UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT
TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of Earliest Event Reported): February 22, 2016
AVALANCHE BIOTECHNOLOGIES, INC.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-36579 |
|
20-5258327 |
| (State or Other Jurisdiction
of Incorporation) |
|
(Commission
File No.) |
|
(I.R.S. Employer
Identification No.) |
1035 O’Brien Drive, Suite A
Menlo Park, CA 94025
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (650) 272-6269
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the
following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| x |
Soliciting material pursuant to Rule 14a–12 under the Exchange Act (17 CFR 240.14a–12) |
| ¨ |
Pre-commencement communication pursuant to Rule 14d–2(b) under the Exchange Act (17 CFR 240.14d–2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e–4(c) under the Exchange Act (17 CFR 240.13e–4(c)) |
Item 8.01 Other Events.
On February 22, 2016, Avalanche Biotechnologies, Inc. (the “Company”) made available on its website a presentation
outlining certain scientific matters relating to the gene therapy programs of Annapurna Therapeutic SAS (“Annapurna”), which presentation was made on February 17, 2016 by Ronald Crystal, M.D., and Mitch Finer, Ph.D. Dr. Crystal
is Chairman of Genetic Medicine, the Bruce Webster Professor of Internal Medicine and a Professor of Genetic Medicine and of Medicine at Weill Cornell Medicine, and a co-founder of Annapurna. Dr. Finer is a managing director of MPM Capital, and a
co-founder of and distinguished research fellow at the Company. The presentation materials and a transcript of the presentation are attached as Exhibit 99.1 and 99.2, respectively, to this Current Report on Form 8-K and are incorporated by reference
herein.
By filing the information in Item 8.01 of this Current Report on Form 8-K, the Company makes no admission as to the
materiality of any information contained herein. The information contained herein is intended to be considered in the context of the Company’s filings with the SEC and other public announcements that the Company makes, by press release or
otherwise, from time to time. The Company undertakes no duty or obligation to publicly update or revise the information contained in this Current Report on Form 8-K.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
| Exhibit No. |
|
Description |
|
|
| 99.1 |
|
Materials for presentation made by Drs. Ron Crystal and Mitch Finer on February 17, 2016. |
|
|
| 99.2 |
|
Transcript of presentation made by Drs. Ron Crystal and Mitch Finer on February 17, 2016. |
Additional Information and Where to Find It
This Current Report on Form 8-K does not constitute a solicitation of any vote or approval. In connection with the Company’s previously
announced proposed acquisition of Annapurna, the Company intends to file with the SEC a proxy statement of the Company, as well as other relevant documents concerning the proposed transaction. INVESTORS AND SECURITYHOLDERS OF THE COMPANY ARE URGED
TO READ THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION WHEN IT BECOMES AVAILABLE AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY WILL CONTAIN IMPORTANT
INFORMATION. A free copy of the proxy statement and other filings containing information about the Company may be obtained at the SEC website at www.sec.gov. You will also be able to obtain these documents, free of charge, from the Company by
directing a written request to: Avalanche Biotechnologies, Inc., 1035 O’Brien Drive, Suite A, Menlo Park, CA 94025, Attention: Investor Relations. Investors and securityholders are urged to read the proxy statement and the other relevant
materials when they become available before making any voting decision with respect to the issuance of the Company’s common stock or any other matters relating to the proposed transaction.
Certain Information Regarding Participants
The Company and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of
the Company in connection with the proposed transaction and the issuance of the Company’s common stock. Information regarding the special interests of these
directors and executive officers in the proposed transaction will be included in the proxy statement referred to above. Additional information regarding the directors and executive officers of
the Company is also included in Avalanche’s Annual Report on Form 10-K for the year ended December 31, 2014 and the proxy statement for the Company’s 2015 Annual Meeting of Stockholders. These documents are available free of charge at
the SEC web site at www.sec.gov and from Investor Relations at the Company at the address set forth above.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: February 22, 2016 |
|
|
|
AVALANCHE BIOTECHNOLOGIES, INC. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Paul B. Cleveland |
|
|
|
|
|
|
Paul B. Cleveland, Chief Executive Officer |

The Science Behind Annapurna R. Crystal
Department of Genetic Medicine Weill Cornell Medical College 2-17-16 Exhibit 99.1

Cautionary Statements Regarding
Forward-Looking Statements Statements contained in this document regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Examples of
such statements include, but are not limited to, statements regarding plans, potential opportunities, expectations, projections, goals, objectives, milestones, strategies, product pipeline, the occurrence or effect of the proposed transaction
between Avalanche Biotechnologies, Inc. (“Avalanche”) and Annapurna Therapeutics SAS (“Annapurna”), the sufficiency of the combined company’s resources to fund the advancement of any development program or the
completion of any clinical trials, and the safety, efficacy and projected development timeline and commercial potential of products under development by Avalanche and Annapurna, all of which are based on certain assumptions made by us based on
current conditions, expected future developments and other factors we believe are appropriate in the circumstances. Actual results, the timing of events, product development programs, performance or achievements could differ materially from those
anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties inherent in the product development and the regulatory approval process, delays in clinical
trials and other matters that could affect the availability or commercial potential of product candidates, the ability to consummate the proposed transaction with Annapurna, the ability to project future cash utilization, the availability of
sufficient resources to conduct or continue planned development programs, and the ability to successfully develop any of Avalanche’s or Annapurna’s product candidates. Risks and uncertainties facing Avalanche are described more fully in
Avalanche’s periodic reports filed with the SEC. All forward-looking statements contained in this document speak only as of the date on which they were made. Avalanche undertakes no obligation to update such statements to reflect events that
occur or circumstances that exist after the date on which they were made. This document contains estimates, projections and other information concerning Avalanche’s and Annapurna’s industry, business and the markets for certain drugs,
including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject
to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports,
research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by,
and should not be construed as representations made by, Avalanche or Annapurna.

Additional Information and Where To
Find It This document not constitute a solicitation of any vote or approval. In connection with the proposed transaction, Avalanche intends to file with the SEC a proxy statement of Avalanche, as well as other relevant documents concerning the
proposed transaction. INVESTORS AND SECURITYHOLDERS OF AVALANCHE ARE URGED TO READ THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION WHEN IT BECOMES AVAILABLE AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR
SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. A free copy of the proxy statement and other filings containing information about Avalanche may be obtained at the SEC website at www.sec.gov. You will also be able to
obtain these documents, free of charge, from Avalanche by directing a written request to: Avalanche Biotechnologies, Inc., 1035 O’Brien Drive, Suite A, Menlo Park, CA 94025, Attention: Investor Relations. Investors and security holders are
urged to read the proxy statement and the other relevant materials when they become available before making any voting decision with respect to the issuance of shares of the Avalanche common stock in connection with the proposed transaction and any
other matters relating to the proposed transaction. Certain Information Regarding Participants Avalanche and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Avalanche in
connection with the proposed transaction and the issuance of additional Avalanche common stock. Information regarding the special interests of these directors and executive officers in the proposed transaction will be included in the proxy statement
referred to above. Additional information regarding the directors and executive officers of Avalanche is also included in Avalanche’s Annual Report on Form 10-K for the year ended December 31, 2014 and the proxy statement for the
Company’s 2015 Annual Meeting of Stockholders. Additional information about the interests of potential participants will be contained in the proxy statement (when filed) and other relevant materials to be filed with the SEC in connection with
the proposed transaction. These documents may be obtained free of charge at the SEC web site at www.sec.gov and from Investor Relations at Avalanche in the manner set forth above.

Why Gene Therapy ? Gene Modify gene
expression Modify phenotype Advantages Versatile protein delivery system Persistent expression Local delivery

Basic Concept of Gene Therapy
Therapeutic gene Human genome m m m m m m m m m m m m m m m m m m Delivery vehicle (vector) Target organ m m m m m m m m m m m m m m m m m m

Challenges of Gene Therapy Challenges
Target choice – need, feasibility Targeting to site of disease Level of expression required Robust phenotype, clear demonstration of efficacy using FDA-acceptable parameters Safety – risk/benefit, dose-limiting immune related toxicity,
lack of control of expression and inability to reverse Manufacturing Registration

Annapurna Programs Alpha 1-antitrypsin
deficiency Hereditary angioedema Friedreich’s ataxia cardiac disease Severe allergy

AAVrh.10 Vectors ITR y ITR ITR CAG
promoter Therapeutic gene (cDNA) Genome Capsid serotype AAVrh.10 AAV2 AAV2 Therapeutic genes Alpha 1-antitrypsin C1-esterase inhibitor Frataxin Anti-IgE

Why Gene Therapy? Alpha 1-antitrypsin
deficiency, hereditary angioedema, severe allergy Reduced treatment burden Ideal pharmacokinetics - constant levels For hereditary angioedema and severe allergy, eliminate risk for attacks without need for immediate medical care Friedreich’s
ataxia cardiac disease Intracellular delivery of the deficient protein in the target organ Unmet medical need for a fatal disease

Alpha 1-Antitrypsin Deficiency
Common autosomal recessive disorder characterized by a marked reduction in serum a1AT levels Estimated 90,000 affected individuals in US Emphysema develops at ages 35-45 in cigarette smokers, 55-65 in non-smokers Small % develop liver cirrhosis a1AT
normally protects the lung from the destructive potential of neutrophil elastase; if a1AT levels are low, neutrophil elastase slowly destroys the lung parenchyma Current therapy to protect the lung - weekly intravenous infusions of 4 gm of human
a1AT purified from pooled plasma
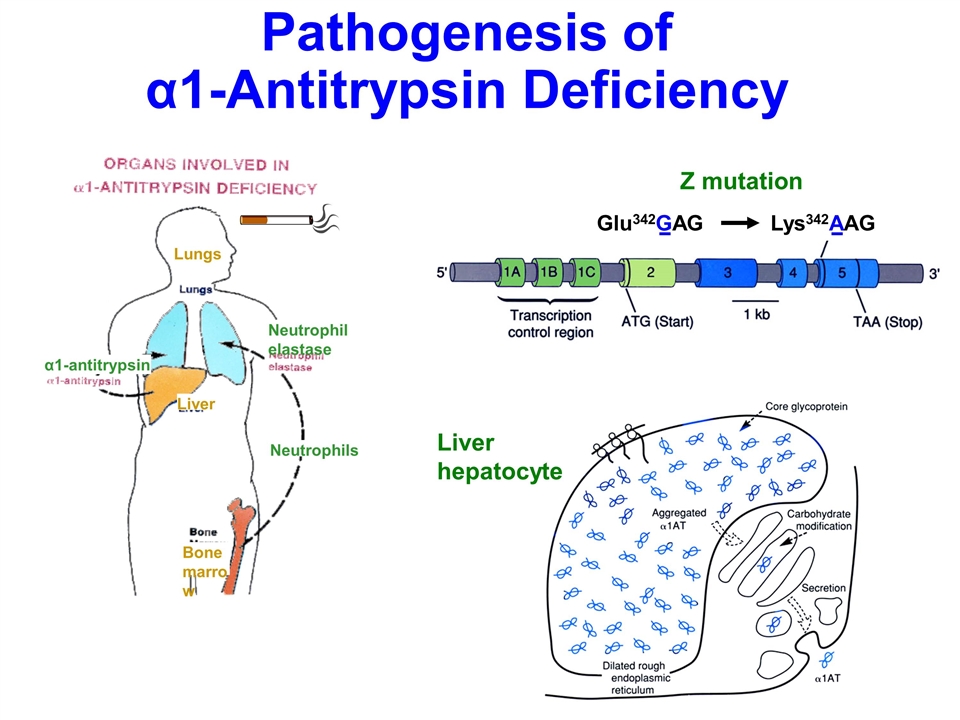
Pathogenesis of α1-Antitrypsin
Deficiency α1-antitrypsin Neutrophil elastase Lungs Liver Neutrophils Bone marrow Glu342GAG Lys342AAG Z mutation Liver hepatocyte

Augmentation Therapy with Purified
Human a1-Antitrypsin Developed by Gadek and Crystal in 1981 FDA approved in 1988 Administration IV 60 mg/kg (~4 g), once weekly Safe - minimal adverse reactions Cost ~$100,000/yr

Lungs Liver Bone marrow Neutrophils
Neutrophil elastase α1-antitrypsin A. Pathogenesis D.Epithelial lining fluid anti-neutrophil elastase capacity before and after augmentation therapy Normal ZZ pre-therapy ZZ 6 days post- therapy 1 2 3 4 5 ELF anti-neutrophil elastase capacity
(mM) C.Serum α1AT following augmentation therapy with intravenous human α1AT pathogenesis Pulmonary capillary Interstitium Air Plasma proteins Endothelial cell Epithelial cell B. Alveolar endothelial-epithelial “barriers” ELF
Logic Underlying “Biochemical Efficacy” for the Lung Manifestations of α1-antitrypsin Deficiency Serum α1-antitrypsin level (mg/dl) Serum α1-antitrypsin level (μM) Days

Association of a1-Antitrypsin Serum
Levels and Risk for Emphysema MM MS SS MZ SZ ZZ Threshold 11 mM Null 0 10 20 30 40 50 a1AT serum level (mM) Phenotype At risk for emphysema Background risk

unidirectional valve parietal
extrapleural interstitium parietal lymphatic pleural space stoma microvilli basal lamina parietal pleural visceral pleural capillary pulmonary interstitum alveolus pulmonary lymphatic A. Anatomy of the human pleura AAV vector coding for normal human
a1AT B.Intrapleural gene therapy strategy Local production of α1AT AAVha1AT a1AT Mesothelial cells Lung Intravascular AAVα1AT AAVα1AT from the pleura via lymphatics to the venous circulation Hepatocyte expression of α1AT
α1AT secreted into blood diffuses across the alveoli Logic for Intrapleural Gene Transfer to Treat α1-antitrypsin Deficiency

Time post-injection (wk) Serum human
a1-antitrypsin levels (% of maximum) 0.1 1 10 100 0 1 2 3 4 Undetectable AAVch.5 AAVhu.11 AAVhu.41 AAVhu.47 AAVrh.34 AAVhu.1 AAVhu.13 AAVrh.16 AAVrh.24 AAVrh.22 AAVrh.21 AAVrh.13 AAVbb.2 AAV2 AAVcy.5 AAV7 AAVrh.2 AAVrh.8 AAVrh.43 AAVrh.20 AAVhu.37
AAV5 AAV9 AAV8 AAVrh.10 Vector Clade/ clone Species of origin E Rhesus macaque E Rhesus macaque E-D Rhesus macaque E-D Rhesus macaque E-D Rhesus macaque Serum Human a1AT Levels Following Intrapleural Administration to C57Bl/6 Mice of 25 Different
Serotypes of AAV Expressing a1AT E Rhesus macaque F Human AAV5 Human E Human E Rhesus macaque E Rhesus macaque Rh.8 Rhesus macaque E Rhesus macaque D Rhesus macaque D Cynomolgus macaque B Human B Baboon B Human D Human C Human rh.34 Rhesus macaque B
Human E Human C Human Ch.5 Chimpanzee

0 1 2 4 6 12 1 10 102 103 104 24
Time post-injection (wk) Human a1AT level in serum (mg/ml) Naive AAVrh.10hα1AT 0-24 wk Evaluate AAVrh.10hα1AT 1011 genome copies (gc) Intrapleural Human α1AT level in serum (ELISA) C57BL/6 mice n = 4/group Time Course of Serum Human
α1AT Levels Following Intrapleural Administration of AAVrh.10hα1AT Therapeutic target 11 μM

12 wk Evaluate Serum and lavage
human α1AT (ELISA) C57BL/6 mice n = 4/group Human α1AT Levels in Bronchoalveolar Lavage Fluid Compared to Serum Following Intrapleural Administration of AAVrh.10hα1AT Human a1AT (mg/mg protein) Naive AAVrh.10hα1AT Serum Serum
Lavage Lavage 0 10 20 30 40 AAVrh.10hα1AT 1011 gc, intrapleural

Ultrasonography-guided Intrapleural
Administration of AAVrh.10hα1AT to African Green Monkeys Single intrapleural injection of 1012 or 1013 genome copies of AAVrh.10ha1AT to African Green Monkeys (n=36) Quantify human α1AT mRNA in chest wall up to 1 yr All safety / toxicology
parameters normal at all time points Days after AAVrh.10hα1AT administration 107 105 104 103 102 28 90 360 0 Limit of detection 1013 gc AAVrh.10hα1AT 1012 gc AAVrh.10hα1AT PBS 106 hα1AT copies/mg of total RNA

Hereditary Angioedema Autosomal
dominant disorder associated with episodic attacks of swelling of face, extremities, genitals, GI tract and upper airways Airway edema can be life threatening Triggered by trauma, surgery, dental work, menstruation, medications, viral illness,
stress Affects 1 in 10,000-50,000 15,000-30,000 emergency room visits/yr in the US

Hereditary Angioedema (2) Caused by
mutations of the SERPING1 gene, coding for C1 esterase inhibitor (C1EI) that regulates the complement system C1EI - single chain, 510 residue, 105 kDa, glycosylated serine anti-protease expressed 10 by hepatocytes SERPING1 mutations result in
reduced serum levels in C1EI (80-85% cases) or functional C1EI deficiency (15-20%) C1EI deficiency results in up-regulation of bradykinin, causing edema via leaky vessels 6-8,000 patients in the US Approved/reimbursed prophylactic recombinant C1EI
therapy reduces number and severity of attacks, but requires 2x/week infusion and costs $500,000/yr

Gene Therapy for Hereditary
Angioedema Proof-of-concept studies cannot be discussed at this time due to patent filings in progress

Friedreich Ataxia Approximately
5,000 patients in the US, 5,000-10,000 in Europe Autosomal recessive, results from variants in the frataxin (FXN) gene, a nuclear gene coding for a mitochondrial protein associated with iron sulfur clusters Impairs dorsal root ganglia, spinal cord
and cerebellum resulting in gait ataxia, gradually worsening and spreading to the arms and the trunk, slurred and slowed speech Hypertrophic cardiomyopathy, >60% of patients die from cardiac events No effective therapy

MCK Mouse Model Recapitulating the
Cardiomyopathy of Friedrich Ataxia Absence of the frataxin protein in myocardium and skeletal muscle MCK 4 5 7 11 0 Death 8 Progressive left ventricle (LV) systolic dysfunction Cell death and fibrosis Progressive LV hypertrophy and dilation
Progressive cardiomyopathy Iron sulfur cluster deficit in heart Mitochondrial anomalies and cardiac iron deposits Biochemical features Cardiac hypertrophy (10 wk) Cardiac fibrosis (10 wk) Time (wk) Mutant Control Age (wk) CII/CIV activity (% WT)
Succinate dehydrogenase activity 2 3 4 5 6 7 8 9 10 11 25 125 100 75 50 0 *

3 MCK Evaluation of survival and
multiple cardiac phenotypes 5 7 Intravenous AAVrh10.hFXN 5.4x1013 vg/kg) 22 35 60 Time (wk) MCK Mice in Heart Failure Treated with AAVrh.10.hFXN Advanced heart failure

Untreated AAVrh10hFXN-treated Normal
A. Correction of hypertrophy B. Correction of cardiac function C. Survival Some treated mice die from late myopathy (MCK mice, not patients, have myopathy) AAVrh10hFXN Treatment of MCK Mice with Heart Failure at 7 wk - Rapid Normalization 22 18 14
10 20 16 12 8 0 80 60 40 20 Wk Left ventricle mass (mg) 40 30 20 10 0 22 18 14 10 20 16 12 8 Wk Shortening fraction (%) 22 18 14 6 10 2 Wk 100 80 40 20 0 60 Survival (%) Untreated AAVrh10hFXN-treated Normal Untreated AAVrh10hFXN-treated
Normal

Identifying a Robust Cardiac
Phenotype for Cardiac Freidreich’s Ataxia Gene Therapy Studies to identify cardiac parameters Department of Genetic Medicine, Weill Cornell Hôpital Universitaire Pitié-Salpêtrière, Paris Parameters Genetic Neurologic
Cardiac Serology Cardiac echo Cardiac mri Exercise CDG PET CT

Severe Allergy – Example
Peanut Allergy Common food allergy, manifests with itchiness, urticaria, swelling, eczema, asthma, abdominal pain, hypotension and anaphylaxis Can be fatal 0.4–0.6% US population, 4,000 diagnosed/yr (11/day); most common cause of anaphylaxis
in children presenting to the emergency ward Significant adverse effect on the quality of life No definitive therapy Strict avoidance Epinephrine Desensitization Omalizumab (Xolair) anti-IgE

Anti-IgE Therapy of Peanut Allergy
Omalizumab (Xolair) is a humanized IgG1 anti-IgE monoclonal that binds to Fc portion of circulating IgE, preventing the IgE from binding to, and triggering, mast cells Mast cell mediators Y Y Y Y Peanut-specific IgE Y Y Mast cell Exposure to peanuts
+ Peanut antigens IgE anti-peanut-peanut antigen immune complex Y Y Y Y Therapy with anti-IgE (Xolair) IgG-anti-IgE Y Y Y Y Y Y Mast cell Y Y Y Y Y Y Y Y Y Y Y Y Y Y

Characterization of NOD-scid IL2
Rgammanull Immunodeficient Mice Humanized with Immune Cells from Peanut Allergic and Control Donors PBMC from donor with no peanut allergy D. Post-peanut challenge PBMC from donor with peanut allergy PBMC from donor with no peanut allergy IgE
response pre-/post-peanut sensitization (wk) A. Serum peanut-specific IgE Serum human peanut-specific IgE (IU/ml) 0 1 2 3 4 5 6 7 1 2 3 PBMC from donor with peanut allergy PBMC from donor with no peanut allergy B. Histamine C. Anaphylaxis score PBMC
from donor with peanut allergy PBMC from donor with no peanut allergy PBMC from donor with peanut allergy 30 min post-peanut challenge Plasma histamine level ng/ml 0 500 1000 1500 2000 2500 3000 Anaphylaxis score p<0.02 p<0.04 * 0 2 3 4 1 *
undetectable 5

Expression of AAVrh.10Anti-hIgE in
NOD-scid IL2 Rgammanull Mice 2-44 wk Evaluate AAVrh.10anti-hIgE AAVrh.10IgGcontrol 1011 gc, intravenous Serum anti-hIgE antibody titer (every 2 wk) Nodscid IL2rγnull (n=4F/group) (6-8 wk) Time post-injection (wk) Serum anti-hIgE (μg/ml)
AAVrh.10anti-hIgE AAVrh.10IgGcontrol PBS 0 50 100 150 200 250 300 350 0 2 4 6 8 10 12 14 16 18 20 22 24 44

Treatment of Peanut Antigen-Induced
Systemic Anaphylaxis by Treatment with AAVrh.10anti-hIgE; Clinical and Molecular Phenotype A. Post-peanut (PN) challenge clinical phenotype Donor with PN allergy + omalizumab Donor with PN allergy + AAVrh.10anti-hIgE wk 10 (5 wk after therapy) wk 10
(5 wk after therapy) wk 10 (5 wk after therapy) B. Locomotor activity Cumulative distance traveled (cm) AAVrh.10IgGcontrol AAVrh.10anti-hIgE Omalizumab 0 100 200 300 400 500 wk 7 (2 wk after therapy) All AAVrh.10- IgGcontrol mice died * * p<0.001
p<0.001 p<0.5 p<0.001 C. Anaphylaxis score Anaphylaxis score (1-5) All AAVrh.10IgGcontrol mice died 0 1 2 3 4 5 AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab * wk 7 (2 wk after
therapy) wk 10 (5 wk after therapy) * p<0.001 p<0.001 p>0.5 p<0.001 p<0.001 p>0.5 0 1 2 AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab AAVrh.10- IgGcontrol AAVrh.10-anti-hIgE Omali-zumab * wk 6 (1 wk after therapy) wk 9 (4 wk
after therapy) D. Histamine Anaphylaxis score (1-5) All AAVrh.10IgGcontrol mice died 3 4 5 * p<0.001 p<0.001 p<0.5 p<0.01 p<0.5 p<0.01 E. Passive cutaneous anaphylaxis Mouse serum: PBMC donor PN allergy + omalizumab Mouse serum:
PBMC donor PN allergy + AAVrh.10anti-hIgE Human serum: PN allergic donor Human serum: non-PN allergic donor wk 7 (2 wk after therapy) wk 10 (5 wk after therapy)

Mouse Survival Following Treatment
with AAVrh.10anti-hIgE, Xolair Alone or Control Vector p<0.03 p<0.01 p<0.01 AAVrh.10IgGcontrol Omalizumab AAVrh.10anti-hIgE 100 60 40 20 0 80 0 30 20 10 40 Survival (%) Days after therapy

Gene Therapy Gene Modify gene
expression Modify phenotype
Exhibit 99.2
Transcript of Presentation made by Drs. Ronald Crystal and Mitchell Finer on February 17, 2016
Participants
Lauren Glaser, Avalanche Biotechnologies,
Inc., Head of Investor Relations
Ronald Crystal, MD., Chairman of Genetic Medicine, the Bruce Webster Professor of
Internal Medicine and a Professor of Genetic Medicine and of Medicine at Weill Cornell Medicine, and a co-founder of Annapurna Therapeutics SAS
Mitchell Finer, Ph.D., Managing Director of MPM Capital and a co-founder of Avalanche Biotechnologies, Inc.
LAUREN GLASER: Hi, everyone. Just wanted to welcome you all to our discussion today around the science behind
Annapurna.
Of course, I have to let you know that this presentation may contain forward-looking statements, and of course urge you to our
Risk Factors, which are contained within our files with the SEC.
Also, this conversation is being recorded, and will be posted
subsequently to our website. And with that, I’d like to introduce Dr. Mitchell Finer.
DR. MITCHELL
FINER: Great, thanks Lauren. Good afternoon. I’m Mitchell Finer, and I’ve been asked to make some introductory remarks prior to today’s Science Teach-in by Dr. Ronald Crystal.
I’m currently a founder and distinguished research fellow at Avalanche, and I’m a member of the Scientific Advisory Board. My primary
responsibility now, outside of Avalanche, is as a managing director, working at MPM Capital in Boston.
Some of you may know me from me
previous role as co-founder and Chief Scientific Officer at bluebird, where I led the R&D team, and completed the scientific and strategic build of one of the leading gene therapy companies today.
My experience in this field has included process development, manufacturing, pre-clinical for both orphan disease and oncology products
delivered by gene insertion, or genome engineering. Over the last 30 years, I’ve spent my time developing over a dozen gene-based therapeutic companies, Avalanche Biotechnologies being one them.
The management and the board of Avalanche undertook an exhaustive evaluation over the past six months of their internal programs and technology
platform, and have looked across the AAV drug development space to identify opportunities with the most significant synergies that Avalanche could build with its own pre-clinical programs, and with new, additional pre-clinical programs.
As you are all aware, on Monday,
February 1st, Paul Cleveland, the CEO of Avalanche, and Dr. Amber Salzman, the CEO of Annapurna, described to the market a proposed merger between Avalanche and Annapurna. This proposed
transaction brings together synergies between these two companies. The leading pipelines of Annapurna, which we’ll spend a lot of time this afternoon.
So, at Avalanche, there is robust AAV vector capabilities, manufacturing platform, quality assurance, and development potential under the
leadership of Mehdi Gasmi, who’s here in the audience.
As you know, the successful gene therapy product development really starts
with the basest development and manufacturing capabilities; being able to make a reproducible viral product of significant purity and potency, and through the development of analytics. This is what Mehdi and his team have brought from the Avalanche
side.
So, now, what you’ll hear today is combined with the robust pipeline of Annapurna, largely developed by Dr. Ronald Crystal
at the Wilde Cornell Medical School, together with the research and development team of Annapurna, led by Dr. Amber Salzman, we’ll create a combined entity that is really the first gene therapy entity that addresses both orphan diseases
and significant, prevalent diseases across the spectrum of human disease.
Today, we have an excellent opportunity to dig into the
scientific rationale and development strategy, as well as the progress towards IND initiation for Annapurna’s lead programs. The discussion will be led by Dr. Ronald Crystal, a pioneer in the development of gene therapy, vector platforms,
and clinical development of gene therapy.
The beauty of Ron’s work is that he has developed many first in — indications across a
number of vector platforms, across a number of disease indications, some of which you’ll hear about today.
Dr. Crystal will be
focused on the unmet medical need and shortcomings, and the current standard of care for the lead programs that Annapurna has chosen, and why Annapurna’s gene therapy approaches will provide the best possible solution for patients with these
diseases.
Dr. Crystal will also highlight key differentiators such as local regional delivery, and AAT gene therapy. Sustained protein
delivery for improved safety in the treatment of Hereditary Angeoedema, and efficient delivery to the myocardia for the treatment of the major cause of morbidity and mortality in Friedreich’s Ataxia.
These aspects, trying to focus on key differentiators and improving the probability of success of
the pre-clinical programs, together with Avalanche’s manufacturing and drug delivery capabilities, are the rationale that we believe these programs have the highest chance for success.
From Ron’s presentation and the questions to follow, I think we can convince you of the significant synergies between the Avalanche
program and pipeline, and the Annapurna pipeline, and a product engine that we believe can continue to deliver IND candidates over the next several years. These programs have been a part of, over the last 30 years, Ron’s development of gene
therapy products.
And now, I will turn it over to Ron to walk you through, in details, of the product pipeline at Annapurna. Ron?
DR. RONALD CRYSTAL: Thank you, Mitch. Nice to see many of you again. I had the opportunity to meet several of you over
the years. Dr. Finer and I are both probably very lucky that we’ve been in the field of gene therapy since the beginning, and have seen it evolve, and now, of course, with the anticipation of significant success.
So, let me tell you about some of the programs that we have planned. First, cautionary statements. So, first, why gene therapy? The whole idea
of gene therapy is to modify gene expressions somewhere in the body, and therefore, modify the disease phenotype.
The advantages of gene
therapy is, that, first, it’s a versatile protein delivery system. It can be used for some other things, but that’s really its primary use. The second is persistent expression. And third is local delivery, and we’ll show you how we
can take advantage of several of those some of the programs.
So, the gene therapists sort of look at the genome, the human genome, with
25,000 genes. It’s like a big bag of M&Ms. And what we do as gene therapists, is we choose our M&Ms, the genes we like, we put it in a delivery vehicle—that is, in this case, the Adeno-associated virus—and then we put that in
our target organ, or systemically, depending on what the approach is, and as you can see, we then have a — is being cured.
So, what
are the challenges that we have in terms of gene therapy? First, we have to look at our targets very carefully, and evaluate them for need and feasibility. We want to target to the site of disease; some are systemic, but some are local. We need to
determine the level of expression, and that’s one of the challenges in the field, to be able to get the level of expressions that we need.
But we also have to have robust phenotypes, something that we can measure easily and convince the regulatory agencies that, in terms to accept
it. And we have to use clear demonstration of efficacy, using acceptable parameters to the regulatory groups.
From a safety point of view, we always have to think about risk and benefit. There are
dose-limiting immune toxicities that we have to be concerned about against the viruses that we use. And we have issues of lack of control of expression. And of course, once you do it, you can’t reverse it, and so we have to be very sure of what
we’re doing.
And then, as you’ve heard from Dr. Finer, manufacturing is an issue, and eventually, what we have to do for
registration. So, the through — core programs I’ll tell you about is, one is Alpha 1 Antitrypsin Deficiency. Second, Hereditary Angioedema. The third is Friedrich’s Ataxia, the cardiac disease associated with it. And finally, severe
allergy.
For all of these, I’m going to show you data with the same vector, and this is a vector called RH10, originally identified
at the University of Pennsylvania. And that’s the outside of the virus, that’s the capsid of the virus. I’ll show you in just a little bit why we chose that.
The construct that we use is basically the same. At the ends of the gene are some genetic material from serotype AB2. We use a very active
promoter in the field called CHE [phonetic] promoter. We put in our therapeutic gene, which is the coding sequences of the protein we’re interested in, and then we have the stop codons [phonetic].
The therapeutic genes are Alpha 1 Antitrypsin, and a1 Antitrypsin Deficiency, or Hereditary
Angioedema, it’s C1- esterase Deficiency—C1- esterase inhibitor, or Friedreich’s Ataxia, it’s frataxin. And for the allergy it’s Anti-IgE and I’ll show you for each of those.
So, the obvious question is, why gene therapy? Particularly, for some of these disease indications we’re going after, is that for several
of them, there already are products on the market. And so the argument is pretty obvious.
For Alpha 1 Antitrypsin Deficiency, Hereditary
Angioedema, and severe allergy, there’s a reduced treatment burden for the patient. These patients have to get, for Alpha 1 Antitrypsin, weekly infusions. Right now, for Hereditary Angioedema, even more frequently than that.
Gene therapy has ideal pharma kinetics. It gives constant levels, rather than when you give a — approach, it goes way up and it goes way
down with the half-life. And for Hereditary Angioedema and severe allergy, it eliminates, if it works, the risk for attacks without immediate—without need for immediate medical care.
And we’ve talked to several people, or parents, for instance, of children who have Hereditary Angioedema or severe allergy. They live in
areas close to medical facilities that are of high quality because of the risk of that. So, if we could give a constant level, and cure the disease, that would eliminate that.
Friedreich’s Ataxia is a different problem for the cardiac disease, because for there, we
want to deliver the gene to the heart. The other indications are all secreted proteins.
But for Friedreich’s Ataxia, the frataxin is
an intercellular protein, and so we need intracellular delivery of the deficient protein in the target organ. And of course, for Friedreich’s, it’s a little different, because it’s an unmet medical need for a fatal disease.
The first, Alpha 1 Antitrypsin Deficiency. This is a common, autosomal, recessive disorder. One out of 50 Caucasians carry the gene, so you
have to have both genes of your parents. It’s characterized by marked reduction in serum levels of Alpha 1 Antitrypsin. It’s estimated in the United States, from one Banks study, says there’s 90,000 people affected with the disease.
Emphysema develops in cigarette smokers in ages 35 to 45, and in non-smokers, about 20 years later, from about 55 to 65. A much, much
smaller proportion develop liver cirrhosis.
Alpha 1 Antitrypsin’s function is to protect the lung from elastase. It’s a very
potent protease that’s produced by neutrophils. I’ll show that in just a second. And if Alpha 1 Antitrypsin are low, the neutrophil elastase slowly just destroys the lung parenchyma.
And on the right is a scan electron micrograph of a lung of a patient with Alpha 1 Antitrypsin Deficiency. It looks like Swiss cheese. Those
big holes? That’s destroyed lung; that’s what emphysema is all about.
The current therapy to protect the lung is weekly
infusions of programs of human Alpha 1 Antitrypsin purified by pure plasma. So, this is the concept of the pathogenicity.
Alpha 1
Antitrypsin is produced by the liver, and of course, the neutrophils come from the bone marrow. And it turns out that all the neutrophils circulating in your body, one third of them are marginating in your lungs. So, they’re sitting there, and
they’re a potential time bomb for the lung parenchyma, which is very, very fragile.
Alpha 1 Antitrypsin’s sole role is to
protect the lung from neutrophil elastase. And it’s also an interesting disorder, and also somewhat unusual for hereditary disorders, in that there’s one mutation called the Z mutation, which is responsible for 90% to 95% of the cases, in
contrast, to, say, Cystic Fibrosis, where there’s a thousand different mutations. And so, it’s basically this one mutation called the Z mutation.
When that occurs, the single amino acid substitution, as the protein is made in the liver, and rather than folding in its three-dimensional
configuration normally, it stays open, and adjacent molecules glom onto each other, and they stay in the liver, in the rough endoplasmic reticulum. As a result, the molecules don’t get out to the serum. That’s why you have low serum levels
of Alpha 1 Antitrypsin. So, that’s the pathogenesis.
One of my post-doctoral fellows and I, back in the early 1980s, we got the idea of why not purify
Alpha 1 Antitrypsin from plasma, and give it back? And that was approved by the FDA and ADA, and it’s administered once weekly.
It’s safe, and there’s minimal adverse reactions. It costs about $100,000 a year, but it’s a significant burden for the
patients; they have to get this weekly infusion once a week.
And so, this is how it was approved. So, we carried out the studies, then
about three, four years later, one of the plasma fractionating companies came to us and said, here, we have a lot of Alpha 1 Antitrypsin, will you do the trial?
We carried out the trial, and the whole concept of efficacy is shown on the top right. That is one of your air sacs blown up. And so
you’re looking at the capillary on the left, and on the right side is the air. The plasma protein, Alpha 1 Antitrypsin, fuses what’s called the interstitium, or the wall of the air sacs.
So, what we did is showed that we could increase the levels on the plasma side. But we also did bronchoscopy, and washed out the lung, and
showed that on the air side that we could also get increased levels. And it was on that basis that the FDA made the approval. This is actually the data from the original study.
When you give Alpha 1 Antitrypsin, it goes up, and it comes down; it goes up, and then it comes down, and so on. And this is the levels in the
epithelial lining fluid. These are the normal individuals. These are the ZZ homozygotes, and these are the individuals that are treated with the purified protein six days after therapy.
It was on that graph that the FDA approved it in about 15 patients, back in 1987. So, that’s the basic concept. If we can show biochemical
equivalency of that, we know we have a product.
So, the other issue is, how much do you need? And we looked at the genetics of the
disease, because there are various forms of it, and they’re associated with different levels.
What we realized was that at 11
micromolar—which is about 80 milligrams per deciliter—at 11 micromolar, that the risk of disease was below that. Almost all those patients are ZZ homozygotes, there’s very nulls, and then there’s another mutation called S, and a
few of those patients had the disease.
And so that 11 micromolar was what we proposed, and that has stood up for 25-plus years.
There’s now four plasma fractionating companies that produce it, and all have used biochemical equivalence to get approval.
So, what
is the gene therapy approach? And so we tried for years to try to put viruses down the lung to make it work. And the problem with that is the lung—the epithelia surface of your lung has figured out, over millions of years, how to protect
itself, and so it just doesn’t work. And neither we nor anyone else could really get it to work.
But then we realized that we might
be able to do an outside-in kind of approach, and that is, put it in your portal space. So, your portal space is between your lung and chest wall; it’s a virtual space. We can easily put fluid there.
And here on the left is a schematic of your portal. And so this is the chest wall. This is the lung, and it’s covered by mesothelial
cells. But there’s another interesting anatomic fact that we take advantage of; and that is, on the chest wall side, there are lymphatics. There are schemata opened to the lymphatic system, which drain directly into the vena system.
So, we hypothesized that if we could put an adnoassociative [phonetic] associative virus gene vector on the portal surface, that we’d not
only get the mesothelial cells to be positive—and therefore get local delivery—but also, some of the vector would go out through the lymphatics, to the vena system, and therefore go to the liver, which is what happens when you put —
vectors intravenously.
So, the concept is shown on the right. Here’s the lung. You take an adnoassociative vector coating for normal
Alpha 1 Antitrypsin. For a pulmonary doctor, this is about a five-minute, outpatient procedure.
The mesothelial cells locally produce the
Alpha 1 Antitrypsin, but a significant amount of the vector also slowly leaks out, goes to the liver, and you’ve got the liver producing as well. So, you’ve got both the blood levels, and you’ve got the lung levels to go up.
So, why are the AVRH10? So, we did a study of 25 different serotypes, every adnoassociate virus that’s out there. And this is the serum
Alpha 1 Antitrypsin levels, and the ordinate is given as 100% here, because we wanted to compare all of them.
These are all of the
viruses, and those of you in the gene therapy field will recognize the ones that are most popular: AVRH10, AV8, AV9, AV5. And in fact, those four are the highest in terms of level, but you can see that HR10 was the highest. And that’s why we
chose it.
So, we went forward with that. Here’s a study. You take a mouse, and you give it intrapleural to the mouse, and then you
measure him in Alpha 1 Antitrypsin levels, and by the — relate to amino acid.
The human Alpha 1 Antitrypsin levels are awarded; that’s a logarithmic scale. This is the 11
micromolar, therapeutic threshold. These are naïve animals here. And this is data out a half a year, for six months, and you can see the levels go up, and they just stay constant. And they stay constant for the life of the animals, no matter
what kinds of animals they do the study in.
Then, to do the same study that we did with the humans in the protein therapy, we also washed
out the lungs, and that’s this data. So, the vector, the adnoassociative virus, or RH10, was given intrapleural.
The doses are 10 to
11th genome copies. When you multiply that by the weight of a human, compared to a mouse, it’s about 1000 molar difference. So, that’s well within the range that we can easily give to
humans.
We evaluate the status at 12 weeks, and we look at the serum, and then we washed out the lungs to look at the epithelial lining
fluid as well. Here’s the human Alpha 1 Antitrypsin levels. Naïve animals, there’s no human protein. Here’s the serum levels, 12 weeks later. And this is the epithelial lining fluid levels.
So, we’re getting both sides of the lungs: the air side, and the blood side. And that was what the original approval from the FDA was, in
terms of that. And so that’s really the goal, in terms of the studies.
We also carried out studies in non-human primates. We
can’t separate out the human versus the non-human primate, because there’s only one or two amino acid differences between us and monkeys for Alpha 1 Antitrypsin, but we were able to measure the messenger RNA levels in 36 monkeys.
And this is, again, on a log scale. This is human Alpha 1 Antitrypsin messenger RNA, out to a year. And this is the 10th to the 12th genome copies. In terms of the 13th genome copies, the — just stays
constant over the period of a year.
All the safety and toxicology parameters were clean, and we have approval from the FDA to move ahead,
in terms of an IND. So, that’s Alpha 1 Antitrypsin Deficiency.
The next is Hereditary Angioedema, and Hereditary Angioedema is a
disorder, in contrast to Alpha 1 Antitrypsin, which is a slowly degenerating disease. Hereditary Angioedema is one that is intermittently acute, and can be fatal.
It’s an autosomal dominant disorder. It’s associated with these episodic attacks of swelling of the face, the extremities, the
genitals, the GI tract and upper airways. This is an example of a hand, a tongue; this is the same woman, as you can see, in terms of various amounts of facial edema.
And the airway edema, if the trachea gets involved in the large airways, it can be life-threatening. It’s triggered by trauma, surgery,
dental work, menstruation, medications, viral illness, and stress. It affects 1 in 10,000 to 1 in 50,000, and it’s responsible in the U.S. for about 50,000 emergency room visits per year.
It’s an excellent target for gene therapy because the levels are about one eighth to one
tenth of that for Alpha 1 Antitrypsin, and so those are levels that we think we can quite easily achieve.
It’s caused by mutations of
what’s called the SERPING1 gene. SERPIN, the groups of gene in the SERPIN, Alpha 1 Antitrypsin was also in the same class. And this gene, the SERPING1, codes for C1 esterase inhibitor that regulates the complement system, and the immune system.
It’s a single chain. It’s bigger than Alpha 1 Antitrypsin — residues; it’s 105 kilodaltons. It has sugars on it. And
the — like Alpha 1 Antitrypsin, primarily by your liver.
The mutations result in either suppress serum levels in about 80% to 85% of
cases, or a functional deficiency of about 15% to 20%. And because of this deficiency, there is an upregulation of a molecule called brichochynin [phonetic] that induces leak in vessels, and that’s the pathogenesis.
They say that for approved patients in the U.S. that there’s an approved prophylactic recombinant therapy. Requires about twice a week
infusions, and costs about $500,000 a year. I can’t say much about the proof of concept studies because of patent filings, but, as I said, this is a target that’s about one tenth to one eighth the levels of Alpha 1 Antitrypsin.
So, the third one is Friedreich’s Ataxia. Now, Friedreich’s Ataxia is thought of as a neurological disease, but in fact, 60% of the
patients die from cardiac disease. And so, approximately 5,000 patients in the U.S., and 5,000 to 10,000 estimated in Europe.
It’s an
autosomal recessive; you have to get it from both parents. Your results from variants in the frataxin gene, and this is a nuclear gene that’s coded by the genome in the nucleus, but it has signals that take it to the mitochondria. So, it
functions in the mitochondria and it is associated with what are called iron-sulphur clusters.
The function of frataxin is to generate
power within the mitochondria, and when you’re deficient in frataxin, my analogy is—let’s say, a hot, July day in Manhattan and there’s a brown-out, because everybody’s losing their power. That’s the problem.
There’s a brown-out in the heart, and in the brain, and so the cells can’t generate enough power.
But they’re still alive,
and that’s very important, because if we can give it back the normal gene, we should be able to then cure it, and I’ll show you that in a mouse model. In the brain, in the central nervous system, it impairs dorsal root ganglia and spinal
cord cerebellum.
And these people, around age 10 or 12, they start stumbling around a little bit. By about age 18,
they’re in wheelchairs; they have slurred and slowed speech. But 60% die from cardio events associated with the hypertrophic cardiomyopathy. And there is no therapy available for Friedreich’s Ataxia.
So, one of our colleagues that collaborates with Annapurna, Helene Puccio [phonetic] in Strasbourg, France created a mouse model, which was an
absence of the frataxin protein the myocardial — scale of the muscle.
And over time—this is a normal looking heart on the mouse,
and that’s the mutant. See, it’s bigger. And also, the heart eventually develops fibrosis, as you can see. And it has a variety of biochemical effects.
These mice develop enlargement of their hearts, and begins to dilate, and they develop heart failure, and they die, around, say, even to eight
weeks. So, that was target. The approach was to administer, again, an AVRH10 vector, but this time, coding for frataxin for the animals who already had advanced heart failure.
So, you can do echocardiograms. Just like you can them in humans, you can do them in mice, and the mice have heart failure, and the results are
really quite remarkable.
So, here’s the data. On the left-hand side is left ventricular mass; that’s the hypertrophy. And you
can see that in the red—these are the untreated animals—it stops, there’s no more data, because they die at this point. But if you treat the animals with the heart failure, they’re exactly the same as normal, so they remain the
same as normal.
Likewise, if you look at what’s called short fraction, which is essentially your ejection fraction, how much blood
comes out of the heart for each time it pumps—you can see in the untreated animals, it’s very low when they die. The treated animals, it improves over a period from this heart failure, improves over the series of four to six weeks, and
then normalizes.
Also, the survival. These mice die at about 10 to 11 weeks, and they live out to more than a year. Eventually, they die
from some skeletal muscle problems, because the gene is also deficient in the skeletal muscle. That doesn’t happen in the humans.
So,
it’s really quite remarkable for gene therapy. So, for gene therapy for Alpha 1 Antitrypsin, we’re preventing deterioration. Here, we’re reversing a significant clinical abnormality. And so that’s for Friedreich’s Ataxia.
We, and also colleagues in the University — in Paris are carrying out some clinical studies now, where we’re taking individuals
with Friedreich’s Ataxia, looking their genetic and neurologic aspects, but determine what would be the best parameters to evaluate?
Whether it be serology, cardio echocardiograms, cardio MRIs, exercise studies—we have people
doing them that are in wheelchairs, but they can do hand-cranking to be able to assess their exercise—and how much sugar, glucose, using CAT scans.
As you can imagine, because of the brown-out problem in terms of trying to make more energy, they’re using up more sugar in their heart
for any given amount of energy, and so that’s another parameter that we’re considering using.
So, finally, let me tell you about
the fourth problem. Does anybody here know anybody with peanut allergy, or any other sever allergy? When I was a kid, I didn’t know anybody. With now, you get on an airplane, and they announce nobody can eat peanuts because there may be a kid
on a plane with a peanut allergy.
So, this is not just peanut allergy. It’s also severe allergies. It can be shrimp; it can be bee
stings. And these people with severe allergies all walk around with these EpiPens, because these can be fatal, in terms of their responses.
So, I’ll show you an example, as a common peanut allergy. So, it’s a common food allergy. It manifests with itchiness, rash,
swelling. You can develop asthma, abdominal pain, low blood pressure. And it can be anaphylactic. They can die; it can be fatal.
It’s
0.04% to 0.6% of the U.S. population. There’s about 4,000 diagnosed per year. The most common cause of anaphylaxis in children presented to the emergency ward. And it significantly adversely affects the quality of life. There is no definitive
therapy, except for strict avoidance. Like I say, they all carry around these EpiPens.
There are programs for desensitization. But there
is a significant literature where people have used Xolair, the anti-IGE produced by Genitech and Roche. And so that’s the approach that we decided to use, and saw where it was going off-patent.
This is the concept of the pathogenesis of the disease. So, in sensitized individuals, when they’re exposed to peanuts—or you could
think of it, if there’s any severe allergy—there is an allergen-specific IgE that combines with the allergen to form an immune complex.
And this immune complex, so, its FC receptors bind to the mass cells, and release these mass cell meditators, which hare nasty mediators, which
cause the phenotype that I showed you.
The way Xolair works, the anti-IgE works, it’s an IGG, anti-IgE. It binds to the immune
complex and prevents them from binding to the mass cells. So, Xolair is a humanized, IgG 1, anti-IgE monochonal that binds the FC [phonetic] portion of the circulating IG, preventing the IgE from binding to and triggering the mass cells.
So, that’s the idea, instead except of giving the monochonal—as you have to frequently,
because of the half-life—is to give it one time. To do that, we created an allergic mouse, a peanut-allergic mouse.
So, we took
immunodeficient mice, so they have no immune system, and we took humans that were allergic to peanuts, and others that were controls. We took their blood and immune cells, and administered them to the mice. So now, we have humanized, immunodeficient
mice that are allergic to peanuts.
You can see, in the top panel here, this is human peanut-specific IgE, and these mice have increasing
levels of that, is that if you feed them peanuts, they release histamine, one of the mediators for the mass cells. They go into anaphylaxis. We have a score for mice, called anaphylaxis score, that we developed.
You can see it here. So, here’s a normal mouse, and here’s a peanut-allergic mouse that we’ve fed peanuts to. You see how his
eyes are really, sort of partially shut? And see how his snout is sort of much wider? These mice just don’t run around, they’re unhappy, and eventually, you keep feeding them peanuts, they will die.
So, if we take an adnoassociative virus, RH10, and we put in this coding sequence for Xolair, and we go out here to 44 weeks, single
administration goes up, and just levels off and stays there. So, that’s basically the concept.
What happens now, if you treat the
animals? So, we waited until the animals were sensitized; that is, they develop all these symptoms when you feed them peanuts. We then—so, equivalent to the human situation—we then gave them the AVRH10 coding for anti-IgE, and we compared
it to Xolair.
So, Xolair, of course is a monochonal, and it has a half-life of about three weeks or so—three to four weeks. When we
waited out, we went out to ten weeks, the animals that had received Xolair now had the phenotypes. See their eyes, closed or shut? Whereas the animals that were treated with gene therapy, were happy-looking, non-allergic.
And likewise, when we looked at locomotor activity and anaphylaxis score, for histamine, in all cases, the gene therapy stayed—not only
cured the animals, but stayed there as a function of time, whereas the Xolair, one administration of Xolair, it disappeared. It didn’t matter what kind of parameter we used to evaluate it.
That’s the advantage of gene therapy. Gene therapy is a strategy that can provide persistent levels of the therapy, and why it would be an
advantage against the existing therapies. And these animals, also, if you keep feeding them peanuts, the survival—and this is a control gene therapy. This is Xolair given once, and this is the gene therapy. So, that’s the advantage, in
terms of gene therapy.
So, in summary, the whole idea of gene therapy is to modify gene expression, and modify the
phenotype. And the programs I have showed you, I think, all of which have a real possibility of significantly affecting people’s life in appositive way.
So, thank you, and pleased to take any questions. Yes, sir?
MALE VOICE 1: Very excited to see your program. What kind of cardiac phenotypes are you looking at?
DR. CRYSTAL: Good question. So, let me repeat it. I understand it’s being recorded, so let me repeat it. The
question is, For Friedreich’s Ataxia, what kind of cardiac phenotypes are we looking at?
I think the ones that the FDA will approve,
using parameters that they will approve, is—probably echo would be number one. Ejection fraction and wall thickness would be, probably, the best two parameters.
Cardiac MRI would be the same. You can use ejection fraction. You can also measure wall thickness and left ventricular mass. In addition, with
cardiac MRI you can estimate extracellular volume, which is loss of cells, and also fibrosis.
We’re also exercising these people to
try to understand whether or not we can separate out the neurologic component, in terms of exercise, versus the cardiac component. That may be more difficult. We just have to do more to see it.
I think if I had to make a decision now, in terms of the parameters, it would be echocardiography. Yes, sir?
MALE VOICE 2: — Antitrypsin program. I think company is also evaluating an intravenous delivery.
DR. CRYSTAL: Yes.
MALE VOICE 2: What are your thoughts on that?
DR. CRYSTAL: The way that we and, of course, many others use AV vectors, to deliver it to the liver, is intravenously.
If you give it an AV vector—it doesn’t matter which serotype—90%, approximately, will go to the liver. The challenge of giving it intravenously, is as you go up in doses, you also begin to have the risk of immunity against the capsid,
because it’s foreign proteins.
The advantage of giving it intraportally is that the longus is somewhat protected from all that. In
fact, if I, as a pulmonary doctor, see a patient who has a pneumothoraxic, collapsed lung, what we do, is we often—after putting a chest tube in—we put talc in the pleural, just to cause inflammation, to cause the lung to adhere to itself.
So, inflammation immunity in the pleural doesn’t affect the function of the lung.
So, we think by giving him the pleura [phonetic] that we will get not only local amounts, but
also in terms of the liver, and we can do so more safely. However, the reason we put intravenous into the studies that we’re going to do is to compare, so we know which is the best. That’s the reason.
We don’t know until you do the human studies. The experimental animals, I think the intrapleural is better, and probably safer, but until
you do the human studies, you don’t know. So, that’s why we set up the protocol that way.
MALE VOICE 2:
And similar doses between the two?
DR. CRYSTAL: Huh?
MALE VOICE 2: Similar dosing between the two — ?
DR. CRYSTAL: Yes. Identical dosing, yes. I’ll ask you guys questions. Yes, sir?
MALE VOICE 3: On the IgE — what do think the risks are with the — ?
DR. CRYSTAL: So, that’s a good question. So, the question is, are there risks for using gene therapy to decrease
IgE? First, anti-IgE has been given to large numbers of patients. I mean, as you know, it’s approved for asthma, and it’s been used often, but in a variety of studies for allergic disorders.
There are a couple of risks. First is, IgE is thought of as being to protect us against parasites, and so that is a theoretical risk. The
second is that in driving down IgE, does IgE have other functions that we don’t know about?
There have been a few patients reported
in the literature that are IgE-null; that is, they just genetically don’t produce any IgE and seem to be normal. But it’s a question that’s a valid question, and one we just have to—until we do the human studies, we’re just
not going to know.
Personally, I think that from all the experience with Xolair, I think that, probably, it’s not going to be an
issue. But until you do the studies, you don’t know. I don’t think I would go swimming in the Nile where there might be a parasite or something, but other than that, I don’t think there’s a risk.
Lauren Glaser: — . Thank you so much.
#
# #
Avalanche Biotechnologies, Inc. (“Avalanche”) has made considerable efforts to provide an accurate transcription, there may be
material errors, omissions, or inaccuracies in the reporting of the substance of presentation. This transcript is being made available for information purposes only.
Forward-Looking Statements
Statements contained in this communication regarding matters that are not historical facts are “forward-looking statements” within
the meaning of the
Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding the nature, strategy and focus of the combined company and the safety,
efficacy and projected development timeline and commercial potential of any product candidates. Avalanche may not consummate the proposed acquisition, or any plans or product development goals in a timely manner, or at all, or otherwise carry out
the intentions or meet the expectations or projections disclosed in our forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results and the timing of events could differ materially from
those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with stockholder approval of the issuance of Avalanche common stock and the
ability to consummate the proposed acquisition, the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources for combined company operations
and to conduct or continue planned development programs and the ability to successfully develop any of Annapurna’s product candidates. Risks and uncertainties facing Avalanche are described more fully in Avalanche’s periodic reports filed
with the SEC. All forward-looking statements contained in this communication speak only as of the date on which they were made. Avalanche undertakes no obligation to update such statements to reflect events that occur or circumstances that exist
after the date on which they were made.
Additional Information about the Proposed Acquisition and Where to Find It
This communication is not intended to and does not constitute a solicitation of any vote or approval. In connection with the proposed
transaction, Avalanche intends to file relevant materials with the Securities and Exchange Commission, or the SEC, including a proxy statement. INVESTORS AND SECURITY HOLDERS OF AVALANCHE ARE URGED TO READ THESE MATERIALS WHEN THEY BECOME AVAILABLE
BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT AVALANCHE, ANNAPURNA AND THE PROPOSED ACQUISITION AND ISSUANCE OF AVALANCHE COMMON STOCK. The proxy statement and other relevant materials (when they become available), and any other documents
filed by Avalanche with the SEC, may be obtained free of charge at the SEC web site at www.sec.gov. In addition, investors and security holders may obtain free copies of the documents filed with the SEC by Avalanche by directing a written request
to: Avalanche Biotechnologies, Inc., 1035 O’Brien Drive, Suite A, Menlo Park, CA 94025, Attention: Investor Relations. Investors and security holders are urged to read the proxy statement and the other relevant materials when they become
available before making any voting decision with respect to the issuance of Avalanche common stock and other matters relating to the proposed acquisition.
Participants in the Solicitation
Avalanche and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of
Avalanche in connection with the proposed transaction and common stock issuance in connection therewith. Information regarding the special interests of these directors and executive officers in the proposed transaction will be included in the proxy
statement referred to above. Additional information regarding the directors and executive officers of Avalanche is also included in Avalanche’s Annual Report on Form 10-K for the year ended December 31, 2014 and the proxy statement for
Avalanche’s 2015 Annual Meeting of Stockholders. These documents are available free of charge at the SEC web site at www.sec.gov and from Investor Relations at Avalanche at the address set forth above.
Adverum Biotechnologies (NASDAQ:ADVM)
Historical Stock Chart
From Feb 2025 to Mar 2025
Adverum Biotechnologies (NASDAQ:ADVM)
Historical Stock Chart
From Mar 2024 to Mar 2025