UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of July 2023
Commission File Number: 001-38097
ARGENX SE
(Translation of registrant’s name into English)
Laarderhoogtweg 25
1101 EB Amsterdam, the Netherlands
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F x
Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K
in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Indicate by check mark if the registrant is submitting the Form 6-K
in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
argenx SE
On July 17, 2023, argenx SE (the “Company”)
issued a press release, a copy of which is attached hereto as Exhibit 99.1, and an investor presentation, a copy of which is filed hereto
as Exhibit 99.2, each of which is incorporated by reference herein.
The information contained in this Current Report on Form 6-K, including
the Exhibits, is incorporated by reference into the Company’s Registration Statements on F-3 (File No. 333-258251) and S-8
(File Nos. 333-225375 and 333-258253).
EXHIBITS
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
ARGENX SE |
| |
|
| Date: July 17, 2023 |
By: |
/s/ Hemamalini (Malini) Moorthy |
| |
|
Hemamalini (Malini) Moorthy |
| |
|
General Counsel |
Exhibit 99.1
argenx Reports Positive Topline Data from ADHERE
Study of VYVGART Hytrulo in Patients with Chronic Inflammatory Demyelinating Polyneuropathy
- Study met primary
endpoint (p=0.000039); VYVGART® Hytrulo demonstrated 61% reduction (HR: 0.39 95% CI: 0.25; 0.61) in risk of relapse versus
placebo
- IgG autoantibodies
shown to play significant role in underlying CIDP disease biology
- Favorable
safety and tolerability profile consistent with previous clinical trials and confirmed safety profile of VYVGART®
- Conference call
scheduled for today, July 17, 2023 at 8:30am ET (2:30pm CET)
Regulated information – Inside information
Amsterdam, The Netherlands – July 17,
2023 01:00 ET – argenx SE (Euronext & Nasdaq: ARGX), a global immunology company committed to improving the lives
of people suffering from severe autoimmune diseases, today announced positive topline results from the ADHERE study evaluating
VYVGART Hytrulo (efgartigimod alfa and hyaluronidase-qvfc) in adults with chronic inflammatory demyelinating polyneuropathy (CIDP).
The study met its primary endpoint (p=0.000039), demonstrating a significantly lower risk of relapse with VYVGART Hytrulo compared
to placebo. Detailed data from ADHERE will be presented at an upcoming medical meeting.
ADHERE Highlights
| ● | Primary endpoint met (p=0.000039); VYVGART Hytrulo demonstrated 61% reduction (HR: 0.39 95% CI: 0.25;
0.61) in the risk of relapse versus placebo |
| ● | 67% of patients in open-label Stage A demonstrated evidence of clinical improvement (ECI), indicating
that IgG autoantibodies play a significant role in the underlying biology of CIDP |
| ● | Safety and tolerability profile consistent with confirmed safety profile of VYVGART |
| ● | 91% (226/249) of eligible patients continued to the ADHERE-Plus open-label extension study |
"CIDP is a chronic, progressive autoimmune disease that can cause
substantial disability in those affected, often leading to impaired ambulation or difficulty completing normal daily tasks without help.
The positive ADHERE data show that VYVGART Hytrulo may represent a new patient-forward treatment option that can prevent symptom deterioration
while minimizing side effects and treatment burden,” commented Jeffrey Allen, M.D., Associate Professor, Department of Neurology,
University of Minnesota. “With ADHERE, argenx has set a new standard for innovative CIDP studies that more broadly inform the neuromuscular
community. The findings from the trial indicate we may have a novel weapon to combat this debilitating condition in our ongoing efforts
to improve the lives of individuals affected by CIDP.”
“People living with CIDP often experience
significant challenges with daily function including fatigue, numbness, tingling, pain and weakness while facing a future with limited
mobility or independence. The promising ADHERE data bring hope to the CIDP community of a brighter future where they could experience
more positive moments doing the things that make them most happy,” said Lisa Butler, Executive Director of the GBS-CIDP Foundation
International.
“With these positive ADHERE data, we have
generated strong clinical evidence that CIDP has a significant IgG-driven pathogenesis component and that VYVGART Hytrulo can meaningfully
improve and stabilize disease symptoms with a favorable safety profile and a simple route of administration,” commented Luc Truyen,
M.D., Ph.D., Chief Medical Officer of argenx. “We are very grateful to the patients participating in the ADHERE trial and their
supporters, the investigators, our collaborators and our argenx colleagues for the success of this innovative trial. Together, we are
moving one step closer to transforming the treatment of autoimmunity.”
Detailed ADHERE Results
ADHERE is the largest clinical trial of CIDP patients
to date, enrolling adults who were treatment naïve (not on active treatment within the past six months) or currently on immunoglobulin
therapy or corticosteroids. The trial consisted of a run-in period where current treatment was stopped followed by an open-label Stage
A, after which responders to VYVGART Hytrulo advanced to a randomized, placebo-controlled Stage B.
322 patients enrolled in Stage A and received
treatment with VYVGART Hytrulo
| · | 67% (214/322) demonstrated evidence of clinical
improvement (ECI) after a run-in withdrawal period based on the Inflammatory Neuropathy Cause and Treatment (INCAT) Disability Score,
the Inflammatory Rasch-built Overall Disability Scale (I-RODS) or grip strength |
| · | 70% (214/304) demonstrated ECI excluding patients
ongoing in Stage A at the time of the 88th event who did not have the full opportunity to achieve a response |
| · | 78% (214/275) demonstrated ECI in a sensitivity
analysis of patients who received at least four injections to reach the full IgG-lowering effect of VYVGART Hytrulo |
| · | Response rates similar across all prior CIDP
medication subgroups with consistent efficacy on INCAT, I-RODS and grip strength. |
221 responders from Stage A entered Stage B, where
the primary endpoint was the relative risk of relapse based on time to relapse on the INCAT Disability Score
| · | VYVGART Hytrulo significantly reduced the risk
of CIDP relapse compared to placebo |
| o | Primary endpoint was met (p=0.000039); VYVGART Hytrulo demonstrated a 61% reduction (HR: 0.39 95% CI:
0.25; 0.61) in the risk of relapse compared to placebo based on time to the first adjusted INCAT deterioration of ≥1 point |
| o | VYVGART Hytrulo patients had a lower relapse rate compared to placebo at Week 24 (26% versus 54%) and
Week 48 (34% versus 60%) |
| o | VYVGART Hytrulo patients experienced longer time to relapse compared to those on placebo with a rapid
separation of the Kaplan–Meier curves beginning at Week 4 and sustained through Week 48 |
| o | VYVGART Hytrulo patients demonstrated a clinically meaningful mean improvement of 7.7 points on I-RODS
and 12.3kPa on grip strength in Stage A. This clinically meaningful benefit was maintained in Stage B by treated patients and lost in
placebo patients. |
| o | Clinical benefit observed across all efficacy scales and patient subgroups, regardless of prior therapy. |
VYVGART Hytrulo was well-tolerated with a safety
profile that is consistent with prior clinical trials and the known profile of VYVGART. The most frequent treatment-related adverse event
was injection site reactions (ISRs), which occurred in a lower percentage of patients than previous VYVGART Hytrulo trials (20% in Stage
A; 10% in Stage B). All ISRs were mild to moderate and resolved over time.
Conference Call Details
argenx will host a conference call today at 2:30pm CET (8:30am ET) to discuss the ADHERE results. A webcast of the live call and replay may be accessed on the Investors section of the
argenx website.
Dial-in Numbers:
Please dial in 15 minutes prior to the live
call.
| Belgium |
32 800 50 201 |
| France |
33 800 943355 |
| Netherlands |
31 20 795 1090 |
| United Kingdom |
44 800 358 0970 |
| United States |
1 888 415 4250 |
| Japan |
81 3 4578 9081 |
| Switzerland |
41 43 210 11 32 |
About ADHERE Trial Design
The ADHERE trial was a multicenter,
randomized, double-blind, placebo-controlled trial evaluating VYVGART® Hytrulo (efgartigimod alfa and
hyaluronidase-qvfc) for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP). ADHERE enrolled 322 adult
patients with CIDP who were treatment naïve (not on active treatment within the past six months or newly diagnosed) or being
treated with immunoglobulin therapy or corticosteroids. The trial consisted of an open-label Stage A followed by a randomized,
placebo-controlled Stage B. In order to be eligible for the trial, the diagnosis of CIDP was confirmed by an independent panel of
experts. Patients entered a run-in stage, where any ongoing CIDP treatment was stopped and in order to be eligible for Stage A had
to demonstrate active disease, with clinically meaningful worsening on at least one CIDP clinical assessment tool, including
INCAT, I-RODS, or mean grip strength. Treatment naïve patients were able to skip the run-in period with proof of recent
worsening. To advance to Stage B, patients needed to demonstrate evidence of clinical improvement (ECI) with VYVGART Hytrulo. ECI
was achieved through improvement of the INCAT score, or improvement on I-RODS or mean grip strength if those scales had demonstrated
worsening during the run-in period. In Stage B, patients were randomized to either VYVGART Hytrulo or placebo for up to 48 weeks.
The primary endpoint was measured once 88 total relapses or events were achieved in Stage B and was based on the hazard ratio for
the time to first adjusted INCAT deterioration (i.e. relapse). After Stage B, all patients had the option to roll-over to an
open-label extension study to receive VYVGART Hytrulo.
argenx has an exclusive license agreement with
Zai Lab for the development and commercialization of VYVGART and VYVGART Hytrulo in Greater China. Through this agreement, Zai Lab recruited
Chinese patients into the ADHERE trial.
About Chronic Inflammatory Demyelinating Polyneuropathy
Chronic inflammatory demyelinating polyneuropathy
(CIDP) is a rare and serious autoimmune disease of the peripheral nervous system. Although confirmation of disease pathophysiology is
still emerging, there is increasing evidence that IgG antibodies play a key role in the damage to the peripheral nerves. People with CIDP
experience fatigue, muscle weakness and a loss of feeling in their arms and legs that can get worse over time or may come and go. These
symptoms can significantly impair a person's ability to function in their daily lives. Without treatment, one-third of people living with
CIDP will need a wheelchair.
About VYVGART® Hytrulo
VYVGART Hytrulo is a subcutaneous combination
of efgartigimod alfa, a human IgG1 antibody fragment marketed for intravenous use as VYVGART®, and recombinant human hyaluronidase
PH20 (rHuPH20), Halozyme’s ENHANZE® drug delivery technology to facilitate
subcutaneous injection delivery of biologics. In binding to the neonatal Fc receptor (FcRn), VYVGART Hytrulo results in the reduction
of circulating IgG. It is the first-and-only approved FcRn blocker administered by subcutaneous injection.
VYVGART Hytrulo is the proprietary name in the
U.S. for subcutaneous efgartigimod alfa and recombinant human hyaluronidase PH20. It may be marketed under different proprietary names
following approval in other regions.
See Important Safety Information below and full
Prescribing Information for VYVGART Hytrulo for additional information
Important Safety Information
What is VYVGART® HYTRULO (efgartigimod
alfa and hyaluronidase-qvfc)?
VYVGART HYTRULO is a prescription medicine used
to treat a condition called generalized myasthenia gravis, which causes muscles to tire and weaken easily throughout the body, in adults
who are positive for antibodies directed toward a protein called acetylcholine receptor (anti-AChR antibody positive).
IMPORTANT SAFETY INFORMATION
What is the most important information I should
know about VYVGART HYTRULO?
VYVGART HYTRULO may cause serious side effects,
including:
| · | Infection. VYVGART HYTRULO may increase
the risk of infection. The most common infections for efgartigimod alfa-fcab-treated patients were urinary tract and respiratory tract
infections. More patients on efgartigimod alfa-fcab vs placebo had below normal levels for white blood cell counts, lymphocyte counts,
and neutrophil counts. The majority of infections and observed lower white blood cell counts were mild to moderate in severity. Your healthcare
provider should check you for infections before starting treatment, during treatment, and after treatment with VYVGART HYTRULO. Tell your
healthcare provider if you have any history of infections. Tell your healthcare provider right away if you have signs or symptoms of an
infection during treatment with VYVGART HYTRULO such as fever, chills, frequent and/or painful urination, cough, pain and blockage of
nasal passages/sinus, wheezing, shortness of breath, fatigue, sore throat, excess phlegm, nasal discharge, back pain, and/or chest pain.
If a serious infection occurs, your doctor will treat your infection and may even stop your VYVGART HYTRULO treatment until the infection
has resolved. |
| · | Undesirable immune reactions (hypersensitivity
reactions). VYVGART HYTRULO and efgartigimod alfa-fcab can cause the immune system to have undesirable reactions such as rashes, swelling
under the skin, and shortness of breath. Hives were also observed in patients treated with VYVGART HYTRULO. In clinical studies, the reactions
were mild or moderate and occurred within 1 hour to 3 weeks of administration, and the reactions did not lead to VYVGART HYTRULO discontinuation.
Your healthcare provider should monitor you during and after treatment and discontinue VYVGART HYTRULO if needed. Tell your healthcare
provider immediately about any undesirable reactions to VYVGART HYTRULO. |
Before taking VYVGART HYTRULO, tell your healthcare
provider about all of your medical conditions, including if you:
| · | Have a history of infection or you think you
have an infection. |
| · | Have received or are scheduled to receive a vaccine
(immunization). Discuss with your healthcare provider whether you need to receive age-appropriate immunizations before initiation of a
new treatment cycle with VYVGART HYTRULO. The use of vaccines during VYVGART HYTRULO treatment has not been studied, and the safety with
live or live-attenuated vaccines is unknown. Administration of live or live-attenuated vaccines is not recommended during treatment with
VYVGART HYTRULO. |
| · | Are pregnant or plan to become pregnant and are
breastfeeding or plan to breastfeed. |
Tell your healthcare provider about all the medicines
you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the common side effects of VYVGART
HYTRULO?
The most common side effects of efgartigimod alfa-fcab-treated
patients were respiratory tract infection, headache, and urinary tract infection. Additional common side effects of VYVGART HYTRULO are
injection site reactions, including rash, redness of the skin, itching sensation, bruising, pain, and hives.
These are not all the possible side effects of
VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to the US Food and Drug Administration
at 1-800-FDA-1088.
Please see the full Prescribing Information
for VYVGART HYTRULO and talk to your doctor.
About argenx
argenx is a global immunology company committed
to improving the lives of people suffering from severe autoimmune diseases. Partnering with leading academic researchers through its Immunology
Innovation Program (IIP), argenx aims to translate immunology breakthroughs into a world-class portfolio of novel antibody-based medicines.
argenx developed and is commercializing the first approved neonatal Fc receptor (FcRn) blocker in the U.S., Japan, Israel, the EU,
the UK and China. The Company is evaluating efgartigimod in multiple serious autoimmune diseases and advancing several earlier stage experimental
medicines within its therapeutic franchises. For more information, visit www.argenx.com and follow
us on LinkedIn, Twitter, and Instagram.
For further information, please contact:
Media:
Erin Murphy
emurphy@argenx.com
Investors:
Alexandra Roy (US)
ARoy@argenx.com
Lynn Elton (EU)
LElton@argenx.com
Forward-looking Statements
The contents of this announcement include statements
that are, or may be deemed to be, “forward-looking statements.” These forward-looking statements can be identified by the
use of forward-looking terminology, including the terms “may,” “will,” or “should” and include those
that argenx makes concerning the benefits and safety profile of VYVGART and VYVGART Hytrulo; the expected availability of VYVGART Hytrulo;
the safety profile and efficacy signals from the ADHERE study; and the prospects of VYVGART Hytrulo as a treatment for chronic inflammatory
demyelinating polyneuropathy (“CIDP”), including its ability to transform CIDP treatment for patients and the therapeutic
potential and patient treatment experience of VYVGART Hytrulo for the treatment of CIDP. By their nature, forward-looking statements involve
risks and uncertainties and readers are cautioned that any such forward-looking statements are not guarantees of future performance. argenx’s
actual results may differ materially from those predicted by the forward-looking statements as a result of various important factors.
A further list and description of these risks, uncertainties and other risks can be found in argenx’s U.S. Securities and Exchange
Commission (“SEC”) filings and reports, including in argenx’s most recent annual report on Form 20-F filed with
the SEC as well as subsequent filings and reports filed by argenx with the SEC. Given these uncertainties, the reader is advised not to
place any undue reliance on such forward-looking statements. These forward-looking statements speak only as of the date of publication
of this press release. argenx undertakes no obligation to publicly update or revise the information in this press release, including any
forward-looking statements, except as may be required by law.
Exhibit 99.2

ADHERE Study T opline Results July 17 , 2023

Forward Looking Statements This presentation has been prepared by argenx se (“argenx” or the “company”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or the company or any director, employee, agent, or adviser of the company. This presentation does not purport to be all - inclusive or to contain all of the information you may desire. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third - party sources and the company’s own internal estimates and research. While argenx believes these third - party studies, publications, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, no independent source has evaluated the reasonableness or accuracy of argenx’s internal estimates or research and no reliance should be made on any information or statements made in this presentation rela tin g to or based on such internal estimates and research. Certain statements contained in this presentation, other than present and historical facts and conditions independently verif iab le at the date hereof, may constitute forward - looking statements. Examples of such forward - looking statements include those argenx makes concerning the benefits and safety pr ofile of VYVGART and VYVGART Hytrulo ; the expected availability of VYVGART Hytrulo; the safety profile and efficacy signals from the ADHERE study; and the prospects of VYVGART Hytrulo as a treatment for chronic inflammatory demyelinating polyneuropathy (“CIDP”), including its ability to transform CIDP treatment for patients and the th era peutic potential and patient treatment experience of VYVGART Hytrulo for the treatment of CIDP . By their nature, forward - looking statements involve risks and uncertainties and readers are cautioned that any such forward - looking statements are not guarantees of future performance. A further list and description of these risks, uncertaintie s and other risks can be found in argenx’s U.S. Securities and Exchange Commission (“SEC”) filings and reports, including in argenx’s most recent annual report on Form 20 - F filed with the SEC as well as subsequent filings and reports filed by argenx with the SEC. Given these uncertainties, the reader is advised not to place any undue reliance on suc h f orward - looking statements. These forward - looking statements speak only as of the date of publication of this presentation. argenx undertakes no obligation to publicly update or revise the information in this press release, including any forward - looking statements, except as may be required by law. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their resp ect ive owners. 2
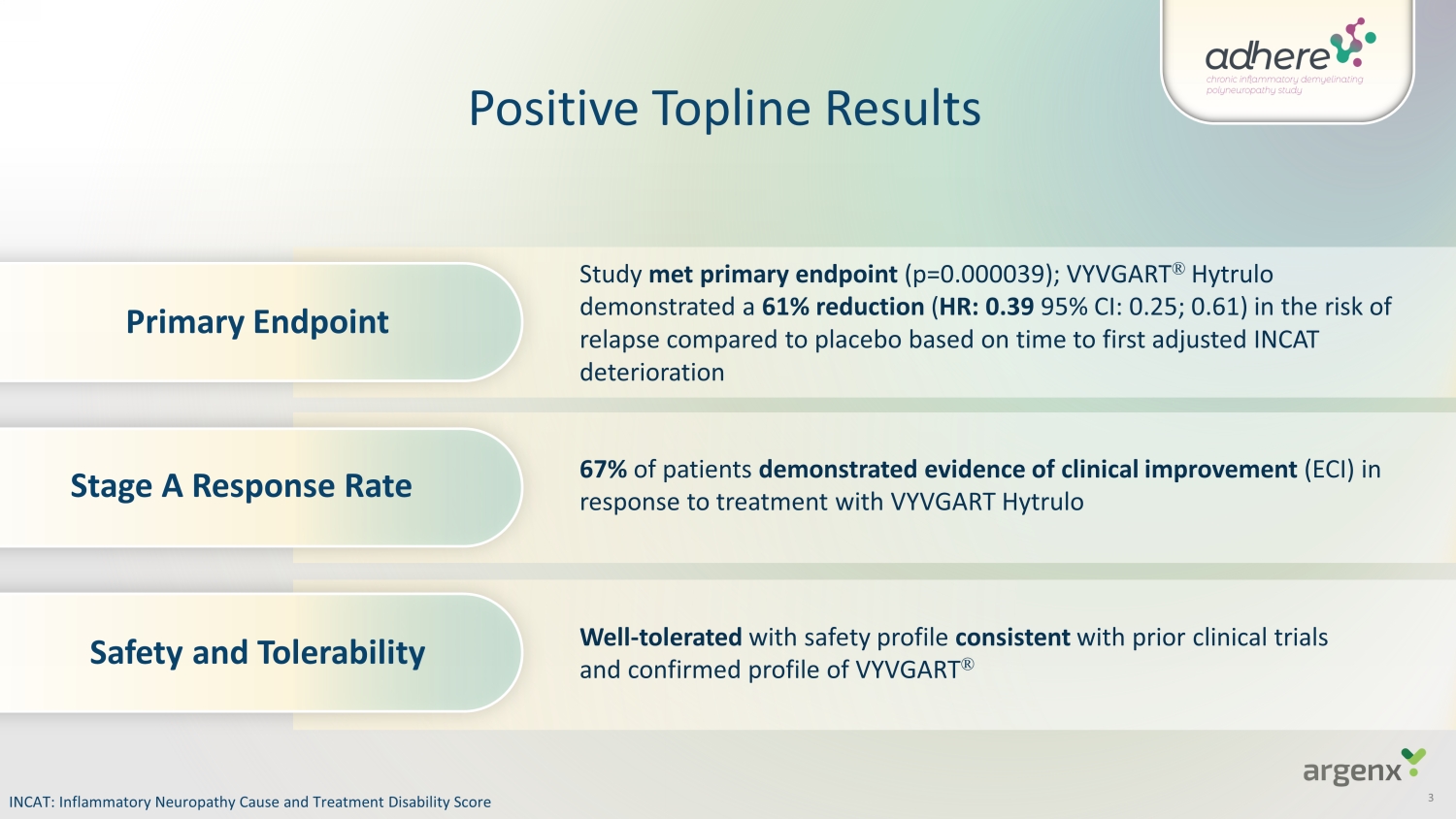
Stage A Response Rate Primary Endpoint Safety and Tolerability Study met primary endpoint (p=0.000039); VYVGART ® Hytrulo demonstrated a 61% reduction ( HR: 0.39 95% CI: 0.25; 0.61) in the risk of relapse compared to placebo based on time to first adjusted INCAT deterioration 67% of patients demonstrated evidence of clinical improvement (ECI) in response to treatment with VYVGART Hytrulo Well - tolerated with safety profile consist e nt with prior clinical trials and confirmed profile of VYVGART ® Positive Topline Results INCAT: Inflammatory Neuropathy Cause and Treatment Disability Score 3

4 Our mission is to transform severe autoimmunity Raising expectations for what ‘well - controlled’ means for patients Redefining autoimmune diseases as IgG - mediated

Bringing Hope to Patients of New Treatment Option A Historic Day for CIDP Patients I was the type of woman that would run first thing in the morning before work, and then CIDP hit, and it was like hitting the wall at a hundred miles an hour. Crystal Living With CIDP Largest Global CIDP Trial New standard set for innovative trial design Unlocking New Disease Biology Insights IgG shown to play significant role in underlying biology of CIDP Potential new treatment modality First Innovation in 30+ Years 5
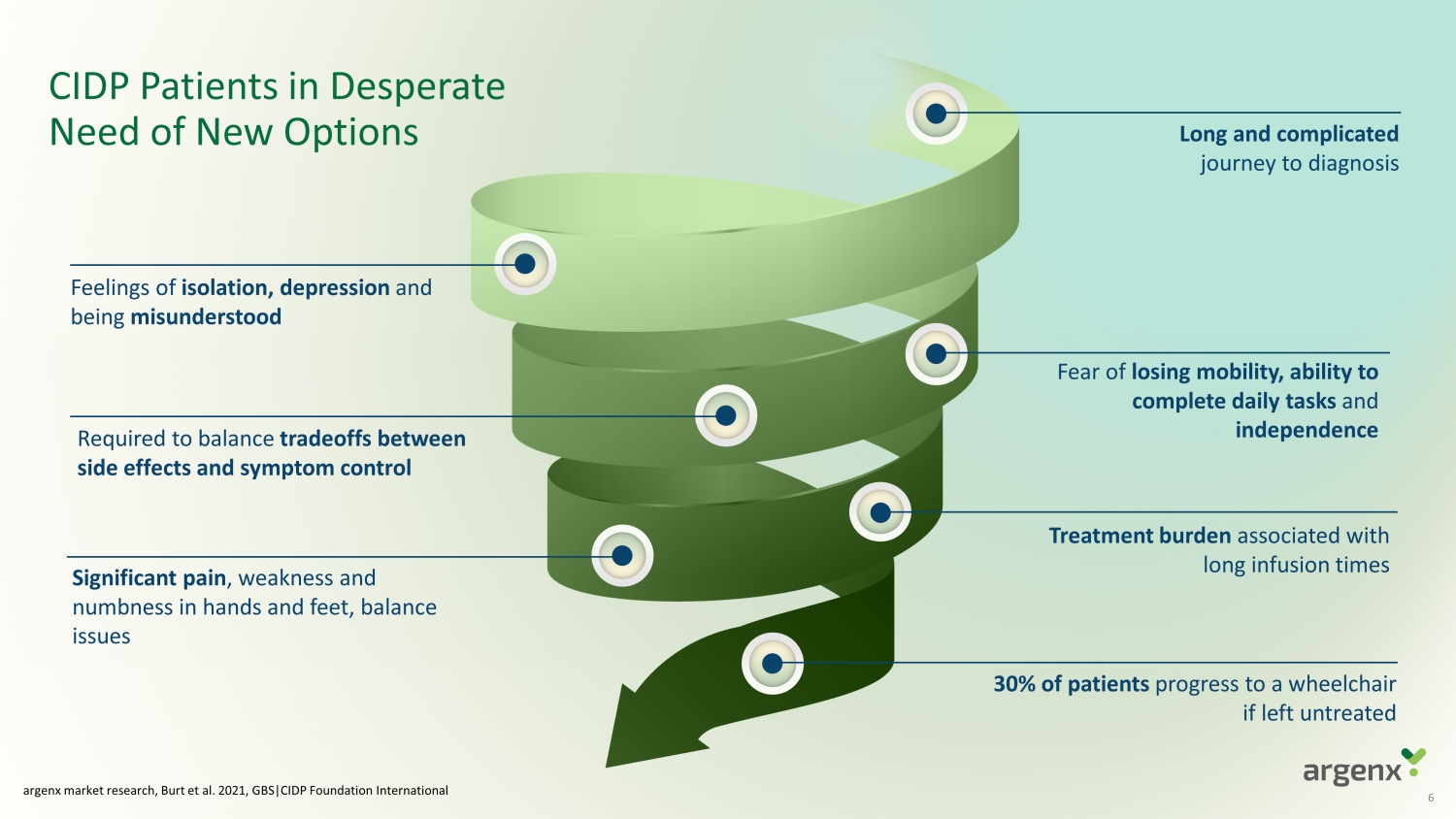
CIDP Patients in Desperate Need of New Options argenx market research, Burt et al. 2021, GBS |CIDP Foundation International 30% of patients progress to a wheelchair if left untreated Feelings of i solation , depression and being misunderstood Required to balance tradeoffs between side effects and symptom control Fear o f losing mobility , ability to complete daily tasks and independence Significant pain , weakness and numbness in hands and feet, balance issues Long and complicated journey to diagnosis Treatment burden associated with long infusion times 6
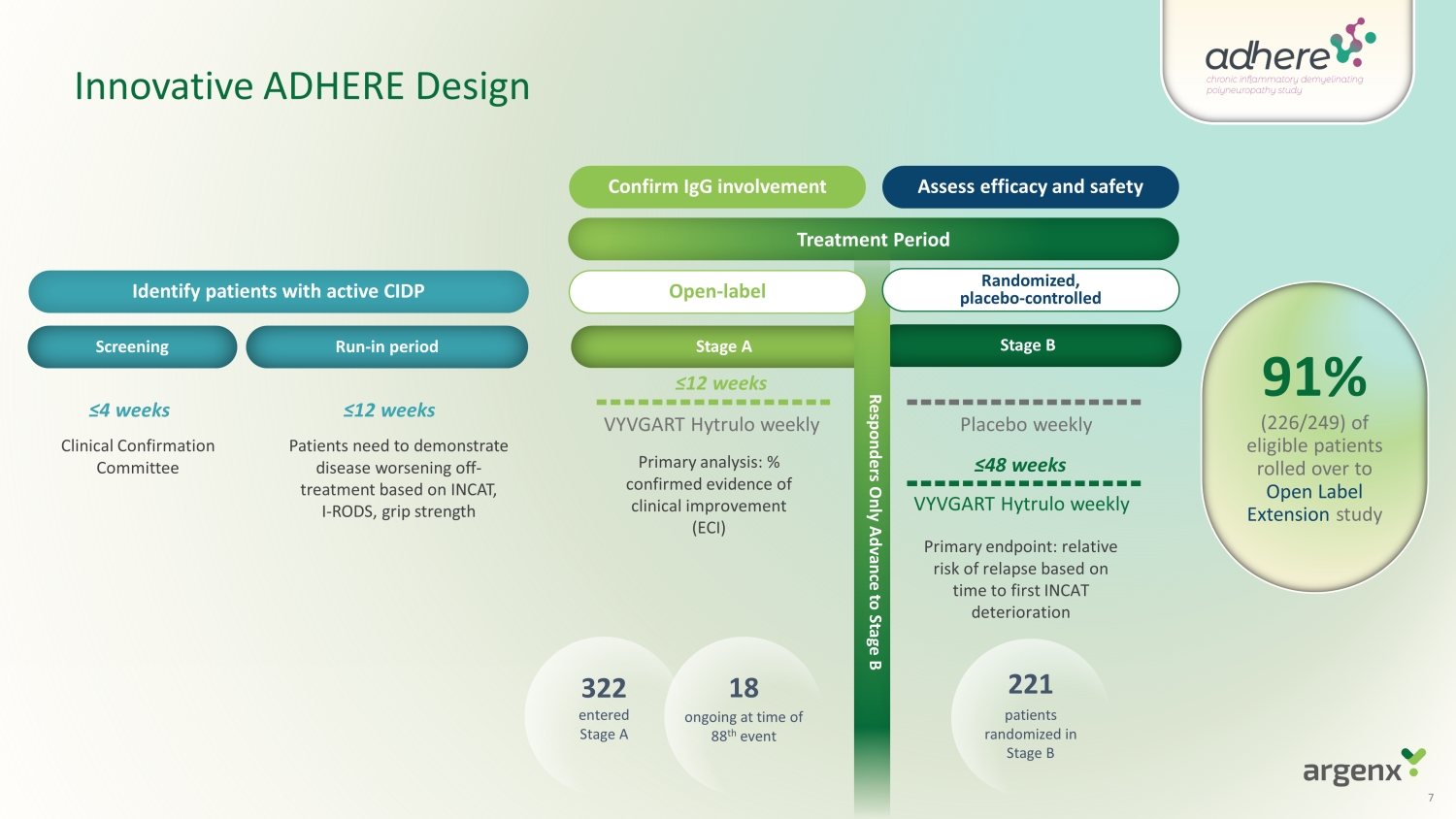
Innovative ADHERE Design Patients need to demonstrate disease worsening off - treatment based on INCAT, I - RODS, grip strength Clinical Confirmation Committee Treatment Period Confirm IgG involvement Assess e fficacy and safety Run - in period Stage A Stage B Screening ≤ 12 weeks ≤ 4 weeks VYVGART Hytrulo w e e k l y Placebo w e e k l y VYVGART Hytrulo weekly ≤12 weeks ≤48 weeks Responders Only Advance to Stage B Primary analysis: % confirmed evidence of clinical improvement (ECI) Primary endpoint: relative risk of relapse based on time to first INCAT deterioration 91 % (226/249) of eligible patients rolled over to Open Label Extension study 322 entered Stage A 18 ongoing at time of 88 th event 221 patients randomized in Stage B Open - label Randomized, placebo - controlled Ide n t i f y patients with active CIDP 7
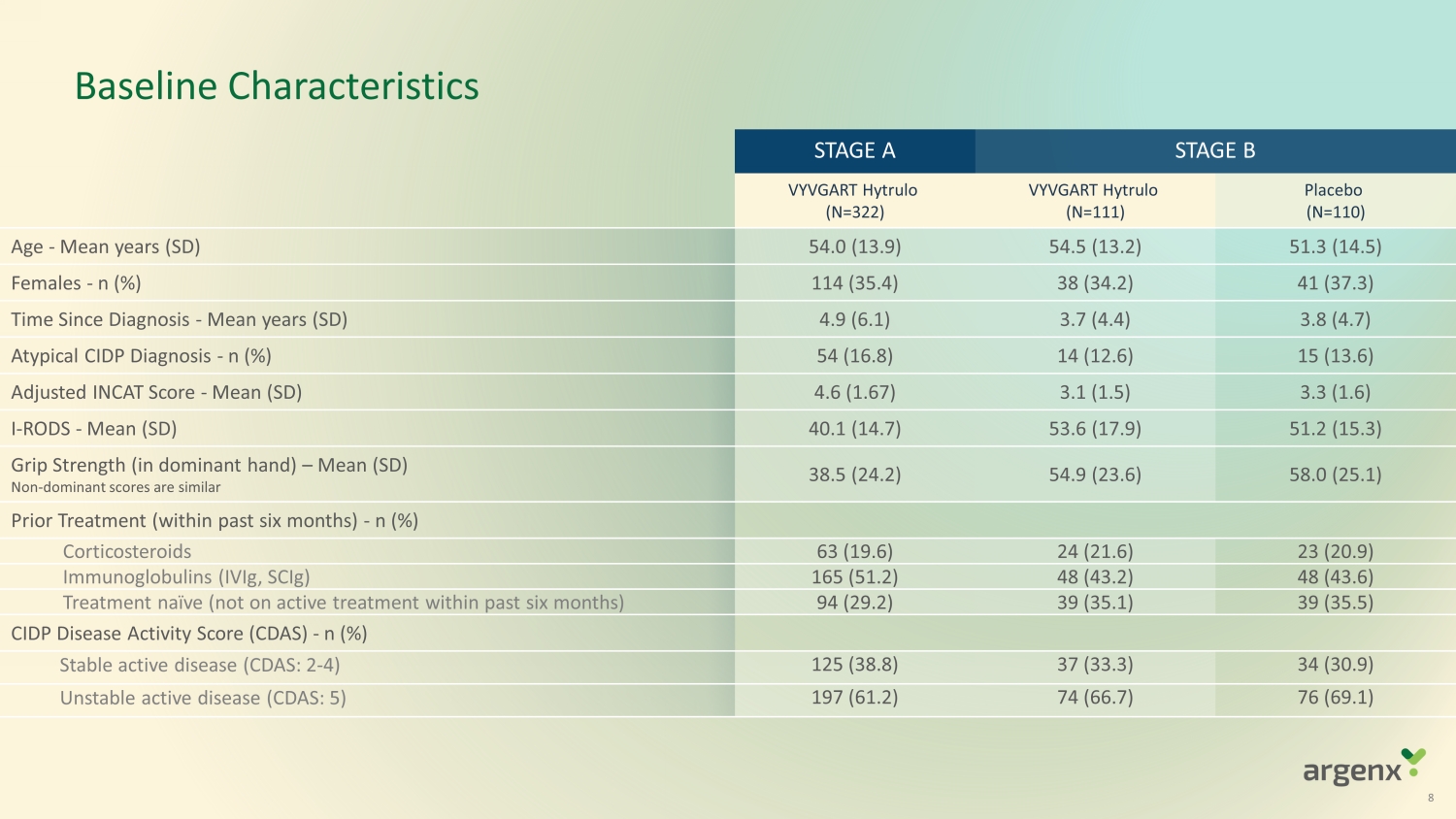
Baseline Characteristics STAGE A STAGE B VYVGART Hytrulo (N=322) VYVGART Hytrulo (N=111) Placebo (N=110) Age - Mean years (SD) 54.0 (13.9) 54.5 (13. 2 ) 51.3 (14. 5 ) Females - n (%) 114 (35.4) 38 (34.2) 41 (37.3) Time Since Diagnosis - Mean years (SD) 4.9 (6. 1 ) 3.7 (4.4) 3.8 (4. 7 ) Atypical CIDP Diagnosis - n (%) 54 (16.8) 14 (12.6) 15 (13.6) Adjusted INCAT Score - Mean (SD) 4.6 (1.67) 3.1 (1.5) 3.3 (1. 6 ) I - RODS - Mean (SD) 40.1 (14. 7 ) 53.6 (17.9) 51.2 (15.3) Grip Strength (in dominant hand) – Mean (SD) Non - dominant scores are similar 38.5 (24. 2 ) 54.9 (23.6) 58.0 (25. 1 ) Prior Treatment (within past six months) - n (%) Corticosteroids 63 (19.6) 24 (21.6) 23 (20.9) Immunoglobulins (IVIg, SCIg) 165 (51.2) 48 (43.2) 48 (43.6) Treatment naïve (not on active treatment within past six months) 94 (29.2) 39 (35.1) 39 (35.5) CIDP Disease Activity Score (CDAS) - n (%) Stable active disease (CDAS: 2 - 4) 125 (38.8) 37 (33.3) 34 (30.9) Unstable active disease (CDAS: 5) 197 (61.2) 74 (66.7) 76 (69.1) 8
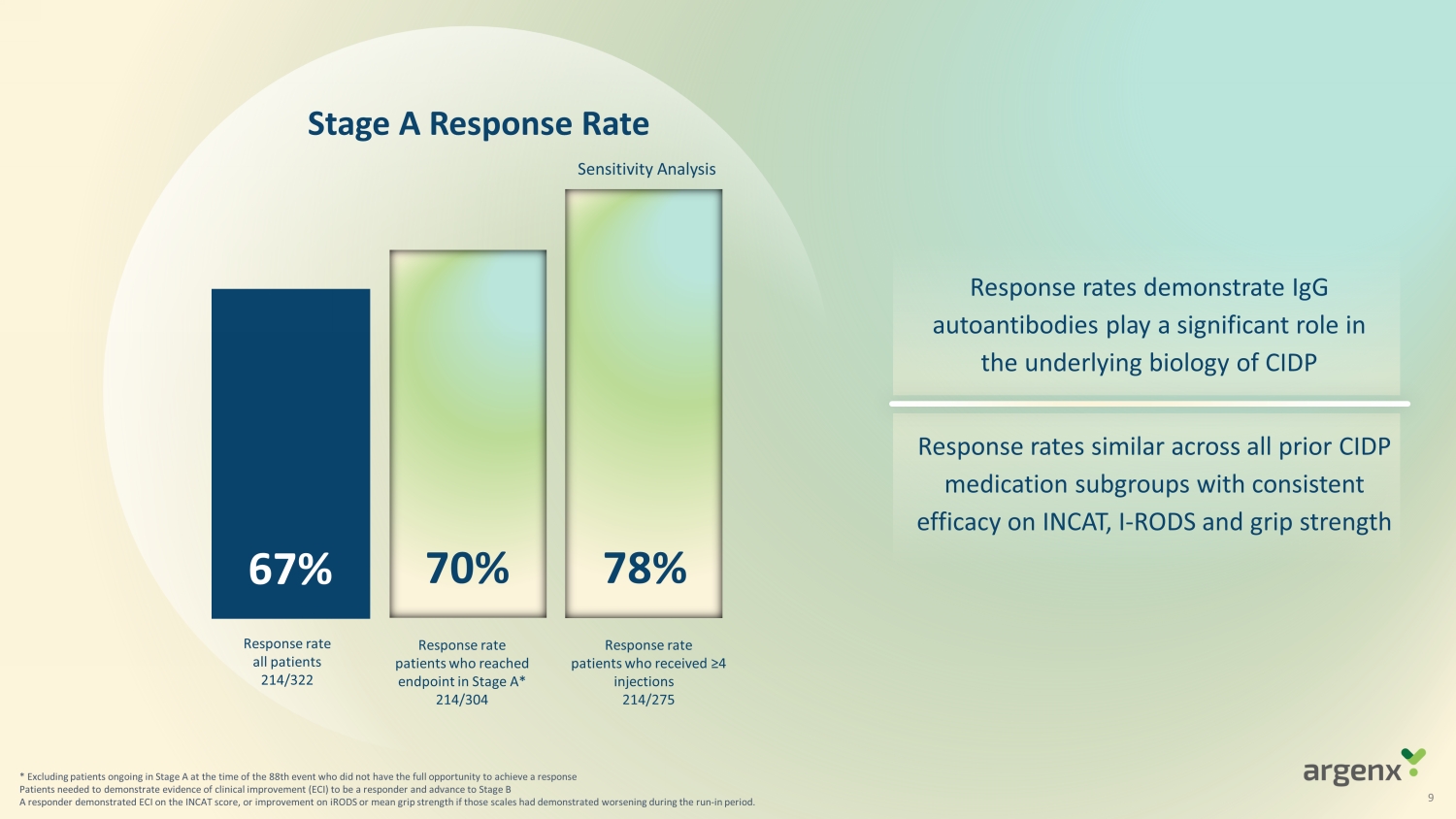
* Excluding patients ongoing in Stage A at the time of the 88th event who did not have the full opportunity to achieve a resp ons e Patients needed to demonstrate evidence of clinical improvement (ECI) to be a responder and advance to Stage B A responder demonstrated ECI on the INCAT score, or improvement on iRODS or mean grip strength if those scales had demonstrat ed worsening during the run - in period. S t age A Response Rate 67% 70% R e sponse rate patients who reached endpoint in S tage A * 214/304 Response rate all patients 214 /322 Response rate patients who received ≥4 injections 214/275 S ensitivity Analysis 7 8 % Response rates similar across all prior CIDP medication subgroups with consistent efficacy on INCAT, I - RODS and grip strength Response rates demonstrate IgG autoantibodies play a significant role in the underlying biology of CIDP 9
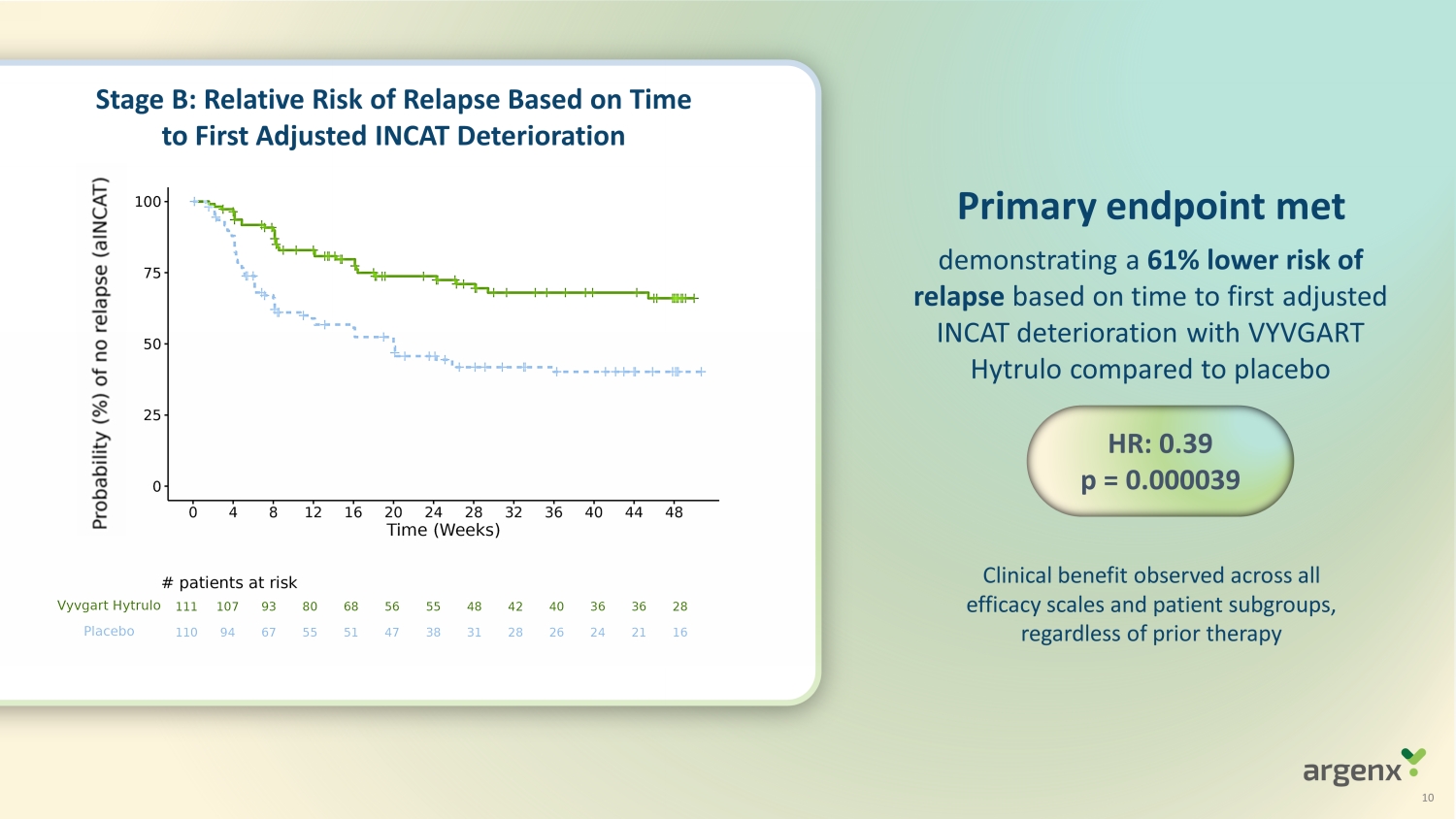
Clinical benefit observed across all efficacy scales and patient subgroups, regardless of prior therapy P rimary endpoint met HR: 0.39 p = 0.000039 S t age B: Relative Risk of Relapse Based on Time to First Adjusted INCAT Deterioration demonstrat ing a 61% lower risk of relapse based on time to first adjusted INCAT deterioration with VYVGART Hytrulo compared to placebo 10
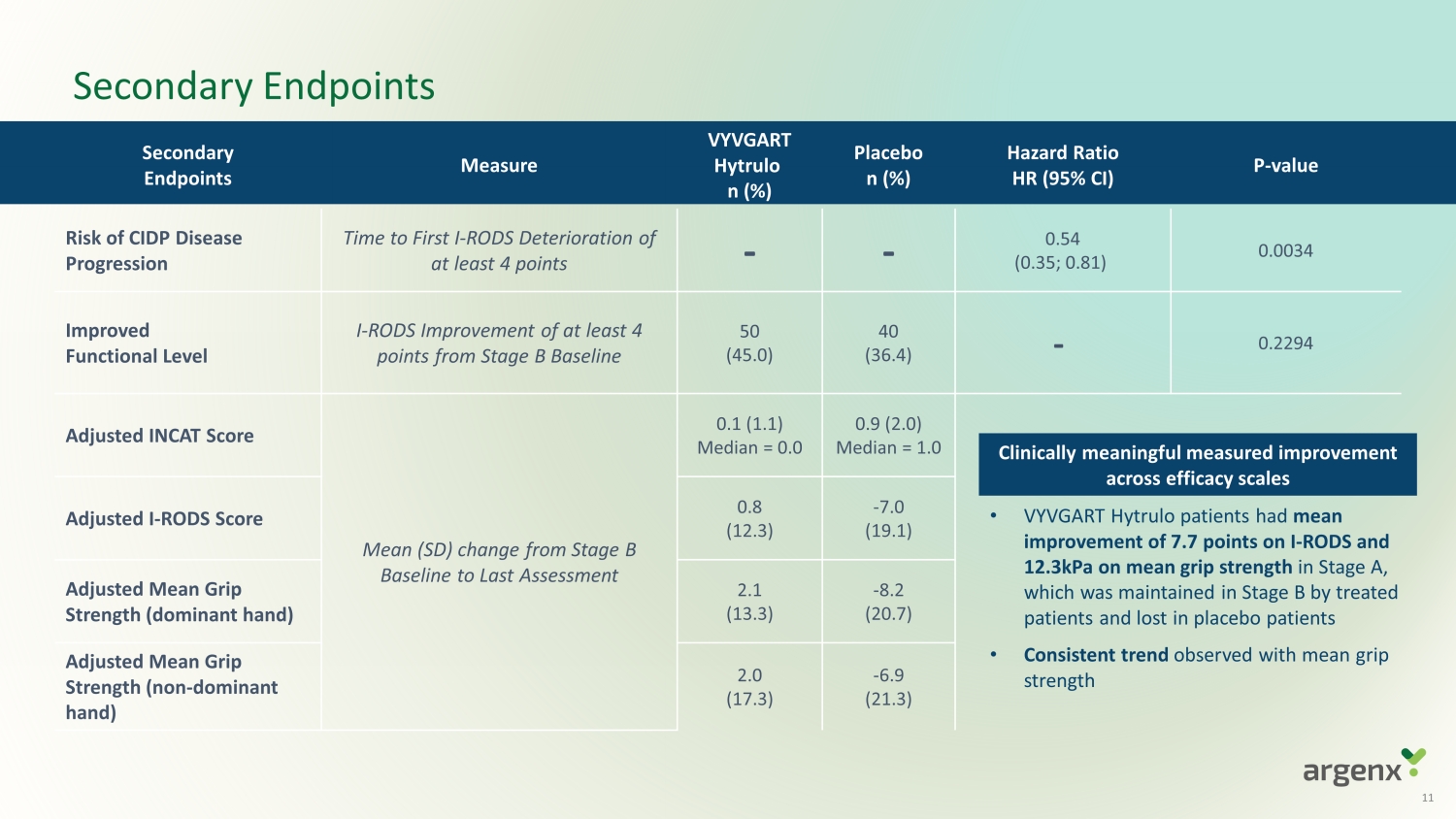
Secondary Endpoints Measure VYVGART Hytrulo n (%) Placebo n (%) Hazard Ratio HR (95% CI) P - value Risk of CIDP Disease Progression Time to First I - RODS Deterioration of at least 4 points - - 0.5 4 (0.35; 0.81) 0.0034 Improved Functional Level I - RODS Improvement of at least 4 points from Stage B Baseline 50 (45.0) 40 (36.4) - 0.2294 Adjusted INCAT Score Mean (SD) change from Stage B Baseline to Last Assessment 0.1 ( 1. 1 ) Median = 0.0 0.9 ( 2.0 ) Median = 1.0 Adjusted I - RODS Score 0.8 ( 12.3 ) - 7.0 (19.1) Adjusted Mean Grip Strength (dominant hand) 2.1 ( 13. 3 ) - 8.2 (20. 7 ) Adjusted Mean Grip Strength (non - dominant hand) 2.0 ( 17.3 ) - 6.9 (21.3) Secondary Endpoints Clinically meaningful measured improvement across efficacy scales • VYVGART Hytrulo patients had mean improvement of 7.7 points on I - RODS and 12.3kPa on mean grip strength in Stage A, which was maintained in Stage B by treated patients and lost in placebo patients • Consistent trend observed with mean grip strength 11
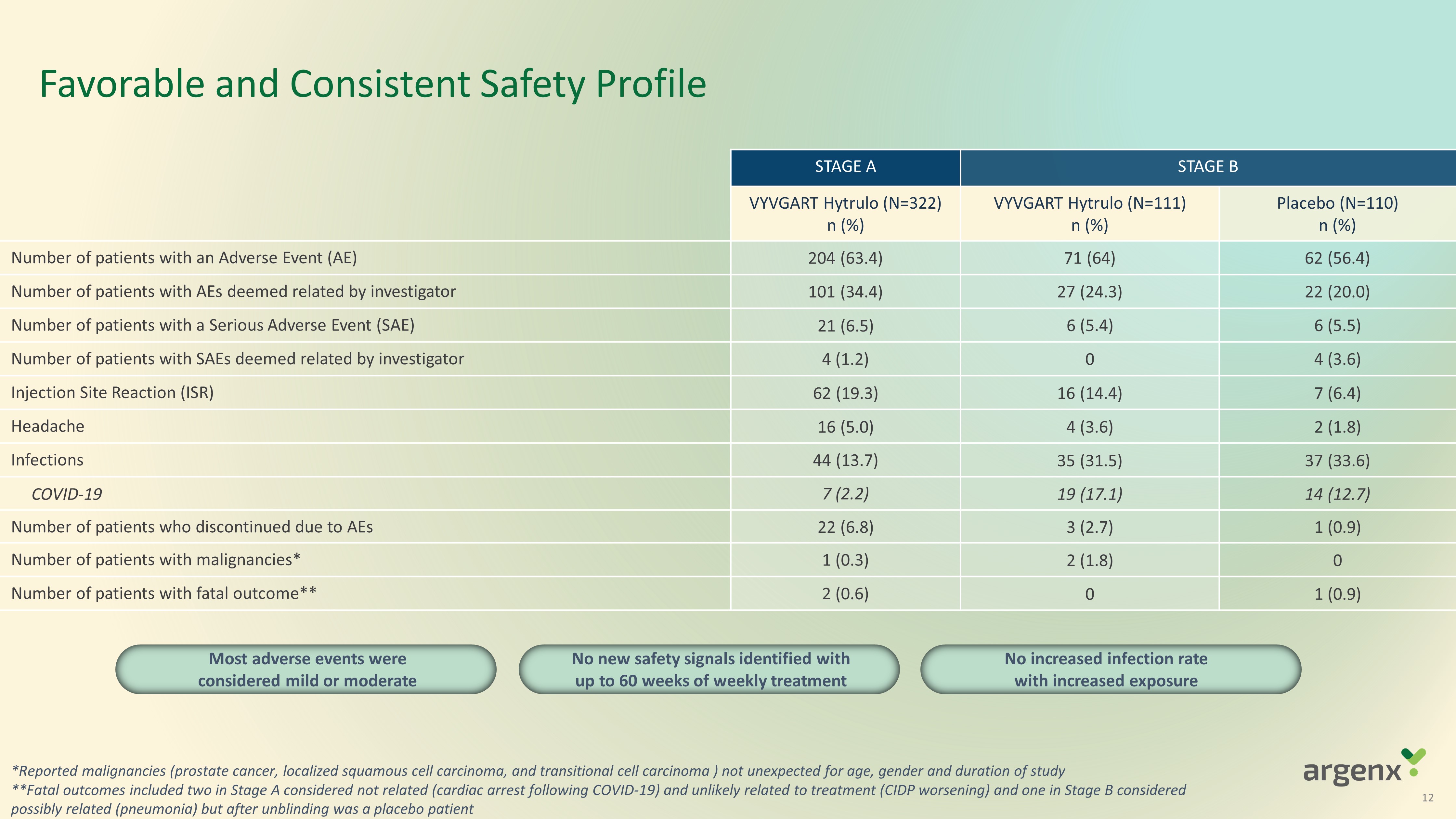
Favorable and Consistent Safety Profile 12 STAGE A STAGE B VYVGART Hytrulo (N= 322 ) n (%) VYVGART Hytrulo (N= 111 ) n (%) Placebo (N= 110 ) n (%) Number of patients with an Adverse Event (AE) 204 (63.4) 71 (64) 62 (56.4) Number of patients with AEs deemed related by investigator 101 (34.4) 27 (24.3) 22 (20.0) Number of patients with a Serious Adverse Event (SAE) 21 (6.5) 6 (5.4) 6 (5.5) Number of patients with S AEs deemed related by investigator 4 (1.2) 0 4 (3.6) Injection Site Reaction (ISR) 62 (19.3) 16 (14.4) 7 (6.4) Headache 16 (5.0) 4 (3.6) 2 (1.8) Infections 44 (13.7) 35 (31.5) 37 (33.6) COVID - 19 7 (2.2) 19 (17.1) 14 (12.7) Number of patients who discontinued due to AEs 22 (6.8) 3 (2.7) 1 (0.9) Number of patients with malignancies * 1 (0.3) 2 (1.8) 0 Number of patients with fatal outcome ** 2 (0.6) 0 1 (0.9) Most adverse events were considered mild or moderate * Reported malignancies ( prostate cancer, localized squamous cell carcinoma, and transitional cell carcinoma ) not unexpected for age, gender and duration of study **F atal outcomes included two in Stage A considered not related (cardiac arrest following COVID - 19) and unlikely related to treatment (CIDP worsening) and one in Stage B considered possibly related (pneumonia) but after unblinding was a placebo patient N o new safety signals identified with up to 60 weeks of weekly treatment No increased infection rate with increased exposure
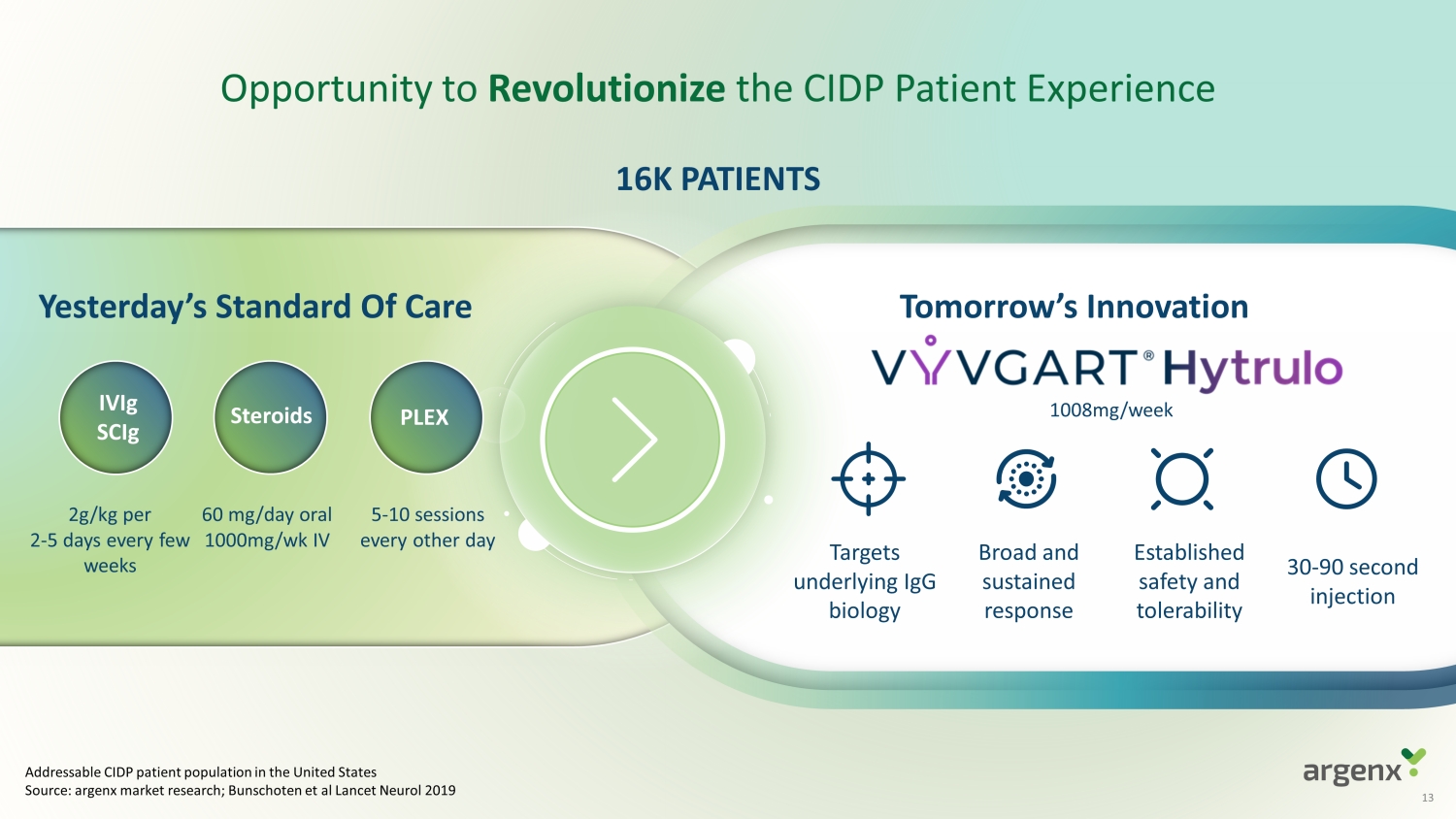
Opportunity to Revolutionize the CIDP Patient Experience Tomorrow’s Innovation Broad and sustained response Established safety and tolerability 30 - 90 sec ond injection Yesterday’s Standard Of Care 16K PATIENTS Steroids IVIg SCIg PLEX 2g/kg per 2 - 5 days every few weeks 60 mg/day oral 1000mg/ wk IV 5 - 10 sessions every other day Addressable CIDP patient population in the United States Source: argenx market research; Bunschoten et al Lancet Neurol 2019 1008mg/week Targets underlying IgG biology 13

Building Leadership Within Neuromuscular 14
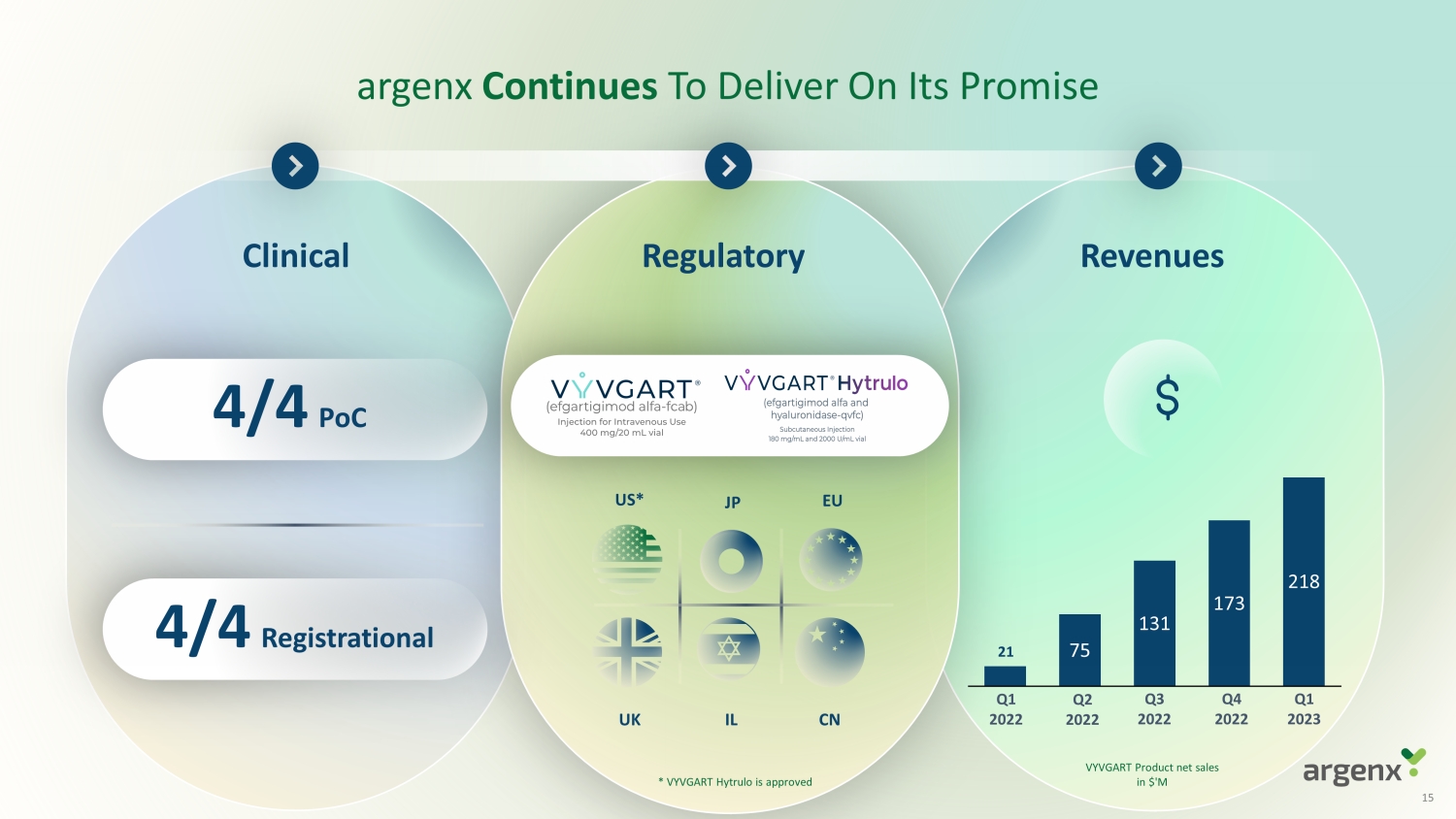
argenx Continues To Deliver On Its Promise 75 131 173 218 21 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 US* Regulatory Clinical R evenues EU JP UK IL CN * * VYVGART Hytrulo is approved 4/4 PoC 4/4 Registrational VYVGART Product net sales in $'M 15

16 Our mission continues… Humility Innovation Excellence Co - Creation Empowerment
argenx (NASDAQ:ARGX)
Historical Stock Chart
From Apr 2024 to May 2024
argenx (NASDAQ:ARGX)
Historical Stock Chart
From May 2023 to May 2024