0001808805FALSE2023FY00018088052023-01-012023-12-3100018088052023-06-30iso4217:USD00018088052024-02-15xbrli:shares00018088052023-12-3100018088052022-12-31iso4217:USDxbrli:shares00018088052022-01-012022-12-310001808805us-gaap:CommonStockMember2021-12-310001808805us-gaap:AdditionalPaidInCapitalMember2021-12-310001808805us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001808805us-gaap:RetainedEarningsMember2021-12-3100018088052021-12-310001808805us-gaap:CommonStockMember2022-01-012022-12-310001808805us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001808805us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310001808805us-gaap:RetainedEarningsMember2022-01-012022-12-310001808805us-gaap:CommonStockMember2022-12-310001808805us-gaap:AdditionalPaidInCapitalMember2022-12-310001808805us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001808805us-gaap:RetainedEarningsMember2022-12-310001808805us-gaap:CommonStockMember2023-01-012023-12-310001808805us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001808805us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001808805us-gaap:RetainedEarningsMember2023-01-012023-12-310001808805us-gaap:CommonStockMember2023-12-310001808805us-gaap:AdditionalPaidInCapitalMember2023-12-310001808805us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001808805us-gaap:RetainedEarningsMember2023-12-310001808805us-gaap:PrivatePlacementMember2021-06-092021-06-090001808805us-gaap:PrivatePlacementMember2021-06-0900018088052021-06-012021-06-30naut:Segment0001808805srt:MinimumMembernaut:LaboratoryEquipmentMember2023-12-310001808805srt:MaximumMembernaut:LaboratoryEquipmentMember2023-12-310001808805naut:PrototypeEquipmentMember2023-12-310001808805us-gaap:ComputerEquipmentMember2023-12-310001808805us-gaap:FurnitureAndFixturesMember2023-12-310001808805us-gaap:OfficeEquipmentMembersrt:MinimumMember2023-12-310001808805srt:MaximumMemberus-gaap:OfficeEquipmentMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MutualFundMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MutualFundMemberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ShortTermInvestmentsMemberus-gaap:MutualFundMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MutualFundMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:ShortTermInvestmentsMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ShortTermInvestmentsMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMemberus-gaap:ShortTermInvestmentsMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMember2023-12-310001808805us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310001808805us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ShortTermInvestmentsMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ShortTermInvestmentsMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ShortTermInvestmentsMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CashAndCashEquivalentsMember2023-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ShortTermInvestmentsMember2023-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MutualFundMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MutualFundMemberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ShortTermInvestmentsMemberus-gaap:MutualFundMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MutualFundMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:ShortTermInvestmentsMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ShortTermInvestmentsMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMemberus-gaap:ShortTermInvestmentsMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMember2022-12-310001808805us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2022-12-310001808805us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ShortTermInvestmentsMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ShortTermInvestmentsMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ShortTermInvestmentsMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CashAndCashEquivalentsMember2022-12-310001808805us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ShortTermInvestmentsMember2022-12-310001808805naut:LongTermInvestmentsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001808805us-gaap:USTreasurySecuritiesMember2023-12-310001808805us-gaap:CommercialPaperMember2023-12-310001808805us-gaap:CorporateDebtSecuritiesMember2023-12-310001808805us-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310001808805us-gaap:USTreasurySecuritiesMember2022-12-310001808805us-gaap:CommercialPaperMember2022-12-310001808805us-gaap:CorporateDebtSecuritiesMember2022-12-310001808805us-gaap:USGovernmentAgenciesDebtSecuritiesMember2022-12-310001808805naut:LaboratoryEquipmentMember2023-12-310001808805naut:LaboratoryEquipmentMember2022-12-310001808805us-gaap:LeaseholdImprovementsMember2023-12-310001808805us-gaap:LeaseholdImprovementsMember2022-12-310001808805us-gaap:ComputerEquipmentMember2022-12-310001808805naut:FurnitureFixturesAndOfficeEquipmentMember2023-12-310001808805naut:FurnitureFixturesAndOfficeEquipmentMember2022-12-310001808805naut:PrototypeEquipmentMember2022-12-310001808805us-gaap:ConstructionInProgressMember2023-12-310001808805us-gaap:ConstructionInProgressMember2022-12-310001808805naut:A2021EquityIncentivePlanMember2023-12-310001808805naut:A2021EquityIncentivePlanMember2022-12-310001808805naut:A2021EmployeeStockPurchasePlanMember2023-12-310001808805naut:A2021EmployeeStockPurchasePlanMember2022-12-310001808805us-gaap:DomesticCountryMember2023-12-310001808805us-gaap:StateAndLocalJurisdictionMember2023-12-310001808805us-gaap:DomesticCountryMemberus-gaap:ResearchMember2023-12-310001808805us-gaap:ResearchMemberus-gaap:StateAndLocalJurisdictionMember2023-12-310001808805naut:A2021EmployeeStockPurchasePlanMemberus-gaap:EmployeeStockMember2023-01-012023-12-31xbrli:pure0001808805naut:A2021EmployeeStockPurchasePlanMember2023-01-012023-12-310001808805naut:A2021EmployeeStockPurchasePlanMember2022-01-012022-12-310001808805naut:A2021EquityIncentivePlanMember2023-01-012023-12-310001808805us-gaap:EmployeeStockOptionMembersrt:MinimumMember2023-01-012023-12-310001808805srt:MaximumMemberus-gaap:EmployeeStockOptionMember2023-01-012023-12-310001808805us-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-01-012022-12-310001808805srt:MaximumMemberus-gaap:EmployeeStockOptionMember2022-01-012022-12-310001808805us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001808805us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001808805naut:StockOptionsAndEmployeeStockPurchasePlanMemberus-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-12-310001808805naut:StockOptionsAndEmployeeStockPurchasePlanMemberus-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-12-310001808805us-gaap:GeneralAndAdministrativeExpenseMembernaut:StockOptionsAndEmployeeStockPurchasePlanMember2023-01-012023-12-310001808805us-gaap:GeneralAndAdministrativeExpenseMembernaut:StockOptionsAndEmployeeStockPurchasePlanMember2022-01-012022-12-310001808805naut:StockOptionsAndEmployeeStockPurchasePlanMember2023-01-012023-12-310001808805naut:StockOptionsAndEmployeeStockPurchasePlanMember2022-01-012022-12-3100018088052021-08-310001808805naut:October2021ToOctober2031Member2020-12-310001808805naut:October2022ToOctober2031Member2021-12-310001808805srt:MaximumMembernaut:SanCarlosMember2021-12-310001808805naut:SanCarlosMember2021-12-310001808805naut:SanDiegoMember2022-11-3000018088052020-12-3100018088052022-11-300001808805us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001808805us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001808805naut:EmployeeStockPurchasePlanMember2023-01-012023-12-310001808805naut:EmployeeStockPurchasePlanMember2022-01-012022-12-3100018088052023-10-012023-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
__________________________________________________________________________
FORM 10-K
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2023
OR
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to ___________
Commission file number 001-39434
NAUTILUS BIOTECHNOLOGY, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | |
Delaware | | 98-1541723 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
2701 Eastlake Avenue East Seattle, Washington |
|
| 98102 |
(Address of Principal Executive Offices) | | (Zip Code) |
Registrant's telephone number, including area code: (206) 333-2001
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | NAUT | The Nasdaq Stock Market LLC |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | |
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
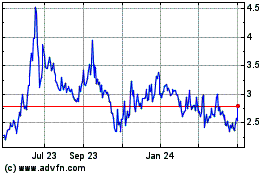
The aggregate market value of the registrant’s common stock held by non-affiliates, based upon the closing price of the common stock on June 30, 2023, as reported by The Nasdaq Stock Market LLC, was $219 million. Shares of common stock held by each executive officer and director and by each other person who is deemed to be an affiliate of the registrant have been excluded from such computation. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The registrant had outstanding 125,098,080 shares of common stock as of February 15, 2024.
DOCUMENTS INCORPORATED BY REFERENCE
None.
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are based on our management’s beliefs and assumptions and on information currently available to our management. The forward-looking statements are contained principally in the section entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Forward-looking statements include, but are not limited to, statements concerning the following:
•our dependence on the success of our proteomics platform (the “Nautilus platform”), which remains in the development stage and subject to scientific and technical validation;
•our expectations regarding the timing and progress of the development of the Nautilus platform and any commercialization timelines;
•our expectations regarding the functionality of the Nautilus platform;
•our estimates of our addressable market, market growth, future revenue, key performance indicators, expenses, capital requirements and needs for additional financing;
•our expectations regarding the rate and degree of market acceptance of the Nautilus platform;
•the impact of the Nautilus platform on the field of proteomics and the size and growth of the addressable proteomics market;
•our ability to manage and grow our business and commercialize our Nautilus platform;
•our ability to successfully implement our three phase commercial launch plan;
•the implementation of our business model and strategic plans for the Nautilus platform;
•our ability to establish and maintain intellectual property protection for our products or avoid or defend claims of infringement;
•our expectations regarding the use of proceeds from the Business Combination;
•the performance of third-party partners, manufacturers and suppliers;
•changes in applicable laws or regulations;
•our ability to raise financing in the future;
•our success in retaining or recruiting, or changes required in, our officers, key employees or directors or other key personnel;
•the volatility of the trading price of our common stock;
•our ability to develop and commercialize new products;
•our expectations about market trends;
•the impact of local, regional, national and international economic conditions and events including the COVID-19 pandemic, the conflicts in Eastern Europe and the Middle East, increasing interest rates, instability in the global financial markets and general economic downturns, on the foregoing; and
•other factors including but not limited to those detailed under the section entitled “Risk Factors.”
Forward-looking statements include statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” or similar expressions and the negatives of those terms.
Forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. We discuss these risks in greater detail in Part I, Item 1A, “Risk Factors,” elsewhere in this Annual Report on Form 10-K. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this Annual Report on Form 10-K may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
The forward-looking statements made in this Annual Report on Form 10-K relate only to events as of the date on which the statements are made. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
This Annual Report on Form 10-K also contains estimates, projections and other information concerning our industry, our business, and market opportunity, including data regarding the estimated size of the market. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources.
This Annual Report on Form 10-K contains references to trademarks and service marks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this Annual Report on Form 10-K may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of it by, any other companies.
Part I
Item 1. Business
BACKGROUND OF BUSINESS COMBINATION
On June 9, 2021 (the “Closing Date”), Nautilus Biotechnology, Inc., a Delaware corporation (f/k/a ARYA Sciences Acquisition Corp III (“ARYA”)), consummated the business combination (the “Business Combination”) pursuant to the terms of that certain Business Combination Agreement, dated as of February 7, 2021 (the “Business Combination Agreement”), by and among ARYA, Mako Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of ARYA (“Mako Merger Sub”), and Nautilus Subsidiary, Inc., a Delaware corporation (f/k/a Nautilus Biotechnology, Inc.) (“Legacy Nautilus”). The Business Combination Agreement provided for, among other things, (i) the domestication of ARYA as a corporation incorporated under the laws of the State of Delaware, upon which ARYA changed its name to “Nautilus Biotechnology, Inc.” (together with its consolidated subsidiary, the “Company” “New Nautilus” or “Nautilus”) and (ii) the merger of Mako Merger Sub with and into Legacy Nautilus (the “Merger”), with Legacy Nautilus as the surviving company in the Merger and, after giving effect to such Merger, Legacy Nautilus becoming a wholly-owned subsidiary of New Nautilus.
Concurrently with the execution of the Business Combination Agreement, ARYA entered into Subscription Agreements (each, a “Subscription Agreement”) with certain investors (each, a “PIPE Investor”), pursuant to which the PIPE Investors subscribed for and purchased, and ARYA issued and sold to the PIPE Investors, on the Closing Date, an aggregate of 20,000,000 shares of common stock of New Nautilus (“Common Stock”) for aggregate gross proceeds of $200,000,000 (the “PIPE Financing”).
As of the open of trading on June 10, 2021, the Common Stock began trading on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “NAUT.”
Unless expressly indicated or the context requires otherwise, the terms “Nautilus,” “New Nautilus,” the “Company,” the “Registrant,” “we,” “us” and “our” in this Form 10-K refer to Nautilus Biotechnology, Inc., the parent entity formerly named ARYA Sciences Acquisition Corp III, and where appropriate, our wholly-owned subsidiaries (including Legacy Nautilus).
OVERVIEW
We are a development stage life sciences company creating a platform technology for quantifying and unlocking the complexity of the proteome. Our mission is to transform the field of proteomics by democratizing access to the proteome and enabling fundamental advancements across human health and medicine. We were founded on the belief that incremental advancements of existing technologies are inadequate, and that a bold scientific leap would be required to radically reinvent proteomics and revolutionize precision medicine. Our vision is to integrate our breakthrough innovations in computer science, engineering, and biochemistry to develop and commercialize a proteomic analysis technology of extreme sensitivity and scale. To accomplish this, we have built a prototype of a proteome analysis system, an instrument to perform massively parallel single protein molecule measurements which will be further developed to deliver the speed, simplicity, accuracy, and versatility that we believe is necessary to establish a new gold standard in the field.
The human proteome, the make-up of all the proteins in a human, is among the most dynamic and valuable sources of biological insight in modern-day science. Unlike the genome, which is largely unchanging throughout an individual’s lifetime, the proteome is an ever-changing source of biological information. Our proteins directly control and determine the functions of our cells, yet we lack the ability to measure all of them with the ease, breadth and sensitivity that is used to measure DNA today. We believe that deep characterization of the proteome will have the potential to unveil an entirely new layer of complexity and valuable biological information that may have significant implications across life sciences, healthcare and drug development. Approximately 95% of FDA-approved drug targets are proteins, and yet today we still lack the ability to routinely read and quantify all of the proteins in our cells, or to fully map the downstream changes and modifications to those proteins which may define their biological function.
By leveraging our novel design coupled with advanced machine learning software, we believe our Nautilus platform, which includes our end-to-end solution comprised of the proteome analysis system, consumables, and software, has the potential to rapidly and reproducibly identify up to approximately 95% of proteins in a sample from virtually any organism, and could have the ability to detect and map the diverse landscape of modifications on those proteins. We believe that unlocking proteomics has the potential to create a long-term transformation of basic science, translational research, and healthcare.
Current proteomics platforms for broadly quantifying the abundance of proteins within samples generally fall into two classes: affinity-based and mass spectrometry-based methods. For years, these methods have facilitated novel drug development and improved diagnostics. As with most technology platforms however, these also suffer from distinct limitations that make
simple, high-throughput, ultra-deep characterization of the proteome challenging. Mass spectrometry approaches have tremendous flexibility and thus have been applied to a wide range of applications, however their use requires a trade-off to be made between either depth or throughput; meaning that a researcher can either look at one sample in a deep analysis or at many samples in a shallow analysis. Additionally, challenges in ease of use and sensitivity have limited the ability of mass spectrometry-based methods from easily, broadly and quickly characterizing the entirety of the proteome. Affinity-based approaches use the binding attraction of antibodies to proteins to capture and measure protein targets in parallel. These technologies can provide greater sensitivity, however this approach is directly dependent on the availability of high quality, highly specific and sensitive affinity reagents, which can limit the scale, reproducibility and accuracy. Consequently, we believe researchers are forced into an unattractive trade-off between the number of samples in a study and the depth and breadth of the analysis. These trade-offs limit researchers’ ability to advance characterization of the proteome to match the current, and highly valuable, characterization of the genome. We believe the limitations of both platforms have prevented progress towards achieving comprehensive proteome and deep proteoform characterization. If detecting and quantifying the complexity of the human proteome were as simple and easy as detecting an entire human genome, we believe a new set of questions could be asked:
•Down to the very low frequencies of expressed proteins, how are healthy tissue cells different from diseased cells?
•What will a comprehensive map of nearly all proteins by organ tissue type tell us about our biology?
•What specific patterns of protein modifications are present in disease, and why?
•What happens to our proteome when we get sick, and how does it change with treatment?
We believe that our Nautilus platform has the potential to position us to answer these questions, and many others that have not previously been possible to fully investigate. Due to the extensive applications and broad potential of large-scale proteomic characterization, we believe the proteomics market is currently among one of the largest untapped opportunities in the biological sciences today. According to BCC Research, the existing proteomics research market is currently estimated to be approximately $27 billion annual spend as of 2022, made up primarily of mass spectrometry and affinity-based quantification methods. Over the longer-term, the proteomics market is expected to reach approximately $55 billion by 2027, representing a compound annual growth rate, or CAGR, of 15% over the five-year period. Further, we believe there are substantial adjacent opportunities across translational research in drug target discovery, precision medicine development, clinical diagnostics, and other disciplines such as food and environmental science.
We plan to initially target the life sciences proteomics research market and are currently entering the first phase of our product development and commercialization strategy. In this first phase, we are focused on developing partnerships with key biopharma companies and leading academic institutions to create a founding group of collaborators that will gain experience with our technology, jointly publish research using our Nautilus platform, and generally help validate our initial applications. As of the date of filing this Annual Report, we have partnerships with Genentech, Amgen, The University of Texas MD Anderson Cancer Research Center, and the Translational Genomics Research Institute, among others. In the second phase we plan to launch an early access program to an expanded group of customers. We believe these customers will become important reference sites and key influencers that aid in the market adoption of our Nautilus platform, and will help us build a strong value proposition ahead of full commercial launch. In our third phase of commercialization, we intend to execute a broad commercial launch of our Nautilus platform including the introduction of our proteome analysis system, which is an integrated fluidics and optics system for massively parallel single protein molecule detection, accompanied by consumable reagents and analysis software, in direct sales to customers across academia and industry. The launch of our proteome analysis system is expected to be done with a multi-year product roadmap of system enhancements and new applications designed to help our customers achieve their research objectives and expand the utility of our Nautilus platform. We also plan to leverage our machine learning software to build a data analysis and insights engine that improves over time as we grow our data sources and the analysis learns to deliver better accuracy and identify new potential discoveries. We believe by following this methodical pathway, we can optimize the development of our Nautilus platform, establish a steady flow of validating publications, appropriately scale our operations, deliver exceptional customer experiences, and help ensure we are delivering long term value and revenue growth.
Since inception in 2016, we have worked diligently to secure a strong intellectual property portfolio, and we have successfully filed and obtained numerous key patents. Our management team also brings a unique combination of experiences from the fields of technology and life sciences, with a proven track record of building successful businesses based on novel technology. Our company is a highly interdisciplinary organization, and as of December 31, 2023 we were comprised of approximately 167 employees, with 59 of such employees holding a Ph.D. Our organization is driven by the pursuit of deep, hard science, and our Scientific Advisory Board is comprised of world-renowned scientific leaders that support our vision.
OUR STRENGTHS
•Highly disruptive proteomics technology. Our Nautilus proteome analysis platform is designed to be a disruptive, single protein molecule analysis technology of extreme sensitivity, scale, and ease-of-use. Leveraging a novel system architecture, advanced machine learning and algorithms, we believe our Nautilus platform has the potential to identify substantially all proteins in a sample from almost any organism. We have designed our Nautilus platform technology by substantially reimagining methods of protein analysis, rather than an incremental or evolutionary advancement. We refer to Nautilus’ framework for what we believe to be a fundamentally different approach to protein analysis as Protein Identification by Short-epitope Mapping (PrISM). We believe that the prototype of our Nautilus proteome analysis system has also demonstrated the ability to detect the patterns of modifications made to proteins, while preserving the context of those modifications on the molecule where they exist, a capability that we do not believe is possible with existing affinity-based or shotgun mass spectrometry-based methods.
•Novel end-to-end proteomics detection platform of extreme sensitivity. We aim to be the first commercially available proteomics detection platform technology and end-to-end solution to decode and quantify virtually the entire proteome, including the variations and modifications of proteins. Our Nautilus platform consists of instruments, reagent consumables and software that we believe has the potential to deliver broad proteomic profiling to the market and potentially unlock the vast, dynamic, and valuable biological information contained in the proteome. With each instrument sale, there is expected to be accompanying recurring revenue comprised of reagent consumable sales, instrumentation service, support, and software that creates a comprehensive proteomics solution.
•Open, flexible and customizable technology platform. Our Nautilus platform is designed to allow our customers to analyze their samples in a variety of run configurations by utilizing flow cells which each contain 4 discrete and independent lanes, analyzed on an instrument that can be loaded with up to 3 flow cells per run. At launch, our platform is designed to either maximize analysis depth by running all 12 lanes on 1 sample, or maximize analysis throughput by running a single sample per lane. This design is also highly compatible with molecular barcoding, which may be introduced in the future to multiplex increasingly larger sample numbers per run. Further, with the introduction of an affinity binding reagent labeling kit, our platform is also designed to be able to incorporate off-the-shelf affinity binding reagents that have already been created by biopharma, academia, or commercial affinity reagent manufacturers. We believe that the ability to customize the type of analysis performed by adding or combining these affinity binding reagents with our Nautilus provided reagent kits could provide a very powerful and desirable analysis for our customers.
•Immense data production capacity coupled with machine learning can unlock new proteomic insights. We have designed the Nautilus platform to create and process a vast amount of proteomic data. The Nautilus platform is expected to generate up to approximately 20 terabytes of digital protein data per run, which will then be decoded using our proprietary machine learning algorithms and cloud-based data processing infrastructure. As we expand and enrich our database with increasing amounts of digital proteomic data over time, we plan to deploy our machine learning algorithms to continuously improve and benefit from each new experiment generated with our Nautilus platform. We believe that this feedback loop has the potential to deliver future value to our customers through the continuous improvements in our analytics, thereby encouraging the analysis, and re-analysis, of more samples through our Nautilus platform to benefit from these advancements.
•Commercial model with clear market entry point, designed to support a wide variety of customers and applications. Many successful life sciences research tools companies with disruptive technology have employed a business model similar to our planned commercial model. However, we believe a key advantage for us is the near-term commercial opportunity of capitalizing on the existing mass spectrometry-based proteomics marketplace estimated at over 16,000 installed systems. Our price point is expected to be in-line with mass spectrometry system budgets allocated for broad scale proteomics applications, and thus with a premium instrumentation average selling price, we plan to operate with a very efficient sales model. Further, since the early days of our product development, we have consulted with biopharma companies, academic institutions and research organizations to inform our product development plan and specifically address our target customer needs.
•Our Nautilus platform technology could position us as a leader in a large initial life sciences research market and provide a path to the clinical diagnostics. The global proteomics market is estimated to be approximately $27 billion annually as of 2022 and is expected to grow at an estimated 15% CAGR from 2022 to 2027 according to BCC Research. Furthermore, we believe our Nautilus platform has the potential to facilitate a broader transformation across life sciences and healthcare, and therefore significantly augment our total addressable market over time. We believe there are multiple high-value research applications in precision and personalized medicine, drug discovery, and clinical diagnostics that can be unlocked by accurate, reproduceable, and cost effective proteomic profiling. As the proteomics
market continues to mature, and if our technology is validated across translational research applications, we believe our Nautilus platform could transfer well into the clinical setting prior technologies have thus far been unable reach.
•Our experienced, multidisciplinary team brings together a group of individuals with diverse backgrounds to disrupt the field of proteomics. Nautilus’ leadership team represents a unique and valuable hybrid of technology and biotech experience. Several members of the executive team and board of directors held leadership roles at Illumina and Isilon, and helped to guide strategy and manage execution both before and throughout the rapid growth and success for those businesses. We view the core design thesis behind the Nautilus platform technology development as a non-traditional approach to new product development within life sciences that requires thinking at the intersection of three unique disciplines not often found together—life sciences, computer and data sciences, and physical sciences and engineering. As such, we have assembled a team of individuals with experience across many different disciplines, including protein biochemists, nano-fabrication engineers, software and machine learning engineers, single-molecule biophysicists, optical engineers and others, all working together toward our common goal.
OUR STRATEGY
•Drive adoption of our Nautilus platform by providing the life sciences industry with access to the proteome. We believe our Nautilus platform has the potential to provide value across the life sciences ecosystem as the first end-to-end solution capable of substantially quantifying the proteome. The utility and potential applications are expected to be broad and serve basic research and discovery, translational and clinical research, and ultimately enable clinical diagnostic market segments. We intend to drive adoption of our Nautilus platform through a three-phase commercial strategy that begins with an initial partnership and collaboration phase with biopharma companies (such as our existing relationships with Genentech and Amgen), academic institutions (such as our existing relationship with The University of Texas MD Anderson Cancer Center, the Translational Genomics Research Institute and others) and research organizations where we aim to jointly publish data and validate our Nautilus platform, followed by a pre-sales or early access program to drive awareness and demand, and finally culminating in a full commercial launch.
•Continuously innovate and scale our Nautilus platform’s capabilities to enable further advancement of proteomic research. Through both internal R&D projects and external collaborations with our customers and partners, we plan to continuously innovate and develop new products, applications, workflows, and analysis tools that simplify and accelerate the ability for our customers to generate new sources of proteomic data and drive novel biological insights. We believe our sustainable advantage could come from a continued stream of development and commercialization of new products and applications using our core technology to help our customers succeed in their research endeavors. We believe if our customers win, we all win.
•Multiple pathways to build and expand our manufacturing capacity to support our commercial launch and the sustained growth of our business. Our technology is comprised of many off the shelf component parts that help to create efficient sourcing and manufacturing processes. We have established a manufacturing process for our technology utilizing a combination of both external contract manufacturers and internal resources based in our San Carlos, CA facility, with the ability to support substantially all of our current core activities during development. We believe there are many potential options we can use in order to increase the manufacturing and production capabilities for our products, including expanding our outsourced manufacturing and supply to multiple suppliers to ensure our quality and production capacity will meet our commercial plan.
•Build long-term value by leveraging the open design of our Nautilus platform to create an ecosystem of products and services based on our core technology. Our Nautilus platform is compatible with a wide variety of protein affinity binding reagents, which we believe will allow us to create a broad menu of applications compatible with our technology. Our Nautilus platform is also designed to be highly customizable, which we believe will allow us to create an infrastructure that enables our customers to design their own custom solutions and applications. We believe that commercializing our technology with a set of standard product applications, alongside the ability to maintain a flexible approach for designing new applications with our customers, could potentially lead to an entire ecosystem of products and services leveraging our core technology.
•Expand adoption of our Nautilus platform into new markets. Our market entry strategy involves identifying markets that are constrained by their inability to access comprehensive proteomic information, which we believe can be addressed by our Nautilus platform. We recognize that these opportunities extend into ancillary markets across life sciences, including clinical and translational research, and clinical diagnostics, where we believe there are substantial unmet needs our Nautilus platform can address in the future. We expect to drive expansion into these adjacent markets by developing and validating new product configurations and workflows targeting high impact applications, either by
adapting our existing workflows or by partnering with leaders in those markets to develop workflows that address their immediate needs and will provide broader general value for other customers in that market segment.
A PRIMER ON PROTEOMICS
Over the past decade, the study of genomics, or DNA, and transcriptomics, or RNA, have been central to drug development and healthcare. Proteomics is the next step in the study of biological information systems and is believed by many to be one of the most important disciplines for exposing disease-causing protein pathways, uncovering new drug targets, highlighting novel therapeutic indications and identifying clinically relevant biomarkers for use in precision medicine.
Molecular profiling techniques, such as next generation sequencing (NGS), have led to widespread genomic characterization and unprecedented access to genomic information. While this information has certainly enhanced our knowledge of complex biological systems, there is still a tremendous amount of detail at the protein level that remains largely unknown. The field of proteomics seeks to address this gap, and is an area of scientific research that involves the identification, characterization, and quantification of proteins in whole cells, tissues, or body fluids. To date, there has been very little technological advancement in how to physically measure a protein, and this has been a major impediment towards creating the same level of access to proteomic detail as we have with genomic detail today.
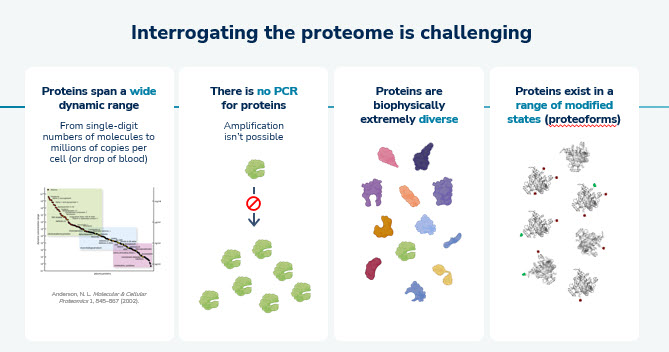
The proteome ultimately drives the function of a cell and tissue, and therefore it dictates the physically observable characteristics known as the phenotype. The proteome undergoes dynamic changes as it continuously responds to chemical signals, blood-borne mediators, temperature, drug treatment, and developing disease over time. This complex interplay of factors contributes to the complexity of proteomics research. However, the detailed and complex information provided from proteomics has the potential to help in identifying novel and causal drug targets and to enable more efficient and effective drug development. A few examples of the way proteomics may lead to novel insights in research are highlighted below.
•Better understanding of biology. Protein research contributes to a better understanding of how molecular information controls and influences an individual’s physiology.
•Identification of novel drug targets. Cellular function and dysfunction is driven by our proteins; increasing our ability to directly measure even the rarest proteins involved in disease may increase the likelihood of identifying new drug targets.
•Patient stratification. The separation of patients into groups with similar molecular features that may be more likely to respond to specific therapeutic treatments.
•Prediction of disease and treatment outcome. The identification of biomarkers that can assist in the early diagnosis of diseases, inform prognosis or monitor the efficacy and safety of ongoing treatments.
•Wellness: from health to disease. Biomarkers can monitor and guide individuals to tailor lifestyle choices to maximize health and avoid the onset of diseases before they develop.
Not only would advancements in the field of proteomics have the potential to directly unlock new insights on their own, but they would also have the potential to increase the value of data and insights generated in related fields such as genetics, gene expression, and metabolism.
OUR MARKET OPPORTUNITY
We believe that our Nautilus platform has the potential to be uniquely positioned in the proteomics market. In our mission to democratize proteomics, we see initial research applications in precision and personalized medicine, with a natural growth path into clinical diagnostics, as well as in machine-learning powered drug discovery. However, we believe that the opportunity could extend far beyond this.
Market Environment
At Nautilus, we recognize the need for a radical breakthrough in proteomics.
Since 2002, global R&D expenditure has increased close to three-fold and is expected to reach approximately $289 billion by 2026 according to EvaluatePharma’s 2023 report. Despite such investments, the number of new drugs approved each year has failed to increase proportionally. Additionally, it takes more than 10 years to bring a drug to market, and the cost has grown significantly in the past decade from approximately $1.2 billion in 2010 to approximately $2.0 billion in 2019. The increasing cost, time and complexity of drug development have driven down the rate of return on R&D to less than 2% in 2019 for the 12 leading biopharmaceutical companies analyzed in a 2020 report by the Deloitte Center for Health Solutions.
Approximately 95% of FDA-approved drug targets are proteins, and most other drugs interact with, or are influenced by signal transduction cascades mediated by proteins. As such, an understanding of the proteome is paramount to understanding pharmacology.
As existing approaches only allow us to routinely quantify a fraction of the proteome, biopharmaceutical companies have become increasingly adept at identifying possible targets within what is currently observable, and as such, many viable targets have been exhausted. Despite the many hundreds of thousands of biomarker research studies estimated to have been published to date, there are currently only approximately 168 unique pharmacogenomic biomarkers with FDA approval for use with therapies today. This number of approved biomarkers is alarmingly low, and further highlights the shortfall of attempting to predict a protein biomarker’s expression level and function primarily from genetic data. Unfortunately, researchers have been forced to use this method, given the availability of powerful tools in genomics without the corresponding power and breadth of tools available in proteomics. With an advancement such as the Nautilus platform, we believe researchers will have the power to deeply and comprehensively measure the physical proteins at the root of disease, dramatically increasing the potential to identify more clinically meaningful biomarkers with greater precision in the practice of medicine. We believe a breakthrough increase in throughput would enable researchers to more deeply measure large cohorts, thereby powering studies at the scale required to quickly and cost-effectively discover new critically important biomarkers.
The inability to easily and reliably quantify the proteins that drive every aspect of human physiology has been a fundamental hindrance to a greater understanding of cellular and molecular biology. With this in mind, we aim to democratize proteomics to make it possible for the broader scientific community to undertake a wider range of high-value scientific inquiries, thereby accelerating research and ultimately enhancing our fundamental understanding of biology and the mechanisms of disease.
The Missing Piece: The Proteome
Improvements in NGS technology have greatly enhanced the understanding of the genome, but when contemplating the number of proteins that can arise from a single gene and their role in the regulation of biological processes, both physiological and pathological, we believe that a better understanding of DNA is simply insufficient. Beyond the genome lies a vast multi-level network of biological interactions with important ramifications across the organism that remains coded and hidden within unique protein patterns. Many scientific and industry leaders believe these patterns may hold the key to a deeper understanding of biological processes at both a molecular and a systems level.
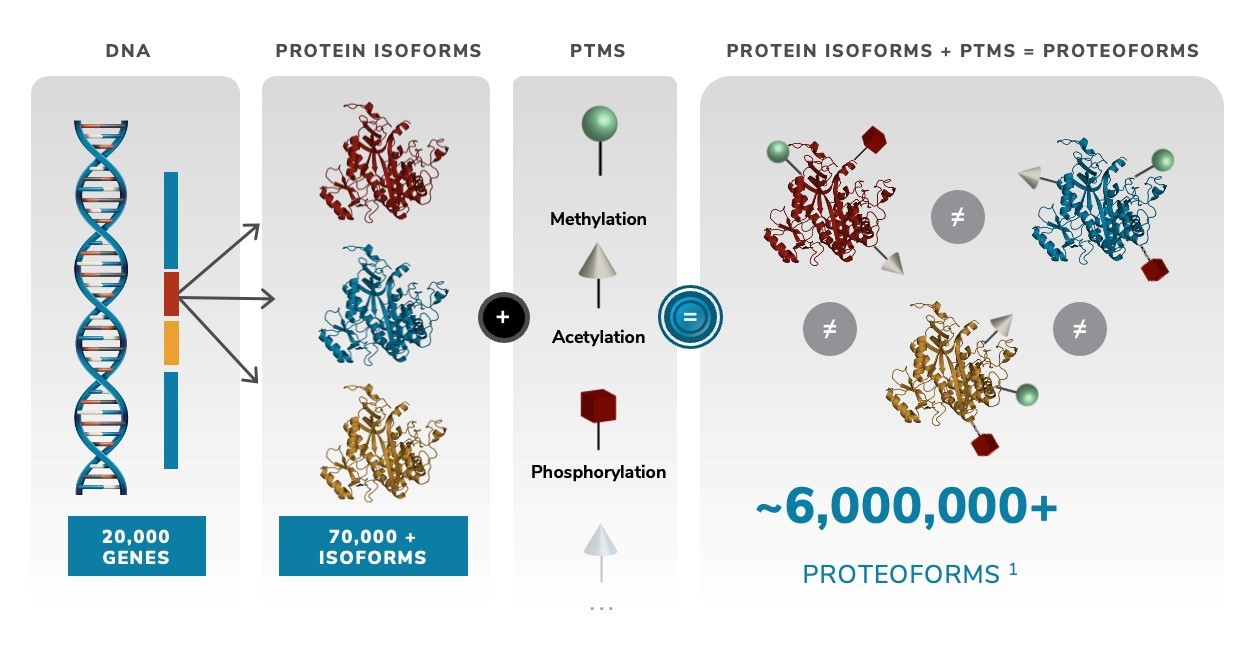
From the day we are born to the day we die, proteins are responsible for regulating all aspects of our physiology. The genome, which represents the complete set of genes within each organism, remains largely unchanged throughout the course of life. Over the years, it has been estimated that humans possess approximately 20,000 protein-encoding genes, many of which have been well characterized. However, to coordinate the myriad of processes that occur within organisms at all times, the genome has evolved multiple ways to generate further biological complexity. DNA genes are expressed in the form of RNA transcripts, which control the expression and regulation of these different genes in the cell. These RNA transcripts are then translated into individual proteins, and protein isoforms, which are subtle variations of the individual proteins themselves.
Scientists have estimated that there may be as many as 70,000 or more human protein isoforms. The resulting proteome is not only highly dynamic and in a constant state of flux by regulating the quantity and type of each protein isoform, but it also exhibits great diversity across cells and tissues. This complexity, which governs all biological processes, both healthy and sick, cannot be captured or characterized routinely by current methods.
However, the molecular complexity of our proteome doesn’t stop here, it actually grows dramatically even beyond the abundance of protein isoforms that are dynamically rising and falling. After a protein isoform has been translated, it can be modified further by biological processes that more precisely control that protein isoform’s location, specific activity, or interaction partners, and these downstream changes are together called post-translational modifications. There are a wide variety of post-translational modifications known today, which result in a tremendous increase in molecular complexity by creating different “forms” of the same basic protein, known as “proteoforms”. In total, our original 20,000 protein-coding genes are estimated to produce as many as 6,000,000 different proteoforms, as illustrated in the figure below. The existence of these proteoforms indicates that there may actually be well over two orders of magnitude (or 100 times) more complexity present across our proteome than there is across our genome. It is strongly suspected that it is within this proteoform space of molecular information that fundamental biological processes are present that govern our cells, and our molecular health.
Post-Translational Modifications Create Multiple Forms of Proteins That Are Estimated to Contain Over 100 Times More Information Complexity Than the Coding Genes in the Genome
While the past several decades have seen advances in proteomics technologies, typical solutions only capture a fraction of the proteome in samples derived from blood or cells, as illustrated in the figure below. On the left, using mass spectrometry-based methods, approximately 8% of proteins are routinely detectable from blood and approximately 30% are routinely detectable from cells. On the right, there is currently no method to easily detect and map the landscape of proteoforms, which would allow for the exploration of the estimated 6,000,000 different forms and patterns of modified proteins serving some biological function. Furthermore, shortfalls in the ability of bioinformatics to predict the existence as well as the function of genes have further illustrated the need for enhanced protein analysis techniques. Today, we believe the field of proteomics is at the very beginning of a significant growth phase. We are of the firm belief that every scientist should have access to the proteome, including proteoforms, in the same way that access to the genome has been made broadly available over recent years.
Current Technologies are Unable to Routinely Access the Full Proteome or Detect Proteoforms
Market Opportunity
Due to the extensive applications and broad potential, we believe that the proteomics market represents one of the largest untapped opportunities in the biological sciences today. According to BCC Research, the global proteomics market was valued at approximately $27 billion as of 2022. This encompasses only the fraction of the proteomics market that is currently available to us via mass spectrometry and other quantification methods and does not include diagnostics. The overall proteomics market is projected to reach approximately $55 billion in 2027, representing a CAGR of 15% for the five-year period.
We believe that as the proteomics market evolves, substantial adjacent opportunities will arise due to the potential applications in not only precision and personalized medicine, clinical diagnostics, and machine-learning powered drug discovery, as well as other disciplines such as food and environmental science. Within the biomedical sciences, the application of proteomic technologies to clinical specimens has the potential to revolutionize multiple aspects of the diagnosis and treatment of many diseases, propelled by biomarker discovery and validation of personalized therapies which we believe will greatly increase the power of prediction, diagnosis and prognosis.
Existing Proteomics Technologies and Shortfalls
Over the past decade, the importance of proteomics in the field of diagnosis and drug research & development has increased dramatically due to the direct biological relevance of analyzing the interaction of proteins in living organisms. However, the analysis of the proteome is substantially more complex than the analysis of the genome or transcriptome. Unlike DNA and RNA, proteins themselves cannot be amplified. Consequently, measurement tools must address the challenges of sensitively detecting the minute quantities of low frequency expressed targets. This challenge is exacerbated by the exceptionally large dynamic range of proteins in both cells and in blood spanning more than seven orders of magnitude. For example, some critical and influential proteins such as transcription factors may be present with only a few copies per cell, whereas abundant proteins such as cytoskeleton or ribosomal proteins may be present in millions of copies per cell. Quantifying both the low frequency and the abundant proteins within a single sample is very challenging and stands in stark contrast to genome or transcriptome analysis which only contends with a dynamic range of approximately three orders of magnitude. Furthermore, the biochemical and physical diversity of proteins far exceeds that of DNA or RNA as proteins are created from 20 highly distinct amino acids, whereas genes and transcripts are created from only 4 distinct nucleotides. These inherent complexities have hampered progress in the development of life science tools that can sensitively and comprehensively quantify the proteome. Additionally, the ability to identify unique proteoform composition and frequency in a single complex sample is not achievable today. Currently available tools can be broadly segmented into mass spectrometry-based and affinity-based methods.
Mass Spectrometry-based Approaches
Mass spectrometry is a powerful tool for the measurement of proteins and has progressed the field of proteomics immensely, similar to the impact that Sanger sequencing had to the founding of large-scale genomics research. However, for the powerful data that is generated, current mass spectrometry workflows still remain complex and time consuming. The mass-spectrometry workflows and processes are not fully automated requiring skilled professionals to prepare samples and operate instruments, which limits the impact of these powerful technologies. Mass spectrometry is also known to have poor sensitivity to detect proteins present at low frequencies within biological samples, which is where many believe important drivers of biology exist. Lastly, the most widespread approach called shotgun mass spectrometry, requires proteins are first broken apart into small pieces called peptides in order to measure them. This detection method can only measure the individual protein fragments, and is therefore unable to identify specific patterns of post-translational modifications and proteoforms visible on intact proteins across a sample. Despite these challenges, there has still been a strong appetite for protein data given its importance in biology and drug development, and the proteomics research marketplace is estimated to have over 16,000 installed mass spectrometry systems.
Limitations with Affinity-based Approaches
Where mass spectrometry-based approaches have been widely used for broad scale protein discovery applications, affinity-based approaches have generally been used for targeted protein measurements. Affinity-based protein detection commonly utilizes affinity binding reagents that are designed to be very specific to an individual protein target that is already known to the researcher. Additionally, the ability of an affinity reagent to selectively bind to its target may also be impacted by protein specific factors, such as the protein’s folded structure and orientation. Lastly, affinity-based approaches intended to target more than one protein at once in a sample commonly require a different affinity binding reagent for each target. Despite decades of ongoing efforts, there are still nowhere near the number of affinity-reagents in existence today to attempt to measure the full proteome. In general, affinity-based approaches are most useful when the end user has a relatively small pre-defined set of targets they want to measure, and because the affinity reagents themselves only detect a small portion of the intended target, this method is also not capable of resolving unique proteoform patterns at the single protein molecule level today.
THE NAUTILUS APPROACH
Our Guiding Principles
Nautilus is driven by a desire to enable the research community to rapidly and comprehensively access and quantify the proteome, thereby transforming our ability to examine disease mechanisms, and develop new therapeutics and diagnostics. This mission is guided by a recognition that major advances in proteomics have generally lagged behind genomics, primarily due to a lack of available tools for measuring the proteome as easily or completely as one can measure the genome and transcriptome.
We believe that evolutionary or incremental improvements to existing technologies will not suffice; that a fundamentally new approach is required to unlock this large opportunity in biological science. In pursuit of that mission, we are developing our innovative Nautilus platform to be an end-to-end single protein molecule analysis solution composed of instrumentation, reagent consumables, and software that processes a sample and returns valuable and unique biological data and insight. We have designed the Nautilus platform to enable extreme sensitivity and scale, without compromising on ease of use. Leveraging
a unique architecture and advanced machine learning software, we believe our Nautilus platform has the potential to identify substantially all proteins in a sample from almost any organism.
We view many of the core ideas underlying the Nautilus platform as “counterintuitive”, as it required innovations at the intersection of three distinct disciplines not often found in harmony: life sciences, computer and data sciences, and physical sciences and engineering. We have designed the Nautilus platform to integrate a variety of both computational and experimental approaches, diverse measurement modalities, and the best available analytical tools to accelerate biomarker discovery and precision medicine. Several Nautilus platform technology elements (e.g., cloud computing and machine learning) are disciplines that have now sufficiently matured to create this timely opportunity for Nautilus to pursue the deep, hard science required to bring to market a potentially revolutionary capability that we believe will help democratize access to proteomics data.
Our Nautilus Platform Design Criteria
To achieve our ambitious goals, and to meet the unmet needs of scientists and researchers, we recognized early on that we would need to tackle the deep, hard, novel science required to innovate and commercialize a fundamentally new detection technology capable of reading and quantifying the proteome and associated proteoforms. As such, we designed the Nautilus platform – from the ground up – to accomplish the following goals:
A core design criterion was that the Nautilus platform needed to be comprehensive. One of the largest challenges with existing “shotgun” proteomics technologies is that replicate analyses are likely to sample different subsets of the proteome.
Next, the Nautilus platform needed to be ultra-sensitive. Unlike NGS technologies, where one can leverage natural processes and enzymes (e.g., polymerases) to amplify DNA and RNA, proteins cannot be amplified from the original molecule. To achieve the goal of measuring the complete proteome, scientists and researchers needed a new analysis method with unprecedented sensitivity.
The Nautilus platform was designed to cover a very large dynamic range in a simple workflow in order to scale to the full proteome.
In addition, the process needed to be reproducible and robust, maximizing the chance that the results derived in one experiment are the same as the results derived in subsequent experiments.
Importantly, the Nautilus platform needed to be fast and able to analyze tens of thousands of samples in a reasonable time period.
It also needed to be sufficiently easy-to-use so that virtually any lab could benefit from using it, not just labs that are explicitly focused on proteomics or analytical chemistry.
In order to fully support the wide-ranging needs of our future customers, the platform can also support multiple run configurations. Our design accommodates both low throughput and high throughput run configurations, and will also employ
in-lane sample multiplexing to enable our customers to meet the high-volume data production needs of large-scale studies or core facilities.
With these objectives identified as our core design criteria, we set out to create a transformative technology with the potential to achieve all of these criteria. The resulting Nautilus platform has embodied many technical innovations across sample preparation, reagent consumables, instrumentation, and downstream protein analysis. However, we believe there are four critically important key technical innovations that, when brought together, make the achievement of our Nautilus platform design specifications and benefits possible:
A Single Protein Molecule Flow Cell
The Proteome Analysis System: An Integrated Multi-cycle Optical and Fluidics Instrument
A Novel Class of Affinity Reagents for Efficient Whole Proteome Analysis
Machine Learning Protein Decoding Analytics
Key Innovations
1.Single Protein Molecule Flow Cell
The vast majority of protein analysis tools, such as affinity-based methods like an ELISA (Enzyme-Linked Immunosorbent Assay), typically measure proteins in bulk. This approach works well for measuring small numbers of proteins, however, it quickly becomes very challenging when measuring hundreds to thousands of proteins. Additionally, going through multiple intermediaries to assess a protein’s concentration (such as protein capture, secondary detection, calibration between fluorescent output and concentration) places limitations on the accuracy, sensitivity, dynamic range and reproducibility. Genomic studies are able to get around these limitations by amplifying DNA or RNA, but unfortunately, there is no equivalent approach for amplifying proteins available. Consequently, the limit of detection for most immunoassays has been bounded primarily by the signal-to-noise ratio provided by the instrument used to detect antibody-antigen binding and by non-specific binding, which in a 50uL sample could represent tens-of-thousands of molecules. Accordingly, the dynamic range of such platforms are typically about 1-order of magnitude, though this can be scaled through dilution at the upper end.
Nautilus recognized early on that in order to achieve its goals for creating extreme protein detection sensitivity it would require measuring proteins whose frequency in a sample might vary from only a few, to hundreds of millions of molecules in a sample. In our view, it was clear that any bulk measurement technology would struggle to cover this immense dynamic range, and that a single protein molecule detection approach would be required to overcome a problem that has long been a barrier to major advancement in the field. Additionally, transitioning from bulk protein measurements to single protein molecule measurements fundamentally changes the nature of the protein quantification problem where the challenges of protein identification and quantification converge. If one is able to identify each protein molecule, quantification arises simply from counting those identifications, and furthermore, single protein molecule counters are by definition the most sensitive detection modalities available.
To break through these barriers, we have designed our Nautilus platform to measure billions of individual protein molecules at a time, in a massively parallel and efficient workflow. We refer to Nautilus’ framework for what we believe to be a fundamentally different approach to protein analysis as Protein Identification by Short-epitope Mapping (PrISM). Our internal testing has demonstrated that our hyper-dense single-molecule protein nanoarray contains 10 billion landing pads. Our team has developed a process for manufacturing our nanoarray as the foundational component of our flow cell consumable. The flow cell itself is comprised of a nanometer-scale fabricated chip that holds the individual protein molecules in place on the surface in a landing pad, encapsulated by a fluidics channel that allows for reagents to flow across the surface. Our design includes the isolation of individual proteins in a protein library preparation by binding them to a much larger scaffold which has been created to hold exactly one protein molecule.
These scaffolds can be reliably made to precise sizes, and the flow cell nanoarray surface can then be generated by well understood manufacturing processes to create surface features, which we call landing pads, that match the dimension of the scaffold. As each landing pad can only hold one scaffold, and each scaffold can only hold one protein molecule, the introduction of scaffold-protein complex onto the nanoarray surface generates a self-assembling, high-density single protein molecule array (as seen in the above and below illustrations). The attachment between the scaffold and the nanoarray surface is extremely robust, enabling scaffolds to persist through extensive reagent washing across many cycles.
Nautilus Single Protein Molecule Flow Cell Designed to Capture One Individual Scaffold-Protein Complex per Landing Pad
As discussed above, our flow cell is designed with the capability to capture up to tens of billions of individual, intact protein molecules. The single protein molecule nature of the Nautilus platform is designed to enable extreme sensitivity, which we have observed in our internal testing as shown in the “Nautilus Platform Sensitivity” section below, and the sheer scale of proteins captured enables the measurement of proteins across an exceptionally wide dynamic range. Flow cells with loaded protein libraries can then be introduced into our proteome analysis system for the analysis and quantitation of the captured protein library.
Nautilus Single Protein Molecule Flow Cell is Designed at Nanometer-Scale to Cover the Information Density Needed to Measure Approximately 95% of the Human Proteome
2.Our Proteome Analysis System: An Integrated Multi-cycle Optical and Fluidics Instrument
Typically, protein measurement approaches, like the ELISA described earlier, are designed to perform a single measurement of the proteins in a sample, after which the sample is either damaged, destroyed or discarded. However, if proteins captured in a sample can be repeatedly probed, it becomes possible to gain far more insight on the individual molecules. With our platform, each protein molecule has a unique coordinate address on the flow cell, and repeated probing enables deeper characterization of each individual molecule with each cycle, unlocking the ability to characterize proteoforms and ultimately decode approximately 95% of the proteome.
Nautilus Platform Multi-Cycle Affinity Probe Reagent Binding, Imaging, Washing, and Re-Binding
To achieve extreme sensitivity and scale, we have designed a novel instrument that integrates reagent fluidics with a sensitive high-resolution optical imaging system to cyclically measure all single protein molecules captured on the flow cell. Our affinity reagents are labeled with proprietary fluorescent labels that help improve both the signal-to-noise and speed of our assay chemistry. The instrument introduces labeled affinity reagents into our flow cell, allowing them to briefly incubate, then rinse off unbound molecules, and then rapidly images the entire surface. During the imaging process, a laser system is used to excite and illuminate the fluorescent labels. The high-resolution imaging components allow resolution sufficient to characterize each individual protein molecule, generating data as shown in the above illustration.
Once an imaging pass is complete, the instrument then washes the flow cell, leaving the proteins fully immobilized, and rinses out the wash reagent before pursuing additional cycles. Samples may be multiplexed in a variety of ways to enable higher sample throughput and to reduce the cost per sample. A typical full scale proteome run will generate approximately 20 terabytes of data, which is then compressed to a digital binding matrix for downstream analysis by our cloud-based software-as-a-service, SaaS, analytics suite.
3.A Novel Class of Multi-Affinity Probe Reagents for Efficient Whole Proteome Analysis
Our Nautilus platform technology is designed with fundamentally different principles of how to use and exploit the properties of affinity binding reagents compared to prior methods. Historically, affinity binding reagents have been qualified for use based on their specificity to a given protein target, and showing the ability to bind strongly to that specific target. In order to see and measure a single protein target, a researcher would require an affinity reagent of sufficient specificity to detect it. These high specificity affinity reagents are commonly used for bulk measurements, and are typically only used for one single bulk measurement event (or cycle) and then discarded.
By using these same high specificity reagents in our system, we believe it is possible to detect each specific protein target now at the single-molecule level, enabling digital quantitation. We further believe it is possible to expand this concept, and use our Nautilus platform with a wide variety of “off-the-shelf” affinity reagents that are highly specific to multiple individual protein targets. Also and of particular importance, is these off-the-shelf affinity reagents can often also target very specific sites on the protein itself, such as post-translational modification sites. Using reagents that target very specific locations and features of proteins will allow the Nautilus platform to detect and quantify the different patterns and varieties of post-translational modifications (i.e. the proteoforms).
In a highly innovative and counterintuitive way, our Nautilus platform has also been designed to exploit low specificity affinity reagents. Identifying the tens-of-thousands of different proteins in a proteome would require a prohibitively large number of traditional highly specific affinity reagents. We therefore explored the possibility of using affinity reagents that bind short, linear epitopes (e.g., target protein sequences of 3-4 amino acids each) with moderate specificity, such that each affinity reagent probes and binds to many different proteins that contain the short linear epitope target, each we describe as a “multi-affinity probe”. While the binding of a single multi-affinity probe is not sufficient to identify a given protein, sequential binding events using a series of multi-affinity probes can create enough information that it is sufficiently powerful to accurately identify an exceptionally broad number of proteins present in a sample. In this approach, each new multi-affinity probe that is introduced in a cycle of binding and imaging provides additional evidence and gradually narrows the list of possible protein identities. Hereafter, we refer to our proposed approach as Protein Identification by Short-epitope Mapping (PrISM). Our Nautilus platform technology is estimated to achieve the detection of the vast majority of proteins in the proteome using a combination of approximately 300 unique multi-affinity probes.
Nautilus Platform Technology Protein Identification with Increasing Multi-Affinity Probe Cycles
Source: A theoretical framework for proteome-scale single-molecule protein identification using multi-affinity protein binding reagents. Jarrett D. Egertson, Dan DiPasquo, Alana Killeen, Vadim Lobanov, Sujal Patel, Parag Mallick bioRxiv 2021.10.11.463967.
4.Machine Learning Protein Identification Software
Among the most unique aspects of our Nautilus platform is the integration of a proprietary machine learning-based protein identification analysis software engineered to work with the type of data our system generates. As discussed, more typical measurements for high specificity reagents can be used in our system to identify, and thereby quantify, each protein from a single binding and imaging step. These high specificity affinity reagents can provide a lot of information about a small number of proteins, and as such it would take an exceedingly large number of highly specific affinity reagents and therefore an exceedingly large number of cycles to measure every protein in the proteome. To enable broad protein identification on our system, we instead use our multi-affinity probes that can bind to hundreds or even thousands of individual proteins in a given cycle.
Our proprietary algorithm is thereby trained using experimental data from our multi-affinity probe development process that provides a baseline estimate of how likely each probe is to bind to each protein in a reference proteome database. As data is collected, a binding matrix is generated for each protein coordinate. For example, a given coordinate [2,1] may have bound probes during cycles [4, 11, 25, 26, 27, 65, and 201]. This data is then fed into our machine learning protein identification analysis to determine which protein is most compatible with the observed pattern of binding. The illustration below provides a view of our machine learning protein identification analysis at work by observing the confidence the algorithm has with respect to each protein as additional cycles of data are collected. On average, it takes roughly 15 cycles of multi-affinity probe binding events to uniquely identify a protein. Prior to 15 cycles, there is a lot of variability in which protein is likely to be at a given spot, but then after 15 cycles, the algorithm locks in on a precise protein and becomes increasingly more confident in its identification. Further, with each additional cycle the other potential proteins become increasingly less likely.
Nautilus Platform Technology Can Identify a Protein by Analyzing Data from Multiple Cycles of Multi-Affinity Probe Binding Event with High Probability.
Source: Internal Data
The machine learning protein identification analysis is run for each of the 10 billion protein molecules captured on the flow cell in parallel to identify each protein molecule present. Following this, each identification is counted to produce a cumulative, absolute quantification. As the algorithm learns more and more about each multi-affinity probe’s binding characteristics, both within and across Nautilus platform data sets, it is able to adapt and update its confidence in each protein identification, essentially getting “smarter” over time. As a result, the machine learning protein identification analysis is able to re-analyze data collected in the past and continuously improve upon its ability to identify proteins within that data.
Our Technology Workflow
From the earliest stages of developing the Nautilus platform, we set out to integrate the four key innovations (listed in the above section) into a single, cohesive proteomics workflow, creating an end-to-end solution designed for ease-of-use, speed, scale and performance. We believed that doing so could unlock the potential to democratize proteomics and make it possible for the broader scientific community to undertake a wider range of new, high-value scientific inquiries, thereby accelerating research and ultimately impacting healthcare and the development of precision medicine.
The Nautilus workflow is designed to consist of five major steps, beginning with sample preparation and concluding with the machine learning analytics that yields high-value proteomic data.
•Step 1 – Sample Preparation
The Nautilus sample preparation process attaches a label to extracted proteins and then attaches them to a proprietary scaffold to isolate them individually, thereby creating a library of single protein molecules. This process was designed to be simple, robust, and rapid. Internal tests demonstrate that substantially all of the proteins are attached to the scaffold during the library preparation process, which requires approximately 2-3 hours of hands-on time over a 2-day protocol.
•Step 2 – Sample Deposition onto the Flow Cell
The protein library is then deposited onto the flow cell capable of holding up to 10 billion intact single protein molecules. The landing pads on the flow cell are matched to the size of the protein-attached scaffold, thus allowing only one protein to be deposited per site. This element of the process was specifically designed to enable massively parallel, rapid, single-molecule sampling of proteins, as shown in the flow cell occupancy figure below.
•Step 3 – Integrated Imaging and Fluidics System Processing Multiple Cycles of Affinity Reagents
Following protein library deposition, the proteome analysis system then initiates the multi-cycle interrogation of each single-molecule bound to the surface of the flow cell. This process entails introducing fluorescent dye labeled multi-affinity probes into the flow cell, rinsing out the unbound fraction, imaging the surface area, and then stripping and washing the multi-affinity probe away. This entire process is then repeated sequentially to collect data on the desired number of cycles in the system run, where each cycle is a unique batch of multi-affinity probes intended to identify a set of target epitopes.
•Step 4 – Processing of Digitalized Proteomic Data
After the proteins on the flow cell have been iteratively imaged over the determined number of cycles, the resulting raw images are converted into a coordinate map with corresponding illumination signals indicating positive multi-affinity probe binding events, effectively digitizing up to approximately 20 terabytes of raw image analyzed proteomic data.
•Step 5 – Machine Learning Analytics - Decoding, Protein Identification, and Quantity
In the final step of the workflow, the digital proteomic data is analyzed by our cloud-based machine learning protein identification analysis software. The data is converted to protein identities during this analysis, evaluating the characteristics of each multi-affinity probe binding event at each location to determine protein identity and quantity.
Nautilus Platform Technology Performance
Simple and Robust Sample Handling
Nautilus’ straightforward sample protein library preparation is designed to convert protein samples into a format optimized for single-molecule deposition on our flow cell. The process has been designed to be accessible to virtually any life sciences researcher. In addition, the protocol is highly compatible with standard lab automation equipment and workflows, which is expected to further minimize the effort required while increasing laboratory processing throughput. Also, unlike existing shotgun proteomics methods, no sample protein digestion is required in our method which in turn makes the workflow very simple. The result is a process that is expected to effectively prepare a sample into a library ready to load on the flow cell with approximately 2-3 hours of hands-on time completed over a 2 day period.
A key feature of our Nautilus platform is the large scale (up to billions) of protein molecules that we believe can be measured massively in parallel on our single-molecule flow cell. An analysis of nearly 1,000 flow cells showed typical sample loading of single protein libraries yielded near complete flow cell occupancy, which demonstrates the speed and efficiency of our sample handling process.
Nautilus Platform Stability in Multi-Cycle Experiments
We believe we have designed a technology with direct applicability in research use settings as well as having the potential to translate discoveries into healthcare practice. A critical aspect of any molecular detection technology with translational and clinical potential is robustness and reproducibility. To understand how stable our measurement process was, we tested the durability of our flow cell with a loaded protein library to ensure that proteins remained present on the surface over multiple cycles in a proteome analysis system run. In our studies evaluating stability over numerous cycles, we observed substantially less than 1% of proteins were lost from the flow cell. To examine both the effectiveness of our wash buffer and the ability of proteins to be probed after being washed, we first examined the detection ability of the protein on a first cycle. We next demonstrated that our wash buffer successfully eliminated remaining signal. Last, we demonstrated that after extended exposure to washing, and rinsing, that the protein detection remained nearly identical to the initial measurement. These results suggest
that our wash conditions are highly effective and that our process of reagent cycling does not significantly damage the protein and thereby interfere with the probability of its measurement accuracy.
Protein Library Remains Bound to Flow Cell During Repetitive Probe Binding and Wash Cycles
Nautilus Platform Sensitivity
Our Nautilus platform is designed both to be extremely sensitive (by virtue of being a single protein molecule platform) and to have an extremely wide dynamic range of detection (by virtue of measuring a very large number of molecules). In single protein molecule assays, dynamic range is defined by the total number of molecules measured. Consequently, a platform measuring a million molecules will have a smaller dynamic range than a platform measuring 10-million molecules. On our Nautilus platform, we project we will be able to reach sensitivity down to 1 molecule out of 10 billion. We are also able to use this sensitivity in flexible way, for example we can perform an extremely deep analysis of a single protein library sample across all lanes of a flow cell, or we can perform a multiplexed analysis by processing a batch of samples together (each sample protein library individually barcoded) during one proteome analysis system run.
Development and Characterization of Multi-Affinity Probes
Multi-affinity probe discovery and development pipelines have been built that generate multi-affinity probe candidates that target short peptides, typically three amino acids in length. These pipelines employ multiple candidate discovery methods to identify and select antibody, antibody fragment, or nucleic acid based candidate multi-affinity probes. Promising candidates are further screened in assays to determine EC50 values off-platform (example results for two candidate probes shown below), and then leads are evaluated in detail for their performance on the Nautilus proteome analysis platform.
Multi-Affinity Probe Discovery and Development Pipeline
Mapping Proteoforms
We believe there are likely millions of different proteoforms that define cellular activity, cellular localization and biochemical function. With peptide-centric approaches (detecting only small pieces of proteins), such as “shotgun” mass spectrometry, it is simply impossible to differentiate proteoforms. Using phosphorylation as an example for a post-translational modification of a protein, consider the case of two samples as shown in the figure below. On the left, one protein sample contains a single protein molecule with a triple phosphorylation (red) and two unmodified proteins versus a second sample on the right, in which each protein molecule contains one phosphorylation each at a single different site. These two samples would likely appear identical to one another in a shotgun mass-spectrometry analysis.
The Nautilus Platform Detection of Proteoform Patterns
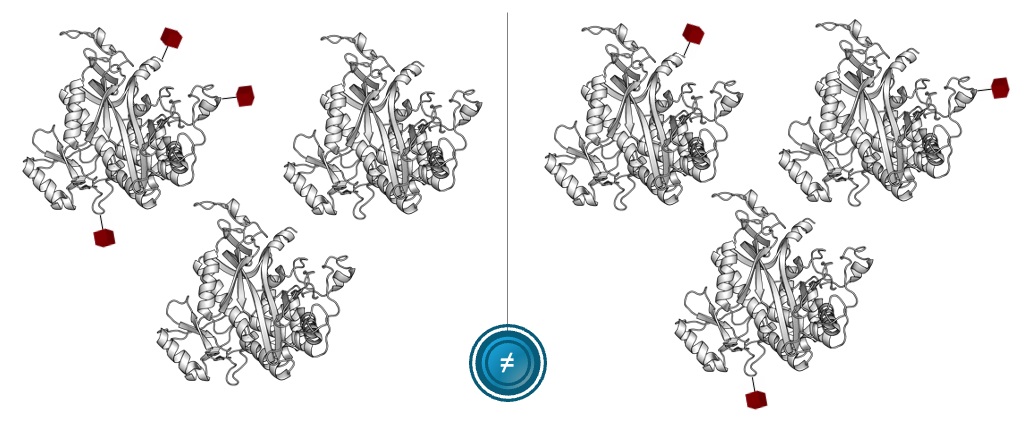
On our Nautilus platform, we are able to use existing commercially available affinity reagents to perform detailed mapping of proteoform patterns at the single protein molecule level. We do this by measuring individual proteins at each specific post-translational modification site over multiple cycles, each cycle targeting a different specific site or feature of the protein. Illustrating this concept in the diagram below, during the first cycle we use an anti Tau affinity reagent to identify all of the Tau proteins present on the flow cell. Then over the next 4 cycles we detect each Tau molecule again, only now with a slightly different affinity reagent that targets a different isoform or phosphorylation modification on the Tau protein. Looking at this data together over 5 cycles, our technology is potentially able to distinguish up to 16 different proteoforms (in this case, different isoform and phosphorylated patterns) of the same Tau protein.
Workflow for the Detection of Tau Proteoform Patterns
The protein counts in the image above are for illustration purposes only.
Nautilus Platform Development Plan Key Areas of Focus
In order to achieve our goal of broad commercialization in 2025, we plan to advance the development of our Nautilus platform across all components including chemistries, reagents, consumables, instrumentation and analysis software. The prototype of our proteome analysis system has generated all of our internal data to date, and we are continuing the development process to optimize, improve upon, and validate the final designs, formulations, protocols, manufacturing processes, and software code comprising our Nautilus platform.
Our development plan will build upon the foundational achievements our prototype technology has made in several key areas, with the goal of ultimately allowing us to fully realize the potential of our technology. We plan to focus on the continued improvement of our flow cell designs. Having initially demonstrated that prototype versions of our flow cell can functionally achieve 10 billion discrete single protein molecule landing pads, we plan to further optimize the landing pad spacing, density, manufacturing process and chemistries of the first commercially available flow cells. We also intend to focus on the completion of the final engineering design of our proteome analysis system, where we plan to complete the development of manufacturing processes to integrate and test all completed sub-systems including the high-speed optical subsystem, fluorophore excitation laser, and micro-fluidics system in combination with our flow cell. We also plan to continue expanding the number of affinity binding reagents and chemistries that can be used within our proteome analysis system for both broad scale proteomics quantification and target quantification of proteoforms at the single molecule level. Our aim is to create a broad portfolio of affinity binding reagents through in-house reagent development efforts and through strategic partnerships where we qualify already developed reagents for compatibility with our technology. Lastly, we intend to continue the development of our analysis software, where we expect improvements to our algorithms and analysis that will help with the speed, accuracy, and reliability of our commercial proteome analysis system performance.
Assuming the completion of our development across these focal areas on our currently anticipated timeline, as well as additional related development activities, we believe we will be in position to achieve our goal of broad commercialization in 2025.
APPLICATIONS OF OUR TECHNOLOGY
The Nautilus platform technology is an open platform that is designed to leverage a wide variety of reagents to read and quantify the proteome and proteoforms
We believe that our Nautilus platform technology is designed to represent one of the first truly novel technologies for the detection and quantitation of proteins and proteoforms by leveraging the creation of our single protein molecule flow cell in combination with a broad range of affinity binding reagents. By design, our Nautilus platform technology is open to the use of virtually any affinity binding reagent, where each reagent can be efficiently chemically labeled and used in our multi-cycle process to identify and quantify a protein library. We further believe one of the inherent strengths of the open design of our Nautilus platform is the ability to use reagents across a range of different binding profiles to create unique applications that unlock different types of important biological information.
On one end of the spectrum (above left), our technology is designed to harness the power of low specificity multi-affinity binding reagents that will potentially allow us to detect substantially all of the proteome. On the other end of the spectrum (above right), we believe we can apply high specificity affinity binding reagents that detect and quantify individual target proteins of interest, and the post-translational modifications of these target proteins to detect and quantify the various proteoforms that may exist. We believe it is this inherent flexibility of reagent applications on our Nautilus platform that will enable a broad suite of uses across research, discovery, translational and clinical applications. Because of this inherent flexibility, we also believe our Nautilus platform will spark the creation of new and unforeseen applications, in a similar market expansion and innovation trend that was experienced in the years following the launch of open and flexible NGS platform technologies.
The open nature of the Nautilus platform creates the opportunity to partner with third-parties on the development and supply of affinity reagents for use on our instrument.
Basic Research and Discovery Applications
The Discovery Potential of Our Nautilus Platform
One of the long-standing challenges to accelerating the discovery and understanding of protein biological function has been the overwhelming dynamic range of proteins present in a cell or a biospecimen. We believe that a sensitivity of detecting 1 protein molecule in as little as 1,000 cells will be required to identify the exceptionally rare but biologically significant proteins in a sample. Our Nautilus platform is designed with this extreme sensitivity in mind, which we believe makes it ideally suited for capturing and cataloging the variation of the proteome in a comprehensive way, both in human and non-human species.
Further, we believe speed, scale, and single protein molecule data quality will be required to enable research projects with aims to create new species-specific, tissue-specific, or disease-specific reference datasets that have the potential to accelerate discovery across academic and industry research communities. We believe our customers could embrace our Nautilus platform for these applications broadly. Comparatively, during the initial market adoption of NGS, as the instrumentation and methods improved in speed and data production scale, projects increased dramatically in size. Sample cohorts grew from dozens of samples to hundreds, and then to thousands in an effort to use the speed and data production capacity to improve the statistical power required to make new discoveries. We believe our Nautilus platform technology could experience a similar trajectory of utilization for research and discovery applications, making very large sample size studies that were not feasible using prior proteomic detection methods now practical for our customers to implement.
A deeper level of detail and molecular complexity also clearly exists beyond the estimated 20,000 proteins in the human proteome, and we expect our customers to utilize proteoform specific reagents for the profiling, mapping, and characterization of post-translational modification patterns on proteins of interest. It is estimated there are as many as 6,000,000 different proteoforms produced through protein modification pathways that hold critical biological and contextual information on the function and purpose of the proteins in our cells. We believe our customers could show strong interest in this important field of research given the lack of technologies and tools in existence today capable of mapping multiple features on a single protein in one analysis workflow. We believe discovery focused proteoform specific reagents could be used in combination with our multi-affinity broad protein detection method to enhance the output of our analyses.
Multi-Omic Systems Biology and Proteogenomics
We believe the creation of matched DNA, RNA and protein data sets for integrated multi-omic (DNA, RNA and protein) analyses will enable a more complete understanding of the path of information transfer from gene, to transcript, to protein. It is estimated that at most only 40% of protein expression can be predicted by gene expression data. Integrated multi-omic data sets are expected to have far greater potential for better understanding this discordance, its biological origin, and ultimately its impact on cell function with deeper and more complete proteomic data. We expect the creation of workflows with matched NGS and proteomic data will become standard practice in the community, further driving the utility and value of our Nautilus platform technology.
Proteogenomics is an emerging area of research, with the goal of identifying brand new proteins or proteoforms not currently captured in the protein reference sequence. In proteogenomics, individual protein sequence databases are generated using matched transcriptomic and genomic data to aid in the identification of novel peptides and proteins detected but not yet mapped within the reference databases of known proteins. In this area of research, the integration of genomics and gene expression data enhances the predictive capability to determine what new proteins are present in a sample, and further brings functional context to genomic information and gene expression patterns. Our Nautilus platform represents an entirely new single protein molecule data source for proteogenomics, which we believe could contribute significantly to the field by increasing the scale of proteomic data accessible for these analyses, and ultimately increasing the discovery potential of the integrated dataset. Given the current level of access to genomic and transcriptomic information enabled by NGS, we believe the
research community could rapidly integrate data from our Nautilus platform technology into these studies to leverage matched genomic and proteomic data.
Translational Research and Discovery Applications
Biomarker Discovery
It has been published that approximately 95% of FDA-approved drug targets are proteins. The Human Protein Atlas collaborative research project identified that FDA-approved drugs are targeting up to 854 separate human proteins and that there are 4,906 genes in the UniProt database that have experimental evidence for being involved in disease. We believe that the drug development and diagnostic industries have suffered from an inability to access the low frequency and rare proteins present in biological samples due to the tremendous dynamic range present across proteins in a specimen. As already described, we believe that our Nautilus platform technology is designed with the scale to adequately overcome the dynamic range problem in proteomics, and provide researchers with access to the rare, but biologically important protein detection where biomarkers are believed to exist. We believe our Nautilus platform’s sensitivity targeting the detection of events as rare as 1 protein molecule in 1,000 cells will be critically important and may unlock the potential for many new biomarkers to accelerate the development of precision medicine diagnostics and therapeutics.
Proteoform Patterns as Biomarkers and Mechanism of Action Studies
We believe the study of proteoform patterns, proteoform frequency, and proteoform diversity of critically important drug targets will be a widely used application of our Nautilus platform. Which drugs work on specific protein drug targets is not just a result of the total number of post-translational modifications, but instead by how combinations of specific post-translational modification are operating together. Our technology is designed to enable the research community to see these proteoform patterns, and to measure their relationship to one another. Every disease is the result of a dysregulation of molecular functions that create biological consequences compared to normal healthy function. Given the inability to detect proteoform patterns today, we believe this will become an essential application of our technology used to investigate important drug targets and molecular disease pathways. We believe this application has the potential to advance precision medicine by making an entire layer of molecular complexity and information available to researchers for the first time.
Longitudinal Monitoring of Proteome Dynamics
The study of proteome composition, protein and proteoform frequency, patterns, and variations over time represents an opportunity to survey and understand the biological changes resulting from environmental factors that influence our health and wellness. Individual or small panel protein surveillance tools have existed in the healthcare market for decades using traditional assay methods across a range of biospecimen, all of which have the same inherent limitations as those in the research space. Also, cell-free nucleic acid methods have emerged recently as amongst the first molecular surveillance tools in oncology for the emergence of disease progression post treatment or surgery, and may also prove to enable the detection of disease at earlier stages in some cancers where cell-free nucleic acids are present at higher levels. However, the same fundamental challenges exist in this setting. Nucleic acids are still only a proxy for measuring the biological consequences of the functional proteins, and further the sensitivity needed to find early-onset molecular features of disease before it presents clinically is incredibly high. We believe the routine surveillance of proteins at sufficient breadth and depth to capture even the exceptionally low-frequency changes will be a key area of interest in the future. This application has implications across not only oncology, but across virtually any human disease where the molecular underpinnings driving that disease may one day be revealed and then tracked to identify that disease earlier, measure the response to treatments, and create a comprehensive and dynamic view of our overall molecular health.
Diagnostics, Clinical Research and Drug Development Applications
Transitioning from Discovery into Clinical Application
We believe one of the largest and most impactful applications for our technology in the future will be the development of diagnostics that leverage the sensitivity, speed, stability, and ease of use we are designing our system to achieve. Significant technical and practical barriers have existed with prior high-throughput proteomic technologies preventing them from accessing the clinic. Despite advances in sample preparation methods, we believe the detection of enriched and modified protein samples by mass spectrometry will continue to experience challenges in the effort to transition to the clinic. We believe our novel protein detection method embodies the performance characteristics and design criteria that will be desirable for clinical applications. We further believe there will be opportunities to identify and develop content for proteomic clinical diagnostic tools as a result of the more direct nature of measuring the individual proteins at the source of biological function, as opposed to inferring biological function from genomic or gene expression measurements.
We also believe there will be an opportunity to leverage the proteoform pattern detection methods established in a translational research setting into the development of clinical tests in the future. We expect that once our technology is validated in a translational research setting for the identification of proteoform patterns which are themselves biomarkers of disease, we could potentially be in the position of being the only technology capable of physically detecting such patterns. We believe this presents an opportunity to use our Nautilus platform to continue to advance these applications and methods of proteoform pattern biomarker detection from discovery all the way through to future diagnostic using our technology. As we work to build evidence with our customers and partners on the utility of new proteoform patterns as translational and clinical biomarkers, we believe such applications of our Nautilus platform could have a profound impact on precision medicine.
Precision Medicine Development & Clinical Trial Support
We believe there is tremendous demand for broad scale proteomic data across the continuum of preclinical and clinical drug development. Starting at the earliest stages of therapeutic asset development, the ability to strategically inform and prioritize experimental compounds with deep proteomic data will provide a much more comprehensive view of cellular responses and resistance mechanisms. This data may also create a new perspective on how to modify experimental therapies to interact with molecular pathways in much more specific and intentional ways. We believe these types of applications present a very compelling use-case for our Nautilus platform.
We first expect adoption of our Nautilus platform could occur in the preclinical and clinical retrospective settings, where we believe single-molecule proteomic and proteoform composition and frequency will become essential tools in building a more complete picture of how experimental medicines are interacting in complex molecular pathways. Each individual tissue type offers its own unique profile of expressed proteins and functions, where advances in proteomic data breadth and depth may elucidate how and where a compound is interacting within these different cell types. We also believe this type of comprehensive proteomic analysis could become an important tool for improving our understanding of drug toxicities, metabolism and distribution. For this application, our technology has the potential to substantially improve visibility to the entire landscape of drug-target interactions, and consequently may help to improve the probability of creating strong therapeutic responses while minimizing detrimental or off-target effects. As these new insights become available, we further believe our customers may engage in very large-scale studies to catalog the frequency of target proteins and proteomic patterns across large and diverse biobanks that represent the intent to treat populations of interest, which will help inform and prioritize the development strategy and the potential impact of their experimental therapy pipelines.
We believe that as these advances in the application of large-scale proteomic data are realized in preclinical and retrospective settings, a natural transition will occur where our customers and partners will seek to apply their learnings in prospective settings. In the prospective clinical development environment, we believe the same design features which make our Nautilus platform desirable in a research setting can be fully realized. Prior proteomic profiling technologies have struggled to make an impact in prospective clinical settings due to a lack of run-to-run data reproducibility, slow turn-around-time, and overall complexity of practical implementation. We believe our Nautilus platform design is ideally suited for the quality, stability, and speed required to fully realize the value of accessing deep proteomic profiling data to identify biomarkers that stratify patients for clinical trials and improve drug development.
OUR PRODUCTS
Overview
Our primary business model is anticipated to be focused on the commercialization of our Nautilus platform through the sale of instrumentation, reagent consumables, and software. Our proteome analysis system is our detection instrument at the center of our product suite, supported by reagent consumables for the preparation and analysis of proteins, and followed by sophisticated machine learning software architecture for the analysis and reporting of our data in the cloud.
Proteome Analysis System
Our proteome analysis system is a high-resolution optical imaging system coupled with integrated fluidics and liquid handling sub-systems. The system is designed to deposit protein libraries onto a flow cell and to process labeled multi-affinity probe binding and imaging cycles rapidly in order to decode and quantify the vast majority of proteins present in biological samples. After the reagent kits, samples (protein libraries), and flow cells are loaded onto the system, the remainder of the workflow is automated.
Reagent Kits
System run reagent kits are comprised of four main components: sample preparation, flow cell(s), multi-affinity probe reagents, and instrument buffers used to perform multi-cycle analysis runs.
Our proprietary sample preparation kits will be designed for the isolation and preparation of a library of proteins from a variety of input materials including cell cultures, tissues and biospecimen. The library preparation includes an automatable workflow consisting of chemically labeling target proteins and attaching them to a scaffold used to deposit proteins on our flow cell. Given the breadth and depth of data output capability planned for our proteome analysis system, it is not expected that additional protein sample enrichment, enhancement or pre-treatments of samples will be required for processing, however we do intend to be compatible with such pre-treated samples. We also expect our customers and partners may wish to design their own custom process to target specific proteins prior to creating a library with them, and we intend to ensure our kits will be compatible with pre-treated or enriched protein samples. Our protein library preparation process is designed to be simple, efficient, and robust, all features which are expected to allow for easy automated processing for high throughput applications.
In an effort to provide maximum flexibility, our initial flow cell design includes four physically separated and independent fluid channels, or “lanes,” such that a customer can introduce a unique biological sample in to each lane for multi-cycle analysis. Our proteome analysis system is designed to hold and concurrently analyze up to three flow cells in a single system run, for a total of 12 lanes. Additional sample throughput may also be achieved by the use of a molecular barcode in our reagent kits that will enable the multiplexing of more than one barcoded sample library together within a single lane for analysis in the future.
Affinity binding reagents will also be included as a reagent kit. Kits will be offered in configurations that cover a catalog of proteomic content. We intend to supply a standardized set of multi-affinity probe reagents for the broadscale detection of proteins, or “proteome kits,” as well as protein-specific or proteoform-specific kits, or “targeted proteoform kits,” focused on high interest protein targets in key disease areas. Additionally, custom affinity reagent labeling kits are expected to be supplied in the future to enable customers to label their own in-house developed or purchased affinity reagents to be compatible with Nautilus workflows for use on the system.
Software & Analysis
Our machine learning analysis software suite also is expected to be utilized as the analysis engine to decode the proteome system raw data into protein identifications and counts. Our software is expected to be a SaaS based service, utilizing Nautilus’ machine learning computational algorithms required to identify and quantify the proteins or proteoforms present in a sample run on the system. Our software is a learning and evolving system, which we are designing to improve in accuracy over time as the multi-affinity probe binding profiles are refined and trained across a growing database. We expect our software enhancements in performance will also be accessible to customers who wish to re-analyze prior run data with later versions to deliver new insight and discovery value.
SALES & MARKETING
Commercial Strategy
The primary business model we intend to implement is to directly commercialize our entire end-to-end Nautilus platform technology solution through the sale and installation of our proteome analysis system at customer sites; the ongoing sale of consumables covering a broad suite of applications run on our system; a SaaS analytics and insights software subscription to capture long term value created by our machine learning-based analysis enhancements; and a service warranty plan to maintain our install base and support our customers in the field. We believe a comprehensive solution could offer a compelling value proposition across multiple market segments due to the substantial enhancements it will create in speed and scale of data creation, single protein molecule quantitation, sensitivity, and reproducibility.
We initially intend to target customers with a history of strong performance in proteomic research, and a substantial annual research budget allocation for proteomics technologies and proteomic data. We expect many of our customers will already have high complexity molecular analysis laboratories which include high throughput proteomic or genomic analysis capabilities on site. We believe these customers represent a segment of the greater than 16,000 system install base of mass spectrometry detection systems already in use, many of which are dedicated to proteomic analyses. Our early customers are expected to include large pharma and biotech research groups, sophisticated proteomic translational research laboratories in academia, and large-scale commercial and academic multi-omics research laboratories. As our Nautilus platform is introduced into these customer segments, we further intend to expand our commercialization into clinical settings, where our target customers are expected to include pharma and biotech clinical development groups, contract research organizations, and ultimately, diagnostic laboratories.
Our proteome analysis system is expected to be priced in-line with mass spectrometry peptide detection equipment, or high-throughput NGS equipment, making the capital expense for our system within the budget for our initial customers. Our consumables are expected be priced at a level that provides comparable market value for full proteome analysis. The use of multiplexed run configurations will continue to grow over time, which we believe will help to reduce our costs and the price-
per-sample of our reagent kits such that we are able to support customers with very high-throughput applications of our systems. We expect these high-throughput run configurations and economics to accelerate the initiation of large-scale proteomics and multi-omics research projects, and also to be much more compatible with centralized core lab facility operations with requirements for proteomic data generation that can support an entire organization or user base.
Because we believe our unique approach to protein and proteoform detection is a significant deviation from any prior method, we believe it is critical to provide the market with peer reviewed publications describing our technology and its performance capabilities, and to demonstrate its ability to deliver new biological insight. Our publication strategy is a key component of our overall go-to-market plan, and we expect to spend considerable time and resources building these fundamental proof-sources to accelerate adoption of our proteome analysis system. We further believe that once our proteome analysis system is launched, a key performance indicator of our success will be the rate of new publications generated using our technology. We intend to track this closely, and we expect to invest both internally and externally to accelerate the pace of new research and publications leveraging our Nautilus platform pre- and post- proteome analysis system launch.
Go-To-Market Strategy
We expect our proteome analysis system technology will be highly disruptive to the current proteomics technology and market landscape, and as a result, we have designed our go-to-market plan with a similar strategy to the highly successful NGS platform technology introduction and commercialization in genomics. We also believe that engaging with the market early is a critically important activity in building confidence and awareness of our technology and its capabilities.
Our planned go-to-market strategy is organized into 3 phases:
1)Collaborations & Partnerships
2)Early Access Program
3)Proteome Analysis System Launch & Commercial Scale Up
We have mapped the phases of our go-to-market strategy against specific technology development milestones which we believe will allow us to build the value proposition of our technology early, and to grow it in conjunction with our Nautilus platform enhancements over time. Our strategy to utilize our Nautilus platform early in its formal development cycle through partnerships is an important component, and in part can be attributed to the inherent flexibility we have to employ commercially available reagents for targeted applications of single-molecule proteomics that drive new and significant discovery value. Further, as we advance from low-cycle targeted applications towards longer runs with increasing data output, we believe each of the milestones on our development plan are potentially new and unprecedented advancements we can leverage to build commercial momentum.
Aligning Go-to-Market Strategy with Research and Development Milestone
Collaborations & Partnerships
We believe that directly engaging the market early, well before system launch, has the potential to be a very important differentiator to raise awareness of our novel Nautilus platform as it matures throughout the formal development process, and to build credibility as we educate the community on our scientific approach through the value of our data. We believe the most effective way to engage our future customers now is through partnerships and collaborations, with the primary purpose of driving new and meaningful biological insights while demonstrating our technology’s performance, unique characteristics, and capabilities. We have launched a formal partnering program with the goal of establishing multiple research collaborations generating data and publications in high impact research areas. We also believe we can use these collaborations to improve the performance characteristics of our technology during development, and we can shape the system and run parameters to more precisely meet our customer’s needs. We intend to target projects with these engagements that will help to define and validate our product applications, which we expect will further aid in the rapid adoption of such applications once directly commercialized as products.
Early Access Program
Following the important Collaboration and Partnership work necessary to lay a foundation of publications describing our technology and the initial product applications, we believe we will have a body of scientific evidence sufficient to start building demand for our technology and the single-molecule proteomic data it generates. We then intend to initiate our pre-sales activities, which include the launch of an Early Access Program comprised of a service offering that will generate data on customer biological samples using our prototype systems run by Nautilus staff in our facilities. Using this Early Access Program, we intend to begin building a pipeline of customer engagements, supporting their evaluations of our technology through proof of concept and pilot studies, and giving them access to our data to begin establishing interest in our proteome analysis system leading up to launch.
We further intend to build on the momentum of our Early Access Program by expanding it to include a small group of target customers for the sales and subsequent testing of our first generation proteome analysis system on site at their facilities. Our goal in the planned proteome analysis system Early Access Program is to establish this influential group of customers as reference sites ahead of our broader commercial launch, and to integrate the learnings from our system performance outside of our own laboratories to improve the performance and robustness of our system and process design.
Proteome Analysis System Launch and Commercial Scale Up
At full commercial proteome analysis system launch, we expect to continue offering our Proteomic Data Early Access Program, and to maintain the laboratory services to continue supporting proof of concept and pilot projects for an extended
period of time to continue building our sales funnel. Our proteome analysis system launch is expected to be coupled with a substantial scale up of our commercial sales and marketing workforce. We intend to employ a “land and expand” sales model to promote high value cutting edge technology adoption, where we will first establish a presence in key accounts across our customer demographics, then work to broaden and expand our value and contributions across those key account organizations while concurrently growing our customer base through an increasing salesforce. We expect to commercialize directly in the United States, and in the future to expand commercial operations to the Asia Pacific and European regions. Following our initial proteome analysis system commercial launch, we expect future system upgrades and enhancements periodically over time that will further drive discovery potential and our business with each incremental advancement.
Our planned commercialization strategy and technology are designed to offer a highly differentiated and defensible position in the market we intend to capitalize on. We believe we will have significant competitive advantages if we are able to execute on the following opportunities:
•Being first to market with a novel protein and proteoform detection platform;
•Demonstrating the ability to unlock new sources of primary biological information with proteoform mapping and rare protein detection;
•Providing immense data production capacity, driving discovery by enabling large scale studies and building our database to become a strategic asset;
•Implementing a proven commercial model with an efficient direct salesforce; and
•Expanding our impact in translational research for clinical applications, precision medicine and diagnostics.
Partnerships
In December of 2020, we signed a research collaboration agreement with Genentech to engage in a pilot study using our technology. We are collaborating with Genentech using our proteome analysis system to analyze and map the proteoform landscape of a Genentech protein target of interest. In October 2021, we entered into a research collaboration agreement with Amgen in which the Nautilus platform will be used across a number of projects to investigate proteins and proteoforms of interest to the both organizations. In October 2021, we signed a research agreement with The University of Texas MD Anderson Cancer Center. In this research collaboration the Nautilus platform will be applied in measuring the quantity and patterns of post-translational modifications on specific oncology protein targets of interest across different settings, such as pre- and post treatment. And, in January of 2023, we entered into a research collaboration with the Translational Genomics Research Institute, which is a Phoenix, Arizona-based nonprofit organization dedicated to conducting groundbreaking research with life-changing results. Translational Genomics Research Institute is part of City of Hope, a world-renowned independent research and treatment center for cancer, diabetes and other life-threatening diseases. All of the above research collaborations are consistent with our objectives in Phase I of our commercial go-to-market strategy to establish external collaborations and relationships that produce data and publications exploring the utility and strengths of our Nautilus platform technology.
Commercial Organization
We plan to build out a world-class commercial organization, focused on delivering value and support through every stage of the sales cycle. Our company is driven by the advancement of science and the improvement of human health, and we anticipate our commercial organization to be scientifically oriented to align with the goals and objectives of our customers. We believe strongly in building an exceptional support infrastructure, which we believe will be particularly important for our customers given the scale and novelty of data we anticipate our systems will provide. We aim to build long-term loyalty with our customers by enhancing their individual research programs, enabling their successes, and driving growth within their organizations through their successful use of our technologies.
MANUFACTURING AND SUPPLY
Reagent and Flow Cell Consumables
We have designed and sourced our consumables primarily from third-party suppliers. While some of these components are sourced from a single supplier, we have qualified second sources for several of our critical reagents. We currently source base nanoarray chips and flow cell components, sample preparation and assay reagents. We believe that our suppliers have sufficient capacity to meet our near-term development needs through to commercialization. We believe it may be advantageous to have multiple sources for our consumable components and reagents in the future, to help reduce the risk of production delays or quality issues that may cause a disruption to our development timelines or pre-commercial activities. For further discussion of the risks relating to our third-party suppliers, see the section titled “Risk Factors— Risks Related to our Business.”
Instrumentation
Our proteome analysis system instrumentation automates the Nautilus assay chemistry concurrent with rapid optical imaging of the flow cell. The current system is an early-stage design, used for optimization of the function and design of each component. We currently source components for our systems from external manufacturers and assemble them in-house at our San Carlos, CA facility or at our manufacturing partner facilities. Once development is completed, we will determine the most appropriate path for high volume production. This may consist of a process developed by contract manufacturing of major system components with final assembly and testing in-house, or fully outsourced production, or some combination of both.
COMPETITION
The life sciences market is highly competitive. There are other companies, both established and early-stage, that have indicated that they are designing, manufacturing and marketing products for, among other things, multiplexed or high-throughput proteomic analysis. Nautilus currently competes with technology and diagnostic companies that supply components, products, and services to customers engaged in proteomics analysis. These companies include Agilent Technologies; Becton, Dickinson and Company; Bruker Corporation; Danaher; Luminex; Olink Proteomics; Quanterix; Standard Biotools (formerly known as SomaLogic); Quantum-Si; and Thermo Fisher Scientific. Nautilus also competes with a number of emerging companies that are developing proteomic products and solutions. Some of these companies may be further along in their commercial and operating plans than we are, including actively commercializing products and growing established marketing and sales forces. Other competitors are earlier than us, and in the process of developing their technologies for the life sciences market which may lead to products that rival or replace our products.
However, we believe we are substantially differentiated from our competitors for many reasons, including our novel approach to high throughput and massively parallel proteomic technology, the unique and proprietary nature of our technologies, the novel detail of protein modification mapping our platform can achieve, our rigorous product development processes and quality of science, our multidisciplinary teams, and our access to an immediate growing market with opportunities to expand into adjacent translational and clinical markets. We believe our customers will favor our products and company because of these differentiators.
GOVERNMENT REGULATION
The development, testing, manufacturing, marketing, post-market surveillance, distribution, advertising and labeling of certain medical devices are subject to regulation in the United States by the Center for Devices and Radiological Health of the U.S. Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act (FDC Act) and comparable state and international agencies. FDA defines a medical device as an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent or other similar or related article, including any component part or accessory, which is (i) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or (ii) intended to affect the structure or any function of the body of man or other animals and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. Medical devices to be commercially distributed in the United States must receive from the FDA either clearance of a premarket notification, known as 510(k), or premarket approval pursuant to the FDC Act prior to marketing, unless subject to an exemption.
We intend to label and sell our products for research purposes only (RUO) and expect to sell them to academic institutions, life sciences and research laboratories that conduct research, and biopharmaceutical and biotechnology companies for non-diagnostic and non-clinical purposes. Our products are not intended or promoted for use in clinical practice in the diagnosis of disease or other conditions, and they are labeled for research use only, not for use in diagnostic procedures. Accordingly, we believe our products, as we intend to market them, are not subject to regulation by FDA. Rather, while FDA regulations require that research use only products be labeled with – “For Research Use Only. Not for use in diagnostic procedures.” – the regulations do not subject such products to the FDA’s jurisdiction or the broader pre- and post-market controls for medical devices.
In November 2013, the FDA issued a final guidance on products labeled RUO, which, among other things, reaffirmed that a company may not make any clinical or diagnostic claims about an RUO product, stating that merely including a labeling statement that the product is for research purposes only will not necessarily render the device exempt from the FDA’s clearance, approval, or other regulatory requirements if the totality of circumstances surrounding the distribution of the product indicates that the manufacturer knows its product is being used by customers for diagnostic uses or the manufacturer intends such a use. These circumstances may include, among other things, written or verbal marketing claims regarding a product’s performance in clinical diagnostic applications and a manufacturer’s provision of technical support for such activities. If FDA were to determine, based on the totality of circumstances, that our products labeled and marketed for RUO are intended for diagnostic purposes, they would be considered medical devices that will require clearance or approval prior to
commercialization. Further, sales of devices for diagnostic purposes may subject us to additional healthcare regulation. We continue to monitor the changing legal and regulatory landscape to ensure our compliance with any applicable rules, laws and regulations.
In the future, certain of our products or related applications could become subject to regulation as medical devices by the FDA. If we wish to label and expand product lines to address the diagnosis of disease, regulation by governmental authorities in the United States and other countries will become an increasingly significant factor in development, testing, production, and marketing. Products that we may develop in the molecular diagnostic markets, depending on their intended use, may be regulated as medical devices or in vitro diagnostic products (IVDs) by the FDA and comparable agencies in other countries. In the U.S., if we market our products for use in performing clinical diagnostics, such products would be subject to regulation by the FDA under pre-market and post-market control as medical devices, unless an exemption applies, we would be required to obtain either prior 510(k) clearance or prior premarket approval from the FDA before commercializing the product.
The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risk to the patient are placed in either class I or II, which, unless an exemption applies, requires the manufacturer to submit a pre-market notification requesting FDA clearance for commercial distribution pursuant to Section 510(k) of the FDC Act. This process, known as 510(k) clearance, requires that the manufacturer demonstrate that the device is substantially equivalent to a previously cleared and legally marketed 510(k) device or a “pre-amendment” class III device for which pre-market approval applications (PMAs) have not been required by the FDA. This FDA review process typically takes from four to twelve months, although it can take longer. Most class I devices are exempted from this 510(k) premarket submission requirement. If no legally marketed predicate can be identified for a new device to enable the use of the 510(k) pathway, the device is automatically classified under the FDC Act as class III, which generally requires PMA approval. However, FDA can reclassify or use “de novo classification” for a device that meets the FDC Act standards for a class II device, permitting the device to be marketed without PMA approval. To grant such a reclassification, FDA must determine that the FDC Act’s general controls alone, or general controls and special controls together, are sufficient to provide a reasonable assurance of the device’s safety and effectiveness. The de novo classification route is generally less burdensome than the PMA approval process.
Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices, or those deemed not substantially equivalent to a legally marketed predicate device, are placed in class III. Class III devices typically require PMA approval. To obtain PMA approval, an applicant must demonstrate the reasonable safety and effectiveness of the device based, in part, on data obtained in clinical studies. All clinical studies of investigational medical devices to determine safety and effectiveness must be conducted in accordance with FDA’s investigational device exemption (IDE) regulations, including the requirement for the study sponsor to submit an IDE application to FDA, unless exempt, which must become effective prior to commencing human clinical studies. PMA reviews generally last between one and two years, although they can take longer. Both the 510(k) and the PMA processes can be expensive and lengthy and may not result in clearance or approval. If we are required to submit our products for pre-market review by the FDA, we may be required to delay marketing and commercialization while we obtain premarket clearance or approval from the FDA. There would be no assurance that we could ever obtain such clearance or approval.
All medical devices, including IVDs, that are regulated by the FDA are also subject to the quality system regulation. Obtaining the requisite regulatory approvals, including the FDA quality system inspections that are required for PMA approval, can be expensive and may involve considerable delay. The regulatory approval process for such products may be significantly delayed, may be significantly more expensive than anticipated, and may conclude without such products being approved by the FDA. Without timely regulatory approval, we will not be able to launch or successfully commercialize such diagnostic products. Changes to the current regulatory framework, including the imposition of additional or new regulations, could arise at any time during the development or marketing of our products. This may negatively affect our ability to obtain or maintain FDA or comparable regulatory clearance or approval of our products in the future. In addition, regulatory agencies may introduce new requirements that may change the regulatory requirements for us or our customers, or both.
As noted above, although our products are currently labeled and sold for research purposes only, the regulatory requirements related to marketing, selling, and supporting such products could be uncertain and depend on the totality of circumstances. This uncertainty exists even if such use by our customers occurs without our consent. If the FDA or other regulatory authorities assert that any of our RUO products are subject to regulatory clearance or approval, our business, financial condition, or results of operations could be adversely affected.
For example, in some cases, our customers may use our RUO products in their own laboratory-developed tests (LDTs) or in other FDA-regulated products for clinical diagnostic use. The FDA has historically exercised enforcement discretion in not enforcing the medical device regulations against LDTs and LDT manufacturers. However, on October 3, 2014, the FDA issued two draft guidance documents that set forth the FDA’s proposed risk-based framework for regulating LDTs, which are designed, manufactured, and used within a single laboratory. The FDA has issued warning letters to genomics labs for illegally
marketing genetic tests that claim to predict patients’ responses to specific medications, noting that the FDA has not created a legal “carve-out” for LDTs and retains discretion to take action when appropriate, such as when certain genomic tests raise significant public health concerns. Legislative and administrative proposals to amend the FDA's oversight of LDTs have been introduced in recent years, including the Verifying Accurate Leading-edge IVCT Development Act of 2021 (VALID Act), which aims to create a new category of medical products separate from medical devices called “in vitro clinical tests,” or IVCTs, and bring all such products within the scope of the FDA’s oversight. To date, Congress has not passed the VALID Act, but may revisit the VALID Act or similar policy riders and enact other FDA programmatic reforms in the future. In October 2023, through rulemaking, the FDA proposed to amend the definition of “in vitro diagnostic products” in FDA regulations to include laboratories that manufacture such products and to phase out the FDA’s general enforcement discretion approach for LDTs so that IVDs manufactured by a laboratory would be regulated similarly to IVDs. It is unclear when the FDA will finalize and begin enforcing such rule and how current and future judicial challenges and legislation by the federal and state governments will impact the industry, including our business and that of our customers. As laboratories and manufacturers develop more complex genetic tests and diagnostic software, FDA may increase its regulation of LDTs. Any future legislative or administrative rule making or oversight of LDTs and LDT manufacturers, if and when finalized, may impact the sales of our products and how customers use our products, and may require us to change our business model in order to maintain compliance with these laws. We would become subject to additional FDA requirements if our products are determined to be medical devices or if we elect to seek 510(k) clearance or premarket approval. If our products become subject to FDA regulation as medical devices, we would need to invest significant time and resources to ensure ongoing compliance with FDA quality system regulations and other post-market regulatory requirements.
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. In the future, if we decide to distribute or market our diagnostic products as IVDs in Europe, such products will be subject to regulation under the IVD Medical Device Regulation (IVDR) European Union (EU) 2017/746. The EU IVDR was entered into application on May 26, 2022, which replaced the IVD Directive and aims to improve the quality, safety and reliability of in vitro diagnostic medical devices with a new risk-based device classification system, provide for more detailed and stringent rules on the evaluation of device performance, and to enhance vigilance and post-market surveillance, among other changes. Outside of the EU, regulatory authorization needs to be sought on a country-by-country basis in order to market medical devices. Although there is a trend towards harmonization of quality system, standards and regulations in each country may vary substantially which can affect timelines of introduction.
In the future, to the extent we develop any clinical diagnostic assays, we may pursue payment for such products through a diverse and broad range of channels and seek coverage and reimbursement by government health insurance programs and commercial third-party payors for such products. In the United States, there is no uniform coverage for clinical laboratory tests. The extent of coverage and rate of payment for covered services or items vary from payor to payor. Obtaining coverage and reimbursement for such products can be uncertain, time-consuming, and expensive, and, even if favorable coverage and reimbursement status were attained for our tests, to the extent applicable, less favorable coverage policies and reimbursement rates may be implemented in the future. Changes in healthcare regulatory policies could also increase our costs and subject us to additional regulatory requirements that may interrupt commercialization of our products, decrease our revenue and adversely impact sales of, and pricing of and reimbursement for, our products.
For further discussion of the risks we face relating to regulation, see the section titled “Risk factors— Risks Related to our Business— Risks Related to Regulatory and Legal Compliance Matters.”
The federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH), and their implementing regulations, which impose obligations, including mandatory contractual terms, with respect to safeguarding the transmission, security and privacy of protected health information by covered entities subject to HIPAA, such as health plans, health care clearinghouses and healthcare providers, and their respective business associates that access protected health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates in some cases, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
In addition, in the U.S., numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws, govern the collection, use, disclosure, and protection of health-related and other personal information. For example, in June 2018, the State of California enacted the CCPA, which came into effect on January 1, 2020 and provides new data privacy rights for consumers and new operational requirements for companies. The California Privacy Rights Act (CPRA), whose substantive provisions go into effect in 2023, revises and expands the CCPA. While we are not currently subject to the CCPA, we may in the future be required to comply with the CCPA, which may increase our compliance costs and potential liability. Furthermore, the CCPA could mark the
beginning of a trend toward more stringent state privacy legislation in the U.S., which could increase our potential liability and adversely affect our business.
Furthermore, the collection, use, storage, disclosure, transfer, or other processing of personal data regarding individuals in the European Economic Area (EEA), including personal health data, is subject to the GDPR, which became effective on May 25, 2018. The GDPR is wide-ranging in scope and imposes numerous requirements on companies that process personal data, including requirements relating to processing health and other sensitive data, obtaining consent of the individuals to whom the personal data relates, providing information to individuals regarding data processing activities, implementing safeguards to protect the security and confidentiality of personal data, providing notification of data breaches, and taking certain measures when engaging third-party processors. The GDPR also imposes strict rules on the transfer of personal data to countries outside the EEA, including the United States, and permits data protection authorities to impose large penalties for violations of the GDPR, including potential fines of up to €20 million or 4% of annual global revenues, whichever is greater. The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. In addition, the GDPR includes restrictions on cross-border data transfers. The GDPR may increase our responsibility and liability in relation to personal data that we process where such processing is subject to the GDPR, and we may be required to put in place additional mechanisms to ensure compliance with the GDPR, including as implemented by individual countries. Compliance with the GDPR will be a rigorous and time-intensive process that may increase our cost of doing business or require us to change our business practices, and despite those efforts, there is a risk that we may be subject to fines and penalties, litigation, and reputational harm in connection with our European activities.
Further, with the end of the United Kingdom’s transition period to leave the European Union, or the Brexit transition period, on December 31, 2020, there is uncertainty with regard to medical device and data protection regulations as well as other regulations that may apply to our industry in the United Kingdom, including new guidance, rules, and regulations by the Medicines and Healthcare products Regulatory Agency (MHRA).
Our research and development processes involve the controlled use of hazardous materials, including select chemicals that may be flammables, toxic or corrosives, which subject us to a variety of federal, state and local environmental and safety laws and regulations. Some of the regulations governing hazardous materials under the current regulatory structure provide for strict liability, holding a party potentially liable without regard to fault or negligence. We could be held liable for damages, remediation costs, and fines as a result of our, or our agents’ or contractors’, business operations should contamination of the environment or individual exposure to hazardous materials occur. We cannot predict how changes in laws or development of new regulations will affect our business operations or the cost of compliance.
For further discussion of the risks we face relating to regulation, see the section titled “Risk factors— Risks Related to our Business— Risks Related to Regulatory and Legal Compliance Matters.”
Intellectual Property
Patents
We strive to obtain and maintain intellectual protection for our products and technology by using a variety of intellectual protection strategies, such as patents, trademarks, trade secrets and other methods of protecting proprietary information.
As of December 31, 2023, we owned seventeen issued U.S. patents, approximately thirty-eight pending U.S. non-provisional patent applications, approximately twenty-three pending U.S. provisional patent applications, approximately four granted foreign patents, and approximately eighty-two pending foreign patent applications, including nineteen international patent applications filed under the Patent Cooperation Treaty (PCT application). Our owned patents and patent applications, if issued, are expected to expire between 2037 and 2044, in each case absent any patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Our solely owned patents and patent applications contain, among others, claims directed to our core platform technology, such as compositions, methods, and systems directed to identifying and quantifying proteins utilizing probes that can bind different epitopes of the proteins with different degrees of binding non-specificity; reagents and materials; instruments; arrays and other consumables; sample preparation; high throughput decoding algorithms, and algorithms for secondary analysis of proteins and proteomes, amongst other things.
Trade Secrets
In addition to patents, we utilize trade secrets and proprietary know-how to boost our competitive position. Specifically, we rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for,
patent protection. We protect trade secrets and know-how by establishing confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and partners. These agreements generally provide that all confidential information developed or made known during the course of an individual or entity’s relationship with us must be kept confidential during and after the relationship. These agreements also generally provide that all inventions resulting from work performed for us or relating to our business and conceived or completed during the period of employment or assignment, as applicable, shall be our exclusive property.
Trademarks
As of December 31, 2023, we owned approximately nine registered trademarks in Australia, China, Israel, the United Kingdom, and Europe. In addition, we have approximately thirty-four pending trademark applications covering seven different marks in the U.S., Australia, Brazil, Canada, China, the European Union, India, Israel, Japan, Korea, Mexico, Singapore, Switzerland, and the United Kingdom.
Collaboration Agreements
We have entered into research collaboration agreements with Genentech in December 2020, with Amgen in October 2021, with The University of Texas MD Anderson Cancer Center in October 2021, and with Translational Genomics Research Institute in January 2023. Under each of these agreements, respective research collaboration teams are using the Nautilus platform to analyze and map proteoforms of interest to the specific collaborator. These agreements are for research only and we will not generate any revenue under the agreements.
Scientific Advisory Board
We have assembled a highly qualified scientific advisory board composed of advisors who have deep expertise in the fields of proteomics, medicine, regulatory compliance and data science. Our scientific advisory board is composed of:
Ruedi Aebersold, Ph.D.
Dr. Aebersold is Professor of Systems Biology at the Institute of Molecular Systems Biology in ETH Zurich (IMSB). He is widely considered a pioneer in the field of proteomics and has served as the head of the biology/disease branch of the human proteome project.
Lee Hartwell, Ph.D.
Dr. Hartwell is the President and Director Emeritus of the Fred Hutchinson Cancer Research Center. He is a 2001 Co-recipient Nobel Prize in Physiology and Medicine for his discovery of the protein molecules that control the division of cells.
Joshua LaBaer, MD, Ph.D.
Dr. LaBaer is the Executive Director of the Biodesign Institute at Arizona State University. He is a leading researcher in cancer and personalized medicine and the inventor of the novel protein microarray technology, Nucleic Acid Programmable Protein Array (NAPPA), which has been used widely for biomedical research.
Emma Lundberg, Ph.D.
Dr. Lundberg is a Professor in cell biology proteomics at KTH Royal Institute of Technology, Sweden, and Director of the Cell Atlas of the Human Protein Atlas, an international proteomics and cell mapping project. Dr. Lundberg also holds the positions of Director of the Cell Profiling facility at the Science for Life Laboratory (SciLifeLab) in Sweden.
Employees and Human Capital
As of December 31, 2023, we had 167 employees, all based in the United States, 59 of whom hold doctorate degrees. Of these employees, 120 were engaged in research and development activities, and 47 were engaged in general and administrative activities. None of our employees are represented by a labor union or covered under a collective bargaining agreement.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Corporate and Available Information
We were incorporated as a Cayman Islands exempted company in March 2020 as a blank check company under the name ARYA Sciences Acquisition Corp III. On June 9, 2021, we consummated the Business Combination pursuant to the terms of the Business Combination Agreement. Pursuant to the terms of the Business Combination Agreement, on the Closing Date, (i) we changed our jurisdiction of incorporation by deregistering as a Cayman Islands exempted company and continuing and domesticating as a corporation incorporated under the laws of the State of Delaware, upon which we changed our name to Nautilus Biotechnology, Inc.
Our principal executive offices are located at 2701 Eastlake Avenue East Seattle, Washington, 98102, and our telephone number is (206) 333-2001. Our investor relations website is located at https://investors.nautilus.bio/. Information contained on the website is not incorporated by reference into this Form 10-K or any other filings we make with the SEC.
We use our investor relations website to post important information for investors, including news releases, analyst presentations, and supplemental financial information, and as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Accordingly, investors should monitor our investor relations website, in addition to following press releases, SEC filings and public conference calls and webcasts. We also make available, free of charge, on our investor relations website under “Financial Information—SEC Filings,” our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to these reports as soon as reasonably practicable after electronically filing or furnishing those reports to the SEC.
Item 1A. Risk Factors
You should consider carefully the following information about the risks described below, together with the other information contained in this Annual Report on Form 10-K and in our other public filings, in evaluating our business. If any of the following risks actually occurs, our business, financial condition, results of operations, and future growth prospects would likely be materially and adversely affected. In these circumstances, the market price of our common stock would likely decline.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties that you should consider before investing in our company, as more fully described below. The principal factors and uncertainties that make investing in our company risky include, among others:
Risks Related to Our Business
•We are a development stage company that has incurred net losses in every period to date, has not yet commercialized any products, and expects to continue to incur significant losses as we develop our business.
•Our business is entirely dependent on the successful development and commercialization of our proteomics platform (the “Nautilus platform”), which remains in the development stage and could be subject to delays, technical challenges and market acceptance challenges.
•We may not compete successfully with our initial or future products in the highly competitive life sciences technology market.
•We are dependent upon third parties for certain aspects of the development and commercialization of the Nautilus platform.
•Our business depends significantly on research and development spending by pharmaceutical companies as well as by academic institutions and other research institutions and any reduction in spending could limit demand for our products.
•We may not be able to launch our Nautilus platform successfully and even if it is successful, we may experience material delays in our commercialization program relative to current expectations.
•Our operating results may fluctuate significantly in the future, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or our guidance.
•We may need to raise additional capital to fund our development and commercialization plans.
Risks Related to Our Intellectual Property
•We may be unable to obtain and maintain sufficient intellectual property protection for our products and technology, or if the scope of our intellectual property protection obtained is not sufficiently broad, competitors could develop and commercialize products similar or identical to ours.
•We may not be able to protect our intellectual property and proprietary rights throughout the world.
Risks Related to Litigation
•We may become involved in litigation to enforce or defend our intellectual property rights, or to defend ourselves from claims that we infringe the intellectual property rights of others.
•We may face liability and/or negative publicity for any unknown defects or errors in our products.
Risks Related to Regulatory and Legal Compliance Matters
•Our products may, in the future, be subject to regulation by the FDA or other regulatory authorities.
•We are currently subject to, and may in the future become subject to additional, U.S. federal and state laws and regulations, as well as the laws and regulations of other countries, relating to how we collect, store and process personal information.
•Future expansion of our development and commercialization activities outside of the United States, may subject us to an increased risk of inadvertently conducting activities in a manner that violates the U.S. Foreign Corrupt Practices Act and similar laws.
•Environmental and health safety laws, including any failure to comply with such laws, may result in liabilities, expenses and restrictions on our operations.
•Our employees, independent contractors, consultants, commercial partners, distributors and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
Risks Related to our Operations
•We may experience a significant disruption in our information technology systems or breaches of data security.
•We are highly dependent on our key personnel, and if we are unable to recruit and retain key executives and scientists, we may not be able to achieve our goals.
•Our operations and financial results could be adversely impacted by global and national events, such as the COVID-19 pandemic, conflicts in Eastern Europe and the Middle East, and general economic downturns.
•Global supply chain interruptions may negatively impact the development and commercialization of our products.
Risks Related to Our Common Stock
•The price of and market for our Common Stock may be volatile, which could result in substantial losses for investors and/or an inability to readily trade in our Common Stock.
General Risk Factors
•We will continue to incur significant increased costs and management resources as a result of operating as a public company.
•Reports published by analysts, including projections in those reports that differ from our actual results, could adversely affect the price and trading volume of our common shares.
•Our ability to timely and accurately report our financial results and projections as a public company may be impacted by the effectiveness of our internal controls, and our estimates and judgments relating to critical accounting policies.
Our risk factors are not guarantees that no such conditions exist as of the date of this report and should not be interpreted as an affirmative statement that such risks or conditions have not materialized, in whole or in part.
Risks Related to Our Business
We are a development stage company that has incurred net losses in every period to date, has not yet commercialized any products, and expects to continue to incur significant losses as we develop our business. We may never achieve profitability.
We are a development stage company that has incurred net losses in each quarterly and annual period since inception and that has not yet generated any revenue. We expect to incur increasing costs as we continue to devote substantially all of our resources towards the development and anticipated future commercialization of our Nautilus platform, which includes our end-to-end solution comprised of instruments, consumables, and software analysis. We cannot be certain if we will ever generate revenue or if or when we will produce sufficient revenue from operations to support our costs. Even if profitability is achieved, we may not be able to sustain profitability. We incurred net losses of $63.7 million and $57.9 million during the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $202.2 million. These losses and accumulated deficit were primarily due to the substantial investments we made in the scientific and technological development of our Nautilus platform. We expect to incur substantial losses and negative cash flows for the foreseeable future. In addition, as a public company, we will continue to incur significant legal, accounting, and other expenses that we did not incur as a private company. These increased expenses will make it harder for us to achieve and sustain future profitability. We may incur significant losses in the future for a number of reasons, many of which are beyond our control, including the other risks described in this Annual Report on Form 10-K.
Our business is entirely dependent on the success of our Nautilus platform, which remains in the development stage and subject to scientific and technical validation. If we are unable to develop and commercialize our Nautilus platform successfully and in a manner that provides currently anticipated functionality and levels of performance, we may never be able to recognize any revenue, and our business, operating results, and financial condition will suffer.
Our future success is entirely dependent on our ability to successfully develop and commercialize our Nautilus platform, which is based on innovative yet complex and unproven technologies and which is anticipated to be used in demanding scientific research that requires substantial levels of accuracy and precision. We are investing substantially all of our management efforts and financial resources in the development and commercialization of our Nautilus platform. Additionally, in developing our platform technology, we currently rely on co-development partners to assist us in the development of certain component technologies in our platform. We have experienced difficulties with some of these partners successfully delivering these component technologies on time and to our specifications, and these partners may not be successful in delivering these component technologies on time, to our specifications, or at all, in the future, which could have an adverse impact on our ability to meet our development timelines, and/or our products’ level of currently anticipated functionality and performance. While our goal is to leverage our Nautilus platform to comprehensively measure the human proteome, the human proteome is dynamic and far more complex and diverse in structure, composition and number of variants than either the genome or transcriptome. If we cannot successfully complete platform development, if we are unable to achieve our goals for mapping the proteome, if our products fail to deliver currently anticipated functionality and levels of performance, if our products are found by a court of law to infringe the intellectual property of another party, or if we are unable to obtain broad scientific and market acceptance of our products and technologies, we may never recognize material revenue and may be unable to continue our operations.
We have not yet commercially launched our Nautilus platform. We may not be able to launch our Nautilus platform successfully and even if it is successful, we may experience material delays in our commercialization program relative to current expectations.
We anticipate commercializing our Nautilus platform in three phases involving first collaboration with biopharmaceutical companies and key opinion leaders to validate the performance and utility of our product, during which we do not expect to recognize significant revenue, if any; secondly an early access limited release phase in which we expect to recognize limited revenue; and finally a broader commercial launch phase. We are currently in the collaboration phase during which we have entered into collaborations with a small number of research customers, including with biopharmaceutical companies and key opinion leaders in proteomics whose assessment and validation of our products can significantly influence other researchers in their respective markets and/or fields. We do not anticipate that these activities will result in any material revenue. During the second, early access phase, we expect to work closely with early access customers to demonstrate a unique value proposition for our Nautilus platform. During this phase, we plan to provide early access program partners with broad-scale analysis and profiling of samples analyzed in our facility and shared via a cloud platform. We anticipate early access engagements and associated revenue in 2025. We expect this second phase to lead into the third phase of broad commercialization and launch of our proteome analysis platform in 2025. Voice of customer studies have suggested that there is market demand for a proteomics platform with specifications that are initially lower than what we have previously disclosed, for example, around characteristics such as sample input and proteome coverage. Consequently, as we balance our time to market goals with our evolving view of
customer requirements, we are refining our initial launch specifications. We believe that subsequent consumable releases will enable our platform to meet or exceed our previously announced product specifications.
Achieving the scientific and commercial objectives identified above within currently anticipated timelines will require substantial investments in our technologies and in the underlying science. Scientific and technological development of the nature being undertaken by us is extraordinarily complex, and there can be no assurances that any of these phases of commercial development will be successful or that they will be completed within the timelines currently anticipated. Given the scientific and technical complexity of our products, we could experience material delays in product development and commercial launch. If our research and product development efforts do not result in commercially viable products within the anticipated timelines, our business, operating results, and financial condition will be adversely affected.
The commercialization of our products will require us to establish relationships and successfully collaborate with leading life science companies and research institutions, initially to test and validate our products and subsequently as we seek to expand the markets for our products. We may be unable to establish sufficient collaborations of this nature, and such collaborations could result in agreements that limit or otherwise impair our flexibility to pursue other strategic opportunities.
As noted above, establishing collaborations and partnerships with large pharmaceutical and biotechnology companies and with major research institutions is a material element of our commercialization strategy. While early collaborations are expected to focus on the assessment and validation of our Nautilus platform with a focus in part on publication of results in peer-reviewed scientific journals, we also intend to pursue additional, potentially revenue-generating collaborations in areas of biological interest. Among other examples, we may pursue collaborations relating to the development and commercialization of therapeutic product candidates targeting proteins identified by our Nautilus platform.
There can be no assurance that we will be successful in developing or maintaining collaborations or that, if established, these collaborations will achieve the desired objectives. Establishing collaborations is difficult and time-consuming. Discussions may not lead to collaborations on favorable terms, if at all, and particularly where we are negotiating against major pharmaceutical companies, we may have relatively less leverage in negotiating favorable terms. To the extent we agree to work exclusively with a party in a given field, our opportunities to collaborate with others in that field would be limited. Certain parties may seek to partner with other companies in addition to us in connection with a project. This, in turn, may limit the commercial potential of any products that are the subject of such collaborations. Potential collaborators may elect not to work with us based upon their assessment of our financial, regulatory, commercial or intellectual property position.
Even if we are successful in entering into collaborations, the success of such collaborations will depend heavily on the efforts and activities of our collaborators.
Scientific collaborations of the nature we propose to pursue are subject to numerous risks, including that:
•collaborators may have significant discretion in determining the efforts and resources that they will apply to a specific project;
•collaborators may not pursue development and commercialization of products or may elect not to continue or renew development or commercialization programs based on trial or test results, changes in their strategic focus due to the acquisition of competitive products, availability of funding, or other external factors such as a business combination that diverts resources or creates competing priorities;
•collaborators may own intellectual property covering products that result from our collaboration with them, and in such cases, we would not have the right to develop or commercialize such intellectual property;
•collaborators may co-own intellectual property covering products that result from our collaboration with them, and in such cases, we would not have the right to exclude others from developing or commercializing such intellectual property;
•collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with product candidates that are being developed under the collaboration with us;
•a collaborator with marketing, manufacturing, and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities;
•we could grant exclusive rights to our collaborators that would prevent us from collaborating with others;
•collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
•disputes may arise between us and a collaborator that cause the delay or termination of the research, development, or commercialization of products or that result in costly litigation or arbitration that diverts management attention and resources;
•collaborations may be terminated, and, if terminated, in addition to reducing our revenue, may reduce exposure to research and clinical trials that facilitate the collection and incorporation of new information into our platform; and
•a collaborator’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings.
In addition, before obtaining marketing approval from regulatory authorities for the sale of product candidates subject to future collaborations, our collaborators must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates. If clinical trials of product candidates resulting from collaborations are prolonged or delayed, collaborators may be unable to obtain required regulatory approvals and therefore be unable to commercialize product candidates on a timely basis or at all, which may have a material impact on the revenue recognized from such collaborations.
Even if we are able to complete development of our Nautilus platform, we may not achieve or maintain significant commercial market acceptance.
Even if we are able to complete development of our Nautilus platform, the platform will be subject to market forces and adoption curves common to new technologies. The market for novel proteomics technologies and products like those being developed by us is in the early stages of development. While these technologies present the potential to displace legacy products, changing long-standing scientific workflows with new instruments requiring substantial capital expenditures will require us to invest substantial financial and management resources to educate potential customers on the benefits of our Nautilus platform relative to existing technologies and to validate our Nautilus platform’s ability to meet customer requirements. In that regard, we anticipate that our initial market focus will be pharmaceutical development and associated research, which are characterized by demanding and exacting requirements for product performance and accuracy. If widespread adoption of our Nautilus platform takes longer than anticipated or does not occur, our business will be materially and adversely affected.
More specifically, the successful introduction of new technologies in life science markets requires substantial engagement with the scientific community in order to encourage community acceptance of the utility, performance, and cost of the technology relative to its benefits in the applicable field or fields of research. The life sciences scientific community is often led by a small number of early adopters and key opinion leaders who significantly influence the larger community through publications in peer-reviewed journals. In these journal publications, the researchers describe not only their discoveries but also the methods and typically the products used to fuel these discoveries. We expect that references to the use of our Nautilus platform in peer-reviewed journal publications will be critical to our ability to obtain widespread acceptance within the scientific community. In addition, continuing collaborative relationships with key opinion leaders will be vital to maintaining any market acceptance we achieve. If too few researchers describe the use of our products, too many researchers shift to a competing product and publish research outlining their use of that product, or too many researchers negatively describe the use of our products in publications, customers may be less willing to engage with us concerning our products, which could materially delay our commercialization plan and/or substantially extend our sales cycles. Moreover, these customers may ultimately be less willing to purchase our products, which would adversely affect our business and future revenue.
Specific, material factors that will influence our ability to achieve market acceptance include the following:
•the ability of our marketing and engagement initiatives to increase awareness of the capabilities of our Nautilus platform;
•the ability of our Nautilus platform to demonstrate reliable performance in intended use applications, in particular, when the platform is used by customers in their own research;
•our ability to demonstrate that the functionality and performance of our Nautilus platform relative to alternative products and technologies justifies the substantial anticipated cost of the platform;
•the willingness of prospective customers to adopt new products and workflows;
•the ease of use of our Nautilus platform and whether it reliably provides significant advantages over alternative products and technologies;
•the rate of adoption of our Nautilus platform by biopharmaceutical companies, laboratories, academic institutions and others;
•the prices at which we will be able to sell our Nautilus platform instruments and consumables;
•our ability to develop new products, workflows, and solutions that meet customer requirements;
•the introduction or development and commercialization by competitors of new products or enhancements to existing products with functionality and/or performance similar to our Nautilus platform; and
•the impact of our investments in product innovation and commercial growth.
We cannot assure you that we will be successful in addressing any of these criteria or any additional criteria that might affect the market acceptance of our products. If we are unsuccessful in achieving and maintaining market acceptance of our Nautilus platform, our business, financial condition and results of operations would be adversely affected.
We have no experience in manufacturing our products at commercial scale. If we are unable to establish manufacturing capacity by ourselves or with partners in a timely manner after completing development, commercialization of our Nautilus platform would be delayed, which would result in lost revenue and harm our business.
In order for us to commercialize our Nautilus platform in volume, we will need to establish internal manufacturing capacity or to contract with one or more manufacturing partners, or both. Our technology is complex, and the manufacturing process for our products will be similarly complex, involving a large number of unique precision parts in addition to the production of various reagents and antibodies. We may encounter unexpected difficulties in manufacturing our Nautilus platform, including our proteome analysis system and related consumables. Among other factors, we will need to develop reliable supply chains for the various components in our platform instruments and consumables to support large-scale commercial production. In connection with our Nautilus platform, we may utilize long lead time instrument system components, such as cameras and lasers, and as a result, it may impact our ability to consistently source such components. Additionally, we intend to utilize over 300 complex reagents and various antibodies in order to generate deep proteomic information at the speed and scale which we expect our Nautilus platform to perform. Such reagents and antibodies are expected to be more difficult to manufacture and more expensive to procure. There are no assurances that we will be able to build manufacturing or consumable production capacity internally or find one or more suitable manufacturing or production partners, or both, to meet the volume and quality requirements necessary to be successful in the proteomics market. In addition, in connection with establishing third party relationships or sourcing component supplies, including with respect to instrument components, reagents and antibodies, we may incur costs that are higher than currently expected and that may adversely affect our gross margins and operating results following commercialization. Assuming we complete development of our Nautilus platform, we may experience manufacturing and product quality issues as we increase the scale of our production. Any delay or inability in establishing or expanding our manufacturing capacity could diminish our ability to develop or sell our products, result in increased or unanticipated costs, result in lost revenue, and seriously harm our business, results of operations and financial condition.
If we are unable to establish an effective commercial organization, including effective distribution channels and sales and marketing functions, we may not be successful in commercializing our Nautilus platform.
We are only beginning to establish an internal organization focused specifically on the commercialization of our Nautilus platform. Our initial hiring has focused on senior commercial leadership, and although this leadership has considerable industry experience, in order to achieve substantial revenue growth and profitability, we will be required to develop sales, marketing, distribution, customer service, and customer support capabilities. Staffing of these functions will frequently require individuals with the requisite technical and scientific expertise to establish and support sales of a sophisticated and complex platform for life sciences experimentation. We will be required to expend substantial financial resources to hire personnel and develop our commercial operations prior to commercial launch of our Nautilus platform. Accordingly, these initiatives will adversely affect our operating expenses prior to us having material off-setting revenue, if any.
To develop these functions successfully, we will face a number of additional risks, including:
•our ability to attract, retain, and manage the sales, marketing, customer service, and customer support force necessary to commercialize and gain market acceptance for our technology, with the additional challenge that many of these new hires will require specific scientific and technological expertise that may be more difficult to find; and
•the time and cost of establishing a specialized sales, marketing and customer service and support force.
In addition to our internal organization, we may seek to enlist one or more third parties to assist with sales, distribution, and customer service and support globally or in certain regions of the world. In certain markets, we could seek to establish
partnerships with larger market participants to provide access to their distribution channels and which could also involve scientific or technological collaboration. There is no guarantee, if we do seek to enter into any of these arrangements, that we will be successful in attracting desirable partners or that we will be able to enter into such arrangements on commercially favorable terms. If our commercialization efforts, or those of any third-party partners, are not successful, our Nautilus platform may not gain market acceptance, which could materially impact our business and results of operations.
The size of the markets for our Nautilus platform may be smaller than estimated, and new market opportunities may not develop as quickly as we expect, or at all, limiting our ability to successfully sell our products.
The market for proteomics technologies and products is evolving, making it difficult to predict with any accuracy the size of the markets for our current and future products, including our Nautilus platform. Our estimates of the total addressable market for our current and future products, including with respect to the proteomics market, the diagnostic market, and the mass spectrometry market, are based on a number of internal and third-party estimates and assumptions. In particular, our estimates are based on our expectations that researchers in the market for certain life sciences research tools and technologies will view our products as competitive alternatives to, or better options than, existing tools and technologies. We also expect researchers will recognize the ability of our products to complement, enhance and enable new applications of their current tools and technologies. We expect them to recognize the value proposition offered by our products enough to purchase our products in addition to the tools and technologies they already own. Underlying each of these expectations are a number of estimates and assumptions that may be incorrect, including the assumptions that government or other sources of funding will continue to be available to life sciences researchers at times and in amounts necessary to allow them to purchase our products and that researchers have sufficient samples and an unmet need for performing proteomics studies at scale across thousands of samples. In addition, sales of new products into new market opportunities may take years to develop and mature and we cannot be certain that these market opportunities will develop as we expect. New life sciences technology may not be adopted until the consistency and accuracy of such technology, method or device has been proven. As a result, the sizes of the annual total addressable market for new markets and new products are even more difficult to predict. Our product is an innovative new product, and while we draw comparisons between the evolution and growth of the genomics market, the proteomics market may develop more slowly or differently. In addition, our Nautilus platform may not impact the field of proteomics in the same manner or degree, or within the same time frame, that NGS technologies have impacted the field of genomics, or at all. While we believe our assumptions and the data underlying our estimates of the total addressable market for our products are reasonable, these assumptions and estimates may not be correct and the conditions supporting our assumptions or estimates, or those underlying the third-party data we have used, may change at any time, thereby reducing the accuracy of our estimates. As a result, our estimates of the total addressable market for our products may be incorrect.
The future growth of the market for our current and future products depends on many factors beyond our control, including recognition and acceptance of our products by the scientific community and the growth, prevalence and costs of competing products and solutions. Such recognition and acceptance may not occur in the near term, or at all. If the markets for our current and future products are smaller than estimated or do not develop as we expect, our growth may be limited and our business, financial condition and operational results of operations could be adversely affected.
We are dependent on single source suppliers for some of the components and materials used in our Nautilus platform, and the loss of any of these suppliers could harm our business.
We rely on single source suppliers for certain components and materials used in our Nautilus platform, including our click-reagent modified oligos, glass or silicon that is nano-fabricated into our biochips and high-speed stage used in the instrument. The loss of any of these single source suppliers would require us to expend significant time and effort to locate and qualify an alternative source of supply for these components. Though we do not currently have contracts for third parties to provide manufacturing capabilities for each component of our Nautilus platform for which we expect to employ such third party manufacturers, if we are successful in reaching the point of manufacturing our products for commercialization, we may rely on a single company for such manufacturing. Any contractual disputes between us and such manufacturer or loss of manufacturing ability by such manufacturer could similarly require significant time, effort and expense to locate and qualify an alternative source of manufacturing, which could materially harm our business.
We also rely, and expect to continue to rely, on third-party manufacturers and, in many cases, single third-party manufacturers for the production of certain reagents and antibodies needed to generate the deep proteomic information at the speed and scale which we expect our Nautilus platform to perform. With respect to any antibodies or reagents that are single sourced, the loss of any suppliers would require significant time and effort to locate and qualify an alternative source of supply. Such reagents and antibodies may also become scarce, more expensive to procure, or not meet quality standards, and we may not be able to obtain favorable terms in agreements with suppliers. Given their complexity, our suppliers may not be able to provide these reagents and antibodies in a cost-effective manner or in a time frame that is consistent with our expected future needs. If our suppliers cease or interrupt production or if suppliers fail to supply materials, products or services to us for any
reason, such interruption could delay development, or interrupt the commercial supply, with the potential for additional costs and lost revenue. If this were to occur, we might also need to seek alternative means to fulfill our manufacturing needs. Any such transition would require significant efforts in testing and validation and could result in delays or other issues, which could materially harm our business.
The life sciences technology market is highly competitive. If we fail to compete effectively, our business and results of operation will suffer.
We face significant competition in the life sciences technology market. We currently compete with technology and diagnostic companies that supply components, products, and services to customers engaged in proteomics analysis. These companies include Agilent Technologies; Becton, Dickinson and Company; Bruker Corporation; Danaher; Luminex; Olink Proteomics; Quanterix; Standard Biotools (formerly known as SomaLogic); Quantum-Si; and Thermo Fisher Scientific. We also compete with a number of emerging companies that are developing proteomic products and solutions.
Some of our current competitors are large publicly-traded companies, or are divisions of large publicly-traded companies, and enjoy a number of competitive advantages over us, including:
•greater name and brand recognition;
•greater financial and human resources;
•broader product lines;
•larger sales forces and more established distributor networks;
•substantial intellectual property portfolios;
•larger and more established customer bases and relationships; and
•better established, larger scale and lower cost manufacturing capabilities.
We cannot assure investors that our products will compete favorably or that we will be successful in the face of increasing competition from products and technologies introduced by our existing or future competitors or by companies entering our markets or that are developed by our customers internally. In addition, we cannot assure investors that our competitors do not have or will not develop products or technologies that currently or in the future will enable them to produce competitive products with superior functionality or performance or at lower costs than ours or that are able to run comparable experiments at a lower total experiment cost. Any failure to compete effectively could materially and adversely affect our business, financial condition and operating results.
Even if our Nautilus platform is commercialized and achieves broad scientific and market acceptance, if we fail to improve it or introduce compelling new products, our revenue and our prospects could be harmed.
The life sciences industry is characterized by rapid and significant technological changes, frequent new product introductions and enhancements and evolving industry standards. Even if we are able to commercialize our Nautilus platform and achieve broad scientific and market acceptance, our ability to attract new customers and increase revenue from existing customers will depend in large part on our ability to enhance and improve our Nautilus platform and to introduce compelling new products. The success of any enhancement to our Nautilus platform or introduction of new products depends on several factors, including timely completion and delivery, competitive pricing, adequate quality testing, integration with existing technologies, freedom from intellectual property encumbrance, appropriately timed and staged introduction and overall market acceptance. Any new product or enhancement to our Nautilus platform that we develop may not be introduced in a timely or cost-effective manner, may contain defects, errors, vulnerabilities or bugs, or may not achieve the market acceptance necessary to generate significant revenue.
The typical development cycle of new life sciences products can be lengthy and complicated, and may require new scientific discoveries or advancements, considerable resources and complex technology and engineering. Such developments may involve external suppliers and service providers, making the management of development projects complex and subject to risks and uncertainties regarding timing, timely delivery of required components or services and satisfactory technical performance of such components or assembled products. If we do not achieve the required technical specifications or successfully manage new product development processes, or if development work is not performed according to schedule, then such new technologies or products may be adversely impacted. If we are unable to successfully develop new products, enhance our proteomics product platform to meet customer requirements, compete with alternative products, or otherwise gain and maintain market acceptance, our business, results of operations and financial condition could be harmed.
We rely on third parties for development of certain aspects of the Nautilus platform, and any failure of these third parties to perform their respective obligations in a timely manner or to our specifications could negatively impact our timelines, costs or product performance.
We are engaged with a number of third party collaborators who assist us in co-development of certain aspects of the Nautilus platform, including, for example, certain affinity reagents and array chip substrates. Our agreements with these third party collaborators include obligations for these third parties to deliver certain aspects of technology to be used in the Nautilus platform in accordance with certain defined timelines, in accordance with defined specifications, and in accordance with certain cost limitations. We have also sought to include redundancy and contingency planning with respect to the efforts of our third party collaborators where practicable. Despite our contractual assurances and contingency planning, it is possible that one or more of our third party collaborators may fail to deliver their respective technologies to us on time or in accordance with our specifications, and such failure could negatively impact the timing of the commercialization of the Nautilus platform, its performance, or its cost.
Our business will depend significantly on research and development spending by pharmaceutical companies as well as by academic and other research institutions. Any reduction in spending could limit demand for our products and adversely affect our business, results of operations, financial condition and prospects.
We expect that our revenue in the foreseeable future will be derived primarily from sales of our Nautilus platform to biotechnology companies and life science laboratories worldwide, and to a lesser extent, academic institutions and non-profit organizations. Our success will depend upon demand for and use of our products. Accordingly, the spending policies of these customers could have a significant effect on the demand for our technology. These policies may be based on a wide variety of factors, including the resources available to make purchases, the spending priorities among various types of equipment, policies regarding spending during recessionary periods and changes in the political climate. In addition, academic, governmental and other research institutions that fund research and development activities may be subject to stringent budgetary constraints that could result in spending reductions, reduced allocations or budget cutbacks, which could jeopardize the ability of these customers to purchase our products. Our operating results may fluctuate substantially due to reductions and delays in research and development expenditures by these customers. For example, reductions in capital expenditures by these customers may result in lower than expected system sales and, similarly, reductions in operating expenditures by these customers could result in lower than expected sales of our Nautilus platform. These reductions and delays may result from factors that are not within our control, such as:
•decreases in government funding of research and development;
•changes in economic conditions, including recessionary effects, inflationary pressures and instability in the global financial markets, including with respect to any future financial institution failures;
•changes in government programs that provide funding to research institutions and companies, including changes in the amount of funds allocated to different areas of research or changes that have the effect of increasing the length of time of the funding process;
•changes in the regulatory environment affecting life science and Ag-Bio companies engaged in research and commercial activities;
•differences in budget cycles across various geographies and industries;
•market-driven pressures on companies to consolidate operations and reduce costs;
•mergers and acquisitions in the life science and Ag-Bio industries; and
•other factors affecting research and development spending.
Any decrease in our customers’ budgets or expenditures or in the size, scope or frequency of capital or operating expenditures as a result of the foregoing or other factors could materially and adversely affect our business, results of operations, financial condition, and prospects.
Our operating results may fluctuate significantly in the future, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide.
Our quarterly and annual operating results may fluctuate significantly, which makes it difficult for us to predict our future operating results. In the near term, as we devote substantially all of our resources towards the development and anticipated future commercialization of our Nautilus platform, specific factors that may result in fluctuations include, without limitation:
•the timing and cost of, and level of investment in, research and development and commercialization activities relating to our Nautilus platform;
•our ability to successfully establish and successfully maintain appropriate collaborations and derive revenue from those collaborations;
•the impact that economic inflation may have on our costs for manufacturing our products;
•any litigation or governmental investigations involving us, our industry or both; and
•our ability to successfully develop and commercialize our Nautilus platform on our anticipated timeline.
As we transition from a company with a focus on research and development to a company capable of supporting manufacturing, these fluctuations may also occur due to a variety of other factors, many of which are outside of our control, including, but not limited to:
•the level of demand for any products we are able to commercialize, particularly our Nautilus platform, which may vary significantly from period to period;
•our ability to drive adoption of our Nautilus platform in our target markets and our ability to expand into any future target markets;
•the impact that economic inflation may have on our costs for manufacturing our products;
•the prices at which we will be able to sell our Nautilus platform;
•the volume and mix of our sales between consumables, instruments and software, or changes in the manufacturing or sales costs related to our products;
•the timing and amount of expenditures that we may incur to develop, commercialize or acquire additional products and technologies or for other purposes, such as the expansion of our facilities;
•changes in governmental funding of life sciences research and development or changes that impact budgets and budget cycles;
•seasonal spending patterns of our customers;
•the timing of when we recognize any revenue;
•future accounting pronouncements or changes in our accounting policies;
•higher than anticipated service, replacement and warranty costs;
•the impact of the COVID-19 pandemic, the conflicts in Eastern Europe and the Middle East, recent and any potential future financial institution failures, and other national and global events on the economy, investment in life sciences and research industries, our business operations, and resources and operations of our customers, suppliers, and distributors; and
•general industry, economic and market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
The cumulative effects of the factors discussed above could result in large fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful. Investors should not rely on our past results as an indication of our future performance.
This variability and unpredictability could also result in us failing to meet the expectations of industry or financial analysts or investors for any period. If we are unable to commercialize products or generate revenue, or if our operating results fall below the expectations of analysts or investors or below any guidance we may provide, or if the guidance we provide is below the expectations of analysts or investors, it could cause the market price of our Common Stock to decline.
We have a limited operating history, which may make it difficult to evaluate our current business and the prospects for our future viability, and to predict our future performance.
We are a life sciences technology company with a limited operating history. We have not completed development of our Nautilus platform or any other products and have not generated any revenue to date. Our operations to date have been limited to
developing our Nautilus platform. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. Consequently, predictions about our future success or viability are highly uncertain and may not be as accurate as they could be if we had a longer operating history or a company history of successfully developing and commercializing products.
In addition, as a business with a limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown obstacles. We will eventually need to transition from a company with a focus on research and development to a company capable of supporting manufacturing and commercial activities as well, and we may not be successful in such a transition. We have encountered in the past, and will encounter in the future, risks and uncertainties frequently experienced by growing companies with limited operating histories in emerging and rapidly changing industries. If our assumptions regarding these risks and uncertainties, which we use to plan and operate our business, are incorrect or change, or if we do not address these risks successfully, our results of operations could differ materially from our expectations, and our business, financial condition and results of operations could be adversely affected.
We may need to raise additional capital to fund our development and commercialization plans.
Based on our current plans, we believe that our available resources and existing cash, cash equivalents and short-term investments, will be sufficient to meet our anticipated cash requirements for at least 12 months from the date of this Annual Report on Form 10-K. If our available resources and existing cash and cash equivalents and short-term investments are insufficient to satisfy our liquidity requirements, including because of the realization of other risks described in this Annual Report on Form 10-K, we may be required to raise additional capital prior to such time through issuances of equity or convertible debt securities, enter into a credit facility or another form of third-party funding or seek other debt financing.
We expect that we will need to raise additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing or acquisition opportunities or for other reasons, including:
•funding development and marketing efforts of our Nautilus platform or any other future products;
•increasing our sales and marketing and other commercialization efforts to drive market adoption of our Nautilus platform, once commercialized;
•expanding our technologies into additional markets;
•preparing, filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
•acquiring, licensing or defending against third party intellectual property rights;
•acquiring or investing in complementary technologies, businesses or assets; and
•financing capital expenditures and general and administrative expenses.
Our present and future funding requirements will depend on many factors, including:
•delays in execution of our development plans;
•the scope and timing of our investment in our sales, marketing, and distribution capabilities;
•changes we may make to our business that affect ongoing operating expenses;
•the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
•changes we may make in our business or commercialization strategy;
•changes we may make in our research and development spending plans;
•the effect of competing technological and market developments;
•our need to implement additional infrastructure and internal systems;
•the impact of the COVID-19 pandemic, the conflicts in Eastern Europe and the Middle East; and
•other items affecting our forecasted level of expenditures and use of cash resources including potential acquisitions.
The various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, dilution to our stockholders could result. If we raise funds by issuing debt securities, those debt securities could have rights, preferences and privileges senior to those of holders of our Common Stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations or licensing arrangements, we might be required to relinquish significant rights to our technologies or products or grant licenses on terms that are not favorable to us.
We may be unable to raise additional funds or to enter into such agreements or arrangements on favorable terms, or at all. Our ability to raise additional funds may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, the credit and financial markets in the United States and worldwide resulting from the ongoing COVID‑19 pandemic, the conflicts in Eastern Europe and the Middle East, and otherwise. If we are unable to obtain adequate financing or financing on terms satisfactory to us, if we require it, our ability to continue to pursue our business objectives and to respond to business opportunities, challenges, or unforeseen circumstances could be significantly limited, and could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, actual events involving limited liquidity or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, the collapse of Silicon Valley Bank and other financial institutions in March 2023 has caused and could continue to cause instability in the global financial markets.
Although we assess our banking relationships as we believe necessary or appropriate, our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, the financial institutions with which we have arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships, but could also include factors involving financial markets or the financial services industry generally. Credit and banking costs, generally, may also be adversely impacted by these factors, resulting in higher costs for the Company. For example, as part of our efforts to diversify our banking and credit arrangements following the collapse of Silicon Valley Bank, we have incurred higher banking related costs.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain sufficient intellectual property protection for our products and technology, or if the scope of our intellectual property protection obtained is not sufficiently broad, competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products may be impaired.
Our commercial success depends in part on our ability to protect our intellectual property and proprietary technologies. We rely on patent protection, where appropriate and available, as well as a combination of copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. If we fail to obtain, maintain and protect our intellectual property, third parties may be able to compete more effectively against us. In addition, we may incur substantial costs related to litigation or other patent proceedings in our attempts to recover or restrict use of our intellectual property.
To the extent our intellectual property offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct competition. If our intellectual property does not provide adequate coverage of our competitors’ products, our competitive position could be adversely affected, as could our business, financial condition, results of operations and prospects. Both the patent application process and the process of managing patent and other intellectual property disputes are generally unpredictable, time-consuming and expensive.
Our success depends in large part on our and any future licensor’s ability to obtain and maintain protection of the intellectual property we may own or license, whether solely or jointly, particularly patents, in the United States and other
countries with respect to our products and technologies. We apply for patents to protect our products, technologies and commercial activities, as we deem appropriate. However, obtaining and enforcing patents is costly, time-consuming and complex, and we may fail to apply for patents on important products and technologies in a timely fashion or at all, or we may fail to apply for patents in potentially relevant jurisdictions. We may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, we may not develop additional proprietary products, methods and technologies that are patentable. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents which may be licensed from or to third parties. In connection with any future licensing arrangements with third parties, these patents and applications may not be prosecuted and enforced by such third parties in a manner consistent with the best interests of our business.
In addition, the patent position of life sciences technology companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. Changes in either the patent laws or in interpretations of patent laws in the United States or other jurisdictions may diminish the value of our intellectual property. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain. It is possible that none of our pending patent applications will result in issued patents in a timely fashion or at all, and even if issued, the patents may not provide a basis for intellectual property protection of commercially viable products or services, may not provide us with any competitive advantages, or may be challenged, narrowed or invalidated by third parties. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. It is possible that third parties will design around our current or future patents such that we cannot prevent such third parties from using similar technologies and commercializing similar products to compete with us. Some of our owned or any future licensed patents or patent applications may be challenged at a future point in time and we may not be successful in defending any such challenges made against our patents or patent applications. Any successful third-party challenge to our patents could result in diminished or lost rights, for example, due to narrowing, unenforceability or invalidity of such patents and increased competition to our business. The outcome of patent litigation or other proceedings is generally uncertain, and any attempt by us to enforce our patent rights against others or to challenge the patent rights of others may not be successful, or, regardless of success, may take substantial time and result in substantial cost, and may divert our efforts and attention from other aspects of our business. Any of the foregoing events could have a material adverse effect on our business, financial condition and results of operations.
The U.S. law relating to the patentability of certain inventions in the life sciences technology industry is uncertain and rapidly changing, which may adversely impact our existing patents or our ability to obtain patents in the future.
Changes in either the patent laws or interpretation of the patent laws in the United States or in other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. In the last decade, the US Congress made sweeping changes to patent law in passing the America Invents Act (AIA). These changes include, among others, allowing third-party submission of prior art to the United States Patent and Trademark Office (USPTO) during patent prosecution and additional procedures to challenge the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review and derivation proceedings. The changes brought about by the AIA have not been extensively tested, and therefore increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Various courts, including the U.S. Supreme Court, have recently rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to our technology and commercial goals. Specifically, these decisions have substantially increased the probability that patent claims will be ruled patent ineligible for reciting a natural phenomenon, law of nature or abstract idea. Furthermore, in view of these decisions, since December 2014, the USPTO has published and continues to publish revised guidelines for patent examiners to apply when examining claims for patent eligibility. Patent claims relating to software algorithms, biologically-derived reagents, methods for analyzing biological systems and other subject matters that underlies our technology and commercial goals are impacted by these changes.
Actions taken by the U.S. Congress, federal courts and USPTO have from time to time narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. Similar changes have been made by authorities in other jurisdictions. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, such changes create uncertainty with respect to the value of patents, once obtained. Depending on decisions by authorities in various jurisdictions, the laws and regulations governing patents could change in unpredictable ways that may have a material adverse effect on our ability to obtain new patents and to defend and enforce our existing patents and patents that we might obtain in the future.
We cannot assure you that our patent portfolio will not be negatively impacted by the current uncertain state of the law, new court rulings or changes in guidance or procedures issued by governments or patent offices around the world. From time to time, the U.S. Supreme Court, other federal courts, the U.S. Congress or the USPTO may change the standards of patentability, scope and validity of patents within the life sciences technology and any such changes, or any similar adverse changes in the patent laws of other jurisdictions, could have a negative impact on our business, financial condition, prospects and results of operations.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our Nautilus platform in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and we and any future licensor may encounter difficulties in protecting and defending such rights in foreign jurisdictions. Consequently, we and any future licensor may not be able to prevent third parties from practicing our inventions in some or all countries outside the United States, or from selling or importing products made using our or any future licensor’s inventions in and into the United States or other jurisdictions. Competitors and other third parties may be able to use our technologies in jurisdictions where we have not obtained patent protection to develop our own products and technologies and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products. We and any future licensor’s patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. In addition, certain countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to other parties. Furthermore, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of any patents.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of many other countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the misappropriation or other violations of our intellectual property rights including infringement of our patents in such countries. The legal systems in certain countries may also favor state-sponsored companies or companies headquartered in particular jurisdictions over our patents and other intellectual property protection. The absence of harmonized intellectual property protection laws and effective enforcement makes it difficult to ensure consistent respect for patent, trade secret, and other intellectual property rights on a worldwide basis. As a result, it is possible that we will not be able to enforce our rights against third parties that misappropriate our proprietary technology in those countries.
Proceedings to enforce our or any future licensor’s patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business, could put our and any future licensor’s patents at risk of being invalidated or interpreted narrowly and our and any future licensor’s patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We and any future licensor may not prevail in any lawsuits that we and any future licensor initiates, or that are initiated against us or any future licensor, and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our products, services and other technologies and the enforcement of intellectual property. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may become involved in lawsuits to defend against third-party claims of infringement, misappropriation or other violations of intellectual property or to protect or enforce our intellectual property, any of which could be expensive, time consuming and unsuccessful, and may prevent or delay our development and commercialization efforts.
Litigation may be necessary for us to enforce our patent and proprietary rights and/or to determine the scope, coverage and validity of others’ proprietary rights. Litigation on these matters has been prevalent in our industry and we expect that this will continue. To determine the priority of inventions, we may have to initiate and participate in interference proceedings declared by the USPTO that could result in substantial legal fees and could substantially affect the scope of our patent protection. Also, our intellectual property may be subject to significant administrative and litigation proceedings such as invalidity, unenforceability, re-examination and opposition proceedings against our patents. The outcome of any litigation or other proceeding is inherently uncertain and might not be favorable to us, and we might not be able to obtain licenses to technology that we require or a competitor may have already obtained an exclusive license to such technology in all fields. Even if such licenses are obtainable, they may not be available at a reasonable cost. We could therefore incur substantial costs related to
royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. In some cases, the outcome of litigation may be to enjoin us from commercializing a patent protected technology. We could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products.
In addition, if we resort to legal proceedings to enforce our intellectual property rights or to determine the validity, scope and coverage of the intellectual property or other proprietary rights of others, the proceedings could be burdensome and expensive, even if we were to prevail.
Our commercial success may depend in part on our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and new participants in the life sciences market and competitors may assert that our products infringe their intellectual property rights as part of a business strategy to impede our successful entry into those markets. Third parties may assert that we are employing our proprietary technology without authorization. We are aware that there are issued third party patents that are in the general proteomics field. Specifically, we are aware of various U.S. patents and U.S. non-provisional applications assigned to Washington University and the National Institute of Health, with claims directed to characterizing and identifying a polypeptide strand.
In addition, our competitors and others may have patents or may in the future obtain patents and may claim that use of our products infringes these patents. For example, we have received and may from time to time in the future receive letters, notices or “invitations to license” related to the use of intellectual property in our products or services, or may become the subject of claims that our products and business operations infringe or violate the intellectual property rights of others. For example, on December 14, 2023, we filed suit in the United States District Court for the Northern District of California against Somalogic, Inc. (Somalogic) which recently merged with and is now part of “Standard Biotools, Inc.”, and the California Institute of Technology. This suit seeks declaratory judgment that we do not infringe any claims of US Patent No. 7,842,793 related to DNA origami structures (the ‘793 Patent), which Somalogic has allegedly licensed from the California Institute of Technology through its purchase of Palamedrix, Inc. We took this action in response to Somalogic’s allegations that our proteome analysis platform infringes the claims of the ‘793 Patent. Notwithstanding our belief as to the strength of our claims, litigation generally, and patent litigation in particular, can be unpredictable. If we do not succeed in establishing that our products or services do not infringe the claims of the ‘793 Patent, it could negatively impact our business by imposing royalties on our products or services, requiring significant redevelopment of certain aspects of our products and/or preventing us from making, using or selling our products or services. Even if we were to prevail in this or any future litigation, litigation could also result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results or financial condition.
As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us, alleging that our products or services infringe, misappropriate or otherwise violate their intellectual property rights, including patents and trade secrets, as a means of slowing or preventing our entry into such markets, or as a means to extract substantial license and royalty payments from us. The defense of these matters can be time consuming, costly to defend in litigation, divert management’s attention and resources, damage our reputation and brand and cause us to incur significant expenses or make substantial payments.
Issued patents covering our products could be found invalid or unenforceable if challenged.
Our owned and any future licensed patents and patent applications may be subject to validity, enforceability and priority disputes. The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability. Some of our patents or patent applications may be challenged at a future point in time in opposition, derivation, reexamination, inter partes review, post-grant review or interference or other similar proceedings. Any successful third-party challenge to our patents in this or any other proceeding could result in the unenforceability or invalidity of such patents, which may lead to increased competition to our business, which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, if we or any future licensor initiates legal proceedings against a third party to enforce a patent covering our products, the defendant could counterclaim that such patent covering our products, as applicable, is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. There are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including, but not limited to, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the relevant patent office, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include ex parte re-examination, inter partes review, post-grant review, derivation and equivalent proceedings in non-U.S. jurisdictions, such as opposition proceedings. Such proceedings could result in revocation of or amendment to our patents in such a way that they no longer cover and protect our products. With respect to the validity of our patents, for example, we cannot be certain that there is no invalidating prior art of
which us, any future licensor, our patent counsel and the patent examiner were unaware during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. If a defendant or other third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection for our products and technologies, which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license intellectual property or develop or commercialize current or future products.
We may not be aware of all third-party intellectual property rights potentially relating to our products. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until approximately 18 months after filing or, in some cases, not until such patent applications issue as patents. We might not have been the first to make the inventions covered by each of our pending patent applications and we might not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings, derivation proceedings or other post-grant proceedings declared by the USPTO, or other similar proceedings in non-U.S. jurisdictions, that could result in substantial cost to us and the loss of valuable patent protection. The outcome of such proceedings is uncertain. No assurance can be given that other patent applications will not have priority over our patent applications. In addition, changes to the patent laws of the United States in the last decade allow for various post-grant opposition proceedings that have not been extensively tested, and their outcome is therefore uncertain. Furthermore, if third parties bring these proceedings against our patents, regardless of the merit of such proceedings and regardless of whether we are successful, we could experience significant costs and our management may be distracted. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, and our business could be harmed.
We rely heavily on trade secrets and confidentiality agreements to protect our unpatented know-how, technology and other proprietary information, including parts of our Nautilus platform, and to maintain our competitive position. However, trade secrets and know-how can be difficult to protect. In particular, we anticipate that with respect to our technologies, these trade secrets and know how will over time be disseminated within the industry through independent development, the publication of journal articles describing the methodology, and the movement of personnel between academic and industry scientific positions.
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into agreements, including confidentiality agreements, non-disclosure agreements and intellectual property assignment agreements, with our employees, consultants, academic institutions, corporate partners and, when needed, our advisers. However, we cannot be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors or other third parties will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure, which could adversely impact our ability to establish or maintain a competitive advantage in the market, business, financial condition, results of operations and prospects.
Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had wrongfully obtained and was using our trade secrets, it would be expensive and time-consuming, it could distract our personnel, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
We also seek to preserve the integrity and confidentiality of our confidential proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. If any of our confidential proprietary information were to be lawfully obtained or independently developed by a competitor or other third party, absent patent protection, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. Competitors or third parties could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, design around our protected technology, develop their own competitive technologies that fall outside the scope of our intellectual property rights or independently develop our technologies without reference to our
trade secrets. If any of our trade secrets were to be disclosed to or independently discovered by a competitor or other third party, it could materially and adversely affect our business, financial condition, results of operations and prospects.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We or any future licensor may be subject to claims that former employees, collaborators or other third parties have an interest in our patents, trade secrets or other intellectual property. For example, us or any future licensor may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our products. In addition, counterparties to our consulting, software development, and other agreements may assert that they have an ownership interest in intellectual property developed under such arrangements. Litigation may be necessary to defend against claims challenging ownership or inventorship of our or any future licensor’s ownership of our patents, trade secrets or other intellectual property. If we or any future licensor fails in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our Nautilus platform, including our software, workflows, consumables and reagent kits. In such an event, we may be required to obtain licenses from third parties and such licenses may not be available on commercially reasonable terms or at all or may be non-exclusive. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture or commercialization of our products and technologies. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees, and certain customers or partners may defer engaging with us until the particular dispute is resolved. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may not be able to protect and enforce our trademarks and trade names or build name recognition in our markets of interest thereby harming our competitive position.
The registered or unregistered trademarks or trade names that we own may be challenged, infringed, circumvented, declared generic, lapsed or determined to be infringing on or dilutive of other marks. We may not be able to protect our rights in these trademarks and trade names, which we need in order to build name recognition. In addition, third parties have filed, and may in the future file, for registration of trademarks similar or identical to our trademarks, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Further, we have and may in the future enter into agreements with owners of such third-party trade names or trademarks to avoid potential trademark litigation which may limit our ability to use our trade names or trademarks in certain fields of business. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business, financial condition, results of operations and prospects may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources. Any of the foregoing events could have a material adverse effect on our business, financial condition and results of operations.
Patent terms may be inadequate to protect our competitive position on our Nautilus platform for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. While extensions may be available, the life of a patent, and the protection it affords, is limited. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. Even if patents covering our products are obtained, once the patent life has expired, we may be open to competition from competitive products. If one of our products requires extended development, testing and/or regulatory review, patents protecting such products might expire before or shortly after such products are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours, which could have a material adverse effect on our business, financial condition and results of operations.
Obtaining and maintaining our patent protection depends on compliance with various required procedures, document submissions, fee payments and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States at several stages over the lifetime of the patents and/or applications. The USPTO and various non-U.S. governmental patent agencies require
compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In certain circumstances, we may rely on any future licensor to pay these fees due to the U.S. and non-U.S. patent agencies and to take the necessary action to comply with these requirements with respect to any future licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors may be able to enter the market without infringing our patents and this circumstance would have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed trade secrets of our former employers.
We have employed and expect to employ individuals who were previously employed at universities or other companies, including, for example, our competitors or potential competitors. Although we try to ensure that our employees, consultants, advisors and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that our employees, advisors, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information of their former employers or other third parties, or to claims that we have improperly used or obtained such trade secrets. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights and face increased competition to our business. Any such litigation or the threat thereof may adversely affect our ability to hire employees or contract with advisors, contractors and consultants. A loss of key research personnel work product could hamper or prevent our ability to commercialize potential products, which could harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. This type of litigation or proceeding could substantially increase our operating losses and reduce our resources available for development activities. Some of our competitors may be able to sustain the costs of this type of litigation or proceedings more effectively than we can because of their substantially greater financial resources.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing, or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. Furthermore, individuals executing agreements with us may have pre-existing or competing obligations to a third party, such as an academic institution, and thus an agreement with us may be ineffective in perfecting ownership of inventions developed by that individual, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Furthermore, we or any future licensor may in the future be subject to claims by former or current employees, consultants or other third parties asserting an ownership right or inventorship in our owned, or any future licensed, patents or patent applications. For example, our Founder and Chief Scientist is employed by Stanford University and a member of the Stanford Cancer Institute. Stanford University and the Stanford Cancer Institute may assert an ownership right in any of our owned patents or patent applications. We may have other consultants that are or have been employed by third parties, which may assert an ownership right in any of our owned patents or patent applications. In addition, we are aware that we might not be able to obtain ownership of or seek a license to any intellectual property developed during a research collaboration with a third party. An adverse determination in any such proceeding may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar technology, without payment to us, or could limit the duration of the patent protection covering our technology and products. Such challenges may also result in our inability to develop, manufacture or commercialize our products without infringing third-party patent rights. Any of the foregoing could harm our business, financial condition, results of operations and prospects.
If we cannot license rights to use technologies on reasonable terms, we may not be able to commercialize new products in the future.
We may identify third-party technology that we may need to license or acquire in order to develop or commercialize our products or technologies, including our Nautilus platform. However, we may be unable to secure such licenses or acquisitions. The licensing or acquisition of third-party intellectual property rights is a competitive area, and several more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources, or
greater development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us.
We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment or at all. In return for the use of a third party’s technology, we may agree to pay the licensor royalties based on sales of our products or services. Royalties are a component of cost of products or technologies and affect the margins on our products. We may also need to negotiate licenses to patents or patent applications before or after introducing a commercial product. We may not be able to obtain necessary licenses to patents or patent applications, and our business may suffer if we are unable to enter into the necessary licenses on acceptable terms or at all, if any necessary licenses are subsequently terminated, if the licensor fails to abide by the terms of the license or fails to prevent infringement by third parties, or if the licensed intellectual property rights are found to be invalid or unenforceable.
Our use of open source software and failure to comply with the terms of the underlying open source software licenses could impose limitations on our ability to commercialize our products and provide third parties to our proprietary software.
Our products utilize open source software that contain modules licensed for use from third-party authors under open source licenses. In particular, some of the software may be provided under license arrangements that allow use of the software for research or other noncommercial purposes. Use and distribution of open source software may entail greater risks than use of third-party commercial software, as open source software licensors generally do not provide warranties or other contractual protections regarding infringement claims or the quality of the code. Some open source software licenses contain requirements that the licensee make its source code publicly available if the licensee creates modifications or derivative works using the open source software, depending on the type of open source software the licensee uses and how the licensee uses it. If we combine our proprietary software with open source software in a certain manner, we could, under certain open source software licenses, be required to release the source code of our proprietary software to the public for free. This would allow our competitors and other third parties to create similar products with less development effort and time and ultimately could result in a loss of our product sales and revenue, which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, some companies that use third-party open source software have faced claims challenging their use of such open source software and their compliance with the terms of the applicable open source license. We may be subject to suits by third parties claiming ownership of what we believe to be open source software or claiming non-compliance with the applicable open source licensing terms. Use of open source software may also present additional security risks because the public availability of such software may make it easier for hackers and other third parties to compromise or attempt to compromise our technology platform and systems.
Although we review and monitors our use of open source software to avoid subjecting our proprietary software to conditions we do not intend, the terms of many open source software licenses have not been interpreted by United States courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to commercialize our products and proprietary software. Moreover, we cannot assure investors that our processes for monitoring and controlling our use of open source software in our products will be effective. If we are held to have breached the terms of an open source software license, we could be subject to damages, required to seek licenses from third parties to continue offering our products on terms that are not economically feasible, to re-engineer our products, to discontinue the sale of our products if re-engineering could not be accomplished on a timely basis, or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to make products that are similar to products and technologies we may develop or may be able to utilize similar technologies that are not covered by the claims of the patents that we own or licenses now or in the future;
•we, or any future licensor(s), might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or may own in the future;
•we, or any future licensor(s), might not have been the first to file patent applications covering certain of our or their inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing, misappropriating or otherwise violating our owned or future licensed intellectual property rights;
•it is possible that our pending patent applications or those that we may license or own in the future will not lead to issued patents;
•issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors;
•our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable;
•the patents of others may harm our business; and
•we may choose not to file a patent for certain trade secrets or know-how, and a third party may independently derive, use, commercialize, publish or patent such intellectual property.
Should any of these events occur, they could materially adversely affect our business, financial condition, results of operations and prospects.
Risks Related to Litigation
We may become involved in litigation to enforce or defend our intellectual property rights, or defend ourselves from claims that we infringe the intellectual property rights of others, which litigation could consume significant resources and management time, and in which an adverse result could result in loss of our intellectual property rights, a requirement that we pay significant damages, and could prevent us from selling our products.
The life sciences industry is highly competitive, and companies in this industry routinely engage in litigation and governmental proceedings to enforce and defend the intellectual property rights that they believe they possess. We may become involved in litigation or governmental and/or administrative proceedings to enforce or defend our intellectual property rights. Additionally, we have and may continue to become involved in litigation and/or governmental or administrative proceedings to defend ourselves from claims that our products or services infringe the intellectual property rights of others, or to challenge the claimed intellectual property rights of others where we believe they may not be entitled to such rights. Such litigation and governmental proceedings are inherently unpredictable and costly, and can require significant time and attention of management. In addition to the costs and distraction of litigation, if we are unsuccessful in enforcing our intellectual property rights, or in defending our intellectual property rights from challenges of others, wit could result in our loss of our ability to exclude others from practicing aspects of our technology which could lead to greater competition for our products and services. Additionally, if we are unable to successfully defend ourselves from claims that we infringe the intellectual property rights of others and are unable to develop non-infringing alternative approaches for our products and services, we may be required to pay significant damages and ongoing royalties, or we may be prohibited from selling our products and services. Our success depends upon our ability to successfully enforce and defend our own intellectual property rights, and to defend ourselves from claims that we infringe the intellectual property rights of others.
Our products could have unknown defects or errors, which may give rise to claims against us and adversely affect market adoption of our Nautilus platform.
Our Nautilus platform utilizes novel and complex technology applied on a microscopic scale, using key components that are not amenable to full characterization or quality assessment using conventional techniques or instrumentation, and such systems may develop or contain undetected defects or errors. We cannot assure you that material performance problems, defects or errors will not arise, and as we increase the density and integration of our Nautilus platform, these risks may increase. We expect to provide warranties that our Nautilus platform will meet performance expectations or be free from defects. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating margins.
In manufacturing our Nautilus platform, we depend upon third parties for the supply of various components. Many of these components require a significant degree of technical expertise to produce. If our suppliers fail to produce components to specification, or if the suppliers, or we, use defective materials or workmanship in the manufacturing process, the reliability and performance of our products will be compromised.
If our products contain defects, we may experience:
•a failure to achieve market acceptance or expansion of our product sales;
•loss of customer orders and delay in order fulfillment;
•damage to our brand reputation;
•increased cost of our warranty program due to product repair or replacement;
•product recalls or replacements;
•inability to attract new customers;
•diversion of resources from our manufacturing and research and development departments into our service department; and
•legal claims against us, including product liability claims, which could be costly and time consuming to defend and result in substantial damages.
The occurrence of any one or more of the foregoing could negatively affect our business, financial condition and results of operations.
If we are sued for product liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of our products could lead to the filing of product liability claims were someone to allege that our products identified inaccurate or incomplete information regarding the proteins analyzed or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of or inappropriate reliance upon, the information we provide in the ordinary course of our business activities. A product liability claim could result in substantial damages and be costly and time-consuming for us to defend. We maintain product liability insurance, but this insurance may not fully protect us from the financial impact of defending against product liability claims. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation, or cause current customers to terminate existing agreements and potential partners to seek other partners, any of which could adversely impact our business, financial condition and results of operations.
Risks Related to Regulatory and Legal Compliance Matters
Although our products currently are not labeled or intended for any use which would subject us to regulation by the FDA or other regulatory authorities, if we elect to label and promote any of our products as clinical or medical device products, we would be subject to regulation in the future and would be required to obtain prior approval or clearance by the FDA or other regulatory authorities, which could take significant time and expense and could fail to result in FDA clearance or approval for the intended uses we believe are commercially attractive.
Our products are currently labeled and promoted, and are, and in the near-future will be, sold primarily to research companies and academic and research institutions as research use only (“RUO”) products, and are not currently intended to be used, for clinical diagnostic tests or as medical devices. If we elect to label and market our products for use as, or in the performance of, clinical diagnostics in the United States, thereby subjecting them to FDA regulation as medical devices, we would be required to obtain premarket 510(k) clearance or premarket approval from the FDA, unless an exception applies.
We may in the future register with the FDA as a medical device manufacturer and list some of our products with the FDA pursuant to an FDA Class I listing for general purpose laboratory equipment. While this regulatory classification is exempt from certain FDA requirements, such as the need to submit a premarket notification commonly known as a 510(k) application, and some of the requirements of the FDA’s Quality System Regulations (the “QSRs”), we would be subject to ongoing FDA “general controls,” which include compliance with FDA regulations for labeling, inspections by the FDA, complaint evaluation, corrections and removals reporting, promotional restrictions, reporting adverse events or malfunctions for our products, and general prohibitions against misbranding and adulteration.
In addition, we may in the future submit 510(k) premarket notification applications to the FDA to obtain FDA clearance of certain of our products on a selective basis. It is possible, in the event we elect to submit 510(k) applications for certain of our products, that the FDA would take the position that a more burdensome premarket application, such as a premarket approval application (“PMA”) or a de novo application is required for some of our products. If such applications were required, greater time and investment would be required to obtain FDA approval. Even if the FDA agreed that a 510(k) was appropriate, FDA clearance can be expensive and time consuming. It can take a significant amount of time to prepare and submit a 510(k) application, including conducting appropriate testing on our products, and several months to years for the FDA to review a submission. Notwithstanding the effort and expense, FDA clearance or approval could be denied for some or all of our products for which we choose to market as a medical device or a clinical diagnostic device. Even if we were to seek and obtain regulatory approval or clearance, it may not be for the intended uses we request or that we believe are important or
commercially attractive. There can be no assurance that future products for which we may seek premarket clearance or approval will be cleared or approved by the FDA or a comparable foreign regulatory authority on a timely basis, if at all, nor can there be assurance that labeling claims will be consistent with our anticipated claims or adequate to support continued adoption of such products. Compliance with FDA or comparable foreign regulatory authority regulations will require substantial costs, and subject us to heightened scrutiny by regulators and substantial penalties for failure to comply with such requirements or the inability to market our products. The lengthy and unpredictable premarket clearance or approval process, as well as the unpredictability of the results of any required clinical studies, may result in our failing to obtain regulatory clearance or approval to market such products, which would significantly harm our business, results of operations, reputation, and prospects.
If we sought and received regulatory clearance or approval for certain of our products, we would be subject to ongoing FDA obligations and continued regulatory oversight and review, including the general controls listed above and the FDA’s QSRs for our development and manufacturing operations. In addition, we may be required to obtain a new 510(k) clearance before we could introduce subsequent modifications or improvements to such products. We could also be subject to additional FDA post-marketing obligations for such products, any or all of which would increase our costs and divert resources away from other projects. If we sought and received regulatory clearance or approval and are not able to maintain regulatory compliance with applicable laws, we could be prohibited from marketing our products for use as, or in the performance of, clinical diagnostics and/or could be subject to enforcement actions, including warning letters and adverse publicity, fines, injunctions, and civil penalties; recall or seizure of products; operating restrictions; and criminal prosecution.
In addition, we could decide to seek regulatory clearance or approval for certain of our products in countries outside of the United States. Sales of such products outside the United States will likely be subject to foreign regulatory requirements, which can vary greatly from country to country. As a result, the time required to obtain clearances or approvals outside the United States may differ from that required to obtain FDA clearance or approval and we may not be able to obtain foreign regulatory approvals on a timely basis or at all. Following Brexit, medical device products entering the U.K. market will have to comply with the regulatory requirements of the Medicines and Healthcare products Regulatory Agency (the “MHRA”), including the new UK Medical Device Regulations, which are scheduled to go into effect by July 2024. In July 2023, MHRA updated its guidance to reflect that the government now aims to delay implementation of the core aspects of the regulations to July 2025. These foreign regulations and any future requirements that may be implemented by regulatory authorities will increase the difficulty of obtaining and maintaining regulatory approvals and compliance in Europe in the future. In addition, the FDA regulates exports of medical devices. Failure to comply with these regulatory requirements or obtain and maintain required approvals, clearances or certifications could impair our ability to commercialize our products for diagnostic use outside of the United States.
Our products could become subject to government regulation as medical devices by the FDA and other regulatory agencies even if we do not elect to seek regulatory clearance or approval to market our products for diagnostic purposes, which would adversely impact our ability to market and sell our products and harm our business. If our products become subject to FDA regulation, the regulatory clearance or approval and the maintenance of continued and post-market regulatory compliance for such products will be expensive, time-consuming, and uncertain both in timing and in outcome.
We do not currently expect our Nautilus platform to be subject to the clearance or approval of the FDA, as it is not intended to be used for the diagnosis, treatment or prevention of disease. However, as we expand our product line and the applications and uses of our current or products into new fields, certain of our future products could become subject to regulation by the FDA, or comparable international agencies, including requirements for regulatory clearance or approval of such products before they can be marketed. Also, even if our products are labeled, promoted, and intended as RUO, the FDA or comparable agencies of other countries could disagree with our conclusion that our products are intended for research use only or deem our sales, marketing and promotional efforts as being inconsistent with RUO products. For example, our customers may independently elect to use our RUO labeled products in their own laboratory developed tests (“LDTs”) for clinical diagnostic use. While the FDA has traditionally exercised enforcement discretion with LDTs, the FDA could take the view that our sale of our RUO labeled products were made with the knowledge that the products will be used as medical devices, and could therefore subject our products to government regulation, and the regulatory clearance or approval and maintenance process for such products may be uncertain, expensive, and time-consuming. Regulatory requirements related to marketing, selling, and distribution of RUO products could change or be uncertain, even if clinical uses of our RUO products by our customers were done without our consent. If the FDA or other regulatory authorities assert that any of our RUO products are subject to regulatory clearance or approval, our business, financial condition, or results of operations could be adversely affected.
The FDA has historically exercised enforcement discretion in not enforcing the medical device regulations against laboratories offering LDTs. FDA recently issued a proposed rule that, if finalized, will amend FDA’s regulations to make explicit that in vitro diagnostis (“IVDs”) are devices under the Federal Food, Drug, and Cosmetic Act, including when the manufacturer of the IVD is a laboratory and will phase out its enforcement discretion for LDTs. It is unclear how this proposed rule, if finalized, as well as future legislation by the government will impact the industry, including our business and that of our
customers. Any restrictions on LDTs by the FDA, Congress, or regulatory authorities may decrease the demand for our products. The adoption of new restrictions on RUO products, whether by the FDA or Congress, could adversely affect demand for our specialized reagents and instruments. Further, we could be required to obtain premarket clearance or approval before we can sell our products to certain customers.
Further, sales of devices for diagnostic purposes may subject us to additional healthcare regulation and enforcement by the applicable government agencies. Such laws include, without limitation, state and federal anti-kickback or anti-referral laws, healthcare fraud and abuse laws, false claims laws, privacy and security laws, the Physician Payments Sunshine Act and related transparency and manufacturer reporting laws, and other laws and regulations applicable to medical device manufacturers. If our operations are found to be in violation of any applicable FDA or healthcare laws and regulations, we may be subject to penalties, monetary damages, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain clearance or approvals from the FDA, fees from regulators, fines, significant settlements or judgments, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, or other restrictions on our operations, any of which could adversely impact our financial results. Any action against us for an alleged or suspected violation by a private party or governmental agency could cause us to incur significant legal expenses, adversely impact our reputation, and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
Additionally, on November 25, 2013, the FDA issued Final Guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only.” This guidance emphasizes that the FDA will review the totality of the circumstances when it comes to evaluating whether equipment and testing components are properly labeled as RUO. This guidance states that merely including a labeling statement that the product is for research purposes only will not necessarily render the device exempt from the FDA’s clearance, approval, and other regulatory requirements if the circumstances surrounding the distribution, marketing and promotional practices indicate that the manufacturer knows its products are, or intends for its products to be, used for clinical diagnostic purposes. These circumstances may include written or verbal sales and marketing claims or links to articles regarding a product’s performance in clinical applications and a manufacturer’s provision of technical support for clinical applications.
Even if the FDA does not modify its policy of enforcement discretion, whether due to changes in FDA policy or legislative action, the FDA may disagree with the marketing of our current products in the United States. We may also be required to conduct clinical studies to support our currently marketed products or planned product launches. If we are required to conduct such clinical trials or to obtain regulatory authorization, delays in the commencement of our product launches or our changes to our current marketing strategy could significantly increase our costs and delay our commercialization plans, which could harm our financial prospects.
We are currently subject to, and may in the future become subject to additional, U.S. federal and state laws and regulations imposing obligations on how we collect, store and processes personal information. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our future customer base, and thereby decrease our revenue.
In the ordinary course of our business, we currently, and, in the future, will, collect, store, transfer, use or process sensitive data, including personally identifiable information of employees, and intellectual property and proprietary business information owned or controlled by us and other parties. The secure processing, storage, maintenance, and transmission of this critical information are vital to our operations and business strategy. We are, and may increasingly become, subject to various laws and regulations, as well as contractual obligations, relating to data privacy and security in the jurisdictions in which we operate. The regulatory environment related to data privacy and security is increasingly rigorous, with new and constantly changing requirements applicable to our business, and enforcement practices are likely to remain uncertain for the foreseeable future. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible that they will be interpreted and applied in ways that may have a material adverse effect on our business, financial condition, results of operations and prospects.
In the United States, various federal and state regulators, including governmental agencies like the Consumer Financial Protection Bureau and the Federal Trade Commission, have adopted, or are considering adopting, laws and regulations concerning personal information and data security. Certain state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to personal information than federal, international or other state laws, and such laws may differ from each other, all of which may complicate compliance efforts. For example, the California Consumer Privacy Act (the “CCPA”), which increases privacy rights for California residents and imposes obligations on companies that process their personal information, came into effect on January 1, 2020. Among other things, the CCPA requires covered companies to provide new disclosures to California consumers and provide such consumers new data protection and privacy rights, including
the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information. This private right of action may increase the likelihood of, and risks associated with, data breach litigation. In November 2020, California passed the California Privacy Rights Act (the “CPRA”), which amended and expanded the CCPA as of January 1, 2023. Although the CCPA includes exemptions for certain clinical trial data, the law may increase our compliance costs and potential liability with respect to other personal information we collect about California customers. It is possible that these consumer, health-related and data protection laws may be interpreted and applied in a manner that is inconsistent with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business. In addition to the CCPA, numerous other states’ legislatures are considering or have enacted similar data privacy laws. For example, Virginia, Colorado, Utah and Connecticut have each passed laws similar to but different from the CCPA and CPRA that have taken effect in 2023; Florida, Montana, Oregon and Texas have enacted similar laws that go into effect in 2024; Tennessee, Delaware and Iowa have enacted similar laws that go into effect in 2025; and Indiana has enacted a similar law that will go into effect in 2026. Additionally, certain other state laws govern the privacy and security of health information in certain circumstances, such as Washington’s My Health, My Data Act, which contains a private right of action. These new state laws will require ongoing compliance efforts and investment. In addition, laws in all 50 U.S. states require businesses to provide notice to consumers whose personal information has been disclosed as a result of a data breach. State laws are changing rapidly and there is discussion in the U.S. Congress of a new comprehensive federal data privacy law to which we would become subject if it is enacted.
Furthermore, regulations promulgated pursuant to the Health Insurance Portability and Accountability Act of 1996 (the “HIPAA”), establish privacy and security standards that limit the use and disclosure of certain individually identifiable health information defined in HIPAA as “protected health information” and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. Determining whether protected health information has been handled in compliance with applicable privacy standards and our contractual obligations can require complex factual and statistical analyses and may be subject to changing interpretation. Although we take measures to protect sensitive data from unauthorized access, use or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached, subject to a security incident, or otherwise compromised or disrupted due to various causes, including employee error, malfeasance or other malicious or inadvertent actions. Any such breach, incident, compromise or disruption could compromise our networks and the information stored there could be accessed by unauthorized parties, manipulated, publicly disclosed, lost, stolen, corrupted, made unavailable, or misused or otherwise processed without authorization. Any such access, breach or other loss of information could result in legal claims or proceedings, and liability under federal or state laws that protect the privacy of personal information, such as the HIPAA, the Health Information Technology for Economic and Clinical Health Act, and regulatory penalties. Notice of breaches must be made to affected individuals, the Secretary of the Department of Health and Human Services, and for extensive breaches, notice may need to be made to the media or State Attorneys General. Such a notice could harm our reputation and our ability to compete.
We are in the process of evaluating compliance needs but do not currently have in place formal policies and procedures related to the storage, collection and processing of information, and have not conducted any internal or external data privacy audits to fully assess our compliance with all applicable data protection laws and regulations. Additionally, we do not currently have policies and procedures in place for assessing our third-party vendors’ compliance with applicable data protection laws and regulations. All of these evolving compliance and operational requirements impose significant costs, such as costs related to organizational changes, implementing additional protection technologies, training employees and engaging consultants, which are likely to increase over time. In addition, such requirements may require us to modify our data processing practices and policies, distract management or divert resources from other initiatives and projects, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Any failure or perceived failure by us or our third-party vendors, collaborators, contractors and consultants to comply with any applicable federal, state or similar foreign laws and regulations, contractual obligations or any other actual or asserted obligations relating to data privacy and security could result in damage to our reputation, as well as claims, demands, proceedings or litigation by governmental agencies or other third parties, including class action privacy litigation in certain jurisdictions, which would subject us to significant fines, sanctions, awards, penalties, judgments and other liabilities, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Our use of artificial intelligence and machine learning technologies may result in reputational harm or liability.
We have incorporated and may continue to incorporate additional artificial intelligence and machine learning, or AIML, technologies into our platform, including within our decoding pipelines and otherwise within our business, and these solutions and features are key elements of our technological approach and to our future growth over time. We rely and expect to rely on AIML technologies in our platform, but there can be no assurance that we will realize the desired or anticipated benefits from AIML or any at all. We may also fail to properly implement or utilize AIML technologies. Our competitors or other third
parties may incorporate AIML into their products and services or otherwise within their business more quickly or more successfully than us, which could impair our ability to compete effectively and adversely affect our results of operations. Additionally, our use of AIML technologies may expose us to additional claims, demands and proceedings by private parties and regulatory authorities and subject us to legal liability as well as brand and reputational harm. For example, if output from AIML technologies or that they assist in producing are or are alleged to be deficient, inaccurate, or biased, or for such output, or such technologies or their development or deployment, including the collection, use, or other processing of data used to train or create such AIML technologies, to alleged to infringe upon or to have misappropriated third-party intellectual property rights or to violate applicable laws, regulations, or other actual or asserted legal obligations to which we are or may become subject, then our business, financial condition, and results of operations may be adversely affected. The legal, regulatory, and policy environments around AIML are evolving rapidly, and we may become subject to new and evolving legal and other obligations. These and other developments may require us to make significant changes to our use of AIML, including by limiting or restricting our use of AIML, and may require us to make significant changes to our policies and practices, which may necessitate expenditure of significant time, expense, and other resources, AIML also presents emerging ethical issues, and if our use of AIML becomes controversial, we may experience brand or reputational harm.
If we commercialize our Nautilus platform outside of the United States, our international business could expose us to business, tax, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
If we commercialize our Nautilus platform outside of the United States, our international business may be adversely affected by changing economic, political and regulatory conditions in foreign countries. Engaging in international business inherently involves a number of difficulties and risks, including:
•required compliance with existing and changing foreign regulatory requirements and laws;
•required compliance with U.S. laws such as the Foreign Corrupt Practices Act, and other U.S. federal laws and regulations established by the office of Foreign Asset Control;
•export or import restrictions;
•laws and business practices favoring local companies;
•foreign currency exchange, longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;
•political and economic instability;
•changes in social, economic, and political conditions or in laws, regulations and policies governing foreign trade, intellectual property, manufacturing, research and development, and investment both domestically as well as in the other countries and jurisdictions in which we operate and into which we may sell our products including as a result of the separation of the United Kingdom from the European Union (Brexit);
•potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements and other trade barriers;
•difficulties and costs of staffing and managing foreign operations, including compliance with diverse and complex local employment laws and practices; and
•difficulties protecting, maintaining, enforcing or procuring intellectual property rights.
If one or more of these risks occurs, it could require us to dedicate significant resources to remedy such occurrence, and if we are unsuccessful in finding a solution, our financial results will suffer.
In addition, if we commercialize our Nautilus platform outside of the United States, we may rely on distributors for sales of our Nautilus platform and related products. To do so we must attract distributors and maintain distributors to maximize the commercial opportunity for our platform. There is no guarantee that we will be successful in attracting or retaining desirable sales and distribution partners or that we will be able to enter into such arrangements on favorable terms. Distributors may not commit the necessary resources to market and sell our Nautilus platform and related products to the level of our expectations or may choose to favor marketing the products of our competitors. If current or future distributors do not perform adequately, or we are unable to enter into effective arrangements with distributors in particular geographic areas, we may not realize long-term international revenue growth and our financial results will suffer.
If we expand our development and commercialization activities outside of the United States, we will be subject to an increased risk of conducting activities in a manner that violates the U.S. Foreign Corrupt Practices Act and similar laws. If that occurs, we may be subject to civil or criminal penalties and other adverse consequences which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We are subject to the U.S. Foreign Corrupt Practices Act, or the FCPA, and similar anti-corruption laws which generally prohibit companies, their employees, agents, representatives, business partners, and third-party intermediaries from authorizing, offering, or providing, directly or indirectly, improper payments or benefits to recipients in the public or private sector. Specifically, the FCPA which prohibits corporations and individuals from paying, offering to pay, or authorizing the payment of anything of value to any foreign government official, government staff member, political party, or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. We are also subject to the UK Bribery Act, which prohibits both domestic and international bribery, as well as bribery across both public and private sectors.
If we choose to establish and expand our commercial operations outside of the United States we will need to comply with non-U.S. regulatory requirements, may need to establish and expand business relationships with various third parties, and we, our employees, agents, representatives, business partners and third-party intermediaries may interact more frequently with foreign officials, including regulatory authorities, and we may be held liable for the corrupt or other illegal activities of these employees, agents, representatives, business partners or third-party intermediaries, even if we do not explicitly authorize such activities. Any interactions with any such parties or individuals where improper payments are provided that are found to be in violation of such laws could result in substantial fines and penalties and could materially harm our business. We cannot assure you that all of our employees, agents, representatives, business partners and third-party intermediaries will not take actions in violation of applicable law for which we may ultimately be held responsible.
These laws also require that we keep accurate books and records and maintain internal controls and compliance procedures designed to prevent any such actions. While we have policies and procedures to address compliance with such laws, we cannot assure you that none of our employees, agents, representatives, business partners or third-party intermediaries will take actions in violation of our policies and applicable law, for which we may be ultimately held responsible. Further, as we increase our international sales and business, our risks under these laws may increase and expanded programs to maintain compliance with such laws may be costly and may not be effective.
Furthermore, any finding of a violation under one country’s laws may increase the likelihood that we will be prosecuted and be found to have violated another country’s laws. If our business practices are alleged to be or are found to be in violation of the FCPA, UK Bribery Act or other similar anti-corruption laws, we may be subject to whistleblower complaints, sanctions, settlements, prosecution, enforcement actions, fines, damages, adverse media coverage, investigations, loss of export privileges, significant civil and criminal penalties, or suspension or debarment from government contracts, all of which could have a material adverse effect on our reputation, financial condition and results of operations. Responding to any investigation or action will likely result in materially significant diversion of management’s attention and resources and significant defense costs and other professional fees.
Environmental and health safety laws may result in liabilities, expenses and restrictions on our operations. Failure to comply with environmental laws and regulations could subject us to significant liability.
Federal, state, local and foreign laws regarding environmental protection, hazardous substances and human health and safety may adversely affect our business. Our research and development operations involve the use of hazardous substances and are subject to a variety of federal, state, local and foreign environmental laws and regulations relating to the storage, use, discharge, disposal, remediation of, and human exposure to, hazardous substances and the sale, labeling, collection, recycling, treatment and disposal of products containing hazardous substances. These operations are permitted by regulatory authorities, and the resultant waste materials are disposed of in material compliance with environmental laws and regulations. Using hazardous substances in our operations exposes us to the risk of accidental injury, contamination or other liability from the use, storage, importation, handling or disposal of hazardous materials. If we or our suppliers’ operations result in the contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and fines, and any liability could significantly exceed our insurance coverage and have a material adverse effect on our business, financial condition and results of operations. Liability under environmental laws and regulations can be joint and several and without regard to fault or negligence. Compliance with environmental laws and regulations may be expensive and noncompliance could result in substantial liabilities, fines and penalties, personal injury and third-party property damage claims and substantial investigation and remediation costs. Environmental laws and regulations could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations. We cannot assure you that violations of these laws and regulations will not occur in the future or have not occurred in the past as a result of human error, accidents,
equipment failure or other causes. The expense associated with environmental regulation and remediation could harm our financial condition and operating results.
Our employees, independent contractors, consultants, commercial partners, distributors and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, consultants, commercial collaborators, distributors, suppliers and vendors may engage in misconduct or other improper activities. Misconduct by these parties could include failures to comply with applicable FDA regulations, provide accurate information to the FDA, comply with federal and state health care fraud and abuse laws and regulations, accurately report financial information or data or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the health care industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by these parties could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter misconduct by these parties, and the precautions we take to detect and prevent such misconduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending our self or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant penalties, including civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings and the curtailment or restructuring of our operations.
Demand for our technology could be reduced by legal, social and ethical concerns surrounding the use of genetic information and biological materials.
Our products may be used to provide genetic information or analyze biological materials from humans and other living organisms. The information obtained from our products could be used in a variety of applications, which may have underlying legal, social and ethical concerns, including the genetic engineering or modification of agricultural products, testing for genetic predisposition for certain medical conditions and stem cell research. Governmental authorities could, for safety, social or other purposes, call for limits on or impose regulations on the use of genetic testing or the use of certain biological materials. Such concerns or governmental restrictions could limit the use of our products, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to our Operations
If we experience a significant disruption in our information technology systems or breaches of data security, our business could be adversely affected.
We rely on information technology systems to keep financial records, facilitate our research and development initiatives, manage our manufacturing operations, maintain quality control, fulfill customer orders, process customer data and information, maintain corporate records, communicate with staff and external parties and operate other critical functions. Our information technology systems and those of our vendors and partners are potentially vulnerable to disruption, failure and compromise due to breakdown, malicious intrusion and computer viruses, ransomware or other malicious software, or other disruptive events, including, but not limited to, natural disasters and catastrophes. Like other life sciences technology companies, we have experienced cybersecurity incidents in the past, and we may experience them again in the future. For example, in January 2024, we experienced an incident involving unauthorized access to an employee account. The Company has worked, and continues to work with external cybersecurity experts, in detecting, blocking, containing, remediating, and investigating any cyber security incidents and risks, and in further enhancing our cybersecurity safeguards. This incident did not, and other incidents have not impacted the availability of our systems, materially disrupted our operations, or had any material impact on our financial or operating results. Cyber security risks are constantly evolving, and as such, we anticipate additional work and expense in the future as we continue to further enhance our security processes and initiatives to meet the changing landscape of cyber security risks. While cyber insurance may be available for companies, any available insurance for cyber events, may be limited in amount, subject to deductibles, and may not be adequate to cover us for all costs arising from cyber security incidents. Given increasing cyber security risks, cyber insurance may not be available to us in the future, or may not be available on commercially reasonable terms.
Cyberattacks and other malicious internet-based activity continue to increase and cloud-based platform providers of services have been and are expected to continue to be targeted. Furthermore, there may be a heightened risk of potential cyberattacks by state actors or others since the escalation of the conflicts in Eastern Europe and intensified conflicts in the
Middle East. Methods of attacks on information technology systems and attempting or effecting data security breaches and incidents change frequently, are increasingly complex and sophisticated, including social engineering and phishing scams, and can originate from a wide variety of sources. In addition to traditional computer “hackers,” malicious code, such as viruses and worms, employee theft or misuse, denial-of-service attacks and sophisticated nation-state and nation-state supported actors now engage in attacks, including advanced persistent threat intrusions. Despite our efforts to create security barriers to such threats, it is virtually impossible for us to entirely mitigate these risks. Despite any of our current or future efforts to protect against cybersecurity attacks and data security breaches, there is no guarantee that our efforts are adequate to safeguard against all such attacks and breaches. Moreover, it is possible that we may not be able to anticipate, detect, appropriately react and respond to, or implement effective preventative measures against, all cybersecurity incidents.
If our security measures, or those of our vendors and partners, are compromised due to cybersecurity attacks or security breaches or incidents, including as a result of third-party action, employee or customer error, malfeasance, stolen or fraudulently obtained log-in credentials or otherwise, or if any of these events is perceived to have occurred, our reputation could be damaged, our business and reputation may be harmed, we could become subject to claims, demands and litigation by private parties, and regulatory investigations and other proceedings, and we could incur significant liability. If we were to experience a prolonged system disruption in our information technology systems or those of certain of our vendors and partners, it could negatively impact our ability to serve our customers, which could adversely impact our business, financial condition, results of operations and prospects. If operations at our facilities were disrupted, it may cause a material disruption in our business if we are not capable of restoring functionality on an acceptable timeframe. In addition, our information technology systems, and those of our vendors and partners, are potentially vulnerable to data security breaches and incidents, whether by internal bad actors, such as employees or other third parties with legitimate access to our or our third-party providers’ systems, or external bad actors, which could lead to the exposure of personal data, sensitive data and confidential information to unauthorized persons. Any such data security breaches or incidents could lead to the loss of trade secrets or other intellectual property, or could lead to the loss, unavailability, exposure, unauthorized modification, alteration or other processing of personal information, including sensitive personal information, of our employees, customers and others, any of which could have a material adverse effect on our business, reputation, financial condition and results of operations.
In addition, any such access, disclosure or other loss or unauthorized use of information or data could result in legal claims or proceedings, regulatory investigations or actions, and other types of liability under laws that protect the privacy and security of personal information, including federal, state and foreign data protection and privacy regulations, violations of which could result in significant penalties and fines. Furthermore, defending a suit, regardless of its merit, could be costly, divert management’s attention and harm our reputation. In addition, although we seek to detect and investigate data security incidents, security breaches and other incidents of unauthorized access to our information technology systems and data can be difficult to detect and any delay in identifying such breaches or incidents may lead to increased harm and legal exposure of the type described above. Moreover, there could be public announcements regarding any actual or perceived cybersecurity incidents and any steps we take to respond to or remediate such incidents, and if securities analysts or investors perceive these announcements to be negative, it could, among other things, have a material adverse effect on the price of our Common Stock.
The cost of protecting against, investigating, mitigating and responding to potential breaches of our information technology systems and data security breaches and incidents and complying with applicable breach notification obligations to individuals, regulators, partners and others can be significant. As cybersecurity incidents continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any information security vulnerabilities. The inability to implement, maintain and upgrade adequate safeguards could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may be unable to manage our anticipated growth effectively.
Our anticipated growth will place significant strains on our management, operational and manufacturing systems and processes, sales and marketing team, financial systems and internal controls and other aspects of our business. We must upgrade our internal business processes and capabilities to create the scalability that a growing business demands. As of December 31, 2023, we had 167 employees. To execute our anticipated growth successfully, we must continue to attract and retain qualified personnel and manage and train them effectively. Developing and commercializing our Nautilus platform will require us to hire and retain scientific, sales and marketing, software, manufacturing, customer service, distribution and quality assurance personnel. In addition, we expect that we will need to hire additional accounting, finance and other personnel as a public company. As a public company, our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements and effectively manage these growth activities. We may face challenges integrating, developing and motivating our rapidly growing employee base.
Further, our anticipated growth will place additional strain on our suppliers and manufacturing facilities, resulting in an increased need for us to carefully monitor quality assurance. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.
Our ability to successfully manage our expected growth is uncertain given the fact that we have been in operation only since 2016. As we continue to grow, we will be required to implement more complex organizational management structures and may find it increasingly difficult to maintain the benefits of our corporate culture, including our ability to quickly develop and launch new and innovative products. If we do not successfully manage our anticipated growth, our business, results of operations, financial condition and prospects will be harmed.
If we are unable to recruit and retain key executives and scientists, we may be unable to achieve our goals.
Our performance is substantially dependent on the performance of our senior management and key scientific and technical personnel, particularly Sujal Patel, one of our founders and our Chief Executive Officer, and Parag Mallick, one of our founders and our Chief Scientist.
The loss of the services of any member of our senior management or our scientific or technical staff might significantly delay or prevent the development of our products or achievement of other business objectives by diverting management’s attention to transition matters and identification of suitable replacements, if any, and could have a material adverse effect on our business. We do not maintain fixed term employment contracts with any of our employees and do not maintain key man life insurance on any of our employees.
In addition, our research and product development efforts could be delayed or curtailed if we are unable to attract, train and retain highly skilled employees, particularly, senior scientists and engineers. To expand our research and product development efforts, we need additional people skilled in areas such as molecular and cellular biology, biochemistry, surface chemistry, software, bioinformatics, assay development, mechanical engineering, electrical engineering, optics, fluidics and manufacturing. Competition for these people is intense. Because of the complex and technical nature of our system and the dynamic market in which we compete, any failure to attract and retain a sufficient number of qualified employees could materially harm our ability to develop and commercialize our technology. As part of our retention and incentive efforts, in addition to salary and cash incentives, we have issued stock options that vest over time. The value to employees of stock options that vest over time may be significantly affected by decreases in our stock price (whether or not related to or proportional to our operating performance) and may at any time be insufficient to counteract more lucrative offers from other companies. We may face challenges in retaining and recruiting such individuals due to sustained declines in our stock price that could reduce the retention value of equity awards.
We may acquire other companies or technologies, which could divert our management’s attention, result in additional dilution to our stockholders and otherwise disrupt our operations and harm our operating results.
We may in the future seek to acquire or invest in businesses, applications or technologies that we believe could complement or expand our Nautilus platform or future products, enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of our management and cause us to incur various costs and expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated. We may not be able to identify desirable acquisition targets or be successful in entering into an agreement with any particular target or obtain the expected benefits of any acquisition or investment.
We have limited experience in acquiring other businesses or technologies. We may not be able to successfully integrate acquired personnel, operations and technologies, or effectively manage the combined business following an acquisition. Acquisitions could also result in dilutive issuances of equity securities, the use of our available cash, or the incurrence of debt, which could harm our operating results. In addition, if an acquired business fails to meet our expectations, our operating results, business and financial condition may suffer.
Unfavorable U.S. or global economic conditions as a result of multiple global events, including the COVID-19 pandemic, the conflicts in Eastern Europe and the Middle East, increasing interest rates, instability in the global financial markets, and general economic downturns, could adversely affect our ability to raise capital and our business, results of operations and financial condition.
While the potential economic impact brought by multiple adverse global circumstances, such as the COVID-19 pandemic, conflicts in Eastern Europe and the Middle East, potential uncertainty related to Taiwan and its relationship with China, increasing interest rates and general economic downturns, and the collapse of Silicon Valley Bank and other financial institutions in March 2023, and related instability in the global financial markets, are difficult to assess or predict, both as to magnitude and duration, these events have resulted in, and may continue to result in, extreme volatility and disruptions in the
capital and credit markets, reducing our ability to raise additional capital through equity, equity-linked or debt financings, which could negatively impact our short-term and long-term liquidity and our ability to operate in accordance with our operating plan, or at all. Additionally, these events have resulted, and in the future may result, in disruptions in our supply chains or the supply chains of those entities providing services or products to us, restrictions in our ability to deploy our workforce in our own facilities, locally, nationally or internationally, restrictions on the operating capacity of the laboratories or research facilities of our customers, decreases in government funding of research and development, or changes to programs that provide funding to research laboratories that may have the impact of redirecting funding to other areas of research, or prolonging or delaying funding cycles, any of which could adversely impact our ability to manufacture and sell our products. Moreover, our results of operations could be adversely affected by general conditions in the global economy and financial markets. A severe or prolonged economic downturn could result in a variety of risks to our business, including weakened demand for our Nautilus platform and our ability to raise additional capital when needed on favorable terms, if at all. A weak or declining economy could strain our customers’ budgets or cause delays in their payments to us. As a result of such events, we or our contractors, partners and/or suppliers could experience shortages, business disruptions or delays for materials sourced or manufactured in countries affected by such events, and their ability to supply us with services or components may be adversely affected. In addition, our contractors and suppliers have raised and may continue to raise prices for goods and services we employ in our research and development efforts and for components or materials used in our Nautilus platform.
Additionally, there is ongoing uncertainty regarding the federal budget and federal spending levels, including the possible impacts of a failure to increase the “debt ceiling.” Any U.S. government default on its debt could have broad macroeconomic effects that could, among other things, disrupt access to capital markets and deepen recessionary conditions. Further, as of December 31, 2023, we had cash, cash equivalents and investments of $264.1 million, consisting of U.S. treasury securities, mutual funds, commercial paper, corporate debt securities, and agency securities. Any default by the U.S. government or credit downgrade of the securities we hold could impact the liquidity or valuation of our investments.
Any of the foregoing could harm our business, financial condition and results of operations, and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our ability to raise capital, business, results of operations and financial condition.
Global supply chain interruptions could adversely affect our ability to develop and commercialize our products.
We may be subject to supply chain interruptions. Current or future supply chain interruptions that could be exacerbated by global political tensions, such as the situation in Eastern Europe, increased conflict in the Middle East, uncertainty related to Taiwan and its relationship with China, and resurgent or new global pandemics could negatively impact our ability to further develop our products or to manufacture and deliver our products or services, which could negatively impact our timelines and business results. For example, we have experienced some supply disruptions due to the COVID-19 pandemic, including closures at certain chip manufacturers, which led to extended lead times for certain chips; diversion of certain lab materials needed to support COVID-19 relief efforts; and lower availability of certain reagents, and delays similar to those we experienced during the COVID-19 pandemic could impact us if they recur or are exacerbated due to the situations in Eastern Europe and/or the Middle East.
If our facilities become unavailable or inoperable, our research and development program and commercialization launch plan could be adversely impacted and manufacturing of our instruments and consumables could be interrupted.
Our Seattle, Washington, facility primarily houses our corporate executive team and our software development operations, while our facilities in San Carlos and San Diego, California primarily house our research and development teams.
Our facilities in Seattle, San Carlos and San Diego are vulnerable to natural disasters, public health crises, including the impact of the COVID-19 pandemic, and other catastrophic events. For example, our San Carlos and San Diego facilities are located near earthquake fault zones and are vulnerable to damage from earthquakes as well as other types of disasters, including fires, floods, power loss, communications failures and similar events. If any disaster, public health crisis or catastrophic event were to occur, our ability to operate our business would be seriously, or potentially completely, impaired. If our facilities become unavailable for any reason, we cannot provide assurances that we will be able to secure alternative facilities with the necessary capabilities and equipment on acceptable terms, if at all. We may encounter particular difficulties in replacing our San Carlos facilities given the specialized equipment housed within it. The inability to manufacture our instruments or consumables, combined with our limited inventory of manufactured instruments and consumables, may result in the loss of future customers or harm our reputation, and we may be unable to re-establish relationships with those customers in the future.
If our research and development program or planned commercialization program were disrupted by a disaster or catastrophe, the launch of new products, including our Nautilus platform, and the timing of improvements to our products could be significantly delayed and could adversely impact our ability to compete with other available products and solutions. If our capabilities are impaired, we may not be able to manufacture and ship our products in a timely manner, which would adversely
impact our business. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
We use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development processes involve the controlled use of hazardous materials, including select chemicals that may be flammables, toxic or corrosives, as well as potential biohazard materials. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. In addition, our Nautilus platform involves the use of a high-powered laser system, which could result in injury. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials. We do not currently maintain separate environmental liability coverage and any such contamination or discharge could result in significant cost to us in penalties, damages and suspension of our operations.
Risks Related to Our Common Stock
An active trading market for our Common Stock may never develop or be sustained.
Prior to the Business Combination, there was no public trading market for Legacy Nautilus’ Common Stock. Although our Common Stock is listed on the Nasdaq Global Select Market, the market for our shares has demonstrated varying levels of trading activity. If an active trading market does not develop, or develops but is not maintained, you may have difficulty selling any of our Common Stock due to the limited public float. We cannot predict the prices at which our Common Stock will trade. It is possible that in one or more future periods our results of operations and progression of our product pipeline may not meet the expectations of public market analysts and investors, and, as a result of these and other factors, the price of our Common Stock may fall. Accordingly, we cannot assure you of your ability to sell your shares of our Common Stock when desired or at prices at or above the price you paid for your shares or at all.
The market price of our Common Stock has been and may continue to be volatile, which could result in substantial losses for investors.
The market price of our Common Stock has been and may continue to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control.
The market price of our Common Stock may fluctuate due to a variety of factors, including:
•the timing of the launch and commercialization of our products and degree to which such launch and commercialization meets the expectations of securities analysts and investors;
•actual or anticipated fluctuations in our operating results, including fluctuations in our quarterly and annual results;
•operating expenses being more than anticipated;
•the failure or discontinuation of any of our product development and research programs;
•changes in the structure or funding of research at academic and research laboratories and institutions, including changes that would affect their ability to purchase our instruments or consumables;
•the success of existing or new competitive businesses or technologies;
•announcements about new research programs or products of our competitors;
•developments or disputes concerning patent applications, issued patents or other proprietary rights;
•the recruitment or departure of key personnel;
•litigation and governmental investigations involving us, our industry or both;
•regulatory or legal developments in the United States and other countries;
•volatility and variations in market conditions in the life sciences technology sector generally, or the proteomics or genomics sectors specifically;
•investor perceptions of us or our industry;
•the level of expenses related to any of our research and development programs or products;
•actual or anticipated changes in our estimates as to our financial results or development timelines, variations in our financial results or those of companies that are perceived to be similar to us or changes in estimates or recommendations by securities analysts, if any, that cover our Common Stock or companies that are perceived to be similar to us;
•whether our financial results meet the expectations of securities analysts or investors;
•the announcement or expectation of additional financing efforts;
•sales of our Common Stock by us or by our insiders or other stockholders;
•general economic, industry and market conditions; and
•the COVID-19 pandemic, natural disasters or major catastrophic events.
Recently, stock markets in general, and the market for life sciences technology companies in particular, have experienced significant price and volume fluctuations that have often been unrelated or disproportionate to changes in the operating performance of the companies whose stock is experiencing those price and volume fluctuations, particularly in light of the current COVID-19 pandemic. Broad market and industry factors may seriously affect the market price of our Common Stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our Common Stock. Following periods of such volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Because of the potential volatility of our Common Stock price, we may become the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and resources from our business.
There can be no assurance that we will be able to comply with the continued listing standards of Nasdaq.
If Nasdaq delists our shares of Common Stock from trading on its exchange for failure to meet Nasdaq’s listing standards, we and our stockholders could face significant material adverse consequences including:
•a limited availability of market quotations for our securities;
•reduced liquidity for our securities;
•a determination that our Common Stock is a “penny stock” which will require brokers trading in our Common Stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities;
•a limited amount of new and analyst coverage; and
•a decreased ability to issue additional securities or obtain additional financing in the future.
Our principal stockholders and management own a significant percentage of our Common Stock and will be able to exercise significant influence over matters subject to stockholder approval.
As of December 31, 2023, our directors, executive officers, holders of more than 5% of our outstanding shares of Common Stock and their respective affiliates beneficially owned, collectively, approximately 68.8% of the outstanding shares of Common Stock. As a result, these stockholders, if they act together, may significantly influence all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control of our company that our other stockholders may believe is in their best interests. This in turn could have a material adverse effect on our stock price and may prevent attempts by our stockholders to replace or remove the board of directors or management.
The sale or the perception of future sales of a substantial number of shares of our Common Stock could cause the market price of our Common Stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our Common Stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our Common Stock.
Pursuant to the Amended and Restated Registration Rights and Lock-Up Agreement (the “Registration Rights and Lock-Up Agreement”) and the Subscription Agreements entered into in connection with the PIPE Financing, we have filed resale
registration statements to provide for the resale of the shares issued in the PIPE Financing and the shares of our Common Stock held by the parties to the Registration Rights and Lock-Up Agreement. The market price of our Common Stock could decline if the holders whose shares are registered under such registration statements sell their shares or are perceived by the market as intending to sell their shares.
We do not expect to pay any dividends for the foreseeable future. Investors may never obtain a return on their investment.
You should not rely on an investment in our Common Stock to provide dividend income. We do not anticipate that we will pay any dividends to holders of our Common Stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations, fund our research and development programs and continue to invest in our commercial infrastructure. In addition, any future credit facility or financing we obtain may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our Common Stock. Accordingly, investors must rely on sales of our Common Stock after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not purchase our Common Stock.
Our bylaws designate a state or federal court located within the State of Delaware as the exclusive forum for substantially all disputes between us and our stockholders, and also provide that the federal district courts will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act of 1933, as amended, each of which could limit our stockholders’ ability to choose the judicial forum for disputes with us or our directors, officers, stockholders, or employees.
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum (an “Alternative Forum Consent”), the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, another state court in Delaware or the federal district court for the District of Delaware) will, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, stockholders or other employees to us or our stockholders, (iii) any action arising pursuant to any provision of the Delaware General Corporation Law or our certificate of incorporation or bylaws (each, as may be amended from time to time), or (iv) any action asserting a claim governed by the internal affairs doctrine of the State of Delaware, except for any claim as to which the court does not have jurisdiction over an indispensable party to that claim. The foregoing shall not apply to any claims under the Exchange Act or the Securities Act of 1933, as amended (the “Securities Act”). In addition, unless we give an Alternative Forum Consent, the federal district courts of the United States shall be the sole and exclusive forum for resolving any action asserting a claim arising under the Securities Act against any person in connection with any offering of the Company’s securities, including any auditor, underwriter, expert, control person or other defendant.
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our bylaws also provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act.
Any person or entity purchasing or otherwise acquiring or holding or owning (or continuing to hold or own) any interest in any of our securities shall be deemed to have notice of and consented to the foregoing bylaw provisions. Although we believe these exclusive forum provisions benefit us by providing increased consistency in the application of Delaware law and federal securities laws in the types of lawsuits to which each applies, the exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum of its choosing for disputes with us or any of our directors, officers, stockholders, or other employees, which may discourage lawsuits with respect to such claims against us and our current and former directors, officers, stockholders, or other employees. In addition, a stockholder that is unable to bring a claim in the judicial forum of its choosing may be required to incur additional costs in the pursuit of actions which are subject to the exclusive forum provisions described above. Our stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder as a result of our exclusive forum provisions. Further, in the event a court finds either exclusive forum provision contained in our bylaws to be unenforceable or inapplicable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our results of operations.
Delaware law and provisions in our certificate of incorporation and bylaws might discourage, delay or prevent a change in control of our company or changes in our management and, therefore, depress the trading price of our Common Stock.
Our status as a Delaware corporation and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested
stockholder for a period of three years after the person becomes an interested stockholder without the approval of holders of 66 2/3% of the voting power of our stockholders other than the interested stockholder, even if a change of control would be beneficial to our existing stockholders. In addition, our certificate of incorporation and bylaws contain provisions that may make the acquisition of our company more difficult, including the following:
•our board of directors is classified into three classes of directors with staggered three-year terms and directors are only able to be removed from office for cause by the affirmative vote of holders of at least two-thirds of the voting power of our then outstanding capital stock;
•certain amendments to our certificate of incorporation require the approval of stockholders holding two-thirds of the voting power of our then outstanding capital stock;
•any stockholder-proposed amendment to certain provisions of our bylaws require the approval of stockholders holding two-thirds of the voting power of our then outstanding capital stock;
•our stockholders are only able to take action at a meeting of stockholders and are not able to take action by written consent for any matter;
•vacancies on our board of directors are able to be filled only by our board of directors and not by stockholders;
•only the chair of our board of directors, our chief executive officer, our president or a majority of our board of directors are authorized to call a special meeting of stockholders;
•certain litigation against us can only be brought in Delaware;
•our certificate of incorporation authorizes undesignated preferred stock, the terms of which may be established by our Board and shares of which may be issued, without the approval of the holders of our capital stock; and
•advance notice procedures apply for stockholders to nominate candidates for election as directors or to bring matters before an annual meeting of stockholders.
These anti-takeover defenses could discourage, delay, or prevent a transaction involving our change in control. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors of their choosing and to cause us to take other corporate actions they desire, any of which, under certain circumstances, could limit the opportunity for our stockholders to receive a premium for their shares of our capital stock.
General Risk Factors
We will continue to incur significant increased costs and management resources as a result of operating as a public company.
As a public company, we will continue to incur significant legal, accounting, compliance and other expenses that we did not incur as a private company and these expenses may increase even more after we are no longer an “emerging growth company.” Our management and other personnel will need to devote a substantial amount of time and incur significant expense in connection with compliance initiatives. As a public company, we will continue to bear all of the internal and external costs of preparing and distributing periodic public reports in compliance with our obligations under the securities laws.
In addition, regulations and standards relating to corporate governance and public disclosure, including the SOX, and the related rules and regulations implemented by the SEC and The Nasdaq Stock Market LLC, have increased legal and financial compliance costs and will make some compliance activities more time-consuming. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment will result in increased general and administrative expenses and may divert management’s time and attention from our other business activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us, and our business may be harmed. In the future, it may be more expensive or more difficult for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members for our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
We have broad discretion in the use of the net proceeds from the Business Combination and the PIPE Financing and may not use them effectively.
We cannot specify with certainty the particular uses of the net proceeds we received from the Business Combination and the PIPE Financing. Our management will have broad discretion in the application of the net proceeds. Our management may spend a portion or all of the net proceeds in ways that our stockholders may not desire or that may not yield a favorable return. The failure by our management to apply these funds effectively could harm our business, financial condition, results of operations and prospects. Pending their use, we may invest the net proceeds from the Business Combination and the PIPE Financing in a manner that does not produce income or that loses value.
Our ability to use net operating losses and certain other tax attributes to offset future taxable income may be subject to certain limitations.
Our U.S. federal and state net operating loss carryforwards, or NOLs, may be unavailable to offset future taxable income because of restrictions under U.S. federal and/or state law. U.S. federal NOLs that arose in tax years ending on or prior to December 31, 2017, are only permitted to be carried forward for 20 years. U.S. federal NOLs that arose in tax years beginning after December 31, 2017, may be carried forward indefinitely, but for taxable years beginning after December 31, 2020, the deductibility of such U.S. federal NOLs will be limited to 80% of our current year taxable income. Our state NOLs may be subject to similar or different limitations. As of December 31, 2023, we had U.S. federal NOLs of $71.2 million, of which $70.6 million do not expire, and state NOLs of $95.5 million that will begin to expire in 2037.
In addition, Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, may limit the NOLs we may use in any year for U.S. federal income tax purposes in the event of certain changes in our ownership. A Code Section 382 “ownership change” generally occurs if one or more stockholders or groups of stockholders who own at least 5% of a company’s stock increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. Similar rules may apply under state tax laws. We have not conducted a Code Section 382 study to determine whether the use of our NOLs is impaired. We may have previously undergone an “ownership change.” In addition, future issuances or sales of our stock, including certain transactions involving our stock that are outside of our control, could result in future “ownership changes.” “Ownership changes” that have occurred in the past or that may occur in the future could result in the imposition of an annual limit on the amount of pre-ownership change NOLs and other tax attributes we can use to reduce our taxable income, potentially increasing and accelerating our liability for income taxes, and also potentially causing those tax attributes to expire unused. States may impose other limitations on the use of our NOLs and other tax attributes. Any limitation on using NOLs could, depending on the extent of such limitations and the NOLs previously used, result in our retaining less cash after payment of U.S. federal and state income taxes during any year in which we have taxable income, rather than losses, than we would be entitled to retain if such NOLs were available as an offset against such taxable income for U.S. federal and state income tax reporting purposes, which could adversely impact our operating results.
Changes in tax laws could have a material adverse effect on our future business, cash flows, results of operations or financial condition.
We may in the future be subject to the tax laws, regulations, and policies of several taxing jurisdictions. Changes in tax laws, as well as other factors, could cause us to experience fluctuations in our tax obligations and effective tax rates and otherwise adversely affect our tax positions and/or our tax liabilities. For example, many countries and local jurisdictions and organizations such as the Organisation for Economic Co-operation and Development have proposed or implemented new tax laws or changes to existing tax laws, including additional taxes on payroll or employees and a proposed 15% global minimum tax (Pillar 2) that is being adopted by several countries with implementation beginning in 2024. Any new or changes to tax laws could adversely affect our future effective tax rate, operating results, tax credits or incentives or tax payments, which could have a material adverse effect on our future business, cash flows, results of operations or financial condition if we expand internationally. For example, the United States recently enacted the Inflation Reduction Act of 2022, which implements, among other changes, a 1% non-deductible excise tax on certain stock buybacks effective January 1, 2023. Further, on January 1, 2022, a provision of the Tax Cuts and Jobs Act of 2017 went into effect that eliminates the option to deduct research and development costs in the year incurred and instead requires taxpayers to amortize such costs over five years for domestic and 15 years for foreign costs. Such changes, among others, may adversely affect our future effective tax rate, business, cash flows, results of operations and financial condition.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results in a timely manner or prevent fraud, which would harm our business.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations
in a timely manner, or at all. In addition, any testing by us conducted in connection with Section 404(a) of the Sarbanes-Oxley Act of 2002 (“SOX”) or any subsequent testing by our independent registered public accounting firm in connection with Section 404(b) of SOX, may reveal deficiencies in our internal controls over financial reporting that are deemed to be significant deficiencies or material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Ineffective internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our Common Stock.
We will be required to disclose material changes made in our internal controls over financing reporting and procedures on a quarterly basis and our management will be required to assess the effectiveness of these controls annually. We will be required to make a formal assessment of the effectiveness of our internal control over financial reporting, and once we cease to be an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), we will be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, for as long as we are an “emerging growth company,” our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404(b) of SOX.
To achieve compliance with Section 404(a) of SOX within the prescribed period, we have engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a plan to assess and document the adequacy of our internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are designed and operating effectively and implement a continuous reporting and improvement process for internal control over financial reporting.
An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not identify. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
If our estimates or judgments relating to our critical accounting policies are based on assumptions that change or prove to be incorrect, our results of operation could fall below our publicly announced guidance or the expectations of securities analysts and investors, resulting in a decline in the market price of our Common Stock.
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. We base our estimates on historical experience and estimates and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets, liabilities, equity, and expenses that are not readily apparent from other sources. For example, in connection with the implementation of the new revenue accounting standard if and when we have product sales, management makes judgments and assumptions based on our interpretation of the new standard. The new revenue standard is principle-based, and interpretation of those principles may vary from company to company based on their unique circumstances. It is possible that interpretation, industry practice and guidance may evolve as we apply the new standard. If our assumptions underlying our estimates and judgements relating to our critical accounting policies change or if actual circumstances differ from our assumptions, estimates or judgements, our operating results may be adversely affected and could fall below our publicly announced guidance or the expectations of securities analysts and investors, resulting in a decline in the market price of our Common Stock.
We are an “emerging growth company” and a “smaller reporting company” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For so long as we remain an emerging growth company, we are permitted by SEC rules and plan to rely on exemptions from certain disclosure requirements that are applicable to other SEC registered public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of SOX, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, the information we provide stockholders will be different than the information that is available with respect to other public companies that are not emerging growth companies. To the extent that we continue to qualify as a “smaller reporting company,” as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), after we cease to qualify as an emerging growth company, we will continue to be permitted to make
certain reduced disclosures in our periodic reports and other documents that we file with the SEC. We cannot predict whether investors will find our Common Stock less attractive if we rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will not be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Reports published by analysts, including projections in those reports that differ from our actual results, could adversely affect the price and trading volume of our common shares.
Securities research analysts may establish and publish their own periodic projections for us. These projections may vary widely and may not accurately predict the results we actually achieve. The share price of our Common Stock may decline if our actual results do not match the projections of these securities research analysts. Similarly, if one or more of the analysts who write reports on us downgrades our stock or publishes inaccurate or unfavorable research about our business, the share price of our Common Stock could decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, the share price or trading volume of our Common Stock could decline.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
We have established policies and processes for assessing, identifying, and managing risks from cybersecurity threats, and have integrated these processes into our overall risk management systems and processes. We routinely assess risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through our information systems that may result in adverse effects on the confidentiality, integrity, or availability of our information systems or any information residing therein.
We conduct periodic risk assessments to identify cybersecurity threats, as well as assessments in the event of a material change in our business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems, and safeguards in place to manage such risks.
Following these risk assessments, we evaluate whether and how to re-design, implement, and maintain reasonable safeguards to minimize identified risks; reasonably address any identified gaps in existing safeguards; and regularly monitor the effectiveness of our safeguards. We devote significant resources and designate high-level personnel, including our Head of IT, who reports to our Chief Financial Officer, to manage the risk assessment and mitigation process.
As part of our overall risk management system, we monitor our safeguards and train our employees on these safeguards, in collaboration with human resources, IT, and management. Personnel at all levels and departments are made aware of our cybersecurity policies through trainings integrated into new employee onboarding processes and annual employee re-training.
We engage consultants, experts, or other third parties in connection with our risk assessment processes. These third parties assist us in designing and implementing our cybersecurity policies and procedures, as well as in monitoring and testing our safeguards.
We require each third-party service provider who may have access to our systems and/or our sensitive data to attest that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company.
We have not experienced any cybersecurity incidents that have been determined to be material in the past, however, like other life sciences technology companies, we have experienced cybersecurity incidents and may continue to experience them in the future. For additional information regarding whether any risks from cybersecurity threats, including as a result of any previous cybersecurity incidents, materially affected or are reasonably likely to materially affect our company, including our business strategy, results of operations, or financial condition, please refer to Item 1A, “Risk Factors,” in this annual report on Form 10-K, including, for example, the risk factor entitled “If we experience a significant disruption in our information technology systems or breaches of data security, our business could be adversely affected.”
Governance
One of the key functions of our board of directors is informed oversight of our risk management process, including risks from cybersecurity threats. Our board of directors is responsible for monitoring and assessing strategic risk exposure, and our executive officers are responsible for the day-to-day management of the material risks we face. Our board of directors administers its cybersecurity risk oversight function directly as a whole, as well as through the audit committee.
Our Head of IT and our management committee on cybersecurity, which includes representatives of the Company’s legal department, finance department and IT department, who collectively possess significant experience in evaluating, managing and mitigating security and other risks, including cybersecurity risks, are primarily responsible to assess and manage our material risks from cybersecurity threats.
Our Head of IT and our management committee on cybersecurity oversee our cybersecurity policies and processes, including those described in “Risk Management and Strategy” above. The processes by which our Head of IT and representatives from our management committee on cybersecurity are informed about and monitor the prevention, detection, mitigation, and remediation of cybersecurity incidents includes the following:
•monitoring of Company computer and information systems for potential malware, ransomware and other malicious activity, and remediation of identified issues, including mitigation of identified risks and containment and elimination of any malicious software;
•mandatory cybersecurity training as part of new employee onboarding along with required annual employee cybersecurity re-training;
•automated monitoring of systems and network infrastructure through event log analysis by security information and event management application;
•prompt incident reporting directly to the Company’s CFO and General Counsel; and
◦escalation to the Company’s audit committee and board of directors as warranted based upon the nature of the identified issue.
Our Head of IT and/or representatives from our management committee on cybersecurity provide periodic briefings to the audit committee regarding our company’s cybersecurity risks and activities, including any recent cybersecurity incidents and related responses, cybersecurity systems testing, activities of third parties, and the like. Our audit committee provides regular updates to the board of directors on such reports.
Item 2. Properties
Our current corporate headquarter is located at 2701 Eastlake Avenue East, Seattle, Washington and our current research and development facilities, and manufacturing centers are located at 835 Industrial Rd, San Carlos, California. The Seattle facility is approximately 14,800 square feet. The 835 Industrial Rd, San Carlos, California lease is for approximately 45,338 square feet of office and research space. We lease approximately 19,957 square feet of additional research and development and manufacturing space at 1561 Industrial Rd, San Carlos, California. In addition, we lease office and research space located at 4475 Executive Drive, San Diego, California. The San Diego facility is approximately 7,064 square feet. We do not own real property and believe that our current facilities are sufficient to meet our ongoing needs and that, if we require additional space, we will be able to obtain additional facilities on commercially reasonable terms.
Item 3. Legal Proceedings
U.S. District Court Proceedings
On December 14, 2023, we filed suit in the United States District Court for the Northern District of California against Somalogic, Inc. (Somalogic) which recently merged with and is now part of “Standard Biotools, Inc.”, and the California
Institute of Technology. This suit seeks declaratory judgment that Nautilus does not infringe any claims of US Patent No. 7,842,793 related to DNA origami structures (the ‘793 Patent), which Somalogic has allegedly licensed from the California Institute of Technology through its purchase of Palamedrix, Inc. We have taken this action in response to Somalogic’s allegations that the Nautilus proteome analysis platform infringes the claims of the ‘793 Patent. We believe that it does not use any technology related to what the ‘793 Patent purports to claim in any of the products or services that we are developing. Nor do we have any interest or plans to use such technology in any of our future products or services. We intend to vigorously pursue our claims in this litigation.
Other Proceedings
From time to time, we may become involved in various claims and legal proceedings. Regardless of outcome, litigation and other legal and administrative proceedings can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 4. Mine Safety Disclosures
Not applicable.
Part II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market information for common stock
Our common stock has traded on the Nasdaq Global Select Market under the symbol “NAUT” since June 10, 2021. Prior to June 10, 2021 and before completion of the Business Combination, the Class A ordinary shares of ARYA Sciences Acquisition Corp III traded on The Nasdaq Capital Market under the symbol “ARYA” and there was no public trading market for Legacy Nautilus’ equity.
Holders
As of February 15, 2024, there were 35 registered stockholders of record of our common stock. Because most of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial stockholders represented by these holders of record.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We intend to retain any future earnings and do not expect to pay any dividends in the foreseeable future. In addition, future debt instruments we issue may materially restrict our ability to pay dividends on our common stock. Payment of future cash dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, the requirements of then-existing debt instruments and other factors our board of directors deems relevant.
Equity Compensation Plan
Our equity plan information required by this Item is incorporated by reference to the information in Part III, Item 12 of this Annual Report on Form 10-K.
Recent Sales of Unregistered Securities
None.
Issuer Purchases of Equity Securities
None.
Item 6. [ Reserved ]
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with the financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. Some of the information in this discussion and analysis contains forward-looking statements reflecting our current expectations and involves risk and uncertainties. For example, statements regarding our expectations as to our plans and strategy for our business, future financial performance, expense levels and liquidity sources are forward-looking statements. Our actual results and the timing of events could differ materially from those discussed in our forward-looking statements as a result of many factors, including those set forth under the “Risk Factors” section and elsewhere in this Annual Report on Form 10-K. Please also see the section entitled “Special Note Regarding Forward-Looking Statements.”
Unless otherwise indicated or the context otherwise requires, references in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section to “Nautilus,” “we,” “us,” “our” and other similar terms refer to the business and operations of Legacy Nautilus prior to the Business Combination and to New Nautilus and its consolidated subsidiary following the Business Combination.
Overview
We are a development stage life sciences company creating a platform technology for quantifying and unlocking the complexity of the proteome. Our mission is to transform the field of proteomics by democratizing access to the proteome and enabling fundamental advancements across human health and medicine. We were founded on the belief that incremental advancements of existing technologies are inadequate, and that a bold scientific leap would be required to radically reinvent proteomics and revolutionize precision medicine. Our vision is to integrate our breakthrough innovations in computer science, engineering, and biochemistry to develop and commercialize a proteomic analysis technology of extreme sensitivity and scale. To accomplish this, we have built a prototype of a proteome analysis system, an instrument to perform massively parallel single protein molecule measurements which will be further developed to deliver the speed, simplicity, accuracy, and versatility that we believe is necessary to establish a new gold standard in the field.
Since our incorporation in 2016, we have devoted substantially all of our resources to research and development activities, including with respect to our proteomics platform, or Nautilus platform, business planning, establishing and maintaining our intellectual property portfolio, hiring personnel, raising capital and providing general and administrative support for these operations. We do not have any products available for commercial sale, and we have not generated any revenue from our Nautilus platform or other sources since inception. Our ability to generate revenue sufficient to achieve profitability, if ever, will depend on the successful development and eventual commercialization of our Nautilus platform, which we expect, if it ever occurs, will take a number of years. Our Nautilus platform, which includes our end-to-end solution comprised of instruments, consumables, and software analysis, is currently under development and will require significant additional research and development efforts, including extensive testing prior to commercialization. These efforts require significant amounts of additional capital and adequate personnel infrastructure. There can be no assurance that our research and development activities will be successfully completed, or that our Nautilus platform will be commercially viable.
In order to commercialize our Nautilus platform in volume, we will need to establish internal manufacturing capacity or to contract with one or more manufacturing partners, or both. Our technology is complex, and the manufacturing process for our products will be similarly complex, involving a large number of unique precision parts in addition to the production of various reagents and antibodies. We may encounter unexpected difficulties in manufacturing our Nautilus platform, instruments, and related consumables. Among other factors, we will need to develop reliable supply chains for the various components in our Nautilus platform, instruments, and consumables to support large-scale commercial production. In connection with our Nautilus platform, we intend to utilize over 300 complex reagents and various antibodies in order to generate deep proteomic information at the speed and scale which we expect our Nautilus platform to perform. Such reagents and antibodies are expected to be more difficult to manufacture and more expensive to procure. There is no assurance that we will be able to build manufacturing or consumable production capacity internally or find one or more suitable manufacturing or production partners, or both, to meet the volume and quality requirements necessary to be successful in the proteomics market.
Given our stage of development, we have not yet established a commercial organization or distribution capabilities. We do intend to build a commercial infrastructure to support sales of our products. We expect to manage sales, marketing and distribution through both internal resources and third-party relationships. We plan to commercialize our proteomics platform using a three-phase plan that has been shown to be effective and optimal for introducing disruptive products in numerous life sciences technology markets. The first phase is expected to involve collaboration with biopharmaceutical companies and key opinion leaders to validate the performance and utility of Nautilus’ product, during which we do not expect to recognize significant revenue, if any. The second phase will include an early access limited release phase in which we expect to recognize limited revenue. Finally, the third phase is anticipated to include a broader commercial launch. We are currently in the
collaboration phase during which we have entered into collaborations with a small number of research customers, including with biopharmaceutical companies and key opinion leaders in proteomics whose assessment and validation of our products can significantly influence other researchers in their respective markets and/or fields. During the early access limited release phase, we plan to leverage our publications to drive awareness and customer demand to pre-sell instruments and reagents to select customers performing large-scale proteomics research. We do not anticipate that these activities will result in any material revenue. During the second phase, we expect to work closely with early access customers to demonstrate a unique value proposition for our Nautilus platform. During this phase, we plan to provide early access program partners with broad-scale analysis and profiling of samples analyzed in our facility and shared via a cloud platform. We anticipate early access engagements and associated revenue in 2025. We expect this second phase to lead into the third phase of broad commercialization and launch of our proteome analysis platform in 2025. Voice of customer studies have suggested that there is market demand for a proteomics platform with specifications that are initially lower than what we have previously disclosed, for example, around characteristics such as sample input and proteome coverage. Consequently, as we balance our time to market goals with our evolving view of customer requirements, we are refining our initial launch specifications. We believe that subsequent consumable releases will enable our platform to meet or exceed our previously announced product specifications.
We intend to commercialize our Nautilus platform through a direct sales channel in the United States, and through both direct and distributor sales channels in regions outside the United States. Given our stage of development, we currently have limited marketing, sales, commercial product distribution or service and support capabilities. We intend to build the necessary infrastructure for these activities in the United States, European Union, the United Kingdom, and potentially other countries and regions, including Asia-Pacific, as we execute on our three phase commercial launch strategy for our Nautilus platform.
On June 9, 2021, Nautilus Biotechnology, Inc., a Delaware corporation (f/k/a ARYA Sciences Acquisition Corp. III, a Cayman Islands exempted company and the Company’s predecessor company (“ARYA”)), consummated the business combination (the “Business Combination”) pursuant to the terms of that certain Business Combination Agreement, dated as of February 7, 2021 (the “BCA”), by and among ARYA, Mako Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of ARYA (“Mako Merger Sub”), and Nautilus Subsidiary, Inc., a Delaware corporation (f/k/a Nautilus Biotechnology, Inc.) (“Legacy Nautilus”). As a result of the Business Combination, ARYA changed its name to “Nautilus Biotechnology, Inc.” and Mako Merger Sub merged with and into Legacy Nautilus with Legacy Nautilus surviving as the surviving company and becoming a wholly-owned subsidiary of ARYA (the “Merger” and, collectively with the other transactions described in the BCA, the “Reverse Recapitalization”).
In addition, in conjunction with the completion of the Business Combination, certain investors (“PIPE Investors”) subscribed for the purchase of an aggregate of 20,000,000 shares of common stock of the Company (“New Nautilus Common Stock”) at a price of $10.00 per share for aggregate gross proceeds of $200.0 million (“PIPE Financing”).
Prior to the Business Combination, we financed our operations primarily through private placements of convertible preferred stock and had raised aggregate net proceeds of $108.4 million from these private placements. In connection with the consummation of the Business Combination and PIPE Financing, we received additional gross proceeds of approximately $345.5 million from PIPE Investors and the Business Combination, offset by approximately $18.2 million of transaction costs and underwriters’ fees relating to the closing of the Business Combination. As of December 31, 2023, we had cash, cash equivalents and short-term investments of $173.4 million. Based on this, we believe that our existing cash, cash equivalents, and short-term investments will enable us to fund our planned operating expenses and capital expenditures through at least the next 12 months.
We have incurred significant losses since the commencement of our operations. Our net loss was $63.7 million during the year ended December 31, 2023, and we expect to continue to incur significant losses for the foreseeable future as we continue our research and development activities and planned commercialization of our proteomics platform. As of December 31, 2023, we had an accumulated deficit of $202.2 million. These losses have resulted primarily from costs incurred in connection with research and development activities and to a lesser extent from general and administrative costs associated with our operations. We expect to incur significant and increasing expenses and operating losses for the foreseeable future. Our net losses may fluctuate significantly from period to period, depending on the timing of and expenditures on our planned commercialization and research and development activities.
We expect our expenses and capital requirements will increase substantially in connection with our ongoing activities as we:
•continue our research and development activities, including with respect to our Nautilus platform;
•undertake activities to establish sales, marketing and distribution capabilities for our Nautilus platform;
•incur setup costs related to production tooling and required testing;
•maintain, protect and expand our intellectual property portfolio, including patents, trade secrets and know how;
•implement operational, financial and management information systems;
•attract, hire and retain additional management, scientific and administrative personnel; and
•operate as a public company.
As a result, we will require substantial additional funding to develop our products and support our continuing operations. Until such time that we can generate significant revenue from product sales, if ever, we expect to finance our operations through the sale of equity, debt financings or other capital sources, which could include income from collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties or from grants. We may be unable to raise additional funds or to enter into such agreements or arrangements on favorable terms, or at all. Our ability to raise additional funds may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, the credit and financial markets in the United States and worldwide resulting from the ongoing COVID‑19 pandemic, recent and any potential future financial institution failures, the conflicts in Eastern Europe, the Middle East and in other countries, and otherwise. Our failure to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on our business, results of operations or financial condition, and could force us to delay, reduce or eliminate our product development or future commercialization efforts. We may also be required to grant rights to develop and market products that we would otherwise prefer to develop and market ourselves. The amount and timing of our future funding requirements will depend on many factors, including the pace and results of our development efforts. We cannot assure you that we will ever be profitable or generate positive cash flow from operating activities.
Impact of Negative Global or National Events
Businesses have been and will continue to be impacted by a number of challenging global and national events and circumstances that continue to evolve, including the COVID-19 pandemic, extreme weather conditions, increased economic uncertainty, inflation, rising interest rates, recent and any potential future financial institution failures, and conflicts in Eastern Europe, the Middle East and in other countries. The extent of the impact of these events and circumstances on our business, operations and development timelines and plans remains uncertain, and will depend on certain developments, including the duration and scope of the events and their impact on our development activities, third-party manufacturers, and other third parties with whom we do business, as well as its impact on regulatory authorities and our key scientific and management personnel. We have been and continue to actively monitor the potential impacts that these various events and circumstances may have on our business and we take steps, where warranted, to minimize any potential negative impacts on our business resulting from these events and circumstances. For example, as the COVID-19 pandemic has developed, we have taken numerous steps to help ensure the health and safety of our employees. We continue to employ hygiene protocols; controls for social distancing; enhanced cleaning, disinfecting, decontamination, and ventilation protocols; health policies; and usage of personal protective equipment, in all cases where appropriate. While we have resumed normal operations, any resurgence or worsening of the COVID-19 pandemic may cause us to reinstitute certain measures to protect employee safety, including staggered work hours or reduced in-person staffing, that could result in additional disruption and/or delays in our ability to conduct development activities.
We have been and continue to actively monitor our supply chain in light of these challenging global and national events and circumstances, including our third-party materials suppliers. We have, in the past, experienced some supply disruptions due to the COVID-19 pandemic, including closures at certain chip manufacturers, which led to extended lead times for certain chips; diversion of certain lab materials needed to support COVID-19 relief efforts; and lower availability of certain reagents. While certain of these disruptions have been resolved since the start of the COVID-19 pandemic, we are continuing to monitor our supply chain and contingency planning is ongoing with our partners to reduce the possibility of an interruption to our development activities or the availability of necessary materials.
The ultimate impact of these global and national events and circumstances, either individually or in the aggregate, is highly uncertain and subject to change. In April 2023, President Biden signed legislation that ended the COVID-19 national emergency on May 11, 2023. The full impact of this termination of the national emergency and the wind-down of the public health emergency on FDA and other regulatory policies and operations are unclear. To the extent possible, we are conducting business as usual, with necessary or advisable modifications to mitigate potentially negative impacts to our business. For example, during the COVID-19 pandemic, we made certain modifications to employee travel, with masking and vaccination requirements in our offices, and with our employees working remotely fully or intermittently as able from March 2020 until August 2022. We will continue to actively monitor the rapidly evolving situation related to these global and national events, and may take further actions to mitigate potential negative impacts to our business, and that may alter our operations, including those that may be required by federal, state or local authorities, or that we determine are in the best interests of our employees and other third parties with whom we do business. At this point, the extent to which these global or national events and
circumstances may affect our future business, operations and development timelines and plans, including the resulting impact on our expenditures and capital needs, remains uncertain.
Components of Our Results of Operations
Revenue
To date, we have not generated any revenue and we may not generate any revenue from the sale of products or from other sources in the near future.
Operating Expenses
Research and Development Expense
Research and development expenses account for a significant portion of our operating expenses and consist primarily of salaries, related benefits and stock-based compensation expense of product development personnel, laboratory supplies and equipment, depreciation and amortization, external costs of vendors engaged to conduct research and development activities, and allocated expenses for technology and facilities. We expense research and development expenses in the periods in which they are incurred.
We plan to continue to invest in our research and development efforts and to increase our investment in research and development efforts related to our product development. As a result, we expect research and development expenses to increase in absolute dollars as we continue to advance our product development, hire additional personnel and retain existing personnel, purchase supplies and materials and allocate expense to our research and development facilities.
General and Administrative Expenses
General and administrative expenses consist of salaries, related benefits, and stock-based compensation expense for personnel in executive, operations, legal, human resources, finance, marketing, commercial, IT personnel and administrative functions, professional fees for legal, patent, consulting, accounting and audit services, directors and officers insurance, and allocated expenses for technology and facilities. We expense general and administrative expenses in the periods in which they are incurred.
We expect that our general and administrative expenses will increase substantially over the next several years as we hire additional personnel to support the growth in research and development activities for our products and commercial activities supporting the growth of our business. We also anticipate that we will incur higher expenses as a result of expenses related to accounting, audit, legal, regulatory, insurance, compliance with the rules and regulations of the SEC, Sarbanes-Oxley Act and those of any national securities exchange on which our securities are traded, investor and public relations, and other administrative and professional services.
Other Income (Expense)
Other income (expense) consists primarily of interest income on our cash, cash equivalents and investments (including accretion and amortization of discounts and premiums on marketable debt securities), gains and losses on foreign currency transactions, and other miscellaneous non-recurring expenses such as gains or losses on disposal of property and equipment.
Results of Operations
Comparison of Fiscal Years Ended December 31, 2023 to December 31, 2022
The following table shows our consolidated statements of operations for the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, | | 2023 to 2022 | | |
| 2023 | | 2022 | | | | Change
($) | | Change
(%) | | | | |
| (in thousands) | | | | |
| Operating expenses: | | | | | | | | | | | | | |
| Research and development | $ | 47,251 | | | $ | 37,672 | | | | | $ | 9,579 | | | 25 | % | | | | |
| General and administrative | 28,901 | | | 25,946 | | | | | 2,955 | | | 11 | % | | | | |
| Total operating expenses | 76,152 | | | 63,618 | | | | | 12,534 | | | 20 | % | | | | |
| Other income (expense): | | | | | | | | | | | | | |
| Interest income | 12,550 | | | 5,816 | | | | | 6,734 | | | 116 | % | | | | |
| Other expense | (73) | | | (122) | | | | | 49 | | | (40) | % | | | | |
| Total other income (expense) | $ | 12,477 | | | $ | 5,694 | | | | | $ | 6,783 | | | 119 | % | | | | |
| Net loss | $ | 63,675 | | | $ | 57,924 | | | | | $ | 5,751 | | | 10 | % | | | | |
Research and Development Expenses
Research and development expenses were $47.3 million for the year ended December 31, 2023, compared to $37.7 million for the year ended December 31, 2022, an increase of $9.6 million, or 25%. The increase was primarily due to a $5.6 million increase in salaries, related benefits, and stock-based compensation, a $3.2 million increase in facilities cost, a $2.8 million increase in laboratory supplies and equipment expense, and a $0.5 million increase in depreciation expense. These increases were primarily caused by increased hiring and new leases commencing during the period. These increases were partially offset by a $2.7 million decrease in costs for external development services as more development efforts were performed by the Company.
General and Administrative Expenses
General and administrative expenses were $28.9 million for the year ended December 31, 2023, compared to $25.9 million for the year ended December 31, 2022, an increase of $3.0 million, or 11%. The increase was primarily due to a $4.1 million increase in salaries, related benefits, and stock-based compensation due to an increase in headcount to support general and administrative activities and supply chain and manufacturing readiness, and a $0.2 million increase in facilities costs driven by new leases commencing during the period. These increases were partially offset by a $1.1 million decrease in insurance costs, and a $0.7 million decrease in professional services, including accounting and recruiting services, as more of these activities were completed by the Company.
Other Income (Expense)
Other income (expense) for the year ended December 31, 2023 as compared to the year ended December 31, 2022 changed primarily due to higher interest income from marketable debt securities driven by higher interest rates.
Liquidity and Capital Resources
Sources of Liquidity
Since our inception, we have not generated any revenue from product sales and have incurred significant operating losses and negative cash flows from our operations. Our net loss was $63.7 million for the year ended December 31, 2023. As of December 31, 2023, we had an accumulated deficit of $202.2 million. Prior to the Business Combination, we funded our operations primarily with proceeds from the sale of convertible preferred stock. Prior to the Business Combination, we had raised net proceeds of $108.4 million from these private placements of our convertible preferred stock. In June 2021, in conjunction with the consummation of the Business Combination with ARYA, we received additional gross proceeds of approximately $345.5 million from PIPE Investors and the Business Combination, offset by approximately $18.2 million of transaction costs and underwriters’ fees relating to the closing of the Business Combination. As of December 31, 2023, we had cash, cash equivalents, and investments of $264.1 million.
Our primary uses of cash to date have been to fund our research and development activities, business planning, establishing and maintaining our intellectual property portfolio, hiring personnel, raising capital, and providing general and administrative support for these operations.
Funding Requirements
To date, we have not generated any revenue and we may not generate any revenue from the sale of products or from other sources in the near future. We expect our expenses and capital requirements will increase substantially in connection with our ongoing activities as we:
•continue our research and development activities, including with respect to our Nautilus platform;
•undertake activities to establish sales, marketing and distribution capabilities for our Nautilus platform;
•incur setup costs related to production tooling and required testing;
•maintain, protect and expand our intellectual property portfolio, including patents, trade secrets and know how;
•implement operational, financial and management information systems;
•attract, hire and retain additional management, scientific and administrative personnel; and
•operate as a public company.
Based on our planned operations, we expect our current cash, cash equivalents, and short-term investments will be sufficient to fund our operating expenses and capital expenditures for at least the next 12 months. We continue to face challenges and uncertainties and, as a result, our available capital resources may be consumed more rapidly than currently expected due to: delays in execution of our development plans; the scope and timing of our investment in our sales, marketing, and distribution capabilities; changes we may make to the business that affect ongoing operating expenses; the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; changes we may make in our business or commercialization strategy; changes we may make in our research and development spending plans; our need to implement additional infrastructure and internal systems; the impact of the COVID-19 pandemic, the conflicts in Eastern Europe the Middle East and in other countries; and other items affecting our forecasted level of expenditures and use of cash resources including potential acquisitions.
Until such time as we can generate significant revenue from commercialization of our products, if ever, we will continue to require substantial additional capital to develop our proteomics platform and fund operations for the foreseeable future. We intend to obtain such capital through public or private equity offerings or debt financings, credit or loan facilities or a combination of one or more of these funding sources. We may also seek additional financing opportunistically. We may be unable to raise additional funds on favorable terms or at all. Our ability to raise additional funds may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, the credit and financial markets in the United States and worldwide resulting from the ongoing COVID‑19 pandemic, recent and any potential future financial institution failures, the conflicts in Eastern Europe, the Middle East and in other countries, and otherwise. Our failure to raise additional capital, if needed, would have a negative impact on our financial condition and our ability to execute our business plan.
Our expected future capital requirements depend on many factors including expansion of our product portfolio and the timing and extent of spending on sales and marketing and the development of our technology. If we raise additional funds by issuing equity securities, our stockholders will experience dilution. Any future debt financing into which we enter may impose upon us additional covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our common stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders.
Historical Cash Flows
For the Fiscal Years Ended December 31, 2023 and 2022
The following table summarizes our cash flows for the periods indicated:
| | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | |
| (in thousands) |
| Net cash used in operating activities | $ | (51,711) | | | $ | (45,806) | | | |
| Net cash used in investing activities | (43,735) | | | (25,740) | | | |
| Net cash provided by financing activities | 368 | | | 562 | | | |
| Net increase in cash, cash equivalents and restricted cash | $ | (95,078) | | | $ | (70,984) | | | |
Operating Activities
During the year ended December 31, 2023, net cash used in operating activities was $51.7 million, primarily resulting from our net loss of $63.7 million, a decrease in the net changes in assets and liabilities aggregating $3.2 million. The net loss and decrease in net changes in assets and liabilities was partially offset by $15.2 million of non-cash expenses. The non-cash expenses included $12.1 million of stock-based compensation, $3.9 million amortization of operating lease right-of-use assets, $1.8 million in depreciation, partially offset by $2.7 million in accretion of discounts of investments.
During the year ended December 31, 2022, net cash used in operating activities was $45.8 million, primarily resulting from our net loss of $57.9 million and a decrease in the changes in assets and liabilities aggregating $0.8 million. The net loss and decrease in net changes in assets and liabilities was partially offset by $12.9 million of non-cash expenses. The non-cash expenses included $10.4 million of stock-based compensation, $2.2 million of amortization of operating lease right-of-use assets, $1.2 million of depreciation, partially offset by $0.9 million of accretion of discounts of investments.
Investing Activities
During the year ended December 31, 2023, net cash used in investing activities was $43.7 million, resulting from $112.9 million in purchases of securities and $2.4 million in purchases of property and equipment, partially offset by $71.6 million in proceeds from the maturities of securities.
During the year ended December 31, 2022, net cash used in investing activities was $25.7 million, resulting from $186.6 million in purchases of securities and $2.3 million in purchases of property and equipment, partially offset by $163.2 million in proceeds from the maturities of securities.
Financing Activities
During the year ended December 31, 2023, net cash provided by financing activities was $0.4 million, primarily from proceeds from the exercise of stock options and issuance of common stock under the employee stock purchase plan.
During the year ended December 31, 2022, net cash provided by financing activities was $0.6 million, primarily from proceeds from the exercise of stock options and issuance of common stock under the employee stock purchase plan.
Contractual Obligations and Commitments
For a discussion of our contractual obligations and commitments, refer to Part II, Item 8, Note 8, “Commitments and Contingencies” to the consolidated financial statements in this Annual Report on Form 10-K.
Critical Accounting Policies and Estimates
Our discussion and analysis of financial condition results of operations are based upon our financial statements included in Part II, Item 8 of this Annual Report on Form 10-K. The preparation of our financial statements in accordance with GAAP requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, and expenses.
We base our estimates on past experience and other assumptions that we believe are reasonable under the circumstances, and we evaluate these estimates on an ongoing basis. Actual results may differ from those estimates.
Our critical accounting policies are those that materially affect our financial statements and involve difficult, subjective or complex judgments by management. A thorough understanding of these critical accounting policies is essential when reviewing
our financial statements. We believe that the critical accounting policies listed below are the most difficult management decisions as they involve the use of significant estimates and assumptions as described above.
Research and Development
Costs for research and development activities are expensed in the period in which they are incurred. Research and development expenses consist of costs incurred in performing research and development activities, including salaries, related bonuses, stock-based compensation, employee benefits, facilities costs, laboratory supplies and equipment, depreciation and amortization, external costs of vendors engaged to conduct research and development activities, and allocated expenses for technology and facilities.
As part of the process of preparing our financial statements, we estimate our accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on our behalf and estimating the level of services performed and the associated cost incurred for services for which we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses at the end of each reporting period based on the facts and circumstances known to us at that time. The significant estimates in our accrued research and development expenses relate to expenses incurred with respect to academic research centers and other vendors in connection with research and development activities for which we have not yet been invoiced.
Stock Based Compensation
We maintain a stock-based compensation plan as a long-term incentive for employees, non-employee directors and consultants. The plan allows for the issuance of incentive stock options, non-qualified stock options, restricted stock units, and other forms of equity awards. Our stock-based compensation programs include shares issued under our 2021 Equity Incentive Plan and 2021 Employee Stock Purchase Plan.
We recognize stock-based compensation expense for stock options on a straight-line basis over the requisite service period and account for forfeitures as they occur. Our stock-based compensation costs are based upon the grant date fair value of options estimated using the Black-Scholes option pricing model. To the extent any stock option grants are made subject to the achievement of a performance-based milestone, management evaluates when the achievement of any such performance-based milestone is probable based on the relative satisfaction of the performance conditions as of the reporting date.
The Black-Scholes option pricing model utilizes inputs which are highly subjective assumptions and generally require significant judgment. These assumptions include:
•Risk-Free Interest Rate. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for zero-coupon U.S. Treasury notes with maturities corresponding to the expected term of the awards.
•Expected Volatility. Historically, we have been a private company and lacked company-specific historical and implied volatility information for our common stock. Therefore, the expected volatility of our common stock was determined by using an average of historical volatilities of selected industry peers deemed to be comparable to our business corresponding to the expected term of the awards and we expect to continue to do so until such time we have adequate historical data regarding the volatility of our traded common stock price.
•Expected Term. The expected term of stock options represents the weighted-average period that the stock options are expected to remain outstanding. We do not have sufficient historical exercise and post-vesting termination activity to provide accurate data for estimating the expected term of options and have opted to use the “simplified method,” whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option.
•Expected Dividend Yield. The expected dividend rate is zero as we have no history or expectation of declaring dividends on our common stock.
Certain assumptions we used in applying the Black-Scholes option pricing model to determine the estimated fair value of our stock options involve inherent uncertainties and the application of significant judgment. As a result, if factors or expected outcomes change and we use significantly different assumptions or estimates, our stock-based compensation could be materially different.
Recent Accounting Pronouncements
For a description of recent accounting pronouncements, including the expected dates of adoption and estimated effects, if any, on our consolidated financial statements, see Part II, Item 8, Note 2, “Significant Accounting Policies” to the consolidated financial statements in this Annual Report on Form 10-K.
Emerging Growth Company Accounting Election
The Jumpstart Our Business Startups Act of 2012, or the JOBS Act, permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have elected to use this extended transition period under the JOBS Act until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our financial statements may not be comparable to the financial statements of issuers who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies, which may make comparison of our financials to those of other public companies more difficult.
We will cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross revenue of $1.235 billion or more, (ii) the last day of our fiscal year following the fifth anniversary of the date of the closing of ARYA’s initial public offering, (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
Further, even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
We had cash, cash equivalents and investments of $264.1 million as of December 31, 2023. The primary goals of our investment policy are liquidity and capital preservation. We do not enter into investments for trading or speculative purposes. The carrying amount of our cash equivalents reasonably approximates fair value, due to the short maturities of these instruments. Our investments are exposed to market risk due to a fluctuation in interest rates, which may affect the fair market value of our investments in marketable securities. As of December 31, 2023, the effect of a hypothetical 1.00% (100 basis point) change in interest rates would have changed the fair value of our marketable securities by $2.3 million. Such change would only be realized if we sold the marketable securities prior to maturity.
Inflation Risk
Inflation generally affects us by increasing our cost of labor and goods and services. We believe that inflation has had some effect on our financial results during the periods presented. If we experience continued or future inflationary pressure, it may impact the costs of our operations as well as the costs to manufacture, sell and distribute our products and provide our services in the future. We may not be able to fully offset those increased costs through reduced spending or price increases to our products and services.
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Nautilus Biotechnology, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Nautilus Biotechnology, Inc. and its subsidiary (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of operations, of comprehensive loss, of stockholders’ equity and of cash flows for the years then ended, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
San Jose, California
February 28, 2024
We have served as the Company’s auditor since 2020.
| | |
Nautilus Biotechnology, Inc. Consolidated Balance Sheets At December 31, 2023 and 2022 |
| | | | | | | | | | | |
| (in thousands, except share and per share amounts) | December 31, 2023 | | December 31, 2022 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 19,397 | | | $ | 114,523 | |
| Short-term investments | 154,021 | | | 69,948 | |
| Prepaid expenses and other current assets | 3,419 | | | 2,738 | |
| Total current assets | 176,837 | | | 187,209 | |
| Property and equipment, net | 4,267 | | | 3,700 | |
| Operating lease right-of-use assets | 32,634 | | | 28,866 | |
| Long-term investments | 90,647 | | | 129,169 | |
| Other long-term assets | 1,180 | | | 1,108 | |
| Total assets | $ | 305,565 | | | $ | 350,052 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 1,639 | | | $ | 1,272 | |
| Accrued expenses and other liabilities | 3,945 | | | 3,528 | |
| Current portion of operating lease liability | 3,538 | | | 1,991 | |
| Total current liabilities | 9,122 | | | 6,791 | |
| Operating lease liability, net of current portion | 31,090 | | | 28,337 | |
| Total liabilities | 40,212 | | | 35,128 | |
| | | |
Commitments and contingencies (Note 8) | | | |
| | | |
| Stockholders’ equity: | | | |
Preferred stock, $0.0001 par value, 200,000,000 authorized as of December 31, 2023 and December 31, 2022, respectively; 0 shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | — | | | — | |
Common stock, $0.0001 par value, 1,000,000,000 shares authorized as of December 31, 2023 and 2022, respectively; 125,068,601 and 124,865,485 shares issued and outstanding as of December 31, 2023 and 2022, respectively | 13 | | | 12 | |
| Additional paid-in capital | 467,834 | | | 455,330 | |
| Accumulated other comprehensive loss | (255) | | | (1,854) | |
| Accumulated deficit | (202,239) | | | (138,564) | |
| Total stockholders’ equity | 265,353 | | | 314,924 | |
| Total liabilities and stockholders’ equity | $ | 305,565 | | | $ | 350,052 | |
The accompanying notes are an integral part of these consolidated financial statements.
| | |
Nautilus Biotechnology, Inc. Consolidated Statements of Operations Years Ended December 31, 2023 and 2022 |
| | | | | | | | | | | | | |
| (in thousands, except share and per share amounts) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Operating expenses: | | | | | |
| Research and development | $ | 47,251 | | | $ | 37,672 | | | |
| General and administrative | 28,901 | | | 25,946 | | | |
| Total operating expenses | 76,152 | | | 63,618 | | | |
| Other income (expense): | | | | | |
| Interest income | 12,550 | | | 5,816 | | | |
| Other expense | (73) | | | (122) | | | |
| Total other income | $ | 12,477 | | | $ | 5,694 | | | |
| Net loss | $ | (63,675) | | | $ | (57,924) | | | |
| Net loss per share attributable to common stockholders, basic and diluted | $ | (0.51) | | | $ | (0.46) | | | |
| Weighted-average shares used in computing net loss per share attributable to common stockholders, basic and diluted | 124,919,144 | | | 124,589,555 | | | |
The accompanying notes are an integral part of these consolidated financial statements.
| | |
Nautilus Biotechnology, Inc. Consolidated Statements of Comprehensive Loss Years Ended December 31, 2023 and 2022 |
| | | | | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Net loss | $ | (63,675) | | | $ | (57,924) | | | |
Other comprehensive income (loss): | | | | | |
Unrealized gain (loss) on securities available-for-sale | 1,599 | | | (1,670) | | | |
Total other comprehensive income (loss) | 1,599 | | | (1,670) | | | |
| Comprehensive loss | $ | (62,076) | | | $ | (59,594) | | | |
The accompanying notes are an integral part of these consolidated financial statements.
| | |
Nautilus Biotechnology, Inc. Consolidated Statements of Stockholders’ Equity Years Ended December 31, 2023 and 2022 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional Paid-in Capital | | Accumulated Other Comprehensive Income (Loss) | | Accumulated Deficit | | Total Stockholders’ Equity |
| | | | |
| (in thousands, except share amounts) | Shares | | Amount | | | | |
Balances at December 31, 2021 | 124,303,083 | | | $ | 12 | | | $ | 444,388 | | | $ | (184) | | | $ | (80,640) | | | $ | 363,576 | |
| Issuance of common stock upon exercise of vested stock options | 463,207 | | | — | | | 330 | | | — | | | — | | | 330 | |
| Issuance of common stock under employee stock purchase plan | 99,195 | | | — | | | 232 | | | — | | | — | | | 232 | |
| Stock-based compensation expense | — | | | — | | | 10,380 | | | — | | | — | | | 10,380 | |
| Other comprehensive loss | — | | | — | | | — | | | (1,670) | | | — | | | (1,670) | |
| Net loss | — | | | — | | | — | | | — | | | (57,924) | | | (57,924) | |
Balances at December 31, 2022 | 124,865,485 | | | 12 | | | $ | 455,330 | | | $ | (1,854) | | | $ | (138,564) | | | $ | 314,924 | |
| Issuance of common stock upon exercise of vested stock options | 70,865 | | | — | | | 104 | | | — | | | — | | | 104 | |
| Issuance of common stock under employee stock purchase plan | 132,251 | | | 1 | | | 263 | | | — | | | — | | | 264 | |
| | | | | | | | | | | |
| Stock-based compensation expense | — | | | — | | | 12,137 | | | — | | | — | | | 12,137 | |
Other comprehensive income | — | | | — | | | — | | | 1,599 | | | — | | | 1,599 | |
| Net loss | — | | | — | | | — | | | — | | | (63,675) | | | (63,675) | |
Balances at December 31, 2023 | 125,068,601 | | | 13 | | | $ | 467,834 | | | $ | (255) | | | $ | (202,239) | | | $ | 265,353 | |
The accompanying notes are an integral part of these consolidated financial statements.
| | |
Nautilus Biotechnology, Inc. Consolidated Statements of Cash Flows Years Ended December 31, 2023 and 2022 |
| | | | | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Cash flows from operating activities | | | | | |
| Net loss | $ | (63,675) | | | $ | (57,924) | | | |
| Adjustments to reconcile net loss to net cash used in operating activities | | | | | |
| Stock-based compensation | 12,137 | | | 10,380 | | | |
| Amortization of operating lease right-of-use assets | 3,856 | | | 2,199 | | | |
| Depreciation | 1,849 | | | 1,217 | | | |
| Amortization (accretion) of premiums (discount) on securities, net | (2,659) | | | (890) | | | |
| | | | | |
| Changes in operating assets and liabilities: | | | | | |
| Prepaid expenses and other assets | (821) | | | 756 | | | |
| Accounts payable | 393 | | | (561) | | | |
| Accrued expenses and other liabilities | 417 | | | 409 | | | |
| Operating lease liabilities | (3,208) | | | (1,392) | | | |
| Net cash used in operating activities | (51,711) | | | (45,806) | | | |
| Cash flows from investing activities | | | | | |
| Purchases of securities | (112,892) | | | (186,591) | | | |
| Purchases of property and equipment | (2,442) | | | (2,324) | | | |
| Proceeds from maturities of securities | 71,599 | | | 163,175 | | | |
| Net cash used in investing activities | (43,735) | | | (25,740) | | | |
| Cash flows from financing activities | | | | | |
| Proceeds from exercise of stock options | 104 | | | 330 | | | |
| Proceeds from issuance of common stock under employee stock purchase plan | 264 | | | 232 | | | |
| | | | | |
| | | | | |
| Net cash provided by financing activities | 368 | | | 562 | | | |
Net decrease in cash, cash equivalents and restricted cash | (95,078) | | | (70,984) | | | |
| | | | | |
| Cash, cash equivalents and restricted cash at beginning of period | 115,477 | | | 186,461 | | | |
| Cash, cash equivalents and restricted cash at end of period | $ | 20,399 | | | $ | 115,477 | | | |
| | | | | |
| Supplementary cash flow information on non-cash activities: | | | | | |
| Right-of-use assets obtained in exchange for operating lease liability | $ | 7,623 | | | $ | 1,688 | | | |
| Acquisitions of property and equipment included in accounts payable | $ | 148 | | | $ | 174 | | | |
| | | | | |
| | | | | |
| | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
| | |
Nautilus Biotechnology, Inc.
Notes to Consolidated Financial Statements |
1.Description of Business and Basis of Presentation
Nautilus Biotechnology, Inc. (the “Company”) is a biotechnology company incorporated in 2016 with corporate headquarters in Seattle, Washington and research and development headquarters in San Carlos, California. Since the Company’s incorporation in 2016, the Company has devoted substantially all of its resources to research and development activities, including with respect to its proteomics platform, business planning, establishing and maintaining its intellectual property portfolio, hiring personnel, raising capital and providing general and administrative support for these operations.
On June 9, 2021, Nautilus Biotechnology, Inc. a Delaware corporation (f/k/a ARYA Sciences Acquisition Corp. III, a Cayman Islands exempted company and the Company’s predecessor company (“ARYA”)), consummated the business combination (the “Business Combination”) pursuant to the terms of that certain Business Combination Agreement, dated as of February 7, 2021 (the “BCA”), by and among ARYA, Mako Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of ARYA (“Mako Merger Sub”), and Nautilus Subsidiary, Inc., a Delaware corporation (f/k/a Nautilus Biotechnology, Inc.) (“Legacy Nautilus”). As a result of the Business Combination, ARYA changed its name to “Nautilus Biotechnology, Inc.” and Mako Merger Sub merged with and into Legacy Nautilus with Legacy Nautilus surviving as the surviving company and becoming a wholly-owned subsidiary of ARYA (the “Merger” and, collectively with the other transactions described in the BCA, the “Reverse Recapitalization”).
In addition, in conjunction with the completion of the Business Combination, certain investors (“PIPE Investors”) subscribed for the purchase of an aggregate of 20,000,000 shares of common stock of the Company (“New Nautilus Common Stock”) at a price of $10.00 per share for aggregate gross proceeds of $200.0 million (“PIPE Financing”).
Basis of Presentation
The consolidated financial statements and accompanying notes were prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and regulations of the U.S. Securities and Exchange Commission. The accompanying financial statements are consolidated for the years ended December 31, 2023 and 2022 and include the accounts of Nautilus Biotechnology, Inc. and its wholly-owned subsidiary, Nautilus Subsidiary, Inc. All intercompany transactions and balances have been eliminated upon consolidation. The Company’s reporting currency is the U.S. dollar. Certain prior period amounts were reclassified to conform to the current year presentation in Note 6, “Income Taxes.”
Going Concern
The Company’s consolidated financial statements have been prepared on the basis of continuity of operations, the realization of assets, and the satisfaction of liabilities in the ordinary course of business. Since inception, the Company has been engaged in developing its technology, raising capital, and recruiting personnel. The Company’s operating plan may change as a result of many factors currently unknown and there can be no assurance that the current operating plan will be achieved in the time frame anticipated by the Company, and it may need to seek additional funds sooner than planned. If adequate funds are not available to the Company on a timely basis, it may be required to delay, limit, reduce, or terminate certain commercial efforts, or pursue merger or acquisition strategies, all of which could adversely affect the holdings or the rights of the Company’s stockholders. The Company has incurred net operating losses and negative cash flows from operations in every year since inception and expects this to continue for the foreseeable future. As of December 31, 2023, the Company had an accumulated deficit of $202.2 million.
The Company has funded its operations primarily with proceeds from the issuance of redeemable convertible preferred stock and common stock. In June 2021, the Company received gross proceeds of approximately $345.5 million from PIPE Investors and the Business Combination offset by approximately $18.2 million of transaction costs and underwriters’ fees relating to the closing of the Business Combination. The Company had cash, cash equivalents, and short-term investments of $173.4 million as of December 31, 2023. As of the date on which these consolidated financial statements were issued, the Company believes that its cash, cash equivalents, and short-term
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
investments will be sufficient to fund its operations for at least the next twelve months following the issuance of the consolidated financial statements. The Company’s actual results could vary as a result of, and its near and long-term future capital requirements will depend on many factors, including its growth rate and the timing and extent of spending to support its research and development efforts. The Company has based its estimates on assumptions that may prove to be wrong, and it could use its available capital resources sooner than it currently expects. The Company may be required to seek additional equity or debt financing. Future liquidity and cash requirements will depend on numerous factors. In the event that additional financing is required, the Company may not be able to raise it on acceptable terms or at all. If the Company is unable to raise additional capital when desired, or if it cannot expand its operations or otherwise capitalize on its business opportunities because it lacks sufficient capital, its business, operating results, and financial condition would be adversely affected.
Impact of the COVID-19 Coronavirus
The COVID-19 pandemic has already had an adverse effect on the global economy. Additionally, concerns over the economic impact of COVID-19 have caused extreme volatility in financial and other capital markets, which may adversely affect the Company’s ability to access capital markets in the future. The level and nature of the disruption caused by COVID-19 is unpredictable, may be cyclical and long-lasting, and may again in the future adversely affect the Company's operating results.
2.Significant Accounting Policies
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include determining the estimated lives of property and equipment, stock-based compensation, research and development accruals, and the valuation allowance for deferred tax assets. These estimates and assumptions are based on management’s best estimates and judgment. Management evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors, including the current economic environment, which management believes to be reasonable under the circumstances. The Company adjusts such estimates and assumptions when facts and circumstances dictate. Changes in those estimates resulting from continuing changes in the economic environment will be reflected in the financial statements in future periods. As future events and their effects cannot be determined with precision, actual results could materially differ from those estimates and assumptions.
Concentrations of Credit Risk and Other Risks and Uncertainties
Credit risk represents the accounting loss that would be recognized as of the reporting date if counterparties failed to perform as contracted.
Financial instruments, which potentially subject the Company to concentration of credit risk, consist of cash balances maintained in excess of federal depository insurance limits and investments in marketable debt securities that are not federally insured. The Company has not experienced any losses in such accounts and believes it is not exposed to significant credit risk on cash or investments. The Company relies, and expects to continue to rely, on a number of vendors to provide services, supplies and materials related to its research and development programs. The Company relies on single source suppliers for certain components and materials used in the Nautilus platform. The loss of any of these single source suppliers would require the Company to expend significant time and effort to locate and qualify an alternative source of supply for these components. The Company also relies, and expects to continue to rely, on third-party manufacturers and, in many cases, single third-party manufacturers for the production of certain reagents and antibodies. These programs could be adversely affected by a significant interruption in these services or the availability of materials.
The Company is subject to risks similar to those of pre-clinical stage companies in the biopharmaceutical industry, including dependence on key individuals, the need to develop commercially viable products, competition from other companies, many of whom are larger and better capitalized, the impact of negative global and national
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
events and the need to obtain adequate additional financing to fund the development of its products. There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection for the Company’s intellectual property will be maintained, that any products developed will obtain required regulatory approval or that any approved products will be commercially viable. Even if the Company’s development efforts are successful, it is uncertain when, if ever, the Company will generate significant revenue from the sale of its products.
Segment Reporting
Operating segments are defined as components of an entity where discrete financial information is evaluated regularly by the chief operating decision maker (“CODM”) in deciding how to allocate resources and in assessing performance. The Company’s Chief Executive Officer is its CODM. The Company’s CODM reviews financial information presented on a consolidated basis for the purposes of making operating decisions, allocating resources and evaluating financial performance. As such, the Company has determined that it operates in one operating and one reportable segment. The Company’s long-lived assets are entirely based in the United States.
Cash and Cash Equivalents
The Company considers all highly-liquid investments with an original maturity of three months or less as of the date of acquisition to be cash equivalents.
Investments
The Company considers investments with an original maturity greater than three months and remaining maturities less than one year to be short-term investments. The Company classifies those investments that are not required for use in current operations and that mature in more than 12 months as long-term investments.
The Company classifies its marketable debt securities as available for sale and reports them at fair value, with unrealized gains and losses recorded in accumulated other comprehensive income (loss). For investments sold prior to maturity, the cost of investments sold is based on the specific identification method. Realized gains and losses on the sale of investments are recorded in other income (expense), net in the consolidated statement of operations.
If the estimated fair value of a marketable debt security is below its amortized cost basis, the Company evaluates whether it is more likely than not that the Company will be required to sell the security before its anticipated recovery in market value and whether credit losses exist for the related securities. Credit-related losses are recognized as an allowance for credit losses on the balance sheet with a corresponding adjustment to earnings. Unrealized gains and losses that are unrelated to credit deterioration are reported in accumulated other comprehensive income (loss). No credit-related losses or allowance for credit losses were necessary during the periods presented.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value.
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets;
Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
The carrying amounts of cash and cash equivalents, prepaid expenses and other current assets, accounts payable and accrued expenses and other liabilities approximate their respective fair values due to their short-term nature.
Property and Equipment, net
Property and equipment, net, consisting primarily of laboratory equipment, computers, furniture and fixtures, and office equipment are recorded at cost and are depreciated on a straight-line basis over their estimated useful life.
Useful lives assigned to property and equipment are as follows:
| | | | | |
| Laboratory equipment | 3 years to 5 years |
| Prototype equipment | 3 years |
| Computer hardware | 3 years |
| Furniture and fixtures | 3 years |
| Office equipment | 3 years to 5 years |
Leasehold improvements | Shorter of estimated useful life or remaining lease term |
When assets are retired or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any resulting gain or loss is recognized as income or loss for the period.
Maintenance and repairs are charged to operating expense in the period incurred.
Impairment of Long-Lived Assets
The Company periodically reviews its long-lived assets, including property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable.
With respect to property and equipment, the Company compares the carrying value of the long-lived assets with the estimated future net undiscounted cash flows expected to result from the use and eventual disposition of the asset (or asset group). Should the sum of the estimated future net undiscounted cash flows be less than the carrying value, the Company would recognize an impairment loss as of that date. An impairment loss would be measured by comparing the amount by which the carrying value exceeds the fair value of the long-lived assets. No impairment of long-lived assets was recorded in any of the periods presented.
Leases
The Company determines if an arrangement includes a lease at inception by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset for a period of time in exchange for consideration. Operating leases with a term of more than one year are included in operating lease right-of-use (“ROU”) assets and operating lease liabilities on the Company's consolidated balance sheets. ROU assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the obligation to make lease payments. Operating lease ROU assets and liabilities are recognized on the lease commencement date based on the present value of the future minimum lease payments over the lease term. The Company uses the incremental borrowing rate commensurate with the lease term based on the information available at the lease commencement date in determining the present value of the lease payments as the Company's leases generally do not provide an implicit rate. ROU assets initially equal the lease liability, adjusted for any prepaid lease payments and initial direct costs incurred, less any lease incentives received. Certain of the Company's leases
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
include renewal options which allow the Company to, at its election, renew or extend the lease for a fixed or indefinite period of time. These renewal periods are included in the lease terms when the Company is reasonably certain the options will be exercised. Lease expense is recognized on a straight-line basis over the lease term when leases are operating leases. If it is considered a finance lease, expense is recognized over the lease term within interest expense and amortization in the Company’s consolidated statements of operations. The Company also has lease arrangements with lease and non-lease components. The Company elected the practical expedient not to separate non-lease components from lease components for the Company's facility leases and to account for the lease and non-lease components as a single lease component. The Company also elected to apply the short-term lease measurement and recognition exemption in which ROU assets and lease liabilities are not recognized for leases with terms of 12 months or less.
Income Taxes
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the net deferred tax assets will not be realized. In making such a determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and recent results of operations, primarily over the most recent three-year period.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained upon an audit. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being recognized. Changes in recognition or measurement are reflected in the period in which the change in judgement occurs.
Research and Development
Costs for research and development activities are expensed in the period in which they are incurred. Research and development expenses consist of costs incurred in performing research and development activities, including salaries, bonuses, stock-based compensation, and employee benefits for product development personnel, laboratory supplies and equipment, depreciation and amortization, external costs of vendors engaged to conduct research and development activities, and allocated expenses for technology and facilities.
As part of the process of preparing its financial statements, the Company estimates its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of services performed and the associated cost incurred for services for which the Company has not yet been invoiced or otherwise notified of the actual cost. The majority of the Company’s service providers invoice monthly in arrears for services performed or when contractual milestones are met. The Company makes estimates of its accrued expenses at the end of each reporting period based on the facts and circumstances known to the Company at that time. The significant estimates in the Company’s accrued research and development expenses relate to expenses incurred with respect to academic research centers and other vendors in connection with research and development activities for which the Company has not yet been invoiced.
Stock-based Compensation
The Company accounts for stock-based compensation expense by calculating the estimated fair value of each employee and non-employee award at the grant date or modification date by applying the Black-Scholes option pricing model (the “model”). The model utilizes the NASDAQ closing price of the Company’s common stock at the measurement date, the expected or contractual term of the option, the expected stock price volatility, risk-free interest rates, and expected dividend yield of the common stock. Stock-based compensation expense is recognized on a straight-line basis over the requisite service period, which is generally the vesting period. Forfeitures are
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
recognized in the period in which the forfeiture occurs. The Company classifies stock-based compensation expense in its statement of operations in the same manner in which the award recipient’s payroll costs are classified or in which the award’s recipient’s service payments are classified. The Company’s stock-based compensation programs include stock options grants, as well as shares issued under its 2021 Employee Stock Purchase Plan.
The Company calculates the expected term as the mid-point between the requisite service period and the contractual term of the award.
The Company bases its estimate of expected volatility on the historical volatility of comparable public companies from a representative peer group selected based on industry, financial, and market capitalization data.
The Company has never declared or paid any dividends and does not currently expect to do so in the future. The risk-free interest rate used in the model is based on the implied yield currently available in the U.S. Treasury securities at maturity with an equivalent term.
Comprehensive Loss
Comprehensive loss consists of net loss and other gains or losses affecting stockholders’ equity that, under U.S. GAAP are excluded from net loss. For the years ended December 31, 2023 and 2022, unrealized gains and losses on marketable debt securities were included as a component of comprehensive income (loss).
Net Loss per Share Attributable to Common Stockholders
Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common stock outstanding during the period, without consideration of potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common stock and potentially dilutive securities outstanding for the period. For the purposes of the diluted net loss per share calculation, stock options and shares to be purchased from the Employee Stock Purchase Plan are considered to be potentially dilutive securities. The net loss is attributed entirely to the Company’s common stockholders. Since the Company has reported a net loss for all periods presented, diluted net loss per common share is the same as basic net loss per common share for those periods.
Accounting Pronouncements
The Company is provided the option to adopt new or revised accounting guidance as an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) either (1) within the same periods as those otherwise applicable to public business entities, or (2) within the same time periods as non-public business entities, including early adoption when permissible. With the exception of standards the Company elected to early adopt, when permissible, the Company has elected to adopt new or revised accounting guidance within the same time period as non-public business entities, as indicated below.
Recently Adopted Accounting Standards
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments- Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which amends existing guidance on the impairment of financial assets and adds an impairment model that is based on expected losses rather than incurred losses and requires an entity to recognize as an allowance its estimate of expected credit losses for its financial assets. An entity will apply this guidance through a cumulative-effect adjustment to retained earnings upon adoption (a modified-retrospective approach) while a prospective transition approach is required for debt securities for which an other-than-temporary impairment had been recognized before the effective date. This ASU is effective for the Company for its fiscal year ending December 31, 2023. Early adoption is permitted. The Company adopted this ASU on January 1, 2023 and the adoption did not have a material impact on its consolidated financial statements.
Recently Issued Accounting Pronouncements
In November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures,” which amends existing guidance on segment reporting to improve reportable
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
segment disclosure requirements through enhanced disclosures about significant segment expenses. The ASU is effective for the Company for the fiscal year ending December 31, 2024 and for interim periods beginning after December 31, 2024. The Company is in the process of evaluating the impact of the adoption of this Accounting Standards Update on its consolidated financial statements and related disclosures.
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” to enhance the transparency and decision usefulness of income tax disclosures. The ASU is effective for the Company for the fiscal year ending December 31, 2025. The Company is in the process of evaluating the impact of the adoption of this Accounting Standards Update on its consolidated financial statements and related disclosures. The Company does not anticipate adoption to have a material impact on its consolidated financial statements and related disclosures.
3. Fair Value Measurements
The following table details the assets carried at fair value and measured on a recurring basis within the three levels of fair value as of December 31, 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | | | Gross Unrealized | | | | Reported as: |
December 31, 2023 | | Amortized Cost | | Gains | | Losses | | Fair Value | | Cash equivalents | | Short-term investments | | Long-term investments |
| Level 1 | | | | | | | | | | | | | | |
| Mutual funds | | 590 | | | — | | | — | | | 590 | | | 590 | | | — | | | — | |
| U.S. treasury securities | | 74,115 | | | 104 | | | (247) | | | 73,972 | | | — | | | 50,638 | | | 23,334 | |
| Total Level 1 | | 74,705 | | | 104 | | | (247) | | | 74,562 | | | 590 | | | 50,638 | | | 23,334 | |
| Level 2 | | | | | | | | | | | | | | |
| Commercial paper | | 18,653 | | | — | | | (9) | | | 18,644 | | | 18,644 | | | — | | | — | |
| Corporate debt securities | | 6,950 | | | — | | | (37) | | | 6,913 | | | — | | | 6,913 | | | — | |
Agency securities | | 163,849 | | | 358 | | | (424) | | | 163,783 | | | — | | | 96,470 | | | 67,313 | |
| Total Level 2 | | 189,452 | | | 358 | | | (470) | | | 189,340 | | | 18,644 | | | 103,383 | | | 67,313 | |
| Total Level 1 and Level 2 | | $ | 264,157 | | | $ | 462 | | | $ | (717) | | | $ | 263,902 | | | $ | 19,234 | | | $ | 154,021 | | | $ | 90,647 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | | | Gross Unrealized | | | | Reported as: |
December 31, 2022 | | Amortized Cost | | Gains | | Losses | | Fair Value | | Cash equivalents | | Short-term investments | | Long-term investments |
| Level 1 | | | | | | | | | | | | | | |
| Mutual funds | | 1,121 | | | — | | | — | | | 1,121 | | | 1,121 | | | — | | | — | |
U.S. treasury securities | | 52,686 | | | 4 | | | (774) | | | 51,916 | | | — | | | 2,873 | | | 49,043 | |
| Total Level 1 | | 53,807 | | | 4 | | | (774) | | | 53,037 | | | 1,121 | | | 2,873 | | | 49,043 | |
| Level 2 | | | | | | | | | | | | | | |
| Commercial paper | | 156,419 | | | 3 | | | (266) | | | 156,156 | | | 113,402 | | | 42,754 | | | — | |
| Corporate debt securities | | 14,154 | | | — | | | (71) | | | 14,083 | | | — | | | 7,224 | | | 6,859 | |
Agency securities | | 91,114 | | | 33 | | | (783) | | | 90,364 | | | — | | | 17,097 | | | 73,267 | |
| Total Level 2 | | 261,687 | | | 36 | | | (1,120) | | | 260,603 | | | 113,402 | | | 67,075 | | | 80,126 | |
| Total Level 1 and Level 2 | | $ | 315,494 | | | $ | 40 | | | $ | (1,894) | | | $ | 313,640 | | | $ | 114,523 | | | $ | 69,948 | | | $ | 129,169 | |
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
Short-term investments have a contractual maturity date that is one year or less from the respective balance sheet date. Long-term investments have a contractual maturity date that is more than one year, but less than two years from the respective balance sheet date.
The unrealized losses and fair values of available-for-sale securities that have been in an unrealized loss position for a period of less than and greater than 12 months as of December 31, 2023 and December 31, 2022 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | Securities in Unrealized Loss Position Less than 12 months | | Securities in Unrealized Loss Position Greater than 12 months | | Total |
December 31, 2023 | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value |
| U.S. treasury securities | | $ | 85 | | | $ | 20,408 | | | $ | 162 | | | $ | 30,230 | | | $ | 247 | | | $ | 50,638 | |
| Commercial paper | | 9 | | | 18,644 | | | — | | | — | | | 9 | | | 18,644 | |
| Corporate debt securities | | 37 | | | 6,913 | | | — | | | — | | | 37 | | | 6,913 | |
Agency securities | | 229 | | | 85,039 | | | 195 | | | 25,704 | | | 424 | | | 110,743 | |
| Total | | $ | 360 | | | $ | 131,004 | | | $ | 357 | | | $ | 55,934 | | | $ | 717 | | | $ | 186,938 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | Securities in Unrealized Loss Position Less than 12 months | | Securities in Unrealized Loss Position Greater than 12 months | | Total |
December 31, 2022 | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value |
| U.S. treasury securities | | $ | 774 | | | $ | 49,114 | | | $ | — | | | $ | — | | | $ | 774 | | | $ | 49,114 | |
| Commercial paper | | 266 | | | 151,354 | | | — | | | — | | | 266 | | | 151,354 | |
| Corporate debt securities | | 14 | | | 6,859 | | | 57 | | | 7,224 | | | 71 | | | 14,083 | |
Agency securities | | 670 | | | 50,531 | | | 113 | | | 8,887 | | | 783 | | | 59,418 | |
| Total | | $ | 1,724 | | | $ | 257,858 | | | $ | 170 | | | $ | 16,111 | | | $ | 1,894 | | | $ | 273,969 | |
The Company reviewed its investment portfolio based on the underlying risk profile of the securities and has no loss expectation for these investments. The Company also reviewed the securities in an unrealized loss position and evaluated the current expected credit loss by considering factors such as historical experience, market data, issuer-specific factors, and current economic conditions. The Company recognized no credit losses during the years ended December 31, 2023 and 2022, and had no allowance for credit losses as of December 31, 2023.
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
4. Composition of Certain Consolidated Financial Statement Line Items
Property and Equipment, Net
Property and equipment consisted of the following:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Laboratory equipment | $ | 6,337 | | | $ | 4,892 | |
| Leasehold improvements | 118 | | | 13 | |
| Computer hardware | 222 | | | 166 | |
| Furniture, fixtures and office equipment | 324 | | | 25 | |
| Prototype equipment | 996 | | | 332 | |
| Construction in progress | 904 | | | 1,235 | |
| 8,901 | | | 6,663 | |
| Less: Accumulated depreciation | (4,634) | | | (2,963) | |
| Total | $ | 4,267 | | | $ | 3,700 | |
The Company recorded $1.8 million and $1.2 million of depreciation expense for the years ended December 31, 2023 and 2022, respectively, which was primarily allocated to research and development expense.
Accrued Expenses and Other Liabilities
Accrued expenses and other liabilities consisted of the following:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Employee compensation | $ | 2,590 | | | $ | 1,669 | |
| Accrued research and development | 731 | | | 970 | |
| Accrued professional and consulting fees | 245 | | | 451 | |
| Other | 379 | | | 438 | |
| Total | 3,945 | | | 3,528 | |
Cash, Cash Equivalents and Restricted Cash
Cash, cash equivalents and restricted cash consisted of the following:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Cash and cash equivalents | 19,397 | | | 114,523 | |
Restricted cash included in other long-term assets (Note 8) | 1,002 | | | 954 | |
| Total | 20,399 | | | 115,477 | |
Other long-term assets consisted of $1.0 million of restricted cash and $0.2 million of deposits as of December 31, 2023, and $0.9 million of restricted cash and $0.2 million of deposits as of December 31, 2022.
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
5. Common Stock
There were 125,068,601 shares issued and outstanding as of December 31, 2023.
Common Stock Reserved for Future Issuance
Shares of common stock reserved for future issuance on an as-if converted basis, were as follows:
| | | | | | | | | | | |
| December 31,
2023 | | December 31,
2022 |
| Shares available for grant under 2021 Equity Incentive Plan | 20,279,560 | | | 17,298,043 | |
| Stock options issued and outstanding | 14,676,335 | | | 11,485,443 | |
| Shares available for grant under 2021 Employee Stock Purchase Plan | 3,505,138 | | | 2,388,735 | |
| Total shares of common stock reserved | 38,461,033 | | | 31,172,221 | |
6. Income Taxes
The Company is liable for income taxes in the United States. For the years ended December 31, 2023 and 2022, the Company did not have any income for income tax purposes and therefore, no tax liability or expense has been recorded in these financial statements. The difference between the tax at the statutory federal tax rate and no tax provision recorded by the Company is primarily due to the Company’s full valuation allowance against its deferred tax assets.
A reconciliation between the expected income tax provision at the federal statutory rate and the reported income tax provision is approximately as follows:
| | | | | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Federal income tax at statutory rate | $ | (13,372) | | | $ | (12,164) | | | |
| State income tax, net of federal benefit | (4,027) | | | (3,126) | | | |
Equity-based Compensation | 2,423 | | | 290 | | | |
| | | | | |
| Tax credits generated in current year | (2,075) | | | (2,607) | | | |
| Valuation allowance change | 17,011 | | | 17,127 | | | |
| Other | 40 | | | 480 | | | |
| Total | $ | — | | | $ | — | | | |
As of December 31, 2023, the Company had federal net operating loss carryforwards of $0.5 million that begin to expire in 2037 and federal net operating loss carryforwards of $70.6 million that arose after the 2017 tax year that will carryforward indefinitely and will be subject to the 80% of taxable income limitation. The Company has state net operating loss carryforwards of $95.5 million that will begin to expire in 2037.
As of December 31, 2023, the Company had research and development tax credit carryover of $6.8 million and $4.7 million for federal and state tax purposes, respectively. If not utilized, the federal carryforward will expire in various amounts beginning in 2039. The California credits can be carried forward indefinitely.
The Company has evaluated the positive and negative evidence bearing upon its ability to realize the deferred tax assets. Management has considered the Company’s history of cumulative net losses incurred since inception and its lack of revenue since inception and has concluded that it is more likely than not that the Company will not realize the benefits of the deferred tax assets. Accordingly, the Company has provided a full valuation allowance against the
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
net deferred tax assets. The valuation allowance increased by $17.0 million during the year ended December 31, 2023. Management reevaluates the positive and negative evidence at each reporting period.
Deferred income taxes reflect the net tax effects of loss and credit carryforwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Components of the Company’s deferred tax assets are as follows:
| | | | | | | | | | | |
| (in thousands) | December 31, 2023 | | December 31, 2022 |
| Deferred tax assets | | | |
| Depreciation and amortization | $ | 6,508 | | | $ | 11,142 | |
| Capitalized research and development | 16,502 | | | 7,607 | |
| Loss carryforwards | 21,617 | | | 12,402 | |
| Lease liabilities | 9,213 | | | 8,005 | |
| Tax credit carryforwards | 8,438 | | | 5,408 | |
| Equity-based compensation | 4,196 | | | 4,015 | |
| Other accruals and reserves | 424 | | | 246 | |
| Total deferred tax assets | 66,898 | | | 48,825 | |
| Valuation allowance for deferred tax assets | (58,215) | | | (41,205) | |
| Total deferred tax assets, net of valuation allowance | $ | 8,683 | | | $ | 7,620 | |
| | | |
| Deferred tax liability | | | |
| Right-of-use assets | (8,683) | | | (7,620) | |
| Net deferred tax assets (liability) | $ | — | | | $ | — | |
The Company began to file income tax returns in the United States in 2017. All tax years are open to examination.
The Tax Cuts and Jobs Act contained a provision which requires the capitalization of Section 174 costs incurred in years beginning on or after January 1, 2022. Section 174 costs are expenditures which represent research and development costs that are incident to the development or improvement of a product, process, formula, invention, computer software, or technique. This provision changes the treatment of Section 174 costs such that the expenditures are no longer allowed as an immediate deduction but rather must be capitalized and amortized. We have included the impact of this provision, which results in a deferred tax asset of approximately $16.5 million as of December 31, 2023.
A valuation allowance of $58.2 million and $41.2 million at December 31, 2023 and 2022, respectively, has been recognized to offset net deferred tax assets where realization of such net deferred tax assets is determined to be more likely than not to not be realized. The valuation allowance increased by $17.0 million in 2023 and increased by $17.1 million in 2022, which was primarily due to changes in our deferred tax asset balances. The increases in the valuation allowance in 2023 and 2022 was primarily due to the net operating loss and tax credit generation, capitalized research and development expense and share-based compensation.
The Company had an unrecognized tax benefit balance of $2.4 million and $1.5 million related to research and development credits and California net operating loss carryforward as of December 31, 2023 and 2022, respectively. No amount of unrecognized tax benefits as of December 31, 2023, if recognized, would reduce the Company’s effective tax rate because the benefits would be in the form of tax credit carryforwards, which would be reduced to $0 by a full valuation allowance. There are no provisions for which it is reasonably possible that the total amounts of unrecognized tax benefits will significantly increase or decrease within 12 months of the reporting date. Because the statute of limitations does not expire until after the net operating loss and credit carryforwards are actually used, the statutes are still open on calendars years ending 2017 forward for federal and state purposes.
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
A reconciliation of the beginning and ending amount of the liability for uncertain tax positions, excluding potential interest and penalties, is as follows:
| | | | | | | | |
| (in thousands) | December 31, 2023 | December 31, 2022 |
| Beginning balance | $ | 1,544 | | $ | 837 | |
| Increase based on current year tax positions | 812 | | 616 | |
| Increase for prior year tax positions | 9 | | 91 | |
| | |
| Ending balance | $ | 2,365 | | $ | 1,544 | |
Net operating loss and tax credit carry-forwards are subject to review and possible adjustment by the Internal Revenue Service (the “IRS”) and may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three-year period in excess of 50% as defined under Sections 382 and 383 in the Code, which could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the Company’s value immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has not, as yet, conducted a study to determine if any such changes have occurred that could limit its ability to use the net operating loss and tax credit carryforwards.
7. Equity Incentive Plans and Stock-based Compensation
On June 8, 2021, the stockholders of the Company approved the 2021 Equity Incentive Plan (“2021 Plan”) and the 2021 Employee Stock Purchase Plan (“2021 ESPP”). As of December 31, 2023, 20,279,560 and 3,505,138 shares were available for grant under the 2021 Plan and 2021 ESPP, respectively.
2021 Employee Stock Purchase Plan
Under the 2021 ESPP, participants are permitted to purchase shares of Common Stock, up to the IRS allowable limit, through contributions (in the form of payroll deductions or otherwise to the extent permitted by the administrator) of up to 15% of their eligible compensation. Participants are permitted to purchase shares of the Company’s Common Stock at 85% of the lower of the fair market value of the Company’s Common Stock on the first trading day of an offering period or on the last trading date in each purchase period. Participants may end their participation at any time during an offering and will be paid their accrued contributions that have not yet been used to purchase shares. Participation ends automatically upon termination of employment with the Company. The number of shares of common stock available for issuance under the 2021 ESPP will be increased on the first day of each fiscal year beginning on January 1, 2022, in an amount equal to the least of (i) 3,734,500 shares of common stock, (ii) a number of shares of common stock equal to one percent (1%) of the total number of shares of all classes of common stock of the Company on the last day of the immediately preceding fiscal year, or (iii) such number of shares determined by the Administrator no later than the last day of the immediately preceding fiscal year.
The first offering period was from October 1, 2021 through June 1, 2022. For subsequent offering periods, the Company offers a six month purchase period. During the years ended December 31, 2023 and 2022, 132,251 and 99,195 shares of common stock, respectively, were purchased under the 2021 ESPP.
2021 Equity Incentive Plan
Under the 2021 Plan, the Company can grant incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units and performance awards to employees, non-employee directors and consultants. Options generally expire ten years after the date of grant. The number of shares available for issuance under the 2021 Plan will be increased on the first day of each fiscal year, beginning on January 1, 2022, in an amount equal to the least of (i) 18,672,200 shares, (ii) a number of shares equal to five percent (5%) of the total number of shares of all classes of common stock of the Company outstanding on the last day of the immediately preceding fiscal year, or (iii) such number of shares determined by the Administrator no later than the last day of the immediately preceding fiscal year.
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
2017 Equity Incentive Plan
At the time of adoption of the 2021 Plan and the 2021 ESPP, no further awards will be granted under the 2017 Equity Incentive Plan (“2017 Plan”). Stock-based awards forfeited or cancelled from the 2017 Plan are returned to the pool of shares of Common Stock available for issuance under the 2021 Plan.
Grant Date Fair Value of Stock Options
In determining the compensation cost of the option awards, the fair value for each option award has been estimated using the Black Scholes model. The significant assumptions used in these calculations are summarized as follows:
| | | | | | | | | | | | | |
| Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Expected term (in years) | 5.3 - 6.4 | | 5.3 - 6.1 | | |
| Expected volatility | 102.7% - 107.5% | | 105.2% - 110.4% | | |
| Expected dividend rate | 0.0% | | 0.0% | | |
| Risk free interest rate | 3.5% - 4.8% | | 1.7% - 4.4% | | |
| | | | | |
Expected term: The expected term of stock options represents the weighted-average period the stock options are expected to remain outstanding. The Company does not have sufficient historical exercise and post-vesting termination activity to provide accurate data for estimating the expected term of options and has opted to use the “simplified method,” whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option.
Expected volatility: Historically, the Company has been a private company and lacked company‑specific historical and implied volatility information for its common stock. Therefore, the expected volatility of the Company’s common stock was determined by using an average of historical volatilities of selected industry peers deemed to be comparable to the Company’s business corresponding to the expected term of the awards and the Company expects to continue to do so until such time as the Company has adequate historical data regarding the volatility of its traded common stock price.
Expected dividend yield: The expected dividend rate is zero as the Company has no history or expectation of declaring dividends on its common stock.
Risk-free interest rate: The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for zero-coupon U.S. Treasury notes with maturities corresponding to the expected term of the awards.
The following table summarizes option award activity during the year ended December 31, 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of Stock Option Awards | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Life (Years) | | Aggregate Intrinsic Value
(in thousands) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Outstanding as of December 31, 2022 | 11,485,443 | | | $ | 4.12 | | | | | |
| | | | | | | |
| | | | | | | |
| Granted | 4,009,775 | | | $ | 2.42 | | | | | |
| Exercised | (70,865) | | | $ | 1.46 | | | | | |
| Forfeited | (748,018) | | | $ | 4.97 | | | | | |
Outstanding as of December 31, 2023 | 14,676,335 | | | $ | 3.63 | | | 7.8 | | $ | 11,451 | |
Options vested and expected to vest as of December 31, 2023 | 14,676,335 | | | $ | 3.63 | | | | | |
Vested and exercisable at December 31, 2023 | 7,488,649 | | | $ | 3.90 | | | 7.1 | | $ | 7,366 | |
As of December 31, 2023, there was $19.1 million of total unrecognized compensation expense expected to be recognized over a weighted average-period of 2.1 years. The total intrinsic value of options exercised during the
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
years ended December 31, 2023 and 2022 was $0.1 million and $1.2 million, respectively. Aggregate intrinsic value represents the difference between the fair market value of the common stock and the exercise price of outstanding, in-the-money options.
The weighted-average grant-date fair value of options granted during the years ended December 31, 2023 and 2022 was $1.98 and $2.98 per share, respectively.
Stock-based Compensation Expense
The following sets forth the total stock-based compensation expense for the Company’s stock options and ESPP included in the Company’s consolidated statements of operations:
| | | | | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Research and development | $ | 4,199 | | | $ | 3,840 | | | |
| General and administrative | 7,938 | | | 6,540 | | | |
| Total stock-based compensation expense | $ | 12,137 | | | $ | 10,380 | | | |
8. Commitments and Contingencies
Purchase Commitments
Open purchase commitments are for the purchase of goods and services related to, but not limited to, research and development, facilities, and professional services under non-cancellable contracts. They were not recorded as liabilities on the consolidated balance sheet as of December 31, 2023 as the Company had not yet received the related goods or services. As of December 31, 2023, the Company had open purchase commitments for goods and services of $2.5 million, of which $1.8 million are expected to be received through the next 12 months.
Legal Proceedings
From time to time, the Company may become involved in litigation relating to claims arising from the ordinary course of business where the expense, distraction and/or the ultimate disposition could have a material adverse effect on the Company’s results of operations, financial condition or cash flows. The Company filed suit in the United States District Court for the Northern District of California against Somalogic (now known as Standard Biotools, Inc.), and the California Institute of Technology seeking declaratory judgment that the Company does not infringe any claims of the ‘793 Patent, allegedly licensed from the California Institute of Technology through Somalogic’s purchase of Palamedrix, Inc. While the Company believes that it will be able to prevail as to its claims in this litigation, the Company may incur significant expense and management distraction in prosecuting this litigation. Moreover, notwithstanding management’s belief as to the strength of our claims in this matter, litigation generally, and patent litigation in particular, can be unpredictable. As such, management cannot be certain that the Company will prevail in this litigation.
Leases
The Company is obligated under certain non-cancellable operating leases for office space and laboratory space. This space includes operating leases in Seattle, Washington, San Carlos, California, and San Diego, California.
Seattle Lease
In July 2021, the Company entered into a 7-year non-cancellable operating lease, which commenced in August 2021, for office space in Seattle, Washington. Total non-cancellable payments under this lease aggregate $4.5 million through June 2028.
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
San Carlos Leases
In December 2020, the Company entered into a new lease in San Carlos, California for ten years which commenced in October 2021 and expiring in October 2031 with total minimum lease payments of $40.7 million.
In December 2021, the Company entered into another lease in San Carlos, California for nine years commencing in March 2023. The Company can terminate this lease after five years from the commencement date without bearing any significant termination penalties and therefore the Company concluded that the lease term is five years with total minimum lease payments of $7.2 million. The Company utilized $2.0 million from the landlord with an interest rate of 7% to finance its tenant improvements. The principal and interest payments are included in the payments used to measure the lease liability.
San Diego Lease
In November 2022, the Company entered into a lease in San Diego, California for 39 months commencing in December 2022. Total non-cancellable payments under this lease aggregate $2.1 million through March 2026.
The components of lease costs which were included in operating expenses in the consolidated statements of operations were as follows: | | | | | | | | | | | |
| (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 |
| Fixed operating lease costs | $ | 6,893 | | | $ | 4,751 | |
| Variable operating lease costs | 2,432 | | | 1,655 | |
| Short-term lease costs | — | | | 25 | |
| Total lease costs | $ | 9,325 | | | $ | 6,431 | |
For the years ended December 31, 2023 and 2022, cash paid for amounts included in the measurement of operating lease liabilities included in cash flows used in operating activities was $6.2 million and $3.9 million, respectively.
As of December 31, 2023, the weighted-average remaining lease term and weighted-average discount rate for operating leases was 6.7 years and 9.1%, respectively.
The following table summarizes the Company's future principal contractual obligations for operating lease commitments as of December 31, 2023:
| | | | | |
| (in thousands) | |
| Year Ended December 31, | Lease Obligations |
| 2024 | 6,414 | |
| 2025 | 7,186 | |
| 2026 | 6,878 | |
| 2027 | 6,893 | |
| 2028 and thereafter | 19,028 | |
| Total future minimum lease payments | 46,399 | |
| Less: Imputed interest | (11,771) | |
| Total operating lease liabilities | $ | 34,628 | |
Guarantees and Indemnifications
In the ordinary course of business, the Company enters into agreements that may include indemnification provisions. Pursuant to such agreements, the Company may indemnify, hold harmless and defend an indemnified party for losses suffered or incurred by the indemnified party. Some of the provisions will limit losses to those
| | |
Nautilus Biotechnology, Inc. Notes to Consolidated Financial Statements—(Continued) |
arising from third-party actions. In some cases, the indemnifications will continue after the termination of the agreement. The maximum potential amount of future payments the Company could be required to make under these provisions is not determinable. The Company has never incurred material costs to defend lawsuits or settle claims related to these indemnification provisions.
The Company has also agreed to indemnify its directors and executive officers for costs associated with any fees, expenses, judgments, fines and settlement amounts incurred by them in any action or proceeding to which any of them are, or are threatened to be, made a party by reason of their service as a director or officer. The Company maintains director and officer insurance coverage that would generally enable it to recover a portion of any future amounts paid. The Company may be subject to indemnification obligation by law with respect to the actions of its employees under certain circumstances and in certain jurisdictions.
Letters of Credit
In conjunction with the San Carlos lease agreement entered in December 2020, the Company issued a cash-collateralized letter of credit in lieu of security deposit of $0.6 million. In conjunction with the San Carlos lease agreement entered in December 2021, the Company issued a cash-collateralized letter of credit in lieu of security deposit of $0.2 million. In conjunction with the San Diego lease agreement entered in November 2022, the Company issued a cash-collateralized letter of credit in lieu of a security deposit of $0.1 million.
9. Basic and Diluted Net Loss per Share
The following tables set forth the computation of the Company’s basic and diluted net loss per share attributable to common stockholders for the years ended December 31, 2023 and 2022:
| | | | | | | | | | | | | |
| (in thousands, except share and per share data) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Numerator: | | | | | |
| Net loss attributable to common stockholders | $ | (63,675) | | | $ | (57,924) | | | |
| Denominator: | | | | | |
| | | | | |
| | | | | |
| Weighted average shares used in computing net loss per share attributable to common stockholders, basic and diluted | 124,919,144 | | 124,589,555 | | |
| Net loss per share attributable to common stockholders, basic and diluted: | $ | (0.51) | | | $ | (0.46) | | | |
The potential shares of common stock that were excluded from the computation of diluted net loss per share attributable to common stockholders for the periods presented because including them would have had an antidilutive effect were as follows:
| | | | | | | | | | | | | |
| Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | |
| Options to purchase common stock | 14,676,335 | | 11,485,443 | | |
| Employee stock purchase plan | 141,720 | | 63,246 | | |
| Total potentially dilutive common share equivalents | 14,818,055 | | 11,548,689 | | |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosures
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time period specified in the SEC's rules and forms, and that such information is accumulated and communicated to management including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Our management, with the participation and supervision of our Chief Executive Officer and our Chief Financial Officer, have evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of such date, our disclosure controls and procedures were, in design and operation, effective at a reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Under the supervision of and with the participation of our principal executive officer and principal financial officer, our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in “Internal Control-Integrated Framework” (2013). Based on this assessment, management concluded that our internal control over financial reporting was effective as of December 31, 2023.
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm on our internal control over financial reporting due to an exemption established by the JOBS Act for “emerging growth companies.”
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Securities Exchange Act of 1934 that occurred during the quarter ended December 31, 2023, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating, and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, in designing and evaluating the disclosure controls and procedures, management recognizes that any system of internal control over financial reporting, including ours, no matter how well designed and operated, can only provide reasonable, not absolute assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs. Moreover, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. We intend to continue to monitor and upgrade our internal controls as necessary or appropriate for our business but cannot assure you that such improvements will be sufficient to provide us with effective internal control over financial reporting.
Item 9B. Other Information
Not applicable.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
Part III
Item 10. Directors, Executive Officers and Corporate Governance
Board of Directors
Our board of directors currently consists of nine directors, seven of whom are independent under the listing standards of The Nasdaq Stock Market LLC, or Nasdaq. Our board of directors is divided into three classes with staggered three-year terms. Thus, at each annual meeting of stockholders, a class of directors will be elected for a three-year term to succeed the class whose term is then expiring.
The following table sets forth the names, ages as of February 28, 2024, and certain other information for each of our current directors:
| | | | | | | | | | | | | | | | | | | |
| Name | | Age | | Position(s) | | | | | |
Sujal Patel | | 49 | | Chief Executive Officer, President, Secretary and Director | | | | | |
Parag Mallick | | 47 | | Chief Scientist and Director | | | | | |
Farzad Nazem (2) | | 62 | | Director | | | | | |
Karen Akinsanya(3) | | 56 | | Director | | | | | |
Matthew L. Posard (1) (2) | | 56 | | Chairperson | | | | | |
Matthew McIlwain (1) (3) | | 59 | | Director | | | | | |
Melissa Epperly (1) | | 46 | | Director | | | | | |
Michael Altman (2) | | 42 | | Director | | | | | |
Vijay Pande (3) | | 53 | | Director | | | | | |
___________________
| | | | | |
| (1) | Member of audit committee |
| (2) | Member of compensation committee |
| (3) | Member of nominating and governance committee |
Sujal Patel. Sujal Patel has served as our Chief Executive Officer, President, Secretary and a member of our board of directors since June 2021. Mr. Patel co-founded Legacy Nautilus and served as its Chief Executive Officer and a member of its board of directors from January 2017 through the closing of the Business Combination. Previously, from January 2001 to December 2010, Mr. Patel founded and served in various executive positions at Isilon Systems, Inc., an enterprise data storage company, including as Chief Executive Officer from 2007 until Isilon Systems was acquired by EMC Corporation, an enterprise storage systems and software company, in December 2010. Following the acquisition, Mr. Patel served as the President of EMC’s Isilon Storage Division until October 2012. Mr. Patel has been a Strategic Director at Madrona Venture Group, a Seattle venture capital firm, since January 2015. Mr. Patel holds a B.S. in Computer Science from the University of Maryland, College Park.
We believe Mr. Patel is qualified to serve on our board of directors because of his deep knowledge of our business, and strategy, and his extensive executive leadership and operational experience.
Parag Mallick. Parag Mallick has served as our Chief Scientist and a member of our board of directors since June 2021. Dr. Mallick co-founded Legacy Nautilus and served as its Chief Scientist and a member of its board of directors from December 2016 through the closing of the Business Combination. Dr. Mallick served as Assistant Professor at Stanford University from 2011 to 2017 and as Associate Professor at Stanford University from 2017 to the present. Previously, he served as Adjunct Assistant Professor at the University of California, Los Angeles from 2005 to 2011 and at the University of Southern California from 2009 to 2011. Dr. Mallick served as Director of Clinical Proteomics at Cedars-Sinai from 2005 to 2009. Dr. Mallick completed his post-doctoral fellowship in clinical proteomics and systems biology at the Institute for Systems Biology from 2002 to 2004. Dr. Mallick holds a B.S. in Computer Science from Washington University in St. Louis and a Ph.D. in Chemistry and Biochemistry from the University of California, Los Angeles.
We believe Dr. Mallick is qualified to serve on our board of directors because of his scientific expertise and his deep understanding of our business, operations and strategy.
Farzad Nazem. Farzad Nazem has served on our board of directors since June 2021 and served on the Legacy Nautilus board of directors from June 2017 through the closing of the Business Combination. Mr. Nazem spent over 11 years at Yahoo! Inc., a web services provider, where he served as Chief Technology Officer, and over 10 years at Oracle Corporation, a database management company, where he spent time as Vice President of the Web and Media Server Division. Mr. Nazem holds a B.S. in Computer Science from California Polytechnic State University–San Luis Obispo.
We believe Mr. Nazem is qualified to serve on our board of directors because of his technical expertise, extensive industry background and management experience.
Karen Akinsanya. Karen Akinsanya has served on our board of directors since March 2022. Dr. Akinsanya has served as President of R&D, Therapeutics of Schrödinger, Inc. (NASDAQ: SDGR), a life sciences company, since February 2022, and its Executive Vice President and Chief Biomedical Scientist since January 2020 and previously served as its Senior Vice President and Chief Biomedical Scientist from April 2018 to December 2019. Prior to joining Schrödinger, Dr. Akinsanya spent 12 years at Merck & Co., Inc., or Merck, a pharmaceutical company, beginning in 2005, where she held a variety of positions across Merck Research Labs, including Associate Vice President, Early Scientific Assessment Lead, Business Development & Licensing from December 2013 to July 2017, Collaboration Lead and Executive Director, Cardiovascular Research from January 2010 to December 2013, and Associate Director of Clinical Pharmacology from October 2005 to December 2009. Prior to Merck, Dr. Akinsanya held a number of roles in drug discovery at Ferring Pharmaceuticals in the United Kingdom and the United States from 1997 to 2005. In 2007, Dr. Akinsanya founded Envision Science Group LLC, or Envision, a translational science consulting company, where she currently serves as President. Dr. Akinsanya provided consulting services on behalf of Envision to companies in the pharmaceutical industry between July 2017 and April 2018. Dr. Akinsanya received a B.Sc. in Biochemistry from Queen Mary College, University of London, a Ph.D. in Endocrine Physiology from the Imperial College and completed postdoctoral studies at the Ludwig Institute for Cancer Research, University College, London.
We believe Dr. Akinsanya is qualified to serve on our board of directors because of her extensive industry background and experience in the life sciences industry.
Matthew L. Posard. Matthew L. Posard has served on our board of directors and as Chairperson since June 2021 and served on the Legacy Nautilus board of directors from February 2019 through the closing of the Business Combination. Mr. Posard currently serves as Founding Partner at Explore-DNA, a life sciences and diagnostics consulting firm. Previously, Mr. Posard served as the President and Chief Commercial Officer of GenePeeks, Inc., a genetic information company, from February 2017 to April 2018, and as Executive Vice President and Chief Commercial Officer at Trovagene Inc. (now Cardiff Oncology, Inc.), an oncology therapeutics company, from March 2015 to April 2016. Mr. Posard also held multiple executive leadership roles at Illumina, Inc., a biotechnology company, from 2006 to 2015. Mr. Posard currently serves on multiple boards including Halozyme Therapeutics (NASDAQ: HALO), Talis Biomedical (NASDAQ: TLIS), and DermTech (NASDAQ CM: DMTK). Mr. Posard holds a B.A. in Management Science from the University of California, San Diego.
We believe Mr. Posard is qualified to serve on our board of directors because of his extensive experience as an executive and serving on various boards of directors of companies in the life sciences industry.
Matthew McIlwain. Matthew McIlwain has served on our board of directors since June 2021 and served on the Legacy Nautilus board of directors from January 2021 through the closing of the Business Combination. Mr. McIlwain currently serves as a Managing Director at Madrona Venture Group, a venture capital firm, a position he has held since June 2002, and joined as a Venture Partner in June 2000. Prior to joining Madrona Venture Group, Mr. McIlwain served as Vice President of Business Process at Genuine Parts Company from January 1996 to May 2000, a consultant at McKinsey & Company from August 1992 to December 1995, and in investment banking at Credit Suisse First Boston from July 1987 to July 1989. Mr. McIlwain currently serves as a director on the boards of directors of Smartsheet Inc. (NYSE: SMAR). Mr. McIlwain holds a B.A. in Government and Economics from Dartmouth College, an M.P.P. in Public Policy from Harvard University’s Kennedy School of Government, and an M.B.A. from Harvard Business School.
We believe Mr. McIlwain is qualified to serve on our board of directors due to his extensive industry background and experience investing in the technology and life sciences industry.
Melissa Epperly. Melissa Epperly has served on our board of directors since June 2021 and served on the Legacy Nautilus board of directors from January 2021 through the closing of the Business Combination. Ms. Epperly currently serves as Chief Financial Officer of Zentalis Pharmaceuticals, Inc. (NASDAQ: ZNTL), a clinical-stage cancer company, a position she has held since September 2019. From June 2018 to August 2019, Ms. Epperly served as Chief Financial Officer of PsiOxus Therapeutics Ltd., a clinical-stage gene therapy cancer company, where she led the company’s financial operations. Prior to joining PsiOxus, Ms. Epperly served as Chief Financial Officer and Head of Business Development at R-Pharm US, a commercial-stage oncology company, from October 2015 to June 2018, where she led the company’s financial operations and business development. Ms. Epperly served as a Director at Anchorage Capital Group, a credit-focused hedge fund, from August 2012 to
September 2015. Previously, Ms. Epperly was a Vice President at Goldman Sachs in equity research in New York and London, a management consultant with Bain & Company, and a healthcare investment banker at Morgan Stanley. Ms. Epperly currently serves on the board of directors of Kinnate Biopharma Inc. (NASDAQ: KNTE) and Roivant Sciences Ltd (NASDAQ: ROIV). Ms. Epperly holds a B.A. in Biochemisty and Economics from the University of Virginia and an M.B.A from Harvard Business School.
We believe Ms. Epperly is qualified to serve on our board of directors because of her extensive experience as a senior financing executive in the life sciences industry.
Michael Altman. Michael Altman, CFA, has served on our board of directors since June 2021 and served on the ARYA board of directors from inception until the closing of the Business Combination. Mr. Altman also served as ARYA’s Chief Financial Officer from inception until the closing of the Business Combination. Mr. Altman has served as a Managing Director on the investment team of Perceptive Advisors since 2007, and is a member of the internal investment committee of Perceptive Advisors’ credit opportunities fund. Mr. Altman’s focus is on medical devices, diagnostics, digital health and specialty pharmaceuticals. Mr. Altman also serves on the board of directors of Lyra Therapeutics (Nasdaq: LYRA), which is a portfolio company of Perceptive Advisors. Mr. Altman also serves as the Chief Financial Officer and on the board of directors of ARYA Sciences Acquisition Corp IV (Nasdaq: ARYD) and ARYA Sciences Acquisition Corp V (Nasdaq: ARYE). Mr. Altman graduated from the University of Vermont with a BS in Business Administration.
We believe Mr. Altman is qualified to serve on our board of directors because of his broad operational and transactional experience.
Vijay Pande. Vijay Pande has served on our board of directors since June 2021, and served on the Legacy Nautilus board of directors from May 2018 through the closing of the Business Combination. Since September 2015, Dr. Pande has served as a general partner at Andreessen Horowitz, a venture capital fund, where he focuses on investments in biopharma and healthcare companies. From October 1999 to February 2019, Dr. Pande served as founding director of Folding@home, a distributed computing project for disease research. Dr. Pande holds a B.S. in Physics from Princeton University and a Ph.D. in Physics from Massachusetts Institute of Technology.
We believe Dr. Pande is qualified to serve on our board of directors because of his extensive industry experience and board experience with healthcare companies.
Executive Officers
The following table sets forth certain information about our executive officers as of February 28, 2024.
| | | | | | | | | | | | | | |
Name | | Age | | Position |
Sujal Patel | | 49 | | Chief Executive Officer, President, Secretary and Director |
Parag Mallick | | 47 | | Chief Scientist and Director |
Anna Mowry | | 46 | | Chief Financial Officer and Treasurer |
Nick Nelson | | 41 | | Chief Business Officer and Senior Vice President, Business Development |
Subra Sankar | | 64 | | Senior Vice President, Product Development |
Matthew Murphy | | 59 | | General Counsel |
Gwen Weld | | 66 | | Chief People Officer |
Mary Godwin | | 65 | | Senior Vice President, Operations |
For the biography of each of Mr. Patel and Dr. Mallick, please see “Directors, Executive Officers and Corporate Governance – Board of Directors.”
Anna Mowry. Anna Mowry has served as our Chief Financial Officer and Treasurer since June 2021. Ms. Mowry served as Legacy Nautilus’ Chief Financial Officer and Treasurer from January 2021 through the closing of the Business Combination. Previously, Ms. Mowry served as Vice President of Finance and Operations at Igneous, Inc., an unstructured data management solutions company, from April 2018 to December 2020. From August 2014 to March 2018, Ms. Mowry served at ExtraHop Networks, Inc., a cloud-native network detection and response company, initially as Director of Finance and subsequently, as Senior Director of Finance and Sales Operations. From January 2014 to July 2014, Ms. Mowry served as Senior Manager of Worldwide Operations, Commercial Sales at Amazon Web Services, a provider of cloud computing services. Ms. Mowry held a variety of finance and operational roles at Isilon Systems, an enterprise data storage company, from November 2006 until Isilon Systems was acquired by EMC Corporation, an enterprise storage systems and software company, in December 2010. Following the acquisition, Ms. Mowry served as Director of Sales Finance and Operation of EMC’s Isilon Storage Division until December 2013. Ms. Mowry holds a B.S. in Biochemistry from Western Washington University and a master’s in business administration in finance from the University of Washington.
Nick Nelson. Nick Nelson has served as our Chief Business Officer and Senior Vice President, Business Development since June 2021. Mr Nelson served as Legacy Nautilus’ Chief Business Officer and Senior Vice President, Business Development from October 2020 through the closing of the Business Combination. Previously, Mr. Nelson served as Chief Business Officer and Senior Vice President at Caris Life Sciences, Inc., an oncology precision medicine diagnostic company, from May 2018 to July 2020. Prior to Caris, Mr. Nelson served as Vice President of Corporate Development at Trovagene, Inc. (now Cardiff Oncology, Inc.), an oncology diagnostic and therapeutics company, from March 2015 to May 2018. Prior to joining Trovagene, Mr. Nelson spent 10 years at Illumina, Inc., a biotechnology company, in positions spanning research and development, technical service, sales, market development and business management. Mr. Nelson holds a B.S. in Cellular and Molecular Biology from San Diego State University.
Subra Sankar. Subra Sankar has served as our Senior Vice President, Product Development since June 2021. Dr. Sankar served as Legacy Nautilus’ Senior Vice President, Product Development from December 2020 through the closing of the Business Combination. Previously, Dr. Sankar served at GenapSys Inc., a DNA sequencing technology company, as Senior Vice President, Product Development from November 2019 to December 2020 and Vice President, Product Development from July 2015 to November 2019. Prior to joining GenapSys, Dr. Sankar served as the Vice President, Engineering and Development at LumaSense Technologies, Inc., a temperature and gas sensing solutions company, from January 2014 to July 2015. Dr. Sankar holds a B.Tech. in Aeronautical Engineering from the Indian Institute of Technology, Madras, India, a Master of Science degree in Aerospace Engineering from Georgia Institute of Technology, a Ph.D. in Aerospace Engineering from Georgia Institute of Technology, and a Master of Business Administration degree from Haas School of Business, University of California, Berkeley.
Matthew Murphy. Matthew Murphy has served as our General Counsel since June 2021. Mr. Murphy served as Legacy Nautilus’ General Counsel from April 2021 through the closing of the Business Combination. Prior to joining us, from August 2020 to March 2021, Mr. Murphy provided advisory services to various life sciences companies and, from July 2018 to August 2020, Mr. Murphy served as a Strategic Consultant and Consulting General Counsel to various life sciences and technology companies. From August 2016 to July 2018, Mr. Murphy served as General Counsel at BioElectron Technology Corporation, a clinical-stage biotechnology company. From February 2014 to July 2016, Mr. Murphy served as Vice President, General Counsel and Secretary at 10X Genomics, Inc., a biotechnology company that designs and manufactures gene sequencing technology used in scientific research. Mr. Murphy also previously served in Vice President and General Counsel roles at various companies including Siluria Technologies, Inc. and Pacific Biosciences of California, Inc. Mr. Murphy holds a B.S. in Fermentation Science from the University of California at Davis and a J.D. from the University of San Francisco School of Law.
Gwen Weld. Gwen Weld has served as our Chief People Officer since April 2022. Ms. Weld served as a human resources consultant for us and Legacy Nautilus from September 2020 to April 2022. Prior to joining us, from April 2019 to April 2022, Ms. Weld served as Interim Chief People Officer at Amperity, a software company. From April 2021 to April 2022, Ms. Weld served as a human resources consultant for OctoML, a software development company. From June 2006 to December 2011, Ms. Weld served as Vice President of Global People and Infrastructure at Isilon Systems, an enterprise data storage company, and previously served as General Manager of Human Resources at Microsoft Corporation, a technology company, for nearly twenty years, leading Microsoft’s human resources agenda for its various groups and overseeing worldwide recruiting and alternative staffing. Ms. Weld studied business administration at Pace University.
Mary Godwin. Mary Godwin has served as our Senior Vice President, Operations since July 2022. Ms. Godwin previously served as our Vice President, Operations from January 2020 to June 2022. Prior to joining us, from January 2014 to November 2018, Ms. Godwin served as Vice President, Operations at Qumulo, Inc., a data storage company. Ms. Godwin holds a B.S. in Plastics Engineering from the University of Massachusetts, Lowell and a Master of Business Administration degree from Santa Clara University.
Family Relationships
There are no family relationships among any of our directors or executive officers.
Director Independence
Our common stock is listed on Nasdaq. As a company listed on Nasdaq, we are required under Nasdaq listing rules to maintain a board comprised of a majority of independent directors as determined affirmatively by our board. Under Nasdaq listing rules, a director will only qualify as an independent director if, in the opinion of that listed company’s board of directors, the director does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In addition, the Nasdaq listing rules require that, subject to specified exceptions, each member of our audit, compensation and nominating and governance committees be independent.
Audit committee members must also satisfy the additional independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Nasdaq listing rules applicable to audit committee members. Compensation committee members must also satisfy the additional independence criteria set forth in Rule 10C-1 under the Exchange Act and Nasdaq listing rules applicable to compensation committee members.
Our board of directors has undertaken a review of the independence of each of our directors. Based on information provided by each director concerning his or her background, employment and affiliations, our board of directors has determined that none of Mr. Pande, Mr. Altman, Mr. Nazem, Mr. McIlwain, Ms. Epperly, Mr. Posard and Ms. Akinsanya, representing seven of our nine directors, has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is an “independent director” as defined under the listing standards of Nasdaq. Sujal Patel is not considered an independent director because of his position as our Chief Executive Officer, President and Secretary and Parag Mallick is not considered an independent director because of his position as our Chief Scientist.
In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances that our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director, and the transactions involving them described in the section titled “Certain Relationships and Related Transactions, and Director Independence.”
Board Leadership Structure
Our corporate governance framework provides our board flexibility to determine the appropriate leadership structure for the company, and whether the roles of chairperson and chief executive officer should be separated or combined. In making this determination, our board considers many factors, including the needs of the business, our board’s assessment of its leadership needs from time to time and the best interests of our stockholders. If the role of chairperson is filled by a director who does not qualify as an independent director, then our corporate governance guidelines provide that one of our independent directors may serve as our lead independent director.
Our board believes that it is currently appropriate to separate the roles of chairperson and chief executive officer. The chief executive officer is responsible for day-to-day leadership, while our chairperson, along with the rest of our independent directors, ensures that our board’s time and attention is focused on providing independent oversight of management and matters critical to our company. The board believes that Mr. Posard’s deep knowledge of the company and industry, as well as strong leadership and governance experience, enable Mr. Posard to lead our board effectively and independently.
Role of Board in Risk Oversight Process
One of the key functions of our board of directors is informed oversight of the risk management process. Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee has the responsibility to consider and discuss major financial risk exposures as well as risks and exposures associated with cybersecurity, information security and privacy matters, and the steps our management takes to monitor and control such exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The audit committee also monitors compliance with legal and regulatory requirements. Our compensation committee is responsible for overseeing the management of risks relating to executive compensation plans and arrangements. The compensation committee also assesses and monitors whether compensation plans, policies and programs comply with applicable legal and regulatory requirements.
Board Committees
Our board of directors has established the following standing committees of the board: audit committee; compensation committee; and nominating and governance committee. The composition and responsibilities of each of the committees of our board of directors is described below.
Audit Committee
The current members of our audit committee are Ms. Epperly, Mr. McIlwain and Mr. Posard. Ms. Epperly is the chairperson of our audit committee. Our board of directors has determined that each member of our audit committee meets the requirements for independence of audit committee members under the rules and regulations of the SEC and the listing standards of Nasdaq, and also meets the financial literacy requirements of the listing standards of Nasdaq. Our board of directors has
determined that each of Ms. Epperly and Mr. McIlwain is an audit committee financial expert within the meaning of Item 407(d) of Regulation S-K. Our audit committee is responsible for, among other things:
•selecting, retaining, compensating, evaluating, overseeing and, where appropriate, terminating our independent registered public accounting firm;
•reviewing and approving the scope and plans for the audits and the audit fees and approving all non-audit and tax services to be performed by the independent auditor;
•evaluating the independence and qualifications of our independent registered public accounting firm;
•reviewing our financial statements, and discussing with management and our independent registered public accounting firm the results of the annual audit and the quarterly reviews;
•reviewing and discussing with management and our independent registered public accounting firm the quality and adequacy of our internal controls and our disclosure controls and procedures;
•discussing with management our procedures regarding the presentation of our financial information, and reviewing earnings press releases and guidance;
•overseeing the design, implementation and performance of our internal audit function, if any;
•setting hiring policies with regard to the hiring of employees and former employees of our independent auditor and overseeing compliance with such policies;
•reviewing, approving and monitoring related party transactions;
•reviewing and monitoring compliance with our code of business conduct and ethics, and reviewing conflicts of interest of our board members and officers;
•adopting and overseeing procedures to address complaints regarding accounting, internal accounting controls and auditing matters, including confidential, anonymous submissions by our employees of concerns regarding questionable accounting or auditing matters;
•reviewing and discussing with management and our independent auditor the adequacy and effectiveness of our legal, regulatory and ethical compliance programs; and
•reviewing and discussing with management and our independent auditor our guidelines and policies to identify, monitor and address enterprise risks, including major financial risks exposures and and risks and exposures associated with cybersecurity, information security and privacy matters.
Our audit committee operates under a written charter that satisfies the applicable rules and regulations of the SEC and the listing standards of Nasdaq. A copy of the charter of our audit committee is available on our website at http://investors.nautilus.bio/. During 2023, our audit committee held four meetings.
Compensation Committee
The current members of our compensation committee are Mr. Posard, Mr. Altman and Mr. Nazem. Mr. Posard is the chairperson of our compensation committee. Our board of directors has determined that each member of our compensation committee meets the requirements for independence for compensation committee members under the rules and regulations of the SEC and the listing standards of Nasdaq. Each member of the compensation committee is also a non-employee director, as defined pursuant to Rule 16b-3 promulgated under the Exchange Act. Our compensation committee is responsible for, among other things:
•reviewing, approving or making recommendations to our board of directors regarding the compensation for our executive officers, including our chief executive officer;
•reviewing, approving and administering our employee benefit and equity incentive plans;
•establishing and reviewing the compensation plans and programs of our employees, and ensuring that they are consistent with our general compensation strategy;
•monitoring compliance with any stock ownership guidelines;
•approving or making recommendations to our board of directors regarding the creation or revision of any clawback policy; and
•making recommendations to our board of directors regarding non-employee director compensation.
Our compensation committee operates under a written charter that satisfies the applicable rules and regulations of the SEC and the listing standards of Nasdaq. A copy of the charter of our compensation committee is available on our website at http://investors.nautilus.bio/. During 2023, our compensation committee held four meetings.
Nominating and Governance Committee
The current members of our nominating and governance committee are Mr. McIlwain, Mr. Pande and Ms. Akinsanya. Mr. McIlwain is the chairperson of our nominating and governance committee. Our board of directors has determined that each member of our nominating and governance committee meets the requirements for independence for nominating and governance committee members under the listing standards of Nasdaq. Our nominating and governance committee is responsible for, among other things:
•reviewing and assessing and making recommendations to our board of directors regarding desired qualifications, expertise and characteristics sought of board members;
•identifying, evaluating, selecting or making recommendations to our board of directors regarding nominees for election to our board of directors;
•developing policies and procedures for considering stockholder nominees for election to our board of directors;
•reviewing our succession planning process for our chief executive officer and any other members of our executive management team;
•reviewing and making recommendations to our board of directors regarding the composition, organization and governance of our board of directors and its committees;
•reviewing and making recommendations to our board of directors regarding our corporate governance guidelines and corporate governance framework;
•overseeing director orientation for new directors and continuing education for our directors;
•overseeing the evaluation of the performance of our board of directors and its committees; and
•administering policies and procedures for communications with the non-management members of our board of directors.
Our nominating and governance committee operates under a written charter that satisfies the applicable listing standards of Nasdaq. A copy of the charter of our nominating and governance committee is available on our website at http://investors.nautilus.bio/. During 2023, our nominating and governance committee held four meetings.
Director Diversity
Our nominating and governance committee is committed to continuing to identify and recruit highly qualified director candidates with diverse experiences, perspectives, and backgrounds to join our board of directors. The table below provides certain information regarding the composition of our board of directors. Each of the categories listed in the below table has the meaning as it is used in Nasdaq Rule 5605(f). As shown below in the board diversity matrix, the Company is currently in compliance with the diversity requirements of Nasdaq Rule 5605(f).
Board Diversity Matrix (as of February 28, 2024)
| | | | | | | | | | | | | | |
| Board Size |
| Total Number of Directors | 9 |
| Part I: Gender Identity | Male | Female | Non-Binary | Did Not Disclose Gender |
| Number of directors based on gender identity | 7 | 2 | | |
| Part II: Demographic Background |
| African American or Black | | | | |
| Alaskan Native or Native American | | | | |
| Asian | 3 | | | |
| Hispanic or Latinx | | | | |
| Native Hawaiian or Pacific Islander | | | | |
| White | 4 | 1 | | |
| Two or More Races or Ethnicities | | 1 | | |
| LGBTQ+ | | | | |
| Did Not Disclose Demographic Background | | | | |
Attendance at Board and Stockholder Meetings
During our fiscal year ended December 31, 2023, our board of directors held four meetings (including regularly scheduled and special meetings), and each director attended at least 75% of the aggregate of (1) the total number of meetings of the board of directors held during the period for which he or she was a director and (2) the total number of meetings held by all committees on which he or she served during the periods that he or she served as a director.
Although we do not have a formal policy regarding attendance by members of our board of directors at the annual meetings of stockholders, we encourage, but do not require, directors to attend. six members of our board of directors attended our 2023 annual meeting of stockholders.
Executive Sessions of Non-Employee Directors
To encourage and enhance communication among non-employee directors, and as required under applicable Nasdaq rules, our corporate governance guidelines provide that the non-employee directors will meet in executive sessions without management directors or management present on a periodic basis but no less than two times a year. In addition, if any of our non-employee directors are not independent directors, then our independent directors will also meet in executive session on a periodic basis but no less than two times a year.
Compensation Committee Interlocks and Insider Participation
During 2023, the members of our compensation committee were Mr. Posard, Mr. Altman and Mr. Nazem. None of the members of our compensation committee has ever been an executive officer or employee of Nautilus. Mr. Altman served as ARYA’s Chief Financial Officer and director from its inception until the closing of the Business Combination in 2021. None of our executive officers currently serve, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers that serves as a member of the board of directors or our compensation committee.
Considerations in Evaluating Director Nominees
Our nominating and governance committee uses a variety of methods for identifying and evaluating potential director nominees. In its evaluation of director candidates, including the current directors eligible for re-election, our nominating and governance committee will consider the current size and composition of our board of directors and the needs of our board of directors and the respective committees of our board of directors and other director qualifications. While our board has not established minimum qualifications for board members, some of the factors that our nominating and governance committee considers in assessing director nominee qualifications include, without limitation, issues of character, professional ethics and integrity, judgment, business experience and diversity, and with respect to diversity, such factors as race, ethnicity, gender, differences in professional background, age and geography, as well as other individual qualities and attributes that contribute to the total mix of viewpoints and experience represented on our board. Although our board of directors does not maintain a specific policy with respect to board diversity, our board of directors believes that the board should be a diverse body, and the nominating and governance committee considers a broad range of perspectives, backgrounds and experiences.
If our nominating and governance committee determines that an additional or replacement director is required, then the committee may take such measures as it considers appropriate in connection with its evaluation of a director candidate, including candidate interviews, inquiry of the person or persons making the recommendation or nomination, engagement of an outside search firm to gather additional information, or reliance on the knowledge of the members of the committee, board or management.
After completing its review and evaluation of director candidates, our nominating and governance committee recommends to our full board of directors the director nominees for selection. Our nominating and governance committee has discretion to decide which individuals to recommend for nomination as directors and our board of directors has the final authority in determining the selection of director candidates for nomination to our board.
Stockholder Recommendations and Nominations to our Board of Directors
Our nominating and governance committee will consider recommendations and nominations for candidates to our board of directors from stockholders in the same manner as candidates recommended to the committee from other sources, so long as such recommendations and nominations comply with our amended and restated certificate of incorporation and amended and restated bylaws, all applicable company policies and all applicable laws, rules and regulations, including those promulgated by the SEC. Our nominating and governance committee will evaluate such recommendations in accordance with its charter, our bylaws and corporate governance guidelines and the director nominee criteria described above.
A stockholder that wants to recommend a candidate to our board of directors should direct the recommendation in writing by letter to our corporate secretary at Nautilus Biotechnology, Inc., 2701 Eastlake Avenue East, Seattle, Washington 98102, Attention: Corporate Secretary. Such recommendation must include the candidate’s name, home and business contact information, detailed biographical data, relevant qualifications, a signed letter from the candidate confirming willingness to serve, information regarding any relationships between the candidate and us and evidence of the recommending stockholder’s ownership of our capital stock. Such recommendation must also include a statement from the recommending stockholder in support of the candidate. Our nominating and governance committee has discretion to decide which individuals to recommend for nomination as directors.
Under our amended and restated bylaws, stockholders may also directly nominate persons for our board of directors. Any nomination must comply with the requirements set forth in our amended and restated bylaws and the rules and regulations of the SEC, including but not limited to Rule 14a-8 and Rule 14a-19 under the Exchange Act, and should be sent in writing to our corporate secretary at the address above.
Communications with the Board of Directors
Stockholders and other interested parties wishing to communicate directly with our non-management directors, may do so by writing and sending the correspondence to our Chief Financial Officer or General Counsel by mail to our principal executive offices at Nautilus Biotechnology, Inc., 2701 Eastlake Avenue East, Seattle, Washington 98102. Our Chief Financial Officer or General Counsel, in consultation with appropriate directors as necessary, will review all incoming communications and screen for communications that (1) are solicitations for products and services, (2) relate to matters of a personal nature not relevant for our stockholders to act on or for our board to consider and (3) matters that are of a type that are improper or irrelevant to the functioning of our board or our business, for example, mass mailings, job inquiries and business solicitations. If appropriate, our Chief Financial Officer or General Counsel will route such communications to the appropriate director(s) or, if none is specified, then to the chairperson of the board or the lead independent director (if one is appointed). These policies and procedures do not apply to communications to non-management directors from our officers or directors who are stockholders or stockholder proposals submitted pursuant to Rule 14a-8 under the Exchange Act.
Policy Prohibiting Hedging or Pledging of Securities
Under our insider trading policy, our employees, including our executive officers, and the members of our board of directors are prohibited from, directly or indirectly, among other things, (1) engaging in short sales, (2) trading in publicly-traded options, such as puts and calls, and other derivative securities with respect to our securities (other than stock options, restricted stock units and other compensatory awards issued to such individuals by us), (3) purchasing financial instruments (including prepaid variable forward contracts, equity swaps, collars and exchange funds), or otherwise engaging in transactions that hedge or offset, or are designed to hedge or offset, any decrease in the market value of equity securities granted to them by us as part of their compensation or held, directly or indirectly, by them, (4) pledging any of our securities as collateral for any loans and (5) holding our securities in a margin account.
Corporate Governance Guidelines and Code of Business Conduct and Ethics
Our board of directors has adopted corporate governance guidelines. These guidelines address, among other items, the qualifications and responsibilities of our directors and director candidates, the structure and composition of our board of directors and corporate governance policies and standards applicable to us in general. In addition, our board of directors has adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including our chief executive officer, chief financial officer and other executive and senior financial officers. The full text of our corporate governance guidelines and code of business conduct and ethics are available on our website at http://investors.nautilus.bio/. We will post amendments to our code of business conduct and ethics or any waivers of our code of business conduct and ethics for directors and executive officers on the same website.
Director Compensation
Director Compensation Policy
Our board of directors reviews director compensation periodically to ensure that director compensation remains competitive such that we are able to recruit and retain qualified directors.
In 2020, the compensation committee of the Legacy Nautilus board of directors retained Compensia, a third-party compensation consultant, to provide the Legacy Nautilus board of directors and its compensation committee with an analysis of publicly available market data regarding practices and compensation levels at comparable companies and assistance in determining compensation to be provided to our non-employee directors. Based on the discussions with and assistance from the compensation consultant, in connection with the Business Combination, our board of directors adopted an Outside Director Compensation Policy that provides for certain compensation to our non-employee directors. The Outside Director Compensation Policy originally became effective in connection with the closing of the Business Combination in June 2021, and was subsequently amended, effective as of February 15, 2023, to set upper limits on the number of shares of common stock subject to each Initial Award and Annual Award (as described below) granted under the policy.
Cash Compensation
The Outside Director Compensation Policy provides for the following cash compensation program for our non-employee directors:
•$40,000 per year for service as a non-employee director;
•$40,000 per year for service as non-employee chair of our board of directors;
•$20,000 per year for service as chair of our audit committee;
•$10,000 per year for service as a member of our audit committee;
•$14,000 per year for service as chair of our compensation committee;
•$7,000 per year for service as a member of our compensation committee;
•$10,000 per year for service as chair of our nominating and governance committee; and
•$5,000 per year for service as a member of our nominating and governance committee.
Each non-employee director who serves as a committee chair of our board of directors receives the cash retainer fee as the chair of the committee but not the cash retainer fee as a member of that committee, provided that the non-employee director who serves as the non-employee chair of our board of directors receives the annual retainer fees for such role as well as the
annual retainer fee for service as a non-employee director. These fees to our non-employee directors are paid quarterly in arrears on a prorated basis. The above-listed fees for service as non-employee chair of our board of directors or a chair or member of any committee are payable in addition to the non-employee director retainer. Under our Outside Director Compensation Policy, we also reimburse our non-employee directors for reasonable travel expenses to attend meetings of our board of directors and its committees.
Equity Compensation
Initial Award. Pursuant to our Outside Director Compensation Policy, each person who first becomes a non-employee director after the effective date of such policy will receive, on the first trading day on or after the date that the person first becomes a non-employee director, an initial award of stock options to purchase shares of our common stock (the “Initial Award”). The Initial Award will cover a number of shares of common stock equal to the lesser of (a) such number of shares of common stock that results in the Initial Award having an aggregate grant date fair value (determined in accordance with U.S. GAAP) of $370,000 (but with any resulting fractional share rounded down to the nearest whole share), or (b) 90,000 shares of common stock. Prior to the amendment of the Outside Director Compensation Policy in February 2023, each Initial Award covered a number of shares of common stock that resulted in the Initial Award having an aggregate grant date fair value (determined in accordance with U.S. GAAP) of $370,000. Each Initial Award will be scheduled to vest in equal installments as to 1/36th of the shares of our common stock subject to the Initial Award on a monthly basis following the Initial Award’s grant date, on the same day of the month as the grant date, subject to continued service to us through the applicable vesting dates. If the person was a member of our board of directors and also an employee, then becoming a non-employee director due to termination of employment will not entitle the person to an Initial Award.
Annual Award. Each non-employee director automatically will receive, on the first trading day immediately after the date of each annual meeting of our stockholders (an “Annual Meeting”) that occurs following the effective date of our Outside Director Compensation Policy, an annual award of stock options to purchase shares of our common stock (the “Annual Award”). Each Annual Award will cover a number of shares of common stock equal to the lesser of (a) such number of shares of common stock that results in the Annual Award having an aggregate grant date fair value (determined in accordance with U.S. GAAP) of $185,000 (but with any resulting fractional share rounded down to the nearest whole share), or (b) 45,000 shares of our common stock, except if an individual began service as a non-employee director after the date of the Annual Meeting that occurred immediately prior to such Annual Meeting, then the Annual Award granted to such non-employee director will cover a number of shares of common stock determined in the manner previously described, but prorated based on the number of whole months that the individual served as a non-employee director prior to the Annual Award’s grant date during the 12 month period immediately preceding such Annual Meeting. Prior to the amendment of the Outside Director Compensation Policy in February 2023, each Annual Award covered a number of shares of common stock that resulted in the Annual Award having an aggregate grant date fair value (determined in accordance with U.S. GAAP) of $185,000, subject to the same proration as set forth above for any director that began service as a non-employee director after the date of the preceding Annual Meeting (or the Business Combination in the case of the first Annual Meeting thereafter). Each Annual Award will be scheduled to vest as to 1/12th of the shares of our common stock subject to such award on a monthly basis following the Annual Award’s grant date, on the same day of the month as the grant date, subject to continued service to us through the applicable vesting dates.
Change in Control. In the event of our change in control, as defined in the 2021 Equity Incentive Plan (“2021 Plan”), each non-employee director’s then outstanding equity awards covering shares of our common stock that were granted to him or her while a non-employee director will accelerate vesting in full, provided that he or she remains a non-employee director through the date of such change in control.
Other Award Terms. Each Initial Award and Annual Award will be granted under the 2021 Plan (or its successor plan, as applicable) and form of award agreement under such plan. These awards will have a maximum term to expiration of ten years from their grant and a per share exercise price equal to 100% of the fair market value of a share of our common stock on the award’s grant date.
Director Compensation Limits. Our Outside Director Compensation Policy and the 2021 Plan provide that in any fiscal year, a non-employee director may be paid cash compensation and granted equity awards with an aggregate value of no more than $1,000,000 (with the value of equity awards based on its grant date fair value determined in accordance with U.S. GAAP for purposes of this limit). Equity awards granted or other compensation provided to a non-employee director for services provided as an employee or consultant (other than a non-employee director), or provided before the closing of the Business Combination, will not count toward this annual limit.
Director Compensation for Fiscal Year 2023
The following table sets forth information regarding the compensation earned for service on our board of directors during the year ended December 31, 2023 by non-employee directors. Neither Mr. Patel nor Dr. Mallick received any additional compensation for their service as a director in 2023. Mr. Patel and Dr. Mallick’s compensation as a named executive officer is set forth below under “— Summary Compensation Table.”
| | | | | | | | | | | | | | | | | | | | |
Name and principal position | | Fees earned or paid in cash ($) | | Option awards ($)(1) | | Total ($) |
Karen Akinsanya(2) | | 45,000 | | | 142,976 | | | 187,976 | |
Michael Altman(3) | | 47,000 | | | 142,976 | | | 189,976 | |
Melissa Epperly(4) | | 60,000 | | | 142,976 | | | 202,976 | |
Matthew McIlwain(5) | | 60,000 | | | 142,976 | | | 202,976 | |
Farzad Nazem(6) | | 47,000 | | | 142,976 | | | 189,976 | |
Vijay Pande(7) | | 45,000 | | | 142,976 | | | 187,976 | |
Matthew Posard(8) | | 104,000 | | | 142,976 | | | 246,976 | |
_________________ | | | | | |
| (1) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the stock option awards granted during 2023, computed in accordance with FASB ASC 718. These amounts do not reflect the actual economic value that will be realized by the director upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options. |
| (2) | As of December 31, 2023, Ms. Akinsanya holds outstanding options to purchase 165,409 shares. |
| (3) | As of December 31, 2023, Mr. Altman holds outstanding options to purchase 175,902 shares. |
| (4) | As of December 31, 2023, Ms. Epperly holds outstanding options to purchase 204,028 shares. |
| (5) | As of December 31, 2023, Mr. McIlwain holds outstanding options to purchase 167,747 shares. |
| (6) | As of December 31, 2023, Mr. Nazem holds outstanding options to purchase 167,747 shares. |
| (7) | As of December 31, 2023, Mr. Pande holds outstanding options to purchase 167,747 shares. |
| (8) | As of December 31, 2023, Mr. Posard holds outstanding options to purchase 399,943 shares. |
Item 11. Executive Compensation
To achieve our goals, we have designed, and intend to modify as necessary, our compensation and benefits program to attract, retain, incentivize and reward deeply talented and qualified executives who share our philosophy and desire to work towards achieving these goals.
We believe our compensation program should promote the success of the company and align executive incentives with the long-term interests of our stockholders. As our needs evolve, we intend to continue to evaluate our philosophy and compensation programs as circumstances require.
This section provides an overview of our executive compensation programs, including a narrative description of the material factors necessary to understand the information disclosed in the summary compensation table below. As used in this section, “Nautilus,” the “Company,” “we,” “us” and “our” refer to Legacy Nautilus prior to the closing of the Business Combination and New Nautilus after the closing of the Business Combination.
Our board of directors, with input from our Chief Executive Officer (other than with respect to his own compensation), has historically determined the compensation for our named executive officers. The following table provides information regarding the compensation of our principal executive officer, our Chief Scientist, and our next most highly compensated executive officer who was serving as an executive officer as of December 31, 2023, the end of our last completed fiscal year. We refer to these executive officers in this Annual Report as our named executive officers. For the year ended December 31, 2023, our named executive officers were:
•Sujal Patel, Chief Executive Officer, President, and Secretary;
•Parag Mallick, Chief Scientist; and
•Anna Mowry, Chief Financial Officer and Treasurer.
Summary Compensation Table for the Fiscal Year Ended December 31, 2023
The following table shows the compensation earned by our named executive officers for the fiscal years ended December 31, 2023 and December 31, 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and principal position | | Year | | Salary ($) | | Option awards ($)(1) | | Non-equity incentive plan compensation ($)(2) | | All other compensation ($) | | | Total ($) |
| Sujal Patel | | | | | | | | | | | | | |
| President, Chief Executive Officer and Secretary | | 2023 | | 535,000 | | | 1,065,622 | | | 291,040 | | | 1,200 | | (3) | | 1,892,862 | |
| | 2022 | | 500,000 | | | 1,256,572 | | | — | | | 1,200 | | (3) | | 1,757,772 | |
| Parag Mallick | | | | | | | | | | | | | |
| Chief Scientist | | 2023 | | 420,000 | | | 459,037 | | | 142,800 | | | — | | | | 1,021,837 | |
| | 2022 | | 400,000 | | | 879,600 | | | — | | | — | | | | 1,279,600 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Anna Mowry | | | | | | | | | | | | | |
| Chief Financial Officer and Treasurer | | 2023 | | 386,000 | | | 262,307 | | | 132,012 | | | 1,200 | | (3) | | 781,519 | |
| | 2022 | | 364,000 | | | 879,600 | | | 102,375 | | | 1,200 | | (3) | | 1,347,175 | |
________________________ | | | | | |
| (1) | In accordance with SEC rules, this column reflects the aggregate grant date fair value of the stock option awards granted during 2022 or 2023, computed in accordance with FASB ASC 718. These amounts do not reflect the actual economic value that will be realized by the named executive officer upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options. For a discussion of valuation assumptions, see Note 7 to our audited financial statements included in this Annual Report on Form 10-K for the fiscal year ended December 31, 2023. |
| (2) | These amounts represent performance-based cash bonuses paid under the Incentive Plan (as defined below) for the years ended December 31, 2023 and December 31, 2022, respectively, each of which was paid in the subsequent fiscal year. Each of Mr. Patel and Dr. Mallick voluntarily declined any payments under the Incentive Plan for the year ended December 31, 2022. If Mr. Patel and Dr. Mallick had received cash bonuses under the Incentive Plan based on the same determinations with respect to cash bonuses for our other NEOs, Mr. Patel would have received a payment of $125,000 and Dr. Mallick would have received a payment of $100,000. The Incentive Plan is more fully described below under the section titled “—Non-Equity Incentive Plan Compensation.” |
| (3) | The amounts reported represent $1,200 in parking benefits. |
| |
| |
| |
| |
| |
| |
| |
Equity Incentive Plan Compensation
We maintain the Executive Incentive Compensation Plan (the “Incentive Plan”), which allows our board of directors or compensation committee to grant incentive awards, generally payable in cash, to employees selected by our compensation committee, including our executive officers, based upon performance goals established by our compensation committee. In February 2023, our board of directors approved performance goals under the Incentive Plan for 2023 (the “2023 Bonus Plan”).
The performance goals under the 2023 Bonus Plan applied to each of our named executive officers and consisted of five financial or strategic goals with respect to Nautilus (or “corporate performance goals”) for the 2023 year related to certain profiling milestones, certain instrument and consumable operational and manufacturing readiness, certain commercial engagement, and financial metrics related to cash management. Each corporate performance goal was provided a specific weighting, and the extent of achievement of each such goal was expressed as a percentage (with 100% representing target achievement, provided that achievement could be higher or lower depending on actual performance) determined the payout with respect to the portion of the cash incentive opportunity allocated to such goal. Each of Mr. Patel and Dr. Mallick’s cash incentive opportunities for 2023 were based 100% on the corporate performance goals.
For Ms. Mowry, the 2023 Bonus Plan additionally provided for an individual performance component based on corporate and strategic objectives specific to Ms. Mowry as established by the compensation committee of our board of directors. Ms. Mowry’s total cash incentive opportunity for 2023 was based 75% on achievement of the corporate performance goals described above and 25% on individual performance achievement.
In February 2024, our board of directors completed a review of the Company’s performance against the corporate performance goals under the 2023 Bonus Plan. In its review, our board of directors evaluated the Company’s progress against these corporate performance goals, determining that certain goals were achieved or exceeded, that certain goals were not achieved, and that certain goals were partially achieved. Based upon this evaluation and application of the weightings, our board of directors approved payment of a cash bonus to each of Mr. Patel, Dr. Mallick and Ms. Mowry in amounts that resulted
from 68% achievement of the corporate performance goals under the 2023 Bonus Plan, and, for Ms. Mowry only, in combination with her level of achievement of her individual performance component. Each cash bonus was paid to Mr. Patel, Dr. Mallick and Ms. Mowry in early 2024.
The amounts in the Summary Compensation Table under the column titled “Non-equity incentive plan compensation” for 2023 for each of Mr. Patel and Dr. Mallick is equal to 100% of his target bonus opportunity, multiplied by 68% for the corporate performance component, and for Ms. Mowry are equal to the sum of (i) 75% of her target bonus opportunity, multiplied by 68% for the corporate performance component, and (ii) 25% of her target bonus opportunity allocated to the individual performance component, multiplied by an aggregate achievement of 100% for 2023. For 2023, the cash incentive bonus under the 2023 Bonus Plan for our named executive officers was $291,040 for Mr. Patel, $142,800 for Dr. Mallick, and $132,012 for Ms. Mowry (of which $88,587 was paid out based on achievement of the corporate performance goals and $43,425 was paid out based on achievement of her individual performance goals).
Outstanding Equity Awards at Fiscal Year-End December 31, 2023
The following table sets forth certain information regarding equity awards granted to the named executive officers that remained outstanding as of December 31, 2023. The number of shares subject to each award granted prior to the Business Combination and, where applicable, the exercise price per share, reflect all changes as a result of our capitalization adjustments in connection with the Business Combination.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Option Awards |
| Name | | Grant Date | | Number of securities underlying unexercised options (#) exercisable | | Number of securities underlying unexercised options (#) unexercisable (1) | | Option exercise price ($) (2) | | Option expiration date |
| Sujal Patel | | 1/31/2021 | (3) | | 575,090 | | | 213,643 | | (4) | | 10.00 | | | 1/31/2031 |
| | 2/25/2022 | (5) | | 183,333 | | | 216,667 | | (6) | | 3.78 | | | 2/25/2032 |
| | 2/27/2023 | (5) | | — | | | 650,000 | | (8) | | 2.00 | | | 2/27/2033 |
| Parag Mallick | | 1/31/2021 | (3) | | 338,346 | | | 125,673 | | (4) | | 10.00 | | | 1/31/2031 |
| | 2/25/2022 | (5) | | 128,333 | | | 151,667 | | (6) | | 3.78 | | | 2/25/2032 |
| | 2/27/2023 | (5) | | — | | | 280,000 | | (8) | | 2.00 | | | 2/27/2033 |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Anna Mowry | | 12/3/2020 | (3) | | 319,863 | | | 115,505 | | (7) | | 1.14 | | | 12/3/2030 |
| | 2/25/2022 | (5) | | 128,333 | | | 151,667 | | (6) | | 3.78 | | | 2/25/2032 |
| | 2/27/2023 | (5) | | — | | | 160,000 | | (8) | | 2.00 | | | 2/27/2033 |
________________________
| | | | | |
| (1) | The unvested portions of these awards may be subject to vesting acceleration under certain circumstances, described below under “—Potential Payments upon Termination or Change in Control.” |
| (2) | The stock option awards that were granted by Legacy Nautilus to Mr. Patel, Dr. Mallick and Ms. Mowry prior to the closing of the Business Combination were granted with a per share exercise price equal to the fair market value of one share of Legacy Nautilus Common Stock on the date of grant, as determined in good faith by Legacy Nautilus’ board of directors. The stock option granted following the closing of the Business Combination was granted with a per share exercise price equal to the closing price of the Company’s common stock in trading on the Nasdaq Global Select Market on the date of grant. |
| (3) | Option granted under and subject to the terms of the Legacy Nautilus 2017 Equity Incentive Plan (the “2017 Plan”).” |
| (4) | Twenty-five percent of the shares subject to this option vested on January 31, 2022, and one thirty-sixth (1/36th) of the remaining shares subject to this option shall vest each month thereafter, subject to the optionee continuing to be a service provider to Nautilus through each such date. |
| (5) | Option granted under and subject to the terms of the 2021 Plan. |
| (6) | Twenty-five percent of the shares subject to this option vested on February 25, 2023, and one thirty-sixth (1/36th) of the remaining shares subject to this option shall vest each month thereafter, subject to the optionee continuing to be a service provider to Nautilus through each such date. |
| (7) | 13/49th of the shares subject to this option vested on December 3, 2021, and one forty-ninth (1/49th) of the shares subject to this option shall vest each month thereafter, subject to the optionee continuing to be a service provider to Nautilus through each such date. |
| (8) | Twenty-five percent of the shares subject to this option vested on February 27, 2024, and one thirty-sixth (1/36th) of the remaining shares subject to this option shall vest each month thereafter, subject to the optionee continuing to be a service provider to Nautilus through each such date. |
| |
| |
Employment Arrangements with Named Executive Officers
Sujal Patel
We have entered into an amended and restated confirmatory employment letter with Mr. Patel, our Chief Executive Officer, effective as of July 31, 2023. The confirmatory employment letter has no specific term and provides that Mr. Patel is an at-will employee. The confirmatory employment letter superseded any prior agreements and understandings that Mr. Patel may have entered into concerning his employment relationship with us. For 2023, Mr. Patel’s annual base salary was $535,000 and he was eligible for a target annual cash bonus opportunity equal to 80% of his annual base salary. On February 13, 2024, our board of directors, upon recommendation of the compensation committee, determined not to adjust Mr. Patel’s base salary for 2024 and approved an increase in Mr. Patel’s cash bonus opportunity for 2024 to 85% of his annual base salary, effective retroactive to January 1, 2024.
Parag Mallick
We have entered into an amended and restated confirmatory employment letter with Dr. Mallick, our Chief Scientist, effective as of July 31, 2023. The confirmatory employment letter has no specific term and provides that Dr. Mallick is an at-will employee. The confirmatory employment letter superseded any prior agreements and understandings that Dr. Mallick may have entered into concerning his employment relationship with us. For 2023, Dr. Mallick’s annual base salary was $420,000 and he was eligible for a target annual cash bonus opportunity equal to 50% of his annual base salary. On February 13, 2024, our board of directors, upon recommendation of the compensation committee, determined not to adjust Dr. Mallick’s base salary for 2024 and approved an increase in Dr. Mallick’s target annual cash bonus opportunity for 2024 to 55% of his annual base salary, effective retroactive to January 1, 2024.
Anna Mowry
We have entered into an amended and restated confirmatory employment letter with Ms. Mowry, our Chief Financial Officer, effective as of July 31, 2023. The confirmatory employment letter has no specific term and provides that Ms. Mowry is an at-will employee. The confirmatory employment letter superseded any prior agreements and understandings that Ms. Mowry may have entered into concerning her employment relationship with us. For 2023, Ms. Mowry’s annual base salary was $386,000 and she was eligible for a target annual cash bonus opportunity equal to 45% of her annual base salary. On February 13, 2024, our board of directors, upon recommendation of the compensation committee, approved an increase in Ms. Mowry’s base salary to $400,000, and an increase in Ms. Mowry’s target annual cash bonus opportunity for 2024 to 50% of her annual base salary, both effective retroactive to January 1, 2024.
Equity Based Incentive Awards
Our equity-based incentive awards are designed to more closely align the interests of our stockholders with those of our employees and consultants, including our named executive officers. Our board of directors is responsible for approving equity grants to our employees and consultants, including our named executive officers. In 2023, stock option awards were the only form of equity awards we granted to our named executive officers. Following the closing of the Business Combination, we have granted equity incentive awards under the terms of our 2021 Equity Incentive Plan (the “2021 Plan”). Options under the 2021 Plan are granted with a per share exercise price equal to the closing price of a share of the Company’s common stock in trading on the Nasdaq Global Select Market on the date of grant.
Our stock option awards generally vest over a four-year period and may be subject to acceleration of vesting and exercisability under certain termination and change in control events. See “—Outstanding Equity Awards at Fiscal Year-End December 31, 2023” and “—Potential Payments Upon Termination or Change of Control.”
Potential Payments Upon Termination or Change of Control
Regardless of the manner in which a named executive officer’s service terminates, that named executive officer is entitled to receive amounts earned during his or her term of service, including unpaid salary and unused vacation, as applicable.
Each of our named executive officers holds stock options granted subject to the general terms of the 2017 Plan or the 2021 Plan. Each such equity incentive plan generally provides that, in the event of a change in control (as defined in each such plan), equity awards will be treated as the administrator of such plan determines, provided that, in the event that the successor corporation does not assume or substitute for an equity award (or portion thereof), then the equity award (or portion thereof) not assumed or substituted for will fully vest and become fully exercisable and, with respect to awards subject to performance-based vesting, the performance goals will be deemed achieved at target levels, unless for equity awards granted under the 2021 Plan, such treatment is specifically provided otherwise by written agreement.
Each named executive officer is party to an amended and restated change in control and severance agreement (each, a “CIC Agreement”) entered into with us and Nautilus Subsidiary, Inc. (the “Employer”) in July 2023, which provides for certain severance and change in control benefits.
The Agreements replaced and superseded any prior agreements or arrangements providing such named executive officers with severance or change in control payments and benefits, including replacing and superseding the change in control and severance agreements previously entered into with such named executive officers.
The CIC Agreements have an initial term of three years commencing on July 31, 2023. On the three-year anniversary of the effective date of each CIC Agreement, the agreement will renew automatically for additional one year terms unless either party provides the other party with written notice of nonrenewal at least ninety (90) days prior to the date of automatic renewal. However, if a change in control (as defined in the applicable CIC Agreement) occurs when there are fewer than twelve months remaining during the initial term or during an additional term, the term of the CIC Agreement will extend automatically through the date that is twelve months following the date of the change in control.
The CIC Agreements provide that if, other than during the period beginning three months before a change in control through the one-year anniversary of a change in control, or the CIC Period, either (x) the named executive officer’s employment is terminated without cause (as defined in the CIC Agreement, and excluding by reason of his or her death or disability) or (y) the named executive officer resigns for good reason (as defined in the CIC Agreement), then the named executive officer will receive the following severance payments and benefits:
•A lump sum cash payment equal to 100% for Mr. Patel, or 50% for Dr. Mallick and Ms. Mowry, of the named executive officer’s base salary as in effect immediately before such termination; and
•Payment by us (or our applicable subsidiary) of the employer-paid portion of the premiums required for continued coverage pursuant to COBRA under the Employer’s group health, dental and vision care plans for the named executive officer and his or her eligible dependents for up to twelve months for Mr. Patel, or six months for Dr. Mallick and Ms. Mowry.
If, during the CIC Period, either (x) the named executive officer’s employment is terminated without cause (as defined in the CIC Agreement, and excluding by reason of his or her death or disability) or (y) the named executive officer resigns for good reason (as defined in the CIC Agreement), the named executive officer will receive the following severance payments and benefits:
•A lump sum cash payment equal to 150% for Mr. Patel, or 100% for Dr. Mallick and Ms. Mowry, of the named executive officer’s base salary as in effect immediately before such termination or if greater, the base salary in effect immediately before the change in control;
•A lump sum cash payment equal to 150% for Mr. Patel, or 100% for Dr. Mallick and Ms. Mowry, of the named executive officer’s target bonus opportunity as in effect immediately before such termination or if greater, the target bonus opportunity in effect immediately before the change in control;
•Payment by us (or our applicable subsidiary) of the employer-paid premiums required for continued coverage pursuant to COBRA under our (or our applicable subsidiary’s) group health, dental and vision care plans for the named executive officer and his or her eligible dependents for up to eighteen months for Mr. Patel, or twelve months for Dr. Mallick and Ms. Mowry; and
•100% accelerated vesting and exercisability of the outstanding and unvested equity awards (other than equity awards subject to performance-based vesting criteria) granted to the named executive officer.
Any severance payments and benefits under a CIC Agreement are subject to the named executive officer timely executing and not revoking a separation agreement and release of claims in favor of the Company and any of its subsidiaries (the “Company Group”). Each CIC Agreement provides that, if any of the amounts provided for under a CIC Agreement or otherwise payable to the named executive officer would constitute “parachute payments” within the meaning of Internal Revenue Code Section 280G and could be subject to the related excise tax, the named executive officer would receive (to the extent he or she is entitled to such receipt) either the full payment of benefits under the named executive officer’s CIC Agreement or such lesser amount that would result in no portion of the payments and benefits being subject to the excise tax, whichever results in the greater amount of after-tax benefits to the named executive officer. The CIC Agreements do not provide for any tax gross-ups in connection with a change in control.
The following definitions generally apply for purposes of each CIC Agreement:
•“cause” generally means (a) a named executive officer’s failure to substantially perform his or her material duties and obligations as an employee (for reasons other than a named executive officer’s death or disability); (b) a named executive officer’s failure or refusal to comply with the policies, standards and regulations established by the Company Group from time to time; (c) any act of personal dishonesty, moral turpitude, fraud, embezzlement, misrepresentation, or other unlawful act committed by a named executive officer that results in harm to the Company or any other member of the Company Group, or any of their respective affiliates, including financial or reputational; (d) a named executive officer’s violation of a federal or state law or regulation applicable to the business of the Company or any other member of the Company Group, or any of their respective affiliates; (e) a named executive officer being convicted of, or entering a plea of nolo contendere or guilty to, a felony under the laws of the United States or its equivalent in the jurisdiction in which the act that constituted the felony occurred; (f) a named executive officer’s material breach of the terms of his or her applicable CIC Agreement or any other agreement between a named executive officer and any member of the Company Group (or any affiliate of the Company Group); or (g) the Company’s or the Employer’s economic duress or necessity. With respect to clauses (a) and (b) above only, a named executive officer will have 10 days to cure following written notice of a named executive officer’s failure or refusal to perform or comply.
•“change in control” generally means the first occurrence of any of the following events on or after the Effective Date:
◦A change in the ownership of the Company which occurs on the date that any one person, or more than one person acting as a group (“Person”), acquires ownership of the stock of the Company that, together with the stock held by such Person, constitutes more than 50% of the total voting power of the stock of the Company; provided, however, the acquisition of additional stock by any one Person, who is considered to own more than 50% of the total voting power of the stock of the Company will not be considered a change in control; provided, further, that any change in the ownership of the stock of the Company as a result of a private financing of the Company that is approved by our board of directors also will not be considered a change in control. Further, if the stockholders of the Company immediately before such change in ownership continue to retain immediately after the change in ownership, in substantially the same proportions as their ownership of shares of the Company’s voting stock immediately prior to the change in ownership, direct or indirect beneficial ownership of 50% or more of the total voting power of the stock of the Company or of the ultimate parent entity of the Company, such event shall not be considered a change in control.
◦If the Company has a class of securities registered pursuant to Section 12 of the U.S. Securities Exchange Act of 1934, as amended, a change in the effective control of the Company which occurs on the date that a majority of members of the board of directors is replaced during any 12 month period by directors whose appointment or election is not endorsed by a majority of the members of the board of directors prior to the date of the appointment or election.
◦A change in the ownership of a substantial portion of the Company’s assets which occurs on the date that any Person acquires (or has acquired during the 12 month period ending on the date of the most recent acquisition by such Person or Persons) assets from the Company that have a total gross fair market value equal to or more than 50% of the total gross fair market value of all of the assets of the Company immediately prior to such acquisition or acquisitions; provided, however, that the following will not constitute a change in the ownership of a substantial portion of the Company’s assets: (a) a transfer to an entity that is controlled by the Company’s stockholders immediately after the transfer, or (b) a transfer of assets by the Company to: (i) a stockholder of the Company (immediately before the asset transfer) in exchange for or with respect to the Company’s stock, (ii) an entity, 50% or more of the total value or voting power of which is owned, directly or indirectly, by the Company, (iii) a Person, that owns, directly or indirectly, 50% or more of the total value or voting power of all the outstanding stock of the Company, or (iv) an entity, at least 50% of the total value or voting power of which is owned, directly or indirectly, by a Person previously described in this definition.
•“disability” generally means total and permanent disability as defined in Section 22(e)(3) of the Internal Revenue Code.
•“good reason” generally means a named executive officer’s termination of his or her employment with the Company Group within 90 days following the expiration of the Company’s cure period (as defined below) following the occurrence of any of the following without a named executive officer’s written consent: (a) a material reduction in a named executive officer’s responsibilities, provided that neither a mere change in title nor reassignment following a change in control to a position that is substantially similar to the position held prior to the change in control will constitute a material reduction in job responsibilities; (b) relocation of a named executive officer’s principal work location to a principal work location more than 40 miles from a named executive officer’s principal work location immediately before such relocation; or (c) a reduction in a named executive officer then current base salary by at least 10%, provided that an across-the-board reduction in the salary level
of all other similarly situated employees by the same percentage amount as part of a general salary level reduction will not constitute such a reduction under this clause (c). In order for an event to qualify as good reason, a named executive officer must not terminate his or her employment without first providing the Company Group with written notice of the acts or omissions constituting the grounds for “good reason” within 90 days following the initial existence of the grounds for “good reason” and a cure period of 30 days following the date of such notice (the “cure period”).
Executive Incentive Compensation Plan
On June 9, 2021, we adopted the Incentive Plan. The Incentive Plan is administered by our board of directors or a committee appointed by our board of directors. The Incentive Plan allows us to grant incentive awards, generally payable in cash, to employees selected by the administrator, including our executive officers, based upon any performance goals that may be established by the administrator.
The administrator of the Incentive Plan, in its sole discretion and at any time prior to the actual payment of an award, may increase, reduce or eliminate a participant’s actual award, and/or increase, reduce or eliminate the amount allocated to any bonus pool for a particular performance period. The administrator may determine any such increase, reduction, or elimination on the basis of such factors as it deems relevant, and the administrator is not required to establish any allocation or weighting with respect to the factors it considers. Actual awards generally will be paid in cash (or its equivalent) only after they are earned, and, unless otherwise determined by the administrator, a participant must be employed with us through the date the actual award is paid. The administrator of the Incentive Plan may settle an actual award with a grant of an equity award under its then-current equity compensation plan, which equity award may have such terms and conditions, including vesting, as determined by the administrator. Payment of awards occurs as soon as administratively practicable after they are earned, but no later than the dates set forth in the Incentive Plan.
Awards under the Incentive Plan are subject to any clawback policy we or our parent or subsidiary corporations may establish or amend from time to time to comply with applicable laws, including without limitation, the listing standards of any national securities exchange or association on which our securities are listed. The administrator also may impose such other clawback, reduction, recovery, forfeiture, recoupment, reimbursement or reacquisition provisions with respect an award under the Incentive Plan as the administrator determines necessary or appropriate, including for example, reduction, cancellation, forfeiture or recoupment upon a termination of a participant’s status as an employee or other service provider for cause, or any specified action or inaction occurring before or after such termination of employment or other service, that would constitute cause for termination of a participant’s status as an employee or other service provider. Certain participants may be required to reimburse us for certain amounts paid under an award under the Incentive Plan in connection with certain accounting restatements we may be required to prepare due to our material noncompliance with any financial reporting requirements under applicable securities laws, as a result of misconduct.
The administrator of the Incentive Plan has the authority to amend, alter, suspend or terminate the Incentive Plan or any part of the Incentive Plan, at any time and for any reason, provided such action does not materially alter or materially impair the existing rights or obligations of any participant with respect to any earned awards. The Incentive Plan will remain in effect until terminated in accordance with its terms.
Compensation Recovery Policy
We have adopted a compensation recovery policy, effective as of October 2, 2023, that complies with the new SEC rules under the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Clawback Policy”). Subject to the terms of the Clawback Policy, the Clawback Policy requires us to recover certain cash or equity-based incentive compensation payments or awards made or granted to an executive officer in the event we are required to prepare an accounting restatement due to our material noncompliance with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
Benefits and Perquisites
We provide benefits to our executive officers on the same basis as provided to all of our employees, including health, dental and vision insurance; life insurance; accidental death and dismemberment insurance; short-and long-term disability insurance; a flexible spending account; and a tax-qualified Section 401(k) plan for which no match is provided by us. We do not maintain any executive-specific benefit or perquisite programs.
Retirement Benefits
We maintain a 401(k) retirement savings plan, for the benefit of employees, including our executive officers, who satisfy certain eligibility requirements. The 401(k) plan provides eligible employees with an opportunity to save for retirement on a tax-advantaged basis. Under the 401(k) plan, eligible employees may elect to defer a portion of their compensation, within the limits prescribed by the Internal Revenue Code and the applicable limits under the 401(k) plan, on a pre-tax or after-tax (Roth) basis, through contributions to the 401(k) plan. All of a participant’s contributions into the 401(k) plan are 100% vested when contributed. The 401(k) plan is intended to qualify under Sections 401(a) and 501(a) of the Internal Revenue Code. As a tax-qualified retirement plan, pre-tax contributions to the 401(k) plan and earnings on those pre-tax contributions are not taxable to the employees until distributed from the 401(k) plan, and earnings on Roth contributions are not taxable when distributed from the 401(k) plan. We do not provide a match for participants’ elective contributions to the 401(k) plan, nor do we provide to employees, including our executive officers, any other retirement benefits, including without limitation any tax-qualified defined benefit plans, supplemental executive retirement plans and nonqualified defined contribution plans.
Legacy Nautilus 2017 Equity Incentive Plan
The 2017 Plan allowed Legacy Nautilus to provide incentive stock options, within the meaning of Section 422 of the Code, nonstatutory stock options, stock appreciation rights, restricted stock awards and restricted stock units (each, an “award” and the recipient of such award, a “participant”) to any of Legacy Nautilus’ eligible employees, directors, and consultants of and any parent or subsidiary of Legacy Nautilus. The 2017 Plan was terminated as of one day prior to the closing of the Business Combination and we will not grant any additional awards under the 2017 Plan. However, the 2017 Plan will continue to govern the terms and conditions of the outstanding awards previously granted under the 2017 Plan and assumed by us at the closing of the Business Combination.
As of December 31, 2023, stock options covering 5,678,766 shares of Common Stock were outstanding under the 2017 Plan.
Purposes of the 2017 Plan
The purposes of the 2017 Plan were to attract and retain personnel for positions with us, any parent or subsidiary; to provide additional incentive to employees, directors, and consultants; and to promote the success of our business. These incentives were provided through the grant of equity awards, including stock options, as the administrator of the 2017 Plan determined.
Eligibility. The 2017 Plan provided for the grant of incentive stock options, within the meaning of Section 422 of the Code, to Legacy Nautilus’ employees and any of its parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, restricted stock units, and stock appreciation rights to any of Legacy Nautilus’ employees, directors and consultants and any of its parents or subsidiaries.
Plan Administration. The 2017 Plan is administered by our board of directors or one or more of its committees. Different committees may administer the 2017 Plan with respect to different service providers. The administrator has all authority and discretion necessary or appropriate to administer the 2017 Plan and to control its operation, including the authority to construe and interpret the terms of the 2017 Plan and the awards granted under the 2017 Plan. The administrator’s decisions are final and binding on all participants and any other persons holding awards.
The administrator’s powers under the 2017 Plan include the power to determine the fair market value of stock subject to awards granted under the 2017 Plan, select the service providers to whom awards may be granted, determine the number of shares covered by each award, approve forms of award agreements for use under the 2017 Plan, determine the terms and conditions of awards (including, but not limited to, the exercise price, the time or times at which awards may be exercised, any vesting acceleration or waiver or forfeiture restrictions, and any restriction or limitation regarding any award or the shares relating thereto), prescribe, amend and rescind rules relating to the 2017 Plan including creating sub-plans, modify or amend each award, and allow a participant to defer the receipt of payment of cash or the delivery of shares that otherwise would be due to such participant under an award. The administrator’s powers also include the power to institute an exchange program under which (i) outstanding awards are surrendered or cancelled in exchange for awards of the same type (which may have higher or lower exercise prices and different terms), awards of a different type and/or cash, (ii) participants would have the opportunity to transfer any outstanding awards to a financial institution or other person or entity selected by the administrator or (iii) the exercise price of an outstanding award is reduced or increased.
Stock Options. Stock options have been granted under the 2017 Plan. The exercise price of options granted under the 2017 Plan generally must be equal to at least 100% of the fair market value of a share of our Common Stock on the date of grant. The term of an option may not exceed ten years. With respect to any participant who owns more than 10% of the voting power of all
classes of our (or any of its parent’s or subsidiary’s) outstanding stock, the term of an incentive stock option granted to such participant must not exceed five years and the per share exercise price must equal at least 110% of the fair market value of a share of our Common Stock on the grant date. The administrator determines the methods of payment of the exercise price of an option, which may include cash, certain shares of our Common Stock, cashless exercise, net exercise, as well as other types of consideration permitted by applicable law. After the cessation of service of an employee, director or consultant, he or she may exercise his or her option for the period of time stated in his or her option agreement; provided generally that if such cessation is due to death or disability, the option will remain exercisable for at least six months and in all other cases, for at least 30 days, following the cessation of service. An option, however, may not be exercised later than the expiration of its term. Subject to the provisions of the 2017 Plan, the administrator determines the terms of options.
Non-Transferability of Awards. Unless the administrator provides otherwise, the 2017 Plan generally will not allow for the transfer of awards other than by will or the laws of descent and distribution, and only the recipient of an award may exercise an award during his or her lifetime. If the administrator makes an award transferable, such award will contain such additional terms and conditions as the administrator deems appropriate, subject to the limitations of the 2017 Plan.
Certain Adjustments. If any dividend or other distribution (whether in cash, shares, other securities, or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase, or exchange of shares or other of our securities, issuance of warrants or other rights to acquire our securities, other change in our corporate structure affecting the shares, or any similar equity restructuring transaction affecting the shares occurs, the administrator of the 2017 Plan, to prevent diminution or enlargement of the benefits or potential benefits intended to be provided under the 2017 Plan, will adjust the number and class of shares that may be delivered under the 2017 Plan and the number, class, and price of shares covered by each outstanding award. In the case of awards issued to California residents, the administrator will make such adjustments to an award required by Section 25102(o) of the California Corporations Code to the extent Nautilus is relying upon the exemption afforded thereby with respect to the award.
Dissolution or Liquidation. If there is a proposed liquidation or dissolution of our Company, the administrator will notify participants at such time before the effective date of such event as the administrator determines and all awards, to the extent that they have not been previously exercised, will terminate immediately before the consummation of such event.
Merger or Change or Control. The 2017 Plan provides that in the event of our merger or change in control, as defined in the 2017 Plan, each outstanding award will be treated as the administrator determines, without a participant’s consent. The administrator may provide that awards granted under the 2017 Plan will be assumed or substituted by substantially equivalent awards, be terminated immediately before the merger or change in control, become vested and exercisable or payable and be terminated in connection with the merger or change in control, be terminated in exchange for cash, other property or other consideration or any combination of the above. The administrator is not required to treat all awards, all awards held by a participant, or all awards of the same type, similarly.
In the event that the successor corporation does not assume or substitute for an award (or portion thereof), the participant will fully vest in and have the right to exercise all of his or her outstanding options and stock appreciation rights, including shares as to which such awards would not otherwise be vested or exercisable, all restrictions on restricted stock and restricted stock units will lapse, and, with respect to awards with performance-based vesting, all performance goals or other vesting criteria will be deemed achieved at 100% of target levels and all other terms and conditions met. In addition, if an option or stock appreciation right is not assumed or substituted in the event of a merger or change in control, the administrator will notify the participant in writing or electronically that the option or stock appreciation right will be exercisable for a period of time determined by the administrator in its sole discretion, and the option or stock appreciation right will terminate upon the expiration of such period.
Amendment and Termination. As noted above, as of the day immediately prior to the closing of the Business Combination, the 2017 Plan terminated and we will not grant any additional awards under the 2017 Plan.
2021 Equity Incentive Plan
The following paragraphs provide a summary of the principal features of our 2021 Plan and its operation. However, this summary is not a complete description of all of the provisions of the 2021 Plan and is qualified in its entirety by the specific language of the 2021 Plan.
As of December 31, 2023, stock options covering 8,997,569 shares of Common Stock were outstanding under the 2021 Plan.
Purposes of the 2021 Plan
The purposes of the 2021 Plan are to attract and retain personnel for positions with us or any parent or subsidiary of ours; to provide additional incentive to employees, directors, and consultants; and to promote the success of our business. These incentives are provided through the grant of stock options, stock appreciation rights, restricted stock, restricted stock units, and performance awards as the administrator of the 2021 Plan may determine.
Eligibility
The 2021 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and any of its parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights and performance awards to our employees, directors and consultants of and employees and consultants of any of our parents or subsidiaries.
Authorized Shares
Subject to the adjustment provisions contained in the 2021 Plan and the evergreen provision described below, a total of 16,182,600 shares of our Common Stock were initially reserved for issuance pursuant to the 2021 Plan. In addition, the shares reserved for issuance under the 2021 Plan include any shares of our Common Stock subject to awards of stock options or other awards that were assumed in the Business Combination (or “assumed awards”) that, on or after the effective date of the Business Combination, are terminated, canceled, expire or otherwise terminate without having been exercised in full, are tendered to or withheld by us for payment of an exercise price or for tax withholding obligations, or are forfeited to or repurchased by us due to failure to vest (provided that the maximum number of shares that may be added to the 2021 Plan pursuant to this sentence is 7,500,000 shares). The number of shares available for issuance under the 2021 Plan also includes an annual increase, or the evergreen feature, on the first day of each of our fiscal years, beginning with our fiscal year 2022, equal to the least of:
•18,672,200 shares of our Common Stock;
•a number of shares equal to 5% of the outstanding shares of our Common Stock as of the last day of the immediately preceding fiscal year; or
•such number of shares as our board of directors or its designated committee may determine no later than the last day of our immediately preceding fiscal year.
On January 1, 2024, the number of shares available under the 2021 Plan increased by 6,253,430 shares pursuant to this feature.
Shares issuable under the 2021 Plan will be authorized, but unissued, or reacquired shares of our Common Stock. If an award expires or becomes unexercisable without having been exercised in full, is surrendered pursuant to an exchange program (as described below), or, with respect to restricted stock, restricted stock units, or performance awards, is forfeited to or repurchased due to failure to vest, the unpurchased shares (or for awards other than stock options or stock appreciation rights, the forfeited or repurchased shares) will become available for future grant or sale under the 2021 Plan. With respect to stock appreciation rights, only the net shares actually issued will cease to be available under the 2021 Plan and all remaining shares under stock appreciation rights will remain available for future grant or sale under the 2021 Plan. Shares that actually have been issued under the 2021 Plan under any award will not be returned to the 2021 Plan; except if shares issued pursuant to awards of restricted stock, restricted stock units, or performance awards are repurchased or forfeited, such shares will become available for future grant under the 2021 Plan. Shares used to pay the exercise price of an award or satisfy the tax liabilities or withholding obligations related to an award (which withholdings may be in amounts greater than the minimum statutory amount required to be withheld as determined by the administrator of the 2021 Plan) will become available for future grant or sale under the 2021 Plan. To the extent an award is paid out in cash rather than shares, such cash payment will not result in a reduction in the number of shares available for issuance under the 2021 Plan.
If any dividend or other distribution (whether in cash, shares, other securities, or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, reclassification, repurchase, or exchange of our shares or other securities, issuance of warrants or other rights to acquire our securities, other change in our corporate structure affecting the shares, or any similar equity restructuring transaction affecting our shares occurs (other than any ordinary dividends or other ordinary distributions), the administrator of the 2021 Plan, to prevent diminution or enlargement of the benefits or potential benefits intended to be provided under the 2021 Plan, will adjust the number and class of shares that may be delivered under the 2021 Plan; the number, class, and price of shares covered by each outstanding award; and the numerical share limits contained in the 2021 Plan.
Plan Administration
Our board of directors or one or more committees appointed by our board of directors has authority to administer the 2021 Plan. The compensation committee of our board of directors initially will administer the 2021 Plan. In addition, to the extent it is desirable to qualify transactions under the 2021 Plan as exempt under Rule 16b-3 of the Exchange Act, such transactions will be structured to satisfy the requirements for exemption under Rule 16b-3. Subject to the provisions of the 2021 Plan, the administrator has the power to administer the 2021 Plan and make all determinations deemed necessary or advisable for administering the 2021 Plan, including but not limited to, the power to determine the fair market value of our Common Stock, select the service providers to whom awards may be granted, determine the number of shares or dollar amounts covered by each award, approve forms of award agreements for use under the 2021 Plan, determine the terms and conditions of awards (including, but not limited to, the exercise price, the time or times at which awards may be exercised, any vesting acceleration or waiver or forfeiture restrictions and any restriction or limitation regarding any award or the shares relating thereto), construe and interpret the terms of the 2021 Plan and awards granted under it, prescribe, amend and rescind rules relating to the 2021 Plan, including creating sub-plans, modify or amend each award, and allow a participant to defer the receipt of payment of cash or the delivery of shares that otherwise would be due to such participant under an award. The administrator also has the authority to allow participants the opportunity under an exchange program to transfer outstanding awards granted under the 2021 Plan to a financial institution or other person or entity selected by the administrator, and to institute an exchange program by which outstanding awards granted under the 2021 Plan may be surrendered or cancelled in exchange for awards of the same type, which may have a higher or lower exercise price and/or different terms, awards of a different type and/or cash, or by which the exercise price of an outstanding award granted under the 2021 Plan is increased or reduced. The administrator’s decisions, interpretations and other actions are final and binding on all participants and will be given the maximum deference permitted by applicable law.
Stock Options
Stock options may be granted under the 2021 Plan. The exercise price of options granted under the 2021 Plan generally must be equal to at least 100% of the fair market value of a share of our Common Stock on the date of grant. The term of an option may not exceed ten years. With respect to any participant who owns more than 10% of the voting power of all classes of our (or any of our parent’s or subsidiary’s) outstanding stock, the term of an incentive stock option granted to such participant must not exceed five years and the per share exercise price must equal at least 110% of the fair market value of a share of our Common Stock on the grant date. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, certain shares of our Common Stock, cashless exercise, net exercise, as well as other types of consideration permitted by applicable law. After the cessation of service of an employee, director or consultant, he or she may exercise his or her option for the period of time stated in his or her option agreement. In the absence of a specified time in an award agreement, if such cessation is due to death or disability, the option will remain exercisable for six months. In all other cases, in the absence of a specified time in an award agreement, the option will remain exercisable for three months following the cessation of service. An option, however, may not be exercised later than the expiration of its term. Subject to the provisions of the 2021 Plan, the administrator determines the terms of options.
Stock Appreciation Rights
Stock appreciation rights may be granted under the 2021 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our Common Stock between the exercise date and the date of grant. The term of a stock appreciation right may not exceed ten years. After the cessation of service of an employee, director or consultant, he or she may exercise his or her stock appreciation right for the period of time stated in his or her stock appreciation rights agreement. In the absence of a specified time in an award agreement, if such cessation is due to death or disability, the stock appreciation rights will remain exercisable for six months. In all other cases, in the absence of a specified time in an award agreement, the stock appreciation rights will remain exercisable for three months following the cessation of service. However, in no event may a stock appreciation right be exercised later than the expiration of its term. Subject to the provisions of the 2021 Plan, the administrator determines the terms of stock appreciation rights, including when such rights become exercisable and whether to pay any increased appreciation in cash or with shares of our Common Stock, or a combination of both, except that the per-share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right generally will be no less than 100% of the fair market value per share on the date of grant.
Restricted Stock
Restricted stock may be granted under the 2021 Plan. Restricted stock awards are grants of shares of our Common Stock that may have vesting requirements under any such terms and conditions established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee, director or consultant and, subject to the provisions of the 2021 Plan, will determine the terms and conditions of such awards. The administrator may impose whatever vesting conditions (if any) it determines to be appropriate (for example, the administrator may set restrictions based on the achievement of specific performance goals or continued service to us). The administrator, in its sole discretion, may accelerate
the time at which any restrictions will lapse or be removed. The administrator may determine that an award of restricted stock will not be subject to any period of restriction and consideration for such award is paid for by past services rendered as a service provider. Recipients of restricted stock awards generally will have voting and dividend rights with respect to such shares upon grant, unless the administrator provides otherwise. If such dividends are paid in shares, the shares will be subject to the same restrictions on transferability and forfeitability as the share of restricted stock with respect to which they were paid. Shares of restricted stock that do not vest are subject to the right of repurchase or forfeiture.
Restricted Stock Units
Restricted stock units (or “RSUs”) may be granted under the 2021 Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one share of our Common Stock. Subject to the provisions of the 2021 Plan, the administrator determines the terms and conditions of restricted stock units, including any vesting criteria and the form and timing of payment. The administrator may set vesting criteria based upon the achievement of company-wide, divisional, business unit, or individual goals (including, but not limited to, continued employment or service), applicable federal or state securities laws or any other basis determined by the administrator in its discretion. The administrator, in its sole discretion, may pay earned restricted stock units in the form of cash, shares, or a combination of both. Notwithstanding the foregoing, the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Performance Awards
Performance awards may be granted under the 2021 Plan. Performance awards are awards that may be earned in whole or in part on the attainment of performance goals or other vesting criteria that the administrator may determine, and that may be denominated in cash or stock. Each performance award will have an initial value that is determined by the administrator. Subject to the terms and conditions of the 2021 Plan, the administrator determines the terms and conditions of performance awards, including any vesting criteria and form and timing of payment. The administrator may set vesting criteria based upon the achievement of company-wide, divisional, business unit, or individual goals (including, but not limited to, continued employment or service), applicable federal or state securities laws or any other basis determined by the administrator in its discretion. The administrator, in its sole discretion, may pay earned performance awards in the form of cash, shares, or a combination of both. Notwithstanding the foregoing, the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Non-Employee Directors
All outside (non-employee) directors are eligible to receive all types of awards (except for incentive stock options) under the 2021 Plan. The 2021 Plan provides that in any given fiscal year, no outside director may be granted any equity awards (including equity awards under the 2021 Plan) (the value of which will be based on their grant date fair value) and be provided any other compensation (including without limitation any cash retainers and fees) that in the aggregate exceed $750,000, provided that in the fiscal year of the individual’s initial service as a non-employee director, such amount is increased to $1,000,000. For the purposes of this maximum limit provision, the grant date fair values of awards granted under the 2021 Plan will be determined according to U.S. GAAP. Any awards or other compensation provided to an individual for his or her services as an employee or a consultant (other than an outside director), will not count toward this limit. This maximum limit provision does not reflect the intended size of any potential grants or a commitment to make grants to the outside directors under the 2021 Plan in the future.
Non-Transferability of Awards
Unless the administrator provides otherwise, the 2021 Plan generally will not allow for the transfer of awards other than by will or the laws of descent and distribution, and only the recipient of an award may exercise an award during his or her lifetime. If the administrator makes an award transferable, such award will contain such additional terms and conditions as the administrator deems appropriate.
Dissolution or Liquidation
If there is a proposed liquidation or dissolution of our company, the administrator will notify participants at such time before the effective date of such event as the administrator determines and all awards, to the extent that they have not been previously exercised, will terminate immediately before the consummation of such event.
Merger or Change in Control
The 2021 Plan provides that in the event of a merger or change in control, as defined in the 2021 Plan, each outstanding award will be treated as the administrator determines, without a participant’s consent. The administrator may provide that awards granted under the 2021 Plan will be assumed or substituted by substantially equivalent awards, be terminated
immediately before the merger or change in control, become vested and exercisable or payable and be terminated in connection with the merger or change in control, be terminated in exchange for cash, other property or other consideration or any combination of the above. The administrator is not required to treat all awards, all awards held by a participant, all portions of awards, or all awards of the same type, similarly.
If a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award (or a portion of such award), then such award (or its applicable portion) will fully vest, all restrictions on such award (or its applicable portion) will lapse, all performance goals or other vesting criteria applicable to such award (or its applicable portion) will be deemed achieved at 100% of target levels and such award (or its applicable portion) will become fully exercisable, if applicable, for a specified period before the transaction, unless specifically provided otherwise under the applicable award agreement or other written agreement authorized by the administrator with the participant. The award (or its applicable portion) will then terminate upon the expiration of the specified period of time. If an option or stock appreciation right is not assumed or substituted, the administrator will notify the participant that such option or stock appreciation right will be exercisable for a period of time determined by the administrator in its sole discretion and the option or stock appreciation right will terminate upon the expiration of such period.
If awards granted to a non-employee director while such individual was a non-employee director are assumed or substituted for in the merger or change in control and the service of such non-employee director is terminated (other than upon his or her voluntary resignation that does not include a resignation at the request of the acquirer) on or following the merger or change in control, all such awards will fully vest, all restrictions on such awards will lapse, all performance goals or other vesting criteria applicable to such awards will be deemed achieved at 100% of target levels and such awards will become fully exercisable, if applicable, unless specifically provided otherwise under the applicable award agreement or other written agreement authorized by the administrator with the non-employee director.
Forfeiture and Clawback
Awards will be subject to any clawback policy of which we are required to adopt to comply with the listing standards of any national securities exchange or association on which our securities are listed or as is otherwise required by applicable laws. The administrator also may specify in an award agreement that the participant’s rights, payments and benefits with respect to an award will be subject to reduction, cancellation, forfeiture or recoupment upon the occurrence of certain specified events. The administrator may require a participant to forfeit, return or reimburse us for all or a portion of the award and any amounts paid under the award in order to comply with any of our clawback policies as described in the first sentence of this paragraph or with applicable laws.
Amendment or Termination
The 2021 Plan became effective on June 9, 2021 and will continue in effect until terminated by the administrator. However, no incentive stock options may be granted after the ten year anniversary of the adoption of the 2021 Plan by our board of directors, and the evergreen feature of the 2021 Plan will terminate following the increase on the first day of the 2031 fiscal year. In addition, the administrator has the authority to amend, suspend, or terminate the 2021 Plan or any part of the 2021 Plan, at any time and for any reason, but such action generally may not materially impair the rights of any participant without his or her written consent.
2021 Employee Stock Purchase Plan
The following is a summary of the principal features of our 2021 Employee Stock Purchase Plan (the “ESPP”) and its operation.
Purpose
The purpose of the ESPP is to provide eligible employees with an opportunity to purchase shares of our Common Stock through accumulated contributions, which generally will be made through payroll deductions. The ESPP permits the administrator of the ESPP to grant purchase rights that qualify for preferential tax treatment under Section 423 of the Code. In addition, the ESPP authorizes the grant of purchase rights that do not qualify under Code Section 423 pursuant to rules, procedures or sub-plans adopted by the administrator that are designed to achieve desired tax or other objectives.
Shares Available for Issuance
The maximum number of shares of our Common Stock that are available for issuance under the ESPP was initially 1,244,900 shares of our Common Stock. The number of shares of our Common Stock available for issuance under the ESPP will be increased on the first day of each fiscal year beginning with the 2022 fiscal year in an amount equal to the least of (a) 3,734,500 shares of our Common Stock, (b) a number of shares of our Common Stock equal to 1% of the outstanding shares of
all classes of our Common Stock on the last day of the immediately preceding fiscal year, or (c) an amount determined by the administrator. Shares issuable under the ESPP will be authorized, but unissued, or reacquired shares of our Common Stock. On January 1, 2024, the number of shares available under the ESPP increased by 1,250,686 shares pursuant to this feature.
Administration
Our board of directors or a committee appointed by our board has authority to administer the ESPP. Unless and until determined otherwise by our board of directors, the compensation committee of our board of directors will administer the ESPP. The administrator will have full and exclusive discretionary authority to construe, interpret and apply the terms of the ESPP, delegate ministerial duties to any of our employees, designate separate offerings under the ESPP, designate our subsidiaries as participating in the ESPP, determine eligibility, adjudicate all disputed claims filed under the ESPP and establish procedures that it deems necessary or advisable for the administration of the ESPP, including, but not limited to, adopting such procedures, sub-plans and appendices to the enrollment agreement as are necessary or appropriate to permit participation in the ESPP by employees who are non-U.S. nationals or employed outside the U.S. The administrator’s findings, decisions and determinations will be final and binding on all participants to the maximum extent permitted by law.
Eligibility
Generally, any of our employees will be eligible to participate in our ESPP if they are customarily employed by us or any of our participating subsidiaries for at least 20 hours per week and more than five months in any calendar year. The administrator, in its discretion, before an enrollment date for all options granted on such enrollment date in an offering, may determine that an employee who (a) has not completed at least two years of service (or a lesser period of time determined by the administrator) since the employee’s last hire date, (b) customarily works not more than 20 hours per week (or a lesser period of time determined by the administrator), (c) customarily works not more than five months per calendar year (or a lesser period of time determined by the administrator), (d) is a highly compensated employee within the meaning of Code Section 414(q) or (e) is a highly compensated employee within the meaning of Code Section 414(q) with compensation above a certain level or who is an officer or subject to disclosure requirements under Section 16(a) of the Exchange Act, is not eligible to participate in an offering. However, an employee may not be granted an option to purchase stock under our ESPP if the employee (a) immediately after the grant, would own stock and/or hold outstanding options to purchase such stock possessing 5% or more of the total combined voting power or value of all classes of capital stock of ours or any parent or subsidiary of ours; or (b) holds rights to purchase stock under all of our employee stock purchase plans that accrue at a rate that exceeds $25,000 worth of stock for each calendar year during which his or her right to purchase shares is outstanding at any time.
Participants may end their participation at any time during an offering period and will be paid their accrued contributions that have not yet been used to purchase shares of our Common Stock. Participation ends automatically upon termination of employment with us (or our participating subsidiaries).
Offering Periods and Purchase Periods
The ESPP includes a component, or the “423 Component,” that is intended to qualify as an “employee stock purchase plan” under Code Section 423, and a component that does not comply with Code Section 423, or the “Non-423 Component.” For purposes of this summary, a reference to the ESPP generally will mean the terms and operations of the 423 Component.
The ESPP provides for offering periods with a duration and start and end dates as determined by the administrator, provided that no offering period will have a duration exceeding 27 months. Unless determined otherwise by the administrator, each offering period will have one purchase period with the same duration as the offering period. The administrator is authorized to change the duration of future offering periods and purchase periods under the ESPP, including the starting and ending dates of offering periods and purchase periods and the number of purchase periods in any offering periods. Unless determined otherwise by the administrator and to the extent an offering period provides for more than one purchase date in such offering period, if the fair market value of a share of our Common Stock on a purchase date is less than the fair market value of a share of our Common Stock on the first trading day of the offering period, participants in that offering period will be withdrawn from that offering period following their purchase of shares on such purchase date and automatically will be enrolled in a new offering period.
Contributions
The ESPP permits participants to purchase shares of our Common Stock through payroll deductions of up to 15% of their eligible compensation, which includes a participant’s base straight time gross earnings but excludes payments for overtime and shift premium, incentive compensation, bonuses, commissions, equity compensation and other similar compensation. The administrator may change the compensation eligible for contribution under the ESPP on a uniform and nondiscriminatory basis for future offering periods.
Exercise of Purchase Right
Amounts deducted and accumulated by a participant under the ESPP are used to purchase shares of our Common Stock at the end of each purchase period. The purchase price of the shares will be 85% of the lower of (a) the fair market value of a share of our Common Stock on the first trading day of the offering period or (b) the fair market value of a share of our Common Stock on the exercise date. A participant will be permitted to purchase a maximum of 1,250 shares during each offering period, provided that the administrator may increase or decrease such maximum number of shares for each purchase period or offering period. Until shares of our Common Stock are issued (as evidenced by the appropriate entry on our books or the books of a duly authorized transfer agent of ours) to a participant, the participant will have only rights of an unsecured creditor with respect to such shares, and no right to vote or receive dividends or any other rights as a stockholder with respect to such shares.
Termination of Participation
Participation in the ESPP generally will terminate when a participating employee’s employment with us or a participating subsidiary ceases for any reason, the employee withdraws from the ESPP or we terminate or amend the ESPP such that the employee no longer is eligible to participate. An employee may withdraw his or her participation in the ESPP at any time in accordance with procedures, and prior to any applicable deadline, specified by the administrator. Upon withdrawal from the ESPP, in general the employee will receive all amounts credited to his or her account without interest (unless otherwise required under applicable law) and his or her payroll withholdings or contributions under the ESPP will cease.
Non-Transferability
A participant will not be permitted to transfer the contributions credited to his or her ESPP account or rights granted under the ESPP, other than by will or the laws of descent and distribution.
Certain Adjustments
The ESPP provides that in the event that any dividend or other distribution (whether in the form of cash, our Common Stock, other securities or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, reclassification, repurchase or exchange of our Common Stock or other of our securities or other change in our corporate structure affecting our Common Stock occurs (other than any ordinary dividends or other ordinary distributions), the administrator will make adjustments to the number and class of shares that may be delivered under the ESPP and/or the purchase price per share and number of shares covered by each option granted under the ESPP that has not yet been exercised, and the numerical share limits under the ESPP.
Dissolution or Liquidation
In the event of our proposed dissolution or liquidation, any offering period in progress will be shortened by setting a new purchase date and will terminate immediately before the completion of such proposed transaction, unless determined otherwise by the administrator.
Merger or Change in Control
In the event of a merger or our change in control, as defined in the ESPP, a successor corporation may assume or substitute for each outstanding option. If the successor corporation does not assume or substitute for the options, the offering period then in progress under the ESPP will be shortened, and a new exercise date will be set to occur before the date of the proposed merger or change in control. The administrator will notify each participant that the exercise date has been changed and that the participant’s option will be exercised automatically on the new exercise date unless prior to such date the participant has withdrawn from the offering period.
Amendment; Termination
The administrator will have the authority to modify, amend, suspend or terminate the ESPP except that, subject to certain exceptions described in the ESPP, no such action may adversely affect any outstanding rights to purchase shares of our Common Stock under the ESPP. The ESPP will terminate automatically on June 9, 2041, unless we terminate it earlier.
Equity Compensation Plan Information
The following table provides information as of December 31, 2023 with respect to shares of our common stock that may be issued under our existing equity compensation plans.
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | (a) Number of
Securities to be
Issued Upon Exercise
of Outstanding
Options, Warrants
and Rights | | (b) Weighted-
Average Exercise
Price of Outstanding
Options, Warrants
and Rights | | (c) Number of Securities
Remaining Available for
Future Issuance Under
Equity Compensation
Plans (Excluding
Securities Reflected in
Column (a)) |
Equity compensation plans approved by security holders | | | | | | |
2017 Plan (1) | | 5,678,766 | | $3.56 | | 0 |
2021 Plan (2) | | 8,997,569 | | $3.67 | | 20,279,560 |
ESPP (3) | | — | | — | | 3,505,138 |
Equity compensation plans not approved by security holders | | — | | — | | — |
Total | | 14,676,335 | | $3.63 | | 23,784,698 |
________________________ | | | | | |
| (1) | The Legacy Nautilus board of directors adopted, and Legacy Nautilus stockholders approved, the 2017 Plan. The 2017 Plan was terminated as of one day prior to the closing of the Business Combination and we will not grant any additional awards under the 2017 Plan. However, the 2017 Plan will continue to govern the terms and conditions of the outstanding awards previously granted under the 2017 Plan and assumed by us at the closing of the Business Combination. |
| (2) | Our board of directors adopted, and our stockholders approved, the 2021 Plan. The 2021 Plan provides that the number of shares available for issuance under the 2021 Plan will be increased on the first day of each fiscal year beginning with the 2022 fiscal year, in an amount equal to the least of (i) 18,672,000 shares, (ii) five percent (5%) of the outstanding shares of common stock on the last day of the immediately preceding fiscal year or (iii) such other amount as our board of directors may determine. On January 1, 2024, the number of shares available under the 2021 Plan increased by 6,253,430 shares pursuant to this feature. In addition, the shares reserved for issuance under the 2021 Plan include any shares of our Common Stock subject to awards of stock options or other awards that were assumed in the Business Combination (or “assumed awards”) that, on or after the effective date of the Business Combination, are terminated, canceled, expire or otherwise terminate without having been exercised in full, are tendered to or withheld by us for payment of an exercise price or for tax withholding obligations, or are forfeited to or repurchased by us due to failure to vest (provided that the maximum number of shares that may be added to the 2021 Plan pursuant to this provision is 7,500,000 shares). |
| (3) | Our board of directors adopted, and our shareholders approved, the ESPP. The ESPP provides that the number of shares available for issuance under the ESPP will be increased on the first day of each fiscal year beginning with the 2022 fiscal year, in an amount equal to the least of (i) 3,734,500 shares, (ii) one percent (1%) of the outstanding shares of common stock on the last day of the immediately preceding fiscal year or (iii) such other amount as the administrator may determine. On January 1, 2024, the number of shares available under the ESPP increased by 1,250,686 shares pursuant to this feature. |
Item 12. Security Ownership of Certain Beneficial Owner and Management and Related Stockholder Matters
The following table sets forth information regarding the beneficial ownership of Common Stock as of February 15, 2024 by:
•each person known by us to be the beneficial owner of more than 5% of our outstanding Common Stock;
•each of our named executive officers and our directors; and
•all of our executive officers and directors as a group.
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security. Under those rules, beneficial ownership includes securities that the individual or entity has the right to acquire, such as through the exercise of stock options, within 60 days of February 15, 2024. Shares subject to options that are currently exercisable or exercisable within 60 days of February 15, 2024 are considered outstanding and beneficially owned by the person holding such options for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Except as noted by footnote, and subject to community property laws where applicable, based on the information provided to us, we believe that the persons and entities named in the table below have sole voting and investment power with respect to all shares shown as beneficially owned by them. Unless otherwise noted, the business address of each of the directors and executive officers of Nautilus is 2701 Eastlake Avenue East, Seattle, WA 98102. The percentage of beneficial ownership of Nautilus is calculated based on 125,098,080 shares of Common Stock outstanding as of February 15, 2024.
| | | | | | | | | | | | | | |
| Name and Address of Beneficial Owners | | Number of Shares | | % |
Sujal Patel(1) | | 18,144,849 | | 14.4% |
Parag Mallick(2) | | 21,280,570 | | 16.9% |
| | | | |
Anna Mowry(3) | | 611,736 | | * |
Melissa Epperly(4) | | 196,528 | | * |
Matthew McIlwain(5) | | 6,640,092 | | 5.3% |
Farzad Nazem(6) | | 2,048,393 | | 1.6% |
Vijay Pande(7) | | 160,247 | | * |
Matthew L. Posard(8) | | 535,643 | | * |
Michael Altman(9) | | 331,793 | | * |
Karen Akinsanya(10) | | 122,576 | | * |
All directors and officers as a group (15 persons)(11) | | 53,024,146 | | 40.4% |
| Five Percent Holders: | | | | |
Perceptive Life Sciences Master Fund Ltd.(12) | | 12,594,211 | | 10.1% |
Entities affiliated with Andreessen Horowitz(13) | | 17,653,917 | | 14.1% |
Entities affiliated with Cercano Management(14) | | 7,172,985 | | 5.7% |
Entities affiliated with Madrona Ventures(15) | | 6,640,092 | | 5.3% |
________________________
| | | | | |
* | Less than 1% |
| (1) | Consists of (i) 10,014,488 shares held by Mr. Patel, (ii) 5,280,476 shares held by PFV I, LLC, (iii) 1,814,035 shares held by the Sujal Patel 2020 Children's Trust, u/a/d December 3, 2020 (the “Patel Trust”) and (iv) 1,035,850 shares subject to options held by Mr. Patel exercisable withing 60 days of February 15, 2024. Mr. Patel is the manager of PFV I, LLC and a trustee of the Patel Trust and as such has voting and investment control over the shares held by PFV I, LLC and the Patel Trust. Mr. Patel disclaims, for purposes of Section 16 of the Securities Exchange Act of 1934, beneficial ownership of the securities held by the Patel Trust, except to the extent of any pecuniary interest therein, and this report shall not be deemed an admission that Mr. Patel is the beneficial owner of such securities for purposes of Section 16 or for any other purposes. |
| (2) | Consists of (i) 20,479,892 shares held by Dr. Mallick, (ii) 200,000 shares held by The Dream Finder Foundation, and (iii) 600,678 shares subject to options held by Dr. Mallick exercisable within 60 days of February 15, 2024. Dr. Mallick has voting and investment control over the shares held by The Dream Finder Foundation. Dr. Mallick disclaims, for purposes of Section 16 of the Securities Exchange Act of 1934, beneficial ownership of the securities held by The Dream Finder Foundation, except to the extent of any pecuniary interest therein, and this report shall not be deemed an admission that Dr. Mallick is the beneficial owner of such securities for purposes of Section 16 or for any other purposes. |
| (3) | Consists of (i) 60,500 shares held by Ms. Mowry and (ii) 551,236 shares subject to options held by Ms. Mowry exercisable within 60 days of February 15, 2024. |
| (4) | Consists of shares subject to options held by Ms. Epperly exercisable within 60 days of February 15, 2024. |
| (5) | Consists of the shares set forth in footnote 15 below. |
| (6) | Consists of (i) 1,888,146 shares held by HAND Capital, LLC and (ii) 160,247 shares subject to options held by Mr. Nazem exercisable within 60 days of February 15, 2024. Mr. Nazem is the manager of HAND Capital, LLC and as such has voting and investment power over the shares held by HAND Capital, LLC. |
| (7) | Consists of shares subject to options held by Mr. Pande exercisable within 60 days of February 15, 2024. Mr. Pande has no voting or investment control over the shares held by entities affiliated with Andreessen Horowitz that are included in footnote 13 below. |
| (8) | Consists of (i) 50,000 shares held by Mr. Posard, (ii) 100,000 shares held by the Matthew and Elizabeth Posard Trust and (iii) 385,643 shares subject to options held by Mr. Posard exercisable within 60 days of February 15, 2024. Mr. Posard is the trustee of the Matthew and Elizabeth Posard Trust and as such has voting and investment control over shares held by the Matthew and Elizabeth Posard Trust. |
| (9) | Consists of (i) 165,860 shares held by Mr. Altman and (ii) 165,933 shares subject to options held by Mr. Altman exercisable within 60 days of February 15, 2024. |
| (10) | Consists of shares subject to options held by Ms. Akinsanya exercisable within 60 days of February 15, 2024. |
| (11) | Consists of (i) 46,713,632 shares beneficially owned by the directors and officers and (ii) 6,310,514 shares subject to options held by the directors and officers exercisable within 60 days of February 15, 2024. |
| (12) | Consists of (i) 12,428,351 shares held by Perceptive Life Sciences Master Fund Ltd. (the “Master Fund”) and (ii) 165,860 shares held by C2 Life Sciences LLC (“C2 Life Sciences”). Perceptive Advisors LLC (the “Advisor”) serves as the investment manager of Master Fund and C2 Life Sciences. Joseph Edelman is the managing member of the Advisor. Each of Mr. Edelman and the Advisor disclaims, for purposes of Section 16 of the Securities Exchange Act of 1934, beneficial ownership of such securities, except to the extent of his/its indirect pecuniary interest therein, and this report shall not be deemed an admission that either Mr. Edelman or the Advisor is the beneficial owner of such securities for purposes of Section 16 or for any other purposes. The address for the persons and entities set forth herein is 51 Astor Place, 10th Floor, New York, NY 10003. |
| | | | | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| (13) | Consists of (i) 16,298,006 shares held by AH Bio Fund II, L.P., for itself and as nominee for AH Bio Fund II-B, L.P. (collectively, the “AH Bio Fund II Entities”) and (ii) 1,355,911 shares held by Andreessen Horowitz LSV Fund II, L.P., for itself and as nominee for Andreessen Horowitz LSV Fund II-B, L.P. and Andreessen Horowitz LSV Fund II-Q, L.P. (collectively, the “AH LSV Fund II Entities”). AH Equity Partners Bio II, L.L.C. (“AH EP Bio II”), the general partner of the AH Bio Fund II Entities, may be deemed to have sole voting and dispositive power over the shares held by the AH Bio Fund II Entities. AH Equity Partners LSV II, L.L.C. (“AH EP LSV II”), the general partner of the AH LSV Fund II Entities, may be deemed to have sole voting and dispositive power over the shares held by the AH LSV Fund II Entities. The managing members of each of AH EP Bio II and AH EP LSV II are Marc Andreessen and Ben Horowitz, and each of them may be deemed to hold shared voting and dispositive power over the shares held by the AH Bio Fund II Entities and AH LSV Fund II Entities. Shares held by each of these entities include shares that may be subsequently sold by each of Marc Andreessen, Ben Horowitz and Vijay Pande, a member of Nautilus’ board of directors, following in-kind distributions of shares by these entities. The address for the persons and entities set forth herein is 2865 Sand Hill Road, Suite 101, Menlo Park, CA 94025. |
| (14) | Consists of (i) 3,586,493 shares held by Vulcan Capital Holdings Columbia LLC (“VCHC”) and (ii) 3,586,492 shares held by VCVC V LLC (“VCVC”). Based solely on the most recently available Schedule 13G filed with the SEC on February 13, 2023, Cercano Management LLC (“Cercano”) may be deemed to be the beneficial owner of such shares as Cercano acts as an investment adviser to VCHC and VCVC pursuant to investment management agreements whereby all voting and investment discretion has been contractually allocated to Cercano, and such discretion may not be revoked with less than 61 days’ notice, and Christopher N. Ordnorff may also be deemed to be the beneficial owner of such shares because he controls Cercano in his position as managing member of Cercano. Both Cercano and Mr. Orndorff disclaim, for purposes of Section 16 of the Securities Exchange Act of 1934, beneficial ownership of these securities, except to the extent of their respective pecuniary interests therein, and this report shall not be deemed an admission that either of Cercano or Mr. Orndorff is the beneficial owner of such securities for purposes of Section 16 or for any other purposes. The address for the foregoing entities is 1110 112th Avenue NE, Suite 202, Bellevue, WA 98004. |
| (15) | Consists of (i) 5,798,394 shares held by Madrona Venture Fund VI, LP (“Madrona Fund VI”), (ii) 222,376 shares held by Madrona Venture Fund VI-A, LP (“Madrona Fund VI-A”), (iii) 459,075 shares held by Mr. McIlwain and (iv) 160,247 shares subject to options held by Mr. McIlwain exercisable within 60 days of February 15, 2024. Madrona Investment Partners VI, L.P. (“Madrona Partners VI”) is the general partner of each of Madrona Fund VI and Madrona Fund VI-A, and Madrona VI General Partner, LLC (“Madrona VI LLC”) is the general partner of Madrona Partners VI. Matthew McIlwain, together with Paul Goodrich, Scott Jacobson, Len Jordon, Tim Porter, Soma Somasegar, Hope Cochran, and Steve Singh are the managing members of Madrona VI LLC, and each may be deemed to share voting and investment power over the securities held by Madrona Fund VI and Madrona Fund VI-A. Each of such individuals disclaims beneficial ownership over such securities except to the extent of his pecuniary interest therein. The address for the persons and entities set forth herein is 999 Third Avenue, 34th Floor, Seattle, WA. |
Please see the sections titled “Executive Compensation” and “Certain Relationships and Related Transactions, and Director Independence” appearing elsewhere in this Annual Report for information regarding material relationships with our principal securityholders within the past two years.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Described below are any transactions occurring since January 1, 2022 and any currently proposed transactions in which we have been or are a party, and in which:
•the amounts involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years; and
•a director, executive officer, holder of more than 5% of our outstanding capital stock, or any member of such person’s immediate family had or will have a direct or indirect material interest.
Our Policies and Procedures for Related Party Transactions
Our board of directors has adopted a written Related Person Transactions Policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related person transactions.” For purposes of our policy only, a “related person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we or any of our subsidiaries are participants involving an amount that exceeds $120,000, in which any “related person” has a material interest.
Transactions involving compensation for services provided to us as an employee, consultant or director will not be considered related person transactions under this policy. A related person is any executive officer, director, nominee to become a director or a holder of more than 5% of any class of our voting securities (including our Common Stock), including any of their immediate family members and affiliates, including entities owned or controlled by such persons.
Under the policy, the related person in question or, in the case of transactions with a holder of more than 5% of any class of our voting securities, an officer with knowledge of a proposed transaction, must present information regarding the proposed related person transaction to our audit committee (or, where review by our audit committee would be inappropriate, to another independent body of our board of directors) for review. To identify related person transactions in advance, we will rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related person transactions, our audit committee will take into account the relevant available facts and circumstances, which may include, but are not limited to:
•the risks, costs, and benefits to us;
•the impact on a director’s independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated;
•the terms of the transaction;
•the availability of other sources for comparable services or products; and
•the terms available to or from, as the case may be, unrelated third parties.
Our audit committee will approve only those transactions that it determines are fair to us and in our best interests.
Item 14. Principal Accountant Fees and Services
The following table presents fees for professional audit services and other services rendered by PricewaterhouseCoopers LLP to us for our fiscal years ended December 31, 2023 and 2022.
| | | | | | | | | | | |
| 2023 | | 2022 |
Audit Fees(1) | $ | 913,000 | | | $ | 863,500 | |
Audit-Related Fees | — | | | — | |
Tax Fees | — | | | — | |
All Other Fees(2) | 2,900 | | | 3,078 | |
Total Fees | $ | 915,900 | | | $ | 866,578 | |
________________________ | | | | | |
| (1) | “Audit Fees” consist of fees billed for professional services rendered in connection with the audit of our financial statements, reviews of our quarterly financial statements and related accounting consultations and services that are normally provided by the independent registered public accountants in connection with statutory and regulatory filings or engagements for those fiscal years. |
| (2) | “All Other Fees” consist of the cost of a subscription accounting research software. |
Auditor Independence
In 2023, there were no other professional services provided by PricewaterhouseCoopers LLP, other than those listed above, that would have required our audit committee to consider their compatibility with maintaining the independence of PricewaterhouseCoopers LLP.
Part IV
Item 15. Exhibits and Financial Statement Schedules
(a) 1. Financial Statements
See Index to consolidated financial statements in Part II, Item 8 of this Annual Report on Form 10-K, which is incorporated herein by reference.
2. Financial Statement Schedules
All financial statement schedules have been omitted because they are either not applicable or the required information is shown in the consolidated financial statements or notes thereto.
3. Exhibits
See the Exhibit Index which precedes the signature page of this Annual Report, which is incorporated herein by reference.
(b) Exhibits
See Item 15(a)(3) above.
(c) Financial Statement Schedules
See Item 15(a)(2) above.
EXHIBITS
| | | | | | | | |
Exhibit Number | | Description |
| 2.1 | | |
| 3.1 | | |
| 3.2 | | |
| 4.1 | | |
4.2 | | |
| 10.1 | | |
| 10.2 | | |
10.3* | | |
10.4+ | | |
10.5+ | | |
10.6+ | | |
10.7+ | | |
10.8+ | | |
10.9+ | | |
10.10+ | | |
10.11+ | | |
10.12+ | | |
10.13+ | | |
10.14+ | | |
10.15+ | | |
| | | | | | | | |
10.16+ | | |
10+17+ | | |
10.18+ | | |
10.19+ | | |
10.20+ | | |
10.21+ | | |
10.22+ | | |
10.23+ | | |
10.24+ | | |
10.25+ | | |
10.26+ | | |
10.27+ | | |
| 21.1 | | |
| 23.1* | | |
| 31.1* | | |
| 31.2* | | |
32.1*† | | |
32.2*† | | |
97.1* | | |
| 101.INS | | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document |
101.SCH | | Inline XBRL Taxonomy Extension Schema Document |
101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document |
| | | | | | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| 104 | | Cover page Interactive Data File (embedded with the Inline XBRL document) |
__________________
* Filed herewith.
+ Indicates management contract or compensatory plan.
† The certifications attached as Exhibit 32.1 and 32.2 that accompany this Annual Report on Form 10-K are deemed furnished and not filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Nautilus Biotechnology, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing.
Item 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act the Registrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| NAUTILUS BIOTECHNOLOGY, INC. |
| |
| By: | /s/ Sujal Patel |
| Sujal Patel |
| Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Sujal Patel and Anna Mowry, and each of them, as his or her true and lawful attorney-in-fact and agent with full power of substitution, for him or her in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact, proxy and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorney-in-fact, proxy and agent, or his or her substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed by the following persons in the capacities and on the dates indicated:
| | | | | | | | | | | | | | | | | |
Signature | | Title | | Date |
| | | | | |
/s/ Sujal Patel | | Chief Executive Officer, President and Director | | February 28, 2024 |
| Sujal Patel | | (Principal Executive Officer) | | |
| | | | | |
/s/ Anna Mowry | | Chief Financial Officer | | February 28, 2024 |
| Anna Mowry | | (Principal Financial and Accounting Officer) | | |
| | | | | |
/s/ Michael Altman | | Director | | February 28, 2024 |
| Michael Altman | | | | |
| | | | | |
/s/ Melissa Epperly | | Director | | February 28, 2024 |
| Melissa Epperly | | | | |
| | | | | |
/s/ Parag Mallick | | Director | | February 28, 2024 |
| Parag Mallick | | | | |
| | | | | |
/s/ Matthew McIlwain | | Director | | February 28, 2024 |
| Matthew McIlwain | | | | |
| | | | | |
/s/ Farzad Nazem | | Director | | February 28, 2024 |
| Farzad Nazem | | | | |
| | | | | |
/s/ Vijay Pande | | Director | | February 28, 2024 |
Vijay Pande | | | | |
| | | | | |
/s/ Matthew L. Posard | | Director | | February 28, 2024 |
| Matthew L. Posard | | | | |
| | | | | |
/s/ Karen Akinsanya | | Director | | February 28, 2024 |
| Karen Akinsanya | | | | |
| | | | | |
| | | | | |
NAUTILUS BIOTECHNOLOGY, INC.
AMENDMENT NO. 1 TO
AMENDED AND RESTATED REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment No. 1 (this “Amendment”) to the Amended and Restated Registration Rights and Lock-Up Agreement, dated February 7, 2021 (the “Rights Agreement”), is made and entered into as of February 23, 2024, by and among Nautilus Biotechnology, Inc., a Delaware corporation (f/k/a ARYA Sciences Acquisition Corp III) (the “Company”) and the Holders identified on the signature pages to this Amendment. Capitalized terms used and not otherwise defined in this Amendment shall have the meanings ascribed to them in the Rights Agreement.
WHEREAS, the Company and the undersigned Holders desire to amend the Rights Agreement as set forth in this Amendment;
WHEREAS, Section 7.10 of the Rights Agreement provides that the Rights Agreement may be amended by a written instrument signed by (i) the Company, (ii) Perceptive Holders holding at least a majority in interest of the then-outstanding number of Registrable Securities held by all Perceptive Holders in their capacity as Perceptive Holders (provided the Perceptive Holders or their Permitted Transferees hold Registrable Securities at the time of such amendment), and (iii) New Holders holding at least a majority in interest of the then-outstanding number of Registrable Securities held by all New Holders in their capacity as New Holders (provided the New Holders or their Permitted Transferees hold Registrable Securities at the time of such amendment) (together, the “Requisite Parties”); and
WHEREAS, the undersigned parties constitute the Requisite Parties.
NOW THEREFORE, in consideration of the foregoing, the parties hereby agree as follows:
1.Amendments.
(a)The definition for “Registrable Security” and “Registrable Securities” set forth in Section 1.1 of the Rights Agreement is hereby amended and restated in its entirety to read as follows:
“Registrable Security”, “Registrable Securities” shall mean (a) any outstanding share of Common Stock or any other equity security (including the shares of Common Stock issued or issuable upon the exercise of any other equity security) of the Company held by a Holder as of the Closing Date (including, without limitation, the PIPE Shares and any shares of Common Stock issued pursuant to the Business Combination Agreement) and (b) any other equity security of the Company issued or issuable with respect to any such share of Common Stock included in clause (a) above by way of a stock dividend or stock split or in connection with a combination of shares, recapitalization, merger, consolidation or reorganization; provided, however, that, as to any particular Registrable Security, such securities shall cease to be Registrable Securities when: (A) a Registration Statement with respect to the sale of such securities shall have become effective under the Securities Act and such securities shall have been sold, transferred, disposed of or exchanged in accordance with such Registration Statement; (B) such securities shall have been otherwise transferred, new certificates for such securities not bearing a legend restricting further transfer shall have been delivered by the Company to the transferee, and subsequent public distribution of such securities shall not require registration under the Securities Act; (C) such securities shall have ceased to be outstanding; (D) such securities have been sold to, or through, a broker, dealer or underwriter in a public distribution or other public securities transaction or (E) such securities may be immediately sold without registration pursuant to Rule 144 under the Securities Act (or any successor rule promulgated by the Commission) without volume limitations or manner-of-sale restrictions during a three-month period.
(b)Section 2.4 of the Rights Agreement is hereby amended by adding a new sentence at the end thereof that reads as follows:
Notwithstanding anything to the contrary stated herein, the piggyback registration rights under Section 2.2 (including, without limitation, the right to receive notice from the Company of the proposed filing of a Registration Statement and the right to include Registrable Securities in a Piggyback Registration) shall be suspended with respect to a Holder’s Registrable Securities for as long as a Resale Registration Statement covering the resale of such Registrable Securities has been declared effective and remains effective and available.
(c)Section 7.3 of the Rights Agreement is hereby amended by adding a new sentence at the end thereof that reads as follows:
A Permitted Transferee shall not be entitled to any rights under this Agreement unless and until such Permitted Transferee has executed a written instrument, in form and substance reasonably satisfactory to the Company, agreeing to be bound by the terms and provisions of this Agreement.
(d)Section 7.11 of the Rights Agreement is hereby amended and restated in its entirety to read as follows:
Section 7.11. Waiver. At any time, (i) the Company may (a) extend the time for the performance of any obligation or other act of any Holder, (b) waive any inaccuracy in the representations and warranties of any Holder contained herein or in any document delivered by such Holder pursuant hereto and (c) waive compliance with any agreement of such Holder or any condition to its own obligations contained herein. At any time, (i) the Holders may (a) extend the time for the performance of any obligation or other act of the Company, (b) waive any inaccuracy in the representations and warranties of the Company contained herein or in any document delivered by the Company pursuant hereto and (c) waive compliance with any agreement of the Company or any condition to their own obligations contained herein. Any such extension or waiver by the Company shall be valid if set forth in an instrument in writing signed by the Company, and any such extension or waiver by the Holders shall be valid if set forth in an instrument in writing signed by either (x) the Holder(s) to be bound thereby or (y) for a waiver or extension applicable to all Holders, (1) Perceptive Holders holding at least a majority in interest of the then-outstanding number of Registrable Securities held by all Perceptive Holders in their capacity as Perceptive Holders (provided the Perceptive Holders or their Permitted Transferees hold Registrable Securities at the time of such extension or waiver), and (2) New Holders holding at least a majority in interest of the then-outstanding number of Registrable Securities held by all New Holders in their capacity as New Holders (provided the New Holders or their Permitted Transferees hold Registrable Securities at the time of such extension or waiver).
2.Miscellaneous.
(a)Continuing Agreement. Except as specifically amended hereby, all of the terms of the Rights Agreement shall remain and continue in full force and effect and are hereby confirmed in all respects.
(b)Governing Law. This Amendment shall be governed by the internal law of the State of Delaware.
(c)Successors and Assigns. Except as otherwise provided herein, the provisions hereof shall inure to the benefit of and be binding upon the respective successors and assigns of the parties hereto.
(d)Entire Agreement; Amendment. This Amendment and the Rights Agreement constitute the full and entire understanding and agreement between the parties with regard to the subject matter hereof and thereof.
(e)Counterparts. This Amendment may be executed and delivered (including by facsimile or portable document format (pdf) transmission) in counterparts, and by the different parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
| | | | | | | | |
| | |
COMPANY: |
|
| NAUTILUS BIOTECHNOLOGY, INC. |
| |
| By: | | /s/ Sujal Patel |
| Name: | | Sujal Patel |
| Title: | | Chief Executive Officer |
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Matthew and Elizabeth Posard Trust
By: /s/ Matthew Posard__________________
Name: Matthew Posard
Title: Trustee
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Matthew Posard
By: /s/ Matthew Posard__________________
Name: Matthew Posard
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
HAND Capital, LLC
By: /s/ Farzad Nazem__________________
Name Farzad Nazem
Title: Manager
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Farzad Nazem
By: /s/ Farzad Nazem__________________
Name Farzad Nazem
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Melissa Epperly
By: /s/ Melissa Epperly
Name Melissa Epperly
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Sujal Patel
By: /s/ Sujal Patel
Name Sujal Patel
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
PFV I, LLC
By: /s/ Sujal Patel
Name Sujal Patel
Title: Manager
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Sujal Patel 2020 Children’s Trust, u/a/d December 3, 2020
By: /s/ Sujal Patel
Name Sujal Patel
Title: Investment Trustee
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Matthew McIlwain
By: /s/ Matthew McIlwain
Name Matthew McIlwain
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Madrona Venture Fund VI-A, LP
By: Madrona Investment Partners, VI,
its General Partner
By: Madrona VI General Partner, LLC, its General Partner
By: /s/ Matthew McIlwain
Name Matthew McIlwain
Title: Managing Director
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Madrona Venture Fund VI, LP
By: Madrona Investment Partners, VI,
its General Partner
By: Madrona VI General Partner, LLC, its General Partner
By: /s/ Matthew McIlwain
Name Matthew McIlwain
Title: Managing Director
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
The Dream Finder Foundation
By: /s/ Parag Mallick
Name Parag Mallick
Title: President
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Parag Mallick
By: /s/ Parag Mallick
Name Parag Mallick
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
AH BIO FUND II, L.P.
For itself and as nominee for
AH Bio Fund II-B, L.P.
By: AH Equity Partners Bio II, L.L.C.
Its general partner
By: /s/ Phil Hathaway
Name Phil Hathaway
Title: Chief Operating Officer
HOLDERS:
Andreessen Horowitz LSV Fund II, L.P.
For itself and as nominee for
Andreessen Horowitz LSV Fund II-B, L.P. and
Andreessen Horowitz LSV Fund II-Q, L.P. and
By: AH Equity Partners LSV II, L.L.C.
General partner
By: /s/ Phil Hathaway
Name Phil Hathaway
Title: Chief Operating Officer
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Vijay Pande
By: /s/ Vijay Pande
Name Vijay Pande
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
This Amendment is hereby acknowledged and agreed on behalf of the undersigned:
HOLDERS:
Perceptive Life Sciences Master Fund Ltd.
By: /s/ James Mannix
Name James Mannix
Title: Chief Operating Officer
SIGNATURE PAGE TO AMENDMENT OF REGISTRATION RIGHTS AND LOCK-UP AGREEMENT
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (No. 333-258100) and Form S-8 (Nos. 333-258684, 333-258686, 333-262992, and 333-269950) of Nautilus Biotechnology, Inc of our report dated February 28, 2024 relating to the financial statements, which appears in this Form 10-K.
/s/ PricewaterhouseCoopers LLP
San Jose, California
February 28, 2024
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Sujal Patel, certify that:
1.I have reviewed this Annual Report on Form 10-K of Nautilus Biotechnology, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
| | | | | | | | |
Date: February 28, 2024 | By: | /s/ Sujal Patel |
| | Sujal Patel |
| | President and Chief Executive Officer |
| | (Principal Executive Officer) |
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Anna Mowry, certify that:
1.I have reviewed this Annual Report on Form 10-K of Nautilus Biotechnology, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
| | | | | | | | |
Date: February 28, 2024 | By: | /s/ Anna Mowry |
| | Anna Mowry |
| | Chief Financial Officer |
| | (Principal Financial and Accounting Officer) |
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Nautilus Biotechnology, Inc. (the “Company”) on Form 10-K for the period ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Sujal Patel, hereby certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| | | | | | | | |
Date: February 28, 2024 | By: | /s/ Sujal Patel |
| | Sujal Patel |
| | President and Chief Executive Officer |
| | (Principal Executive Officer) |
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Nautilus Biotechnology, Inc. (the “Company”) on Form 10-K for the period ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Anna Mowry, hereby certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| | | | | | | | |
Date: February 28, 2024 | By: | /s/ Anna Mowry |
| | Anna Mowry |
| | Chief Financial Officer |
| | (Principal Financial and Accounting Officer) |
NAUTILUS BIOTECHNOLOGY, INC.
COMPENSATION RECOVERY POLICY
As adopted on October 24, 2023
Nautilus Biotechnology, Inc. (the “Company”) is committed to strong corporate governance. As part of this commitment, the Compensation Committee (the “Committee”) of the Company’s Board of Directors (the “Board”) has adopted this clawback policy called the Compensation Recovery Policy (the “Policy”). The Policy is intended to further the Company’s pay-for-performance philosophy and to comply with applicable laws by providing rules relating to the reasonably prompt recovery of certain compensation received by Covered Executives in the event of an Accounting Restatement. The application of the Policy to Covered Executives is not discretionary, except to the limited extent provided below, and applies without regard to whether a Covered Executive was at fault. Capitalized terms used in the Policy are defined below, and the definitions have substantive impact on its application so reviewing them carefully is important to your understanding.
The Policy is intended to comply with, and will be interpreted in a manner consistent with, Section 10D of the Securities Exchange Act of 1934 (the “Exchange Act”), with Exchange Act Rule 10D-1 and with the listing standards of the national securities exchange (the “Exchange”) on which the securities of the Company are listed, including any interpretive guidance thereunder.
Persons Covered by the Policy
The Policy is binding and enforceable against all “Covered Executives.” A Covered Executive is each individual who is or ever was designated as an “officer” by the Board in accordance with Exchange Act Rule 16a-1(f) (a “Section 16 Officer”). The Committee may (but is not obligated to) request or require a Covered Executive to sign and return to the Company an acknowledgement that such Covered Executive will be bound by the terms and comply with the Policy. The Policy is binding on each Covered Executive whether or not the Covered Executive signs and/or returns any acknowledgment.
Administration of the Policy
The Committee has full delegated authority to administer the Policy. The Committee is authorized to interpret and construe the Policy and to make all determinations necessary, appropriate, or advisable for the administration of the Policy. In addition, if determined in the discretion of the Board, the Policy may be administered by the independent members of the Board or another committee of the Board consisting of independent members of the Board, in which case all references to the Committee will be deemed to refer to the independent members of the Board or such other Board committee. All determinations of the Committee will be final and binding and will be given the maximum deference permitted by law.
Accounting Restatements Requiring Application of the Policy
If the Company is required to prepare an accounting restatement due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (an “Accounting Restatement”), then the Committee must determine the Excess Compensation, if any, that
must be recovered. The Company’s obligation to recover Excess Compensation is not dependent on if or when restated financial statements are filed.
Compensation Covered by the Policy
The Policy applies to certain Incentive-Based Compensation (certain terms used in this Section are defined below) that is Received on or after October 2, 2023 (the “Effective Date”), during the Covered Period while the Company has a class of securities listed on a national securities exchange. Such Incentive-Based Compensation is considered “Clawback Eligible Incentive-Based Compensation” if the Incentive-Based Compensation is Received by a person after such person became a Section 16 Officer and the person served as a Section 16 Officer at any time during the performance period for the Incentive-Based Compensation. “Excess Compensation” means the amount of Clawback Eligible Incentive-Based Compensation that exceeds the amount of Clawback Eligible Incentive-Based Compensation that otherwise would have been Received had such Clawback Eligible Incentive-Based Compensation been determined based on the restated amounts. Excess Compensation must be computed without regard to any taxes paid and is referred to in the listings standards as “erroneously awarded compensation.”
To determine the amount of Excess Compensation for Incentive-Based Compensation based on stock price or total shareholder return, where it is not subject to mathematical recalculation directly from the information in an Accounting Restatement, the amount must be based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was Received and the Company must maintain documentation of the determination of that reasonable estimate and provide that documentation to the Exchange.
“Incentive-Based Compensation” means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a Financial Reporting Measure. For the avoidance of doubt, no compensation that is potentially subject to recovery under the Policy will be earned until the Company’s right to recover under the Policy has lapsed.
“Financial Reporting Measures” are measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures that are derived wholly or in part from such measures. Stock price and total shareholder return are also Financial Reporting Measures. A Financial Reporting Measure need not be presented within the financial statements or included in a filing with the Securities and Exchange Commission.
Incentive-Based Compensation is “Received” under the Policy in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-Based Compensation award is attained, even if the payment, vesting, settlement or grant of the Incentive-Based Compensation occurs after the end of that period. For the avoidance of doubt, the Policy does not apply to Incentive-Based Compensation for which the Financial Reporting Measure is attained prior to the Effective Date.
“Covered Period” means the three completed fiscal years immediately preceding the Accounting Restatement Determination Date. In addition, Covered Period can include certain transition periods resulting from a change in the Company’s fiscal year.
“Accounting Restatement Determination Date” means the earliest to occur of: (a) the date the Board, a committee of the Board, or one or more of the officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company
is required to prepare an Accounting Restatement; and (b) the date a court, regulator, or other legally authorized body directs the Company to prepare an Accounting Restatement.
Repayment of Excess Compensation
The Company must recover Excess Compensation reasonably promptly and Covered Executives are required to repay Excess Compensation to the Company. Subject to applicable law, the Company may recover Excess Compensation by requiring the Covered Executive to repay such amount to the Company by direct payment to the Company or such other means or combination of means as the Committee determines to be appropriate (these determinations do not need to be identical as to each Covered Executive). These means include (but are not limited to):
a.requiring reimbursement of cash Incentive-Based Compensation previously paid;
b.seeking recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any equity-based awards (including, but not limited to, time-based vesting awards), without regard to whether such awards are Incentive-Based Compensation or vest based on the achievement of performance goals;
c.offsetting the amount to be recovered from any unpaid or future compensation to be paid by the Company or any affiliate of the Company to the Covered Executive, including (but not limited to) payments of severance that might otherwise be due in connection with a Covered Executive’s termination of employment and without regard to whether such amounts are Incentive-Based Compensation;
d.cancelling outstanding vested or unvested equity awards (including, but not limited to, timebased vesting awards), without regard to whether such awards are Incentive-Based Compensation; and/or
e.taking any other remedial and recovery action permitted by law, as determined by the Committee.
The repayment of Excess Compensation must be made by a Covered Executive notwithstanding any Covered Executive’s belief (whether or not legitimate) that the Excess Compensation had been previously earned under applicable law and therefore is not subject to clawback.
In addition to its rights to recovery under the Policy, the Company or any affiliate of the Company may take any legal actions it determines appropriate to enforce a Covered Executive’s obligations to the Company or to discipline a Covered Executive. Failure of a Covered Executive to comply with their obligations under the Policy result in (without limitation) termination of that Covered Executive’s employment, institution of civil proceedings, reporting of misconduct to appropriate governmental authorities, reduction of future compensation opportunities or change in role. The decision to take any actions described in the preceding sentence will not be subject to the approval of the Committee and can be made by the Board, any committee of the Board, or any duly authorized officer of the Company or of any applicable affiliate of the Company. For avoidance of doubt, any decisions of the Company or the Covered Executive’s employer to discipline a Covered Executive or terminate the employment of a Covered Executive are independent of determinations under this Policy. For example, if a Covered Executive was involved in activities that led to an Accounting Restatement, the Company’s decision as to whether or not to terminate such Covered Executive’s employment would be made under its employment arrangements with such Covered Executive and the requirement to apply this no-fault and
non-discretionary clawback policy will not be determinative of whether any such termination is for cause, although failure to comply with the Policy could result in a termination for cause depending on the terms of such arrangements.
Limited Exceptions to the Policy
The Company must recover the Excess Compensation in accordance with the Policy except to the limited
extent that any of the conditions set forth below is met, and the Committee determines that recovery of the Excess Compensation would be impracticable:
a.The direct expense paid to a third party to assist in enforcing the Policy would exceed the amount to be recovered. Before reaching this conclusion, the Company must make a reasonable attempt to recover such Excess Compensation, document such reasonable attempt(s) to recover, and provide that documentation to the Exchange; or
b.Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the legal requirements as such.
Other Important Information in the Policy
The Policy is in addition to the requirements of Section 304 of the Sarbanes-Oxley Act of 2002 that are applicable to the Company’s Chief Executive Officer and Chief Financial Officer, as well as any other applicable laws, regulatory requirements, rules, or pursuant to the terms of any existing Company policy or agreement providing for the recovery of compensation.
Notwithstanding the terms of any of the Company’s organizational documents (including, but not limited to, the Company’s bylaws), any corporate policy or any contract (including, but not limited to, any indemnification agreement), neither the Company nor any affiliate of the Company will indemnify or provide advancement for any Covered Executive against any loss of Excess Compensation. Neither the Company nor any affiliate of the Company will pay for or reimburse insurance premiums for an insurance policy that covers potential recovery obligations. In the event that the Company is required to recover Excess Compensation pursuant to the Policy from a Covered Executive who is no longer an employee, the Company will be entitled to seek recovery in order to comply with applicable law, regardless of the terms of any release of claims or separation agreement that individual may have signed.
The Committee or Board may review and modify the Policy from time to time.
If any provision of the Policy or the application of any such provision to any Covered Executive is adjudicated to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability will not affect any other provisions of the Policy or the application of such provision to another Covered Executive, and the invalid, illegal or unenforceable provisions will be deemed amended to the minimum extent necessary to render any such provision or application enforceable.
The Policy will terminate and no longer be enforceable when the Company ceases to be a listed issuer within the meaning of Section 10D of the Exchange Act.
ACKNOWLEDGEMENT
•I acknowledge that I have received and read the Compensation Recovery Policy (the “Policy”) of Nautilus Biotechnology, Inc. (the “Company”).
•I understand and acknowledge that the Policy applies to me, and all of my beneficiaries, heirs, executors, administrators or other legal representatives and that the Company’s right to recovery in order to comply with applicable law will apply, regardless of the terms of any release of claims or separation agreement I have signed or will sign in the future.
• I agree to be bound by and to comply with the Policy and understand that determinations of the Committee (as such term is used in the Policy) will be final and binding and will be given the maximum deference permitted by law.
•I understand and agree that my current indemnification rights, whether in an individual agreement or the Company’s organizational documents, exclude the right to be indemnified for amounts required to be recovered under the Policy.
•I understand that my failure to comply in all respects with the Policy is a basis for termination of my employment with the Company and any affiliate of the Company as well as any other appropriate discipline.
• I understand that my failure to comply in all respects with the Policy is a basis for termination of my employment with the Company and any affiliate of the Company as well as any other appropriate discipline.
•I understand that neither the Policy, nor the application of the Policy to me, gives rise to a resignation for good reason (or similar concept) by me under any applicable employment agreement or arrangement.
•I acknowledge that if I have questions concerning the meaning or application of the Policy, it is my responsibility to seek guidance from the Chief People Officer, Human Resources or my own personal advisers.
•I acknowledge that neither this Acknowledgement nor the Policy is meant to constitute an employment contract.
Please review, sign and return this form to Human Resources.
Covered Executive
| | | | | |
(print name)
| |
(signature)
| |
(date)
| |
Cover - USD ($)
$ in Millions |
12 Months Ended |
|
|
Dec. 31, 2023 |
Feb. 15, 2024 |
Jun. 30, 2023 |
| Cover [Abstract] |
|
|
|
| Document Type |
10-K
|
|
|
| Document Annual Report |
true
|
|
|
| Document Period End Date |
Dec. 31, 2023
|
|
|
| Current Fiscal Year End Date |
--12-31
|
|
|
| Document Transition Report |
false
|
|
|
| Entity File Number |
001-39434
|
|
|
| Entity Registrant Name |
NAUTILUS BIOTECHNOLOGY, INC.
|
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
|
| Entity Tax Identification Number |
98-1541723
|
|
|
| Entity Address, Address Line One |
2701 Eastlake Avenue East
|
|
|
| Entity Address, City or Town |
Seattle
|
|
|
| Entity Address, State or Province |
WA
|
|
|
| Entity Address, Postal Zip Code |
98102
|
|
|
| City Area Code |
206
|
|
|
| Local Phone Number |
333-2001
|
|
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
|
|
| Trading Symbol |
NAUT
|
|
|
| Security Exchange Name |
NASDAQ
|
|
|
| Entity Well-known Seasoned Issuer |
No
|
|
|
| Entity Voluntary Filers |
No
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
|
| Entity Small Business |
true
|
|
|
| Entity Emerging Growth Company |
true
|
|
|
| Entity Ex Transition Period |
false
|
|
|
| ICFR Auditor Attestation Flag |
false
|
|
|
| Document Financial Statement Error Correction [Flag] |
false
|
|
|
| Entity Shell Company |
false
|
|
|
| Entity Public Float |
|
|
$ 219
|
| Entity Common Stock, Shares Outstanding |
|
125,098,080
|
|
| Documents Incorporated by Reference |
None.
|
|
|
| Entity Central Index Key |
0001808805
|
|
|
| Amendment Flag |
false
|
|
|
| Document Fiscal Year Focus |
2023
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDocuments incorporated by reference. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-23
| Name: |
dei_DocumentsIncorporatedByReferenceTextBlock |
| Namespace Prefix: |
dei_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_IcfrAuditorAttestationFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Balance Sheets - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 19,397
|
$ 114,523
|
| Short-term investments |
154,021
|
69,948
|
| Prepaid expenses and other current assets |
3,419
|
2,738
|
| Total current assets |
176,837
|
187,209
|
| Property and equipment, net |
4,267
|
3,700
|
| Operating lease right-of-use assets |
32,634
|
28,866
|
| Long-term investments |
90,647
|
129,169
|
| Other long-term assets |
1,180
|
1,108
|
| Total assets |
305,565
|
350,052
|
| Current liabilities: |
|
|
| Accounts payable |
1,639
|
1,272
|
| Accrued expenses and other liabilities |
3,945
|
3,528
|
| Current portion of operating lease liability |
3,538
|
1,991
|
| Total current liabilities |
9,122
|
6,791
|
| Operating lease liability, net of current portion |
31,090
|
28,337
|
| Total liabilities |
40,212
|
35,128
|
| Commitments and contingencies (Note 8) |
|
|
| Stockholders’ equity: |
|
|
| Preferred stock, $0.0001 par value, 200,000,000 authorized as of December 31, 2023 and December 31, 2022, respectively; 0 shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively |
0
|
0
|
| Common stock, $0.0001 par value, 1,000,000,000 shares authorized as of December 31, 2023 and 2022, respectively; 125,068,601 and 124,865,485 shares issued and outstanding as of December 31, 2023 and 2022, respectively |
13
|
12
|
| Additional paid-in capital |
467,834
|
455,330
|
| Accumulated other comprehensive loss |
(255)
|
(1,854)
|
| Accumulated deficit |
(202,239)
|
(138,564)
|
| Total stockholders’ equity |
265,353
|
314,924
|
| Total liabilities and stockholders’ equity |
$ 305,565
|
$ 350,052
|
| X |
- DefinitionAccrued Liabilities And Other Liabilities, Current
| Name: |
naut_AccruedLiabilitiesAndOtherLiabilitiesCurrent |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecuritiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecuritiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Dec. 31, 2023 |
Dec. 31, 2022 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred stock, par value (in dollars per share) |
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares authorized (in shares) |
200,000,000
|
200,000,000
|
| Preferred stock, shares issued (in shares) |
0
|
0
|
| Preferred stock, shares outstanding (in shares) |
0
|
0
|
| Common stock, par value (in dollars per share) |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized (in shares) |
1,000,000,000
|
1,000,000,000
|
| Common stock, shares issued (in shares) |
125,068,601
|
124,865,485
|
| Common stock, shares outstanding (in shares) |
125,068,601
|
124,865,485
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Statements of Operations - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Operating expenses: |
|
|
| Research and development |
$ 47,251
|
$ 37,672
|
| General and administrative |
28,901
|
25,946
|
| Total operating expenses |
76,152
|
63,618
|
| Other income (expense): |
|
|
| Interest income |
12,550
|
5,816
|
| Other expense |
(73)
|
(122)
|
| Total other income |
12,477
|
5,694
|
| Net loss |
$ (63,675)
|
$ (57,924)
|
| Net loss per share attributable to common stockholders, basic (in dollars per share) |
$ (0.51)
|
$ (0.46)
|
| Net loss per share attributable to common stockholders, diluted (in dollars per share) |
$ (0.51)
|
$ (0.46)
|
| Weighted average shares used in computing net loss per share attributable to common stockholders, basic (in shares) |
124,919,144
|
124,589,555
|
| Weighted average shares used in computing net loss per share attributable to common stockholders, diluted (in shares) |
124,919,144
|
124,589,555
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net amount of nonoperating interest income (expense).
| Name: |
us-gaap_InterestIncomeExpenseNonoperatingNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherIncomeAndExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Statements of Comprehensive Loss - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Statement of Comprehensive Income [Abstract] |
|
|
| Net loss |
$ (63,675)
|
$ (57,924)
|
| Other comprehensive income (loss): |
|
|
| Unrealized gain (loss) on securities available-for-sale |
1,599
|
(1,670)
|
| Total other comprehensive income (loss) |
1,599
|
(1,670)
|
| Comprehensive loss |
$ (62,076)
|
$ (59,594)
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after tax and reclassification adjustment, of gain (loss) in value of unsold investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), attributable to parent. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeAvailableforsaleSecuritiesAdjustmentNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPeriodIncreaseDecreaseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfIncomeAndComprehensiveIncomeAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit) - USD ($)
$ in Thousands |
Total |
Common Stock |
Additional Paid-in Capital |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
| Beginning balance (in shares) at Dec. 31, 2021 |
|
124,303,083
|
|
|
|
| Beginning balance at Dec. 31, 2021 |
$ 363,576
|
$ 12
|
$ 444,388
|
$ (184)
|
$ (80,640)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
| Issuance of common stock upon exercise of vested stock options (in shares) |
|
463,207
|
|
|
|
| Issuance of common stock upon exercise of vested stock options |
330
|
|
330
|
|
|
| Issuance of common stock under employee stock purchase plan (in shares) |
|
99,195
|
|
|
|
| Issuance of common stock under employee stock purchase plan |
232
|
|
232
|
|
|
| Stock-based compensation expense |
10,380
|
|
10,380
|
|
|
| Other comprehensive (loss) income |
(1,670)
|
|
|
(1,670)
|
|
| Net loss |
(57,924)
|
|
|
|
(57,924)
|
| Ending balance (in shares) at Dec. 31, 2022 |
|
124,865,485
|
|
|
|
| Ending balance at Dec. 31, 2022 |
$ 314,924
|
$ 12
|
455,330
|
(1,854)
|
(138,564)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
| Issuance of common stock upon exercise of vested stock options (in shares) |
70,865
|
70,865
|
|
|
|
| Issuance of common stock upon exercise of vested stock options |
$ 104
|
|
104
|
|
|
| Issuance of common stock under employee stock purchase plan (in shares) |
|
132,251
|
|
|
|
| Issuance of common stock under employee stock purchase plan |
264
|
$ 1
|
263
|
|
|
| Stock-based compensation expense |
12,137
|
|
12,137
|
|
|
| Other comprehensive (loss) income |
1,599
|
|
|
1,599
|
|
| Net loss |
(63,675)
|
|
|
|
(63,675)
|
| Ending balance (in shares) at Dec. 31, 2023 |
|
125,068,601
|
|
|
|
| Ending balance at Dec. 31, 2023 |
$ 265,353
|
$ 13
|
$ 467,834
|
$ (255)
|
$ (202,239)
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_IncreaseDecreaseInStockholdersEquityRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of other comprehensive income (loss) attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued during the period as a result of an employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesEmployeeStockPurchasePlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate change in value for stock issued during the period as a result of employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueEmployeeStockPurchasePlan |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Consolidated Statements of Cash Flows - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Cash flows from operating activities |
|
|
| Net loss |
$ (63,675)
|
$ (57,924)
|
| Adjustments to reconcile net loss to net cash used in operating activities |
|
|
| Stock-based compensation |
12,137
|
10,380
|
| Amortization of operating lease right-of-use assets |
3,856
|
2,199
|
| Depreciation |
1,849
|
1,217
|
| Amortization (accretion) of premiums (discount) on securities, net |
(2,659)
|
(890)
|
| Changes in operating assets and liabilities: |
|
|
| Prepaid expenses and other assets |
(821)
|
756
|
| Accounts payable |
393
|
(561)
|
| Accrued expenses and other liabilities |
417
|
409
|
| Operating lease liabilities |
(3,208)
|
(1,392)
|
| Net cash used in operating activities |
(51,711)
|
(45,806)
|
| Cash flows from investing activities |
|
|
| Purchases of securities |
(112,892)
|
(186,591)
|
| Purchases of property and equipment |
(2,442)
|
(2,324)
|
| Proceeds from maturities of securities |
71,599
|
163,175
|
| Net cash used in investing activities |
(43,735)
|
(25,740)
|
| Cash flows from financing activities |
|
|
| Proceeds from exercise of stock options |
104
|
330
|
| Proceeds from issuance of common stock under employee stock purchase plan |
264
|
232
|
| Net cash provided by financing activities |
368
|
562
|
| Net decrease in cash, cash equivalents and restricted cash |
(95,078)
|
(70,984)
|
| Cash, cash equivalents and restricted cash at beginning of period |
115,477
|
186,461
|
| Cash, cash equivalents and restricted cash at end of period |
20,399
|
115,477
|
| Supplementary cash flow information on non-cash activities: |
|
|
| Right-of-use assets obtained in exchange for operating lease liability |
7,623
|
1,688
|
| Acquisitions of property and equipment included in accounts payable |
$ 148
|
$ 174
|
| X |
- DefinitionThe sum of the periodic adjustments of the differences between securities' face values and purchase prices that are charged against earnings. This is called accretion if the security was purchased at a discount and amortization if it was purchased at premium. As a noncash item, this element is an adjustment to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_AccretionAmortizationOfDiscountsAndPremiumsInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in accrued expenses, and obligations classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilitiesAndOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of fixed assets that an Entity acquires in a noncash (or part noncash) acquisition. Noncash is defined as information about all investing and financing activities of an enterprise during a period that affect recognized assets or liabilities but that do not result in cash receipts or cash payments in the period. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_NoncashOrPartNoncashAcquisitionFixedAssetsAcquired1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow to acquire investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-11
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-11
| Name: |
us-gaap_PaymentsToAcquireAvailableForSaleSecuritiesDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from sale, maturity, prepayment and call of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-11
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from the stock plan during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromStockPlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for operating lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_SupplementalCashFlowElementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Description of Business and Basis of Presentation
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Description of Business and Basis of Presentation |
Description of Business and Basis of Presentation Nautilus Biotechnology, Inc. (the “Company”) is a biotechnology company incorporated in 2016 with corporate headquarters in Seattle, Washington and research and development headquarters in San Carlos, California. Since the Company’s incorporation in 2016, the Company has devoted substantially all of its resources to research and development activities, including with respect to its proteomics platform, business planning, establishing and maintaining its intellectual property portfolio, hiring personnel, raising capital and providing general and administrative support for these operations. On June 9, 2021, Nautilus Biotechnology, Inc. a Delaware corporation (f/k/a ARYA Sciences Acquisition Corp. III, a Cayman Islands exempted company and the Company’s predecessor company (“ARYA”)), consummated the business combination (the “Business Combination”) pursuant to the terms of that certain Business Combination Agreement, dated as of February 7, 2021 (the “BCA”), by and among ARYA, Mako Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of ARYA (“Mako Merger Sub”), and Nautilus Subsidiary, Inc., a Delaware corporation (f/k/a Nautilus Biotechnology, Inc.) (“Legacy Nautilus”). As a result of the Business Combination, ARYA changed its name to “Nautilus Biotechnology, Inc.” and Mako Merger Sub merged with and into Legacy Nautilus with Legacy Nautilus surviving as the surviving company and becoming a wholly-owned subsidiary of ARYA (the “Merger” and, collectively with the other transactions described in the BCA, the “Reverse Recapitalization”). In addition, in conjunction with the completion of the Business Combination, certain investors (“PIPE Investors”) subscribed for the purchase of an aggregate of 20,000,000 shares of common stock of the Company (“New Nautilus Common Stock”) at a price of $10.00 per share for aggregate gross proceeds of $200.0 million (“PIPE Financing”). Basis of Presentation The consolidated financial statements and accompanying notes were prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and regulations of the U.S. Securities and Exchange Commission. The accompanying financial statements are consolidated for the years ended December 31, 2023 and 2022 and include the accounts of Nautilus Biotechnology, Inc. and its wholly-owned subsidiary, Nautilus Subsidiary, Inc. All intercompany transactions and balances have been eliminated upon consolidation. The Company’s reporting currency is the U.S. dollar. Certain prior period amounts were reclassified to conform to the current year presentation in Note 6, “Income Taxes.” Going Concern The Company’s consolidated financial statements have been prepared on the basis of continuity of operations, the realization of assets, and the satisfaction of liabilities in the ordinary course of business. Since inception, the Company has been engaged in developing its technology, raising capital, and recruiting personnel. The Company’s operating plan may change as a result of many factors currently unknown and there can be no assurance that the current operating plan will be achieved in the time frame anticipated by the Company, and it may need to seek additional funds sooner than planned. If adequate funds are not available to the Company on a timely basis, it may be required to delay, limit, reduce, or terminate certain commercial efforts, or pursue merger or acquisition strategies, all of which could adversely affect the holdings or the rights of the Company’s stockholders. The Company has incurred net operating losses and negative cash flows from operations in every year since inception and expects this to continue for the foreseeable future. As of December 31, 2023, the Company had an accumulated deficit of $202.2 million. The Company has funded its operations primarily with proceeds from the issuance of redeemable convertible preferred stock and common stock. In June 2021, the Company received gross proceeds of approximately $345.5 million from PIPE Investors and the Business Combination offset by approximately $18.2 million of transaction costs and underwriters’ fees relating to the closing of the Business Combination. The Company had cash, cash equivalents, and short-term investments of $173.4 million as of December 31, 2023. As of the date on which these consolidated financial statements were issued, the Company believes that its cash, cash equivalents, and short-term investments will be sufficient to fund its operations for at least the next twelve months following the issuance of the consolidated financial statements. The Company’s actual results could vary as a result of, and its near and long-term future capital requirements will depend on many factors, including its growth rate and the timing and extent of spending to support its research and development efforts. The Company has based its estimates on assumptions that may prove to be wrong, and it could use its available capital resources sooner than it currently expects. The Company may be required to seek additional equity or debt financing. Future liquidity and cash requirements will depend on numerous factors. In the event that additional financing is required, the Company may not be able to raise it on acceptable terms or at all. If the Company is unable to raise additional capital when desired, or if it cannot expand its operations or otherwise capitalize on its business opportunities because it lacks sufficient capital, its business, operating results, and financial condition would be adversely affected. Impact of the COVID-19 Coronavirus The COVID-19 pandemic has already had an adverse effect on the global economy. Additionally, concerns over the economic impact of COVID-19 have caused extreme volatility in financial and other capital markets, which may adversely affect the Company’s ability to access capital markets in the future. The level and nature of the disruption caused by COVID-19 is unpredictable, may be cyclical and long-lasting, and may again in the future adversely affect the Company's operating results.
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Significant Accounting Policies
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Significant Accounting Policies |
Significant Accounting Policies Use of Estimates The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include determining the estimated lives of property and equipment, stock-based compensation, research and development accruals, and the valuation allowance for deferred tax assets. These estimates and assumptions are based on management’s best estimates and judgment. Management evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors, including the current economic environment, which management believes to be reasonable under the circumstances. The Company adjusts such estimates and assumptions when facts and circumstances dictate. Changes in those estimates resulting from continuing changes in the economic environment will be reflected in the financial statements in future periods. As future events and their effects cannot be determined with precision, actual results could materially differ from those estimates and assumptions. Concentrations of Credit Risk and Other Risks and Uncertainties Credit risk represents the accounting loss that would be recognized as of the reporting date if counterparties failed to perform as contracted. Financial instruments, which potentially subject the Company to concentration of credit risk, consist of cash balances maintained in excess of federal depository insurance limits and investments in marketable debt securities that are not federally insured. The Company has not experienced any losses in such accounts and believes it is not exposed to significant credit risk on cash or investments. The Company relies, and expects to continue to rely, on a number of vendors to provide services, supplies and materials related to its research and development programs. The Company relies on single source suppliers for certain components and materials used in the Nautilus platform. The loss of any of these single source suppliers would require the Company to expend significant time and effort to locate and qualify an alternative source of supply for these components. The Company also relies, and expects to continue to rely, on third-party manufacturers and, in many cases, single third-party manufacturers for the production of certain reagents and antibodies. These programs could be adversely affected by a significant interruption in these services or the availability of materials. The Company is subject to risks similar to those of pre-clinical stage companies in the biopharmaceutical industry, including dependence on key individuals, the need to develop commercially viable products, competition from other companies, many of whom are larger and better capitalized, the impact of negative global and national events and the need to obtain adequate additional financing to fund the development of its products. There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection for the Company’s intellectual property will be maintained, that any products developed will obtain required regulatory approval or that any approved products will be commercially viable. Even if the Company’s development efforts are successful, it is uncertain when, if ever, the Company will generate significant revenue from the sale of its products. Segment Reporting Operating segments are defined as components of an entity where discrete financial information is evaluated regularly by the chief operating decision maker (“CODM”) in deciding how to allocate resources and in assessing performance. The Company’s Chief Executive Officer is its CODM. The Company’s CODM reviews financial information presented on a consolidated basis for the purposes of making operating decisions, allocating resources and evaluating financial performance. As such, the Company has determined that it operates in one operating and one reportable segment. The Company’s long-lived assets are entirely based in the United States. Cash and Cash Equivalents The Company considers all highly-liquid investments with an original maturity of three months or less as of the date of acquisition to be cash equivalents. Investments The Company considers investments with an original maturity greater than three months and remaining maturities less than one year to be short-term investments. The Company classifies those investments that are not required for use in current operations and that mature in more than 12 months as long-term investments. The Company classifies its marketable debt securities as available for sale and reports them at fair value, with unrealized gains and losses recorded in accumulated other comprehensive income (loss). For investments sold prior to maturity, the cost of investments sold is based on the specific identification method. Realized gains and losses on the sale of investments are recorded in other income (expense), net in the consolidated statement of operations. If the estimated fair value of a marketable debt security is below its amortized cost basis, the Company evaluates whether it is more likely than not that the Company will be required to sell the security before its anticipated recovery in market value and whether credit losses exist for the related securities. Credit-related losses are recognized as an allowance for credit losses on the balance sheet with a corresponding adjustment to earnings. Unrealized gains and losses that are unrelated to credit deterioration are reported in accumulated other comprehensive income (loss). No credit-related losses or allowance for credit losses were necessary during the periods presented. Fair Value of Financial Instruments Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include: Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement. The carrying amounts of cash and cash equivalents, prepaid expenses and other current assets, accounts payable and accrued expenses and other liabilities approximate their respective fair values due to their short-term nature. Property and Equipment, net Property and equipment, net, consisting primarily of laboratory equipment, computers, furniture and fixtures, and office equipment are recorded at cost and are depreciated on a straight-line basis over their estimated useful life. Useful lives assigned to property and equipment are as follows: | | | | | | | Laboratory equipment | 3 years to 5 years | | Prototype equipment | 3 years | | Computer hardware | 3 years | | Furniture and fixtures | 3 years | | Office equipment | 3 years to 5 years | Leasehold improvements | Shorter of estimated useful life or remaining lease term |
When assets are retired or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any resulting gain or loss is recognized as income or loss for the period. Maintenance and repairs are charged to operating expense in the period incurred. Impairment of Long-Lived Assets The Company periodically reviews its long-lived assets, including property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. With respect to property and equipment, the Company compares the carrying value of the long-lived assets with the estimated future net undiscounted cash flows expected to result from the use and eventual disposition of the asset (or asset group). Should the sum of the estimated future net undiscounted cash flows be less than the carrying value, the Company would recognize an impairment loss as of that date. An impairment loss would be measured by comparing the amount by which the carrying value exceeds the fair value of the long-lived assets. No impairment of long-lived assets was recorded in any of the periods presented. Leases The Company determines if an arrangement includes a lease at inception by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset for a period of time in exchange for consideration. Operating leases with a term of more than one year are included in operating lease right-of-use (“ROU”) assets and operating lease liabilities on the Company's consolidated balance sheets. ROU assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the obligation to make lease payments. Operating lease ROU assets and liabilities are recognized on the lease commencement date based on the present value of the future minimum lease payments over the lease term. The Company uses the incremental borrowing rate commensurate with the lease term based on the information available at the lease commencement date in determining the present value of the lease payments as the Company's leases generally do not provide an implicit rate. ROU assets initially equal the lease liability, adjusted for any prepaid lease payments and initial direct costs incurred, less any lease incentives received. Certain of the Company's leases include renewal options which allow the Company to, at its election, renew or extend the lease for a fixed or indefinite period of time. These renewal periods are included in the lease terms when the Company is reasonably certain the options will be exercised. Lease expense is recognized on a straight-line basis over the lease term when leases are operating leases. If it is considered a finance lease, expense is recognized over the lease term within interest expense and amortization in the Company’s consolidated statements of operations. The Company also has lease arrangements with lease and non-lease components. The Company elected the practical expedient not to separate non-lease components from lease components for the Company's facility leases and to account for the lease and non-lease components as a single lease component. The Company also elected to apply the short-term lease measurement and recognition exemption in which ROU assets and lease liabilities are not recognized for leases with terms of 12 months or less. Income Taxes The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the net deferred tax assets will not be realized. In making such a determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and recent results of operations, primarily over the most recent three-year period. The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained upon an audit. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being recognized. Changes in recognition or measurement are reflected in the period in which the change in judgement occurs. Research and Development Costs for research and development activities are expensed in the period in which they are incurred. Research and development expenses consist of costs incurred in performing research and development activities, including salaries, bonuses, stock-based compensation, and employee benefits for product development personnel, laboratory supplies and equipment, depreciation and amortization, external costs of vendors engaged to conduct research and development activities, and allocated expenses for technology and facilities. As part of the process of preparing its financial statements, the Company estimates its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of services performed and the associated cost incurred for services for which the Company has not yet been invoiced or otherwise notified of the actual cost. The majority of the Company’s service providers invoice monthly in arrears for services performed or when contractual milestones are met. The Company makes estimates of its accrued expenses at the end of each reporting period based on the facts and circumstances known to the Company at that time. The significant estimates in the Company’s accrued research and development expenses relate to expenses incurred with respect to academic research centers and other vendors in connection with research and development activities for which the Company has not yet been invoiced. Stock-based Compensation The Company accounts for stock-based compensation expense by calculating the estimated fair value of each employee and non-employee award at the grant date or modification date by applying the Black-Scholes option pricing model (the “model”). The model utilizes the NASDAQ closing price of the Company’s common stock at the measurement date, the expected or contractual term of the option, the expected stock price volatility, risk-free interest rates, and expected dividend yield of the common stock. Stock-based compensation expense is recognized on a straight-line basis over the requisite service period, which is generally the vesting period. Forfeitures are recognized in the period in which the forfeiture occurs. The Company classifies stock-based compensation expense in its statement of operations in the same manner in which the award recipient’s payroll costs are classified or in which the award’s recipient’s service payments are classified. The Company’s stock-based compensation programs include stock options grants, as well as shares issued under its 2021 Employee Stock Purchase Plan. The Company calculates the expected term as the mid-point between the requisite service period and the contractual term of the award. The Company bases its estimate of expected volatility on the historical volatility of comparable public companies from a representative peer group selected based on industry, financial, and market capitalization data. The Company has never declared or paid any dividends and does not currently expect to do so in the future. The risk-free interest rate used in the model is based on the implied yield currently available in the U.S. Treasury securities at maturity with an equivalent term. Comprehensive Loss Comprehensive loss consists of net loss and other gains or losses affecting stockholders’ equity that, under U.S. GAAP are excluded from net loss. For the years ended December 31, 2023 and 2022, unrealized gains and losses on marketable debt securities were included as a component of comprehensive income (loss). Net Loss per Share Attributable to Common Stockholders Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common stock outstanding during the period, without consideration of potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common stock and potentially dilutive securities outstanding for the period. For the purposes of the diluted net loss per share calculation, stock options and shares to be purchased from the Employee Stock Purchase Plan are considered to be potentially dilutive securities. The net loss is attributed entirely to the Company’s common stockholders. Since the Company has reported a net loss for all periods presented, diluted net loss per common share is the same as basic net loss per common share for those periods. Accounting Pronouncements The Company is provided the option to adopt new or revised accounting guidance as an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) either (1) within the same periods as those otherwise applicable to public business entities, or (2) within the same time periods as non-public business entities, including early adoption when permissible. With the exception of standards the Company elected to early adopt, when permissible, the Company has elected to adopt new or revised accounting guidance within the same time period as non-public business entities, as indicated below. Recently Adopted Accounting Standards In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments- Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which amends existing guidance on the impairment of financial assets and adds an impairment model that is based on expected losses rather than incurred losses and requires an entity to recognize as an allowance its estimate of expected credit losses for its financial assets. An entity will apply this guidance through a cumulative-effect adjustment to retained earnings upon adoption (a modified-retrospective approach) while a prospective transition approach is required for debt securities for which an other-than-temporary impairment had been recognized before the effective date. This ASU is effective for the Company for its fiscal year ending December 31, 2023. Early adoption is permitted. The Company adopted this ASU on January 1, 2023 and the adoption did not have a material impact on its consolidated financial statements. Recently Issued Accounting Pronouncements In November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures,” which amends existing guidance on segment reporting to improve reportable segment disclosure requirements through enhanced disclosures about significant segment expenses. The ASU is effective for the Company for the fiscal year ending December 31, 2024 and for interim periods beginning after December 31, 2024. The Company is in the process of evaluating the impact of the adoption of this Accounting Standards Update on its consolidated financial statements and related disclosures. In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” to enhance the transparency and decision usefulness of income tax disclosures. The ASU is effective for the Company for the fiscal year ending December 31, 2025. The Company is in the process of evaluating the impact of the adoption of this Accounting Standards Update on its consolidated financial statements and related disclosures. The Company does not anticipate adoption to have a material impact on its consolidated financial statements and related disclosures.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Fair Value Measurements
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value Measurements |
Fair Value Measurements The following table details the assets carried at fair value and measured on a recurring basis within the three levels of fair value as of December 31, 2023 and 2022: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | | | Gross Unrealized | | | | Reported as: | December 31, 2023 | | Amortized Cost | | Gains | | Losses | | Fair Value | | Cash equivalents | | Short-term investments | | Long-term investments | | Level 1 | | | | | | | | | | | | | | | | Mutual funds | | 590 | | | — | | | — | | | 590 | | | 590 | | | — | | | — | | | U.S. treasury securities | | 74,115 | | | 104 | | | (247) | | | 73,972 | | | — | | | 50,638 | | | 23,334 | | | Total Level 1 | | 74,705 | | | 104 | | | (247) | | | 74,562 | | | 590 | | | 50,638 | | | 23,334 | | | Level 2 | | | | | | | | | | | | | | | | Commercial paper | | 18,653 | | | — | | | (9) | | | 18,644 | | | 18,644 | | | — | | | — | | | Corporate debt securities | | 6,950 | | | — | | | (37) | | | 6,913 | | | — | | | 6,913 | | | — | | Agency securities | | 163,849 | | | 358 | | | (424) | | | 163,783 | | | — | | | 96,470 | | | 67,313 | | | Total Level 2 | | 189,452 | | | 358 | | | (470) | | | 189,340 | | | 18,644 | | | 103,383 | | | 67,313 | | | Total Level 1 and Level 2 | | $ | 264,157 | | | $ | 462 | | | $ | (717) | | | $ | 263,902 | | | $ | 19,234 | | | $ | 154,021 | | | $ | 90,647 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | | | Gross Unrealized | | | | Reported as: | December 31, 2022 | | Amortized Cost | | Gains | | Losses | | Fair Value | | Cash equivalents | | Short-term investments | | Long-term investments | | Level 1 | | | | | | | | | | | | | | | | Mutual funds | | 1,121 | | | — | | | — | | | 1,121 | | | 1,121 | | | — | | | — | | U.S. treasury securities | | 52,686 | | | 4 | | | (774) | | | 51,916 | | | — | | | 2,873 | | | 49,043 | | | Total Level 1 | | 53,807 | | | 4 | | | (774) | | | 53,037 | | | 1,121 | | | 2,873 | | | 49,043 | | | Level 2 | | | | | | | | | | | | | | | | Commercial paper | | 156,419 | | | 3 | | | (266) | | | 156,156 | | | 113,402 | | | 42,754 | | | — | | | Corporate debt securities | | 14,154 | | | — | | | (71) | | | 14,083 | | | — | | | 7,224 | | | 6,859 | | Agency securities | | 91,114 | | | 33 | | | (783) | | | 90,364 | | | — | | | 17,097 | | | 73,267 | | | Total Level 2 | | 261,687 | | | 36 | | | (1,120) | | | 260,603 | | | 113,402 | | | 67,075 | | | 80,126 | | | Total Level 1 and Level 2 | | $ | 315,494 | | | $ | 40 | | | $ | (1,894) | | | $ | 313,640 | | | $ | 114,523 | | | $ | 69,948 | | | $ | 129,169 | |
Short-term investments have a contractual maturity date that is one year or less from the respective balance sheet date. Long-term investments have a contractual maturity date that is more than one year, but less than two years from the respective balance sheet date. The unrealized losses and fair values of available-for-sale securities that have been in an unrealized loss position for a period of less than and greater than 12 months as of December 31, 2023 and December 31, 2022 are as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | Securities in Unrealized Loss Position Less than 12 months | | Securities in Unrealized Loss Position Greater than 12 months | | Total | December 31, 2023 | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | U.S. treasury securities | | $ | 85 | | | $ | 20,408 | | | $ | 162 | | | $ | 30,230 | | | $ | 247 | | | $ | 50,638 | | | Commercial paper | | 9 | | | 18,644 | | | — | | | — | | | 9 | | | 18,644 | | | Corporate debt securities | | 37 | | | 6,913 | | | — | | | — | | | 37 | | | 6,913 | | Agency securities | | 229 | | | 85,039 | | | 195 | | | 25,704 | | | 424 | | | 110,743 | | | Total | | $ | 360 | | | $ | 131,004 | | | $ | 357 | | | $ | 55,934 | | | $ | 717 | | | $ | 186,938 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | Securities in Unrealized Loss Position Less than 12 months | | Securities in Unrealized Loss Position Greater than 12 months | | Total | December 31, 2022 | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | U.S. treasury securities | | $ | 774 | | | $ | 49,114 | | | $ | — | | | $ | — | | | $ | 774 | | | $ | 49,114 | | | Commercial paper | | 266 | | | 151,354 | | | — | | | — | | | 266 | | | 151,354 | | | Corporate debt securities | | 14 | | | 6,859 | | | 57 | | | 7,224 | | | 71 | | | 14,083 | | Agency securities | | 670 | | | 50,531 | | | 113 | | | 8,887 | | | 783 | | | 59,418 | | | Total | | $ | 1,724 | | | $ | 257,858 | | | $ | 170 | | | $ | 16,111 | | | $ | 1,894 | | | $ | 273,969 | |
The Company reviewed its investment portfolio based on the underlying risk profile of the securities and has no loss expectation for these investments. The Company also reviewed the securities in an unrealized loss position and evaluated the current expected credit loss by considering factors such as historical experience, market data, issuer-specific factors, and current economic conditions. The Company recognized no credit losses during the years ended December 31, 2023 and 2022, and had no allowance for credit losses as of December 31, 2023.
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Composition of Certain Consolidated Financial Statement Line Items
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Composition of Certain Financial Statement Line Items |
Composition of Certain Consolidated Financial Statement Line Items Property and Equipment, Net Property and equipment consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Laboratory equipment | $ | 6,337 | | | $ | 4,892 | | | Leasehold improvements | 118 | | | 13 | | | Computer hardware | 222 | | | 166 | | | Furniture, fixtures and office equipment | 324 | | | 25 | | | Prototype equipment | 996 | | | 332 | | | Construction in progress | 904 | | | 1,235 | | | 8,901 | | | 6,663 | | | Less: Accumulated depreciation | (4,634) | | | (2,963) | | | Total | $ | 4,267 | | | $ | 3,700 | |
The Company recorded $1.8 million and $1.2 million of depreciation expense for the years ended December 31, 2023 and 2022, respectively, which was primarily allocated to research and development expense. Accrued Expenses and Other Liabilities Accrued expenses and other liabilities consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Employee compensation | $ | 2,590 | | | $ | 1,669 | | | Accrued research and development | 731 | | | 970 | | | Accrued professional and consulting fees | 245 | | | 451 | | | Other | 379 | | | 438 | | | Total | 3,945 | | | 3,528 | |
Cash, Cash Equivalents and Restricted Cash Cash, cash equivalents and restricted cash consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Cash and cash equivalents | 19,397 | | | 114,523 | | Restricted cash included in other long-term assets (Note 8) | 1,002 | | | 954 | | | Total | 20,399 | | | 115,477 | |
Other long-term assets consisted of $1.0 million of restricted cash and $0.2 million of deposits as of December 31, 2023, and $0.9 million of restricted cash and $0.2 million of deposits as of December 31, 2022.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for supplemental balance sheet disclosures, including descriptions and amounts for assets, liabilities, and equity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//210/tableOfContent
| Name: |
us-gaap_SupplementalBalanceSheetDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Common Stock
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Equity [Abstract] |
|
| Common Stock |
Common Stock There were 125,068,601 shares issued and outstanding as of December 31, 2023. Common Stock Reserved for Future Issuance Shares of common stock reserved for future issuance on an as-if converted basis, were as follows: | | | | | | | | | | | | | December 31,
2023 | | December 31,
2022 | | Shares available for grant under 2021 Equity Incentive Plan | 20,279,560 | | | 17,298,043 | | | Stock options issued and outstanding | 14,676,335 | | | 11,485,443 | | | Shares available for grant under 2021 Employee Stock Purchase Plan | 3,505,138 | | | 2,388,735 | | | Total shares of common stock reserved | 38,461,033 | | | 31,172,221 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Income Taxes
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Income Taxes The Company is liable for income taxes in the United States. For the years ended December 31, 2023 and 2022, the Company did not have any income for income tax purposes and therefore, no tax liability or expense has been recorded in these financial statements. The difference between the tax at the statutory federal tax rate and no tax provision recorded by the Company is primarily due to the Company’s full valuation allowance against its deferred tax assets. A reconciliation between the expected income tax provision at the federal statutory rate and the reported income tax provision is approximately as follows: | | | | | | | | | | | | | | | (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Federal income tax at statutory rate | $ | (13,372) | | | $ | (12,164) | | | | | State income tax, net of federal benefit | (4,027) | | | (3,126) | | | | Equity-based Compensation | 2,423 | | | 290 | | | | | | | | | | | Tax credits generated in current year | (2,075) | | | (2,607) | | | | | Valuation allowance change | 17,011 | | | 17,127 | | | | | Other | 40 | | | 480 | | | | | Total | $ | — | | | $ | — | | | |
As of December 31, 2023, the Company had federal net operating loss carryforwards of $0.5 million that begin to expire in 2037 and federal net operating loss carryforwards of $70.6 million that arose after the 2017 tax year that will carryforward indefinitely and will be subject to the 80% of taxable income limitation. The Company has state net operating loss carryforwards of $95.5 million that will begin to expire in 2037. As of December 31, 2023, the Company had research and development tax credit carryover of $6.8 million and $4.7 million for federal and state tax purposes, respectively. If not utilized, the federal carryforward will expire in various amounts beginning in 2039. The California credits can be carried forward indefinitely. The Company has evaluated the positive and negative evidence bearing upon its ability to realize the deferred tax assets. Management has considered the Company’s history of cumulative net losses incurred since inception and its lack of revenue since inception and has concluded that it is more likely than not that the Company will not realize the benefits of the deferred tax assets. Accordingly, the Company has provided a full valuation allowance against the net deferred tax assets. The valuation allowance increased by $17.0 million during the year ended December 31, 2023. Management reevaluates the positive and negative evidence at each reporting period. Deferred income taxes reflect the net tax effects of loss and credit carryforwards and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Components of the Company’s deferred tax assets are as follows: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Deferred tax assets | | | | | Depreciation and amortization | $ | 6,508 | | | $ | 11,142 | | | Capitalized research and development | 16,502 | | | 7,607 | | | Loss carryforwards | 21,617 | | | 12,402 | | | Lease liabilities | 9,213 | | | 8,005 | | | Tax credit carryforwards | 8,438 | | | 5,408 | | | Equity-based compensation | 4,196 | | | 4,015 | | | Other accruals and reserves | 424 | | | 246 | | | Total deferred tax assets | 66,898 | | | 48,825 | | | Valuation allowance for deferred tax assets | (58,215) | | | (41,205) | | | Total deferred tax assets, net of valuation allowance | $ | 8,683 | | | $ | 7,620 | | | | | | | Deferred tax liability | | | | | Right-of-use assets | (8,683) | | | (7,620) | | | Net deferred tax assets (liability) | $ | — | | | $ | — | |
The Company began to file income tax returns in the United States in 2017. All tax years are open to examination. The Tax Cuts and Jobs Act contained a provision which requires the capitalization of Section 174 costs incurred in years beginning on or after January 1, 2022. Section 174 costs are expenditures which represent research and development costs that are incident to the development or improvement of a product, process, formula, invention, computer software, or technique. This provision changes the treatment of Section 174 costs such that the expenditures are no longer allowed as an immediate deduction but rather must be capitalized and amortized. We have included the impact of this provision, which results in a deferred tax asset of approximately $16.5 million as of December 31, 2023. A valuation allowance of $58.2 million and $41.2 million at December 31, 2023 and 2022, respectively, has been recognized to offset net deferred tax assets where realization of such net deferred tax assets is determined to be more likely than not to not be realized. The valuation allowance increased by $17.0 million in 2023 and increased by $17.1 million in 2022, which was primarily due to changes in our deferred tax asset balances. The increases in the valuation allowance in 2023 and 2022 was primarily due to the net operating loss and tax credit generation, capitalized research and development expense and share-based compensation. The Company had an unrecognized tax benefit balance of $2.4 million and $1.5 million related to research and development credits and California net operating loss carryforward as of December 31, 2023 and 2022, respectively. No amount of unrecognized tax benefits as of December 31, 2023, if recognized, would reduce the Company’s effective tax rate because the benefits would be in the form of tax credit carryforwards, which would be reduced to $0 by a full valuation allowance. There are no provisions for which it is reasonably possible that the total amounts of unrecognized tax benefits will significantly increase or decrease within 12 months of the reporting date. Because the statute of limitations does not expire until after the net operating loss and credit carryforwards are actually used, the statutes are still open on calendars years ending 2017 forward for federal and state purposes. A reconciliation of the beginning and ending amount of the liability for uncertain tax positions, excluding potential interest and penalties, is as follows: | | | | | | | | | | (in thousands) | December 31, 2023 | December 31, 2022 | | Beginning balance | $ | 1,544 | | $ | 837 | | | Increase based on current year tax positions | 812 | | 616 | | | Increase for prior year tax positions | 9 | | 91 | | | | | | Ending balance | $ | 2,365 | | $ | 1,544 | |
Net operating loss and tax credit carry-forwards are subject to review and possible adjustment by the Internal Revenue Service (the “IRS”) and may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three-year period in excess of 50% as defined under Sections 382 and 383 in the Code, which could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the Company’s value immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has not, as yet, conducted a study to determine if any such changes have occurred that could limit its ability to use the net operating loss and tax credit carryforwards.
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Equity Incentive Plans and Stock-based Compensation
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Equity Incentive Plans and Stock-based Compensation |
Equity Incentive Plans and Stock-based Compensation On June 8, 2021, the stockholders of the Company approved the 2021 Equity Incentive Plan (“2021 Plan”) and the 2021 Employee Stock Purchase Plan (“2021 ESPP”). As of December 31, 2023, 20,279,560 and 3,505,138 shares were available for grant under the 2021 Plan and 2021 ESPP, respectively. 2021 Employee Stock Purchase Plan Under the 2021 ESPP, participants are permitted to purchase shares of Common Stock, up to the IRS allowable limit, through contributions (in the form of payroll deductions or otherwise to the extent permitted by the administrator) of up to 15% of their eligible compensation. Participants are permitted to purchase shares of the Company’s Common Stock at 85% of the lower of the fair market value of the Company’s Common Stock on the first trading day of an offering period or on the last trading date in each purchase period. Participants may end their participation at any time during an offering and will be paid their accrued contributions that have not yet been used to purchase shares. Participation ends automatically upon termination of employment with the Company. The number of shares of common stock available for issuance under the 2021 ESPP will be increased on the first day of each fiscal year beginning on January 1, 2022, in an amount equal to the least of (i) 3,734,500 shares of common stock, (ii) a number of shares of common stock equal to one percent (1%) of the total number of shares of all classes of common stock of the Company on the last day of the immediately preceding fiscal year, or (iii) such number of shares determined by the Administrator no later than the last day of the immediately preceding fiscal year. The first offering period was from October 1, 2021 through June 1, 2022. For subsequent offering periods, the Company offers a six month purchase period. During the years ended December 31, 2023 and 2022, 132,251 and 99,195 shares of common stock, respectively, were purchased under the 2021 ESPP. 2021 Equity Incentive Plan Under the 2021 Plan, the Company can grant incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units and performance awards to employees, non-employee directors and consultants. Options generally expire ten years after the date of grant. The number of shares available for issuance under the 2021 Plan will be increased on the first day of each fiscal year, beginning on January 1, 2022, in an amount equal to the least of (i) 18,672,200 shares, (ii) a number of shares equal to five percent (5%) of the total number of shares of all classes of common stock of the Company outstanding on the last day of the immediately preceding fiscal year, or (iii) such number of shares determined by the Administrator no later than the last day of the immediately preceding fiscal year. 2017 Equity Incentive Plan At the time of adoption of the 2021 Plan and the 2021 ESPP, no further awards will be granted under the 2017 Equity Incentive Plan (“2017 Plan”). Stock-based awards forfeited or cancelled from the 2017 Plan are returned to the pool of shares of Common Stock available for issuance under the 2021 Plan. Grant Date Fair Value of Stock Options In determining the compensation cost of the option awards, the fair value for each option award has been estimated using the Black Scholes model. The significant assumptions used in these calculations are summarized as follows: | | | | | | | | | | | | | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Expected term (in years) | 5.3 - 6.4 | | 5.3 - 6.1 | | | | Expected volatility | 102.7% - 107.5% | | 105.2% - 110.4% | | | | Expected dividend rate | 0.0% | | 0.0% | | | | Risk free interest rate | 3.5% - 4.8% | | 1.7% - 4.4% | | | | | | | | |
Expected term: The expected term of stock options represents the weighted-average period the stock options are expected to remain outstanding. The Company does not have sufficient historical exercise and post-vesting termination activity to provide accurate data for estimating the expected term of options and has opted to use the “simplified method,” whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. Expected volatility: Historically, the Company has been a private company and lacked company‑specific historical and implied volatility information for its common stock. Therefore, the expected volatility of the Company’s common stock was determined by using an average of historical volatilities of selected industry peers deemed to be comparable to the Company’s business corresponding to the expected term of the awards and the Company expects to continue to do so until such time as the Company has adequate historical data regarding the volatility of its traded common stock price. Expected dividend yield: The expected dividend rate is zero as the Company has no history or expectation of declaring dividends on its common stock. Risk-free interest rate: The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for zero-coupon U.S. Treasury notes with maturities corresponding to the expected term of the awards. The following table summarizes option award activity during the year ended December 31, 2023: | | | | | | | | | | | | | | | | | | | | | | | | | Number of Stock Option Awards | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Life (Years) | | Aggregate Intrinsic Value
(in thousands) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Outstanding as of December 31, 2022 | 11,485,443 | | | $ | 4.12 | | | | | | | | | | | | | | | | | | | | | | | Granted | 4,009,775 | | | $ | 2.42 | | | | | | | Exercised | (70,865) | | | $ | 1.46 | | | | | | | Forfeited | (748,018) | | | $ | 4.97 | | | | | | Outstanding as of December 31, 2023 | 14,676,335 | | | $ | 3.63 | | | 7.8 | | $ | 11,451 | | Options vested and expected to vest as of December 31, 2023 | 14,676,335 | | | $ | 3.63 | | | | | | Vested and exercisable at December 31, 2023 | 7,488,649 | | | $ | 3.90 | | | 7.1 | | $ | 7,366 | |
As of December 31, 2023, there was $19.1 million of total unrecognized compensation expense expected to be recognized over a weighted average-period of 2.1 years. The total intrinsic value of options exercised during the years ended December 31, 2023 and 2022 was $0.1 million and $1.2 million, respectively. Aggregate intrinsic value represents the difference between the fair market value of the common stock and the exercise price of outstanding, in-the-money options. The weighted-average grant-date fair value of options granted during the years ended December 31, 2023 and 2022 was $1.98 and $2.98 per share, respectively. Stock-based Compensation Expense The following sets forth the total stock-based compensation expense for the Company’s stock options and ESPP included in the Company’s consolidated statements of operations: | | | | | | | | | | | | | | | (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Research and development | $ | 4,199 | | | $ | 3,840 | | | | | General and administrative | 7,938 | | | 6,540 | | | | | Total stock-based compensation expense | $ | 12,137 | | | $ | 10,380 | | | |
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Commitments and Contingencies
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Commitments and Contingencies Purchase Commitments Open purchase commitments are for the purchase of goods and services related to, but not limited to, research and development, facilities, and professional services under non-cancellable contracts. They were not recorded as liabilities on the consolidated balance sheet as of December 31, 2023 as the Company had not yet received the related goods or services. As of December 31, 2023, the Company had open purchase commitments for goods and services of $2.5 million, of which $1.8 million are expected to be received through the next 12 months. Legal Proceedings From time to time, the Company may become involved in litigation relating to claims arising from the ordinary course of business where the expense, distraction and/or the ultimate disposition could have a material adverse effect on the Company’s results of operations, financial condition or cash flows. The Company filed suit in the United States District Court for the Northern District of California against Somalogic (now known as Standard Biotools, Inc.), and the California Institute of Technology seeking declaratory judgment that the Company does not infringe any claims of the ‘793 Patent, allegedly licensed from the California Institute of Technology through Somalogic’s purchase of Palamedrix, Inc. While the Company believes that it will be able to prevail as to its claims in this litigation, the Company may incur significant expense and management distraction in prosecuting this litigation. Moreover, notwithstanding management’s belief as to the strength of our claims in this matter, litigation generally, and patent litigation in particular, can be unpredictable. As such, management cannot be certain that the Company will prevail in this litigation. Leases The Company is obligated under certain non-cancellable operating leases for office space and laboratory space. This space includes operating leases in Seattle, Washington, San Carlos, California, and San Diego, California. Seattle Lease In July 2021, the Company entered into a 7-year non-cancellable operating lease, which commenced in August 2021, for office space in Seattle, Washington. Total non-cancellable payments under this lease aggregate $4.5 million through June 2028. San Carlos Leases In December 2020, the Company entered into a new lease in San Carlos, California for ten years which commenced in October 2021 and expiring in October 2031 with total minimum lease payments of $40.7 million. In December 2021, the Company entered into another lease in San Carlos, California for nine years commencing in March 2023. The Company can terminate this lease after five years from the commencement date without bearing any significant termination penalties and therefore the Company concluded that the lease term is five years with total minimum lease payments of $7.2 million. The Company utilized $2.0 million from the landlord with an interest rate of 7% to finance its tenant improvements. The principal and interest payments are included in the payments used to measure the lease liability. San Diego Lease In November 2022, the Company entered into a lease in San Diego, California for 39 months commencing in December 2022. Total non-cancellable payments under this lease aggregate $2.1 million through March 2026. The components of lease costs which were included in operating expenses in the consolidated statements of operations were as follows: | | | | | | | | | | | | | (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Fixed operating lease costs | $ | 6,893 | | | $ | 4,751 | | | Variable operating lease costs | 2,432 | | | 1,655 | | | Short-term lease costs | — | | | 25 | | | Total lease costs | $ | 9,325 | | | $ | 6,431 | |
For the years ended December 31, 2023 and 2022, cash paid for amounts included in the measurement of operating lease liabilities included in cash flows used in operating activities was $6.2 million and $3.9 million, respectively. As of December 31, 2023, the weighted-average remaining lease term and weighted-average discount rate for operating leases was 6.7 years and 9.1%, respectively. The following table summarizes the Company's future principal contractual obligations for operating lease commitments as of December 31, 2023: | | | | | | | (in thousands) | | | Year Ended December 31, | Lease Obligations | | 2024 | 6,414 | | | 2025 | 7,186 | | | 2026 | 6,878 | | | 2027 | 6,893 | | | 2028 and thereafter | 19,028 | | | Total future minimum lease payments | 46,399 | | | Less: Imputed interest | (11,771) | | | Total operating lease liabilities | $ | 34,628 | |
Guarantees and Indemnifications In the ordinary course of business, the Company enters into agreements that may include indemnification provisions. Pursuant to such agreements, the Company may indemnify, hold harmless and defend an indemnified party for losses suffered or incurred by the indemnified party. Some of the provisions will limit losses to those arising from third-party actions. In some cases, the indemnifications will continue after the termination of the agreement. The maximum potential amount of future payments the Company could be required to make under these provisions is not determinable. The Company has never incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. The Company has also agreed to indemnify its directors and executive officers for costs associated with any fees, expenses, judgments, fines and settlement amounts incurred by them in any action or proceeding to which any of them are, or are threatened to be, made a party by reason of their service as a director or officer. The Company maintains director and officer insurance coverage that would generally enable it to recover a portion of any future amounts paid. The Company may be subject to indemnification obligation by law with respect to the actions of its employees under certain circumstances and in certain jurisdictions. Letters of Credit In conjunction with the San Carlos lease agreement entered in December 2020, the Company issued a cash-collateralized letter of credit in lieu of security deposit of $0.6 million. In conjunction with the San Carlos lease agreement entered in December 2021, the Company issued a cash-collateralized letter of credit in lieu of security deposit of $0.2 million. In conjunction with the San Diego lease agreement entered in November 2022, the Company issued a cash-collateralized letter of credit in lieu of a security deposit of $0.1 million.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Basic and Diluted Net Loss per Share
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Earnings Per Share [Abstract] |
|
| Basic and Diluted Net Loss per Share |
Basic and Diluted Net Loss per Share The following tables set forth the computation of the Company’s basic and diluted net loss per share attributable to common stockholders for the years ended December 31, 2023 and 2022: | | | | | | | | | | | | | | | (in thousands, except share and per share data) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Numerator: | | | | | | | Net loss attributable to common stockholders | $ | (63,675) | | | $ | (57,924) | | | | | Denominator: | | | | | | | | | | | | | | | | | | | Weighted average shares used in computing net loss per share attributable to common stockholders, basic and diluted | 124,919,144 | | 124,589,555 | | | | Net loss per share attributable to common stockholders, basic and diluted: | $ | (0.51) | | | $ | (0.46) | | | |
The potential shares of common stock that were excluded from the computation of diluted net loss per share attributable to common stockholders for the periods presented because including them would have had an antidilutive effect were as follows: | | | | | | | | | | | | | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Options to purchase common stock | 14,676,335 | | 11,485,443 | | | | Employee stock purchase plan | 141,720 | | 63,246 | | | | Total potentially dilutive common share equivalents | 14,818,055 | | 11,548,689 | | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Significant Accounting Policies (Policies)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation |
The consolidated financial statements and accompanying notes were prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and regulations of the U.S. Securities and Exchange Commission. The accompanying financial statements are consolidated for the years ended December 31, 2023 and 2022 and include the accounts of Nautilus Biotechnology, Inc. and its wholly-owned subsidiary, Nautilus Subsidiary, Inc. All intercompany transactions and balances have been eliminated upon consolidation. The Company’s reporting currency is the U.S. dollar.
|
| Reclassifications |
Certain prior period amounts were reclassified to conform to the current year presentation in Note 6, “Income Taxes.”
|
| Use of Estimates |
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements, and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include determining the estimated lives of property and equipment, stock-based compensation, research and development accruals, and the valuation allowance for deferred tax assets. These estimates and assumptions are based on management’s best estimates and judgment. Management evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors, including the current economic environment, which management believes to be reasonable under the circumstances. The Company adjusts such estimates and assumptions when facts and circumstances dictate. Changes in those estimates resulting from continuing changes in the economic environment will be reflected in the financial statements in future periods. As future events and their effects cannot be determined with precision, actual results could materially differ from those estimates and assumptions.
|
| Concentrations of Credit Risk and Other Risks and Uncertainties |
Credit risk represents the accounting loss that would be recognized as of the reporting date if counterparties failed to perform as contracted. Financial instruments, which potentially subject the Company to concentration of credit risk, consist of cash balances maintained in excess of federal depository insurance limits and investments in marketable debt securities that are not federally insured. The Company has not experienced any losses in such accounts and believes it is not exposed to significant credit risk on cash or investments. The Company relies, and expects to continue to rely, on a number of vendors to provide services, supplies and materials related to its research and development programs. The Company relies on single source suppliers for certain components and materials used in the Nautilus platform. The loss of any of these single source suppliers would require the Company to expend significant time and effort to locate and qualify an alternative source of supply for these components. The Company also relies, and expects to continue to rely, on third-party manufacturers and, in many cases, single third-party manufacturers for the production of certain reagents and antibodies. These programs could be adversely affected by a significant interruption in these services or the availability of materials. The Company is subject to risks similar to those of pre-clinical stage companies in the biopharmaceutical industry, including dependence on key individuals, the need to develop commercially viable products, competition from other companies, many of whom are larger and better capitalized, the impact of negative global and national events and the need to obtain adequate additional financing to fund the development of its products. There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection for the Company’s intellectual property will be maintained, that any products developed will obtain required regulatory approval or that any approved products will be commercially viable. Even if the Company’s development efforts are successful, it is uncertain when, if ever, the Company will generate significant revenue from the sale of its products.
|
| Segment Reporting |
Operating segments are defined as components of an entity where discrete financial information is evaluated regularly by the chief operating decision maker (“CODM”) in deciding how to allocate resources and in assessing performance. The Company’s Chief Executive Officer is its CODM. The Company’s CODM reviews financial information presented on a consolidated basis for the purposes of making operating decisions, allocating resources and evaluating financial performance. As such, the Company has determined that it operates in one operating and one reportable segment. The Company’s long-lived assets are entirely based in the United States.
|
| Cash and Cash Equivalents |
The Company considers all highly-liquid investments with an original maturity of three months or less as of the date of acquisition to be cash equivalents.
|
| Investments |
The Company considers investments with an original maturity greater than three months and remaining maturities less than one year to be short-term investments. The Company classifies those investments that are not required for use in current operations and that mature in more than 12 months as long-term investments. The Company classifies its marketable debt securities as available for sale and reports them at fair value, with unrealized gains and losses recorded in accumulated other comprehensive income (loss). For investments sold prior to maturity, the cost of investments sold is based on the specific identification method. Realized gains and losses on the sale of investments are recorded in other income (expense), net in the consolidated statement of operations. If the estimated fair value of a marketable debt security is below its amortized cost basis, the Company evaluates whether it is more likely than not that the Company will be required to sell the security before its anticipated recovery in market value and whether credit losses exist for the related securities. Credit-related losses are recognized as an allowance for credit losses on the balance sheet with a corresponding adjustment to earnings. Unrealized gains and losses that are unrelated to credit deterioration are reported in accumulated other comprehensive income (loss). No credit-related losses or allowance for credit losses were necessary during the periods presented.
|
| Fair Value of Financial Instruments |
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include: Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement. The carrying amounts of cash and cash equivalents, prepaid expenses and other current assets, accounts payable and accrued expenses and other liabilities approximate their respective fair values due to their short-term nature.
|
| Property and Equipment, net |
Property and equipment, net, consisting primarily of laboratory equipment, computers, furniture and fixtures, and office equipment are recorded at cost and are depreciated on a straight-line basis over their estimated useful life. Useful lives assigned to property and equipment are as follows: | | | | | | | Laboratory equipment | 3 years to 5 years | | Prototype equipment | 3 years | | Computer hardware | 3 years | | Furniture and fixtures | 3 years | | Office equipment | 3 years to 5 years | Leasehold improvements | Shorter of estimated useful life or remaining lease term |
When assets are retired or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any resulting gain or loss is recognized as income or loss for the period. Maintenance and repairs are charged to operating expense in the period incurred.
|
| Impairment of Long-Lived Assets |
The Company periodically reviews its long-lived assets, including property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. With respect to property and equipment, the Company compares the carrying value of the long-lived assets with the estimated future net undiscounted cash flows expected to result from the use and eventual disposition of the asset (or asset group). Should the sum of the estimated future net undiscounted cash flows be less than the carrying value, the Company would recognize an impairment loss as of that date. An impairment loss would be measured by comparing the amount by which the carrying value exceeds the fair value of the long-lived assets.
|
| Leases |
The Company determines if an arrangement includes a lease at inception by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset for a period of time in exchange for consideration. Operating leases with a term of more than one year are included in operating lease right-of-use (“ROU”) assets and operating lease liabilities on the Company's consolidated balance sheets. ROU assets represent the Company's right to use an underlying asset for the lease term and lease liabilities represent the obligation to make lease payments. Operating lease ROU assets and liabilities are recognized on the lease commencement date based on the present value of the future minimum lease payments over the lease term. The Company uses the incremental borrowing rate commensurate with the lease term based on the information available at the lease commencement date in determining the present value of the lease payments as the Company's leases generally do not provide an implicit rate. ROU assets initially equal the lease liability, adjusted for any prepaid lease payments and initial direct costs incurred, less any lease incentives received. Certain of the Company's leases include renewal options which allow the Company to, at its election, renew or extend the lease for a fixed or indefinite period of time. These renewal periods are included in the lease terms when the Company is reasonably certain the options will be exercised. Lease expense is recognized on a straight-line basis over the lease term when leases are operating leases. If it is considered a finance lease, expense is recognized over the lease term within interest expense and amortization in the Company’s consolidated statements of operations. The Company also has lease arrangements with lease and non-lease components. The Company elected the practical expedient not to separate non-lease components from lease components for the Company's facility leases and to account for the lease and non-lease components as a single lease component. The Company also elected to apply the short-term lease measurement and recognition exemption in which ROU assets and lease liabilities are not recognized for leases with terms of 12 months or less.
|
| Income Taxes |
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the net deferred tax assets will not be realized. In making such a determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and recent results of operations, primarily over the most recent three-year period. The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained upon an audit. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being recognized. Changes in recognition or measurement are reflected in the period in which the change in judgement occurs.
|
| Research and Development |
Costs for research and development activities are expensed in the period in which they are incurred. Research and development expenses consist of costs incurred in performing research and development activities, including salaries, bonuses, stock-based compensation, and employee benefits for product development personnel, laboratory supplies and equipment, depreciation and amortization, external costs of vendors engaged to conduct research and development activities, and allocated expenses for technology and facilities. As part of the process of preparing its financial statements, the Company estimates its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of services performed and the associated cost incurred for services for which the Company has not yet been invoiced or otherwise notified of the actual cost. The majority of the Company’s service providers invoice monthly in arrears for services performed or when contractual milestones are met. The Company makes estimates of its accrued expenses at the end of each reporting period based on the facts and circumstances known to the Company at that time. The significant estimates in the Company’s accrued research and development expenses relate to expenses incurred with respect to academic research centers and other vendors in connection with research and development activities for which the Company has not yet been invoiced.
|
| Stock-based Compensation |
The Company accounts for stock-based compensation expense by calculating the estimated fair value of each employee and non-employee award at the grant date or modification date by applying the Black-Scholes option pricing model (the “model”). The model utilizes the NASDAQ closing price of the Company’s common stock at the measurement date, the expected or contractual term of the option, the expected stock price volatility, risk-free interest rates, and expected dividend yield of the common stock. Stock-based compensation expense is recognized on a straight-line basis over the requisite service period, which is generally the vesting period. Forfeitures are recognized in the period in which the forfeiture occurs. The Company classifies stock-based compensation expense in its statement of operations in the same manner in which the award recipient’s payroll costs are classified or in which the award’s recipient’s service payments are classified. The Company’s stock-based compensation programs include stock options grants, as well as shares issued under its 2021 Employee Stock Purchase Plan. The Company calculates the expected term as the mid-point between the requisite service period and the contractual term of the award. The Company bases its estimate of expected volatility on the historical volatility of comparable public companies from a representative peer group selected based on industry, financial, and market capitalization data. The Company has never declared or paid any dividends and does not currently expect to do so in the future. The risk-free interest rate used in the model is based on the implied yield currently available in the U.S. Treasury securities at maturity with an equivalent term.
|
| Comprehensive loss |
Comprehensive loss consists of net loss and other gains or losses affecting stockholders’ equity that, under U.S. GAAP are excluded from net loss. For the years ended December 31, 2023 and 2022, unrealized gains and losses on marketable debt securities were included as a component of comprehensive income (loss).
|
| Net Loss per Share Attributable to Common Stockholders |
Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common stock outstanding during the period, without consideration of potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common stock and potentially dilutive securities outstanding for the period. For the purposes of the diluted net loss per share calculation, stock options and shares to be purchased from the Employee Stock Purchase Plan are considered to be potentially dilutive securities. The net loss is attributed entirely to the Company’s common stockholders. Since the Company has reported a net loss for all periods presented, diluted net loss per common share is the same as basic net loss per common share for those periods.
|
| Accounting Pronouncements |
The Company is provided the option to adopt new or revised accounting guidance as an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) either (1) within the same periods as those otherwise applicable to public business entities, or (2) within the same time periods as non-public business entities, including early adoption when permissible. With the exception of standards the Company elected to early adopt, when permissible, the Company has elected to adopt new or revised accounting guidance within the same time period as non-public business entities, as indicated below. Recently Adopted Accounting Standards In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments- Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which amends existing guidance on the impairment of financial assets and adds an impairment model that is based on expected losses rather than incurred losses and requires an entity to recognize as an allowance its estimate of expected credit losses for its financial assets. An entity will apply this guidance through a cumulative-effect adjustment to retained earnings upon adoption (a modified-retrospective approach) while a prospective transition approach is required for debt securities for which an other-than-temporary impairment had been recognized before the effective date. This ASU is effective for the Company for its fiscal year ending December 31, 2023. Early adoption is permitted. The Company adopted this ASU on January 1, 2023 and the adoption did not have a material impact on its consolidated financial statements. Recently Issued Accounting Pronouncements In November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures,” which amends existing guidance on segment reporting to improve reportable segment disclosure requirements through enhanced disclosures about significant segment expenses. The ASU is effective for the Company for the fiscal year ending December 31, 2024 and for interim periods beginning after December 31, 2024. The Company is in the process of evaluating the impact of the adoption of this Accounting Standards Update on its consolidated financial statements and related disclosures. In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” to enhance the transparency and decision usefulness of income tax disclosures. The ASU is effective for the Company for the fiscal year ending December 31, 2025. The Company is in the process of evaluating the impact of the adoption of this Accounting Standards Update on its consolidated financial statements and related disclosures. The Company does not anticipate adoption to have a material impact on its consolidated financial statements and related disclosures.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for comprehensive income.
| Name: |
us-gaap_ComprehensiveIncomePolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480981/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for investment in financial asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(3)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(f)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 12
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-12
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_InvestmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for reclassification affecting comparability of financial statement. Excludes amendment to accounting standards, other change in accounting principle, and correction of error. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 205
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483504/205-10-50-1
| Name: |
us-gaap_PriorPeriodReclassificationAdjustmentDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482785/280-10-55-47
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for award under share-based payment arrangement. Includes, but is not limited to, methodology and assumption used in measuring cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.C.Q3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.1.Q5)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.3.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.2.Q6)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationOptionAndIncentivePlansPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Significant Accounting Policies (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Useful Lives Assigned to Property and Equipment |
Useful lives assigned to property and equipment are as follows: | | | | | | | Laboratory equipment | 3 years to 5 years | | Prototype equipment | 3 years | | Computer hardware | 3 years | | Furniture and fixtures | 3 years | | Office equipment | 3 years to 5 years | Leasehold improvements | Shorter of estimated useful life or remaining lease term |
Property and equipment consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Laboratory equipment | $ | 6,337 | | | $ | 4,892 | | | Leasehold improvements | 118 | | | 13 | | | Computer hardware | 222 | | | 166 | | | Furniture, fixtures and office equipment | 324 | | | 25 | | | Prototype equipment | 996 | | | 332 | | | Construction in progress | 904 | | | 1,235 | | | 8,901 | | | 6,663 | | | Less: Accumulated depreciation | (4,634) | | | (2,963) | | | Total | $ | 4,267 | | | $ | 3,700 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Fair Value Measurements (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of Assets Carried at Fair Value and Measured on a Recurring Basis |
The following table details the assets carried at fair value and measured on a recurring basis within the three levels of fair value as of December 31, 2023 and 2022: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | | | Gross Unrealized | | | | Reported as: | December 31, 2023 | | Amortized Cost | | Gains | | Losses | | Fair Value | | Cash equivalents | | Short-term investments | | Long-term investments | | Level 1 | | | | | | | | | | | | | | | | Mutual funds | | 590 | | | — | | | — | | | 590 | | | 590 | | | — | | | — | | | U.S. treasury securities | | 74,115 | | | 104 | | | (247) | | | 73,972 | | | — | | | 50,638 | | | 23,334 | | | Total Level 1 | | 74,705 | | | 104 | | | (247) | | | 74,562 | | | 590 | | | 50,638 | | | 23,334 | | | Level 2 | | | | | | | | | | | | | | | | Commercial paper | | 18,653 | | | — | | | (9) | | | 18,644 | | | 18,644 | | | — | | | — | | | Corporate debt securities | | 6,950 | | | — | | | (37) | | | 6,913 | | | — | | | 6,913 | | | — | | Agency securities | | 163,849 | | | 358 | | | (424) | | | 163,783 | | | — | | | 96,470 | | | 67,313 | | | Total Level 2 | | 189,452 | | | 358 | | | (470) | | | 189,340 | | | 18,644 | | | 103,383 | | | 67,313 | | | Total Level 1 and Level 2 | | $ | 264,157 | | | $ | 462 | | | $ | (717) | | | $ | 263,902 | | | $ | 19,234 | | | $ | 154,021 | | | $ | 90,647 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | | | Gross Unrealized | | | | Reported as: | December 31, 2022 | | Amortized Cost | | Gains | | Losses | | Fair Value | | Cash equivalents | | Short-term investments | | Long-term investments | | Level 1 | | | | | | | | | | | | | | | | Mutual funds | | 1,121 | | | — | | | — | | | 1,121 | | | 1,121 | | | — | | | — | | U.S. treasury securities | | 52,686 | | | 4 | | | (774) | | | 51,916 | | | — | | | 2,873 | | | 49,043 | | | Total Level 1 | | 53,807 | | | 4 | | | (774) | | | 53,037 | | | 1,121 | | | 2,873 | | | 49,043 | | | Level 2 | | | | | | | | | | | | | | | | Commercial paper | | 156,419 | | | 3 | | | (266) | | | 156,156 | | | 113,402 | | | 42,754 | | | — | | | Corporate debt securities | | 14,154 | | | — | | | (71) | | | 14,083 | | | — | | | 7,224 | | | 6,859 | | Agency securities | | 91,114 | | | 33 | | | (783) | | | 90,364 | | | — | | | 17,097 | | | 73,267 | | | Total Level 2 | | 261,687 | | | 36 | | | (1,120) | | | 260,603 | | | 113,402 | | | 67,075 | | | 80,126 | | | Total Level 1 and Level 2 | | $ | 315,494 | | | $ | 40 | | | $ | (1,894) | | | $ | 313,640 | | | $ | 114,523 | | | $ | 69,948 | | | $ | 129,169 | |
|
| Schedule of Unrealized Losses and Fair Values of Available-for-Sale Securities in an Unrealized Loss Position |
The unrealized losses and fair values of available-for-sale securities that have been in an unrealized loss position for a period of less than and greater than 12 months as of December 31, 2023 and December 31, 2022 are as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | Securities in Unrealized Loss Position Less than 12 months | | Securities in Unrealized Loss Position Greater than 12 months | | Total | December 31, 2023 | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | U.S. treasury securities | | $ | 85 | | | $ | 20,408 | | | $ | 162 | | | $ | 30,230 | | | $ | 247 | | | $ | 50,638 | | | Commercial paper | | 9 | | | 18,644 | | | — | | | — | | | 9 | | | 18,644 | | | Corporate debt securities | | 37 | | | 6,913 | | | — | | | — | | | 37 | | | 6,913 | | Agency securities | | 229 | | | 85,039 | | | 195 | | | 25,704 | | | 424 | | | 110,743 | | | Total | | $ | 360 | | | $ | 131,004 | | | $ | 357 | | | $ | 55,934 | | | $ | 717 | | | $ | 186,938 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (in thousands) | | Securities in Unrealized Loss Position Less than 12 months | | Securities in Unrealized Loss Position Greater than 12 months | | Total | December 31, 2022 | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | Gross Unrealized Losses | | Fair Market Value | | U.S. treasury securities | | $ | 774 | | | $ | 49,114 | | | $ | — | | | $ | — | | | $ | 774 | | | $ | 49,114 | | | Commercial paper | | 266 | | | 151,354 | | | — | | | — | | | 266 | | | 151,354 | | | Corporate debt securities | | 14 | | | 6,859 | | | 57 | | | 7,224 | | | 71 | | | 14,083 | | Agency securities | | 670 | | | 50,531 | | | 113 | | | 8,887 | | | 783 | | | 59,418 | | | Total | | $ | 1,724 | | | $ | 257,858 | | | $ | 170 | | | $ | 16,111 | | | $ | 1,894 | | | $ | 273,969 | |
|
| X |
- DefinitionTabular disclosure of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-9
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, by class that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsMeasuredOnRecurringBasisTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Composition of Certain Consolidated Financial Statement Line Items (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Property and Equipment, Net |
Useful lives assigned to property and equipment are as follows: | | | | | | | Laboratory equipment | 3 years to 5 years | | Prototype equipment | 3 years | | Computer hardware | 3 years | | Furniture and fixtures | 3 years | | Office equipment | 3 years to 5 years | Leasehold improvements | Shorter of estimated useful life or remaining lease term |
Property and equipment consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Laboratory equipment | $ | 6,337 | | | $ | 4,892 | | | Leasehold improvements | 118 | | | 13 | | | Computer hardware | 222 | | | 166 | | | Furniture, fixtures and office equipment | 324 | | | 25 | | | Prototype equipment | 996 | | | 332 | | | Construction in progress | 904 | | | 1,235 | | | 8,901 | | | 6,663 | | | Less: Accumulated depreciation | (4,634) | | | (2,963) | | | Total | $ | 4,267 | | | $ | 3,700 | |
|
| Accrued Expenses |
Accrued expenses and other liabilities consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Employee compensation | $ | 2,590 | | | $ | 1,669 | | | Accrued research and development | 731 | | | 970 | | | Accrued professional and consulting fees | 245 | | | 451 | | | Other | 379 | | | 438 | | | Total | 3,945 | | | 3,528 | |
|
| Other Liabilities |
Accrued expenses and other liabilities consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Employee compensation | $ | 2,590 | | | $ | 1,669 | | | Accrued research and development | 731 | | | 970 | | | Accrued professional and consulting fees | 245 | | | 451 | | | Other | 379 | | | 438 | | | Total | 3,945 | | | 3,528 | |
|
| Cash, Cash Equivalents |
Cash, cash equivalents and restricted cash consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Cash and cash equivalents | 19,397 | | | 114,523 | | Restricted cash included in other long-term assets (Note 8) | 1,002 | | | 954 | | | Total | 20,399 | | | 115,477 | |
|
| Restricted Cash |
Cash, cash equivalents and restricted cash consisted of the following: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Cash and cash equivalents | 19,397 | | | 114,523 | | Restricted cash included in other long-term assets (Note 8) | 1,002 | | | 954 | | | Total | 20,399 | | | 115,477 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of other current liabilities.
| Name: |
us-gaap_OtherCurrentLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of cash and cash equivalents.
| Name: |
us-gaap_ScheduleOfCashAndCashEquivalentsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of cash and cash equivalents restricted as to withdrawal or usage. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_ScheduleOfRestrictedCashAndCashEquivalentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Common Stock (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Equity [Abstract] |
|
| Schedule of Common Stock Reserved for Future Issuance |
Shares of common stock reserved for future issuance on an as-if converted basis, were as follows: | | | | | | | | | | | | | December 31,
2023 | | December 31,
2022 | | Shares available for grant under 2021 Equity Incentive Plan | 20,279,560 | | | 17,298,043 | | | Stock options issued and outstanding | 14,676,335 | | | 11,485,443 | | | Shares available for grant under 2021 Employee Stock Purchase Plan | 3,505,138 | | | 2,388,735 | | | Total shares of common stock reserved | 38,461,033 | | | 31,172,221 | |
|
| X |
- DefinitionSchedule Of Common Stock Reserved For Future Issuance
| Name: |
naut_ScheduleOfCommonStockReservedForFutureIssuanceTableTextBlock |
| Namespace Prefix: |
naut_ |
| Data Type: |
dtr-types1:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Income Taxes (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Schedule of Effective Income Tax Rate Reconciliation |
A reconciliation between the expected income tax provision at the federal statutory rate and the reported income tax provision is approximately as follows: | | | | | | | | | | | | | | | (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Federal income tax at statutory rate | $ | (13,372) | | | $ | (12,164) | | | | | State income tax, net of federal benefit | (4,027) | | | (3,126) | | | | Equity-based Compensation | 2,423 | | | 290 | | | | | | | | | | | Tax credits generated in current year | (2,075) | | | (2,607) | | | | | Valuation allowance change | 17,011 | | | 17,127 | | | | | Other | 40 | | | 480 | | | | | Total | $ | — | | | $ | — | | | |
|
| Schedule of Deferred Tax Assets and Liabilities |
Components of the Company’s deferred tax assets are as follows: | | | | | | | | | | | | | (in thousands) | December 31, 2023 | | December 31, 2022 | | Deferred tax assets | | | | | Depreciation and amortization | $ | 6,508 | | | $ | 11,142 | | | Capitalized research and development | 16,502 | | | 7,607 | | | Loss carryforwards | 21,617 | | | 12,402 | | | Lease liabilities | 9,213 | | | 8,005 | | | Tax credit carryforwards | 8,438 | | | 5,408 | | | Equity-based compensation | 4,196 | | | 4,015 | | | Other accruals and reserves | 424 | | | 246 | | | Total deferred tax assets | 66,898 | | | 48,825 | | | Valuation allowance for deferred tax assets | (58,215) | | | (41,205) | | | Total deferred tax assets, net of valuation allowance | $ | 8,683 | | | $ | 7,620 | | | | | | | Deferred tax liability | | | | | Right-of-use assets | (8,683) | | | (7,620) | | | Net deferred tax assets (liability) | $ | — | | | $ | — | |
|
| Schedule of Unrecognized Tax Benefits Roll Forward |
A reconciliation of the beginning and ending amount of the liability for uncertain tax positions, excluding potential interest and penalties, is as follows: | | | | | | | | | | (in thousands) | December 31, 2023 | December 31, 2022 | | Beginning balance | $ | 1,544 | | $ | 837 | | | Increase based on current year tax positions | 812 | | 616 | | | Increase for prior year tax positions | 9 | | 91 | | | | | | Ending balance | $ | 2,365 | | $ | 1,544 | |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the change in unrecognized tax benefits. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 217
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482663/740-10-55-217
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
| Name: |
us-gaap_ScheduleOfUnrecognizedTaxBenefitsRollForwardTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Equity Incentive Plans and Stock-based Compensation (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Schedule of Significant Assumptions |
The significant assumptions used in these calculations are summarized as follows: | | | | | | | | | | | | | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Expected term (in years) | 5.3 - 6.4 | | 5.3 - 6.1 | | | | Expected volatility | 102.7% - 107.5% | | 105.2% - 110.4% | | | | Expected dividend rate | 0.0% | | 0.0% | | | | Risk free interest rate | 3.5% - 4.8% | | 1.7% - 4.4% | | | | | | | | |
|
| Schedule of Stock Option Activity |
The following table summarizes option award activity during the year ended December 31, 2023: | | | | | | | | | | | | | | | | | | | | | | | | | Number of Stock Option Awards | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Life (Years) | | Aggregate Intrinsic Value
(in thousands) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Outstanding as of December 31, 2022 | 11,485,443 | | | $ | 4.12 | | | | | | | | | | | | | | | | | | | | | | | Granted | 4,009,775 | | | $ | 2.42 | | | | | | | Exercised | (70,865) | | | $ | 1.46 | | | | | | | Forfeited | (748,018) | | | $ | 4.97 | | | | | | Outstanding as of December 31, 2023 | 14,676,335 | | | $ | 3.63 | | | 7.8 | | $ | 11,451 | | Options vested and expected to vest as of December 31, 2023 | 14,676,335 | | | $ | 3.63 | | | | | | Vested and exercisable at December 31, 2023 | 7,488,649 | | | $ | 3.90 | | | 7.1 | | $ | 7,366 | |
|
| Schedule of Stock Based Compensation Expense |
The following sets forth the total stock-based compensation expense for the Company’s stock options and ESPP included in the Company’s consolidated statements of operations: | | | | | | | | | | | | | | | (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Research and development | $ | 4,199 | | | $ | 3,840 | | | | | General and administrative | 7,938 | | | 6,540 | | | | | Total stock-based compensation expense | $ | 12,137 | | | $ | 10,380 | | | |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the change in stock options.
| Name: |
us-gaap_ScheduleOfStockOptionsRollForwardTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Commitments and Contingencies (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Components of Lease Cost |
The components of lease costs which were included in operating expenses in the consolidated statements of operations were as follows: | | | | | | | | | | | | | (in thousands) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Fixed operating lease costs | $ | 6,893 | | | $ | 4,751 | | | Variable operating lease costs | 2,432 | | | 1,655 | | | Short-term lease costs | — | | | 25 | | | Total lease costs | $ | 9,325 | | | $ | 6,431 | |
|
| Schedule of Future Contractual Obligations for Operating Lease Commitments |
The following table summarizes the Company's future principal contractual obligations for operating lease commitments as of December 31, 2023: | | | | | | | (in thousands) | | | Year Ended December 31, | Lease Obligations | | 2024 | 6,414 | | | 2025 | 7,186 | | | 2026 | 6,878 | | | 2027 | 6,893 | | | 2028 and thereafter | 19,028 | | | Total future minimum lease payments | 46,399 | | | Less: Imputed interest | (11,771) | | | Total operating lease liabilities | $ | 34,628 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Basic and Diluted Net Loss per Share (Tables)
|
12 Months Ended |
Dec. 31, 2023 |
|---|
| Earnings Per Share [Abstract] |
|
| Schedule of Earnings Per Share, Basic and Diluted Net Loss per Share |
The following tables set forth the computation of the Company’s basic and diluted net loss per share attributable to common stockholders for the years ended December 31, 2023 and 2022: | | | | | | | | | | | | | | | (in thousands, except share and per share data) | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Numerator: | | | | | | | Net loss attributable to common stockholders | $ | (63,675) | | | $ | (57,924) | | | | | Denominator: | | | | | | | | | | | | | | | | | | | Weighted average shares used in computing net loss per share attributable to common stockholders, basic and diluted | 124,919,144 | | 124,589,555 | | | | Net loss per share attributable to common stockholders, basic and diluted: | $ | (0.51) | | | $ | (0.46) | | | |
|
| Schedule of Antidilutive Securities Excluded from Computation of Earnings per Share |
The potential shares of common stock that were excluded from the computation of diluted net loss per share attributable to common stockholders for the periods presented because including them would have had an antidilutive effect were as follows: | | | | | | | | | | | | | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | | | Options to purchase common stock | 14,676,335 | | 11,485,443 | | | | Employee stock purchase plan | 141,720 | | 63,246 | | | | Total potentially dilutive common share equivalents | 14,818,055 | | 11,548,689 | | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Description of Business and Basis of Presentation - Narrative (Details) - USD ($)
|
|
1 Months Ended |
|
|
Jun. 09, 2021 |
Jun. 30, 2021 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
| Accumulated deficit |
|
|
$ 202,239,000
|
$ 138,564,000
|
| Proceeds from sale of stock and reverse recapitalization transaction |
|
$ 345,500,000
|
|
|
| Payments of transaction costs |
|
$ 18,200,000
|
|
|
| Cash, cash equivalents, and short-term investments |
|
|
$ 173,400,000
|
|
| Private Placement |
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
| Shares issued in transaction (in shares) |
20,000,000
|
|
|
|
| Price per share (in dollars per share) |
$ 10.00
|
|
|
|
| Consideration received on transaction |
$ 200,000,000
|
|
|
|
| X |
- DefinitionPayments of Reverse Recapitalization Transaction Costs
| Name: |
naut_PaymentsOfReverseRecapitalizationTransactionCosts |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds From Sale Of Stock And Reverse Recapitalization Transaction
| Name: |
naut_ProceedsFromSaleOfStockAndReverseRecapitalizationTransaction |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCash includes currency on hand as well as demand deposits with banks or financial institutions. It also includes other kinds of accounts that have the general characteristics of demand deposits in that the customer may deposit additional funds at any time and effectively may withdraw funds at any time without prior notice or penalty. Cash equivalents, excluding items classified as marketable securities, include short-term, highly liquid Investments that are both readily convertible to known amounts of cash, and so near their maturity that they present minimal risk of changes in value because of changes in interest rates. Generally, only investments with original maturities of three months or less qualify under that definition. Original maturity means original maturity to the entity holding the investment. For example, both a three-month US Treasury bill and a three-year Treasury note purchased three months from maturity qualify as cash equivalents. However, a Treasury note purchased three years ago does not become a cash equivalent when its remaining maturity is three months. Short-term investments, exclusive of cash equivalents, generally consist of marketable securities intended to be sold within one year (or the normal operating cycle if longer) and may include trading securities, available-for-sale securities, or held-to-maturity securities (if maturing within one year), as applicable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashCashEquivalentsAndShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCash received on stock transaction after deduction of issuance costs.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedOnTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of allowance for credit loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-9
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleAllowanceForCreditLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in allowance for credit loss of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-9
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleAllowanceForCreditLossPeriodIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of write-downs for impairments recognized during the period for long lived assets held for use (including those held for disposal by means other than sale). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-4
| Name: |
us-gaap_ImpairmentOfLongLivedAssetsHeldForUse |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of operating segments. An operating segment is a component of an enterprise: (a) that engages in business activities from which it may earn revenues and incur expenses (including revenues and expenses relating to transactions with other components of the same enterprise), (b) whose operating results are regularly reviewed by the enterprise's chief operating decision maker to make decisions about resources to be allocated to the segment and assess its performance, and (c) for which discrete financial information is available. An operating segment may engage in business activities for which it has yet to earn revenues, for example, start-up operations may be operating segments before earning revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfOperatingSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfReportableSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Significant Accounting Policies - Useful Lives (Details)
|
Dec. 31, 2023 |
| Prototype equipment |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
3 years
|
| Computer hardware |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
3 years
|
| Furniture and fixtures |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
3 years
|
| Minimum | Laboratory equipment |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
3 years
|
| Minimum | Office equipment |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
3 years
|
| Maximum | Laboratory equipment |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
5 years
|
| Maximum | Office equipment |
|
| Property, Plant and Equipment [Line Items] |
|
| Property and equipment, useful life |
5 years
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=naut_PrototypeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=naut_LaboratoryEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_OfficeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Fair Value Measurements - Assets Carried at Fair Value and Measured on a Recurring Basis (Details) - Fair Value, Recurring - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
$ 264,157
|
$ 315,494
|
| Gross Unrealized Gains |
462
|
40
|
| Gross Unrealized Losses |
(717)
|
(1,894)
|
| Fair Value |
263,902
|
313,640
|
| Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
19,234
|
114,523
|
| Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
154,021
|
69,948
|
| Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
90,647
|
129,169
|
| Level 1 |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
74,705
|
53,807
|
| Gross Unrealized Gains |
104
|
4
|
| Gross Unrealized Losses |
(247)
|
(774)
|
| Fair Value |
74,562
|
53,037
|
| Level 1 | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
590
|
1,121
|
| Level 1 | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
50,638
|
2,873
|
| Level 1 | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
23,334
|
49,043
|
| Level 1 | Mutual funds |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
590
|
1,121
|
| Gross Unrealized Gains |
0
|
0
|
| Gross Unrealized Losses |
0
|
0
|
| Fair Value |
590
|
1,121
|
| Level 1 | Mutual funds | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
590
|
1,121
|
| Level 1 | Mutual funds | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
0
|
| Level 1 | Mutual funds | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
0
|
| Level 1 | U.S. treasury securities |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
74,115
|
52,686
|
| Gross Unrealized Gains |
104
|
4
|
| Gross Unrealized Losses |
(247)
|
(774)
|
| Fair Value |
73,972
|
51,916
|
| Level 1 | U.S. treasury securities | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
0
|
| Level 1 | U.S. treasury securities | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
50,638
|
2,873
|
| Level 1 | U.S. treasury securities | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
23,334
|
49,043
|
| Level 2 |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
189,452
|
261,687
|
| Gross Unrealized Gains |
358
|
36
|
| Gross Unrealized Losses |
(470)
|
(1,120)
|
| Fair Value |
189,340
|
260,603
|
| Level 2 | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
18,644
|
113,402
|
| Level 2 | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
103,383
|
67,075
|
| Level 2 | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
67,313
|
80,126
|
| Level 2 | Commercial paper |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
18,653
|
156,419
|
| Gross Unrealized Gains |
0
|
3
|
| Gross Unrealized Losses |
(9)
|
(266)
|
| Fair Value |
18,644
|
156,156
|
| Level 2 | Commercial paper | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
18,644
|
113,402
|
| Level 2 | Commercial paper | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
42,754
|
| Level 2 | Commercial paper | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
0
|
| Level 2 | Corporate debt securities |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
6,950
|
14,154
|
| Gross Unrealized Gains |
0
|
0
|
| Gross Unrealized Losses |
(37)
|
(71)
|
| Fair Value |
6,913
|
14,083
|
| Level 2 | Corporate debt securities | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
0
|
| Level 2 | Corporate debt securities | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
6,913
|
7,224
|
| Level 2 | Corporate debt securities | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
6,859
|
| Level 2 | Agency securities |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
163,849
|
91,114
|
| Gross Unrealized Gains |
358
|
33
|
| Gross Unrealized Losses |
(424)
|
(783)
|
| Fair Value |
163,783
|
90,364
|
| Level 2 | Agency securities | Cash equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
0
|
0
|
| Level 2 | Agency securities | Short-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
96,470
|
17,097
|
| Level 2 | Agency securities | Long-term investments |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Fair Value |
$ 67,313
|
$ 73,267
|
| X |
- DefinitionAmount, before tax, of unrealized gain in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before tax, of unrealized loss in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=us-gaap_CashAndCashEquivalentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=us-gaap_ShortTermInvestmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_BalanceSheetLocationAxis=naut_LongTermInvestmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_MutualFundMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USTreasurySecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_CommercialPaperMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_CorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USGovernmentAgenciesDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Fair Value Measurements - Unrealized Losses and Fair Values of Available-for-Sale Securities in an Unrealized Loss Position (Details) - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Gross Unrealized Losses |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
$ 360
|
$ 1,724
|
| Securities in Unrealized Loss Position Greater than 12 months |
357
|
170
|
| Total |
717
|
1,894
|
| Fair Market Value |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
131,004
|
257,858
|
| Securities in Unrealized Loss Position Greater than 12 months |
55,934
|
16,111
|
| Total |
186,938
|
273,969
|
| U.S. treasury securities |
|
|
| Gross Unrealized Losses |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
85
|
774
|
| Securities in Unrealized Loss Position Greater than 12 months |
162
|
0
|
| Total |
247
|
774
|
| Fair Market Value |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
20,408
|
49,114
|
| Securities in Unrealized Loss Position Greater than 12 months |
30,230
|
0
|
| Total |
50,638
|
49,114
|
| Commercial paper |
|
|
| Gross Unrealized Losses |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
9
|
266
|
| Securities in Unrealized Loss Position Greater than 12 months |
0
|
0
|
| Total |
9
|
266
|
| Fair Market Value |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
18,644
|
151,354
|
| Securities in Unrealized Loss Position Greater than 12 months |
0
|
0
|
| Total |
18,644
|
151,354
|
| Corporate debt securities |
|
|
| Gross Unrealized Losses |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
37
|
14
|
| Securities in Unrealized Loss Position Greater than 12 months |
0
|
57
|
| Total |
37
|
71
|
| Fair Market Value |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
6,913
|
6,859
|
| Securities in Unrealized Loss Position Greater than 12 months |
0
|
7,224
|
| Total |
6,913
|
14,083
|
| Agency securities |
|
|
| Gross Unrealized Losses |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
229
|
670
|
| Securities in Unrealized Loss Position Greater than 12 months |
195
|
113
|
| Total |
424
|
783
|
| Fair Market Value |
|
|
| Securities in Unrealized Loss Position Less than 12 months |
85,039
|
50,531
|
| Securities in Unrealized Loss Position Greater than 12 months |
25,704
|
8,887
|
| Total |
$ 110,743
|
$ 59,418
|
| X |
- DefinitionDebt Securities, Available-For-Sale, Unrealized Loss Position, Fair Value
| Name: |
naut_DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueAbstract |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AvailableForSaleSecuritiesContinuousUnrealizedLossPositionAccumulatedLossAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for more than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479081/326-30-55-8
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-7
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-5
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLonger |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for 12 months or longer, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-7
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-5
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerAccumulatedLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479081/326-30-55-8
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-7
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-5
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-7
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-5
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsAccumulatedLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position without allowance for credit loss. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479081/326-30-55-8
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPosition |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position, without allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionAccumulatedLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USTreasurySecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_CommercialPaperMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_CorporateDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USGovernmentAgenciesDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- DefinitionAmount of allowance for credit loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-9
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleAllowanceForCreditLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in allowance for credit loss of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-9
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleAllowanceForCreditLossPeriodIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Composition of Certain Consolidated Financial Statement Line Items - Property and Equipment, net (Details) - USD ($)
$ in Thousands |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
$ 8,901
|
$ 6,663
|
| Less: Accumulated depreciation |
(4,634)
|
(2,963)
|
| Total |
4,267
|
3,700
|
| Depreciation |
1,849
|
1,217
|
| Laboratory equipment |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
6,337
|
4,892
|
| Leasehold improvements |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
118
|
13
|
| Computer hardware |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
222
|
166
|
| Furniture, fixtures and office equipment |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
324
|
25
|
| Prototype equipment |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
996
|
332
|
| Construction in progress |
|
|
| Property, Plant and Equipment [Line Items] |
|
|
| Property, plant and equipment, gross |
$ 904
|
$ 1,235
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=naut_LaboratoryEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_LeaseholdImprovementsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=naut_FurnitureFixturesAndOfficeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=naut_PrototypeEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ConstructionInProgressMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- DefinitionAccrued Liabilities And Other Liabilities, Current
| Name: |
naut_AccruedLiabilitiesAndOtherLiabilitiesCurrent |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAccrued Research And Development
| Name: |
naut_AccruedResearchAndDevelopment |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedProfessionalFeesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionObligations not otherwise itemized or categorized in the footnotes to the financial statements that are due within one year or operating cycle, if longer, from the balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 10
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481573/470-10-45-10
| Name: |
us-gaap_OtherSundryLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Composition of Certain Consolidated Financial Statement Line Items - Cash, Cash Equivalents and Restricted Cash (Details) - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
|
|
| Cash and cash equivalents |
$ 19,397
|
$ 114,523
|
|
| Restricted cash included in other long-term assets (Note 8) |
1,002
|
954
|
|
| Total |
$ 20,399
|
$ 115,477
|
$ 186,461
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents restricted as to withdrawal or usage. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_RestrictedCashAndCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.0.1
| X |
- DefinitionCarrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositsAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.0.1
Common Stock - Narrative (Details) - shares
|
Dec. 31, 2023 |
Dec. 31, 2022 |
| Equity [Abstract] |
|
|
| Common stock, shares outstanding (in shares) |
125,068,601
|
124,865,485
|
| Common stock, shares issued (in shares) |
125,068,601
|
124,865,485
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Common Stock - Common Stock Reserved for Future Issuance (Details) - shares
|
Dec. 31, 2023 |
Dec. 31, 2022 |
| Class of Stock [Line Items] |
|
|
| Stock options issued and outstanding (in shares) |
14,676,335
|
11,485,443
|
| Total shares of common stock reserved (in shares) |
38,461,033
|
31,172,221
|
| 2021 Equity Incentive Plan |
|
|
| Class of Stock [Line Items] |
|
|
| Shares available for grant (in shares) |
20,279,560
|
17,298,043
|
| 2021 Employee Stock Purchase Plan |
|
|
| Class of Stock [Line Items] |
|
|
| Shares available for grant (in shares) |
3,505,138
|
2,388,735
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=naut_A2021EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=naut_A2021EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Income Taxes - Narrative (Details) - USD ($)
|
12 Months Ended |
|
Dec. 31, 2023 |
Dec. 31, 2022 |
Dec. 31, 2021 |
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Tax liability |
$ 0
|
$ 0
|
|
| Income tax expense |
0
|
0
|
|
| Increase in valuation allowance |
17,000,000
|
17,100,000
|
|
| Capitalized research and development |
16,502,000
|
7,607,000
|
|
| Valuation allowance |
58,215,000
|
41,205,000
|
|
| Unrecognized tax benefits |
2,365,000
|
$ 1,544,000
|
$ 837,000
|
| Unrecognized tax benefits that would impact effective tax rate |
0
|
|
|
| Tax credit carryforward, offset by valuation allowance |
0
|
|
|
| Decrease in unrecognized tax benefits, reasonably possible |
0
|
|
|
| Increase in unrecognized tax benefits, reasonably possible |
0
|
|
|
| Domestic Tax Authority |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Operating loss carryforwards, subject to expiration |
500,000
|
|
|
| Operating loss carryforwards, not subject to expiration |
70,600,000
|
|
|
| Domestic Tax Authority | Research Tax Credit Carryforward |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Tax credit carryforward, amount |
6,800,000
|
|
|
| State and Local Jurisdiction |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Operating loss carryforwards, subject to expiration |
95,500,000
|
|
|
| State and Local Jurisdiction | Research Tax Credit Carryforward |
|
|
|
| Operating Loss Carryforwards [Line Items] |
|
|
|
| Tax credit carryforward, amount |
$ 4,700,000
|
|
|
| X |
- DefinitionOperating Loss Carryforwards, Not Subject To Expiration
| Name: |
naut_OperatingLossCarryforwardsNotSubjectToExpiration |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionOperating Loss Carryforwards, Subject To Expiration
| Name: |
naut_OperatingLossCarryforwardsSubjectToExpiration |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionTax Credit Carryforward, Offset By Valuation Allowance
| Name: |
naut_TaxCreditCarryforwardOffsetByValuationAllowance |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of decrease reasonably possible in the next twelve months for the unrecognized tax benefit. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 15
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15
| Name: |
us-gaap_DecreaseInUnrecognizedTaxBenefitsIsReasonablyPossible |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from in-process research and development costs expensed in connection with a business combination. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsInProcessResearchAndDevelopment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase reasonably possible in the next twelve months for the unrecognized tax benefit. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 15
-Subparagraph (d)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15
| Name: |
us-gaap_IncreaseInUnrecognizedTaxBenefitsIsReasonablyPossible |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OperatingLossCarryforwardsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of the tax credit carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_TaxCreditCarryforwardAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of unrecognized tax benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-10B
| Name: |
us-gaap_UnrecognizedTaxBenefits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe total amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
| Name: |
us-gaap_UnrecognizedTaxBenefitsThatWouldImpactEffectiveTaxRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in the valuation allowance for a specified deferred tax asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ValuationAllowanceDeferredTaxAssetChangeInAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=us-gaap_ResearchMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Income Taxes - Income Tax Reconciliation (Details) - USD ($)
|
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Income Tax Disclosure [Abstract] |
|
|
| Federal income tax at statutory rate |
$ (13,372,000)
|
$ (12,164,000)
|
| State income tax, net of federal benefit |
(4,027,000)
|
(3,126,000)
|
| Equity-based compensation |
2,423,000
|
290,000
|
| Tax credits generated in current year |
(2,075,000)
|
(2,607,000)
|
| Valuation allowance change |
17,011,000
|
17,127,000
|
| Other |
40,000
|
480,000
|
| Total |
$ 0
|
$ 0
|
v3.24.0.1
Income Taxes - Deferred Tax Assets and Liabilities (Details) - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Deferred tax assets |
|
|
| Depreciation and amortization |
$ 6,508
|
$ 11,142
|
| Capitalized research and development |
16,502
|
7,607
|
| Loss carryforwards |
21,617
|
12,402
|
| Lease liabilities |
9,213
|
8,005
|
| Tax credit carryforwards |
8,438
|
5,408
|
| Equity-based compensation |
4,196
|
4,015
|
| Other accruals and reserves |
424
|
246
|
| Total deferred tax assets |
66,898
|
48,825
|
| Valuation allowance for deferred tax assets |
(58,215)
|
(41,205)
|
| Total deferred tax assets, net of valuation allowance |
8,683
|
7,620
|
| Deferred tax liability |
|
|
| Right-of-use assets |
(8,683)
|
(7,620)
|
| Net deferred tax assets (liability) |
$ 0
|
$ 0
|
| X |
- DefinitionDeferred Tax Assets, Lease Liability
| Name: |
naut_DeferredTaxAssetsLeaseLiability |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredTaxAssetsGrossAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from in-process research and development costs expensed in connection with a business combination. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsInProcessResearchAndDevelopment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from property, plant, and equipment.
| Name: |
us-gaap_DeferredTaxAssetsPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of a valuation allowances, of deferred tax assets attributable to deductible tax credit carryforwards including, but not limited to, research, foreign, general business, alternative minimum tax, and other deductible tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from reserves and accruals. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseReservesAndAccruals |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredTaxLiabilitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from leasing arrangements. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxLiabilitiesLeasingArrangements |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ReconciliationOfUnrecognizedTaxBenefitsExcludingAmountsPertainingToExaminedTaxReturnsRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of unrecognized tax benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-10B
| Name: |
us-gaap_UnrecognizedTaxBenefits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase in unrecognized tax benefits resulting from tax positions that have been or will be taken in current period tax return. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
| Name: |
us-gaap_UnrecognizedTaxBenefitsIncreasesResultingFromCurrentPeriodTaxPositions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in unrecognized tax benefits resulting from tax positions taken in prior period tax returns. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
| Name: |
us-gaap_UnrecognizedTaxBenefitsIncreasesResultingFromPriorPeriodTaxPositions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.0.1
Equity Incentive Plans and Stock-based Compensation - Narrative (Details) - USD ($)
$ / shares in Units, $ in Millions |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Unrecognized compensation expense |
$ 19.1
|
|
| Unrecognized compensation expense, period for recognition |
2 years 1 month 6 days
|
|
| Intrinsic value of options exercised |
$ 0.1
|
$ 1.2
|
| Weighted average grant date fair value (in dollars per share) |
$ 1.98
|
$ 2.98
|
| 2021 Equity Incentive Plan |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Options available for grant (in shares) |
20,279,560
|
17,298,043
|
| Additional shares authorized (in shares) |
18,672,200
|
|
| Percentage of outstanding stock maximum |
5.00%
|
|
| Award expiration period |
10 years
|
|
| 2021 Employee Stock Purchase Plan |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Options available for grant (in shares) |
3,505,138
|
2,388,735
|
| Additional shares authorized (in shares) |
3,734,500
|
|
| Percentage of outstanding stock maximum |
1.00%
|
|
| Offering purchase period |
6 months
|
|
| Common stock purchased (in shares) |
132,251
|
99,195
|
| Employee stock purchase plan | 2021 Employee Stock Purchase Plan |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Purchase price of common stock, percent |
85.00%
|
|
| Options |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Expected dividend rate |
0.00%
|
0.00%
|
| X |
- DefinitionShare-Based Compensation Arrangement By Share-Based Payment Award, Offering Purchase Period
| Name: |
naut_ShareBasedCompensationArrangementByShareBasedPaymentAwardOfferingPurchasePeriod |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of additional shares authorized for issuance under share-based payment arrangement.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated difference between fair value of underlying shares on dates of exercise and exercise price on options exercised (or share units converted) into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum number of shares that may be issued in accordance with the plan as a proportion of outstanding capital stock.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardPercentageOfOutstandingStockMaximum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesIssuedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPurchase price of common stock expressed as a percentage of its fair value.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardPurchasePriceOfCommonStockPercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=naut_A2021EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=naut_A2021EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the maximum percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRateMaximum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the minimum percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRateMinimum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum risk-free interest rate assumption that is used in valuing an option on its own shares.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRateMaximum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe minimum risk-free interest rate assumption that is used in valuing an option on its own shares.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRateMinimum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Equity Incentive Plans and Stock-based Compensation - Stock Option Activity (Details)
$ / shares in Units, $ in Thousands |
12 Months Ended |
|
Dec. 31, 2023
USD ($)
$ / shares
shares
|
|---|
| Number of Stock Option Awards |
|
| Outstanding, beginning balance (in shares) | shares |
11,485,443
|
| Granted (in shares) | shares |
4,009,775
|
| Exercised (in shares) | shares |
(70,865)
|
| Forfeited (in shares) | shares |
(748,018)
|
| Outstanding, ending balance (in shares) | shares |
14,676,335
|
| Options vested and expected to vest (in shares) | shares |
14,676,335
|
| Vested and exercisable (in shares) | shares |
7,488,649
|
| Weighted Average Exercise Price |
|
| Outstanding, beginning balance (in dollars per share) | $ / shares |
$ 4.12
|
| Granted (in dollars per share) | $ / shares |
2.42
|
| Exercised (in dollars per share) | $ / shares |
1.46
|
| Forfeited (in dollars per share) | $ / shares |
4.97
|
| Outstanding, ending balance (in dollars per share) | $ / shares |
3.63
|
| Options vested and expected to vest (in dollars per share) | $ / shares |
3.63
|
| Vested and exercisable (in dollars per share) | $ / shares |
$ 3.90
|
| Options outstanding, weighted average remaining contractual life |
7 years 9 months 18 days
|
| Vested and exercisable, weighted average remaining contractual life |
7 years 1 month 6 days
|
| Options outstanding, aggregate intrinsic value | $ |
$ 11,451
|
| Vested and exercisable, aggregate intrinsic value | $ |
$ 7,366
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of fully vested and expected to vest exercisable options that may be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest exercisable or convertible options. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for fully vested and expected to vest exercisable or convertible options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=naut_StockOptionsAndEmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
Commitments and Contingencies - Narrative (Details) - USD ($)
$ in Thousands |
12 Months Ended |
|
|
|
|
Dec. 31, 2023 |
Dec. 31, 2022 |
Nov. 30, 2022 |
Dec. 31, 2021 |
Aug. 31, 2021 |
Dec. 31, 2020 |
| Lessee, Lease, Description [Line Items] |
|
|
|
|
|
|
| Open purchase commitment |
$ 2,500
|
|
|
|
|
|
| Open purchase commitment to be received through next 12 months |
1,800
|
|
|
|
|
|
| Term of contract |
|
|
|
|
7 years
|
|
| Total minimum lease payments |
46,399
|
|
|
|
$ 4,500
|
|
| Cash paid for lease liabilities included in operating activities |
$ 6,200
|
$ 3,900
|
|
|
|
|
| Weighted average remaining lease term |
6 years 8 months 12 days
|
|
|
|
|
|
| Weighted average discount rate |
9.10%
|
|
|
|
|
|
| Letters of credit |
|
|
$ 100
|
$ 200
|
|
$ 600
|
| San Carlos |
|
|
|
|
|
|
| Lessee, Lease, Description [Line Items] |
|
|
|
|
|
|
| Interest rate on financing for tenant improvements |
|
|
|
7.00%
|
|
|
| San Carlos | Maximum |
|
|
|
|
|
|
| Lessee, Lease, Description [Line Items] |
|
|
|
|
|
|
| Tenant improvement financing |
|
|
|
$ 2,000
|
|
|
| San Diego |
|
|
|
|
|
|
| Lessee, Lease, Description [Line Items] |
|
|
|
|
|
|
| Term of contract |
|
|
39 months
|
|
|
|
| Total minimum lease payments |
|
|
$ 2,100
|
|
|
|
| October 2021 to October 2031 |
|
|
|
|
|
|
| Lessee, Lease, Description [Line Items] |
|
|
|
|
|
|
| Term of contract |
|
|
|
|
|
10 years
|
| Total minimum lease payments |
|
|
|
|
|
$ 40,700
|
| October 2022 to October 2031 |
|
|
|
|
|
|
| Lessee, Lease, Description [Line Items] |
|
|
|
|
|
|
| Term of contract |
|
|
|
9 years
|
|
|
| Total minimum lease payments |
|
|
|
$ 7,200
|
|
|
| Lease term with option to terminate |
|
|
|
5 years
|
|
|
| X |
- DefinitionLessee, Operating Lease, Option To Terminate, Lease Term
| Name: |
naut_LesseeOperatingLeaseOptionToTerminateLeaseTerm |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLessee, Operating Lease, Tenant Improvement Financing
| Name: |
naut_LesseeOperatingLeaseTenantImprovementFinancing |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLessee, Operating Lease, Tenant Improvement Financing, Interest Rate
| Name: |
naut_LesseeOperatingLeaseTenantImprovementFinancingInterestRate |
| Namespace Prefix: |
naut_ |
| Data Type: |
dtr-types1:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeLeaseDescriptionLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe total amount of the contingent obligation under letters of credit outstanding as of the reporting date.
| Name: |
us-gaap_LettersOfCreditOutstandingAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe minimum amount the entity agreed to spend under the long-term purchase commitment.
| Name: |
us-gaap_LongTermPurchaseCommitmentAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of purchase arrangement to be paid in next fiscal year following current fiscal year. Includes, but is not limited to, recorded and unrecorded purchase obligations, long-term purchase commitment, and short-term purchase commitment. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
us-gaap_PurchaseObligationDueInNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=naut_SanCarlosMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=naut_SanDiegoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LeaseContractualTermAxis=naut_October2021ToOctober2031Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_LeaseContractualTermAxis=naut_October2022ToOctober2031Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.0.1
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lease cost recognized by lessee for lease contract. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of short-term lease cost, excluding expense for lease with term of one month or less. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_ShortTermLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of variable lease cost, excluded from lease liability, recognized when obligation for payment is incurred for finance and operating leases. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_VariableLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.0.1
Commitments and Contingencies - Future Contractual Obligations for Operating Lease Commitments (Details) - USD ($)
$ in Thousands |
Dec. 31, 2023 |
Aug. 31, 2021 |
| Commitments and Contingencies Disclosure [Abstract] |
|
|
| 2024 |
$ 6,414
|
|
| 2025 |
7,186
|
|
| 2026 |
6,878
|
|
| 2027 |
6,893
|
|
| 2028 and thereafter |
19,028
|
|
| Total future minimum lease payments |
46,399
|
$ 4,500
|
| Less: Imputed interest |
(11,771)
|
|
| Total operating lease liabilities |
$ 34,628
|
|
| X |
- DefinitionLessee Operating Lease Liability Payments Due After Year Four
| Name: |
naut_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFour |
| Namespace Prefix: |
naut_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.0.1
Basic and Diluted Net Loss per Share - Basic and Diluted Net Loss per Share (Details) - USD ($)
$ / shares in Units, $ in Thousands |
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Earnings Per Share [Abstract] |
|
|
| Net loss attributable to common stockholders, basic |
$ (63,675)
|
$ (57,924)
|
| Net loss attributable to common stockholders, diluted |
$ (63,675)
|
$ (57,924)
|
| Weighted average shares used in computing net loss per share attributable to common stockholders, basic (in shares) |
124,919,144
|
124,589,555
|
| Weighted average shares used in computing net loss per share attributable to common stockholders, diluted (in shares) |
124,919,144
|
124,589,555
|
| Net loss per share attributable to common stockholders, basic (in dollars per share) |
$ (0.51)
|
$ (0.46)
|
| Net loss per share attributable to common stockholders, diluted (in dollars per share) |
$ (0.51)
|
$ (0.46)
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-5
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-11
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities, and addition from assumption of issuance of common shares for dilutive potential common shares; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-40
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-40
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-40
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 40
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-40
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.0.1
Basic and Diluted Net Loss per Share - Antidilutive Securities Excluded from Computation of Earnings per Share (Details) - shares
|
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total potentially dilutive common share equivalents |
14,818,055
|
11,548,689
|
| Options to purchase common stock |
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total potentially dilutive common share equivalents |
14,676,335
|
11,485,443
|
| Employee stock purchase plan |
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total potentially dilutive common share equivalents |
141,720
|
63,246
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=naut_EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Nautilus Biotechnology (NASDAQ:NAUT)
Historical Stock Chart
From Oct 2024 to Nov 2024
Nautilus Biotechnology (NASDAQ:NAUT)
Historical Stock Chart
From Nov 2023 to Nov 2024