As filed with the Securities and Exchange
Commission on September 29, 2023.
Registration Statement No. 333-274562
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Amendment No. 1
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Avenue Therapeutics, Inc.
(Exact name of registrant as specified in its
charter)
| Delaware |
|
2834 |
|
47-4113275 |
|
(State or Other Jurisdiction of
Incorporation or Organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification Number) |
1111 Kane Concourse, Suite 301
Bay Harbor Islands, Florida 33154
(781) 652-4500
(Address, including zip code, and telephone
number, including area code, of registrant’s principal executive offices)
Alexandra MacLean, M.D.
Chief Executive Officer
1111 Kane Concourse, Suite 301
Bay Harbor Islands, Florida 33154
(781) 652-4500
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
Copies to:
|
Rakesh Gopalan
David S. Wolpa
McGuireWoods LLP
201 N. Tryon Street, Suite 3000
Charlotte, North Carolina 28202 |
Barry I. Grossman
Sarah Williams
Matthew Bernstein
Ellenoff Grossman & Schole LLP
1345 Avenue of the Americas
New York, New York 10105 |
Approximate
date of commencement of proposed sale to the public: From time to time after this registration statement becomes effective.
If any of the securities being registered on
this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the
following box: x
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act,
please check the following box and list the Securities Act registration statement number of the earlier effective registration statement
for the same offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box
and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box
and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
¨ |
Accelerated filer |
¨ |
| Non-accelerated filer |
x |
Smaller reporting company |
x |
| |
|
Emerging growth company |
¨ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant hereby amends this registration
statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which
specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the
Securities Act or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting
pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not
complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange
Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these
securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED
SEPTEMBER 29, 2023
Preliminary Prospectus
Up to 21,352,313 Units, Each Consisting
of One Share of Common Stock and One Warrant to Purchase One Share of Common Stock
or
Up to 21,352,313 Pre-Funded Units, Each
Consisting of One Pre-funded Warrant to Purchase One Share of Common Stock and One Warrant to Purchase One Share of Common Stock
Up to 21,352,313 Shares of Common Stock
Underlying the Warrants
Up to 21,352,313 Shares of Common Stock
Underlying the Pre-funded Warrants

We are offering on a best efforts basis up to 21,352,313 units,
each consisting of one share of our common stock, par value $0.0001 per share (“Common Stock”), and one warrant to purchase
one share of our Common Stock, at an assumed offering price of $0.562 per unit (which is the last reported sale price of our Common Stock
on The Nasdaq Capital Market on September 25, 2023). Each warrant will have an exercise price of $ (100% of the public offering
price per unit) per share of Common Stock, will be exercisable immediately, and will expire five years from the date of issuance.
We are also offering to those purchasers, if any, whose purchase of
units in this offering would otherwise result in such purchaser, together with its affiliates and certain related parties, beneficially
owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of Common Stock immediately following the
consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded units in lieu of units that would
otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding
shares of Common Stock. Each pre-funded unit consists of one pre-funded warrant to purchase one share of Common Stock and one warrant
to purchase one share of Common Stock. The purchase price of each pre-funded unit will be equal to the price per unit being sold to the
public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant included in the pre-funded units will be $0.0001
per share. The pre-funded warrants included in the pre-funded units will be immediately exercisable (subject to the beneficial ownership
cap) and may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrant included in the pre-funded
unit is in the same form as the warrant included in the unit.
For each pre-funded unit we sell, the number of units including a share
of Common Stock we are offering will be decreased on a one-for-one basis. The units and the pre-funded units will not be issued or certificated.
The shares of Common Stock or pre-funded warrants and the accompanying warrants can only be purchased together in this offering, but the
securities contained in the units or pre-funded units will be immediately separable upon issuance and will be issued separately. The shares
of Common Stock issuable from time to time upon exercise of the warrants and the pre-funded warrants are also being offered by this prospectus.
The units will be offered at a fixed price and are expected to
be issued in a single closing. The offer will terminate on November 14, 2023, unless completed sooner or unless we decide to terminate
the offering (which we may do at any time in our discretion) prior to that date. We expect this offering to be completed not later
than two business days following the commencement of sales in this offering (after the effective date of the registration statement
of which this prospectus forms a part), and we will deliver all securities to be issued in connection with this offering delivery
versus payment/ receipt versus payment upon receipt by us of investor funds. Accordingly, neither we nor the placement agents (as
defined below) have made any arrangements to place investor funds in an escrow account or trust account since the placement agents
will not receive investor funds in connection with the sale of the securities offered hereunder.
We have engaged Maxim Group LLC and Lake Street Capital Markets,
LLC (the “placement agents”) to act as our placement agents in connection with this offering. The placement agents have agreed
to use their reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agents are not
purchasing or selling any of the securities we are offering, and the placement agents are not required to arrange the purchase or sale
of any specific number or dollar amount of securities. We have agreed to pay to the placement agents the placement agent fees set forth
in the table below, which assumes that we sell all of the securities offered by this prospectus. We will bear all costs associated with
the offering. See “Plan of Distribution” beginning on page 35 of this prospectus for more information regarding
these arrangements.
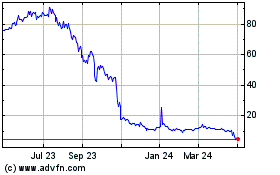
Our Common Stock is quoted for trading under the symbol “ATXI”
on The Nasdaq Capital Market. On September 25, 2023, the closing price of our Common Stock was $0.562 per share. The actual public offering
price per unit or pre-funded unit, as the case may be, in this offering will be determined between us, the placement agents and the investors
in this offering at the time of pricing, and may be at a discount to the current market price for our Common Stock. Therefore, the recent
market price used throughout this preliminary prospectus as an assumed per unit offering price may not be indicative of the final offering
price. There is no established public trading market for the warrants or the pre-funded warrants, and we do not expect such a market
to develop. In addition, we do not intend to apply for a listing of the warrants or the pre-funded warrants on any national securities
exchange or other nationally recognized trading system.
Investing in our securities involves risks. You should
review carefully the risks and uncertainties described under the heading “Risk Factors” contained in this
prospectus and under similar headings in the other documents that are incorporated by reference into this prospectus, as described
beginning on page 40 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities
commission has approved or disapproved of the securities to be issued under this prospectus or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
| | |
Per Unit | | |
Per Pre- Funded Unit | | |
Total | |
| Public Offering Price | |
$ | | | |
$ | | | |
$ | | |
| Placement agent fees(1) | |
$ | | | |
$ | | | |
$ | | |
| Proceeds, before expenses, to us(2) | |
$ | | | |
$ | | | |
$ | | |
| (1) | See “Plan of Distribution” for additional disclosure regarding compensation payable to the placement agents. |
| (2) | Because there is no minimum number of securities or amount of proceeds required as a condition to closing of this offering, the actual
public offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less
than the total maximum offering amounts set forth above. See “Plan of Distribution” for more information. |
We expect that delivery of the securities against payment therefor
will be made on or about , 2023.
Placement Agents
| Maxim
Group LLC |
Lake
Street |
The date of this
prospectus is , 2023
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of the registration statement that we filed
with the Securities and Exchange Commission, or the “SEC,” pursuant to which we may, from time to time, offer and sell or
otherwise dispose of the securities covered by this prospectus. As permitted by the rules and regulations of the SEC, the registration
statement filed by us includes additional information not contained in this prospectus.
This prospectus and the documents incorporated by reference into this
prospectus include important information about us, the securities being offered and other information you should know before investing
in our securities. You should not assume that the information contained in this prospectus is accurate on any date subsequent to the date
set forth on the front cover of this prospectus or that any information we have incorporated by reference is correct on any date subsequent
to the date of the document incorporated by reference, even though this prospectus is delivered or securities are sold or otherwise disposed
of on a later date. It is important for you to read and consider all information contained in this prospectus, including the documents
incorporated by reference therein, in making your investment decision. You should also read and consider the information in the documents
to which we have referred you under “Where You Can Find More Information” and “Incorporation of Certain Information
by Reference” in this prospectus.
You should rely only on this prospectus and the information incorporated
or deemed to be incorporated by reference in this prospectus. We have not authorized anyone to give any information or to make any representation
to you other than those contained or incorporated by reference in this prospectus. If anyone provides you with different or inconsistent
information, you should not rely on it. This prospectus does not constitute an offer to sell or the solicitation of an offer to buy securities
in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
Unless otherwise indicated, information contained or incorporated by
reference in this prospectus concerning our industry, including our general expectations and market opportunity, is based on information
from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted
by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions
based on such information and knowledge, which we believe to be reasonable. In addition, assumptions and estimates of our and our industry’s
future performance are necessarily uncertain due to a variety of factors, including those described in section of this prospectus titled
“Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions
and estimates.
We take no responsibility for, and can provide no assurance as to the
reliability of, any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby
and only under circumstances and in jurisdictions where it is lawful to do so. No dealer, salesperson or other person is authorized to
give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement or any free writing
prospectuses prepared by or on behalf of us or to which we have referred you or are incorporated by reference. This prospectus is not
an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale is not permitted.
For investors outside the United States: we have not done anything
that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is
required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves
about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus outside the United
States.
This prospectus contains summaries of certain provisions contained
in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries
are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed
or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain
copies of those documents as described in this prospectus under “Where You Can Find More Information.”
This prospectus contains references to trademarks, trade names and
service marks belonging to other entities. Solely for convenience, trademarks, trade names and service marks referred to in this prospectus
may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable licensor
will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use
or display of other entities’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship
of us by, any other entities.
PROSPECTUS SUMMARY
This summary highlights selected information from this prospectus
and does not contain all of the information that may be important to you in making an investment decision. This summary is qualified in
its entirety by the more detailed information included elsewhere in this prospectus and/or incorporated by reference herein. Before making
your investment decision with respect to our securities, you should carefully read this entire prospectus, including the information in
our filings with the SEC incorporated by reference into this prospectus.
References in this prospectus to the “Company,” “we,”
“us,” “our” and similar words refer to Avenue Therapeutics, Inc.
Our Business
Overview and Product Candidate Development
We
are a specialty pharmaceutical company focused on the development and commercialization of therapies for the treatment of neurologic
diseases. Our product candidates include AJ201 for the treatment of spinal and bulbar muscular atrophy (“SBMA”), intravenous
tramadol (“IV tramadol”) for the treatment of post-operative acute pain, and BAER-101 for the treatment of epilepsy and
panic disorders.
In November 2022, we completed the transactions
contemplated under a Share Contribution Agreement, dated May 11, 2022 (“Contribution Agreement”), with Fortress Biotech, Inc.
(“Fortress”) to acquire shares in Baergic Bio, Inc. (“Baergic”), which is developing BAER-101, a novel α2/3–subtype-selective
gammaaminobutyric acid (“GABA”) A positive allosteric modulator (“PAM”). As a result, Baergic is a majority-controlled
and owned subsidiary company of the Company.
In February 2023, we entered into a license
agreement with AnnJi Pharmaceuticals Co. Ltd. (“AnnJi”) whereby the Company obtained an exclusive license from AnnJi to intellectual
property rights pertaining to the molecule known as JM17, which activates Nrf1 and Nrf2, enhances androgen receptor degradation and underlies
AJ201, a clinical product candidate currently in a Phase 1b/2a clinical trial in the United States (“U.S.”) for the treatment
of SBMA, also known as Kennedy’s Disease.
AJ201
In February 2023, the Company licensed intellectual
property rights pertaining to the molecule known as JM17, which actives Nrf1 and Nrf2, enhances androgen receptor degradation and underlies
AJ201 from AnnJi. AJ201 is currently in a Phase 1b/2a clinical trial in the U.S. for the treatment of SMBA. SBMA is a rare, inherited,
X-linked genetic neuromuscular disease primarily affecting men. The condition is caused by a polyglutamine expansion in the androgen receptor
(“AR”) which leads to production of an abnormal AR protein that forms aggregates responsible for muscle atrophy focused in
the spinal-bulbar region of the body. The weakening of the bulbar muscles affects chewing, speech and swallowing, with patients prone
to choking or inhaling foods or liquids, resulting in airway infection. SBMA also affects muscles in the limbs, leading to difficulty
walking and injury caused by falling. Currently, there is no effective treatment for SBMA.
AJ201 was designed to modify SBMA through multiple
mechanisms including degradation of the abnormal AR protein and by stimulating Nrf1 and Nrf2, which are involved in protecting cells from
oxidative stress which can lead to cell death. AJ201 completed a Phase 1 clinical trial in 2021, which demonstrated the safety of the
molecule. It is currently being studied in a Phase 1b/2a multicenter, randomized, double-blind clinical trial in six clinical sites across
the U.S., and screening of patients with SBMA has begun. This study aims to evaluate the safety and clinical response of AJ201 in patients
suffering from SBMA. AJ201 has been granted Orphan Drug Designation by the U.S. Food and Drug Administration (“FDA”) for the
indications of SBMA, Huntington’s Disease and Spinocerebellar Ataxia.
In July 2023, we announced the first patient
was dosed in the Phase 1b/2a trial of AJ201 for the treatment of SBMA. The 12-week, multicenter, randomized, double-blind trial is expected
to enroll approximately 24 patients, randomly assigned to AJ201 (600mg/day) or placebo. The primary endpoint of the study is to assess
safety and tolerability of AJ201 in subjects with clinically and genetically defined SBMA. Secondary endpoints include pharmacodynamic
data measuring change from baseline in mutant androgen receptor protein levels in skeletal muscle and changes in the fat and muscle composition
as seen on MRI scans. Further details on the study can be found using the ClinicalTrials.gov identifier NCT05517603. Information on clinicaltrials.gov
does not constitute part of this Form S-1.
IV tramadol
In February 2022, we had our Advisory Committee
meeting with the FDA regarding IV tramadol. In the final part of the public meeting, the Advisory Committee voted yes or no on the following
question: “Has the Applicant submitted adequate information to support the position that the benefits of their product outweigh
the risks for the management of acute pain severe enough to require an opioid analgesic in an inpatient setting?” The results were
8 yes votes and 14 no votes. In March 2022, we received an Appeal Denied Letter from the OND in response to the FDRR. In August 2022,
the Company participated in a Type A Meeting with the FDA Division of Anesthesia, Analgesia, and Addiction Products (“DAAAP”)
regarding a briefing document submitted that presented a study design the Company believed would have the potential to address the comments
and deficiencies noted in the Letter. The meeting on August 9, 2022 was a collaborative discussion on the study design and potential
path forward. We incorporated the FDA’s suggestions from the meeting minutes and submitted a detailed study protocol.
The Company participated in a Type C meeting with
the FDA in March 2023 to discuss a proposed study protocol to assess the risk of respiratory depression related to opioid stacking
on IV tramadol relative to an approved opioid analgesic. We announced in April 2023 that the Company has received official meeting
minutes from the Type C meeting with the FDA. The Type C meeting minutes indicate that the FDA and the Company are in agreement with a
majority of the proposed protocol items and are in active discussion about remaining open items. The minutes indicate that the FDA also
agrees that a successful study will support the submission of a complete response to the second Complete Response Letter for IV tramadol
pending final agreement on a statistical analysis plan and a full review of the submitted data in the complete response as well as concurrence
from the DAAAP.
In July 2023, the Company announced alignment
with the FDA on key elements of the Phase 3 safety study, including the primary endpoint and statistical analysis approach. The non-inferiority
study is designed to assess the theoretical risk of opioid-induced respiratory depression related to opioid stacking on IV tramadol compared
to IV morphine.
The study will randomize post bunionectomy
patients to IV tramadol or IV morphine for pain relief administered during a 48-hour post-operative period. Patients will have access
to IV hydromorphone, a Schedule II opioid, for rescue of breakthrough pain. The primary endpoint is a composite of elements indicative
of respiratory depression.
We submitted the revised protocol to the FDA
including the statistical plan, which reflects the study design previously discussed, for final review.
BAER-101 (novel α2/3–subtype-selective
GABA A PAM)
Baergic is a clinical-stage pharmaceutical company
founded in December 2019 that focuses on the development of pharmaceutical products for the treatment of neurologic disorders. Baergic
was acquired by the Company pursuant to the Contribution Agreement with Fortress, in order to strategically align with Avenue’s
goals of building a rare and neurologic pipeline. Baergic’s pipeline currently consists of a single compound, BAER-101, a novel
α2/3–subtype-selective GABA A positive allosteric modulator. BAER-101 (formally known as AZD7325) was originally developed
by AstraZeneca and has an established safety profile in early clinical trials including over 700 patients.
In August 2023, we reported preclinical data
for BAER-101 from an in vivo evaluation in SynapCell’s Genetic Absence Epilepsy Rate from Strasbourg (“GAERS”) model
of absence epilepsy. The GAERS model mimics behavioral, electrophysiological and pharmacological features of human absence seizures and
has shown to be an early informative indicator of efficacy in anti-seizure drug development. In the model, BAER-101 demonstrated full
suppression of seizure activity with a minimal effective dose of 0.3 mg/kg administered orally.
Relationship with Fortress
We were incorporated in Delaware on February 9,
2015, as a wholly owned subsidiary of Fortress, to develop and market pharmaceutical products for the acute care setting in the United
States. In 2017, we completed an initial public offering of shares of our Common Stock. Fortress continues to control a voting majority
of our capital stock pursuant to its ownership of a class of preferred stock. We anticipate remaining a majority controlled subsidiary
of Fortress after the completion of this offering.
Corporate Information
We are a majority-controlled subsidiary of Fortress.
Baergic is our sole subsidiary. Avenue Therapeutics, Inc. was incorporated in Delaware on February 9, 2015. Our executive offices
are located at 1111 Kane Concourse, Suite 301, Bay Harbor Islands, Florida 33154. Our telephone number is (781) 652-4500, and our
email address is info@avenuetx.com. Information on our website, or any other website, is not incorporated by reference in this prospectus.
We have included our website address in this prospectus solely as an inactive textual reference.
THE OFFERING
| Units Offered by Us |
Up
to 21,352,313 units on a “reasonable best efforts” basis, each unit consisting of one share of Common Stock and one warrant,
each warrant exercisable for one share of Common Stock. The shares of Common Stock and warrants that are part of the units are immediately
separable and will be issued separately in this offering. The warrants included within the units are exercisable immediately, have
an exercise price equal to $ (100%
of the public offering price per unit), and expire five years after the date of issuance. This prospectus also relates to
the offering of the shares of Common Stock issuable upon exercise of the warrants. For more information regarding the warrants, you
should carefully read the section titled “Description of Securities to be Registered” in this prospectus.
|
| Pre-Funded Units Offered by Us |
We are also offering to those purchasers, if any, whose purchase of
units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially
owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of Common Stock immediately following the
consummation of this offering, the opportunity to purchase, if such purchasers so choose, pre-funded units (each pre-funded unit consisting
of one pre-funded warrant to purchase one share of Common Stock and one warrant to purchase one share of Common Stock), in lieu of units
that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding shares of Common Stock.
The purchase price of each pre-funded unit will be equal to the price
per unit being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant included in the pre-funded
units will be $0.0001 per share. The pre-funded warrants included in the pre-funded units will be immediately exercisable and may be exercised
at any time, and from time to time, until all of the pre-funded warrants are exercised in full.
For each pre-funded unit we sell, the number of units we are offering
will be decreased on a one-for-one basis. This prospectus also relates to the offering of the shares of Common Stock issuable upon exercise
of the pre-funded warrants. For more information regarding the pre-funded warrants, you should carefully read the section titled “Description
of Securities to be Registered” in this prospectus.
|
Reasonable Best Efforts Offering |
We have agreed to issue and sell the securities offered hereby
to the purchasers through the placement agents. The placement agents are not required to buy or sell any specific number or dollar
amount of the securities offered hereby, but will use their reasonable best efforts to solicit offers to purchase the securities
offered by this prospectus. See “Plan of Distribution” beginning on page 35 of this prospectus.
|
Shares of Common Stock Outstanding Prior to this Offering
|
8,964,222 shares of Common Stock |
Shares of Common Stock Outstanding Following this Offering(1)
|
30,316,535 shares of Common Stock (assuming all
of the units we are offering under this prospectus are sold, that we do not issue any pre-funded units in the offering and that none
of the holders of warrants issued in the offering exercise their warrants)
|
|
Nasdaq Capital Market Ticker Symbol of our Common Stock
|
ATXI |
| Use of Proceeds |
Assuming all of the units we are offering under
this prospectus are sold at an assumed public offering price of $0.562, we estimate that we will receive approximately $10.7 million
in net proceeds from this offering, after deducting the estimated placement agent fees and estimated offering expenses. However,
this is a reasonable best efforts offering with no minimum number of securities or amount of proceeds as a condition to closing,
and we may not sell all or any of the securities offered pursuant to this prospectus; as a result, we may receive significantly less
in net proceeds.
The net proceeds from this offering will be used for general
corporate purposes and working capital requirements, which may include, among other things, the advancement of our product candidates
to obtain regulatory approval from the FDA. However, we will have broad discretion to allocate the net proceeds of this offering.
See “Use of Proceeds” for additional information.
|
| Lock-up |
We, all of our directors, officers and the holders of 10% or more of
our outstanding shares of Common Stock have agreed with the placement agents, subject to certain exceptions, not to sell, transfer or
dispose of, directly or indirectly, any of our Common Stock or securities convertible into or exercisable or exchangeable for our Common
Stock for a period of 180 days after the date of the final closing of this offering. See “Plan of Distribution” for
more information.
|
| Risk Factors |
Any investment in the Common Stock offered hereby is speculative
and involves a high degree of risk. You should carefully consider the information set forth under “Risk Factors” in
this prospectus and under similar headings in the other documents that are incorporated by reference into this prospectus. |
| (1) | The
number of shares of Common Stock to be outstanding after this offering is based on 8,964,222
shares of our Common Stock outstanding as of September 26, 2023, and excludes: |
| · | 6,078,132 shares of Common Stock issuable upon exercise of outstanding warrants having a weighted-average exercise price of $1.55
per share; |
| · | 85,000 shares of
Common Stock issuable upon the vesting and settlement of outstanding restricted stock award/units; |
| · | 3,352,489 shares of Common Stock reserved for issuance and available for future grant under our 2015 Incentive Plan; |
| · | 1,685,000 shares of Common Stock issuable upon the exercise of stock options with a weighted-average exercise price of $1.14 per share; |
| · | 16,666 shares of
Common Stock issuable upon conversion of the Class A Preferred Stock, at the holders’
election; |
| · | up to 21,352,313
shares of Common Stock issuable upon exercise of the warrants included in the units and the
pre-funded units; and |
| · | excludes 533,808
shares of Common Stock (assuming all of the units we are offering under this prospectus are
sold at the assumed offering price) issuable to Fortress, pursuant to the Amended and Restated
Founders Agreement between Fortress and the Company (the “Founders Agreement”),
following the closing of this offering. |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains predictive or “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of
current or historical fact contained in this prospectus, including statements that express our intentions, plans, objectives, beliefs,
expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions are
forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,”
“expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,”
“should,” “would” and similar expressions, as they relate to us, are intended to identify forward-looking statements.
These statements are based on current expectations,
estimates and projections made by management about our business, our industry and other conditions affecting our financial condition,
results of operations or business prospects. These statements are not guarantees of future performance and involve risks, uncertainties
and assumptions that are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed or
forecasted in, or implied by, the forward-looking statements due to numerous risks and uncertainties. Factors that could cause such outcomes
and results to differ include, but are not limited to, risks and uncertainties arising from:
| · | the fact that we currently have no drug products for sale and that our success is dependent on our product candidates receiving regulatory
approval and being successfully commercialized; |
| · | the possibility that serious adverse or unacceptable side effects are identified during the development of our current or future product
candidates, such that we would need to abandon or limit development of some of our product candidates; |
| · | our ability to successfully integrate Baergic Bio, Inc. or develop BAER-101 or AJ201; |
| · | the substantial doubt raised about our ability to continue as a going concern, which may hinder our ability to obtain future financing; |
| · | the significant losses we have incurred since inception and our expectation that we will continue to incur losses for the foreseeable
future; |
| · | our need for substantial additional funding, which may not be available to us on acceptable terms, or at all, which unavailability
could force us to delay, reduce or eliminate our product development programs or commercialization efforts; |
| · | our reliance on third parties for several aspects of our operations; |
| · | our reliance on clinical data and results obtained by third parties that could ultimately prove to be inaccurate or unreliable; |
| · | the possibility that we may not receive regulatory approval for any or all of our product candidates, or that such approval may be
significantly delayed due to scientific or regulatory reasons; |
| · | the fact that even if one or more of our product candidates receives regulatory approval, they will remain subject to substantial
regulatory scrutiny; |
| · | the effects of current and future laws and regulations relating to fraud and abuse, false claims, transparency, health information
privacy and security and other healthcare laws and regulations; |
| · | the effects of competition for our product candidates and the potential for new products to emerge that provide different or better
therapeutic alternatives for our targeted indications; |
| · | the possibility that the government or third-party payors fail to provide adequate coverage and payment rates for our product candidates
or any future products; |
| · | our ability to establish sales and marketing capabilities or to enter into agreements with third parties to market and sell our product
candidates; |
| · | our exposure to potential product liability claims; |
| · | our ability to secure adequate protection of our intellectual property and our potential inability to maintain sufficient patent protection
for our technology and products; |
| · | our ability to maintain compliance with the obligations under our intellectual property licenses and funding arrangements with third
parties, without which licenses and arrangements we could lose rights that are important to our business; |
| · | the fact that Fortress controls a voting majority of our Common Stock and has rights to receive significant share grants annually; |
| · | our ability to comply with the applicable listing standards and maintain our current listing for our Common Stock on The Nasdaq Capital
Market; and |
| · | those risks discussed or referred to in “Risk Factors” elsewhere in this prospectus, as well as those described
in any other filings which we make with the SEC. |
Any forward-looking statements speak only
as of the date on which they are made, and we undertake no obligation to publicly update or revise any forward-looking statements to reflect
events or circumstances that may arise after the date of this prospectus, except as required by applicable law. Investors should evaluate
any statements made by us in light of these important factors.
MARKET AND INDUSTRY DATA AND FORECASTS
We obtained the industry and market data used throughout this prospectus
and in the other documents incorporated by reference into this prospectus from our own internal estimates and research, as well as from
independent market research, industry and general publications and surveys, governmental agencies, publicly available information and
research, surveys and studies conducted by third parties. Internal estimates are derived from publicly available information released
by industry analysts and third-party sources, our internal research and our industry experience, and are based on assumptions made by
us based on such data and our knowledge of our industry and market, which we believe to be reasonable. In some cases, we do not expressly
refer to the sources from which this data is derived. In addition, while we believe the industry and market data included in this prospectus
is reliable and based on reasonable assumptions, such data involve material risks and other uncertainties and are subject to change based
on various factors, including those discussed in this prospectus in the section titled “Risk Factors,” as well as those
described in the documents incorporated by reference into this prospectus. These and other factors could cause results to differ materially
from those expressed in the estimates made by the independent parties or by us.
RISK FACTORS
Our business, results of operations and financial condition and
the industry in which we operate are subject to various risks. Accordingly, investing in our securities involves a high degree of risk.
This prospectus does not describe all of those risks. You should consider the risk factors described in this prospectus below, as well
as those described under the caption “Risk Factors” in the documents incorporated by reference herein, including our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, together with the other information contained or incorporated
by reference in this prospectus.
We have described below and in the documents incorporated by reference
herein the most significant risk factors applicable to us, but they do not constitute all of the risks that may be applicable to us. New
risks may emerge from time to time, and it is not possible for us to predict all potential risks or to assess the likely impact of all
risks. Before making an investment decision, you should carefully consider these risks as well as other information we include or incorporate
by reference in this prospectus and any prospectus supplement. This prospectus also contains forward-looking statements that involve risks
and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of
a number of factors, including the risks described below. See the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to this Offering
This is a reasonable best efforts offering, with no minimum amount
of securities required to be sold, and we may sell fewer than all of the securities offered hereby.
The placement agents have agreed to use their reasonable best efforts
to solicit offers to purchase the securities in this offering. The placement agents have no obligation to buy any of the securities from
us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum number
of securities that must be sold as a condition to completion of this offering, and there can be no assurance that the offering contemplated
hereby will ultimately be consummated. Even if we sell securities offered hereby, because there is no minimum offering amount required
as a condition to closing of this offering, the actual offering amount is not presently determinable and may be substantially less than
the maximum amount set forth on the cover page of this prospectus. We may sell fewer than all of the securities offered hereby, which
may significantly reduce the amount of proceeds received by us. Thus, we may not raise the amount of capital we believe is required for
our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable to
us.
If the price of our Common Stock fluctuates significantly, your
investment could lose value.
Although our Common Stock is listed on Nasdaq, we cannot assure you
that an active public market will continue for our Common Stock. If an active public market for our Common Stock does not continue, the
trading price and liquidity of our Common Stock will be materially and adversely affected. If there is a thin trading market or “float”
for our stock, the market price for our Common Stock may fluctuate significantly more than the stock market as a whole. Without a large
float, our Common Stock would be less liquid than the stock of companies with broader public ownership and, as a result, the trading prices
of our Common Stock may be more volatile. In addition, in the absence of an active public trading market, investors may be unable to liquidate
their investment in us. Furthermore, the stock market is subject to significant price and volume fluctuations, and the price of our Common
Stock could fluctuate widely in response to several factors, including:
| · | our quarterly or annual operating results; |
| · | changes in our earnings estimates; |
| · | investment recommendations by securities analysts following our business or our industry; |
| · | additions or departures of key personnel; |
| · | our failure to achieve operating results consistent with securities analysts’ projections; and |
| · | changes in industry, general market or economic conditions. |
The stock market has experienced extreme price and volume fluctuations
in recent years that have significantly affected the quoted prices of the securities of many companies, including companies in our industry.
The changes often appear to occur without regard to specific operating performance. The price of our Common Stock could fluctuate based
upon factors that have little or nothing to do with our company and these fluctuations could materially reduce our stock price.
We will have broad discretion in the use of proceeds of this
offering designated for working capital and general corporate purposes.
Our management will have broad discretion over the use and investment
of the net proceeds of this offering. Accordingly, investors in this offering have only limited information concerning our management’s
specific intentions and will need to rely upon the judgment of our management with respect to the use of proceeds. The
failure by our management to apply these funds effectively could result in financial losses that could have a material adverse
effect on our business and cause the price of our securities to decline. Pending the application of these funds, we may invest the net
proceeds from this offering in a manner that does not produce income or that loses value.
We do not intend to pay dividends on our Common Stock, so any
returns will be limited to increases, if any, in our stock’s value. Your ability to achieve a return on your investment will depend
on appreciation, if any, in the price of our Common Stock.
We currently anticipate that we will retain future earnings for the
development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable
future. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on, among
other factors, our financial condition, operating results, capital requirements, general business conditions and other factors that our
board of directors may deem relevant. Any return to stockholders will therefore be limited to the appreciation in the value of their stock,
if any.
The warrants are speculative in nature.
The warrants included in the units and pre-funded units offered
hereby do not confer any rights of Common Stock ownership on their holders, such as voting rights or the right to receive dividends,
but rather merely represent the right to acquire shares of our Common Stock at a fixed price. Specifically, commencing on the date of
issuance, holders of the warrants may exercise their right to acquire the shares of our Common Stock and pay an exercise price of $
, equal to the public offering price per unit or pre-funded unit. Moreover, following this offering, the market value of the warrants
is uncertain and there can be no assurance that the market value of the warrants will equal or exceed their exercise price. Furthermore,
each warrant will expire five years from the original issuance date. In the event the price of our Common Stock does not exceed the exercise
price of the warrants during the period when the warrants are exercisable, the warrants may not have any value. There is no established
public trading market for warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend
to apply to list the warrants on any securities exchange or nationally recognized trading system, including Nasdaq. Without an active
market, the liquidity of the warrants will be limited.
Holders of the warrants or pre-funded warrants will have no rights
as a common stockholder until they acquire shares of our Common Stock.
Until you acquire shares of our Common Stock upon exercise of your
warrants or pre-funded warrants, you will have no rights with respect to shares of Common Stock issuable upon exercise of such warrants.
Upon exercise of your warrants or pre-funded warrants, you will be entitled to exercise the rights of a holder of our Common Stock as
to the security exercised only as to matters for which the record date occurs after the exercise.
Provisions of the warrants and pre-funded warrants offered by
this prospectus could discourage an acquisition of us by a third party.
In addition to the provisions of our amended and restated certificate
of incorporation and bylaws discussed elsewhere in this prospectus, certain provisions of the warrants and pre-funded warrants offered
by this prospectus could make it more difficult or expensive for a third party to acquire us. The warrants and pre-funded warrants prohibit
us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving
entity assumes our obligations under the warrants. These and other provisions of the warrants and pre-funded warrants offered by this
prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
If you purchase shares of our Common Stock included as part of
the units in this offering, you will incur immediate and substantial dilution in the book value of your shares.
Investors purchasing shares of our Common Stock included as
part of the units in this offering will pay a price per unit that substantially exceeds the pro forma as adjusted net tangible book
value per share. As a result, investors purchasing shares of our Common Stock included as part of the units in this offering will
incur immediate dilution of $0.40 per share, representing the difference between the public offering price of $0.562 per unit
and our pro forma as adjusted net tangible book value per share as of June 30, 2023. To the extent outstanding options or warrants
to purchase our Common Stock are exercised and to the extent that we issue to Fortress the shares of Common Stock issuable pursuant
to the Founders Agreement following the closing of this offering, new investors may incur further dilution. For more information on
the dilution you may experience as a result of investing in this offering, see the section of this prospectus entitled
“Dilution.”
If we sell Common Stock or preferred stock in future financings,
stockholders may experience immediate dilution and, as a result, our stock price may decline.
We may from time to time issue additional shares of Common Stock or
preferred stock at a discount from the current trading price of our Common Stock. As a result, our stockholders would experience immediate
dilution upon the purchase of any shares sold at such discount. In addition, as opportunities present themselves, we may enter into financing
or similar arrangements in the future, including the issuance of debt securities, Common Stock or preferred stock. If we issue Common
Stock or securities convertible into Common Stock, the holders of our Common Stock would experience additional dilution and, as a result,
our stock price may decline.
There is substantial doubt about our ability
to continue as a going concern, which may hinder our ability to obtain future financing.
We are not yet generating revenue, have incurred substantial
operating losses since our inception and expect to continue to incur significant operating losses for the foreseeable future as we
execute on our product development plan and may never become profitable. As of December 31, 2022, we had cash and cash
equivalents of $6.7 million and an accumulated deficit of $80.6 million, and, as of June 30, 2023, we had cash and cash
equivalents of $1.6 million and an accumulated deficit of $92.1 million. We do not believe that our cash is sufficient for the next
twelve months. As a result, there is substantial doubt about our ability to continue as a going concern. Our ability to continue as
a going concern will depend on our ability to obtain additional funding, as to which no assurances can be given. This offering is
being conducted on a best efforts basis and we may sell fewer than all of the securities offered hereby and may receive
significantly less in net proceeds from this offering than the maximum amount set forth on the cover page of this prospectus.
Furthermore, even if we sell all of the securities offered hereby and raise the maximum amount of proceeds, we may need to raise
additional capital to fund our operations and continue to support our planned development and commercialization activities. If we
are unable to obtain funds when needed or on acceptable terms, we may be required to curtail our current development programs, cut
operating costs, forgo future development and other opportunities or even terminate our operations.
If we fail to satisfy applicable listing standards of The Nasdaq
Capital Market, our Common Stock could be delisted from The Nasdaq Capital Market.
On May 19, 2023, we received a deficiency letter (the “Nasdaq
Stockholders’ Equity Letter”) from the Listing Qualifications Department (the “Staff”) of The Nasdaq Stock Market
LLC, notifying us that we are not in compliance with Nasdaq Listing Rule 5550(b)(1), which requires us to maintain a minimum of $2,500,000
in stockholders’ equity for continued listing on The Nasdaq Capital Market (the “Stockholders’ Equity Requirement”),
nor in compliance with either of the alternative listing standards, market value of listed securities of at least $35 million or net
income of $500,000 from continuing operations in the most recently completed fiscal year, or in two of the three most recently completed
fiscal years. Our failure to comply with the Stockholders’ Equity Requirement was based on the filing of our Quarterly Report on
Form 10-Q for the quarter ended March 31, 2023, reporting the stockholders’ equity of negative $2,157,000. Pursuant to the Nasdaq
Stockholders’ Equity Letter, we had 45 calendar days from the date of the Nasdaq Stockholders’ Equity Letter to submit a
plan to regain compliance. On July 3, 2023, we submitted a compliance plan (the “Compliance Plan”). On
July 17, 2023, the Staff granted the Company’s request for an extension of the deadline to regain compliance with the
Rule to November 15, 2023.
Additionally, on September 27, 2023, we received a deficiency letter
(the “Nasdaq Minimum-Bid Price Letter”) from the Staff of the Nasdaq Capital Market LLC, stating that the bid price of our
Common Stock had closed below $1.00 per share for 30 consecutive business days and that, therefore, we are not in compliance with Nasdaq
Listing Rule 5550(a)(2) (the “Minimum-Bid Price Requirement”). We have a 180-calendar day grace period, through March 25,
2024, to regain compliance with the Minimum-Bid Price Requirement. Compliance can be achieved by evidencing a closing bid price of at
least $1.00 per share for a minimum of ten (10) consecutive business days, although the Staff may, in its discretion, require compliance
for a longer period of time (generally no more than 20 consecutive business days) during the 180-calendar day grace period. If we do
not regain compliance with the Minimum-Bid Price Requirement by March 25, 2024, we may be eligible for an additional 180-day compliance
period so long as we satisfy the criteria for initial listing
on The Nasdaq Stock Market, other than the market value of publicly held shares requirement, and the continued listing requirement for
market value of publicly held shares and we provide written notice of our intention to cure the deficiency during the second compliance
period by effecting a reverse stock split, if necessary. In the event we are not eligible for the second grace period, the Staff will
provide written notice that our Common Stock is subject to delisting; however, we may request a hearing before the Nasdaq Hearings Panel
(the “Panel”), which request, if timely made, would stay any further suspension or delisting action by the Staff pending
the conclusion of the hearing process and expiration of any extension that may be granted by the Panel. We intend to closely monitor
the closing bid price of the Common Stock and consider all available options to remedy the bid price deficiency, but no decision regarding
any action has yet been made.
We
intend to take all reasonable measures available to regain compliance under the Nasdaq Listing Rules and remain listed on The Nasdaq
Capital Market. However, there can be no assurance that The Nasdaq Stock Market LLC will approve the Compliance Plan or that we will ultimately
regain compliance with all applicable requirements for continued listing. If our Common Stock were delisted from The Nasdaq Stock Market,
it could severely limit the liquidity of our Common Stock and your ability to sell our securities on the secondary market. Delisting
from The Nasdaq Capital Market could adversely affect our ability to raise additional financing through the public or private sale of
equity securities, would significantly affect the ability of investors to trade our securities and would negatively affect the value and
liquidity of our Common Stock. Delisting could also have other negative results, including the potential loss of confidence by employees,
the loss of institutional investor interest and fewer business development opportunities. If our Common Stock is delisted from The Nasdaq
Capital Market, the price of our Common Stock may decline and our Common Stock may be eligible to trade on the OTC Bulletin Board, another
over-the-counter quotation system, or on the pink sheets where an investor may find it more difficult to dispose of their Common Stock
or obtain accurate quotations as to the market value of our Common Stock. Further, if we are delisted, we would incur additional costs
under requirements of state “blue sky” laws in connection with any sales of our securities. These requirements could severely
limit the market liquidity of our Common Stock and the ability of our stockholders to sell our Common Stock in the secondary market.
Purchasers who purchase our securities in
this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit
of a securities purchase agreement.
In addition to rights and remedies available to
all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement
will also be able to bring claims for breach of contract against us. The ability to pursue a claim for breach of contract provides those
investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including timely
delivery of shares and indemnification for breach of contract.
CAPITALIZATION
The following table sets forth our cash and capitalization as of June 30,
2023, as follows:
| · | on an as adjusted
basis to reflect the issuance and sale by us of 21,352,313 units in this offering at
an assumed public offering price of $0.562 per unit (which is the last reported sale price
of our Common Stock on The Nasdaq Capital Market on September 25, 2023), after deducting
the estimated offering expenses payable by us and assuming no sale of any pre-funded units
in the offering, but before applying any of the net proceeds as described below under
“Use of Proceeds,” including any amounts payable to InvaGen Pharmaceuticals Inc. (“InvaGen”). |
The as adjusted information below is illustrative only, and our capitalization
following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering
determined at pricing. You should read this information in conjunction with our financial statements and the “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” section in our Quarterly Report on Form 10-Q for the period ended June 30, 2023, which is incorporated by reference in this prospectus.
| |
|
June 30, 2023 |
|
| |
|
(unaudited) |
|
| ($
in thousands) |
|
Actual |
|
|
As Adjusted |
|
| Cash and cash equivalents |
|
$ |
1,571 |
|
|
$ |
12,301 |
|
| |
|
|
|
|
|
|
|
|
| Stockholders’ Equity (Deficit) |
|
|
|
|
|
|
|
|
| Preferred Stock ($0.0001 par
value), 2,000,000 shares authorized |
|
|
|
|
|
|
|
|
| Class A Preferred
Stock – 250,000 shares issued and outstanding |
|
|
— |
|
|
|
— |
|
| Common Stock ($0.0001 par value), 75,000,000 shares
authorized |
|
|
|
|
|
|
|
|
| Common stock –
7,920,485 issued and outstanding |
|
|
1 |
|
|
|
3 |
|
| Additional paid-in
capital |
|
|
86,757 |
|
|
|
97,485 |
|
| Accumulated deficit |
|
|
(92,094 |
) |
|
|
(92,094 |
) |
| Total stockholders’
equity (deficit) attributed to the Company |
|
|
(5,336 |
) |
|
|
5,394 |
|
| Non-controlling
interests |
|
|
(810 |
) |
|
|
(810 |
) |
| Total Capitalization |
|
$ |
(6,146 |
) |
|
$ |
4,584 |
|
Each $0.10 increase or decrease in the assumed public offering
price of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market on September 25, 2023)
would increase or decrease each of cash and cash equivalents, total stockholders’ equity and total capitalization on an as adjusted
basis by approximately $2.0 million, assuming the maximum number of units offered, as set forth on the cover page of this prospectus,
remains the same, and after deducting the estimated placement agent fees and estimated offering expenses payable by us and assuming no
sale of any pre-funded units in the offering.
Each 1,000,000 unit increase or decrease in the number of units
offered by us in this offering would increase or decrease each of cash and cash equivalents, total stockholders’ equity and total
capitalization on an as adjusted basis by approximately $0.5 million, assuming that the price per unit for the offering remains
at $0.562 (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market on September 25, 2023), and after
deducting the estimated placement agent fees and estimated offering expenses payable by us.
The
number of shares of Common Stock to be outstanding after this offering is based on 7,920,485 shares of our Common Stock outstanding
as of June 30, 2023, and:
| · | excludes 767,085 shares of Common Stock issued and sold on September 8, 2023 in a private placement transaction; |
| | · | excludes 276,652 shares of Common Stock issued to AnnJi
on September 26, 2023, pursuant to the Company’s license agreement with AnnJi, upon
the enrollment of the eighth (8th) participant in the first Phase 1b/2a Clinical Trial in
the United States; |
| | | |
| · | excludes 6,078,132 shares of Common Stock issuable upon exercise of outstanding warrants having a weighted-average exercise price
of $1.55 per share; |
| · | excludes 85,000
shares of Common Stock issuable upon the vesting and settlement of outstanding restricted
stock award/units; |
| · | excludes 3,352,489 shares of Common Stock reserved for issuance and available for future grant under our 2015 Incentive Plan; |
| · | excludes 1,685,000 shares of Common Stock issuable upon the exercise of stock options with a weighted-average exercise price of $1.14
per share; |
| · | excludes 16,666
shares of Common Stock issuable upon conversion of the Class A Preferred Stock, at the
holders’ election; |
| · | excludes up to
21,352,313 shares
of Common Stock issuable upon exercise of the warrants included in the units; and |
| · | excludes 533,808
shares of Common Stock (assuming all of the units we are offering under this prospectus are
sold at the assumed offering price) issuable to Fortress, pursuant to the Founders Agreement,
following the closing of this offering. |
USE OF PROCEEDS
We estimate that we will receive net proceeds from this offering
of approximately $10.7 million (assuming the sale of the maximum number of units offered hereby), based upon an assumed public offering
price of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market on September 25, 2023),
after deducting the estimated placement agent fees and estimated offering expenses payable by us and assuming no exercise of the warrants
included in the units or pre-funded units. We will only receive additional proceeds from the exercise of the warrants included in the
units and pre-funded units we are selling in this offering if the warrants are exercised for cash. However, because this is a reasonable
best efforts offering with no minimum number of securities or amount of proceeds as a condition to closing, the actual offering amount,
placement agent fees, and net proceeds to us are not presently determinable and may be substantially less than the maximum amounts set
forth on the cover page of this prospectus, and we may not sell all or any of the securities we are offering. As a result, we may receive
significantly less in net proceeds. Based on the assumed offering price set forth above, we estimate that our net proceeds from the sale
of 75%, 50%, and 25% of the units offered in this offering would be approximately $8.0 million, $5.2 million, and $2.45 million, respectively,
after deducting the estimated placement agent fees and estimated offering expenses payable by us, and assuming no exercise of the warrants
included in the units or pre-funded units.
Each $0.10 increase (decrease) in the assumed public offering price
of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market on September 25, 2023)
would increase (decrease) the net proceeds to us from this offering by approximately $2.0 million, assuming the number of units
offered, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated offering expenses payable
by us and assuming no exercise of the warrants included in the units and no sale of any pre-funded units in the offering. Each 1,000,000
share increase (decrease) in the number of units offered by us in this offering would increase (decrease) the net proceeds to us from
this offering by approximately $0.5 million, assuming that the price per unit for the offering remains at $0.562 (which is the last
reported sale price of our Common Stock on The Nasdaq Capital Market on September 25, 2023), and after deducting the estimated offering
expenses payable by us and assuming no exercise of the warrants included in the units and no sale of any pre-funded units in the offering.
The net proceeds from this offering will be used for general
corporate purposes and working capital requirements, which may include, among other things, the advancement of our product
candidates to obtain regulatory approval from the FDA. Additionally, under the agreement with InvaGen under which we repurchased all
of InvaGen’s shares in the Company, until we have paid an aggregate of $4 million to InvaGen, we are contractually bound to
pay 7.5% of the net proceeds, before expenses, of any public or private financing to InvaGen. Accordingly, assuming the sale of the
maximum number of units offered hereby, we estimate we will pay InvaGen approximately $803,000 of the net proceeds. We have not
determined the amounts we plan to spend on the areas listed above or the timing of these expenditures, and we have no current plans
with respect to acquisitions as of the date of this prospectus. The timing and amounts of our actual expenditures will depend on
several factors. As a result, our management will have broad discretion to allocate the net proceeds of this offering. Pending their
ultimate use, we intend to invest the net proceeds in a variety of securities, including commercial paper, government and
non-government debt securities and/or money market funds that invest in such securities.
DILUTION
Purchasers
of the securities offered by this prospectus will suffer immediate and substantial dilution in the net tangible book value per share of
the Common Stock included in the units they purchase. Net tangible book value per share represents the amount of total tangible assets
less total liabilities, divided by the number of shares of our Common Stock outstanding as of June 30, 2023. Our net tangible book
value as of June 30, 2023 was approximately $(6.1) million, or $(0.78)
per share of our Common Stock.
Dilution in net tangible book value per share represents the
difference between the amount per share paid by purchasers in this offering and the net tangible book value per share of our Common
Stock immediately after this offering. After giving effect to the sale of the maximum number of units offered hereby at an assumed
public offering price of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market on
September 25, 2023), and after deducting the estimated placement agent fees and the estimated expenses payable by us and assuming no
sale of any pre-funded units in this offering and excluding the issuance of shares of common Stock issuable
to Fortress, pursuant to the Founders Agreement, following the closing of this offering, our net tangible book value as of June 30, 2023 would have been approximately
$4.6 million, or $0.16 per share of Common Stock. This represents an immediate increase in net book value of $0.94 per
share to our existing stockholders and an immediate dilution in net tangible book value of $0.40 per share to new investors
participating in this offering.
The following table illustrates this calculation on a per share basis:
| Offering price per share | |
| | | |
$ | 0.562 | |
| | |
| | | |
| | |
| Net tangible book value per share as of June 30, 2023 | |
$ | (0.78 | ) | |
| | |
| Increase per share attributable to the offering | |
$ | 0.94 | | |
| | |
| As-adjusted net tangible book value per share after giving
effect to the offering | |
| | | |
$ | 0.16 | |
| Dilution in net tangible book value per share to new investors | |
| | | |
$ | 0.40 | |
If we sell only 75% of the units offered in this offering, at an
assumed public offering price of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market
on September 25, 2023), and after deducting the estimated placement agent fees and the estimated expenses payable by us and assuming
no sale of any pre-funded units in this offering, our net tangible book value as of June 30, 2023 would have been approximately $1.8 million,
or $0.08 per share of Common Stock. This represents an immediate increase in net book value of $0.86 per share to our existing stockholders
and an immediate dilution in net tangible book value of $0.48 per share to new investors participating in this offering.
If we sell only 50% of the units offered in this offering, at an
assumed public offering price of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market
on September 25, 2023), and after deducting the estimated placement agent fees and the estimated expenses payable by us and assuming
no sale of any pre-funded units in this offering, our net tangible book value as of June 30, 2023 would have been approximately $(0.9) million,
or $(0.05) per share of Common Stock. This represents an immediate increase in net book value of $0.73 per share to our existing stockholders
and an immediate dilution in net tangible book value of $0.61 per share to new investors participating in this offering.
If we sell only 25% of the units offered in this offering, at an
assumed public offering price of $0.562 per unit (which is the last reported sale price of our Common Stock on The Nasdaq Capital Market
on September 25, 2023), and after deducting the estimated placement agent fees and the estimated expenses payable by us and assuming
no sale of any pre-funded units in this offering, our net tangible book value as of June 30, 2023 would have been approximately $(3.7)
million, or $(0.28) per share of Common Stock. This represents an immediate increase in net book value of $0.50 per share to our
existing stockholders and an immediate dilution in net tangible book value of $0.84 per share to new investors participating in
this offering.
The
number of shares of Common Stock to be outstanding after this offering is based on 7,920,485 shares of our Common Stock outstanding
as of June 30, 2023, and:
| · | excludes 767,085 shares of Common Stock issued and sold on September 8, 2023 in a private placement transaction; |
| | · | excludes 276,652 shares of Common Stock issued to AnnJi
on September 26, 2023, pursuant to the Company’s license agreement with AnnJi, upon
the enrollment of the eighth (8th) participant in the first Phase 1b/2a Clinical Trial in
the United States; |
| | | |
| · | excludes 6,078,132 shares of Common Stock issuable upon exercise of outstanding warrants having a weighted-average exercise price
of $1.55 per share; |
| · | excludes 85,000
shares of Common Stock issuable upon the vesting and settlement of outstanding restricted
stock award/units; |
| · | excludes 3,352,489 shares of Common Stock reserved for issuance and available for future grant under our 2015 Incentive Plan; |
| · | excludes 1,685,000 shares of Common Stock issuable upon the exercise of stock options with a weighted-average exercise price of $1.14
per share; |
| · | excludes 16,666
shares of Common Stock issuable upon conversion of the Class A Preferred Stock, at the
holders’ election; |
| · | excludes up to
21,352,313 shares of Common Stock issuable upon exercise of the warrants included in
the units; and |
| · | excludes 533,808
shares of Common Stock (assuming all of the units we are offering under this prospectus are
sold at the assumed offering price) issuable to Fortress, pursuant to the Founders Agreement,
following the closing of this offering. |
DIVIDEND POLICY
We currently intend to retain all available funds and any future earnings
to fund the growth and development of our business. We have never declared or paid any cash dividends on our capital stock. We do not
intend to pay cash dividends on our Common Stock in the foreseeable future. Investors should not purchase our common stock with the expectation
of receiving cash dividends.
Any future determination to declare dividends will be made at the discretion
of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions,
and other factors that our board of directors may deem relevant.
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
STATEMENTS
The following unaudited pro forma condensed combined consolidated financial
information is presented to illustrate the effects of the acquisition of Baergic Bio, Inc. by Avenue Therapeutics, Inc. based
on the historical financial position and results of operations of Avenue Therapeutics, Inc. and Baergic Bio, Inc. The unaudited
pro forma condensed combined statements of operations for the periods presented give effect to the acquisition of Baergic as if it took
place on January 1, 2021.
The unaudited pro forma condensed combined consolidated statement of
operations for the years ended December 31, 2022, and 2021 were prepared based on (i) the historical audited consolidated statement
of operations of the Company for the years ended December 31, 2022, and 2021 and (ii) the historical audited statement of operations
of Baergic for the year ended December 31, 2021 and the historical unaudited statement of operations of Baergic for the six months
ended June 30, 2022.
Our historical consolidated financial information has been derived
from the consolidated audited and unaudited financial statements of the Company and accompanying notes to the financial statements incorporated
by reference into this prospectus. The historical consolidated financial information of Baergic has been derived from the consolidated
audited and unaudited financial statements of Baergic and accompanying notes to the financial statements incorporated by reference into
this prospectus.
The unaudited pro forma condensed combined consolidated financial information
was prepared in accordance with Article 11 of SEC Regulation S-X.
See the accompanying notes to the Unaudited Pro Forma Consolidated
Financial Information for a discussion of assumptions made.
The unaudited pro forma condensed combined financial information has
been presented for informational purposes only and is not necessarily indicative of what the combined company’s financial position
or results of operations actually would have been had the Company and Baergic been a combined company as of the date indicated. In addition,
the unaudited pro forma condensed combined financial information does not purport to project the future financial position or operating
results of the combined company. The historical consolidated financial information has been adjusted in the accompanying unaudited pro
forma condensed combined consolidated financial information to give effect to unaudited pro forma events.
UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
STATEMENT OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2022
| | |
| | |
January 1, 2022 | | |
| | |
| |
| |
| | |
Year Ended | | |
through | | |
| | |
| |
Year Ended | |
| | |
December 31,
2022 | | |
November 7,
2022 | | |
| | |
| |
December 31,
2022 | |
| | |
| | |
| | |
Transaction | | |
| |
| |
| | |
Avenue | | |
Baergic | | |
Accounting | | |
| |
Pro Forma | |
| | |
(Historical) | | |
(Historical) | | |
Adjustments | | |
Notes | |
Combined | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| |
| | |
| Research and development | |
$ | 2,698 | | |
$ | 290 | | |
| (208 | ) | |
6(a) | |
$ | 2,780 | |
| General and administrative | |
| 5,345 | | |
| 609 | | |
| (208 | ) | |
6(a) | |
| 5,746 | |
| Loss from operations | |
| (8,043 | ) | |
| (899 | ) | |
| 416 | | |
| |
| (8,526 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Interest income | |
| (20 | ) | |
| - | | |
| | | |
| |
| (20 | ) |
| Interest expense | |
| - | | |
| 282 | | |
| | | |
| |
| 282 | |
| Financing costs - warrant liabilities | |
| 1,160 | | |
| - | | |
| | | |
| |
| 1,160 | |
| Change in fair value of warrant liabilities | |
| (5,580 | ) | |
| - | | |
| | | |
| |
| (5,580 | ) |
| Net loss | |
$ | (3,603 | ) | |
$ | (1,181 | ) | |
$ | 416 | | |
| |
$ | (4,368 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Net loss attributable to non-controlling interests | |
| 51 | | |
| - | | |
| | | |
| |
| 51 | |
| Net loss attributable to common stockholders | |
$ | (3,552 | ) | |
$ | (1,181 | ) | |
$ | 416 | | |
| |
$ | (4,317 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Net loss per common share attributable to common stockholders, basic and diluted | |
$ | (1.63 | ) | |
| | | |
| | | |
| |
$ | (2.29 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Weighted average number of common shares outstanding, basic and diluted | |
| 2,185,159 | | |
| | | |
| | | |
| |
| 1,883,638 | |
UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
STATEMENT OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2021
| | |
Year Ended | | |
Year Ended | | |
| | |
| |
Year Ended | |
| | |
December 31,
2021 | | |
December 31,
2021 | | |
| | |
| |
December 31,
2021 | |
| | |
| | |
| | |
Transaction | | |
| |
| |
| | |
Avenue | | |
Baergic | | |
Accounting | | |
| |
Pro Forma | |
| | |
(Historical) | | |
(Historical) | | |
Adjustments | | |
Notes | |
Combined | |
| | |
| | |
| | |
| | |
| |
| |
| Operating expenses: | |
| | | |
| | | |
| | | |
| |
| | |
| Research and development | |
$ | 1,254 | | |
$ | 342 | | |
| (250 | ) | |
6(a) | |
$ | 1,346 | |
| General and administrative | |
| 2,484 | | |
| 363 | | |
| (250 | ) | |
6(a) | |
| 2,597 | |
| Loss from operations | |
| (3,738 | ) | |
| (705 | ) | |
| 500 | | |
| |
| (3,943 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Interest income | |
| (7 | ) | |
| - | | |
| | | |
| |
| (7 | ) |
| Interest expense | |
| - | | |
| 307 | | |
| | | |
| |
| 307 | |
| Financing costs – warrant liabilities | |
| - | | |
| - | | |
| | | |
| |
| - | |
| Change in fair value of warrant liabilities | |
| - | | |
| - | | |
| | | |
| |
| - | |
| Net loss | |
$ | (3,731 | ) | |
$ | (1,012 | ) | |
$ | 500 | | |
| |
$ | (4,243 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Net loss attributable to non-controlling interests | |
| - | | |
| - | | |
| | | |
| |
| - | |
| Net loss attributable to common stockholders | |
$ | (3,731 | ) | |
$ | (1,012 | ) | |
$ | 500 | | |
| |
$ | (4,243 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Net loss per common share attributable to common stockholders, basic and diluted | |
$ | (3.29 | ) | |
| | | |
| | | |
| |
$ | (5.71 | ) |
| | |
| | | |
| | | |
| | | |
| |
| | |
| Weighted average number of common shares outstanding, basic and diluted | |
| 1,133,170 | | |
| | | |
| | | |
| |
| 743,242 | |
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED
FINANCIAL INFORMATION
| 1. | Description of the Merger |
On May 11, 2022, Avenue entered into a stock
contribution agreement (the “Contribution Agreement”) with Fortress, pursuant to which Fortress agreed to transfer its ownership
of a majority of the outstanding shares (common and preferred) in a private subsidiary company of Fortress, Baergic Bio, Inc. (“Baergic
Bio”), to Avenue. Under the Contribution Agreement, Fortress also agreed to assign to Avenue certain intercompany agreements existing
between Fortress and Baergic, including a Founders Agreement and Management Services Agreement. Consummation of the transactions contemplated
by the Contribution Agreement were subject to the satisfaction of certain conditions precedent, including: (i) the closing of an
equity financing by Avenue resulting in gross proceeds of no less than $7.5 million, (ii) the agreement by InvaGen to (A) have
100% of its shares in Avenue repurchased by Avenue and (B) terminate certain of the agreements into which it entered with Avenue
and/or Fortress in connection with InvaGen’s 2019 equity investment in Avenue, which will eliminate certain negative consent rights
of InvaGen over Avenue and restore certain rights and privileges of Fortress in Avenue, and (iii) the sustained listing of Avenue’s
Common Stock on Nasdaq. On October 11, 2022, Avenue consummated the transactions contemplated by the Share Repurchase Agreement with
InvaGen, pursuant to which Avenue repurchased 100% of the shares in Avenue held by InvaGen for a purchase price of $3 million. In connection
with the closing of the Share Repurchase Agreement, which occurred on October 31, 2022, all of the rights retained by InvaGen pursuant
to the Stockholders Agreement entered into by and among the Company, InvaGen and Fortress on November 12, 2018, were terminated.
The acquisition was completed on November 8, 2022, at which time Baergic Bio became a consolidated subsidiary of Avenue.
2. Reverse Stock Split
On September 22, 2022, Avenue filed a Certificate
of Amendment to its Third Amended and Restated Certificate of Incorporation (the “Amendment”) with the Secretary of State
of the State of Delaware to (i) effect a one-for-fifteen reverse stock split (the “Reverse Stock Split”) of the Company’s
shares of common stock, $0.0001 par value (the “Common Stock”), and (ii) effect a related reduction in the number of
the Company’s authorized shares from 50,000,000 to 20,000,000 (the “Authorized Share Reduction”). All share and per
share information has been retroactively adjusted to give effect to the reverse stock split for all periods presented, unless otherwise
indicated.
As a result of the Reverse Stock Split, every
fifteen shares of the Company’s pre-reverse split Common Stock were combined and reclassified as one share of Common Stock. Proportionate
voting rights and other rights of common stockholders were not affected by the reverse split, other than as a result of the payment for
fractional shares. No fractional shares were issued in connection with the Reverse Stock Split. Stockholders who would otherwise hold
a fractional share of Common Stock received (upon surrender to the exchange agent of certificates representing such shares), a cash payment
in lieu thereof, without interest or deduction, rounded to the nearest cent, in an amount equal to the product obtained by multiplying
(a) the closing price per share of our common stock as reported on the Nasdaq Stock Market as of September 22, 2022, the effective
date of the Reverse Stock Split, by (b) the fraction of one share owned by the stockholder. The total amount paid in consideration
for the fractional shares was approximately $10,000.
Proportionate adjustments were made to the per
share exercise price and/or the number of shares issuable upon the exercise or vesting of all restricted stock award/units and warrants
outstanding at September 22, 2022, which resulted in a proportional decrease in the number of shares of the Company’s common
stock reserved for issuance upon exercise or vesting of such restricted stock award/units and warrants, and, in the case of warrants,
a proportional increase in the exercise price of all such stock options and warrants.
3. Basis of Presentation
The unaudited pro forma condensed combined financial
information is prepared in accordance with Article 11 of SEC Regulation S-X. The historical financial information has been adjusted
in the accompanying unaudited pro forma condensed combined financial information to give effect to unaudited pro forma events that are:
| |
● |
directly attributable to the transaction; |
| |
● |
factually supportable; and |
| |
● |
with respect to the unaudited pro forma condensed combined consolidated statement of operations, expected to have a continuing impact on the results of operations of the combined company. |
The transaction was accounted as a transaction
between entities under common control such that Avenue recognized the assets and liabilities of Baergic Bio received in the transaction
at their historical carrying amounts, as reflected in the historical consolidated financial statements of Baergic Bio. No Goodwill or
intangibles were recognized.
The unaudited pro forma condensed combined financial
information is presented solely for informational purposes and is not necessarily indicative of the combined results of operations that
might have been achieved for the periods or dates indicated, nor is it necessarily indicative of the future results of the combined company.
The unaudited pro forma condensed combined financial information has not been adjusted to give effect to certain expected financial benefits
of the merger, such as tax savings, cost synergies or revenue synergies, or the anticipated costs to achieve these benefits, including
the cost of integration activities. The unaudited pro forma condensed combined financial information does not reflect possible adjustments
related to restructuring or integration activities that have yet to be determined or transaction or other costs following the combination
that are not expected to have a continuing impact on the business of the combined company.
4. Accounting Policies
The unaudited pro forma condensed combined consolidated
financial information has been compiled in a manner consistent with the accounting policies of Avenue. Following the acquisition, the
combined company conducted a review of accounting policies of Baergic Bio in an effort to determine if differences in accounting policies
required further reclassification of results of operations or reclassification of assets or liabilities to conform to Avenue’s accounting
policies and classifications. As a result of that review, no significant differences were identified among the accounting policies of
the companies that, when conformed, had a material impact on the unaudited pro forma condensed combined consolidated financial information.
5. Loss per Share
Represents the net loss per share calculated using
the historical weighted average shares outstanding, adjusted for the shares repurchased by Avenue pursuant to the Share Repurchase Agreement,
which occurred on October 31, 2022. The pro forma net loss per share calculation assumes the shares had been repurchased on January 1,
2021, the beginning of the earliest period presented.
6. Transaction Accounting Adjustments
The following provides explanations of the various
adjustments to the unaudited pro forma condensed combined balance sheet:
| (a) | Represents eliminations through consolidation of operating expenses from assignment of Master Services
Agreement from Fortress to Avenue for Baergic. |
DESCRIPTION OF SECURITIES TO BE REGISTERED
Avenue Therapeutics has one class of securities registered under
Section 12 of the Securities Act of 1934, as amended: our Common Stock. The following description of our Common Stock is a summary
and is qualified in its entirety by reference to our Third Amended and Restated Certificate of Incorporation, as amended, and our Amended
and Restated By-Laws (the “By-Laws”), which are included as exhibits to the registration statement on Form S-1 of which
this prospectus forms a part. We encourage you to read the Certificate of Incorporation and By-Laws as well as the applicable provisions
of the General Corporation Law of the State of Delaware, as amended (the “DGCL”), for more information.
Authorized Capital Stock
Our authorized capital stock consists of 75,000,000 shares of Common
Stock, with $0.0001 par value, and 2,000,000 shares of Preferred Stock, with $0.0001 par value, of which 250,000 have been designated
as Class A Preferred Stock and the remainder of which are undesignated Preferred Stock.
As of September 26, 2023, there were 8,964,222 shares of our Common
Stock outstanding held by 32 record stockholders.
Common Stock
Voting Rights
Holders of our Common Stock are entitled to one vote for each share
held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders
shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of Common Stock are
entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights
of outstanding preferred stock.
Liquidation and Other Rights
In the event of our liquidation or dissolution, the holders of Common
Stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and
other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of Common Stock have no preemptive, subscription,
redemption or conversion rights. The rights, preferences and privileges of holders of Common Stock are subject to, and may be adversely
affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Listing
Our Common Stock is traded on The Nasdaq Capital Market under the symbol
“ATXI.” The transfer agent and registrar for our Common Stock is VStock Transfer, LLC.
Dividends
Holders of Common Stock are entitled to receive
proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of outstanding
preferred stock. Pursuant to the certificate of designation relating to the Class A Preferred Stock, we are prohibited from paying dividends
on our Common Stock until all dividends required to be paid to the holders of our Class A Preferred Stock have been paid or declared
and set apart for payment.
Anti-Takeover Effects of Various Provisions
of Delaware Law and Avenue Therapeutics’ Certificate of Incorporation and By-Laws
Provisions of the DGCL and our Certificate
of Incorporation and By-Laws could make it more difficult to acquire Avenue Therapeutics by means of a tender offer, a proxy contest or
otherwise, or to remove incumbent officers and directors. These provisions, including those summarized below, may encourage certain types
of coercive takeover practices and takeover bids.
Delaware
Anti-Takeover Statute. In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation
from engaging in a “business combination” with an “interested stockholder” for a period of three years following
the time the person became an interested stockholder, unless the business combination or the acquisition of shares that resulted in a
stockholder becoming an interested stockholder is approved in a prescribed manner. Generally, a “business combination” includes
a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Generally, an “interested
stockholder” is a person who, together with affiliates and associates, owns (or within three years prior to the determination of
interested stockholder status did own) 15% or more of a corporation’s voting stock. However, our Certificate of Incorporation provides
that we are not subject to the anti-takeover provisions of Section 203 of the DGCL.
Removal.
Subject to the rights of any holders of any outstanding series of our Preferred Stock, stockholders may remove our directors with or without
cause. Removal will require the affirmative vote of holders of a majority of our voting stock.
Size
of Board and Vacancies. Our By-Laws provide that the number of directors be fixed exclusively by the board
of directors. Any vacancies created on its board of directors resulting from any increase in the authorized number of directors or
the death, resignation, retirement, disqualification, removal from office or other cause will be filled by a majority of the board
of directors then in office, even if less than a quorum is present, or by a sole remaining director. Any director appointed to fill
a vacancy on our board of directors will hold office until the next annual meeting and until his or her successor has been
elected and qualified.
Requirements
for Advance Notification of Stockholder Nominations and Proposals. Our By-Laws establish advance notice procedures
with respect to stockholder proposals and nomination of candidates for election as directors other than nominations made by or at the
direction of its board of directors or a committee of our board of directors.
Undesignated
Preferred Stock. Our board of directors is authorized to issue up to 2,000,000 shares of preferred stock without
additional stockholder approval, which preferred stock could have voting rights or conversion rights that, if exercised, could adversely
affect the voting power of the holders of Common Stock. The issuance of shares of preferred stock may have the effect of delaying, deferring
or preventing a change in control of the Company without any action by the Company’s stockholders.
Limitation on Liability of Directors and Indemnification of Directors
and Officers
Elimination
of Liability of Directors. The DGCL authorizes corporations to limit or eliminate the personal liability of directors
to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties as directors, and our Certificate
of Incorporation includes such an exculpation provision. Our Certificate of Incorporation provides that, to the fullest extent permitted
by the DGCL, no director will be personally liable to us or to our stockholders for monetary damages for breach of fiduciary duty as a
director. While our Certificate of Incorporation provides directors with protection from awards for monetary damages for breaches of their
duty of care, it does not eliminate this duty. Accordingly, our Certificate of Incorporation has no effect on the availability of equitable
remedies such as an injunction or rescission based on a director’s breach of his or her duty of care. The provisions apply to an
officer of Avenue Therapeutics only if he or she is a director of Avenue Therapeutics and is acting in his or her capacity as director,
and do not apply to officers of Avenue Therapeutics who are not directors. Additionally, our Certificate of Incorporation provides that,
to the fullest extent permitted by law, we renounce any interest or expectancy in a transaction or matter that may be a corporate opportunity
for us if it was presented to, or acquired, created or developed by, or which otherwise comes into the possession of, (i) any director
on our board of directors who is not an employee of the Company or any of its subsidiaries, or (ii) any holder of our Class A
Preferred Stock or any affiliate or other related person of any such holder, other than someone who is an employee of the Company or any
of its subsidiaries, and no person shall have any duty to present such corporate opportunity to us and will not be liable to us for pursuing
or acquiring such opportunity, or referring such opportunity to a third party.
Indemnification
of Directors, Officers and Employees. Our By-Laws require us to indemnify any person who was or is a party or is threatened
to be made a party to, or was otherwise involved in, a legal proceeding by reason of the fact that he or she is or was a director, officer
or employee of Avenue Therapeutics or, while a director, officer or employee of Avenue Therapeutics, is or was serving at our request
in a fiduciary capacity with another enterprise (including any corporation, partnership, limited liability company, joint venture, trust,
association or other unincorporated organization or other entity and any employee benefit plan, to the fullest extent authorized by the
DGCL, as it exists or may be amended, against all expense, liability and loss (including attorneys’ fees, judgments, fines, U.S.
Employee Retirement Income Security Act of 1974, as amended, excise taxes or penalties and amounts paid in settlement by or on behalf
of such person) actually and reasonably incurred in connection with such service. We are authorized under our By-Laws to carry directors’
and officers’ insurance protecting us, any director, officer or employee of ours or, against any expense, liability or loss, whether
or not we have the power to indemnify the person under the DGCL. We may, to the extent authorized from time to time, indemnify any of
our agents to the fullest extent permitted with respect to directors, officers and employees in our By-Laws.
The limitation of liability and indemnification
provisions in our Certificate of Incorporation and By-Laws may discourage stockholders from bringing a lawsuit against our directors for
breach of fiduciary duty. These provisions also may reduce the likelihood of derivative litigation against our directors and officers,
even though such an action, if successful, might otherwise benefit us and our stockholders. By its terms, the indemnification provided
for in our By-Laws is not exclusive of any other rights that the indemnified party may be or become entitled to under any law, agreement,
vote of stockholders or directors, provisions of our Certificate of Incorporation or By-Laws or otherwise. Any amendment, alteration or
repeal of our By-Laws’ indemnification provisions is, by the terms of our By-Laws, prospective only and will not adversely affect
the rights of any indemnity in effect at the time of any act or omission occurring prior to such amendment, alteration or repeal.
Warrants to be Issued in this Offering
The following summary of certain terms and
provisions of the warrants included in the units offered hereby is not complete and is subject to, and qualified in its entirety by the
provisions of the form of Warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective
investors should carefully review the terms and provisions set forth in the form of Warrant.
Exercisability.
The warrants are exercisable immediately and at any time up to the date that is five years after their original issuance. The warrants
will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any
time a registration statement registering the offer and sale of the shares of Common Stock underlying the warrants under the Securities
Act is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act is available
for the issuance of such shares, by payment in full in immediately available funds for the number of shares of Common Stock purchased
upon such exercise. If a registration statement registering the offer and sale of the shares of Common Stock underlying the warrants under
the Securities Act is not effective or available and an exemption from registration under the Securities Act is not available for the
issuance of such shares, the holder may elect to exercise the warrant through a cashless exercise, in which case the holder would receive
upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the warrant. No fractional
shares of Common Stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will pay the holder
an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise
Limitation. A holder will not have the right to exercise any portion of the warrant if the holder (together with its affiliates
and certain related parties) would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding immediately
after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. However,
any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such
percentage shall not be effective until 61 days following notice from the holder to us.
Exercise
Price. The exercise price per whole share of Common Stock purchasable upon exercise of the warrants is equal to $
(100% of the public offering price per unit). The exercise price is subject to appropriate adjustment in the event of certain stock dividends
and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any
distributions of assets, including cash, stock or other property to our stockholders.
Transferability.
Subject to applicable laws, the warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange
Listing. We do not intend to list the warrants on any securities exchange or nationally recognized trading system.
Warrant
Agent. The warrants will be issued in registered form under a warrant agency agreement between VStock Transfer, LLC, as warrant
agent, and us. The warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian
on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed
by DTC.
Fundamental
Transactions. In the event of a fundamental transaction, as described in the warrants and generally including any
reorganization, recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or
substantially all of our properties or assets, our consolidation or merger with or into another person, or any person or group,
other than Fortress, becoming the beneficial owner of 50% of the voting power represented by our outstanding capital stock, the
holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other
property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction.
Rights
as a Stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our
Common Stock, the holder of a warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights,
until the holder exercises the warrant.
Governing
Law. The warrants and the warrant agency agreement are governed by New York law.
Pre-funded Warrants to be Issued in this
Offering
The following summary of certain terms and
provisions of the pre-funded warrants included in the pre-funded units offered hereby is not complete and is subject to, and qualified
in its entirety by the provisions of the form of pre-funded warrant, which is filed as an exhibit to the registration statement of which
this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of pre-funded
warrant.
Exercisability.
The pre-funded warrants are exercisable immediately and may be exercised at any time, and from time
to time, until the pre-funded warrants are exercised in full. The pre-funded warrants will be exercisable, at the option of each
holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering
the offer and sale of the shares of Common Stock underlying the pre-funded warrants under the Securities Act is effective and available
for the issuance of such shares, or an exemption from registration under the Securities Act is available for the issuance of such shares,
by payment in full in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration
statement registering the offer and sale of the shares of Common Stock underlying the pre-funded warrants under the Securities Act is
not effective or available and an exemption from registration under the Securities Act is not available for the issuance of such shares,
the holder may elect to exercise the pre-funded warrants through a cashless exercise, in which case the holder would receive upon such
exercise the net number of shares of Common Stock determined according to the formula set forth in the warrant. No fractional shares of
Common Stock will be issued in connection with the exercise of a pre-funded warrants. In lieu of fractional shares, we will pay the holder
an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise
Limitation. A holder will not have the right to exercise any portion of the pre-funded warrant if the holder (together with
its affiliates and certain related parties) would beneficially own in excess of 4.99% of the number of shares of our Common Stock outstanding
immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded
warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any
increase in such percentage shall not be effective until 61 days following notice from the holder to us.
Exercise
Price. The exercise price per whole share of Common Stock purchasable upon exercise of the pre-funded warrants is $0.0001.
The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock
combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including cash,
stock or other property to our stockholders.
Transferability.
Subject to applicable laws, the pre-funded warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange
Listing. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system.
Warrant Agent. The pre-funded warrants
will be issued in registered form under a warrant agency agreement between VStock Transfer, LLC, as warrant agent, and us. The pre-funded
warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of
The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental
Transactions. In the event of a fundamental transaction, as described in the pre-funded warrants and generally including
any reorganization, recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or
substantially all of our properties or assets, our consolidation or merger with or into another person, or any person or group,
other than Fortress, becoming the beneficial owner of 50% of the voting power represented by our outstanding capital stock, the
holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of
securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior
to such fundamental transaction.
Rights
as a Stockholder. Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of
shares of our Common Stock, the holder of a pre-funded warrant does not have the rights or privileges of a holder of our Common Stock,
including any voting rights, until the holder exercises the pre-funded warrant.
Governing
Law. The pre-funded warrants and the warrant agency agreement are governed by New York law.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a discussion of certain material U.S. federal income
tax consequences of the acquisition, ownership and disposition of our shares of common units (each consisting of one share of our common
stock and one warrant to purchase one share of our common stock) and our pre-funded units (each consisting of one pre-funded warrant to
purchase one share of our common stock and one warrant to purchase one share of our common stock), which we refer to as our securities,
that are purchased in this offering by U.S. Holders (as defined below) and Non-U.S. Holders (as defined below). Because the components
of a common unit and a pre-funded unit are generally separable at the option of the holder, the holder of a common unit or pre-funded
unit generally should be treated, for U.S. federal income tax purposes, as the owner of the underlying share of our common stock and one
warrant to purchase one share of our common stock in the case of a common unit and one pre-funded warrant and one warrant to purchase
one share of our common stock in the case of a pre-funded unit. As a result, the discussion below with respect to holders of shares of
our common stock, pre-funded warrants and warrants should also apply to holders of common units or pre-funded units (as the deemed owners
of the underlying common stock, pre-funded warrants and warrants that constitute the units).
This discussion applies only to securities that are held as capital
assets for U.S. federal income tax purposes and is applicable only to initial holders who are receiving our securities in this offering.
This discussion is a summary only and does not describe all of the
tax consequences that may be relevant to you in light of your particular circumstances, including but not limited to the alternative minimum
tax, the Medicare tax on certain investment income and the different consequences that may apply if you are subject to special rules that
apply to certain types of investors (such as the effects of Section 451 of the federal income tax code (the “Code”),
including but not limited to:
| · | bank and other financial institutions or financial services entities; |
| · | retirement plans, individual retirement accounts or other tax-deferred accounts; |
| · | governments or agencies or instrumentalities thereof; |
| · | regulated investment companies; |
| · | “controlled foreign corporations,” “passive foreign investment companies,” “qualified foreign pension
funds,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| · | real estate investment trusts; |
| · | expatriates or former long-term residents of the United States; |
| · | persons that actually or constructively own five percent or more of our voting shares; |
| · | taxpayers subject to a mark-to-market method of accounting rules; |
| · | persons holding the securities as part of a “straddle,” constructive sale, hedge, conversion or other integrated or similar
transaction; |
| · | U.S. holders (as defined below) whose functional currency is not the U.S. dollar; |
| · | persons subject to alternative minimum tax; |
| · | partnerships or other pass-through entities for U.S. federal income tax purposes and any beneficial owners of such entities; |
| · | tax-exempt entities; and |
| · | persons that acquired our securities pursuant to an exercise of employee share options, in connection with employee share incentive
plans or otherwise as compensation or in connection with services. |
This discussion is based on the Code, and administrative pronouncements,
judicial decisions and final, temporary and proposed Treasury regulations as of the date hereof, which are subject to change, possibly
on a retroactive basis, and changes to any of which subsequent to the date of this prospectus may affect the tax consequences described
herein. This discussion does not address any aspect of state, local or non-U.S. taxation, or any U.S. federal taxes (e.g., gift and estate
taxes) other than income taxes.
We have not sought, and will not seek, a ruling from the IRS as to
any U.S. federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be
upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions
will not adversely affect the accuracy of the statements in this discussion. You are urged to consult your tax advisor with respect to
the application of U.S. federal tax laws to your particular situation, as well as any tax consequences arising under the laws of any state,
local or foreign jurisdiction.
This discussion does not consider the tax treatment of partnerships
or other pass-through entities or persons who hold our securities through such entities. If a partnership (or other entity or arrangement
classified as a partnership or other pass-through entity for United States federal income tax purposes) is the beneficial owner of our
securities, the United States federal income tax treatment of a partner or member in the partnership or other pass-through entity generally
will depend on the status of the partner or member and the activities of the partnership or other pass-through entity. If you are a partner
or member of a partnership or other pass-through entity holding our securities, we urge you to consult your own tax advisor.
THIS DISCUSSION IS ONLY A SUMMARY OF CERTAIN UNITED STATES FEDERAL
INCOME TAX CONSIDERATIONS ASSOCIATED WITH THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES. EACH PROSPECTIVE INVESTOR IN OUR
SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION,
OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY UNITED STATES FEDERAL NON-INCOME, STATE,
LOCAL, AND NON-U.S. TAX LAWS.
Allocation of Purchase Price and Characterization of a Unit
No statutory, administrative or judicial authority directly addresses
the treatment of a unit or instruments similar to a unit for U.S. federal income tax purposes, and therefore, that treatment is not entirely
clear. The acquisition of a common unit or pre-funded unit should be treated for U.S. federal income tax purposes as the acquisition of
one share of our common stock and one warrant in the case of a common unit and one pre-funded warrant and one warrant in the case of a
pre-funded unit, and we intend to treat the acquisition of a unit in this manner. For U.S. federal income tax purposes, each holder of
a unit must allocate the purchase price paid by such holder for such unit among the underlying securities based on the relative fair market
value of each at the time of issuance. Under U.S. federal income tax law, each investor must make its own determination of such value
based on all the relevant facts and circumstances. Therefore, we strongly urge each investor to consult its tax advisor regarding the
determination of value for these purposes. The price allocated to each share of our common stock, warrants and/or pre-funded warrants
should constitute the holder’s initial tax basis in such share, warrant and/or pre-funded warrant, respectively. Any disposition
of a Unit should be treated for U.S. federal income tax purposes as a disposition of the share of our common stock and warrant or pre-funded
warrant and warrant comprising the Unit, and the amount realized on the disposition should be allocated among the underlying securities
based on their respective relative fair market values at the time of disposition.
The foregoing treatment of the securities and a holder’s purchase
price allocation are not binding on the IRS or the courts. Because there are no authorities that directly address instruments that are
similar to the units, no assurance can be given that the IRS or the courts will agree with the characterization described above or the
discussion below. Accordingly, each prospective investor is urged to consult its tax advisor regarding the tax consequences of an investment
in a unit (including alternative characterizations of a unit). The balance of this discussion assumes that the characterization of the
units described above is respected for U.S. federal income tax purposes.
U.S. Holders
This section applies to you if you are a “U.S. holder.”
A U.S. holder is a beneficial owner of our shares of Common Stock who or that is, for U.S. federal income tax purposes:
| · | an individual who is a citizen or resident of the United States; |
| · | a corporation (or other entity taxable as a corporation) that is created or organized (or treated as created or organized) in or under
the laws of the United States, any state thereof or the District of Columbia; or |
| · | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
| · | a trust, if (i) a court within the United States is able to exercise primary supervision over the administration of the trust
and one or more U.S. persons (as defined in the Code) have authority to control all substantial decisions of the trust or (ii) it
has a valid election in effect under Treasury Regulations to be treated as a U.S. person. |
Taxation
of Distributions. If we pay distributions in cash or other property (other than certain distributions of our stock or rights
to acquire our stock) to U.S. holders of shares of our Common Stock, such distributions generally will constitute dividends for U.S. federal
income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax
principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will first
be applied against and reduce (but not below zero) the U.S. holder’s adjusted tax basis in our Common Stock. Any remaining excess
will be treated as gain realized on the sale or other disposition of the Common Stock and will be treated as described under “U.S.
Holders — Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Common Stock” below.
Dividends we pay to a U.S. holder that is a taxable corporation generally
will qualify for the dividends received deduction if the requisite holding period is satisfied. With certain exceptions (including, but
not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain
holding period requirements are met, dividends we pay to a non-corporate U.S. holder may constitute “qualified dividends”
that will be subject to tax at the maximum tax rate accorded to long-term capital gains. If the holding period requirements are not satisfied,
then a corporation may not be able to qualify for the dividends received deduction and would have taxable income equal to the entire dividend
amount, and non-corporate holders may be subject to tax on such dividend at regular ordinary income tax rates instead of the preferential
rate that applies to qualified dividend income.
Gain
or Loss on Sale, Taxable Exchange or Other Taxable Disposition of our Securities. Upon a sale or other taxable disposition
of our shares of Common Stock, warrants or pre-funded warrants, a U.S. holder generally will recognize capital gain or loss in an amount
equal to the difference between the amount realized and the U.S. holder’s adjusted tax basis in such shares of Common Stock, warrants
or pre-funded warrants. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding
period for the Common Stock, warrants or pre-funded warrants so disposed of exceeds one year. If the holding period requirements are not
satisfied, any gain on a sale or taxable disposition of our securities would be subject to short-term capital gain treatment and would
be taxed at regular ordinary income tax rates. Long-term capital gains recognized by non-corporate U.S. holders will be eligible to be
taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Generally, the amount of gain or loss recognized by a U.S. holder is
an amount equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in
such disposition and (ii) the U.S. holder’s adjusted tax basis in its shares of Common Stock, warrants or pre-funded warrants
disposed. A U.S. holder’s adjusted tax basis in its shares of Common Stock, warrants or pre-funded warrants generally will equal
the U.S. holder’s acquisition cost (that is, the portion of the purchase price of a Unit allocated to a share of our common stock,
warrant or pre-funded warrant, as described above under “— Allocation of Purchase Price and Characterization of a Unit”)
reduced, in the case of a share of Common Stock, by any prior distributions treated as a return of capital. .
Information
Reporting and Backup Withholding. In general, information reporting requirements may apply to dividends paid to a U.S. holder
and to the proceeds of the sale or other disposition of our securities, unless the U.S. holder is an exempt recipient. Backup withholding
may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has
been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Any amounts withheld under the backup withholding rules generally
should be allowed as a refund or a credit against a U.S. holder’s U.S. federal income tax liability provided the required information
is timely furnished to the IRS.
Non-U.S. Holders
This section applies to you if you are a “Non-U.S. holder.”
As used herein, the term “Non-U.S. holder” means a beneficial owner of our common units or pre-funded units who is not a U.S.
Holder or any other person that is for U.S. federal income tax purposes:
| · | a non-resident alien individual (other than certain former citizens and residents of the U.S. subject to U.S. tax as expatriates), |
| · | a foreign corporation, or |
| · | an estate or trust that is not a U.S. holder. |
The term “Non-U.S. Holder” generally does not include a
U.S. Holder or a partnership or other entity classified as a partnership for U.S. federal income tax purposes and does not include an
individual who is present in the United States for 183 days or more in the taxable year of disposition of the securities. If you are such
an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the acquisition, ownership or
sale or other disposition of our securities.
Taxation
of Distributions. In general, any distributions we make to a Non-U.S. holder of shares of our Common Stock, to the extent paid
out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends
for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the Non-U.S. holder’s conduct
of a trade or business within the United States, we will be required to withhold tax from the gross amount of the dividend at a rate of
30%, unless such Non-U.S. holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides
proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting
a dividend will be treated first as reducing (but not below zero) the Non-U.S. holder’s adjusted tax basis in its shares of our
Common Stock and, to the extent such distribution exceeds the Non-U.S. holder’s adjusted tax basis, as gain realized from the sale
or other disposition of the Common Stock, which will be treated as described under “Non-U.S. Holders — Gain on Sale, Taxable
Exchange or Other Taxable Disposition of Our Securities” below. If we are unable to determine, at a time reasonably close to the
date of payment of a distribution on our Common Stock, what portion, if any, of the distribution will constitute a dividend, then we may
withhold U.S. federal income tax on the basis of assuming that the full amount of the distribution will be a dividend. If we or another
withholding agent apply over-withholding, a non-U.S. holder may be entitled to a refund or credit of any excess tax withheld by timely
filing an appropriate claim with the IRS. In addition, if we determine that we are or are likely to be classified as a “United States
real property holding corporation” (see “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition
of Our Securities” below), we will withhold 15% of any distribution that exceeds our current and accumulated earnings and profits,
including a distribution in redemption of shares of our Common Stock.
The withholding tax does not apply to dividends paid to a Non-U.S.
holder who provides a Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. holder’s conduct
of a trade or business within the United States. Instead, the effectively connected dividends will be subject to regular U.S. income tax
as if the Non-U.S. holder were a U.S. resident, subject to an applicable income tax treaty providing otherwise. A Non-U.S. corporation
receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30%
(or a lower treaty rate).
Any documentation provided to an applicable withholding agent may need
to be updated in certain circumstances. The certification requirements described above also may require a non-U.S. holder to provide its
U.S. taxpayer identification number.
Gain
on Sale, Taxable Exchange or Other Taxable Disposition of Common Stock. A Non-U.S. holder generally will not be subject to
U.S. federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of our Common
Stock, warrants or pre-funded warrants, in each case without regard to whether such securities were held as part of a unit, unless:
| · | the gain is effectively connected with the conduct of a trade or business by the Non-U.S. holder within the United States (and, under
certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. holder); |
| · | the non-U.S. holder is a nonresident alien individual who is present in the United States for a period or periods aggregating 183
days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject
to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the amount by which the non-U.S. holder’s
capital gains allocable to U.S. sources exceed capital losses allocable to U.S. sources during the taxable year of the disposition (without
taking into account any capital loss carryovers); or |
| · | we are or have been a “U.S. real property holding corporation” for U.S. federal income tax purposes at any time during
the shorter of the five-year period ending on the date of disposition or the period that the Non-U.S. holder held our Common Stock, and,
in the case where shares of our Common Stock are regularly traded on an established securities market, the Non-U.S. holder has owned,
directly or constructively, more than 5% of our Common Stock at any time within the shorter of the five-year period preceding the disposition
or such Non-U.S. holder’s holding period for the shares of our Common Stock. There can be no assurance that our Common Stock will
be treated as regularly traded on an established securities market for this purpose. Generally, a corporation is a U.S. real property
holding corporation if the fair market value of its U.S. real property interests, as defined in the Code and applicable U.S. Treasury
Regulations, equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets
used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real
property holding corporation for U.S. federal income tax purposes, or that we are likely to become one in the future. These rules may
be modified for Non-U.S. Holders of warrants or pre-funded warrants. If we are or have been a “United States real property holding
corporation” and you own warrants or pre-funded warrants, you are urged to consult your own tax advisor regarding the application
of these rules. |
Unless an applicable treaty provides otherwise, gain described in the
first bullet point above will be subject to tax at generally applicable U.S. federal income tax rates as if the Non-U.S. holder were a
U.S. resident. Any gains described in the first bullet point above of a Non-U.S. holder that is a foreign corporation may also be subject
to an additional “branch profits tax” at a 30% rate (or lower treaty rate).
If the third bullet point above applies to a Non-U.S. holder, gain
recognized by such holder on the sale, exchange or other disposition of our Common Stock, warrants or pre-funded warrants, will generally
be subject to tax at applicable U.S. federal income tax rates as if the Non-U.S. Holder were a U.S. resident. In addition, a buyer of
our Common Stock, warrants or pre-funded warrants from any such holder may be required to withhold U.S. income tax at a rate of 15% of
the amount realized upon such disposition if our Common Stock is not treated as regularly traded on an established securities market.
We cannot determine whether we will be a United States real property holding corporation in the future. In general, we would be classified
as a United States real property holding corporation if the fair market value of our “United States real property interests”
equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other assets used or held
for use in a trade or business, as determined for U.S. federal income tax purposes.
Information
Reporting and Backup Withholding. Information returns will be filed with the IRS in connection with payments of dividends and
the proceeds from a sale or other disposition of our shares of Common Stock, warrants or pre-funded warrants. A Non-U.S. holder may have
to comply with certification procedures to establish that it is not a United States person in order to avoid information reporting and
backup withholding requirements. The certification procedures required to claim a reduced rate of withholding under a treaty will satisfy
the certification requirements necessary to avoid the backup withholding as well. The amount of any backup withholding from a payment
to a Non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder
to a refund, provided that the required information is timely furnished to the IRS.
FATCA
Withholding Taxes. Provisions commonly referred to as “FATCA” impose withholding of 30% on payments of dividends
(including constructive dividends) on our Common Stock to “foreign financial institutions” (which is broadly defined for this
purpose and in general includes investment vehicles) and certain other Non-U.S. entities unless various U.S. information reporting and
due diligence requirements (generally relating to ownership by U.S. persons of interests in or accounts with those entities) have been
satisfied by, or an exemption applies to, the payee (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E).
If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution will be entitled to a refund of any amounts
withheld by filing a U.S. federal income tax return (which may entail significant administrative burden). Foreign financial institutions
located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under certain circumstances, a Non-U.S. holder might be eligible for refunds or credits of such withholding taxes, and a Non-U.S. holder
might be required to file a U.S. federal income tax return to claim such refunds or credits. Prospective investors should consult their
tax advisers regarding the effects of FATCA on their investment in our securities.
The preceding discussion of material U.S. federal tax considerations
is for general information only. It is not tax advice. You should consult your own tax advisors regarding the particular U.S. federal,
state, local and non-U.S. tax consequences of purchasing, holding and disposing of our Common Stock, including the consequences of any
proposed changes in applicable laws.
PLAN OF DISTRIBUTION
We are offering up to 21,352,313 units,
each consisting of one share of Common Stock and one warrant to purchase one share of Common Stock, for gross proceeds of up to $12.0
million before deduction of placement agent commissions and offering expenses, in a best-efforts offering. There is no minimum amount
of proceeds that is a condition to closing of this offering. The actual amount of gross proceeds, if any, in this offering could vary
substantially from the gross proceeds from the sale of the maximum amount of securities being offered in this prospectus.
Pursuant to a placement agency agreement,
dated as of , 2023, we have engaged Maxim Group LLC and Lake Street Capital Markets, LLC to act as our exclusive placement agents to solicit
offers to purchase the securities offered by this prospectus. The placement agents are not purchasing or selling any securities, nor are
they required to arrange for the purchase and sale of any specific number or dollar amount of securities, other than to use their “reasonable
best efforts” to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of securities being
offered. We will enter into a securities purchase agreement directly with certain of the institutional investors, at the investor’s
option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely
on this prospectus in connection with the purchase of our securities in this offering. The placement agents may engage one or more subagents
or selected dealers in connection with this offering.
The placement agency agreement provides that
the placement agents’ obligations are subject to conditions contained in the placement agency agreement.
We will deliver the securities being issued
to the investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. We expect to deliver
the securities being offered pursuant to this prospectus on , 2023.
Placement Agent Fees, Commissions and Expenses
Upon the closing of this offering, we will
pay the placement agents a cash transaction fee equal to 8% of the aggregate gross cash proceeds to us from the sale of the securities
in the offering (reduced to 4% of aggregate gross cash proceeds received from our affiliates). In addition, we will reimburse the placement
agents for their out-of-pocket expenses incurred in connection with this offering, including the fees and expenses of the counsel for
the placement agents, up to $100,000.
The following table shows the public offering
price, placement agent fees and proceeds, before expenses, to us, assuming the purchase of all the securities we are offering.
| | |
Per Unit | | |
Per Pre- Funded Unit | | |
Total | |
| Public Offering Price | |
$ | | | |
$ | | | |
$ | | |
| Placement agent fees | |
$ | | | |
$ | | | |
$ | | |
| Proceeds, before expenses, to us | |
$ | | | |
$ | | | |
$ | | |
We estimate that the total expenses of
the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding placement
agent fees, will be approximately $310,000, all of which are payable by us. This figure includes the placement agents’ accountable
expenses, including, but not limited to, legal fees for placement agents’ legal counsel, that we have agreed to pay at the closing
of the offering up to an aggregate expense reimbursement of $100,000.
The Financial Industry Regulatory Authority, Inc.
has reviewed the proposed terms and arrangements of the compensation to be paid to the placement agents in connection with this offering.
Lock-Up Agreements
We, each of our officers and directors and
any other holders of 10% or more of our outstanding shares of Common Stock as of the date of this prospectus have agreed, subject to certain
exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any of our
shares or other securities convertible into or exercisable or exchangeable for our shares of Common Stock for a period of 180 days after
this offering is completed without the prior written consent of the placement agents.
The placement agents may in their sole discretion
and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the lock-up
period. When determining whether or not to release shares from the lock-up agreements, the placement agents will consider, among other
factors, the security holder’s reasons for requesting the release, the number of shares for which the release is being requested
and market conditions at the time.
Indemnification
We have agreed to indemnify the placement
agents against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the placement agents
may be required to make for these liabilities.
Determination of Offering
Price and Exercise Price
The actual public offering price of the securities
we are offering, and the exercise price of the warrants and pre-funded warrants included in the units and the pre-funded units that we
are offering, were negotiated between us and the investors in the offering based on the trading of our Common Stock prior to the offering,
among other things. Other factors considered in determining the public offering price of the securities we are offering, as well as the
exercise price of the warrants included in the units and pre-funded warrants that we are offering include our history and prospects, the
stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment
of our management, the general conditions of the securities markets at the time of the offering and such other factors as were deemed
relevant.
Regulation M
The placement agents each may be deemed to
be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit
realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions
under the Securities Act. As an underwriter, each placement agent would be required to comply with the requirements of the Securities
Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and
regulations may limit the timing of purchases and sales of our securities by the placement agents acting as principal. Under these rules and
regulations, the placement agents (i) may not engage in any stabilization activity in connection with our securities and (ii) may
not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted
under the Exchange Act, until it has completed its participation in the distribution.
Electronic Distribution
A prospectus in electronic format may be made
available on a website maintained by the placement agents. In connection with the offering, the placement agents or selected dealers may
distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe®
PDF will be used in connection with this offering.
Other than the prospectus in electronic format,
the information on each of the placement agents’ websites and any information contained in any other website maintained by the placement
agents is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or
endorsed by us or either placement agent in its capacity as placement agent and should not be relied upon by investors.
Certain Relationships
Each placement agent and its affiliates have
and may in the future provide, from time to time, investment banking and financial advisory services to us in the ordinary course of business,
for which they may receive customary fees and commissions.
Selling Restrictions
Canada.
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors,
as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario),
and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant
Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the
prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada
may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto) contains
a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed
by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions
of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting
Conflicts (NI 33-105), the placement agents are not required to comply with the disclosure requirements of NI 33-105 regarding
placement agents conflicts of interest in connection with this offering.
European
Economic Area . In relation to each Member State of the European Economic Area which has implemented the Prospectus
Directive (each, a “Relevant Member State”) an offer to the public of any securities may not be made in that Relevant Member
State, except that an offer to the public in that Relevant Member State of any securities may be made at any time under the following
exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| |
● |
to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| |
|
|
| |
● |
to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives for any such offer; or |
| |
|
|
| |
● |
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by us or any placement agents of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of this provision, the expression an “offer
to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of
sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase any
securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State,
the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive,
to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State,
and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Israel.
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed
with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed only to, and is directed
only at, and any offer of the shares is directed only at, investors listed in the first addendum, or the Addendum, to the Israeli Securities
Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment
advisors, members of the Tel Aviv Stock Exchange, placement agents, venture capital funds, entities with equity in excess of NIS 50 million
and “qualified individuals”, each as defined in the Addendum (as it may be amended from time to time), collectively referred
to as qualified investors (in each case purchasing for their own account or, where permitted under the Addendum, for the accounts of their
clients who are investors listed in the Addendum). Qualified investors will be required to submit written confirmation that they fall
within the scope of the Addendum, are aware of the meaning of same and agree to it.
United
Kingdom. Each placement agent has represented and agreed that:
| |
● |
it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (the FSMA) received by it in connection with the issue or sale of the securities in circumstances in which Section 21(1) of the FSMA does not apply to us; and |
| |
|
|
| |
● |
it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom. |
Switzerland.
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the SIX) or on any other stock
exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for
issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses
under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in
Switzerland. Neither this document nor any other offering or marketing material relating to the securities or the offering may be publicly
distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material
relating to the offering, or the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular,
this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority
FINMA, and the offer of securities has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes
(CISA). Accordingly, no public distribution, offering or advertising, as defined in CISA, its implementing ordinances and notices, and
no distribution to any non-qualified investor, as defined in CISA, its implementing ordinances and notices, shall be undertaken in or
from Switzerland, and the investor protection afforded to acquirers of interests in collective investment schemes under CISA does not
extend to acquirers of securities.
Australia.
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities
and Investments Commission (ASIC), in relation to the offering.
This prospectus does not constitute a prospectus, product disclosure
statement or other disclosure document under the Corporations Act 2001 (the Corporations Act) and does not purport to include the information
required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the securities may only be made to persons
(the Exempt Investors) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act),
“professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more
exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the securities without disclosure to investors
under Chapter 6D of the Corporations Act.
The securities applied for by Exempt Investors in Australia must not
be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where
disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the
Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations
Act. Any person acquiring securities must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take
account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities
recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information
in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice
to Prospective Investors in the Cayman Islands. No invitation, whether directly or indirectly, may be made to the public
in the Cayman Islands to subscribe for our securities.
Taiwan.
The securities have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities
laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes
an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory
Commission of Taiwan. No person or entity in Taiwan has been authorized to offer, sell, give advice regarding or otherwise intermediate
the offering and sale of the securities in Taiwan.
Notice
to Prospective Investors in Hong Kong. The contents of this prospectus have not been reviewed by any regulatory authority
in Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this
prospectus, you should obtain independent professional advice. Please note that (i) our shares may not be offered or sold in Hong
Kong, by means of this prospectus or any document other than to “professional investors” within the meaning of Part I
of Schedule 1 of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) (SFO) and any rules made thereunder, or in other
circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32,
Laws of Hong Kong) (CO) or which do not constitute an offer or invitation to the public for the purpose of the CO or the SFO, and (ii) no
advertisement, invitation or document relating to our shares may be issued or may be in the possession of any person for the purpose of
issue (in each case whether in Hong Kong or elsewhere) which is directed at, or the contents of which are likely to be accessed or read
by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to the shares
which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the
meaning of the SFO and any rules made thereunder.
Notice
to Prospective Investors in the People’s Republic of China. This prospectus may not be circulated or distributed
in the PRC and the shares may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly
to any resident of the PRC except pursuant to applicable laws, rules and regulations of the PRC. For the purpose of this paragraph
only, the PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
LEGAL MATTERS
McGuireWoods LLP, Charlotte, North Carolina, will pass upon the validity
of the securities we are offering by this prospectus. The placement agents are being represented in connection with this offering by Ellenoff
Grossman & Schole LLP.
EXPERTS
The consolidated financial statements of Avenue Therapeutics, Inc.
as of December 31, 2022 and for the year ended December 31, 2022, have been incorporated by reference herein and in the registration
statement in reliance on the report of KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and
upon the authority of said firm as experts in auditing and accounting. The audit report covering the December 31, 2022 consolidated
financial statements contains an explanatory paragraph that states the Company has incurred substantial operating losses since its inception
and expects to continue to incur significant operating losses for the foreseeable future that raise substantial doubt about the Company’s
ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the
outcome of that uncertainty.
The financial statements of Avenue Therapeutics, Inc. as of
December 31, 2021 and for the year ended December 31, 2021 incorporated by reference in this prospectus and in the
registration statement have been so incorporated in reliance on the report of BDO USA, LLP (n/k/a BDO USA, P.C.), an independent
registered public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and
accounting. The report on the financial statements contains an explanatory paragraph regarding the Company's ability to continue as
a going concern.
The financial statements of Baergic Bio, Inc. as of December 31,
2021 and 2020 and for each of the years in the two-year period ended December 31, 2021, have been incorporated by reference herein
and in the registration statement in reliance on the report of KPMG LLP, independent registered public accounting firm, incorporated by
reference herein, and upon the authority of said firm as experts in auditing and accounting. The audit report covering the December 31,
2021 financial statements contains an emphasis of matter paragraph that states that the Company’s recurring losses from operations
raise substantial doubt about the entity’s ability to continue as a going concern. The financial statements do not include any adjustments
that might result from the outcome of that uncertainty.
WHERE YOU CAN FIND MORE INFORMATION
We file reports and proxy statements with the SEC. These filings include
our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and proxy statements on Schedule
14A, as well as any amendments to those reports and proxy statements, which are available free of charge through our website as soon as
reasonably practicable after we file them with, or furnish them to, the SEC. Our Internet website address is www.avenuetx.com. Our website
and the information contained on, or that can be accessed through, the website will not be deemed to be incorporated by reference in,
and are not considered part of, this prospectus. You should not rely on any such information in making your decision whether to purchase
our securities. The SEC also maintains a website at www.sec.gov that contains reports, proxy and information statements and other information
regarding us and other issuers that file electronically with the SEC.
We have filed with the SEC a registration statement on Form S-1
under the Securities Act relating to the securities being offered by this prospectus. This prospectus, which constitutes part of that
registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules
which are part of the registration statement. For further information about us and the securities offered, see the registration statement
and the exhibits and schedules thereto. Statements contained in this prospectus regarding the contents of any contract or any other document
to which reference is made are not necessarily complete, and, in each instance where a copy of a contract or other document has been filed
as an exhibit to the registration statement, reference is made to the copy so filed, each of those statements being qualified in all respects
by the reference.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this
prospectus the information we file with the SEC in other documents, which means that we can disclose important information to you by referring
you to those documents instead of having to repeat the information in this prospectus. The information incorporated by reference is considered
to be part of this prospectus, and later information that we file with the SEC will automatically update and supersede such information.
We incorporate by reference the documents listed below and any future information filed (rather than furnished) with the SEC under Sections
13(a), 13(c), 14 or 15(d) of the Exchange Act between the date of this prospectus and the date all securities to which this prospectus
relates have been sold or the offering is otherwise terminated and also between the date of the initial registration statement and prior
to effectiveness of the registration statement, provided, however, that we are not incorporating any information furnished under Item
2.02 or Item 7.01 of any Current Report on Form 8-K:
| · | our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2023, filed with the SEC on May 12, 2023 and for
the quarter ended June 30, 2022, filed with the SEC on August 11, 2023; and |
| · | our Current Reports
on Form 8-K and Form 8-K/A filed with the SEC on January 25,
2023, February 1,
2023, February 3,
2023, February
10, 2023, March 2,
2023, March 8,
2023, March 30,
2023, April 17,
2023, May 12,
2023, May 22,
2023, June 26,
2023, July 5,
2023, July 21,
2023, August 10,
2023, September 8, 2023, September 27 and September 28, 2023. |
We will furnish without charge to you a copy of any or all of the documents
incorporated by reference, including exhibits to these documents, upon written or oral request. Direct your written request to: Corporate
Secretary, Avenue Therapeutics, Inc., 1111 Kane Concourse, Suite 301, Bay Harbor Islands, Florida 33154, or (781) 652-4500.
A statement contained in a document incorporated by reference into
this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained
in this prospectus, any prospectus supplement or in any other subsequently filed document which is also incorporated in this prospectus
modifies or replaces such statement. Any statements so modified or superseded shall not be deemed, except as so modified or superseded,
to constitute a part of this prospectus.

Up to 21,352,313 Units, Each Consisting
of One Share of Common Stock and One Warrant to Purchase One Share of Common Stock
or
Up to 21,352,313 Pre-Funded Units,
Each Consisting of One Pre-funded Warrant to Purchase One Share of Common Stock and One Warrant to Purchase One Share of Common Stock
Up to 21,352,313 Shares of Common Stock
Underlying the Warrants
Up to 21,352,313 Shares of Common Stock
Underlying the Pre-funded Warrants
PROSPECTUS
, 2023
Placement Agents
| Maxim
Group LLC |
Lake
Street |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table indicates the expenses to be incurred in connection
with the offering described in this registration statement, other than placement agent fees, all of which will be paid by us. All amounts
are estimates except the SEC registration fee and the FINRA filing fee.
| | |
Amount | |
| SEC registration fee | |
$ | 2,644.80 | |
| FINRA filing fee | |
$ | 4,100.00 | |
| Accounting fees and expenses | |
$ | 200,000.00 | |
| Legal fees and expenses | |
$ | 110,000.00 | |
| Miscellaneous fees and expenses | |
$ | 0.00 | |
| Placement agents’ reimbursable expenses | |
$ | 25,000.00 | |
| Total expenses | |
$ | 341,744.80 | |
Item 14. Indemnification of Directors and Officers
Under the General Corporation Law of the State of Delaware (“DGCL”),
a corporation may include provisions in its certificate of incorporation that will relieve its directors of monetary liability for breaches
of their fiduciary duty to the corporation, except under certain circumstances, including a breach of the director’s duty of loyalty,
acts or omissions of the director not in good faith or which involve intentional misconduct or a knowing violation of law, the approval
of an improper payment of a dividend or an improper purchase by the corporation of stock or any transaction from which the director derived
an improper personal benefit. The Company’s Amended and Restated Certificate of Incorporation eliminates the personal liability
of directors to the Company or its stockholders for monetary damages for breach of fiduciary duty as a director with certain limited exceptions
set forth in the DGCL.
Section 145 of the DGCL grants to corporations the power to indemnify
each officer and director against liabilities and expenses incurred by reason of the fact that he or she is or was an officer or director
of the corporation if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best
interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct
was unlawful. The Company’s Amended and Restated Certificate of Incorporation and By-Laws provide for indemnification of each officer
and director of the Company to the fullest extent permitted by the DGCL. Section 145 of the DGCL also empowers corporations to purchase
and maintain insurance on behalf of any person who is or was an officer or director of the corporation against liability asserted against
or incurred by him in any such capacity, whether or not the corporation would have the power to indemnify such officer or director against
such liability under the provisions of Section 145 of the DGCL.
Item 15. Recent Sales of Unregistered Securities.
On September 8, 2023, we entered into an
unwritten agreement with Fortress Biotech, Inc., the holder of a majority of the voting power of the Company’s capital stock,
and Dr. Lindsay A. Rosenwald, a director on the board of directors of the Company (Dr. Rosenwald and Fortress Biotech, Inc.,
together, the “Investors”), pursuant to which we agreed to issue and sell 767,085 shares (the “Shares”) of Common
Stock of the Company, for an aggregate purchase price of approximately $550,000 in a private placement transaction (the “Private
Placement”). The Shares were offered and sold in reliance on the exemption from registration provided by Section 4(a)(2) of
the Securities Act. The Shares were purchased by the Investors at a price per share of $0.717, which was the “consolidated closing
bid price” of the Common Stock on the Nasdaq Capital Market as of September 7, 2023, in compliance with Nasdaq Listing Rule 5365(c).
The gross proceeds to the Company from the Private Placement, before
deducting estimated offering expenses payable by the Company, were approximately $550,000. We did not incur any underwriting or placement
agent fees associated with the Private Placement. We intend to use the net proceeds from the Private Placement for working capital and
other general corporate purposes.
Item 16. Exhibits and Financial Statement Schedules
| |
|
Incorporated
by Reference |
|
| Exhibit
No. |
Description |
Form |
File
Number |
Date |
Exhibit
No. |
Filed
herewith |
| 1.1 |
Underwriting
Agreement, dated October 6, 2022, by and between Avenue Therapeutics, Inc. and Aegis Capital Corp. |
8-K |
001-38114 |
October
12, 2022 |
1.1 |
|
| 3.1 |
Third
Amended and Restated Certificate of Incorporation of Avenue Therapeutics, Inc. |
8-K |
001-38114 |
June 27,
2017 |
3.1 |
|
| 3.2 |
Certificate
of Amendment of the Third Amended and Restated Certificate of Incorporation of Avenue Therapeutics, Inc. |
10-Q |
001-38114 |
August 14,
2018 |
3.1 |
|
| 3.3 |
Certificate
of Amendment of the Third Amended and Restated Certificate of Incorporation of Avenue Therapeutics, Inc. |
8-K |
001-38114 |
September 22,
2022 |
3.1 |
|
| 3.4 |
Certificate
of Amendment of the Third Amended and Restated Certificate of Incorporation of Avenue Therapeutics, Inc. |
8-K |
001-38114 |
February 3,
2023 |
3.1 |
|
| 3.5 |
Amended
and Restated Bylaws of Avenue Therapeutics, Inc. |
8-K |
000-38114 |
February 11,
2019 |
3.1 |
|
| 3.6 |
Second
Amended and Restated Bylaws of Avenue Therapeutics, Inc. |
8-K |
000-38114 |
February 10,
2023 |
3.1 |
|
| 4.1 |
Specimen
certificate evidencing shares of Common Stock |
10-12G |
000-55556 |
January 12,
2017 |
4.1 |
|
| 4.2 |
Form
of warrant (2017) |
10-12G |
000-55556 |
January 12,
2017 |
4.2 |
|
| 4.3 |
Form
of Warrant (October 2022) |
8-K |
001-38114 |
October
12, 2022 |
4.1 |
|
| 4.4 |
Form
of Pre-funded Warrant (October 2022) |
8-K |
001-38114 |
October
12, 2022 |
4.2 |
|
| 4.5 |
Form
of Pre-funded Warrant (Registered Offering) (January 2023) |
8-K |
001-38114 |
February
1, 2023 |
10.3 |
|
| 4.6 |
Form
of PIPE Warrant (PIPE) (January 2023) |
8-K |
001-38114 |
February
1, 2023 |
10.4 |
|
| 4.7 |
Warrant
Agent Agreement, dated October 6, 2022, by and between Avenue Therapeutics, Inc. and VStock Transfer, LLC |
8-K |
001-38114 |
October
12, 2022 |
4.3 |
|
| 4.8 |
Form of Warrant* |
|
|
|
|
|
| 4.9 |
Form of
Pre-funded Warrant |
|
|
|
|
X |
| 4.10 |
Form of
Warrant Agent Agreement |
|
|
|
|
X |
| 5.1 |
Opinion of McGuireWoods LLP* |
|
|
|
|
|
| 10.1 |
Asset
Transfer and License Agreement between Fortress Biotech, Inc. and Revogenex Ireland Limited dated February 17, 2015*** |
10-12G/A |
000-55556 |
March 13,
2017 |
10.1 |
|
| 10.2 |
First
Amendment to Asset Transfer and License Agreement between Fortress Biotech, Inc. and Revogenex Ireland Limited dated June 23,
2016 |
10-12G/A |
000-55556 |
March 13,
2017 |
10.11 |
|
| 10.3 |
Second
Amendment to Asset Transfer and License Agreement between Fortress Biotech, Inc. and Revogenex Ireland Limited dated May 4,
2017 |
S-1/A |
333-217552 |
May 22,
2017 |
10.3 |
|
| 10.4 |
Amended
and Restated Founders Agreement between Fortress Biotech, Inc. and Avenue Therapeutics, Inc. dated September 13, 2016 |
10-12G |
000-55556 |
January 12,
2017 |
10.2 |
|
| 10.5 |
Management
Services Agreement between Fortress Biotech, Inc. and Avenue Therapeutics, Inc. effective as of February 17, 2015 |
10-12G |
000-55556 |
January 12,
2017 |
10.5 |
|
| 10.6 |
Avenue
Therapeutics, Inc. 2015 Incentive Plan |
10-12G |
000-55556 |
January 12,
2017 |
10.7 |
|
| 10.7 |
Stock
Contribution Agreement between Avenue Therapeutics, Inc. and Fortress Biotech, Inc., dated May 11, 2022 |
10-Q |
001-38114 |
August 15,
2022 |
10.1 |
|
| 10.8 |
Form of
Securities Purchase Agreement (Registered Offering), dated January 27, 2023, by and among Avenue Therapeutics, Inc. and
the purchaser party thereto |
8-K |
001-38114 |
February 1,
2023 |
10.1 |
|
| 10.9 |
Form of
Securities Purchase Agreement (PIPE), dated January 27, 2023, by and among Avenue Therapeutics, Inc. and the purchaser
party thereto |
8-K |
001-38114 |
February 1,
2023 |
10.2 |
|
| 10.10 |
Form of
Registration Rights Agreement, dated January 27, 2023, by and among Avenue Therapeutics, Inc. and the purchaser party thereto |
8-K |
001-38114 |
February 1,
2023 |
10.5 |
|
| 10.11 |
Placement
Agent Agreement entered into by and between Avenue Therapeutics, Inc. and Aegis Capital Corp., dated January 27, 2023 |
8-K |
001-38114 |
February 1,
2023 |
10.7 |
|
| 10.12 |
License
Agreement entered into by and between AnnJi Pharmaceutical Co. Ltd. and Avenue Therapeutics, Inc., dated February 28, 2023 |
8-K |
001-38114 |
May 12,
2023 |
10.9 |
|
| 10.13 |
Registration
Rights Agreement, dated as of February 28, 2023, by and among Avenue Therapeutics, Inc. and AnnJi Pharmaceutical Co. Ltd. |
8-K |
001-38114 |
May 12,
2023 |
10.9 |
|
| 10.14 |
Form
of Avenue Therapeutics, Inc. Stock Option Agreement |
8-K |
001-38114 |
July
5, 2023 |
10.1 |
|
| 10.15 |
Registration
Rights Letter Agreement, dated as of September 8, 2023, by and among Avenue Therapeutics, Inc. and the purchaser parties
thereto |
8-K |
001-38114 |
September 8,
2023 |
10.1 |
|
| 10.16 |
Form of Placement Agency Agreement* |
|
|
|
|
|
| 10.17 |
Form of Securities Purchase Agreement* |
|
|
|
|
|
| 21.1 |
Subsidiaries
of Avenue Therapeutics, Inc. |
10-K |
001-38114 |
March 31,
2023 |
21.1 |
|
| 23.1 |
Consent
of Independent Registered Public Accounting Firm, KPMG LLP as to Avenue Therapeutics, Inc., for year ended December 31,
2022 |
|
|
|
|
X |
| 23.2 |
Consent
of Independent Registered Public Accounting Firm, BDO USA, P.C. as to Avenue Therapeutics, Inc., for year ended December 31,
2021 |
|
|
|
|
X |
| 23.3 |
Consent
of Independent Registered Public Accounting Firm, KPMG LLP, as to Baergic Bio, Inc. |
|
|
|
|
X |
| 23.4 |
Consent of McGuireWoods LLP (to be included in Exhibit
5.1)* |
|
|
|
|
|
| 24.1 |
Power
of Attorney** |
|
|
|
|
|
| 107 |
Filing
Fee Table |
|
|
|
|
X |
* To be filed by amendment.
** Previously filed.
*** Subject to a request for confidential treatment.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| A. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth
in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar
value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum
offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the
changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation
of Registration Fee” table in the effective registration statement. |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement; |
Provided, however, that Paragraphs (i), (ii), and (iii) of
this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in
reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange
Act of 1934 that are incorporated by reference in this registration statement,
| B. | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to
be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof. |
| C. | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| D. | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as
part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses
filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first
used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration
statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is
part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify
any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such
document immediately prior to such date of first use. |
| E. | The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing
of the registrant's annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where
applicable, each filing of an employee benefit plan's annual report pursuant to section 15(d) of the Securities Exchange Act of 1934)
that is incorporated by reference in this registration statement shall be deemed to be a new registration statement relating to the securities
offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| F. | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the
SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that
a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director,
officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director,
officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel
the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant to the requirements of the Securities
Act, the Registrant has duly caused this Amendment No. 1 to Registration Statement to be signed on its behalf by the undersigned, thereunto
duly authorized, in New York, New York, on September 29, 2023.
| |
Avenue Therapeutics, Inc. |
| |
|
| |
By: |
/s/ Alexandra MacLean, M.D. |
| |
Name: Alexandra MacLean, M.D. |
| |
Title: Chief Executive Officer |
Pursuant to the requirements of the Securities
Act, this Amendment No. 1 to Registration Statement has been signed by the following persons in the capacities and on the dated indicated.
| Signature | |
Title | |
Date |
| | |
| |
|
| /s/ Alexandra MacLean, M.D. | |
Chief Executive Officer | |
September 29, 2023 |
| Alexandra MacLean, M.D. | |
(Principal Executive Officer) | |
|
| | |
| |
|
| * | |
Chief Operating Officer and Interim Chief Financial Officer | |
September 29, 2023 |
| David Jin | |
(Principal Financial Officer, Principal Accounting Officer) | |
|
| | |
| |
|
| * | |
Chairman of the Board | |
September 29, 2023 |
| Jay Kranzler, M.D., Ph.D. | |
| |
|
| | |
| |
|
| * | |
Director | |
September 29, 2023 |
| Faith Charles | |
| |
|
| | |
| |
|
| * | |
Director | |
September 29, 2023 |
| Neil Herskowitz | |
| |
|
| | |
| |
|
| * | |
Director | |
September 29, 2023 |
| Curtis Oltmans | |
| |
|
| | |
| |
|
| * | |
Director | |
September 29, 2023 |
| Lindsay A. Rosenwald, M.D. | |
| |
|
| | |
| |
|
| * |
By: |
/s/ Alexandra MacLean, M.D. | |
| |
|
| |
|
Alexandra MacLean, M.D. | |
| |
|
| |
|
Attorney-in-Fact | |
| |
|
Exhibit 4.9
PREFUNDED COMMON STOCK PURCHASE WARRANT
AVENUE
THERAPEUTICS, INC.
| Warrant Shares: [•] |
|
Issuance Date: [·], 2023 |
| |
|
|
| |
|
Initial Exercise Date: [·], 2023 |
THIS PREFUNDED COMMON STOCK
PURCHASE WARRANT (the “Warrant”) certifies that Cede & Co. or its registered assigns (the “Holder”)
is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after
the date hereof (the “Initial Exercise Date”) until this Warrant is exercised in full (the “Termination Date”)
but not thereafter, to subscribe for and purchase from Avenue Therapeutics, Inc., a Delaware corporation (the “Company”),
up to [·] shares of Common Stock (as subject to adjustment hereunder, the “Warrant
Shares”). The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined
in Section 2(b). This Warrant shall initially be issued and maintained in the form of a security held in book-entry form
and the Depository Trust Company or its nominee (“DTC”) shall initially be the sole registered holder of this Warrant,
subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to the terms of the Warrant Agent Agreement,
in which case this sentence shall not apply.
Section 1. Definitions.
In addition to the terms defined elsewhere in this Warrant, the following terms have the meanings indicated in this Section 1:
“Affiliate”
means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control
with a Person, as such terms are used in and construed under Rule 405 under the Securities Act.
“Bid Price”
means, for any date, the price determined by the first of the following clauses that applies: (a) if the shares of Common Stock
are then listed or quoted on a Trading Market, the bid price of a share of Common Stock for the time in question (or the nearest preceding
date) on the Trading Market on which the shares of Common Stock are then listed or quoted as reported by Bloomberg L.P. (based on a Trading
Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market,
the volume weighted average per share price of the shares of Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX
as applicable, (c) if the shares of Common Stock are not then listed or quoted for trading on OTCQB or OTCQX and if prices for the
shares of Common Stock are then reported on the Pink Open Market (or a similar organization or agency succeeding to its functions of
reporting prices), the most recent bid price per share of Common Stock so reported, or (d) in all other cases, the fair market value
of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest
of the Warrants then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“Board
of Directors” means the board of directors of the Company.
“Business
Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized
or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed
to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential
employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental
authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York
generally are open for use by customers on such day.
“Commission”
means the United States Securities and Exchange Commission.
“Common
Stock” means the common stock, par value $0.0001 per share, of the Company, and any other class of securities into which such
securities may hereafter be reclassified or changed.
“Common
Stock Equivalents” means any securities of the Company which would entitle the holder thereof to acquire at any time shares
of Common Stock, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is at any
time convertible into or exercisable or exchangeable for shares of Common Stock, or otherwise entitles the holder thereof to receive,
shares of Common Stock.
“Exchange
Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Person”
means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability
company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind.
“Registration
Statement” means the Company’s registration statement on Form S-1 (File No. 333-274562), as amended.
“Securities
Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Trading
Day” means a day on which the shares of Common Stock are traded on a Trading Market.
“Trading
Market” means any of the following markets or exchanges on which the shares of Common Stock are listed or quoted for trading
on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the
New York Stock Exchange, OTCQB or OTCQX (or any successors to any of the foregoing).
“Transfer
Agent” means VStock Transfer, LLC, and any successor transfer agent of the Company.
“VWAP”
means, for any date, the price determined by the first of the following clauses that applies: (a) if the shares of Common Stock
are then listed or quoted on a Trading Market, the daily volume weighted average price per share of the shares of Common Stock for such
date (or the nearest preceding date) on the Trading Market on which the shares of Common Stock are then listed or quoted as reported
by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if
OTCQB or OTCQX is not a Trading Market, the volume weighted average price per share of shares of Common Stock for such date (or the nearest
preceding date) on OTCQB or OTCQX as applicable, (c) if the shares of Common Stock are not then listed or quoted for trading on
OTCQB or OTCQX and if prices for shares of Common Stock are then reported on the OTC Pink Open Market (or a similar organization or agency
succeeding to its functions of reporting prices), the most recent bid price per share of Common Stock so reported, or (d) in all
other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the
holders of a majority in interest of the Warrants then outstanding and reasonably acceptable to the Company, the fees and expenses of
which shall be paid by the Company.
“Warrant
Agent Agreement” means that certain warrant agent agreement, dated on or about the Initial Exercise Date, between the Company
and the Warrant Agent.
“Warrant
Agent” means the Transfer Agent and any successor warrant agent of the Company.
“Warrants”
means this Warrant and other common stock purchase warrants issued to investors by the Company pursuant to the Registration Statement.
Section 2. Exercise.
a) Exercise of Warrant. Subject to the provisions of Section 2(e) herein,
exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial
Exercise Date and on or before the Termination Date, by delivery to the Company of a duly executed PDF copy submitted by e-mail (or e-mail
attachment) of the Notice of Exercise in the form annexed hereto as Annex A (the “Notice of Exercise”), and, unless
the cashless exercise procedure specified in Section 2(c) below is specified in the applicable Notice of Exercise, delivery
within the Standard Settlement Period of the aggregate Exercise Price of the Warrant Shares specified in the applicable Notice of Exercise
as specified in this Section 2(a). Within the earlier of (i) two (2) Trading Days and (ii) the number of Trading
Days comprising the Standard Settlement Period (as defined in Section 2(d)(i) herein) following the date of the Notice
of Exercise as aforesaid, the Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice
of Exercise by wire transfer of immediately available funds or cashier’s check drawn on a United States bank unless the cashless
exercise procedure specified in Section 2(c) below is specified in the applicable Notice of Exercise. No ink-original
Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of
Exercise be required. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this
Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised
in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of
the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases
of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant
Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall
maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection
to any Notice of Exercise within one (1) Trading Day of receipt of such notice. The Holder and any assignee, by acceptance of
this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant
Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated
on the face hereof.
Notwithstanding
the foregoing in this Section 2(a), a holder whose interest in this Warrant is a beneficial interest in certificate(s) representing
this Warrant held in book-entry form through DTC (or another established clearing corporation performing similar functions) shall effect
exercises made pursuant to this Section 2(a) by delivering to DTC (or such other clearing corporation, as applicable)
the appropriate instruction form for exercise, complying with the procedures to effect exercise that are required by DTC (or such other
clearing corporation, as applicable), subject to a Holder’s right to elect to receive a Warrant in certificated form pursuant to
the terms of the Warrant Agent Agreement, in which case this sentence shall not apply.
b) Exercise
Price. The aggregate exercise price of this Warrant, except for a nominal exercise price of $0.0001 per Warrant Share, was pre-funded
to the Company on or prior to the Initial Exercise Date and, consequently, no additional consideration (other than the nominal exercise
price of $0.0001 per Warrant Share) shall be required to be paid by the Holder to any Person to effect any exercise of this Warrant.
The Holder shall not be entitled to the return or refund of all, or any portion, of such pre-paid aggregate exercise price under any
circumstance or for any reason whatsoever, including in the event this Warrant shall not have been exercised prior to the Termination
Date. The remaining unpaid exercise price per share of Common Stock under this Warrant shall be $0.0001, subject to adjustment hereunder
(the “Exercise Price”).
c) Cashless
Exercise. The Company shall use commercially reasonable efforts to cause the Registration Statement to remain effective with a current
prospectus and to maintain the registration of the shares of Common Stock and of the Warrants under the Exchange Act. If at any time
after the Initial Exercise Date there is no effective registration statement registering, or no current prospectus available for, the
issuance of the Warrant Shares to the Holder, then this Warrant may also be exercised, in whole or in part, at such time by means of
a “cashless exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained
by dividing [(A-B) (X)] by (A), where:
| |
(A)= |
as applicable: (i) the VWAP on the Trading Day immediately preceding the date of
the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof
on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a
Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b) of Regulation NMS promulgated
under the federal securities laws) on such Trading Day, (ii) at the option of the Holder, either (y) the VWAP on the Trading
Day immediately preceding the date of the applicable Notice of Exercise or (z) the Bid Price of the shares of Common Stock on
the principal Trading Market as reported by Bloomberg L.P. as of the time of the Holder’s execution of the applicable Notice
of Exercise if such Notice of Exercise is executed during “regular trading hours” on a Trading Day and is delivered within
two (2) hours thereafter (including until two (2) hours after the close of “regular trading hours” on a Trading
Day) pursuant to Section 2(a) hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if
the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof
after the close of “regular trading hours” on such Trading Day; |
| |
|
|
| |
(B)= |
the Exercise Price of this Warrant, as adjusted hereunder; and |
| |
|
|
| |
(X)= |
the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with
the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise. |
If Warrant Shares
are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities
Act, the Warrant Shares shall take on the registered characteristics of the Warrants being exercised. The Company agrees not to take
any position contrary to this Section 2(c).
d) Mechanics
of Exercise.
i. Delivery
of Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer
Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust
Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such
system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale of
the Warrant Shares by Holder or (B) this Warrant is being exercised via cashless exercise, and otherwise by physical delivery of
a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant
Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by
the date that is the earliest of (i) two (2) Trading Days after the delivery to the Company of the Notice of Exercise and (ii) the
number of Trading Days comprising the Standard Settlement Period after the delivery to the Company of the Notice of Exercise (such date,
the “Warrant Share Delivery Date”). Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate
purposes to have become the holder of record of the Warrant Shares with respect to which this Warrant has been exercised, irrespective
of the date of delivery of the Warrant Shares, provided that payment of the aggregate Exercise Price (other than in the case of a cashless
exercise) is received within the earlier of (i) two (2) Trading Days and (ii) the number of Trading Days comprising the
Standard Settlement Period following delivery of the Notice of Exercise. If the Company fails for any reason to deliver to the Holder
the Warrant Shares subject to a Notice of Exercise by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash,
as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the shares
of Common Stock on the date of the applicable Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the third
Trading Day after the Warrant Share Delivery Date) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares
are delivered or Holder rescinds such exercise. The Company agrees to maintain a transfer agent that is a participant in the FAST program
so long as this Warrant remains outstanding and exercisable. As used herein, “Standard Settlement Period” means the
standard settlement period, expressed in a number of Trading Days, on the Company’s primary Trading Market with respect to the
shares of Common Stock as in effect on the date of delivery of the Notice of Exercise. Notwithstanding the foregoing, with respect to
any Notice(s) of Exercise delivered on or prior to 12:00 p.m. (New York City time) on the Initial Exercise Date, which may
be delivered at any time after the time of execution of the Purchase Agreement, the Company agrees to deliver the Warrant Shares subject
to such notice(s) by 4:00 p.m. (New York City time) on the Initial Exercise Date and the Initial Exercise Date shall be the
Warrant Share Delivery Date for purposes hereunder, provided that payment of the aggregate Exercise Price (other than in the case of
a cashless exercise) is received by such Warrant Share Delivery Date.
ii. Delivery
of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and
upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing
the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other
respects be identical with this Warrant.
iii. Rescission
Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by
the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise; provided, however,
that the Holder shall be required to return any Warrant Shares subject to any such rescinded exercise notice concurrently with the return
to Holder of the aggregate Exercise Price paid to the Company for such Warrant Shares and the restoration of Holder’s right to
acquire such Warrant Shares pursuant to this Warrant (including, issuance of a replacement warrant certificate evidencing such restored
right).
iv. Compensation
for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if
the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above
pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to
purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock
to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a
“Buy-In”), then the Company shall (A) pay in cash to the Holder the amount, if any, by which (x) the Holder’s
total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the amount
obtained by multiplying (1) the number of Warrant Shares that the Company was required to deliver to the Holder in connection with
the exercise at issue times (2) the price at which the sell order giving rise to such purchase obligation was executed, and (B) at
the option of the Holder, either reinstate the portion of the Warrant and equivalent number of Warrant Shares for which such exercise
was not honored (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock
that would have been issued had the Company timely complied with its exercise and delivery obligations hereunder. For example, if the
Holder purchases shares of Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise
of shares of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, under clause (A) of the
immediately preceding sentence the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice
indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such
loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including,
without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver
shares of Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
v. No Fractional Shares or Scrip. No fractional shares
or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder
would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect
of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
vi. Charges,
Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other
incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and
such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however,
that, in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for
exercise shall be accompanied by the Assignment Form attached hereto as Annex B duly executed by the Holder and the Company may
require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company
shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to the Depository Trust Company
(or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
vii. Closing
of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant,
pursuant to the terms hereof.
e) Holder’s
Exercise Limitations. The Company shall not effect any exercise of this Warrant, and a Holder shall not have the right to exercise
any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect to such issuance
after exercise as set forth on the applicable Notice of Exercise, the Holder (together with the Holder’s Affiliates, and any other
Persons acting as a group together with the Holder or any of the Holder’s Affiliates (such Persons, “Attribution Parties”)),
would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of the foregoing sentence, the
number of shares of Common Stock beneficially owned by the Holder and its Affiliates and Attribution Parties shall include the number
of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being made, but shall exclude
the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, non-exercised portion of this Warrant
beneficially owned by the Holder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion of the unexercised
or non-converted portion of any other securities of the Company (including, without limitation, any other shares of Common Stock Equivalents)
subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the Holder or any
of its Affiliates or Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e),
beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations
promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation
is in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to
be filed in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination
of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with any Affiliates and Attribution
Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice
of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities
owned by the Holder together with any Affiliates and Attribution Parties) and of which portion of this Warrant is exercisable, in each
case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such
determination. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of
the Exchange Act and the rules and regulations promulgated thereunder. For purposes of this Section 2(e), in determining
the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected
in (A) the Company’s most recent periodic or annual report filed with the Commission, as the case may be, (B) a more
recent public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth
the number of shares of Common Stock outstanding. Upon the written or oral request of a Holder, the Company shall within one Trading
Day confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding
shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including
this Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of
Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% (or, upon election by a Holder prior
to the issuance of any Warrants, 9.99%) of the number of shares of Common Stock outstanding immediately after giving effect to the issuance
of shares of Common Stock issuable upon exercise of this Warrant. The Holder, upon notice to the Company, may increase or decrease the
Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation in no
event exceeds 9.99% of the number of shares of Common Stock outstanding immediately after giving effect to the issuance of shares of
Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue
to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice
is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict
conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which may be defective
or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable
to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
Section 3. Certain
Adjustments.
a) Stock
Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise
makes a distribution or distributions on its shares of Common Stock or any other equity or equity equivalent securities payable in shares
of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this
Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way
of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of
shares of Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction
of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before
such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the
number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this
Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately
after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective
immediately after the effective date in the case of a subdivision, combination or re-classification.
b) Subsequent
Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants,
issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all (or
substantially all) of the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the
Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder
could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without
regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the
date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as
of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided,
however, that, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding
the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial
ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall
be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial
Ownership Limitation).
c) Pro Rata Distributions.
During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets
(or rights to acquire its assets) to all (or substantially all) of holders of shares of Common Stock, by way of return of capital or
otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend,
spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”),
at any time after the issuance of this Warrant, then, in each such case, the Holder shall be entitled to participate in such Distribution
to the same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable
upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial
Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the
date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, however,
that, to the extent that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial
Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial
ownership of any shares of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be
held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding
the Beneficial Ownership Limitation).
d) Fundamental
Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related
transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company, directly or indirectly,
effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of its assets in
one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (approved
or recommended by the Board of Directors or a committee thereof) is completed pursuant to which holders of shares of Common Stock are
permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of 50%
or more of the voting power of the capital stock of the Company, (iv) the Company, directly or indirectly, in one or more related
transactions effects any reclassification, reorganization or recapitalization of shares of Common Stock or any compulsory share exchange
pursuant to which the shares of Common Stock are effectively converted into or exchanged for other securities, cash or property, or (v) the
Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business
combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another
Person or group of Persons whereby such other Person or group, other than Fortress Biotech, Inc., acquires more than 50% or more
of the voting power of the capital stock in the Company (each a “Fundamental Transaction”), then, upon any subsequent
exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such
exercise immediately prior to the occurrence of such Fundamental Transaction (without regard to any limitation in Section 2(e) on
the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it
is the surviving corporation, and any additional consideration (together, the “Alternate Consideration”) receivable
as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable
immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on the exercise
of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply
to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such
Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner
reflecting the relative value of any different components of the Alternate Consideration. If holders of shares of Common Stock are given
any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same
choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction.
The Company shall
cause any successor entity in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”)
to assume in writing all of the obligations of the Company under this Warrant in accordance with the provisions of this Section 3(e) pursuant
to written agreements in form and substance reasonably satisfactory to the Warrant Agent and the holders of Warrants representing at
least a majority of the shares of Common Stock underlying the Warrants then outstanding (the “Required Holders”) and
approved by the Required Holders (without unreasonable delay) prior to such Fundamental Transaction and shall, at the option of the Holder,
deliver to the Holder in exchange for this Warrant a security of the Successor Entity evidenced by a written instrument substantially
similar in form and substance to this Warrant which is exercisable for a corresponding number of shares of capital stock of such Successor
Entity (or its parent entity) equivalent to the shares of Common Stock acquirable and receivable upon exercise of this Warrant (without
regard to any limitations on the exercise of this Warrant) prior to such Fundamental Transaction, and with an exercise price which applies
the exercise price hereunder to such shares of capital stock (but taking into account the relative value of the shares of Common Stock
pursuant to such Fundamental Transaction and the value of such shares of capital stock, such number of shares of capital stock and such
exercise price being for the purpose of protecting the economic value of this Warrant immediately prior to the consummation of such Fundamental
Transaction), and which is reasonably satisfactory in form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction,
the Successor Entity shall be added to the term “Company” under this Warrant (so that from and after the occurrence or consummation
of such Fundamental Transaction, each and every provision of this Warrant referring to the “Company” shall refer instead
to each of the Company and the Successor Entity or Successor Entities, jointly and severally), and the Successor Entity or Successor
Entities, jointly and severally with the Company may exercise every right and power of the Company prior thereto and the Successor Entity
or Successor Entities shall assume all of the obligations of the Company prior thereto under this Warrant with the same effect as if
the Company and such Successor Entity or Successor Entities, jointly and severally, had been named as the Company herein.
e) Calculations.
All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may
be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given
date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
f) Notice
to Holder.
i. Adjustment
to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company
shall promptly deliver to the Holder a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to
the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice
to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the
shares of Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the shares of Common
Stock, (C) the Company shall authorize the granting to all holders of the shares of Common Stock rights or warrants to subscribe
for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall
be required in connection with any reclassification of the shares of Common Stock, any consolidation or merger to which the Company (or
any of its subsidiaries) is a party, any sale or transfer of all or substantially all of its assets, or any compulsory share exchange
whereby the shares of Common Stock are converted into other securities, cash or property, or (E) the Company shall authorize the
voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall
cause to be delivered by facsimile or email to the Holder at its last facsimile number or email address as it shall appear upon the Warrant
Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice (unless
such information is filed with the Commission, in which case a notice shall not be required) stating (x) the date on which a record
is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the
date as of which the holders of the shares of Common Stock of record to be entitled to such dividend, distributions, redemption, rights
or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share
exchange is expected to become effective or close, and the date as of which it is expected that holders of the shares of Common Stock
of record shall be entitled to exchange their shares of Common Stock for securities, cash or other property deliverable upon such reclassification,
consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver such notice or any defect therein or in
the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that
any notice provided in this Warrant constitutes, or contains, material, non-public information regarding the Company or any of the subsidiaries,
the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Holder shall
remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering
such notice except as may otherwise be expressly set forth herein.
h) Voluntary
Adjustment by Company. Subject to the rules and regulations of the Trading Market, the Company may at any time during the term
of this Warrant, subject to the prior written consent of the Holder, reduce the then current Exercise Price to any amount and for any
period of time deemed appropriate by the board of directors of the Company.
Section 4. Transfer
of Warrant.
a) Transferability.
This Warrant and all rights hereunder are transferable, in whole or in part, upon surrender of this Warrant at the principal office of
the Company or its designated agent, together with a written assignment of this Warrant substantially in the form attached hereto duly
executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer.
Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the
assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall
issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled.
Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company
unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company within three
(3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning this Warrant in full. The
Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having
a new Warrant issued.
b) New
Warrants. If this Warrant is not held in global form through DTC (or any successor depositary), this Warrant may be divided or combined
with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names
and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a),
as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants
in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or
exchanges shall be dated the initial issuance date of this Warrant and shall be identical with this Warrant except as to the number of
Warrant Shares issuable pursuant thereto.
c) Warrant
Register. The Warrant Agent shall register this Warrant, upon records to be maintained by the Warrant Agent for that purpose (the
“Warrant Register”), in the name of the record Holder hereof from time to time. The Company and the Warrant Agent
may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution
to the Holder, and for all other purposes, absent actual notice to the contrary.
Section 5. Miscellaneous.
a) No Rights
as Stockholder until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or
other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly
set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise”
pursuant to Section 2(c) or to receive cash payments pursuant to Section 2(d)(i) and Section 2(d)(iv) herein,
including if the Company is for any reason unable to issue and deliver Warrant Shares upon exercise of this Warrant as required pursuant
to the terms hereof, in no event shall the Company be required to net cash settle an exercise of this Warrant or cash settle in any other
form.
b) Loss,
Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory
to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case
of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include
the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make
and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays,
Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or
granted herein shall not be a Business Day, then such action may be taken or such right may be exercised on the next succeeding Business
Day.
d) Authorized
Shares.
The Company covenants
that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued shares of Common Stock a sufficient
number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company
further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of
issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable
action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law
or regulation, or of any requirements of the Trading Market upon which the shares of Common Stock may be listed. The Company covenants
that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise
of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly
issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof
(other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the
extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate
of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or
any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all
times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate
to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the
Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately
prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may
validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially
reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof,
as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any
action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price,
the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory
body or bodies having jurisdiction thereof.
e) Governing
Law. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be governed by and
construed in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof.
Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated
by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers, shareholders, partners,
members, employees or agents) shall be commenced exclusively in the state and federal courts sitting in the City of New York. Each party
hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the City of New York, Borough of
Manhattan, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed
herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally
subject to the jurisdiction of any such court, that such suit, action or proceeding is improper or is an inconvenient venue for such
proceeding. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action
or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party
at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient service
of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner
permitted by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant, the prevailing
party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees and other
costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. Notwithstanding the foregoing,
nothing in this paragraph shall limit or restrict the federal district court in which a Holder may bring a claim under the U.S. federal
securities laws.
f) Restrictions.
The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, and the Holder does not
utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
g) Non-Waiver
and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as
a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies. No provision of this Warrant shall be construed
as a waiver by the Holder of any rights which the Holder may have under the U.S. federal securities laws and the rules and regulations
of the Commission thereunder. Without limiting any other provision of this Warrant, if the Company willfully and knowingly fails to comply
with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts
as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those
of appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights,
powers or remedies hereunder.
h) Notices.
Any and all notices or other communications or deliveries to be provided by the Holders hereunder including, without limitation, any
Notice of Exercise, shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service,
addressed to (A) the Company, at 2 Gansevoort Street, 9th Floor, New York, NY 10014, Attention: Chief Operating
Officer, email address: djin@fortressbiotech.com, or such other email address or address as the Company may specify for such purposes
by notice to the Holders; and (B) the Warrant Agent, at VStock Transfer, LLC, 18 Lafayette Pl., Woodmere, NY 11598, Attention: Transaction
Processing Dept., email address: info@vstocktransfer.com. Any and all notices or other communications or deliveries to be provided by
the Company hereunder shall be in writing and delivered personally, by e-mail, or sent by a nationally recognized overnight courier service
addressed to each Holder at the e-mail address or address of such Holder appearing on the books of the Company. Any notice or other communication
or deliveries hereunder shall be deemed given and effective on the earliest of (i) the time of transmission, if such notice or communication
is delivered via e-mail at the e-mail address set forth in this Section prior to 5:30 p.m. (New York City time) on any date,
(ii) the next Trading Day after the time of transmission, if such notice or communication is delivered via e-mail at the e-mail
address set forth in this Section on a day that is not a Trading Day or later than 5:30 p.m. (New York City time) on any Trading
Day, (iii) the second Trading Day following the date of mailing, if sent by U.S. nationally recognized overnight courier service,
or (iv) upon actual receipt by the party to whom such notice is required to be given. To the extent that any notice provided hereunder
constitutes, or contains, material, non-public information regarding the Company or any subsidiaries, the Company shall simultaneously
file such notice with the Commission pursuant to a Current Report on Form 8-K.
i) Limitation
of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant
Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase
price of any shares of Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors
of the Company.
j) Remedies.
The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific
performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss
incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any
action for specific performance that a remedy at law would be adequate.
k) Successors
and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the
benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder.
The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable
by the Holder or holder of Warrant Shares.
l) Amendment
and Waiver. Except as otherwise provided herein, the provisions of this Warrant may be amended and the Company may take any action
herein prohibited, or omit to perform any act herein required to be performed by it, only if the Company has obtained the written consent
of the Required Holders. Any such amendment shall apply to all Warrants outstanding and be binding upon all registered holders of such
Warrants.
m) Severability.
Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law,
but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the
extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings.
The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this
Warrant.
o) Warrant
Agent Agreement. If this Warrant is held in global form through DTC (or any successor depositary), this Warrant is issued subject
to the Warrant Agent Agreement. To the extent any provision of this Warrant conflicts with the express provisions of the Warrant Agent
Agreement, the provisions of this Warrant shall govern and be controlling.
********************
(Signature Page Follows)
IN WITNESS WHEREOF, the Company
has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
{01291517.DOCX.2}
| |
AVENUE THERAPEUTICS, INC. |
| |
|
|
| |
By: |
|
| |
|
Alexandra MacLean, M.D. |
| |
|
Chief Executive Officer |
| |
VSTOCK TRANSFER, LLC |
| |
|
|
| |
By: |
|
| |
|
Yoel Goldfeder |
| |
|
Chief Executive Officer |
ANNEX A
NOTICE OF EXERCISE
TO: AVENUE THERAPEUTICS, INC.
(1) The undersigned hereby
elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full),
and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable
box):
| |
¨ |
in lawful money of the United States; or |
| |
¨ |
if permitted the cancellation of such number of Warrant Shares as is necessary, in accordance
with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable
pursuant to the cashless exercise procedure set forth in subsection 2(c). |
(3) Please issue said
Warrant Shares in the name of the undersigned or in such other name as is specified below:
The Warrant Shares shall be delivered to the
following DWAC Account Number:
[SIGNATURE
OF HOLDER]
| Name of Investing Entity: |
|
| Signature of Authorized Signatory of Investing Entity: |
|
| Name of Authorized Signatory: |
|
| Title of Authorized Signatory: |
|
ANNEX B
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute
this form and supply required information. Do not use this form to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced
thereby are hereby assigned to:
| Name: |
| |
| Address: |
| |
| Phone Number: |
| |
| Email Address: |
| |
| Dated: _______________ __, ______ |
| Holder’s Signature: |
|
|
| |
|
|
| Holder’s Address: |
|
|
| (Signature Guaranteed): |
Date: |
___________________, _____ |
Signature to be guaranteed by an authorized officer of a chartered
bank, trust company or medallion guaranteed by an investment dealer who is a member of a recognized stock exchange.
Exhibit 4.10
Warrant
Agent Agreement
This WARRANT AGENT AGREEMENT
(this “Warrant Agreement”) dated as of [•], 2023 is by and between Avenue Therapeutics, Inc, a Delaware
corporation (the “Company”), and VStock Transfer, LLC (the “Warrant Agent”).
WHEREAS,
the Company is selling in a public offering up to (i) [•]units (the “Units”), with each Unit consisting of
one (1) share of common stock, par value $0.0001 per share (“Common Stock”), and one (1) warrant to purchase
one share of Common Stock at an exercise price of $[•] (each, a “Warrant” and collectively, the “Warrants”),
(ii) [•] pre-funded units (the “Pre-funded Units”), with each Pre-funded Unit consisting of one (1) Warrant
and one (1) pre-funded warrant to purchase one (1) share of Common Stock at an exercise price of $0.0001 per share of Common
Stock (each, a “Pre-funded Warrant” and collectively, the “Pre-funded Warrants”).
WHEREAS,
the Company has filed, with the Securities and Exchange Commission, a registration statement on Form S-1 (Registration No. 333-274562)
(as the same may be amended from time to time, the “Registration Statement”), for the registration, under the Securities
Act of 1933, as amended, of the offer and sale of the Units, Pre-funded Units, Common Stock, Warrants, Pre-funded Warrants, and Common
Stock underlying Pre-funded Warrants and Warrants, and such Registration Statement was declared effective on [•], 2023;
WHEREAS,
the Company desires the Warrant Agent to act on behalf of the Company, and the Warrant Agent is willing to so act, in accordance with
the terms set forth in this Warrant Agreement in connection with the issuance, registration, transfer, exchange and exercise of the Warrants
and the Pre-funded Warrants;
WHEREAS,
the Company desires to provide for the provisions of the Warrants and the Pre-funded Warrants, the terms upon which they shall be issued
and exercised, and the respective rights, limitation of rights, and immunities of the Company, the Warrant Agent, and the holders of the
Warrants and the Pre-funded Warrants; and
WHEREAS,
all acts and things have been done and performed which are necessary to make the Warrants and the Pre-funded Warrants the valid, binding
and legal obligations of the Company, and to authorize the execution and delivery of this Warrant Agreement;
NOW, THEREFORE,
in consideration of the mutual agreements herein contained, the parties hereto agree as follows:
1. Appointment
of Warrant Agent. The Company hereby appoints the Warrant Agent to act as agent for the Company with respect to the Warrants and the
Pre-funded Warrants, and the Warrant Agent hereby accepts such appointment and agrees to perform the same in accordance with the express
terms and conditions set forth in this Warrant Agreement (and no implied terms or conditions).
2. Warrants.
2.1. Form of
Warrants. The Warrants and the Pre-funded Warrants shall each be registered securities and shall be evidenced by a global warrant
(each, a “Global Warrant”) in the forms of Exhibit A and Exhibit B to
this Warrant Agreement, which shall be deposited on behalf of the Company with a custodian for The Depository Trust Company (“DTC”)
and registered in the name of Cede & Co., a nominee of DTC. The terms of the Global Warrants are incorporated herein by reference.
If DTC subsequently ceases to make its book-entry settlement system available for the Warrants or the Pre-funded Warrants, the Company
may instruct the Warrant Agent regarding making other arrangements for book-entry settlement. In the event that the Warrants or the Pre-funded
Warrants are not eligible for, or it is no longer necessary to have such instruments available in, book-entry form, the Company may instruct
the Warrant Agent to provide written instructions to DTC to deliver to the Warrant Agent for cancellation the applicable Global Warrant,
and the Company shall instruct the Warrant Agent to deliver to DTC separate certificates evidencing Warrants or the Pre-funded Warrants
(“Definitive Certificates” and, together with the Global Warrants, “Warrant Certificates”)
registered as requested through the DTC system.
2.2. Issuance
and Registration of Warrants.
2.2.1. Warrant
Register. The Warrant Agent shall maintain books (“Warrant Register”) for the registration of original issuance
and the registration of transfer of the Warrants and the Pre-funded Warrants.
2.2.2. Issuance
of Warrants. Upon the initial issuance of the Warrants and the Pre-funded Warrants, the Warrant Agent shall issue the Global Warrants
and deliver the Warrants and the Pre-funded Warrants in the DTC book-entry settlement system in accordance with written instructions delivered
to the Warrant Agent by the Company. Ownership of security entitlements in the Warrants and the Pre-funded Warrants shall be shown on,
and the transfer of such ownership shall be effected through, records maintained (i) by DTC and (ii) by institutions that have
accounts with DTC (each, a “Participant”).
2.2.3. Beneficial
Owner; Holder. Prior to due presentment for registration of transfer of any Warrant or Pre-funded Warrant, the Company and the Warrant
Agent may deem and treat the person in whose name that Warrant or Pre-funded Warrant shall be registered on the Warrant Register (the “Holder”)
as the absolute owner of such security for purposes of any exercise thereof, and for all other purposes, and neither the Company nor the
Warrant Agent shall be affected by any notice to the contrary; provided, that the rights of beneficial owners in a Warrant or Pre-funded
Warrant evidenced by a Global Warrant shall be exercised by the Holder or a Participant through the DTC system. Notwithstanding the foregoing,
nothing herein shall prevent the Company, the Warrant Agent or any agent of the Company or the Warrant Agent from giving effect to any
written certification, proxy or other authorization furnished by DTC governing the exercise of the rights of a holder of a beneficial
interest in any Warrant or Pre-funded Warrant.
2.2.4. Delivery
of Warrant Certificate. A Holder has the right to elect at any time or from time to time a Warrant Exchange (as defined below) pursuant
to a Warrant Certificate Request Notice (as defined below). Upon written notice by a Holder to the Warrant Agent for the exchange of some
or all of such Holder’s Global Warrants for a Warrant Certificate evidencing the same number of Warrants or Pre-funded Warrants,
which request shall be in the form attached hereto as Exhibit C (a “Warrant Certificate Request Notice”) and
the deemed surrender upon delivery by the Holder of a number of Global Warrants for the same number of Warrants or Pre-funded Warrants
evidenced by a Warrant Certificate, a “Warrant Exchange”), the Warrant Agent shall promptly effect the Warrant
Exchange and shall promptly issue and deliver to the Holder a Warrant Certificate for such number of Warrants or Pre-funded Warrants in
the name set forth in the Warrant Certificate Request Notice. Such Warrant Certificate shall be dated the date of issuance of the Warrant
Certificate, shall include the initial exercise date of the Warrants or Pre-funded Warrants, shall be executed by an authorized signatory
of the Company and shall be reasonably acceptable in all respects to such Holder. In connection with a Warrant Exchange, the Company agrees
to deliver, or to direct the Warrant Agent to deliver, the Warrant Certificate to the Holder within three (3) Business Days of the
Warrant Certificate Request Notice pursuant to the delivery instructions in the Warrant Certificate Request Notice. The Company covenants
and agrees that, upon the date of delivery of the Warrant Certificate Request Notice, the Holder shall be deemed to be the holder of the
Warrant Certificate and, notwithstanding anything to the contrary set forth herein, the Warrant Certificate shall be deemed for all purposes
to contain all of the terms and conditions of the Warrants or Pre-funded Warrants evidenced by such Warrant Certificate and the terms
of this Agreement.
2.2.5. Execution.
The Warrant Certificates shall be executed on behalf of the Company by any authorized officer of the Company (an “Authorized
Officer”), which need not be the same authorized signatory for all of the Warrant Certificates, either manually or by facsimile
signature. The Warrant Certificates shall be countersigned by an authorized signatory of the Warrant Agent, which need not be the same
signatory for all of the Warrant Certificates, and no Warrant Certificate shall be valid for any purpose unless so countersigned. In case
any Authorized Officer of the Company that signed any of the Warrant Certificates ceases to be an Authorized Officer of the Company before
countersignature by the Warrant Agent and issuance and delivery by the Company, such Warrant Certificates, nevertheless, may be countersigned
by the Warrant Agent, issued and delivered with the same force and effect as though the person who signed such Warrant Certificates had
not ceased to be such officer of the Company; and any Warrant Certificate may be signed on behalf of the Company by any person who, at
the actual date of the execution of such Warrant Certificate, shall be an Authorized Officer of the Company authorized to sign such Warrant
Certificate, although at the date of the execution of this Warrant Agreement any such person was not such an Authorized Officer.
2.2.6. Registration
of Transfer. At any time at or prior to the Termination Date (as defined below), a transfer of any Warrants or Pre-funded Warrants
may be registered and any Warrant Certificate or Warrant Certificates may be split up, combined or exchanged for another Warrant Certificate
or Warrant Certificates evidencing the same number of Warrants or Pre-funded Warrants as the Warrant Certificate or Warrant Certificates
surrendered. Any Holder desiring to register the transfer of Warrants or Pre-funded Warrants or to split up, combine or exchange any Warrant
Certificate shall make such request in writing delivered to the Warrant Agent, and shall surrender to the Warrant Agent the Warrant Certificate
or Warrant Certificates evidencing the Warrants or Pre-funded Warrants the transfer of which is to be registered or that is or are to
be split up, combined or exchanged and, in the case of registration of transfer, shall provide a signature guarantee. Thereupon, the Warrant
Agent shall countersign and deliver to the person entitled thereto a Warrant Certificate or Warrant Certificates, as the case may be,
as so requested; provided, however that Warrants and Pre-funded Warrants may not be combined in the same
Warrant Certificate. The Company and the Warrant Agent may require payment, by the Holder requesting a registration of transfer of Warrants
or Pre-funded Warrants or a split-up, combination or exchange of a Warrant Certificate (but, for purposes of clarity, not upon the exercise
of the Warrants and issuance of Warrant Shares to the Holder), of a sum sufficient to cover any tax or governmental charge that may be
imposed in connection with such registration of transfer, split-up, combination or exchange, together with reimbursement to the Company
and the Warrant Agent of all reasonable expenses incidental thereto.
2.2.7. Loss,
Theft and Mutilation of Warrant Certificates. Upon receipt by the Company and the Warrant Agent of evidence reasonably satisfactory
to them of the loss, theft, destruction or mutilation of a Warrant Certificate, and, in case of loss, theft or destruction, of indemnity
or security in customary form and amount, and reimbursement to the Company and the Warrant Agent of all reasonable expenses incidental
thereto, and upon surrender to the Warrant Agent and cancellation of the Warrant Certificate if mutilated, the Warrant Agent shall, on
behalf of the Company, countersign and deliver a new Warrant Certificate of like tenor to the Holder in lieu of the Warrant Certificate
so lost, stolen, destroyed or mutilated. The Warrant Agent may charge the Holder an administrative fee for processing the replacement
of lost Warrant Certificates. The Warrant Agent may receive compensation from the surety companies or surety agents for administrative
services provided to them.
2.2.8. Proxies.
The Holder of a Warrant or Pre-funded Warrant may grant proxies or otherwise authorize any person, including the Participants and beneficial
holders that may own interests through the Participants, to take any action that a Holder is entitled to take under this Agreement or
the Warrants or Pre-funded Warrants; provided, however, that at all times that Warrants or Pre-funded Warrants
are evidenced by a Global Warrant, exercise of those Warrants or Pre-funded Warrants shall be effected on their behalf by Participants
through DTC in accordance the procedures administered by DTC.
3. Terms
and Exercise of Warrants.
3.1. Exercise
Price. Each Warrant shall entitle the Holder, subject to the provisions of the applicable Warrant Certificate and of this Warrant
Agreement, to purchase from the Company the number of shares of Common Stock stated therein, at the price of $[•] per share of Common
Stock, subject to the subsequent adjustments provided in the Global Warrant. Each Pre-funded Warrant shall entitle the Holder, subject
to the provisions of the applicable Warrant Certificate and of this Warrant Agreement to purchase from the Company the number of shares
of Common Stock stated therein, at the price of $0.0001 per share of Common Stock, subject to the subsequent adjustments provided in the
Global Warrant. The term “Exercise Price” as used in this Warrant Agreement refers to the price per
share at which shares of Common Stock may be purchased at the time a Warrant or Pre-funded Warrant is exercised.
3.2. Duration
of Warrants. A Warrant may be exercised only during the period (“Exercise Period”) commencing on the date of issuance
and ending on the Termination Date. For purposes of this Warrant Agreement, the “Termination Date” shall
have the meaning set forth in the Global Warrant. Each Warrant not exercised on or before the Termination Date shall become void, and
all rights thereunder and all rights in respect thereof under this Agreement shall cease at the close of business on the Termination Date.
The Pre-funded Warrants do not expire.
3.3. Exercise
of Warrants.
3.3.1. Exercise.
Subject to the provisions of each Global Warrant, a Holder (or a Participant or a designee of a Participant acting on behalf of a Holder)
may exercise Warrants or Pre-funded Warrants by delivering to the Warrant Agent, not later than 5:00 P.M., Eastern Time, on any business
day during the Exercise Period a notice of exercise of the Warrants or Pre-funded Warrants to be exercised (i) in the form attached
to the Global Warrant or (ii) via an electronic warrant exercise through the DTC system (each, an “Election to Purchase”)
and, unless the cashless exercise procedure is specified in the applicable Election to Purchase, delivery of the aggregate Exercise Price
of the Warrant Shares specified in the applicable Election to Purchase. All other requirements for the exercise of a Warrant or Pre-funded
Warrant shall be as set forth in the Warrant or Pre-funded Warrant, respectively. Notwithstanding anything herein to the contrary, the
Holder shall not be required to physically surrender a Warrant Certificate to the Warrant Agent until the Holder has purchased all of
the Warrant Shares available thereunder and the applicable Warrant Certificate has been exercised in full, in which case, the Holder shall
surrender the Warrant Certificate to the Warrant Agent for cancellation within three (3) Trading Days of the date on which the final
Election to Purchase is delivered to the Warrant Agent. Partial exercises of a Warrant Certificate resulting in purchases of a portion
of the total number of Warrant Shares available thereunder shall have the effect of lowering the outstanding number of Warrant Shares
purchasable thereunder in an amount equal to the applicable number of Warrant Shares purchased.
3.3.2. The
Warrant Agent shall, by 5:00 P.M., Eastern Time, on the Trading Day following the Exercise Date (as defined below) of any Warrant or Pre-funded
Warrant, advise the Company, the transfer agent and registrar for the Company’s Common Stock, in respect of (i) the number
of Warrant Shares indicated on the Notice of Exercise as issuable upon such exercise with respect to such exercised Warrants, (ii) the
instructions of the Holder or Participant, as the case may be, provided to the Warrant Agent with respect to the delivery of the Warrant
Shares and the number of Warrants or Pre-Funded Warrants that remain outstanding after such exercise and (iii) such other information
as the Company or such transfer agent and registrar shall reasonably request. The Company shall issue the Warrant Shares in compliance
with the terms of the Warrant or Pre-funded Warrant, as applicable.
3.3.3. Valid
Issuance. All Warrant Shares issued by the Company upon the proper exercise of a Warrant or Pre-funded Warrant in conformity with
this Warrant Agreement shall be validly issued, fully paid and non-assessable.
3.3.4. No
Fractional Exercise. Notwithstanding any provision contained in this Warrant Agreement to the contrary, no fractional shares or scrip
representing fractional shares shall be issued upon the exercise of the Warrant or Pre-funded Warrant. As to any fraction of a share which
the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment
in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
3.3.5. No
Transfer Taxes. The Company shall not be required to pay any stamp or other tax or governmental charge required to be paid in connection
with any transfer involved in the issue of the Warrant Shares upon the exercise of Warrants or Pre-funded Warrants; and in the event that
any such transfer is involved, the Company shall not be required to issue or deliver any Warrant Shares until such tax or other charge
shall have been paid or it has been established to the Company’s satisfaction that no such tax or other charge is due.
3.3.6. Date
of Issuance. The Company will treat an exercising Holder as a beneficial owner of the Warrant Shares as of the time of delivery of
a Notice of Exercise (as defined in the Warrant Certificate) to the Warrant Agent or the Company, and for purposes of Regulation SHO,
a holder whose interest in the Warrant or Pre-funded Warrant is a beneficial interest in certificate(s) representing the Warrant
or Pre-funded Warrant held in book-entry form through DTC shall be deemed to have exercised its interest in the Warrant or Pre-funded
Warrant upon instructing its broker that is a DTC participant to exercise its interest in the Warrant or Pre-funded Warrant (the date
of any such exercise, an “Exercise Date”), except that, if the Exercise Date is a date when the stock transfer books
of the Company are closed, such person shall be deemed to have become the holder of such shares at the open of business on the next succeeding
date on which the stock transfer books are open.
4. Adjustments.
Upon every adjustment of the Exercise Price or the number of Warrant Shares issuable upon exercise of a Warrant or Pre-funded Warrant,
the Company shall give written notice thereof to the Warrant Agent, which notice shall state the Exercise Price resulting from such adjustment
and the increase or decrease, if any, in the number of Warrant Shares purchasable at such price upon the exercise of a Warrant or Pre-funded
Warrant, setting forth in reasonable detail the method of calculation and the facts upon which such calculation is based. Upon the occurrence
of any event specified in Section 3 of the Warrant or Pre-funded Warrant, then, in any such event, the Company shall give written
notice to the Warrant Agent. Failure to give such notice, or any defect therein, shall not affect the legality or validity of such event.
The Warrant Agent shall be entitled to rely conclusively on, and shall be fully protected in relying on, any certificate, notice or instructions
provided by the Company with respect to any adjustment of the Exercise Price or the number of shares issuable upon exercise of a Warrant
or Pre-funded Warrant, or any related matter, and the Warrant Agent shall not be liable for any action taken, suffered or omitted to be
taken by it in accordance with any such certificate, notice or instructions or pursuant to this Warrant Agreement. The Warrant Agent shall
not be deemed to have knowledge of any such adjustment unless and until it shall have received written notice thereof from the Company.
5. Restrictive
Legends; Fractional Warrants. In the event that a Warrant Certificate surrendered for transfer bears a restrictive legend, the Warrant
Agent shall not register that transfer until the Warrant Agent has received an opinion of counsel for the Company stating that such transfer
may be made and indicating whether the Warrants or Pre-funded Warrants must also bear a restrictive legend upon that transfer. The Warrant
Agent shall not be required to effect any registration of transfer or exchange which will result in the transfer of or delivery of a Warrant
Certificate for a fraction of a Warrant or Pre-funded Warrant.
6. Other
Provisions Relating to Rights of Holders of Warrants.
6.1. No
Rights as Stockholder. Except as otherwise specifically provided herein, a Holder, solely in its capacity as a holder of Warrants
or Pre-funded Warrants, shall not be entitled to vote or receive dividends or be deemed the holder of share capital of the Company for
any purpose, nor shall anything contained in this Warrant Agreement be construed to confer upon a Holder, solely in its capacity as the
registered holder of Warrants or Pre-funded Warrants, any of the rights of a stockholder of the Company or any right to vote, give or
withhold consent to any corporate action (whether any reorganization, issue of stock, reclassification of share capital, consolidation,
merger, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights or rights to participate in new
issues of shares, or otherwise, prior to the issuance to the Holder of the Warrant Shares which it is then entitled to receive upon the
due exercise of Warrants or Pre-funded Warrants.
6.2. Reservation
of Common Stock. The Company shall at all times reserve and keep available a number of its authorized but unissued shares of Common
Stock that will be sufficient to permit the exercise in full of all outstanding Warrants and Pre-funded Warrants issued pursuant to this
Warrant Agreement.
7. Concerning
the Warrant Agent and Other Matters.
7.1. Any
instructions given to the Warrant Agent orally, as permitted by any provision of this Warrant Agreement, shall be confirmed in writing
by the Company as soon as practicable. The Warrant Agent shall not be liable or responsible and shall be fully authorized and protected
for acting, or failing to act, in accordance with any oral instructions which do not conform with the written confirmation received in
accordance with this Section 7.1.
7.2. (a) Whether
or not any Warrants or Pre-funded Warrants are exercised, for the Warrant Agent’s services as agent for the Company hereunder, the
Company shall pay to the Warrant Agent such fees as may be separately agreed between the Company and Warrant Agent. (b) All amounts
owed by the Company to the Warrant Agent under this Warrant Agreement are due within 30 days of the invoice date. Delinquent payments
are subject to a late payment charge of one and one-half percent (1.5%) per month commencing 45 days from the invoice date. The Company
agrees to reimburse the Warrant Agent for any attorney’s fees and any other costs associated with collecting delinquent payments.
(c) No provision of this Warrant Agreement shall require Warrant Agent to expend or risk its own funds or otherwise incur any financial
liability in the performance of any of its duties under this Warrant Agreement or in the exercise of its rights.
7.3. As
agent for the Company hereunder the Warrant Agent: (a) shall have no duties or obligations other than those specifically set forth
herein or as may subsequently be agreed to in writing by the Warrant Agent and the Company; (b) shall be regarded as making no representations
and having no responsibilities as to the validity, sufficiency, value, or genuineness of the Warrants, Pre-funded Warrants or any Warrant
Shares; (c) shall not be obligated to take any legal action hereunder; if, however, the Warrant Agent determines to take any legal
action hereunder, and where the taking of such action might, in its judgment, subject or expose it to any expense or liability it shall
not be required to act unless it has been furnished with an indemnity reasonably satisfactory to it; (d) may rely on and shall be
fully authorized and protected in acting or failing to act upon any certificate, instrument, opinion, notice, letter or other document
or security delivered to the Warrant Agent and believed by it to be genuine and to have been signed by the proper party or parties; (e) shall
not be liable or responsible for any recital or statement contained in the Registration Statement or any other documents relating thereto;
(f) shall not be liable or responsible for any failure on the part of the Company to comply with any of its covenants and obligations
relating to the Warrants and Pre-funded Warrants, including without limitation obligations under applicable securities laws; (g) may
rely on and shall be fully authorized and protected in acting or failing to act upon the written, telephonic or oral instructions with
respect to any matter relating to its duties as Warrant Agent covered by this Warrant Agreement (or supplementing or qualifying any such
actions) of officers of the Company, and is hereby authorized and directed to accept instructions with respect to the performance of its
duties hereunder from the Company or counsel to the Company, and may apply to the Company, for advice or instructions in connection with
the Warrant Agent’s duties hereunder, and the Warrant Agent shall not be liable for any delay in acting while waiting for those
instructions; any applications by the Warrant Agent for written instructions from the Company may, at the option of the Warrant Agent,
set forth in writing any action proposed to be taken or omitted by the Warrant Agent under this Warrant Agreement and the date on or after
which such action shall be taken or such omission shall be effective; the Warrant Agent shall not be liable for any action taken by, or
omission of, the Warrant Agent in accordance with a proposal included in such application on or after the date specified in such application
(which date shall not be less than five business days after the date such application is sent to the Company, unless the Company shall
have consented in writing to any earlier date) unless prior to taking any such action, the Warrant Agent shall have received written instructions
in response to such application specifying the action to be taken or omitted; (h) may consult with counsel satisfactory to the Warrant
Agent, including its in-house counsel; (i) may perform any of its duties hereunder either directly or by or through nominees, correspondents,
designees, or subagents, and it shall not be liable or responsible for any misconduct or negligence on the part of any nominee, correspondent,
designee, or subagent appointed with reasonable care by it in connection with this Warrant Agreement; (j) is not authorized, and
shall have no obligation, to pay any brokers, dealers, or soliciting fees to any person; and (k) shall not be required hereunder
to comply with the laws or regulations of any country other than the United States of America or any political subdivision thereof.
7.4. (a) In
the absence of gross negligence or willful or illegal misconduct on its part, the Warrant Agent shall not be liable for any action taken,
suffered, or omitted by it or for any error of judgment made by it in the performance of its duties under this Warrant Agreement. Anything
in this Warrant Agreement to the contrary notwithstanding, in no event shall Warrant Agent be liable for special, indirect, incidental,
consequential or punitive losses or damages of any kind whatsoever (including but not limited to lost profits, liquidated damages or buy-in
claims), even if the Warrant Agent has been advised of the possibility of such losses or damages and regardless of the form of action.
Any liability of the Warrant Agent will be limited in the aggregate to the amount of fees paid by the Company hereunder. The Warrant Agent
shall not be liable for any failures, delays or losses, arising directly or indirectly out of conditions beyond its reasonable control
including, but not limited to, acts of government, exchange or market ruling, suspension of trading, work stoppages or labor disputes,
fires, civil disobedience, riots, rebellions, storms, electrical or mechanical failure, computer hardware or software failure, communications
facilities failures including telephone failure, war, terrorism, insurrection, earthquakes, floods, acts of God or similar occurrences.
(b) In the event any question or dispute arises with respect to the proper interpretation of the Warrants or the Warrant Agent’s
duties under this Warrant Agreement or the rights of the Company or of any Holder, the Warrant Agent shall not be required to act and
shall not be held liable or responsible for its refusal to act until the question or dispute has been judicially settled (and, if appropriate,
it may file a suit in interpleader or for a declaratory judgment for such purpose) by final judgment rendered by a court of competent
jurisdiction, binding on all persons interested in the matter which is no longer subject to review or appeal, or settled by a written
document in form and substance satisfactory to Warrant Agent and executed by the Company and each such Holder. In addition, the Warrant
Agent may require for such purpose, but shall not be obligated to require, the execution of such written settlement by all the Holders
and all other persons that may have an interest in the settlement.
7.5. The
Company covenants to indemnify the Warrant Agent and hold it harmless from and against any loss, liability, claim or expense (“Loss”)
arising out of or in connection with the Warrant Agent’s duties under this Warrant Agreement, including the costs and expenses of
defending itself against any Loss, unless such Loss shall have been determined by a court of competent jurisdiction to be a result of
the Warrant Agent’s gross negligence or willful or illegal misconduct.
7.6. Unless
terminated earlier by the parties hereto, this Agreement shall terminate 90 days after the later of the Termination Date (as defined in
the Warrants and Pre-funded Warrants) and the date on which no Warrants remain outstanding (the “Termination Date”).
On the business day following the Termination Date, the Warrant Agent shall deliver to the Company any entitlements, if any, held by the
Warrant Agent under this Warrant Agreement. The Agent’s right to be reimbursed for fees, charges and out-of-pocket expenses as provided
in this Section 8 shall survive the termination of this Warrant Agreement.
7.7. If
any provision of this Warrant Agreement shall be held illegal, invalid, or unenforceable by any court, this Warrant Agreement shall be
construed and enforced as if such provision had not been contained herein and shall be deemed an agreement among the parties to it to
the full extent permitted by applicable law.
7.8. The
Company represents and warrants that: (a) it is duly incorporated and validly existing under the laws of its jurisdiction of incorporation;
(b) the offer and sale of the Warrants and Pre-funded Warrants and the execution, delivery and performance of all transactions contemplated
thereby (including this Warrant Agreement) have been duly authorized by all necessary corporate action and will not result in a breach
of or constitute a default under the articles of association, bylaws or any similar document of the Company or any indenture, agreement
or instrument to which it is a party or is bound; (c) this Warrant Agreement has been duly executed and delivered by the Company
and constitutes the legal, valid, binding and enforceable obligation of the Company; (d) the Warrants and Pre-funded Warrants will
comply in all material respects with all applicable requirements of law; and (e) to the best of its knowledge, there is no litigation
pending or threatened as of the date hereof in connection with the offering of the Warrants or Pre-funded Warrants.
7.9. In
the event of inconsistency between this Warrant Agreement and the terms set forth in the Warrant Certificate with respect to a Warrant
or Pre-funded Warrant, as it may from time to time be amended, the terms of the Warrant Certificate shall control.
7.10. Set
forth in Exhibit D hereto is a list of the names and specimen signatures of the persons authorized to act for the
Company under this Warrant Agreement (the “Authorized Representatives”). The Company shall, from time to time,
certify to the Warrant Agent the names and signatures of any other persons authorized to act for the Company under this Warrant Agreement.
7.11. Except
as expressly set forth elsewhere in this Warrant Agreement, all notices, instructions and communications under this Agreement shall be
in writing, shall be effective upon receipt and shall be addressed, if to the Company, to its address set forth beneath its signature
to this Agreement, or, if to the Warrant Agent, to VStock Transfer, LLC, 18 Lafayette Place, Woodmere, NY 11598, or to such other address
of which a party hereto has notified the other party.
7.12. (a) This
Warrant Agreement shall be governed by and construed in accordance with the laws of the State of New York. All actions and proceedings
relating to or arising from, directly or indirectly, this Warrant Agreement may be litigated in courts located within the Borough of Manhattan
in the City and State of New York. The Company hereby submits to the personal jurisdiction of such courts and consents that any service
of process may be made by certified or registered mail, return receipt requested, directed to the Company at its address last specified
for notices hereunder. (b) This Warrant Agreement shall inure to the benefit of and be binding upon the successors and assigns of
the parties hereto. This Warrant Agreement may not be assigned, or otherwise transferred, in whole or in part, by either party without
the prior written consent of the other party, which the other party will not unreasonably withhold, condition or delay; except that (i) consent
is not required for an assignment or delegation of duties by Warrant Agent to any affiliate of Warrant Agent and (ii) any reorganization,
merger, consolidation, sale of assets or other form of business combination by the Warrant Agent or the Company shall not be deemed to
constitute an assignment of this Warrant Agreement. (c) No provision of this Warrant Agreement may be amended, modified or waived,
except in a written document signed by both parties.
7.13. Payment
of Taxes. The Company will from time to time promptly pay all taxes and charges that may be imposed upon the Company or the Warrant
Agent in respect of the issuance or delivery of Warrant Shares upon the exercise of Warrants and Pre-funded Warrants, but the Company
may require the Holders to pay any transfer taxes in respect of the Warrants or such shares. The Warrant Agent may refrain from registering
any transfer of Warrants or Pre-funded Warrants or any delivery of any Warrant Shares unless or until the persons requesting the registration
or issuance shall have paid to the Warrant Agent for the account of the Company the amount of such tax or charge, if any, or shall have
established to the reasonable satisfaction of the Company and the Warrant Agent that such tax or charge, if any, has been paid.
7.14. Resignation
of Warrant Agent.
7.14.1. Appointment
of Successor Warrant Agent. The Warrant Agent, or any successor to it hereafter appointed, may resign its duties and be discharged
from all further duties and liabilities hereunder after giving thirty (30) days’ notice in writing to the Company, or such shorter
period of time agreed to by the Company. The Company may terminate the services of the Warrant Agent, or any successor Warrant Agent,
after giving thirty (30) days’ notice in writing to the Warrant Agent or successor Warrant Agent, or such shorter period of time
as agreed. If the office of the Warrant Agent becomes vacant by resignation, termination or incapacity to act or otherwise, the Company
shall appoint in writing a successor Warrant Agent in place of the Warrant Agent. If the Company shall fail to make such appointment within
a period of 30 days after it has been notified in writing of such resignation or incapacity by the Warrant Agent, then the Warrant Agent
or any Holder may apply to any court of competent jurisdiction for the appointment of a successor Warrant Agent at the Company’s
cost. Pending appointment of a successor to such Warrant Agent, either by the Company or by such a court, the duties of the Warrant Agent
shall be carried out by the Company. Any successor Warrant Agent (but not including the initial Warrant Agent), whether appointed by the
Company or by such court, shall be a person organized and existing under the laws of any state of the United States of America, in good
standing, and authorized under such laws to exercise corporate trust powers and subject to supervision or examination by federal or state
authority. After appointment, any successor Warrant Agent shall be vested with all the authority, powers, rights, immunities, duties,
and obligations of its predecessor Warrant Agent with like effect as if originally named as Warrant Agent hereunder, without any further
act or deed, and except for executing and delivering documents as provided in the sentence that follows, the predecessor Warrant Agent
shall have no further duties, obligations, responsibilities or liabilities hereunder, but shall be entitled to all rights that survive
the termination of this Warrant Agreement and the resignation or removal of the Warrant Agent, including but not limited to its right
to indemnity hereunder. If for any reason it becomes necessary or appropriate or at the request of the Company, the predecessor Warrant
Agent shall execute and deliver, at the expense of the Company, an instrument transferring to such successor Warrant Agent all the authority,
powers, and rights of such predecessor Warrant Agent hereunder; and upon request of any successor Warrant Agent the Company shall make,
execute, acknowledge, and deliver any and all instruments in writing for more fully and effectually vesting in and confirming to such
successor Warrant Agent all such authority, powers, rights, immunities, duties, and obligations.
7.14.2. Notice
of Successor Warrant Agent. In the event a successor Warrant Agent shall be appointed, the Company shall give notice thereof to the
predecessor Warrant Agent and the transfer agent for the Common Stock not later than the effective date of any such appointment.
7.14.3. Merger
or Consolidation of Warrant Agent. Any person into which the Warrant Agent may be merged or converted or with which it may be consolidated
or any person resulting from any merger, conversion or consolidation to which the Warrant Agent shall be a party or any person succeeding
to the shareowner services business of the Warrant Agent or any successor Warrant Agent shall be the successor Warrant Agent under this
Warrant Agreement, without any further act or deed. For purposes of this Warrant Agreement, “person” shall mean any individual,
firm, corporation, partnership, limited liability company, joint venture, association, trust or other entity, and shall include any successor
(by merger or otherwise) thereof or thereto.
8. Miscellaneous
Provisions.
8.1. Persons
Having Rights under this Warrant Agreement. Nothing in this Warrant Agreement expressed and nothing that may be implied from any of
the provisions hereof is intended, or shall be construed, to confer upon, or give to, any person or corporation other than the parties
hereto any right, remedy, or claim under or by reason of this Warrant Agreement or of any covenant, condition, stipulation, promise, or
agreement hereof.
8.2. Examination
of the Warrant Agreement. A copy of this Warrant Agreement shall be available at all reasonable times at the office of the Warrant
Agent designated for such purpose for inspection by any Holder. Prior to such inspection, the Warrant Agent may require any such holder
to provide reasonable evidence of its interest in the Warrants or the Pre-funded Warrants.
8.3. Counterparts.
This Warrant Agreement may be executed in any number of original, facsimile or electronic counterparts and each of such counterparts shall
for all purposes be deemed to be an original, and all such counterparts shall together constitute but one and the same instrument.
8.4. Effect
of Headings. The Section headings herein are for convenience only and are not part of this Warrant Agreement and shall not affect
the interpretation thereof.
9. Certain
Definitions. As used herein, the following terms shall have the following meanings:
| (a) | “Trading Day” means any day on which the Common Stock is traded on the Trading
Market, or, if the Trading Market is not the principal trading market for the Common Stock, then on the principal securities exchange
or securities market in the United States on which the Common Stock are then traded, provided that “Trading Day” shall
not include any day on which the Common Stock is scheduled to trade on such exchange or market for less than 4.5 hours or any day that
the Common Stock are suspended from trading during the final hour of trading on such exchange or market (or if such exchange or market
does not designate in advance the closing time of trading on such exchange or market, then during the hour ending at 4:00 P.M., Eastern
Time). |
| (b) | “Trading Market” means NYSE American, the Nasdaq Capital Market, the Nasdaq Global
Market, the Nasdaq Global Select Market or the New York Stock Exchange. |
[Signature Page Follows]
IN WITNESS WHEREOF, this Warrant
Agent Agreement has been duly executed by the parties hereto as of the day and year first above written
| |
AVENUE THERAPEUTICS, INC. |
| |
|
|
| |
By: |
|
| |
Name: |
Alexandra MacLean, M.D. |
| |
Title: |
Chief Executive Officer |
| |
Address: |
1111 Kane Concourse, Suite 301 |
| |
|
Bay Harbor Islands, Florida 33154 |
| |
|
|
| |
VSTOCK TRANSFER, LLC |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
[Signature
Page to Warrant Agent Agreement]
EXHIBIT A
GLOBAL
WARRANT
[See
attached.]
EXHIBIT B
GLOBAL
PRE-FUNDED WARRANT
[See
attached.]
EXHIBIT C
WARRANT CERTIFICATE REQUEST NOTICE
To: ___________ as Warrant Agent for __________
(the “Company”)
The undersigned Holder of Common Stock Purchase
Warrants (“Warrants”) in the form of Global Warrants issued by the Company hereby elects to receive a Warrant Certificate
evidencing the Warrants held by the Holder as specified below:
| 1. |
Name of Holder of Warrants in form of Global Warrants: _____________________________ |
| |
|
| 2. |
Name of Holder in Warrant Certificate (if different from name of Holder of Warrants in form of Global Warrants): ________________________________ |
| |
|
| 3. |
Number of Warrants in name of Holder in form of Global Warrants: ___________________ |
| |
|
| 4. |
Number of Warrants for which Warrant Certificate shall be issued: __________________ |
| |
|
| 5. |
Number of Warrants in name of Holder in form of Global Warrants after issuance of Warrant Certificate, if any: ___________ |
| |
|
| 6. |
Warrant Certificate shall be delivered to the following address: |
______________________________
______________________________
______________________________
______________________________
The undersigned hereby acknowledges and agrees
that, in connection with this Warrant Exchange and the issuance of the Warrant Certificate, the Holder is deemed to have surrendered the
number of Warrants in form of Global Warrants in the name of the Holder equal to the number of Warrants evidenced by the Warrant Certificate.
[SIGNATURE
OF HOLDER]
Name of Investing Entity: ____________________________________________________
Signature
of Authorized Signatory of Investing Entity: ______________________________
Name of Authorized Signatory: ________________________________________________
Title of Authorized Signatory: _________________________________________________
Date: _______________________________________________________________
EXHIBIT D
AUTHORIZED REPRESENTATIVES
Exhibit 23.1
Consent of Independent Registered Public Accounting
Firm
We consent to the use of our report dated March 31, 2023,
with respect to the consolidated financial statements of Avenue Therapeutics, Inc., incorporated herein by reference, and to the
reference to our firm under the heading "Experts" in the prospectus.
/s/ KPMG LLP
New York, New York
September 29, 2023
Exhibit 23.2
Consent
of Independent Registered Public Accounting Firm
Avenue Therapeutics, Inc.
Bay Harbor Islands, FL
We hereby consent to the incorporation by reference in the Prospectus constituting a part of this Registration Statement of our report
dated March 25, 2022, relating to the financial statements of Avenue Therapeutics, Inc. appearing in the Company’s Annual Report
on Form 10-K for the year ended December 31, 2022. Our report contains an explanatory paragraph regarding the Company’s ability
to continue as a going concern.
We also consent to the reference to us under the caption “Experts” in the Prospectus.
/s/
BDO USA, P.C.
New
York, New York
September 29, 2023
Exhibit 23.3
Consent of Independent Registered Public Accounting
Firm
We consent to the use of our report dated August 31, 2022,
with respect to the financial statements of Baergic Bio, Inc., incorporated herein by reference, and to the reference to our firm
under the heading "Experts" in the prospectus.
/s/ KPMG LLP
New York, New York
September 29, 2023
Exhibit 107
Calculation of Filing Fee Tables
Form
S-1
(Form Type)
Avenue
Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward
Securities
| |
Security
Type |
|
Security
Class
Title |
|
Fee
Calculation
or Carry
Forward Rule |
|
|
Amount
Registered |
|
|
Proposed
Maximum
Offering Price
Per Unit(1)(2) |
|
|
Maximum
Aggregate
Offering Price |
|
|
Fee Rate |
|
Amount of
Registration Fee |
|
| |
|
|
Newly Registered Securities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fees to Be Paid |
Equity |
|
Units, each consisting of one share of Common Stock, par value $0.0001 per share, and one Warrant to purchase one share of Common Stock, and Pre-funded Units, each consisting of one Pre-funded Warrant to purchase one share of Common Stock, and one Warrant to purchase one share of Common Stock (3) |
|
|
457 |
(o) |
|
|
|
|
|
|
|
|
|
$ |
12,000,000 |
|
|
$110.20 per $1,000,000 |
|
$ |
1,322.40 |
|
| Fees to Be Paid |
Equity |
|
Common Stock included as part of the Units |
|
|
457 |
(g) |
|
|
|
|
|
|
|
|
|
|
-- |
(4) |
|
|
|
|
|
|
| Fees to Be Paid |
Equity |
|
Warrants to purchase Common Stock included as part of the Units and Pre-funded Units |
|
|
457 |
(g) |
|
|
|
|
|
|
|
|
|
|
-- |
(4) |
|
|
|
|
|
|
| Fees to Be Paid |
Equity |
|
Common Stock underlying Warrants included in the Units and Pre-funded Units |
|
|
457 |
(o) |
|
|
|
|
|
|
|
|
|
$ |
12,000,000 |
|
|
$110.20 per $1,000,000 |
|
$ |
1,322.40 |
|
| Fees to Be Paid |
Equity |
|
Pre-funded Warrants to purchase Common Stock included as part of the Pre-funded Units |
|
|
457 |
(g) |
|
|
|
|
|
|
|
|
|
|
-- |
(4) |
|
|
|
|
|
|
| Fees to Be Paid |
Equity |
|
Common Stock underlying Pre-funded Warrants included in the Pre-funded Units |
|
|
457 |
(g) |
|
|
|
|
|
|
|
|
|
|
-- |
(4) |
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total Offering Amounts |
|
|
|
|
|
|
|
$ |
24,000,000 |
|
|
$110.20 per $1,000,000 |
|
$ |
2,644.80 |
|
| |
Total Fees Previously Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,644.80 |
|
| |
Total Fee Offsets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-- |
|
| |
Net Fee Due |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
0 |
|
| (1) | Estimated solely for the purpose of calculating the registration
fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | Pursuant to Rule 416 under the Securities Act, the securities
being registered hereunder include such indeterminate number of additional securities as may be issuable to prevent dilution resulting
from stock splits, dividends or similar transactions. |
| (3) | The proposed maximum offering price of the units proposed to
be sold in the offering will be reduced on a dollar-for-dollar basis based on the offering price of any pre-funded units
offered and sold in the offering, and as such the proposed aggregate maximum offering price of the units together with the pre-funded units
(including the Common Stock issuable upon exercise of the pre-funded warrants), if any, is $12,000,000.00. |
| (4) | No separate fee is required pursuant to Rule 457(g) under the
Securities Act. |
Avenue Therapeutics (NASDAQ:ATXI)
Historical Stock Chart
From Oct 2024 to Nov 2024
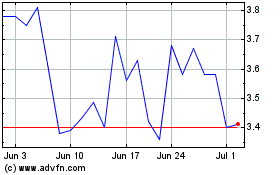
Avenue Therapeutics (NASDAQ:ATXI)
Historical Stock Chart
From Nov 2023 to Nov 2024