Filed pursuant to
Rule 424(b)(3)
Registration
No. 333-276662

21,366,700 Shares of Common Stock
This
prospectus relates to the resale of up to 21,366,700 shares of common stock of One World Products, Inc., a Nevada corporation, which
may be resold by Tysadco Partners, LLC (which we refer to as Tysadco or the selling stockholder), consisting of up to 20,000,000 shares
of common stock issuable pursuant to an equity financing facility established by the terms of the Purchase Agreement described in this
prospectus, and 1,366,700 shares of common stock issuable to Tysadco upon the conversion into common stock of 13,667 shares of Series
B Preferred Stock we issued to Tysadco as a commitment fee for entering into the Purchase Agreement with us. We may draw on the equity
financing facility from time to time, as and when we determine appropriate in accordance with the terms and conditions of the Purchase
Agreement, by delivering “Request Notices” to Tysadco.
We
are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of the shares of our common
stock by the selling stockholder. We will, however, receive proceeds from the sale of common stock directly to Tysadco pursuant to the
Purchase Agreement. When we put shares of our common stock to Tysadco, the per-share purchase price that Tysadco will pay to us in respect
of the put will be equal to 88% of the of the lowest daily volume weighted average price of our common stock during the
period of 10 trading days beginning five trading days preceding the day we deliver the applicable put notice to Tysadco.
Tysadco
is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act of 1933. Tysadco may sell the shares of
common stock described in this prospectus at fixed prices, at prevailing market prices at the time of sale or at prices negotiated with
purchasers, to or through one or more underwriters, dealers or agents, or through any other means described in this prospectus under
“Plan of Distribution”.
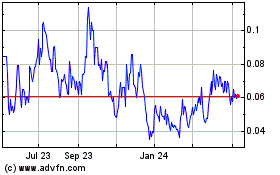
Our
common stock is quoted on the OTCQB tier of the OTC Markets under the symbol “OWPC”. On February 28, 2024, our common
stock closed at $0.0485 per share.
These
are speculative securities. Investing in these securities involves significant risks. You should purchase these securities only if you
can afford a complete loss of your investment. You should carefully consider the risk factors beginning on page 4 of this prospectus
before purchasing any of the shares offered by this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Prospectus
dated February 29, 2024.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
You
should rely only on the information contained in or incorporated by reference in this prospectus. We have not authorized any person to
provide you with different or inconsistent information. If anyone provides you with different or inconsistent information, you should
not rely on it. This is not an offer to sell or seeking an offer to buy these securities in any jurisdiction where the offer or sale
is not permitted. You should assume that the information appearing in this prospectus and the documents incorporated by reference is
accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed since
such dates.
We
further note that the representations, warranties and covenants made by us in any document that is filed as an exhibit to the registration
statement of which this prospectus is a part and in any document that is incorporated by reference herein were made solely for the benefit
of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements,
and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants
were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately
representing the current state of our affairs.
Unless
the context otherwise requires, the terms “One World Products”, the “Company”, “we”, “us”,
“our” and similar terms used in this prospectus refer to One World Products, Inc. and its subsidiaries, including One World
Pharma SAS, a Colombian company.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference in this prospectus “forward-looking statements” about our business,
financial condition and prospects based on our current expectations, assumptions, estimates, and projections about us and our industry.
All statements other than statements of historical fact are “forward-looking statements”, including, but not limited to,
any projections of earnings, revenue or other financial items; any statements of the plans, strategies and objections of management for
future operations; any statements concerning proposed new services or developments; any statements regarding future economic conditions
or performance; any statements or belief; and any statements of assumptions underlying any of the foregoing.
Forward-looking
statements may include the words “may,” “could,” “estimate,” “intend,” “continue,”
“believe,” “expect” or “anticipate” or other similar words. These forward-looking statements present
our estimates and assumptions only as of the date of this report. Unless otherwise required by law, we do not intend, and undertake no
obligation, to update any forward-looking statement.
Although
we believe that the expectations reflected in any of our forward-looking statements are reasonable, actual results could differ materially
from those projected or assumed in any of our forward-looking statements. Our future financial condition and results of operations, as
well as any forward-looking statements, are subject to change and inherent risks and uncertainties.
You
should read the matters described in “Risk Factors” below and disclosed in the documents incorporated by reference in this
prospectus and the other cautionary statements made in this prospectus and in the documents incorporated by reference in this prospectus
as being applicable to all related forward-looking statements wherever they appear in this prospectus and in the documents incorporated
by reference in this prospectus. We cannot assure you that the forward-looking statements in this prospectus and in the documents incorporated
by reference in this prospectus will prove to be accurate and therefore prospective investors are encouraged not to place undue reliance
on forward-looking statements.
PROSPECTUS
SUMMARY
This
summary highlights certain information described in greater detail elsewhere or incorporated by reference in this prospectus. Before
deciding to invest in our securities you should read the entire prospectus carefully, including the “Risk Factors” section
contained in this prospectus, and our consolidated financial statements and the related notes, “Management’s Discussion and
Analysis of Financial Condition and Results of Operations” and the other documents incorporated by reference into this prospectus.
Company
Overview
One
World Products, Inc., a Nevada corporation (the “Company,” “we” or “us”) plans
to be a producer of raw cannabis and hemp plant ingredients for both medical and industrial uses across the globe. The Company is
a holding company and conducts its business in Colombia through One World Products Pharma SAS, its wholly-owned subsidiary (“OWP-Colombia”)
The Company, through OWP Colombia, has received licenses from Colombian regulators to cultivate, produce and distribute the
raw ingredients of the cannabis and hemp plant for medicinal, scientific and industrial purposes in the town of Esmeralda-Popayan,
Cauca, Colombia.
We
are in the process of acquiring another Colombian subsidiary within the Bogota free trade zone, which has all requisite
licenses for the cultivation, production, distribution and export of cannabis and hemp infused products, and will serve as the
Company’s primary base of operations in the Colombian market. Establishing operations within the free trade zone provides
favorable import/export commercial terms and taxation, and will improve the logistics and overall operating efficiencies for the
Company due to the close proximity of El Dorado International Airport and the commercial, economic and cultural center of the city
of Bogota itself.
We
planted our first crop of cannabis in Popayan, Colombia in 2018, and began initial harvesting in the first quarter of 2019 for the purpose
of further research and development activities and quality control testing of the cannabis produced. We commenced limited shipping
of non-psychoactive products to customers in May of 2020.
Our
first cultivation site is located in Popayan, Colombia, encompasses approximately 30 acres and includes a covered greenhouse
built specifically to cultivate high-grade cannabis and hemp. In addition, we entered into an agreement with a local farming
co-operative, under which they will cultivate cannabis on up to approximately
140 acres of land using our seeds and propagation techniques, and sell their harvested products to us on an exclusive basis.
We
employ modern propagation and cultivation techniques drawn from U.S. practices that allow us to rapidly multiply the cells of a specific
plant strain to produce large numbers of genetically consistent progeny plants using our own plant tissue culture method. We believe
this technique allows us to cultivate plants which are stable, robust and able to produce genetically superior cannabis and hemp derived
products. We intend to have our processes and products certified as compliant with international standards, including Good Agricultural
Practices (“GAP”), Good Manufacturing Practice (“GMP”) and the standards set forth in EU Pharmacopoeia, a publication
that sets forth quality standards applicable to the European pharmaceutical industry.
We intend to build out
an extraction and production facility in the Bogota free trade zone extension adjacent to the El Dorado international airport in Bogota
after we execute a lease for this location. Once the extraction equipment is placed in service,
we will be one of the few companies in Colombia to both hold licenses and possess the capability to extract high-quality CBD and THC
oils.
For
strategic and operational reasons, OWP-Colombia filed for protection under Colombian Law 1116 of 2006, which is the primary legislation
governing business insolvency proceedings (restructuring and liquidation) (“Reorganization Proceedings”) in Colombia on December
22, 2023. Subject to court approval, OWP-Colombia intends to continue its normal operations, which consists of providing cannabinoids
in bulk for the domestic and international markets, including the raw material for our brands and affiliate companies. In addition,
OWP-Colombia intends to satisfy its financial obligations to creditors of approximately $1.2 million during the Reorganization Proceedings.
At this time, the Company cannot predict the length of time of the Reorganization Proceeding.
We
expect to start exporting products in 2024, including CBD flower and distillate oil, while developing white label commercial agreements
with partners in Europe, USA, and Latin America. Our product pipeline also includes premium coffee certified by the Colombian National
Coffee Federation infused with CBD, teas infused with CBD and a series of wellness products, including sports CBD energy drinks for optimum
performance, CBD facial and body creams for anti-inflammatory and anti-aging use. We recently entered into strategic partnerships with
Smokiez Edibles and Stephen Marley’s Kx Family Care. There can be no assurances that these strategic partnerships will generate
revenues or be profitable for the Company.
Isiah L. Thomas III was
appointed to serve as our Chief Executive Officer on June 3, 2020. Mr. Thomas was a 12-time NBA All Star, two-time NBA champion,
and is an accomplished international business executive. In 2021, through ISIAH International, LLC, of which he is the sole member, Mr.
Thomas purchased $3,000,000 of our Series B Preferred Stock in installments over a period of time ending in July 2021.
Our principal offices are
located at 6605 Grand Montecito Pkwy, Suite 100, Las Vegas, Nevada 89149. Our telephone number is (800) 605-3210.
We maintain a website at www.oneworldproducts.com. Information contained on our website does not constitute part of this prospectus.
Tysadco
Purchase Agreement
This
prospectus relates to the resale of shares of our common stock that Tysadco has committed to purchase from us following our delivery
to Tysadco of “Request Notices” from time to time under the terms of a Purchase Agreement we entered into on September 1,
2022. Pursuant to the Purchase Agreement, subject to the effectiveness of the registration statement that includes this prospectus and
our compliance with other terms set forth therein, Tysadco has committed to purchase up to $10,000,000 of our common stock upon our delivery
of Request Notices, at a price equal to 88% of the lowest daily volume weighted average
price of our common stock during the period of 10 trading days beginning five trading days preceding
the applicable Request Notice. Each purchase under the Purchase Agreement will
be in a minimum amount of $25,000 and a maximum amount equal to the lesser of (i) $1,000,000 and (ii) 500% of the average daily trading
value of our common stock over the seven trading days preceding the delivery of the applicable Request Notice. Tysadco’s
commitment to purchase common stock under the Purchase Agreement will terminate approximately 36 months after we have satisfied all of
the conditions to effecting sales of common stock under the Purchase Agreement, which conditions include obtaining the effectiveness
of the registration statement that includes this prospectus. The Purchase Agreement also provides that Tysadco is not required to purchase
common stock to the extent that following such purchase, Tysadco would beneficially own in excess of 4.99% of our outstanding shares
of common stock.
In
connection with the Purchase Agreement, we issued to Tysadco as a commitment fee 13,667 shares of our Series B Preferred Stock convertible
into 1,366,667 shares of common stock. The resale of the shares we may issue to Tysadco upon conversion of the Series B Preferred Stock
issued to Tysadco under the Purchase Agreement is also covered by this prospectus.
The
Offering
The
following summary contains basic information about the offering and the securities being registered hereunder and is not intended to
be complete. It does not contain all the information that is important to you. For a more complete understanding of the securities we
are offering, please refer to the sections of this prospectus titled “Description of Capital Stock.”
| Securities
Being Registered: |
|
21,366,700
Shares of common stock. |
| |
|
|
| Shares
of Common Stock Outstanding Before the Offering: |
|
79,827,618 |
| |
|
|
| Shares
of Common Stock Outstanding After the Offering: |
|
101,194,318 |
| |
|
|
| Use
of Proceeds: |
|
The
shares offered by this prospectus will be sold by the selling stockholder. We will not receive any proceeds from the sale of shares
by the selling stockholder. However, we will receive proceeds from the sale of shares of our common stock to Tysadco under the Purchase
Agreement. These proceeds would be used for general working capital purposes. |
| |
|
|
| Risk
Factors: |
|
An
investment in our securities involves a high degree of risk and could result in the loss of your entire investment. Prior to making
an investment decision, you should carefully consider all of the information in this prospectus and, in particular, you should evaluate
the risk factors set forth under the caption “Risk Factors” beginning on page 4 of this prospectus. |
| |
|
|
| OTCQB
Trading Symbol: |
|
OWPC |
RISK
FACTORS
Investment
in our securities involves a high degree of risk. You should carefully consider the risks described below, as well as the other information
in this prospectus. Each of the risks could adversely affect our business, financial condition, results of operations and prospects,
and could result in a complete loss of your investment. This prospectus also contains forward-looking statements that involve risks and
uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain
factors, including the risks mentioned above.
Risks
Relating to our Company
Limited
Operating History
We
are an early stage company that has generated minimal revenues and we have a limited operating history upon which our business and future
prospects may be evaluated. We are subject to all of the business risks and uncertainties associated with any new business enterprise
in the cannabis industry, including the risk that we will not achieve our operating goals. In order for us to meet future operating
requirements, we will need to successfully grow, harvest and sell our cannabis products. Until such time as we are able to fund our business
from operations, we will be required to raise funds through various sources, including the sale of equity and debt securities, Failure
to generate cash from operations and to reach profitability may adversely affect our success.
We
have had a history of losses, we expect losses in the future, and there can be no assurance that we will become profitable in the future.
We have experienced operating losses on an ongoing
basis. For the nine months ended September 30, 2023, and for fiscal years ended December 31, 2022
and 2021, we incurred net losses of $1,513,374, $3,059,477 and $3,784,562, respectively. As of such dates, we had accumulated
deficits of $24,489,739, $22,976,365 and $19,916,888, respectively. We expect our losses to continue for the foreseeable future.
These continuing losses may be greater than current levels. If our revenues do not increase substantially or if our expenses exceed our
expectations, we may never become profitable. Even if we do achieve profitability, we may not sustain profitability on a quarterly or
annual basis in the future.
Our
auditor has given us a “going concern” qualification, which questions our ability to continue as a going concern without
additional financing.
Our
independent certified public accountant has added an emphasis paragraph to its report on our financial statements for the year ended
December 31, 2022 regarding our ability to continue as a going concern. Key to this determination is our recurring net losses,
an accumulated deficit, and a working capital deficiency. In the event sales do not materialize at expected levels, management
would seek additional financing or would conserve cash by further reducing expenses. No assurance
can be given that any future financing will be available or, if available, that it will be on terms that are satisfactory to us. Even
if we are able to obtain additional financing, it may contain undue restrictions on our operations or cause substantial dilution for
our stockholders. If we are unable to obtain additional funds, our ability to carry out and implement our planned business objectives
and strategies will be significantly delayed, limited or may not occur. We cannot guarantee that we will become profitable.
Our
Wholly-owned Colombian Subsidiary, OWP-Colombia, is Operating under Court Supervision Pursuant to a Reorganization Proceeding
OWP-Colombia
experienced significant operational and managerial challenges over the past several years, resulting in the accumulation of financial obligations
of approximately $1.2 million, which are substantially past due. OWP-Colombia filed for protection under Colombian Law 1116 of 2006,
which is the primary legislation governing business insolvency proceedings (restructuring and liquidation) (“Reorganization Proceedings”)
in Colombia on December 22, 2023. There are many risks attendant to the Reorganization Proceeding, including OWP-Colombia’s
ability to obtain approval from the court to conduct its normal business operations, maintain its cannabis licenses, satisfy its financial
obligations to its creditors, the availability of operating capital during the pendency of its Restructuring Proceeding, the length of
time that the Company will operate in the Reorganization Proceedings and the possibility that it may be unable to obtain any additional
funding. Failure to achieve any of these objectives could have a material adverse effect on the business of the Company and OWP-Colombia.
Change
of Cannabis Laws, Regulations and Guidelines
Cannabis
laws and regulations in Colombia and other jurisdictions where we intend to transact business are dynamic and subject to evolving
interpretations which could require us to incur substantial costs associated with compliance or alter certain aspects of our business
plan. Regulations may be enacted in the future that will be directly applicable to certain aspects of our cultivation, manufacturing
and exporting businesses for our cannabis and hemp related products. We cannot predict the nature of any future laws, regulations,
interpretations or applications, nor can we determine what effect additional governmental regulations or administrative policies and
procedures, when and if promulgated, could have on our business. Management expects that the legislative and regulatory environment in
the cannabis industry in Colombia and internationally will continue to be dynamic and will require innovative solutions to comply
with this changing legal landscape in this nascent industry for the foreseeable future. Compliance with any such legislation may have
a material adverse effect on our business, financial condition and results of operations.
Public
opinion can also exert a significant influence over the regulation of the cannabis industry. A negative shift in the public’s perception
of the cannabis industry could affect future legislation or regulation in different jurisdictions.
Reliance
on Colombian Licenses, Authorizations and Quotas
Our
ability to import seeds, grow, manufacture, distribute and sell cannabis and hemp in Colombia or internationally is dependent
on our ability to sustain and/or obtain the necessary licenses and authorizations by certain authorities in Colombia and/or the importing
jurisdiction. The licenses and authorizations are subject to ongoing compliance and reporting requirements and our ability to obtain,
sustain or renew any such licenses and authorizations on acceptable terms is subject to changes in regulations and policies and to the
discretion of the applicable authorities or other governmental agencies in foreign jurisdictions. Failure to comply with the requirements
of the licenses or authorizations or any failure to maintain the licenses or authorizations would have a material adverse impact on our
business, financial condition and operating results. In addition, Colombian regulators limit the cultivation and sale of psychoactive
cannabis by quotas issued on an annual basis to licensed producers.
Although
we believe that we will meet the requirements to obtain, sustain or renew the necessary licenses and authorizations, there can be no
guarantee that the applicable authorities will issue these licenses or authorizations. In addition, to date we have not been issued quotas
from Colombian regulatory authorities that would allow us to commence the commercial sale of psychoactive cannabis products in
Colombia. Should the authorities fail to issue the necessary licenses or authorizations, including required quotas, we may
be curtailed or prohibited from the production and/or distribution of cannabis and hemp or from proceeding with the development of our
operations as currently proposed and our business, financial condition and results of the operation may be materially adversely affected.
Regulatory
Compliance Risks
Achievement
of our business objectives of becoming a producer of raw cannabis and hemp related products is contingent, in part, upon compliance
with regulatory requirements enacted by applicable governmental authorities and obtaining all regulatory approvals, where necessary,
for the sale of our products in Colombia and other jurisdictions where we intend to distribute and sell our products. We will incur ongoing
costs and obligations related to regulatory compliance. Failure to comply with applicable laws, regulations and permitting requirements
may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease
or be curtailed, and may include corrective measures requiring capital expenditures, installation of additional equipment, or remedial
actions. Civil or criminal fines or penalties may be imposed on us for violations of applicable laws or regulations. Vigorous enforcement
of these laws could require extensive changes to our operations, increase our compliance costs or give rise to material liabilities,
which could have a material adverse effect on our business, results of operations and financial condition.
Competition
There
are many companies engaged in the cannabis business who we will compete with, including larger and more established companies with substantially
greater marketing, financial, human and other resources than we have. These companies include PharmaCielo, CannaVida, Empresa Colombiana
de Cannabis, Khiron Life Sciences Corp., MedCan, Canopy Growth Corporation, and Clever Leaves. Although we believe we are competitively
positioned to be a leader in the medicinal cannabis industry given our early entry into the market, the management team’s expertise
in medical product branding, marketing, quality control, and market relationships, competition in the medical cannabis industry is growing
quickly. As more competitors enter the market, prices may be reduced. We believe our approach in creating brand loyalty will allow us
to effectively compete in the market but there is no assurance that will be the case, and our competitors may adopt a similar or identical
approach. To date, we have obtained four licenses in Colombia that authorize us to engage in cannabis activities, and there are currently
few authorized Colombian producers. However, Colombia offers an open process to apply for licenses and there are no significant
barriers to entry. As a result, our ability to generate revenues and earnings may be reduced as competition intensifies, thereby causing
a material adverse effect on our business and financial condition.
Ability
to Establish and Maintain Bank Accounts
Many
banking institutions in countries where we or our prospective customers operate will not accept payments related to the cannabis industry
due to domestic laws and regulations or pressure exerted by the United States on banks with laws subject to the laws of the United
States (including, the Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001
(USA PATRIOT Act)). Failure to conduct our business through normal banking channels may impede our ability to make payments for goods
and services and transact business in the ordinary course. Failure to operate in normal banking channels may also increase our cost of
doing business and adversely impact our business. In the event financial service providers do not accept accounts or transactions
related to the cannabis industry, it is possible that we may be required to seek alternative payment solutions. If the industry was to
move toward alternative payment solutions, we would have to adopt policies and protocols to manage the Company’s exposure
to foreign exchange and interest rate risks. Our inability to effectively manage such risks may adversely affect our operations
and financial performance.
Anti-money
Laundering Laws and Regulations
We
are subject to a variety of laws and regulations within Colombia and internationally that are designed to prevent money laundering
and proceeds of crime through strict financial recordkeeping. In the event that any of our investments, or any proceeds thereof,
any dividends or distributions therefrom, or any profits or revenues accruing from such investments are found to be in violation of money
laundering legislation or otherwise, such transactions may be viewed as proceeds of crime under applicable legislation. Money laundering
laws could restrict or otherwise jeopardize our ability to declare or pay dividends, effect other distributions or subsequently cause
the repatriation of such funds to the United States or to any shareholders’ jurisdiction of residence. Furthermore, while we have
no current intention to declare or pay dividends on our common stock in the foreseeable future, in the event that a determination was
made that the revenues from our cannabis operations could reasonably be shown to constitute proceeds of crime, we may decide or be required
to suspend the declaration, and or, payment of dividends without advance notice and for an extended or indefinite period
of time.
Foreign
Trade Policies
Our
international operations are subject to inherent risks, including changes in the regulations governing the flow of cannabis products
between countries, fluctuations in cross-currency rates, discriminatory fiscal policies, unexpected changes in local regulations
and laws and the uncertainty of enforcement of remedies in foreign jurisdictions. In addition, foreign jurisdictions could impose tariffs,
quotas, trade barriers and other similar restrictions on our international sales and subsidize competing cannabis products. All of these
risks could result in increased costs or a reduction in revenues.
United
States Regulation
Although
we do not believe that our limited U.S. activity will subject us to regulation under U.S. federal or state laws applicable to the sale
of cannabis and marijuana products, we cannot assure you that current or future U.S. laws and regulations will not detrimentally
affect our business. Local, state and federal cannabis laws and regulations in the United States are constantly changing and they are
subject to evolving interpretations, which could require us to incur substantial costs associated with compliance or to alter one or
more of our product or service offerings. In addition, violations of these laws, or allegations of such violations, could disrupt our
business and result in a material adverse effect on our revenues, profitability, and financial condition. We cannot predict the nature
of any future laws, regulations, interpretations or applications, nor can we determine what effect additional governmental regulations
or administrative policies and procedures, when and if promulgated, could have on our business.
Liability,
Enforcement, Complaints, etc.
Our
participation in the cannabis and hemp industries may lead to litigation, formal or informal complaints, enforcement actions, and inquiries
by third parties, other companies and/or various governmental authorities against us. Litigation, complaints, and enforcement actions
involving us could consume considerable amounts of financial and other corporate resources, which could have an adverse effect on our
future cash flows, earnings, results of operations and financial condition.
Legal
Proceedings
From
time to time, we may be a party to legal and regulatory proceedings, including matters involving governmental agencies, entities with
whom we transact business and other proceedings arising in the ordinary course of business. We will evaluate our exposure to these
legal and regulatory proceedings and establish reserves for the estimated liabilities in accordance with generally accepted accounting
principles. Assessing and predicting the outcome of these matters involves substantial uncertainties. Unexpected outcomes in these legal
proceedings, or changes in management’s evaluations or predictions and accompanying changes in established reserves, could have
an adverse impact on our financial results.
Environmental
Regulations
We
are subject to Colombian environmental laws governing the use of natural resources, which prohibit such use that causes harm to the interests
of the community or of third parties. Parties that cause environmental damage while acting under the authority of a permit and, or
license, are responsible for incurring the costs to rectify the damage. The imposition of environmental sanctions in addition
to civil and criminal penalties may be imposed. Environmental damage caused while a party is acting without a license may lead to
the imposition of sanctions, in addition to civil or criminal proceedings. Parties that cause environmental damage, in addition to sanctions
or penalties that apply, are also required to carry out studies to assess the characteristics of the damage. Colombian environmental
authorities may investigate potential claims, authorize preventative measures, or impose sanctions on parties breaching environmental
law. Any such measures imposed on us could have a material adverse effect on our business.
Demand
for Cannabis and Derivate Products
The
global sale of cannabis and hemp products is a new industry as a result of recent legal and regulatory changes. Although we expect demand
for licensed cannabis to exceed supply produced by licensed producers, there is a risk that such demand does not develop as anticipated.
Further, there is a risk that the adoption rate by pharmacies to sell medical cannabis is lower than expected or that such adoption rate
may take longer than anticipated. There is also a risk that the international export market for medicinal cannabis and extracts, such
as CBD, CBG and CBC, will not materialize as projected or not be commercially viable. Should any of such events materialize, the result
may have a material adverse effect on our business, operations and financial condition.
Weather,
Climate Change and Risks Inherent in an Agricultural Business
Our
business involves growing cannabis, which is an agricultural product. Although our medical cannabis is intended to be grown in greenhouses,
hemp used as feedstock for medicinal extracts and derivatives will be grown both outdoors and in greenhouses. Further, our prospective
Colombian medicinal cannabis operations will initially focus on outdoor production. The occurrence of severe adverse weather conditions,
especially droughts, hail, floods or frost, is unpredictable and may have a potentially devastating impact on agricultural production
and may otherwise adversely affect the supply of cannabis and hemp. Adverse weather conditions may be exacerbated by the effects of climate
change and may result in the introduction and increased frequency of pests and diseases. The effects of severe adverse weather conditions
may reduce our yields or require us to increase our level of investment to maintain yields. Additionally, higher than average temperatures
and rainfall can contribute to an increased presence of insects and pests, which could negatively affect cannabis crops. Future droughts
could reduce the yield and quality of our cannabis production, which could materially and adversely affect our business, financial condition
and results of operations.
The
occurrence and effects of plant disease, insects and pests can be unpredictable and devastating to agriculture, potentially rendering
all or a substantial portion of the affected harvests unsuitable for sale. Even if only a portion of the crop and, or,
production is damaged, our results of operations could be adversely affected as all or a substantial portion of the production
costs may have been incurred. Although some plant diseases are treatable, the cost of treatment can be high and such events could adversely
affect our operating results and financial condition. Furthermore, if we fail to control a given plant disease and the production is
threatened, we may be unable to supply our customers, which could adversely affect our business, financial condition and results of operations.
There can be no assurance that natural elements will not have a material adverse effect on any such production.
Product
Liability
As
a manufacturer and distributor of cannabis products designed to be ingested or inhaled by humans, we face an inherent risk of
exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused damages, loss or injury.
In addition, the sale of our cannabis products involves the risk of injury to consumers due to tampering by unauthorized
third parties or product contamination. Adverse reactions resulting from human consumption of our cannabis products alone or in
combination with other medications or substances could occur. We may be subject to various product liability claims, including, among
others, that our products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning
health risks, possible side effects or interactions with other substances. A product liability claim or regulatory action against us
could result in increased costs, could adversely affect our reputation with our clients and consumers generally, and could have a material
adverse effect on our results of operations and financial condition. There can be no assurances that we will be able to obtain or maintain
product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive
and may not be available in the future on acceptable terms, or at all.
Energy
Prices and Supply
We
require substantial amounts of diesel and electric energy and other resources for our harvest activities and to transport cannabis and
hemp. We rely upon third parties for our supply of energy resources used in our operations. The prices for and availability of energy
resources may be subject to change or curtailment, respectively, due to, among other things, new laws or regulations, imposition of new
taxes or tariffs, interruptions in production by suppliers, imposition of restrictions on energy supply by government, worldwide price
levels and market conditions. If our energy supply is curtailed for an extended period of time and we are unable to find replacement
sources at comparable prices, or at all, our business, financial condition and results of operations would be materially and adversely
affected.
Retention
and Acquisition of Skilled Personnel
We
will be required to attract and retain top quality talent to compete in the marketplace. We believe our future growth and success
will depend in part on our abilities to attract and retain highly skilled managerial, product development, sales and marketing, and finance
personnel. There can be no assurance of success in attracting and retaining such personnel. Shortages in qualified personnel could limit
our ability to be successful. At present and for the near future, we will depend upon a relatively small number of employees primarily
in Colombia to develop, manufacture, market, sell and distribute our products. As the size of our business increases, we will seek to
hire additional employees in other jurisdictions. Expansion of marketing and distribution of our products will require us to find, hire
and retain additional capable employees who can understand, explain, market and sell our products and/or our ability to enter into satisfactory
logistic arrangements to sell our products. There is intense competition for capable personnel in all of these areas and we may not be
successful in attracting, training, integrating, motivating, or retaining new personnel or subcontractors for these required functions.
Emerging
Market Risks
Emerging
market investment generally poses a greater degree of risk than investment in more mature market economies as developing market
economies are more susceptible to destabilization resulting from domestic and international developments.
Colombia’s
legal and regulatory requirements in connection with companies conducting agricultural activities, banking system and controls as well
as local business culture and practices are different from those in the United States. Our officers and directors must rely, to a great
extent, on our local legal counsel and local consultants retained by us in order to keep abreast of material legal, regulatory and governmental
developments as they pertain to and affect our business operations, and to assist us with our governmental relations. We also rely on the advice of local experts and professionals
in connection with current and new regulations that develop in respect of banking, financing and tax matters. Any developments or changes
in such legal, regulatory or governmental requirements or in local business practices are beyond our control and may adversely affect
our business.
We
also bear the risk that changes can occur to the Government in Colombia and a new government may void or change the laws and regulations
that we are relying upon. Currently, there are no restrictions on the repatriation from Colombia of earnings to foreign entities and
Colombia has never imposed such restrictions. However, there can be no assurance that restrictions on repatriation of earnings will not
be imposed in the future. Exchange control regulations for Colombia require that any proceeds in foreign currency originated on exports
of goods from Colombia be repatriated to Colombia. However, purchase of foreign currency is allowed through Colombian authorized financial
entities for purposes of payments to foreign suppliers, repayment of foreign debt, payment of dividends to foreign stockholders and other
foreign expenses.
Due
to our location in Colombia, our business, financial position and results of operations may be affected by the general conditions of
the Colombian economy, price instabilities, currency fluctuations, inflation, interest rates, regulatory changes, taxation changes, social
instabilities, political unrest and other developments in or affecting Colombia, over which we do not have control.
Risks
Related to Conducting Operations in Colombia
We
were recently granted medicinal cannabis licenses in Colombia. Over the past 10 to 15 years, the Government of Colombia has made
strides in improving the social, political, economic, legal and fiscal regimes. However, operations in Colombia remain subject
to risk due to the potential for social, political, economic, legal and fiscal instability. The Government of Colombia faces ongoing
problems including, but not limited to, unemployment and inequitable income distribution and unstable neighboring countries. The instability
in neighboring countries could result in an influx of immigrants resulting in a humanitarian crisis and/or increased illegal activities.
Colombia is also home to a number of insurgency groups and large swaths of the countryside are under guerrilla influence. In addition,
Colombia experiences narcotics-related violence, a prevalence of kidnapping, extortion and thefts and civil unrest in certain areas of
the country. Such instability may require us to suspend operations on our properties.
Other
risks exist relating to the conduct of business in Colombia. These risks include the future imposition of special taxes or similar charges,
as well as foreign exchange fluctuations and currency convertibility and controls. Other risks of doing business in Colombia include
our ability to enforce our contractual rights or the taking or nationalization of property without fair compensation, restrictions on
the use of expatriates in our operations, renegotiation or nullification of existing concessions, licenses, permits and contracts, changes
in taxation policies, or other matters.
The
Government of Colombia recently reached a peace accord with the country’s largest guerrilla group. The Government of Colombia also
entered into and dissolved formal discussions with the country’s second largest guerrilla group due to their unwillingness to cease
criminal and violent crimes. There is no certainty that the agreements will be adhered to by all of the members of the guerrilla groups
or that a peace agreement will be ultimately reached with the country’s second largest guerrilla group. There is a risk that any
peace agreement might contain new laws or change existing laws that could have a material adverse effect on us. Furthermore, the achievement
of peace with the country’s guerrilla groups could create additional social or political instability in the immediate aftermath,
which could have a material adverse effect on our operations.
Global
Economy
Financial
and commodity markets in Colombia are influenced by the economic and market conditions in other countries, including other South American
and emerging market countries and other global markets. Although economic conditions in these countries may differ significantly from
economic conditions in Colombia, investors’ reactions to developments in these other countries, such as the recent developments
in the global financial markets, may substantially affect the capital flows into, and the market value of securities of issuers with
operations in Colombia.
Insurance
Coverage
Our
production is, in general, subject to different risks and hazards, including adverse weather conditions, fires, plant diseases and pest
infestations, other natural phenomena, industrial accidents, labor disputes, changes in the legal and regulatory framework applicable
to us, and environmental contingencies. We will endeavor to obtain appropriate insurance covering these risks in amounts sufficient to
support a downturn in the sale of our products due to these potential production risks. The cost of such insurance may be high and we
may not be able to obtain sufficient amount of insurance to cover these risks.
Operations
in Spanish
As
a result of our conducting most of our operations in Colombia, our regulatory licenses and books and records, including key documents
such as material contracts and financial documentation, are principally negotiated and entered into in the Spanish language and English
translations may not exist or be readily available.
General
Business Risks
Inability
to Manage Growth
We
may not be able to effectively manage our growth as a producer, manufacturer and exporter of cannabis and hemp products. Our strategy
envisions growing our business. We plan to expand our production and manufacturing capability and create a global distribution
network. Any growth in or expansion of our business is likely to continue to place a strain on our management and administrative
resources, infrastructure and systems. As with other growing businesses, we expect that we will need to further refine and expand our
business development capabilities, our systems and processes and our access to financing sources. We also will need to hire, train, supervise
and manage new employees. These processes are time consuming and expensive, will increase management responsibilities and will divert
management attention. We cannot assure you that we will be able to:
| |
● |
cultivate our cannabis crops in Colombia and expand our manufacturing processes and systems in our facilities in
Colombia; |
| |
● |
execute
and perform under our current manufacturing and distribution agreements with Smokiez Edibles and Kx Family Care; |
| |
● |
raise additional capital to fund our operations; |
| |
● |
identify and hire qualified
employees or retain valued employees; or |
| |
● |
obtain and maintain necessary
licenses in relevant jurisdictions |
Our
inability, or failure to effectively manage, our growth and expansion could harm our business and materially
and adversely affect our operating results and financial condition.
Speculative
Forecasts
Any
forecasts we provide will be highly speculative in nature and we cannot predict results in a development stage company with a high degree
of accuracy. Any financial projections, especially those based on ventures with minimal operating history, are inherently subject to
a high degree of uncertainty, and their ultimate achievement depends on the timing and occurrence of a complex series of future events,
both internal and external to the enterprise. There can be no assurance that potential revenues or expenses we project will be accurate.
Limited
Management Team
Our
limited senior management team size may hamper our ability to effectively manage a publicly traded company while operating our business.
Our management team has experience in the management of publicly traded companies and complying with federal securities laws, including
compliance with recently adopted disclosure requirements on a timely basis. They realize it will take significant resources to meet these
requirements while simultaneously working on cultivating, developing and distributing our cannabis and hemp related products. Our management will be required
to design and implement appropriate programs and policies in responding to increased legal, regulatory compliance and reporting requirements,
and any failure to do so could lead to the imposition of fines and penalties and harm our business.
Risks
Related To Our Common Stock
The
issuance of such additional shares of common stock may depress the price of our common stock.
We
have outstanding obligations to issue additional shares of common stock in the future. These include the following:
| |
● |
We
may sell and issue to Tysadco up to $10,000,000 of shares of common stock under the Purchase Agreement; |
| |
● |
There
are 14,011,650 shares of common stock issuable pursuant to common stock warrants outstanding as of December 31, 2023; |
| |
● |
There
are 9,973,300 shares of common stock issuable upon conversion of our Series A Preferred Stock as of December 31, 2023; |
| |
● |
There
are 23,850,100 shares of common stock issuable upon conversion of our Series B Preferred Stock as of December 31, 2023; |
| |
● |
There
are 5,000,000 shares of common stock issuable pursuant to convertible debt instruments outstanding as of December 31, 2023, consisting of $750,000 of principal that is convertible into 50,000 shares
of Series B Preferred Stock; |
| |
● |
There are 10,892,000 shares of common stock issuable
pursuant to common stock options outstanding as of December 31, 2023. |
Any
shares of common stock issued pursuant to these securities would further dilute the percentage ownership of existing stockholders. The
terms upon which we may obtain additional capital during the life of these securities may be adversely affected due
to such potential dilution. Finally, we may issue additional shares in the future other than as listed above. There are no preemptive
rights in connection with our common stock. Thus, the percentage ownership of existing stockholders may be diluted if we issue additional
shares in the future. Future issuances of additional shares pursuant to grants, options, warrants other convertible securities
could cause immediate and substantial dilution to the net tangible book value of shares of common stock issued and outstanding immediately
before such issuances. Any future decrease in the net tangible book value of such issued and outstanding shares could materially and
adversely affect the market value of the shares.
Limited
Trading
Although
prices for shares of our common stock are quoted on the OTCQB tier of the OTC Markets, there is limited trading and no assurance
can be given that an active public trading market will develop or, if developed, that it will be sustained. The OTC Markets is generally
regarded as a less efficient and less prestigious trading market than other national markets. There is no assurance if or when our common
stock will be quoted on another more prestigious exchange or market. The market price of our common stock is may be volatile as
there will likely be a limited trading market for the stock, which may cause transactions of small blocks of stock
to have a disproportionate impact on the stock price.
We
may issue additional stock without stockholder consent.
Our
Board of Directors has authority, without action or vote of the stockholders, to issue all or part of our authorized but unissued shares.
Additional shares may be issued in connection with future financing, acquisitions, employee stock plans, or otherwise. Any such issuance
will dilute the percentage ownership of existing stockholders. The Board of Directors can also issue preferred stock in one or more series
and fix the terms of such stock without stockholder approval. Preferred stock may include the right to vote as a series on particular
matters, preferences as to dividends and liquidation, conversion and redemption rights and sinking fund provisions. The issuance of preferred
stock could adversely affect the rights of the holders of common stock and reduce the value of the common stock. In addition, specific
rights granted to holders of preferred stock could discourage, delay or prevent a transaction involving a change in control of our company,
even if doing so would benefit our stockholders. Such issuance could also discourage proxy contests and make it more difficult for you
and other stockholders to elect directors of your choosing and to cause us to take other corporate actions you desire.
Broker-dealers
may be discouraged from effecting transactions in our common stock because it is considered a penny stock and is subject to the penny
stock rules.
Our
common stock currently constitutes “penny stock.” Subject to certain exceptions, for the purposes relevant to us, “penny
stock” includes any equity security that has a market price of less than $5.00 per share. Rules 15g-1 through 15g-9 promulgated
under the Securities Exchange Act of 1934, as amended, impose sales practice and disclosure requirements on certain brokers-dealers who
engage in certain transactions involving a “penny stock.” In particular, a broker-dealer selling penny stock to anyone other
than an established customer or “accredited investor” (generally, an individual with net worth in excess of $1,000,000 or
an annual income exceeding $200,000, or $300,000 together with his or her spouse), must make a special suitability determination for
the purchaser and must receive the purchaser’s written consent to the transaction prior to sale, unless the broker-dealer or the
transaction is otherwise exempt. In addition, the penny stock regulations require the broker-dealer to deliver, prior to any transaction
involving a penny stock, a disclosure schedule prepared by the Securities and Exchange Commission relating to the penny stock market,
unless the broker-dealer or the transaction is otherwise exempt. A broker-dealer is also required to disclose commissions payable to
the broker-dealer and the registered representative and current quotations for the securities. Finally, a broker-dealer is required to
send monthly statements disclosing recent price information with respect to the penny stock held in a customer’s account and information
with respect to the limited market in penny stocks.
The
additional sales practice and disclosure requirements imposed upon broker-dealers may discourage broker-dealers from effecting transactions
in our shares, which could severely limit the market liquidity of the shares and impede the sale of our shares in the secondary market.
Because
our Board of Directors does not intend to pay dividends on our common stock in the foreseeable future, stockholders may have to sell
their shares of our common stock to realize a return on their investment in the company.
Holders
of our common stock are entitled to receive dividends if, and when, declared by our Board of Directors out of funds
legally available. To date, we have paid no dividends. Our Board of Directors does not intend to declare any dividends in the foreseeable
future, but instead intends to retain all earnings, if any, for use in our business operations. Accordingly, a return on an investment
in shares of our common stock may be realized only through a sale of such shares, if at all.
Control
of Common Stock will Influence Decision Making
Our
officers, directors and principal stockholders are able to exert significant influence over us and may make decisions that are not in
the best interests of all stockholders. Our officers, directors and principal stockholders (greater than 5% stockholders) collectively
own approximately 87.0% of our fully-diluted common stock. As a result of such ownership, these stockholders are able to affect
the outcome of, or exert significant influence over, all matters requiring stockholder approval, including the election and removal of
directors and any change in control. In particular, this concentration of ownership of our common stock could have the effect of delaying
or preventing a change of control of our company or otherwise discouraging or preventing a potential acquirer from attempting to obtain
control of our company. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our
stockholders from realizing a premium over the market prices for their shares of our common stock.
We
are an Emerging Growth Company Within the Meaning of the Securities Act.
We
are an “emerging growth company” within the meaning of the Securities Act, as modified by the JOBS Act, and we may take advantage
of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth
companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the
Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden
parachute payments not previously approved. As a result, our stockholders may not have access to certain information they may deem important.
We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including
if the market value of our common stock held by non-affiliates exceeds $700 million as of the end of any second quarter of a fiscal year,
in which case we would no longer be an emerging growth company as of the end of such fiscal year. We cannot predict whether investors
will find our securities less attractive because we will rely on these exemptions. If some investors find our securities less attractive
as a result of our reliance on these exemptions, the trading prices of our securities may be lower than they otherwise would be, there
may be a less active trading market for our securities and the trading prices of our securities may be more volatile.
Further,
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting
standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do
not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting
standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements
that apply to non-emerging growth companies but any such election to opt out is irrevocable. We have elected not to opt out of such extended
transition period which means that when a standard is issued or revised and it has different application dates for public or private
companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised
standard. This may make comparison of our financial statements with another public company which is neither an emerging growth company
nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential
differences in accountant standards used.
Antitakeover
Protections
Anti-takeover
provisions may limit the ability of another party to acquire us, which could cause our stock price to decline. Our articles of incorporation,
as amended, bylaws and Nevada law contain provisions that could discourage, delay or prevent a third party from acquiring us, even if
doing so may be beneficial to our stockholders. In addition, these provisions could limit the price investors would be willing to pay
in the future for shares of our common stock.
Risks
Relating to Our Agreements with Tysadco Partners, LLC
The
sale of our common stock to Tysadco may cause dilution, and the sale of the shares of common stock acquired by Tysadco, or the perception
that such sales may occur, could cause the price of our common stock to fall.
Pursuant
to the Purchase Agreement, Tysadco has committed to purchase up to an aggregate of $10,000,000 of our common stock. The shares that may
be sold pursuant to the Purchase Agreement in the future may be sold by us to Tysadco at our discretion from time to time, commencing
after the SEC has declared effective the registration statement that includes this prospectus and until approximately three years after
such date. The per share purchase price for the shares that we may sell to Tysadco under the Purchase Agreement will fluctuate based
on the price of our common stock, and will be equal to 88% of the of the lowest daily volume weighted average price of our common stock
during the period of 10 trading days beginning five trading days preceding the day we deliver the
applicable put notice to Tysadco. Depending on market liquidity at the time, sales of shares of common stock to Tysadco may cause
the trading price of our common stock to fall.
We
generally have the right to control the timing and amount of any sales of our shares to Tysadco, except that, pursuant to the Purchase
Agreement, we may not sell shares to Tysadco if the sale would result in its beneficial ownership of more than 4.99% of our outstanding
common stock. Tysadco may ultimately purchase all, some or none of the shares of our common stock that may be sold pursuant to the Purchase
Agreement and, after it has acquired shares, Tysadco may sell all, some or none of those shares. Therefore, sales to Tysadco by us could
result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial number
of shares of our common stock to Tysadco, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related
securities in the future at a time and at a price that we might otherwise wish to effect sales.
Tysadco
will pay less than the then-prevailing market price for our common stock for purchases under the Purchase Agreement.
The
common stock to be issued to Tysadco pursuant to the Purchase Agreement will be purchased at a 12% discount to the lowest volume weighted
average price of our common stock during the during the period of 10 trading days beginning five
trading days preceding the day we deliver the applicable put notice to Tysadco. Tysadco has a financial incentive to sell our
common stock immediately upon receiving the shares to realize the profit equal to the difference between the discounted price and the
market price. If Tysadco sells the shares, the price of our common stock could decrease. If our stock price decreases, Tysadco may have
a further incentive to sell the shares of our common stock that it holds. These sales may have a further impact on our stock price.
We
may not be able to put to Tysadco all $10,000,000 of shares available under the Purchase Agreement.
The
Purchase Agreement provides for the purchase by Tysadco of up to $10,000,000 of shares of our common stock. Our ability to draw down
funds and sell shares under the Purchase Agreement requires the satisfaction of a number of conditions, including that the registration
statement of which this prospectus is a part be declared effective by the SEC and continue to be effective at the time of the put, as
well as Tysadco’s compliance with its obligations under the Purchase Agreement. Accordingly, there can be no guarantee that we
will be able to draw down all or any portion of the $10,000,000 available to us under the Purchase Agreement.
USE
OF PROCEEDS
The
Shares offered by this prospectus will be sold by the selling stockholder. We will not receive any proceeds from the sale of common stock
by the selling stockholder. However, we will receive proceeds from the sale of shares of our common stock to Tysadco under the Purchase
Agreement, and upon the exercise of warrants held by the selling stockholder. These proceeds would be used for general working capital
purposes.
SELLING
STOCKHOLDER
This prospectus relates to the
possible resale from time to time by the selling stockholder of our common stock, including shares of common stock that may be issued
by us to Tysadco under the Purchase Agreement, and upon the conversion of shares of Series B Preferred Stock we issued to Tysadco under
the Purchase Agreement. In addition, on September 1, 2022, we entered into a Securities Purchase
Agreement with Tysadco (the “SPA”) under which Tysadco agreed to purchase an aggregate of 20,000 shares of our Series B Preferred
Stock for a total purchase price of $300,000 in two closings of 10,000 Series B Preferred Shares each. The first closing of 10,000 Series
B Shares occurred following the execution of the SPA. On October 12, 2022, we sold the second tranche of 10,000
shares of Series B Preferred Stock to Tysadco for proceeds of $150,000. We paid $15,000 out of the proceeds of the investment
to Garden State Securities, Inc. as financing costs. Except for the transactions contemplated by the SPA and the Purchase Agreement,
including our obligations under the related Registration Rights Agreement pursuant to which we have filed the registration statement
of which this prospectus is a part, Tysadco has not had any material relationship with us within the past three years.
The
table below presents information regarding the selling stockholder and the shares of common stock that they may offer from time to time
under this prospectus. This table is prepared based on information supplied to us by the selling stockholder, and reflects holdings as
of December 31, 2023. As used in this prospectus, the term “selling stockholder” includes the selling stockholder
named below and any donees, pledgees, transferees or other successors in interest selling shares received after the date of this prospectus
from the selling stockholder as a gift, pledge, or other non-sale related transfer. The number of shares in the column “Maximum
Number of Shares of common stock to be Offered Pursuant to this prospectus” represents all of the shares of common stock that the
selling stockholder may offer under this prospectus. The selling stockholder may sell some, all or none of its shares in this Offering.
We do not know how long the selling stockholder will hold the shares before selling them, and we currently have no agreements, arrangements
or understandings with the selling stockholder regarding the sale of any of the shares.
Beneficial
ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act, and includes shares of common
stock with respect to which the selling stockholder has voting and investment power. The percentage of shares of common stock beneficially
owned by the selling stockholder prior to the Offering shown in the table below is based on an aggregate of 79,827,618 shares
of our common stock outstanding on December 31, 2023. The fourth column assumes the sale of all of the shares offered by the selling
stockholder pursuant to this prospectus.
| | |
Beneficially Owned
Prior to Offering | | |
Number of Shares Being Offered by Selling | | |
Beneficially Owned
After Offering | |
| Selling Stockholder | |
Number of Shares | | |
Percent | | |
Stockholder in Offering | | |
Number of Shares(1) | | |
Percent | |
| Tysadco Partners,
LLC (2) | |
| 3,000,000 | (3) | |
| 3.80 | % | |
| 21,366,700 | | |
| 2,000,000 | | |
| 2.00 | % |
| * |
Less
than one percent. |
| |
|
| (1) |
Assumes
the sale of all shares being offered pursuant to this prospectus. Shares owned after the offering consist of shares of common stock
issuable upon conversion of 20,000 shares of Series B Preferred Stock. |
| |
|
| (2) |
The
business address of Tysadco Partners, LLC is 210 West 77th Street, #7W, New York, NY 10024. Tysadco’s principal
business is that of a private investment firm. We have been advised that Tysadco is not a member of FINRA, or an independent broker-dealer,
and that neither Tysadco nor any of its affiliates is an affiliate or an associated person of any FINRA member or independent broker-dealer.
We have been further advised that Brian Loper is the Managing Member of Tysadco, and that Mr. Loper has definitive
power to vote or to direct the vote and definitive power to dispose or to direct the disposition of all securities owned directly
by Tysadco. |
| |
|
| (3) |
Includes
shares of common stock issuable upon conversion of shares of Series B Preferred Stock described above, which are subject to the limitation
that Tysadco may not convert such securities to the extent that Tysadco would beneficially own more than 4.99% of our outstanding common
stock. In accordance with Rule 13d-3(d) under the Exchange Act, we have excluded from the number of shares beneficially owned prior to
the Offering all of the shares that Tysadco may be required to purchase under the Purchase Agreement, because the issuance of such shares
is solely at our discretion and is subject to certain conditions, the satisfaction of all of which are outside of Tysadco’s control,
including the Registration Statement of which this prospectus is a part becoming and remaining effective. |
This
prospectus also covers any additional shares of our common stock which become issuable in connection with the shares being registered
by reason of any stock dividend, stock split, recapitalization or other similar transaction effected without the receipt of consideration
which results in an increase in the number of our outstanding shares of common stock.
PLAN
OF DISTRIBUTION
This
prospectus relates to the resale of up to 21,366,700 shares of our common stock by the selling stockholder.
The
selling stockholder and any of its pledgees, assignees and successors-in-interest may, from time to time, sell any or all of the shares
covered hereby on any stock exchange, market or trading facility on which our common stock is traded or in private transactions. These
sales may be at fixed or negotiated prices. The selling stockholder may use any one or more of the following methods when selling shares:
| |
● |
ordinary
brokerage transactions and transactions in which the broker dealer solicits purchasers; |
| |
|
|
| |
● |
block
trades in which the broker dealer will attempt to sell the shares as agent but may position and resell a portion of the block as
principal to facilitate the transaction; |
| |
|
|
| |
● |
purchases
by a broker dealer as principal and resale by the broker dealer for its account; |
| |
|
|
| |
● |
an exchange
distribution in accordance with the rules of the applicable exchange; |
| |
|
|
| |
● |
privately
negotiated transactions; |
| |
|
|
| |
● |
settlement
of short sales entered into after the effective date of the registration statement of which this prospectus is a part; |
| |
|
|
| |
● |
in transactions
through broker dealers that agree with the selling stockholder to sell a specified number of shares at a stipulated price per security; |
| |
|
|
| |
● |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
|
|
| |
● |
a combination
of any such methods of sale; or |
| |
|
|
| |
● |
any other
method permitted pursuant to applicable law. |
The
selling stockholder may also sell Shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker
dealers engaged by the selling stockholder may arrange for other brokers dealers to participate in sales. Broker dealers may receive
commissions or discounts from the selling stockholder (or, if any broker dealer acts as agent for the purchaser of shares, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or
markdown in compliance with FINRA IM-2440.
In
connection with the sale of the shares or interests therein, the selling stockholder may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the shares in the course of hedging the positions they assume.
The selling stockholder may also enter into option or other transactions with broker-dealers or other financial institutions or create
one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by
this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented
or amended to reflect such transaction).
Tysadco
is an “underwriter” within the meaning of the Securities Act and any broker-dealers or agents that are involved in selling
the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In
such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may
be deemed to be underwriting commissions or discounts under the Securities Act. Tysadco has informed us that it does not have any written
or oral agreement or understanding, directly or indirectly, with any person to distribute the shares. In no event shall any broker-dealer
receive fees, commissions and markups which, in the aggregate, would exceed eight percent (8%).
The
selling stockholder may from time to time pledge or grant a security interest in some or all of the shares owned by them and, if they
default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares from time to time
under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities
Act amending the list of selling stockholder to include the pledgee, transferee or other successors in interest as selling stockholder
under this prospectus.
The
selling stockholder also may transfer the shares in other circumstances, in which case the transferees, pledgees or other successors
in interest will be the selling beneficial owners for purposes of this prospectus and may sell the shares from time to time under this
prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act
amending the list of selling stockholder to include the pledgee, transferee or other successors in interest as selling stockholder under
this prospectus.
Because
Tysadco is an “underwriter” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements
of the Securities Act including Rule 172 thereunder.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the shares may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the selling stockholder will be subject to applicable provisions of the Exchange
Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of our securities
by the selling stockholder or any other person. We will make copies of this prospectus available to the selling stockholder and have
informed it of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance
with Rule 172 under the Securities Act).
MARKET
FOR OUR COMMON STOCK
There
is a limited public market for our common stock. Shares of our common stock trade on the over-the-counter market and are quoted on the
OTCQB tier of the OTC Markets under the symbol “OWPC”.
The
following table sets forth, for the fiscal quarters indicated, the high and low bid information for our common stock, as reported on
the OTC Markets. The following quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent
actual transactions.
| | |
High | | |
Low | |
| Fiscal Year Ending December
31, 2023 | |
| | | |
| | |
| First Quarter | |
$ | 0.13 | | |
$ | 0.06 | |
| Second Quarter | |
$ | 0.10 | | |
$ | 0.05 | |
| Third Quarter | |
$ | 0.12 | | |
$ | 0.06 | |
| Fourth Quarter | |
$ | 0.10 | | |
$ | 0.03 | |
| Fiscal Year Ended December
31, 2022 | |
| | | |
| | |
| First Quarter | |
$ | 0.14 | | |
$ | 0.08 | |
| Second Quarter | |
$ | 0.24 | | |
$ | 0.07 | |
| Third Quarter | |
$ | 0.19 | | |
$ | 0.09 | |
| Fourth Quarter | |
$ | 0.13 | | |
$ | 0.07 | |
| | |
| | | |
| | |
| Fiscal Year Ended December
31, 2021 | |
| | | |
| | |
| First Quarter | |
$ | 1.00 | | |
$ | 0.10 | |
| Second Quarter | |
$ | 0.32 | | |
$ | 0.27 | |
| Third Quarter | |
$ | 0.30 | | |
$ | 0.10 | |
| Fourth Quarter | |
$ | 0.14 | | |
$ | 0.07 | |
As
of December 31, 2023, there were 79,827,618 shares of our common stock held by approximately 112 shareholders of record.
Such number does not include any shareholders holding shares in nominee or “street name”.
DIVIDEND
POLICY
We
have not declared or paid any dividends on our common stock since our inception and do not anticipate paying dividends for the foreseeable
future. The payment of dividends is subject to the discretion of our board of directors and depends, among other things, upon our earnings,
our capital requirements, our financial condition, and other relevant factors. We intend to reinvest any earnings in the development
and expansion of our business. Any cash dividends in the future to common shareholders will be payable when, as and if declared by our
board of directors, based upon the board’s assessment of our financial condition and performance, earnings, need for funds, capital
requirements, prior claims of preferred stock to the extent issued and outstanding, and other factors, including income tax consequences,
restrictions and applicable laws. There can be no assurance, therefore, that any dividends on our common stock will ever be paid.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth, as of December 31, 2023, certain information with regard to the record and beneficial ownership of
the Company’s common stock by (i) each person known to the Company to be the record or beneficial owner of 5% or more of the Company’s
common stock, (ii) each director of the Company, (iii) each of the named executive officers, and (iv) all executive officers and directors
of the Company as a group. The address of each of our directors and executive officers named in the table is c/o One World Products,
Inc., 6605 Grand Montecito Pkwy, Suite 100, Las Vegas, Nevada 89149:
| | |
| | |
Series A | | |
Series B | |
|
|
|
| | |
Common Stock | | |
Preferred Stock | | |
Preferred Stock | |
|
Percent of |
|
| Name of Beneficial Owner(1) | |
Number of Shares | | |
% of Class(2) | | |
Number of Shares | | |
% of Class | | |
Number of Shares | | |
% of Class | |
|
Combined
Voting Power(6) |
|
| Officers and Directors: | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
|
|
|
|
| Isiah Thomas, III, Chairman and CEO(3) | |
| 26,000,000 | | |
| 24.7 | % | |
| - | | |
| - | | |
| 200,000 | | |
| 83.9 | % |
|
|
17.7 |
% |
| Dr. Kenneth Perego II, Vice Chairman(4) | |
| 11,100,000 | | |
| 13.6 | % | |
| 11,000 | | |
| 11.0 | % | |
| - | | |
| - | |
|
|
55.3 |
% |
| Joerg Sommer, President | |
| 1,500,000 | | |
| 1.9 | % | |
| - | | |
| - | | |
| - | | |
| - | |
|
|
1.3 |
% |
| Timothy Woods, Chief Financial Officer | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
|
|
- |
|
| Terry L. Buffalo, Director | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
|
|
- |
|
| Directors and Officers as a Group (4 persons) | |
| 38,600,000 | | |
| 36.0 | % | |
| - | | |
| - | | |
| - | | |
| - | |
|
|
74.2 |
% |
| 5% Shareholders | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
|
|
|
|
| ISIAH International, LLC(3) | |
| 26,000,000 | | |
| 24.7 | % | |
| - | | |
| - | | |
| 200,000 | | |
| 83.9 | % |
|
|
17.7 |
% |
| Dr. John McCabe(5) | |
| 15,000,180 | | |
| 18.8 | % | |
| 3,000 | | |
| 3.0 | % | |
| 20,000 | | |
| 8.4 | % |
|
|
12.8 |
% |
*
less than 1%
| (1) |
Except as indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock owned by such person. |
| |
|
| (2) |
Percentage of beneficial ownership is based upon 79,827,618
shares of common stock, 99,733 shares of Series A Preferred Stock and 238,501 and shares of Series B Preferred Stock
outstanding as of December 31, 2023. For each named person, this percentage includes common stock that the person has the
right to acquire either currently or within 60 days of December 31, 2023, including through the exercise of an option; however,
such common stock is not deemed outstanding for the purpose of computing the percentage owned by any other person. |
| |
|
| (3) |
Includes 5,500,000 shares of common stock that may be acquired upon exercise of a vested option, and 20,000,000 shares of common stock that may be acquired upon conversion of Series B Preferred Stock currently held by Isiah International, LLC. Mr. Thomas is the sole member and Chief Executive Officer of ISIAH International. |
| |
|
| (4) |
Includes 7,000,000 shares of common stock held by CB Medical, LLC, of which Dr. Kenneth Perego, II is the controlling member. Includes 350,000 shares of common stock that may be acquired under an option, and 550,000 shares of common stock that may be acquired under a warrant. In addition, includes 11,000 shares of Series A Preferred Stock, convertible into 1,100,000 shares of common stock with each share of preferred carrying 50 voting rights. |
| |
|
| (5) |
Includes 150,000 shares of common stock that may be acquired upon
exercise of a vested warrant, and 300,000 shares of common stock that may be acquired upon conversion of Series A Preferred Stock,
and 2,000,000 shares of common stock that may be acquired upon conversion of Series B Preferred Stock. The address for Mr. McCabe is 160 Kincaid Lane, Boyce, LA 71409. |
| |
|
| (6) |
Each share of common stock is entitled to one vote per share, and each share of Preferred Stock
carries a number of votes equal to the number of shares of common stock into which such Preferred Stock may then be converted. The
Preferred Stock generally will vote together with the common stock and not as a separate class. |
MANAGEMENT’S
DISCUSSION AND ANALYSIS
OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We plan to be a producer of raw cannabis and hemp
plant ingredients for both medical and industrial uses across the globe. The Company is a holding company and conducts its business
in Colombia through OWP-Colombia, its wholly-owned subsidiary. OWP-Colombia has received licenses from the Colombian government
to cultivate, produce and distribute the raw ingredients of the cannabis and hemp plant for medicinal, scientific and industrial
purposes in the town of Esmeralda-Popayan, Cauca, Colombia.
We are in
the process of acquiring another Colombian subsidiary within the Bogota free trade zone, which has all requisite licenses
for the cultivation, production, distribution and export of cannabis and hemp infused products, and will serve as the Company’s
primary base of operations in the Colombian market. Establishing operations within the free trade zone provides favorable import/export
commercial terms and taxation, and will improve logistics and the overall operating efficiencies for the Company due to the close proximity
of El Dorado International Airport and the commercial, economic and cultural center of the city of Bogota itself.
OWP-Colombia owns approximately 30 acres and
has a covered greenhouse built specifically to cultivate high-grade cannabis and hemp. In addition, we entered into agreements
with a local farming co-operative, under which they will cultivate cannabis on up to approximately 140 acres of land using our
seeds and propagation techniques, and sell their harvested products to us on an exclusive basis. We planted our first crop of cannabis
in 2018, which we harvested in the first quarter of 2019 for the purpose of further research and development activities and quality
control testing of the cannabis we have produced.
We have been generating revenue from the sale
of our seeds since the second quarter of 2020. Between August 2021 and March 2022, we made payments of approximately $1,400,000 for
the purchase of a state of the art distillation machine that cleared customs and is currently located in a warehouse near Bogota. We intend to build out an extraction and production facility in the Bogota
free trade zone adjacent to the El Dorado international airport in Bogota after we execute a lease for this location. Once
the extraction equipment is placed in service, we will be one of the few companies in Colombia to both hold licenses and possess the capability
to extract high-quality CBD and THC oils.
Due to challenging economic conditions and under prior management, OWP-Colombia
experienced significant operational and managerial challenges in 2022 and 2023, resulting its accumulation of financial obligations of
approximately $1.2 million, which are substantially past due. Without adequate resources and in an effort to forestall the imposition
of interest, late charges, fines and any court-mandated order(s) to cease operations, OWP-Colombia filed for protection under Colombian
Law 1116 of 2006, which is the primary legislation governing business insolvency proceedings (restructuring and liquidation) (“Reorganization
Proceedings”) in Colombia on December 22, 2023. During the Reorganization Proceeding, management intends to satisfy OWP-Colombia’s
continuing financial obligations through the negotiation and/or settlement with creditors and the Colombian governmental authorities.
Subject to court approval, the Company intends to continue normal operations, which consists of providing cannabinoids in bulk for the
domestic and international markets, including the raw material for our brands and affiliate companies. At this time, the Company
cannot predict the length of time of the Reorganization Proceeding.
We expect to start exporting products in
2024, including CBD flower and distillate oil. Our product pipeline also includes pipeline premium coffee
certified by the Colombian National Coffee Federation infused with CBD, teas infused with CBD and a series of wellness products,
including sports CBD energy drinks for optimum performance, CBD facial and body creams for anti-inflammatory and anti-aging use and
white label commercial agreements with partners in Europe, USA, and Latin America. We recently entered into strategic partnerships
with Smokiez Edibles in Colombia and Stephen Marley’s Kx Family Care. There can be no assurances that these strategic
partnerships will generate revenues or be profitable for the Company.
Results
of Operations for the Three Months Ended September 30, 2023 and 2022
The
following table summarizes selected items from the statement of operations for the three months ended September 30, 2023
and 2022.
| | |
Three
Months Ended September 30, | | |
Increase
/ | |
| | |
2023 | | |
2022 | | |
(Decrease) | |
| Revenues | |
$ | 5,906 | | |
$ | 33,373 | | |
$ | (27,467 | ) |
| Cost of goods
sold | |
| 767 | | |
| 23,969 | | |
| (23,202 | ) |
| Gross
profit (loss) | |
| 5,139 | | |
| 9,404 | | |
| (4,265 | ) |
| | |
| | | |
| | | |
| | |
| Operating
expenses: | |
| | | |
| | | |
| | |
| General
and administrative | |
| 142,464 | | |
| 378,910 | | |
| (236,446 | ) |
| Professional
fees | |
| 236,139 | | |
| 95,946 | | |
| 140,193 | |
| Depreciation
expense | |
| 9,274 | | |
| 9,883 | | |
| (609 | ) |
| Total
operating expenses: | |
| 387,877 | | |
| 484,739 | | |
| (96,862 | ) |
| | |
| | | |
| | | |
| | |
| Operating
loss | |
| (382,738 | ) | |
| (475,335 | ) | |
| (92,597 | ) |
| | |
| | | |
| | | |
| | |
| Total
other expense | |
| (67,568 | ) | |
| (518,808 | ) | |
| (451,240 | ) |
| | |
| | | |
| | | |
| | |
| Net
loss | |
$ | (450,306 | ) | |
$ | (994,143 | ) | |
$ | (543,837 | ) |
Revenues
Revenues during the three months ended September
30, 2023 were $5,906, compared to $33,373 during the three months ended September 30, 2022, a decrease of $27,467, or 82%. Revenues decreased
as we transitioned to new management at our operating facility.
Cost
of Goods Sold
Cost of goods sold for the three months ended
September 30, 2023 were $767, compared to $23,969 for the three months ended September 30, 2022, a decrease of $23,202, or 97%. Cost
of goods sold consists primarily of labor, agricultural raw materials, depreciation and overhead. Costs of goods sold decreased as we
transitioned to new management at our operating facility.
General and Administrative Expenses
General and administrative expenses for the three
months ended September 30, 2023 were $142,464, compared to $378,910 during the three months ended September 30, 2022, a decrease of $236,446,
or 62%. The expenses for the current period consisted primarily of compensation expenses, office rent, and travel costs. General and
administrative expenses decreased primarily due to decreased salaries and wages and lease expenses in Colombia over the comparative period,
as we transitioned to new management. General and administrative expenses included non-cash, stock-based compensation of $29,347 during
the each of the three months ended September 30, 2023 and 2022.
Professional Fees
Professional fees for the three months ended September
30, 2023 were $236,139, compared to $95,946 during the three months ended September 30, 2022, an increase of $140,193, or 146%. Professional
fees included non-cash, stock-based compensation of $147,367 and $11,833 during the three months ended September 30, 2023 and 2022, respectively.
Professional fees increased primarily due to increased stock-based compensation issued to consultants during the current period.
Depreciation Expense
Depreciation expense for the three months ended
September 30, 2023 was $9,274, compared to $9,883 during the three months ended September 30, 2022, a decrease of $609, or 6%. Depreciation
expense decreased due to the prior year disposal of office equipment.
Other Income (Expense)
Other expenses, on a net basis, for the three
months ended September 30, 2023 were $67,568, compared to other expenses, on a net basis, of $518,808 during the three months ended September
30, 2022, a decrease in net expenses of $451,240, or 87%. Other expenses consisted of $67,571 of interest expense, including $12,684
of stock-based finance costs on the amortization of debt discounts, as partially offset by $3 of interest income, for the three months
ended September 30, 2023, compared to $529,915 of interest expense, including $109,969 of stock-based finance costs on the amortization
of debt discounts, $339,133 of stock-based commitment fees on debt and equity financing, and a loss on the sale of fixed assets of $9,041,
as partially offset by a gain of $20,148 on the early extinguishment of our extraction facility lease in Colombia, during the three months
ended September 30, 2022.
Net Loss
Net loss for the three months ended September
30, 2023 was $450,306, or $0.01 per share, compared to $994,143, or $0.02 per share, during the three months ended September 30, 2022,
a decrease of $543,837, or 55%. The net loss decreased primarily due to decreased compensation and office expenses during the current
period as we transitioned to new management over our Colombian subsidiary.
Results
of Operations for the Nine Months Ended September 30, 2023 and 2022
The
following table summarizes selected items from the statement of operations for the nine months ended September 30, 2023
and 2022.
| | |
Nine
Months Ended September 30, | | |
Increase
/ | |
| | |
2023 | | |
2022 | | |
(Decrease) | |
| Revenues | |
$ | 8,082 | | |
$ | 76,384 | | |
$ | (68,302 | ) |
| Cost
of goods sold | |
| 1,866 | | |
| 54,765 | | |
| (52,899 | ) |
| Gross
profit | |
| 6,216 | | |
| 21,619 | | |
| (15,403 | ) |
| | |
| | | |
| | | |
| | |
| Operating
expenses: | |
| | | |
| | | |
| | |
| General
and administrative | |
| 907,595 | | |
| 1,148,100 | | |
| (240,505 | ) |
| Professional
fees | |
| 413,651 | | |
| 380,801 | | |
| 32,850 | |
| Depreciation
expense | |
| 25,578 | | |
| 34,540 | | |
| (8,962 | ) |
| Total
operating expenses: | |
| 1,346,824 | | |
| 1,563,441 | | |
| (216,617 | ) |
| | |
| | | |
| | | |
| | |
| Operating
loss | |
| (1,340,608 | ) | |
| (1,541,822 | ) | |
| (201,214 | ) |
| | |
| | | |
| | | |
| | |
| Total
other expense | |
| (172,766 | ) | |
| (753,317 | ) | |
| (580,551 | ) |
| | |
| | | |
| | | |
| | |
| Net
loss | |
$ | (1,513,374 | ) | |
$ | (2,295,139 | ) | |
$ | (781,765 | ) |
Revenues
Revenues during the nine months ended September
30, 2023 were $8,082, compared to $76,384 during the nine months ended September 30, 2022, a decrease of $68,302, or 89%. Revenues decreased
as we transitioned to new management at our operating facility.
Cost of Goods Sold
Cost of goods sold for the nine months ended September
30, 2023 were $1,866, compared to $54,765 for the nine months ended September 30, 2022, a decrease of $52,899, or 97%. Cost of goods
sold consists primarily of labor, agricultural raw materials, depreciation and overhead. Costs of goods sold decreased as we transitioned
to new management at our operating facility.
General and Administrative Expenses
General and administrative expenses for the nine
months ended September 30, 2023 were $907,595, compared to $1,148,100 during the nine months ended September 30, 2022, a decrease of
$240,505, or 21%. The expenses for the current period consisted primarily of compensation expenses, office rent, and travel costs. General
and administrative expenses decreased primarily due to decreased salaries and wages and lease expenses in Colombia over the prior year,
as we transitioned to new management. General and administrative expenses included non-cash, stock-based compensation of $177,891 and
$88,041 during the nine months ended September 30, 2023 and 2022, respectively.
Professional Fees
Professional fees for the nine months ended September
30, 2023 were $177,512, compared to $284,855 during the nine months ended September 30, 2022, a decrease of $107,343, or 38%. Professional
fees included non-cash, stock-based compensation of $207,011 and $35,399 during the nine months ended September 30, 2023 and 2022, respectively.
Professional fees decreased primarily due to decreased legal fees during the current period.
Depreciation Expense
Depreciation expense for the nine months ended
September 30, 2023 was $25,578, compared to $34,540 during the nine months ended September 30, 2022, a decrease of $8,962, or 26%. Depreciation
expense decreased due to the prior year disposal of office equipment.
Other
Income (Expense)
Other expenses, on a net basis, for the nine months
ended September 30, 2023 were $172,766, compared to other expenses, on a net basis, of $753,317 during the nine months ended September
30, 2022, a decrease in net expenses of $580,551, or 77%. Other expenses consisted of $177,169 of interest expense, including $12,684
of stock-based finance costs on the amortization of debt discounts, as partially offset by a gain on early extinguishment of debt of
$4,397 on the termination of long term leases and $6 of interest income, for the nine months ended September 30, 2023, compared to $886,837
of interest expense, including $361,921 of stock-based finance costs on the amortization of debt discounts, $339,133 of stock-based commitment
fees on debt and equity financing, and a loss on the sale of fixed assets of $9,041, as partially offset by a gain of $20,148 on the
early extinguishment of our extraction facility lease in Colombia, $1,000 of sublet income on our office space, a gain on early extinguishment
of debt of $121,372 on the forgiveness of a PPP Loan and $41 of interest income, during the nine months ended September 30, 2022.
Net
Loss
Net loss for the nine months ended September 30,
2023 was $1,513,374, or $0.02 per share, compared to $2,295,139, or $0.03 per share, during the nine months ended September 30, 2022,
a decrease of $781,765, or 34%. The net loss decreased primarily due to decreased interest expense on our debt financing.
Results
of Operations for the Years ended December 31, 2022 and 2021
The
following table summarizes selected items from the statement of operations for the years ended December 31, 2022 and 2021.
| | |
For
the Years Ended | | |
| |
| | |
December
31, | | |
Increase
/ | |
| | |
2022 | | |
2021 | | |
(Decrease) | |
| | |
| | |
| | |
| |
| Revenues | |
$ | 125,662 | | |
$ | 38,264 | | |
$ | 87,398 | |
| Cost of goods
sold | |
| 300,757 | | |
| 19,744 | | |
| 281,013 | |
| Gross
profit (loss) | |
| (175,095 | ) | |
| 18,520 | | |
| 281,013 | |
| | |
| | | |
| | | |
| | |
| Operating
expenses: | |
| | | |
| | | |
| | |
| General
and administrative | |
| 1,587,017 | | |
| 2,924,284 | | |
| (707,267 | ) |
| Professional
fees | |
| 431,737 | | |
| 915,217 | | |
| (483,480 | ) |
| Depreciation
expense | |
| 42,287 | | |
| 40,321 | | |
| 1,966 | |
| Total
operating expenses: | |
| 2,061,041 | | |
| 3,249,822 | | |
| (1,188,781 | ) |
| | |
| | | |
| | | |
| | |
| Operating
loss | |
| (2,236,136 | ) | |
| (3,231,302 | ) | |
| (995,166 | ) |
| | |
| | | |
| | | |
| | |
| Total
other expense | |
| (823,341 | ) | |
| (553,260 | ) | |
| 270,081 | |
| | |
| | | |
| | | |
| | |
| Net loss | |
$ | (3,059,477 | ) | |
$ | (3,784,562 | ) | |
$ | (725,085 | ) |
Revenues
Revenues for the year ended December 31, 2022
were $125,662, compared to $38,264 during the year ended December 31, 2021, an increase of $87,398, or 228%.
Revenues increased as we continued to shift our focus toward producing and selling CBD and THC oils.
Cost of Goods Sold
Cost of goods sold for the year ended December 31,
2022 were $300,757, compared to $19,744 during the year ended December 31, 2021, an increase of $281,013,
or 1,423%. Cost of goods sold consists primarily of write offs of obsolete inventory, labor, depreciation and maintenance
on cultivation and production equipment, and supplies consumed in our operations. Our gross margins were approximately negative 139%
for the year ended December 31, 2022, compared to 48% during the year ended December 31, 2021. Our current year
gross margins were less than the prior year due primarily to a significant write down of obsolete inventory, compared to
the prior year.
General
and Administrative Expenses
General and administrative expenses for the year
ended December 31, 2022 were $1,587,017, compared to $2,294,284 for the year ended December 31, 2021, a decrease
of $707,267, or 31%. General and administrative expenses decreased primarily due to decreased stock-based compensation. The expenses
for the current period consisted primarily of compensation expenses, office rent, and travel costs, including $117,388 of stock-based
compensation, which consisted entirely of expense related to stock options that were issued to our officers. The expenses for the prior
period included $638,036 of stock-based compensation, of which $55,234, consisting of 673,582 shares, were issued as compensation
payment in lieu of cash to our former CFO, and $582,802 of expense related to stock options that were issued to our officers. Stock-based
compensation decrease by $520,648, or 82%, for the year ended December 31, 2022, compared to the year ended December 31, 2021.
Professional
Fees
Professional fees for the year ended December 31,
2022 were $431,737, compared to $915,217 during the year ended 2021, a decrease of $483,480, or 53%.
Professional fees included non-cash stock-based compensation of $47,232, consisting entirely of stock options expense, during the
year ended December 31, 2022, compared to $496,553, consisting of $78,521 on issuances of common stock expense and $418,032 of stock
options expense, during the year ended December 31, 2021, a decrease of $449,321, or 90%. Professional fees decreased primarily
due to decreased stock-based compensation during the current period.
Depreciation Expense
We had $42,287 of depreciation expense for the
year ended December 31, 2022, compared to $40,321 of depreciation expense for the year ended December 31, 2021, an increase of $1,966,
or 5%. Depreciation expense increased during the current period as additional assets have been placed in service.
Other Income (Expense)
Other expenses, on a net basis, for the year ended
December 31, 2022 were $823,341, compared to other expenses, on a net basis, of $553,260 for the year ended December
31, 2021. Other expense during the year ended December 31, 2022 consisted of a loss on disposal of fixed assets of $9,041,
and $956,858 of interest expense, including $339,133 on shares of series B preferred stock and common stock issued as commitment fees
to Tysadco Partners on debt financing arrangements, as partially offset by $1,000 of sublease income, a gain on early extinguishment
of leases of $20,148, a gain on forgiveness of PPP loan of $121,372 and $38 of interest income. Other expenses for the year ended
December 31, 2021 consisted of a loss on disposal of fixed assets of $71,487 and $511,131 of interest expense, as partially offset by
$27,000 of sublease income and $2,358 of interest income.
Net Loss
Net loss for the year ended December 31, 2022
was $3,059,477, or $0.05 per share, compared to $3,784,562, or $0.06 per share, during the year ended December 31, 2021,
a decrease of $725,085, or 19%. The net loss for the year ended December 31, 2022 included non-cash expenses consisting of $42,287
of depreciation, a $9,041 loss on disposal of fixed assets, $503,753 of stock-based compensation, and $956,858 of interest expense, including
$412,673 on the amortization of debt discounts and $339,133 on shares of series B preferred stock and common stock issued as commitment
fees to Tysadco Partners on debt financing arrangements. The net loss for the year ended December 31, 2021 included non-cash expenses
consisting of $40,321 of depreciation, a $71,487 loss on disposal of fixed assets, $1,134,589 of stock-based compensation, and $511,131
of interest expense, including $456,656 on the amortization of debt discounts.
Liquidity
and Capital Resources
The
following table summarizes our total current assets, liabilities and working capital at September 30, 2023, December 31,
2022 and 2021.
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | | |
2021 | |
| Current Assets | |
$ | 361,100 | | |
$ | 123,467 | | |
$ | 496,989 | |
| | |
| | | |
| | | |
| | |
| Current Liabilities | |
$ | 4,513,253 | | |
$ | 2,977,435 | | |
$ | 1,523,593 | |
| | |
| | | |
| | | |
| | |
| Working Capital | |
$ | (4,152,153 | ) | |
$ | (2,853,968 | ) | |
$ | (1,026,604 | ) |
The following table summarizes our cash flows during
the nine months ended September 30, 2023 and the years ended December 31, 2022 and 2021, respectively.
| | |
For the Nine Months | | |
For the Year Ended | |
| | |
Ended | | |
December 31, | |
| | |
September 30, 2023 | | |
2022 | | |
2021 | |
| Net cash used in operating activities | |
$ | (1,043,238 | ) | |
$ | (1,380,483 | ) | |
$ | (3,728,702 | ) |
| Net cash used in investing activities | |
$ | (5,046 | ) | |
| (36,851 | ) | |
| (388,001 | ) |
| Net cash provided by financing activities | |
$ | 874,500 | | |
| 1,317,581 | | |
| 4,218,938 | |
| Effect of exchange rate changes on cash | |
$ | 172,920 | | |
| (8,909 | ) | |
| (11,477 | ) |
| | |
| | | |
| | | |
| | |
| Net change in cash | |
$ | (864 | ) | |
$ | (108,662 | ) | |
$ | 90,758 | |
The decrease in funds used in operating activities
for the year ended December 31, 2022, compared to the year ended December 31, 2021, was primarily due to decreased
operations in the current year as capital resources tightened. During the nine months ended September 30, 2023, net cash used in operating
activities was $1,043,238, compared to net cash used in operating activities of $1,124,967 for the nine months ended September 30, 2022. The
cash used in operating activities was primarily attributable to our net loss.
The decrease in funds used in investing activities
for the year ended December 31, 2022, compared to the year ended December 31, 2021, was due primarily to decreased
purchases of fixed assets in the year ended December 31, 2022. During the nine months ended September 30, 2023, net cash used in investing
activities was $5,046, compared to net cash used in investing activities of $36,851 for the nine months ended September 30, 2022. The
cash used in investing activities during the current period consisted entirely of purchases of fixed assets, as compared to the purchases
of fixed assets, as partially offset by proceeds received on the sale of fixed assets in the comparative period.
The decrease in funds provided by financing
activities for the year ended December 31, 2022, compared to the year ended December 31, 2021, was due primarily to $3,227,505
of decreased proceeds received from the sale of our securities, as partially offset by $326,148 of increased net debt
financing proceeds received during the year ended December 31, 2022. During the nine months ended September 30, 2023, net
cash provided by financing activities was $874,500, compared to net cash provided by financing activities of $1,117,581 for
the nine months ended September 30, 2022. The current period consisted of $250,000 of proceeds received on the sale of preferred stock, $300,000 from the sale of common stock and
$262,500 of proceeds received on debt financing and $62,000 of proceeds received on debt financing received by related parties, compared
to $1,117,581 of proceeds received on debt financing and $150,000 of proceeds received on the sale of preferred stock, as partially offset
by $750,000 of repayments on debt, compared to $947,000 of proceeds received on debt financing and $3,577,505 received on the sale of
preferred and common stock, less debt repayments of $455,567, during the nine months ended September 30, 2022.
Ability
to Continue as a Going Concern
As
of September 30, 2023, our balance of cash on hand was $10,152, and we had negative working capital of $4,152,153
and an accumulated deficit of $24,489,739. We do not currently have sufficient funds to fund our operations at their current
levels for the next twelve months. As we implement our cannabis cultivation business and attempt to expand operational activities, we
expect to continue to experience net negative cash flows from operations in amounts not now determinable, and will be required to obtain
additional financing to fund operations. Our ability to continue as a going concern is dependent upon our ability to raise additional
capital and to achieve sustainable revenues and profitable operations. Since inception, we have raised funds primarily through the sale
of equity securities. We will need, and are currently seeking, additional funds to operate our business. No assurance can be given that
any future financing will be available or, if available, that it will be on terms that are satisfactory to us. Even if we are able to
obtain additional financing, it may contain undue restrictions on our operations or cause substantial dilution for our stockholders.
If we are unable to obtain additional funds, our ability to carry out and implement our planned business objectives and strategies will
be significantly delayed, limited or may not occur. We cannot guarantee that we will become profitable. Even if we achieve profitability,
given the competitive and evolving nature of the industry in which we operate, we may not be able to sustain or increase profitability
and our failure to do so would adversely affect our business, including our ability to raise additional funds.
Our
financial statements included in this prospectus do not include any adjustments that might result from the outcome of any uncertainty
as to the Company’s ability to continue as a going concern. Our financial statements included in this prospectus also do not include
any adjustments relating to the recoverability and classification of recorded asset amounts, or amounts and classifications of liabilities
that might be necessary should the Company be unable to continue as a going concern. Our ability to scale production and distribution
capabilities and further increase the value of our brands, is largely dependent on our success in raising additional capital.
Off-Balance
Sheet Arrangements
We
have no outstanding off-balance sheet guarantees, interest rate swap transactions or foreign currency contracts. We do not engage in
trading activities involving non-exchange traded contracts.
Critical
Accounting Policies and Estimates
The
preparation of financial statements in conformity with accounting principles generally accepted in the United States requires our management
to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of
commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our
financial statements. These accounting policies are important for an understanding of our financial condition and results of operations.
Critical accounting policies are those that are most important to the presentation of our financial condition and results of operations
and require management’s subjective or complex judgment, often as a result of the need to make estimates about the effect of matters
that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of
their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly
from management’s current judgments.
While
our significant accounting policies are more fully described in notes to our financial statements appearing elsewhere in this prospectus,
we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our reported
financial results and affect the more significant judgments and estimates that we used in the preparation of our financial statements.
Revenue
Recognition
The
Company recognizes revenue in accordance with ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes
revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies by applying the following
steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction
price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance
obligation is satisfied. The Company’s sales to date have primarily consisted of the sale of seeds. These sales include multi-element
arrangements whereby the Company collects 50% of the sale upon delivery of the sales, and the remaining 50% upon the completion of the
harvest, whether the seeds result in a successful crop, or not. In addition, the Company has a right of first refusal to purchase products
resulting from the harvest. At September 30, 2023, the Company had $11,545 of deferred revenues and $6,760
of deferred cost of goods sold, as included in other current assets on the balance sheet, that are expected to be recognized upon the
customers’ completion of their future harvests.
Inventory
Inventories
are stated at the lower of cost or market. Cost is determined on a standard cost basis that approximates the first-in, first-out (FIFO)
method. Market is determined based on net realizable value. Appropriate consideration is given to obsolescence, excessive levels, deterioration,
and other factors in evaluating net realizable value. Our cannabis products consist of cannabis flower grown in-house, along with produced
extracts.
Stock-Based
Compensation
The
Company accounts for equity instruments issued to employees and non-employees in accordance with the provisions of ASC 718 Stock Compensation
(ASC 718). All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted
for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably
measurable. The measurement date of the fair value of the equity instrument issued is the earlier of the date on which the counterparty’s
performance is complete or the date at which a commitment for performance by the counterparty to earn the equity instruments is reached
because of sufficiently large disincentives for nonperformance.
DESCRIPTION
OF OUR BUSINESS
Overview
On
February 21, 2019, we entered into an Agreement and Plan of Merger (“Merger Agreement”) with OWP Merger Subsidiary, Inc.
(“OWP Merger Sub), our wholly-owned subsidiary, and OWP Ventures, Inc. (“OWP Ventures”). Under the Merger Agreement,
the acquisition of OWP Ventures by us was effected by the merger of OWP Merger Sub with and into OWP Ventures, with OWP Ventures being
the surviving entity as our wholly-owned subsidiary (the “Merger”). The closing (the “Closing”) of the Merger
occurred on February 21, 2019.
Immediately
prior to the Closing, we were a public “shell” company with nominal assets. As of the Closing, we are no longer a public
shell. As a result of the Merger, we are engaged in OWP Ventures’ business, including the business of its wholly-owned subsidiary,
One World Pharma, S.A.S., a Colombian company (“OWP Colombia”). On November 23, 2021,
we changed our name from One World Pharma, Inc. to One World Products, Inc. through the merger of One World Products, Inc., a recently
formed Nevada corporation wholly-owned by us, with and into us. This merger was effected solely to effect the change of our name, and
had no effect on our officers, directors, operations, assets or liabilities.
On
June 3, 2020, Isiah L. Thomas III was appointed to serve as our Chief Executive Officer and Vice Chairman. Mr. Thomas was a 12-time NBA
All Star, two-time NBA champion, and is an accomplished international business executive. In 2021, through ISIAH International, LLC,
of which he is the sole member, Mr. Thomas purchased $3,000,000 of our Series B Preferred Stock in installments over a period of time
ending in July 2021.
We
plan to be a producer of raw cannabis and hemp plant ingredients for both medical and industrial uses across the globe. The Company
is a holding company and conducts its business in Colombia through OWP-Colombia, its wholly-owned subsidiary, The Company, through OWP-Colombia,
received licenses from Colombian regulators to cultivate, produce and distribute the raw ingredients of the cannabis and hemp plant
for medicinal, scientific and industrial purposes in the town of Esmeralda-Popayan, Cauca, Colombia.
We are in
the process of acquiring another Colombian subsidiary within the Bogota free trade zone, which has all requisite licenses
for the cultivation, production, distribution and export of cannabis and hemp infused products, and will serve as the Company’s
primary base of operations in the Colombian market. Establishing operations within the free trade zone provides favorable import/export
commercial terms and taxation, and will improve logistics and the overall operating efficiencies for the Company due to the close proximity
of El Dorado International Airport and the commercial, economic and cultural center of the city of Bogota itself.
We
planted our first crop of cannabis in Popayan, Colombia in 2018, and began initial harvesting in the first quarter of 2019 for the purpose
of further research and development activities and quality control testing of the cannabis we have produced. We commenced limited shipping
of non-psychoactive products to customers in May of 2020. Although we hold the four Colombian Licenses, we will need to obtain additional
approvals from Colombian regulators before we can fully execute our business plan, particularly with respect to the sale psychoactive
products. As described further under “Regulation” below,
| |
● |
We
will need to obtain quota approvals from the Colombian authorities before we can commence commercial sale of our psychoactive products
under our Cannabis Manufacturing License and Psychoactive Cultivation License; |
| |
|
|
| |
● |
We
have successfully registered three non-psychoactive distinct cannabis strains and have received the certification required by Colombia’s
National Registrar as of April 2020; and |
| |
|
|
| |
● |
We
have been issued the sanitary registrations needed to sell our products intended for human consumption; and |
| |
|
|
| |
● |
We
have successfully registered eight psychoactive distinct cannabis strains and have received the certifications required by Columbia’s
National Registrar as of December 2020; and |
| |
|
|
| |
● |
We
will proceed to get quota approvals for 2024. |
Our
first cultivation site is located in Popayan, Colombia. Our cultivation facility encompasses approximately 30 acres and includes a covered
greenhouse built specifically to cultivate high-grade cannabis and hemp. In addition, we have entered into an agreements with
a local farming co-operative that will cultivate cannabis
on up to approximately 140 acres of land using our seeds and propagation techniques, and sell their harvested products to us on an exclusive
basis.
We
employ modern propagation and cultivation techniques drawn from U.S. practices that allow us to rapidly multiply the cells of a specific
plant strain to produce large numbers of genetically consistent progeny plants using our own plant tissue culture method. We believe
this technique allows us to cultivate plants which are stable, robust and able to produce genetically superior cannabis and hemp derived
products. We intend to have our processes and products certified as compliant with international standards, including Good Agricultural
Practices (“GAP”), Good Manufacturing Practice (“GMP”) and the standards set forth in EU Pharmacopoeia, a publication
that sets forth quality standards applicable to the European pharmaceutical industry.
We have been generating revenue from the sale
of our seeds since the second quarter of 2020. Between August 2021 and March 2022, we made payments of approximately
$1,400,000 for the purchase of a state of the art distillation machine that cleared customs. We intend to build out an extraction and production facility in the Bogota
free trade zone extension, after we execute a lease for this location. The installation of this equipment makes
us one of the only companies in Colombia to both, hold licenses and possess the capability to extract high-quality CBD and THC oils.
For strategic and operational reasons, OWP-Colombia
filed for protection under Colombian Law 1116 of 2006, which is the primary legislation governing business insolvency proceedings (restructuring
and liquidation) (“Reorganization Proceedings”) in Colombia on December 22, 2023. Subject to court approval, the Company
intends on continuing its normal operations during the Reorganization Proceeding. Our normal operations consist or providing cannabinoids
in bulk for the domestic and international markets, including the raw material for our brands and affiliate companies. At this time,
the Company cannot predict the length of time of the Reorganization Proceeding.
We expect to start exporting products in
2024, including CBD flower and distillate oil, while developing white label commercial agreements with partners in Europe, USA, and
Latin America. Our product pipeline includes premium coffee certified by the Colombian National Coffee Federation infused with CBD,
teas infused with CBD and a series of wellness products, including sports CBD energy drinks for optimum performance, CBD facial and
body creams for anti-inflammatory and anti-aging use. In September and December 2023, we entered into strategic partnerships
with Smokiez Edibles to distribute Smokiez Gummies in Colombia and other countries in Latin America. In December 2023, we entered
into a strategic partnership with Kx Family Care, which honors the legacy of Bob Marley to distribute Kx Family Care products in
Columbia and other countries in Latin There can be no assurances that these strategic partnerships will generate revenue or be
profitable for the Company.
History and Background
One World Pharma S.A.S., is a Colombian company (“OWP
Colombia”), incorporated on July 14, 2017 with the goal of procuring the following Colombian Licenses.
On December 20, 2017, the Colombian Ministry of Health,
by means of resolution No. 5251 of 2017, granted OWP Colombia its license for the production of cannabis derivatives for domestic use
and export, allowing OWP Colombia to extract high tetrahydrocannabinol (“THC”) compounds (“Cannabis Manufacturing License”).
This license will expire on December 20, 2027.
On December 26, 2017, the Colombian Ministry of Justice,
by means of resolution No. 1087 of 2017, granted OWP Colombia its license to use seeds for sowing for sale or delivery of seeds and/or
for scientific research purposes, allowing for genetic and seed bank registration (“Cannabis Seed Possession License”). This
license will expire on December 26, 2027.
On December 26, 2017, the Colombian Ministry of Justice,
by means of resolution No. 1088 of 2017, granted OWP Colombia its license to grow non-psychoactive cannabis plants (less than 1.0% THC).
Under this license, OWP Colombia can produce seeds for planting, deliver and make sales of the cannabis crop in order to produce cannabis
derivatives and deliver and make sales of the cannabis crop for industrial purposes (“Cannabis Non-Psychoactive Cultivation License”).
This license will expire on December 26, 2027.
On January 4, 2018, the Colombian Ministry of Justice,
by means of resolution No. 0015 of 2018, granted OWP Colombia its license to grow psychoactive cannabis plants (greater than 1.0% THC)
(“Psychoactive Cultivation License”). Under this license, OWP Colombia can produce seeds for planting, and deliver and make
sales of the cannabis crop in order to produce cannabis derivatives. This license will expire on January 4, 2028.
Six months prior to the expiration of each of the
Licenses, we can apply for successive renewals for additional five-year periods. In each renewal application, the corresponding Ministry
will assess compliance with all the relevant requirements in determining whether or not to renew the License.
On March 27, 2018, OWP Ventures, Inc. was formed as
a Delaware corporation for the purpose of acquiring OWP Colombia.
On May 30, 2018, OWP Ventures entered into a Stock
Purchase Agreement with the shareholders of OWP Colombia whereby the shareholders of OWP Colombia transferred their shares in OWP Colombia
to OWP Ventures in exchange for 10,200,000 shares of common stock of OWP Ventures.
Products
We
are focused on cultivating, processing and supplying crude cannabis oil, distillate and isolate to customers’ specification. We
plan to sell as a wholesaler to industrial companies making cannabis related products. We also plan on supplying the hemp plant bio-mass
remaining after our extraction process to industry participants that utilize hemp in the manufacture of their products. Hemp is used
to make a variety of commercial and industrial products, including rope, textiles, clothing, shoes, food, paper, bioplastics, insulation
and biofuel.
We
are currently in the process of cultivating medicinal cannabis at our facility in Popayan, Colombia for a variety of medical conditions.
We have registered 25 varieties or strains of cannabis with the Colombian Ministry of Health. See “Strains of Cannabis” below.
The development of these strains enables us to select mother plants and identify the concentrations of cannabinoids required for the
products which we intend to distribute. The cannabis will be produced in accordance with GMP Standards. We are committed to developing
final products consistent with medicinal cannabis industry standards and pharmaceutical procedures. Our products will include a variety
of cannabinoids and terpenes designed to address specific medical conditions. The composition of the strains will include a wide range
of THC and CBD ratios.
Industry
Medicinal
cannabis refers to the use of cannabis and its constituent cannabinoids and terpenes to treat disease or ameliorate symptoms such as
pain, muscle spasticity, nausea and other indications. Cannabinoid is a blanket term covering a family of complex chemicals, both natural
and man-made, that bind with cannabinoid receptors (protein molecules on the surface of cells) and effect a wide number of responses.
Cannabinoid receptors in the human body are part of a system called the endocannabinoid system. This system produces chemicals called
endocannabinoids, which also bind with cannabinoid receptors. Cannabinoid receptors are found in the brain and throughout the body. Scientists
have found that cannabinoid receptors in the endocannabinoid system are involved in a vast array of functions in our bodies, including
helping to modulate brain and nerve activity (including memory and pain), energy metabolism, heart function, the immune system and even
reproduction. While there are a large number of active cannabinoids found in cannabis, the two most common currently used for medical
purposes are tetrahydrocannabinol and cannabidiol. Although no clinical trials have been completed in the United States to validate the
effectiveness of tetrahydrocannabinol or cannabidiol in managing disease and improving symptoms, scientific studies have identified that
they, alone and/or in combination, may potentially provide treatment benefits for a large number of medical conditions. For example,
tetrahydrocannabinol, a psychotropic cannabinoid, has been shown to activate pathways in the central nervous system which work to block
pain signals and has shown potential to assist patients with Post Traumatic Stress Disorder (PTSD) and stimulate appetite in patients
following chemotherapy. Cannabidiol, on the other hand, is non-psychotropic and has shown potential to relieve convulsion and inflammation,
and is the active ingredient in Epidolex, which in June 2018 was approved by the FDA for the treatment of two rare and severe forms of
epilepsy.
Regulation
Our
active business operations are currently conducted solely within Colombia, and as such, the discussion below is limited to Colombian
laws and regulations applicable to our business, which require us to hold the relevant licenses, quotas and other permits, as described
below. Our activities in the United States consist solely of corporate administrative activities at our Las Vegas headquarters, including
accounting, finance and SEC compliance functions. We believe that our current activities in the United States will not subject us to
regulation under the U.S. Controlled Substances Act or other applicable U.S. federal or state laws with respect to our proposed business
plans. All export activities will be conducted from Colombia, and we do not intend to export any of our products to jurisdictions where
such sales are not legal under local law. Accordingly, we do not currently intend to export our products to the United States to the
extent such products may be subject to regulation under the U.S. Controlled Substances Act or other applicable U.S. federal or state
regulations.
Regulatory
Authorities
Several
authorities interact in the Colombian cannabis industry. The Ministry of Health is in charge of granting the Cannabis Manufacturing and
Distribution License and exercises administrative control over the production of cannabis derivatives. The Ministry of Justice, through
the subsection for the Control and Supervision of Chemical Substances and Narcotic Drugs, is the competent authority for issuing the
Cannabis Seeds Possession License, the Cannabis Psychoactive Cultivation License and the Cannabis Non-Psychoactive Cultivation License
and for exercising administrative control over cannabis operations and cultivation. The National Narcotics Fund (“FNE”) exercises
administrative and operational control over activities related to the management of psychoactive and non-psychoactive cannabis and its
derivatives. The National Food and Drug Surveillance Institute (“INVIMA”) is in charge of issuing and monitoring compliance
under the health and phytosanitary registrations that may be applicable to products containing cannabis derivatives. The Colombian Agricultural
Institute (“ICA”) is responsible for maintaining the registry of the Genetic Pool or ¨Fuente Semillera”
and the registration of cannabis seeds and strains under the “Registro Nacional de Cultivares Comerciales”.
In
exercising the administrative and operational control activities discussed above the Ministry of Justice, Ministry of Health, ICA and
FNE are required to coordinate their activities to the extent necessary, according to their competencies, with the Ministry of Agriculture
and Rural Development through ICA, as well as with the National Police.
Licenses
Under
Colombian law, there are four types of cannabis licenses that authorize different activities concerning the various stages of the production
line of the medical cannabis industry: (i) the Cannabis Seeds Possession License; which is required for the domestic sale and delivery
of seeds (but not export) and for scientific research purposes; (ii) the Cannabis Psychoactive Cultivation License, which is required
for the production of seeds for sowing; for grain production; production of cannabis derivatives; for scientific research purposes, for
storage, and for final disposal; (iii) the Cannabis Non-Psychoactive Cultivation License, which is required for the production of grain
and seeds for sowing; production of cannabis derivatives; for industrial purposes; for scientific research purposes; for storage; and
for final disposal; and (iv) the Cannabis Manufacturing and Distribution License, which is required for the production of cannabis derivatives
for domestic use; production of cannabis derivatives for scientific research purposes; and production of cannabis derivatives for exportation.
OWP Colombia holds all of these licenses.
The
legal framework currently in force in Colombia regarding medical cannabis is established in Law 1787 of 2016 (the “Law”)
and the Decree 613 of 2017 (the “Decree”). Cannabis licenses must be issued by the Ministry of Health or the Ministry of
Justice in an estimated time of 60 days, however, in practice, this process can take between four and six months. In accordance with
Colombia’s international obligations, there is a limit in the amount of Cannabis allowed for fabrication or cultivation assigned
by the Colombian Government (specific crop or manufacturing quotas) that must be requested by each licensee when applying for a Cannabis
Psychoactive Cultivation License or a Cannabis Manufacturing License. The activities of cultivation and manufacturing can only be started
once the specific quotas have been granted to the licensee.
Duration
of Licenses
The
Cannabis Seeds Possession License, the Cannabis Psychoactive Cultivation License, the Cannabis Non-Psychoactive License, and the Cannabis
Manufacturing and Distribution License are granted by the Ministry of Justice and/or the Ministry of Health (as applicable), when the
applicant fulfills the general criteria described in Article 2.8.11.2.1.5 of the Decree, and the specific requirements for each type
of license. Each of these licenses is valid for up to five years. The Ministry of Justice and the Ministry of Health (as applicable)
maintain the right to monitor the activities performed by the corresponding licensee, and in the event of a breach by the licensee of
the obligations and duties set forth in the Decree, the licenses may be revoked. The relevant Ministry may renew these licenses for additional
and successive five-year periods. In each renewal application, the Ministry will assess compliance with all the relevant requirements
in determining whether or not to renew the license.
Quotas
As
described above, regulations of cannabis in Colombia provides an additional requirement applicable to the Cannabis Psychoactive Cultivation
License and Cannabis Manufacturing License, which require the grant of crop and manufacturing quotas (the “Quotas”). According
to Article 2.8.11.2.6.2 of the Decree, the assignment of Quotas is collectively made by the Ministry of Health, the Ministry of Justice,
the ICA, the INVIMA, and the FNE.
According
to Article 2.8.11.2.6.5 of the Decree, there are two types of Quotas: (i) crop quotas for psychoactive cannabis (for holders of the Cannabis
Psychoactive Cultivation License) which are granted by the Ministry of Justice; and (ii) the manufacturing quotas for psychoactive cannabis
(for holders of the Cannabis Manufacturing License) which are granted by the Ministry of Health.
These
Quotas are requested by the licensees no later than the last calendar day of April of each year, and, if they are granted by the corresponding
authority, they can only be used by the licensees during the next calendar year (for instance, if a licensee requests a specific crop
Quota in March, 2018, and this Quota is granted by the Ministry of Justice, the licensee will be allowed to use the Quota from January
1, 2019 to December 31, 2019). In extraordinary events, the licensees can request a supplementary Quota that will apply to the calendar
year requested (the issuance of these Quotas depends on the special circumstances defined by the Colombian governmental authorities).
On
December 3, 2018, by means of resolution 1256 of 2018, Colombia´s Ministry of Justice granted OWP Colombia a supplementary Quota
for growing psychoactive mother plants; six for each of 13 varieties, for a total of 78 “mother” plants. However, before
we commence the commercial sale of our psychoactive products (greater than 1% THC content), we will need to obtain Quotas from the Ministry
of Health. This will require us to conduct successful agricultural characterization tests approved by and registered with the ICA/Ministry
of Agriculture and Rural Development, and stabilized extracts characterization tests approved by INVIMA/Ministry of Health, of product
samples grown by us under Quotas obtained from the Ministry of Justice. We have already requested from the Ministry of Health and Justice
our annual Quotas for the export sale of psychoactive ingredients in 2022, and are awaiting the issuance of such Quotas in order to start
our production process.
Strains
of Cannabis
Strains
of cannabis are registered in Colombia in two manners:
| |
● |
Registration
of the Genetic Pool or “Fuente Semillera”: Under Article 2.8.11.11.1 of the Decree, licensed producers of cannabis
had until December 31, 2018 to register the genetics of strains of cannabis with the ICA. Under this transitory article, the government
allowed a limited period for licensed producers of cannabis to source genetics currently available in Colombia and register these
as their “fuente semillera”. We registered 25 varieties under this article. This registration enables us to grow our
own strains of cannabis as opposed to having to purchase registered strains from other licensed producers. |
| |
|
|
| |
● |
Registration
Under the “Registro Nacional de Cultivares Comerciales”: Licensed producers of cannabis have to be granted a breeding/research
license to be able to develop, select and trial stabilized cannabis cultivars. This registration allows licensed producers to register
unique and stable varieties of cannabis for commercial production within Colombia. We were granted such license in the first quarter
of 2018. Licensed producers can then request from ICA a registration trial, which is a field flowering trial with the supervision
of ICA officials. The data collected in these trials can lead to registration of the cultivar in the National Registrar. Only registered
varieties will be allowed to be produced commercially. We have received full registration for 3 non-psychoactive high CBD strains
which have been approved for sale. We have also received permission to take 13 psychoactive THC strains through this process and
anticipate the completion of such by year end 2021. |
Sanitary
Registration
The
commercialization of cannabis-based finished products intended for human consumption requires the issuance of sanitary registrations
by the INVIMA, and in the case of products intended for animal consumption, by the ICA.
Environmental
Under
Colombian law, general principles of environmental law are set out in Law 99 of 1993 and Article 9 of the National Code of Natural Resources
and Protection of the Environment. These laws establish principles governing the use of natural resources, including that use must occur
without causing harm to the interests of the community or of third parties. Parties that cause environmental damage while acting under
the authority of a permit are responsible for incurring the costs to rectify the damage. The imposition of environmental sanctions is
in addition to civil and criminal penalties that may be imposed. Environmental damage caused while a party is acting without a license
constitutes a breach of Law 99 of 1993 and may lead to the imposition of sanctions, in addition to civil or criminal proceedings that
may result. Parties that cause environmental damage, in addition to sanctions or penalties that apply, will also be required to carry
out studies to assess the characteristics of the damage. Under Colombian law, liability for environmental damage creates a presumption
of liability in case of a: (i) breach of environmental laws; (ii) environmental damage; and (iii) breach of environmental license or
any other administrative act from the environmental authorities. The Environmental Authorities may investigate potential claims, authorize
preventative measures, or impose sanctions on parties breaching environmental law.
Competition
The
market for medicinal cannabis is characterized by unsatisfied patient demand, with few authorized producers. Although competition in
the market is growing and Colombia offers an open process to apply for the licenses, we believe we are competitively positioned to satisfy
the demand for medicinal cannabis given our early entry into the market, the management team’s expertise in medical product branding,
marketing, quality control and domestic market relationships. In addition, the Colombian government has published for comment a draft
decree that requires any applicant for any of the four Licenses to furnish evidence that it has completed the seed registration process
before the ICA and obtained the corresponding technical sheet for the cannabis plants and varieties. If enacted, this new regulation
will result in stricter requirements on potential competitors seeking a Colombian License.
Cultivation
in Colombia has natural cost advantages. However, management believes the more sustainable competitive advantage is to create patient
loyalty and brand preference, as opposed to the distribution of more homogeneous products. Domestically our competition consists of PharmaCielo,
CannaVida, Empresa Colombiana de Cannabis, Khiron Life Sciences Corp., MedCan, Canopy Growth Corporation, and Clever Leaves.
Intellectual
Property
Our
success depends, at least in part, on our ability to protect our core technology and intellectual property. To accomplish this, we rely
on trade secrets, including know-how, employee and third-party nondisclosure agreements and other contractual rights to establish and
protect our proprietary rights in our technology.
Seasonality
Colombia
and its vertical offering of microclimates is the ideal country for year-round growing and processing of all possible varieties of cannabis
in a natural, environmentally friendly manner.
Principal
Executive Offices
Our principal executive offices are located at 6605
Grand Montecito Pkwy, Suite 100, Las Vegas, Nevada 89149. Our telephone number is (800) 605-3210. We believe our facilities
are adequate to meet our current and near-term needs.
Employees
As
of December 31, 2023, we had 17 full-time employees. Since inception, we have never had a work stoppage, other than
due to the Covid-19 quarantine from March 2020 through May 25, 2020, and our employees are not represented by labor unions. We consider
our relationship with our employees to be positive.
DESCRIPTION
OF PROPERTY
Our
principal executive offices are located at 6605 Grand Montecito Pkwy, Suite 100, Las Vegas, Nevada 89149, Telephone
No.: (800) 605-3210. Our leased premises are utilized for corporate business offices. Our Nevada premises are subject to a month-to-month
lease agreement.
We
believe that our current facilities are adequate for our current needs. We intend to secure new facilities or expand existing facilities
as necessary to support future growth. We believe that suitable additional space will be available on commercially reasonable terms as
needed to accommodate our operations.
LEGAL
PROCEEDINGS
There
are no material pending legal proceedings to which we are a party or to which any of our property is subject, nor are there any such
proceedings known to be contemplated by governmental authorities. None of our directors, officers or affiliates is involved in a proceeding
adverse to our business or has a material interest adverse to our business.
DESCRIPTION
OF CAPITAL STOCK
The
following is a brief description of our capital stock. This summary does not purport to be complete in all respects. The brief description
is based upon our Articles of Incorporation, including the Certificate of Amendment to our Articles of Incorporation, (as amended, our
“Articles of Incorporation”), our Bylaws (our “Bylaws”), and provisions of applicable Nevada law. This summary
does not purport to be complete and is subject to, and qualified in its entirety by, the full text of our Articles of Incorporation and
Bylaws, copies of which have been filed with the SEC, and by Nevada law.
General
Our Articles of Incorporation authorizes us to issue
up to 310,000,000 shares of capital stock, consisting of 300,000,000 shares of common stock, par value $0.001 common stock (“common
stock”), and 10,000,000 shares of preferred stock, par value $0.001 per share, of which 500,000 shares have been designated Series
A Preferred Stock and 600,000 shares have been designated Series B Preferred Stock, with the remaining 8,900,000 shares of preferred
stock available for designation from time to time by the Board as set forth below. As of December 31, 2023, we had outstanding
79,827,618 shares of common stock, 99,733 shares of Series A Preferred Stock and 238,501 shares of Series B Preferred
Stock. Our Articles of Incorporation authorizes our Board of Directors (our “Board”) to determine any number of series into
which the undesignated shares of preferred stock may be divided and to determine, at any time and from time to time, the rights, preferences,
privileges and restrictions granted to any series of such preferred stock, as described below.
Common
Stock
Dividend
Rights
Subject
to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our Common Stock
are entitled to receive dividends out of funds legally available at the times and in the amounts that our board of directors may determine.
Voting
Rights
Each
holder of our Common Stock is entitled to one vote for each share of our Common Stock held on all matters submitted to a vote of stockholders.
Cumulative voting for the election of directors is not provided for in our articles of incorporation, as amended, which means that the
holders of a majority of the voting shares voted can elect all of the directors then standing for election.
No
Preemptive or Similar Rights
Holders
of our Common Stock do not have preemptive rights, and our Common Stock is not convertible or redeemable.
Right
to Receive Liquidation Distributions
Upon
our dissolution, liquidation or winding-up, the assets legally available for distribution to our stockholders are distributable ratably
among the holders of our Common Stock, subject to the preferential rights and payment of liquidation preferences, if any, on any outstanding
shares of preferred stock.
Preferred
Stock
Series
A Preferred Stock
Each
share of Series A Preferred Stock is currently convertible into one hundred shares of common stock. The conversion price is subject
to equitable adjustment in the event of stock splits and other adjustments in the Company’s capitalization, and is subject to reduction
to the price at which the Company sells common stock in the future, subject to customary exceptions. Additional terms of the Series A
Preferred Stock include the following:
● The
Series A Preferred Stock accrues dividends at the rate of 6% per annum, payable annually in cash or additional shares of Series A Preferred
Stock, at the Company’s election.
● Upon
the liquidation or dissolution of the Company, or any merger or sale of all or substantially all of the assets, the shares of Series
A Preferred Stock are entitled to receive, prior to any distribution to the holders of common stock, 100% of the purchase price per share
of Series A Preferred Stock plus all accrued but unpaid dividends.
● Each
share of Series A Preferred Stock carries a number of votes equal to the number of shares of common stock into which such Series A Preferred
Stock may then be converted. The Series A Preferred Stock generally will vote together with the common stock and not as a separate class.
Series
B Preferred Stock
Each
share of Series B Preferred Stock is currently convertible into 100 shares of common stock. The conversion price is subject to equitable
adjustment in the event of stock splits and other adjustments in the Company’s capitalization. Additional terms of the Series B
Preferred Stock include the following:
● The
shares of Series B Preferred Stock are entitled to dividends when, as and if declared by the Board as to the shares of the common stock
of the Company into which such Series B Preferred Stock may then be converted.
● Upon
the liquidation or dissolution of the Company, or any merger or sale of all or substantially all of the assets, the shares of Series
B Preferred Stock are entitled to receive, prior to any distribution to the holders of common stock, but after distributions to the holders
of Series A Preferred Stock, 100% of the purchase price per share of Series B Preferred Stock plus all accrued but unpaid dividends.
● Each
share of Series B Preferred Stock carries a number of votes equal to the number of shares of common stock into which such Series B Preferred
Stock may then be converted. The Series B Preferred Stock generally will vote together with the common stock and not as a separate class.
Blank
Check Preferred Stock
The
remaining 8,900,000 shares of preferred stock may be issued in series, and shall have such voting powers, full or limited, or no voting
powers, and such designations, preferences and relative participating, optional or other special rights, and qualifications, limitations
or restrictions thereof, as shall be stated and expressed in the resolution or resolutions providing for the issuance of such stock adopted
from time to time by the Board. The Board is expressly vested with the authority to determine and fix in the resolution or resolutions
providing for the issuances of preferred stock the voting powers, designations, preferences and rights, and the qualifications, limitations
or restrictions thereof, of each such series to the full extent now or hereafter permitted by the laws of the State of Nevada.
Anti-takeover
Provisions
Certain
provisions of our articles of incorporation, as amended, and Nevada law may have the effect of delaying, deferring or discouraging another
person from acquiring control of our company.
Nevada
Law
In
addition, Nevada has enacted the following legislation that may deter or frustrate takeovers of Nevada corporations:
Authorized
but Unissued Stock – The authorized but unissued shares of our Common Stock are available for future issuance without stockholder
approval. These additional shares may be used for a variety of corporate purposes, including future public offerings to raise additional
capital, corporate acquisitions and employee benefit plans. The existence of authorized but unissued shares of common stock may enable
our board of directors to issue shares of stock to persons friendly to existing management.
Evaluation
of Acquisition Proposals – The Nevada Revised Statutes expressly permit our board of directors, when evaluating any proposed
tender or exchange offer, any merger, consolidation or sale of substantially all of our assets, or any similar extraordinary transaction,
to consider all relevant factors including, without limitation, the social, legal, and economic effects on our employees, customers,
suppliers, and other relevant interest holders, and on the communities and geographical areas in which they operate. Our board of directors
may also consider the amount of consideration being offered in relation to the then current market price of our outstanding shares of
capital stock and our then current value in a freely negotiated transaction.
Control
Share Acquisitions – Nevada has adopted a control share acquisitions statute designed to afford stockholders of public corporations
in Nevada protection against acquisitions in which a person, entity or group seeks to gain voting control. With enumerated exceptions,
the statute provides that shares acquired within certain specific ranges will not possess voting rights in the election of directors
unless the voting rights are approved by a majority vote of the public corporation’s disinterested stockholders. Disinterested
shares are shares other than those owned by the acquiring person or by a member of a group with respect to a control share acquisition,
or by any officer of the corporation or any employee of the corporation who is also a director. The specific acquisition ranges that
trigger the statute are: acquisitions of shares possessing one-fifth or more but less than one-third of all voting power; acquisitions
of shares possessing one-third or more but less than a majority of all voting power; or acquisitions of shares possessing a majority
or more of all voting power. Under certain circumstances, the statute permits the acquiring person to call a special stockholders’
meeting for the purpose of considering the grant of voting rights to the holder of the control shares. The statute also enables a corporation
to provide for the redemption of control shares with no voting rights under certain circumstances.
Transfer
Agent and Registrar
The
transfer agent and registrar for our Common Stock is vStock Transfer, LLC. Its mailing address is 18 Lafayette Place, Woodmere, NY 11598,
its telephone number is (212) 828-8436, and its facsimile number is (646) 536-3179.
MANAGEMENT
Set
forth below are the present directors and executive officers of the Company as of December 31, 2023. There are no arrangements or understandings between
any of the directors, officers and other persons pursuant to which such person was selected as a director or an
officer.
| Name |
|
Age |
|
Position |
| Isiah Thomas, III |
|
62 |
|
Chief Executive Officer, Chairman of the Board |
| Dr. Kenneth Perego, II |
|
54 |
|
Vice Chairman of the Board |
| Terry L. Buffalo |
|
59 |
|
Director |
| Timothy Woods |
|
57 |
|
Chief Financial Officer |
| Joerg Sommer |
|
57 |
|
President |
Biographies
Set
forth below are brief accounts of the business experience of each director and executive officer of the Company.
Isiah
Thomas, III has been our Chief Executive Officer since June 2020, and our Chairman of the Board since December 2021. Mr. Thomas has
also been the Chairman and Chief Executive Officer of Isiah International, LLC, a holding company with interests in a diversified portfolio
of businesses, since 2011. Mr. Thomas also has been a Commentator and Analyst for NBA TV, since 2014, and Turner Sports, since 2012.
He previously served as the President & Alternate Governor of the New York Liberty of the Women’s National Basketball Association
from 2015 to February 2019, the Head Basketball Coach at Florida International University, from 2009 to 2012, the General Manager, President
of Basketball Operation and Head Coach of the New York Knicks of the National Basketball Association (“NBA”), from 2006 to
2008, the Head Coach of the Indiana Pacers of the NBA from 2000 to 2003, the Owner of the Continental Basketball Association from 1998
to 2000, Minority Owner & Executive Vice President of the Toronto Raptors of the NBA from 1994 to 1998 and point guard for the Detroit
Pistons of the NBA from 1981 to 1994. Mr. Thomas has served as a director of Get in Chicago, an organization focused on stopping gun
and related violence in Chicago, since 2013, and as a director of Madison Square Garden Entertainment Corp. since April 2020. He is also
the Founder of Mary’s Court Foundation, a charitable organization established in 2010. We believe that Mr. Thomas’s business
experience qualifies him to serve as our chairman and CEO.
Dr.
Kenneth Perego, II was a director of OWP Ventures prior to the Merger and was appointed to our Board of Directors pursuant to the
Merger Agreement, before being appointed Vice Chairman of the Board on December 7, 2021. He has been a practicing urologic surgeon in
private practice since 2001 with an emphasis in urologic oncology and reconstructive urology. He has a strong clinical background in
research and is focused on new drug discovery. We believe that Dr. Perego’s medical experience qualifies him to serve as our director.
Terry Buffalo was appointed to serve as
a director of One World Products on September 1, 2022. Mr. Buffalo established Buffalo Cannabis Advisors in 2022 and currently
serves as its President, was the Chief
Executive Officer and a director of American Cannabis Company from 2015 to 2021, and was the Chief Executive of First Midwest
Securities, Inc. from 2002 to 2013. Prior to that, Mr. Buffalo was the President of American Investment Services
from 1997 to 2002. In addition, Mr. Buffalo currently serves on the advisory board of Element Apothec since 2020. We believe that
Mr. Buffalo’s investment banking and cannabis management experience qualifies him to serve as our director.
Timothy
Woods was appointed to serve as the Company’s Chief Financial Officer on February 14, 2022. From 2015 until his appointment
as our Chief Financial Officer, Mr. Woods served as the Director of Business Development and General Sales Manager of Lithia Motors,
Inc., one of the largest automotive retailers in the United States. Prior to his tenure with Lithia Motors, Mr. Woods was the Chief Financial
Officer of Spend Consciously, a technology-based start-up. Mr. Woods also served as the Chief Financial Officer and energy services division
Vice President of Finance for WGL Holdings Inc., providing high-level financial functions and advanced reporting, including the generation
of quarterly and annual SEC filings, Sarbanes-Oxley compliance, and benefit plans. Earlier in his career, Mr. Woods was VP of Finance
for Freddie Mac; a divisional CFO & North American controller for Stanley Works; and assistant global controller for General Electric’s
Lighting Division. Mr. Woods holds a Bachelor of Business Administration in Accounting from Cleveland State University and is a graduate
of the GE Financial Management program. He has achieved multiple honors, including being named “Business Leader of the Year”
by the National Association of Black Accountants.
Joerg Sommer
was appointed to serve as the Company’s President on May 23, 2023. Mr. Sommer served on the Company’s Advisory Board
from August 2020 through May 23, 2023, and as a consultant to the Company from January 2021 to June 2022. Since July 2022, Mr. Sommer has been the
Managing Partner of peach ventures management GmbH, an investor in energy and mobility startups based in Berlin, Germany, and since
November 2021, he has served as a Venture Partner of bmp Ventures AG, a venture capital investment firm based in Berlin, Germany.
Previously, Mr. Summer was the Chief Operating Officer of StreetScooter of Aachen, German, from March 2019 until February 2020; and
the Chief Operating Officer of Chanje Energy Inc. of Los Angeles, CA, from March 2017 until February 2019. Prior to February 2019,
Mr. Sommer held various top management positions in the automotive industry with Mercedes, Renault Group and Volkswagen Group in
Europe and in the US. Mr. Sommer holds a Masters of Business Administration (M.B.A.) from the MIT Sloan School of Management, and
dual Masters of Mechanical Engineering and Business Administration from Technische Universität Berlin.
Director
Independence
Our
board of directors currently consists of Isiah Thomas, III, our Chief Executive Officer and Chairman, Dr. Kenneth Perego, II, our Vice
Chairman, and Terry L. Buffalo. As an executive officer, Mr. Thomas does not qualify as “independent” under standards of
independence set forth by national securities exchanges. Our Board of Directors has determined that Dr. Kenneth Perego, II and Terry
L. Buffalo are “independent” in accordance with the NASDAQ Global Market’s requirements. As our common stock is currently
quoted on the OTCQB, we are not currently subject to corporate governance standards of listed companies.
Board
Committees and Audit Committee Financial Expert
We
do not currently have a standing audit, nominating or compensation committee of the board of directors, or any committee performing similar
functions. Our board of directors performs the functions of audit, nominating and compensation committees. We believe that Mr. Buffalo
qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities
Act.
Code
of Ethics
We
have adopted a code of ethics that applies to our principal executive officers, principal financial officer, principal accounting officer
or controller, or persons performing similar functions. A copy of our code of ethics may be obtained free of charge by contacting us
at the address or telephone number listed on the cover page hereof.
EXECUTIVE
COMPENSATION
Summary Compensation Table
The
following summary compensation table sets forth the aggregate compensation we paid or accrued during the fiscal years ended December
31, 2023 and 2022 to Isiah Thomas, III, our Chief Executive Officer (our “Named Executive Officer”), who was
our only executive officer that received total compensation in excess of $100,000 during 2023.
| Name and | |
Fiscal | |
| | |
Option | | |
| |
| Financial
Position | |
Year | |
Salary | | |
Awards1 | | |
Total | |
| | |
| |
| | |
| | |
| |
| Isiah Thomas,
III, | |
2023 | |
$ | 120,000 | (1) | |
$ | - | | |
$ | 120,000 | |
| Chief Executive Officer and
Chairman | |
2022 | |
$ | 120,000 | (1) | |
$ | - | | |
$ | 120,000 | |
| | |
| |
| | | |
| | | |
| | |
| Timothy Woods, | |
2023 | |
$ | 90,000 | (2) | |
$ | - | | |
$ | 90,000 | |
| Chief Financial Officer
| |
2022 | |
$ | 78,750 | (2) | |
$ | - | | |
$ | 78,750 | |
| | |
| |
| | | |
| | | |
| | |
| Joerg Sommer | |
2023 | |
$ | 41,000 | (3) | |
$ | 89,850 | (4) | |
$ | 130,850 | |
| President | |
| |
| | | |
| | | |
| | |
(1) Consists of $120,000 and $120,000
of accrued salary for the years ended December 31, 2023 and 2022, respectively, not yet paid.
(2) Consists of $78,750 and $90,000 of accrued salary for the years ended December 31, 2023 and
2022, respectively, not yet paid. Mr. Woods’ employment agreement
provides for an annual base salary of $90,000.
(3) Consists of $41,000 of accrued
salary owed to Mr. Sommer from his appointment date in April 2023 through December 31, 2023, not yet paid. Mr. Sommer’s employment
agreement provides for an annual base salary of $60,000.
(4) On June 15, 2023, we awarded Mr.
Sommer 1,500,000 shares of common stock. The fair value of the common stock was $89,850, based on the closing stock price on the grant
date. The amounts reflected in this column represent the aggregate grant date fair value of options, computed in accordance with ASC
Topic 718.
Outstanding
Equity Awards at Fiscal Year End
As
of December 31, 2023, our Named Executive Officer had outstanding unexercised options as set forth below.
| Name | |
Number of securities underlying unexercised options (#) exercisable | | |
Number of securities underlying unexercised options (#) unexercisable | | |
Option Exercise Price ($) | | |
Option Expiration Date ($) (2) |
| | |
| | | |
| | | |
| | | |
|
| Isiah Thomas, III | |
| 5,500,000 | | |
| - | | |
$ | 0.13 | | |
June 30, 2030 |
On January 21, 2021, the Company granted Mr. Thomas
options to purchase 5,500,000 shares of our common stock, with half of the options vesting immediately on the grant date and the remaining
2,750,000 options vesting quarterly in increments of 250,000 options per quarter.
Director
Compensation
The Company’s non-employee directors, Dr.
Perego and Mr. Buffalo, did not receive any compensation for serving as directors during the year ended December 31, 2023.
However,
they are entitled to reimbursement for reasonable
travel and other out-of-pocket expenses incurred in connection with attendance at meetings of our board of directors.
As of December 31, 2023, Dr. Perego held
options to acquire 350,000 shares and, Mr. Buffalo did not own any outstanding options. As of December 31, 2023, no directors held any outstanding
restricted stock units or other stock awards.
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
Certain
Relationships and Related Party Transactions
Other
than the transactions described below, there has not been, nor is there currently proposed, any transaction or series of similar transactions
to which we were or will be a party:
| |
● |
in
which the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last
two completed fiscal years; and |
| |
|
|
| |
● |
in
which any director, executive officer, stockholders who beneficially owns more than 5% of our common stock or any member of their
immediate family had or will have a direct or indirect material interest. |
Advances
by and repayments to Dr. Kenneth Perego, II, M.D.
On September 11, 2023, the Company received an
advance of $52,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board, pursuant to an unsecured promissory note due on
demand that carried a 10% interest rate.
On August 31, 2023, the Company received an advance
of $4,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On August 14, 2023, the Company received an advance
of $6,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On August 5, 2022, the Company received an advance
of $50,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On July 7, 2022, the Company received an advance of
$5,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On May 5, 2022, the Company received an advance of
$20,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On December 29, 2021, the Company received an advance
of $200,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured promissory note due January 1, 2024 that carried an 8% interest
rate.
On September 14, 2020, the Company received an advance
of $26,000 from Dr. Kenneth Perego, II, M.D., pursuant to an unsecured demand note that carried a 6% interest rate. A total of $27,201,
consisting of $26,000 of principal and $1,201 of interest, was repaid on March 29, 2021.
Advances
by and Repayments to Isiah Thomas, III and Affiliated Entities
On August 2, 2022, the Company received an advance
of $4,500 from Isiah Thomas, III, our Chairman of the Board and CEO, pursuant to an unsecured promissory note due on demand that carried
a 6% interest rate.
On June 3, 2022, the Company received an advance of
$10,000 from Isiah Thomas, III pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On May 5, 2022, the Company received an advance of
$10,000 from Isiah Thomas, III pursuant to an unsecured promissory note due on demand that carried a 6% interest rate.
On October 19, 2021, a total of $52,918, consisting
of $50,000 of principal and $2,918 of interest, was repaid to Mr. Isiah Thomas, III in settlement of a demand note that had originated
on October 28, 2020.
On September 15, 2021, a total of $130,610, consisting
of $125,000 of principal and $5,610 of interest, was repaid to Mr. Isiah Thomas, III in settlement of a demand note that had originated
on December 16, 2020.
Series
B Preferred Stock Sales to Isiah Thomas, III
On
February 7, 2021, the Company and ISIAH International, LLC (“ISIAH International”), an entity controlled by Isiah Thomas, our CEO and Chairman of the Board, entered into a Securities Purchase
Agreement (the “Purchase Agreement”) under which ISIAH International agreed to purchase from the Company, on the dates provided
for in the Purchase Agreement, an aggregate of 200,000 shares of the Company’s newly designated Series B Preferred Stock (“Series
B Preferred Stock”), convertible into an aggregate of 20,000,000 shares of the Company’s common stock, for a purchase price
of $15 per share of Preferred Stock, and an aggregate purchase price of $3 million. Each share of Series B Preferred Stock has a Stated
Value of $15 and is convertible into common stock at a conversion price equal to $0.15. Isiah Thomas, the Company’s Chief Executive
Officer, is the sole member and Chief Executive Officer of ISIAH International. Pursuant to the Purchase Agreement, ISIAH International
purchased the 200,000 shares of Series B Preferred Stock from the Company according to the following schedule:
| Date | |
Shares | | |
Purchase Price | |
| Initial Closing Date | |
| 16,666 | | |
$ | 249,990 | |
| February 22, 2021 | |
| 16,667 | | |
| 250,005 | |
| March 8, 2021 | |
| 16,667 | | |
| 250,005 | |
| March 22, 2021 | |
| 16,667 | | |
| 250,005 | |
| April 5, 2021 | |
| 16,666 | | |
| 249,990 | |
| April 19, 2021 | |
| 16,667 | | |
| 250,005 | |
| May 17, 2021 | |
| 33,334 | | |
| 500,010 | |
| June 14, 2021 | |
| 33,333 | | |
| 499,995 | |
| July 12, 2021 | |
| 33,333 | | |
| 499,995 | |
| Total | |
| 200,000 | | |
$ | 3,000,000 | |
On
various dates in May, 2021, the Company also received total proceeds of $50,010 from the sale of an aggregate of 3,334 shares of Series
B Preferred Stock at a price of $15 per share to trusts whose beneficiaries are adult children of Isiah L. Thomas III. Mr. Thomas disclaims
beneficial ownership of the shares held by these trusts.
Debt and Common Stock Issued to Joerg
Sommers
On October 5, 2023, the Company received an advance of $25,000 from Joerg Sommers, our President, pursuant to an
unsecured promissory note due on demand that carried a 6% interest rate.
On June 15, 2023, the Company issued 1,500,000
shares of common stock to the Company’s President, Joerg Sommer, for services provided. The
aggregate fair value of the common stock was $89,850, based on the closing price of the Company’s common stock on the date of grant.
Common Stock Issued to John McCabe
On February 14, 2023, the Company sold 3,000,000
shares of common stock at a price of $0.10 per share to Dr. John McCabe, a greater than 10% stockholder, for total proceeds of $300,000.
DISCLOSURE
OF COMMISSION POSITION ON
INDEMNIFICATION FOR SECURITIES LIABILITIES
Our
articles of incorporation have eliminated our directors’ and officers’ personal liability for damages for breaches of fiduciary
duty but do not eliminate or limit the liability of a director officer for (a) acts or omissions which involve intentional misconduct,
fraud or a knowing violation of the law, or (b) the payment of dividends in violation of applicable law. The effect of this provision
of our articles of incorporation is to eliminate our rights and those of our stockholders to recover damages against a director or officer
for breach of the fiduciary duty of care as a director or officer (including breaches resulting from negligent or grossly negligent behavior),
except as provided above or under certain situations defined by statute. We believe that the indemnification provisions in our articles
of incorporation are necessary to attract and retain qualified persons as directors and officers.
Insofar
as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers or persons
controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification
is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
LEGAL
MATTERS
Certain
legal matters relating to the validity of our securities offered by this prospectus will be passed upon for us by Fox Rothschild LLP,
New York, York.
EXPERTS
Our
consolidated financial statements as of December 31, 2022 and 2021 and for the years then ended included in this prospectus
have been audited by M&K CPAS, PLLC, an independent registered public accounting firm, given on the authority of said firm as
experts in auditing and accounting.
AVAILABLE
INFORMATION
We
are filing with the SEC this registration statement on Form S-1 under the Securities Act with respect to the common stock offered hereby.
This prospectus, which constitutes part of the registration statement, does not contain all of the information set forth in the registration
statement and the exhibits and schedule thereto, certain parts of which are omitted in accordance with the rules and regulations of the
SEC. For further information regarding our common stock and our company, please review the registration statement, including exhibits,
schedules and reports filed as a part thereof. Statements in this prospectus as to the contents of any contract or other document filed
as an exhibit to the registration statement, set forth the material terms of such contract or other document but are not necessarily
complete, and in each instance, reference is made to the copy of such document filed as an exhibit to the registration statement,
each such statement being qualified in all respects by such reference.
We
file annual, quarterly, and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public over the Internet at the SEC’s web site at www.sec.gov and on the investor relations page of our website at www.oneworldproducts.com.
Information on our web site is not part of this prospectus. You may also read and copy any document we file with the SEC at its public
reference facilities at 100 F Street N.E., Washington, D.C. 20549. You can also obtain copies of the documents upon the payment of a
duplicating fee to the SEC. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
ONE
WORLD PRODUCTS, INC.
CONSOLIDATED
FINANCIAL STATEMENTS
Table
of Contents
| |
|
Page |
| Condensed Consolidated Balance Sheets as of September 30, 2023 (Unaudited) and December 31, 2022 |
|
F-2 |
| |
|
|
| Condensed Consolidated Statements of Operations and Comprehensive Loss for the Three and Nine Months Ended September 30, 2023 and 2022 (Unaudited) |
|
F-3 |
| |
|
|
| Consolidated Statements of Changes in Stockholders’ Equity (Deficit) for the Three and Nine Months Ended September 30, 2023 and 2022 (Unaudited) |
|
F-4 |
| |
|
|
| Condensed Consolidated Statements of Cash Flows for the Nine Months Ended September 30, 2023 and 2022 (Unaudited) |
|
F-6 |
| |
|
|
| Notes to the Condensed Consolidated Financial Statements |
|
F-7 |
| |
|
|
| Report of Independent Registered Public Accounting Firm, M&K CPAS, PLLC (PCAOB ID: 2738) |
|
F-21 |
| |
|
|
| Consolidated
Balance Sheets at December 31, 2022 and December 31, 2021 |
|
F-22 |
| |
|
|
| Consolidated
Statements of Operations and Comprehensive Loss for the years ended December 31, 2022 and 2021 |
|
F-23 |
| |
|
|
| Consolidated
Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2022 and 2021 |
|
F-24 |
| |
|
|
| Consolidated
Statements of Cash Flows for the years ended December 31, 2022 and 2021 |
|
F-25 |
| |
|
|
| Notes to the Consolidated Financial Statements |
|
F-26 |
ONE
WORLD PRODUCTS, INC.
CONDENSED
CONSOLIDATED BALANCE SHEETS
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| | |
(Unaudited) | | |
| |
| Assets | |
| | | |
| | |
| | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 10,152 | | |
$ | 11,016 | |
| Accounts receivable | |
| 12,488 | | |
| 12,355 | |
| Inventory | |
| 312,736 | | |
| 54,153 | |
| Other current assets | |
| 25,724 | | |
| 45,943 | |
| Total current assets | |
| 361,100 | | |
| 123,467 | |
| | |
| | | |
| | |
| Other assets | |
| 213,593 | | |
| 179,927 | |
| Right-of-use assets | |
| - | | |
| 425,969 | |
| Security deposits | |
| 85,000 | | |
| 1,449,808 | |
| Fixed assets, net | |
| 2,367,417 | | |
| 988,536 | |
| | |
| | | |
| | |
| Total Assets | |
$ | 3,027,110 | | |
$ | 3,167,707 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Equity (Deficit) | |
| | | |
| | |
| | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 815,841 | | |
$ | 798,067 | |
| Accrued expenses | |
| 1,238,479 | | |
| 948,458 | |
| Deferred revenues | |
| 11,545 | | |
| 11,808 | |
| Dividends payable | |
| 181,651 | | |
| 137,843 | |
| Current portion of lease liabilities | |
| - | | |
| 86,235 | |
| Convertible notes payable, net of $-0- and $412,673 of debt discounts at December 31, 2022 and 2021, respectively | |
| | | |
| | |
| Convertible note payable, related party, current maturities | |
| 750,000 | | |
| - | |
| Notes payable, related parties, current maturities | |
| 1,061,500 | | |
| 99,500 | |
| Notes payable, net of $53,442 of debt discounts at September 30, 2023 | |
| 454,237 | | |
| 145,524 | |
| Notes payable | |
| 454,237 | | |
| 145,524 | |
| Total current liabilities | |
| 4,513,253 | | |
| 2,227,435 | |
| | |
| | | |
| | |
| Long-term lease liability | |
| - | | |
| 341,680 | |
| Notes payable, long-term portion | |
| | | |
| | |
| Convertible note payable, related party | |
| - | | |
| 750,000 | |
| Notes payable, related parties, long-term portion | |
| - | | |
| 900,000 | |
| | |
| | | |
| | |
| Total Liabilities | |
| 4,513,253 | | |
| 4,219,115 | |
| | |
| | | |
| | |
| Series A convertible preferred stock, $0.001 par value, 500,000 shares authorized; 99,733 and | |
| | | |
| | |
| 70,233 shares issued and outstanding at September 30, 2023 and December 31, 2022, respectively | |
| 997,330 | | |
| 702,330 | |
| Series B convertible preferred stock, $0.001 par value, 300,000 shares authorized; 248,501 and | |
| | | |
| | |
| 272,168 shares issued and outstanding at September 30, 2023 and December 31, 2022, respectively | |
| 3,727,515 | | |
| 4,082,520 | |
| Convertible
preferred stock value | |
| 3,727,515 | | |
| 4,082,520 | |
| | |
| | | |
| | |
| Stockholders’ Equity (Deficit): | |
| | | |
| | |
| Preferred stock, $0.001 par value, 9,200,000 shares authorized; no shares issued and outstanding at
September 30, 2023 and December 31, 2022, respectively | |
| - | | |
| - | |
| Common stock, $0.001 par value, 300,000,000 shares authorized; 76,736,274 and 67,202,907 shares issued
and outstanding at September 30, 2023 and December 31, 2022, respectively | |
| 76,736 | | |
| 67,203 | |
| Additional paid-in capital | |
| 18,062,344 | | |
| 17,123,603 | |
| Subscriptions payable | |
| 45,000 | | |
| - | |
| Accumulated other comprehensive income (loss) | |
| 94,671 | | |
| (50,699 | ) |
| Accumulated (deficit) | |
| (24,489,739 | ) | |
| (22,976,365 | ) |
| Total Stockholders’ Equity (Deficit) | |
| (6,210,988 | ) | |
| (5,836,258 | ) |
| | |
| | | |
| | |
| Total Liabilities and Stockholders’ Equity (Deficit) | |
$ | 3,027,110 | | |
$ | 3,167,707 | |
See
accompanying notes to financial statements.
ONE
WORLD PRODUCTS, INC.
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Unaudited)
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
For the Three Months Ended | | |
For the Nine Months Ended | |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | 5,906 | | |
$ | 33,373 | | |
$ | 8,082 | | |
$ | 76,384 | |
| Cost of goods sold | |
| 767 | | |
| 23,969 | | |
| 1,866 | | |
| 54,765 | |
| Gross profit (loss) | |
| 5,139 | | |
| 9,404 | | |
| 6,216 | | |
| 21,619 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
| 142,464 | | |
| 378,910 | | |
| 907,595 | | |
| 1,148,100 | |
| Professional fees | |
| 236,139 | | |
| 95,946 | | |
| 413,651 | | |
| 380,801 | |
| Depreciation expense | |
| 9,274 | | |
| 9,883 | | |
| 25,578 | | |
| 34,540 | |
| Total operating expenses | |
| 387,877 | | |
| 484,739 | | |
| 1,346,824 | | |
| 1,563,441 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating loss | |
| (382,738 | ) | |
| (475,335 | ) | |
| (1,340,608 | ) | |
| (1,541,822 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Sublease income | |
| - | | |
| - | | |
| - | | |
| 1,000 | |
| Loss on sale of fixed assets | |
| - | | |
| (9,041 | ) | |
| - | | |
| (9,041 | ) |
| Gain on early extinguishment of lease | |
| - | | |
| 20,148 | | |
| 4,397 | | |
| 20,148 | |
| Gain on forgiveness of PPP loan | |
| | | |
| | | |
| | | |
| | |
| Gain on early extinguishment of debt | |
| - | | |
| - | | |
| - | | |
| 121,372 | |
| Interest income | |
| 3 | | |
| - | | |
| 6 | | |
| 41 | |
| Interest expense | |
| (67,571 | ) | |
| (529,915 | ) | |
| (177,169 | ) | |
| (886,837 | ) |
| Total other expense | |
| (67,568 | ) | |
| (518,808 | ) | |
| (172,766 | ) | |
| (753,317 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
$ | (450,306 | ) | |
$ | (994,143 | ) | |
$ | (1,513,374 | ) | |
$ | (2,295,139 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other comprehensive loss: | |
| | | |
| | | |
| | | |
| | |
| Gain (loss) on foreign currency translation | |
$ | (32,831 | ) | |
$ | 2,334 | | |
$ | 145,370 | | |
$ | 6,806 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net other comprehensive loss | |
$ | (483,137 | ) | |
$ | (991,809 | ) | |
$ | (1,368,004 | ) | |
$ | (2,288,333 | ) |
| Series A convertible preferred stock declared ($0.60 per share) | |
| (15,083 | ) | |
| (9,866 | ) | |
| (43,808 | ) | |
| (28,971 | ) |
| Net loss attributable to common shareholders | |
$ | (498,220 | ) | |
$ | (1,001,675 | ) | |
$ | (1,411,812 | ) | |
$ | (2,317,304 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average number of common shares | |
| | | |
| | | |
| | | |
| | |
| outstanding - basic and diluted | |
| 74,958,373 | | |
| 66,080,317 | | |
| 71,436,129 | | |
| 65,850,852 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.01 | ) | |
$ | (0.02 | ) | |
$ | (0.02 | ) | |
$ | (0.04 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Dividends declared per share of common stock | |
$ | 0.00 | | |
$ | 0.00 | | |
$ | 0.00 | | |
$ | 0.00 | |
See
accompanying notes to financial statements.
ONE
WORLD PRODUCTS, INC.
CONSOLIDATED
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(Unaudited)
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Payable | | |
Income (Loss) | | |
Deficit | | |
Equity (Deficit) | |
| | |
For the Three Months Ended
September 30, 2023 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
Series A Convertible | | |
Series B Convertible | | |
| | |
| | |
Additional | | |
| | |
Other | | |
| | |
Total | |
| | |
Preferred Stock | | |
Preferred Stock | | |
Common Stock | | |
Paid-In | | |
Subscriptions | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Payable | | |
Income (Loss) | | |
Deficit | | |
Equity (Deficit) | |
| Balance, June 30, 2023 | |
| 99,733 | | |
$ | 997,330 | | |
| 272,168 | | |
$ | 4,082,520 | | |
| 73,369,574 | | |
$ | 73,370 | | |
$ | 17,594,074 | | |
$ | - | | |
$ | 127,502 | | |
$ | (24,039,433 | ) | |
$ | (6,244,487 | ) |
| Series A preferred stock to be issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 45,000 | | |
| - | | |
| - | | |
| 45,000 | |
| Series B preferred stock conversions | |
| - | | |
| - | | |
| (23,667 | ) | |
| (355,005 | ) | |
| 2,366,700 | | |
| 2,366 | | |
| 352,639 | | |
| - | | |
| - | | |
| - | | |
| 355,005 | |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,000,000 | | |
| 1,000 | | |
| 83,000 | | |
| - | | |
| - | | |
| - | | |
| 84,000 | |
| Amortization of common stock options issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 47,714 | | |
| - | | |
| - | | |
| - | | |
| 47,714 | |
| Series A convertible preferred stock dividend declared ($0.60 per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (15,083 | ) | |
| - | | |
| - | | |
| - | | |
| (15,083 | ) |
| Loss on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (32,831 | ) | |
| - | | |
| (32,831 | ) |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (450,306 | ) | |
| (450,306 | ) |
| Balance, September 30, 2023 | |
| 99,733 | | |
$ | 997,330 | | |
| 248,501 | | |
$ | 3,727,515 | | |
| 76,736,274 | | |
$ | 76,736 | | |
$ | 18,062,344 | | |
$ | 45,000 | | |
$ | 94,671 | | |
$ | (24,489,739 | ) | |
$ | (6,210,988 | ) |
| | |
For the Three Months Ended
September 30, 2022 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
Series A Convertible | | |
Series B Convertible | | |
| | |
| | |
Additional | | |
| | |
Other | | |
| | |
Total | |
| | |
Preferred Stock | | |
Preferred Stock | | |
Common Stock | | |
Paid-In | | |
Subscriptions | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Payable | | |
Income (Loss) | | |
Deficit | | |
Equity (Deficit) | |
| Balance, June 30, 2022 | |
| 65,233 | | |
$ | 652,330 | | |
| 238,501 | | |
$ | 3,577,515 | | |
| 65,861,631 | | |
$ | 65,862 | | |
$ | 16,928,274 | | |
$ | - | | |
$ | (59,875 | ) | |
$ | (21,217,884 | ) | |
$ | (4,283,623 | ) |
| Series B Convertible Preferred Stock sold for cash | |
| - | | |
| - | | |
| 10,000 | | |
| 150,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Series B Convertible Preferred Stock issued as a commitment fee on ELOC | |
| - | | |
| - | | |
| 13,667 | | |
| 205,005 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,341,276 | | |
| 1,341 | | |
| 132,787 | | |
| - | | |
| - | | |
| - | | |
| 134,128 | |
| Amortization of common stock options issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 41,180 | | |
| - | | |
| - | | |
| - | | |
| 41,180 | |
| Series A convertible preferred stock dividend declared ($0.60 per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (9,866 | ) | |
| - | | |
| - | | |
| - | | |
| (9,866 | ) |
| Gain on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,334 | | |
| - | | |
| 2,334 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (994,143 | ) | |
| (994,143 | ) |
| Balance, September 30, 2022 | |
| 65,233 | | |
$ | 652,330 | | |
| 262,168 | | |
$ | 3,932,520 | | |
| 67,202,907 | | |
$ | 67,203 | | |
$ | 17,092,375 | | |
$ | - | | |
$ | (57,541 | ) | |
$ | (22,212,027 | ) | |
$ | (5,109,990 | ) |
| | |
For the Nine Months Ended
September 30, 2023 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
Series A Convertible | | |
Series B Convertible | | |
| | |
| | |
Additional | | |
| | |
Other | | |
| | |
Total | |
| | |
Preferred Stock | | |
Preferred Stock | | |
Common Stock | | |
Paid-In | | |
Subscriptions | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Payable | | |
Income (Loss) | | |
Deficit | | |
Equity (Deficit) | |
| Balance, December 31, 2022 | |
| 70,233 | | |
$ | 702,330 | | |
| 272,168 | | |
$ | 4,082,520 | | |
| 67,202,907 | | |
$ | 67,203 | | |
$ | 17,123,603 | | |
$ | - | | |
$ | (50,699 | ) | |
$ | (22,976,365 | ) | |
$ | (5,836,258 | ) |
| Series A Convertible Preferred Stock sold for cash | |
| 25,000 | | |
| 250,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Series A Convertible Preferred Stock issued for services | |
| 4,500 | | |
| 45,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 45,000 | | |
| - | | |
| - | | |
| 45,000 | |
| Series B preferred stock conversions | |
| - | | |
| - | | |
| (23,667 | ) | |
| (355,005 | ) | |
| 2,366,700 | | |
| 2,366 | | |
| 352,639 | | |
| - | | |
| - | | |
| - | | |
| 355,005 | |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,500,000 | | |
| 2,500 | | |
| 171,350 | | |
| - | | |
| - | | |
| - | | |
| 173,850 | |
| Commitment shares issued pursuant to promissory note | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,666,667 | | |
| 1,667 | | |
| 40,508 | | |
| - | | |
| - | | |
| - | | |
| 42,175 | |
| Common stock sold for cash | |
| - | | |
| - | | |
| - | | |
| - | | |
| 3,000,000 | | |
| 3,000 | | |
| 297,000 | | |
| - | | |
| - | | |
| - | | |
| 300,000 | |
| Amortization of common stock options issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 121,052 | | |
| - | | |
| - | | |
| - | | |
| 121,052 | |
| Series A convertible preferred stock dividend declared ($0.60 per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (43,808 | ) | |
| - | | |
| - | | |
| - | | |
| (43,808 | ) |
| Loss on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 145,370 | | |
| - | | |
| 145,370 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,513,374 | ) | |
| (1,513,374 | ) |
| Balance, September 30, 2023 | |
| 99,733 | | |
$ | 997,330 | | |
| 248,501 | | |
$ | 3,727,515 | | |
| 76,736,274 | | |
$ | 76,736 | | |
$ | 18,062,344 | | |
$ | 45,000 | | |
$ | 94,671 | | |
$ | (24,489,739 | ) | |
$ | (6,210,988 | ) |
| | |
For the Nine Months Ended
September 30, 2022 | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
Series A Convertible | | |
Series B Convertible | | |
| | |
| | |
Additional | | |
| | |
Other | | |
| | |
Total | |
| | |
Preferred Stock | | |
Preferred Stock | | |
Common Stock | | |
Paid-In | | |
Subscriptions | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Payable | | |
Income (Loss) | | |
Deficit | | |
Equity (Deficit) | |
| Balance, December 31, 2021 | |
| 65,233 | | |
$ | 652,330 | | |
| 238,501 | | |
$ | 3,577,515 | | |
| 65,599,565 | | |
$ | 65,600 | | |
$ | 16,843,656 | | |
$ | 21,725 | | |
$ | (64,347 | ) | |
$ | (19,916,888 | ) | |
$ | (3,050,254 | ) |
| Balance | |
| 65,233 | | |
$ | 652,330 | | |
| 238,501 | | |
$ | 3,577,515 | | |
| 65,599,565 | | |
$ | 65,600 | | |
$ | 16,843,656 | | |
$ | 21,725 | | |
$ | (64,347 | ) | |
$ | (19,916,888 | ) | |
$ | (3,050,254 | ) |
| Series B Convertible Preferred Stock sold for cash | |
| - | | |
| - | | |
| 10,000 | | |
| 150,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Series B Convertible Preferred Stock issued as a commitment fee on ELOC | |
| - | | |
| - | | |
| 13,667 | | |
| 205,005 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Common stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,603,342 | | |
| 1,603 | | |
| 154,250 | | |
| (21,725 | ) | |
| - | | |
| - | | |
| 134,128 | |
| Amortization of common stock options issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 123,440 | | |
| - | | |
| - | | |
| - | | |
| 123,440 | |
| Series A convertible preferred stock dividend declared ($0.60 per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (28,971 | ) | |
| - | | |
| - | | |
| - | | |
| (28,971 | ) |
| Gain on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 6,806 | | |
| - | | |
| 6,806 | |
| Gain on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 6,806 | | |
| - | | |
| 6,806 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,295,139 | ) | |
| (2,295,139 | ) |
| Balance, September 30, 2022 | |
| 65,233 | | |
$ | 652,330 | | |
| 262,168 | | |
$ | 3,932,520 | | |
| 67,202,907 | | |
$ | 67,203 | | |
$ | 17,092,375 | | |
$ | - | | |
$ | (57,541 | ) | |
$ | (22,212,027 | ) | |
$ | (5,109,990 | ) |
| Balance | |
| 65,233 | | |
$ | 652,330 | | |
| 262,168 | | |
$ | 3,932,520 | | |
| 67,202,907 | | |
$ | 67,203 | | |
$ | 17,092,375 | | |
$ | - | | |
$ | (57,541 | ) | |
$ | (22,212,027 | ) | |
$ | (5,109,990 | ) |
See
accompanying notes to financial statements.
ONE
WORLD PRODUCTS, INC.
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| | |
2023 | | |
2022 | |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Cash flows from operating activities | |
| | | |
| | |
| Net loss | |
$ | (1,513,374 | ) | |
$ | (2,295,139 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Bad debts expense | |
| | | |
| | |
| Depreciation and amortization expense | |
| 25,578 | | |
| 34,540 | |
| Loss on disposal of fixed assets | |
| - | | |
| 9,041 | |
| Gain on early extinguishment of lease | |
| (4,397 | ) | |
| (20,148 | ) |
| Gain on forgiveness of PPP loan | |
| | | |
| | |
| Gain on early extinguishment of debt | |
| - | | |
| (121,372 | ) |
| Amortization of debt discounts | |
| 26,233 | | |
| 412,673 | |
| Stock-based compensation | |
| | | |
| | |
| Amortization of options issued for services | |
| | | |
| | |
| Series A preferred stock issued for services | |
| 90,000 | | |
| - | |
| Common stock issued for services | |
| 173,850 | | |
| 339,133 | |
| Stock options issued for services | |
| 121,052 | | |
| 123,440 | |
| Decrease (increase) in assets: | |
| | | |
| | |
| Accounts receivable | |
| (133 | ) | |
| (11,216 | ) |
| Inventory | |
| (258,583 | ) | |
| (120,625 | ) |
| Other current assets | |
| 20,219 | | |
| 109,578 | |
| Other assets | |
| (33,666 | ) | |
| (33,327 | ) |
| Right-of-use assets | |
| 34,391 | | |
| 84,667 | |
| Security deposits | |
| - | | |
| (194,020 | ) |
| Increase (decrease) in liabilities: | |
| | | |
| | |
| Accounts payable | |
| 17,774 | | |
| 259,049 | |
| Accrued expenses | |
| 290,021 | | |
| 357,650 | |
| Deferred revenues | |
| (263 | ) | |
| 5,176 | |
| Lease liability | |
| (31,940 | ) | |
| (64,067 | ) |
| Net cash used in operating activities | |
| (1,043,238 | ) | |
| (1,124,967 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities | |
| | | |
| | |
| Proceeds received on sale of fixed assets | |
| - | | |
| 6,350 | |
| Purchase of fixed assets | |
| (5,046 | ) | |
| (43,201 | ) |
| Net cash used in investing activities | |
| (5,046 | ) | |
| (36,851 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities | |
| | | |
| | |
| Proceeds received on convertible note payable | |
| - | | |
| 750,000 | |
| Repayment of convertible note payable | |
| - | | |
| (750,000 | ) |
| Proceeds from notes payable, related parties | |
| 62,000 | | |
| 99,500 | |
| Proceeds from notes payable | |
| 262,500 | | |
| 868,081 | |
| Repayment of notes payable | |
| | | |
| | |
| Proceeds from sale of preferred and common stock | |
| 550,000 | | |
| 150,000 | |
| Net cash provided by financing activities | |
| 874,500 | | |
| 1,117,581 | |
| | |
| | | |
| | |
| Effect of exchange rate changes on cash | |
| 172,920 | | |
| (6,820 | ) |
| | |
| | | |
| | |
| Net increase (decrease) in cash | |
| (864 | ) | |
| (51,057 | ) |
| Cash - beginning | |
| 11,016 | | |
| 119,678 | |
| Cash - ending | |
$ | 10,152 | | |
$ | 68,621 | |
| | |
| | | |
| | |
| Supplemental disclosures: | |
| | | |
| | |
| Interest paid | |
$ | 40,693 | | |
$ | 79,269 | |
| Income taxes paid | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-cash investing and financing transactions: | |
| | | |
| | |
| Initial recognition of right-of-use assets and lease liabilities | |
| | | |
| | |
| Cost of preferred shares exchanged for conversion to common
stock | |
| | | |
| | |
| Dividends payable | |
$ | 43,808 | | |
$ | 28,971 | |
| Initial recognition of right-of-use assets and lease liabilities | |
$ | - | | |
$ | 1,535,706 | |
| Deposit on equipment settled with note payable | |
$ | 35,000 | | |
$ | - | |
| Value of debt discounts attributable to commitment shares | |
$ | 42,175 | | |
$ | - | |
See
accompanying notes to financial statements.
ONE
WORLD PRODUCTS, INC.
Notes
to Condensed Consolidated Financial Statements
(Unaudited)
Note
1 – Nature of Business and Significant Accounting Policies
Nature
of Business
One
World Products, Inc. (the “Company,” “we,” “our” or “us”) was incorporated in Nevada
on September 2, 2014. On February 21, 2019, we entered into an Agreement and Plan of Merger with OWP Merger Subsidiary, Inc., our wholly-owned
subsidiary, and OWP Ventures, Inc. (“OWP Ventures”), which is the parent company of One World Pharma SAS, a Colombian company
(“OWP Colombia”). Pursuant to the Merger Agreement, we acquired OWP Ventures (and indirectly, OWP Colombia) by the merger
of OWP Merger Subsidiary with and into OWP Ventures, with OWP Ventures being the surviving entity as our wholly-owned subsidiary (the
“Merger”). As a result of the Merger (a) holders of the outstanding capital stock of OWP Ventures received an aggregate of
39,475,398 shares of our common stock; (b) options to purchase 825,000 shares of common stock of OWP Ventures at an exercise price of
$0.50 automatically converted into options to purchase 825,000 shares of our common stock at an exercise price of $0.50; (c) the outstanding
principal and interest under a $300,000 convertible note issued by OWP Ventures became convertible, at the option of the holder, into
shares of our common stock at a conversion price equal to the lesser of $0.424 per share or 80% of the price we sell our common stock
in a future “Qualified Offering”; (d) 875,000 shares of our common stock owned by OWP Ventures prior to the Merger were cancelled;
and (e) OWP Ventures’ chief operating officer became our chief operating officer and two of OWP Ventures’ directors became
members of our board of directors. The Company’s headquarters are located in Las Vegas, Nevada, and all of its customers are expected
to be outside of the United States. On January 10, 2019, the Company changed its name from Punto Group, Corp. to One World Pharma, Inc.,
and on November 23, 2021, the Company changed its name to One World Products, Inc. through the merger of One World Products, Inc., a
recently formed Nevada corporation wholly-owned by the Company, with and into the Company (the “Name Change Merger”) pursuant
to the applicable provisions of the Nevada Revised Statutes (“NRS”). As permitted by the NRS, the articles of merger filed
with the Secretary of State of the state of Nevada to effect the Name Change Merger amended Article I of the Company’s Articles
of Incorporation to change the Company’s name to “One World Products, Inc.” The Name Change Merger was effected solely
to effect the change of the Company’s name, and had no effect on the Company’s officers, directors, operations, assets or
liabilities.
OWP
Ventures is a holding company formed in Delaware on March 27, 2018 to enter and support the cannabis industry, and on May 30, 2018,
it acquired OWP Colombia. OWP Colombia is a licensed cannabis cultivation, production and distribution (export) company located in
Popayán, Colombia (nearest major city is Cali). We plan to be a producer of raw cannabis and hemp plant ingredients for both
medical and industrial uses across the globe. We have received licenses to cultivate, produce and distribute the raw ingredients of
the cannabis and hemp plant for medicinal, scientific and industrial purposes. Specifically, we are one of the few companies in
Colombia to receive all four licenses, including seed use, cultivation of non-psychoactive cannabis, cultivation of psychoactive
cannabis, and manufacturing allowing for extraction and export. Currently, we own approximately 30 acres and have a covered
greenhouse built specifically to cultivate high-grade cannabis and hemp. In addition, we have entered into agreements with local
farming cooperatives that include small farmers and indigenous tribe members, under which they will cultivate cannabis on up to
approximately 140 acres of land using our seeds and propagation techniques, and sell their harvested products to us on an exclusive
basis. We began harvesting cannabis in the first quarter of 2019 for the purpose of further research and development activities,
quality control testing and extraction. We have been generating revenue from the sale of our seeds since the second quarter of 2020.
During the first quarter of 2022, we made payments of approximately $1,400,000 for
a state-of-the-art distillation machine that was placed in service during the second quarter of 2023 within our vertically
integrated extraction facility.
Basis
of Presentation
The
accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the
United States of America (U.S. GAAP) and the rules of the Securities and Exchange Commission (SEC). Intercompany accounts and transactions
have been eliminated.
The
unaudited condensed consolidated financial statements of the Company and the accompanying notes included in this Quarterly Report on
Form 10-Q are unaudited. In the opinion of management, all adjustments necessary for a fair presentation of the Condensed Consolidated
Financial Statements have been included. Such adjustments are of a normal, recurring nature. The Condensed Consolidated Financial Statements,
and the accompanying notes, are prepared in accordance with GAAP and do not contain certain information included in the Company’s
Annual Report on Form 10-K for the fiscal year ended December 31, 2022. The interim Condensed Consolidated Financial Statements should
be read in conjunction with that Annual Report on Form 10-K. Results for the interim periods presented are not necessarily indicative
of the results that might be expected for the entire fiscal year.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Principles
of Consolidation
The
accompanying consolidated financial statements include the accounts of the following entities, all of which were under common control
and ownership at September 30, 2023:
Schedule
of Common Control and Ownership Interest
| |
|
State
of |
|
|
| Name
of Entity |
|
Incorporation |
|
Relationship |
| One
World Products, Inc.(1) |
|
Nevada |
|
Parent |
| OWP
Ventures, Inc.(2) |
|
Delaware |
|
Subsidiary |
| One
World Pharma S.A.S.(3) |
|
Colombia |
|
Subsidiary |
| Colombian
Hope, S.A.S.(4) |
|
Colombia |
|
Subsidiary |
| Agrobase,
S.A.S.(5) |
|
Colombia |
|
Subsidiary |
| (1) |
Holding company in the form of a corporation. |
| (2) |
Holding company in the form of a corporation and wholly-owned subsidiary of One World Products, Inc. |
| (3) |
Wholly-owned subsidiary of OWP Ventures, Inc. since May 30, 2018, located in Colombia and legally constituted as a simplified stock company registered in the Chamber of Commerce of Bogotá on July 18, 2017. Its headquarters are located in Bogotá. |
| (4) |
Wholly-owned subsidiary of OWP Ventures, Inc., acquired
on November 19, 2019, located in Colombia and legally constituted as a simplified stock company. This company has yet to incur any
substantive income or expenses. |
| (5) |
Wholly-owned subsidiary of OWP Ventures, Inc., formed
on September 12, 2019, located in Colombia and legally constituted as a simplified stock company. This company commenced operations
during 2023. |
The
consolidated financial statements herein contain the operations of the wholly-owned subsidiaries listed above. The Company’s headquarters
are located in Las Vegas, Nevada and substantially all of its production efforts are within Popayán, Colombia.
Reclassifications
Certain
reclassifications have been made to the prior years’ financial statements to conform to current year presentation. These reclassifications
had no effect on previously reported results of operations or retained earnings.
Foreign
Currency Translation
The
functional currency of the Company is Colombian Peso (COP). The Company has maintained its financial statements using the functional
currency, and translated those financial statements to the US Dollar (USD) throughout this report. Monetary assets and liabilities denominated
in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance
sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at
the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are
included in the determination of net income (loss) for the respective periods.
Comprehensive
Income
The
Company has adopted the Financial Accounting Standards Boards (“FASB”) Accounting Standards Codification (“ASC”)
220, Reporting Comprehensive Income, which establishes standards for reporting and displaying comprehensive income, its components, and
accumulated balances in a full-set of general-purpose financial statements. Accumulated other comprehensive income represents the accumulated
balance of foreign currency translation adjustments.
Use
of Estimates
The
preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires
management to make estimates and assumptions that may affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting
period. Actual results could differ from these estimates.
Segment
Reporting
ASC
Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment reporting. The management
approach model is based on the way a company’s management organizes segments within the company for making operating decisions
and assessing performance. The Company operates as a single segment and will evaluate additional segment disclosure requirements as it
expands its operations.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Fair
Value of Financial Instruments
The
Company discloses the fair value of certain assets and liabilities in accordance with ASC 820 – Fair Value Measurement and Disclosures
(ASC 820). Under ASC 820-10-05, the FASB establishes a framework for measuring fair value in generally accepted accounting principles
and expands disclosures about fair value measurements. This Statement reaffirms that fair value is the relevant measurement attribute.
The adoption of this standard did not have a material effect on the Company’s financial statements as reflected herein. The carrying
amounts of cash, accounts receivable, accounts payable and accrued expenses reported on the balance sheets are estimated by management
to approximate fair value primarily due to the short-term nature of the instruments.
Cash
in Excess of FDIC Insured Limits
The
Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. Accounts are guaranteed by
the Federal Deposit Insurance Corporation (FDIC) up to $250,000, under current regulations. The Company did not have any cash in excess
of FDIC insured limits at September 30, 2023, and has not experienced any losses in such accounts.
Revenue
Recognition
The
Company recognizes revenue in accordance with ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes
revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies by applying the following
steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction
price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance
obligation is satisfied. The Company’s sales to date have primarily consisted of the sale of seeds. These sales include multi-element
arrangements whereby the Company collects 50% of the sale upon delivery of the sales, and the remaining 50% upon the completion of the
harvest, whether the seeds result in a successful crop, or not. In addition, the Company has a right of first refusal to purchase products
resulting from the harvest. At September 30, 2023, the Company had $11,545 of deferred revenues and $6,760 of deferred cost of goods
sold, as included in other current assets on the balance sheet, that are expected to be recognized upon the customers’ completion
of their future harvests.
Inventory
Inventories
are stated at the lower of cost or net realizable value. Cost is determined on a standard cost basis that approximates the first-in,
first-out (FIFO) method. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating
net realizable value. Our cannabis products consist of cannabis flower grown in-house, along with produced extracts.
Stock-Based
Compensation
The
Company accounts for equity instruments issued to employees and non-employees in accordance with the provisions of ASC 718 Stock Compensation
(ASC 718). All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted
for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably
measurable. The measurement date of the fair value of the equity instrument issued is the earlier of the date on which the counterparty’s
performance is complete or the date at which a commitment for performance by the counterparty to earn the equity instruments is reached
because of sufficiently large disincentives for nonperformance.
Basic
and Diluted Loss Per Share
The
basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding. Diluted
net loss per common share is computed by dividing the net loss adjusted on an “as if converted” basis, by the weighted average
number of common shares outstanding plus potential dilutive securities. For the periods presented, potential dilutive securities had
an anti-dilutive effect and were not included in the calculation of diluted net loss per common share.
Recent
Accounting Pronouncements
From
time to time, new accounting pronouncements are issued by the FASB that are adopted by the Company as of the specified effective date.
If not discussed, management believes that the impact of recently issued standards, which are not yet effective, will not have a material
impact on the Company’s financial statements upon adoption.
In
July 2023, the FASB issued Accounting Standards Update (“ASU”) 2023-03 to amend various SEC paragraphs in the Accounting
Standards Codification to primarily reflect the issuance of SEC Staff Accounting Bulletin No. 120. ASU No. 2023-03, “Presentation
of Financial Statements (Topic 205), Income Statement—Reporting Comprehensive Income (Topic 220), Distinguishing Liabilities from
Equity (Topic 480), Equity (Topic 505), and Compensation—Stock Compensation (Topic 718): Amendments to SEC Paragraphs Pursuant
to SEC Staff Accounting Bulletin No. 120, SEC Staff Announcement at the March 24, 2022 EITF Meeting, and Staff Accounting Bulletin Topic
6.B, Accounting Series Release 280—General Revision of Regulation S-X: Income or Loss Applicable to Common Stock.” ASU
2023-03 amends the ASC for SEC updates pursuant to SEC Staff Accounting Bulletin No. 120; SEC Staff Announcement at the March 24, 2022
Emerging Issues Task Force (“EITF”) Meeting; and Staff Accounting Bulletin Topic 6.B, Accounting Series Release 280 - General
Revision of Regulation S-X: Income or Loss Applicable to Common Stock. These updates were immediately effective and did not have a material
impact on our financial statements.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
In
October 2021, the FASB issued ASU 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities
from Contracts with Customers, which creates an exception to the general recognition and measurement principle for contract assets
and contract liabilities from contracts with customers acquired in a business combination. The new guidance will require companies to
apply the definition of a performance obligation under accounting standard codification ASC Topic 606 to recognize and measure contract
assets and contract liabilities (i.e., deferred revenue) relating to contracts with customers that are acquired in a business combination.
Under current GAAP, an acquirer in a business combination is generally required to recognize and measure the assets it acquires and the
liabilities it assumes at fair value on the acquisition date. The new guidance will result in the acquirer recording acquired contract
assets and liabilities on the same basis that would have been recorded by the acquiree before the acquisition under ASC Topic 606. These
amendments are effective for fiscal years beginning after December 15, 2022, with early adoption permitted. The adoption of ASU 2021-08
did not have a material impact on the Company’s financial statements or related disclosures.
In
May 2021, the FASB issued ASU No. 2021-04, Earnings Per Share (Topic 260), Debt – Modifications and Extinguishments
(Subtopic 470-50), Compensation (Topic 718), and Derivatives and Hedging – Contracts in Entity’s Own Equity
(Subtopic 815-40) Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity Classified Written Call
Options. ASU 2021-04 addresses issuer’s accounting for certain modifications or exchanges of freestanding equity-classified
written call options. ASU 2021-04 is effective for fiscal years beginning after December 15, 2021 and interim periods within those fiscal
years, with early adoption permitted. The adoption of ASU 2021-04 has not had a material impact on the Company’s financial statements
or related disclosures.
In
March 2020, the FASB issued ASU 2020-04 establishing Topic 848, Reference Rate Reform. ASU 2020-04 contains practical expedients
for reference rate reform related activities that impact debt, leases, derivatives and other contracts. The pronouncement provides temporary
optional expedients and exceptions to the current guidance on contract modifications and hedge accounting to ease the financial reporting
burdens related to the expected market transition from the London Interbank Offered Rate (“LIBOR”) and other interbank offered
rates to alternative reference rates. The guidance was effective upon issuance and may be applied prospectively to contract modifications
made and hedging relationships entered into or evaluated on or before December 31, 2022. The adoption of ASU 2020-04 did not have a material
impact on the Company’s consolidated financial statements, as we transitioned from the London Interbank Offered Rate, commonly
referred to as LIBOR, to alternative references rates, as well as utilizing the aforementioned expedients and exceptions provided in
ASU 2020-04.
In
August 2020, the FASB issued ASU No. 2020-06, Debt–Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and
Hedging–Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s
Own Equity (ASU 2020-06), which simplifies the accounting for convertible instruments by reducing the number of accounting models available
for convertible debt instruments. This guidance also eliminates the treasury stock method to calculate diluted earnings per share for
convertible instruments and requires the use of the if converted method. The new guidance is effective for all entities for annual periods,
and interim periods within those annual periods, beginning after December 15, 2021, with early adoption permitted. The adoption of ASU
2020-06 has not had a material impact on the Company’s financial statements or related disclosures.
No
other new accounting pronouncements, issued or effective during the period ended September 30, 2023, have had or are expected to have
a significant impact on the Company’s financial statements.
Note
2 –Going Concern
As
shown in the accompanying condensed consolidated financial statements as of September 30, 2023, our balance of cash on hand was $10,152,
and we had negative working capital of $4,152,153 and an accumulated deficit of $24,489,739. We are too early in our development stage
to project future revenue levels, and may not be able to generate sufficient funds to sustain our operations for the next twelve months.
Accordingly, we may need to raise additional cash to fund our operations. These factors raise substantial doubt about the Company’s
ability to continue as a going concern.
In
the event sales do not materialize at the expected rates, management would seek additional financing and would attempt to conserve cash
by further reducing expenses. There can be no assurance that we will be successful in achieving these objectives; therefore, without
sufficient financing it would be unlikely for the Company to continue as a going concern.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The
condensed consolidated financial statements do not include any adjustments that might result from the outcome of any uncertainty as to
the Company’s ability to continue as a going concern. The condensed consolidated financial statements also do not include any adjustments
relating to the recoverability and classification of recorded asset amounts, or amounts and classifications of liabilities that might
be necessary should the Company be unable to continue as a going concern. Our ability to scale production and distribution capabilities
and further increase the value of our brands, is largely dependent on our success in raising additional capital.
Note
3 – Related Party Transactions
Common
Stock Issued for Services, Related Party
On
June 15, 2023, the Company issued 1,500,000 shares of common stock to the Company’s President, Joerg Sommer, for services provided.
The aggregate fair value of the common stock was $89,850, based on the closing price of the Company’s common stock on the date
of grant. The shares were expensed upon issuance.
Note
4 – Fair Value of Financial Instruments
Under
FASB ASC 820-10-5, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date (an exit price). The standard outlines a valuation framework and creates
a fair value hierarchy in order to increase the consistency and comparability of fair value measurements and the related disclosures.
Under GAAP, certain assets and liabilities must be measured at fair value, and FASB ASC 820-10-50 details the disclosures that are required
for items measured at fair value.
The
Company has certain financial instruments that must be measured under the new fair value standard. The Company’s financial assets
and liabilities are measured using inputs from the three levels of the fair value hierarchy. The three levels are as follows:
Level
1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access
at the measurement date.
Level
2 - Inputs include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets
or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (e.g.,
interest rates, yield curves, etc.), and inputs that are derived principally from or corroborated by observable market data by correlation
or other means (market corroborated inputs).
Level
3 - Unobservable inputs that reflect our assumptions about the assumptions that market participants would use in pricing the asset or
liability.
The
following schedule summarizes the valuation of financial instruments at fair value on a recurring basis in the balance sheet as of September
30, 2023 and December 31, 2022, respectively:
Schedule of Valuation of Financial Instruments at Fair Value on a Recurring Basis
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| | |
Fair Value Measurements at September 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets | |
| | | |
| | | |
| | |
| Cash | |
$ | 10,152 | | |
$ | - | | |
$ | - | |
| Total assets | |
| 10,152 | | |
| - | | |
| - | |
| Liabilities | |
| | | |
| | | |
| | |
| Convertible note payable, related party | |
| - | | |
| 750,000 | | |
| - | |
| Notes payable, related parties | |
| - | | |
| 1,061,500 | | |
| - | |
| Notes payable , net of $53,442 of debt discounts at September 30, 2023 | |
| - | | |
| 454,237 | | |
| - | |
| Total liabilities | |
| - | | |
| (2,265,737 | ) | |
| - | |
| Total assets and liabilities | |
$ | 10,152 | | |
$ | (2,265,737 | ) | |
$ | - | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| | |
Fair Value Measurements at December 31, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets | |
| | | |
| | | |
| | |
| Cash | |
$ | 11,016 | | |
$ | - | | |
$ | - | |
| Right-of-use asset | |
| - | | |
| - | | |
| 425,969 | |
| Total assets | |
| 11,016 | | |
| - | | |
| 425,969 | |
| Liabilities | |
| | | |
| | | |
| | |
| Lease liabilities | |
| | | |
| - | | |
| 427,915 | |
| Convertible notes payable | |
| - | | |
| 750,000 | | |
| - | |
| Notes payable | |
| - | | |
| 145,524 | | |
| - | |
| Notes payable, related parties | |
| - | | |
| 999,500 | | |
| - | |
| Total liabilities | |
| - | | |
| (1,895,024 | ) | |
| (427,915 | ) |
| Total assets and liabilities | |
$ | 11,016 | | |
$ | (1,895,024 | ) | |
$ | (1,946 | ) |
There
were no transfers of financial assets or liabilities between Level 1, Level 2 and Level 3 inputs for the nine months ended September
30, 2023 or the year ended December 31, 2022.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note
5 – Inventory
Inventories
are stated at the lower of cost or net realizable value. Cost is determined on a standard cost basis that approximates the first-in,
first-out (FIFO) method. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating
net realizable value. Our cannabis products consist of cannabis flower grown in-house, along with produced extracts. Inventory consisted
of the following at September 30, 2023 and December 31, 2022, respectively.
Schedule of Inventory
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Raw materials | |
$ | 21,934 | | |
$ | 18,580 | |
| Work in progress | |
| 30,174 | | |
| 1,464 | |
| Finished goods | |
| 316,100 | | |
| 80,858 | |
| Inventory gross | |
| 368,208 | | |
| 100,902 | |
| Less obsolescence | |
| (55,472 | ) | |
| (46,749 | ) |
| Total inventory | |
$ | 312,736 | | |
$ | 54,153 | |
Note
6 – Other Current Assets
Other
current assets included the following as of September 30, 2023 and December 31, 2022, respectively:
Schedule of Other Current Assets
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Prepaid expenses | |
$ | 18,964 | | |
$ | 39,288 | |
| Deferred cost of goods sold | |
| 6,760 | | |
| 6,655 | |
| Total | |
$ | 25,724 | | |
$ | 45,943 | |
Note
7 – Other Assets
Other
assets consist entirely of VAT receivables in the amounts of $213,593 and $179,927 at September 30, 2023 and December 31, 2022, respectively,
which will be repaid to the Company by the applicable taxing authority upon the successful export of the products for which the taxes
were originally paid.
Note
8 – Security Deposits
Security
deposits included the following as of September 30, 2023 and December 31, 2022, respectively:
Schedule of Security Deposits
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Refundable deposit on equipment purchase | |
$ | 85,000 | | |
$ | 50,000 | |
| Down payment on distillation equipment | |
| - | | |
| 1,399,413 | |
| Security deposits on leases held in Colombia | |
| - | | |
| 395 | |
| Security
deposits | |
$ | 85,000 | | |
$ | 1,449,808 | |
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note
9 – Fixed Assets
Fixed
assets consist of the following at September 30, 2023 and December 31, 2022, respectively:
Schedule
of Fixed Assets
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Land | |
$ | 138,248 | | |
$ | 138,248 | |
| Buildings | |
| 473,971 | | |
| 473,971 | |
| Office equipment | |
| 30,902 | | |
| 30,902 | |
| Furniture and fixtures | |
| 6,495 | | |
| 6,495 | |
| Equipment and machinery | |
| 1,828,006 | | |
| 423,547 | |
| Fixed assets, gross | |
| 2,477,622 | | |
| 1,073,163 | |
| Less: accumulated depreciation | |
| (110,205 | ) | |
| (84,627 | ) |
| Total | |
$ | 2,367,417 | | |
$ | 988,536 | |
Depreciation
and amortization expense totaled $25,578 and $34,540 for the nine months ended September 30, 2023 and 2022, respectively.
Note
10 – Accrued Expenses
Accrued
expenses consisted of the following at September 30, 2023 and December 31, 2022, respectively:
Schedule of Accrued Expenses
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Accrued payroll | |
$ | 771,199 | | |
$ | 613,569 | |
| Accrued withholding taxes and employee benefits | |
| 42,764 | | |
| 31,632 | |
| Accrued ICA fees and contributions | |
| 178,053 | | |
| 167,037 | |
| Accrued interest | |
| 246,463 | | |
| 136,220 | |
| Accrued expenses | |
$ | 1,238,479 | | |
$ | 948,458 | |
Note
11 – Deferred Revenues
Arrangements
with customers include multiple deliverables, consisting of an initial delivery of seeds and a contingent portion of the purchase price
that is payable on the customer’s future harvest of the plants grown from such seeds. Deferred revenues associated with these multiple-element
arrangements were $11,545 and $11,808 at September 30, 2023 and December 31, 2022, respectively. Related deferred cost of goods sold
were $6,760 and $6,655 at September 30, 2023 and December 31, 2022, respectively, resulting in deferred gross margins of $4,785 and $5,153
at September 30, 2023 and December 31, 2022, respectively, that is expected to be recognized upon the customers’ completion of
their harvests in future periods.
Note
12 – Leases
On
April 28, 2023, the Company leased commercial property for its extraction facility under a commercial lease contract at a monthly lease
rate of 3,000,000 COP (approximately $645) over a one-year term. The lease shall be automatically extended for another one year period
with respect to a mutually agreed upon lease rate at the time of extension. Either party can terminate the lease three months prior to
the expiration of the lease term.
In
addition, the Company leases its corporate offices and operational facility in Colombia under short-term non-cancelable real property
lease agreements that expire within a year. The Company doesn’t have any other office or equipment leases that would require capitalization.
The office lease contains provisions requiring payment of property taxes, utilities, insurance, maintenance and other occupancy costs
applicable to the leased premise. In the locations in which it is economically feasible to continue to operate, management expects to
enter into a new lease upon expiration. The extraction facility lease contained provisions requiring payment of property taxes, utilities,
insurance, maintenance and other occupancy costs applicable to the leased premise. As the Company’s leases do not provide implicit
discount rates, the Company uses an incremental borrowing rate based on the information available at the commencement date in determining
the present value of lease payments.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Terminated
Leases
The
Company leased its 12,400 square foot extraction facility under a non-cancelable real property lease agreement that commenced on January
1, 2022 and was to expire on December 31, 2027, at a monthly lease rate of 57,339,000 COP (approximately $15,290). The Company terminated
the lease on September 30, 2022, resulting in termination fees of approximately $7,700. A gain of $20,148 was recognized on the early
extinguishment of the lease for the year ended December 31, 2022.
On
October 1, 2022, the Company entered into a five-year non-cancelable property lease, with an automatic five year extension, for a new
extraction facility with combined office space, at a monthly lease term of 29,000,000 COP plus VAT and administration fees (approximately
$6,300 in the aggregate), with annual escalation of lease payments equal to the consumer price index, plus 2%. The Company terminated
the lease on May 23, 2023, resulting in a gain of $3,825 on the early extinguishment of the lease for the nine months ended September
30, 2023.
The
Company also leased a residential premise under a non-cancelable real property lease agreement that commenced on September 1, 2021 that
was to expire on August 31, 2024, at a monthly lease term of 3,800,000 COP (approximately $1,013), with approximately a 3% annual escalation
of lease payments commencing September 1, 2022. The Company terminated the lease on April 1, 2023, resulting in a gain of $372 on the
early extinguishment of the lease for the nine months ended September 30, 2023.
The
Company leased another residential premise under a non-cancelable real property lease agreement that commenced on June 1, 2022 and expires
on May 30, 2024, at a monthly lease term of 1,900,000 COP (approximately $507), with an 8% annual escalation of lease payments commencing
June 1, 2023. The Company terminated the lease on April 1, 2023, resulting in a gain of $200 on the early extinguishment of the lease
for the nine months ended September 30, 2023.
The
components of lease expense were as follows:
Schedule of Components of Lease Expense
| | |
2023 | | |
2022 | |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Operating lease cost: | |
| | | |
| | |
| Amortization of right-of-use assets | |
$ | 34,391 | | |
$ | 33,431 | |
| Interest on lease liabilities | |
| 11,379 | | |
| 26,463 | |
| Lease payments on short term leases | |
| 1,290 | | |
| 12,590 | |
| Total operating lease cost | |
$ | 47,060 | | |
$ | 72,484 | |
Supplemental
balance sheet information related to leases was as follows:
Schedule of Supplemental Balance Sheet Information Related to Leases
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Operating lease: | |
| | | |
| | |
| Operating lease assets | |
$ | - | | |
$ | 425,969 | |
| | |
| | | |
| | |
| Current portion of operating lease liabilities | |
$ | - | | |
| 86,235 | |
| Noncurrent operating lease liabilities | |
| - | | |
| 341,680 | |
| Total operating lease liability | |
$ | - | | |
$ | 427,915 | |
| | |
| | | |
| | |
| Weighted average remaining lease term: | |
| | | |
| | |
| Operating leases | |
| None | | |
| 4.25 years | |
| | |
| | | |
| | |
| Weighted average discount rate: | |
| | | |
| | |
| Operating lease | |
| 6.75 | % | |
| 6.75 | % |
Supplemental
cash flow and other information related to operating leases was as follows:
Schedule of Supplemental Cash Flow
Related to Operating Leases
| | |
2023 | | |
2022 | |
| | |
For
the Nine Months Ended | |
| | |
September
30, | |
| | |
2023 | | |
2022 | |
| Cash
paid for amounts included in the measurement of lease liabilities: | |
| | | |
| | |
| Operating
cash flows used for operating leases | |
$ | 31,940 | | |
$ | 38,725 | |
| | |
| | | |
| | |
| Leased
assets obtained in exchange for lease liabilities: | |
| | | |
| | |
| Total
operating lease liabilities | |
$ | - | | |
$ | 1,535,706 | |
| | |
| | | |
| | |
| Gain
on early extinguishment of debt: | |
$ | 4,397 | | |
$ | - | |
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note
13 – Convertible Note Payable, Related Party
Convertible
note payable, related party consists of the following at September 30, 2023 and December 31, 2022, respectively:
Schedule
of Convertible Note Payable Related Party
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| On September 27, 2022 | |
| 750,000 | | |
| 750,000 | |
| On September 27, 2022, the Company completed the sale of a Convertible Promissory Note in the principal amount of $750,000 (the “Convertible McCabe Note”) to Dr. John McCabe, an affiliate investor. The unsecured note matures on September 16, 2024 (the “Maturity Date”), bears interest at a rate of 8% per annum, and the principal and interest is convertible into shares of the Company’s convertible Series B common stock at a conversion price of $15 per share. | |
$ | 750,000 | | |
$ | 750,000 | |
| | |
| | | |
| | |
| Total convertible note payable, related party | |
| 750,000 | | |
| 750,000 | |
| Less: current maturities | |
| - | | |
| - | |
| Convertible note payable, related party, long-term portion | |
$ | 750,000 | | |
$ | 750,000 | |
The
Company recorded interest expense pursuant to the stated interest rates on the convertible note, related party in the amount of $44,877
and $43,899 for the nine months ended September 30, 2023 and 2022, respectively.
Note
14 – Notes Payable, Related Parties
Notes
payable, related party, consists of the following at September 30, 2023 and December 31, 2022, respectively:
Schedule
of Notes Payable Related Party
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| On September 11, 2023, the Company received an advance of $52,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 10% interest rate. | |
$ | 52,000 | | |
$ | - | |
| | |
| | | |
| | |
| On August 31, 2023, the Company received an advance of $4,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 4,000 | | |
| - | |
| | |
| | | |
| | |
| On August 14, 2023, the Company received an advance of $6,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 6,000 | | |
| - | |
| | |
| | | |
| | |
| On August 5, 2022, the Company received an advance of $50,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 50,000 | | |
| 50,000 | |
| | |
| | | |
| | |
| On August 2, 2022, the Company received an advance of $4,500 from Isiah Thomas, III, our Chairman of the Board and CEO, pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 4,500 | | |
| 4,500 | |
| | |
| | | |
| | |
| On June 13, 2022, the Company, through its wholly-owned subsidiary, OWP Ventures, Inc., received an advance of $100,000 from Dr. John McCabe, an affiliate investor, pursuant to an unsecured promissory note, maturing on January 1, 2024, that carries an 8% interest rate. | |
| 100,000 | | |
| 100,000 | |
| | |
| | | |
| | |
| On July 7, 2022, the Company received an advance of $5,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 5,000 | | |
| 5,000 | |
| | |
| | | |
| | |
| On June 3, 2022, the Company received an advance of $10,000 from Isiah Thomas, III, our Chairman of the Board and CEO, pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 10,000 | | |
| 10,000 | |
| | |
| | | |
| | |
| On May 5, 2022, the Company received an advance of $10,000 from Isiah Thomas, III, our Chairman of the Board and CEO, pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 10,000 | | |
| 10,000 | |
| | |
| | | |
| | |
| On May 5, 2022, the Company received an advance of $20,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6% interest rate. | |
| 20,000 | | |
| 20,000 | |
| | |
| | | |
| | |
| On March 1, 2022, the Company, through its wholly-owned subsidiary, OWP Ventures, Inc., received an advance of $400,000 from Dr. John McCabe, an affiliate investor, pursuant to an unsecured promissory note, maturing on January 1, 2024, that carries an 8% interest rate. | |
| 400,000 | | |
| 400,000 | |
| | |
| | | |
| | |
| On February 15, 2022, the Company, through its wholly-owned subsidiary, OWP Ventures, Inc., received an advance of $200,000 from Dr. John McCabe, an affiliate investor, pursuant to an unsecured promissory note, maturing on January 1, 2024, that carries an 8% interest rate. | |
| 200,000 | | |
| 200,000 | |
| | |
| | | |
| | |
| On December 29, 2021, the Company received an advance of $200,000 from Dr. Kenneth Perego, II, M.D., our Vice Chairman of the Board pursuant to an unsecured promissory note due January 1, 2024 that carries an 8% interest rate. | |
| 200,000 | | |
| 200,000 | |
| | |
| | | |
| | |
| Total notes payable, related party | |
| 1,061,500 | | |
| 999,500 | |
| Less: current maturities | |
| 1,061,500 | | |
| 99,500 | |
| Notes payable, related party, long-term portion | |
$ | - | | |
$ | 900,000 | |
The
Company recorded interest expense pursuant to the stated interest rates on the notes payable, related parties, in the amount of $58,804
and $43,763 for the nine months ended September 30, 2023 and 2022, respectively.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note
15 – Notes Payable
Schedule
of Notes Payable
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| On August 18, 2023, the Company, through its wholly-owned subsidiary, OWP Ventures, Inc., issued an unsecured promissory note of $35,000 to LDL8 Consulting, LLC for the purchase of equipment from another vendor. The promissory note bears interest at 10% per annum and is due on demand. In the event of default, the interest rate increases to 15% until repayment. | |
$ | 35,000 | | |
$ | - | |
| | |
| | | |
| | |
On June 23, 2023, the Company completed the sale of a Promissory Note in the principal amount of $300,000 (the “Third AJB Note”) to AJB Capital Investments LLC (“AJB Capital”) for an aggregate purchase price of $276,000, pursuant to a Securities Purchase Agreement between the Company and AJB Capital (the “Purchase Agreement”). The Company received net proceeds of $262,500 after deduction of an original issue discount of $24,000, $7,500 of legal fees and a $6,000 of broker fee, which are being amortized as a debt discount over the life of the loan.
The Third AJB Note matures on March 23, 2024 (the “Maturity Date”), bears interest at a rate of 12% per annum, and, following an event of default only, is convertible into shares of the Company’s common stock at a conversion price equal to the lesser of the Volume Weighted Average Price (“VWAP”) during (i) the 10 trading day period preceding the issuance date of the note, or (ii) the 10 trading day period preceding date of conversion of the Note. The Note is also subject to covenants, events of defaults, penalties, default interest and other terms and conditions customary in transactions of this nature.
Pursuant to the Purchase Agreement, the Company paid a commitment fee to AJB Capital in the amount of $100,000 (the “Commitment Fee”) in the form of 1,666,667 shares of the Company’s common stock (the “Commitment Fee Shares”). During the period commencing on the six-month anniversary of the closing date and ending on the five-year anniversary of the closing date, AJB Capital is entitled to be issued additional shares of common stock or receive a cash payment to the extent AJB Capital’s sale of the Commitment Fee Shares has resulted in net proceeds in an amount less than the Commitment Fee. The Commitment Fee Shares resulted in a debt discount of $42,175 that is being amortized over the life of the loan.
In connection with the issuance of the Third AJB Note and Commitment Fee Shares, the Company entered into a Registration Rights Agreement with AJB Capital in which the Company agreed to file a registration statement with the SEC within 180 days of June 23, 2023, registering the shares of common stock issuable under the Third AJB Note and Purchase Agreement. | |
| 300,000 | | |
| - | |
| | |
| | | |
| | |
| On September 15, 2022, the Company, through its wholly-owned subsidiary, One World Pharma, SAS, received proceeds of 55,488,000 COP, or approximately $12,243, on a loan with a face value of 70,000,000 COP, or approximately $15,445, from an individual pursuant to an unsecured promissory note, bearing interest at 4% per month, or 48% per annum, due on demand. The debt discount of $3,202 was expensed as finance costs at the time of origination. The face value of the note has been adjusted by $1,823 due to foreign currency translation adjustments. | |
| 17,268 | | |
| 14,552 | |
| | |
| | | |
| | |
| On June 17, 2022, the Company, through its wholly-owned subsidiary, One World Pharma, SAS, received proceeds of 230,400,000 COP, or approximately $55,821, on a loan with a face value of 240,000,000 COP, or approximately $58,147, from an individual pursuant to an unsecured promissory note, bearing interest at 4% per month, or 48% per annum, due on demand. The debt discount of $2,326 was expensed as finance costs at the time of origination. The face value of the note has been adjusted by $3,383 due to foreign currency translation adjustments. | |
| 59,204 | | |
| 49,894 | |
| | |
| | | |
| | |
| On May 31, 2022, the Company, through its wholly-owned subsidiary, One World Pharma, SAS, received proceeds of 314,640,000 COP, or approximately $76,231, on a loan with a face value of 360,000,000 COP, or approximately $87,220, from an individual pursuant to promissory note, security by equipment, bearing interest at 2.1% per month, or 25% per annum, which matured on November 28, 2022 and is currently past due. The debt discount of $10,990 was expensed as finance costs at the time of origination. The face value of the note has been adjusted by $1,586 due to foreign currency translation adjustments. | |
| 88,806 | | |
| 74,841 | |
| | |
| | | |
| | |
| On May 30, 2022, the Company, through its wholly-owned subsidiary, One World Pharma, SAS, received a non-interest bearing loan of 20,000,000 COP, or approximately $4,846, from an individual pursuant to an unsecured promissory note, due on demand. The face value of the note has been adjusted by $88 due to foreign currency translation adjustments. | |
| 4,934 | | |
| 4,158 | |
| | |
| | | |
| | |
| On April 29, 2022, the Company, through its wholly-owned subsidiary, One World Pharma, SAS, received a non-interest bearing loan of 10,000,000 COP, or approximately $2,423, from an individual pursuant to an unsecured promissory note, due on demand. The face value of the note has been adjusted by $44 due to foreign currency translation adjustments. | |
| 2,467 | | |
| 2,079 | |
| | |
| | | |
| | |
| Total notes payable | |
| 507,679 | | |
| 145,524 | |
| Less: unamortized debt discounts | |
| 53,442 | | |
| - | |
| Notes payable, net of discounts | |
| 454,237 | | |
| 145,524 | |
| Less: current maturities | |
| 454,237 | | |
| 145,524 | |
| Notes payable, long-term portion | |
$ | - | | |
$ | - | |
The
Company recognized aggregate debt discounts on the notes payable to AJB Capital for the nine months ended September 30, 2023, as follows:
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Schedule
of Notes Payable Debt Discounts
| | |
September 30, | |
| | |
2023 | |
| | |
| |
| Fair value of 1,666,667 commitment shares of common stock | |
$ | 42,175 | |
| Original issue discounts | |
| 24,000 | |
| Legal and brokerage fees | |
| 13,500 | |
| Total debt discounts | |
| 79,675 | |
| Amortization of debt discounts | |
| 26,233 | |
| Unamortized debt discounts | |
$ | 53,442 | |
The
aggregate debt discounts of $79,675, for the nine months ended September 30, 2023, are being amortized over the life of the loan using
the straight-line method, which approximates the effective interest method. The Company recorded finance expense in the amount of $26,233
and $-0- on the amortization of these discounts for the nine months ended September 30, 2023 and 2022, respectively.
The
convertible note limits the maximum number of shares that can be owned by the note holder as a result of the conversions to common stock
to 4.99% of the Company’s issued and outstanding shares.
The
Company recorded interest expense pursuant to the stated interest rates on the notes payable in the amount of $47,255 and $21,120 for
the nine months ended September 30, 2023 and 2022, respectively.
The
Company recognized interest expense for the nine months ended September 30, 2023 and 2022, as follows:
Schedule of Interest Expenses
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Finance cost on equity line of credit | |
$ | - | | |
$ | 15,000 | |
| Interest on convertible notes, related party | |
| 44,877 | | |
| 43,899 | |
| Interest on notes payable, related parties | |
| 58,804 | | |
| 43,763 | |
| Interest on notes payable | |
| 47,255 | | |
| 21,120 | |
| Amortization of debt discounts | |
| 13,549 | | |
| 50,753 | |
| Amortization of debt discounts, common stock | |
| 12,684 | | |
| 106,894 | |
| Amortization of debt discounts, warrants | |
| - | | |
| 255,026 | |
| Series B preferred stock issued as a commitment on an ELOC | |
| - | | |
| 205,005 | |
| Common stock issued as a commitment on the 2nd AJB Note | |
| - | | |
| 134,128 | |
| Interest on accounts payable | |
| - | | |
| 11,249 | |
| Total interest expense | |
$ | 177,169 | | |
$ | 886,837 | |
Note
16 – Convertible Preferred Stock
Preferred
Stock
The
Company has 10,000,000 authorized shares of $0.001 par value “blank check” preferred stock, of which 500,000 shares have
been designated Series A Preferred Stock and 600,000 shares have been designated Series B Preferred Stock, as amended on August 2, 2022.
The shares of Series A Preferred Stock and Series B Preferred Stock are each currently convertible into one hundred (100) shares of the
Company’s common stock. The Series A Preferred Stock accrues dividends at the rate of 6% per annum, payable in cash as and when
declared by the Board or upon a liquidation. The shares of Series B Preferred Stock are not entitled to dividends, other than the right
to participate in dividends payable to holders of common stock on an as-converted basis. As of September 30, 2023, there were 99,733
and 248,501 shares of Series A Preferred Stock and Series B Preferred Stock, respectively, issued and outstanding. The Series A and B
Preferred Stock are presented as mezzanine equity on the balance sheet due because they carry a stated value of $10 and $15 per share,
respectively, and a deemed liquidation clause, which entitles the holders thereof to receive proceeds thereof in an amount equal to the
stated value per share, plus any accrued and unpaid dividends, before any payment may be made to holders of common stock. Each share
of Preferred Stock carries a number of votes equal to the number of shares of common stock into which such Preferred Stock may then be
converted. The Preferred Stock generally will vote together with the common stock and not as a separate class.
The
Series A and B Preferred Stock have been classified outside of permanent equity and liabilities. the Series A Preferred Stock embodies
conditional obligations that the Company may settle by issuing a variable number of equity shares, and in both the Series A and B Preferred
Stock, monetary value of the obligation is based on a fixed monetary amount known at inception.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Series
A Preferred Stock Sales
On
various dates between January 4, 2023 and April 3, 2023, the Company received total proceeds from four accredited investors of $250,000
from the sale of 25,000 units, consisting in the aggregate of 25,000 shares of series A preferred stock and five-year warrants to purchase
an aggregate 2,500,000 shares of common stock at an exercise price of $0.25 per share. The proceeds received were allocated between the
Series A Preferred Stock and warrants on a relative fair value basis.
Series
A Preferred Stock Payable, Consultants
On
July 1, 2023, the Company was obligated to issue 4,500 shares of series A preferred stock in consideration of consulting services. The
fair value of the shares was $45,000, based on recent sales prices of the Company’s series A preferred stock on the date of grant.
The fair value of the shares have been recognized as a subscription payable, as the shares have not yet been issued.
Series
A Preferred Stock Issued for Services, Consultants
On
January 1, 2023, the Company issued 4,500 shares of series A preferred stock in consideration of consulting services. The fair value
of the shares was $45,000, based on recent sales prices of the Company’s series A preferred stock on the date of grant.
Preferred
Stock Dividends
The
Series A Preferred Stock accrues dividends at the rate of 6% per annum, payable in cash as and when declared by the Board or upon a liquidation.
The Company recognized $43,808 and $28,971 for the nine months ended September 30, 2023 and 2022, respectively. A total of $181,651 of
dividends had accrued as of September 30, 2023.
Series
B Preferred Stock Issuances
On
September 12, 2023, a shareholder converted 10,000 shares of Series B Preferred Stock into 1,000,000 shares of common stock.
On
July 7, 2023, a shareholder converted 13,667 shares of Series B Preferred Stock into 1,366,700 shares of common stock.
Note
17 – Commitments and Contingencies
Equity
Line of Credit
On
September 1, 2022, the Company entered into a Purchase Agreement (the “ELOC Purchase Agreement”) with Tysadco Partners, LLC
(“Tysadco”). Pursuant to the ELOC Purchase Agreement, Tysadco has agreed to purchase from the Company, from time to time
upon delivery by the Company to Tysadco of “Request Notices,” and subject to the other terms and conditions set forth in
the ELOC Purchase Agreement, up to an aggregate of $10,000,000 of the Company’s common stock. The purchase price of the shares of common stock to be purchased under the Purchase Agreement will be equal to 88% of the lowest daily “VWAP” during the period
of 10 trading days beginning five trading days preceding the applicable Request. Each purchase under the Purchase Agreement will be in
a minimum amount of $25,000 and a maximum amount equal to the lesser of (i) $1,000,000 and (ii) 500% of the average daily trading value
of the common stock over the seven trading days preceding the delivery of the applicable Request Notice.
In
connection with the ELOC Purchase Agreement, the Company entered into a Registration Rights Agreement with Tysadco under which the Company
agreed to file a registration statement with the Securities and Exchange Commission covering the shares of common stock issuable under
the ELOC Purchase Agreement and conversion of the Commitment Fee Shares (the “Registration Rights Agreement”). There have
not been any advances on this arrangement to date.
Contingent
Compensation
On
August 22, 2023, the Company entered into an advisor agreement with an individual to provide consulting and business advisory services
to the Company. Pursuant to the agreement, the Company has agreed to compensate the consultant a fee of $5,000 per month, which is to
be deferred until the Company completes the of sale of its equity securities in a transaction, or related series of transactions, resulting
in aggregate gross proceeds to the Company of at least $5,000,000, while the Advisor is providing Services (the “Qualified Offering”).
Within 60 days of the closing of a Qualified Offering, the Company shall pay to Advisor a cash bonus of up to $200,000. The advisor shall
have no participation in any manner or form with any Qualified Offering.
On
May 23, 2023, the Company appointed Joerg Sommer to be the Company’s President. In connection
with his appointment, the Company entered into an offer letter with Mr. Sommer (the “Offer Letter”) under which he will initially
be paid an annual base salary of $60,000, which will increase to $240,000 upon the closing of an offering of the Company’s equity
securities that results in gross proceeds to the Company of at least $5,000,000. Mr. Sommer received 1,500,000 shares of the Company’s
common stock upon his appointment as President; and is entitled to be issued an additional 1,500,000 shares of the Company’s common
stock within 60 days of the closing of a Qualified Offering. Mr. Sommer will also be entitled
to a bonus of up $380,000 upon the sale of the Company’s equity securities during the term of his employment, as set forth below;
$200,000
upon the Company raising $2 million
$80,000
upon the Company raising an additional $1 million
$60,000
upon the Company raising an additional $1 million
$40,000
upon the Company raising an additional $1 million
Note
18 – Changes in Stockholders’ Equity
Common
Stock
The
Company is authorized to issue an aggregate of 300,000,000 shares of common stock with a par value of $0.001. As of September 30, 2023,
there were 76,736,274 shares of common stock issued and outstanding.
Common
Stock Sales
On
February 14, 2023, the Company sold 3,000,000 shares of common stock at a price of $0.10 per share for total cash proceeds of $300,000.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Common
Stock Issued as a Commitment Fee
On
June 23, 2023, the Company paid a commitment fee to AJB Capital in the form of 1,666,667 shares of common stock in connection with the
issuance of the Third AJB Note (defined above). The aggregate fair value of the common stock was $42,175, based on the closing price
of the Company’s common stock on the date of grant. The shares are being amortized as a debt discount over the life of the loan.
Common
Stock Issued for Services, Related Party
On
June 15, 2023, the Company issued 1,500,000 shares of common stock to the Company’s President, Joerg Sommer, for services provided.
The aggregate fair value of the common stock was $89,850, based on the closing price of the Company’s common stock on the date
of grant. The shares were expensed upon issuance.
Common
Stock Issued for Services
On
September 18, 2023, the Company issued 1,000,000 shares of common stock to ClearThink Capital Partners, LLC, for services provided. The
aggregate fair value of the common stock was $84,000, based on the closing price of the Company’s common stock on the date of grant.
The shares were expensed upon issuance.
Amortization
of Stock-Based Compensation
A
total of $121,052 and $123,440 of stock-based compensation expense was recognized from the amortization of options to purchase common
stock over their vesting period during the nine months ended September 30, 2023 and 2022, respectively.
Note
19 – Common Stock Options
Stock
Incentive Plan
On
February 12, 2020, the Company’s stockholders approved our 2019 Stock Incentive Plan (the “2019 Plan”), which had been
adopted by the Company’s Board of Directors (the “Board”) as of December 10, 2019. The 2019 Plan provides for the issuance
of up to 10,000,000 shares of common stock to the Company and its subsidiaries’ employees, officers, directors, consultants and
advisors, stock options (non-statutory and incentive), restricted stock awards, stock appreciation rights (“SARs”), restricted
stock units (“RSUs”) and other performance stock awards. Options granted under the 2019 Plan may either be intended to qualify
as incentive stock options under the Internal Revenue Code of 1986, or may be non-qualified options, and are exercisable over periods
not exceeding ten years from date of grant. Unless sooner terminated in accordance with its terms, the Stock Plan will terminate on December
10, 2029.
Outstanding
Options
Options
to purchase an aggregate total of 10,392,000 shares of common stock at a weighted average strike price of $0.14, exercisable over a weighted
average life of 7.37 years were outstanding as of September 30, 2023.
Options
Granted
On
August 22, 2023, the Company awarded options to purchase 250,000 shares of common stock under the 2019 Plan at an exercise price equal
to $0.10 per share, exercisable over a ten year period to a consultant. The estimated value using the Black-Scholes Pricing Model, based
on a volatility rate of 145% and a call option value of $0.0735, was $18,367. The options were fully vested, resulting in $18,367 of
stock-based compensation expense during the nine months ending September 30, 2023.
The
Company recognized a total of $121,052, and $123,440 of compensation expense during the nine months ended September 30, 2023 and 2022,
respectively, related to common stock options issued in the prior year to officers, directors, and employees that are being amortized
over the implied service term, or vesting period, of the options. The remaining unamortized balance of these options is $29,342 as of
September 30, 2023.
Note
20 – Warrants
Outstanding
Warrants
Warrants
to purchase an aggregate total of 14,011,650 shares of common stock at a weighted average strike price of $0.29, exercisable over a weighted
average life of 2.16 years were outstanding as of September 30, 2023.
Warrants
Granted
On
April 3, 2023, the Company received proceeds of $100,000 from the sale of 10,000 units, consisting of 10,000 shares of Series A Preferred
Stock and five-year warrants to purchase 1,000,000 shares of common stock at an exercise price of $0.25 per share from an accredited
investor. The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis. The
aggregate estimated value of the warrants using the Black-Scholes Pricing Model, based on a weighted average volatility rate of 146%
and a weighted average call option value of $0.0635, was $63,508.
ONE WORLD PRODUCTS, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
On
January 27, 2023, the Company received proceeds of $100,000 from the sale of 10,000 units, consisting of 10,000 shares of Series A Preferred
Stock and five-year warrants to purchase 1,000,000 shares of common stock at an exercise price of $0.25 per share from an accredited
investor. The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis. The
aggregate estimated value of the warrants using the Black-Scholes Pricing Model, based on a weighted average volatility rate of 148%
and a weighted average call option value of $0.0672, was $67,180.
On
January 9, 2023, the Company received proceeds of $25,000 from the sale of 2,500 units, consisting of 2,500 shares of Series A Preferred
Stock and five-year warrants to purchase 250,000 shares of common stock at an exercise price of $0.25 per share from an accredited investor.
The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis. The aggregate
estimated value of the warrants using the Black-Scholes Pricing Model, based on a weighted average volatility rate of 152% and a weighted
average call option value of $0.0550, was $13,757.
On
January 4, 2023, the Company received proceeds of $25,000 from the sale of 2,500 units, consisting of 2,500 shares of Series A Preferred
Stock and five-year warrants to purchase 250,000 shares of common stock at an exercise price of $0.25 per share from an accredited investor.
The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis. The aggregate
estimated value of the warrants using the Black-Scholes Pricing Model, based on a weighted average volatility rate of 156% and a weighted
average call option value of $0.0559, was $13,970.
Note
21 – Income Taxes
The
Company accounts for income taxes under FASB ASC 740-10, which requires use of the liability method. FASB ASC 740-10-25 provides that
deferred tax assets and liabilities are recorded based on the differences between the tax bases of assets and liabilities and their carrying
amounts for financial reporting purposes, referred to as temporary differences.
For
the nine months ended September 30, 2023, and the year ended December 31, 2022, the Company incurred a net operating loss and, accordingly,
no provision for income taxes has been recorded. In addition, no benefit for income taxes has been recorded due to the uncertainty of
the realization of any tax assets. At September 30, 2023, the Company had approximately $9,746,000 of federal net operating losses. The
net operating loss carry forwards, if not utilized, will begin to expire in 2025.
Based
on the available objective evidence, including the Company’s history of its loss, management believes it is more likely than not
that the net deferred tax assets will not be fully realizable. Accordingly, the Company provided for a full valuation allowance against
its net deferred tax assets at September 30, 2023 and December 31, 2022, respectively.
In
accordance with FASB ASC 740, the Company has evaluated its tax positions and determined there are no uncertain tax positions.
Note
22 – Subsequent Events
The
Company evaluates events that have occurred after the balance sheet date through the date these financial statements were issued.
Related
Party Debt Financing
On
October 11, 2023, the Company received an advance of $25,000 from the Company’s President, Joerg Sommer, pursuant to an unsecured
promissory note due on demand that carries a 10% interest rate.
Common
Stock Sales
On
October 2, 2023, the Company sold 1,000,000 shares of common stock at a price of $0.10 per share for total cash proceeds of $100,000.
Common
Stock Issued for Services
On
October 4, 2023, the Company issued 572,083 shares of common stock to ClearThink Capital Partners, LLC, for services provided. The aggregate
fair value of the common stock was $51,487, based on the closing price of the Company’s common stock on the date of grant.

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and
Stockholders
of One World Products, Inc. and subsidiaries
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of One World Products, Inc. and subsidiaries (the Company) as of December 31,
2022 and 2021, and the related consolidated statements of operations and comprehensive loss, consolidated stockholders’ equity
(deficit) and consolidated cash flows for each of the years in the two-year period ended December 31, 2022, and the related notes (collectively
referred to as the consolidated financial statements). In our opinion, the financial statements present fairly, in all material respects,
the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each
of the years in the two-year period ended December 31, 2022, in conformity with accounting principles generally accepted in the United
States of America.
Going
Concern
The
accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed
in Note 2 to the consolidated financial statements, the Company suffered a net loss from operations and has a net capital deficiency,
which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are
also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of
this uncertainty.
Basis
for Opinion
These
financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s
financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities
laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits,
we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error
or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits
provide a reasonable basis for our opinion.
Critical
Audit Matter
The
critical audit matter communicated below is a matter arising from the current period audit of the financial statements that were communicated
or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial
statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter
does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit
matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Convertible
Preferred Stock
As
discussed in Note 17, the Company has complex financial instruments due to the issued and outstanding preferred stock, resulting in the
classification of the financial instruments outside of permanent equity due to the terms of the instruments. Given the factors, the related
audit effort in evaluating management’s judgments in determining the appropriate classification was extensive and required a high
degree of auditor judgment.
We
tested the Company’s classification of the financial instruments by examining and evaluating the agreements along with management’s
evaluation of the key terms and management’s disclosure of the transactions.
/s/
M&K CPAS, PLLC
We
have served as the Company’s auditor since 2018.
Houston,
TX
May
15, 2023
ONE
WORLD PRODUCTS, INC.
CONSOLIDATED
BALANCE SHEETS
| | |
December 31, | | |
December 31, | |
| | |
2022 | | |
2021 | |
| Assets | |
| | | |
| | |
| | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash | |
$ | 11,016 | | |
$ | 119,678 | |
| Accounts receivable | |
| 12,355 | | |
| 19,880 | |
| Inventory | |
| 54,153 | | |
| 198,595 | |
| Other current assets | |
| 45,943 | | |
| 158,836 | |
| Total current assets | |
| 123,467 | | |
| 496,989 | |
| | |
| | | |
| | |
| Other assets | |
| 179,927 | | |
| 147,194 | |
| Right-of-use assets | |
| 425,969 | | |
| - | |
| Security deposits | |
| 1,449,808 | | |
| 1,255,988 | |
| Fixed assets, net | |
| 988,536 | | |
| 1,003,013 | |
| | |
| | | |
| | |
| Total Assets | |
$ | 3,167,707 | | |
$ | 2,903,184 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Equity (Deficit) | |
| | | |
| | |
| | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 798,067 | | |
$ | 480,146 | |
| Accrued expenses | |
| 948,458 | | |
| 457,762 | |
| Deferred revenues | |
| 11,808 | | |
| 30,164 | |
| Dividends payable | |
| 137,843 | | |
| 98,920 | |
| Current portion of lease liabilities | |
| 86,235 | | |
| - | |
| Convertible notes payable, net of $-0-
and $412,673 of debt
discounts at December 31, 2022 and 2021, respectively | |
| 750,000 | | |
| 337,327 | |
| Notes payable, current maturities | |
| 145,524 | | |
| 119,274 | |
| Notes payable, related parties, current maturities | |
| 99,500 | | |
| - | |
| Total current liabilities | |
| 2,977,435 | | |
| 1,523,593 | |
| | |
| | | |
| | |
| Long-term lease liability | |
| 341,680 | | |
| - | |
| Notes payable, long-term portion | |
| 700,000 | | |
| - | |
| Notes payable, related parties, long-term portion | |
| 200,000 | | |
| 200,000 | |
| Notes payable | |
| | | |
| | |
| | |
| | | |
| | |
| Total Liabilities | |
| 4,219,115 | | |
| 1,723,593 | |
| | |
| | | |
| | |
| Series A convertible preferred stock, $0.001
par value, 500,000
shares authorized; 70,233
and 65,233
shares issued and outstanding at December 31, 2022 and 2021, respectively | |
| 702,330 | | |
| 652,330 | |
| Series B convertible preferred stock, $0.001
par value, 300,000
shares authorized; 272,168
and 238,501
shares issued and outstanding at December 31, 2022 and 2021, respectively | |
| 4,082,520 | | |
| 3,577,515 | |
| Convertible preferred stock value | |
| 4,082,520 | | |
| 3,577,515 | |
| | |
| | | |
| | |
| Stockholders’ Equity (Deficit): | |
| | | |
| | |
| Preferred stock, $0.001
par value, 9,200,000 shares
authorized; no shares issued
and outstanding at December 31, 2022 and 2021, respectively | |
| - | | |
| - | |
| Common stock, $0.001
par value, 300,000,000 shares
authorized; 67,202,907 and 65,599,565
shares issued and outstanding at December 31, 2022 and 2021, respectively | |
| 67,203 | | |
| 65,600 | |
| Additional paid-in capital | |
| 17,123,603 | | |
| 16,843,656 | |
| Subscriptions payable, consisting of -0-
and 262,066 shares at December 31,
2022 and 2021, respectively | |
| - | | |
| 21,725 | |
| Accumulated other comprehensive loss | |
| (50,699 | ) | |
| (64,347 | ) |
| Accumulated (deficit) | |
| (22,976,365 | ) | |
| (19,916,888 | ) |
| Total Stockholders’ Equity (Deficit) | |
| (5,836,258 | ) | |
| (3,050,254 | ) |
| | |
| | | |
| | |
| Total Liabilities and Stockholders’ Equity (Deficit) | |
$ | 3,167,707 | | |
$ | 2,903,184 | |
The accompanying notes are an integral part of these consolidated financial
statements.
ONE
WORLD PRODUCTS, INC.
CONSOLIDATED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| | |
| | |
| |
| | |
For the Year Ended | |
| | |
December 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Revenues | |
$ | 125,662 | | |
$ | 38,264 | |
| Cost of goods sold | |
| 300,757 | | |
| 19,744 | |
| Gross profit (loss) | |
| (175,095 | ) | |
| 18,520 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| General and administrative | |
| 1,587,017 | | |
| 2,294,284 | |
| Professional fees | |
| 431,737 | | |
| 915,217 | |
| Depreciation expense | |
| 42,287 | | |
| 40,321 | |
| Total operating expenses | |
| 2,061,041 | | |
| 3,249,822 | |
| | |
| | | |
| | |
| Operating loss | |
| (2,236,136 | ) | |
| (3,231,302 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Sublease income | |
| 1,000 | | |
| 27,000 | |
| Loss on disposal of fixed assets | |
| (9,041 | ) | |
| (71,487 | ) |
| Gain on early extinguishment of lease | |
| 20,148 | | |
| - | |
| Gain on forgiveness of PPP loan | |
| 121,372 | | |
| - | |
| Interest income | |
| 38 | | |
| 2,358 | |
| Interest expense | |
| (956,858 | ) | |
| (511,131 | ) |
| Total other expense | |
| (823,341 | ) | |
| (553,260 | ) |
| | |
| | | |
| | |
| Net loss | |
$ | (3,059,477 | ) | |
$ | (3,784,562 | ) |
| | |
| | | |
| | |
| Other comprehensive loss: | |
| | | |
| | |
| Gain (loss) on foreign currency translation | |
$ | 13,648 | | |
$ | (11,477 | ) |
| | |
| | | |
| | |
| Net other comprehensive loss | |
$ | (3,045,829 | ) | |
$ | (3,796,039 | ) |
| Series A convertible preferred stock declared ($0.60
per share) | |
| (38,923 | ) | |
| (61,684 | ) |
| Net loss attributable to common shareholders | |
$ | (3,084,752 | ) | |
$ | (3,857,723 | ) |
| | |
| | | |
| | |
| Weighted average number of common shares outstanding - basic and diluted | |
| 66,191,644 | | |
| 60,600,548 | |
| | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.05 | ) | |
$ | (0.06 | ) |
| | |
| | | |
| | |
| Dividends declared per share of common stock | |
$ | 0.00 | | |
$ | 0.00 | |
The accompanying notes are an integral part of these consolidated financial
statements.
ONE
WORLD PRODUCTS, INC.
CONSOLIDATED
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
Series
A Convertible | | |
Series
B Convertible | | |
| | |
| | |
Additional | | |
| | |
Accumulated
Other | | |
| | |
Total
Stockholders’ | |
| | |
Preferred
Stock | | |
Preferred
Stock | | |
Common
Stock | | |
Paid-In | | |
Subscriptions | | |
Comprehensive | | |
Accumulated | | |
Equity | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Payable | | |
Income
(Loss) | | |
Deficit | | |
(Deficit) | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance,
December 31, 2020 | |
| 150,233 | | |
$ | 1,502,330 | | |
| - | | |
$ | - | | |
| 53,085,305 | | |
$ | 53,085 | | |
$ | 14,103,672 | | |
$ | 75,000 | | |
$ | (52,870 | ) | |
$ | (16,132,326 | ) | |
$ | (1,953,439 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
B convertible preferred stock sold for cash to our CEO | |
| - | | |
| - | | |
| 203,334 | | |
| 3,050,010 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
B convertible preferred stock sold for cash | |
| - | | |
| - | | |
| 35,167 | | |
| 527,505 | | |
| - | | |
| - | | |
| (10 | ) | |
| - | | |
| - | | |
| - | | |
| (10 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common
stock sold for cash | |
| - | | |
| - | | |
| - | | |
| - | | |
| 750,000 | | |
| 750 | | |
| 74,250 | | |
| (75,000 | ) | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Conversion
of series A convertible preferred stock | |
| (85,000 | ) | |
| (850,000 | ) | |
| - | | |
| - | | |
| 8,500,000 | | |
| 8,500 | | |
| 841,500 | | |
| - | | |
| - | | |
| - | | |
| 850,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common
stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 954,260 | | |
| 955 | | |
| 111,075 | | |
| 21,725 | | |
| - | | |
| - | | |
| 133,755 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Commitment
shares issued pursuant to promissory note | |
| - | | |
| - | | |
| - | | |
| - | | |
| 2,250,000 | | |
| 2,250 | | |
| 416,062 | | |
| - | | |
| - | | |
| - | | |
| 418,312 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Exercise
of cashless options | |
| - | | |
| - | | |
| - | | |
| - | | |
| 60,000 | | |
| 60 | | |
| (60 | ) | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Warrants
issued as a debt discount | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 358,017 | | |
| - | | |
| - | | |
| - | | |
| 358,017 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Amortization
of common stock options issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,000,834 | | |
| - | | |
| - | | |
| - | | |
| 1,000,834 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
A convertible preferred stock dividend declared ($0.60
per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (61,684 | ) | |
| - | | |
| - | | |
| - | | |
| (61,684 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss
on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (11,477 | ) | |
| - | | |
| (11,477 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,784,562 | ) | |
| (3,784,562 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
December 31, 2021 | |
| 65,233 | | |
$ | 652,330 | | |
| 238,501 | | |
$ | 3,577,515 | | |
| 65,599,565 | | |
$ | 65,600 | | |
$ | 16,843,656 | | |
$ | 21,725 | | |
$ | (64,347 | ) | |
$ | (19,916,888 | ) | |
$ | (3,050,254 | ) |
| Balance | |
| 65,233 | | |
$ | 652,330 | | |
| 238,501 | | |
$ | 3,577,515 | | |
| 65,599,565 | | |
$ | 65,600 | | |
$ | 16,843,656 | | |
$ | 21,725 | | |
$ | (64,347 | ) | |
$ | (19,916,888 | ) | |
$ | (3,050,254 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
A convertible preferred stock sold for cash | |
| 5,000 | | |
| 50,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
B convertible preferred stock sold for cash | |
| - | | |
| - | | |
| 20,000 | | |
| 300,000 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
B convertible preferred stock issued as commitment fee on ELOC | |
| - | | |
| - | | |
| 13,667 | | |
| 205,005 | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common
stock issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| 1,603,342 | | |
| 1,603 | | |
| 154,250 | | |
| (21,725 | ) | |
| - | | |
| - | | |
| 134,128 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Amortization
of common stock options issued for services | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 164,620 | | |
| - | | |
| - | | |
| - | | |
| 164,620 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Series
A convertible preferred stock dividends declared ($0.60
per share) | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (38,923 | ) | |
| - | | |
| - | | |
| - | | |
| (38,923 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss
on foreign currency translation | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| 13,648 | | |
| - | | |
| 13,648 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,059,477 | ) | |
| (3,059,477 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
December 31, 2022 | |
| 70,233 | | |
$ | 702,330 | | |
| 272,168 | | |
$ | 4,082,520 | | |
| 67,202,907 | | |
$ | 67,203 | | |
$ | 17,123,603 | | |
$ | - | | |
$ | (50,699 | ) | |
$ | (22,976,365 | ) | |
$ | (5,836,258 | ) |
| Balance | |
| 70,233 | | |
$ | 702,330 | | |
| 272,168 | | |
$ | 4,082,520 | | |
| 67,202,907 | | |
$ | 67,203 | | |
$ | 17,123,603 | | |
$ | - | | |
$ | (50,699 | ) | |
$ | (22,976,365 | ) | |
$ | (5,836,258 | ) |
The
accompanying notes are an integral part of these consolidated financial statements.
ONE
WORLD PRODUCTS, INC.
CONSOLIDATED
STATEMENTS OF CASH FLOWS
| | |
2022 | | |
2021 | |
| | |
For the Year Ended | |
| | |
December 31, | |
| | |
2022 | | |
2021 | |
| Cash flows from operating activities | |
| | | |
| | |
| Net loss | |
$ | (3,059,477 | ) | |
$ | (3,784,562 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Bad debts expense | |
| - | | |
| 2,062 | |
| Depreciation and amortization expense | |
| 42,287 | | |
| 40,321 | |
| Loss on disposal of fixed assets | |
| 9,041 | | |
| 71,487 | |
| Gain on early extinguishment of lease | |
| (20,148 | ) | |
| - | |
| Gain on forgiveness of PPP loan | |
| (121,372 | ) | |
| - | |
| Amortization of debt discounts | |
| 412,673 | | |
| 456,656 | |
| Stock-based compensation | |
| 339,133 | | |
| 133,755 | |
| Amortization of options issued for services | |
| 164,620 | | |
| 1,000,834 | |
| Decrease (increase) in assets: | |
| | | |
| | |
| Accounts receivable | |
| 7,525 | | |
| (16,306 | ) |
| Inventory | |
| 144,442 | | |
| 68,557 | |
| Other current assets | |
| 112,893 | | |
| (187,119 | ) |
| Other assets | |
| (32,733 | ) | |
| - | |
| Right-of-use assets | |
| 118,347 | | |
| 195,029 | |
| Security deposits | |
| (193,820 | ) | |
| (1,190,874 | ) |
| Increase (decrease) in liabilities: | |
| | | |
| | |
| Accounts payable | |
| 317,921 | | |
| (254,408 | ) |
| Accrued expenses | |
| 492,794 | | |
| (92,773 | ) |
| Deferred revenues | |
| (18,356 | ) | |
| 30,164 | |
| Lease liability | |
| (96,253 | ) | |
| (201,525 | ) |
| Net cash used in operating activities | |
| (1,380,483 | ) | |
| (3,728,702 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities | |
| | | |
| | |
| Proceeds received on disposal of fixed assets | |
| 6,350 | | |
| 5,125 | |
| Purchase of fixed assets | |
| (43,201 | ) | |
| (393,126 | ) |
| Net cash used in investing activities | |
| (36,851 | ) | |
| (388,001 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities | |
| | | |
| | |
| Proceeds from convertible note payable | |
| 750,000 | | |
| - | |
| Repayment of convertible note payable | |
| (750,000 | ) | |
| - | |
| Proceeds from notes payable | |
| 868,081 | | |
| 1,147,000 | |
| Repayment of notes payable | |
| - | | |
| (505,567 | ) |
| Proceeds from notes payable, related parties | |
| 99,500 | | |
| - | |
| Proceeds from sale of preferred and common stock | |
| 350,000 | | |
| 3,577,505 | |
| Net cash provided by financing activities | |
| 1,317,581 | | |
| 4,218,938 | |
| | |
| | | |
| | |
| Effect of exchange rate changes on cash | |
| (8,909 | ) | |
| (11,477 | ) |
| | |
| | | |
| | |
| Net increase (decrease) in cash | |
| (108,662 | ) | |
| 90,758 | |
| Cash - beginning | |
| 119,678 | | |
| 28,920 | |
| Cash - ending | |
$ | 11,016 | | |
$ | 119,678 | |
| | |
| | | |
| | |
| Supplemental disclosures: | |
| | | |
| | |
| Interest paid | |
$ | 117,112 | | |
$ | 48,252 | |
| Income taxes paid | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-cash investing and financing transactions: | |
| | | |
| | |
| Initial recognition of right-of-use assets and lease liabilities | |
$ | 1,962,998 | | |
$ | - | |
| Cost of preferred shares exchanged for conversion to common
stock | |
$ | - | | |
$ | 850,000 | |
| Value of commitment shares issued as a debt discount | |
$ | - | | |
$ | 418,312 | |
| Value of warrants issued as a debt discount | |
$ | - | | |
$ | 358,017 | |
| Dividends payable | |
$ | 38,923 | | |
$ | 61,684 | |
| Par value of cashless exercise of common stock options | |
$ | - | | |
$ | 60 | |
The accompanying notes are an integral part of these consolidated financial
statements.
ONE WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
Note
1 – Nature of Business and Significant Accounting Policies
Nature
of Business
One
World Products, Inc. (the “Company,” “we,” “our” or “us”) was incorporated in Nevada
on September 2, 2014. On February 21, 2019, One World Pharma, Inc. (“One World Pharma”) entered into an Agreement and Plan
of Merger with OWP Merger Subsidiary, Inc., our wholly-owned subsidiary, and OWP Ventures, Inc. (“OWP Ventures”), which is
the parent company of One World Pharma SAS, a Colombian company (“OWP Colombia”). Pursuant to the Merger Agreement, we acquired
OWP Ventures (and indirectly, OWP Colombia) by the merger of OWP Merger Subsidiary with and into OWP Ventures, with OWP Ventures being
the surviving entity as our wholly-owned subsidiary (the “Merger”). As a result of the Merger (a) holders of the outstanding
capital stock of OWP Ventures received an aggregate of 39,475,398
shares of our common stock; (b) options
to purchase 825,000
shares of common stock of OWP Ventures
at an exercise price of $0.50
automatically converted into options to
purchase 825,000
shares of our common stock at an exercise
price of $0.50;
(c) the outstanding principal and interest under a $300,000
convertible note issued by OWP Ventures
became convertible, at the option of the holder, into shares of our common stock at a conversion price equal to the lesser of $0.424
per share or 80% of the price we sell
our common stock in a future “Qualified Offering”; (d) 875,000
shares of our common stock owned by OWP
Ventures prior to the Merger were cancelled; and (e) OWP Ventures’ chief operating officer became our chief operating officer and
two of OWP Ventures’ directors became members of our board of directors. The Company’s headquarters are located in Las Vegas,
Nevada, and all of its customers are expected to be outside of the United States. On January 10, 2019, the Company changed its name from
Punto Group, Corp. to One World Pharma, Inc., and on November 23, 2021, the Company changed its name to One World Products, Inc. through
the merger of One World Products, Inc., a recently formed Nevada corporation wholly-owned by the Company, with and into the Company (the
“Name Change Merger”) pursuant to the applicable provisions of the Nevada Revised Statutes (“NRS”). As permitted
by the NRS, the articles of merger filed with the Secretary of State of the state of Nevada to effect the Name Change Merger amended
Article I of the Company’s Articles of Incorporation to change the Company’s name to “One World Products, Inc.”
The Name Change Merger was effected solely to effect the change of the Company’s name, and had no effect on the Company’s
officers, directors, operations, assets or liabilities.
OWP
Ventures is a holding company formed in Delaware on March 27, 2018 to enter and support the cannabis industry, and on May 30, 2018,
it acquired OWP Colombia. OWP Colombia is a licensed cannabis cultivation, production and distribution (export) company located in
Popayán, Colombia (nearest major city is Cali). We plan to be a producer of raw cannabis and hemp plant ingredients for both
medical and industrial uses across the globe. We have received licenses to cultivate, produce and distribute the raw ingredients of
the cannabis and hemp plant for medicinal, scientific and industrial purposes. Specifically, we are one of the few companies in
Colombia to receive all four licenses, including seed use, cultivation of non-psychoactive cannabis, cultivation of psychoactive
cannabis, and manufacturing allowing for extraction and export. Currently, we own approximately 30 acres and have a covered
greenhouse built specifically to cultivate high-grade cannabis and hemp. In addition, we have entered into agreements with a local
farming co-operative that include small farmers and indigenous tribe members, under which they will cultivate cannabis on up to
approximately 140 acres of land using our seeds and propagation techniques, and sell their harvested products to us on an exclusive
basis. We began harvesting cannabis in the first quarter of 2019 for the purpose of further research and development activities,
quality control testing and extraction. We have been generating revenue from the sale of our seeds since the second quarter of 2020.
During the first quarter of 2022, we made payments of approximately $1,400,000 for
a state of the art distillation machine that we expect to be placed in service during the second quarter of 2023 within our
vertically integrated extraction facility in which we entered into a 5-year
lease on October 1, 2022, where we have combined our office and extraction facilities into the same building.
Basis
of Presentation
The
accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States
of America and the rules of the Securities and Exchange Commission (“SEC”). All references to Generally Accepted Accounting
Principles (“GAAP”) are in accordance with The Financial Accounting Standards Board (“FASB”) Accounting Standards
Codification (“ASC”) and the Hierarchy of Generally Accepted Accounting Principles.
These
statements reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for
fair presentation of the information contained therein.
ONE WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
Principles
of Consolidation
The
accompanying consolidated financial statements include the accounts of the following entities, all of which were under common control
and ownership at December 31, 2022:
Schedule
of Common Control and Ownership Interest
| |
|
State
of |
|
|
| Name
of Entity |
|
Incorporation |
|
Relationship |
| One
World Products, Inc.(1) |
|
Nevada |
|
Parent |
| OWP Ventures, Inc.(2) |
|
Delaware |
|
Subsidiary |
| One
World Pharma S.A.S.(3) |
|
Colombia |
|
Subsidiary |
| Colombian
Hope, S.A.S.(4) |
|
Colombia |
|
Subsidiary |
| Agrobase,
S.A.S.(5) |
|
Colombia |
|
Subsidiary |
| (1) |
Holding
company in the form of a corporation. |
| (2) |
Holding
company in the form of a corporation and wholly-owned subsidiary of One World Products, Inc. |
| (3) |
Wholly-owned
subsidiary of OWP Ventures, Inc. since May 30, 2018, located in Colombia and legally constituted as a simplified stock company registered
in the Chamber of Commerce of Bogotá on July 18, 2017. Its headquarters are located in Bogotá. |
| (4) |
Wholly-owned
subsidiary of OWP Ventures, Inc., acquired on November 19, 2019, located in Colombia and legally constituted as a simplified stock
company. This company has yet to incur any substantive income or expenses. |
| (5) |
Wholly-owned
subsidiary of OWP Ventures, Inc., formed on September 12, 2019, located in Colombia and legally constituted as a simplified stock company.
This company has yet to incur any substantive income or expenses. |
The
consolidated financial statements herein contain the operations of the wholly-owned subsidiaries listed above. The Company’s headquarters
are located in Las Vegas, Nevada and substantially all of its production efforts are within Popayán, Colombia.
Reclassifications
Certain
reclassifications have been made to the prior years’ financial statements to conform to current year presentation. These reclassifications
had no effect on previously reported results of operations or retained earnings.
Foreign
Currency Translation
The
functional currency of the Company is Columbian Peso (COP). The Company has maintained its financial statements using the functional
currency, and translated those financial statements to the US Dollar throughout this report. Monetary assets and liabilities denominated
in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance
sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at
the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are
included in the determination of net income (loss) for the respective periods.
Comprehensive
Income
The
Company has adopted ASC 220, Reporting Comprehensive Income, which establishes standards for reporting and displaying comprehensive income,
its components, and accumulated balances in a full-set of general-purpose financial statements. Accumulated other comprehensive income
represents the accumulated balance of foreign currency translation adjustments.
Use
of Estimates
The
preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires
management to make estimates and assumptions that may affect the reported amounts of assets and liabilities and disclosure of contingent
assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting
period. Actual results could differ from these estimates.
Segment
Reporting
ASC
Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment reporting. The management
approach model is based on the way a company’s management organizes segments within the company for making operating decisions
and assessing performance. The Company operates as a single segment and will evaluate additional segment disclosure requirements as it
expands its operations.
ONE WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
Fair
Value of Financial Instruments
The
Company discloses the fair value of certain assets and liabilities in accordance with ASC 820, Fair Value Measurements and Disclosures
(ASC 820). ASC 820 defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances
disclosure requirements for fair value measures. The three levels are defined as follows:
| |
- |
Level
1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| |
- |
Level
2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that
are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| |
- |
Level
3 inputs to valuation methodology are unobservable and significant to the fair measurement. |
The
carrying value of cash, accounts receivable, accounts payables and accrued expenses are estimated by management to approximate fair value
primarily due to the short-term nature of the instruments.
Cash
in Excess of FDIC Insured Limits
The
Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. Accounts are guaranteed by
the Federal Deposit Insurance Corporation (FDIC) up to $250,000,
under current regulations. The Company did not
have any cash in excess of FDIC insured limits at December
31, 2022, and has not experienced any losses in such accounts.
Inventory
Inventories
are stated at the lower of cost or net realizable value. Cost is determined on a standard cost basis that approximates the first-in,
first-out (FIFO) method. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating
net realizable value. Our cannabis products consist of cannabis flower grown in-house, along with produced extracts.
Fixed
Assets
Fixed
assets are stated at the lower of cost or estimated net recoverable amount. The cost of property, plant and equipment is depreciated
using the straight-line method based on the lesser of the estimated useful lives of the assets or the lease term based on the following
life expectancy:
Schedule
of Estimated Useful Lives of Fixed Assets
| Buildings |
15
years |
| Office
equipment |
5
years |
| Furniture
and fixtures |
7
years |
| Equipment
and machinery |
7
years |
| Leasehold
improvements |
Term
of lease |
Repairs
and maintenance expenditures are charged to operations as incurred. Major improvements and replacements, which have extended the useful
life of an asset, are capitalized and depreciated over the remaining estimated useful life of the asset. When assets are retired or sold,
the cost and related accumulated depreciation and amortization are eliminated and any resulting gain or loss is reflected in operations.
Revenue
Recognition
The
Company recognizes revenue in accordance with ASC 606 — Revenue from Contracts with Customers. Under ASC 606, the Company recognizes
revenue from the commercial sales of products, licensing agreements and contracts to perform pilot studies by applying the following
steps: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction
price; (4) allocate the transaction price to each performance obligation in the contract; and (5) recognize revenue when each performance
obligation is satisfied. The Company’s sales to date have primarily consisted of the sale of seeds. These sales include multi-element
arrangements whereby the Company collects 50% of the sale upon delivery of the sales, and the remaining 50% upon the completion of the
harvest, whether the seeds result in a successful crop, or not. In addition, the Company has a right of first refusal to purchase products
resulting from the harvest. At December 31, 2022, the Company had $11,808
of deferred revenues and $6,655
of deferred cost of goods sold, as included
in other current assets on the balance sheet, that are expected to be recognized upon the customers’ completion of their harvests
in 2023.
Advertising
Costs
The
Company expenses the cost of advertising and promotions as incurred. Advertising and promotions expense was $80,498
and $137,915
for the years ended December 31, 2022
and 2021, respectively.
ONE WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
Basic
and Diluted Loss Per Share
The
basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding. Diluted
net loss per common share is computed by dividing the net loss adjusted on an “as if converted” basis, by the weighted average
number of common shares outstanding plus potential dilutive securities. For the years ended December 31, 2022 and 2021, potential dilutive
securities had an anti-dilutive effect and were not included in the calculation of diluted net loss per common share.
Stock-Based
Compensation
The
Company accounts for equity instruments issued to employees and non-employees in accordance with the provisions of ASC 718 Stock Compensation
(ASC 718). All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted
for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably
measurable. The measurement date of the fair value of the equity instrument issued is the earlier of the date on which the counterparty’s
performance is complete or the date at which a commitment for performance by the counterparty to earn the equity instruments is reached
because of sufficiently large disincentives for nonperformance.
Income
Taxes
The
Company recognizes deferred tax assets and liabilities based on differences between the financial reporting and tax basis of assets and
liabilities using the enacted tax rates and laws that are expected to be in effect when the differences are expected to be recovered.
The Company provides a valuation allowance for deferred tax assets for which it does not consider realization of such assets to be more
likely than not.
Uncertain
Tax Positions
In
accordance with ASC 740, “Income Taxes” (“ASC 740”), the Company recognizes the tax benefit from an uncertain
tax position only if it is more likely than not that the tax position will be capable of withstanding examination by the taxing authorities
based on the technical merits of the position. These standards prescribe a recognition threshold and measurement attribute for the financial
statement recognition and measurement of a tax position taken or expected to be taken in a tax return. These standards also provide guidance
on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition.
Various
taxing authorities periodically audit the Company’s income tax returns. These audits include questions regarding the Company’s
tax filing positions, including the timing and amount of deductions and the allocation of income to various tax jurisdictions. In evaluating
the exposures connected with these various tax filing positions, including state and local taxes, the Company records allowances for
probable exposures. A number of years may elapse before a particular matter, for which an allowance has been established, is audited
and fully resolved. The Company has not yet undergone an examination by any taxing authorities.
The
assessment of the Company’s tax position relies on the judgment of management to estimate the exposures associated with the Company’s
various filing positions.
Recent
Accounting Pronouncements
From
time to time, new accounting pronouncements are issued by the FASB that are adopted by the Company as of the specified effective date.
If not discussed, management believes that the impact of recently issued standards, which are not yet effective, will not have a material
impact on the Company’s financial statements upon adoption.
In
October 2021, the FASB issued ASU 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities
from Contracts with Customers, which creates an exception to the general recognition and measurement principle for contract assets
and contract liabilities from contracts with customers acquired in a business combination. The new guidance will require companies to
apply the definition of a performance obligation under accounting standard codification ASC Topic 606 to recognize and measure contract
assets and contract liabilities (i.e., deferred revenue) relating to contracts with customers that are acquired in a business combination.
Under current GAAP, an acquirer in a business combination is generally required to recognize and measure the assets it acquires and the
liabilities it assumes at fair value on the acquisition date. The new guidance will result in the acquirer recording acquired contract
assets and liabilities on the same basis that would have been recorded by the acquiree before the acquisition under ASC Topic 606. These
amendments are effective for fiscal years beginning after December 15, 2022, with early adoption permitted. The adoption of ASU 2021-08
is not expected to have a material impact on the Company’s financial statements or related disclosures.
ONE
WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
In
May 2021, the FASB issued ASU No. 2021-04, Earnings Per Share (Topic 260), Debt – Modifications and Extinguishments
(Subtopic 470-50), Compensation (Topic 718), and Derivatives and Hedging – Contracts in Entity’s Own Equity
(Subtopic 815-40) Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity Classified Written Call
Options. ASU 2021-04 addresses issuer’s accounting for certain modifications or exchanges of freestanding equity-classified
written call options. ASU 2021-04 is effective for fiscal years beginning after December 15, 2021 and interim periods within those fiscal
years, with early adoption permitted. The adoption of ASU 2021-04 has not had a material impact on the Company’s financial statements
or related disclosures.
In
March 2020, the FASB issued ASU 2020-04 establishing Topic 848, Reference Rate Reform. ASU 2020-04 contains practical expedients
for reference rate reform related activities that impact debt, leases, derivatives and other contracts. The pronouncement provides temporary
optional expedients and exceptions to the current guidance on contract modifications and hedge accounting to ease the financial reporting
burdens related to the expected market transition from the London Interbank Offered Rate (“LIBOR”) and other interbank offered
rates to alternative reference rates. The guidance was effective upon issuance and may be applied prospectively to contract modifications
made and hedging relationships entered into or evaluated on or before December 31, 2022. The adoption of ASU 2020-04 did not have a material
impact on the Company’s consolidated financial statements, as we transitioned from the London Interbank Offered Rate, commonly
referred to as LIBOR, to alternative references rates, as well as utilizing the aforementioned expedients and exceptions provided in
ASU 2020-04.
In
August 2020, the FASB issued ASU No. 2020-06, Debt–Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and
Hedging–Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s
Own Equity (ASU 2020-06), which simplifies the accounting for convertible instruments by reducing the number of accounting models available
for convertible debt instruments. This guidance also eliminates the treasury stock method to calculate diluted earnings per share for
convertible instruments and requires the use of the if converted method. The new guidance is effective for all entities for annual periods,
and interim periods within those annual periods, beginning after December 15, 2021, with early adoption permitted. The adoption of ASU
2020-06 has not had a material impact on the Company’s financial statements or related disclosures.
There
are no other recently issued accounting pronouncements that the Company has yet to adopt that are expected to have a material effect
on its financial position, results of operations, or cash flows.
Note
2 – Going Concern
As
shown in the accompanying financial statements, the Company had $2,853,968
of negative working capital as of December
31, 2022, has incurred recurring losses from operations resulting in an accumulated deficit of $22,976,365
as of December 31, 2022, and its cash
on hand may not be sufficient to sustain operations. These factors raise substantial doubt about the Company’s ability to continue
as a going concern. Management is actively pursuing new customers to increase revenues. In addition, the Company is currently seeking
additional sources of capital to fund short term operations. Management believes these factors will contribute toward achieving profitability.
The accompanying financial statements do not include any adjustments that might be necessary if the Company is unable to continue as
a going concern.
The
financial statements do not include any adjustments that might result from the outcome of any uncertainty as to the Company’s ability
to continue as a going concern. These financial statements also do not include any adjustments relating to the recoverability and classification
of recorded asset amounts, or amounts and classifications of liabilities that might be necessary should the Company be unable to continue
as a going concern.
ONE WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
Note
3 – Related Party Transactions
Expense
Reimbursements Owed to Officers and Directors
As
of December 31, 2022, the Company owed a total of $65,382
to one of our Directors, Dr. Kenneth Perego,
II, M.D., and $2,505
to the Company’s CFO, Mr. Timothy
Woods, for unreimbursed expenses, as presented within accounts payable on the Balance Sheet.
Debt
Repayments, Related Party
On
October 18, 2021, the Company repaid a total of $52,918,
consisting of $50,000
of principal and $2,918
of interest, to Isiah Thomas, the Company’s
Chief Executive Officer.
On
September 15, 2021, the Company repaid a total of $130,610,
consisting of $125,000
of principal and $5,610
of interest, to Isiah Thomas, the Company’s
Chief Executive Officer.
On
March 29, 2021, the Company repaid a total of $27,201
of indebtedness owed to the Company’s
Chairman of the Board, Dr. Kenneth Perego, II, M.D., consisting of $26,000
of principal and $1,201
of interest.
Series
B Preferred Stock Sales
On
February 7, 2021, the Company and ISIAH International, LLC (“ISIAH International”), entered into a Securities Purchase Agreement
(the “Purchase Agreement”) under which ISIAH International agreed to purchase from the Company, on the dates provided for
in the Purchase Agreement, an aggregate of 200,000
shares of the Company’s newly designated
Series B Preferred Stock (“Series B Preferred Stock”), convertible into an aggregate of 20,000,000
shares of the Company’s common stock,
for a purchase price of $15
per share of Preferred Stock, and an aggregate
purchase price of $3
million. Each share of Series B Preferred
Stock has a Stated Value of $15
and is convertible into common stock at
a conversion price equal to $0.15.
Isiah Thomas, the Company’s Chief Executive Officer, is the sole member and Chief Executive Officer of ISIAH International. Pursuant
to the Purchase Agreement, ISIAH International purchased the 200,000
shares of Series B Preferred Stock from
the Company according to the following schedule:
Schedule
of Agreement to Purchase Shares of Preferred Stock
| Date | |
Shares | | |
Purchase Price | |
| Initial Closing Date | |
| 16,666 | | |
$ | 249,990 | |
| February 22, 2021 | |
| 16,667 | | |
| 250,005 | |
| March 8, 2021 | |
| 16,667 | | |
| 250,005 | |
| March 22, 2021 | |
| 16,667 | | |
| 250,005 | |
| April 5, 2021 | |
| 16,666 | | |
| 249,990 | |
| April 19, 2021 | |
| 16,667 | | |
| 250,005 | |
| May 17, 2021 | |
| 33,334 | | |
| 500,010 | |
| June 14, 2021 | |
| 33,333 | | |
| 499,995 | |
| July 12, 2021 | |
| 33,333 | | |
| 499,995 | |
| Total | |
| 200,000 | | |
$ | 3,000,000 | |
On
various dates in May, 2021, the Company also received total proceeds of $50,010
from the sale of an aggregate of 3,334
shares of Series B Preferred Stock at
a price of $15
per share to trusts whose beneficiaries
are adult children of Isiah L. Thomas III. Mr. Thomas disclaims beneficial ownership of the shares held by these trusts.
Common
Stock Issued for Services
On
January 1, 2021, the Company awarded options to purchase 5,500,000
shares of common stock at an exercise
price equal to $0.13
per share to Isiah L. Thomas III, the
Company’s Chief Executive Officer and Vice Chairman. The options were issued outside of the 2019 Plan and are exercisable over
a ten
year period. The options vested immediately
as to 2,750,000
shares, and vest as to the remaining 2,750,000
shares quarterly in 250,000
increments over the following eleven quarters.
The estimated value using the Black-Scholes Pricing Model, based on a volatility rate of 192%
and a call option value of $0.1174,
was $645,624.
The options are being expensed over the vesting period, resulting in $117,388
and $410,853
of stock-based compensation expense during
the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, a total of $117,383
of unamortized expenses are expected to
be expensed over the vesting period.
ONE
WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
On
January 1, 2021, the Company awarded options to purchase 350,000
shares of common stock under the 2019
Plan at an exercise price equal to $0.13
per share, exercisable over a ten
year period to the Company’s Vice
Chairman of the Board, Dr. Ken Perego. The options vest in equal quarterly installments over one year. The estimated value using the
Black-Scholes Pricing Model, based on a volatility rate of 192%
and a call option value of $0.1170,
was $40,943.
The options were expensed over the vesting period, resulting in $40,943
of stock-based compensation expense during
the year ended December 31, 2021.
Note
4 – Fair Value of Financial Instruments
Under
FASB ASC 820-10-5, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date (an exit price). The standard outlines a valuation framework and creates
a fair value hierarchy in order to increase the consistency and comparability of fair value measurements and the related disclosures.
Under GAAP, certain assets and liabilities must be measured at fair value, and FASB ASC 820-10-50 details the disclosures that are required
for items measured at fair value.
The
Company has certain financial instruments that must be measured under the new fair value standard. The Company’s financial assets
and liabilities are measured using inputs from the three levels of the fair value hierarchy. The three levels are as follows:
Level
1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access
at the measurement date.
Level
2 - Inputs include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets
or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (e.g.,
interest rates, yield curves, etc.), and inputs that are derived principally from or corroborated by observable market data by correlation
or other means (market corroborated inputs).
Level
3 - Unobservable inputs that reflect our assumptions about the assumptions that market participants would use in pricing the asset or
liability.
ONE WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
The
following schedule summarizes the valuation of financial instruments at fair value on a recurring basis in the balances sheet as of December
31, 2022 and 2021:
Schedule of Valuation
of Financial Instruments at Fair Value on a Recurring Basis
| | |
Level
1 | | |
Level
2 | | |
Level
3 | |
| | |
Fair Value Measurements at December 31, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets | |
| | | |
| | | |
| | |
| Cash | |
$ | 11,016 | | |
$ | - | | |
$ | - | |
| Right-of-use asset | |
| - | | |
| - | | |
| 425,969 | |
| Total assets | |
| 11,016 | | |
| - | | |
| 425,969 | |
| Liabilities | |
| | | |
| | | |
| | |
| Lease liabilities | |
| | | |
| - | | |
| 427,915 | |
| Convertible notes payable, net of $412,673 of debt discounts | |
| - | | |
| 337,327 | | |
| - | |
| Convertible notes payable | |
| - | | |
| 750,000 | | |
| - | |
| Notes payable | |
| - | | |
| 845,524 | | |
| - | |
| Notes payable, related parties | |
| - | | |
| 299,500 | | |
| - | |
| Total liabilities | |
| - | | |
| (1,895,024 | ) | |
| (427,915 | ) |
| Total assets and liabilities | |
$ | 11,016 | | |
$ | (1,895,024 | ) | |
$ | (1,946 | ) |
| | |
Level
1 | | |
Level
2 | | |
Level
3 | |
| | |
Fair Value Measurements at December 31, 2021 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Assets | |
| | | |
| | | |
| | |
| Cash | |
$ | 119,678 | | |
$ | - | | |
$ | - | |
| Total assets | |
| 119,678 | | |
| - | | |
| - | |
| Liabilities | |
| | | |
| | | |
| | |
| Convertible notes payable, net of $412,673
of debt discounts | |
| - | | |
| 337,327 | | |
| - | |
| Notes payable | |
| - | | |
| 119,274 | | |
| - | |
| Notes payable, related parties | |
| - | | |
| 200,000 | | |
| - | |
| Total liabilities | |
| - | | |
| (656,601 | ) | |
| - | |
| Total assets and liabilities | |
$ | 119,678 | | |
$ | (656,601 | ) | |
$ | - | |
There
were no transfers of financial assets or liabilities between Level 1 and Level 2 inputs for the years ended December 31, 2022 or 2021.
Note
5 – Major Customers and Accounts Receivable
The
Company had certain customers whose revenue individually represented 10% or more of the Company’s total revenue, or whose accounts
receivable balances individually represented 10% or more of the Company’s total accounts receivable, as follows:
For
the year ended December 31, 2022, no customers accounted for more than 10%
of the Company’s total revenue,
and for the year ended December 31, 2021, four customers accounted for 60%
of revenue, respectively.
At
December 31, 2022 and 2021, one customer accounted for 67%
and 75%
of accounts receivable, respectively.
ONE
WORLD PRODUCTS, INC.
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
Note
6 – Inventory
Inventories
are stated at the lower of cost or net realizable value. Cost is determined on a standard cost basis that approximates the first-in,
first-out (FIFO) method. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating
net realizable value. Our cannabis products consist of cannabis flower grown in-house, along with produced extracts. Inventory consisted
of the following at December 31, 2022 and 2021, respectively.
Schedule
of Inventory
| | |
December 31, | | |
December 31, | |
| | |
2022 | | |
2021 | |
| Raw materials | |
$ | 18,580 | | |
$ | 31,233 | |
| Work in progress | |
| 1,464 | | |
| 81,182 | |
| Finished goods | |
| 80,858 | | |
| 108,246 | |
| Inventory gross | |
| 100,902 | | |
| 220,661 | |
| Less obsolescence | |
| (46,749 | ) | |
| (22,066 | ) |
| Total
inventory | |
$ | 54,153 | | |
$ | 198,595 | |
Note
7 – Other Current Assets
Other
current assets included the following as of December 31, 2022 and 2021, respectively:
Schedule
of Other Current Assets
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| Prepaid expenses | |
$ | 39,288 | | |
$ | 29,366 | |
| Deferred cost of goods
sold | |
| 6,655 | | |
| 19,470 | |
| Other
receivables | |
| - | | |
| 110,000 | |
| Total | |
$ | 45,943 | | |
$ | 158,836 | |
Note
8 – Other Assets
Other
assets consist entirely of a $179,927
and $147,194
VAT receivable at December 31, 2022 and
2021, respectively, which will be returned upon the successful export of the products purchased in which the taxes were originally paid.
Note
9 – Security Deposits
Security
deposits included the following as of December 31, 2022 and 2021, respectively:
Schedule
of Security Deposits
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| Utility
deposits | |
$ | - | | |
$ | 1,090 | |
| Refundable deposit on
equipment purchase | |
| 50,000 | | |
| 50,000 | |
| Down payment on distillation
equipment | |
| 1,399,413 | | |
| 1,155,000 | |
| Security deposits on
leases held in Colombia | |
| 395 | | |
| 35,869 | |
| Security
deposit on office lease | |
| - | | |
| 14,029 | |
| Security
deposits | |
$ | 1,449,808 | | |
$ | 1,255,988 | |
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
Note
10 – Fixed Assets
Fixed
assets consist of the following at December 31, 2022 and 2021, respectively:
Schedule
of Fixed Assets
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| Land | |
$ | 138,248 | | |
$ | 138,248 | |
| Buildings | |
| 473,971 | | |
| 473,971 | |
| Office equipment | |
| 30,902 | | |
| 56,502 | |
| Furniture and fixtures | |
| 6,495 | | |
| 34,409 | |
| Equipment
and machinery | |
| 423,547 | | |
| 383,829 | |
| Fixed assets, gross | |
| 1,073,163 | | |
| 1,086,959 | |
| Less:
accumulated depreciation | |
| (84,627 | ) | |
| (83,946 | ) |
| Total | |
$ | 988,536 | | |
$ | 1,003,013 | |
On
August 15, 2022, the Company, through its wholly-owned subsidiary, OWP Ventures, Inc., sold its office furniture and equipment with a
net book value of $15,391
for gross proceeds of $6,350,
resulting in a loss on the disposal of fixed assets of $9,041,
which represented the proceeds received, less the net book value at the time of disposal.
On
November 30, 2021, the Company disposed of a building that was damaged in a storm at the Popayán farm. No
proceeds were received on the disposal,
resulting in a loss on disposal of fixed assets of $53,925,
which represented the net book value at the time of disposal.
On
July 27, 2021, the Company sold a truck previously used at the Popayán farm. The Company received proceeds of $5,125
on the sale, resulting in a loss on disposal
of fixed assets of $2,064,
which represented the net book value at the time of disposal.
On
July 1, 2021, the Company disposed of equipment used at the Popayán farm that is no longer in service. No
proceeds were received on the disposals,
resulting in a loss on disposal of fixed assets of $15,498,
which represented the net book value at the time of disposal.
Depreciation
and amortization expense totaled $42,287
and $40,321
for the years ended December 31, 2022
and 2021, respectively.
Note
11 – Accrued Expenses
Accrued
expenses consisted of the following at December 31, 2022 and 2021, respectively:
Schedule
of Accrued Expenses
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| Accrued
payroll | |
$ | 613,569 | | |
$ | 261,044 | |
| Accrued withholding
taxes and employee benefits | |
| 31,632 | | |
| 9,162 | |
| Accrued ICA fees and
contributions | |
| 167,037 | | |
| 129,856 | |
| Accrued
interest | |
| 136,220 | | |
| 57,700 | |
| Accrued expenses | |
$ | 948,458 | | |
$ | 457,762 | |
Note
12 – Deferred Revenues
Arrangements
with customers include multiple deliverables, consisting of an initial delivery of seeds and a contingent portion of the sale that is
dependent on the customers future harvest of the seeds. Deferred revenues associated with these multiple-element arrangements were $11,808
and $30,164
at December 31, 2022 and 2021, respectively.
Related deferred cost of goods sold were $6,655
and $19,470
at December 31, 2022 and 2021, respectively,
resulting in deferred gross margins of $5,153
and $10,964
at December 31, 2022 and 2021, respectively,
that is expected to be recognized upon the customers’ completion of their harvests in future periods.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
Note
13 – Leases
The
Company leased its 12,400 square
foot extraction facility under a non-cancelable real property lease agreement that commenced on January 1, 2022 and was to expire on
December 31, 2027, at a monthly lease rate of 57,339,000
COP, or approximately $15,290.
The Company terminated the lease on September 30, 2022, resulting in termination fees of approximately $7,700.
A gain of $20,148
was recognized on the early extinguishment of the lease for
the year ended December 31, 2022.
On
October 1, 2022, the Company entered into a five-year non-cancelable property lease, with an automatic five year extension, for a new
extraction facility with combined office space, at a monthly lease term of 29,000,000
COP plus VAT and administration fees,
or approximately $6,300,
with annual escalation of lease payments equal to the Consumer Price Index, plus 2%.
The
Company also leases a residential premise under a non-cancelable real property lease agreement that commenced on September 1, 2021 and
expires on August 31, 2024, at a monthly lease term of 3,800,000
COP, or approximately $1,013,
with approximately a 3%
annual escalation of lease payments commencing September 1,
2022.
The
Company leases another residential premise under a non-cancelable real property lease agreement that commenced on June 1, 2022 and expires
on May 30, 2024, at a monthly lease term of 1,900,000
COP, or approximately $507with
an 8% annual
escalation of lease payments commencing June 1, 2023.
In
addition, the Company leases its corporate offices and operational facility in Colombia under short-term non-cancelable real property
lease agreements that expire within a year. The Company doesn’t have any other office or equipment leases that would require capitalization.
The office lease contains provisions requiring payment of property taxes, utilities, insurance, maintenance and other occupancy costs
applicable to the leased premise. In the locations in which it is economically feasible to continue to operate, management expects to
enter into a new lease upon expiration. The extraction facility lease contained provisions requiring payment of property taxes, utilities,
insurance, maintenance and other occupancy costs applicable to the leased premise. As the Company’s leases do not provide implicit
discount rates, the Company uses an incremental borrowing rate based on the information available at the commencement date in determining
the present value of lease payments.
The
components of lease expense were as follows:
Schedule of Components
of Lease Expense
| | |
2022 | | |
2021 | |
| | |
For the Year
Ended | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Operating lease cost: | |
| | | |
| | |
| Amortization
of right-of-use assets | |
$ | 114,907 | | |
$ | 87,276 | |
| Interest
on lease liabilities | |
| 83,702 | | |
| 3,035 | |
| Lease
payments on short term leases | |
| 23,811 | | |
| - | |
| Total
operating lease cost | |
$ | 222,420 | | |
$ | 90,311 | |
Supplemental
balance sheet information related to leases was as follows:
Schedule of Supplemental
Balance Sheet Information Related to Leases
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| Operating lease: | |
| | | |
| | |
| Operating
lease assets | |
$ | 425,969 | | |
$ | - | |
| | |
| | | |
| | |
| Current
portion of operating lease liabilities | |
$ | 86,235 | | |
| - | |
| Noncurrent
operating lease liabilities | |
| 341,680 | | |
| - | |
| Total
operating lease liability | |
$ | 427,915 | | |
$ | - | |
| | |
| | | |
| | |
| Weighted average remaining
lease term: | |
| | | |
| | |
| Operating
leases | |
| 4.25
years | | |
| - | |
| | |
| | | |
| | |
| Weighted
average discount rate: | |
| | | |
| | |
| Operating
lease | |
| 6.75 | % | |
| - | |
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
Supplemental
cash flow and other information related to operating leases was as follows:
Schedule
of Supplemental Cash Flow Related to Operating Leases
| | |
2022 | | |
2021 | |
| | |
For the Year
Ended | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Cash paid for amounts
included in the measurement of lease liabilities: | |
| | |
| |
| Operating
cash flows used for operating leases | |
$ | 96,253 | | |
$ | 201,525 | |
| | |
| | | |
| | |
| Early
extinguishment of lease: | |
| | | |
| | |
| Lease
liability terminated | |
$ | 1,438,830 | | |
$ | - | |
| Right-of
use asset terminated | |
| (1,418,682 | ) | |
| - | |
| Gain
on early extinguishment of lease | |
$ | 20,148 | | |
$ | - | |
| | |
| | | |
| | |
| Leased assets obtained
in exchange for lease liabilities: | |
| | | |
| | |
| Total
operating lease liabilities | |
$ | 1,962,998 | | |
$ | - | |
Our
anticipated future lease commitments on a calendar year basis in US dollars, excluding common area maintenance fees, under non-cancelable
operating leases are as follows:
Schedule of Operating Lease Liability
Maturity
| | |
Minimum | |
| Year Ending | |
Lease | |
| December 31, | |
Commitments | |
| 2023 | |
$ | 112,508 | |
| 2024 | |
| 107,632 | |
| 2025 | |
| 99,186 | |
| 2026 | |
| 102,162 | |
| 2027 | |
| 78,336 | |
| Total future minimum lease liabilities | |
$ | 499,824 | |
Note
14 – Convertible Note Payable
Convertible
note payable consists of the following at December 31, 2022 and 2021, respectively:
Schedule
of Convertible Note Payable
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| On September 27, 2022, | |
$ | 750,000 | | |
$ | - | |
| On September 27, 2022, the Company completed the sale of a Convertible Promissory Note in the
principal amount of $750,000
(the “Convertible McCabe Note”) to Dr. John McCabe. The unsecured note matures on 16, 2024 (the “Maturity
Date”), bears interest at a rate of 8%
per annum, and the principal and interest is convertible into shares of the Company’s convertible Series B common stock at
a conversion price of $15
per share. | |
$ | 750,000 | | |
$ | - | |
| | |
| | | |
| | |
On
September 24, 2021, the Company completed the sale of a (i) Promissory Note in the principal
amount of $750,000
(the
“Second AJB Note”) to AJB Capital Investments LLC (“AJB Capital”),
(ii) a three-year
warrant to purchase 1,500,000
shares
of the Company’s common stock at an initial exercise price of $0.25
per
share, and (iii) a three-year
warrant to purchase 2,000,000
shares
of the Company’s common stock at an initial exercise price of $0.50
per
share, for an aggregate purchase price of $705,000,
pursuant to a Securities Purchase Agreement between the Company and AJB Capital (the “Purchase
Agreement”). The aggregate estimated value using the Black-Scholes Pricing Model, based
on a volatility rate of 197%
and a call option value of $0.1053
and
$0.1001,
respectively, was $358,017,
and is being amortized as a debt discount over the life of the loan. The Company received
net proceeds of $678,750
after
deductions of debt discounts, consisting of $45,000
pursuant
to an original issue discount, $15,000
of
legal fees and $11,250
of
brokerage fees.
The
Note matures on September
24, 2022 (the
“Maturity Date”), bears interest at a rate of 8%
per annum, and, following an event of default only, is convertible into shares of the Company’s
common stock at a conversion price equal to the lesser of 90% of the lowest trading price
during (i) the 20
trading
day period preceding the issuance date of the note, or (ii) the 20 trading day period preceding
date of conversion of the Note. The Note is also subject to covenants, events of defaults,
penalties, default interest and other terms and conditions customary in transactions of this
nature.
Pursuant
to the Purchase Agreement, the Company paid a commitment fee to AJB Capital in the amount
of $250,000
(the
“Commitment Fee”) in the form of 1,250,000
shares
of the Company’s common stock (the “Commitment Fee Shares”). During the
six month period following the six month anniversary of the closing date, AJB Capital shall
be entitled to be issued additional shares of common stock of the Company to the extent AJB
Capital’s sale of the Commitment Fee Shares has resulted in net proceeds in an amount
less than the Commitment Fee. The Commitment Fee Shares resulted in a debt discount of $150,062
that
is being amortized over the life of the loan.
The
obligations of the Company to AJB Capital under the Note and the Purchase Agreement are secured
by a lien on the Company’s assets pursuant to a Security Agreement between the Company
and AJB Capital. The note was repaid on September 27, 2022. | |
$ | - | | |
$ | 750,000 | |
| | |
| | | |
| | |
| Total convertible notes
payable | |
| 750,000 | | |
| 750,000 | |
| Less:
unamortized debt discounts | |
| - | | |
| 412,673 | |
| Convertible
note payable, net of discounts | |
$ | 750,000 | | |
$ | 337,327 | |
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
The
Company recognized debt discounts for the years ended December 31, 2022 and 2021, as follows:
Schedule
of Convertible Debt Discounts
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Fair value
of 3,250,000
commitment shares of common stock | |
$ | 106,894 | | |
$ | 418,312 | |
| Fair value of warrants
to purchase 3,500,000
shares of common stock | |
| 255,026 | | |
| 358,017 | |
| Original issue discounts | |
| 32,055 | | |
| 53,700 | |
| Legal
and brokerage fees | |
| 18,698 | | |
| 39,300 | |
| Total
debt discounts | |
| 412,673 | | |
| 869,329 | |
| Amortization
of debt discounts | |
| 412,673 | | |
| 456,656 | |
| Unamortized
debt discounts | |
$ | - | | |
$ | 412,673 | |
The
aggregate debt discounts of $869,329 incurred
during the year ended December 31, 2021, were amortized over the life of the loans using the straight-line method, which approximated
the effective interest method. The Company recorded finance expense in the amount of $412,673
and $456,656
on the amortization of these discounts
for the years ended December 31, 2022 and 2021, respectively.
The
convertible note limits the maximum number of shares that can be owned by the note holder as a result of the conversions to common stock
to 4.99% of
the Company’s issued and outstanding shares.
The
Company recorded interest expense pursuant to the stated interest rates on the convertible notes in the amount of $59,023
and $36,243
for the years ended December 31, 2022
and 2021, respectively. In addition, the Company recognized $412,673
and $456,656
of interest expense related to the debt
discounts for the years ended December 31, 2022 and 2021, respectively.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
Note
15 – Notes Payable
Notes
payable consists of the following at December 31, 2022 and 2021, respectively:
Schedule
of Notes Payable
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| On September
15, 2022, the Company, through its wholly-owned subsidiary, One World Pharma, SAS, received proceeds of 55,488,000
COP, or approximately $12,243,
on a loan with a face value of 70,000,000
COP, or approximately $15,445,
from an individual pursuant to an unsecured promissory note, bearing interest at 4%
per month, or 48% per annum, due on
demand. The debt discount of $3,202
was expensed as finance costs at the
time of origination. The face value of the note has been adjusted by $893
due to foreign currency translation
adjustments. | |
$ | 14,552 | | |
$ | - | |
| | |
| | | |
| | |
| On June 17, 2022, the
Company, through its wholly-owned subsidiary, One World Pharma, SAS, received proceeds of 230,400,000
COP, or approximately $55,821,
on a loan with a face value of 240,000,000
COP, or approximately $58,147,
from an individual pursuant to an unsecured promissory note, bearing interest at 4%
per month, or 48% per annum, due on
demand. The debt discount of $2,326
was expensed as finance costs at the
time of origination. The face value of the note has been adjusted by $8,253
due to foreign currency translation
adjustments. | |
| 49,894 | | |
| - | |
| | |
| | | |
| | |
| On June 13, 2022, the
Company, through its wholly-owned subsidiary, OWP Ventures, Inc., received an advance of $100,000
from an individual pursuant to an
unsecured promissory note, maturing on January
1, 2024, that carries an 8%
interest rate. | |
| 100,000 | | |
| - | |
| | |
| | | |
| | |
| On May 31, 2022, the
Company, through its wholly-owned subsidiary, One World Pharma, SAS, received proceeds of 314,640,000
COP, or approximately $76,231,
on a loan with a face value of 360,000,000
COP, or approximately $87,220,
from an individual pursuant to promissory note, security by equipment, bearing interest at 2.1%
per month, or 25% per annum, maturing
on November 28, 2022. The debt discount of $10,990
was expensed as finance costs at the
time of origination. The face value of the note has been adjusted by $12,380
due to foreign currency translation
adjustments. | |
| 74,841 | | |
| - | |
| | |
| | | |
| | |
| On May 30, 2022, the
Company, through its wholly-owned subsidiary, One World Pharma, SAS, received a non-interest bearing loan of 20,000,000
COP, or approximately $4,846,
from an individual pursuant to an unsecured promissory note, due on demand. The face value of the note has been adjusted by $688
due to foreign currency translation
adjustments. | |
| 4,158 | | |
| - | |
| | |
| | | |
| | |
| On April 29, 2022, the
Company, through its wholly-owned subsidiary, One World Pharma, SAS, received a non-interest bearing loan of 10,000,000
COP, or approximately $2,423,
from an individual pursuant to an unsecured promissory note, due on demand. The face value of the note has been adjusted by $344
due to foreign currency translation
adjustments. | |
| 2,079 | | |
| - | |
| | |
| | | |
| | |
| On March 1, 2022, the
Company, through its wholly-owned subsidiary, OWP Ventures, Inc., received an advance of $400,000
from an individual pursuant to an
unsecured promissory note, maturing on January
1, 2024, that carries an 8%
interest rate. | |
| 400,000 | | |
| - | |
| | |
| | | |
| | |
| On February 15, 2022,
the Company, through its wholly-owned subsidiary, OWP Ventures, Inc., received an advance of $200,000
from an individual pursuant to an
unsecured promissory note, maturing on January
1, 2024, that carries an 8%
interest rate. | |
| 200,000 | | |
| - | |
| | |
| | | |
| | |
On
May 4, 2020, the Company, through its wholly-owned subsidiary OWP Ventures, Inc., borrowed $119,274
from Customers Bank (“Lender”),
pursuant to a Promissory Note issued by OWP Ventures to Lender (the “PPP Note”). The loan was made pursuant to the Payroll
Protection Program established as part of the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”). The
PPP Note carried interest at 1.00%
per annum, payable monthly beginning
December 4, 2020, and was due on May
4, 2022. The PPP Note could have been
repaid at any time without penalty.
Under the Payroll Protection Program, the Company was eligible for loan forgiveness
up to the full amount of the PPP Note and any accrued interest. The forgiveness amount was equal to the amount that the Company spent
during the 24-week period beginning May 4, 2020 on payroll costs, payment of rent on any leases in force prior to February 15, 2020
and payment on any utility for which service began before February 15, 2020. The maximum amount of loan forgiveness for non-payroll
expenses was 40%
of the amount of the PPP Note. A total
of $121,372,
consisting of $119,274
of principal and $2,098
of interest, was forgiven on February
11, 2022. | |
| - | | |
| 119,274 | |
| | |
| | | |
| | |
| Notes payable | |
| - | | |
| 119,274 | |
| Total notes payable | |
| 845,524 | | |
| 119,274 | |
| Less:
current maturities | |
| 145,524 | | |
| 119,274 | |
| Notes
payable, long-term portion | |
$ | 700,000 | | |
$ | - | |
The
Company recorded interest expense in the amount of $85,653
and $18,945
for the years ended December 31, 2022
and 2021, respectively.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
Notes
Payable, Related Parties
Note
16 – Notes Payable, Related Party
Notes
payable, related party, consists of the following at December 31, 2022 and 2021, respectively:
Schedule
of Notes Payable Related Party
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| On August
5, 2022, the Company received an advance of $50,000
from Dr. Kenneth Perego, II, M.D.,
our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6%
interest rate. | |
$ | 50,000 | | |
$ | - | |
| | |
| | | |
| | |
| On August 2, 2022, the
Company received an advance of $4,500
from Isiah Thomas, III, our Chairman
of the Board and CEO, pursuant to an unsecured promissory note due on demand that carries a 6%
interest rate. | |
| 4,500 | | |
| - | |
| | |
| | | |
| | |
| On July 7, 2022, the
Company received an advance of $5,000
from Dr. Kenneth Perego, II, M.D.,
our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6%
interest rate. | |
| 5,000 | | |
| - | |
| | |
| | | |
| | |
| On June 3, 2022, the
Company received an advance of $10,000
from Isiah Thomas, III, our Chairman
of the Board and CEO, pursuant to an unsecured promissory note due on demand that carries a 6%
interest rate. | |
| 10,000 | | |
| - | |
| | |
| | | |
| | |
| On May 5, 2022, the
Company received an advance of $10,000
from Isiah Thomas, III, our Chairman
of the Board and CEO, pursuant to an unsecured promissory note due on demand that carries a 6%
interest rate. | |
| 10,000 | | |
| - | |
| | |
| | | |
| | |
| On May 5, 2022, the
Company received an advance of $20,000
from Dr. Kenneth Perego, II, M.D.,
our Vice Chairman of the Board pursuant to an unsecured promissory note due on demand that carries a 6%
interest rate. | |
| 20,000 | | |
| - | |
| | |
| | | |
| | |
| On
December 29, 2021, the Company received an advance of $200,000
from Dr. Kenneth Perego, II, M.D.,
our Vice Chairman of the Board pursuant to an unsecured promissory note due January
1, 2024 that carries an 8%
interest rate. | |
| 200,000 | | |
| 200,000 | |
| | |
| | | |
| | |
| Total notes payable.
related party | |
| 299,500 | | |
| 200,000 | |
| Less:
current maturities | |
| 99,500 | | |
| - | |
| Notes
payable, related party, long-term portion | |
$ | 200,000 | | |
$ | 200,000 | |
The
Company recorded interest expense pursuant to the stated interest rates on the notes payable, related party, in the amount of $19,127
and $9,729
for the years ended December 31, 2022
and 2021, respectively.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
The
Company recognized interest expense for the year ended December 31, 2022 and 2021, respectively, as follows:
Schedule
of Interest Expenses
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Interest
on convertible notes | |
$ | 59,023 | | |
$ | 17,260 | |
| Interest on notes payable | |
| 85,653 | | |
| 18,945 | |
| Interest on notes payable,
related parties | |
| 19,127 | | |
| 9,729 | |
| Amortization of debt
discounts on convertible notes | |
| 50,753 | | |
| 42,247 | |
| Amortization of debt
discounts on convertible notes, common stock | |
| 106,894 | | |
| 311,418 | |
| Amortization of debt
discounts on convertible notes, warrants | |
| 255,026 | | |
| 102,991 | |
| Finance cost on equity
line of credit, issuances of series B preferred stock | |
| 205,005 | | |
| - | |
| Finance cost on equity
line of credit, issuances of common stock | |
| 134,128 | | |
| - | |
| Finance cost on equity
line of credit | |
| 30,000 | | |
| - | |
| Interest
on accounts payable | |
| 11,249 | | |
| 8,541 | |
| Total
interest expense | |
$ | 956,858 | | |
$ | 511,131 | |
Note
17 – Convertible Preferred Stock
Preferred
Stock
The
Company has 10,000,000
authorized shares of $0.001
par value “blank check” preferred
stock, of which 500,000
shares have been designated Series A Preferred
Stock and 300,000
shares have been designated Series B Preferred
Stock. The shares of Series A Preferred Stock and Series B Preferred Stock are each currently convertible into one hundred (100) shares
of the Company’s common stock. The
Series A Preferred Stock accrues dividends at the rate of 6%
per annum, payable in cash as and when declared by the Board or upon a liquidation. The
shares of Series B Preferred Stock are not entitled to dividends, other than the right to participate in dividends payable to holders
of common stock on an as-converted basis. As of December 31, 2022, there were 70,233
and 272,168
shares of Series A Preferred Stock and
Series B Preferred Stock, respectively, issued and outstanding, respectively. The Series A and B Preferred Stock are presented as mezzanine
equity on the balance sheet because they carry a stated value of $10
and $15
per share, respectively, and a deemed
liquidation clause, which entitles the holders thereof to receive proceeds in an amount equal to the stated value per share, plus any
accrued and unpaid dividends, before any payment may be made to holders of common stock. Each share of Preferred Stock carries a number
of votes equal to the number of shares of common stock into which such Preferred Stock may then be converted. The Preferred Stock generally
will vote together with the common stock and not as a separate class.
The
Series A and B Preferred Stock have been classified outside of permanent equity and liabilities. the Series A Preferred Stock embodies
conditional obligations that the Company may settle by issuing a variable number of equity shares, and in both the Series A and B Preferred
Stock, monetary value of the obligation is based on a fixed monetary amount known at inception.
Series
A Preferred Stock Sales
On
various dates between December 20, 2022 and December 21, 2022, the Company received total proceeds of $50,000
from the sale of 5,000
units, consisting in the aggregate of
5,000
shares of Series A Preferred Stock and
five-year
warrants to purchase 500,000
shares of common stock at an exercise
price of $0.25
per share to twenty-two accredited investors.
The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis.
Series
A Preferred Stock Conversions
On
November 15, 2021, a shareholder converted 30,000
shares of Series A Preferred Stock into
3,000,000
shares of common stock. The note was converted
in accordance with the conversion terms; therefore, no gain or loss has been recognized.
On
April 6, 2021, a shareholder converted 30,000
shares of Series A Preferred Stock into
3,000,000
shares of common stock. The note was converted
in accordance with the conversion terms; therefore, no gain or loss has been recognized.
On
March 24, 2021, a shareholder converted 10,000
shares of Series A Preferred Stock into
1,000,000
shares of common stock. The shares of
common stock were subsequently issued on April 7, 2021. The note was converted in accordance with the conversion terms; therefore, no
gain or loss has been recognized.
On
January 26, 2021, a shareholder converted 5,000
shares of Series A Preferred Stock into
500,000
shares of common stock. The note was converted
in accordance with the conversion terms; therefore, no gain or loss has been recognized.
On
January 12, 2021, a shareholder converted 10,000
shares of Series A Preferred Stock into
1,000,000
shares of common stock. The note was converted
in accordance with the conversion terms; therefore, no gain or loss has been recognized.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
Preferred
Stock Dividends
The
Series A Preferred Stock accrues dividends at the rate of 6%
per annum, payable in cash as and when declared by the Board or upon a liquidation. The Company recognized $38,923
and $61,684
for years ended December 31, 2022 and
2021, respectively. A total of $137,843
of dividends had accrued as of December
31, 2022.
Series
B Preferred Stock Sales
On
September 1, 2022, the Company and Tysadco Partners, LLC (“Tysadco”), entered into a Securities Purchase Agreement (the “Purchase
Agreement”) under which Tysadco agreed to purchase from the Company, 20,000
shares of the Company’s Series B
Preferred Stock for a purchase price of $15
per share of Series B Preferred Stock,
and an aggregate purchase price of $300,000.
On September 12, 2022, Tysadco purchased the first 10,000
shares of Series B Preferred Stock under
the Purchase Agreement for $150,000,
and on October 12, 2022, Tysadco purchased the second 10,000
shares of Series B Preferred Stock under
the Purchase Agreement for $150,000.
The Company paid $15,000
out of the proceeds of each investment to Garden State Securities,
Inc. as financing costs. In addition, the Company paid a commitment fee to Tysadco, consisting of 13,667
shares of Series B Preferred Stock. The
issuance of the commitment fee shares resulted in $205,005
of finance expense during the year ended
December 31, 2022.
On
February 7, 2021, the Company and ISIAH International entered into a Securities Purchase Agreement under which ISIAH International agreed
to purchase from the Company, on the dates provided for in the Purchase Agreement, an aggregate of 200,000
shares of the Company’s newly designated
Series B Preferred Stock, convertible into an aggregate of 20,000,000
shares of common stock, for a purchase
price of $15
per share of Preferred Stock, and an aggregate
purchase price of $3
million. Each share of Series B Preferred
Stock has a Stated Value of $15
and is convertible into common stock at
a conversion price equal to $0.15.
Isiah Thomas, the Company’s Chief Executive Officer, is the sole member and Chief Executive Officer of ISIAH International. Pursuant
to the Purchase Agreement, ISIAH International purchased the 200,000
shares of Series B Preferred Stock from
the Company according to the following schedule:
Schedule
to Purchase Shares of Preferred Stock
| Date | |
Shares | | |
Purchase
Price | |
| Initial Closing
Date | |
| 16,666 | | |
$ | 249,990 | |
| February 22, 2021 | |
| 16,667 | | |
| 250,005 | |
| March 8, 2021 | |
| 16,667 | | |
| 250,005 | |
| March 22, 2021 | |
| 16,667 | | |
| 250,005 | |
| April 5, 2021 | |
| 16,666 | | |
| 249,990 | |
| April 19, 2021 | |
| 16,667 | | |
| 250,005 | |
| May 17, 2021 | |
| 33,334 | | |
| 500,010 | |
| June 14, 2021 | |
| 33,333 | | |
| 499,995 | |
| July 12, 2021 | |
| 33,333 | | |
| 499,995 | |
| Total | |
| 200,000 | | |
$ | 3,000,000 | |
In
addition to the shares sold to ISIAH International, the Company received total proceeds of $527,520
on various dates between March 9, 2021
and April 22, 2021 from the sale of an additional 35,167
shares of Series B Preferred Stock at
a price of $15
per share to seven accredited investors,
including proceeds of $50,010
from the sale of an aggregate of 3,334
shares of Series B Preferred Stock at
a price of $15
per share to trusts whose beneficiaries
are adult children of Isiah L. Thomas III. Mr. Thomas disclaims beneficial ownership of the shares held by these trusts.
Note
18 – Commitments and Contingencies
Equity
Line of Credit
On
September 1, 2022, the Company entered into a Purchase Agreement (the “ELOC Purchase Agreement”) with Tysadco. Pursuant to
the ELOC Purchase Agreement, Tysadco has agreed to purchase from the Company, from time to time upon delivery by the Company to Tysadco
of “Request Notices,” and subject to the other terms and conditions set forth in the ELOC Purchase Agreement, up to an aggregate
of $10,000,000
of the Company’s common stock. The
purchase price of the shares of common stock to be purchased under the Purchase Agreement will be equal to 88% of the lowest daily “VWAP”
during the period of 10 trading days beginning five trading days preceding the applicable Request. Each purchase under the Purchase Agreement
will be in a minimum amount of $25,000 and a maximum amount equal to the lesser of (i) $1,000,000 and (ii) 500% of the average daily
trading value of the common stock over the seven trading days preceding the delivery of the applicable Request Notice.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
In
connection with the ELOC Purchase Agreement, the Company entered into a Registration Rights Agreement with Tysadco under which the Company
agreed to file a registration statement with the Securities and Exchange Commission covering the shares of common stock issuable under
the ELOC Purchase Agreement and conversion of the Commitment Fee Shares (the “Registration Rights Agreement”). There have
not been any advances on this arrangement to date.
Commitment
for the Sale of Series B Preferred Stock
On
October 3, 2022, the Company and ISIAH International, LLC (“ISIAH International”), an entity in which the Company’s
CEO, Isiah L. Thomas, III, is the sole member, entered into a securities purchase agreement under which ISIAH International has agreed
to purchase from the Company an aggregate of 33,333
shares of the Company’s Series B
Preferred Stock (the “Series B Shares”), initially convertible into an aggregate of three million three hundred thirty three
thousand three hundred (3,333,300)
shares of the Company’s common stock, for a total purchase price of $499,995.
To date, no purchases under this agreement have occurred.
Note
19 – Stockholders’ Equity
Preferred
Stock
The
Company has 10,000,000
authorized shares of $0.001
par value “blank check” preferred
stock, of which 500,000
shares have been designated Series A Preferred
Stock and 300,000
shares have been designated Series B Preferred
Stock, See Note 17 above for a description of the features and issuances of the Series A Preferred Stock and Series B Preferred Stock.
Common
Stock
The
Company is authorized to issue an aggregate of 300,000,000
shares of common stock with a par value
of $0.001.
As of December 31, 2022, there were 67,202,907
shares of common stock issued and outstanding.
Common
Stock Issued on Subscriptions Payable
On
March 29, 2022, the Company issued 262,066
shares of common stock on a Subscriptions
Payable for the December 1, 2021 award of common stock to COR IR for services.
Common
Stock Issued as a Promissory Note Commitment
As
disclosed in Note 14 above, the Company paid a commitment fee to AJB Capital of $250,000
in the form of 1,250,000
shares of the Company’s common stock
(“Commitment Fee Shares”) in connection with the issuance of the Second AJB Note, which was repaid on September 27, 2022.
The issuance of these commitment fee shares resulted in a debt discount of $150,062
that was amortized over the life of the
loan, resulting in $106,894
and $43,168
of finance expense during the years ended
December 31, 2022 and 2021, respectively. During the six month period following the six-month anniversary of the closing date, AJB Capital
was entitled to be issued additional shares of common stock of the Company to the extent AJB Capital’s sale of the Commitment Fee
Shares has resulted in net proceeds in an amount less than the Commitment Fee. As a result, the Company issued an additional 1,341,276
shares of common stock to AJB Capital
on September 15, 2022. The fair value of the shares was $134,128,
based on the closing price of the Company’s common stock on the date of grant.
Also,
as disclosed in Note 14 above, the Company paid a commitment fee to AJB Capital of $200,000
in the form of 2,000,000
shares of the Company’s common stock
in connection with the issuance of the First AJB Note, which was repaid on September 17, 2021. The issuance of the commitment fee shares
resulted in a debt discount of $268,250
that was amortized over the life of the
loan, resulting in $268,250
of finance expense during the year ended
December 31, 2021. On October 15, 2021, pursuant to the early repayment terms of the promissory note, one million of these shares were
redeemed and cancelled for a nominal aggregate purchase price of $1.00.
Common
Stock Options Exercised
On
July 26, 2021, a total of 60,000
shares of common stock were issued upon
exercise on a cashless basis of options to purchase 125,000
shares of common stock at a price $0.13
per share.
Common
Stock Issued for Services, Employees and Consultants
On
May 25, 2021, the Company awarded a total of 50,000
shares of common stock pursuant for consulting
services to two individuals. The aggregate fair value of the shares was $8,500,
based on the closing price of the Company’s common stock on the date of grant.
On
May 12, 2021, the Company entered into a Settlement Agreement with COR. Pursuant to the Settlement Agreement, the Company issued COR
118,150
shares of common stock. The
fair value of the shares was $29,538,
based on the closing price of the Company’s common stock on the date of grant.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
On
June 1, 2021, the Company entered into a new agreement with COR and issued another 112,528
shares of common stock to COR. The
fair value of the shares was $18,758,
based on the closing price of the Company’s common stock on the date of grant.
On December 1, 2021, the Company owed COR another 262,066
shares of common stock, which were subsequently
issued on March 29, 2022. The fair value of the shares was $21,725,
based on the closing price of the Company’s common stock on the date of grant.
Common
Stock Issued for Services, Officers and Directors
On
December 31, 2021, the Company issued 673,582
shares of common stock in lieu of cash
compensation to its former Chief Financial Officer, Vahé Gabriel. The aggregate fair value
of the shares was $55,234,
based on the closing price of the Company’s common stock on the date of grant.
Note
20 – Common Stock Options
Stock
Incentive Plan
On
February 12, 2020, the Company’s stockholders approved our 2019 Stock Incentive Plan (the “2019 Plan”), which had been
adopted by the Company’s Board of Directors (the “Board”) as of December 10, 2019. The 2019 Plan provides for the issuance
of up to 10,000,000
shares of common stock to the Company
and its subsidiaries’ employees, officers, directors, consultants and advisors, stock options (non-statutory and incentive), restricted
stock awards, stock appreciation rights (“SARs”), restricted stock units (“RSUs”) and other performance stock
awards. Options granted under the 2019 Plan may either be intended to qualify as incentive stock options under the Internal Revenue Code
of 1986, or may be non-qualified options, and are exercisable over periods not exceeding ten years from date of grant. Unless sooner
terminated in accordance with its terms, the Stock Plan will terminate on December 10, 2029.
Common
Stock Options Issued for Services
On
May 28, 2021, the Company awarded options to purchase 1,000,000
shares of common stock under the 2019
Plan at an exercise price equal to $0.1782
per share, exercisable over a ten
year period to the Company’s CFO
and COO, Vahé Gabriel. The options vested immediately as to 500,000
shares, and vest as to the remaining 500,000
shares quarterly in 250,000
increments over the following two quarters.
The estimated value using the Black-Scholes Pricing Model, based on a volatility rate of 183%
and a call option value of $0.1719,
was $171,949.
The options were expensed over the vesting period, resulting in $171,949
of stock-based compensation expense during
the year ended December 31, 2021.
On
May 25, 2021, the Company awarded options to purchase an aggregate 425,000
shares of common stock under the 2019
Plan at an exercise price equal to $0.17
per share, exercisable over a ten
year period to three advisory board members.
The options vest in equal quarterly installments over two years. The aggregate estimated value using the Black-Scholes Pricing Model,
based on a volatility rate of 183%
and a call option value of $0.1653,
was $70,269.
The options are being expensed over the vesting period, resulting in $35,132
and $20,493
of stock-based compensation expense during
the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, a total of $14,644
of unamortized expenses are expected to
be expensed over the vesting period.
On
January 1, 2021, the Company awarded options to purchase 5,500,000
shares of common stock at an exercise
price equal to $0.13
per share to Isiah L. Thomas III, the
Company’s Chief Executive Officer and Vice Chairman. The options were issued outside of the 2019 Plan and are exercisable over
a ten
year period. The options vested immediately
as to 2,750,000
shares, and vest as to the remaining 2,750,000
shares quarterly in 250,000
increments over the following eleven quarters.
The estimated value using the Black-Scholes Pricing Model, based on a volatility rate of 192%
and a call option value of $0.1174,
was $645,624.
The options are being expensed over the vesting period, resulting in $117,388
and $410,853
of stock-based compensation expense during
the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, a total of $117,383
of unamortized expenses are expected to
be expensed over the vesting period.
On
January 1, 2021, the Company awarded options to purchase 350,000
shares of common stock under the 2019
Plan at an exercise price equal to $0.13
per share, exercisable over a ten
year period to the Company’s Chairman
of the Board, Dr. Ken Perego. The options vest in equal quarterly installments over one year. The estimated value using the Black-Scholes
Pricing Model, based on a volatility rate of 192%
and a call option value of $0.1170,
was $40,943.
The options were expensed over the vesting period, resulting in $40,943
of stock-based compensation expense during
the year ended December 31, 2021.
On
January 1, 2021, the Company awarded options to purchase 475,000
shares of common stock under the 2019
Plan at an exercise price equal to $0.13
per share, exercisable over a ten
year period to Bruce Raben, one of the
Company’s Directors. The options vest in equal quarterly installments over one year. The estimated value using the Black-Scholes
Pricing Model, based on a volatility rate of 192%
and a call option value of $0.1170,
was $55,565.
The options were expensed over the vesting period, resulting in $55,565
of stock-based compensation expense during
the year ended December 31, 2021.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
On
January 1, 2021, the Company awarded options to purchase an aggregate 1,842,000
shares of common stock under the 2019
Plan at an exercise price equal to $0.13
per share, exercisable over a ten
year period to seven consultants and employees.
The options vest in equal quarterly installments over one year. The aggregate estimated value using the Black-Scholes Pricing Model,
based on a volatility rate of 192%
and a call option value of $0.1170,
was $215,475.
The options were expensed over the vesting period, resulting in $215,475
of stock-based compensation expense during
the year ended December 31, 2021.
Common
Stock Options Exercised
On
July 26, 2021, a total of 60,000
shares of common stock were issued upon
exercise on a cashless basis of options to purchase 125,000
shares of common stock at a price $0.13
per share.
Common
Stock Options Expired
On
January 28, 2022, options to purchase a total of 500,000
shares of common stock at a price $0.50
per share expired.
The
following is a summary of information about the Stock Options outstanding at December 31, 2022.
Schedule
of Option Exercise Price Range
| | | |
Shares Underlying | |
| Shares
Underlying Options Outstanding | | |
Options
Exercisable | |
| | | |
| | |
Weighted | | |
| | |
| | |
| |
| | | |
Shares | | |
Average | | |
Weighted | | |
Shares | | |
Weighted | |
| | | |
Underlying | | |
Remaining | | |
Average | | |
Underlying | | |
Average | |
| Range of | | |
Options | | |
Contractual | | |
Exercise | | |
Options | | |
Exercise | |
| | Exercise
Prices | | |
| Outstanding | | |
| Life | | |
| Price | | |
| Exercisable | | |
| Price | |
| $ | 0.13
-
$0.56 | | |
| 10,242,000 | | |
| 8.05
years | | |
$ | 0.15 | | |
| 9,113,528 | | |
$ | 0.15 | |
The
following is a summary of activity of outstanding stock options:
Schedule
of Option Activity
| | |
| | |
Weighted | |
| | |
| | |
Average | |
| | |
Number | | |
Exercise | |
| | |
of
Shares | | |
Prices | |
| Balance,
December 31, 2020 | |
| 1,275,000 | | |
$ | 0.36 | |
| Options
granted | |
| 9,592,000 | | |
| 0.14 | |
| Options
exercised | |
| (125,000 | ) | |
| (0.13 | ) |
| Balance, December 31,
2021 | |
| 10,742,000 | | |
| 0.16 | |
| Options
granted | |
| - | | |
| - | |
| Options
expired | |
| (500,000 | ) | |
| (0.50 | ) |
| Balance,
December 31, 2022 | |
| 10,242,000 | | |
$ | 0.15 | |
| | |
| | | |
| | |
| Exercisable,
December 31, 2022 | |
| 9,113,528 | | |
$ | 0.15 | |
Note
21 – Common Stock Warrants
Warrants
to purchase a total of 11,511,650
shares of common stock were outstanding as of December 31,
2022.
On
December 21, 2022, the Company received proceeds of $50,000
from the sale of 2,500
units, consisting of 2,500
shares of Series A Preferred Stock and
five-year
warrants to purchase 250,000
shares of common stock at an exercise
price of $0.25
per share from an accredited investor.
The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis. The aggregate
estimated value of the warrants using the Black-Scholes Pricing Model, based on a weighted average volatility rate of 157%
and a weighted average call option value of $0.0699,
was $17,468.
On
December 20, 2022, the Company received proceeds of $50,000
from the sale of 2,500
units, consisting of 2,500
shares of Series A Preferred Stock and
five-year
warrants to purchase 250,000
shares of common stock at an exercise
price of $0.25
per share from another accredited investor.
The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis. The aggregate
estimated value of the warrants using the Black-Scholes Pricing Model, based on a weighted average volatility rate of 157%
and a weighted average call option value of $0.07,
was $17,499.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
The
following is a summary of information about our warrants to purchase common stock outstanding at December 31, 2022.
Schedule
of Warrants to Purchase Common Stock Outstanding
| Shares
Underlying Warrants Outstanding | | |
Shares
Underlying
Warrants
Exercisable | |
| | Range
of Exercise Prices | | |
| Shares Underlying Warrants Outstanding | | |
| Weighted Average Remaining Contractual Life | | |
| Weighted Average Exercise Price | | |
| Shares Underlying Warrants Exercisable | | |
| Weighted Average Exercise Price | |
| $ | 0.25-$0.50 | | |
| 11,511,650 | | |
| 2.43
years | | |
$ | 0.25-$0.50 | | |
| 11,511,650 | | |
$ | 0.25-$0.50 | |
The
fair value of each warrant grant is estimated on the date of grant using the Black-Scholes option pricing model with the following weighted-average
assumptions used for grants under the fixed option plan:
Schedule
of Fair value Assumption of Warrants
| | |
December
31, | | |
December
31, | |
| | |
2022 | | |
2021 | |
| | |
| | | |
| | |
| Average
risk-free interest rates | |
| 3.79 | % | |
| 0.47 | % |
| Average expected life
(in years) | |
| 5.00 | | |
| 3.00 | |
| Volatility | |
| 157 | % | |
| 197 | % |
The
weighted average fair value of warrants granted with exercise prices at the current fair value of the underlying stock was approximately
$0.29
and $0.10
per warrant for the years ended December
31, 2022 and 2021, respectively.
The
following is a summary of activity of outstanding common stock warrants:
Schedule
of Warrants Activity
| | |
| | |
Weighted | |
| | |
| | |
Average | |
| | |
Number | | |
Exercise | |
| | |
of
Shares | | |
Prices | |
| Balance,
December 31, 2020 | |
| 7,511,650 | | |
$ | 0.25 | |
| Warrants
granted | |
| 3,500,000 | | |
| 0.39 | |
| Balance, December 31,
2021 | |
| 11,011,650 | | |
| 0.30 | |
| Warrants
granted | |
| 500,000 | | |
| 0.25 | |
| Balance,
December 31, 2022 | |
| 11,511,650 | | |
$ | 0.29 | |
| | |
| | | |
| | |
| Exercisable,
December 31, 2022 | |
| 11,511,650 | | |
$ | 0.29 | |
Note
22 - Income Tax
Income
Taxes
The
Company accounts for income taxes under FASB ASC 740-10, which requires use of the liability method. FASB ASC 740-10-25 provides that
deferred tax assets and liabilities are recorded based on the differences between the tax bases of assets and liabilities and their carrying
amounts for financial reporting purposes, referred to as temporary differences.
For
the years ended December 31, 2022 and 2021, the Company incurred a net operating loss and, accordingly, no provision for income taxes
has been recorded. In addition, no benefit for income taxes has been recorded due to the uncertainty of the realization of any tax assets.
At December 31, 2022, the Company had approximately $9,285,000
of federal net operating losses. The
net operating loss carry forwards, if not utilized, will begin to expire in 2025.
ONE
WORLD PRODUCTS, INC.
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
The
provision (benefit) for income taxes for the years ended December 31, 2022 and 2021 were assuming a 21% effective tax rate. The effective
income tax rate for the years ended December 31, 2022 and 2021 consisted of the following:
Schedule
of Effective Income Tax Rate
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Federal statutory
income tax rate | |
| 21 | % | |
| 21 | % |
| State income taxes | |
| - | % | |
| - | % |
| Change
in valuation allowance | |
| (21 | %) | |
| (21 | %) |
| Net
effective income tax rate | |
| - | | |
| - | |
The
components of the Company’s deferred tax asset are as follows:
Schedule
of Deferred Tax Asset
| | |
2022 | | |
2021 | |
| | |
December
31, | |
| | |
2022 | | |
2021 | |
| Deferred tax assets: | |
| | | |
| | |
| Net
operating loss carry forwards | |
$ | 1,950,000 | | |
$ | 1,535,000 | |
| | |
| | | |
| | |
| Net deferred tax assets
before valuation allowance | |
$ | 1,950,000 | | |
$ | 1,535,000 | |
| Less:
Valuation allowance | |
| (1,950,000 | ) | |
| (1,535,000 | ) |
| Net
deferred tax assets | |
$ | - | | |
$ | - | |
Based
on the available objective evidence, including the Company’s history of its loss, management believes it is more likely than not
that the net deferred tax assets will not be fully realizable. Accordingly, the Company provided for a full valuation allowance against
its net deferred tax assets at December 31, 2022 and 2021, respectively.
In
accordance with FASB ASC 740, the Company has evaluated its tax positions and determined there are no uncertain tax positions.
Note
23 – Subsequent Events
Series
A Preferred Stock Sales
On
various dates between January 4, 2023 and April 3, 2023, the Company received total proceeds of $250,000
from the sale of 25,000
units, consisting in the aggregate of
25,000
shares of series A preferred stock and
five-year
warrants to purchase an aggregate 2,500,000
shares of common stock at an exercise
price of $0.25
per share to four accredited investors.
The proceeds received were allocated between the Series A Preferred Stock and warrants on a relative fair value basis.
Common
Stock Sales
On
February 14, 2023, the Company sold 3,000,000
shares of common stock at a price of $0.10
per share for total cash proceeds of $300,000.
Common
Stock Issued for Services, Consultants
On
January 1, 2023, the Company issued 4,500
shares of series A preferred stock in
consideration of consulting services. The fair value of the shares was $45,000,
based on recent sales prices of the Company’s series A preferred stock on the date of grant.

21,366,700
SHARES OF COMMON STOCK
OF
ONE WORLD PRODUCTS, INC.
PROSPECTUS
YOU
SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS DOCUMENT OR THAT WE HAVE REFERRED YOU TO. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE
YOU WITH INFORMATION THAT IS DIFFERENT. THIS PROSPECTUS IS NOT AN OFFER TO SELL COMMON STOCK AND IS NOT SOLICITING AN OFFER TO BUY COMMON
STOCK IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
The
date of this prospectus is February 29, 2024
One World Products (QB) (USOTC:OWPC)
Historical Stock Chart
From Jan 2025 to Feb 2025
One World Products (QB) (USOTC:OWPC)
Historical Stock Chart
From Feb 2024 to Feb 2025