As filed with the Securities and Exchange Commission on December 29, 2023.
Registration No.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-10
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
CYBIN INC.
(Exact name of Registrant as specified in its charter)
Ontario, Canada
(Province or other jurisdiction of incorporation or organization)
2834
(Primary Standard Industrial Classification Code Number, if applicable)
N/A
(I.R.S. Employer Identification No., if applicable)
100 King Street West, Suite 5600
Toronto, Ontario, Canada M5X 1C9
(908) 764-8385
(Address and telephone number of Registrant's principal executive offices)
C T Corporation System
1015 15th Street N.W., Suite 1000
Washington, DC 20005
(202) 572-3133
(Name, address (including zip code) and telephone number (including area code) of agent for service in the United States)
Copies to:
|
Douglas Drysdale
Cybin, Inc.
100 King Street West, Suite 5600
Toronto, Ontario, Canada
M5X 1C9
(866) 292-4601
|
Richard Raymer
Dorsey & Whitney LLP
TD Canada Trust Tower
Brookfield Place, 161 Bay
Street, Suite 4310 Toronto, Ontario
Canada, M5J 2S1
(416) 367-7388
|
Approximate date of commencement of proposed sale of the securities to the public:
From time to time after this Registration Statement becomes effective.
Province of Ontario, Canada
(Principal jurisdiction regulating this offering)
It is proposed that this filing shall become effective (check appropriate box below):
|
A.
|
|
☐
|
|
upon filing with the Commission pursuant to Rule 467(a) (if in connection with an offering being made contemporaneously in the United States and Canada).
|
|
B.
|
|
☒
|
|
at some future date (check the appropriate box below):
|
|
|
|
1.
|
|
☐
|
|
pursuant to Rule 467(b) on ( ) at ( ) (designate a time not sooner than 7 calendar days after filing).
|
|
|
|
2.
|
|
☐
|
|
pursuant to Rule 467(b) on ( ) at ( ) (designate a time 7 calendar days or sooner after filing) because the securities regulatory authority in the review jurisdiction has issued a receipt or notification of clearance on ( ).
|
|
|
|
3.
|
|
☒
|
|
pursuant to Rule 467(b) as soon as practicable after notification of the Commission by the Registrant or the Canadian securities regulatory authority of the review jurisdiction that a receipt or notification of clearance has been issued with respect hereto.
|
|
|
|
4.
|
|
☐
|
|
after the filing of the next amendment to this Form (if preliminary material is being filed).
|
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to the home jurisdiction's shelf prospectus offering procedures, check the following box. ☒
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registration Statement shall become effective as provided in Rule 467 under the Securities Act or on such date as the Commission, acting pursuant to Section 8(a) of the Securities Act, may determine.
Pursuant to Rule 429 under the Securities Act, the prospectus contained in this Registration Statement relates to Registration Statement 333-272706.
PART I
INFORMATION REQUIRED TO BE DELIVERED TO OFFEREES OR PURCHASERS
Amendment No. 1 dated December 22, 2023 to the Short Form Base Shelf Prospectus dated August 17, 2023
The short form base shelf prospectus dated August 17, 2023, as amended by this amendment, is referred to as the base shelf prospectus and has been filed under legislation in each of the provinces and the territories of Canada that permits certain information about these securities to be determined after the short form prospectus dated August 17, 2023, as amended by this amendment, has become final and that permits the omission from the prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities, except in cases where an exemption from such delivery requirements is available.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. The short form base shelf prospectus dated August 17, 2023, as amended by this amendment, constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities. Information has been incorporated by reference in this short form base shelf prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of Cybin Inc. at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9, telephone 1-866-292-4601, and are also available electronically at www.sedarplus.ca.
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the United States Securities and Exchange Commission (the "SEC"). These securities may not be sold nor may offers to buy be accepted prior to the time the registration statement becomes effective. The prospectus shall not constitute an offer to sell or the solicitation of an offer to buy nor shall be any sale of these securities in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
December 22, 2023

CYBIN INC.
$400,000,000
Common Shares
Warrants
Units
Debt Securities
Subscription Receipts
The short form base shelf prospectus dated August 17, 2023 ("Prospectus") of Cybin Inc. (the "Corporation" or "Cybin") is hereby amended by this Amendment No. 1 to increase the aggregate amount of Securities that may be offered from time to time under the Prospectus from $160,000,000 to $400,000,000. Capitalized terms used but not otherwise defined in this Amendment No. 1 have the meanings ascribed thereto in the Prospectus.
_____________________________
The Corporation is a foreign private issuer under United States securities laws and is permitted under the multijurisdictional disclosure system adopted by the United States and Canada to prepare the Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. The Corporation has prepared its financial statements, included or incorporated herein by reference, in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board and its consolidated financial statements are subject to Canadian generally accepted auditing standards and auditor independence standards. As a result, they may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the purchase of Securities may have tax consequences that may not be fully described in the Prospectus or in any Prospectus Supplement, and should carefully review the tax discussion, if any, in the applicable Prospectus Supplement and in any event consult with a tax advisor.
An investor's ability to enforce civil liabilities under the United States federal securities laws may be affected adversely because the Corporation is incorporated in Canada, most of the officers and directors named in the Prospectus are not residents of the United States, and some of the Corporation's assets and all or a substantial portion of the assets of such persons are located outside of the United States. See "Risk Factors - Enforcement of Civil Liabilities" in the Prospectus and in this Amendment No. 1.
See "Cautionary Statement Regarding Forward-Looking Statements" and "Risk Factors" beginning on pages 1 and 29 of the Prospectus, respectively, and the documents incorporated or deemed to be incorporated by reference therein and the applicable Prospectus Supplement, for a discussion of certain risks that you should consider in connection with an investment in these Securities.
NEITHER THE SEC OR ANY CANADIAN SECURITIES REGULATOR, NOR ANY STATE SECURITIES REGULATOR, HAS APPROVED OR DISAPPROVED THE SECURITIES OFFERED HEREBY OR PASSED UPON THE ACCURACY OR ADEQUACY OF THE PROSPECTUS OR DETERMINED IF THE PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
_____________________________
Specifically, the Prospectus is amended by deleting all references to "$160,000,000" contained on the face page of the Prospectus and substituting "$400,000,000" therefor. The first paragraph of the text on the face page of the Prospectus, as so amended, is deleted and replaced with the following:
"This short form base shelf prospectus ("Prospectus") relates to the offering for sale from time to time (each, an "Offering") by Cybin Inc. (the "Corporation" or "Cybin") during the 25-month period commencing on August 17, 2023, that this Prospectus, including any amendments, remains valid, of up to $400,000,000 in the aggregate of: (i) common shares ("Common Shares") of the Corporation; (ii) warrants ("Warrants") to purchase other Securities (as defined below) of the Corporation; (iii) units ("Units") comprising of one or more of the other Securities, (iv) senior and subordinated unsecured debt securities (collectively, "Debt Securities"), including debt securities convertible or exchangeable into other securities of the Corporation, and (v) subscription receipts ("Subscription Receipts" and together with the Common Shares, Warrants, Units and Debt Securities, collectively referred to herein as the "Securities"). The Securities may be offered separately or together, in amounts, at prices and on terms determined based on market conditions at the time of the sale and as set forth in an accompanying prospectus supplement ("Prospectus Supplement")."
The Prospectus is further amended by deleting the reference to "$160,000,000" contained in the first sentence of the first paragraph under the heading "Plan of Distribution" on page 28, so that such sentence, as so amended, reads as follows:
"The Corporation may from time to time during the 25-month period commencing on August 17, 2023, that this Prospectus, including any amendments and supplements hereto, remains valid, offer for sale and sell up to an aggregate of $400,000,000 in Securities hereunder."
The Prospectus is further amended by deleting the third paragraph on page iv of the Prospectus, and replacing with the following:
“Douglas Drysdale, an officer of the Corporation, and George Tziras, a director and officer of the Corporation, each reside outside of Canada. They have appointed Maxims CS Inc., Suite 1800, 181 Bay Street, Toronto, Ontario, M5J 2T9, as agent for service of process in Ontario. Prospective purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process, see “Risk Factors – Risks Related to an Offering – Enforcement of Civil Liabilities”.”
The Prospectus is further amended by deleting the second paragraph under the section titled “Documents Incorporated by Reference” and replacing it with the following:
“The following documents of the Corporation filed with the securities commission or similar authorities in Canada are incorporated by reference in this Prospectus:
1. annual information form of the Corporation dated June 27, 2023 for the year ended March 31, 2023 (the “Annual Information Form”);
2. the audited consolidated financial statements of the Corporation and the notes thereto as at and for the fiscal year ended March 31, 2023 and 2022, together with the auditor’s report thereon;
3. management’s discussion and analysis of the Corporation for the year ended March 31, 2023;
4. the unaudited interim condensed consolidated financial statements of the Corporation and the notes thereto for the three and six months ended September 30, 2023 (the “Interim Financial Statements”);
5. management’s discussion and analysis of the Corporation for the three and six months ended September 30, 2023 (the “Interim MD&A”);
6. management information circular of the Corporation dated September 13, 2023 relating to an annual and special meeting of shareholders of the Corporation held on October 12, 2023;
7. material change report dated May 31, 2023 relating to the sale of up to US$30,000,000 of Common Shares to Lincoln Park Capital Fund, LLC, upon the terms and subject to the conditions contained in the purchase agreement dated May 30, 2023 (the “Purchase Agreement”), and made pursuant to and qualified by a prospectus supplement dated May 30, 2023, to the Corporation’s short form base shelf prospectus dated July 5, 2021 (the “2021 Base Shelf Prospectus”);
8. material change report dated August 8, 2023 relating to the public offering of 24,264,706 units of the Corporation at a price of US$0.34 per unit for gross proceeds of US$8,250,000 pursuant to a prospectus supplement dated August 1, 2023 to the 2021 Base Shelf Prospectus;
9. material change report dated August 23, 2023 relating to the renewal of the Corporation’s previously established at-the-market equity program (the “2023 ATM Program”) that allows the Corporation to issue and sell up to US$35,000,000 of Common Shares to the public from time to time, pursuant to a prospectus supplement dated August 23, 2023 to the Prospectus;
10. material change report dated August 23, 2023 with respect to the requalification of the Purchase Agreement pursuant to a prospectus supplement dated August 23, 2023 to the Prospectus (the “August 2023 Prospectus Supplement”);
11. material change report dated September 7, 2023 with respect to the acquisition of Small Pharma Inc. (“Small Pharma”) by the Corporation pursuant to the terms of the definitive agreement under which the Corporation will acquire all of the issued and outstanding common shares of Small Pharma pursuant to a court-approved plan of arrangement (the “Arrangement”);
12. material change report dated October 25, 2023 with respect to the closing of the Arrangement with Small Pharma;
13. material change report dated November 20, 2023 relating to the firm commitment underwritten offering of units of the Corporation at a price of US$0.45 per unit for gross proceeds of US$30,000,000 pursuant to a prospectus supplement dated November 10, 2023 to the Prospectus; and
14. business acquisition report dated October 27, 2023 relating to the Arrangement.”
The Prospectus is further amended by deleting the section titled “Select Recent Developments” and replacing it with the following:
“Other than as set forth below and generally in this Prospectus or the documents incorporated by reference therein, there have been no material developments in the business of the Corporation since November 14, 2023, the date of the Interim Financial Statements and Interim MD&A.
On November 30, 2023, the Corporation announced positive Phase 2 topline safety and efficacy data for CYB0003, its proprietary deuterated psilocybin analog, being developed for the treatment of major depressive disorder (“MDD”).
On December 6, 2023, the Corporation announced that the United States Patent and Trademark Office had granted U.S. patent 11,834,410 in support of its CYB003 program. The patent, which is expected to provide exclusivity until at least 2041, includes composition of matter claims to pharmaceutical compositions within the Corporation’s proprietary deuterated psilocybin analog program, CYB003, as well as claims directed toward the therapeutic treatment of MDD, treatment-resistant depression, and alcohol use disorder.”
The Prospectus is further amended by deleting the section titled “Use of Proceeds” and replacing it with the following:
“The net proceeds from any Offering of Securities and the proposed use of those proceeds will be set forth in the applicable Prospectus Supplement relating to that Offering of Securities. Notwithstanding, the Corporation’s management has broad discretion in the application of proceeds of an Offering of Securities. On the basis of results obtained or for other sound business reasons, the Corporation may re-allocate funds as required. Accordingly, the Corporation’s actual use of proceeds may vary significantly from any proposed use of proceeds disclosed in any applicable Prospectus Supplement. See “Risk Factors – Risks Related to an Offering – Discretion over the Use of Proceeds”.
Sources and Uses of Capital
As at December 21, 2023, the Corporation had cash resources, being cash and an HST receivable, of approximately $39,290,000. As at December 21, 2023, the Corporation anticipates that it will require approximately $39,290,000 to continue operations over the next 12 months, including funding for the achievement of the next significant milestone under the Corporation’s Deuterated Psilocybin Analog Program (CYB003) and the Deuterated Dimethyltryptamine Program (CYB004).
The Corporation may be required to reduce its general and administrative expenses if it does not have sufficient capital to meet its below stated objectives, or it may allocate its financial resources differently than listed below. The expected reduction in future expenditures, absent any capital injections, compared to the Corporation’s historic expenditures would be achieved as follows: (i) a reduction in marketing activities; (ii) a reduction in workforce and/or compensation; (iii) a reduction in public relations and investor relations activities; and (iv) a reduction of planned expenses under the Deuterated Dimethyltryptamine Program and Phenethylamine Derivatives Program, which would also result in a delay of anticipated timing for completion of the next significant milestones under each of these programs.
| Program(1) |
|
Anticipated
12 Month Use
of Funds(2)(3)
($) |
|
|
Anticipated
Timeline for
Completion(4) |
|
| Deuterated Psilocybin Analog Program |
|
|
|
|
|
|
| Complete Phase 1/2a topline safety and efficacy data readout for CYB003 in MDD |
|
- |
|
|
Complete |
|
| Complete FDA submission of Phase 1/2a data |
|
893,000 |
|
|
Complete |
|
| Phase 2 data assessing durability of effect of CYB003 at 12 weeks(5) |
|
- |
|
|
Q1 2024 |
|
| Initiate a Phase 3 study of CYB003 in MDD |
|
8,940,000 |
|
|
Q1 2024 |
|
| Deuterated Dimethyltryptamine Program |
|
|
|
|
|
|
| Provide topline data from Phase 1 CYB004-E trial |
|
215,000 |
|
|
Q1 2024 |
(6) |
| Complete FDA IND submission |
|
181,000 |
|
|
Q1 2024 |
|
| Initiate a Phase 2 proof-of-concept study |
|
1,239,000 |
|
|
Q1 2024 |
|
| Initiate a subcutaneous formulation study |
|
685,000 |
|
|
Q1 2024 |
|
| Phenethylamine Derivatives Program |
|
|
|
|
- |
|
| Complete preclinical development of phenethylamine drug candidate |
|
55,000 |
|
|
Q1 2024 |
(7) |
| Technology Programs |
|
- |
|
|
- |
|
| Working Capital, and General Corporate Purposes(8) |
|
27,082,000 |
|
|
N/A |
|
| Total |
|
39,290,000 |
|
|
|
|
Notes:
(1) Please see the Corporation’s Interim MD&A for a description of the Corporation’s programs. All milestones are subject to receipt of all necessary approvals, including the academic and scientific organizations with which the Corporation is working.
(2) Certain amounts have been converted from USD to CAD at an exchange rate of 1.37:1.
(3) Represents proceeds from private placements and offerings previously disclosed, as well proceeds from the 2023 ATM Program and August 2023 Prospectus Supplement.
(4) Anticipated timeline for completion is based on the calendar year. Anticipated spending and timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation.
(5) This milestone, being the receipt of Phase 2 data assessing durability of the effect of CYB003 at 12 weeks, is derived from the results of the Corporation’s Phase 1/2a study and, therefore, the Corporation has not allocated additional funds for this milestone.
(6) The Corporation has updated the anticipated timeline for completion of this milestone. The Corporation had previously expected it would complete this milestone in Q3/Q4 2023. The Corporation now expects to receive the topline data readout in Q1 2024, due to certain timing issues.
(7) The Corporation has updated the anticipated timeline for completion of this milestone. The Corporation had previously expected it would complete this milestone in Q4 2023. Due to the Corporation prioritizing the progression of its Deuterated Psilocybin Analog Program and Deuterated Dimethyltryptamine Program, it now expects to complete preclinical development of a phenethylamine drug candidate in Q1 2024.
(8) Includes personnel costs, professional services, overhead expenses and general expenses to be incurred by the Corporation in the normal course of business. In addition, the Corporation intends to use a portion of these proceeds to continue funding both its Deuterated Psilocybin Analog Program and Deuterated Dimethyltryptamine Program. The allocation between and specific milestones within these programs have not yet been determined.
For detailed information in respect of the Corporation’s business objectives and milestones, and the application of proceeds from prior offerings by the Corporation, prospective purchasers of Securities should carefully consider the information described in the Annual Information Form and Interim MD&A of the Corporation, and the documents incorporated by reference herein, including the applicable Prospectus Supplement.
As at December 21, 2023, the Corporation’s current financial resources, absent any capital injections, will be sufficient to meet the Corporation’s short-term liquidity requirements and to fund its operations for the coming 12 months. The Corporation makes this statement based on its cash resources, being cash and an HST receivable, as of December 21, 2023 in the amount of approximately $39,290,000.
The Corporation’s expectation is based on certain assumptions and risks as set forth in the section “Cautionary Note Regarding Forward Looking Information” and “Risk Factors” in this Prospectus.
The expected uses of capital represents the Corporation’s current intentions based upon its present plans and business condition, which could change in the future as its plans and business conditions evolve. The amounts and timing of the actual use of available capital will depend on multiple factors and there may be circumstances where, for sound business reasons, a reallocation of capital, or termination of a program objective, may be necessary in order for the Corporation to achieve its program objectives. The Corporation may also require additional funds in order to fulfill its expenditure requirements to meet existing and any new business objectives, and the Corporation expects to either issue additional securities or incur debt to do so. The material factors or assumptions used to develop the estimated amounts for the 12 months period disclosed above are included in the “Cautionary Note Regarding Forward-Looking Information” section above. The actual amount that the Corporation spends in connection with each of the identified uses and programs will depend on a number of factors, including those listed under “Risk Factors” in, or incorporated by reference in, this Prospectus.
Negative Cash Flow From Operations
Since inception, the Corporation has financed its operations primarily from the issuance of equity and interest income on funds available for investment. To date, the Corporation has raised approximately C$156,500,000 in gross proceeds through private placement and prospectus offerings. The Corporation has experienced operating losses and cash outflows from operations since incorporation and will require ongoing financing to continue its research and development activates. As the Corporation has not yet achieved profitability, there are uncertainties regarding its ability to continue as a going concern. The Corporation has not earned any revenue or reached successful commercialization of any products. The Corporation’s success is dependent upon the ability to finance its cash requirements to continue its activities. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained, or at all. To the extent that the Corporation has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Corporation will be required to raise additional funds through the issuance of additional equity securities, through loan financing, or other means, such as through partnerships with other companies and research and development reimbursements. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained. See “Risk Factors – Risks Related to an Offering - Negative Operating Cash Flow and Going Concern”.”
The Prospectus is further amended by adding the following as the second paragraph under the section titled “Auditors, Transfer Agent and Registrar”:
MNP LLP, Chartered Professional Accountants, are the former auditors of Small Pharma and have confirmed that they are independent of Small Pharma within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation. Neither MNP LLP nor any designated professional thereof, had any registered or beneficial interest in any securities or other property of Small Pharma at the time they prepared the relevant financial statements incorporated by reference in this Prospectus or at any time thereafter.”
The Prospectus must be read together with this amendment, any documents incorporated or deemed to be incorporated by reference therein from time to time and any supplements relating to an offering of Securities thereunder. The statements contained in the Prospectus or in a document incorporated or deemed to be incorporated by reference therein on or subsequent to August 17, 2023 are modified or superseded for the purposes of this amendment to the extent that a statement contained in any subsequently filed document, which is also or is deemed to be incorporated by reference therein, modifies or superseded that statement.
RISK FACTORS
An investment in the Securities involves a high degree of risk and must be considered speculative due to the nature of the Corporation's business and present stage of development. Before making an investment decision, prospective purchasers of Securities should carefully consider the information described in the Prospectus and the documents incorporated or deemed to be incorporated by reference therein, including the applicable Prospectus Supplement.
Enforcement of Civil Liabilities
Certain of the Corporation's subsidiaries and assets are located outside of Canada. Accordingly, it may be difficult for investors to enforce within Canada any judgments obtained against the Corporation, including judgments predicated upon the civil liability provisions of applicable Canadian securities laws or otherwise. Consequently, investors may be effectively prevented from pursuing remedies against the Corporation under Canadian securities laws or otherwise.
The Corporation has subsidiaries incorporated in the United States. It may not be possible for shareholders to effect service of process outside of Canada against the directors and officers of the Corporation who are not resident in Canada. In the event a judgment is obtained in a Canadian court against one or more of such persons for violations of Canadian securities laws or otherwise, it may not be possible to enforce such judgment against persons not resident in Canada. Additionally, it may be difficult for an investor, or any other person or entity, to assert Canadian securities law or other claims in original actions instituted in the United States. Courts in such jurisdiction may refuse to hear a claim based on a violation of Canadian securities laws or otherwise on the grounds that such jurisdiction is not the most appropriate forum to bring such a claim. Even if a foreign court agrees to hear a claim, it may determine that the local law, and not Canadian law, is applicable to the claim. If Canadian law is found to be applicable, the content of applicable Canadian law must be proven as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by foreign law.
Most of the officers and directors named in the Prospectus are not residents of the United States, and some of the Corporation's assets and all or a substantial portion of the assets of such person are located outside of the United States.
The Corporation has been advised that, subject to certain limitations, a judgment of a United States court predicated solely upon civil liability under United States federal securities laws may be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. The Corporation has also been advised, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of liability predicated solely upon United States federal securities laws or any such state securities or "blue sky" laws.
The Corporation is filing with the SEC, concurrently with a registration statement of which this Prospectus forms a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, the Corporation appointed C T Corporation System as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving the Corporation in a United States court, arising out of or related to or concerning the offering of the Securities.
This short form base shelf prospectus has been filed under legislation in each of the provinces and the territories of Canada that permits certain information about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities, except in cases where an exemption from such delivery requirements is available.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form base shelf prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities.
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the United States Securities and Exchange Commission (the "SEC"). These securities may not be sold nor may offers to buy be accepted prior to the time the registration statement becomes effective. This prospectus shall not constitute an offer to sell or the solicitation of an offer to buy nor shall be any sale of these securities in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
Information has been incorporated by reference in this short form base shelf prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of Cybin Inc. at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9, telephone 1-866-292-4601, and are also available electronically at www.sedarplus.ca.
| New Issue |
August 17, 2023 |
SHORT FORM BASE SHELF PROSPECTUS

CYBIN INC.
$160,000,000
Common Shares
Warrants
Units
Debt Securities
Subscription Receipts
This short form base shelf prospectus ("Prospectus") relates to the offering for sale from time to time (each, an "Offering") by Cybin Inc. (the "Corporation" or "Cybin") during the 25-month period that this Prospectus, including any of amendments thereto, remains valid, of up to $160,000,000 in the aggregate of: (i) common shares ("Common Shares") of the Corporation; (ii) warrants ("Warrants") to purchase other Securities (as defined below) of the Corporation; (iii) units ("Units") comprising of one or more of the other Securities, (iv) senior and subordinated unsecured debt securities (collectively, "Debt Securities"), including debt securities convertible or exchangeable into other securities of the Corporation, and (v) subscription receipts ("Subscription Receipts" and together with the Common Shares, Warrants, Units and Debt Securities, collectively referred to herein as the "Securities"). The Securities may be offered separately or together, in amounts, at prices and on terms determined based on market conditions at the time of the sale and as set forth in an accompanying prospectus supplement ("Prospectus Supplement").
All shelf information permitted under applicable laws to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus, except in cases where an exemption from such delivery requirements is available. Each Prospectus Supplement containing the specific terms of any Securities will be incorporated by reference into this Prospectus for the purposes of securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains.
The specific terms of any Securities offered will be described in a Prospectus Supplement, including: (i) in the case of Common Shares, the number of Common Shares offered, the offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event the offering is a non-fixed price distribution) and any other specific terms; (ii) in the case of Warrants, the number of Warrants being offered, the offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event the offering is a non-fixed price distribution), the designation, number and terms of the other Securities purchasable upon exercise of the Warrants, and any procedures that will result in the adjustment of those numbers, the exercise price, the dates and periods of exercise and any other specific terms; (iii) in the case of Units, the number of Units offered, the offering price, the designation, number and terms of the other Securities comprising the Units, and any other specific terms; (iv) in the case of Debt Securities, the specific designation of the Debt Securities, whether such Debt Securities are senior or subordinate, the aggregate principal amount of the Debt Securities being offered, the currency or currency unit in which the Debt Securities may be purchased, authorized denominations, any limit on the aggregate principal amount of the Debt Securities of the series being offered, the issue and delivery date, the maturity date, the offering price (at par, at a discount or at a premium), the interest rate or method of determining the interest rate, the interest payment date(s), any conversion or exchange rights that are attached to the Debt Securities, any redemption provisions, any repayment provisions and any other specific terms; and (v) in the case of Subscription Receipts, the number of Subscription Receipts being offered, the offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event the offering is a non-fixed price distribution), the terms, conditions and procedures for the conversion of the Subscription Receipts into other Securities, the designation, number and terms of such other Securities, and any other specific terms. A Prospectus Supplement relating to a particular Offering of Securities may include terms pertaining to the Securities being offered thereunder that are not within the terms and parameters described in this Prospectus.
The Securities may be sold through underwriters or dealers, directly by the Corporation pursuant to applicable statutory exemptions, or through designated agents from time to time. See "Plan of Distribution". The Prospectus Supplement relating to a particular Offering of Securities will identify each underwriter, dealer or agent, as the case may be, engaged by the Corporation in connection with the offering and sale of the Securities, and will set forth the terms of the Offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection with the Offering, the method of distribution of the Securities, the initial issue price (in the event that the offering is a fixed price distribution), the net proceeds to the Corporation and any other material terms of the plan of distribution.
The Securities may be sold from time to time in one or more transactions at a fixed price or prices or at non-fixed prices. This Prospectus may qualify an "at-the-market distribution", as defined in National Instrument 44-102 - Shelf Distributions ("NI 44-102"). If offered on a non-fixed price basis, the Securities may be offered at market prices prevailing at the time of sale, at prices determined by reference to the prevailing price of a specified security in a specified market or at prices to be negotiated with purchasers including sales in transactions that are deemed to be "at-the-market distributions", including sales made directly on the Neo Exchange Inc., now operating as Cboe Canada (the "NEO") or other existing trading markets for the Securities, and as set forth in an accompanying Prospectus Supplement, in which case the compensation payable to an underwriter, dealer or agent in connection with any such sale will be decreased by the amount, if any, by which the aggregate price paid for the Securities by the purchasers is less than the gross proceeds paid by the underwriter, dealer or agent to the Corporation. The price at which the Securities will be offered and sold may vary from purchaser to purchaser and during the period of distribution. See "Plan of Distribution".
This Prospectus does not qualify the issuance of Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to one or more underlying interests including, for example, an equity or debt security, a statistical measure of economic or financial performance including, but not limited to, any currency, consumer price or mortgage index, or the price or value of one or more commodities, indices or other items, or any other item or formula, or any combination or basket of the foregoing items. For greater certainty, this Prospectus may qualify for issuance Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to published rates of a central banking authority or one or more financial institutions, such as a prime rate or bankers' acceptance rate, or to recognized market benchmark interest rates such as the Canadian Overnight Repo Rate Average (CORRA), Secured Overnight Financing Rate (SOFR), Euro Inter-Bank Offered Rate (EURIBOR) or an alternative United States federal fund rate.
ii
No underwriter or dealer involved in an "at-the-market distribution" under this Prospectus, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot securities in connection with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the offered Securities or securities of the same class as the Securities distributed under the "at-the-market distribution", including selling an aggregate number or principal amount of Securities that would result in the underwriter creating an over-allocation position in the Securities.
In connection with any Offering of the Securities, subject to applicable laws and other than an "at-the-market distribution", the underwriters or agents may over-allot or effect transactions that stabilize or maintain the market price of the offered Securities at a level above that which might otherwise prevail on the open market. Such transactions, if commenced, may be interrupted or discontinued at any time. See "Plan of Distribution".
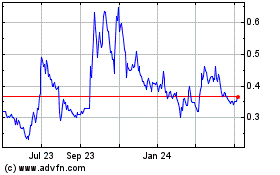
The Common Shares are listed on the NEO under the trading symbol “CYBN”, and in the United States on the NYSE American LLC stock exchange (the “NYSE American”) under the symbol “CYBN”. On August 16, 2023, the last trading day prior to the filing of this Prospectus, the closing prices of the Common Shares listed on the NEO and the NYSE American were $0.44 and US$0.323, respectively.
Unless specified in the applicable Prospectus Supplement, there is no market through which the Subscription Receipts, Warrants, Units and Debt Securities may be sold and purchasers may not be able to resell the Subscription Receipts, Warrants, Units and Debt Securities purchased under this Prospectus and the Prospectus Supplement. This may affect the pricing of the Subscription Receipts, Warrants, Units and Debt Securities in the secondary market, the transparency and availability of trading prices, the liquidity of the Subscription Receipts, Warrants, Units and Debt Securities and the extent of issuer regulation. See "Risk Factors".
The Corporation is a foreign private issuer under United States securities laws and is permitted under the multijurisdictional disclosure system adopted by the United States and Canada to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. The Corporation has prepared its financial statements, included or incorporated herein by reference, in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board and its consolidated financial statements are subject to Canadian generally accepted auditing standards and auditor independence standards. As a result, they may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the purchase of Securities may have tax consequences that may not be fully described in this Prospectus or in any Prospectus Supplement, and should carefully review the tax discussion, if any, in the applicable Prospectus Supplement and in any event consult with a tax advisor.
An investors ability to enforce civil liabilities under the United States federal securities laws may be affected adversely because the Corporation is incorporated in Canada, most of the officers and directors named in this Prospectus are not residents of the United States, and some of the Corporation's assets and all or a substantial portion of the assets of such persons are located outside of the United States. See "Risk Factors - Enforcement of Civil Liabilities".
An investment in the Securities is subject to a number of risks, including those risks described in this Prospectus and documents incorporated by reference into this Prospectus. See “Risk Factors” in this Prospectus and in the Corporation’s Annual Information Form and Interim MD&A (each as defined herein) incorporated by reference herein.
iii
No person is authorized by the Corporation to provide any information or to make any representation other than as contained in this Prospectus in connection with the issue and sale of the Securities offered hereunder.
No underwriter has been involved in the preparation of this Prospectus or performed any review of the contents hereof.
NEITHER THE SEC OR ANY CANADIAN SECURITIES REGULATOR, NOR ANY STATE SECURITIES REGULATOR, HAS APPROVED OR DISAPPROVED THE SECURITIES OFFERED HEREBY OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
Douglas Drysdale, an officer of the Corporation, resides outside of Canada. He has appointed Maxims CS Inc., Suite 1800, 181 Bay Street, Toronto, Ontario, M5J 2T9, as agent for service of process in Ontario. Prospective purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process, see "Risk Factors - Risks Related to an Offering - Enforcement of Civil Liabilities".
In this Prospectus, references to the "Corporation", "Cybin", "we", "us" and "our" refer to Cybin Inc. and/or, as applicable, one or more of its subsidiaries. The Corporation's registered and head office is located at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9.
|
The Corporation's current business focuses on conducting research and development of psychedelic therapeutics that aim to address unmet needs in the treatment of mental health conditions. This comprehensive development work is predicated on structural modifications of known tryptamine and phenethylamine derivatives to improve their pharmacokinetic properties while maintaining their respective pharmacology. Like most pharmaceutical companies, the Corporation's business is focused on research and development, see "Use of Proceeds".
The Canadian and United States federal governments regulate drugs through the Controlled Drugs and Substances Act (Canada) (the "CDSA") and the Controlled Substances Act (21 U.S.C. § 811) (the "CSA"), respectively, which place controlled substances in a schedule. Under the CDSA, psilocybin is currently a Schedule III drug. Under the CSA, psilocybin is currently a Schedule I drug.
In both Canada and the United States, the applicable federal government is responsible for regulating, among other things, the approval, import, sale and marketing of drugs, including any psychedelic substances, whether natural or novel. Health Canada, and the United States Food and Drug Administration ("FDA"), have not approved psilocybin as a drug for any indication. It is illegal to possess such substances without a prescription. The Corporation does not directly engage in any activities that would trigger the need to comply with any federal laws related to psychedelic substances. See "Regulatory Overview - Research and Development".
The Corporation does not deal with psychedelic substances except in jurisdictions where such activity is not illegal and then only within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Corporation currently does not handle controlled or restricted substances under the CDSA or CSA. If the Corporation were to conduct this work without reliance on third parties, it would need to obtain the required licenses, approvals and authorizations from Health Canada, the FDA or other applicable regulatory bodies. The Corporation does not have any direct or indirect involvement with illegal selling, production or distribution of any substances in jurisdictions in which it operates.
|
iv
|
The Corporation's operations are conducted in strict compliance with local laws where such activities are permissible and do not require any specific legal or regulatory approvals.
Given the early stage of its prescription drug product development, the Corporation can make no assurance that its research and development program will result in regulatory approval or commercially viable products. To achieve profitable operations, the Corporation, alone or with others, must successfully develop, gain regulatory approval for, and market its future products. The Corporation currently has no products that have been approved by Health Canada, the FDA, or any similar regulatory authority. To obtain regulatory approvals for its prescription drug product candidates being developed and to achieve commercial success, clinical trials must demonstrate that the prescription drug product candidate are safe for human use and that they demonstrate efficacy. See "Risk Factors" herein and "Risk Factors" in the Annual Information Form.
Certain statements throughout this Prospectus regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds have not been evaluated by Health Canada, the FDA or other similar regulatory authorities, nor has the efficacy of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds been confirmed by approved research. There is no assurance that psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds can be used to diagnose, treat, cure or prevent any disease or condition and robust scientific research and clinical trials are needed. There are multiple risk factors regarding the ability to successfully commercially scale a chemically synthesized process to obtain psilocybin and other analogues, including, but not limited to, regulatory changes or other changes in law, and risks related to drug development. See "Risk Factors" herein and "Risk Factors" in the Annual Information Form.
The Corporation oversees and monitors compliance with applicable laws in each jurisdiction in which it operates. In addition to the Corporation's senior executives and the employees responsible for overseeing compliance, the Corporation has local regulatory/compliance counsel engaged in every jurisdiction in which it operates. See "Compliance Program". Additionally, the Corporation has received legal opinions or advice in each jurisdiction where it currently operates regarding (a) compliance with applicable regulatory frameworks and (b) potential exposure and implications arising from applicable laws in jurisdictions where the Corporation has operations or intends to operate.
For these reasons, the Corporation may be (a) subject to heightened scrutiny by regulators, stock exchanges, clearing agencies and other authorities, (b) susceptible to regulatory changes or other changes in law, and (c) subject to risks related to drug development, among other things. There are a number of risks associated with the business of the Corporation. See "Risk Factors" herein and "Risk Factors" in the Annual Information Form.
An investment in the Securities is highly speculative and involves a high degree of risk that should be considered by potential purchasers. An investment in the Securities is suitable only for those purchasers who are willing to risk a loss of some or all of their investment and who can afford to lose some or all of their investment. A prospective purchaser should therefore review this Prospectus and the documents incorporated by reference herein in their entirety, including the Annual Information Form, and carefully consider the risk factors described under the section "Risk Factors" in this Prospectus, prior to investing in the Securities. See "Cautionary Note Regarding Forward-Looking Information" and "Risk Factors".
|
v
TABLE OF CONTENTS
Page
GENERAL MATTERS
Investors should rely only on the information contained in or incorporated by reference into this Prospectus or any applicable Prospectus Supplement. The Corporation has not authorized anyone to provide investors with different information. Information contained on the Corporation's website shall not be deemed to be a part of this Prospectus or incorporated by reference herein or in any applicable Prospectus Supplement and may not be relied upon by prospective investors for the purpose of determining whether to invest in the Securities qualified for distribution under this Prospectus. The Corporation is not making an offer of these Securities in any jurisdiction where the offer is not permitted. Investors should not assume that the information contained in this Prospectus is accurate as of any date other than the date on the front of this Prospectus or the date of the relevant document incorporated by reference. The Corporation's business, operating results, financial condition and prospects may have changed since that date.
The information contained on www.cybin.com is not intended to be included in or incorporated by reference herein, and prospective purchasers should not rely on such information when deciding whether or not to invest in any Securities.
In this Prospectus, unless stated otherwise or the context requires otherwise, all dollar amounts are expressed in Canadian dollars.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus, and in certain documents incorporated by reference herein, constitute "forward-looking information" and "forward-looking statements," within the meaning of applicable securities laws (collectively, "forward-looking statements"). All statements other than statements of historical fact, including, without limitation, those regarding the Corporation's future financial position, business strategy, budgets, research and development, plans and objectives of management for future operations, and any statements preceded by, followed by or that include the words "expect," "likely", "may," "will," "should," "intend," or "anticipate," "potential," "proposed," "estimate" and other similar words, including negative and grammatical variations thereof, or statements that certain events or conditions "may" or "will" happen, or by discussions of strategy, are forward-looking statements.
These statements are not historical facts but instead represent only the Corporation's expectations, estimates and projections regarding future events. These statements are not guarantees of future performance and involve assumptions, risks and uncertainties that are difficult to predict. Therefore, actual results may differ materially from what is expressed, implied or forecasted in such forward-looking statements. Additional factors that could cause actual results, performance or achievements to differ materially include, but are not limited to, those discussed under "Risk Factors" in the Annual Information Form and in this Prospectus and in other documents incorporated by reference in this Prospectus. Management provides forward-looking statements because it believes they provide useful information to readers when considering their investment objectives and cautions readers that the information may not be appropriate for other purposes. Consequently, all of the forward-looking statements made in this Prospectus and in documents incorporated by reference in this Prospectus are qualified by these cautionary statements and other cautionary statements or factors contained herein, and there can be no assurance that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Corporation. These forward-looking statements are made as of the date of this Prospectus and the Corporation assumes no obligation to update or revise them to reflect subsequent information, events or circumstances or otherwise, except as required by law.
The forward-looking statements in this Prospectus and in documents incorporated by reference in this Prospectus are based on numerous assumptions regarding the Corporation's present and future business strategies and the environment in which the Corporation will operate in the future, including assumptions regarding business and operating strategies, and the Corporation's ability to operate on a profitable basis.
Some of the risks which could affect future results and could cause results to differ materially from those expressed in the forward-looking statements contained herein include: the ongoing impact of the novel coronavirus COVID-19; limited operating history; achieving publicly announced milestones; speculative nature of investment risk; early stage of the industry and product development; regulatory risks and uncertainties; "foreign private issuer" status under the U.S. securities laws; plans for growth; limited products; limited marketing and sales capabilities; no assurance of commercial success; no profits or significant revenues; reliance on third parties for clinical development activities; risks related to third party relationships; reliance on contract manufacturers; safety and efficacy of products; clinical testing and commercializing products; completion of clinical trials; commercial grade product manufacturing; nature of regulatory approvals; unfavourable publicity or consumer perception; social media; biotechnology and pharmaceutical market competition; reliance on key executives and scientists; employee misconduct; business expansion and growth; negative results of external clinical trials or studies; product liability; enforcing contracts; product recalls; distribution and supply chain interruption; difficulty to forecast; promoting the brand; product viability; success of quality control systems; reliance on key inputs; liability arising from fraudulent or illegal activity; operating risk and insurance coverage; costs of operating as public company; management of growth; conflicts of interest; foreign operations; cybersecurity and privacy risk; environmental regulation and risks; decriminalisation of psychedelics; forward-looking statements may prove to be inaccurate; effects of inflation; political and economic conditions; application and interpretation of tax laws; enforcement of civil liabilities; Risks Related to Intellectual Property: trademark protection; trade secrets; patent law reform; patent litigation and intellectual property; protection of intellectual property; third-party licences; Financial and Accounting Risks: substantial number of authorized but unissued Common Shares; dilution; negative cash flow from operating activities; additional capital requirements; lack of significant product revenue; estimates or judgments relating to critical accounting policies; inadequate internal controls; Risks related to the Common Shares: market for the Common Shares; significant sales of Common Shares; volatile market price for the Common Shares; tax issues; no dividends; Risks related to an Offering and the Corporation: an investment in the Securities is highly speculative; completion of an Offering; receipt of all regulatory and stock exchange approvals in respect of an Offering; forward-looking statements may prove to be inaccurate; potential need for additional financing; negative operating cash flow and going concern; discretion over the use of proceeds; potential dilution; trading market; significant sales of Common Shares; positive return not guaranteed; management of growth; the Common Shares are subject to market price volatility; no history of payment of cash dividends; limited operating history as a public company; and risks relating to research and development objectives and milestones.
Although the forward-looking statements contained in this Prospectus are based upon what management currently believes to be reasonable assumptions, the Corporation cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. In particular, the Corporation has made assumptions regarding, among other things:
• substantial fluctuation of losses from quarter to quarter and year to year due to numerous external risk factors, and anticipation that the Corporation will continue to incur significant losses in the future;
• uncertainty as to the Corporation's ability to raise additional funding to support operations;
• the Corporation's ability to access additional funding;
• the fluctuation of foreign exchange rates;
• the risks associated with the development of the Corporation's product candidates which are at early stages of development;
• reliance upon industry publications as the Corporation's primary sources for third-party industry data and forecasts;
• reliance on third parties to plan, conduct and monitor the Corporation's preclinical studies and clinical trials;
• reliance on third party contract manufacturers to deliver quality clinical and preclinical materials;
• the Corporation's product candidates may fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or may not otherwise produce positive results;
• risks related to filing investigational new drug applications to commence clinical trials and to continue clinical trials if approved;
• the risks of delays and inability to complete clinical trials due to difficulties enrolling patients;
• competition from other biotechnology and pharmaceutical companies;
• the Corporation's reliance on the capabilities and experience of the Corporation's key executives and scientists and the resulting loss of any of these individuals;
• the Corporation's ability to fully realize the benefits of acquisitions;
• the Corporation's ability to adequately protect the Corporation's intellectual property and trade secrets;
• the risk of patent-related or other litigation; and
• the risk of unforeseen changes to the laws or regulations in the United States, Canada, the United Kingdom, the Netherlands, Ireland and other jurisdictions in which the Corporation operates.
Drug development involves long lead times, is very expensive and involves many variables of uncertainty. Anticipated timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation. Every patient treated on future studies can change those assumptions either positively (to indicate a faster timeline to new drug applications and other approvals) or negatively (to indicate a slower timeline to new drug applications and other approvals). This Prospectus and the documents incorporated by reference herein contain certain forward-looking statements regarding anticipated or possible drug development timelines. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assumes the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Corporation's development efforts to date.
In addition to the factors set out above and those identified under the heading "Risk Factors" in the Annual Information Form and in this Prospectus, other factors not currently viewed as material could cause actual results to differ materially from those described in the forward-looking statements. Although the Corporation has attempted to identify important risks and factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors and risks that cause actions, events or results not to be anticipated, estimated or intended. Accordingly, readers should not place any undue reliance on forward-looking statements.
Many of these factors are beyond the Corporation's ability to control or predict. These factors are not intended to represent a complete list of the general or specific factors that may affect the Corporation. The Corporation may note additional factors elsewhere in this Prospectus and in any documents incorporated by reference into this Prospectus. All forward-looking statements speak only as of the date made. All subsequent written and oral forward-looking statements attributable to the Corporation, or persons acting on the Corporation's behalf, are expressly qualified in their entirety by the cautionary statements. Except as required by law, the Corporation undertakes no obligation to update any forward-looking statement.
The forward-looking statements contained in this Prospectus and the documents incorporated by reference herein are expressly qualified in their entirety by the foregoing cautionary statement. Investors should read this entire Prospectus, including the Annual Information Form, the documents incorporated by reference herein, and each applicable Prospectus Supplement, and consult their own professional advisers to ascertain and assess the income tax and legal risks and other aspects associated with holding Securities.
TRADEMARKS AND SERVICE MARKS
This Prospectus includes trademarks, trade names and service marks which are protected under applicable intellectual property laws for use in connection with the operation of the Corporation's business, and which are the property of the Corporation. All other trade names, trademarks or service marks appearing in this prospectus that are not identified as marks owned by the Corporation are the property of their respective owners. Solely for convenience, trademarks, service marks and trade names referred to in this prospectus may be listed without the ®, (TM) and (sm) symbols, however, we will assert, to the fullest extent under applicable law, the Corporation's applicable rights in these trademarks, service marks and trade names.
MARKETING MATERIALS
Any template version of marketing materials (as such terms are defined in National Instrument 41-101 - General Prospectus Requirements) that are utilized in connection with the distribution of Securities will be filed under the Corporation's profile on www.sedarplus.ca (SEDAR+). In the event that such marketing materials are filed after the date of the applicable Prospectus Supplement for the offering and before termination of the distribution of such Securities, such filed versions of the marketing materials will be deemed to be incorporated by reference into the applicable Prospectus Supplement for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains.
MARKET AND INDUSTRY DATA
Market and industry data contained and incorporated by reference in this Prospectus or any applicable Prospectus Supplement concerning economic and industry trends is based upon good faith estimates of the Corporation's management or derived from information provided by industry sources. The Corporation believes that such market and industry data is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of such information and we have not independently verified the assumptions upon which projections of future trends are based. While the Corporation is not aware of any misstatements regarding the industry data presented herein, the Corporation's estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under "Cautionary Note Regarding Forward-Looking Information" and "Risk Factors" in this Prospectus.
For the avoidance of doubt, nothing stated in this paragraph operates to relieve the Corporation or the underwriters/agents from liability for any misrepresentation in this Prospectus or under applicable Canadian securities laws.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of the Corporation at 100 King Street West, Suite 5600, Toronto, Ontario M5X 1C9, telephone (908) 764-8385, and are also available electronically on SEDAR+.
The following documents of the Corporation filed with the securities commissions or similar authorities in Canada are incorporated by reference in this Prospectus:
1. annual information form of the Corporation dated June 27, 2023 for the year ended March 31, 2023 (the “Annual Information Form”);
2. the audited consolidated financial statements of the Corporation and the notes thereto as at and for the fiscal year ended March 31, 2023, together with the auditor’s report thereon;
3. management’s discussion and analysis of the Corporation for the year ended March 31, 2023;
4. the unaudited interim condensed consolidated financial statements of the Corporation and the notes thereto for the three months ended June 30, 2023 (the “Interim Financial Statements”);
5. management’s discussion and analysis of the Corporation for the three months ended June 30, 2023 (the “Interim MD&A”);
6. management information circular of the Corporation dated July 13, 2022 relating to an annual meeting of shareholders of the Corporation held on August 15, 2022;
7. material change report dated May 31, 2023 relating to the sale of up to US$30,000,000 of Common Shares to Lincoln Park Capital Fund, LLC (the “Investor”), upon the terms and subject to the conditions contained in the purchase agreement dated May 30, 2023 (the “Purchase Agreement”), and made pursuant to and qualified by a prospectus supplement dated May 30, 2023 (the “May 2023 Prospectus Supplement”), to the Corporation’s short form base shelf prospectus dated July 5, 2021 (the “2021 Base Shelf Prospectus”); and
8. material change report dated August 8, 2023 relating to the public offering of 24,264,706 units of the Corporation (the “Units”) at a price of US$0.34 per Unit for gross proceeds of US$8,250,000 pursuant to a prospectus supplement dated August 1, 2023 to the 2021 Base Shelf Prospectus (the “Offering”).
Any document of the type referred to in Item 11.1 of Form 44-101F1 - Short Form Prospectus filed by the Corporation with a securities commission or similar regulatory authority in Canada after the date of this Prospectus and prior to 25 months from the date hereof shall be deemed to be incorporated by reference in this Prospectus.
In addition, to the extent any such document is included in any annual report on Form 40-F or 20-F (or any respective successor form) filed with the SEC subsequent to the date of this Prospectus and prior to 25 months from the date hereof, such document shall be deemed to be incorporated by reference as exhibits to the Registration Statement on Form F-10 (the "Registration Statement") of which this Prospectus forms a part. In addition, any other report on Form 6-K and the exhibits thereto filed or furnished by the Corporation with the SEC, and any other reports filed, under the United States Securities Exchange Act of 1934, as amended (the "Exchange Act") from the date of this Prospectus and prior to 25 months from the date hereof shall be deemed to be incorporated by reference as exhibits to the Registration Statement of which this Prospectus forms a part, but only if and to the extent expressly so provided in any such report.
Copies of the documents incorporated by reference herein will be available electronically on the Corporation's SEDAR+ profile, which can be accessed at www.sedarplus.ca, and from the Corporation's EDGAR profile at www.sec.gov. The Corporation's filings through SEDAR+ and EDGAR are not incorporated by reference in the Prospectus except as specifically set out herein.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded for the purposes of this Prospectus to the extent that a statement contained herein or in any subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus modifies or supersedes that statement. Any statement so modified or superseded shall not constitute a part of this Prospectus except as so modified or superseded. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
Upon filing of a new annual information form and related annual financial statements with, and where required, accepted by, the applicable securities regulatory authorities during the currency of this Prospectus, the previous annual information form, including all amendments thereto, the previous annual financial statements and all interim financial statements (including any interim period management's discussion and analysis related thereto), material change reports and management information circulars filed prior to the commencement of the fiscal year in which the new annual information form is filed, shall be deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of Securities hereunder.
A Prospectus Supplement containing the specific terms of any Securities offered thereunder will be delivered to purchasers of such Securities together with this Prospectus to the extent required under applicable securities laws and will be deemed to be incorporated by reference into this Prospectus as of the date of such Prospectus Supplement solely for the purposes of the Securities offered hereunder and thereunder.
DOCUMENTS FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have been, or will be, filed with the SEC as part of the Registration Statement of which this Prospectus forms a part: (i) the documents listed under "Documents Incorporated by Reference"; (ii) powers of attorney from certain of the Corporation's directors and officers (included on the signature page to the Registration Statement); (iii) the consent of Zeifmans LLP; and (iv) the form of indenture for any Debt Securities issued hereunder. A copy of any applicable form of warrant indenture, subscription receipt agreement or statement of eligibility of trustee on Form T-1, as applicable, will be filed by post-effective amendment or by incorporation by reference to documents filed or furnished with the SEC under the Exchange Act.
THE CORPORATION
This summary does not contain all the information that may be important to you in deciding whether to invest in the Securities. You should read the entire Prospectus, including the section entitled "Risk Factors", the applicable Prospectus Supplement, and the documents incorporated by reference herein, including the Annual Information Form, before making such decision.
Summary of the Business
The Corporation is a clinical-stage biopharmaceutical company on a mission to create safe and effective psychedelic-based therapeutics to address the unmet need for new and innovative treatment options for people who suffer from mental health conditions. The Corporation’s goal of revolutionizing mental healthcare is supported by a network of world-class partners and internationally recognized scientists aimed at progressing proprietary drug discovery platforms, innovative drug delivery systems, and novel formulation approaches and treatment regimens.1
The Corporation's research and development work focuses on a three-pillar strategy that leverages the Corporation's core competencies in preclinical innovation and clinical development. This strategy supports the creation of intellectual property ("IP") focused on developing the Corporation's platform technology, the progression of clinical development programs including CYB003, a deuterated psilocybin analog, CYB004, a deuterated version of N, N-dimethyltryptamine ("DMT"), CYB005, phenethylamine derivatives, and an expansive list of preclinical molecules to facilitate future drug development opportunities.
The Corporation currently has more than 50 pending patent applications across six patent families and spanning seven research programs through a combination of internal filings and external licensing agreements.
Advancement of Mental Healthcare
The Corporation is conducting research and development of psychedelic therapeutics that aim to address unmet needs in the treatment of mental health conditions. This comprehensive development work is predicated on structural modifications of known tryptamine and phenethylamine derivatives to improve their pharmacokinetic properties while maintaining their respective pharmacology.
Across its extensive research and development programs, the Corporation is evaluating a wide array of novel, synthetic psychedelic active pharmaceutical ingredients intended to be delivered through innovative drug delivery systems including via inhalation, via intravenous ("IV"), and intramuscular, or subcutaneous ("SC") administration.2
The Corporation intends to apply for regulatory approval for therapies targeting indications such as major depressive disorder, alcohol use disorder, generalized anxiety disorder and potentially other various mental health conditions3. The Corporation is also developing compounds that may have the potential to address neuroinflammation4, central nervous system disorders, and psychiatric disorders5.
For additional information in respect of the Corporation and its operation, please see the Corporation’s Annual Information Form and Interim MD&A incorporated by reference into this Prospectus.
_____________________________________________
1 This is a forward-looking statement that involves material assumptions by the Corporation. Drug development involves long lead times, is very expensive and involves many variables of uncertainty. Anticipated timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assumes the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Corporation's development efforts to date.
2 See footnote 1.
3 See footnote 1.
Inter-Corporate Relationships
As at the date of this Prospectus, the Corporation's corporate structure includes the following material wholly-owned subsidiaries:
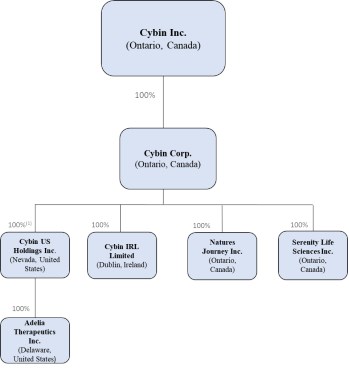
Note:
(1) The shareholders of Adelia Therapeutics Inc. ("Adelia") hold certain non-voting securities of Cybin U.S. issued in connection with the acquisition of Adelia on December 14, 2020 (the "Adelia Transaction"). For additional information in respect of the Adelia Transaction, please see the Annual Information Form incorporated by reference in this Prospectus Supplement.
Select Recent Developments
Other than as set forth below and generally in this Prospectus or the documents incorporated by reference herein, there have been no material developments in the business of the Corporation since August 14, 2023, the date of the Interim Financial Statements and Interim MD&A.
On May 30, 2023, the Corporation announced it had entered into the Purchase Agreement with the Investor, pursuant to which the Investor agreed to purchase from the Corporation, at the Corporation’s direction from time to time, in the Corporation’s sole discretion, from and after the date that the conditions to the Investor’s purchase obligations set forth in the Purchase Agreement are satisfied, including the filing of the May 2023 Prospectus Supplement, and over a period of up to 36 months thereafter, Common Shares having a total maximum aggregate purchase price to the Investor of US$30,000,000 (subject to certain limitations contained in the Purchase Agreement), upon the terms and subject to the conditions contained in the Purchase Agreement. On July 31, 2023, the Corporation issued a notice of suspension of the Purchase Agreement to the Investor in the context of announcing the Offering. The Corporation sold a total of US$465,273 under the Purchase Agreement out of the total US$30,000,000 qualified under the May 2023 Prospectus Supplement, using the proceeds for the advancement of its research and development programs and for general corporate purposes. The Corporation has suspended all sales under the Purchase Agreement allowing it to use the balance of US$29,534,727 available under the 2021 Base Shelf Prospectus for the Offering. Subject to receipt of all necessary regulatory approvals, the Corporation intends to file a prospectus supplement under this Prospectus with respect to the Purchase Agreement.
On June 29, 2023, the Corporation announced the appointment of Aaron Bartlone as Chief Operating Officer, effective July 1, 2023. Mr. Bartlone has served as Chief Operating Officer of Cybin’s U.S. subsidiary, Cybin US Holdings, Inc., since March 2021. Mr. Bartlone is a United States resident and currently holds 701,070 Common Shares and options exercisable into 1,315,000 Common Shares.
On August 5, 2023, the Corporation’s at-the-market equity program to issue and sell up to US$35,000,000 of Common Shares (the “ATM Program”) automatically terminated in accordance with the terms of the equity distribution agreement entered into on August 8, 2022, due to the Corporation’s prior base shelf prospectus, dated July 5, 2021, lapsing on August 5, 2023, being 25 months after the effective date. Subject to receipt of all necessary approvals and the entering into of a new equity distribution agreement, the Corporation intends to file a prospectus supplement under this Prospectus in order to qualify the issuance and sale of Common Shares pursuant to an at-the-market equity program.
On August 15, 2023, the Corporation announced that the U.S. Patent and Trademark Office has granted U.S. patent 11,724,985, to a deuterated psilocybin analog in the Corporation’s CYB003, investigational drug program. The patent, which is expected to provide exclusivity until 2041, includes composition of matter claims to deuterated tryptamines in support of the Corporation’s clinical-stage programs, CYB003, a proprietary deuterated psilocybin analog, and CYB004, a proprietary deuterated dimethyltryptamine, in addition to other of the Corporation’s pre-clinical programs, as well as claims directed towards methods of treating major depressive disorder (“MDD”) and treatment-resistant depression.
On August 17, 2023, the Corporation announced that it has initiated preparations for good manufacturing practices (“GMP”) production of a capsule formulation of CYB003, its proprietary deuterated psilocybin analog in development for the potential treatment of MDD.
REGULATORY OVERVIEW
A summary of the applicable regulatory framework for the Corporation's various business segments and proposed business activity are set forth below.
Canada
In Canada, oversight of healthcare is divided between the federal and provincial governments. The federal government is responsible for regulating, among other things, the approval, import, sale, and marketing of drugs such as psilocybin and other psychedelic substances, whether natural or novel. The provincial/territorial level of government has authority over the delivery of health care services, including regulating health facilities, administering health insurance plans such as the Ontario Health Insurance Plan, distributing prescription drugs within the province, and regulating health professionals such as doctors, psychologists, psychotherapists and nurse practitioners. Regulation is generally overseen by various colleges formed for that purpose, such as the College of Physicians and Surgeons of Ontario.
Certain psychoactive compounds, such as psilocybin, are considered controlled substances under Schedule III of the CDSA. In order to conduct any scientific research, including pre-clinical and clinical trials, using psychoactive compounds listed as controlled substances under the CDSA, an exemption under Section 56 of the CDSA (a "Section 56 Exemption") is required. This exemption allows the holder to possess and use the controlled substance without being subject to the restrictions set out in the CDSA.
Health Canada has not approved psilocybin as a drug for any indication. However, there are legal routes through which psilocybin may be accessed for medical or scientific purposes. The Canadian Minister of Health can grant Section 56 Exemptions if it is deemed to be necessary for a medical or scientific purpose or is otherwise in the public interest. The Corporation has not applied for a Section 56 Exemption from Health Canada.
Health Canada's Special Access Program ("SAP") was designed to provide Canadians to access certain restricted drugs before they are formally approved for use in Canada. In January 2022, certain amendments to the SAP came into force to permit medical practitioners treating patients with serious or life-threatening conditions to request access to restricted drugs that have not yet been approved for sale in Canada when conventional therapies have failed, are unsuitable, or unavailable in Canada. Such amendments create a means of legally accessing psilocybin through the SAP. The Corporation has not applied for access under the SAP.
The possession, sale or distribution of controlled substances is prohibited unless specifically permitted by the government. A party may seek government approval for a Section 56 Exemption to allow for the possession, transport or production of a controlled substance for medical or scientific purposes. Products that contain a controlled substance such as psilocybin cannot be made, transported or sold without proper authorization from the government. A party can apply for a Dealer's License under the Food and Drug Regulations (Part J). In order to qualify as a licensed dealer, a party must meet all regulatory requirements mandated by the regulations including having compliant facilities, compliant materials and staff that meet the qualifications under the regulations of a senior person in charge and a qualified person in charge. Assuming compliance with all relevant laws (CDSA, Food and Drugs Regulations) and subject to any restrictions placed on the license by Health Canada, an entity with a Dealer's License may produce, assemble, sell, provide, transport, send, deliver, import or export a restricted drug (as listed in Part J in the Food and Drugs Regulations - which includes psilocybin and psilocin) (see s. J.01.009 (1) of the Food and Drug Regulations).
The Corporation intends to sponsor and work with licensed third parties to conduct any clinical trials and research and does not handle controlled substances. If the Corporation were to conduct this work without the reliance on third parties, it would need to obtain additional licenses and approvals described above.
Please see "Description of the Business - Research and Development" in the Annual Information Form for additional information concerning the regulation applicable to the process required before prescription drug product candidates may be marketed in Canada.
United States
The FDA and other federal, state, local and foreign regulatory agencies impose substantial requirements upon the clinical development, approval, labeling, manufacture, marketing and distribution of drug products. These agencies regulate, among other things, research and development activities and the testing, approval, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, advertising and promotion of any prescription drug product candidates or commercial products. The regulatory approval process is generally lengthy and expensive, with no guarantee of a positive result. Moreover, failure to comply with applicable FDA or other requirements may result in civil or criminal penalties, recall or seizure of products, injunctive relief including partial or total suspension of production, or withdrawal of a product from the market. The Corporation intends to file an investigational new drug application (“IND”) related to its Deuterated Psilocybin Analog Program upon completion of its pre-clinical studies and CMC development.6 Anticipated timelines related to regulatory filings are based on reasonable assumptions informed by current knowledge and information available to the Corporation.
Psilocybin, psilocin, dimethyltryptamine, and 5-Methoxy-N-N-dimethyltryptamine are strictly controlled under the CSA as Schedule I substances. Schedule I substances by definition have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. Schedule I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements and criteria for importation. Anyone wishing to conduct research on substances listed in Schedule I under the CSA must register with the United States Drug Enforcement Administration ("DEA") and obtain DEA approval of the research proposal. A majority of state laws in the United States also classify psilocybin and psilocin as Schedule I controlled substances. For any product containing psilocybin or any Schedule I substance to be available for commercial marketing in the United States, such substance must be rescheduled, or the product itself must be scheduled, by the DEA to Schedule II, III, IV or V. Scheduling determinations by the DEA are dependent on FDA approval of a substance or a specific formulation of a substance.
See "Description of the Business - Research and Development" in the Annual Information Form for additional information concerning the regulation applicable to the process required before prescription drug product candidates may be marketed in the United States.
Europe (Netherlands)
The International Narcotics Control Board ("INCB"), a United Nations ("UN") entity, monitors enforcement of restrictions on controlled substances. The INCB's authority is defined by three international UN treaties - the UN Single Convention on Narcotic Drugs of 1961, the UN Psychotropic Convention of 1971 (referred to herein as the UN71), and the UN Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988, which contains provisions related to the control of controlled substance precursors. EU Member States, including the Netherlands, that have agreed to abide by the provisions of these treaties, each create responsible agencies and enact laws or regulations to implement the requirements of these conventions.
Specific EU legislation establishing different classes of controlled substances is limited to EU regulations that define classes of precursors, or substances used in the illicit manufacture of controlled substances, including Regulation (EC) No. 273/2004 of the European Parliament and the Council of February 11, 2004 and the Council Regulation (EC) No. 111/2005 of December 22, 2004. While EU legislation does not establish different classes of narcotic drugs or psychotropic substances, the Council Decision 2005/387/JHA of May 10, 2005 can provoke a Council Decision requiring EU member states to put a drug under national controls equivalent to those of the INCB. DMT is currently classified as a Schedule I substance under the UN71; the EU member states that are party to the UN71, including the Netherlands, have agreed to the following in respect of Schedule I substances:
_____________________________________________
6 This statement is based on the following material assumption: drug development involves long lead times, is very expensive and involves many variables of uncertainty. Anticipated timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation. As of the date hereof, it has not yet completed the aforementioned items. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assumes the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Corporation’s development efforts to date. See “Risk Factors”.
- prohibit all use except for scientific and very limited medical purposes by duly authorized persons, in medical or scientific establishments which are directly under the control of their Governments or specifically approved by them;
- require that manufacture, trade, distribution and possession be under a special licence or prior authorization;
- provide for close supervision of the activities and acts mentioned in paragraphs (a) and (b);
- restrict the amount supplied to a duly authorized person to the quantity required for his authorized purpose;
- require that persons performing medical or scientific functions keep records concerning the acquisition of the substances and the details of their use, such records to be preserved for at least two years after the last use recorded therein; and
- prohibit export and import except when both the exporter and importer are the competent authorities or agencies of the exporting and importing country or region, respectively, or other persons or enterprises which are specifically authorized by the competent authorities of their country or region for the purpose.
As classification of controlled substances may vary among different EU member states, sponsors must be aware of the prevailing legislation in each country where a clinical trial may be conducted. Prior to operating or conducting any preclinical or clinical studies in any other EU member state, Cybin will investigate the specific regulatory requirements of such EU member state. As referenced above, a licence is required for individuals and entities who wish to produce, dispense, import, or export Schedule I substances (including DMT), but the specific requirements vary from country to country. Currently, DMT is classified in the Netherlands as a List 1 Drug under the Dutch Opium Act (Opiumwet) (the "Dutch Opium Act") and as such, subject to express authorization being obtained, the production, trade and possession of DMT are prohibited.
In addition to the Dutch Opium Act, two other Dutch Acts may be relevant when it comes to drugs: the Medicines Act and the Commodities Act.
The specific regulatory processes and approvals required may vary among different EU member states and are set forth in the respective legislation of each country. For The Netherlands, there are specific regulatory requirements for the approval of clinical trials that need to be met. Firstly, a CTA (Clinical Trial Application) dossier containing the preclinical and any clinical information along with the proposed clinical trial design must be submitted to an accredited Ethics Committee and to the Central Commission on Research in Humans (the "CCMO"), which is also known as the Competent Authority in The Netherlands. In Dutch, the CCMO is called the 'Centrale Commissie Mensgebonden Onderzoek'. In cases where the study involves a substance subject to the Dutch Opium Act (such as DMT), an official exemption by Farmatec is needed, which needs to be included in the CTA.
Specific rules for the submission, assessment and conduct of clinical trials with medicinal products are set out in, among others, the EU Clinical Trial Regulation 536/2014 (CTR), which is applicable in the EU as of January 31, 2022 and the Medical Research (Human Subjects) Act (Wet medisch-wetenschappelijk onderzoek met mensen).
Ireland
In Ireland, psilocin is a controlled substance under the Misuse of Drugs Act, 1977, 1984 and 2015 (the "Ireland MDA"), the Misuse of Drugs Regulations 2017 (the "Ireland MDR") and the Criminal Justice (Psychoactive Substances) Act 2010. These are the primary legislative instruments which govern controlled substances in Ireland. This legislation regulates the use, possession, supply, licensing, and administration of listed scheduled substances and establishes the offences and penalties for anything done contrary to the legislation. Any substance, product or preparation (whether natural or otherwise) including a fungus of any kind or description, which contains psilocin or an ester of psilocin is controlled as a Schedule 1 controlled substance under the Ireland MDA and the Ireland MDR. The Ireland MDR includes "any substance, product or preparation including fungi of any kind or description, containing psilocin or an ester of psilocin (which are commonly described as 'magic mushrooms')" within the strict regime of control that applies to those substances in Schedule 1 of the Ireland MDR. Accordingly, psilocin will qualify as a Schedule 1 controlled substance and is subject to the strict regime of control that applies. As a Schedule 1 controlled substance under the Ireland MDA, unlawful manufacturing, production, preparation, importation, exportation, supply, or distribution of psilocin carries onerous obligations and harsh punishments for contravention; this include fines and/or terms of imprisonment of up to 14 years. Pursuant to the Ireland MDA, in certain circumstances, the Minister for Health "may grant licences or issue permits or authorizations for any of the purposes of this Act, attach conditions to any such licence, permit or authorization, vary such conditions and revoke any such licence, permit or authorization". Where licences are granted, there are very strict conditions imposed on licence holders. For example, strict conditions can be placed regarding the security, storage and documenting controlled substances.
The Corporation does not currently engage in any activities in Ireland that are regulated by such laws. If the Corporation were to engage in such activities, it would need to obtain the appropriate licences and authorization to do so. The Corporation intends to constantly review its Irish operations to ensure compliance with all applicable laws as the operations evolve.
United Kingdom
In the United Kingdom, there are two main "layers" of regulation with which products containing controlled substances must comply. These are: (i) controlled drugs legislation, which applies to all products irrespective of the type of product, and (ii) the regulatory framework applicable to a specific category of products, in this case, pharmaceuticals and food/food supplements.
The main controlled drugs legislation in the United Kingdom is the Misuse of Drugs Act 1971 ("MDA") and the Misuse of Drugs Regulations 2001 ("MDR"), each as amended. The MDA sets out the penalties for unlawful production, possession and supply of controlled drugs based on three classes of risk (A, B and C). The MDR sets out the permitted uses of controlled drugs based on which Schedule (1 to 5) they fall within.
In the United Kingdom, "Fungus (of any kind) which contains psilocin or an ester of psilocin" is controlled as a Class A drug under the MDA and Schedule 1 drug under the MDR. As psilocybin is a phosphate ester of psilocin, even if it is isolated from psilocin, it will still be treated as a Class A drug under the MDA and as a Schedule 1 drug under the MDR.
In the United Kingdom, Class A drugs are deemed to be the most dangerous, and so carry the harshest punishments for unlawful manufacture, production, possession and supply. Schedule 1 drugs can only be lawfully manufactured, produced, possessed and supplied under a controlled drugs domestic licence issued by the UK Home Office. While exemptions do exist, none are applicable to the API.
The Corporation previously disclosed that it intended to file a clinical trial application with the United Kingdom Medicines and Healthcare products Regulatory Agency (the "MHRA") related to the Deuterated Psilocybin Analog Program upon completion of its pre-clinical studies and CMC development. The Corporation has since decided that it will first proceed in the United States and will re-evaluate other applications at a later date. Anticipated timelines related to regulatory filings are based on reasonable assumptions informed by current knowledge and information available to the Corporation.
Licensing Requirements
The Corporation obtains CYB003 API from a pharmaceutical ingredient provider who is FDA-registered and based in the United States. The API itself has been manufactured and packaged in FDA-registered facilities in the United States. The API is expected to be sent directly to the Corporation's partners for research and development purposes in the United States, Canada and the United Kingdom and to its clinical trial site in the U.S. As a part of the Corporation's acquisition of a Phase 1 DMT study from Entheon Biomedical Corp. in July 2022 (the "Asset Acquisition"), the Corporation also acquired API. The CYB004-E API was manufactured in the Netherlands by a pharmaceutical ingredient provider that is United States FDA-inspected.7
_____________________________________________
7 As a result of the Asset Acquisition, including the existing API, the Corporation did not direct the manufacturing of the API for CYB004-E and proceeded in reliance upon the representations of Entheon Biomedical Corp. and the Corporation's acquisition diligence. While the Corporation believes the CYB004- E API meets all required specifications, the Corporation did not oversee or direct the manufacture of the DMT API being used in CYB004-E.
Although the facilities in the UK are currently FDA-registered, this would not be sufficient to ensure the existence of valid marketing activities at this site. As mentioned above, in order to produce, possess and supply the API, the UK-based facility must also hold a domestic licence issued by the Home Office covering the manufacture, production, possession and supply of a controlled substance, as well as an export licence for each API shipment. The export application must include details of the importer and any import licence required by the local authorities in the United States. Moreover, as set out below in more detail under the heading "Pharmaceutical Products", depending on how the API is developed, certain authorizations and licences from the MHRA may be required to authorize some of the activities carried on at the UK-based facilities in relation to the API.
All premises that are licensed in connection with the possession, supply, manufacture and/or production of controlled drugs are required to adhere to detailed security standards.8
Typically, when controlled drugs are being transported between licensees, responsibility for their security remains with the owner and does not transfer to either the courier or the customer until the drugs arrive at their destination and are signed for. However, where a third party is involved in the transit and/or storage of controlled drugs, even if they are not the legal owners, this party also carries responsibility for their security by virtue of being 'in possession' of them. Under the Home Office guidance, each organisation involved in the movement of controlled drugs should have a standard operating procedure covering their responsibilities, record keeping, reconciliation and reporting of thefts/losses.9
Pharmaceutical Products
A product is regulated as a "medicinal product" under UK legislation (the Human Medicines Regulations 2012) if (i) it is a substance or combination of substances presented as having properties of preventing or treating disease in human beings (e.g., in marketing claims) or (ii) it is a substance or combination of substances that may be used by or administered to human beings with a view to (a) restoring, correcting or modifying a physiological function by exerting a pharmacological, immunological or metabolic action, or (b) making a medical diagnosis.
Whether a specific product restores, corrects or modifies a physiological function by exerting a pharmacological, immunological or metabolic action will depend on factors such as the concentration of the psilocybin/psilocin and the mode of action of any psilocybin/psilocin absorbed in the body.
If a product is a medicinal product, a marketing authorization for the product is required before the product can be placed on the market in the UK. The process for obtaining a marketing authorization involves submitting preclinical and clinical data as well as quality and manufacturing information in the form of a common technical document. In addition to a marketing authorization for the product itself, companies carrying out activities involving medicinal products, such as manufacturing, distribution and wholesaling, need to meet defined standards GMP and/or Good Distribution Practice ("GDP")) and to hold a related licence from the MHRA
How the API is subsequently processed will determine the licences that the UK-based facility must hold. In particular:
-
if the API is just one 'ingredient' of the investigational medicinal product ("IMP") which is used in the clinical trial then the UK-based facility must register with the MHRA and provide the MHRA with 60 days' notice of the intended start of manufacture/distribution, and comply with GMP and GDP for active substances; and
-
conversely, if the API will itself constitute the IMP, the manufacturer must, except in certain limited circumstances, hold a Manufacturer's Authorisations for IMPs licence ("MIA(IMP)"). In this scenario, an MIA(IMP) would be required regardless of whether the IMP is for use in the UK, another EEA Member State or a third country (such as the United States or Canada).
_____________________________________________
8 Home Office guidance; Security guidance for all existing or prospective Home Office Controlled Drug Licensees and/or Precursor Chemical Licensees or Registrants; 2022; https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1125889/ Security_Guidance_for_all_Businesses_and_Other_Organisations_v1.5_Nov_2022.pdf.
9 Home Office guidance; Guidelines for Standard Operating Procedures (SOPs); https://assets.publishing.service.gov.uk/government/uploads/ system/uploads/attachment_data/file/480572/StandardOpProcedure.pdf.
Some products fall on the borderline between medicines and another category such as medical devices, cosmetics or food supplements. The regulatory status of the product will be determined by i) the actual effect of the product on the body; and ii) any claims made about the effect of the product. Where a product is potentially both a medicinal product and another category of product, the legal position in the UK and EU is that it will be regulated as a medicinal product.
Research and Development
The Corporation is focused on development of psychedelic medicines and other products, through research and development of novel chemical compounds and delivery mechanisms and study of such compounds in clinical environments around the world. The Corporation anticipates growing its pipeline of psychedelic pharmaceutical products through its internal research, development, proprietary discovery programs, mergers and acquisitions, joint ventures and collaborative development agreements. For the time being, the Corporation maintains intellectual property generated by its R&D programs through patent filings and as trade secrets. The Corporation anticipates that as these programs mature more patent applications will be filed and more details about these programs will be disclosed at such time.
As a result of COVID-19, certain institutions have implemented certain facility procedures and are utilizing technology in an effort to mitigate the effects of the pandemic, specifically by moving patient interactions to remote status wherever possible. The Corporation cannot guarantee that the continued effects of COVID-19 will not impact patient recruiting for clinical trials and institutional processes at institutions involved in pharmaceutical product development.
Psychedelics are a class of drug whose primary action is to trigger psychedelic experiences by way of serotonin receptor agonism, causing thought, visual and auditory changes, and altered state of consciousness. Major psychedelic drugs include mescaline, LSD, psilocybin, and DMT. Psilocybin is a naturally occurring psychedelic prodrug compound produced by more than 200 species of mushrooms, collectively known as psilocybin mushrooms. The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. As a prodrug, psilocybin is quickly converted by the body to psilocin, which has mind-altering effects.
The pharmacokinetics, pharmacology and human metabolism of psilocybin are well known and well characterized. In conjunction with psychotherapy, psilocybin has been utilized broadly in phase II clinical trials.
Psilocybin found in certain species of mushrooms is a non-habit forming naturally occurring psychedelic compound. Once ingested, psilocybin is rapidly metabolized to psilocin, which then acts on serotonin receptors in the brain.
Cybin has commenced research and development on the delivery of synthetic psilocybin and other psychedelics through mechanisms such as sublingual film delivery, IV, and by way of inhalation.
The Corporation's research and development must be conducted in strict compliance with the regulations of federal, state, local and regulatory agencies in Canada, the United States, and the United Kingdom and the equivalent regulatory agencies in the other jurisdictions in which the Corporation operates. These regulatory authorities regulate, among other things, the research, manufacture, promotion and distribution of drugs in specific jurisdictions under applicable laws and regulations.
Canada
The process required before a prescription drug product candidate may be marketed in Canada generally involves:
- Chemical and Biological Research - Laboratory tests are carried out on tissue cultures and with a variety of small animals to determine the effects of the drug. If the results are promising, the manufacturer will proceed to the next step of development.
-
Pre-Clinical Development - Animals are given the drug in varying amounts over differing periods of time. If it can be shown that the drug causes no serious or unexpected harm at the doses required to have an effect, the manufacturer will proceed to clinical trials.
-
Clinical Trials - Phase I - The first administration in humans is to test if people can tolerate the drug. If this testing is to take place in Canada, the manufacturer must prepare a clinical trial application for the Therapeutic Products Directorate of Health Canada (the "TPD"). This includes the results of the first two steps and a proposal for testing in humans. If the information is sufficient, the Health Products and Food Branch of Health Canada (the "HPFB") grants permission to start testing the drug, generally first on healthy volunteers.
-
Clinical Trials - Phase II - Phase II trials are carried out on people with the target condition, who are usually otherwise healthy, with no other medical condition. Trials carried out in Canada must be approved by the TPD. In phase II, the objective of the trials is to continue to gather information on the safety of the drug and begin to determine its effectiveness.
-
Clinical Trials - Phase III - If the results from phase II show promise, the manufacturer provides an updated clinical trial application to the TPD for phase III trials. The objectives of phase III include determining whether the drug can be shown to be effective, and have an acceptable side effect profile, in people who better represent the general population. Further information will also be obtained on how the drug should be used, the optimal dosage regimen and the possible side effects.
-
New Drug Submission - If the results from phase III continue to be favourable, the drug manufacturer can submit a new drug submission ("NDS") to the TPD. A drug manufacturer can submit an NDS regardless of whether the clinical trials were carried out in Canada. The TPD reviews all the information gathered during the development of the drug and assesses the risks and benefits of the drug. If it is judged that, for a specific patient population and specific conditions of use, the benefits of the drug outweigh the known risks, the HPFB will approve the drug by issuing a notice of compliance.
United States
Because psilocybin, psilocin, DMT, and 5-Methoxy-DMT are listed as Schedule I substances under the CSA, for any product containing psilocybin or any Schedule I substance to be available for commercial marketing in the United States, such substance must be rescheduled, or the product itself must be scheduled, by the DEA to Schedule II, III, IV or V.
The process required before a prescription drug product candidate may be marketed in the United States generally involves:
-
completion of extensive non-clinical laboratory tests, animal studies and formulation studies, all performed in accordance with the FDA's Good Laboratory, Good Clinical and/or Manufacturing Practice regulations;
-
submission to the FDA of an IND, which must become effective before human clinical trials may begin;
-
approval by an institutional review board or independent ethics committee at each clinical trial site before each trial may be initiated;
-
for some products, performance of adequate and well-controlled human clinical trials in accordance with the FDA's regulations, including Good Clinical Practices, to establish the safety and efficacy of the prescription drug product candidate for each proposed indication;
-
submission to the FDA of a New Drug Application ("NDA"); and
-
FDA review and approval of the NDA prior to any commercial marketing, sale or shipment of the drug.
The testing and approval process requires substantial time, effort and financial resources, and the Corporation cannot be certain that the DEA will schedule or reschedule any Schedule I substance or product candidate to Schedule II, III, IV, or V, or that approvals for its prescription drug product candidates will be granted on a timely basis, if at all.
Non-clinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals and other animal studies. The results of non-clinical tests, together with manufacturing information and analytical data, are submitted as part of an IND to the FDA. Some non-clinical testing may continue even after an IND is submitted. The IND also includes one or more protocols for the initial clinical trial or trials and an investigator's brochure. An IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions relating to the proposed clinical trials as outlined in the IND and places the clinical trial on a clinical hold. In such cases, the IND sponsor and the FDA must resolve any outstanding concerns or questions before any clinical trials can begin. Clinical trial holds also may be imposed at any time before or during studies due to safety concerns or non-compliance with regulatory requirements.
An independent institutional review board ("IRB"), at each of the clinical centers proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center. An IRB considers, among other things, whether the risks to individuals participating in the trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the consent form signed by the trial participants and must monitor the study until completed. The FDA, the IRB, or the sponsor may suspend or discontinue a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trials to public registries.
The FDA offers a number of regulatory mechanisms that provide expedited or accelerated approval procedures for selected drugs and indications which are designed to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These include programs such as Breakthrough Therapy designations, Fast Track designations, Priority Review and Accelerated Approval, which the Corporation may need to rely upon in order to receive timely approval or to be competitive.
The Corporation may plan to seek orphan drug designation for certain indications qualified for such designation. The United States, European Union and other jurisdictions may grant orphan drug designation to drugs intended to treat a "rare disease or condition," which, in the US, is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or 200,000 or more individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the United States for this type of disease or condition will be recovered from sales of the product. In the EU, orphan drug designation can be granted if: the disease is life threatening or chronically debilitating and affects no more than 50 in 100,000 persons in the EU; without incentive it is unlikely that the drug would generate sufficient return to justify the necessary investment; and no satisfactory method of treatment for the condition exists or, if it does, the new drug will provide a significant benefit to those affected by the condition. Orphan drug designation must be requested before submitting an NDA. If a product that has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for a period of seven years in the United States and 10 years in the EU. Orphan drug designation does not prevent competitors from developing or marketing different drugs for the same indication or the same drug for different indications. After orphan drug designation is granted, the identity of the therapeutic agent and its potential orphan use are publicly disclosed. Orphan drug designation does not convey an advantage in, or shorten the duration of, the development, review and approval process. However, this designation provides an exemption from marketing and authorization (NDA) fees.
Drugs manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, reporting of adverse experiences with the product, and complying with promotion and advertising requirements. The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-market testing, including phase IV clinical trials, and surveillance to further assess and monitor the product's safety and effectiveness after commercialization. In addition, drug manufacturers and their subcontractors involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including current Good Manufacturing Practices, which impose certain procedural and documentation requirements. Failure to comply with statutory and regulatory requirements may subject a manufacturer to legal or regulatory action, such as warning letters, suspension of manufacturing, product seizures, injunctions, civil penalties or criminal prosecution. There is also a continuing, annual prescription drug product program user fee.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information, requirements for post-market studies or clinical trials to assess new safety risks, or imposition of distribution or other restrictions under a risk evaluation and mitigation strategy.
In the United States, pharmaceutical manufacturers are subject to complex laws and regulations pertaining to healthcare "fraud and abuse," including, but not limited to, the Anti-Kickback Statute, the federal False Claims Act (the "FCA"), and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid.
The FCA prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes "any request or demand" for money or property presented to the U.S. government. Violations of the FCA can result in very significant monetary penalties and treble damages. The federal government is using the FCA, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multibillion dollar settlements under the FCA in addition to individual criminal convictions under applicable criminal statutes. In addition, the federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers' and manufacturers' compliance with applicable fraud and abuse laws.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. In addition, a similar federal requirement Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the Affordable Care Act, commonly referred to as the "Physician Payments Sunshine Act" requires applicable manufacturers to track and report to the federal government certain payments and "transfers of value" made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, made in the previous calendar year. There are a number of states that have various types of additional reporting requirements.
Controlled Substances
The CSA and its implementing regulations establish a "closed system" of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA. The DEA is responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
The DEA categorizes controlled substances into one of five schedules - Schedule I, II, III, IV or V - with varying qualifications for listing in each schedule. Schedule I substances by definition have a high potential for abuse, have no currently accepted medical use in treatment in the United States and lack accepted safety for use under medical supervision. For any product containing a Schedule I substance, such as psilocybin, to be available for commercial marketing in the United States, such substance must be rescheduled, or the product itself must be scheduled, by the DEA to Schedule II, III, IV or V. Scheduling determinations by the DEA are dependent on FDA approval of a substance or a specific formulation of a substance.
Facilities that manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s). For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized.
The DEA inspects all manufacturing facilities to review security, recordkeeping, reporting and handling prior to issuing a controlled substance registration and periodically to ensure continued compliance. The specific security requirements vary by the type of business activity and the schedule and quantity of controlled substances handled. The most stringent requirements apply to manufacturers of Schedule I and Schedule II substances. Required security measures commonly include background checks on employees and physical control of controlled substances through storage in approved vaults, safes and cages, and through use of alarm systems and surveillance cameras. Once registered, manufacturing facilities must maintain records documenting the manufacture, receipt and distribution of all controlled substances. Manufacturers must submit periodic reports to the DEA of the distribution of Schedule I and II controlled substances, Schedule III narcotic substances, and other designated substances. Registrants must also report any controlled substance thefts or significant losses, and must obtain authorization to destroy or dispose of controlled substances. Imports of Schedule I and II controlled substances for commercial purposes are generally restricted to substances not already available from a domestic supplier or where there is not adequate competition among domestic suppliers. In addition to an importer or exporter registration, importers and exporters must obtain a permit for every import or export of a Schedule I and II substance or Schedule III, IV and V narcotic, and submit import or export declarations for Schedule III, IV and V non-narcotics.
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within Schedules I and II that may be manufactured or produced in the United States based on the DEA's estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. The quotas apply equally to the manufacturing of the active pharmaceutical ingredient and production of dosage forms. The DEA may adjust aggregate production quotas a few times per year, and individual manufacturing or procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments for individual companies.
Individual U.S. states also establish and maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. A majority of state laws in the United States classify psilocybin and psilocin as Schedule I controlled substances. State authorities, including boards of pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on the Corporation's business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
Netherlands
Regulation (EU) No 536/2014 on Clinical Trials on Medicinal Products for Human Use ( the "CTR") is applicable as of January 31, 2022, harmonizing the laws, regulations and administrative provisions of the EU Member States relating to the implementation of Good Clinical Practice in the conduct of clinical trials on medicinal products for human use. EU Member States have transformed the requirements outlined in the Clinical Trials Directive into the respective national laws. Pursuant to the CTR, as of January 31, 2023 sponsors are obliged to use the Clinical Trials Information System (CTSI) for regularity submission, authorization and supervision of clinical trials in the EU and the EEA. CTIS will thus serve as the single-entry point for submissions by sponsors and for regulatory assessment. In addition to this obligation, sponsors must transfer any ongoing (approved) trials under the CTR to CTIS by January 2025.
The IMPD is one of several regulatory documents required for conducting a clinical trial of a pharmacologically API intended for one or more EU Member States. The IMPD includes summaries of information related to the quality, manufacture and control of any Investigational Medicinal Product (including reference product and placebo) ("IMP"), and data from non-clinical and clinical studies. Guidance concerning IMPDs is based on the CTR and on the approximation of laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use (also commonly referred to as the "Clinical Trials Directive").
The content of the IMPD may be adapted to the existing level of knowledge and the product's phase of development. When applying for a clinical trial authorization, a full IMPD is required when little or no information about an API has been previously submitted to competent authorities, when it is not possible to cross-refer to data submitted by another sponsor and/or when there is no authorization for sale in the EU. However, a simplified IMPD may be submitted if information has been assessed previously as part of a Marketing Authorization or a clinical trial to that competent authority. Although the format is not obligatory, the components of an IMPD are largely equivalent to clinical trial applications in Canada and the United States. The IMPD need not be a large document as the amount of information to be contained in the dossier is dependent on various factors such as product type, indication, development phase etc.
The assessment of an IMPD is focused on patient safety and any risks associated with the IMP. Whenever any potential new risks are identified the IMPD must be amended to reflect the changes. Certain amendments are considered substantial in which case the competent authority must be informed of the substantial amendment. This may be the case for changes in IMP impurities, microbial contamination, viral safety, transmissible spongiform encephalopathies (e.g. mad cow disease) and in some particular cases to stability when toxic degradation products may be generated.
With the completion of the Asset Acquisition, the Corporation has an ongoing phase I study to obtain preliminary evidence of the safety and efficacy of infused DMT. Prior to the Asset Acquisition, an investigator's brochure (including prior safety, preclinical and clinical data), and an IMPD document that includes CMC information and a clinical study protocol and supporting information had been prepared. Approval by the Dutch ethics committee of the Phase 1 Study, planned to be conducted by CHDR will be based on the vast amount of published human and animal studies of DMT. Prior to the Asset Acquisition, preclinical data was not provided as part of the application package; however, limited additional in vivo and in vitro data to support the rationale for human dosing and safety had been included. CHDR and its partner GMP-licensed pharmacy that will be involved in the Phase 1 Study, the Leiden University Medical Center, have all the required approvals to possess and handle DMT for the Phase 1 Study.
Failure of the Corporation to receive the necessary regulatory approvals required to conduct the Phase 1 Study would have an adverse impact on its business plans and financial condition for a number of reasons including, without limitation: (i) it would cause delays in the Corporation's research and development plans; (ii) it may require the Corporation to expend additional financial and human resources on revising its application package or creating a new one; or (iii) it may require the Corporation to approach an entirely different regulatory authority in a new jurisdiction, in which case the Corporation would have to expend a substantial amount of capital and other resources on engaging the appropriate research and development partners and creating an application package that complies with the regulations of that new jurisdiction. Additionally, the Corporation would be required to spend capital on transferring the DMT materials to the new jurisdiction. All of the foregoing would likely have a negative impact on the Corporation's business and financial condition.
Pharmaceutical Products
In accordance with the Dutch Medicines Act (Geneesmiddelenwet), "medicinal products" are defined as: a substance or a combination of substances that is intended to be administered or used for, or is presented in any way as being suitable for, use: (i) the cure or prevention of any disease, defect, wound or pain in human beings, (ii) the making of a medical diagnosis in human beings, or (iii) restoring, improving or otherwise modifying physiological functions in humans by exerting a pharmacological, immunological or metabolic effect.
If a product constitutes a medicinal product, a marketing authorization for the product is required before the product may be placed on the market in the Netherlands. In the EU, marketing authorizations may be obtained through the Centralized procedure, the Decentralized procedure and/or the national procedure. The Centralized procedure is compulsory for medicines intended to treat i.e. cancer, AIDS, neurodegenerative diseases and diabetes and optional (only) for medicines comprising of new active substances not previously approved for the EEA. When applying for a marketing authorization through the Centralized procedure, applications are submitted with the European Medicines Agency (the "EMA"). Where the Centralized procedure is not available but a medicinal product is intended for several EU/EEA Member States, an application for a marketing authorization may be submitted with the competent authority of a single EU/EEA Member State in accordance with the Decentralized procedure. When the assessment of the application results in a decision to grant the marketing authorization, this decision will be mutually recognized by the competent authorities of the other Member States for which the marketing authorization is applied. Finally, should a medicinal product be intended for the Netherlands only, then the national procedure may be followed as well by submitting an application with the Dutch Medicines Evaluation Board. It may be remarked that the national procedure is unavailable in case the Centralized procedure is compulsory or in case an applicant has already submitted an application for and/or obtained a marketing authorization in another Member State. In that case, applications must follow the mutual recognition procedure instead.
Companies that manufacture or trade in medicinal products and/or active pharmaceutical ingredients in the Netherlands require a manufacturing authorization or a wholesale distribution authorization. A manufacturing authorization is required for the preparation, trading in, import and export of medicinal products and/or active substances. Here, 'preparation' means the total or partial manufacture of medicinal products and/or active substances or the packaging or labelling thereof. 'Importing' means the import of medicinal products or active substances from a country outside the EEA into the Dutch territory, while 'exporting' means the export of medicinal products or active substances from the Dutch territory to a country outside the EEA. A wholesale distribution authorization is required for one or more activities within the wholesale business, such as procuring, holding, supplying, delivering or exporting medicinal products or active substances which are prepared or imported by a third party. It may be noted that holders of wholesale distribution authorization, other than holders of marketing authorizations, are not authorized to import medicinal products from countries outside the EEA.
Only a natural or legal person established in the Netherlands may obtain either a Dutch marketing authorization or a wholesale distribution authorization. These authorizations concern national permits, meaning that these authorizations are not automatically valid in other EU Member States. Furthermore, in the Netherlands applicants of marketing authorizations and wholesale distributions authorizations must be registered with Farmatec and comply with GDP norms.
Market Authorization Regulatory Process
Under the Centralized procedure, pharmaceutical companies submit a single marketing authorization application to the EMA, which provides the basis of a legally binding recommendation that will be provided by the EMA to the European Commission, the authorizing body for all centrally authorized products. This allows the marketing authorization holder to market the medicine and make it available to patients and healthcare professionals throughout the EU on the basis of a single marketing authorization. EMA's Committee for Medicinal products for Human Use or Committee for Medicinal Products for Veterinary Use carry out a scientific assessment of the application and give a recommendation on whether the medicine should be marketed or not, under any particular dosing regime. Although, under EU law, the EMA has no authority to permit marketing in the different EU countries, the European Commission is the authorizing body for all centrally authorized products, who takes a legally binding decision based on EMA's recommendation. Once granted by the European Commission, the centralized marketing authorization is valid in all EU Member States as well as in the European Economic Area countries Iceland, Liechtenstein and Norway. European Commission decisions are published in the Community Register of medicinal products for human use. Once a medicine has been authorized for use in the EU, the EMA and the EU Member States constantly monitor its safety and take action if new information indicates that the medicine is no longer as safe and effective as previously thought. The safety monitoring of medicines involves a number of routine activities ranging from: assessing the way risks associated with a medicine will be managed and monitored once it is authorized; continuously monitoring suspected side effects reported by patients and healthcare professionals, identified in new clinical studies or reported in scientific publications; regularly assessing reports submitted by the Corporation holding the marketing authorization on the benefit-risk balance of a medicine in real life; and assessing the design and results of post-authorization safety studies which were required at the time of authorization. The EMA can also carry out a review of a medicine or a class of medicines upon request of a Member State or the European Commission. These are called EU referral procedures; they are usually triggered by concerns in relation to a medicine's safety, the effectiveness of risk minimization measures or the benefit-risk balance of the medicine. The EMA has a dedicated committee responsible for assessing and monitoring the safety of medicines, the Pharmacovigilance Risk Assessment Committee. This ensures that EMA and the EU Member States can move very quickly once an issue is detected and take any necessary action, such as amending the information available to patients and healthcare professionals, restricting use or suspending a medicine, in a timely manner in order to protect patients.
Besides the Centralized procedure, pharmaceutical companies may also submit marketing authorization applications through the Decentralized procedure with the competent authority of a Member State. As the Centralized procedure is compulsory for medicines intended to treat specified diseases i.e. cancer, AIDS, neurodegenerative diseases and diabetes and only optional for medicines comprising of new active substances not previously approved for the EU/ EEA, in all other circumstances the Decentralized procedure should be used instead if a marketing authorization is to be obtained for several EU/EEA Member States. When following the Decentralized procedure, the applicant requests one country to be the Reference Member State ("RMS") in the procedure. After having shared draft assessment reports to which both the applicant and the competent authorities of other Member States may respond, the to be granted marketing authorization will eventually go through the Mutual recognition procedure. In the Mutual recognition procedure other Member States generally adopt the RMS's assessment, unless there are important objections on the grounds of a potentially serious risk to public health. In such situations, further discussions will also be held in the Co-ordination group for Mutual recognition and Decentralised procedures ("CMDh"). When all Member States involved decide on a positive opinion on products in the CMDh, Dutch translations of the summary of product characteristics, package leaflet, labelling texts and mock-ups are submitted and a national marketing authorization is issued.
Patent Cooperation Treaty
The Patent Cooperation Treaty (the "PCT") facilitates filing for patent recognition in multiple jurisdictions simultaneously using a single uniform patent application. 157 countries, including Canada and the United States have ratified the PCT.
Ultimately, patents are still granted in each country individually. As such, the PCT procedure consists of two phases: filing of an international application, and national evaluation under the patent laws in force in each country where a patent is sought.
Within 12 months of filing a provisional patent application at the United States Patent and Trademark Office, the Corporation may elect to file a regular utility patent application in the United States in tandem with filing a PCT application with the World Intellectual Property Office, in each case claiming priority to the provisional patent application. Within 30 months of the provisional filing date, deadlines begin for a PCT application to enter the national phase in desired jurisdictions globally, such as Canada (30 months) and Europe (31 months), in each case claiming priority to the provisional patent application.
While the Corporation is focused on programs using psychedelic-inspired compounds, the Corporation does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Corporation is exploring drug development within approved laboratory clinical trial settings conducted within approved regulatory frameworks. Though highly speculative, should any prescription drug product be developed by the Corporation (which, if it does occur, would not be for several years), such drug product will not be commercialized prior to receipt of applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended use(s) is successfully developed. The Corporation may also employ non-prescription drugs, where appropriate.
COMPLIANCE PROGRAM
The Corporation oversees and monitors compliance with applicable laws in each jurisdiction in which it operates. In addition to the Corporation's senior executives and the employees responsible for overseeing compliance, the Corporation has local counsel engaged in every jurisdiction in which it operates and has received legal opinions or advice in each of these jurisdictions regarding (a) compliance with applicable regulatory frameworks, and (b) potential exposure to, and implications arising from, applicable laws in jurisdictions in which the Corporation has operations or intends to operate.
The Corporation works with third parties who require regulatory licensing to handle scheduled drugs. The Corporation continuously updates its compliance and channel programs to maintain regulatory standards set for drug development. The Corporation also works with clinical research organizations who maintain batch records and data storage for the Corporation's clinical programs.
Additionally, the Corporation has established a Medical & Clinical Advisory Team, a Research, Clinical and Regulatory Team and a Government Relations and Communications Team with cross-functional expertise in business, neuroscience, pharmaceuticals, mental health and psychedelics to advise management.
In conjunction with the Corporation's human resources and operations departments, the Corporation oversees and implements training on the Corporation's protocols. The Corporation will continue to work closely with external counsel and other compliance experts, and is evaluating the engagement of one or more independent third party providers to further develop, enhance and improve its compliance and risk management and mitigation processes and procedures in furtherance of continued compliance with the laws of the jurisdictions in which the Corporation operates.
The programs currently in place include monitoring by executives of the Corporation to ensure that operations conform to and comply with required laws, regulations and operating procedures. The Corporation is currently in compliance with the laws and regulations in all jurisdictions and the related licencing framework applicable to its business activities.
The Corporation and, to its knowledge, each of its third-party researchers, suppliers and manufacturers have not received any non-compliance, citations or notices of violation which may have an impact on the Corporation's licences, business activities or operations.
The Corporation conducts due diligence on third-party researchers, medical professionals, clinics, cultivators, processors and others as applicable, with whom it engages. Such due diligence includes but is not limited to the review of necessary licenses and the regulatory framework enacted in the jurisdiction of operation. Further, the Corporation generally obtains, under its contractual arrangements, representations and warranties from such third parties pertaining to compliance with applicable licensing requirements and the regulatory framework enacted in the jurisdiction of operation.
CONSOLIDATED CAPITALIZATION
There have been no material changes to the share and loan capitalization of the Corporation since the date of the Interim Financial Statements.
The applicable Prospectus Supplement will describe any material changes, and the effect of such material changes, on the share and loan capitalization of the Corporation that will result from the issuance of Securities pursuant to each Prospectus Supplement.
USE OF PROCEEDS
The net proceeds from any Offering of Securities and the proposed use of those proceeds will be set forth in the applicable Prospectus Supplement relating to that Offering of Securities. Notwithstanding, the Corporation's management has broad discretion in the application of proceeds of an Offering of Securities. On the basis of results obtained or for other sound business reasons, the Corporation may re-allocate funds as required. Accordingly, the Corporation's actual use of proceeds may vary significantly from any proposed use of proceeds disclosed in any applicable Prospectus Supplement. See "Risk Factors - Risks Related to an Offering - Discretion over the Use of Proceeds".
Sources and Uses of Capital
As at August 8, 2023, the Corporation had cash resources, being cash and an HST receivable, of approximately $21,082,000. As at August 8, 2023, the Corporation anticipates that it will require approximately $21,082,000 to continue operations over the next 12 months, including funding for the achievement of the next significant milestone under the Corporation’s Deuterated Psilocybin Analog Program (CYB003) and the Deuterated Dimethyltryptamine Program (CYB004).
The Corporation may be required to reduce its general and administrative expenses if it does not have sufficient capital to meet its below stated objectives, or it may allocate its financial resources differently than listed below. The expected reduction in future expenditures, absent any capital injections, compared to the Corporation’s historic expenditures would be achieved as follows: (i) a reduction in marketing activities; (ii) a reduction in workforce and/or compensation; (iii) a reduction in public relations and investor relations activities; and (iv) a reduction of planned expenses under the Deuterated Dimethyltryptamine Program and Phenethylamine Derivatives Program, which would also result in a delay of anticipated timing for completion of the next significant milestones under each of these programs.
| Program(1) |
Anticipated
12 Month Use
of Funds(2)(3)
($) |
|
Anticipated
Timeline for
Completio(4) |
| Deuterated Psilocybin Analog Program |
|
|
|
| Provide topline data readout from the Phase 1/2a study |
3,880,627 |
|
Q3/Q4 2023 |
| Complete FDA submission of Phase 1/2a data |
2,500,200 |
|
Q4 2023 |
| Deuterated Dimethyltryptamine Program |
-
|
|
-
|
| Provide topline data from Phase 1 CYB004-E trial |
1,269,000 |
|
Q3/Q4 2023 |
| Complete FDA IND submission |
1,372,950 |
|
Q1 2024 |
| Phenethylamine Derivatives Program |
-
|
|
-
|
| Technology Programs |
-
|
|
-
|
| Working Capital, and General Corporate Purposes(5) |
12,059,223
|
|
N/A
|
| Total |
21,082,000
|
|
|
Notes:
(1) Please see the Corporation’s Interim MD&A for a description of the Corporation’s programs. All milestones are subject to receipt of all necessary approvals, including the academic and scientific organizations with which the Corporation is working.
(2) Certain amounts have been converted from USD to CAD at an exchange rate of 1.35:1.
(3) Represents proceeds from private placements and offerings previously disclosed, as well proceeds from the ATM Program and May 2023 Prospectus.
(4) Anticipated timeline for completion is based on the calendar year. Anticipated spending and timelines regarding drug development are based on reasonable assumptions informed by current knowledge and information available to the Corporation.
(5) Includes personnel costs, professional services, overhead expenses and general expenses to be incurred by the Corporation in the normal course of business. In addition, the Corporation intends to use a portion of these proceeds to continue funding both its Deuterated Psilocybin Analog Program and Deuterated Dimethyltryptamine Program. The allocation between and specific milestones within these programs have not yet been determined.
For detailed information in respect of the Corporation’s business objectives and milestones, and the application of proceeds from prior offerings by the Corporation, prospective purchasers of Securities should carefully consider the information described in the Annual Information Form and Interim MD&A of the Corporation, and the documents incorporated by reference herein, including the applicable Prospectus Supplement.
As at August 8, 2023, the Corporation’s current financial resources, absent any capital injections, will be sufficient to meet the Corporation’s short-term liquidity requirements and to fund its operations for the coming 12 months. The Corporation makes this statement based on its cash resources, being cash and an HST receivable, as of August 8, 2023 in the amount of approximately $21,082,000.
The anticipated spend and cash burn do not account for funds that the Corporation had previously disclosed as being required to progress its Phenethylamine Derivatives Program listed in the table above. As disclosed in its Interim MD&A, the Corporation anticipated spending approximately $900,000 under its Phenethylamine Derivatives Program by September 30, 2023 to complete preclinical development of a phenethylamine drug candidate, of which approximately $42,000 was spent during the three months ended June 30, 2023, and approximately $782,000 was spent during the financial year ended March 31, 2023, resulting in an approximate remaining spend as of June 30, 2023 of $76,000 by September 30, 2023.10 If the Corporation does not raise additional funds in the coming 12 months, it anticipates that it will need to reduce the amounts spent on the Phenethylamine Derivatives Program programs and adjust the corresponding timelines disclosed in its Interim MD&A.
_____________________________________________
10 Reflects actual spend during the financial year ended March 31, 2023, the three months ended June 30, 2023, and expected spend during the period from July 1, 2023 until the achievement of preclinical development of a phenethylamine drug candidate by September 30, 2023. The Company had previously estimated that its spending to complete preclinical development of a phenethylamine drug candidate would be $1,283,000. Anticipated timing and spending regarding drug development is based on reasonable assumptions informed by current knowledge and information available to the Corporation. Such statements are informed by, among other things, regulatory guidelines for developing a drug with safety studies, proof of concept studies, and pivotal studies for new drug application submission and approval, and assumes the success of implementation and results of such studies on timelines indicated as possible by such guidelines, other industry examples, and the Corporation’s development efforts to date.
The Corporation's expectation is based on certain assumptions and risks as set forth in the section "Cautionary Note Regarding Forward Looking Information" and "Risk Factors" in this Prospectus.
The expected uses of capital represents the Corporation's current intentions based upon its present plans and business condition, which could change in the future as its plans and business conditions evolve. The amounts and timing of the actual use of available capital will depend on multiple factors and there may be circumstances where, for sound business reasons, a reallocation of capital, or termination of a program objective, may be necessary in order for the Corporation to achieve its program objectives. The Corporation may also require additional funds in order to fulfill its expenditure requirements to meet existing and any new business objectives, and the Corporation expects to either issue additional securities or incur debt to do so. The material factors or assumptions used to develop the estimated amounts for the 12 months period disclosed above are included in the "Cautionary Note Regarding Forward-Looking Information" section above. The actual amount that the Corporation spends in connection with each of the identified uses and programs will depend on a number of factors, including those listed under "Risk Factors" in, or incorporated by reference in, this Prospectus.
Negative Cash Flow From Operations
Since inception, the Corporation has financed its operations primarily from the issuance of equity and interest income on funds available for investment. To date, the Corporation has raised approximately C$156,500,000 in gross proceeds through private placement and prospectus offerings. The Corporation has experienced operating losses and cash outflows from operations since incorporation and will require ongoing financing to continue its research and development activates. As the Corporation has not yet achieved profitability, there are uncertainties regarding its ability to continue as a going concern. The Corporation has not earned any revenue or reached successful commercialization of any products. The Corporation's success is dependent upon the ability to finance its cash requirements to continue its activities. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained, or at all. To the extent that the Corporation has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Corporation will be required to raise additional funds through the issuance of additional equity securities, through loan financing, or other means, such as through partnerships with other companies and research and development reimbursements. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favourable to the Corporation as those previously obtained. See "Risk Factors - Risks Related to an Offering - Negative Operating Cash Flow and Going Concern".
DIVIDEND POLICY
The Corporation has never declared nor paid dividends on the Common Shares. Currently, the Corporation intends to retain its future earnings, if any, to fund the development and growth of its business, and the Corporation does not anticipate declaring or paying any dividends on the Common Shares in the near future, although the Corporation reserves the right to pay dividends if and when it is determined to be advisable by the board of directors of the Corporation. As a result, shareholders will have to rely on capital appreciation, if any, to earn a return on investment in the Common Shares in the foreseeable future. See "Risk Factors - Risks Related to an Offering - Speculative Nature of Investment Risk".
DESCRIPTION OF SECURITIES BEING DISTRIBUTED
The following is a brief summary of certain general terms and provisions of the Securities that may be offered pursuant to this Prospectus. This summary does not purport to be complete. The particular terms and provisions of the Securities as may be offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement pertaining to such Offering of Securities, and the extent to which the general terms and provisions described below may apply to such Securities will be described in the applicable Prospectus Supplement.
Common Shares
The Corporation is authorized to issue an unlimited number of Common Shares and an unlimited number of preferred shares. As at August 16, 2023, the Corporation had 234,683,234 Common Shares and nil preferred shares issued and outstanding.
Each Common Share entitles the holder thereof to one vote at meetings of shareholders of the Corporation other than meetings of the holders of another class of shares. Each holder of Common Shares is also entitled to receive dividends if, as and when declared by the board of directors of the Corporation. Holders of Common Shares are entitled to participate in any distribution of the Corporation's net assets upon liquidation, dissolution or winding-up on an equal basis per share. There are no pre-emptive, redemption, retraction, purchase or conversion rights attaching to the Common Shares.
Common Shares may be sold separately or together with certain other Securities under this Prospectus. Common Shares may also be issuable on conversion, exchange, exercise or maturity of certain other Securities qualified for issuance under this Prospectus.
Warrants
Warrants may be offered separately or together with other Securities, as the case may be. Each series of Warrants may be issued under a separate warrant indenture or warrant agency agreement to be entered into between the Corporation and one or more banks or trust companies acting as Warrant agent or may be issued as stand-alone contracts. The applicable Prospectus Supplement will include details of the Warrant agreements, if any, governing the Warrants being offered. The Warrant agent, if any, will be expected to act solely as the agent of the Corporation and will not assume a relationship of agency with any holders of Warrant certificates or beneficial owners of Warrants. The following sets forth certain general terms and provisions of the Warrants that may be offered under this Prospectus. The specific terms of the Warrants, and the extent to which the general terms described in this section apply to those Warrants, will be set forth in the applicable Prospectus Supplement.
A copy of any warrant indenture or any warrant agency agreement relating to an offering of Warrants will be filed by the Corporation with the relevant securities regulatory authorities in Canada after it has been entered into by the Corporation.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Warrants being offered thereby, which may include, without limitation, the following (where applicable):
-
the designation of the Warrants;
-
the aggregate number of Warrants offered and the offering price;
-
the designation, number and terms of the other Securities purchasable upon exercise of the Warrants, and procedures that will result in the adjustment of those numbers;
-
the exercise price of the Warrants;
-
the dates or periods during which the Warrants are exercisable;
-
the designation and terms of any securities with which the Warrants are issued;
-
if the Warrants are issued as a unit with another Security, the date on and after which the Warrants and the other Security will be separately transferable;
-
any minimum or maximum amount of Warrants that may be exercised at any one time;
-
whether such Warrants will be listed on any securities exchange;
-
any terms, procedures and limitations relating to the transferability, exchange or exercise of the Warrants;
-
certain material Canadian tax consequences of owning the Warrants; and
-
any other material terms and conditions of the Warrants.
Units
The Corporation may issue Units comprised of one or more of the other Securities described herein in any combination. Each Unit may be issued so that the holder of the Unit is also the holder of each Security included in the Unit; thus, the holder of a Unit may have the rights and obligations of a holder of each included Security. Any Unit agreement under which a Unit may be issued may provide that the Securities included in the Unit may not be held or transferred separately at any time or at any time before a specified date.
Each applicable Prospectus Supplement will set forth the terms and other information with respect to the Units being offered thereby, which may include, without limitation, the following (where applicable):
-
the designation, number and terms of the Units and of the Securities comprising the Units, including whether and under what circumstances those Securities may be held or transferred separately;
-
any provisions for the issuance, payment, settlement, transfer or exchange of the Units or of the Securities comprising the Units;
-
certain material Canadian tax consequences of owning the Securities comprising the Units; and
-
any other material terms and conditions of the Units.
Debt Securities
The Debt Securities may be issued in one or more series under an indenture (the "Indenture") to be entered into between the Corporation and one or more trustees (the "Trustee") that may be named in a Prospectus Supplement for a series of Debt Securities. To the extent applicable, the Indenture will be subject to and governed by the United States Trust Indenture Act of 1939, as amended. A copy of the form of the Indenture to be entered into has been or will be filed with the SEC as an exhibit to the Registration Statement of which this Prospectus forms a part and will be filed with the securities commissions or similar authorities in Canada when it is entered into. The Corporation may issue Debt Securities, separately or together, with Common Shares, Warrants, Units or Subscription Receipts or any combination thereof, as the case may be.
The description of certain provisions of the Indenture in this section do not purport to be complete and are subject to, and are qualified in their entirety by reference to, the provisions of the Indenture. The following sets forth certain general terms and provisions of the Debt Securities. The particular terms and provisions of a series of Debt Securities offered pursuant to this Prospectus will be set forth in the applicable Prospectus Supplement, and the extent to which the general terms and provisions described below may apply to such Debt Securities will be described in the applicable Prospectus Supplement. This description may include, but may not be limited to, any of the following, if applicable:
-
the title of the Debt Securities;
-
any limit on the aggregate principal amount of the Debt Securities;
-
the date or dates, if any, on which the Debt Securities will mature and the portion (if less than all of the principal amount) of the Debt Securities to be payable upon declaration of acceleration of maturity;
-
the rate or rates (whether fixed or variable) at which the Debt Securities will bear interest, if any, the date or dates from which any such interest will accrue and on which any such interest will be payable and the record dates for any interest payable on the Debt Securities;
-
the terms and conditions under which the Corporation may be obligated to redeem, repay or purchase the Debt Securities pursuant to any sinking fund or analogous provisions or otherwise;
-
the terms and conditions upon which the Corporation may redeem the Debt Securities, in whole or in part, at its option;
-
the covenants applicable to the Debt Securities;
-
the terms and conditions for any conversion or exchange of the Debt Securities for any other securities;
-
the extent and manner, if any, to which payment on or in respect of the Debt Securities of the series will be senior or will be subordinated to the prior payment of other liabilities and obligations of the Corporation;
-
whether the Debt Securities will be secured or unsecured;
-
whether the Debt Securities will be issuable in the form of global securities ("Global Securities"), and, if so, the identity of the depositary for such Global Securities;
-
the denominations in which Debt Securities will be issuable, if other than denominations of US$1,000 or integral multiples of US$1,000;
-
each office or agency where payments on the Debt Securities will be made and each office or agency where the Debt Securities may be presented for registration of transfer or exchange;
-
if other than United States dollars, the currency in which the Debt Securities are denominated or the currency in which we will make payments on the Debt Securities;
-
material Canadian federal income tax consequences and United States federal income tax consequences of owning the Debt Securities; and
-
any other terms, conditions, rights or preferences of the Debt Securities which apply solely to the Debt Securities.
If the Corporation denominates the purchase price of any of the Debt Securities in a currency or currencies other than United States dollars or a non-United States dollar unit or units, or if the principal of and any premium and interest on any Debt Securities is payable in a currency or currencies other than United States dollars or a non-United States dollar unit or units, the Corporation will provide investors with information on the restrictions, elections, general tax considerations, specific terms and other information with respect to that issue of Debt Securities and such non-United States dollar currency or currencies or non-United States dollar unit or units in the applicable Prospectus Supplement.
This Prospectus does not qualify for issuance Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to one or more underlying interests including, for example, an equity or debt security, a statistical measure of economic or financial performance including, but not limited to, any currency, consumer price or mortgage index, or the price or value of one or more commodities, indices or other items, or any other item or formula, or any combination or basket of the foregoing items. For greater certainty, this Prospectus may qualify for issuance Debt Securities in respect of which the payment of principal and/or interest may be determined, in whole or in part, by reference to published rates of a central banking authority or one or more financial institutions, such as a prime rate or bankers’ acceptance rate, or to recognized market benchmark interest rates such as CORRA, SOFR, EURIBOR or an alternative United States federal funds rate.
Each series of Debt Securities may be issued at various times with different maturity dates, may bear interest at different rates and may otherwise vary.
The terms on which a series of Debt Securities may be convertible into or exchangeable for Common Shares or other securities of the Corporation will be described in the applicable Prospectus Supplement. These terms may include provisions as to whether conversion or exchange is mandatory, at the option of the holder or at the option of the Corporation, and may include provisions pursuant to which the number of Common Shares or other securities to be received by the holders of such series of Debt Securities would be subject to adjustment.
To the extent any Debt Securities are convertible into Common Shares or other securities of the Corporation, prior to such conversion the holders of such Debt Securities will not have any of the rights of holders of the securities into which the Debt Securities are convertible, including the right to receive payments of dividends or the right to vote such underlying securities.
The Corporation may, from time to time, issue Debt Securities and incur additional indebtedness other than through the issue of Debt Securities pursuant to this Prospectus
Subscription Receipts
Subscription Receipts may be offered separately or together with other Securities, as the case may be. The Subscription Receipts may be issued under a subscription receipt agreement.
The applicable Prospectus Supplement will include details of any subscription receipt agreement covering the Subscription Receipts being offered. A copy of any subscription receipt agreement relating to an offering of Subscription Receipts will be filed by the Corporation with the relevant securities regulatory authorities in Canada after the Corporation has entered into it. The specific terms of the Subscription Receipts, and the extent to which the general terms described in this section apply to those Subscription Receipts, will be set forth in the applicable Prospectus Supplement. This description may include, without limitation, the following (where applicable):
-
the number of Subscription Receipts;
-
the price at which the Subscription Receipts will be offered;
-
the terms, conditions and procedures for the conversion of the Subscription Receipts into other Securities;
-
the designation, number and terms of the other Securities that may be exchanged upon conversion of each Subscription Receipt;
-
the designation, number and terms of other Securities with which the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security;
-
terms applicable to the gross or net proceeds from the sale of the Subscription Receipts plus any interest earned thereon;
-
certain material Canadian tax consequences of owning the Subscription Receipts; and
-
any other material terms and conditions of the Subscription Receipts.
PLAN OF DISTRIBUTION
General
The Corporation may from time to time during the 25-month period that this Prospectus, including any amendments and supplements hereto, remains valid, offer for sale and sell up to an aggregate of $160,000,000 in Securities hereunder.
The Securities may be sold by the Corporation (i) directly pursuant to applicable statutory exemptions, (ii) to or through underwriters or dealers, or (iii) through designated agents. The Prospectus Supplement relating to a particular Offering of Securities will identify any underwriter, dealer or agent engaged in connection with the offering and sale of such Securities, and will set forth the terms of the offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection with the offering, the method of distribution of the Securities, the purchase price of the Securities (or the manner of determination thereof if offered on a non-fixed price basis), the net proceeds to the Corporation and any other material terms of the plan of distribution (including sales in transactions that are deemed to be "at-the-market distributions" as defined in NI 44-102). Any initial offering price and discounts, concessions or commissions allowed or re-allowed or paid to underwriters, dealers or agents may be changed from time to time. Only underwriters named in the Prospectus Supplement are deemed to be underwriters in connection with the Corporation's Securities offered by that Prospectus Supplement.
The Securities may be sold from time to time in one or more transactions at a fixed price or prices or at non-fixed prices. If offered on a non-fixed price basis, the Securities may be offered at market prices prevailing at the time of sale, at prices determined by reference to the prevailing price of a specified security in a specified market or at prices to be negotiated with purchasers including sales in transactions that are deemed to be "at-the-market" distributions, including sales made directly on the NEO or other existing trading markets for the Securities, in which case the compensation payable to an underwriter, dealer or agent in connection with any such sale will be decreased by the amount, if any, by which the aggregate price paid for the Securities by the purchasers is less than the gross proceeds paid by the underwriter, dealer or agent to the Corporation. The price at which the Securities will be offered and sold may vary from purchaser to purchaser and during the period of distribution.
Sales of Securities under an "at-the-market distribution", if any, will be made pursuant to an accompanying Prospectus Supplement. Sales of Securities under any "at-the-market" program will be made in transactions that are "at-the-market distributions" as defined in NI 44-102. The volume and timing of any "at-the-market distributions" will be determined at the Corporation's sole discretion.
No underwriter or dealer involved in an "at-the-market distribution" under this Prospectus, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot securities in connection with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the offered Securities or securities of the same class as the Securities distributed under the "at-the-market distribution", including selling an aggregate number or principal amount of Securities that would result in the underwriter creating an over-allocation position in the Securities.
In connection with the sale of the Securities, underwriters, dealers or agents may receive compensation from the Corporation including in the form of underwriters', dealers' or agents' fees, commissions or concessions.
Underwriters, dealers and agents that participate in the distribution of the Securities may be deemed to be underwriters for the purposes of applicable Canadian securities legislation and any such compensation that they receive from the Corporation and any profit that they make on the resale of the Securities, may be deemed to be underwriting commissions.
Underwriters, dealers or agents who participate in the distribution of the Securities may be entitled, under agreements to be entered into with the Corporation to indemnification by the Corporation against certain liabilities, including liabilities under Canadian securities legislation, or to contribution with respect to payments, which such underwriters, dealers or agents may be required to make in respect thereof. Such underwriters, dealers and agents may be customers of, engage in transactions with, or perform services for, the Corporation in the ordinary course of business.
In connection with any Offering of Securities, subject to applicable laws and other than an "at-the-market distribution", the underwriters, dealers or agents, as the case may be, may over-allot or effect transactions which stabilize, maintain or otherwise affect the market price of the offered Securities at a level other than those which otherwise might prevail on the open market. Such transactions may be commenced, interrupted or discontinued at any time.
Unless specified in the applicable Prospectus Supplement, there is no market through which the Subscription Receipts, Warrants, Units and Debt Securities may be sold and purchasers may not be able to resell the Subscription Receipts, Warrants, Units and Debt Securities purchased under this Prospectus and the Prospectus Supplement. This may affect the pricing of the Subscription Receipts, Warrants, Units and Debt Securities in the secondary market, the transparency and availability of trading prices, the liquidity of the Subscription Receipts, Warrants, Units and Debt Securities and the extent of issuer regulation. See "Risk Factors".
RISK FACTORS
An investment in the Securities involves a high degree of risk and must be considered speculative due to the nature of the Corporation’s business and present stage of development. Before making an investment decision, prospective purchasers of Securities should carefully consider the information described in this Prospectus and the documents incorporated by reference herein, including the applicable Prospectus Supplement. There are certain risks inherent in an investment in the Securities, including the factors described below and under the heading “Risk Factors” in the Annual Information Form and under the heading “Risks and Uncertainties” in the Interim MD&A, and any other risk factors described herein or in a document incorporated by reference herein, which investors should carefully consider before investing. Additional risk factors relating to a specific Offering of Securities will be described in the applicable Prospectus Supplement. Some of the factors described herein, in the documents incorporated by reference herein, and/or the applicable Prospectus Supplement are interrelated and, consequently, investors should treat such risk factors as a whole. If any of the risk factors described herein, in the Annual Information Form, in another document incorporated by reference herein or in the applicable Prospectus Supplement occur, it could have a material adverse effect on the business, financial condition and results of operations of the Corporation. Additional risks and uncertainties of which the Corporation currently is unaware or that are unknown or that it currently deems to be immaterial could have a material adverse effect on the Corporation’s business, financial condition and results of operation. The Corporation cannot assure purchasers that it will successfully address any or all of these risks. There is no assurance that any risk management steps taken will avoid future loss due to the occurrence of the risks described herein, in the Annual Information Form, in the other documents incorporated by reference herein or in the applicable Prospectus Supplement or other unforeseen risks.
Risks Related to the Business of the Corporation
Ongoing Impact of the Novel Coronavirus "COVID-19"
The outbreak of COVID-19 and its eventual declaration as a pandemic by the World Health Organization ("WHO") on March 11, 2020, resulted in governments worldwide enacting emergency measures to combat the spread of the virus. These measures, which include the implementation of travel bans, self-imposed quarantine periods and social distancing, have caused material disruption to businesses globally resulting in an economic slowdown. Global equity markets have experienced significant volatility and weakness. Governments and central banks have reacted with significant monetary and fiscal interventions designed to stabilize economic conditions. The duration and impact of the COVID-19 outbreak is still unknown at this time, as is the efficacy of the government and central bank interventions. It is not possible to reliably estimate the length and severity of these developments and the impact on the financial results and condition of the Corporation in future periods. The Corporation is not currently aware of any changes in laws, regulations or guidelines, including tax and accounting requirements, arising from COVID-19 which would be reasonably anticipated to materially affect the Corporation's business.
Regulatory Risks and Uncertainties
In Canada, certain psychedelic drugs, including psilocybin, are classified as Schedule III drugs under the CDSA and as such, medical and recreational use is illegal under Canadian federal laws. In the United States, certain psychedelic drugs, including psilocybin, psilocin, DMT, and 5-Methoxy-DMT, are classified as Schedule I drugs under the CSA and the Controlled Substances Import and Export Act and as such, medical and recreational use is illegal under the U.S. federal laws. Anyone wishing to conduct research on substances listed in Schedule I under the CSA must register with the DEA and obtain DEA approval of the research proposal. The EU member states currently classify DMT as a Schedule I substance under the UN 71 and, as such, a licence is required to produce, dispense, import or export any Schedule I substances, but the specific requirements vary from country to country. Currently in the Netherlands, DMT is classified as a List 1 Drug under the Dutch Opium Act and, as such, subject to express authorization being obtained, the production, trade and possession of DMT are prohibited. In the United Kingdom, "Fungus (of any kind) which contains psilocin or an ester of psilocin" is controlled as a Class A drug under the MDA and Schedule 1 drug under the MDR. As psilocybin is a phosphate ester of psilocin, even if it is isolated from psilocin, it will still be treated as a Class A drug under the MDA and as a Schedule 1 drug under the MDR. Schedule 1 drugs can only be lawfully manufactured, produced, possessed and supplied under a controlled drugs domestic licence issued by the UK Home Office.
There is no guarantee that psychedelic drugs or psychedelic inspired drugs will ever be approved as medicines in any jurisdiction in which the Corporation operates. All activities involving such substances by or on behalf of the Corporation are conducted in accordance with applicable federal, provincial, state and local laws. Further, all facilities engaged with such substances by or on behalf of the Corporation do so under current licences and permits issued by appropriate federal, provincial and local governmental agencies. While the Corporation is focused on programs using psychedelic inspired compounds, the Corporation does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates and does not intend to have any such involvement. However, the laws and regulations generally applicable to the industry in which the Corporation is involved in may change in ways currently unforeseen. Any amendment to or replacement of existing laws or regulations, including the classification or re-classification of the substances the Corporation is developing or working with, which are matters beyond the Corporation's control, may cause the Corporation's business, financial condition, results of operations and prospects to be adversely affected or may cause the Corporation to incur significant costs in complying with such changes or it may be unable to comply therewith. A violation of any applicable laws and regulations of the jurisdictions in which the Corporation operates could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings initiated by either government entities in the jurisdictions in which the Corporation operates, or private citizens or criminal charges.
The loss of the necessary licences and permits for any of the above scheduled drugs could have an adverse effect on the Corporation's operations.
The psychedelic drug industry is a fairly new industry and the Corporation cannot predict the impact of the ever-evolving compliance regime in respect of this industry. Similarly, the Corporation cannot predict the time required to secure all appropriate regulatory approvals for future products, or the extent of testing and documentation that may, from time to time, be required by governmental authorities. The impact of compliance regimes, any delays in obtaining, or failure to obtain regulatory approvals may significantly delay or impact the development of markets, its business and products, and sales initiatives and could have a material adverse effect on the business, financial condition and operating results of the Corporation.
The success of the Corporation's business is dependent on the reform of controlled substances laws pertaining to psilocybin. If controlled substances laws are not favourably reformed in Canada, the United States, the Netherlands, the United Kingdom, and other global jurisdictions, the commercial opportunity that the Corporation is pursuing may be highly limited.
The Corporation makes no medical, treatment or health benefit claims about the Corporation's proposed products. The FDA, Health Canada, the EMA or other similar regulatory authorities have not evaluated claims regarding psilocybin, DMT, psilocybin analogues, or other psychedelic compounds. The efficacy of such products have not been confirmed by approved research. There is no assurance that the use of psilocybin, DMT, psilocybin analogues, or other psychedelic compounds can diagnose, treat, cure or prevent any disease or condition. Vigorous scientific research and clinical trials are needed. The Corporation has not conducted clinical trials for the use of its proposed products. Any references to quality, consistency, efficacy and safety of potential products do not imply that the Corporation verified such in clinical trials or that the Corporation will complete such trials. If the Corporation cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on the Corporation's performance and operations.
"Foreign Private Issuer" Status Under the U.S. Securities Laws
The Corporation is a "foreign private issuer", under applicable U.S. federal securities laws, and is, therefore, not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, the Corporation is subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. As a result, the Corporation does not file the same reports that a U.S. domestic issuer would file with the SEC, although the Corporation is required to file with or furnish to the SEC the continuous disclosure documents that it is required to file in Canada under Canadian securities laws. In addition, the Corporation's officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act. Therefore, the Corporation's shareholders may not know on as timely a basis when the Corporation's officers, directors and principal shareholders purchase or sell Common Shares, as the reporting periods under the corresponding Canadian insider reporting requirements are longer.
As a foreign private issuer, the Corporation is exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements. The Corporation is also exempt from Regulation FD, which prohibits issuers from making selective disclosures of material non-public information. While the Corporation complies with the corresponding requirements relating to proxy statements and disclosure of material non-public information under Canadian securities laws, these requirements differ from those under the Exchange Act and Regulation FD and shareholders should not expect to receive the same information at the same time as such information is provided by U.S. domestic companies. In addition, the Corporation may not be required under the Exchange Act to file annual and quarterly reports with the SEC as promptly as U.S. domestic companies whose securities are registered under the Exchange Act.
Risks Related to an Offering
Speculative Nature of Investment Risk
An investment in the Common Shares and the Corporation's prospects generally are speculative due to the risky nature of its business and the present stage of its development. Investors may lose their entire investment. There is no assurance that risk management steps taken will avoid future loss due to the occurrence of the risks described or incorporated by reference herein, or other unforeseen risks. If any of such risks actually occur, then the Corporation's business, financial condition and operating results could be adversely affected. Investors should carefully consider all risks and consult with their professional advisors to assess any investment in the Corporation.
Negative Operating Cash Flow and Going Concern
The Corporation has had negative cash flow from operating activities since inception. Drug development involves long lead times, is very expensive and involves many variables of uncertainty. As such, significant capital investment will be required to achieve the Corporation's existing plans. The Corporation's net losses have had and will continue to have an adverse effect on, among other things, shareholder equity, total assets and working capital. The Corporation expects that losses may fluctuate from quarter to quarter and year to year, and that such fluctuations may be substantial based on the stage of development of its principal programs. The Corporation cannot predict when it will become profitable, if at all. Accordingly, the Corporation may be required to obtain additional financing in order to meet its future cash commitments.
The threat of the Corporation's ability to continue as a going concern will be removed only when, in the opinion of the Corporation's auditor, the Corporation's revenues have reached a level that is able to sustain its business operations. If the Corporation is unable to obtain additional financing from outside sources and eventually generate enough revenues, the Corporation may be forced to sell a portion or all of the Corporation's assets, or curtail or discontinue the Corporation's operations. If any of these events happen, shareholders could lose all or part of their investment. The Corporation's financial statements do not include any adjustments to the Corporation's recorded assets or liabilities that might be necessary if the Corporation becomes unable to continue as a going concern. See "Risk Factors - Risks Related to the Offering - Potential Need for Additional Financing".
Discretion over the Use of Proceeds
While detailed information regarding the use of proceeds from the sale of the Securities will be described in the applicable Prospectus Supplement, the Corporation will have broad discretion over the use of net proceeds from an offering by the Corporation of its Securities. There may be circumstances where, for sound business reasons, a reallocation of funds may be deemed prudent or necessary. In such circumstances, the net proceeds will be reallocated at the Corporation's sole discretion.
Management will have discretion concerning the use of proceeds ascribed in the applicable Prospectus Supplement as well as the timing of their expenditures. As a result, an investor will be relying on the judgment of management for the application of the proceeds. Management may use the net proceeds described in a Prospectus Supplement in ways that an investor may not consider desirable. The results and the effectiveness of the application of the proceeds are uncertain. If the proceeds are not applied effectively, the Corporation's results of operations may suffer. See "Use of Proceeds".
Need for Additional Financing
The continued development of the Corporation will require additional financing. The Corporation's activities do have scope for flexibility in terms of the amount and timing of expenditures, and expenditures may be adjusted accordingly. However, further operations will require additional capital and will depend on the Corporation's ability to obtain financing through debt, equity or other means. The Corporation's ability to meet its obligations and maintain operations may be contingent upon successful completion of additional financing arrangements. There is no assurance that the Corporation will be successful in obtaining the required financing in the future or that such financing will be available on terms acceptable to the Corporation. In addition, any future financing may also be dilutive to existing shareholders of the Corporation. See "Risk Factors - Risks Related to an Offering - Negative Operating Cash Flow and Going Concern" and "- Potential Dilution".
Volatile Market Price of Corporation's Common Shares
The securities market in Canada has recently experienced a high level of price and volume volatility, and the market prices of securities of many companies have experienced wide fluctuations in price which have not necessarily been related to the operating performance, underlying asset values or prospects of such companies. There can be no assurance that continual fluctuations in price will not occur. It may be anticipated that any market for the Common Shares will be subject to market trends generally, notwithstanding any potential success of the Corporation. The value of the Common Shares distributed hereunder will be affected by such volatility.
The volatility of the Common Shares may affect the ability of holders to sell the Common Shares at an advantageous price or at all. Market price fluctuations in the Common Shares may be adversely affected by a variety of factors relating to the Corporation's business, including fluctuations in the Corporation's operating and financial results, such results failing to meet the expectations of securities analysts or investors and downward revisions in securities analysis' estimates in connection therewith, sales of additional Common Shares, governmental regulatory action, adverse change in general market conditions or economic trends, acquisitions, dispositions or other material public announcements by the Corporation or its competitors, along with a variety of additional factors, including, without limitation, those set forth under the heading "Cautionary Note Regarding Forward-Looking Information". In addition, the market price for securities on stock markets, including the Neo is subject to significant price and trading fluctuations. These fluctuations have resulted in volatility in the market prices of securities that often has been unrelated or disproportionate to changes in operating performance. These broad market fluctuations may materially adversely affect the market price of the Corporation.
Additionally, the value of the Common Shares is subject to market value fluctuations based upon factors that influence the Corporation's operations, such as legislative or regulatory developments, competition, technological change and changes in interest rates or foreign exchange rates. There can be no assurance that the market price of the Common Shares will not experience significant fluctuations in the future, including fluctuations that are unrelated to the Corporation's performance. As at the date of this Prospectus, only the Common Shares are listed on a securities exchange and may be purchased in the secondary market.
Potential Dilution
The Corporation's articles of incorporation and by-laws allow it to issue an unlimited number of Common Shares for such consideration and on such terms and conditions as established by the board of directors of the Corporation, in many cases, without the approval of the Corporation's shareholders. The Corporation cannot predict the size of future issuances of Common Shares or other Securities or the effect that future issuances and sales of Common Shares or other Securities will have on the market price of the Corporation's Securities. Issuances of a substantial number of additional Securities, or the perception that such issuances could occur, may adversely affect prevailing market prices for the Common Shares. With any additional issuance of Common Shares, investors will suffer dilution to their voting power and the Corporation may experience dilution in its earnings per share. "Risk Factors - Risks Related to an Offering - Potential Need for Additional Financing".
Market for Securities
There is currently no market through which the Securities, other than the Common Shares, may be sold and, unless otherwise specified in the applicable Prospectus Supplement, such unlisted Securities may not be listed on any securities or stock exchange or any automated dealer quotation system. As a consequence, purchasers may not be able to resell such unlisted Securities purchased under this Prospectus. This may affect the pricing of the Corporation's Securities, other than the Common Shares, in the secondary market, the transparency and availability of trading prices, the liquidity of these Securities and the extent of issuer regulation. There can be no assurance that an active trading market for the Securities, other than the Common Shares, will develop or, if developed, that any such market, including for the Common Shares, will be sustained.
Enforcement of Civil Liabilities
Certain of the Corporation's subsidiaries and assets are located outside of Canada. Accordingly, it may be difficult for investors to enforce within Canada any judgments obtained against the Corporation, including judgments predicated upon the civil liability provisions of applicable Canadian securities laws or otherwise. Consequently, investors may be effectively prevented from pursuing remedies against the Corporation under Canadian securities laws or otherwise.
The Corporation has subsidiaries incorporated in the United States. It may not be possible for shareholders to effect service of process outside of Canada against the directors and officers of the Corporation who are not resident in Canada. In the event a judgment is obtained in a Canadian court against one or more of such persons for violations of Canadian securities laws or otherwise, it may not be possible to enforce such judgment against persons not resident in Canada. Additionally, it may be difficult for an investor, or any other person or entity, to assert Canadian securities law or other claims in original actions instituted in the United States. Courts in such jurisdiction may refuse to hear a claim based on a violation of Canadian securities laws or otherwise on the grounds that such jurisdiction is not the most appropriate forum to bring such a claim. Even if a foreign court agrees to hear a claim, it may determine that the local law, and not Canadian law, is applicable to the claim. If Canadian law is found to be applicable, the content of applicable Canadian law must be proven as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by foreign law.
Most of the officers and directors named in this Prospectus are not residents of the United States, and some of the Corporation's assets and all or a substantial portion of the assets of such person are located outside of the United States.
The Corporation has been advised that, subject to certain limitations, a judgment of a United States court predicated solely upon civil liability under United States federal securities laws may be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. The Corporation has also been advised, however, that there is substantial doubt whether an action could be brought in Canada in the first instance on the basis of liability predicated solely upon United States federal securities laws or any such state securities or "blue sky" laws.
The Corporation is filing with the SEC, concurrently with the Registration Statement, an appointment of agent for service of process on Form F-X. Under the Form F-X, the Corporation appointed C T Corporation System as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving the Corporation in a United States court, arising out of or related to or concerning the offering of the Securities.
CERTAIN FEDERAL INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement will include a general summary of certain Canadian federal income tax consequences which may be applicable to a purchaser of Securities offered thereunder. The applicable Prospectus Supplement may also describe certain United States federal income tax consequences which may be applicable to a purchaser of Securities hereunder by an initial investor who is a United States person (within the meaning of the United States Internal Revenue Code of 1986, as amended). Investors should read the tax discussion in any Prospectus Supplement with respect to a particular offering and consult their own tax advisors with respect to their own particular circumstances.
LEGAL MATTERS
Certain legal matters in connection with the offering of the Securities will be passed upon by Aird & Berlis LLP on behalf of the Corporation. As at the date of this Prospectus, the designated professionals of Aird & Berlis LLP, as a group, beneficially own, directly or indirectly, less than one percent of the securities of the Corporation.
AUDITORS, TRANSFER AGENT AND REGISTRAR
Zeifmans LLP, Chartered Professional Accountants, are the auditors of the Corporation and have confirmed that they are independent of the Corporation within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation. Neither Zeifmans LLP nor any designated professional thereof, had any registered or beneficial interest in any securities or other property of the Corporation at the time they prepared the relevant financial statements incorporated by reference in this Prospectus or at any time thereafter
The registrar and transfer agent of the Common Shares is Odyssey Trust Company at its principal office in Calgary, Alberta.
PART II
INFORMATION NOT REQUIRED TO BE DELIVERED TO
OFFEREES OR PURCHASERS
Indemnification of Directors and Officers
Under the Business Corporations Act (Ontario), the Registrant may indemnify a director or officer of the Registrant, a former director or officer of the Registrant or another individual who acts or acted at the Registrant's request as a director or officer, or an individual acting in a similar capacity, of another entity (each of the foregoing, an "individual"), against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the Registrant or other entity, on the condition that (i) such individual acted honestly and in good faith with a view to the best interests of the Registrant or, as the case may be, to the best interests of the other entity for which the individual acted as a director or officer or in a similar capacity at the Registrant's request; and (ii) if the matter is a criminal or administrative action or proceeding that is enforced by a monetary penalty, the Registrant shall not indemnify the individual unless the individual had reasonable grounds for believing that his or her conduct was lawful.
Further, the Registrant may, with the approval of a court, indemnify an individual in respect of an action by or on behalf of the Registrant or other entity to obtain a judgment in its favor, to which the individual is made a party because of the individual's association with the Registrant or other entity as a director or officer, a former director or officer, an individual who acts or acted at the Registrant's request as a director or officer, or an individual acting in a similar capacity, against all costs, charges and expenses reasonably incurred by the individual in connection with such action, if the individual fulfills the conditions in (i) and (ii) above. Such individuals are entitled to indemnification from the Registrant in respect of all costs, charges and expenses reasonably incurred by the individual in connection with the defense of any civil, criminal, administrative, investigative or other proceeding to which the individual is subject because of the individual's association with the Registrant or other entity as described above, provided the individual seeking an indemnity: (A) was not judged by a court or other competent authority to have committed any fault or omitted to do anything that the individual ought to have done; and (B) fulfills the conditions in (i) and (ii) above.
The by-laws of the Registrant provide that, subject to the Business Corporations Act (Ontario), the Registrant shall indemnify a director or officer of the Registrant, a former director or officer of the Registrant or another individual who acts or acted at the Registrant's request as a director or officer, or an individual acting in a similar capacity, or another entity, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the Registrant or other entity, if: (i) the individual acted honestly and in good faith with a view to the best interests of the Registrant or, as the case may be, to the best interest of the other entity for which the individual acted as a director or officer or in a similar capacity at the Registrant's request and (ii) in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the person had reasonable grounds for believing that the individual's conduct was lawful.
The Registrant maintains directors' and officers' liability insurance which insures directors and officers for losses as a result of claims against the directors and officers of the Registrant in their capacity as directors and officers and also reimburses the Registrant for payments made pursuant to the indemnity provisions under the by-laws of the Registrant and the Business Corporations Act (Ontario).
* * *
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing provisions, the Registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable.
EXHIBIT INDEX
|
Exhibit
Number
|
|
Description
|
|
4.1
|
|
The annual information form of the Registrant dated June 27, 2023 for the year ended March 31, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Annual Report on Form 40-F, filed with the Commission on June 27, 2023)
|
| |
|
|
|
4.2
|
|
The audited consolidated financial statements of the Registrant and the notes thereto as at and for the fiscal year ended March 31, 2023, together with the auditor's report thereon (incorporated by reference from Exhibit 99.2 of the Registrant's Annual Report on Form 40-F, filed with the Commission on June 27, 2023)
|
|
|
|
|
|
4.3
|
|
Management's discussion and analysis of the Registrant for the year ended March 31, 2023 (incorporated by reference from Exhibit 99.3 of the Registrant's Annual Report on Form 40-F, filed with the Commission on June 27, 2023)
|
|
|
|
|
|
4.4
|
|
The unaudited interim condensed consolidated financial statements of the Registrant and the notes thereto for the three and six months ended September 30, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on November 15, 2023)
|
|
|
|
|
|
4.5
|
|
Management's discussion and analysis of the Registrant for the three and six months ended September 30, 2023 (incorporated by reference from Exhibit 99.2 of the Registrant's Form 6-K, furnished to the Commission on November 15, 2023)
|
|
|
|
|
|
4.6
|
|
The management information circular of the Registrant dated September 13, 2023 relating to an annual and special meeting of shareholders of the Registrant held on October 12, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on September 14, 2023)
|
|
|
|
|
|
4.7
|
|
The material change report of the Registrant dated November 20, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on November 21, 2023)
|
|
|
|
|
|
4.8
|
|
The material change report of the Registrant dated October 25, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on October 25, 2023)
|
|
|
|
|
|
4.9
|
|
The material change report of the Registrant dated September 7, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on September 12, 2023)
|
|
|
|
|
|
4.10
|
|
The material change report of the Registrant dated August 23, 2023 (incorporated by reference from Exhibit 99.5 of the Registrant's Form 6-K, furnished to the Commission on August 24, 2023)
|
|
|
|
|
|
4.11
|
|
The material change report of the Registrant dated August 8, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on August 9, 2023)
|
|
|
|
|
|
4.12
|
|
The material change report of the Registrant dated May 31, 2023 (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on June 1, 2023)
|
|
|
|
|
|
4.13
|
|
The business acquisition report (incorporated by reference from Exhibit 99.1 of the Registrant's Form 6-K, furnished to the Commission on October 27, 2023)
|
|
|
|
|
|
5.1*
|
|
Consent of Zeifmans LLP
|
|
|
|
|
|
5.2*
|
|
Consent of MNP LLP
|
|
|
|
|
|
6.1*
|
|
Powers of Attorney (contained on the signature page hereto)
|
|
|
|
|
|
7.1*
|
|
Form of Debt Indenture
|
| |
|
|
| 107* |
|
Filing Fee Table |
* Filed herewith
PART III
UNDERTAKING AND CONSENT TO SERVICE OF PROCESS
Item 1. Undertaking
The Registrant undertakes to make available, in person or by telephone, representatives to respond to inquiries made by the Commission staff, and to furnish promptly, when requested to do so by the Commission staff, information relating to the securities registered pursuant to Form F-10 or to transactions in said securities.
Item 2. Consent to Service of Process
A written Appointment of Agent for Service of Process and Undertaking on Form F-X for the Registrant and its agent for service of process is being filed concurrently herewith.
Any change to the name or address of the agent for service of process of the Registrant shall be communicated promptly to the Commission by amendment to Form F-X referencing the file number of this Registration Statement on Form F-10.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-10 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Toronto, Country of Canada on December 29, 2023.
|
|
CYBIN INC.
|
| |
|
|
| |
By: |
/s/ Greg Cavers |
|
|
|
Name:
|
Greg Cavers
|
|
|
|
Title:
|
Chief Financial Officer
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Douglas Drysdale and Greg Cavers or any of them, his or her true and lawful attorneys-in-fact and agents, each of whom may act alone, with full powers of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any or all amendments to this Registration Statement, including post-effective amendments, registration statements filed pursuant to Rule 429 under the Securities Acts of 1933, as amended, and any and all additional registration statements (including amendments and post-effective amendments thereto) in connection with any increase in the amount of securities registered with the Securities and Exchange Commission, and to file the same, with all exhibits thereto, and other documents and in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, and hereby ratifies and confirms all his or her said attorneys-in-fact and agents or any of them or his or her substitute or substitutes may lawfully do or cause to be done by virtue hereof.
This Power of Attorney may be executed in multiple counterparts, each of which shall be deemed an original, but which taken together shall constitute one instrument.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed below by the following persons in the capacities indicated and on the dates indicated.
| Signature |
|
Capacity |
|
Date |
| |
|
|
|
|
| /s/ Douglas Drysdale |
|
|
|
|
| Douglas Drysdale |
|
Chief Executive Officer |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Greg Cavers |
|
|
|
|
| Greg Cavers |
|
Chief Financial Officer |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Eric So |
|
|
|
|
| Eric So |
|
Director and Executive Chairman and President |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Eric Hoskins |
|
|
|
|
| Eric Hoskins |
|
Director |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Grant Froese |
|
|
|
|
| Grant Froese |
|
Director |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Mark Lawson |
|
|
|
|
| Mark Lawson |
|
Director |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Theresa Firestone |
|
|
|
|
| Theresa Firestone |
|
Director |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ Paul Glavine |
|
|
|
|
| Paul Glavine |
|
Director |
|
December 29, 2023 |
| |
|
|
|
|
| /s/ George Tziras |
|
|
|
|
| George Tziras |
|
Director |
|
December 29, 2023 |
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of Section 6(a) of the Securities Act of 1933, as amended, the undersigned has signed this Registration Statement, in the capacity of the duly authorized representative of the Registrant in the United States, on December 29, 2023.
| |
/s/ Douglas Drysdale |
| |
Name: Douglas Drysdale |
| |
Title: Chief Executive Officer |

We consent to the incorporation by reference into the Registration Statement on Form F-10 (the "Form F-10") of Cybin Inc. (the "Company") being filed with the United States Securities and Exchange Commission, and any amendments thereto, of our report, dated June 27, 2023, with respect to the consolidated statements of financial position of the Company as of March 31, 2023 and 2022, and the consolidated statements of loss and comprehensive loss, changes in shareholders' equity and cash flows for the years then ended, and notes to the consolidated financial statements, including a summary of significant accounting policies.
We also consent to the reference to us under the caption "Auditor, Transfer Agent and Registrar" in the short-form base shelf prospectus contained in the Form F-10 and the caption "Interest of Experts in the management information circular of the Company dated September 13, 2023 that is incorporated by reference into the Form F-10.
| |
/s/ Zeifmans LLP |
| Toronto, Canada |
Chartered Professional Accountants |
| December 29, 2023 |
Licensed Public Accountants |
|
201 Bridgeland Avenue | Toronto
Ontario | M6A 1Y7 | Canada
|
zeifmans.ca
T: 416.256.4000
|
|

|

Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference into the Registration Statement on Form F-10 (the "Form F-10") of Cybin Inc. (the "Company") being filed with the United States Securities and Exchange Commission, and any amendments thereto, of our report, dated June 28, 2023 to the shareholders of Small Pharma Inc. on the consolidated financial statements of Small Pharma Inc., which comprises of the consolidated statements of financial position as at February 28, 2023 and 2022, and the consolidated statements of operations and comprehensive loss, changes in equity (deficit), cash flows, and notes to the consolidated financial statements, including a summary of significant accounting policies, for the years then ended.
We also consent to the reference to us under the caption “Auditor, Transfer Agent and Registrar” in the short-form base shelf prospectus contained in the Form F-10 and “Interest of Experts in the management information circular of the Company dated September 13, 2023 that is incorporated by reference into the Form F-10.
/s/ MNP LLP
Chartered Professional Accountants
Licensed Public Accountants
Toronto, Canada
December 29, 2023
________________________________________________________
CYBIN INC.
as Issuer
and
[ ]
as U.S. Trustee
and
[ ]
as Canadian Trustee
Indenture
Dated as of [ ]
________________________________________________________
TABLE OF CONTENTS
i
ii
iii
iv
CROSS-REFERENCE TABLE
TIA
Section |
Indenture
Section |
| 310 |
(a) |
6.08(1) |
| |
(b) |
6.09 |
| |
(c) |
Not Applicable |
| 311 |
(a) |
6.05 |
| |
(b) |
6.05 |
| |
(c) |
Not Applicable |
| 312 |
(a) |
7.05 |
| |
(b) |
7.03 |
| |
(c) |
7.03 |
| 313 |
(a) |
7.04 |
| |
(b) |
7.04 |
| |
(c) |
7.04 |
| |
(d) |
7.05 |
| 314 |
(a) |
7.05 |
| |
(a)(4) |
10.04 |
| |
(b) |
Not Applicable |
| |
(c)(1) |
1.01 |
| |
(c)(2) |
1.01 |
| |
(d) |
Not Applicable |
| |
(e) |
1.01 |
| |
(f) |
Not Applicable |
| 315 |
(a) |
6.02 |
| |
(b) |
6.01 |
| |
(c) |
6.02 |
| |
(d) |
6.02 |
| |
(e) |
5.15 |
| 316 |
(a)(last sentence) |
1.02 ("Outstanding") |
| |
(a)(1)(A) |
5.12 |
| |
(a)(1)(B) |
5.02, 5.13 |
| |
(a)(2) |
Not Applicable |
| |
(b) |
5.08 |
| |
(c) |
1.04(e) |
| 317 |
(a)(1) |
5.03 |
| |
(a)(2) |
5.04 |
| |
(b) |
10.03 |
| 318 |
(a) |
1.16 |
Note: This Cross-Reference Table shall not, for any purpose, be deemed to be part of this Indenture.
Cybin Inc., a corporation duly continued and existing under the laws of the Province of Ontario, Canada (herein called the "Company"), having its principal office at 100 King Street West, Suite 5600, Toronto, Ontario, Canada, M5X 1C9, and ______________________, a ______________________, organized under the laws of ______________________, as U.S. trustee (herein called the "U.S. Trustee"), and ______________________, a ______________________, organized under the laws of ______________________, as Canadian trustee (the "Canadian Trustee" and, together with the U.S. Trustee, the "Trustees").
RECITALS
The Company has duly authorized the execution and delivery of this Indenture to provide for the issuance from time to time of its debentures, notes, bonds or other evidences of indebtedness (herein called the "Securities"), which may be convertible into or exchangeable for any securities of any Person (including the Company), to be issued in one or more series as in this Indenture provided.
This Indenture is subject to the provisions of Trust Indenture Legislation that are required to be part of this Indenture and shall, to the extent applicable, be governed by such provisions.
All things necessary to make this Indenture a valid agreement of the Company, in accordance with its terms, have been done.
NOW, THEREFORE, THIS INDENTURE WITNESSETH:
For and in consideration of the premises and the purchase of the Securities by the Holders thereof, it is mutually covenanted and agreed, for the equal and proportionate benefit of all Holders of the Securities or of series thereof, as follows:
ARTICLE ONE
DEFINITIONS AND OTHER PROVISIONS OF GENERAL APPLICATION
SECTION 1.01 Definitions.
"Act," when used with respect to any Holder, has the meaning specified in Section 1.04.
"Affiliate" of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For the purposes of this definition, "control" when used with respect to any specified Person means the power to direct the management and policies of such Person, directly or indirectly, whether through the ownership of voting securities, by contract or otherwise; and the terms "controlling" and "controlled" have meanings correlative to the foregoing.
"Authenticating Agent" means any Person authorized by the applicable Trustee pursuant to Section 6.12 to act on behalf of such Trustee to authenticate Securities.
"Base Currency" has the meaning specified in Section 1.14.
"Board of Directors" means the board of directors of the Company or any duly authorized committee thereof.
"Board Resolution" means a copy of a resolution certified by the Corporate Secretary or an Assistant Secretary of the Company to have been duly adopted by the Board of Directors and to be in full force and effect on the date of such certification, and delivered to the Trustees.
"Business Day," when used with respect to any Place of Payment or any other particular location referred to in this Indenture or in the Securities, means, unless otherwise specified with respect to any Securities pursuant to Section 3.01, any day other than Saturday, Sunday or any other day on which commercial banking institutions in that Place of Payment or other location are permitted or required by any applicable law, regulation or executive order to close.
"calculation period" has the meaning specified in Section 3.11.
"Canadian Trustee" means the Person named as the "Canadian Trustee" in the first paragraph of this Indenture until a successor Canadian Trustee shall have become such pursuant to the applicable provisions of this Indenture, and thereafter "Canadian Trustee" shall mean or include each Person who is then a Canadian Trustee hereunder; provided, however, that if at any time there is more than one such Person, "Canadian Trustee" as used with respect to the Securities of any series shall mean only the Canadian Trustee with respect to Securities of that series.
"Commission" means the U.S. Securities and Exchange Commission, as from time to time constituted, created under the Exchange Act, or, if at any time after the execution of this Indenture such Commission is not existing and performing the duties now assigned to it under the Trust Indenture Act, then the body performing such duties at such time.
"Company" means the Person named as the "Company" in the first paragraph of this Indenture until a successor Person shall have become such pursuant to the applicable provisions of this Indenture, and thereafter "Company" shall mean such successor Person.
"Company Request" or "Company Order" means a written request or order signed in the name of the Company by an Officer and delivered to the Trustees.
"Component Currency" has the meaning specified in Section 3.12(h).
"Conversion Date" has the meaning specified in Section 3.12(d).
"Conversion Event" means the cessation of use of (i) a Foreign Currency (other than the Euro or other Currency unit) both by the government of the country which issued such Currency and by a central bank or other public institution of or within the international banking community for the settlement of transactions, (ii) the Euro or (iii) any currency unit (or composite currency) other than the Euro for the purposes for which it was established.
"Corporate Trust Office" means the principal corporate trust office of the U.S. Trustee or the Canadian Trustee, as applicable, at which at any particular time its corporate trust business may be administered, such an office on the date of execution of this Indenture of the U.S. Trustee is located at _________________________, Attention: _______________________, and of the Canadian Trustee is located at ______________________, Attention: ____________________________, except that with respect to presentation of Securities for payment or for registration of transfer or exchange, such term shall mean the office or agency of the U.S. Trustee or the Canadian Trustee, as applicable, designated in writing to the Company at which, at any particular time, its corporate agency business shall be conducted.
"covenant defeasance" has the meaning specified in Section 14.03.
"Currency" means any currency or currencies, composite currency or currency unit or currency units, including, without limitation, the Euro, issued by the government of one or more countries or by any recognized confederation or association of such governments.
"Default" means any event which is, or after notice or passage of time or both would be, an Event of Default.
"Defaulted Interest" has the meaning specified in Section 3.07.
"defeasance" has the meaning specified in Section 14.02.
"Depositary" means, with respect to the Securities of any series issuable or issued in global form, the Person designated as Depositary by the Company pursuant to Section 3.05 until a successor Depositary shall have become such pursuant to the applicable provisions of this Indenture, and thereafter "Depositary" shall mean or include each Person who is then a Depositary hereunder, and, if at any time there is more than one such Person, "Depositary" as used with respect to the Securities of any such series shall mean the Depositary with respect to the Securities of that series.
"Dollar" or "$" means a dollar or other equivalent unit in such coin or currency of the United States of America as at the time shall be legal tender for the payment of public and private debts.
"Dollar Equivalent of the Currency Unit" has the meaning specified in Section 3.12(g).
"Dollar Equivalent of the Foreign Currency" has the meaning specified in Section 3.12(f).
"Election Date" has the meaning specified in Section 3.12(h).
"Euro" means the single currency of the participating member states from time to time of the European Union described in legislation of the European Counsel for the operation of a single unified European currency (whether known as the Euro or otherwise).
"Event of Default" has the meaning specified in Section 5.01.
"Exchange Act" means the United States Securities Exchange Act of 1934, as amended.
"Exchange Date" has the meaning specified in Section 3.04.
"Exchange Rate Agent" means, with respect to Securities of or within any series, unless otherwise specified with respect to any Securities pursuant to Section 3.01, a New York clearing house bank, designated pursuant to Section 3.01 or Section 3.13.
"Exchange Rate Officer's Certificate" means a tested telex or a certificate setting forth (i) the applicable Market Exchange Rate and (ii) the Dollar or Foreign Currency amounts of principal, premium (if any) and interest (if any) (on an aggregate basis and on the basis of a Security having the lowest denomination principal amount determined in accordance with Section 3.02 in the relevant Currency), payable with respect to a Security of any series on the basis of such Market Exchange Rate, sent (in the case of a telex) or signed (in the case of a certificate) by the Chief Executive Officer, President or Chief Financial Officer of the Company.
"Extension Notice" has the meaning specified in Section 3.08.
"Extension Period" has the meaning specified in Section 3.08.
"Final Maturity" has the meaning specified in Section 3.08.
"First Currency" has the meaning specified in Section 1.15.
"Foreign Currency" means any Currency other than Currency of the United States.
"GAAP" means generally accepted accounting principles in Canada in effect from time to time, unless the Person's most recent audited or quarterly financial statements are not prepared in accordance with generally accepted accounting principles in Canada, in which case "GAAP" shall mean generally accepted accounting principles in the United States in effect from time to time.
"Government Obligations" means, unless otherwise specified with respect to any series of Securities pursuant to Section 3.01, securities which are (i) direct obligations of the government which issued the Currency in which the Securities of a particular series are payable or (ii) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the government which issued the Currency in which the Securities of such series are payable, the payment of which is unconditionally guaranteed by such government, which, in either case, are full faith and credit obligations of such government payable in such Currency and are not callable or redeemable at the option of the issuer thereof and shall also include a depository receipt issued by a bank or trust company as custodian with respect to any such Government Obligation or a specific payment of interest on or principal of any such Government Obligation held by such custodian for the account of the holder of a depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Obligation or the specific payment of interest or principal of the Government Obligation evidenced by such depository receipt.
"Holder" means the Person in whose name a Security is registered in the Security Register.
"Indenture" means this instrument as originally executed and as it may from time to time be supplemented or amended by one or more indentures supplemental hereto entered into pursuant to the applicable provisions hereof, and shall include the terms of particular series of Securities established as contemplated by Section 3.01; provided, however, that, if at any time more than one Person is acting as Trustee under this instrument, "Indenture" shall mean, with respect to any one or more series of Securities for which such Person is Trustee, this instrument as originally executed or as it may from time to time be supplemented or amended by one or more indentures supplemental hereto entered into pursuant to the applicable provisions hereof and shall include the terms of the particular series of Securities for which such Person is Trustee established as contemplated by Section 3.01, exclusive, however, of any provisions or terms which relate solely to other series of Securities for which such Person is not Trustee, regardless of when such terms or provisions were adopted, and exclusive of any provisions or terms adopted by means of one or more indentures supplemental hereto executed and delivered after such Person had become such Trustee but to which such Person, as such Trustee, was not a party.
"Indexed Security" means a Security the terms of which provide that the principal amount thereof payable at Stated Maturity may be more or less than the principal face amount thereof at original issuance.
"interest," when used with respect to an Original Issue Discount Security which by its terms bears interest only after Maturity, means interest payable after Maturity at the rate prescribed in such Original Issue Discount Security.
"Interest Payment Date," when used with respect to any Security, means the Stated Maturity of an installment of interest on such Security.
"Judgment Currency" has the meaning specified in Section 1.14.
"Lien" means any mortgage, pledge, hypothecation, charge, assignment, deposit arrangement, encumbrance, security interest, lien (statutory or other), or preference, priority or other security or similar agreement or preferential arrangement of any kind or nature whatsoever (including, without limitation, any agreement to give or grant a Lien or any lease, conditional sale or other title retention agreement having substantially the same economic effect as any of the foregoing).
"mandatory sinking fund payment" has the meaning specified in Section 12.01.
"Market Exchange Rate" means, unless otherwise specified with respect to any Securities pursuant to Section 3.01, (i) for any conversion involving a Currency unit on the one hand and Dollars or any Foreign Currency on the other, the exchange rate between the relevant Currency unit and Dollars or such Foreign Currency calculated by the method specified pursuant to Section 3.01 for the Securities of the relevant series, (ii) for any conversion of Dollars into any Foreign Currency, the noon (New York City time) buying rate for such Foreign Currency for cable transfers quoted in New York City as certified for customs purposes by the Federal Reserve Bank of New York and (iii) for any conversion of one Foreign Currency into Dollars or another Foreign Currency, the spot rate at noon local time in the relevant market at which, in accordance with normal banking procedures, the Dollars or Foreign Currency into which conversion is being made could be purchased with the Foreign Currency from which conversion is being made from major banks located in New York City, Toronto, London or any other principal market for Dollars or such purchased Foreign Currency, in each case determined by the Exchange Rate Agent. Unless otherwise specified with respect to any Securities pursuant to Section 3.01, in the event of the unavailability of any of the exchange rates provided for in the foregoing clauses (i), (ii) and (iii), the Exchange Rate Agent shall use, in its sole discretion and without liability on its part, such quotation of the Federal Reserve Bank of New York as of the most recent available date, or quotations from one or more major banks in New York City, Toronto, London or another principal market for the Currency in question, or such other quotations as the Exchange Rate Agent shall deem appropriate. Unless otherwise specified by the Exchange Rate Agent, if there is more than one market for dealing in any Currency by reason of foreign exchange regulations or otherwise, the market to be used in respect of such Currency shall be that upon which a non-resident issuer of securities designated in such Currency would purchase such Currency in order to make payments in respect of such securities.
"Maturity," when used with respect to any Security, means the date on which the principal of such Security or an installment of principal becomes due and payable as therein or herein provided, whether at the Stated Maturity or by declaration of acceleration, notice of redemption, notice of option to elect repayment or otherwise.
"Notice of Default" has the meaning specified in Section 6.01.
"Officer" means the Chair of the Board of Directors, the Chief Executive Officer, the President, the Chief Financial Officer, the Chief Operating Officer, any Executive Vice President, any Vice President, the Treasurer or the Corporate Secretary of the Company or, in the event that the Company is a partnership or a limited liability company that has no such officers, a person duly authorized under applicable law by the general partner, managers, members or a similar body to act on behalf of the Company.
"Officer's Certificate" means a certificate, which shall comply with this Indenture, signed by an Officer and delivered to the Trustees.
"Opinion of Counsel" means a written opinion of counsel, who may be counsel for the Company, including an employee of the Company, who shall be acceptable to the Trustees, which opinion may contain customary exceptions and qualifications as to the matters set forth therein.
"Optional Reset Date" has the meaning specified in Section 3.07.
"optional sinking fund payment" has the meaning specified in Section 12.01.
"Original Issue Discount Security" means any Security which provides for an amount less than the principal amount thereof to be due and payable upon a declaration of acceleration of the Maturity thereof pursuant to Section 5.02.
"Original Stated Maturity" has the meaning specified in Section 3.08.
"Outstanding," when used with respect to Securities, means, as of the date of determination, all Securities theretofore authenticated and delivered under this Indenture, except:
(i) Securities theretofore cancelled by either Trustee or delivered to either Trustee for cancellation;
(ii) Securities, or portions thereof, for whose payment or redemption or repayment at the option of the Holder, money in the necessary amount has been theretofore deposited with either Trustee or any Paying Agent (other than the Company) in trust or set aside and segregated in trust by the Company (if the Company shall act as its own Paying Agent) for the Holders of such Securities; provided that, if such Securities are to be redeemed, notice of such redemption has been duly given pursuant to this Indenture or provision therefor satisfactory to the Trustees has been made;
(iii) Securities, except to the extent provided in Section 14.02 and Section 14.03, with respect to which the Company has effected defeasance and/or covenant defeasance as provided in Article Fourteen; and
(iv) Securities which have been paid pursuant to Section 3.06 or in exchange for or in lieu of which other Securities have been authenticated and delivered pursuant to this Indenture, other than any such Securities in respect of which there shall have been presented to the Trustees proof satisfactory to them that such Securities are held by a bona fide purchaser in whose hands such Securities are valid obligations of the Company;
provided, however, that in determining whether the Holders of the requisite principal amount of the Outstanding Securities have given any request, demand, authorization, direction, notice, consent or waiver hereunder, and for the purpose of making the calculations required by TIA Section 313, (i) the principal amount of an Original Issue Discount Security that may be counted in making such determination or calculation and that shall be deemed to be Outstanding for such purpose shall be equal to the amount of principal thereof that would be (or shall have been declared to be) due and payable, at the time of such determination, upon a declaration of acceleration of the maturity thereof pursuant to Section 5.02, (ii) the principal amount of any Security denominated in a Foreign Currency that may be counted in making such determination or calculation and that shall be deemed Outstanding for such purpose shall be equal to the Dollar equivalent, determined as of the date such Security is originally issued by the Company as set forth in an Exchange Rate Officer's Certificate delivered to the Trustees, of the principal amount (or, in the case of an Original Issue Discount Security, the Dollar equivalent as of such date of original issuance of the amount determined as provided in clause (i) above) of such Security, (iii) the principal amount of any Indexed Security that may be counted in making such determination or calculation and that shall be deemed outstanding for such purpose shall be equal to the principal face amount of such Indexed Security at original issuance, unless otherwise provided with respect to such Security pursuant to Section 3.01, and (iv) Securities owned by the Company or any other obligor upon the Securities or any Affiliate of the Company or of such other obligor shall be disregarded and deemed not to be Outstanding, except that, in determining whether the Trustees shall be protected in making such calculation or in relying upon any such request, demand, authorization, direction, notice, consent or waiver, only Securities which the Trustees know to be so owned shall be so disregarded. Securities so owned which have been pledged in good faith may be regarded as Outstanding if the pledgee establishes to the satisfaction of the Trustees the pledgee's right so to act with respect to such Securities and that the pledgee is not the Company or any other obligor upon the Securities or any Affiliate of the Company or such other obligor.
"Paying Agent" means any Person (including the Company acting as Paying Agent) authorized by the Company to pay the principal of, premium (if any) or interest (if any) on any Securities on behalf of the Company. Such Person must be capable of making payment in the Currency of the issued Security.
"Person" means any individual, corporation, body corporate, partnership, limited partnership, limited liability partnership, joint venture, limited liability company, unlimited liability company, association, joint-stock company, trust, unincorporated organization or government or any agency or political subdivision thereof.
"Place of Payment" means, when used with respect to the Securities of or within any series, each place where the principal of, premium (if any) and interest (if any) on such Securities are payable as specified as contemplated by Sections 3.01 and 10.02.
"Predecessor Security" of any particular Security means every previous Security evidencing all or a portion of the same debt as that evidenced by such particular Security; and, for the purposes of this definition, any Security authenticated and delivered under Section 3.06 in exchange for or in lieu of a mutilated, destroyed, lost or stolen Security shall be deemed to evidence the same debt as the mutilated, destroyed, lost or stolen Security.
"Privacy Laws" has the meaning specified in Section 6.14.
"rate(s) of exchange" has the meaning specified in Section 1.14.
"Redemption Date," when used with respect to any Security to be redeemed, in whole or in part, means the date fixed for such redemption by or pursuant to this Indenture.
"Redemption Price," when used with respect to any Security to be redeemed, in whole or in part, means the price at which it is to be redeemed pursuant to this Indenture, plus accrued and unpaid interest thereon to the Redemption Date.
"Regular Record Date" for the interest payable on any Interest Payment Date on the Securities of or within any series means the date specified for that purpose as contemplated by Section 3.01.
"Repayment Date" means, when used with respect to any Security to be repaid at the option of the Holder, the date fixed for such repayment pursuant to this Indenture.
"Reset Notice" has the meaning specified in Section 3.07.
"Responsible Officer," when used with respect to a Trustee, means any vice president, secretary, any assistant secretary, treasurer, any assistant treasurer, any senior trust officer, any trust officer, the controller within the corporate trust administration division of a Trustee or any other officer of a Trustee customarily performing functions similar to those performed by any of the above-designated officers, and also means, with respect to a particular corporate trust matter, any other officer to whom such matter is referred because of his knowledge of and familiarity with the particular subject.
"Securities" has the meaning stated in the first recital of this Indenture and more particularly means any Securities authenticated and delivered under this Indenture; provided, however, that if at any time there is more than one Person acting as Trustee under this Indenture, "Securities" with respect to the Indenture as to which such Person is Trustee shall have the meaning stated in the first recital of this Indenture and shall more particularly mean Securities authenticated and delivered under this Indenture, exclusive, however, of Securities of any series as to which such Person is not Trustee.
"Security Register" and "Security Registrar" have the respective meanings specified in Section 3.05.
"Special Record Date" for the payment of any Defaulted Interest on the Securities of or within any series means a date fixed by the Trustees pursuant to Section 3.07.
"Specified Amount" has the meaning specified in Section 3.12(h).
"Stated Maturity," when used with respect to any Security or any installment of principal thereof or interest thereon, means the date specified in such Security as the fixed date on which the principal of such Security or such installment of principal or interest is due and payable, as such date may be extended pursuant to the provisions of Section 3.08 (if applicable).
"Subsequent Interest Period" has the meaning specified in Section 3.07.
"Trust Indenture Act" or "TIA" means the United States Trust Indenture Act of 1939, as amended, as in force at the date as of which this Indenture was executed, except as provided in Section 9.05.
"Trust Indenture Legislation" means, at any time, the provisions of (i) any applicable statute of Canada or any province or territory thereof and the regulations thereunder as amended or re-enacted from time to time, but only to the extent applicable, or (iii) the Trust Indenture Act and regulations thereunder, but only to the extent applicable, in each case relating to trust indentures and to the rights, duties and obligations of trustees under trust indentures and of corporations issuing debt obligations under trust indentures, to the extent that such provisions are at such time in force and applicable to this Indenture or the Company or the Trustees.
"Trustee" or "Trustees" means the U.S. Trustee and the Canadian Trustee. If a Canadian Trustee is not appointed under this Indenture, or resigns or is removed and, pursuant to Section 6.09, the Company is not required to appoint a successor Trustee to the Canadian Trustee, "Trustee," "Trustees" and any reference to "either Trustee," "both of the Trustees" or such similar references shall mean the Person named as the U.S. Trustee or any successor thereto appointed pursuant to the applicable provisions of this Indenture. Except to the extent otherwise indicated, "Trustees" shall refer to the Canadian Trustee (if appointed and still serving) and the U.S. Trustee, both jointly and individually.
"U.S. Federal Bankruptcy Code" means the Bankruptcy Act of Title 11 of the United States Code, as amended from time to time.
"U.S. Trustee" means the Person named as the "U.S. Trustee" in the first paragraph of this Indenture until a successor U.S. Trustee shall have become such pursuant to the applicable provisions of this Indenture, and thereafter "U.S. Trustee" shall mean or include each Person who is then a U.S. Trustee hereunder; provided, however, that if at any time there is more than one such Person, "U.S. Trustee" as used with respect to the Securities of any series shall mean only the U.S. Trustee with respect to Securities of that series.
"United States" means, unless otherwise specified with respect to any Securities pursuant to Section 3.01, the United States of America (including the states and the District of Columbia), its territories, its possessions and other areas subject to its jurisdiction.
"United States person" means, unless otherwise specified with respect to any Securities pursuant to Section 3.01, an individual who is a citizen or resident of the United States, a corporation, partnership (including any entity treated as a corporation or as a partnership for United States federal income tax purposes) or other entity created or organized in or under the laws of the United States, any state thereof or the District of Columbia, an estate the income of which is subject to United States federal income taxation regardless of its source, or a trust if (A) it is subject to the primary supervision of a court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust or (B) it has a valid election in effect under applicable United States Treasury Regulations to be treated as a United States person.
"Valuation Date" has the meaning specified in Section 3.12(c).
"Writing" has the meaning specified in Section 6.13.
"Yield to Maturity" means the yield to maturity, computed at the time of issuance of a Security (or, if applicable, at the most recent redetermination of interest on such Security) and as set forth in such Security in accordance with generally accepted United States bond yield computation principles.
SECTION 1.02 Rules of Construction.
For all purposes of this Indenture, except as otherwise expressly provided or unless the context otherwise requires:
(1) the terms defined in this Indenture have the meanings assigned to them herein and include the plural as well as the singular;
(2) all terms used herein which are defined in the Trust Indenture Act, either directly or by reference therein, have the meanings assigned to them therein, and the terms "cash transaction" and "self-liquidating paper," as used in TIA Section 319, shall have the meanings assigned to them in the rules of the Commission adopted under the Trust Indenture Act;
(3) the words "herein," "hereof" and "hereunder" and other words of similar import refer to this Indenture as a whole and not to any particular Article, Section or other subdivision;
(4) "or" is not exclusive;
(5) words implying any gender shall apply to all genders;
(6) the words Subsection, Section and Article refer to the Subsections, Sections and Articles, respectively, of this Indenture unless otherwise noted; and
(7) "include," "includes" or "including" means include, includes or including, in each case, without limitation.
SECTION 1.03 Compliance Certificates and Opinions.
Upon any application or request by the Company to the Trustees to take any action under any provision of this Indenture, the Company shall furnish to the Trustees an Officer's Certificate stating that all conditions precedent, if any, provided for in this Indenture (including any covenant compliance with which constitutes a condition precedent) relating to the proposed action have been complied with and an Opinion of Counsel stating that in the opinion of such counsel all such conditions precedent, if any, have been complied with, except that in the case of any such application or request as to which the furnishing of such documents is specifically required by any provision of this Indenture relating to such particular application or request, no additional certificate or opinion need be furnished.
Every certificate or opinion with respect to compliance with a covenant or condition provided for in this Indenture (other than pursuant to Section 10.04) shall include:
(1) a statement that each individual signing such certificate or opinion has read such covenant or condition and the definitions herein relating thereto;
(2) a brief statement as to the nature and scope of the examination or investigation upon which the statements or opinions contained in such certificate or opinion are based;
(3) a statement that, in the opinion of each such individual, he has made such examination or investigation as is necessary to enable him to express an informed opinion as to whether or not such covenant or condition has been complied with; and
(4) a statement as to whether, in the opinion of each such individual, such covenant or condition has been complied with.
SECTION 1.04 Form of Documents Delivered to Trustees.
In any case where several matters are required to be certified by, or covered by an opinion of, any specified Person, it is not necessary that all such matters be certified by, or covered by the opinion of, only one such Person, or that they be so certified or covered by only one document, but one such Person may certify or give an opinion with respect to some matters and one or more other such Persons may certify or give an opinion with respect to other matters, and any such Person may certify or give an opinion as to such matters in one or several documents.
Any certificate or opinion of an officer of the Company may be based, insofar as it relates to legal matters, upon an Opinion of Counsel, a certificate of, or representations by, counsel, unless such officer knows, or in the exercise of reasonable care should know, that the certificate or opinion or representations with respect to the matters upon which his certificate or opinion is based are erroneous. Any such certificate or Opinion of Counsel may be based, insofar as it relates to factual matters, upon a certificate or opinion of, or representations by, an officer or officers of the Company stating that the information with respect to such factual matters is in the possession of the Company, unless such counsel knows, or in the exercise of reasonable care should know, that the certificate or opinion or representations with respect to such matters are erroneous.
Any certificate or opinion of an officer of the Company or counsel may be based, insofar as it relates to accounting matters, upon a certificate or opinion of, or representations by, an accountant or firm of accountants in the employ of the Company, unless such officer or counsel, as the case may be, knows, or in the exercise of reasonable care should know, that the certificate or opinion or representations with respect to the accounting matters upon which such certificate or opinion may be based are erroneous. Any certificate or opinion of any independent firm of public accountants filed with the Trustees shall contain a statement that such firm is independent.
Where any Person is required to make, give or execute two or more applications, requests, consents, certificates, statements, opinions or other instruments under this Indenture, they may, but need not, be consolidated and form one instrument.
SECTION 1.05 Acts of Holders.
(a) Any request, demand, authorization, direction, notice, consent, waiver or other action provided by this Indenture to be given or taken by Holders of the Outstanding Securities of all series or one or more series, as the case may be, may be embodied in and evidenced by one or more instruments of substantially similar tenor signed by such Holders in person or by agents duly appointed in writing. Except as herein otherwise expressly provided, such action shall become effective when such instrument or instruments or record or both are delivered to the Trustees and, where it is hereby expressly required, to the Company. Such instrument or instruments and any such record (and the action embodied therein and evidenced thereby) are herein sometimes referred to as the "Act" of the Holders signing such instrument or instruments or so voting at any such meeting. Proof of execution of any such instrument or of a writing appointing any such agent, or of the holding by any Person of a Security, shall be sufficient for any purpose of this Indenture and conclusive in favor of the Trustees and the Company, if made in the manner provided in this Section 1.05. The Trustees may make reasonable rules for action by or at a meeting of Holders.
(b) The fact and date of the execution by any Person of any such instrument or writing may be proved by the affidavit of a witness of such execution or by a certificate of a notary public or other officer authorized by law to take acknowledgments of deeds, certifying that the individual signing such instrument or writing acknowledged to him the execution thereof. Where such execution is by a signer acting in a capacity other than his individual capacity, such certificate or affidavit shall also constitute sufficient proof of authority. The fact and date of the execution of any such instrument or writing, or the authority of the Person executing the same, may also be proved in any other manner which the Trustees deem sufficient.
(c) The ownership of the Securities, including the principal amount and serial numbers, and the date of holding the same, shall be proved by the Security Register.
(d) If the Company shall solicit from the Holders of Securities any request, demand, authorization, direction, notice, consent, waiver or other Act, the Company may, at its option, by or pursuant to a Board Resolution, fix in advance a record date for the determination of Holders entitled to give such request, demand, authorization, direction, notice, consent, waiver or other Act, but the Company shall have no obligation to do so. Notwithstanding Trust Indenture Legislation, including TIA Section 316(c), such record date shall be the record date specified in or pursuant to such Board Resolution, which shall be a date not earlier than the date 30 days prior to the first solicitation of Holders generally in connection therewith and not later than the date such solicitation is completed. If such a record date is fixed, such request, demand, authorization, direction, notice, consent, waiver or other Act may be given before or after such record date, but only the Holders of record at the close of business on such record date shall be deemed to be Holders for the purposes of determining whether Holders of the requisite proportion of Outstanding Securities have authorized or agreed or consented to such request, demand, authorization, direction, notice, consent, waiver or other Act, and for that purpose the Outstanding Securities shall be computed as of such record date; provided that no such authorization, agreement or consent by the Holders on such record date shall be deemed effective unless it shall become effective pursuant to the provisions of this Indenture not later than eleven months after the record date.
(e) Any request, demand, authorization, direction, notice, consent, waiver or other Act of the Holder of any Security shall bind every future Holder of the same Security and the Holder of every Security issued upon the registration of transfer thereof or in exchange therefor or in lieu thereof in respect of anything done, omitted or suffered to be done by the Trustees or the Company in reliance thereon, whether or not notation of such action is made upon such Security.
SECTION 1.06 Notices, Etc. to Trustees and Company.
Any request, demand, authorization, direction, notice, consent, waiver or Act of Holders or other documents provided or permitted by this Indenture to be made upon, given or furnished to, or filed with:
(1) the U.S. Trustee, by the Canadian Trustee, any Holder or by the Company shall be sufficient for every purpose hereunder if made, given, furnished or filed in writing to or with the U.S. Trustee at its Corporate Trust Office, Attention: ________________, or
(2) the Canadian Trustee, by the U.S. Trustee, any Holder or by the Company shall be sufficient for every purpose hereunder if made, given, furnished or filed in writing to or with the Canadian Trustee at its Corporate Trust Office, Attention: ________________, or
(3) the Company by either Trustee or any Holder shall be sufficient for every purpose hereunder (unless otherwise herein expressly provided) if in writing and mailed, first-class postage prepaid, or sent by overnight courier, to the Company at 100 King Street West, Suite 5600, Toronto, Ontario, Canada, M5X 1C9, Attention: Corporate Secretary or such other address and/or officer as the Company may designate on written notice to the Trustees
SECTION 1.07 Notice to Holders; Waiver.
Where this Indenture provides for notice of any event to Holders of Securities by the Company or the Trustees, such notice shall be sufficiently given (unless otherwise herein expressly provided) if in writing and mailed, first-class postage prepaid, to each such Holder affected by such event, at his address as it appears in the Security Register. In any case where notice to Holders of Securities is given by mail, neither the failure to mail such notice, nor any defect in any notice so mailed, to any particular Holder shall affect the sufficiency of such notice with respect to other Holders of Securities. Any notice mailed to a Holder in the manner herein prescribed shall be conclusively deemed to have been received by such Holder, whether or not such Holder actually receives such notice.
In case, by reason of the suspension of or irregularities in regular mail service or by reason of any other cause, it shall be impractical to mail notice of any event to Holders of Securities when such notice is required to be given pursuant to any provision of this Indenture, then any manner of giving such notice as shall be satisfactory to the Trustees shall be deemed to be sufficient giving of such notice for every purpose hereunder.
Any request, demand, authorization, direction, notice, consent or waiver required or permitted under this Indenture shall be in the English language, except that any published notice may be in an official language of the country of publication.
Where this Indenture provides for notice in any manner, such notice may be waived in writing by the Person entitled to receive such notice, either before or after the event, and such waiver shall be the equivalent of such notice. Waivers of notice by Holders shall be filed with the Trustees, but such filing shall not be a condition precedent to the validity of any action taken in reliance upon such waiver.
SECTION 1.08 Effect of Headings and Table of Contents.
The Article and Section headings herein and the Table of Contents are for convenience only and shall not affect the construction hereof.
SECTION 1.09 Successors and Assigns.
All covenants and agreements in this Indenture by the Company and the Trustees shall bind their successors and assigns, whether so expressed or not.
SECTION 1.10 Severability Clause.
In case any provision in this Indenture or in any Security shall be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
SECTION 1.11 Benefits of Indenture.
Nothing in this Indenture or in the Securities, express or implied, shall give to any Person, other than the parties hereto, any Authenticating Agent, any Paying Agent, any Securities Registrar and their successors hereunder and the Holders of Securities, any benefit or any legal or equitable right, remedy or claim under this Indenture. Subject to Section 1.16, at all times in relation to this Indenture and any action to be taken hereunder, the Company and the Trustees each shall observe and comply with Trust Indenture Legislation and the Company, the Trustees and each Holder of a Security shall be entitled to the benefits of Trust Indenture Legislation.
SECTION 1.12 Governing Law.
This Indenture and the Securities shall be governed by and construed in accordance with the law of the State of New York, but without giving effect to applicable principles of conflicts of law to the extent that the application of the law of another jurisdiction would be required thereby. Notwithstanding the preceding sentence, the exercise, performance or discharge by the Canadian Trustee of any of its rights, powers, duties or responsibilities hereunder shall be construed in accordance with the laws of the Province of Ontario and the federal laws of Canada applicable thereto. This Indenture is subject to the provisions of Trust Indenture Legislation that are required to be part of this Indenture and shall, to the extent applicable, be governed by such provisions. Each Trustee and the Company agrees to comply with all provisions of Trust Indenture Legislation applicable to or binding upon it in connection with this Indenture and any action to be taken hereunder.
SECTION 1.13 Legal Holidays.
In any case where any Interest Payment Date, Redemption Date, sinking fund payment date or Stated Maturity or Maturity of any Security shall not be a Business Day at any Place of Payment or other location contemplated hereunder, then (notwithstanding any other provision of this Indenture or of any Security other than a provision in the Securities of any series which specifically states that such provision shall apply in lieu of this Section 1.13), payment of principal, premium (if any) or interest (if any), need not be made at such Place of Payment or other location contemplated hereunder on such date, but may be made on the next succeeding Business Day at such Place of Payment or other location contemplated hereunder with the same force and effect as if made on the Interest Payment Date or Redemption Date or sinking fund payment date, or at the Stated Maturity or Maturity; provided that no interest shall accrue for the period from and after such Interest Payment Date, Redemption Date, sinking fund payment date, Stated Maturity or Maturity, as the case may be.
SECTION 1.14 Agent for Service; Submission to Jurisdiction; Waiver of Immunities.
By the execution and delivery of this Indenture, the Company (i) acknowledges that it has irrevocably designated and appointed _____________________ as its authorized agent upon which process may be served in any suit, action or proceeding arising out of or relating to the Securities or this Indenture that may be instituted in any United States federal or New York state court located in The Borough of Manhattan, The City of New York, or brought by the Trustees (whether in their individual capacity or in their capacity as Trustees hereunder), (ii) irrevocably submits to the non-exclusive jurisdiction of any such court in any such suit or proceeding, and (iii) agrees that service of process upon _____________________ and written notice of said service to the Company (mailed or delivered to the Company at 100 King Street West, Suite 5600, Toronto, Ontario, Canada, M5X 1C9, Attention: Corporate Secretary or such other address and/or officer as the Company may designate on written notice to the Trustees), shall be deemed in every respect effective service of process upon the Company in any such suit or proceeding. The Company further agrees to take any and all action, including the execution and filing of any and all such documents and instruments, as may be necessary to continue such designation and appointment of _____________________ in full force and effect so long as this Indenture shall be in full force and effect.
To the extent that the Company has or hereafter may acquire any immunity from jurisdiction of any court or from any legal process (whether through service of notice, attachment prior to judgment, attachment in aid of execution, execution or otherwise) with respect to itself or its property, the Company hereby irrevocably waives such immunity in respect of its obligations under this Indenture and the Securities, to the extent permitted by law.
The Company irrevocably and unconditionally waives, to the fullest extent permitted by law, any objection that it may now or hereafter have to the laying of venue of any such action, suit or proceeding in any such court or any appellate court with respect thereto. The Company irrevocably waives, to the fullest extent permitted by law, the defense of an inconvenient forum to the maintenance of any such action, suit or proceeding in any such court.
SECTION 1.15 Conversion of Judgment Currency.
(a) The Company covenants and agrees that the following provisions shall apply to conversion of Currency in the case of the Securities and this Indenture, to the fullest extent permitted by applicable law:
(i) If for the purposes of obtaining judgment in, or enforcing the judgment of, any court in any country, it becomes necessary to convert into a Currency (the "Judgment Currency") an amount due or contingently due in any other Currency under the Securities of any series and this Indenture (the "Base Currency"), then the conversion shall be made at the rate of exchange prevailing on the Business Day before the day on which the final judgment is given or the order of enforcement is made, as the case may be (unless a court shall otherwise determine).
(ii) If there is a change in the rate of exchange prevailing between the Business Day before the day on which the judgment referred to in (i) above is given or an order of enforcement is made, as the case may be (or such other date as a court shall determine), and the date of receipt of the amount due, the Company shall pay such additional (or, as the case may be, such lesser) amount, if any, as may be necessary so that the amount paid in the Judgment Currency when converted at the rate of exchange prevailing on the date of receipt will produce the amount in the Base Currency originally due.
(b) In the event of the winding-up of the Company at any time while any amount or damages owing under the Securities and this Indenture, or any judgment or order rendered in respect thereof, shall remain outstanding, the Company shall indemnify and hold the Holders and the Trustees harmless against any deficiency arising or resulting from any variation in rates of exchange between (1) the date as of which the equivalent of the amount in the Base Currency due or contingently due under the Securities and this Indenture (other than under this Subsection (b)) is calculated for the purposes of such winding-up and (2) the final date for the filing of proofs of claim in such winding-up. For the purpose of this Subsection (b) the final date for the filing of proofs of claim in the winding-up of the Company shall be the date fixed by the liquidator or otherwise in accordance with the relevant provisions of applicable law as being the latest practicable date as at which liabilities of the Company may be ascertained for such winding-up prior to payment by the liquidator or otherwise in respect thereto.
(c) The obligations contained in Subsections (a)(ii) and (b) of this Section 1.15 shall constitute separate and independent obligations of the Company from its other obligations under the Securities and this Indenture, shall give rise to separate and independent causes of action against the Company, shall apply irrespective of any waiver or extension granted by any Holder or the Trustees from time to time and shall continue in full force and effect notwithstanding any judgment or order or the filing of any proof of claim in the winding up of the Company for a liquidated sum in respect of amounts due hereunder (other than under Subsection (b) above) or under any such judgment or order. Any such deficiency as aforesaid shall be deemed to constitute a loss suffered by the Holders or the Trustees, as the case may be, and no proof or evidence of any actual loss shall be required by the Company or its liquidator. In the case of Subsection (b) above, the amount of such deficiency shall not be deemed to be increased or reduced by any variation in rates of exchange occurring between the said final date and the date of any liquidating distribution.
The term "rate(s) of exchange" shall mean the rate of exchange quoted by a Canadian chartered bank as may be designated in writing by the Company to the Trustees from time to time, at its central foreign exchange desk in its main office in Toronto at 12:00 noon (Toronto time) on the relevant date for purchases of the Base Currency with the Judgment Currency and includes any premiums and costs of exchange payable. The Trustees shall have no duty or liability with respect to monitoring or enforcing this Section 1.15.
SECTION 1.16 Currency Equivalent.
Except as otherwise provided in this Indenture, for purposes of the construction of the terms of this Indenture or of the Securities, in the event that any amount is stated herein in the Currency of one nation (the "First Currency"), as of any date such amount shall also be deemed to represent the amount in the Currency of any other relevant nation which is required to purchase such amount in the First Currency at the Bank of Canada daily average exchange rate as reported by Telerate on screen 3194 (or such other means of reporting the Bank of Canada daily average exchange rate as may be agreed upon by each of the parties to this Indenture) on the date of determination.
SECTION 1.17 Conflict with Trust Indenture Legislation.
If and to the extent that any provision of this Indenture limits, qualifies or conflicts with any mandatory requirement of Trust Indenture Legislation, such mandatory requirement shall control. If and to the extent that any provision hereof modifies or excludes any provision of Trust Indenture Legislation that may be so modified or excluded, the latter provision shall be deemed to apply hereof as so modified or to be excluded, as the case may be.
SECTION 1.18 Incorporators, Shareholders, Officers and Directors of the Company Exempt from Individual Liability.
No recourse under or upon any obligation, covenant or agreement contained in this Indenture, or in any Security, or because of any indebtedness evidenced thereby, shall be had against any incorporator, as such, or against any past, present or future shareholder, officer or director, as such, of the Company or of any successor, either directly or through the Company or any successor, under any rule of law, statute or constitutional provision or by the enforcement of any assessment or by any legal or equitable proceeding or otherwise, all such liability being expressly waived and released by the acceptance of the Securities by the Holders and as part of the consideration for the issue of the Securities.
SECTION 1.19 Waiver of Jury Trial.
Each of the Company and the Trustees hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any legal proceeding arising out of or relating to this Indenture, the Securities or the transactions contemplated hereby.
SECTION 1.20 Counterparts.
This Indenture may be executed in any number of counterparts (either by facsimile or by original manual signature), each of which so executed shall be deemed to be an original, but all such counterparts shall together constitute but one and the same Indenture.
SECTION 1.21 Force Majeure.
Except for the payment obligations of the Company contained herein, neither the Company nor the Trustees shall be liable to each other, or held in breach of this Indenture, if prevented, hindered, or delayed in the performance or observance of any provision contained herein by reason of act of God, riots, terrorism, acts of war, epidemics, governmental action or judicial order, earthquakes, or any other similar causes (including, but not limited to, mechanical, electronic or communication interruptions, disruptions or failures). Performance times under this Indenture shall be extended for a period of time equivalent to the time lost because of any delay that is excusable under this Section 1.21.
ARTICLE TWO
SECURITIES FORMS
SECTION 2.01 Forms Generally.
The Securities of each series shall be in substantially the forms as shall be established by or pursuant to a Board Resolution or in one or more indentures supplemental hereto, in each case with such appropriate insertions, omissions, substitutions and other variations as are required or permitted by this Indenture, and may have such letters, numbers or other marks of identification and such legends or endorsements placed thereon as may be required to comply with the rules of any securities exchange or as may, consistently herewith, be determined by the Officer executing such Securities , as evidenced by the execution of such Securities by such Officer. If the forms of Securities of any series are established by action taken pursuant to a Board Resolution, a copy of an appropriate record of such action shall be certified by the Corporate Secretary or an Assistant Secretary of the Company and delivered to the Trustees at or prior to the delivery of the Company Order contemplated by Section 3.03 for the authentication and delivery of such Securities. Any portion of the text of any Security may be set forth on the reverse thereof, with an appropriate reference thereto on the face of the Security.
Either Trustee's certificate of authentication shall be in substantially the form set forth in this Article Two.
SECTION 2.02 Form of Trustee's Certificate of Authentication.
Subject to Section 6.12, either Trustee's certificate of authentication shall be in substantially the following form:
TRUSTEE'S CERTIFICATE OF AUTHENTICATION
(Certificate of Authentication may be executed by either Trustee)
Dated: ____________
_______________________, as U.S. Trustee, certifies that this is one of the Securities of the series designated therein referred to in the within-mentioned Indenture.
| |
, |
| |
as U.S. Trustee |
| |
|
|
| |
By: |
|
| |
|
Authorized Officer |
OR
Dated: ____________
____________________, as Canadian Trustee, certifies that this is one of the Securities of the series designated therein referred to in the within-mentioned Indenture.
| |
, |
| |
as Canadian Trustee |
| |
|
|
| |
By: |
|
| |
|
Authorized Officer |
SECTION 2.03 Securities Issuable in Global Form.
If Securities of or within a series are issuable in global form, as specified and contemplated by Section 3.01, then any such Security shall represent such of the Outstanding Securities of such series as shall be specified therein and may provide that it shall represent the aggregate amount of Outstanding Securities of such series from time to time endorsed thereon and that the aggregate amount of Outstanding Securities of such series represented thereby may from time to time be increased or decreased to reflect exchanges. Any endorsement of a Security in global form to reflect the amount, or any increase or decrease in the amount, of Outstanding Securities represented thereby shall be made by the Trustees in such manner and upon instructions given by the Holder or its nominee as shall be specified therein or in the Company Order to be delivered to the Trustees pursuant to Section 3.03 or 3.04. Subject to the provisions of Sections 3.03 and 3.04 (if applicable), the Trustees shall deliver and redeliver any Security in global form in the manner and upon instructions given by the Holder or its nominee as shall be specified therein or in the applicable Company Order. If a Company Order pursuant to Section 3.03 or Section 3.04 has been, or simultaneously is, delivered, any instructions by the Company with respect to endorsement or delivery or redelivery of a Security in global form shall be in writing but need not comply with Section 1.03 and need not be accompanied by an Opinion of Counsel.
Notwithstanding the provisions of Section 3.07, unless otherwise specified as contemplated by Section 3.01, payment of principal of, premium (if any) and interest (if any) on any Security in permanent global form shall be made to the Holder or its nominee specified therein.
Notwithstanding Section 3.09 and except as provided in the preceding paragraph, the Company, the Trustees and any agent of the Company and the Trustees shall treat as the Holder of such principal amount of Outstanding Securities represented by a permanent global Security, the Holder of such permanent global Security.
ARTICLE THREE
THE SECURITIES
SECTION 3.01 Issuable in Series.
The aggregate principal amount of Securities which may be authenticated and delivered under this Indenture is unlimited.
The Securities may be issued in one or more series and may be denominated and payable in Dollars or any Foreign Currency. There shall be established in one or more Board Resolutions or pursuant to authority granted by one or more Board Resolutions and set forth in, or determined in the manner provided in, an Officer's Certificate, or established in one or more indentures supplemental hereto, prior to the issuance of Securities of any series, any or all of the following, as applicable:
(1) the title of the Securities of the series (which shall distinguish the Securities of such series from the Securities of all other series);
(2) the aggregate principal amount of the Securities of the series and any limit upon the aggregate principal amount of the Securities of the series that may be authenticated and delivered under this Indenture (except for Securities authenticated and delivered upon registration of transfer (including any restriction or condition on the transferability of the Securities of such series) of, or in exchange for, or in lieu of, other Securities of the series pursuant to Section 3.04, 3.05, 3.06, 9.06, 11.07 or 13.05) and, in the event that no limit upon the aggregate principal amount of the Securities of that series is specified, the Company shall have the right, subject to any terms, conditions or other provisions specified pursuant to this Section 3.01 with respect to the Securities of such series, to re-open such series for the issuance of additional Securities of such series from time to time;
(3) the extent and manner, if any, to which payment on or in respect of the Securities of the series will be senior or will be subordinated to the prior payment of other liabilities and obligations of the Company, and whether the payment of principal, premium (if any) and interest (if any) will be guaranteed by any other Person;
(4) the percentage or percentages of principal amount at which the Securities of the series will be issued;
(5) the date or dates, or the method by which such date or dates will be determined or extended, on which the Securities of the series may be issued and the date or dates, or the method by which such date or dates will be determined or extended, on which the principal of and premium (if any) on the Securities of the series is payable;
(6) the rate or rates at which the Securities of the series shall bear interest, whether fixed or variable (if any), or the method by which such rate or rates shall be determined, whether such interest shall be payable in cash or additional Securities of the same series or shall accrue and increase the aggregate principal amount outstanding of such series, the date or dates from which such interest shall accrue, or the method by which such date or dates shall be determined, the Interest Payment Dates on which such interest shall be payable and the Regular Record Date, if any, for the interest payable on any Security on any Interest Payment Date, or the method by which such date or dates shall be determined, and the basis upon which interest shall be calculated if other than on the basis of a 360-day year of twelve 30-day months;
(7) the place or places, if any, other than or in addition to the Borough of Manhattan, The City of New York, where the principal of, premium (if any) and interest (if any) on Securities of the series shall be payable, where any Securities of the series may be surrendered for registration of transfer, where Securities of the series may be surrendered for exchange, where Securities of the series that are convertible or exchangeable may be surrendered for conversion or exchange, as applicable and, if different than the location specified in Section 1.06, the place or places where notices or demands to or upon the Company in respect of the Securities of the series and this Indenture may be served;
(8) the period or periods within which, the date or dates on which, the price or prices at which, the Currency in which, and other terms and conditions upon which Securities of the series may be redeemed, in whole or in part, at the option of the Company, if the Company is to have that option;
(9) the obligation, if any, of the Company to redeem, repay or purchase Securities of the series pursuant to any sinking fund, amortization or analogous provisions or at the option of a Holder thereof, and the period or periods within which, the price or prices at which, the Currency in which, and other terms and conditions upon which Securities of the series shall be redeemed, repaid or purchased, in whole or in part, pursuant to such obligation;
(10) if other than denominations of $1,000 and any integral multiple thereof, the denomination or denominations in which any Securities of the series shall be issuable;
(11) the identity of each Security Registrar and/or Paying Agent;
(12) if other than the principal amount thereof, the portion of the principal amount of Securities of the series that shall be payable upon declaration of acceleration of the Maturity thereof pursuant to Section 5.02 or the method by which such portion shall be determined;
(13) if other than Dollars, the Foreign Currency in which payment of the principal of, premium (if any) or interest (if any) on the Securities of the series shall be payable or in which the Securities of the series shall be denominated and the particular provisions applicable thereto in accordance with, in addition to or in lieu of any of the provisions of Section 3.12;
(14) whether the amount of payments of principal of, premium (if any) or interest (if any) on the Securities of the series may be determined with reference to an index, formula or other method (which index, formula or method may be based, without limitation, on one or more Currencies, commodities, equity indices or other indices), and the manner in which such amounts shall be determined;
(15) whether the principal of, premium (if any) or interest (if any) on the Securities of the series are to be payable, at the election of the Company or a Holder thereof, in a Currency other than that in which such Securities are denominated or stated to be payable, the period or periods within which (including the Election Date), and the terms and conditions upon which, such election may be made, and the time and manner of determining the exchange rate between the Currency in which such Securities are denominated or stated to be payable and the Currency in which such Securities are to be so payable, in each case in accordance with, in addition to or in lieu of any of the provisions of Section 3.12;
(16) the designation of the initial Exchange Rate Agent, if any;
(17) the applicability, if any, of Sections 14.02 and/or 14.03 to the Securities of the series and any provisions in modification of, in addition to or in lieu of any of the provisions of Article Fourteen that shall be applicable to the Securities of the series;
(18) provisions, if any, granting special rights to the Holders of Securities of the series upon the occurrence of such events as may be specified;
(19) any deletions from, modifications of or additions to the Events of Default or covenants (including any deletions from, modifications of or additions to Section 10.09) of the Company with respect to Securities of the series, whether or not such Events of Default or covenants are consistent with the Events of Default or covenants set forth herein;
(20) any restrictions applicable to the offer, sale or delivery of Securities of the series, whether any Securities of the series are to be issuable initially in temporary global form and whether any Securities of the series are to be issuable in permanent global form and, if so, whether beneficial owners of interests in any such permanent global Security may exchange such interests for Securities of such series and of like tenor of any authorized form and denomination and the circumstances under which any such exchanges may occur, if other than in the manner provided in Section 3.05, and the circumstances under which and the place or places where any such exchanges may be made and, if Securities of the series are to be issuable in global form, the designation of any Depositary therefor;
(21) the date as of which any temporary global Security of the series shall be dated if other than the date of original issuance of the first Security of the series to be issued;
(22) the Person to whom any interest on any Security of the series shall be payable, if other than the Person in whose name that Security (or one or more Predecessor Securities) is registered at the close of business on the Regular Record Date for such interest, and the extent to which, or the manner in which, any interest payable on a temporary global Security on an Interest Payment Date will be paid if other than in the manner provided in Section 3.04;
(23) if Securities of the series are to be issuable in definitive form (whether upon original issue or upon exchange of a temporary Security of such series) only upon receipt of certain certificates or other documents or satisfaction of other conditions, the form and/or terms of such certificates, documents or conditions;
(24) if the Securities of the series are to be issued upon the exercise of warrants or subscription receipts, the time, manner and place for such Securities to be authenticated and delivered;
(25) if the Securities of the series are to be convertible into or exchangeable for any securities or property of any Person (including the Company), the terms and conditions upon which such Securities will be so convertible or exchangeable, and any additions or changes to permit or facilitate such conversion or exchange;
(26) provisions as to modification, amendment or variation of any rights or terms attaching to the Securities;
(27) whether the Securities will be secured or unsecured and the nature and priority of any security; and
(28) any other terms, conditions, rights and preferences (or limitations on such rights and preferences) relating to the series (which terms shall not be inconsistent with the requirements of Trust Indenture Legislation or the provisions of this Indenture).
All Securities of any one series shall be substantially identical except as to denomination and except as may otherwise be provided in or pursuant to such Board Resolution (subject to Section 3.03) and set forth in such Officer's Certificate or in any such indenture supplemental hereto. Not all Securities of any one series need be issued at the same time, and, unless otherwise provided, a series may be reopened for issuances of additional Securities of such series.
If any of the terms of the series are established by action taken pursuant to one or more Board Resolutions, such Board Resolutions shall be delivered to the Trustees at or prior to the delivery of the Officer's Certificate setting forth the terms of the series.
SECTION 3.02 Denominations.
The Securities of each series shall be issuable in such denominations as shall be specified as contemplated by Section 3.01. With respect to Securities of any series denominated in Dollars, in the absence of any such provisions, the Securities of such series, other than Securities issued in global form (which may be of any denomination), shall be issuable in denominations of $1,000 and any integral multiple thereof.
SECTION 3.03 Execution, Authentication, Delivery and Dating.
The Securities shall be executed on behalf of the Company by an Officer. The signature of an Officer on the Securities may be the manual or facsimile signatures of the present or any future such authorized officer and may be imprinted or otherwise reproduced on the Securities.
Securities bearing the manual or facsimile signatures of individuals who were at any time the proper officers of the Company shall bind the Company, notwithstanding that such individuals or any of them have ceased to hold such offices prior to the authentication and delivery of such Securities or did not hold such offices at the date of such Securities.
At any time and from time to time after the execution and delivery of this Indenture, the Company may deliver Securities of any series, executed by the Company to the applicable Trustee for authentication, together with a Company Order for the authentication and delivery of such Securities, and the applicable Trustee in accordance with such Company Order shall authenticate and deliver such Securities. If not all the Securities of any series are to be issued at one time and if the Board Resolution or supplemental indenture establishing such series shall so permit, such Company Order may set forth procedures acceptable to the Trustees for the issuance of such Securities and determining terms of particular Securities of such series such as interest rate, Stated Maturity, date of issuance and date from which interest shall accrue.
In authenticating such Securities, and accepting the additional responsibilities under this Indenture in relation to such Securities, the Trustees shall be entitled to receive, and (subject to Trust Indenture Legislation, including TIA Sections 315(a) through 315(d)) shall be fully protected in relying upon, an Opinion of Counsel stating:
(a) that the form or forms of such Securities have been established in conformity with the provisions of this Indenture;
(b) that the terms of such Securities have been established in conformity with the provisions of this Indenture;
(c) that such Securities, when completed by appropriate insertions and executed and delivered by the Company to the applicable Trustee for authentication in accordance with this Indenture, authenticated and delivered by the applicable Trustee in accordance with this Indenture and issued by the Company in the manner and subject to any conditions specified in such Opinion of Counsel, will constitute the legal, valid and binding obligations of the Company, enforceable in accordance with their terms;
(d) the execution and delivery by the Company of such Securities and any supplemental indenture will not contravene the articles of incorporation or continuance, or such other constating documents then in effect, if any, or the by-laws of the Company, or violate applicable laws; and
(e) that the Company has the corporate power to issue such Securities, and has duly taken all necessary corporate action with respect to such issuance.
Notwithstanding the provisions of Section 3.01 and of the preceding two paragraphs, if not all the Securities of any series are to be issued at one time, it shall not be necessary to deliver the Officer's Certificate otherwise required pursuant to Section 3.01 or the Company Order and Opinion of Counsel otherwise required pursuant to the preceding two paragraphs prior to or at the time of issuance of each Security, if such documents are delivered prior to or at the time of issuance of the first Security of such series and with respect to all Securities of such series.
The Trustees shall not be required to authenticate and deliver any such Securities if the issue of such Securities pursuant to this Indenture will affect the Trustees' own rights, duties or immunities under the Securities and this Indenture or otherwise in a manner which is not reasonably acceptable to the Trustees.
Each Security shall be dated the date of its authentication.
No Security shall entitle a Holder to any benefit under this Indenture or be valid or obligatory for any purpose unless there appears on such Security a certificate of authentication substantially in the form provided for herein duly executed by the applicable Trustee by manual signature of an authorized officer thereof, and such certificate upon any Security shall be conclusive evidence, and the only evidence, that such Security has been duly authenticated and delivered hereunder and is entitled to the benefits of this Indenture. Notwithstanding the foregoing, if any Security shall have been authenticated and delivered hereunder but never issued and sold by the Company, and the Company shall deliver such Security to the Trustees for cancellation as provided in Section 3.10 together with a written statement (which need not comply with Section 1.03 and need not be accompanied by an Opinion of Counsel) stating that such Security has never been issued and sold by the Company, for all purposes of this Indenture such Security shall be deemed never to have been authenticated and delivered hereunder and shall never entitle a Holder to the benefits of this Indenture.
SECTION 3.04 Temporary Securities.
Pending the preparation of definitive Securities of any series, the Company may execute, and upon Company Order the applicable Trustee shall authenticate and deliver, temporary Securities which are printed, lithographed, typewritten, mimeographed or otherwise produced, in any authorized denomination, substantially of the tenor of the definitive Securities in lieu of which they are issued, in registered form, and with such appropriate insertions, omissions, substitutions and other variations as the Officer executing such Securities may determine, as conclusively evidenced by their execution of such Securities. Such temporary Securities may be in global form.
Except in the case of temporary Securities in global form (which shall be exchanged in accordance with the provisions of the following paragraphs), if temporary Securities of any series are issued, the Company will cause definitive Securities of that series to be prepared without unreasonable delay. After the preparation of definitive Securities of such series, the temporary Securities of such series shall be exchangeable for definitive Securities of such series upon surrender of the temporary Securities of such series at the office or agency of the Company in a Place of Payment for that series, without charge to the Holder. Upon surrender for cancellation of any one or more temporary Securities of any series, the Company shall execute and the applicable Trustee shall authenticate and deliver in exchange therefor a like principal amount of definitive Securities of the same series of authorized denominations and of like tenor and evidencing the same indebtedness. Until so exchanged the temporary Securities of any series shall in all respects be entitled to the same benefits under this Indenture as definitive Securities of such series.
If temporary Securities of any series are issued in global form, any such temporary global Security shall, unless otherwise provided therein, be delivered to the office of the Depositary for credit to the respective accounts of the beneficial owners of such Securities (or to such other accounts as they may direct).
Without unnecessary delay, but in any event not later than the date specified in, or determined pursuant to the terms of, any such temporary global Security (the "Exchange Date"), the Company shall deliver to the Trustees definitive Securities, in aggregate principal amount equal to the principal amount of such temporary global Security and of like tenor and evidencing the same indebtedness, executed by the Company. On or after the Exchange Date, such temporary global Security shall be surrendered by the Depositary to the Trustees, as the Company's agent for such purpose, to be exchanged, in whole or from time to time in part, for definitive Securities without charge and the applicable Trustee shall authenticate and deliver, in exchange for each portion of such temporary global Security, an equal aggregate principal amount of definitive Securities of the same series of authorized denominations and of like tenor and evidencing the same indebtedness as the portion of such temporary global Security to be exchanged. The definitive Securities to be delivered in exchange for any such temporary global Security shall be in registered form or permanent global registered form, or any combination thereof, as specified as contemplated by Section 3.01, and, if any combination thereof is so specified, as requested by the beneficial owner thereof; provided, however, that, unless otherwise specified in such temporary global Security, upon such presentation by the Depositary, such temporary global Security is accompanied by a certificate dated the Exchange Date or a subsequent date and signed by the Depositary as to the portion of such temporary global Security held for its account then to be exchanged and a certificate dated the Exchange Date or a subsequent date, each in the form set forth in Exhibit A-2 to this Indenture (or in such other form as may be established pursuant to Section 3.01).
Unless otherwise specified in such temporary global Security, the interest of a beneficial owner of Securities of a series in a temporary global Security shall be exchanged for definitive Securities of the same series and of like tenor and evidencing the same indebtedness following the Exchange Date when the account holder instructs the Depositary to request such exchange on his behalf and delivers to the Depositary a certificate in the form set forth in Exhibit A-1 to this Indenture (or in such other form as may be established pursuant to Section 3.01), dated no earlier than 15 days prior to the Exchange Date, copies of which certificate shall be available from the offices of the Depositary, the Trustees, any Authenticating Agent appointed for such series of Securities and each Paying Agent. Unless otherwise specified in such temporary global Security, any such exchange shall be made free of charge to the beneficial owners of such temporary global Security, except that a Person receiving definitive Securities must bear the cost of insurance, postage, transportation and the like in the event that such Person does not take delivery of such definitive Securities in person at the offices of the Depositary.
Until exchanged in full as hereinabove provided, the temporary Securities of any series shall in all respects be entitled to the same benefits under this Indenture as definitive Securities of the same series and of like tenor and evidencing the same indebtedness authenticated and delivered hereunder, except that, unless otherwise specified as contemplated by Section 3.01, interest payable on a temporary global Security on an Interest Payment Date for Securities of such series occurring prior to the applicable Exchange Date shall be payable to the Depositary on such Interest Payment Date upon delivery by the Depositary to the Trustees of a certificate or certificates in the form set forth in Exhibit A-2 to this Indenture (or in such other form as may be established pursuant to Section 3.01), for credit without further interest thereon on or after such Interest Payment Date to the respective accounts of the Persons who are the beneficial owners of such temporary global Security on such Interest Payment Date and who have each delivered to the Depositary a certificate dated no earlier than 15 days prior to the Interest Payment Date occurring prior to such Exchange Date in the form set forth in Exhibit A-1 to this Indenture (or in such other form as may be established pursuant to Section 3.01). Notwithstanding anything to the contrary herein contained, the certifications made pursuant to this paragraph shall satisfy the certification requirements of the preceding two paragraphs of this Section 3.04 and of the third paragraph of Section 3.03 and the interests of the Persons who are the beneficial owners of the temporary global Security with respect to which such certification was made will be exchanged for definitive Securities of the same series and of like tenor and evidencing the same indebtedness on the Exchange Date or the date of certification if such date occurs after the Exchange Date, without further act or deed by such beneficial owners. Except as otherwise provided in this paragraph, no payments of principal of, premium (if any) or interest (if any) owing with respect to a beneficial interest in a temporary global Security will be made unless and until such interest in such temporary global Security shall have been exchanged for an interest in a definitive Security. Any interest so received by the Depositary and not paid as herein provided shall be returned to the Trustees immediately prior to the expiration of two years after such Interest Payment Date in order to be repaid to the Company in accordance with Section 10.03.
SECTION 3.05 Registration, Registration of Transfer and Exchange.
So long as required by Trust Indenture Legislation, the Company shall cause to be kept at the Corporate Trust Offices of the applicable Trustee a register for each series of Securities (the registers maintained in the Corporate Trust Offices of the Trustees and in any other office or agency of the Company in a Place of Payment being herein sometimes collectively referred to as the "Security Register") in which, subject to such reasonable regulations as it may prescribe, the Company shall provide for the registration of the Holders of Securities and of transfers of Securities. The Security Register shall be in written form or any other form capable of being converted into written form within a reasonable time. At all reasonable times, the Security Register shall be open to inspection by the Trustees. The Trustees are hereby initially appointed as security registrar (the "Security Registrar") for the purpose of registering Securities and transfers of Securities as herein provided. The Company shall have the right to remove and replace from time to time the Security Registrar for any series of Securities; provided, however, that, no such removal or replacement shall be effective until a successor Security Registrar with respect to such series of Securities shall have been appointed by the Company and shall have accepted such appointment by the Company. In the event that the Trustees shall not be or shall cease to be the Securities Registrar with respect to a series of Securities, they shall have the right to examine the Security Register for such series at all reasonable times. There shall be only one Securities Register for such series of Securities.
Upon surrender for registration of transfer of any Security of any series at the office or agency in a Place of Payment for that series, the Company shall execute, and the applicable Trustee shall authenticate and deliver, in the name of the designated transferee, one or more new Securities of the same series, of any authorized denominations and of a like aggregate principal amount and tenor and evidencing the same indebtedness.
For Canadian Securities, the Security must be duly endorsed for transfer or in a duly endorsed transferable form as applicable and must comply with the current industry practice in accordance with the Securities Transfer Association of Canada.
At the option of the Holder, Securities of any series may be exchanged for other Securities of the same series, of any authorized denomination and of a like aggregate principal amount and tenor and evidencing the same indebtedness, upon surrender of the Securities to be exchanged at such office or agency. Whenever any Securities are so surrendered for exchange, the Company shall execute, and the applicable Trustee shall authenticate and deliver, the Securities which the Holder making the exchange is entitled to receive.
Whenever any Securities are so surrendered for exchange, the Company shall execute, and the applicable Trustee shall authenticate and deliver, the Securities which the Holder making the exchange is entitled to receive.
Notwithstanding the foregoing, except as otherwise specified as contemplated by Section 3.01, any permanent global Security shall be exchangeable only as provided in this Section. If any beneficial owner of an interest in a permanent global Security is entitled to exchange such interest for Securities of such series and of like tenor and principal amount of another authorized form and denomination, as contemplated by Section 3.01 and provided that any applicable notice provided in the permanent global Security shall have been given to the Company, the Trustees and the Depositary, then without unnecessary delay but in any event not later than the earliest date on which such interest may be so exchanged, the Company shall deliver to the applicable Trustee definitive Securities in aggregate principal amount equal to the principal amount of such beneficial owner's interest in such permanent global Security, executed by the Company. On or after the earliest date on which such interests may be so exchanged, such permanent global Security shall be surrendered by the Depositary or such other depositary as shall be specified in the Company Order with respect thereto to the applicable Trustee, as the Company's agent for such purpose, to be exchanged in whole or from time to time in part, for definitive Securities without charge, and the applicable Trustee shall authenticate and deliver, in exchange for each portion of such permanent global Security, an equal aggregate principal amount of definitive Securities of the same series of authorized denominations and of like tenor as the portion of such permanent global Security to be exchanged. If a Security is issued in exchange for any portion of a permanent global Security after the close of business at the office or agency where such exchange occurs on (i) any Regular Record Date and before the opening of business at such office or agency on the relevant Interest Payment Date, or (ii) any Special Record Date and before the opening of business at such office or agency on the related proposed date for payment of Defaulted Interest, interest or Defaulted Interest, as the case may be, will not be payable on such Interest Payment Date or proposed date for payment, as the case may be, in respect of such Security, but will be payable on such Interest Payment Date or proposed date for payment, as the case may be, only to the Person to whom interest in respect of such portion of such permanent global Security is payable in accordance with the provisions of this Indenture.
Transfers of global Securities shall be limited to transfers in whole, but not in part, to the Depositary, its successors or their respective nominees. If at any time the Depositary for Securities of a series notifies the Company that it is unwilling, unable or no longer qualifies to continue as Depositary for Securities of such series or if at any time the Depositary for such series shall no longer be registered or in good standing under the Exchange Act, or other applicable statute or regulation, the Company shall appoint a successor Depositary for the Securities of such series. If a successor to the Depositary for Securities of such series is not appointed by the Company within 90 days after the Company receives such notice or becomes aware of such condition, as the case may be, the Company's election pursuant to Section 3.01 shall no longer be effective with respect to the Securities for such series and the Company will execute, and the applicable Trustee, upon receipt of a Company Order for the authentication and delivery of definitive Securities of such series, will authenticate and deliver Securities of such series in definitive form, in authorized denominations, and in an aggregate principal amount equal to the principal amount of the global Security or Securities representing such series and evidencing the same indebtedness in exchange for such global Security or Securities.
The Company may at any time and in its sole discretion determine that the Securities of any series issued in the form of one or more global Securities shall no longer be represented by such global Security or Securities. In such event the Company will execute, and the applicable Trustee, upon receipt of a Company Order for the authentication and delivery of definitive Securities of such series, will authenticate and deliver Securities of such series in definitive form, in authorized denominations, and in an aggregate principal amount equal to the principal amount of the global Security or Securities representing such series and evidencing the same indebtedness in exchange for such global Security or Securities.
Upon the exchange of a global Security for Securities in definitive form, such global Security shall be cancelled by the applicable Trustee. Securities issued in exchange for a global Security pursuant to this Section 3.05 shall be registered in such names and in such authorized denominations as the Depositary for such global Security, pursuant to instructions from its direct or indirect participants or otherwise, shall instruct the applicable Trustee in writing. The applicable Trustee shall deliver such Securities to the Persons in whose names such Securities are so registered.
All Securities issued upon any registration of transfer or exchange of Securities shall be the valid obligations of the Company, evidencing the same debt, and entitled to the same benefits under this Indenture, as the Securities surrendered upon such registration of transfer or exchange.
Every Security presented or surrendered for registration of transfer or for exchange shall (if so required by the Company or the Security Registrar or applicable securities transfer industry practices) be duly endorsed, or be accompanied by a written instrument of transfer, in form satisfactory to the Company and the Security Registrar, duly executed by the Holder thereof or his attorney duly authorized in writing.
Any registration of transfer or exchange of Securities may be subject to service charges by the Securities Registrar and the Company may require payment of a sum sufficient to cover any tax or other governmental charge that may be imposed in connection with any registration of transfer or exchange of Securities, other than exchanges pursuant to Section 3.04, 9.06, 11.07 or 13.05 not involving any transfer.
The Company shall not be required (i) to issue, register the transfer of or exchange Securities of any series in definitive form during a period beginning at the opening of business 15 days before the day of the selection for redemption of Securities of that series under Section 11.03 or 12.03 and ending at the close of business on the day of the mailing of the relevant notice of redemption or (ii) to register the transfer of or exchange any Security in definitive form so selected for redemption in whole or in part, except the unredeemed portion of any Security being redeemed in part, or (iii) to issue, register the transfer of or exchange any Security in definitive form which has been surrendered for repayment at the option of the Holder, except the portion, if any, of such Security not to be so repaid.
SECTION 3.06 Mutilated, Destroyed, Lost and Stolen Securities.
If any mutilated Security is surrendered to the applicable Trustee, the Company shall execute and the applicable Trustee shall authenticate and deliver in exchange therefor a new Security of the same series and of like tenor and principal amount and evidencing the same indebtedness and bearing a number not contemporaneously outstanding, or, in case any such mutilated Security has become or is about to become due and payable, the Company in its discretion may, instead of issuing a new Security, , pay such Security. If there shall be delivered to the Company and to the Trustees (i) evidence to their satisfaction of the destruction, loss or theft of any Security and (ii) such security (or surety in the case of the Canadian Trustee) or indemnity as may be required by them to save each of them and any agent of either of them harmless, then, in the absence of notice to the Company or the Trustees that such Security has been acquired by a bona fide purchaser , the Company shall execute and upon Company Order the applicable Trustee shall authenticate and deliver, in lieu of any such destroyed, lost or stolen Security, a new Security of the same series and of like tenor and principal amount and evidencing the same indebtedness and bearing a number not contemporaneously outstanding.
Notwithstanding the provisions of the previous two paragraphs, in case any such mutilated, destroyed, lost or stolen Security has become or is about to become due and payable, the Company in its discretion may, instead of issuing a new Security appertaining to such mutilated, destroyed, lost or stolen Security, pay such Security.
Upon the issuance of any new Security under this Section 3.06, the Company may require the payment of a sum sufficient to cover any tax or other governmental charge that may be imposed in relation thereto and any other expenses (including the fees and expenses of the Trustees) connected therewith.
Every new Security of any series issued pursuant to this Section 3.06 in lieu of any mutilated, destroyed, lost or stolen Security, shall constitute an original additional contractual obligation of the Company, whether or not the mutilated, destroyed, lost or stolen Security shall be at any time enforceable by anyone, and the Holders of such Security shall be entitled to all the benefits of this Indenture equally and proportionately with the Holders of any and all other Securities of that series duly issued hereunder.
The provisions of this Section 3.06 as amended or supplemented pursuant to this Indenture with respect to a particular series of Securities or generally are exclusive and shall preclude (to the extent lawful) all other rights and remedies with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities.
SECTION 3.07 Payment of Principal, Premium and Interest; Interest Rights Preserved; Optional Interest Reset.
(a) Unless otherwise provided as contemplated by Section 3.01 with respect to any series of Securities, interest (if any) on any Security which is payable, and is punctually paid or duly provided for, on any Interest Payment Date shall be paid by the Paying Agent to the Person in whose name such Security (or one or more Predecessor Securities) is registered at the close of business on the Regular Record Date for such interest at the office or agency of the Company maintained for such purpose pursuant to Section 10.02; provided, however, that each installment of interest (if any) on any Security may at the Company's option be paid by (i) mailing a check for such interest, payable to or upon the written order of the Person entitled thereto pursuant to Section 3.09, to the address of such Person as it appears on the Security Register, (ii) wire transfer to an account located in the United States maintained by the Person entitled to such payment as specified in the Security Register, or (iii) as otherwise specified pursuant to Section 3.01 for the Securities of such series. Unless otherwise provided as contemplated by Section 3.01 with respect to any series of Securities, principal and premium (if any) paid in relation to any Security shall be paid to the Holder of such Security only upon presentation and surrender of such Security at the office or agency of the Company maintained for such purpose pursuant to Section 10.02.
Unless otherwise provided as contemplated by Section 3.01, every permanent global Security will provide that interest (if any) payable on any Interest Payment Date will be paid to the Depositary with respect to that portion of such permanent global Security held for its account by the Depositary, for the purpose of permitting the Depositary to credit the interest (if any) received by it in respect of such permanent global Security to the accounts of the beneficial owners thereof.
Any interest on any Security of any series which is payable, but is not punctually paid or duly provided for, on any Interest Payment Date shall forthwith cease to be payable to the Holder on the relevant Regular Record Date by virtue of having been such Holder, and such defaulted interest and, if applicable, interest on such defaulted interest (to the extent lawful) at the rate specified in the Securities of such series (such defaulted interest and, if applicable, interest thereon herein collectively called "Defaulted Interest") must be paid by the Company as provided for in either clause (1) or (2), at the Company's election:
(1) The Company may elect to make payment of any Defaulted Interest to the Persons in whose names the Securities of such series (or their respective Predecessor Securities) are registered at the close of business on a Special Record Date for the payment of such Defaulted Interest, which shall be fixed in the following manner. The Company shall notify the Trustees in writing of the amount of Defaulted Interest proposed to be paid on each Security of such series and the date of the proposed payment, and at the same time the Company shall deposit with the applicable Trustee an amount of money in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)) equal to the aggregate amount proposed to be paid in respect of such Defaulted Interest or shall make arrangements satisfactory to the Trustees for such deposit on or prior to the date of the proposed payment, such money when deposited to be held in trust for the benefit of the Persons entitled to such Defaulted Interest as in this clause provided. Thereupon the Trustees shall fix a Special Record Date for the payment of such Defaulted Interest which shall be not more than 15 days and not less than 10 days prior to the date of the proposed payment and not less than 10 days after the receipt by the Trustees of the notice of the proposed payment. The Trustees shall promptly notify the Company of such Special Record Date and, in the name and at the expense of the Company, shall cause notice of the proposed payment of such Defaulted Interest and the Special Record Date therefor to be given in the manner provided in Section 1.07, not less than 10 days prior to such Special Record Date. Notice of the proposed payment of such Defaulted Interest and the Special Record Date therefor having been so given, such Defaulted Interest shall be paid to the Persons in whose name the Securities of such series (or their respective Predecessor Securities) are registered at the close of business on such Special Record Date and shall no longer be payable pursuant to the following clause (2).
(2) The Company may make payment of any Defaulted Interest on the Securities of any series in any other lawful manner not inconsistent with the requirements of any securities exchange on which such Securities may be listed, and, upon such notice as may be required by such exchange, if, after notice given by the Company to the Trustees of the proposed payment pursuant to this clause, such manner of payment shall be deemed practicable by the Trustees.
(b) The provisions of this Section 307(b) may be made applicable to any series of Securities pursuant to Section 3.01 (with such modifications, additions or substitutions as may be specified pursuant to such Section 3.01). The interest rate (or the spread or spread multiplier used to calculate such interest rate, if applicable) on any Security of such series may be reset by the Company on the date or dates specified on the face of such Security (each an "Optional Reset Date"). The Company may exercise such option with respect to such Security by notifying the Trustees of such exercise at least 50 but not more than 60 days prior to an Optional Reset Date for such Security. Not later than 40 days prior to each Optional Reset Date, the Trustees shall transmit, in the manner provided for in Section 1.07, to the Holder of any such Security a notice (the "Reset Notice") indicating whether the Company has elected to reset the interest rate (or the spread or spread multiplier used to calculate such interest rate, if applicable), and if so (i) such new interest rate (or such new spread or spread multiplier, if applicable) and (ii) the provisions, if any, for redemption during the period from such Optional Reset Date to the next Optional Reset Date or if there is no such next Optional Reset Date, to the Stated Maturity of such Security (each such period a "Subsequent Interest Period"), including the date or dates on which or the period or periods during which and the price or prices at which such redemption may occur during the Subsequent Interest Period.
Notwithstanding the foregoing, not later than 20 days prior to the Optional Reset Date, the Company may, at its option, revoke the interest rate (or the spread or spread multiplier used to calculate such interest rate, if applicable) provided for in the Reset Notice and establish an interest rate (or the spread or spread multiplier, if applicable) that is higher than the interest rate (or the spread or spread multiplier, if applicable) provided for in the Reset Notice, for the Subsequent Interest Period by causing the Trustees to transmit, in the manner provided for in Section 1.07, notice of such higher interest rate (or such higher spread or spread multiplier, if applicable) to the Holder of such Security. Such notice shall be irrevocable. All Securities with respect to which the interest rate (or the spread or spread multiplier used to calculate such interest rate, if applicable) is reset on an Optional Reset Date, and with respect to which the Holders of such Securities have not tendered such Securities for repayment (or have validly revoked any such tender) pursuant to the next succeeding paragraph, will bear such higher interest rate (or such higher spread or spread multiplier, if applicable).
The Holder of any such Security will have the option to elect repayment by the Company of the principal of such Security on each Optional Reset Date at a price equal to the principal amount thereof plus interest accrued to such Optional Reset Date. In order to obtain repayment on an Optional Reset Date, the Holder must follow the procedures set forth in Article Thirteen for repayment at the option of Holders except that the period for delivery or notification to the Trustees shall be at least 25 but not more than 35 days prior to such Optional Reset Date and except that, if the Holder has tendered any Security for repayment pursuant to the Reset Notice, the Holder may, by written notice to the Trustees, revoke such tender or repayment until the close of business on the tenth day before such Optional Reset Date.
Subject to the foregoing provisions of this Section 3.07 and Section 3.05, each Security delivered under this Indenture upon registration of transfer of or in exchange for or in lieu of any other Security shall carry the rights to interest accrued and unpaid, and to accrue, which were carried by such other Security.
SECTION 3.08 Optional Extension of Stated Maturity.
The provisions of this Section 3.08 may be made applicable to any series of Securities pursuant to Section 3.01 (with such modifications, additions or substitutions as may be specified pursuant to such Section 3.01). The Stated Maturity of any Security of such series may be extended at the option of the Company for the period or periods specified on the face of such Security (each an "Extension Period") up to but not beyond the date (the "Final Maturity") set forth on the face of such Security. The Company may exercise such option with respect to any Security by notifying the Trustees of such exercise at least 50 but not more than 60 days prior to the Stated Maturity of such Security in effect prior to the exercise of such option (the "Original Stated Maturity"). If the Company exercises such option, the Trustees shall transmit, in the manner provided for in Section 1.07, to the Holder of such Security not later than 40 days prior to the Original Stated Maturity a notice (the "Extension Notice") indicating (i) the election of the Company to extend the Stated Maturity, (ii) the new Stated Maturity, (iii) the interest rate (if any) applicable to the Extension Period and (iv) the provisions, if any, for redemption during such Extension Period. Upon the Trustees' transmittal of the Extension Notice, the Stated Maturity of such Security shall be extended automatically and, except as modified by the Extension Notice and as described in the next paragraph, such Security will have the same terms as prior to the transmittal of such Extension Notice.
Notwithstanding the foregoing, not later than 20 days before the Original Stated Maturity of such Security, the Company may, at its option, revoke the interest rate provided for in the Extension Notice and establish a higher interest rate for the Extension Period by causing the Trustees to transmit, in the manner provided for in Section 1.07, notice of such higher interest rate to the Holder of such Security. Such notice shall be irrevocable. All Securities with respect to which the Stated Maturity is extended will bear such higher interest rate.
If the Company extends the Maturity of any Security, the Holder will have the option to elect repayment of such Security by the Company on the Original Stated Maturity at a price equal to the principal amount thereof, plus interest accrued to such date. In order to obtain repayment on the Original Stated Maturity once the Company has extended the Maturity thereof, the Holder must follow the procedures set forth in Article Thirteen for repayment at the option of Holders, except that the period for delivery or notification to the Trustees shall be at least 25 but not more than 35 days prior to the Original Stated Maturity and except that, if the Holder has tendered any Security for repayment pursuant to an Extension Notice, the Holder may by written notice to the Trustees revoke such tender for repayment until the close of business on the tenth day before the Original Stated Maturity.
SECTION 3.09 Persons Deemed Owners.
Prior to due presentment of a Security for registration of transfer, the Company, the Trustees and any agent of the Company or the Trustees may treat the Person in whose name such Security is registered as the owner of such Security for the purpose of receiving payment of principal of, premium (if any) and (subject to Sections 3.05 and 3.07) interest (if any) on such Security and for all other purposes whatsoever, whether or not such Security be overdue, and none of the Company, the Trustees or any agent of the Company or the Trustees shall be affected by notice to the contrary.
The Depositary for Securities may be treated by the Company, the Trustees, and any agent of the Company or the Trustees as the owner of such global Security for all purposes whatsoever. None of the Company, the Trustees, any Paying Agent or the Security Registrar will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests of a Security in global form or for maintaining, supervising or reviewing any records relating to such beneficial ownership interests.
Notwithstanding the foregoing, with respect to any global Security, nothing herein shall prevent the Company, the Trustees, or any agent of the Company or the Trustees, from giving effect to any written certification, proxy or other authorization furnished by any Depositary, as a Holder, with respect to such global Security or impair, as between such Depositary and owners of beneficial interests in such global Security, the operation of customary practices governing the exercise of the rights of such Depositary (or its nominee) as Holder of such global Security.
SECTION 3.10 Cancellation.
All Securities surrendered for payment, redemption, repayment at the option of the Holder, registration of transfer or exchange or for credit against any current or future sinking fund payment shall, if surrendered to any Person other than a Trustee, be delivered to either Trustee. All Securities so delivered to either Trustee shall be promptly cancelled by such Trustee. The Company may at any time deliver to a Trustee for cancellation any Securities previously authenticated and delivered hereunder which the Company may have acquired in any manner whatsoever, and may deliver to either Trustee (or to any other Person for delivery to such Trustee) for cancellation any Securities previously authenticated hereunder which the Company has not issued and sold, and all Securities so delivered shall be promptly cancelled by such Trustee. If the Company shall so acquire any of the Securities, however, such acquisition shall not operate as a redemption or satisfaction of the indebtedness represented by such Securities unless and until the same are surrendered to either Trustee for cancellation. No Securities shall be authenticated in lieu of or in exchange for any Securities cancelled as provided in this Section 3.10, except as expressly permitted by this Indenture. All cancelled Securities held by either Trustee shall be disposed of by such Trustee in accordance with its customary procedures and certification of their disposal delivered to the Company unless by Company Order the Company shall direct that cancelled Securities be returned to it.
SECTION 3.11 Computation of Interest.
Except as otherwise specified as contemplated by Section 3.01 with respect to any Securities, interest (if any) on the Securities of each series shall be computed on the basis of a 360-day year of twelve 30-day months. For the purposes of disclosure under the Interest Act (Canada), the yearly rate of interest to which interest calculated under a Security for any period in any calendar year (the "calculation period") is equivalent, is the rate payable under a Security in respect of the calculation period multiplied by a fraction the numerator of which is the actual number of days in such calendar year and the denominator of which is the actual number of days in the calculation period.
SECTION 3.12 Currency and Manner of Payments in Respect of Securities.
(a) With respect to Securities of any series not permitting the election provided for in paragraph (b) below or the Holders of which have not made the election provided for in paragraph (b) below, payment of the principal of, premium (if any) and interest (if any) on such Security of such series will be made in the Currency in which such Security is payable. The provisions of this Section 3.12 may be modified or superseded with respect to any Securities pursuant to Section 3.01.
(b) It may be provided pursuant to Section 3.01 with respect to Securities of any series that Holders shall have the option, subject to paragraphs (d) and (e) below, to receive payments of principal of, premium (if any) or interest (if any) on such Securities in any of the Currencies which may be designated for such election by delivering to the Trustees a written election with signature guarantees and in the applicable form established pursuant to Section 3.01, not later than the close of business on the Election Date immediately preceding the applicable payment date. If a Holder so elects to receive such payments in any such Currency, such election will remain in effect for such Holder or any transferee of such Holder until changed by such Holder or such transferee by written notice to the Trustees (but any such change must be made not later than the close of business on the Election Date immediately preceding the next payment date to be effective for the payment to be made on such payment date and no such change of election may be made with respect to payments to be made on any Security of such series with respect to which an Event of Default has occurred or with respect to which the Company has deposited funds pursuant to Article Four or Fourteen or with respect to which a notice of redemption has been given by the Company or a notice of option to elect repayment has been sent by such Holder or such transferee). Any Holder of any such Security who shall not have delivered any such election to the Trustees not later than the close of business on the applicable Election Date will be paid the amount due on the applicable payment date in the relevant Currency as provided in Section 3.12(a). The Trustees shall notify the Exchange Rate Agent as soon as practicable after the Election Date of the aggregate principal amount of Securities for which Holders have made such written election.
(c) Unless otherwise specified pursuant to Section 3.01, if the election referred to in paragraph (b) above has been provided for pursuant to Section 3.01, then, unless otherwise specified pursuant to Section 3.01, not later than the fourth Business Day after the Election Date for each payment date for Securities of any series, the Exchange Rate Agent will deliver to the Company a written notice specifying, in the Currency in which Securities of such series are payable, the respective aggregate amounts of principal of, premium (if any) and interest (if any) on the Securities to be paid on such payment date, specifying the amounts in such Currency so payable in respect of the Securities as to which the Holders of Securities of such series shall have elected to be paid in another Currency as provided in paragraph (b) above. If the election referred to in paragraph (b) above has been provided for pursuant to Section 3.01 and if at least one Holder has made such election, then, unless otherwise specified pursuant to Section 3.01, on the second Business Day preceding such payment date the Company will deliver to the Trustees for such series of Securities an Exchange Rate Officer's Certificate in respect of the Dollar or Foreign Currency payments to be made on such payment date. Unless otherwise specified pursuant to Section 3.01, the Dollar or Foreign Currency amount receivable by Holders of Securities who have elected payment in a Currency as provided in paragraph (b) above shall be determined by the Company on the basis of the applicable Market Exchange Rate in effect on the third Business Day (the "Valuation Date") immediately preceding each payment date, and such determination shall be conclusive and binding for all purposes, absent manifest error.
(d) If a Conversion Event occurs with respect to a Foreign Currency in which any of the Securities are denominated or payable other than pursuant to an election provided for pursuant to paragraph (b) above, then, with respect to each date for the payment of principal of, premium (if any) and interest (if any) on the applicable Securities denominated or payable in such Foreign Currency occurring after the last date on which such Foreign Currency was used (the "Conversion Date"), the Dollar shall be the Currency of payment for use on each such payment date. Unless otherwise specified pursuant to Section 3.01, the Dollar amount to be paid by the Company to the Trustees and by the Trustees or any Paying Agent to the Holders of such Securities with respect to such payment date shall be, in the case of a Foreign Currency other than a currency unit, the Dollar Equivalent of the Foreign Currency or, in the case of a currency unit, the Dollar Equivalent of the Currency Unit, in each case as determined by the Exchange Rate Agent in the manner provided in paragraph (f) or (g) below.
(e) Unless otherwise specified pursuant to Section 3.01, if the Holder of a Security denominated in any Currency shall have elected to be paid in another Currency as provided in paragraph (b) above, and a Conversion Event occurs with respect to such elected Currency, such Holder shall receive payment in the Currency in which payment would have been made in the absence of such election; and if a Conversion Event occurs with respect to the Currency in which payment would have been made in the absence of such election, such Holder shall receive payment in Dollars as provided in paragraph (d) above.
(f) The "Dollar Equivalent of the Foreign Currency" shall be determined by the Exchange Rate Agent and shall be obtained for each subsequent payment date by converting the specified Foreign Currency into Dollars at the Market Exchange Rate on the Conversion Date.
(g) The "Dollar Equivalent of the Currency Unit" shall be determined by the Exchange Rate Agent and subject to the provisions of paragraph (h) below shall be the sum of each amount obtained by converting the Specified Amount of each Component Currency into Dollars at the Market Exchange Rate for such Component Currency on the Valuation Date with respect to each payment.
(h) For purposes of this Section 3.12 the following terms shall have the following meanings:
A "Component Currency" shall mean any Currency which, on the Conversion Date, was a component currency of the relevant currency unit, including, but not limited to, the Euro.
A "Specified Amount" of a Component Currency shall mean the number of units of such Component Currency or fractions thereof which were represented in the relevant currency unit, including, but not limited to, the Euro, on the Conversion Date. If after the Conversion Date the official unit of any Component Currency is altered by way of combination or subdivision, the Specified Amount of such Component Currency shall be divided or multiplied in the same proportion. If after the Conversion Date two or more Component Currencies are consolidated into a single currency, the respective Specified Amounts of such Component Currencies shall be replaced by an amount in such single Currency equal to the sum of the respective Specified Amounts of such consolidated Component Currencies expressed in such single Currency, and such amount shall thereafter be a Specified Amount and such single Currency shall thereafter be a Component Currency. If after the Conversion Date any Component Currency shall be divided into two or more currencies, the Specified Amount of such Component Currency shall be replaced by amounts of such two or more currencies, having an aggregate Dollar Equivalent value at the Market Exchange Rate on the date of such replacement equal to the Dollar Equivalent value of the Specified Amount of such former Component Currency at the Market Exchange Rate immediately before such division and such amounts shall thereafter be Specified Amounts and such currencies shall thereafter be Component Currencies. If, after the Conversion Date of the relevant currency unit, including, but not limited to, the Euro, a Conversion Event (other than any event referred to above in this definition of "Specified Amount") occurs with respect to any Component Currency of such currency unit and is continuing on the applicable Valuation Date, the Specified Amount of such Component Currency shall, for purposes of calculating the Dollar Equivalent of the Currency Unit, be converted into Dollars at the Market Exchange Rate in effect on the Conversion Date of such Component Currency.
"Election Date" shall mean the date for any series of Securities as specified pursuant to clause (15) of Section 3.01 by which the written election referred to in paragraph (b) above may be made.
All decisions and determinations of the Exchange Rate Agent regarding the Dollar Equivalent of the Foreign Currency, the Dollar Equivalent of the Currency Unit, the Market Exchange Rate and changes in the Specified Amounts as specified above shall be in its sole discretion and shall, in the absence of manifest error, be conclusive for all purposes and irrevocably binding upon the Company, the Trustees and all Holders of such Securities denominated or payable in the relevant Currency. The Exchange Rate Agent shall promptly give written notice to the Company and the Trustees of any such decision or determination.
In the event that the Company determines in good faith that a Conversion Event has occurred with respect to a Foreign Currency, the Company will immediately give written notice thereof to the Trustees and to the Exchange Rate Agent (and the Trustees will promptly thereafter give notice in the manner provided for in Section 1.07 to the affected Holders) specifying the Conversion Date. In the event the Company so determines that a Conversion Event has occurred with respect to the Euro or any other currency unit in which Securities are denominated or payable, the Company will immediately give written notice thereof to the Trustees and to the Exchange Rate Agent (and the Trustees will promptly thereafter give notice in the manner provided for in Section 1.07 to the affected Holders) specifying the Conversion Date and the Specified Amount of each Component Currency on the Conversion Date. In the event the Company determines in good faith that any subsequent change in any Component Currency as set forth in the definition of Specified Amount above has occurred, the Company will similarly give written notice to the Trustees and the Exchange Rate Agent.
The Trustees shall be fully justified and protected in relying and acting upon information received by it from the Company and the Exchange Rate Agent and shall not otherwise have any duty or obligation to determine the accuracy or validity of such information independent of the Company or the Exchange Rate Agent.
SECTION 3.13 Appointment and Resignation of Successor Exchange Rate Agent.
(a) Unless otherwise specified pursuant to Section 3.01, if and so long as the Securities of any series (i) are denominated in a Currency other than Dollars or (ii) may be payable in a Currency other than Dollars, or so long as it is required under any other provision of this Indenture, then the Company will maintain with respect to each such series of Securities, or as so required, at least one Exchange Rate Agent. The Company will cause the Exchange Rate Agent to make the necessary foreign exchange determinations at the time and in the manner specified pursuant to Section 3.01 for the purpose of determining the applicable rate of exchange and, if applicable, for the purpose of converting the issued Currency into the applicable payment Currency for the payment of principal, premium (if any) and interest (if any) pursuant to Section 3.12.
(b) The Company shall have the right to remove and replace from time to time the Exchange Rate Agent for any series of Securities. No resignation of the Exchange Rate Agent and no appointment of a successor Exchange Rate Agent pursuant to this Section 3.13 shall become effective until the acceptance of appointment by the successor Exchange Rate Agent as evidenced by a written instrument delivered to the Company and the Trustees.
(c) If the Exchange Rate Agent shall resign, be removed or become incapable of acting, or if a vacancy shall occur in the office of the Exchange Rate Agent for any cause with respect to the Securities of one or more series, the Company, by or pursuant to a Board Resolution, shall promptly appoint a successor Exchange Rate Agent or Exchange Rate Agents with respect to the Securities of that or those series (it being understood that any such successor Exchange Rate Agent may be appointed with respect to the Securities of one or more or all of such series and that, unless otherwise specified pursuant to Section 3.01, at any time there shall only be one Exchange Rate Agent with respect to the Securities of any particular series that are originally issued by the Company on the same date and that are initially denominated and/or payable in the same Currency).
ARTICLE FOUR
SATISFACTION AND DISCHARGE
SECTION 4.01 Satisfaction and Discharge of Indenture.
This Indenture shall upon Company Request cease to be of further effect with respect to any series of Securities specified in such Company Request (except as to any surviving rights of registration of transfer or exchange of Securities of such series expressly provided for herein or pursuant hereto and the rights of Holders of such series of Securities to receive, solely from the trust fund described in subclause (b) of clause (1) of this Section 4.01, payments in respect of the principal of, premium (if any) and interest (if any) on such Securities when such payments are due and except as provided in the last paragraph of this Section 4.01) and the Trustees, at the expense of the Company, shall execute proper instruments acknowledging satisfaction and discharge of this Indenture as to such series when
(1) either
(a) all Securities of such series theretofore authenticated and delivered (other than Securities of such series for whose payment money has theretofore been deposited in trust with either Trustee or any Paying Agent or segregated and held in trust by the Company and thereafter repaid to the Company, as provided in Section 10.03) have been delivered to either Trustee for cancellation; or
(b) all Securities of such series not theretofore delivered to either Trustee for cancellation
(i) have become due and payable, or
(ii) will become due and payable at their Stated Maturity within one year, or
(iii) if redeemable at the option of the Company, are to be called for redemption within one year under arrangements satisfactory to the Trustees for the giving of notice of redemption by the Trustees in the name, and at the expense, of the Company,
and the Company, in the case of (i), (ii) or (iii) above, has irrevocably deposited or caused to be deposited with either Trustee as trust funds in trust for such purpose an amount in the Currency in which the Securities of such series are payable, sufficient to pay and discharge the entire indebtedness on such Securities not theretofore delivered to such Trustee for cancellation, for principal, premium (if any) and interest (if any) to the date of such deposit (in the case of Securities which have become due and payable) or to the Stated Maturity or Redemption Date, as the case may be;
(2) the Company has paid or caused to be paid all other sums payable hereunder by the Company; and
(3) the Company has delivered to the Trustees an Officer's Certificate and an Opinion of Counsel, each stating that all conditions precedent herein provided for relating to the satisfaction and discharge of this Indenture as to such series have been complied with.
Notwithstanding the satisfaction and discharge of this Indenture, the obligations of the Company to the Trustees under Section 6.07, the obligations of the Trustees to any Authenticating Agent under Section 6.12 and, if money shall have been deposited with the Trustees pursuant to subclause (b) of clause (1) of this Section 4.01, the obligations of the Trustees under Section 4.02, Section 6.07(3) and the last paragraph of Section 10.03 shall survive.
SECTION 4.02 Application of Trust Money.
Subject to the provisions of the last paragraph of Section 10.03, all money deposited with the Trustees pursuant to Section 4.01 shall be held in trust and applied by it, in accordance with the provisions of the Securities and this Indenture, to the payment, either directly or through any Paying Agent (including the Company acting as its own Paying Agent) as the Trustees may determine, to the Persons entitled thereto, of the principal, premium (if any) and interest (if any) for whose payment such money has been deposited with the Trustees; but such money need not be segregated from other funds except to the extent required by law.
ARTICLE FIVE
REMEDIES
SECTION 5.01 Events of Default.
"Event of Default," wherever used herein with respect to Securities of any series, means any one of the following events (whatever the reason for such Event of Default and whether it shall be voluntary or involuntary or be effected by operation of law or pursuant to any judgment, decree or order of any court or any order, rule or regulation of any administrative or governmental body), unless such event is specifically deleted or modified in or pursuant to a supplemental indenture, Board Resolution or Officer's Certificate establishing the terms of such series pursuant to Section 3.01 of this Indenture:
(1) default in the payment of any interest due on any Security of that series, when such interest becomes due and payable, and continuance of such default for a period of 30 days; or
(2) default in the payment of the principal or premium (if any) in respect of any Security of that series at its Maturity; or
(3) default in the deposit of any sinking fund, amortization or analogous payment when due by the terms of any Security of that series and Article Twelve; or
(4) default in the performance, or breach, of any covenant or agreement of the Company in this Indenture which affects or is applicable to the Securities of that series (other than a covenant or agreement, a default in whose performance or whose breach is elsewhere in this Section 5.01 specifically dealt with), and continuance of such default or breach for a period of 60 days after there has been given (and 120 days with respect to a default or breach under Section 7.05), by registered or certified mail, to the Company by the Trustees or to the Company and the Trustees by the Holders of at least 25% in principal amount of all Outstanding Securities of that series a written notice specifying such default or breach and requiring it to be remedied and stating that such notice is a "Notice of Default" hereunder; or
(5) the entry of a decree or order by a court having jurisdiction in the premises adjudging the Company bankrupt or insolvent, or approving as properly filed a petition seeking reorganization, arrangement, adjustment or composition of or in respect of the Company under or subject to the Bankruptcy and Insolvency Act (Canada), the Companies' Creditors Arrangement Act (Canada), the U.S. Federal Bankruptcy Code or any other federal, provincial, state or foreign bankruptcy, insolvency or analogous laws, or the issuance of a sequestration order or the (appointment of a receiver, liquidator, assignee, trustee, sequestrator (or other similar official) of the Company or in receipt of any substantial part of the property of the Company, and any such decree, order or appointment continues unstayed and in effect for a period of 90 consecutive days; or
(6) the institution by the Company of proceedings to be adjudicated bankrupt or insolvent, or the consent by it to the institution of bankruptcy or insolvency proceedings against it, or the filing by it of a petition or answer or consent seeking reorganization or relief under or subject to the Bankruptcy and Insolvency Act (Canada), the Companies' Creditors Arrangement Act (Canada), the U.S. Federal Bankruptcy Code or any other federal, provincial, state or foreign bankruptcy, insolvency or analogous laws or the consent by it to the filing of any such petition or to the appointment of a receiver, liquidator, assignee, trustee, sequestrator (or other similar official) of the Company or of any substantial part of its property, or the making by it of a general assignment for the benefit of creditors, or the admission by it in writing of its inability to pay its debts generally as they become due or the taking by it of corporate action in furtherance of any of the aforesaid purposes; or
(7) any other Event of Default provided with respect to Securities of that series.
SECTION 5.02 Acceleration of Maturity; Rescission and Annulment.
If an Event of Default described in clause (1), (2), (3), (4) or (7) of Section 5.01 with respect to Securities of any series at the time Outstanding occurs and is continuing, then in every such case, either Trustee or the Holders of not less than 25% in principal amount of the Outstanding Securities of that series, may declare the principal amount (or, if the Securities of that series are Original Issue Discount Securities or Indexed Securities, such portion of the principal amount as may be specified in the terms of that series) of all of the Securities of that series and all interest thereon to be due and payable immediately, by a notice in writing to the Company (and to the Trustees if given by Holders), and upon any such declaration such principal amount (or specified portion thereof) shall become immediately due and payable. If an Event of Default specified in clause (5) or (6) of Section 5.01 occurs and is continuing, then the principal amount of all the Securities shall ipso facto become and be immediately due and payable without any declaration or other act on the part of the Trustees or any Holder.
At any time after such a declaration of acceleration with respect to Securities of any series (or of all series, as the case may be) has been made and before a judgment or decree for payment of the money due has been obtained by either Trustee as hereinafter provided in this Article Five, the Holders of a majority in principal amount of the Outstanding Securities of that series (or of all series, as the case may be), by written notice to the Company and the Trustees, may rescind and annul such declaration and its consequences if:
(1) the Company has paid or deposited with either Trustee a sum sufficient to pay in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)),
(a) all overdue interest (if any) on all Outstanding Securities of that series (or of all series, as the case may be),
(b) all unpaid principal of and premium (if any) on any Outstanding Securities of that series (or of all series, as the case may be) which has become due otherwise than by such declaration of acceleration, and interest on such unpaid principal and premium (if any) at the rate or rates prescribed therefor in such Securities,
(c) to the extent that payment of such interest is legally enforceable, interest on overdue interest at the rate or rates prescribed therefor in such Securities, and
(d) all sums paid or advanced by the Trustees hereunder and the reasonable compensation, expenses, disbursements and advances of the Trustees, their agents and counsel; and
(2) all Events of Default with respect to Securities of that series (or of all series, as the case may be), other than the non-payment of amounts of principal of, premium (if any) or interest (if any) on Securities of that series (or of all series, as the case may be) which have become due solely by such declaration of acceleration, have been cured or waived as provided in Section 5.13.
No such rescission shall affect any subsequent default or impair any right consequent thereon.
SECTION 5.03 Collection of Debt and Suits for Enforcement by Trustees.
The Company covenants that if
(1) default is made in the payment of any installment of interest on any Security when such interest becomes due and payable and such default continues for a period of 30 days, or
(2) default is made in the payment of the principal of or premium (if any) any Security at the Maturity thereof,
then the Company will, upon demand of the Trustees, pay to the applicable Trustee for the benefit of the Holders of such Securities , the whole amount then due and payable on such Securities for principal of, premium (if any) and interest (if any) and interest on any overdue principal, overdue premium (if any) and, to the extent lawful, overdue interest (if any), at the rate or rates prescribed therefor in such Securities, and, in addition thereto, such further amount as shall be sufficient to cover the costs and expenses of collection, including the reasonable compensation, expenses, disbursements and advances of the Trustees, their agents and counsel.
If the Company fails to pay such amounts forthwith upon such demand, the Trustees, in their own names as trustees of an express trust, may institute a judicial proceeding for the collection of the sums so due and unpaid, may prosecute such proceeding to judgment or final decree and may enforce the same against the Company or any other obligor upon such Securities and collect the moneys adjudged or decreed to be payable in the manner provided by law out of the property of the Company or any other obligor upon such Securities, wherever situated.
If an Event of Default with respect to Securities of any series (or of all series, as the case may be) occurs and is continuing, either Trustee may in its discretion proceed to protect and enforce its rights and the rights of the Holders of Securities of such series (or of all series, as the case may be) by such appropriate judicial proceedings as such Trustee shall deem most effectual to protect and enforce any such rights, whether for the specific enforcement of any covenant or agreement in this Indenture or in aid of the exercise of any power granted herein, or to enforce any other proper remedy.
SECTION 5.04 Trustees May File Proofs of Claim.
In case of the pendency of any receivership, insolvency, liquidation, bankruptcy, reorganization, arrangement, adjustment, composition or other judicial proceeding relative to the Company or any other obligor upon the Securities or the property of the Company or of such other obligor or their creditors, each Trustee (irrespective of whether the principal of the Securities shall then be due and payable as therein expressed or by declaration or otherwise and irrespective of whether either Trustee shall have made any demand on the Company for the payment of overdue principal, premium (if any) or interest) shall be entitled and empowered, by intervention in such proceeding or otherwise,
(i) to file and prove a claim for the whole amount of principal and premium (if any), or such portion of the principal amount of any series of Original Issue Discount Securities or Indexed Securities as may be specified in the terms of such series, and interest (if any) owing and unpaid in respect of the Securities and to file such other papers or documents as may be necessary or advisable in order to have the claims of such Trustee (including any claim for the reasonable compensation, expenses, disbursements and advances of such Trustee, its agents and counsel) and of the Holders allowed in such judicial proceeding, and
(ii) to collect and receive any moneys or other property payable or deliverable on any such claims and to distribute the same;
and any custodian, receiver, assignee, trustee, liquidator, sequestrator or other similar official in any such judicial proceeding is hereby authorized by each Holder to make such payments to such Trustee and, in the event that such Trustee shall consent to the making of such payments directly to the Holders, to pay to such Trustee any amount due to it for the reasonable compensation, expenses, disbursements and advances of each Trustee, its agents and counsel, and any other amounts due to such Trustee under Section 6.07.
Nothing herein contained shall be deemed to authorize the Trustees to authorize or consent to or accept or adopt on behalf of any Holder any plan of reorganization, arrangement, adjustment or composition affecting the Securities or the rights of any Holder thereof or to authorize the Trustees to vote in respect of the claim of any Holder in any such proceeding.
SECTION 5.05 Trustees May Enforce Claims Without Possession of Securities.
All rights of action and claims under this Indenture, the Securities may be prosecuted and enforced by the Trustees without the possession of any of the Securities or the production thereof in any proceeding relating thereto, and any such proceeding instituted by either Trustee shall be brought in its own name as trustee of an express trust, and any recovery of judgment shall, after provision for the payment of the reasonable compensation, expenses, disbursements and advances of such Trustee, its agents and counsel, be for the ratable benefit of the Holders of the Securities in respect of which such judgment has been recovered.
SECTION 5.06 Application of Money Collected.
Any money collected by either Trustee pursuant to this Article Five shall be applied in the following order, at the date or dates fixed by the Trustees and, in case of the distribution of such money on account of principal of, premium (if any) or interest (if any) upon presentation of the Securities, and the notation thereon of the payment if only partially paid and upon surrender thereof if fully paid:
First: to the payment of all amounts due the Trustees under Section 6.07;
Second: to the payment of the amounts then due and unpaid for principal of, premium (if any) and interest (if any), on the Securities in respect of which or for the benefit of which such money has been collected, ratably, without preference or priority of any kind, according to the amounts due and payable on such Securities for principal, premium (if any) and interest (if any), respectively; and
Third: the balance, if any, to the Person or Persons entitled thereto.
SECTION 5.07 Limitation on Suits.
No Holder of any Security of any series shall have any right to institute any proceeding, judicial or otherwise, with respect to this Indenture or the Securities, or for the appointment of a receiver or trustee, or for any other remedy hereunder, unless
(1) such Holder has previously given written notice to the Trustees of a continuing Event of Default with respect to the Securities of that series;
(2) the Holders of not less than 25% in principal amount of the Outstanding Securities of that series in the case of any Event of Default described in clause (1), (2), (3), (4) or (7) of Section 5.01, or, in the case of any Event of Default described in clause (5) or (6) of Section 5.01, the Holders of not less than 25% in principal amount of all Outstanding Securities, shall have made written request to the Trustees to institute proceedings in respect of such Event of Default in their own names as Trustees hereunder;
(3) such Holder or Holders have offered to the Trustees reasonable indemnity against the costs, expenses and liabilities to be incurred in compliance with such request;
(4) the Trustees for 60 days after their receipt of such notice, request and offer of indemnity have failed to institute any such proceeding; and
(5) no direction inconsistent with such written request has been given to the Trustees during such 60-day period by the Holders of a majority or more in principal amount of the Outstanding Securities of that series in the case of any Event of Default described in clause (1), (2), (3), (4) or (7) of Section 5.01, or in the case of any Event of Default described in clause (5) or (6) of Section 5.01, by the Holders of a majority or more in principal amount of all Outstanding Securities;
it being understood and intended that no one or more of such Holders shall have any right in any manner whatever by virtue of, or by availing of, any provision of this Indenture to affect, disturb or prejudice the rights of any other Holders of Securities of the same series, in the case of any Event of Default described in clause (1), (2), (3), (4) or (7) of Section 5.01, or of Holders of all Securities in the case of any Event of Default described in clause (5) or (6) of Section 5.01, or to obtain or to seek to obtain priority or preference over any other of such Holders or to enforce any right under this Indenture, except in the manner herein provided and for the equal and ratable benefit of all Holders of Securities of the same series, in the case of any Event of Default described in clause (1), (2), (3), (4) or (7) of Section 5.01, or of Holders of all Securities in the case of any Event of Default described in clause (5) or (6) of Section 5.01.
SECTION 5.08 Unconditional Right of Holders to Receive Principal, Premium and Interest.
Notwithstanding any other provision in this Indenture, the Holder of any Security shall have the right, which is absolute and unconditional, to receive payment, as provided herein (including, if applicable, Article Fourteen) and in such Security, of the principal of and premium (if any) and (subject to Section 3.07) interest (if any) on, such Security on the respective Stated Maturities expressed in such Security (or, in the case of redemption, on the Redemption Date or, in the case of repayment at the option of the Holder as contemplated by Article Twelve, on the Repayment Date) and subject to the limitations on a Holder's ability to institute suit contained Section 5.07, to institute suit for the enforcement of any such payment, and such rights shall not be impaired without the consent of such Holder.
SECTION 5.09 Restoration of Rights and Remedies.
If either Trustee or any Holder has instituted any proceeding to enforce any right or remedy under this Indenture and such proceeding has been discontinued or abandoned for any reason, or has been determined adversely to such Trustee or to such Holder, then and in every such case, subject to any determination in such proceeding, the Company, the Trustees and the Holders of Securities shall be restored severally and respectively to their former positions hereunder and thereafter all rights and remedies of the Trustees and the Holders shall continue as though no such proceeding had been instituted.
SECTION 5.10 Rights and Remedies Cumulative.
Except as otherwise provided with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities in the last paragraph of Section 3.06, no right or remedy herein conferred upon or reserved to the Trustees or to the Holders of Securities is intended to be exclusive of any other right or remedy, and every right and remedy shall, to the extent permitted by law, be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at law or in equity or otherwise. The assertion or employment of any right or remedy hereunder, or otherwise, shall not, to the extent permitted by law, prevent the concurrent assertion or employment of any other appropriate right or remedy.
SECTION 5.11 Delay or Omission Not Waiver.
No delay or omission of the Trustees or of any Holder of any Security to exercise any right or remedy accruing upon any Event of Default shall impair any such right or remedy or constitute a waiver of any such Event of Default or an acquiescence therein. Every right and remedy given by this Article Five or by law to the Trustees or to the Holders may be exercised from time to time, and as often as may be deemed expedient, by the Trustees or by the Holders, as the case may be.
SECTION 5.12 Control by Holders.
With respect to the Securities of any series, the Holders of not less than a majority in principal amount of the Outstanding Securities of such series shall have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustees, or exercising any trust or power conferred on the Trustees, relating to or arising under clause (1), (2), (3), (4) or (7) of Section 5.01, and, with respect to all Securities, the Holders of not less than a majority in principal amount of all Outstanding Securities shall have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustees, or exercising any trust or power conferred on the Trustees, not relating to or arising under clause (1), (2), (3), (4) or (7) of Section 5.01, provided that in each case
(1) such direction shall not be in conflict with any rule of law or with this Indenture,
(2) the Trustees may take any other action deemed proper by the Trustees which is not inconsistent with such direction, and
(3) the Trustees need not take any action which might involve them in personal liability or be unjustly prejudicial to the Holders of Securities of such series not consenting.
SECTION 5.13 Waiver of Past Defaults.
Subject to Section 5.02, the Holders of not less than a majority in principal amount of the Outstanding Securities of any series may on behalf of the Holders of all the Securities of such series waive any past Default described in clause (1), (2), (3), (4) or (7) of Section 5.01 (or, in the case of a Default described in clause (5) or (6) of Section 5.01, the Holders of not less than a majority in principal amount of all Outstanding Securities may waive any such past Default), and its consequences, except a default
(1) in respect of the payment of the principal of, premium (if any) or interest (if any) on any Security, or
(2) in respect of a covenant or provision herein which under Article Nine cannot be modified or amended without the consent of the Holder of each outstanding Security of such series affected.
Upon any such waiver, any such Default shall cease to exist, and any Event of Default arising therefrom shall be deemed to have been cured, for every purpose of this Indenture; but no such waiver shall extend to any subsequent or other Default or Event of Default or impair any right consequent thereon.
SECTION 5.14 Waiver of Stay or Extension Laws.
The Company covenants (to the extent that it may lawfully do so) that it will not at any time insist upon, or plead, or in any manner whatsoever claim or take the benefit or advantage of, any stay or extension law wherever enacted, now or at any time hereafter in force, which may affect the covenants or the performance of this Indenture; and the Company (to the extent that it may lawfully do so) hereby expressly waives all benefit or advantage of any such law and covenants that it will not hinder, delay or impede the execution of any power herein granted to the Trustees, but will suffer and permit the execution of every such power as though no such law had been enacted.
SECTION 5.15 Undertaking for Costs.
In any suit for the enforcement of any right or remedy under this Indenture, or in any suit against either Trustee for any action taken, suffered or omitted by it as Trustee, a court may require any party litigant in such suit to file an undertaking to pay the costs of such suit, and may assess costs against any such party litigant, in the manner and to the extent provided in Trust Indenture Legislation; provided, however, that neither this Section 5.15 nor the provisions of TIA Section 315(e) shall apply to any suit instituted by either Trustee or by any Holder or group of Holders holding more than 10% in principal amount of all Outstanding Securities or by any Holder of any Security on any suit for the enforcement of the right to receive the principal of and interest on any such Securities.
ARTICLE SIX
THE TRUSTEES
SECTION 6.01 Notice of Defaults.
Each Trustee shall promptly give the other Trustee notice of any Default or Event of Default known to it. Within a reasonable time, but no more than 30 days after either Trustee has knowledge of any Default hereunder with respect to the Securities of any series, one or both of the Trustees shall transmit in the manner and to the extent provided in Trust Indenture Legislation, including TIA Section 313(c), notice to the Holders of such Default hereunder known to either Trustee, unless such Default shall have been cured or waived (and, in the case where such Default shall have been cured, the Trustees shall notify the Holders in writing of such cure in writing within a reasonable time, but not exceeding 30 days, after the Trustees have become aware that the Default has been cured); provided, however, that, except in the case of a Default in the payment of the principal of, premium (if any) or interest (if any) on any Security of such series or in the payment of any sinking fund installment with respect to Securities of such series, the Trustees shall be protected in withholding such notice if and so long as the board of directors, the executive committee or a trust committee of directors and/or Responsible Officers of each Trustee in good faith determine that the withholding of such notice is in the interest of the Holders of Securities of such series; provided further that in the case of any Default of the character specified in clause (4) of Section 5.01 with respect to Securities of such series, no such notice to Holders shall be given until at least 30 days after the occurrence thereof.
SECTION 6.02 Certain Duties and Responsibilities of Trustees.
(a) The Trustees, prior to the occurrence of an Event of Default and after the curing of all Events of Default that may have occurred, shall undertake to perform with respect to the Securities of any series such duties and only such duties as are specifically set forth in this Indenture, and no implied covenants shall be read into this Indenture against the Trustees.
(b) In all instances, in the exercise of the powers, rights, duties and discharge of obligations prescribed or conferred by the terms of this Indenture, each Trustee shall act honestly and in good faith with a view to the best interests of the Holders and exercise that degree of care, diligence and skill that a reasonably prudent trustee in respect of indentures for the purpose of issuing corporate debt obligations would exercise in comparable circumstances.
(c) No provision of this Indenture shall be construed to relieve each Trustee from liability for its own actions or failure to act in accordance with Subsection 6.02(b), except that:
(i) prior to the occurrence of an Event of Default and after the curing or waiving of all such Events of Default that may have occurred:
(A) the duties and obligations of each Trustee with respect to the Securities of any series shall be determined solely by the express provisions of this Indenture, and the Trustees shall not be liable except for the performance of such duties and obligations as are specifically set forth in this Indenture, and no implied covenants or obligations shall be read into this Indenture against the Trustees; and
(B) in the absence of bad faith on the part of either Trustee, such Trustee may conclusively rely, as to the truth of the statements and the correctness of the opinions expressed therein, upon any certificates or opinions furnished to the Trustees and conforming to the requirements of this Indenture and Trust Indenture Legislation; but in the case of any such certificates or opinions that by any provision hereof are specifically required to be furnished to the Trustees, the Trustees shall be under a duty to examine the same to determine whether or not they conform to the requirements of this Indenture; provided, however, the Canadian Trustee shall not be required to determine whether the certificates or opinions presented to it conform to the Trust Indenture Act and the U.S. Trustee shall not be required to determine whether the certificates or opinions presented to it conform to Canadian Trust Indenture Legislation.
(ii) the Trustees shall not be liable with respect to any action taken or omitted to be taken by them in good faith in accordance with the direction of the Holders of not less than a majority in principal amount of the Securities of any series at the time Outstanding relating to the time, method and place of conducting any proceeding for any remedy available to the Trustees, or exercising any trust or power conferred upon the Trustees under this Indenture;
(iii) none of the provisions contained in this Indenture shall require either Trustee to expend or risk their own funds or otherwise incur personal or any financial liability in the performance of any of their duties or in the exercise of any of their rights or powers; and
(iv) whether or not therein expressly so provided, except to the extent expressly provided herein to the contrary, every provision of this Indenture relating to the conduct or effecting the liability or affording protection to the Trustees shall be subject to the provisions of this Section 6.02.
(d) Notwithstanding the provisions of this Section 6.02 or any provision in this Indenture or in the Securities, the Trustees will not be charged with knowledge of the existence of any Event of Default or any other fact that would prohibit the making of any payment of monies to or by the Trustees, or the taking of any other action by the Trustees, unless and until the Trustees have received written notice thereof from the Company or any Holder.
SECTION 6.03 Certain Rights of Trustees.
Subject to the provisions of TIA Sections 315(a) through 315(d):
(1) the Trustees may rely and shall be protected in acting or refraining from acting upon any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order, bond, debenture, note, other evidence of indebtedness or other paper or document believed by them to be genuine and to have been signed or presented by the proper party or parties;
(2) any request or direction of the Company mentioned herein shall be sufficiently evidenced by a Company Request or Company Order and any resolution of the Board of Directors may be sufficiently evidenced by a Board Resolution;
(3) whenever in the administration of this Indenture the Trustees shall deem it desirable that a matter be proved or established prior to taking, suffering or omitting any action hereunder, each Trustee (unless other evidence be herein specifically prescribed) may, in the absence of bad faith on its part, rely upon an Officer's Certificate;
(4) the Trustees may consult with counsel and the written advice of such counsel or any opinion of Counsel shall be full and complete authorization and protection in respect of any action taken, suffered or omitted by them hereunder in good faith and in reliance thereon;
(5) the Trustees shall be under no obligation to exercise any of the rights or powers vested in it by this Indenture at the request or direction of any of the Holders of Securities of any series pursuant to this Indenture, unless such Holders shall have offered to the Trustees reasonable security or indemnity against the costs, expenses and liabilities which might be incurred by them in compliance with such request or direction;
(6) the Trustees shall not be bound to make any investigation into the facts or matters stated in any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order, bond, debenture, note, other evidence of indebtedness or other paper or document, but the Trustees, in their discretion, may make such further inquiry or investigation into such facts or matters as they may see fit, and, if the Trustees shall determine to make such further inquiry or investigation, they shall be entitled to examine the books, records and premises of the Company, personally or by agent or attorney;
(7) in an Event of Default, the Trustees' powers shall not be infringed upon so long as they act in accordance with Section 6.02(b);
(8) the Trustees may execute any of the trusts or powers hereunder or perform any duties hereunder either directly or by or through agents or attorneys and the Trustees shall not be responsible for any misconduct or negligence on the part of any agent or attorney appointed with due care by them hereunder; and
(9) the Trustees shall not be liable for any action taken, suffered or omitted by them in good faith and believed by them to be authorized or within the discretion or rights or powers conferred upon them by this Indenture, so long as they act in accordance with this Section 6.02(b).
SECTION 6.04 Trustees Not Responsible for Recitals or Issuance of Securities.
The recitals contained herein and in the Securities, except for a Trustee's certificate of authentication, shall be taken as the statements of the Company, and neither Trustee nor any Authenticating Agent assumes any responsibility for their correctness. The Trustees make no representations as to the validity or sufficiency of this Indenture or of the Securities , except that the Trustees represent that they are duly authorized to execute and deliver this Indenture, authenticate the Securities and perform their obligations hereunder and that the statements made by the U.S. Trustee in a Statement of Eligibility on Form T-1 supplied to the Company are true and accurate, subject to the qualifications set forth therein. Neither Trustee nor any Authenticating Agent shall be accountable for the use or application by the Company of Securities or the proceeds thereof. Nothing herein contained will impose on either Trustee any obligation to see to, or to require evidence of, the registration or filing (or renewal thereof) of this Indenture or any supplemental indenture. The Trustees shall not be bound to give notice to any person of the execution hereof.
SECTION 6.05 May Hold Securities.
The Trustees, any Authenticating Agent, any Paying Agent, any Security Registrar or any other agent of the Company or of the Trustees, in their individual or any other capacity, may become the owner or pledgee of Securities and, subject to TIA Sections 310(b) and 311, may otherwise deal with the Company, including, without limitation, as a creditor of the Company, with the same rights they would have if they were not Trustees, Authenticating Agent, Paying Agent, Security Registrar or such other agent. A Trustee that has resigned or is removed shall remain subject to TIA Section 311(a) to the extent provided therein.
SECTION 6.06 Money Held in Trust.
Money held by the Trustees in trust hereunder need not be segregated from other funds except to the extent required by law. The Trustees shall be under no liability for interest on any money received by them hereunder except as otherwise agreed with the Company.
SECTION 6.07 Compensation and Reimbursement.
The Company agrees:
(1) to pay to the Trustees from time to time reasonable compensation for all services rendered by them hereunder (which compensation shall not be limited by any provision of law in regard to the compensation of a trustee of an express trust);
(2) except as otherwise expressly provided herein, to reimburse the Trustees upon their request for all reasonable expenses, disbursements and advances incurred or made by the Trustees in accordance with any provision of this Indenture (including the reasonable compensation and the expenses and disbursements of their agents and counsel), except any such expense, disbursement or advance as may be attributable to the U.S. Trustee's gross negligence or bad faith or the Canadian Trustee's gross negligence or willful misconduct, respectively; and
(3) to indemnify the Trustees for, and to hold them and their directors, officers, agents, representatives, successors, assigns and employees harmless against, any loss, liability or expense incurred without gross negligence or bad faith on the part of the U.S. Trustee, or gross negligence or willful misconduct on the part of the Canadian Trustee, respectively, arising out of or in connection with the acceptance or administration of the trust or trusts hereunder, including reasonable attorneys' fees and other reasonable costs and expenses of defending themselves against any claim or liability in connection with the exercise or performance of any of their powers or duties hereunder.
The obligations of the Company under this Section 6.07 to compensate the Trustees, to pay or reimburse the Trustees for expenses, disbursements and advances and to indemnify and hold harmless the Trustees shall constitute additional indebtedness hereunder and shall survive the satisfaction and discharge of this Indenture and the resignation or removal of the Trustee. As security for the performance of such obligations of the Company, the Trustees shall have a claim prior to the Securities upon all property and funds held or collected by the Trustees as such, except funds held in trust for the payment of principal of, premium (if any) or interest (if any) on particular Securities.
When the Trustees incur expenses or render services in connection with an Event of Default specified in clause (5) or (6) of Section 5.01, the expenses (including reasonable charges and expense of its counsel) of and the compensation for such services are intended to constitute expenses of administration under any applicable United States or Canadian federal, state or provincial bankruptcy, insolvency or other similar law.
The provisions of this Section 6.07 shall survive the termination of this Indenture.
SECTION 6.08 Corporate Trustees Required; Eligibility.
(1) There shall be at all times a U.S. Trustee hereunder which shall be eligible to act as Trustee under TIA Section 310(a)(1) and, together with its immediate parent, shall have a combined capital and surplus of at least $50,000,000. If the U.S. Trustee publishes reports of condition at least annually, pursuant to law or to the requirements of United States federal, state, territorial or District of Columbia supervising or examining authority, then for the purposes of this Section 6.08, the combined capital and surplus of U.S. Trustee shall be deemed to be its combined capital and surplus as set forth in its most recent report of condition so published. If at any time the U.S. Trustee shall cease to be eligible in accordance with the provisions of this Section 6.08, it shall resign immediately in the manner and with the effect hereinafter specified in this Article Six.
(2) For so long as required by Trust Indenture Legislation, there shall be a Canadian Trustee under this Indenture. The Canadian Trustee shall at all times be a resident or authorized to do business in the Province of Ontario and any other province in Canada where Holders may be resident from time to time. The Canadian Trustee represents and warrants that no material conflict of interest exists in the Canadian Trustee's role as a fiduciary hereunder and agrees that in the event of a material conflict of interest arising hereafter it will, within 30 days after ascertaining that it has such material conflict of interest, either eliminate the same or resign its trust hereunder. If any such material conflict of interests exists or hereafter shall exist, the validity and enforceability of this Indenture shall not be affected in any manner whatsoever by reason thereof.
(3) The Trustees will not be required to give any bond or security in respect of the execution of the trusts and powers set out in this Indenture or otherwise in respect of the premises.
(4) Neither Trustee nor any Affiliate of either Trustee shall be appointed a receiver or receiver and manager or liquidator of all or any part of the assets or undertaking of the Company.
SECTION 6.09 Resignation and Removal; Appointment of Successor.
(1) No resignation or removal of either Trustee and no appointment of a successor Trustee pursuant to this Article Six shall become effective until the acceptance of appointment by the successor Trustee in accordance with the applicable requirements of Section 6.10.
(2) Either Trustee may resign at any time with respect to the Securities of one or more series by giving written notice thereof to the Company. If the instrument of acceptance by a successor Trustee required by Section 6.10 shall not have been delivered to such Trustee within 30 days after the giving of such notice of resignation, the resigning Trustee may petition any court of competent jurisdiction for the appointment of a successor Trustee with respect to the Securities of such series.
(3) Either Trustee may be removed following 30 days notice at any time with respect to the Securities of any series by Act of the Holders of not less than a majority in principal amount of the Outstanding Securities of such series, delivered to such Trustee and to the Company.
(4) If at any time:
(i) either Trustee shall acquire any conflicting interest as defined in TIA Section 310(b) and fail to comply with the provisions of TIA Section 310(b)(i), or
(ii) either Trustee shall fail to comply with the provisions of TIA Section 310(b) after written request therefor by the Company or by any Holder who has been a bona fide Holder of a Security for at least six months, or
(iii) either Trustee shall cease to be eligible under Section 6.08 and shall fail to resign after written request therefor by the Company or by any Holder who has been a bona fide Holder of a Security for at least six months, or
(iv) either Trustee shall become incapable of acting or shall be adjudged a bankrupt or insolvent or a receiver of such Trustee or of its property shall be appointed or any public officer shall take charge or control of such Trustee or of its property or affairs for the purpose of rehabilitation, conservation or liquidation,
then, in any such case, (i) the Company, by a Board Resolution, may remove such Trustee with respect to all Securities, or (ii) subject to TIA Section 315(e), any Holder who has been a bona fide Holder of a Security of such series for at least six months may, on behalf of himself and all others similarly situated, petition any court of competent jurisdiction for the removal of such Trustee with respect to all Securities of such series and the appointment of a successor Trustee or Trustees.
(5) If either Trustee shall resign, be removed or become incapable of acting, or if a vacancy shall occur in the office of the U.S. Trustee or the Canadian Trustee for any cause, with respect to the Securities of one or more series, the Company, by a Board Resolution, shall promptly appoint a successor Trustee or Trustees with respect to the Securities of that or those series (it being understood that any such successor Trustee may be appointed with respect to the Securities of one or more or all of such series) provided, however, that the Company shall not be required to appoint a successor Trustee to the Canadian Trustee if the Canadian Trustee resigns or is removed and a Canadian Trustee under this Indenture is no longer required under Trust Indenture Legislation. If, within one year after such resignation, removal or incapability, or the occurrence of such vacancy, a successor Trustee with respect to the Securities of any series shall be appointed by Act of the Holders of a majority in principal amount of the Outstanding Securities of such series delivered to the Company and the retiring Trustee, the successor Trustee so appointed shall, forthwith upon its acceptance of such appointment, become the successor Trustee with respect to the Securities of such series and to that extent supersede the successor Trustee appointed by the Company. If no successor Trustee with respect to the Securities of any series shall have been so appointed by the Company or the Holders and accepted appointment in the manner hereinafter provided, any Holder who has been a bona fide Holder of a Security of such series for at least six months may, on behalf of himself and all others similarly situated, petition any court of competent jurisdiction for the appointment of a successor Trustee with respect to the Securities of such series.
(6) The Company shall give notice of each resignation and each removal of a Trustee with respect to the Securities of any series and each appointment of a successor Trustee with respect to the Securities of any series to the Holders of Securities of such series in the manner provided for in Section 1.07. Each notice shall include the name of the successor Trustee with respect to the Securities of such series and the address of its Corporate Trust Office.
(7) If a Canadian Trustee under this Indenture is no longer required by Trust Indenture Legislation, then the Company by a Board Resolution may remove the Canadian Trustee.
SECTION 6.10 Acceptance of Appointment by Successor.
(1) In case of the appointment hereunder of a successor Trustee with respect to all Securities, every such successor Trustee so appointed shall execute, acknowledge and deliver to the Company and to the retiring Trustee an instrument accepting such appointment, and thereupon the resignation or removal of the retiring Trustee shall become effective and such successor Trustee, without any further act, deed or conveyance, shall become vested with all the rights, powers, trusts and duties of the retiring Trustee; but, on the request of the Company or the successor Trustee, such retiring Trustee shall, upon payment of its charges, execute and deliver an instrument transferring to such successor Trustee all the rights, powers and trusts of the retiring Trustee and shall duly assign, transfer and deliver to such successor Trustee all property and money held by such retiring Trustee hereunder.
(2) In case of the appointment hereunder of a successor Trustee with respect to the Securities of one or more (but not all) series, the Company, the retiring Trustee and each successor Trustee with respect to the Securities of one or more series shall execute and deliver an indenture supplemental hereto wherein each successor Trustee shall accept such appointment and which (1) shall contain such provisions as shall be necessary or desirable to transfer and confirm to, and to vest in, each successor Trustee all the rights, powers, trusts and duties of the retiring Trustee with respect to the Securities of that or those series to which the appointment of such successor Trustee relates, (2) if the retiring Trustee is not retiring with respect to all Securities, shall contain such provisions as shall be deemed necessary or desirable to confirm that all the rights, powers, trusts and duties of the retiring Trustee with respect to the Securities of that or those series as to which the retiring Trustee is not retiring shall continue to be vested in the retiring Trustee, and (3) shall add to or change any of the provisions of this Indenture as shall be necessary to provide for or facilitate the administration of the trusts hereunder by more than one Trustee, it being understood that nothing herein or in such supplemental indenture shall constitute such Trustees co-trustees of the same trust and that each such Trustee shall be trustee of a trust or trusts hereunder separate and apart from any trust or trusts hereunder administered by any other such Trustee; and upon the execution and delivery of such supplemental indenture the resignation or removal of the retiring Trustee shall become effective to the extent provided therein and each such successor Trustee, without any further act, deed or conveyance, shall become vested with all the rights, powers, trusts and duties of the retiring Trustee with respect to the Securities of that or those series to which the appointment of such successor Trustee relates; but, on request of the Company or any successor Trustee, such retiring Trustee shall duly assign, transfer and deliver to such successor Trustee all property and money held by such retiring Trustee hereunder with respect to the Securities of that or those series to which the appointment of such successor Trustee relates. Whenever there is a successor Trustee with respect to one or more (but less than all) series of Securities issued pursuant to this Indenture, the terms "Indenture" and "Securities" shall have the meanings specified in the provisos to the respective definitions of those terms in Section 1.01 which contemplate such situation.
(3) Upon reasonable request of any such successor Trustee, the Company shall execute any and all instruments for more fully and certainly vesting in and confirming to such successor Trustee all rights, powers and trusts referred to in paragraph (1) or (2) of this Section 6.10, as the case may be.
(4) No successor Trustee shall accept its appointment unless at the time of such acceptance such successor Trustee shall be qualified and eligible under this Article Six.
SECTION 6.11 Merger, Conversion, Consolidation or Succession to Business.
Any corporation into which either Trustee or its corporate trust business may be merged or converted or with which it may be consolidated, or any corporation resulting from any merger, conversion or consolidation to which either Trustee shall be a party, or any corporation succeeding to all or substantially all the corporate trust business of either Trustee, shall be the successor of such Trustee hereunder, provided such corporation shall be otherwise qualified and eligible under this Article Six, without the execution or filing of any paper or any further act on the part of any of the parties hereto. In case any Securities shall have been authenticated, but not delivered, by a Trustee then in office, any successor by merger, conversion or consolidation to such authenticating Trustee may adopt such authentication and deliver the Securities so authenticated with the same effect as if such successor Trustee had itself authenticated such Securities. In case any of the Securities shall not have been authenticated by such predecessor Trustee, any successor Trustee may authenticate such Securities either in the name of any predecessor hereunder or in the name of the successor Trustee. In all such cases such certificates shall have the full force and effect which this Indenture provides for the certificate of authentication of such Trustee; provided, however, that the right to adopt the certificate of authentication of any predecessor Trustee or to authenticate Securities in the name of any predecessor Trustee shall apply only to its successor or successors by merger, conversion or consolidation.
SECTION 6.12 Appointment of Authenticating Agent.
At any time when any of the Securities remain outstanding, the Trustees may appoint an Authenticating Agent or Agents, with respect to one or more series of Securities which shall be authorized to act on behalf of the Trustees to authenticate Securities of such series and the Trustees shall give written notice of such appointment to all Holders of Securities of the series with respect to which such Authenticating Agent will serve, in the manner provided for in Section 1.07. Securities so authenticated shall be entitled to the benefits of this Indenture and shall be valid and obligatory for all purposes as if authenticated by the applicable Trustee hereunder. Any such appointment shall be evidenced by an instrument in writing signed by a Responsible Officer of the Trustees, and a copy of such instrument shall be promptly furnished to the Company. Wherever reference is made in this Indenture to the authentication and delivery of Securities by the Trustees or either Trustee's certificate of authentication, such reference shall be deemed to include authentication and delivery on behalf of the Trustees by an Authenticating Agent and a certificate of authentication executed on behalf of the Trustees by an Authenticating Agent. Each Authenticating Agent shall be acceptable to the Company and shall at all times be a corporation organized and doing business under the laws of the United States of America, any state thereof or the District of Columbia or the laws of Canada or any province thereof, authorized under such laws to act as Authenticating Agent, having a combined capital and surplus of not less than $50,000,000 and subject to supervision or examination by United States federal or state or Canadian federal or provincial authority. If such corporation publishes reports of condition at least annually, pursuant to law or to the requirements of said supervising or examining authority, then for the purposes of this Section 6.12, the combined capital and surplus of such corporation shall be deemed to be its combined capital and surplus as set forth in its most recent report of condition so published. If at any time an Authenticating Agent shall cease to be eligible in accordance with the provisions of this Section 6.12, it shall resign immediately in the manner and with the effect specified in this Section 6.12.
Any corporation into which an Authenticating Agent may be merged or converted or with which it may be consolidated, or any corporation resulting from any merger, conversion or consolidation to which such Authenticating Agent shall be a party, or any corporation succeeding to the corporate agency or corporate trust business of an Authenticating Agent, shall continue to be an Authenticating Agent, provided such corporation shall be otherwise eligible under this Section 6.12, without the execution or filing of any paper or any further act on the part of the Trustees or the Authenticating Agent.
An Authenticating Agent may resign at any time by giving written notice thereof to the Trustees and to the Company. The Trustees may at any time terminate the agency of an Authenticating Agent by giving written notice thereof to such Authenticating Agent and to the Company. Upon receiving such a notice of resignation or upon such a termination, or in case at any time such Authenticating Agent shall cease to be eligible in accordance with the provisions of this Section 6.12, the Trustees may appoint a successor Authenticating Agent which shall be acceptable to the Company and shall give written notice of such appointment to all Holders of Securities of the series with respect to which such Authenticating Agent will serve, in the manner provided for in Section 1.07. Any successor Authenticating Agent upon acceptance of its appointment hereunder shall become vested with all the rights, powers and duties of its predecessor hereunder, with like effect as if originally named as an Authenticating Agent. No successor Authenticating Agent shall be appointed unless eligible under the provisions of this Section 6.12.
The Trustees agree to pay to each Authenticating Agent from time to time reasonable compensation for its services under this Section 6.12, and the Trustees shall be entitled to be reimbursed for such payments, subject to the provisions of Section 6.07.
If an appointment with respect to one or more series is made pursuant to this Section 6.12, the Securities of such series may have endorsed thereon, in addition to either Trustee's certificate of authentication, an alternate certificate of authentication in the following form:
(Certificate of Authentication may be executed by either Trustee)
_____________________, as U.S. Trustee, certifies that this is one of the Securities of the series designated therein referred to in the within-mentioned Indenture.
Dated: ____________
| |
, |
| |
as U.S. Trustee |
| |
|
|
| |
By: |
|
| |
|
As Authenticating Agent |
| |
|
|
| |
By: |
|
| |
|
Authorized Officer |
_____________________, as Canadian Trustee, certifies that this is one of the Securities of the series designated therein referred to in the within-mentioned Indenture.
Dated: ____________
| |
, |
| |
as Canadian Trustee |
| |
|
|
| |
By: |
|
| |
|
As Authenticating Agent |
| |
|
|
| |
By: |
|
| |
|
Authorized Officer |
SECTION 6.13 Joint Trustees.
The rights, powers, duties and obligations conferred and imposed upon the Trustees are conferred and imposed upon and shall be exercised and performed by the U.S. Trustee and the Canadian Trustee individually, except to the extent the Trustees are required under Trust Indenture Legislation to perform such acts jointly, and neither Trustee shall be liable or responsible for the acts or omissions of the other Trustee. If the U.S. Trustee and Canadian Trustee are unable to agree jointly to act or refrain from acting, the applicable Trustee shall make the decision in accordance with its applicable legislation. Unless the context implies or requires otherwise, any written notice, request, direction, certificate, instruction, opinion or other document (each such document, a "Writing") delivered pursuant to any provision of this Indenture to any of the U.S. Trustee or the Canadian Trustee shall be deemed for all purposes of this Indenture as delivery of such Writing to the Trustee. Each such Trustee in receipt of such Writing shall notify such other Trustee of its receipt of such Writing within two Business Days of such receipt provided, however, that any failure of such trustee in receipt of such Writing to so notify such other Trustee shall not be deemed as a deficiency in the delivery of such Writing to the Trustee.
SECTION 6.14 Other Rights of Trustees.
Each Trustee shall retain the right not to act and shall not be liable for refusing to act if, due to a lack of information or for any other reason whatsoever, either Trustee, in its sole judgment, determines that such act might cause it to be in non-compliance with any applicable anti-money laundering or anti-terrorist legislation, regulation or guideline. Further, should either Trustee, in its sole judgment, determine at any time that its acting under this Indenture has resulted in its being in non-compliance with any applicable anti-money laundering or anti-terrorist legislation, regulation or guideline, then it shall have the right to resign on 10 days written notice to all parties provided (i) that such Trustee's written notice shall describe the circumstances of such non-compliance; and (ii) that if such circumstances are rectified to such Trustee's satisfaction within such 10 day period, then such resignation shall not be effective.
The parties hereto acknowledge that Canadian federal and provincial legislation addressing the protection of individuals' personal information (collectively, "Privacy Laws") applies to obligations and activities under this Indenture. Despite any other provision of this Indenture, neither party shall take or direct any action that would contravene, or cause the other to contravene, applicable Privacy Laws. The Company, prior to transferring, or causing to be transferred, personal information to the Canadian Trustee, shall obtain and retain required consents of the relevant individuals to the collection, use and disclosure of their personal information, or shall have determined that such consents either have been previously given and can be relied on or are not required under Privacy Laws. The Canadian Trustee shall use commercially reasonable efforts to ensure that its services hereunder comply with Privacy Laws. Specifically, the Trustee agrees to (i) have designated a chief privacy officer; (ii) maintain policies and procedures to protect personal information and to receive and respond to any privacy complaint or inquiry; (iii) use personal information solely for the purposes of providing its services under or ancillary to this Indenture and not to use it for any other purpose except with the consent and direction of the Company; (iv) not sell or otherwise improperly disclose personal information to any third party; and (v) use employee administrative, physical and technological safeguards to reasonably secure and protect personal information against loss, theft or unauthorized access, use or modification.
It is expressly acknowledged and agreed that the Canadian Trustee may, in the course of providing services hereunder, collect or receive, use and disclose financial and other personal information about such parties and/or their representatives, as individuals, or about other individuals related to the subject matter hereof, and use such information for the following purposes:
(i) to provide the services required under this Indenture and other services that may be requested from time to time;
(ii) to help the Canadian Trustee manage its servicing relationships with such individuals;
(iii) to meet the Canadian Trustee's legal and regulatory requirements; and
(iv) if social insurance numbers are collected by the Canadian Trustee, to perform tax reporting and to assist in verification of an individual's identity for security purposes.
Further, each party agrees that it shall not provide or cause to be provided to the Canadian Trustee any personal information relating to an individual who is not a party to this Indenture unless that party has assured itself that such individual understands and has consented to the aforementioned uses and disclosures. Notwithstanding anything to the contrary herein, the Company and the Trustees may, without liability, disclose information about the Holders and beneficial owners or potential Holders or potential beneficial owners of the Securities pursuant to subpoena or other order issued by a court of competent jurisdiction or when otherwise required by applicable law.
Each Trustee hereby accepts the trusts in this Indenture declared and provided for and agrees to perform the same upon the terms and conditions herein set forth and to hold all rights, privileges and benefits conferred hereby and by law in trust for the various persons who shall from time to time be holders, subject to all the terms and conditions herein set forth.
ARTICLE SEVEN
HOLDERS' LISTS AND REPORTS BY TRUSTEE AND COMPANY
SECTION 7.01 Company to Furnish Trustees Names and Addresses of Holders.
The Company will furnish or cause to be furnished to the Trustees (1) not more than 15 days after each Regular Record Date, or such lesser time as required by the Trustees, a list, in such form as the Trustees may reasonably require, of the names and addresses of Holders as of such Regular Record Date; provided, however, that the Company shall not be obligated to furnish or cause to be furnished such list at any time that the list shall not differ in any respect from the most recent list furnished to the Trustees by the Company or at such times as either Trustee is acting as Security Registrar for the applicable series of Securities and (2) at such other times as the Trustees may request in writing within 30 days after the receipt by the Company of any such request, a list of similar form and content as of a date not more than 15 days prior to the time such list is furnished.
SECTION 7.02 Preservation of List of Names and Addresses of Holders.
The Trustees shall preserve, in as current a form as is reasonably practicable, all information as to the names and addresses of the Holders contained in the most recent list furnished to them as provided in Section 7.01 and as to the names and addresses of Holders received by either Trustee in its capacity as Security Registrar for the applicable series of Securities (if acting in such capacity).
The Trustees may destroy any list furnished as provided in Section 7.01 upon receipt of a new list so furnished.
Holders may communicate as provided in TIA Section 312(b) with other Holders with respect to their rights under this Indenture or under the Securities.
SECTION 7.03 Disclosure of Names and Addresses of Holders.
Every Holder of Securities , by receiving and holding the same, agrees with the Company and the Trustees that none of the Company or the Trustees or any agent of either of them shall be held accountable by reason of the disclosure of any such information as to the names and addresses of the Holders in accordance with TIA Section 312, regardless of the source from which such information was derived, and that the Trustees shall not be held accountable by reason of mailing any material pursuant to a request made under TIA Section 312(b).
SECTION 7.04 Reports by Trustees.
(1) Within 60 days after May 15 of each year commencing with the first year after the first issuance of Securities pursuant to this Indenture, the U.S. Trustee shall transmit to the Holders of Securities, in the manner and to the extent provided in TIA Section 313(c), a brief report dated as of such reporting date, if required by TIA Section 313(a).
(2) The U.S. Trustee shall comply with TIA Sections 313(b) and 313(c).
(3) A copy of such report shall, at the time of such transmission to the Holders, be filed by the U.S. Trustee with the Company, with each securities exchange upon which any of the Securities are listed (if so listed) and also with the Commission. The Company agrees to notify the Trustees when the Securities become listed on any securities exchange.
SECTION 7.05 Reports by the Company.
(1) The Company will file with the Trustees, within 20 days after filing with or furnishing to the Commission, copies of its annual reports and of the information, documents and other reports (or copies of such portions of any of the foregoing as the Commission may by rules and regulations prescribe) which the Company is required to file or furnish with the Commission pursuant to Section 13 or 15(d) of the Exchange Act or, if the Company is not required to file information, documents or reports pursuant to either of such sections, then to file with the Trustees and the Commission, in accordance with rules and regulations prescribed by the Commission, such of the supplementary and periodic information, documents and reports which may be required pursuant to Section 13 of the Exchange Act in respect of a security listed and registered on a national securities exchange as may be prescribed in such rules and regulations; provided that any such reports, information or documents filed with the Commission pursuant to its Electronic Data Gathering, Analysis and Retrieval (EDGAR) system shall be deemed filed with the Trustees.
(2) The Company will transmit to all Holders, in the manner and to the extent provided in TIA Section 313(c), within 30 days after the filing thereof with the Trustees, such summaries of any information, documents and reports required to be filed by the Company pursuant to paragraph (1) of this Section 7.05 as may be required by rules and regulations prescribed from time to time by the Commission.
(3) If at any time the Securities are guaranteed by a direct or indirect parent of the Company, and such parent has furnished the reports required by this Section 7.05 with respect to parent as required by this Section 7.05 as if parent were the Company (including any financial information required hereby), the Company shall be deemed to be in compliance with this Section 7.05.
ARTICLE EIGHT
CONSOLIDATION, MERGER, CONVEYANCE, TRANSFER OR LEASE
SECTION 8.01 Company May Consolidate, etc., only on Certain Terms.
The Company shall not amalgamate or consolidate with or merge into or enter into any statutory arrangement with any other Person, or, directly or indirectly, convey, transfer or lease all or substantially all of its properties and assets to any Person, unless:
(1) the Person formed by or continuing from such amalgamation or consolidation or into which the Company is merged or with which it enters into such statutory arrangement or the Person which acquires by operation of law or by conveyance or transfer, or which leases, all or substantially all of the properties and assets of the Company shall be a corporation, partnership or trust organized and validly existing under the laws of Canada or any province or territory thereof, the United States of America or any state thereof or the District of Columbia or, if such amalgamation, consolidation, merger, statutory arrangement or other transaction would not impair the rights of Holders, any other country, and, unless the Company is the continuing corporation, shall expressly assume, by an indenture supplemental hereto, executed and delivered to the Trustees, in form satisfactory to the Trustees, the Company's obligation for the due and punctual payment of the principal of, premium (if any) and interest (if any) on all the Securities and the performance and observance of every covenant of this Indenture on the part of the Company to be performed or observed;
(2) immediately after giving effect to such transaction, no Default or Event of Default shall have happened and be continuing; and
(3) the Company or such Person shall have delivered to the Trustees an Officer's Certificate and an Opinion of Counsel, each stating that such amalgamation, consolidation, merger, statutory arrangement or other transaction and such supplemental indenture comply with this Article Eight and that all conditions precedent herein provided for relating to such transaction have been complied with.
Notwithstanding the above, the Company may consolidate with, amalgamate with, undergo an arrangement with, merge with or into an Affiliate of the Company solely for the purpose of reincorporating the Company in a state of the United States or the District of Columbia or in another province or territory of Canada.
This Section 8.01 shall only apply to a merger, consolidation or amalgamation in which the Company is not the surviving Person and to conveyances, leases and transfers by the Company as transferor or lessor.
SECTION 8.02 Successor Person Substituted.
Upon any amalgamation or consolidation by the Company with or merger by the Company into any other corporation or a statutory arrangement or any conveyance, transfer or lease of all or substantially all of the properties and assets of the Company to any Person in accordance with Section 8.01, the successor Person formed by such amalgamation or consolidation or into which the Company is merged or statutory arrangement, or to which such conveyance, transfer or lease is made shall succeed to, and be substituted for, and may exercise every right and power of, the Company under this Indenture with the same effect as if such successor Person had been named as the Company herein, and in the event of any such conveyance or transfer, the Company (which term shall for this purpose mean the Person named as the "Company" in the first paragraph of this Indenture or any successor Person which shall theretofore become such in the manner described in Section 8.01), except in the case of a lease, shall be discharged of all obligations and covenants under this Indenture and the Securities and may be dissolved and liquidated.
ARTICLE NINE
SUPPLEMENTAL INDENTURES
SECTION 9.01 Supplemental Indentures Without Consent of Holders.
Notwithstanding Section 9.02, without the consent of any Holders, the Company, when authorized by or pursuant to a Board Resolution, and the Trustees, at any time and from time to time, may enter into one or more indentures supplemental hereto, in form satisfactory to the Trustees, for any of the following purposes:
(1) to evidence the succession of another Person to the Company and the assumption by any such successor of the covenants of the Company contained herein and in the Securities; or
(2) to add to the covenants of the Company for the benefit of the Holders of all or any series of Securities (and if such covenants are to be for the benefit of less than all series of Securities, stating that such covenants are being included solely for the benefit of such series) or to surrender any right or power herein conferred upon the Company; or
(3) to add any additional Events of Default (and if such Events of Default are to be for the benefit of less than all series of Securities, stating that such Events of Default are being included solely for the benefit of such series); or
(4) to delete or modify any Events of Default with respect to a series of the Securities, the form and terms of which are being established pursuant to such supplemental indenture as permitted in Section 3.01; or
(5) to change or eliminate any of the provisions of this Indenture; provided that any such change or elimination shall become effective only when there is no Security Outstanding of any series created prior to the execution of such supplemental indenture which is entitled to the benefit of such provision; or
(6) to establish the form or terms of Securities of any series as permitted by Sections 2.01 and 3.01; or
(7) to evidence and provide for the acceptance of appointment hereunder by a successor Trustee with respect to the Securities of one or more series and to add to or change any of the provisions of this Indenture as shall be necessary to provide for or facilitate the administration of the trusts hereunder by more than one Trustee, pursuant to the requirements of Section 6.10; or
(8) to close this Indenture with respect to the authentication and delivery of additional series of Securities; or
(9) to cure any ambiguity or to correct or supplement any provision contained herein or in any indenture supplemental hereto which may be defective or inconsistent with any other provision contained herein or in any supplemental indenture or to conform the terms hereof, as amended and supplemented, that are applicable to the Securities of any series to the description of the terms of such Securities in the offering memorandum, prospectus supplement or other offering document applicable to such Securities at the time of initial sale thereof; or
(10) to make any change in any series of Securities that does not adversely affect in any material respect the rights of the Holders of such Securities; or
(11) to add to or change or eliminate any provision of this Indenture as shall be necessary or desirable in accordance with any amendments to the Trust Indenture Act; or
(12) to supplement any of the provisions of this Indenture to such extent as shall be necessary to permit or facilitate the defeasance and discharge of any series of Securities pursuant to Sections 4.01, 14.02 and 14.03; provided that any such action shall not adversely affect the interests of the Holders of Securities of such series or any other series of Securities in any material respect; or
(13) to modify, eliminate or add to the provisions of this Indenture to such extent as shall be necessary to effect the qualifications of this Indenture under any applicable law of the United States and Canada or of any province or territory thereof to the extent they do not conflict with the applicable law of the United States heretofore or hereafter enacted.
SECTION 9.02 Supplemental Indentures with Consent of Holders.
Except as provided in Section 9.01 and this Section 9.02, with the consent of the Holders of not less than a majority in principal amount of all Outstanding Securities affected by such supplemental indenture, by Act of said Holders delivered to the Company and the Trustees, the Company, when authorized by or pursuant to a Board Resolution, and the Trustees may enter into an indenture or indentures supplemental hereto for the purpose of adding any provisions to or changing in any manner or eliminating any of the provisions of this Indenture which affect such series of Securities or of modifying in any manner the rights of the Holders of Securities of such series under this Indenture; provided, however, that no such supplemental indenture shall, without the consent of the Holder of each Outstanding Security of such series,
(1) change the Stated Maturity of the principal of, premium (if any) or any installment of interest (if any) on any Security of such series, or reduce the principal amount thereof, premium (if any) or the rate of interest (if any) thereon, or reduce the amount of the principal of an Original Issue Discount Security of such series that would be due and payable upon a declaration of acceleration of the Maturity thereof pursuant to Section 5.02 or the amount thereof provable in bankruptcy pursuant to Section 5.04, or adversely affect any right of repayment at the option of any Holder of any Security of such series, or change any Place of Payment where, or the Currency in which, any Security of such series or any premium or interest thereon is payable, or impair the right to institute suit for the enforcement of any such payment on or after the Stated Maturity thereof (or, in the case of redemption or repayment at the option of the Holder, on or after the Redemption Date or Repayment Date, as the case may be), or adversely affect any right to convert or exchange any Security as may be provided pursuant to Section 3.01 herein, or
(2) reduce the percentage in principal amount of the Outstanding Securities of such series required for any such supplemental indenture, or the consent of whose Holders is required for any waiver of compliance with certain provisions of this Indenture which affect such series or certain defaults applicable to such series hereunder and their consequences provided for in this Indenture, or
(3) modify any of the provisions of this 9.02 Section, Section 5.13 or Section 10.09, except to increase any such percentage or to provide that certain other provisions of this Indenture which affect such series cannot be modified or waived without the consent of the Holder of each Outstanding Security of such series.
A supplemental indenture which changes or eliminates any covenant or other provision of this Indenture which has expressly been included solely for the benefit of one or more particular series of Securities, or which modifies the rights of the Holders of Securities of such series with respect to such covenant or other provision, shall be deemed not to affect the rights under this Indenture of the Holders of Securities of any other series. Any such supplemental indenture adding any provisions to or changing in any manner or eliminating any of the provisions of this Indenture, or modifying in any manner the rights of the Holders of Securities of such series, shall not affect the rights under this Indenture of the Holders of Securities of any other series.
It shall not be necessary for any Act of Holders under this 9.02 Section to approve the particular form of any proposed supplemental indenture, but it shall be sufficient if such Act shall approve the substance thereof.
SECTION 9.03 Execution of Supplemental Indentures.
In executing, or accepting the additional trusts created by, any supplemental indenture permitted by this Article Nine or the modifications thereby of the trusts created by this Indenture, the Trustees shall be entitled to receive, and shall be fully protected in relying upon, an Opinion of Counsel stating that the execution of such supplemental indenture is authorized or permitted by this Indenture. Each Trustee may, but shall not be obligated to, enter into any such supplemental indenture which affects such Trustee's own rights, duties or immunities under this Indenture or otherwise.
SECTION 9.04 Effect of Supplemental Indentures.
Upon the execution of any supplemental indenture under this Article Nine, this Indenture shall be modified in accordance therewith, and such supplemental indenture shall form a part of this Indenture for all purposes; and every Holder of Securities theretofore or thereafter authenticated and delivered hereunder shall be bound thereby.
SECTION 9.05 Conformity with Trust Indenture Legislation.
Every supplemental indenture executed pursuant to this Article Nine shall conform to the requirements of Trust Indenture Legislation as then in effect.
SECTION 9.06 Reference in Securities to Supplemental Indentures.
Securities of any series authenticated and delivered after the execution of any supplemental indenture pursuant to this Article Nine may, and shall if required by the Trustees, bear a notation in form approved by the Trustees as to any matter provided for in such supplemental indenture. If the Company shall so determine, new Securities of any series so modified as to conform, in the opinion of the Trustees and the Company, to any such supplemental indenture may be prepared and executed by the Company and authenticated and delivered by the Trustees in exchange for outstanding Securities of such series.
SECTION 9.07 Notice of Supplemental Indentures.
Promptly after the execution by the Company and the Trustees of any supplemental indenture pursuant to the provisions of Section 9.02, the Company shall give notice thereof to the Holders of each outstanding Security affected, in the manner provided for in Section 1.07, setting forth in general terms the substance of such supplemental indenture.
ARTICLE TEN
COVENANTS
SECTION 10.01 Payment of Principal, Premium and Interest.
The Company covenants and agrees for the benefit of the Holders of each series of Securities that it will duly and punctually pay the principal of, premium (if any) and interest (if any), on the Securities of that series in accordance with the terms of the Securities and this Indenture.
SECTION 10.02 Maintenance of Office or Agency.
(1) The Company will maintain in each Place of Payment for any series of Securities an office or agency where Securities of that series may be presented or surrendered for payment, where Securities of that series may be surrendered for registration of transfer or exchange, where Securities of that series that are convertible or exchangeable may be surrendered for conversion or exchange, as applicable, and where notices and demands to or upon the Company in respect of the Securities of that series and this Indenture may be served .
(2) The Company will give prompt written notice to the Trustees of the location, and any change in the location, of such office or agency. If at any time the Company shall fail to maintain any such required office or agency or shall fail to furnish the Trustees with the address thereof, such presentations, surrenders, notices and demands may be made or served at the Corporate Trust Offices of the Trustees.
(3)
(4) The Company may also from time to time designate one or more other offices or agencies where the Securities of one or more series may be presented or surrendered for any or all such purposes and may from time to time rescind any such designation; provided, however, that no such designation or rescission shall in any manner relieve the Company of its obligation to maintain an office or agency in accordance with the requirements set forth above for Securities of any series for such purposes. The Company will give prompt written notice to the Trustees of any such designation or rescission and of any change in the location of any such other office or agency. Unless otherwise specified with respect to any Securities as contemplated by Section 3.01 with respect to a series of Securities, the Company hereby initially appoints the U.S. Trustee at its Corporate Trust Office as Paying Agent in such city and as its agent to receive all such presentations, surrenders, notices and demands.
(5) Unless otherwise specified with respect to any Securities pursuant to Section 3.01, if and so long as the Securities of any series (i) are denominated in a Currency other than Dollars or (ii) may be payable in a Currency other than Dollars, or so long as it is required under any other provision of the Indenture, then the Company will maintain with respect to each such series of Securities, or as so required, at least one Exchange Rate Agent.
SECTION 10.03 Money for Securities Payments to Be Held in Trust.
If the Company shall at any time act as its own Paying Agent with respect to any series of Securities , it will, on or before each due date of the principal of, premium (if any) or interest (if any) on any of the Securities of that series, segregate and hold in trust for the benefit of the Persons entitled thereto a sum in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)) sufficient to pay the principal of, premium (if any) or interest (if any) on Securities of such series so becoming due until such sums shall be paid to such Persons or otherwise disposed of as herein provided and will promptly notify the Trustees of its action or failure so to act.
Whenever the Company shall have one or more Paying Agents for any series of Securities, it will, prior to or on each due date of the principal of, premium (if any) or interest (if any) on any Securities of that series, deposit with a Paying Agent a sum (in the Currency described in the preceding paragraph) sufficient to pay the principal, premium (if any) or interest (if any) so becoming due, such sum to be held in trust for the benefit of the Persons entitled to such principal, premium or interest, and (unless such Paying Agent is a Trustee) the Company will promptly notify the Trustees of its action or failure so to act.
The Company will cause each Paying Agent (other than the Trustees) for any series of Securities to execute and deliver to the Trustees an instrument in which such Paying Agent shall agree with the Trustees, subject to the provisions of this 10.03 Section, that such Paying Agent will:
(1) hold all sums held by it for the payment of the principal of, premium (if any) and interest (if any) on Securities of such series in trust for the benefit of the Persons entitled thereto until such sums shall be paid to such Persons or otherwise disposed of as herein provided;
(2) give the Trustees notice of any default by the Company (or any other obligor upon the Securities of such series) in the making of any payment of principal of, premium (if any) or interest (if any) on the Securities of such series; and
(3) at any time during the continuance of any such default, upon the written request of the Trustees, forthwith pay to the Trustees all sums so held in trust by such Paying Agent.
The Company may at any time, for the purpose of obtaining the satisfaction and discharge of this Indenture or for any other purpose, pay, or by Company Order direct any Paying Agent to pay, to the Trustees all sums held in trust by the Company or such Paying Agent, such sums to be held by the Trustees upon the same trusts as those upon which sums were held by the Company or such Paying Agent; and, upon such payment by any Paying Agent to the Trustees, such Paying Agent shall be released from all further liability with respect to such sums.
Except as provided in the Securities of any series, any money deposited with the Trustees or any Paying Agent, or then held by the Company, in trust for the payment of the principal of, premium (if any) or interest (if any) on any Security of any series, and remaining unclaimed for two years after such principal, premium or interest has become due and payable shall be paid to the Company on Company Request, or (if then held by the Company) shall be discharged from such trust; and the Holder of such Security shall thereafter, as an unsecured general creditor, look only to the Company for payment thereof, and all liability of the Trustees or such Paying Agent with respect to such trust money, and all liability of the Company as trustee thereof, shall thereupon cease.
SECTION 10.04 Statement as to Compliance.
The Company shall deliver to the Trustees, on or before 120 days after the end of the Company's fiscal year, an Officer's Certificate stating that a review of the activities of the Company during such fiscal year has been made under the supervision of the signing Officer with a view to determining whether the Company has kept, observed, performed and fulfilled its obligations under this Indenture, and further stating, as to such Officer, that the Company has kept, observed, performed and fulfilled each and every covenant contained in this Indenture and is not in default in the performance or observance of any of the terms, provisions and conditions hereof (or, if a Default or Event of Default shall have occurred and is continuing, describing all such Defaults or Events of Default of which he or she may have knowledge and what action the Company is taking or propose to take with respect thereto). The Company shall deliver to the Trustees upon demand evidence in such form as the Trustees may require as to compliance by the Company with any condition or covenant of the Company set out herein relating to any action required or permitted to be taken by the Company under this Indenture or as a result of any obligation imposed by this Indenture. For purposes of this Section 10.04, such compliance shall be determined without regard to any period of grace or requirement of notice under this Indenture.
SECTION 10.05 Payment of Taxes and Other Claims.
The Company will pay or discharge or cause to be paid or discharged, before the same shall become delinquent, (1) all material taxes, assessments and governmental charges levied or imposed upon the Company or upon the income, profits or property of the Company, and (2) all material lawful claims for labor, materials and supplies which, if unpaid, might by law become a Lien upon any property or assets of the Company; provided, however, that the Company shall not be required to pay or discharge or cause to be paid or discharged any such tax, assessment, charge or claim whose amount, applicability or validity is being contested in good faith by appropriate proceedings.
SECTION 10.06 Corporate Existence.
Subject to Article Eight, the Company will do or cause to be done all things necessary to preserve and keep in full force and effect its corporate existence and the rights (charter and statutory) and franchises of the Company; provided, however, that the Company shall not be required to preserve any such right or franchise if the Company shall determine that the preservation thereof is no longer desirable in the conduct of the business of the Company.
SECTION 10.07 Waiver of Certain Covenants.
The Company may, with respect to any series of Securities, omit in any particular instance to comply with any term, provision or condition which affects such series set forth in Sections 10.06 and 10.07, or, as specified pursuant to Section 3.01(19) for Securities of such series, in any covenants of the Company added to this Article Ten pursuant to Section 3.01(19) in connection with Securities of such series, if before the time for such compliance the Holders of at least a majority in principal amount of all Outstanding Securities of any series, by Act of such Holders, waive such compliance in such instance with such term, provision or condition, but no such waiver shall extend to or affect such term, provision or condition except to the extent so expressly waived, and, until such waiver shall become effective, the obligations of the Company and the duties of the Trustees to Holders of Securities of such series in respect of any such term, provision or condition shall remain in full force and effect.
ARTICLE ELEVEN
REDEMPTION OF SECURITIES
SECTION 11.01 Applicability of Article.
Securities of any series which are redeemable before their Stated Maturity shall be redeemable in accordance with the terms of such Securities and (except as otherwise specified as contemplated by Section 3.01 for Securities of any series) in accordance with this Article Eleven.
SECTION 11.02 Election to Redeem; Notice to Trustees.
The election of the Company to redeem any Securities shall be evidenced by or pursuant to a Board Resolution. In case of any redemption at the election of the Company, the Company shall, at least 60 days prior to the Redemption Date fixed by the Company (unless a shorter notice shall be satisfactory to the Trustees), notify the Trustees of such Redemption Date and of the principal amount of Securities of such series to be redeemed and, in the case of certificated Securities, shall deliver to the Trustees such documentation and records as shall enable the Trustees to select the Securities to be redeemed pursuant to Section 11.03. In the case of any redemption of Securities prior to the expiration of any restriction on such redemption provided in the terms of such Securities or elsewhere in this Indenture, the Company shall furnish to the Trustees an Officer's Certificate evidencing compliance with such restriction.
SECTION 11.03 Selection by Trustees of Securities to Be Redeemed.
If less than all the Securities of any series are to be redeemed, the particular Securities to be redeemed shall be selected not more than 60 days prior to the Redemption Date by the Trustees, from the Outstanding Securities of such series not previously called for redemption, in the case of certificated Securities, by such method as the Trustees shall deem fair and appropriate and which may provide for the selection for redemption of portions of the principal of Securities of such series, or in the case of Securities in global form in accordance with the policies and procedures of the applicable Depositary; provided, however, that no such partial redemption shall reduce the portion of the principal amount of a Security not redeemed to less than the minimum authorized denomination for Securities of such series established pursuant to Section 3.01.
The Trustees shall promptly notify the Company in writing of the Securities selected for redemption and, in the case of any Securities selected for partial redemption, the principal amount thereof to be redeemed.
For all purposes of this Indenture, unless the context otherwise requires, all provisions relating to the redemption of Securities shall relate, in the case of any Security redeemed or to be redeemed only in part, to the portion of the principal amount of such Security which has been or is to be redeemed.
SECTION 11.04 Notice of Redemption.
Except as otherwise specified as contemplated by Section 3.01, notice of redemption shall be given in the manner provided for in Section 1.07 not less than 30 nor more than 60 days prior to the Redemption Date, to each Holder of Securities to be redeemed. Failure to give notice in the manner provided in Section 1.07 to the Holder of any Securities designated for redemption as a whole or in part, or any defect in the notice to any such Holder, shall not affect the validity of the proceedings for the redemption of any other Securities or portion thereof.
All notices of redemption shall state:
(1) the Redemption Date,
(2) the Redemption Price and the amount of accrued interest to the Redemption Date payable as provided in Section 11.06, if any,
(3) if less than all the Outstanding Securities of any series are to be redeemed, the identification (and, in the case of partial redemption, the principal amounts) of the particular Securities to be redeemed,
(4) in case any Security is to be redeemed in part only, the notice which relates to such Security shall state that on and after the Redemption Date, upon surrender of such Security, the Holder will receive, without charge, a new Security or Securities of authorized denominations for the principal amount thereof remaining unredeemed,
(5) that on the Redemption Date, the Redemption Price and accrued interest (if any) to the Redemption Date payable as provided in Section 11.06 will become due and payable upon each such Security, or the portion thereof, to be redeemed and, if applicable, that interest thereon will cease to accrue on and after said date,
(6) the Place or Places of Payment where such Securities are to be surrendered for payment of the Redemption Price and accrued interest (if any),
(7) that the redemption is for a sinking fund, if such is the case, and
(8) if applicable, any condition to such redemption.
Notice of redemption of Securities to be redeemed at the election of the Company shall be given by the Company or, at the Company's request, by the Trustees in the name and at the expense of the Company.
SECTION 11.05 Deposit of Redemption Price.
Prior to any Redemption Date, the Company shall deposit with a Trustee or with a Paying Agent (or, if the Company is acting as its own Paying Agent, segregate and hold in trust as provided in Section 10.03) an amount of money in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)) sufficient to pay the Redemption Price of, and accrued interest (if any) on, all the Securities which are to be redeemed on that date.
SECTION 11.06 Securities Payable on Redemption Date.
Notice of redemption having been given as aforesaid, the Securities so to be redeemed shall, on the Redemption Date, become due and payable at the Redemption Price therein specified in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)) (together with accrued interest (if any) to the Redemption Date), and from and after such date (unless the Company shall default in the payment of the Redemption Price and accrued interest (if any)) such Securities shall, if the same were interest-bearing, cease to bear interest. Upon surrender of any such Security for redemption in accordance with said notice, such Security shall be paid by the Company at the Redemption Price, together with accrued interest (if any), to the Redemption Date; provided, however, that installments of interest on Securities whose Stated Maturity is on or prior to the Redemption Date shall be payable to the Holders of such Securities, or one or more Predecessor Securities, registered as such at the close of business on the relevant record dates according to their terms and the provisions of Section 3.07.
If any Security called for redemption shall not be so paid upon surrender thereof for redemption, the principal and premium (if any) shall, until paid, bear interest from the Redemption Date at the rate of interest or Yield to Maturity (in the case of Original Issue Discount Securities) set forth in such Security.
SECTION 11.07 Securities Redeemed in Part.
Any Security which is to be redeemed only in part (pursuant to the provisions of this Article Eleven or of Article Twelve) shall be surrendered at a Place of Payment therefor (with, if the Company or the Trustees so requires, due endorsement by, or a written instrument of transfer in form satisfactory to the Company and the Trustees duly executed by, the Holder thereof or such Holder's attorney duly authorized in writing), and the Company shall execute, and the applicable Trustee shall authenticate and deliver to the Holder of such Security without service charge, a new Security or Securities of the same series, of any authorized denomination as requested by such Holder, in aggregate principal amount equal to and in exchange for the unredeemed portion of the principal of the Security so surrendered.
ARTICLE TWELVE
SINKING FUNDS
SECTION 12.01 Applicability of Article.
Retirements of Securities of any series pursuant to any sinking fund shall be made in accordance with the terms of such Securities and (except as otherwise specified as contemplated by Section 3.01 for Securities of any series) in accordance with this Article Twelve.
The minimum amount of any sinking fund payment provided for by the terms of Securities of any series is herein referred to as a "mandatory sinking fund payment," and any payment in excess of such minimum amount provided for by the terms of Securities of any series is herein referred to as an "optional sinking fund payment". If provided for by the terms of Securities of any series, the cash amount of any mandatory sinking fund payment may be subject to reduction as provided in Section 12.02. Each sinking fund payment shall be applied to the redemption of Securities of any series as provided for by the terms of Securities of such series.
SECTION 12.02 Satisfaction of Sinking Fund Payments with Securities.
Subject to Section 12.03, in lieu of making all or any part of any mandatory sinking fund payment with respect to any Securities of a series in cash, the Company may at its option (1) deliver to the Trustees Outstanding Securities of a such series (other than any previously called for redemption) theretofore purchased or otherwise acquired by the Company, and/or (2) receive credit for the principal amount of Securities of such series which have been previously delivered to the Trustees by the Company or redeemed either at the election of the Company pursuant to the terms of such Securities or through the application of permitted optional sinking fund payments pursuant to the terms of such Securities, in each case in satisfaction of all or any part of any mandatory sinking fund payment with respect to the Securities of the same series required to be made pursuant to the terms of such Securities as provided for by the terms of such series; provided, however, that such Securities have not been previously so credited. Such Securities shall be received and credited for such purpose by the Trustees at the Redemption Price specified in such Securities for redemption through operation of the sinking fund and the amount of such mandatory sinking fund payment shall be reduced accordingly.
SECTION 12.03 Redemption of Securities for Sinking Fund.
Not less than 60 days prior to each sinking fund payment date for any series of Securities, the Company will deliver to the Trustees an Officer's Certificate specifying the amount of the next ensuing sinking fund payment for that series pursuant to the terms of that series, the portion thereof, if any, which is to be satisfied by payment of cash in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)) and the portion thereof, if any, which is to be satisfied by delivering or crediting Securities of that series pursuant to Section 12.02 (which Securities will, if not previously delivered, accompany such certificate) and whether the Company intends to exercise its right to make a permitted optional sinking fund payment with respect to such series.
Such certificate shall be irrevocable and upon its delivery the Company shall be obligated to make the cash payment or payments therein referred to, if any, on or before the next succeeding sinking fund payment date. In the case of the failure of the Company to deliver such certificate, the sinking fund payment due on the next succeeding sinking fund payment date for that series shall be paid entirely in cash and shall be sufficient to redeem the principal amount of such Securities subject to a mandatory sinking fund payment without the option to deliver or credit Securities as provided in Section 12.02 and without the right to make any optional sinking fund payment, if any, with respect to such series.
Not more than 60 days before each such sinking fund payment date the Trustees shall select the Securities to be redeemed upon such sinking fund payment date in the manner specified in Section 11.03 and cause notice of the redemption thereof to be given in the name of and at the expense of the Company in the manner provided in Section 11.04. Such notice having been duly given, the redemption of such Securities shall be made upon the terms and in the manner stated in Sections 11.06 and 11.07.
Prior to any sinking fund payment date, the Company shall pay to the Trustees or a Paying Agent (or, if the Company is acting as its own Paying Agent, segregate and hold in trust as provided in Section 10.03) in cash a sum equal to any interest that will accrue to the date fixed for redemption of Securities or portions thereof to be redeemed on such sinking fund payment date pursuant to this 12.03 Section.
Notwithstanding the foregoing, with respect to a sinking fund for any series of Securities, if at any time the amount of cash to be paid into such sinking fund on the next succeeding sinking fund payment date, together with any unused balance of any preceding sinking fund payment or payments for such series, does not exceed in the aggregate $100,000, the Trustees, unless requested by the Company, shall not give the next succeeding notice of the redemption of Securities of such series through the operation of the sinking fund. Any such unused balance of moneys deposited in such sinking fund shall be added to the sinking fund payment for such series to be made in cash on the next succeeding sinking fund payment date or, at the request of the Company, shall be applied at any time or from time to time to the purchase of Securities of such series, by public or private purchase, in the open market or otherwise, at a purchase price for such Securities (excluding accrued interest and brokerage commissions, for which the Trustees or any Paying Agent will be reimbursed by the Company) not in excess of the principal amount thereof.
ARTICLE THIRTEEN
REPAYMENT AT OPTION OF HOLDERS
SECTION 13.01 Applicability of Article.
Repayment of Securities of any series before their Stated Maturity at the option of Holders thereof shall be made in accordance with the terms of such Securities and (except as otherwise specified as contemplated by Section 3.01 for Securities of any series) in accordance with this Article Thirteen.
SECTION 13.02 Repayment of Securities.
Securities of any series subject to repayment in whole or in part at the option of the Holders thereof will, unless otherwise provided in the terms of such Securities, be repaid at a price equal to the principal amount thereof, together with interest (if any) thereon accrued to the Repayment Date specified in or pursuant to the terms of such Securities. The Company covenants that, with respect to such Securities, on or before the Repayment Date it will deposit with a Trustee or with a Paying Agent (or, if the Company is acting as its own Paying Agent, segregate and hold in trust as provided in Section 10.03) an amount of money in the Currency in which the Securities of such series are payable (except as otherwise specified pursuant to Section 3.01 for the Securities of such series and except, if applicable, as provided in Sections 3.12(b), 3.12(d) and 3.12(e)) sufficient to pay the principal (or, if so provided by the terms of the Securities of any series, a percentage of the principal) of and (except if the Repayment Date shall be an Interest Payment Date) accrued interest (if any) on, all the Securities or portions thereof, as the case may be, to be repaid on such date.
SECTION 13.03 Exercise of Option.
Securities of any series subject to repayment at the option of the Holders thereof will contain an "Option to Elect Repayment" form on the reverse of such Securities. To be repaid at the option of the Holder, any Security so providing for such repayment, with the "Option to Elect Repayment" form on the reverse of such Security duly completed by the Holder (or by the Holder's attorney duly authorized in writing), must be received by the Company at the Place of Payment therefor specified in the terms of such Security (or at such other place or places which the Company shall from time to time notify the Holders of such Securities) not earlier than 45 days nor later than 30 days prior to the Repayment Date. If less than the entire principal amount of such Security is to be repaid in accordance with the terms of such Security, the principal amount of such Security to be repaid, in increments of the minimum denomination for Securities of such series, and the denomination or denominations of the Security or Securities to be issued to the Holder for the portion of the principal amount of such Security surrendered that is not to be repaid, must be specified. The principal amount of any Security providing for repayment at the option of the Holder thereof may not be repaid in part if, following such repayment, the unpaid principal amount of such Security would be less than the minimum authorized denomination of Securities of the series of which such Security to be repaid is a part. Except as otherwise may be provided by the terms of any Security providing for repayment at the option of the Holder thereof, exercise of the repayment option by the Holder shall be irrevocable unless waived by the Company.
SECTION 13.04 When Securities Presented for Repayment Become Due and Payable.
If Securities of any series providing for repayment at the option of the Holders thereof shall have been surrendered as provided in this Article Thirteen and as provided by or pursuant to the terms of such Securities, such Securities or the portions thereof, as the case may be, to be repaid shall become due and payable and shall be paid by the Company on the Repayment Date therein specified, and on and after such Repayment Date (unless the Company shall default in the payment of such Securities on such Repayment Date) such Securities shall, if the same were interest- bearing, cease to bear interest. Upon surrender of any such Security for repayment in accordance with such provisions, the principal amount of such Security so to be repaid shall be paid by the Company, together with accrued interest (if any) to the Repayment Date; provided, however, that, in the case of Securities, installments of interest (if any) whose Stated Maturity is on or prior to the Repayment Date shall be payable to the Holders of such Securities, or one or more Predecessor Securities, registered as such at the close of business on the relevant Record Dates according to their terms and the provisions of Section 3.07.
If any Security surrendered for repayment shall not be so repaid upon surrender thereof for repayment, the principal amount and premium (if any) shall, until paid, bear interest from the Repayment Date at the rate of interest or Yield to Maturity (in the case of Original Issue Discount Securities) set forth in such Security.
SECTION 13.05 Securities Repaid in Part.
Upon surrender of any Security which is to be repaid in part only, the Company shall execute and the applicable Trustee shall authenticate and deliver to the Holder of such Security, without service charge and at the expense of the Company, a new Security or Securities of the same series, of any authorized denomination specified by the Holder, in an aggregate principal amount equal to and in exchange for the portion of the principal of such Security so surrendered which is not to be repaid.
ARTICLE FOURTEEN
DEFEASANCE AND COVENANT DEFEASANCE
SECTION 14.01 Company's Option to Effect Defeasance or Covenant Defeasance.
Except as otherwise specified as contemplated by Section 3.01 for Securities of any series, the provisions of this Article Fourteen shall apply to each series of Securities, and the Company may, at its option, effect defeasance of the Securities of or within a series under Section 14.02, or covenant defeasance of or within a series under Section 14.03 in accordance with the terms of such Securities and in accordance with this Article Fourteen.
SECTION 14.02 Defeasance and Discharge.
Upon the Company's exercise of the above option applicable to this Section 14.02 with respect to any Securities of or within a series, the Company shall be deemed to have been discharged from its obligations with respect to such Securities on the date the conditions set forth in Section 14.04 are satisfied (hereinafter, "defeasance"). For this purpose, such defeasance means that the Company shall be deemed to have paid and discharged the entire indebtedness represented by such Securities, which shall thereafter be deemed to be "Outstanding" only for the purposes of Section 14.05 and the other Sections of this Indenture referred to in (A) and (B) below, and to have satisfied all of its other obligations under such Securities and this Indenture insofar as such Securities are concerned (and the Trustees, at the expense of the Company, shall execute proper instruments acknowledging the same), except for the following which shall survive until otherwise terminated or discharged hereunder: (A) the rights of Holders of such Securities to receive, solely from the trust fund described in Section 14.04 and as more fully set forth in such Section, payments in respect of the principal of, premium (if any) and interest (if any) on such Securities when such payments are due, (B) the Company's obligations with respect to such Securities under Sections 3.05, 3.06, 10.02 and 10.03, (C) the rights, powers, trusts, duties and immunities of the Trustees hereunder and (D) this Article Fourteen. Subject to compliance with this Article Fourteen, the Company may exercise its option under this Section 14.02 notwithstanding the prior exercise of its option under Section 14.03 with respect to such Securities.
SECTION 14.03 Covenant Defeasance.
Upon the Company's exercise of the above option applicable to this Section 14.03 with respect to any Securities of or within a series, the Company shall be released from its obligations under Sections 10.05 and 10.06, and, if specified pursuant to Section 3.01, its obligations under any other covenant, with respect to such Securities on and after the date the conditions set forth in Section 14.04 are satisfied (hereinafter, "covenant defeasance"), and such Securities shall thereafter be deemed not to be "Outstanding" for the purposes of any direction, waiver, consent or declaration or Act of Holders (and the consequences of any thereof) in connection with such covenants, but shall continue to be deemed "Outstanding" for all other purposes hereunder. For this purpose, such covenant defeasance means that, with respect to such Securities, the Company may omit to comply with and shall have no liability in respect of any term, condition or limitation set forth in any such covenant, whether directly or indirectly, by reason of any reference elsewhere herein to any such covenant or by reason of reference in any such covenant to any other provision herein or in any other document and such omission to comply shall not constitute a Default or an Event of Default under clauses (4) or (7) of Section 5.01 or otherwise but, except as specified above, the remainder of this Indenture and such Securities shall be unaffected thereby.
SECTION 14.04 Conditions to Defeasance or Covenant Defeasance.
The following shall be the conditions to application of either Section 14.02 or Section 14.03 to any Securities of or within a series:
(1) The Company shall irrevocably have deposited or caused to be deposited with either Trustee (or another trustee satisfying the requirements of Section 6.08 who shall agree to comply with the provisions of this Article Fourteen applicable to it) as trust funds in trust for the purpose of making the following payments, specifically pledged as security for, and dedicated solely to, the benefit of the Holders of such Securities, (A) an amount (in such Currency in which such Securities are then specified as payable at Stated Maturity), or (B) Government Obligations applicable to such Securities (determined on the basis of the Currency in which such Securities are then specified as payable at Stated Maturity) which through the scheduled payment of principal and interest in respect thereof in accordance with their terms will provide, not later than one day before the due date of any payment of principal of and premium (if any) and interest (if any) under such Securities, money in an amount, or (C) a combination thereof, sufficient, in the opinion of a nationally recognized firm of independent public accountants expressed in a written certification thereof delivered to the Trustees, to pay and discharge, and which shall be applied by the Trustees (or another trustee satisfying the requirements of Section 6.08 who shall agree to comply with the provisions of this Article Fourteen) to pay and discharge, (i) the principal of, premium (if any) and interest (if any) on such Securities on the Stated Maturity (or Redemption Date, if applicable) of such principal of, premium (if any) or installment of interest (if any), (ii) any mandatory sinking fund payments or analogous payments applicable to such Securities on the day on which such payments are due and payable in accordance with the terms of this Indenture and of such Securities, and (iii) all amounts due the Trustees under Section 6.07; provided that the Trustees shall have been irrevocably instructed to apply such money or the proceeds of such Government Obligations to said payments with respect to such Securities. Before such a deposit, the Company may give to the Trustees, in accordance with Section 11.02, a notice of its election to redeem all or any portion of such Securities at a future date in accordance with the terms of such Securities and Article Eleven hereof, which notice shall be irrevocable. Such irrevocable redemption notice, if given, shall be given effect in applying the foregoing.
(2) No Default or Event of Default with respect to such Securities shall have occurred and be continuing on the date of such deposit or, insofar as clauses (5) and (6) of Section 5.01 are concerned, at any time during the period ending on the 91st day after the date of such deposit (it being understood that this condition shall not be deemed satisfied until the expiration of such period).
(3) Such defeasance or covenant defeasance shall not result in a breach or violation of, or constitute a Default or an Event of Default under, this Indenture or any default under any material agreement or instrument to which the Company is a party or by which it is bound.
(4) In the case of an election under Section 14.02, the Company shall have delivered to the Trustees an Opinion of Counsel in the United States stating that (x) the Company has received from, or there has been published by, the Internal Revenue Service a ruling, or (y) since the date of execution of this Indenture, there has been a change in the applicable United States federal income tax law, in either case to the effect that, and based thereon such opinion shall confirm that, the Holders of such Securities will not recognize income, gain or loss for United States federal income tax purposes as a result of such defeasance and will be subject to United States federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such defeasance had not occurred.
(5) In the case of an election under Section 14.03, the Company shall have delivered to the Trustees an Opinion of Counsel in the United States to the effect that the Holders of such Securities will not recognize income, gain or loss for United States federal income tax purposes as a result of such covenant defeasance and will be subject to United States federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such covenant defeasance had not occurred.
(6) The Company shall have delivered to the Trustees an Opinion of Counsel in Canada or a ruling from the Canada Revenue Agency to the effect that the Holders of such Securities will not recognize income, gain or loss for Canadian federal, provincial or territorial income tax or other tax purposes as a result of such defeasance or covenant defeasance, as applicable, and will be subject to Canadian federal, provincial or territorial income tax and other tax on the same amounts, in the same manner and at the same times as would have been the case had such defeasance or covenant defeasance, as applicable, not occurred (and for the purposes of such opinion, such Canadian counsel shall assume that Holders of such Securities include Holders who are not resident in Canada).
(7) The Company is not an "insolvent person" within the meaning of the Bankruptcy and Insolvency Act (Canada) on the date of such deposit or at any time during the period ending on the 91st day after the date of such deposit (it being understood that this condition shall not be deemed satisfied until the expiration of such period).
(8) Notwithstanding any other provisions of this Section 14.04, such defeasance or covenant defeasance shall be effected in compliance with any additional or substitute terms, conditions or limitations in connection therewith pursuant to Section 3.01.
(9) The Company shall have delivered to the Trustees an Officer's Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for, relating to either the defeasance under Section 14.02 or the covenant defeasance under Section 14.03 (as the case may be), have been complied with.
SECTION 14.05 Deposited Money and Government Obligations to Be Held in Trust; Other Miscellaneous Provisions.
Subject to the provisions of the last paragraph of Section 10.03, all money and Government Obligations (or other property as may be provided pursuant to Section 3.01) (including the proceeds thereof) deposited with a Trustee (or another trustee satisfying the requirements of Section 6.08 who shall agree to comply with the provisions of this Article Fourteen) pursuant to Section 14.04 in respect of such Securities shall be held in trust and applied by such Trustee, in accordance with the provisions of such Securities and this Indenture, to the payment, either directly or through any Paying Agent (including the Company acting as its own Paying Agent), to the Holders of such Securities of all sums due and to become due thereon in respect of principal, premium (if any) and interest (if any) on such Securities but such money need not be segregated from other funds except to the extent required by law.
Unless otherwise specified with respect to any Security pursuant to Section 3.01, if, after a deposit referred to in Section 14.04(1) has been made, (a) the Holder of a Security in respect of which such deposit was made is entitled to, and does, elect pursuant to Section 3.12(b) or the terms of such Security to receive payment in a Currency other than that in which the deposit pursuant to Section 14.04(1) has been made in respect of such Security, or (b) a Conversion Event occurs as contemplated in Section 3.12(d) or 3.12(e) or by the terms of any Security in respect of which the deposit pursuant to Section 14.04(1) has been made, the indebtedness represented by such Security shall be deemed to have been, and will be, fully discharged and satisfied through the payment of the principal of, premium (if any) and interest (if any) on such Security as they become due out of the proceeds yielded by converting (from time to time as specified below in the case of any such election) the amount or other property deposited in respect of such Security into the Currency in which such Security becomes payable as a result of such election or Conversion Event based on the applicable Market Exchange Rate for such Currency in effect on the third Business Day prior to each payment date, except, with respect to a Conversion Event, for such Currency in effect (as nearly as feasible) at the time of the Conversion Event.
The Company shall pay and indemnify such Trustee against any tax, fee or other charge imposed on or assessed against the Government Obligations deposited pursuant to Section 14.04 or the principal and interest received in respect thereof other than any such tax, fee or other charge which by law is for the account of the Holders of such Securities.
Anything in this Article Fourteen to the contrary notwithstanding, such Trustee shall deliver or pay to the Company from time to time upon Company Request any money or Government Obligations (or other property and any proceeds therefrom) held by it as provided in Section 14.04 which, in the opinion of a nationally recognized firm of independent public accountants expressed in a written certification thereof delivered to such Trustee, are in excess of the amount thereof which would then be required to be deposited to effect an equivalent defeasance or covenant defeasance, as applicable, in accordance with this Article Fourteen.
SECTION 14.06 Reinstatement.
If a Trustee or any Paying Agent is unable to apply any money in accordance with Section 14.05 by reason of any order or judgment of any court or governmental authority enjoining, restraining or otherwise prohibiting such application, then the Company's obligations under this Indenture and such Securities shall be revived and reinstated as though no deposit had occurred pursuant to Section 14.02 or 14.03, as the case may be, until such time as such Trustee or Paying Agent is permitted to apply all such money in accordance with Section 14.05; provided, however, that if the Company makes any payment of principal of, premium (if any) or interest (if any) on any such Security following the reinstatement of its obligations, the Company shall be subrogated to the rights of the Holders of such Securities to receive such payment from the money held by such Trustee or Paying Agent.
IN WITNESS WHEREOF, the parties hereto have caused this Indenture to be duly executed, all as of the day and year first above written.
| |
CYBIN INC. |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
| |
|
|
| |
, |
| |
as U.S. Trustee |
| |
|
|
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
| |
|
|
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
| |
|
|
| |
, |
| |
as Canadian Trustee |
| |
|
|
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
Authorized Signing Officer |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
Authorized Signing Officer |
EXHIBIT A-1
FORM OF CERTIFICATE TO BE GIVEN BY
PERSON ENTITLED TO OBTAIN INTEREST PAYABLE PRIOR
TO THE EXCHANGE DATE
CERTIFICATE
CYBIN INC.
_____% Notes due _________________
This is to certify that as of the date hereof, and except as set forth below, the above-captioned Securities held by you for our account (i) are owned by any person(s) that is not a citizen or resident of the United States; a corporation or partnership (including any entity treated as a corporation or partnership for United States federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia unless, in the case of a partnership, United States Treasury Regulations provide otherwise; any estate whose income is subject to United States federal income tax regardless of its source; or a trust if (A) a United States court can exercise primary supervision over the trust's administration and one or more United States persons are authorized to control all substantial decisions of the trust or (B) it was in existence on August 20, 1996 and has a valid election in effect under applicable United States Treasury Regulations to be treated as a United States person ("United States persons(s)"), (ii) are owned by United States person(s) that are (a) foreign branches of United States financial institutions (financial institutions, as defined in United States. United States Treasury Regulation Section 1.165-12(c)(1)(iv) are herein referred to as "financial institutions") purchasing for their own account or for resale, or (b) United States person(s) who acquired the Securities through foreign branches of United States financial institutions and who hold the Securities through such United States financial institutions on the date hereof (and in either case (a) or (b), each such United States financial institution hereby agrees, on its own behalf or through its agent, that you may advise Cybin Inc. or its agent that such financial institution will comply with the requirements of Section 165(j)(3)(A), (B) or (C) of the United States Internal Revenue Code of 1986, as amended, and the regulations thereunder), or (iii) are owned by United States or foreign financial institution(s) for purposes of resale during the restricted period (as defined in United States Treasury Regulation Section 1.163-5(c)(2)(i)(D)(7)), and, in addition, if the owner is a United States or foreign financial institution described in clause (iii) above (whether or not also described in clause (i) or (ii)), this is to further certify that such financial institution has not acquired the Securities for purposes of resale directly or indirectly to a United States person or to a person within the United States or its possessions.
As used herein, "United States" means the United States of America (including the states and the District of Columbia); and its "possessions" include Puerto Rico, the U.S. Virgin Islands, Guam, American Samoa, Wake Island and the Northern Mariana Islands.
We undertake to advise you promptly in writing on or prior to the date on which you intend to submit your certification relating to the above-captioned Securities held by you for our account in accordance with your operating procedures if any applicable statement herein is not correct on such date, and in the absence of any such notification it may be assumed that this certification applies as of such date.
This certificate excepts and does not relate to U.S. $__________ of such interest in the above-captioned Securities in respect of which we are not able to certify and as to which we understand an exchange for an interest in a permanent global security or an exchange for and delivery of definitive Securities (or, if relevant, collection of any interest) cannot be made until we do so certify.
We understand that this certificate may be required in connection with certain tax legislation in the United States. If administrative or legal proceedings are commenced or threatened in connection with which this certificate is or would be relevant, we irrevocably authorize you to produce this certificate or a copy thereof to any interested party in such proceedings.
Dated:__________________
[To be dated no earlier than the 15th day prior to
(i) the Exchange Date or (ii) the relevant Interest
Payment Date occurring prior to the Exchange
Date, as applicable]
| |
[Name of Person Making Certification] |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
EXHIBIT A-2
FORM OF CERTIFICATE TO BE GIVEN BY THE DEPOSITARY
IN CONNECTION WITH THE EXCHANGE OF A PORTION OF A
TEMPORARY GLOBAL SECURITY OR TO OBTAIN INTEREST
PAYABLE PRIOR TO THE EXCHANGE DATE
CERTIFICATE
CYBIN INC.
_____% Notes due _________________
This is to certify that based solely on written certifications that we have received in writing or by electronic transmission from each of the persons appearing in our records as persons entitled to a portion of the principal amount set forth below (our "Member Organizations") substantially in the form attached hereto, as of the date hereof, U.S. $__________ principal amount of the above-captioned Securities (i) is owned by any person(s) that is not a citizen or resident of the United States; a corporation or partnership (including any entity treated as a corporation or partnership for United States federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia unless, in the case of a partnership, United States Treasury Regulations provide otherwise; any estate whose income is subject to United States federal income tax regardless of its source; or a trust if (A) a United States court can exercise primary supervision over the trust's administration and one or more United States persons are authorized to control all substantial decisions of the trust or (B) it was in existence on August 20, 1996 and has a valid election in effect under applicable United States Treasury Regulations to be treated as a United States person ("United States person(s)"), (ii) is owned by United States person(s) that are (a) foreign branches of United States financial institutions (financial institutions, as defined in United States Treasury Regulation Section 1.165-12(c)(1)(iv) are herein referred to as "financial institutions") purchasing for their own account or for resale, or (b) United States person(s) who acquired the Securities through foreign branches of United States financial institutions and who hold the Securities through such United States financial institutions on the date hereof (and in either case (a) or (b), each such financial institution has agreed, on its own behalf or through its agent, that we may advise Cybin Inc. or its agent that such financial institution will comply with the requirements of Section 165(j)(3)(A), (B) or (C) of the Internal Revenue Code of 1986, as amended, and the regulations thereunder), or (iii) is owned by United States or foreign financial institution(s) for purposes of resale during the restricted period (as defined in United States Treasury Regulation Section 1.163-5(c)(2)(i)(D)(7)) and, to the further effect, that financial institutions described in clause (iii) above (whether or not also described in clause (i) or (ii)) have certified that they have not acquired the Securities for purposes of resale directly or indirectly to a United States person or to a person within the United States or its possessions.
As used herein, "United States" means the United States of America (including the states and the District of Columbia); and its "possessions" include Puerto Rico, the U.S. Virgin Islands, Guam, American Samoa, Wake Island and the Northern Mariana Islands.
We further certify that (i) we are not making available herewith for exchange (or, if relevant, collection of any interest) any portion of the temporary global Security representing the above-captioned Securities excepted in the above-referenced certificates of Member Organizations and (ii) as of the date hereof we have not received any notification from any of our Member Organizations to the effect that the statements made by such Member Organizations with respect to any portion of the part submitted herewith for exchange (or, if relevant, collection of any interest) are no longer true and cannot be relied upon as of the date hereof.
We understand that this certification is required in connection with certain tax legislation in the United States. If administrative or legal proceedings are commenced or threatened in connection with which this certificate is or would be relevant, we irrevocably authorize you to produce this certificate or a copy thereof to any interested party in such proceedings.
Dated:_____________
[To be dated as of (i) the Exchange Date or
(ii) the relevant Interest Payment Date occurring
prior to the Exchange Date, as applicable]
| |
[INSERT NAME OF DEPOSITARY] |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
EX-FILING FEES
Calculation of Filing Fee Tables
FORM F-10
(Form Type)
CYBIN INC.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| |
|
Security Type |
|
Security
Class Title |
|
Fee
Calculation
or Carry
Forward
Rule |
|
Amount
Registered(1)(3) |
|
Proposed
Maximum
Offering
Price
Per Unit |
|
|
Maximum
Aggregate
Offering Price(1)(2) |
|
|
Fee Rate |
|
|
Amount of
Registration Fee(1)(3) |
|
| Newly Registered Securities |
|
| Fees to be Paid |
|
Unallocated (Universal) Shelf |
|
Common Shares, Warrants, Units, Debt Securities
Subscription Receipts |
|
Rule 457(o) |
$ |
181,641,445 |
|
(1) |
|
$ |
181,641,445 |
|
$ |
0.00014760 |
|
$ |
26,811 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Total Offering Amounts |
|
|
|
$ |
181,641,445 |
|
|
|
|
$ |
26,811 |
|
| |
|
Total Fees Previously Paid |
|
|
|
|
|
|
|
|
|
$ |
0 |
|
| |
|
Total Fee Offsets |
|
|
|
|
|
|
|
|
|
$ |
0 |
|
| |
|
Net Fee Due |
|
|
|
|
|
|
|
|
|
$ |
26,811 |
|
(1) There are being registered under this Registration Statement such indeterminate number of common shares, warrants, units, debt securities and subscription receipts of the Registrant, and a combination of such securities, separately or as units, as may be sold by the Registrant from time to time, which collectively shall have an aggregate initial offering price not to exceed $300,503,343 (converted from CAD$400,000,000 at an exchange rate of CAD$1.00 = $1.3311, which was the daily average exchange rate reported by the Bank of Canada on December 21, 2023). The securities registered hereunder also include such indeterminate number of each class of identified securities as may be issued upon conversion, exercise or exchange of any other securities that provide for such conversion into, exercise for or exchange into such securities. Separate consideration may or may not be received for securities that are issuable on exercise, conversion or exchange of other securities. In addition, pursuant to Rule 416 under the Securities Act of 1933, as amended (the "Securities Act"), the common shares being registered hereunder include such indeterminate number of common shares as may be issuable with respect to the common shares being registered hereunder as a result of stock splits, stock dividends, distributions or similar transactions. The proposed maximum initial offering price per security will be determined, from time to time, by the Registrant in connection with the sale of the securities under this Registration Statement.
(2) Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(o) of the Securities Act.
(3) This Registration Statement relates to an aggregate of US$300,503,343 of securities (converted from CAD$400,000,000 at an exchange rate of CAD$1.00 = $1.3311, which was the daily average exchange rate reported by the Bank of Canada on December 21, 2023), including, pursuant to Rule 429 under the Securities Act, US$118,861,898 (converted from CAD$160,000,000 at an exchange rate of CAD$1.00 = $1.3461, which was the daily average exchange rate reported by the Bank of Canada on August 14, 2023) of securities that were previously registered under the Registrant's Registration Statement on Form F-10 (File No. 333-272706), initially filed on June 16, 2023, as amended on August 17, 2023 and which became effective on August 17, 2023.
Cybin (AMEX:CYBN)
Historical Stock Chart
From Dec 2024 to Jan 2025
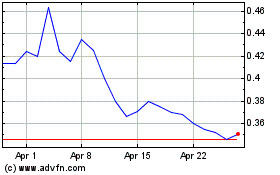
Cybin (AMEX:CYBN)
Historical Stock Chart
From Jan 2024 to Jan 2025