direct sales to drive top-line growth.
General and administrative
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2023 | | 2022 | | Change | | % Change |
| General and administrative | | $ | 49,613 | | | $ | 77,596 | | | $ | (27,983) | | | (36.1) | % |
| Percentage of revenue | | 75.3 | % | | 105.7 | % | | | | |
General and administrative expenses decreased by $28.0 million, or 36.1%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. This decrease was primarily driven by reductions of $22.0 million in
personnel costs resulting from our reductions in force over the past year and $4.9 million in professional service fees for legal and other administrative services.
Other
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| (in thousands) | | 2023 | | 2022 | | Change | | % Change |
| Other | | $ | 18,164 | | | $ | 7,346 | | | $ | 10,818 | | | 147.3 | % |
| Percentage of revenue | | 27.6 | % | | 10.0 | % | | | | |
Other increased by $10.8 million for the year ended December 31, 2023 compared to the year ended December 31, 2022. This increase was primarily driven by $6.7 million of higher employee severance and benefits costs resulting from our reductions in force in 2023 and $4.1 million of higher legal costs due to litigation and other legal matters. These costs are not representative of our ongoing operations.
Liquidity and Capital Resources
Since our inception, our primary sources of liquidity are cash flows from operations and proceeds from stock issuances and the Business Combination. Our primary uses of liquidity are operating expenses, working capital requirements, and capital expenditures.
During the year ended December 31, 2024, the Company utilized $45.9 million of cash and cash equivalents. As of December 31, 2024, our cash and cash equivalents balance was $88.8 million. On January 31, 2025, we raised an additional $86.9 million, before underwriting costs and expenses, through the issuance and sale in a public offering of 27.6 million shares of our Class A common stock. Our future spending on capital resources may vary from those currently planned and will depend on various factors, including our rate of revenue growth and the timing and extent of spending on strategic business initiatives.
We have restricted cash of $4.0 million as of December 31, 2024 to secure a letter of credit for one of our leases, which is expected to be maintained as a security deposit for the duration of the lease.
Our material cash requirements include contractual obligations with third parties for office leases, technology licensing agreements, and inventory supply agreements. Our fixed office lease payment obligations were $28.0 million as of December 31, 2024, with $3.7 million payable within the next 12 months. Our fixed technology license payment obligations were $14.0 million as of December 31, 2024, with $3.5 million payable within the next 12 months. Our fixed purchase obligations for inventory supply agreements, net of vendor advances, were $3.8 million as of December 31, 2024, all of which is payable within the next 12 months.
As of December 31, 2024, we had no obligations, assets or liabilities, which would be considered off-balance sheet arrangements.
Cash Flows
The following table summarizes our sources and uses of cash for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, |
| (in thousands) | | 2024 | | 2023 | | 2022 |
| Net cash used in operating activities | | $ | (41,707) | | | $ | (98,820) | | | $ | (169,115) | |
| Net cash provided by (used in) investing activities | | (2,658) | | | 70,414 | | | (93,779) | |
| Net cash provided by (used in) financing activities | | (1,495) | | | 228 | | | 2,881 | |
| Net decrease in cash, cash equivalents, and restricted cash | | $ | (45,860) | | | $ | (28,178) | | | $ | (260,013) | |
Comparison of the period for the years ended December 31, 2024 and 2023
Cash flows used in operating activities
Net cash used in operating activities represents the cash receipts and disbursements related to our activities other than investing and financing activities. We expect cash provided by historical financing activities will continue to be our primary source of funds to support operating needs and capital expenditures for the foreseeable future.
Net cash used in operating activities decreased by $57.1 million, or 57.8%, for the year ended December 31, 2024 compared to the year ended December 31, 2023. The decrease was comprised of improvements of $61.2 million in net loss and $15.3 million in net working capital cash usage. These improvements were partially offset by a decrease in noncash adjustments that resulted in $19.4 million less in add-backs to net loss, largely due to the $21.1 million write-down of inventories in 2023 that didn't recur in 2024. The decrease in net working capital cash usage was driven by reductions of $18.8 million in cash used for changes in our inventory and the related vendor advances and accrued purchase commitments and $8.4 million in cash used for changes in accounts payable and accrued expenses. These reductions were partially offset by an $8.3 million increase in cash used for changes in accounts receivable, a $1.8 million decrease in cash provided by changes in deferred revenue, and a $1.7 million decrease in cash provided by changes in prepaid expenses and other assets.
Cash flows provided by investing activities
Net cash provided by investing activities decreased by $73.1 million for the year ended December 31, 2024 compared to the year ended December 31, 2023. The decrease was primarily due to the sale of our marketable securities in 2023.
Cash flows provided by financing activities
Net cash provided by financing activities decreased by $1.7 million for the year ended December 31, 2024 compared to the year ended December 31, 2023. The decrease was primarily due to $2.0 million of payments made in connection with financing activities in 2024 that did not occur in 2023, partially offset by $0.5 million of proceeds from our employee stock purchase plan that began in 2024.
Comparison of the period for the years ended December 31, 2023 and 2022
Cash flows used in operating activities
Net cash used in operating activities represents the cash receipts and disbursements related to our activities other than investing and financing activities. We expect cash provided by historical financing activities will continue to be our primary source of funds to support operating needs and capital expenditures for the foreseeable future.
Net cash used in operating activities decreased by $70.3 million, or 41.6%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. The decrease was driven by a $9.3 million decrease in net working capital cash usage and a $61.0 million decrease in net loss adjusted for certain noncash items, primarily driven by the loss on excess inventory, the change in fair value of warrant liabilities, and net income. The decrease in net working capital cash usage was driven by a $17.7 million reduction in cash used for changes in our inventory and the related vendor advances and accrued purchase commitments, partially offset by a $4.5 million increase in cash used by accrued expenses and other liabilities, $2.9 million increase in cash used by operating lease assets and liabilities, and a $0.8 million increase in cash used by prepaid expenses and other assets.
Cash flows used in investing activities
Net cash used in investing activities decreased by $164.2 million, or 175.1%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. The decrease was primarily due to the purchase of marketable securities in 2022, and the subsequent sale of those securities in 2023. Additionally, there was a $12.5 million decrease in purchases of fixed assets.
Cash flows provided by financing activities
Net cash provided by financing activities decreased by $2.7 million, or 92.1%, for the year ended December 31, 2023 compared to the year ended December 31, 2022. The decrease was primarily due to the non-recurrence of net proceeds from exercise of stock options and warrants of $2.7 million, partially offset by $0.1 million from other financing activities.
Critical Accounting Policies and Estimates
Our consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The process of preparing financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the reported amounts of certain assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported amounts of revenue and expense during the period. We base our assumptions, judgments, and estimates on historical experience and various other factors that we believe to be reasonable under the circumstances. Actual results could differ materially from these estimates under different assumptions or conditions. We evaluate our assumptions, judgments, and estimates on a regular basis. Historically, our assumptions, judgments, and estimates relative to our critical accounting policies have not differed materially from actual results.
While our significant accounting policies are described in more detail in Note 2 “Summary of Significant Accounting Policies” in our consolidated financial statements, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our financial statements.
Revenue recognition
We generate revenue from the sale of products and software and other services. Our contracts with customers often include multiple performance obligations. Generally, we have identified the following performance obligations can be promised in our contracts with customers:
•Hardware devices and accessories;
•Software subscriptions, including renewal subscriptions, which represent an obligation to provide the customer with ongoing access to our cloud-hosted software applications on a continuous basis throughout the subscription period;
•Implementation and integration services;
•Extended warranties; and
•SDKs, either perpetual or term-based.
Transaction price is allocated to all identified performance obligations based on relative standalone selling prices of the underlying goods or services. Each sale of a hardware device, accessory, or perpetual SDK is a performance obligation satisfied at a point in time when control of the good transfers from us to the customer or when we provide the SDK to the customer. Our software subscriptions and extended warranties are stand-ready obligations that are satisfied over time, and our term-based SDKs are performance obligations satisfied over time through our continued provision of access to the customer. We use the time-elapsed (i.e., straight-line) measure of progress to recognize revenue for these services. Our implementation and integration services are a performance obligation satisfied over time, and we use costs incurred as inputs into the measure of progress to recognize revenue for these services.
We account for the warranty as an assurance-type warranty. When product revenue is recognized, an estimate of future warranty costs is recognized as cost of product revenue and accrued expenses. Factors that affect the estimate of future warranty costs include historical and current product failure rates, service delivery costs incurred in correcting product failures, and warranty policies and business practices.
Our contracts with customers include variable consideration in the form of refunds and credits for product returns and price concessions. We estimate variable consideration based on the individual contract characteristics or may apply a portfolio approach depending on the circumstances.
Stock-based compensation
Our stock-based compensation program includes stock option grants and restricted stock unit ("RSU") grants to our employees, directors, and consultants as well as an employee stock purchase plan ("ESPP"). Stock options are granted at exercise prices not less than the fair market value ("FMV") of our common stock on the grant date. The ESPP sets purchase prices as 85% of the lower of (i) the FMV of the stock on the offering date, or (ii) the FMV of the stock on the purchase date.
The grant date fair values of stock option grants and ESPP options are estimated using a Black-Scholes option-pricing model. Key inputs and assumptions include the underlying stock price, the exercise price, the risk-free interest rate, the expected dividend yield, the expected term of the option, and the expected stock price volatility. Many of the assumptions require significant judgment, and changes in assumptions could have a significant impact in the determination of stock-based compensation expense.
The grant date fair values of time-based and performance-based RSU grants are calculated as the FMV of our common stock on the grant date. The grant date fair values of market-based RSU grants are estimated using a Monte Carlo simulation with similar risk-free interest rate, expected dividend yield, and expected stock price volatility assumptions as those used in estimating the grant date fair value of our stock option grants and ESPP options.
Stock-based compensation expense is generally recognized evenly over the requisite service periods of awards, which is typically three to four years for RSU and stock option grants and typically two years for ESPP options. Stock-based compensation expense for performance-based and market-based RSU grants is generally recognized using the accelerated attribution method. We do not apply a forfeiture rate assumption to our awards.
No related tax benefits of the stock-based compensation expense have been recognized, and no related tax benefits have been realized from the exercise of stock options due to our net operating loss carryforwards.
Inventory and inventory valuation
Inventories are stated at the lower of actual cost, determined using the average cost method, or net realizable value (“NRV”). We routinely evaluate quantities and value of our inventories in light of current market conditions and market trends, and record a write-down against the cost of inventories for NRV below cost. NRV is based upon an estimated average selling price reduced by the estimated costs of completion, disposal, and transportation. The determination of NRV involves numerous judgments including estimating selling prices, existing customer orders, and estimated costs of completion, disposal, and transportation. If actual market conditions differ from our estimates, future results of operations could be materially affected. We reduce the value of our inventory for estimated obsolescence or lack of marketability by the difference between the cost of the affected inventory and the estimated market value.
The valuation of inventory also requires us to estimate excess and obsolete inventory. We periodically review the age, condition and turnover of our inventory to determine whether any inventory has become obsolete or has declined in value, and we incur a charge to operations for known and anticipated inventory obsolescence. We also consider the rate at which new products will be accepted in the marketplace and how quickly customers will transition from older products to newer products, including whether older products can be re-manufactured into new products. The evaluation also takes into consideration new product development schedules, the effect that new products might have on the sale of existing products, product obsolescence, product merchantability, and other factors. Market conditions are subject to change, and, if actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required, which would have a negative impact on gross margin.
Losses expected to arise from firm, non-cancelable, and unhedged commitments for the future purchase of inventory items are recognized unless the losses are recoverable through firm sales contracts or other means. We consider a variety of factors and data points when determining the existence and scope of a loss on the minimum purchase commitment. The factors and data points include Company-specific forecasts which are reliant on our limited sales history, agreement-specific provisions, macroeconomic factors, and market and industry trends. Determining the loss is subjective and requires significant management judgment and estimates. Future events may differ from those assumed in our assessment, and therefore the loss may change in the future.
We capitalize manufacturing overhead expenditures as part of inventory costs. Capitalized costs primarily include management’s best estimate and allocation of the direct labor, materials costs, and other overhead costs incurred related to
inventory acquired or produced but not sold during the respective period. Manufacturing overhead costs are capitalized to inventory and are recognized as cost of revenues in future periods based on our rate of inventory turnover.
Recently Adopted Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 “Summary of Significant Accounting Policies – Recent Accounting Pronouncements Issued but Not Yet Adopted” to our consolidated financial statements contained in this Annual Report on Form 10-K.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
We did not have any floating rate debt as of December 31, 2024. Our cash and cash equivalents are comprised primarily of bank deposits and money market accounts. The primary objective of our investments is the preservation of capital to fulfill liquidity needs. We do not enter into investments for trading or speculative purposes. Due to the short-term nature and low risk profile of these investments, we do not expect cash flows to be affected to any significant degree by a sudden change in market interest rates, including an immediate change of 100 basis points, or one percentage point. Declines in interest rates, however, would reduce future investment income.
Inflation Risk
We do not believe that inflation has had a material effect on our business, financial condition, or results of operations, other than its impact on the general economy. Nonetheless, to the extent our costs are subject to inflationary pressures, we may not be able to fully offset such higher costs through price increases or manufacturing efficiencies. Our inability or failure to do so could harm our business, financial condition, and results of operations.
Foreign Exchange Risk
We operate our business primarily within the United States and currently execute the majority of our transactions in U.S. dollars. We have not utilized hedging strategies with respect to our foreign exchange exposure. This limited foreign currency translation risk is not expected to have a material impact on our consolidated financial statements.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Our audited consolidated financial statements, together with the reports of our independent registered public accounting firm, for the years ended December 31, 2024, 2023, and 2022 appear beginning on page F-1 of this Annual Report on Form 10-K.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
Item 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the "Exchange Act").
Disclosure controls and procedures are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is
accumulated and communicated to management, including our Chief Executive Officer and Chief Financial & Operations Officer, to allow timely decisions regarding required disclosure. Based on the evaluation of our disclosure controls and procedures, our Chief Executive Officer and Chief Financial & Operations Officer concluded that our disclosure controls and procedures were effective as of December 31, 2024.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2024 based on the guidelines established in the Internal Control—Integrated Framework (2013 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Internal control over financial reporting includes policies and procedures that provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. GAAP.
Based on the results of its evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2024.
Attestation Report of the Registered Public Accounting Firm Regarding Internal Control Over Financial Reporting
This Annual Report on Form 10-K does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting because we are not an “accelerated filer” or “large accelerated filer.”
Changes in Internal Controls
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by Rules 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the fourth quarter ended December 31, 2024, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION
Rule 10b5-1 Trading Arrangements
During the quarter ended December 31, 2024, no director or officer (as defined in Rule 16a-1(f) of the Exchange Act) of the Company adopted, modified, or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408 of Regulation S-K.
Ratification of Prior Shares Issued
On January 28, 2025, our board of directors adopted resolutions (the “Resolutions”) approving, among other things, the ratification of the issuance of 16 shares of our Class A common stock in connection with the exercise of certain awards granted under the BFLY Operations, Inc. 2012 Employee, Director and Consultant Equity Incentive Plan, as amended, which may constitute shares of putative stock pursuant to Section 204 of the Delaware General Corporation Law (the “Ratification”).
The issuance of such shares occurred on such dates as set forth on Exhibit 99.1 attached hereto, resulting from the exercise by certain of our service providers of shares of our Class A common stock in excess of the number of shares underlying options to purchase shares of our Class A common stock approved by our board of directors to be issued in exchange for options of Legacy Butterfly upon the consummation of the Business Combination on February 12, 2021. The public filing of this document with the SEC constitutes the notice required to be given under Section 204 of the General Corporation Law of the State of Delaware (the “DGCL”) in connection with the Ratification. Any claim that any defective corporate act or putative stock ratified pursuant to the Ratification is void or voidable due to the failure of authorization specified in the Resolutions, or that the Delaware Court of Chancery should declare in its discretion that the Ratification in accordance with Section 204 of the DGCL not be effective or be effective only on certain conditions, must be brought within 120 days from the giving of this notice (which is deemed given on the date that this Annual Report on Form 10-K is filed with the SEC).
Item 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required under this item is incorporated herein by reference to the Proxy Statement.
Code of Business Conduct
We have adopted a code of business conduct that applies to all of our directors, officers, and employees, including our principal executive officer, principal financial officer, and principal accounting officer, which is available on our website at https://www.butterflynetwork.com under About Us – Investors – Governance – Corporate Governance. Our code of business conduct is a “code of ethics,” as defined in Item 406(b) of Regulation S-K. Please note that our Internet website address is provided as an inactive textual reference only. We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of this Code of Business Conduct and Ethics by posting such information on our website, at the address and location specified above and, to the extent required by the listing standards of the New York Stock Exchange, by filing a Current Report on Form 8-K with the SEC, disclosing such information.
Item 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference to the Proxy Statement.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated by reference to the Proxy Statement.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated by reference to the Proxy Statement.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Our independent public accounting firm is Deloitte & Touche LLP, New York, NY, USA, PCAOB Auditor ID 34.
The information required by this item is incorporated by reference to the Proxy Statement.
PART IV
Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| | | | | |
| Item 15(a). | The following documents are filed as part of this Annual Report on Form 10-K: |
| |
Item 15(a)(1)
and (2) | See “Index to Consolidated Financial Statements and Financial Statement Schedules” at Item 8 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto. |
| |
| Item 15(a)(3) | Exhibits |
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Exhibit Description | | Filed
Herewith | | Incorporated by
Reference herein
from
Form or Schedule | Filing Date | SEC File/
Reg. Number |
| 3.1 | | | | | | Form 8-K (Exhibit 3.1) | 6/13/2024 | 001-39292 |
| 3.2 | | | | | | Form 8-K (Exhibit 3.2) | 2/16/2021 | 001-39292 |
| 4.1 | | | | | | Form 10-K/A (Exhibit 4.1) | 3/28/2022 | 001-39292 |
| 4.2 | | | | | | Form 8-K (Exhibit 4.1) | 2/16/2021 | 001-39292 |
| 4.3 | | | | | | Form 8-K (Exhibit 4.1) | 5/27/2020 | 001-39292 |
| 10.1.1@ | | | | | | Form S-4 (Exhibit 10.13.1) | 11/27/2020 | 333-250995 |
| 10.1.2@ | | | | | | Form S-4 (Exhibit 10.13.2) | 11/27/2020 | 333-250995 |
| 10.2.1@ | | | | | | Form S-4 (Exhibit 10.14.1) | 11/27/2020 | 333-250995 |
| 10.2.2@ | | | | | | Form S-4 (Exhibit 10.14.2) | 11/27/2020 | 333-250995 |
| 10.2.3@ | | | | | | Form 10-K/A (Exhibit 10.6.3) | 5/12/2021 | 001-39292 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.3@ | | | | | | Form S-4 (Exhibit 10.15) | 11/27/2020 | 333-250995 |
| 10.4.1@ | | | | | | Form S-4/A (Exhibit 10.17.1) | 1/6/2021 | 333-250995 |
| 10.4.2@ | | | | | | Form S-4/A (Exhibit 10.17.2) | 1/6/2021 | 333-250995 |
| 10.5 | | | | | | Form S-4/A (Exhibit 10.18) | 1/6/2021 | 333-250995 |
10.6.1 | | | | | | Form 8-K (Exhibit 10.1) | 5/28/2021 | 001-39292 |
10.6.2 | | | | | | Form 8-K (Exhibit 99.2) | 1/29/2025 | 001-39292 |
| 10.7+ | | | | | | Form 10-Q (Exhibit 10.3) | 8/9/2021 | 001-39292 |
| 10.8+ | | | | | | Form 10-Q (Exhibit 10.1) | 5/6/2022 | 001-39292 |
| 10.9.1+ | | | | | | Form 8-K (Exhibit 10.1) | 4/24/2023 | 001-39292 |
| 10.9.2+ | | | | | | Form 10-Q (Exhibit 10.2) | 8/4/2023 | 001-39292 |
10.9.3+ | | | | | | Form 10-Q (Exhibit 10.2) | 8/1/2024 | 001-39292 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.10+ | | | | | | Form 10-Q (Exhibit 10.1) | 11/1/2024 | 001-39292 |
| 10.11+ | | | | | | Form 10-Q (Exhibit 10.3) | 11/15/2021 | 001-39292 |
| 10.12+ | | | | | | Form 10-K (Exhibit 10.25) | 2/16/2021 | 001-39292 |
10.13.1+ | | | | | | Form 10-K (Exhibit 10.19.1) | 3/29/2021 | 001-39292 |
10.13.2+ | | | | | | Form 8-K (Exhibit 10.15.2) | 2/16/2021 | 001-39292 |
10.13.3+ | | | | | | Form S-8 (Exhibit 99.3) | 5/12/2021 | 333-256044 |
10.14.1+ | | | | | | Form 10-K (Exhibit 10.20.1) | 3/29/2021 | 001-39292 |
10.14.2+ | | | | | | Form 8-K (Exhibit 10.16.2) | 2/16/2021 | 001-39292 |
10.14.3+ | | | | | | Form 8-K (Exhibit 10.16.3) | 2/16/2021 | 001-39292 |
| 10.15+ | | | | | | Form 10-Q (Exhibit 10.1) | 8/1/2024 | 001-39292 |
| 10.16+ | | | | | | Form 10-Q (Exhibit 10.4) | 8/3/2022 | 001-39292 |
| 10.17+ | | | | | | Form 8-K (Exhibit 10.18) | 2/16/2021 | 001-39292 |
| 10.18 | | | | | | Form 8-K (Exhibit 10.19) | 2/16/2021 | 001-39292 |
| 19.1 | | | | X | | | | |
| 21.1 | | | | | | Form 8-K (Exhibit 21.1) | 2/16/2021 | 001-39292 |
| 23.1 | | | | X | | | | |
| 31.1 | | | | X | | | | |
| 31.2 | | | | X | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 32.1 | | | | X* | | | | |
| 97.1+ | | | |
| | Form 10-K (Exhibit 97.1) | 3/4/2024 | 001-39292 |
| 99.1 | | | | X | | | | |
| 101.INS | | XBRL Instance Document. | | | | | | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document. | | | | | | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document. | | | | | | |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document. | | | | | | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document. | | | | | | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document. | | | | | | |
| 104 | | Cover Page Interactive Date File (formatted as Inline XBRL and contained in Exhibit 101). | | | | | | |
*Furnished herewith.
†Certain of the exhibits and schedules to this Exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Registrant agrees to furnish a copy of all omitted exhibits and schedules to the SEC upon its request.
+Management contract or compensatory plan or arrangement.
@Certain confidential portions of this Exhibit were omitted by means of marking such portions with brackets (“[***]”) because the identified confidential portions (i) are not material and (ii) would be competitively harmful if publicly disclosed.
Item 16. FORM 10-K SUMMARY
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| BUTTERFLY NETWORK, INC. |
| | | |
Date: February 28, 2025 | By: | /s/ Heather C. Getz, CPA |
| | | Heather C. Getz, CPA |
| | | Executive Vice President and Chief Financial & Operations Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | | | | |
| Signatures | | Title | | Date |
| | | | | |
| By: | /s/ Joseph DeVivo | | President, Chief Executive Officer, and Chairman of the Board (Principal Executive Officer) | | February 28, 2025 |
| Joseph DeVivo | |
| | |
| | | | | |
| | | | | |
| By: | /s/ Heather C. Getz, CPA | | Executive Vice President and Chief Financial & Operations Officer (Principal Financial Officer and Principal Accounting Officer) | | February 28, 2025 |
| Heather C. Getz, CPA | |
| | |
| | | | | |
| | | | | |
| By: | /s/ Dawn Carfora | | Director | | February 28, 2025 |
| Dawn Carfora | | | | |
| | | | | |
| By: | /s/ Elazer Edelman, M.D., Ph.D. | | Director | | February 28, 2025 |
| Elazer Edelman, M.D., Ph.D. | | | | |
| | | | | |
| By: | /s/ S. Louise Phanstiel | | Director | | February 28, 2025 |
| S. Louise Phanstiel | | | | |
| | | | | |
| By: | /s/ Larry Robbins | | Director | | February 28, 2025 |
| Larry Robbins | | | | |
| | | | | |
| By: | /s/ Jonathan M. Rothberg, Ph.D. | | Director | | February 28, 2025 |
| Jonathan M. Rothberg, Ph.D. | | | | |
| | | | | |
| By: | /s/ Erica Schwartz, M.D., J.D., M.P.H. | | Director | | February 28, 2025 |
| Erica Schwartz, M.D., J.D., M.P.H. | | | | |
INDEX TO FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of Butterfly Network, Inc. and subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Butterfly Network, Inc. and subsidiaries (the "Company") as of December 31, 2024 and 2023, the related consolidated statements of operations and comprehensive loss, changes in stockholders' equity, and cash flows, for each of the three years in the period ended December 31, 2024, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Inventory Raw Materials Valuation – Refer to “Note 2. Summary of Significant Accounting Policies and Note 5. Inventories” to the financial statements
Critical Audit Matter Description
The Company purchases inventory under long-term supply agreements with third-party manufacturing vendors. The agreements require minimum purchases of inventory by the Company, which represent firm commitments to take or pay for the product.
The Company compares the on-hand inventory plus minimum commitments remaining, to their projected future sales of product and determines whether they would be able to sell the product for greater than cost, prior to any estimated obsolescence period or changes in technology, and establishes inventory write-downs for any losses on projected excess quantities of raw materials on hand as well as liabilities for any excess quantities not on hand but that they are committed to purchase. Projections of future sales of inventory are based on a number of factors, including new product development
schedules, the effect that new products might have on the sale of existing products, product obsolescence, product merchantability, and whether older products can be remanufactured into new products, among other factors. Significant judgments and estimates are made by the Company in evaluating the projected sales and evaluating expected losses on excess quantities, if any, at the end of each reporting period.
We identified the valuation of raw materials inventory as a critical audit matter because of the complexity associated with the assumptions used and judgments made to determine the excess and obsolescence reserve. Auditing such assumptions and judgments involved a high degree of auditor judgment and an increased extent of audit effort.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the management’s evaluation of the valuation of raw materials inventory included the following, among others:
•With respect to management’s evaluation of the valuation of raw materials inventory, we performed the following procedures:
-Read relevant contracts and compared key provisions of the contracts to the Company’s analysis.
-Recalculated the Company’s analysis of the valuation of raw materials inventory, including future purchase commitments, by comparing on-hand inventory used in the analysis to inventory observed via annual physical counts and confirmations with third parties, as well as comparing the remaining fixed minimum purchases used in management’s analysis to the third-party contracts.
-Obtained and evaluated the Company’s projected sales of inventory by performing the following:
▪Compared management’s prior-year assumptions of expected future sales to actual sales during the current year to identify potential bias in the determination of the reserves.
▪Compared projections to recent sales history and related trends.
▪Compared projections to industry information, market data, and peer group data.
▪Inspected minutes of the board of directors, regulatory and other public filings, and investor communications to identify any evidence that may contradict management’s assertions.
▪Obtained evidence, including executed third-party contracts used by management to support sales strategies reflected in the analysis.
▪Inquired of sales and operations personnel regarding projections and strategies to determine whether it supported or contradicted the conclusions reached by management in the analysis.
▪Inquired of operations personnel as to projected technology obsolescence, if any, to determine whether it supported or contradicted the conclusions reached by management in the analysis.
/s/ Deloitte & Touche LLP
New York, New York
February 28, 2025
We have served as the Company’s auditor since 2020.
BUTTERFLY NETWORK, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| | | | | | | | | | | |
| December 31, |
| 2024 | | 2023 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 88,775 | | | $ | 134,437 | |
| | | |
Accounts receivable, net of allowance for doubtful accounts of $2,583 and $1,787 at December 31, 2024 and December 31, 2023, respectively | 20,793 | | | 13,418 | |
| Inventories | 70,789 | | | 73,022 | |
| Current portion of vendor advances | 5,547 | | | 2,815 | |
| Prepaid expenses and other current assets | 6,709 | | | 7,571 | |
| Total current assets | 192,613 | | | 231,263 | |
| Property and equipment, net | 19,518 | | | 25,321 | |
| Intangible assets, net | 8,916 | | | 10,317 | |
| Non-current portion of vendor advances | 15,042 | | | 15,276 | |
| Operating lease assets | 14,233 | | | 15,675 | |
| Other non-current assets | 5,760 | | | 6,422 | |
| Total assets | $ | 256,082 | | | $ | 304,274 | |
| Liabilities and stockholders’ equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 4,250 | | | $ | 5,090 | |
| Deferred revenue, current | 16,139 | | | 15,625 | |
| Accrued purchase commitments, current | 131 | | | 131 | |
| Accrued expenses and other current liabilities | 27,695 | | | 23,425 | |
| Total current liabilities | 48,215 | | | 44,271 | |
| Deferred revenue, non-current | 7,315 | | | 7,394 | |
| Warrant liabilities | 2,685 | | | 826 | |
| Operating lease liabilities | 20,398 | | | 22,835 | |
| Other non-current liabilities | 8,637 | | | 8,895 | |
| Total liabilities | 87,250 | | | 84,221 | |
Commitments and contingencies (Note 17) | | | |
| Stockholders’ equity: | | | |
Class A common stock $.0001 par value; 600,000,000 shares authorized at December 31, 2024 and December 31, 2023; 188,626,154 and 181,221,794 shares issued and outstanding at December 31, 2024 and December 31, 2023, respectively | 19 | | | 18 | |
Class B common stock $.0001 par value; 27,000,000 shares authorized at December 31, 2024 and December 31, 2023; 26,426,937 shares issued and outstanding at December 31, 2024 and December 31, 2023 | 3 | | | 3 | |
| Additional paid-in capital | 970,940 | | | 949,670 | |
| Accumulated deficit | (802,130) | | | (729,638) | |
| Total stockholders’ equity | 168,832 | | | 220,053 | |
| Total liabilities and stockholders’ equity | $ | 256,082 | | | $ | 304,274 | |
The accompanying notes are an integral part of these consolidated financial statements.
BUTTERFLY NETWORK, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except shares and per share amounts)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Revenue: | | | | | |
| Product | $ | 54,200 | | | $ | 40,036 | | | $ | 50,263 | |
| Software and other services | 27,856 | | | 25,864 | | | 23,127 | |
| Total revenue | 82,056 | | | 65,900 | | | 73,390 | |
| Cost of revenue: | | | | | |
| Product | 24,380 | | | 40,655 | | | 26,804 | |
| Software and other services | 8,845 | | | 8,389 | | | 7,126 | |
| | | | | |
| Total cost of revenue | 33,225 | | | 49,044 | | | 33,930 | |
| Gross profit | 48,831 | | | 16,856 | | | 39,460 | |
| Operating expenses: | | | | | |
| Research and development | 37,800 | | | 55,616 | | | 88,044 | |
| Sales and marketing | 41,567 | | | 39,073 | | | 59,494 | |
| General and administrative | 39,810 | | | 49,613 | | | 77,596 | |
| Other | 4,065 | | | 18,164 | | | 7,346 | |
| Total operating expenses | 123,242 | | | 162,466 | | | 232,480 | |
| Loss from operations | (74,411) | | | (145,610) | | | (193,020) | |
| Interest income | 5,020 | | | 7,450 | | | 3,384 | |
| Interest expense | (1,261) | | | — | | | (2) | |
| Change in fair value of warrant liabilities | (1,859) | | | 4,544 | | | 20,859 | |
| Other income (expense), net | (13) | | | (2) | | | 98 | |
| Loss before provision for income taxes | (72,524) | | | (133,618) | | | (168,681) | |
| Provision (benefit) for income taxes | (32) | | | 82 | | | 42 | |
| Net loss and comprehensive loss | $ | (72,492) | | | $ | (133,700) | | | $ | (168,723) | |
| Net loss per common share attributable to Class A and B common stockholders, basic and diluted | $ | (0.34) | | | $ | (0.65) | | | $ | (0.84) | |
| Weighted-average shares used to compute net loss per share attributable to Class A and B common stockholders, basic and diluted | 211,682,760 | | 205,385,544 | | 199,848,386 |
The accompanying notes are an integral part of these consolidated financial statements.
BUTTERFLY NETWORK, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Class A
Common
Stock | | Class B
Common
Stock | | Additional
Paid-In
Capital | | Accumulated
Deficit | | Total Stockholders’ Equity |
| | | | | | Shares | | Amount | | Shares | | Amount | | | |
| December 31, 2021 | | | | | | 171,613,049 | | $ | 17 | | | 26,426,937 | | $ | 3 | | | $ | 874,886 | | | $ | (427,215) | | | $ | 447,691 | |
| Net loss | | | | | | — | | — | | | — | | — | | | — | | | (168,723) | | | (168,723) | |
| Common stock issued upon exercise of stock options and warrants | | | | | | 1,081,313 | | — | | | — | | — | | | 2,982 | | | — | | | 2,982 | |
| Common stock issued upon vesting of restricted stock units, net | | | | | | 1,765,594 | | — | | | — | | — | | | (106) | | | — | | | (106) | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Stock-based compensation expense | | | | | | — | | — | | | — | | — | | | 43,516 | | | — | | | 43,516 | |
| December 31, 2022 | | | | | | 174,459,956 | | 17 | | | 26,426,937 | | 3 | | | 921,278 | | | (595,938) | | | 325,360 | |
| Net loss | | | | | | — | | — | | | — | | — | | | — | | | (133,700) | | | (133,700) | |
| Common stock issued upon exercise of stock options | | | | | | 180,467 | | — | | | — | | — | | | 228 | | | — | | | 228 | |
| Common stock issued upon vesting of restricted stock units, net | | | | | | 6,581,371 | | 1 | | | — | | — | | | — | | | — | | | 1 | |
| Stock-based compensation expense | | | | | | — | | — | | | — | | — | | | 28,164 | | | — | | | 28,164 | |
| December 31, 2023 | | | | | | 181,221,794 | | 18 | | | 26,426,937 | | 3 | | | 949,670 | | | (729,638) | | | 220,053 | |
| Net loss | | | | | | — | | — | | | — | | — | | | — | | | (72,492) | | | (72,492) | |
| Common stock issued upon exercise of stock options | | | | | | 36,340 | | — | | | — | | — | | | 64 | | | — | | | 64 | |
| Common stock issued upon vesting of restricted stock units | | | | | | 6,696,585 | | 1 | | | — | | — | | | (739) | | | — | | | (738) | |
Common stock issued for employee stock purchase plan | | | | | | 671,435 | | — | | | — | | — | | | 495 | | | — | | | 495 | |
| Stock-based compensation expense | | | | | | — | | — | | | — | | — | | | 21,450 | | | — | | | 21,450 | |
| December 31, 2024 | | | | | | 188,626,154 | | $ | 19 | | | 26,426,937 | | $ | 3 | | | $ | 970,940 | | | $ | (802,130) | | | $ | 168,832 | |
The accompanying notes are an integral part of these consolidated financial statements.
BUTTERFLY NETWORK, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (72,492) | | | $ | (133,700) | | | $ | (168,723) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation, amortization, and impairments | 10,342 | | | 10,574 | | | 5,935 | |
| | | | | |
Non-cash interest expense | 1,256 | | | — | | | — | |
| Write-down of inventories | 15 | | | 21,083 | | | 783 | |
| Stock-based compensation expense | 21,032 | | | 27,480 | | | 42,531 | |
| Change in fair value of warrant liabilities | 1,859 | | | (4,544) | | | (20,859) | |
| Gain on lease termination | — | | | (214) | | | — | |
| Other | 1,102 | | | 633 | | | 615 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable | (8,503) | | | (162) | | | (3,063) | |
| Inventories | 2,218 | | | (34,135) | | | (24,510) | |
| Prepaid expenses and other assets | 1,304 | | | 2,979 | | | 3,819 | |
| Vendor advances | (2,498) | | | 17,091 | | | 5,100 | |
| Accounts payable | (841) | | | (1,875) | | | 1,216 | |
| Deferred revenue | 435 | | | 2,206 | | | 2,266 | |
| Accrued purchase commitments | — | | | (2,015) | | | (17,383) | |
| Change in operating lease assets and liabilities | (750) | | | (635) | | | 2,257 | |
| Accrued expenses and other liabilities | 3,814 | | | (3,586) | | | 901 | |
| Net cash used in operating activities | (41,707) | | | (98,820) | | | (169,115) | |
| | | | | |
| Cash flows from investing activities: | | | | | |
| Purchases of marketable securities | — | | | (297) | | | (75,534) | |
| Sales of marketable securities | — | | | 76,484 | | | — | |
| Purchases of property, equipment, and intangible assets, including capitalized software | (2,694) | | | (5,783) | | | (18,302) | |
| Sales of property and equipment | 36 | | | 10 | | | 57 | |
| Net cash provided by (used in) investing activities | (2,658) | | | 70,414 | | | (93,779) | |
| | | | | |
| Cash flows from financing activities: | | | | | |
| Proceeds from exercise of stock options and warrants | 64 | | | 228 | | | 2,982 | |
Proceeds from employee stock purchase plan | 495 | | | — | | | — | |
Payments to tax authorities for restricted stock units withheld | (739) | | | — | | | (101) | |
Payments on technology license commitment | (1,315) | | | — | | | — | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Net cash provided by (used in) financing activities | (1,495) | | | 228 | | | 2,881 | |
| Net decrease in cash, cash equivalents, and restricted cash | (45,860) | | | (28,178) | | | (260,013) | |
| Cash, cash equivalents, and restricted cash, beginning of period | 138,650 | | | 166,828 | | | 426,841 | |
| Cash, cash equivalents, and restricted cash, end of period | $ | 92,790 | | | $ | 138,650 | | | $ | 166,828 | |
| | | | | |
| Supplementary disclosure of non-cash investing and financing activities | | | | | |
| Acquisition of property, equipment, and intangible assets, including capitalized software | $ | 470 | | | $ | 9,247 | | | $ | 4,501 | |
The accompanying notes are an integral part of these consolidated financial statements.
BUTTERFLY NETWORK, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Organization and Description of Business
Butterfly Network, Inc., formerly known as Longview Acquisition Corp., was incorporated in Delaware on February 4, 2020. The Company is an innovative digital health business transforming care through a unique combination of portable, semiconductor-based ultrasound technology, intuitive software, services and educational offerings that can make medical imaging more accessible than ever before. Butterfly’s solution enables the practical application of ultrasound information into the clinical workflow through affordable hardware that fits in a healthcare professional’s pocket and is paired with cloud-connected software that is easily accessed through a mobile application.
The Company operates wholly-owned subsidiaries in Australia, Germany, the Netherlands, Taiwan, and the United Kingdom.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements include the accounts of BFLY Operations, Inc. (formerly Butterfly Network, Inc.) and its wholly-owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the accounting disclosure rules and regulations of the Securities and Exchange Commission (the “SEC”). All intercompany balances and transactions have been eliminated in consolidation.
Functional Currency
The Company’s worldwide operations utilize the U.S. dollar (“USD”) as the functional currency considering the significant dependency of each subsidiary on the Company. Subsidiary operations are financed through the funding received from the Company in USD. For foreign entities where the USD is the functional currency, all foreign currency-denominated monetary assets and liabilities are remeasured at end-of-period exchange rates. Exchange gains and losses arising from the remeasurement of foreign currency-denominated monetary assets and liabilities are included in other income (expense), net in the consolidated statements of operations and comprehensive loss.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash and cash equivalents and accounts receivable. As of December 31, 2024 and 2023, substantially all of the Company’s cash and cash equivalents were invested in money market accounts with one financial institution. The Company also maintains balances in various operating accounts above federally insured limits. The Company has not experienced any significant losses on such accounts and does not believe it is exposed to any significant credit risk on cash and cash equivalents.
As of December 31, 2024 and 2023, one customer and no customers accounted for more than 10% of the Company’s accounts receivable, respectively. For the years ended December 31, 2024, 2023, and 2022, no customer accounted for more than 10% of the Company’s total revenue.
Segment Information
The Company has determined that it operates in one reportable segment, which includes all activities related to the development, manufacture, and sale of the Company's products, software, and other services. The Company’s chief operating decision maker ("CODM"), its chief executive officer ("CEO"), regularly reviews the Company's consolidated net loss, which is reported as net loss and comprehensive loss on the consolidated statements of operations and comprehensive loss, for purposes of evaluating the Company's financial performance, including reviewing budget versus actual results, and determining changes in the Company's allocation of resources across the Company's strategic initiatives. The Company's measure of segment assets is total assets, as reported on the consolidated balance sheets, and substantially all of the Company’s long-lived assets are located in the United States.
In addition to the operating expenses presented on the consolidated statements of operations and comprehensive loss, the CODM also reviews certain significant segment expenses. The following table summarizes the Company's segment revenue and significant segment expenses included in consolidated net loss (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
Revenue | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | |
Less: | | | | | |
Cost of revenue | 33,225 | | | 49,044 | | | 33,930 | |
Payroll operating expenses | 57,334 | | | 67,241 | | | 111,361 | |
Stock-based compensation expense | 21,032 | | | 27,480 | | | 42,531 | |
Non-payroll operating expenses | 40,811 | | | 49,581 | | | 71,242 | |
Other | 4,065 | | | 18,164 | | | 7,346 | |
Other segment items | (1,919) | | | (11,910) | | | (24,297) | |
Net loss | $ | (72,492) | | | $ | (133,700) | | | $ | (168,723) | |
Other segment items include interest income, interest expense, the change in fair value of warrant liabilities, other income (expense), net, and the provision (benefit) for income taxes.
Because the Company operates in one reportable segment, other required segment disclosures are included on the Company's consolidated financial statements. Interest income, interest expense, and the provision (benefit) for income taxes are included on the consolidated statements of operations and comprehensive loss. Depreciation, amortization, and impairments; write-down of inventories; and purchases of property, equipment, and intangible assets, including capitalized software, are included on the consolidated statements of cash flows.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires the Company to make estimates and assumptions about future events that affect the amounts reported in its consolidated financial statements and accompanying notes. Future events and their effects cannot be determined with certainty. On an ongoing basis, management evaluates these estimates and assumptions. Significant estimates and assumptions include:
•revenue recognition, including determination of the timing and pattern of satisfaction of performance obligations and determination of the standalone selling price (“SSP”) of performance obligations;
•measurement and allocation of capitalized costs, the net realizable value (the selling price as well as estimated costs of completion, disposal and transportation) of inventory, and demand and future use of inventory;
•assumptions underlying the capitalization of costs to develop or obtain software for internal use;
•assumptions underlying incremental borrowing rate calculations;
•assumptions underlying the measurement of contingent losses; and
•assumptions underlying the fair value used in the stock-based compensation expense calculation.
The Company bases these estimates on historical and anticipated results and trends and on various other assumptions that the Company believes are reasonable under the circumstances, including assumptions about future events. Changes in estimates are recorded in the period in which they become known. Actual results could differ from these estimates, and any such differences may be material to the Company’s consolidated financial statements.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“Topic 606”). Revenue is recognized when or as a customer obtains control of the promised goods and services. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to in exchange for these goods and services. To achieve this core principle, the Company applies the following five steps:
•Step 1: Identify Contracts with Customers: The Company typically enters into contracts with customers through direct sales executed via signed contracts with payment terms of 30 days or less. Multi-year software subscriptions typically require advance payment for each annual subscription period.
•Step 2: Identify Performance Obligations: The Company’s contracts with customers often include multiple performance obligations. The Company has identified the following performance obligations in its contracts with customers:
•Hardware devices and accessories;
•Software subscriptions, including renewal subscriptions, which represent an obligation to provide the customer with ongoing access to the Company’s cloud-hosted software applications on a continuous basis throughout the subscription period;
•Implementation and integration services;
•Extended warranties; and
•SDKs, either perpetual or term-based.
•Step 3: Determine Transaction Price: The Company’s contracts with customers include variable consideration in the form of refunds and credits for product returns and price concessions. The Company estimates variable consideration based on the individual contract circumstances or by using the expected value method based on a portfolio of data from similar contracts, depending on the circumstances.
•Step 4: Allocate Transaction Price to Performance Obligations: The Company allocates transaction price to the performance obligations in contracts with customers based on the relative SSPs of the underlying goods and services which the Company has a history of selling to customers on a standalone basis.
•Step 5: Recognize Revenue as Performance Obligations are Satisfied: Each sale of a hardware device, accessory, or perpetual SDK is a performance obligation satisfied at a point in time when control of the good transfers from the Company to the customer based on shipping terms or when the SDK is provided to the customer. The Company’s software subscriptions and extended warranties are stand-ready performance obligations that are satisfied over time by providing the customer with ongoing access to the Company’s resources. Term-based SDK sales are performance obligations that are satisfied over time through continued provision of access to the customer. The Company uses the time-elapsed (i.e., straight-line) measure of progress to recognize revenue as these performance obligations are satisfied evenly over the respective service periods. The implementation and integration services are a performance obligation satisfied over time, and the Company uses the costs incurred as inputs into the measure of progress to recognize revenue as it satisfies the performance obligation.
Deferred Revenue
Deferred revenue primarily consists of billings or payments received in advance of revenue recognition from software subscriptions and other services and is reduced as the revenue recognition criteria are met. Deferred revenue is classified as current or non-current on the consolidated balance sheets based on the expected timing of revenue recognition. The deferred revenue that will be recognized as revenue within the next twelve months is classified as current, and the deferred revenue that will be recognized thereafter is classified as non-current.
Warranties
The Company offers a standard product warranty that its products will function according to standard specifications and free of significant defects for a period ranging from one year to three years, depending on the product, from when control is transferred to the customer. The Company evaluated the warranty liability under ASC Topic 606 and determined that it is an assurance-type warranty. When product revenue is recognized, an estimate of future warranty costs is recognized as cost of product revenue on the consolidated statements of operations and comprehensive loss and accrued expenses on the consolidated balance sheets. Factors that affect the estimate of future warranty costs include historical and current product failure rates, service delivery costs incurred in correcting product failures, and warranty policies and business practices.
Cash and Cash Equivalents
All highly liquid investments purchased with a maturity of three months or less are considered to be cash equivalents. As of December 31, 2024 and 2023, cash and cash equivalents consist principally of cash and money market accounts.
Trade Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recognized as the original amount invoiced less an allowance for doubtful accounts based on the probability of future collection. In accordance with ASC Topic 326, Financial Instruments-Credit Losses, the Company estimates its allowance for doubtful accounts based on historical loss patterns, the number of days that billings are past due, current market conditions, and reasonable and supportable forecasts of future economic conditions. Accounts receivable are written off when deemed uncollectible and collection of the receivable is no longer being actively pursued. The following table summarizes the allowance for doubtful accounts activity:
| | | | | |
| (in thousands) | Fair Value |
| Allowance for doubtful accounts as of December 31, 2022 | $ | 528 | |
| Additions | 1,446 | |
| Deductions – write offs | (187) | |
| Allowance for doubtful accounts as of December 31, 2023 | 1,787 | |
| Additions | 1,128 | |
| Deductions – write offs | (332) | |
| Allowance for doubtful accounts as of December 31, 2024 | $ | 2,583 | |
Inventories
Inventories primarily consist of raw materials, work-in-progress, and finished goods which are purchased and held by the Company’s third-party contract manufacturers. Inventories are stated at the lower of actual cost, determined using the average cost method, or net realizable value. Actual cost includes all direct and indirect production costs to convert materials into a finished product. Net realizable value is based upon an estimated average selling price reduced by the estimated costs of completion, disposal, and transportation. The determination of net realizable value involves certain judgments including estimating average selling prices. The Company reduces the value of inventory for estimated obsolescence or lack of marketability by the difference between the cost of the affected inventory and the net realizable value.
The valuation of inventories also requires the Company to estimate excess and obsolete inventory. The Company considers new product development schedules, the effect that new products might have on the sale of existing products, product obsolescence, product merchantability, and whether older products can be remanufactured into new products, among other factors.
Losses expected to arise from firm, non-cancelable and unhedged commitments for the future purchase of inventories are recognized unless the losses are recoverable through firm sales contracts or other means.
Restricted Cash
Restricted cash includes deposits in financial institutions restricted according to an agreement and used to secure a lease agreement. The Company classifies the amount restricted according to an agreement as prepaid expenses and other current assets on the consolidated balance sheets as the Company expects the deposit to be released from restriction within the next twelve months. The Company classifies the amount used to secure a lease agreement within other non-current assets on the consolidated balance sheets as the lease is long-term. The amount shown as restricted cash is included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown in the consolidated statements of cash flows.
Vendor Advances
Vendor advances represent amounts paid to third-party vendors for future services to be received related to production of the Company’s inventories. The amounts are presented net of write offs. The classification of vendor advances as current or non-current is based on the estimated timing of inventory delivery.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation expense is computed using the straight-line method over the estimated useful lives of the related assets. Leasehold improvements are amortized on a straight-line basis over the shorter of the remaining lease term or the estimated useful lives of related improvements.
Useful lives for property and equipment are as follows:
| | | | | | | | |
| Property and Equipment | | Estimated Useful Life |
| Software | | 3 years |
| Machinery and equipment | | 3 – 5 years |
| Furniture and fixtures | | 5 – 7 years |
| Leasehold improvements | | Lesser of estimated useful life or remaining lease term |
Expenditures for major renewals and improvements are capitalized. Expenditures for repairs and maintenance are expensed as incurred. When assets are retired or otherwise disposed of, the cost of those assets and the related accumulated depreciation and amortization is eliminated from the balance sheet, and any resulting gains or losses are included in the consolidated statements of operations and comprehensive loss in the period of disposal.
Capitalized Software Development Costs
Costs to develop or obtain software for internal use are capitalized and recorded as capitalized software development costs on the consolidated balance sheets as a component of property and equipment. The Company capitalizes qualifying costs associated with internal-use software incurred during the application development stage if management with relevant authority authorizes the project, it is probable the project will be completed, and the software will be used to perform the function intended. Costs incurred during the preliminary project and post-implementation stages, including training and maintenance, are expensed as incurred. Capitalized costs are amortized on a project-by-project basis using the straight-line method over the estimated economic life of the software, which is three years, beginning when the software is substantially ready for use. Amortization expense is classified in the consolidated statements of operations and comprehensive loss based on the nature of the software.
Leases
The Company primarily enters into leases for office space that are classified as operating leases. The Company determines if an agreement is or contains a lease at inception. The Company accounts for leases in accordance with ASC Topic 842, Leases, by recognizing right-of-use assets and lease liabilities. The Company classifies right-of-use assets as operating lease assets on the consolidated balance sheets. The Company classifies the current portion of lease liabilities, representing lease payments due within the next twelve months, as accrued expenses and other current liabilities on the consolidated balance sheets. The Company classifies the non-current portion of lease liabilities as operating lease liabilities on the consolidated balance sheets. The lease term includes the non-cancelable period of the lease plus any additional periods covered by an option to extend that the Company is reasonably certain to exercise. Generally, the Company may terminate its leases with the notice required in the lease agreement and upon payment of a termination fee, if required. The Company’s leases do not include substantial variable payments based on indexes or rates. The Company’s lease agreements do not contain any significant residual value guarantees or restrictive covenants.
The Company’s leases do not provide a readily determinable implicit discount rate. The Company’s incremental borrowing rate is estimated to approximate an interest rate on a collateralized basis with similar terms and payments and in similar economic environments. Operating lease right-of-use assets and liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. The Company recognizes a single lease cost on a straight-line basis over the lease term, and the Company includes all cash payments within cash flows from operating activities as the change in operating lease assets and liabilities in the consolidated statements of cash flows.
The Company does not have any finance leases as of December 31, 2024 and 2023.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets, including its property and equipment, definite-lived intangible assets, and operating lease assets, for impairment at least annually or whenever events or changes in business circumstances indicate that the carrying amount of assets may not be fully recoverable. Each impairment test is based on a comparison of the undiscounted cash flows to the recorded value of the asset. If the recorded value of the asset is less than the undiscounted cash flows, the asset is written down to its estimated fair value.
Warrant Liability
The Company’s outstanding warrants include publicly-traded warrants (the “Public Warrants”), which were issued as one-third of a warrant per unit during the Company’s initial public offering on May 26, 2020 (the “IPO”), and warrants sold in a private placement to Longview’s sponsor (the “Private Warrants”). The Company evaluated its warrants under ASC Subtopic 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, and concluded that they do not meet the criteria to be classified in stockholders’ equity. Since the Public Warrants and Private Warrants meet the definition of a derivative under ASC Topic 815, Derivatives and Hedging, the Company recorded these warrants as non-current liabilities on the consolidated balance sheets at fair value upon the closing of the Business Combination, with subsequent changes in their respective fair values recognized in the consolidated statements of operations and comprehensive loss at each reporting date. See Note 15 "Warrants" for additional information about our warrants.
Cost of Revenue
Cost of product revenue includes manufacturing costs, personnel costs and benefits, inbound freight, packaging, warranty replacement costs, payment processing fees, and inventory obsolescence and write-offs. Cost of software and other services revenue includes personnel costs, cloud hosting costs, amortization of capitalized software development costs, and payment processing fees.
Research and Development
R&D expenses primarily consist of personnel costs and benefits, facilities expenses, consulting and professional fees, fabrication services, software, and other outsourcing expenses. Substantially all of the Company’s R&D expenses are related to developing new products and services and improving existing products and services. R&D expenses are expensed as incurred.
Sales and Marketing
Sales and marketing expenses primarily consist of personnel costs and benefits, third-party logistics, fulfillment and outbound shipping costs, facilities expenses, advertising, and travel and entertainment. Advertising expenses are expensed as incurred. For the years ended December 31, 2024, 2023, and 2022, advertising expenses were $4.3 million, $4.3 million and $5.8 million, respectively.
General and Administrative
General and administrative expenses primarily consist of personnel costs and benefits, insurance, patent fees, software costs, facilities costs, and outside services. Outside services consist of professional services, legal fees, and other professional fees.
Operating Expenses – Other
The Company classifies certain operating expenses that are not representative of its ongoing operations as other on the consolidated statements of operations and comprehensive loss. These include costs related to the Company’s business transformation initiative, reductions in force, litigation, and legal settlements.
The following table summarizes the types of expenses classified as other in the Company’s consolidated statements of operations and comprehensive loss (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Employment-related expenses | $ | 1,341 | | | $ | 8,701 | | | $ | 2,019 | |
| Legal-related expenses | 2,724 | | | 9,463 | | | 5,327 | |
| Total other | $ | 4,065 | | | $ | 18,164 | | | $ | 7,346 | |
On July 1, 2024, the Company entered into an agreement with a third-party global technology and business transformation partner to optimize and lower the cost of certain non-specialized technical functions. As part of the transition into this new partnership, a portion of the Company's workforce will be in lower-cost geographies. The Company incurred $0.7 million of severance costs for impacted employees that continued providing transition services to the Company through the end of 2024. These costs were recognized as other in the consolidated statements of operations and comprehensive loss during the year ended December 31, 2024. As of December 31, 2024, a total of $0.7 million of accrued severance related to our global workforce optimization is included in accrued expenses and other current liabilities and other non-current liabilities on the consolidated balance sheets.
Net Loss per Common Share
We compute net loss per share of Class A and Class B common stock using the two-class method. Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of each class of the Company’s common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all of the Company’s potential common shares outstanding of the Company’s common stock to the extent the potential shares are dilutive. Basic and diluted net loss per share were the same for each period presented in the consolidated statements of operations and comprehensive loss as the inclusion of all potential shares of the Company’s common stock would have been anti-dilutive. Refer to Note 12 “Net Loss Per Share” for further discussion.
Stock-Based Compensation Expense
The measurement of stock-based compensation expense for all stock-based payment awards, including stock options and RSUs granted to employees, directors, and nonemployees as well as the Company's ESPP, is based on the estimated fair value of the awards on the grant date.
The Company recognizes stock-based compensation expense for its awards on a straight-line basis over the requisite service period of the individual grants, which is generally the vesting period. Generally, stock option and RSU awards fully vest three to four years from the grant date, and stock options have a contractual term of 10 years. ESPP options generally vest over two years. The Company recognizes the effect of forfeitures in stock-based compensation expense based on actual forfeitures when they occur.
The Company granted performance-based restricted stock units during the years ended December 31, 2024, 2023, and 2022. The Company accounted for these awards according to the relevant provisions of ASC Topic 718, Compensation-Stock Compensation. For performance-based awards, the Company recognizes expense using the accelerated attribution method. Refer to Note 11 “Stock-Based Compensation” for further discussion about the nature of the Company's stock-based compensation transactions.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax
expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more likely than not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
Recent Accounting Pronouncements Adopted
In November 2023, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which introduced new guidance on disclosures for reportable segments and significant segment expenses, including for entities with a single reportable segment. The Company retrospectively adopted this guidance during the year ended December 31, 2024. The adoption did not have a significant impact on the consolidated financial statements, other than the newly required disclosures.
Recent Accounting Pronouncements Issued but Not Yet Adopted
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which introduced new guidance on disclosures for income taxes, including enhancements to the rate reconciliation and income taxes paid disclosures. This guidance is effective for the Company for annual reporting periods beginning January 1, 2025. The Company is currently evaluating the impact that the adoption of this pronouncement will have on the Company’s consolidated financial statements and disclosures.
In November 2024, the FASB issued ASU 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, which introduced new guidance on disclosures of specified information about certain costs and expenses included within expenses presented on the face or the income statements, such as purchases of inventory and employee compensation. This guidance is effective for the Company for annual reporting periods beginning January 1, 2027 and interim reporting periods beginning January 1, 2028. The Company is currently evaluating the impact that the adoption of this pronouncement will have on the Company's consolidated financial statements and disclosures.
Note 3. Revenue Recognition
Disaggregation of Revenue
The Company disaggregates revenue from contracts with customers by product type and by geographical market. The Company believes that these categories aggregate the payor types by nature, amount, timing, and uncertainty of its revenue streams. The following table summarizes the Company’s disaggregated revenues (in thousands) for the years ended December 31:
| | | | | | | | | | | | | | | | | | | | | | | |
| Pattern of
Recognition | | 2024 | | 2023 | | 2022 |
| By product type: | | | | | | | |
| Devices and accessories | Point-in-time | | $ | 54,200 | | | $ | 40,036 | | | $ | 50,263 | |
| Software and other services | Over time | | 27,856 | | | 25,864 | | | 23,127 | |
| Total revenue | | | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | |
| | | | | | | |
| By geographical market: | | | | | | | |
| United States | | | $ | 63,423 | | | $ | 52,116 | | | $ | 51,072 | |
| International | | | 18,633 | | | 13,784 | | | 22,318 | |
| Total revenue | | | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | |
Contract Balances
Contract balances represent amounts presented in the consolidated balance sheets when the Company has either transferred goods or services to the customer or the customer has paid consideration to the Company under the contract. These contract balances include trade accounts receivable and deferred revenue. The Company recognizes a receivable when it has an unconditional right to payment, and payment terms are typically 30 days for product and software and other services sales on credit. The amount of revenue recognized during the years ended December 31, 2024 and 2023 that was included in the deferred revenue balance at the beginning of the period was $15.6 million and $14.9 million, respectively.
Transaction Price Allocated to Remaining Performance Obligations
As of December 31, 2024, the Company had $33.3 million of remaining performance obligations. The Company expects to recognize approximately 64% of its remaining performance obligations as revenue in the next twelve months and approximately 36% thereafter.
Costs of Obtaining or Fulfilling Contracts
The Company incurs incremental costs of obtaining contracts and costs of fulfilling contracts with customers. Incremental costs of obtaining contracts, which include commissions and referral fees paid to third parties as a result of obtaining contracts with customers, are capitalized to the extent that the Company expects to recover such costs. Costs of fulfilling contracts that relate specifically to a contract with a customer, which result from activities that generate resources for the Company and enable the Company to satisfy its performance obligations in the contract with the customer, are capitalized to the extent that the Company expects to recover such costs. Capitalized costs are amortized in a pattern that is consistent with the Company’s transfer of the related goods and services to the customer. The Company had $0.7 million and $1.4 million of capitalized costs of obtaining or fulfilling contracts as of December 31, 2024 and 2023, respectively. The Company’s amortization costs for capitalized costs of obtaining or fulfilling contracts were $0.7 million and $0.6 million for the years ended December 31, 2024 and 2023. The Company’s amortization costs for capitalized costs of obtaining or fulfilling contracts was not significant for the year ended December 31, 2022.
Practical Expedients and Accounting Policy Elections
In determining the transaction price of its contracts with customers, the Company estimates variable consideration using a portfolio of data from similar contracts.
As a practical expedient, the Company does not adjust the transaction price for the effects of a significant financing component in contracts in which the period between when the Company transfers the promised good or service to the customer and when the customer pays for that good or service is a year or less.
The Company has made an accounting policy election to exclude all sales taxes from the transaction price of its contracts with customers. Accordingly, sales taxes collected from customers and remitted to government authorities are not included in revenue and are accounted for as a liability until they have been remitted to the respective government authority.
As a practical expedient, the Company does not capitalize incremental costs of obtaining contracts when the amortization period would be one year or less. Such incremental costs of obtaining contracts are recognized as expense when incurred.
Note 4. Fair Value of Financial Instruments
Fair value estimates of financial instruments are made at a specific point in time, based on relevant information about financial markets and specific financial instruments. As these estimates are subjective in nature, involving uncertainties and matters of significant judgment, they cannot be determined with precision. Changes in assumptions can significantly affect estimated fair value.
The Company measures fair value as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The Company utilizes a three-tier hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
•Level 1 — Valuations based on quoted prices in active markets for identical assets or liabilities that an entity has the ability to access.
•Level 2 — Valuations based on quoted prices for similar assets or liabilities, quoted prices for identical assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially the full term of the assets or liabilities.
•Level 3 — Valuations based on inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. The Company has no assets or liabilities valued with Level 3 inputs.
The carrying value of cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses approximates their fair values due to the short-term or on demand nature of these instruments.
There were no transfers between fair value measurement levels during the years ended December 31, 2024 and 2023.
The Company determined the fair value of its Public Warrants as Level 1 financial instruments, as they are traded in active markets. Because any transfer of Private Warrants from the initial holder of the Private Warrants would result in the Private Warrants having substantially the same terms as the Public Warrants, management determined that the fair value of each Private Warrant is the same as that of a Public Warrant. Accordingly, the Private Warrants are classified as Level 2 financial instruments.
The following table summarizes the Company’s assets and liabilities that are measured at fair value on a recurring basis, by level, within the fair value hierarchy (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | | Fair Value Measurement Level |
| Total | | Level 1 | | Level 2 | | Level 3 |
| December 31, 2024: | | | | | | | |
| Warrants: | | | | | | | |
| Public Warrants | $ | 1,794 | | | $ | 1,794 | | | $ | — | | | $ | — | |
| Private Warrants | 891 | | | — | | | 891 | | | — | |
| Total liabilities at fair value on a recurring basis | $ | 2,685 | | | $ | 1,794 | | | $ | 891 | | | $ | — | |
| | | | | | | |
| December 31, 2023: | | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Warrants: | | | | | | | |
| Public Warrants | $ | 552 | | | $ | 552 | | | $ | — | | | $ | — | |
| Private Warrants | 274 | | | — | | | 274 | | | — | |
| Total liabilities at fair value on a recurring basis | $ | 826 | | | $ | 552 | | | $ | 274 | | | $ | — | |
Note 5. Inventories
A summary of inventories is as follows at December 31 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Raw materials | $ | 47,642 | | | 49,366 | |
| Work-in-progress | 4,736 | | | 3,384 | |
| Finished goods | 18,411 | | | 20,272 | |
| Total inventories | $ | 70,789 | | | $ | 73,022 | |
Work-in-progress represents inventory items in intermediate stages of production by third party manufacturers. For the years ended December 31, 2024, 2023, and 2022, net realizable value inventory adjustments and excess and obsolete inventory charges were not significant, $21.1 million, and $0.8 million, respectively, and were recognized in cost of product revenue.
Note 6. Restricted Cash
A reconciliation of cash, cash equivalents and restricted cash from the consolidated balance sheets to the consolidated statements of cash flows as of December 31, 2024 and 2023 is as follows (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Reconciliation of cash, cash equivalents and restricted cash: | | | |
| Cash and cash equivalents | $ | 88,775 | | | $ | 134,437 | |
| Restricted cash included within prepaid expenses and other current assets | — | | | 199 | |
| Restricted cash included within other non-current assets | 4,015 | | | 4,014 | |
| Total cash, cash equivalents and restricted cash shown in the condensed consolidated statements of cash flows | $ | 92,790 | | | $ | 138,650 | |
Restricted cash included within prepaid expenses and other current assets is restricted by an agreement with the Gates Foundation. The restriction on these funds lapses as the Company fulfills its obligations in the agreement. As of December 31, 2024, the Company has fulfilled all of its obligations in the agreement, and all of the restrictions on these funds have lapsed. Restricted cash included within other non-current assets is held as collateral to secure a letter of credit for one of our office leases and is expected to be maintained as a security deposit throughout the duration of the lease.
Note 7. Other Non-Current Assets
Other non-current assets consist of the following at December 31 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Security deposits | $ | 982 | | | $ | 989 | |
| Restricted cash | 4,015 | | | 4,014 | |
| Other long-term assets | 763 | | | 1,419 | |
| Total other non-current assets | $ | 5,760 | | | $ | 6,422 | |
Note 8. Property, Equipment, and Intangible Assets
Property and Equipment
Property and equipment, net, are recorded at historical cost and consist of the following at December 31 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Capitalized internally developed software | $ | 20,677 | | | $ | 17,722 | |
| Leasehold improvements | 11,120 | | | 11,102 | |
| Machinery and equipment | 10,043 | | | 9,930 | |
| Furniture and fixtures | 2,152 | | | 2,152 | |
| Construction in progress | 2,394 | | | 2,566 | |
| Other | 29 | | | 44 | |
| 46,415 | | | 43,516 | |
| Less: accumulated depreciation and amortization | (26,897) | | | (18,195) | |
| Property and equipment, net | $ | 19,518 | | | $ | 25,321 | |
Total depreciation and amortization expense related to property and equipment amounted to $8.9 million, $8.7 million, and $5.9 million for the years ended December 31, 2024, 2023, and 2022, respectively. For the Company’s capitalized internally developed software assets, accumulated amortization was $15.5 million and $9.4 million as of December 31, 2024 and 2023, respectively. Amortization expense recognized on these capitalized internally developed software assets was $6.1 million, $5.5 million, and $3.3 million for the years ended December 31, 2024, 2023 and 2022, respectively.
Intangible Assets
The Company's intangible assets consist of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Gross Carrying
Amount | | Accumulated
Amortization | | Net Carrying
Amount |
| December 31, 2024: | | | | | |
| Technology licenses | $ | 10,317 | | | $ | (1,401) | | | $ | 8,916 | |
| | | | | |
| December 31, 2023: | | | | | |
| Technology licenses | $ | 10,317 | | | $ | — | | | $ | 10,317 | |
For the Company's commitment related to technology licenses acquired in a prior period, the current and non-current portions are included on the consolidated balance sheets in accrued expenses and other current liabilities and other non-current liabilities, respectively. As of December 31, 2024, the current portion was $3.1 million, and the non-current portion was $5.6 million. As of December 31, 2023, the current portion was $1.3 million, and the non-current portion was $7.6 million.
Estimated amortization expense for the Company’s capitalized internally developed software assets and intangible assets over the next five years ended December 31 is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2025 | | 2026 | | 2027 | | 2028 | | 2029 |
| $ | 4,989 | | | $ | 2,737 | | | $ | 1,583 | | | $ | 1,120 | | | $ | 1,120 | |
Impairment
During the year ended December 31, 2023, the Company fully impaired its leasehold improvements related to a lease that was terminated during the third quarter of 2023. The Company recognized an impairment loss of $1.8 million for the year ended December 31, 2023 in operating expenses on the consolidated statements of operations and comprehensive loss. See Note 16, “Leases” for further discussion of the terminated lease. The Company did not recognize any impairment losses for the years ended December 31, 2024 and 2022.
Note 9. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following at December 31 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Employee compensation | $ | 11,192 | | | $ | 9,442 | |
| Customer deposits | 1,909 | | | 1,613 | |
| Accrued warranty liability | 498 | | | 297 | |
| Non-income tax | 2,356 | | | 1,197 | |
| Professional fees | 2,015 | | | 2,481 | |
| Current portion of operating lease liabilities | 2,437 | | | 2,192 | |
| Other | 7,288 | | | 6,203 | |
| Total accrued expenses and other current liabilities | $ | 27,695 | | | $ | 23,425 | |
Warranty expense activity for the years ended December 31 is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Balance, beginning of period | $ | 697 | | | $ | 873 | | | $ | 1,116 | |
| Warranty provision charged to operations | 1,224 | | | 276 | | | 296 | |
| Warranty claims | (898) | | | (452) | | | (539) | |
| Balance, end of period | $ | 1,023 | | | $ | 697 | | | $ | 873 | |
The Company classifies its accrued warranty liability based on the timing of expected warranty activity. The future costs of expected activity greater than one year is recorded within other non-current liabilities on the consolidated balance sheet.
Note 10. Stockholders’ Equity
The Company's outstanding shares consist of two classes of common stock, Class A and Class B.
Dividends
Holders of the Company’s Class A and Class B common stock are not entitled to receive dividends unless declared by the Board. Any such dividends would be subject to the preferential dividend rights of the holders of the then outstanding preferred stock or any other series stock having preferential rights. Holders of the Class A and Class B common stock will share ratably, if and when any dividend is declared, out of funds legally available. There have been no dividends declared to date.
Voting rights
The holders of shares of the Class A common stock are entitled to 1 vote per share on all matters on which the shares shall be entitled to vote. The holders of shares of the Class B common stock are entitled to 20 votes per share on all matters on which the shares shall be entitled to vote. Generally, holders of all classes of common stock vote together as a single class.
Liquidation Rights
On the liquidation, dissolution, distribution of assets, or winding up of the Company, each holder of Class A and Class B common stock will be entitled, pro rata on a per share basis, to all assets of the Company of whatever kind available for distribution to the holders of common stock, subject to the designations, preferences, limitations, restrictions, and relative rights of any other class or series of preferred stock of the Company then outstanding and unless disparate or different treatment of the shares of Class A common stock and Class B common stock is approved by the affirmative vote of the holders of a majority of the outstanding shares of Class A common stock and Class B common stock, each voting separately as a class.
Other Matters
Holders of shares of Class A common stock do not have subscription, redemption or conversion rights.
Holders of Class B common stock have the right to convert shares of their Class B common stock into fully paid and non-assessable shares of Class A common stock, on a one-to-one basis, at the option of the holder at any time upon written notice to the Company. Holders of Class B common stock will have their Class B common stock automatically converted into Class A common stock, on a one-to-one basis, upon the occurrence of any of the events described below:
(1)Any sale, assignment, transfer, conveyance, hypothecation, or other transfer or disposition, directly or indirectly, of any Class B common stock or any legal or beneficial interest in such share, whether or not for value and whether voluntary or involuntary or by operation of law (including by merger, consolidation, or otherwise), including, without limitation the transfer of a share of Class B common stock to a broker or other nominee or the transfer of, or entering into a binding agreement with respect to, voting control over such share by proxy or otherwise, other than a permitted transfer.
(2)Upon the first date on which Dr. Rothberg, together with all other qualified stockholders, collectively cease to beneficially own at least 20% of the number of shares of Class B common stock (as such number of shares is equitably adjusted in respect of any reclassification, stock dividend, subdivision, combination, or recapitalization of the Class B common stock) collectively beneficially owned by Dr. Rothberg and permitted transferees of Class B common stock as of the effective time of the Business Combination.
(3)Upon the date specified by the affirmative vote of the holders of at least two-thirds (2/3) of the then outstanding shares of Class B common stock, voting as a separate class.
(4)If not converted earlier, whether optionally or automatically, on February 12, 2028.
Note 11. Stock-Based Compensation
Equity incentive plans
The Company’s 2012 Employee, Director and Consultant Equity Incentive Plan (the "2012 Plan") was approved by the Board and the Company’s stockholders in March 2012. In connection with the closing of the Business Combination, the Company has not granted and will not grant any additional awards under the 2012 Plan. However, the 2012 Plan will continue to govern the terms and conditions of the outstanding awards previously granted thereunder. As of December 31, 2024, the number of shares of common stock reserved for issuance under the 2012 Plan was 4.8 million.
The Butterfly Network, Inc. Amended and Restated 2020 Equity Incentive Plan (the “2020 Plan”, and together with the 2012 Plan, the “Plans”) was approved by the Board in the fourth quarter of 2020 and by the Company's stockholders in the first quarter of 2021. The 2020 Plan is administered by the Board. The Board may grant stock-based awards, restricted stock, and options to purchase shares either as incentive stock options or non-qualified stock options. The restricted stock and options grants are subject to certain terms and conditions, option periods and conditions, exercise rights, and privileges and are fully discussed in the 2020 Plan. Grants under the Plans are included in the tables below.
As of December 31, 2024, the number of shares of common stock reserved for issuance under the 2020 Plan was 53.5 million and 17.2 million common shares remain available for issuance under the 2020 Plan.
Stock options
Each stock option grant carries varying vesting schedules whereby the options may be exercised at the participant’s sole discretion provided they are an employee, director or consultant of the Company on the applicable vesting date. Each option shall terminate not more than ten years from the grant date.
A summary of the stock option activity under the Plans is presented in the table below:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of
Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term | | Aggregate
Intrinsic
Value
(in thousands) |
| Outstanding at December 31, 2022 | 12,571,912 | | 7.67 | | | 5.62 | | 1,342 | |
| Granted | — | | — | | | | | |
| Exercised | (180,467) | | 1.26 | | | | | |
| Forfeited | (4,952,258) | | 10.14 | | | | | |
| Outstanding at December 31, 2023 | 7,439,187 | | 6.17 | | | 4.68 | | — | |
| Granted | — | | — | | | | | |
| Exercised | (36,340) | | 1.77 | | | | | |
| Forfeited | (842,111) | | 8.98 | | | | | |
| Outstanding at December 31, 2024 | 6,560,736 | | 5.88 | | | 3.56 | | 1,963 | |
| Options exercisable at December 31, 2023 | 6,537,433 | | 5.88 | | | 4.37 | | — | |
| Options exercisable at December 31, 2024 | 6,222,073 | | 5.94 | | | 3.48 | | 1,799 | |
The total intrinsic value excludes those options whereby the stock price does not exceed the exercise price of the option.
Additional information about the Company’s stock option activity during the years ended December 31, 2024, 2023 and 2022 is presented in the table below:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Cash proceeds from the exercise of stock options (in millions) | $ | 0.1 | | | $ | 0.2 | | | $ | 3.0 | |
| Total intrinsic value of stock options exercised (in millions) | — | | | 0.2 | | | 3.6 | |
| Weighted average grant date fair value of options granted | — | | | — | | | 2.79 | |
The intrinsic value of a stock option that’s been exercised is the amount by which the stock price exceeds the exercise price of the option on the date of exercise.
Restricted stock units
A summary of the restricted stock unit activity under the Plans is presented in the table below:
| | | | | | | | | | | |
| Number of
Restricted
Stock Units | | Weighted
Average
Grant Date
Fair Value |
| Outstanding at December 31, 2022 | 9,961,291 | | 4.55 | |
| Granted | 16,082,613 | | 2.23 | |
| Vested | (5,418,832) | | 4.09 | |
| Forfeited | (5,055,089) | | 3.65 | |
| Outstanding at December 31, 2023 | 15,569,983 | | 2.61 | |
| Granted | 15,838,283 | | 1.10 | |
| Vested | (6,906,452) | | 2.69 | |
| Forfeited | (3,251,584) | | 2.34 | |
| Outstanding at December 31, 2024 | 21,250,230 | | 1.52 | |
The total fair value of the restricted stock units vested was $10.4 million, $7.3 million, and $10.7 million during the years ended December 31, 2024, 2023, and 2022, respectively.
Included in the table above are performance-based restricted stock units that include certain service conditions in the award. In 2022, the Company granted 0.2 million performance-based restricted stock units to certain executives. The service condition for these awards is satisfied by providing service to the Company based on the defined service period per the award agreement. The performance-based conditions are objective performance metrics defined in the award agreement. An insignificant amount of expense was recognized for these awards during the years ended December 31, 2024, 2023, and 2022.
Also included in the table above are market-based restricted stock units that include a service condition. In 2024 and 2023, the Company granted 1.0 million and 1.8 million, respectively, of these awards to certain executives. The market-based conditions for these awards are objective metrics related to the Company’s stock price defined in the award agreement. The service condition for these awards is satisfied by providing service to the Company through the achievement date of the market-based conditions. The grant date fair value of the awards is recognized as stock-based compensation expense over the derived service period. The grant date fair value and derived service period were determined by using a Monte Carlo simulation with the risk-free interest rate, expected dividend yield, and expected volatility assumptions detailed below. The Company recognized $2.0 million and $2.5 million of expense for these awards during the years ended December 31, 2024 and 2023.
Valuation assumptions
In accordance with ASC Topic 718, Compensation-Stock Compensation, the Company estimates and records the compensation cost associated with the grants described above with an offsetting entry to paid-in capital. As described in Note 2 “Summary of Significant Accounting Policies”, the Company selected the Black-Scholes option pricing model for determining the estimated fair value of its service-based stock option awards. The Black-Scholes model requires the use of subjective assumptions which determine the fair value of stock-based awards. These assumptions are also used to determine the fair value of market-based awards via Monte Carlo simulations. The assumptions used to value stock option and market-based grants to employees were as follows:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Risk-free interest rate | 3.6% | | 3.6% – 3.9% | | 1.7% – 3.0% |
| Expected dividend yield | 0% | | 0% | | 0% |
| Expected term | 5.0 years | | 5.0 years | | 5.8 years – 6.5 years |
| Expected volatility | 89% | | 76% | | 70% – 73% |
The Company did not grant any stock options during the years ended December 31, 2024 and 2023. The Company did not grant any stock options or market-based awards to non-employees during the years ended December 31, 2024, 2023, and 2022.
Risk-free interest rate
The risk-free interest rate for periods within the expected term of the awards is based on the U.S. Treasury yield curve in effect on the grant date.
Expected dividend yield
The Company has never declared or paid any cash dividends and does not expect to pay any cash dividends in the foreseeable future.
Expected term
For employee stock option awards, the Company calculates the expected term using the “simplified” method, which is the simple average of the vesting period and the contractual term. The simplified method is applied as the Company does not have sufficient historical data to provide a reasonable basis for an estimate of the expected term. The Company calculates the expected term for employee stock option awards that take into account the effects of employee’s expected exercise and post-vesting employment termination behavior. For non-employee stock option awards, the expected term is determined on an award by award basis. For market-based awards, the expected term is based on the length of the award's performance period.
Expected volatility
The Company considers the historical volatility of its stock price and the implied stock price volatility derived from the price of exchange-traded options on the Company's stock. Because Legacy Butterfly was privately held from its inception until the closing of the Business Combination in 2021, there was no specific historical or implied volatility information available prior to 2021. Accordingly, when the Company calculates the historical stock price volatility for awards with expected terms greater than the length of time since the closing of the Business Combination, the Company supplements its own historical stock price volatility with the historical stock price volatility of a group of publicly-traded peer companies that are publicly traded over a period equivalent to the expected term of the stock-based awards.
Exercise price
The exercise price is taken directly from the grant notice issued to employees and non-employees.
Award accelerations and modifications
On December 30, 2022, the Company’s then CEO resigned from his position. Pursuant to the separation agreement between the CEO and the Company, he received equity-based compensation including the acceleration of vesting of 1.7 million of the CEO’s service-based stock options and service-based restricted stock units. This acceleration was pursuant to the original award agreements. As a modification to the original award agreements, 0.1 million performance-based restricted stock units had an acceleration of vesting, and 0.3 million service-based stock options had their post-employment exercise period extended. The Company recognized a total of $7.8 million of incremental stock-based compensation expense during the year ended December 31, 2022 related to the acceleration of these awards pursuant to the original award agreements and the modifications to the original award agreements. The incremental stock-based compensation expense resulting from the modifications was not significant.
Employee stock purchase plan
The Company’s 2024 Employee Stock Purchase Plan (the “ESPP”) was approved by the Board and the Company’s stockholders in the second quarter of 2024, with 4.2 million shares of common stock initially reserved and available for issuance. Under the ESPP, each eligible employee is granted an option to purchase shares of common stock, with the purchase price paid through payroll deductions, subject to the plan’s limitations on the number and value of shares purchasable. Each offering period under the ESPP has an expected duration of 24 months, divided into four six-month purchase periods, with purchases occurring in June and December. The purchase price per share is equal to the lower of 85% of the closing market price on the first day of the offering period, or 85% of the closing market price on the applicable purchase date. Proceeds received from the issuance of shares are credited to stockholders’ equity in the period that the shares are issued. The grant date fair value of the awards is recognized as stock-based compensation expense over the requisite service period. The grant date fair value was determined using similar methods and assumptions as those used for the Company’s stock option awards granted under its equity incentive plans. During the year ended December 31, 2024, the Company's employees purchased 671,435 shares of newly issued common stock through the ESPP.
Stock-based compensation expense
The Company’s stock-based compensation expense for the periods presented was as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Research and development | $ | 6,950 | | | $ | 9,772 | | | 12,834 | |
| Sales and marketing | 4,871 | | | 4,260 | | | 5,974 | |
| General and administrative | 9,211 | | | 13,448 | | | 23,723 | |
| Total stock-based compensation expense | $ | 21,032 | | | $ | 27,480 | | | $ | 42,531 | |
No related tax benefits of the stock-based compensation expense have been recognized and no related tax benefits have been realized from the exercise of stock options due to the Company’s net operating loss carryforwards and valuation allowance. The Company has capitalized $0.4 million, $0.7 million and $1.0 million of stock-based compensation expense as part of the cost of its capitalized internally developed software assets during the years ended December 31, 2024, 2023 and 2022, respectively.
Total unrecognized stock-based compensation expense as of December 31, 2024 was $21.1 million which will be recognized over the remaining weighted average vesting period of 1.9 years.
Note 12. Net Loss Per Share
We compute net loss per share of Class A and Class B common stock using the two-class method. Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of each class of the Company’s common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all potential shares of the Company’s common stock, including those presented in the table below, to the extent dilutive. Basic and diluted net loss per share were the same for each period presented as the inclusion of all potential shares of the Company’s common stock outstanding would have been anti-dilutive.
As the Company uses the two-class method required for companies with multiple classes of common stock, the following table presents the calculation of basic and diluted net loss per share for each class of the Company’s common stock outstanding (in thousands, except share and per share amounts):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, 2024 | | | | | |
| Class A | | Class B | | Total
Common Stock |
| Numerator: | | | | | |
| Allocation of undistributed earnings | $ | (63,442) | | | $ | (9,050) | | | $ | (72,492) | |
| Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (63,442) | | | $ | (9,050) | | | $ | (72,492) | |
| Denominator: | | | | | |
| Weighted-average common shares outstanding | 185,255,823 | | 26,426,937 | | 211,682,760 |
| Denominator for basic and diluted net loss per share – weighted-average common stock | 185,255,823 | | 26,426,937 | | 211,682,760 |
| Basic and diluted net loss per share | $ | (0.34) | | | $ | (0.34) | | | $ | (0.34) | |
| | | | | | | | | | | | | | | | | |
| Year ended December 31, 2023 | | | | | |
| Class A | | Class B | | Total
Common Stock |
| Numerator: | | | | | |
| Allocation of undistributed earnings | $ | (116,497) | | | $ | (17,203) | | | $ | (133,700) | |
| Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (116,497) | | | $ | (17,203) | | | $ | (133,700) | |
| Denominator: | | | | | |
| Weighted-average common shares outstanding | 178,958,607 | | 26,426,937 | | 205,385,544 |
| Denominator for basic and diluted net loss per share – weighted-average common stock | 178,958,607 | | 26,426,937 | | 205,385,544 |
| Basic and diluted net loss per share | $ | (0.65) | | | $ | (0.65) | | | $ | (0.65) | |
| | | | | | | | | | | | | | | | | |
| Year ended December 31, 2022 | | | | | |
| Class A | | Class B | | Total
Common Stock |
| Numerator: | | | | | |
| Allocation of undistributed earnings | $ | (146,412) | | | $ | (22,311) | | | $ | (168,723) | |
| Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (146,412) | | | $ | (22,311) | | | $ | (168,723) | |
| Denominator: | | | | | |
| Weighted-average common shares outstanding | 173,421,449 | | 26,426,937 | | 199,848,386 |
| Denominator for basic and diluted net loss per share – weighted-average common stock | 173,421,449 | | 26,426,937 | | 199,848,386 |
| Basic and diluted net loss per share | $ | (0.84) | | | $ | (0.84) | | | $ | (0.84) | |
For the periods presented above, the net loss per share amounts are the same for Class A and Class B common stock because the holders of each class are entitled to equal per-share dividends or distributions in liquidation in accordance with the Company’s certificate of incorporation, as amended and restated. The undistributed earnings for each year are allocated based on the contractual participation rights of the Class A and Class B common stock as if the earnings for the year had been distributed. As the liquidation and dividend rights are identical, the undistributed earnings are allocated on a proportionate basis.
Anti-dilutive common equivalent shares were as follows:
| | | | | | | | | | | | | | | | | |
| December 31, |
| 2024 | | 2023 | | 2022 |
| Outstanding options to purchase common stock | 6,560,736 | | 7,439,187 | | 12,571,912 |
| Outstanding restricted stock units | 21,250,230 | | 15,569,983 | | 9,961,291 |
Outstanding employee stock purchase plan options | 1,948,409 | | — | | — |
| Outstanding warrants | 20,652,690 | | 20,652,690 | | 20,652,690 |
| Total anti-dilutive common equivalent shares | 50,412,065 | | 43,661,860 | | 43,185,893 |
Note 13. Income Taxes
Income (loss) before provision for income taxes consisted of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Federal | $ | (72,923) | | | $ | (133,961) | | | $ | (169,122) | |
| Foreign | 399 | | | 343 | | | 441 | |
| Loss before provision for income taxes | $ | (72,524) | | | $ | (133,618) | | | $ | (168,681) | |
The Company recorded a tax benefit of $0.03 million, a tax provision of $0.08 million, and a tax provision of $0.04 million for the years ended December 31, 2024, 2023 and 2022, respectively, due to foreign income and return to provision adjustments. Due to the Company’s loss position domestically, the Company has not recorded a significant federal tax provision for the years ended December 31, 2024, 2023, and 2022.
A reconciliation of the Company’s statutory income tax rate to the Company’s effective income tax rate is as follows:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Income at U.S. statutory rate | 21.00 | % | | 21.00 | % | | 21.00 | % |
| State taxes, net of federal benefit | 0.39 | | | 4.65 | | | 2.21 | |
| Stock compensation | (6.41) | | | (2.57) | | | (5.01) | |
| Change in fair value of warrants | (0.54) | | | 0.71 | | | 2.60 | |
| Tax credits | 2.58 | | | 1.74 | | | 2.16 | |
| Valuation allowance | (11.93) | | | (25.47) | | | (22.91) | |
Return to provision | (3.45) | | | — | | | — | |
| Other | (1.60) | | | (0.12) | | | (0.08) | |
| 0.04 | % | | (0.06) | % | | (0.03) | % |
Net deferred tax assets as of December 31, 2024 and 2023 consisted of the following (in thousands):
| | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 |
| Deferred tax assets | | | |
| Net operating loss carryforwards | $ | 158,097 | | | $ | 151,230 | |
| Tax credits | 18,114 | | | 16,288 | |
| Stock compensation | 3,203 | | | 4,812 | |
| Accruals and reserves | 4,106 | | | 2,061 | |
| Inventory reserve | 15,114 | | | 15,207 | |
| Lease liability | 5,562 | | | 6,114 | |
| Depreciation | 99 | | | 2,197 | |
| Capitalized tax R&E | 27,296 | | | 25,844 | |
| Other | 4,183 | | | 1,581 | |
| Total deferred tax assets | 235,774 | | | 225,334 | |
| Valuation allowance | (230,109) | | | (221,454) | |
| Total deferred tax assets | 5,665 | | | 3,880 | |
| Deferred tax liabilities | | | |
| Right-of-use asset | (3,467) | | | (3,829) | |
Software costs | (1,839) | | | — | |
| Net deferred tax assets | $ | 359 | | | $ | 51 | |
As of December 31, 2024 and 2023, the Company had federal net operating loss (“NOL”) carryforwards of approximately $634.8 million and $609.8 million, respectively. As of December 31, 2024 and 2023, the Company had state NOL carryforwards of approximately $446.1 million and $407.8 million, respectively. Of the $634.8 million of federal NOL carryforwards, $73.7 million will begin to expire at various dates in 2031 and $561.2 million may be carried forward indefinitely. The state NOL carryforwards will begin to expire in 2031. As of December 31, 2024, the Company also had federal and state tax credits of $15.4 million and $2.0 million, which will begin to expire in 2032 and 2024, respectively.
The Tax Cuts and Jobs Act resulted in significant changes to the treatment of research and experimental (“R&E”) expenditures under Section 174 of the Internal Revenue Code of 1986, as amended, (“IRC”). For tax years beginning after December 31, 2021, companies are required to capitalize and amortize all R&E expenditures that are paid or incurred in connection with their trade or business. Specifically, costs for U.S. based R&E activities must be amortized over five years. Previously, these expenses could be deducted in the year incurred. The implementation of this provision didn’t increase our cash income tax payment in 2024 due to our significant pre-tax net loss.
Future realization of the tax benefits of existing temporary differences and net operating loss carryforwards ultimately depends on the existence of sufficient taxable income within the carryforward period. As of December 31, 2024 and 2023, the Company performed an evaluation to determine whether a valuation allowance was needed. The Company considered
all available evidence, both positive and negative, which included the results of operations for the current and preceding years. The Company determined that it was not possible to reasonably quantify future taxable income and determined that it is more likely than not that all of the deferred tax assets will not be realized. Accordingly, the Company maintained a full valuation allowance against its net U.S. deferred tax assets as of December 31, 2024 and 2023. The deferred tax asset recognized relates entirely to the Company's foreign entities.
The Company’s valuation allowance increased by $8.7 million and $34.0 million for the years ended December 31, 2024 and 2023, respectively, due primarily to the generation of NOLs.
The utilization of NOLs and tax credit carryforwards to offset future taxable income may be subject to an annual limitation as a result of ownership changes that have occurred previously or may occur in the future. Under IRC Sections 382 and 383, a corporation that undergoes an ownership change may be subject to limitations on its ability to utilize its pre-change NOLs and other tax attributes otherwise available to offset future taxable income and/or tax liability. An ownership change is defined as a cumulative change of 50% or more in the ownership positions of certain stockholders during a rolling three-year period. The Company conducted an ownership analysis under IRC Section 382 based upon publicly available information as of December 31, 2024 and determined that there has not been an ownership change since the last ownership change event on February 12, 2021 that would limit the Company’s utilization of its NOLs and tax credits.
The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations for both federal taxes and the many states in which the Company operates or does business. ASC 740-10 states that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, on the basis of the technical merits.
The Company records uncertain tax positions as liabilities in accordance with ASC 740-10 and adjusts these liabilities when the Company’s judgment changes as a result of the evaluation of new information not previously available. Because of the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from the Company’s current estimate of the unrecognized tax benefit liabilities. These differences will be reflected as increases or decreases to income tax expense in the period in which new information is available. As of December 31, 2024 and 2023, the Company has not recorded any uncertain tax positions in its financial statements.
The Company recognizes interest and penalties related to unrecognized tax benefits within the provision for income taxes on the consolidated statements of operations and comprehensive loss. As of December 31, 2024 and 2023, there was no significant accrued interest or penalties.
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal, state and foreign jurisdictions, where applicable. There are currently no pending tax examinations. The Company’s tax years are still open under statute from December 31, 2020 to the present. Federal and state net operating losses are subject to review by taxing authorities in the year utilized.
From time to time, the Company applies for government assistance in the form of non-income tax refundable credits based on meeting various eligibility criteria. To account for government assistance, where there is limited GAAP guidance for for-profit entities, the Company analogizes to International Accounting Standards 20, Accounting for Government Grants and Disclosures of Government Assistance. Under that standard, the Company recognizes government assistance when there is reasonable assurance that it will comply with the relevant conditions and that the assistance will be received.
During the year ended December 31, 2022, the Company received a tax credit paid in cash of $0.9 million under the state of Massachusetts Life Sciences Tax Incentive Program and recorded the receipt as other income (expense), net on the consolidated statements of operations and comprehensive loss. The government grant is subject to claw-back if the Company fails to meet certain targets in the tax year following the time of the award. During the year ended December 31, 2023, the Company determined that it did not ultimately meet the required targets in 2022 and expects to repay the tax credit received. As a result, the Company has accrued $0.9 million for the expected repayment in other non-current liabilities on the consolidated balance sheets as of December 31, 2024 and 2023, and the Company recognized a corresponding expense in other income (expense), net on the consolidated statements of operations and comprehensive loss for the year ended December 31, 2023.
During the year ended December 31, 2024, the Company received a tax credit paid in cash of $0.5 million for the U.S. government's Employee Retention Credit and recorded the receipt as other income (expense), net on the consolidated statements of operations and comprehensive loss.
Note 14. 401(k) Retirement Plan
The Company sponsors a 401(k) defined contribution plan covering all eligible U.S. employees. Contributions to the 401(k) plan are discretionary. The expense related to the matching contributions was $0.5 million, $0.8 million, and $1.3 million for the years ended December 31, 2024, 2023, and 2022, respectively.
Note 15. Warrants
Public Warrants
The Company issued Public Warrants and Private Warrants in connection with its IPO in 2020. As of December 31, 2024, there were an aggregate of 13,799,357 outstanding Public Warrants, which entitle the holder to acquire Class A common stock. Each whole warrant entitles the registered holder to purchase one share of Class A common stock at an exercise price of $11.50 per share, subject to adjustment as discussed below, beginning on May 26, 2021. The warrants will expire on February 12, 2026 or earlier upon redemption or liquidation. During the years ended December 31, 2024, 2023, and 2022, the amount of exercises of Public Warrants was not significant, and the amount reclassified into equity upon the exercise of the Public Warrants was not significant.
Redemptions
At any time while the warrants are exercisable, the Company may redeem not less than all of the outstanding Public Warrants:
•at a price of $0.01 per warrant;
•upon a minimum of 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder;
•provided that the last reported sale price of the Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like and for certain issuances of Class A common stock and equity-linked securities) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date the Company sends the notice of redemption to the warrant holders; and
•provided that there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants, and a current prospectus relating thereto, available through the 30-day redemption period or the Company has elected to require the exercise of the warrants on a “cashless basis” (as described below).
If the foregoing conditions are satisfied and the Company issues a notice of redemption of the Public Warrants at $0.01 per warrant, each holder of Public Warrants will be entitled to exercise their Public Warrants prior to the scheduled redemption date.
If the Company calls the Public Warrants for redemption for $0.01 as described above, the Board may elect to require any holder that wishes to exercise a Public Warrant to do so on a “cashless basis.” If the Board makes such election, all holders of Public Warrants would pay the exercise price by surrendering their warrants for that number of shares of Class A common stock equal to the quotient obtained by dividing (x) the product of the number of shares of Class A common stock underlying the warrants, multiplied by the excess of the “fair market value” over the exercise price of the warrants by (y) the “fair market value.” For purposes of the redemption provisions of the warrants, the “fair market value” means the average last reported sale price of the Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
The Company may also redeem not less than all of the outstanding Public Warrants and Private Warrants:
•at $0.10 per warrant;
•upon a minimum of 30 days' prior written notice of redemption;
•provided that the last reported sale price of the Class A common stock equals or exceeds $10.00 per share (as adjusted per stock splits, stock dividends, reorganizations, reclassifications, recapitalizations and the like) on the trading day prior to the date on which the Company sends the notice of redemption to the warrant holders;
•provided that the Private Warrants are also concurrently exchanged at the same price (equal to a number of shares of Class A common stock) as the outstanding Public Warrants; and
•provided that there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and a current prospectus relating thereto available throughout the 30-day redemption period.
If the foregoing conditions are satisfied and the Company issues a notice of redemption of the warrants at $0.10 per warrant, each warrant holder will be entitled to exercise their warrant prior to the scheduled redemption date on a cashless basis and receive that number of shares based on the redemption date and the “fair market value” of the Class A common stock, in accordance with a table set forth in the warrant agreement.
The Company evaluated the Public Warrants under ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, in conjunction with the SEC Division of Corporation Finance’s April 12, 2021 Public Statement, Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”), and concluded that they do not meet the criteria to be classified in stockholders’ equity. Specifically, the exercise of the warrants may be settled in cash upon the occurrence of a tender offer or exchange offer in which the maker of the tender offer or exchange offer, upon completion of the tender offer or exchange offer, beneficially owns more than 50% of the outstanding shares of the Company’s Class A common stock, even if it would not result in a change of control of the Company. This provision would preclude the warrants from being classified in equity and thus the warrants are classified as a liability.
Private Warrants
As of December 31, 2024, there were 6,853,333 Private Warrants outstanding. There have been no exercises of the Private Warrants. The Private Warrants are identical to the Public Warrants, except that so long as they are held by Longview Investors LLC (the “Sponsor”) or any of its permitted transferees, (i) the Private Warrants and the shares of Class A common stock issuable upon the exercise of the Private Warrants are not transferable, assignable or saleable until 30 days after the completion of the Business Combination, (ii) the Private Warrants will be exercisable for cash or on a cashless basis, at the holder’s option, and (iii) the Private Warrants are not subject to the Company’s redemption option at the price of $0.01 per warrant. The Private Warrants are subject to the Company’s redemption option at the price of $0.10 per warrant, provided that the other conditions of such redemption are met, as described above. If the Private Warrants are held by a holder other than the Sponsor or any of its permitted transferees, the Private Warrants will be redeemable by the Company in all redemption scenarios applicable to the Public Warrants and exercisable by such holders on the same basis as the Public Warrants.
The Company evaluated the Private Warrants under ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, in conjunction with the SEC Division of Corporation Finance’s April 12, 2021 Public Statement, Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”), and concluded that they do not meet the criteria to be classified in stockholders’ equity. Specifically, the terms of the warrants provide for potential changes to the settlement amounts dependent upon the characteristics of the warrant holder, and, because the holder of a warrant is not an input into the pricing of a fixed-for-fixed option on equity shares, such provision would preclude the warrant from being classified in equity and thus the warrants are classified as a liability.
The Company recognized a loss of $1.9 million, a gain of $4.5 million, and a gain of $20.9 million for the change in fair value of warrant liabilities in the consolidated statements of operations and comprehensive loss for the years ended December 31, 2024, 2023, and 2022, respectively.
Note 16. Leases
The Company primarily enters into leases for office space that are classified as operating leases. Most leases are not cancelable prior to their expiration.
The Company terminated one of its operating leases for office space and modified another during the third quarter of 2023 that increased its lease payments by $0.2 million. The Company recognized a total decrease of $4.2 million to operating lease assets, $0.7 million to the current portion of operating lease liabilities included in accrued expenses and other current
liabilities, and $4.7 million to the non-current portion of operating lease liabilities on the consolidated balance sheets for the lease termination and lease modification. As part of the lease termination, the Company agreed to forfeit a $0.9 million security deposit included in other non-current assets on the consolidated balance sheets. The Company recognized a $0.2 million gain on lease termination within operating expenses on the consolidated statements of operations and comprehensive loss for the year ended December 31, 2023.
The following table presents the components of operating lease cost for the years ended December 31, 2024, 2023, and 2022 (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Operating lease cost | $ | 2,807 | | | $ | 3,206 | | | $ | 4,300 | |
| Short-term lease cost | 101 | | | 73 | | | 249 | |
| Variable lease cost | 321 | | | 317 | | | 353 | |
| Total operating lease cost | $ | 3,229 | | | $ | 3,596 | | | $ | 4,902 | |
The expected maturities related to the Company’s leases with initial non-cancellable lease terms in excess of one year as of December 31, 2024 are as follows:
| | | | | |
| Year ended December 31, | Operating Lease Payments |
| 2025 | $ | 3,664 | |
| 2026 | 3,749 | |
| 2027 | 3,835 | |
| 2028 | 3,921 | |
| 2029 | 3,454 | |
| 2030 and thereafter | 9,354 | |
| Total gross operating lease payments | 27,977 | |
| Less: imputed interest | (5,142) | |
| Total operating lease liabilities, reflecting the present value of net lease payments | $ | 22,835 | |
Additional information related to operating leases is presented as follows:
| | | | | | | | | | | | | | | | | |
| December 31, |
| 2024 | | 2023 | | 2022 |
| Weighted average remaining lease term (in years) | 7.5 | | 8.4 | | 8.8 |
| Weighted average discount rate | 5.9 | % | | 5.9 | % | | 5.5 | % |
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | |
| Operating cash flows from operating leases | $ | 3,557 | | | $ | 3,121 | | | $ | 2,042 | |
| Non-cash activities involving right-of-use assets and lease liabilities: | | | | | |
| | | | | |
| Derecognition of right-of-use assets | — | | | 4,163 | | | — | |
| Derecognition of operating lease liabilities | — | | | 5,401 | | | — | |
Note 17. Commitments and Contingencies
Commitments
Purchase commitments:
The Company enters into inventory purchase commitments with third-party manufacturers in the ordinary course of business, including a non-cancellable inventory supply agreement with a certain third-party manufacturing vendor. The provisions of the agreement allowed the Company, once it reached a certain cumulative purchase threshold in the fourth quarter of 2021, to pay for a portion of the subsequent inventory purchases using an advance previously paid to the vendor. As of December 31, 2024, the aggregate amount of minimum inventory purchase commitments is $6.9 million and the Company has a vendor advance asset of $3.1 million, net of write-downs, and an insignificant accrued purchase commitment liability. The portion of the balances that is expected to be utilized in the next twelve months is included in current assets and current liabilities in the accompanying consolidated balance sheets.
The Company applied the guidance in ASC Topic 330, Inventory, to assess the purchase commitment and related loss, using such factors as Company-specific forecasts which are reliant on the Company’s limited sales history, agreement-specific provisions, macroeconomic factors, and market and industry trends. For the years ended December 31, 2024, 2023, and 2022, the Company did not recognize any additions to the accrued purchase commitment liability, or any related losses, based on its purchase commitment assessment as there were no significant changes to the assessment factors.
The Company reviews its inventory on hand, including inventory acquired under the purchase commitments, for excess and obsolescence (“E&O”) on a quarterly basis. Any E&O inventory acquired that was previously accounted for as a purchase commitment liability accrual or vendor advance write-down is recorded at zero value. During the year ended December 31, 2024, the Company did not acquire such E&O inventory. During the year ended December 31, 2023, the Company utilized $2.0 million of the accrued purchase commitment liability to acquire such E&O inventory.
Contingencies
The Company is involved in litigation and legal matters from time to time, which have arisen in the normal course of business. The Company accrues an estimated liability for legal contingencies when the Company considers a potential loss probable and can reasonably estimate the amount of the potential loss. Although the ultimate results of these matters are not currently determinable, management does not expect that they will have a material effect on the Company’s consolidated balance sheets, statements of operations and comprehensive loss, or statements of cash flows.
On February 16, 2022, a putative class action lawsuit, styled Rose v. Butterfly Network, Inc., et al. was filed in the United States District Court for the District of New Jersey. The claims are against the Company and certain of its directors and previous management as well as members of the board of directors of the Company prior to the completion of the Business Combination, alleging that the defendants made false and misleading statements and/or omissions about its post-Business Combination business and financial prospects. The alleged class consists of all persons or entities who purchased or otherwise acquired the Company’s stock between January 12, 2021 and November 15, 2021, persons who exchanged Longview shares for the Company’s common stock, and persons who purchased Longview stock pursuant, or traceable to, the Proxy/Registration Statement filed with the SEC on November 27, 2020 or any amendment thereto. The Company intends to vigorously defend against this action. The lawsuit seeks unspecified damages, together with interest thereon, as well as the costs and expenses of litigation. There is no assurance that the Company will be successful in the defense of the litigation or that insurance will be available or adequate to fund any potential settlement or judgment or the litigation costs of the action. The Company is unable to predict the outcome or reasonably estimate a range of possible loss at this time.
On June 21, 2022, a stockholder derivative action, styled Koenig v. Todd M. Fruchterman, et al. was filed in the United States District Court for the District of Delaware against the Company’s board of directors and the Company as nominal defendant. On November 28, 2023, a stockholder derivative action, styled Bhavsar v. Todd M. Fruchterman, et al. was filed in the United States District Court for the District of Delaware against the board of directors and the Company as nominal defendant. Both these actions allege violation of Section 14(a) of the Exchange Act, as amended, and Rule 14a-9 promulgated thereunder, and claims for breach of fiduciary duty, contribution and indemnification, aiding and abetting, and gross mismanagement. The lawsuits are premised upon allegedly inadequate internal controls and purportedly misleading representations regarding the Company’s financial condition, business prospects, and the Company’s November 2021 earnings announcement. The Company intends to vigorously defend against these actions. The lawsuit seeks unspecified damages, disgorgement, and restitution, together with interest thereon, as well as the costs and expenses of litigation. There
is no assurance that the Company will be successful in the defense of the litigation or that insurance will be available or adequate to fund any potential settlement or judgment or the litigation costs of the action. The Company is unable to predict the outcome or reasonably estimate a range of possible loss at this time.
Note 18. Subsequent Events
On January 29, 2025, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with TD Securities (USA) LLC and William Blair & Company, L.L.C., as representatives of the several underwriters named therein (collectively, the “Underwriters”), to issue and sell in a public offering up to 27,600,000 shares of Butterfly’s Class A common stock, par value $0.0001 per share, which includes 3,600,000 shares of the Company’s Class A common stock that were subject to an option to purchase additional shares granted to and exercised by the Underwriters, at a public offering price of $3.15 per share (the “Offering”). The Offering closed on January 31, 2025, and the Company issued 27,600,000 shares of Class A common stock, which included the full exercise of the Underwriters' option. The gross proceeds to the Company from the Offering were $86.9 million, before deducting $5.2 million of underwriting discounts and commissions and $0.7 million of estimated Offering expenses payable by the Company. The net proceeds to the Company, after expenses, are expected to be $81.0 million. The net proceeds will be recognized as increases in cash and cash equivalents and stockholders' equity on the consolidated balance sheets.
BUTTERFLY NETWORK, INC.
INSIDER TRADING POLICY
(Effective February 12, 2021, Revised October 25, 2023)
TABLE OF CONTENTS
Page
Butterfly Network, Inc. (the “Company”) has adopted the following policy regarding trading by Company personnel in the Company’s securities (the “Insider Trading Policy,” or this “Policy”). This Policy applies to all Company personnel, including directors, officers, employees and consultants of the Company and its subsidiaries. This Policy also applies to certain family members, other members of a person’s household and entities controlled by Company personnel, as described in Section IV below.
I.The Need for an Insider Trading Policy
This Policy has been developed:
•to educate all Company personnel as to the federal securities laws and the rules of the Securities and Exchange Commission (the “SEC”) on insider trading in public company securities;
•to set forth requirements that apply to Company personnel and other persons covered by this Policy who seek to trade in the Company’s securities;
•to protect the Company and its personnel from legal liability; and
•to preserve the reputation of the Company and its personnel for integrity and ethical conduct.
Because the Company is publicly traded, transactions in the Company’s securities are subject to the federal securities laws and regulations adopted by the SEC. These laws and regulations make it illegal for an individual to buy or sell securities of the Company while in possession of material non-public information. The SEC takes insider trading very seriously and devotes significant resources to uncovering the activity and to prosecuting offenders. Liability may extend not only to the individuals who trade while in possession of material non-public information but also to their “tippers,” people who leak material non-public information to individuals who then trade based on that information. The Company and “controlling persons” of the Company may also be liable for violations by Company employees.
II.What is Material Non-Public Information?
Definition of Material Non-Public Information.
Material information is any information (positive or negative) about the Company or any other company that:
•could reasonably be expected to affect the investment decisions of a stockholder or potential investor; or
•could reasonably be expected to significantly alter the total mix of information in the marketplace about the Company or any other company.
In simple terms, material information is any type of information that could reasonably be expected to affect the market price of the Company’s securities.
Examples. Common examples of information that will frequently be regarded as material include, but are not limited to:
•quarterly or annual earnings results;
•projections of future financial results;
•the gain or loss of a significant customer or supplier;
•pending or proposed mergers, acquisitions, tender offers, joint ventures, divestitures or other significant changes in assets;
•a company restructuring;
•significant news or developments in the Company’s business and/or product development efforts;
•licenses of significant technologies;
•entry into, modification or termination of significant strategic collaborations;
•entry into agreements with significant new customers or for a significant quantity of the Company’s products;
•significant transactions with officers, directors or greater than 5% stockholders;
•dividends;
•stock splits;
•executive management or Board member changes or changes in control;
•the public or private sale of additional securities by the Company;
•pending or threatened significant litigation, arbitrations or similar disputes, or governmental investigations;
•cybersecurity risks and incidents, including the discovery of significant vulnerabilities or breaches;
•establishment by the Company of a program to buy the Company’s own shares;
•entry into, amendment or termination of a material contract;
•the gain or loss of a significant contract, license, registration or collaboration;
•potential restatements of the Company’s financial statements, changes in or disagreements with auditors, or a notification that the auditor’s reports may no longer be relied upon;
•deterioration in the Company’s credit status; or
•other items that require the filing of a Current Report on Form 8-K with the SEC.
Non-Public information is information that has not been disseminated in a manner making it available to investors generally. To demonstrate that information is public, one must be able to point to some fact that establishes that the information has become publicly available, such as the filing of a report with the SEC, the distribution of a press release, publishing the information on our website or posting on social media if those are regular ways we communicate with investors, or by other means that are reasonably designed to provide broad public access.
Before a person with material nonpublic information can trade, the market must have adequate time to absorb the information that has been disclosed. For the purposes of this Insider Trading Policy, information will be considered public after the completion of one full day of trading following our public release of the information. For that purpose, a full day of trading means a session of regular trading hours on the New York Stock Exchange (“NYSE”) or the Nasdaq Stock Market (“Nasdaq”) between 9:30 a.m. and 4:00 p.m. Eastern Time (or such earlier closing time as has been set by exchange rules) has occurred. Thus, if an announcement is made before the market opens on a Monday, Tuesday generally would be the first day on which you may trade. If an announcement is made before the market opens on a Friday, Monday generally would be the first day on which you may trade.
Twenty-Twenty Hindsight. In determining whether information is material, the SEC and other regulators will view the information after-the-fact with the benefit of hindsight. As a result, in determining whether any information is material, we will, and you should, carefully consider whether regulators and others might view the information as being material in hindsight, with the benefit of all relevant information that later becomes available. For example, if there is a significant change in the Company’s stock price following release of certain information, that information will likely be determined to have been material when viewed with the benefit of hindsight.
In addition to addressing the relevant statutes and regulations in this area, we are adopting this Policy to avoid even the appearance of improper conduct on the part of anyone employed by or associated with the Company and certain related persons, not just members of executive management.
III.The Consequences of Insider Trading
The consequences of insider trading violations can be severe:
For individuals who trade while in possession of material non-public information (or tip information to others):
•a civil penalty of up to three times the profit gained or loss avoided;
•a criminal fine (no matter how small the profit) of up to $5 million; and
•a jail term of up to 20 years.
These penalties can apply even if the individual is not a member of the Board of Directors or an officer of the Company. Moreover, if an employee violates this Policy, he or she may also be subject to Company-imposed sanctions, including termination for cause.
For a company (as well as possibly any supervisory person) that fails to take appropriate steps to prevent illegal trading:
•a civil penalty of the greater of $2 million or three times the profit gained or loss avoided as a result of the employee’s violation; and
•a criminal penalty of up to $25 million.
Any of the above consequences, including an SEC investigation that does not result in prosecution, can tarnish the Company’s or an individual’s reputation and irreparably damage a career.
IV.Our Policy
General Prohibition on Trading. Company personnel and Related Persons (as defined below in this Section IV) may not buy or sell securities of the Company while in possession of material non-public information or engage in any other action to take advantage of, or pass on to
others, that information, subject to the specific exceptions noted below in this Section IV under the caption “Exceptions for Certain Transactions.”
Prohibition on Tipping. Providing material nonpublic information about the Company to another person who may trade or advise others to trade on the basis of that information is known as “tipping” and is illegal. You are prohibited from providing material nonpublic information about the Company to a friend, relative, or anyone else who might buy or sell a security or other financial instrument on the basis of that information, whether or not you intend to or actually do realize a profit (or any other benefit) from such tipping. Additionally, you are prohibited from recommending to any person that such person engage in or refrain from engaging in any transaction involving the Company’s securities, or otherwise give trading advice concerning the Company’s securities, if you are in possession of material nonpublic information about the Company.
Transactions or Tipping by Family Members, Others in Your Household and Entities You Control. The restrictions in this Policy also apply to (1) immediate family members who reside with you, (2) others living in your household (whether or not related to you), (3) family members who do not live in your household but whose transactions in the Company’s securities are directed by you or are subject to your influence or control (e.g., parents or children who consult with you before they trade in the Company’s securities) and (4) any entities that you influence or control, including any corporations, limited liability companies, partnerships or trusts (each person or entity identified in clauses (1) - (4), a “Related Person”);
SEC regulations specifically provide that any material non-public information about the Company communicated to any spouse, parent, child or sibling is considered to have been communicated under a duty of trust or confidence; and that any trading in the Company’s securities by such family members while they are aware of such information may, therefore, violate insider trading laws and regulations. Company personnel are expected to be responsible for the compliance of all Related Persons with this Policy. This means that, to the extent such Related Persons of Company personnel intend to trade in the Company’s securities, the Related Persons need to comply with the black-out periods and all other restrictions in this Policy. Furthermore, you should not participate in any investment club (i.e., groups of people who pool their money to make investments) that may invest in the Company’s securities.
Other Companies’ Non-public Information. This Policy also applies with equal force to information relating to any other company, including our customers or suppliers, obtained by Company personnel during the course of their service to or employment by the Company. Specifically, no Company personnel who, in the course of work on behalf of the Company, learns of material non-public information about a company with which the Company does business may trade in the other company’s securities until the information becomes public or is no longer material.
Personal or Independent Reasons Are Not Exceptions. Transactions in the Company’s securities that may be necessary or justifiable for independent reasons (such as the need to raise money for an emergency expenditure) are no exception. Even the appearance of an improper transaction must be avoided to preserve our reputation for adhering to the highest standards of conduct.
Policy Administrator. This Policy shall be administered by the “Policy Administrator,” who shall be the Chief Financial Officer or their designee, and if such person is not available,
then the Legal Counsel or their designee, shall serve as the alternate Policy Administrator. The Policy Administrator may, however, change from time to time.
Prohibited Trading Periods. While it is never permissible to trade based on material nonpublic information, we are implementing the following procedures to help prevent inadvertent violations of this Policy and avoid even the appearance of an improper transaction (which could result, for example, where Company personnel engage in a trade while unaware of a pending major development).
(1)Company-Wide Black-Out Periods Applicable to All Company Personnel. All Company personnel and Related Persons are prohibited from trading in any of the Company’s securities during the following periods:
•from the time each such individual becomes aware of the material information (the black-out start times often vary), until the beginning of the first business day after the day the Company has made a public announcement of material information, including earnings releases, unless the information released is complex, in which case it may be necessary to extend this period and the Policy Administrator will notify you of any such extension of the black-out period; and
•during other specified periods when significant developments or announcements are anticipated, as notified by the Policy Administrator.
You will be notified by e-mail when you may not trade in the Company’s securities during periods when significant developments or announcements are anticipated, in which event you will also be notified when trading restrictions are lifted. Of course, even during periods when trading is permitted, no one, including persons or entities who do not fall within the definition of Related Persons, should trade in the Company’s securities if he or she possesses material non-public information.
(2)Additional Black-Out Periods Applicable to the Board of Directors, Executive Management, Finance Team Members and Designated Employees. In addition to being subject to the trading procedures applicable to all Company personnel (above), members of the Company’s Board of Directors, Executive Management, Finance Team Members, Designated Employees (each as defined below) and Related Persons of such individuals are also subject to additional trading procedures and restrictions during the following periods:
•the periods from 15 days prior to the close of each fiscal quarter until the beginning of the first business day after the release of the Company’s financial results for each quarter and, in the case of the fourth quarter, financial results for the year end; and
•any other periods as determined by the Company.
The following members of management constitute the “Executive Management” of the Company: all Executive (Section 16) Officers and other executives, as listed on Exhibit A hereto, which list may be amended from time to time, without the need to amend this policy, to reflect the then-current group of such individuals.
The following individuals constitute the “Finance Team Members” of the Company: all members of the Company’s finance and accounting team.
The following individuals constitute other “Designated Employees” of the Company: certain members of Company personnel (in addition to Executive Management and Finance Team Members), as listed on Exhibit B hereto, which list may be amended from time to time to reflect the then-current group of such individuals without the need to amend this policy.
The Policy Administrator may, from time to time, amend the list of and/or designate other employees as Executive Management, Finance Team Members or Designated Employees, in which case the Policy Administrator shall notify the affected individuals.
Exceptions for Certain Transactions.
(1)Gifts. Bona fide gifts are not transactions that are subject to this Policy, unless the person making the gift (the donor) has reason to believe that the recipient of the gift intends to sell the Company’s securities while the donor is in possession of material non-public information.
(2)Mutual Funds. Transactions in mutual funds that are invested in the Company’s securities are not transactions subject to this Policy.
(3)Transactions Involving Company Equity Plans. Except as otherwise noted below, this Policy does not apply to the following transactions:
•Stock Option Exercises. This Policy does not apply to the exercise of an employee stock option for cash acquired pursuant to the Company’s equity plans, or to the exercise of a tax withholding right pursuant to which a person has elected to have the Company withhold shares subject to an option to satisfy tax withholding requirements. This Policy does apply, however, to any sale of stock as part of a broker-assisted cashless exercise of an option, or any other market sale of stock for the purpose of generating the cash needed to pay the exercise price and or taxes upon the exercise of an option.
•Restricted Stock Awards and Restricted Stock Unit Awards. This Policy does not apply to the vesting of restricted stock or restricted stock units, the automatic sale of shares received to satisfy tax withholding requirements upon vesting of restricted stock units in accordance with the Company’s “sell-to-cover” option (unless a person has elected to use cash to satisfy a tax withholding requirement) or the withholding of the Company of shares of stock to satisfy applicable tax withholding requirements upon the vesting of any restricted stock or restricted stock unit if (i) the withholding is required by the applicable plan or award agreement; or (ii) the election to exercise such tax withholding right was made in compliance with the Company’s applicable trading procedures. This Policy does apply, however, to any market sale of restricted stock or shares received upon vesting of restricted stock units.
•Employee Stock Purchase Plan. This Policy does not apply to periodic wage withholding contributions by the Company or its employees that are used to purchase the Company’s securities under any employee stock purchase plan of the Company and pursuant to the employee’s advance instructions under the Company’s plan. This Policy does apply, however, to an employee’s election to participate in the plan or alter their instructions regarding the level of withholding or purchase the Company’s
securities under the plan, an employee’s cash contributions to the plan (other than through periodic wage withholdings), and subsequent sales or other transfers of such securities.
•Other Transactions with the Company. Any other purchase of the Company’s securities from the Company or sales of the Company’s securities to the Company are not subject to this Policy.
(4)Rule 10b5-1 Trading Plans. Notwithstanding the restrictions and prohibitions on trading in the Company’s securities set forth in this Policy, persons subject to this Policy are permitted to effect transactions in the Company’s securities pursuant to approved trading plans established under Rule 10b5-1 of the Securities Exchange Act of 1934, as amended (“Trading Plans”), which may include transactions during the prohibited periods discussed above. Please refer to the Company’s separate Rule 10b5-1 Trading Plan Policy for the requirements associated with such Trading Plans. .
Pre-Clearance of All Acquisitions, Sales and Other Transfers by Certain Company Personnel. In order to ensure compliance with this Policy and with any Section 16 reporting requirements, all transactions in the Company’s securities (including acquisitions, sales, gifts and other transfers, whether or not for value), including the execution of Trading Plans, by members of the Company’s Board of Directors, Executive Management, Finance Team Members, Designated Employees and Related Persons, must be pre-cleared by the Policy Administrator. If you are a member of one of the groups listed above and you contemplate a transaction in the Company’s securities, you must complete the Stock Transaction Request Form included in Exhibit C of this Policy, or such other form as the Company may designate, and submit the Form to the Policy Administrator or other designated individual prior to executing the transaction. The Policy Administrator will use his or her reasonable best efforts to provide approval or disapproval within two business days. You must wait until receiving preclearance to execute the transaction. Neither the Company nor the Policy Administrator shall be liable for any delays that may occur due to the pre-clearance process. If the transaction is precleared by the Policy Administrator, it must be executed by the end of the second business day after receipt of pre-clearance. Notwithstanding receipt of pre-clearance of a transaction, if you become aware of material non-public information about the Company after receiving the preclearance but prior to the execution of the transaction, you may not execute the transaction. The responsibility for determining whether you are in possession of material non-public information rests with you, as discussed below in Section V. If you are a Section 16 reporting person, promptly following execution of the transaction, but in no event later than the end of the first business day after the execution of the transaction, you must notify the Policy Administrator and provide details regarding the transaction sufficient to complete the required Section 16 filing.
Employees of the Company who are not Directors, members of Executive Management, Finance Team Members or Designated Employees may, but are not required to, pre-clear transactions in the Company’s securities in the same manner as set forth above. Such employees are not required to notify the Policy Administrator following execution of the transaction.
Please note that pre-clearance does not provide Company personnel with immunity from investigation or suit, for which it is the responsibility of the individual to comply with the federal securities regulations.
V.Individual Responsibility
Persons subject to this Policy have ethical and legal obligations to maintain the confidentiality of information about the Company and to refrain from engaging in transactions in the Company’s securities while in possession of material non-public information. Each individual is responsible for making sure that he or she complies with this Policy, and that any Related Person, whose transactions are subject to this Policy, also comply with this Policy. In all cases, the responsibility for determining whether an individual is in possession of material non-public information rests with that individual, and any action on the part of the Company, the Policy Administrator or any other employee or director pursuant to this Policy (or otherwise) does not in any way constitute legal advice or insulate an individual from liability under applicable securities laws. You may be subject to legal penalties and disciplinary action by law enforcement officials and/or the Company for any conduct prohibited by this Policy or applicable securities laws, as described in Section III above.
Prevention of Insider Trading by Others. If you become aware of a potential insider trading violation, you must immediately advise our Policy Administrator and/or report the matter using the Company’s anonymous whistleblower reporting procedures. You should also take steps, where appropriate, to prevent persons under your supervision and/or control from using material non-public information for trading purposes. Moreover, Company-imposed sanctions, including termination for cause, could result if an employee fails to comply with this Policy.
Confidentiality. Serious problems could be caused for the Company by the unauthorized disclosure of internal information about the Company, whether or not for the purpose of facilitating improper trading in the Company’s securities. Company personnel should not discuss internal Company matters or developments (whether or not you think such information is material) with anyone outside of the Company (including, but not limited to, family, friends, business associates, investors and expert consulting firms), except as required in the performance of regular corporate duties. This prohibition applies specifically (but not exclusively) to inquiries about the Company that may be made by the financial press, investment analysts or others in the financial community and also includes posting material non-public information on any social media outlets such as Facebook, Twitter, etc. It is important that all such communications on behalf of the Company be made only through an authorized officer under carefully controlled circumstances. Unless you are expressly authorized to the contrary, if you receive any inquiries of this nature, you should decline comment and refer the inquirer to the Company’s Chief Financial Officer or their desginee. Please review the Company’s separate Regulation FD Policy, which governs all public communications with people outside the Company.
VI.Additional Prohibited Transactions
Because we believe it is generally improper and inappropriate for Company personnel to engage in short-term or speculative transactions involving the Company’s securities, it is our policy that Company personnel and Related Persons not engage in any of the following activities:
•trading in the Company’s securities on a short-term basis. Any shares of Company common stock purchased in the open market must be held for a minimum of six months and ideally longer;
•purchasing of financial instruments (including prepaid variable forward contracts, equity swaps, puts, calls, straddles, collars and exchange funds) that are designed to hedge or offset any decrease in the market value of the Company’s equity securities and entering into other transactions with the same economic effect, including short sales;
•borrowing or other arrangements involving pledge of securities; and
•selling a security future that establishes a position that increases in value as the value of the underlying equity security decreases.
VII.Post-Termination Transactions
This Policy will no longer apply after termination of service to the Company. However, if an individual is in possession of material non-public information when his or her service terminates, that individual may not trade in the Company’s securities until that information has become public or is no longer material, and it would be prudent for the individual, if he or she is subject to a black-out period upon termination of service, to refrain from trading until those restrictions no longer apply to Company personnel.
VIII.Company Assistance
Any person who has any questions about specific transactions or this Policy in general may obtain additional guidance from the Policy Administrator. Remember, however, the ultimate responsibility for adhering to this Policy and avoiding improper transactions rests with you. In this regard, please use your best judgment when considering a transaction in the Company’s securities.
IX.Certifications
As a condition to employment, all employees will be required to certify their understanding of and intent to comply with this Policy. Members of the Board of Directors, Executive Management and other personnel may be required to certify compliance on an annual basis.
As of May _____, 2023:
Exhibit A
“Executive Management”
All Executive (Section 16) Officers and executive leadership team members, including:
1.Joseph DeVivo, President, Chief Executive Officer, and Chairman of the Board
2.Heather Getz, Executive Vice President and Chief Financial and Operations Officer
3.Andrei Stoica, Chief Technology Officer
4.John Martin. Chief Medical Officer
5.Darius Shahida, Chief Strategy Officer and Chief Business Development Officer
6.John Soto, Senior Vice President – International Sales
Exhibit B
“Designated Employees”
Employees the Company may designate from time to time.
EXHIBIT C
STOCK TRANSACTION REQUEST
Pursuant to Butterfly Network, Inc.’s Insider Trading Policy, I hereby notify Butterfly Network, Inc. (the “Company”) of my intent to trade the securities of the Company as indicated below:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
REQUESTER INFORMATION Insider’s Name: _________________________________________ |
INTENT TO PURCHASE Number of shares: __________________________ Intended trade date: __________________________ |
Means of acquiring shares: | | Acquisition through employee benefit plan (please specify):
___________________________________________________________ |
| | Purchase through a broker on the open market |
| | Other (please specify): ________________________________________ |
INTENT TO SELL Number of shares: __________________________ Intended trade date: __________________________ |
Means of selling shares: | | Sale through employee benefit plan (please specify):
___________________________________________________________ |
| | Sale through a broker on the open market |
| | Other (please specify): ________________________________________ |
SECTION 16 | RULE 144 (Not applicable if transaction requested involves a purchase) |
| I am not subject to Section 16.
To the best of my knowledge, I have not (and am not deemed to have) engaged in an opposite way transaction within the previous 6 months that was not exempt from Section 16(b) of the Exchange Act.
None of the above. |
| | I am not an “affiliate” of the Company and the transaction requested above does not involve the sale of “restricted securities” (as those terms are defined in Rule 144 under the Securities Act of 1933, as amended). |
| To the best of my knowledge, the transaction requested above will meet all of the applicable conditions of Rule 144. |
| The transaction requested will be made pursuant to an effective registration statement covering such transaction. |
| None of the above. |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
CERTIFICATION I hereby certify that I am not (1) in possession of any material nonpublic information concerning the Company, as defined in the Company’s Insider Trading Policy and (2) purchasing any securities of the Company on margin in contravention of the Company’s Trading Procedures. I understand that, if I trade while possessing such information or in violation of such trading restrictions, I may be subject to severe civil and/or criminal penalties and may be subject to discipline by the Company including termination of my employment.
| |
| Insider’s Signature |
| Date |
|
APPROVAL |
| Signature of Compliance Officer (or designee) |
| Date |
|
*NOTE: Multiple lots must be listed on separate forms or broken out.
Certification Under Insider Trading Policy
The undersigned hereby certifies that he/she has read and understands, and agrees to comply with, the Company’s Insider Trading Policy, a copy of which was distributed with this Certification.
Date: Signature
Name:
(Please Print)
Title:
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in Registration Statement Nos. 333-273811 and 333-254836 on Form S-3, and Registration Statement Nos. 333-280218, 333-256044 and 333-263151 on Form S-8 of our report dated February 28, 2025, relating to the financial statements of Butterfly Network, Inc., appearing in this Annual Report on Form 10-K for the year ended December 31, 2024.
/s/ Deloitte & Touche LLP
New York, New York
February 28, 2025
Exhibit 31.1
CERTIFICATIONS UNDER SECTION 302
I, Joseph DeVivo, certify that:
1.I have reviewed this Annual Report on Form 10-K of Butterfly Network, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: February 28, 2025
| | | | | |
| /s/ Joseph DeVivo | |
| Joseph DeVivo | |
| President, Chief Executive Officer, and Chairman of the Board | |
| (Principal Executive Officer) | |
Exhibit 31.2
CERTIFICATIONS UNDER SECTION 302
I, Heather C. Getz, CPA, certify that:
1.I have reviewed this Annual Report on Form 10-K of Butterfly Network, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: February 28, 2025
| | | | | |
| /s/ Heather C. Getz, CPA | |
| Heather C. Getz, CPA | |
| Executive Vice President and Chief Financial & Operations Officer | |
| (Principal Financial Officer) | |
Exhibit 32.1
CERTIFICATIONS UNDER SECTION 906
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), each of the undersigned officers of Butterfly Network, Inc., a Delaware corporation (the “Company”), does hereby certify, to such officer’s knowledge, that:
The Annual Report on Form 10-K for the year ended December 31, 2024 (the “Form 10-K”), of the Company fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of the Company.
| | | | | |
Dated: February 28, 2025 | /s/ Joseph DeVivo |
| Joseph DeVivo |
| President, Chief Executive Officer, and Chairman of the Board |
| (Principal Executive Officer) |
| |
Dated: February 28, 2025 | /s/ Heather C. Getz, CPA |
| Heather C. Getz, CPA |
| Executive Vice President and Chief Financial & Operations Officer |
| (Principal Financial Officer) |
Exhibit 99.1
List of Excess Option Exercises
| | | | | | | | |
Grant Date of Legacy Butterfly Option (Exchanged for Option to Purchase Shares of the Company) | Exercise Date | Option Exercise Overage |
April 23, 2020 | February 23, 2022 | 1 |
December 29, 2016 | February 23, 2022 | 1 |
January 5, 2018 | June 1, 2022 | 1 |
December 29, 2016 | September 20, 2021 | 1 |
April 26, 2016 | December 21, 2021 | 1 |
April 6, 2017 | May 24, 2021 | 2 |
November 8, 2017 | May 24, 2021 | 1 |
January 26, 2016 | August 24, 2021 | 1 |
January 26, 2016 | May 18, 2021 | 2 |
November 8, 2017 | January 4, 2022 | 1 |
April 6, 2017 | August 25, 2021 | 1 |
November 8, 2017 | March 17, 2022 | 1 |
February 16, 2019 | July 16, 2019 | 1 |
September 12, 2012 | June 1, 2021 | 1 |
v3.25.0.1
Cover Page - USD ($)
$ in Millions |
12 Months Ended |
|
|
|
Dec. 31, 2024 |
Feb. 13, 2025 |
Feb. 09, 2025 |
Jun. 30, 2024 |
| Entity Information [Line Items] |
|
|
|
|
| Document Type |
10-K
|
|
|
|
| Document Annual Report |
true
|
|
|
|
| Document Period End Date |
Dec. 31, 2024
|
|
|
|
| Current Fiscal Year End Date |
--12-31
|
|
|
|
| Document Transition Report |
false
|
|
|
|
| Entity File Number |
001-39292
|
|
|
|
| Entity Registrant Name |
Butterfly Network, Inc.
|
|
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
|
|
| Entity Tax Identification Number |
84-4618156
|
|
|
|
| Entity Address, Address Line One |
1600 District Avenue
|
|
|
|
| Entity Address, City or Town |
Burlington
|
|
|
|
| Entity Address, State or Province |
MA
|
|
|
|
| Entity Address, Postal Zip Code |
01803
|
|
|
|
| City Area Code |
781
|
|
|
|
| Local Phone Number |
557-4800
|
|
|
|
| Entity Well-known Seasoned Issuer |
No
|
|
|
|
| Entity Voluntary Filers |
No
|
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
|
|
| Entity Small Business |
true
|
|
|
|
| Entity Emerging Growth Company |
false
|
|
|
|
| ICFR Auditor Attestation Flag |
false
|
|
|
|
| Document Financial Statement Error Correction [Flag] |
false
|
|
|
|
| Entity Shell Company |
false
|
|
|
|
| Entity Public Float |
|
|
|
$ 133.7
|
| Documents Incorporated by Reference |
Portions of the Registrant’s definitive proxy statement for its 2025 Annual Meeting of Stockholders (the “Proxy Statement”), to be filed within 120 days of the Registrant’s fiscal year ended December 31, 2024, are incorporated by reference in Part III of this Annual Report on Form 10-K. Except with respect to information specifically incorporated by reference in this Form 10-K, the Proxy Statement is not deemed to be filed as part of this Annual Report on Form 10-K.
|
|
|
|
| Entity Central Index Key |
0001804176
|
|
|
|
| Document Fiscal Year Focus |
2024
|
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
|
| Amendment Flag |
false
|
|
|
|
| Class A Common Stock |
|
|
|
|
| Entity Information [Line Items] |
|
|
|
|
| Title of 12(b) Security |
Class A common stock, $0.0001 par value per share
|
|
|
|
| Trading Symbol |
BFLY
|
|
|
|
| Security Exchange Name |
NYSE
|
|
|
|
| Entity Common Stock, Shares Outstanding |
|
216,496,214
|
|
|
| Warrants to purchase one share of Class A common stock, each at an exercise price of $11.50 per share |
|
|
|
|
| Entity Information [Line Items] |
|
|
|
|
| Title of 12(b) Security |
Warrants to purchase one share of Class A common stock, each at an exercise price of $11.50 per share
|
|
|
|
| Trading Symbol |
BFLY WS
|
|
|
|
| Security Exchange Name |
NYSE
|
|
|
|
| Class B Common Stock |
|
|
|
|
| Entity Information [Line Items] |
|
|
|
|
| Entity Common Stock, Shares Outstanding |
|
|
26,426,937
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDocuments incorporated by reference. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-23
| Name: |
dei_DocumentsIncorporatedByReferenceTextBlock |
| Namespace Prefix: |
dei_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_IcfrAuditorAttestationFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=bfly_WarrantsToPurchaseCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
CONSOLIDATED BALANCE SHEETS - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 88,775
|
$ 134,437
|
| Accounts receivable, net of allowance for doubtful accounts of $2,583 and $1,787 at December 31, 2024 and December 31, 2023, respectively |
20,793
|
13,418
|
| Inventories |
70,789
|
73,022
|
| Current portion of vendor advances |
5,547
|
2,815
|
| Prepaid expenses and other current assets |
6,709
|
7,571
|
| Total current assets |
192,613
|
231,263
|
| Property and equipment, net |
19,518
|
25,321
|
| Intangible assets, net |
8,916
|
10,317
|
| Non-current portion of vendor advances |
15,042
|
15,276
|
| Operating lease assets |
14,233
|
15,675
|
| Other non-current assets |
5,760
|
6,422
|
| Total assets |
256,082
|
304,274
|
| Current liabilities: |
|
|
| Accounts payable |
4,250
|
5,090
|
| Deferred revenue, current |
16,139
|
15,625
|
| Accrued purchase commitments, current |
131
|
131
|
| Accrued expenses and other current liabilities |
27,695
|
23,425
|
| Total current liabilities |
48,215
|
44,271
|
| Deferred revenue, non-current |
7,315
|
7,394
|
| Warrant liabilities |
2,685
|
826
|
| Operating lease liabilities |
20,398
|
22,835
|
| Other non-current liabilities |
8,637
|
8,895
|
| Total liabilities |
87,250
|
84,221
|
| Commitments and contingencies (Note 17) |
|
|
| Stockholders' equity: |
|
|
| Additional paid-in capital |
970,940
|
949,670
|
| Accumulated deficit |
(802,130)
|
(729,638)
|
| Total stockholders’ equity |
168,832
|
220,053
|
| Total liabilities and stockholders’ equity |
256,082
|
304,274
|
| Class A Common Stock |
|
|
| Stockholders' equity: |
|
|
| Common stock |
19
|
18
|
| Class B Common Stock |
|
|
| Stockholders' equity: |
|
|
| Common stock |
$ 3
|
$ 3
|
| X |
- DefinitionAmount of accrued purchase commitments, classified as current.
| Name: |
bfly_AccruedPurchaseCommitmentsCurrent |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of vendor advances classified as non-current.
| Name: |
bfly_VendorAdvancesNonCurrent |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of warrant liability.
| Name: |
bfly_WarrantLiability |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481990/310-10-45-2
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid nor invoiced, and liabilities classified as other.
| Name: |
us-gaap_AccruedLiabilitiesAndOtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value of capitalized payments made in advance for inventory that is expected to be received within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdvancesOnInventoryPurchases |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of obligation to transfer good or service to customer for which consideration has been received or is receivable, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479837/606-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-8
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479837/606-10-45-2
| Name: |
us-gaap_ContractWithCustomerLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of obligation to transfer good or service to customer for which consideration has been received or is receivable, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479837/606-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-8
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479837/606-10-45-2
| Name: |
us-gaap_ContractWithCustomerLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due after one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
CONSOLIDATED BALANCE SHEETS (Parenthetical) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Accounts receivable, net of allowance for doubtful accounts |
$ 2,583
|
$ 1,787
|
| Class A Common Stock |
|
|
| Common stock, par value (in dollars per share) |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized (in shares) |
600,000,000
|
600,000,000
|
| Common stock, shares issued (in shares) |
188,626,154
|
181,221,794
|
| Common stock, shares outstanding (in shares) |
188,626,154
|
181,221,794
|
| Class B Common Stock |
|
|
| Common stock, par value (in dollars per share) |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized (in shares) |
27,000,000
|
27,000,000
|
| Common stock, shares issued (in shares) |
26,426,937
|
26,426,937
|
| Common stock, shares outstanding (in shares) |
26,426,937
|
26,426,937
|
| X |
- DefinitionAmount of allowance for credit loss on accounts receivable, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479344/326-20-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-4
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Revenue: |
|
|
|
| Total revenue |
$ 82,056
|
$ 65,900
|
$ 73,390
|
| Cost of revenue: |
|
|
|
| Total cost of revenue |
33,225
|
49,044
|
33,930
|
| Gross profit |
48,831
|
16,856
|
39,460
|
| Operating expenses: |
|
|
|
| Research and development |
37,800
|
55,616
|
88,044
|
| Sales and marketing |
41,567
|
39,073
|
59,494
|
| General and administrative |
39,810
|
49,613
|
77,596
|
| Other |
4,065
|
18,164
|
7,346
|
| Total operating expenses |
123,242
|
162,466
|
232,480
|
| Loss from operations |
(74,411)
|
(145,610)
|
(193,020)
|
| Interest income |
5,020
|
7,450
|
3,384
|
| Interest expense |
(1,261)
|
0
|
(2)
|
| Change in fair value of warrant liabilities |
(1,859)
|
4,544
|
20,859
|
| Other income (expense), net |
(13)
|
(2)
|
98
|
| Loss before provision for income taxes |
(72,524)
|
(133,618)
|
(168,681)
|
| Provision (benefit) for income taxes |
(32)
|
82
|
42
|
| Net loss and comprehensive loss |
$ (72,492)
|
$ (133,700)
|
$ (168,723)
|
| Net loss per common share attributable to Class A and B common stockholders - basic (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Net loss per common share attributable to Class A and B common stockholders - diluted (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Weighted-average common shares used to compute net loss per share attributable to Class A and Class B common stockholders - basic (in shares) |
211,682,760
|
205,385,544
|
199,848,386
|
| Weighted-average common shares used to compute net loss per share attributable to Class A and Class B common stockholders - diluted (in shares) |
211,682,760
|
205,385,544
|
199,848,386
|
| Product |
|
|
|
| Revenue: |
|
|
|
| Total revenue |
$ 54,200
|
$ 40,036
|
$ 50,263
|
| Cost of revenue: |
|
|
|
| Total cost of revenue |
24,380
|
40,655
|
26,804
|
| Software and other services |
|
|
|
| Revenue: |
|
|
|
| Total revenue |
27,856
|
25,864
|
23,127
|
| Cost of revenue: |
|
|
|
| Total cost of revenue |
$ 8,845
|
$ 8,389
|
$ 7,126
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CostOfRevenueAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate revenue less cost of goods and services sold or operating expenses directly attributable to the revenue generation activity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 9: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_GrossProfit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense classified as nonoperating. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InterestExpenseNonoperating |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income on nonoperating securities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
| Name: |
us-gaap_InvestmentIncomeInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenuesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total amount of expenses directly related to the marketing or selling of products or services.
| Name: |
us-gaap_SellingAndMarketingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ProductMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ServiceOtherMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY - USD ($)
$ in Thousands |
Total |
Class A Common Stock |
Class B Common Stock |
Common Stock
Class A Common Stock
|
Common Stock
Class B Common Stock
|
Additional Paid-In Capital |
Accumulated Deficit |
| Balance at beginning of the period at Dec. 31, 2021 |
$ 447,691
|
|
|
$ 17
|
$ 3
|
$ 874,886
|
$ (427,215)
|
| Balance at beginning of the period (in shares) at Dec. 31, 2021 |
|
|
|
171,613,049
|
26,426,937
|
|
|
| Increase (Decrease) in Stockholders' Equity |
|
|
|
|
|
|
|
| Net loss |
(168,723)
|
|
|
|
|
|
(168,723)
|
| Common stock issued upon exercise of stock options and warrants |
2,982
|
|
|
|
|
2,982
|
|
| Common stock issued upon exercise of stock options and warrants (in shares) |
|
|
|
1,081,313
|
|
|
|
| Common stock issued upon vesting of restricted stock units, net |
(106)
|
|
|
|
|
(106)
|
|
| Common stock issued upon vesting of restricted stock units, net (in shares) |
|
|
|
1,765,594
|
|
|
|
| Stock-based compensation expense |
43,516
|
|
|
|
|
43,516
|
|
| Balance at end of the period at Dec. 31, 2022 |
325,360
|
|
|
$ 17
|
$ 3
|
921,278
|
(595,938)
|
| Balance at end of the period (in shares) at Dec. 31, 2022 |
|
|
|
174,459,956
|
26,426,937
|
|
|
| Increase (Decrease) in Stockholders' Equity |
|
|
|
|
|
|
|
| Net loss |
(133,700)
|
|
|
|
|
|
(133,700)
|
| Common stock issued upon exercise of stock options and warrants |
$ 228
|
|
|
|
|
228
|
|
| Common stock issued upon exercise of stock options and warrants (in shares) |
180,467
|
|
|
180,467
|
|
|
|
| Common stock issued upon vesting of restricted stock units, net |
$ 1
|
|
|
$ 1
|
|
|
|
| Common stock issued upon vesting of restricted stock units, net (in shares) |
|
|
|
6,581,371
|
|
|
|
| Stock-based compensation expense |
28,164
|
|
|
|
|
28,164
|
|
| Balance at end of the period at Dec. 31, 2023 |
220,053
|
|
|
$ 18
|
$ 3
|
949,670
|
(729,638)
|
| Balance at end of the period (in shares) at Dec. 31, 2023 |
|
181,221,794
|
26,426,937
|
181,221,794
|
26,426,937
|
|
|
| Increase (Decrease) in Stockholders' Equity |
|
|
|
|
|
|
|
| Net loss |
(72,492)
|
|
|
|
|
|
(72,492)
|
| Common stock issued upon exercise of stock options and warrants |
$ 64
|
|
|
|
|
64
|
|
| Common stock issued upon exercise of stock options and warrants (in shares) |
36,340
|
|
|
36,340
|
|
|
|
| Common stock issued upon vesting of restricted stock units, net |
$ (738)
|
|
|
$ 1
|
|
(739)
|
|
| Common stock issued upon vesting of restricted stock units, net (in shares) |
|
|
|
6,696,585
|
|
|
|
| Common stock issued for employee stock purchase plan |
495
|
|
|
|
|
495
|
|
| Common stock issued for employee stock purchase plan (in shares) |
|
|
|
671,435
|
|
|
|
| Stock-based compensation expense |
21,450
|
|
|
|
|
21,450
|
|
| Balance at end of the period at Dec. 31, 2024 |
$ 168,832
|
|
|
$ 19
|
$ 3
|
$ 970,940
|
$ (802,130)
|
| Balance at end of the period (in shares) at Dec. 31, 2024 |
|
188,626,154
|
26,426,937
|
188,626,154
|
26,426,937
|
|
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_IncreaseDecreaseInStockholdersEquityRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period as a result of an employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesEmployeeStockPurchasePlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period related to Restricted Stock Awards, net of any shares forfeited. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardNetOfForfeitures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate change in value for stock issued during the period as a result of employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueEmployeeStockPurchasePlan |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock related to Restricted Stock Awards issued during the period, net of the stock value of such awards forfeited. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueRestrictedStockAwardNetOfForfeitures |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.25.0.1
CONSOLIDATED STATEMENTS OF CASH FLOWS - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Cash flows from operating activities: |
|
|
|
| Net loss |
$ (72,492)
|
$ (133,700)
|
$ (168,723)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
| Depreciation, amortization, and impairments |
10,342
|
10,574
|
5,935
|
| Non-cash interest expense |
1,256
|
0
|
0
|
| Write-down of inventories |
15
|
21,083
|
783
|
| Stock-based compensation expense |
21,032
|
27,480
|
42,531
|
| Change in fair value of warrant liabilities |
1,859
|
(4,544)
|
(20,859)
|
| Gain on lease termination |
0
|
(214)
|
0
|
| Other |
1,102
|
633
|
615
|
| Changes in operating assets and liabilities: |
|
|
|
| Accounts receivable |
(8,503)
|
(162)
|
(3,063)
|
| Inventories |
2,218
|
(34,135)
|
(24,510)
|
| Prepaid expenses and other assets |
1,304
|
2,979
|
3,819
|
| Vendor advances |
(2,498)
|
17,091
|
5,100
|
| Accounts payable |
(841)
|
(1,875)
|
1,216
|
| Deferred revenue |
435
|
2,206
|
2,266
|
| Accrued purchase commitments |
0
|
(2,015)
|
(17,383)
|
| Change in operating lease assets and liabilities |
(750)
|
(635)
|
2,257
|
| Accrued expenses and other liabilities |
3,814
|
(3,586)
|
901
|
| Net cash used in operating activities |
(41,707)
|
(98,820)
|
(169,115)
|
| Cash flows from investing activities: |
|
|
|
| Purchases of marketable securities |
0
|
(297)
|
(75,534)
|
| Sales of marketable securities |
0
|
76,484
|
0
|
| Purchases of property, equipment, and intangible assets, including capitalized software |
(2,694)
|
(5,783)
|
(18,302)
|
| Sales of property and equipment |
36
|
10
|
57
|
| Net cash provided by (used in) investing activities |
(2,658)
|
70,414
|
(93,779)
|
| Cash flows from financing activities: |
|
|
|
| Proceeds from exercise of stock options and warrants |
64
|
228
|
2,982
|
| Proceeds from employee stock purchase plan |
495
|
0
|
0
|
| Payments to tax authorities for restricted stock units withheld |
(739)
|
0
|
(101)
|
| Payments on technology license commitment |
(1,315)
|
0
|
0
|
| Net cash provided by (used in) financing activities |
(1,495)
|
228
|
2,881
|
| Net decrease in cash, cash equivalents, and restricted cash |
(45,860)
|
(28,178)
|
(260,013)
|
| Cash, cash equivalents, and restricted cash, beginning of period |
138,650
|
166,828
|
426,841
|
| Cash, cash equivalents, and restricted cash, end of period |
92,790
|
138,650
|
166,828
|
| Supplementary disclosure of non-cash investing and financing activities |
|
|
|
| Acquisition of property, equipment, and intangible assets, including capitalized software |
$ 470
|
$ 9,247
|
$ 4,501
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, depleting assets, and impairments to periods that benefit from use of the assets.
| Name: |
bfly_DepreciationDepletionAmortizationAndImpairments |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period of accrued purchase commitments.
| Name: |
bfly_IncreaseDecreaseInAccruedPurchaseCommitments |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in vendor advances.
| Name: |
bfly_IncreaseDecreaseInVendorAdvances |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPayments on Technology License Commitment
| Name: |
bfly_PaymentsOnTechnologyLicenseCommitment |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPayments to Tax Authorities for Restricted Stock Units Withheld
| Name: |
bfly_PaymentsToTaxAuthoritiesForRestrictedStockUnitsWithheld |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option and warrants under share-based payment arrangement.
| Name: |
bfly_ProceedsFromExerciseOfStockOptionsAndWarrants |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFuture cash outflow to pay for purchases of fixed assets that have occurred. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-5
| Name: |
us-gaap_CapitalExpendituresIncurredButNotYetPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on termination of lease before expiration of lease term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 40
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479092/842-20-40-1
| Name: |
us-gaap_GainLossOnTerminationOfLease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionChange in recurring obligations of a business that arise from the acquisition of merchandise, materials, supplies and services used in the production and sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableTrade |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in accrued expenses, and obligations classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilitiesAndOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in deferred income and obligation to transfer product and service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInDeferredRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInInventories |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of loss from reductions in inventory due to subsequent measurement adjustments, including, but not limited to, physical deterioration, obsolescence, or changes in price levels. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483080/330-10-50-2
| Name: |
us-gaap_InventoryWriteDown |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionOther cash or noncash adjustments to reconcile net income to cash provided by (used in) operating activities that are not separately disclosed in the statement of cash flows (for example, cash received or cash paid during the current period for miscellaneous operating activities, net change during the reporting period in other assets or other liabilities).
| Name: |
us-gaap_OtherOperatingActivitiesCashFlowStatement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionInterest paid other than in cash for example by issuing additional debt securities. As a noncash item, it is added to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_PaidInKindInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the aggregate amount received by the entity through sale or maturity of marketable securities (held-to-maturity or available-for-sale) during the period.
| Name: |
us-gaap_ProceedsFromSaleAndMaturityOfMarketableSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the sale of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from the stock plan during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromStockPlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-3
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477314/942-235-S99-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 31: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4J
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481175/810-10-55-4K
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-2
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.25.0.1
Organization and Description of Business
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Organization and Description of Business |
Organization and Description of Business Butterfly Network, Inc., formerly known as Longview Acquisition Corp., was incorporated in Delaware on February 4, 2020. The Company is an innovative digital health business transforming care through a unique combination of portable, semiconductor-based ultrasound technology, intuitive software, services and educational offerings that can make medical imaging more accessible than ever before. Butterfly’s solution enables the practical application of ultrasound information into the clinical workflow through affordable hardware that fits in a healthcare professional’s pocket and is paired with cloud-connected software that is easily accessed through a mobile application. The Company operates wholly-owned subsidiaries in Australia, Germany, the Netherlands, Taiwan, and the United Kingdom.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Summary of Significant Accounting Policies
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
Summary of Significant Accounting Policies Basis of Presentation and Principles of Consolidation The accompanying consolidated financial statements include the accounts of BFLY Operations, Inc. (formerly Butterfly Network, Inc.) and its wholly-owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the accounting disclosure rules and regulations of the Securities and Exchange Commission (the “SEC”). All intercompany balances and transactions have been eliminated in consolidation. Functional Currency The Company’s worldwide operations utilize the U.S. dollar (“USD”) as the functional currency considering the significant dependency of each subsidiary on the Company. Subsidiary operations are financed through the funding received from the Company in USD. For foreign entities where the USD is the functional currency, all foreign currency-denominated monetary assets and liabilities are remeasured at end-of-period exchange rates. Exchange gains and losses arising from the remeasurement of foreign currency-denominated monetary assets and liabilities are included in other income (expense), net in the consolidated statements of operations and comprehensive loss. Concentration of Credit Risk Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash and cash equivalents and accounts receivable. As of December 31, 2024 and 2023, substantially all of the Company’s cash and cash equivalents were invested in money market accounts with one financial institution. The Company also maintains balances in various operating accounts above federally insured limits. The Company has not experienced any significant losses on such accounts and does not believe it is exposed to any significant credit risk on cash and cash equivalents. As of December 31, 2024 and 2023, one customer and no customers accounted for more than 10% of the Company’s accounts receivable, respectively. For the years ended December 31, 2024, 2023, and 2022, no customer accounted for more than 10% of the Company’s total revenue. Segment Information The Company has determined that it operates in one reportable segment, which includes all activities related to the development, manufacture, and sale of the Company's products, software, and other services. The Company’s chief operating decision maker ("CODM"), its chief executive officer ("CEO"), regularly reviews the Company's consolidated net loss, which is reported as net loss and comprehensive loss on the consolidated statements of operations and comprehensive loss, for purposes of evaluating the Company's financial performance, including reviewing budget versus actual results, and determining changes in the Company's allocation of resources across the Company's strategic initiatives. The Company's measure of segment assets is total assets, as reported on the consolidated balance sheets, and substantially all of the Company’s long-lived assets are located in the United States. In addition to the operating expenses presented on the consolidated statements of operations and comprehensive loss, the CODM also reviews certain significant segment expenses. The following table summarizes the Company's segment revenue and significant segment expenses included in consolidated net loss (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | Revenue | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | | Less: | | | | | | Cost of revenue | 33,225 | | | 49,044 | | | 33,930 | | Payroll operating expenses | 57,334 | | | 67,241 | | | 111,361 | | Stock-based compensation expense | 21,032 | | | 27,480 | | | 42,531 | | Non-payroll operating expenses | 40,811 | | | 49,581 | | | 71,242 | | Other | 4,065 | | | 18,164 | | | 7,346 | | Other segment items | (1,919) | | | (11,910) | | | (24,297) | | Net loss | $ | (72,492) | | | $ | (133,700) | | | $ | (168,723) | |
Other segment items include interest income, interest expense, the change in fair value of warrant liabilities, other income (expense), net, and the provision (benefit) for income taxes. Because the Company operates in one reportable segment, other required segment disclosures are included on the Company's consolidated financial statements. Interest income, interest expense, and the provision (benefit) for income taxes are included on the consolidated statements of operations and comprehensive loss. Depreciation, amortization, and impairments; write-down of inventories; and purchases of property, equipment, and intangible assets, including capitalized software, are included on the consolidated statements of cash flows. Use of Estimates The preparation of the consolidated financial statements in conformity with U.S. GAAP requires the Company to make estimates and assumptions about future events that affect the amounts reported in its consolidated financial statements and accompanying notes. Future events and their effects cannot be determined with certainty. On an ongoing basis, management evaluates these estimates and assumptions. Significant estimates and assumptions include: •revenue recognition, including determination of the timing and pattern of satisfaction of performance obligations and determination of the standalone selling price (“SSP”) of performance obligations; •measurement and allocation of capitalized costs, the net realizable value (the selling price as well as estimated costs of completion, disposal and transportation) of inventory, and demand and future use of inventory; •assumptions underlying the capitalization of costs to develop or obtain software for internal use; •assumptions underlying incremental borrowing rate calculations; •assumptions underlying the measurement of contingent losses; and •assumptions underlying the fair value used in the stock-based compensation expense calculation. The Company bases these estimates on historical and anticipated results and trends and on various other assumptions that the Company believes are reasonable under the circumstances, including assumptions about future events. Changes in estimates are recorded in the period in which they become known. Actual results could differ from these estimates, and any such differences may be material to the Company’s consolidated financial statements. Revenue Recognition The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“Topic 606”). Revenue is recognized when or as a customer obtains control of the promised goods and services. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to in exchange for these goods and services. To achieve this core principle, the Company applies the following five steps: •Step 1: Identify Contracts with Customers: The Company typically enters into contracts with customers through direct sales executed via signed contracts with payment terms of 30 days or less. Multi-year software subscriptions typically require advance payment for each annual subscription period. •Step 2: Identify Performance Obligations: The Company’s contracts with customers often include multiple performance obligations. The Company has identified the following performance obligations in its contracts with customers: •Hardware devices and accessories; •Software subscriptions, including renewal subscriptions, which represent an obligation to provide the customer with ongoing access to the Company’s cloud-hosted software applications on a continuous basis throughout the subscription period; •Implementation and integration services; •Extended warranties; and •SDKs, either perpetual or term-based. •Step 3: Determine Transaction Price: The Company’s contracts with customers include variable consideration in the form of refunds and credits for product returns and price concessions. The Company estimates variable consideration based on the individual contract circumstances or by using the expected value method based on a portfolio of data from similar contracts, depending on the circumstances. •Step 4: Allocate Transaction Price to Performance Obligations: The Company allocates transaction price to the performance obligations in contracts with customers based on the relative SSPs of the underlying goods and services which the Company has a history of selling to customers on a standalone basis. •Step 5: Recognize Revenue as Performance Obligations are Satisfied: Each sale of a hardware device, accessory, or perpetual SDK is a performance obligation satisfied at a point in time when control of the good transfers from the Company to the customer based on shipping terms or when the SDK is provided to the customer. The Company’s software subscriptions and extended warranties are stand-ready performance obligations that are satisfied over time by providing the customer with ongoing access to the Company’s resources. Term-based SDK sales are performance obligations that are satisfied over time through continued provision of access to the customer. The Company uses the time-elapsed (i.e., straight-line) measure of progress to recognize revenue as these performance obligations are satisfied evenly over the respective service periods. The implementation and integration services are a performance obligation satisfied over time, and the Company uses the costs incurred as inputs into the measure of progress to recognize revenue as it satisfies the performance obligation. Deferred Revenue Deferred revenue primarily consists of billings or payments received in advance of revenue recognition from software subscriptions and other services and is reduced as the revenue recognition criteria are met. Deferred revenue is classified as current or non-current on the consolidated balance sheets based on the expected timing of revenue recognition. The deferred revenue that will be recognized as revenue within the next twelve months is classified as current, and the deferred revenue that will be recognized thereafter is classified as non-current. Warranties The Company offers a standard product warranty that its products will function according to standard specifications and free of significant defects for a period ranging from one year to three years, depending on the product, from when control is transferred to the customer. The Company evaluated the warranty liability under ASC Topic 606 and determined that it is an assurance-type warranty. When product revenue is recognized, an estimate of future warranty costs is recognized as cost of product revenue on the consolidated statements of operations and comprehensive loss and accrued expenses on the consolidated balance sheets. Factors that affect the estimate of future warranty costs include historical and current product failure rates, service delivery costs incurred in correcting product failures, and warranty policies and business practices. Cash and Cash Equivalents All highly liquid investments purchased with a maturity of three months or less are considered to be cash equivalents. As of December 31, 2024 and 2023, cash and cash equivalents consist principally of cash and money market accounts. Trade Accounts Receivable and Allowance for Doubtful Accounts Accounts receivable are recognized as the original amount invoiced less an allowance for doubtful accounts based on the probability of future collection. In accordance with ASC Topic 326, Financial Instruments-Credit Losses, the Company estimates its allowance for doubtful accounts based on historical loss patterns, the number of days that billings are past due, current market conditions, and reasonable and supportable forecasts of future economic conditions. Accounts receivable are written off when deemed uncollectible and collection of the receivable is no longer being actively pursued. The following table summarizes the allowance for doubtful accounts activity: | | | | | | | (in thousands) | Fair Value | | Allowance for doubtful accounts as of December 31, 2022 | $ | 528 | | | Additions | 1,446 | | | Deductions – write offs | (187) | | | Allowance for doubtful accounts as of December 31, 2023 | 1,787 | | | Additions | 1,128 | | | Deductions – write offs | (332) | | | Allowance for doubtful accounts as of December 31, 2024 | $ | 2,583 | |
Inventories Inventories primarily consist of raw materials, work-in-progress, and finished goods which are purchased and held by the Company’s third-party contract manufacturers. Inventories are stated at the lower of actual cost, determined using the average cost method, or net realizable value. Actual cost includes all direct and indirect production costs to convert materials into a finished product. Net realizable value is based upon an estimated average selling price reduced by the estimated costs of completion, disposal, and transportation. The determination of net realizable value involves certain judgments including estimating average selling prices. The Company reduces the value of inventory for estimated obsolescence or lack of marketability by the difference between the cost of the affected inventory and the net realizable value. The valuation of inventories also requires the Company to estimate excess and obsolete inventory. The Company considers new product development schedules, the effect that new products might have on the sale of existing products, product obsolescence, product merchantability, and whether older products can be remanufactured into new products, among other factors. Losses expected to arise from firm, non-cancelable and unhedged commitments for the future purchase of inventories are recognized unless the losses are recoverable through firm sales contracts or other means. Restricted Cash Restricted cash includes deposits in financial institutions restricted according to an agreement and used to secure a lease agreement. The Company classifies the amount restricted according to an agreement as prepaid expenses and other current assets on the consolidated balance sheets as the Company expects the deposit to be released from restriction within the next twelve months. The Company classifies the amount used to secure a lease agreement within other non-current assets on the consolidated balance sheets as the lease is long-term. The amount shown as restricted cash is included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown in the consolidated statements of cash flows. Vendor Advances Vendor advances represent amounts paid to third-party vendors for future services to be received related to production of the Company’s inventories. The amounts are presented net of write offs. The classification of vendor advances as current or non-current is based on the estimated timing of inventory delivery. Property and Equipment Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation expense is computed using the straight-line method over the estimated useful lives of the related assets. Leasehold improvements are amortized on a straight-line basis over the shorter of the remaining lease term or the estimated useful lives of related improvements. Useful lives for property and equipment are as follows: | | | | | | | | | | Property and Equipment | | Estimated Useful Life | | Software | | 3 years | | Machinery and equipment | | 3 – 5 years | | Furniture and fixtures | | 5 – 7 years | | Leasehold improvements | | Lesser of estimated useful life or remaining lease term |
Expenditures for major renewals and improvements are capitalized. Expenditures for repairs and maintenance are expensed as incurred. When assets are retired or otherwise disposed of, the cost of those assets and the related accumulated depreciation and amortization is eliminated from the balance sheet, and any resulting gains or losses are included in the consolidated statements of operations and comprehensive loss in the period of disposal. Capitalized Software Development Costs Costs to develop or obtain software for internal use are capitalized and recorded as capitalized software development costs on the consolidated balance sheets as a component of property and equipment. The Company capitalizes qualifying costs associated with internal-use software incurred during the application development stage if management with relevant authority authorizes the project, it is probable the project will be completed, and the software will be used to perform the function intended. Costs incurred during the preliminary project and post-implementation stages, including training and maintenance, are expensed as incurred. Capitalized costs are amortized on a project-by-project basis using the straight-line method over the estimated economic life of the software, which is three years, beginning when the software is substantially ready for use. Amortization expense is classified in the consolidated statements of operations and comprehensive loss based on the nature of the software. Leases The Company primarily enters into leases for office space that are classified as operating leases. The Company determines if an agreement is or contains a lease at inception. The Company accounts for leases in accordance with ASC Topic 842, Leases, by recognizing right-of-use assets and lease liabilities. The Company classifies right-of-use assets as operating lease assets on the consolidated balance sheets. The Company classifies the current portion of lease liabilities, representing lease payments due within the next twelve months, as accrued expenses and other current liabilities on the consolidated balance sheets. The Company classifies the non-current portion of lease liabilities as operating lease liabilities on the consolidated balance sheets. The lease term includes the non-cancelable period of the lease plus any additional periods covered by an option to extend that the Company is reasonably certain to exercise. Generally, the Company may terminate its leases with the notice required in the lease agreement and upon payment of a termination fee, if required. The Company’s leases do not include substantial variable payments based on indexes or rates. The Company’s lease agreements do not contain any significant residual value guarantees or restrictive covenants. The Company’s leases do not provide a readily determinable implicit discount rate. The Company’s incremental borrowing rate is estimated to approximate an interest rate on a collateralized basis with similar terms and payments and in similar economic environments. Operating lease right-of-use assets and liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. The Company recognizes a single lease cost on a straight-line basis over the lease term, and the Company includes all cash payments within cash flows from operating activities as the change in operating lease assets and liabilities in the consolidated statements of cash flows. The Company does not have any finance leases as of December 31, 2024 and 2023. Impairment of Long-Lived Assets The Company reviews its long-lived assets, including its property and equipment, definite-lived intangible assets, and operating lease assets, for impairment at least annually or whenever events or changes in business circumstances indicate that the carrying amount of assets may not be fully recoverable. Each impairment test is based on a comparison of the undiscounted cash flows to the recorded value of the asset. If the recorded value of the asset is less than the undiscounted cash flows, the asset is written down to its estimated fair value. Warrant Liability The Company’s outstanding warrants include publicly-traded warrants (the “Public Warrants”), which were issued as one-third of a warrant per unit during the Company’s initial public offering on May 26, 2020 (the “IPO”), and warrants sold in a private placement to Longview’s sponsor (the “Private Warrants”). The Company evaluated its warrants under ASC Subtopic 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, and concluded that they do not meet the criteria to be classified in stockholders’ equity. Since the Public Warrants and Private Warrants meet the definition of a derivative under ASC Topic 815, Derivatives and Hedging, the Company recorded these warrants as non-current liabilities on the consolidated balance sheets at fair value upon the closing of the Business Combination, with subsequent changes in their respective fair values recognized in the consolidated statements of operations and comprehensive loss at each reporting date. See Note 15 "Warrants" for additional information about our warrants. Cost of Revenue Cost of product revenue includes manufacturing costs, personnel costs and benefits, inbound freight, packaging, warranty replacement costs, payment processing fees, and inventory obsolescence and write-offs. Cost of software and other services revenue includes personnel costs, cloud hosting costs, amortization of capitalized software development costs, and payment processing fees. Research and Development R&D expenses primarily consist of personnel costs and benefits, facilities expenses, consulting and professional fees, fabrication services, software, and other outsourcing expenses. Substantially all of the Company’s R&D expenses are related to developing new products and services and improving existing products and services. R&D expenses are expensed as incurred. Sales and Marketing Sales and marketing expenses primarily consist of personnel costs and benefits, third-party logistics, fulfillment and outbound shipping costs, facilities expenses, advertising, and travel and entertainment. Advertising expenses are expensed as incurred. For the years ended December 31, 2024, 2023, and 2022, advertising expenses were $4.3 million, $4.3 million and $5.8 million, respectively. General and Administrative General and administrative expenses primarily consist of personnel costs and benefits, insurance, patent fees, software costs, facilities costs, and outside services. Outside services consist of professional services, legal fees, and other professional fees. Operating Expenses – Other The Company classifies certain operating expenses that are not representative of its ongoing operations as other on the consolidated statements of operations and comprehensive loss. These include costs related to the Company’s business transformation initiative, reductions in force, litigation, and legal settlements. The following table summarizes the types of expenses classified as other in the Company’s consolidated statements of operations and comprehensive loss (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Employment-related expenses | $ | 1,341 | | | $ | 8,701 | | | $ | 2,019 | | | Legal-related expenses | 2,724 | | | 9,463 | | | 5,327 | | | Total other | $ | 4,065 | | | $ | 18,164 | | | $ | 7,346 | |
On July 1, 2024, the Company entered into an agreement with a third-party global technology and business transformation partner to optimize and lower the cost of certain non-specialized technical functions. As part of the transition into this new partnership, a portion of the Company's workforce will be in lower-cost geographies. The Company incurred $0.7 million of severance costs for impacted employees that continued providing transition services to the Company through the end of 2024. These costs were recognized as other in the consolidated statements of operations and comprehensive loss during the year ended December 31, 2024. As of December 31, 2024, a total of $0.7 million of accrued severance related to our global workforce optimization is included in accrued expenses and other current liabilities and other non-current liabilities on the consolidated balance sheets. Net Loss per Common Share We compute net loss per share of Class A and Class B common stock using the two-class method. Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of each class of the Company’s common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all of the Company’s potential common shares outstanding of the Company’s common stock to the extent the potential shares are dilutive. Basic and diluted net loss per share were the same for each period presented in the consolidated statements of operations and comprehensive loss as the inclusion of all potential shares of the Company’s common stock would have been anti-dilutive. Refer to Note 12 “Net Loss Per Share” for further discussion. Stock-Based Compensation Expense The measurement of stock-based compensation expense for all stock-based payment awards, including stock options and RSUs granted to employees, directors, and nonemployees as well as the Company's ESPP, is based on the estimated fair value of the awards on the grant date. The Company recognizes stock-based compensation expense for its awards on a straight-line basis over the requisite service period of the individual grants, which is generally the vesting period. Generally, stock option and RSU awards fully vest three to four years from the grant date, and stock options have a contractual term of 10 years. ESPP options generally vest over two years. The Company recognizes the effect of forfeitures in stock-based compensation expense based on actual forfeitures when they occur. The Company granted performance-based restricted stock units during the years ended December 31, 2024, 2023, and 2022. The Company accounted for these awards according to the relevant provisions of ASC Topic 718, Compensation-Stock Compensation. For performance-based awards, the Company recognizes expense using the accelerated attribution method. Refer to Note 11 “Stock-Based Compensation” for further discussion about the nature of the Company's stock-based compensation transactions. Income Taxes The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies. The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more likely than not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties. Recent Accounting Pronouncements Adopted In November 2023, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which introduced new guidance on disclosures for reportable segments and significant segment expenses, including for entities with a single reportable segment. The Company retrospectively adopted this guidance during the year ended December 31, 2024. The adoption did not have a significant impact on the consolidated financial statements, other than the newly required disclosures. Recent Accounting Pronouncements Issued but Not Yet Adopted In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which introduced new guidance on disclosures for income taxes, including enhancements to the rate reconciliation and income taxes paid disclosures. This guidance is effective for the Company for annual reporting periods beginning January 1, 2025. The Company is currently evaluating the impact that the adoption of this pronouncement will have on the Company’s consolidated financial statements and disclosures. In November 2024, the FASB issued ASU 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, which introduced new guidance on disclosures of specified information about certain costs and expenses included within expenses presented on the face or the income statements, such as purchases of inventory and employee compensation. This guidance is effective for the Company for annual reporting periods beginning January 1, 2027 and interim reporting periods beginning January 1, 2028. The Company is currently evaluating the impact that the adoption of this pronouncement will have on the Company's consolidated financial statements and disclosures.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Revenue Recognition
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Revenue from Contract with Customer [Abstract] |
|
| Revenue Recognition |
Revenue Recognition Disaggregation of Revenue The Company disaggregates revenue from contracts with customers by product type and by geographical market. The Company believes that these categories aggregate the payor types by nature, amount, timing, and uncertainty of its revenue streams. The following table summarizes the Company’s disaggregated revenues (in thousands) for the years ended December 31: | | | | | | | | | | | | | | | | | | | | | | | | | Pattern of
Recognition | | 2024 | | 2023 | | 2022 | | By product type: | | | | | | | | | Devices and accessories | Point-in-time | | $ | 54,200 | | | $ | 40,036 | | | $ | 50,263 | | | Software and other services | Over time | | 27,856 | | | 25,864 | | | 23,127 | | | Total revenue | | | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | | | | | | | | | | | By geographical market: | | | | | | | | | United States | | | $ | 63,423 | | | $ | 52,116 | | | $ | 51,072 | | | International | | | 18,633 | | | 13,784 | | | 22,318 | | | Total revenue | | | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | |
Contract Balances Contract balances represent amounts presented in the consolidated balance sheets when the Company has either transferred goods or services to the customer or the customer has paid consideration to the Company under the contract. These contract balances include trade accounts receivable and deferred revenue. The Company recognizes a receivable when it has an unconditional right to payment, and payment terms are typically 30 days for product and software and other services sales on credit. The amount of revenue recognized during the years ended December 31, 2024 and 2023 that was included in the deferred revenue balance at the beginning of the period was $15.6 million and $14.9 million, respectively. Transaction Price Allocated to Remaining Performance Obligations As of December 31, 2024, the Company had $33.3 million of remaining performance obligations. The Company expects to recognize approximately 64% of its remaining performance obligations as revenue in the next twelve months and approximately 36% thereafter. Costs of Obtaining or Fulfilling Contracts The Company incurs incremental costs of obtaining contracts and costs of fulfilling contracts with customers. Incremental costs of obtaining contracts, which include commissions and referral fees paid to third parties as a result of obtaining contracts with customers, are capitalized to the extent that the Company expects to recover such costs. Costs of fulfilling contracts that relate specifically to a contract with a customer, which result from activities that generate resources for the Company and enable the Company to satisfy its performance obligations in the contract with the customer, are capitalized to the extent that the Company expects to recover such costs. Capitalized costs are amortized in a pattern that is consistent with the Company’s transfer of the related goods and services to the customer. The Company had $0.7 million and $1.4 million of capitalized costs of obtaining or fulfilling contracts as of December 31, 2024 and 2023, respectively. The Company’s amortization costs for capitalized costs of obtaining or fulfilling contracts were $0.7 million and $0.6 million for the years ended December 31, 2024 and 2023. The Company’s amortization costs for capitalized costs of obtaining or fulfilling contracts was not significant for the year ended December 31, 2022. Practical Expedients and Accounting Policy Elections In determining the transaction price of its contracts with customers, the Company estimates variable consideration using a portfolio of data from similar contracts. As a practical expedient, the Company does not adjust the transaction price for the effects of a significant financing component in contracts in which the period between when the Company transfers the promised good or service to the customer and when the customer pays for that good or service is a year or less. The Company has made an accounting policy election to exclude all sales taxes from the transaction price of its contracts with customers. Accordingly, sales taxes collected from customers and remitted to government authorities are not included in revenue and are accounted for as a liability until they have been remitted to the respective government authority. As a practical expedient, the Company does not capitalize incremental costs of obtaining contracts when the amortization period would be one year or less. Such incremental costs of obtaining contracts are recognized as expense when incurred.
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenueFromContractWithCustomerAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure of revenue from contract with customer to transfer good or service and to transfer nonfinancial asset. Includes, but is not limited to, disaggregation of revenue, credit loss recognized from contract with customer, judgment and change in judgment related to contract with customer, and asset recognized from cost incurred to obtain or fulfill contract with customer. Excludes insurance and lease contracts. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-9
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-15
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-12
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-12
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-13
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org/606/tableOfContent
| Name: |
us-gaap_RevenueFromContractWithCustomerTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Fair Value of Financial Instruments
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value of Financial Instruments |
Fair Value of Financial Instruments Fair value estimates of financial instruments are made at a specific point in time, based on relevant information about financial markets and specific financial instruments. As these estimates are subjective in nature, involving uncertainties and matters of significant judgment, they cannot be determined with precision. Changes in assumptions can significantly affect estimated fair value. The Company measures fair value as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The Company utilizes a three-tier hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value: •Level 1 — Valuations based on quoted prices in active markets for identical assets or liabilities that an entity has the ability to access. •Level 2 — Valuations based on quoted prices for similar assets or liabilities, quoted prices for identical assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially the full term of the assets or liabilities. •Level 3 — Valuations based on inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. The Company has no assets or liabilities valued with Level 3 inputs. The carrying value of cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses approximates their fair values due to the short-term or on demand nature of these instruments. There were no transfers between fair value measurement levels during the years ended December 31, 2024 and 2023. The Company determined the fair value of its Public Warrants as Level 1 financial instruments, as they are traded in active markets. Because any transfer of Private Warrants from the initial holder of the Private Warrants would result in the Private Warrants having substantially the same terms as the Public Warrants, management determined that the fair value of each Private Warrant is the same as that of a Public Warrant. Accordingly, the Private Warrants are classified as Level 2 financial instruments. The following table summarizes the Company’s assets and liabilities that are measured at fair value on a recurring basis, by level, within the fair value hierarchy (in thousands): | | | | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement Level | | Total | | Level 1 | | Level 2 | | Level 3 | | December 31, 2024: | | | | | | | | | Warrants: | | | | | | | | | Public Warrants | $ | 1,794 | | | $ | 1,794 | | | $ | — | | | $ | — | | | Private Warrants | 891 | | | — | | | 891 | | | — | | | Total liabilities at fair value on a recurring basis | $ | 2,685 | | | $ | 1,794 | | | $ | 891 | | | $ | — | | | | | | | | | | | December 31, 2023: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Warrants: | | | | | | | | | Public Warrants | $ | 552 | | | $ | 552 | | | $ | — | | | $ | — | | | Private Warrants | 274 | | | — | | | 274 | | | — | | | Total liabilities at fair value on a recurring basis | $ | 826 | | | $ | 552 | | | $ | 274 | | | $ | — | |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 107
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-107
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 940
-SubTopic 820
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478119/940-820-50-1
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Inventories
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Inventory Disclosure [Abstract] |
|
| Inventories |
Inventories A summary of inventories is as follows at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Raw materials | $ | 47,642 | | | 49,366 | | | Work-in-progress | 4,736 | | | 3,384 | | | Finished goods | 18,411 | | | 20,272 | | | Total inventories | $ | 70,789 | | | $ | 73,022 | |
Work-in-progress represents inventory items in intermediate stages of production by third party manufacturers. For the years ended December 31, 2024, 2023, and 2022, net realizable value inventory adjustments and excess and obsolete inventory charges were not significant, $21.1 million, and $0.8 million, respectively, and were recognized in cost of product revenue.
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for inventory. Includes, but is not limited to, the basis of stating inventory, the method of determining inventory cost, the classes of inventory, and the nature of the cost elements included in inventory. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/330/tableOfContent
| Name: |
us-gaap_InventoryDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Restricted Cash
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Cash and Cash Equivalents [Abstract] |
|
| Restricted Cash |
Restricted Cash A reconciliation of cash, cash equivalents and restricted cash from the consolidated balance sheets to the consolidated statements of cash flows as of December 31, 2024 and 2023 is as follows (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Reconciliation of cash, cash equivalents and restricted cash: | | | | | Cash and cash equivalents | $ | 88,775 | | | $ | 134,437 | | | Restricted cash included within prepaid expenses and other current assets | — | | | 199 | | | Restricted cash included within other non-current assets | 4,015 | | | 4,014 | | | Total cash, cash equivalents and restricted cash shown in the condensed consolidated statements of cash flows | $ | 92,790 | | | $ | 138,650 | |
Restricted cash included within prepaid expenses and other current assets is restricted by an agreement with the Gates Foundation. The restriction on these funds lapses as the Company fulfills its obligations in the agreement. As of December 31, 2024, the Company has fulfilled all of its obligations in the agreement, and all of the restrictions on these funds have lapsed. Restricted cash included within other non-current assets is held as collateral to secure a letter of credit for one of our office leases and is expected to be maintained as a security deposit throughout the duration of the lease.
|
| X |
- References
+ Details
| Name: |
us-gaap_CashAndCashEquivalentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for cash and cash equivalent footnotes, which may include the types of deposits and money market instruments, applicable carrying amounts, restricted amounts and compensating balance arrangements. Cash and equivalents include: (1) currency on hand (2) demand deposits with banks or financial institutions (3) other kinds of accounts that have the general characteristics of demand deposits (4) short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Generally, only investments maturing within three months from the date of acquisition qualify. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CashAndCashEquivalentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Other Non-Current Assets
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Other Assets, Noncurrent Disclosure [Abstract] |
|
| Other Non-Current Assets |
Other Non-Current Assets Other non-current assets consist of the following at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Security deposits | $ | 982 | | | $ | 989 | | | Restricted cash | 4,015 | | | 4,014 | | | Other long-term assets | 763 | | | 1,419 | | | Total other non-current assets | $ | 5,760 | | | $ | 6,422 | |
|
| X |
- DefinitionThe entire disclosure for other non-current assets.
| Name: |
bfly_OtherAssetsNonCurrentTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherAssetsNoncurrentDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Property, Equipment, and Intangible Assets
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Property, Equipment, and Intangible Assets [Abstract] |
|
| Property, Equipment, and Intangible Assets |
Property, Equipment, and Intangible Assets Property and Equipment Property and equipment, net, are recorded at historical cost and consist of the following at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Capitalized internally developed software | $ | 20,677 | | | $ | 17,722 | | | Leasehold improvements | 11,120 | | | 11,102 | | | Machinery and equipment | 10,043 | | | 9,930 | | | Furniture and fixtures | 2,152 | | | 2,152 | | | Construction in progress | 2,394 | | | 2,566 | | | Other | 29 | | | 44 | | | 46,415 | | | 43,516 | | | Less: accumulated depreciation and amortization | (26,897) | | | (18,195) | | | Property and equipment, net | $ | 19,518 | | | $ | 25,321 | |
Total depreciation and amortization expense related to property and equipment amounted to $8.9 million, $8.7 million, and $5.9 million for the years ended December 31, 2024, 2023, and 2022, respectively. For the Company’s capitalized internally developed software assets, accumulated amortization was $15.5 million and $9.4 million as of December 31, 2024 and 2023, respectively. Amortization expense recognized on these capitalized internally developed software assets was $6.1 million, $5.5 million, and $3.3 million for the years ended December 31, 2024, 2023 and 2022, respectively. Intangible Assets The Company's intangible assets consist of the following (in thousands): | | | | | | | | | | | | | | | | | | | Gross Carrying
Amount | | Accumulated
Amortization | | Net Carrying
Amount | | December 31, 2024: | | | | | | | Technology licenses | $ | 10,317 | | | $ | (1,401) | | | $ | 8,916 | | | | | | | | | December 31, 2023: | | | | | | | Technology licenses | $ | 10,317 | | | $ | — | | | $ | 10,317 | |
For the Company's commitment related to technology licenses acquired in a prior period, the current and non-current portions are included on the consolidated balance sheets in accrued expenses and other current liabilities and other non-current liabilities, respectively. As of December 31, 2024, the current portion was $3.1 million, and the non-current portion was $5.6 million. As of December 31, 2023, the current portion was $1.3 million, and the non-current portion was $7.6 million. Estimated amortization expense for the Company’s capitalized internally developed software assets and intangible assets over the next five years ended December 31 is as follows (in thousands): | | | | | | | | | | | | | | | | | | | | | | | | | | | | 2025 | | 2026 | | 2027 | | 2028 | | 2029 | | $ | 4,989 | | | $ | 2,737 | | | $ | 1,583 | | | $ | 1,120 | | | $ | 1,120 | |
Impairment During the year ended December 31, 2023, the Company fully impaired its leasehold improvements related to a lease that was terminated during the third quarter of 2023. The Company recognized an impairment loss of $1.8 million for the year ended December 31, 2023 in operating expenses on the consolidated statements of operations and comprehensive loss. See Note 16, “Leases” for further discussion of the terminated lease. The Company did not recognize any impairment losses for the years ended December 31, 2024 and 2022.
|
| X |
- References
+ Details
| Name: |
bfly_PropertyEquipmentAndIntangibleAssetsAbstract |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for intangible assets and long-lived, physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, accounting policies and methodology, roll forwards, depreciation, depletion and amortization expense, including composite depreciation, accumulated depreciation, depletion and amortization expense, useful lives and method used, income statement disclosures, assets held for sale and public utility disclosures. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-30/tableOfContent
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-20/tableOfContent
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/360/tableOfContent
| Name: |
us-gaap_PropertyPlantAndEquipmentAndIntangibleAssetsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Accrued Expenses and Other Current Liabilities
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Accrued Expenses and Other Current Liabilities |
Accrued Expenses and Other Current Liabilities Accrued expenses and other current liabilities consist of the following at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Employee compensation | $ | 11,192 | | | $ | 9,442 | | | Customer deposits | 1,909 | | | 1,613 | | | Accrued warranty liability | 498 | | | 297 | | | Non-income tax | 2,356 | | | 1,197 | | | Professional fees | 2,015 | | | 2,481 | | | Current portion of operating lease liabilities | 2,437 | | | 2,192 | | | Other | 7,288 | | | 6,203 | | | Total accrued expenses and other current liabilities | $ | 27,695 | | | $ | 23,425 | |
Warranty expense activity for the years ended December 31 is as follows (in thousands): | | | | | | | | | | | | | | | | | | | 2024 | | 2023 | | 2022 | | Balance, beginning of period | $ | 697 | | | $ | 873 | | | $ | 1,116 | | | Warranty provision charged to operations | 1,224 | | | 276 | | | 296 | | | Warranty claims | (898) | | | (452) | | | (539) | | | Balance, end of period | $ | 1,023 | | | $ | 697 | | | $ | 873 | |
The Company classifies its accrued warranty liability based on the timing of expected warranty activity. The future costs of expected activity greater than one year is recorded within other non-current liabilities on the consolidated balance sheet.
|
| X |
- DefinitionThe entire disclosure for accounts payable, accrued expenses, and other liabilities that are classified as current at the end of the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 720
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483384/720-30-45-1
| Name: |
us-gaap_AccountsPayableAccruedLiabilitiesAndOtherLiabilitiesDisclosureCurrentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Stockholders' Equity
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Stockholders' Equity Note [Abstract] |
|
| Stockholders' Equity |
Stockholders’ Equity The Company's outstanding shares consist of two classes of common stock, Class A and Class B. Dividends Holders of the Company’s Class A and Class B common stock are not entitled to receive dividends unless declared by the Board. Any such dividends would be subject to the preferential dividend rights of the holders of the then outstanding preferred stock or any other series stock having preferential rights. Holders of the Class A and Class B common stock will share ratably, if and when any dividend is declared, out of funds legally available. There have been no dividends declared to date. Voting rights The holders of shares of the Class A common stock are entitled to 1 vote per share on all matters on which the shares shall be entitled to vote. The holders of shares of the Class B common stock are entitled to 20 votes per share on all matters on which the shares shall be entitled to vote. Generally, holders of all classes of common stock vote together as a single class. Liquidation Rights On the liquidation, dissolution, distribution of assets, or winding up of the Company, each holder of Class A and Class B common stock will be entitled, pro rata on a per share basis, to all assets of the Company of whatever kind available for distribution to the holders of common stock, subject to the designations, preferences, limitations, restrictions, and relative rights of any other class or series of preferred stock of the Company then outstanding and unless disparate or different treatment of the shares of Class A common stock and Class B common stock is approved by the affirmative vote of the holders of a majority of the outstanding shares of Class A common stock and Class B common stock, each voting separately as a class. Other Matters Holders of shares of Class A common stock do not have subscription, redemption or conversion rights. Holders of Class B common stock have the right to convert shares of their Class B common stock into fully paid and non-assessable shares of Class A common stock, on a one-to-one basis, at the option of the holder at any time upon written notice to the Company. Holders of Class B common stock will have their Class B common stock automatically converted into Class A common stock, on a one-to-one basis, upon the occurrence of any of the events described below: (1)Any sale, assignment, transfer, conveyance, hypothecation, or other transfer or disposition, directly or indirectly, of any Class B common stock or any legal or beneficial interest in such share, whether or not for value and whether voluntary or involuntary or by operation of law (including by merger, consolidation, or otherwise), including, without limitation the transfer of a share of Class B common stock to a broker or other nominee or the transfer of, or entering into a binding agreement with respect to, voting control over such share by proxy or otherwise, other than a permitted transfer. (2)Upon the first date on which Dr. Rothberg, together with all other qualified stockholders, collectively cease to beneficially own at least 20% of the number of shares of Class B common stock (as such number of shares is equitably adjusted in respect of any reclassification, stock dividend, subdivision, combination, or recapitalization of the Class B common stock) collectively beneficially owned by Dr. Rothberg and permitted transferees of Class B common stock as of the effective time of the Business Combination. (3)Upon the date specified by the affirmative vote of the holders of at least two-thirds (2/3) of the then outstanding shares of Class B common stock, voting as a separate class. (4)If not converted earlier, whether optionally or automatically, on February 12, 2028.
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Stock-Based Compensation
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Stock-Based Compensation |
Stock-Based Compensation Equity incentive plans The Company’s 2012 Employee, Director and Consultant Equity Incentive Plan (the "2012 Plan") was approved by the Board and the Company’s stockholders in March 2012. In connection with the closing of the Business Combination, the Company has not granted and will not grant any additional awards under the 2012 Plan. However, the 2012 Plan will continue to govern the terms and conditions of the outstanding awards previously granted thereunder. As of December 31, 2024, the number of shares of common stock reserved for issuance under the 2012 Plan was 4.8 million. The Butterfly Network, Inc. Amended and Restated 2020 Equity Incentive Plan (the “2020 Plan”, and together with the 2012 Plan, the “Plans”) was approved by the Board in the fourth quarter of 2020 and by the Company's stockholders in the first quarter of 2021. The 2020 Plan is administered by the Board. The Board may grant stock-based awards, restricted stock, and options to purchase shares either as incentive stock options or non-qualified stock options. The restricted stock and options grants are subject to certain terms and conditions, option periods and conditions, exercise rights, and privileges and are fully discussed in the 2020 Plan. Grants under the Plans are included in the tables below. As of December 31, 2024, the number of shares of common stock reserved for issuance under the 2020 Plan was 53.5 million and 17.2 million common shares remain available for issuance under the 2020 Plan. Stock options Each stock option grant carries varying vesting schedules whereby the options may be exercised at the participant’s sole discretion provided they are an employee, director or consultant of the Company on the applicable vesting date. Each option shall terminate not more than ten years from the grant date. A summary of the stock option activity under the Plans is presented in the table below: | | | | | | | | | | | | | | | | | | | | | | | | | Number of
Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term | | Aggregate
Intrinsic
Value
(in thousands) | | Outstanding at December 31, 2022 | 12,571,912 | | 7.67 | | | 5.62 | | 1,342 | | | Granted | — | | — | | | | | | | Exercised | (180,467) | | 1.26 | | | | | | | Forfeited | (4,952,258) | | 10.14 | | | | | | | Outstanding at December 31, 2023 | 7,439,187 | | 6.17 | | | 4.68 | | — | | | Granted | — | | — | | | | | | | Exercised | (36,340) | | 1.77 | | | | | | | Forfeited | (842,111) | | 8.98 | | | | | | | Outstanding at December 31, 2024 | 6,560,736 | | 5.88 | | | 3.56 | | 1,963 | | | Options exercisable at December 31, 2023 | 6,537,433 | | 5.88 | | | 4.37 | | — | | | Options exercisable at December 31, 2024 | 6,222,073 | | 5.94 | | | 3.48 | | 1,799 | |
The total intrinsic value excludes those options whereby the stock price does not exceed the exercise price of the option. Additional information about the Company’s stock option activity during the years ended December 31, 2024, 2023 and 2022 is presented in the table below: | | | | | | | | | | | | | | | | | | | 2024 | | 2023 | | 2022 | | Cash proceeds from the exercise of stock options (in millions) | $ | 0.1 | | | $ | 0.2 | | | $ | 3.0 | | | Total intrinsic value of stock options exercised (in millions) | — | | | 0.2 | | | 3.6 | | | Weighted average grant date fair value of options granted | — | | | — | | | 2.79 | |
The intrinsic value of a stock option that’s been exercised is the amount by which the stock price exceeds the exercise price of the option on the date of exercise. Restricted stock units A summary of the restricted stock unit activity under the Plans is presented in the table below: | | | | | | | | | | | | | Number of
Restricted
Stock Units | | Weighted
Average
Grant Date
Fair Value | | Outstanding at December 31, 2022 | 9,961,291 | | 4.55 | | | Granted | 16,082,613 | | 2.23 | | | Vested | (5,418,832) | | 4.09 | | | Forfeited | (5,055,089) | | 3.65 | | | Outstanding at December 31, 2023 | 15,569,983 | | 2.61 | | | Granted | 15,838,283 | | 1.10 | | | Vested | (6,906,452) | | 2.69 | | | Forfeited | (3,251,584) | | 2.34 | | | Outstanding at December 31, 2024 | 21,250,230 | | 1.52 | |
The total fair value of the restricted stock units vested was $10.4 million, $7.3 million, and $10.7 million during the years ended December 31, 2024, 2023, and 2022, respectively. Included in the table above are performance-based restricted stock units that include certain service conditions in the award. In 2022, the Company granted 0.2 million performance-based restricted stock units to certain executives. The service condition for these awards is satisfied by providing service to the Company based on the defined service period per the award agreement. The performance-based conditions are objective performance metrics defined in the award agreement. An insignificant amount of expense was recognized for these awards during the years ended December 31, 2024, 2023, and 2022. Also included in the table above are market-based restricted stock units that include a service condition. In 2024 and 2023, the Company granted 1.0 million and 1.8 million, respectively, of these awards to certain executives. The market-based conditions for these awards are objective metrics related to the Company’s stock price defined in the award agreement. The service condition for these awards is satisfied by providing service to the Company through the achievement date of the market-based conditions. The grant date fair value of the awards is recognized as stock-based compensation expense over the derived service period. The grant date fair value and derived service period were determined by using a Monte Carlo simulation with the risk-free interest rate, expected dividend yield, and expected volatility assumptions detailed below. The Company recognized $2.0 million and $2.5 million of expense for these awards during the years ended December 31, 2024 and 2023. Valuation assumptions In accordance with ASC Topic 718, Compensation-Stock Compensation, the Company estimates and records the compensation cost associated with the grants described above with an offsetting entry to paid-in capital. As described in Note 2 “Summary of Significant Accounting Policies”, the Company selected the Black-Scholes option pricing model for determining the estimated fair value of its service-based stock option awards. The Black-Scholes model requires the use of subjective assumptions which determine the fair value of stock-based awards. These assumptions are also used to determine the fair value of market-based awards via Monte Carlo simulations. The assumptions used to value stock option and market-based grants to employees were as follows: | | | | | | | | | | | | | | | | | | | 2024 | | 2023 | | 2022 | | Risk-free interest rate | 3.6% | | 3.6% – 3.9% | | 1.7% – 3.0% | | Expected dividend yield | 0% | | 0% | | 0% | | Expected term | 5.0 years | | 5.0 years | | 5.8 years – 6.5 years | | Expected volatility | 89% | | 76% | | 70% – 73% |
The Company did not grant any stock options during the years ended December 31, 2024 and 2023. The Company did not grant any stock options or market-based awards to non-employees during the years ended December 31, 2024, 2023, and 2022. Risk-free interest rate The risk-free interest rate for periods within the expected term of the awards is based on the U.S. Treasury yield curve in effect on the grant date. Expected dividend yield The Company has never declared or paid any cash dividends and does not expect to pay any cash dividends in the foreseeable future. Expected term For employee stock option awards, the Company calculates the expected term using the “simplified” method, which is the simple average of the vesting period and the contractual term. The simplified method is applied as the Company does not have sufficient historical data to provide a reasonable basis for an estimate of the expected term. The Company calculates the expected term for employee stock option awards that take into account the effects of employee’s expected exercise and post-vesting employment termination behavior. For non-employee stock option awards, the expected term is determined on an award by award basis. For market-based awards, the expected term is based on the length of the award's performance period. Expected volatility The Company considers the historical volatility of its stock price and the implied stock price volatility derived from the price of exchange-traded options on the Company's stock. Because Legacy Butterfly was privately held from its inception until the closing of the Business Combination in 2021, there was no specific historical or implied volatility information available prior to 2021. Accordingly, when the Company calculates the historical stock price volatility for awards with expected terms greater than the length of time since the closing of the Business Combination, the Company supplements its own historical stock price volatility with the historical stock price volatility of a group of publicly-traded peer companies that are publicly traded over a period equivalent to the expected term of the stock-based awards. Exercise price The exercise price is taken directly from the grant notice issued to employees and non-employees. Award accelerations and modifications On December 30, 2022, the Company’s then CEO resigned from his position. Pursuant to the separation agreement between the CEO and the Company, he received equity-based compensation including the acceleration of vesting of 1.7 million of the CEO’s service-based stock options and service-based restricted stock units. This acceleration was pursuant to the original award agreements. As a modification to the original award agreements, 0.1 million performance-based restricted stock units had an acceleration of vesting, and 0.3 million service-based stock options had their post-employment exercise period extended. The Company recognized a total of $7.8 million of incremental stock-based compensation expense during the year ended December 31, 2022 related to the acceleration of these awards pursuant to the original award agreements and the modifications to the original award agreements. The incremental stock-based compensation expense resulting from the modifications was not significant. Employee stock purchase plan The Company’s 2024 Employee Stock Purchase Plan (the “ESPP”) was approved by the Board and the Company’s stockholders in the second quarter of 2024, with 4.2 million shares of common stock initially reserved and available for issuance. Under the ESPP, each eligible employee is granted an option to purchase shares of common stock, with the purchase price paid through payroll deductions, subject to the plan’s limitations on the number and value of shares purchasable. Each offering period under the ESPP has an expected duration of 24 months, divided into four six-month purchase periods, with purchases occurring in June and December. The purchase price per share is equal to the lower of 85% of the closing market price on the first day of the offering period, or 85% of the closing market price on the applicable purchase date. Proceeds received from the issuance of shares are credited to stockholders’ equity in the period that the shares are issued. The grant date fair value of the awards is recognized as stock-based compensation expense over the requisite service period. The grant date fair value was determined using similar methods and assumptions as those used for the Company’s stock option awards granted under its equity incentive plans. During the year ended December 31, 2024, the Company's employees purchased 671,435 shares of newly issued common stock through the ESPP. Stock-based compensation expense The Company’s stock-based compensation expense for the periods presented was as follows (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Research and development | $ | 6,950 | | | $ | 9,772 | | | 12,834 | | | Sales and marketing | 4,871 | | | 4,260 | | | 5,974 | | | General and administrative | 9,211 | | | 13,448 | | | 23,723 | | | Total stock-based compensation expense | $ | 21,032 | | | $ | 27,480 | | | $ | 42,531 | |
No related tax benefits of the stock-based compensation expense have been recognized and no related tax benefits have been realized from the exercise of stock options due to the Company’s net operating loss carryforwards and valuation allowance. The Company has capitalized $0.4 million, $0.7 million and $1.0 million of stock-based compensation expense as part of the cost of its capitalized internally developed software assets during the years ended December 31, 2024, 2023 and 2022, respectively. Total unrecognized stock-based compensation expense as of December 31, 2024 was $21.1 million which will be recognized over the remaining weighted average vesting period of 1.9 years.
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Net Loss Per Share
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Net Loss Per Share |
Net Loss Per Share We compute net loss per share of Class A and Class B common stock using the two-class method. Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of each class of the Company’s common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all potential shares of the Company’s common stock, including those presented in the table below, to the extent dilutive. Basic and diluted net loss per share were the same for each period presented as the inclusion of all potential shares of the Company’s common stock outstanding would have been anti-dilutive. As the Company uses the two-class method required for companies with multiple classes of common stock, the following table presents the calculation of basic and diluted net loss per share for each class of the Company’s common stock outstanding (in thousands, except share and per share amounts): | | | | | | | | | | | | | | | | | | | Year ended December 31, 2024 | | | | | | | Class A | | Class B | | Total
Common Stock | | Numerator: | | | | | | | Allocation of undistributed earnings | $ | (63,442) | | | $ | (9,050) | | | $ | (72,492) | | | Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (63,442) | | | $ | (9,050) | | | $ | (72,492) | | | Denominator: | | | | | | | Weighted-average common shares outstanding | 185,255,823 | | 26,426,937 | | 211,682,760 | | Denominator for basic and diluted net loss per share – weighted-average common stock | 185,255,823 | | 26,426,937 | | 211,682,760 | | Basic and diluted net loss per share | $ | (0.34) | | | $ | (0.34) | | | $ | (0.34) | |
| | | | | | | | | | | | | | | | | | | Year ended December 31, 2023 | | | | | | | Class A | | Class B | | Total
Common Stock | | Numerator: | | | | | | | Allocation of undistributed earnings | $ | (116,497) | | | $ | (17,203) | | | $ | (133,700) | | | Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (116,497) | | | $ | (17,203) | | | $ | (133,700) | | | Denominator: | | | | | | | Weighted-average common shares outstanding | 178,958,607 | | 26,426,937 | | 205,385,544 | | Denominator for basic and diluted net loss per share – weighted-average common stock | 178,958,607 | | 26,426,937 | | 205,385,544 | | Basic and diluted net loss per share | $ | (0.65) | | | $ | (0.65) | | | $ | (0.65) | |
| | | | | | | | | | | | | | | | | | | Year ended December 31, 2022 | | | | | | | Class A | | Class B | | Total
Common Stock | | Numerator: | | | | | | | Allocation of undistributed earnings | $ | (146,412) | | | $ | (22,311) | | | $ | (168,723) | | | Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (146,412) | | | $ | (22,311) | | | $ | (168,723) | | | Denominator: | | | | | | | Weighted-average common shares outstanding | 173,421,449 | | 26,426,937 | | 199,848,386 | | Denominator for basic and diluted net loss per share – weighted-average common stock | 173,421,449 | | 26,426,937 | | 199,848,386 | | Basic and diluted net loss per share | $ | (0.84) | | | $ | (0.84) | | | $ | (0.84) | |
For the periods presented above, the net loss per share amounts are the same for Class A and Class B common stock because the holders of each class are entitled to equal per-share dividends or distributions in liquidation in accordance with the Company’s certificate of incorporation, as amended and restated. The undistributed earnings for each year are allocated based on the contractual participation rights of the Class A and Class B common stock as if the earnings for the year had been distributed. As the liquidation and dividend rights are identical, the undistributed earnings are allocated on a proportionate basis. Anti-dilutive common equivalent shares were as follows: | | | | | | | | | | | | | | | | | | | December 31, | | 2024 | | 2023 | | 2022 | | Outstanding options to purchase common stock | 6,560,736 | | 7,439,187 | | 12,571,912 | | Outstanding restricted stock units | 21,250,230 | | 15,569,983 | | 9,961,291 | Outstanding employee stock purchase plan options | 1,948,409 | | — | | — | | Outstanding warrants | 20,652,690 | | 20,652,690 | | 20,652,690 | | Total anti-dilutive common equivalent shares | 50,412,065 | | 43,661,860 | | 43,185,893 |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Income Taxes
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Income Taxes Income (loss) before provision for income taxes consisted of the following (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Federal | $ | (72,923) | | | $ | (133,961) | | | $ | (169,122) | | | Foreign | 399 | | | 343 | | | 441 | | | Loss before provision for income taxes | $ | (72,524) | | | $ | (133,618) | | | $ | (168,681) | |
The Company recorded a tax benefit of $0.03 million, a tax provision of $0.08 million, and a tax provision of $0.04 million for the years ended December 31, 2024, 2023 and 2022, respectively, due to foreign income and return to provision adjustments. Due to the Company’s loss position domestically, the Company has not recorded a significant federal tax provision for the years ended December 31, 2024, 2023, and 2022. A reconciliation of the Company’s statutory income tax rate to the Company’s effective income tax rate is as follows: | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Income at U.S. statutory rate | 21.00 | % | | 21.00 | % | | 21.00 | % | | State taxes, net of federal benefit | 0.39 | | | 4.65 | | | 2.21 | | | Stock compensation | (6.41) | | | (2.57) | | | (5.01) | | | Change in fair value of warrants | (0.54) | | | 0.71 | | | 2.60 | | | Tax credits | 2.58 | | | 1.74 | | | 2.16 | | | Valuation allowance | (11.93) | | | (25.47) | | | (22.91) | | Return to provision | (3.45) | | | — | | | — | | | Other | (1.60) | | | (0.12) | | | (0.08) | | | 0.04 | % | | (0.06) | % | | (0.03) | % |
Net deferred tax assets as of December 31, 2024 and 2023 consisted of the following (in thousands): | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | Deferred tax assets | | | | | Net operating loss carryforwards | $ | 158,097 | | | $ | 151,230 | | | Tax credits | 18,114 | | | 16,288 | | | Stock compensation | 3,203 | | | 4,812 | | | Accruals and reserves | 4,106 | | | 2,061 | | | Inventory reserve | 15,114 | | | 15,207 | | | Lease liability | 5,562 | | | 6,114 | | | Depreciation | 99 | | | 2,197 | | | Capitalized tax R&E | 27,296 | | | 25,844 | | | Other | 4,183 | | | 1,581 | | | Total deferred tax assets | 235,774 | | | 225,334 | | | Valuation allowance | (230,109) | | | (221,454) | | | Total deferred tax assets | 5,665 | | | 3,880 | | | Deferred tax liabilities | | | | | Right-of-use asset | (3,467) | | | (3,829) | | Software costs | (1,839) | | | — | | | Net deferred tax assets | $ | 359 | | | $ | 51 | |
As of December 31, 2024 and 2023, the Company had federal net operating loss (“NOL”) carryforwards of approximately $634.8 million and $609.8 million, respectively. As of December 31, 2024 and 2023, the Company had state NOL carryforwards of approximately $446.1 million and $407.8 million, respectively. Of the $634.8 million of federal NOL carryforwards, $73.7 million will begin to expire at various dates in 2031 and $561.2 million may be carried forward indefinitely. The state NOL carryforwards will begin to expire in 2031. As of December 31, 2024, the Company also had federal and state tax credits of $15.4 million and $2.0 million, which will begin to expire in 2032 and 2024, respectively. The Tax Cuts and Jobs Act resulted in significant changes to the treatment of research and experimental (“R&E”) expenditures under Section 174 of the Internal Revenue Code of 1986, as amended, (“IRC”). For tax years beginning after December 31, 2021, companies are required to capitalize and amortize all R&E expenditures that are paid or incurred in connection with their trade or business. Specifically, costs for U.S. based R&E activities must be amortized over five years. Previously, these expenses could be deducted in the year incurred. The implementation of this provision didn’t increase our cash income tax payment in 2024 due to our significant pre-tax net loss. Future realization of the tax benefits of existing temporary differences and net operating loss carryforwards ultimately depends on the existence of sufficient taxable income within the carryforward period. As of December 31, 2024 and 2023, the Company performed an evaluation to determine whether a valuation allowance was needed. The Company considered all available evidence, both positive and negative, which included the results of operations for the current and preceding years. The Company determined that it was not possible to reasonably quantify future taxable income and determined that it is more likely than not that all of the deferred tax assets will not be realized. Accordingly, the Company maintained a full valuation allowance against its net U.S. deferred tax assets as of December 31, 2024 and 2023. The deferred tax asset recognized relates entirely to the Company's foreign entities. The Company’s valuation allowance increased by $8.7 million and $34.0 million for the years ended December 31, 2024 and 2023, respectively, due primarily to the generation of NOLs. The utilization of NOLs and tax credit carryforwards to offset future taxable income may be subject to an annual limitation as a result of ownership changes that have occurred previously or may occur in the future. Under IRC Sections 382 and 383, a corporation that undergoes an ownership change may be subject to limitations on its ability to utilize its pre-change NOLs and other tax attributes otherwise available to offset future taxable income and/or tax liability. An ownership change is defined as a cumulative change of 50% or more in the ownership positions of certain stockholders during a rolling three-year period. The Company conducted an ownership analysis under IRC Section 382 based upon publicly available information as of December 31, 2024 and determined that there has not been an ownership change since the last ownership change event on February 12, 2021 that would limit the Company’s utilization of its NOLs and tax credits. The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations for both federal taxes and the many states in which the Company operates or does business. ASC 740-10 states that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, on the basis of the technical merits. The Company records uncertain tax positions as liabilities in accordance with ASC 740-10 and adjusts these liabilities when the Company’s judgment changes as a result of the evaluation of new information not previously available. Because of the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from the Company’s current estimate of the unrecognized tax benefit liabilities. These differences will be reflected as increases or decreases to income tax expense in the period in which new information is available. As of December 31, 2024 and 2023, the Company has not recorded any uncertain tax positions in its financial statements. The Company recognizes interest and penalties related to unrecognized tax benefits within the provision for income taxes on the consolidated statements of operations and comprehensive loss. As of December 31, 2024 and 2023, there was no significant accrued interest or penalties. The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal, state and foreign jurisdictions, where applicable. There are currently no pending tax examinations. The Company’s tax years are still open under statute from December 31, 2020 to the present. Federal and state net operating losses are subject to review by taxing authorities in the year utilized. From time to time, the Company applies for government assistance in the form of non-income tax refundable credits based on meeting various eligibility criteria. To account for government assistance, where there is limited GAAP guidance for for-profit entities, the Company analogizes to International Accounting Standards 20, Accounting for Government Grants and Disclosures of Government Assistance. Under that standard, the Company recognizes government assistance when there is reasonable assurance that it will comply with the relevant conditions and that the assistance will be received. During the year ended December 31, 2022, the Company received a tax credit paid in cash of $0.9 million under the state of Massachusetts Life Sciences Tax Incentive Program and recorded the receipt as other income (expense), net on the consolidated statements of operations and comprehensive loss. The government grant is subject to claw-back if the Company fails to meet certain targets in the tax year following the time of the award. During the year ended December 31, 2023, the Company determined that it did not ultimately meet the required targets in 2022 and expects to repay the tax credit received. As a result, the Company has accrued $0.9 million for the expected repayment in other non-current liabilities on the consolidated balance sheets as of December 31, 2024 and 2023, and the Company recognized a corresponding expense in other income (expense), net on the consolidated statements of operations and comprehensive loss for the year ended December 31, 2023. During the year ended December 31, 2024, the Company received a tax credit paid in cash of $0.5 million for the U.S. government's Employee Retention Credit and recorded the receipt as other income (expense), net on the consolidated statements of operations and comprehensive loss.
|
| X |
- DefinitionThe entire disclosure for income tax. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 231
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482663/740-10-55-231
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12C
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477891/740-270-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/740/tableOfContent
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-14
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-21
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
401(k) Retirement Plan
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Retirement Benefits [Abstract] |
|
| 401(k) Retirement Plan |
401(k) Retirement Plan The Company sponsors a 401(k) defined contribution plan covering all eligible U.S. employees. Contributions to the 401(k) plan are discretionary. The expense related to the matching contributions was $0.5 million, $0.8 million, and $1.3 million for the years ended December 31, 2024, 2023, and 2022, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_CompensationAndRetirementDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for defined contribution plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 70
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480794/715-70-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 70
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/715-70/tableOfContent
| Name: |
us-gaap_DefinedContributionPlanTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Warrants
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Warrants and Rights Note Disclosure [Abstract] |
|
| Warrants |
Warrants Public Warrants The Company issued Public Warrants and Private Warrants in connection with its IPO in 2020. As of December 31, 2024, there were an aggregate of 13,799,357 outstanding Public Warrants, which entitle the holder to acquire Class A common stock. Each whole warrant entitles the registered holder to purchase one share of Class A common stock at an exercise price of $11.50 per share, subject to adjustment as discussed below, beginning on May 26, 2021. The warrants will expire on February 12, 2026 or earlier upon redemption or liquidation. During the years ended December 31, 2024, 2023, and 2022, the amount of exercises of Public Warrants was not significant, and the amount reclassified into equity upon the exercise of the Public Warrants was not significant. Redemptions At any time while the warrants are exercisable, the Company may redeem not less than all of the outstanding Public Warrants: •at a price of $0.01 per warrant; •upon a minimum of 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder; •provided that the last reported sale price of the Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like and for certain issuances of Class A common stock and equity-linked securities) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date the Company sends the notice of redemption to the warrant holders; and •provided that there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants, and a current prospectus relating thereto, available through the 30-day redemption period or the Company has elected to require the exercise of the warrants on a “cashless basis” (as described below). If the foregoing conditions are satisfied and the Company issues a notice of redemption of the Public Warrants at $0.01 per warrant, each holder of Public Warrants will be entitled to exercise their Public Warrants prior to the scheduled redemption date. If the Company calls the Public Warrants for redemption for $0.01 as described above, the Board may elect to require any holder that wishes to exercise a Public Warrant to do so on a “cashless basis.” If the Board makes such election, all holders of Public Warrants would pay the exercise price by surrendering their warrants for that number of shares of Class A common stock equal to the quotient obtained by dividing (x) the product of the number of shares of Class A common stock underlying the warrants, multiplied by the excess of the “fair market value” over the exercise price of the warrants by (y) the “fair market value.” For purposes of the redemption provisions of the warrants, the “fair market value” means the average last reported sale price of the Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. The Company may also redeem not less than all of the outstanding Public Warrants and Private Warrants: •at $0.10 per warrant; •upon a minimum of 30 days' prior written notice of redemption; •provided that the last reported sale price of the Class A common stock equals or exceeds $10.00 per share (as adjusted per stock splits, stock dividends, reorganizations, reclassifications, recapitalizations and the like) on the trading day prior to the date on which the Company sends the notice of redemption to the warrant holders; •provided that the Private Warrants are also concurrently exchanged at the same price (equal to a number of shares of Class A common stock) as the outstanding Public Warrants; and •provided that there is an effective registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and a current prospectus relating thereto available throughout the 30-day redemption period. If the foregoing conditions are satisfied and the Company issues a notice of redemption of the warrants at $0.10 per warrant, each warrant holder will be entitled to exercise their warrant prior to the scheduled redemption date on a cashless basis and receive that number of shares based on the redemption date and the “fair market value” of the Class A common stock, in accordance with a table set forth in the warrant agreement. The Company evaluated the Public Warrants under ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, in conjunction with the SEC Division of Corporation Finance’s April 12, 2021 Public Statement, Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”), and concluded that they do not meet the criteria to be classified in stockholders’ equity. Specifically, the exercise of the warrants may be settled in cash upon the occurrence of a tender offer or exchange offer in which the maker of the tender offer or exchange offer, upon completion of the tender offer or exchange offer, beneficially owns more than 50% of the outstanding shares of the Company’s Class A common stock, even if it would not result in a change of control of the Company. This provision would preclude the warrants from being classified in equity and thus the warrants are classified as a liability. Private Warrants As of December 31, 2024, there were 6,853,333 Private Warrants outstanding. There have been no exercises of the Private Warrants. The Private Warrants are identical to the Public Warrants, except that so long as they are held by Longview Investors LLC (the “Sponsor”) or any of its permitted transferees, (i) the Private Warrants and the shares of Class A common stock issuable upon the exercise of the Private Warrants are not transferable, assignable or saleable until 30 days after the completion of the Business Combination, (ii) the Private Warrants will be exercisable for cash or on a cashless basis, at the holder’s option, and (iii) the Private Warrants are not subject to the Company’s redemption option at the price of $0.01 per warrant. The Private Warrants are subject to the Company’s redemption option at the price of $0.10 per warrant, provided that the other conditions of such redemption are met, as described above. If the Private Warrants are held by a holder other than the Sponsor or any of its permitted transferees, the Private Warrants will be redeemable by the Company in all redemption scenarios applicable to the Public Warrants and exercisable by such holders on the same basis as the Public Warrants. The Company evaluated the Private Warrants under ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, in conjunction with the SEC Division of Corporation Finance’s April 12, 2021 Public Statement, Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”), and concluded that they do not meet the criteria to be classified in stockholders’ equity. Specifically, the terms of the warrants provide for potential changes to the settlement amounts dependent upon the characteristics of the warrant holder, and, because the holder of a warrant is not an input into the pricing of a fixed-for-fixed option on equity shares, such provision would preclude the warrant from being classified in equity and thus the warrants are classified as a liability. The Company recognized a loss of $1.9 million, a gain of $4.5 million, and a gain of $20.9 million for the change in fair value of warrant liabilities in the consolidated statements of operations and comprehensive loss for the years ended December 31, 2024, 2023, and 2022, respectively.
|
| X |
- DefinitionEntire disclosure of warrants and rights.
| Name: |
bfly_WarrantsOrRightsTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WarrantsAndRightsNoteDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Leases
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Leases [Abstract] |
|
| Leases |
Leases The Company primarily enters into leases for office space that are classified as operating leases. Most leases are not cancelable prior to their expiration. The Company terminated one of its operating leases for office space and modified another during the third quarter of 2023 that increased its lease payments by $0.2 million. The Company recognized a total decrease of $4.2 million to operating lease assets, $0.7 million to the current portion of operating lease liabilities included in accrued expenses and other current liabilities, and $4.7 million to the non-current portion of operating lease liabilities on the consolidated balance sheets for the lease termination and lease modification. As part of the lease termination, the Company agreed to forfeit a $0.9 million security deposit included in other non-current assets on the consolidated balance sheets. The Company recognized a $0.2 million gain on lease termination within operating expenses on the consolidated statements of operations and comprehensive loss for the year ended December 31, 2023. The following table presents the components of operating lease cost for the years ended December 31, 2024, 2023, and 2022 (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Operating lease cost | $ | 2,807 | | | $ | 3,206 | | | $ | 4,300 | | | Short-term lease cost | 101 | | | 73 | | | 249 | | | Variable lease cost | 321 | | | 317 | | | 353 | | | Total operating lease cost | $ | 3,229 | | | $ | 3,596 | | | $ | 4,902 | |
The expected maturities related to the Company’s leases with initial non-cancellable lease terms in excess of one year as of December 31, 2024 are as follows: | | | | | | | Year ended December 31, | Operating Lease Payments | | 2025 | $ | 3,664 | | | 2026 | 3,749 | | | 2027 | 3,835 | | | 2028 | 3,921 | | | 2029 | 3,454 | | | 2030 and thereafter | 9,354 | | | Total gross operating lease payments | 27,977 | | | Less: imputed interest | (5,142) | | | Total operating lease liabilities, reflecting the present value of net lease payments | $ | 22,835 | |
Additional information related to operating leases is presented as follows: | | | | | | | | | | | | | | | | | | | December 31, | | 2024 | | 2023 | | 2022 | | Weighted average remaining lease term (in years) | 7.5 | | 8.4 | | 8.8 | | Weighted average discount rate | 5.9 | % | | 5.9 | % | | 5.5 | % |
| | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | Operating cash flows from operating leases | $ | 3,557 | | | $ | 3,121 | | | $ | 2,042 | | | Non-cash activities involving right-of-use assets and lease liabilities: | | | | | | | | | | | | | Derecognition of right-of-use assets | — | | | 4,163 | | | — | | | Derecognition of operating lease liabilities | — | | | 5,401 | | | — | |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Commitments and Contingencies
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Commitments and Contingencies Commitments Purchase commitments: The Company enters into inventory purchase commitments with third-party manufacturers in the ordinary course of business, including a non-cancellable inventory supply agreement with a certain third-party manufacturing vendor. The provisions of the agreement allowed the Company, once it reached a certain cumulative purchase threshold in the fourth quarter of 2021, to pay for a portion of the subsequent inventory purchases using an advance previously paid to the vendor. As of December 31, 2024, the aggregate amount of minimum inventory purchase commitments is $6.9 million and the Company has a vendor advance asset of $3.1 million, net of write-downs, and an insignificant accrued purchase commitment liability. The portion of the balances that is expected to be utilized in the next twelve months is included in current assets and current liabilities in the accompanying consolidated balance sheets. The Company applied the guidance in ASC Topic 330, Inventory, to assess the purchase commitment and related loss, using such factors as Company-specific forecasts which are reliant on the Company’s limited sales history, agreement-specific provisions, macroeconomic factors, and market and industry trends. For the years ended December 31, 2024, 2023, and 2022, the Company did not recognize any additions to the accrued purchase commitment liability, or any related losses, based on its purchase commitment assessment as there were no significant changes to the assessment factors. The Company reviews its inventory on hand, including inventory acquired under the purchase commitments, for excess and obsolescence (“E&O”) on a quarterly basis. Any E&O inventory acquired that was previously accounted for as a purchase commitment liability accrual or vendor advance write-down is recorded at zero value. During the year ended December 31, 2024, the Company did not acquire such E&O inventory. During the year ended December 31, 2023, the Company utilized $2.0 million of the accrued purchase commitment liability to acquire such E&O inventory. Contingencies The Company is involved in litigation and legal matters from time to time, which have arisen in the normal course of business. The Company accrues an estimated liability for legal contingencies when the Company considers a potential loss probable and can reasonably estimate the amount of the potential loss. Although the ultimate results of these matters are not currently determinable, management does not expect that they will have a material effect on the Company’s consolidated balance sheets, statements of operations and comprehensive loss, or statements of cash flows. On February 16, 2022, a putative class action lawsuit, styled Rose v. Butterfly Network, Inc., et al. was filed in the United States District Court for the District of New Jersey. The claims are against the Company and certain of its directors and previous management as well as members of the board of directors of the Company prior to the completion of the Business Combination, alleging that the defendants made false and misleading statements and/or omissions about its post-Business Combination business and financial prospects. The alleged class consists of all persons or entities who purchased or otherwise acquired the Company’s stock between January 12, 2021 and November 15, 2021, persons who exchanged Longview shares for the Company’s common stock, and persons who purchased Longview stock pursuant, or traceable to, the Proxy/Registration Statement filed with the SEC on November 27, 2020 or any amendment thereto. The Company intends to vigorously defend against this action. The lawsuit seeks unspecified damages, together with interest thereon, as well as the costs and expenses of litigation. There is no assurance that the Company will be successful in the defense of the litigation or that insurance will be available or adequate to fund any potential settlement or judgment or the litigation costs of the action. The Company is unable to predict the outcome or reasonably estimate a range of possible loss at this time. On June 21, 2022, a stockholder derivative action, styled Koenig v. Todd M. Fruchterman, et al. was filed in the United States District Court for the District of Delaware against the Company’s board of directors and the Company as nominal defendant. On November 28, 2023, a stockholder derivative action, styled Bhavsar v. Todd M. Fruchterman, et al. was filed in the United States District Court for the District of Delaware against the board of directors and the Company as nominal defendant. Both these actions allege violation of Section 14(a) of the Exchange Act, as amended, and Rule 14a-9 promulgated thereunder, and claims for breach of fiduciary duty, contribution and indemnification, aiding and abetting, and gross mismanagement. The lawsuits are premised upon allegedly inadequate internal controls and purportedly misleading representations regarding the Company’s financial condition, business prospects, and the Company’s November 2021 earnings announcement. The Company intends to vigorously defend against these actions. The lawsuit seeks unspecified damages, disgorgement, and restitution, together with interest thereon, as well as the costs and expenses of litigation. There is no assurance that the Company will be successful in the defense of the litigation or that insurance will be available or adequate to fund any potential settlement or judgment or the litigation costs of the action. The Company is unable to predict the outcome or reasonably estimate a range of possible loss at this time.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405-30/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/450/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478522/954-440-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Subsequent Events
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
Subsequent Events On January 29, 2025, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with TD Securities (USA) LLC and William Blair & Company, L.L.C., as representatives of the several underwriters named therein (collectively, the “Underwriters”), to issue and sell in a public offering up to 27,600,000 shares of Butterfly’s Class A common stock, par value $0.0001 per share, which includes 3,600,000 shares of the Company’s Class A common stock that were subject to an option to purchase additional shares granted to and exercised by the Underwriters, at a public offering price of $3.15 per share (the “Offering”). The Offering closed on January 31, 2025, and the Company issued 27,600,000 shares of Class A common stock, which included the full exercise of the Underwriters' option. The gross proceeds to the Company from the Offering were $86.9 million, before deducting $5.2 million of underwriting discounts and commissions and $0.7 million of estimated Offering expenses payable by the Company. The net proceeds to the Company, after expenses, are expected to be $81.0 million. The net proceeds will be recognized as increases in cash and cash equivalents and stockholders' equity on the consolidated balance sheets.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection b
-Paragraph 1
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16
-Subsection J
-Paragraph a
| Name: |
ecd_InsiderTradingPoliciesProcLineItems |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection b
-Paragraph 1
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16
-Subsection J
-Paragraph a
| Name: |
ecd_InsiderTrdPoliciesProcAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Cybersecurity Risk Management and Strategy Disclosure
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Cybersecurity Risk Management, Strategy, and Governance [Line Items] |
|
| Cybersecurity Risk Management Processes for Assessing, Identifying, and Managing Threats [Text Block] |
Butterfly Network, Inc. uses, stores, and processes data for and about our customers, employees, partners, and suppliers. We have implemented a cybersecurity risk management program that is designed to identify, assess, and mitigate risks from cybersecurity threats to this data and our systems. Risk Management Strategy and Governance Under the ultimate direction of our Chief Executive Officer, our Information Security Committee has primary responsibility for overseeing our management of cybersecurity risks. It is chaired by our Chief Information Security Officer ("CISO") who reports directly to our Chief Technology Officer. Other members of the committee include representation from information technology, quality, product, operations, sales, and compliance as well as advisory support from internal audit. Our CISO, working with his team and the Information Security Committee, has primary responsibility for assessing and managing our cybersecurity threat management program. The current CISO has more than 20 years of experience in building and leading security, risk management, and compliance organizations across several industries, including med-tech, healthcare, and financial services, and many of these companies include highly-regulated Fortune 500 companies. The current CISO is an expert in NIST 800-53 (Rev-5), ISO27001, and other national and international security risk management disciplines. The Information Security Committee meets periodically and as circumstances warrant to discuss and monitor prevention, detection, and remediation of risks from cybersecurity threats. When appropriate, cyber or information security incidents would be escalated by the CISO to our executive leadership team and/or our disclosure committee. On a regular basis, the CISO also updates the executive management team on developments within the cybersecurity sphere. The Board of Directors has delegated oversight of the Company’s cybersecurity program to the Audit Committee of the Board of Directors. As provided in the Audit Committee Charter, the Audit Committee is responsible for reviewing reports on data management, security initiatives, significant existing and emerging cybersecurity risks, including cybersecurity incidents, the impact on the Company and its stakeholders of any significant cybersecurity incident, and any disclosure obligations arising from any such incidents. Our CISO meets at least quarterly with the Audit Committee of the Board of Directors to discuss management’s ongoing cybersecurity risk management programs. He provides information about the sources and nature of risks the Company faces, how management assesses such risks – including in terms of likelihood and severity of impact, progress on vulnerability remediation, and current developments in the cybersecurity landscape. In turn, the Chair of the Audit Committee provides a readout to the full Board of Directors that includes a summary of the CISO’s presentation to enable discussion of cybersecurity risk management at the full board level. Although risks from cybersecurity threats have to date not materially affected, and we do not believe they are reasonably likely to materially affect, us, our business strategy, results of operations, or financial condition, we could, from time to time, experience threats and security incidents relating to our and our third party vendors’ information systems. For more information, please see Part I, Item 1A “Risk Factors.” Processes for the Identification of Cybersecurity Threats Our Information Security team is responsible for monitoring our information systems for vulnerabilities and mitigating any issues. It works with other groups in the Company to understand the severity and the likelihood of the potential consequences of a cybersecurity incident and to make decisions about how to prioritize mitigation and other initiatives based on, among other things, materiality to the business. The Information Security team has processes designed to keep the Company apprised of the different threats in the cybersecurity landscape – this includes intelligence networks alerts, working with researchers, discussions with peers at other companies, monitoring social media, reviewing government alerts and other news items, and attending security conferences. The team also regularly monitors our internal network and our customer-facing network to identify security risks. In addition, the team has completed several assessments and threat modeling tabletop exercises, based on “what-if” scenarios. We have a mandatory employee education program that is designed to raise awareness of cybersecurity threats to reduce our vulnerability as well as to encourage consideration of cybersecurity risks across functions. Security training is required upon hire for new employees, and on an annual basis for the rest of the workforce. As part of the assessment of the protections we have in place to mitigate risks from cybersecurity threats, we engage third parties to conduct vendor risk assessments. To assess the effectiveness of our program, we also have engaged consultants to conduct penetration testing and other vulnerability assessment. Before purchasing third-party technology or other solutions that involve exposure to the Company’s assets and electronic information, our Information Technology group requires those companies to complete a security review before being approved to work with the Company. We utilize an external tool to manage critical vendors. Vendors are assessed to determine inherent and residual risk. Annually, the security team conducts a risk assessment that is informed by industry standards. The Risk Assessment consists of: •Listing the vulnerabilities company assets are exposed to; •Identifying the threats that may exploit such vulnerabilities; •Calculating inherent risk rating based on impact and exploitability; and •Calculating residual risk by assessing mitigating controls
|
| Cybersecurity Risk Management Processes Integrated [Flag] |
true
|
| Cybersecurity Risk Management Processes Integrated [Text Block] |
We have implemented a cybersecurity risk management program that is designed to identify, assess, and mitigate risks from cybersecurity threats to this data and our systems.
|
| Cybersecurity Risk Management Third Party Engaged [Flag] |
true
|
| Cybersecurity Risk Third Party Oversight and Identification Processes [Flag] |
true
|
| Cybersecurity Risk Materially Affected or Reasonably Likely to Materially Affect Registrant [Flag] |
false
|
| Cybersecurity Risk Board of Directors Oversight [Text Block] |
Under the ultimate direction of our Chief Executive Officer, our Information Security Committee has primary responsibility for overseeing our management of cybersecurity risks. It is chaired by our Chief Information Security Officer ("CISO") who reports directly to our Chief Technology Officer. Other members of the committee include representation from information technology, quality, product, operations, sales, and compliance as well as advisory support from internal audit. Our CISO, working with his team and the Information Security Committee, has primary responsibility for assessing and managing our cybersecurity threat management program. The current CISO has more than 20 years of experience in building and leading security, risk management, and compliance organizations across several industries, including med-tech, healthcare, and financial services, and many of these companies include highly-regulated Fortune 500 companies. The current CISO is an expert in NIST 800-53 (Rev-5), ISO27001, and other national and international security risk management disciplines. The Information Security Committee meets periodically and as circumstances warrant to discuss and monitor prevention, detection, and remediation of risks from cybersecurity threats. When appropriate, cyber or information security incidents would be escalated by the CISO to our executive leadership team and/or our disclosure committee. On a regular basis, the CISO also updates the executive management team on developments within the cybersecurity sphere. The Board of Directors has delegated oversight of the Company’s cybersecurity program to the Audit Committee of the Board of Directors. As provided in the Audit Committee Charter, the Audit Committee is responsible for reviewing reports on data management, security initiatives, significant existing and emerging cybersecurity risks, including cybersecurity incidents, the impact on the Company and its stakeholders of any significant cybersecurity incident, and any disclosure obligations arising from any such incidents. Our CISO meets at least quarterly with the Audit Committee of the Board of Directors to discuss management’s ongoing cybersecurity risk management programs. He provides information about the sources and nature of risks the Company faces, how management assesses such risks – including in terms of likelihood and severity of impact, progress on vulnerability remediation, and current developments in the cybersecurity landscape. In turn, the Chair of the Audit Committee provides a readout to the full Board of Directors that includes a summary of the CISO’s presentation to enable discussion of cybersecurity risk management at the full board level.
|
| Cybersecurity Risk Board Committee or Subcommittee Responsible for Oversight [Text Block] |
Under the ultimate direction of our Chief Executive Officer, our Information Security Committee has primary responsibility for overseeing our management of cybersecurity risks. It is chaired by our Chief Information Security Officer ("CISO") who reports directly to our Chief Technology Officer. Other members of the committee include representation from information technology, quality, product, operations, sales, and compliance as well as advisory support from internal audit.
|
| Cybersecurity Risk Process for Informing Board Committee or Subcommittee Responsible for Oversight [Text Block] |
It is chaired by our Chief Information Security Officer ("CISO") who reports directly to our Chief Technology Officer. Other members of the committee include representation from information technology, quality, product, operations, sales, and compliance as well as advisory support from internal audit.
|
| Cybersecurity Risk Role of Management [Text Block] |
Our CISO, working with his team and the Information Security Committee, has primary responsibility for assessing and managing our cybersecurity threat management program. The current CISO has more than 20 years of experience in building and leading security, risk management, and compliance organizations across several industries, including med-tech, healthcare, and financial services, and many of these companies include highly-regulated Fortune 500 companies. The current CISO is an expert in NIST 800-53 (Rev-5), ISO27001, and other national and international security risk management disciplines. The Information Security Committee meets periodically and as circumstances warrant to discuss and monitor prevention, detection, and remediation of risks from cybersecurity threats. When appropriate, cyber or information security incidents would be escalated by the CISO to our executive leadership team and/or our disclosure committee. On a regular basis, the CISO also updates the executive management team on developments within the cybersecurity sphere. The Board of Directors has delegated oversight of the Company’s cybersecurity program to the Audit Committee of the Board of Directors. As provided in the Audit Committee Charter, the Audit Committee is responsible for reviewing reports on data management, security initiatives, significant existing and emerging cybersecurity risks, including cybersecurity incidents, the impact on the Company and its stakeholders of any significant cybersecurity incident, and any disclosure obligations arising from any such incidents.
|
| Cybersecurity Risk Management Positions or Committees Responsible [Flag] |
true
|
| Cybersecurity Risk Management Positions or Committees Responsible [Text Block] |
Under the ultimate direction of our Chief Executive Officer, our Information Security Committee has primary responsibility for overseeing our management of cybersecurity risks.
|
| Cybersecurity Risk Management Expertise of Management Responsible [Text Block] |
The current CISO has more than 20 years of experience in building and leading security, risk management, and compliance organizations across several industries, including med-tech, healthcare, and financial services, and many of these companies include highly-regulated Fortune 500 companies. The current CISO is an expert in NIST 800-53 (Rev-5), ISO27001, and other national and international security risk management disciplines.
|
| Cybersecurity Risk Process for Informing Management or Committees Responsible [Text Block] |
Our CISO meets at least quarterly with the Audit Committee of the Board of Directors to discuss management’s ongoing cybersecurity risk management programs.
|
| Cybersecurity Risk Management Positions or Committees Responsible Report to Board [Flag] |
true
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 1
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 1
| Name: |
cyd_CybersecurityRiskBoardCommitteeOrSubcommitteeResponsibleForOversightTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 1
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 1
| Name: |
cyd_CybersecurityRiskBoardOfDirectorsOversightTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 2
-Subparagraph i
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 2
-Subparagraph i
| Name: |
cyd_CybersecurityRiskManagementExpertiseOfManagementResponsibleTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 2
-Subparagraph i
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 2
-Subparagraph i
| Name: |
cyd_CybersecurityRiskManagementPositionsOrCommitteesResponsibleFlag |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 2
-Subparagraph iii
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 2
-Subparagraph iii
| Name: |
cyd_CybersecurityRiskManagementPositionsOrCommitteesResponsibleReportToBoardFlag |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 2
-Subparagraph i
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 2
-Subparagraph i
| Name: |
cyd_CybersecurityRiskManagementPositionsOrCommitteesResponsibleTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection b
-Paragraph 1
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection b
-Paragraph 1
| Name: |
cyd_CybersecurityRiskManagementProcessesForAssessingIdentifyingAndManagingThreatsTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection b
-Paragraph 1
-Subparagraph i
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection b
-Paragraph 1
-Subparagraph i
| Name: |
cyd_CybersecurityRiskManagementProcessesIntegratedFlag |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection b
-Paragraph 1
-Subparagraph i
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection b
-Paragraph 1
-Subparagraph i
| Name: |
cyd_CybersecurityRiskManagementProcessesIntegratedTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
| Name: |
cyd_CybersecurityRiskManagementStrategyAndGovernanceLineItems |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection b
-Paragraph 1
-Subparagraph ii
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection b
-Paragraph 1
-Subparagraph ii
| Name: |
cyd_CybersecurityRiskManagementThirdPartyEngagedFlag |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection b
-Paragraph 2
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection b
-Paragraph 2
| Name: |
cyd_CybersecurityRiskMateriallyAffectedOrReasonablyLikelyToMateriallyAffectRegistrantFlag |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 1
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 1
| Name: |
cyd_CybersecurityRiskProcessForInformingBoardCommitteeOrSubcommitteeResponsibleForOversightTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 2
-Subparagraph ii
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 2
-Subparagraph ii
| Name: |
cyd_CybersecurityRiskProcessForInformingManagementOrCommitteesResponsibleTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection c
-Paragraph 2
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection c
-Paragraph 2
| Name: |
cyd_CybersecurityRiskRoleOfManagementTextBlock |
| Namespace Prefix: |
cyd_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Section 106
-Subsection b
-Paragraph 1
-Subparagraph iii
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Section 16K
-Subsection b
-Paragraph 1
-Subparagraph iii
| Name: |
cyd_CybersecurityRiskThirdPartyOversightAndIdentificationProcessesFlag |
| Namespace Prefix: |
cyd_ |
| Data Type: |
i:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Summary of Significant Accounting Policies (Policies)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation and Principles of Consolidation |
Basis of Presentation and Principles of Consolidation The accompanying consolidated financial statements include the accounts of BFLY Operations, Inc. (formerly Butterfly Network, Inc.) and its wholly-owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the accounting disclosure rules and regulations of the Securities and Exchange Commission (the “SEC”). All intercompany balances and transactions have been eliminated in consolidation.
|
| Functional Currency |
Functional Currency The Company’s worldwide operations utilize the U.S. dollar (“USD”) as the functional currency considering the significant dependency of each subsidiary on the Company. Subsidiary operations are financed through the funding received from the Company in USD. For foreign entities where the USD is the functional currency, all foreign currency-denominated monetary assets and liabilities are remeasured at end-of-period exchange rates. Exchange gains and losses arising from the remeasurement of foreign currency-denominated monetary assets and liabilities are included in other income (expense), net in the consolidated statements of operations and comprehensive loss.
|
| Concentration of Credit Risk |
Concentration of Credit Risk Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash and cash equivalents and accounts receivable. As of December 31, 2024 and 2023, substantially all of the Company’s cash and cash equivalents were invested in money market accounts with one financial institution. The Company also maintains balances in various operating accounts above federally insured limits. The Company has not experienced any significant losses on such accounts and does not believe it is exposed to any significant credit risk on cash and cash equivalents.
|
| Segment Information |
Segment Information The Company has determined that it operates in one reportable segment, which includes all activities related to the development, manufacture, and sale of the Company's products, software, and other services. The Company’s chief operating decision maker ("CODM"), its chief executive officer ("CEO"), regularly reviews the Company's consolidated net loss, which is reported as net loss and comprehensive loss on the consolidated statements of operations and comprehensive loss, for purposes of evaluating the Company's financial performance, including reviewing budget versus actual results, and determining changes in the Company's allocation of resources across the Company's strategic initiatives. The Company's measure of segment assets is total assets, as reported on the consolidated balance sheets, and substantially all of the Company’s long-lived assets are located in the United States. Other segment items include interest income, interest expense, the change in fair value of warrant liabilities, other income (expense), net, and the provision (benefit) for income taxes. Because the Company operates in one reportable segment, other required segment disclosures are included on the Company's consolidated financial statements. Interest income, interest expense, and the provision (benefit) for income taxes are included on the consolidated statements of operations and comprehensive loss. Depreciation, amortization, and impairments; write-down of inventories; and purchases of property, equipment, and intangible assets, including capitalized software, are included on the consolidated statements of cash flows.
|
| Use of Estimates |
Use of Estimates The preparation of the consolidated financial statements in conformity with U.S. GAAP requires the Company to make estimates and assumptions about future events that affect the amounts reported in its consolidated financial statements and accompanying notes. Future events and their effects cannot be determined with certainty. On an ongoing basis, management evaluates these estimates and assumptions. Significant estimates and assumptions include: •revenue recognition, including determination of the timing and pattern of satisfaction of performance obligations and determination of the standalone selling price (“SSP”) of performance obligations; •measurement and allocation of capitalized costs, the net realizable value (the selling price as well as estimated costs of completion, disposal and transportation) of inventory, and demand and future use of inventory; •assumptions underlying the capitalization of costs to develop or obtain software for internal use; •assumptions underlying incremental borrowing rate calculations; •assumptions underlying the measurement of contingent losses; and •assumptions underlying the fair value used in the stock-based compensation expense calculation. The Company bases these estimates on historical and anticipated results and trends and on various other assumptions that the Company believes are reasonable under the circumstances, including assumptions about future events. Changes in estimates are recorded in the period in which they become known. Actual results could differ from these estimates, and any such differences may be material to the Company’s consolidated financial statements.
|
| Revenue Recognition |
Revenue Recognition The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“Topic 606”). Revenue is recognized when or as a customer obtains control of the promised goods and services. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to in exchange for these goods and services. To achieve this core principle, the Company applies the following five steps: •Step 1: Identify Contracts with Customers: The Company typically enters into contracts with customers through direct sales executed via signed contracts with payment terms of 30 days or less. Multi-year software subscriptions typically require advance payment for each annual subscription period. •Step 2: Identify Performance Obligations: The Company’s contracts with customers often include multiple performance obligations. The Company has identified the following performance obligations in its contracts with customers: •Hardware devices and accessories; •Software subscriptions, including renewal subscriptions, which represent an obligation to provide the customer with ongoing access to the Company’s cloud-hosted software applications on a continuous basis throughout the subscription period; •Implementation and integration services; •Extended warranties; and •SDKs, either perpetual or term-based. •Step 3: Determine Transaction Price: The Company’s contracts with customers include variable consideration in the form of refunds and credits for product returns and price concessions. The Company estimates variable consideration based on the individual contract circumstances or by using the expected value method based on a portfolio of data from similar contracts, depending on the circumstances. •Step 4: Allocate Transaction Price to Performance Obligations: The Company allocates transaction price to the performance obligations in contracts with customers based on the relative SSPs of the underlying goods and services which the Company has a history of selling to customers on a standalone basis. •Step 5: Recognize Revenue as Performance Obligations are Satisfied: Each sale of a hardware device, accessory, or perpetual SDK is a performance obligation satisfied at a point in time when control of the good transfers from the Company to the customer based on shipping terms or when the SDK is provided to the customer. The Company’s software subscriptions and extended warranties are stand-ready performance obligations that are satisfied over time by providing the customer with ongoing access to the Company’s resources. Term-based SDK sales are performance obligations that are satisfied over time through continued provision of access to the customer. The Company uses the time-elapsed (i.e., straight-line) measure of progress to recognize revenue as these performance obligations are satisfied evenly over the respective service periods. The implementation and integration services are a performance obligation satisfied over time, and the Company uses the costs incurred as inputs into the measure of progress to recognize revenue as it satisfies the performance obligation. Deferred Revenue Deferred revenue primarily consists of billings or payments received in advance of revenue recognition from software subscriptions and other services and is reduced as the revenue recognition criteria are met. Deferred revenue is classified as current or non-current on the consolidated balance sheets based on the expected timing of revenue recognition. The deferred revenue that will be recognized as revenue within the next twelve months is classified as current, and the deferred revenue that will be recognized thereafter is classified as non-current. Warranties The Company offers a standard product warranty that its products will function according to standard specifications and free of significant defects for a period ranging from one year to three years, depending on the product, from when control is transferred to the customer. The Company evaluated the warranty liability under ASC Topic 606 and determined that it is an assurance-type warranty. When product revenue is recognized, an estimate of future warranty costs is recognized as cost of product revenue on the consolidated statements of operations and comprehensive loss and accrued expenses on the consolidated balance sheets. Factors that affect the estimate of future warranty costs include historical and current product failure rates, service delivery costs incurred in correcting product failures, and warranty policies and business practices.
|
| Cash and Cash Equivalents |
Cash and Cash Equivalents All highly liquid investments purchased with a maturity of three months or less are considered to be cash equivalents. As of December 31, 2024 and 2023, cash and cash equivalents consist principally of cash and money market accounts.
|
| Trade Accounts Receivable and Allowance for Doubtful Accounts |
Trade Accounts Receivable and Allowance for Doubtful Accounts Accounts receivable are recognized as the original amount invoiced less an allowance for doubtful accounts based on the probability of future collection. In accordance with ASC Topic 326, Financial Instruments-Credit Losses, the Company estimates its allowance for doubtful accounts based on historical loss patterns, the number of days that billings are past due, current market conditions, and reasonable and supportable forecasts of future economic conditions. Accounts receivable are written off when deemed uncollectible and collection of the receivable is no longer being actively pursued.
|
| Inventories |
Inventories Inventories primarily consist of raw materials, work-in-progress, and finished goods which are purchased and held by the Company’s third-party contract manufacturers. Inventories are stated at the lower of actual cost, determined using the average cost method, or net realizable value. Actual cost includes all direct and indirect production costs to convert materials into a finished product. Net realizable value is based upon an estimated average selling price reduced by the estimated costs of completion, disposal, and transportation. The determination of net realizable value involves certain judgments including estimating average selling prices. The Company reduces the value of inventory for estimated obsolescence or lack of marketability by the difference between the cost of the affected inventory and the net realizable value. The valuation of inventories also requires the Company to estimate excess and obsolete inventory. The Company considers new product development schedules, the effect that new products might have on the sale of existing products, product obsolescence, product merchantability, and whether older products can be remanufactured into new products, among other factors. Losses expected to arise from firm, non-cancelable and unhedged commitments for the future purchase of inventories are recognized unless the losses are recoverable through firm sales contracts or other means.
|
| Restricted Cash |
Restricted Cash Restricted cash includes deposits in financial institutions restricted according to an agreement and used to secure a lease agreement. The Company classifies the amount restricted according to an agreement as prepaid expenses and other current assets on the consolidated balance sheets as the Company expects the deposit to be released from restriction within the next twelve months. The Company classifies the amount used to secure a lease agreement within other non-current assets on the consolidated balance sheets as the lease is long-term. The amount shown as restricted cash is included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown in the consolidated statements of cash flows.
|
| Vendor Advances |
Vendor Advances Vendor advances represent amounts paid to third-party vendors for future services to be received related to production of the Company’s inventories. The amounts are presented net of write offs. The classification of vendor advances as current or non-current is based on the estimated timing of inventory delivery.
|
| Property and Equipment |
Property and Equipment Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation expense is computed using the straight-line method over the estimated useful lives of the related assets. Leasehold improvements are amortized on a straight-line basis over the shorter of the remaining lease term or the estimated useful lives of related improvements. Expenditures for major renewals and improvements are capitalized. Expenditures for repairs and maintenance are expensed as incurred. When assets are retired or otherwise disposed of, the cost of those assets and the related accumulated depreciation and amortization is eliminated from the balance sheet, and any resulting gains or losses are included in the consolidated statements of operations and comprehensive loss in the period of disposal.
|
| Capitalized Software Development Costs |
Capitalized Software Development Costs Costs to develop or obtain software for internal use are capitalized and recorded as capitalized software development costs on the consolidated balance sheets as a component of property and equipment. The Company capitalizes qualifying costs associated with internal-use software incurred during the application development stage if management with relevant authority authorizes the project, it is probable the project will be completed, and the software will be used to perform the function intended. Costs incurred during the preliminary project and post-implementation stages, including training and maintenance, are expensed as incurred. Capitalized costs are amortized on a project-by-project basis using the straight-line method over the estimated economic life of the software, which is three years, beginning when the software is substantially ready for use. Amortization expense is classified in the consolidated statements of operations and comprehensive loss based on the nature of the software.
|
| Leases |
Leases The Company primarily enters into leases for office space that are classified as operating leases. The Company determines if an agreement is or contains a lease at inception. The Company accounts for leases in accordance with ASC Topic 842, Leases, by recognizing right-of-use assets and lease liabilities. The Company classifies right-of-use assets as operating lease assets on the consolidated balance sheets. The Company classifies the current portion of lease liabilities, representing lease payments due within the next twelve months, as accrued expenses and other current liabilities on the consolidated balance sheets. The Company classifies the non-current portion of lease liabilities as operating lease liabilities on the consolidated balance sheets. The lease term includes the non-cancelable period of the lease plus any additional periods covered by an option to extend that the Company is reasonably certain to exercise. Generally, the Company may terminate its leases with the notice required in the lease agreement and upon payment of a termination fee, if required. The Company’s leases do not include substantial variable payments based on indexes or rates. The Company’s lease agreements do not contain any significant residual value guarantees or restrictive covenants. The Company’s leases do not provide a readily determinable implicit discount rate. The Company’s incremental borrowing rate is estimated to approximate an interest rate on a collateralized basis with similar terms and payments and in similar economic environments. Operating lease right-of-use assets and liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. The Company recognizes a single lease cost on a straight-line basis over the lease term, and the Company includes all cash payments within cash flows from operating activities as the change in operating lease assets and liabilities in the consolidated statements of cash flows.
|
| Impairment of Long-Lived Assets |
Impairment of Long-Lived Assets The Company reviews its long-lived assets, including its property and equipment, definite-lived intangible assets, and operating lease assets, for impairment at least annually or whenever events or changes in business circumstances indicate that the carrying amount of assets may not be fully recoverable. Each impairment test is based on a comparison of the undiscounted cash flows to the recorded value of the asset. If the recorded value of the asset is less than the undiscounted cash flows, the asset is written down to its estimated fair value.
|
| Warrant Liability |
Warrant Liability The Company’s outstanding warrants include publicly-traded warrants (the “Public Warrants”), which were issued as one-third of a warrant per unit during the Company’s initial public offering on May 26, 2020 (the “IPO”), and warrants sold in a private placement to Longview’s sponsor (the “Private Warrants”). The Company evaluated its warrants under ASC Subtopic 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, and concluded that they do not meet the criteria to be classified in stockholders’ equity. Since the Public Warrants and Private Warrants meet the definition of a derivative under ASC Topic 815, Derivatives and Hedging, the Company recorded these warrants as non-current liabilities on the consolidated balance sheets at fair value upon the closing of the Business Combination, with subsequent changes in their respective fair values recognized in the consolidated statements of operations and comprehensive loss at each reporting date.
|
| Cost of Revenue |
Cost of Revenue Cost of product revenue includes manufacturing costs, personnel costs and benefits, inbound freight, packaging, warranty replacement costs, payment processing fees, and inventory obsolescence and write-offs. Cost of software and other services revenue includes personnel costs, cloud hosting costs, amortization of capitalized software development costs, and payment processing fees.
|
| Research and Development |
Research and Development R&D expenses primarily consist of personnel costs and benefits, facilities expenses, consulting and professional fees, fabrication services, software, and other outsourcing expenses. Substantially all of the Company’s R&D expenses are related to developing new products and services and improving existing products and services. R&D expenses are expensed as incurred.
|
| Sales and Marketing |
Sales and Marketing Sales and marketing expenses primarily consist of personnel costs and benefits, third-party logistics, fulfillment and outbound shipping costs, facilities expenses, advertising, and travel and entertainment. Advertising expenses are expensed as incurred.
|
| General and Administrative |
General and Administrative General and administrative expenses primarily consist of personnel costs and benefits, insurance, patent fees, software costs, facilities costs, and outside services. Outside services consist of professional services, legal fees, and other professional fees.
|
| Operating Expenses - Other |
Operating Expenses – Other The Company classifies certain operating expenses that are not representative of its ongoing operations as other on the consolidated statements of operations and comprehensive loss. These include costs related to the Company’s business transformation initiative, reductions in force, litigation, and legal settlements. The following table summarizes the types of expenses classified as other in the Company’s consolidated statements of operations and comprehensive loss (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Employment-related expenses | $ | 1,341 | | | $ | 8,701 | | | $ | 2,019 | | | Legal-related expenses | 2,724 | | | 9,463 | | | 5,327 | | | Total other | $ | 4,065 | | | $ | 18,164 | | | $ | 7,346 | |
On July 1, 2024, the Company entered into an agreement with a third-party global technology and business transformation partner to optimize and lower the cost of certain non-specialized technical functions. As part of the transition into this new partnership, a portion of the Company's workforce will be in lower-cost geographies. The Company incurred $0.7 million of severance costs for impacted employees that continued providing transition services to the Company through the end of 2024. These costs were recognized as other in the consolidated statements of operations and comprehensive loss during the year ended December 31, 2024. As of December 31, 2024, a total of $0.7 million of accrued severance related to our global workforce optimization is included in accrued expenses and other current liabilities and other non-current liabilities on the consolidated balance sheets.
|
| Net Loss per Common Share |
Net Loss per Common Share We compute net loss per share of Class A and Class B common stock using the two-class method. Basic net loss per share is computed by dividing the net loss by the weighted-average number of shares of each class of the Company’s common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all of the Company’s potential common shares outstanding of the Company’s common stock to the extent the potential shares are dilutive.
|
| Stock-Based Compensation Expense |
Stock-Based Compensation Expense The measurement of stock-based compensation expense for all stock-based payment awards, including stock options and RSUs granted to employees, directors, and nonemployees as well as the Company's ESPP, is based on the estimated fair value of the awards on the grant date. The Company recognizes stock-based compensation expense for its awards on a straight-line basis over the requisite service period of the individual grants, which is generally the vesting period. Generally, stock option and RSU awards fully vest three to four years from the grant date, and stock options have a contractual term of 10 years. ESPP options generally vest over two years. The Company recognizes the effect of forfeitures in stock-based compensation expense based on actual forfeitures when they occur. The Company granted performance-based restricted stock units during the years ended December 31, 2024, 2023, and 2022. The Company accounted for these awards according to the relevant provisions of ASC Topic 718, Compensation-Stock Compensation. For performance-based awards, the Company recognizes expense using the accelerated attribution method.
|
| Income Taxes |
Income Taxes The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies. The Company accounts for uncertainty in income taxes recognized in the consolidated financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood that it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more likely than not to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the consolidated financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves, or unrecognized tax benefits, that are considered appropriate as well as the related net interest and penalties.
|
| Recent Accounting Pronouncements Adopted and Not Yet Adopted |
Recent Accounting Pronouncements Adopted In November 2023, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which introduced new guidance on disclosures for reportable segments and significant segment expenses, including for entities with a single reportable segment. The Company retrospectively adopted this guidance during the year ended December 31, 2024. The adoption did not have a significant impact on the consolidated financial statements, other than the newly required disclosures. Recent Accounting Pronouncements Issued but Not Yet Adopted In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which introduced new guidance on disclosures for income taxes, including enhancements to the rate reconciliation and income taxes paid disclosures. This guidance is effective for the Company for annual reporting periods beginning January 1, 2025. The Company is currently evaluating the impact that the adoption of this pronouncement will have on the Company’s consolidated financial statements and disclosures. In November 2024, the FASB issued ASU 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, which introduced new guidance on disclosures of specified information about certain costs and expenses included within expenses presented on the face or the income statements, such as purchases of inventory and employee compensation. This guidance is effective for the Company for annual reporting periods beginning January 1, 2027 and interim reporting periods beginning January 1, 2028. The Company is currently evaluating the impact that the adoption of this pronouncement will have on the Company's consolidated financial statements and disclosures.
|
| X |
- DefinitionDisclosure of accounting policy for basis of presentation and principles of consolidation.
| Name: |
bfly_BasisOfPresentationAndPrinciplesOfConsolidationPolicyTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
bfly_OtherOperatingExpensesPolicyTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for sales and marketing.
| Name: |
bfly_SalesAndMarketingPolicyTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for vendor advances.
| Name: |
bfly_VendorAdvancesPolicyTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for warrant liability.
| Name: |
bfly_WarrantLiabilityPolicyTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEntity's cash and cash equivalents accounting policy with respect to restricted balances. Restrictions may include legally restricted deposits held as compensating balances against short-term borrowing arrangements, contracts entered into with others, or company statements of intention with regard to particular deposits; however, time deposits and short-term certificates of deposit are not generally included in legally restricted deposits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsRestrictedCashAndCashEquivalentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478898/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cost of product sold and service rendered. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Topic 705
-Publisher FASB
-URI https://asc.fasb.org/705/tableOfContent
| Name: |
us-gaap_CostOfSalesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-20
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-1
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs incurred when both (1) the software is acquired, internally developed, or modified solely to meet the entity's internal needs, and (2) during the software's development or modification, no substantive plan exists or is being developed to market the software externally. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 40
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/350-40/tableOfContent
| Name: |
us-gaap_InternalUseSoftwarePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483080/330-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483489/210-10-50-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 330
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478411/912-330-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/330/tableOfContent
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483080/330-10-50-4
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)
-SubTopic 10
-Topic 270
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482989/270-10-45-6
| Name: |
us-gaap_InventoryPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for receivable. Includes, but is not limited to, accounts receivable and financing receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481569/310-20-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
| Name: |
us-gaap_ReceivablesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 36
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-36
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for inclusion of significant items in the selling, general and administrative (or similar) expense report caption. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 720
-SubTopic 35
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483406/720-35-50-1
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpensesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Summary of Significant Accounting Policies (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Schedule of Segment Revenue and Significant Segment Expenses |
The following table summarizes the Company's segment revenue and significant segment expenses included in consolidated net loss (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | Revenue | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | | Less: | | | | | | Cost of revenue | 33,225 | | | 49,044 | | | 33,930 | | Payroll operating expenses | 57,334 | | | 67,241 | | | 111,361 | | Stock-based compensation expense | 21,032 | | | 27,480 | | | 42,531 | | Non-payroll operating expenses | 40,811 | | | 49,581 | | | 71,242 | | Other | 4,065 | | | 18,164 | | | 7,346 | | Other segment items | (1,919) | | | (11,910) | | | (24,297) | | Net loss | $ | (72,492) | | | $ | (133,700) | | | $ | (168,723) | |
|
| Schedule of allowance for doubtful accounts |
The following table summarizes the allowance for doubtful accounts activity: | | | | | | | (in thousands) | Fair Value | | Allowance for doubtful accounts as of December 31, 2022 | $ | 528 | | | Additions | 1,446 | | | Deductions – write offs | (187) | | | Allowance for doubtful accounts as of December 31, 2023 | 1,787 | | | Additions | 1,128 | | | Deductions – write offs | (332) | | | Allowance for doubtful accounts as of December 31, 2024 | $ | 2,583 | |
|
| Schedule of useful life for property and equipment |
Useful lives for property and equipment are as follows: | | | | | | | | | | Property and Equipment | | Estimated Useful Life | | Software | | 3 years | | Machinery and equipment | | 3 – 5 years | | Furniture and fixtures | | 5 – 7 years | | Leasehold improvements | | Lesser of estimated useful life or remaining lease term |
|
| Schedule of other expenses |
The following table summarizes the types of expenses classified as other in the Company’s consolidated statements of operations and comprehensive loss (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Employment-related expenses | $ | 1,341 | | | $ | 8,701 | | | $ | 2,019 | | | Legal-related expenses | 2,724 | | | 9,463 | | | 5,327 | | | Total other | $ | 4,065 | | | $ | 18,164 | | | $ | 7,346 | |
|
| X |
- DefinitionTabular disclosure of other expenses.
| Name: |
bfly_OtherExpensesTableTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of useful life of property and equipment.
| Name: |
bfly_UsefulLifeForPropertyAndEquipmentTableTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allowance for credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
| Name: |
us-gaap_AccountsReceivableAllowanceForCreditLossTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-25
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Revenue Recognition (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Revenue from Contract with Customer [Abstract] |
|
| Schedule of disaggregated revenue |
The following table summarizes the Company’s disaggregated revenues (in thousands) for the years ended December 31: | | | | | | | | | | | | | | | | | | | | | | | | | Pattern of
Recognition | | 2024 | | 2023 | | 2022 | | By product type: | | | | | | | | | Devices and accessories | Point-in-time | | $ | 54,200 | | | $ | 40,036 | | | $ | 50,263 | | | Software and other services | Over time | | 27,856 | | | 25,864 | | | 23,127 | | | Total revenue | | | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | | | | | | | | | | | By geographical market: | | | | | | | | | United States | | | $ | 63,423 | | | $ | 52,116 | | | $ | 51,072 | | | International | | | 18,633 | | | 13,784 | | | 22,318 | | | Total revenue | | | $ | 82,056 | | | $ | 65,900 | | | $ | 73,390 | |
|
| X |
- DefinitionTabular disclosure of disaggregation of revenue into categories depicting how nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factor. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
| Name: |
us-gaap_DisaggregationOfRevenueTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenueFromContractWithCustomerAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Fair Value of Financial Instruments (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of assets and liabilities measured at fair value on a recurring basis |
The following table summarizes the Company’s assets and liabilities that are measured at fair value on a recurring basis, by level, within the fair value hierarchy (in thousands): | | | | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement Level | | Total | | Level 1 | | Level 2 | | Level 3 | | December 31, 2024: | | | | | | | | | Warrants: | | | | | | | | | Public Warrants | $ | 1,794 | | | $ | 1,794 | | | $ | — | | | $ | — | | | Private Warrants | 891 | | | — | | | 891 | | | — | | | Total liabilities at fair value on a recurring basis | $ | 2,685 | | | $ | 1,794 | | | $ | 891 | | | $ | — | | | | | | | | | | | December 31, 2023: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Warrants: | | | | | | | | | Public Warrants | $ | 552 | | | $ | 552 | | | $ | — | | | $ | — | | | Private Warrants | 274 | | | — | | | 274 | | | — | | | Total liabilities at fair value on a recurring basis | $ | 826 | | | $ | 552 | | | $ | 274 | | | $ | — | |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets and liabilities, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Inventories (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Inventory Disclosure [Abstract] |
|
| Summary of inventories |
A summary of inventories is as follows at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Raw materials | $ | 47,642 | | | 49,366 | | | Work-in-progress | 4,736 | | | 3,384 | | | Finished goods | 18,411 | | | 20,272 | | | Total inventories | $ | 70,789 | | | $ | 73,022 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the carrying amount as of the balance sheet date of merchandise, goods, commodities, or supplies held for future sale or to be used in manufacturing, servicing or production process. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483489/210-10-50-1
| Name: |
us-gaap_ScheduleOfInventoryCurrentTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Restricted Cash (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Cash and Cash Equivalents [Abstract] |
|
| Summary of reconciliation of cash, cash equivalents and restricted cash |
A reconciliation of cash, cash equivalents and restricted cash from the consolidated balance sheets to the consolidated statements of cash flows as of December 31, 2024 and 2023 is as follows (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Reconciliation of cash, cash equivalents and restricted cash: | | | | | Cash and cash equivalents | $ | 88,775 | | | $ | 134,437 | | | Restricted cash included within prepaid expenses and other current assets | — | | | 199 | | | Restricted cash included within other non-current assets | 4,015 | | | 4,014 | | | Total cash, cash equivalents and restricted cash shown in the condensed consolidated statements of cash flows | $ | 92,790 | | | $ | 138,650 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashAndCashEquivalentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of cash and cash equivalents restricted as to withdrawal or usage. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_ScheduleOfRestrictedCashAndCashEquivalentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- References
+ Details
| Name: |
us-gaap_OtherAssetsNoncurrentDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of noncurrent assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ScheduleOfOtherAssetsNoncurrentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Property, Equipment, and Intangible Assets (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Property, Equipment, and Intangible Assets [Abstract] |
|
| Schedule of property and equipment, net |
Property and equipment, net, are recorded at historical cost and consist of the following at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Capitalized internally developed software | $ | 20,677 | | | $ | 17,722 | | | Leasehold improvements | 11,120 | | | 11,102 | | | Machinery and equipment | 10,043 | | | 9,930 | | | Furniture and fixtures | 2,152 | | | 2,152 | | | Construction in progress | 2,394 | | | 2,566 | | | Other | 29 | | | 44 | | | 46,415 | | | 43,516 | | | Less: accumulated depreciation and amortization | (26,897) | | | (18,195) | | | Property and equipment, net | $ | 19,518 | | | $ | 25,321 | |
|
| Schedule of company's intangible assets |
The Company's intangible assets consist of the following (in thousands): | | | | | | | | | | | | | | | | | | | Gross Carrying
Amount | | Accumulated
Amortization | | Net Carrying
Amount | | December 31, 2024: | | | | | | | Technology licenses | $ | 10,317 | | | $ | (1,401) | | | $ | 8,916 | | | | | | | | | December 31, 2023: | | | | | | | Technology licenses | $ | 10,317 | | | $ | — | | | $ | 10,317 | |
|
| Schedule of estimated intangible asset amortization expense |
Estimated amortization expense for the Company’s capitalized internally developed software assets and intangible assets over the next five years ended December 31 is as follows (in thousands): | | | | | | | | | | | | | | | | | | | | | | | | | | | | 2025 | | 2026 | | 2027 | | 2028 | | 2029 | | $ | 4,989 | | | $ | 2,737 | | | $ | 1,583 | | | $ | 1,120 | | | $ | 1,120 | |
|
| X |
- References
+ Details
| Name: |
bfly_PropertyEquipmentAndIntangibleAssetsAbstract |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance with a finite life, by either major class or business segment. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the amount of amortization expense expected to be recorded in succeeding fiscal years for finite-lived intangible assets. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleofFiniteLivedIntangibleAssetsFutureAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Accrued Expenses and Other Current Liabilities (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Schedule of accrued expenses and other current liabilities |
Accrued expenses and other current liabilities consist of the following at December 31 (in thousands): | | | | | | | | | | | | | 2024 | | 2023 | | Employee compensation | $ | 11,192 | | | $ | 9,442 | | | Customer deposits | 1,909 | | | 1,613 | | | Accrued warranty liability | 498 | | | 297 | | | Non-income tax | 2,356 | | | 1,197 | | | Professional fees | 2,015 | | | 2,481 | | | Current portion of operating lease liabilities | 2,437 | | | 2,192 | | | Other | 7,288 | | | 6,203 | | | Total accrued expenses and other current liabilities | $ | 27,695 | | | $ | 23,425 | |
|
| Schedule of warranty expense activity |
Warranty expense activity for the years ended December 31 is as follows (in thousands): | | | | | | | | | | | | | | | | | | | 2024 | | 2023 | | 2022 | | Balance, beginning of period | $ | 697 | | | $ | 873 | | | $ | 1,116 | | | Warranty provision charged to operations | 1,224 | | | 276 | | | 296 | | | Warranty claims | (898) | | | (452) | | | (539) | | | Balance, end of period | $ | 1,023 | | | $ | 697 | | | $ | 873 | |
|
| X |
- DefinitionTabular disclosure of accrued liabilities and other current liabilities.
| Name: |
bfly_ScheduleOfAccruedLiabilitiesAndOtherCurrentLiabilitiesTableTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the changes in the guarantor's aggregate product warranty liability, including the beginning balance of the aggregate product warranty liability, the aggregate reductions in that liability for payments made (in cash or in kind) under the warranty, the aggregate changes in the liability for accruals related to product warranties issued during the reporting period, the aggregate changes in the liability for accruals related to preexisting warranties (including adjustments related to changes in estimates), and the ending balance of the aggregate product warranty liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
| Name: |
us-gaap_ScheduleOfProductWarrantyLiabilityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Stock-Based Compensation (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Summary of the stock option activity |
A summary of the stock option activity under the Plans is presented in the table below: | | | | | | | | | | | | | | | | | | | | | | | | | Number of
Options | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual
Term | | Aggregate
Intrinsic
Value
(in thousands) | | Outstanding at December 31, 2022 | 12,571,912 | | 7.67 | | | 5.62 | | 1,342 | | | Granted | — | | — | | | | | | | Exercised | (180,467) | | 1.26 | | | | | | | Forfeited | (4,952,258) | | 10.14 | | | | | | | Outstanding at December 31, 2023 | 7,439,187 | | 6.17 | | | 4.68 | | — | | | Granted | — | | — | | | | | | | Exercised | (36,340) | | 1.77 | | | | | | | Forfeited | (842,111) | | 8.98 | | | | | | | Outstanding at December 31, 2024 | 6,560,736 | | 5.88 | | | 3.56 | | 1,963 | | | Options exercisable at December 31, 2023 | 6,537,433 | | 5.88 | | | 4.37 | | — | | | Options exercisable at December 31, 2024 | 6,222,073 | | 5.94 | | | 3.48 | | 1,799 | |
|
| Summary of additional information about stock option activity |
Additional information about the Company’s stock option activity during the years ended December 31, 2024, 2023 and 2022 is presented in the table below: | | | | | | | | | | | | | | | | | | | 2024 | | 2023 | | 2022 | | Cash proceeds from the exercise of stock options (in millions) | $ | 0.1 | | | $ | 0.2 | | | $ | 3.0 | | | Total intrinsic value of stock options exercised (in millions) | — | | | 0.2 | | | 3.6 | | | Weighted average grant date fair value of options granted | — | | | — | | | 2.79 | |
|
| Summary of the restricted stock unit activity |
A summary of the restricted stock unit activity under the Plans is presented in the table below: | | | | | | | | | | | | | Number of
Restricted
Stock Units | | Weighted
Average
Grant Date
Fair Value | | Outstanding at December 31, 2022 | 9,961,291 | | 4.55 | | | Granted | 16,082,613 | | 2.23 | | | Vested | (5,418,832) | | 4.09 | | | Forfeited | (5,055,089) | | 3.65 | | | Outstanding at December 31, 2023 | 15,569,983 | | 2.61 | | | Granted | 15,838,283 | | 1.10 | | | Vested | (6,906,452) | | 2.69 | | | Forfeited | (3,251,584) | | 2.34 | | | Outstanding at December 31, 2024 | 21,250,230 | | 1.52 | |
|
| Schedule of assumptions used to value option grants to employees and non-employees |
The assumptions used to value stock option and market-based grants to employees were as follows: | | | | | | | | | | | | | | | | | | | 2024 | | 2023 | | 2022 | | Risk-free interest rate | 3.6% | | 3.6% – 3.9% | | 1.7% – 3.0% | | Expected dividend yield | 0% | | 0% | | 0% | | Expected term | 5.0 years | | 5.0 years | | 5.8 years – 6.5 years | | Expected volatility | 89% | | 76% | | 70% – 73% |
|
| Schedule of stock-based compensation expense |
The Company’s stock-based compensation expense for the periods presented was as follows (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Research and development | $ | 6,950 | | | $ | 9,772 | | | 12,834 | | | Sales and marketing | 4,871 | | | 4,260 | | | 5,974 | | | General and administrative | 9,211 | | | 13,448 | | | 23,723 | | | Total stock-based compensation expense | $ | 21,032 | | | $ | 27,480 | | | $ | 42,531 | |
|
| X |
- DefinitionTabular disclosure for additional information about stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value.
| Name: |
bfly_ShareBasedPaymentArrangementOptionActivityAdditionalInformationTableTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the number and weighted-average grant date fair value for restricted stock units that were outstanding at the beginning and end of the year, and the number of restricted stock units that were granted, vested, or forfeited during the year. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Net Loss Per Share (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Schedule of calculation of basic and diluted net loss per share |
As the Company uses the two-class method required for companies with multiple classes of common stock, the following table presents the calculation of basic and diluted net loss per share for each class of the Company’s common stock outstanding (in thousands, except share and per share amounts): | | | | | | | | | | | | | | | | | | | Year ended December 31, 2024 | | | | | | | Class A | | Class B | | Total
Common Stock | | Numerator: | | | | | | | Allocation of undistributed earnings | $ | (63,442) | | | $ | (9,050) | | | $ | (72,492) | | | Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (63,442) | | | $ | (9,050) | | | $ | (72,492) | | | Denominator: | | | | | | | Weighted-average common shares outstanding | 185,255,823 | | 26,426,937 | | 211,682,760 | | Denominator for basic and diluted net loss per share – weighted-average common stock | 185,255,823 | | 26,426,937 | | 211,682,760 | | Basic and diluted net loss per share | $ | (0.34) | | | $ | (0.34) | | | $ | (0.34) | |
| | | | | | | | | | | | | | | | | | | Year ended December 31, 2023 | | | | | | | Class A | | Class B | | Total
Common Stock | | Numerator: | | | | | | | Allocation of undistributed earnings | $ | (116,497) | | | $ | (17,203) | | | $ | (133,700) | | | Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (116,497) | | | $ | (17,203) | | | $ | (133,700) | | | Denominator: | | | | | | | Weighted-average common shares outstanding | 178,958,607 | | 26,426,937 | | 205,385,544 | | Denominator for basic and diluted net loss per share – weighted-average common stock | 178,958,607 | | 26,426,937 | | 205,385,544 | | Basic and diluted net loss per share | $ | (0.65) | | | $ | (0.65) | | | $ | (0.65) | |
| | | | | | | | | | | | | | | | | | | Year ended December 31, 2022 | | | | | | | Class A | | Class B | | Total
Common Stock | | Numerator: | | | | | | | Allocation of undistributed earnings | $ | (146,412) | | | $ | (22,311) | | | $ | (168,723) | | | Numerator for basic and diluted net loss per share – loss available to common stockholders | $ | (146,412) | | | $ | (22,311) | | | $ | (168,723) | | | Denominator: | | | | | | | Weighted-average common shares outstanding | 173,421,449 | | 26,426,937 | | 199,848,386 | | Denominator for basic and diluted net loss per share – weighted-average common stock | 173,421,449 | | 26,426,937 | | 199,848,386 | | Basic and diluted net loss per share | $ | (0.84) | | | $ | (0.84) | | | $ | (0.84) | |
|
| Schedule of anti-dilutive common equivalent shares |
Anti-dilutive common equivalent shares were as follows: | | | | | | | | | | | | | | | | | | | December 31, | | 2024 | | 2023 | | 2022 | | Outstanding options to purchase common stock | 6,560,736 | | 7,439,187 | | 12,571,912 | | Outstanding restricted stock units | 21,250,230 | | 15,569,983 | | 9,961,291 | Outstanding employee stock purchase plan options | 1,948,409 | | — | | — | | Outstanding warrants | 20,652,690 | | 20,652,690 | | 20,652,690 | | Total anti-dilutive common equivalent shares | 50,412,065 | | 43,661,860 | | 43,185,893 |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Income Taxes (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Schedule of income (loss) before provision for income taxes |
Income (loss) before provision for income taxes consisted of the following (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Federal | $ | (72,923) | | | $ | (133,961) | | | $ | (169,122) | | | Foreign | 399 | | | 343 | | | 441 | | | Loss before provision for income taxes | $ | (72,524) | | | $ | (133,618) | | | $ | (168,681) | |
|
| Schedule of reconciliation of the statutory income tax rate to the effective income tax rate |
A reconciliation of the Company’s statutory income tax rate to the Company’s effective income tax rate is as follows: | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Income at U.S. statutory rate | 21.00 | % | | 21.00 | % | | 21.00 | % | | State taxes, net of federal benefit | 0.39 | | | 4.65 | | | 2.21 | | | Stock compensation | (6.41) | | | (2.57) | | | (5.01) | | | Change in fair value of warrants | (0.54) | | | 0.71 | | | 2.60 | | | Tax credits | 2.58 | | | 1.74 | | | 2.16 | | | Valuation allowance | (11.93) | | | (25.47) | | | (22.91) | | Return to provision | (3.45) | | | — | | | — | | | Other | (1.60) | | | (0.12) | | | (0.08) | | | 0.04 | % | | (0.06) | % | | (0.03) | % |
|
| Schedule of net deferred tax assets |
Net deferred tax assets as of December 31, 2024 and 2023 consisted of the following (in thousands): | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | Deferred tax assets | | | | | Net operating loss carryforwards | $ | 158,097 | | | $ | 151,230 | | | Tax credits | 18,114 | | | 16,288 | | | Stock compensation | 3,203 | | | 4,812 | | | Accruals and reserves | 4,106 | | | 2,061 | | | Inventory reserve | 15,114 | | | 15,207 | | | Lease liability | 5,562 | | | 6,114 | | | Depreciation | 99 | | | 2,197 | | | Capitalized tax R&E | 27,296 | | | 25,844 | | | Other | 4,183 | | | 1,581 | | | Total deferred tax assets | 235,774 | | | 225,334 | | | Valuation allowance | (230,109) | | | (221,454) | | | Total deferred tax assets | 5,665 | | | 3,880 | | | Deferred tax liabilities | | | | | Right-of-use asset | (3,467) | | | (3,829) | | Software costs | (1,839) | | | — | | | Net deferred tax assets | $ | 359 | | | $ | 51 | |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 231
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482663/740-10-55-231
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12A
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of income before income tax between domestic and foreign jurisdictions. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_ScheduleOfIncomeBeforeIncomeTaxDomesticAndForeignTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Leases (Tables)
|
12 Months Ended |
Dec. 31, 2024 |
|---|
| Leases [Abstract] |
|
| Schedule of operating lease cost |
The following table presents the components of operating lease cost for the years ended December 31, 2024, 2023, and 2022 (in thousands): | | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Operating lease cost | $ | 2,807 | | | $ | 3,206 | | | $ | 4,300 | | | Short-term lease cost | 101 | | | 73 | | | 249 | | | Variable lease cost | 321 | | | 317 | | | 353 | | | Total operating lease cost | $ | 3,229 | | | $ | 3,596 | | | $ | 4,902 | |
|
| Schedule of operating lease expected future payments |
The expected maturities related to the Company’s leases with initial non-cancellable lease terms in excess of one year as of December 31, 2024 are as follows: | | | | | | | Year ended December 31, | Operating Lease Payments | | 2025 | $ | 3,664 | | | 2026 | 3,749 | | | 2027 | 3,835 | | | 2028 | 3,921 | | | 2029 | 3,454 | | | 2030 and thereafter | 9,354 | | | Total gross operating lease payments | 27,977 | | | Less: imputed interest | (5,142) | | | Total operating lease liabilities, reflecting the present value of net lease payments | $ | 22,835 | |
|
| Schedule of lease term, discount rate and cash flows from operating lease |
Additional information related to operating leases is presented as follows: | | | | | | | | | | | | | | | | | | | December 31, | | 2024 | | 2023 | | 2022 | | Weighted average remaining lease term (in years) | 7.5 | | 8.4 | | 8.8 | | Weighted average discount rate | 5.9 | % | | 5.9 | % | | 5.5 | % |
| | | | | | | | | | | | | | | | | | | Year ended December 31, | | 2024 | | 2023 | | 2022 | | Cash paid for amounts included in the measurement of lease liabilities: | | | | | | | Operating cash flows from operating leases | $ | 3,557 | | | $ | 3,121 | | | $ | 2,042 | | | Non-cash activities involving right-of-use assets and lease liabilities: | | | | | | | | | | | | | Derecognition of right-of-use assets | — | | | 4,163 | | | — | | | Derecognition of operating lease liabilities | — | | | 5,401 | | | — | |
|
| X |
- DefinitionTabular disclosure of weighted average lease term, discount rate and cash flows from lease.
| Name: |
bfly_LeaseWeightedAverageLeaseTermDiscountRateAndCashFlowsTableTextBlock |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- DefinitionThe value of "Public Warrants" which were issued per unit issued during the company's initial public offering (IPO).
| Name: |
bfly_ValueOfPublicWarrantsIssuedPerUnitIssuedDuringIpo |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDuration of company warranty.
| Name: |
bfly_WarrantyPeriod |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount charged to advertising expense for the period, which are expenses incurred with the objective of increasing revenue for a specified brand, product or product line. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 720
-SubTopic 35
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483385/720-35-55-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 720
-SubTopic 35
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483406/720-35-50-1
| Name: |
us-gaap_AdvertisingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFor an entity that discloses a concentration risk in relation to quantitative amount, which serves as the "benchmark" (or denominator) in the equation, this concept represents the concentration percentage derived from the division. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 21
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-21
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 825
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-20
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-18
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-20
| Name: |
us-gaap_ConcentrationRiskPercentage1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of finite-lived intangible assets, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.
| Name: |
us-gaap_FiniteLivedIntangibleAssetUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfReportableSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLiability for amount due employees, in addition to wages and any other money that employers owe employees, when their employment ends through a layoff or other termination. For example, a company may provide involuntarily terminated employees with a lump sum payment equal to one week's salary for every year of employment.
| Name: |
us-gaap_SupplementalUnemploymentBenefitsSeveranceBenefits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=bfly_OneCustomerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bfly_AccountsReceivableBenchmarkMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByTypeAxis=us-gaap_CustomerConcentrationRiskMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=us-gaap_SalesRevenueNetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_MajorCustomersAxis=bfly_NoCustomersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=bfly_EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_RestructuringCostAndReserveAxis=us-gaap_EmployeeSeveranceMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=bfly_AccruedLiabilitiesAndOtherLiabilitiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IndefiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_ComputerSoftwareIntangibleAssetMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Summary of Significant Accounting Policies - Schedule of Segment Revenue and Significant Segment Expenses (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Segment Reporting Information [Line Items] |
|
|
|
| Total revenue |
$ 82,056
|
$ 65,900
|
$ 73,390
|
| Cost of revenue |
33,225
|
49,044
|
33,930
|
| Stock-based compensation expense |
21,032
|
27,480
|
42,531
|
| Other |
4,065
|
18,164
|
7,346
|
| Net loss and comprehensive loss |
(72,492)
|
(133,700)
|
(168,723)
|
| Reportable segment |
|
|
|
| Segment Reporting Information [Line Items] |
|
|
|
| Total revenue |
82,056
|
65,900
|
73,390
|
| Cost of revenue |
33,225
|
49,044
|
33,930
|
| Payroll operating expenses |
57,334
|
67,241
|
111,361
|
| Stock-based compensation expense |
21,032
|
27,480
|
42,531
|
| Non-payroll operating expenses |
40,811
|
49,581
|
71,242
|
| Other |
4,065
|
18,164
|
7,346
|
| Other segment items |
(1,919)
|
(11,910)
|
(24,297)
|
| Net loss and comprehensive loss |
$ (72,492)
|
$ (133,700)
|
$ (168,723)
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate cost of goods produced and sold and services rendered during the reporting period. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_CostOfRevenue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Excludes Selling, General and Administrative Expense.
| Name: |
us-gaap_OperatingCostsAndExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of other expense (income) and loss (gain) calculated as difference between segment revenue and separately disclosed expense category to arrive at segment profit (loss). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 26C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-26C
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 26B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-26B
| Name: |
us-gaap_SegmentReportingOtherItemAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementBusinessSegmentsAxis=bfly_ReportableSegmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
| X |
- DefinitionAmount of allowance for credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479344/326-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivableRollforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of direct write-downs of accounts receivable charged against the allowance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivableWriteOffs |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (reversal of expense) for expected credit loss on accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479319/326-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_ProvisionForDoubtfulAccounts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_SoftwareAndSoftwareDevelopmentCostsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_MachineryAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Summary of Significant Accounting Policies - Operating Expenses - Other (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Accounting Policies [Abstract] |
|
|
|
| Employment-related expenses |
$ 1,341
|
$ 8,701
|
$ 2,019
|
| Legal-related expenses |
2,724
|
9,463
|
5,327
|
| Total other |
$ 4,065
|
$ 18,164
|
$ 7,346
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of other expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses associated with a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OtherRestructuringCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses for special or contractual termination benefits provided to current employees involuntarily terminated under a benefit arrangement associated exit or disposal activities pursuant to an authorized plan. Excludes expenses related to one-time termination benefits, a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_SeveranceCosts1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.25.0.1
Revenue Recognition - Disaggregation of Revenue (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Revenue Recognition |
|
|
|
| Total revenue |
$ 82,056
|
$ 65,900
|
$ 73,390
|
| United States |
|
|
|
| Revenue Recognition |
|
|
|
| Total revenue |
63,423
|
52,116
|
51,072
|
| International |
|
|
|
| Revenue Recognition |
|
|
|
| Total revenue |
18,633
|
13,784
|
22,318
|
| Devices and accessories |
|
|
|
| Revenue Recognition |
|
|
|
| Total revenue |
54,200
|
40,036
|
50,263
|
| Devices and accessories | Point-in-time |
|
|
|
| Revenue Recognition |
|
|
|
| Total revenue |
54,200
|
40,036
|
50,263
|
| Software and other services |
|
|
|
| Revenue Recognition |
|
|
|
| Total revenue |
27,856
|
25,864
|
23,127
|
| Software and other services | Over time |
|
|
|
| Revenue Recognition |
|
|
|
| Total revenue |
$ 27,856
|
$ 25,864
|
$ 23,127
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 91
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479777/606-10-55-91
| Name: |
us-gaap_DisaggregationOfRevenueLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_US |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=us-gaap_NonUsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ProductMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TimingOfTransferOfGoodOrServiceAxis=us-gaap_TransferredAtPointInTimeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ProductOrServiceAxis=us-gaap_ServiceOtherMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TimingOfTransferOfGoodOrServiceAxis=us-gaap_TransferredOverTimeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
| X |
- DefinitionDuration of typical payment terms for product and service sales.
| Name: |
bfly_DurationForPaymentTerms |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized that was previously included in balance of obligation to transfer good or service to customer for which consideration from customer has been received or is due. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-8
| Name: |
us-gaap_ContractWithCustomerLiabilityRevenueRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenueFromContractWithCustomerAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Revenue Recognition - Performance Obligations (Details)
$ in Millions |
Dec. 31, 2024
USD ($)
|
| Revenue Recognition |
|
| Remaining performance obligations |
$ 33.3
|
| Revenue, Remaining Performance Obligation, Expected Timing of Satisfaction, Start Date [Axis]: 2025-01-01 |
|
| Revenue Recognition |
|
| Percentage of remaining performance obligations as revenue |
64.00%
|
| Revenue, remaining performance obligation, expected timing of satisfaction, period |
12 months
|
| Revenue, Remaining Performance Obligation, Expected Timing of Satisfaction, Start Date [Axis]: 2026-01-01 |
|
| Revenue Recognition |
|
| Percentage of remaining performance obligations as revenue |
36.00%
|
| Revenue, remaining performance obligation, expected timing of satisfaction, period |
|
| X |
- DefinitionAmount of transaction price allocated to performance obligation that has not been recognized as revenue. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-SubTopic 10
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-13
| Name: |
us-gaap_RevenueRemainingPerformanceObligation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_RevenueRemainingPerformanceObligationExpectedTimingOfSatisfactionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod in which remaining performance obligation is expected to be recognized as revenue, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)(1)
-SubTopic 10
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-13
| Name: |
us-gaap_RevenueRemainingPerformanceObligationExpectedTimingOfSatisfactionPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of remaining performance obligation to total remaining performance obligation not recognized as revenue. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)(1)
-SubTopic 10
-Topic 606
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-13
| Name: |
us-gaap_RevenueRemainingPerformanceObligationPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_RevenueRemainingPerformanceObligationExpectedTimingOfSatisfactionStartDateAxis=2025-01-01 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_RevenueRemainingPerformanceObligationExpectedTimingOfSatisfactionStartDateAxis=2026-01-01 |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
| X |
- DefinitionAmount of amortization expense for asset recognized from cost incurred to obtain or fulfill contract with customer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479483/340-40-50-3
| Name: |
us-gaap_CapitalizedContractCostAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before accumulated amortization and accumulated impairment loss, of asset recognized from cost incurred to obtain or fulfill contract with customer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479483/340-40-50-3
| Name: |
us-gaap_CapitalizedContractCostGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_RevenueFromContractWithCustomerAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- Definition
+ References
+ Details
| Name: |
bfly_WarrantsFairValueDisclosure |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value of financial and nonfinancial obligations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_LiabilitiesFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=bfly_PrivateWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=bfly_PublicWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Inventories (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Inventory Disclosure [Abstract] |
|
|
|
| Raw materials |
$ 47,642
|
$ 49,366
|
|
| Work-in-progress |
4,736
|
3,384
|
|
| Finished goods |
18,411
|
20,272
|
|
| Total inventories |
70,789
|
73,022
|
|
| Net realizable value inventory adjustments and excess and obsolete inventory charges |
$ 15
|
$ 21,083
|
$ 783
|
| X |
- References
+ Details
| Name: |
us-gaap_InventoryDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of completed merchandise or goods expected to be sold within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryFinishedGoods |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of raw materials expected to be sold, or consumed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryRawMaterials |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before valuation and LIFO reserves of merchandise or goods in the production process expected to be completed within one year or operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(6)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InventoryWorkInProcess |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of loss from reductions in inventory due to subsequent measurement adjustments, including, but not limited to, physical deterioration, obsolescence, or changes in price levels. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 330
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483080/330-10-50-2
| Name: |
us-gaap_InventoryWriteDown |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.25.0.1
Restricted Cash - Reconciliation of Cash, Cash Equivalents and Restricted Cash (Details) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Reconciliation of cash, cash equivalents and restricted cash: |
|
|
|
|
| Cash and cash equivalents |
$ 88,775
|
$ 134,437
|
|
|
| Restricted cash included within prepaid expenses and other current assets |
0
|
199
|
|
|
| Restricted cash included within other non-current assets |
4,015
|
4,014
|
|
|
| Total cash, cash equivalents and restricted cash shown in the condensed consolidated statements of cash flows |
$ 92,790
|
$ 138,650
|
$ 166,828
|
$ 426,841
|
| Restricted Cash and Cash Equivalents, Current, Statement of Financial Position [Extensible Enumeration] |
Prepaid expenses and other current assets
|
Prepaid expenses and other current assets
|
|
|
| Restricted Cash and Cash Equivalents, Noncurrent, Statement of Financial Position [Extensible Enumeration] |
Other non-current assets
|
Other non-current assets
|
|
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents restricted as to withdrawal or usage, classified as current. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_RestrictedCashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionIndicates line item in statement of financial position that includes cash and cash equivalents restricted to withdrawal or usage, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashAndCashEquivalentsCurrentAssetStatementOfFinancialPositionExtensibleList |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477220/954-210-45-5
| Name: |
us-gaap_RestrictedCashAndCashEquivalentsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionIndicates line item in statement of financial position that includes cash and cash equivalents restricted to withdrawal or usage, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashAndCashEquivalentsNoncurrentAssetStatementOfFinancialPositionExtensibleList |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.25.0.1
Other Non-Current Assets (Details) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Other Assets, Noncurrent Disclosure [Abstract] |
|
|
| Security deposits |
$ 982
|
$ 989
|
| Restricted cash |
$ 4,015
|
$ 4,014
|
| Restricted Cash, Noncurrent, Statement of Financial Position [Extensible Enumeration] |
Total other non-current assets
|
Total other non-current assets
|
| Other long-term assets |
$ 763
|
$ 1,419
|
| Total other non-current assets |
$ 5,760
|
$ 6,422
|
| X |
- DefinitionAmount of other miscellaneous assets expected to be realized or consumed after one year or normal operating cycle, if longer.
| Name: |
us-gaap_OtherAssetsMiscellaneousNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherAssetsNoncurrentDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477220/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionIndicates line item in statement of financial position that includes cash restricted to withdrawal or usage, classified as noncurrent. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrentAssetStatementOfFinancialPositionExtensibleList |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of an asset, typically cash, provided to a counterparty to provide certain assurance of performance by the entity pursuant to the terms of a written or oral agreement, such as a lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_SecurityDeposit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.25.0.1
Property, Equipment, and Intangible Assets - Summary of Property and Equipment, Net (Details) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
$ 46,415
|
$ 43,516
|
| Less: accumulated depreciation and amortization |
(26,897)
|
(18,195)
|
| Property and equipment, net |
19,518
|
25,321
|
| Capitalized internally developed software |
|
|
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
20,677
|
17,722
|
| Less: accumulated depreciation and amortization |
(15,500)
|
(9,400)
|
| Leasehold improvements |
|
|
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
11,120
|
11,102
|
| Machinery and equipment |
|
|
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
10,043
|
9,930
|
| Furniture and fixtures |
|
|
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
2,152
|
2,152
|
| Construction in progress |
|
|
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
2,394
|
2,566
|
| Other |
|
|
| Property and Equipment, Net |
|
|
| Property and equipment, gross |
$ 29
|
$ 44
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_SoftwareAndSoftwareDevelopmentCostsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LeaseholdImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_MachineryAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ConstructionInProgressMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_OtherCapitalizedPropertyPlantAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Property, Equipment, and Intangible Assets - Additional Information (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Property, Equipment, and Intangible Assets |
|
|
|
| Depreciation and amortization expense |
$ 8,900
|
$ 8,700
|
$ 5,900
|
| Accumulated amortization |
26,897
|
18,195
|
|
| Operating expenses |
|
|
|
| Property, Equipment, and Intangible Assets |
|
|
|
| Impairments of long-lived assets |
|
1,800
|
|
| Technology licenses |
|
|
|
| Property, Equipment, and Intangible Assets |
|
|
|
| Current portion of commitments related to intangible assets |
3,100
|
1,300
|
|
| Noncurrent portion of commitments related to intangible assets |
5,600
|
7,600
|
|
| Capitalized internally developed software |
|
|
|
| Property, Equipment, and Intangible Assets |
|
|
|
| Accumulated amortization |
15,500
|
9,400
|
|
| Property and equipment included amortization expense |
$ 6,100
|
$ 5,500
|
$ 3,300
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe aggregate amount of recurring noncash expense charged against earnings in the period to allocate the cost of assets over their estimated remaining economic lives. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_AdjustmentForAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483154/926-20-50-5
| Name: |
us-gaap_FiniteLivedIntangibleAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe adjustment to reduce the value of existing agreements that specify the lessee's rights to use the leased property. This expense is charged when the estimates of future profits generated by the leased property are reduced. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482130/360-10-45-4
| Name: |
us-gaap_ImpairmentOfLeasehold |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_OperatingIncomeLossMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IndefiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_TechnologyBasedIntangibleAssetsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_SoftwareAndSoftwareDevelopmentCostsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Property, Equipment, and Intangible Assets - Schedule Of Intangible Assets (Details) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Property, Equipment, and Intangible Assets |
|
|
| Net Carrying Amount |
$ 8,916
|
$ 10,317
|
| Technology licenses |
|
|
| Property, Equipment, and Intangible Assets |
|
|
| Gross Carrying Amount |
10,317
|
10,317
|
| Accumulated Amortization |
(1,401)
|
0
|
| Net Carrying Amount |
$ 8,916
|
$ 10,317
|
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 10
-Name Accounting Standards Codification
-Section S45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480265/350-10-S45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483154/926-20-50-5
| Name: |
us-gaap_FiniteLivedIntangibleAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accumulated amortization of intangible assets, excluding goodwill. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_IntangibleAssetsGrossExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_IndefiniteLivedIntangibleAssetsByMajorClassAxis=us-gaap_TechnologyBasedIntangibleAssetsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482640/350-30-55-40
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482665/350-30-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_FiniteLivedIntangibleAssetsFutureAmortizationExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
Accrued Expenses and Other Current Liabilities - Summary (Details) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Payables and Accruals [Abstract] |
|
|
| Employee compensation |
$ 11,192
|
$ 9,442
|
| Customer deposits |
1,909
|
1,613
|
| Accrued warranty liability |
498
|
297
|
| Non-income tax |
2,356
|
1,197
|
| Professional fees |
2,015
|
2,481
|
| Current portion of operating lease liabilities |
2,437
|
2,192
|
| Other |
$ 7,288
|
$ 6,203
|
| Operating Lease, Liability, Current, Statement of Financial Position [Extensible Enumeration] |
Total accrued expenses and other current liabilities
|
Total accrued expenses and other current liabilities
|
| Total accrued expenses and other current liabilities |
$ 27,695
|
$ 23,425
|
| X |
- DefinitionAmount of expenses incurred but not yet paid nor invoiced, and liabilities classified as other.
| Name: |
us-gaap_AccruedLiabilitiesAndOtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedProfessionalFeesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe current portion, due within one year or one operating cycle, if longer, of deposits held other than customer deposits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionIndicates line item in statement of financial position that includes current operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-2
| Name: |
us-gaap_OperatingLeaseLiabilityCurrentStatementOfFinancialPositionExtensibleList |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for estimated claims under standard and extended warranty protection rights granted to customers. For classified balance sheets, represents the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)(5)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
| Name: |
us-gaap_ProductWarrantyAccrualClassifiedCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.25.0.1
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_MovementInStandardAndExtendedProductWarrantyIncreaseDecreaseRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for estimated claims under standard and extended warranty protection rights granted to customers. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)(5)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
| Name: |
us-gaap_ProductWarrantyAccrual |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of decrease in the standard and extended product warranty accrual from payments made in cash or in kind to satisfy claims under the terms of the standard and extended product warranty. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
| Name: |
us-gaap_ProductWarrantyAccrualPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in the standard and extended product warranty accrual from changes in estimates attributable to preexisting product warranties. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Subparagraph (c)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-8
| Name: |
us-gaap_ProductWarrantyAccrualPreexistingIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- DefinitionThe threshold percent of shareholders voting for conversion of common stock.
| Name: |
bfly_CommonStockConversionAffirmativeVoteOfShareholdersPercent |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of Class A common shares issuable upon conversion for each share of Class B common shares to be converted.
| Name: |
bfly_CommonStockConversionRatio |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:pureItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe threshold percent of beneficial ownership of Class B common stock ceased to be held by the qualified stockholders for conversion of common stock.
| Name: |
bfly_CommonStockConversionThresholdBeneficialOwnershipCeasedToBeHeldByQualifiedShareholdersPercent |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of votes per share.
| Name: |
bfly_CommonStockVotingRightsPerShare |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate dividends declared during the period for each share of common stock outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_CommonStockDividendsPerShareDeclared |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Stock-Based Compensation - Additional Information (Details)
|
|
12 Months Ended |
|
|
Dec. 30, 2022
shares
|
Dec. 31, 2024
USD ($)
purchase_period
shares
|
Dec. 31, 2023
USD ($)
shares
|
Dec. 31, 2022
USD ($)
shares
|
Jun. 30, 2024
shares
|
| Equity Incentive Plan |
|
|
|
|
|
| Term of stock options |
|
10 years
|
|
|
|
| Stock-based compensation expense | $ |
|
$ 21,032,000
|
$ 27,480,000
|
$ 42,531,000
|
|
| Granted |
|
0
|
0
|
|
|
| Tax benefits of the stock-based compensation expense | $ |
|
$ 0
|
|
|
|
| Tax benefits from the exercise of stock options | $ |
|
0
|
|
|
|
| Capitalized stock-based compensation expense | $ |
|
400,000
|
$ 700,000
|
$ 1,000,000.0
|
|
| Stock-based compensation expense | $ |
|
$ 21,100,000
|
|
|
|
| Remaining weighted average vesting period |
|
1 year 10 months 24 days
|
|
|
|
| Class A Common Stock | Common Stock |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Common stock issued for employee stock purchase plan (in shares) |
|
671,435
|
|
|
|
| Nonemployee |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Granted |
|
0
|
0
|
0
|
|
| Service Based Stock Options |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Number of accelerated shares (in shares) |
1,700,000
|
|
|
|
|
| Expense related to acceleration | $ |
|
|
$ 7,800,000
|
|
|
| Service Based Stock Options | Maximum |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Term of stock options |
|
10 years
|
|
|
|
| Service Based Restricted Stock Options |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Number of accelerated shares (in shares) |
300,000
|
|
|
|
|
| Performance Based Restricted Stock Units |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Number of accelerated shares (in shares) |
100,000
|
|
|
|
|
| Performance Based Restricted Stock Units | Employee |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Share-based compensation arrangement by share-based payment award, equity instruments other than options, grants in period (in shares) |
|
|
|
200,000
|
|
| Outstanding restricted stock units |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Total fair value | $ |
|
$ 10,400,000
|
$ 7,300,000
|
$ 10,700,000
|
|
| Share-based compensation arrangement by share-based payment award, equity instruments other than options, grants in period (in shares) |
|
15,838,283
|
16,082,613
|
|
|
| Market-based restricted stock | Employee |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Share-based compensation arrangement by share-based payment award, equity instruments other than options, grants in period (in shares) |
|
1,000,000.0
|
1,800,000
|
|
|
| Stock-based compensation expense | $ |
|
$ 2,000,000.0
|
$ 2,500,000
|
|
|
| 2012 Plan |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Common Stock reserved for issuance (in shares) |
|
4,800,000
|
|
|
|
| 2020 Plan |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Common Stock reserved for issuance (in shares) |
|
53,500,000
|
|
|
|
| Common shares remain available for issuance (in shares) |
|
17,200,000
|
|
|
|
| ESPP 2024 |
|
|
|
|
|
| Equity Incentive Plan |
|
|
|
|
|
| Common stock reserved and available for issuance (in shares) |
|
|
|
|
4,200,000
|
| Length of total offering period |
|
24 months
|
|
|
|
| Number of offering periods | purchase_period |
|
4
|
|
|
|
| Length of individual offering periods |
|
6 months
|
|
|
|
| Purchase price percentage of closing market price on the first day of the offering period |
|
85.00%
|
|
|
|
| Purchase price percentage of closing market price on the day of purchase |
|
85.00%
|
|
|
|
| X |
- DefinitionShare-Based Compensation Arrangement by Share-Based Payment Award, Individual Offering Period
| Name: |
bfly_ShareBasedCompensationArrangementByShareBasedPaymentAwardIndividualOfferingPeriod |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-Based Compensation Arrangement by Share-Based Payment Award, Number of Offering Periods
| Name: |
bfly_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfOfferingPeriods |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-Based Compensation Arrangement by Share-Based Payment Award, Total Offering Period
| Name: |
bfly_ShareBasedCompensationArrangementByShareBasedPaymentAwardTotalOfferingPeriod |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost capitalized for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsCapitalizedAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of tax benefit for recognition of expense of award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationTaxBenefitFromCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of tax benefit from exercise of option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationTaxBenefitFromExerciseOfStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of additional cost recognized for award under share-based payment arrangement from occurrence of event accelerating recognition of cost.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAcceleratedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionDiscount rate from fair value on offering date that participants pay for shares. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardDiscountFromMarketPriceOfferingDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDiscount rate from fair value on purchase date that participants pay for shares. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardDiscountFromMarketPricePurchaseDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of share-based awards for which the grantee gained the right by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodTotalFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares authorized for issuance under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares for which recognition of cost was accelerated for award under share-based payment arrangement.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAcceleratedVestingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period as a result of an employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesEmployeeStockPurchasePlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_GranteeStatusAxis=us-gaap_ShareBasedPaymentArrangementNonemployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=bfly_ServiceBasedRestrictedStockUnitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_GranteeStatusAxis=us-gaap_ShareBasedPaymentArrangementEmployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=bfly_MarketBasedRestrictedStockUnitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=bfly_A2012PlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=bfly_A2020PlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=bfly_EmployeeStockPurchasePlan2024Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Stock-Based Compensation - Stock options (Details) - USD ($)
$ / shares in Units, $ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Number of Options |
|
|
|
| Outstanding at beginning of the period |
7,439,187
|
12,571,912
|
|
| Granted |
0
|
0
|
|
| Exercised |
(36,340)
|
(180,467)
|
|
| Forfeited |
(842,111)
|
(4,952,258)
|
|
| Outstanding at end of the period |
6,560,736
|
7,439,187
|
12,571,912
|
| Options exercisable |
6,222,073
|
6,537,433
|
|
| Weighted Average Exercise Price |
|
|
|
| Outstanding at beginning of year |
$ 6.17
|
$ 7.67
|
|
| Granted |
0
|
0
|
|
| Exercised |
1.77
|
1.26
|
|
| Forfeited |
8.98
|
10.14
|
|
| Outstanding at end of year |
5.88
|
6.17
|
$ 7.67
|
| Options exercisable |
$ 5.94
|
$ 5.88
|
|
| Weighted Average Remaining Contractual Term |
|
|
|
| Outstanding at the end of year |
3 years 6 months 21 days
|
4 years 8 months 4 days
|
5 years 7 months 13 days
|
| Options exercisable |
3 years 5 months 23 days
|
4 years 4 months 13 days
|
|
| Aggregate Intrinsic Value (in thousands) |
|
|
|
| Outstanding at beginning of year |
$ 0
|
$ 1,342
|
|
| Outstanding at end of year |
1,963
|
0
|
$ 1,342
|
| Options exercisable |
$ 1,799
|
$ 0
|
|
| X |
- References
+ Details
| Name: |
bfly_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAggregateIntrinsicValueAbstract |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bfly_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsWeightedAverageRemainingContractualTermAbstract |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of accumulated difference between fair value of underlying shares on dates of exercise and exercise price on options exercised (or share units converted) into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum risk-free interest rate assumption that is used in valuing an option on its own shares.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRateMaximum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe minimum risk-free interest rate assumption that is used in valuing an option on its own shares.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRateMinimum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_GranteeStatusAxis=us-gaap_ShareBasedPaymentArrangementNonemployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_GranteeStatusAxis=us-gaap_ShareBasedPaymentArrangementEmployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Stock-Based Compensation - Restricted stock units (Details) - $ / shares
|
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Outstanding restricted stock units |
|
|
|
| Number of Restricted Stock Units |
|
|
|
| Outstanding at beginning of the period |
15,569,983
|
9,961,291
|
|
| Granted |
15,838,283
|
16,082,613
|
|
| Vested |
(6,906,452)
|
(5,418,832)
|
|
| Forfeited |
(3,251,584)
|
(5,055,089)
|
|
| Outstanding at end of the period |
21,250,230
|
15,569,983
|
9,961,291
|
| Weighted Average Grant Date Fair Value |
|
|
|
| Outstanding at beginning of year |
$ 2.61
|
$ 4.55
|
|
| Granted |
1.10
|
2.23
|
|
| Vested |
2.69
|
4.09
|
|
| Forfeited |
2.34
|
3.65
|
|
| Outstanding at end of year |
$ 1.52
|
$ 2.61
|
$ 4.55
|
| Performance Based Restricted Stock Units | Employee |
|
|
|
| Number of Restricted Stock Units |
|
|
|
| Granted |
|
|
200,000
|
| Market-based restricted stock | Employee |
|
|
|
| Number of Restricted Stock Units |
|
|
|
| Granted |
1,000,000.0
|
1,800,000
|
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that were forfeited during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average fair value as of the grant date of equity-based award plans other than stock (unit) option plans that were not exercised or put into effect as a result of the occurrence of a terminating event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValueRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_GranteeStatusAxis=us-gaap_ShareBasedPaymentArrangementEmployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=bfly_MarketBasedRestrictedStockUnitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Stock-Based Compensation - Stock-based compensation expense (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Equity Incentive Plan |
|
|
|
| Total stock-based compensation expense |
$ 21,032
|
$ 27,480
|
$ 42,531
|
| Research and development |
|
|
|
| Equity Incentive Plan |
|
|
|
| Total stock-based compensation expense |
6,950
|
9,772
|
12,834
|
| Sales and marketing |
|
|
|
| Equity Incentive Plan |
|
|
|
| Total stock-based compensation expense |
4,871
|
4,260
|
5,974
|
| General and administrative |
|
|
|
| Equity Incentive Plan |
|
|
|
| Total stock-based compensation expense |
$ 9,211
|
$ 13,448
|
$ 23,723
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_SellingAndMarketingExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Net Loss Per Share - Summary (Details) - USD ($)
$ / shares in Units, $ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Numerator: |
|
|
|
| Allocation of undistributed earnings |
$ (72,492)
|
$ (133,700)
|
$ (168,723)
|
| Numerator for basic net loss per share – loss available to common stockholders |
(72,492)
|
(133,700)
|
(168,723)
|
| Numerator for diluted net loss per share – loss available to common stockholders |
$ (72,492)
|
$ (133,700)
|
$ (168,723)
|
| Denominator: |
|
|
|
| Weighted-average common shares outstanding (in shares) |
211,682,760
|
205,385,544
|
199,848,386
|
| Denominator for diluted net loss per share - weighted average common stock (in shares) |
211,682,760
|
205,385,544
|
199,848,386
|
| Basic net loss per share (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Diluted net loss per share (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Class A |
|
|
|
| Numerator: |
|
|
|
| Allocation of undistributed earnings |
$ (63,442)
|
$ (116,497)
|
$ (146,412)
|
| Numerator for basic net loss per share – loss available to common stockholders |
(63,442)
|
(116,497)
|
(146,412)
|
| Numerator for diluted net loss per share – loss available to common stockholders |
$ (63,442)
|
$ (116,497)
|
$ (146,412)
|
| Denominator: |
|
|
|
| Weighted-average common shares outstanding (in shares) |
185,255,823
|
178,958,607
|
173,421,449
|
| Denominator for diluted net loss per share - weighted average common stock (in shares) |
185,255,823
|
178,958,607
|
173,421,449
|
| Basic net loss per share (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Diluted net loss per share (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Class B |
|
|
|
| Numerator: |
|
|
|
| Allocation of undistributed earnings |
$ (9,050)
|
$ (17,203)
|
$ (22,311)
|
| Numerator for basic net loss per share – loss available to common stockholders |
(9,050)
|
(17,203)
|
(22,311)
|
| Numerator for diluted net loss per share – loss available to common stockholders |
$ (9,050)
|
$ (17,203)
|
$ (22,311)
|
| Denominator: |
|
|
|
| Weighted-average common shares outstanding (in shares) |
26,426,937
|
26,426,937
|
26,426,937
|
| Denominator for diluted net loss per share - weighted average common stock (in shares) |
26,426,937
|
26,426,937
|
26,426,937
|
| Basic net loss per share (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| Diluted net loss per share (in dollars per share) |
$ (0.34)
|
$ (0.65)
|
$ (0.84)
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities, and addition from assumption of issuance of common shares for dilutive potential common shares; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-40
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-40
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-40
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersDilutedAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of undistributed earnings (loss) allocated to participating securities for the basic earnings (loss) per share or per unit calculation under the two-class method. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 65
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-65
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 66
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-66
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
| Name: |
us-gaap_UndistributedEarningsLossAllocatedToParticipatingSecuritiesBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Net Loss Per Share - Anti-dilutive common equivalent shares (Details) - shares
|
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Net Loss Per Share |
|
|
|
| Total anti-dilutive common equivalent shares (in shares) |
50,412,065
|
43,661,860
|
43,185,893
|
| Outstanding options to purchase common stock |
|
|
|
| Net Loss Per Share |
|
|
|
| Total anti-dilutive common equivalent shares (in shares) |
6,560,736
|
7,439,187
|
12,571,912
|
| Outstanding restricted stock units |
|
|
|
| Net Loss Per Share |
|
|
|
| Total anti-dilutive common equivalent shares (in shares) |
21,250,230
|
15,569,983
|
9,961,291
|
| Outstanding employee stock purchase plan options |
|
|
|
| Net Loss Per Share |
|
|
|
| Total anti-dilutive common equivalent shares (in shares) |
1,948,409
|
0
|
0
|
| Outstanding warrants |
|
|
|
| Net Loss Per Share |
|
|
|
| Total anti-dilutive common equivalent shares (in shares) |
20,652,690
|
20,652,690
|
20,652,690
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
v3.25.0.1
Income Taxes - Additional Information (Details) - USD ($)
|
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Tax provision (benefit) |
$ (30,000.00)
|
$ 80,000.00
|
$ 40,000.00
|
| Federal tax provision |
0
|
0
|
0
|
| Valuation allowance related to net operating losses |
8,700,000
|
34,000,000.0
|
|
| Accrued interest or penalties |
0
|
0
|
|
| Accrued repayment tax credit, noncurrent |
900,000
|
900,000
|
|
| Other income (expense) |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| State of Massachusetts Life Sciences tax credit received |
500,000
|
|
$ 900,000
|
| Domestic |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Net operating loss ("NOL") carryforwards |
634,800,000
|
609,800,000
|
|
| Net operating loss ("NOL") carryforwards, subject to expire |
73,700,000
|
|
|
| Net operating loss ("NOL") carryforwards, carried forward indefinitely |
561,200,000
|
|
|
| Federal and state tax credits |
15,400,000
|
|
|
| State and local |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Net operating loss ("NOL") carryforwards |
446,100,000
|
$ 407,800,000
|
|
| Federal and state tax credits |
$ 2,000,000.0
|
|
|
| X |
- DefinitionThe amount of tax credit expected to be repaid classified as noncurrent.
| Name: |
bfly_AccruedRepaymentTaxCreditNoncurrent |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws, that are not subject to expiration.
| Name: |
bfly_OperatingLossCarryforwardsNotSubjectToExpiration |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws, that are subject to expiration.
| Name: |
bfly_OperatingLossCarryforwardsSubjectToExpiration |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash inflow from tax credit received from the state of Massachusetts Life Sciences Tax Incentive Program.
| Name: |
bfly_ProceedsFromTaxCredit |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of current federal tax expense (benefit) attributable to income (loss) from continuing operations. Includes, but is not limited to, current national tax expense (benefit) for non-US (United States of America) jurisdiction. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 6.I.7)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479360/740-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-SubTopic 10
-Topic 740
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
| Name: |
us-gaap_CurrentFederalTaxExpenseBenefit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of current foreign income tax expense (benefit) pertaining to income (loss) from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(1)(Note 1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-SubTopic 10
-Topic 740
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
| Name: |
us-gaap_CurrentForeignTaxExpenseBenefit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwardsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of the valuation allowance pertaining to the deferred tax asset representing potential future taxable deductions from net operating loss carryforwards for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_OperatingLossCarryforwardsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_OtherNonoperatingIncomeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
v3.25.0.1
Income Taxes - Net deferred tax assets (Details) - USD ($)
$ in Thousands |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Deferred tax assets |
|
|
| Net operating loss carryforwards |
$ 158,097
|
$ 151,230
|
| Tax credits |
18,114
|
16,288
|
| Stock compensation |
3,203
|
4,812
|
| Accruals and reserves |
4,106
|
2,061
|
| Inventory reserve |
15,114
|
15,207
|
| Lease liability |
5,562
|
6,114
|
| Depreciation |
99
|
2,197
|
| Capitalized tax R&E |
27,296
|
25,844
|
| Other |
4,183
|
1,581
|
| Total deferred tax assets |
235,774
|
225,334
|
| Valuation allowance |
(230,109)
|
(221,454)
|
| Total deferred tax assets |
5,665
|
3,880
|
| Deferred tax liabilities |
|
|
| Right-of-use asset |
(3,467)
|
(3,829)
|
| Software costs |
(1,839)
|
0
|
| Net deferred tax assets |
$ 359
|
$ 51
|
| X |
- DefinitionAmount of deferred tax asset attributable to taxable temporary differences from research and development costs.
| Name: |
bfly_DeferredTaxAssetsCapitalizedResearchAndDevelopmentCosts |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from lease obligations.
| Name: |
bfly_DeferredTaxAssetsLeaseLiability |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from inventory. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsInventory |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredTaxAssetsNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of valuation allowance, of deferred tax asset attributable to deductible temporary differences, classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from property, plant, and equipment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of a valuation allowances, of deferred tax assets attributable to deductible tax credit carryforwards including, but not limited to, research, foreign, general business, alternative minimum tax, and other deductible tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from reserves and accruals. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseReservesAndAccruals |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from capitalized software. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxLiabilitiesDeferredExpenseCapitalizedSoftware |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from leasing arrangements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxLiabilitiesLeasingArrangements |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredTaxLiabilitiesNetAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- References
+ Details
| Name: |
us-gaap_CompensationAndRetirementDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of discretionary contributions made by an employer to a defined contribution plan.
| Name: |
us-gaap_DefinedContributionPlanEmployerDiscretionaryContributionAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.25.0.1
Warrants (Details) - USD ($)
$ / shares in Units, $ in Thousands |
12 Months Ended |
Dec. 31, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Warrants |
|
|
|
| Change in fair value of warrant liabilities |
$ (1,859)
|
$ 4,544
|
$ 20,859
|
| Public Warrants |
|
|
|
| Warrants |
|
|
|
| Outstanding warrants |
13,799,357
|
|
|
| Warrants settleable in cash shares held for tender offer percentage |
50.00%
|
|
|
| Private Warrants |
|
|
|
| Warrants |
|
|
|
| Outstanding warrants |
6,853,333
|
|
|
| Warrants exercised |
0
|
|
|
| Fair market value trading days |
30 days
|
|
|
| Class A Common Stock | Public Warrants |
|
|
|
| Warrants |
|
|
|
| Shares called by warrants |
1
|
|
|
| Exercise price |
$ 11.50
|
|
|
| Warrant Redemption $0.01 | Public Warrants |
|
|
|
| Warrants |
|
|
|
| Warrants redemption price per warrant |
$ 0.01
|
|
|
| Warrants redemption period |
30 days
|
|
|
| Warrant Redemption $0.01 | Private Warrants |
|
|
|
| Warrants |
|
|
|
| Warrants redemption price per warrant |
$ 0.01
|
|
|
| Warrant Redemption $0.01 | Class A Common Stock | Public Warrants |
|
|
|
| Warrants |
|
|
|
| Stock price trigger |
$ 18.00
|
|
|
| Threshold trading days |
20 days
|
|
|
| Trading day period |
30 days
|
|
|
| Trading day period for cashless basis redemption |
10 days
|
|
|
| Warrant Redemption $0.10 | Public Warrants |
|
|
|
| Warrants |
|
|
|
| Warrants redemption price per warrant |
$ 0.10
|
|
|
| Warrants redemption period |
30 days
|
|
|
| Warrant Redemption $0.10 | Private Warrants |
|
|
|
| Warrants |
|
|
|
| Warrants redemption price per warrant |
$ 0.10
|
|
|
| Warrant Redemption $0.10 | Class A Common Stock | Public Warrants |
|
|
|
| Warrants |
|
|
|
| Stock price trigger |
$ 10.00
|
|
|
| X |
- DefinitionDuration of trading day period associated with warrant redemption in which holder exercises redemption on a "cashless basis."
| Name: |
bfly_CashlessBasisTradingDayPeriod |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants exercised during the period.
| Name: |
bfly_ClassOfWarrantOrRightExercisedNumber |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThreshold number of specified trading days for fair market value of common stock to trigger redemption.
| Name: |
bfly_ClassOfWarrantOrRightRedemptionFairMarketValueTradingDays |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWarrants redemption period, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days.
| Name: |
bfly_ClassOfWarrantOrRightRedemptionPeriod |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe price per share at which the warrants can be redeemed.
| Name: |
bfly_ClassOfWarrantOrRightRedemptionPricePerShare |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPrice of the entity's common stock which would be required to be attained for the redemption of warrants.
| Name: |
bfly_ClassOfWarrantOrRightRedemptionStockPriceTrigger |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThreshold number of specified trading days that common stock price to must exceed threshold price within a specified consecutive trading period to trigger redemption.
| Name: |
bfly_ClassOfWarrantOrRightRedemptionThresholdTradingDays |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of shares owned for warrants settleable in cash in tender offer.
| Name: |
bfly_PercentageOfSharesOwnedWarrantsSettleableInCash |
| Namespace Prefix: |
bfly_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDuration of trading day period associated with warrant redemption.
| Name: |
bfly_TradingDayPeriod |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ClassOfWarrantOrRightLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of securities into which each warrant or right may be converted. For example, but not limited to, each warrant may be converted into two shares.
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByEachWarrantOrRight |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=bfly_PublicWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=bfly_PrivateWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ScheduleOfSharesSubjectToMandatoryRedemptionBySettlementTermsAxis=bfly_WarrantRedemption0.01Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ScheduleOfSharesSubjectToMandatoryRedemptionBySettlementTermsAxis=bfly_WarrantRedemption0.10Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
| X |
- DefinitionAmount of increase (decrease) in current obligation for operating lease.
| Name: |
bfly_IncreaseDecreaseInCurrentOperatingLeaseLiability |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in noncurrent obligation for operating lease.
| Name: |
bfly_IncreaseDecreaseInNoncurrentOperatingLeaseLiability |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) of operating lease payments.
| Name: |
bfly_IncreaseDecreaseInOperatingLeasePayments |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in operating lease right of use asset.
| Name: |
bfly_IncreaseDecreaseInOperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLessee, Operating Lease, Number Of Leases Terminated
| Name: |
bfly_LesseeOperatingLeaseNumberOfLeasesTerminated |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of an asset, typically cash, provided to a counterparty to provide certain assurance of performance by the entity pursuant to the terms of a written or oral agreement, such as a lease, forfeited during the period.
| Name: |
bfly_SecurityDepositForfeited |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of gain (loss) on termination of lease before expiration of lease term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 40
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479092/842-20-40-1
| Name: |
us-gaap_GainLossOnTerminationOfLease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.25.0.1
| X |
- DefinitionAmount of lease cost recognized by lessee for lease contract. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of short-term lease cost, excluding expense for lease with term of one month or less. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_ShortTermLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of variable lease cost, excluded from lease liability, recognized when obligation for payment is incurred for finance and operating leases. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_VariableLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.25.0.1
Leases - Operating lease payments (Details)
$ in Thousands |
Dec. 31, 2024
USD ($)
|
| Operating lease payments |
|
| 2025 |
$ 3,664
|
| 2026 |
3,749
|
| 2027 |
3,835
|
| 2028 |
3,921
|
| 2029 |
3,454
|
| 2030 and thereafter |
9,354
|
| Total gross operating lease payments |
27,977
|
| Less: imputed interest |
(5,142)
|
| Total operating lease liabilities, reflecting the present value of net lease payments |
$ 22,835
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease due after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingLeaseLiabilitiesPaymentsDueAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.25.0.1
| X |
- Definition
+ References
+ Details
| Name: |
bfly_CashPaidForLeaseLiabilitiesAbstract |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of derecognition of operating lease liability.
| Name: |
bfly_DerecognitionOfOperatingLeaseLiability |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of derecognition of right-of-use assets.
| Name: |
bfly_DerecognitionOfRightOfUseAssets |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionNon Cash Activities Involving Right Of Use Assets and Lease Liabilities
| Name: |
bfly_NonCashActivitiesInvolvingRightOfUseAssetsAndLeaseLiabilitiesAbstract |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.25.0.1
| X |
- DefinitionAmount of prepaid vendor advances net of write downs.
| Name: |
bfly_PrepaidVendorAdvancesNetOfWriteDowns |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount utilized from accrual on purchase commitments accrued as liability.
| Name: |
bfly_UtilizationOfAccrualOnPurchaseCommitmentLiability |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe minimum amount the entity agreed to spend under the long-term purchase commitment.
| Name: |
us-gaap_LongTermPurchaseCommitmentAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_LongTermPurchaseCommitmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_LongTermPurchaseCommitmentByCategoryOfItemPurchasedAxis=us-gaap_InventoriesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.25.0.1
Subsequent Events - (Details) - USD ($)
$ / shares in Units, $ in Millions |
Jan. 29, 2025 |
Jan. 31, 2025 |
Dec. 31, 2024 |
Dec. 31, 2023 |
| Subsequent Event |
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
| Public offering price (in dollars per share) |
|
$ 3.15
|
|
|
| Gross proceeds from the sale of equity |
$ 86.9
|
|
|
|
| Proceeds from Issuance or Sale of Equity |
5.2
|
|
|
|
| Estimated offering expenses |
0.7
|
|
|
|
| Net proceeds |
$ 81.0
|
|
|
|
| Class A Common Stock |
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
| Common stock, par value (in dollars per share) |
|
|
$ 0.0001
|
$ 0.0001
|
| Common stock, shares issued (in shares) |
|
|
188,626,154
|
181,221,794
|
| Class A Common Stock | Subsequent Event |
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
| Issuance of shares (in shares) |
27,600,000
|
|
|
|
| Common stock, par value (in dollars per share) |
$ 0.0001
|
|
|
|
| Common stock, shares issued (in shares) |
3,600,000
|
|
|
|
| X |
- DefinitionGross Proceeds from the Sale of Equity
| Name: |
bfly_GrossProceedsFromTheSaleOfEquity |
| Namespace Prefix: |
bfly_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Butterfly Network (NYSE:BFLY)
Historical Stock Chart
From Feb 2025 to Mar 2025
Butterfly Network (NYSE:BFLY)
Historical Stock Chart
From Mar 2024 to Mar 2025