UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of August 2023
Commission File No. 001-39621
OPTHEA LIMITED
(Translation of registrant’s name into English)
Level 4
650 Chapel Street
South Yarra, Victoria, 3141
Australia
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
Yes ☐ No ☒
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes ☐ No ☒
Indicate by check mark whether the registrant by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ☐ No ☐
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
|
|
OPTHEA LIMITED |
|
(Registrant) |
|
|
|
|
By: |
/s/ Megan Baldwin |
|
Name: |
Megan Baldwin, Ph.D. |
|
Title: |
Chief Executive Officer and Managing Director |
Date: 8/1/2023

Population Pharmacokinetics and Safety of �sozinibercept (OPT-302), an anti-VEGF-C/-D�‘trap’ in Patients with Retinal Vascular Diseases Dante J. Pieramici, MD California Retina Consultants, Santa Barbara, CA ASRS, Seattle, 2023 Exhibit 99.1

Disclosures Presenter’s Financial Disclosures: - Adverum (C), Gemini, Genentech, Inc., Iveric Bio, NGM, Opthea (C), Regeneron, Regenxbio • This presentation will discuss IRB/IEC approved research of an investigational product. (C): Consultant; (S): Stock/shareholder; (R): Grants/Research Support

Sozinibercept (OPT-302): A novel “Trap” Inhibitor of VEGF-C/-D�When used in combination is complementary/agnostic with anti-VEGF-A molecule used In combination with any VEGF-A inhibitor, Sozinibercept (OPT-302) completely blocks VEGFR-2 and VEGFR-3 signaling, inhibiting the most important pathways driving angiogenesis and vascular leakage VEGF-A inhibition elevates VEGF-C and VEGF-D which may contribute Dugel et al 2019, Ophthalmology Retina; ⍭ Lashkari et al , 2013 ARVO Annual Meeting, 4999-A0128; Bevacizumab used “off-label”; *Faricimab also targets Angiopoietin 2 Molecule type ‘Trap’ fusion protein Structure MW 140 kDa Dose 2 mg IVT injection volume 0.05 mL VEGF-A inhibition NO VEGF-C/-D inhibition YES + Antibody fragment ‘Trap’ fusion protein 48 kDa 115 kDa 0.5 mg 2 mg 0.05 mL 0.05 mL YES YES NO NO OPT-302 can also be potentially combined with Bevacizumab, Faricimab, Biosimilars, HD Eylea Sozinibercept (OPT-302)
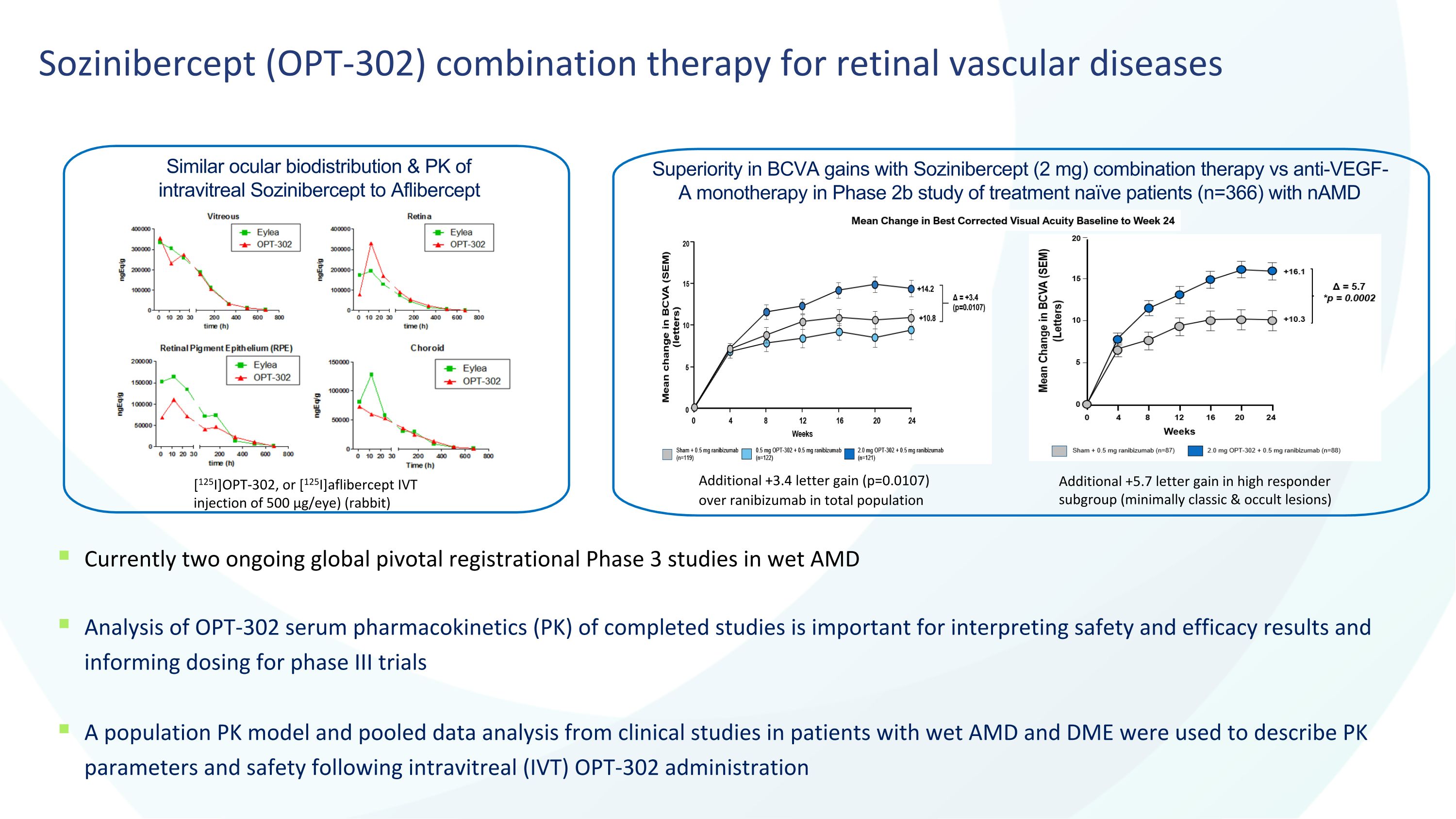
4 Sozinibercept (OPT-302) combination therapy for retinal vascular diseases Currently two ongoing global pivotal registrational Phase 3 studies in wet AMD Analysis of OPT-302 serum pharmacokinetics (PK) of completed studies is important for interpreting safety and efficacy results and informing dosing for phase III trials A population PK model and pooled data analysis from clinical studies in patients with wet AMD and DME were used to describe PK parameters and safety following intravitreal (IVT) OPT-302 administration Similar ocular biodistribution & PK of intravitreal Sozinibercept to Aflibercept Superiority in BCVA gains with Sozinibercept (2 mg) combination therapy vs anti-VEGF-A monotherapy in Phase 2b study of treatment naïve patients (n=366) with nAMD Additional +3.4 letter gain (p=0.0107) over ranibizumab in total population [125I]OPT-302, or [125I]aflibercept IVT injection of 500 µg/eye) (rabbit) Additional +5.7 letter gain in high responder subgroup (minimally classic & occult lesions)
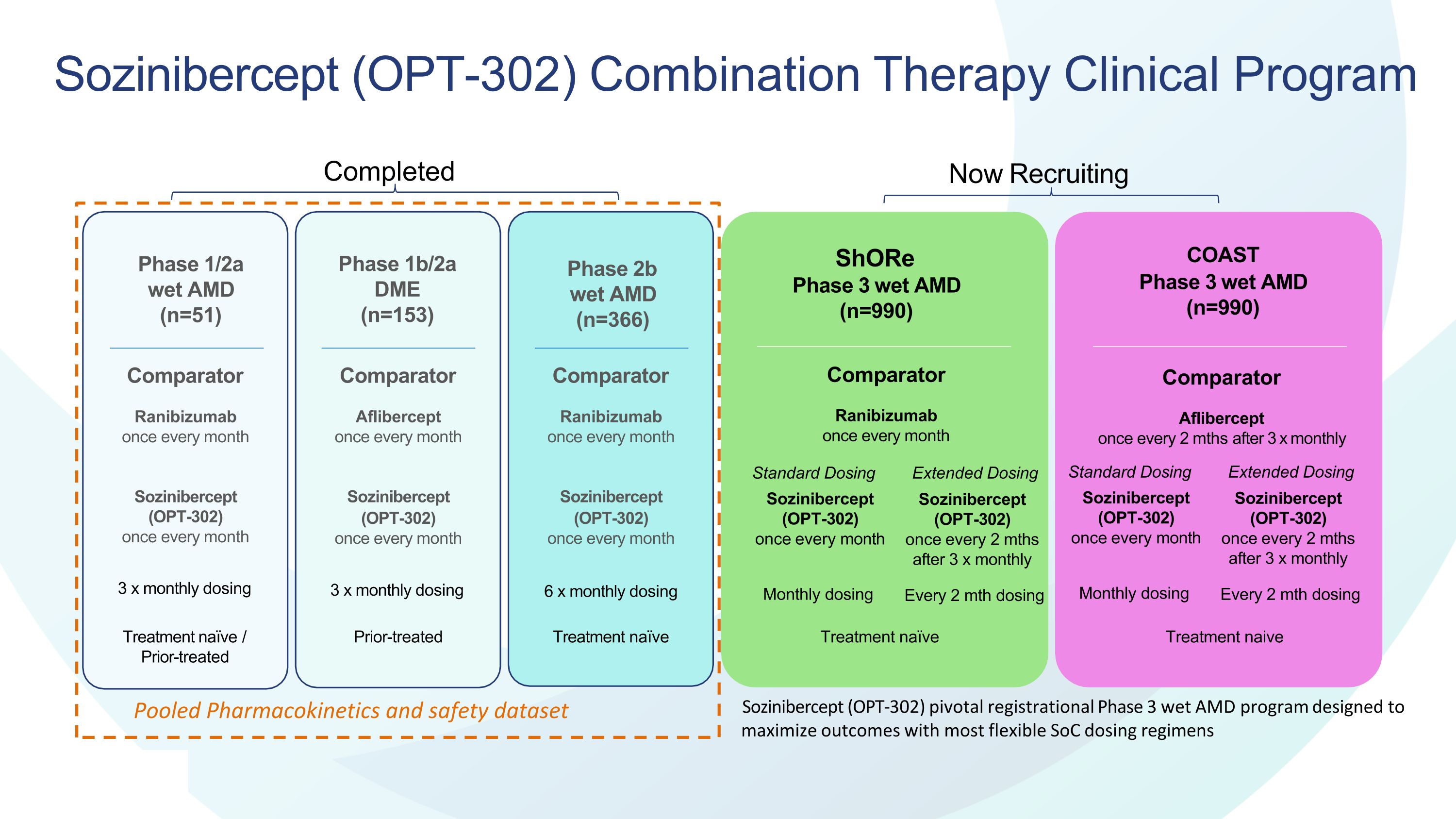
Sozinibercept (OPT-302) Combination Therapy Clinical Program Sozinibercept (OPT-302) pivotal registrational Phase 3 wet AMD program designed to maximize outcomes with most flexible SoC dosing regimens . ShORe Phase 3 wet AMD (n=990) Comparator Ranibizumab once every month COAST Phase 3 wet AMD (n=990) Standard Dosing Sozinibercept (OPT-302) once every month Monthly dosing Extended Dosing Comparator Aflibercept once every 2 mths after 3 x monthly Phase 1/2a wet AMD (n=51) Comparator Ranibizumab once every month Sozinibercept (OPT-302) once every month 3 x monthly dosing Comparator Aflibercept once every month Sozinibercept (OPT-302) once every month 3 x monthly dosing Phase 1b/2a DME (n=153) Comparator Ranibizumab once every month Sozinibercept (OPT-302) once every month 6 x monthly dosing Treatment naïve / Prior-treated Treatment naïve Treatment naïve Treatment naive Prior-treated Phase 2b wet AMD (n=366) Now Recruiting Completed Pooled Pharmacokinetics and safety dataset Sozinibercept (OPT-302) once every 2 mths after 3 x monthly Every 2 mth dosing Standard Dosing Sozinibercept (OPT-302) once every month Monthly dosing Extended Dosing Sozinibercept (OPT-302) once every 2 mths after 3 x monthly Every 2 mth dosing
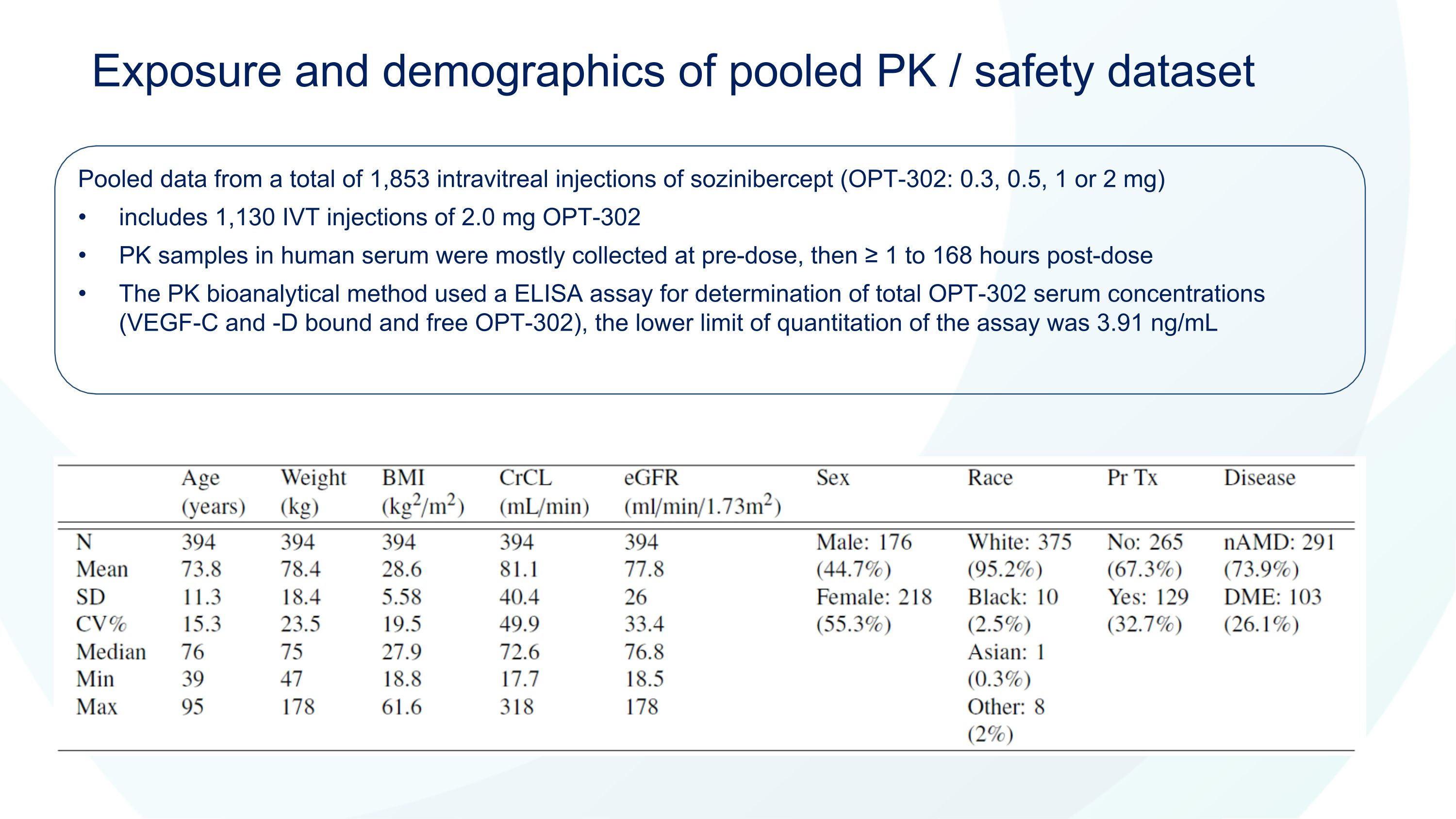
Exposure and demographics of pooled PK / safety dataset Pooled data from a total of 1,853 intravitreal injections of sozinibercept (OPT-302: 0.3, 0.5, 1 or 2 mg) includes 1,130 IVT injections of 2.0 mg OPT-302 PK samples in human serum were mostly collected at pre-dose, then ≥ 1 to 168 hours post-dose The PK bioanalytical method used a ELISA assay for determination of total OPT-302 serum concentrations (VEGF-C and -D bound and free OPT-302), the lower limit of quantitation of the assay was 3.91 ng/mL

Sozinibercept (OPT-302) systemic PK profile & noncompartmental parameters The majority of the PK data for OPT-302 was collected following an IVT dose of 2 mg, where serum (Cmax) occurs ~30 hours after administration. Quantifiable concentrations remained in some subjects at 168 hours post- dose. The interpretation of linear kinetics across dose range studied is challenging given the limited data from low dose groups ≤ 1 mg due to BLQ. . Pooled systemic serum PK data: Cmax ~20 ng/mL [4 - 83] Tmax ~30 hrs [1.8 - 96] T1/2 ~7 days [2 -12]
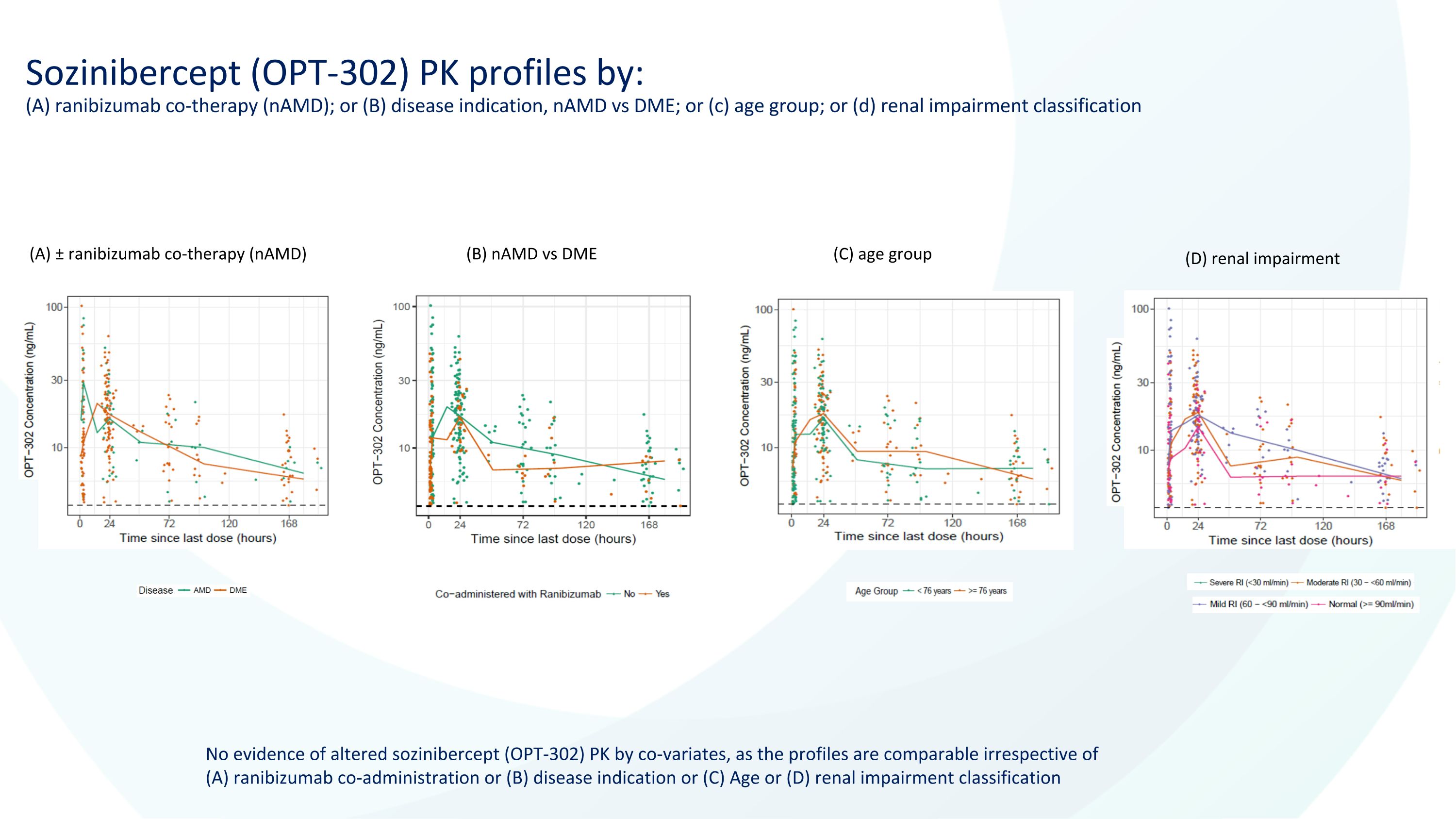
Sozinibercept (OPT-302) PK profiles by:�(A) ranibizumab co-therapy (nAMD); or (B) disease indication, nAMD vs DME; or (c) age group; or (d) renal impairment classification No evidence of altered sozinibercept (OPT-302) PK by co-variates, as the profiles are comparable irrespective of (A) ranibizumab co-administration or (B) disease indication or (C) Age or (D) renal impairment classification (A) ± ranibizumab co-therapy (nAMD) (B) nAMD vs DME (C) age group (D) renal impairment

Vitreous Parameter Estimates from sozinibercept (OPT-302) Population PK Model The PK model retained the single distribution compartment and linear elimination. Absorption from the vitreous space was described by a first-order process. During selected dosing occasions, a small fraction, (≈7%) of the administered OPT-302 bypassed the vitreous compartment into the systemic circulation. This phenomena has previously been described for IVT ranibizumab using a population PK approach. The absorption of OPT-302 was the rate-limiting step, with the PK of OPT-302 via IVT administration described by ‘flip-flop’ kinetics. The model assumes no clearance of OPT-302 in the vitreous compartment. The M3 method was used during estimation given the BLQ data.

N Participants (%) OPT-302 Any dose* N=399 (N=1,842 injections) OPT-302 2.0 mg N=263 (N=1,121 injections) Sham + anti-VEGF-A control N=169 (N=854 injections) Ocular TEAEs - Study Eye – related to study product(s) 41 (10.2%) 22 (8.4%) 20 (11.8%) Ocular TEAEs - Study Eye – Severe 4 (1.0%) 2 (0.8%) 2 (1.2%) Intraocular inflammation – Study Eye 71,2,3 (1.8%) 31 (1.1%) 31 (1.8%) Participants with AEs leading to treatment discontinuation 42,4-6 (1.0%) 14 (0.4%) 27,8 (1.2%) Any APTC event 44,5,9,10 (1.0%) 35,9,10(1.1%) 211,12 (1.2%) Deaths 210,13 (0.5%) 210,13 (0.8%) 214,15 (1.2%) 1Transient anterior chamber cell (trace 1-4 cells); 2 SAE of endophthalmitis, with AE’s of hypopyon and anterior chamber cell (n=1; 0.5 mg); 3 SAE of vitritis (n=1; 0.5 mg); 4Non-fatal myocardial infarction; 5Cerebrovascular accident; 6Enteritis; 7Abdominal pain; 8Increased IOP; 9 Non-fatal angina pectoris; 10Fatal congestive heart failure/myocardial infarction; 11Non-fatal arterial embolism; 12Embolic stroke; 13Metatstaic ovarian cancer; 14 Pneumonia; 15 infective endocarditis * Any dose (OPT-302 0.3 mg, 0.5 mg, 1 mg or 2 mg) Pooled safety for completed sozinibercept (OPT-302) Trials�Combination therapy well-tolerated and comparable to standard of care monotherapy
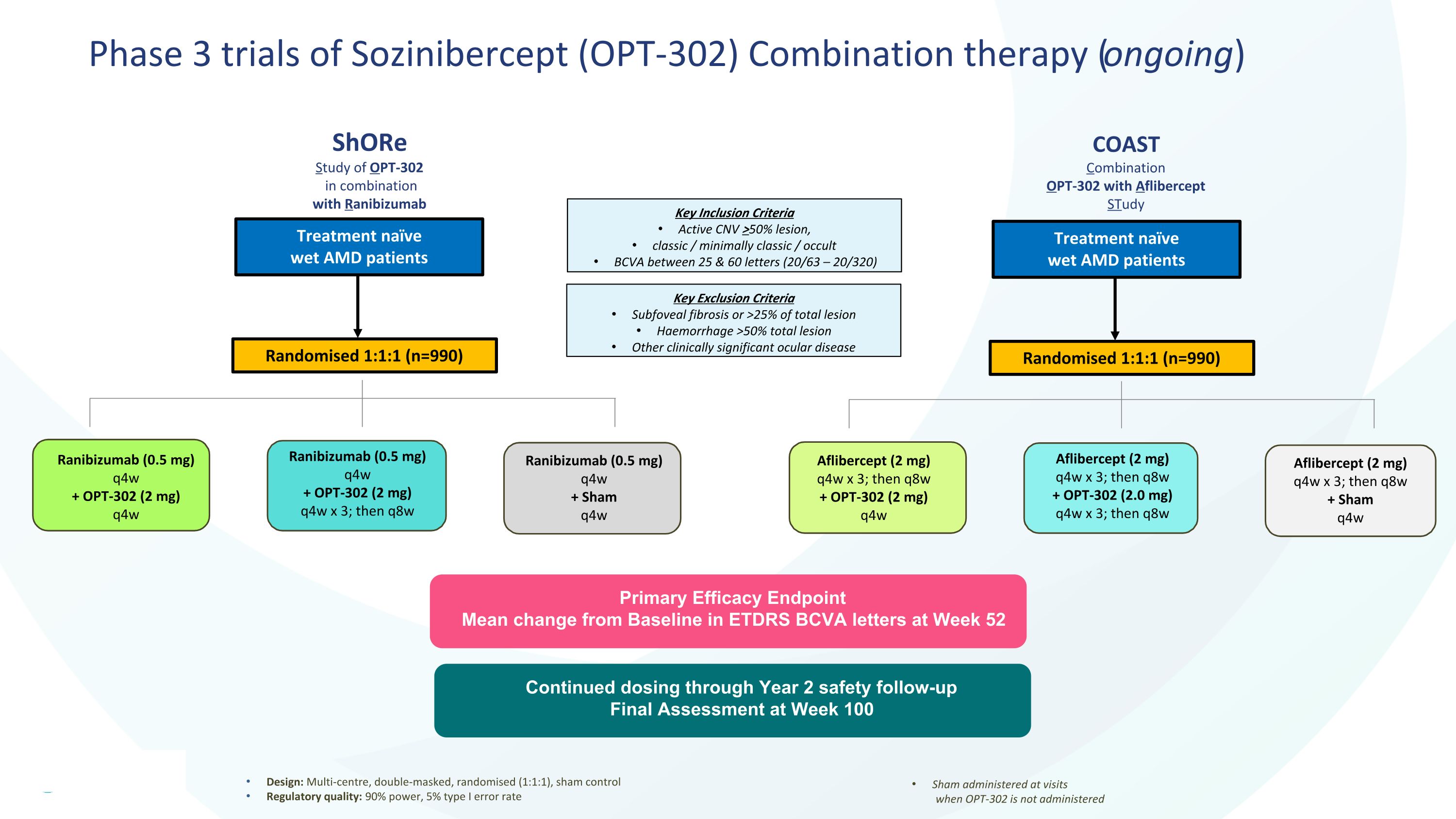
Phase 3 trials of Sozinibercept (OPT-302) Combination therapy (ongoing) Design: Multi-centre, double-masked, randomised (1:1:1), sham control Regulatory quality: 90% power, 5% type I error rate Sham administered at visits when OPT-302 is not administered Primary Efficacy Endpoint Mean change from Baseline in ETDRS BCVA letters at Week 52 Ranibizumab (0.5 mg) q4w + OPT-302 (2 mg) q4w Ranibizumab (0.5 mg) q4w + OPT-302 (2 mg) q4w x 3; then q8w Ranibizumab (0.5 mg) q4w + Sham q4w Treatment naïve wet AMD patients Key Exclusion Criteria Subfoveal fibrosis or >25% of total lesion Haemorrhage >50% total lesion Other clinically significant ocular disease Key Inclusion Criteria Active CNV >50% lesion, classic / minimally classic / occult BCVA between 25 & 60 letters (20/63 – 20/320) Randomised 1:1:1 (n=990) Aflibercept (2 mg) q4w x 3; then q8w + OPT-302 (2.0 mg) q4w x 3; then q8w Aflibercept (2 mg) q4w x 3; then q8w + Sham q4w Treatment naïve wet AMD patients Randomised 1:1:1 (n=990) Aflibercept (2 mg) q4w x 3; then q8w + OPT-302 (2 mg) q4w Continued dosing through Year 2 safety follow-up Final Assessment at Week 100 ShORe Study of OPT-302 in combination with Ranibizumab COAST Combination OPT-302 with Aflibercept STudy

Summary: Sozinibercept (OPT-302) pooled PK and safety The PK profiles of intravitreal sozinibercept (OPT-302) from completed studies in patients with nAMD and DME indicated: low systemic exposure within one week drug levels were mostly no longer detectable in serum estimated vitreous absorption half-life was ~4.6 days [95% CI 3.6-5.8] comparable to other IVT biologics the PK profile was unaltered by anti-VEGF-A co-therapy, disease indication (nAMD vs DME), age or renal function Pooled safety analysis shows soziniberept (OPT-302) combination therapy has a favourable safety and tolerability profile comparable to standard of care anti-VEGF-A monotherapy Promising treatment option for wet AMD currently in two pivotal registrational Phase 3 studies pooled PK and safety data support ShORe and COAST dosing regimens of 2 mg sozinibercept q4w or q8w following 3 loading doses
Opthea (NASDAQ:OPT)
Historical Stock Chart
From Apr 2024 to May 2024
Opthea (NASDAQ:OPT)
Historical Stock Chart
From May 2023 to May 2024