false000071893700007189372024-05-072024-05-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): May 7, 2024
STAAR Surgical Company
(Exact Name of Registrant as Specified in Charter)
|
|
|
Delaware |
0-11634 |
95-3797439 |
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
25651 Atlantic Ocean Drive Lake Forest, California |
|
92630 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: 626-303-7902
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
☐ |
Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communication pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communication pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common |
STAA |
NASDAQ |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1 933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On May 7, 2024, STAAR Surgical Company (the “Company”) published a press release reporting its financial results for the quarter ended March 29, 2024, a copy of which is furnished as Exhibit 99.1 to this report and is incorporated herein by this reference.
Item 7.01 Regulation FD Disclosure.
During a conference call and webcast scheduled to be held at 4:15 p.m. Eastern / 1:15 p.m. Pacific on May 7, 2024, the Company’s Chair of the Board, President and Chief Executive Officer, and the Company's Chief Financial Officer will discuss the Company’s results for the quarter ended March 29, 2024 and the Company’s outlook for fiscal year 2024. The Company’s slide presentation for the conference call and webcast is furnished as Exhibit 99.2 to this Current Report.
The information furnished herewith pursuant to Items 2.02 and 7.01 of this Current Report, including Exhibits 99.1 and 99.2, shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. The information in Items 2.02 and 7.01 of this Current Report shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this Current Report, regardless of any general incorporation language in the filing.
Item 9.01 Financial Statements and Exhibits
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
STAAR Surgical Company |
|
May 7, 2024 |
By: |
/s/ Tom Frinzi |
|
|
Thomas G. Frinzi |
|
|
President and Chief Executive Officer |

STAAR Surgical Reports First Quarter 2024 Results
EVO ICL™ Continues to Outpace Refractive Industry Growth
Record Quarterly U.S. Sales and Multiple Strategic Agreements Secured
Reiterates Fiscal 2024 Net Sales Outlook and Increases Adjusted EBITDA
LAKE FOREST, CA, May 7, 2024 --- STAAR Surgical Company (NASDAQ: STAA), a leading developer, manufacturer and marketer of the EVO family of Implantable Collamer® Lenses (EVO ICL™) for myopia, astigmatism and presbyopia, today reported financial results for the first quarter ended March 29, 2024.
First Quarter 2024 Overview
•Net sales up 5% to $77.4 million and up 7% in constant currency
•ICL sales up 9% and units up 2%
•Gross margin at 78.9% vs. 78.3% year ago
•Net loss of $3.3 million or $0.07 loss per share vs. net income of $2.7 million or $0.05 income per share year ago
•Adjusted EBITDA of $5.3 million or $0.11 per share vs. $10.0 million or $0.20 per share year ago
•Record cash, cash equivalents and investments available for sale of $252.1 million at March 29, 2024
“Our first quarter results illustrate the impact of our commercial focus, which is driving continued market adoption and share gains of our EVO ICL,” said Tom Frinzi, President and CEO of STAAR Surgical. “Our strategic investments in people and processes in recent quarters are now bearing fruit. We are very pleased by the quickening pace of our momentum to start 2024, marked by several significant business milestones, including today’s announcement of the largest-ever practice commitment to EVO ICL in the U.S.”
Mr. Frinzi continued, “In the first quarter, STAAR continued to capture market share during a challenging macroeconomic environment for our surgeon customers and their patients. We saw strong momentum and remain well positioned to capitalize on our growth opportunities. In the U.S., sales were $5 million in the quarter, up 15% year over year and 21% sequentially. In APAC, we generated 9% sales growth, which was above our expectations, including 10% growth in China. Our EMEA region exceeded our expectations during the quarter, generating 11% sales growth with Belgium and the Netherlands joining China and Japan with a 20%+ share of refractive industry procedures1. Spain, one of Europe’s largest markets for refractive vision correction, is also quickly approaching 20% market share. The investments in our European markets started just a few years ago and are now paying off. For fiscal 2024, we are reiterating our net sales outlook range of $335 million to $340 million and expect, based on current trends, to be at the higher end of the range.”
First Quarter 2024 Financial Results
Net sales were $77.4 million for the first quarter of 2024, up 5% compared to $73.5 million reported in the prior year quarter. The sales increase in the first quarter was driven by ICL sales growth of $6.5 million, up 9%, and unit growth of 2% as compared to the prior year period. Cataract IOL and Other Product sales were down $2.7 million as compared to the prior year period. The Company exited its cataract IOL business in fiscal 2023.
Gross profit margin for the first quarter of 2024 was 78.9% of net sales compared to the prior year quarter of 78.3% of net sales. Product and country mix favorably impacted gross margin in the first quarter of 2024 as compared to the prior year quarter.
Operating expenses for the first quarter of 2024 were $63.3 million compared to the prior year quarter of $54.8 million. General and administrative expenses were $23.2 million compared to the prior year quarter of $18.1 million. The increase in general and administrative expenses was due to increased outside services and facilities costs. Selling and marketing expenses were $26.7 million compared to the prior year quarter of $26.4 million. The increase in selling and marketing expenseswas due to increased compensation-related expenses, trade shows and meetings expenses offset by decreased advertising and promotional activities. Research and development expenses were $13.4 million compared to the prior year quarter of $10.3 million. The increase in research and development expenses was due primarily to increased compensation-related expenses.
Operating loss for the first quarter of 2024 was $2.3 million or 2.9% of net sales as compared to operating income of $2.8 million or 3.8% of net sales for the first quarter of 2023.
Net loss for the first quarter of 2024 was $3.3 million or $0.07 loss per share compared with net income of $2.7 million or $0.05 income per share for the prior year quarter. The decrease in net income was attributable to increased SG&A expenses and losses on foreign currency transactions, partially offset by higher gross profit.
Cash, cash equivalents and investments available for sale at March 29, 2024, totaled $252.1 million, compared to $232.4 million at December 29, 2023.
Outlook
The Company reiterated its prior outlook for fiscal year 2024 net sales and increased its outlook for Adjusted EBITDA. The Company now expects the following for fiscal year 2024:
•Net sales of $335 million to $340 million.
•Adjusted EBITDA of approximately $39 million and Adjusted EBITDA per diluted share of approximately $0.75, compared to prior outlook of Adjusted EBITDA of approximately $36 million and Adjusted EBITDA per diluted share of approximately $0.70.
The outlook above contemplates EVO ICL sales growth of approximately 7% in APAC, including 10% in China; 10% growth in the Americas, including 10% in the U.S.; and EMEA sales consistent with fiscal year 2023.
Conference Call
The Company will host a conference call and webcast today, Tuesday, May 7 at 4:15 p.m. Eastern / 1:15 p.m. Pacific to discuss its financial results and operational progress. To access the conference call please dial 833-816-1164 for domestic participants and 412-317-1899 for international participants. No access code is required. Please ask to be joined into the STAAR Surgical Company call. The live webcast can be accessed from the investor relations section of the STAAR website at www.staar.com.
A taped replay of the conference call (Access Code 3030937) will be available for seven days beginning approximately one hour after the call’s conclusion. This replay can be accessed by dialing 877-344-7529 for domestic callers and 412-317-0088 for international callers. An archived webcast will also be available at www.staar.com.
1 Company estimates as of April 6, 2024, includes Spain refractive procedure market share of approximately 18%.
Use of Non-GAAP Financial Measures
To supplement the Company’s financial measures prepared in accordance with U.S. generally accepted accounting principles (GAAP), this press release and the accompanying tables include certain non-GAAP financial measures, including Adjusted EBITDA. Management uses these non-GAAP financial measures in its evaluation of Company operating performance and believes investors will find them useful in evaluating the Company’s operating performance, including cash flow generation, and in analyzing period-to-period financial performance of core business operations and underlying business trends. Non-GAAP financial measures are in addition to, not a substitute for, or superior to, measures of financial performance prepared in accordance with GAAP.
EBITDA is a non-GAAP financial measure, which is calculated by adding interest income and expense, net; provision for income taxes; and depreciation and amortization to net income. In calculating Adjusted EBITDA and Adjusted EBITDA per diluted share, the Company further adjusts for stock-based compensation expense. As stock-based compensation is a non-cash expense that can vary significantly based on the timing, size and nature of awards granted, the Company believes that the exclusion of stock-based compensation expense can assist investors in comparisons of Company operating results with other peer companies because (i) the amount of such expense in any specific period may not directly correlate to the underlying performance of our business operations and (ii) such expense can vary significantly between periods as a result of the timing of grants of new stock-based awards, including inducement grants in connection with hiring. Additionally, the Company believes that excluding stock-based compensation from Adjusted EBITDA and Adjusted EBITDA per diluted share assists management and investors in making meaningful comparisons between the Company’s operating performance and the operating performance of other companies that may use different forms of employee compensation or different valuation methodologies for their stock-based compensation. Investors should note that stock-based compensation is a key incentive offered to employees whose efforts contributed to the operating results in the periods presented and are expected to contribute to operating results in future periods. Investors should also note that such expenses will recur in the future.
The Company also presents certain financial information on a constant currency basis, which is intended to exclude the effects of foreign currency fluctuations. The Company conducts a significant part of its activities outside the U.S. It receives sales revenue and pays expenses principally in U.S. dollars, Swiss francs, Japanese yen and euros. The exchange rates between dollars and non-U.S. currencies can fluctuate greatly and can have a significant effect on the Company’s results when reported in U.S. dollars. In order to compare the Company's performance from period to period without the effect of currency, the Company will apply the same average exchange rate applicable in the prior period, or the “constant currency” rate to sales or expenses in the current period as well.
In the tables provided below, the Company has included a reconciliation of Adjusted EBITDA and Adjusted EBITDA per diluted share to net income and net income per diluted share, the most directly comparable GAAP financial measure, as well as supplemental financial information with net sales expressed in constant currency. The Company has also provided a reconciliation of forward-looking Adjusted EBITDA and Adjusted EBITDA per diluted share to net income and net income per diluted share. This represents forward-looking information, and actual results may vary. Please see the risks and assumptions referred to in the Safe Harbor section of this press release.
About STAAR Surgical
STAAR, which has been dedicated solely to ophthalmic surgery for over 40 years, designs, develops, manufactures and markets implantable lenses for the eye. These lenses are intended to provide visual freedom for patients, lessening or eliminating the reliance on glasses or contact lenses. All of these lenses are foldable, which permits the surgeon to insert them through a small incision. STAAR’s lens used in refractive surgery is called an Implantable Collamer® Lens or “ICL,” which includes the EVO ICL™ product line. More than 3,000,000 ICLs have been sold to date and STAAR markets these lenses in over 75 countries. To learn more about the ICL go to: EVOICL.com. Headquartered
in Lake Forest, CA, the company operates manufacturing and packaging facilities in Aliso Viejo, CA, Monrovia, CA and Nidau, Switzerland. For more information, please visit the Company’s website at www.staar.com.
Safe Harbor
All statements that are not statements of historical fact are forward-looking statements, including statements about any of the following: any financial projections, anticipated financial results, estimates and outlook (including as to net sales, Adjusted EBITDA, and Adjusted EBITDA per diluted share), plans, strategies, and objectives of management for 2024 and beyond or prospects for achieving such plans, expectations for sales, revenue, margin, expenses or earnings, and any statements of assumptions underlying any of the foregoing, including those relating to financial performance in the upcoming quarter, fiscal year 2024 and beyond. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include risks and uncertainties related to global economic conditions, as well as the factors set forth in the Company’s Annual Report on Form 10-K for the year ended December 29, 2023 under the caption “Risk Factors,” which is on file with the Securities and Exchange Commission and available in the “Investor Information” section of the company’s website under the heading “SEC Filings.” We disclaim any intention or obligation to update or revise any financial projections or forward-looking statement due to new information or events. These statements are based on expectations and assumptions as of the date of this press release and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. The risks and uncertainties include the following: global economic conditions; the impact of COVID-19; the discretion of regulatory agencies to approve or reject existing, new or improved products, or to require additional actions before or after approval, or to take enforcement action; international conflicts, trade disputes and substantial dependence on demand from Asia; and the willingness of surgeons and patients to adopt a new or improved product and procedure.
We intend to use our website as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Such disclosures will be included on our website in the ‘Investor Relations’ sections. Accordingly, investors should monitor such portions of our website, in addition to following our press releases, SEC filings and public conference calls and webcasts.
|
|
CONTACT: |
Investors & Media |
|
Brian Moore |
|
Vice President, Investor Relations and Corporate Development |
|
(626) 303-7902, Ext. 3023 |
|
bmoore@staar.com |
|
|
|
|
|
|
|
|
|
Consolidated Balance Sheets |
|
|
|
|
|
|
(in 000's) |
|
|
|
|
|
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS |
|
March 29, 2024 |
|
|
December 29, 2023 |
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
224,024 |
|
|
$ |
183,038 |
|
Investments available for sale |
|
|
21,125 |
|
|
|
37,688 |
|
Accounts receivable trade, net |
|
|
64,604 |
|
|
|
94,704 |
|
Inventories, net |
|
|
38,581 |
|
|
|
35,130 |
|
Prepayments, deposits, and other current assets |
|
|
17,381 |
|
|
|
14,709 |
|
Total current assets |
|
|
365,715 |
|
|
|
365,269 |
|
Investments available for sale |
|
|
6,963 |
|
|
|
11,703 |
|
Property, plant, and equipment, net |
|
|
72,337 |
|
|
|
66,835 |
|
Finance lease right-of-use assets, net |
|
|
146 |
|
|
|
183 |
|
Operating lease right-of-use assets, net |
|
|
34,600 |
|
|
|
34,387 |
|
Goodwill |
|
|
1,786 |
|
|
|
1,786 |
|
Deferred income taxes |
|
|
5,125 |
|
|
|
5,190 |
|
Other assets |
|
|
5,863 |
|
|
|
3,339 |
|
Total assets |
|
$ |
492,535 |
|
|
$ |
488,692 |
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
16,560 |
|
|
$ |
13,557 |
|
Obligations under finance leases |
|
|
166 |
|
|
|
165 |
|
Obligations under operating leases |
|
|
4,403 |
|
|
|
4,202 |
|
Allowance for sales returns |
|
|
6,284 |
|
|
|
6,174 |
|
Other current liabilities |
|
|
35,261 |
|
|
|
40,938 |
|
Total current liabilities |
|
|
62,674 |
|
|
|
65,036 |
|
|
|
|
|
|
|
|
Obligations under finance leases |
|
|
- |
|
|
|
42 |
|
Obligations under operating leases |
|
|
31,126 |
|
|
|
31,425 |
|
Deferred income taxes |
|
|
1,074 |
|
|
|
1,077 |
|
Asset retirement obligations |
|
|
96 |
|
|
|
103 |
|
Pension liability |
|
|
4,777 |
|
|
|
5,055 |
|
Total liabilities |
|
|
99,747 |
|
|
|
102,738 |
|
|
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
Common stock |
|
|
491 |
|
|
|
488 |
|
Additional paid-in capital |
|
|
447,716 |
|
|
|
436,947 |
|
Accumulated other comprehensive loss |
|
|
(4,712 |
) |
|
|
(4,113 |
) |
Accumulated deficit |
|
|
(50,707 |
) |
|
|
(47,368 |
) |
Total stockholders' equity |
|
|
392,788 |
|
|
|
385,954 |
|
Total liabilities and stockholders' equity |
|
$ |
492,535 |
|
|
$ |
488,692 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated Statements of Operations |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in 000's except for per share data) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year to Date |
|
|
|
% of Sales |
|
|
March 29, 2024 |
|
|
% of Sales |
|
|
March 31, 2023 |
|
|
Fav (Unfav) Amount |
|
|
% |
|
Net sales |
|
|
100.0 |
% |
|
$ |
77,356 |
|
|
|
100.0 |
% |
|
$ |
73,528 |
|
|
$ |
3,828 |
|
|
|
5.2 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
|
21.1 |
% |
|
|
16,321 |
|
|
|
21.7 |
% |
|
|
15,966 |
|
|
|
(355 |
) |
|
|
(2.2 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
78.9 |
% |
|
|
61,035 |
|
|
|
78.3 |
% |
|
|
57,562 |
|
|
|
3,473 |
|
|
|
6.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
30.0 |
% |
|
|
23,228 |
|
|
|
24.7 |
% |
|
|
18,098 |
|
|
|
(5,130 |
) |
|
|
(28.3 |
)% |
Selling and marketing |
|
|
34.5 |
% |
|
|
26,708 |
|
|
|
35.8 |
% |
|
|
26,354 |
|
|
|
(354 |
) |
|
|
(1.3 |
)% |
Research and development |
|
|
17.3 |
% |
|
|
13,380 |
|
|
|
14.0 |
% |
|
|
10,310 |
|
|
|
(3,070 |
) |
|
|
(29.8 |
)% |
Total selling, general, and administrative expenses |
|
|
81.8 |
% |
|
|
63,316 |
|
|
|
74.5 |
% |
|
|
54,762 |
|
|
|
(8,554 |
) |
|
|
(15.6 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income (loss) |
|
|
-2.9 |
% |
|
|
(2,281 |
) |
|
|
3.8 |
% |
|
|
2,800 |
|
|
|
(5,081 |
) |
|
|
(181.5 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income, net |
|
|
2.0 |
% |
|
|
1,529 |
|
|
|
2.5 |
% |
|
|
1,822 |
|
|
|
(293 |
) |
|
|
(16.1 |
)% |
Gain (loss) on foreign currency transactions |
|
|
-3.0 |
% |
|
|
(2,297 |
) |
|
|
0.0 |
% |
|
|
34 |
|
|
|
(2,331 |
) |
|
|
(6855.9 |
)% |
Royalty income |
|
|
0.7 |
% |
|
|
508 |
|
|
|
0.0 |
% |
|
|
- |
|
|
|
508 |
|
|
|
0.0 |
% |
Other income, net |
|
|
0.4 |
% |
|
|
330 |
|
|
|
0.1 |
% |
|
|
63 |
|
|
|
267 |
|
|
|
423.8 |
% |
Total other income, net |
|
|
0.1 |
% |
|
|
70 |
|
|
|
2.6 |
% |
|
|
1,919 |
|
|
|
(1,849 |
) |
|
|
(96.4 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before provision for income taxes |
|
|
-2.8 |
% |
|
|
(2,211 |
) |
|
|
6.4 |
% |
|
|
4,719 |
|
|
|
(6,930 |
) |
|
|
(146.9 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
|
1.5 |
% |
|
|
1,128 |
|
|
|
2.7 |
% |
|
|
2,009 |
|
|
|
881 |
|
|
|
43.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
|
-4.3 |
% |
|
|
(3,339 |
) |
|
|
3.7 |
% |
|
|
2,710 |
|
|
|
(6,049 |
) |
|
|
(223.2 |
)% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share - basic |
|
|
|
|
|
(0.07 |
) |
|
|
|
|
|
0.06 |
|
|
|
|
|
|
|
Net income (loss) per share - diluted |
|
|
|
|
|
(0.07 |
) |
|
|
|
|
|
0.05 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding - basic |
|
|
|
|
|
48,907 |
|
|
|
|
|
|
48,247 |
|
|
|
|
|
|
|
Weighted average shares outstanding - diluted |
|
|
|
|
|
48,907 |
|
|
|
|
|
|
49,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated Statements of Cash Flows |
|
|
|
|
|
|
(in 000's) |
|
|
|
|
|
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year to Date |
|
|
|
March 29, 2024 |
|
|
March 31, 2023 |
|
Cash flows from operating activities: |
|
|
|
|
|
|
Net income (loss) |
|
$ |
(3,339 |
) |
|
$ |
2,710 |
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
|
|
|
|
|
|
Depreciation of property and equipment |
|
|
1,237 |
|
|
|
1,113 |
|
Amortization of long-lived intangibles |
|
|
- |
|
|
|
7 |
|
Accretion/Amortization of investments available for sale |
|
|
(120 |
) |
|
|
(983 |
) |
Deferred income taxes |
|
|
61 |
|
|
|
57 |
|
Change in net pension liability |
|
|
(93 |
) |
|
|
(13 |
) |
Stock-based compensation expense |
|
|
6,339 |
|
|
|
6,065 |
|
Provision for sales returns and bad debts |
|
|
128 |
|
|
|
(377 |
) |
Inventory provision |
|
|
646 |
|
|
|
614 |
|
Changes in working capital: |
|
|
|
|
|
|
Accounts receivable |
|
|
29,837 |
|
|
|
(1,110 |
) |
Inventories |
|
|
(4,002 |
) |
|
|
(3,920 |
) |
Prepayments, deposits and other assets |
|
|
(5,485 |
) |
|
|
(4,249 |
) |
Accounts payable |
|
|
1,519 |
|
|
|
(3,168 |
) |
Other current liabilities |
|
|
(5,048 |
) |
|
|
(1,840 |
) |
Net cash provided by (used in) operating activities |
|
|
21,680 |
|
|
|
(5,094 |
) |
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
Acquisition of property and equipment |
|
|
(5,202 |
) |
|
|
(2,901 |
) |
Purchase of investments available for sale |
|
|
- |
|
|
|
(27,445 |
) |
Proceeds from sale or maturity of investments available for sale |
|
|
21,389 |
|
|
|
40,279 |
|
Net provided by investing activities |
|
|
16,187 |
|
|
|
9,933 |
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
Repayment of finance lease obligations |
|
|
(40 |
) |
|
|
(42 |
) |
Repurchase of employee common stock for taxes withheld |
|
|
(1,229 |
) |
|
|
(1,849 |
) |
Proceeds from vested restricted stock and exercise of stock options |
|
|
5,325 |
|
|
|
530 |
|
Net cash provided by (used in) financing activities |
|
|
4,056 |
|
|
|
(1,361 |
) |
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
|
(937 |
) |
|
|
10 |
|
|
|
|
|
|
|
|
Increase in cash and cash equivalents |
|
|
40,986 |
|
|
|
3,488 |
|
Cash and cash equivalents, at beginning of the period |
|
|
183,038 |
|
|
|
86,480 |
|
Cash and cash equivalents, at end of the period |
|
$ |
224,024 |
|
|
$ |
89,968 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of Non-GAAP Financial Measure |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
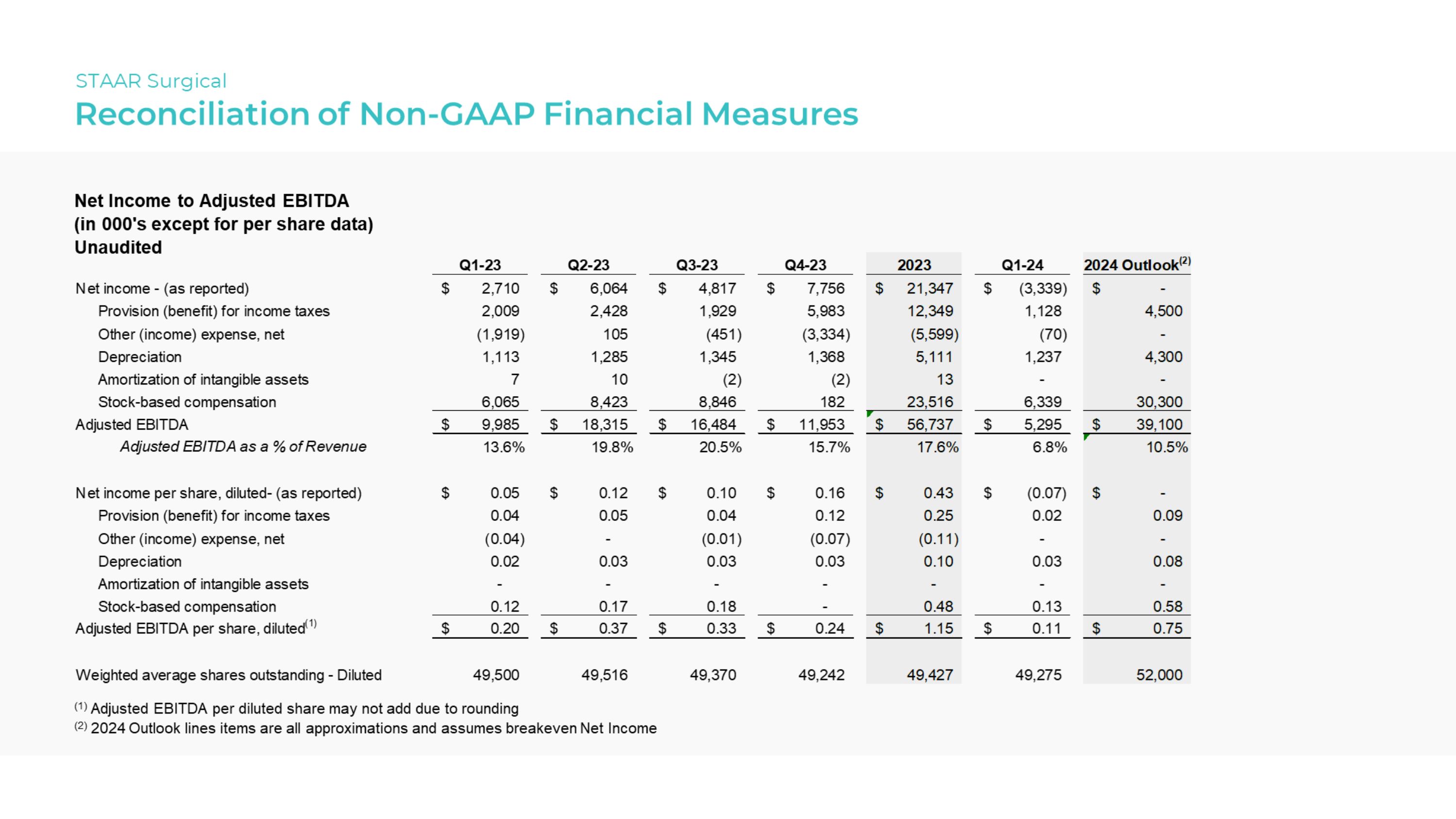
Net Income to Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in 000's except for per share data) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
|
|
Q1-22 |
|
|
Q2-22 |
|
|
Q3-22 |
|
|
Q4-22 |
|
|
2022 |
|
|
Q1-23 |
|
|
Q2-23 |
|
|
Q3-23 |
|
|
Q4-23 |
|
|
2023 |
|
|
Q1-24 |
|
|
2024 Outlook(2) |
|
Net income - (as reported) |
|
$ |
27,511 |
|
|
$ |
9,602 |
|
|
$ |
13,038 |
|
|
$ |
10,262 |
|
|
$ |
6,763 |
|
|
$ |
39,665 |
|
|
$ |
2,710 |
|
|
$ |
6,064 |
|
|
$ |
4,817 |
|
|
$ |
7,756 |
|
|
$ |
21,347 |
|
|
$ |
(3,339 |
) |
|
$ |
- |
|
Provision (benefit) for income taxes |
|
|
3,793 |
|
|
|
1,925 |
|
|
|
2,431 |
|
|
|
2,315 |
|
|
|
(784 |
) |
|
|
5,887 |
|
|
|
2,009 |
|
|
|
2,428 |
|
|
|
1,929 |
|
|
|
5,983 |
|
|
|
12,349 |
|
|
|
1,128 |
|
|
|
4,500 |
|
Other (income) expense, net |
|
|
2,035 |
|
|
|
586 |
|
|
|
1,551 |
|
|
|
1,128 |
|
|
|
(5,015 |
) |
|
|
(1,750 |
) |
|
|
(1,919 |
) |
|
|
105 |
|
|
|
(451 |
) |
|
|
(3,334 |
) |
|
|
(5,599 |
) |
|
|
(70 |
) |
|
|
- |
|
Depreciation |
|
|
3,608 |
|
|
|
994 |
|
|
|
1,030 |
|
|
|
1,077 |
|
|
|
1,380 |
|
|
|
4,481 |
|
|
|
1,113 |
|
|
|
1,285 |
|
|
|
1,345 |
|
|
|
1,368 |
|
|
|
5,111 |
|
|
|
1,237 |
|
|
|
4,300 |
|
Amortization of intangible assets |
|
|
34 |
|
|
|
8 |
|
|
|
7 |
|
|
|
7 |
|
|
|
6 |
|
|
|
28 |
|
|
|
7 |
|
|
|
10 |
|
|
|
(2 |
) |
|
|
(2 |
) |
|
|
13 |
|
|
|
- |
|
|
|
- |
|
Stock-based compensation |
|
|
14,605 |
|
|
|
3,894 |
|
|
|
5,754 |
|
|
|
5,727 |
|
|
|
4,996 |
|
|
|
20,371 |
|
|
|
6,065 |
|
|
|
8,423 |
|
|
|
8,846 |
|
|
|
182 |
|
|
|
23,516 |
|
|
|
6,339 |
|
|
|
30,300 |
|
Adjusted EBITDA |
|
$ |
51,586 |
|
|
$ |
17,009 |
|
|
$ |
23,811 |
|
|
$ |
20,516 |
|
|
$ |
7,346 |
|
|
$ |
68,682 |
|
|
$ |
9,985 |
|
|
$ |
18,315 |
|
|
$ |
16,484 |
|
|
$ |
11,953 |
|
|
$ |
56,737 |
|
|
$ |
5,295 |
|
|
$ |
39,100 |
|
Adjusted EBITDA as a % of Revenue |
|
|
22.4 |
% |
|
|
26.9 |
% |
|
|
29.4 |
% |
|
|
27.0 |
% |
|
|
11.5 |
% |
|
|
24.2 |
% |
|
|
13.6 |
% |
|
|
19.8 |
% |
|
|
20.5 |
% |
|
|
15.7 |
% |
|
|
17.6 |
% |
|
|
6.8 |
% |
|
10.5% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share, diluted- (as reported) |
|
$ |
0.56 |
|
|
$ |
0.19 |
|
|
$ |
0.26 |
|
|
$ |
0.21 |
|
|
$ |
0.14 |
|
|
$ |
0.80 |
|
|
$ |
0.05 |
|
|
$ |
0.12 |
|
|
$ |
0.10 |
|
|
$ |
0.16 |
|
|
$ |
0.43 |
|
|
$ |
(0.07 |
) |
|
$ |
- |
|
Provision (benefit) for income taxes |
|
|
0.08 |
|
|
|
0.04 |
|
|
|
0.05 |
|
|
|
0.05 |
|
|
|
(0.02 |
) |
|
|
0.12 |
|
|
|
0.04 |
|
|
|
0.05 |
|
|
|
0.04 |
|
|
|
0.12 |
|
|
|
0.25 |
|
|
|
0.02 |
|
|
|
0.09 |
|
Other (income) expense, net |
|
|
0.04 |
|
|
|
0.01 |
|
|
|
0.03 |
|
|
|
0.02 |
|
|
|
(0.10 |
) |
|
|
(0.04 |
) |
|
|
(0.04 |
) |
|
|
- |
|
|
|
(0.01 |
) |
|
|
(0.07 |
) |
|
|
(0.11 |
) |
|
|
- |
|
|
|
- |
|
Depreciation |
|
|
0.07 |
|
|
|
0.02 |
|
|
|
0.02 |
|
|
|
0.02 |
|
|
|
0.03 |
|
|
|
0.09 |
|
|
|
0.02 |
|
|
|
0.03 |
|
|
|
0.03 |
|
|
|
0.03 |
|
|
|
0.10 |
|
|
|
0.03 |
|
|
|
0.08 |
|
Amortization of intangible assets |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
Stock-based compensation |
|
|
0.30 |
|
|
|
0.08 |
|
|
|
0.12 |
|
|
|
0.12 |
|
|
|
0.10 |
|
|
|
0.41 |
|
|
|
0.12 |
|
|
|
0.17 |
|
|
|
0.18 |
|
|
|
- |
|
|
|
0.48 |
|
|
|
0.13 |
|
|
|
0.58 |
|
Adjusted EBITDA per share, diluted(1) |
|
$ |
1.04 |
|
|
$ |
0.35 |
|
|
$ |
0.48 |
|
|
$ |
0.41 |
|
|
$ |
0.15 |
|
|
$ |
1.39 |
|
|
$ |
0.20 |
|
|
$ |
0.37 |
|
|
$ |
0.33 |
|
|
$ |
0.24 |
|
|
$ |
1.15 |
|
|
$ |
0.11 |
|
|
$ |
0.75 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding - Diluted |
|
|
49,456 |
|
|
|
49,288 |
|
|
|
49,223 |
|
|
|
49,549 |
|
|
|
49,389 |
|
|
|
49,380 |
|
|
|
49,500 |
|
|
|
49,516 |
|
|
|
49,370 |
|
|
|
49,242 |
|
|
|
49,427 |
|
|
|
49,275 |
|
|
|
52,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Adjusted EBITDA per diluted share may not add due to rounding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2) 2024 Adjusted EBITDA Outlook line items are all approximations and assumes breakeven Net Income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ICL Sales by Geography |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in 000's) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year |
|
|
Three Months Ended |
|
ICL Sales by Region(5) |
|
2021 |
|
|
2022 |
|
|
2023 |
|
|
March 31, 2023 |
|
|
June 30, 2023 |
|
|
September 29, 2023 |
|
|
December 29, 2023 |
|
|
March 29, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Americas(1) |
|
$ |
14,054 |
|
|
$ |
20,114 |
|
|
$ |
22,233 |
|
|
$ |
5,566 |
|
|
$ |
5,954 |
|
|
$ |
5,449 |
|
|
$ |
5,264 |
|
|
$ |
6,260 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EMEA(2) |
|
|
37,343 |
|
|
|
36,715 |
|
|
|
39,318 |
|
|
|
10,180 |
|
|
|
9,782 |
|
|
|
9,253 |
|
|
|
10,103 |
|
|
|
11,299 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
APAC(3) |
|
|
161,508 |
|
|
|
212,883 |
|
|
|
257,876 |
|
|
|
54,879 |
|
|
|
77,376 |
|
|
|
66,367 |
|
|
|
59,254 |
|
|
|
59,592 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global ICL Sales |
|
$ |
212,905 |
|
|
$ |
269,712 |
|
|
$ |
319,427 |
|
|
$ |
70,625 |
|
|
$ |
93,112 |
|
|
$ |
81,069 |
|
|
$ |
74,621 |
|
|
$ |
77,151 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global ICL Sales Growth |
|
|
51 |
% |
|
|
27 |
% |
|
|
18 |
% |
|
|
20 |
% |
|
|
19 |
% |
|
|
13 |
% |
|
|
22 |
% |
|
|
9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global ICL Unit Growth |
|
|
48 |
% |
|
|
33 |
% |
|
|
19 |
% |
|
|
20 |
% |
|
|
21 |
% |
|
|
14 |
% |
|
|
19 |
% |
|
|
2 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year |
|
|
Three Months Ended |
|
ICL Sales by Country(4)(5) |
|
2021 |
|
|
2022 |
|
|
2023 |
|
|
March 31, 2023 |
|
|
June 30, 2023 |
|
|
September 29, 2023 |
|
|
December 29, 2023 |
|
|
March 29, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
China |
|
$ |
107,130 |
|
|
$ |
147,967 |
|
|
$ |
185,404 |
|
|
$ |
35,042 |
|
|
$ |
61,288 |
|
|
$ |
48,262 |
|
|
$ |
40,813 |
|
|
$ |
38,460 |
|
Growth |
|
|
50 |
% |
|
|
38 |
% |
|
|
25 |
% |
|
|
25 |
% |
|
|
33 |
% |
|
|
14 |
% |
|
|
30 |
% |
|
|
10 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Japan |
|
$ |
28,688 |
|
|
$ |
32,623 |
|
|
$ |
36,352 |
|
|
$ |
9,203 |
|
|
$ |
8,563 |
|
|
$ |
9,091 |
|
|
$ |
9,495 |
|
|
$ |
10,227 |
|
Growth |
|
|
56 |
% |
|
|
14 |
% |
|
|
11 |
% |
|
|
6 |
% |
|
|
13 |
% |
|
|
12 |
% |
|
|
16 |
% |
|
|
11 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
South Korea |
|
$ |
15,173 |
|
|
$ |
17,940 |
|
|
$ |
19,853 |
|
|
$ |
6,656 |
|
|
$ |
3,316 |
|
|
$ |
4,886 |
|
|
$ |
4,996 |
|
|
$ |
6,725 |
|
Growth |
|
|
36 |
% |
|
|
18 |
% |
|
|
11 |
% |
|
|
19 |
% |
|
|
(15 |
)% |
|
|
1 |
% |
|
|
39 |
% |
|
|
1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
9,478 |
|
|
$ |
15,070 |
|
|
$ |
17,168 |
|
|
$ |
4,396 |
|
|
$ |
4,446 |
|
|
$ |
4,162 |
|
|
$ |
4,164 |
|
|
$ |
5,039 |
|
Growth |
|
|
58 |
% |
|
|
59 |
% |
|
|
14 |
% |
|
|
71 |
% |
|
|
10 |
% |
|
|
6 |
% |
|
|
(8 |
)% |
|
|
15 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Americas includes the United States, Canada and Latin American countries |
|
|
|
|
|
|
|
(2) EMEA includes Spain, Germany, United Kingdom, European, Middle East and Africa Distributors |
|
|
|
|
|
|
|
(3) APAC includes China, Japan, South Korea, India and the rest of Asia Pacific distributors |
|
|
|
|
|
|
|
(4) ICL Sales by country includes countries representing more than 5% of total ICL sales in the most recently completed fiscal year |
|
|
|
|
|
|
|
(5) ICL sales do not include IOL, injector or other sales. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of Non-GAAP Financial Measure |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Constant Currency Sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in 000's) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year to Date |
|
|
|
|
|
As Reported |
|
|
Constant Currency |
|
Sales |
|
March 29, 2024 |
|
|
Effect of Currency |
|
|
Constant Currency |
|
|
March 31, 2023 |
|
|
$ Change |
|
|
% Change |
|
|
$ Change |
|
|
% Change |
|
ICL |
|
$ |
77,151 |
|
|
$ |
944 |
|
|
$ |
78,095 |
|
|
$ |
70,625 |
|
|
$ |
6,526 |
|
|
|
9.2 |
% |
|
$ |
7,470 |
|
|
|
10.6 |
% |
Cataract IOL |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,476 |
|
|
|
(1,476 |
) |
|
|
(100.0 |
)% |
|
|
(1,476 |
) |
|
|
(100.0 |
)% |
Other |
|
|
205 |
|
|
|
22 |
|
|
|
227 |
|
|
|
1,427 |
|
|
|
(1,222 |
) |
|
|
(85.6 |
)% |
|
|
(1,200 |
) |
|
|
(84.1 |
)% |
Total Sales |
|
$ |
77,356 |
|
|
$ |
966 |
|
|
$ |
78,322 |
|
|
$ |
73,528 |
|
|
$ |
3,828 |
|
|
|
5.2 |
% |
|
$ |
4,794 |
|
|
|
6.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NASDAQ: STAA 1Q 2024
Earnings Call Supplement MAY 7, 2024 Exhibit 99.2
02 Conference Call and Webcast Speakers Investor Contact TOM FRINZI PATRICK WILLIAMS Chair of the Board, President and CEO Chief Financial Officer WEBCAST ARCHIVE: Audio webcast archive will be available at http://investors.staar.com +1 626.303.7902 x3023 Brian Moore VP, Investor Relations and Corporate Development bmoore@staar.com http://investors.staar.com
All statements that are not statements of historical fact are forward-looking statements, including statements about any of the following: any financial projections, anticipated financial results, estimates and outlook (including as to net sales, Adjusted EBITDA, and Adjusted EBITDA per diluted share), plans, strategies, and objectives of management for 2024 and beyond or prospects for achieving such plans, expectations for sales, revenue, margin, expenses or earnings, and any statements of assumptions underlying any of the foregoing, including those relating to financial performance in the upcoming quarter, fiscal year 2024 and beyond. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include risks and uncertainties related to global economic conditions, as well as the factors set forth in the Company’s Annual Report on Form 10-K for the year ended December 29, 2023 under the caption “Risk Factors,” which is on file with the Securities and Exchange Commission and available in the “Investor Information” section of the Company’s website, www.staar.com, under the heading “SEC Filings.” We disclaim any intention or obligation to update or revise any financial projections or forward-looking statement due to new information or events. These statements are based on expectations and assumptions as of the date of this presentation and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. The risks and uncertainties include the following: global economic conditions; the impact of COVID-19; the discretion of regulatory agencies to approve or reject existing, new or improved products, or to require additional actions before or after approval, or to take enforcement action; international conflicts, trade disputes and substantial dependence on demand from Asia; and the willingness of surgeons and patients to adopt a new or improved product and procedure. We intend to use our website as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Such disclosures will be included on our website in the ‘Investor Relations’ sections. Accordingly, investors should monitor such portions of our website, in addition to following our presentations, SEC filings and public conference calls and webcasts. Forward Looking Statements 03

To supplement the Company’s financial measures prepared in accordance with U.S. generally accepted accounting principles (GAAP), this presentation and the accompanying tables include certain non-GAAP financial measures, including Adjusted EBITDA. Management uses these non-GAAP financial measures in its evaluation of Company operating performance and believes investors will find them useful in evaluating the Company’s operating performance, including cash flow generation, and in analyzing period-to-period financial performance of core business operations and underlying business trends. Non-GAAP financial measures are in addition to, not a substitute for, or superior to, measures of financial performance prepared in accordance with GAAP. EBITDA is a non-GAAP financial measure, which is calculated by adding interest income and expense, net; provision for income taxes; and depreciation and amortization to net income. In calculating Adjusted EBITDA and Adjusted EBITDA per diluted share, the Company further adjusts for stock-based compensation expense. As stock-based compensation is a non-cash expense that can vary significantly based on the timing, size and nature of awards granted, the Company believes that the exclusion of stock-based compensation expense can assist investors in comparisons of Company operating results with other peer companies because (i) the amount of such expense in any specific period may not directly correlate to the underlying performance of our business operations and (ii) such expense can vary significantly between periods as a result of the timing of grants of new stock-based awards, including inducement grants in connection with hiring. Additionally, the Company believes that excluding stock-based compensation from Adjusted EBITDA and Adjusted EBITDA per diluted share assists management and investors in making meaningful comparisons between the Company’s operating performance and the operating performance of other companies that may use different forms of employee compensation or different valuation methodologies for their stock-based compensation. Investors should note that stock-based compensation is a key incentive offered to employees whose efforts contributed to the operating results in the periods presented and are expected to contribute to operating results in future periods. Investors should also note that such expenses will recur in the future. In the appendix to this presentation, the Company has included a reconciliation of Adjusted EBITDA and Adjusted EBITDA per diluted share to net income and net income per diluted share, the most directly comparable GAAP financial measure. The Company has also provided a reconciliation of forward-looking Adjusted EBITDA and Adjusted EBITDA per diluted share to net income and net income per diluted share. This represents forward-looking information, and actual results may vary. Please see the risks and assumptions referred to in the Forward Looking Statements section of this presentation. Non-GAAP Financial Information 04
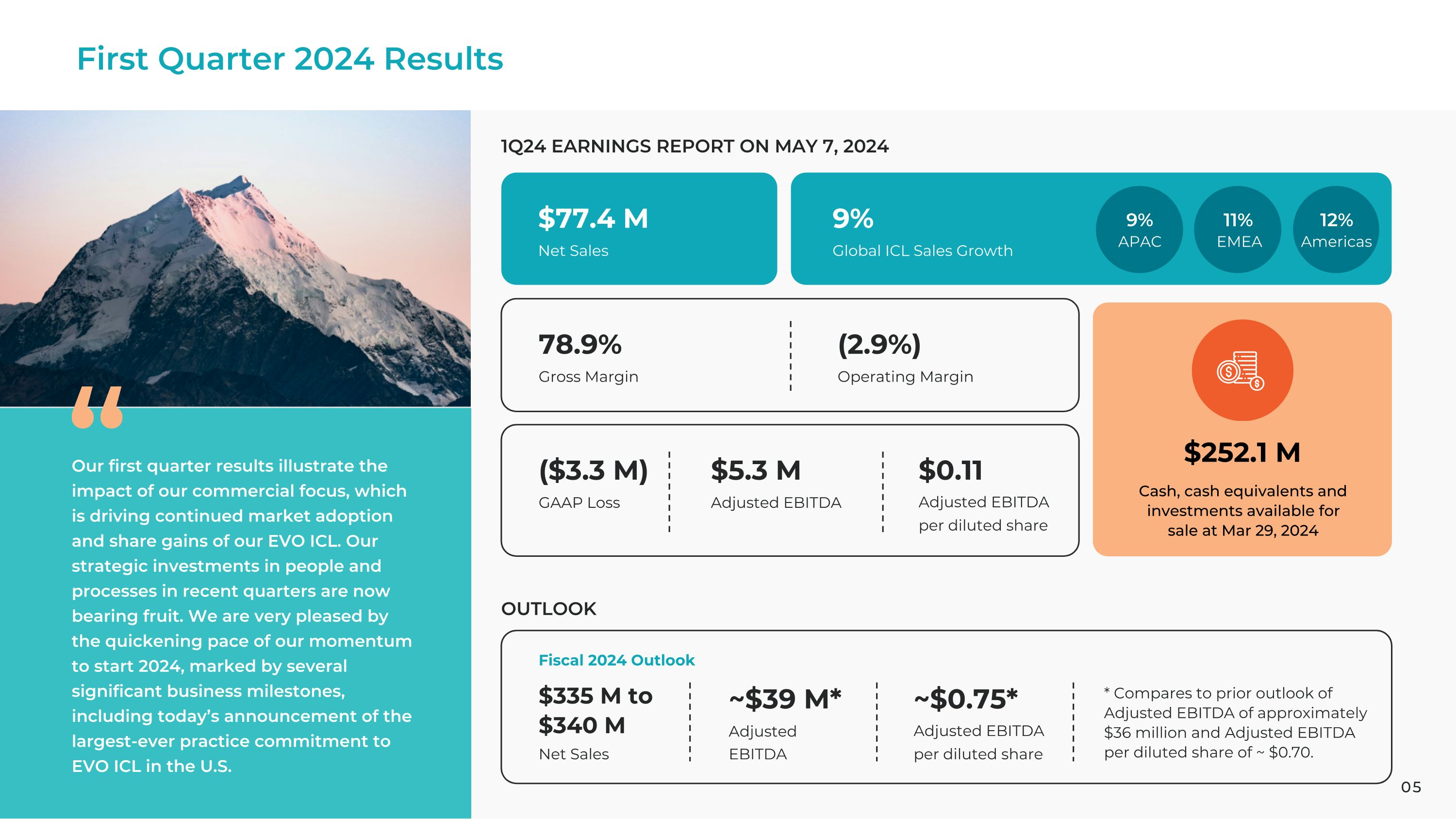
First Quarter 2024 Results 1Q24 EARNINGS REPORT ON MAY 7, 2024 OUTLOOK $77.4 M 9% Global ICL Sales Growth $252.1 M Cash, cash equivalents and investments available for sale at Mar 29, 2024 78.9% Gross Margin (2.9%) Operating Margin Net Sales ($3.3 M) GAAP Loss $335 M to $340 M $5.3 M $0.11 Adjusted EBITDA Adjusted EBITDA �per diluted share Net Sales Fiscal 2024 Outlook ~$39 M* ~$0.75* Adjusted EBITDA Adjusted EBITDA �per diluted share * Compares to prior outlook of Adjusted EBITDA of approximately $36 million and Adjusted EBITDA per diluted share of ~ $0.70. Our first quarter results illustrate the impact of our commercial focus, which is driving continued market adoption and share gains of our EVO ICL. Our strategic investments in people and processes in recent quarters are now bearing fruit. We are very pleased by the quickening pace of our momentum to start 2024, marked by several significant business milestones, including today’s announcement of the largest-ever practice commitment to EVO ICL in the U.S. 11% 9% EMEA 12% APAC Americas 05

Achieved three million total implantable collamer® lenses (ICLs) sold in March 2024. >1.5M lCLs have been sold in just the last three years, i.e. fiscal years 2021-2023. First Quarter 2024 & Recent Business Highlights Enhanced commercial focus is yielding positive results Our APAC, EMEA, Americas regions generated ICL sales growth of 9%, 11% and 12%, respectively in the first quarter vs. fiscal year outlook of 7%, flat and 10%, respectively Generated 21% sequential sales growth in the U.S., achieving record quarterly U.S. ICL sales of $5 million On May 7, 2024, STAAR announced the largest commitment ever to EVO ICL in the U.S. – a strategic agreement with Dr. Robert Lin and IQ Laser Vision as part of our U.S. Highway 93 go-to-market program. The agreement follows the Sharpe Vision agreement announced on January 23, 2024 Two new markets, Belgium and Netherlands, joined China and Japan with a 20%+ share of refractive procedures. Spain, at approximately 18% share, should be the next large market to reach 20% share. ENGAGED HUNDREDS OF U.S. AND APAC SURGEONS AT INDUSTRY MEETINGS IN MARCH & APRIL ASCRS Annual Meeting, Boston and STAAR APAC Experts Summit, Okinawa 44 posters and presentations featuring ICL, including at least two that should meaningfully increase surgeon confidence in the measurement of the eye and ICL lens size selection
Launched STAAR University, our medical science website, at ASCRS for surgeons and other healthcare professionals, featuring clinical data and research, and the ability to apply for grants
Introduced Stella® ordering and planning system 93% of those surveyed “Agreed” or “Strongly Agreed” that, based on the data presented, they were more comfortable recommending EVO ICL for patients -6 and above 06
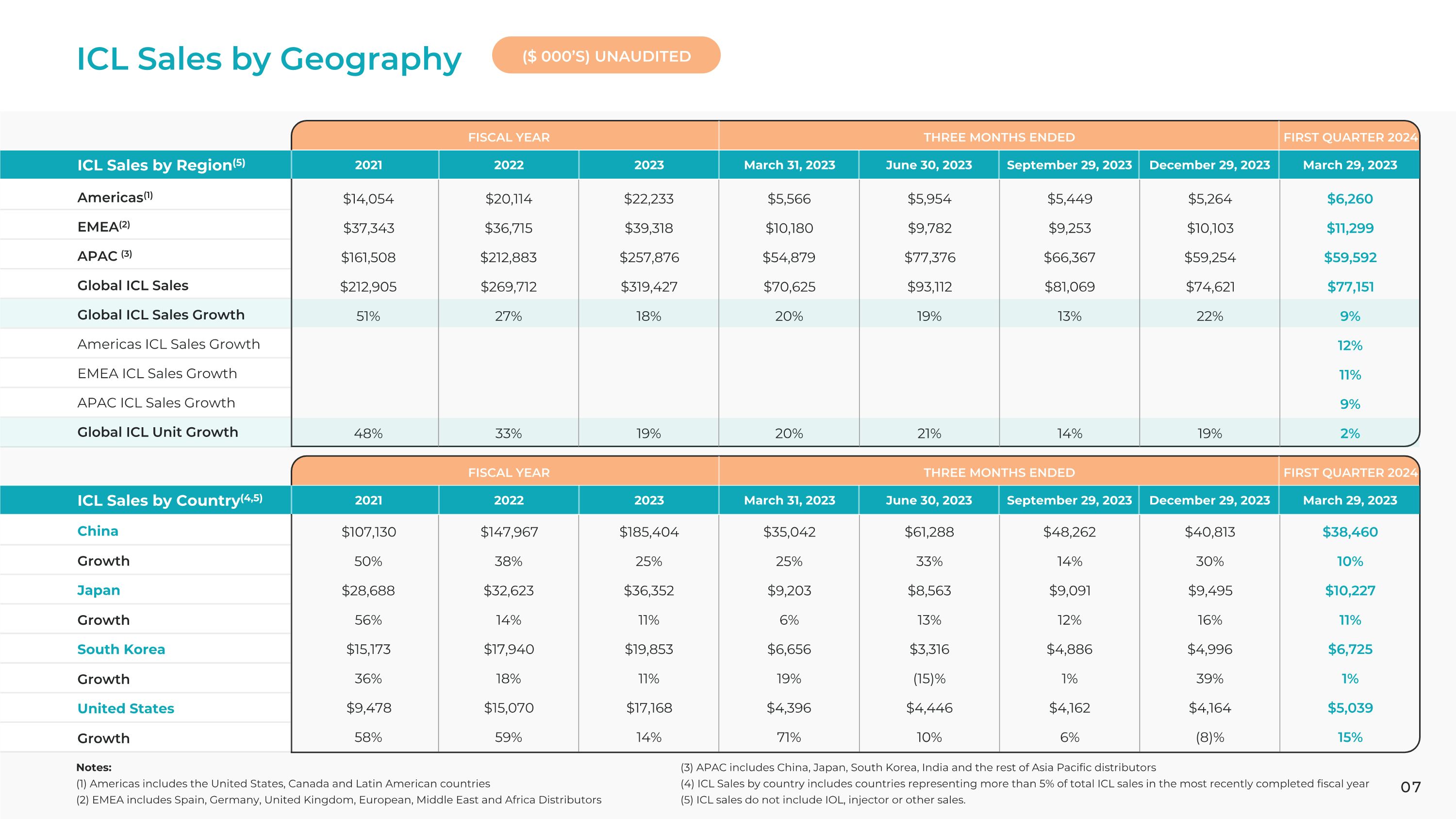
ICL Sales by Geography ICL Sales by Region(5) ICL Sales by Country(4,5) 2021 2021 2022 2022 2023 2023 March 31, 2023 March 31, 2023 June 30, 2023 June 30, 2023 September 29, 2023 September 29, 2023 December 29, 2023 December 29, 2023 March 29, 2023 March 29, 2023 Americas(1)
EMEA(2)
APAC (3)
Global ICL Sales
Global ICL Sales Growth
Americas ICL Sales Growth
EMEA ICL Sales Growth
APAC ICL Sales Growth
Global ICL Unit Growth China
Growth $14,054
$37,343
$161,508
$212,905
51%
48% $107,130
50%
$28,688
56%
$15,173
36%
$9,478
58% $20,114
$36,715
$212,883
$269,712
27%
33% $147,967
38%
$32,623
14%
$17,940
18%
$15,070
59% $22,233
$39,318
$257,876
$319,427
18%
19% $185,404
25%
$36,352
11%
$19,853
11%
$17,168
14% $5,566
$10,180
$54,879
$70,625
20%
20% $35,042
25%
$9,203
6%
$6,656
19%
$4,396
71% $5,954
$9,782
$77,376
$93,112
19%
21% $61,288
33%
$8,563
13%
$3,316
(15)%
$4,446
10% $5,449
$9,253
$66,367
$81,069
13%
14% $48,262
14%
$9,091
12%
$4,886
1%
$4,162
6% $5,264
$10,103
$59,254
$74,621
22%
19% $40,813
30%
$9,495
16%
$4,996
39%
$4,164
(8)% $6,260
$11,299
$59,592
$77,151
9%
12%
11%
9%
2% $38,460
10%
$10,227
11%
$6,725
1%
$5,039
15% FISCAL YEAR FISCAL YEAR THREE MONTHS ENDED THREE MONTHS ENDED FIRST QUARTER 2024 FIRST QUARTER 2024 Japan
Growth South Korea
Growth United States
Growth ($ 000’S) UNAUDITED Notes: (1) Americas includes the United States, Canada and Latin American countries (2) EMEA includes Spain, Germany, United Kingdom, European, Middle East and Africa Distributors (3) APAC includes China, Japan, South Korea, India and the rest of Asia Pacific distributors (4) ICL Sales by country includes countries representing more than 5% of total ICL sales in the most recently completed fiscal year (5) ICL sales do not include IOL, injector or other sales. 07

Significant Market Opportunity for EVO ICL™ MYOPIA Today: Every third person in the world STAAR EVO ICL’s near and long-term market opportunity remains exciting and vast, despite any transient noise (previously COVID and now macroeconomic headwinds associated with a normal business cycle). Myopia is a global pandemic with no known cure. 2050: Every other person in the world * Normal Myopia Key Factors with Myopia Environmental Factors Genetic Predisposition *BHVI, adapted from Holden et al. 2016 Ophthalmology. 36% of studies defined high myopia as -6.0D or more. Billions of People The Bigger Picture 08

EVO ICL™ Procedure and Collamer® Material Advantage 200+ Peer-Reviewed ICL Clinical Papers Quick procedure and recovery Additive / Preserves the Cornea 3 Million+ Lenses Sold Does not cause dry eye syndrome 3,4 UV Protection 30+ Year History of Safety and Effectiveness Reversible Lens Implant Sharp, clear vision Day & Night 1,2 99.4% of EVO patients surveyed would choose EVO again5 COLLAMER COLLAGEN + POLYMER 1) Martínez-Plaza E, López-Miguel A, López-de la Rosa A, et al. Effect of the EVO+ Visian Phakic Implantable Collamer Lens on Visual Performance and Quality of Vision and Life, Am J Ophthalmol 2021;226:117-125. 2) Packer M. Evaluation of the EVO/EVO+ Sphere and Toric Visian ICL: Six month results from the United States Food and Drug Administration clinical trial. Clinical Ophthalmology. 2022;16:1541-53. 3) Ganesh S, Brar S, Pawar A. Matched population comparison of visual outcomes and patient satisfaction between 3 modalities for the correction of low to moderate myopic astigmatism. Clin Ophthalmol. 2017;11:1253-1263. 4) Naves J.S, Carracedo G, Cacho-Babillo I, Diadenosine nucleotid measurements as dry-eye score in patients after LASIK and ICL surgery. Presented at American Society of Cataract and Refractive Surgery (ASCRS) 2012. 5) STAAR Survey. 09 The Bigger Picture

IT’S OUR TIME… IT’S EVO’S TIME!
v3.24.1.u1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
STAAR Surgical (NASDAQ:STAA)
Historical Stock Chart
From Oct 2024 to Nov 2024
STAAR Surgical (NASDAQ:STAA)
Historical Stock Chart
From Nov 2023 to Nov 2024