UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 1-A/A
Dated: July 30, 2024
REGULATION A OFFERING CIRCULAR UNDER THE SECURITIES
ACT OF 1933
Standard Dental
Labs, Inc.
(Exact name of issuer as specified in its charter)
Nevada
(State of other jurisdiction of incorporation or organization)
424 E Central Blvd, Suite 308,
Orlando, Florida, 32801
321-465-9899
(Address, including zip code, and telephone number,
including area code of issuer’s principal executive office)
Jeff Turner
7533 S Center View Ct, #4291
West Jordan, UT 84084
801-810-4465
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
| 3843 |
|
88-0411500 |
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
This Preliminary Offering Circular shall only be qualified
upon order of the Commission, unless a subsequent amendment is filed indicating the intention to become qualified by operation of the
terms of Regulation A.
This Offering Circular is following the Offering
Circular format described in Part II (a)(1)(ii) of Form 1-A.
TABLE OF CONTENTS
PART II – PRELIMINARY OFFERING CIRCULAR
- FORM 1-A: TIER II
An Offering statement pursuant to Regulation
A relating to these securities has been filed with the Securities and Exchange Commission. Information contained in this Preliminary
Offering Circular is subject to completion or amendment. These securities may not be sold nor may offers to buy be accepted before the
Offering statement filed with the Securities and Exchange Commission is qualified. This Preliminary Offering Circular shall not constitute
an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in which such offer,
solicitation or sale would be unlawful before registration or qualification under the laws of any such state. We may elect to satisfy
our obligation to deliver a Final Offering circular by sending you a notice within two business days after the completion of our sale
to you that contains the URL where the Final Offering Circular or the Offering statement in which such Final Offering Circular was filed
may be obtained.
PRELIMINARY OFFERING CIRCULAR
Dated: July 30, 2024
Subject to Completion
PURSUANT TO REGULATION A OF THE SECURITIES ACT OF 1933
Standard Dental Labs Inc.
424 E Central Blvd, Suite 308,
Orlando, Florida, 32801
400,000,000 Shares of Common Stock
at a price range of $0.01 - $0.03 per share
Minimum Investment: $5,000
Maximum Offering: $12,000,000
See The Offering - Page
3 for further details. None of the securities offered are being sold by present security holders.
Upon qualification of this Offering by the Securities and Exchange Commission, the Offering will commence within two business days of
being qualified by the Securities and Exchange Commission (“SEC”) and will terminate 365 days from the date of qualification
by the Securities and Exchange Commission, unless extended or terminated earlier by the Company.
PLEASE REVIEW ALL RISK FACTORS
BEGINNING ON PAGES 4 THROUGH 10 BEFORE MAKING AN INVESTMENT IN THIS COMPANY. AN INVESTMENT IN THIS COMPANY SHOULD ONLY BE MADE IF YOU
ARE CAPABLE OF EVALUATING THE RISKS AND MERITS OF THIS INVESTMENT AND IF YOU HAVE SUFFICIENT RESOURCES TO BEAR THE ENTIRE LOSS OF YOUR
INVESTMENT, SHOULD THAT OCCUR.
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION
DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE
ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SELLING LITERATURE. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION
FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED
HEREUNDER ARE EXEMPT FROM REGISTRATION.
Because these securities are being offered on a “best efforts”
basis, the following disclosures are hereby made:
| | |
Price to Public | | |
Commissions
(1) | | |
Proceeds
to Company
(2) | | |
Proceeds
to Other Persons
(3) | |
| | |
| | |
| | |
| | |
| |
| Per Share | |
$ | 0.01-0.03 | | |
$ | 0 | | |
$ | 0.01-0.03 | | |
None | |
| Minimum Investment | |
$ | 5,000 | | |
$ | 0 | | |
$ | 5,000 | | |
None | |
| Maximum Offering | |
$ | 4,000,000
-12,000,000 | | |
$ | 0 | | |
$ | 4,000,000
-12,000,000 | | |
None | |
| |
(1) |
The Company has not
presently engaged an underwriter for the sale of securities under this Offering. |
| |
(2) |
Does not reflect payment
of expenses of this Offering, which are estimated to not exceed $32,500.00 and which include, among other things, legal fees, accounting
costs, reproduction expenses, due diligence, marketing, consulting, administrative services other costs of blue-sky compliance, and
actual out-of-pocket expenses incurred by the Company selling the Shares. This amount represents the proceeds of the offering to
the Company, which will be used as set out in “USE OF PROCEEDS TO ISSUER.” |
| |
(3) |
There are no finder’s
fees or other fees being paid to third parties from the proceeds. See “Plan of Distribution.” |
This Offering (the “Offering”) consists
of up to 400,000,000 shares of common stock (the “Company Offered Shares”) that are being offered on a “best efforts”
basis, which means that there is no guarantee that any minimum amount will be sold, at a fixed price of $0.01-0.03 per share (the “Offering
Price”) (the Offering Price will be provided via an Offering Circular Supplement within two business days following the earlier
of the date of determination of the offering price or the date such offering circular is first used after qualification in connection
with a public offering or sale in accordance with Rule 253(g)(1)), pursuant to Tier 2 of Regulation A of the United States Securities
and Exchange Commission (the “SEC”). A minimum purchase of $5,000 of the Company Offered Shares is required in this offering;
any additional purchase must be in an amount of at least $1,000. The Company Offered Shares are being offered only by the Company on
a best-efforts basis to an unlimited number of accredited investors and to an unlimited number of non-accredited investors subject to
the limitations of Regulation A. Under Rule 251(d)(2)(i)(C) of Regulation A+, non-accredited,
non-natural investors are subject to the investment limitation and may only invest funds which do not exceed 10% of the greater of the
purchaser’s revenue or net assets (as of the purchaser’s most recent fiscal year end). A non-accredited, natural person may
only invest funds which do not exceed 10% of the greater of the purchaser’s annual income or net worth (please see below on how
to calculate your net worth). The maximum aggregate amount of the Shares that will be offered is 400,000,000 Shares of Common Stock with
a Maximum Offering Price of $4,000,000 to $12,000,000, depending on the final offering price. There is no minimum number of Shares that
needs to be sold in order for funds to be released to the Company and for this Offering to close.
Upon qualification of this offering by the SEC,
the Company may issue Company Offered Shares in satisfaction of outstanding debt obligations including $830,900 of convertible notes
(the “Subject Convertible Notes”) at the Offering Price. (See “Use of Proceeds” and “Plan
of Distribution”).
Please see the “Risk Factors”
section, beginning on page 4, for a discussion of the risks associated with a purchase of the Offered Shares.
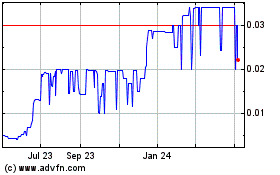
Our common stock is quoted in the over-the-counter
under the symbol “CSSI” in the OTC Pink marketplace of OTC Link. On June 25, 2024, the closing price of our common stock
was $0.0123 per share.
The Company Offered Shares will only be issued
to purchasers who satisfy the requirements set forth in Regulation A. The offering is expected to expire on the first of: (i) all of
the Shares offered are sold; or (ii) the close of business 365 days from the date of qualification by the Commission, unless sooner terminated
or extended by the Company’s CEO. Pending each closing, payments for the Shares will be paid directly to the Company. Funds will
be immediately transferred to the Company where they will be available for use in the operations of the Company’s business in a
manner consistent with the “USE OF PROCEEDS TO ISSUER” in this Offering Circular.
THIS OFFERING CIRCULAR DOES NOT CONSTITUTE
AN OFFER OR SOLICITATION IN ANY JURISDICTION IN WHICH SUCH AN OFFER OR SOLICITATION WOULD BE UNLAWFUL. NO PERSON HAS BEEN AUTHORIZED
TO GIVE ANY INFORMATION OR TO MAKE ANY REPRESENTATIONS CONCERNING THE COMPANY OTHER THAN THOSE CONTAINED IN THIS OFFERING CIRCULAR, AND
IF GIVEN OR MADE, SUCH OTHER INFORMATION OR REPRESENTATION MUST NOT BE RELIED UPON.
PROSPECTIVE INVESTORS ARE NOT TO CONSTRUE THE
CONTENTS OF THIS OFFERING CIRCULAR, OR OF ANY PRIOR OR SUBSEQUENT COMMUNICATIONS FROM THE COMPANY OR ANY OF ITS EMPLOYEES, AGENTS OR
AFFILIATES, AS INVESTMENT, LEGAL, FINANCIAL OR TAX ADVICE.
NO SALE MAY BE MADE TO INVESTORS IF THE AGGREGATE
PURCHASE PRICE IS MORE THAN $75,000,000, PURSUANT TO THE TERMS OF RULE 251 OF REGULATION A TIER II SET FORTH UNDER THE SECURITIES ACT
OF 1933 (THE “ACT”). DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION
THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL
INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO WWW.INVESTOR.GOV (WHICH IS NOT INCORPORATED BY REFERENCE INTO THIS OFFERING CIRCULAR).
THE INFORMATION CONTAINED IN THIS OFFERING CIRCULAR
HAS BEEN SUPPLIED BY THE COMPANY. THIS OFFERING CIRCULAR CONTAINS SUMMARIES OF DOCUMENTS NOT CONTAINED IN THIS OFFERING CIRCULAR. COPIES
OF DOCUMENTS REFERRED TO IN THIS OFFERING CIRCULAR, BUT NOT INCLUDED AS AN EXHIBIT, WILL BE MADE AVAILABLE TO QUALIFIED PROSPECTIVE INVESTORS
UPON REQUEST.
RULE 251(D)(3)(I)(F) DISCLOSURE. RULE 251(D)(3)(I)((F)
PERMITS REGULATION A OFFERINGS TO CONDUCT ONGOING CONTINUOUS OFFERINGS OF SECURITIES FOR MORE THAN THIRTY (30) DAYS AFTER THE QUALIFICATION
DATE IF: (1) THIS OFFERING WILL BEGIN WITHIN TWO (2) DAYS AFTER THE DATE OF QUALIFICATION; (2) THE OFFERING WILL BE MADE ON A CONTINUOUS
AND ONGOING BASIS FOR A PERIOD THAT MAY BE IN EXCESS OF THIRTY (30) DAYS OF THE INITIAL QUALIFICATION DATE; (3) THE OFFERING WILL BE IN
AN AMOUNT THAT, AT THE TIME THE OFFERING CIRCULAR IS QUALIFIED, IS REASONABLY EXPECTED TO BE OFFERED AND SOLD WITHIN ONE (1) YEAR FROM
THE INITIAL QUALIFICATION DATE; AND (4) THE SECURITIES MAY BE OFFERED AND SOLD ONLY IF NOT MORE THAN THREE (3) YEARS HAVE ELAPSED SINCE
THE INITIAL QUALIFICATION DATE OF THE OFFERING, UNLESS A NEW OFFERING CIRCULAR IS SUBMITTED AND FILED BY THE COMPANY PURSUANT TO RULE
251(D)(3)(I)((F) WITH THE SEC COVERING THE REMAINING SECURITIES OFFERED UNDER THE PREVIOUS OFFERING; THEN THE SECURITIES MAY CONTINUE
TO BE OFFERED AND SOLD UNTIL THE EARLIER OF THE QUALIFICATION DATE OF THE NEW OFFERING CIRCULAR OR THE ONE HUNDRED EIGHTY (180) CALENDAR
DAYS AFTER THE THIRD ANNIVERSARY OF THE INITIAL QUALIFICATION DATE OF THE PRIOR OFFERING CIRCULAR.
This Offering is inherently risky. See “Risk
Factors” beginning on page 4.
Sales of these securities will commence within
two business days of being qualified by the SEC. The Offering will be a continuous offering pursuant to Rule 251(d)(3)(i)(F).
The Company is following the “Offering Circular”
format of disclosure under Regulation A.
AN OFFERING STATEMENT PURSUANT TO REGULATION
A RELATING TO THESE SECURITIES HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. INFORMATION CONTAINED IN THIS PRELIMINARY
OFFERING CIRCULAR IS SUBJECT TO COMPLETION OR AMENDMENT. THESE SECURITIES MAY NOT BE SOLD NOR MAY OFFERS TO BUY BE ACCEPTED BEFORE THE
OFFERING STATEMENT FILED WITH THE COMMISSION IS QUALIFIED. THIS PRELIMINARY OFFERING CIRCULAR SHALL NOT CONSTITUTE AN OFFER TO SELL OR
THE SOLICITATION OF AN OFFER TO BUY NOR MAY THERE BE ANY SALES OF THESE SECURITIES IN ANY STATE IN WHICH SUCH OFFER, SOLICITATION OR
SALE WOULD BE UNLAWFUL BEFORE REGISTRATION OR QUALIFICATION UNDER THE LAWS OF SUCH STATE. THE COMPANY MAY ELECT TO SATISFY ITS OBLIGATION
TO DELIVER A FINAL OFFERING CIRCULAR BY SENDING YOU A NOTICE WITHIN TWO BUSINESS DAYS AFTER THE COMPLETION OF THE COMPANY’S SALE
TO YOU THAT CONTAINS THE URL WHERE THE FINAL OFFERING CIRCULAR OR THE OFFERING STATEMENT IN WHICH SUCH FINAL OFFERING CIRCULAR WAS FILED
MAY BE OBTAINED.
NASAA UNIFORM LEGEND
FOR RESIDENTS OF ALL STATES: THE PRESENCE OF
A LEGEND FOR ANY GIVEN STATE REFLECTS ONLY THAT A LEGEND MAY BE REQUIRED BY THAT STATE AND SHOULD NOT BE CONSTRUED TO MEAN AN OFFER OR
SALE MAY BE MADE IN A PARTICULAR STATE. IF YOU ARE UNCERTAIN AS TO WHETHER OR NOT OFFERS OR SALES MAY BE LAWFULLY MADE IN ANY GIVEN STATE,
YOU ARE HEREBY ADVISED TO CONTACT THE COMPANY. THE SECURITIES DESCRIBED IN THIS OFFERING CIRCULAR HAVE NOT BEEN REGISTERED UNDER ANY
STATE SECURITIES LAWS (COMMONLY CALLED ‘BLUE SKY’ LAWS).
IN MAKING AN INVESTMENT DECISION INVESTORS
MUST RELY ON THEIR OWN EXAMINATION OF THE PERSON OR ENTITY CREATING THE SECURITIES AND THE TERMS OF THE OFFERING, INCLUDING THE MERITS
AND RISKS INVOLVED. THESE SECURITIES HAVE NOT BEEN RECOMMENDED BY ANY FEDERAL OR STATE SECURITIES COMMISSION OR REGULATORY AUTHORITY.
FURTHERMORE, THE FOREGOING AUTHORITIES HAVE NOT CONFIRMED THE ACCURACY OR DETERMINED THE ADEQUACY OF THIS DOCUMENT. ANY REPRESENTATION
TO THE CONTRARY IS A CRIMINAL OFFENSE.
NOTICE TO FOREIGN INVESTORS
IF THE PURCHASER LIVES OUTSIDE THE UNITED STATES,
IT IS THE PURCHASER’S RESPONSIBILITY TO FULLY OBSERVE THE LAWS OF ANY RELEVANT TERRITORY OR JURISDICTION OUTSIDE THE UNITED STATES
IN CONNECTION WITH ANY PURCHASE OF THE SECURITIES, INCLUDING OBTAINING REQUIRED GOVERNMENTAL OR OTHER CONSENTS OR OBSERVING ANY OTHER
REQUIRED LEGAL OR OTHER FORMALITIES. THE COMPANY RESERVES THE RIGHT TO DENY THE PURCHASE OF THE SECURITIES BY ANY FOREIGN PURCHASER.
PATRIOT ACT RIDER
The Investor hereby represents and warrants that
Investor is not, nor is it acting as an agent, representative, intermediary or nominee for, a person identified on the list of
blocked persons maintained by the Office of Foreign Assets Control, U.S. Department of Treasury. In addition, the Investor has complied
with all applicable U.S. laws, regulations, directives, and executive orders relating to anti-money laundering , including but not limited
to the following laws: (1) the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism
Act of 2001, Public Law 107-56, and (2) Executive Order 13224 (Blocking Property and Prohibiting Transactions with Persons Who Commit,
Threaten to Commit, or Support Terrorism) of September 23, 2001.
NO DISQUALIFICATION EVENT (“BAD ACTOR”
DECLARATION)
NONE OF THE COMPANY, ANY OF ITS PREDECESSORS,
ANY AFFILIATED ISSUER, ANY DIRECTOR, EXECUTIVE OFFICER, OTHER OFFICER OF THE COMPANY PARTICIPATING IN THE OFFERING CONTEMPLATED HEREBY,
ANY BENEFICIAL OWNER OF 20% OR MORE OF THE COMPANY’S OUTSTANDING VOTING EQUITY SECURITIES, CALCULATED ON THE BASIS OF VOTING POWER,
NOR ANY PROMOTER (AS THAT TERM IS DEFINED IN RULE 405 UNDER THE SECURITIES ACT OF 1933) CONNECTED WITH THE COMPANY IN ANY CAPACITY AT
THE TIME OF SALE (EACH, AN “ISSUER COVERED PERSON”) IS SUBJECT TO ANY OF THE “BAD ACTOR” DISQUALIFICATIONS
DESCRIBED IN RULE 506(D)(1)(I) TO (VIII) UNDER THE SECURITIES ACT OF 1933 (A “DISQUALIFICATION EVENT”), EXCEPT
FOR A DISQUALIFICATION EVENT COVERED BY RULE 506(D)(2) OR (D)(3) UNDER THE SECURITIES ACT. THE COMPANY HAS EXERCISED REASONABLE CARE
TO DETERMINE WHETHER ANY ISSUER COVERED PERSON IS SUBJECT TO A DISQUALIFICATION EVENT.
Continuous Offering
Under Rule 251(d)(3) to Regulation A, the following
types of continuous or delayed Offerings are permitted, among others: (1) securities offered or sold by or on behalf of a person
other than the issuer or its subsidiary or a person of which the issuer is a subsidiary; (2) securities issued upon conversion of other
outstanding securities; or (3) securities that are part of an Offering which commences within two calendar days after the qualification
date. These may be offered on a continuous basis and may continue to be offered for a period in excess of 30 days from the date of initial
qualification. They may be offered in an amount that, at the time the Offering statement is qualified, is reasonably expected to be offered
and sold within one year from the initial qualification date. No securities will be offered or sold “at the market.” The
Shares will be sold at a fixed price of $0.01 per share.
Subscriptions are irrevocable and the purchase
price is non-refundable as expressly stated in this Offering Circular. The Company, by determination of the Board of Directors, in its
sole discretion, may issue the Securities under this Offering for cash, services, in satisfaction of outstanding debt obligation, and/or
other consideration without notice to subscribers. All cash proceeds received by the Company from subscribers for this Offering will
be available for use by the Company upon acceptance of subscriptions for the Securities by the Company.
About This Form 1-A and Offering Circular
In making an investment decision, you should
rely only on the information contained in this Form 1-A and Offering Circular. The Company has not authorized anyone to provide you with
information different from that contained in this Form 1-A and Offering Circular. We are offering to sell, and seeking offers to buy
the Shares only in jurisdictions where offers and sales are permitted. You should assume that the information contained in this Form
1-A and Offering Circular is accurate only as of the date of this Form 1-A and Offering Circular, regardless of the time of delivery
of this Form 1-A and Offering Circular. Our business, financial condition, results of operations, and prospects may have changed since
that date. In compliance with Rule 257(b), the Company will make material updates to the Offering Circular by filing current reports
on Form 1-U, annual reports on Form 1-K, and semi-annual reports on Form 1-SA.
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING
FORWARD-LOOKING STATEMENTS
The information contained in this Offering Circular
includes some statements that are not historical and that are considered forward-looking statements. Such forward-looking statements include,
but are not limited to, statements regarding our development plans for our business; our strategies and business outlook; anticipated
development of our company; and various other matters (including contingent liabilities and obligations and changes in accounting policies,
standards and interpretations). These forward-looking statements express our expectations, hopes, beliefs and intentions regarding the
future. In addition, without limiting the foregoing, any statements that refer to projections, forecasts or other characterizations of
future events or circumstances, including any underlying assumptions, are forward-looking statements. The words anticipates, believes,
continue, could, estimates, expects, intends, may, might, plans, possible, potential, predicts, projects, seeks, should, will, would and
similar expressions and variations, or comparable terminology, or the negatives of any of the foregoing, may identify forward-looking
statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this
Offering Circular are based on current expectations and beliefs concerning future developments that are difficult to predict. We cannot
guarantee future performance, or that future developments affecting our company will be as currently anticipated. These forward-looking
statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual
results or performance to be materially different from those expressed or implied by these forward-looking statements.
All forward-looking statements attributable to
us are expressly qualified in their entirety by these risks and uncertainties. These risks and uncertainties, along with others, are also
described below in the Risk Factors section. Should one or more of these risks or uncertainties materialize, or should any of our assumptions
prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. You should not
place undue reliance on any forward-looking statements and should not make an investment decision based solely on these forward-looking
statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise, except as may be required under applicable securities laws.
OFFERING CIRCULAR SUMMARY,
PERKS AND RISK FACTORS
OFFERING CIRCULAR SUMMARY
The following summary is qualified in its entirety
by the more detailed information appearing elsewhere in this Offering Circular and/or incorporated by reference in this Offering Circular.
For full offering details, please (1) thoroughly review this Form 1-A filed with the Securities and Exchange Commission (2) thoroughly
review this Offering Circular and (3) thoroughly review any attached documents to or documents referenced in, this Form 1-A and Offering
Circular.
Unless otherwise indicated,
the terms “Standard Dental Labs”, “the Company”, “CSSI”, “we”, “our”,
and “us” are used in this Offering Circular to refer to Standard Dental Labs, Inc.
Business Overview
Standard Dental Labs Inc.,
(formerly known as Costas, Inc.) was incorporated in the State of Nevada on December 10, 1998. From inception, our company had pursued
business operations several different industries, including real estate speculation and financial technologies.
In 2017, our current sole
officer and a director, James D. Brooks, commenced an action against the company and was awarded a judgment in the Eighth Judicial District
Court, Clark County, Nevada, against our company for breach of contract, and non-payment of obligations. On September 20, 2017, the court
filed an order effective September 18, 2017, whereby Mr. Brooks, as a creditor of our company, was granted a judgment against us in the
principal amount of $1,114,500. On October 21, 2020, Mr. Brooks filed a motion requesting the appointment of a Receiver over our company.
On March 25, 2021, the Receiver filed a motion with the Court requesting approval to (i) appoint Mr. Brooks as an officer and director
of our company; and (ii) increase our authorized capital and subsequently to issue sufficient common and preferred shares on terms to
be finalized with Mr. Brooks, whereby Mr. Brooks became the controlling stockholder of our company. On February 9, 2022, an Order was
entered by the Eighth Judicial District Court, Clark County, Nevada, Case No. A-17-749977D, terminating the receivership for our company.
Our
primary operations are focused on our dental lab where we manufacture and produce several types of dental prosthetics. We provide dental
lab services to more than 50 dental practices and produce approximately 500 dental prosthetics each month. As the Company’s sole
officer and director, Mr. Brooks oversees the overall direction of the Company in relation to its plans for long-term growth.
”
section for further description of the Company). Though incorporated in 1998, the Company has a limited operating history in our current
business segment and a history of net losses. (See “Risks Related to Our Company” for additional information.)
Offering Summary
| Securities Offered |
|
Company Offered Shares: 400,000,000 shares of
common stock, par value $0.001.
|
| |
|
|
| Offering Price |
|
$0.01-0.03 per Offered Share. |
| |
|
|
|
Shares Outstanding
Before This Offering |
|
465,728,363 shares of common stock issued and outstanding as of
the date hereof. |
| |
|
|
|
Shares Outstanding
After This Offering |
|
865,728,363 shares of common stock issued and outstanding, assuming
the sale of all of the Company Offered Shares hereunder. |
| |
|
|
|
Minimum Number of Shares
to Be Sold in This Offering |
|
None |
| |
|
|
| Investor Suitability Standards |
|
The Offered Shares may only be purchased by investors residing in a state in which this Offering Circular is duly qualified who have either (a) a minimum annual gross income of $70,000 and a minimum net worth of $70,000, exclusive of automobile, home and home furnishings, or (b) a minimum net worth of $250,000, exclusive of automobile, home and home furnishings. |
| |
|
|
| Market for our Common Stock |
|
Our common stock is quoted in the over-the-counter market under the symbol “CSSI” in the OTC Pink marketplace of OTC Link. |
| |
|
|
| Termination of this Offering |
|
This offering will terminate at the earliest of (a) the date on
which the maximum offering has been sold, (b) the date which is one year from this offering circular being qualified by the SEC and
(c) the date on which this offering is earlier terminated by us, in our sole discretion, subject to the limitations set forth in
Rule 251(d)(3)(i)(F) of Regulation A. |
| |
|
|
|
Conversion of the Subject
Convertible Notes |
|
Upon qualification of this offering by the SEC, the Company may
issue Company Offered Shares in satisfaction of the Subject Convertible Notes at the Offering Price. In such an event, we would realize
approximately $830,900 in a reduction of outstanding liabilities rather can cash proceeds. |
| |
|
|
| Use of Proceeds |
|
We will apply the proceeds of this offering for sales and marketing, dental lab equipment, new lab acquisitions, general and administrative expenses, and working capital. (See “Use of Proceeds”). |
| |
|
|
| Risk Factors |
|
An investment in the Offered Shares involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investments. You should carefully consider the information included in the Risk Factors section of this Offering Circular, as well as the other information contained in this Offering Circular, prior to making an investment decision regarding the Offered Shares. |
| |
|
|
| Corporate Information |
|
Our principal executive offices are located at 424 E. Central Boulevard,
Suite 308, Orlando, Florida 32801; our telephone number is (321) 465-9899; our corporate website is located at https://sdl.care.
No information found on our Company’s website is part of this Offering Circular. |
| |
|
|
| Legal Counsel |
|
No independent counsel has been retained to represent the investors
in the Company. Each investor should retain its own counsel and other appropriate advisers as to legal, regulatory and tax matters
affecting investment in shares of common stock and its suitability for such investor. |
The Offering
| Common Stock Outstanding (1) |
|
|
465,728,363 |
|
| Common Stock in this Offering |
|
|
400,000,000 |
|
| Stock to be outstanding after the offering (2) |
|
|
865,728,363 |
|
| |
(1) |
As of the date of this
Offering Circular. |
| |
(2) |
The total number of
Shares of Common Stock assumes that the maximum number of Shares are sold in this Offering. The Company may not be able to sell the
Maximum Offering Amount. The Company will conduct one or more closings on a rolling basis as funds are received from investors. |
Investment Analysis
There is no assurance the Company will be profitable,
or that management’s opinion of the Company’s future prospects will not be outweighed by the unanticipated losses, adverse
regulatory developments and other risks. Investors should carefully consider the various risk factors below before investing in the Shares.
Continuing Reporting Requirements Under Regulation
A
Following this Tier II, Regulation A offering,
we will be required to comply with certain ongoing disclosure requirements under Rule 257 of Regulation A. We will be required to file:
(i) an annual report with the SEC on Form 1-K; (ii) a semi-annual report with the SEC on Form 1-SA; (iii) current reports with the SEC
on Form 1-U; and (iv) a notice under cover of Form 1-Z. The necessity to file current reports will be triggered by certain corporate events,
similar to the ongoing reporting obligation faced by issuers under the Exchange Act, however the requirement to file a Form 1-U is expected
to be triggered by significantly fewer corporate events than that of the Form 8-K. Parts I & II of Form 1-Z will be filed by us if
and when we decide to and are no longer obligated to file and provide annual reports pursuant to the requirements of Regulation A.
As soon as practicable, but in no event later than
one hundred twenty (120) days after the close of our fiscal year, ending December 31st, our board of directors will cause to
be mailed or made available, by any reasonable means, to each stockholder as of a date selected by the board of directors, an annual report
containing financial statements of the Company for such fiscal year, presented in accordance with GAAP, including a balance sheet and
statements of operations, company equity and cash flows, with such statements having been audited by an accountant selected by the board
of directors. The board of directors shall be deemed to have made a report available to each stockholder as required if it has either
(i) filed such report with the SEC via its Electronic Data Gathering, Analysis and Retrieval, or EDGAR, system and such report is publicly
available on such system or (ii) made such report available on any website maintained by the Company and available for viewing by the
stockholders.
Additionally, during the pendency of this offering
and following this offering, we intend to file quarterly and annual financial reports and other supplemental reports with OTC Markets,
which will be available at www.otcmarkets.com.
All of our future periodic reports, whether filed
with OTC Markets or the SEC, will not be required to include the same information as analogous reports required to be filed by companies
whose securities are listed on the NYSE or NASDAQ, for example.
RISK FACTORS
An investment in the Offered Shares involves substantial
risks. You should carefully consider the following risk factors, in addition to the other information contained in this Offering Circular,
before purchasing any of the Offered Shares. The occurrence of any of the following risks might cause you to lose a significant part of
your investment. The risks and uncertainties discussed below are not the only ones we face but do represent those risks and uncertainties
that we believe are most significant to our business, operating results, prospects and financial condition. Some statements in this Offering
Circular, including statements in the following risk factors, constitute forward-looking statements. (See “Cautionary Statement Regarding Forward-Looking Statements”).
Risks Related to Our Company
We have a limited operating
history. Our operating history is limited. There can be no assurance that our proposed plan of business can be realized in the
manner contemplated and, if it cannot be, shareholders may lose all or a substantial part of their investment. There is no guarantee
that we will ever realize any significant operating revenues or that our operations will ever be profitable.
Our company has
a limited operating history and an evolving business model which raises doubt about our ability to achieve profitability or obtain financing.
Our company only has a couple of years of operating history. Moreover, our business model will rely on the ability to make additional
acquisitions of dental labs on commercially viable terms. Our company’s ability to continue as a going concern is dependent upon
our ability to obtain adequate financing and to reach profitable levels of operations and we have a limited history of performance, earnings,
and success. There can be no assurance that we will achieve profitability or obtain future financing. Also, our business depends on our
ability to successfully purchase more advanced equipment and technology from time to time such as 3D printing and modelling technology,
which there is no assurance that we will achieve.
There
is no assurance that we will be able to acquire additional dental labs pursuant to our growth
strategy. In order to grow and achieve the level of operations and margins that we
hope to achieve, one component of our strategy
is the acquisition and integration of additional dental lab operations. While we are confident
in our ability to execute on this, there can be no assurances that we will be able to identify
suitable dental labs for acquisition, or that we will be able to acquire such labs on commercially
viable terms. If we are unable to make such additional acquisitions, or successfully integrate
any acquired labs into existing operations, our growth prospects may be limited.
Conflicts of interest between our company
and our sole officer may result in a loss of business opportunity. Our sole officer is not obligated to commit his full time and
attention to our business and, accordingly, he may encounter a conflict of interest in allocating his time between our future operations
and those of other businesses. In the course of his other business activities, he may become aware of investment and business opportunities
which may be appropriate for presentation to us as well as other entities to which he owes a fiduciary duty. As a result, he may have
conflicts of interest in determining to which entity a particular business opportunity should be presented. He may also in the future
become affiliated with entities, engaged in business activities similar to those we intend to conduct.
In general, officers and directors of a corporation
are required to present business opportunities to a corporation if:
| |
· |
the corporation could financially undertake the opportunity; |
| |
· |
the opportunity is within the corporation’s line of business; and |
| |
· |
it would be unfair to the corporation and its stockholders not to bring the opportunity to the attention of the corporation. |
We plan to adopt a code of ethics that obligates
our directors, officers and employees to disclose potential conflicts of interest and prohibits those persons from engaging in such transactions
without our consent. Despite our intentions, conflicts of interest may nevertheless arise which may deprive our company of a business
opportunity, which may impede the successful development of our business and negatively impact the value of an investment in our company.
The speculative nature of our business plan
may result in the loss of your investment. Our operations are in the start-up or stage only and are unproven. We may not be successful
in implementing our business plan to become profitable. There may be less demand for our services than we anticipate. There is no assurance
that our business will succeed, and you may lose your entire investment.
General economic factors may negatively impact
the market for our dental products. The willingness of dental practices and their patients/consumers to spend money on our products
may be dependent upon general economic conditions; and any material downturn may reduce the likelihood of such parties incurring costs
toward what some consumers may consider a discretionary expense item.
A wide range of economic and logistical factors
may negatively impact our operating results. Our operating results will be affected by a wide variety of factors that could materially
affect revenues and profitability, including the timing and cancellation of customer orders and projects, competitive pressures on pricing,
availability of personnel, and market acceptance of our services. As a result, we may experience material fluctuations in future operating
results on a quarterly and annual basis which could materially affect our business, financial condition and operating results.
If we fail to effectively and efficiently
advertise, the growth of our business may be compromised. The future growth and profitability of our business will be dependent
in part on the effectiveness and efficiency of our advertising and promotional expenditures, including our ability to (i) create greater
awareness of our services, (ii) determine the appropriate creative message and media mix for future advertising expenditures, and (iii)
effectively manage advertising and promotional costs in order to maintain acceptable operating margins. There can be no assurance that
we will experience benefits from advertising and promotional expenditures in the future. In addition, no assurance can be given that our
planned advertising and promotional expenditures will result in increased revenues, will generate levels of service and name awareness
or that we will be able to manage such advertising and promotional expenditures on a cost-effective basis.
Our success is dependent on our unproven
ability to attract qualified personnel. We depend on our ability to attract, retain and motivate our management team, consultants
and advisors. There is strong competition for qualified technical and management personnel in the dental business sector, and it is expected
that such competition will increase. Our planned growth will place increased demands on our existing resources and will likely require
the addition of technical personnel and the development of additional expertise by existing personnel. There can be no assurance that
our compensation packages will be sufficient to ensure the continued availability of qualified personnel who are necessary for the development
of our business.
We have a limited operating history with
losses, and we expect the losses to continue, which raises concerns about our ability to continue as a going concern. We have
generated minimal revenues since our inception and will, in all likelihood, continue to incur operating expenses with minimal revenues
until we are able to successfully develop our business. Our business plan will require us to incur further expenses. We may not be able
to ever become profitable. These circumstances raise concerns about our ability to continue as a going concern. We have a limited operating
history and must be considered in the start-up stage.
Our management has been able, thus far, to finance
the operations through equity financing and cash on hand. There is no assurance that our company will be able to continue to finance our
company on this basis.
Without additional financing to develop our
business plan, our business may fail. Because we have generated only minimal revenue from our business and cannot anticipate when
we will be able to generate meaningful revenue from our business, we will need to raise additional funds to conduct and grow our business.
We do not currently have sufficient financial resources to completely fund the development of our business plan. We anticipate that we
will need to raise further financing. We can provide no assurance to investors that we will be able to find such financing if required.
The most likely source of future funds presently available to us is through the sale of equity capital. Any sale of share capital will
result in dilution to existing security-holders.
We may not be able to obtain all of the licenses
necessary to operate our business, which would cause our business to fail. Our operations require licenses and permits from various
governmental authorities related to the operation of our acquired dental laboratory facilities. We believe that we will be able to obtain
all necessary licenses and permits under applicable laws and regulations for our operations and believe we will be able to comply in all
material respects with the terms of such licenses and permits. However, such licenses and permits are subject to change in various circumstances.
There can be no guarantee that we will be able to obtain or maintain all necessary licenses and permits.
If
we are unable to recruit or retain qualified personnel, it could have a material adverse effect on our operating results and stock price.
Our success depends in large part on the continued services of our sole executive officer and third-party relationships. We currently
do not have key person insurance on these individuals. The loss of these people, especially without advance notice, could have a material
adverse impact on our results of operations and our stock price. It is also very important that we be able to attract and retain highly
skilled personnel, including technical personnel, to accommodate our acquisition and expansion plans and to replace personnel who leave.
Competition for qualified personnel can be intense, and there are a limited number of people with the requisite knowledge and experience.
Under these conditions, we could be unable to recruit, train, and retain employees. If we cannot attract and retain qualified personnel,
it could have a material adverse impact on our operating results and stock price.
If we fail to effectively manage our growth
our future business results could be harmed. As we proceed with our business plan, we expect to experience significant and rapid
growth in the scope and complexity of our business. We will need to add staff to market our services, manage operations, handle sales
and marketing efforts and perform finance and accounting functions. We will be required to hire a broad range of additional personnel
in order to successfully advance our operations. This growth is likely to place a strain on our management and operational resources.
The failure to develop and implement effective systems, or to hire and retain sufficient personnel for the performance of all of the functions
necessary to effectively service and manage our potential business, or the failure to manage growth effectively, could have a materially
adverse effect on our business and financial condition.
Our internal control over financial reporting
may be ineffective. We do not have the internal infrastructure necessary, and are not required, to complete an attestation about
our financial controls that would be required under Section 404 of the Sarbanes-Oxley Act of 2002. There can be no assurances that there
are no significant deficiencies or material weaknesses in the quality of our financial controls. We expect to incur additional expenses
and diversion of management’s time when it becomes necessary to perform the system and process evaluation, testing and remediation
required to comply with the management certification and auditor attestation requirements.
If our techniques for managing risk are ineffective,
we may be exposed to unanticipated losses. In order to manage the significant risks inherent in our business, we must maintain
effective policies, procedures and systems that enable us to identify, monitor and control our exposure to market, operational, legal
and reputational risks. Our risk management methods may prove to be ineffective due to their design or implementation or as a result of
the lack of adequate, accurate or timely information. If our risk management efforts are ineffective, we could suffer losses or face litigation,
particularly from our clients, and sanctions or fines from regulators. Our techniques for managing risks may not fully mitigate the risk
exposure in all economic or market environments, or against all types of risk, including risks that we might fail to identify or anticipate.
Any failures in our risk management techniques and strategies to accurately quantify such risk exposure could limit our ability to manage
risks or to seek positive, risk-adjusted returns. In addition, any risk management failures could cause fund losses to be significantly
greater than historical measures predict. Our more qualitative approach to managing those risks could prove insufficient, exposing us
to unanticipated losses in our net asset value and therefore a reduction in our revenues.
We cannot assure that we will have the resources
to repay all of our liabilities in the future. We have liabilities and may in the future have other liabilities to affiliated
or unaffiliated lenders. These liabilities represent fixed costs, which are required to be paid regardless of the level of business or
profitability experienced by us. We cannot assure that we will not incur debt in the future, that we will have sufficient funds to repay
our indebtedness or that we will not default on our debt, jeopardizing our business viability. Furthermore, we may not be able to borrow
or raise additional capital in the future to meet our needs or to otherwise provide the capital necessary to conduct our business. We
often utilize purchase order financing from third party lenders when we are supplying or distributing consumer goods, which increases
our costs and the risks that we may incur a default, which would harm its business reputation and financial condition. We cannot assure
that we will be able to pay all of our liabilities, or that we will not experience a default on our indebtedness.
Risks Related to Securities Compliance and Regulation
We will not have reporting obligations under
Sections 14 or 16 of the Securities Exchange Act of 1934, nor will any stockholders have reporting requirements of Regulation 13D or 13G,
nor Regulation 14D. So long as our common shares are not registered under the Exchange Act, our directors and executive officers
and beneficial holders of 10% or more of our outstanding common shares will not be subject to Section 16 of the Exchange Act. Section
16(a) of the Exchange Act requires executive officers and directors and persons who beneficially own more than 10% of a registered class
of equity securities to file with the SEC initial statements of beneficial ownership, reports of changes in ownership and annual reports
concerning their ownership of common shares and other equity securities, on Forms 3, 4 and 5, respectively. Such information about our
directors, executive officers and beneficial holders will only be available through periodic reports we file with OTC Markets.
Our common stock is not registered under the Exchange
Act and we do not intend to register our common stock under the Exchange Act for the foreseeable future; provided, however, that we will
register our common stock under the Exchange Act if we have, after the last day of any fiscal year, more than either (1) 2,000 persons;
or (2) 500 stockholders of record who are not accredited investors, in accordance with Section 12(g) of the Exchange Act.
Further, as long as our common stock is not registered
under the Exchange Act, we will not be subject to Section 14 of the Exchange Act, which, among other things, prohibits companies that
have securities registered under the Exchange Act from soliciting proxies or consents from stockholders without furnishing to stockholders
and filing with the SEC a proxy statement and form of proxy complying with the proxy rules.
The reporting required by Section 14(d) of the
Exchange Act provides information to the public about persons other than the company who is making the tender offer. A tender offer is
a broad solicitation by a company or a third party to purchase a substantial percentage of a company’s common stock for a limited
period of time. This offer is for a fixed price, usually at a premium over the current market price, and is customarily contingent on
stockholders tendering a fixed number of their shares.
In addition, as long as our common stock is not
registered under the Exchange Act, our company will not be subject to the reporting requirements of Regulation 13D and Regulation 13G,
which require the disclosure of any person who, after acquiring directly or indirectly the beneficial ownership of any equity securities
of a class, becomes, directly or indirectly, the beneficial owner of more than 5% of the class.
There may be deficiencies with our internal
controls that require improvements. Our company is not required to provide a report on the effectiveness of our internal controls
over financial reporting. We are in the process of evaluating whether our internal control procedures are effective and, therefore, there
is a greater likelihood of undiscovered errors in our internal controls or reported financial statements as compared to issuers that have
conducted such independent evaluations.
Risks Related to Our Organization and Structure
As a non-listed company conducting an exempt
offering pursuant to Regulation A, we are not subject to a number of corporate governance requirements, including the requirements for
independent board members. As a non-listed company conducting an exempt offering pursuant to Regulation A, we are not subject
to a number of corporate governance requirements that an issuer conducting an offering on Form S-1 or listing on a national stock exchange
would be. Accordingly, we are not required to have (a) a board of directors of which a majority consists of independent directors under
the listing standards of a national stock exchange, (b) an audit committee composed entirely of independent directors and a written audit
committee charter meeting a national stock exchange’s requirements, (c) a nominating/corporate governance committee composed entirely
of independent directors and a written nominating/ corporate governance committee charter meeting a national stock exchange’s requirements,
(d) a compensation committee composed entirely of independent directors and a written compensation committee charter meeting the requirements
of a national stock exchange, and (e) independent audits of our internal controls. Accordingly, you may not have the same protections
afforded to stockholders of companies that are subject to all of the corporate governance requirements of a national stock exchange.
Our holding company structure makes us dependent
on our subsidiaries for our cash flow and could serve to subordinate the rights of our stockholders to the rights of creditors of our
subsidiaries, in the event of an insolvency or liquidation of any such subsidiary. Our company acts as a holding company and,
accordingly, substantially all of our operations are conducted through our subsidiaries. Such subsidiaries will be separate and distinct
legal entities. As a result, substantially all of our cash flow will depend upon the earnings of our subsidiaries. In addition, we will
depend on the distribution of earnings, loans or other payments by our subsidiaries. No subsidiary will have any obligation to provide
our company with funds for our payment obligations. If there is an insolvency, liquidation or other reorganization of any of our subsidiaries,
our stockholders will have no right to proceed against their assets. Creditors of those subsidiaries will be entitled to payment in full
from the sale or other disposal of the assets of those subsidiaries before our company, as a stockholder, would be entitled to receive
any distribution from that sale or disposal.
Risks Related to a Purchase of the Offered Shares
There is no minimum offering and no person
has committed to purchase any of the Offered Shares. We have not established a minimum offering hereunder, which means that we
will be able to accept even a nominal amount of proceeds, even if such amount of proceeds is not sufficient to permit us to achieve any
of our business objectives. In this regard, there is no assurance that we will sell any of the Offered Shares or that we will sell enough
of the Offered Shares necessary to achieve any of our business objectives. Additionally, no person is committed to purchase any of the
Offered Shares.
We may seek additional capital that may result
in stockholder dilution or that may have rights senior to those of our common stock. From time to time, we may seek to obtain
additional capital, either through equity, equity-linked or debt securities. The decision to obtain additional capital will depend on,
among other factors, our business plans, operating performance and condition of the capital markets. If we raise additional funds through
the issuance of equity, equity-linked or debt securities, those securities may have rights, preferences or privileges senior to the rights
of our common stock, which could negatively affect the market price of our common stock or cause our stockholders to experience dilution.
You may never realize any economic benefit
from a purchase of Offered Shares. Because the market for our common stock is volatile, there is no assurance that you will ever
realize any economic benefit from your purchase of Offered Shares.
We do not intend to pay dividends on our
common stock. We intend to retain earnings, if any, to provide funds for the implementation of our business strategy. We do not
intend to declare or pay any dividends in the foreseeable future. Therefore, there can be no assurance that holders of our common stock
will receive cash, stock or other dividends on their shares of our common stock, until we have funds which our Board of Directors determines
can be allocated to dividends.
Our shares of common stock are penny stock,
which may impair trading liquidity. Disclosure requirements pertaining to penny stocks may reduce the level of trading activity
in the market for our common stock and investors may find it difficult to sell their shares. Trades of our common stock will be subject
to Rule 15g-9 of the SEC, which rule imposes certain requirements on broker-dealers who sell securities subject to the rule to persons
other than established customers and accredited investors. For transactions covered by the rule, broker-dealers must make a special suitability
determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction prior to sale. The
SEC also has rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks generally are equity
securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or quoted on the
NASDAQ system, provided that current price and volume information with respect to transactions in that security is provided by the exchange
or system). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules,
to deliver a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the
penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation
of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock
held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must
be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or
with the customer’s confirmation.
The market price for our common stock has
been, and may continue to be, highly volatile. The market for low-priced securities is generally less liquid and more volatile
than securities traded on national stock markets. Wide fluctuations in market prices are not uncommon. No assurance can be given that
the market for our common stock will continue. The price of our common stock may be subject to wide fluctuations in response to factors
such as the following, some of which are beyond our control:
| |
· |
quarterly variations in our operating results; |
| |
|
|
| |
· |
operating results that vary from the expectations of investors; |
| |
|
|
| |
· |
changes in expectations as to our future financial performance, including financial estimates by investors; |
| |
|
|
| |
· |
reaction to our periodic filings, or presentations by executives at investor and industry conferences; |
| |
|
|
| |
· |
changes in our capital structure; |
| |
|
|
| |
· |
announcements of innovations or new services by us or our competitors; |
| |
|
|
| |
· |
announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| |
|
|
| |
· |
lack of success in the expansion of our business operations; |
| |
|
|
| |
· |
announcements by third parties of significant claims or proceedings against our company or adverse developments in pending proceedings; |
| |
|
|
| |
· |
additions or departures of key personnel; |
| |
|
|
| |
· |
asset impairment; |
| |
|
|
| |
· |
temporary or permanent inability to offer our products and services; and |
| |
|
|
| |
· |
rumors or public speculation about any of the above factors. |
The terms of this offering were determined
arbitrarily. The terms of this offering were determined arbitrarily by us. The offering price for the Offered Shares does not
necessarily bear any relationship to our company’s assets, book value, earnings or other established criteria of valuation. Accordingly,
the offering price of the Offered Shares should not be considered as an indication of any intrinsic value of such securities. (See “Dilution”).
Our common stock is subject to price volatility
unrelated to our operations. The market price of our common stock could fluctuate substantially due to a variety of factors, including
market perception of our ability to achieve our planned growth, quarterly operating results of other companies in the same industry, trading
volume in our common stock, changes in general conditions in the economy and the financial markets or other developments affecting our
company’s competitors or our company itself. In addition, the over-the-counter stock market is subject to extreme price and volume
fluctuations in general. This volatility has had a significant effect on the market price of securities issued by many companies for reasons
unrelated to their operating performance and could have the same effect on our common stock.
You will suffer dilution in the net tangible
book value of the Offered Shares you purchase in this offering. If you acquire any Offered Shares, you will suffer immediate dilution,
due to the lower book value per share of our common stock compared to the purchase price of the Offered Shares in this offering. (See
“Dilution”).
As an issuer of penny stock, the protection
provided by the federal securities laws relating to forward looking statements does not apply to us. Although federal securities
laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws,
this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection
in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was
misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such
an action could hurt our financial condition.
DILUTION
Dilution in net tangible book value per share to
purchasers of our common stock in this offering represents the difference between the amount per share paid by purchasers of the Offered
Shares in this offering and the net tangible book value per share immediately after completion of this offering. In this offering, dilution
is attributable primarily to our negative net tangible book value per share.
If you purchase Offered Shares in this offering,
your investment will be diluted to the extent of the difference between your purchase price per Offered Share and the net tangible book
value of our common stock after this offering. Our net tangible book value as of September 30, 2023, was $1,710,343 (unaudited), or $(0.00)
per share. Net tangible book value per share is equal to total assets minus the sum of total liabilities and intangible assets divided
by the total number of shares outstanding.
The tables below illustrate the dilution to purchasers
of Company Offered Shares in this offering, on a pro forma basis, assuming 100%, 75%, 50% and 25% of the Offered Shares are sold at a
per share price of $0.02, which represents the mid-point of the price range presented herein.
| Assuming the Sale of 100% of the Company Offered Shares |
| Assumed offering price per share | |
$ | 0.02 | |
| Net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Increase in net tangible book value per share after giving effect to this offering | |
$ | 0.00 | |
| Pro forma net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | |
$ | 0.02 | |
| Assuming the Sale of 75% of the Company Offered Shares |
| Assumed offering price per share | |
$ | 0.02 | |
| Net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Increase in net tangible book value per share after giving effect to this offering | |
$ | 0.00 | |
| Pro forma net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | |
$ | 0.02 | |
| Assuming the Sale of 50% of the Company Offered Shares |
| Assumed offering price per share | |
$ | 0.02 | |
| Net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Increase in net tangible book value per share after giving effect to this offering | |
$ | 0.00 | |
| Pro forma net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | |
$ | 0.02 | |
| Assuming the Sale of 25% of the Company Offered Shares |
| Assumed offering price per share | |
$ | 0.02 | |
| Net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Increase in net tangible book value per share after giving effect to this offering | |
$ | 0.00 | |
| Pro forma net tangible book value per share as of December 31, 2023 (audited) | |
$ | 0.00 | |
| Dilution in net tangible book value per share to purchasers of Offered Shares in this offering | |
$ | 0.02 | |
USE OF PROCEEDS
The table below sets forth the estimated proceeds
we would derive from this offering, assuming the sale of 25%, 50%, 75% and 100% of the Offered Shares and assuming the payment of no sales
commissions or finder’s fees. There is, of course, no guaranty that we will be successful in selling any of the Company Offered
Shares in this offering.
| | |
Assume
Percentage of Company Offered Shares Sold in this Offering | |
| | |
25% | | |
50% | | |
75% | | |
100% | |
| Shares Sold | |
$ | 100,000,000 | | |
| 200,000,000 | | |
| 300,000,000 | | |
| 400,000,000 | |
| Gross Proceeds | |
$ | 1,000,000
to 3,000,000 | | |
| 2,000,000
to 6,000,000 | | |
| 3,000,000
to 9,000,000 | | |
| 4,000,000
to 12,000,000 | |
| Offering Expense | |
$ | 32,500 | | |
| 32,500 | | |
| 32,500 | | |
| 32,500 | |
| Net Proceeds | |
$ | 967,500
to 2,967,500 | | |
| 1,967,500
to 5,967,500 | | |
| 2,967,500
to 8,967,500 | | |
| 3,967,500
to 11,967,500 | |
The table below sets forth
the manner in which we intend to apply the net proceeds derived by us in this offering, assuming the sale of 10%, 25%, 50%, 75% and 100%
of the Offered Shares at a per share price of $0.02, which represents the mid-point of the price range presented herein. All amounts
set forth below are estimates.
| | |
| 25% | | |
| 50% | | |
| 75% | | |
| 100% | |
| Sales and Marketing | |
$ | 196,750 | | |
$ | 396,750 | | |
$ | 596,750 | | |
$ | 796,750 | |
| Dental Lab/Lab Equipment | |
| 787,000 | | |
| 1,587,000 | | |
| 2,387,000 | | |
| 3,187,000 | |
| Lab Business Acquisition 1 | |
| 787,000 | | |
| 1,587,000 | | |
| 2,387,000 | | |
| 3,187,000 | |
| Working Capital | |
| 98,375 | | |
| 198,375 | | |
| 298,375 | | |
| 398,375 | |
| G&A Expense | |
| 98,375 | | |
| 198,375 | | |
| 298,375 | | |
| 398,375 | |
| TOTAL | |
$ | 1,967,500 | | |
$ | 3,967,500 | | |
$ | 5,967,500 | | |
$ | 7,967,500 | |
|
(1) |
Currently, we have not entered into any agreement, oral or written,
or other understanding with respect to the acquisition of any dental lab business. There is no assurance that we will be able to acquire
any other dental lab businesses. To the extent we are unable to acquire another dental lab business, proceeds allocated for such use
would be applied to sales and marketing expenses and to working capital and to expanding the existing business. |
| |
|
We
reserve the right to change the foregoing use of proceeds, should our management believe
it to be in the best interest of our company. The allocations of the proceeds of this offering
presented above constitute the current estimates of our management and are based on our current
plans, assumptions made with respect to the industry in which we operate, general economic
conditions and our future revenue and expenditure estimates. The Company, by the determination
of the Board of Directors, in its sole discretion, may issue the Securities under this Offering
for cash, services, in satisfaction of outstanding debt obligations, and/or other consideration
without notice to subscriber; provided, however, that any Offered Shares issued in
this manner shall be issued at the Offering Price. In the event any Securities are issued
for non-cash consideration, the Company will not recognize net cash proceeds to allocate
towards the uses set forth above.
Investors are cautioned that expenditures may vary
substantially from the estimates presented above. Investors must rely on the judgment of our management, who will have broad discretion
regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous
factors, including market conditions, cash generated by our operations (if any), business developments and the rate of our growth. We
may find it necessary or advisable to use portions of the proceeds of this offering for other purposes.
In the event we do not obtain the entire offering
amount hereunder, we may attempt to obtain additional funds through private offerings of our securities or by borrowing funds. Currently,
we do not have any committed sources of financing.
PLAN OF DISTRIBUTION
In General
Our
company is offering a maximum of 400,000,000 Company Offered Shares on a best-efforts basis,
at a fixed price of $0.01-0.03 per Company Offered Share; any funds derived from this offering
will be immediately available to us for our use. There will be no refunds. This offering
will terminate at the earliest of (a) the date on which the maximum offering has been sold,
(b) the date which is one year from this offering being qualified by the SEC or (c) the date
on which this offering is earlier terminated by us, in our sole discretion, subject to the
limitations set forth in Rule 251(d)(3)(i)(F) of Regulation A.
The
Company, by the determination of the Board of Directors, in its sole discretion, may issue the Securities under this Offering for cash,
services, in satisfaction of outstanding debt obligations, and/or other consideration without notice to subscriber; provided, however,
that any Offered Shares issued in this manner shall be issued at the Offering Price. In the event any Securities are issued for non-cash
consideration, the Company will not recognize net cash proceeds to allocate towards the uses set forth in the Use of Proceeds.
There is no minimum number of Company Offered Shares
that we are required to sell in this offering. All funds derived by us from this offering will be immediately available for use by us,
in accordance with the uses set forth in the Use of Proceeds section of this Offering Circular. No funds will be placed in an escrow account
during the offering period and no funds will be returned, once an investor’s subscription agreement has been accepted by us.
We intend to sell the Company Offered Shares in
this offering through the efforts of our Chief Executive Officer, James D. Brooks. Mr. Brooks will not receive any compensation for offering
or selling the Company Offered Shares. We believe that Mr. Brooks is exempt from registration as a broker-dealer under the provisions
of Rule 3a4-1 promulgated under the Securities Exchange Act of 1934 (the Exchange Act). In particular, Mr. Brooks:
| |
· |
is not subject to a statutory disqualification, as that term is defined in Section 3(a)(39) of the Securities Act; and |
| |
|
|
| |
· |
is not to be compensated in connection with his participation by the payment of commissions or other remuneration based either directly or indirectly on transactions in securities; and |
| |
|
|
| |
· |
is not an associated person of a broker or dealer; and |
| |
|
|
| |
· |
meets the conditions of the following: |
| |
· |
primarily performs, and will perform at the end of this offering, substantial duties for us or on our behalf otherwise than in connection with transactions in securities; and |
| |
|
|
| |
· |
was not a broker or dealer, or an associated person of a broker or dealer, within the preceding 12 months; and |
| |
|
|
| |
· |
did not participate in selling an offering of securities for any issuer more than once every 12 months other than in reliance on paragraphs (a)(4)(i) or (iii) of Rule 3a4-1 under the Exchange Act. |
As of the date of this Offering Circular, we have
not entered into any agreements with selling agents for the sale of the Company Offered Shares. However, we reserve the right to engage
FINRA-member broker-dealers. In the event we engage FINRA-member broker-dealers, we expect to pay sales commissions of up to 8% of the
gross offering proceeds from their sales of the Company Offered Shares. In connection with our appointment of a selling broker-dealer,
we intend to enter into a standard selling agent agreement with the broker-dealer pursuant to which the broker-dealer would act as our
non-exclusive sales agent in consideration of our payment of commissions of up to 8% on the sale of Company Offered Shares effected by
the broker-dealer.
Procedures for Subscribing
If you are interested in subscribing for Company
Offered Shares in this offering, please submit a request for information by e-mail to the company at: info@sdl.care; all relevant information
will be delivered to you by return e-mail.
Thereafter, should you decide to subscribe for
Company Offered Shares, you are required to follow the procedures described therein, which are:
| |
· |
Electronically execute and deliver to us a subscription agreement; and |
| |
|
|
| |
· |
Deliver funds directly by check or by wire or electronic funds transfer via ACH to our specified bank account. |
Acceptance of Subscriptions. Upon
our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the Company Offered Shares subscribed.
Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription
funds. All accepted subscription agreements are irrevocable.
This Offering Circular will be furnished to prospective
investors upon their request via electronic PDF format and will be available for viewing and download 24 hours per day, 7 days per week
on our company’s page on the SEC’s website: www.sec.gov.
An investor will become a stockholder of our company
and the Company Offered Shares will be issued as of the date of settlement. Settlement will not occur until an investor’s funds
have cleared and we accept the investor as a stockholder.
By executing the subscription agreement and paying
the total purchase price for the Company Offered Shares subscribed, each investor agrees to accept the terms of the subscription agreement
and attests that the investor meets certain minimum financial standards. (See “State Qualification and Investor Suitability Standards”
below).
An approved trustee must process and forward to
us subscriptions made through IRAs, Keogh plans and 401(k) plans. In the case of investments through IRAs, Keogh plans and 401(k) plans,
we will send the confirmation and notice of our acceptance to the trustee.
Minimum Purchase Requirements
You must initially purchase at least $5,000 of
the Company Offered Shares in this offering. If you have satisfied the minimum purchase requirement, any additional purchase must be in
an amount of at least $1,000.
State Law Exemption and Offerings to Qualified Purchasers
State Law Exemption. This Offering
Circular does not constitute an offer to sell or the solicitation of an offer to purchase any Offered Shares in any jurisdiction in which,
or to any person to whom, it would be unlawful to do so. An investment in the Offered Shares involves substantial risks and possible loss
by investors of their entire investments. (See “Risk Factors”).
The Offered Shares have not been qualified under
the securities laws of any state or jurisdiction. Currently, we plan to sell the Company Offered Shares in as many states as this offering
is able to be qualified. In the case of each state in which we sell the Company Offered Shares, we will qualify the Company Offered Shares
for sale with the applicable state securities regulatory body or we will sell the Company Offered Shares pursuant to an exemption from
registration found in the applicable state’s securities, or Blue Sky, law.
Certain of our offerees may be broker-dealers registered
with the SEC under the Exchange Act, who may be interested in reselling the Company Offered Shares to others. Any such broker-dealer will
be required to comply with the rules and regulations of the SEC and FINRA relating to underwriters.
Investor Suitability Standards. The
Company Offered Shares may only be purchased by investors residing in a state in which this Offering Circular is duly qualified who have
either (a) a minimum annual gross income of $70,000 and a minimum net worth of $70,000, exclusive of automobile, home and home furnishings,
or (b) a minimum net worth of $250,000, exclusive of automobile, home and home furnishings.
Issuance of the Company Offered Shares
Upon settlement, that is, at such time as an investor’s
funds have cleared and we have accepted an investor’s subscription agreement, we will either issue such investor’s purchased
Company Offered Shares in book-entry form or issue a certificate or certificates representing such investor’s purchased Company
Offered Shares.
Transferability of the Offered Shares
The Offered Shares will be generally freely transferable,
subject to any restrictions imposed by applicable securities laws or regulations.
Advertising, Sales and Other Promotional Materials
In addition to this Offering Circular, subject
to limitations imposed by applicable securities laws, we expect to use additional advertising, sales and other promotional materials in
connection with this offering. These materials may include information relating to this offering, articles and publications concerning
industries relevant to our business operations or public advertisements and audio-visual materials, in each case only as authorized by
us. In addition, the sales material may contain certain quotes from various publications without obtaining the consent of the author or
the publication for use of the quoted material in the sales material. Although these materials will not contain information in conflict
with the information provided by this Offering Circular and will be prepared with a view to presenting a balanced discussion of risk and
reward with respect to the Company Offered Shares, these materials will not give a complete understanding of our company, this offering
or the Company Offered Shares and are not to be considered part of this Offering Circular. This offering is made only by means of this
Offering Circular and prospective investors must read and rely on the information provided in this Offering Circular in connection with
their decision to invest in the Company Offered Shares.
SELLING STOCKHOLDER
The stockholder named in the table below is the
“Selling Stockholder.” The Selling Stockholder intends to sell a total of 37,500,000 shares of our common stock (the Selling
Stockholder Offered Shares) in this offering. The Selling Stockholder is a third party. The Selling Stockholder Offered Shares to be offered
by the Selling Stockholder named herein are “restricted securities” under applicable federal and state securities laws.
We will pay all of the expenses of this offering
(other than the selling commissions payable with respect to the Selling Stockholder Offered Shares sold in this offering) but will not
receive any of the proceeds from the sale of Selling Stockholder Offered Shares in this offering.
The Selling Stockholder is not a broker-dealer
or affiliated with a broker-dealer. The Selling Stockholder may be deemed to be an underwriter of the shares of our common stock offered
by the Selling Stockholder in this offering.
The Selling Stockholder intends to sell the Selling
Stockholder Offered Shares in market transactions or in negotiated private transactions at the per share offering price of the Offered
Shares, $0.01-0.03.
The table below assumes that all of the Company
Offered Shares offered in this offering will be sold.
| |
|
|
|
Prior to this Offering |
|
|
|
After this Offering |
|
| Name of Selling Stockholder |
|
Position, Office or Other Material Relationship |
|
# of Shares Beneficially Owned |
|
% Beneficially Owned (1) |
|
# of Shares to be Offered for the Account of the Selling Stockholder |
|
# of Shares Beneficially Owned |
|
% Beneficially Owned (2) |
|
| John Jonpil Kim |
|
None |
|
37,500,000 |
|
* |
|
37,500,000 |
|
0 |
|
0% |
|
Less than 1%
|
(1) |
Based on 445,728,363 shares outstanding, before this offering. |
|
(2) |
Based on 845,228,363 shares outstanding, assuming the sale of all of the Company Offered Shares, after this offering. |
DESCRIPTION OF SECURITIES
General
Our authorized capital stock consists of 2,000,000,000
shares of common stock, $.001 par value per share.
As of the date of this
Offering Circular, there were 465,728,363 shares of our common stock issued and outstanding held by approximately 75 holders of record.
Common Stock
General. The holders of our common
stock currently have (a) equal ratable rights to dividends from funds legally available therefore, when, as and if declared by our Board
of Directors; (b) are entitled to share ratably in all of our assets available for distribution to holders of common stock upon liquidation,
dissolution or winding up of the affairs of our company; (c) do not have preemptive, subscriptive or conversion rights and there are no
redemption or sinking fund provisions or rights applicable thereto; and (d) are entitled to one non-cumulative vote per share on all matters
on which stockholders may vote. Our Bylaws provide that, at all meetings of the stockholders for the election of directors, a plurality
of the votes cast shall be sufficient to elect. On all other matters, except as otherwise required by Nevada law or our Articles of Incorporation,
as amended, a majority of the votes cast at a meeting of the stockholders shall be necessary to authorize any corporate action to be taken
by vote of the stockholders.
Non-cumulative Voting. Holders of
shares of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares,
voting for the election of directors, can elect all of the directors to be elected, if they so choose, and, in such event, the holders
of the remaining shares will not be able to elect any of our directors.
Pre-emptive Rights. As of the date
of this Offering Circular, no holder of any shares of our capital stock has pre-emptive or preferential rights to acquire or subscribe
for any unissued shares of any class of our capital stock not otherwise disclosed herein.
Anti-takeover Effects of Nevada Law
Business Combinations. The “business
combination” provisions of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes, or NRS, prohibit a Nevada corporation
with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder: for a period
of three years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved
by the board of directors prior to the date the interested stockholder obtained such status; or after the expiration of the three-year
period, unless:
| |
· |
the transaction is approved by the board of directors, or a majority of the voting power held by disinterested stockholders, or |
| |
|
|
| |
· |
if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is defined to include
mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series
of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate
market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all
outstanding shares of the corporation, or (c) 10% or more of the earning power or net income of the corporation.
In general, an “interested stockholder”
is a person who, together with affiliates and associates, owns (or within three years, did own) 10% or more of a corporation’s voting
stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts
to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above
the prevailing market price.
Control Share Acquisitions
The “control share” provisions of Sections
78.378 to 78.3793, inclusive, of the NRS, which apply only to Nevada corporations with at least 200 stockholders, including at least 100
stockholders of record who are Nevada residents, and which conduct business directly or indirectly in Nevada, prohibit an acquirer, under
certain circumstances, from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages,
unless the acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds: one-fifth
or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Once an
acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control
shares” and such control shares are deprived of the right to vote until disinterested stockholders restore the right. These provisions
also provide that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting
power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment
for the fair value of their shares in accordance with statutory procedures established for dissenters” rights.
Dividend Policy
We have never declared or paid any dividends on
our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we do
not anticipate paying any cash dividends in the foreseeable future.
Stockholder Meetings
Our bylaws provide that special meetings of stockholders
may be called only by our Board of Directors, the chairman of the board, or our president, or as otherwise provided under Nevada law.
Transfer Agent
We have retained the services of Transfer Online,
512 SE Salmon Street, Portland, Oregon 97214-3444, as the transfer agent for our common stock. Transfer Online’s website is located
at: www.transferonline.com. No information found on Transfer Online’s website is part of this Offering Circular.
BUSINESS
Corporate History
Standard Dental Labs Inc.
(f/k/a Costas, Inc.) was incorporated in the State of Nevada on December 10, 1998. Standard Dental Labs was a development stage company
that had a primary business plan to acquire, improve, and re-market undeveloped real estate in Las Vegas, Nevada and its surrounding
communities.
In 2017, our current Chief
Executive Officer and Director, James D. Brooks, commenced an action against the Company for breach of contract and non-payment of obligations
and was awarded a judgment in the Eighth Judicial District Court, Clark County, Nevada. The Company satisfied the judgment by issuing
a convertible promissory note in the principal amount of $1,114,500. On October 21, 2020, Mr. Brooks, as a majority shareholder, filed
a motion requesting the appointment of a Receiver over our company. On March 25, 2021, the Receiver filed a motion with the Court requesting
approval to appoint Mr. Brooks as an officer and director of our company and to increase our authorized capital and subsequently to issue
sufficient common and preferred shares on terms to be finalized with Mr. Brooks, whereby Mr. Brooks became the controlling stockholder
of our company.
On February 9, 2022, an Order
was entered by the Eighth Judicial District Court, Clark County, Nevada, Case No. A-17-749977D, terminating the receivership for our
company.
On May 6, 2022, the company
entered into an asset purchase agreement with Standard Dental Labs Inc. (“SDL”), a Wyoming corporation controlled by the
company’s CEO, James Brooks, to acquire certain assets including: (i) a ready to implement business model and platform for the
identification and acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package
created around SDL, including logo, website, presentation materials and corporate name. Under the terms of the acquisition agreement,
assets valued at $75,900 were acquired through the issuance of a total of 31,661,760 shares of the Company’s restricted common
stock to SDL. With the conclusion of this acquisition, the Company planned to operate in the dental lab industry, paving the way for
future acquisitions and consolidations in the industry. The assets acquired from SDL allow the Company to immediately facilitate the
acquisition of small to medium sized dental labs. The assets acquired from SDL did not constitute an operating business, but rather an
immediately actionable plan and branding concept from which the Company identified its first operating dental lab targets.
On August 15, 2022, the Company
announced the completion of a definitive agreement to acquire the assets of Prime Dental Lab, LLC (“Prime Dental” or “PDL”),
an Orlando-based dental lab in operation since 2012. The Purchased Assets consisted of: all client contracts for existing PDL clients;
certain physical assets of PDL including all dental lab equipment, furniture, computers and other office equipment; the assumption of
certain contracts, equipment leases and office leases; certain employees and management of PDL as determined by the Company to be retained
and/or contracted by the Company; and specifically the right to continue to use the name “Prime Dental Lab LLC” along with
certain other rights, trademarks, intellectual property and intangible assets of the PDL in a definitive way. Upon completion of the
asset purchase, and by virtue of the fact the Company acquired all rights to the name and branding associate with Prime Dental Lab, LLC,
PDL changed its name to Smile Dental (all references hereinafter to Prime Dental, Prime Dental Lab, and PDL shall be construed and interpreted
as references to Smile Dental). The Company paid $700,000 in cash and stock for the assets of Prime Dental Lab equivalent to $560,000
in stock (the “Share Consideration”), and $140,000 in cash, of which a final payment of $70,000 is contingent upon the Company
being successful in achieving a registered financing under an S-1 registration statement.
The Share Consideration is
subject to a lock-up agreement for a term of twenty-four months from the issuance date, whereunder Smile Dental shall be entitled to
a release of 12.5% of the total Consideration Shares each quarter.
In order to facilitate ongoing
lab operations and to seamlessly service its acquired customer base, the Company entered into a subcontractor agreement with Smile Dental
on August 31, 2022, as amended April 30, 2023, whereunder Smile Dental agreed to provide ongoing labor, quality control and delivery
services during a period of up to two years from the latest date as a subcontractor to Standard Dental. Currently, all labor in
our dental lab is performed under the Smile Dental subcontractor agreement.
Upon acquisition of the customers
and operational assets of Smile Dental, the Company immediately commenced revenue generating operations effective September 1, 2022,
and reported gross revenues of $173,329 in the four-month period ended December 31, 2022, and associated costs of goods sold totaling
$101,054 for gross profit of $72,275.
On March 24, 2023 the Company
and Smile Dental executed an addendum to the definitive agreement with Smile Dental (the “Smile Dental Amendment”) whereunder
the Cash Consideration as part of Purchase Price was revised so that the first instalment shall be released immediately upon execution
of the Smile Dental Amendment, or March 24, 2023, and the second Cash Instalment shall be payable on the date that is no later than seven
(7) calendar days subsequent to the receipt of proceeds from the financing associated with this application.
The company is now operating
in the dental lab industry and is currently manufacturing dental prosthetics for dentists and dental clinics via its first operational
lab facility. Our existing dental lab supplies dentists and dental clinics with dental prosthetics such as crowns, bridges, and implants,
including other prosthetics.
Corporate Information
Our principal executive offices
are located at 424 E. Central Boulevard, Suite 308, Orlando, Florida 32801; our telephone number is (321) 465-9899; our corporate website
is located at http://sdl.care. No information found on our company’s website is part of this Offering Circular.
Our Current Business
Our current business activities
include operating and developing the Company’s dental lab business.
Standard Dental Labs produces
several kinds of dental prosthetics. The Company uses state-of-the-art equipment such as 3D scanners, printers and 5-axis milling machines,
along with several other types of machinery needed to complete jobs for dental clinics. The Company plans to acquire independent labs
looking to exit the market, or whom may be interested in retirement is part of the company’s growth strategy but focus on growing
the existing business organically is part of the Company’s strategy.
The consolidation of dental
and medical clinics has been an increasing trend over the past two decades, but only recently have investors focused on dental labs.
We see this as an opportunity to apply similar strategies to what has been learned from other consolidations.
The Company operates day-to-day
as a full-service dental lab. With more than 50 dental clinics as clients, we produce roughly 500 dental prosthetics each month. As per
industry standard, we do not have any definitive agreements in place with these dental clinics. The items we manufacture and produce
are shipped using standard carriers.
Presently, the Company has
engaged the labor and manufacturing services of Smile Dental as a contract manufacturer. Our goal is to open a large and modern facility
in Orlando, Florida to accommodate 75 full-time employees, including dental lab technicians. We have not undertaken specific steps at
this time for this facility and would not anticipate doing so until we have acquired at least 3 dental labs. We sell our dental lab products
and market these products to our clients using our acquired tradename Prime Dental Lab LLC and compensate Smile Dental for contract services
provided. Smile Dental in turn reimburses the company for the use of our acquired dental lab equipment.
The 500 orders that are delivered
each month are completed by the contracted dental lab techs. With 60+ techs working in the new facility, we expect tech productivity
to factor on par or better than current levels due to improved support technologies and equipment in our new facility.
Products and Services
Dental labs supply dentists
and dental clinics with dental prosthetics such as crowns, bridges, and implants. Although they manufacture many other prosthetics, these
represent a large majority of what is supplied, and would be called the “bread and butter” of the industry. We have a specific
process to make sure that we comply with the deadlines. Smile Dental is responsible for the distribution of our products and for making
contact with our various suppliers. The resources needed to produce the dental prosthetics are readily available and can be sourced from
a variety of suppliers. As such, we do not have agreements in place with any specific suppliers.
The type of dental labs the
company has and will continue to acquire typically serve 20 to 25 dental clinics each and employ 4 to 6 dental lab technicians who prepare
dental prosthetics for dentists. The dentists are normally responsible for the final fitting with the patient, but most of the work is
done by the lab.
Over the past 5 years, 3D
printing and modelling technology has become more mainstream. As most of this technology has been unaffordable for small business owners,
advanced technology is uncommon in these smaller operations.
The Market
The transition from skilled labor to computer driven
technology in the dental lab industry, although in its infancy, is well underway. The changeover of skilled artisans to 3D printers and
milling equipment is causing a shift in the perception for would-be investors. No one can predict how this technology might evolve, how
long it will take, or how it may impact the industry in the long term.
The
uncertain future of dental labs means there is less interest from investors in the field. The erosion of the one, two, and three person
businesses is certain. (Forbes, Nov. 2019). The result: A generation of lab owners that may not be able to sell their business for retirement
or raise the necessary capital to retool to this advancing technology.
Competition
The biggest competition to the private dental lab
owner in the United States is large, Chinese factory labs operating in southern China. We estimate that a large percentage of dental prosthetics
are now sourced through these offshore manufacturers. This is detrimental to the local industry, and there is very little an individual
lab owner can do to protect himself from the market erosion caused by this competitor.
However, this may represent an opportunity for
the company to lobby the state government to enact legislation requiring the materials be “certified” prior to being used to
supply the industry with the raw materials required to manufacture dental products.
Certified materials could only become certified
through local inspectors which could be funded through a state or national association. So far in the US, the only such association is
The National Association of Dental Laboratories, located in Tallahassee, Florida.
As a start-up company in the dental lab industry,
we will be at a significant disadvantage to other more established and better capitalized companies that we are in competition with for
the acquisition of additional dental labs. As the Company is able to acquire additional labs and enhance its revenue, when appropriate
the company will seek to uplist to the OTCQB Market or a more senior exchange to further enhance liquidity benefits for stockholders and
vendors of dental lab assets.
Compliance with Government Regulation
In a 2009, American Dental Association member survey,
nearly 65 percent of dentists responded that they believe dental technicians and laboratories are regulated in their state. This is not
the case. In fact, only four states in the United States require either certification or continuing education.
However, 12 states require basic minimum standards
be met for laboratories, which include state registration. The most up-to-date regulations were last set in 2019, in Washington State,
but most haven’t updated their requirements for 25 years or longer. We feel that there is an opportunity to contribute to the industry
by proposing new, more current legislation that addresses the importation of materials from foreign countries. New legislation that requires
certification of materials could greatly enhance the safety of the materials for patients, and also contribute to the success of US based
dental labs.
Other than the payment of a modest registration
fee every two years to maintain our dental lab registration with the State of Florida, ongoing continuing education of 18 hours biennially
by one certified lab technician in the lab in accordance with Florida Administrative Code Rule Chapter 64B-5, we do not require any governmental
approvals or authorizations for the operation of our existing dental lab in Florida. Further, we are not aware of any pending or probable
regulations that would have an impact upon our operations.
| |
FL |
IL |
KY |
MN |
MO |
NC |
OH |
OK |
PA |
SC |
TX |
VA |
WA |
| Year of Initial Enactment |
1987 |
1993 |
1977 |
2012 |
1995 |
1962 |
2008 |
1992 |
1987 |
1942 |
1985 |
2012 |
2019 |
| Laboratory Registration Fee |
$200/2
yrs |
N/A |
$150/yr |
$50/yr |
N/A |
N/A |
N/A |
$300/yr |
$25/yr |
$150/2
yrs |
$135/yr |
N/A |
$250 |
| Laboratory Registration |
Yes |
No |
Yes |
Yes |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
| Technician Registration Fee |
No |
No |
No |
No |
No |
No |
No |
No |
No |
$100
Initial,
$150
Renewal |
No |
No |
No |
| Certificate to Perform Dental Technology |
No |
No |
No |
No |
No |
No |
No |
No |
No |
Yes ‡ |
No |
No |
No |
| CDT or Equivalent Required |
No |
No † |
Yes |
No |
No |
No |
No |
No |
No |
Yes |
Yes |
No |
Yes
(2025) |
| State Laws and Rules Exam Required |
No |
No |
No |
No |
No |
No |
No |
No |
No |
Yes |
Yes |
No |
No |
| Out-of-State Laboratories Required to Register |
No |
No |
Yes |
No |
No |
No |
No |
Yes |
No ‡ |
Yes |
Yes |
No |
Yes |
| Dentists Required to Use Registered Laboratory |
No |
No |
Yes |
Yes # |
No |
No |
No |
Yes |
No |
Yes |
Yes |
No |
Yes |
| Materials Disclosure |
Yes |
Yes |
Yes |
Yes |
Yes + |
Yes |
Yes |
No |
No |
Yes |
No |
Yes |
Yes |
| Point of Origin Disclosure |
Yes |
Yes |
Yes |
Yes |
Yes + |
Yes |
Yes |
No |
No |
Yes |
Yes |
Yes |
Yes |
| CE for One Technician in Each Laboratory |
Yes |
No |
Yes |
No |
No |
No |
No |
No |
No |
Yes |
Yes |
No |
Yes |
The company’s current lab operations in Florida
are not subject to OSHA regulations or regulations governing the handling or disposing of medical waste. There are currently no specific
OSHA standards for dentistry, however the labs follow best practices including:
| |
(a) |
having a lab space that is clean and orderly and in good repair, with regard to normal fabrication procedures; |
| |
|
|
| |
(b) |
All waste materials properly disposed of at the end of each day; |
| |
|
|
| |
(c) |
Maintaining on the laboratory premises a copy of the laboratory registration so it is readily available for inspection by Department personnel; |
| |
|
|
| |
(d) |
Maintaining on the laboratory premises, for each separate appliance and for a period of four years, a work order from a licensed dentist authorizing construction or repair of the specified artificial oral appliance; and |
| |
|
|
| |
(e) |
Maintaining on the laboratory premises a written policy and procedure
document on sanitation. Said policy shall include, but not necessarily be limited to: Intake and disinfection procedure for each appliance,
impression, bite, or other material posing a possible contamination risk received by the laboratory.
|
In the State of Florida the prosthetics and other
oral appliances manufactured and repaired by the lab do not for the most part meet the definition of medical waste according to the Florida
Department of Health which includes, “Any solid or liquid waste which may present a threat of infection to humans, including nonliquid
tissue, body parts, blood, blood products, and body fluids from humans and other primates; laboratory and veterinary wastes which contain
human disease-causing agents; and discarded sharps.” Most prosthetics and appliances coming to the lab from a dental office have
been disinfected prior to intake. However, the company’s lab operations follow the recommended procedures for waste including properly
labeled and identified waste bags, proper seals on waste containers and proper disposal of waste in accordance with the State of Florida
department of health chapter 64E-16 of the Florida Administrative Code.
Significant Acquisitions
In May of 2022, Standard Dental Labs Inc. acquired
certain assets of Standard Dental Labs Inc. (SDL), a privately owned Wyoming corporation controlled by our CEO, James Brooks, which is
in the business of providing consulting services, including the creation of business plans for identifying and purchasing operating assets
in various industry sectors. Immediately following the acquisition of the aforementioned assets by the company, including the tradename
“Standard Dental Labs Inc.”, SDL changed its name to “Fastbend Holdings Inc.” in order to continue its ongoing operations.
The Company has since adopted the name “Standard Dental Labs Inc.” effective March 21st, 2024.
The
business model acquired from SDL includes metrics and data in order to allow the company
to quickly identify and purchase privately owned dental lab operations in the United States,
allowing for these operations to be consolidated regionally, and to operate them efficiently
applying economies of scale. To that end, in August of 2022, Standard Dental Labs signed
an agreement to purchase all of the assets, including the revenue, of Prime Dental Lab LLC,
a Florida dental laboratory, and contracted Prime Dental Lab to continue to operate the company’s
assets. This agreement included the purchase of not only every asset but also trademarks
and intellectual properties as described below.
On
August 15, 2022, the company announced the completion of the definitive agreement to acquire
the assets of Prime Dental Lab LLC (now known as Smile Dental), an Orlando-based dental lab
in operation since 2012. The Purchased Assets pursuant to the agreement consisted of: all
client contracts for existing PDL clients; certain physical assets of PDL including all dental
lab equipment, furniture, computers and other office equipment; the assumption of certain
contracts, equipment leases and office leases (if any); certain employees and management
of PDL as determined by the company to be retained and/or contracted by the company; and
specifically the right to continue to use the name “Prime Dental Lab LLC” along
with any corresponding rights, trademarks, intellectual property and intangible assets of
PDL. The assets acquired more specifically consisted of the lab equipment, the client base
and the corporate brand.
In order to facilitate ongoing
lab operations and seamlessly service its acquired customer base the company entered into a subcontractor contract with Smile Dental
on August 31, 2022, as amended April 30, 2023, whereunder Smile Dental will provide ongoing labor, quality control and delivery services
and management oversight during a period of up to two years. Under the terms of the agreement, as amended, with Smile Dental, either
the Company or Smile Dental may terminate the agreement with six (6) months written notice, in order to provide the Company sufficient
time to identify, hire and train a suitable replacement. The company operates using its acquired tradename Prime Dental Labs LLC. The
company did not acquire the building where the manufacturing of Prime Dental Lab appliances and prosthetics takes place. The Company
and Smile Dental have agreed that the cost of manufacturing goods paid by the company to Smile Dental shall include overhead costs including
applied costs on a per item bases for the use of the space where the lab operations take place. There is no separate agreement for the
rental or lease of the space where the prosthetics are manufactured.
Offices
The
mailing address of our company is # 424 E Central Blvd, Suite 308, Orlando, FL, 32801. This
space is leased by our President, James Brooks, for a term of one year from January 2023
through 2024, is approximately 1,500 square feet and has a cost of approximately $3,185 per
month, plus utilities and insurances, which amounts are reimbursed to Mr. Brooks, who uses
this space to work as he lives in Canada and allows him to have a suitable workplace that
meets the minimum requirements of an office, in addition to also serving a place to stay.
This makes it a more attractive option for the Company since it does not have to bear the
expense of a hotel every time Mr. Brooks travels from Canada for business, but also does
not have to pay for an additional physical workspace. The mailing address is a shared office
and residential space. Our main telephone number is 321-465-9899. Our initial lab location
is at 1008 N Pine Hills Rd, Orlando, FL 32808. We do not own or lease our lab location, as
the location is owned by Mr. John Kim, managing member of Smile Dental, our contract manufacturer
which provides ongoing labor, quality control and delivery services and management services
during a period of up to two years. Smile Dental is reimbursed for the use of this lab space
through a usage fee included in the costs of goods sold charged to the company by Smile Dental
for each dental appliance. In addition, Smile Dental remits to the company a usage fee monthly
for access to the lab equipment acquired by the company and used in the manufacturing process.
Our current locations provide adequate space for our purposes at this stage of our development.
Employees
We currently have no employees.
Our sole officer is also a member of our board of directors, James Brooks, our second board member, Ms. Claire Ambrosio.
We do expect material changes in the number of
employees over the next 12-month period, as will be required as we expand with our acquisitions of additional dental lab operations. Also,
we do and will continue to outsource contract employment as needed.
Litigation
We know of no material, existing or pending legal
proceedings against us, nor are we involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings
in which any of our directors, officers or affiliates, or any registered or beneficial stockholder, is an adverse party or has a material
interest adverse to our company.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statements
This registration statement contains forward-looking
statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements relate to future events or
our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may”,
’should”, “expects”, “plans”, “anticipates”, “believes”, “estimates”,
“predicts”, “potential” or “continue” or the negative of these terms or other comparable terminology.
These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the
section entitled “Risk Factors” that may cause our or our industry’s actual results, levels of activity, performance
or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied
by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable,
we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by applicable law, including
the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements
to actual results.
Our financial statements are stated in United
States Dollars ($) except as otherwise indicated and are prepared in accordance with United States Generally Accepted Accounting Principles.
The following discussion should be read in conjunction with our unaudited interim consolidated financial statements and the related notes
that appear elsewhere in this registration statement. The following discussion contains forward-looking statements that reflect our plans,
estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that
could cause or contribute to such differences include, but are not limited to, those discussed below and elsewhere in this registration
statement, particularly in the section entitled “Risk Factors” of this registration statement.
In this registration statement, unless otherwise
specified, all dollar amounts are expressed in United States dollars and all references to “common shares” refer to the common
shares in our capital stock.
Plan of Operation
During the next twelve-month period (beginning
January 1, 2024), we intend to identify and secure sources of equity and/or debt financing for additional acquisitions.
We anticipate that we will incur the following
operating expenses during this period:
Estimated Funding Required During the 12 Months
beginning January 1, 2024
| | |
Amount | |
| Expenses | |
($) | |
| Improvements to planned corporate facility | |
| 300,000 | |
| Executive salaries, including new hires | |
| 540,000 | |
| Administrative support | |
| 150,000 | |
| Professional fees | |
| 240,000 | |
| General and administrative expense including rent, transfer agent, travel, office and sundry | |
| 300,000 | |
| Advertising, marketing, and website costs | |
| 80,000 | |
| Total operating expense | |
| 1,610,000 | |
| Cash required for lab acquisitions | |
| 250,000 | |
| Total | |
| 1,860,000 | |
Results of Operations for our Years Ended
December 31, 2023 and 2022
Our net income (loss) and
comprehensive income (loss) for our year ended December 31, 2023, for our year ended December 31, 2022, and the changes between those
periods for the respective items are summarized as follows:
For the years ended December
31, 2023, and 2022, the Company generated revenues of $339,466 and $173,329, respectively. The Company experienced an increase in revenue
as a result of an increase in the volume of products being manufactured as a result of the PDL acquisition. Accordingly, our cost of
goods sold increased from $101,054 at December 31, 2022, to $197,860 at December 31, 2023.
Operating expenses for
the years ended December 31, 2023, and 2022 were $329,977 and $391,647, respectively, a decrease of $61,670. As summarized in the table
below, selling and marketing expenses increased by $66,380 due to increased efforts to grow and market our dental lab, while general
and administrative expenses were down by $49,348 due to increased operational efficiencies. Professional fees also saw a decrease of
$93,251 due to reduced legal, accounting, and audit expenses.
Net loss for the years
ended December 31, 2023, and 2022 was $(412,386) and $(1,651,897), respectively, a decrease of $1,239,511.
| | |
For the Year Ended | | |
| |
| | |
December 31, | | |
| |
| | |
2023 | | |
2022 | | |
Change | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 339,466 | | |
$ | 173,329 | | |
$ | 166,137 | |
| Cost of goods sold | |
| 197,860 | | |
| 101,054 | | |
| 96,806 | |
| Gross profit | |
| 141,606 | | |
| 72,275 | | |
| 69,331 | |
| | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Selling and marketing expense | |
| 140,773 | | |
| 74,393 | | |
| 66,380 | |
| General and administrative expenses | |
| 83,068 | | |
| 132,416 | | |
| (49,348 | ) |
| Professional fees | |
| 84,314 | | |
| 177,564 | | |
| (93,251 | ) |
| Depreciation | |
| 21,823 | | |
| 7,274 | | |
| 14,549 | |
| Total operating expenses | |
| 329,977 | | |
| 391,647 | | |
| (61,670 | ) |
| | |
| | | |
| | | |
| | |
| Operating loss | |
| (188,371 | ) | |
| (319,372 | ) | |
| 131,001 | |
| | |
| | | |
| | | |
| | |
| | |
| For the Year Ended | | |
| | |
| | |
| December 31, | | |
| | |
| | |
| 2023 | | |
| 2022 | | |
| Change | |
| Other income (expense): | |
| | | |
| | | |
| | |
| Interest expense | |
| (482,249 | ) | |
| (1,339,409 | ) | |
| 857,160 | |
| Gain on settlement of debt and liabilities | |
| 258,234 | | |
| 6,884 | | |
| 251,350 | |
| Total other income (expense) | |
| (224,015 | ) | |
| (1,332,525 | ) | |
| 1,108,510 | |
| | |
| | | |
| | | |
| | |
| Net loss | |
$ | (412,386 | ) | |
$ | (1,651,897 | ) | |
$ | 1,239,511 | |
During the years ended
December 31, 2023, and 2022, respectively the Company reported $339,446 and $173,329 in gross revenue, $197,860 and $101,054 in
costs of goods sold and $141,606 and $72,275 as gross profit. The increases in revenue, cost of goods sold and gross profit were due
to the acquisition of Prime Dental Labs in 2022.
In
the years ended December 31, 2023, and 2022 the Company reported net operating losses of
$188,371 and $319,371, respectively. Losses from operations in the year ended December
31, 2023, consist of professional fees of $84,314, depreciation of $21,823, Selling and marketing
expenses of $140,773 and general and administrative expenses, including amounts paid to the
transfer agent, rent, consultants and filing fees of $83,068. Losses from operations
in the year ended December 31, 2022, consist of professional fees of $177,564, depreciation
of $7,274, Selling and marketing expenses of $74,393 and general and administrative expenses,
including amounts paid to the transfer agent, rent, consultants and filing fees of $132,416.
Professional fees and administrative costs in 2022
can be attributed to the prior increases in both areas, which were associated with the acquisition
of PDL. Accordingly, professional fees and administrative costs in 2023 decreased as there
were no acquisition costs to report.
During the comparative years ended December 31,
2023, and 2022 the Company incurred interest expenses of $482,249 and $1,339,409 respectively as a result of interest accruing on a judgment
payable in favor of our sole officer and director, Mr. James Brooks and an underlying convertible promissory note in the principal amount
of $1,171,727, as well as certain other convertible notes issued during the year ended December 31, 2022. The Company recorded a
gain of $258,234 and $6,884 in the year ended December 31, 2023 and 2022 respectively as a result of a settlement of certain accounts
payable.
Liquidity and Financial Condition
Cash Provided (used in) Operating Activities
During the year ended December 31, 2023 cash used in operating expenses
was $454,565, and consisted of our net loss of $412,386 offset by depreciation of $21,823, a gain on extinguishment of debt of $258,234,
amortization of discounts on convertible debt of $372,692, a decrease to accounts payable – related parties of $89,915, an increase
to accounts payable and other liabilities of $7,286 and a decrease to other current liabilities of $95,831 . During the year ended December
31, 2022, cash used in operating expenses was $202,485, and consisted of our net loss of $1,651,896 offset by depreciation of $7,274,
a gain on extinguishment of debt of $6,884, amortization of discounts on convertible debt of $1,238,291, an increase to accounts payable
– related parties of $72,861 and an increase to accounts payable and other liabilities of $137,870.
Cash Provided by Investing Activities
There was no cash used in investing activities
in the years ended December 31, 2023, and 2022.
Cash Provided by Financing Activities
Cash provided by financing activities totalled
$443,394 for the year ended December 31, 2023, including proceeds from convertible notes payable of $411,000 and advances payable from
related parties of $32,394.
Cash provided by financing activities totalled
$215,608 for the year ended December 31, 2022, including proceeds from convertible notes payable of $232,140 and repayments to advances
from related parties of $16,532.
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements
that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues
or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Critical Accounting Policies
Our financial statements and accompanying notes
are prepared in accordance with generally accepted accounting principles used in the United States of America. Preparing financial statements
requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses.
These estimates and assumptions are affected by management’s application of accounting policies. We believe that understanding the basis
and nature of the estimates and assumptions involved with the following aspects of our financial statements is critical to an understanding
of our financials.
Going Concern
We have suffered recurring losses from operations.
The continuation of our Company as a going concern is dependent upon our Company attaining and maintaining profitable operations and/or
raising additional capital. The financial statements do not include any adjustment relating to the recovery and classification of recorded
asset amounts or the amount and classification of liabilities that might be necessary should our Company discontinue operations.
The continuation of our business is dependent
upon us raising additional financial support and/or attaining and maintaining profitable levels of internally generated revenue. The issuance
of additional equity securities by us could result in a significant dilution in the equity interests of our current stockholders. Obtaining
commercial loans, assuming those loans would be available, will increase our liabilities and future cash commitments.
Recently Issued Accounting Standards
In August 2020, the FASB issued ASU 2020-06, Debt
– Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging – Contracts in Entity’s Own
Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. The new guidance, among
other things, simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible
instruments, and amends existing earnings-per-share (“EPS”) guidance by requiring that an entity use the if converted method
when calculating diluted EPS for convertible instruments. ASU 2020-06 is effective for public business entities that meet the definition
of an SEC filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after
December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal
years beginning after December 15, 2023, including interim periods within those fiscal years. The Company adopted the new guidance effective
from January 1, 2024.
Controls and Procedures
We maintain disclosure controls and procedures
that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of
1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange
Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our president (also
our principal executive officer) and our secretary, treasurer and chief financial officer (also our principal financial and accounting
officer) to allow for timely decisions regarding required disclosure.
As of September 30, 2022, the end of the third
quarter covered by the comparative information of this prospectus, we carried out an evaluation, under the supervision and with the participation
of our president (also our principal executive officer) and our secretary, treasurer and chief financial officer (also our principal financial
and accounting officer), of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing,
our president (also our principal executive officer) and who is also our secretary, treasurer and chief financial officer (also our principal
financial and accounting officer) concluded that our disclosure controls and procedures were effective in providing reasonable assurance
in the reliability of our financial reports as of the end of the period.
Inherent limitations on effectiveness of
controls
Internal control over financial reporting has
inherent limitations which include but is not limited to the use of independent professionals for advice and guidance, interpretation
of existing and/or changing rules and principles, segregation of management duties, scale of organization, and personnel factors. Internal
control over financial reporting is a process which involves human diligence and compliance and is subject to lapses in judgment and breakdowns
resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management
override. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements on a
timely basis, however these inherent limitations are known features of the financial reporting process and it is possible to design into
the process safeguards to reduce, though not eliminate, this risk. Therefore, even those systems determined to be effective can provide
only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness
to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of
compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial
Reporting
There have been no changes in our internal controls over financial
reporting that occurred during the year ended December 31, 2023, that have materially or are reasonably likely to materially affect, our
internal controls over financial reporting.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND
CONTROL PERSONS
Directors and Executive Officers
The following table sets forth certain information
concerning our company’s sole officer and director.
| Name |
|
Age |
|
Position(s) |
| James D. Brooks |
|
53 |
|
President, Chief Financial Officer, Secretary and Director |
| Claire Ambrosio |
|
58 |
|
Director |
Our directors serve until a successor is elected
and qualified. Our officers are elected by the Board of Directors to a term of one (1) year and serves until their successor(s) is duly
elected and qualified, or until they are removed from office. There are no family relationships between our directors.
Our company believes that all of our directors’
respective educational background, operational and business experience give them the qualifications and skills necessary to serve as directors
and officers, respectively, of our company.
Certain information regarding the backgrounds of
our officers and directors is set forth below.
James D. Brooks has served as our
President, Chief Financial Officer, Secretary, Treasurer and Director since December 30, 2021. Mr. Brooks first began working with public
companies in 1998, and has founded and exited from two substantial companies in the transportation logistics sector since. The first,
Urban Dispatch, where Mr. Brooks served as the CEO, COO and a board member was a Canadian logistics company, and employed more than 250
people, operating in 4 provinces in Western Canada. Following his time with Urban Dispatch, Mr. Brooks founded Oveldi Worldwide Shipping
Company, based in Hong Kong, where he served as CEO, COO and a board member until divestiture in 2012.
From 2014 through 2019, Mr. Brooks served as an
officer and director of Oveldi Strategic Capital Ltd. where he also provided services as a business consultant. Oveldi Strategic Capital
assisted small businesses in a variety of industries with corporate structure, governance, business planning, exit strategies, strategic
partnerships and overall corporate objectives and direction. Such clients included companies in moving and storage, transportation logistics,
jewelry distribution, real estate development, clothing manufacturing, and others.
During the Covid-19 pandemic commencing in early
2019, Mr. Brooks repositioned his focus and chose to dedicate his business time exclusively to his next controlled corporation, Fastbend
Holdings Inc. (formerly Standard Dental Labs), where he is the CEO, President, and a director. Fastbend Holdings is a consulting firm
and project incubation facilitator, drawing on the experience gained from his former venture Oveldi Strategic Capital. Upon acquiring
a controlling interest in Standard Dental Labs Inc. in December 2021 and becoming the sole officer and director, Mr. Brooks again reallocated
his time resources to spend 90% of his time developing the ongoing business of Standard Dental Labs, with the remaining 10% allocated
to oversight of his controlled private corporation, Fastbend Holdings.
Given his background and experience building operations
from the ground up, Mr. Brooks has a clear vision of how to identify and acquire target companies for Standard Dental Labs, and how to
execute the company’s business plan. Mr. Brooks has been responsible for advancing the company’s objectives, including the financing the
efforts made to date for Standard Dental Labs Inc., as its largest creditor, stockholder and CEO.
Mr. Brooks currently devotes approximately 90%
of his professional time to the business and intends to continue to devote this amount of time in the future, or more as required.
Claire
Ambrosio has served as a Director since April 20, 2023. Ms. Ambrosio is a member
of The State Bar of California and has practiced as a lawyer in California for the past 22
years. She has served as the Vice President of Legal for 4 Over, LLC, a trade printer, from
2022 to present, and as General Counsel for True Family Enterprises from 2019 through 2022.
She holds a law degree from Southwestern University, School of Law (1991) and has been a
member of the bar in California since 1996. Ms. Ambrosio holds an LL.M., Loyola University
Chicago School of Law, Chicago, Illinois in Healthcare and Compliance (2021). Ms. Ambrosio
has focused her legal practice in the area of Corporate Compliance. Her experience in this
area makes her uniquely qualified to contribute as a board member of a publicly traded company.
Significant Consultant
We
have retained the consulting services of Smile Dental, which is owned and controlled by Jongpil
(John) Kim, under the terms of a subcontractor agreement entered into August 31, 2022, as
amended April 30, 2023, for services including labor and manufacturing expertise, as well
as management services with respect to the servicing of our company’s client list for
dental prosthetics and orthotics.
Mr. Kim immigrated from Seoul, South Korea to Boston,
Massachusetts in 2006, where he studied for 3 years to become a dental lab technician at Mass Dental Technique, a private school in Boston.
In 2008, Mr. Kim received permanent and green card residency and started his first full-time job at Reliable Dental Lab in Andover, MA,
and worked there until 2011. In 2011, Mr. Kim moved to Orlando, FL and after one year of practical clinical experience in Florida at Mount
Dora Modern Dentistry, opened his own lab, Prime Dental Lab LLC in 2012. In 2014, Mr. Kim received US Citizenship. Mr. Kim established
a solid client base and operated Prime Dental until the sale of its material assets in 2021.
Conflicts of Interest
At the present time, we do not foresee any direct
conflict between our officers and directors, and their respective other business interests and their involvement in our company.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors
or executive officers has, during the past ten years:
1. been convicted in a criminal proceeding or been subject
to a pending criminal proceeding (excluding traffic violations and other minor offences);
2. had any bankruptcy petition filed by or against the business
or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer,
either at the time of the bankruptcy filing or within two years prior to that time;
3. been subject to any order, judgment, or decree, not subsequently
reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining,
barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking,
savings and loan, or insurance activities, or to be associated with persons engaged in any such activity;
4. been found by a court of competent jurisdiction in a civil
action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and
the judgment has not been reversed, suspended, or vacated;
5. been the subject of, or a party to, any federal or state
judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement
of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law
or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary
or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or
removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity;
or
6. been the subject of, or a party to, any sanction or order,
not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act
(15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or
any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with
a member.
Audit Committee and Audit Committee Financial Expert
Our board of directors (currently consisting of
two members) also acts as the audit committee and has determined that it does not have a member that qualifies as an “audit committee
financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K . We currently have one “independent” director as
the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act of 1934, as amended.
We believe that our board of directors is capable
of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. We believe
that retaining additional independent directors who would qualify as an “audit committee financial expert” would be overly costly
and burdensome and is not warranted in our circumstances given the early stages of our development and the fact that we have not generated
any material revenues to date. In addition, we currently do not have nominating, compensation or audit committees or committees performing
similar functions nor do we have a written nominating, compensation or audit committee charter. Our directors do not believe that it is
necessary to have such committees because they believe the functions of such committees can be adequately performed by the members of
our board of directors.
Stockholder Communications with Our Board of Directors
Our company welcomes comments and questions from
our stockholders. Stockholders should direct all communications to our President, James D. Brooks, at our executive offices. However,
while we appreciate all comments from stockholders, we may not be able to respond individually to all communications. We attempt to address
stockholder questions and concerns in our press releases and documents filed with OTC Markets, so that all stockholders have access to
information about us at the same time. Mr. Brooks collects and evaluates all stockholder communications. All communications addressed
to our directors and executive officers will be reviewed by those parties, unless the communication is clearly frivolous.
Code of Ethics
Our Board of Directors has not adopted a Code of
Ethics.
EXECUTIVE COMPENSATION
In General
As of the date of this Offering Circular, there
are no annuity, pension or retirement benefits proposed to be paid to officers, directors or employees of our company, pursuant to any
presently existing plan provided by, or contributed to, our company.
Compensation Summary
The following table summarizes information concerning
the compensation awarded, paid to or earned by, our executive officers.
| Name and Principal Position |
|
Year Ended 12/31 |
|
Salary
($) |
|
|
Bonus
($) |
|
|
Stock
Awards
($) |
|
|
Option
Awards
($) |
|
|
Non-Equity Incentive
Plan Compensation ($) |
|
|
Non-qualified
Deferred Compensation Earnings
($) |
|
|
All Other Compensation
($)(2) |
|
|
Total
($) |
|
| James D. Brooks (1) |
|
2023 |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
68,220 |
|
|
|
68,220 |
|
| President |
|
2022 |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
6,370 |
|
|
|
6,370 |
|
| Fred Waid |
|
2023 |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
| Former President |
|
2022 |
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
|
|
– |
|
| |
(1) |
On December 30, 2021, Mr. Brooks was appointed as our President and as a director, replacing Mr. Waid who held those positions due to the company’s receivership proceedings as appointed by the court. |
| |
(2) |
Includes non-taxable reimbursable expense of $3,185/month from November 2022 through December
2023 for the lease payment on shared use office space (See CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS). |
Outstanding Option Awards
The following table provides certain information
regarding unexercised options to purchase common stock, stock options that have not vested and equity-incentive plan awards outstanding
as of the date of this Offering Circular, for each named executive officer.
| |
|
Option Awards |
|
Stock Awards |
| Name |
|
Number
of
Securities
Underlying
Unexercised
Options
(#)
Exercisable |
|
Number
of
Securities
Underlying
Unexercised
Options
(#)
Unexercisable |
|
Equity
Incentive
Plan
Awards:
Number
of
Securities
Underlying
Unexercised
Unearned
Options
(#) |
|
Option
Exercise
Price
($) |
|
Option
Expiration
Date |
|
Number
of
Shares
or
Units
of
Stock
That
Have
Not
Vested
(#) |
|
Market
Value
of
Shares
or
Units
of
Stock
That
Have
Not
Vested
($) |
|
Equity
Incentive
Plan
Awards:
Number
of
Unearned
Shares,
Units
or
Other
Rights
That
Have
Not
Vested
(#) |
|
Equity
Incentive
Plan
Awards:
Market
or
Payout
Value
of
Unearned
Shares,
Units
or
Other
Rights
That Have Not
Vested
($) |
| James D. Brooks |
|
– |
|
– |
|
– |
|
N/A |
|
N/A |
|
– |
|
– |
|
– |
|
– |
Outstanding Equity Awards
During the years ended December 31, 2023 and 2022,
our Board of Directors made no equity awards and no such award is pending.
Long-Term Incentive Plans
We currently have no long-term incentive plans.
Director Compensation
Our directors receive no compensation for their
serving as directors of our company.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND MANAGEMENT
The following table sets forth, as of the date
of this Offering Circular, information regarding beneficial ownership of our common stock by the following: (a) each person, or group
of affiliated persons, known by our company to be the beneficial owner of more than five percent of any class of our voting securities;
(b) each of our directors; (c) each of the named executive officers; and (d) all directors and executive officers as a group. Beneficial
ownership is determined in accordance with the rules of the SEC, based on voting or investment power with respect to the securities. In
computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock underlying
convertible instruments, if any, held by that person are deemed to be outstanding if the convertible instrument is exercisable within
60 days of the date hereof. Unless otherwise indicated, the address of each listed person is in care of our company, 424 E. Central Boulevard,
Suite 308, Orlando, Florida 32801.
| | |
Share Ownership | | |
Share Ownership | |
| | |
| Before This Offering | | |
| After This Offering | |
| | |
| Number of Shares | | |
| % | | |
| Number of Shares | | |
| % | |
| | |
| Beneficially | | |
| Beneficially | | |
| Beneficially | | |
| Beneficially | |
| Name of Stockholder | |
| Owned | | |
| Owned(1) | | |
| Owned | | |
| Owned(2) | |
| Common Stock | |
| | | |
| | | |
| | | |
| | |
| Executive Officers and Directors | |
| | | |
| | | |
| | | |
| | |
| James D. Brooks | |
| 331,663,760 | (3) | |
| 74.41% | | |
| 331,663,760 | (3) | |
| 39.22% | |
| Claire Ambrosio | |
| 0 | | |
| 0% | | |
| 0 | | |
| 0% | |
Officers and directors, as a group(2 persons) | |
| 331,663,760 | | |
| 74.41% | | |
| 331,663,760 | | |
| 39.22% | |
| (1) |
Based on 465,728,363
shares outstanding, before this offering. |
| |
|
| (2) |
Based on 865,728,363
shares outstanding, assuming the sale of all of the Company Offered Shares, after this offering. |
| |
|
| (3) |
Of the shares owned
beneficially by Mr. Brooks, 31,663,760 shares are held in the name of Fastbend Holdings Inc., formerly Standard Dental Labs Inc.,
over which Mr. Brooks has voting and investment authority, and is the 63% stockholder of such entity. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Fred Waid, Former Sole Officer and Director
During the year ended December 31, 2020, Intermountain
Fiduciary Services Inc., a company of which Fred Waid, the former Receiver and our former sole officer and director, is also director
and officer (“IFSI”), advanced a total of $1,725 for legal and professional which remained unpaid at December 31, 2020.
During the fiscal year ended December 31, 2021,
IFSI incurred an additional $13,275 in legal and professional fees, respectively. The Company made cash payment in the amount of $15,000
to Intermountain and no additional invoices were received during the year ended December 31, 2022. At December 31, 2022 and December 31,
2021, $0 and $15,000, respectively, is reflected on the balance sheet as accounts payable - related party.
James Brooks, Sole Officer and a Director, Controlling Stockholder
On December 30, 2021, Mr. James Brooks was appointed
the Company’s sole officer and director in place of Mr. Fred Waid. Immediately prior Mr. Brooks became the Company’s controlling stockholder
upon issuance of 300,000,000 shares of common stock for certain debt in the amount of $175,000. On December 23, 2021, the Company entered
into an 8% Convertible Promissory Note with Brooks in the amount of $1,171,727. The convertible promissory note bears interest rate at
8% per annum for a period of 12 months. The holder has the right to convert any or all of the outstanding principal into shares of the
Company’s common stock at a conversion price of $0.001 per share. On March 2, 2023, Mr. Brooks assigned $10,000 of his convertible note
leaving the principal amount of $1,161,727 due and payable.
Prior to maturity, Mr. Brooks agreed to extend the repayment date of
the note to December 31, 2024.
On
November 7, 2022, Mr. Brooks, for the benefit of the Company, entered into a lease agreement
with MAA Parkside for a term of one year commencing January 2023 for approximately 1,500
square feet at a cost of $3,185 per month, plus insurance and utilities, which is reimbursed
by the Company. This location is used exclusively for business purposes as a shared use office
space with overnight accommodations for business travel as an alternative to incurring hotel
expenses.
Interest expenses associated
with the aggregate convertible notes for the year ended December 31, 2023, was $109,557.
Mr. Brooks was paid a total of $198,805 in the
year ended December 31, 2023, against interest owing on the convertible note, leaving a balance owing of $0 as interest payable to Mr.
Brooks at December 31, 2023 (December 31, 2022 - $89,915), which is reflected on the balance sheets as accounts payable – related
party. (Note 7 above).
During the year ended December 31, 2023, the Company
paid and/or accrued $20,000 to Mr. Brooks in respect to his agreement for a monthly stipend entered into in December 2022.
At December 31, 2023 a total of $32,394 (December
31, 2022 - $0) is reflected on the Company’s balance sheets as advances payable – related party with respect to expenses paid
by Mr. Brooks on behalf of the Company which have not yet been reimbursed.
During the year ended December 31, 2022, the Company acquired certain
assets by way of issuance of 31,663,760 common shares of the Company’s restricted, unregistered common stock to Fastbend Holdings, Inc.
(formerly Standard Dental Labs Inc.) a company controlled by Mr. Brooks.
During the year ended December 31, 2022 the Company paid $30,000 to
Mr. Brooks in respect to a monthly expense allowance of $2,500.
Director Independence
We currently act with two directors, consisting
of James D. Brooks and Claire Ambrosio. We have determined that Mr. Brooks is not an “independent director” as defined in NASDAQ
Marketplace Rule 4200(a)(15).
Currently our audit committee consists of all members
of our board of directors. We currently do not have nominating, compensation committees or committees performing similar functions. There
has not been any defined policy or procedure requirements for stockholders to submit recommendations or nomination for directors.
Our board of directors has determined that it does
not have a member of its audit committee who qualifies as an “audit committee financial expert” as defined in as defined in
Item 407(d)(5)(ii) of Regulation S-K.
From inception to present date, we believe that
the members of our audit committee and the board of directors have been and are collectively capable of analyzing and evaluating our financial
statements and understanding internal controls and procedures for financial reporting.
We do not have a standing compensation or nominating
committee. We believe that our directors are capable of analyzing and evaluating our financial statements and understanding internal controls
and procedures for financial reporting. Our directors do not believe that it is necessary to have an audit committee because we believe
that the functions of an audit committee can be adequately performed by the board of directors. In addition, we believe that retaining
additional independent directors who would qualify as an “audit committee financial expert” would be overly costly and burdensome
and is not warranted in our circumstances given the early stages of our development.
LEGAL MATTERS
Certain legal matters with respect to the Offered
Shares offered by this Offering Circular will be passed upon by Centarus Legal Services Ltd., Schaumburg, Illinois. Centarus Legal Services
Ltd. owns no securities of our company.
WHERE YOU CAN FIND MORE INFORMATION
We have filed an offering statement on Form 1-A
with the SEC under the Securities Act with respect to the common stock offered by this Offering Circular. This Offering Circular, which
constitutes a part of the offering statement, does not contain all of the information set forth in the offering statement or the exhibits
and schedules filed therewith. For further information with respect to us and our common stock, please see the offering statement and
the exhibits and schedules filed with the offering statement. Statements contained in this Offering Circular regarding the contents of
any contract or any other document that is filed as an exhibit to the offering statement are not necessarily complete, and each such statement
is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the offering statement.
The offering statement, including its exhibits and schedules, may be inspected without charge at the public reference room maintained
by the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549, and copies of all or any part of the offering statement
may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further
information about the public reference room. The SEC also maintains an Internet website that contains all information regarding companies
that file electronically with the SEC. The address of the site is www.sec.gov.
INDEX TO FINANCIAL STATEMENTS
COSTAS, INC.
TABLE OF CONTENTS
FOR AUDITED
FINANCIAL STATEMENTS
Year ended December
31, 2023 and 2022

Report of Independent Registered Public Accounting
Firm
The Board of Directors and Stockholders of
STANDARD DENTAL LABS INC.
(f/k/a Costas, Inc.)
Opinion on the Financial Statements
We have audited the accompanying balance sheets
of Standard Dental Labs Inc, Formerly Costas, Inc. (the ‘Company’) as of December 31, 2023, and 2022, and the related statements
of operations and changes in stockholders’ equity and cash flows for each of the two years ended December 31, 2023, and 2022, and
the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present
fairly, in all material respects, the financial position of the Company as of December 31, 2023, and 2022, and the results of its operations
and its cash flows for each of the two years ended December 31, 2023, and 2022, in conformity with accounting principles generally accepted
in the United States of America.
Going Concern
The accompanying consolidated financial statements
have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2, the Company suffered an accumulated
deficit of $(16,943,806), net loss of $(412,386). These matters raise substantial doubt about the Company’s ability to continue
as a going concern. Management’s plans with regards to these matters are also described in Note 2 to the financial statements. These
financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from
the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and
that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging,
subjective, or complex judgments. Communication of critical audit matters does not alter in any way our opinion on the financial statements
taken as a whole and we are not, by communicating the critical audit matters, providing separate opinions on the critical audit matter
or on the accounts or disclosures to which they relate as of December 31, 2023, there are no critical audit matter to communicate.
OLAYINKA OYEBOLA & CO.
(Chartered Accountants)
Lagos, Nigeria
We have served as the Company’s auditor
since August 2022.
April 10, 2024
Costas, Inc.
Balance Sheets
| | |
December 31, |
|
| | |
2023 | | |
2022 | |
| ASSETS | |
| | |
| |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 1,953 | | |
$ | 13,123 | |
| Total current assets | |
| 1,953 | | |
| 13,123 | |
| Property and equipment, net | |
| 48,963 | | |
| 70,786 | |
| Intangible assets | |
| 104,103 | | |
| 104,103 | |
| Total assets | |
$ | 155,019 | | |
$ | 188,012 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
$ | 63,350 | | |
$ | 204,741 | |
| Accounts payable - related party | |
| – | | |
| 89,915 | |
| Advance payable - related party | |
| 32,394 | | |
| – | |
| Convertible notes | |
| 415,925 | | |
| 92,246 | |
| Convertible note-shareholder, net | |
| 1,171,727 | | |
| 1,171,727 | |
| Other current liability | |
| 70,000 | | |
| 140,000 | |
| Total liabilities | |
| 1,753,397 | | |
| 1,698,629 | |
| | |
| | | |
| | |
| Stockholders’ equity (deficit): | |
| | | |
| | |
| Common stock, 2,000,000,000 shares authorized, $0.001 par value, 445,728,363 shares issued and
outstanding as of both December 31, 2023 and 2022 | |
| 445,728 | | |
| 445,728 | |
| Additional paid-in capital | |
| 14,899,700 | | |
| 14,545,075 | |
| Accumulated deficit | |
| (16,943,806 | ) | |
| (16,531,420 | ) |
| Total stockholders’ equity (deficit) | |
| (1,598,378 | ) | |
| (1,540,617 | ) |
| Total liabilities and stockholders’ equity (deficit) | |
$ | 155,019 | | |
$ | 188,012 | |
The accompanying notes are an integral part of
these financial statements.
Costas, Inc.
Statements of Operations
| | |
For the Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Revenue | |
$ | 339,466 | | |
$ | 173,329 | |
| Cost of goods sold | |
| 197,860 | | |
| 101,054 | |
| Gross profit | |
| 141,606 | | |
| 72,275 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| Selling and marketing expense | |
| 140,773 | | |
| 74,393 | |
| General and administrative expenses | |
| 83,068 | | |
| 132,416 | |
| Professional fees | |
| 84,314 | | |
| 177,564 | |
| Depreciation | |
| 21,823 | | |
| 7,274 | |
| Total operating expenses | |
| 329,977 | | |
| 391,647 | |
| | |
| | | |
| | |
| Operating loss | |
| (188,371 | ) | |
| (319,372 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Interest expense | |
| (482,249 | ) | |
| (1,339,409 | ) |
| Gain on settlement of debt and liabilities | |
| 258,234 | | |
| 6,884 | |
| Total other income (expense) | |
| (224,015 | ) | |
| (1,332,525 | ) |
| | |
| | | |
| | |
| Net loss | |
$ | (412,386 | ) | |
$ | (1,651,897 | ) |
| | |
| | | |
| | |
| Net loss per common share - basic and diluted | |
$ | (0.00 | ) | |
$ | (0.00 | ) |
| | |
| | | |
| | |
| Weighted average common shares outstanding - basic and diluted | |
| 445,728,363 | | |
| 445,728,363 | |
The accompanying notes are an integral part of these financial statements
Costas, Inc.
Statements of Stockholders’ Equity
| | |
| | |
| | |
| | |
| | |
| |
| | |
Common Stock | | |
Additional
Paid-in | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| Balance at December 31, 2021 | |
| 413,314,603 | | |
$ | 413,315 | | |
$ | 14,333,185 | | |
$ | (14,879,523 | ) | |
$ | (133,023 | ) |
| Beneficial conversion feature associated with convertible notes | |
| – | | |
| – | | |
| 232,140 | | |
| – | | |
| 232,140 | |
| Shares issued under acquisition agreements | |
| 32,413,760 | | |
| 32,413 | | |
| 9,750 | | |
| – | | |
| 42,163 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| (1,651,897 | ) | |
| (1,651,897 | ) |
| Balance at December 31, 2022 | |
| 445,728,363 | | |
| 445,728 | | |
| 14,575,075 | | |
| (16,531,420 | ) | |
| (1,510,617 | ) |
| Beneficial conversion feature associated with convertible notes | |
| – | | |
| – | | |
| 324,625 | | |
| – | | |
| 324,625 | |
| Net loss | |
| – | | |
| – | | |
| – | | |
| (412,386 | ) | |
| (412,386 | ) |
| Balance at December 31, 2023 | |
| 445,728,363 | | |
$ | 445,728 | | |
$ | 14,899,700 | | |
$ | (16,943,806 | ) | |
$ | (1,598,378 | ) |
The accompanying notes are an integral part of
these financial statements.
Costas, Inc.
Statements of Cash Flows
| | |
For the Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (412,386 | ) | |
$ | (1,651,897 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation | |
| 21,823 | | |
| 7,274 | |
| Amortization of debt discount | |
| 372,692 | | |
| 1,238,291 | |
| Gain on settlement of debt and liabilities | |
| (258,234 | ) | |
| (6,884 | ) |
| Changes in certain assets and liabilities : | |
| | | |
| . | |
| Accounts payable – related party | |
| (89,915 | ) | |
| 72,861 | |
| Accounts payable and other liabilities | |
| 7,286 | | |
| 137,870 | |
| Other current liabilities | |
| (95,831 | ) | |
| – | |
| Net cash used in operating activities | |
| (454,565 | ) | |
| (202,485 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from convertible notes | |
| 411,000 | | |
| 232,140 | |
| Advance payable - related party | |
| 32,394 | | |
| – | |
| Repayment to related party | |
| – | | |
| (16,532 | ) |
| Net cash provided by operating activities | |
| 443,394 | | |
| 215,608 | |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| (11,170 | ) | |
| 13,123 | |
| Cash and cash equivalents at beginning of the year | |
| 13,123 | | |
| – | |
| Cash and cash equivalents at end of the year | |
$ | 1,953 | | |
$ | 13,123 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Beneficial conversion feature associated with convertible notes | |
$ | 324,625 | | |
$ | 232,140 | |
| Property and equipment acquired under asset purchase agreement | |
$ | – | | |
$ | 78,060 | |
| Intangible assets acquired under acquisition agreement | |
$ | – | | |
$ | 31,664 | |
| Other current liability acquired under asset purchase agreement | |
$ | – | | |
$ | 140,000 | |
| Common stock issued under asset purchase agreement | |
$ | – | | |
$ | 10,500 | |
The accompanying notes are an integral part of
these financial statements.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
NOTE 1 - NATURE OF OPERATIONS
Historical Information
The Company was originally organized as Costas, Inc.
under the corporate laws of the State of Nevada on December 10, 1998. On July 1,2010, the Company purchased the technology assets of eJob
Resource, Inc. The purchase included eJob Resources’ online job search and posting site to provide a virtual bridge between the
Indian and U.S. technology job markets; all job search technology, which aggregates job posting from many sites, and make them available
via XML, API.
On July 17, 2014, the Company amended its Articles
of Incorporation by approving a 25 for 1 reverse split.
On January 21, 2015, the Company entered into an agreement
with Mr. James Brooks to provide certain services to the Company in exchange for a salary of $10,000 per month and 2,550,000 common shares
of the Company.
In January 2016 the Company purchased 48% of AuthentaTradeLtd,
a Seychelles based corporation, with operations in Cyprus whose function was building a digital currency exchange platform. The remaining
52% was purchased in January 2018.
On September 20, 2017, Mr. James Brooks, a creditor
of the Company was granted a Judgment against the Issuer in the principal amount of $1,114,500 with respect to unpaid salary and non-issuance
of common shares as required under the original 2015 agreement.
In November 2018, the Company changed its business
model to participate in on-line gaming, which operations ceased during the three months period ended March 31, 2019.
On May 20, 2019, the Company announced the completion
of the acquisition of Nano Creaciones Sapi de C.V., a Mexican company. The Company issued a total of 25,000,000 shares as consideration
for the acquisition. The Company has not received sufficient support from the vendors to confirm ownership of this Mexican entity, and
therefore has not included its operations in these financial statements.
On October 21, 2020, Mr. James Brooks, the creditor
of the Company holding a judgment in the principal amount of $1,114,500 filed a motion requesting the appointment of a Receiver over the
Company. By order filed on November 7, 2020, the Eighth Judicial District Court for Clark County, Nevada appointed Frederick P. Waid as
Receiver for the Company in Case No. A-17-749977-B. Notice of entry of that order was filed on November 9, 2020. Mr. Waid became the sole
officer and director of the Company. The Receiver was not provided any historical accounting documents from former management as part
of the proceedings. As a result of the aforementioned actions, the Court approved an amended opening balance sheet for the Company as
of December 31, 2019, which reflects the debt owing to Mr. Brooks, previously omitted, including accrued interest as well as any other
approved amounts while eliminating any outstanding debts not approved during the receivership.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
On March 25, 2021, the Receiver filed a motion with
the Court requesting approval to appoint Mr. Brooks as an officer and director of the Company and to increase the authorized capital of
the Company and subsequently to issue sufficient common and preferred shares on terms to be finalized between the Receiver and Mr. Brooks,
to settle a total of $175,000 of outstanding debt. Further, subsequent to the March 25, 2021, order, the Receiver sought and received
approval from the Court to eliminate certain unsupported assets, outstanding payables and convertible loans on the financial statements
of the Company as at December 31, 2019. The Receiver further placed an administrative hold on a total of 26,500,000 shares issued in 2019
for the acquisition of Nano Creaciones Sapi de C.V., a Mexican company, and as consideration for services purported to be rendered, due
to the fact that there was no verifiable support for the completion of the acquisition or the provision of services.
Current Information
On January 26, 2022, with an effective date of December
23, 2021, three hundred million (300,000,000) shares of the Company’s common stock were issued to Mr. James Brooks pursuant to a
Court Order entered in the Eighth District Judicial Court, Clark County, Nevada, Case No. A-17-749977-B, resulting in a change of control
of the Company. The issuance of 300,000,000 shares to Mr. Brooks was issued in partial settlement of debt owed to Mr. Brooks. On December
30, 2021, Mr. Brooks was named the sole officer and director of the Company. On February 9, 2022, an Order was entered in the Eighth Judicial
District Judicial Court, Clark County, Nevada, Case No. A-17-749977D by the Appointed Receiver of the Company, Frederick Waid, terminating
the receivership for the Company. Concurrently, the Company changed its operating address to 424 E Central Blvd, Suite 308, Orlando, Florida
32801.
On May 6, 2022, the Company entered into a formal
acquisition agreement with Standard Dental Labs Inc. (“SDL”), a Wyoming corporation controlled by the Company’s CEO,
James Brooks, in order to acquire certain assets including: (i) a ready to implement business model and platform for the identification
and acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package created under SDL,
including logo, website, presentation materials and corporate name. Under the terms of the acquisition agreement, assets valued at $75,900
was acquired through the issuance of a total of 31,663,760 shares of the Company’s unregistered, restricted common stock to SDL.
With the conclusion of this acquisition, the Company intends to operate in the dental lab industry, paving the way for future acquisitions
and consolidations in the industry. The assets acquired from SDL will allow the Company to immediately facilitate the acquisition of small
to medium sized dental labs, of which there are thousands in the United States.
On August 15, 2022 the Company completed a definitive
agreement to acquire the assets of Smile Dental Management LLC (Formerly Prime Dental Lab, LLC) (“Smile Dental”), an Orlando-based
dental lab in operation since 2012. Total consideration was paid to the shareholders of Smile Dental in a combination of cash and registered
shares for the assets, which includes all equipment, customer relationships, and associated revenue. The Company commenced operations
in the dental lab business effective September 1, 2022.
On August 17, 2022 the Company’s board of directors
and majority shareholder increased the Company’s authorized share capital from 1.25bn to 2bn shares of common stock.
During the year ended December 31, 2022, the Company
entered into certain 8% interest convertible note agreements (the “CPNs” with various individual investors for total gross
proceeds of $232,140. Under the terms of the agreements, holders of the CPNs may convert the principal balance of the notes to unregistered,
restricted shares of the Company’s common stock at $0.001 at any time with three (3) days written notice.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
On December 30, 2022 the Company filed a Registration
on Form S-1 with the United States Securities and Exchange Commission for the benefit of certain selling stockholders. (Ref:
Note 12). On October 13, 2023 the Company withdrew its application to the SEC.
During the quarter ended March 31, 2023 the Company
and Smile Dental executed an addendum to the definitive agreement with Smile Dental (Ref: Note 5(2)) (the “Smile Amendment”)
whereunder the Cash Consideration as part of Purchase Price was revised so that the first instalment was released immediately upon execution
of the Smile Amendment, or March 24, 2023, and the second Cash Instalment shall be payable on the date that is no later than seven (7)
calendar days subsequent to the receipt of proceeds from the first payment under the Purchase Agreement with World Amber
(Ref: Note 12).
On April 20, 2023 Ms. Claire Ambrosio, 57, was appointed
to the Board of Directors of the Company. Ms. Ambrosio is a member of The State Bar of California
and has practiced as a lawyer in California for the past 22 years. She has served as the Vice President of Legal for 4 Over, LLC, a trade
printer, from 2022 to present, and as General Counsel for True Family Enterprises from 2019 through 2022. She holds a law degree from
Southwestern University, School of Law (1991) and has been a member of the bar in California since 1996. Ms. Ambrosio holds an LL.M.,
Loyola University Chicago School of Law, Chicago, Illinois in Healthcare and Compliance (2021).
Current operations are focused on our dental lab where
we manufacture and produce several types of dental prosthetics. We provide dental lab services to more than 50 dental practices and produce
approximately 500 dental prosthetics each month.
NOTE 2 – GOING CONCERN
The Company’s financial
statements are prepared in accordance with generally accepted accounting principles applicable to a going concern. This contemplates the
realization of assets and the liquidation of liabilities in the normal course of business. The Company has recently acquired assets including
branding and a detailed business plan to facilitate the acquisition of small to medium sized dental
labs, as well as its first dental lab operation. Presently the Company does not have a source of revenue sufficient to cover all
of its operating costs. While we have recently commenced revenue generating operations, the Company’s sole officer and
director is currently providing capital for operational shortfalls as needed by the Company, and we continue to raise proceeds from the
sale of convertible notes having received gross proceeds of $411,000 in the year ended December 31, 2023, there remains substantial doubt
about our ability to continue as a going concern. As at December 31, 2023, the Company has $1,953 cash on hand, and substantial debt.
As we continue to implement our business plan, the Company may continue to be dependent upon financing from our sole officer and director,
and the raising of additional capital through placement of our common stock or debt financing. There can be no assurance that the Company
will be successful in either situation in order to continue as a going concern.
The ability of the Company
to continue as a going concern is dependent on attaining profitable operations. There are no assurances that the Company will be able
to meet its obligations, raise funds or conclude additional acquisitions of identified businesses. Further upon acquisition of any target
businesses there is no guarantee these operations will reach profitability. The financial statements do not include any adjustments relating
to the recoverability and classification of recorded asset amounts, or amount and classification of liabilities that might cause results
from this uncertainty.
Other factors
Factors which may impact the Company’s ongoing
operations include inflation, the recent war in the Ukraine, ongoing supply chain issues as a result of the recent Covid-19 pandemic,
climate change and others. These events may have serious adverse impact on domestic and foreign economies which may impact the Company’s
operations as a result of a variety of factors including the potential for reduced consumer spending. The Company is unable to predict
the ongoing impact of these factors on the Company’s financial operations.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
NOTE 3 - USE OF ESTIMATES IN THE PREPARATION OF FINANCIAL STATEMENTS
The preparation of financial statements in conformity
with Generally Accepted Accounting Principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts
of assets and liabilities, and disclosure of contingent assets and liabilities, at the date of these financial statements, and the reported
amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
NOTE 4 – SUMMARY OF ACCOUNTING POLICIES
Fiscal Year End
The Company has selected December 31 as its fiscal year end.
Basis of Presentation
The accompanying financial
statements have been prepared in accordance with generally accepted accounting principles (US GAAP). In the opinion of management,
all adjustments considered necessary for a fair presentation have been included. All such adjustments are of a normal recurring
nature.
Cash and Cash Equivalents
The Company considers all highly liquid investments
with an original maturity of three months or less to be cash equivalents.
Beneficial Conversion Feature
For conventional convertible debt where the rate
of conversion is below market value, the Company records any “beneficial conversion feature” (“BCF”) intrinsic
value as additional paid in capital and related debt discount. When the Company records a BCF, the relative fair value of the BCF
is recorded as a debt discount against the face amount of the respective debt instrument. The discount is amortized over the life of the
debt. If a conversion of the underlying debt occurs, a proportionate share of the unamortized amounts is immediately expensed.
Property
and Equipment
Property
and equipment are recorded at cost. Depreciation on property and equipment are determined using the straight-line method over the one
to eight year estimated useful lives of the assets.
Intangible Assets
During the year ended December 31, 2022, the Company
has acquired (i) a ready to implement business model and platform for the identification and acquisition of small to medium sized dental
labs in the United States, and (ii) a fully developed branding package including logo, website, presentation materials and corporate name.
A total of $31,663 has been capitalized in respect to these assets. The Company has also recognized assets for customer relationships
in the amount of $72,440 in respect to a recent asset purchase agreement with Smile Dental, whereunder we acquired assets to commence
operation of our first dental lab. The Company will review these intangible assets for impairment at a minimum of once per year or whenever
events or changes in circumstances suggest a need for evaluation. There is no impairment expense for the intangible assets
as of the year ended December 31, 2023, and 2022.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
Impairment
Our long-lived
assets are subject to an impairment test if there is an indicator of impairment. The carrying value and ultimate realization of these
assets is dependent upon our estimates of future earnings and the benefits that we expect to generate from their use. If our expectations
of future results and cash flows are significantly diminished, other long-lived assets may be impaired and the resulting charge to operations
may be material. When we determine that the carrying value of intangibles or other long-lived assets may not be recoverable based upon
the existence of one or more indicators of impairment, we use the projected undiscounted cash flow method or realizable value to determine
whether an impairment exists, and then measure the impairment using discounted cash flows.
Revenue Recognition
The Company
applies ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes revenue from the sales of products
by applying the following steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract;
(3) determine the transaction price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize
revenue when each performance obligation is satisfied.
The Company
recognizes revenue when the earnings process is complete and persuasive evidence of an arrangement exists. This generally occurs at the
point in time when a purchased product has been delivered to a customer from our lab facility, at which time both title and the risks
and rewards of ownership are transferred to and accepted by the customer, and the selling price has been collected.
Inventory
Inventories, if
maintained, consist of work-in-progress inventory or replacement parts on hand in order to complete customer orders.
Warranty
We do not
record warranty liabilities at the time of sale for the estimated costs that may be incurred under the terms of the applicable limited
warranty as all component parts are covered by our respective industry suppliers. Our products are custom created for the individual client,
and therefore we have no formal return policy or money back guarantee, however, if a product is determined to be defective, we will deliver
a replacement unit to meet expected customer service terms. We assess the need for warranty and return liabilities at each report date.
Cost of Sales
Cost of sales includes actual product cost, labor,
and allocated overheard, which is applied on a per unit basis.
Accounts Receivable
Accounts receivable is trade related. The Company’s
management has established an allowance for bad debt based upon accounts receivable that are more than one year past due. Management reviews
the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current
economic trends and changes in customer payment patterns to evaluate the necessary for reserves for bad debt. Reserves, if required, are
recorded on management’s best estimate of collection. At December 31, 2023 and 2022 there were no outstanding accounts receivable.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
Basic and Diluted Loss Per Share
The Company computed basic and diluted loss per
share amounts pursuant to the ASC 260 “Earnings per Share.” There are no potentially dilutive shares outstanding and, accordingly,
dilutive per share amounts have not been presented in the accompanying statements of operations.
Potential common stock consists of the incremental
common stock issuable upon the exercise of convertible notes (using the if-converted method). The table below reflects the potentially
dilutive securities at each reporting period, which have not been included in the computation of diluted net loss per share due to
their anti-dilutive effect:
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Convertible notes (principal balance) at $0.001 per share | |
| 3,321,400,000 | | |
| 1,403,867,310 | |
| Convertible notes (principal balance) at $0.004 per share | |
| 377,500,000 | | |
| – | |
| Convertible notes (principal balance) at $0.005 per share | |
| 220,000,000 | | |
| – | |
| | |
| 3,918,900,000 | | |
| 1,403,867,310 | |
Income Taxes
Income taxes are recognized in accordance with
ASC 740, “Income Taxes”, whereby deferred income tax liabilities or assets at the end of each period are determined using
the tax rate expected to be in effect when the taxes are actually paid or recovered. A valuation allowance is recognized on deferred tax
assets when it is more likely than not that some or all of these deferred tax assets will not be realized.
Recent Accounting Pronouncements
In August
2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging –
Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own
Equity. The new guidance, among other things, simplifies the accounting for certain financial instruments with characteristics of liabilities
and equity, including convertible instruments, and amends existing earnings-per-share (“EPS”) guidance by requiring that an
entity use the if-converted method when calculating diluted EPS for convertible instruments. ASU 2020-06 is effective for public business
entities that meet the definition of an SEC filer, excluding entities eligible to be smaller reporting companies as defined by the SEC,
for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments
are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. The Company plans
to adopt the new guidance effective January 1, 2024.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
NOTE 5 – ASSET PURCHASE AGREEMENTS
(1) On May 6, 2022, the Company entered into a
formal acquisition agreement with Standard Dental Labs Inc. (“SDL”), a Wyoming corporation controlled by the Company’s
CEO, James Brooks, to acquire certain assets including: (i) a ready to implement business model and platform for the identification and
acquisition of small to medium sized dental labs in the United States, and (ii) a fully developed branding package created under SDL,
including logo, website, presentation materials and corporate name. Under the terms of the acquisition agreement, assets valued at $75,900
were acquired through the issuance of a total of 31,663,760 shares of the Company’s unregistered, restricted common stock to SDL.
The transaction occurred under common control and as a result, the issued shares were valued at par value, or $0.001 per share, and a
total of $31,663 was recorded as intangible assets on the Company’s balance sheet.
(2) On August 15, 2022 the Company (the “Acquiror”)
announced the completion of a definitive agreement to acquire the assets of Smile Dental Management LLC (“Smile Dental”)(Formerly
Prime Dental Lab, LLC), an Orlando-based dental lab in operation since 2012. The Purchased Assets consisted of: all client contracts for
existing Smile Dental clients; certain physical assets of Smile Dental including all dental lab equipment, furniture, computers and other
office equipment; the assumption of certain contracts, equipment leases and office leases (if any); certain employees and management of
Smile Dental as determined by the Acquiror to be retained and/or contracted by the Acquiror; and specifically the right to continue to
use the name “Prime Dental Lab LLC” along with certain other rights, trademarks, intellectual property and intangible assets
of the Seller in a definitive way. The Purchase Price consisted of 750,000 unregistered, restricted Common Shares (the “Consideration
Shares”) of the Acquiror (the “Share Consideration) plus additional cash consideration in the amount of $140,000.00 (the “Cash
Consideration”) payable in two (2) equal instalments of seventy thousand ($70,000.00) dollars (each a “Cash Instalment”).
The first Cash Instalment shall be paid by the Acquiror to Smile Dental no later than fifteen (15) calendar days after receipt by the
Acquiror of a Notice of Effect from the Securities and Exchange Commission of a Form S-1 Registration Statement (the “First Cash
Instalment”). The second Cash Instalment of seventy thousand ($70,000.00) dollars shall be paid by the Acquiror to Smile Dental
on the date that is no later than ninety (90) calendar days subsequent to the payment of the First Cash Instalment (the “Second
Cash Instalment”).
During the quarter ended March 31, 2023, the Company
and Smile Dental executed an addendum to the definitive agreement with Smile Dental (Ref: Note 5(2)) (the “Smile Amendment”)
whereunder the Cash Consideration as part of Purchase Price was revised so that the first instalment was agreed to be released immediately
upon execution of the Smile Amendment, or March 24, 2023, and the second Cash Instalment shall be payable on
the date that is no later than seven (7) calendar days subsequent to the receipt of proceeds from the first payment under the Purchase
Agreement with World Amber (Ref: Note 12).
The Share Consideration is subject to a lock-up agreement for a term of twenty-four
months from the issuance date, whereunder Smile Dental shall be entitled to a release of 12.5% of the total Consideration Shares each
quarter (93,750 shares) provided certain minimum quarterly revenue targets are achieved. Further, the Company has agreed to include such
Consideration Shares in any registration statement filed, and in the event that the Company decides to approve and complete a share consolidation
or share rollback within twelve (12) months after the date of the issuance of the Consideration Shares, such shares shall be protected
from such share consolidation action (on a one-time basis).
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
The Company allocated the acquired assets on the
Company’s balance sheets as of the date of closing as Property and Equipment and Intangible Assets at fair market value. Assets
acquired were as follows:
| 750,000 shares of common stock | |
$ | 10,500 | |
| Cash consideration – other current liability | |
| 140,000 | |
| Total consideration purchase cost | |
$ | 150,500 | |
| | |
| | |
| Allocation: | |
| | |
| Property and equipment | |
$ | 78,060 | |
| Customer relationships | |
| 72,440 | |
| Total purchased assets | |
$ | 150,500 | |
The purchase accounting for the acquisition of
assets from Smile Dental includes an analysis of all available information as at the acquisition date.
NOTE 6 – SUBCONTRACTOR AGREEMENT
Concurrent with the closing of the acquisition of
certain assets from Smile Dental (formerly Prime Dental Lab, LLC) (see Note 5(2)) on August 31, 2022, as amended in April 2023, the Company
entered into a subcontractor agreement with Smile Dental for the provision of certain services including labor, materials, supplies and
manufacturing expertise with respect to the servicing of the Company’s client list for dental prosthetics and orthotics. Smile Dental
shall have available for the Company’s exclusive use certain acquired assets in order to facilitate the provision of finished products.
As consideration under the terms of the agreement, Smile Dental, shall be entitled to retain all gross profits from the sale of such finished
goods, net the cost of use of the production equipment, as management fees. The agreement has an initial term of up to two (2) years and
either the Company or Smile Dental may terminate the agreement with six (6) months’ written notice, in order to provide the Company
sufficient time to identify, hire and train a suitable replacement. For the fiscal years ended December 31, 2023, and 2022, the Company
paid Smile Dental $171,329 and $341,807, respectively, as amounts due and owing under the Subcontractor Agreement.
NOTE 7 – JUDGMENT PAYABLE AND CONVERTIBLE
NOTE
During fiscal 2017, Mr. James Brooks (“Brooks”),
a creditor of the Company, obtained a judgment in the principal amount of $1,114,500. Previously, on January 21, 2015, the Company entered
into an agreement with Mr. Brooks whereunder he would provide certain services to the Company in exchange for a salary of $10,000 per
month and 2,550,000 common shares of the Company. Under the terms of this contract, Mr. Brooks was owed $120,000 in salary and 2,550,000
shares, which consideration was not provided by the Company in accordance with the contract terms. On January 23, 2017 Mr. Brooks filed
a complaint in respect to amounts payable and applicable damages.
The Company failed to respond to the action,
and on August 2, 2017, Mr. Brooks filed a motion for entry of default judgment. On September 6, 2017, the court determined the unpaid
2,550,000 common shares had a market value of $994,500 at the time they were originally deliverable to Mr. Brooks. In addition to the
value of the unpaid shares, unpaid salary of $120,000 resulted in a judgment of $1,114,500. Concurrently, the court granted post-judgment
interest pursuant to Nevada Revised Statute 17.130 which provides that when there is no express contract in writing, interest must be
allowed at a rate equal to the prime rate at the largest bank in Nevada, as ascertained by the Commissioner of Financial Institution
on January 1 or July 1 as the case may be, immediately preceding the date of the transaction, plus 2 percent. The rate must be adjusted
accordingly on each January 1 and July 1 thereafter until the judgment is satisfied. As a result, interest applied on the judgment over
the applicable periods was as follows:
| January 1, 2021 |
5.25% |
July 1, 2020 |
5.25% |
January 1, 2020 |
6.75% |
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
On March 25, 2021, the Court approved the first
proposed settlement of a portion of Brooks’ debt, in the amount of One Hundred Seventy-Five Thousand Dollars ($175,000) (the “Settlement
Debt”) to be paid via the issuance of certain common shares of the Company. On December 6, 2021, Brooks entered into certain Debt
Assignment and Purchase Agreements with several third parties in the accumulated amount of $70,000. (See Note 9). On December 23, 2021,
the Company entered into an 8% Convertible Promissory Note with Brooks, our then sole officer and director, in the amount of $1,171,727.
Concurrently, three hundred million (300,000,000) shares of the Company’s common stock were issued to Brooks pursuant to a Court
Order entered in the Eighth District Judicial Court, Clark County, Nevada, Case No. A-17-749977-B. The Company valued the 300,000,000
shares at the closing price of the Company’s stock as traded on the OTC Markets on the date of issuance and recorded a loss on the
extinguishment of debt of $10,025,000.
The convertible promissory note bears interest
rate at 8% per annum for a period of 12 months. The holder has the right to convert any or all of the outstanding principal into shares
of the Company’s common stock at a conversion price of $0.001 per share. The beneficial conversion
feature associated with the note and realized on issuance date totaled $1,171,727, which amount is being amortized over the term of the
note, or 12 months. Prior to maturity, Mr. Brooks agreed to extend the repayment date of the note to December 31, 2023.
Interest payable included in accounts payable-
related party and the principal outstanding balance of the debt as at each period-end are as follows:
| | |
Interest | | |
Shareholder | | |
Convertible | | |
| |
| | |
Payable | | |
Loan | | |
Note | | |
Total | |
| Balance December 31, 2021 | |
$ | 2,054 | | |
$ | – | | |
$ | 25,682 | | |
$ | 27,736 | |
| Interest expense on convertible note | |
| 93,738 | | |
| – | | |
| – | | |
| 93,738 | |
| Repayments to interest expense | |
| (5,877 | ) | |
| – | | |
| – | | |
| (5,877 | ) |
| Amortized Debt Discount | |
| – | | |
| – | | |
| 1,146,045 | | |
| 1,146,045 | |
| Balance, December 31, 2022 | |
| 89,915 | | |
| – | | |
| 1,171,727 | | |
| 1,261,642 | |
| Advance from shareholder | |
| – | | |
| 139,769 | | |
| – | | |
| 139,769 | |
| Debt assignment and purchase agreement | |
| – | | |
| – | | |
| (10,000 | ) | |
| (10,000 | ) |
| Interest expense on convertible note | |
| 108,890 | | |
| – | | |
| – | | |
| 108,890 | |
| Repayments to interest expense | |
| (198,805 | ) | |
| – | | |
| – | | |
| (198,805 | ) |
| Repayments to shareholder | |
| | | |
| (107,374 | ) | |
| | | |
| (107,374 | ) |
| Balance, December 31, 2023 | |
$ | – | | |
$ | 32,394 | | |
$ | 1,161,727 | | |
$ | 1,194,121 | |
On March 2, 2023, Mr. Brooks assigned $10,000
from his convertible note to a shareholder of the Company. The assigned amount has the same terms and conditions as the Brooks note described
above. Ref Note 8(2) below.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
NOTE 8 – CONVERTIBLE NOTES
(1)
8% convertible notes
During the year ended December 31, 2022, the company
issued convertible promissory notes in the principal amount of $232,140 to several investors bearing interest at 8% per annum for a period
of 12 months. The holders have the right to convert any or all of the outstanding principal into shares of the Company’s common
stock at a conversion price of $0.001 per share. The beneficial conversion feature associated with
the note and realized on issuance date totaled $232,140, which amount is being amortized over the term of the note, or 12 months.
During the
year ended March 31, 2023, the company issued convertible promissory notes in the principal amount of $50,000 to several investors
bearing interest at 8% per annum for a period of 12 months. The holders have the right to convert any or all of the outstanding principal
into shares of the Company’s common stock at a conversion price of $0.001 per share. The beneficial
conversion feature associated with the note and realized on issuance date totaled $50,000, which amount is being amortized over the term
of the note, or 12 months.
During the
year ended December 31, 2023, the company issued convertible promissory notes in the principal amount of $160,000 to several investors
bearing interest at 8% per annum for a period of 12 months. Of the notes received, holders issuing $50,000 have the right to convert any
or all of the outstanding principal into shares of the Company’s common stock at a conversion price of $0.001 per share and holders
issuing $100,000 have the right to convert any or all of the outstanding principal into shares of the Company’s common stock at
a conversion price of $0.005 per share. The beneficial conversion feature associated with the 2023
notes and realized on issuance date totaled $160,000, which amount is being amortized over the term of the note, or 12 months.
In 2023,
the Company determined with the noteholders that interest should not be accrued on the notes and reversed the previously accrued interest
payable.
| | |
Interest | | |
Convertible | | |
| |
| | |
Payable | | |
Note | | |
Total | |
| Balance, December 31, 2021 | |
$ | – | | |
$ | – | | |
$ | – | |
| Proceeds | |
| – | | |
| 232,140 | | |
| 232,140 | |
| Unamortized debt discount | |
| – | | |
| (232,140 | ) | |
| (232,140 | ) |
| Interest expense on convertible notes | |
| 7,380 | | |
| – | | |
| 7,380 | |
| Amortized debt discount | |
| – | | |
| 92,246 | | |
| 92,246 | |
| Balance, December 31, 2022 | |
| 7,380 | | |
| 92,246 | | |
| 99,626 | |
| Proceeds | |
| – | | |
| 160,000 | | |
| 160,000 | |
| Unamortized debt discount | |
| – | | |
| (160,000 | ) | |
| (160,000 | ) |
| Interest expense on convertible notes | |
| 21,689 | | |
| – | | |
| 21,689 | |
| Reversal of interest payable | |
| (29,068 | ) | |
| – | | |
| (29,068 | ) |
| Amortized debt discount | |
| – | | |
| 183,644 | | |
| 183,644 | |
| Balance, December 31, 2023 | |
$ | – | | |
$ | 275,890 | | |
$ | 275,890 | |
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
(2)
0% convertible notes
During the year
ended December 31, 2023, the Company issued convertible promissory notes in the principal amount of $251,000 to several investors bearing
interest at 0% per annum for a period of 12 months. The holders have the right to convert any or all of the outstanding principal into
shares of the Company’s common stock at a conversion price of $0.001 per share as to $100,000 of the proceeds and $0.004 per share
as to $151,000 of the proceeds. The beneficial conversion feature associated with the note and realized
on issuance date totaled $64,625, which amount is being amortized over the term of the note, or 12 months.
| | |
Convertible | | |
| |
| | |
Note | | |
Total | |
| Balance, December 31, 2022 | |
$ | – | | |
$ | – | |
| Proceeds | |
| 251,000 | | |
| 251,000 | |
| Unamortized debt discount | |
| (164,625 | ) | |
| (164,625 | ) |
| Amortized debt discount | |
| 53,660 | | |
| 53,660 | |
| Balance, December 31, 2023 | |
$ | 140,035 | | |
$ | 140,035 | |
(3)
Other convertible notes
On March 2, 2023, Mr. Brooks assigned $10,000
in principal from his outstanding convertible note to a shareholder of the Company. The assigned amount has the same terms and conditions
as the Brooks note described above. (Ref Note 7)
The amount is included in convertible note –
shareholder, net on the balance sheet as of December 31, 2023.
NOTE 9 – DEBT ASSIGNMENTS AND PURCHASE
AGREEMENT
On March 2, 2023, Mr. James Brooks, entered into
a Debt Assignment and Purchase Agreement with a shareholder of the Company and assigned a total of $10,000 to Mr. O’Connor under
the same terms and conditions of his convertible note described under Note 7 and 8 (above).
NOTE 10 – RELATED PARTY TRANSACTIONS
James Brooks, sole officer and director, controlling
shareholder
On December 30, 2021, Mr. James Brooks was appointed
the Company’s sole officer and director in place of Mr. Fred Waid. Immediately prior Mr. Brooks became the Company’s controlling
shareholder upon issuance of 300,000,000 shares of common stock for certain debt in the amount of $175,000. Concurrently the Company and
Mr. Brooks entered into a convertible note with respect to amounts payable totaling an accumulated $1,171,727. On March 2, 2023, Mr. Brooks
assigned $10,000 of his convertible note leaving the principal amount of $1,161,727 due and payable.
Interest expenses associated
with the aggregate convertible notes for the year ended December 31, 2023 was $109,557.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
Mr. Brooks was paid a total of $198,805 in the
year ended December 31, 2023 against interest owing on the convertible note, leaving a balance owing of $0 as interest payable to Mr.
Brooks at December 31, 2023 (December 31, 2022 - $89,915), which is reflected on the balance sheets as accounts payable – related
party. (Note 7 above).
During the year ended December 31, 2023, the Company
paid and/or accrued $20,000 to Mr. Brooks in respect to his agreement for a monthly stipend entered into in December 2022.
At December 31, 2023 a total of $32,394 (December
31, 2022 - $0) is reflected on the Company’s balance sheets as advances payable – related party with respect to expenses paid
by Mr. Brooks on behalf of the Company which have not yet been reimbursed.
During the year ended December 31, 2022, the Company
acquired certain assets by way of issuance of 31,663,760 common shares of the Company’s restricted, unregistered common stock to
a company controlled by Mr. Brooks. (see Note 5(1)).
NOTE 11 – COMMON STOCK
The Company has authorized a total of 2,000,000,000
shares of common stock, $0.001 par value.
On May 6, 2022, 31,663,760 shares were issued
in respect to an asset purchase agreement. (see Note 5(1)).
On August 31, 2022, 750,000 shares were issued
in respect to an asset purchase agreement. (see Note 5(2)).
There were no shares issued during the period
ended December 31, 2023. At December 31, 2023, and December 31, 2022, there was a total of 445,728,363 shares issued and outstanding,
respectively.
NOTE 12 – PURCHASE AGREEMENT WORLD AMBER
CORP.
On November 22, 2022, we entered into a purchase
agreement (the “Purchase Agreement”), and a registration rights agreement (the “Registration Rights Agreement”)
with World Amber Corp. (“World Amber”), a Nevada corporation, pursuant to which World Amber committed to purchase up
to $2,500,000 of our common stock. Under the terms and subject to the conditions of the Purchase Agreement, we have the obligation, to
sell to World Amber, and World Amber is obligated to purchase up to $2,500,000 of shares of our common stock.
Future sales of common stock under the Purchase
Agreement, if any, will be subject to certain limitations, and may occur from time to time, over the 24-month period commencing on the
date that a registration statement is filed with the Securities and Exchange Commission (the “SEC”) pursuant to the
Registration Rights Agreement, and is declared effective by the SEC and a final prospectus in connection therewith is filed and the other
conditions set forth in the Purchase Agreement are satisfied (such date on which all of such conditions are satisfied, the “Commencement
Date”).
After the Commencement Date, for every month over
the term of the Purchase Agreement, the Company has the right, in its sole discretion, to direct World Amber to purchase up to 346,667
shares of common stock per business day, at $0.30 per share (each, a “Regular Purchase”). In each case, World Amber’s
maximum commitment in any single Regular Purchase may not exceed $104,000.
Costas, Inc.
Notes to Financial Statements
For the Period Ended December 31, 2023, and
2022
Pursuant to the terms of the Purchase Agreement,
in no event may the Company issue or sell to World Amber any shares of our common stock under the Purchase Agreement which, when
aggregated with all other shares of common stock then beneficially owned by the World Amber and its affiliates, would result in the beneficial
ownership by World Amber and its affiliates of more than 9.99% of the then issued and outstanding shares of common stock (the
“Beneficial Ownership Limitation”).
The Purchase Agreement and the Registration Rights
Agreement contain customary representations, warranties, agreements, conditions and indemnification obligations of the parties. The Company
has the right to terminate the Purchase Agreement at any time, at no cost or penalty.
The Company filed its initial registration statement
on December 30, 2022, and subsequently, several amendments thereto. The Company withdrew the registration statement in October 2023.
The Company and World Amber are currently negotiating an amendment to the Purchase Agreement.
NOTE 13 - SUBSEQUENT EVENTS
The Company has evaluated subsequent events from
the balance sheet date through the date that the financial statements were issued and determined that there are no additional subsequent
events to disclose.
PART III – EXHIBITS
Index to Exhibits
SIGNATURES
Pursuant to the requirements of Regulation
A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has
duly caused this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Orlando,
State of Florida, on August 1, 2024.
| |
STANDARD DENTAL LABS INC.
By: /s/ James D. Brooks
James D. Brooks
President |
This Offering Statement has been signed by the
following persons in the capacities and on the dates indicated.
| |
By: /s/ James D. Brooks
James D. Brooks
President [Principal Executive Officer}, Chief
Financial Officer [Principal Financial Officer],
Secretary and Director |
| |
|
| |
By: /s/ Claire Ambrosio
Claire Ambrosio
Director |
Exhibit 6.1
SUBCONTRACT AGREEMENT
This Subcontract Agreement (the “Agreement”)
is made and effective this August 31st, 2022,
| BETWEEN: | STANDARD DENTAL LABS INC. (the “Contractor”), a corporation organized and existing under the laws of the State of
Nevada, with its head office located at: 424 E Central Blvd., Orlando, FL 32801 |
| AND: | SMILE DENTAL MANAGEMENT LLC (F/K/A PRIME DENTAL LAB LLC) (the “Subcontractor”), a corporation organized and existing
under the laws of the [STATE/PROVINCE], with its head office located at: 1008 N Pine Hills Road, Orlando, FL 32808 |
WHEREAS Contractor has entered into an Asset Purchase Agreement, henceforth
“The Prime Contract” with PRIME DENTAL LAB LLC, to perform in accordance with various contract documents and specifications
certain work requested by the Contractor’s clients, henceforth “Clients”, and/or to furnish labor, materials, supplies,
labor and/or goods required to manufacture the following dental prosthetics and orthodontics:
The manufacturing of various dental prosthetics, henceforth to be known
as “The Project”, and to be manufactured at the business address of the contractor located at 1008 N Pine Hills Road, Orlando,
FL 32808, and
WHEREAS Contractor desires to retain Subcontractor to perform certain contract
work in accordance with various contract documents and specifications and/or to furnish labor, materials, supplies, labor and/or goods
for The Project;
NOW THEREFORE Contractor and Subcontractor agree as follows:
Subcontractor shall be employed as an independent contractor and shall
provide and furnish all labor, materials, tools, supplies, equipment, services, facilities, supervision, and administration necessary
for the proper and complete performance and acceptance of the following portions of the work, hereinafter
“the Subcontract Work”, for the Project, together with such other
portions of the drawings, specifications and addendum as related thereto:
SEE EXHIBIT A: List of Products and Services.
In consideration of Subcontractor’s performance of this Subcontract, and
at the times and subject to the terms and conditions hereinafter set forth, Contractor shall pay to Subcontractor the total sum of mutually
agreed upon amount relative to the work performed and billed, hereinafter “subcontract price.” Said subcontract price is dependent
upon the conditions set forth in Exhibit A being met. Should said conditions not be met, the subcontract amount shall be modified accordingly.
The Special Conditions to Subcontract are incorporated in this Subcontract
as though fully set forth herein. Subcontractor hereby acknowledges receipt of the Special Conditions.
| 4. | COMMUNICATION AND NOTICE |
| a. | All communications between Subcontractor and Client shall include Contractor, and use the medium provided by Contractor. |
| b. | Subcontractor shall furnish Contractor with periodic progress reports as required by Contractor, including status of material, equipment,
manpower and submittal. |
| c. | Subcontractor shall provide proof of completion and proof of delivery for each job, and furnished to Contractor upon demand. |
| d. | Subcontractor shall be deemed to have received notice of a fact, request, order, or demand when notified, either orally or in writing,
or 5 days after written notice is sent by registered or certified mail addressed to Subcontractor’s last known place of business, whichever
is sooner. |
| e. | Contractor shall be deemed to have received notice of a fact, request, or demand 10 days after written notice is sent by registered
or certified mail addressed to the following address: |
1008 N Pine Hills Road, Orlando, FL 32808
| 5. | GOVERNING LAW AND RULES OF CONSTRUCTION |
| a. | The validity, interpretation, and performance of this Subcontract shall be governed by the laws of the jurisdiction where the Project
is located. |
| b. | Titles, captions, or headings to any provision, article, etc., shall not limit the full contents of the same. These articles have
the full force and effect as if no titles existed. |
| c. | If any term or provision of this Subcontract is determined to be invalid, it shall not affect the validity and enforcement of the
remaining terms and provisions of this Subcontract. |
| d. | This contract shall be binding upon and inure to the benefit of the respective successors, assigns, representatives, and heirs of
the parties herein. |
This Subcontract shall only be amended or modified by written document
executed by authorized representatives of Contractor and Subcontractor. This Subcontract supersedes all prior representations made by
Contractor.
Any and all disputes or claims between the Contractor and the Subcontractor
arising out of this Subcontract shall be resolved by submission of the same to FLORIDA CIRCUIT-CIVIL MEDIATOR SOCIETY, for resolution
by binding arbitration according to Florida’s Rules of Arbitration. In so agreeing the parties expressly waive their right to a jury trial,
if any, on these issues and further agree that the award of the arbitrator shall be final and binding upon them as though rendered by
a court of law and shall be enforceable in any court having
jurisdiction over the same.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as
of the day and year first above written.
| SUBCONTRACTOR |
|
CONTRACTOR |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| Signed - John Kim – Prime Dental Lab LLC |
|
James Brooks – CEO Standard Dental Labs Inc. |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| Print Name and Title |
|
Print Name and Title |
Exhibit A
List of Products and Services
| Name |
|
Material |
|
Labor |
|
Sales Price |
| PFZ (Layer tech) |
|
$35.00 |
|
$25.00 |
|
$109.00 |
| EMAX1 |
|
$35.00 |
|
$25.00 |
|
$139.00 |
| E-MAX |
|
$35.00 |
|
$25.00 |
|
$129.00 |
| Anterior Bruxir |
|
$35.00 |
|
$25.00 |
|
$109.00 |
| |
|
|
|
|
|
|
| N-PFM |
|
$25.00 |
|
$25.00 |
|
$89.00 |
| PFM |
|
$25.00 |
|
$25.00 |
|
$99.00 |
| PFM |
|
$40.00 |
|
$25.00 |
|
$100.00 |
| PFM |
|
$25.00 |
|
$25.00 |
|
$109.00 |
| |
|
|
|
|
|
|
| Zirconia (Bruxer) |
|
$30.00 |
|
$25.00 |
|
$79.00 |
| Zirconia (Bruxir) |
|
$30.00 |
|
$25.00 |
|
$89.00 |
| |
|
|
|
|
|
|
| All Ceramic |
|
$30.00 |
|
$25.00 |
|
$99.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$89.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$99.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$109.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$129.00 |
| All Ceramic Crown |
|
$30.00 |
|
$25.00 |
|
$99.00 |
| |
|
|
|
|
|
|
| Post |
|
$10.00 |
|
$20.00 |
|
$40.00 |
| Post |
|
$10.00 |
|
$20.00 |
|
$50.00 |
| |
|
|
|
|
|
|
| Metal Occlusion |
|
$10.00 |
|
$10.00 |
|
$30.00 |
| Metal collar |
|
$10.00 |
|
$5.00 |
|
$20.00 |
| Metal lingual |
|
$10.00 |
|
$10.00 |
|
$30.00 |
| |
|
|
|
|
|
|
| Full Gold Crown |
|
$25.00 |
|
$25.00 |
|
$99.00 |
| Repair |
|
$15.00 |
|
$50.00 |
|
$89.00 |
| Bridge Connection |
|
$0.00 |
|
$5.00 |
|
$15.00 |
| Porcelain Veneer |
|
$40.00 |
|
$30.00 |
|
$139.00 |
| Fit to Partial |
|
$0.00 |
|
$10.00 |
|
$20.00 |
| Add Clasp |
|
$0.00 |
|
$10.00 |
|
$20.00 |
| Rest |
|
$0.00 |
|
$10.00 |
|
$20.00 |
| Soft Tissue |
|
$5.00 |
|
$10.00 |
|
$20.00 |
| |
|
|
|
|
|
|
| Flipper |
|
$30.00 |
|
$30.00 |
|
$109.00 |
| |
|
|
|
|
|
|
| Rush |
|
$0.00 |
|
$0.00 |
|
$30.00 |
| Late Fee |
|
$0.00 |
|
$0.00 |
|
$35.00 |
| |
|
|
|
|
|
|
| Screw Implant |
|
$100.00 |
|
$70.00 |
|
$299.00 |
| Implnat (Por) |
|
$10.00 |
|
$10.00 |
|
$30.00 |
Exhibit 6.2
SUBCONTRACT AGREEMENT
This Subcontract Agreement (the “Agreement”)
is made and effective this August 31st, 2022,
| BETWEEN: | STANDARD DENTAL LABS INC. (the “Contractor”), a corporation organized and existing under the laws of the State of
Nevada, with its head office located at: 424 E Central Blvd., Orlando, FL 32801 |
| AND: | SMILE DENTAL MANAGEMENT LLC (F/K/A PRIME DENTAL LAB LLC) (the “Subcontractor”), a corporation organized and existing
under the laws of the [STATE/PROVINCE], with its head office located at: 1008 N Pine Hills Road, Orlando, FL 32808 |
WHEREAS Contractor has entered into an Asset Purchase Agreement, henceforth
“The Prime Contract” with PRIME DENTAL LAB LLC, to perform in accordance with various contract documents and specifications
certain work requested by the Contractor’s clients, henceforth “Clients”, and/or to furnish labor, materials, supplies,
labor and/or goods required to manufacture the following dental prosthetics and orthodontics:
The manufacturing of various dental prosthetics, henceforth to be known
as “The Project”, and to be manufactured at the business address of the contractor located at 1008 N Pine Hills Road, Orlando,
FL 32808, and
WHEREAS Contractor desires to retain Subcontractor to perform certain contract
work in accordance with various contract documents and specifications and/or to furnish labor, materials, supplies, labor and/or goods
for The Project;
NOW THEREFORE Contractor and Subcontractor agree as follows:
Subcontractor shall be employed as an independent contractor and shall
provide and furnish all labor, materials, tools, supplies, equipment, services, facilities, supervision, and administration necessary
for the proper and complete performance and acceptance of the following portions of the work, hereinafter
“the Subcontract Work”, for the Project, together with such other
portions of the drawings, specifications and addendum as related thereto:
SEE EXHIBIT A: List of Products and Services.
In consideration of Subcontractor’s performance of this Subcontract, and
at the times and subject to the terms and conditions hereinafter set forth, Contractor shall pay to Subcontractor the total sum of mutually
agreed upon amount relative to the work performed and billed, hereinafter “subcontract price.” Said subcontract price is dependent
upon the conditions set forth in Exhibit A being met. Should said conditions not be met, the subcontract amount shall be modified accordingly.
The Special Conditions to Subcontract are incorporated in this Subcontract
as though fully set forth herein. Subcontractor hereby acknowledges receipt of the Special Conditions.
| 4. | COMMUNICATION AND NOTICE |
| a. | All communications between Subcontractor and Client shall include Contractor, and use the medium provided by Contractor. |
| b. | Subcontractor shall furnish Contractor with periodic progress reports as required by Contractor, including status of material, equipment,
manpower and submittal. |
| c. | Subcontractor shall provide proof of completion and proof of delivery for each job, and furnished to Contractor upon demand. |
| d. | Subcontractor shall be deemed to have received notice of a fact, request, order, or demand when notified, either orally or in writing,
or 5 days after written notice is sent by registered or certified mail addressed to Subcontractor’s last known place of business, whichever
is sooner. |
| e. | Contractor shall be deemed to have received notice of a fact, request, or demand 10 days after written notice is sent by registered
or certified mail addressed to the following address: |
1008 N Pine Hills Road, Orlando, FL 32808
| 5. | GOVERNING LAW AND RULES OF CONSTRUCTION |
| a. | The validity, interpretation, and performance of this Subcontract shall be governed by the laws of the jurisdiction where the Project
is located. |
| b. | Titles, captions, or headings to any provision, article, etc., shall not limit the full contents of the same. These articles have
the full force and effect as if no titles existed. |
| c. | If any term or provision of this Subcontract is determined to be invalid, it shall not affect the validity and enforcement of the
remaining terms and provisions of this Subcontract. |
| d. | This contract shall be binding upon and inure to the benefit of the respective successors, assigns, representatives, and heirs of
the parties herein. |
This Subcontract shall only be amended or modified by written document
executed by authorized representatives of Contractor and Subcontractor. This Subcontract supersedes all prior representations made by
Contractor.
Any and all disputes or claims between the Contractor and the Subcontractor
arising out of this Subcontract shall be resolved by submission of the same to FLORIDA CIRCUIT-CIVIL MEDIATOR SOCIETY, for resolution
by binding arbitration according to Florida’s Rules of Arbitration. In so agreeing the parties expressly waive their right to a jury trial,
if any, on these issues and further agree that the award of the arbitrator shall be final and binding upon them as though rendered by
a court of law and shall be enforceable in any court having
jurisdiction over the same.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as
of the day and year first above written.
| SUBCONTRACTOR |
|
CONTRACTOR |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| Signed - John Kim – Prime Dental Lab LLC |
|
James Brooks – CEO Standard Dental Labs Inc. |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| Print Name and Title |
|
Print Name and Title |
Exhibit A
List of Products and Services
| Name |
|
Material |
|
Labor |
|
Sales Price |
| PFZ (Layer tech) |
|
$35.00 |
|
$25.00 |
|
$109.00 |
| EMAX1 |
|
$35.00 |
|
$25.00 |
|
$139.00 |
| E-MAX |
|
$35.00 |
|
$25.00 |
|
$129.00 |
| Anterior Bruxir |
|
$35.00 |
|
$25.00 |
|
$109.00 |
| |
|
|
|
|
|
|
| N-PFM |
|
$25.00 |
|
$25.00 |
|
$89.00 |
| PFM |
|
$25.00 |
|
$25.00 |
|
$99.00 |
| PFM |
|
$40.00 |
|
$25.00 |
|
$100.00 |
| PFM |
|
$25.00 |
|
$25.00 |
|
$109.00 |
| |
|
|
|
|
|
|
| Zirconia (Bruxer) |
|
$30.00 |
|
$25.00 |
|
$79.00 |
| Zirconia (Bruxir) |
|
$30.00 |
|
$25.00 |
|
$89.00 |
| |
|
|
|
|
|
|
| All Ceramic |
|
$30.00 |
|
$25.00 |
|
$99.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$89.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$99.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$109.00 |
| All Ceramic (layer tech) |
|
$35.00 |
|
$25.00 |
|
$129.00 |
| All Ceramic Crown |
|
$30.00 |
|
$25.00 |
|
$99.00 |
| |
|
|
|
|
|
|
| Post |
|
$10.00 |
|
$20.00 |
|
$40.00 |
| Post |
|
$10.00 |
|
$20.00 |
|
$50.00 |
| |
|
|
|
|
|
|
| Metal Occlusion |
|
$10.00 |
|
$10.00 |
|
$30.00 |
| Metal collar |
|
$10.00 |
|
$5.00 |
|
$20.00 |
| Metal lingual |
|
$10.00 |
|
$10.00 |
|
$30.00 |
| |
|
|
|
|
|
|
| Full Gold Crown |
|
$25.00 |
|
$25.00 |
|
$99.00 |
| Repair |
|
$15.00 |
|
$50.00 |
|
$89.00 |
| Bridge Connection |
|
$0.00 |
|
$5.00 |
|
$15.00 |
| Porcelain Veneer |
|
$40.00 |
|
$30.00 |
|
$139.00 |
| Fit to Partial |
|
$0.00 |
|
$10.00 |
|
$20.00 |
| Add Clasp |
|
$0.00 |
|
$10.00 |
|
$20.00 |
| Rest |
|
$0.00 |
|
$10.00 |
|
$20.00 |
| Soft Tissue |
|
$5.00 |
|
$10.00 |
|
$20.00 |
| |
|
|
|
|
|
|
| Flipper |
|
$30.00 |
|
$30.00 |
|
$109.00 |
| |
|
|
|
|
|
|
| Rush |
|
$0.00 |
|
$0.00 |
|
$30.00 |
| Late Fee |
|
$0.00 |
|
$0.00 |
|
$35.00 |
| |
|
|
|
|
|
|
| Screw Implant |
|
$100.00 |
|
$70.00 |
|
$299.00 |
| Implnat (Por) |
|
$10.00 |
|
$10.00 |
|
$30.00 |
Exhibit 6.3
LOCK-UP AGREEMENT
September 1, 2022
TO:Costas, Inc. (d/b/a Standard Dental Labs
Inc.) (the “Company”)
RE:Lock-up Agreement pursuant to Asset
Acquisition
1.The
undersigned acknowledges that this lock-up agreement (the “Lock-Up Agreement”) is being entered into and delivered
to the Company pursuant to Section 3.1 of the asset purchase agreement dated August 15, 2022 (the “Asset Purchase Agreement”)
between the undersigned and the Company.
2.All
capitalized terms not otherwise defined herein have the meaning given to them in the Asset Purchase Agreement.
3.In
consideration of the issuance of the Share Consideration portion of the Gross Consideration to the undersigned pursuant to the Acquisition,
and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the undersigned agrees that
during the period beginning from the date of issuance of any Share Consideration of the Company to the undersigned pursuant to the Asset
Purchase Agreement (the “Security Issuance Date”) and ending on each quarterly release date (for each applicable quarterly
release of securities, the “Quarterly Lock-Up Release”) during the period that ends twenty-four (24) months following
the Security Issuance Date (the “Restricted Period”) that the undersigned shall not, directly or indirectly, offer,
sell, transfer, pledge, hypothecate, assign, grant an option or right to purchase, make any short sale, enter into any swap, forward,
hedge or any other agreement or arrangement to transfer the economic consequences of or alter the economic exposure to, or otherwise dispose
of, monetize or deal with, or publicly announce any intention to do any of the foregoing, whether through the facilities of a stock exchange,
by private placement or otherwise (the “Lock-Up Restriction”) unless, in each case: (a) the prior written consent of
the Company has been obtained; OR (b) such Share Consideration has satisfied any applicable Quarterly Revenue Targets AND (c) has been
released from the Lock-Up Restrictions set forth herein in accordance with the schedule below:
| Date |
Quarterly Lock-Up Release |
|
The Closing Date:
September 1, 2022 (unless extended) |
0% (0 shares)
|
|
The date that is three (3) months following
the Closing Date:
December 1, 2022 (unless extended) |
12.5% (93,750 shares)
|
|
The date that is six (6) months
following the Closing Date:
March 1, 2023 (unless extended) |
12.5% (93,750 shares)
|
|
The date that is nine (9) months
following the Closing Date:
June 1, 2023 (unless extended) |
12.5% (93,750 shares)
|
|
The date that is twelve (12) months
following the Closing Date:
September 1, 2022 (unless extended) |
12.5% (93,750 shares)
|
|
The date that is fifteen (15) months
following the Closing Date:
December 1, 2023 (unless extended) |
12.5% (93,750 shares) |
|
The date that is eighteen (18) months
following the Closing Date:
March 1, 2024 (unless extended) |
12.5% (93,750 shares) |
|
The date that is twenty-one (21) months
following the Closing Date:
June 1, 2024 (unless extended) |
12.5% (93,750 shares)
|
|
The date that is twenty-four (24) months
following the Closing Date:
September 1, 2024 (unless extended) |
12.5% (93,750 shares)
|
4.Section
3 above shall not apply to: (a) transfers occurring by operation of law, provided, in each case, that any such transferee shall first
execute a lock-up agreement in substantially the form hereof covering the remainder of the Restricted Period for such transferred Share
Consideration, (b) transfers made pursuant to a bona fide take-over bid or similar transaction made to all holders of common shares
of the Company, including without limitation, a merger, arrangement or amalgamation, involving a change of control of the Company and
provided that in the event the take-over or acquisition transaction is not completed, the Consideration Securities shall remain subject
to the restrictions contained in this Lock-Up Agreement, or (c) transfers or other dealings in respect of which the Company has provided
prior written consent, such consent not to be unreasonably withheld.
5.The
Parties recognize that the Company currently has a substantial number of shares outstanding and, in the event that the Company decides
to validly approve and complete a share consolidation or share rollback within twelve (12) months after the date of this Agreement, the
Share Consideration issued in this transaction to the undersigned shall be protected from such share consolidation action (on a one time
basis) and the total number of shares issued as Share Consideration in this transaction shall remain as outlined herein.
6.The
undersigned represents and warrants that it has full power, capacity and authority to enter into this Lock-Up Agreement and understands
that the Company is relying upon this Lock-Up Agreement in proceeding with Closing. The undersigned further understands that this Lock-Up
Agreement is irrevocable and shall be binding upon the undersigned’s legal representatives, successors, and permitted assigns, and
shall enure to the benefit of the Company and its legal representatives, successors and assigns.
7.The
undersigned hereby authorizes the Company and its transfer agent to decline to make any transfer of the Share Consideration if such transfer
would constitute a violation or breach of this Lock-Up Agreement and hereby agrees and consents to the entry of stop transfer restrictions,
or other equivalent measures, with the Company’s transfer agent and registrar, against the transfer of the Share Consideration except
in compliance with this Lock-Up Agreement.
8.This
Lock-Up Agreement will be governed by the laws of the State of Nevada and the federal laws of the United States applicable therein and
may be executed by facsimile or PDF signature and as so executed shall constitute an original.
[Rest of page intentionally left blank; Signature
page follows]
DATED as of the date first written above.
| |
PRIME DENTAL LAB LLC
|
| |
By: |
|
| |
|
Name: |
| |
|
Title: |
Exhibit 6.4
ASSET PURCHASE AGREEMENT
between
PRIME DENTAL LAB LLC
(as Seller)
and
COSTAS, INC.
(D/B/A STANDARD DENTAL LABS INC.)
(as Acquiror)
Dated as of August 16, 2022
TABLE OF CONTENTS
| ARTICLE I PURCHASE AND SALE OF ASSETS; SALES TAXES; DEFINITIONS |
1 |
| 1.1 |
Transfer of Purchased Assets. |
1 |
| 1.2 |
Excluded Assets. |
2 |
| 1.3 |
Assignment of Contracts, Rights Etc. |
2 |
| 1.4 |
Further Assurances. |
3 |
| 1.5 |
Sales Tax. |
3 |
| 1.6 |
Defined Terms. |
3 |
| ARTICLE II LIABILITIES |
3 |
| 2.1 |
Liabilities Being Assumed. |
3 |
| 2.2 |
Liabilities Not Being Assumed. |
3 |
| ARTICLE III CONSIDERATION |
4 |
| 3.1 |
Purchase Price. |
4 |
| 3.2 |
Earn-Out Provisions. |
5 |
| 3.3 |
Allocation of Purchase Price. |
6 |
| 3.4 |
Tax Consequences. |
6 |
| ARTICLE IV CLOSING |
6 |
| 4.1 |
Closing. |
6 |
| ARTICLE V REPRESENTATIONS AND WARRANTIES OF THE SELLER |
7 |
| 5.1 |
Organization; Good Standing; Qualification and Power. |
7 |
| 5.2 |
Authority; Noncontravention; Consents. |
7 |
| 5.3 |
Title to Purchased Assets. |
7 |
| 5.4 |
Transferred Intellectual Property. |
7 |
| 5.5 |
Agreements, No Defaults. |
8 |
| 5.6 |
Litigation Etc. |
8 |
| 5.7 |
Compliance; Permits. |
8 |
| 5.8 |
Taxes. |
8 |
| 5.9 |
Warranties. |
9 |
| 5.10 |
Books and Records. |
9 |
| 5.11 |
Financial Information. |
9 |
| 5.12 |
Brokers. |
9 |
| 5.13 |
Environmental Matters. |
9 |
| 5.14 |
Bankruptcy Etc. |
10 |
| 5.15 |
No Purchased Assets Held by Affiliates. |
10 |
| 5.16 |
Equipment and Property |
10 |
| 5.17 |
Contracts and Commitments. |
10 |
| 5.18 |
Securities Law Matters. |
10 |
| 5.19 |
Relationship with Affiliates. |
11 |
| 5.20 |
Material Adverse Effect. |
11 |
| 5.21 |
Undisclosed Liabilities. |
11 |
| 5.22 |
Compliance with OFAC. |
12 |
| ARTICLE VI REPRESENTATIONS AND WARRANTIES OF THE ACQUIROR |
12 |
| 6.1 |
Organization; Good Standing. |
12 |
| 6.2 |
Authority, Noncontravention; Consents. |
12 |
| 6.3 |
Intentionally Deleted. |
12 |
| 6.4 |
Brokers. |
13 |
| ARTICLE VII CLOSING DELIVERIES |
13 |
| 7.1 |
The Seller’s Deliveries. |
13 |
| 7.2 |
The Acquiror’s Deliveries. |
14 |
| ARTICLE VIII INDEMNIFICATION |
14 |
| 8.1 |
Indemnification Generally; Etc. |
14 |
| 8.2 |
Assertion of Claims. |
15 |
| 8.3 |
Limitations on Indemnification. |
15 |
| 8.4 |
Notice and Defense of Third Person Claims. |
16 |
| 8.5 |
Survival of Representations and Warranties and Covenants. |
17 |
| 8.6 |
Right of Setoff. |
17 |
| 8.7 |
Exclusive Remedy. |
17 |
| ARTICLE IX POST-CLOSING COVENANTS AND OTHER AGREEMENTS |
17 |
| 9.1 |
Transfer of Purchased Assets. |
17 |
| ARTICLE X MISCELLANEOUS PROVISIONS |
17 |
| 10.1 |
No Third Party Beneficiaries. |
17 |
| 10.2 |
Entire Agreement. |
18 |
| 10.3 |
Successors and Assigns. |
18 |
| 10.4 |
Amendment; Waiver. |
18 |
| 10.5 |
Fees and Expenses. |
18 |
| 10.6 |
Notices. |
18 |
| 10.7 |
Governing Law; Arbitration. |
19 |
| 10.8 |
Interpretation; Construction. |
19 |
| 10.9 |
Incorporation of Exhibits. |
20 |
| 10.10 |
Independence of Covenants and Representations and Warranties. |
20 |
| 10.11 |
Counterparts; Electronic Signatures |
20 |
ANNEX AND EXHIBITS
[NTD: TO BE
INSERTED IF UPON CLOSING AS APPLICABLE]
INDEX OF DEFINED TERMS
| TERM |
Defined on Page |
| |
|
| Acquiror |
1 |
| Acquisition |
1 |
| Affiliate |
1 |
| Agreement |
29 |
| Assigned Contracts |
1 |
| Assumed Liabilities |
4 |
| Business |
1 |
| Business Day |
1 |
| Cap |
23 |
| Closing |
8 |
| Closing Date |
8 |
| Code |
1 |
| Competing Business |
16 |
| Contract |
1 |
| Control |
1 |
| Deductible |
23 |
| Earnout Dispute Notice |
7 |
| Earnout Statement |
7 |
| Encumbrances |
1 |
| Environmental, Health and Safety Laws |
1 |
| Excluded Assets |
2 |
| Excluded Liabilities |
4 |
| Financial Statements |
12 |
| Fraud |
2 |
| Fundamental Documents |
2 |
| Fundamental Representations |
22 |
| GAAP |
2 |
| Governmental Entity |
2 |
| Hazardous Materials |
2 |
| Indemnified Persons |
2 |
| Indemnifying Persons |
2 |
| Intellectual Property |
2 |
| Intellectual Property Rights |
2 |
| Inventory |
2 |
| Knowledge |
29 |
| Law |
2 |
| Liability |
3 |
| Litigation Expense |
3 |
| Losses |
3 |
| Material Adverse Effect |
3 |
| Non-Fundamental Representations |
22 |
| OFAC |
16 |
| Orders |
3 |
| Parent |
1 |
| SDL Stock |
3 |
| Parties |
1 |
| Party |
1 |
| Patents |
3 |
| Permits |
3 |
| Person |
3 |
| Proceedings |
4 |
| Products |
4 |
| Purchased Assets |
1 |
| Purchaser Indemnified Persons |
4 |
| Purchaser Indemnifying Persons |
4 |
| Purchaser Losses |
4 |
| Purchasing Parties |
1 |
| Purchasing Party |
1 |
| Related Documents |
19 |
| Representatives |
4 |
| Sanction |
16 |
| Securities Act |
14 |
| Seller |
1 |
| Seller Indemnified Persons |
4 |
| Seller Indemnifying Persons |
4 |
| Seller Losses |
4 |
| Shareholder |
4 |
| Survival Date |
25 |
| Tax |
4 |
| Tax Return |
5 |
| Taxes |
4 |
| Taxing Authority |
5 |
| Third Person Claim |
24 |
| Trade Names |
5 |
| Trademarks |
5 |
| Trading Price |
5 |
| Transaction Taxes |
4 |
| Transferred Permits |
2 |
ASSET PURCHASE AGREEMENT,
dated as of August 16, 2022, between Prime Dental Labs LLC., a Florida limited liability company (“Seller”); and Costas,
Inc. (d/b/a Standard Dental Labs Inc.), a Nevada corporation (“SDL” or the “Acquiror”). The Acquiror
and Seller are sometimes individually referred to herein as a “Party”, and collectively as the “Parties”.
PREAMBLE
The Acquiror wishes to acquire
from Seller, and Seller wishes to transfer to Acquiror certain assets of Seller in consideration for the Purchase Price as a combination
of Acquiror’s Class A Common Shares and certain cash or other consideration as otherwise outlined herein (the “Acquisition”).
ARTICLE
I
PURCHASE AND SALE OF ASSETS; SALES TAXES; DEFINITIONS
1.1
Transfer of Purchased Assets.
On the terms and subject to
the conditions contained in this Agreement, at the Closing the Seller shall sell, transfer, convey and assign to the Acquiror, free and
clear of all Encumbrances, and the Acquiror shall purchase and acquire from Seller, all of Seller’s right, title and interest in,
to and under substantially all of Seller’s assets, properties, interests in properties and rights, whether tangible or intangible
and whether real, personal or mixed, as the same shall exist immediately prior to the Closing, but excluding the Excluded Assets (collectively,
the “Purchased Assets”).
The Purchased Assets shall
include, but are not limited to: all client contracts for existing PDL clients; certain physical assets of the Seller that include all
dental lab equipment, furniture, computers and other office equipment; the assumption of certain contracts, equipment leases, and office
leases; certain employees and management of the Seller as determined to be retained by the Acquiror; and specifically the right to continue
to use the name “Prime Dental Lab LLC” along with certain other rights, trademarks, intellectual property and intangible assets
of the Seller. More specifically and subject to Section 1.2, the Purchased Assets include, but are not limited to the following
assets, properties and rights of the Seller as of the Closing Date:
(a)
all Accounts Receivable of the Seller, as listed on Schedule 1.1(a);
(b)
all Inventory of the Seller, as listed on Schedule 1.1(b), other than Excluded Assets of the Seller as listed on Schedule
1.2;
(c)
all deposits, advances, pre-paid expenses and credits relating to prepaid packages;
(d)
all furniture, fixtures, machinery, equipment, computer hardware and software, and all other tangible assets and personal property
of the Seller as listed on Schedule 1.1(d);
(e)
all rights and benefits of the Seller under the Contracts as listed on Schedule 1.1(e) (the “Assigned Contracts”);
(f)
all goodwill, going concern value, patents, patent applications, patent rights, copyrights, copyright applications, Websites, URL’s,
domain names, methods, know-how, software, technical documentation, computer programs, engineering drawings, product concepts, and ideas
under development, processes, process charts, procedures, inventions, trade secrets, trademarks, trade names, trade dress, logos, business
names (and specifically the right to continue to use the name “Prime Dental Lab LLC”), telephone numbers, confidential information,
franchises, customer lists, customer files, , marketing materials, advertising records, advertising rights with respect to all media,
service marks, service names, registered user names, technology, research records, data, designs, plans, drawings, manufacturing know-how
and formulas, whether patentable or unpatentable, and other intellectual or proprietary rights (and all rights thereto and applications
therefor), including the Intellectual Property;
(g)
all rights to causes of action, lawsuits, judgments, claims, and demands of any nature available to or being pursued by the Seller,
including all rights and claims against manufacturers and vendors of the Seller, relating to the subject matter of this Agreement, whether
arising by way of counterclaim or otherwise;
(h)
all rights in and under all express or implied guarantees, warranties, representations, covenants, indemnities, and similar rights
in favor of the Seller related to the subject matter of this Agreement;
(i)
all Permits, licenses or similar rights to the extent that they are assignable, including those listed on Schedule 1.1(i)
(the “Transferred Permits”); and
(j)
all information, files, databases, correspondence, records, data, plans, reports, and Contracts, including any and all information
and records relating to investment, insurance and other current and past customers, client files, customer, supplier, price and mailing
lists, business contacts, and investment, underwriting, and claims files together with all usual and customary records in connection therewith,
and all accounting or other books and records of Seller in whatever media retained or stored, including computer programs and disks.
1.2
Excluded Assets.
The Purchased Assets exclude,
and the Acquiror shall not purchase or acquire hereunder, any right, title or interest in, to and under any of the excluded assets of
the Seller as outlined on Schedule 1.2 (collectively, the “Excluded Assets”).
(a)
all ownership and other rights with respect to Seller employee benefit plans;
(b)
all rights of Seller under any excluded Contracts listed on Schedule 1.2;
(c)
all accounts payable outstanding;
(d)
any Permit or license that by its terms is not transferable to Acquiror;
(e)
the charter documents, minute books, stock ledgers, Tax Returns, books of account and other constituent records relating to the
corporate organization of the Seller; and
(f)
any other items listed on Schedule 1.2.
1.3
Assignment of Contracts and Rights.
Notwithstanding anything in
this Agreement to the contrary, this Agreement does not constitute an agreement or an attempted agreement to sell, transfer, sublease
or assign any Assigned Contract (or any claim or right or any benefit arising thereunder or resulting therefrom) if the attempted transfer,
sublease or assignment thereof, without the consent of any other party thereto, would constitute a breach thereof or in any way affect
the rights of the Acquiror or the Seller thereunder. The Seller shall use its commercially reasonable efforts to obtain the consent of
the other party to any Assigned Contract to the transfer, sublease or assignment thereof to the Acquiror in all cases in which such consent
is required for the transfer, sublease or assignment of any such Assigned Contract. If any such consent is not obtained and the Closing
occurs, the Seller shall use its commercially reasonable efforts to provide for the Acquiror the benefits of such Assigned Contract.
1.4
Further Assurances.
The Seller shall, from time
to time after the Closing, upon the request of Acquiror, do, execute, acknowledge and deliver, and cause to be done, executed, acknowledged
or delivered, all such further acts, deeds, assignments, transfers, conveyances or assurances as may be reasonably required to transfer,
assign, convey, grant and confirm to the Acquiror, or to aid and assist in the reducing to possession by the Acquiror of the Purchased
Assets, or to vest in the Acquiror good and marketable title to the Purchased Assets.
1.5
Sales Tax.
The Seller shall pay to applicable
Taxing Authorities any and all sales Taxes, use Taxes, transfer Taxes, license Taxes, documentary charges, recording fees or similar Taxes,
charges or fees (other than income Taxes of the Seller) that may become payable in connection with the sale, transfer and conveyance of
the Purchased Assets to the Acquiror (the “Transaction Taxes”). The Seller and the Acquiror shall coordinate with each
other the filing of any forms required in connection with such Taxes, charges or fees. The parties shall reasonably cooperate to minimize
the amount of any Transaction Taxes imposed in connection with the sale of the Purchased Assets to the Acquiror.
1.6
Defined Terms.
Certain capitalized terms used in this Agreement
are defined on Annex I attached hereto.
ARTICLE
II
LIABILITIES
2.1
Liabilities Being Assumed.
On the terms and subject to
the conditions contained in this Agreement, effective as of the Closing, and from and after the Closing, the Acquiror shall pay or assume,
perform and discharge when due, the following Liabilities of the Seller (collectively, the “Assumed Liabilities”):
(a)
Liabilities accruing after the Closing Date under each Assigned Contract.
2.2
Liabilities Not Being Assumed.
The Acquiror is not assuming
and shall not be obligated to pay or satisfy, and the Seller shall remain responsible for, any Liabilities other than the Assumed Liabilities
(collectively, the “Excluded Liabilities”), including the following:
(a)
any Liabilities relating to or arising out of the operation or conduct of the Business;
(b)
any accounts or notes payable of the Seller;
(c)
any Liabilities accruing under any Assigned Contract on or prior to the Closing Date;
(d)
any Liabilities arising out of any claim, irrespective of the legal theory asserted, related to the development, commercialization,
manufacture, packaging, import, marketing, distribution, sale or use of the Products or the use of the Purchased Assets;
(e)
any Liabilities arising out of any claim, irrespective of the legal theory asserted, related to the operation of the Business;
(f)
any Liabilities related to Taxes payable in connection with the operation of the Business, the ownership, leasing, possession or
use of the Purchased Assets or the sale of Products;
(g)
any Liabilities for pollution or contamination of the environment or damage to natural resources arising out of or related to the
conduct of the Business, including any manufacture, generation, refining, processing, distribution, use, sale, treatment, recycling, receipt,
storage, disposal, transportation, handling, emission, discharge, leaching, release or threatened release of any Hazardous Material in
connection with the conduct of the Business;
(h)
any Liabilities under any Environmental, Health and Safety Laws arising out of or related to the conduct of the Business; and
(i)
any Liabilities of the Seller to the employees of the Seller, including deferred compensation, and any Liabilities of the Seller
to such employees arising from the termination of such employees’ employment with the Seller.
(j)
any Liabilities of the Seller based upon Seller’s acts or omission occurring after the Closing.
ARTICLE
III
CONSIDERATION
3.1
Purchase Price.
The Purchase Price will be up to $700,000.00 (the
“Gross Consideration”), consisting of the equivalent trading value of $560,000.00 of the Class A Common Shares of the
Acquiror. The equivalent per share value assigned to each Class A Common Share to be issued shall be determined based on the final closing
trading price on the date of the Closing (the “Share Consideration) plus additional cash consideration in the amount of $140,000.00
(the “Cash Consideration”) payable as outlined herein, plus the Stock Option Consideration, which Gross Consideration is payable
as described herein.
Provided however; the timing of issuance, the
pricing, and the number of shares to be authorized and issued as Share Consideration shall also be determined in accordance with the various
regulations restricting the issuance of shares by a public company, including but not limited to, any restrictions imposed by the Securities
Exchange Commission (the “SEC”), the OTC Exchange (or any subsequent stock exchange as applicable) (the “Exchange”)
and any other regulatory authorities as appropriate.
Further, the Acquiror will provide the equivalent
of up to an additional ten (10%) percent of the total Share Consideration to employees of the Seller (as determined on the Closing Date)
in the form of a grant of stock option shares, also subject to the terms and conditions of the Acquiror’s approved Stock Option
Plan and any restrictions imposed by the SEC, the Exchange and any other regulatory authorities as appropriate (the “Stock Option
Consideration”).
Subject to any restrictions imposed by the SEC,
the Exchange and any other regulatory authorities, the number of shares issuable as Share Consideration shall be based on the final closing
trading price of the Acquiror’s shares on the date of the Closing. The Share Consideration shall be specifically subject to the
further Lock-Up restrictions outlined below in Section 3.1(c) and elsewhere in this Agreement.
The Cash Consideration shall be payable in two
(2) equal instalments of seventy thousand ($70,000.00) dollars (each a “Cash Instalment”). The first instalment of
seventy thousand ($70,000.00) dollars shall be paid by the Acquiror to the Seller at the Closing (the “First Cash Instalment”).
The second instalment of seventy thousand ($70,000.00) dollars shall be paid by the Acquiror to the Seller on the date that is six (6)
months subsequent to the Closing (the “Second Cash Instalment”).
The number of shares issuable as Stock Option
Consideration shall also be based on the final closing trading price of the Acquiror’s shares on the date of the Closing. Each qualified
employee of the Seller that is determined to be eligible participate in the Acquiror’s stock option plan shall receive their allocated
portion of the Stock Option Consideration at the Closing, subject to the pricing, vesting, and other terms of the Acquiror’s stock
option plan and further subject to any restrictions imposed by the SEC, the Exchange and any other regulatory authorities as appropriate
For clarity, the sum of the Cash Consideration,
the Share Consideration and the Stock Option Consideration is collectively known as the Gross Consideration.
a.
Closing. Upon Closing, the Acquiror will issue to Seller Share Consideration equal in value to five hundred and sixty
thousand ($560,000.00) dollars, less any Gross Consideration previously provided by Acquiror to Seller, including any deposits or other
advance payments, and any funds owed in connection with prior agreements between the Parties, (the “Closing”) with the
number of shares issuable as Share Consideration shall be based on the final closing trading price of the Acquiror’s shares on the
date of the Closing. Irrespective of any contrary terms and conditions outlined in this Agreement, at the time of issuance, the Share
Consideration shall be subject to any regulations restricting the issuance of shares by a public company, including but not limited to,
any restrictions imposed by the SEC, the Exchange and any other regulatory authorities as appropriate.
b.
[Intentionally Deleted]
c.
Share Lock-Up. Seller acknowledges that all Share Consideration issued pursuant to this Agreement shall be subject to
a contractual lock-up (in addition to any restrictions imposed by the SEC, the Exchange and any other regulatory authorities as appropriate)
restricting the transfer of the Share Consideration during such lock-up period. Any Share Consideration issued pursuant to this Agreement
will be locked-up for a total of twenty-four (24) months from the date of Closing, during which twelve and one half (12.5%) percent of
the total issued Share Consideration will be released from such lock-up on each three (3) month anniversary (i.e., quarterly) of the issuance
of such Share Consideration (the “Lock-Up”) pursuant to the terms and conditions of a lock-up agreement to be
entered into between the Seller and the Acquiror (the “Lock-Up Agreement”). The Lock-Up Agreement will provide for certain
exemptions to the transfer restrictions, to be mutually agreed between the parties, provided that such transferred Share Consideration
will remain subject to the Lock-Up following such transfer.
d.
Asset Verification. Seller shall provide such tags or other information as necessary or reasonably requested for Acquiror’s
auditors to verify the existence and location of all of Seller’s tangible assets as well as to confirm ownership of all of Seller’s intellectual
property and intangible assets. The Parties reaffirm that the agreement will be for the purchase of all of Seller’s assets, tangible and
intangible, except those assets, contracts, and agreements that are specifically excluded as elected by Purchasers, and such excluded
assets and obligations shall remain the separate property and/or separate obligation of Seller. If required, the Seller hereby agrees
to provide full access to its books and records related to the Assets in order for the Acquiror to discharge its continuous disclosure
obligations under applicable securties laws.
For a certain period to be determined
after the Closing, the Managing Member of the Seller (the “Contractor”) shall continue to receive as compensation substantially
all (up to one hundred (100%) percent) of the revenues directly generated by the Seller’s client base provided that the Contractor
continues to complete all contracted work. Any chargebacks or costs related to returns or repairs will be deducted accordingly from the
total compensation to the Contractor as appropriate.
This “Contractor Period”
will continue until terminated in writing by the Acquiror, in its sole discretion, with no less than thirty (30) days writted notice to
the Contractor; provided however, the Acquiror may proceed in its sole discretion to negotiate employment contracts as appropriate with
any employees of the Seller during such written notice period.
3.2
[Intentionally Deleted].
(a)
[Intentionally Deleted].
(b)
[Intentionally Deleted].
(c)
[Intentionally Deleted].
(d)
[Intentionally Deleted].
3.3
Allocation of Purchase Price.
The Parties agree that the
Purchase Price shall be allocated, for Tax purposes, among the Purchased Assets in a manner consistent with the provisions of Section
1060 of the Code and all regulations promulgated thereunder. Within thirty (30) days after the Closing, the Seller shall:
(a)
complete and execute Form 8594 Asset Acquisition Statement Under Section 1060, consistent with the Statement of Allocation; and
(b)
deliver copies of such form to the Purchaser.
The Parties shall file a copy of the above-referenced
form applicable to it with its Tax Returns for the period which includes the Closing Date.
If there is an increase or
decrease in consideration (the Purchase Price under this Agreement) within the meaning of Section 1.1060-1(e)(1)(ii)(B) of the Treasury
Regulations after the parties have completed the Statement of Allocation or have filed their initial Form 8594 Asset Acquisition Statement,
the Parties shall allocate such increase or decrease in consideration as required by and consistent with Section 1060 of the Code and
the applicable Treasury Regulations.
3.4
Tax Consequences.
The Parties intend that the
Acquisition will constitute a “reorganization” within the meaning of Section 368(a) of the Code, and that this Agreement constitutes
a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a). Notwithstanding
anything herein to the contrary, each Party hereby acknowledges and agrees that such Party is relying solely upon its own tax advisors
and counsel for advice concerning the Tax consequences of the Acquisition, and that no Party provides assurances to any other Party concerning
such Tax consequences.
ARTICLE
IV
CLOSING
4.1
Closing.
The closing of the sale of
the Purchased Assets to the Acquiror, and the transactions contemplated hereby (the “Closing”), shall take place at
the offices of the Acquiror (or at any other location determined with the mutual agreement of the Parties) on a mutually agreed date following
the full execution of this Agreement (the “Closing Date”). The parties hereto and their respective Representatives
may participate in the Closing via electronic means.
ARTICLE
V
REPRESENTATIONS AND WARRANTIES OF THE SELLER
The Seller hereby represents
and warrants to the Purchaser as follows:
5.1
Organization; Good Standing; Qualification and Power.
The Seller is limited liability
company duly organized, validly existing and in good standing under the Laws of State of Florida and has all requisite power and authority
to own, lease and operate its properties and carry on its business relating to the Products and the Purchased Assets as it is now conducting
that business. The Seller is duly licensed or qualified to transact business and in good standing to do business in each jurisdiction
in which the nature of its current operations related to the Business, including its ownership of any of the Purchased Assets and ownership
or leasing of properties used in connection with the Business, makes such qualification necessary, except to the extent that the
Seller’s failure to be so licensed or qualified and in good standing would not have a Material Adverse Effect on the operation of
the Business after the Closing or the transfer of the Purchased Assets to the Acquiror.
5.2
Authority; Noncontravention; Consents.
(a)
The Seller has all requisite power and authority to enter into this Agreement and each Related Document to which it is a party
and to perform its obligations hereunder and thereunder and to consummate the transactions contemplated hereby and thereby. The execution
and delivery by the Seller of this Agreement and each Related Document to which the Seller is a party, and the performance by the Seller
of its obligations hereunder and thereunder, have been duly and validly authorized by all necessary action on the part of the Seller.
This Agreement and each Related Document to which the Seller is a party have been duly and validly executed and delivered by the Seller
and are the valid and binding obligations of the Seller, enforceable against the Seller in accordance with their terms.
(b)
Neither the execution and delivery by the Seller of this Agreement and each Related Document to which the Seller is a party nor
the performance by the Seller of its obligations hereunder and thereunder (i) materially conflicts with, or results in a material violation
of, or causes a material breach or default (with or without notice or lapse of time, or both) under, or gives rise to a right of termination,
amendment, cancellation or acceleration of any material obligation contained in or the loss of any material benefit under, or results
in the creation of any Encumbrance upon any of the Purchased Assets under, any term, condition or provision of (y) the Seller’s
Fundamental Documents or (z) any Assigned Contract or any other material Contract to which the Seller is a party or by which the Purchased
Assets are bound, or (ii) violates any Laws applicable to the Seller or any of the Purchased Assets.
(c)
No material (i) consent, (ii) approval, (iii) Order or authorization of, (iv) registration, declaration or filing with, or (v)
notification to any Governmental Entity or any other third Person is required in connection with the execution and delivery by the Seller
of this Agreement or the Related Documents to which the Seller is a party, the performance by it of its obligations hereunder or thereunder
or the consummation by it of the transactions contemplated hereby or thereby.
5.3
Title to Purchased Assets.
The Seller has good title
to all Purchased Assets free and clear of any Encumbrances.
5.4
Transferred Intellectual Property.
(a)
The Seller owns, has the right to use, sell, license and dispose of, and has the right to bring actions for the infringement of,
the Intellectual Property.
(b)
Prior to Closing Seller shall provide to the Acquiror a complete list of all Intellectual Property held by Seller;
(c)
There are no royalties, honoraria, fees or other payments payable to any Person or claimed by any Person by reason of the ownership,
use, license, sale or disposition of the Intellectual Property or the manufacture or sale of the Products.
(d)
The Seller has not received from any Person in the past five (5) years any written notice, charge, complaint, claim or assertion
that its activities or contemplated activities with respect to the Products infringe or would infringe any Intellectual Property Rights
of any Person, and no such claim is impliedly threatened by an offer to license from another Person under a claim of use.
(e)
The Seller has not sent to any Person in the past five (5) years, or otherwise communicated to any Person, any written notice,
charge, complaint, claim or other assertion of any present, impending or threatened infringement by or misappropriation of, or other conflict
with, any Intellectual Property by such other Person.
(f)
The Seller has not licensed any of the Intellectual Property to any third Person, other than implied licenses granted by the Seller
in connection with the sale of its products.
(g)
Other than the Intellectual Property already disclosed to the Acquiror, the Seller does not own or license any Intellectual Property
Rights.
5.5
Agreements, No Defaults.
(a)
The Seller is not a party to any material Contracts other than the Assigned Contracts.
(b)
Prior to closing Seller shall provide a complete and accurate list of all the Assigned Contracts.
(c)
All Assigned Contracts are in full force and effect, constitute legal, valid and binding obligations of the Seller and, to the
Knowledge of the Seller, the other parties thereto, and are, subject to bankruptcy and other laws relating to or generally affecting the
enforcement of creditors’ rights, enforceable in accordance with their respective terms against the Seller, and to the Knowledge
of the Seller, each other party thereto. The Seller has in all material respects performed all of the obligations required to be performed
by it to date under each such Contract, and there exists no default by the Seller or, to the Knowledge of the Seller, any other party,
or any event that upon the giving of notice or the passage of time, or both, would give rise to a claim of a default in the performance
by the Seller or, to the Knowledge of the Seller, any other party, to any of their respective obligations thereunder. The Seller has made
available to the Purchaser correct and complete copies of all written Assigned Contracts.
5.6
Litigation Etc.
There are no (i) Proceedings
pending or, to the Knowledge of the Seller, threatened against the Seller, whether at law or in equity, or before or by any Governmental
Entity or arbitrator or (ii) Orders of any Governmental Entity or arbitrator naming the Seller.
5.7
Compliance; Permits.
(a)
The Business is not being conducted in violation in any material respect of any Law, Order or Permit applicable in any jurisdiction
in which the Seller operates the Business, including Environmental, Health and Safety Laws. To the Knowledge of the Seller, no investigation
or review by any Governmental Entity with respect to the Products or the Business is pending or threatened, nor has any Governmental Entity
notified the Seller of its intention to conduct the same. The Seller (a) possesses all Permits required under applicable Laws for the
Seller’s conduct of the Business as it is currently conducted, a list of which shall be provided by Seller to the Acquiror prior
to Closing, and each such Permit is valid and in full force and effect, (b) is in compliance, in all material respects, with each such
Permit and (c) no Proceeding is pending or, to the Knowledge of the Seller, threatened to revoke, modify, suspend or limit any such Permit.
5.8
Taxes.
(a)
All material Tax Returns that are required to be filed by or on behalf of the Seller with respect to the Business, the Purchased
Assets and otherwise have been filed with the applicable Taxing Authorities and (b) all Taxes shown as due and payable on such Tax Returns
have been paid. No Tax Return filed by the Seller is currently being examined by any Taxing Authority, and there are no outstanding agreements
or waivers extending the statute of limitations applicable to any such Tax Return. The Seller is not a “foreign person” as
that term is defined in Section 1445 of the Code.
(b)
Seller has delivered to Acquiror copies of all Tax Returns filed since 2019.
5.9
Warranties.
Prior to closing Seller shall
provide to the Acquiror a complete copy of each form of product warranty and guaranty issued by the Seller with respect to the Products
that has not expired. No Person has asserted in the past five (5) years any Proceeding against the Seller under any Law relating to unfair
competition, false advertising or other similar claims arising out of warranties, guarantees, specifications, manuals or brochures or
other advertising materials used in connection with the Business.
5.10
Books and Records.
The books of account and other
records of Seller, which have been made available to Purchaser, are complete and correct in all material respects.
5.11
Financial Information.
Prior to Closing, Seller shall
provide to the Acquiror complete copies of (a) the unaudited balance sheets of Seller for the fiscal years ended December 31, 2020 and
December 31, 2021 and the related statements of income for the fiscal years then ended and the related notes thereto, and (b) the unaudited
balance sheets of Sellers as of May 31, 2022 and the statement of income for the five (5) month period ended May 31, 2022 (collectively,
the “Financial Statements”). The Financial Statements (i) present fairly, in all material respects, the financial condition
and results of operations of Sellers as of the dates thereof or for the periods covered thereby, as the case may be, and (ii) are in conformity
with GAAP.
5.12
Brokers.
Neither the Seller nor any
of its officers, directors, equity owners or employees nor any other Person acting on its or their behalf has employed any broker or finder
or incurred any Liability for any brokerage fees, commissions or finders’ fees in connection with the transactions contemplated
hereby. To the extent that the Seller has incurred any Liability for any brokerage fees, commissions or finder’s fees in connection
with the transactions contemplated hereby, the Seller will be solely responsible for the payment of such commission or fee.
5.13
Environmental Matters.
(a)
The Seller is not, subject to or the subject of any Order or Contract arising under any Environmental, Health and Safety Laws,
nor, to the Knowledge of the Seller, is any Proceeding pending or threatened against the Seller under any Environmental, Health and Safety
Laws.
(b)
The Seller has not received written notice from any Person (i) that it is potentially responsible under any Environmental, Health
and Safety Laws for Hazardous Material or response costs or natural resource damages, as those terms are defined under the Environmental,
Health and Safety Laws, (ii) alleging that it is not in compliance with any Environmental, Health and Safety Laws or (iii) seeking penalties,
damages or injunctive relief for past non-compliance with any Environmental, Health and Safety Laws, in each case related to the Business.
(c)
The Seller is in Compliance with all environmental requirements of its Permits.
5.14
Bankruptcy.
The Seller is not involved
in any Proceeding by or against it as a debtor before any Governmental Entity under Title 11 of the United States Bankruptcy Code or any
other insolvency or debtors’ relief Law, whether state, federal or foreign, or for the appointment of a trustee, receiver, liquidator,
assignee, sequestrator or other similar official for any part of the Seller’s property.
5.15
No Purchased Assets Held by Affiliates.
There are no assets, properties,
interests in assets or properties or rights owned or held by any Affiliate of the Seller that would constitute Purchased Assets if owned
or held by the Seller.
5.16
Equipment and Property
All equipment, property, and
tangible assets purchased pursuant to this Agreement are in good repair and good operating condition, ordinary wear and tear excepted,
is suitable for immediate use in the ordinary course of business and is free from latent and patent defects. No equipment, property, and
tangible assets purchased pursuant to this Agreement are in need of repair or replacement other than as part of routine maintenance in
the ordinary course of business.
5.17
Contracts and Commitments.
The Seller is not a party
to or otherwise obligated under any of the following Contracts, whether written or oral:
(a)
Any revocable or irrevocable power of attorney relating to the Purchased Assets or the Business granted to any person, firm or
corporation for any purpose whatsoever; or
(b)
Any Contract or option relating to the acquisition or sale of any of the Purchased Assets.
5.18
Securities Law Matters.
(a)
The Seller acknowledges and understands that it is acquiring the Stock Consideration under this Agreement under a private placement
in reliance upon the exemption from the registration requirement of the U.S. Securities Act of 1933, as amended (the “Securities
Act”), provided by Section 4(a)(2) of the Securities Act and Rule 506(b) thereunder and similar exemptions under applicable
state securities laws.
(b)
Seller is an “accredited investor,” as defined in Rule 501(a) of Regulation D under Section 4(a)(2) of the Securities
Act.
(c)
Seller is acquiring the Stock Consideration under this Agreement for its own account and not with a view to its distribution in
violation of the Securities Act.
(d)
The Seller acknowledges and understands that the shares of Acquiror’s stock are “restricted securities” as defined
in Rule 144(a)(3) under the Securities Act, and the Seller will not offer, sell, pledge or otherwise transfer any of such securities,
directly or indirectly, unless the offer, sale, pledge or transfer is in a transaction that does not require registration under the U.S.
Securities Act or any applicable state securities laws; or pursuant to an effective registration statement under the Securities Act.
(e)
The Seller acknowledges and understands that until such time as the same is no longer required under the requirements of the Securities
Act or applicable state securities laws, the certificates representing the Stock Consideration, and all certificates representing any
securities issued in exchange thereof or in substitution therefor, will bear the following legend:
“UNLESS PERMITTED UNDER
SECURITIES LEGISLATION, THE HOLDER OF THIS SECURITY MUST NOT TRADE THE SECURITY BEFORE [NTD: INSERT END OF RESTRICTION DATE AFTER THE
DISTRIBUTION DATE].”
“THE SECURITIES REPRESENTED BY THIS
CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “U.S. SECURITIES ACT”),
OR ANY STATE SECURITIES LAWS. THE HOLDER HEREOF, BY PURCHASING THESE SECURITIES, AGREES FOR THE BENEFIT OF STANDARD DENTAL LABS INC. (THE
“CORPORATION”) THAT SUCH SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY: (A) TO THE CORPORATION, (B)
OUTSIDE THE UNITED STATES IN ACCORDANCE WITH RULE 904 OF REGULATION S UNDER THE U.S. SECURITIES ACT (“REGULATION S”), (C)
IN ACCORDANCE WITH (1) RULE 144A UNDER THE U.S. SECURITIES ACT OR (2) RULE 144 UNDER THE U.S. SECURITIES ACT, IF AVAILABLE, OR (D) PURSUANT
TO ANOTHER EXEMPTION OR EXCLUSION FROM REGISTRATION UNDER THE U.S. SECURITIES ACT, AND IN EACH CASE, IN ACCORDANCE WITH ALL APPLICABLE
STATE SECURITIES LAWS, AFTER, IN THE CASE OF TRANSFERS PURSUANT TO CLAUSE (C)(2) OR (D) (OR IF REQUIRED BY THE CORPORATION, OR ITS TRANSFER
AGENT, CLAUSE (B)) ABOVE, THE HOLDER HAS PROVIDED TO THE CORPORATION A LEGAL OPINION OF COUNSEL OF RECOGNIZED STANDING OR OTHER EVIDENCE,
REASONABLY SATISFACTORY TO THE CORPORATION, TO THE EFFECT THAT THE SALE OF SUCH SECURITIES IS NOT REQUIRED TO BE REGISTERED UNDER THE
U.S. SECURITIES ACT OR APPLICABLE STATE SECURITIES LAWS.”
5.19
Relationship with Affiliates.
Except as disclosed by Seller,
neither Seller nor any Affiliates has, since the first date of the next to last completed fiscal year, had any interest in any property
(whether real, personal or mixed and whether tangible or intangible) used in or pertaining to the Business. Neither Seller, nor any Shareholder
nor any Affiliates of any of them owns, since the first date of the next to last completed fiscal year, has owned of record or as beneficial
owner, an equity interest or any other financial or profit interest in any Person that has (a) had business dealings or a material financial
interest in any transaction with Seller other than business dealings or transaction disclosed by Seller to the Acquiror, each of which
has been conducted in the ordinary course of business with Seller at substantially prevailing market prices and on substantially prevailing
market terms or (b) engaged in competition with Seller with respect to any line of products or services of Seller (“Competing
Business”) in any market presently served by Seller, except for ownership of less than one percent of the outstanding capital
stock of any Competing Business that is publicly traded on any recognized exchange or in the over-the-counter market. Except as disclosed
by Seller to the Acquiror, neither Seller nor any Shareholder nor any Affiliates of any of them is a party to any Contract with, or has
any claim or right against, Seller.
5.20
Material Adverse Effect.
To the Knowledge of the Seller,
since the date of the last balance sheet for the last fiscal year, no event or series of events has occurred that could have, or has had,
a Material Adverse Effect on the Business or the Seller.
5.21
Undisclosed Liabilities.
Seller has no Liability except
for Liabilities reflected or reserved against in the balance sheets comprising the Financial Statements and current liabilities incurred
in the Ordinary Course of Business of Seller since the date of the most recent balance sheet comprising the Financial Statements.
5.22
Compliance with OFAC.
Neither the Seller nor, to
the Knowledge of the Seller, any director, manager, officer, agent, employee or Affiliate of the Seller is a Person that is, or is owned
or controlled by a Person that is, currently the subject or target of any sanctions administered or enforced by the U.S. government (including,
without limitation, the Office of Foreign Assets Control of the U.S. Treasury Department (“OFAC”) or the U.S. Department
of State and including, without limitation, the designation as a “specially designated national” or “blocked person”),
the United Nations Security Council, or other relevant sanctions authority (collectively, “Sanction”). No Seller has
knowingly engaged in and is not now knowingly engaged in any dealings or transactions with any Person that at the time of the dealing
or transaction is or was the subject or the target of Sanctions or with any country or territory that is the subject or the target of
Sanctions, including, without limitation, Cuba, Iran, North Korea, Sudan, and Syria.
ARTICLE
VI
REPRESENTATIONS AND WARRANTIES OF THE ACQUIROR
The Acquiror hereby
represents and warrants to the Seller as follows:
6.1
Organization; Good Standing.
The Acquiror is a corporation
duly organized, validly existing and in good standing under the Laws of State of Nevada.
6.2
Authority, Noncontravention; Consents.
(a)
The Acquiror has all requisite corporate power and authority to enter into this Agreement and each Related Document to which it
is a party and to perform its obligations hereunder and thereunder and to consummate the transactions contemplated hereby and thereby.
The execution and delivery by the Acquiror of this Agreement and each Related Document to which the Acquiror is a party, and the performance
by the Acquiror of its obligations hereunder and thereunder, have been duly and validly authorized by all necessary corporate action on
the part of the Acquiror. This Agreement and each Related Document to which the Acquiror is a party have been duly and validly executed
and delivered by the Acquiror and are the valid and binding obligations of the Acquiror, enforceable against the Acquiror in accordance
with their terms.
(b)
Neither the execution and delivery by the Acquiror of this Agreement and each Related Document to which the Acquiror is a party
nor the performance by the Acquiror of its obligations hereunder and thereunder (i) materially conflicts with, or results in a material
violation of, or causes a material breach or default (with or without notice or lapse of time, or both) under, or gives rise to a right
of termination, amendment, cancellation or acceleration of any material obligation contained in or the loss of any material benefit under,
any term, condition or provision of (y) the Acquiror’s Fundamental Documents or (z) any material Contract to which the Acquiror
is a party or by which its assets are bound, or (ii) violates any Laws applicable to the Acquiror or any of its assets.
(c)
No material (i) consent, (ii) approval, (iii) Order or authorization of, (iv) registration, declaration or filing with, or (v)
notification to any Governmental Entity or any other third Person is required in connection with the execution and delivery by the Acquiror
of this Agreement or the Related Documents to which the Purchaser is a party or the consummation by it of the transactions contemplated
hereby or thereby.
6.3
Valid Issuance.
The shares of Stock Consideration
to be issued to Seller pursuant to the terms of this Agreement, when issued as provided in this Agreement, will be duly authorized and
validly issued, fully paid and nonassessable, and will be free of restrictions on transfer other than restrictions under applicable securities
Laws in the United States.
6.4
Brokers.
Neither the Acquiror nor any
of its officers, directors, equity owners or employees nor any other Person acting on its or their behalf has employed any broker or finder
or incurred any Liability for any brokerage fees, commissions or finders’ fees in connection with the transactions contemplated
hereby. To the extent that the Acquiror has incurred any Liability for any brokerage fees, commissions or finder’s fees in connection
with the transactions contemplated hereby, the Acquiror will be solely responsible for the payment of such commission or fee.
ARTICLE
VII
CLOSING DELIVERIES
7.1
The Seller’s Deliveries.
(a)
Related Documents. At the Closing, the Seller shall execute and deliver to the Acquiror each of the documents set forth
below (collectively, the “Related Documents):
(i)
Any document necessary for the completion of the transaction in the following categories: Bill of Sale, Assignment and Assumption
Agreement;
(ii)
any Trademarks owned by Seller to be attached as Exhibit A;
(iii)
a copyright assignment for any copyright held by Seller;
(iv)
a Patent assignment for any Patents held by Seller;
(v)
a list of Seller Shareholders;
(vi)
all other documents required to be entered into by Seller pursuant to this Agreement or reasonably requested by Acquiror to convey
the Purchased Assets to Acquiror or to otherwise consummate the transactions contemplated by this Agreement.
(b)
Permits. The transferable permits held by Seller to be transferred to the Acquiror if applicable;
(c)
Related Certificates. At the Closing, the Seller shall deliver the certificates set forth below to the Acquiror, executed
by the Person set forth below:
(i)
all written consents (or waivers with respect to thereto) for the assignment to Acquiror of the Assigned Contracts;
(ii)
evidence of satisfaction of all obligations for the Indebtedness, including true, correct, and complete payoff letters or UCC termination
statements with respect to the Indebtedness and satisfactory evidence that all Liens affecting the Purchased Assets have been released
(iii)
a certificate of an officer of the Seller dated as of the Closing Date, certifying (A) as to the incumbency and genuineness of
the signatures of each officer of the Seller executing this Agreement or any of the Related Documents on behalf of the Seller; and (B)
the genuineness of the resolutions (attached thereto) of the Seller’s Board of Directors authorizing the execution, delivery and
performance of this Agreement and the Related Documents to which the Seller is a party and the consummation of the transactions contemplated
hereby and thereby; and
(iv)
a certificate of the Florida Secretary of State certifying as to the good standing of the Seller, dated as of a date not more than
five (5) Business Days prior to the Closing Date.
7.2
The Acquiror’s Deliveries.
(a)
Related Documents. At the Closing, the Acquiror shall execute and deliver to the Seller each Related Document.
(b)
Related Certificates. At the Closing, the Acquiror shall deliver the certificates set forth below to the Seller, executed
by the Person set forth below:
(i)
a certificate of an officer of the Acquiror dated as of the Closing Date, certifying (A) as to the incumbency and genuineness of
the signatures of each officer of the Acquiror executing this Agreement or any of the Related Documents on behalf of the Acquiror; and
(B) the genuineness of the resolutions (attached thereto) of the Acquiror’s Board of Directors or similar governing body authorizing
the execution, delivery and performance of this Agreement and the Related Documents to which the Acquiror is a party and the consummation
of the transactions contemplated hereby and thereby; and
(ii)
a certificate of the Nevada Secretary of State certifying as to the good standing of the Acquiror, dated as of a date not more
than five (5) Business Days prior to the Closing Date.
(c)
Share Consideration. At the Closing, the Acquiror shall deliver to the Seller the Share Consideration.
ARTICLE
VIII
INDEMNIFICATION
8.1
Indemnification Generally; Etc.
(a)
The Seller Indemnifying Persons shall indemnify, defend, and hold harmless the Purchaser Indemnified Persons from, against and
in respect of any and all claims, Liabilities, losses (whether or not involving a third party claim), costs, expenses, penalties, fines
and judgments (at equity or at law), and damages whenever arising or incurred (including amounts paid in settlement, costs of investigation
and reasonable attorneys’ fees and expenses) arising out of, relating to or in connection with:
(i)
the untruth, inaccuracy or breach of any representation or warranty of the Seller contained in this Agreement or in any Related
Document to which the Seller is a party (or any facts or circumstances constituting any such untruth, inaccuracy or breach);
(ii)
the breach of any agreement or covenant of the Seller contained in this Agreement or in any Related Document to which the Seller
is a party;
(iii)
the Excluded Liabilities;
(iv)
any sales, value added, excise or other Taxes payable in connection with sales of the Products and the operation of the Business
on or prior to the Closing Date; and
(v)
all claims, damages, liabilities, losses and expenses, including reasonable attorneys’ fees, that arise out of negligent
business and operational decisions made by Seller;
(b)
Acquiror shall indemnify, defend, and hold harmless the Seller Indemnified Persons from, against and in respect of any and all
claims, Liabilities, losses (whether or not involving a third party claim), costs, expenses, penalties, fines and judgments (at equity
or at law), and damages whenever arising or incurred (including amounts paid in settlement, costs of investigation and reasonable attorneys’
fees and expenses) (arising out of, relating to or in connection with:
(i)
the untruth, inaccuracy or breach of any representation or warranty of the Acquiror in this Agreement, or in any Related Document
to which the Acquiror is a party (or any facts or circumstances constituting any such untruth, inaccuracy or breach);
(ii)
the breach of any agreement or covenant of the Acquiror contained in this Agreement or in any Related Document to which the Acquiror
is a party; and
(iii)
the Assumed Liabilities.
8.2
Assertion of Claims.
All claims under Section
8.1 must be brought within a period of time after the Closing Date equal to the maximum statute of limitations to which the Parties
can contractually agree under applicable law with respect to any Fundamental Representation or Fraud, and within twenty-four (24) months
after the Closing Date with respect to any Non-Fundamental Representation. The “Fundamental Representations” are those
representations contained in Sections 5.1, (Organization; Good Standing; Qualification and Power), 5.3 (Title
to Purchased Assets), 5.8 (Taxes), 5.11 (Financial Information), 5.12 (Brokers),
5.13 (Environmental Matters), 5.14 (Bankruptcy Etc.), 5.18 (Securities Law Matters), 5.19
(Relationships with Affiliates), 6.1 (Organization; Good Standing), and 6.4 (Brokers). “Non-Fundamental
Representations” are all representations that are not Fundamental Representations.
8.3
Limitations on Indemnification.
(a)
Indemnity Limitations for the Seller Indemnifying Persons. Except in the case of Fraud:
(i)
the Purchaser Indemnified Persons shall not have the right to be indemnified pursuant to Section 8.1(a)(i) unless and until
the Purchaser Indemnified Persons (or any of them) shall have incurred on a cumulative basis aggregate Losses in an amount exceeding $5,000.00
(the “Deductible”), in which event the Purchaser Indemnified Persons’ right to be indemnified shall apply only
to the extent such Losses exceed the Deductible; and
(ii)
the sum of all Losses pursuant to which indemnification is payable by the Seller Indemnifying Persons pursuant to Section 8.1(a)
shall not exceed $1,000,000.00 (the “Cap”).
(b)
Indemnity Limitations for the Purchaser Indemnifying Persons. Except in the case of Fraud:
(i)
the Seller Indemnified Persons shall not have the right to be indemnified pursuant to Section 8.1(b)(i) unless and until
the Seller Indemnified Persons (or any of them) shall have incurred on a cumulative basis aggregate Losses in an amount exceeding the
Deductible, in which event the Seller Indemnified Persons’ right to be indemnified shall apply only to the extent such Losses exceed
the Deductible; and
(ii)
the sum of all Losses pursuant to which indemnification is payable by the Purchaser Indemnifying Persons pursuant to Section
8.1(b) shall not exceed the Cap.
(c)
Section 8(a) and 8(b) will not apply to any claim for indemnification if the representation and warrant to which
the claim related is a Fundamental Representation.
(d)
If a Party would have a claim for indemnification under this Article VII if the representation and warranty to which the
claim relates did not include a materiality qualifier and the aggregate amount of all such claims exceeds the Deductible, then the Indemnified
Person shall be entitled to indemnification for the amount of such claims in excess of the Deductible in the aggregate notwithstanding
the materiality qualification in the relevant provisions of this Agreement.
(e)
DAMAGES LIMITATION. IN NO EVENT SHALL ANY PARTY TO THIS AGREEMENT BE LIABLE TO ANY OTHER PARTY TO THIS AGREEMENT, UNDER
THIS AGREEMENT OR OTHERWISE IN CONNECTION WITH THE SUBJECT MATTER OF THIS AGREEMENT, FOR CONSEQUENTIAL, INDIRECT, INCIDENTAL, PUNITIVE
OR SPECIAL DAMAGES OR LOST PROFITS, DIMINUTION IN VALUE OF THE BUSINESS OR THE PURCHASED ASSETS, MULTIPLIERS OF DIRECT DAMAGES OR DAMAGE
TO REPUTATION OR GOODWILL, EXCEPT, IN EACH CASE, (i) IN THE EVENT OF ACTUAL FRAUD AND (ii) TO THE EXTENT THAT AN INDEMNIFIED PERSON
IS REQUIRED TO PAY SUCH DAMAGES OR OTHER ITEMS TO A THIRD PERSON IN CONNECTION WITH A MATTER FOR WHICH SUCH INDEMNIFIED PERSON IS ENTITLED
TO INDEMNIFICATION UNDER THIS ARTICLE VIII.
8.4
Notice and Defense of Third Person Claims.
The obligations and liabilities
of an Indemnifying Person with respect to Losses resulting from the assertion of liability by third Persons (each, a “Third Person
Claim”) shall be subject to the following terms and conditions:
(a)
An Indemnified Person shall promptly give written notice to the Indemnifying Persons of any Third Person Claim that might give
rise to any Losses by the Indemnified Persons, stating the nature and basis of such Third Person Claim, and the amount thereof to the
extent known; provided, however, that no delay on the part of the Indemnified Persons in notifying any Indemnifying Persons
shall relieve the Indemnifying Persons from any liability or obligation hereunder unless (and then solely to the extent that) the Indemnifying
Person is prejudiced by the delay. Such notice shall be accompanied by copies of all relevant documentation with respect to such Third
Person Claim, including any summons, complaint or other pleading which may have been served, any written demand or any other related document
or instrument.
(b)
If the Indemnifying Persons acknowledge in a writing delivered to the Indemnified Persons that the Indemnifying Persons shall be
obligated under the terms of their indemnification obligations hereunder in connection with such Third Person Claim, then the Indemnifying
Persons shall have the right to assume the defense of any Third Person Claim at their own expense and by their own counsel, which counsel
shall be reasonably satisfactory to the Indemnified Persons.
(c)
If the Indemnifying Persons shall assume the defense of a Third Person Claim, the Indemnifying Persons shall not be responsible
for any legal or other defense costs subsequently incurred by the Indemnified Persons in connection with the defense thereof and the Indemnifying
Persons shall nevertheless be entitled to participate in such defense with their own counsel and at their own expense. If the Indemnifying
Persons do not exercise their right to assume the defense of a Third Person Claim by giving the written acknowledgement referred to in
Section 8.4(b), the Indemnified Persons may defend the Third Person Claim and seek indemnity from the Indemnifying Persons for
Litigation Expenses incurred in connection with such defense.
(d)
If the Indemnifying Persons exercise their right to assume the defense of a Third Person Claim, they shall not make any settlement
of any claims without the written consent of the Indemnified Persons, which consent shall not be unreasonably withheld; provided,
however, that if the Indemnifying Persons propose the settlement of any claim that is capable of settlement by the payment of money
only and the Indemnified Persons do not consent thereto within twenty (20) days after the receipt of written notice thereof, any Losses
incurred by the Indemnified Persons in excess of such proposed settlement shall be at the sole expense of the Indemnified Persons.
8.5
Survival of Representations and Warranties and Covenants.
(a)
The representations and warranties of the Seller contained in this Agreement or in any certificate delivered in connection with
this Agreement shall survive the Closing Date and shall terminate on the second anniversary of the Closing Date, provided that
the Fundamental Representations of the Seller shall survive the Closing until the expiration of the applicable statute of limitations.
The covenants and other agreements of the Seller in this Agreement shall survive the Closing Date unless and until they terminate in accordance
with their own terms.
(b)
The representations and warranties of the Acquiror contained in this Agreement or in any certificate delivered in connection with
this Agreement shall survive the Closing Date and shall terminate on the second anniversary of the Closing Date, provided that
the representations and warranties of the Acquiror contained in Section 6.4 shall survive the Closing until the expiration of the
applicable statute of limitations. The covenants and other agreements of the Acquiror contained in this Agreement shall survive the Closing
Date unless and until they terminate in accordance with their own terms.
(c)
For convenience of reference, the date upon which any representation, warranty, covenant or agreement contained herein shall terminate,
if any, is referred to herein as the “Survival Date”.
8.6
Right of Setoff.
Upon Notice to Seller specifying
in reasonable detail the basis therefor, the Acquiror may set off any amount to which it may be entitled under this Article VIII
against any amounts otherwise payable under Section 3.4. Neither the exercise of not the failure to exercise such right of set
off will constitute an election of remedies or limit the Acquiror in any manner in the enforcement of any other remedies that may be available
to it.
8.7
Exclusive Remedy.
Except in the case of Fraud,
the rights and remedies provided for in this Article VIII shall be the sole and exclusive rights and remedies of the Indemnified
Persons with respect to any matter relating to this Agreement, or otherwise relating to the transactions contemplated hereby or arising
under or in connection with this Agreement. Neither party shall avoid the limitations on liability set forth in this Article VIII
by seeking monetary damages for tort or pursuant to any other theory of liability other than a claim for indemnification under this Article
VIII. To the extent that the rights and remedies provided for in this Article VIII are determined by a court of competent jurisdiction
not to be exclusive under circumstances in which no exception to exclusivity contained in this Section 8.7 applies, the provisions
of Sections 8.3 and 8.5 shall apply to any remedy available to the Parties.
ARTICLE
IX
POST-CLOSING COVENANTS AND OTHER AGREEMENTS
9.1
Transfer of Purchased Assets.
The Seller shall cooperate
with the Acquiror to facilitate the efficient and expeditious transfer to the Acquiror of the Purchased Assets.
ARTICLE
X
MISCELLANEOUS PROVISIONS
10.1
No Third Party Beneficiaries.
This Agreement shall not confer
any rights or remedies upon any Person, other than the Parties and their respective successors and permitted assigns.
10.2
Entire Agreement.
This Agreement and the Related
Documents (including the schedules and the exhibits attached hereto) contain all of the agreements between the parties hereto with respect
to the transactions contemplated hereby and supersede all prior agreements or understandings, whether written or oral, between the Seller
and the Acquiror.
10.3
Successors and Assigns.
All the terms and provisions
of this Agreement shall be binding upon and inure to the benefit of the Parties and their respective successors and permitted assigns.
Notwithstanding anything herein to the contrary, no party shall assign this Agreement without the prior written consent of the other Parties,
provided that the Acquiror may assign this Agreement to any of its Affiliates or a successor in interest to substantially all of its business.
10.4
Amendment; Waiver.
This Agreement shall not be
altered or otherwise amended except pursuant to an instrument in writing signed by each Party. No obligation of the Seller to the Acquiror
shall be waived except by means of a writing signed by the Acquiror, and no obligation of the Acquiror to the Seller shall be waived except
by means of a writing signed by the Seller. No waiver by any Party of any default, misrepresentation, or breach of warranty or covenant
hereunder, whether intentional or not, shall be deemed to extend to any prior or subsequent default, misrepresentation, or breach of warranty
or covenant hereunder or affect in any way any rights arising by virtue of any prior or subsequent such occurrence.
10.5
Fees and Expenses.
Subject to Article VIII,
each Party hereto shall bear its own fees and expenses incurred in connection with this Agreement and the Related Documents and the transactions
contemplated hereby and thereby, including the legal, accounting and due diligence fees, costs and expenses incurred by such Party.
10.6
Notices.
All notices, amendments, waivers,
or other communications pursuant to this Agreement shall be in writing and shall be deemed to be sufficient if delivered personally, faxed,
sent by e-mail, sent by nationally-recognized overnight courier or mailed by registered or certified mail (return receipt requested),
postage prepaid, to the parties at the following addresses (or at such other address for a party as shall be specified by like notice):
To Acquiror:Costas, Inc (d/b/a Standard
Dental Labs Inc.)
Suite 308
424 E Central Blvd.
Orlando, FL 32801
Attention: James D. Brooks
Email: james.brooks@standarddentallabs.com
To Seller:Prime Dental Lab LLC
1008 N. Pine Hills
Road
Orlando, FL 32808
Attention: John (Jong Pil)
Kim
Email: primedentallabllc@gmail.com
All such notices and other
communications shall be deemed to have been delivered and received (i) in the case of personal delivery or delivery by e-mail, on the
date of such delivery if delivered during business hours on a Business Day or, if not delivered during business hours on a Business Day,
the first Business Day thereafter, (ii) in the case of delivery by nationally-recognized overnight courier, on the Business Day delivered,
and (iii) in the case of mailing, on the fifth Business Day following such mailing. A copy of any notice or other communication sent by
e-mail shall also be sent on the same day by registered or certified mail (return receipt requested) or by nationally-recognized overnight
courier.
10.7
Governing Law; Arbitration.
(a)
Governing Law. To the extent not prohibited by state law all questions concerning the construction, interpretation and validity
of this Agreement and all claims or causes of action (whether in contract or tort) that may be based upon, arise out of or relate to this
Agreement or the negotiation, execution or performance of this Agreement shall be governed by and construed and enforced in accordance
with the domestic Laws of the State of Nevada], without giving effect to any choice or conflict of Law provision or rule, whether in the
State of Nevada or any other jurisdiction, that would cause the Laws of any jurisdiction other than the State of Nevada to apply. In furtherance
of the foregoing, the internal Law of the State of Nevada shall control the interpretation and construction of this Agreement, even if
under the State of Nevada’s choice of Law or conflict of Law analysis, the substantive Law of some other jurisdiction would ordinarily
or necessarily apply.
(b)
Arbitration. Except to the extent prohibited by law, any dispute or controversy or claim between Seller and Acquiror arising
out of or relating to this Agreement, or the breach thereof, shall be submitted to arbitration in accordance with the commercial rules
of the American Arbitration Association. The site of the arbitration shall be Florida. The arbitration shall be conducted in accordance
with the Rules of the Commercial Arbitration Association prevailing at the time the demand for arbitration is made hereunder. Judgment
upon any award rendered by the arbitrator(s) may be entered in any court of competent jurisdiction and shall be binding and final. The
costs of the arbitration, including administrative and arbitrator’s fees, shall be fully paid by the non-prevailing party. Notwithstanding
the foregoing, the parties shall bear the expense of their own attorneys’ fees in accordance with this section.
10.8
Interpretation; Construction.
(a)
“Agreement” means this agreement together with all schedules and exhibits hereto, as the same may from time
to time be amended, modified, supplemented or restated in accordance with the terms hereof. “Knowledge” of any Person
means the actual knowledge of such Person. When used in the case of the Seller, the term “Knowledge” shall mean actual
or constructive knowledge. The use in this Agreement of the term “including” or “include” means
“including, without limitation” or “include, without limitation.” The words “herein”,
“hereof”, “hereunder”, “hereby”, “hereto”, “hereinafter”,
and other words of similar import refer to this Agreement as a whole, including the schedules and exhibits, as the same may from time
to time be amended, modified, supplemented or restated, and not to any particular article, section, subsection, paragraph, subparagraph
or clause contained in this Agreement. All references to articles, sections, subsections, clauses, paragraphs, schedules and exhibits
mean such provisions of this Agreement and the schedules and exhibits attached to this Agreement, except where otherwise stated. The title
of and the article, section and paragraph headings in this Agreement are for convenience of reference only and shall not govern or affect
the interpretation of any of the terms or provisions of this Agreement. The use herein of the masculine, feminine or neuter forms shall
also denote the other forms, as in each case the context may require.
(b)
Where specific language is used to clarify by example a general statement contained herein, such specific language shall not be
deemed to modify, limit or restrict in any manner the construction of the general statement to which it relates. The language used in
this Agreement has been chosen by the parties to express their mutual intent, and no rule of strict construction shall be applied against
any party. Accounting terms used but not otherwise defined herein shall have the meanings given to them under GAAP. Unless expressly provided
otherwise, the measure of a period of one month or year for purposes of this Agreement shall be that date of the following month or year
corresponding to the starting date, provided that if no corresponding date exists, the measure shall be that date of the following month
or year corresponding to the next day following the starting date. For example, one month following February 18 is March 18, and one month
following March 31 is May 1.
10.9
Incorporation of Exhibits.
The Exhibits and Annexes identified
in this Agreement are incorporated herein by reference and made a part hereof.
10.10
Independence of Covenants and Representations and Warranties.
All covenants hereunder shall
be given independent effect so that if a certain action or condition constitutes a default under a certain covenant, the fact that such
action or condition is permitted by another covenant shall not affect the occurrence of such default, unless expressly permitted under
an exception to such initial covenant. In addition, all representations and warranties hereunder shall be given independent effect so
that if a particular representation or warranty proves to be incorrect or is breached, the fact that another representation or warranty
concerning the same or similar subject matter is correct or is not breached shall not affect the incorrectness of or a breach of a representation
and warranty hereunder.
10.11
Counterparts; Electronic Signatures
This Agreement may be executed
in two (2) or more counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same
instrument. Electronic counterpart signatures to this Agreement shall be deemed to be original and shall be acceptable and binding.
[Signature Page Follows]
WITNESS WHEREOF, each of the undersigned has executed
this Agreement as of the date first written above.

ANNEX I
CERTAIN DEFINITIONS
“Affiliate”
means, with respect to any Person (i) a director, officer or 5% or greater shareholder of such Person, (ii) a spouse, parent, sibling
or descendant of such Person (or spouse, parent, sibling or descendant of any director or executive officer of such Person), and (iii)
any other Person that, directly or indirectly through one or more intermediaries, Controls, or is Controlled by, or is under common Control
with, such Person.
“Business”
means the business of the Seller, as it exists now or may exist in the future.
“Business Day”
means any day that is not a Saturday, Sunday or a day on which banking institutions in New York, New York are not required to be open.
“Code” means
the Internal Revenue Code of 1986, as amended.
“Contract”
means any loan or credit agreement, note, bond, mortgage, indenture, lease, sublease, purchase order or other agreement, commitment, instrument,
permit, concession, franchise or license.
“Control”
means, with respect to any Person, the possession, directly or indirectly, of the power to direct or cause the direction of the management
or policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Encumbrances”
means any security interests, mortgages, deeds of trust, liens, pledges, charges, claims, easements, reservations, restrictions, clouds,
equities, rights of way, options, rights of first refusal, grants of power to confess judgment, conditional sales and title retention
agreements (including any lease in the nature thereof) and all other encumbrances, whether or not relating to the extension of credit
or the borrowing of money.
“Environmental, Health
and Safety Laws” means all Laws, Permits and Contracts with Governmental Entities relating to or addressing pollution or protection
of the environment or natural resources, releases of Hazardous Materials, public health and safety or employee health and safety, including
the Solid Waste Disposal Act, as amended, 42 U.S.C. §6901, et seq., the Clean Air Act, as amended, 42 U.S.C. §7401 et
seq., the Federal Water Pollution Control Act, as amended, 33 U.S.C. §1251 et seq., the Emergency Planning and Community
Right-to-Know Act, as amended, 42 U.S.C. §11001 et seq., the Comprehensive Environmental Response, Compensation, and Liability
Act, as amended, 42 U.S.C. §9601 et seq., the Hazardous Materials Transportation Uniform Safety Act, as amended, 49 U.S.C.
§1804 et seq., the Occupational Safety and Health Act of 1970, as amended, the regulations promulgated thereunder, and any
similar Laws and other requirements having the force or effect of Law, and all Orders issued or promulgated thereunder, and all related
common law theories.
“Fraud” means
any material misrepresentation made with the intention of misleading the Party alleging any breach of a representation or warranty.
“Fundamental Documents”
means the documents by which any Person (other than an individual) establishes its legal existence or which govern its internal affairs.
For example, the “Fundamental Documents” of a corporation are its certificate of incorporation and by-laws.
“GAAP” means
United States generally accepted accounting principles as consistently applied by the Seller.
“Governmental Entity”
means any federal, state, local, foreign, political subdivision, court, administrative agency, commission or department or other governmental
authority or instrumentality.
“Hazardous Materials”
means any hazardous or toxic chemicals, materials or substances, pollutants, contaminants or crude oil or any fraction thereof (including
as such terms are defined under any Environmental, Health and Safety Law).
“Indemnified Persons”
means and includes the Seller Indemnified Persons or the Purchaser Indemnified Persons, as the case may be.
“Indemnifying Persons”
means and includes the Seller Indemnifying Persons or the Purchaser Indemnifying Persons, as the case may be.
“Intellectual Property”
means the Intellectual Property Rights of the Seller.
“Intellectual Property
Rights” means all intellectual property rights including all Patents, Trademarks, Trade Names, domain names, websites, internet
addresses and applications for any of the foregoing, copyrights, copyright rights, manufacturing and other know-how, trade secrets, proprietary
processes, formulae and information, computer software, confidential information, franchises, licenses, inventions, marketing materials
and other intellectual property, and all documentation and media constituting, describing or relating to the foregoing, including software,
manuals, memoranda and records of a Person.
“Inventory” means inventory, including inventories of raw materials, work
in process, and finished goods.
“Law” means
any law, statute, treaty, rule, directive, regulation, guideline or Order of any Governmental Entity.
“Liability”
means any liability or obligation, whether known or unknown, asserted or unasserted, absolute or contingent, accrued or unaccrued, liquidated
or unliquidated and whether due or to become due, regardless of when asserted.
“Litigation Expense”
means any expenses incurred in connection with investigating, defending or asserting any claim, legal or administrative action, suit or
Proceeding incident to any matter indemnified against under Article VIII, including court filing fees, court costs, arbitration
fees or costs, witness fees and fees and disbursements of legal counsel, investigators, expert witnesses, accountants and other professionals.
“Losses”
means any and all losses, claims, shortages, damages, Liabilities, expenses (including reasonable attorneys’ and accountants’
and other professionals’ fees and Litigation Expenses), assessments, Tax deficiencies, and Taxes (including interest or penalties
thereon) arising from or in connection with any such matter that is the subject of indemnification under Article VIII net of any
amounts recovered by the Indemnified Persons under insurance policies with respect to such Loss.
“Material Adverse Effect”'
means any event, occurrence, fact, condition or change that is, or could reasonably be expected to become, individually or in the aggregate,
materially adverse to (a) the Business, results of operations, prospects, condition (financial or otherwise) or assets of Seller, or (b)
the ability of Seller to consummate the transactions contemplated hereby on a timely basis.
“Orders”
means judgments, writs, decrees, compliance agreements, injunctions or orders of any Governmental Entity or arbitrator.
“Patents”
means all pending, abandoned, expires, completed and issued U.S. and foreign patents and applications therefor, including all reissues,
re-examinations, divisions, continuations, continuations-in-part and extensions thereof, foreign equivalents thereto and provisional and
non-provisional applications, including Patent Cooperation Treaty (PCT) and regional patent applications.
“Permits”
means all permits, licenses, authorizations, registrations, franchises, approvals, certificates, variances and similar rights obtained,
or required to be obtained, from Governmental Entities.
“Person”
shall be construed broadly and shall include an individual, a partnership, a corporation, a limited liability company, an association,
a joint stock company, a trust, a joint venture, an unincorporated organization or another entity including a Governmental Entity.
“Proceedings”
means actions, suits, claims, investigations or legal or administrative or arbitration proceedings.
“Products”
means all goods and services produced, distributed, sold, offered or provided by the Seller.
“Purchaser Indemnified
Persons” means the Purchaser, its Affiliates, successors and assigns, and the shareholders, directors, officers, managers, members,
partners, trustees, subsidiaries, employees, contractors, subcontractors, attorneys, intermediaries, brokers or other agents, or representatives
of the foregoing.
“Purchaser Indemnifying
Persons” means the Purchaser and its successors and assigns.
“Purchaser Losses”
means any and all Losses sustained, suffered or incurred by any of the Purchaser Indemnified Persons.
“Representatives”
means officers, directors, employees, agents, attorneys, accountants and financial advisors of the Purchaser or the Seller, as the case
may be.
“Seller Indemnified
Persons” means the Seller and its Affiliates, successors and assigns and the officers and directors of each of the foregoing.
“Seller Indemnifying
Persons” means the Seller and its successors and assigns.
“Seller Losses”
means any and all Losses sustained, suffered or incurred by any Seller Indemnified Person.
“Shareholder”
means any individual or entity holding shares or membership units of a company or corporation.
“Stock Consideration”
means the Acquiror’s Class A Common Shares.
“Tax” or
“Taxes” means, with respect to any Person, (i) all income taxes (including any tax on or based upon net income, gross
income, income as specially defined, earnings, profits or selected items of income, earnings or profits) and all gross receipts, sales,
use, ad valorem, transfer, franchise, license, withholding, payroll, employment, excise, environmental (including taxes under Code Section
59A), social security, severance, stamp, occupation, premium, property or windfall profits taxes, alternative or add-on minimum taxes,
customs duties and other taxes, fees, assessments or charges of any kind whatsoever, together with all interest and penalties, additions
to tax and other additional amounts imposed by any Taxing Authority (domestic or foreign) on such Person (if any) and (ii) any liability
for the payment of any amount of the type described in clause (i) above as a result of (A) being a “transferee” (within
the meaning of Section 6901 of the Code or any other applicable Law) of another Person, (B) being a member of an affiliated, combined
or consolidated group or (C) a contractual arrangement or otherwise.
“Tax Return”
means any return, declaration, report, claim for refund, or information return or statement relating to Taxes, including any schedule
or attachment thereto, and including any amendment thereof.
“Taxing Authority”
means any Governmental Entity that has the authority to determine the amount of or collect any Taxes.
“Trademarks”
means all pending, expired, abandoned, registered, unregistered, and common law U.S. and foreign trademark applications and trademarks,
service mark applications and service marks, designs, logos, and trade dress, including the goodwill related to the foregoing, and all
federal and state registrations thereof.
“Trade Names”
means (i) names, (ii) brand names, (iii) business names and (iv) logos and all other names and slogans.
“Trading Price”
means (i) as to the Stock Consideration, the ten (10) day volume weighted average trading price for shares of Share Consideration for
the ten (10) Business Day period immediately preceding the Closing Date (the “Closing Trading Price”);
“Trailing 12 Months
(TTR) Revenue” shall mean the total Product sales of the Seller product lines into any market and the wholesale product
sales from all Seller Products.
SCHEDULE 1.1
ACCOUNTS RECEIVABLE
SCHEDULE 1.1(a)
INVENTORY
SCHEDULE 1.1(b)
EXCLUDED INVENTORY
SCHEDULE 1.1(e)
ASSIGNED CONTRACTS & CONSENTS TO TRANSFER
SCHEDULE 1.1(i)
TRANSFERRED PERMITS
SCHEDULE 1.2
ADDITIONAL EXCLUDED ASSETS
SCHEDULE 5.4
INTELLECTUAL PROPERTY
SCHEDULE 5.8
PERMITS
SCHEDULE 5.10
WARRANTIES
SCHEDULE 5.19
ACCOUNTS RECEIVABLE
SCHEDULE 5.21
Relationship With Affiliates
Addendum No. 1
Dated March 24, 2023 to that certain Asset
Purchase Agreement (the “Agreement”) originally dated as of the 15th day of August, 2022 by and between Prime
Dental Lab LLC (“Prime”), a company incorporated under the laws of the State of Florida and Costas Inc. (dba Standard
Dental Labs Inc.) (“Costas”), a company incorporated under the laws of the State of Nevada, individually referred
to as “Party” and collectively as the “Parties”.
Whereas subsequent to the closing of the
Agreement the Parties hereto have agreed to amend Article III – Consideration, Item 3.1 Purchase Price, subheading Cash Consideration
in order to revise the payment terms for the cash instalments.
Notwithstanding anything contained in the Agreement
to the contrary, the provisions set forth below will be deemed to be a part of the Agreement and shall supersede any contrary provision
in the Agreement. All references in the Agreement and in this Addendum shall be construed to mean the Agreement as amended and supplemented
by this Addendum. Any inconsistency between the Agreement and this Addendum shall be resolved in favour of the provisions of this Addendum.
| 1. | Defined Terms: All defined terms used in this Addendum, unless specifically defined in this Addendum,
shall have the same meaning as such terms have in the Agreement; |
| 2. | Modification of the Agreement: |
Article III – Consideration,
Item 3.1 Purchase Price, subheading Cash Consideration be replaced in its entirety with the following:
Cash Consideration – The Cash Consideration
shall be payable in two (2) equal instalments of seventy thousand ($70,000.00) dollars (each a “Cash Instalment”).
The first Cash Instalment shall be paid by the Acquiror to the Seller no later than fifteen (15) calendar days after receipt by the Acquiror
of the Notice of Effect from the SEC of its Form S-1 filing (the “First Cash Instalment”), or on such earlier
date as may be agreed between the Parties in writing.
The second Cash Instalment of seventy thousand
($70,000.00) dollars shall be paid by the Acquiror to the Seller on the date that is no later than seven (7) calendar days subsequent
to the first payment from the ELOC referenced in the company’s current S-1 registration notice (the “Second Cash Instalment”).
| 3. | Effect to Amendment: Except as expressively modified in this Addendum, all terms, conditions and covenants
set forth in the Agreement shall remain in full force and effect among the Parties. |
| 4. | Amendment: This Addendum may be amended, supplemented or modified only by a written instrument duly executed
by or on behalf of each party hereto. |
| 5. | Governing Law: This Addendum shall be governed by and construed and enforced in accordance with the laws
of the State of Nevada. |
| 6. | Counterparts: This Addendum maybe executed in any number of counterparts, each of which shall be an original
but all of which together, shall constitute one instrument. A facsimile or other electronic transmission of this signed Addendum shall
be legal and binding on all parties hereto. |
This Addendum No. 1 shall be appended to and form
a part of the Agreement and shall become effective on March 24, 2023.
******Signature page to follow******
IN WITNESS WHEREOF, each of the undersigned has
caused this Addendum No. 1 to be duly executed and delivered as of the date first written above.
|
THE ACQUIROR:
Costas, Inc. (dba Standard Dental Labs Inc.)
By: __________________________
Name: James D. Brooks
Title: CEO, President
|
|
THE SELLER:
PRIME DENTAL LAB LLC
By:__________________________
Name: John (Jong Pil) Kim
Title: Managing Member |
Exhibit 6.5
THIS CONVERTIBLE PROMISSORY NOTE
HAS NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933 (THE “ACT”), OR THE SECURITIES LAWS OF ANY JURISDICTION. SUCH SECURITIES
MAY NOT BE OFFERED, SOLD, HYPOTHECATED, GIVEN, BEQUEATHED, TRANSFERRED, ASSIGNED PLEDGED, ENCUMBERED, OR OTHERWISE DISPOSED OF (“TRANSFERRED”)
EXCEPT PURSUANT TO (I) A REGISTRATION STATEMENT WITH RESPECT TO SUCH SECURITIES THAT IS EFFECTIVE UNDER THE ACT AND APPLICABLE STATE SECURITIES
LAW, OR (II) ANY EXEMPTION FROM REGISTRATION UNDER THE ACT, AND APPLICABLE STATE SECURITIES LAW, PROVIDED THAT AN OPINION OF COUNSEL IS
FURNISHED TO THE COMPANY, IN FORM AND SUBSTANCE REASONABLY SATISFACTORY TO THE COMPANY, TO THE EFFECT THAT AN EXEMPTION FROM THE REGISTRATION
REQUIREMENTS OF THE ACT AND/OR APPLICABLE STATE SECURITIES LAW IS AVAILABLE.
COSTAS,
INC.
8% CONVERTIBLE PROMISSORY NOTE
| Dated: 23 December 2021 |
Principal Balance: US $1,171,727.31 |
WHEREAS,
On September 20, 2017, an Order was entered into by the Eighth District Judicial Court, Clarke County, Nevada, Case Number A-17-749977-B
wherein the Court ordered debt payable by Costas Inc. (the “Company”) to Brooks memorialized in the amount of One Million
One Hundred Fourteen Thousand Five Hundred Dollars ($1,114,500.00) (the (“Order”)). Subsequently, on March 25, 2021, the Court
approved the first proposed settlement of a portion of Brooks’ debt, in the amount of One Hundred Seventy-Five Thousand Dollars
($175,000) (the “Settlement Debt”) to be paid via the issuance of certain common shares. Subsequently on December 23, 2021
the court entered a further order approving the issuance of 300,000,000 shares of the Common stock of the Company to Brooks’ for
the Settlement Debt pursuant to Section 3(a)(10) of the Securities Act, and further memorializing the remaining debt payable to Mr. Brooks
in the form of a convertible note in the amount set out above and convertible into shares of the Common stock of the Company at $0.001
per share and pursuant to the exemption of Section 3(a)(10) of the Securities Act of 1933 (the “Settlement Order”). The Order
and the Settlement Order are attached hereto.
FOR VALUE
RECEIVED, the undersigned, Costas, Inc., a company organized under the laws of the State of Nevada (herein called the “Company”),
hereby promises to pay to James Brooks, an individual residing in the United States (the “Holder”), the amount
set forth above (the “Principal Balance”) no later than December 31, 2022 (the “Maturity Date”).
1.
Interest. Simple interest shall accrue on this Note at a rate of Eight Percent (8%) per annum and shall be payable on the
Maturity Date.
2.
Repayment. (a) At the Holder’s discretion, the Company may pre-pay all, or any portion, of this Note and any interest
accrued thereon at any time upon Thirty (30) days written notice to the Holder, without penalty.
| 3. | Conversion. The Holder has the right, in its sole discretion, at any time, with three |
(3) days written notice, to convert
any part of this Note and accrued interest thereon into shares of the Company's common stock at a conversion rate of par value or $0.001
per share pursuant to the exemption of Section 3(a)(10) of the Securities Act of 1933. The Holder shall exercise his right to convert
this Note or any part thereof, by delivering to the Company a Notice of Election to Convert in the form attached hereto as Exhibit A (the
“Notice of Election to Convert”). The Holder shall provide this original Note to the Company along with such Notice of Election
to Convert. In the case that the Holder elects to convert the entire outstanding balance of this Note, then the Note shall be cancelled
in whole. In the event that Holder elects to convert less than the entire outstanding balance of this Note, then a new Note shall be reissued
reflecting the remaining balance of this Note and delivered to the Holder along with the common stock issued as a result of the Holder's
Notice of Election to Convert.
4.
Anti-dilution. In the event that the Holder elects to convert the principal amount of this Note into shares of the Company’s
common stock, then no re-capitalization, forward split or reverse split of the Company’s common stock to take effect after the date
hereof but prior to the date of conversion shall have a dilutive effect on the number of shares that are to be issued as a result of such
conversion.
5.
Affiliate. Holder acknowledges that he is an Affiliate of the Company and will be subject to applicable resale rules with
respect to all common shares issued under this Note, and more specifically, Holder, as long as he remains an Affiliate of the Company,
will be limited to selling 1% of the issued and outstanding common stock every ninety days (or every quarter).
6.
Unregistered. This Note has not been registered under the Act, or the securities laws of any state and:
| (a) | In the case of a U.S. Person (as such term is defined in Rule 902 of Regulation
S under the Act) or any entity or individual that is a resident of the U.S., this Note is being offered and sold to a limited number of
U.S. accredited investors in transactions not requiring registration under the Act; and |
| (b) | In the case of a non-U.S. Person, this Note is being offered and sold to such non-U.S.
Person pursuant to Regulation S in transactions not requiring registration under the Act. |
Accordingly,
this Note, upon issuance, will be a “restricted security” in the United States within the meaning of Rule 144(a)(3) of the
Act.
If the Note is
not repaid in full on the Maturity Date, an Event of Default occurs. If an Event of Default is noticed by the Holder and is not remedied
within thirty (30) days, any remaining Principal of the Note and any accrued, but unpaid interest may be immediately converted into common
shares as set forth in Item 3 herein.
7.
Non-circumvention. The Company hereby covenants and agrees that the Company will not, by amendment of its Articles of Incorporation,
Bylaws or through any reorganization, transfer of assets, consolidation, merger, scheme of arrangement, dissolution, issue or sale of
securities, or any other voluntary action, avoid or seek to avoid the observance or
performance of any of the terms of
this Note, and will at all times in good faith carry out all of the provisions of this Note and take all action as may be required to
protect the rights of the Holder of this Note.
8.
Waiver. To the extent permitted by law, the Company hereby waives demand, notice, protest and all other demands and notices
in connection with the delivery, acceptance, performance, default or enforcement of this Note and the Note Purchase Agreement.
9.
Enforcement Fees. If (a) this Note is placed in the hands of an attorney for collection or enforcement or is collected or
enforced through any legal proceeding or the Holder otherwise takes action to collect amounts due under this Note or to enforce the provisions
of this Note or (b) there occurs any bankruptcy, reorganization, receivership of the Company or other proceedings affecting Company creditors'
rights and involving a claim under this Note, then the Company shall pay the costs incurred by the Holder for such collection, enforcement
or action or in connection with such bankruptcy, reorganization, receivership or other proceeding, including, but not limited to, financial
advisory fees and attorneys' fees and disbursements.
10.
Governing Law, Jurisdiction; Severability; Jury Trial. This Note shall be construed and enforced in accordance with, and
all questions concerning the construction, validity, interpretation and performance of this Note shall be governed by, the internal laws
of the State of Nevada, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Nevada
or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of Nevada. The Company
hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the State of Nevada, for the adjudication
of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably
waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of
any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding
is improper. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. In
the event that any provision of this Note is invalid or unenforceable under any applicable statute or rule of law, then such provision
shall be deemed inoperative to the extent that it may conflict therewith and shall be deemed modified to conform with such statute or
rule of law. Any such provision which may prove invalid or unenforceable under any law shall not affect the validity or enforceability
of any other provision of this Note. Nothing contained herein shall be deemed or operate to preclude the Holder from bringing suit or
taking other legal action against the Company in any other jurisdiction to collect on the Company’s obligations to the Holder, to
realize on any collateral or any other security for such obligations, or to enforce a judgment or other court ruling in favor of the Holder.
***** Signature Page to Follow*****
The Company has caused this Note to be duly executed on the
date first written above.
| COSTAS, INC. |
JAMES BROOKS |
EXHIBIT A
FORM OF NOTICE OF ELECTION
TO CONVERT
NOTICE OF ELECTION TO CONVERT
DEBT
TO COSTAS, INC.
The undersigned Holder of that certain
debt owed to it (the "Debt") by COSTAS, INC. a Nevada corporation ("CSSI"), hereby irrevocably exercises the option
to convert $ of the Debt into shares of Common Stock of CSSI, at the conversion price of $0.001 per share for a total of _____
shares and directs that the shares of the Common Stock issuable and deliverable upon the conversion be issued and delivered to the Holder
hereof.
Signature
Dated: __________________, 20__
Signature Guaranteed
Fill in for name in which Shares are to be issued:
(Please print name and address, including zip code)
Social Security or other Taxpayer Identification Number
Exhibit 6.6
THIS CONVERTIBLE PROMISSORY NOTE HAS NOT BEEN
REGISTERED UNDER THE SECURITIES ACT OF 1933 (THE “ACT”), OR THE SECURITIES LAWS OF ANY JURISDICTION. SUCH SECURITIES MAY NOT
BE OFFERED, SOLD, HYPOTHECATED, GIVEN, BEQUEATHED, TRANSFERRED, ASSIGNED PLEDGED, ENCUMBERED, OR OTHERWISE DISPOSED OF (“TRANSFERRED”)
EXCEPT PURSUANT TO (I) A REGISTRATION STATEMENT WITH RESPECT TO SUCH SECURITIES THAT IS EFFECTIVE UNDER THE ACT AND APPLICABLE STATE SECURITIES
LAW, OR (II) ANY EXEMPTION FROM REGISTRATION UNDER THE ACT, AND APPLICABLE STATE SECURITIES LAW, PROVIDED THAT AN OPINION OF COUNSEL IS
FURNISHED TO THE COMPANY, IN FORM AND SUBSTANCE REASONABLY SATISFACTORY TO THE COMPANY, TO THE EFFECT THAT AN EXEMPTION FROM THE REGISTRATION
REQUIREMENTS OF THE ACT AND/OR APPLICABLE STATE SECURITIES LAW IS AVAILABLE.
COSTAS,
INC.
0% CONVERTIBLE
PROMISSORY Note
| Dated: March 4, 2024 |
Principal Balance: US $10,000 |
For Value
Received, the undersigned, Costas, Inc., a company organized under the laws of the State of Nevada (herein called the “Company”),
hereby promises to pay to XXXXX XXXXXX, an individual residing at YOUR ADDRESS HERE (the “Holder”), the
amount set forth above (the “Principal Balance”) no later than December 31, 2024 (the “Maturity Date”).
1. Interest.
Simple interest shall accrue on this Note at a rate of Zero (0%) per annum and shall be payable on the Maturity Date.
2. Repayment.
(a) At the Company’s discretion, the Company may pre-pay all, or any portion, of this Note and any interest accrued thereon at any
time upon Thirty (30) days written notice to the Holder, without penalty. Upon issuance of such notice to the Holder by the Company, any
rights to convert either all or any portion of the shares of the Company’s common stock under this Note shall respectively be immediately
null and void as appropriate.
3.Conversion.
Only after a period of one hundred and eighty (180) days from the date of this Note, the Holder has the right, in its sole discretion,
at any time, with thirty (30) days written notice, to convert any part of this Note and accrued interest thereon into shares of the Company's
common stock at a conversion rate of par value or $0.005 per share. The Holder shall exercise his right to convert this Note or any part
thereof, by delivering to the Company a Notice of Election to Convert in the form attached hereto as Exhibit A (the “Notice of Election
to Convert”). The Holder shall provide this original Note to the Company along with such Notice of Election to Convert. In the case
that the Holder elects to convert the entire outstanding balance of this Note, then the Note shall be cancelled in whole. In the event
that Holder elects to convert less than the entire outstanding balance of this Note, then a new Note shall be reissued reflecting the
remaining balance of this Note and delivered to the Holder along with the common stock issued as a result of the Holder's Notice of Election
to Convert. Upon receipt of any Notice of Election to Convert from the Holder, the Company, in its sole discretion, retains its right
of Repayment as outlined in Section 2(a) above as an alternative to any such conversion request.
4.Anti-dilution.
In the event that the Holder elects to convert the principal amount of this Note into shares of the Company’s common stock, then
no re-capitalization, forward split or reverse split of the Company’s common stock to take effect after the date hereof but prior
to the date of conversion shall have a dilutive effect on the number of shares that are to be issued as a result of such conversion.
5.Unregistered.
Although this Note has not been registered under the Act, nor the securities laws of any state, however the Company hereby agrees
to, within ninety (90) days of the date hereof: either (i) submit a new registration statement (the “Registration Statement”)
for this Note or (ii) include this Note for registration by amendment in the Form S-1 application currently pending in the approval process
with the Securities and Exchange Commission (“SEC”) and further to exercise its best efforts to register this Note as appropriate,
and:
| (a) | In the case of a U.S. Person (as such term is defined in Rule 902 of Regulation S under the Act) or any
entity or individual that is a resident of the U.S., this Note is being offered and sold to a limited number of U.S. accredited investors
in transactions not requiring registration under the Act; and |
| (b) | In the case of a non-U.S. Person, this Note is being offered and sold to such non-U.S. Person pursuant
to Regulation S in transactions not requiring registration under the Act. |
Accordingly, this Note, upon
issuance, will be a “restricted security” in the United States within the meaning of Rule 144(a)(3) of the Act.
If the Note is not repaid
in full on the Maturity Date, an Event of Default occurs. If an Event of Default is noticed by the Holder and is not remedied within thirty
(30) days, any remaining Principal of the Note and any accrued, but unpaid interest may be immediately converted into common shares as
set forth in Item 3 herein.
6. Non-circumvention.
The Company hereby covenants and agrees that the Company will not, by amendment of its Articles of Incorporation, Bylaws or through any
reorganization, transfer of assets, consolidation, merger, scheme of arrangement, dissolution, issue or sale of securities, or any other
voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Note, and will at all times in good
faith carry out all of the provisions of this Note and take all action as may be required to protect the rights of the Holder of this
Note.
7.Waiver.
To the extent permitted by law, the Company hereby waives demand, notice, protest and all other demands and notices in connection with
the delivery, acceptance, performance, default or enforcement of this Note and the Note Purchase Agreement.
8.Enforcement
Fees. If (a) this Note is placed in the hands of an attorney for collection or enforcement or is collected or enforced through any
legal proceeding or the Holder otherwise takes action to collect amounts due under this Note or to enforce the provisions of this Note
or (b) there occurs any bankruptcy, reorganization, receivership of the Company or other proceedings affecting Company creditors' rights
and involving a claim under this Note, then the Company shall pay the costs incurred by the Holder for such collection, enforcement or
action or in connection with such bankruptcy, reorganization, receivership or other proceeding, including, but not limited to, financial
advisory fees and attorneys' fees and disbursements.
9.Governing
Law, Jurisdiction; Severability; Jury Trial. This Note shall be construed and enforced in accordance with, and all questions concerning
the construction, validity, interpretation and performance of this Note shall be governed by, the internal laws of the State of Nevada,
without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Nevada or any other jurisdictions)
that would cause the application of the laws of any jurisdictions other than the State of Nevada. The Company hereby irrevocably submits
to the exclusive jurisdiction of the state and federal courts sitting in the State of Nevada, for the adjudication of any dispute hereunder
or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not
to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such
suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Nothing
contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. In the event that any
provision of this Note is invalid or unenforceable under any applicable statute or rule of law, then such provision shall be deemed inoperative
to the extent that it may conflict therewith and shall be deemed modified to conform with such statute or rule of law. Any such provision
which may prove invalid or unenforceable under any law shall not affect the validity or enforceability of any other provision of this
Note. Nothing contained herein shall be deemed or operate to preclude the Holder from bringing suit or taking other legal action against
the Company in any other jurisdiction to collect on the Company’s obligations to the Holder, to realize on any collateral or any
other security for such obligations, or to enforce a judgment or other court ruling in favor of the Holder.
***** Signature Page to Follow*****
The Company has caused this
Note to be duly executed on the date first written above.
| COSTAS, INC. |
|
XXXXX XXXXXX |
| |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| By: |
|
|
By: |
|
| |
James Brooks, CEO |
|
|
XXXXX XXXXXX |
| |
|
|
|
|
| |
|
|
|
|
EXHIBIT A
FORM OF NOTICE OF ELECTION TO CONVERT
NOTICE OF ELECTION TO CONVERT
DEBT
TO COSTAS,
INC.
The undersigned Holder of that
certain debt owed to it (the "Debt") by COSTAS, INC. a Nevada corporation ("CSSI"),
hereby irrevocably exercises the option to convert $_____________ of the Debt into shares of Common Stock of CSSI, at the conversion price
of $0.001 per share for a total of ___________ shares and directs that the shares of the Common Stock issuable and deliverable upon the
conversion be issued and delivered to the Holder hereof.
Signature
Dated: ______________, 20__
Signature Guaranteed
Fill in for name in which Shares are to be issued:
(Please print name and address, including zip
code)
Social Security or other Taxpayer Identification
Number
Exhibit 11.1

CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
The Shareholders and Board of Directors of Standard Dental Labs Inc., (f/k/a Costas, Inc).
We consent to the use in the offering circular
of Standard Dental Labs Inc., a Nevada corporation f/k/a Costas, Inc. constituting a part of this offering statement on Form 1-A/A, as
it may be amended, of our independent Auditors Report dated April 10th, 2024, of the balance sheet and the related statements of operations,
stockholders’ equity, and cashflows for the years ended December 31, 2023 and 2022.
/S/ Olayinka Oyebola
OLAYINKA OYEBOLA & CO
Chartered Accountant
PCAOB No:5968
Lagos, Nigeria
July 31, 2024
Exhibit 12.1

Jeffrey Turner – Attorney at Law
7533 S. Center View Ct, #4291
West. Jordan, Utah 84084
(801) 810-4465
Admitted in the State of Utah
July 30, 2024
James Brooks
Chief Executive Officer
Standard Dental Labs, Inc.
424 E Central Blvd, Suite 308
Orlando, FL 32801
Dear Mr. Brooks:
I have acted, at your request,
as special counsel to Standard Dental Labs, Inc. (f/k/a Costas, Inc.), a Nevada corporation (the “Company”), for the purpose
of rendering an opinion as to the legality of 400,000,000 shares of Company common stock, par value $0.001, offered by the Company at
a price range of $0.01-$0.03 per share of Company common stock to be offered and distributed by Company (the “Shares”), pursuant
to a Tier 2 Offering Statement filed under Regulation A of the Securities Act of 1933, as amended, by Company with the U.S. Securities
and Exchange Commission (the “SEC”) on Form 1-A, for the purpose of registering the offer and sale of the Shares (“Offering
Statement”).
In rendering this opinion, I have
reviewed (a) statutes of the State of Nevada, to the extent I deem relevant to the matter opined upon herein; (b) true copies of the Articles
of Incorporation of Company and all amendments thereto; (c) the By-Laws of Company; (d) selected proceedings of the board of directors
of Company authorizing the issuance of the Shares; (e) certificates of officers of Company and of public officials; (f) and such other
documents of Company and of public officials as I have deemed necessary and relevant to the matter opined upon herein.
I have assumed (a)
the Offering Statement filed on Form 1-A and all corresponding exhibits (collectively, the “Documents”) have been duly authorized
and executed (except as it relates to the Company in which case the Documents have in fact been duly authorized and executed); (b) the
persons who executed the Documents had the legal capacity to do so; and (c) the persons identified as officers are actually serving as
such and that any shares issued under and pursuant to the Offering Statement will be properly authorized by one or more such persons.
Based upon my review described
herein, it is my opinion the Shares are duly authorized and when/if issued and delivered by Company against payment therefore, as described
in the offering statement, will be validly issued, fully paid, and non-assessable.
I have not been engaged to examine,
nor have I examined, the Offering Statement for the purpose of determining the accuracy or completeness of the information included therein
or the compliance and conformity thereof with the rules and regulations of the SEC or the requirements of Form 1-A, and I express no opinion
with respect thereto. The forgoing opinion is strictly limited to matters of Nevada corporation law; and, I do not express an opinion
on the federal law of the United States of America or the law of any state or jurisdiction therein other than Nevada, as specified herein.
I hereby consent to the filing
of this opinion as Exhibit 12.1 to the Offering Statement and to the reference to our firm under the caption “Legal Matters”
in the Offering Circular constituting a part of the Offering Statement. We assume no obligation to update or supplement any of the opinion
set forth herein to reflect any changes of law or fact that may occur following the date hereof.
Sincerely,
JDT Legal
/s/ Jeffrey Turner
Jeffrey Turner
Costas (PK) (USOTC:CSSI)
Historical Stock Chart
From Jan 2025 to Feb 2025
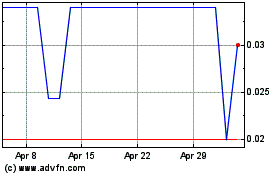
Costas (PK) (USOTC:CSSI)
Historical Stock Chart
From Feb 2024 to Feb 2025