UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the Month of January 2025
Commission File Number: 001-38104
IMMURON LIMITED
(Name of Registrant)
Level 3, 62 Lygon Street, Carlton South,
Victoria, 3053, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form
40-F ☐
Indicate by check mark whether by furnishing the information contained
in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities
Exchange Act of 1934.
Yes ☐ No
☒
If “Yes” is marked, indicate below the file number assigned
to the registrant in connection with Rule 12g3-2(b): 82-
IMMURON LIMITED
EXPLANATORY NOTE
Immuron Limited (the “Company”) published
two announcements (the “Public Notices”) to the Australian Securities Exchange on January 15, 2025 titled:
| |
- |
“Immuron Announces Monash AMR Research Collaboration” |
| |
|
|
| |
- |
“Proposed issue of securities – IMC” |
A copy of the Public Notices are attached as an exhibit to this report
on Form 6-K.
This report on Form 6-K (including the exhibit
hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”) and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, except as shall be
expressly set forth by specific reference in such filing.
EXHIBITS
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
IMMURON LIMITED |
| |
|
|
| Date: January 15, 2025 |
By: |
/s/ Phillip Hains |
| |
|
Phillip Hains |
| |
|
Company Secretary |
3
Exhibit 99.1

Immuron Announces New Research Collaboration
targeting Antimicrobial Resistance
Highlights:
| ● | New Research Collaboration with Monash University
|
| ● | One proposal will target Antimicrobial Resistant
Pathways to develop broad spectrum therapeutic drug products |
| ● | Second project proposal will focus on the
Develop of new therapeutic drug candidates against Vancomycin-resistant enterococci (VRE) |
Melbourne, Australia, January 15, 2025: Immuron
Limited (ASX: IMC; NASDAQ: IMRN), an Australian based and globally integrated biopharmaceutical company is pleased to announce a new research
collaboration agreement with Monash University.
The major objective of this research collaboration
is to develop new therapeutic drug candidates which target antimicrobial resistant pathogens. This work will utilize the Immuron technology
platform, and the extensive experience of the Biomedicine Discovery Institute research team lead by Professor Dena Lyras.
The research collaboration is effective and will
continue whilst there are relevant research activities being performed under the research plan. No additional or new funding is required
for the initial activities by Immuron for the strategic collaboration. The funding of Immuron’s research activities is allowed for
in the Company’s existing research budget.
After the results from this research agreement
are known, the parties will negotiate in good faith (and without obligation) whether to jointly develop or commercialise the outcomes
of these research collaborations on commercially reasonable terms.
The first project proposal will focus on the underlying
mechanisms which bacteria utilise to share and transfer their DNA. A process which can rapidly alter the functional capacity and characteristics
of a bacterium, resulting in the emergence of antimicrobial resistance (AMR) with the aim to develop broad spectrum therapeutic drug products.
Antimicrobial resistance (AMR) poses a significant
threat to healthcare systems worldwide. AMR can lead to more severe and harder-to-treat infections in healthcare settings, such as hospitals
and nursing homes. These infections often result in longer hospital stays, higher medical costs, and increased mortality rates. In the
U.S., the estimated national cost to treat these infections exceeds $4.6 billion annually (CDC Antimicrobial Resistance Facts and Stats:
https://www.cdc.gov/antimicrobial-resistance/data-research/facts-stats/index.html).


The second project proposal will specifically
target Vancomycin-resistant enterococci (VRE) and as the name suggests VRE are bacteria that are resistant to the antibiotic vancomycin.
VRE are opportunistic nosocomial pathogens that have emerged as a major healthcare problem worldwide. The two most clinically significant
enterococci, Enterococcus faecalis and Enterococcus faecium, are associated with a range of nosocomial infections in elderly
and immunosuppressed patients. VRE complicates outcomes for at-risk patients, increasing their risk of developing subsequent infections
and/or transmitting VRE to other patients. VRE colonisation has been associated with an increased risk of bacteremia, infections at other
body sites and can also lead, in severe cases, to mortality.
The global market for antibiotics is projected
to reach $57.0 billion by 2026 with a compound annual growth rate (CAGR) of 4.0%. The rising prevalence of drug-resistant infections,
including VRE, is expected to drive the demand for new and innovative treatments in this space.
This release has been authorised by the directors
of Immuron Limited.
- - - END - - -
|
COMPANY CONTACT:
Steven Lydeamore
Chief Executive Officer
steve@immuron.com |
|
|
About Immuron
Immuron Limited (ASX:
IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal
antibodies for the treatment of infectious diseases.
About Travelan®
Travelan® is an
orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea, a
digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a highly
purified tabletized preparation of hyper immune bovine antibodies and other factors, which when taken with meals bind to diarrhea-causing
bacteria and prevent colonization and the pathology associated with travelers’ diarrhea. In Australia, Travelan® is a listed
medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Travelers’ Diarrhea,
reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product
(NPN 80046016) and is indicated to reduce the risk of Travelers’ Diarrhea. In the U.S., Travelan® is sold as a dietary supplement
for digestive tract protection.
Travelers’
diarrhea (TD)
TD is generally defined
as the passage of ≥ 3 unformed stools per 24 hours plus at least one additional symptom (such as nausea, vomiting, abdominal cramps,
fever, blood/mucus in the stools, or fecal urgency) that develop while abroad or within 10 days of returning from any resource-limited
destinations (Leung et al., 2006). Diarrhea continues to be the most frequent health problem among travelers to destinations in lower-
and middle-income regions (Steffen, 2017). Deployed US military personnel, essentially representing a long-term traveller population,
are particularly affected given their population dynamics and the context in which they seek care and treatment (Connor et al., 2012).
Diarrhea is the leading infectious disease threat to the overall health and preparedness of deployed US armed forces, with diarrheagenic
E. coli, Campylobacter spp., and Shigella spp. among the most commonly reported etiologies (Riddle et al., 2006).


Immuron Platform Technology
Immuron’s proprietary
technology is based on polyclonal immunoglobulins (IgG) derived from engineered hyper-immune bovine colostrum. Immuron has the capability
of producing highly specific immunoglobulins to any enteric pathogen and our products are orally active. Bovine IgG can withstand the
acidic environment of the stomach and is resistant to proteolysis by the digestive enzymes found in the Gastrointestinal (GI) tract. Bovine
IgG also possesses this unique ability to remain active in the human GI tract delivering its full benefits directly to the bacteria found
there. The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of infectious
diseases. The platform can be used to block viruses or bacteria at mucosal surfaces such as the Gastrointestinal tract and neutralize
the toxins they produce.
IMM-124E (Travelan®)
IMM-124E was developed
using Immuron’s platform technology. IMM-124E is produced from the colostrum of birthing cattle that have been immunised during
pregnancy with a vaccine containing the outer antigens of multiple human derived ETEC. A total of 13 ETEC strains are used in the vaccine
to produce high levels of antibodies against selected surface antigens from the most common strains of ETEC.
The resultant hyperimmune
colostrum IMM-124E from ETEC vaccinated cows contains significant levels of polyclonal antibodies specific for ETEC antigens LPS, CFA-I
and Flagellin (Sears et al., 2017).
The antibodies produced
in IMM-124E have been found to have a stronger binding and neutralizing activity (than the antibodies of unvaccinated cattle) against
a wide range of LPS antigens including both the variable O-polysaccharide region and the preserved oligosaccharide core ‘R’
region of LPS from the 13 serotypes used in the ETEC vaccine.
IMM-124E is manufactured
into a tablet form referred to as Travelan®.
IMM-529
Immuron is developing IMM-529 as an adjunctive
therapy in combination with standard of care antibiotics for the prevention and/or treatment of recurrent Clostridioides difficile infection
(CDI). IMM-529 antibodies targeting Clostridioides difficile (C. diff) may help to clear CDI infection and promote a quicker re-establishment
of normal gut flora, providing an attractive oral preventative for recurrent CDI.
Immuron is collaborating with Dr. Dena Lyras
and her team at Monash University, Australia to develop vaccines to produce bovine colostrum-derived antibodies. Dairy cows were immunised
to generate hyperimmune bovine colostrum (HBC) that contains antibodies targeting three essential C. diff virulence components. IMM-529
targets Toxin B (TcB), the spores and the surface layer proteins of the vegetative cells.
This unique 3-target approach has yielded
promising results in pre-clinical infection and relapse models, including (1) Prevention of primary disease (80% P =0.0052); (2) Protection
of disease recurrence (67%, P <0.01) and (3) Treatment of primary disease (78.6%, P<0.0001; TcB HBC). Importantly IMM-529 antibodies
cross-react with whole cell lysates of many different human strains of C. diff including hypervirulent strains.
To our knowledge, IMM-529 is, to date, the
only investigational drug that has shown therapeutic potential in all three phases of the disease (Hutton et al., 2017).


References
Connor P, Porter CK,
Swierczewski B and Riddle MS. Diarrhea during military deployment: current concepts and future directions. Curr Opin Infect Dis. 25(5):
546-54; 2012.
Hutton, M.L., Cunningham,
B.A., Mackin, K.E. et al. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative.
Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598-017-03982-5
Leung AK, Robson WL,
Davies HD. Travelers’ diarrhea. Adv Ther. Jul-Aug; 23(4): 519-27; 2006
Riddle MS, Sanders JW,
Putnam SD, and Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers’ (US military and similar populations):
A systematic review. American Journal of Tropical Medicine and Hygiene. 74(5): 891-900; 2006.
Sears KT, Tennant SM,
Reymann MK, Simon R, Konstantopolos N, Blackwelder WC, Barry EM and Pasetti MF. Bioactive Immune Components of Anti-Diarrheagenic Enterotoxigenic
Escherichia coli Hyperimmune Bovine Colostrum products. Clinical and Vaccine Immunology. 24 (8) 1-14; 2017.
Steffen R. Epidemiology
of travelers’ diarrhea. J Travel Med. 24(suppl_1): S2-S5; 2017.
For more information
visit: https://www.immuron.com.au/ and https://www.travelan.com
Subscribe for Immuron
News: Here
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934,
each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development
programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations
and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition, and stock
value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth
strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the
results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties
relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key
personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and
intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation
or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in
our expectations or any changes in events, conditions, or circumstances on which any such statement is based, except as required by law.
crobiological and Biomedical Laboratories.

Exhibit 99.2
 |
Appendix
3B - Proposed issue of securities |
Entity name
IMMURON LIMITED
Announcement Type
New announcement
Date of this announcement
15/1/2025
The Proposed issue is:
A placement or other type of issue
Total number of +securities proposed to be issued for
a placement or other type of issue
| ASX +security code |
Security description |
Maximum Number of
+securities to be issued
|
| |
|
|
| IMC |
ORDINARY FULLY PAID |
1,801,680 |
Proposed +issue date
15/1/2025
Refer to next page for full details of the announcement
| Appendix 3B - Proposed issue of securities | 1/5 |
 | Appendix 3B - Proposed issue of securities |
| Part 1 - Entity and announcement details |
|
| |
|
1.1 Name of +Entity
IMMURON LIMITED
We (the entity named above) give ASX the following information
about a proposed issue of +securities and, if ASX agrees to +quote any of the +securities (including any rights) on a +deferred settlement
basis, we agree to the matters set out in Appendix 3B of the ASX Listing Rules.
If the +securities are being offered under a +disclosure
document or +PDS and are intended to be quoted on ASX, we also apply for quotation of all of the +securities that may be issued under
the +disclosure document or +PDS on the terms set out in Appendix 2A of the ASX Listing Rules (on the understanding that once the final
number of +securities issued under the +disclosure document or +PDS is known, in accordance with Listing Rule 3.10.3C, we will complete
and lodge with ASX an Appendix 2A online form notifying ASX of their issue and applying for their quotation).
| |
1.2 Registered Number Type |
Registration Number |
| |
|
|
| |
ACN |
063114045 |
1.3 ASX issuer code
IMC
1.4 The announcement is
New announcement
1.5 Date of this announcement
15/1/2025
1.6 The Proposed issue is:
A placement or other type of issue
| Appendix 3B - Proposed issue of securities | 2/5 |
 | Appendix 3B - Proposed issue of securities |
| Part 7 - Details of proposed placement or other issue |
|
| |
|
7A.1 Do any external approvals need to be obtained or
other conditions satisfied before the placement or other type of issue can proceed on an unconditional basis?
No
| |
Is the proposed security a ‘New class’
(+securities in a class that is not yet quoted or recorded by ASX) or an ‘Existing class’ (additional securities in a class that is already
quoted or recorded by ASX)?
|
|
Will the proposed issue of this+security include an offer of attaching +securities? |
|
| |
|
|
|
|
| |
Existing class |
|
No |
|
Details of +securities proposed to be issued
ASX +security code and description
IMC : ORDINARY FULLY PAID
Number of +securities proposed to
be issued
1,801,680
Offer price details
Are the +securities proposed to be
issued being issued for a cash consideration?
Yes
| |
In what currency is the cash consideration being paid? |
What is the issue price per+security? |
| |
|
|
| |
AUD - Australian Dollar |
AUD 0.08470 |
Will these +securities rank equally
in all respects from their issue date with the existing issued +securities in that class?
Yes
| Appendix 3B - Proposed issue of securities | 3/5 |
 | Appendix 3B - Proposed issue of securities |
7C.1 Proposed +issue date
15/1/2025
| Part 7D - Listing Rule requirements |
|
| |
|
7D.1 Has the entity obtained, or
is it obtaining, +security holder approval for the entire issue under listing rule 7.1?
No
7D.1b Are any of the +securities
proposed to be issued without +security holder approval using the entity’s 15% placement capacity under listing rule 7.1?
No
7D.1c Are any of the +securities
proposed to be issued without +security holder approval using the entity’s additional 10% placement capacity under listing rule 7.1A
(if applicable)?
Yes
7D.1c ( i ) How many +securities
are proposed to be issued without +security holder approval using the entity’s additional 10% placement capacity under listing rule 7.1A?
1,801,680
7D.1c ( ii ) Please explain why the
entity has chosen to do a placement rather than a +pro rata issue or an offer under a +security purchase plan in which existing ordinary
+security holders would have been eligible to participate
Due to the costs and timing benefits of completing the placement.
7D.2 Is a party referred to in listing
rule 10.11 participating in the proposed issue?
No
7D.3 Will any of the +securities
to be issued be +restricted securities for the purposes of the listing rules?
No
7D.4 Will any of the +securities
to be issued be subject to +voluntary escrow?
No
| Part 7E - Fees and expenses |
|
| |
|
7E.1 Will there be a lead manager
or broker to the proposed issue?
No
7E.2 Is the proposed issue to be
underwritten?
No
7E.4 Details of any other material
fees or costs to be incurred by the entity in connection with the proposed issue
| Appendix 3B - Proposed issue of securities | 4/5 |
 | Appendix 3B - Proposed issue of securities |
| Part 7F - Further Information |
|
| |
|
7F.01 The purpose(s) for which the
entity is issuing the securities
To further research and development
activities and provide on-going working capital.
7F.1 Will the entity be changing
its dividend/distribution policy if the proposed issue proceeds?
No
7F.2 Any other information the entity
wishes to provide about the proposed issue
Securities issued under an ATM
trade in line with the Form F-3 and ATM Prospectus lodged on 3 July 2024.
7F.3 Any on-sale of the +securities
proposed to be issued within 12 months of their date of issue will comply with the secondary sale provisions in sections 707(3) and 1012C(6)
of the Corporations Act by virtue of:
The publication of a cleansing notice under section 708A(5),
708AA(2)(f), 1012DA(5) or 1012DAA(2)(f)
| Appendix 3B - Proposed issue of securities | 5/5 |
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Dec 2024 to Jan 2025
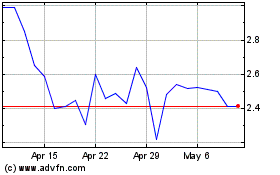
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Jan 2024 to Jan 2025