Milestone Scientific Announces 510(k) FDA Clearance for the Use of CompuFlo Epidural System in Thoracic Indications
February 27 2023 - 11:00AM

Milestone Scientific Inc. (NYSE:MLSS), a leading
developer of computerized drug delivery instruments that provides
painless and precise injections, today announced that its CompuFlo®
Epidural System has received 510(k) FDA clearance for use in the
thoracic region of the spine, including the cervical thoracic
junction. This approval expands upon the Company’s prior approval
of CompuFlo for use within the lumbar region of the spine, where
the focus has been on labor and delivery.
The use of the CompuFlo Epidural System, with its patented DPS
Dynamic Pressure Sensing Technology® in these newly expanded
indications, provides anesthesiologists and pain management
providers the ability to navigate challenging anatomical regions in
the thoracic region and cervical thoracic junction in real-time,
confirming needle placement both audibly and visually, thereby
making the epidural procedure easier and safer to perform. The
clinical and safety benefits of the CompuFlo Epidural System are
backed by extensive published clinical data demonstrating
significant reductions in dural punctures, as well as complication
rates, and contribute to savings of time on the part of providers.
An independent study has shown that the CompuFlo Epidural System
has the potential to reduce costs associated with morbidity,
providing a direct economic benefit to healthcare institutions.
Dr. Ronny Hertz, MD, an anesthesiologist and pain management
physician, as well as an advisor to Milestone Scientific, stated,
“Each year spinal cord injury cases are reported as a result of
epidural procedures, especially in the thoracic and cervical
regions. We believe this approval will be of benefit to physicians
who have been trained in the placement of epidurals in the thoracic
and cervical regions of the spine, including anesthesiologists and
pain management providers due to the difficulties accessing the
epidural space, especially in the higher thoracic cervical regions
of the spine. The use of the CompuFlo Epidural System in these
regions will add another level of safety, efficiency and confidence
as it audibly and visually measures tissue pressures in real time,
allowing a provider to confirm the needle placement. I believe it
will also be of tremendous assistance for spinal cord stimulator
cases.”
Dr. Hertz added, “The CompuFlo device should also be beneficial
in teaching residents and nurse anesthetists the proper placement
of epidural needles since it provides the instructor and student
audible and visual confirmation when the tip of the needle is in
the correct location. Every medical technique has risks, but it is
the job of the healthcare provider to try to reduce those risks,
and I believe CompuFlo has the potential to become the new standard
of care.”
Arjan Haverhals, Chief Executive Officer of
Milestone Scientific, noted, “We are delighted to receive marketing
clearance from the FDA for this new thoracic indication. Given the
extensive published clinical data supporting successful epidural
placement, we are pleased to expand the scope of indications beyond
labor and delivery into challenging thoracic and cervical epidural
procedures, where the incidence rates of morbidity are believed to
be much higher, at 17% and 30%, respectively. The FDA clearance
represents a major milestone as it broadens the market opportunity
for CompuFlo Epidural System, with approximately 11 million
epidural procedures performed in the U.S. on an annual basis. We
look forward to expanding the use of our technology within the area
of pain management and remain encouraged by the interest in our
epidural instruments by anesthesiologists and pain management
providers, especially for patients with complex anatomy and
difficult cases that involve the thoracic and cervical thoracic
junction.”
About Milestone Scientific
Inc.
Milestone Scientific Inc. (MLSS), a technology-focused medical
research and development company that patents, designs and develops
innovative injection technologies and instruments for medical,
dental and cosmetic applications. Milestone Scientific’s
computer-controlled systems are designed to make injections
precise, efficient and increase the overall patient comfort and
safety. Their proprietary DPS Dynamic Pressure Sensing Technology®
instruments is the platform to advance the development of
next-generation devices, regulating flow rate and monitoring
pressure from the tip of the needle, through platform extensions of
subcutaneous drug delivery, including local anesthetic. To learn
more, view the MLSS brand video or visit
milestonescientific.com.
Safe Harbor Statement This press release
contains forward-looking statements regarding the timing and
financial impact of Milestone's ability to implement its business
plan, expected revenues, timing of regulatory approvals and future
success. These statements involve a number of risks and
uncertainties and are based on assumptions involving judgments with
respect to future economic, competitive and market conditions,
future business decisions and regulatory developments, all of which
are difficult or impossible to predict accurately and many of which
are beyond Milestone's control. Some of the important factors that
could cause actual results to differ materially from those
indicated by the forward-looking statements are general economic
conditions, failure to achieve expected revenue growth, changes in
our operating expenses, adverse patent rulings, FDA or legal
developments, competitive pressures, changes in customer and market
requirements and standards, and the risk factors detailed from time
to time in Milestone's periodic filings with the Securities and
Exchange Commission, including without limitation, Milestone's
Annual Report for the year ended December 31, 2021. The
forward-looking statements in this press release are based upon
management's reasonable belief as of the date hereof. Milestone
undertakes no obligation to revise or update publicly any
forward-looking statements for any reason.
Contact:Crescendo Communications, LLCEmail:
mlss@crescendo-ir.comTel: 212-671-1020
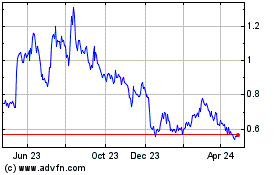
Milestone Scientific (AMEX:MLSS)
Historical Stock Chart
From Nov 2024 to Dec 2024
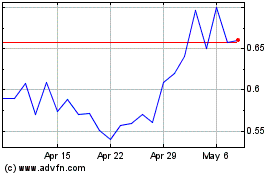
Milestone Scientific (AMEX:MLSS)
Historical Stock Chart
From Dec 2023 to Dec 2024