UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16 under the
Securities Exchange Act of 1934
February
25, 2025
Commission
File Number 001-14978
SMITH & NEPHEW plc
(Registrant’s
name)
Building 5, Croxley Park, Hatters Lane
Watford, England, WD18 8YE
(Address
of principal executive office)
Indicate
by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form
20-F ✓
Form 40-F
__
Smith+Nephew
Fourth Quarter and Full Year 2024 Results
Transformative 12-Point Plan delivering higher revenue growth,
margin expansion, strong cash flow and better returns; further
step-up in performance expected in 2025
25
February 2025
Smith+Nephew
(LSE:SN, NYSE:SNN), the global medical technology business, reports
results for the fourth quarter and full year ended 31 December
2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
31 Dec
|
|
31 Dec
|
|
Reported
|
|
Underlying
|
|
|
|
2024
|
|
2023
|
|
growth
|
|
growth
|
|
|
|
$m
|
|
$m
|
|
%
|
|
%
|
|
Fourth Quarter Results1,2
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
1,571
|
|
1,458
|
|
7.8
|
|
8.3
|
|
|
|
|
|
|
|
|
|
|
|
Full Year Results1,2
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
5,810
|
|
5,549
|
|
4.7
|
|
5.3
|
|
Operating
profit
|
|
657
|
|
425
|
|
54.6
|
|
|
|
Operating
profit margin (%)
|
|
11.3
|
|
7.7
|
|
|
|
|
|
EPS
(cents)
|
|
47.2
|
|
30.2
|
|
56.3
|
|
|
|
Cash
generated from operations
|
|
1,245
|
|
829
|
|
50.2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trading
profit
|
|
1,049
|
|
970
|
|
8.2
|
|
|
|
Trading
profit margin (%)
|
|
18.1
|
|
17.5
|
|
|
|
|
|
EPSA
(cents)
|
|
84.3
|
|
82.8
|
|
1.7
|
|
|
|
Free
cash flow
|
|
551
|
|
129
|
|
327.1
|
|
|
Deepak Nath, Chief Executive Officer, said:
“Smith+Nephew’s transformation remains on track with
the 12-Point Plan increasingly delivering better financial
performance. Revenue growth is consistently above historical levels
following operational and commercial improvements. Changes to our
organisational structure are driving increased accountability at
the Business Unit level. Operating leverage and productivity
improvements are supporting margin expansion despite significant
sector-wide headwinds. Working capital discipline and asset
utilisation have driven strong cash flow generation and better
returns.
“We finished the year strongly and US Reconstruction was
again sequentially better. Our innovation continued to deliver,
with more than 60% of revenue growth in 2024 coming from products
launched in the last five years. We have launched nearly 50 new
products over the last three years and have an exciting pipeline
for 2025.
“There
is much more to be done, but we have made solid progress fixing the
foundations and expect a step-up in returns in 2025, including
significant margin expansion. We are confident that this will be
the year when transformation starts to unlock substantial value for
our shareholders.”
Full Year
Highlights1,2
●
12-Point Plan
actions driving strong revenue growth and second year of trading
margin expansion, offsetting headwinds from inflation and
China
●
Around
410bps of incremental costs savings and a near 9% net reduction in
total workforce delivered across 2023 and 2024
●
2024 revenue of
$5,810 million (2023: $5,549 million), with underlying revenue
growth of 5.3%. Reported growth of 4.7% was after -60bps FX
headwind
●
Recent product
launches were a major contributor to higher growth, with many at
early stages of roll-out, and exciting pipeline to
come
●
Trading profit grew
8.2% to $1,049 million (2023: $970 million) with 18.1% trading
profit margin, up 60bps (2023: 17.5%). Reported operating profit
improved to $657 million (2023: $425 million)
●
Significant
improvements in cash generated from operations at $1,245 million
(2023: $829 million), trading cash flow at $999 million (2023: $635
million) and trading cash conversion, up to 95% (2023: 65%). Free
cash flow increased to $551 million (2023: $129
million)
●
Adjusted
Return on Invested Capital (ROIC) improved to 7.4% (2023: 5.9%),
with further progress expected in 2025
●
Restructuring
charges down significantly to $123 million (2023: $220
million)
●
Increase
in EPSA to 84.3¢ (2023: 82.8¢) and EPS to 47.2¢
(2023: 30.2¢)
●
Full
year dividend of 37.5¢ per share (2023: 37.5¢ per
share)
Q4 Trading
Highlights1,2
●
Q4 revenue of
$1,571 million (2023: $1,458 million),
with underlying revenue growth of 8.3%, which includes the
benefit of two extra trading days.
Reported growth of 7.8% was after -50bps FX
headwind
●
Strong finish to
year in all regions except China
●
US Reconstruction
performance continued to improve. Underlying and reported revenue
growth was 5.4% for Knee Implants and 7.6% for Hip
Implants
●
China headwind
sustained across Sports Medicine Joint Repair and Reconstruction,
as expected, reducing the Group
underlying revenue growth rate by -280bps
Outlook1,2
●
For
2025 we are targeting another year of strong revenue growth and a
significant step-up in trading profit margin
●
2025 underlying
revenue growth expected to be around 5% (reported growth of around 4.8%); first
quarter underlying revenue growth expected to be in the range of 1%
to 2% primarily due to continued China headwinds and one less
trading day, with acceleration thereafter
●
Full year trading
profit margin expected in the range of 19.0% to 20.0%; with margin
stronger in the second half than the first as impact of China
headwinds reduce and operational savings are delivered
●
Continued momentum
and efficiency gains expected to drive further margin expansion
beyond 2025
Analyst conference call
An analyst conference call to discuss Smith+Nephew’s fourth
quarter and full year results will be held 8.30am GMT / 3.30am EST
on 25 February 2025, details of which can be found on the
Smith+Nephew website at https://www.smith-nephew.com/en/about-us/investors.
Enquiries
|
|
|
|
Investors
|
|
|
Andrew Swift
|
+44 (0) 1923 477433
|
|
Smith+Nephew
|
|
|
|
|
|
Media
|
|
|
Charles Reynolds
|
+44 (0) 1923 477314
|
|
Smith+Nephew
|
|
|
|
|
|
Susan Gilchrist / Ayesha Bharmal
|
+44 (0) 20 7404 5959
|
|
Brunswick
|
|
Notes
1.
Unless
otherwise specified as ‘reported’ all revenue growth
throughout this document is ‘underlying’ after
adjusting for the effects of currency translation and including the
comparative impact of acquisitions and excluding disposals. All
percentages compare to the equivalent 2023 period.
‘Underlying
revenue growth’ reconciles to reported revenue growth, the
most directly comparable financial measure calculated in accordance
with IFRS, by making two adjustments, the ‘constant currency
exchange effect’ and the ‘acquisitions and disposals
effect’, described below. See Other Information on
pages 37 to 43 for a
reconciliation of underlying revenue growth to reported revenue
growth.
The
‘constant currency exchange effect’ is a measure of the
increase/decrease in revenue resulting from currency movements on
non-US Dollar sales and is measured as the difference between: 1)
the increase/decrease in the current year revenue translated into
US Dollars at the current year average exchange rate and the prior
revenue translated at the prior year rate; and 2) the
increase/decrease being measured by translating current and prior
year revenues into US Dollars using the prior year closing
rate.
The
‘acquisitions and disposals effect’ is the measure of
the impact on revenue from newly acquired material business
combinations and recent material business disposals. This is
calculated by comparing the current year, constant currency actual
revenue (which includes acquisitions and excludes disposals from
the relevant date of completion) with prior year, constant currency
actual revenue, adjusted to include the results of acquisitions and
exclude disposals for the commensurate period in the prior year.
These sales are separately tracked in the Group’s internal
reporting systems and are readily identifiable.
2.
Certain
items included in ‘trading results’, such as trading
profit, trading profit margin, tax rate on trading results, trading
cash flow, trading profit to trading cash conversion ratio, free
cash flow, adjusted ROIC, EPSA, leverage ratio and underlying
growth are non-IFRS financial measures. The non-IFRS financial
measures reported in this announcement are explained in Other
Information on pages 37 to 43 and are reconciled to the most
directly comparable financial measure prepared in accordance with
IFRS. Reported results represent IFRS financial measures as shown
in the Condensed Consolidated Financial Statements.
Smith+Nephew Fourth Quarter Trading and Full Year 2024
Results
Smith+Nephew’s transformative 12-Point Plan
is delivering revenue growth consistently above historical levels,
improved trading profit margin in the face of significant
headwinds, strong and substantially improved cash generation and
increased ROIC. Much of the 12-Point Plan is complete, and we have
addressed the structural weaknesses that were holding back the
Group. There is much more to be done to drive productivity and
asset efficiency to their full potential, with significant
additional benefit expected to follow in 2025 and
beyond.
Delivering higher revenue growth
Group revenue in 2024 was $5,810 million (2023: $5,549 million),
reflecting underlying revenue growth of 5.3%. Reported growth of
4.7% reflected a -60bps headwind from foreign exchange primarily
due to the strength of the US Dollar.
We delivered a strong finish to the year, with fourth quarter
underlying revenue growth of 8.3% and revenue of $1,571 million
(2023: $1,458 million). Fourth quarter reported revenue growth was
7.8% after a -50bps foreign exchange headwind.
The fourth quarter performance was ahead of our expectations driven
by a strong finish to the year, particularly in US Sports Medicine,
ENT and Advanced Wound Bioactives, and the benefit of two extra
trading days. The Group is now operationally and commercially more
able to benefit from better demand, as we saw at the end of the
quarter. As a result, we realised more of a benefit to our surgical
businesses from the two extra trading days than we expected to see
during the holiday season. US Hip Implants and US Knee Implants
both delivered a sequential improvement in performance in absolute
terms and on an adjusted day sales basis. China remained a headwind
in the quarter, as expected, reducing the fourth quarter Group
underlying revenue growth rate by -280bps.
Improved trading profit margin, cash generation and
ROIC
Trading profit for 2024 was up
8.2% to $1,049 million (2023: $970 million). The trading profit
margin was 18.1% (2023: 17.5%), a 60bps improvement on the prior
year. Operating profit increased to $657 million (2023: $425
million).
Over the last two years we have delivered an 80bps uplift in
trading profit margin. This was achieved as productivity savings of
around 410bps and operating leverage of around 390bps offset major
headwinds of around -490bps from input cost inflation and merit,
around -140bps from foreign exchange and around -90bps from
China.
Improving cash flow has been an area of specific focus, with good
progress made in 2024. Cash generated from operations was $1,245
million (2023: $829 million) and trading cash flow was $999 million
(2023: $635 million), with significantly better trading cash
conversion of 95% (2023: 65%). We have also reduced restructuring
costs year-on-year to $123 million (2023: $220 million). Free cash
flow increased to $551 million (2023: $129 million).
We have increased visibility and focus on improving our ROIC at the
Business Unit level through allocation of central costs and our
drive to improve working capital. ROIC increased year-on-year by
150bps to 7.4%, reflecting the progress made under the 12-Point
Plan. Going forward, we will continue to focus on driving further
improvement in our ROIC.
Delivering our Strategy for Growth and 12-Point Plan
Smith+Nephew’s Strategy for Growth is based on three
pillars:
●
First, Strengthen the foundations of
Smith+Nephew. A solid base in
commercial and manufacturing will enable us to serve customers
sustainably and efficiently, and deliver the best from our core
portfolio.
●
Second, Accelerate our growth
profitably, through more robust
prioritisation of resources and investment, and with continuing
customer focus.
●
Third, continue to Transform ourselves for higher
long-term growth, through
continued investment in innovation and
acquisitions.
In July 2022 we announced our 12-Point Plan to fundamentally change
the way Smith+Nephew operates, accelerating delivery of our
Strategy for Growth and transforming to a consistently
higher-growth company. The 12-Point Plan supports the first two
pillars of the Strategy for Growth and is focused on:
●
Fixing
Orthopaedics, to regain
momentum across hip and knee implants, robotics and trauma, and win
share with our differentiated technology;
●
Improving
productivity, to support
trading profit margin expansion; and
●
Further accelerating
growth in our already
well-performing Advanced Wound Management and Sports Medicine &
ENT business units.
There is clear evidence of the expected operational and financial
outcomes coming through across the Group. Revenue growth is
consistently above historical levels, built on improved product
supply and better commercial execution. Productivity is improving
with two years of margin expansion reflecting operating leverage
and efficiency savings which have more than offset major headwinds.
We have improved our capital intensity and cash conversion
supported by the better profitability, improving inventory
management and reduced restructuring costs. We have reengineered
our ability to match production to demand and are reducing capacity
in our manufacturing network. We have delivered a near 9% net
reduction in our total workforce since the start of the 12-Point
Plan. More than 1,000 of these role reductions were in 2024, with
the majority taking place in the final quarter of the
year.
The transformation programme remains on track and there is much
more to focus on, with further financial benefits supporting
trading margin expansion expected to follow this year and
beyond.
12-Point Plan: Delivering higher revenue growth
Through delivery of the 12-Point Plan we have transformed the
revenue growth profile of Smith+Nephew. This progress has been
achieved against some major headwinds, including the
underperformance in our US Orthopaedics business and the pressures
of Volume Based Procurement (VBP) programmes in China across both
our Reconstruction and Sports Medicine Joint Repair
segments.
The progress was underpinned by 12-Point Plan initiatives that
addressed a number of key issues that were holding back
performance. We have significantly improved product and instrument
set availability, which were far below industry standards, and have
now exceeded target levels. Overdue orders have improved
significantly, falling by 90% since 2022. The percentage of sets
that are available reached target at the start of the year, and
improved further during 2024. We also made significant progress
simplifying our portfolio, with a third of global hip and knee
brands now phased out.
We have also improved our commercial execution. We have turned
around performance in Trauma, which is now a significant growth
driver built upon our new EVOS◊
Plating System, and improved
Orthopaedics outside the US. US Orthopaedics is now also on a clear
improvement path. Here we have introduced new management, customer
service and satisfaction levels have improved, new growth-oriented
incentive plans are in place and employee turnover has returned to
low-levels. We strengthened our position in robotics with a series
of new features, and the installed base now exceeds 1,000
systems.
Sports Medicine has been outperforming its market for many years
built on sustainable and fundamental factors including commercial
excellence, a steady stream of innovation across procedures, new
segment development in tissue regeneration and successful
integration of acquired assets. Whilst we continued to face
significant VBP headwinds in China in 2024, the overall trajectory
for Sports Medicine remains encouraging.
In Advanced Wound Management we have delivered improved performance
in recent years based on better commercial execution focused on our
differentiated strengths, such as our unique portfolio breadth and
evidence-based selling. Performance in 2024 was driven by our
leading position in the high-growth Negative Pressure Wound Therapy
(NPWT) segment.
12-Point Plan: Improving organisational effectiveness
In 2023, we reorganised our global commercial operating model
around our three business units of Orthopaedics, Sports Medicine
& ENT, and Advanced Wound Management in order to drive more
agile decision making and greater accountability. In 2024, central
costs attributable to business units were directly allocated to
each business unit, with the objective of driving greater business
unit accountability and efficiency, with each business unit having
full profit and loss and capital accountability. These decisions
are already driving more informed investment decisions in areas
such as IT. A small proportion of the corporate costs continue to
be held centrally, reflecting the centralised infrastructure
required to support the Group and run a publicly listed
company.
These changes will support our drive to improve our ROIC at the
business unit level through allocation of central costs and
improved working capital.
12-Point Plan: Creating value through innovation
Smith+Nephew’s innovation pipeline is a significant
contributor to our transformation to being a higher growth
business. In 2024, more than 60% of underlying revenue growth came
from products launched in the last five years. In 2023, new
products accounted for around half of our underlying revenue
growth.
We maintained our recent high cadence of launches, with 16 new
products in 2024, bringing our total of new products to nearly 50
over the last three years. Many of these new platforms are driving
growth today and have multi-year runways still ahead of them as we
expand indication and applications and launch in new
markets.
Major launches in 2024 included the CATALYSTEM◊
Primary Hip System designed to address
the evolving demands of primary hip surgery, including the
increased adoption of anterior approach procedures. We moved to
full commercial launch of the AETOS◊
Shoulder System in the US, enabling us
to compete effectively in the fast-growing $1.7 billion shoulder
market, and continued to build out the platform adding planning
software and a stemless anatomic total shoulder option. We
announced new CORIOGRAPH◊
Pre-Operative Planning and Modelling
Services for the CORI◊
Surgical System, making it the only
orthopaedic robotic-assisted system to offer either intraoperative
image-free or image-based registration for knee implants. This is
one of a number of unique features for CORI, including supporting
revision knee procedures, a first-of-its-kind digital tensioner for
robotics-assisted knee surgery for soft tissue balancing and
offering both burr and saw cutting options.
In Sports Medicine, we completed the acquisition of CartiHeal, the
developer of the CARTIHEAL AGILI-C◊
Cartilage Repair Implant, a novel
sports medicine technology for cartilage regeneration in the knee.
We have made good progress on market development activities in the
first year of ownership, including clinical strategy and
reimbursement milestones. We have shown with
REGENETEN◊ that
we have the market development and commercialisation expertise to
acquire regenerative technologies and successfully establish a new
standard of care. In ENT, we launched the ARIS◊
COBLATION◊
Turbinate Reduction Wand. This
utilises our advanced COBLATION Plasma Technology to provide a
minimally invasive way to reduce hypertrophic turbinates, a
condition that requires 350,000 procedures per annum in the
US.
In Advanced Wound Management, we launched the
RENASYS◊
EDGE NPWT System. This is designed to
reduce inefficiency and complexity and features an improved user
interface for enhanced intuitiveness and simplicity and a durable
pump built to offer virtually maintenance free use. We also
continued our high cadence of incremental innovation in skin
substitutes, with the launch of GRAFIX◊
PLUS in the second quarter, an
easier-to-handle new version in our lead product family, targeting
the growing post-acute market.
An exciting pipeline of further innovation
In 2025 we expect to launch a number of exciting new products.
These include next-generation digital video-based navigation in the
arthroscopic tower, a new intramedullary nail and further
extensions to the CORI Surgical System and AETOS Shoulder
System.
Investing in clinical evidence
Clinical, scientific, and real-world evidence continue to play a
critical role in our go-to-market strategy, with compelling and
differentiating data supporting key brands in 2024. This included
the first randomised controlled trial with the
REGENETEN◊
Bioinductive Implant, market-leading
20-year survivorship data for OXINIUM◊
with highly cross-linked polyethylene
among all total hip replacement bearing combinations, and
impressive early results for the OR3O◊
Dual Mobility Hip, LEGION
CONCELOC◊
Cementless Knee, and revision total
knee arthroplasty using CORI. A systematic review and meta-analysis
showed that meniscal repair in selected patients aged ≥40
years had good success rates and patient-reported outcomes, similar
to those in patients aged <40 years, supporting our leading
meniscal repair business. We also published new data supporting the
use of PICO◊
Single Use Negative Pressure Wound
Therapy (NPWT) to reduce surgical site infections and using the
ALLEVYN◊
LIFE Dressing in a pressure injury
prevention protocol.
12-Point Plan: Cost efficiency and margin expansion
We have made significant productivity improvements through the
12-Point Plan, delivering around 410bps of incremental costs
savings across 2023 and 2024. Our trading profit margin has
expanded by 80 bps since 2022, driven by revenue leverage from the
higher revenue growth and operational efficiencies, successfully
making progress despite major headwinds from inflation, foreign
exchange and China.
Since 2022 we have improved our Sales, Inventory & Operations
Planning (SIOP) process. This is a dynamic process that has brought
better alignment of production plans and commercial delivery.
Further productivity improvements are expected to come through in
2025 as we benefit from costs savings from our decision to shut
four smaller Orthopaedics manufacturing facilities and our
reductions in workforce.
Looking beyond 2025, we expect our work to better align production
and commercial delivery along with capacity reduction, and the
timing of lower costs passing through inventory, to support further
margin expansion.
12-Point Plan: Improved cash generation and ROIC
In 2024 we made good progress improving both our trading and free
cash flow by reducing our capital expenditure, working capital and
restructuring costs, and we expect to make further progress in
2025. Through the 12-Point Plan we have improved our order to cash
and asset utilisation, and started to address our high inventory.
By the end of 2024, we had reduced our Day Sales of Inventory (DSI)
by 20 days year-on-year, with DSI down across all business units.
Further inventory improvements will be an area of continued focus
in 2025 to further enhance working capital and ROIC.
Group ROIC increased year-on-year by 150bps to 7.4% in 2024,
reflecting progress made under the 12-Point Plan. We anticipate
further improvement in 2025. We will continue to prioritise
investment in areas where we expect to see the highest incremental
returns on invested capital.
Better working capital movements resulted in significant
improvement in trading cash flow to $999 million (2023: $635
million), and the trading profit to cash conversion ratio improved
to 95% (2023: 65%). We reduced our restructuring costs to $123
million, down from $220 million in 2023, and expect a further
reduction in 2025 as 12-Point Plan charges come to an end. This was
a driver behind the significant increase in free cash flow to $551
million in 2024 (2023: $129 million).
Fourth Quarter 2024 Trading Update
Our fourth quarter revenue was $1,571 million (2023: $1,458
million), with underlying revenue growth of 8.3% (reported growth
of 7.8% after -50bps foreign exchange headwind). There were 62
trading days in the quarter, two more than Q4 2023. As stated
above, the fourth quarter performance was ahead of our expectations
driven by a strong finish to the year.
Geographically, our Established Markets underlying revenue growth
was 10.6% (reported growth 10.5%). Within this, the US underlying
revenue growth was 11.9% (reported growth 11.9%) and Other
Established Markets underlying revenue growth was 8.2% (reported
growth 7.9%). The Emerging Markets underlying revenue decline of
-2.3% (reported decline -5.1%) reflected the headwinds in China in
Reconstruction and Sports Medicine Joint Repair.
Fourth Quarter Consolidated Revenue Analysis
|
|
|
31
December
|
|
31
December
|
|
Reported
|
|
Underlying
|
|
Acquisitions
|
|
Currency
|
|
|
|
2024
|
|
2023
|
|
growth
|
|
growth(i)
|
|
/disposals
|
|
impact
|
|
Consolidated revenue by business unit by product
|
|
$m
|
|
$m
|
|
%
|
|
%
|
|
%
|
|
%
|
|
Orthopaedics
|
|
608
|
|
576
|
|
5.5
|
|
6.0
|
|
-
|
|
(0.5)
|
|
Knee
Implants
|
|
246
|
|
242
|
|
1.7
|
|
2.4
|
|
-
|
|
(0.7)
|
|
Hip
Implants
|
|
161
|
|
155
|
|
4.0
|
|
4.7
|
|
-
|
|
(0.7)
|
|
Other Reconstruction(ii)
|
|
39
|
|
31
|
|
23.7
|
|
23.9
|
|
-
|
|
(0.2)
|
|
Trauma
& Extremities
|
|
162
|
|
148
|
|
9.4
|
|
9.5
|
|
-
|
|
(0.1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sports Medicine & ENT
|
|
494
|
|
462
|
|
7.1
|
|
7.8
|
|
-
|
|
(0.7)
|
|
Sports
Medicine Joint Repair
|
|
267
|
|
256
|
|
4.4
|
|
5.3
|
|
-
|
|
(0.9)
|
|
Arthroscopic
Enabling Technologies
|
|
173
|
|
161
|
|
7.9
|
|
8.5
|
|
-
|
|
(0.6)
|
|
ENT
(Ear, Nose and Throat)
|
|
54
|
|
45
|
|
19.6
|
|
19.4
|
|
-
|
|
0.2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Advanced Wound Management
|
|
469
|
|
420
|
|
11.7
|
|
12.2
|
|
-
|
|
(0.5)
|
|
Advanced
Wound Care
|
|
187
|
|
185
|
|
1.2
|
|
1.9
|
|
-
|
|
(0.7)
|
|
Advanced
Wound Bioactives
|
|
179
|
|
149
|
|
20.1
|
|
20.3
|
|
-
|
|
(0.2)
|
|
Advanced
Wound Devices
|
|
103
|
|
86
|
|
19.9
|
|
20.6
|
|
-
|
|
(0.7)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
1,571
|
|
1,458
|
|
7.8
|
|
8.3
|
|
-
|
|
(0.5)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated revenue by geography
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US
|
|
881
|
|
788
|
|
11.9
|
|
11.9
|
|
-
|
|
-
|
|
Other Established Markets(iii)
|
|
453
|
|
420
|
|
7.9
|
|
8.2
|
|
-
|
|
(0.3)
|
|
Total Established Markets
|
|
1,334
|
|
1,208
|
|
10.5
|
|
10.6
|
|
-
|
|
(0.1)
|
|
Emerging Markets(iv)
|
|
237
|
|
250
|
|
(5.1)
|
|
(2.3)
|
|
-
|
|
(2.8)
|
|
Total
|
|
1,571
|
|
1,458
|
|
7.8
|
|
8.3
|
|
-
|
|
(0.5)
|
(i)
Underlying growth
is defined in Note 1 on page 3
(ii)
Other
Reconstruction includes robotics capital sales and bone
cement
(iii)
Other Established
Markets are Europe, Canada, Japan, Australia and New
Zealand
Fourth Quarter Business Unit Performance
Orthopaedics
Our Orthopaedics
business unit delivered underlying
revenue growth of 6.0% (reported growth 5.5%) in the
quarter.
Knee Implants underlying
revenue growth was 2.4% (reported growth 1.7%) and
Hip Implants
underlying revenue growth was 4.7%
(reported growth 4.0%). Knee Implants growth was driven by our
JOURNEY II◊
Total Knee System and by our
cementless and revision systems. Hip Implants growth was led by our
POLAR3◊
Total Hip Solution and
R3◊
Acetabular System.
Our US Reconstruction business continued to build momentum as we
increasingly benefit from the 12-Point Plan actions to improve
product and set availability and commercial execution. US Knee
Implants underlying revenue growth was 5.4% (reported growth 5.4%)
and US Hip Implants underlying revenue growth was 7.6% (reported
growth 7.6%) in the quarter, both sequentially improving over the
third quarter.
Outside the US, Knee Implants underlying revenue decline was -1.3%
(reported decline -2.8%) and Hip Implants underlying revenue growth
was 1.2% (reported decline -0.6%). As expected, China remained a
significant headwind to overall growth. Here, our distribution
partners have continued to reduce their holdings of implants,
following slow end-customer demand earlier in the year. Inventory
in the channel has come down significantly, but is not yet at
normalised levels, and as indicated previously, the largely-paused
ordering is likely to continue through the first quarter of 2025.
Other Established Markets continued its recent good momentum,
delivering its best quarterly performance of the year.
Other Reconstruction delivered
another good quarter of growth, with underlying revenue growth of
23.9% (reported growth 23.7%). This included strong growth across
robotics including another record quarter of placements of our CORI
Surgical System.
Trauma & Extremities underlying revenue growth was 9.5% (reported
growth 9.4%), returning to its recent stronger growth profile
following a slower third quarter, as expected. Performance
continued to be driven by the EVOS Plating System with a growing
contribution to growth coming from the roll-out of the new AETOS
Shoulder System.
Sports Medicine & ENT
Our Sports
Medicine & ENT business
unit delivered underlying revenue growth of 7.8% (reported growth
7.1%). Excluding China, Sports Medicine & ENT underlying
revenue growth was 14.0% (reported growth 13.1%). The segment
continued to face a headwind from the Sports Medicine Joint Repair
VBP programme in China, which commenced in May 2024. We expect this
VBP headwind to persist throughout the first half of 2025, as
previously flagged. We also expect an additional VBP process on
mechanical resection blades and COBLATION wands within Arthroscopic
Enabling Technologies in the second half of 2025, which we expect
to be around a $25 million revenue headwind in 2025 from price
impact and channel adjustments.
Sports Medicine Joint Repair underlying revenue growth was 5.3% (reported
growth 4.4%) led by strong double-digit growth from REGENETEN.
Excluding China, Sports Medicine Joint Repair underlying revenue
growth was 15.9% (reported growth 15.0%).
Arthroscopic Enabling Technologies underlying revenue growth was 8.5% (reported
growth 7.9%), with good growth from our COBLATION resection range
and patient positioning portfolio.
ENT underlying revenue growth
was 19.4% (reported growth 19.6%), a significant increase from the
previous quarter. This acceleration reflects some catch-up after
slow procedure volumes in the third quarter and a more normal
comparator. Growth was led by our tonsil and adenoid
business.
Advanced Wound Management
Our Advanced
Wound Management business unit
delivered underlying revenue growth of 12.2% (reported growth
11.7%), its strongest quarter of growth in
2024.
Advanced Wound Care underlying
revenue growth was 1.9% (reported growth 1.2%) with good growth
across our foam dressing and infection management portfolios offset
by skin care.
Advanced Wound Bioactives delivered underlying revenue growth of 20.3%
(reported growth 20.1%). The growth rate reflects typical quarterly
volatility in the category with strong double-digit growth in skin
substitutes following the launch of GRAFIX PLUS. We delivered
mid-single digit growth from SANTYL◊.
Advanced Wound Devices underlying revenue growth was 20.6% (reported
growth 19.9%) driven by both our traditional RENASYS Negative
Pressure Wound Therapy System and our single-use PICO Negative
Pressure Wound Therapy System.
Full Year Trading
Group revenue in 2024 was $5,810 million (2023: $5,549 million),
with underlying revenue growth of 5.3%. Reported growth of 4.7%
reflected a -60bps headwind from foreign exchange primarily due to
the strength of the US Dollar.
Geographically, our Established Markets underlying revenue growth
was 5.5% (reported growth 5.2%). Within this, US underlying revenue
growth was 4.8% (reported growth 4.8%) and Other Established
Markets underlying revenue growth was 6.7% (reported growth 6.0%).
Emerging Markets underlying revenue growth of 4.3% (reported growth
2.2%) included the impacts of the China headwinds described
above.
Full Year Consolidated Revenue Analysis
|
|
|
31
December
|
|
31
December
|
|
Reported
|
|
Underlying
|
|
Acquisitions
|
|
Currency
|
|
|
|
2024
|
|
2023
|
|
growth
|
|
Growth(i)
|
|
/disposals
|
|
impact
|
|
Consolidated revenue by business unit by product
|
|
$m
|
|
$m
|
|
%
|
|
%
|
|
%
|
|
%
|
|
Orthopaedics
|
|
2,305
|
|
2,214
|
|
4.1
|
|
4.6
|
|
-
|
|
(0.5)
|
|
Knee
Implants
|
|
947
|
|
940
|
|
0.7
|
|
1.3
|
|
-
|
|
(0.6)
|
|
Hip
Implants
|
|
619
|
|
599
|
|
3.2
|
|
4.0
|
|
-
|
|
(0.8)
|
|
Other Reconstruction(ii)
|
|
131
|
|
111
|
|
18.2
|
|
18.5
|
|
-
|
|
(0.3)
|
|
Trauma
& Extremities
|
|
608
|
|
564
|
|
7.9
|
|
8.1
|
|
-
|
|
(0.2)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sports Medicine & ENT
|
|
1,824
|
|
1,729
|
|
5.5
|
|
6.2
|
|
-
|
|
(0.7)
|
|
Sports
Medicine Joint Repair
|
|
982
|
|
945
|
|
4.0
|
|
4.8
|
|
-
|
|
(0.8)
|
|
Arthroscopic
Enabling Technologies
|
|
632
|
|
588
|
|
7.4
|
|
8.2
|
|
-
|
|
(0.8)
|
|
ENT
(Ear, Nose and Throat)
|
|
210
|
|
196
|
|
6.9
|
|
7.3
|
|
-
|
|
(0.4)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Advanced Wound Management
|
|
1,681
|
|
1,606
|
|
4.7
|
|
5.1
|
|
-
|
|
(0.4)
|
|
Advanced
Wound Care
|
|
735
|
|
725
|
|
1.4
|
|
2.0
|
|
-
|
|
(0.6)
|
|
Advanced
Wound Bioactives
|
|
581
|
|
553
|
|
5.1
|
|
5.1
|
|
-
|
|
-
|
|
Advanced
Wound Devices
|
|
365
|
|
328
|
|
11.5
|
|
12.2
|
|
-
|
|
(0.7)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
5,810
|
|
5,549
|
|
4.7
|
|
5.3
|
|
-
|
|
(0.6)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated revenue by geography
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US
|
|
3,123
|
|
2,979
|
|
4.8
|
|
4.8
|
|
-
|
|
-
|
|
Other Established Markets(iii)
|
|
1,707
|
|
1,611
|
|
6.0
|
|
6.7
|
|
-
|
|
(0.7)
|
|
Total Established Markets
|
|
4,830
|
|
4,590
|
|
5.2
|
|
5.5
|
|
-
|
|
(0.3)
|
|
Emerging Markets(iv)
|
|
980
|
|
959
|
|
2.2
|
|
4.3
|
|
-
|
|
(2.1)
|
|
Total
|
|
5,810
|
|
5,549
|
|
4.7
|
|
5.3
|
|
-
|
|
(0.6)
|
(i)
Underlying growth
is defined in Note 1 on page 3
(ii)
Other
Reconstruction includes robotics capital sales and bone
cement
(iii)
Other Established
Markets are Europe, Canada, Japan, Australia and New
Zealand
Full Year Business Unit Performance
Orthopaedics
Our Orthopaedics business unit delivered underlying revenue growth
of 4.6% (reported growth 4.1%) for the full year.
Knee Implants underlying
revenue growth was 1.3% (reported growth 0.7%) and
Hip Implants
underlying revenue growth was 4.0%
(reported growth 3.2%).
Knee Implants and Hip Implant growth was driven by performance in
Other Established Markets and a significant improvement in the US
over the course of the year. These changes reflected the
operational progress in product supply and sharper commercial
execution following the 12-Point Plan. Performance was held back by
China, where we saw reduced end-customer demand in the second half
of the year, resulting in orders from our distribution partners
significantly slowing as they reduced stock-levels in response.
2024 Knee Implants growth was driven by our JOURNEY II Total Knee
System and by our cementless and revision systems. Hip growth was
led by our POLAR3 Total Hip Solution and R3 Acetabular
System.
Other Reconstruction underlying
revenue growth was 18.5% (reported growth 18.2%) for the full year.
Performance principally reflects sales of our robotics-assisted
CORI Surgical System and consumables.
Trauma & Extremities underlying revenue growth was 8.1% (reported
growth 7.9%) for the full year. Following 12-Point Plan actions to
improve product supply and turnaround performance in Trauma &
Extremities, this business was a significant growth driver in 2024.
Progress was driven by our investment to build out the EVOS Plating
System. We also continued to successfully roll-out the launch of
the AETOS Shoulder System, entering a high growth category in
Orthopaedics.
Sports Medicine & ENT
Our Sports
Medicine & ENT business
unit delivered underlying revenue growth of 6.2% (reported growth
5.5%) for the full year. Sports Medicine & ENT underlying
revenue growth was 10.0% (reported growth 9.3%) excluding China,
where the implementation of VBP was a headwind, as noted
above.
Sports Medicine Joint Repair underlying revenue growth was 4.8% (reported
growth 4.0%) for the full year. Outside of China, Sports Medicine
Joint Repair had another strong year driven by our knee repair
portfolio and the REGENETEN Bioinductive Implant. Excluding China,
Sports Medicine Joint Repair underlying revenue growth was 11.3%
(reported growth 10.6%).
Arthroscopic Enabling Technologies underlying revenue growth was 8.2% (reported
growth 7.4%). This was significantly ahead of the prior year,
driven by our arthroscopic tower and COBLATION
technologies.
ENT underlying revenue growth
was 7.3% (reported growth 6.9%). Growth was led by our tonsil and
adenoid business.
Advanced Wound Management
Our Advanced
Wound Management business unit
delivered underlying revenue growth of 5.1% (reported growth 4.7%)
in 2024.
Advanced Wound Care underlying
revenue growth was 2.0% (reported growth 1.4%). Growth was driven
by good performances in foam dressings and infection management
categories.
Advanced Wound Bioactives underlying revenue growth was 5.1% (reported
growth 5.1%). SANTYL◊
delivered growth for the full year,
although we continued to see quarter-to-quarter variability, a
long-term feature of this product. We delivered double-digit growth
from our skin substitutes business following the launch of GRAFIX
PLUS.
Advanced Wound Devices underlying revenue growth was 12.2% (reported
growth 11.5%). This was driven by both our traditional RENASYS
Negative Pressure Wound Therapy System and our single-use PICO
Negative Pressure Wound Therapy System, and from our
LEAF◊
Patient Monitoring System as we
continued to expand the market in pressure injury
prevention.
Full Year 2024 Consolidated Analysis
Smith+Nephew
results for the year ended 31 December 2024:
|
|
|
|
|
|
|
Reported
|
|
|
|
2024
|
|
2023
|
|
growth
|
|
|
|
$m
|
|
$m
|
|
%
|
|
Revenue
|
|
5,810
|
|
5,549
|
|
4.7
|
|
Operating profit
|
|
657
|
|
425
|
|
54.6
|
|
Acquisition
and disposal related items
|
|
94
|
|
60
|
|
|
|
Restructuring
and rationalisation costs
|
|
123
|
|
220
|
|
|
|
Amortisation
and impairment of acquisition intangibles
|
|
187
|
|
207
|
|
|
|
Legal
and other
|
|
(12)
|
|
58
|
|
|
|
Trading profit(i)
|
|
1,049
|
|
970
|
|
8.2
|
|
|
|
¢
|
|
¢
|
|
|
|
Earnings per share ('EPS')
|
|
47.2
|
|
30.2
|
|
|
|
Acquisition
and disposal related items
|
|
11.2
|
|
7.3
|
|
|
|
Restructuring
and rationalisation costs
|
|
10.8
|
|
20.7
|
|
|
|
Amortisation
and impairment of acquisition intangibles
|
|
16.6
|
|
18.6
|
|
|
|
Legal
and other
|
|
(1.5)
|
|
6.0
|
|
|
|
Adjusted Earnings per share ('EPSA')(i)
|
|
84.3
|
|
82.8
|
|
7.7
|
(i)
See Other Information on pages 37 to
43
Full Year 2024 Analysis
Group revenue for 2024 was $5,810 million (2023: $5,549 million),
reflecting underlying revenue growth of 5.3%. Reported growth of
4.7% reflected a -60bps headwind from foreign exchange primarily
due to the strength of the US Dollar.
The gross profit was $3,996 million (2023: $3,819 million) with a
gross profit margin of 69.6% (2023: 68.8%). Operating profit
increased to $657 million (2023: $425 million) after acquisition
and disposal related items, restructuring and rationalisation
costs, amortisation and impairment of acquisition intangibles and
legal and other items (see Other Information on pages 37 to
43).
Trading profit was up 8.2% to $1,049 million (2023: $970 million),
with a trading profit margin of 18.1% (2023: 17.5%). The 60bps
margin expansion reflects the benefits of revenue leverage and
manufacturing, distribution and operating expense savings offset by
input cost inflation and China VBP (see Note 2 to the Financial
Statements for global business unit trading profit).
Acquisition and disposal-related items primarily relate to
impairment of BHR goodwill, discontinuation of certain products and
integration costs relating to the CartiHeal acquisition (see Note 2
to the Financial Statements).
Restructuring costs significantly decreased year-on-year to $123
million (2023: $220 million), and included costs related to the
efficiency and productivity work underway across the Group under
the 12-Point Plan.
The net interest charge within reported results was $121 million
(2023: $98 million) which reflects the recent maturities of low
coupon debt and the increased interest rate
environment.
Reported tax for the year to 31 December 2024 was a charge of $86
million (2023: $27 million). The tax rate on trading results was
19.1% (2023: 16.2%) (see Note 3 to the Financial Statements and
Other Information on pages 37 to 43 for further details on
taxation).
Adjusted earnings per share (‘EPSA’) increased to
84.3¢ (168.6¢ per ADS) (2023: 82.8¢ per share).
Basic earnings per share (‘EPS’) was 47.2¢
(94.4¢ per ADS) (2023: 30.2¢ per share), reflecting
restructuring costs, acquisition and disposal related items,
amortisation and impairment of acquisition intangibles and legal
and other items incurred.
Cash generated from operations was up significantly to $1,245
million (2023: $829 million) and trading cash flow was up to $999
million (2023: $635 million). The increase was primarily driven by
lower working capital costs, including in inventory, and higher
payables. Capital expenditure was also lower versus an elevated
level of investment in 2023 (see Other Information on pages 37 to
43 for a reconciliation between cash generated from operations and
trading cash flow). As a result of the working capital movement,
the trading profit to cash conversion ratio improved to 95% (2023:
65%).
The Group’s net debt, excluding lease liabilities, was $2,513
million at 31 December 2024, with access to committed facilities of
$4.1 billion (see Note 6 to the Financial Statements). Our adjusted
leverage ratio for 2024 was 1.9x.
Dividend and Capital Allocation Framework
The Board is recommending a Final Dividend of 23.1¢ per share
(46.2¢ per ADS) (2023: 23.1¢ per share). Together with
the Interim Dividend of 14.4¢ per share (28.8¢ per ADS),
this will give a total distribution of 37.5¢ per share
(75.0¢ per ADS), unchanged from 2023. Subject to confirmation
at our Annual General Meeting, the Final Dividend will be paid on
28 May 2025 to shareholders on the register at the close of
business on 28 March 2025.
The appropriate use of capital on behalf of shareholders is
important to Smith+Nephew. In July 2024, we announced an updated
capital allocation framework to prioritise the use of cash and
inform our investment decisions.
Our first priority remains investing in the business to drive
organic growth and meet our sustainability targets. The second
priority is also unchanged, and is to invest in acquisitions,
targeting new technologies in high growth segments with a strong
strategic fit that meet our financial criteria. The third priority
is to maintain an optimal balance sheet and appropriate dividend.
Here we will continue to target investment grade credit ratings
with a target leverage ratio of around 2x net debt to adjusted
EBITDA. We have a progressive dividend policy and from 2025 onwards
we expect a payout of around 35% to 40% of EPSA. The interim
payment will be 40% of the prior full year. Our final priority
remains to return any surplus capital to shareholders, via a share
buyback subject to the above balance sheet metrics.
2025 Outlook
For 2025 we are targeting another year of revenue growth above
historical levels and a significant step-up in trading profit
margin.
For revenue, we expect to deliver underlying revenue growth of
around 5%. Within this, we expect ongoing improvement from US
Reconstruction and continued strong growth from Sports Medicine
outside of China, ENT and Advanced Wound Management offset by the
impact of the anticipated China VBP extension into Arthroscopic
Enabling Technologies (estimated $25 million revenue headwind in
2025). There will be one fewer trading day in 2025 than in 2024.
The guidance equates to reported growth of around 4.8% based on
exchange rates prevailing on 19 February 2025.
In terms of phasing, we expect the headwinds from China to continue
in Reconstruction for the first quarter, and in Sports Medicine
Joint Repair into the second quarter as we lap VBP implementation.
We expect that some of the strong finish to 2024, particularly in
US Sports Medicine and Advanced Wound Bioactives, will normalise in
the first quarter. In addition, we have one fewer trading day in
each of the first and second quarters versus 2024, then one extra
day in the fourth quarter. As a
result of these factors, we expect first quarter underlying revenue
growth to be in the range of 1% to 2% and then be higher across the
remainder of the year.
We expect to deliver a trading profit margin of between 19% to 20%.
This significant step-up will be driven by operating leverage, cost
reductions and the benefits of our network optimisation programme.
These benefits are expected to more than offset headwinds from
China and cost inflation.
We expect trading profit margin to be stronger in the second half
than the first as the impact of China headwinds reduce and
operational savings are delivered.
We expect trading cash conversion of 80% to 90% and restructuring
costs of around $45 million in 2025.
The tax rate on trading results for 2025 is forecast to be in the
range of 19% to 20%, subject to any material changes to tax law or
other one-off items.
Forward calendar
The Q1 2025 Trading Report will be released on 30 April
2025.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused on
the repair, regeneration and replacement of soft and hard tissue.
We exist to restore people’s bodies and their self-belief by
using technology to take the limits off living. We call this
purpose ‘Life Unlimited’. Our 17,000 employees deliver
this mission every day, making a difference to patients’
lives through the excellence of our product portfolio, and the
invention and application of new technologies across our three
global business units of Orthopaedics, Sports Medicine & ENT
and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in around 100
countries, and generated annual sales of $5.8 billion in 2024.
Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN).
The terms ‘Group’ and ‘Smith+Nephew’ are
used to refer to Smith & Nephew plc and its consolidated
subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit
www.smith-nephew.com and
follow us on X, LinkedIn,
Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or
may not prove accurate. For example, statements regarding expected
revenue growth and trading profit margins, market trends and our
product pipeline are forward-looking statements. Phrases such as
"aim", "plan", "intend", "anticipate", "well-placed", "believe",
"estimate", "expect", "target", "consider" and similar expressions
are generally intended to identify forward-looking statements.
Forward-looking statements involve known and unknown risks,
uncertainties and other important factors that could cause actual
results to differ materially from what is expressed or implied by
the statements. For Smith+Nephew, these factors include: conflicts
in Europe and the Middle East, economic and financial conditions in
the markets we serve, especially those affecting healthcare
providers, payers and customers; price levels for established and
innovative medical devices; developments in medical technology;
regulatory approvals, reimbursement decisions or other government
actions; product defects or recalls or other problems with quality
management systems or failure to comply with related regulations;
litigation relating to patent or other claims; legal and financial
compliance risks and related investigative, remedial or enforcement
actions; disruption to our supply chain or operations or those of
our suppliers; competition for qualified personnel; strategic
actions, including acquisitions and disposals, our success in
performing due diligence, valuing and integrating acquired
businesses; disruption that may result from transactions or other
changes we make in our business plans or organisation to adapt to
market developments; relationships with healthcare professionals;
reliance on information technology and cybersecurity; disruptions
due to natural disasters, weather and climate change related
events; changes in customer and other stakeholder sustainability
expectations; changes in taxation regulations; effects of foreign
exchange volatility; and numerous other matters that affect us or
our markets, including those of a political, economic, business,
competitive or reputational nature. Please refer to the documents
that Smith+Nephew has filed with the U.S. Securities and Exchange
Commission under the U.S. Securities Exchange Act of 1934, as
amended, including Smith+Nephew's most recent annual report on Form
20-F, which is available on the SEC’s website at www.
sec.gov, for a discussion of certain of these factors. Any
forward-looking statement is based on information available to
Smith+Nephew as of the date of the statement. All written or oral
forward-looking statements attributable to Smith+Nephew are
qualified by this caution. Smith+Nephew does not undertake any
obligation to update or revise any forward-looking statement to
reflect any change in circumstances or in Smith+Nephew's
expectations.
◊
Trademark of Smith+Nephew. Certain marks registered in US Patent
and Trademark Office.
2024 CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Group Income Statement for the year ended 31 December
2024
|
|
|
|
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
Notes
|
|
$m
|
|
$m
|
|
Revenue
|
|
2
|
|
5,810
|
|
5,549
|
|
Cost
of goods sold
|
|
|
|
(1,764)
|
|
(1,730)
|
|
Gross profit
|
|
|
|
4,046
|
|
3,819
|
|
Selling,
general and administrative expenses
|
|
|
|
(3,100)
|
|
(3,055)
|
|
Research
and development expenses
|
|
|
|
(289)
|
|
(339)
|
|
Operating profit
|
|
2
|
|
657
|
|
425
|
|
Interest
income
|
|
|
|
24
|
|
34
|
|
Interest
expense
|
|
|
|
(145)
|
|
(132)
|
|
Other
finance costs
|
|
|
|
(28)
|
|
(7)
|
|
Share
of results of associates
|
|
|
|
(10)
|
|
(30)
|
|
Profit before taxation
|
|
|
|
498
|
|
290
|
|
Taxation
|
|
3
|
|
(86)
|
|
(27)
|
|
Attributable profit for the yearA
|
|
|
|
412
|
|
263
|
|
Earnings per ordinary shareA
|
|
|
|
|
|
|
|
Basic
|
|
|
|
47.2
|
|
30.2
|
|
Diluted
|
|
|
|
47.0
|
|
30.1
|
Group Statement of Comprehensive Income for the year ended 31
December 2024
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Attributable profit for the yearA
|
|
412
|
|
263
|
|
Other comprehensive income
|
|
|
|
|
|
Items that will not be reclassified to income
statement
|
|
|
|
|
|
Remeasurement
of net retirement benefit obligations
|
|
16
|
|
(89)
|
|
Taxation
on other comprehensive income
|
|
(1)
|
|
18
|
|
Total
items that will not be reclassified to income
statement
|
|
15
|
|
(71)
|
|
|
|
|
|
|
|
Items that may be reclassified subsequently to income
statement
|
|
|
|
|
|
Cash
flow hedges - forward exchange contracts
|
|
|
|
|
|
Gains
arising in the year
|
|
38
|
|
23
|
|
Gains
recycled to income statement in the year
|
|
(1)
|
|
(25)
|
|
Exchange
differences on translation of foreign operations
|
|
(124)
|
|
56
|
|
Taxation
on other comprehensive income
|
|
(5)
|
|
-
|
|
Total
items that may be reclassified subsequently to income
statement
|
|
(92)
|
|
54
|
|
Other comprehensive loss for the year, net of taxation
|
|
(77)
|
|
(17)
|
|
Total comprehensive income for the yearA
|
|
335
|
|
246
|
A.
Attributable to the
equity holders of the Company and wholly derived from continuing
operations.
Group Balance Sheet as at 31 December 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
31
December
|
|
31
December
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
Notes
|
|
$m
|
|
$m
|
|
ASSETS
|
|
|
|
|
|
|
|
Non-current assets
|
|
|
|
|
|
|
|
Property,
plant and equipment
|
|
|
|
1,422
|
|
1,470
|
|
Goodwill
|
|
|
|
3,026
|
|
2,992
|
|
Intangible
assets
|
|
|
|
1,032
|
|
1,110
|
|
Investments
|
|
|
|
9
|
|
8
|
|
Investments
in associates
|
|
|
|
7
|
|
16
|
|
Other
non-current assets
|
|
|
|
24
|
|
18
|
|
Retirement
benefit assets
|
|
|
|
63
|
|
69
|
|
Deferred
tax assets
|
|
|
|
350
|
|
274
|
|
|
|
|
|
5,933
|
|
5,957
|
|
Current assets
|
|
|
|
|
|
|
|
Inventories
|
|
|
|
2,387
|
|
2,395
|
|
Trade
and other receivables
|
|
|
|
1,381
|
|
1,300
|
|
Current
tax receivable
|
|
|
|
34
|
|
33
|
|
Cash
and cash equivalents
|
|
6
|
|
619
|
|
302
|
|
|
|
|
|
4,421
|
|
4,030
|
|
TOTAL ASSETS
|
|
|
|
10,354
|
|
9,987
|
|
|
|
|
|
|
|
|
|
EQUITY AND LIABILITIES
|
|
|
|
|
|
|
|
Equity attributable to owners of the Company
|
|
|
|
|
|
|
|
Share
capital
|
|
|
|
175
|
|
175
|
|
Share
premium
|
|
|
|
615
|
|
615
|
|
Capital
redemption reserve
|
|
|
|
20
|
|
20
|
|
Treasury
shares
|
|
|
|
(66)
|
|
(94)
|
|
Other
reserves
|
|
|
|
(497)
|
|
(405)
|
|
Retained
earnings
|
|
|
|
5,018
|
|
4,906
|
|
Total equity
|
|
|
|
5,265
|
|
5,217
|
|
|
|
|
|
|
|
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
Long-term
borrowings and lease liabilities
|
|
6
|
|
3,258
|
|
2,319
|
|
Retirement
benefit obligations
|
|
|
|
79
|
|
88
|
|
Other
payables
|
|
|
|
95
|
|
35
|
|
Provisions
|
|
|
|
95
|
|
48
|
|
Deferred
tax liabilities
|
|
|
|
31
|
|
9
|
|
|
|
|
|
3,558
|
|
2,499
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
Bank
overdrafts, borrowings, loans and lease liabilities
|
|
6
|
|
63
|
|
765
|
|
Trade
and other payables
|
|
|
|
1,128
|
|
1,055
|
|
Provisions
|
|
|
|
108
|
|
233
|
|
Current
tax payable
|
|
|
|
232
|
|
218
|
|
|
|
|
|
1,531
|
|
2,271
|
|
Total liabilities
|
|
|
|
5,089
|
|
4,770
|
|
TOTAL EQUITY AND LIABILITIES
|
|
|
|
10,354
|
|
9,987
|
Group Cash Flow Statement for the year ended 31 December
2024
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Cash flows from operating activities
|
|
|
|
|
|
Profit
before taxation
|
|
498
|
|
290
|
|
Net
interest expense
|
|
121
|
|
98
|
|
Depreciation,
amortisation and impairment
|
|
645
|
|
683
|
|
Loss
on disposal of property, plant and equipment and
software
|
|
22
|
|
18
|
|
Share-based
payments expense (equity-settled)
|
|
40
|
|
39
|
|
Share
of results of associates
|
|
10
|
|
30
|
|
Pension
costs less cash paid
|
|
16
|
|
3
|
|
Increase
in inventories
|
|
(42)
|
|
(178)
|
|
Increase
in trade and other receivables
|
|
(81)
|
|
(49)
|
|
Decrease/(increase)
in trade and other payables and provisions
|
|
16
|
|
(105)
|
|
Cash
generated from operations
|
|
1,245
|
|
829
|
|
Interest
received
|
|
22
|
|
8
|
|
Interest
paid
|
|
(140)
|
|
(104)
|
|
Income
taxes paid
|
|
(140)
|
|
(125)
|
|
Net
cash inflow from operating activities
|
|
987
|
|
608
|
|
|
|
|
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
Acquisitions,
net of cash acquired
|
|
(186)
|
|
(21)
|
|
Capital
expenditure
|
|
(381)
|
|
(427)
|
|
Purchase
of investments
|
|
(1)
|
|
-
|
|
Investment
in associates
|
|
(1)
|
|
-
|
|
Net
cash used in investing activities
|
|
(569)
|
|
(448)
|
|
|
|
|
|
|
|
Cash flows from financing activities
|
|
|
|
|
|
Payment
of capital element of lease liabilities
|
|
(55)
|
|
(52)
|
|
Proceeds
from borrowings due within one year
|
|
-
|
|
326
|
|
Settlement
of borrowings due within one year
|
|
(705)
|
|
(151)
|
|
Proceeds
from borrowings due after one year
|
|
1,000
|
|
-
|
|
Proceeds
from own shares
|
|
1
|
|
-
|
|
Settlement
of currency swaps
|
|
-
|
|
4
|
|
Equity
dividends paid
|
|
(327)
|
|
(327)
|
|
Net
cash used in financing activities
|
|
(86)
|
|
(200)
|
|
|
|
|
|
|
|
Net increase/(decrease) in cash and cash equivalents
|
|
332
|
|
(40)
|
|
Cash
and cash equivalents at beginning of year
|
|
300
|
|
344
|
|
Exchange
adjustments
|
|
(15)
|
|
(4)
|
|
Cash and cash equivalents at end of yearB
|
|
617
|
|
300
|
B
Cash and cash
equivalents at the end of the year are net of bank overdrafts of
$2m (2023: $2m).
Group Statement of Changes in Equity for the year ended 31 December
2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital
|
|
|
|
|
|
|
|
|
|
|
|
Share
|
|
Share
|
|
redemption
|
|
Treasury
|
|
Other
|
|
Retained
|
|
Total
|
|
|
|
capital
|
|
premium
|
|
reserve
|
|
shares
|
|
reservesC
|
|
earningsD
|
|
equity
|
|
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
At
1 January 2024
|
|
175
|
|
615
|
|
20
|
|
(94)
|
|
(405)
|
|
4,906
|
|
5,217
|
|
Attributable profit for the
yearA
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
412
|
|
412
|
|
Other comprehensive incomeA
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(92)
|
|
15
|
|
(77)
|
|
Equity
dividends declared and paid
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(327)
|
|
(327)
|
|
Share-based
payments recognised
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
40
|
|
40
|
|
Taxation
on share-based payments
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(1)
|
|
(1)
|
|
Cost
of shares transferred to beneficiaries
|
|
-
|
|
-
|
|
-
|
|
28
|
|
-
|
|
(27)
|
|
1
|
|
At 31 December 2024
|
|
175
|
|
615
|
|
20
|
|
(66)
|
|
(497)
|
|
5,018
|
|
5,265
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital
|
|
|
|
|
|
|
|
|
|
|
|
Share
|
|
Share
|
|
redemption
|
|
Treasury
|
|
Other
|
|
Retained
|
|
Total
|
|
|
|
capital
|
|
premium
|
|
reserve
|
|
shares
|
|
reservesC
|
|
earningsD
|
|
equity
|
|
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
At
1 January 2023
|
|
175
|
|
615
|
|
20
|
|
(118)
|
|
(459)
|
|
5,026
|
|
5,259
|
|
Attributable profit for the
yearA
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
263
|
|
263
|
|
Other comprehensive incomeA
|
|
-
|
|
-
|
|
-
|
|
-
|
|
54
|
|
(71)
|
|
(17)
|
|
Equity
dividends declared and paid
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(327)
|
|
(327)
|
|
Share-based
payments recognised
|
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
39
|
|
39
|
|
Cost
of shares transferred to beneficiaries
|
|
-
|
|
-
|
|
-
|
|
24
|
|
-
|
|
(24)
|
|
-
|
|
At 31 December 2023
|
|
175
|
|
615
|
|
20
|
|
(94)
|
|
(405)
|
|
4,906
|
|
5,217
|
A.
Attributable to the
equity holders of the Company and wholly derived from continuing
operations.
C.
Other reserves
comprises gains and losses on cash flow hedges, foreign exchange
differences on translation of foreign operations and net changes on
fair value of trade investments. The cumulative translation loss
within other reserves at 31 December 2024 was $520m (2023: $396m,
2022: $452m).
D.
Within retained
earnings is a non-distributable capital reserve of $2,266m (2023:
$2,266m, 2022: $2,266m) which arose as a result of the
Group’s reorganisation in 2008.
Notes to the Condensed Consolidated Financial
Statements
1. Basis
of preparation and accounting policies
Smith
& Nephew plc (the ‘Company’) is a public limited
company incorporated in England and Wales. In these condensed
consolidated financial statements (‘Financial
Statements’), ‘Group’ means the Company and all
its subsidiaries. The financial information herein has been
prepared on the basis of the accounting policies as set out in the
Annual Report of the Group for the year ended 31 December 2024. The
Group has prepared its accounts in accordance with UK-adopted
International Accounting Standards. The Group has also prepared its
accounts in accordance with International Financial Reporting
Standards (IFRS Accounting Standards) as issued by the
International Accounting Standards Board (IASB) effective as at 31
December 2024. IFRS as adopted in the UK differs in certain
respects from IFRS Accounting Standards as issued by the IASB.
However, the differences have no impact for the periods
presented.
The
preparation of accounts in conformity with IFRS requires management
to use estimates and assumptions that affect the reported amounts
of assets and liabilities and disclosure of contingent assets and
liabilities at the date of the accounts and the reported amounts of
revenues and expenses during the year. The accounting policies
requiring management to use significant estimates and assumptions
are discussed below. Although these estimates are based on
management’s best knowledge of current events and actions,
actual results ultimately may differ from those estimates.
Estimates and underlying assumptions are reviewed on an ongoing
basis. Revisions to estimates are recognised
prospectively.
The
uncertainties as to the future impact on the financial performance
and cash flows of the Group as a result of the current economic
environment have been considered as part of the Group’s
adoption of the going concern basis in these financial statements,
in which context the Directors reviewed cash flow forecasts
prepared for a period of at least 12 months from the date of
approval of these financial statements. Having carefully
reviewed those forecasts, the Directors concluded that it was
appropriate to adopt the going concern basis of accounting in
preparing these financial statements for the reasons set out
below.
The
Group had access to $617m of cash and cash equivalents at 31
December 2024. The Group’s net debt, excluding lease
liabilities, at 31 December 2024 was $2,513m with access to
committed facilities of $4.1bn with an average maturity of 5.5
years.
At the
date of approving these financial statements the funding position
of the Group has remained unchanged and the cash position is not
materially different. The Group does not have any debt that is due
for repayment in 2025.
$625m
of private placement debt is subject to financial covenants. The
principal covenant on the private placement debt is a leverage
ratio of <3.5 which is measured on a rolling 12-month basis at
half year and year end. There are no financial covenants in any of
the Group’s other facilities.
The
Directors have considered various scenarios in assessing the impact
of the economic environment on future financial performance and
cash flows, including the impact of a significant global economic
downturn, leading to lower healthcare spending across both public
and private systems. Throughout these scenarios, which include a
severe but plausible outcome, the Group continues to have
headroom on its borrowing facilities and financial
covenants.
The
Directors have a reasonable expectation that the Company and the
Group are well placed to manage their business risks, have
sufficient funds to continue to meet their liabilities as they fall
due and to continue in operational existence for a period of at
least 12 months from the date of the approval of the financial
statements. The financial statements have therefore been prepared
on a going concern basis.
Accordingly, the
Directors continue to adopt the going concern basis (in accordance
with the guidance ‘Guidance on Risk Management, Internal
Control and Related Financial and Business Reporting’ issued
by the FRC) in preparing these financial statements.
The
principal risks that the Group is exposed to will be disclosed in
the Group’s 2024 Annual Report. These are: strategy and
commercial execution; cybersecurity; global supply chain; legal and
compliance; mergers and acquisitions; new product innovation,
design and development including intellectual property; political
and economic; pricing and reimbursement; quality and regulatory;
talent management; and financial markets.
The
financial information contained in this document does not
constitute statutory financial statements as defined in sections
434 and 435 of the Companies Act 2006 for the years ended 31
December 2024 or 2023 but is derived from those accounts. Statutory
accounts for 2023 have been delivered to the registrar of companies
and those for 2024 will be delivered in due course. The auditor has
reported on those accounts; their report was (i) unqualified, (ii)
did not include a reference to any matters to which the auditor
drew attention by way of emphasis without qualifying their report
and (iii) did not contain a statement under section 498 (2) or (3)
of the Companies Act 2006.
New
accounting standards effective 2024
A
number of new amendments to standards are effective from 1 January
2024 but they do not have a material effect on the Group’s
financial statements.
The
Group is adopting the mandatory temporary exception from the
recognition and disclosure of deferred taxes arising from the
jurisdictional implementation of the Pillar Two model rules which
took effect for the Group from 1 January 2024.
Accounting
standards issued but not yet effective
A
number of new standards and amendments to standards are effective
for annual periods beginning after 1 January 2025 and earlier
application is permitted; however, the Group has not adopted them
early in preparing these financial statements.
Critical
judgements and estimates
The
Group prepares its consolidated financial statements in accordance
with IFRS Accounting Standards as issued by the IASB and IFRS
adopted in the UK, the application of which often requires
judgements and estimates to be made by management when
formulating the Group’s financial position and results. Under
IFRS, the Directors are required to adopt those accounting policies
most appropriate to the Group’s circumstances for the
purpose of presenting fairly the Group’s financial position,
financial performance and cash flows.
Management
regularly reviews, and revises as necessary, the accounting
judgements that significantly impact the amounts recognised in the
financial statements and the estimates that are considered to be
critical estimates due to their potential to give rise to material
adjustments in the Group’s financial statements in the next
financial year. The Group’s accounting policies do not
include any critical judgements. The critical accounting estimate
with a significant risk of a material change to the carrying value
of assets and liabilities within the next year is impairment of
Orthopaedics CGU as outlined below. In addition, other estimates
have also been identified that are not considered to be critical in
respect of the provision for excess and obsolete inventory and
liability provisioning for legal disputes relating to
metal-on-metal cases.
Management have
considered the impact of the uncertainties around the current
economic environment below.
Impairment
In
carrying out impairment reviews of intangible assets and goodwill,
a number of significant assumptions have to be made when preparing
cash flow projections. These include the future rate of market
growth, discount rates, the market demand for the products
acquired, the future profitability of acquired businesses or
products, levels of reimbursement and success in obtaining
regulatory approvals. If actual results should differ or changes in
expectations arise, impairment charges may be required which would
adversely impact operating results. The Orthopaedics CGU is
sensitive to a reasonably possible change in assumptions, in
particular the projected trading profit margin. For other
intangible assets and goodwill CGUs, this critical estimate is not
considered to have a significant risk of material adjustment in
2025 or thereafter based on sensitivity analyses undertaken (as
outlined below).
Current
economic environment impact assessment: Management have assessed
the non-current assets held by the Group at 31 December 2024 to
identify any indicators of impairment as a result of current
economic environment. Where an impairment indicator has arisen,
impairment reviews have been undertaken by comparing the expected
recoverable value of the asset to the carrying value of the asset.
The recoverable amounts are based on cash flow projections using
the Group’s base case scenario in its going concern models,
which was reviewed and approved by the Board.
Climate
change considerations
The
impact of climate change has been considered as part of the
assessment of estimates and judgements in preparing the Group
accounts. The climate change scenario analyses undertaken this year
in line with TCFD recommendations did not identify
any material financial impact. The following considerations
were made in respect of the financial statements:
a.
The impact of
climate change on the going concern assessment and the viability of
the Group over the next three years.
b.
The impact of
climate change on the cash flow forecasts used in the impairment
assessments of non-current assets including goodwill.
c.
The impact of
climate change on the carrying value and useful economic lives of
property, plant and equipment.
While
there is currently no material medium term impact expected, the
Group closely monitors climate-related risks given the changing
nature of these risks and management consider the impact of climate
change as part of the decision making process and continue to
assess the impact on judgements and estimates, and on preparation
of the consolidated financial statements.
2. Business
segment information
The
Group’s operating structure is organised around four global
business units (Orthopaedics, Sports Medicine, ENT and Advanced
Wound Management) and the chief operating decision maker monitors
performance, makes operating decisions and allocates resources on a
global business unit basis. Business unit presidents have
responsibility for upstream marketing, driving product portfolio
and technology acquisition decisions, full commercial
responsibility and for the implementation of their business unit
strategy globally. Accordingly, the Group consists of four
operating segments.
The
Group has concluded that Sports Medicine and ENT meet the
aggregation criteria and therefore, these operating segments have
been aggregated into a single operating segment. In applying the
aggregation criteria prescribed by IFRS 8 Operating Segments,
management made certain judgements pertaining to the economic
indicators relating to these operating segments including those
relating to the similarities in the expected long-term market
growth rates, the geographic and operational risks and the
competitive landscape that these segments operate in. Therefore, in
accordance with IFRS 8, the Group has three operating segments
which are also reportable segments.
The
Executive Committee (‘ExCo’) comprises the Chief
Financial Officer (‘CFO’), the business unit presidents
and certain heads of function, and is chaired by the Chief
Executive Officer (‘CEO’). ExCo is the body through
which the CEO uses the authority delegated to him by the Board of
Directors to manage the operations and performance of the Group.
All significant operating decisions regarding the allocation and
prioritisation of the Group’s resources and assessment of the
Group’s performance are made by ExCo, and while the members
have individual responsibility for the implementation of decisions
within their respective areas, it is at the ExCo level that these
decisions are made. Accordingly, ExCo is considered to be the
Group’s chief operating decision maker as defined by IFRS 8
Operating
Segments.
In
making decisions about the prioritisation and allocation of the
Group’s resources, ExCo reviews financial information for the
business units and determines the best allocation of resources to
the business units. This information is prepared substantially on
the same basis as the Group’s IFRS financial statements aside
from the adjustments described in Note 2b. In 2024, the Group
changed the segment trading profit measure presented to the ExCo by
allocating directly attributable corporate costs to business units.
Financial information for corporate costs relating to centalised
infrastructure costs such as compliance and group functions is
presented on a Group-wide basis. The ExCo is not provided with
total assets and liabilities by segment, and therefore these
measures are not included in the disclosures below. The results of
the segments are shown below.
2a. Revenue by business segment and
geography
Revenue
is recognised as the performance obligations to deliver products or
services are satisfied and is recorded based on the amount of
consideration expected to be received in exchange for satisfying
the performance obligations. Revenue is recognised primarily when
control is transferred to the customer, which is generally when the
goods are shipped or delivered in accordance with the contract
terms, with some transfer of services taking place over time.
Substantially all performance obligations are fulfilled within one
year. There is no significant revenue associated with the provision
of services.
Payment
terms to our customers are based on commercially reasonable terms
for the respective markets while also considering a
customer’s credit rating. Appropriate provisions for returns,
trade discounts and rebates are deducted from revenue. Rebates
primarily comprise chargebacks and other discounts granted to
certain customers. Chargebacks are discounts that occur when a
third-party purchases product from a wholesaler at its agreed price
plus a mark-up. The wholesaler in turn charges the Group for the
difference between the price initially paid by the wholesaler and
the agreed price. The provision for chargebacks is based on
expected sell-through levels by the Group’s wholesalers to
such customers, as well as estimated wholesaler inventory
levels.
Orthopaedics
and Sports Medicine & ENT (Ear, Nose & Throat)
Orthopaedics and
Sports Medicine & ENT consists of the following businesses:
Knee Implants, Hip Implants, Other Reconstruction, Trauma &
Extremities, Sports Medicine Joint Repair, Arthroscopic Enabling
Technologies and ENT. Sales of inventory located at customer
premises and available for customers’ immediate use are
recognised when notification is received that the product has been
implanted or used. Substantially all other revenue is recognised
when control is transferred to the customer, which is generally
when the goods are shipped or delivered in accordance with the
contract terms. Revenue is recognised for the amount of
consideration expected to be received in exchange for transferring
the products or services.
In
general our business in Established Markets is direct to hospitals
and ambulatory surgery centers whereas in the Emerging Markets we
generally sell through distributors.
Advanced
Wound Management
Advanced Wound
Management consists of the following businesses: Advanced Wound
Care, Advanced Wound Bioactives and Advanced Wound Devices.
Substantially all revenue is recognised when control is transferred
to the customer, which is generally when the goods are shipped or
delivered in accordance with the contract terms. Revenue is
recognised for the amount of consideration expected to be received
in exchange for transferring the products or services. Appropriate
provisions for returns, trade discounts and rebates are deducted
from revenue, as explained above.
The
majority of our Advanced Wound Management business, and in
particular products used in community and homecare facilities, is
through wholesalers and distributors. When
control is transferred to a wholesaler or distributor, revenue is
recognised accordingly. The proportion of sales direct to hospitals
is higher in our Advanced Wound Devices business in Established
Markets.
Segment
revenue reconciles to statutory revenue from continuing operations
as follows:
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Reportable segment revenue
|
|
|
|
|
|
Orthopaedics
|
|
2,305
|
|
2,214
|
|
Sports
Medicine & ENT
|
|
1,824
|
|
1,729
|
|
Advanced
Wound Management
|
|
1,681
|
|
1,606
|
|
Revenue
from external customers
|
|
5,810
|
|
5,549
|
|
|
|
|
|
|
Disaggregation
of revenue
The
following table shows the disaggregation of Group revenue by
product by business unit:
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Knee
Implants
|
|
947
|
|
940
|
|
Hip
Implants
|
|
619
|
|
599
|
|
Other
Reconstruction
|
|
131
|
|
111
|
|
Trauma
& Extremities
|
|
608
|
|
564
|
|
Orthopaedics
|
|
2,305
|
|
2,214
|
|
Sports
Medicine Joint Repair
|
|
982
|
|
945
|
|
Arthroscopic
Enabling Technologies
|
|
632
|
|
588
|
|
ENT
(Ear, Nose and Throat)
|
|
210
|
|
196
|
|
Sports Medicine & ENT
|
|
1,824
|
|
1,729
|
|
Advanced
Wound Care
|
|
735
|
|
725
|
|
Advanced
Wound Bioactives
|
|
581
|
|
553
|
|
Advanced
Wound Devices
|
|
365
|
|
328
|
|
Advanced Wound Management
|
|
1,681
|
|
1,606
|
|
Total
|
|
5,810
|
|
5,549
|
The
following table shows the disaggregation of Group revenue by
geographic market and product category. The disaggregation of
revenue into the two product categories below reflects that in
general the products in the Advanced Wound Management business unit
are sold to wholesalers and intermediaries, while products in the
other business units are sold directly to hospitals, ambulatory
surgery centers and distributors. The further disaggregation of
revenue by Established Markets and Emerging Markets reflects that
in general our products are sold through distributors and
intermediaries in the Emerging Markets while in the Established
Markets, with the exception of the Advanced Wound Care and
Bioactives products, which are in general sold direct to hospitals
and ambulatory surgery centers. The disaggregation by Established
Markets and Emerging Markets also reflects their differing economic
factors including volatility in growth and outlook.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
Established
MarketsE
|
|
Emerging
Markets
|
|
Total
|
|
Established
MarketsE
|
|
Emerging
Markets
|
|
Total
|
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
Orthopaedics,
Sports Medicine & ENT
|
3,366
|
|
763
|
|
4,129
|
|
3,184
|
|
759
|
|
3,943
|
|
Advanced
Wound Management
|
1,464
|
|
217
|
|
1,681
|
|
1,406
|
|
200
|
|
1,606
|
|
Total
|
4,830
|
|
980
|
|
5,810
|
|
4,590
|
|
959
|
|
5,549
|
E
Established Markets
comprises the US, Australia, Canada, Europe, Japan and New
Zealand.
Sales
are attributed to the country of destination. US revenue for 2024
was $3,123m (2023: $2,979m), China revenue for 2024 was $210m
(2023: $275m) and UK revenue for 2024 was $226m (2023:
$201m).
No
single customer generates revenue greater than 10% of the
consolidated revenue.
2b. Trading profit by business
segment
The
segment profit measure presented to the ExCo is the segment trading
profit. The Group has identified the following items, where
material, as those to be excluded from operating profit when
arriving at segment trading profit: corporate costs; acquisition
and disposal-related items; significant restructuring programmes;
amortisation and impairment of acquisition intangibles; gains and
losses arising from legal disputes; and other significant
items.
In
2024, the Group changed the segment trading profit measure
presented to the ExCo by allocating directly attributable corporate
costs to business units except for corporate costs relating to
centralised infrastructure costs such as compliance and group
functions. Accordingly, 2023 operating segment results have been
restated for comparative purposes.
Segment
trading profit is reconciled to the statutory measure
below:
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Segment profit
|
|
|
|
|
|
Orthopaedics
|
|
265
|
|
251
|
|
Sports
Medicine & ENT
|
|
437
|
|
394
|
|
Advanced
Wound Management
|
|
399
|
|
372
|
|
Segment
trading profit
|
|
1,101
|
|
1,017
|
|
Corporate costs1
|
|
(52)
|
|
(47)
|
|
Acquisition and disposal related
items2
|
|
(94)
|
|
(60)
|
|
Restructuring
and rationalisation expenses
|
|
(123)
|
|
(220)
|
|
Amortisation and impairment of acquisition
intangibles2
|
|
(187)
|
|
(207)
|
|
Legal and other2
|
|
12
|
|
(58)
|
|
Operating
profit
|
|
657
|
|
425
|
|
Interest
income
|
|
24
|
|
34
|
|
Interest
expense
|
|
(145)
|
|
(132)
|
|
Other
finance costs
|
|
(28)
|
|
(7)
|
|
Share
of results of associates
|
|
(10)
|
|
(30)
|
|
Profit
before taxation
|
|
498
|
|
290
|
|
|
|
|
|
|
1
In 2024 and 2023,
corporate costs include centralised infrastructure costs such as
compliance and group functions.
2
During 2024, the
Group announced its intention to close the Warwick manufacturing
site that manufactures Birmingham Hip Resurfacing (BHR) products.
As a result, a total of $68m of BHR assets and liabilities were
written off, which mainly includes goodwill of $63m (included in
acquisition and disposal-related items).
During
2023, management evaluated the commercial viability of Engage
products and concluded that they should be discontinued. A total of
$109m of Engage’s assets and liabilities were written off as
a result of this action, which includes goodwill of $84m (included
in acquisition and disposal-related items), intangible assets of
$37m (included in amortisation and impairment of acquisition
intangibles), inventory of $21m (included in legal and other),
partially offset by remeasurement of contingent consideration of
$33m (included in acquisition and disposal-related
items).
Acquisition and disposal-related items
For
the year ended 31 December 2024, costs primarily relate to
impairment of BHR goodwill, disposal of certain products and
integration costs relating to CartiHeal.
For
the year ended 31 December 2023, costs primarily relate to the
acquisition of CartiHeal and impairment of Engage goodwill,
partially offset by credits relating to remeasurement of
contingent consideration for prior year acquisitions.
Restructuring and rationalisation costs
For
the years ended 31 December 2024 and 2023, these costs include
efficiency and productivity elements of the 12-Point Plan and the
Operations and Commercial Excellence programme. These costs
primarily consist of severance, business advisory services, asset
write-offs, contractual terminations and integration and dual
running costs.
Amortisation and impairment of acquisition intangibles
For
the years ended 31 December 2024 and 2023, these costs relate to
the amortisation and impairment of intangible assets acquired in
material business combinations.
Legal and other
For
the year ended 31 December 2024, the credit mainly relates to a
$28m reduction in the provision for ongoing metal-on-metal hip
claims as a result of decrease in the present value of the
estimated costs to resolve all known and anticipated metal-on-metal
hip claims, partially offset by legal expenses for ongoing
metal-on-metal hip claims.
For
the year ended 31 December 2023, charges primarily relate to legal
expenses for ongoing metal-on-metal hip claims partially offset by
a decrease of $8m in the provision that reflects the decrease in
the present value of the estimated costs to resolve all other known
and anticipated metal-on-metal hip claims and by the release of a
provision for an intellectual property dispute.
The
years ended 31 December 2024 and 2023 also include costs for
implementing the requirements of the EU Medical Device Regulation
which came into effect in May 2021 with a transition period to May
2024.
3. Taxation
Reported
tax for the year ended 31 December 2024 was a charge of $86m (2023:
$27m charge). The reported tax charge is higher than 2023 due to an
increase in reported profits.
Pillar Two
The
OECD Pillar Two GloBE Rules (Pillar Two) introduce a global minimum
corporation tax rate of 15% applicable to multinational enterprise
groups with global revenue over €750m. The Pillar Two Rules
applied to the Group for its accounting period
ended
31 December 2024 and the Pillar Two current tax charge for the
period is approximately $8m.
The
Group is adopting the IAS12 mandatory temporary exception from the
recognition and disclosure of deferred taxes arising from the
jurisdictional implementation of the Pillar Two model
rules.
The
Group does not meet the threshold for application of the Pillar One
transfer pricing rules.
4. Dividends
The
2023 final dividend of 23.1 US cents per ordinary share totalling
$202m was paid on 22 May 2024. The 2024 interim dividend of 14.4 US
cents per ordinary share totalling $125m was paid on 8 November
2024.
A final
dividend for 2024 of 23.1 US cents per ordinary share has been
proposed by the Board and will be paid, subject to shareholder
approval, on 28 May 2025 to shareholders whose names appear on the
Register of Members on 28 March 2025. The sterling equivalent per
ordinary share will be set following the record date. The
ex-dividend date is 27 March 2025 and the final day for currency
and dividend reinvestment plan (‘DRIP’) elections is 6
May 2025.
5. Acquisitions
Year
ended 31 December 2024
On 9
January 2024, the Group completed the acquisition of 100% of the
share capital of CartiHeal (2009) Ltd (CartiHeal), the developer of
CARTIHEAL AGILI-C, a novel Sports Medicine technology for cartilage
regeneration in the knee. The acquisition of this disruptive
technology supports our strategy to invest behind our successful
Sports Medicine & ENT business unit.
The
fair value of the consideration amounted to $231m. This is
comprised of contingent consideration of $49m, which represents the
discounted value of $150m of consideration contingent upon the
achievement of a single future financial performance milestone in
the next 10 years, and initial cash consideration of $180m adjusted
for cash acquired and other liabilities assumed, of which $18m was
transferred in to escrow to be released in equal instalments to the
seller in 12 and 18 months from completion.
The
fair value of assets acquired and liabilities assumed is set out
below:
|
|
|
|
|
|
|
CartiHeal
(2009) Ltd
|
|
|
|
$m
|
|
Intangible
assets - product-related and trade name
|
|
84
|
|
Inventory
|
|
1
|
|
Cash
|
|
6
|
|
Other
liabilities
|
|
(2)
|
|
Trade
and other payables
|
|
(1)
|
|
Net
deferred tax liability
|
|
(3)
|
|
Net
assets
|
|
85
|
|
Goodwill
|
|
146
|
|
Consideration
|
|
231
|
The
product-related intangible assets and the trade name were valued
using a relief-from-royalty methodology with the key inputs being
revenue, profit and discount rate.
The
cash outflow from acquisitions in 2024 of $186m (2023: $21m)
comprises payments of consideration of $177m net of cash acquired
(2023: $nil) relating to acquisitions in the current year and
payments of deferred and contingent consideration of $9m relating
to acquisitions completed in prior years.
The
goodwill represents the control premium, acquired workforce and the
synergies expected from integrating CartiHeal into the
Group’s existing business.
The
carrying value of goodwill increased from $2,992m at 31 December
2023 to $3,026m at 31 December 2024. The acquisition in the year
ended 31 December 2024 increased goodwill by $146m, this was
partially offset by goodwill impairment of $65m and foreign
exchange movements of $47m.
For the
year ended 31 December 2024, the contribution from CartiHeal to the
Group’s revenue and profit was immaterial. If the business
combination had occurred at the beginning of the year the
contribution to revenue and profit would not have been materially
different.
Year
ended 31 December 2023
No
acquisitions were completed in the year ended 31 December
2023.
During
2023, management evaluated the commercial viability of Engage
products and concluded that they should be discontinued. Refer to
note 2b for further details.
6. Net
debt
Net
debt comprises borrowings and credit balances on currency swaps
less cash and cash equivalents.
|
|
|
|
|
|
|
|
|
31 December
|
|
31 December
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Bank overdrafts, borrowings and loans - current
|
|
2
|
|
710
|
|
Corporate bond
|
|
2,498
|
|
1,550
|
|
Private placement notes
|
|
625
|
|
625
|
|
Borrowings
|
|
3,125
|
|
2,885
|
|
Cash and cash equivalents
|
|
(619)
|
|
(302)
|
|
Credit balance on derivatives - currency swaps
|
|
1
|
|
1
|
|
Credit/(debit) balance on derivatives - interest rate
swaps
|
|
6
|
|
(7)
|
|
Net debt excluding lease liabilities
|
|
2,513
|
|
2,577
|
|
Non-current lease liabilities
|
|
135
|
|
144
|
|
Current lease liabilities
|
|
61
|
|
55
|
|
Net debt
|
|
2,709
|
|
2,776
|
|
|
|
|
|
|
The
Group has available committed facilities of $4.1bn
(2023: $3.6bn). At the date
of
approving these financial statements the funding position of the
Group has remained unchanged and the cash position is not
materially different.
The
Group does not have any debt that is due for repayment in
2025.
7a. Financial
instruments
The
following table shows the carrying amounts and fair values of
financial assets and financial liabilities, including their levels
in the fair value hierarchy.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount
|
|
Fair value
|
|
|
|
|
|
2024
|
|
2023
|
|
2024
|
|
2023
|
|
Fair value
|
|
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
level
|
|
Financial assets measured at fair value
|
|
|
|
|
|
|
|
|
|
|
|
Forward foreign exchange contacts
|
|
46
|
|
25
|
|
46
|
|
25
|
|
Level 2
|
|
Investments
|
|
9
|
|
8
|
|
9
|
|
8
|
|
Level 3
|
|
Contingent consideration receivable
|
|
-
|
|
18
|
|
-
|
|
18
|
|
Level 3
|
|
Interest rate swaps
|
|
10
|
|
7
|
|
10
|
|
7
|
|
Level 2
|
|
Currency swaps
|
|
1
|
|
2
|
|
1
|
|
2
|
|
Level 2
|
|
|
|
66
|
|
60
|
|
66
|
|
60
|
|
|
|
Financial assets not measured at fair value
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
1,190
|
|
1,163
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
619
|
|
302
|
|
|
|
|
|
|
|
|
|
1,809
|
|
1,465
|
|
|
|
|
|
|
|
Total financial assets
|
|
1,875
|
|
1,525
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial liabilities measured at fair value
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition consideration - contingent
|
|
(84)
|
|
(32)
|
|
(84)
|
|
(32)
|
|
Level 3
|
|
Forward foreign exchange contracts
|
|
(16)
|
|
(25)
|
|
(16)
|
|
(25)
|
|
Level 2
|
|
Interest rate swaps
|
|
(16)
|
|
-
|
|
(16)
|
|
-
|
|
Level 2
|
|
Currency swaps
|
|
(2)
|
|
(3)
|
|
(2)
|
|
(3)
|
|
Level 2
|
|
|
|
(118)
|
|
(60)
|
|
(118)
|
|
(60)
|
|
|
|
Financial liabilities not measured at fair value
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition consideration - deferred
|
|
(21)
|
|
(4)
|
|
|
|
|
|
|
|
Bank overdrafts
|
|
(2)
|
|
(2)
|
|
|
|
|
|
|
|
Bank loans
|
|
-
|
|
(303)
|
|
|
|
|
|
|
|
Corporate bond not in a hedge relationship
|
|
(1,492)
|
|
(995)
|
|
|
|
|
|
|
|
Corporate bond in a hedge relationship
|
|
(1,006)
|
|
(555)
|
|
|
|
|
|
|
|
Private placement debt not in a hedge relationship
|
|
(625)
|
|
(1,030)
|
|
|
|
|
|
|
|
Trade and other payables
|
|
(1,084)
|
|
(1,026)
|
|
|
|
|
|
|
|
|
|
(4,230)
|
|
(3,915)
|
|
|
|
|
|
|
|
Total financial liabilities
|
|
(4,348)
|
|
(3,975)
|
|
|
|
|
|
|
At 31
December 2024, the book value and market value of the 2020 USD
corporate bond were $995m and $836m respectively (2023: $995m and
$826m), the book value and market value of the $650m 2024 USD
corporate bond maturing in 2034 were $628m and $642m respectively,
the book value and market value of the $350m 2024 USD corporate
bond maturing in 2027 were $348m and $352m respectively, the book
value and market value of the EUR Corporate bond were $527m and
$547m respectively (2023: $555m and $585m). The book value and fair
value of the private placement debt were $625m and $573m
respectively (2023: $1,030m and $959m).
There
were no transfers between Levels 1, 2 and 3 during the year ended
31 December 2024 and the year ended 31 December 2023. For cash and
cash equivalents, short-term loans and receivables, overdrafts and
other short-term liabilities which have a maturity of less than
three months, the book values approximate the fair values because
of their short-term nature.
Long-term
borrowings are measured in the balance sheet at amortised cost. The
corporate bonds issued in October 2020, October 2022 and March 2024
are publicly listed and a market price is available. The
Group’s other long-term borrowings are not quoted publicly,
their fair values are estimated by discounting future contractual
cash flows to net present values at the current market interest
rates available to the Group for similar financial instruments as
at the year end. The fair value of the private placement notes is
determined using a discounted cash flow model based on prevailing
market rates.
The
fair value of forward exchange contracts is calculated by reference
to quoted market forward exchange rates for contracts with similar
maturity profiles. The fair value of interest rate swaps is
determined by reference to quoted market interest rates. The fair
value of currency swaps is determined by reference to quoted market
spot rates. As a result, foreign forward exchange contracts,
interest rate swaps and currency swaps are classified as Level 2
within the fair value hierarchy.
The
fair value of contingent acquisition consideration is estimated
using a discounted cash flow model. The valuation model considers
the present value of expected payment, discounted using a
risk-adjusted discount rate. The expected payment is determined by
considering the possible scenarios, which relate to the achievement
of established milestones and targets, the amount to be paid under
each scenario and the probability of each scenario. As a result,
contingent acquisition consideration is classified as Level 3
within the fair value hierarchy.
The
fair value of investments is based upon third party pricing models
for share issues. As a result, investments are considered Level 3
in the fair value hierarchy. The movements in the year ended 31
December 2024 and the year ended 31 December 2023 for financial
instruments measured using Level 3 valuation methods are presented
below:
|
|
|
|
|
|
2024
|
2023
|
|
|
$m
|
$m
|
|
Investments
|
|
|
|
At 1 January
|
8
|
12
|
|
Additions
|
1
|
-
|
|
Fair value remeasurement
|
-
|
(4)
|
|
At 31 December
|
9
|
8
|
|
|
|
|
|
Contingent consideration receivable
|
|
|
|
At 1 January
|
18
|
18
|
|
Transferred to receivables
|
(18)
|
-
|
|
At 31 December
|
-
|
18
|
|
|
|
|
|
Contingent acquisition consideration liability
|
|
|
|
At 1 January
|
(32)
|
(78)
|
|
Arising on acquisitions
|
(49)
|
-
|
|
Payments
|
6
|
13
|
|
Remeasurements
|
(9)
|
33
|
|
At 31 December
|
(84)
|
(32)
|
7b. Retirement
benefit obligations
The
discount rate applied to the future pension liabilities of the UK
plan is based on the yield on bonds that have a credit rating of AA
denominated in the currency in which the benefits are expected to
be paid with a maturity profile approximately the same as the
obligations. The UK discount rate has increased since 31 December
2023 by 100bps to 5.5%. The remeasurement gain of $16m recognised
in Other Comprehensive Income (OCI) was principally made up of a
$15m gains on remeasurement of plan obligations in the UK, US,
Germany and Switzerland.
In
October 2022, US Pension Plan members were notified that Smith
& Nephew Inc. (SNI) would begin the termination process for the
US Plan. In December 2023, Fidelity & Guaranty Life was
selected to take over responsibility for the remaining US Pension
Plan obligation and administration upon termination. A premium
amount of $245m was paid in cash by the US Plan on 4 January 2024.
Certain active employees and terminated vested participants elected
to receive a lump sum in exchange for their plan benefit of $80m.
This resulted in $4m of settlement costs which were recognised in
2023, representing the difference between defined benefit
obligation (DBO) and the lump sums paid to members in December
2023.
Following the US
buyout, members move to having a direct relationship with Fidelity
& Guaranty Life with SNI no longer retaining any obligation for
the settlement of accrued member benefits.
8. Exchange
rates
The
exchange rates used for the translation of currencies into US
Dollars that have the most significant impact on the Group results
were:
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
Average rates
|
|
|
|
|
|
Sterling
|
|
1.28
|
|
1.24
|
|
Euro
|
|
1.08
|
|
1.08
|
|
Swiss
Franc
|
|
1.14
|
|
1.11
|
|
Japanese
Yen
|
|
0.0066
|
|
0.0071
|
|
Year end rates
|
|
|
|
|
|
Sterling
|
|
1.25
|
|
1.27
|
|
Euro
|
|
1.04
|
|
1.10
|
|
Swiss
Franc
|
|
1.10
|
|
1.19
|
|
Japanese
Yen
|
|
0.0064
|
|
0.0071
|
9. Post
balance sheet events
There
have been no events between the balance sheet date, and the date on
which the financial statements were approved by the Board, which
would require adjustment to the financial statements or any
additional disclosures.
Other
information
These
financial statements include financial measures that are not
prepared in accordance with International Financial Reporting
Standards (IFRS). This additional information presented is not
uniformly defined by all companies including those in the
Group’s industry. Accordingly, it may not be comparable with
similarly titled measures and disclosures by other companies.
Additionally, certain information presented is derived from amounts
calculated in accordance with IFRS but is not itself a measure
defined under IFRS. Such measures should not be viewed in isolation
or as an alternative to the equivalent GAAP measure. The non-IFRS
measures
discussed
in this document are set out below.
|
|
|
|
|
|
|
Performance measures
|
|
Non-IFRS
measure
|
Purpose
|
Definition
|
Closest
equivalent
IFRS
measure
|
Reconciled
on
|
|
Underlying revenue growth
|
Underlying
revenue growth is used to compare revenue in a given year to the
previous year on a like-for-like basis. This measure is used by
both management and the investor community.
|
Underlying
revenue growth reconciles to reported revenue growth, the most
directly comparable financial measure calculated in accordance with
IFRS, by making two adjustments, the ‘constant currency
exchange effect’ and the ‘acquisitions and disposals
effect’.
The
‘constant currency exchange effect’ is a measure of the
increase/decrease in revenue resulting from currency movements on
non-US Dollar sales and is measured as the difference between: 1)
the increase/decrease in the current year revenue translated into
US Dollars at the current year average exchange rate and the prior
year revenue translated at the prior year rate; and 2) the
increase/decrease being measured by translating current and prior
year revenues into US Dollars using the prior year closing
rate.
The
‘acquisitions and disposals effect’ is the measure of
the impact on revenue from newly acquired material business
combinations and recent material business disposals. This is
calculated by comparing the current year, constant currency actual
revenue (which includes acquisitions and excludes disposals from
the relevant date of completion) with prior year, constant currency
actual revenue, adjusted to include the results of acquisitions and
exclude disposals for the commensurate period in the prior year.
These sales are separately tracked in the Group’s internal
reporting systems and are readily identifiable.
|
Revenue
growth
|
39
|
|
Trading profit
|
Trading
profit is used in conjunction with operating profit to assess the
performance and profitability of the Group. It is a key internal
and external metric used by the investor community to assess our
performance. It is our segment performance measure in accordance
with IFRS 8 Operating Segments.
|
Trading
profit is operating profit excluding the impact of acquisition and
disposal related items arising in connection with business
combinations, including amortisation of acquisition intangible
assets, impairments and integration costs; restructuring events;
and gains and losses resulting from legal disputes and uninsured
losses. In addition to these items, gains and losses that
materially impact the Group’s profitability on a short-term
or one-off basis are excluded.
|
Operating
profit
|
40
|
|
Trading profit margin
|
This
measure is used to assess the performance and profitability of the
Group. It is a key external metric used by the investor community
to assess our performance.
|
Trading
profit margin is trading profit divided by revenue.
|
Operating
profit margin
|
40
|
|
Performance measures (continued)
|
|
Non-IFRS
measure
|
Purpose
|
Definition
|
Closest
equivalent
IFRS
measure
|
Reconciled
on
|
|
Trading profit before tax
|
Trading
profit before tax is used in conjunction with profit before tax to
assess performance and profitability of the Group. This measure is
intended to enable the users to assess the performance of the Group
by excluding items that impact the short-term profitability of the
Group.
|
Trading
profit before tax is profit before tax excluding impact of
acquisition and disposal related items arising in connection with
business combinations, including amortisation of acquisition
intangible assets, impairments and integration costs; restructuring
events; and gains and losses resulting from legal disputes and
uninsured losses. In addition to these items, gains and losses that
materially impact the Group’s profitability on a short-term
or one-off basis are excluded.
|
Profit
before tax
|
40
|
|
Trading taxation
|
Trading
taxation is used in conjunction with taxation to assess taxation
that corresponds to trading profit before tax. This metric is used
by both management and the investor community.
|
Trading
taxation is taxation excluding the impact of acquisition and
disposal related items arising in connection with business
combinations, including amortisation of acquisition intangible
assets, impairments and integration costs; restructuring events;
and gains and losses resulting from legal disputes and uninsured
losses. In addition to these items, gains and losses that
materially impact the Group’s profitability on a short-term
or one-off basis are excluded.
|
Taxation
|
40
|
|
Trading attributable profit
|
This
metric is used in the calculation of adjusted basic earnings per
share.
|
Trading
attributable profit is attributable profit excluding the impact of
acquisition and disposal related items arising in connection with
business combinations, including amortisation of acquisition
intangible assets, impairments and integration costs; restructuring
events; and gains and losses resulting from legal disputes and
uninsured losses. In addition to these items, gains and losses that
materially impact the Group’s profitability on a short-term
or one-off basis are excluded.
|
Attributable
profit
|
40
|
|
Adjusted earnings per share (‘EPSA’)
|
EPSA is
a trend measure. The Group presents this measure to assist
investors in their understanding of trends.
|
Adjusted
earnings per share is trading attributable profit divided by the
weighted average number of shares outstanding. This is the same
denominator used when calculating basic earnings per
share.
|
Basic
earnings per share
|
40
|
|
Trading cash flow
|
Trading
cash flow is used in conjunction with cash generated from
operations to assess the conversion of trading profit into cash. It
is key external metric used by the investor community and is a key
performance measure for management.
|
Trading
cash flow is cash generated from operations excluding the impact of
acquisition and disposal related items arising in connection with
business combinations, including integration costs; restructuring
events; and gains and losses resulting from legal disputes and
uninsured losses. In addition to these items, gains and losses that
materially impact the Group’s cash flows on a short-term or
one-off basis are excluded. Trading cash flow includes payment of
capital element of lease liabilities and capital expenditure as
presented in the Group cash flow statement.
|
Cash
generated from operations
|
40
|
|
Trading cash conversion
|
This
measure is used to assess the conversion of trading profit into
cash. It is a key external metric used by the investor community
and is a key performance measure for management.
|
Trading
cash conversion is trading cash flow divided by trading
profit.
|
Cash
generated from operations
|
40
|
|
|
|
|
|
|
|
Other measures
|
|
Non-IFRS
measure
|
Purpose
|
Definition
|
Closest
equivalent
IFRS
measure
|
Reconciled
on
|
|
Free cash flow
|
Free cash flow is a measure of the cash generated for the Group to
use after capital expenditure according to its Capital Allocation
Framework. This metric is used by both management and investor
community.
|
Free cash flow is cash generated from operations less capital
expenditure, payment of lease liabilities and cash flows from
interest and income taxes.
|
Cash
generated from operations
|
41
|
|
Adjusted EBITDA
|
Adjusted EBITDA is used in the calculation of adjusted leverage
ratio.
|
Adjusted EBITDA is attributable profit excluding taxation, share of
results of associates, other finance costs, interest expense,
interest income, acquisition and disposal related items,
restructuring and rationalisation costs, amortisation and
impairment of acquisition intangibles, legal and other costs,
depreciation and impairment of property, plant and equipment and
amortisation and impairment of other intangible
assets.
|
Attributable
profit
|
42
|
|
Adjusted leverage ratio
|
Adjusted leverage ratio is used in the calculation relating to debt
covenants.
|
We calculate adjusted leverage ratio by dividing net debt by
adjusted EBITDA. Net debt is defined as total borrowings less cash
and cash equivalents in the statement of financial position. Total
borrowings include bank overdrafts, borrowings, loans and lease
liabilities and long-term borrowings and lease
liabilities.
|
Leverage
ratio (using IFRS measures)
|
42
|
|
Adjusted return on invested capital (‘Adjusted
ROIC’)
|
Adjusted ROIC is a metric used by investor community and is a
measure of the return generated on capital invested by the Group.
It provides a metric for long-term value creation and encourages
compounding reinvestment within the business and discipline around
acquisitions with low returns and long payback. Adjusted ROIC is a
key performance measure under the Performance Share
Program.
|
Adjusted ROIC is defined as operating profit (before amortisation
and impairment of acquisition intangibles) less adjusted
taxes/((opening net operating assets + closing net operating
assets)/2).
|
Return
on invested capital (‘ROIC’) (using IFRS
measures)
|
43
|
Underlying revenue
Reported
revenue growth, the most directly comparable financial measure
calculated in accordance with IFRS, reconciles to underlying
revenue growth as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciling Items
|
|
|
|
|
|
|
|
Reported
|
|
Underlying
|
|
Acquisitions
|
|
Currency
|
|
|
|
2024
|
|
2023
|
|
growth
|
|
growth
|
|
& disposals
|
|
impact
|
|
|
|
$m
|
|
$m
|
|
%
|
|
%
|
|
%
|
|
%
|
|
Segment revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Orthopaedics
|
|
2,305
|
|
2,214
|
|
4.1
|
|
4.6
|
|
-
|
|
(0.5)
|
|
Sports
Medicine & ENT
|
|
1,824
|
|
1,729
|
|
5.5
|
|
6.2
|
|
-
|
|
(0.7)
|
|
Advanced
Wound Management
|
|
1,681
|
|
1,606
|
|
4.7
|
|
5.1
|
|
-
|
|
(0.4)
|
|
Revenue
from external customers
|
|
5,810
|
|
5,549
|
|
4.7
|
|
5.3
|
|
-
|
|
(0.6)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
|
|
|
|
|
|
Profit
|
|
|
|
|
|
generated
|
|
|
|
|
|
Operating
|
|
before
|
|
|
|
Attributable
|
|
from
|
|
Earnings
|
|
|
|
profit1
|
|
tax2
|
|
Taxation3
|
|
profit4
|
|
operations5
|
|
per share6
|
|
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
¢
|
|
2024 Reported
|
|
657
|
|
498
|
|
(86)
|
|
412
|
|
1,245
|
|
47.2
|
|
Acquisition and disposal related
items8
|
|
94
|
|
106
|
|
(9)
|
|
97
|
|
3
|
|
11.2
|
|
Restructuring
and rationalisation costs
|
|
123
|
|
123
|
|
(29)
|
|
94
|
|
151
|
|
10.8
|
|
Amortisation and impairment of acquisition
intangibles8
|
|
187
|
|
187
|
|
(42)
|
|
145
|
|
-
|
|
16.6
|
|
Legal and other7,8
|
|
(12)
|
|
(6)
|
|
(7)
|
|
(13)
|
|
36
|
|
(1.5)
|
|
Lease
liability payments
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(55)
|
|
-
|
|
Capital
expenditure
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(381)
|
|
-
|
|
2024 Non-IFRS
|
|
1,049
|
|
908
|
|
(173)
|
|
735
|
|
999
|
|
84.3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
|
|
|
|
|
|
Profit
|
|
|
|
|
|
generated
|
|
|
|
|
|
Operating
|
|
before
|
|
|
|
Attributable
|
|
from
|
|
Earnings
|
|
|
|
profit1
|
|
tax2
|
|
Taxation3
|
|
profit4
|
|
operations5
|
|
per share6
|
|
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
$m
|
|
¢
|
|
2023 Reported
|
|
425
|
|
290
|
|
(27)
|
|
263
|
|
829
|
|
30.2
|
|
Acquisition and disposal related
items8
|
|
60
|
|
78
|
|
(14)
|
|
64
|
|
16
|
|
7.3
|
|
Restructuring
and rationalisation costs
|
|
220
|
|
223
|
|
(42)
|
|
181
|
|
124
|
|
20.7
|
|
Amortisation and impairment of acquisition
intangibles8
|
|
207
|
|
207
|
|
(45)
|
|
162
|
|
-
|
|
18.6
|
|
Legal and other7,8
|
|
58
|
|
64
|
|
(12)
|
|
52
|
|
145
|
|
6.0
|
|
Lease
liability payments
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(52)
|
|
-
|
|
Capital
expenditure
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(427)
|
|
-
|
|
2023 Non-IFRS
|
|
970
|
|
862
|
|
(140)
|
|
722
|
|
635
|
|
82.8
|
1
Represents a
reconciliation of operating profit to trading profit.
2
Represents a
reconciliation of reported profit before tax to trading profit
before tax.
3
Represents a
reconciliation of reported tax to trading tax.
4
Represents a
reconciliation of reported attributable profit to trading
attributable profit.
5
Represents a
reconciliation of cash generated from operations to trading cash
flow.
6
Represents a
reconciliation of basic earnings per ordinary share to adjusted
earnings per ordinary share (EPSA).
7
The ongoing funding
of defined benefit pension schemes that are closed to future
accrual is not included in management’s definition of trading
cash flow as there is no defined benefit service cost for these
schemes.
8
During 2024, the
Group announced its intention to close the Warwick manufacturing
site that manufactures Birmingham Hip Resurfacing (BHR) products.
As a result, a total of $68m of BHR assets and liabilities were
written off, which mainly includes goodwill of $63m (included in
acquisition and disposal-related items).
During
2023, management evaluated the commercial viability of Engage
products and concluded that they should be discontinued. A total of
$109m of Engage’s assets and liabilities were written off as
a result of this action, which includes goodwill of $84m (included
in acquisition and disposal-related items), intangible assets of
$37m (included in amortisation and impairment of acquisition
intangibles), inventory of $21m (included in legal and other),
partially offset by remeasurement of contingent consideration of
$33m (included in acquisition and disposal-related
items).
Acquisition and disposal related items
For the year ended 31 December 2024, costs primary related to
impairment of BHR goodwill,
disposal of certain products and integration costs relating to
integration of CartiHeal. Trading profit before tax additionally
excludes losses related to the Group’s shareholding in
Bioventus. This primarily includes the Group’s share of loss
recognised by Bioventus in its financial statements.
For the year ended 31 December 2023, costs primary related to
the acquisition of CartiHeal and impairment of Engage goodwill,
partially offset by credits relating to remeasurement of contingent
consideration for prior year acquisitions. Trading profit before
tax additionally excludes losses of $18m related to the
Group’s shareholding in Bioventus. This
primarily
includes the Group’s share of loss recognised by Bioventus in
its financial statements.
Restructuring and rationalisation costs
For the year ended 31 December 2024 and 2023, these
costs relate to the efficiency and productivity elements of the
12-Point Plan and the Operations and Commercial Excellence
programme. These costs primarily consist of severance, business
advisory services, asset write-offs, contractual terminations and
integration and dual running costs.
In 2023, trading profit before tax additionally excludes $3m of
restructuring costs related to the Group’s share of results
of associates.
Amortisation and impairment of acquisition intangibles
For the years ended 31 December 2024 and 2023, these costs relate
to the amortisation and impairment of intangible assets acquired in
material business combinations.
Legal and other
For the year ended 31 December 2024, the credit mainly relates to a
$28m reduction in the provision for ongoing metal-on-metal hip
claims as a result of decrease in the present value of the
estimated costs to resolve all known and anticipated metal-on-metal
hip claims, partially offset by legal expenses for ongoing
metal-on-metal hip claims.
Trading profit before tax additionally excludes $6m of finance
costs for the unwind of discount relating to the provision for
metal-on-metal hip claims.
For the year ended 31 December 2023, charges primarily relate to
legal expenses for ongoing metal-on-metal hip claims partially
offset by a decrease of $8m in the provision that reflects the
present value of the estimated cost to resolve all other known and
anticipated metal-on-metal hip claims, and by the release of a
provision for an intellectual property dispute.
For the years ended 31 December 2024 and 2023, charges also include
the costs for implementing the requirements of the EU Medical
Device Regulation that was effective from May 2021 with a
transition period to May 2024.
Free cash flow
A reconciliation from cash generated from operations, the most
comparable IFRS measure, to free cash flow is set out
below:
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
$m
|
|
$m
|
|
Cash generated from operations
|
1,245
|
|
829
|
|
Capital expenditure
|
(381)
|
|
(427)
|
|
Interest received
|
22
|
|
8
|
|
Interest paid
|
(140)
|
|
(104)
|
|
Payment of lease liabilities
|
(55)
|
|
(52)
|
|
Income taxes paid
|
(140)
|
|
(125)
|
|
Free cash flow
|
551
|
|
129
|
Adjusted leverage ratio
The calculation of the adjusted leverage ratio is set out below.
Adjusted leverage ratio is calculated using metrics similar to
those used in the debt covenant calculation.
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
$m
|
|
$m
|
|
Net debt including lease liabilities
|
2,709
|
|
2,776
|
|
|
|
|
|
|
Attributable profit
|
412
|
|
263
|
|
Taxation
|
86
|
|
27
|
|
Share of results of associates
|
10
|
|
30
|
|
Other finance costs
|
28
|
|
7
|
|
Interest expense
|
145
|
|
132
|
|
Interest income
|
(24)
|
|
(34)
|
|
Acquisition and disposal-related items
|
94
|
|
60
|
|
Restructuring and rationalisation costs
|
123
|
|
220
|
|
Amortisation and impairment of acquisition intangibles
|
187
|
|
207
|
|
Legal and other
|
(12)
|
|
58
|
|
Depreciation of property, plant and equipment
|
325
|
|
306
|
|
Impairment and amortisation of other intangible assets and
impairment of property, plant and equipment
|
67
|
|
51
|
|
Adjusted EBITDA
|
1,441
|
|
1,327
|
|
Adjusted leverage ratio
|
1.9
|
|
2.1
|
Leverage ratio (using closest equivalent IFRS
measures)
The leverage ratio using closest equivalent IFRS measures is not
based on measures used in the calculation of debt covenants and is
not used by management internally. This measure is not used for the
Company’s covenant in its private placement
debt.
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Bank overdrafts, borrowings, loans and lease
liabilities
|
|
63
|
|
765
|
|
Long-term borrowings and lease liabilities
|
|
3,258
|
|
2,319
|
|
Total borrowings
|
|
3,321
|
|
3,084
|
|
|
|
|
|
|
|
Attributable profit
|
|
412
|
|
263
|
|
Leverage ratio
|
|
8.1
|
|
11.7
|
Adjusted return on invested capital
The calculation of adjusted return on invested capital and is set
out below:
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Attributable profit for the year
|
|
412
|
|
263
|
|
Share of results of associates
|
|
10
|
|
30
|
|
Other finance costs
|
|
28
|
|
7
|
|
Interest expense
|
|
145
|
|
132
|
|
Interest income
|
|
(24)
|
|
(34)
|
|
Amortisation and impairment of acquisition intangibles
|
|
187
|
|
207
|
|
Taxation adjustment1
|
|
(73)
|
|
(77)
|
|
Operating profit before amortisation and impairment of acquisition
intangibles less adjusted taxes
|
|
685
|
|
528
|
|
|
|
|
|
|
|
Total equity
|
|
5,265
|
|
5,217
|
|
Accumulated amortisation and impairment of acquisition intangibles
net of associated tax
|
|
1,470
|
|
1,365
|
|
Retirement benefit assets
|
|
(63)
|
|
(69)
|
|
Investments
|
|
(9)
|
|
(8)
|
|
Investments in associates
|
|
(7)
|
|
(16)
|
|
Right-of-use assets
|
|
(173)
|
|
(185)
|
|
Cash and cash equivalents
|
|
(619)
|
|
(302)
|
|
Long-term borrowings and lease liabilities
|
|
3,258
|
|
2,319
|
|
Retirement benefit obligations
|
|
79
|
|
88
|
|
Bank overdrafts, borrowings, loans and lease
liabilities
|
|
63
|
|
765
|
|
Net operating assets
|
|
9,264
|
|
9,174
|
|
Average net operating assets2
|
|
9,219
|
|
8,907
|
|
Adjusted return on invested capital
|
|
7.4%
|
|
5.9%
|
1
Being the taxation
on amortisation and impairment of acquisition intangibles, interest
income, interest expense, other finance costs and share of results
of associates.
2
(Opening net
operating assets + closing net operating assets)/2.
Return on invested capital (using closest equivalent IFRS
measures)
The calculation of return on invested capital using closest
equivalent IFRS measures is set out below:
|
|
|
|
|
|
|
|
|
2024
|
|
2023
|
|
|
|
$m
|
|
$m
|
|
Attributable profit
|
|
412
|
|
263
|
|
Long term borrowings and lease liabilities
|
|
3,258
|
|
2,319
|
|
Bank overdrafts, borrowings, loans and lease
liabilities
|
|
63
|
|
765
|
|
Investments
|
|
(9)
|
|
(8)
|
|
Investments in associates
|
|
(7)
|
|
(16)
|
|
Retirement benefit assets
|
|
(63)
|
|
(69)
|
|
Retirement benefit obligations
|
|
79
|
|
88
|
|
Total equity
|
|
5,265
|
|
5,217
|
|
Invested capital at end of the year
|
|
8,586
|
|
8,296
|
|
Average invested capital for the year
|
|
8,441
|
|
8,149
|
|
Return on invested capital using IFRS measures
|
|
4.9%
|
|
3.2%
|
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the
registrant has duly caused this report to be signed on its behalf
by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
Smith & Nephew plc
|
|
|
|
(Registrant)
|
|
|
|
|
|
|
|
|
|
Date:
February 25, 2025
|
By:
|
/s/
Helen Barraclough
|
|
|
|
Helen
Barraclough
|
|
|
|
Company
Secretary
|
Smith and Nephew (PK) (USOTC:SNNUF)
Historical Stock Chart
From Feb 2025 to Mar 2025
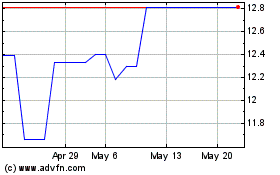
Smith and Nephew (PK) (USOTC:SNNUF)
Historical Stock Chart
From Mar 2024 to Mar 2025