U.
S. SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-KSB
[X]
ANNUAL REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the
fiscal year ended
December 31,
2007
[X]
TRANSITIONAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the
transition period from ___________ to _____________
Commission
File Number:
000-32917
PROTOKINETIX,
INC.
(Name of
small business issuer as specified in its charter)
|
Nevada
|
94-3355026
|
|
(State
or other jurisdiction of
incorporation
or organization)
|
(I.R.S.
Employer
Identification
No.)
|
|
Suite
1500-885 West Georgia Street
Vancouver,
British Columbia Canada V6C 3E8
________________________________________________________________________
(Address
of principal executive offices, including zip
code)
|
Registrant’s
telephone number, including area
code:
604-687-9887
Securities
registered pursuant to Section 12(b) of the
Act:
None
Securities
registered pursuant to Section 12(g) of the
Act:
$.001 par value common
stock
___________________
Check
whether the issuer (1) filed all reports required to be filed by
Section 13 or 15(d) of the Exchange Act of 1934 during the past 12 months
(or for such shorter period that the registrant was required to file such
reports), and (2) has been subject to such filing requirements for the past
90 days.Yes
X
No
Check if
disclosure of delinquent filers pursuant to Item 405 of Regulation S-B is not
contained in this form, and no disclosure will be contained, to the best of
registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-KSB or any amendment to
this Form 10-KSB. [ ]
The
issuer’s revenues for the most recent fiscal year were $0.
The
aggregate market value of the voting and non-voting common equity held by
non-affiliates of the registrant was approximately $16,464,950 based upon the
closing price of our common stock which was $0.33 on April 10,
2008. Shares of common stock held by each officer and director and by
each person or group who owns 10% or more of them outstanding common stock
amounting to 620,000 shares have been excluded in that such persons or groups
may be deemed to be affiliates. This determination of affiliate status is not
necessarily a conclusive determination for other purposes.
As of
April 10, 2008, there were 50,513,788 shares of our common stock were issued and
outstanding.
Documents
Incorporated by Reference: None.
Transitional
Small Business Disclosure Format: No.
INTRODUCTION
The
following discussion should be read in conjunction with our audited financial
statements and notes thereto. Because we desire to take advantage of,
the "safe harbor" provisions of the Private Securities Litigation Reform Act of
1995, we caution readers regarding certain forward looking statements in the
following discussion and elsewhere in this report and in any other statement
made by, or on our behalf, whether or not in future filings with the Securities
and Exchange Commission. Forward looking statements are statements
not based on historical information and which relate to future operations,
strategies, financial results or other developments. Forward looking
statements are necessarily based upon estimates and assumptions that are
inherently subject to significant business, economic and competitive
uncertainties and contingencies, many of which are beyond our control and many
of which, with respect to future business decisions, are subject to change.
These uncertainties and contingencies can affect actual results and could cause
actual results to differ materially from those expressed in any forward looking
statements made by, or our behalf. We disclaim any obligation to
update forward looking statements.
Forward
looking statements involve known and unknown risks, uncertainties and other
factors that may cause our actual results, levels of activity, performance, or
achievements to be materially different from any future results, levels of
activity, performance, or achievement expressed or implied by such
forward-looking statements. In some cases, you can identify forward-looking
statements by terminology such as "may," "will," "should," "could," "intend,"
"expects," "plan," "anticipates," "believes," "estimates," "predicts,"
"potential," or "continue" or the negative of such terms or other comparable
terminology. Although we believe that the expectations reflected in the
forward-looking statements are reasonable, we cannot guarantee future results,
levels of activity, performance, or achievements. Moreover, neither we nor any
other person assumes responsibility for the accuracy and completeness of such
statements.
WE ARE A
DEVELOPMENT STAGE BUSINESS AND AN INVESTMENT IN OUR COMPANY IS
EXTREMELY
RISKY.
TABLE
OF CONTENTS
FORM
10-KSB ANNUAL REPORT
_________________________
PROTOKINETIX,
INC.
|
Section
|
Heading
|
|
Part
I
|
|
|
Item
1
|
Description
of Business
|
|
Item
2
|
Description
of Property
|
|
Item
3
|
Legal
Proceedings
|
|
Item
4
|
Submission
of Matters to a Vote of Security Holders
|
|
Part
II
|
|
|
Item
5
|
Market
for the Registrant's Common Equity and Related Stockholder
Matters
|
|
Item
6
|
Management's
Discussion and Analysis of Financial Condition and Results of Operations
or Plan of Operation
|
|
Item
6A
|
Quantitative
and Qualitative Disclosures About Market Risk
|
|
Item
7
|
Financial
Statements
|
|
Item
8
|
Changes
in and Disagreements on Accounting and Financial
Disclosure
|
|
Item
8A
|
Controls
and Procedures
|
|
Item
8B
|
Other
Information
|
|
Part
III
|
|
|
Item
9
|
Directors,
Executive Officers, Promoters and Control Persons, Compliance with Section
16(a) of the Exchange Act
|
|
Item
10
|
Executive
Compensation
|
|
Item
11
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
|
Item
12
|
Certain
Relationships and Related Transactions
|
|
Part
IV
|
|
|
Item
13
|
Exhibits
and Reports on Form 8-K
|
|
Item
14
|
Principal
Accountant Fees and Services
|
|
|
Certifications
and Signatures
|
PART
I
ITEM
1. DESCRIPTION
OF THE BUSINESS
Important
Disclosures and Disclaimers
.
Please
note that ProtoKinetix, Inc. (the "Company") is a research and product
development stage company that has not yet sold
any
products. The Company had $0 in revenues for the year ended December
31, 2007.
It
is important to understand that although the Company (as is discussed below) is
focused on various promising scientific and business development efforts, to
date, we have not yet marketed a product. Ongoing testing of the AAGP™ molecule
with three amino acids joined to a monosaccharide by a gemdifluride bond
continues to show that there is significant promise in the field of medicine of
preserving cells, tissue and organs from various stresses. The antiaging
properties and the protective effect of AAGP™ also is of significant interest to
the cosmetic and skin care industries. Tests have confirmed that the AAGP™
molecule improves the harvest of cells from cryopreservation by 30% to 120%. We
believe there is a market for AAGP™ to preserve cells, particularly various stem
cells, and we will continue testing with potential customers. At the same time
we are taking steps to improve the manufacturing process to reduce costs and
improve purity and biochemical activity.
Our
progress to date has been achieved notwithstanding the inherent risks relating
to the science, applications, market opportunities and commercial relationships.
The progress of the business has and will continue to be dependant on having
appropriate human and sufficient financial resources which have and will be
uncertain.
About
ProtoKinetix
ProtoKinetix
owns the world-wide rights to a family of anti-aging glycoproteins, trademarked
as AAGPs™. In scientific tests AAGPs™ have demonstrated the ability to
enhance the health and extend the life of biologically sensitive cells which
have been subjected to severe stress conditions under laboratory controlled test
conditions. AAGPs™ are stable and non-toxic.
Since
2005, ProtoKinetix has primarily focused on scientific research, but the company
has recently been in the process of directing major efforts to the practical
side of commercial validation. The commercial applications for AAGPs™ in large
markets such as skincare/cosmetic products and targeted health care solutions
are numerous, and ProtoKinetix is currently working with researchers, business
leaders and advisors and commercial entities to bring AAGP™ to
market.
Background
Native
AFGP Compound
AFGP
(Anti-Freeze Glycoprotein) is found in nature as a compound produced by some
fish, insects, reptiles, bacteria and plants that enable survival in freezing
temperatures.
One of
the many accomplishments from pioneering research of the U.S. Antarctic Program
was the discovery, in the early sixties, that fish living year-long in subzero
temperature are extremely resistant to freezing. The substances that
prevent these fish from freezing were isolated, characterized and designated as
antifreeze glycoproteins or AFGP. Various kinds of AFGP were isolated
from many species of fishes, and in some amphibians, plants and
insects. All of the AFGPs share a common characteristic that prevents
ice crystals from growing and connecting to each other. Research has
also confirmed a cell membrane stabilizing characteristics of native
AFGP.
There has
been much scientific research done in an attempt to synthetically replicate
AFGPs in research institutions because the protective properties of AFGPs could
have commercial applications, primarily in food and crop preservation at
freezing temperatures. The native antifreeze glycoproteins are very large
molecules that are often made up of a repeating series of smaller molecules,
glycoproteins. Glycoproteins are often very biologically active, but they are
inherently quite unstable. The oxygen-glycosidic link is readily cleaved by
glycosidases, resulting in a low bio-availability of these glycoconjugate based
molecules.
Scientific
research prior to AAGP has focused on building a stable and more efficient
compound with a strong bond.
AAGP™
– The Core Technology of ProtoKinetix
AAGP™
Invention
Dr.
Geraldine Castelot-Deliencourt, along with Dr. Jean-Charles Quirion at the
Research Institute of Organic Chemistry in Rouen, France, developed a patented
process to stabilize the oxygen-glycosidic bond in these sugar based molecules.
This patented process replaces the weaker oxygen bond with a C-F2 mimetic. The
resultant molecules are biologically active and stable over a pH range of 2 to
13. They are not broken down by glycosidases.
AAGP™ Toxicity
Tests
Tests
have shown cells that have been exposed to AAGP™ at low and high concentrations
have remained viable. A common viability test used on cell cultures using trypan
blue dye exclusion method has been used to show AAGP™ non-toxicity.
AAGP™ Stability
Tests
AAGP™
molecules have remained stable when subjected to three tests:
|
1.
|
pH
ranging from a strong acid level of 1.8 (stronger than stomach acid) to a
strong alkali level of 13.8. (the pH scale is calibrated from 1, highly
acidic, to 14, highly alkali);
|
|
2.
|
Enzymatic
action using protease, which targets the amino acid bonds, and
glycosidase, which targets the amino acid bonds, and glycosidase, which
targets the sugar molecules; and
|
|
3.
|
Temperatures
ranging from -196°C (cryopreservation) to +37°C (body
temperature).
|
Stress Tests on 12 Different
Cell Lines
Cell
lines are selected for their high level of sensitivity. Cell lines are also
selected for their potential role in adding value in medical applications,
enhancing health and extending life. All tests are designed to explore how cells
from different cell lines act biologically in the presence of AAGP™ when
subjected to health and life threatening inflammatory stress conditions
and agents.
Cells Lines
Tested
|
§
Stem
cells (human)
|
§
Adult
skin fibroblast cells
|
|
§
Whole
blood cells
|
§
Heart
cells (cardiac myocites)
|
|
§
Blood
Platelet cells
|
§
Liver
cells (hepatocites)
|
|
§
Heart
tissue
|
§
Embryonic
skin fibroblast cells
|
|
§
Hela
(cancer) cells
|
§
Islet
cells (pancreatic)
|
|
§
Kidney
(KB and vero) cells
|
§
Stem
cells (mouse)
|
Stress Conditions and
Agents
Temperature
|
§
|
temperatures
ranging from -80° C to +37°
|
UV-C
Radiation
|
§
|
harsh
sterilizing radiation
|
|
§
|
254
nanometer wavelength
|
Oxidation
|
§
|
hydrogen
peroxide (H2O20
|
Starvation
|
§
|
serum
free culture media
|
|
§
|
food/growth/nutrients
factors (fetal bovine serum)
withheld
|
Inflammation
|
§
|
Interleukin
1 Beta, a standard agent for stimulating inflammation in cell
testing
|
|
§
|
All
of the above tests are also considered to cause
inflammation
|
Bio-Screening Control Lab
Testing
AAGP™
testing is conducted to international standards in outsourced research
laboratories in North America and Europe. All tests are designed to explore both
the safety and effectiveness of AAGP™ when challenged to enhance the health
and extend the life of cells.
Test
Results Summary
Cells
that were tested in the presence of AAGP™ had a higher survival and viability
rate than the controls. The overall effect of AAGP™ is to protect, preserve and
in some cases to repair. Anti-inflammatory effects appear to be at work,
although the mechanism and pathways of action are not yet
determined. AAGP™ appears to enhance heath and extend cell
life.
The test
results are considered preliminary. The limited number of samples and extent of
the tests are designed to investigate the potential attributes of AAGP™ and
should not be considered as statistically or scientifically conclusive.
Notwithstanding, we feel the results are sufficient to justify further tests by
commercial entities in health care.
AAGP™
Commercial Applications
The
extent of the value of the ProtoKinetix family of AAGPs™ is being investigated
by companies and the Company is targeting commercial entities specializing in
regenerative medicine, cellular and tissue therapies, organ transplantation,
trauma, blood product banking, anti- inflammation and cosmetics/skin
care.
Skincare and
Cosmetics
Industry
sources estimate that the skincare market in the USA, including both mass and
prestige, will reach $7.2 billion by 2010, driven in part by expected
double-digit growth of anti-aging products, which is likely to become the second
largest category behind hand & body lotions in the industry.
According
to the Johnson and Johnson 2003 Annual Report, the global skin care and
cosmetics market is already running easily in the tens of billions at some $43
billion dollars per year.
In the
skin care business it’s about healthier, younger looking skin. The two major
causes of dry, wrinkled, less elastic or even diseased skin are inflammation and
oxidation. The main culprits are the sun (UV rays and free radicals) and other
environmental and physiological stresses that also cause inflammation and
oxidation.
When
AAGP™ is combined with Coenzyme Q10 a powerful anti-oxidant effect is achieved
that not only protects but also seems to help the cells repair previously
existing damage. In vitro laboratory tests have shown the AAGP™ molecules can
protect in vitro skin cells from damage and death that would otherwise occur
from UV rays and free radicals. To the extent of the laboratory tests conducted,
AAGP™ appears to protect in vitro skin cells from cold temperatures, oxidation,
UV irradiation and pH variations.
Health
Care
Acute
medical problems are increasingly reliant on, and benefit from, solutions that
can deal with the fundamental factors of inflammation and oxidation. Both are
well-known causes of life-threatening conditions and diseases, and accelerated
aging. In addition many acute medical problems are benefiting from cell
therapies and transplantation of cells, tissues and time sensitive
organs.
Health
Care Applications of AAGP™ fall into two main categories: (i) harvesting,
storage and transplanting cells, tissues and organs; and (ii) treatments for
conditions and disease caused by stress factors, including UV radiation,
oxidation and inflammation. These are all areas that expand into many
sub-categories of existing and future health care solutions.
Intellectual
Property
Because
it is difficult and costly to protect our proprietary rights, we may not be able
to ensure their protection. Our commercial success will depend in part on
maintaining patent protection and trade secret protection for our products, as
well as successfully defending these patents against third-party challenges. We
will only be able to protect our technologies from unauthorized use by third
parties to the extent that valid and enforceable patents or trade secrets cover
them.
The
patent positions of pharmaceutical and biotechnology companies can be highly
uncertain and involve complex legal and factual questions for which important
legal principles remain unresolved. No consistent policy regarding the breadth
of claims allowed in pharmaceutical or biotechnology patents has emerged to date
in the United States. The patent situation outside the United States is even
more uncertain. Changes in either the patent laws or in interpretations of
patent laws in the United States and other countries may diminish the value of
our intellectual property. Accordingly, we cannot predict the breadth of claims
that may be allowed or enforced in our patents or in third-party
patents.
Patents
As of the
date of this Report, our development agents, including the parties we have
licensed AAGP™ technologies from, have applied to receive patents for
technologies we have licensed and continue to primarily base our research
efforts on. At present, we have engaged the patent law firm of Cabinet-Moutard
of Versaille, France, and have filed a number of international patent
applications. These patent applications include:
WO
2004/014928 A2 (19 February 2004)
PCT Int.
Appl. (2006), 87 pp. WO2006059227 A1 20060608 AN 2006:538719
Patent
application: Fr 03 May 2006, 06 03952
Consistent
with our agreements with the licensors of various technologies we license, we
have no finished commercial product or products, and have received no final
patents awards or FDA approvals for any product or diagnostic
procedures. We are focused on the research and development of one
primary compound known as AAGP™, which we have filed a trademark application
for.
Subject
to our available financial resources, our intellectual property strategy
is: (1) to pursue licenses, trade secrets, and know-how within our
primary research areas, and (2) to develop and acquire proprietary positions to
reagents and new platforms for the development of products related to these
technologies.
Trade
Secrets and Know-How
The
Company has developed a substantial body of trade secrets and know-how relating
to the development, use and manufacture of AAGP™, including but not limited to
the optimization of materials for efforts, and how to maximize sensitivity,
speed-to-result, specificity, stability, purity and
reproducibility.
Super
Antibody and Catalytic Antibody Platform Technologies
The
Company continues to own the rights to both the Super Antibody and the Catalytic
Antibody platform technologies. The Company plans to, as a secondary priority
and subject to available resources, search for a patentable receptor sites that
exist on cancer cells.
Competition
The
markets that the Company is focusing on are multi-billion dollar international
industries. They are intensely competitive. Many of the
Company’s competitors are substantially larger and have greater financial,
research, manufacturing, and marketing resources.
Industry
competition in general is based on the following:
|
§
|
Scientific
and technological capability;
|
|
§
|
The
ability to develop and market products and
processes;
|
|
§
|
The
ability to obtain FDA or other required regulatory
approvals;
|
|
§
|
The
ability to manufacture products that meet applicable FDA requirements,
(i.e. FDA’s Quality System Regulations) see Governmental Regulation
section;
|
|
§
|
Access
to adequate capital;
|
|
§
|
The
ability to attract and retain qualified personnel;
and
|
|
§
|
The
availability of patent protection.
|
The
Company believes its scientific and technological capabilities are
significant.
The
Company’s ability to develop its research is in large measure dependent on
having sufficient and additional resources and/or collaborative
relationships.
The
Company’s access to capital is more challenging, relative to most of its
competitors. This is a competitive disadvantage. The
Company believes however that its access to capital may increase as it gets
closer to the development of a commercially viable product.
The
Company believes that its research has enabled it to attract and retain
qualified consultants. Because of the greater financial resources of
many of its competitors, the Company may not be able to complete effectively for
the same individuals to the extent that a competitor uses its substantial
resources to attract any such individuals.
Governmental
Regulation
The
Company’s AAGPs™ have commercial applications in markets and circumstances that
fall under government regulations ranging from none to limited to
extensive.
Although
there is no such immediate need to make any regulatory filing in the United
States or other jurisdictions, the Company has limited or no experience with
regard to obtaining FDA or other required regulatory approvals. The Company
intends to retain the services of appropriately experiences consultants. For
this reason, should our research efforts continue to show promise, we will need
to hire consultants to assist the Company with such governmental
regulations.
As the
Company continues to conduct research and testing programs, in collaboration
with commercial entities, to expand and confirm the potential medical
applications of AAGP™ in the a number of fields, including regenerative
medicine, cell therapy, blood products, transplants and skin care/cosmetics, the
Company intends to utilize the regulatory expertise of others, whether they are
consultants or commercial entities involved on collaborative development
programs with the Company.
The
following discussion relates to factors that may come into play when and if the
Company has a commercially viable product in an area which requires regulatory
approval. These products may be regulated by the European regulatory
agencies, FDA, U.S. Department of Agriculture, certain state and local agencies,
and/or comparable regulatory bodies in other countries (collectively, these
agencies shall be referred to as the "Agencies"). Government
regulation affects almost all aspects of development, production, and marketing,
including product testing, authorizations to market, labeling, promotion,
manufacturing, and record keeping. The FDA and U.S. Department of
Agriculture regulated products require some form of action by that agency before
they can be marketed in the United States, and, after approval or clearance, the
products must continue to comply with other FDA requirements applicable to
marketed products. Both before and after approval or clearance,
failure to comply with the FDA’s requirements can lead to significant penalties.
The Company's proposed AAGP™ products will require government regulatory
approval as a biologic agent. Such regulatory approval will be granted only
after the appropriate preclinical and clinical studies are conducted to confirm
efficacy and safety.
Every
company that manufactures biologic products or medical devices distributed in
the United States must comply with the FDA’s Quality System
Regulations. These regulations govern the manufacturing process,
including design, manufacture, testing, release, packaging, distribution,
documentation, and purchasing. Compliance with the Quality System
Regulations is required before the FDA will approve an application. These
requirements also apply to marketed products. Companies are also
subject to other post-market and general requirements, including compliance with
restrictions imposed on marketed products, compliance with promotional
standards, record keeping, and reporting of certain adverse reactions or
events. The FDA regularly inspects companies to determine compliance
with the Quality System Regulations and other post-approval
requirements. Failure to comply with statutory requirements and the
FDA’s regulations can lead to substantial penalties, including monetary
penalties, injunctions, product recalls, seizure of products, and criminal
prosecution.
The
Clinical Laboratory Improvement Act of 1988 prohibits laboratories from
performing in vitro tests for the purpose of providing information for the
diagnosis, prevention or treatment of any disease or impairment of, or the
assessment of, the health of human beings unless there is in effect for such
laboratories a certificate issued by the U.S. Department of Health and Human
Services applicable to the category of examination or procedure
performed. Although a certificate is not required for ProtoKinetix,
ProtoKinetix considers the applicability of the requirements of the Clinical
Laboratory Improvement Act in the potential design and development of its
products.
The
Company is also subject to regulations in foreign countries governing products,
human clinical trials and marketing, and may need to obtain approval or
evaluations by international public health agencies, such as the World Health
Organization, in order to sell products in certain
countries. Approval processes vary from country to country, and the
length of time required for approval or to obtain other clearances may in some
cases be longer than that required for U.S. governmental
approvals. The extent of potentially adverse governmental regulation
affecting ProtoKinetix that might arise from future legislative or
administrative action cannot be predicted.
Environmental
Laws
To date,
the Company has not encountered any costs relating to compliance with any
environmental laws.
ITEM
2. DESCRIPTION
OF PROPERTY
The
Company does not own any real property. The Company is currently
paying a rental fee where it is located.
ITEM
3. LEGAL
PROCEEDINGS
There are
currently no legal matters pending.
ITEM
4. SUBMISSION
OF MATTERS TO A VOTE OF SECURITY HOLDERS
No
shareholder meetings were held during the year ended December 31,
2007.
PART
II
|
ITEM
5.
|
MARKET
FOR THE REGISTRANT'S COMMON EQUITY AND RELATED STOCKHOLDER
MATTERS
|
Trades of
our common stock are subject to Rule 15g-9 of the Securities and Exchange
Commission, known as the Penny Stock Rule. This rule imposes
requirements on broker/dealers who sell securities subject to the rule to
persons other than established customers and accredited
investors. For transactions covered by the rule, brokers/dealers must
make a special suitability determination for purchasers of the securities and
receive the purchaser’s written agreement to the transaction prior to
sale. The Securities and Exchange Commission also has rules that
regulate broker/dealer practices in connection with transactions in "penny
stocks." Penny stocks generally are equity securities with a price of
less than $5.00 (other than securities registered on certain national securities
exchanges or quoted on the NASDAQ system, provided that current price and volume
information with respect to transactions in that security is provided by the
exchange or system). The Penny Stock Rules requires a broker/ dealer,
prior to a transaction in a penny stock not otherwise exempt from the rules, to
deliver a standardized risk disclosure document prepared by the Commission that
provides information about penny stocks and the nature and level of risks in the
penny stock market. The broker/dealer also must provide the customer
with current bid and offer quotations for the penny stock, the compensation of
the broker/dealer and its salesperson in the transaction, and monthly account
statements showing the market value of each penny stock held in the customer’s
account. The bid and offer quotations, and the broker/dealer and
salesperson compensation information, must be given to the customer orally or in
writing prior to effecting the transaction and must be given to the customer in
writing before or with the customer’s confirmation. These disclosure
requirements have the effect of reducing the level of trading activity in the
secondary market for our common stock. As a result of these rules,
investors may find it difficult to sell their shares.
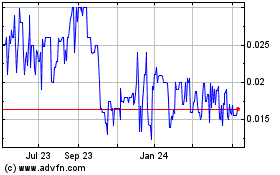
The Company's Common Stock is quoted
on the over-the-counter market and
quoted on the National Association
of Securities Dealers Electronic Bulletin
Board ("OTC Bulletin Board") under the
symbol "PKTX". The high and low bid prices for the Common Stock, as
reported by the National Quotation Bureau, Inc., are indicated for the periods
described below. Such prices are inter-dealer prices without retail
markups, markdowns or commissions, and may not necessarily represent actual
transactions.
|
2007
|
Low
|
High
|
|
First
Quarter
|
$.36
|
$.50
|
|
Second
Quarter
|
.32
|
.45
|
|
Third
Quarter
|
.24
|
.42
|
|
Fourth
Quarter
|
.10
|
.26
|
|
|
|
|
|
2006
|
Low
|
High
|
|
First
Quarter
|
$.65
|
$.68
|
|
Second
Quarter
|
.62
|
.65
|
|
Third
Quarter
|
.51
|
.57
|
|
Fourth
Quarter
|
.40
|
.46
|
Holders
As of
April 10, 2008, there were approximately 60 shareholders of record of the
company's Common Stock.
Dividends
We have
never paid cash dividends and have no plans to do so in the foreseeable
future. Our future dividend policy will be determined by our board of
directors and will depend upon a number of factors, including our financial
condition and performance, our cash needs and expansion plans, income tax
consequences, and the restrictions that applicable laws, our current preferred
stock instruments, and our future credit arrangements may then
impose.
Recent
Sales of Unregistered Securities; Use of Proceeds From Registered
Securities
There
have been no sales of unregistered securities during calendar 2007 which would
be required to be disclosed pursuant to Item 701 of Regulation S-B, except for
the following:
On
January 2, 2007, we issued 84,906 common shares to two consultants in connection
with a consulting agreement. These issuances were made in lieu of cash payments
for services rendered and were considered exempt transactions under Section 4(2)
of the Securities Act of 1933, as amended.
On
January 8, 2007, we issued 133,928 common shares to two consultants in
connection with a consulting agreement. These issuances were made in lieu of
cash payments for services rendered and were considered exempt transactions
under Section 4(2) of the Securities Act of 1933, as amended.
On March
20, 2007, we issued 104,652 common shares to two consultants in connection with
a consulting agreement. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
On April
16, 2007, we issued 187,500 common shares to two consultants in connection with
a consulting agreement. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
On June
11, 2007, we issued 112,500 common shares to two consultants in connection with
a consulting agreement. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
On July
11, 2007, we issued 100,000 common shares to a consultant in connection with a
consulting agreement. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
On July
18, 2007, we issued 191,812 common shares to two consultants in connection with
a consulting agreement. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
On August
15, 2007, we issued a total of 860,000 common shares to several directors,
officers and consultants in connection with services provided by such directors,
officers and consultants. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
On
September 17, 2007, we issued a total of 1,400,000 common shares to several
consultants in connection with services provided by such directors, officers and
consultants. These issuances were made in lieu of cash payments for services
rendered and were considered exempt transactions under Section 4(2) of the
Securities Act of 1933, as amended
On
September 17, 2007, we issued 116,275 common shares to two consultants in
connection with a consulting agreement. These issuances were made in lieu of
cash payments for services rendered and were considered exempt transactions
under Section 4(2) of the Securities Act of 1933, as amended.
On
October 10, 2007, we issued 250,000 common shares to two consultants in
connection with a consulting agreement. These issuances were made in lieu of
cash payments for services rendered and were considered exempt transactions
under Section 4(2) of the Securities Act of 1933, as amended.
On
December 4, 2007, we issued 535,716 common shares to two consultants in
connection with a consulting agreement. These issuances were made in lieu of
cash payments for services rendered and were considered exempt transactions
under Section 4(2) of the Securities Act of 1933, as amended.
On
December 19, 2007, we issued 100,000 common shares to a former director in
connection with services provided to the Company. These issuances were made in
lieu of cash payments for services rendered and were considered exempt
transactions under Section 4(2) of the Securities Act of 1933, as
amended.
On
February 8, 2008, we issued 278,846 common shares to a consultant in connection
with a consulting agreement. These issuances were made in lieu of cash payments
for services rendered and were considered exempt transactions under Section 4(2)
of the Securities Act of 1933, as amended.
On March
20, 2008, we issued a total of 1,700,000 common shares to several investors in
connection with a private placement for a total sales price of $255,000. These
issuances were considered exempt transactions under Section 4(2) of the
Securities Act of 1933, as amended.
On March
26, 2008, we issued 90,500 common shares to two consultants in connection with a
consulting agreement. These issuances were made in lieu of cash payments for
services rendered and were considered exempt transactions under Section 4(2) of
the Securities Act of 1933, as amended.
Warrants
During
2007, in lieu of payment for advisory services rendered to the Company, the
Company issued the following parties warrants to purchase common shares of the
Company's stock:
|
|
|
Date
|
|
Number
of
|
|
Exercise
|
|
Closing
price
|
|
Expiration
|
|
|
|
Issued
|
|
Warrants
|
|
Price
|
|
on
Issuance
|
|
Date
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Simon
Shah
|
|
6/1/2007
|
|
350,000
|
|
$ 0.50
|
|
$ 0.41
|
|
6/1/2012
|
|
Ravi
Chiruvolu
|
|
6/1/2007
|
|
250,000
|
|
$ 0.50
|
|
$ 0.41
|
|
6/1/2012
|
|
Chardan
Capital Markets
|
|
6/1/2007
|
|
350,000
|
|
$ 0.50
|
|
$ 0.41
|
|
6/1/2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bruce
Dorfman
|
|
7/12/2007
|
|
500,000
|
|
$ 0.50
|
|
$ 0.35
|
|
7/12/2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fred
Whittaker
|
|
8/1/2007
|
|
400,000
|
|
$ 0.50
|
|
$ 0.35
|
|
8/1/2012
|
|
Maximilien
Arella
|
|
8/1/2007
|
|
400,000
|
|
$ 0.50
|
|
$ 0.35
|
|
8/1/2012
|
|
Mark
Ralston
|
|
8/1/2007
|
|
2,050,000
|
|
$ 0.50
|
|
$ 0.35
|
|
8/1/2012
|
|
Blair
Henderson
|
|
8/1/2007
|
|
250,000
|
|
$ 0.50
|
|
$ 0.35
|
|
8/1/2012
|
|
Grant
Young
|
|
8/1/2007
|
|
1,500,000
|
|
$ 0.50
|
|
$ 0.35
|
|
8/1/2012
|
|
Dr.
John Todd
|
|
8/1/2007
|
|
500,000
|
|
$ 0.50
|
|
$ 0.35
|
|
8/1/2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Randy
Anderson
|
|
12/1/2007
|
|
250,000
|
|
$ 0.20
|
|
$ 0.16
|
|
12/1/2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wall
Street Communications
|
|
12/4/2007
|
|
250,000
|
|
$ 0.12
|
|
$ 0.15
|
|
12/4/2009
|
|
Wall
Street Communications
|
|
12/4/2007
|
|
250,000
|
|
$ 0.24
|
|
$ 0.15
|
|
12/4/2009
|
|
Wall
Street Communications
|
|
12/4/2007
|
|
250,000
|
|
$ 0.36
|
|
$ 0.15
|
|
12/4/2009
|
|
Wall
Street Communications
|
|
12/4/2007
|
|
250,000
|
|
$ 0.50
|
|
$ 0.15
|
|
12/4/2009
|
Disclosure
Related to Form S-8 Issuances
Prior to
issuing any common shares under Form S-8, the Company requests and receives an
executed verification from all issuees stating that the issuee is a natural
person and that: (a) the shares being issued are not being provided to create or
sustain a market for the Company's securities, and (b) that the shares are not
being issued as a part of a capital raising transaction. All
consultants to the Company are required to provide work product as a part of and
condition to their relationship with the Company. Consultant work
product is delivered in accordance with the terms and conditions of each
respective Consultant’s agreement.
ITEM
6. MANAGEMENT'S
DISCUSSION AND ANALYSIS OR PLAN OF OPERATION
This
discussion and analysis should be read in conjunction with the accompanying
Financial Statements and related notes. Our discussion and analysis
of our financial condition and results of operations are based upon our
financial statements, which have been prepared in accordance with accounting
principles generally accepted in the United States. The preparation of financial
statements in conformity with accounting principles generally accepted in the
United States of America requires us to make estimates and assumptions that
affect the reported amounts of assets and liabilities, disclosure of any
contingent liabilities at the financial statement date and reported amounts of
revenue and expenses during the reporting period. On an on-going basis we review
our estimates and assumptions. Our estimates were based on our historical
experience and other assumptions that we believe to be reasonable under the
circumstances. Actual results are likely to differ from those estimates under
different assumptions or conditions, but we do not believe such differences will
materially affect our financial position or results of operations. Our critical
accounting policies, the policies we believe are most important to the
presentation of our financial statements and require the most difficult,
subjective and complex judgments, are outlined below in "Critical Accounting
Policies," and have not changed significantly.
In
addition, certain statements made in this report may constitute "forward-looking
statements." These forward-looking statements involve known or
unknown risks, uncertainties and other factors that may cause the actual
results, performance, or achievements of the Company to be materially different
from any future results, performance or achievements expressed or implied by the
forward-looking statements. Specifically, 1) our ability to obtain
necessary regulatory approvals for our products; and 2) our ability to
increase revenues and operating income, is dependent upon our ability to develop
and sell our products, general economic conditions, and other factors. You can
identify forward-looking statements by terminology such as "may," "will,"
"should," "expects," "intends," "plans," "anticipates," "believes," "estimates,"
"predicts," "potential," "continues" or the negative of these terms or other
comparable terminology. Although we believe that the expectations reflected-in
the forward-looking statements are reasonable, we cannot guarantee future
results, levels of activity, performance or achievements.
Critical
Accounting Policies
Our
critical and significant accounting policies, including the assumptions and
judgments underlying them, are disclosed in the Notes to the Financial
Statements. These policies have been consistently applied in all
material respects and address such matters as revenue recognition and
depreciation methods. The preparation of the financial statements in
conformity with generally accepted accounting principles in the United States
requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and
liabilities at the date of the financial statements and the reported amounts of
revenues and expenses during the reported period. Actual results
could differ from those estimates. The accounting treatment of a
particular transaction is specifically dictated by accounting principles,
generally accepted in the United States of America, with no need for
management’s judgment in their application. There are also areas in
which management’s judgment in selecting any viable alternative would not
produce a materially different result. See our audited financial
statements and notes thereto which contain accounting policies and other
disclosures required by accounting principles, generally accepted in the United
States of America.
Plan
of Operation
Our
current operations are centered around the Company's relationships with various
research and development consultants who are conducting research on behalf of
the company at discrete and established laboratories in various parts of the
world. The Company intends to continue these efforts throughout
2008.
Sales
and Marketing
The
Company is currently not selling or marketing any products.
Liquidity
and Capital Resources
At
December 31, 2007, we had $37,350 in cash and $147,350 in total current
assets. As of the date of this report, we require additional capital
investments or borrowed funds to meet cash flow projections and carry forward
our business objectives. There can be no assurance that we will be
able to raise capital from outside sources in sufficient amounts to fund our new
business.
The
failure to secure adequate outside funding would have an adverse affect on our
plan of operation and results therefrom and a corresponding negative impact on
shareholder liquidity.
Inflation
Although
management expects that our operations will be influenced by general economic
conditions, we do not believe that inflation had a material effect on our
results of operations during the year ending December 31, 2007.
Going
Concern
The
accompanying financial statements have been prepared in conformity with
generally accepted accounting principles, which contemplate continuation of the
Company as a going concern. The history of losses and the inability
for the Company to make a profit from selling a good or service has raised
substantial doubt about our ability to continue as a going concern. In spite of
the fact that the current cash obligations of the Company are relatively
minimal, given the cash position of the Company, we have very little cash to
operate. We intend to fund the Company and attempt to meet corporate obligations
by selling common stock. However the Company's common stock is at a
low price and is not actively traded.
Results
of Operations for the Year Ended December 31, 2007.
We had $0
in net revenues.
We had a
$2,728,269 net loss from operations for 2007.
Our
expenses in 2007 were $2,728,269 which consisted of $418,724 in professional
legal and accounting expenses. We operate the company by hiring outside
consultants to assist us with management, strategic planning, organization and
daily operations. These professional consulting fees amounted to
$1,134,276. These professional consulting services related to
marketing and investment banking services including financing, capitalization
and merger opportunities. Additional professional consulting fees have been
included in product research and development totaling $996,538.
ITEM
6A QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We face
exposure to fluctuations in the price of our common stock due to the very
limited cash resources we have. For example, the Company has very
limited resources to pay legal and accounting professionals. If we
are unable to pay a legal or accounting professional in order to perform various
professional services for the company, it may be difficult, if not impossible,
for the Company to maintain its reporting status under the '34 Exchange
Act. If the Company felt that it was likely that it would not be able
to maintain its reporting status, it would make a disclosure by filing a Form
8-K with the SEC. In any case, if the Company was not able to
maintain its reporting status, it would become "delisted" and this would
potentially cause an investor or an existing shareholder to lose all or part of
his investment.
ITEM
7. FINANCIAL
STATEMENTS
C O N T E N T S
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
FINANCIAL
STATEMENTS
BALANCE SHEET
STATEMENT OF
OPERATIONS
STATEMENTS OF
STOCKHOLDERS' EQUITY
STATEMENTS OF CASH
FLOWS
NOTES TO FINANCIAL
STATEMENTS
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors and Shareholders
ProtoKinetix,
Incorporated
We have
audited the accompanying balance sheet of ProtoKinetix, Incorporated
(a development stage company) ("the Company") as of December 31, 2007,
and the related statements of operations, stockholders' equity (deficit), and
cash flows for the years ended December 31, 2007 and 2006, and for the
period from December 23, 1999 (date of inception) to December 31,
2007. These financial statements are the responsibility of the
Company's management. Our responsibility is to express an opinion on
these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. The
Company has determined that it is not required to have, nor were we engaged to
perform, an audit of its internal control over financial
reporting. Our audits included consideration of internal control over
financial reporting as a basis for designing audit procedures that are
appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company's internal control over financial
reporting. Accordingly, we express no such opinion. An
audit includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis
for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of ProtoKinetix, Incorporated (a
development stage company) as of December 31, 2007, and the results of its
operations and its cash flows for the years ended December 31, 2007 and 2006,
and for the period from December 23, 1999 (date of inception) through
December 31, 2007, in conformity with accounting principles generally
accepted in the United States.
The
accompanying financial statements have been prepared assuming the Company will
continue as a going concern. As discussed in Note 1 to the
financial statements, the Company has experienced recurring losses from
operations since inception, has a working capital deficit, and has a deficit
accumulated during the development stage. These conditions raise
substantial doubt about the Company's ability to continue as a going
concern. Management's plans regarding these matters are also
described in Note 1. The financial statements do not include any
adjustments that might result from the outcome of this uncertainty.
/s/
PETERSON SULLIVAN PLLC
April 10,
2008
Seattle,
Washington
PROTOKINETIX,
INCORPORATED
BALANCE
SHEET
December 31,
2007
|
|
|
|
|
ASSETS
|
|
|
Current
Assets
|
|
|
|
Cash
|
$ 37,350
|
|
|
Prepaid
expenses
|
110,000
|
|
|
Total
current assets
|
147,350
|
|
Computer
equipment, net of accumulated depreciation of $2,963
|
426
|
|
|
|
|
|
|
$ 147,776
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
Current
Liabilities
|
|
|
|
Accounts
payable
|
$ 108,825
|
|
|
Convertible
note payable
|
300,000
|
|
|
|
|
|
Total
current liabilities
|
408,825
|
|
Stockholders'
Equity (Deficit)
|
|
|
|
Common
stock, $.0000053 par value; 100,000,000 common
|
|
|
|
|
shares
authorized; 48,444,442 shares issued and outstanding
|
262
|
|
|
Common
stock issuable; 1,190,000 shares
|
6
|
|
|
Additional
paid-in capital
|
19,323,715
|
|
|
Deficit
accumulated during the development stage
|
(19,585,032)
|
|
|
|
|
|
|
(261,049)
|
|
|
|
|
|
|
$ 147,776
|
PROTOKINETIX,
INCORPORATED
STATEMENTS
OF OPERATIONS
December 31,
2007
For the
Years Ended December 31, 2007 and 2006, and for the Period from
December
23, 1999 (Date of Inception) to December 31, 2007
|
|
|
|
|
|
|
|
|
|
Cumulative
|
|
|
|
|
|
|
|
|
|
|
During
the
|
|
|
|
|
|
|
|
|
|
|
Development
|
|
|
|
|
|
|
2007
|
|
2006
|
|
Stage
|
|
Revenues
|
$ -
|
|
$ 2,000
|
|
$ 2,000
|
|
Expenses
|
|
|
|
|
|
|
|
Licenses
|
|
|
|
|
3,379,756
|
|
|
Professional
fees
|
418,724
|
|
386,095
|
|
3,231,512
|
|
|
Consulting
fees
|
1,134,276
|
|
1,196,124
|
|
10,368,079
|
|
|
Research
and development
|
996,538
|
|
180,709
|
|
1,797,429
|
|
|
General
and administrative
|
166,731
|
|
192,836
|
|
706,628
|
|
|
Interest
|
12,000
|
|
11,869
|
|
60,162
|
|
|
|
|
|
|
2,728,269
|
|
1,967,633
|
|
19,543,566
|
|
|
|
|
|
Loss
from continuing operations
|
(2,728,269)
|
|
(1,965,633)
|
|
(19,541,566)
|
|
Discontinued
Operations
|
|
|
|
|
|
|
|
Loss
from operations of the discontinued segment
|
-
|
|
-
|
|
(43,466)
|
|
|
|
|
|
Net
loss
|
$
(2,728,269)
|
|
$(1,965,633)
|
|
$(19,585,032)
|
|
Net
Loss per Common Share (basic and
|
|
|
|
|
|
|
|
fully
diluted)
|
$ (0.06)
|
|
$ (0.05)
|
|
|
|
Weighted
average number of common
|
|
|
|
|
|
|
|
shares
outstanding
|
45,749,464
|
|
43,233,617
|
|
|
PROTOKINETIX,
INCORPORATED
STATEMENTS
OF STOCKHOLDERS' EQUITY (DEFICIT)
For the
Period from December 23, 1999 (Date of Inception) to December 31,
2007
|
|
|
|
|
|
|
|
|
|
|
|
Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Common
Stock
|
Additional
|
Stock
|
During
the
|
|
|
|
|
Common
Stock
|
|
Issuable
|
Paid-in
|
Subscriptions
|
Development
|
|
|
|
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
Capital
|
Receivable
|
Stage
|
Total
|
|
Issuance
of common stock, December 1999
|
9,375,000
|
|
$ 50
|
|
-
|
|
$ -
|
$ 4,950
|
$ -
|
$ -
|
$ 5,000
|
|
Net
loss for period
|
|
|
|
|
|
|
|
|
|
(35)
|
(35)
|
|
Balance,
December 31, 2000
|
9,375,000
|
|
50
|
|
-
|
|
-
|
4,950
|
-
|
(35)
|
4,965
|
|
Issuance
of common stock, April 2001
|
5,718,750
|
|
30
|
|
|
|
|
15,220
|
|
|
15,250
|
|
Net
loss for year
|
|
|
|
|
|
|
|
|
|
(16,902)
|
(16,902)
|
|
Balance,
December 31, 2001
|
15,093,750
|
|
80
|
|
-
|
|
-
|
20,170
|
-
|
(16,937)
|
3,313
|
|
Net
loss for year
|
|
|
|
|
|
|
|
|
|
(14,878)
|
(14,878)
|
|
Balance,
December 31, 2002
|
15,093,750
|
|
80
|
|
-
|
|
-
|
20,170
|
-
|
(31,815)
|
(11,565)
|
|
Issuance
of common stock for services:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July
2003
|
2,125,000
|
|
11
|
|
|
|
|
424,989
|
|
|
425,000
|
|
|
August
2003
|
300,000
|
|
2
|
|
|
|
|
14,998
|
|
|
15,000
|
|
|
September
2003
|
1,000,000
|
|
5
|
|
|
|
|
49,995
|
|
|
50,000
|
|
|
October
2003
|
1,550,000
|
|
8
|
|
|
|
|
619,992
|
|
|
620,000
|
|
Issuance
of common stock for licensing rights
|
14,000,000
|
|
74
|
|
|
|
|
2,099,926
|
|
|
2,100,000
|
|
Common
stock issuable for licensing rights
|
|
|
|
|
2,000,000
|
|
11
|
299,989
|
|
|
300,000
|
|
Shares
cancelled on September 30, 2003
|
(9,325,000)
|
|
(49)
|
|
|
|
|
49
|
|
|
-
|
|
Net
loss for year
|
|
|
|
|
|
|
|
|
|
(3,662,745)
|
(3,662,745)
|
|
Balance,
December 31, 2003
|
24,743,750
|
|
131
|
|
2,000,000
|
|
11
|
3,530,108
|
-
|
(3,694,560)
|
(164,310)
|
|
Issuance
of common stock for services:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March
2004
|
1,652,300
|
|
9
|
|
|
|
|
991,371
|
|
|
991,380
|
|
|
May
2004
|
500,000
|
|
3
|
|
|
|
|
514,997
|
|
|
515,000
|
|
|
July
2004
|
159,756
|
|
1
|
|
|
|
|
119,694
|
|
|
119,695
|
|
|
August
2004
|
100,000
|
|
1
|
|
|
|
|
70,999
|
|
|
71,000
|
|
|
October
2004
|
732,400
|
|
4
|
|
|
|
|
479,996
|
|
|
480,000
|
|
|
November
2004
|
650,000
|
|
4
|
|
|
|
|
454,996
|
|
|
455,000
|
|
|
December
2004
|
255,000
|
|
1
|
|
|
|
|
164,425
|
|
|
164,426
|
|
Common
stock issuable for AFGP license
|
|
|
|
|
1,000,000
|
|
5
|
709,995
|
|
|
710,000
|
|
Common
stock issuable for Recaf License
|
|
|
|
|
400,000
|
|
2
|
223,998
|
|
|
224,000
|
|
Warrants
granted (for 3,450,000 shares) for services,
|
|
|
|
|
|
|
|
|
|
|
|
|
October
2004
|
|
|
|
|
|
|
|
1,716,253
|
|
|
1,716,253
|
|
Options
granted for services, October 2004
|
|
|
|
|
|
|
|
212,734
|
|
|
212,734
|
|
Stock
subscriptions receivable
|
|
|
|
|
1,800,000
|
|
10
|
329,990
|
(330,000)
|
|
-
|
|
Warrants
exercised:
|
|
|
|
|
|
|
|
|
|
|
-
|
|
|
August
2004
|
|
|
|
|
50,000
|
|
|
15,000
|
|
|
15,000
|
|
|
October
2004
|
|
|
|
|
600,000
|
|
3
|
134,997
|
|
|
135,000
|
|
|
December
2004
|
|
|
|
|
1,000,000
|
|
5
|
224,995
|
|
|
225,000
|
|
Options
exercised, December 2004
|
|
|
|
|
100,000
|
|
1
|
29,999
|
|
|
30,000
|
|
Net
loss for year
|
|
|
|
|
|
|
|
|
|
(6,368,030)
|
(6,368,030)
|
|
Balance,
December 31, 2004
|
28,793,206
|
|
$ 154
|
|
6,950,000
|
|
$ 37
|
$9,924,547
|
$
(330,000)
|
$(10,062,590)
|
$(467,852)
|
PROTOKINETIX,
INCORPORATED
STATEMENTS
OF STOCKHOLDERS' EQUITY
(Continued)
For the
Period from December 23, 1999 (Date of Inception) to December 31,
2007
|
|
|
|
|
|
|
|
|
|
|
Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Common
Stock
|
Additional
|
Stock
|
During
the
|
|
|
|
|
|
Common
Stock
|
Issuable
|
|
|
Paid-in
|
Subscriptions
|
Development
|
|
|
|
|
|
Shares
|
|
Amount
|
Shares
|
|
Amount
|
Capital
|
Receivable
|
Stage
|
|
Total
|
|
Issuance
of stock subscriptions receivable
|
-
|
|
$ -
|
-
|
|
$ -
|
$ -
|
$ 240,000
|
$ -
|
|
$ 240,000
|
|
Issuance
of common stock for licensing rights
|
2,000,000
|
|
11
|
(2,000,000)
|
|
(11)
|
|
|
|
|
-
|
|
Issuance
of stock for warrants exercised
|
2,050,000
|
|
10
|
(2,050,000)
|
|
(10)
|
|
|
|
|
-
|
|
Options
exercised,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
February
2005
|
|
|
|
35,000
|
|
1
|
10,499
|
|
|
|
10,500
|
|
|
May
2005
|
200,000
|
|
1
|
|
|
|
59,999
|
|
|
|
60,000
|
|
Note
payable conversion, February 2005
|
|
|
|
285,832
|
|
1
|
85,749
|
|
|
|
85,750
|
|
Issuance
of common stock for Note payable conversion
|
|
|
|
|
|
|
|
|
|
|
|
|
April
2005
|
285,832
|
|
1
|
(285,832)
|
|
(1)
|
|
|
|
|
-
|
|
|
May
2005
|
353,090
|
|
2
|
|
|
|
105,925
|
|
|
|
105,927
|
|
Issuance
of common stock for AFGP license
|
1,000,000
|
|
5
|
(1,000,000)
|
|
(5)
|
|
|
|
|
-
|
|
Issuance
of common stock for stock subscriptions received
|
1,400,000
|
|
6
|
(1,400,000)
|
|
(6)
|
|
90,000
|
|
|
90,000
|
|
Issuance
of stock for options exercised
|
135,000
|
|
2
|
(135,000)
|
|
(2)
|
|
|
|
|
-
|
|
Issuance
of common stock for services:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
April
2005
|
30,000
|
|
1
|
|
|
|
14,999
|
|
|
|
15,000
|
|
|
May
2005
|
3,075,000
|
|
15
|
|
|
|
3,320,985
|
|
|
|
3,321,000
|
|
|
June
2005
|
50,000
|
|
1
|
|
|
|
50,499
|
|
|
|
50,500
|
|
|
August
2005
|
(250,000)
|
|
(1)
|
|
|
|
(257,499)
|
|
|
|
(257,500)
|
|
|
August
2005
|
111,111
|
|
1
|
(92,593)
|
|
(1)
|
15,000
|
|
|
|
15,000
|
|
|
October
2005
|
36,233
|
|
1
|
(36,233)
|
|
(1)
|
-
|
|
|
|
-
|
|
|
November
2005
|
|
|
|
|
|
|
|
|
|
|
|
|
|
November
2005
|
311,725
|
|
2
|
(245,000)
|
|
(1)
|
36,249
|
|
|
|
36,250
|
|
|
December
2005
|
1,220,000
|
|
8
|
|
|
|
756,392
|
|
|
|
756,400
|
|
Common
stock issuable for services rendered
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June
2005
|
|
|
|
200,000
|
|
1
|
149,999
|
|
|
|
150,000
|
|
|
August
2005
|
|
|
|
36,233
|
|
1
|
21,739
|
|
|
|
21,740
|
|
|
September
2005
|
|
|
|
125,000
|
|
1
|
74,999
|
|
|
|
75,000
|
|
|
September
2005(Proteocell)
|
|
|
|
100,000
|
|
1
|
57,999
|
|
|
|
58,000
|
|
|
December
2005
|
|
|
|
120,968
|
|
1
|
74,999
|
|
|
|
75,000
|
|
Net
loss for the year
|
|
|
|
|
|
|
|
|
(4,826,540)
|
|
(4,826,540)
|
|
Balance,
December 31, 2005
|
40,801,197
|
|
$ 220
|
608,375
|
|
$ 6
|
$14,503,079
|
$ -
|
$(14,889,130)
|
|
$ (385,825)
|
PROTOKINETIX,
INCORPORATED
STATEMENTS
OF STOCKHOLDERS' EQUITY
(Continued)
For the
Period from December 23, 1999 (Date of Inception) to December 31,
2007
|
|
|
|
|
|
|
|
|
|
|
Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Common
Stock
|
Additional
|
Stock
|
During
the
|
|
|
|
|
|
Common
Stock
|
Issuable
|
|
|
Paid-in
|
Subscriptions
|
Development
|
|
|
|
|
|
Shares
|
|
Amount
|
Shares
|
|
Amount
|
Capital
|
Receivable
|
Stage
|
|
Total
|
|
February
2006 private placement (issued June 2006)
|
900,000
|
|
$ 5
|
|
|
$ -
|
$ 352,142
|
$ -
|
$ -
|
|
$ 352,147
|
|
Warrants
granted from private placement (450,000)
|
|
|
|
|
|
|
97,853
|
|
|
|
97,853
|
|
Issuance
of common stock for Note payable conversion
|
529,279
|
|
3
|
|
|
|
158,780
|
|
|
|
158,783
|
|
Issuance
of common stock for services:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
February/March
2006 services
|
|
|
|
20,000
|
|
1
|
10,499
|
|
|
|
10,500
|
|
|
March
2006
|
166,359
|
|
1
|
(108,375)
|
|
(1)
|
36,750
|
|
|
|
36,750
|
|
|
April
2006
|
(1,200,000)
|
|
(6)
|
|
|
|
6
|
|
|
|
-
|
|
|
May
2006
|
1,266,278
|
|
7
|
(70,000)
|
|
(1)
|
792,750
|
|
|
|
792,756
|
|
|
June
2006
|
27,056
|
|
|
1,200,000
|
|
6
|
718,244
|
|
|
|
718,250
|
|
|
July
2006
|
1,200,000
|
|
6
|
(1,200,000)
|
|
(6)
|
|
|
|
|
-
|
|
|
August
2006
|
100,000
|
|
1
|
|
|
|
64,999
|
|
|
|
65,000
|
|
|
September
2006
|
369,984
|
|
2
|
(50,000)
|
|
|
209,998
|
|
|
|
210,000
|
|
|
November
2006
|
100,000
|
|
1
|
|
|
|
48,999
|
|
|
|
49,000
|
|
|
December
2006
|
7,000
|
|
|
|
|
|
3,010
|
|
|
|
3,010
|
|
Warrants
issued (for 700,000 shares) for services
|
|
|
|
|
|
|
58,658
|
|
|
|
58,658
|
|
Net
loss for the year
|
|
|
|
|
|
|
|
|
(1,967,633)
|
|
(1,967,633)
|
|
Balance,
December 31, 2006
|
44,267,153
|
|
240
|
400,000
|
|
5
|
17,055,767
|
-
|
(16,856,763)
|
|
199,249
|
|
Issuance
of common stock for services:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January
2007
|
218,834
|
|
1
|
|
|
|
119,999
|
|
|
|
120,000
|
|
|
March
2007
|
104,652
|
|
1
|
|
|
|
44,999
|
|
|
|
45,000
|
|
|
April
2007
|
187,500
|
|
1
|
|
|
|
74,999
|
|
|
|
75,000
|
|
|
June
2007
|
112,500
|
|
1
|
|
|
|
44,999
|
|
|
|
45,000
|
|
|
July
2007
|
291,812
|
|
2
|
|
|
|
112,998
|
|
|
|
113,000
|
|
|
August
2007
|
860,000
|
|
5
|
|
|
|
257,995
|
|
|
|
258,000
|
|
|
September
2007
|
1,516,275
|
|
8
|
|
|
|
457,492
|
|
|
|
457,500
|
|
|
October
2007
|
250,000
|
|
1
|
|
|
|
37,499
|
|
|
|
37,500
|
|
|
December
2007
|
535,716
|
|
1
|
|
|
|
74,999
|
|
|
|
75,000
|
|
Warrants
issued for services
|
|
|
|
|
|
|
825,476
|
|
|
|
825,476
|
|
Cancellation
of issuable stock for Recaf License
|
|
|
|
(400,000)
|
|
(5)
|
|
|
|
|
(5)
|
|
Warrant
exercised-December 2007
|
100,000
|
|
1
|
|
|
|
43,999
|
|
|
|
44,000
|
|
Issuable
common stock from Private Placement
|
|
|
|
1,190,000
|
|
6
|
172,494
|
|
|
|
172,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the year
|
|
|
|
|
|
|
|
|
$
(2,728,269)
|
|
(2,728,269)
|
|
Balance,
December 31, 2007
|
48,444,442
|
|
$ 262
|
1,190,000
|
|
$ 6
|
$19
,323,715
|
-
|
$(19,585,032)
|
|
$ (261,049)
|
PROTOKINETIX,
INCORPORATED
STATEMENTS
OF CASH FLOWS
For the
Years Ended December 31, 2007 and 2006, and for the Period from
December
23, 1999 (Date of Inception) to December 31, 2007
|
|
|
|
|
|
2007
|
|
2006
|
|
Cumulative
During the Development Stage
|
|
Cash
Flows from Operating Activities
|
|
|
|
|
|
|
|
Net
loss for year
|
$(2,728,269)
|
|
$(1,967,633)
|
|
$(19,585,032)
|
|
|
Adjustments
to reconcile net loss to net cash
|
|
|
|
|
|
|
|
|
used
in operating activities
|
|
|
|
|
|
|
|
|
Depreciation
expense
|
1,018
|
|
1,017
|
|
2,962
|
|
|
Issuance
of common stock for services
|
|
|
|
|
|
|
|
|
and
expenses
|
1,225,979
|
|
1,885,266
|
|
14,668,136
|
|
|
Warrants
issued for consulting services
|
825,476
|
|
58,658
|
|
2,600,387
|
|
|
Stock
options issued for consulting services
|
|
|
|
|
212,734
|
|
|
Changes
in operating assets and liabilities
|
|
|
|
|
|
|
|
|
Accounts
receivable
|
6,391
|
|
148
|
|
-
|
|
|
|
Prepaid
expenses
|
330,000
|
|
(433,800)
|
|
(110,000)
|
|
|
|
Amounts
due to outside
|
|
|
|
|
|
|
|
|
|
management
consultants
|
(306,892)
|
|
|
|
|
|
|
|
Accounts
payable
|
1,032
|
|
75,888
|
|
108,007
|
|
|
|
Interest
payable
|
|
|
|
|
36,294
|
|
|
|
|
|
Net
cash used in operating activities
|
(645,265)
|
|
(380,456)
|
|
(2,066,512)
|
|
Cash
Flows from Investing Activity
|
|
|
|
|
|
|
|
Purchase
of computer equipment
|
|
|
|
|
(3,388)
|
|
|
|
|
|
Net
cash used in investing activities
|
-
|
|
-
|
|
(3,388)
|
|
Cash
Flows from Financing Activities
|
|
|
|
|
|
|
|
Warrants
exercised
|
44,000
|
|
|
|
749,000
|
|
|
Stock
options exercised
|
|
|
|
|
100,500
|
|
|
Issuance
of common stock for cash
|
172,500
|
|
450,000
|
|
642,750
|
|
|
Loan
proceeds
|
300,000
|
|
|
|
615,000
|
|
|
|
|
|
Net
cash provided by
|
|
|
|
|
|
|
|
|
|
|
financing
activities
|
516,500
|
|
450,000
|
|
2,107,250
|
|
|
|
|
|
Net
change in cash
|
(128,765)
|
|
69,544
|
|
37,350
|
|
Cash,
beginning of period
|
166,115
|
|
96,571
|
|
|
|
Cash,
end of period
|
$ 37,350
|
|
$ 166,115
|
|
$ 37,350
|
|
Cash
paid for interest
|
$ 12,000
|
|
$ 11,869
|
|
$ 12,703
|
|
Cash
paid for income taxes
|
$ -
|
|
$ -
|
|
$ -
|
|
Supplementary
information - Non-cash Transactions:
|
|
|
|
|
|
|
Stock
subscriptions received
|
|
|
$ -
|
|
$ 330,000
|
|
|
Note
payable converted to common stock
|
$ -
|
|
$ 158,783
|
|
350,457
|
NOTES TO FINANCIAL
STATEMENTS
Note
1. Basis of Presentation – Going Concern Uncertainties
ProtoKinetix,
Incorporated (the "Company"), a development stage company, was incorporated
under the laws of the State of Nevada on December 23, 1999. The
Company is a medical research company whose mission is the advancement of human
health care.
In 2003,
the Company entered into an assignment of license agreement (the "Agreement")
with BioKinetix, Inc., an Alberta, Canada, corporation. The Agreement
provided the Company with an exclusive assignment of all of the rights (the
"Rights") that BioKinetix possessed relating to proprietary technologies that
are being developed for the creation and commercialization of "superantibodies,"
an enhancement of antibody technology that makes ordinary antibodies much more
lethal. In consideration, the Company's Board of Directors authorized
the Company to issue 16,000,000 shares of its common stock to the shareholders
of BioKinetix.
The
Company is also currently researching the benefits and feasibility of
proprietary synthesized Antifreeze Glycoproteins ("AFGP"). In
preliminary studies, AFGP has demonstrated an ability to protect and preserve
human cells at temperatures below freezing.
The
Company's financial statements are prepared consistent with accounting
principles generally accepted in the United States applicable to a going
concern.
As shown
in the financial statements, the Company has not developed a commercially viable
product, has not generated any significant revenue to date, and has incurred
losses since inception, resulting in a net accumulated deficit at
December 31, 2007. These factors raise substantial doubt about
the Company's ability to continue as a going concern.
The
Company needs additional working capital to continue its medical research or to
be successful in any future business activities and continue to pay its
liabilities. Therefore, continuation of the Company as a going
concern is dependent upon obtaining the additional working capital necessary to
accomplish its objective. Management is presently engaged in seeking
additional working capital.
The
accompanying financial statements do not include any adjustments to the recorded
assets or liabilities that might be necessary should the Company fail in any of
the above objectives and is unable to operate for the coming year.
Note
2 – Summary of Significant Accounting Policies
Principles of
Accounting
These
financial statements are stated in U.S. Dollars and have been prepared in
accordance with accounting principles generally accepted in the United States of
America.
Use of
Estimates
Preparation
of financial statements in conformity with generally accepted accounting
principles in the United States of America requires management to make estimates
and assumptions that affect reported amounts of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial
statements and reported amounts of revenue and expenses during the reporting
period. Actual results could differ from those
estimates. The more significant accounting estimates inherent in the
preparation of the Company's financial statements include estimates as to
valuation of equity related instruments issued.
Reclassification
Certain
prior period amounts have been reclassified to conform to current year
presentation.
Cash
Cash
consists of funds held in checking accounts. Cash balances may exceed
federally insured limits from time to time.
Prepaid
Expenses
Prepaid
expenses consist of the unamortized value of shares issued for services related
to contract research and development to be performed through April of
2008.
Computer
Equipment
Computer
equipment is stated at cost and is depreciated using straight-line methods over
their estimated useful lives.
Convertible Note
Payable
On July
1, 2007, the Company executed a loan agreement under which the Company issued to
a corporation an 8% convertible promissory note in exchange for
$300,000. The noteholder has the right to demand payment of
outstanding principal and interest at any time with a 30-day grace
period. The note is due and payable no later than June 30, 2012, and
is convertible into shares of the Company's common stock at $0.25 per
share. No beneficial conversion feature was applicable to this
convertible note.
Fair Value of Financial
Instruments
Financial
instruments consist of cash, accounts payable, accrued interest, and convertible
promissory note payable. The fair value of these financial
instruments approximates the carrying amounts due to the short-term nature and
the market rate of interest on the convertible note.
Revenue
Recognition
The
Company recognizes revenue when a sale is made, the fee is fixed or
determinable, collectibility is probable, and no significant company obligations
remain.
Income
Taxes
The
Company accounts for income taxes under an asset and liability approach that
requires the recognition of deferred tax assets and liabilities for expected
future tax consequences of events that have been recognized in the Company's
financial statements or tax returns. In estimating future tax
consequences, the Company generally considers all expected future events other
than enactments of changes in the tax laws or rates.
Research and Development
Costs
Research
and development costs are expensed as incurred.
Earnings per Share and
Potentially Dilutive Securities
Basic
loss per share is computed by dividing the net loss available to common
stockholders by the weighted average number of common shares outstanding in the
period. The Company's stock split 1:75 on August 24,
2001. In April 2002, the Board of Directors approved a 2.5 for 1
split of the Company's stock. The accompanying financial statements
are presented on a post-split basis. The loss per share for the years
ended December 31, 2007 and 2006, have been adjusted
accordingly. Diluted loss per share takes into consideration common
shares outstanding (computed under basic earnings per share) and potentially
dilutive securities. The effect of debt convertible into common
shares was not included in the computation of diluted earnings per share for all
periods presented because it was anti-dilutive due to the Company's
losses. Common stock issuable is considered outstanding as of the
original approval date for purposes of earnings per share
computations.
Share-Based
Compensation
The
Company has granted warrants to purchase shares of the Company's common stock to
various parties for consulting services. The fair values of the
warrants issued have been estimated using the Black-Scholes option valuation
model.
The
Company accounts for stock-based compensation under SFAS No. 123(R) "Share-Based
Payment," which requires measurement of compensation cost for all stock-based
awards at fair value on the date of grant and recognition of compensation over
the service period for awards expected to vest. The fair value of
stock options is determined using the Black-Scholes valuation
model.
Related Party
Transactions
A related
party is generally defined as (i) any person that holds 10% or more of the
Company's securities and their immediate families, (ii) the Company's
management, (iii) someone that directly or indirectly controls, is controlled by
or is under common control with the Company, or (iv) anyone who can
significantly influence the financial and operating decisions of the
Company. A transaction is considered to be a related party
transaction when there is a transfer of resources or obligations between related
parties.
Recent Accounting
Pronouncements
In June
2006, the Financial Accounting Standards Board ("FASB") issued FASB
Interpretation No. 48,
Accounting for Uncertainty in Income
Taxes as amended),
("FIN 48"). FIN 48 clarifies the accounting
for uncertainty in income taxes and prescribes a recognition threshold and
measurement attribute for the financial statement recognition and measurement of
a tax position taken or expected to be taken in a tax return. FIN 48
is effective for financial statements as of January 1, 2007. The
Company has not yet determined the impact of applying FIN 48.
In
December 2007, the FASB issued SFAS No. 141(R),
"Business Combinations"
("SFAS 141(R)"), which replaces SFAS No. 141. SFAS No.
141(R) establishes principles and requirements for how an acquirer recognizes
and measures in its financial statements the identifiable assets acquired, the
liabilities assumed, any non-controlling interest in the acquiree and the
goodwill acquired. The Statement also establishes disclosure
requirements which will enable users to evaluate the nature and financial
effects of the business combination. SFAS 141(R) is effective
for fiscal years beginning after December 15, 2008. The adoption of
SFAS 141(R) will have an impact on accounting for business combinations
once adopted, but the effect is dependent upon acquisitions at that
time.
In
September 2006, the FASB issued Statement of Financial Accounting Standards No.
157,
Fair Value Measurements
(as amended)
("FAS 157"). FAS 157 defines fair value,
establishes a framework for measuring fair value and expands disclosures about
fair value measurements but does not require any new fair value
measurements. FAS 157 is effective for financial statements
issued for fiscal years beginning after November 15, 2007, and interim periods
within those fiscal years. The Company has not yet determined the
impact of applying FAS 157.
In
September 2006, the FASB issued Statement of Financial Accounting Standards No.
158,
Employers' Accounting for
Defined Benefit Pension and Other Postretirement Plans (as amended)
,
("FAS 158"). FAS 158 requires an employer to recognize the overfunded
or underfunded status of a defined benefit postretirement plan (other than a
multiemployer plan) as an asset or liability in its statement of financial
position and to recognize changes in that funded status in the year in which the
changes occur through comprehensive income. FAS 158 is effective
for financial statements issued for fiscal years ending after December 31,
2006. The Company does not expect any material impact from applying
FAS 158.
In
February 2007, the FASB issued FAS No. 159,
"The Fair Value Option for Financial
Assets and Financial Liabilities - Including an amendment of FASB Statement No.
115"
, ("FAS 159") which permits entities to choose to measure many
financial instruments and certain other items at fair value at specified
election dates. A business entity is required to report unrealized gains
and losses on items for which the fair value option has been elected in earnings
at each subsequent reporting date. This statement is expected to expand
the use of fair value measurement. FAS 159 is effective for
financial statements issued for fiscal years beginning after November 15,
2007, and interim periods within those fiscal years. The Company has not
yet determined the impact of applying FAS 159.
In
December 2007, the FASB issued SFAS No. 160,
"Noncontrolling Interests in
Consolidated Financial Statements – an amendment of Accounting Research Bulletin
No. 51"
("SFAS 160"), which establishes accounting and reporting
standards for ownership interests in subsidiaries held by parties other than the
parent, the amount of consolidated net income attributable to the parent and to
the noncontrolling interest, changes in a parent's ownership interest and the
valuation of retained non-controlling equity investments when a subsidiary is
deconsolidated. The Statement also establishes reporting requirements that
provide sufficient disclosures that clearly identify and distinguish between the
interests of the parent and the interests of the non-controlling
owners. SFAS 160 is effective for fiscal years beginning after
December 15, 2008. The Company does not expect the adoption of SFAS
160 to have an impact on its financial statements.
In June
2007, the Emerging Issues Task Force of the FASB issued EITF Issue No. 07-3,
Accounting for Nonrefundable
Advance Payments for Goods or Services to be Used in Future Research and
Development Activities
, ("EITF 07-3") which is effective for fiscal years
beginning after December 15, 2007. EITF 07-3 requires that nonrefundable
advance payments for future research and development activities be deferred and
capitalized. Such amounts will be recognized as an expense as the
goods are delivered or the related services are performed. The Company
does not expect the adoption of EITF 07-3 to have a material impact on the
financial results of the Company.
Note
3. Income Taxes
The
Company is liable for taxes in the United States. As of
December 31, 2007, the Company did not have any income for tax purposes and
therefore, no tax liability or expense has been recorded in these financial
statements.
The
Company has tax losses of approximately $19,500,000 available to reduce future
taxable income. The tax loss expires in years between 2022 and
2028.
The
deferred tax asset associated with the tax loss carry forward is approximately
$6,600,000. The Company has provided a full valuation allowance
against the deferred tax asset since it is more likely than not tat the asset
will not be realized. The valuation allowance increased by $900,000
and $574,000 for 2007 and 2006, respectively.
The
difference between the Company's statutory income tax rate of (34%) and its
effective rate of zero is primarily attributable to the valuation allowance
provided on deferred taxes arising from net operating loss
carryforwards.
Note
4. Discontinued Operations
In 2003,
the Company signed the licensing agreement described in Note 1. This
agreement changed the Company's business plan to that of a medical research
company. Accordingly, the operating results related to the
internet-based real estate listing segment have been presented as discontinued
operations in these financial statements for all periods
presented. There were no revenues for the cumulative period presented
in losses from discontinued operations.
Note
5. Share-Based Compensation
In 2003,
the Company adopted its 2003 and 2004 Stock Incentive Plans. Each
plan provides for the issuance of incentive and non-qualified shares of the
Company's stock to officers, directors, employees, and
non-employees. The Board of Directors determines the terms of the
shares or options to be granted, including the number of shares or options, the
exercise price, and the vesting schedule, if applicable. In 2006 and
2007, the Company issued common shares from both plans to non-employee
consultants for services rendered as follows:
|
2006
|
|
Number
of
Shares
|
|
Value
per
Share
|
|
February/March
|
|
20,000
|
|
$0.53
|
|
March
|
|
77,984
|
|
0.61
|
|
May
|
|
1,196,278
|
|
0.66
|
|
June
|
|
1,227,056
|
|
0.59
|
|
August
|
|
100,000
|
|
0.65
|
|
September
|
|
319,984
|
|
0.66
|
|
November
|
|
100,000
|
|
0.49
|
|
December
|
|
7,000
|
|
0.43
|
|
Total
2006
|
|
3,048,302
|
|
|
|
2007
|
|
Number
of Shares
|
|
Value
per Share
|
|
January
|
|
84,906
|
|
$0.47
|
|
January
|
|
133,928
|
|
0.45
|
|
March
|
|
104,652
|
|
0.36
|
|
April
|
|
187,500
|
|
0.35
|
|
June
|
|
112,500
|
|
0.43
|
|
July
|
|
100,000
|
|
0.38
|
|
July
|
|
191,812
|
|
0.39
|
|
August
|
|
860,000
|
|
0.30
|
|
September
|
|
1,516,275
|
|
0.30
|
|
October
|
|
250,000
|
|
0.15
|
|
December
|
|
635,716
|
|
0.15
|
|
Total
2007
|
|
4,177,289
|
|
|
The
Company has not issued stock options in 2007 or 2006. There are no
options outstanding as of December 31, 2007 or 2006.
Note
6. Warrants
During
2007, the Company issued 7,800,000 warrants to purchase common stock at exercise
prices from $0.12 to $0.50 per share for services, these warrants expire in two
to five years. The cost of the warrants has been expensed in 2007 as
compensation. The fair value of the warrants was
$825,476. The warrants were valued using the Black-Scholes method
including the following assumptions:
|
Risk
free interest rate
|
|
3.11%
to 4.96%
|
|
Expected
life of conversion feature in years
|
|
1
to 2.5
|
|
Expected
volatility
|
|
64.63%
to 84.89%
|
|
Dividend
per share
|
|
$0.00
|
|
ITEM
8.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
|
Not
applicable.
ITEM
8A. CONTROLS
AND PROCEDURES
We
evaluated the effectiveness of the design and operation of our disclosure
controls and procedures as of the end of the period covered by this Annual
Report on Form 10-K. Disclosure controls and procedures are designed to ensure
that information required to be disclosed in our reports filed under the
Exchange Act, such as this Annual Report on Form 10-K is recorded, processed,
summarized and reported within the time periods specified by the SEC. Disclosure
controls are also designed to ensure that such information is accumulated and
communicated to our management, including the CEO and CFO, as appropriate, to
allow timely decisions regarding required
disclosure.
Based on
the evaluation, our President and Chief Executive Officer, after evaluating the
effectiveness of our “disclosure controls and procedures” has concluded that, ,
as of December 31, 2007, our disclosure controls and procedures were not
effective due to the existence of several material weaknesses in our internal
control over financial reporting, as discussed below.
Material
Weaknesses Identified
In
connection with the preparation of our financial statements for the year ended
December 31, 2007, certain significant deficiencies in internal control became
evident to management that, in the aggregate, represent material weaknesses,
including,
Insufficient
segregation of duties in our finance and accounting functions due to limited
personnel. During the year ended December 31, 2007, the company used
outside services to perform all aspects of our financial reporting
process, including, but not limited to, access to the underlying accounting
records and systems, the ability to post and record journal entries and
responsibility for the preparation of the financial statements. This
creates a lack of review over the financial reporting process that would likely
result in a failure to detect errors in spreadsheets, calculations, or
assumptions used to compile the financial statements and related disclosures as
filed with the SEC. These control deficiencies could result in a material
misstatement to our interim or annual financial statements that would not be
prevented or detected.
Insufficient
corporate governance policies. Although we have a code of ethics which
provides broad guidelines for corporate governance, our corporate governance
activities and processes are not always formally documented. Specifically,
decisions made by the board to be carried out by management should be documented
and communicated on a timely basis to reduce the likelihood of any
misunderstandings regarding key decisions affecting our operations and
management.
Plan
for Remediation of Material Weaknesses
We intend
to take appropriate and reasonable steps to make the necessary improvements to
remediate these deficiencies.
We intend
to consider the results of our remediation efforts and related testing as part
of our year-end 2008 assessment of the effectiveness of our internal control
over financial reporting.
ITEM
8B. OTHER
INFORMATION
Not
applicable.
PART
III
|
ITEM
9.
|
DIRECTORS,
EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS; COMPLIANCE WITH SECTION
16(A) OF THE EXCHANGE ACT
|
As of
April 10, 2008, the Company's current officers and directors consist of the
following persons:
|
Name
|
Age
|
Office
|
Since
|
|
Ross
L. Senior, LLB
|
59
|
Chairman
of the Board, President, CEO and CFO
|
2007
|
|
Mr.
C. Fred Whittaker
|
67
|
Director
|
2005
|
|
Dr.
Maximilien Arella, PhD
|
54
|
Director
|
2007
|
Ross L. Senior,
LLB
Mr.
Senior is our President and Chief Executive Officer. In 2005, Mr. Senior
co-founded Rowan All Natural Skin Care, Inc., a Canadian-based provider of skin
care products. In 1988, Mr. Senior founded Ross L. Senior and Associates, a
business consulting firm, where he maintained his position as principal of the
firm from 1988 to 2005. Mr. Senior brings to ProtoKinetix a combination of
business, organizational and legal experience through consultation roles in
technology research and development institutions and a wide range of businesses
including health care, property development, electronics distribution,
manufacturing, natural resources, educational institutions and social
enterprises.
C. Fred
Whittaker
Mr. C.
Fred Whittaker is one of our directors. Mr. Whittaker has been in the accounting
profession for over 40 years. Mr. Whittaker received his Chartered Accounting
designation in 1967, and has worked for various accounting firms, including
KPMG, as well as for himself at different times in the past. For the last 15
years, he has worked exclusively for Whittaker & Associates, a regional
accounting firm which he founded located in Vancouver, British Columbia.
Currently, Mr. Whittaker is a senior partner at the accounting firm of Whittaker
& Associates and has been for the past 30 years.
Dr. Maximilien Arella,
PhD
Dr.
Arella is one of our Directors. He is not a full time employee and has other
outside commitments. For the past twenty years, Dr. Arella has acted as a
private consultant advising clients and businesses with technological and
scientific development, innovative technology transfer and commercial
development from university bench top to commercial developments.
Since
1993, Dr. Arella has carried out two mandates as chairman of the Virology
Research Center of the Armand-Frappier Institute/University of Quebec (the
“IAF”) during which he held the responsibility of managing both the research and
the teaching programs (M.Sc. and Ph.D.) consisting of a team of 20 researchers
combined with approximately 100 students and support employees. From 1984 to
1993 Dr. Arella was scholar, assistant professor and professor of Virology at
IAF as well as adjunct professor at the School of Graduate Studies of the
University of Montreal. He also served as president of the professor
association from 1989 to 1992. His academic research is mainly based in the
fields of molecular biology, fundamental aspects and applications of the
double-stranded RNA virus, as well as amplification systems for the analysis of
human and animal viruses, and cancer markers. Throughout his career, he has
written 76 scientific publications, 24 scientific reports for research contracts
as well as 28 chapters in books and summaries of techniques. He has been invited
to give 49 conferences, has presented 198 scientific communications and has
submitted 3 patents. Mr. Arella is fluent in English, French and Italian. In
addition to his position with ProtoKinetix, Dr. Arella sits on the scientific
advisory boards of two addition publicly traded companies, Biophage, Inc. and
Viropro, Inc.
Section
16(a) Beneficial Ownership Reporting Compliances
Section
16(a) of the Securities Exchange Act of 1934, as amended (the "Exchange Act"),
requires the Company’s directors, executive officers and holders of more than
10% of the Company’s common stock to file with the Securities and Exchange
Commission initial reports of ownership and reports of changes in ownership of
common stock and other equity securities of the Company. The Company
believes that during the year ended December 31, 2007, its officers, directors
and holders of more than 10% of the Company’s common stock complied with all
Section 16(a) filing requirements.
Code
of Ethics
Effective
March 31, 2006, our board of directors adopted the ProtoKinetix, Inc. Code of
Business Conduct and Ethics. The board of directors believes that our
Code of Business Conduct and Ethics provides standards that are reasonably
designed to deter wrongdoing and to promote the following:
(1)
honest and ethical conduct, including the ethical handling of actual or apparent
conflicts of interest between personal and professional relationships;
(2) full, fair, accurate, timely, and understandable disclosure in
reports and documents that we file with, or submits to, the Securities and
Exchange Commission
; (3) compliance with applicable governmental
laws, rules and regulations;
the prompt internal reporting of
violations of the Code of Business Conduct and Ethics to an appropriate person
or persons; and
(4) accountability for adherence to the Code of
Business Conduct and Ethics.
Identification
of Audit Committee; Audit Committee Financial Expert
The
Company currently does not have an audit committee and has not made a
determination of whether there is a financial expert. The Company
plans to establish an audit committee during the third quarter of the current
fiscal year.
ITEM
10. EXECUTIVE
COMPENSATION
The
following table summarizes the annual compensation paid to ProtoKinetix’s named
executive officers for the two years ended December 31, 2007, and
2006:
|
|
|
Annual
Compensation
|
|
Long-Term
Compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
Shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted
|
|
Underlying
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
Options
|
|
All
|
|
|
|
|
|
|
|
|
|
Other
Annual
|
|
Awards
|
|
Granted
|
|
Other
|
|
Name
and Position
|
|
Year
|
|
Salary
|
|
Bonus
|
|
Compensation
|
|
(#
of Shares)
|
|
(#
Shares)
|
|
Compensation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr.
John Todd
|
|
2007
|
|
$0
|
|
-0-
|
|
-0-
|
|
100,000
|
|
------
|
|
-0-
|
|
Former
President, Chief
|
|
2006
|
|
0
|
|
-0-
|
|
-0-
|
|
-0-
|
|
------
|
|
-0-
|
|
Executive
Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and
Director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mark
L. Baum
|
|
2007
|
|
$0
|
|
-0-
|
|
-0-
|
|
400,000
|
|
------
|
|
-0-
|
|
Former
Interim President
|
|
2006
|
|
0
|
|
-0-
|
|
-0-
|
|
-0-
|
|
------
|
|
-0-
|
|
and
Director
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ross
L. Senior, LLB
|
|
2007
|
|
$0
|
|
-0-
|
|
-0-
|
|
60,000
|
|
------
|
|
-0-
|
|
President,
Chief
|
|
2006
|
|
0
|
|
-0-
|
|
-0-
|
|
-0-
|
|
------
|
|
-0-
|
|
Executive
Officer and
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief
Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mr.
C. Fred Whittaker
|
|
2007
|
|
$0
|
|
-0-
|
|
-0-
|
|
200,000
|
|
------
|
|
-0-
|
|
Director
|
|
2006
|
|
0
|
|
-0-
|
|
-0-
|
|
-0-
|
|
------
|
|
-0-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr.
Maximilien Arella
|
|
2007
|
|
$0
|
|
-0-
|
|
-0-
|
|
200,000
|
|
------
|
|
-0-
|
|
Director
|
|
2006
|
|
0
|
|
-0-
|
|
-0-
|
|
-0-
|
|
------
|
|
-0-
|
Options/SAR
Grants in the Last Fiscal Year
Employment
Agreements
Pursuant
to the terms of his employment agreement with the Company, our Chief Executive
Officer Ross L. Senior receives a quarterly payment of $20,000 payable in shares
of the Company’s restricted Common Stock. The calculation of the number of
shares issued to Mr. Senior for each quarterly payment is based upon the closing
price of the Company’s Common Stock on each payment date.
Chief
Executives Officer’s compensation
During
fiscal year 2007, the only compensation issued to our Chief Executive Officer
Ross L. Senior, LLB was 60,000 shares of the Company’s restricted Common
Stock.
Compensation
of Directors
Directors
receive no remuneration for their services as directors at this
time. The Company has adopted no retirement, pension, profit sharing
or other similar programs.
|
ITEM
11.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS
|
The
following table sets forth certain information regarding the beneficial
ownership of the Company’s Common Stock as of December 31, 2007 based on
information available to the Company by (i) each person who is known by the
Company to own more than 5% of the outstanding Common Stock based upon reports
filed by such persons within the Securities and Exchange Commission; (ii) each
of the Company’s directors; (iii) each of the Named Executive Officers; and (iv)
all officers and directors of the Company as a group.
|
Name
and Address
|
Shares
Beneficially Owned
|
Percent
of Class
|
|
Ross
L. Senior
(1)
|
60,000
|
Less
than 1%
|
|
Mr.
C. Fred Whittaker
(2)
|
320,000
|
Less
than 1%
|
|
Dr.
Maximilien Arella
(3)
|
300,000
|
Less
than 1%
|
|
TOTAL
|
680,000
|
1.3%
|
(1)
The
address is 1500-885 Georgia Street, Vancouver, BC V6C 3E8 Canada
(2)
The
address is 1500-885 Georgia Street, Vancouver, BC V6C 3E8 Canada
(3)
The
address is 1500-885 Georgia Street, Vancouver, BC V6C 3E8 Canada
A person is deemed to be the beneficial
owner of securities that can be acquired by such person within 60 days from the
date of the registration statement upon the exercise of options or warrants.
Each beneficial owner's percentage ownership is determined by assuming that
options or warrants that are held by such person and which are exercisable
within 60 days of the date of this registration statement have been exercised.
Unless otherwise indicated, the company believes that all persons named in the
table have voting and investment power with respect to all shares of common
stock beneficially owned by them.
ITEM
12. CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
ITEM
13. EXHIBITS
AND REPORTS ON FORM 8-K
(a)
Exhibits.
|
Exhibit
#
|
|
Description
|
|
|
|
|
|
3.1(i)
|
|
Certificate
of Incorporation filed as an exhibit to the Company's registration
statement on Form 10-SB/A filed on July 24, 2001 and incorporated herein
by reference.
|
|
|
|
|
|
3.1(ii)
|
|
By-Laws
filed as an exhibit to the Company's registration statement on Form
10-SB/A filed on July 24, 2001 and incorporated herein by
reference.
|
|
|
|
|
|
14.1
|
|
ProtoKinetix,
Inc. Code of Ethics filed as an exhibit to the Company's Form 10-KSB filed
on April 13, 2006 and incorporated herein by reference.
|
|
|
|
|
|
31.1
|
|
Rule
13a-12(a)/15d-14(a) Certification
|
|
|
|
|
|
32.1
|
|
Section
1350 Certification attached.
|
ITEM
14. PRINCIPAL
ACCOUNTANT FEES AND SERVICES
Audit
Fees
For the
years ended December 31, 2007 and December 31, 2006, Peterson Sullivan PLLC, the
Company’s principal accountants, billed the Company $39,700 and $40,000,
respectively, for fees for the audit of the Company’s annual financial
statements and review of financial statements included in the Company’s Forms
10-QSB.
Audit-Related
Fees
For the
years ended December 31, 2007 and December 31, 2006, Peterson Sullivan PLLC did
not provide the Company with any assurances or related services reasonably
related to the performance of the audit or review of the Company’s financial
statements and are not reported above under "Audit Fees."
Tax
Fees
For the
years ended December 31, 2007 and December 31, 2006, Peterson Sullivan PLLC did
not bill for professional services for tax compliance, tax advice, and tax
planning.
All
Other Fees
For the
years ended December 31, 2007 and December 31, 2006, Peterson Sullivan PLLC did
not bill the Company for fees associated with the preparation and filing of the
Company’s registration statements, the creation of pro forma financial
statements and other related matters.
Audit
Committee Pre-Approval Policies
The
Company currently does not have an audit committee. The Company’
Board of Directors currently approves in advance all audit and non-audit related
services performed by the Company’s principal accountants.
Signatures
In
accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the
registrant caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
PROTOKINETIX,
INC.
/s/ Ross
L. Senior
By:
Ross L. Senior, LLP
Its:
Chief Executive Officer and Chief Financial
Officer
|
Pursuant
to the requirements of the Securities Exchange Act of 1934, as amended, this
report has been signed below by the following persons on behalf of the
registrant and in the capacities indicated as of the date of this
report.
|
|
/s/ Ross L.
Senior
By:
Ross L. Senior, LLP
Its:
Chief Executive Officer and Chief Financial Officer
|
|
|
/s/ C. Fred
Whittaker
By:
C. Fred Whittaker
Its:
Director
|
|
|
/s/ Maximilien
Arella
By:
Dr. Maximilien Arella, PhD
Its:
Director
|
Protokinetix (PK) (USOTC:PKTX)
Historical Stock Chart
From Oct 2024 to Nov 2024
Protokinetix (PK) (USOTC:PKTX)
Historical Stock Chart
From Nov 2023 to Nov 2024