Stryker Launches New Gamma4 Hip Fracture Nailing System
September 01 2022 - 8:07AM
Business Wire
New System to Strengthen the Gamma Legacy and Streamline
Procedure Workflows
Stryker (NYSE:SYK) has launched the Gamma4 System, strengthening
the product’s 30-year legacy of continuous innovation and clinical
history. The newest Gamma System will provide surgeons with the
next generation of Stryker’s intramedullary nailing system.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20220901005173/en/
Stryker's Gamma System will provide
surgeons with the next generation of Stryker’s intramedullary
nailing system. (Photo: Business Wire)
“Since 2004, the Gamma3 System has been the proven work horse of
our Trauma business,” said Eric Tamweber, Vice President and
General Manager, Stryker’s Trauma business unit. “But we aren’t
proven because we have a legacy; we have a legacy because we are
proven. That’s why we’re so excited to introduce Gamma4—an
enhanced, modern product that is designed to fulfill our customers’
hip fracture needs.1”
The Gamma4 System is indicated for the treatment of stable and
unstable fractures as well as for stabilization of bones and
correction of bone deformities in the intracapsular, trochanteric,
subtrochanteric and shaft regions of the femur (including
osteoporotic and osteopenic bone). The system features:
- Precision Pin™: With the Precision Pin™, the
potential for skiving is reduced by 66% compared to a standard ∅3.2
k-wire.2
- A redefined nail design: The SOMA-designed nail features
length-dependent RoC, a shortened proximal body and a chamfered
distal tip with a pre-inserted set screw.
- An integrated instrument platform: The streamlined
portfolio of all Stryker nails now works off the existing IMN Basic
set.
“Our design team spent the last decade working to understand how
we could enhance the Gamma System based on surgeon experience and
feedback,” said James Maxey, M.D., orthopaedic surgeon and a design
surgeon for Gamma4. “Our goal when designing the Gamma4 system was
to make it easier for the surgeon—and better for the patient. Being
one of the most commonly used devices to repair a hip fracture, I’m
confident that we met our goal of reshaping patient hip fracture
care for many years to come.”
“The Gamma4 System features the Precision Pin™, which allows me
to control the placement of the Lag Screw while having familiar
instrumentation to the T2 Alpha System,” said David Forsh, M.D., an
orthopaedic surgeon at Mount Sinai Hospital in New York. “The
instrumentation is sleek, the nail design and geometry have been
enhanced and the nail facilitates the ease of insertion, which is
one of the biggest benefits I have experienced firsthand in the
operating room.”
About Stryker
Stryker is one of the world’s leading medical technology
companies and, together with its customers, is driven to make
healthcare better. The company offers innovative products and
services in Medical and Surgical, Neurotechnology, Orthopaedics and
Spine that help improve patient and healthcare outcomes. Alongside
its customers around the world, Stryker impacts more than 100
million patients annually. More information is available at
www.stryker.com.
References
- Regling, Matthias. EPS Final Report (D0000205786), Stryker
Trauma Kiel, Germany
- Internal test report D0000099352
Drs. Maxey and Forsh are paid consultants of Stryker
Orthopaedics. The opinions expressed by Drs. Maxey and Forsh are
those of Drs. Maxey and Forsh and not necessarily those of Stryker.
Individual experiences may vary.
A surgeon must always rely on his or her own professional
clinical judgment when deciding whether to use a particular product
when treating a particular patient. Stryker does not dispense
medical advice and recommends that surgeons be trained in the use
of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth
of Stryker’s product offerings. A surgeon must always refer to the
package insert, product label and/or instructions for use before
using any of Stryker’s products. Products may not be available in
all markets because product availability is subject to the
regulatory and/or medical practices in individual markets. Please
contact your sales representative if you have questions about the
availability of products in your area.
G4-LT-1 07-2022
View source
version on businesswire.com: https://www.businesswire.com/news/home/20220901005173/en/
Andrea Sampson President/CEO, Sampson Public Relations Group
asampson@sampsonprgroup.com 562.304.0301
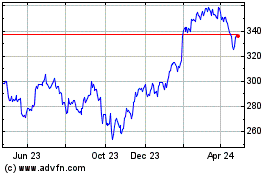
Stryker (NYSE:SYK)
Historical Stock Chart
From Oct 2024 to Nov 2024
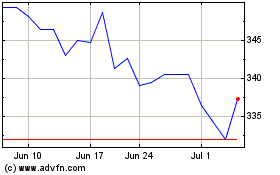
Stryker (NYSE:SYK)
Historical Stock Chart
From Nov 2023 to Nov 2024