|
PROSPECTUS SUPPLEMENT
|
Filed Pursuant to Rule 424(b)(5)
|
|
(To Prospectus dated March 1, 2024)
|
Registration No. 333-277585
|

GeoVax Labs, Inc.
1,360,731 Shares of Common Stock
339,269 Pre-Funded Warrants to Purchase up to 339,269 Shares of Common Stock
339,269 Shares of Common Stock underlying such Pre-Funded Warrants
We are offering 1,360,731 shares of our common stock, par value $0.001 per share, and prefunded warrants (the “Pre-Funded Warrants”) to purchase 339,269 shares of common stock to investors pursuant to this prospectus supplement and the accompanying prospectus. The per share offering price of the common stock is $5.00 and the offering price per Pre-Funded Warrant is $4.99999 (and each share of common stock and Pre-Funded Warrant shall be coupled with one Common Warrant (as defined below), each to purchase one share of our common stock).
We are offering Pre-Funded Warrants to investors whose purchase of shares of common stock in this offering would otherwise result in such purchasers, together with their affiliates and certain related parties, beneficially owning more than 9.99% of our outstanding shares of common stock immediately following the closing of this offering. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 9.99% of the number of shares of common stock outstanding immediately after giving effect to such exercise. This offering also relates to the shares of common stock issuable upon exercise of any Pre-Funded Warrants sold in this offering. Each Pre-Funded Warrant is immediately exercisable for one share of common stock at an exercise price of $0.00001 per share and may be exercised at any time until exercised in full.
In a concurrent private placement, we are also offering to the investors common warrants to purchase an aggregate of up to 1,700,000 shares of our common stock (the “Common Warrants”) at an exercise price of $5.00 per share. The Common Warrants are immediately exercisable and will expire five years from the date of issuance. Subject to limited exceptions, a holder of Common Warrants will not have the right to exercise any portion of its Common Warrants if the holder, together with its affiliates, would beneficially own in excess of 9.99% of the number of shares of common stock outstanding immediately after giving effect to such exercise. The Common Warrants and the shares of common stock issuable upon exercise of such warrants are not being registered under the Securities Act of 1933, as amended (the “Securities Act”), and are not being offered pursuant to this prospectus supplement and accompanying prospectus and are being offered pursuant to an exemption from the registration requirements of the Securities Act provided in Section 4(a)(2) of the Securities Act and Rule 506(b) promulgated thereunder.
Our common stock is listed on the Nasdaq Capital Market under the symbol “GOVX.” On August 19, 2024, the last reported sale price for our common stock on the Nasdaq Capital Market was $7.15 per share. We do not intend to apply for the listing of the Pre-Funded Warrants on any national securities exchange or other trading market.
As of August 19, 2024, the aggregate market value of our outstanding common stock held by non-affiliates was approximately $41,501,524, which was calculated based on 5,804,409 shares of outstanding common stock held by non-affiliates, at a price per share of $7.15, the closing price of our common stock on August 19, 2024, the highest closing price of the Company's common stock on the Nasdaq Capital Market during the preceding sixty (60) day trading period. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell the securities described in this prospectus in a public primary offering with a value exceeding more than one-third (1/3) of the aggregate market value of our common stock held by non-affiliates in any twelve (12)-month period, so long as the aggregate market value of our outstanding common stock held by non-affiliates remains below $75,000,000. During the twelve (12) calendar months prior to and including the date of this prospectus supplement, we have offered and sold aggregate gross proceeds of approximately $4,547,686 of our securities pursuant to General Instruction I.B.6 of Form S-3.
| |
|
Per Share
|
|
|
Per Pre-Funded
Warrant
|
|
|
Total
|
|
|
Offering Price
|
|
$ |
5.00 |
|
|
$ |
4.99999 |
|
|
$ |
8,499,996.61 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Placement Agent Fees (1)
|
|
$ |
0.35 |
|
|
$ |
0.35 |
|
|
$ |
595,000.00 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds to us before offering expenses
|
|
$ |
4.65 |
|
|
$ |
4.64999 |
|
|
$ |
7,904,996.61 |
|
|
(1)
|
Includes a cash fee of 7.0% of the aggregate gross proceeds in this offering. In addition, we have agreed to pay up to $75,000 in expenses (with supporting invoices/receipts) and customary out-of-pocket expense and to reimburse certain expenses of the placement agent. See “Plan of Distribution” beginning on page S-27 of this prospectus supplement for additional information with respect to the compensation we will pay the placement agent.
|
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” appearing on page S-9 of this prospectus supplement and elsewhere in this prospectus supplement and the accompanying base prospectus for a discussion of information that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus supplement. Any representation to the contrary is a criminal offense.
We have engaged Roth Capital Partners, LLC (the “Placement Agent”), as our exclusive placement agent in connection with this offering. The Placement Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay the Placement Agent the placement agent fees set forth in the table above. See “Plan of Distribution” beginning on page S-27 of this prospectus supplement for more information regarding these arrangements.
Delivery of the securities being offered pursuant to this prospectus supplement and the accompanying prospectus is expected to be made on or about August 21, 2024, subject to satisfaction of customary closing conditions.
Roth Capital Partners
The date of this prospectus supplement is August 20, 2024
TABLE OF CONTENTS
Prospectus Supplement
|
ABOUT THIS PROSPECTUS SUPPLEMENT
|
S-1
|
|
CAUTIONARY STATEMENTS REGARDING FORWARD-LOOKING INFORMATION
|
S-2
|
|
PROSPECTUS SUPPLEMENT SUMMARY
|
S-3
|
|
RISK FACTORS
|
S-9
|
|
USE OF PROCEEDS
|
S-21
|
|
CAPITALIZATION
|
S-22
|
|
DILUTION
|
S-23
|
|
DESCRIPTION OF SECURITIES WE ARE OFFERING
|
S-24
|
|
PLAN OF DISTRIBUTION
|
S-27
|
|
CONCURRENT PRIVATE PLACEMENT
|
S-27
|
|
LEGAL MATTERS
|
S-28
|
|
EXPERTS
|
S-28
|
|
INTEREST OF NAMED EXPERTS AND COUNSEL
|
S-28
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
S-29
|
|
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
|
S-29
|
Prospectus
|
ABOUT THIS PROSPECTUS
|
1
|
|
CAUTIONARY STATEMENT CONCERNING FORWARD LOOKING STATEMNTS
|
2
|
|
PROSPECTUS SUMMARY
|
3
|
|
RISK FACTORS
|
7
|
|
USE OF PROCEEDS
|
7
|
|
DESCRIPTION OF SECURITIES
|
8
|
|
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
14
|
|
THE SECURITIES WE MAY OFFER
|
15
|
|
DILUTION
|
17
|
|
SELLING STOCKHOLDERS
|
17
|
|
PLAN OF DISTRIBUTION
|
19
|
|
LEGAL MATTERS
|
22
|
|
EXPERTS
|
22
|
|
INTERESTS OF NAMED EXPERTS AND COUNSEL
|
22
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
22
|
|
INCORPORATION OF CERTAIN DOCUMENTS REFERENCE
|
22
|
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is this prospectus supplement, which adds to and updates information contained in the accompanying prospectus. The second part, the prospectus, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus, you should rely on the information in this prospectus supplement.
This prospectus supplement and the accompanying prospectus relate to the offering of shares of our common stock and warrants to purchase shares of our common stock. Before buying the shares of common stock and warrants to buy shares of common stock offered hereby, we urge you to carefully read this prospectus supplement and the accompanying prospectus, together with the information incorporated herein by reference as described under the headings “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.” These documents contain important information that you should consider when making your investment decision. This prospectus supplement contains information about the common stock and warrants offered hereby and may add, update or change information in the accompanying prospectus.
You should rely only on the information that we have provided or incorporated by reference in this prospectus supplement and the accompanying prospectus and in any free writing prospectus we have authorized for use in connection with this offering. Neither we nor the placement agent (or any of our or its respective affiliates) have authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it.
We and the placement agent are not making offers to sell or solicitations to buy our securities in any jurisdiction in which an offer or solicitation is not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information in this prospectus supplement and the accompanying prospectus or any related free writing prospectus we have authorized for use in connection with this offering is accurate only as of the date on the front of the document and that any information that we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus supplement, the accompanying prospectus or such related free writing prospectus, or any sale of a security.
This prospectus supplement and the accompanying prospectus contain summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been or will be filed as exhibits to the registration statement of which this prospectus supplement is a part or as exhibits to documents incorporated by reference herein, and you may obtain copies of those documents as described below under the headings “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
When used herein, “GeoVax,” “we,” “us” or “our” refers to GeoVax Labs, Inc., a Delaware corporation, and our subsidiaries.
CAUTIONARY STATEMENT CONCERNING FORWARD LOOKING STATEMENTS
Some of the statements in this prospectus supplement, the base prospectus, and in the documents incorporated herein by reference contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond our ability to control or predict and that may cause actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these identifying words. Our forward-looking statements may include, among other things, statements about:
| |
●
|
our ability to continue as a going concern and our history of losses;
|
| |
●
|
our ability to obtain additional financing;
|
| |
●
|
our use of the net proceeds from this offering;
|
| |
●
|
our ability to prosecute, maintain or enforce our intellectual property rights;
|
| |
●
|
the accuracy of our estimates regarding expenses, future revenues and capital requirements;
|
| |
●
|
the implementation of our business model and strategic plans for our business and technology;
|
| |
●
|
the successful development and regulatory approval of our technologies and products;
|
| |
●
|
the potential markets for our products and our ability to serve those markets;
|
| |
●
|
the rate and degree of market acceptance of our products and any future products;
|
| |
●
|
our ability to retain key management personnel; and
|
| |
●
|
regulatory developments and our compliance with applicable laws.
|
Forward-looking statements are inherently subject to risks and uncertainties, many of which we cannot predict with accuracy and some of which we might not even anticipate. Although we believe that the expectations reflected in such forward-looking statements are based upon reasonable assumptions at the time made, we can give no assurance that such expectations will be achieved. Actual events or results may differ materially. Readers are cautioned not to place undue reliance on forward-looking statements. We have no duty to update or revise any forward-looking statements after the date of this prospectus or to conform them to actual results, new information, future events or otherwise.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of these forward-looking statements.
You should read the risk factors and the other cautionary statements made in this prospectus as being applicable to all related forward-looking statements wherever they appear in this prospectus. If one or more of these factors materialize, or if any underlying assumptions prove incorrect, our actual results, performance or achievements may vary materially from any future results, performance or achievements expressed or implied by these forward-looking statements. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
PROSPECTUS SUPPLEMENT SUMMARY
The following information below is only a summary of more detailed information included elsewhere in, or incorporated by reference in, this prospectus supplement and the accompanying base prospectus, and should be read together with the information contained or incorporated by reference in other parts of this prospectus supplement and the accompanying base prospectus. This summary highlights selected information about us and this offering. This summary may not contain all of the information that may be important to you. Before making a decision to invest in our common stock, you should read carefully all of the information contained in or incorporated by reference into this prospectus supplement and the accompanying base prospectus, including the information set forth under the caption “Risk Factors” in this prospectus supplement and the accompanying base prospectus as well as the documents incorporated herein by reference, which are described under “Where you can Find More Information” and “Incorporation of Certain Documents by Reference” in this prospectus supplement.
Company Overview
GeoVax Labs, Inc. (“GeoVax” or “the Company”) is a clinical-stage biotechnology company developing human vaccines and immunotherapies against infectious diseases and solid tumor cancers using novel proprietary platforms. GeoVax’s product pipeline includes ongoing human clinical trials for a next-generation COVID-19 vaccine and a gene-directed therapy against advanced head and neck cancers. Additional research and development programs include preventive vaccines against Mpox (formerly known as monkeypox) and smallpox, hemorrhagic fever viruses (Ebola Zaire, Ebola Sudan and Marburg), Zika virus and malaria, as well as immunotherapies for multiple solid tumors. The Company’s portfolio of wholly owned, co-owned, and in-licensed intellectual property, stands at over 155 granted or pending patent applications spread over 24 patent families.
Our Product Development Pipeline
The table below summarizes the status of our product development programs, which are in various stages of development, the most significant of which are described further below along with recent developments.
Clinical Development Programs
|
Product
|
Indication
|
Clinical Trial
|
Status
|
|
|
|
BARDA Project NextGen
10,000 Patient Comparison Study
|
Phase 2b
Initiation pending
|
| GEO-CM04S1 |
COVID-19 |
Primary Vaccine for
Immunocompromised/Stem Cell Transplant Patients (NCT04977024)
|
Phase 2
Currently enrolling
|
| |
|
Booster Vaccine for
Immunocompromised/Chronic Lymphocytic Patients (NCT05672355)
|
Phase 2
Currently enrolling
|
| |
|
Booster Vaccine for
Healthy Adults (NCT04639466)
|
Phase 2
Enrollment closed
|
|
Gedeptin®
|
Advanced Head &
Neck Cancer*
|
Effect on Targeted Tumors (NCT03754933)
|
Phase 1b/2a
Completed
|
| |
Squamous Cell Head & Neck Cancer
|
First Recurrence Therapy in Combination
with Immune Checkpoint Inhibitor
|
Phase 2
Planning
|
Preclinical Development Programs
|
Product
|
Indication
|
Status
|
|
GEO-MVA-MUC1
|
Solid Tumor Cancers
|
Humanized Mouse Model (completed)
|
|
GEO-CM02
|
Pan-Coronavirus Vaccine
|
Humanized Mouse Model (completed)
|
|
GEO-EM01-Z
|
Ebola Zaire Vaccine**
|
Non-Human Primate (completed)
|
|
GEO-EM01-S
|
Ebola Sudan Vaccine**
|
Non-Human Primate (completed)
|
|
GEO-MM01
|
Marburg Vaccine**
|
Non-Human Primate (completed)
|
|
GEO-ZM02
|
Zika Vaccine**
|
Mouse Model (completed)
|
|
GEO-MVA
|
Mpox & Smallpox Vaccine
|
Regulatory Discussions &
Manufacturing Scale-up
|
-------
* Orphan Drug status granted
** Indication within FDA Priority Review Voucher program
Our programs are in various stages of development, the most significant of which are summarized below along with recent developments. We have not generated any revenues from the sale of the products we are developing, and we do not expect to generate any such revenues for at least the next several years. Our product candidates will require significant additional research and development efforts, including extensive preclinical and clinical testing. All product candidates that we advance to clinical testing will require regulatory approval prior to commercial use and will require significant costs for commercialization. We may not be successful in our research and development efforts, and we may never generate sufficient product revenue to be profitable.
Strategy
Our corporate strategy is to advance, protect and exploit our differentiated vaccine and oncology platforms leading to the successful development of preventive and therapeutic interventions against infectious diseases and various cancers. With our design and development capabilities, we are progressing and validating an array of cancer treatment and infectious disease vaccine product candidates. Our goal is to advance products through to human clinical testing, registration and commercialization in the U.S, and to seek partnership or licensing arrangements for achieving regulatory approval and commercialization outside of the U.S. We also leverage third party resources through collaborations and partnerships for preclinical and clinical testing with multiple government, academic and corporate entities.
Recent Development – BARDA Project NextGen Award – GEO-CM04S1 Phase 2b Trial
On June 18, 2024, we announced our receipt of an award through the Rapid Response Partnership Vehicle (RRPV) to advance development of GEO-CM04S1, our dual-antigen next-generation COVID-19 vaccine, in a Phase 2b clinical trial Under the agreement, GeoVax will sponsor a 10,000-participant, randomized, Phase 2b double-blinded study to assess the clinical efficacy, safety, and immunogenicity of GEO-CM04S1 compared with a U.S. Food and Drug Administration (FDA)-approved mRNA COVID-19 vaccine. The RRPV is a Consortium funded by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) in the U.S. Department of Health and Human Services (HHS).
Preparations for the Phase 2b study are underway, and execution of the study will be fully funded by BARDA under its Clinical Studies Network. The direct award to GeoVax of $24.3 million, which may increase to as much as $45 million, will fund the manufacturing of clinical materials and support for the Phase 2b clinical trial, including regulatory activities. BARDA has made a separate award of $343 million from the Project NextGen program to Allucent, a global clinical research organization (CRO), to execute the clinical trial as part of BARDA’s Clinical Studies Network. The combined value of the awards to GeoVax and Allucent toward the clinical evaluation of GEO-CM04S1 is expected to be $367-388
million.
GEO-CM04S1 – Immunocompromised/Cell Transplant Phase 2 Trial
| |
●
|
GEO-CM04S1 is undergoing a Phase 2 multi-site clinical trial (ClinicalTrials.gov Identifier: NCT04977024), evaluating its safety and efficacy, compared to either the Pfizer/BioNTech or Moderna mRNA-based vaccine, as a preventive COVID-19 vaccine in high-risk immunocompromised patients (e.g. patients with blood cancers who have previously received either an allogeneic hematopoietic cell transplant, an autologous hematopoietic cell transplant or chimeric antigen receptor (CAR) T cell therapy). Data published from the open-label safety lead-in portion of the trial indicates that GEO-CM04S1 is highly immunogenic, inducing broad and durable neutralizing antibody and T cell responses.
|
GEO-CM04S1 – Healthy Adult Booster Phase 2 Trial
| |
●
|
A Phase 2 trial of GEO-CM04S1 (ClinicalTrials.gov Identifier: NCT04639466), evaluating two vaccine does levels as a heterologous COVID-19 booster vaccine to current FDA-approved mRNA vaccines from Pfizer/BioNTech and Moderna
|
| |
●
|
In September 2023, we announced completion of enrollment. The trial protocol requires the subjects be followed for 12 months and the last data collection points are scheduled towards the end September 2024. Safety and immune response readouts from this study should be available towards the end of 2024 or early 2025.
|
| |
●
|
In February 2024, we announced positive interim safety and immune responses findings following vaccine administration. Consolidated data (blinded to vaccine dose) from all subjects tested one-month post-vaccination, documented statistically significant increases in neutralizing antibody responses against multiple SARS-CoV-2 variants, ranging from the original Wuhan strain through Delta and Omicron XBB 1.5.
|
GEO-CM04S1 – Immunocompromised/CLL Trial Phase 2 Trial
| |
●
|
GEO-CM04S1 is undergoing an investigator-initiated Phase 2 clinical trial (ClinicalTrials.gov Identifier: NCT05672355), evaluating its use as a COVID-19 vaccine booster in patients with chronic lymphocytic leukemia (CLL), compared to the Pfizer/BioNTech mRNA-based vaccine. Interim data results are scheduled for Q3 2024.
|
Gedeptin® – Advanced Head and Neck Cancer Phase 1b/2a Trial
| |
●
|
Gedeptin® recently completed a Phase 1b/2a clinical trial (PNP-002) (ClinicalTrials.gov Identifier: NCT03754933) for treatment of patients with advanced head and neck squamous cell carcinoma (HNSCC). This trial was being funded in part by the U.S. Food & Drug Administration (FDA) pursuant to its Orphan Products Clinical Trials Grants Program.
|
| |
●
|
We recently convened a special clinical advisory board to conduct a comprehensive review of the PNP-002 trial results, together with the previously completed Phase 1 trial (PNP-001). This review concluded that Gedeptin demonstrated an acceptable safety and efficacy profile to support continued development. In addition, the therapy has demonstrated sufficient tumor stabilization/reduction activity to support plans to advance clinical development of Gedeptin therapy in an expanded Phase 2 clinical trial.
|
| |
●
|
We have initiated activities in support of a Phase 2 trial in first-recurrence head and neck cancer. The primary goal of this trial will be to establish efficacy of neoadjuvant Gedeptin therapy combined with an immune checkpoint inhibitor in squamous cell head and neck cancer. This trial is anticipated to be a single cycle trial with surgery to follow in approximately 36 patients with pathologic response rate as the primary endpoint. We have initiated the necessary planning activities, including protocol development, manufacturing and CRO selection, with the trial activation anticipated during the first half of 2025.
|
MVA-Based Vaccine Manufacturing Process Development
| |
●
|
In March 2024, we announced a significant milestone toward implementation of a validated chicken embryonic fibroblast (CEF) based production system for our MVA-based vaccines, with the release of the first lot of GEO-CM04S1 produced with a commercial manufacturing platform. This marked the successful completion of the transfer and scale-up of manufacturing to Oxford Biomedica, the Company’s cGMP (current Good Manufacturing Procedures) manufacturing partner.
|
Intellectual Property Development
| |
●
|
In January 2024, the U.S. Patent and Trademark Office issued Patent No. 11,857,611 to GeoVax, pursuant to patent application No. 17/726,254 titled “Compositions and Methods for Generating an Immune Response to Treat or Prevent Malaria”. The allowed claims cover compositions comprising GeoVax’s modified vaccinia Ankara (MVA) vector expressing Plasmodium antigens and methods of inducing an immune response to malaria utilizing the compositions. The compositions and methods covered in the allowed claims are useful both prophylactically and therapeutically and may be used to prevent and/or treat malaria.
|
| |
●
|
In February 2024, the U.S. Patent and Trademark Office issued Patent No. 11,896,657 to GeoVax, pursuant to patent application No. 17/584,231 titled “Replication Deficient Modified Vaccina Ankara (MVA) Expressing Marburg Virus Glycoprotein (GP) and Matrix Protein (VP40).” The allowed claims generally cover GeoVax’s vector platform for expressing Marburg virus antigens in virus-like particles (VLPs) utilizing an MVA viral vector.
|
| |
●
|
In February 2024, the U.S Patent and Trademark Office issued Patent No. 11,897,919 to GeoVax, pursuant to patent application No. 17/409,574 titled “Multivalent HIV Vaccine Boost Compositions and Methods of Use.” The allowed claims generally cover a priming vaccination with a DNA vector encoding multiple HIV antigens in virus-like particles (VLPs), followed by a boost vaccination with GeoVax’s vector platform for expressing HIV-1 antigens in VLPs utilizing an MVA viral vector.
|
| |
●
|
In February 2024, the Japanese Patent Office issued a Decision of Grant notifying GeoVax of the allowance of the Company’s Patent Application No. 2022-153352 titled “Compositions and Methods for Generating an Immune Response to a Tumor Associated Antigen.” The allowed claims are directed to recombinant MVA viral vectors comprising specific MUC-1 nucleic sequences used in GeoVax’s MUC-1 tumor-associated antigen immunotherapy program. Pharmaceutical compositions for inducing immune responses, preventing or reducing neoplasm growth, or treating cancer are also covered by the granted claims. This represents an extension of the GeoVax MVA-VLP platform that was originally developed for vaccines targeting infectious diseases.
|
Recent Developments
Registered Direct Offering
On July 11, 2024, the Company entered into a placement agency agreement (the “Placement Agency Agreement”) with Roth Capital Partners, LLC (“Roth”) and a securities purchase agreement (the “Purchase Agreement”) with a purchaser pursuant to which the Company agreed to sell, in a registered direct offering (the “Registered Direct Offering”), an aggregate of (i) 458,632 shares of the Company’s common stock, and (ii) pre-funded warrants to purchase up to an aggregate of 626,368 shares of common stock (the “July Pre-Funded Warrants,” and the Shares issuable upon exercise thereof, the “July Pre-Funded Warrant Shares”). In a concurrent private placement, the Company offered Common Warrants to the purchaser, with each warrant exercisable to purchase one share of Common Stock (the “July Common Warrants”), with two July Common Warrants to accompany each share of common stock or July Pre-Funded Warrant sold in the Offering, and to purchase in the aggregate up to 2,170,000 shares of common stock (the “July Common Warrant Shares”). The public offering price for each share was $2.86 and the public offering price for each July Pre-Funded Warrant was $2.85999. The July Pre-Funded Warrants have an exercise price of $0.0001 per share, are exercisable immediately and may be exercised at any time until exercised in full. The July Common Warrants have an exercise price of $2.86 per share, are immediately exercisable upon stockholder approval and will expire five years from the date of such stockholder approval. The net proceeds of the offering, after deducting Roth’s fees and expenses and other offering expenses payable by the Company and excluding the net proceeds, if any, from the exercise of the July Common Warrants, is approximately $2.8 million. The Company intends to use the net proceeds from the offering for working capital and general corporate purposes. The offering closed on July 12, 2024.
Resale of Common Stock by Selling Stockholder
On August 6, 2024, the Company filed a registration statement on Form S-1 for the resale of the July Common Warrant Shares, issuable upon the exercise of the July Common Warrants issued in a private placement on July 12, 2024 to a purchaser (the “Selling Stockholder”). We will not receive any proceeds from the sale of the July Common Warrant Shares covered by that prospectus by the Selling Stockholder. All net proceeds from the sale of the July Common Warrant Shares covered by that prospectus will go to the Selling Stockholder. However, we will receive the proceeds from any cash exercise of the July Common Warrants.
Summary of Risk Factors
Any investment in our securities involves a high degree of risk. You should consider carefully the risks described below, and the more detailed information at “Risk Factors” on page S-9 of this prospectus supplement, together with all of the other information contained in or incorporated by reference into this prospectus and the applicable prospectus supplement, before you decide whether to purchase our securities:
Risks Related to Our Business and Capital Requirements
| |
●
|
We have a history of operating losses, and we expect losses to continue for the foreseeable future.
|
| |
●
|
We have received a going concern opinion from our auditors.
|
| |
●
|
Our business will require continued funding. If we do not receive adequate funding, we may not be able to continue our operations.
|
| |
●
|
Significant disruptions of information technology systems or breaches of information security systems could adversely affect our business.
|
Risks Related to Development and Commercialization of Product Candidates and Dependence on Third Parties
| |
●
|
Our products are still being developed and are unproven. These products may not be successful.
|
| |
●
|
We depend upon key personnel who may terminate their employment with us at any time. If we were to lose the services of any of these individuals, our business and operations may be adversely affected.
|
| |
●
|
Regulatory and legal uncertainties could result in significant costs or otherwise harm our business.
|
| |
●
|
We face intense competition and rapid technological change that could result in products that are superior to, or earlier to the market than, the products we will be commercializing or developing.
|
| |
●
|
Our product candidates are based on new medical technology and, consequently, are inherently risky. Concerns about the safety and efficacy of our products could limit our future success.
|
| |
●
|
We may experience delays in our clinical trials that could adversely affect our financial results and our commercial prospects.
|
| |
●
|
Failure to obtain timely regulatory approvals required to exploit the commercial potential of our products could increase our future development costs or impair our future sales.
|
| |
●
|
State pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
|
| |
●
|
Changes in healthcare law and implementing regulations, as well as changes in healthcare policy, may impact our business in ways that we cannot currently predict, and may have a significant adverse effect on our business and results of operations.
|
| |
●
|
We may not be successful in establishing collaborations for product candidates we seek to commercialize, which could adversely affect our ability to discover, develop, and commercialize products.
|
| |
●
|
We do not have manufacturing, sales or marketing experience.
|
| |
●
|
Our products under development may not gain market acceptance.
|
| |
●
|
We may be required to defend lawsuits or pay damages for product liability claims.
|
| |
●
|
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for our products, it is less likely that they will be widely used.
|
Risks Related to Our Intellectual Property
| |
●
|
Our success depends on our ability to obtain, maintain, protect and enforce our intellectual property and our proprietary technologies.
|
| |
●
|
We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our licensors.
|
| |
●
|
Other parties may claim that we infringe their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling products.
|
| |
●
|
Any inability to protect our or our licensors’ intellectual property rights in the United States and foreign countries could limit our ability to prevent others from manufacturing or selling our products.
|
| |
●
|
Changes in United States patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
|
| |
●
|
The patent protection and patent prosecution for our product candidates is dependent in part on third parties.
|
Risks Related to Our Common Stock
| |
●
|
The market price of our common stock is highly volatile.
|
| |
●
|
The sale or issuance of additional shares of our common stock or other equity securities could result in additional dilution to our stockholders.
|
| |
●
|
Certain provisions of our certificate of incorporation which authorize the issuance of shares of preferred stock may make it more difficult for a third party to effect a change in control.
|
| |
●
|
We have never paid dividends and have no plans to do so.
|
| |
●
|
Public company compliance may make it more difficult for us to attract and retain officers and directors.
|
| |
●
|
Our Certificate of Incorporation and Bylaws may be amended by the affirmative vote of a majority of our stockholders.
|
| |
●
|
Broker-dealers may be discouraged from effecting transactions in shares of our common stock if we are considered to be a penny stock and thus subject to the penny stock rules.
|
| |
●
|
We may be delisted from the Nasdaq Capital Market LLC due to noncompliance with Nasdaq Listing Rules.
|
Corporate Information
We are incorporated under the laws of the State of Delaware. Our principal corporate offices are located at 1900 Lake Park Drive, Suite 380, Smyrna, Georgia 30080 (metropolitan Atlanta). Our telephone number is (678) 384-7220. The address of our website is www.geovax.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports, are available to you free of charge through the “Investors” section of our website as soon as reasonably practicable after such materials have been electronically filed with or furnished to the Securities and Exchange Commission (“SEC”). Information contained on our website does not form a part of this prospectus.
Summary of the Offering
|
Issuer:
|
|
GeoVax Labs, Inc.
|
| |
|
|
|
Common Stock offered:
|
|
1,360,731 shares of our common stock
|
| |
|
|
|
Pre-Funded Warrants offered
|
|
We are also offering the Pre-Funded Warrants to purchase up to 339,269 shares of Common Stock in lieu of shares of common stock because the purchase of shares of Common Stock in this offering would otherwise result in the investor, together with its affiliates, beneficially owning more than 9.99% of our outstanding common stock immediately following the consummation of this offering. Each Pre-Funded Warrant is exercisable for one share of our common stock at an exercise price of $0.00001 per share. The Pre-Funded Warrants are exercisable immediately upon issuance and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. This prospectus supplement and the accompanying base prospectus also relate to the offering of the shares of common stock issuable upon exercise of the Pre-Funded Warrants.
|
| |
|
|
|
Concurrent Private Placement
|
|
In a concurrent private placement, we are selling to the purchasers of shares of our common stock and Pre-Funded Warrants in this offering Common Warrants to purchase 1,700,000 shares of our common stock at an exercise price of $5.00 per share. We will receive gross proceeds from the concurrent private placement transaction solely to the extent such warrants are exercised for cash. The Common Warrants and the shares of our common stock issuable upon the exercise of such warrants are not being offered pursuant to this prospectus and are being offered pursuant to the exemption provided in Section 4(a)(2) under the Securities Act and Rule 506(b) promulgated thereunder. See “Private Placement Transaction.”
|
| |
|
|
|
Shares of common stock outstanding prior to the offering:
|
|
5,849,098 shares
|
| |
|
|
|
Shares of common stock outstanding after the offering:
|
|
7,209,829 shares (assuming no exercise of the Pre-Funded Warrants). Assuming all of the Pre-Funded Warrants were immediately exercised, there would be 7,549,098 shares of our common stock outstanding after this offering (and no exercise of the Common Warrants issued in the concurrent private placement).
|
| |
|
|
|
Trading symbols:
|
|
Our common stock and warrants are listed on The Nasdaq Capital Market under the symbols “GOVX” and “GOVXW”, respectively. There is no established trading market for the Pre-Funded Warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Pre-Funded Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Pre-Funded Warrants will be limited.
|
| |
|
|
|
Use of proceeds:
|
|
We estimate that we will receive net proceeds of approximately $7,855,000 from this offering, after deducting placement agent fees and estimated offering expenses payable by us. We intend to use the net proceeds from this offering to advance our product candidates, including research and technical development, manufacturing, clinical studies, capital expenditures, and working capital. We may also use the net proceeds from this offering to acquire and invest in complementary products, technologies or businesses; however, we currently have no agreements or commitments to complete any such transaction.
|
| |
|
|
|
Risk factors:
|
|
Investing in our securities involves substantial risks. You should carefully review and consider the “Risk Factors” section of this prospectus supplement beginning on page S-9 and page 7 of the accompanying prospectus, and the other information in this prospectus supplement for a discussion of the factors you should consider before you decide to invest in this offering.
|
|
(1)
|
The number of shares of our common stock outstanding after the completion of this offering is based on 5,849,098 shares of our common stock outstanding as of August 19, 2024, and excludes the following:
|
| |
●
|
339,269 shares of common stock issuable upon the exercise of the Pre-Funded Warrants with an exercise price of $0.00001 per share;
|
| |
●
|
1,700,000 shares of common stock issuable upon the exercise of the Common Warrants with an exercise price of $5.00 per share;
|
| |
●
|
3,942,137 shares of common stock issuable upon the exercise of other outstanding warrants with a weighted average exercise price of $5.63 per share; and
|
| |
●
|
333,648 shares of common stock which are reserved for issuance under our 2020 and 2023 Stock Incentive Plans, of which 328,648 shares of common stock are issuable upon exercise of outstanding options at a weighted average exercise price of $12.83 per share.
|
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider and evaluate all of the information contained in this prospectus supplement, the accompanying base prospectus and in the documents we incorporate by reference into this prospectus supplement and the accompanying base prospectus before you decide to purchase our securities. In particular, you should carefully consider and evaluate the risks and uncertainties described under the heading “Risk Factors” in this prospectus supplement and the accompanying base prospectus. Any of the risks and uncertainties set forth in this prospectus supplement and the accompanying base prospectus, as updated by annual, quarterly and other reports and documents that we file, or we are deemed to have filed, with the SEC and incorporate by reference into this prospectus supplement or the accompanying base prospectus could materially and adversely affect our business, results of operations and financial condition, which in turn could materially and adversely affect the value of our common stock. As a result, you could lose all or part of your investment.
Risks Related to this Offering of Securities
We have broad discretion in determining how to use the proceeds from this offering and we cannot assure you that we will be successful in spending the proceeds in ways which increase our profitability or market value, or otherwise yield favorable returns.
We plan to utilize the proceeds of this offering for general working capital. Nevertheless, we will have broad discretion in determining specific expenditures. You will be entrusting your funds to our management, upon whose judgment you must depend, with limited information concerning the purposes to which the funds will ultimately be applied. We may not be successful in spending the proceeds of this offering in ways which increase our profitability or market value, or otherwise yield favorable returns.
Fluctuations in the price of our common stock, including as a result of actual or anticipated sales of shares by stockholders, may make our common stock more difficult to resell.
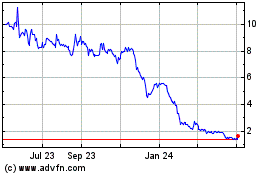
The market price and trading volume of our common stock have been and may continue to be subject to significant fluctuations due not only to general stock market conditions, but also to changes in sentiment in the market regarding the industry in which we operate, our operations, business prospects or liquidity or this offering. In addition to the risk factors discussed in our periodic reports and in this prospectus supplement, the price and volume volatility of our common stock may be affected by actual or anticipated sales of common stock by existing stockholders, including of shares purchased in this offering, whether in the market or in subsequent public offerings. Stock markets in general may experience extreme volatility that is unrelated to the operating performance of listed companies. These broad market fluctuations may adversely affect the trading price of our common stock, regardless of our operating results. As a result, these fluctuations in the market price and trading volume of our common stock may make it difficult to predict the market price of our common stock in the future, cause the value of your investment to decline and make it more difficult to resell our common stock.
If we are not able to comply with the applicable continued listing requirements or standards of the Nasdaq Capital Market, Nasdaq could delist our common stock and the related warrants.
Our common stock (GOVX) and related warrants (GOVXW) are currently listed on the Nasdaq Capital Market. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements. There can be no assurances that we will be able to continue to comply with the applicable listing standards.
On May 23, 2024, the Company received a notice from the Listing Qualifications Department of Nasdaq notifying the Company that it no longer complied with the $2,500,000 minimum stockholders’ equity required for continued listing pursuant to Nasdaq Listing Rule 5550(b)(1) (the “Stockholders’ Equity Requirement”), because the Company’s stockholders’ equity as reported in its Form 10-Q for the period ended March 31, 2024 did not meet the required minimum, and as of the date of the Notice, the Company did not meet the alternatives of market value of listed securities or net income from continuing operations (together with the Stockholders’ Equity Requirement, the “Listing Rule”).
We are currently reviewing potential transactions that, if implemented, could remedy the shortfall in our stockholders’ equity. We do not know at this time, however, whether we will be able to remedy the non-compliance. If we are unable to maintain compliance with these Nasdaq Capital Market requirements, our common stock will be delisted from the Nasdaq Capital Market. In that event, and if our common stock is not then eligible for quotation on another market or exchange, trading of our common stock could be conducted in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the OTC Pink. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on an exchange.
Investors will incur immediate and substantial dilution as a result of this offering.
Investors purchasing securities in this offering will incur immediate and substantial dilution in net tangible book value per share. Based on the per share common stock offering price of $5.00, purchasers of the shares will effectively incur dilution of approximately $4.06 per share in the net tangible book value of their purchased shares of common stock, or approximately 81% at the offering price of the shares. Furthermore, you may experience further dilution to the extent that shares of our common stock are issued upon the exercise of outstanding warrants or stock options. See “Dilution.”
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our common stock adversely, our common stock price and trading volume could decline.
The trading market for our shares of common stock will be influenced by many factors, including without limitation, the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our common stock adversely, or provide more favorable relative recommendations about our competitors, our share price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our common stock price or trading volume to decline.
In making your investment decision, you should understand that we and the underwriter have not authorized any other party to provide you with information concerning us or this offering.
You should carefully evaluate all of the information in this prospectus supplement before investing in our company. We may receive media coverage regarding our company, including coverage that is not directly attributable to statements made by our officers, that incorrectly reports on statements made by our officers or employees, or that is misleading as a result of omitting information provided by us, our officers or employees. We and the underwriter have not authorized any other party to provide you with information concerning us or this offering, and you should not rely on this information in making an investment decision.
An active, liquid trading market for our common stock may not develop, which may cause our common stock to trade at a discount from the offering price and make it difficult for you to sell the common stock you purchase.
Our common stock is currently listed on the Nasdaq Capital Market. However, there can be no assurance that there will be an active market for our common stock either now or in the future. If an active and liquid trading market does not develop or if developed cannot be sustained, you may have difficulty selling any of our common stock that you purchase. The market price of our common stock may decline below the initial offering price, and you may not be able to sell your shares of our common stock at or above the price you paid, or at all.
Risks Related to Our Business and Capital Requirements
We have a history of operating losses, and we expect losses to continue for the foreseeable future.
As a research and development-focused company, we have had no product revenue to date and revenues from our government grants and other collaborations have not generated sufficient cash flows to cover operating expenses. Since our inception, we have incurred operating losses each year due to costs incurred in connection with research and development activities and general and administrative expenses associated with our operations. We incurred a net loss of approximately $26 million for the year ended December 31, 2023 and of approximately $10.9 million for the six months ended June 30, 2024. We expect to incur additional operating losses and expect cumulative losses to increase as our research and development, preclinical, clinical, and manufacturing efforts expand. Our ability to generate revenue and achieve profitability depends on our ability to successfully complete the development of our product candidates, conduct preclinical tests and clinical trials, obtain the necessary regulatory approvals, and manufacture and market or otherwise commercialize our products. Unless we are able to successfully meet these challenges, we will not be profitable and may not remain in business.
We have received a going concern opinion from our auditors.
We have received a "going concern" opinion from our independent registered public accounting firm, reflecting substantial doubt about our ability to continue as a going concern. Our consolidated financial statements contemplate that we will continue as a going concern and do not contain any adjustments that might result if we were unable to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to raise additional capital and implement our business plan. If we are unable to achieve or sustain profitability or to secure additional financing on acceptable terms, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our stockholders losing their entire investment. There is no guarantee that we will become profitable or secure additional financing on acceptable terms.
Our business will require continued funding. If we do not receive adequate funding, we may not be able to continue our operations.
To date, we have financed our operations principally through the sale of our equity securities and through government grants and clinical trial support. We will require substantial additional financing at various intervals for our operations, including clinical trials, operating expenses, intellectual property protection and enforcement, for pursuit of regulatory approvals, and for establishing or contracting out manufacturing, marketing and sales functions. There is no assurance that such additional funding will be available on terms acceptable to us or at all. If we are not able to secure the significant funding that is required to maintain and continue our operations at current levels, or at levels that may be required in the future, we may be required to delay clinical studies or clinical trials, curtail operations, or obtain funds through collaborative arrangements that may require us to relinquish rights to some of our products or potential markets.
We may pursue additional support from the federal government for our vaccine and immunotherapy development programs; however, as we progress to the later stages of our development activities, government financial support may be more difficult to obtain, or may not be available at all. Therefore, it will be necessary for us to look to other sources of funding to finance our development activities.
We will need to raise additional funds to significantly advance our vaccine development programs and to continue our operations. In order to meet our operating cash flow needs we plan to seek sources of non-dilutive capital through government grant programs and clinical trial support. We may also plan additional offerings of our equity securities, debt, or convertible debt instruments. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could have a material adverse effect on our business, operating results, financial condition and prospects.
Significant disruptions of information technology systems or breaches of information security systems could adversely affect our business.
We rely upon a combination of information technology systems and traditional recordkeeping to operate our business. In the ordinary course of business, we collect, store, and transmit confidential information (including, but not limited to, personal information and intellectual property). We have also outsourced elements of our operations to third parties, including elements of our information technology systems and, as a result, we manage a number of independent vendor relationships with third parties who may or could have access to our confidential information. Our information technology and information security systems and records are potentially vulnerable to security breaches, service interruptions, or data loss from inadvertent or intentional actions by our employees or vendors. Our information technology and information security systems and records are also potentially vulnerable to malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of expertise and motives (including, but not limited to, financial crime, industrial espionage, and market manipulation).
While we have invested, and continue to invest, a portion of our limited funds in our information technology and information security systems, there can be no assurance that our efforts will prevent security breaches, service interruptions, or data losses. Any security breaches, service interruptions, or data losses could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business, and reputational harm to us or allow third parties to gain material, inside information that they may use to trade in our securities.
Risks Related to Development and Commercialization of Product Candidates and Dependence on Third Parties
Our products are still being developed and are unproven. These products may not be successful.
To become profitable, we must generate revenue through sales of our products. However, our products are in varying stages of development and testing. Our products have not been proven in human clinical trials and have not been approved by any government agency for sale. If we cannot successfully develop and prove our products and processes, or if we do not develop other sources of revenue, we will not become profitable and at some point, we would discontinue operations.
We depend upon key personnel who may terminate their employment with us at any time. If we were to lose the services of any of these individuals, our business and operations may be adversely affected.
The success of our business strategy will depend to a significant degree upon the continued services of key management, technical and scientific personnel and our ability to attract and retain additional qualified personnel and managers. Competition for qualified personnel is intense among companies, academic institutions and other organizations. The ability to attract and retain personnel is adversely affected by our financial challenges. If we are unable to attract and retain key personnel and advisors, it may negatively affect our ability to successfully develop, test, commercialize and market our products and product candidates.
Regulatory and legal uncertainties could result in significant costs or otherwise harm our business.
To manufacture and sell our products, we must comply with extensive domestic and international regulation. In order to sell our products in the United States, approval from the U.S. Food and Drug Administration (the “FDA”) is required. Satisfaction of regulatory requirements, including FDA requirements, typically takes many years, and if approval is obtained at all, it is dependent upon the type, complexity and novelty of the product, and requires the expenditure of substantial resources. We cannot predict whether our products will be approved by the FDA. Even if they are approved, we cannot predict the time frame for approval. Foreign regulatory requirements differ from jurisdiction to jurisdiction and may, in some cases, be more stringent or difficult to meet than FDA requirements. As with the FDA, we cannot predict if or when we may obtain these regulatory approvals. If we cannot demonstrate that our products can be used safely and successfully in a broad segment of the patient population on a long-term basis, our products would likely be denied approval by the FDA and the regulatory agencies of foreign governments.
We face intense competition and rapid technological change that could result in products that are superior to, or earlier to the market than, the products we will be commercializing or developing.
The market for vaccines that protect against or treat human infectious diseases is intensely competitive and is subject to rapid and significant technological change. We have numerous competitors in the United States and abroad, including, among others, large companies with substantially greater resources than us. If any of our competitors develop products with efficacy or safety profiles significantly better than our products, we may not be able to commercialize our products, and sales of any of our commercialized products could be harmed. Some of our competitors and potential competitors have substantially greater product development capabilities and financial, scientific, marketing and human resources than we do. Competitors may develop products earlier, obtain FDA approvals for products more rapidly, or develop products that are more effective than those under development by us. We will seek to expand our technological capabilities to remain competitive; however, research and development by others may render our technologies or products obsolete or noncompetitive or result in treatments or cures superior to ours.
Our product candidates are based on new medical technology and, consequently, are inherently risky. Concerns about the safety and efficacy of our products could limit our future success.
We are subject to the risks of failure inherent in the development of product candidates based on new medical technologies. These risks include the possibility that the products we create will not be effective, that our product candidates will be unsafe or otherwise fail to receive the necessary regulatory approvals, and that our product candidates will be difficult to manufacture on a large scale or will be uneconomical to market.
Many pharmaceutical products cause multiple potential complications and side effects, not all of which can be predicted with accuracy and many of which may vary from patient to patient. Long-term follow-up data may reveal previously unidentified complications associated with our products. The responses of potential physicians and others to information about complications could materially adversely affect the market acceptance of our products, which in turn would materially harm our business.
We may experience delays in our clinical trials that could adversely affect our financial results and our commercial prospects.
We do not know whether planned pre-clinical and clinical trials will begin on time or whether we will complete any of our trials on schedule, if at all. Product development costs will increase if we have delays in testing or approvals, or if we need to perform more or larger clinical trials than planned. Significant delays may adversely affect our financial results and the commercial prospects for our products and delay our ability to become profitable.
We rely heavily on independent clinical investigators, vaccine manufacturers, and other third-party service providers for successful execution of our clinical trials, but do not control many aspects of their activities. We are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Third parties may not complete activities on schedule or may not conduct our clinical trials in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations could delay or prevent the development, approval and commercialization of our product candidates.
Failure to obtain timely regulatory approvals required to exploit the commercial potential of our products could increase our future development costs or impair our future sales.
None of our vaccines are approved by the FDA for sale in the United States or by other regulatory authorities for sale in foreign countries. To exploit the commercial potential of our technologies, we are conducting and planning to conduct additional pre-clinical studies and clinical trials. This process is expensive and can require a significant amount of time. Failure can occur at any stage of testing, even if the results are favorable. Failure to adequately demonstrate safety and efficacy in clinical trials could delay or preclude regulatory approval and restrict our ability to commercialize our technology or products. Any such failure may severely harm our business. In addition, any approvals we obtain may not cover all of the clinical indications for which approval is sought or may contain significant limitations in the form of narrow indications, warnings, precautions or contraindications with respect to conditions of use, or in the form of onerous risk management plans, restrictions on distribution, or post-approval study requirements.
State pharmaceutical marketing compliance and reporting requirements may expose us to regulatory and legal action by state governments or other government authorities.
Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs and file periodic reports on sales, marketing, pricing and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and available guidance is limited. Unless we are in full compliance with these laws, we could face enforcement action, fines, and other penalties and could receive adverse publicity, all of which could harm our business.
Changes in healthcare law and implementing regulations, as well as changes in healthcare policy, may impact our business in ways that we cannot currently predict, and may have a significant adverse effect on our business and results of operations.
In the United States and foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval. Among policy makers and payors in the United States and elsewhere, including in the European Union, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the “Affordable Care Act”), substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry. The Affordable Care Act includes a number of provisions that are intended to lower healthcare costs, including provisions relating to prescription drug prices and government spending on medical products.
Since its enactment, there have also been judicial and Congressional challenges to certain aspects of the Affordable Care Act, as well as efforts by the former Trump administration to repeal or replace certain aspects of the statute. We continue to evaluate the effect that the Affordable Care Act and subsequent changes to the statute has on our business. It is uncertain the extent to which any such changes may impact our business or financial condition.
There has also been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products. There have been several Congressional inquiries and proposed bills, as well as state efforts, designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. In June 2017, the FDA issued a Drug Competition Action plan intended to lower prescription drug prices by encouraging competition from generic versions of existing products. In July 2018, the FDA issued a Biosimilar Action Plan, intended to similarly promote competition to prescription biologics from biosimilars.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures. For example, in September 2017, the California State Assembly approved SB17, which requires pharmaceutical companies to notify health insurers and government health plans at least 60 days before any scheduled increases in the prices of their products if they exceed 16% over a two-year period, and further requiring pharmaceutical companies to explain the reasons for such increase. Effective in 2016, Vermont passed a law requiring certain manufacturers identified by the state to justify their price increases.
We expect that these, and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and lower reimbursement, and in downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our drugs, once marketing approval is obtained.
We may not be successful in establishing collaborations for product candidates we seek to commercialize, which could adversely affect our ability to discover, develop, and commercialize products.
We expect to seek collaborations for the development and commercialization of product candidates in the future. The timing and terms of any collaboration will depend on the evaluation by prospective collaborators of the clinical trial results and other aspects of a product’s safety and efficacy profile. If we are unable to reach agreements with suitable collaborators for any product candidate, we will be forced to fund the entire development and commercialization of such product candidates, ourselves, and we may not have the resources to do so. If resource constraints require us to enter into a collaboration agreement early in the development of a product candidate, we may be forced to accept a more limited share of any revenues the product may eventually generate. We face significant competition in seeking appropriate collaborators. Moreover, these collaboration arrangements are complex and time-consuming to negotiate and document. We may not be successful in our efforts to establish collaborations or other alternative arrangements for any product candidate. Even if we are successful in establishing collaborations, we may not be able to ensure fulfillment by collaborators of their obligations or our expectations.
We do not have manufacturing, sales, or marketing experience.
We do not have experience in manufacturing, selling, or marketing. To obtain the expertise necessary to successfully manufacture, market, and sell our products, we must develop our own commercial infrastructure and/or collaborative commercial arrangements and partnerships. Our ability to execute our current operating plan is dependent on numerous factors, including, the performance of third-party collaborators with whom we may contract.
Our products under development may not gain market acceptance.
Our products may not gain market acceptance among physicians, patients, healthcare payers and the medical community. Significant factors in determining whether we will be able to compete successfully include:
| |
•
|
the efficacy and safety of our products;
|
| |
•
|
the time and scope of regulatory approval;
|
| |
•
|
reimbursement coverage from Medicare, Medicaid, insurance companies and others;
|
| |
•
|
the price and cost-effectiveness of our products, especially as compared to any competitive products; and
|
| |
•
|
the ability to maintain patent protection.
|
We may be required to defend lawsuits or pay damages for product liability claims.
Product liability is a major risk in testing and marketing biotechnology and pharmaceutical products. We may face substantial product liability exposure in human clinical trials and for products that we sell after regulatory approval. We carry product liability insurance and we expect to continue such policies. However, product liability claims, regardless of their merits, could exceed policy limits, divert management’s attention, and adversely affect our reputation and demand for our products.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for our products, it is less likely that they will be widely used.
Market acceptance of products we develop, if approved, will depend on reimbursement policies and may be affected by, among other things, future healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will cover and establish payment levels. We cannot be certain that reimbursement will be available for any products that we may develop. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for our products. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize products that we develop.
Risks Related to Our Intellectual Property
Our success depends on our ability to obtain, maintain, protect, and enforce our intellectual property and our proprietary technologies.
In general, our commercial success will depend in part on our and our licensors’ ability to obtain, maintain, protect, and enforce our intellectual property and proprietary technologies, including patent protection and trade secret protection for our product candidates, proprietary technologies and their uses as well as our ability to operate without infringing, misappropriating, or otherwise violating the intellectual property rights of others. If we or our licensors are unable to obtain, maintain, protect, or enforce our intellectual property rights or if our intellectual property rights are inadequate for our technology or our product candidates, our competitive position could be harmed, which could have a material adverse impact on our business, results of operations, financial conditions, and prospects. There can be no assurance that our patent applications will result in patents being issued or that issued patents will afford sufficient protection against competitors with similar technologies, nor can there be any assurance that the patents if issued will not be infringed, misappropriated, violated, designed around or invalidated by third parties. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our intellectual property is uncertain. Only limited protection may be available and may not adequately obtain, maintain, protect, and enforce our rights or permit us to gain or keep any competitive advantage. These uncertainties and/or limitations in our ability to properly obtain, maintain, protect, and enforce the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operations.
We cannot be certain that the claims in our in-licensed pending patent applications will be considered patentable by the U.S. Patent and Trademark Office, or USPTO, courts in the United States or by the patent offices and courts in foreign countries, nor can we be certain that claims that may ultimately issue from our patent applications will not be found invalid or unenforceable if challenged. If we or our licensors are unable to obtain or maintain patent protection with respect to our product candidates, our business, financial condition, results of operations, and prospects could be materially harmed.
We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our licensors.
Our rights to significant parts of the technology we use in our products are licensed from third parties and are subject to termination if we do not fulfill our contractual obligations to our licensors. Termination of intellectual property rights under any of our license agreements could adversely impact our ability to produce or protect our products. Our obligations under our license agreements include requirements that we make milestone payments to our licensors upon the achievement of clinical development and regulatory approval milestones, royalties as we sell commercial products, and reimbursement of patent filing and maintenance expenses. Should we become bankrupt or otherwise unable to fulfill our contractual obligations, our licensors could terminate our rights to critical technology that we rely upon.
Other parties may claim that we infringe their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling products.
Our success will depend in part on our ability to operate without infringing the patents and proprietary rights of third parties. The manufacture, use and sale of biologic products have been subject to substantial patent litigation in the biopharmaceutical industry. Such lawsuits often relate to the validity or infringement of patents or other proprietary rights of third parties. Pharmaceutical companies, biotechnology companies, universities, research institutions or other third parties may have filed patent applications or may have been granted patents that cover aspects of our products or our licensors’ products, product candidates or other technologies.
Future or existing patents issued to third parties may contain patent claims that cover our products or their use or manufacture. In particular, the patent landscape in the COVID-19 vaccine space is crowded, and a large number of patent applications have been filed by numerous entities since January 2020, including for the use of certain SARS-CoV-2 antigens and antigenic combinations, including from Moderna, Janssen Pharmaceuticals, Inc., Sementis LTD., VaxBio, Inc., Oxford University, BioNTech, Ichan School of Medicine at Mount Sinai, Diosynvax LTD., The University of Alberta, University of Texas, and Tonix Pharmaceuticals. If a third party were to assert an infringement claim against us in the future with respect to our current products or with respect to products that we may develop or license, such litigation or interference proceedings could force us to:
| |
•
|
stop or delay selling, manufacturing or using products that incorporate, or are made using the challenged intellectual property;
|
| |
•
|
pay damages; or
|
| |
•
|
enter into licensing or royalty agreements that may not be available on acceptable terms, if at all.
|
Any litigation or interference proceedings, regardless of their outcome, may delay the regulatory approval process, be costly and require significant time and attention of our key management and technical personnel.
Any inability to protect our or our licensors’ intellectual property rights in the United States and foreign countries could limit our ability to prevent others from manufacturing or selling our products.
We will rely on trade secrets, unpatented proprietary know-how, continuing technological innovation and, in some cases, patent protection to preserve our competitive position. Our patents and licensed patent rights may be challenged, invalidated, infringed or circumvented, and the rights granted in those patents may not provide proprietary protection or competitive advantages to us. We and our licensors may not be able to develop patentable products with acceptable patent protection. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us. If patents containing competitive or conflicting claims are issued to third parties, we may be prevented from commercializing the products covered by such patents or may be required to obtain or develop alternate technology. In addition, other parties may duplicate, design around or independently develop similar or alternative technologies.
Some of our patent families and our in-licensed patent families are in an early stage of prosecution and cannot be enforced against third parties practicing the technology claimed in such applications unless, and until, patents are issued from such applications, and then only to the extent the issued claims cover the third-party technology. There can be no assurance that our patent applications will result in patents being issued or that issued patents will afford sufficient protection against competitors with similar technologies. There can be no assurance that the patents if issued will not be infringed, misappropriated, violated, designed around or invalidated by third parties. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our intellectual property is uncertain. Only limited protection may be available and may not adequately obtain, maintain, protect, and enforce our rights or permit us to gain or keep any competitive advantage. These uncertainties and/or limitations in our ability to properly obtain, maintain, protect, and enforce the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operations.
We may not be able to prevent third parties from infringing or using our intellectual property, and the parties from whom we may license intellectual property may not be able to prevent third parties from infringing or using the licensed intellectual property. We generally attempt to control and limit access to, and the distribution of, our product documentation and other proprietary information. Despite efforts to protect this proprietary information, unauthorized parties may obtain and use information that we may regard as proprietary. Other parties may independently develop similar know-how or may even obtain access to these technologies.
The laws of some foreign countries do not protect proprietary information to the same extent as the laws of the United States, and many companies have encountered significant problems and costs in protecting their proprietary information in these foreign countries.
Furthermore, even if our or our licensors’ patent applications are granted, the patent term may be inadequate to protect our competitive position on our product candidates for an adequate amount of time. Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest United States non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates have been or are obtained, once the patent life has expired, we may be open to competition from competitive products. Given the amount of time required for the development, testing, and regulatory review of product candidates, patents protecting our product candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing product candidates similar or identical to ours.
Changes in United States patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing biotechnology patents is costly, time-consuming, and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property and may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. In addition, Congress or other foreign legislative bodies may pass patent reform legislation that is unfavorable to us.
For example, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, signed into law on September 16, 2011, includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art and provide more efficient and cost-effective avenues for competitors to challenge the validity of patents. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Further, because of a lower evidentiary standard in these USPTO post-grant proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Thus, the Leahy-Smith Act and its implementation can increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects. After March 2013, under the Leahy-Smith Act, the United States transitioned to a first inventor to file system in which, assuming that the other statutory requirements are met, the first inventor to file a patent application is entitled to the patent on an invention regardless of whether a third-party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before we file an application covering the same invention, could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Because patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either (i) file any patent application related to our product candidates and other proprietary technologies we may develop or (ii) invent any of the inventions claimed in our or our licensor’s patents or patent applications. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing the claimed invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent United States Supreme Court and Federal Circuit rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. For example, recent Federal Circuit rulings such as Ariad Pharms., Inc. v. Eli Lilly & Co., 598 F.3d 1336, 1340 (Fed. Cir. 2010) (en banc), Wyeth & Cordis Corp. v. Abbott Labs, 720 F.3d 1380 (Fed. Cir. 2013), Enzo Life Scis., Inc. v. Roche Molecular Sys., 928 F.3d 1340 (Fed. Cir. 2019), and Idenix Pharms. LLC v. Gilead Scis. Inc., 941 F.3d 1149 (Fed. Cir. 2019), and Amgen Inc. v. Sanofi, 598 U.S. 594 (2023) have significantly heightened the standard for securing broad claims to pharmaceutical and biological products. In addition, recent Federal Circuit rulings such as In re Cellect, 81 F.4th 1216 (Fed. Cir. 2023) have expanded the bases for invalidating a patent under the judicially created doctrine of obviousness-type double patenting.
In addition to heightened patentability requirements, recent Supreme Court and Federal Circuit cases relating to biosimilar product approval under the BPCIA, have held that the “patent dance” provisions of the statute, which are intended to resolve any patent infringement issues before the approval of a biosimilar, are discretionary, and a biosimilar applicant can opt out by refusing to provide a copy of its application and manufacturing information to the biologic sponsor (see Sandoz Inc. v. Amgen Inc.,137 S. Ct. 1664 (2017). It may be that we do not learn of a biosimilar application until after FDA publishes its approval (see Immunex v. Samsung Bioepsis, 2:19-cv-117555-CCC-MF (D.N.J. Apr. 30, 2019). In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by Congress, the United States federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future.
The patent protection and patent prosecution for our product candidates is dependent in part on third parties.
We or our licensors may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, we may miss potential opportunities to strengthen our patent position. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example, with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments. If we or our licensors, fail to establish, maintain, or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our licensors are not fully cooperative or disagree with us as to the prosecution, maintenance, or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form, preparation, prosecution, or enforcement of our patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
We rely on third parties to file and prosecute patent applications and maintain patents and otherwise protect the licensed intellectual property under some of our license agreements. We have not had and do not have primary control over these activities for some of our in-licensed patents or patent applications and other intellectual property rights. We cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents or other intellectual property rights. Pursuant to the terms of the license agreements with some of our licensors, the licensors may have the right to control enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents and even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors. We cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents. Even if we are not a party to these legal actions, an adverse outcome could harm our business because it might prevent us from continuing to license intellectual property that we may need to operate our business. If any of our licensors fail to appropriately prosecute and maintain patent protection for patents covering our product candidates, our ability to develop and commercialize those product candidates may be adversely affected and we may not be able to prevent competitors from making, using, and selling competing products.
In addition, even where we have the right to control patent prosecution of patents and patent applications we have acquired or licensed from third parties, we may still be adversely affected or prejudiced by actions or inactions of our predecessors or licensors and their counsel that took place prior to us assuming control over patent prosecution.
Our technology acquired or licensed from various third parties may be subject to retained rights. Our predecessors or licensors often retain certain rights under their agreements with us, including the right to use the underlying technology for non-commercial academic and research use, to publish general scientific findings from research related to the technology, and to make customary scientific and scholarly disclosures of information relating to the technology. It is difficult to monitor whether our predecessors or licensors limit their use of the technology to these uses, and we could incur substantial expenses to enforce our rights to our licensed technology in the event of misuse.
In addition, the research resulting in certain of our in-licensed patent rights and technology was funded in part by the United States government. As a result, the government may have certain rights, or march-in rights, to such patent rights and technology. When new technologies are developed with government funding, the government generally obtains certain rights in any resulting patents, including a nonexclusive license authorizing the government to use the invention for noncommercial purposes. These rights may permit the government to disclose our confidential information to third parties and to exercise march-in rights to use or allow third parties to use our licensed technology. The United States government also has the right to take title to these inventions if the applicable licensor fails to disclose the invention to the government or fails to file an application to register the intellectual property within specified time limits. The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to United States industry. In addition, our rights in such inventions may be subject to certain requirements to manufacture products embodying such inventions in the United States. Any exercise by the government of such rights could harm our competitive position, business, financial condition, results of operations, and prospects.
Risks Related to Our Common Stock
The market price of our common stock is highly volatile.
The market price of our common stock has been, and is expected to continue to be, highly volatile. Certain factors, including announcements of new developments by us or other companies, regulatory matters, new or existing medicines or procedures, concerns about our financial position, operating results, litigation, government regulation, developments or disputes relating to agreements, patents or proprietary rights, may have a significant impact on the market price of our stock. In addition, potential dilutive effects of future sales of shares of common stock by us, and subsequent sales of common stock by the holders of our options and warrants could have an adverse effect on the market price of our shares.
In addition, the securities markets from time-to-time experience significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
The sale or issuance of additional shares of our common stock or other equity securities could result in additional dilution to our stockholders.
In order to meet our operating cash flow needs, we may plan additional offerings of our equity securities, debt, or convertible debt instruments. The sale of additional equity securities could result in significant additional dilution to our stockholders. The incurrence of indebtedness could result in debt service obligations and operating and financing covenants that would restrict our operations. We cannot assure investors that financing will be available in amounts or on terms acceptable to us, if at all.
We are obligated to issue additional shares of our common stock in connection with our outstanding warrants if the warrant holders choose to exercise them. In addition to the Pre-Funded Warrants and the Common Warrants which are exercisable for 1,700,000 shares at exercise prices of $5.00 per share, there are other outstanding warrants exercisable for 3,942,137 shares at exercise prices ranging from $1.68 to $195 per share. The exercise of these warrants will cause us to issue additional shares of our common stock and will dilute the percentage ownership of our shareholders.
Certain provisions of our certificate of incorporation which authorize the issuance of shares of preferred stock may make it more difficult for a third party to effect a change in control.
Our certificate of incorporation authorizes our Board of Directors to issue up to 10,000,000 shares of preferred stock. The shares of preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our Board of Directors without further action by the stockholders. These terms may include voting rights, including the right to vote as a series on particular matters, preferences as to dividends and liquidation, conversion rights, redemption rights and sinking fund provisions. The issuance of any newly issued preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of our common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of our Board of Directors to issue preferred stock could make it more difficult, delay, discourage, prevent or make it costlier to acquire or effect a change-in-control, which in turn could prevent the stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the market price of our common stock.
We have never paid dividends and have no plans to do so.
Holders of shares of our common stock are entitled to receive such dividends as may be declared by our Board of Directors. To date, we have paid no cash dividends on our shares of common stock and we do not expect to pay cash dividends on our common stock in the foreseeable future. We intend to retain future earnings, if any, to provide funds for operations of our business. Therefore, any potential return investors may have in our common stock will be in the form of appreciation, if any, in the market value of their shares of common stock.
Public company compliance may make it more difficult for us to attract and retain officers and directors.
The Sarbanes-Oxley Act, the Dodd-Frank Act, the JOBS Act, the FAST Act, and rules subsequently implemented by the SEC have required changes in corporate governance practices of public companies. As a public company, we expect these rules and regulations, and amendments to them, to contribute to our compliance costs and to make certain activities more time consuming and costly. As a public company, we also expect that these rules and regulations may make it difficult and expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
Our Certificate of Incorporation and Bylaws may be amended by the affirmative vote of a majority of our stockholders.
Under the Delaware General Corporation Law, a corporation’s certificate of incorporation may be amended by the affirmative vote of the holders of a majority of the outstanding shares entitled to vote, and a majority of the outstanding shares of each class entitled to vote as a class, unless the articles require the vote of a larger percentage of shares. Our Certificate of Incorporation, as amended, does not require the vote of a larger percentage of shares. As permitted under the Delaware General Corporation Law, our Bylaws give our board of directors the power to adopt, amend, or repeal our Bylaws. Our stockholders entitled to vote have concurrent power to adopt, amend, or repeal our Bylaws.
Broker-dealers may be discouraged from effecting transactions in shares of our common stock if we are considered to be a penny stock and thus subject to the penny stock rules.
The SEC has adopted a number of rules to regulate “penny stocks” that restrict transactions involving stock which is deemed to be penny stock. Such rules include Rules 3a51-1, 15g-1, 15g-2, 15g-3, 15g-4, 15g-5, 15g-6, 15g-7, and 15g-9 under the Exchange Act. These rules may have the effect of reducing the liquidity of penny stocks. “Penny stocks” generally are equity securities with a price of less than $5.00 per share (other than securities registered on certain national securities exchanges or quoted on Nasdaq if current price and volume information with respect to transactions in such securities is provided by the exchange or system). Our securities have in the past constituted, and may again in the future, if we are delisted from Nasdaq, constitute, “penny stock” within the meaning of the rules. The additional sales practice and disclosure requirements imposed upon U.S. broker-dealers may discourage broker-dealers from effecting transactions in shares of our common stock, which could severely limit the market liquidity of such shares and impede their sale in the secondary market.
A U.S. broker-dealer selling penny stock to anyone other than an established customer or “accredited investor” (generally, an individual with net worth in excess of $1,000,000 (exclusive of personal residence) or an annual income exceeding $200,000, or $300,000 together with his or her spouse) must make a special suitability determination for the purchaser and must receive the purchaser’s written consent to the transaction prior to sale, unless the broker-dealer or the transaction is otherwise exempt. In addition, the “penny stock” regulations require the U.S. broker-dealer to deliver, prior to any transaction involving a “penny stock”, a disclosure schedule prepared in accordance with SEC standards relating to the “penny stock” market, unless the broker-dealer or the transaction is otherwise exempt. A U.S. broker-dealer is also required to disclose commissions payable to the U.S. broker-dealer and the registered representative and current quotations for the securities. Finally, a U.S. broker-dealer is required to submit monthly statements disclosing recent price information with respect to the “penny stock” held in a customer’s account and information with respect to the limited market in “penny stocks”.
Stockholders should be aware that, according to the SEC, the market for “penny stocks” has suffered in recent years from patterns of fraud and abuse. Such patterns include (i) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (ii) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (iii) “boiler room” practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (iv) excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and (v) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, resulting in investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities.
USE OF PROCEEDS
We estimate that we will receive net proceeds of approximately $7,855,000 from this offering, after deducting placement agent fees and estimated offering expenses payable by us.
We intend to use the net proceeds from this offering to advance our product candidates, including research and technical development, manufacturing, clinical studies, capital expenditures, and working capital. We may also use the net proceeds from this offering to acquire and invest in complementary products, technologies or businesses; however, we currently have no agreements or commitments to complete any such transaction.
CAPITALIZATION
The following table sets forth our capitalization as of June 30, 2024:
| |
●
|
on an actual basis; and
|
| |
●
|
on an as adjusted basis to give effect to (a) the issuance and sale by us of 1,360,731 shares of common stock and pre-funded warrants exercisable for 339,269 shares of Common Stock in this offering at the offering price of $5.00 per share, after deducting the estimated private placement fee and commissions and estimated offering expenses payable by us, and the use of proceeds therefrom; and (b) the issuance and sale by us of common warrants exercisable for 1,700,000 shares of Common Stock in the concurrent private placement to this offering at the offering price of $5.00 per share, after deducting the estimated private placement fee and commissions and estimated offering expenses payable by us, and the use of proceeds therefrom.
|
You should read this table together with our financial statements and the related notes, and our most recent “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which have been incorporated by reference into this prospectus supplement.
| |
|
As of June 30, 2024
(unaudited)
|
|
| |
|
|
|
|
|
|
|
|
| |
|
Actual
|
|
|
As Adjusted
|
|
|
Cash and cash equivalents
|
|
$ |
1,561,712 |
|
|
|
9,416,712 |
|
|
Total liabilities
|
|
|
6,404,296 |
|
|
|
6,404,296 |
|
|
Stockholder’s equity:
|
|
|
|
|
|
|
|
|
|
Common stock
|
|
|
4,179 |
|
|
|
5,879 |
|
|
Additional paid-in capital
|
|
|
112,964,554 |
|
|
|
120,817,854 |
|
|
Accumulated deficit
|
|
|
(115,277,959 |
) |
|
|
(115,277,959 |
) |
|
Total stockholders’ equity
|
|
$ |
(2,309,226 |
) |
|
|
5,545,774 |
|
The table and discussion above are based on 4,178,700 shares of common stock outstanding as of June 30, 2024, and 5,878,700 shares as adjusted, and do not include, as of that date:
|
●
|
1,700,000 of common stock issuable upon the exercise of the Common Warrants with an exercise price of $5.00 per share;
|
|
●
|
3,942,137 shares of common stock issuable upon the exercise of other outstanding warrants with a weighted average exercise price of $5.63 per share; and
|
|
●
|
333,648 shares of common stock which are reserved for issuance under our 2020 and 2023 Stock Incentive Plans, of which 328,648 shares of common stock are issuable upon exercise of outstanding options at an average exercise price of $12.83 per share.
|
DILUTION
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the offering price per share of common stock and the as adjusted net tangible book value per share of common stock immediately after this offering.
Our net tangible book value is the amount of our total tangible assets less our total liabilities. Net tangible book value per share is our net tangible book value divided by the number of shares of common stock. Our net tangible book value as of June 30, 2024 was $(2,309,226), or $(0.55) per share, based on 4,178,700 shares of our common stock outstanding at that date.
After giving effect to the issuance and sale by us of $8,500,000 of (i) shares of common stock and (ii) pre-funded warrants exercisable for shares of common stock in this offering at the offering price of $5.00 per share, after deducting the estimated private placement fee and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2024 would have been approximately $5,545,774, or $0.94 per share of common stock. This represents an immediate increase in net tangible book value of $1.49 per share to our existing holders of common stock and an immediate dilution of $4.06 per share to investors purchasing shares in this offering.
The following table illustrates this dilution on a per share basis:
|
Offering price per share
|
|
$ |
5.00 |
|
|
Net tangible book value per share as of June 30, 2024
|
|
$ |
(0.55 |
) |
|
Increase in net tangible book value per share attributable to investors in this Offering
|
|
$ |
1.49 |
|
|
As adjusted net tangible book value per share after this Offering
|
|
$ |
0.94 |
|
|
Dilution per share to investors in this Offering
|
|
$ |
4.06 |
|
The foregoing discussion and table do not take into account further dilution to investors in this Offering that could occur upon the exercise of outstanding warrants having a per share exercise or conversion price less than the per share offering price to the public in this offering. We may also choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The table and discussion above are based on 4,178,700 shares of common stock outstanding as of June 30, 2024, and 5,878,700 shares as adjusted, and do not include, as of that date:
|
●
|
1,700,000 of common stock issuable upon the exercise of the Common Warrants with an exercise price of $5.00 per share;
|
|
●
|
3,942,137 shares of common stock issuable upon the exercise of other outstanding warrants with a weighted average exercise price of $5.63 per share; and
|
|
●
|
333,648 shares of common stock which are reserved for issuance under our 2020 and 2023 Stock Incentive Plans, of which 328,648 shares of common stock are issuable upon exercise of outstanding options at an average exercise price of $12.83 per share.
|
DESCRIPTION OF SECURITIES WE ARE OFFERING
In this offering, we are offering 1,360,731 shares of our common stock and pre-funded warrants to purchase up to 339,269 shares of common stock.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Securities-Common Stock” starting on page 8 of the accompanying base prospectus.
Pre-Funded Warrants
The following summary of certain terms and provisions of the Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Pre-Funded Warrant, the form of which will be filed as an exhibit to our Current Report on Form 8-K. Prospective investors should carefully review the terms and provisions of the form of Pre-Funded Warrant for a complete description of the terms and conditions of the Pre-Funded Warrants.
The term “pre-funded” refers to the fact that the purchase price of our common stock in this offering includes almost the entire exercise price that will be paid under the Pre-Funded Warrants, except for a nominal remaining exercise price of $0.00001. The purpose of the Pre-Funded Warrants is to enable investors that may have restrictions on their ability to beneficially own more than 9.99% of our outstanding common stock following the consummation of this offering the opportunity to make an investment in the Company without triggering their ownership restrictions, by receiving Pre-Funded Warrants in lieu of our common stock which would result in such ownership of more than 9.99%, and receive the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at such nominal price at a later date.
Duration and Exercise Price. Each Pre-Funded Warrant offered hereby has an initial exercise price per share equal to $0.00001. The Pre-Funded Warrants are immediately exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability. The Pre-Funded Warrants are exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of the Pre-Funded Warrants in this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the issuance of the Pre-Funded Warrants at closing to have their Pre-Funded Warrants exercised immediately upon issuance and receive shares of common stock underlying the Pre-Funded Warrants upon closing of this offering. A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrant to the extent that the holder would own more than 9.99% of the outstanding common stock. No fractional shares of common stock will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. In lieu of making the cash payment otherwise contemplated to be made to us upon exercise of a Pre-Funded Warrant in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants.
Transferability. Subject to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate instruments of transfer.
Exchange Listing. There is no trading market available for the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system.
Trading Market. There is no trading market available for the Pre-Funded Warrants on any securities exchange or nationally recognized trading system, and we do not expect a trading market to develop. We do not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading market. Without a trading market, the liquidity of the Pre-Funded Warrants will be extremely limited. The shares of common stock issuable upon exercise of the Pre-Funded Warrants are currently traded on the Nasdaq Capital Market.
Right as a Stockholder. Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the Pre-Funded Warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Pre-Funded Warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Waivers and Amendments
The Pre-Funded Warrant may be modified or amended or the provisions of the Pre-Funded Warrant waived with our and the holder’s written consent.
PRIVATE PLACEMENT TRANSACTION
Concurrently with the sale of common stock in this offering, we will issue and sell to the investors in this offering Common Warrants to purchase up to an aggregate of 1,700,000 shares of common stock at an exercise price equal to $5.00 per share.
The Common Warrants and the shares of common stock issuable upon the exercise of such warrants are not being registered under the Securities Act, are not being offered pursuant to this prospectus supplement and the accompanying prospectus and are being offered pursuant to the exemption provided in Section 4(a)(2) under the Securities Act and Rule 506(b) promulgated thereunder. Accordingly, purchasers may only sell shares of common stock issued upon exercise of the Common Warrants pursuant to an effective registration statement under the Securities Act covering the resale of those shares, an exemption under Rule 144 under the Securities Act or another applicable exemption under the Securities Act.
In connection with the concurrent private placement, we are obligated to file a registration statement with the SEC to register the shares underlying the Common Warrants sold in the private placement under the Securities Act, within 30 days of the closing of this offering (the “Filing Deadline”) and have such registration statement declared effective by the SEC within 60 days of such closing, or in the case of full review of the applicable registration statement by the SEC, within 120 days of the Filing Deadline (the “Event Date”). If such registration statement is not so filed or declared effective, on each applicable monthly anniversary for which such registration even is not achieved or cured, we are required to pay to the holder an amount in cash, as partial liquidated damages and not as a penalty, equal to the product of 1.0% multiplied by the product of the most recent closing price of our common stock on the applicable Event Date, and the number of shares underlying such Common Warrants, until the shares underlying such Common Warrants are freely tradeable under Rule 144 of the Securities Act or we regain compliance with the registration rights. If we fail to pay partial liquidated damages required thereby within seven (7) days after the date payable, we are required to pay interest thereon at a rate of 10% per annum (or such lesser maximum amount that is permitted to be paid by applicable law) to the holder, accruing daily from the date such partial liquidated damages are due until such amounts, plus all such interest thereon, are paid in full. The partial liquidated damages provisions apply on a daily pro rata basis for any applicable portion of a month.
The following is a summary of the material terms and provisions of the Common Warrants that are being offered in the concurrent private placement. This summary is subject to and qualified in its entirety by the form of Common Warrant, which has been provided to the investors in this offering and which will be filed with the SEC as an exhibit to a Current Report on Form 8-K in connection with this offering and incorporated by reference into the registration statement of which this prospectus supplement forms a part.
Duration and Exercise Price
The Common Warrants will have an exercise price of $5.00 per share. The Common Warrants will be immediately exercisable and will be exercisable for five years from the date of issuance. The exercise price and number of shares of common stock issuable upon exercise are subject to appropriate adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our shares of common stock. The Common Warrants will be issued separately from the common stock or Pre-Funded Warrants, respectively, and may be transferred separately immediately thereafter. The Common Warrants will be issued in certificated form only.
Exercisability
The Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s warrants to the extent that the holder would own more than 9.99% of our outstanding shares of common stock immediately after exercise, except that prior to the issuance of the Common Warrants, the holder may elect to increase the amount of ownership of outstanding shares of common stock after exercising the holder’s Common Warrants up to 9.99% of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Common Warrants.
Cashless Exercise
If at the time of exercise of the Common Warrant there is no effective registration statement registering, or the prospectus contained therein is not available for the resale of the shares of common stock issuable upon exercise of the Common Warrant, then the Common Warrants will only be exercisable on a “cashless exercise” basis under which the holder will receive upon such exercise a net number of common shares determined according to a formula set forth in the Common Warrants.
Fundamental Transactions
In the event of any fundamental transaction, as described in the Common Warrants and generally including any merger with or into another entity (but excluding the potential Acquisition), sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common stock, then upon any subsequent exercise of a Common Warrant, the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of our common stock for which the Common Warrant is exercisable immediately prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the Common Warrants have the right to require us or a successor entity to redeem the Common Warrants for cash in the amount of the Black-Scholes Value (as defined in each common warrant) of the unexercised portion of the Common Warrants concurrently with or within 30 days following the consummation of a fundamental transaction.
However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our board of directors, the holders of the Common Warrants will only be entitled to receive from us or our successor entity, as of the date of consummation of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the common warrant that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction. If the holders of our common stock are not offered or paid any consideration in such fundamental transaction, then such holders will be deemed to have received common stock.
Transferability
In accordance with its terms and subject to applicable laws, a Common Warrant may be transferred at the option of the holder upon surrender of the Common Warrant to us together with the appropriate instruments of transfer and payment of funds sufficient to pay any transfer taxes (if applicable).
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the Common Warrants. Rather, the number of shares of common stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading market for the Common Warrants, and we do not expect a market to develop. We do not intend to apply for a listing for the Common Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Common Warrants will be limited.
Rights as a Shareholder
Except as otherwise provided in the Common Warrants or by virtue of the holder’s ownership of shares of our common stock, such holder of Common Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until such holder exercises such holder’s Common Warrants. The Common Warrants will provide that the holders of the Common Warrants have the right to participate in distributions or dividends paid on our shares of common stock.
PLAN OF DISTRIBUTION
We engaged Roth Capital Partners, LLC (“Roth” or the “placement agent”) to act as our exclusive placement agent in connection with this offering. Roth is not purchasing or selling any of the securities offered by us in this offering, and is not required to arrange for the sale of any specific number or dollar amount of securities, other than to use its reasonable best efforts to arrange for the sale of such securities by us. The terms of this offering were subject to market conditions and negotiations between us, Roth and the prospective investor.
We are entering into separate securities purchase agreements directly with investors in connection with this offering of common stock and Pre-Funded Warrants pursuant to this prospectus supplement and accompanying prospectus under which we will sell the securities offered hereby directly to such investors.
Roth will have no authority to bind us. Further, Roth does not guarantee that it will be able to raise new capital in any prospective offering.
Delivery of the securities offered hereby is expected to occur on or about August 21, 2024, subject to satisfaction or waiver of customary closing conditions.
We have agreed to pay Roth a cash fee equal to 7.0% of the gross proceeds received from the investor who purchased securities in the offering. We have also agreed to reimburse Roth for accountable expenses, including, but not limited to, legal fees for placement agent’s legal counsel, that we have agreed to pay at the closing of the offering up to an aggregate expense reimbursement of $75,000. We estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent’s fees and expenses, will be approximately $25,000.
We have agreed to indemnify the placement agent against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the placement agent may be required to make for these liabilities.
Under the terms of the securities purchase agreement, from the date of such agreements until fifteen (15) days after the closing of this offering, neither we nor any subsidiary shall (i) issue, enter into any agreement to issue or announce the issuance or proposed issuance of any shares of common stock or common stock equivalents, or (ii) file any registration statement or prospectus, or any amendment or supplement thereto, subject to certain exceptions.
We have also agreed, subject to certain exceptions, until the six month anniversary of the closing of this offering, not to (i) issue or sell any debt or equity securities that are convertible into, exchangeable or exercisable for, or include the right to receive, additional shares of common stock either (A) at a conversion price, exercise price or exchange rate or other price that is based upon, and/or varies with, the trading prices of or quotations for the shares of common stock at any time after the initial issuance of such debt or equity securities or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly related to our business or the market for our common stock or (ii) enter into, or effect a transaction under, any agreement, including, but not limited to, an equity line of credit, whereby we may issue securities at a future determined price, subject to certain exceptions including following 38 days after the closing of this offering, an “at the market” offering shall be permitted.
Roth may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, Wainwright would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of common shares by Roth acting as principal. Under these rules and regulations, Roth:
|
●
|
may not engage in any stabilization activity in connection with our securities; and
|
|
●
|
may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
|
The securities purchase agreement is included as an exhibit to a Current Report on Form 8-K that we have filed with the SEC and that is incorporated by reference into the registration statement of which this prospectus supplement forms a part.
The transfer agent for our shares of common stock is American Stock Transfer & Trust Company.
Our common stock is listed on the Nasdaq Capital Market under the symbol “GOVX.”
Other Relationships
The placement agent may, from time to time, engage in transactions with or perform services for us in the ordinary course of its business and may continue to receive compensation from us for such services.
LEGAL MATTERS
The validity of the issuance of the shares of common stock covered by this prospectus will be passed upon for us by Womble Bond Dickinson (US) LLP.
EXPERTS
Our consolidated financial statements as of and for the years ended December 31, 2023 and December 31, 2022 incorporated by reference in this prospectus and elsewhere in the registration statement have been audited by Wipfli LLP, an independent registered public accounting firm, as indicated in their report with respect thereto, and have been so incorporated by reference in reliance upon the authority of said firm as experts in auditing and accounting in giving said report.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No named expert or counsel was hired on a contingent basis, will receive a direct or indirect interest in the issuer, or was a promoter, underwriter, voting trustee, director, officer, or employee of GeoVax.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the registration statement and any document we file with the SEC. The SEC maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The address of the SEC’s website is www.sec.gov. The information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive textual references only.
This prospectus supplement is part of a registration statement on Form S-3 that we filed with the SEC to register the securities to be offered hereby. This prospectus supplement does not contain all of the information included in the registration statement, including certain exhibits and schedules. In addition to the foregoing, we maintain a website at www.geovax.com. Our website content is made available for informational purposes only. It should neither be relied upon for investment purposes nor is it incorporated by reference into this prospectus supplement. We make available at www.geovax.com copies of our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and any amendments to such document as soon as practicable after we electronically file such material with or furnish such documents to the SEC.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus supplement. Information that is incorporated by reference is considered to be part of this prospectus supplement and you should read it with the same care that you read this prospectus supplement. Later information that we file with the SEC will automatically update and supersede the information that is either contained, or incorporated by reference, in this prospectus supplement and the base prospectus and will be considered to be a part of this prospectus supplement and the base prospectus from the date those documents are filed. We have filed with the SEC, and incorporate by reference the following in this prospectus supplement and the base prospectus:
| |
●
|
our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2024 and June 30, 2024, filed on May 14, 2024 and August 6, 2024, respectively;
|
| |
●
|
our Current Reports on Form 8-K filed on January 31, 2024, February 16, 2024, May 21, 2024, May 23, 2024, May 24, 2024, June 18, 2024, June 21, 2024 and July 12, 2024; and
|
| |
●
|
our Preliminary Proxy Statement on Schedule 14A as filed with the SEC on April 3, 2024 and our Definitive Proxy Statement on Schedule 14A, as filed with the SEC on April 15, 2024;
|
| |
●
|
the description of our common stock which is registered under Section 12 of the Exchange Act, in our registration statement on S-1, filed with the SEC on July 20, 2020, including any amendment or reports filed for the purposes of updating this description.
|
In addition, all documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities and Exchange Act of 1934, as amended, after the date of the initial registration statement of which this prospectus supplement is a part as well as all such documents that we file with the SEC after the date of this prospectus supplement and before the termination of the offering of our securities shall be deemed incorporated by reference into this prospectus supplement and to be a part of this prospectus supplement from the respective dates of filing such documents. Unless specifically stated to the contrary, none of the information that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K that we may from time to time furnish to the SEC will be incorporated by reference into, or otherwise included in, this prospectus supplement.
You may request a copy of any or all of the documents incorporated by reference but not delivered with this prospectus supplement, at no cost, by writing or telephoning us at the following address and number: GeoVax Labs, Inc., 1900 Lake Park Drive, Suite 380, Smyrna, Georgia 30080, telephone (678) 384-7220. We will not, however, send exhibits to those documents, unless the exhibits are specifically incorporated by reference in those documents.
PROSPECTUS

GEOVAX LABS, INC.
$100,000,000
Common Stock
Preferred Stock
Warrants
Units
We may, from time to time, offer and sell common stock, preferred stock, or warrants, either separately or in units, in one or more offerings. The preferred stock and warrants may be convertible into or exercisable or exchangeable for common or preferred stock. Selling stockholders may sell common stock. We will specify in the accompanying prospectus supplement more specific information about any such offering. The aggregate initial offering price of all securities sold under this prospectus will not exceed $100,000,000, including the U.S. dollar equivalent if the public offering of any such securities is denominated in one or more foreign currencies, foreign currency units or composite currencies.
We and any selling stockholders may offer these securities independently or together in any combination for sale directly to investors or through underwriters, dealers or agents. We will set forth the names of any underwriters, dealers or agents and their compensation in the accompanying prospectus supplement.
This prospectus may not be used to sell any of these securities unless accompanied by a prospectus supplement.
Our common stock and warrants previously issued by the Company to investors in its September 2020 public offering (the “2020 Warrants”) are presently traded on the Nasdaq Capital Market under the symbols “GOVX” and “GOVXW,” respectively. On February 23, 2024, the last reported sale price of our common stock on the Nasdaq Capital Market was $2.172 per share and the last reported sale price of 2020 Warrants was $0.028 per warrant. As of February 29, 2024, the aggregate market value of our outstanding common stock held by non-affiliates was approximately $11,980,621, which was calculated based on 2,135,583 shares of outstanding common stock held by non-affiliates, at a price per share of $5.61, the closing price of our common stock on January 3, 2024, the highest closing price of the Company's common stock on the Nasdaq Capital Market during the preceding sixty (60) day trading period. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell the securities described in this prospectus in a public primary offering with a value exceeding more than one-third (1/3) of the aggregate market value of our common stock held by non-affiliates in any twelve (12)-month period, so long as the aggregate market value of our outstanding common stock held by non-affiliates remains below $75,000,000. During the twelve (12) calendar months prior to and including the date of this prospectus, we have not offered or sold any securities pursuant to General Instruction I.B.6 of Form S-3.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 6 and other information included or incorporated by reference in this prospectus and any prospectus supplement for a discussion of factors you should carefully consider before you make your investment decision.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Subject to completion, the date of this prospectus is , 2024.
TABLE OF CONTENTS
Page
|
ABOUT THIS PROSPECTUS
|
1
|
|
CAUTIONARY STATEMENT CONCERNING FORWARD LOOKING STATEMENTS
|
1
|
|
PROSPECTUS SUMMARY
|
3
|
|
RISK FACTORS
|
7
|
|
USE OF PROCEEDS
|
7
|
|
DESCRIPTION OF SECURITIES
|
7
|
|
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
11
|
|
THE SECURITIES WE MAY OFFER
|
11
|
|
DILUTION
|
13
|
|
PLAN OF DISTRIBUTION
|
13
|
|
LEGAL MATTERS
|
14
|
|
EXPERTS
|
14
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
14
|
|
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
|
15
|
You should rely only on the information incorporated by reference or provided in this prospectus, any prospectus supplement and the registration statement. We have not authorized anyone else to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any state where the offer or sale is not permitted. You should assume that the information in this prospectus and any prospectus supplement, or incorporated by reference, is accurate only as of the dates of those documents. Our business, financial condition, results of operations and prospects may have changed since those dates.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration, or continuous offering, process. Under this shelf registration process, we may, from time to time, issue and sell any combination of preferred stock, common stock or warrants, either separately or in units, in one or more offerings with a maximum aggregate offering price of $100,000,000, including the U.S. dollar equivalent if the public offering of any such securities is denominated in one or more foreign currencies, foreign currency units or composite currencies. Common stock may also be sold by selling stockholders.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement that will contain specific information about the terms of that offering and the offered securities. Any prospectus supplement may also add, update or change information contained in this prospectus. Any statement that we make in this prospectus will be modified or superseded by any inconsistent statement made by us in a prospectus supplement. The registration statement we filed with the SEC includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC and any prospectus supplement, together with additional information described under the heading “Where You Can Find More Information,” before making your investment decision.
THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SECURITIES UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
Neither we, nor any agent, underwriter or dealer has authorized any person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus, any applicable prospectus supplement prepared by or on behalf of us or to which we have referred you. This prospectus or any applicable supplement to this prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus or any applicable supplement to this prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You should not assume that the information contained in this prospectus or any applicable prospectus supplement is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus or any applicable prospectus supplement is delivered, or securities are sold, on a later date.
CAUTIONARY STATEMENT CONCERNING FORWARD LOOKING STATEMENTS
Some of the statements in this prospectus and in the documents incorporated herein by reference contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond our ability to control or predict and that may cause actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these identifying words. Our forward-looking statements may include, among other things, statements about:
| |
●
|
our ability to continue as a going concern and our history of losses;
|
| |
●
|
our ability to obtain additional financing;
|
| |
●
|
our use of the net proceeds from this offering;
|
| |
●
|
our ability to prosecute, maintain or enforce our intellectual property rights;
|
| |
●
|
the accuracy of our estimates regarding expenses, future revenues and capital requirements;
|
| |
●
|
the implementation of our business model and strategic plans for our business and technology;
|
| |
●
|
the successful development and regulatory approval of our technologies and products;
|
| |
●
|
the potential markets for our products and our ability to serve those markets;
|
| |
●
|
the rate and degree of market acceptance of our products and any future products;
|
| |
●
|
our ability to retain key management personnel; and
|
| |
●
|
regulatory developments and our compliance with applicable laws.
|
Forward-looking statements are inherently subject to risks and uncertainties, many of which we cannot predict with accuracy and some of which we might not even anticipate. Although we believe that the expectations reflected in such forward-looking statements are based upon reasonable assumptions at the time made, we can give no assurance that such expectations will be achieved. Actual events or results may differ materially. Readers are cautioned not to place undue reliance on forward-looking statements. We have no duty to update or revise any forward-looking statements after the date of this prospectus or to conform them to actual results, new information, future events or otherwise.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of these forward-looking statements.
You should read the risk factors and the other cautionary statements made in this prospectus as being applicable to all related forward-looking statements wherever they appear in this prospectus. If one or more of these factors materialize, or if any underlying assumptions prove incorrect, our actual results, performance or achievements may vary materially from any future results, performance or achievements expressed or implied by these forward-looking statements. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus or incorporated by reference. This summary does not contain all of the information you should consider before buying shares of our common stock, preferred stock, warrants, or units or any combination of these securities. You should read the entire prospectus carefully, especially the risks of investing in our securities that we describe under “Risk Factors” and our consolidated financial statements appearing in our annual and periodic reports incorporated in this prospectus by reference, before deciding to invest in our securities. References in this prospectus to “we,” “us,” “our,” “GeoVax,” and “Company” refer to GeoVax Labs, Inc. and its subsidiaries. You should read both this prospectus and any prospectus supplement together with additional information described below under the heading “Where You Can Find More Information.”
Overview
GeoVax Labs, Inc. (“GeoVax” or “the Company”) is a clinical-stage biotechnology company developing human vaccines and immunotherapies against infectious diseases and solid tumor cancers using novel proprietary platforms. GeoVax’s product pipeline includes ongoing human clinical trials for a next-generation Covid-19 vaccine and a gene-directed therapy against advanced head and neck cancers. Additional research and development programs include preventive vaccines against Mpox (formerly known as monkeypox) and smallpox, hemorrhagic fever viruses (Ebola Zaire, Ebola Sudan and Marburg), Zika virus and malaria, as well as immunotherapies for multiple solid tumors. The Company’s portfolio of wholly owned, co-owned, and in-licensed intellectual property, stands at over 155 granted or pending patent applications spread over 24 patent families.
Our Product Development Pipeline
The table below summarizes the status of our product development programs as of the date of this prospectus.
|
Indication
|
Current Status
|
|
Coronavirus Vaccines
|
|
|
COVID-19 (Booster to mRNA vaccines)
|
Clinical – Phase 2
|
|
COVID-19 (Primary vaccine for immunocompromised patients)
|
Clinical – Phase 2
|
|
COVID-19 (Booster for Chronic Lymphocytic Leukemia patients)
|
Clinical – Phase 2 (Investigator Initiated Trial)
|
|
Cancer Therapy
|
|
|
Solid Tumors (Advanced Head and Neck Cancer)*
|
Clinical – Phase 1/2
|
|
Solid Tumors (MUC1)
|
Preclinical/IND-Enabling
|
|
Other Infectious Disease Vaccines
|
|
|
Mpox & smallpox
|
Preclinical/IND-Enabling
|
|
Hemorrhagic Fever Viruses
|
Preclinical/IND-Enabling
|
|
Ebola Zaire**
|
|
|
Ebola Sudan**
|
|
|
Marburg**
|
|
|
Zika**
|
Preclinical/IND-Enabling
|
|
Malaria**
|
Exploratory
|
* Orphan Drug status granted
** Indication within FDA Priority Review Voucher program
Our programs are in various stages of development, the most significant of which are summarized below along with recent developments:
GEO-CM04S1 – Immunocompromised Trial
● GEO-CM04S1 is currently undergoing a Phase 2 clinical trial (ClinicalTrials.gov Identifier: NCT04977024), evaluating its safety and efficacy, compared to either the Pfizer/BioNTech or Moderna mRNA-based vaccine, as a preventive COVID-19 vaccine in high-risk immunocompromised patients (e.g. patients who have previously received either an allogeneic hematopoietic cell transplant, an autologous hematopoietic cell transplant or chimeric antigen receptor (CAR) T cell therapy).
● In September 2023, the journal, Vaccines, published data from the open-label safety portion of the trial indicating that GEO-CM04S1 is highly immunogenic, inducing both antibody responses, including neutralizing antibodies, and T cell responses.
● In September 2023, preclinical vaccine efficacy data for GEO-CM02 were presented during the Keystone Symposia on Molecular and Cellular Biology, Vaccinology During and After COVID-19, demonstrating that our multi-antigen SARS-CoV-2 vaccine, GEO-CM02, induced efficacious immune responses against the original Wuhan strain and BA.1 Omicron variant with a single dose. The data generated in the GEO-CM02 studies validate our hypothesis that vaccines such as GEO-CM04S1, which are designed to induce both antibodies and T-cells to multiple viral structural proteins, can address the issue of viral variation and escape from the immune system.
● In October 2023, we announced commencement of the planned site expansion for this trial to accelerate patient enrollment. In addition to study enrollments completed at the City of Hope Medical Center (Duarte, California), the trial is now open to eligible patients at Wake Forest Baptist Medical Center (Winston Salem, North Carolina), the University of Massachusetts Medical Center (Worcester, Massachusetts), and the Fred Hutchinson Cancer Center (Seattle, Washington).
GEO-CM04S1 – Healthy Booster Trial
● GEO-CM04S1 is undergoing the Phase 2 portion of a Phase 1/2 trial (ClinicalTrials.gov Identifier: NCT04639466), evaluating its use as a universal COVID-19 booster vaccine to current FDA-approved two-shot mRNA vaccines from Pfizer/BioNTech and Moderna.
● In September 2023, we announced the completion of patient enrollment for this trial.
GEO-CM04S1 – CLL Trial
● In July 2023, an investigator-initiated Phase 2 clinical trial (ClinicalTrials.gov Identifier: NCT05672355) of GEO-CM04S1 began, evaluating its use as a COVID-19 booster vaccine in patients with chronic lymphocytic leukemia (CLL), compared to the Pfizer/BioNTech mRNA-based vaccine.
Gedeptin® – Advanced Head and Neck Cancer Trial
● Gedeptin® is currently undergoing a Phase 1/2 clinical trial (ClinicalTrials.gov Identifier: NCT03754933) for treatment of patients with advanced head and neck squamous cell carcinoma (HNSCC). This trial is being funded in part by the U.S. Food & Drug Administration (FDA) pursuant to its Orphan Products Clinical Trials Grants Program. The trial is designed to inform the design of a larger patient trial that also may involve patients with other anatomically accessible oral and pharyngeal cancers, including cancers of the lip, tongue, gum, floor of mouth, salivary gland and other oral cavities.
● In July 2023, interim data were presented at the American Association for Cancer Research (AACR) and the American Head and Neck Society (AHNS) joint Head and Neck Cancer Conference, indicating that administration of Gedeptin® is safe and feasible, with observation of tumor growth impairment in a majority of the patients.
Advanced Vaccine Manufacturing Process Development
● In September 2023, GeoVax and ProBioGen AG announced the signing of a commercial license agreement for ProBioGen’s AGE1.CR.pIX® suspension cell line. The agreement enhances our manufacturing capabilities for our entire Modified Vaccinia Ankara (MVA)-based vaccine portfolio. This follows the May 2023 execution of a Master Services Agreement with Advanced Bioscience Laboratories, Inc. (ABL) to support current Good Manufacturing Practices (cGMP) production of our vaccine candidates through late-stage development toward eventual commercialization. These agreements move the Company toward implementing a continuous cell line manufacturing system to provide lower-cost, scalable versatility for our entire MVA-based vaccine portfolio.
Intellectual Property Development
● In October 2023, the U.S. Patent and Trademark Office issued a Notice of Allowance to GeoVax for Patent Application No. 17/584,231 titled “Replication Deficient Modified Vaccina Ankara (MVA) Expressing Marburg Virus Glycoprotein (GP) and Matrix Protein (VP40).” The allowed claims generally cover GeoVax’s vector platform for expressing Marburg virus antigens in virus-like particles (VLPs) utilizing an MVA viral vector.
● In October 2023, the U.S Patent and Trademark Office issued a Notice of Allowance to GeoVax for Patent Application No. 17/409,574 titled “Multivalent HIV Vaccine Boost Compositions and Methods of Use.” The allowed claims generally cover a priming vaccination with a DNA vector encoding multiple HIV antigens in virus-like particles (VLPs), followed by a boost vaccination with GeoVax’s vector platform for expressing HIV-1 antigens in VLPs utilizing an MVA viral vector.
● In August 2023, the U.S. Patent and Trademark Office issued a Notice of Allowance to GeoVax for Patent Application No. 17/726,254 titled “Compositions and Methods for Generating an Immune Response to Treat or Prevent Malaria”. The allowed claims cover compositions comprising GeoVax’s modified vaccinia Ankara (MVA) vector expressing Plasmodium antigens and methods of inducing an immune response to malaria utilizing the compositions. The compositions and methods covered in the allowed claims are useful both prophylactically and therapeutically and may be used to prevent and/or treat malaria.
● In July 2023, the U.S. Patent and Trademark Office issued Patent No. 11,701,418 B2 to GeoVax, pursuant to the Company’s patent application No. 15/543,139 titled “Replication-Deficient Modified Vaccinia Ankara (MVA) and Matrix Protein (VP40), covering GeoVax’s vector platform for expressing ebolavirus antigens in virus-like particles (VLPs) utilizing an MVA viral vector. The claims encompass multiple ebolavirus strains, including Sudan ebolavirus, Zaire ebolavirus, Taï Forest ebolavirus, and Reston ebolavirus.
December 2023 Private Placement
On December 2, 2023, we entered into a common stock warrant exercise inducement offer letter (the “Inducement Letter”) with the holder (the “Holder”) of existing warrants to purchase shares of the Company’s common stock at an exercise price of $48.90 per share, issued on January 19, 2022 and warrants to purchase shares of the Company’s common stock at an exercise price of $24.75 per share issued on May 27, 2022 (together, the “Existing Warrants”), pursuant to which the Holder agreed to exercise for cash its Existing Warrants to purchase an aggregate of 704,499 shares of the Company’s common stock, at a reduced exercised price of $6.21 per share, in consideration for the Company’s agreement to issue a warrant (the “2023 Common Warrant”) to purchase up to 1,408,998 common shares with an exercise price of $6.21 share, exercisable at any on or after six months from the date of issuance and will expire five and one-half (5 ½) years following the date of issuance.
The Securities That May Be Offered
We may offer or sell common stock, preferred stock, warrants and units in one or more offerings and in any combination. The aggregate offering price of the securities we sell pursuant to this prospectus will not exceed $100,000,000. Each time securities are offered with this prospectus, we will provide a prospectus supplement that will describe the specific amounts, prices and terms of the securities being offered and the net proceeds we expect to receive from that sale.
The securities may be sold to or through underwriters, dealers or agents or directly to purchasers or as otherwise set forth in the section titled “Plan of Distribution.” Each prospectus supplement will set forth the names of any underwriters, dealers, agents or other entities involved in the sale of securities described in that prospectus supplement and any applicable fee, commission or discount arrangements with them.
Common Stock
We may offer shares of our common stock, par value $0.001 per share, either alone or underlying other registered securities convertible into our common stock. Holders of our common stock are entitled to receive dividends declared by our board of directors out of funds legally available for the payment of dividends, subject to rights, if any, of preferred stockholders. We have not paid dividends in the past and have no current plans to pay dividends. Each holder of common stock is entitled to one vote per share. The holders of common stock have no preemptive rights.
Preferred Stock
Our board of directors has the authority, subject to limitations prescribed by Delaware law, to issue preferred stock in one or more series, to establish from time to time the number of shares to be included in each series, and to fix the designation, powers, preferences and rights of the shares of each series and any of its qualifications, limitations or restrictions, in each case without further vote or action by our stockholders. Each series of preferred stock offered by us will be more fully described in the particular prospectus supplement that will accompany this prospectus, including redemption provisions, rights in the event of our liquidation, dissolution or winding up, voting rights and rights to convert into common stock.
Warrants
We may offer warrants for the purchase of common stock or preferred stock. We may offer warrants independently or together with other securities.
Units
We may offer units comprised of one or more of the other classes of securities described in this prospectus in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit.
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully review the risks and uncertainties described under the heading “Risk Factors” contained in the applicable prospectus supplement, and under similar headings in our Annual Report on Form 10-K for the year ended December 31, 2023, as updated by our annual, quarterly and other reports and documents that are incorporated by reference into this prospectus, before deciding whether to purchase any of the securities being registered pursuant to the registration statement of which this prospectus is a part. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities, and the occurrence of any of these risks might cause you to lose all or part of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations.
USE OF PROCEEDS
Unless we state otherwise in the accompanying prospectus supplement, we intend to use the net proceeds from the sale of the securities offered by this prospectus for general corporate purposes. General corporate purposes may include additions to working capital, research and development, financing of capital expenditures, and future acquisitions, collaborations, and strategic investment opportunities. Pending the application of net proceeds, we expect to invest the net proceeds in interest-bearing securities.
We will not receive any of the proceeds in the event the selling stockholders sell common stock.
DESCRIPTION OF SECURITIES
Capital Stock
The following description of our capital stock is summarized from, and qualified in its entirety by reference to, our certificate of incorporation, as amended, including the certificates of designation, as amended, setting forth the terms of our Preferred Stock. This summary is not intended to give full effect to provisions of statutory or common law. We urge you to review the following documents because they, and not this summary, define the rights of a holder of shares of common stock and Preferred Stock:
| |
•
|
the General Corporation Law of the State of Delaware, or the “DGCL”, as it may be amended from time to time;
|
| |
•
|
our certificate of incorporation, as it may be amended or restated from time to time; and
|
| |
•
|
our bylaws, as they may be amended or restated from time to time.
|
General
As of the date of this prospectus, our authorized capital stock currently consists of 160,000,000 shares, which are divided into two classes consisting of 150,000,000 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.01 per share.
As of February 29, 2024, there were 2,172,272 shares of common stock outstanding and no shares of preferred stock outstanding. As of February 29, 2024, there are outstanding warrants to purchase 1,970,781 shares of common stock with a weighted average exercise price of $11.81 per share. An additional 334,609 shares of common stock are reserved for issuance under our 2020 and 2023 Stock Incentive Plans, of which 134,609 shares of common stock are issuable upon exercise of outstanding options at an average exercise price of $28.41 per share.
Common Stock
Our common stock is listed and traded on the Nasdaq Capital Market under the symbol “GOVX.” Holders of our common stock are entitled to one vote for each share held in the election of directors and in all other matters to be voted on by the stockholders. There is no cumulative voting in the election of directors. Holders of common stock are entitled to receive dividends as may be declared from time to time by our Board of Directors out of funds legally available therefor. In the event of liquidation, dissolution or winding up of the Company, holders of common stock are to share in all assets remaining after the payment of liabilities. Holders of common stock have no pre-emptive or conversion rights and are not subject to further calls or assessments. There are no redemption or sinking fund provisions applicable to the common stock. The rights of the holders of the common stock are subject to any rights that may be fixed in the future for holders of preferred stock. All of the outstanding shares of common stock are fully paid and non-assessable.
Undesignated Preferred Stock
Our Board of Directors has the authority to issue up to 10,000,000 shares of preferred stock in one or more series and fix the number of shares constituting any such series, the voting powers, designations, preferences and relative, participating, optional or other special rights and qualifications, limitations or restrictions thereof, including the dividend rights, dividend rate, terms of redemption (including sinking fund provisions), redemption price or prices, conversion rights and liquidation preferences of the shares constituting any series, without any further vote or action by the stockholders. For example, the Board of Directors is authorized to issue preferred stock that would have the right to vote, separately or with any other stockholder of preferred stock, on any proposed amendment to our certificate of incorporation, or on any other proposed corporate action, including business combinations and other transactions. We will not offer preferred stock unless the offering is approved by a majority of our independent directors. The independent directors will have access, at our expense, to our counsel or independent counsel.
The 2020 Warrants
Overview. The 2020 Warrants are listed and traded on the Nasdaq Capital Market under the symbol “GOVXW.” The 2020 Warrants were issued in September 2020 pursuant to a Warrant Agent Agreement dated as of September 24, 2020 (the “Warrant Agent Agreement”), between us and American Stock Transfer & Trust Company, LLC, as the warrant agent (the “Warrant Agent”) as part of our September 2020 public offering. Certain provisions of the 2020 Warrants are set forth herein but are only a summary and are qualified in their entirety by the relevant provisions of the Warrant Agent Agreement which is filed as an exhibit to the registration statement of which this prospectus is a part.
The 2020 Warrants entitle the registered holder to purchase one share of our common stock at a price equal to $75.00 per share subject to adjustment as discussed below, immediately following the issuance of such warrant and terminating at 5:00 p.m., New York City time, five years from the date of issuance, or September 29, 2025.
Exercisability. The 2020 Warrants are exercisable at any time after their original issuance and at any time up to the date that is five (5) years after their original issuance. The 2020 Warrants may be exercised by delivering a duly executed exercise notice on or prior to the expiration date at the offices of the Warrant Agent, accompanied by full payment of the exercise price, by certified or official bank check payable to the Warrant Agent, for the number of warrants being exercised. Under the terms of the Warrant Agreement, we must use our best efforts to maintain the effectiveness of the related registration statement and current prospectus relating to common stock issuable upon exercise of the 2020 Warrants until the expiration of the warrants. If we fail to maintain the effectiveness of the registration statement and current released prospectus relating to the common stock issuable upon exercise of the 2020 Warrants, the holders shall have the right to exercise them solely via a cashless exercise feature provided for in the 2020 Warrants, until such time as there is an effective registration statement and current prospectus.
Exercise Limitation. A holder may not exercise any portion of a September Warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of the outstanding common stock after exercise, as such percentage ownership is determined in accordance with the terms of the warrant, except that upon at least 61 days’ prior notice from the holder to us, the holder may waive such limitation up to a percentage not in excess of 9.99%.
Exercise Price. The exercise price per whole share of common stock purchasable upon exercise of the 2020 Warrants is $75.00 per share. The exercise price is subject to adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders. However, the 2020 Warrants will not be adjusted for issuances of common stock at prices below the exercise price.
Fractional Shares. No fractional shares of common stock will be issued upon exercise of the 2020 Warrants. If, upon exercise of the September Warrant, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such fraction in an amount equal to such fraction multiplied by the exercise price. If multiple 2020 Warrants are exercised by the holder at the same time, we shall pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Transferability. Subject to applicable laws, the 2020 Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. Our 2020 Warrants are listed on the Nasdaq Capital Market under the symbol “GOVXW.”
Warrant Agent Agreement. The 2020 Warrants are represented only by one or more global warrants deposited with the Warrant Agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC. The Warrant Agent is Equiniti Trust Company, LLC, 48 Wall Street, New York, NY 10005, telephone (800) 468-9716.
Fundamental Transactions. In the event of a fundamental transaction, as described in the 2020 Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, upon any subsequent exercise of the 2020 Warrants, the holders will be entitled to receive the kind and amount of securities, cash or other property that the holders would have received had they exercised the 2020 Warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. The holders of 2020 Warrants do not have the rights or privileges of holders of common stock or any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the 2020 Warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Governing Law. The 2020 Warrants and the Warrant Agent Agreement are governed by New York law.
Delaware Anti-Takeover Law
We have elected not to be subject to certain provisions of Delaware law that could make it more difficult to acquire us by means of a tender offer, a proxy contest, open market purchases, removal of incumbent directors and otherwise. These provisions, summarized below, are expected to discourage types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to first negotiate with our Board of Directors.
In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation from engaging in various “business combination” transactions with any interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
| |
●
|
the transaction is approved by the corporation’s board of directors prior to the date the interested stockholder obtained interested stockholder status;
|
| |
●
|
upon consummation of the transaction that resulted in the stockholder’s becoming an interested stockholder, the stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding those shares owned by (a) persons who are directors and also officers and (b) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or;
|
| |
●
|
on or subsequent to the date the business combination is approved by the corporation’s board of directors and authorized at an annual or special meeting of stockholders by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder.
|
A “business combination” is defined to include mergers, asset sales and other transactions resulting in financial benefit to a stockholder. In general, an “interested stockholder” is a person who, together with affiliates and associates, owns or within three years, did own, 15% or more of a corporation’s voting stock.
Section 203 applies to Delaware corporations that have a class of voting stock that is listed on a national securities exchange or held of record by more than 2,000 stockholders; provided, however, the restrictions of this statute will not apply to a corporation if:
| |
●
|
the corporation’s original charter contains a provision expressly electing not to be governed by the statute;
|
| |
●
|
the corporation’s board of directors adopts an amendment to the corporation’s bylaws within 90 days of the effective date of the statute expressly electing not to be governed by it;
|
| |
●
|
the stockholders of the corporation adopt an amendment to its charter or bylaws expressly electing not to be governed by the statute (so long as such amendment is approved by the affirmative vote of a majority of the shares entitled to vote);
|
| |
●
|
a stockholder becomes an interested stockholder inadvertently and as soon as practicable divests himself of ownership of a sufficient number of shares so that he ceases to be an interested stockholder, and during the three-year period immediately prior to a business combination, would not have been an interested stockholder but for the inadvertent acquisition;
|
| |
●
|
the business combination is proposed prior to the consummation or abandonment of a merger or consolidation, a sale, lease, exchange, mortgage, pledge, transfer or other disposition of assets of the corporation or a proposed tender or exchange offer for 50% or more of the outstanding voting shares of the corporation; or
|
| |
●
|
the business combination is with an interested stockholder who became an interested stockholder at a time when the restrictions contained in the statutes did not apply.
|
Our certificate of incorporation includes a provision electing not to be governed by Section 203 of the DCGL. Accordingly, our board of directors does not have the power to reject certain business combinations with interested stockholders based on Section 203 of the DCGL.
Transfer Agent, Warrant Agent and Registrar
The transfer agent, warrant agent and registrar for our common stock and 2020 Warrants is Equiniti Trust Company, LLC, 48 Wall Street, New York, NY 10005, telephone (800) 468-9716.
Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “GOVX.”
DISCLOSURE OF COMMISSION POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
THE SECURITIES WE MAY OFFER
Common Stock
The Common Stock we may offer is described above in “Description of Securities-Capital Stock - Common Stock”.
Preferred Stock
This section describes the general terms of our preferred stock to which any prospectus supplement may relate. A prospectus supplement will describe the terms relating to any preferred stock to be offered by us in greater detail and may provide information that is different from terms described in this prospectus. A copy of our certificate of incorporation, as amended, and of our bylaws, are exhibits to the registration statement of which this prospectus forms a part. See “Description of Securities - Capital Stock - Undesignated Preferred Stocks” for additional information. A certificate of designation or amendment to our certificate of incorporation, as amended, will specify the terms of the preferred stock being offered, and will be filed or incorporated by reference as an exhibit to the registration statement before the preferred stock is issued. The following description of our preferred stock, and any description of the preferred stock in a prospectus supplement may not be complete and is subject to, and qualified in its entirety by reference to, Delaware law and the actual terms and provisions contained in our certificate of incorporation and our bylaws, each as amended from time to time.
Under our certificate of incorporation, as amended, our board of directors is authorized, without action by the stockholders, to issue preferred stock from time to time with such dividend, liquidation, conversion, voting, redemption, sinking fund and other rights and restrictions as it may determine. All shares of any one series of our preferred stock will be identical, except that shares of any one series issued at different times may differ as to the dates from which dividends may be cumulative, as described in the applicable prospectus supplement.
Unless provided in a prospectus supplement, the shares of our preferred stock to be issued will have no preemptive rights. Any prospectus supplement offering our preferred stock will furnish the following information with respect to the preferred stock offered by that prospectus supplement:
| |
●
|
the distinctive designation of each series and the number of shares that will constitute the series;
|
| |
●
|
the voting rights, if any, of shares of the series and the terms and conditions of the voting rights;
|
| |
●
|
the dividend rate, if any, on the shares of the series, the dates on which dividends are payable, any restriction, limitation or condition upon the payment of dividends, whether dividends will be cumulative, and the dates from and after which dividends shall accumulate;
|
| |
●
|
the prices at which, and the terms and conditions on which, the shares of the series may be redeemed, if the shares are redeemable;
|
| |
●
|
the terms and conditions of a sinking or purchase fund for the purchase or redemption of shares of the series, if such a fund is provided;
|
| |
●
|
any preferential amount payable upon shares of the series in the event of the liquidation, dissolution or winding up of, or upon the distribution of any of our assets; and
|
| |
●
|
the prices or rates of conversion or exchange at which, and the terms and conditions on which, the shares of the series may be converted or exchanged into other securities, if the shares are convertible or exchangeable.
|
If our Board of Directors decides to issue any shares of preferred stock, that issuance may discourage or make more difficult a merger, tender offer, business combination or proxy contest, assumption of control by a holder of a large block of our securities, or the removal of incumbent management, even if these events were favorable to the interests of stockholders. Our Board of Directors, without stockholder approval, may issue preferred stock with voting and conversion rights and dividend and liquidation preferences that may adversely affect the holders of our other equity or debt securities.
The particular terms of any series of preferred stock, and the transfer agent and registrar for that series, will be described in a prospectus supplement. All preferred stock offered, when issued, will be fully paid and nonassessable. Any material United States federal income tax consequences and other special considerations with respect to any preferred stock offered under this prospectus will also be described in the applicable prospectus supplement.
Warrants
We may issue warrants for the purchase of preferred stock, common stock, or any combination thereof. We may issue warrants independently or together with any other securities offered by any prospectus supplement and may be attached to or separate from the other offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into by us with a warrant agent. The warrant agent will act solely as our agent in connection with the warrants and will not assume any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants. Further terms of the warrants and the applicable warrant agreements will be set forth in the applicable prospectus supplement.
The applicable prospectus supplement relating to any particular issue of warrants will describe the terms of the warrants, including, as applicable, the following:
| |
●
|
the title of the warrants;
|
| |
●
|
the aggregate number of the warrants;
|
| |
●
|
the price or prices at which the warrants will be issued;
|
| |
●
|
the designation, terms and number of shares of preferred stock or common stock purchasable upon exercise of the warrants;
|
| |
●
|
the designation and terms of the offered securities, if any, with which the warrants are issued and the number of the warrants issued with each offered security;
|
| |
●
|
the date, if any, on and after which the warrants and the related preferred stock or common stock will be separately transferable;
|
| |
●
|
the price at which each share of preferred stock or common stock purchasable upon exercise of the warrants may be purchased;
|
| |
●
|
the date on which the right to exercise the warrants shall commence and the date on which that right shall expire;
|
| |
●
|
the minimum or maximum amount of the warrants which may be exercised at any one time;
|
| |
●
|
information with respect to book-entry procedures, if any;
|
| |
●
|
a discussion of certain U.S. federal income tax considerations; and
|
| |
●
|
any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants.
|
We and the warrant agent may amend or supplement the warrant agreement for a series of warrants without the consent of the holders of the warrants issued thereunder to effect changes that are not inconsistent with the provisions of the warrants and that do not materially and adversely affect the interests of the holders of the warrants.
Units
The following description, together with the additional information we include in any applicable prospectus supplement, summarizes the material terms and provisions of the units that we may offer under this prospectus. Units may be offered independently or together with common or preferred stock, and warrants offered by any prospectus supplement, and may be attached to or separate from those securities. While the terms we have summarized below will generally apply to any future units that we may offer under this prospectus, we will describe the particular terms of any series of units that we may offer in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below.
We will incorporate by reference into the registration statement of which this prospectus forms a part the form of unit agreement, including a form of unit certificate, if any, that describes the terms of the series of units we are offering before the issuance of the related series of units. The following summaries of material provisions of the units and the unit agreements are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement applicable to a particular series of units. We urge you to read the applicable prospectus supplements related to the units that we sell under this prospectus, as well as the complete unit agreements that contain the terms of the units.
We may issue units consisting of one or more shares of common stock or preferred stock, warrants or any combination of such securities. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit.
Additionally, we will describe in the applicable prospectus supplement the terms of the series of units, including the following:
| |
●
|
the designation and terms of the units and the securities included in the units;
|
| |
●
|
any provision for the issuance, payment, settlement, transfer or exchange of the units;
|
| |
●
|
the date, if any, on and after which the securities included in the units may be transferable separately;
|
| |
●
|
whether we will apply to have the units traded on a securities exchange or securities quotation system;
|
| |
●
|
any material United States federal income tax consequences; and
|
| |
●
|
how, for United States federal income tax purposes, the purchase price paid for the units is to be allocated among the component securities.
|
DILUTION
We will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests of investors purchasing securities in an offering under this prospectus:
| |
●
|
the net tangible book value per share of our equity securities before and after the offering;
|
| |
●
|
the amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering; and
|
| |
●
|
the amount of the immediate dilution from the public offering price which will be absorbed by such purchasers.
|
PLAN OF DISTRIBUTION
We or any selling stockholder may sell the securities offered by this prospectus in the manner described below.
By the Company
We may sell the securities from time to time pursuant to underwritten public offerings, direct sales to the public, negotiated transactions, block trades or a combination of these methods. We may sell the securities to or through underwriters or dealers, through agents, directly to one or more purchasers, or through any combination of these methods. The distribution of the securities may be effected from time to time in one or more transactions at a fixed price or prices, which may be changed, at market prices prevailing at the time of sale, at prices related to the prevailing market prices or at negotiated prices.
A prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities, including, to the extent applicable:
| |
●
|
the name or names of any underwriters or dealers, if any;
|
| |
●
|
the purchase price of the securities and the proceeds we will receive from the sale;
|
| |
●
|
any over-allotment options under which underwriters may purchase additional securities from us;
|
| |
●
|
any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation;
|
| |
●
|
any public offering price;
|
| |
●
|
any discounts or concessions allowed or reallowed or paid to dealers; and
|
| |
●
|
any securities exchange or market on which the securities may be listed.
|
Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement. Underwriters, dealers and agents that participate in the distribution of the securities may be underwriters as defined in the applicable securities laws and any discounts or commissions they receive from us and any profit on their resale of the securities may be treated as underwriting discounts and commissions under the applicable securities laws. We will identify in the applicable prospectus supplement any underwriters, dealers or agents and will describe their compensation. We may have agreements with the underwriters, dealers and agents to indemnify them against specified civil liabilities, including liabilities under the applicable securities laws
By Underwriters. If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement. Any public offering price and any discounts or concessions allowed or reallowed may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
During and after an offering through underwriters, the underwriters may purchase and sell the securities in the open market. These transactions may include overallotment and stabilizing transactions and purchases to cover syndicate short positions created in connection with the offering. The underwriters may also impose a penalty bid, which means that selling concessions allowed to syndicate members or other broker-dealers for the offered securities sold for their account may be reclaimed by the syndicate if the offered securities are repurchased by the syndicate in stabilizing or covering transactions. These activities may stabilize, maintain or otherwise affect the market price of the offered securities, which may be higher than the price that might otherwise prevail in the open market. If commenced, the underwriters may discontinue these activities at any time
By Dealers. If a dealer is utilized in the sale of any securities offered by this prospectus, we will sell those securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. We will set forth the names of the dealers and the terms of the transaction in the applicable prospectus supplement.
LEGAL MATTERS
The validity of any securities offered by this prospectus will be passed upon for us by Womble Bond Dickinson (US) LLP.
EXPERTS
Our consolidated financial statements as of and for our year ended December 31, 2023 and 2022 incorporated by reference into this prospectus and elsewhere in the registration statement have been audited by Wipfli LLP, an independent registered public accounting firm, as indicated in their report with respect thereto, and are included herein in reliance upon the authority of said firm as experts in auditing and accounting in giving said report.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the registration statement and any document we file with the SEC. The SEC maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The address of the SEC’s website is www.sec.gov. The information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive textual references only.
This prospectus is part of a registration statement on Form S-3 that we filed with the SEC to register the securities to be offered hereby. This prospectus does not contain all of the information included in the registration statement, including certain exhibits and schedules. In addition to the foregoing, we maintain a website at www.geovax.com. Our website content is made available for informational purposes only. It should neither be relied upon for investment purposes nor is it incorporated by reference into this prospectus. We make available at www.geovax.com copies of our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and any amendments to such document as soon as practicable after we electronically file such material with or furnish such documents to the SEC.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the same care that you read this prospectus. Later information that we file with the SEC will automatically update and supersede the information that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from the date those documents are filed. We have filed with the SEC, and incorporate by reference the following in this prospectus:
| |
●
|
our Annual Report on Form 10-K for the year ended December 31, 2023, filed on February 29, 2024;
|
| |
●
|
our Current Reports on Form 8-K filed on January 16, 2024, January 31, 2024 and February 16, 2024;
|
| |
●
|
our Definitive Proxy Statements on Schedule 14A, as filed with the SEC on June 12, 2023 and November 27, 2023; and
|
| |
●
|
our Registration Statement on Form 8-A as filed with the SEC on September 24, 2020.
|
In addition, all documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities and Exchange Act of 1934, as amended, after the date of the initial registration statement of which this prospectus is a part and prior to the effectiveness of the registration statement as well as all such documents that we file with the SEC after the date of this prospectus and before the termination of the offering of our securities shall be deemed incorporated by reference into this prospectus and to be a part of this prospectus from the respective dates of filing such documents. Unless specifically stated to the contrary, none of the information that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K that we may from time to time furnish to the SEC will be incorporated by reference into, or otherwise included in, this prospectus.
You may request a copy of any or all of the documents incorporated by reference but not delivered with this prospectus, at no cost, by writing or telephoning us at the following address and number: GeoVax Labs, Inc., 1900 Lake Park Drive, Suite 380, Smyrna, Georgia 30080, telephone (678) 384-7220. We will not, however, send exhibits to those documents, unless the exhibits are specifically incorporated by reference in those documents.

1,360,731 Shares of Common Stock
339,269 Pre-Funded Warrants to Purchase up to 339,269 Shares of Common Stock
339,269 Shares of Common Stock underlying such Pre-Funded Warrants
PROSPECTUS SUPPLEMENT
Roth Capital Partners
August 20, 2024
GeoVax Labs (NASDAQ:GOVX)
Historical Stock Chart
From Oct 2024 to Nov 2024
GeoVax Labs (NASDAQ:GOVX)
Historical Stock Chart
From Nov 2023 to Nov 2024