RegenxBio Gets FDA Fast-Track Designation for RGX-202 to Treat Duchenne Muscular Dystrophy
April 11 2023 - 7:54AM
Dow Jones News
By Chris Wack
RegenxBio Inc. said Tuesday that the Food and Drug
Administration has granted fast-track designation for RGX-202, a
potential one-time gene therapy to treat Duchenne muscular
dystrophy.
The designation aims to speed up the development and expedite
the review of new therapeutics that are intended to treat serious
or life-threatening conditions and that demonstrate the potential
to address unmet medical needs, the Rockville, Md.-based company
said.
Therapies granted the fast-track designation have the
opportunity for more frequent interactions with the agency and may
qualify for priority review, the company said.
The FDA already granted RGX-202 orphan drug designation and rare
pediatric disease designation, RegenxBio said.
The company said it plans to report initial data from its
clinical trial of RGX-202 in the second half of 2023.
RegenxBio shares were up 5% to $18.13 in premarket trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
April 11, 2023 07:39 ET (11:39 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
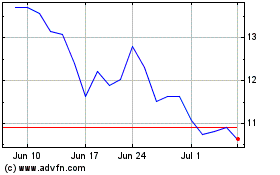
REGENXBIO (NASDAQ:RGNX)
Historical Stock Chart
From Jun 2024 to Jul 2024
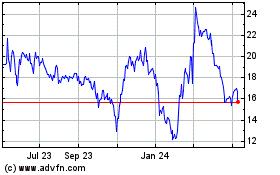
REGENXBIO (NASDAQ:RGNX)
Historical Stock Chart
From Jul 2023 to Jul 2024