false
0001708599
0001708599
2024-07-29
2024-07-29
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (date of earliest event reported): July 29, 2024
Serina
Therapeutics, Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
1-38519 |
|
82-1436829 |
| (State
or other jurisdiction |
|
(Commission |
|
(IRS
Employer |
| of
incorporation) |
|
File
Number) |
|
Identification
No.) |
601
Genome Way, Suite 2001
Huntsville,
Alabama 35806
(Address
of principal executive offices)
(256)
327-9630
(Registrant’s
telephone number, including area code)
Not
applicable
(Former
name or former address, if changed since last report)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol |
|
Name
of exchange on which registered |
| Common
Stock, par value $0.0001 per share |
|
SER |
|
NYSE
American |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☒
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item
7.01 Regulation FD Disclosure
On
July 29, 2024, Serina Therapeutics, Inc. (the “Company”) issued a press release announcing that Randall Moreadith,
M.D., Ph.D., the Company's Chief Development Officer, will participate in the 4th Annual mRNA-Based Therapeutics Summit
held between July 29 and July 31, 2024 (“Therapeutics Summit”) in Boston, Massachusetts and will be using the materials
furnished as Exhibit 99.1 to this report (the “Presentation”) in connection with the Therapeutics Summit. The Presentation
is incorporated into this Item 7.01 by reference. A copy of the press release is furnished as Exhibit 99.2 hereto and is incorporated
into this Item 7.01 by reference.
The
information in this Item 7.01 is being furnished, not filed, pursuant to Regulation FD. Accordingly, the information in Item 7.01 of
this report will not be incorporated by reference into any registration statement filed by the Company under the Securities Act of 1933,
as amended, unless specifically identified therein as being incorporated therein by reference. The furnishing of the information in this
report is not intended to, and does not, constitute a determination or admission by the Company that the information in this report is
material or complete, or that investors should consider this information before making an investment decision with respect to any security
of the Company or any of its affiliates.
Item
9.01 Financial Statements and Exhibits
(d)
Exhibits
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
SERINA
THERAPEUTICS, INC. |
| |
|
|
| Date:
July 29, 2024 |
By: |
/s/
Steven Ledger |
| |
|
Interim
Chief Executive Officer |
Exhibit 99.1





























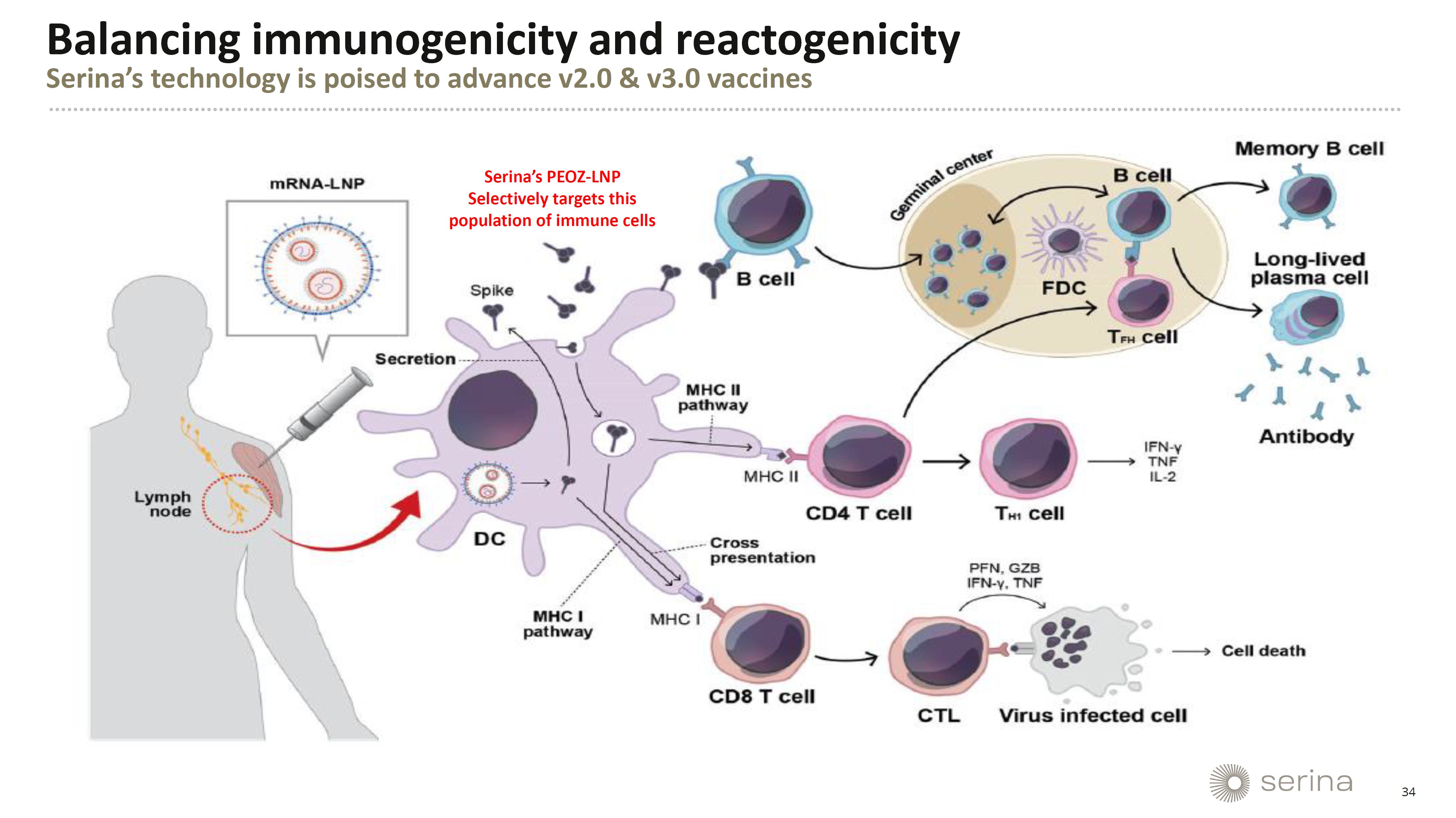
Exhibit
99.2

Serina
Therapeutics to Present Novel Data on POZ-Lipid Technology Potentially Enhancing Safety and Efficacy of mRNA LNP Formulations at the
4th mRNA-Based Therapeutics Summit
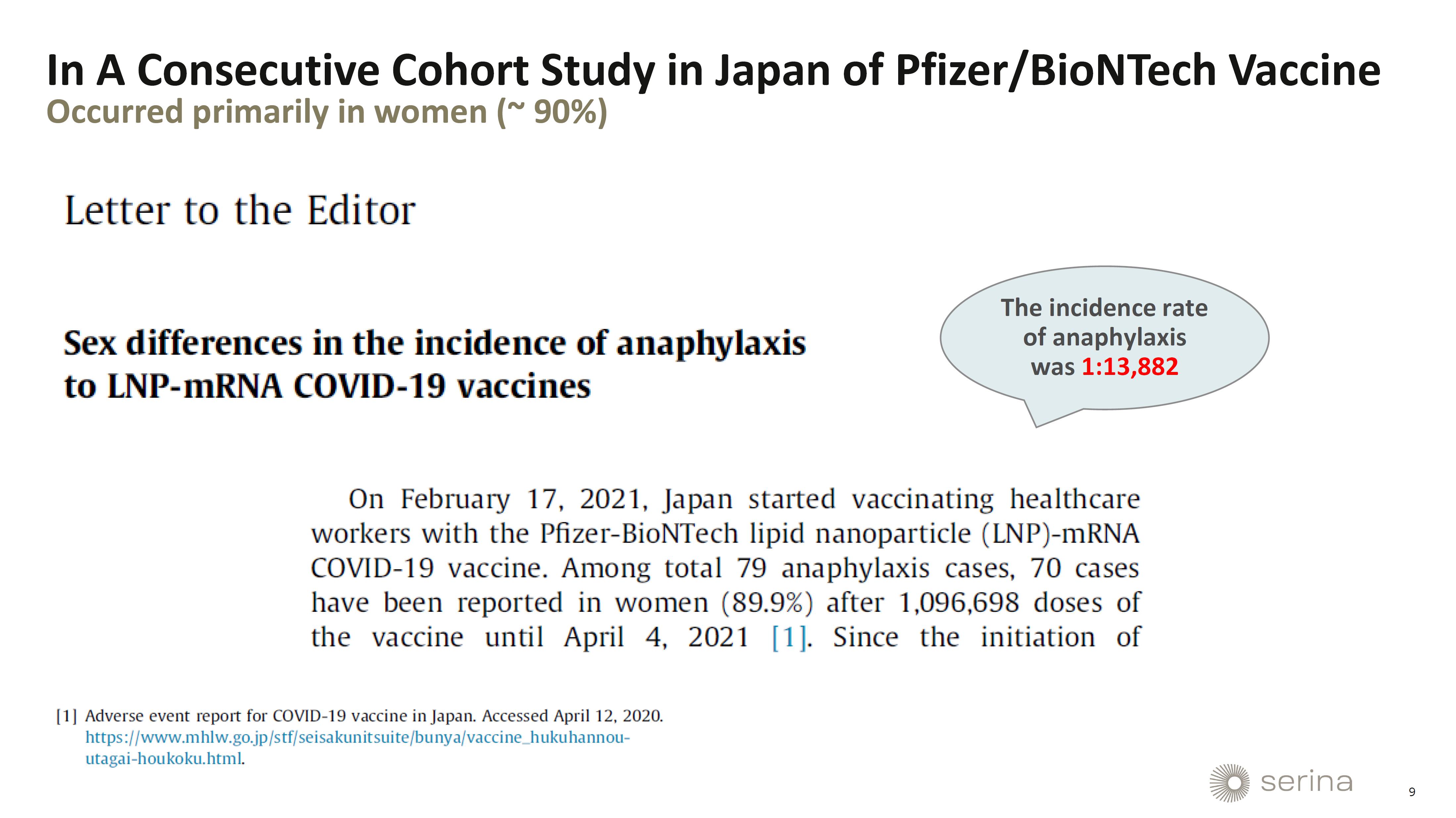
HUNTSVILLE,
Jul. 29, 2024 (GLOBE NEWSWIRE) — Serina Therapeutics (“Serina”) (NYSE American: SER), a clinical-stage biotechnology
company developing its proprietary POZ PlatformTM drug delivery technology, today announced that Randall Moreadith, MD, Ph.D.,
Chief Development Officer, will present at the 4th Annual mRNA-Based Therapeutics Summit. The conference will take place from July 29-31,
2024, in Boston, MA.
Dr.
Moreadith’s presentation, titled “Overcoming Anti-PEG Antibody Responses to Increase Potency & Decrease Adverse Side
Effects of mRNA-LNP Formulations”, is scheduled for 9:00 AM ET on Monday, July 29. In this talk, Dr. Moreadith will present novel
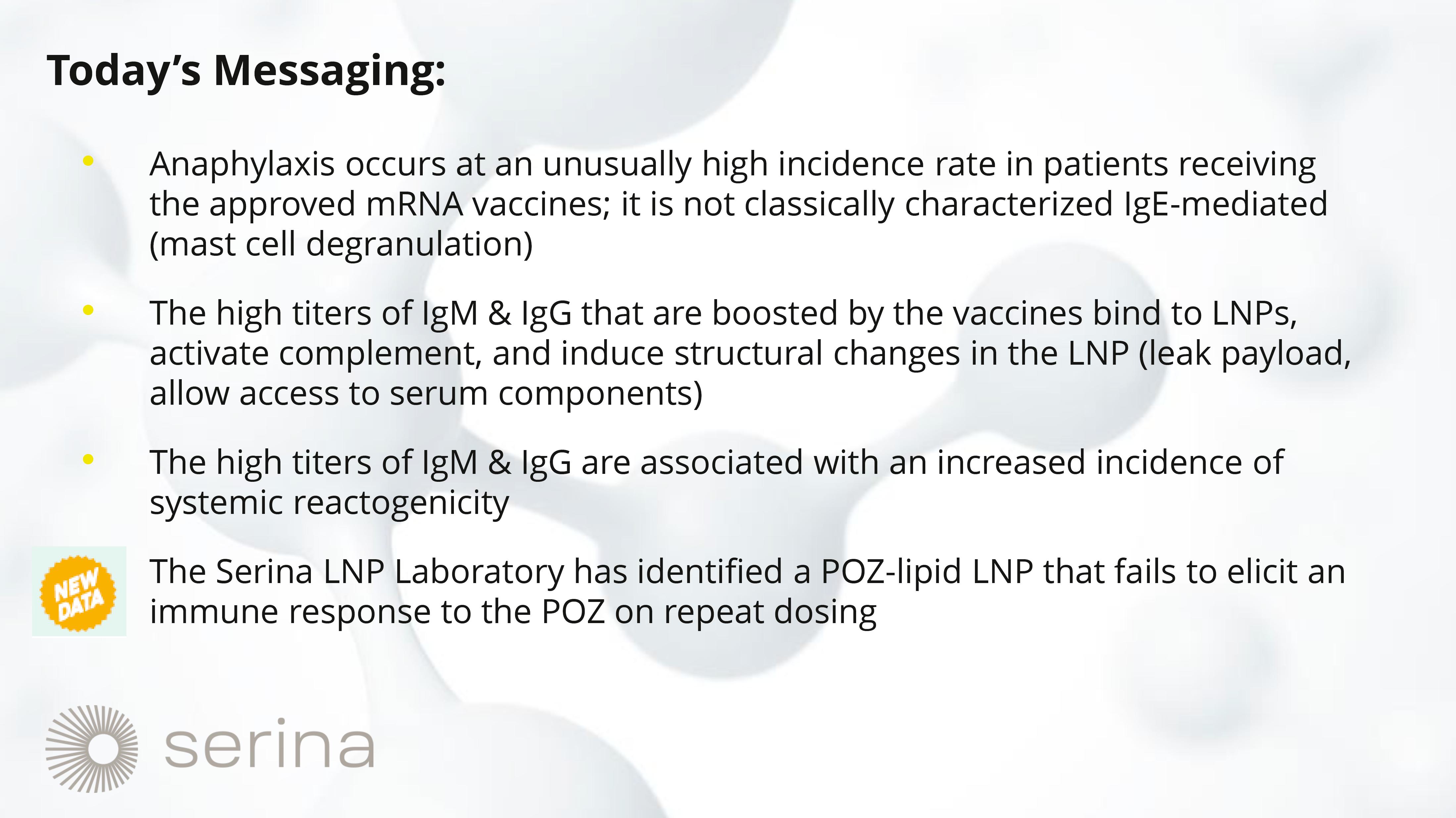
data on Serina Therapeutics’ innovative POZ-lipid, a proprietary technology that aims to significantly advance the safety and efficacy
of mRNA-LNP formulations. These data show that POZ-lipid does not induce an IgM or IgG antibody response, even with repeated dosing in
in vivo models.
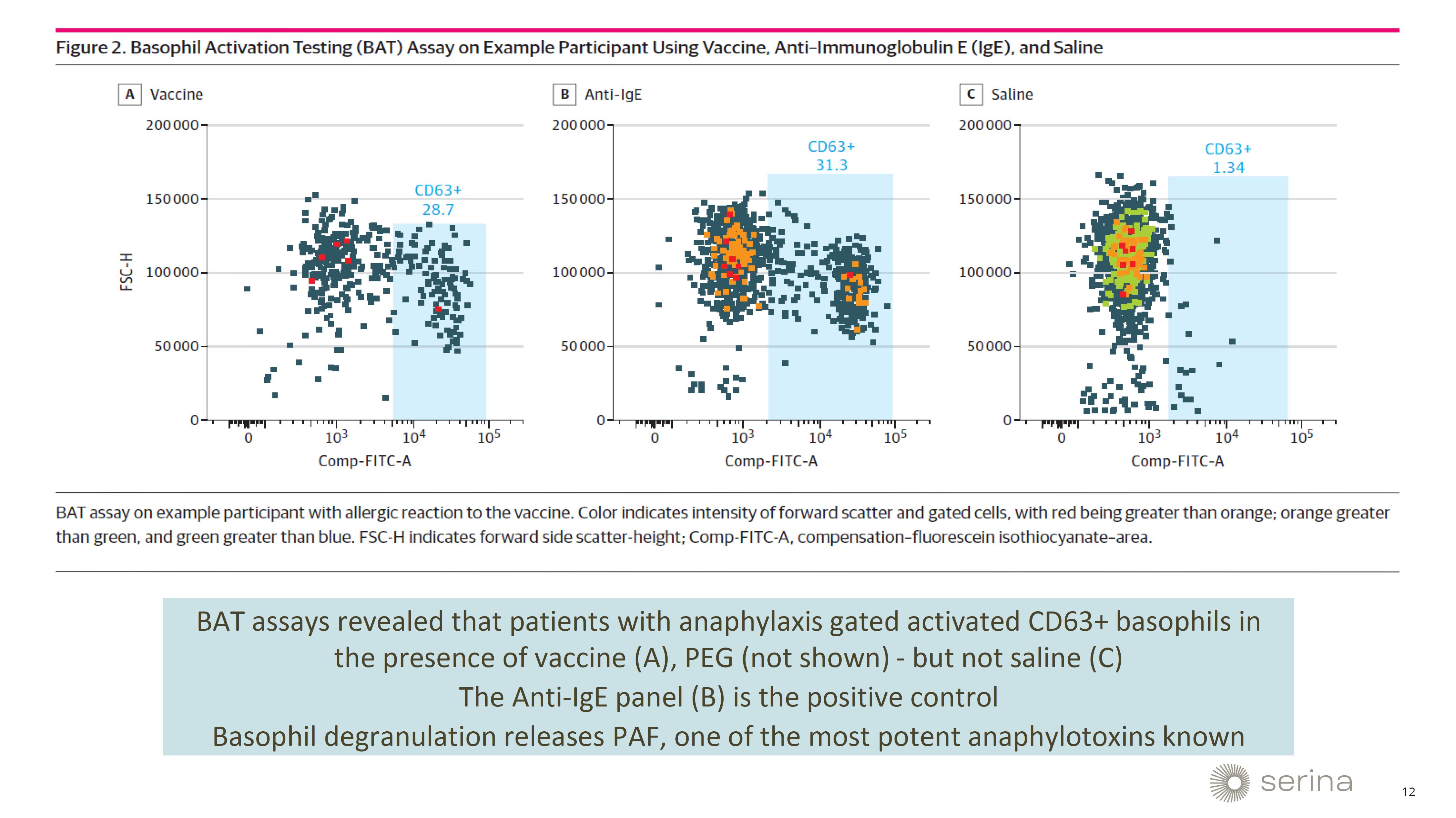
This
finding is crucial because the PEG-lipids currently used in mRNA vaccines can elicit an anti-PEG antibody response. This response is
associated with serious adverse events, including anaphylaxis, which can pose a life-threatening risk to patients. The presentation will
provide insights into how Serina’s POZ-lipid technology can potentially mitigate these adverse effects, thereby enhancing the safety
and efficacy profile of mRNA-LNP medicines.
Conference
Details:
Event:
4th Annual mRNA-Based Therapeutics Summit “Turbocharging New Frontiers of mRNA Medicine to Patients Faster”
Date:
July 29-31, 2024
Location:
Boston, MA
Presentation
Title: “Overcoming Anti-PEG Antibody Responses to Increase Potency & Decrease Adverse Side Effects of mRNA-LNP Formulations”
Presenter:
Randall Moreadith, MD, PhD, Chief Development Officer, Serina Therapeutics, Inc.
Time:
9:00 AM ET, Monday, July 29
For
more information about the conference and to view the agenda, please visit mRNA-Based
Therapeutics Summit Agenda.
About
Serina Therapeutics
Serina
is a clinical-stage biotechnology company developing a pipeline of wholly owned drug product candidates to treat neurological diseases,
cancer, and other indications. Serina’s POZ PlatformTM provides the potential to improve the integrated efficacy and
safety profile of multiple modalities including small molecules, RNA-based therapeutics and antibody-based drug conjugates (ADCs). Serina
is headquartered in Huntsville, Alabama on the campus of the HudsonAlpha Institute of Biotechnology.
For
more information, please visit https://serinatherapeutics.com.
Cautionary
Statement Regarding Forward-Looking Statement
This
release contains forward-looking statements within the meaning of federal securities laws. These statements are based on management’s
current expectations, plans, beliefs or forecasts for the future, and are subject to uncertainty and changes in circumstances. Any express
or implied statements in this press release that are not statements of historical fact, including statements about the potential of Serina’s
POZ polymer technology, are forward-looking statements that involve substantial risks and uncertainties that could cause actual results
to differ materially from those expressed or implied by such statements. Risks and uncertainties include, among other things, the uncertainties
inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates
for clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as the possibility of unfavorable
new clinical data and further analyses of existing clinical data; the risk that clinical trial data are subject to differing interpretations
and assessments by regulatory authorities; whether regulatory authorities will be satisfied with the design of and results from our clinical
studies; whether and when any applications may be filed for any drug or vaccine candidates in any jurisdictions; whether and when regulatory
authorities may approve any potential applications that may be filed for any drug or vaccine candidates in any jurisdictions, which will
depend on a myriad of factors, including making a determination as to whether the product’s benefits outweigh its known risks and
determination of the product’s efficacy and, if approved, whether any such drug or vaccine candidates will be commercially successful;
decisions by regulatory authorities impacting labeling, manufacturing processes, safety and/or other matters that could affect the availability
or commercial potential of any drug or vaccine candidates; and competitive developments. These risks as well as other risks are more
fully discussed in Serina’s Annual Report on Form 10-K for the year ended December 31, 2023, Serina’s Current Report on Form
8-K that was filed with the SEC on April 1, 2024, and Serina’s other periodic reports and documents filed from time to time with
the SEC.
The
information contained in this release is as of the date hereof, and Serina assumes no obligation to update forward-looking statements
contained in this release as the result of new information or future events or developments.
For
inquiries, please contact:
Investor.relations@serinatherapeutics.com
(256) 327-9630
v3.24.2
Cover
|
Jul. 29, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jul. 29, 2024
|
| Entity File Number |
1-38519
|
| Entity Registrant Name |
Serina
Therapeutics, Inc.
|
| Entity Central Index Key |
0001708599
|
| Entity Tax Identification Number |
82-1436829
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
601
Genome Way
|
| Entity Address, Address Line Two |
Suite 2001
|
| Entity Address, City or Town |
Huntsville
|
| Entity Address, State or Province |
AL
|
| Entity Address, Postal Zip Code |
35806
|
| City Area Code |
(256)
|
| Local Phone Number |
327-9630
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common
Stock, par value $0.0001 per share
|
| Trading Symbol |
SER
|
| Security Exchange Name |
NYSEAMER
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
true
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Serina Therapeutics (AMEX:SER)
Historical Stock Chart
From Nov 2024 to Dec 2024

Serina Therapeutics (AMEX:SER)
Historical Stock Chart
From Dec 2023 to Dec 2024
