false
0001726711
0001726711
2024-10-28
2024-10-28
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 OR 15(d) of the
Securities
Exchange Act of 1934
Date
of Report (Date of earliest event reported): October 28, 2024
Aditxt, Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-39336 |
|
82-3204328 |
(State
or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS
Employer
Identification No.) |
| 2569 Wyandotte Street, Suite 101, Mountain View, CA |
|
94043 |
| (Address of principal executive
offices) |
|
(Zip Code) |
Registrant’s
telephone number, including area code: (650) 870-1200
N/A
(Former
name or former address, if changed since last report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written communications
pursuant to Rule 425 under the Securities Act (17 CFR 230.425 ) |
| |
|
| ☐ |
Soliciting material pursuant
to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title of
each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common Stock, par value $0.001 |
|
ADTX |
|
The
Nasdaq Stock Market LLC |
Indicate
by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933
(§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☒
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item
1.01 Entry Into a Material Definitive Agreement
On
October 28, 2024 (the “Closing Date”), Aditxt, Inc. (the “Company”) entered into a Securities Purchase
Agreement (the “Series F-1 Securities Purchase Agreement”) with Evofem Biosciences, Inc. (“Evofem”),
pursuant to which the Company purchased 2,280 shares of Evofem Series F-1 Convertible Preferred Stock (the “Evofem Series F-1
Preferred Stock”) for an aggregate purchase price of $2,280,000. In connection with the Series F-1 Securities Purchase Agreement,
the Company and Evofem entered into a Registration Rights Agreement (the “Registration Rights Agreement”), pursuant
to which Evofem agreed to file with the SEC a registration statement covering the resale of the shares of its common stock issuable upon
conversion of the Evofem Series F-1 Preferred Stock within 300 days of the Closing Date and to have such registration statement declared
effective by the SEC the earlier of the (i) 90th calendar day after the Closing Date and (ii) 2nd Business
Day after the date Evofem is notified (orally or in writing, whichever is earlier) by the SEC that such registration statement will not
be reviewed or will not be subject to further review.
The
foregoing descriptions of the Series F-1 Securities Purchase Agreement and Registration Rights Agreement are not complete and are qualified
in their entirety by reference to the full text of the forms of the Series F-1 Securities Purchase Agreement and Registration Rights
Agreement, copies of which are filed as Exhibit 10.1 and Exhibit 10.2, respectively to this Current Report
on Form 8-K and are incorporated by reference herein.
Item
7.01 Regulation FD Disclosure.
On October 28, 2024, the Company hosted a fireside chat and Q&A
session led by Amro Albanna, its Chief Executive Officer, and Saundra Pelletier, Chief Executive Officer of Evofem. The fireside chat
was moderated by Dr. Drew Pinsky. A copy of the transcript of the fireside chat is furnished as Exhibit 99.1 to this Current Report on
Form 8-K and is incorporated herein by reference.
The
information contained in this item, including that incorporated by reference, is being furnished to the Securities and Exchange Commission.
Such information shall not be deemed "filed" for purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise
subject to the liabilities of that Section. The information shall not be deemed incorporated by reference into any registration statement
or other document filed pursuant to the Securities Act of 1933, except as expressly set forth by specific reference in such filing.
Cautionary
Note on Forward-Looking Statements
This
Current Report on Form 8-K contains certain forward-looking statements within the meaning of the “safe harbor “provisions
under the United States Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained
in this Current Report on Form 8-K,
including statements regarding the Company’s future results of operations and financial position are forward-looking statements.
These forward-looking statements generally are identified by the words “believe,” “project,” “expect,”
“anticipate,” “estimate,” “target,” “intend,” “strategy,” “future,”
“opportunity,” “plan,” “may,” “should,” “will,” “would,” “will
be,” “will continue,” “will likely result,” and similar expressions. These statements are based
on various assumptions, whether or not identified in this Current Report on Form 8-K, and on the current expectations of the management
team of the Company and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes
only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive
statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions.
Many actual events and circumstances are beyond the control of the Company.
These
forward-looking statements are subject to a number of risks including, but not limited to, the following risks relating to the proposed
transactions: (1) the risk that the proposed transactions may not be completed in a timely manner or at all, which may adversely affect
the price of the Company’s securities; (2) the failure to satisfy the conditions to the closing, including the approval by the
stockholders of the Company; (3) the ability to realize the anticipated benefits of the proposed transactions; and
(4) other risks and uncertainties indicated from time to time in the Company’s public filings with the SEC. If any of these risks
materialize or the Company’s assumptions prove incorrect, actual results could differ materially from the results implied by these
forward-looking statements. You should carefully consider the risks and uncertainties described in the “Risk Factors” section
of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and other documents we filed, or will file, including the
proxy statement/prospectus, with the SEC. There may be additional risks that the Company does not presently know, or that the Company
currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements.
In addition, forward-looking statements reflect the Company’s expectations, plans or forecasts of future events and views as of
the date of this Current Report on Form 8-K. The Company anticipates that subsequent events and developments will cause the Company’s
assessments to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the
Company specifically disclaim any obligation to do so, except as otherwise required by law. These forward-looking statements should not
be relied upon as representing the Company’s assessments of any date subsequent to the date of this Current Report on Form 8-K.
Accordingly, undue reliance should not be placed upon the forward-looking statements.
Disclaimer:
The
information contained in the transcript furnished as Exhibit 99.1 is a textual representation of an audio recording of the fireside chat
and while efforts are made to provide an accurate transcription, there may be material errors, omissions or inaccuracies in the reporting
of the substance of the audio recording. The Company does not assume any responsibility for any investment or other decisions made based
upon the information provided in this transcript. Users are advised to review the audio recording and the Company’s SEC filings
before making any investment or other decisions. An archived recording of the fireside chat will be available for 30 days on the “Investor
Relations” section of the Company’s website at www.aditxt.com.
Item
9.01. Financial Statements and Exhibits.
(d)
Exhibits.
SIGNATURE
Pursuant
to the requirements of the Securities and Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf
by the undersigned hereunto duly authorized.
Dated:
October 30, 2024
| |
Aditxt,
Inc. |
| |
|
|
| |
By: |
/s/
Amro Albanna |
| |
Name: |
Amro Albanna |
| |
Title: |
Chief
Executive Officer |
-
3 -
Exhibit 10.1
Execution Version
SECURITIES
PURCHASE AGREEMENT
This
SECURITIES PURCHASE AGREEMENT (the “Agreement”), dated as of October 28, 2024 is by and among Evofem Biosciences,
Inc., a Delaware corporation with offices located at 7770 Regents Road, Suite 113-618, San Diego, CA 92122 (the “Company”),
and Aditxt, Inc. (the “Buyer”).
RECITALS
A. The
Company and the Buyer are executing and delivering this Agreement in reliance upon the exemption from securities registration afforded
by Section 4(a)(2) of the Securities Act of 1933, as amended (the “1933 Act”).
B. The
Company has authorized a new series of convertible preferred stock of the Company designated as Series F-1 Convertible Preferred Stock,
$0.0001 par value, the terms of which are set forth in the amended and restated certificate of designation for such series of Preferred
Stock (the “Certificate of Designations”) in the form attached hereto as Exhibit A (together with any
convertible preferred shares issued in replacement thereof in accordance with the terms thereof, the “Series F-1 Preferred Stock”),
which Series F-1 Preferred Stock shall be convertible into shares of its Common Stock, par value $0.0001 per share (“Common
Stock”, and such shares of Common Stock issuable pursuant to the terms of the Certificate of Designations, including, without
limitation, upon conversion or otherwise, collectively, the “Conversion Shares”), in accordance with the terms of
the Certificate of Designations.
C. The
Buyer wishes to purchase, and the Company wishes to sell, upon the terms and conditions stated in this Agreement, (i) the aggregate number
of shares of Series F-1 Preferred Stock (the “Preferred Shares”) set forth opposite the Buyer’s name in column
(3) on the Schedule of Buyers.
D. At
the Closing, the parties hereto shall execute and deliver a Registration Rights Agreement, in the form attached hereto as Exhibit
C (the “Registration Rights Agreement”), pursuant to which the Company has agreed to provide certain registration
rights with respect to the Registrable Securities (as defined in the Registration Rights Agreement), under the 1933 Act and the rules
and regulations promulgated thereunder, and applicable state securities laws.
D. The
Preferred Shares and the Conversion Shares are collectively referred to herein as the “Securities.”
AGREEMENT
NOW,
THEREFORE, in consideration of the premises and the mutual covenants contained herein and for other good and valuable consideration,
the receipt and sufficiency of which are hereby acknowledged, the Company and the Buyer hereby agree as follows:
1.
PURCHASE AND SALE OF PREFERRED SHARES.
(a)
Purchase of Preferred Shares . Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6 and 7 below,
on the date hereof (the “Closing Date”) the Company shall issue and sell to the Buyer, and the Buyer agrees to purchase from
the Company on the Closing Date the aggregate number of Preferred Shares as is set forth opposite the Buyer’s name in column (3)
on the Schedule of Buyers.
(b)
Closing. The closing (the “Closing”) of the purchase of the Preferred Shares by the Buyer shall occur at the
offices of Sheppard, Mullin, Richter & Hampton LLP, 30 Rockefeller Plaza, New York, NY 10112-0015 on the Closing Date. As used herein
“Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New
York are authorized or required by law to remain closed; provided, however, for clarification,
commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”,
“non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations
at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial
banks in The City of New York generally are open for use by customers on such day.
(c)
Purchase Price. The aggregate purchase price for the Preferred Shares to be purchased by the Buyer (the “Purchase Price”)
shall be the amount set forth opposite the Buyer’s name in column (5) on the Schedule of Buyers.
(d)
Form of Payment. On the Closing Date, (i) the Buyer shall pay its Purchase Price to the Company for the Preferred Shares to be
issued and sold to the Buyer at the Closing, by wire transfer of immediately available funds in accordance with the Flow of Funds Letter
(as defined below) and (ii) the Company shall deliver to the Buyer (A) the aggregate number of Preferred Shares as is set forth
opposite the Buyer’s name in column (3) of the Schedule of Buyers, duly executed on behalf of the Company and registered in the
name of the Buyer or its designee.
2.
BUYER’S REPRESENTATIONS AND WARRANTIES.
The
Buyer represents and warrants to the Company that as of the date hereof:
(a)
Organization; Authority. The Buyer is an entity duly organized, validly existing and in good standing under the laws of the jurisdiction
of its organization with the requisite power and authority to enter into and to consummate the transactions contemplated by the Transaction
Documents (as defined below) to which it is a party and otherwise to carry out its obligations hereunder and thereunder.
(b)
No Public Sale or Distribution. The Buyer (i) is acquiring its Preferred Shares, (ii) upon conversion of its Preferred Shares
will acquire the Conversion Shares issuable upon conversion thereof, for its own account and not with a view towards, or for resale in
connection with, the public sale or distribution thereof in violation of applicable securities laws, except pursuant to sales registered
or exempted under the 1933 Act; provided, however, by making the representations herein, the Buyer does not agree, or make any representation
or warranty, to hold any of the Securities for any minimum or other specific term and reserves the right to dispose of the Securities
at any time in accordance with or pursuant to a registration statement or an exemption from registration under the 1933 Act. The Buyer
does not presently have any agreement or understanding, directly or indirectly, with any Person to distribute any of the Securities in
violation of applicable securities laws. For purposes of this Agreement, “Person” means an individual, a limited liability
company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization, any other entity and any Governmental
Entity or any department or agency thereof.
(c)
Omitted.
(d)
Reliance on Exemptions. The Buyer understands that the Securities are being offered and sold to it in reliance on specific exemptions
from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the
truth and accuracy of, and such Buyer’s compliance with, the representations, warranties, agreements, acknowledgments and understandings
of the Buyer set forth herein in order to determine the availability of such exemptions and the eligibility of the Buyer to acquire the
Securities.
(e)
Information. The Buyer and its advisors, if any, have been furnished with all materials relating to the business, finances and
operations of the Company and materials relating to the offer and sale of the Securities that have been requested by such Buyer and/or
included in the SEC Documents (as hereinafter defined). The Buyer and its advisors, if any, have been afforded the opportunity to ask
questions of the Company. Neither such inquiries nor any other due diligence investigations conducted by the Buyer or its advisors, if
any, or its representatives shall modify, amend or affect the Buyer’s right to rely on the Company’s representations and
warranties contained herein. The Buyer understands that its investment in the Securities involves a high degree of risk. The Buyer has
sought such accounting, legal and tax advice as it has considered necessary to make an informed investment decision with respect to its
acquisition of the Securities.
(f)
No Governmental Review. The Buyer understands that no United States federal or state agency or any other government or governmental
agency has passed on or made any recommendation or endorsement of the Securities or the fairness or suitability of the investment in
the Securities nor have such authorities passed upon or endorsed the merits of the offering of the Securities.
(g)
Transfer or Resale. The Buyer understands that except as provided in the Registration Rights Agreement and Section 4(h) hereof:
(i) the Securities have not been and are not being registered under the 1933 Act or any state securities laws, and may not be offered
for sale, sold, assigned or transferred unless (A) subsequently registered thereunder, (B) the Buyer shall have delivered to the Company
(if requested by the Company) an opinion of counsel, in a form reasonably acceptable to the Company, to the effect that such Securities
to be sold, assigned or transferred may be sold, assigned or transferred pursuant to an exemption from such registration, or (C) the
Buyer provides the Company with reasonable assurance that such Securities can be sold, assigned or transferred pursuant to Rule 144 or
Rule 144A promulgated under the 1933 Act (or a successor rule thereto) (collectively, “Rule 144”); (ii) any sale of
the Securities made in reliance on Rule 144 may be made only in accordance with the terms of Rule 144, and further, if Rule 144 is not
applicable, any resale of the Securities under circumstances in which the seller (or the Person through whom the sale is made) may be
deemed to be an underwriter (as that term is defined in the 1933 Act) may require compliance with some other exemption under the 1933
Act or the rules and regulations of the United States Securities Commission (the “SEC”) promulgated thereunder; and
(iii) neither the Company nor any other Person is under any obligation to register the Securities under the 1933 Act or any state securities
laws or to comply with the terms and conditions of any exemption thereunder.
(h)
Validity; Enforcement. This Agreement and the Registration Rights have been duly and validly authorized, executed and delivered
on behalf of the Buyer and shall constitute the legal, valid and binding obligations of the Buyer enforceable against the Buyer in accordance
with their respective terms, except as such enforceability may be limited by general principles of equity or to applicable bankruptcy,
insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable
creditors’ rights and remedies.
(i)
No Conflicts. The execution, delivery and performance by such Buyer of this Agreement and the Registration Rights Agreement and
the consummation by such Buyer of the transactions contemplated hereby and thereby will not (i) result in a violation of the organizational
documents of such Buyer, or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would
become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture
or instrument to which such Buyer is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree
(including federal and state securities laws) applicable to such Buyer, except in the case of clauses (ii) and (iii) above, for such
conflicts, defaults, rights or violations which could not, individually or in the aggregate, reasonably be expected to have a material
adverse effect on the ability of such Buyer to perform its obligations hereunder.
3.
REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
The
Company represents and warrants to the Buyer that as of the date hereof:
(a)
Organization and Qualification. Each of the Company and each of its Subsidiaries are entities duly organized and validly existing
and in good standing under the laws of the jurisdiction in which they are formed, and have the requisite power and authority to own their
properties and to carry on their business as now being conducted and as presently proposed to be conducted. Each of the Company and each
of its Subsidiaries is duly qualified as a foreign entity to do business and is in good standing in every jurisdiction in which its ownership
of property or the nature of the business conducted by it makes such qualification necessary, except to the extent that the failure to
be so qualified or be in good standing would not reasonably be expected to have a Material Adverse Effect (as defined below). As used
in this Agreement, “Material Adverse Effect” means any material adverse effect on (i) the business, properties, assets,
liabilities, operations (including results thereof), condition (financial or otherwise) or prospects of the Company or any Subsidiary,
individually or taken as a whole, (ii) the transactions contemplated hereby or in any of the other Transaction Documents or any other
agreements or instruments to be entered into in connection herewith or therewith or (iii) the authority or ability of the Company or
any of its Subsidiaries to perform any of their respective obligations under any of the Transaction Documents (as defined below). Other
than the Persons (as defined below) set forth on Schedule 3(a), the Company has no Subsidiaries. “Subsidiaries”
means any Person in which the Company, directly or indirectly, (I) owns any of the outstanding capital stock or holds any equity or similar
interest of such Person or (II) controls or operates all or any part of the business, operations or administration of such Person, and
each of the foregoing, is individually referred to herein as a “Subsidiary.”
(b)
Authorization; Enforcement; Validity. The Company has the requisite power and authority to enter into and perform its obligations
under this Agreement and the other Transaction Documents and to issue the Securities in accordance with the terms hereof and thereof.
Each Subsidiary has the requisite power and authority to enter into and perform its obligations under the Transaction Documents to which
it is a party. The execution and delivery of this Agreement and the other Transaction Documents by the Company, and the consummation
by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Preferred Shares
and the reservation for issuance and issuance of the Conversion Shares issuable upon conversion of the Preferred Shares and the reservation
for issuance) have been duly authorized by the Company’s board of directors or other governing body, as applicable, and (other
than the filing with the SEC of one or more Registration Statements in accordance with the requirements of the Registration Rights Agreement,
a Form D with the SEC and any filings as may be required by any state securities agencies) no further filing, consent or authorization
is required by the Company, its Subsidiaries, their respective boards of directors or their stockholders or other governing body. This
Agreement has been, and the other Transaction Documents to which it is a party will be prior to the Closing, duly executed and delivered
by the Company, and each constitutes the legal, valid and binding obligations of the Company, enforceable against the Company in accordance
with its respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency,
reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’
rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities law. The
Certificate of Designations in the form attached hereto as Exhibit A has been filed with the Secretary of State of the State of Delaware
and is in full force and effect, enforceable against the Company in accordance with its terms and has not have been amended. “Transaction
Documents” means, collectively, this Agreement, the Preferred Shares, the Certificate of Designations, the Registration Rights
Agreement, the Irrevocable Transfer Agent Instructions (as defined below) and each of the other agreements and instruments entered into
or delivered by any of the parties hereto in connection with the transactions contemplated hereby and thereby, as may be amended from
time to time.
(c)
Issuance of Securities. The issuance of the Preferred Shares are duly authorized and upon issuance in accordance with the terms
of the Transaction Documents shall be validly issued, fully paid and non-assessable and free from all preemptive or similar rights, mortgages,
defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances (collectively
“Liens”) with respect to the issuance thereof. As of the Closing, the Company shall have reserved from its duly authorized
capital stock not less than the sum of (i) 150% of the maximum number of Conversion Shares issuable upon conversion of the Preferred
Shares (assuming for purposes hereof that (x) the Preferred Shares are convertible at the Alternate Conversion Price (as defined in the
Certificate of Designations) assuming an Alternate Conversion Date (as defined in the Certificate of Designations) as of the date hereof,
and (y) any such conversion shall not take into account any limitations on the conversion of the Preferred Shares set forth in the Certificate
of Designations). Upon issuance or conversion in accordance with the Preferred, the Conversion Shares, when issued, will be validly issued,
fully paid and nonassessable and free from all preemptive or similar rights or Liens with respect to the issue thereof, with the holders
being entitled to all rights accorded to a holder of Common Stock. Subject to the accuracy of the representations and warranties of the
Buyer in this Agreement, the offer and issuance by the Company of the Securities is exempt from registration under the 1933 Act.
(d)
No Conflicts. Except as disclosed in the SEC Documents and/or Schedule 3(d), the execution, delivery and performance of the Transaction
Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without
limitation, the issuance of the Preferred Shares, the Conversion Shares and the reservation for issuance of the Conversion Shares will
not (i) result in a violation of the Certificate of Incorporation (as defined below) (including, without limitation, any certificate
of designation contained therein), Bylaws (as defined below), certificate of formation, memorandum of association, articles of association,
bylaws or other organizational documents of the Company or any of its Subsidiaries, or any capital stock or other securities of the Company
or any of its Subsidiaries, (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would
become a default) in any respect under, or give to others any rights of termination, amendment, acceleration or cancellation of, any
agreement, indenture or instrument to which the Company or any of its Subsidiaries is a party, or (iii) result in a violation of any
law, rule, regulation, order, judgment or decree (including, without limitation, foreign, federal and state securities laws and regulations
and the rules and regulations of OTC Markets Group (the “Principal Market”) and including all applicable foreign,
federal and state laws, rules and regulations) applicable to the Company or any of its Subsidiaries or by which any property or asset
of the Company or any of its Subsidiaries is bound or affected.
(e)
Consents. Except for disclosed on the SEC Documents and/or Schedule 3(e), neither the Company nor any Subsidiary is required to
obtain any consent from, authorization or order of, or make any filing or registration with (other than the filing with the SEC of one
or more Registration Statements in accordance with the requirements of the Registration Rights Agreement, a Form D with the SEC and any
other filings as may be required by any state securities agencies), any Governmental Entity (as defined below) or any regulatory or self-regulatory
agency or any other Person in order for it to execute, deliver or perform any of its respective obligations under or contemplated by
the Transaction Documents, in each case, in accordance with the terms hereof or thereof. All consents, authorizations, orders, filings
and registrations which the Company or any Subsidiary is required to obtain pursuant to the preceding sentence have been or will be obtained
or effected on or prior to the Closing Date, and neither the Company nor any of its Subsidiaries are aware of any facts or circumstances
which might prevent the Company or any of its Subsidiaries from obtaining or effecting any of the registration, application or filings
contemplated by the Transaction Documents. Excepts as disclosed in the SEC Documents and the Schedules hereto, the Company is not in
violation of the requirements of the Principal Market and has no knowledge of any facts or circumstances which could reasonably lead
to delisting or suspension of the Common Stock in the foreseeable future. “Governmental Entity” means any nation,
state, county, city, town, village, district, or other political jurisdiction of any nature, federal, state, local, municipal, foreign,
or other government, governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department,
official, or entity and any court or other tribunal), multi-national organization or body; or body exercising, or entitled to exercise,
any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature or instrumentality
of any of the foregoing, including any entity or enterprise owned or controlled by a government or a public international organization
or any of the foregoing.
(f)
Acknowledgment Regarding Buyer’s Purchase of Securities. The Company acknowledges and agrees that the Buyer is acting solely
in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated hereby
and thereby and that the Buyer is not (i) an officer or director of the Company or any of its Subsidiaries, (ii) an “affiliate”
(as defined in Rule 144) of the Company or any of its Subsidiaries or (iii) to its knowledge, a “beneficial owner” of more
than 10% of the shares of Common Stock (as defined for purposes of Rule 13d-3 of the Securities Exchange Act of 1934, as amended (the
“1934 Act”)). The Company further acknowledges that the Buyer is not acting as a financial advisor or fiduciary of
the Company or any of its Subsidiaries (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated
hereby and thereby, and any advice given by the Buyer or any of its representatives or agents in connection with the Transaction Documents
and the transactions contemplated hereby and thereby is merely incidental to the Buyer’s purchase of the Securities. The Company
further represents to the Buyer that the Company’s and each Subsidiary’s decision to enter into the Transaction Documents
to which it is a party has been based solely on the independent evaluation by the Company, each Subsidiary and their respective representatives.
(g)
No General Solicitation; No Placement Agent’s Fees. Neither the Company, nor any of its Subsidiaries or affiliates, nor
any Person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning
of Regulation D) in connection with the offer or sale of the Securities. The Company shall be responsible for the payment of any placement
agent’s fees, financial advisory fees, or brokers’ commissions (other than for Persons engaged by the Buyer or its investment
advisor) relating to or arising out of the transactions contemplated hereby. The Company shall pay, and hold the Buyer harmless against,
any liability, loss or expense (including, without limitation, attorney’s fees and out-of-pocket expenses) arising in connection
with any such claim. Neither the Company nor any of its Subsidiaries has engaged any placement agent or other agent in connection with
the offer or sale of the Securities.
(h)
No Integrated Offering. None of the Company, its Subsidiaries or any of their affiliates, nor any Person acting on their behalf
has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances
that would require registration of the issuance of any of the Securities under the 1933 Act, whether through integration with prior offerings
or otherwise, or cause this offering of the Securities to require approval of stockholders of the Company for purposes of the 1933 Act
or under any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of any exchange
or automated quotation system on which any of the securities of the Company are listed or designated for quotation. None of the Company,
its Subsidiaries, their affiliates nor any Person acting on their behalf will take any action or steps that would require registration
of the issuance of any of the Securities under the 1933 Act or cause the offering of any of the Securities to be integrated with other
offerings of securities of the Company.
(i)
Dilutive Effect. The Company understands and acknowledges that the number of Conversion Shares will increase in certain circumstances.
The Company further acknowledges that its obligation to issue the Conversion Shares pursuant to the terms of the Preferred Shares in
accordance with this Agreement and the Certificate of Designation in accordance with this Agreement, in each case, absolute and unconditional
regardless of the dilutive effect that such issuance may have on the ownership interests of other stockholders of the Company.
(j)
Omitted.
(k)
SEC Documents; Financial Statements. Except as disclosed on Schedule 3(k), during the two (2) years prior to the date hereof,
the Company has timely filed all reports, schedules, forms, proxy statements, statements and other documents required to be filed by
it with the SEC pursuant to the reporting requirements of the 1934 Act (all of the foregoing filed prior to the date hereof and all exhibits
and appendices included therein and financial statements, notes and schedules thereto and documents incorporated by reference therein
being hereinafter referred to as the “SEC Documents”); reports filed in compliance with the time periods specified
in Rule 12b-25 promulgated under the 1934 Act shall be considered timely for this purpose. When requested, the Company has delivered
or has made available to the Buyer or its representatives true, correct and complete copies of each of the SEC Documents not available
on the EDGAR system. As of their respective dates, the SEC Documents complied in all material respects with the requirements of the 1934
Act and the rules and regulations of the SEC promulgated thereunder applicable to the SEC Documents, and none of the SEC Documents, at
the time they were filed with the SEC, contained any untrue statement of a material fact or omitted to state a material fact required
to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made,
not misleading. As of their respective dates, the financial statements of the Company included in the SEC Documents complied in all material
respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto as in effect
as of the time of filing. Such financial statements have been prepared in accordance with generally accepted accounting principles (“GAAP”),
consistently applied, during the periods involved (except (i) as may be otherwise indicated in such financial statements or the notes
thereto, or (ii) in the case of unaudited interim statements, to the extent they may exclude footnotes or may be condensed or summary
statements) and fairly present in all material respects the financial position of the Company as of the dates thereof and the results
of its operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal year-end audit adjustments
which will not be material, either individually or in the aggregate). The reserves, if any, established by the Company or the lack of
reserves, if applicable, are reasonable based upon facts and circumstances known by the Company on the date hereof and there are no loss
contingencies that are required to be accrued by the Statement of Financial Accounting Standard No. 5 of the Financial Accounting Standards
Board which are not provided for by the Company in its financial statements or otherwise. No other information provided by or on behalf
of the Company to the Buyer which is not included in the SEC Documents (including, without limitation, information referred to in Section 2(e)
of this Agreement or in the disclosure schedules to this Agreement) contains any untrue statement of a material fact or omits to state
any material fact necessary in order to make the statements therein not misleading, in the light of the circumstance under which they
are or were made. The Company is not currently contemplating to amend or restate any of the financial statements (including, without
limitation, any notes or any letter of the independent accountants of the Company with respect thereto) included in the SEC Documents
(the “Financial Statements”), nor is the Company currently aware of facts or circumstances which would require the
Company to amend or restate any of the Financial Statements, in each case, in order for any of the Financials Statements to be in compliance
with GAAP and the rules and regulations of the SEC. The Company has not been informed by its independent accountants that they recommend
that the Company amend or restate any of the Financial Statements or that there is any need for the Company to amend or restate any of
the Financial Statements.
(l)
Absence of Certain Changes. Except as set forth in the SEC Documents and Schedules hereto, since the date of the Company’s
most recent audited financial statements contained in its Form 10-K, there has been no material adverse change and no material adverse
development in the business, assets, liabilities, properties, operations (including results thereof), condition (financial or otherwise)
or prospects of the Company or any of its Subsidiaries. Except as set forth in the SEC Documents, since the date of the Company’s
most recent audited financial statements contained in a Form 10-K, neither the Company nor any of its Subsidiaries has (i) declared or
paid any dividends, (ii) sold any assets, individually or in the aggregate, outside of the ordinary course of business or (iii) made
any capital expenditures, individually or in the aggregate, outside of the ordinary course of business.
(m)
No Undisclosed Events, Liabilities, Developments or Circumstances. Except as set forth in the Schedules hereto, the SEC Documents
or as otherwise disclosed in writing to the Buyer, no event, liability, development or circumstance has occurred or exists, or is reasonably
expected to exist or occur with respect to the Company, any of its Subsidiaries or any of their respective businesses, properties, liabilities,
prospects, operations (including results thereof) or condition (financial or otherwise), that (i) would be required to be disclosed by
the Company under applicable securities laws on a registration statement on Form S-1 filed with the SEC relating to an issuance and sale
by the Company of its Common Stock and which has not been publicly announced, (ii) has had, or would be reasonably expected to have,
a material adverse effect on the Buyer’s investment hereunder or (iii) has had, or would be reasonably expected to have a Material
Adverse Effect.
(n)
Conduct of Business; Regulatory Permits. Neither the Company nor any of its Subsidiaries is in violation of any term of or in
default under its Certificate of Incorporation, any certificate of designation, preferences or rights of any other outstanding series
of preferred stock of the Company or any of its Subsidiaries or Bylaws or their organizational charter, certificate of formation, memorandum
of association, articles of association, Certificate of Incorporation or certificate of incorporation or bylaws, respectively. Neither
the Company nor any of its Subsidiaries is in violation of any judgment, decree or order or any statute, ordinance, rule or regulation
applicable to the Company or any of its Subsidiaries, and neither the Company nor any of its Subsidiaries will conduct its business in
violation of any of the foregoing, except in all cases for violations which have not had, and would not reasonably be expected to have,
individually or in the aggregate, a Material Adverse Effect. Without limiting the generality of the foregoing, and other than as disclosed
on the Schedules hereto, the Company is not in violation of any of the rules, regulations or requirements of the Principal Market and
has no knowledge of any facts or circumstances that could reasonably lead to delisting or suspension of the Common Stock by the Principal
Market in the foreseeable future. During the two years prior to the date hereof, (i) the Common Stock has been listed or designated for
quotation on the Principal Market, (ii) trading in the Common Stock has not been suspended by the SEC or the Principal Market and (iii)
except as disclosed in the SEC Documents, the Company has received no communication, written or oral, from the SEC or the Principal Market
regarding the suspension or delisting of the Common Stock from the Principal Market. The Company and each of its Subsidiaries possess
all certificates, authorizations and permits issued by the appropriate regulatory authorities necessary to conduct their respective businesses,
except where the failure to possess such certificates, authorizations or permits would not have, individually or in the aggregate, a
Material Adverse Effect, and neither the Company nor any such Subsidiary has received any notice of proceedings relating to the revocation
or modification of any such certificate, authorization or permit. Excepts as provided on Schedule 3(n) hereto, there is no agreement,
commitment, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries or to which the Company or any
of its Subsidiaries is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any
business practice of the Company or any of its Subsidiaries, any acquisition of property by the Company or any of its Subsidiaries or
the conduct of business by the Company or any of its Subsidiaries as currently conducted other than such effects, individually or in
the aggregate, which have not had and would not reasonably be expected to have a Material Adverse Effect on the Company or any of its
Subsidiaries.
(o)
Foreign Corrupt Practices. Neither the Company, the Company’s subsidiary or any director, officer, agent, employee, nor, to
the knowledge of the Company, any other person acting for or on behalf of the foregoing (individually and collectively, a “Company
Affiliate”) have violated the U.S. Foreign Corrupt Practices Act (the “FCPA”) or any other applicable anti-bribery
or anti-corruption laws, nor has any Company Affiliate offered, paid, promised to pay, or authorized the payment of any money, or offered,
given, promised to give, or authorized the giving of anything of value, to any officer, employee or any other person acting in an official
capacity for any Governmental Entity to any political party or official thereof or to any candidate for political office (individually
and collectively, a “Government Official”) or to any person under circumstances where such Company Affiliate knew
or was aware of a high probability that all or a portion of such money or thing of value would be offered, given or promised, directly
or indirectly, to any Government Official, for the purpose of:
(i)
(A) influencing any act or decision of such Government Official in his/her official capacity, (B) inducing such Government Official to
do or omit to do any act in violation of his/her lawful duty, (C) securing any improper advantage, or (D) inducing such Government Official
to influence or affect any act or decision of any Governmental Entity, or
(ii)
assisting the Company or its Subsidiaries in obtaining or retaining business for or with, or directing business to, the Company or its
Subsidiaries.
(p)
Sarbanes-Oxley Act. The Company and each Subsidiary is in compliance with any and all applicable requirements of the Sarbanes-Oxley
Act of 2002, as amended, and any and all applicable rules and regulations promulgated by the SEC thereunder.
(q)
Transactions With Affiliates. Except as disclosed on Schedule 3(q) or in the SEC Disclosures, no current or former employee, partner,
director, officer or stockholder of the Company or its Subsidiaries, or, to the knowledge of the Company, any affiliate of any thereof,
or, to the knowledge of the Company, any member of the immediate family of any of the foregoing, is presently (or in the last twelve
months has been) (i) a party to any transaction with the Company or its Subsidiaries (including any contract, agreement or other arrangement
providing for the furnishing of services by, or rental of real or personal property from, or otherwise requiring payments to, any such
director, officer or stockholder or such associate or affiliate or relative Subsidiaries (other than for ordinary course services as
employees, officers or directors of the Company or any of its Subsidiaries)) or (ii) the direct or indirect owner of an interest in any
corporation, firm, association or business organization which is a competitor, supplier or customer of the Company or its Subsidiaries
(except for a passive investment (direct or indirect) in less than 5% of the common stock of a company whose securities are traded on
or quoted through an Eligible Market (as defined in the Certificate of Designations)), nor does any such Person receive income from any
source other than the Company or its Subsidiaries which relates to the business of the Company or its Subsidiaries or should properly
accrue to the Company or its Subsidiaries. No employee, officer, stockholder or director of the Company or any of its Subsidiaries or
member of his or her immediate family is indebted to the Company or its Subsidiaries, as the case may be, nor is the Company or any of
its Subsidiaries indebted (or committed to make loans or extend or guarantee credit) to any of them, other than (i) for payment of salary
or consulting or director fees for services rendered, (ii) reimbursement for reasonable expenses incurred on behalf of the Company, and
(iii) for other standard employee benefits made generally available to all employees or executives (including in connection with the
administration of the Company’s employee stock purchase plan and stock option agreements outstanding under any stock option plan
approved by the board of directors of the Company).
(r)
Equity Capitalization.
(i)
Definitions:
(A) “Common
Stock” means (x) the Company’s shares of common stock, $0.0001 par value per share, and (y) any capital stock into
which such common stock shall have been changed or any share capital resulting from a reclassification of such common stock.
(B) “Preferred
Stock” means (x) the Company’s blank check preferred stock, $0.0001 par value per share, the terms of which may be designated
by the board of directors of the Company in a certificate of designations and (y) any capital stock into which such preferred stock shall
have been changed or any share capital resulting from a reclassification of such preferred stock (other than a conversion of such preferred
stock into Common Stock in accordance with the terms of such certificate of designations).
(C)
“Series A Preferred Stock” means (x) the Company’s shares of Series
A Preferred Stock as designated by that certain Certificate of Designation filed with the Secretary of State of the State of Delaware
on March 24, 2020 and (y) any capital stock into which such Series A Preferred Stock shall have been changed or any share capital resulting
from a reclassification of such Series A Preferred Stock.
(D)
“Series B-1 Preferred Stock” means (x) the Company’s shares of Series
B-1 Convertible Preferred Stock as designated by that certain Certificate of Designation of Preferences, Rights and Limitations filed
with the Secretary of State of the State of Delaware on October 11, 2021 and (y) any capital stock into which such Series B-1 Preferred
Stock shall have been changed or any share capital resulting from a reclassification of such Series B-1 Preferred Stock.
(E)
“Series B-2 Preferred Stock” means (x) the Company’s shares of Series
B-2 Convertible Preferred Stock as designated by that certain Certificate of Designation of Preferences, Rights and Limitations filed
with the Secretary of State of the State of Delaware on October 11, 2021 and (y) any capital stock into which such Series B-2 Preferred
Stock shall have been changed or any share capital resulting from a reclassification of such Series B-2 Preferred Stock.
(F)
“Series C Preferred Stock” means (x) the Company’s shares of Series
C Convertible Preferred Stock as designated by that certain Certificate of Designation of Preferences, Rights and Limitations filed with
the Secretary of State of the State of Delaware on March 24, 2022 and (y) any capital stock into which such Series C Preferred Stock
shall have been changed or any share capital resulting from a reclassification of such Series C Preferred Stock. (G) “Series
D Preferred Stock” means (x) the Company’s shares of Series D Non-Convertible Preferred Stock as designated by that certain
Certificate of Designations filed with the Secretary of State of the State of Delaware on December 16, 2022 and (y) any capital stock
into which such Series D Preferred Stock shall have been changed or any share capital resulting from a reclassification of such Series
D Preferred Stock.
(G)
“Series E-1 Preferred Stock” means (x) the Company’s shares of Series
E-1 Convertible Preferred Stock as designated by that certain Certificate of Designations filed with the Secretary of State of the State
of Delaware August 7, 2023 and (y) any capital stock into which such Series E-1 Preferred Stock shall have been changed or any share
capital resulting from a reclassification of such Series E-1 Preferred Stock.
(H)
“Series F-1 Preferred Stock” means (x) the Company’s shares of Series
F-1 Convertible Preferred Stock as designated by that certain Certificate of Designations filed with the Secretary of State of the State
of Delaware December 11, 2023, and Amended and Restated Certificate of Designations filed with the Secretary of the State of Delaware
June 18, 2024 and (y) any capital stock into which such Series F-1 Preferred Stock shall have been changed or any share capital resulting
from a reclassification of such Series F-1 Preferred Stock.
(ii)
Authorized and Outstanding Capital Stock. As of October 28, 2024, the authorized capital stock of the Company consists of (A)
3,000,000,000 shares of Common Stock, of which, 100,328,686 are issued and outstanding and 953,695,341 shares are reserved for issuance
pursuant to Convertible Securities (as defined below) (other than the Preferred Shares) exercisable or exchangeable for, or convertible
into, shares of Common Stock and (B) 5,000,000 shares of Preferred Stock of which 1,000 shares have been designated as Series A Preferred
Stock, none of which are issued and outstanding; 5,000 shares have been designated as Series B-1 Preferred Stock, none of which are issued
and outstanding; 5,000 shares have been designated as Series B-2 Preferred Stock, none of which are issued and outstanding; 1,700 shares
have been designated as Series C Preferred Stock, none of which are issued and outstanding, 70 shares have been designated as Series
D Preferred Stock, none of which are issued and outstanding; 2,300 have been designated as Series E-1 Preferred Stock, 1,920 of which
are issued and outstanding; 95,000 shares have been designated as Series F-1 Preferred Stock, 24,000 of which are issued and outstanding.
There are no shares of Common Stock are held in the treasury of the Company. “Convertible Securities” means any capital
stock or other security of the Company or any of its Subsidiaries that is at any time and under any circumstances directly or indirectly
convertible into, exercisable or exchangeable for, or which otherwise entitles the holder thereof to acquire, any capital stock or other
security of the Company (including, without limitation, Common Stock) or any of its Subsidiaries.
(iii)
Valid Issuance; Available Shares; Affiliates. All of such outstanding shares are duly authorized and have been, or upon issuance
will be, validly issued and are fully paid and nonassessable. Schedule 3(r)(iii) sets forth the number of shares of Common Stock
that are (A) reserved for issuance pursuant to Convertible Securities (as defined below) (other than the Preferred Shares) and (B) that
are, as of the date hereof, owned by Persons who are “affiliates” (as defined in Rule 405 of the 1933 Act and calculated
based on the assumption that only officers, directors and holders of at least 10% of the Company’s issued and outstanding Common
Stock are “affiliates” without conceding that any such Persons are “affiliates” for purposes of federal securities
laws) of the Company or any of its Subsidiaries. To the Company’s knowledge, no Person owns 10% or more of the Company’s
issued and outstanding shares of Common Stock (calculated based on the assumption that all Convertible Securities, whether or not presently
exercisable or convertible, have been converted, taking account of any limitations on conversion (including “blockers”)
contained therein without conceding that such identified Person is a 10% stockholder for purposes of federal securities laws).
(iv)
Existing Securities; Obligations. Except as disclosed in the SEC Documents: (A) none of the Company’s or any Subsidiary’s
shares, interests or capital stock is subject to preemptive rights or any other similar rights or Liens suffered or permitted by the
Company or any Subsidiary; (B) there are no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any
character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests
or capital stock of the Company or any of its Subsidiaries, or contracts, commitments, understandings or arrangements by which the Company
or any of its Subsidiaries is or may become bound to issue additional shares, interests or capital stock of the Company or any of its
Subsidiaries or options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities
or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries;
(C) there are no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any
of their securities under the 1933 Act (except pursuant to the Registration Rights Agreement); (D) there are no outstanding securities
or instruments of the Company or any of its Subsidiaries which contain any redemption or similar provisions, and there are no contracts,
commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to redeem a security
of the Company or any of its Subsidiaries; (E) there are no securities or instruments containing anti-dilution or similar provisions
that will be triggered by the issuance of the Securities; and (F) neither the Company nor any Subsidiary has any stock appreciation rights
or “phantom stock” plans or agreements or any similar plan or agreement.
(v)
Organizational Documents. The Company has furnished to the Buyer true, correct and complete copies of the Company’s Certificate
of Incorporation, as amended and as in effect on the date hereof (the “Certificate of Incorporation”), and the Company’s
bylaws, as amended and as in effect on the date hereof (the “Bylaws”), and the terms of all Convertible Securities
and the material rights of the holders thereof in respect thereto.
(s)
Indebtedness and Other Contracts. Neither the Company nor any of its Subsidiaries, (i) except as disclosed on Schedule 3(r)
or in the SEC Documents, has any outstanding debt securities, notes, credit agreements, credit facilities or other agreements, documents
or instruments evidencing Indebtedness of the Company or any of its Subsidiaries or by which the Company or any of its Subsidiaries is
bound, (ii) is a party to any contract, agreement or instrument, any reasonably expected violation of which, or reasonably expected default
under which, by the other party(ies) to such contract, agreement or instrument would reasonably be expected to result in a Material Adverse
Effect, (iii) has any financing statements securing obligations in any amounts filed in connection with the Company or any of its Subsidiaries;
(iv) is in violation of any term of, or in default under, any contract, agreement or instrument relating to any Indebtedness, except
where such violations and defaults would not be reasonably likely to result, individually or in the aggregate, in a Material Adverse
Effect, or (v) is a party to any contract, agreement or instrument relating to any Indebtedness, the performance of which, in the judgment
of the Company’s officers, has or is reasonably likely to have a Material Adverse Effect. Neither the Company nor any of its Subsidiaries
have any liabilities or obligations required to be disclosed in the SEC Documents which are not so disclosed in the SEC Documents, other
than those incurred in the ordinary course of the Company’s or its Subsidiaries’ respective businesses and which, individually
or in the aggregate, would not be reasonably likely to have a Material Adverse Effect. For purposes of this Agreement: (x) “Indebtedness”
of any Person means, without duplication (A) all indebtedness for borrowed money, (B) all obligations issued, undertaken or assumed as
the deferred purchase price of property or services (including, without limitation, “capital leases” in accordance with GAAP)
(other than trade payables entered into in the ordinary course of business consistent with past practice), (C) all reimbursement or payment
obligations with respect to letters of credit, surety bonds and other similar instruments, (D) all obligations evidenced by notes, bonds,
debentures or similar instruments, including obligations so evidenced incurred in connection with the acquisition of property, assets
or businesses, (E) all indebtedness created or arising under any conditional sale or other title retention agreement, or incurred as
financing, in either case with respect to any property or assets acquired with the proceeds of such indebtedness (even though the rights
and remedies of the seller or bank under such agreement in the event of default are limited to repossession or sale of such property),
(F) all monetary obligations under any leasing or similar arrangement which, in connection with GAAP, consistently applied for the periods
covered thereby, is classified as a capital lease, (G) all indebtedness referred to in clauses (A) through (F) above secured by (or for
which the holder of such Indebtedness has an existing right, contingent or otherwise, to be secured by) any Lien upon or in any property
or assets (including accounts and contract rights) owned by any Person, even though the Person which owns such assets or property has
not assumed or become liable for the payment of such indebtedness, and (H) all Contingent Obligations in respect of indebtedness or obligations
of others of the kinds referred to in clauses (A) through (G) above; and (y) “Contingent Obligation” means, as to
any Person, any direct or indirect liability, contingent or otherwise, of that Person with respect to any Indebtedness, lease, dividend
or other obligation of another Person if the primary purpose or intent of the Person incurring such liability, or the primary effect
thereof, is to provide assurance to the obligee of such liability that such liability will be paid or discharged, or that any agreements
relating thereto will be complied with, or that the holders of such liability will be protected (in whole or in part) against loss with
respect thereto.
(t)
Litigation. There is no action, suit, arbitration, proceeding, inquiry or investigation before or by the Principal Market, any
court, public board, other Governmental Entity, self-regulatory organization or body pending or, to the knowledge of the Company, threatened
against or affecting the Company or any of its Subsidiaries, the Common Stock or any of the Company’s or its Subsidiaries’
officers or directors, whether of a civil or criminal nature or otherwise, in their capacities as such that if adversely determined would
have a Material Adverse Effect, except as set forth in Schedule 3(t) or in the SEC Documents. To the knowledge of the Company,
no director, officer or employee of the Company or any of its subsidiaries has willfully violated 18 U.S.C. §1519 or engaged in
spoliation in reasonable anticipation of litigation. Without limitation of the foregoing, there has not been, and to the knowledge of
the Company, there is not pending or contemplated, any investigation by the SEC involving the Company, any of its Subsidiaries or any
current or former director or officer of the Company or any of its Subsidiaries. The SEC has not issued any stop order or other order
suspending the effectiveness of any registration statement filed by the Company under the 1933 Act or the 1934 Act. The Company is not
aware of any fact which might result in or form the basis for any such action, suit, arbitration, investigation, inquiry or other proceeding.
Neither the Company nor any of its Subsidiaries is subject to any order, writ, judgment, injunction, decree, determination or award of
any Governmental Entity.
(u)
Insurance. The Company and each of its Subsidiaries are insured against such losses and risks and in such amounts as management
of the Company believes to be prudent and customary in the businesses in which the Company and its Subsidiaries are engaged. Neither
the Company nor any such Subsidiary has been refused any insurance coverage sought or applied for, and neither the Company nor any such
Subsidiary has any reason to believe that it will be unable to renew its existing insurance coverage as and when such coverage expires
or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not have a Material
Adverse Effect.
(v)
Employee Relations. Neither the Company nor any of its Subsidiaries is a party to any collective bargaining agreement or employs
any member of a union. Except as discussed in the Schedules hereto, the Company and its Subsidiaries believe that their relations with
their employees are good. Except as set forth in the SEC Documents, no executive officer (as defined in Rule 501(f) promulgated under
the 1933 Act) of the Company or any of its Subsidiaries has notified the Company or any such Subsidiary that such officer intends to
leave the Company or any such Subsidiary or otherwise terminate such officer’s employment with the Company or any such Subsidiary.
No executive officer of the Company or any of its Subsidiaries is, or is expected to be at this time, in violation of any material term
of any employment contract, confidentiality, disclosure or proprietary information agreement, non-competition agreement, or any other
contract or agreement or any restrictive covenant, and the continued employment of each such executive officer does not subject the Company
or any of its Subsidiaries to any liability with respect to any of the foregoing matters. The Company and its Subsidiaries are in compliance
with all federal, state, local and foreign laws and regulations respecting labor, employment and employment practices and benefits, terms
and conditions of employment and wages and hours, except where failure to be in compliance would not, either individually or in the aggregate,
reasonably be expected to result in a Material Adverse Effect.
(w)
Title.
(i)
Real Property. Each of the Company and its Subsidiaries holds good title to all real property, leases in real property, facilities
or other interests in real property owned or held by the Company or any of its Subsidiaries (the “Real Property”)
owned by the Company or any of its Subsidiaries (as applicable). The Real Property is free and clear of all Liens and is not subject
to any rights of way, building use restrictions, exceptions, variances, reservations, or limitations of any nature except for (a) Liens
for current taxes not yet due and (b) zoning laws and other land use restrictions that do not impair the present or anticipated use of
the property subject thereto. Any Real Property held under lease by the Company or any of its Subsidiaries are held by them under valid,
subsisting and enforceable leases with such exceptions as are not material and do not interfere with the use made and proposed to be
made of such property and buildings by the Company or any of its Subsidiaries.
(ii)
Fixtures and Equipment. Each of the Company and its Subsidiaries (as applicable) has good title to, or a valid leasehold interest
in, the tangible personal property, equipment, improvements, fixtures, and other personal property and appurtenances that are used by
the Company or its Subsidiary in connection with the conduct of its business (the “Fixtures and Equipment”). The Fixtures
and Equipment are structurally sound, are in good operating condition and repair, are adequate for the uses to which they are being put,
are not in need of maintenance or repairs except for ordinary, routine maintenance and repairs and are sufficient for the conduct of
the Company’s and/or its Subsidiaries’ businesses (as applicable) in the manner as conducted prior to the Closing. Each of
the Company and its Subsidiaries owns all of its Fixtures and Equipment free and clear of all Liens except for (a) liens for current
taxes not yet due and (b) zoning laws and other land use restrictions that do not impair the present or anticipated use of the property
subject thereto.
(x)
Intellectual Property Rights. The Company and its Subsidiaries own or possess adequate rights or licenses to use all trademarks,
trade names, service marks, service mark registrations, service names, original works of authorship, patents, patent rights, copyrights,
inventions, licenses, approvals, governmental authorizations, trade secrets and other intellectual property rights and all applications
and registrations therefor (“Intellectual Property Rights”) necessary to conduct their respective businesses as now
conducted and presently proposed to be conducted. Each of the patents both (x) owned by the Company or any of its Subsidiaries and (y)
currently used (or proposed to be used) in the business of the Company or any of its Subsidiaries is listed on Schedule 3(x)(i) (the
“Material Intellectual Property Rights”). Except as set forth in Schedule 3(x)(ii) or in the SEC Documents, none of
the Company’s Material Intellectual Property Rights have expired or terminated or have been abandoned or are expected to expire
or terminate or are expected to be abandoned, within three years from the date of this Agreement other than any such expirations or terminations
that, individually or in the aggregate, are not reasonably likely to have a Material Adverse Effect. The Company does not have any knowledge
of any infringement by the Company or its Subsidiaries of Material Intellectual Property Rights of others which infringement is reasonably
likely to have a Material Adverse Effect. There is no claim, action or proceeding being made or brought, or to the knowledge of the Company
or any of its Subsidiaries, being threatened, against the Company or any of its Subsidiaries regarding its Intellectual Property Rights,
which claim, action or proceeding would reasonably be expected to result in a Material Adverse Effect. Neither the Company nor any of
its Subsidiaries is aware of any facts or circumstances which might give rise to any of the foregoing infringements or claims, actions
or proceedings. The Company and its Subsidiaries have taken reasonable security measures to protect the secrecy, confidentiality and
value of all of the Material Intellectual Property Rights.
(y)
Environmental Laws(i). (i) The Company and its Subsidiaries (A) are in compliance with any and all Environmental Laws (as defined
below), (B) have received all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their
respective businesses and (C) are in compliance with all terms and conditions of any such permit, license or approval where, in each
of the foregoing clauses (A), (B) and (C), the failure to so comply or so receive such approvals would be reasonably expected to have,
individually or in the aggregate, a Material Adverse Effect. The term “Environmental Laws” means all federal, state,
local or foreign laws relating to pollution or protection of human health or the environment (including, without limitation, ambient
air, surface water, groundwater, land surface or subsurface strata), including, without limitation, laws relating to emissions, discharges,
releases or threatened releases of chemicals, pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous
Materials”) into the environment, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage,
disposal, transport or handling of Hazardous Materials, as well as all authorizations, codes, decrees, demands or demand letters, injunctions,
judgments, licenses, notices or notice letters, orders, permits, plans or regulations issued, entered, promulgated or approved thereunder.
(ii)
No Hazardous Materials:
(A)
have been disposed of or otherwise released from any Real Property of the Company or any of its Subsidiaries in violation of any Environmental
Laws; or
(B)
are present on, over, beneath, in or upon any Real Property or any portion thereof in quantities that would constitute a violation of
any Environmental Laws. No prior use by the Company or any of its Subsidiaries of any Real Property has occurred that violates any Environmental
Laws, which violation would be reasonably expected to have a Material Adverse Effect.
(iii)
Neither the Company nor any of its Subsidiaries knows of any other person who or entity which has stored, treated, recycled, disposed
of or otherwise located on any Real Property any Hazardous Materials, including, without limitation, such substances as asbestos and
polychlorinated biphenyls.
(iv)
None of the Real Properties are on any federal or state “Superfund” list or Liability Information System (“CERCLIS”)
list or any state environmental agency list of sites under consideration for CERCLIS, nor subject to any environmental related Liens.
(z)
Subsidiary Rights. The Company or one of its Subsidiaries has the unrestricted right to vote, and (subject to limitations imposed
by applicable law) to receive dividends and distributions on, all capital securities of its Subsidiaries as owned by the Company or such
Subsidiary.
(aa)
Tax Status. The Company and each of its Subsidiaries (i) has timely made or filed all foreign, federal and state income and all
other tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has timely paid all taxes and other
governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations,
except those being contested in good faith and (iii) has set aside on its books provision reasonably adequate for the payment of all
taxes for periods subsequent to the periods to which such returns, reports or declarations apply in each case except as would not reasonably
be expected to have a Material Adverse Effect. There are no unpaid taxes in any material amount claimed to be due by the taxing authority
of any jurisdiction, and the officers of the Company and its Subsidiaries know of no basis for any such claim. The Company is not operated
in such a manner as to qualify as a passive foreign investment company, as defined in Section 1297 of the Internal Revenue Code of 1986,
as amended (the “Code”). So long as such Investor together with the other Attribution Parties (as defined in the Notes)
collectively do not own in excess of the Maximum Percentage (as defined in the Notes) of the shares of Common Stock outstanding, the
net operating loss carryforwards (“NOLs”) for United States federal income tax purposes of the consolidated group
of which the Company is the common parent, if any, shall not be adversely effected by the transactions contemplated hereby, and the transactions
contemplated hereby do not constitute an “ownership change” within the meaning of Section 382 of the Code, thereby preserving
the Company’s ability to utilize such NOLs.
(bb)
Internal Accounting and Disclosure Controls. As disclosed in the SEC Documents, the Company and each of its Subsidiaries have
not maintained internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the 1934 Act) that is effective
in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles, including that (i) transactions are executed in accordance with
management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial
statements in conformity with GAAP and to maintain asset and liability accountability, (iii) access to assets or incurrence of liabilities
is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability for assets
and liabilities is compared with the existing assets and liabilities at reasonable intervals and appropriate action is taken with respect
to any difference. The Company has failed to maintain disclosure controls and procedures (as such term is defined in Rule 13a-15(e) under
the 1934 Act) that are effective in ensuring that information required to be disclosed by the Company in the reports that it files or
submits under the 1934 Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms
of the SEC, including, without limitation, controls and procedures designed to ensure that information required to be disclosed by the
Company in the reports that it files or submits under the 1934 Act is accumulated and communicated to the Company’s management,
including its principal executive officer or officers and its principal financial officer or officers, as appropriate, to allow timely
decisions regarding required disclosure. Neither the Company nor any of its Subsidiaries has received any notice or correspondence from
any accountant, Governmental Entity or other Person relating to any potential material weakness or significant deficiency (which significant
deficiency has not been subsequently resolved) in any part of the internal controls over financial reporting of the Company or any of
its Subsidiaries.
(cc)
Off Balance Sheet Arrangements. There is no transaction, arrangement, or other relationship between the Company or any of its
Subsidiaries and an unconsolidated or other off balance sheet entity that is required to be disclosed by the Company in its 1934 Act
filings and is not so disclosed or that otherwise would be reasonably likely to have a Material Adverse Effect.
(dd)
Investment Company Status. The Company is not, and upon consummation of the sale of the Securities will not be, an “investment
company,” an affiliate of an “investment company,” a company controlled by an “investment company” or an
“affiliated person” of, or “promoter” or “principal underwriter” for, an “investment company”
as such terms are defined in the Investment Company Act of 1940, as amended.
(ee)
Acknowledgement Regarding Buyer’s Trading Activity. It is understood and acknowledged by the Company that (i) following
the public disclosure of the transactions contemplated by the Transaction Documents, in accordance with the terms thereof, the Buyer
has not been asked by the Company or any of its Subsidiaries to agree, nor has any Buyer agreed with the Company or any of its Subsidiaries,
to desist from effecting any transactions in or with respect to (including, without limitation, purchasing or selling, long and/or short)
any securities of the Company, or “derivative” securities based on securities issued by the Company or to hold any of the
Securities for any specified term; (ii) the Buyer, and counterparties in “derivative” transactions to which the Buyer is
a party, directly or indirectly, presently may have a “short” position in the Common Stock which was established prior to
the Buyer’s knowledge of the transactions contemplated by the Transaction Documents; (iii) each Buyer shall not be deemed to have
any affiliation with or control over any arm’s length counterparty in any “derivative” transaction; and (iv) the Buyer
may rely on the Company’s obligation to timely deliver shares of Common Stock upon conversion or exchange, as applicable, of the
Securities as and when required pursuant to the Transaction Documents for purposes of effecting trading in the Common Stock of the Company.
The Company further understands and acknowledges that following the public disclosure of the transactions contemplated by the Transaction
Documents pursuant to the 8-K Filing (as defined below) the Buyer may engage in hedging and/or trading activities (including, without
limitation, the location and/or reservation of borrowable shares of Common Stock) at various times during the period that the Securities
are outstanding, including, without limitation, during the periods that the value and/or number of the Conversion Shares, deliverable
with respect to the Securities are being determined and such hedging and/or trading activities (including, without limitation, the location
and/or reservation of borrowable shares of Common Stock), if any, can reduce the value of the existing stockholders’ equity interest
in the Company both at and after the time the hedging and/or trading activities are being conducted. The Company acknowledges that such
aforementioned hedging and/or trading activities do not constitute a breach of this Agreement, the Certificate of Designations, or any
other Transaction Document or any of the documents executed in connection herewith or therewith.
(ff)
Manipulation of Price. Other than disclosed in the SEC Document or in Schedule 3(ff) hereto, neither the Company nor any of its
Subsidiaries has, and, to the knowledge of the Company, no Person acting on their behalf has, directly or indirectly, (i) taken any action
designed to cause or to result in the stabilization or manipulation of the price of any security of the Company or any of its Subsidiaries
to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases
of, any of the Securities, (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities
of the Company or any of its Subsidiaries or (iv) paid or agreed to pay any Person for research services with respect to any securities
of the Company or any of its Subsidiaries.
(gg)
U.S. Real Property Holding Corporation. Neither the Company nor any of its Subsidiaries is, or has ever been, and so long as any
of the Securities are held by the Buyer, shall become, a U.S. real property holding corporation within the meaning of Section 897
of the Code, and the Company and each Subsidiary shall so certify upon the Buyer’s request.
(hh)
Omitted.
(ii)
Transfer Taxes. On the Closing Date, all stock transfer or other taxes (other than income or similar taxes) which are required
to be paid in connection with the issuance, sale and transfer of the Securities to be sold to the Buyer hereunder will be, or will have
been, fully paid or provided for by the Company, and all laws imposing such taxes will be or will have been complied with.
(jj)
Bank Holding Company Act. Neither the Company nor any of its Subsidiaries is subject to the Bank Holding Company Act of 1956,
as amended (the “BHCA”) and to regulation by the Board of Governors of the Federal Reserve System (the “Federal
Reserve”). Neither the Company nor any of its Subsidiaries or affiliates owns or controls, directly or indirectly, five percent
(5%) or more of the outstanding shares of any class of voting securities or twenty-five percent (25%) or more of the total equity of
a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Subsidiaries
or affiliates exercises a controlling influence over the management or policies of a bank or any entity that is subject to the BHCA and
to regulation by the Federal Reserve.
(kk)
Shell Company Status. The Company is not, and has never been, an issuer identified in, or subject to, Rule 144(i).
(ll)
Illegal or Unauthorized Payments; Political Contributions. Neither the Company nor any of its Subsidiaries nor, to the best of
the Company’s knowledge (after reasonable inquiry of its officers and directors), any of the officers, directors, employees, agents
or other representatives of the Company or any of its Subsidiaries or any other business entity or enterprise with which the Company
or any Subsidiary is or has been affiliated or associated, has, directly or indirectly, made or authorized any payment, contribution
or gift of money, property, or services, whether or not in contravention of applicable law, (i) as a kickback or bribe to any Person
or (ii) to any political organization, or the holder of or any aspirant to any elective or appointive public office except for personal
political contributions not involving the direct or indirect use of funds of the Company or any of its Subsidiaries.
(mm)
Money Laundering. The Company and its Subsidiaries are in compliance with, and have not previously violated, the USA Patriot Act
of 2001 and all other applicable U.S. and non-U.S. anti-money laundering laws and regulations, including, without limitation, the laws,
regulations and Executive Orders and sanctions programs administered by the U.S. Office of Foreign Assets Control, including, but not
limited, to (i) Executive Order 13224 of September 23, 2001 entitled, “Blocking Property and Prohibiting Transactions With Persons
Who Commit, Threaten to Commit, or Support Terrorism” (66 Fed. Reg. 49079 (2001)); and (ii) any regulations contained in 31 CFR,
Subtitle B, Chapter V.
(nn)
Management. Except as set forth in Schedule 3(nn) hereto, during the past two year period, no current or, to the knowledge
of the Company, former officer or director or, to the knowledge of the Company, no current ten percent (10%) or greater stockholder of
the Company or any of its Subsidiaries has been the subject of:
(i)
a petition under bankruptcy laws or any other insolvency or moratorium law or the appointment by a court of a receiver, fiscal agent
or similar officer for such Person, or any partnership in which such person was a general partner at or within two years before the filing
of such petition or such appointment, or any corporation or business association of which such person was an executive officer at or
within two years before the time of the filing of such petition or such appointment;
(ii)
a conviction in a criminal proceeding or a named subject of a pending criminal proceeding (excluding traffic violations that do not relate
to driving while intoxicated or driving under the influence);
(iii)
any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or
temporarily enjoining any such person from, or otherwise limiting, the following activities:
(1)
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage
transaction merchant, any other person regulated by the United States Commodity Futures Trading Commission or an associated person of
any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director
or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct
or practice in connection with such activity;
(2)
Engaging in any particular type of business practice; or
(3)
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of
securities laws or commodities laws;
(iv)
any order, judgment or decree, not subsequently reversed, suspended or vacated, of any authority barring, suspending or otherwise limiting
for more than sixty (60) days the right of any such person to engage in any activity described in the preceding sub paragraph, or to
be associated with persons engaged in any such activity;
(v)
a finding by a court of competent jurisdiction in a civil action or by the SEC or other authority to have violated any securities law,
regulation or decree and the judgment in such civil action or finding by the SEC or any other authority has not been subsequently reversed,
suspended or vacated; or
(vi)
a finding by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any
federal commodities law, and the judgment in such civil action or finding has not been subsequently reversed, suspended or vacated.
(oo)
Stock Option Plans(b). Except as set forth in the SEC Documents, each stock option granted by the Company was granted (i) in accordance
with the terms of the applicable stock option plan of the Company and (ii) with an exercise price at least equal to the fair market value
of the Common Stock on the date such stock option would be considered granted under GAAP and applicable law. No stock option granted
under the Company’s stock option plan has been backdated. The Company has not knowingly granted, and there is no and has been no
policy or practice of the Company to knowingly grant, stock options prior to, or otherwise knowingly coordinate the grant of stock options
with, the release or other public announcement of material information regarding the Company or its Subsidiaries or their financial results
or prospects.
(pp)
No Disagreements with Accountants and Lawyers(c). Excepts as disclosed in the SEC Documents and/or Schedule 3(pp) there are no
material disagreements of any kind presently existing, or reasonably anticipated by the Company to arise, between the Company and the
accountants and lawyers formerly or presently employed by the Company and the Company is current with respect to any fees owed to its
accountants and lawyers which would be reasonably likely to affect the Company’s ability to perform any of its obligations under
any of the Transaction Documents. In addition, on or prior to the date hereof, the Company had discussions with its accountants about
its financial statements previously filed with the SEC. Based on those discussions, the Company has no reason to believe that it will
need to restate any such financial statements or any part thereof.
(qq)
No Disqualification Events(d). With respect to Securities to be offered and sold hereunder in reliance on Rule 506(b) under the
1933 Act (“Regulation D Securities”), none of the Company, any of its predecessors, any affiliated issuer, any director,
executive officer, other officer of the Company participating in the offering contemplated hereby, any beneficial owner of 20% or more
of the Company’s outstanding voting equity securities, calculated on the basis of voting power, nor any promoter (as that term
is defined in Rule 405 under the 1933 Act) connected with the Company in any capacity at the time of sale (each, an “Issuer
Covered Person” and, together, “Issuer Covered Persons”) is subject to any of the “Bad Actor”
disqualifications described in Rule 506(d)(1)(i) to (viii) under the 1933 Act (a “Disqualification Event”), except
for a Disqualification Event covered by Rule 506(d)(2) or (d)(3). The Company has exercised reasonable care to determine whether any
Issuer Covered Person is subject to a Disqualification Event. The Company has complied, to the extent applicable, with its disclosure
obligations under Rule 506(e), and has furnished to the Buyer a copy of any disclosures provided thereunder.
(rr)
Other Covered Persons(e). The Company is not aware of any Person that has been or will be paid (directly or indirectly) remuneration
for solicitation of Buyer or potential purchasers in connection with the sale of any Regulation D Securities.
(ss)
No Additional Agreements. The Company does not have any agreement or understanding with the Buyer with respect to the transactions
contemplated by the Transaction Documents other than as specified in the Transaction Documents.
(tt)
Public Utility Holding Act. None of the Company nor any of its Subsidiaries is a “holding company,” or an “affiliate”
of a “holding company,” as such terms are defined in the Public Utility Holding Act of 2005.
(uu)
Federal Power Act. None of the Company nor any of its Subsidiaries is subject to regulation as a “public utility”
under the Federal Power Act, as amended.
(vv)
Omitted.
(ww)
Potential Products; FDA; EMEA.
(i)
The Company possesses all certificates, authorizations and permits issued by the appropriate federal, state or foreign regulatory authorities
necessary to conduct its business as currently conducted, including without limitation all such certificates, authorizations and permits
required by the United States Food and Drug Administration (the “FDA”) or any other federal, state or foreign agencies
or bodies engaged in the regulation of pharmaceuticals or biohazardous materials, except where the failure to so possess such certificates,
authorizations and permits, individually or in the aggregate, would not result in a Material Adverse Effect. The Company has not received
any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit which, individually
or in the aggregate, if the subject of an unfavorable decision, ruling or finding, would have a Material Adverse Effect.
(ii)
The Company has not received any written notices or statements from the FDA, the European Medicines Agency (the “EMEA”)
or any other governmental agency, and otherwise has no knowledge or reason to believe, that (i) any drug or other product candidate of
the Company (each a “Potential Product”) may or will be rejected or determined to be non-approvable; (ii) a delay
in time for review and/or approval of a marketing authorization application or marketing approval application in any jurisdiction for
any Potential Product is or may be required, requested or being implemented; (iii) one or more clinical studies for any Potential Product
shall or may be requested or required in addition to the clinical studies submitted to the FDA prior to the date hereof as a precondition
to or condition of issuance or maintenance of a marketing approval for any Potential Product; (iv) any license, approval, permit or authorization
to conduct any clinical trial of or market any product or Potential Product of the Company has been, will be or may be suspended, revoked,
modified or limited, except in the cases of clauses (i), (ii), (iii) and (iv) where such rejections, determinations, delays, requests,
suspensions, revocations, modifications or limitations might not reasonably be expected to have, individually or in the aggregate, a
Material Adverse Effect.
(iii)
To the Company’s knowledge, the preclinical and clinical testing, application for marketing approval of, manufacture, distribution,
promotion and sale of the products and Potential Products of the Company is in compliance, in all material respects, with all laws, rules
and regulations applicable to such activities, including without limitation applicable good laboratory practices, good clinical practices
and good manufacturing practices, except for such non-compliance as would not, individually or in the aggregate, have a Material Adverse
Effect. The Company is not aware of any studies, tests or trial the results of which reasonably call into question the results of the
tests and trials conducted by or on behalf of the Company. The Company has not received notice of adverse finding, warning letter or
clinical hold notice from the FDA or any non-U.S. counterpart of any of the foregoing, or any untitled letter or other correspondence
or notice from the FDA or any other governmental authority or agency or any institutional or ethical review board alleging or asserting
noncompliance with any law, rule or regulation applicable in any jurisdiction, except notices, letters, and correspondences and non-U.S.
counterparts thereof alleging or asserting such noncompliance as would not, individually or in the aggregate, have a Material Adverse
Effect. The Company has not, either voluntarily or involuntarily, initiated, conducted or issued, or caused to be initiated, conducted
or issued, any recall, field correction, market withdrawal or replacement, safety alert, warning, “dear doctor” letter, investigator
notice, or other notice or action relating to an alleged or potential lack of safety or efficacy of any product or Potential Product
of the Company, any alleged product defect of any product or Potential Product of the Company, or any violation of any material applicable
law, rule, regulation or any clinical trial or marketing license, approval, permit or authorization for any product or potential product
of the Company, and the Company is not aware of any facts or information that would cause it to initiate any such notice or action and
has no knowledge or reason to believe that the FDA, the EMEA or any other governmental agency or authority or any institutional or ethical
review board or other non-governmental authority intends to impose, require, request or suggest such notice or action.
(xx)
Cybersecurity. The Company and its Subsidiaries’ information technology assets and equipment, computers, systems, networks,
hardware, software, websites, applications, and databases (collectively, “IT Systems”) are adequate for, and operate
and perform in all material respects as required in connection with the operation of the business of the Company and its subsidiaries
as currently conducted, free and clear of all material bugs, errors, defects, Trojan horses, time bombs, malware and other corruptants
that would reasonably be expected to have a Material Adverse Effect on the Company’s business. The Company and its Subsidiaries
have implemented and maintained commercially reasonable physical, technical and administrative controls, policies, procedures, and safeguards
to maintain and protect their material confidential information and the integrity, continuous operation, redundancy and security of all
IT Systems and data, including “Personal Data,” used in connection with their businesses. “Personal Data”
means (i) a natural person’s name, street address, telephone number, e-mail address, photograph, social security number or tax
identification number, driver’s license number, passport number, credit card number, bank information, or customer or account number;
(ii) any information which would qualify as “personally identifying information” under the Federal Trade Commission Act,
as amended; (iii) “personal data” as defined by the European Union General Data Protection Regulation (“GDPR”)
(EU 2016/679); (iv) any information which would qualify as “protected health information” under the Health Insurance Portability
and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act (collectively, “HIPAA”);
and (v) any other piece of information that allows the identification of such natural person, or his or her family, or permits the collection
or analysis of any data related to an identified person’s health or sexual orientation. There have been no breaches, violations,
outages or unauthorized uses of or accesses to same, except for those that have been remedied without material cost or liability or the
duty to notify any other person or such, nor any incidents under internal review or investigations relating to the same except in each
case, where such would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect. The
Company and its Subsidiaries are presently in compliance with all applicable laws or statutes and all judgments, orders, rules and regulations
of any court or arbitrator or governmental or regulatory authority, internal policies and contractual obligations relating to the privacy
and security of IT Systems and Personal Data and to the protection of such IT Systems and Personal Data from unauthorized use, access,
misappropriation or modification except in each case, where such would not, either individually or in the aggregate, reasonably be expected
to result in a Material Adverse Effect.
(yy)
Compliance with Data Privacy Laws. The Company and its Subsidiaries are, and at all prior times were, in compliance with all applicable
state and federal data privacy and security laws and regulations, including without limitation HIPAA, and the Company and its Subsidiaries
have taken commercially reasonable actions to prepare to comply with, and since May 25, 2018, have been and currently are in compliance
with, the GDPR (EU 2016/679) (collectively, the “Privacy Laws”) except in each case, where such would not, either
individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect. To ensure compliance with the Privacy
Laws, the Company and its Subsidiaries have in place, comply with, and take appropriate steps reasonably designed to ensure compliance
in all material respects with their policies and procedures relating to data privacy and security and the collection, storage, use, disclosure,
handling, and analysis of Personal Data (the “Policies”). The Company and its Subsidiaries have at all times made
all disclosures to users or customers required by applicable laws and regulatory rules or requirements, and none of such disclosures
made or contained in any Policy have, to the knowledge of the Company, been inaccurate or in violation of any applicable laws and regulatory
rules or requirements in any material respect. The Company further certifies that neither it nor any Subsidiary: (i) has received notice
of any actual or potential liability under or relating to, or actual or potential violation of, any of the Privacy Laws, and has no knowledge
of any event or condition that would reasonably be expected to result in any such notice; (ii) is currently conducting or paying for,
in whole or in part, any investigation, remediation, or other corrective action pursuant to any Privacy Law; or (iii) is a party to any
order, decree, or agreement that imposes any obligation or liability under any Privacy Law.
(zz)
Registration Rights. Except as disclosed in the SEC Documents and/or the Schedules hereto, no holder of securities of the Company
has rights to the registration of any securities of the Company because of the filing of the Registration Statement or the issuance of
the Securities hereunder that could expose the Company to material liability or the Buyer to any liability or that could impair the Company’s
ability to consummate the issuance and sale of the Securities in the manner, and at the times, contemplated hereby, which rights have
not been waived by the holder thereof as of the date hereof.
(aaa)
Disclosure. The Company confirms that neither it nor any other Person acting on its behalf has provided the Buyer or their agents
or counsel with any information that constitutes or could reasonably be expected to constitute material, non-public information concerning
the Company or any of its Subsidiaries, other than the existence of the transactions contemplated by this Agreement and the other Transaction
Documents. The Company understands and confirms that the Buyer will rely on the foregoing representations in effecting transactions in
securities of the Company. All disclosure provided to the Buyer regarding the Company and its Subsidiaries, their businesses and the
transactions contemplated hereby, including the schedules to this Agreement, furnished by or on behalf of the Company or any of its Subsidiaries
is true and correct as of the date furnished and does not contain any untrue statement of a material fact or omit to state any material
fact as of the date furnished necessary in order to make the statements made therein, in the light of the circumstances under which they
were made, not misleading. All of the written information furnished after the date hereof by or on behalf of the Company or any of its
Subsidiaries to the Buyer pursuant to or in connection with this Agreement and the other Transaction Documents, taken as a whole, will
be true and correct in all material respects as of the date on which such information is so provided and will not contain any untrue
statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light
of the circumstances under which they were made, not misleading. Each press release issued by the Company or any of its Subsidiaries
during the twelve (12) months preceding the date of this Agreement did not at the time of release contain any untrue statement of a material
fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light
of the circumstances under which they are made, not misleading. No event or circumstance has occurred or information exists with respect
to the Company or any of its Subsidiaries or its or their business, properties, liabilities, prospects, operations (including results
thereof) or conditions (financial or otherwise), which, under applicable law, rule or regulation, requires public disclosure at or before
the date hereof or announcement by the Company but which has not been so publicly disclosed. The Company acknowledges and agrees that
no Buyer makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically
set forth in Section 2.
4.
COVENANTS.
(a)
Best Efforts. The Buyer shall use its best efforts to timely satisfy each of the covenants hereunder and conditions to be satisfied
by it as provided in Section 6 of this Agreement. The Company shall use its best efforts to timely satisfy each of the covenants hereunder
and conditions to be satisfied by it as provided in Section 7 of this Agreement.
(b)
Blue Sky. The Company shall, on or before the Closing Date, take such action as the Company shall reasonably determine is necessary
in order to obtain an exemption for, or to, qualify the Securities for sale to the Buyers at the Closing pursuant to this Agreement under
applicable securities or “Blue Sky” laws of the states of the United States (or to obtain an exemption from such qualification),
and shall provide evidence of any such action so taken to the Buyers on or prior to the Closing Date. Without limiting any other obligation
of the Company under this Agreement, the Company shall timely make all filings and reports relating to the offer and sale of the Securities
required under all applicable securities laws (including, without limitation, all applicable federal securities laws and all applicable
“Blue Sky” laws), and the Company shall comply with all applicable foreign, federal, state and local laws, statutes, rules,
regulations and the like relating to the offering and sale of the Securities to the Buyers.
(c)
Reporting Status. Until the date on which the Buyer shall have sold all of the Securities (the “Reporting Period”),
the Company shall timely file all reports required to be filed with the SEC pursuant to the 1934 Act, and the Company shall not terminate
its status as an issuer required to file reports under the 1934 Act even if the 1934 Act or the rules and regulations thereunder would
no longer require or otherwise permit such termination. From the time Form S-3 is available to the Company for the registration of the
Registrable Securities, the Company shall take all actions necessary to maintain its eligibility to register the Registrable Securities
for resale by the Buyers on Form S-3.
(d)
Use of Proceeds. The Company will use the proceeds from the sale of the Securities for general corporate purposes, but not, directly
or indirectly, for (i) except as set forth on Schedule 4(d), the satisfaction of any indebtedness of the Company or any of its Subsidiaries,
(ii) the redemption or repurchase of any securities of the Company or any of its Subsidiaries, or (iii) the settlement of any outstanding
litigation.
(e)
Financial Information. The Company agrees to send the following to the Buyer (as defined in the Registration Rights Agreement)
during the Reporting Period (i) unless the following are filed with the SEC through EDGAR and are available to the public through the
EDGAR system, within one (1) Business Day after the filing thereof with the SEC, a copy of its Annual Reports on Form 10-K and Quarterly
Reports on Form 10-Q, any interim reports or any consolidated balance sheets, income statements, stockholders’ equity statements
and/or cash flow statements for any period other than annual or quarterly, any Current Reports on Form 8-K and any registration statements
(other than on Form S-8) or amendments filed pursuant to the 1933 Act, (ii) unless the following are either filed with the SEC through
EDGAR or are otherwise widely disseminated via a recognized news release service (such as PR Newswire), on the same day as the release
thereof, e-mail copies of all press releases issued by the Company or any of its Subsidiaries and (iii) unless the following are filed
with the SEC through EDGAR, copies of any notices and other information made available or given to the stockholders of the Company generally,
contemporaneously with the making available or giving thereof to the stockholders.
(f)
Omitted
(g)
Fees. Except as otherwise set forth in the Transaction Documents, each party to this Agreement shall bear its own expenses in
connection with the sale of the Securities to the Buyer.
(h)
Omitted.
(i)
Disclosure of Transactions and Other Material Information.
(i)
Disclosure of Transaction. On or before 9:00 a.m., New York time, on or before the fourth (4th) Business Day after
the date of this Agreement, the Company shall file a Current Report on Form 8-K describing all the material terms of the transactions
contemplated by the Transaction Documents in the form required by the 1934 Act and attaching all the material Transaction Documents (including,
without limitation, this Agreement (and all schedules to this Agreement), and the form of Certificate of Designations and the form of
the Registration Rights Agreement) (including all attachments, the “8-K Filing”). From and after the filing of the
8-K Filing, the Company shall have disclosed all material, non-public information (if any) provided to the Buyers by the Company or any
of its Subsidiaries or any of their respective officers, directors, employees or agents in connection with the transactions contemplated
by the Transaction Documents. In addition, effective upon the filing of the 8-K Filing, the Company acknowledges and agrees that any
and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries
or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and the Buyer or any of its affiliates,
on the other hand, with the exception of the Agreement and Plan of Merger dated December 11, 2023, as amended, Transaction Agreements
(defined therein) and related disclosures, shall terminate.
(j)
Reservation of Shares. So long as any of the Preferred Shares remain outstanding, the Company shall take all action necessary
to at all times have authorized, and reserved for the purpose of issuance, not less than (i) 100% of the maximum number of shares of
Common Stock issuable upon conversion of all the Preferred Shares then outstanding (assuming for purposes hereof that (x) the Preferred
Shares are convertible at the Alternate Conversion Price assuming an Alternate Conversion Date as of such applicable date of determination,
and (y) any such conversion shall not take into account any limitations on the conversion of the Preferred Shares set forth in the Certificate
of Designations), (collectively, the “Required Reserve Amount”); provided that at no time shall the number of shares
of Common Stock reserved pursuant to this Section 4(l) be reduced other than proportionally in connection with any conversion, or redemption,
as applicable of Preferred Shares. If at any time the number of shares of Common Stock authorized and reserved for issuance is not sufficient
to meet the Required Reserve Amount, the Company will promptly take all corporate action necessary to authorize and reserve a sufficient
number of shares, including, without limitation, calling a special meeting of stockholders to authorize additional shares to meet the
Company’s obligations pursuant to the Transaction Documents, in the case of an insufficient number of authorized shares, obtain
stockholder approval of an increase in such authorized number of shares, and voting the management shares of the Company in favor of
an increase in the authorized shares of the Company to ensure that the number of authorized shares is sufficient to meet the Required
Reserve Amount.
(k)
Conduct of Business. The business of the Company and its Subsidiaries shall not be conducted in violation of any law, ordinance
or regulation of any Governmental Entity, except where such violations would not reasonably be expected to result, either individually
or in the aggregate, in a Material Adverse Effect.
(l)
Other Preferred Shares; Variable Securities. So long as any Preferred Shares remain outstanding, the Company and each Subsidiary
shall be prohibited from effecting or entering into an agreement to effect any Subsequent Placement involving a Variable Rate Transaction.
“Variable Rate Transaction” means a transaction in which the Company or any Subsidiary (i) issues or sells any Convertible
Securities either (A) at a conversion or exchange rate or other price that is based upon and/or varies with the trading prices of or
quotations for the shares of Common Stock at any time after the initial issuance of such Convertible Securities, or (B) with a conversion
or exchange price that is subject to being reset at some future date after the initial issuance of such Convertible Securities or upon
the occurrence of specified or contingent events directly or indirectly related to the business of the Company or the market for the
Common Stock, other than pursuant to a customary “weighted average” anti-dilution provision or (ii) enters into any agreement
(including, without limitation, an equity line of credit or an “at-the-market” offering) whereby the Company or any Subsidiary
may sell securities at a future determined price (other than standard and customary “preemptive” or “participation”
rights). Each Buyer shall be entitled to obtain injunctive relief against the Company and its Subsidiaries to preclude any such issuance,
which remedy shall be in addition to any right to collect damages.
(m)
Dilutive Issuances. For so long as any Preferred Shares remain outstanding, the Company shall not, in any manner, enter into or
affect any Dilutive Issuance (as defined in the Certificate of Designations) if the effect of such Dilutive Issuance is to cause the
Company to be required to issue upon conversion of any Preferred Shares any shares of Common Stock in excess of that number of shares
of Common Stock which the Company may issue upon conversion of the Preferred Shares without breaching the Company’s obligations
under the rules or regulations of the Principal Market.
(n)
Passive Foreign Investment Company. The Company shall conduct its business, and shall cause its Subsidiaries to conduct their
respective businesses, in such a manner as will ensure that the Company will not be deemed to constitute a passive foreign investment
company within the meaning of Section 1297 of the Code.
(o)
Restriction on Redemption and Cash Dividends. So long as any Preferred Shares are outstanding, the Company shall not, directly
or indirectly, redeem, or declare or pay any cash dividend or distribution on, any securities of the Company without the prior express
written consent of the Buyer (other than as required by the Certificate of Designations).
(p)
Corporate Existence. So long as the Buyer beneficially owns any Preferred Shares, the Company shall not be party to any Fundamental
Transaction (as defined in the Certificate of Designations) unless the Company is in compliance with the applicable provisions governing
Fundamental Transactions set forth in the Certificate of Designations.
(q)
Omitted.
(r)
Conversion Procedures. Each of the form of Conversion Notice (as defined in the Certificate of Designations) included in the Certificate
of Designations set forth the totality of the procedures required of the Buyer in order to convert the Preferred Shares. Except as provided
in Section 5(d), no additional legal opinion, other information or instructions shall be required of the Buyers to convert their Preferred
Shares. The Company shall honor conversions of the Preferred Shares and shall deliver the Conversion Shares in accordance with the terms,
conditions and time periods set forth in the Certificate of Designations. Without limiting the preceding sentences, no ink-original Conversion
Notice shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Conversion Notice form
be required in order to convert the Preferred Shares.
(s)
General Solicitation. None of the Company, any of its affiliates (as defined in Rule 501(b) under the 1933 Act) or any person
acting on behalf of the Company or such affiliate will solicit any offer to buy or offer or sell the Securities by means of any form
of general solicitation or general advertising within the meaning of Regulation D, including: (i) any advertisement, article, notice
or other communication published in any newspaper, magazine or similar medium or broadcast over television or radio; and (ii) any seminar
or meeting whose attendees have been invited by any general solicitation or general advertising.
(t)
Integration. None of the Company, any of its affiliates (as defined in Rule 501(b) under the 1933 Act), or any person acting
on behalf of the Company or such affiliate will sell, offer for sale, or solicit offers to buy or otherwise negotiate in respect of any
security (as defined in the 1933 Act) which will be integrated with the sale of the Securities in a manner which would require the registration
of the Securities under the 1933 Act or require stockholder approval under the rules and regulations of the Principal Market and the
Company will take all action that is appropriate or necessary to assure that its offerings of other securities will not be integrated
for purposes of the 1933 Act or the rules and regulations of the Principal Market, with the issuance of Securities contemplated hereby.
(u)
Notice of Disqualification Events. The Company will notify the Buyer in writing, prior to the Closing Date of (i) any Disqualification
Event relating to any Issuer Covered Person and (ii) any event that would, with the passage of time, become a Disqualification Event
relating to any Issuer Covered Person.
5.
REGISTER; TRANSFER AGENT INSTRUCTIONS; LEGEND.
(a)
Register. The Company shall maintain at its principal executive offices (or such other office or agency of the Company as it may
designate by notice to each holder of Securities), a register for the Preferred Shares in which the Company shall record the name and
address of the Person in whose name the Preferred Shares have been issued (including the name and address of each transferee), the aggregate
number of Preferred Shares held by such Person, the number of Conversion Shares issuable pursuant to the terms of the Preferred Shares
held by such Person. The Company shall keep the register open and available at all times during business hours for inspection of the
Buyer or its legal representatives.
(b)
Transfer Agent Instructions. The Company shall issue irrevocable instructions to its transfer agent and any subsequent transfer
agent (as applicable, the “Transfer Agent”) in a form acceptable to the Buyer (the “Irrevocable Transfer
Agent Instructions”) to issue certificates or credit shares to the applicable balance accounts at The Depository Trust Company
(“DTC”), registered in the name of the Buyer or its respective nominee(s), for the Conversion Shares in such amounts
as specified from time to time by the Buyer to the Company upon conversion of the Preferred Shares. The Company represents and warrants
that no instruction other than the Irrevocable Transfer Agent Instructions referred to in this Section 5(b), and stop transfer instructions
to give effect to Section 2(g) hereof, will be given by the Company to its transfer agent with respect to the Securities, and that the
Securities shall otherwise be freely transferable on the books and records of the Company, as applicable, to the extent provided in this
Agreement and the other Transaction Documents. If the Buyer effects a sale, assignment or transfer of the Securities in accordance with
Section 2(g), the Company shall permit the transfer and shall promptly instruct its transfer agent to issue one or more certificates
or credit shares to the applicable balance accounts at DTC in such name and in such denominations as specified by the Buyer to effect
such sale, transfer or assignment. In the event that such sale, assignment or transfer involves Conversion Shares sold, assigned or transferred
pursuant to an effective registration statement or in compliance with Rule 144, the transfer agent shall issue such shares to the Buyer,
assignee or transferee (as the case may be) without any restrictive legend in accordance with Section 5(d) below. The Company acknowledges
that a breach by it of its obligations hereunder will cause irreparable harm to a Buyer. Accordingly, the Company acknowledges that the
remedy at law for a breach of its obligations under this Section 5(b) will be inadequate and agrees, in the event of a breach or
threatened breach by the Company of the provisions of this Section 5(b), that the Buyer shall be entitled, in addition to all other
available remedies, to an order and/or injunction restraining any breach and requiring immediate issuance and transfer, without the necessity
of showing economic loss and without any bond or other security being required. Any fees (with respect to the transfer agent, counsel
to the Company or otherwise) associated with the issuance of such opinion or the removal of any legends on any of the Securities shall
be borne by the Company.
(c)
Legends. The Buyer understands that the Securities have been issued (or will be issued in the case of the Conversion Shares) pursuant
to an exemption from registration or qualification under the 1933 Act and applicable state securities laws, and except as set forth below,
the Securities shall bear any legend as required by the “blue sky” laws of any state and a restrictive legend in substantially
the following form (and a stop-transfer order may be placed against transfer of such stock certificates):
NEITHER
THE ISSUANCE AND SALE OF THE SECURITIES REPRESENTED BY THIS CERTIFICATE NOR THE SECURITIES INTO WHICH THESE SECURITIES ARE
CONVERTIBLE [HAVE BEEN] [THE SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN] REGISTERED UNDER THE SECURITIES ACT OF 1933,
AS AMENDED, OR APPLICABLE STATE SECURITIES LAWS. THE SECURITIES MAY NOT BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED (I) IN
THE ABSENCE OF (A) AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR (B) AN
OPINION OF COUNSEL TO THE HOLDER (IF REQUESTED BY THE COMPANY), IN A FORM REASONABLY ACCEPTABLE TO THE COMPANY, THAT REGISTRATION IS
NOT REQUIRED UNDER SAID ACT OR (II) UNLESS SOLD OR ELIGIBLE TO BE SOLD PURSUANT TO RULE 144 OR RULE 144A UNDER SAID ACT.
NOTWITHSTANDING THE FOREGOING, THE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR
FINANCING ARRANGEMENT SECURED BY THE SECURITIES.
(d)
Removal of Legends. Certificates evidencing Securities shall not be required to contain the legend set forth in Section 5(c)
above or any other legend (i) while a registration statement (including a Registration Statement) covering the resale of such Securities
is effective under the 1933 Act, (ii) following any sale of such Securities pursuant to Rule 144 (assuming the transferor is not an affiliate
of the Company), (iii) if such Securities are eligible to be sold, assigned or transferred under Rule 144 (provided that the Buyer provides
the Company with reasonable assurances that such Securities are eligible for sale, assignment or transfer under Rule 144 which shall
not include an opinion of Buyer’s counsel), (iv) in connection with a sale, assignment or other transfer (other than under Rule
144), provided that the Buyer provides the Company with an opinion of counsel to the Buyer, in a generally acceptable form, to the effect
that such sale, assignment or transfer of the Securities may be made without registration under the applicable requirements of the 1933
Act or (v) if such legend is not required under applicable requirements of the 1933 Act (including, without limitation, controlling judicial
interpretations and pronouncements issued by the SEC). If a legend is not required pursuant to the foregoing, the Company shall no later
than two (2) Trading Days (or such earlier date as required pursuant to the 1934 Act or other applicable law, rule or regulation for
the settlement of a trade initiated on the date the Buyer delivers such legended certificate representing such Securities to the Company)
following the delivery by the Buyer to the Company or the transfer agent (with notice to the Company) of a legended certificate representing
such Securities (endorsed or with stock powers attached, signatures guaranteed, and otherwise in form necessary to affect the reissuance
and/or transfer, if applicable), together with any other deliveries from the Buyer as may be required above in this Section 5(d),
as directed by the Buyer, either: (A) provided that the Company’s transfer agent is participating in the DTC Fast Automated Securities
Transfer Program (“FAST”) and such Securities are Conversion Shares, credit the aggregate number of shares of Common
Stock to which the Buyer shall be entitled to the Buyer’s or its designee’s balance account with DTC through its Deposit/Withdrawal
at Custodian system or (B) if the Company’s transfer agent is not participating in FAST, issue and deliver (via reputable overnight
courier) to the Buyer, a certificate representing such Securities that is free from all restrictive and other legends, registered in
the name of the Buyer or its designee (the date by which such credit is so required to be made to the balance account of the Buyer’s
or the Buyer’s designee with DTC or such certificate is required to be delivered to the Buyer pursuant to the foregoing is referred
to herein as the “Required Delivery Date”, and the date such shares of Common Stock are actually delivered without
restrictive legend to the Buyer or the Buyer’s designee with DTC, as applicable, the “Share Delivery Date”).
The Company shall be responsible for any transfer agent fees or DTC fees with respect to any issuance of Securities or the removal of
any legends with respect to any Securities in accordance herewith.
(e)
Failure to Timely Deliver; Buy-In. If the Company fails, for any reason or for no reason, to issue and deliver (or cause to be
delivered) to the Buyer (or its designee) by the Required Delivery Date, either (I) if the Transfer Agent is not participating in FAST,
a certificate for the number of Conversion Shares to which the Buyer is entitled and register such Conversion Shares on the Company’s
share register or, if the Transfer Agent is participating in FAST, to credit the balance account of the Buyer or the Buyer’s designee
with DTC for such number of Conversion Shares (as the case may be) submitted for legend removal by the Buyer pursuant to Section 5(d)
above or (II) if a registration statement covering the resale of the Conversion Shares (as the case may be) submitted for legend removal
by such Buyer pursuant to Section 5(d) above (the “Unavailable Shares”) is not available for the resale of such Unavailable
Shares and the Company fails to promptly, but in no event later than as required pursuant to the Registration Rights Agreement (x) so
notify the Buyer and (y) deliver the Conversion Shares, electronically without any restrictive legend by crediting such aggregate number
of Conversion Shares submitted for legend removal by the Buyer pursuant to Section 5(d) above to the Buyer’s or its designee’s
balance account with DTC through its Deposit/Withdrawal At Custodian system (the event described in the immediately foregoing clause
(II) is hereinafter referred as a “Notice Failure” and together with the event described in clause (I) above, a “Delivery
Failure”), then, in addition to all other remedies available to the Buyer, the Company shall pay in cash to the Buyer on each
day after the Share Delivery Date and during such Delivery Failure an amount equal to 2% of the product of (A) the sum of the number
of shares of Common Stock not issued to the Buyer on or prior to the Required Delivery Date and to which the Buyer is entitled, and (B)
any trading price of the Common Stock selected by the Buyer in writing as in effect at any time during the period beginning on the date
of the delivery by the Buyer to the Company of the applicable Conversion Shares and ending on the applicable Share Delivery Date. In
addition to the foregoing, if on or prior to the Required Delivery Date either (I) if the Transfer Agent is not participating in FAST,
the Company shall fail to issue and deliver a certificate to the Buyer and register such shares of Common Stock on the Company’s
share register or, if the Transfer Agent is participating in FAST, credit the balance account of the Buyer or the Buyer’s designee
with DTC for the number of shares of Common Stock to which the Buyer submitted for legend removal by the Buyer pursuant to Section 5(d)
above (ii) below or (II) a Notice Failure occurs, and if on or after such Trading Day the Buyer acquires (in an open market transaction,
stock loan or otherwise) shares of Common Stock to deliver in satisfaction of a sale by the Buyer of shares of Common Stock submitted
for legend removal by the Buyer pursuant to Section 5(d) above that the Buyer is entitled to receive from the Company (a “Buy-In”),
then the Company shall, within two (2) Trading Days after the Buyer’s request and in the Buyer’s discretion, either (i) pay
cash to the Buyer in an amount equal to the Buyer’s total purchase price (including brokerage commissions, stock loan costs and
other out-of-pocket expenses, if any) for the shares of Common Stock so acquired (including, without limitation, by any other Person
in respect, or on behalf, of the Buyer) (the “Buy-In Price”), at which point the Company’s obligation to so
deliver such certificate or credit the Buyer’s balance account shall terminate and such shares shall be cancelled, or (ii) promptly
honor its obligation to so deliver to the Buyer a certificate or certificates or credit the balance account of the Buyer or such Buyer’s
designee with DTC representing such number of shares of Common Stock that would have been so delivered if the Company timely complied
with its obligations hereunder and pay cash to the Buyer in an amount equal to the excess (if any) of the Buy-In Price over the product
of (A) such number of shares of Conversion Shares that the Company was required to deliver to the Buyer by the Required Delivery Date
multiplied by (B) the lowest Closing Sale Price of the Common Stock on any Trading Day during the period commencing on the date of the
delivery by the Buyer to the Company of the applicable Conversion Shares and ending on the date of such delivery and payment under this
clause (ii). Nothing shall limit the Buyer’s right to pursue any other remedies available to it hereunder, at law or in equity,
including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure
to timely deliver certificates representing shares of Common Stock (or to electronically deliver such shares of Common Stock) as required
pursuant to the terms hereof. Notwithstanding anything herein to the contrary, with respect to any given Notice Failure and/or Delivery
Failure, this Section 5(e) shall not apply to the Buyer the extent the Company has already paid such amounts in full to the Buyer with
respect to such Notice Failure and/or Delivery Failure, as applicable, pursuant to the analogous sections of the Certificate of Designations
with respect to the Preferred Shares then held by such Buyer.
6.
CONDITIONS TO THE COMPANY’S OBLIGATION TO SELL.
(a)
The obligation of the Company hereunder to issue and sell the Preferred Shares to the Buyer at the Closing is subject to the satisfaction,
at or before the Closing Date, of each of the following conditions, provided that these conditions are for the Company’s sole benefit
and may be waived by the Company at any time in its sole discretion by providing the Buyer with prior written notice thereof:
(i)
The Buyer shall have executed each of the other Transaction Documents to which it is a party and delivered the same to the Company.
(ii)
The Buyer shall have delivered to the Company the Purchase for the Preferred Shares being purchased by the Buyer at the Closing by wire
transfer of immediately available funds in accordance with the Flow of Funds Letter.
7.
CONDITIONS TO THE BUYER’S OBLIGATION TO PURCHASE.
(a)
The obligation of the Buyer hereunder to purchase its Preferred Shares at the Closing is subject to the satisfaction, at or before the
Closing Date, of each of the following conditions, provided that these conditions are for the Buyer’s sole benefit and may be waived
by the Buyer at any time in its sole discretion by providing the Company with prior written notice thereof:
(i)
The Company shall have duly executed and delivered to the Buyer each of the Transaction Documents to which it is a party and the Company
shall have duly executed and delivered to such Buyer (A) such aggregate number of Preferred Shares as set forth across from the Buyer’s
name in column (3) of the Schedule of Buyers, , as being purchased by the Buyer at the Closing pursuant to this Agreement.
(ii)
The Company shall have delivered to the Buyer a copy of the Irrevocable Transfer Agent Instructions, in the form acceptable to the Buyer,
which instructions shall have been delivered to and acknowledged in writing by the Company’s transfer agent.
(iii)
The Company shall have delivered to the Buyer a letter from the Company’s transfer agent certifying the number of shares of Common
Stock outstanding on the Closing Date immediately prior to the Closing.
(iv)
The Common Stock (A) shall be designated for quotation or listed (as applicable) on the Principal Market and (B) shall not have been
suspended, as of the Closing Date, by the SEC or the Principal Market from trading on the Principal Market nor shall suspension by the
SEC or the Principal Market have been threatened, as of the Closing Date, either (I) in writing by the SEC or the Principal Market or
(II) by falling below the minimum maintenance requirements of the Principal Market
(v)
The Company shall have obtained all governmental, regulatory or third party consents and approvals, if any, necessary for the sale of
the Securities, including without limitation, those required by the Principal Market, if any.
(vi)
No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated or endorsed
by any court or Governmental Entity of competent jurisdiction that prohibits the consummation of any of the transactions contemplated
by the Transaction Documents.
(vii)
The Company shall have obtained approval of the Principal Market to list or designate for quotation the Conversion Shares.
(viii)
The Buyer shall have received a letter on the letterhead of the Company, duly executed by the Chief Executive Officer of the Company,
setting forth the wire amounts of the Buyer and the wire transfer instructions of the Company (the “Flow of Funds Letter”).
(ix)
The Company and its Subsidiaries shall have delivered to the Buyer such other documents, instruments or certificates relating to the
transactions contemplated by this Agreement as such Buyer or its counsel may reasonably request.
8.
OMITTED
9.
MISCELLANEOUS.
(a)
Governing Law; Jurisdiction; Jury Trial. All questions concerning the construction, validity, enforcement and interpretation of
this Agreement shall be governed by the internal laws of the State of New York, without giving effect to any choice of law or conflict
of law provision or rule (whether of the State of New York or any other jurisdictions) that would cause the application of the laws of
any jurisdictions other than the State of New York. The Company hereby irrevocably submits to the exclusive jurisdiction of the state
and federal courts sitting in The City of New York, Borough of Manhattan, for the adjudication of any dispute hereunder or in connection
herewith or under any of the other Transaction Documents or with any transaction contemplated hereby or thereby, and hereby irrevocably
waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of
any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding
is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit,
action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that
such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to
limit in any way any right to serve process in any manner permitted by law. Nothing contained herein shall be deemed or operate to preclude
the Buyer from bringing suit or taking other legal action against the Company in any other jurisdiction to collect on the Company’s
obligations to the Buyer or to enforce a judgment or other court ruling in favor of the Buyer. EACH PARTY HEREBY IRREVOCABLY WAIVES
ANY RIGHT IT MAY HAVE TO, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR UNDER ANY OTHER TRANSACTION
DOCUMENT OR IN CONNECTION WITH OR ARISING OUT OF THIS AGREEMENT, ANY OTHER TRANSACTION DOCUMENT OR ANY TRANSACTION CONTEMPLATED HEREBY
OR THEREBY.
(b)
Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the
same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party. In the event
that any signature is delivered by facsimile transmission or by an e-mail which contains a portable document format (.pdf) file of an
executed signature page, such signature page shall create a valid and binding obligation of the party executing (or on whose behalf such
signature is executed) with the same force and effect as if such signature page were an original thereof.
(c)
Headings; Gender. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation
of, this Agreement. Unless the context clearly indicates otherwise, each pronoun herein shall be deemed to include the masculine, feminine,
neuter, singular and plural forms thereof. The terms “including,” “includes,” “include” and words
of like import shall be construed broadly as if followed by the words “without limitation.” The terms “herein,”
“hereunder,” “hereof” and words of like import refer to this entire Agreement instead of just the provision in
which they are found.
(d)
Severability; Maximum Payment Amounts. If any provision of this Agreement is prohibited by law or otherwise determined to be invalid
or unenforceable by a court of competent jurisdiction, the provision that would otherwise be prohibited, invalid or unenforceable shall
be deemed amended to apply to the broadest extent that it would be valid and enforceable, and the invalidity or unenforceability of such
provision shall not affect the validity of the remaining provisions of this Agreement so long as this Agreement as so modified continues
to express, without material change, the original intentions of the parties as to the subject matter hereof and the prohibited nature,
invalidity or unenforceability of the provision(s) in question does not substantially impair the respective expectations or reciprocal
obligations of the parties or the practical realization of the benefits that would otherwise be conferred upon the parties. The parties
will endeavor in good faith negotiations to replace the prohibited, invalid or unenforceable provision(s) with a valid provision(s),
the effect of which comes as close as possible to that of the prohibited, invalid or unenforceable provision(s). Notwithstanding anything
to the contrary contained in this Agreement or any other Transaction Document (and without implication that the following is required
or applicable), it is the intention of the parties that in no event shall amounts and value paid by the Company and/or any of its Subsidiaries
(as the case may be), or payable to or received by the Buyer, under the Transaction Documents (including without limitation, any amounts
that would be characterized as “interest” under applicable law) exceed amounts permitted under any applicable law. Accordingly,
if any obligation to pay, payment made to the Buyer, or collection by the Buyer pursuant the Transaction Documents is finally judicially
determined to be contrary to any such applicable law, such obligation to pay, payment or collection shall be deemed to have been made
by mutual mistake of the Buyer, the Company and its Subsidiaries and such amount shall be deemed to have been adjusted with retroactive
effect to the maximum amount or rate of interest, as the case may be, as would not be so prohibited by the applicable law. Such adjustment
shall be effected, to the extent necessary, by reducing or refunding, at the option of the Buyer, the amount of interest or any other
amounts which would constitute unlawful amounts required to be paid or actually paid to the Buyer under the Transaction Documents. For
greater certainty, to the extent that any interest, charges, fees, expenses or other amounts required to be paid to or received by the
Buyer under any of the Transaction Documents or related thereto are held to be within the meaning of “interest” or another
applicable term to otherwise be violative of applicable law, such amounts shall be pro-rated over the period of time to which they relate.
(e)
Entire Agreement; Amendments. This Agreement, the other Transaction Documents and the schedules and exhibits attached hereto and
thereto and the instruments referenced herein and therein supersede all other prior oral or written agreements between the Buyer, the
Company, its Subsidiaries, their affiliates and Persons acting on their behalf, including, without limitation, any transactions by the
Buyer with respect to Common Stock or the Securities, and the other matters contained herein and therein, and this Agreement, the other
Transaction Documents, the schedules and exhibits attached hereto and thereto and the instruments referenced herein and therein contain
the entire understanding of the parties solely with respect to the matters covered herein and therein; provided, however, nothing contained
in this Agreement or any other Transaction Document shall (or shall be deemed to) (i) have any effect on any agreements the Buyer has
entered into with, or any instruments the Buyer has received from, the Company or any of its Subsidiaries prior to the date hereof with
respect to any prior investment made by the Buyer in the Company or (ii) waive, alter, modify or amend in any respect any obligations
of the Company or any of its Subsidiaries, or any rights of or benefits to the Buyer or any other Person, in any agreement entered into
prior to the date hereof between or among the Company and/or any of its Subsidiaries and the Buyer, or any instruments the Buyer received
from the Company and/or any of its Subsidiaries prior to the date hereof, and all such agreements and instruments shall continue in full
force and effect. Except as specifically set forth herein or therein, neither the Company nor the Buyer makes any representation, warranty,
covenant or undertaking with respect to such matters. For clarification purposes, the Recitals are part of this Agreement. No provision
of this Agreement may be amended other than by an instrument in writing signed by the Company and the. No waiver shall be effective unless
it is in writing and signed by an authorized representative of the waiving party No consideration (other than reimbursement of legal
fees) shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of any of the Transaction
Documents unless the same consideration also is offered to all of the parties to the Transaction Documents, all holders of the Preferred
Shares. As a material inducement for each Buyer to enter into this Agreement, the Company expressly acknowledges and agrees that (x)
no due diligence or other investigation or inquiry conducted by the Buyer, any of its advisors or any of its representatives shall affect
the Buyer’s right to rely on, or shall modify or qualify in any manner or be an exception to any of, the Company’s representations
and warranties contained in this Agreement or any other Transaction Document and (y) unless a provision of this Agreement or any other
Transaction Document is expressly preceded by the phrase “except as disclosed in the SEC Documents,” nothing contained in
any of the SEC Documents shall affect the Buyer’s right to rely on, or shall modify or qualify in any manner or be an exception
to any of, the Company’s representations and warranties contained in this Agreement or any other Transaction Document.
(f)
Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement
must be in writing and will be deemed to have been delivered: (i) upon receipt, when delivered personally; (ii) upon receipt, when sent
by electronic mail (provided that such sent email is kept on file (whether electronically or otherwise) by the sending party and the
sending party does not receive an automatically generated message from the recipient’s email server that such e-mail could not
be delivered to such recipient); or (iii) one (1) Business Day after deposit with an overnight courier service with next day delivery
specified, in each case, properly addressed to the party to receive the same. The mailing addresses and e-mail addresses for such communications
shall be:
If
to the Company:
Evofem
Biosciences, Inc.
7770 Regents Road, Suite 113-618
San
Diego, CA 92122
Telephone: (858) 550-1900
Attention: Chief Financial Officer
E-Mail: Izhang@evofem.com
With
a copy (for informational purposes only) to:
Procopio
Cory Hargreaves & Savitch, LLP
12544 High Bluff Drive
Suite 400
San Diego, CA 92130
Telephone: (858) 523-4305
E-Mail: paul.johnson@procopio.com
If
to the Transfer Agent:
Pacific
Stock Transfer
6725 Via Austi Pkwy, Suite 300
Las Vegas, NV 89119
Telephone: (702) 323-0033
Attention: Joslyn Claiborne
E-Mail: jclairborne@pacificstocktransfer.com
If
to the Buyer, to its mailing address and e-mail address set forth on the Schedule of Buyers, with copies to the Buyer’s representatives
as set forth on the Schedule of Buyers,
Aditxt,
Inc.
737
Fifth Street, Suite 200
Richmond,
VA 23219
Attention:
Amro Albanna, CEO
E-mail:
aalbanna@aditxt.com
with
a copy (for informational purposes only) to:
Sheppard,
Mullin Richter & Hampton LLP
30 Rockefeller Plaza
New York, NY 10112-0015
Telephone: 1-212-634-3073
Attention: John R. Hempill, Esq.
E-mail:
JHempill@sheppardmullin.com
or
to such other mailing address and/or e-mail address and/or to the attention of such other Person as the recipient party has specified
by written notice given to each other party five (5) days prior to the effectiveness of such change, provided that Sheppard, Mullin Richter
& Hampton LLP shall only be provided copies of notices sent to the Buyer. Written confirmation of receipt (A) given by the recipient
of such notice, consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s e-mail containing
the time, date and recipient’s e-mail or (C) provided by an overnight courier service shall be rebuttable evidence of personal
service, receipt by e-mail or receipt from an overnight courier service in accordance with clause (i), (ii) or (iii) above, respectively.
(g)
Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors
and assigns, including any purchasers of any of the Preferred Shares. The Company shall not assign this Agreement or any rights or obligations
hereunder without the prior written consent of the Buyer, including, without limitation, a Fundamental Transaction (as defined in the
Certificate of Designations) (unless the Company is in compliance with the applicable provisions governing Fundamental Transactions set
forth in the Certificate of Designations). The Buyer may assign some or all of its rights hereunder in connection with any transfer of
any of its Securities without the consent of the Company, in which event such assignee shall be deemed to be a Buyer hereunder with respect
to such assigned rights.
(h)
No Third Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted
successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, other than the
Indemnitees referred to in Section 9(k).
(i)
Survival. The representations, warranties, agreements and covenants shall survive the Closing.
(j)
Further Assurances. Each party shall do and perform, or cause to be done and performed, all such further acts and things, and
shall execute and deliver all such other agreements, certificates, instruments and documents, as any other party may reasonably request
in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated
hereby.
(k)
Indemnification. In consideration of the Buyer’s execution and delivery of the Transaction Documents and acquiring the Securities
thereunder and in addition to all of the Company’s other obligations under the Transaction Documents, the Company shall defend,
protect, indemnify and hold harmless the Buyer and each holder of any Securities and all of their stockholders, partners, members, officers,
directors, employees and direct or indirect investors and any of the foregoing Persons’ agents or other representatives (including,
without limitation, those retained in connection with the transactions contemplated by this Agreement) (collectively, the “Indemnitees”)
from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities and damages, and expenses
in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification hereunder is
sought), and including reasonable attorneys’ fees and disbursements (the “Indemnified Liabilities”), incurred
by any Indemnitee as a result of, or arising out of, or relating to (i) any misrepresentation or breach of any representation or warranty
made by the Company or any Subsidiary in any of the Transaction Documents, (ii) any breach of any covenant, agreement or obligation of
the Company or any Subsidiary contained in any of the Transaction Documents or (iii) any cause of action, suit, proceeding or claim brought
or made against such Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company or
any Subsidiary) or which otherwise involves such Indemnitee that arises out of or results from (A) the execution, delivery, performance
or enforcement of any of the Transaction Documents, (B) any transaction financed or to be financed in whole or in part, directly or indirectly,
with the proceeds of the issuance of the Securities, (C) any disclosure properly made by the Buyer pursuant to Section 4(i), or
(D) the status of the Buyer or holder of the Securities either as an investor in the Company pursuant to the transactions contemplated
by the Transaction Documents or as a party to this Agreement (including, without limitation, as a party in interest or otherwise in any
action or proceeding for injunctive or other equitable relief). To the extent that the foregoing undertaking by the Company may be unenforceable
for any reason, the Company shall make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities
which is permissible under applicable law. Except as otherwise set forth herein, the mechanics and procedures with respect to the rights
and obligations under this Section 9(k) shall be the same as those set forth in Section 6 of the Registration Rights Agreement.
(l)
Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual
intent, and no rules of strict construction will be applied against any party. No specific representation or warranty shall limit the
generality or applicability of a more general representation or warranty. Each and every reference to share prices, shares of Common
Stock and any other numbers in this Agreement that relate to the Common Stock shall be automatically adjusted for any stock splits, stock
dividends, stock combinations, recapitalizations or other similar transactions that occur with respect to the Common Stock after the
date of this Agreement. Notwithstanding anything in this Agreement to the contrary, for the avoidance of doubt, nothing contained herein
shall constitute a representation or warranty against, or a prohibition of, any actions with respect to the borrowing of, arrangement
to borrow, identification of the availability of, and/or securing of, securities of the Company in order for the Buyer (or its broker
or other financial representative) to effect short sales or similar transactions in the future.
(m)
Remedies. The Buyer and in the event of assignment by Buyer of its rights and obligations hereunder, each holder of Securities,
shall have all rights and remedies set forth in the Transaction Documents and all rights and remedies which such holders have been granted
at any time under any other agreement or contract and all of the rights which such holders have under any law. Any Person having any
rights under any provision of this Agreement shall be entitled to enforce such rights specifically (without posting a bond or other security),
to recover damages by reason of any breach of any provision of this Agreement and to exercise all other rights granted by law. Furthermore,
the Company recognizes that in the event that it or any Subsidiary fails to perform, observe, or discharge any or all of its or such
Subsidiary’s (as the case may be) obligations under the Transaction Documents, any remedy at law would inadequate relief to the
Buyer. The Company therefore agrees that the Buyer shall be entitled to specific performance and/or temporary, preliminary and permanent
injunctive or other equitable relief from any court of competent jurisdiction in any such case without the necessity of proving actual
damages and without posting a bond or other security. The remedies provided in this Agreement and the other Transaction Documents shall
be cumulative and in addition to all other remedies available under this Agreement and the other Transaction Documents, at law or in
equity (including a decree of specific performance and/or other injunctive relief). In addition to the other remedies set forth herein
and in the other Transaction Documents, if (a) this Agreement or any other Transaction Document is placed in the hands of an attorney
for collection of amounts due thereunder or enforcement or is enforced (or such collections are sought) through any legal proceeding
or the holder otherwise takes action to collect amounts due under this Agreement or any other Transaction Document or to enforce the
provisions of this Agreement or any other Transaction Document or (b) there occurs any bankruptcy, reorganization, receivership of the
company or other proceedings affecting company creditors’ rights and involving a claim under this Agreement or any other Transaction
Document, then the Company shall pay the costs incurred by the Buyer or holder of Securities, as applicable, for such collection, enforcement
or action or in connection with such bankruptcy, reorganization, receivership or other proceeding, including, without limitation, attorneys’
fees and disbursements.
(n)
Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) the Transaction
Documents, whenever the Buyer exercises a right, election, demand or option under a Transaction Document and the Company or any Subsidiary
does not timely perform its related obligations within the periods therein provided, then the Buyer may rescind or withdraw, in its sole
discretion from time to time upon written notice to the Company or such Subsidiary (as the case may be), any relevant notice, demand
or election in whole or in part without prejudice to its future actions and rights.
(o)
Payment Set Aside; Currency. To the extent that the Company makes a payment or payments to the Buyer hereunder or pursuant to
any of the other Transaction Documents or the Buyer enforces or exercises its rights hereunder or thereunder, and such payment or payments
or the proceeds of such enforcement or exercise or any part thereof are subsequently invalidated, declared to be fraudulent or preferential,
set aside, recovered from, disgorged by or are required to be refunded, repaid or otherwise restored to the Company, a trustee, receiver
or any other Person under any law (including, without limitation, any bankruptcy law, foreign, state or federal law, common law or equitable
cause of action), then to the extent of any such restoration the obligation or part thereof originally intended to be satisfied shall
be revived and continued in full force and effect as if such payment had not been made or such enforcement or setoff had not occurred.
Unless otherwise expressly indicated, all dollar amounts referred to in this Agreement and the other Transaction Documents are in United
States Dollars (“U.S. Dollars”), and all amounts owing under this Agreement and all other Transaction Documents shall
be paid in U.S. Dollars. All amounts denominated in other currencies (if any) shall be converted into the U.S. Dollar equivalent amount
in accordance with the Exchange Rate on the date of calculation. “Exchange Rate” means, in relation to any amount
of currency to be converted into U.S. Dollars pursuant to this Agreement, the U.S. Dollar exchange rate as published in the Wall Street
Journal on the relevant date of calculation.
(p)
Judgment Currency.
(i)
If for the purpose of obtaining or enforcing judgment against the Company in connection with this Agreement or any other Transaction
Document in any court in any jurisdiction it becomes necessary to convert into any other currency (such other currency being hereinafter
in this Section 9(p) referred to as the “Judgment Currency”) an amount due in US Dollars under this Agreement,
the conversion shall be made at the Exchange Rate prevailing on the Trading Day immediately preceding:
(1)
the date actual payment of the amount due, in the case of any proceeding in the courts of New York or in the courts of any other jurisdiction
that will give effect to such conversion being made on such date: or
(2)
the date on which the foreign court determines, in the case of any proceeding in the courts of any other jurisdiction (the date as of
which such conversion is made pursuant to this Section 9(p)(i)(2) being hereinafter referred to as the “Judgment Conversion
Date”).
(ii)
If in the case of any proceeding in the court of any jurisdiction referred to in Section 9(p)(i)(2) above, there is a change in
the Exchange Rate prevailing between the Judgment Conversion Date and the date of actual payment of the amount due, the applicable party
shall pay such adjusted amount as may be necessary to ensure that the amount paid in the Judgment Currency, when converted at the Exchange
Rate prevailing on the date of payment, will produce the amount of US Dollars which could have been purchased with the amount of Judgment
Currency stipulated in the judgment or judicial order at the Exchange Rate prevailing on the Judgment Conversion Date.
(iii)
Any amount due from the Company under this provision shall be due as a separate debt and shall not be affected by judgment being obtained
for any other amounts due under or in respect of this Agreement or any other Transaction Document.
[signature
pages follow]
IN
WITNESS WHEREOF, the Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of
the date first written above.
| |
COMPANY: |
| |
|
| |
EVOFEM BIOSCIENCES, INC. |
| |
|
|
| |
By: |
/s/Saundra Pelletier |
| |
|
Name: Saundra Pelletier |
| |
|
Title: Chief Executive Officer |
IN
WITNESS WHEREOF, the Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of
the date first written above.
| |
BUYER: |
| |
|
| |
ADITXT, INC. |
| |
|
|
| |
By: |
/s/Amro Albanna |
| |
|
Name: Amro Albanna |
| |
|
Title: Chief Executive Officer |
SCHEDULE
OF BUYERS
| (1) | | |
(2) | |
| (3) | | |
| (5) | | |
(9) |
| | | |
| |
| | | |
| | | |
|
| Buyer | | |
Mailing Address and E-mail Address | |
| Aggregate
Number of
Preferred Shares | | |
| Purchase Price | | |
Legal Representative’s
Mailing Address and E-mail Address |
| | | |
| |
| | | |
| | | |
|
| Aditxt, Inc. | | |
737 Fifth Street, Suite 200
Richmond, VA 23219
E-mail: aalbanna@aditxt.com | |
| 2,280 | | |
$ | 2,280,000 | | |
Sheppard, Mullin Richter & Hampton LLP
30 Rockefeller Plaza
New York, NY 10112-0015
Telephone: 212-634-3073
Attention: John R. Hempill, Esq. |
| | | |
| |
| | | |
| | | |
|
| TOTAL | | |
| |
| 2,280 | | |
$ | 2,280,000 | | |
|
Exhibit 10.2
Execution Version
REGISTRATION
RIGHTS AGREEMENT
This
REGISTRATION RIGHTS AGREEMENT (this “Agreement”), dated as of October 28, 2024, is by and among Evofem Biosciences,
Inc., a Delaware corporation with offices located at 7770 Regents Road, Suite 113-618, San Diego, CA 92122 (the “Company”),
and the undersigned buyer (“Buyer”).
RECITALS
A. In
connection with the Securities Purchase Agreement by and among the parties hereto, dated as of October 28, 2024 (the “Securities
Purchase Agreement”), the Company has agreed, upon the terms and subject to the conditions of the Securities Purchase Agreement,
to issue and sell to the Buyer the Preferred Shares (as defined in the Securities Purchase Agreement) which will be convertible into
Conversion Shares (as defined in the Securities Purchase Agreement) in accordance with the terms of the Amended and Restated Certificate
of Designations (as defined in the Securities Purchase Agreement).
B. To
induce the Buyer to consummate the transactions contemplated by the Securities Purchase Agreement, the Company has agreed to provide
certain registration rights under the Securities Act of 1933, as amended, and the rules and regulations thereunder, or any similar successor
statute (collectively, the “1933 Act”), and applicable state securities laws.
AGREEMENT
NOW,
THEREFORE, in consideration of the premises and the mutual covenants contained herein and for other good and valuable consideration,
the receipt and sufficiency of which are hereby acknowledged, the Company and each of the Buyers hereby agree as follows:
1. Definitions.
Capitalized
terms used herein and not otherwise defined herein shall have the respective meanings set forth in the Securities Purchase Agreement.
As used in this Agreement, the following terms shall have the following meanings:
(a) “Business
Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized
or required by law to remain closed; provided, however, for clarification, commercial
banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”,
“non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations
at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial
banks in The City of New York generally are open for use by customers on such day.
(b) “Closing
Date” shall have the meaning set forth in the Securities Purchase Agreement.
(c) “Effective
Date” means the date that the applicable Registration Statement has been declared effective by the SEC.
(d) “Effectiveness
Deadline” means (i) with respect to the initial Registration Statement required to be filed pursuant to Section 2(a),
the earlier of the (A) 90th calendar day after the Closing Date and (B) 2nd Business Day after the date the Company
is notified (orally or in writing, whichever is earlier) by the SEC that such Registration Statement will not be reviewed or will not
be subject to further review and (ii) with respect to any additional Registration Statements that may be required to be filed by the
Company pursuant to this Agreement, the earlier of the (A) 90th calendar day following the date on which the Company was required
to file such additional Registration Statement and (B) 2nd Business Day after the date the Company is notified (orally or
in writing, whichever is earlier) by the SEC that such Registration Statement will not be reviewed or will not be subject to further
review.
(e) “Filing
Deadline” means (i) with respect to the initial Registration Statement required to be filed pursuant to Section 2(a),
the 300th calendar day after the Closing Date and (ii) with respect to any additional Registration Statements that may
be required to be filed by the Company pursuant to this Agreement, the date on which the Company was required to file such additional
Registration Statement pursuant to the terms of this Agreement.
(f) “Investor”
means the Buyer or any transferee or assignee of any Registrable Securities or Preferred Shares, as applicable, to whom Buyer assigns
its rights under this Agreement and who agrees to become bound by the provisions of this Agreement in accordance with Section 9
and any transferee or assignee thereof to whom a transferee or assignee of any Registrable Securities or Preferred Shares as applicable,
assigns its rights under this Agreement and who agrees to become bound by the provisions of this Agreement in accordance with Section 9.
(g) “Person”
means an individual, a limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization
or a government or any department or agency thereof.
(h) “register,”
“registered,” and “registration” refer to a registration effected by preparing and filing one or
more Registration Statements in compliance with the 1933 Act and pursuant to Rule 415 and the declaration of effectiveness of such Registration
Statement(s) by the SEC.
(i) “Registrable
Securities” means (i) the Conversion Shares, and (ii) any capital stock of the Company issued or issuable with respect to the
Conversion Shares, or the Preferred Shares including, without limitation, (1) as a result of any stock split, stock dividend, recapitalization,
exchange or similar event or otherwise and (2) shares of capital stock of the Company into which the shares of Common Stock (as defined
in the Certificate of Designations) into which the shares of Common Stock are converted or exchanged, in each case, without regard to
any limitations on conversion of the Preferred Shares.
(j) “Registration
Statement” means a registration statement or registration statements of the Company filed under the 1933 Act covering Registrable
Securities.
(l) “Required
Registration Amount” means, as of any time of determination, the sum of (i) 150% of the maximum number of Conversion Shares
issuable upon conversion of the Preferred Shares (assuming for purposes hereof that (x) the Preferred Shares are convertible at the Alternate
Conversion Price (as defined in the Certificate of Designations) assuming an Alternate Conversion Date (as defined in the Certificate
of Designations) as of such applicable date of determination, and (y) any such conversion shall not take into account any limitations
on the conversion of the Preferred Shares set forth in the Certificate of Designations) as of such time of determination, subject to
adjustment as provided in Section 2(d) and/or Section 2(f).
(m) “Rule
144” means Rule 144 promulgated by the SEC under the 1933 Act, as such rule may be amended from time to time, or any other
similar or successor rule or regulation of the SEC that may at any time permit the Investors to sell securities of the Company to the
public without registration.
(n) “Rule
415” means Rule 415 promulgated by the SEC under the 1933 Act, as such rule may be amended from time to time, or any other
similar or successor rule or regulation of the SEC providing for offering securities on a continuous or delayed basis.
(o) “SEC”
means the United States Securities and Exchange Commission or any successor thereto.
2. Registration.
(a) Mandatory
Registration. The Company shall prepare and, as soon as practicable, but in no event later than the Filing Deadline, file with the
SEC an initial Registration Statement on Form S-3 covering the resale of all of the Registrable Securities, provided that such initial
Registration Statement shall register for resale at least the number of shares of Common Stock equal to the Required Registration Amount
as of the date such Registration Statement is initially filed with the SEC; provided further that if Form S-3 is unavailable for such
a registration, the Company shall use such other form as is required by Section 2(c). Such initial Registration Statement, and each other
Registration Statement required to be filed pursuant to the terms of this Agreement, shall contain (except if otherwise directed by the
Investor) the “Selling Stockholders” and “Plan of Distribution” sections in substantially the form
attached hereto as Exhibit B. The Company shall use its best efforts to have such initial Registration Statement, and each other
Registration Statement required to be filed pursuant to the terms of this Agreement, declared effective by the SEC as soon as practicable,
but in no event later than the applicable Effectiveness Deadline for such Registration Statement.
(b) omitted.
(c) Ineligibility
to Use Form S-3. In the event that Form S-3 is not available for the registration of the resale of Registrable Securities hereunder,
the Company shall (i) register the resale of the Registrable Securities on Form S-1 or another appropriate form reasonably acceptable
to the Investor and (ii) undertake to register the resale of the Registrable Securities on Form S-3 as soon as such form is available,
provided that the Company shall maintain the effectiveness of all Registration Statements then in effect until such time as a Registration
Statement on Form S-3 covering the resale of all the Registrable Securities has been declared effective by the SEC and the prospectus
contained therein is available for use.
(d) Sufficient
Number of Shares Registered. In the event the number of shares available under any Registration Statement is insufficient to cover
all of the Registrable Securities required to be covered by such Registration Statement or an Investor’s allocated portion of the
Registrable Securities pursuant to Section Error! Reference source not found., the Company shall amend such Registration
Statement (if permissible), or file with the SEC a new Registration Statement (on the short form available therefor, if applicable),
or both, so as to cover at least the Required Registration Amount as of the Trading Day (as defined in the Certificate of Designations)
immediately preceding the date of the filing of such amendment or new Registration Statement, in each case, as soon as practicable, but
in any event not later than fifteen (15) days after the necessity therefor arises (but taking account of any Staff position with respect
to the date on which the Staff will permit such amendment to the Registration Statement and/or such new Registration Statement (as the
case may be) to be filed with the SEC). The Company shall use its best efforts to cause such amendment to such Registration Statement
and/or such new Registration Statement (as the case may be) to become effective as soon as practicable following the filing thereof with
the SEC, but in no event later than the applicable Effectiveness Deadline for such Registration Statement. For purposes of the foregoing
provision, the number of shares available under a Registration Statement shall be deemed “insufficient to cover all of the Registrable
Securities” if at any time the number of shares of Common Stock available for resale under the applicable Registration Statement
is less than the product determined by multiplying (i) the Required Registration Amount as of such time by (ii) 0.90. The calculation
set forth in the foregoing sentence shall be made without regard to any limitations on conversion, amortization and/or redemption of
the Preferred Shares (and such calculation shall assume (A) that the Preferred Shares are then convertible in full into shares of Common
Stock at the then prevailing Conversion Rate (as defined in the Certificate of Designations), and (B) the initial outstanding number
of Preferred Shares remains outstanding through the first anniversary of the date hereof and no redemptions of the Preferred Shares occur
prior thereto.
(e) Effect
of Failure to File and Obtain and Maintain Effectiveness of any Registration Statement. If (i) a Registration Statement covering
the resale of all of the Registrable Securities required to be covered thereby (disregarding any reduction pursuant to Section 2(f))
and required to be filed by the Company pursuant to this Agreement is (A) not filed with the SEC on or before the Filing Deadline for
such Registration Statement (a “Filing Failure”) (it being understood that if the Company files a Registration Statement
without affording Investor the opportunity to review and comment on the same as required by Section 3(c) hereof, the Company shall
be deemed to not have satisfied this clause (i)(A) and such event shall be deemed to be a Filing Failure) or (B) not declared effective
by the SEC on or before the Effectiveness Deadline for such Registration Statement (an “Effectiveness Failure”) (it
being understood that if on the Business Day immediately following the Effective Date for such Registration Statement the Company shall
not have filed a “final” prospectus for such Registration Statement with the SEC under Rule 424(b) in accordance with Section 3(b)
(whether or not such a prospectus is technically required by such rule), the Company shall be deemed to not have satisfied this clause
(i)(B) and such event shall be deemed to be an Effectiveness Failure), (ii) other than during an Allowable Grace Period (as defined below),
on any day after the Effective Date of a Registration Statement sales of all of the Registrable Securities required to be included on
such Registration Statement (disregarding any reduction pursuant to Section 2(f)) cannot be made pursuant to such Registration Statement
(including, without limitation, because of a failure to keep such Registration Statement effective, a failure to disclose such information
as is necessary for sales to be made pursuant to such Registration Statement, a suspension or delisting of (or a failure to timely list)
the shares of Common Stock on the Principal Market (as defined in the Securities Purchase Agreement) or any other limitations imposed
by the Principal Market, or a failure to register a sufficient number of shares of Common Stock or by reason of a stop order) or the
prospectus contained therein is not available for use for any reason (a “Maintenance Failure”), or (iii) if a Registration
Statement is not effective for any reason or the prospectus contained therein is not available for use for any reason, and either (x)
the Company fails for any reason to satisfy the requirements of Rule 144(c)(1), including, without limitation, the failure to satisfy
the current public information requirement under Rule 144(c) or (y) the Company has ever been an issuer described in Rule 144(i)(1)(i)
or becomes such an issuer in the future, and the Company shall fail to satisfy any condition set forth in Rule 144(i)(2) (a “Current
Public Information Failure”) as a result of which any of the Investors are unable to sell Registrable Securities without restriction
under Rule 144 (including, without limitation, volume restrictions), then, as partial relief for the damages to any holder by reason
of any such delay in, or reduction of, its ability to sell the underlying shares of Common Stock (which remedy shall not be exclusive
of any other remedies available at law or in equity, including, without limitation, specific performance), the Company shall pay to each
holder of Registrable Securities relating to such Registration Statement an amount in cash equal to two percent (2%) of such Investor’s
Purchase Price (as defined in the Securities Purchase Agreement) (1) on the date of such Filing Failure, Effectiveness Failure, Maintenance
Failure or Current Public Information Failure, as applicable, and (2) on every thirty (30) day anniversary of (I) a Filing Failure until
such Filing Failure is cured; (II) an Effectiveness Failure until such Effectiveness Failure is cured; (III) a Maintenance Failure
until such Maintenance Failure is cured; and (IV) a Current Public Information Failure until the earlier of (i) the date such Current
Public Information Failure is cured and (ii) such time that such public information is no longer required pursuant to Rule 144 (in
each case, pro rated for periods totaling less than thirty (30) days). The payments to which a holder of Registrable Securities shall
be entitled pursuant to this Section 2(e) are referred to herein as “Registration Delay Payments.” Following
the initial Registration Delay Payment for any particular event or failure (which shall be paid on the date of such event or failure,
as set forth above), without limiting the foregoing, if an event or failure giving rise to the Registration Delay Payments is cured prior
to any thirty (30) day anniversary of such event or failure, then such Registration Delay Payment shall be made on the third (3rd)
Business Day after such cure. In the event the Company fails to make Registration Delay Payments in a timely manner in accordance with
the foregoing, such Registration Delay Payments shall bear interest at the rate of two percent (2%) per month (prorated for partial months)
until paid in full. Notwithstanding the foregoing, no Registration Delay Payments shall be owed to an Investor (other than with respect
to a Maintenance Failure resulting from a suspension or delisting of (or a failure to timely list) the shares of Common Stock on the
Principal Market) with respect to any period during which all of such Investor’s Registrable Securities may be sold by such Investor
without restriction under Rule 144 (including, without limitation, volume restrictions) and without the need for current public information
required by Rule 144(c)(1) (or Rule 144(i)(2), if applicable).
(f) Offering.
Notwithstanding anything to the contrary contained in this Agreement, but subject to the payment of the Registration Delay Payments pursuant
to Section 2(e), in the event the staff of the SEC (the “Staff”) or the SEC seeks to characterize any offering
pursuant to a Registration Statement filed pursuant to this Agreement as constituting an offering of securities by, or on behalf
of, the Company, or in any other manner, such that the Staff or the SEC do not permit such Registration Statement to become
effective and used for resales in a manner that does not constitute such an offering and that permits the continuous resale at the market
by the Investors participating therein (or as otherwise may be acceptable to each Investor) without being named therein as
an “underwriter,” then the Company shall reduce the number of shares to be included in such Registration Statement by all
Investors until such time as the Staff and the SEC shall so permit such Registration Statement to become effective as aforesaid.
In making such reduction, the Company shall reduce the number of shares to be included by all Investors on a pro rata basis (based upon
the number of Registrable Securities otherwise required to be included for each Investor) unless the inclusion of shares by a particular
Investor or a particular set of Investors are resulting in the Staff or the SEC’s “by or on behalf of the Company”
offering position, in which event the shares held by such Investor or set of Investors shall be the only shares subject to reduction
(and if by a set of Investors on a pro rata basis by such Investors or on such other basis as would result in the exclusion of the least
number of shares by all such Investors); provided, that, with respect to such pro rata portion allocated to any Investor, such Investor
may elect the allocation of such pro rata portion among the Registrable Securities of such Investor. In addition, in the event that the
Staff or the SEC requires any Investor seeking to sell securities under a Registration Statement filed pursuant to this Agreement to
be specifically identified as an “underwriter” in order to permit such Registration Statement to become effective, and such
Investor does not consent to being so named as an underwriter in such Registration Statement, then, in each such case, the Company shall
reduce the total number of Registrable Securities to be registered on behalf of such Investor, until such time as the
Staff or the SEC does not require such identification or until such Investor accepts such identification and the manner thereof. Any
reduction pursuant to this paragraph will first reduce all Registrable Securities other than those issued pursuant to the Securities
Purchase Agreement, including those securities set forth on Schedule 2(i). In the event of any reduction in Registrable
Securities pursuant to this paragraph, an affected Investor shall have the right to require, upon delivery of a written request
to the Company signed by such Investor, the Company to file a registration statement within twenty (20) days of such request (subject
to any restrictions imposed by Rule 415 or required by the Staff or the SEC) for resale by such Investor in a manner acceptable
to such Investor, and the Company shall following such request cause to be and keep effective such registration statement in the
same manner as otherwise contemplated in this Agreement for registration statements hereunder, in each case until such time
as: (i) all Registrable Securities held by such Investor have been registered and sold pursuant to an effective Registration Statement
in a manner acceptable to such Investor or (ii) all Registrable Securities may be resold by such Investor without restriction
(including, without limitation, volume limitations) pursuant to Rule 144 (taking account of any Staff position with respect to “affiliate”
status) and without the need for current public information required by Rule 144(c)(1) (or Rule 144(i)(2), if applicable) or (iii) such
Investor agrees to be named as an underwriter in any such Registration Statement in a manner acceptable to such Investor as to all Registrable
Securities held by such Investor and that have not theretofore been included in a Registration Statement under this Agreement (it being
understood that the special demand right under this sentence may be exercised by an Investor multiple times and with respect to limited
amounts of Registrable Securities in order to permit the resale thereof by such Investor as contemplated above).
(g) Piggyback
Registrations. Without limiting any obligation of the Company hereunder or under the Securities Purchase Agreement, if there is not
an effective Registration Statement covering all of the Registrable Securities or the prospectus contained therein is not available for
use and the Company shall determine to prepare and file with the SEC a registration statement or offering statement relating to an offering
for its own account or the account of others under the 1933 Act of any of its equity securities (other than on Form S-4 or Form S-8 (each
as promulgated under the 1933 Act) or their then equivalents relating to equity securities to be issued solely in connection with any
acquisition of any entity or business or equity securities issuable in connection with the Company’s stock option or other employee
benefit plans), then the Company shall deliver to each Investor a written notice of such determination and, if within fifteen (15)
days after the date of the delivery of such notice, any such Investor shall so request in writing, the Company shall include in such
registration statement or offering statement all or any part of such Registrable Securities such Investor requests to be registered;
provided, however, the Company shall not be required to register any Registrable Securities pursuant to this Section 2(g) that are
eligible for resale pursuant to Rule 144 without restriction (including, without limitation, volume restrictions) and without the need
for current public information required by Rule 144(c)(1) (or Rule 144(i)(2), if applicable) or that are the subject of a then-effective
Registration Statement.
3. Related
Obligations.
The
Company shall use its best efforts to effect the registration of the Registrable Securities in accordance with the intended method of
disposition thereof, and, pursuant thereto, the Company shall have the following obligations:
(a) The
Company shall promptly prepare and file with the SEC a Registration Statement with respect to all the Registrable Securities (but in
no event later than the applicable Filing Deadline) and use its best efforts to cause such Registration Statement to become effective
as soon as practicable after such filing (but in no event later than the Effectiveness Deadline). Subject to Allowable Grace Periods,
the Company shall keep each Registration Statement effective (and the prospectus contained therein available for use) pursuant to Rule
415 for resales by the Investors on a delayed or continuous basis at then-prevailing market prices (and not fixed prices) at all times
until the earlier of (i) the date as of which all of the Investors may sell all of the Registrable Securities required to be covered
by such Registration Statement (disregarding any reduction pursuant to Section 2(f)) without restriction pursuant to Rule 144 (including,
without limitation, volume restrictions) and without the need for current public information required by Rule 144(c)(1) (or Rule 144(i)(2),
if applicable) or (ii) the date on which the Investors shall have sold all of the Registrable Securities covered by such Registration
Statement (the “Registration Period”). Notwithstanding anything to the contrary contained in this Agreement, the Company
shall ensure that, when filed and at all times while effective, each Registration Statement (including, without limitation, all amendments
and supplements thereto) and the prospectus (including, without limitation, all amendments and supplements thereto) used in connection
with such Registration Statement (1) shall not contain any untrue statement of a material fact or omit to state a material fact required
to be stated therein, or necessary to make the statements therein (in the case of prospectuses, in the light of the circumstances in
which they were made) not misleading and (2) will disclose (whether directly or through incorporation by reference to other SEC filings
to the extent permitted) all material information regarding the Company and its securities. The Company shall submit to the SEC, within
one (1) Business Day after the later of the date that (i) the Company learns that no review of a particular Registration Statement will
be made by the Staff or that the Staff has no further comments on a particular Registration Statement (as the case may be) and (ii) the
consent of Legal Counsel is obtained pursuant to Section 3(c) (which consent shall be immediately sought), a request for acceleration
of effectiveness of such Registration Statement to a time and date not later than twenty-four (24) hours after the submission of
such request. The Company shall respond in writing to comments made by the SEC in respect of a Registration Statement as soon as practicable,
but in no event later than fifteen (15) days after the receipt of comments by or notice from the SEC that an amendment is required in
order for a Registration Statement to be declared effective.
(b) Subject
to Section 3(r) of this Agreement, the Company shall prepare and file with the SEC such amendments (including, without limitation,
post-effective amendments) and supplements to each Registration Statement and the prospectus used in connection with each such Registration
Statement, which prospectus is to be filed pursuant to Rule 424 promulgated under the 1933 Act, as may be necessary to keep each such
Registration Statement effective at all times during the Registration Period for such Registration Statement, and, during such period,
comply with the provisions of the 1933 Act with respect to the disposition of all Registrable Securities of the Company required to be
covered by such Registration Statement until such time as all of such Registrable Securities shall have been disposed of in accordance
with the intended methods of disposition by the seller or sellers thereof as set forth in such Registration Statement; provided, however,
by 8:30 a.m. (New York time) on the Business Day immediately following each Effective Date, the Company shall file with the SEC in accordance
with Rule 424(b) under the 1933 Act the final prospectus to be used in connection with sales pursuant to the applicable Registration
Statement (whether or not such a prospectus is technically required by such rule). In the case of amendments and supplements to any Registration
Statement which are required to be filed pursuant to this Agreement (including, without limitation, pursuant to this Section 3(b))
by reason of the Company filing a report on Form 8-K, Form 10-Q or Form 10-K or any analogous report under the Securities Exchange Act
of 1934, as amended (the “1934 Act”), the Company shall, if permitted under the applicable rules and regulations of
the SEC, have incorporated such report by reference into such Registration Statement, if applicable, or shall file such amendments or
supplements with the SEC on the same day on which the 1934 Act report is filed which created the requirement for the Company to amend
or supplement such Registration Statement.
(c) The
Company shall permit legal counsel for Investor to review and comment upon (i) each Registration Statement at least three (3) Business
Days prior to its filing with the SEC and (ii) all amendments and supplements to each Registration Statement (including, without limitation,
the prospectus contained therein) (except for Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K,
and any similar or successor reports) within a reasonable number of days prior to their filing with the SEC, The Company shall promptly
furnish to legal counsel for Investor, without charge, (i) copies of any correspondence from the SEC or the Staff to the Company or its
representatives relating to each Registration Statement, provided that such correspondence shall not contain any material, non-public
information regarding the Company or any of its Subsidiaries (as defined in the Securities Purchase Agreement), (ii) after the same
is prepared and filed with the SEC, one (1) copy of each Registration Statement and any amendment(s) and supplement(s) thereto, including,
without limitation, financial statements and schedules, all documents incorporated therein by reference, if requested by Investor, and
all exhibits and (iii) upon the effectiveness of each Registration Statement, one (1) copy of the prospectus included in such Registration
Statement and all amendments and supplements thereto. The Company shall reasonably cooperate with legal counsel for Investor in performing
the Company’s obligations pursuant to this Section 3.
(d) The
Company shall promptly furnish to each Investor whose Registrable Securities are included in any Registration Statement, without charge,
(i) after the same is prepared and filed with the SEC, at least one (1) copy of each Registration Statement and any amendment(s) and
supplement(s) thereto, including, without limitation, financial statements and schedules, all documents incorporated therein by reference,
if requested by an Investor, all exhibits and each preliminary prospectus, (ii) upon the effectiveness of each Registration Statement,
ten (10) copies of the prospectus included in such Registration Statement and all amendments and supplements thereto (or such other
number of copies as such Investor may reasonably request from time to time) and (iii) such other documents, including, without limitation,
copies of any preliminary or final prospectus, as such Investor may reasonably request from time to time in order to facilitate the disposition
of the Registrable Securities owned by such Investor.
(e) The
Company shall use its best efforts to (i) register and qualify, unless an exemption from registration and qualification applies, the
resale by Investors of the Registrable Securities covered by a Registration Statement under such other securities or “blue sky”
laws of all applicable jurisdictions in the United States, (ii) prepare and file in those jurisdictions, such amendments (including,
without limitation, post-effective amendments) and supplements to such registrations and qualifications as may be necessary to maintain
the effectiveness thereof during the Registration Period, (iii) take such other actions as may be necessary to maintain such registrations
and qualifications in effect at all times during the Registration Period, and (iv) take all other actions reasonably necessary or advisable
to qualify the Registrable Securities for sale in such jurisdictions; provided, however, the Company shall not be required in connection
therewith or as a condition thereto to (x) qualify to do business in any jurisdiction where it would not otherwise be required to qualify
but for this Section 3(e), (y) subject itself to general taxation in any such jurisdiction, or (z) file a general consent to service
of process in any such jurisdiction. The Company shall promptly notify legal counsel for Investor who holds Registrable Securities of
the receipt by the Company of any notification with respect to the suspension of the registration or qualification of any of the Registrable
Securities for sale under the securities or “blue sky” laws of any jurisdiction in the United States or its receipt of actual
notice of the initiation or threatening of any proceeding for such purpose.
(f) The
Company shall notify legal counsel for Investor and in writing of the happening of any event, as promptly as practicable after becoming
aware of such event, as a result of which the prospectus included in a Registration Statement, as then in effect, may include an untrue
statement of a material fact or omission to state a material fact required to be stated therein or necessary to make the statements therein,
in the light of the circumstances under which they were made, not misleading (provided that in no event shall such notice contain any
material, non-public information regarding the Company or any of its Subsidiaries), and, subject to Section 3(r), promptly prepare
a supplement or amendment to such Registration Statement and such prospectus contained therein to correct such untrue statement or omission
and deliver ten (10) copies of such supplement or amendment to legal counsel for Investor. The Company shall also promptly notify legal
counsel for Investor in writing (i) when a prospectus or any prospectus supplement or post-effective amendment has been filed, when a
Registration Statement or any post-effective amendment has become effective (notification of such effectiveness shall be delivered to
legal counsel for Investor by e-mail on the same day of such effectiveness and by overnight mail), and when the Company receives written
notice from the SEC that a Registration Statement or any post-effective amendment will be reviewed by the SEC, (ii) of any request by
the SEC for amendments or supplements to a Registration Statement or related prospectus or related information, (iii) of the Company’s
reasonable determination that a post-effective amendment to a Registration Statement would be appropriate; and (iv) of the receipt of
any request by the SEC or any other federal or state governmental authority for any additional information relating to the Registration
Statement or any amendment or supplement thereto or any related prospectus. The Company shall respond as promptly as practicable to any
comments received from the SEC with respect to each Registration Statement or any amendment thereto (it being understood and agreed that
the Company’s response to any such comments shall be delivered to the SEC no later than fifteen (15) Business Days after the
receipt thereof).
(g) The
Company shall (i) use its best efforts to prevent the issuance of any stop order or other suspension of effectiveness of each Registration
Statement or the use of any prospectus contained therein, or the suspension of the qualification, or the loss of an exemption from qualification,
of any of the Registrable Securities for sale in any jurisdiction and, if such an order or suspension is issued, to obtain the withdrawal
of such order or suspension at the earliest possible moment and (ii) notify legal counsel for Investor who holds Registrable Securities
of the issuance of such order and the resolution thereof or its receipt of actual notice of the initiation or threat of any proceeding
for such purpose.
(h) If
any Investor may be required under applicable securities law to be described in any Registration Statement as an underwriter and such
Investor consents to so being named an underwriter, at the request of any Investor, the Company shall furnish to such Investor, on the
date of the effectiveness of such Registration Statement and thereafter from time to time on such dates as an Investor may reasonably
request (i) a letter, dated such date, from the Company’s independent certified public accountants in form and substance as is
customarily given by independent certified public accountants to underwriters in an underwritten public offering, addressed to the Investors,
and (ii) an opinion, dated as of such date, of counsel representing the Company for purposes of such Registration Statement, in form,
scope and substance as is customarily given in an underwritten public offering, addressed to the Investors.
(i) If
any Investor may be required under applicable securities law to be described in any Registration Statement as an underwriter and such
Investor consents to so being named an underwriter, upon the written request of such Investor, the Company shall make available for inspection
by (i) such Investor, (ii) legal counsel for such Investor and (iii) one (1) firm of accountants or other agents retained by such Investor
(collectively, the “Inspectors”), all pertinent financial and other records, and pertinent corporate documents and
properties of the Company (collectively, the “Records”), as shall be reasonably deemed necessary by each Inspector,
and cause the Company’s officers, directors and employees to supply all information which any Inspector may reasonably request;
provided, however, each Inspector shall agree in writing to hold in strict confidence and not to make any disclosure (except to such
Investor) or use of any Record or other information which the Company’s board of directors determines in good faith to be confidential,
and of which determination the Inspectors are so notified, unless (1) the disclosure of such Records is necessary to avoid or correct
a misstatement or omission in any Registration Statement or is otherwise required under the 1933 Act, (2) the release of such Records
is ordered pursuant to a final, non-appealable subpoena or order from a court or government body of competent jurisdiction, or (3) the
information in such Records has been made generally available to the public other than by disclosure in violation of this Agreement or
any other Transaction Document (as defined in the Securities Purchase Agreement). Such Investor agrees that it shall, upon learning that
disclosure of such Records is sought in or by a court or governmental body of competent jurisdiction or through other means, give prompt
notice to the Company and allow the Company, at its expense, to undertake appropriate action to prevent disclosure of, or to obtain a
protective order for, the Records deemed confidential. Nothing herein (or in any other confidentiality agreement between the Company
and such Investor, if any) shall be deemed to limit any Investor’s ability to sell Registrable Securities in a manner which is
otherwise consistent with applicable laws and regulations.
(j) The
Company shall hold in confidence and not make any disclosure of information concerning an Investor provided to the Company unless (i)
disclosure of such information is necessary to comply with federal or state securities laws, (ii) the disclosure of such information
is necessary to avoid or correct a misstatement or omission in any Registration Statement or is otherwise required to be disclosed in
such Registration Statement pursuant to the 1933 Act, (iii) the release of such information is ordered pursuant to a subpoena or other
final, non-appealable order from a court or governmental body of competent jurisdiction, or (iv) such information has been made generally
available to the public other than by disclosure in violation of this Agreement or any other Transaction Document. The Company agrees
that it shall, upon learning that disclosure of such information concerning an Investor is sought in or by a court or governmental body
of competent jurisdiction or through other means, give prompt written notice to such Investor and allow such Investor, at such Investor’s
expense, to undertake appropriate action to prevent disclosure of, or to obtain a protective order for, such information.
(k) Without
limiting any obligation of the Company under the Securities Purchase Agreement, the Company shall use its best efforts either to (i)
cause all of the Registrable Securities covered by each Registration Statement to be listed on each securities exchange on which securities
of the same class or series issued by the Company are then listed, if any, if the listing of such Registrable Securities is then permitted
under the rules of such exchange, (ii) secure designation and quotation of all of the Registrable Securities covered by each Registration
Statement on an Eligible Market (as defined in the Securities Purchase Agreement), or (iii) if, despite the Company’s best efforts
to satisfy the preceding clauses (i) or (ii) the Company is unsuccessful in satisfying the preceding clauses (i) or (ii), without
limiting the generality of the foregoing, to use its best efforts to arrange for at least two market makers to register with the Financial
Industry Regulatory Authority (“FINRA”) as such with respect to such Registrable Securities. In addition, the Company
shall cooperate with each Investor and any broker or dealer through which any such Investor proposes to sell its Registrable Securities
in effecting a filing with FINRA pursuant to FINRA Rule 5110 as requested by such Investor. The Company shall pay all fees and expenses
in connection with satisfying its obligations under this Section 3(k).
(l) The
Company shall cooperate with the Investors who hold Registrable Securities being offered and, to the extent applicable, facilitate the
timely preparation and delivery of certificates (not bearing any restrictive legend) representing the Registrable Securities to be offered
pursuant to a Registration Statement and enable such certificates to be in such denominations or amounts (as the case may be) as the
Investors may reasonably request from time to time and registered in such names as the Investors may request.
(m) If
requested by an Investor, the Company shall as soon as practicable after receipt of notice from such Investor and subject to Section 3(r)
hereof, (i) incorporate in a prospectus supplement or post-effective amendment such information as an Investor reasonably requests to
be included therein relating to the sale and distribution of Registrable Securities, including, without limitation, information with
respect to the number of Registrable Securities being offered or sold, the purchase price being paid therefor and any other terms of
the offering of the Registrable Securities to be sold in such offering; (ii) make all required filings of such prospectus supplement
or post-effective amendment after being notified of the matters to be incorporated in such prospectus supplement or post-effective amendment;
and (iii) supplement or make amendments to any Registration Statement or prospectus contained therein if reasonably requested by an Investor
holding any Registrable Securities.
(n) The
Company shall use its best efforts to cause the Registrable Securities covered by a Registration Statement to be registered with or approved
by such other governmental agencies or authorities as may be necessary to consummate the disposition of such Registrable Securities.
(o) The
Company shall make generally available to its security holders as soon as practical, but not later than ninety (90) days after the close
of the period covered thereby, an earnings statement (in form complying with, and in the manner provided by, the provisions of Rule 158
under the 1933 Act) covering a twelve-month period beginning not later than the first day of the Company’s fiscal quarter next
following the applicable Effective Date of each Registration Statement.
(p) The
Company shall otherwise use its best efforts to comply with all applicable rules and regulations of the SEC in connection with any registration
hereunder.
(q) Within
one (1) Business Day after a Registration Statement which covers Registrable Securities is declared effective by the SEC, the Company
shall deliver, and shall cause legal counsel for the Company to deliver, to the transfer agent for such Registrable Securities (with
copies to the Investors whose Registrable Securities are included in such Registration Statement) confirmation that such Registration
Statement has been declared effective by the SEC in the form attached hereto as Exhibit A.
(r) Notwithstanding
anything to the contrary herein (but subject to the last sentence of this Section 3(r)), at any time after the Effective Date of
a particular Registration Statement, the Company may delay the disclosure of material, non-public information concerning the Company
or any of its Subsidiaries the disclosure of which at the time is not, in the good faith opinion of the board of directors of the Company,
in the best interest of the Company and, in the opinion of counsel to the Company, otherwise required (a “Grace Period”),
provided that the Company shall promptly notify the Investors in writing of the (i) existence of material, non-public information giving
rise to a Grace Period (provided that in each such notice the Company shall not disclose the content of such material, non-public information
to any of the Investors) and the date on which such Grace Period will begin and (ii) date on which such Grace Period ends, provided
further that (I) no Grace Period shall exceed ten (10) consecutive days and during any three hundred sixty five (365) day period all
such Grace Periods shall not exceed an aggregate of thirty (30) days, (II) the first day of any Grace Period must be at least five (5)
Trading Days after the last day of any prior Grace Period and (III) except as described below, no Grace Period may exist during the sixty
(60) Trading Day period immediately following the Effective Date of such Registration Statement (provided that such sixty (60) Trading
Day period shall be extended by the number of Trading Days during such period and any extension thereof contemplated by this proviso
during which such Registration Statement is not effective or the prospectus contained therein is not available for use) (each, an “Allowable
Grace Period”). Notwithstanding the foregoing, any Registration Delay Payments due and payable as a result of an Effectiveness
Failure will accrue but remain unpaid for sixty (60) calendar days after such Effectiveness Failure (the “Effectiveness Cure
Period”). In the event the Company cures Effectiveness Failure the Effectiveness Cure Period, the accrued but unpaid Registration
Delay Payments shall be forgiven. A failure to cure the Effectiveness Failure in the Effectiveness Cure Period shall result in all Registration
Delay Payments accrued and unpaid since the Effectiveness Failure to be due and payable. For purposes of determining the length of a
Grace Period above, such Grace Period shall begin on and include the date the Investors receive the notice referred to in clause (i)
above and shall end on and include the later of the date the Investors receive the notice referred to in clause (ii) above and the date
referred to in such notice. The provisions of Section 3(g) hereof shall not be applicable during the period of any Allowable Grace
Period. Upon expiration of each Grace Period, the Company shall again be bound by the first sentence of Section 3(f) with respect
to the information giving rise thereto unless such material, non-public information is no longer applicable. Notwithstanding anything
to the contrary contained in this Section 3(r), the Company shall cause its transfer agent to deliver unlegended shares of Common
Stock to a transferee of an Investor in accordance with the terms of the Securities Purchase Agreement in connection with any sale of
Registrable Securities with respect to which such Investor has entered into a contract for sale, and delivered a copy of the prospectus
included as part of the particular Registration Statement to the extent applicable, prior to such Investor’s receipt of the notice
of a Grace Period and for which the Investor has not yet settled.
(s) The
Company shall take all other reasonable actions necessary to expedite and facilitate disposition by each Investors of its Registrable
Securities pursuant to each Registration Statement.
(t) Neither
the Company nor any Subsidiary or affiliate thereof shall identify any Investor as an underwriter in any public disclosure or filing
with the SEC, the Principal Market or any Eligible Market and any Buyer being deemed an underwriter by the SEC shall not relieve the
Company of any obligations it has under this Agreement or any other Transaction Document (as defined in the Securities Purchase Agreement);
provided, however, that the foregoing shall not prohibit the Company from including the disclosure found in the “Plan of Distribution”
section attached hereto as Exhibit B in the Registration Statement.
(u) Neither
the Company nor any of its Subsidiaries has entered, as of the date hereof, nor shall the Company or any of its Subsidiaries, on or after
the date of this Agreement, enter into any agreement with respect to its securities, that would have the effect of impairing the rights
granted to the Buyers in this Agreement or otherwise conflicts with the provisions hereof.
4. Obligations
of the Investors.
(a) At
least five (5) Business Days prior to the first anticipated filing date of each Registration Statement, the Company shall notify each
Investor in writing of the information the Company requires from each such Investor with respect to such Registration Statement. It shall
be a condition precedent to the obligations of the Company to complete the registration pursuant to this Agreement with respect to the
Registrable Securities of a particular Investor that such Investor shall furnish to the Company such information regarding itself, the
Registrable Securities held by it and the intended method of disposition of the Registrable Securities held by it, as shall be reasonably
required to effect and maintain the effectiveness of the registration of such Registrable Securities and shall execute such documents
in connection with such registration as the Company may reasonably request.
(b) Each
Investor, by such Investor’s acceptance of the Registrable Securities, agrees to cooperate with the Company as reasonably requested
by the Company in connection with the preparation and filing of each Registration Statement hereunder, unless such Investor has notified
the Company in writing of such Investor’s election to exclude all of such Investor’s Registrable Securities from such Registration
Statement.
(c) Each
Investor agrees that, upon receipt of any notice from the Company of the happening of any event of the kind described in Section 3(g)
or the first sentence of 3(f), such Investor will immediately discontinue disposition of Registrable Securities pursuant to any Registration
Statement(s) covering such Registrable Securities until such Investor’s receipt of the copies of the supplemented or amended prospectus
contemplated by Section 3(g) or the first sentence of Section 3(f) or receipt of notice that no supplement or amendment is
required. Notwithstanding anything to the contrary in this Section 4(c), the Company shall cause its transfer agent to deliver unlegended
shares of Common Stock to a transferee of an Investor in accordance with the terms of the Securities Purchase Agreement in connection
with any sale of Registrable Securities with respect to which such Investor has entered into a contract for sale prior to the Investor’s
receipt of a notice from the Company of the happening of any event of the kind described in Section 3(g) or the first sentence of
Section 3(f) and for which such Investor has not yet settled.
5. Expenses
of Registration.
(a) All
reasonable expenses, other than underwriting discounts and commissions, incurred in connection with registrations, filings or qualifications
pursuant to Sections 2 and 3, including, without limitation, all registration, listing and qualifications fees, printers and accounting
fees, FINRA filing fees (if any) and fees and disbursements of counsel for the Company shall be paid by the Company. The Company shall
reimburse Legal Counsel for its fees and disbursements in connection with registration, filing or qualification pursuant to Sections 2
and 3 of this Agreement which amount shall be limited to $10,000 for each such registration, filing or qualification.
6. Indemnification.
(a) To
the fullest extent permitted by law, the Company will, and hereby does, indemnify, hold harmless and defend each Investor and each of
its directors, officers, shareholders, members, partners, employees, agents, advisors, representatives (and any other Persons with a
functionally equivalent role of a Person holding such titles notwithstanding the lack of such title or any other title) and each Person,
if any, who controls such Investor within the meaning of the 1933 Act or the 1934 Act and each of the directors, officers, shareholders,
members, partners, employees, agents, advisors, representatives (and any other Persons with a functionally equivalent role of a Person
holding such titles notwithstanding the lack of such title or any other title) of such controlling Persons (each, an “Indemnified
Person”), against any losses, obligations, claims, damages, liabilities, contingencies, judgments, fines, penalties, charges,
costs (including, without limitation, court costs, reasonable attorneys’ fees and costs of defense and investigation), amounts
paid in settlement or expenses, joint or several, (collectively, “Claims”) incurred in investigating, preparing or
defending any action, claim, suit, inquiry, proceeding, investigation or appeal taken from the foregoing by or before any court or governmental,
administrative or other regulatory agency, body or the SEC, whether pending or threatened, whether or not an Indemnified Person is or
may be a party thereto (“Indemnified Damages”), to which any of them may become subject insofar as such Claims (or
actions or proceedings, whether commenced or threatened, in respect thereof) arise out of or are based upon: (i) any untrue statement
or alleged untrue statement of a material fact in a Registration Statement or any post-effective amendment thereto or in any filing made
in connection with the qualification of the offering under the securities or other “blue sky” laws of any jurisdiction in
which Registrable Securities are offered (“Blue Sky Filing”), or the omission or alleged omission to state a material
fact required to be stated therein or necessary to make the statements therein not misleading, (ii) any untrue statement or alleged untrue
statement of a material fact contained in any preliminary prospectus if used prior to the effective date of such Registration Statement,
or contained in the final prospectus (as amended or supplemented, if the Company files any amendment thereof or supplement thereto with
the SEC) or the omission or alleged omission to state therein any material fact necessary to make the statements made therein, in light
of the circumstances under which the statements therein were made, not misleading or (iii) any violation or alleged violation by the
Company of the 1933 Act, the 1934 Act, any other law, including, without limitation, any state securities law, or any rule or regulation
thereunder relating to the offer or sale of the Registrable Securities pursuant to a Registration Statement or (iv) any violation of
this Agreement (the matters in the foregoing clauses (i) through (iv) being, collectively, “Violations”). Subject
to Section 6(c), the Company shall reimburse the Indemnified Persons, promptly as such expenses are incurred and are due and payable,
for any legal fees or other reasonable expenses incurred by them in connection with investigating or defending any such Claim. Notwithstanding
anything to the contrary contained herein, the indemnification agreement contained in this Section 6(a): (i) shall not apply to
a Claim by an Indemnified Person arising out of or based upon a Violation which occurs in reliance upon and in conformity with information
furnished in writing to the Company by such Indemnified Person for such Indemnified Person expressly for use in connection with the preparation
of such Registration Statement or any such amendment thereof or supplement thereto, if such prospectus was timely made available by the
Company pursuant to Section 3(d); and (ii) shall not apply to amounts paid in settlement of any Claim if such settlement is effected
without the prior written consent of the Company, which consent shall not be unreasonably withheld or delayed. Such indemnity shall remain
in full force and effect regardless of any investigation made by or on behalf of the Indemnified Person and shall survive the transfer
of any of the Registrable Securities by any of the Investors pursuant to Section 9.
(b) In
connection with any Registration Statement in which an Investor is participating, such Investor agrees to severally and not jointly indemnify,
hold harmless and defend, to the same extent and in the same manner as is set forth in Section 6(a), the Company, each of its directors,
each of its officers who signs the Registration Statement and each Person, if any, who controls the Company within the meaning of the
1933 Act or the 1934 Act (each, an “Indemnified Party”), against any Claim or Indemnified Damages to which any of
them may become subject, under the 1933 Act, the 1934 Act or otherwise, insofar as such Claim or Indemnified Damages arise out of or
are based upon any Violation, in each case, to the extent, and only to the extent, that such Violation occurs in reliance upon and in
conformity with written information furnished to the Company by such Investor expressly for use in connection with such Registration
Statement; and, subject to Section 6(c) and the below provisos in this Section 6(b), such Investor will reimburse an Indemnified
Party any legal or other expenses reasonably incurred by such Indemnified Party in connection with investigating or defending any such
Claim; provided, however, the indemnity agreement contained in this Section 6(b) and the agreement with respect to contribution
contained in Section 7 shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior
written consent of such Investor, which consent shall not be unreasonably withheld or delayed, provided further that such Investor shall
be liable under this Section 6(b) for only that amount of a Claim or Indemnified Damages as does not exceed the net proceeds to
such Investor as a result of the applicable sale of Registrable Securities pursuant to such Registration Statement. Such indemnity shall
remain in full force and effect regardless of any investigation made by or on behalf of such Indemnified Party and shall survive the
transfer of any of the Registrable Securities by any of the Investors pursuant to Section 9.
(c) Promptly
after receipt by an Indemnified Person or Indemnified Party (as the case may be) under this Section 6 of notice of the commencement
of any action or proceeding (including, without limitation, any governmental action or proceeding) involving a Claim, such Indemnified
Person or Indemnified Party (as the case may be) shall, if a Claim in respect thereof is to be made against any indemnifying party under
this Section 6, deliver to the indemnifying party a written notice of the commencement thereof, and the indemnifying party shall
have the right to participate in, and, to the extent the indemnifying party so desires, jointly with any other indemnifying party similarly
noticed, to assume control of the defense thereof with counsel mutually satisfactory to the indemnifying party and the Indemnified Person
or the Indemnified Party (as the case may be); provided, however, an Indemnified Person or Indemnified Party (as the case may be) shall
have the right to retain its own counsel with the fees and expenses of such counsel to be paid by the indemnifying party if: (i) the
indemnifying party has agreed in writing to pay such fees and expenses; (ii) the indemnifying party shall have failed promptly to assume
the defense of such Claim and to employ counsel reasonably satisfactory to such Indemnified Person or Indemnified Party (as the case
may be) in any such Claim; or (iii) the named parties to any such Claim (including, without limitation, any impleaded parties) include
both such Indemnified Person or Indemnified Party (as the case may be) and the indemnifying party, and such Indemnified Person or such
Indemnified Party (as the case may be) shall have been advised by counsel that a conflict of interest is likely to exist if the same
counsel were to represent such Indemnified Person or such Indemnified Party and the indemnifying party (in which case, if such Indemnified
Person or such Indemnified Party (as the case may be) notifies the indemnifying party in writing that it elects to employ separate counsel
at the expense of the indemnifying party, then the indemnifying party shall not have the right to assume the defense thereof and such
counsel shall be at the expense of the indemnifying party), provided further that in the case of clause (iii) above the indemnifying
party shall not be responsible for the reasonable fees and expenses of more than one (1) separate legal counsel for such Indemnified
Person or Indemnified Party (as the case may be). The Indemnified Party or Indemnified Person (as the case may be) shall reasonably cooperate
with the indemnifying party in connection with any negotiation or defense of any such action or Claim by the indemnifying party and shall
furnish to the indemnifying party all information reasonably available to the Indemnified Party or Indemnified Person (as the case may
be) which relates to such action or Claim. The indemnifying party shall keep the Indemnified Party or Indemnified Person (as the case
may be) reasonably apprised at all times as to the status of the defense or any settlement negotiations with respect thereto. No indemnifying
party shall be liable for any settlement of any action, claim or proceeding effected without its prior written consent; provided, however,
the indemnifying party shall not unreasonably withhold, delay or condition its consent. No indemnifying party shall, without the prior
written consent of the Indemnified Party or Indemnified Person (as the case may be), consent to entry of any judgment or enter into any
settlement or other compromise which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such
Indemnified Party or Indemnified Person (as the case may be) of a release from all liability in respect to such Claim or litigation,
and such settlement shall not include any admission as to fault on the part of the Indemnified Party. Following indemnification as provided
for hereunder, the indemnifying party shall be subrogated to all rights of the Indemnified Party or Indemnified Person (as the case may
be) with respect to all third parties, firms or corporations relating to the matter for which indemnification has been made. The failure
to deliver written notice to the indemnifying party within a reasonable time of the commencement of any such action shall not relieve
such indemnifying party of any liability to the Indemnified Person or Indemnified Party (as the case may be) under this Section 6,
except to the extent that the indemnifying party is materially and adversely prejudiced in its ability to defend such action.
(d) The
indemnification required by this Section 6 shall be made by periodic payments of the amount thereof during the course of the investigation
or defense, as and when bills are received or Indemnified Damages are incurred.
(e) The
indemnity and contribution agreements contained herein shall be in addition to (i) any cause of action or similar right of the Indemnified
Party or Indemnified Person against the indemnifying party or others, and (ii) any liabilities the indemnifying party may be subject
to pursuant to the law.
7. Contribution.
To
the extent any indemnification by an indemnifying party is prohibited or limited by law, the indemnifying party agrees to make the maximum
contribution with respect to any amounts for which it would otherwise be liable under Section 6 to the fullest extent permitted
by law; provided, however: (i) no contribution shall be made under circumstances where the maker would not have been liable for indemnification
under the fault standards set forth in Section 6 of this Agreement, (ii) no Person involved in the sale of Registrable Securities
which Person is guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the 1933 Act) in connection with such
sale shall be entitled to contribution from any Person involved in such sale of Registrable Securities who was not guilty of fraudulent
misrepresentation; and (iii) contribution by any seller of Registrable Securities shall be limited in amount to the amount of net
proceeds received by such seller from the applicable sale of such Registrable Securities pursuant to such Registration Statement. Notwithstanding
the provisions of this Section 7, no Investor shall be required to contribute, in the aggregate, any amount in excess of the amount
by which the net proceeds actually received by such Investor from the applicable sale of the Registrable Securities subject to the Claim
exceeds the amount of any damages that such Investor has otherwise been required to pay, or would otherwise be required to pay under
Section 6(b), by reason of such untrue or alleged untrue statement or omission or alleged omission.
8. Reports
Under the 1934 Act.
With
a view to making available to the Investors the benefits of Rule 144, the Company agrees to:
(a) make
and keep public information available, as those terms are understood and defined in Rule 144;
(b) file
with the SEC in a timely manner all reports and other documents required of the Company under the 1933 Act and the 1934 Act so long as
the Company remains subject to such requirements (it being understood and agreed that nothing herein shall limit any obligations of the
Company under the Securities Purchase Agreement) and the filing of such reports and other documents is required for the applicable provisions
of Rule 144; and
(c) furnish
to each Investor so long as such Investor owns Registrable Securities, promptly upon request, (i) a written statement by the Company,
if true, that it has complied with the reporting, submission and posting requirements of Rule 144, the 1933 Act and the 1934 Act, (ii)
a copy of the most recent annual or quarterly report of the Company and such other reports and documents so filed by the Company with
the SEC if such reports are not publicly available via EDGAR, and (iii) such other information as may be reasonably requested to
permit the Investors to sell such securities pursuant to Rule 144 without registration.
9. Assignment
of Registration Rights.
All
or any portion of the rights under this Agreement shall be automatically assignable by each Investor to any transferee or assignee (as
the case may be) of all or any portion of such Investor’s Registrable Securities or Preferred Shares if: (i) such Investor agrees
in writing with such transferee or assignee (as the case may be) to assign all or any portion of such rights, and a copy of such agreement
is furnished to the Company within a reasonable time after such transfer or assignment (as the case may be); (ii) the Company is, within
a reasonable time after such transfer or assignment (as the case may be), furnished with written notice of (a) the name and address of
such transferee or assignee (as the case may be), and (b) the securities with respect to which such registration rights are being transferred
or assigned (as the case may be); (iii) immediately following such transfer or assignment (as the case may be) the further disposition
of such securities by such transferee or assignee (as the case may be) is restricted under the 1933 Act or applicable state securities
laws if so required; (iv) at or before the time the Company receives the written notice contemplated by clause (ii) of this sentence
such transferee or assignee (as the case may be) agrees in writing with the Company to be bound by all of the provisions contained herein;
(v) such transfer or assignment (as the case may be) shall have been made in accordance with the applicable requirements of the Securities
Purchase Agreement and the Preferred Shares ;and (vi) such transfer or assignment (as the case may be) shall have been conducted in accordance
with all applicable federal and state securities laws.
10. Amendment
of Registration Rights.
Provisions
of this Agreement may be amended and the observance thereof may be waived (either generally or in a particular instance and either retroactively
or prospectively), only with the written consent of the Company and the Investor; Any amendment or waiver effected in accordance with
this Section 10 shall be binding upon Investor and the Company. No waiver shall be effective unless it is in writing and signed by an
authorized representative of the waiving party. No consideration shall be offered or paid to any Person to amend or consent to a waiver
or modification of any provision of this Agreement unless the same consideration (other than the reimbursement of legal fees) also is
offered to all of the parties to this Agreement.
11. Miscellaneous.
(a) Solely
for purposes of this Agreement, a Person is deemed to be a holder of Registrable Securities whenever such Person owns, or is deemed to
own, of record such Registrable Securities. If the Company receives conflicting instructions, notices or elections from two or more Persons
with respect to the same Registrable Securities, the Company shall act upon the basis of instructions, notice or election received from
such record owner of such Registrable Securities.
(b) Any
notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing
and will be deemed to have been delivered: (i) upon receipt, when delivered personally; (ii) upon receipt, when sent by electronic mail
(provided that such sent email is kept on file (whether electronically or otherwise) by the sending party and the sending party does
not receive an automatically generated message from the recipient’s email server that such e-mail could not be delivered to such
recipient); or (iii) one (1) Business Day after deposit with an overnight courier service with next day delivery specified, in each case,
properly addressed to the party to receive the same. The mailing addresses and e-mail addresses for such communications shall be:
If
to the Company:
Evofem
Biosciences, Inc.
7770 Regents Road, Suite 113-618
San
Diego, CA 92122
Telephone: (858) 550-1900
Attention: Chief Financial Officer
E-Mail: Izhang@evofem.com
With
a copy (for informational purposes only) to:
Procopio
Cory Hargreaves & Savitch, LLP
12544 High Bluff Drive
Suite 400
San Diego, CA 92130
Telephone: (858) 523-4305
E-Mail: paul.johnson@procopio.com
If
to the Transfer Agent:
Pacific
Stock Transfer
6725 Via Austi Pkwy, Suite 300
Las Vegas, NV 89119
Telephone: (702) 323-0033
Attention: Joslyn Claiborne
E-Mail: jclairborne@pacificstocktransfer.com
If
to Investor:
Aditxt,
Inc.
737
Fifth Street, Suite 200
Richmond,
VA 23219
Attention:
Amro Albanna, CEO
E-mail:
aalbanna@aditxt.com
With
a copy to:
Sheppard,
Mullin Richter & Hampton LLP
30 Rockefeller Plaza
New York, NY 10112-0015
Telephone: 1-212-634-3073
Attention: John R. Hempill, Esq.
E-mail:
JHempill@sheppardmullin.com
If
to a Buyer, to its mailing address and/or email address set forth on the Schedule of Buyers attached to the Securities Purchase Agreement,
with copies to such Buyer’s representatives as set forth on the Schedule of Buyers, or to such other mailing address and/or email
address and/or to the attention of such other Person as the recipient party has specified by written notice given to each other party
five (5) days prior to the effectiveness of such change, Written confirmation of receipt (A) given by the recipient of such notice,
consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s e-mail containing the
time, date and recipient’s e-mail or (C) provided by a courier or overnight courier service shall be rebuttable evidence of personal
service, receipt by e-mail or receipt from a nationally recognized overnight delivery service in accordance with clause (i), (ii)
or (iii) above, respectively.
(c) Failure
of any party to exercise any right or remedy under this Agreement or otherwise, or delay by a party in exercising such right or remedy,
shall not operate as a waiver thereof. The Company and each Investor acknowledge and agree that irreparable damage would occur in the
event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached.
It is accordingly agreed that each party hereto shall be entitled to an injunction or injunctions to prevent or cure breaches of the
provisions of this Agreement by any other party hereto and to enforce specifically the terms and provisions hereof (without the necessity
of showing economic loss and without any bond or other security being required), this being in addition to any other remedy to which
any party may be entitled by law or equity.
(d) All
questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws
of the State of New York, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of New
York or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of New York.
Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in The City of New York,
Borough of Manhattan, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby
or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is
not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum
or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and
consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such
notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof.
Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. EACH PARTY HEREBY
IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE TO, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR
IN CONNECTION HEREWITH OR ARISING OUT OF THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY.
(e) If
any provision of this Agreement is prohibited by law or otherwise determined to be invalid or unenforceable by a court of competent jurisdiction,
the provision that would otherwise be prohibited, invalid or unenforceable shall be deemed amended to apply to the broadest extent that
it would be valid and enforceable, and the invalidity or unenforceability of such provision shall not affect the validity of the remaining
provisions of this Agreement so long as this Agreement as so modified continues to express, without material change, the original intentions
of the parties as to the subject matter hereof and the prohibited nature, invalidity or unenforceability of the provision(s) in question
does not substantially impair the respective expectations or reciprocal obligations of the parties or the practical realization of the
benefits that would otherwise be conferred upon the parties. The parties will endeavor in good faith negotiations to replace the prohibited,
invalid or unenforceable provision(s) with a valid provision(s), the effect of which comes as close as possible to that of the prohibited,
invalid or unenforceable provision(s).
(f) This
Agreement, the other Transaction Documents, the schedules and exhibits attached hereto and thereto and the instruments referenced herein
and therein constitute the entire agreement among the parties hereto and thereto solely with respect to the subject matter hereof and
thereof. There are no restrictions, promises, warranties or undertakings, other than those set forth or referred to herein and therein.
This Agreement, the other Transaction Documents, the schedules and exhibits attached hereto and thereto and the instruments referenced
herein and therein supersede all prior agreements and understandings among the parties hereto solely with respect to the subject matter
hereof and thereof; provided, however, nothing contained in this Agreement or any other Transaction Document shall (or shall be deemed
to) (i) have any effect on any agreements any Investor has entered into with the Company or any of its Subsidiaries prior to the date
hereof with respect to any prior investment made by such Investor in the Company, (ii) waive, alter, modify or amend in any respect any
obligations of the Company or any of its Subsidiaries or any rights of or benefits to any Investor or any other Person in any agreement
entered into prior to the date hereof between or among the Company and/or any of its Subsidiaries and any Investor and all such agreements
shall continue in full force and effect or (iii) limit any obligations of the Company under any of the other Transaction Documents.
(g) Subject
to compliance with Section 9 (if applicable), this Agreement shall inure to the benefit of and be binding upon the permitted successors
and assigns of each of the parties hereto. This Agreement is not for the benefit of, nor may any provision hereof be enforced by, any
Person, other than the parties hereto, their respective permitted successors and assigns and the Persons referred to in Sections 6
and 7 hereof.
(h) The
headings in this Agreement are for convenience of reference only and shall not limit or otherwise affect the meaning hereof. Unless the
context clearly indicates otherwise, each pronoun herein shall be deemed to include the masculine, feminine, neuter, singular and plural
forms thereof. The terms “including,” “includes,” “include” and words of like import shall be construed
broadly as if followed by the words “without limitation.” The terms “herein,” “hereunder,” “hereof”
and words of like import refer to this entire Agreement instead of just the provision in which they are found.
(i) This
Agreement may be executed in two or more identical counterparts, each of which shall be deemed an original, but all of which shall be
considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the
other party. In the event that any signature is delivered by an email which contains a portable document format (.pdf) file of an executed
signature page, such signature page shall create a valid and binding obligation of the party executing (or on whose behalf such signature
is executed) with the same force and effect as if such signature page were an original thereof.
(j) Each
party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such
other agreements, certificates, instruments and documents as any other party may reasonably request in order to carry out the intent
and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
(k) The
language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent and no rules of
strict construction will be applied against any party. Notwithstanding anything to the contrary set forth in Section 10, terms used in
this Agreement but defined in the other Transaction Documents shall have the meanings ascribed to such terms on the Closing Date in such
other Transaction Documents unless otherwise consented to in writing by each Investor.
(l) All
consents and other determinations required to be made by the Investor pursuant to this Agreement shall be made as determined as if all
of the outstanding Preferred Shares then held by the Investor have been converted for Registrable Securities without regard to any limitations
on redemption, amortization and/or conversion of the Preferred Shares.
(m) This
Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the
benefit of, nor may any provision hereof be enforced by, any other Person.
[signature
page follows]
IN
WITNESS WHEREOF, Buyer and the Company have caused their respective signature page to this Registration Rights Agreement to be duly
executed as of the date first written above.
| |
COMPANY: |
| |
|
| |
EVOFEM BIOSCIENCES, INC. |
| |
|
|
| |
By: |
|
| |
|
Name: Saundra Pelletier |
| |
|
Title: CEO |
IN
WITNESS WHEREOF, Buyer and the Company have caused their respective signature page to this Registration Rights Agreement to be duly
executed as of the date first written above.
| |
BUYER: |
| |
|
| |
ADITXT, INC. |
| |
|
|
| |
By: |
|
| |
|
Name: Amro Albana |
| |
|
Title: CEO |
EXHIBIT
A
FORM
OF NOTICE OF EFFECTIVENESS
OF REGISTRATION STATEMENT
______________________
______________________
______________________
Attention: _____________
| Re: | Evofem
Biosciences, Inc. |
Ladies
and Gentlemen:
[We
are][I am] counsel to Evofem Biosciences, Inc., a Delaware corporation (the “Company”), and have represented the Company
in connection with that certain Securities Purchase Agreement (the “Securities Purchase Agreement”) entered into by
and among the Company and the buyers named therein (collectively, the “Holders”) pursuant to which the Company issued
to the Holders of Series F-1 Convertible Preferred Stock (the “Preferred Shares”) convertible into the Company’s
shares of common stock, $0.0001 par value per share (the “Common Stock”).Pursuant to the Securities Purchase Agreement,
the Company also has entered into a Registration Rights Agreement with the Holders (the “Registration Rights Agreement”)
pursuant to which the Company agreed, among other things, to register the Registrable Securities (as defined in the Registration Rights
Agreement), including the shares of Common Stock issuable upon conversion of the Preferred Shares, under the Securities Act of 1933,
as amended (the “1933 Act”). In connection with the Company’s obligations under the Registration Rights Agreement,
on ____________ ___, 20__, the Company filed a Registration Statement on Form [S-1][S-3] (File No. 333-_____________) (the “Registration
Statement”) with the Securities and Exchange Commission (the “SEC”) relating to the Registrable Securities
which names each of the Holders as a selling stockholder thereunder.
In
connection with the foregoing, [we][I] advise you that [a member of the SEC’s staff has advised [us][me] by telephone that [the
SEC has entered an order declaring the Registration Statement effective under the 1933 Act at [ENTER TIME OF EFFECTIVENESS] on [ENTER
DATE OF EFFECTIVENESS]] [an order declaring the Registration Statement effective under the 1933 Act at [ENTER TIME OF EFFECTIVENESS]
on [ENTER DATE OF EFFECTIVENESS]] has been posted on the web site of the SEC at www.sec.gov] and [we][I] have no knowledge, after a review
of information posted on the website of the SEC at http://www.sec.gov/litigation/stoporders.shtml, that any stop order suspending its
effectiveness has been issued or that any proceedings for that purpose are pending before, or threatened by, the SEC and the Registrable
Securities are available for resale under the 1933 Act pursuant to the Registration Statement.
This
letter shall serve as our standing opinion to you that the shares of Common Stock underlying the Preferred Shares are freely transferable
by the Holders pursuant to the Registration Statement. You need not require further letters from us to effect any future legend-free
issuance or reissuance of such shares of Common Stock to the Holders as contemplated by the Company’s Irrevocable Transfer Agent
Instructions dated _________ __, 20__.
| |
Very truly yours, |
| |
|
| |
[ISSUER’S COUNSEL] |
| |
|
|
| |
By: |
|
EXHIBIT
B
SELLING
STOCKHOLDERS
The
shares of common stock being offered by the selling stockholders are those issuable to the selling stockholders upon conversion of the
preferred shares. For additional information regarding the issuance of the preferred shares, see “Private Placement of Preferred
Shares” above. We are registering the shares of common stock in order to permit the selling stockholders to offer the shares for
resale from time to time.
The
table below lists the selling stockholders and other information regarding the beneficial ownership (as determined under Section 13(d)
of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder) of the shares of common stock held by each
of the selling stockholders. The second column lists the number of shares of common stock beneficially owned by the selling stockholders,
based on their respective ownership of shares of common stock, preferred shares and warrants, as of ________, 20__, assuming conversion
of the preferred shares held by each such selling stockholder on that date but taking account of any limitations on conversion and exercise
set forth therein.
The
third column lists the shares of common stock being offered by this prospectus by the selling stockholders and does not take in account
any limitations on (i) conversion of the preferred shares set forth therein or (ii) exercise of the warrants set forth therein.
In
accordance with the terms of a registration rights agreement with the holders of the preferred shares, this prospectus generally covers
the resale of the sum of (i) 150% of the maximum number of shares of common stock issued or issuable upon conversion of the preferred
shares (assuming for purposes hereof that the preferred shares are convertible at the alternate conversion price assuming an alternate
conversion date as of the date of filing of the registration statement this prospectus forms a part of determined as if the outstanding
preferred shares were converted in full (without regard to any limitations on conversion contained in the certificate of designations
or any limitations on exercise contained in the warrants, solely for the purpose of such calculation) at an alternate conversion price
or exercise price (as the case may be) calculated as of the trading day immediately preceding the date this registration statement was
initially filed with the SEC. Because the conversion price and alternate conversion price of the preferred shares may be adjusted, the
number of shares that will actually be issued may be more or less than the number of shares being offered by this prospectus. The fourth
column assumes the sale of all of the shares offered by the selling stockholders pursuant to this prospectus.
Under
the terms of the preferred shares, a selling stockholder may not convert the preferred shares to the extent (but only to the extent)
such selling stockholder or any of its affiliates would beneficially own a number of shares of our common stock which would exceed 4.99%
of the outstanding shares of the Company. The number of shares in the second column reflects these limitations. The selling stockholders
may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
Name
of Selling Stockholder | |
Number of Shares of Common Stock Owned Prior to Offering | | |
Maximum Number of Shares of Common Stock to be Sold Pursuant to this Prospectus | | |
Number of Shares of Common Stock of Owned After Offering | |
| Aditxt, Inc.(1) | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | |
PLAN
OF DISTRIBUTION
We
are registering the shares of common stock issuable upon conversion of the preferred shares to permit the resale of these shares of common
stock by the holders of the preferred shares from time to time after the date of this prospectus. We will not receive any of the proceeds
from the sale by the selling stockholders of the shares of common stock.. We will bear all fees and expenses incident to our obligation
to register the shares of common stock.
The
selling stockholders may sell all or a portion of the shares of common stock held by them and offered hereby from time to time directly
or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers,
the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common
stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices
determined at the time of sale or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block
transactions, pursuant to one or more of the following methods:
| ● | on
any national securities exchange or quotation service on which the securities may be listed
or quoted at the time of sale; |
| ● | in
the over-the-counter market; |
| ● | in
transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| ● | through
the writing or settlement of options, whether such options are listed on an options exchange
or otherwise; |
| ● | ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block
trades in which the broker-dealer will attempt to sell the shares as agent but may position
and resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an
exchange distribution in accordance with the rules of the applicable exchange; |
| ● | privately
negotiated transactions; |
| ● | short
sales made after the date the Registration Statement is declared effective by the SEC; |
| ● | broker-dealers
may agree with a selling security holder to sell a specified number of such shares at a stipulated
price per share; |
| ● | a
combination of any such methods of sale; and |
| ● | any
other method permitted pursuant to applicable law. |
The
selling stockholders may also sell shares of common stock under Rule 144 promulgated under the Securities Act of 1933, as amended,
if available, rather than under this prospectus. In addition, the selling stockholders may transfer the shares of common stock by other
means not described in this prospectus. If the selling stockholders effect such transactions by selling shares of common stock to or
through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts,
concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may
act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers
or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common
stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short
sales of the shares of common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares
of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed
shares in connection with such short sales. The selling stockholders may also loan or pledge shares of common stock to broker-dealers
that in turn may sell such shares.
The
selling stockholders may pledge or grant a security interest in some or all of the preferred shares, warrants or shares of common stock
owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell
the shares of common stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or
other applicable provision of the Securities Act amending, if necessary, the list of selling stockholders to include the pledgee, transferee
or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate
the shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will
be the selling beneficial owners for purposes of this prospectus.
To
the extent required by the Securities Act and the rules and regulations thereunder, the selling stockholders and any broker-dealer participating
in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities
Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions
or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement,
if required, will be distributed, which will set forth the aggregate amount of shares of common stock being offered and the terms of
the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation
from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
Under
the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers
or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified
for sale in such state or an exemption from registration or qualification is available and is complied with.
There
can be no assurance that any selling stockholder will sell any or all of the shares of common stock registered pursuant to the registration
statement, of which this prospectus forms a part.
The
selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Securities
Exchange Act of 1934, as amended, and the rules and regulations thereunder, including, without limitation, to the extent applicable,
Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the
selling stockholders and any other participating person. To the extent applicable, Regulation M may also restrict the ability of any
person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of
common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity
to engage in market-making activities with respect to the shares of common stock.
We
will pay all expenses of the registration of the shares of common stock pursuant to the registration rights agreement, estimated to be
$[ ] in total, including, without limitation, Securities and Exchange Commission filing fees and expenses
of compliance with state securities or “blue sky” laws; provided, however, a selling stockholder will pay all underwriting
discounts and selling commissions, if any. We will indemnify the selling stockholders against liabilities, including some liabilities
under the Securities Act in accordance with the registration rights agreements or the selling stockholders will be entitled to contribution.
We may be indemnified by the selling stockholders against civil liabilities, including liabilities under the Securities Act that may
arise from any written information furnished to us by the selling stockholder specifically for use in this prospectus, in accordance
with the related registration rights agreements or we may be entitled to contribution.
Once
sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the
hands of persons other than our affiliates.
Exhibit 99.1
Dr. Drew Pinsky (00:26):
Alright, welcome everyone. Thank you so much for being here. I am truly
excited. This is a pleasure to be a part of this project. First, I want to tell you about the players. Aditxt is a social innovation platform
that has to say socially owned, dedicated to accelerating promising health innovations, Aditxt ecosystem of research institutions, industry
partners and shareholders collaboratively drive the mission to make promising innovations possible together. Understand innovations don’t
always get to the public and to the patients the way they should. That’s the Aditxt intention to get them there. The innovation platform
is the cornerstone of the Aditxt strategy, where multiple disciplines drive disruptive growth and address significant societal changes.
Aditxt operates a unique model that democratizes innovation and ensures every stakeholder’s voice is heard and valued and empowers collective
progress. Aditxt currently operates two programs, operated and focused on immune health and precision health.
(01:29):
The company will introduce two additional programs dedicated to public
health and women’s health. For these Aditxt has entered into an arrangement agreement with Appili Therapeutics, which focuses on infectious
diseases. That is APLIF on the OTC Pink and a merger agreement with Evofem Biosciences Inc. That is EVFM. Each program will be designed
to function autonomously while collectively advancing Aditxt’s mission of discovering, developing and deploying innovative health
solutions. To tackle some of the most urgent health challenges, I want to emphasize that getting innovative products that patients need,
doctors need, the public should have. That is what Aditxt is going to help us do. Evofem Inc is a commercializing innovative products
organization to address unmet needs in women’s health and reproductive health. It was launched in September, 2020. They launched Phexxi®
the first and only FDA approved hormone free birth control. I was very excited to see when this product came around and when Aditxt
got involved in the world of Phexxi.®
(02:38):
I was extremely excited. In July, 2024, they
broadened their commercial offering with the acquisition of SOLOSEC® an FDA approved single dose oral agent for the
treatment of two common sexual health infections, trichomoniasis, and bacterial vaginosis. Those of you that are clinicians know these
are very common problems and can be a frustrating issue for many patients. Saundra Pelletier is the CEO. She’s the force behind driving
true innovations to market for women’s health. She’s here with me. She’s the heart of the Evofem organization, having spent her entire
career defending women’s rights and advocating for every woman to have access to game changing, project changing products that positively
impact their daily lives. I cannot tell you what a privilege it is to be a part of this, to have these two here with me. Today. We’re
going to enter into a little conversation about what the goals are, what is available, what we’re going to do. I’m going to just open
it with Amro to give him to kick off the conversation.
Amro Albanna (03:38):
Thank you Dr. Drew, and I appreciate the fact that you joined us today.
You and I have had discussions over the years, and today really is more of a conversation. It’s more of a discussion. Look, we want to
make sure that we talk about women’s health, the stage of women’s health, and how desperately this field requires new innovations. We
want to talk about Evofem as a foundation for and how we see it as a foundation for accelerating women’s health in the field of diagnostics,
treatment, and prevention. And finally, to really touch on the point that you just brought up, which is how do we bring these promising
innovations to the world? I mean, we all need health innovations. We all need to address some of the most challenging health needs that
we have.
Dr. Drew Pinsky (04:29):
I want to interrupt and just say, let’s shine a hang lantern on that,
so to speak. People think that magically good things are invented and then we get them is not how it works. And Aditxt wants to make sure
you do get them. And this is why I’m so excited this morning.
Amro Albanna (04:46):
Yeah, absolutely. I mean, look, that’s really the key. I mean, unless
you’re involved in the industry, you really don’t realize that just because it’s a good idea does not mean it’s going to make it to the
market. And there are all kinds of challenges that I know Saundra and I can talk for days about. But reality is promising innovations,
in fact, the vast number of promising innovations, we’ll never see the light of day.
Dr. Drew Pinsky (05:09):
Fantastic, so hang on. That is nuts. That’s crazy when you say things
like that. I think about the electric car a hundred years ago and like, oh, okay. Well, people kind of know that story, but they imagine
that in healthcare ethics would dictate that good things would get to patients, but it’s just not how it works. So maybe we had to let
Saundra kind of kick in here. I see her chomping at the bit.
Saundra Pelletier (05:32):
I’m well, thank you, by the way. And so look, as it relates to women’s
health, birth control was introduced in 1960. Okay? So imagine this. Since that time, women have been told all they get is synthetic hormones,
but they could just have them delivered in different ways. You could have them in a pill, in a patch, in an IUD, in a ring. But no one
has really said, what do women want? What’s good for women are women suffering from side effects. And by the way, women don’t have sex
every day. I realize that that might be a big statement, but to take a drug every day that you don’t need if you’re having side effects,
is crazy.
Dr. Drew Pinsky (06:13):
Well, but not only that, hang on, Saundra. I mean, any physician, any
healthcare provider, any woman that takes hormonal contraceptives knows that not having side effects is sort of unusual.
Saundra Pelletier (06:25):
A hundred percent. A hundred percent,
Saundra Pelletier (06:28):
Yes. And by the way, there’s so many women right now in the United
States, 23 million women will tell you, Dr. Drew, that they will not use hormones. That they have tried all of these different choices,
that the headaches and the bleeding and the weight gain and the emotional highs and lows, they’re not doing it anymore.
Dr. Drew Pinsky (06:45):
Well, not only that, doctors don’t sit down. This is the part that
drives me crazy. I did a radio show for 30 years where I was having to explain to young people like, no, this is the hormonal contraceptive
that your doctor didn’t sit down and explain to you that vaginal dryness, that lack of libido, that sleep, they just don’t. And these
are routine, particularly with high progestational agents. It’s routine now to see those side effects. So again, that’s why I spotted
your product. It’s like, oh, why did it take so long? Right?
Saundra Pelletier (07:18):
Yes. Well, and by the way, when Amro said, some of these innovations
never see the light of day. Only 30 to 40% of drugs get approved after they do phase one, phase two, phase three. So I’d love to show
you the product. I mean, I know you know it, but okay. So this innovation that I think we all agree is pretty game changing. It’s called
Phexxi® it is the first and the only non-hormonal birth control that women use on demand only when they have sex, only
when they need it, never when they don’t. Men have had condoms for 150 years, right? A man can go out with a condom in his pocket, he
can protect himself, but now women are going to be empowered with the same thing. And a prescription is a box of 12. So a woman will get
12 of these prefilled applicators.
(08:06):
When you open this up, which you can put in your purse or your pocket,
this is the applicator. Any woman who’s used a tampon or any kind of applicator, it’s very, very easy. But here’s why I like to show,
like to show everybody. By the way, sometimes I get invited extra to dinner parties because I bring this with me. But if you can see it,
this gel, it’s five milliliters in each applicator. But here’s what matters. Women are not going to use something that leaks out. But
what women is that it’s lubricating, it’s viscous. It stays inside the vaginal cavity. It creates lubrication if you want it and you need
it. And even young women today say to me, they’re using lubrication as part of intimacy. So it’s sort of a no brainer.
Dr. Drew Pinsky (08:47):
That’s because they’re having side effects from the hormonal contraceptive
difficulties, which is so, so common. But let’s dig in a little bit. So I’d like to hear the mechanism of action and how it’s different.
And tell us more.
Saundra Pelletier (09:01):
Okay. So Rush University developed this, and they developed it in the
early nineties. They were looking for something for HIV prevention, and here’s what they recognized. They recognized that a normal vaginal
pH for all women is 3.5 to 4.5. That’s it. When semen enters, pH rises up to seven or eight and women get pregnant. Same thing that happens
when pathogens like chlamydia and gonorrhea enter. But what Rush realized is that this product, it helps a woman’s body maintain her natural
pH making it inhospitable to semen. So it’s pretty amazing that the ingredients are simple. Food grade, lactic acid, citric acid, potassium
by tartrate. But when combined together, they create this mechanism of action that prevents pregnancy, and it’s really
Dr. Drew Pinsky (09:48):
Efficacy, efficacy rates.
Saundra Pelletier (09:49):
So two different things. So in our clinical study, there were 25,000
acts of sex. We had less than 1% of pregnancies. But when we did our clinical study, this is the other thing that I think is always challenging.
The FDA insisted that we count failures of women who didn’t use the drug at all, or women who used it after sex. So in the label it says
93% and 86%. But what we’ve seen is being on the market for almost over two years, we’ve had less than 1% of pregnancies, to be honest.
Dr. Drew, you’ll know this better than most, the most effective method for women is the one she’s going to use consistently. And if a
woman has had side effects, and if a woman says, I’m worried about what taking a synthetic hormone is going to do to me. Will I be able
to get pregnant when I want to? What will happen to me? Will there be some other unintended consequence they feel really good about using
Phexxi®.
Dr. Drew Pinsky (10:41):
These are just naive questions, but I’m going to ask because people
have them and I have them. Back in the day, we used to always talk about adding a second barrier. If you want to really protect yourself,
does that work with other barriers?
Saundra Pelletier (10:54):
It does. It does. And the interesting thing is that sometimes women
who are breastfeeding, right, they don’t want to have hormones in their breast milk. So usually a lot of people are using this as spacing.
They might be on a long acting method, but they want to take a break. Maybe they have a partner that’s away, or maybe they’ve had a baby,
they’re going to space their pregnancies. Also, think about this. 900,000 women get cancer every year. Those women are usually told they
should not use a synthetic hormone again.
(11:23):
But the big usage, I’ll be honest, is young women who say, I don’t
have sex every day. I want to have sex on demand. I don’t want to have emotional highs and lows. I was put on an antidepressant. I was
put on an anti-anxiety drug. No one thought it was my birth control. So I have to say,
Dr. Drew Pinsky (11:42):
Oh, don’t even start me with that. It is not just anxiety, meds, mood,
stable, everything gets, can’t even go there. It’s just so frustrating that doctors don’t go, are you on a hormone? Or we just ignore
that completely.
Saundra Pelletier (11:56):
It’s crazy. And the final thing, the final thing I want to say before
I get off of this sort of soapbox that I’m on, is that one of the huge fastest growing categories of drugs are GLP-1s. So Mounjaro, Ozempic.
And in some of the labels, it says that if you were on oral birth control, you must use a backup method to your exact point, because the
mechanism of action of the GLP- 1s, it makes oral birth control less effective. So it says, you must use a backup method.
Dr. Drew Pinsky (12:25):
Nobody knows that knows that. I didn’t know that you just said it.
Saundra Pelletier (12:30):
And every time your dose is titrated up, you need a backup method.
So you see all over social media, Ozempic babies, all these babies, because the last thing most women want is when they’re trying to use
that to lose weight or for their diabetes, they don’t want to get pregnant. So it’s been a fascinating thing to see that the more people
who learn about Phexxi I think the more protected women can be, and the more physicians that understand that there’s so many women on
GLP- 1s that if they had access to Phexxi I think they’d feel more protected.
Dr. Drew Pinsky (13:00):
Amro, I’m sorry that, and I are completely taking over this conversation,
but I’m excited. So please jump in….
Amro Albanna (13:09):
No, no. This is why we set it up.
Dr. Drew Pinsky (13:11):
It’s really cool. And I knew when I saw the product, of course, I didn’t
think of all the ways that it was so important. I just knew it would be important. But it’s FDA approved,
Saundra Pelletier (13:23):
It’s FDA approved, so it’s FDA approved. You can get it through telemedicine,
you can get it through any pharmacy. And under Obamacare, ACA, if a woman has health insurance, many women are getting it at zero out-of-pocket
pay. And so it’s been really exciting, but not enough people know about Phexxi.
Dr. Drew Pinsky
I know that. I know, including myself. That’s why when Aditxt
grabbed it. I was like, oh, here we go. But how many come? Is it supplied in boxes of 30 or something? Or how does that work?
Saundra Pelletier (13:58):
No. So 12 pre-filled applicators. There’s a four year shelf life. So
women can, and by the way, typically a prescription one box is a month prescription. But if a woman says she has sex more often, her monthly
prescription could be two boxes of 12.
Dr. Drew Pinsky (14:12):
Yeah, of course.
Saundra Pelletier 14:14):
But yeah, I mean, look, the big thing to say, when I met Amro, I will
tell you this. It was one of the things that he said to me was that he watched a TED talk that I did, where I said, I want to ask the
audience a question. I said, how many of you have sons? And I said, well, what if I told you your son was going to take a medication every
single day of every month, year after year, he was probably going to have side effects. He didn’t need the medication every day. What
would you say? You’d say, I was out of my mind. So why is that okay that we ask our daughters to do that? And Amro’s like Saundra,
because we have had a really difficult time as a small little company trying to, sure, this innovation really gets elevated and escalated.
(14:55):
And when I met Amro, I was like, you are too good to be true. He’s
like, my entire platform is to help companies like yours stay in existence, elevate what you’re doing, because it’s truly innovative.
It’s not about a me too product in a crowded category. I’m not saying, let me deliver you a synthetic hormone this in a different way.
So that’s why it’s been great to find a partner that instead of trying to put me out of business, which a lot of big pharma wants to Amro’s
like I want you to stay in business and grow.
Dr. Drew Pinsky(15:26):
So now we can talk to Amro. He can have his way with us. I have more
questions, but go ahead, Amro.
Amro Albanna (15:31):
Yeah, yeah. No, I mean, Dr. Drew, again, this is really why we want
to make sure we shine a light on what Evofem and Saundra and team are doing. I mean, it is critical to make sure that Phexxi as a product
and beyond Phexxi, we really bring attention to the products. And that’s what Aditxt is all about. That’s our DNA, no pun intended. And
you and I have had these conversations over the years as far as innovations and what it takes to bring these innovations to market.
Dr. Drew Pinksy (16:00):
I have worked with you over the years here and there, and just when
I’m not working, I still have the same enthusiasm for what you’re doing. I watch the news feed on you every single, literally, every single
day, my friend. So I know what you’re doing, and I know who you are. I know who your team is. And so Saundra, you’re very lucky to be
a part of this. I know you see it right there, who they say they are, which it is rare these days when people are who they say they are.
Right?
Saundra Pelletier (16:25):
Without question. And by the way, and not just to care about the domestic
difference about the global difference, which is also because a lot of people will say it for a halo effect, right? Oh, we care about
social responsibility. But people who really genuinely want to make a difference, it’s big.
Dr. Drew Pinsky (16:42):
And I want to contextualize things too, because our purpose is not
to bash oral contraceptives. That is not what we are doing. What bothers me is that the practice of medicine has become less reflective,
and we don’t think about the options, and we don’t think about the particular impact of what we’re doing. The risk reward. I mean, we
seem to have lost track of that completely. And so to sit down and tell a patient, hey, look, I’m not sure. Are you the kind of person
that we can rely on yourself to do this and inject this? And you have, you’re comfortable with it, as opposed to taking a hormone every
day, which may cause some dryness and libido changes. And by the way, and can be really beneficial in certain situations. There’s no doubt
that OCP is the choice, but doctors need to make those choices consciously rather than automatically. And then I will just say the part
we have not talked about, I have been concerned for a long time that so many of the relational changes that are going on these days, whether
it’s fertility or in any interpersonal setting, same sex, heterosexual, whatever it is, these hormones are going to have an effect. And
we don’t think about it. And I’m just saying, let’s just think about it, especially now that we have an option. But I’ll let you go on,
Saundra.
Saundra Pelletier (18:05):
Well, no, I just wanted to say I am grateful that you brought that
up, right? Look, I know that Aditxt’s DNA, Evofem’s, DNA, we want women to have choices. We know that women go through different
stages and phases. We know that women will use three or four different kinds of contraception. We want to be an option for the women who
say we want something non-hormonal that we can use on demand.
(18:28):
But the other thing, the reality that not a lot of people talk about
in the United States right now, United States, half of all pregnancies every year, almost 3 million are unplanned. But that doesn’t mean
they’re necessarily unwanted, but they’re unplanned. Unintended. And the United States of America ready has the highest adolescent teen
pregnancy rate out of any developed country in the world. So if there was a silver bullet that was working, those rates wouldn’t be so
high. I mean, it’s madness to think that. It’s madness to think, people said to me, why are you coming out with birth control? There’s
hundreds of choices that exist. And I’m like, if the choices that existed were going to solve the problem, well then we wouldn’t have
so many unintended pregnancy rights. And then the final thing I want to say is when I talked to Amro about the global impact, I said,
look, he and I agreed on this, that people talk about global warming and clean water, and all those things matter, but the very best way
to eliminate poverty is less people on the planet by choice. Choice. But you got to make it available. You got to give these women product
that they’re going to feel good on. But yes, your whole point about relationships and how just to think and be mindful and thoughtful,
I love that comment. It’s so rare, by the way.
Dr. Drew Pinsky (19:46):
I know I’ve had to see it. I’ve seen it. I’ve been worried. I’ve been
in this space in and out for 20 years, and I’ve not looked at the unplanned pregnancy rates lately, but it’s been a half pregnancies being
unplanned as long as I’ve been working in this area. And so it’s not really changing very much. And that’s a disaster. And back to, I
just want to make clear, we live in this world where people are exercised about certain topics. This is not a board of face, and this
is not interfere with any of those issues that people worry about sometimes.
Saundra Pelletier (20:20):
In fact, not only is it not, Phexxi is actually the only product that
does not, when you’re on birth control, hormonal birth control, your body thinks it’s pregnant. So that’s why you typically don’t get
pregnant because we have no systemic activity in the body. Once you use it and then you’re done using it, it’s no longer in your body.
So that’s what’s really fascinating. It doesn’t impact a woman’s natural god-given body and the no hormones, no systemic activity. It
matter what a woman’s weight is, it doesn’t matter what other medication she’s on. That’s also a peace of mind too. For physicians who
love Phexxi, they say, look, we don’t have to worry all the other things that are impacting these women’s lives.
It makes it really a safe choice regardless of what they’re on, which
Dr Drew Pinsky (21:03):
Is, and let me say, one of the things that used to really trouble me
was there is a group of people, and I am a radical moderate in all things, and join me in the middle. It’s quite nice here. You can see
more clearly from the middle of both sides. But one of the groups that of course is out there, is a group that’s very, very concerned
about aborta phases, about when life begins and these kinds of things. And listen, if you are using a hormonal contraceptive or an IUD,
IUDs work primarily by preventing implantation. And for a large percentage of people, they consider that an abortion, and they should
be aware that’s what they have in place. Same thing is true with hormonal contraceptives. There is a finite effect, not all of the primary
effect of those hormones, but there is an effect on the uterine lining that affects implantation should the hormonal contraceptive fail.
And you ovulate and people are not aware of that. And this takes that away completely.
Saundra Pelletier (22:09):
It does. You’re right. And by the way, kids aren’t even learning about
their bodies and how they work. Girls aren’t learning.
Dr. Drew Pinsky (22:15):
Well, everybody that’s very bent out of shape about this topic, they
need to understand their biology. If they really want to get this right, they can just study biology and good for you. Go get it right,
but don’t get confused about this. Understand how our bodies work. And this is a great, great way to address that. When it comes to some
of these, when I hear your data on efficacy, almost always it’s proper use that interferes with its, and it sounds like the proper use
in reality is when people actually use it in the marketplace, they’re using it properly because its so easy to use
Saundra Pelletier (22:56):
Without question, and they’re motivated to do so, right? They’re motivated
to do so. And I think they not only feel good physically, I think they feel good psychologically, which I think is really important. And
look, the other thing is that in the opening, and thank you, we talked about that. We have Phexxi, but one of the interesting parts about,
one of the interesting parts about being acquired by Aditxt is that some of the other organizations that Amro is looking at also will
provide us access to look at other serious unmet needs for women like early detection.
Dr. Drew Pinsky (23:26):
Tell us about that. Go ahead, Amro. This is you. We’re done with our
love fest for the second.
Saundra Pelletier
Well, no, I’m not done.
Amro Albanna (23:34):
No, please don’t be done. I mean, look, this is really what it’s all
about. I mean, this specific session is about Evofem, and again, I really do appreciate you joining us and helping out with the word.
I mean, look, we just did endometriosis, which we announced for Evofem. And when Saundra and I connected, and we’ve been really looking
at how do we accelerate and how do we take Evofem to that next step? And we both see the same vision of diversifying the offerings by
making sure that we’re not only focusing on prevention, but we’re looking at treatments as well as diagnostics and monitoring. So when
it comes to monitoring endometriosis, huge problem, undiagnosed difficult to diagnose. And with Pearsanta, which is one of our subsidiaries
actually is bringing to market by mid 2025, a new test blood-based noninvasive test to detect endometriosis. And Saundra, maybe you can
shed some light on the test and how excited you and your team are as far as endometriosis diagnostic is.
Saundra Pelletier (24:37):
Yeah. Well, at the end of the day, and Dr. Drew this better than probably
anybody I’ve spoken to, is that there has been a shift in patients wanting more demanding, more patient-centered care
Dr. Drew Pinksy
Let me even amplify that further. It goes on the heels of kind of,
they want free, they want to have access at their, I call it medical freedom essentially. And we are on the threshold of really telehealth
becoming extraordinarily effective at delivering not just therapeutics and assessment, but diagnostics. So have at it.
Saundra Pelletier (25:17):
Yes, by the way, and look, early detection changes the entire health
trajectory for a woman early detection of something like endometriosis. And by the way, also things that are the silent killers like ovarian
cancer, which the same test over time with more clinical work could have early detection. And so what we’re really trying to do is, we
are trying to be part of a bigger organization that says what you’re doing, it matters. And doing it alone, it will take us two lifetimes
to speed up the trajectory of offering these real innovations to women. And the one thing that I really valued that Amro said, it’s also
about the people who are the people leading the charge to bring these innovations to market. Do they have the grit and the backbone to
make it happen? They’re not going to give up easy, but really saying, now, let’s go to the next thing.
(26:13):
Let’s build the portfolio. Let’s keep moving forward and continuing
to find these products that are underserved. And we have a real chance to do it. But I will tell you that a lot of the reasons some of
these products don’t see light is because they can’t get the support, they can’t get the funding, and they get stomped out of existence,
particularly if they’re a threat to what is the status quo, right? Threat. And instead of saying, Hey, let’s rise up and give women more
access, or men too to innovation. Unfortunately, not everybody thinks that way, but I like your meet in the middle because meet in the
middle is people should have access to choose what they want, what’s right for them.
Dr. Drew Pinksy(26:56):
They do not discriminate. Do not discriminate. We try to meet the needs
and to be careful, assess what people’s specific needs are, and you need to be discriminatory. But we are not. We’re not going to discriminate
from our position. Amro, in the intro, I mentioned trichomoniasis and bacterial vaginosis. What’s going on there?
Amro Albanna (27:23):
SOLOSEC®. SOLOSEC®
is another product that, and I know Saundra’s ready to go. I mean, it’s another product that we brought in and we supported
Evofem to bring it on board. And this really fits the treatment category. As you recall. We talked about prevention with Phexxi diagnostics
and monitoring with endometriosis and the Pearsanta test, and now we’re talking about SOLOSEC®. So again, Saundra
and team went after it. We supported their efforts, and now we have, and Evofem really has three different products in these three different
categories, and Saundra can certainly shed some light on SOLOSEC® and why it’s a great opportunity to launch into
the market.
Saundra Pelletier (27:59):
So for us, as you stated, bacterial vaginosis
is just so staggering for women. They have so much stigma with it. Trichomoniasis is so pervasive. Bottom line is this product, SOLOSEC®.,
why we wanted it so badly is that it’s one dose, one and done. It’s a packet of granules that you put in applesauce, yogurt,
pudding, and that’s it. To have one dose when right now, a lot of the historical treatment regimen has been twice a day for seven
to 14 days. So when we had a chance to bring this product into our portfolio, we were so used to operating on such a shoestring budget,
so used to operating out of desperation. I was like, I just don’t think it’s possible. And I called Amro and he’s like,
Saundra, be a possibility thinker. We’ll help you make this happen. And so I’ve really been able to shed all of that, what
we can’t have to, anything’s possible. And so he supported us to bring this product into the portfolio, same call point,
OBGYN. And so we’re really able to show that this company that’s very passionate about delivering good things to women can
make it happen with this.
Dr. Drew Pinsky(29:07):
Talk about mechanism of action and the spectrum of care with that product.
Saundra Pelletier (29:12):
So what it looks like. And so really, so BV and Trich are…..
Dr. Drew Pinsky(29:20):
Hold it closer. I can’t quite see it. There we go.
Saundra Pelletier (29:23):
And it’s also about vaginal pH, by the way. So what’s fascinating is
that our team, our sales team, we have a very small but mighty sales team of 16 people. They already talk about vaginal pH, right? They’re
very comfortable talking about it, men and women, and they feel really, really good that the ease of the conversation. Physicians love
this product. It was out of promotion for a couple of years before we brought it into our portfolio, but it was approved for bacterial
vaginosis, but then it got approved during Covid for trichomoniasis. And so the exciting part is that the fact that it’s just a packet
of granules, and it is so effective, it’s just been an interesting thing to be able to say, just because a product has been around for
a while, maybe it needs to be dusted off and get a little bit of a facelift. And that’s what’s cool too, to be able to bring things
back to light that deserve it. A little bit of a dusting off.
Dr. Drew Pinksy(30:25):
It is the same issue that we were talking about at the outset, which
is just because something is good doesn’t mean it gets used or deployed or distributed. And so why not bring out a good idea that’s not
getting the right attention? It’s the same philosophy.
Saundra Pelletier (30:40):
Totally. Totally.
Dr. Drew Pinsky (30:41):
So I’m just looking it up. What is the mechanism of action here?
Saundra Pelletier (30:47):
So it’s the same way it actually impacts the vaginal pH. So when women
have BV, they have odor, they have discharge, they have pain by the way. And so it actually goes in and it helps a woman’s body reregulate
her pH. And so then she just takes the one dose. And the one other thing too that I feel like I can say to you that’s been fascinating
is women, even women who have never been taught or know about their body or their pH, everybody seems to understand pH. They understand
why, what it means, why it matters. And I do think though, that as we embark on really leaning into women’s health, that we’ll also have
a chance to educate women, to educate women and girls about not just their bodies and how they work, but about how empowering it is to
be able to say, I deserve sex on demand. For example, there was a lot of people when we launched that said, oh, women can’t be trusted
to use something on demand. They have to have a fix and forget method.
Dr. Drew Pinsky (31:52):
No, no, those days are gone. Everything has to be….we have to
empower the patient because our system doesn’t help the patients this way, it should. So the patients have to take over their care.
Saundra Pelletier (32:05):
They really do. And the other thing is that healthcare plans too, I
think are being forced to do better. They’re being forced to do better and really support companies and not all of them. But I do think
that that’s what’s been fascinating about the Affordable Care Act in Obamacare, because they did put out a legislative mandate saying
that one product in each category of contraception must be covered because the cost of pregnancy is so much more than the cost of contraception,
right? It’s crazy not to cover contraception.
Dr. Drew Pinsky (32:39):
There’s a lot of craziness in our system that is one of many. So I
would say I was surprised, but I’m not. So I’m interested in the product. So it sounds like it’s in the same class as Metronidazole, the
old fashioned way of doing this, and as you said, that was a week to two weeks that had medicine has loads of side effects, is its same
mechanism as the old Flagyl?
Saundra Pelletier (33:04):
Yep, it is. You’re right. Metronidazole, yes. And the beauty of it
is that women, it’s very, very well tolerated.
Dr. Drew Pinsky (33:13):
I see that.
Saundra Pelletier (33:14):
And the other thing truthfully is that when women have vaginal infections
versus putting something vaginally administered, there are products out there that aren’t vaginally administered, taking something oral….
Dr. Drew Pinsky (33:28):
It is a complicated landscape. So the intravaginal creams for this,
I can’t be alone that pretty much every physician that prescribes echoes, I hope this works because it’s not as efficacious as taking
the pills. But we don’t want to give the pills a week or two weeks of really a medication that causes depression and headache and sleep
disturbances and oral ulcers and things. I mean, if you need it, great, but if it’s not needed, I mean, we want to do something different.
This is a single dose now of a medication. I’d much rather do that. Amro, I’m going to challenge you here. Again, I want to live in a
day when patients can do this whole thing from home where they could send in some sort of diagnostic kit to a telehealth company or a
provider and just boom and just do a quick online access and get the treatment. Do you have any therapeutic plans out there for this kind
of thing? To me that’s much like the endometriosis screen. There are things you could do on your own if you knew about it.
Amro Albanna (34:37):
Yeah, well, we’re working on multiple programs right now. You know
about Adimune. Adimune is truly the ADI platform is being designed and we’re advancing that forward towards clinical trials where we could
potentially retrain the immune system rather than suppress the immune system. And you can imagine the potential and the opportunity. Of
course, we’re still preclinical. We can’t predict efficacy, but imagine really changing what we’ve been doing for the last 50 years when
it comes to autoimmunity, when it comes to organ rejection, even severe allergies. So this is one platform which frankly deserves its
own fireside chat, but this is one of the programs we have. The other, which was Pearsanta, we discuss endometriosis, but MDNA, which
relies on mitochondrial DNA, where we could potentially or early detect cancer starting with prostate cancer, ovarian cancer, lung cancer,
and so on. So yeah, we’ve got a lot in the pipeline and women’s health and Evofem is currently, we’re still in the process of acquisition,
just to make sure that’s clear, that we’re still not done with the transaction. We still have few things to get done before…
Dr. Drew Pinsky (35:50):
With Pearsanta?
Amro Albanna (35:52):
So Pearsanta and Adimune are our own wholly own subsidiaries.
Dr. Drew Pinsky (35:57):
Got it.
Amro Albanna (35:58):
And Evofem and Appili are still in the process of being acquired.
Dr. Drew Pinsky (36:03):
Got it. And I just want to make sure I heard you clearly. So Pearsanta™
will have applications outside of women’s health. Obviously I’m a 12 year post prostatectomy, maybe not 13 or 14 years prostate cancer
patient. So I’m working that world a lot. So I’m interested in talking more about other applications of Pearsanta for the future. And
did I hear that correctly? They will have other applications?
Amro Albanna (36:27):
You did. Yeah. So we’re starting with endometriosis and prostate cancer
followed by ovarian cancer.
Dr. Drew Pinsky (36:32):
Amazing. Amazing. Saundra, you wanted to jump in there? I could tell
Saundra Pelletier (36:36):
No. Well, no, no. Look, I guess I wanted to say too, as people are
listening to this, one of the things that I’ve tried to say to people to understand the Aditxt platform is that Aditxt is therapeutic
area agnostic. What do I mean by that? Is that we are women’s healthcare. We want to care about everything from puberty through menopause.
We want to care about everything that impacts women, birth control and geland oral antibiotic and endometriosis. But the other subsidiaries
of Aditxt also are all about innovation, right? Game changing therapeutics and diagnostics that deliver in areas that also have this huge
unmet need. Because sometimes people are like, oh, well, why aren’t you all in women’s health? Or Why aren’t you all? It’s by design,
right? It’s a purposeful thing about let’s identify innovative organizations and let’s support them because on their own, they’re struggling.
(37:36):
And that’s been the interesting thing is the struggle, by the way.
And look, I don’t want to be a crybaby or a whiner, but it has been harder than you’d ever believe When people hear about our innovations
and they look at me and they’re like, how come everybody doesn’t know about this? How come every woman doesn’t know about this? Why doesn’t
every doctor know about this? And you think to yourself, it’s true. Why don’t they? But small companies have a hard time getting the kind
of support that they need to be a household name. And by the way, we almost were in a place where we were no longer existing, literally
eliminated from the platform, from the planet, and sincerely, and that’s not even an exaggeration at all. And to do all this work to bring
a product like this to market, and then you think to yourself, and I know that founders and innovators, their hearts in it, but we’ve
been able to build a team of people that would fight, kill, and die for these products.
(38:41):
And to think there were so many people on the firing squad ready to
say, you know what? We’re just going to take you out of the game. So that’s been hard too. Final thing is, my crybaby part was that you
hear a lot of people don’t want to support women’s health because it only impacts half the population. But come on, men are part of this
too. There’s so many good men that want to support women, and they really understand that even pregnancy, it’s a two way street. And so
I think those days are over. But I do think that it’s so important that when people want to innovate, they should do it and not worry
that they’re not going to make it. Because I think there’s going to be more opportunities with groups like Aditxt to help companies like
mine stay alive.
Dr. Drew Pinsky (39:26):
And I want to speak directly to anybody listening that you need to
know that changing physician behavior is really hard. It’s more difficult than changing the public’s behavior, frankly. It’s why pharmaceutical
companies, they advertise directly to you. So you’ll go to the doctor’s center, I want that, blah, blah, blah. We can talk about the ethics
of that. That’s a different issue. But the fact, that’s why they do it, because us as physicians, we have our way of doing things and
we’ve done it 10,000 times and we have confidence with it, and we know the risk reward of every decision in that context. Bringing something
new in is a record scratch for us. Even when it’s simple with no side effects, it’s hard for us. And so I’m sympathetic to my peers that
we don’t do this, but I’m telling you, the future is patient centered care.
(40:21):
We all talk about the patient specific care and the individualized
services and stuff, but I think it’s more than that. We need to shift the sort of way we think about our own healthcare on We should be
in control of things at all times. And if we’re not getting things the way we want, we should find ways to do so because we live in a
time when it’s all accessible. It’s all there. And again, some of it needs to be ironed out, some of the interstate things and all the,
it’s coming. It’s really going to be here. And you’re going to be able to do diagnostics at home. You’re going to be able to do it all
through telehealth. You’re going to be able to use Phexxi with no side effects. I have one more question about the bacterial vaginosis
treatment. Bacterial vaginosis in pregnancy is a serious problem. I’m imagining you can’t use this medication in pregnancy. Is that correct?
Saundra Pelletier (41:14):
To be candid, I’m going to have to tell you that. I don’t know if you
can use it
Dr. Drew Pinsky (41:20):
I’m going to guess not because it’s a complicated zone, but we got
to look into it. We ought to figure out where we fit that. Yeah, we got to definitely look into it. And I should have the answer to that.
It’s a complicated and sort of believe it or not, a controversial zone. Some people just leave it alone by way.
Saundra Pelletier (41:39):
I’m going to tell you right now. Wait, hold on.
Dr. Drew Pinsky (41:42):
But I don’t think you can use much of anything during pregnancy, really,
particularly early in it. It may be late in third trimester you might, but…
Saundra Pelletier (41:49):
Nope. Can be used. Can be used in pregnancy.
Dr. Drew Pinsky (41:51):
It’s not a category C, it’s just a full out?
Saundra Pelletier (41:54):
No, no can be used in pregnancy. Okay, so that’s great.
Dr. Drew Pinsky (41:56):
That might be a breakthrough. So let’s look at that and see if that,
I’m not an obstetrician, I can’t comment, but that’s kind of interesting. It’d be an opportunity maybe if that’s one of the only options
or good options out there, or safer options out there. We should be really raising awareness among obstetricians, if that’s true. I don’t
know the answer. I don’t know. I’m just guessing.
Saundra Pelletier (42:21):
Yeah, no, no, no. But yes, yes. Used in pregnancy. And as you probably
know better, it’s very pervasive.
Dr. Drew Pinsky (42:26):
Let, let’s be very careful. I don’t let pregnant women take anything.
And so until we get the obstetrical world to sign on to it, let’s say we are going to look into this further, but there might be a real
Aron. You agree with me on that one?
Amro Albanna (42:40):
Absolutely.
Dr. Drew Pinsky (42:41):
And let me say it again. I don’t let pregnant women take anything I
don’t. Maybe Tylenol maybe. And that’s about it. So I’m not comfortable with saying
(42:50):
It’s anything safe, but if the OB world says it is, then we can more
comfortably say so. Okay. Well, this has been a great conversation. I wonder if you have any last comments. Look, Saundra and I are, we’ve
only met briefly before today, and we are in mind meld in terms of our priorities and how we see the landscape of the world. And the fact
that Saundra has to say something like it only affects half of humanity. The fact those words have to come out of your mouth is bizarre.
We worry about illnesses that affect 0.01% of humanity. We get very worried about, concerned about it. This is half of humanity. Let’s
be clear. There’s a lot of people, and as you say, men of course are in the game too with this. But Amro, let’s finish this up.
Amro Albanna (43:34):
Yeah, no, this is great. Thank you again for giving us this opportunity
and joining us. I mean, Dr. Drew, you know what Aditxt is, our DNA and our mission and today’s session was really focused on making sure
that our stakeholders and frankly future stakeholders get to learn and understand better why. Why we’re pursuing this opportunity and
why we want to make sure that fixie and other products make it to the world. So thank you again.
Dr. Drew Pinsky (44:01):
And to be fair to you, both Saundra and I are here to represent that
Amro who he says he is, Aditxt is who they say they are. They walk the walk, they talk the talk. I’ve never seen anything other than the
highest level ethics and judgment and the way you guys do things. I could not be happier than to be here chatting with you guys. Saundra,
I’ll let you sort of wrap up as well.
Saundra Pelletier (44:30):
Thank you. Well, thank you very much for having us as guests, and thank
you Amro for allowing me to showcase Evofem and why women’s health is not just important because it’s half the population, but because
there really is a value proposition and an investment proposition, investing in women. I mean, we know women are the healthcare decision
makers. We decide for ourselves, for our families, usually our parents. And at the end of the day, if women feel often, there’s still
that old mindset. Women don’t put themselves first. Everybody else gets to be before them, but, but when women have access to products
that are innovative, that can make them feel as good as they should because they choose them, it’s just going to be a different world
for women. And so I just want to thank you for caring about this. By the way, if I could clone you, Dr. Drew, I would do it immediately
because you’re understanding.
Dr. Drew Pinsky (45:24):
Don’t want to deal with that. Ask my wife.
Saundra Pelletier (45:27):
But yeah, it’s amazing though your understanding of patient-centered
care and how critical it’s, and so I really appreciate it.
Dr. Drew Pinsky (45:35):
My whole career. I’ve been about trying to, I just naturally moved
to what I think is important. I was deep in the AIDS pandemic, I was treating lots of those patients, and then I sort of got into the
effects of childhood trauma and then addiction. And now it’s about getting the control of medicine back into the hands of patients. That’s
where I am right now. And you guys are clearly part of that. Let me just put out some specifics so everybody’s clear. If you’re just listening,
Aditxt, Inc. Is A-D-I-T-X-T. If you’re watching it, you see it over Amro’s head there. You screwed me up. Or you said Evofem. You told
me it’s Evofem from evolution, right?
Saundra Pelletier (46:17):
It’s by the way, truthfully, it’s like Sandra. Saundra.
Dr. Drew Pinsky (46:22):
Good. So I screw that up too. We went everyone on the boat either way
or get on the ship. Evofem Biosciences is EVFM on OTCQB. Am I getting that correct?
Saundra Pelletier (46:35):
That’s correct, yep.
Dr. Drew Pinsky (46:36):
Anything else? The SOLOSEC. Anything else we should be highlighting,
Amro as we exit here?
Amro Albanna (46:46):
Yeah, just making sure that obviously the acquisition is not completed.
We want to make sure that’s very clear. We still have many things to other closing conditions, one of which is a senior creditor and a
senior loan that has to be addressed.
Dr. Drew Pinsky (46:59):
Well, it needs to happen. So let’s all just jump on board as best we
can to support and make sure it does. And we thank you, Saundra, for fighting the good fight. I thank you, Amro, for being the figurehead
of this organization, Aditxt that I’ve been a fan of for four years now, five years. I’ve been watching you guys regularly, and I appreciate
it very much. It’s my pleasure to be here. And I hope you all got something out of this. I hope you’re listening. I hope you’re doing
what we’re suggesting you ought to do, which is take control of your own care and look at these products very carefully and make decisions.
Make it with your physicians where it’s appropriate access, telehealth where it’s appropriate, but it’s a new age and we should all be
a part of it. So thank you all for being here and look forward to future events with Aditxt work, and they figure can they find that Amro?
Is there a site or anything going to announce any future fireside chats?
Amro Albanna (47:53):
Yeah, we will be. We’re doing it through our press releases and links
will be submitted as well.
Dr. Drew Pinsky (47:58):
Okay. So check that out and we’ll see you all soon, hopefully. Thank
you.
16
v3.24.3
Cover
|
Oct. 28, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 28, 2024
|
| Entity File Number |
001-39336
|
| Entity Registrant Name |
Aditxt, Inc.
|
| Entity Central Index Key |
0001726711
|
| Entity Tax Identification Number |
82-3204328
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
2569 Wyandotte Street
|
| Entity Address, Address Line Two |
Suite 101
|
| Entity Address, City or Town |
Mountain View
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94043
|
| City Area Code |
650
|
| Local Phone Number |
870-1200
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.001
|
| Trading Symbol |
ADTX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Aditxt (NASDAQ:ADTX)
Historical Stock Chart
From Nov 2024 to Dec 2024
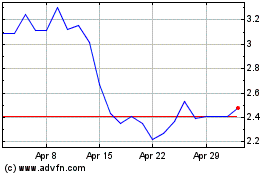
Aditxt (NASDAQ:ADTX)
Historical Stock Chart
From Dec 2023 to Dec 2024