0001447028 False 0001447028 2023-11-09 2023-11-09 iso4217:USD xbrli:shares iso4217:USD xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________
FORM 8-K
_________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 9, 2023
_______________________________
Arbutus Biopharma Corporation
(Exact name of registrant as specified in its charter)
_______________________________
| British Columbia, Canada | 001-34949 | 98-0597776 |
| (State or Other Jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) |
701 Veterans Circle
Warminster, Pennsylvania 18974
(Address of Principal Executive Offices) (Zip Code)
(267) 469-0914
(Registrant's telephone number, including area code)
(Former name or former address, if changed since last report)
_______________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Shares, without par value | ABUS | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On November 9, 2023, Arbutus Biopharma Corporation (the "Company") issued a press release announcing that the Company and Barinthus Biotherapeutics plc (Nasdaq: BRNS), formerly Vaccitech plc, today announced a late breaking poster presentation at The American Association for the Study of Liver Diseases (AASLD) – The Liver Meeting® 2023. The poster contains preliminary data from the Phase 2a clinical trial (AB-729-202) combining Arbutus’ RNAi therapeutic, imdusiran (AB-729), with Barinthus Bio's T-cell stimulating immunotherapeutic, VTP-300, and standard-of-care nucleos(t)ide analogue (NA) therapy. A copy of the press release is filed herewith as Exhibit 99.1 and is incorporated by reference herein.
On November 9, 2023, the Company posted an updated corporate presentation on its website at www.arbutusbio.com. A copy of the presentation is filed herewith as Exhibit 99.2 and is incorporated by reference herein.
Item 9.01. Financial Statements and Exhibits.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | Arbutus Biopharma Corporation |
| | | |
| | | |
| Date: November 9, 2023 | By: | /s/ David C. Hastings |
| | | David C. Hastings |
| | | Chief Financial Officer |
| | | |
EXHIBIT 99.1
Arbutus Biopharma and Barinthus Bio Present Preliminary Data from Phase 2a Clinical Trial Combining Imdusiran with VTP-300 at AASLD - The Liver Meeting®
The combination of imdusiran and VTP-300 provides a meaningful reduction of HBsAg levels that are maintained well below baseline
Preliminary data in subset of patients given imdusiran and then VTP-300 show early signs of immune activation
WARMINSTER, Pa. and OXFORD, United Kingdom, Nov. 09, 2023 (GLOBE NEWSWIRE) -- Arbutus Biopharma Corporation (Nasdaq: ABUS), a clinical-stage biopharmaceutical company leveraging its extensive virology expertise to develop a cure for people with chronic hepatitis B virus (cHBV) infection, and Barinthus Biotherapeutics plc (Nasdaq: BRNS), formerly Vaccitech plc, a clinical-stage biopharmaceutical company developing novel T cell immunotherapeutic candidates designed to guide the immune system to overcome chronic infectious diseases, autoimmunity and cancer, today announced a late breaking poster presentation at The American Association for the Study of Liver Diseases (AASLD) – The Liver Meeting® 2023. The poster contains preliminary data from the Phase 2a clinical trial (AB-729-202) combining Arbutus’ RNAi therapeutic, imdusiran (AB-729), with Barinthus Bio's T-cell stimulating immunotherapeutic, VTP-300, and standard-of-care nucleos(t)ide analogue (NA) therapy.
Professor Man-Fung Yuen, D.Sc., M.D., Ph.D., Chief of the Division of Gastroenterology and Hepatology, University of Hong Kong, and a lead investigator of this Phase 2a clinical trial, stated, “These preliminary data are very encouraging and we look forward to additional follow-up of these patients which will provide insight into the possibility of a functional cure with this combination treatment regimen. It is well accepted that a combination therapy that can both reduce HBV surface antigen and stimulate an HBV-specific host immune response will be needed to provide a functional cure for patients with chronic HBV. These promising initial data show that the combination of imdusiran followed by VTP-300 reduces surface antigen from baseline and, importantly, the reduced levels are maintained compared to placebo controls. Although the patient numbers are small and the data are early to see the expected clinical effect of VTP-300 on surface antigen, it is impressive to see that some patients have more robust immune activation post-VTP-300 treatment compared to controls.”
Clinical trial AB-729-202 enrolled 40 non-cirrhotic, virally suppressed cHBV patients that were on stable NA therapy. The patients initially received imdusiran (60mg every 8 weeks) for 24 weeks and were then randomized to receive either VTP-300 or placebo at week 26 and 30 (and conditionally at week 38 if they experienced a >0.5 log10 decline in HBsAg between weeks 26 and 34), in addition to ongoing NA therapy. The preliminary data include a subset of patients that received the two dose VTP-300 regimen (28/40 patients) and available follow-up data to week 48 (12/40 patients) and showed the following:
- Robust reductions of HBsAg were seen during the imdusiran treatment period (-1.86 log10 mean reduction from baseline after 24 weeks of treatment). This decline in HBsAg is comparable to the declines seen with imdusiran in other clinical trials conducted to date.
- 97% of the imdusiran treated patients (33/34) had HBsAg <100 IU/mL at the time of the first VTP-300/placebo dose.
- VTP-300 treatment appears to contribute to the maintenance of low HBsAg levels in the early post-treatment period, as the mean HBsAg levels in the placebo group begin to increase starting ~12 weeks after the last dose of imdusiran.
- All VTP-300 treated patients have maintained HBsAg <100 IU/mL through week 48, 60% have maintained HBsAg <10 IU/mL, and all have qualified to stop NA therapy.
- Preliminary immunology data suggests HBV-specific T cell IFN-γ production is enhanced in patients receiving imdusiran plus VTP-300 compared to placebo.
The preliminary safety data from this trial demonstrate that imdusiran and VTP-300 were both safe and well-tolerated. There were no serious adverse events, Grade 3 or 4 adverse events or treatment discontinuations.
Dr. Karen Sims, Chief Medical Officer of Arbutus Biopharma, commented, “Imdusiran consistently delivers compelling efficacy and safety data in multiple Phase 2a populations and combinations. In this trial, all but one patient reached surface antigen levels below 100 IU/mL and one reached <LLOQ with 24 weeks of imdusiran plus NA therapy alone, which is a meaningful achievement as we believe lowering surface antigen is key to promoting host HBV-specific immune reawakening. As we continue to dose and follow these patients, I look forward to seeing the potential that imdusiran, VTP-300, and NA therapy can have on achieving a functional cure for patients with cHBV.”
Bill Enright, Chief Executive Officer of Barinthus Bio, added, “Although these are preliminary data, we can already see that VTP-300 appears to show a meaningful impact on sustaining low HBsAg in patients after imdusiran treatment, with clear differences shown between placebo and VTP-300. It’s very positive that we are seeing that all participants treated with imdusiran and VTP-300 have qualified to stop NA therapy, which really highlights VTP-300’s potential as an important component of a functional cure regimen.”
The above poster presentation can be accessed through the Publications section of the Arbutus Biopharma website at https://www.arbutusbio.com/publications/ or via Barinthus Bio website here https://investors.barinthusbio.com/events-presentations.
About imdusiran (AB-729), Arbutus’ Lead RNAi Therapeutic
Imdusiran is an RNA interference (RNAi) therapeutic specifically designed to reduce all HBV viral proteins and antigens including hepatitis B surface antigen, which is thought to be a key prerequisite to enable reawakening of a patient’s immune system to respond to the virus. Imdusiran targets hepatocytes using Arbutus’ novel covalently conjugated N-Acetylgalactosamine (GalNAc) delivery technology enabling subcutaneous delivery. Clinical data generated thus far has shown single and multiple doses of imdusiran to be generally safe and well-tolerated, while also providing meaningful reductions in hepatitis B surface antigen and hepatitis B DNA. Imdusiran is currently in multiple Phase 2a clinical trials.
About Barinthus Bio’s VTP-300
VTP-300 is an immunotherapeutic candidate consisting of an initial dose using the ChAdOx platform and a secondary dose(s) using MVA, both encoding multiple hepatitis B antigens, including full-length surface, modified polymerase and core antigens. VTP-300 is the first antigen-specific immunotherapy that has been shown to induce sustained reductions in HBsAg. Barinthus Bio is studying VTP-300 in combination with other agents, including siRNA and low-dose anti-PD-1 antibodies, to control the infection and counterbalance the immune suppression and T cell exhaustion in the liver caused by chronic HBV infection.
About HBV
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). HBV can cause chronic infection which leads to a higher risk of death from cirrhosis and liver cancer. Chronic HBV infection represents a significant unmet medical need. The World Health Organization estimates that over 290 million people worldwide suffer from chronic HBV infection, while other estimates indicate that approximately 2.4 million people in the United States suffer from chronic HBV infection. Approximately 820,000 people die every year from complications related to chronic HBV infection despite the availability of effective vaccines and current treatment options.
About Arbutus
Arbutus Biopharma Corporation (Nasdaq: ABUS) is a clinical-stage biopharmaceutical company leveraging its extensive virology expertise to identify and develop novel therapeutics with distinct mechanisms of action, which can be combined to provide a functional cure for patients with chronic hepatitis B virus (cHBV). We believe the key to success in developing a functional cure involves suppressing HBV DNA, reducing surface antigen, and boosting HBV-specific immune responses. Our pipeline of internally developed, proprietary compounds includes an RNAi therapeutic, imdusiran (AB-729), and an oral PD-L1 inhibitor, AB-101. Imdusiran has generated meaningful clinical data demonstrating an impact on both surface antigen reduction and reawakening of the HBV-specific immune response. Imdusiran is currently in two Phase 2a combination clinical trials. AB-101 is currently being evaluated in a Phase 1a/1b clinical trial. Additionally, we have identified compounds in our internal PD-L1 portfolio that could also be used in oncology indications. For more information, visit www.arbutusbio.com.
About Barinthus Bio
Barinthus Bio is a clinical-stage biopharmaceutical company developing novel T cell immunotherapeutic candidates designed to guide the immune system to overcome chronic infectious diseases, autoimmunity and cancer. The company stands apart through its broad pipeline, built around four proprietary platform technologies; ChAdOx, MVA, SNAP-TI and SNAP-CI. Barinthus Bio is advancing a pipeline of five product candidates across a diverse range of therapeutic areas, including: VTP-300, an immunotherapeutic candidate designed as a potential component of a functional cure for chronic hepatitis B viral (HBV) infection; VTP-200, a non-surgical product candidate for persistent high-risk human papillomavirus (HPV); VTP-1000, an autoimmune candidate designed to utilize the SNAP-TI platform to treat patients with celiac disease; VTP-850, a second-generation immunotherapeutic candidate designed to treat recurrent prostate cancer; VTP-1100, a preclinical cancer candidate designed to utilize the SNAP-CI platform to treat patients with HPV-related cancer. Barinthus Bio’s proven scientific expertise, high-value portfolio and focus on product development uniquely positions the company to navigate towards delivering treatments for patients with infectious diseases, autoimmunity and cancers that have a significant impact on their lives every day. For more information, visit www.barinthusbio.com.
Arbutus’ Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and forward-looking information within the meaning of Canadian securities laws (collectively, forward-looking statements). Forward-looking statements in this press release include statements about our belief that the key to success in developing a functional cure for cHBV involves suppressing HBV DNA, reducing surface antigen, and boosting HBV-specific immune responses; our future development plans for our product candidates; our program updates; the expected cost, timing and results of our clinical development plans and clinical trials with respect to our product candidates; our expectations with respect to clinical trial design and the release of data from our clinical trials and the expected timing thereof; our expectations and goals for our collaborations with third parties and any potential benefits related thereto, including with respect to the Phase 2a clinical trial combining imdusiran with VTP-300; and the potential for our product candidates to achieve success in clinical trials.
With respect to the forward-looking statements contained in this press release, Arbutus has made numerous assumptions regarding, among other things: the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the continued demand for Arbutus’ assets; and the stability of economic and market conditions. While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies, including uncertainties and contingencies related to the ongoing patent litigation matters.
Additionally, there are known and unknown risk factors which could cause Arbutus’ actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements contained herein. Known risk factors include, among others: the risk that anticipated pre-clinical studies and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested product candidate; Arbutus may elect to change its strategy regarding its product candidates and clinical development activities; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus’ products; economic and market conditions may worsen; uncertainties associated with litigation generally and patent litigation specifically; Arbutus and its collaborators may never realize the expected benefits of the collaborations, including with Barinthus Bio; and market shifts may require a change in strategic focus; and risks related to the sufficiency of Arbutus’ cash resources and its ability to obtain adequate financing in the future for its foreseeable and unforeseeable operating expenses and capital expenditures.
A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus’ Annual Report on Form 10-K, Arbutus’ Quarterly Reports on Form 10-Q and Arbutus’ continuous and periodic disclosure filings, which are available at www.sedar.com and at www.sec.gov. All forward-looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward-looking statements or to publicly announce the result of any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, except as required by law.
Barinthus Bio’s Forward-Looking Statements
This press release contains forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, which can generally be identified as such by use of the words “may,” “will,” “plan,” “forward,” “encouraging,” “believe,” “potential,” and similar expressions, although not all forward-looking statements contain these identifying words. These forward-looking statements include, without limitation, express or implied statements regarding: the company’s plans and strategy with respect to its pipeline and product candidates, including VTP-300 and the HBV003 clinical trial, and the potential benefits of VTP-300 for the treatment of chronic HBV. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to numerous risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, risks and uncertainties related to the success, cost and timing of the Company’s pipeline development activities and planned and ongoing clinical trials, the company’s ability to execute on its strategy, regulatory developments, the risk that the company may not realize the benefits related to its rebranding and name change, the company’s ability to fund its operations and access capital, global economic uncertainty, the conflict in Ukraine, and the conflict in Israel and Gaza, including disruptions in the banking industry, and other risks identified in the company’s filings with the Securities and Exchange Commission (the “SEC”), including its Annual Report on Form 10-K for the year ended December 31, 2022, its Quarterly Reports on Form 10-Q and subsequent filings with the SEC. The Company cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. The Company expressly disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
Arbutus - Investor and Media Contact
Lisa M. Caperelli
Vice President, Investor Relations
Phone: 215-206-1822
Email: lcaperelli@arbutusbio.com
Barinthus Bio Contacts
Investor Contacts:
Christopher M. Calabrese
LifeSci Advisors
+1 917-680-5608
ccalabrese@lifesciadvisors.com
Kevin Gardner
LifeSci Advisors
+1 617-283-2856
kgardner@lifesciadvisors.com
Media contact:
Audra Friis
Sam Brown, Inc.
+1 917-519-9577
audrafriis@sambrown.com
Company contact:
Jonothan Blackbourn
IR & PR Manager
Barinthus Biotherapeutics
ir@barinthusbio.com
Exhibit 99.2

NASDAQ: ABUS www.arbutusbio.com November 9, 2023 Corporate Presentation © 2023 Arbutus Biopharma, Inc.

Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 and Canadian securities laws . All statements that are not historical facts are hereby identified as forward - looking statements for this purpose and include, among others, statements relating to : the potential market opportunity for HBV ; Arbutus’ ability to meet a significant unmet medical need ; the sufficiency of Arbutus’ cash and cash equivalents for the anticipated durations ; the expected cost, timing and results of Arbutus’ clinical development plans and clinical trials, including its clinical collaborations with third parties ; the potential for Arbutus’ product candidates to achieve their desired or anticipated outcomes ; Arbutus’ expectations regarding the timing and clinical development of Arbutus’ product candidates, including its articulated clinical objectives ; the timeline to a combination cure for HBV ; Arbutus’ expectations regarding its technology licensed to third parties ; the expected timing and payments associated with strategic and/or licensing agreements ; the patent infringement lawsuits ; and other statements relating to Arbutus’ future operations, future financial performance, future financial condition, prospects or other future events . With respect to the forward - looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things : the timely receipt of expected payments ; the effectiveness and timeliness of pre - clinical studies and clinical trials, and the usefulness of the data ; the timeliness of regulatory approvals ; the continued demand for Arbutus’ assets ; and the stability of economic and market conditions . While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties, and contingencies including uncertainties and contingencies related to patent litigation matters . Forward - looking statements herein involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward - looking statements . Such factors include, among others : anticipated pre - clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate ; changes in Arbutus’ strategy regarding its product candidates and clinical development activities ; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus' products ; economic and market conditions may worsen ; uncertainties associated with litigation generally and patent litigation specifically ; market shifts may require a change in strategic focus ; and the parties may never realize the expected benefits of the collaborations . A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus' Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and Arbutus' periodic disclosure filings, which are available at www . sec . gov and at www . sedar . com . All forward - looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward - looking statements or to publicly announce the result of any revisions to any of the forward - looking statements contained herein to reflect future results, events or developments, except as required by law . 2 © 2023 Arbutus Biopharma, Inc.

Our Strategy for Value Creation Leverage the proven track record of success established with our team's expertise in understanding and treating viral infections by discovering and developing a differentiated pipeline of therapies targeting chronic HBV. Develop a combination therapy that includes antivirals and immunologics to provide a finite duration treatment for people with cHBV that results in >20% functional cure rate. HBV: Hepatitis B Virus | cHBV: chronic HB V 3 © 2023 Arbutus Biopharma, Inc.
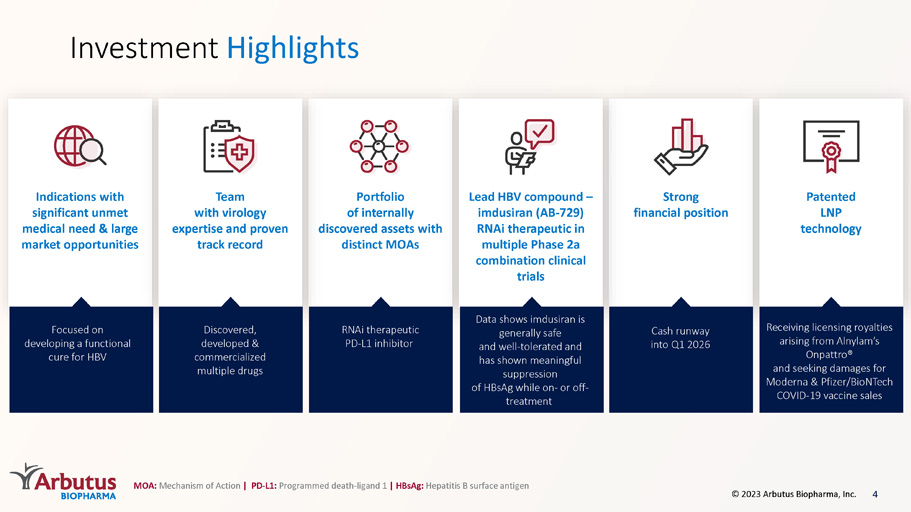
Investment Highlights Strong financial position Indications with significant unmet medical need & large market opportunities Patented LNP technology Portfolio of internally discovered assets with distinct MOAs Lead HBV compound – imdusiran (AB - 729) RNAi therapeutic in multiple Phase 2a combination clinical trials Team with virology expertise and proven track record Focused on developing a functional cure for HBV Cash runway into Q1 2026 Data shows imdusiran is generally safe and well - tolerated and has shown meaningful suppression of HBsAg while on - or off - treatment RNAi therapeutic PD - L1 inhibitor Receiving licensing royalties arising from Alnylam’s Onpattro ® and seeking damages for Moderna & Pfizer/BioNTech COVID - 19 vaccine sales Discovered, developed & commercialized multiple drugs MOA: Mechanism of Action | PD - L1: Programmed death - ligand 1 | HBsAg: Hepatitis B surface antigen 4 © 2023 Arbutus Biopharma, Inc.

Pipeline AB - 101 cHBV NA: Nucleoside Analogue 5 Imdusiran (AB - 729) cHBV AB - 729 - 201 Combo trial (imdusiran + Peg - IFN α - 2a + NA) AB - 729 - 202 Combo trial (imdusiran + vaccine + NA +/ - checkpoint inhibitor) AB - 729 - 001 single - ascending dose / multiple - ascending dose RNAi Therapeutic PD - L1 Inhibitor AB - 101 - 001 single - and multiple - ascending dose © 2023 Arbutus Biopharma, Inc. Lead Optimization IND Enabling Phase 1 Phase 2 Phase 3 Marketed

Africa 60M E Mediterranean 21M SE Asia 39M W Pacific 115M EU 15M Americas 7M ~820k people die every year as a consequence despite the availability of effective vaccines and antivirals. people are chronically infected with HBV, globally. >290M >290M Chronic HBV Sources: https://www.who.int/news - room/fact - sheets/detail/hepatitis - b https://www.hepb.org/what - is - hepatitis - b/what - is - hepb/facts - and - figures/ HBV Presents a Significant Unmet Medical Need 30M 6.6M 2.3% Treated Low due to sub - optimal SOC cure rate and asymptomatic nature of disease. 10.5% Diagnosed 2M USA 15M Europ e 90M China 6 SOC: Standard of Care © 2023 Arbutus Biopharma, Inc.

HBV Overview Life - threatening liver infection caused by hepatitis B virus (HBV) Transmitted through body fluids and from mother to child Long - term chronic infection ( cHBV ) leads to higher risk of cirrhosis and/or liver cancer Cause & Symptoms Diagnosis HBsAg detection Additional biomarkers necessary to determine stage of disease Treatments NA therapy – lifelong daily therapy, aimed at reducing HBV DNA and risk of cirrhosis and/or HCC Peg - IFN α – administered weekly; poorly tolerated <5% of patients achieve functional cure Rationale Need for finite and more efficacious HBV treatments that further improve long - term outcomes and increase functional cure rate Combination therapy with different MOAs will be required to reduce HBsAg, suppress HBV DNA, and boost immune system Sources for all data on slide: 1 Hepatitis B Fact Sheet, WHO https://www.who.int/news - room/fact - sheets/detail/hepatitis - b ; Hep B Foundation link https://www.hepb.org/what - is - hepatitis - b/what - is - hepb/facts - and - figures/ ; Kowdley et al. Hepatology (2012) Prevalence of Chronic Hepatitis B Among Foreign - Born Persons Living in the US by Country of Origin 2 Pegasys , PEG - Intron, Baraclude and Viread Package Inserts HBsAg : HBV Surface Antigen | HCC: Hepatocellular carcinoma 7 © 2023 Arbutus Biopharma, Inc.
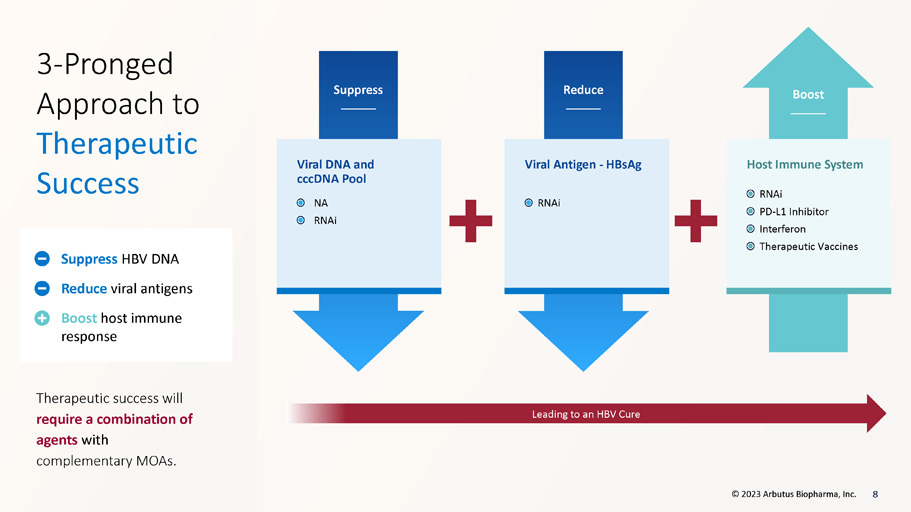
Suppress Reduce Boost Viral DNA and cccDNA Pool Viral Antigen - HBsAg Host Immune System Leading to an HBV Cure 3 - Pronged Approach to Therapeutic Success Therapeutic success will require a combination of agents with complementary MOAs. Suppress HBV DNA Reduce viral antigens Boost host immune response 8 NA RNAi RNAi RNAi PD - L1 Inhibitor Interferon Therapeutic Vaccines © 2023 Arbutus Biopharma, Inc.

9 RNAi Therapeutic

Proprietary GalNAc - conjugate delivery technology provides liver targeting and enables subcutaneous dosing Single trigger RNAi agent targeting all HBV transcripts Inhibits HBV replication and lowers all HBV antigens Pan - genotypic activity across HBV genotypes Demonstrated complementarity with other agents Actively targets the liver Active against cccDNA derived and integrated HBsAg transcripts Clean profile in long term preclinical safety studies RNAi Therapeutic Imdusiran GalNAc n Linker Polymerase, Core Ag, eAg , pgRNA sAg sAg HBx 10 © 2023 Arbutus Biopharma, Inc.
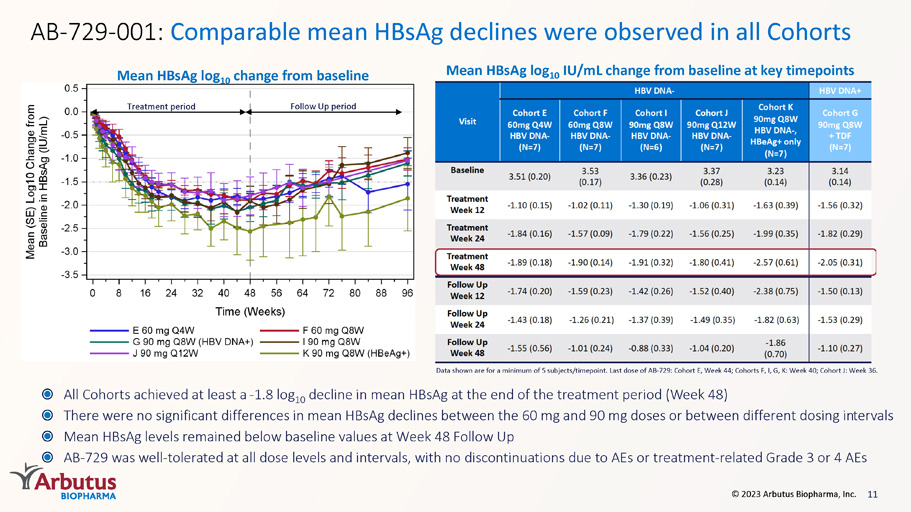
11 © 2023 Arbutus Biopharma, Inc. AB - 729 - 001: Comparable mean HBsAg declines were observed in all Cohorts All Cohorts achieved at least a - 1.8 log 10 decline in mean HBsAg at the end of the treatment period (Week 48) There were no significant differences in mean HBsAg declines between the 60 mg and 90 mg doses or between different dosing in ter vals Mean HBsAg levels remained below baseline values at Week 48 Follow Up AB - 729 was well - tolerated at all dose levels and intervals, with no discontinuations due to AEs or treatment - related Grade 3 or 4 AEs Data shown are for a minimum of 5 subjects/timepoint. Last dose of AB - 729: Cohort E, Week 44; Cohorts F, I, G, K: Week 40; Cohort J: Week 36. Mean HBsAg log 10 change from baseline Mean HBsAg log 10 IU/mL change from baseline at key timepoints Treatment period Follow Up period Data shown as mean (SE) log 10 IU/mL; minimum of 5 subjects/timepoint. Last AB-729 dose Cohort E: Week 44, Cohorts F, I, G, K: Week 40, Cohort J: Week 36; HBsAg Assay LLOQ = 0.07 IU/mL; *N=6; # N=5 Visit HBV DNA- HBV DNA+ CohortE 60mg Q4W HBV DNA- (N=7) Cohort F 60mg Q8W HBV DNA- (N=7) Cohort I 90mg Q8W HBV DNA- (N=6) Cohort J 90mg Q12W HBV DNA- (N=7) Cohort K 90mg Q8W HBV DNA-, HBeAg+ only (N=7) CohortG 90mg Q8W + TDF (N=7) Baseline 3.51 (0.20) 3.53 (0.17) 3.36 (0.23) 3.37 (0.28) 3.23 (0.14) 3.14 (0.14) Treatment Week 12 -1.10 (0.15) -1.02 (0.11) -1.30 (0.19) -1.06 (0.31) -1.63 (0.39) -1.56 (0.32) Treatment Week 24 -1.84 (0.16) -1.57 (0.09) -1.79 (0.22) -1.56 (0.25) -1.99 (0.35) -1.82 (0.29) Treatment Week 48 -1.89 (0.18) -1.90 (0.14) -1.91 (0.32) -1.80 (0.41) -2.57 (0.61) -2.05 (0.31) Follow Up Week 12 -1.74 (0.20) -1.59 (0.23) -1.42 (0.26) -1.52 (0.40) -2.38 (0.75) -1.50 (0.13) Follow Up Week 24 -1.43 (0.18) -1.26 (0.21) -1.37 (0.39) -1.49 (0.35) -1.82 (0.63) -1.53 (0.29) Follow Up Week 48 -1.55 (0.56) -1.01 (0.24) -0.88 (0.33) -1.04 (0.20) -1.86 (0.70) -1.10 (0.27)

AB - 729 - 001 : HBV Markers and ALT Levels Remain Low Long After Imdusiran Treatment in cHBV Patients Who Stopped All Therapy 7 of 9 (78%) subjects remain off NA therapy for 44 - 64 weeks and all completed imdusiran treatment over 1½ years ago Most subjects have maintained low HBV DNA levels off treatment 12 Data presented at GHS 2023 © 2023 Arbutus Biopharma, Inc. Patient 46 (Cohort E) Baseline HBsAg = 1392 IU/mL Patient 51 (Cohort F) Patient 61 (Cohort I) Baseline HBsAg = 2021 IU/mL HBsAg (IU/mL), ALT (U/L) HBV DNA (IU/mL) Patient 52 (Cohort F) Baseline HBsAg = 1888 IU/mL Patient 59 (Cohort G) Baseline HBsAg = 1338 IU/mL Patient 60 (Cohort G) Baseline HBsAg = 1128 IU/mL Patient 56 (Cohort G) Baseline HBsAg = 277 IU/mL HBsAg remains between - 0.8 and - 1.6 log 10 IU/mL below baseline values NA discontinuation post - imdusiran treatment appears well tolerated with no ALT flares HBV DNA (IU/mL) Baseline HBsAg = 6765 IU/mL
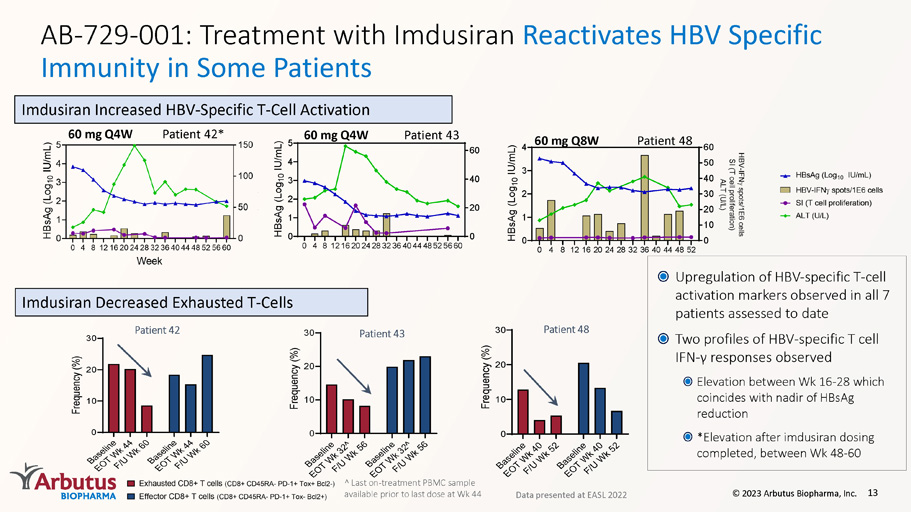
AB - 729 - 001 : Treatment with Imdusiran Reactivates HBV Specific Immunity in Some Patients 13 0 4 812162024283236404448525660 0 1 2 3 4 5 0 20 40 60 H B s A g ( L o g 1 0 I U / m L ) Patient 43 60 mg Q4W 0 4 8 1216202428323640444852 0 1 2 3 4 0 10 20 30 40 50 60 H B s A g ( L o g 1 0 I U / m L ) Patient 48 60 mg Q8W Upregulation of HBV - specific T - cell activation markers observed in all 7 patients assessed to date Two profiles of HBV - specific T cell IFN - γ responses observed Elevation between Wk 16 - 28 which coincides with nadir of HBsAg reduction *Elevation after imdusiran dosing completed, between Wk 48 - 60 Data presented at EASL 2022 Patient 42* 60 mg Q4W Imdusiran Increased HBV - Specific T - Cell Activation Imdusiran Decreased Exhausted T - Cells B a s e l i n e E O T W k 3 2 ^ F / U W k 5 6 B a s e l i n e E O T W k 3 2 ^ F / U W k 5 6 0 10 20 30 F r e q u e n c y ( % ) Patient 43 ^ Last on - treatment PBMC sample available prior to last dose at Wk 44 B a s e l i n e E O T W k 4 0 F / U W k 5 2 B a s e l i n e E O T W k 4 0 F / U W k 5 2 0 10 20 30 F r e q u e n c y ( % ) Patient 48 B a s e l i n e E O T W k 4 4 F / U W k 6 0 B a s e l i n e E O T W k 4 4 F / U W k 6 0 0 10 20 30 F r e q u e n c y ( % ) Patient 42 © 2023 Arbutus Biopharma, Inc.

AB - 729 - 001 Clinical Trial Key Takeaways Imdusiran provided robust and comparable HBsAg declines regardless of dose, dosing interval, HBeAg or DNA status Discontinuation of both imdusiran and NA - therapy results in sustained reduction in HBsAg and HBV DNA in 7 of 9 patients Imdusiran was generally safe and well - tolerated after completing dosing in 41 patients Imdusiran results in HBV - specific T - cell immune restoration and decrease of exhausted T - cells in some patients 14 * Data previously presented © 2023 Arbutus Biopharma, Inc.
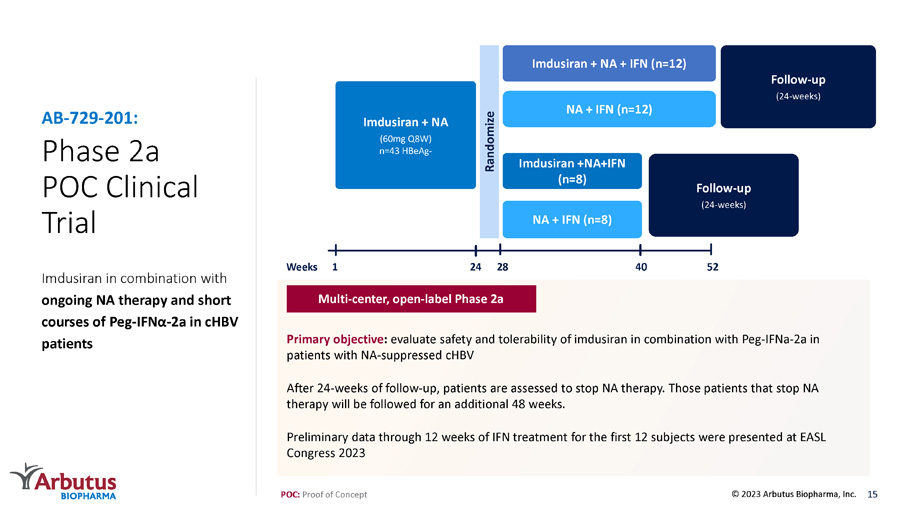
Phase 2a POC Clinical Trial Imdusiran in combination with ongoing NA therapy and short courses of Peg - IFN α - 2 a in cHBV patients AB - 729 - 201: Follow - up (24 - weeks) Imdusiran + NA + IFN (n=12) NA + IFN (n=12) Imdusiran +NA+IFN (n=8) NA + IFN (n=8) Imdusiran + NA (60mg Q8W) n=43 HBeAg - Randomize Follow - up (24 - weeks) 1 52 28 24 40 Weeks POC: Proof of Concept Primary objective : evaluate safety and tolerability of imdusiran in combination with Peg - IFNa - 2a in patients with NA - suppressed cHBV After 24 - weeks of follow - up, patients are assessed to stop NA therapy. Those patients that stop NA therapy will be followed for an additional 48 weeks. Preliminary data through 12 weeks of IFN treatment for the first 12 subjects were presented at EASL Congress 2023 Multi - center, open - label Phase 2a 15 © 2023 Arbutus Biopharma, Inc.

Individual and Mean HBsAg Results by Cohort Over Time 16 © 2023 Arbutus Biopharma, Inc. AB - 729 - 201: Imdusiran and IFN Treatment Led to Consistent HBsAg Declines After 24 Weeks Mean (SE) HBsAg log 10 Change from Baseline at Key Timepoints Data presented at EASL 2023 Preliminary results: Treatment was generally well tolerated with continued HBsAg declines in some patients during the IFN treatment period Mean HBsAg decline during lead - in phase was 1.6 log 10 at week 24 of treatment 93% of patients (38 of 41 randomized) had HBsAg levels <100 IU/mL during treatment period 4 patients reached HBsAg levels <LLOQ during IFN treatment

Phase 2a POC Clinical Trial POC Phase 2a clinical trial evaluating imdusiran in combination with Barinthus Bio’s (formerly Vaccitech ) immunotherapeutic, VTP - 300, NA, and with or without low dose nivolumab AB - 729 - 202: Primary objective: evaluate safety and reactogenicity of imdusiran followed by VTP - 300 or placebo At Week 48 all participants who are eligible to discontinue NA therapy will be followed for an additional 48 weeks Expanded the clinical trial to include an additional arm with nivolumab ( Opdivo ® ), and dosed first patient in this arm in the first half of 2023 Preliminary results presented at AASLD The Liver Meeting 2023 Full rights retained by the Companies of their respective product candidates and all costs split equally 17 Follow - up (24 - 48 weeks) VTP - 300 + NA (n=20) NA + placebo (n=20) 1 Imdusiran + NA (60mg Q8W) n=40 Randomize Weeks Imdusiran + NA (60mg Q8W) n=20 24 VTP - 300 + NA + Nivo 1 48 26 24 Weeks 48 Follow - up (24 - 48 weeks) © 2023 Arbutus Biopharma, Inc.

18 © 2023 Arbutus Biopharma, Inc. AB - 729 - 202: HBsAg Levels were Reduced and Sustained with Imdusiran and VTP - 300 Treatment Mean HBsAg Change from Baseline and Key Milestones Mean HBsAg Change from Baseline by Treatment Group Data presented at AASLD 2023 Preliminary results: Robust reductions of HBsAg were seen during the imdusiran treatment period, with 33/34 (97%) of patients <100 IU/mL at the time of VTP - 300/placebo administration VTP - 300 appears to maintain low HBsAg levels in the early post - treatment period, as the mean HBsAg levels in the placebo group begin to rebound starting ~12 weeks after the last dose of imdusiran All VTP - 300 treated patients have maintained HBsAg <100 IU/mL through Week 48, 60% have maintained HBsAg <10 IU/mL, and all have qualified to stop NA therapy

19 © 2023 Arbutus Biopharma, Inc. AB - 729 - 202: HBV - Specific T Cell Responses and Soluble Immune Biomarkers increased after VTP - 300 dosing Preliminary results: Elevations in HBV - specific T cell IFN - γ production were observed during imdusiran lead - in and after vaccination for n=7 patients profiled thus far Enhanced HBV - specific T cell responses were observed against HBsAg, PreS1/S2 peptides in VTP - 300 treated patients (n=4) Transient increases in other plasma immune biomarkers were also observed during imdusiran lead - in and vaccination period Data presented at AASLD 2023 Patient 30 (Group A/VTP - 300) experienced HBsAg decline and enhanced IFN - γ production (via ELISpot) after VTP - 300 through Week 48

Strategic Collaboration Exclusive Licensing* and Strategic Partnership Develop, manufacture and commercialize imdusiran in mainland China, Hong Kong, Macau and Taiwan Imdusiran Upfront payment (received in 2022) $40M Equity investment (received in 2022) $15M Commercialization and milestone payments Up to $245M Tiered royalties on annual sales Double - digit up to low twenties % *ABUS retains the non - exclusive right to develop and manufacture in the Qilu territory for exploiting AB - 729 in the rest of the world Deal economics for Arbutus: Qilu Pharmaceutical: One of the leading pharmaceutical companies in China, provides development, manufacturing, and commercialization expertise to this partnership China 20 © 2023 Arbutus Biopharma, Inc.

Oral PD - L1 Inhibitor 21

AB - 101: Oral PD - L1 Inhibitor for HBV Immune Reactivation PD - 1: Programmed death ligand protein | Abs: Antibodies Currently in a Phase 1a/1b clinical trial Rationale • HBV immune tolerance is a critical driver of cHBV infection • PD - 1:PD - L1 checkpoint axis plays a key role in immune tolerization in cHBV • PD - L1 expression upregulated during HBV infection • PD - 1 upregulated on HBV - specific T - and B - cells • Inhibition associated with HBsAg loss in some cHBV patients AB - 101 • Blocks PD - L1/PD - 1 interaction at sub - nM concentrations • Activates HBV - specific immune responses in T - cells from cHBV patients in vitro • Novel MOA identified • Demonstrates a robust checkpoint mediated in vivo effect • Improves HBV - specific T - and B - cell responses ex vivo Small - Molecule Inhibitor Approach • Allows controlled checkpoint blockade • Enables oral dosing • Designed to reduce systemic safety issues seen with Abs 22 © 2023 Arbutus Biopharma, Inc.

AB - 101: Small - Molecule Oral PD - L1 Inhibitor for HBV AB - 101 is highly potent and activates HBV specific immune cells from chronic HBV patients AB - 101 reinvigorates HBV - specific cHBV patient T - cells PBMCs N= cells from 9 cHBV patients *p< - 0.05 PBMC: P eripheral Blood Mononuclear Cells PDL1 * Inactive AB - 101 * 0 3 2 1 IFN - y Fold Increase Over HBV peptide alone 23 © 2023 Arbutus Biopharma, Inc. Once daily oral administration of AB - 101 resulted in profound tumor reduction Study Day MC38 Tumor Mouse Model Tumor Volume (mm 3 ) Data presented at EASL 2022

24 © 2023 Arbutus Biopharma, Inc. Part 1: SAD (n=8/cohort – 6:2) 1C: Dose 3 1B: Dose 2 1A: Dose 1 Part 2: MAD (n=10/cohort – 8:2) 2A: Do se ≤ dose tested in Part 1; interval TBD 2B: Dose/interval TBD 3A: Dose ≤ dose tested in Part 2; interval TBD x 28d 3B: Dose/interval TBD x 28d 3C: Dose/interval TBD x 28d AB - 101 - 001: Phase 1a/1b Clinical Trial with AB - 101 Parts 1 & 2 – Healthy Subjects Part 3 – cHBV Patients (n=12/cohort – 10:2) Virally suppressed Additional optional dose panels may be used.

2023 Key Milestones Cash balance * of $145M as of September 30, 2023, cash runway into Q1 2026; 2023 net cash burn of between $90M and $95M *Consists of cash, cash equivalents and marketable securities 25 Anticipated Timing 2023 Milestone 1H Imdusiran : Dose f irst patient in the imdusiran+VTP - 300+Nivo arm of the ongoing Phase 2a Vaccitech trial 1H Imdusiran: Preliminary IFN data from patients in the AB - 729 - 201 clinical trial 1H Imdusiran: Follow - up off - treatment data from AB - 729 - 001 clinical trial Q4 Imdusiran: Preliminary data from Phase 2a POC clinical trial with imdusiran+VTP - 300+NA therapy 2H AB - 101: Initiate single - ascending dose portion of Phase 1 clinical trial in healthy subjects © 2023 Arbutus Biopharma, Inc.

Thank You © 2023 Arbutus Biopharma, Inc.
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Arbutus Biopharma (NASDAQ:ABUS)
Historical Stock Chart
From Oct 2024 to Nov 2024
Arbutus Biopharma (NASDAQ:ABUS)
Historical Stock Chart
From Nov 2023 to Nov 2024