UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
DC 20549
SCHEDULE
14C INFORMATION
Information
Statement Pursuant to Section 14(c) of the
Securities
Exchange Act of 1934
Check
the appropriate box: ☐
| ☒ |
Preliminary
Information Statement |
| |
|
| ☐ |
Confidential,
for Use of the Commission Only (as permitted by Rule 14c-5(d)(2)) |
| |
|
| ☐ |
Definitive
Information Statement |
AYALA
PHARMACEUTICALS, INC.
(Name
of Registrant as Specified in Its Charter)
Payment
of Filing Fee (Check the appropriate box):
| ☐ |
No
fee required. |
| |
|
| ☐ |
Fee
paid previously with preliminary materials. |
| |
|
| ☒ |
Fee
computed on table in exhibit required by Item 25(b) of Schedule 14A (17 CFR 240.14a-101) per Item 1 of this Schedule and Exchange
Act Rules 14c-5(g) and 0-11. |
PRELIMINARY
COPY - SUBJECT TO COMPLETION
AYALA
PHARMACEUTICALS, INC.
9
Deer Park Drive, Suite K-1
Monmouth
Junction, NJ
NOTICE
OF WRITTEN CONSENT
AND
INFORMATION
STATEMENT
WE
ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO
SEND
US A PROXY.
To
our Stockholders:
This
notice of action by written consent and information statement is being furnished to the holders of common stock, par value $0.001 per
share (the “Common Stock”), of Ayala Pharmaceuticals, Inc. (the “Company,” “our,” “we,”
or “Ayala”) in connection with the Asset Purchase Agreement, dated as of February 5, 2024 (the “Asset Purchase Agreement”),
a copy of which is attached as Annex A to this information statement, by and between the Company and Immunome, Inc. (the “Purchaser”).
Pursuant to the Asset Purchase Agreement, the Purchaser will acquire certain of the Company’s assets and liabilities related to
the Company’s AL101 and AL102 programs (the “Asset Sale”), which constitute substantially all of the Company’s
assets. As consideration for the Asset Sale, the Purchaser will pay to the Company (i) an aggregate upfront purchase price of approximately
$50,000,000, of which $20,000,000 is payable in cash at the closing of the Asset Sale (the “Closing”), subject to certain
adjustments, 2,175,489 shares of Purchaser’s common stock, $0.0001 par value per share (the “Purchaser Common Stock”)
issued at Closing, and (ii) up to $37,500,000 in cash due upon the achievement of certain development and commercial milestone
events set forth in the Asset Purchase Agreement. The Purchaser is also assuming certain specified liabilities of Purchaser, including
outstanding liabilities to vendors and the costs of the ongoing Phase 3 clinical trial of AL102 and manufacturing costs and Ayala’s
obligations to Bristol-Meyers Squibb Company, or BMS, under its license agreement.
In
connection with the execution of the Asset Purchase Agreement, certain of the Company’s officers, directors and stockholders who
collectively are the record or beneficial holders of more than a majority of the issued and outstanding shares of capital stock of the
Company (the “Consenting Stockholders”) entered into a stockholder support agreement in favor of the Purchaser (the “Support
Agreement”) providing, among other things, that such officers, directors and stockholders will, among other things, (i) deliver
a written consent (the “Written Consent”) authorizing, approving and adopting the Asset Purchase Agreement and the transactions
contemplated thereby, including the Asset Sale and other transactions contemplated by the Asset Purchase Agreement and the related transaction
documents as defined therein; (ii) vote against any proposal made in opposition to, or in competition with, the Asset Purchase Agreement
or the Asset Sale and (iii) vote against any acquisition proposal involving a third party.
On
February 5, 2024, the board of directors of Ayala (the “Board”): (i) determined that the Asset Purchase Agreement, the Asset
Sale and the other transactions contemplated thereby are expedient and in the best interests of the Company and its
stockholders; (ii) approved the Asset Purchase Agreement, the Asset Sale and the other transactions contemplated thereby; and (iii) approved
the execution, delivery and performance by the Company of the Asset Purchase Agreement and, subject to obtaining the required approval
of the Consenting Stockholders, the consummation of the Asset Sale and the other transactions contemplated thereby.
On
February 7, 2024, the holders of certain notes and warrants that we had previously issued in 2023, including certain of the Consenting
Stockholders, exercised in full their conversion rights under their notes and warrants (the latter on a cashless basis), utilizing a
conversion price, in accordance with the terms of the notes and warrants, equal to 50% of the Common Stock’s price per share as
of market close on November 16, 2023. As a result, an aggregate of 30,736,555 shares of Common Stock were issued by the Company to these
noteholders and warrant holders.
On
February 8, 2024, the Consenting Stockholders delivered the Written Consent authorizing, approving and adopting in all respects the Asset
Purchase Agreement and the transactions contemplated thereby, including the Asset Sale and other transactions contemplated by the Asset
Purchase Agreement and the related transaction documents as defined therein, and voting all of the shares of the Common Stock held by
them in favor of such authorization, approval and adoption. As a result, no further action by any stockholder of the Company is required
under applicable law, the Company’s certificate of incorporation or bylaws or the Asset Purchase Agreement (or otherwise) to adopt
the Asset Purchase Agreement, and the Company will not be soliciting your vote to adopt the Asset Purchase Agreement and will not call
a stockholders meeting for purposes of voting on the adoption of the Asset Purchase Agreement. This notice and the accompanying information
statement shall constitute notice to you from the Company of the Written Consent contemplated by Section 228 of the General Corporation
Law of the State of Delaware (the “DGCL”).
WE
ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY. NO ACTION IN CONNECTION WITH THIS INFORMATION STATEMENT
IS REQUIRED BY YOU.
The
information statement accompanying this letter provides you with more specific information concerning the Asset Purchase Agreement, the
Asset Sale and the other transactions contemplated by the Asset Purchase Agreement and the related transaction documents.
| BY
ORDER OF THE BOARD OF DIRECTORS, |
|
| |
|
| /s/
Kenneth A. Berlin |
|
| Kenneth
A. Berlin |
|
| President
and Chief Executive Officer |
|
Neither
the United States Securities and Exchange Commission nor any state securities commission has approved or disapproved of the Asset Sale,
passed upon the merits of the Asset Sale or passed upon the adequacy or accuracy of the information contained in this information statement
and any documents incorporated by reference. Any representation to the contrary is a criminal offense.
This
information statement is dated February , 2024 and is first being mailed to stockholders on or about February , 2024.
TABLE
OF CONTENTS
SUMMARY
TERM SHEET
This
summary highlights selected information from this information statement and may not contain all of the information that is important
to you to understand the asset sale (the “Asset Sale”) contemplated by the Asset Purchase Agreement, dated as of February
5, 2024 (the “Asset Purchase Agreement”), by and between Ayala Pharmaceuticals, Inc. (the “Company” or “Ayala”)
and Immunome, Inc. (the “Purchaser” or “Immunome”). For a more complete description of the legal terms of the
Asset Sale, you should carefully read this entire information statement, the annexes attached to this information statement and the documents
referred to or incorporated by reference in this information statement. Any document or agreement referred to in this information statement
is qualified in its entirety by reference to the full text of such document or agreement. In this information statement, the terms “we,”
“us” and “our” refer to the Company. All references in this information statement to terms defined in the notice
to which this information statement is attached have the meanings provided in that notice. All references to capitalized terms not defined
herein or in the notice to which this information statement is attached have the meanings ascribed to them in the Asset Purchase Agreement,
a copy of which is attached as Annex A to this information statement.
The
Parties to the Asset Purchase Agreement (page 10)
Ayala
Pharmaceuticals, Inc.
We
are primarily a clinical-stage oncology company focused on developing and commercializing small molecule therapeutics for patients suffering
from rare and aggressive cancers, primarily in genetically defined patient populations. Our differentiated development approach is predicated
on identifying and addressing tumorigenic drivers of cancer, through a combination of our bioinformatics platform and next-generation
sequencing to deliver targeted therapies to underserved patient populations. Our current portfolio of product candidates, AL101 and AL102,
targets the aberrant activation of the Notch pathway using gamma secretase inhibitors. Gamma secretase is the enzyme responsible for
Notch activation and, when inhibited, turns off the Notch pathway activation. Aberrant activation of the Notch pathway has long been
implicated in multiple solid tumor and hematological cancers and has often been associated with more aggressive cancers. In cancers,
Notch is known to serve as a critical facilitator in processes such as cellular proliferation, survival, migration, invasion, drug resistance
and metastatic spread, all of which contribute to a poorer patient prognosis. AL101 and AL102 are designed to address the underlying
key drivers of tumor growth, and our initial Phase 2 clinical data of AL101 suggest that our approach may address shortcomings of existing
treatment options. We believe that our novel product candidates, if approved, have the potential to transform treatment outcomes for
patients suffering from rare and aggressive cancers. In addition to AL101 and AL102, which are our primary product candidates, we also
own assets relating to programs on which we have not recently placed substantial emphasis, including assets acquired from Biosight relating
to its programs to develop Aspacytarabine (“BST-236”), which is a novel proprietary anti-metabolite, prodrug of cytarabine,
covalently bound to asparagine, as well as assets relating to former Advaxis’ operations as a clinical-stage biotechnology company
focused on the development and commercialization of proprietary Listeria monocytogenes (“Lm”)-based antigen delivery products.
Our
product candidates, AL101 and AL102, are being developed as potent, selective, small molecule gamma secretase inhibitors, or GSIs. We
obtained an exclusive, worldwide license to develop and commercialize AL101 and AL102 from Bristol-Myers Squibb Company, or BMS, in November
2017. BMS evaluated AL101 in three Phase 1 studies involving more than 200 total subjects and AL102 in a single Phase 1 study involving
36 subjects with various cancers who had not been prospectively characterized for Notch activation, and to whom we refer to as unselected
subjects. While these Phase 1 studies did not report statistically significant overall results, clinical activity was observed across
these studies in cancers in which Notch has been implicated as a tumorigenic driver.
The
Company is headquartered in Monmouth Junction, NJ. Ayala’s common stock, par value $0.001 per share (the “Common Stock”),
is quoted on the OTCQX under the symbol “ADXS”. Additional information regarding the Company is contained in our filings
with the Securities and Exchange Commission (the “SEC”), copies of which may be obtained without charge by following the
instructions in the section entitled “Where You Can Find More Information.”
Immunome,
Inc.
Immunome
is a biotechnology company dedicated to developing first-in-class and best-in-class targeted cancer therapies. Immunome selects its targets
with a modality appropriate to its biology, including antibody-drug conjugates, or ADCs; radioligand therapies, or RLTs; and immunotherapies.
Immunome believes that pursuing underexplored targets with appropriate drug modalities leads to transformative therapies. Immunome’s
proprietary memory B cell hybridoma technology allows for the rapid screening and functional characterization of novel antibodies and
targets.
Immunome
has taken a dual-track approach to developing its current pipeline: organic pipeline expansion driven by its proprietary internal discovery
programs and expansion through disciplined strategic transactions. As a result, Immunome is currently advancing three programs with investigational
new drug application submissions expected in Q1 2025: IM-1021, an ADC targeting ROR1; an undisclosed RLT targeting FAP, or fibroblast
activation protein; and IM-4320, an immunotherapy targeting interleukin 38.
Immunome
is headquartered in Bothell, Washington. Immunome’s common stock is listed on The Nasdaq Capital Market under the trading symbol
“IMNM” and its website is www.immunome.com.
The
Asset Sale (page 11)
On
February 5, 2024, we entered into an Asset Purchase Agreement with Immunome pursuant to which the Purchaser will acquire certain of the
Company’s assets and assumed certain of the Company’s liabilities, in each case, related to the Company’s AL101 and
AL102 programs, as contemplated by and more fully described in the Asset Purchase Agreement, a copy of which is attached as Annex
A to this information statement, which under Delaware law constitutes a sale of substantially all of our assets.
Consideration
for the Asset Sale (page 42)
Pursuant
to the Asset Purchase Agreement, the Purchaser will pay to the Company (i) at the closing of the Asset Sale (the “Closing”)
an aggregate purchase price of approximately $50,000,000 consisting of $20,000,000 in cash, subject to certain adjustments and 2,175,489
shares of Purchaser’s common stock, $0.0001 par value (the “Purchaser Common Stock”) issued at Closing, and (ii) up to an aggregate of $37,500,000 in cash due upon
the achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement. The Purchaser will
also assume certain outstanding liabilities of the Company and reimburse the Company for certain expenditures between signing and Closing
and assume the ongoing expenses of the development, manufacturing and commercialization of AL102 from and after Closing.
Recommendation
of the Board; Reasons for the Asset Sale (page 28)
After
consideration of various factors, at a meeting on February 5, 2024, the board of directors of the Company (the “Board”) (i)
determined that the Asset Purchase Agreement, the Asset Sale and the other transactions contemplated thereby are expedient and in the best interests of the Company and its stockholders; (ii) approved the Asset Purchase Agreement, the Asset Sale and
the other transactions contemplated thereby; and (iii) approved the execution, delivery and performance by the Company of the Asset Purchase
Agreement and, subject to obtaining the required approval of the Consenting Stockholders, the consummation of the Asset Sale and the
other transactions contemplated thereby.
Required
Stockholder Approval for the Asset Sale (page 47)
Under
Delaware law and the Company’s certificate of incorporation and bylaws, the adoption of the Asset Purchase Agreement required the
written consent of the holders of the Common Stock representing a majority of the issued and outstanding shares of capital stock entitled
to vote thereon. As of February 8, 2024, the record date for determining stockholders of the Company entitled to vote on the adoption
of the Asset Purchase Agreement, there were 42,633,400 shares of the Common Stock outstanding. Holders of the Common Stock are entitled
to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including adoption of
the Asset Purchase Agreement.
On
February 7, 2024, the holders of certain notes and warrants that we had previously issued in 2023, including certain of the Consenting
Stockholders, exercised in full their conversion rights under their notes and warrants (the latter on a cashless basis), utilizing a
conversion price, in accordance with the terms of the notes and warrants, equal to 50% of the Common Stock’s price per share as
of market close on November 16, 2023. As a result, an aggregate of 30,736,555 shares of Common Stock were issued by the Company to these
noteholders and warrant holders. On February 8, 2024, the Consenting Stockholders, who collectively held approximately 78.4% of the voting
power of the outstanding shares of Common Stock at the close of business on such date, which was the record date established by the Board
with respect to the consent of stockholders, delivered the Written Consent authorizing, approving and adopting in all respects the Asset
Purchase Agreement and the transactions contemplated thereby and voting all of the shares of Common Stock held by them in favor of such
authorization, approval and adoption. As a result, no further action by any stockholder of the Company is required under applicable law,
the Company’s certificate of incorporation or bylaws or the Asset Purchase Agreement (or otherwise) to adopt the Asset Purchase
Agreement, and the Company will not be soliciting your vote to adopt the Asset Purchase Agreement and will not call a stockholders meeting
for purposes of voting on the adoption of the Asset Purchase Agreement.
When
actions are taken by written consent of less than all of the stockholders entitled to vote on a matter, Delaware law requires notice
of the action to those stockholders who did not consent in writing and who, if the action had been taken at a meeting, would have been
entitled to notice of the meeting. This information statement and the notice attached hereto constitute notice to you of action by written
consent as required by Delaware law.
Opinion
of A.G.P./Alliance Global Partners (page 30 and Annex B)
In
connection with the Asset Sale, A.G.P./Alliance Global Partners (“A.G.P.”) rendered to the Company’s Board an opinion,
dated February 5, 2024, as to the fairness, from a financial point of view and as of the date of the opinion, to the Company of the Aggregate
Consideration (which was defined in A.G.P.’s opinion as consisting of $20,000,000 in cash, 2,175,489 newly-issued unregistered
shares of Immunome common stock and the contingent payments of $10,000,000, $17,500,000 and $10,000,000 in cash due upon
the achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement) to be paid to the Company
for certain of the Company’s assets and liabilities related to the Company’s AL101 and AL102 programs in the Asset Sale.
The
full text of A.G.P.’s written opinion, dated February 5, 2024, which describes the assumptions made, procedures followed, matters
considered and limitations on the review undertaken, is attached to this information statement as Annex B and is incorporated by reference
in its entirety. A.G.P.’s opinion was provided for the information and assistance of the Company’s Board (in its capacity
as such) in connection with its consideration of the Asset Sale. A.G.P.’s opinion does not constitute a recommendation to the Company’s
Board, the Company, its stockholders or any other person as to how any of them should vote or act with respect to the Asset Sale or any
other matter. A.G.P.’s opinion does not address the underlying business decision of the Company to engage in the Asset Sale, or
the relative merits of the Asset Sale as compared to any strategic alternatives that may be available to the Company.
The
Asset Purchase Agreement (page 40 and Annex A)
Conditions
to Closing
The
closing of the Asset Sale is subject to the satisfaction or, to the extent permissible under applicable law or pursuant to the Asset
Purchase Agreement, waiver of certain conditions on or prior to the closing. Such conditions include:
Conditions
for both Parties:
● No
temporary restraining order, preliminary or permanent injunction, or other order preventing the consummation of the Asset Sale shall
have been issued by any court of competent jurisdiction and remain in effect, and no material law shall have been enacted that makes
consummation of the Asset Sale illegal.
● Each
of Ayala and Purchaser shall have performed and complied with, in all material respects, all of its covenants contained in the Asset
Purchase Agreement at or before the Closing (to the extent that such covenants require performance by such party at or before the Closing).
Conditions
for Purchaser:
●
The fundamental representations and warranties of Ayala set forth in the Asset Purchase Agreement shall be true and correct in all respects
as of the date of the Asset Purchase Agreement and as of the Closing Date (provided that the accuracy of representations and warranties
that by their terms speak of a specified date will be determined as of such date), and all other representations and warranties
of Ayala set forth in the Asset Purchase Agreement (without giving effect to materiality qualifications) shall be true and correct in
all material respects, except where the failure of such representations and warranties to be so true and correct would not reasonably
be expected to, individually or in the aggregate, have a material adverse effect.
● There
shall not have occurred and be continuing a material adverse effect since the date of the Asset Purchase Agreement.
● Ayala
shall have delivered a certificate to the effect that each of the conditions specified in Sections 7.1, 7.2 and 7.4
of the Asset Purchase Agreement is satisfied in all respects as of the Closing.
● Ayala
shall have delivered to Purchaser each of the documents and materials contemplated to be delivered by Section 4.2(a) of the Asset
Purchase Agreement.
● At
least twenty (20) calendar days shall have elapsed from the date the definitive information statement was mailed to the Company’s
stockholders in accordance with Rule 14c-2 of the Exchange Act.
Conditions
for Ayala:
● The
representations and warranties of Purchaser set forth in the Asset Purchase Agreement (without giving effect to materiality qualifications)
shall be true and correct in all material respects, except where the failure of such representations and warranties to be so true and
correct would not reasonably be expected to, individually or in the aggregate, prevent or materially delay the consummation of the Asset
Sale.
● Purchaser
shall have delivered a certificate to the effect that each of the conditions specified in Sections 8.1 and 8.2 of the Asset
Purchase Agreement is satisfied in all respects as of the Closing.
● Ayala
shall have delivered to Purchaser each of the documents and materials contemplated to be delivered by Section 4.2(b) of the Asset
Purchase Agreement.
Seller
Stockholder Approval
On
February 7, 2024, the holders of certain notes and warrants that we had previously issued in 2023, including certain of the Consenting
Stockholders, exercised in full their conversion rights under their notes and warrants (the latter on a cashless basis), utilizing a
conversion price, in accordance with the terms of the notes and warrants, equal to 50% of the Common Stock’s price per share as
of market close on November 16, 2023. As a result, an aggregate of 30,736,555 shares of Common Stock were issued by the Company to these
noteholders and warrant holders. On February 8, 2024, Ayala stockholders who collectively held approximately 78.4% of the voting power
of the outstanding shares of Common Stock at the close of business on such date, which was the record date established by the Board with
respect to the consent of stockholders, delivered the Written Consent, in the form attached to the Asset Purchase Agreement as Exhibit
F, authorizing, approving and adopting in all respects the Asset Purchase Agreement and the transactions contemplated thereby, including
the Asset Sale and other transactions contemplated by the Asset Purchase Agreement and the related transaction documents as defined therein.
The Written Consent constituted the necessary action by Ayala’s stockholders to authorize, approve and adopt the Asset Sale under
applicable law (including Section 271 of the Delaware General Corporation Law) and Ayala’s certificate of incorporation and bylaws.
As
a result of the execution of the Written Consent on February 8, 2024, the requisite stockholder approval has been obtained and, as such,
the Company will not be soliciting your vote to adopt the Asset Purchase Agreement and will not call a stockholders meeting for purposes
of voting on the adoption of the Asset Purchase Agreement.
Termination
Ayala
and Purchaser may terminate the Asset Purchase Agreement by mutual written consent. In addition, there are certain other circumstances
under which the Asset Purchase Agreement may be terminated prior to the Closing, including:
●
by either Ayala or Purchaser if any government authority of competent jurisdiction issues an order permanently restraining, enjoining,
or otherwise prohibiting the consummation of the Asset Sale, and such order becomes final and non-appealable (provided that a party shall
not be entitled to exercise such termination right if, at the time of such termination, such party is in breach of any of its representations,
warranties or covenants under the Asset Purchase Agreement, which breach has caused or resulting in the imposition of such order or the
failure of such order to be resisted, resolved or lifted);
●
by Purchaser if Ayala has breached any of its representations, warranties or covenants under the Asset Purchase Agreement such that an
applicable condition to Closing shall not be satisfied and which breach is not cured by Ayala within the shorter of 30 days after Ayala
receives written notice of such breach from Purchaser or by the date that is six months following the date of the Asset Purchase Agreement
(such date, the “Outside Date”) (provided that Purchaser shall not be entitled to exercise such termination right if, at
the time of such termination, Purchaser is in breach of any of its representations, warranties or covenants under the Asset Purchase
Agreement, such that an applicable condition to Closing shall not be satisfied);
●
by Ayala if Purchaser has breached any of its representations, warranties or covenants under the Asset Purchase Agreement such that an
applicable condition to Closing shall not be satisfied and which breach is not cured by Purchaser within the shorter of 30 days after
Purchaser receives written notice of such breach from Ayala or by the Outside Date (provided that Ayala shall not be entitled to exercise
such termination right if, at the time of such termination, Ayala is in breach of any of its representations, warranties or covenants
under the Asset Purchase Agreement, such that an applicable condition to Closing shall not be satisfied);
● by
either party if the Closing shall not have occurred on or before the Outside Date (provided that a party shall only be entitled to exercise
such termination right if the terminating party is not in breach of any representation, warranty or covenant such that an applicable
condition to Closing shall not be satisfied); or
● by
Purchaser, if Ayala fails to deliver, by 11:59 p.m. Eastern Time on the date that is three (3) Business Days after the date of the Asset
Purchase Agreement, the Written Consent.
Interests
of Our Directors and Executive Officers in the Asset Sale (page 19)
You
should be aware that the Company’s directors and executive officers have interests in the Asset Sale that are different from or
in addition to your interests as a stockholder and that may present actual or potential conflicts of interest. The Board was aware of
these interests and considered them, among other matters, in approving the Asset Purchase Agreement, Asset Sale, and the transactions
contemplated thereby. You should be aware of these and other interests of our directors and executive officers that are described in
this information statement.
U.S.
Federal Income Tax Consequences of the Asset Sale (page 39)
The
Asset Sale will be treated for U.S. federal income tax purposes as a taxable transaction upon which we will recognize gain or loss. The
amount of gain or loss we recognize with respect to the sale of a particular asset will be measured by the difference between the amount
realized by us on the sale of that asset and our tax basis in that asset. The determination of whether we will recognize gain or loss
will be made with respect to each of the assets to be sold. Accordingly, we may recognize gain on the sale of certain assets and loss
on the sale of certain others, depending on the amount of consideration allocated to an asset as compared with the basis of that asset.
Further, the sale of certain assets may result in ordinary income or loss, depending on the nature of the asset. To the extent the Asset
Sale results in us recognizing a net gain for U.S. federal income tax purposes, we expect to incur income tax with respect to such gain,
which may be offset to a limited degree by available net operating loss carryforwards.
Regulatory
Approvals (page 30)
The
Company is not required to obtain any regulatory approvals in order to consummate the Asset Sale and the Asset Sale is not conditioned
upon any such approvals.
Appraisal
Rights (page 51)
No
appraisal or dissenters’ rights are available to our stockholders under Delaware law or our certificate of incorporation or bylaws
in connection with the Asset Sale.
Anticipated
Accounting Treatment (page 39)
Under
generally accepted accounting principles, upon completion of the Asset Sale, we will remove the net assets and assumed liabilities sold
and add the proceeds from the Asset Sale to our consolidated balance sheet, which we anticipate would result in recording a gain from
the Asset Sale.
Use
of Proceeds (page 39)
Assuming
that the Asset Sale is consummated in accordance with the terms of the Asset Purchase Agreement, Ayala intends to use substantially all
of the after-tax proceeds for general corporate purposes.
Market
Price of Our Stock (page 50)
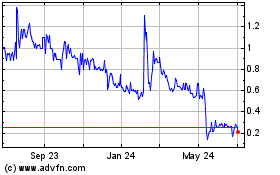
Ayala’s
Common Stock is quoted on the OTCQX under the symbol “ADXS”.
The
closing price of shares of the Common Stock on February 5, 2024, the trading day immediately prior to the public announcement of the
Asset Sale on February 6, 2024, as reported on the OTCQX, was $0.5899 per share. Any over-the-counter market quotations reflect inter-dealer
prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
QUESTIONS
AND ANSWERS ABOUT THE ASSET SALE
The
following questions and answers are intended to briefly address commonly asked questions as they pertain to the Asset Purchase Agreement
and the Asset Sale. These questions and answers may not address all questions that may be important to you as an Ayala stockholder. Please
refer to the section entitled “Summary Term Sheet” and the more detailed information contained elsewhere in this information
statement, the annexes to this information statement and the documents referred to or incorporated by reference in this information statement,
each of which you should read carefully. You may obtain information incorporated by reference in this information statement without charge
by following the instructions in the section entitled “Where You Can Find More Information.”
| Q: |
Why
am I receiving this information statement? |
| A: |
On
February 5, 2024, following approval of such action by the Board, the Company entered into an Asset Purchase Agreement with
Immunome. On February 7, 2024, the holders of certain notes and warrants that we had previously issued in 2023, including certain
of the Consenting Stockholders, exercised in full their conversion rights under their notes and warrants (the latter on a cashless
basis), utilizing a conversion price, in accordance with the terms of the notes and warrants, equal to 50% of the Common Stock’s
price per share as of market close on November 16, 2023. As a result, an aggregate of 30,736,555 shares of Common Stock were issued
by the Company to these noteholders and warrant holders. On February 8, 2024, the Consenting Stockholders, who collectively held
approximately 78.4% of the voting power of the outstanding shares of Common Stock at the close of business on such date, which was
the record date established by the Board with respect to the consent of stockholders, delivered the Written Consent authorizing,
approving and adopting in all respects the Asset Purchase Agreement and the transactions contemplated thereby and voting all of the
shares of Common Stock held by them in favor of such authorization, approval and adoption. Applicable provisions of Delaware law
and certain securities regulations require us to provide you with information regarding the Asset Sale, even though your vote or
consent is neither required nor requested to adopt the Asset Purchase Agreement or to complete the Asset Sale. |
| Q: |
What
is the Asset Sale? |
| A: |
Pursuant
to the Asset Purchase Agreement, the Purchaser, Immunome, will acquire certain of the Company’s assets and assume certain of
the Company’s liabilities, in each case, related to the Company’s AL101 and AL102 programs, which constitute substantially
all of the Company’s assets. |
| Q: |
Who
is buying substantially all of the Company’s assets and what is the aggregate purchase price for the assets? |
| A: |
Immunome,
Inc. is the purchaser in the Asset Sale.
Pursuant
to the Asset Purchase Agreement, Immunome will pay to the Company (i) an aggregate purchase price of approximately $50,000,000 at
Closing, consisting of $20,000,000 in cash (subject to certain adjustments) and 2,175,489 shares of Purchaser Common Stock issued
at Closing, and (ii) up to $37,500,000 in cash due upon the achievement of certain development and commercial milestone events
set forth in the Asset Purchase Agreement. Immunome will also assume certain outstanding liabilities of the Company and reimburse
the Company for certain expenditures between signing and Closing and assume the ongoing expenses of the development, manufacturing
and commercialization of AL102 from and after Closing. |
| Q: |
When
do you expect the Asset Sale to be completed? |
| A: |
We
are working to complete the Asset Sale as quickly as possible. We currently expect to complete the Asset Sale promptly after all
of the conditions to the Asset Sale have been satisfied or waived and subject to the other terms and conditions in the Asset Purchase
Agreement. Completion of the Asset Sale is currently expected to occur in late Q1 or early Q2 of 2024, although the Company cannot
assure completion by any particular date, if at all. |
| Q: |
What
happens if the Asset Sale is not completed? |
| A: |
If
the Asset Sale is not completed for any reason, the Company will not receive any payment for the assets and liabilities related to
the Company’s AL101 and AL102 programs, nor will Immunome assume any of the related liabilities, and the Company would be in
a similar position to that of the Company prior to the execution of the Asset Purchase Agreement. |
| Q: |
Why
am I not being asked to vote on the Asset Sale? |
| A: |
No
vote or other action of our stockholders is required by applicable law, our certificate of incorporation or our bylaws or otherwise
in order for the Company to consummate the Asset Purchase Agreement and other transactions contemplated thereby. Therefore, your
vote is not required and is not being sought. We are not asking you for a proxy, and you are requested not to send us a proxy. |
| Q: |
Did
the Board authorize and recommend the Asset Sale and the Asset Purchase Agreement? |
| A: |
Yes.
After fully considering the terms and conditions of the Asset Sale, the Asset Purchase Agreement and the transactions contemplated
thereby, the Board (i) determined that the Asset Purchase Agreement, the Asset Sale and the other transactions contemplated thereby
are expedient and in the best interests of the Company and its stockholders; (ii) approved the Asset Purchase
Agreement, the Asset Sale and the other transactions contemplated thereby; and (iii) approved the execution, delivery and performance
by the Company of the Asset Purchase Agreement and, subject to obtaining the required approval of the Consenting Stockholders, the
consummation of the Asset Sale and the other transactions contemplated thereby. |
| Q: |
What
are the material terms of the Asset Sale Proposal? |
| A: |
In
addition to the cash and equity consideration to be paid and issued at Closing and the cash payable upon the achievement of certain
development and commercial milestone events set forth in the Asset Purchase Agreement, and customary representations and warranties,
the Asset Purchase Agreement contains other important terms and provisions, including: |
| |
● |
By
11:59 p.m. Eastern Time on February 8, 2024, the Consenting Stockholders were required to execute and deliver to the Company the
Written Consent authorizing, approving and adopting the Asset Purchase Agreement and the transactions contemplated thereby, including
the Asset Sale and other transactions contemplated by the Asset Purchase Agreement and the related transaction documents as defined
therein, and vote all the shares of the Common Stock held by them in favor of such authorization, approval and adoption. |
| |
|
|
| |
● |
The
Company has agreed to continue to conduct business in the ordinary course and subject to certain other restrictions during the period
prior to completion of the Asset Sale and other transactions contemplated by the Asset Purchase Agreement. |
| |
|
|
| |
● |
The
obligations of the parties to the Asset Purchase Agreement to close the Asset Sale and other transactions contemplated by the Asset
Purchase Agreement subject thereto are subject to several closing conditions, including the authorization of the Asset Sale by our
stockholders. |
| |
|
|
| |
● |
The
Asset Purchase Agreement may be terminated by the Purchaser or the Company in certain circumstances, in which case the Asset Sale
and other transactions contemplated by the Asset Purchase Agreement will not be completed. |
| Q: |
Am
I entitled to exercise appraisal or dissenters’ rights? |
| A: |
No
appraisal or dissenters’ rights are available to our stockholders under Delaware law or under our certificate of incorporation
or bylaws in connection with the types of actions contemplated under the Asset Sale and other transactions contemplated by the Asset
Purchase Agreement. |
| Q: |
What
are the U.S. federal income tax consequences of the Asset Sale to stockholders of the Company? |
| A: |
The
Asset Sale and other transactions contemplated by the Asset Purchase Agreement are a corporate action of the Company. Therefore,
the Asset Sale will not be a taxable event (for federal income tax purposes) to our stockholders. |
| Q: |
Do
any of the Company’s directors or executive officers have interests in the Asset Sale that may differ from those of Company
stockholders generally? |
| A: |
You
should be aware that the Company’s directors and executive officers have interests in the Asset Sale that may be different
from, or in addition to, the interests of the Company stockholders generally. These interests are described in more detail in the
section entitled “Interests of Our Directors and Executive Officers in the Asset Sale.” The Board was aware of
these interests and considered them, among other matters, in evaluating and approving the Asset Purchase Agreement. |
| Q: |
Where
can I find more information about the Company? |
| A: |
We
file periodic reports and other information with the SEC. You may read and copy this information at the SEC’s public reference
facilities. Please call the SEC at (800) SEC-0330 for information about these facilities. This information is also available on the
website maintained by the SEC at www.sec.gov. For a more detailed description of the available information, please refer to
the section entitled “Where You Can Find More Information.” |
| Q: |
Who
can help answer my other questions? |
| A: |
If
you have any questions about the Asset Sale, this information statement or your ownership of our common stock, please contact Roy
Golan, our Chief Financial Officer, by mail at Ayala Pharmaceuticals, Inc., 9 Deer Park Drive, Suite K-1, Monmouth Junction, NJ 08852,
by telephone at (908)-215-2787 or by e-mail at Roy.g@ayalapharma.com. |
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
To
the extent that statements contained in this information statement are not descriptions of historical facts, they are forward-looking
statements reflecting the current beliefs and expectations of the Company made pursuant to the safe harbor provisions of the Private
Securities Litigation Reform Act of 1995, as amended. Forward-looking statements include, but are not limited to, statements that represent
the Company’s beliefs concerning future operations, strategies, financial results or other developments, and contain words and
phrases such as “may,” “expects,” “should,” “believes,” “anticipates,” “estimates,”
“intends” or similar expressions. Such forward-looking statements involve substantial risks and uncertainties that could
cause the Company’s future results, performance or achievements to differ significantly from those expressed or implied by the
forward-looking statements.
The
proposed Asset Sale is subject to risks and uncertainties and factors that could cause the Company’s actual results to differ,
possibly materially, from those in the specific projections, goals, assumptions and statements herein which include, but are not limited
to:
| |
● |
the ability of the parties to consummate the Asset Sale and other transactions contemplated by the Asset Purchase Agreement; |
| |
|
|
| |
● |
satisfaction of closing conditions precedent to the consummation of the Asset Sale and other transactions contemplated by the Asset Purchase Agreement; |
| |
|
|
| |
● |
potential delays in consummating the Asset Sale and other transactions contemplated by the Asset Purchase Agreement; |
| |
|
|
| |
● |
our execution costs in connection with the Asset Sale and other transactions contemplated by the Asset Purchase Agreement; |
| |
|
|
| |
● |
the occurrence of any event, change or other circumstance that could give rise to the termination of the Asset Purchase Agreement; |
| |
|
|
| |
● |
whether
the milestone conditions are satisfied; |
| |
|
|
| |
● |
the possibility that the Purchaser Common Stock issued at Closing diminishes in value; |
| |
|
|
| |
● |
risks related to disruption of management’s attention from the Company’s ongoing business operations due to the Asset Sale; |
| |
|
|
| |
● |
the effect of the announcement of the Asset Sale on the Company’s relationships with its vendors and employees, and its operating results and business generally; |
| |
|
|
| |
● |
the application of, and any changes in, applicable tax laws, regulations, administrative practices, principles and interpretations; and |
| |
|
|
| |
● |
the outcome of any legal proceedings to the extent initiated against the Company or others following the announcement of the Asset Sale, as well as Company management’s response to any of the aforementioned factors. |
The
Company undertakes no obligation to update or revise any forward-looking statements. Forward-looking statements should not be relied
upon as representing the Company’s views as of any date subsequent to the date hereof. For a further description of the risks and
uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating
to the Company’s business in general and ownership of shares of Common Stock, see the “Risk Factors” section of the
Company’s Annual Report on Form 10-K for the year ended October 31, 2022, and the other reports the Company files with the SEC,
as well under the heading “Risk Factors” in the Form 10-K for the fiscal year ended December 31, 2022 of Old Ayala, Inc.
(f/k/a Ayala Pharmaceuticals, Inc.).
THE
PARTIES TO THE ASSET PURCHASE AGREEMENT
The
Company
Ayala
Pharmaceuticals, Inc.
9
Deer Park Drive, Suite K-1
Monmouth
Junction, NJ
Phone:
908-215-2787
We
are a clinical-stage oncology company, incorporated in the state of Delaware, focused on developing and commercializing small molecule
therapeutics for patients suffering from rare and aggressive cancers, primarily in genetically defined patient populations. Our differentiated
development approach is predicated on identifying and addressing tumorigenic drivers of cancer, through a combination of our bioinformatics
platform and next-generation sequencing to deliver targeted therapies to underserved patient populations. Our current portfolio of product
candidates, AL101 and AL102, targets the aberrant activation of the Notch pathway using gamma secretase inhibitors. Gamma secretase is
the enzyme responsible for Notch activation and, when inhibited, turns off the Notch pathway activation. Aberrant activation of the Notch
pathway has long been implicated in multiple solid tumor and hematological cancers and has often been associated with more aggressive
cancers. In cancers, Notch is known to serve as a critical facilitator in processes such as cellular proliferation, survival, migration,
invasion, drug resistance and metastatic spread, all of which contribute to a poorer patient prognosis. AL101 and AL102 are designed
to address the underlying key drivers of tumor growth, and our initial Phase 2 clinical data of AL101 suggest that our approach may address
shortcomings of existing treatment options. We believe that our novel product candidates, if approved, have the potential to transform
treatment outcomes for patients suffering from rare and aggressive cancers. In addition to AL101 and AL102, which are our primary product
candidates, we also own assets relating to programs on which we have not recently placed substantial emphasis, including assets acquired
from Biosight relating to its programs to develop Aspacytarabine (“BST-236”), which is a novel proprietary anti-metabolite,
prodrug of cytarabine, covalently bound to asparagine, as well as assets relating to former Advaxis’ operations as a clinical-stage
biotechnology company focused on the development and commercialization of proprietary Listeria monocytogenes (“Lm”)-based
antigen delivery products.
We
are headquartered in Monmouth Junction, NJ. Ayala’s Common Stock is quoted on the OTCQX under the symbol “ADXS”. Additional
information regarding the Company is contained in our filings with the SEC, copies of which may be obtained without charge by following
the instructions in the section entitled “Where You Can Find More Information.”
The
Purchaser
Immunome,
Inc.
665
Stockton Drive, Suite 300
Exton,
PA 19341
Phone:
(610) 321-3700
The
Purchaser is a biotechnology company, incorporated in the state of Delaware, dedicated to developing first-in-class and best-in-class
targeted cancer therapies. Purchaser selects targets with a modality appropriate to its biology, including immunotherapies, targeted
effectors, radioligand therapies and ADCs. The Purchaser believes that pursuing underexplored targets with appropriate drug modalities
leads to transformative therapies. The Purchaser’s proprietary memory B cell hybridoma technology allows for the rapid screening
and functional characterization of novel antibodies and targets.
The
Purchaser is headquartered in Bothell, Washington. The Purchaser Common Stock is listed on The Nasdaq Capital Market under the trading
symbol “IMNM.” The Purchaser’s website is www.immunome.com.
THE
ASSET SALE
Background
of the Asset Sale
The
following chronology summarizes the key communications and events that led to the signing of the Asset Purchase Agreement. This chronology
is not, and does not purport to be, a catalogue of every interaction among the Company, Immunome, their respective advisors or any other
applicable parties.
The
Company is a clinical stage oncology company primarily focused on developing and commercializing small molecule therapeutics for people
living with rare tumors and aggressive cancers, with several product candidates under development, including AL102 and related drug candidate
AL101. AL102 is an investigational small molecule gamma secretase inhibitor currently being evaluated in the randomized Phase 3 RINGSIDE
international trial for the treatment of desmoid tumors.
The
Company’s board of directors (the “Board”), together with members of the Company’s senior management (“Company
Management”), and with the assistance of the Company’s advisors at the relevant time, regularly evaluate the Company’s
strategic direction and ongoing business plans with the purpose of strengthening the Company’s business and enhancing stockholder
value. As part of this evaluation, the Board has, from time to time, considered various potential strategic and financing alternatives.
References in this Background of the Transaction to the Board and to Company Management refer to the board of directors and management
of the Company at the time contemplated by such reference.
In
early 2022, the Board, in consultation with Company Management, decided, given the Company’s then-current cash position, the early-stage
nature of its clinical programs and lack of near-term value creating milestones, to explore potential strategic opportunities with companies
that had more mature clinical programs. With the assistance of its financial advisor at that time, Company Management and the Board reviewed
and considered a number of potential strategic opportunities for the Company, which at that time was named “Advaxis, Inc.”,
and following such review and consideration, determined that it was in the best interests of the Company and its stockholders to pursue
a merger (the “Old Ayala Merger”) with Old Ayala, Inc., known at such time as Ayala Pharmaceuticals, Inc. (“Old Ayala”).
It was understood at the time that Old Ayala’s late-stage AL102 and AL101 programs would require the investment of amounts greater
than either the Company or Old Ayala was bringing to the merged company. As a result, Company Management, in consultation with the Board,
determined to continue to pursuing strategic and financing opportunities to raise capital.
On
October 18, 2022, the Company and Old Ayala entered into that certain Agreement and Plan of Merger, dated as of October 18, 2022, by
and among the Company, Old Ayala and a wholly owned subsidiary of the Company.
In
late October and early November 2022, Company Management engaged in discussions with several potential investors in connection with a
potential private investment in public equity transaction (the “Potential PIPE”) to obtain additional financing.
On
January 19, 2023, the Company and Old Ayala consummated the Old Ayala Merger. Following the Old Ayala Merger, the Company changed its
name to “Ayala Pharmaceuticals, Inc.”
Following
the consummation of the Old Ayala Merger, Company Management, in consultation with the Board, determined that, given the addition of
Old Ayala’s late-stage program following the consummation of the Old Ayala Merger, the Company would require additional financing.
As a result, in consultation with the Board, Company Management decided to continue to pursue potential financing alternatives, including
combining with a company with clinical stage assets and additional investors who could invest in the combined company and improve the
ability of the Company to raise additional capital. Company Management and the Board reviewed and considered a number of potential strategic
opportunities, and following such review and consideration, determined to engage in discussions with several of such parties, including
Biosight, Ltd. (“Biosight”), a private Phase 2 clinical-stage biotechnology company developing an investigational product,
aspacytarabine (BST-236), as an innovative, proprietary anti-metabolite therapeutic that seeks to address unmet medical needs for hematological
malignancies and disorders. On July 4, 2021, the Company and Biosight had entered into a merger agreement pursuant to which Biosight
would have merged with a wholly owned subsidiary of the Company and survived such merger as a wholly owned subsidiary of Advaxis. Such
transaction was not consummated at that time.
In
the middle of 2023, the Company engaged an additional financial advisor, to reach out to several potential investors in connection with
the Potential PIPE, and Company Management engaged in discussions with approximately ten prospective investors as part of such process.
On
June 14, 2023, representatives of Company Management and representatives of the management of Party A (“Party A Management”)
had an initial call to discuss a potential strategic investment or transaction between the parties (the “Potential Party A Transaction”).
During
the spring and summer of 2023, the Company and Biosight engaged in discussions regarding a potential strategic transaction, including
among other things negotiating potential terms thereof and conducting due diligence.
On
June 21, 2023, Party A commenced due diligence in connection with the Potential Party A Transaction.
On
June 22, 2023, representatives of Company Management provided representatives of Party A Management with access to an online data room
(the “Data Room”), to allow Party A to continue to progress due diligence in connection with the Potential Party A Transaction.
On
July 25, 2023, representatives of Company Management and representatives of Party A Management had a due diligence call to discuss clinical
operations and regulatory related issues.
On
July 26, 2023, the Board determined that it was in the best interests of the Company and its stockholders to pursue a merger (the “Biosight
Merger”) with Biosight, and the Company and Biosight entered into that certain Agreement and Plan of Merger, dated as of July 26,
2023, by and among the Company, Advaxis Israel Ltd. and Biosight Ltd (the “Biosight Merger Agreement”). Following entry into
the Biosight Merger Agreement, the Company continued to explore strategic and financing options for the combined company following the
consummation of the Biosight Merger.
On
August 7, 2023, in order to obtain temporary financing as it pursued its ongoing efforts to achieve longer-term financing, the Company
issued Senior Secured Convertible Promissory Notes to two of its existing stockholders, Israel Biotech Fund I, L.P. (“IBF I”)
and Israel Biotech Fund II, L.P. (“IBF II,” and together with IBF I, “IBF”), in the aggregate principal amount
of $2 million (the “August Secured Convertible Notes”).
On
September 6, 2023, Dr. Pini Orbach, a member of the Board (“Dr. Orbach”), provided an introduction over email between representatives
of Company Management and representatives of the management of Party B (the “Party B Management”) to discuss a potential
strategic transaction between the Company and Party B (the “Potential Party B Transaction”).
On
September 8, 2023, the Chief Business Officer of Party B (the “Party B CBO”) emailed representatives of Company Management,
proposing that the Company and Party B enter into a confidentiality agreement (the “Party B NDA”) in furtherance of Party
B’s due diligence of the Company in connection with the Potential Party B Transaction.
On
September 12, 2023, representatives of Party B Management provided representatives of Company Management with a draft of the Party B
NDA.
On
September 15, 2023, the Company and Party B entered into the Party B NDA.
Also
on September 15, 2023, in furtherance of its ongoing financing efforts, the Company entered into that certain Simple Agreement for Future
Equity, by and among the Company and the investor parties thereto.
On
September 20, 2023, Dr. Orbach provided an introduction over email between representatives of Company Management and representatives
of the management of Party C (“Party C Management”) to discuss a potential strategic transaction between the Company and
Party C (the “Potential Party C Transaction”).
Also
on September 20, 2023, Mr. Roy Golan, Chief Financial Officer of the Company (“Mr. Golan”), provided representatives of Party
C Management with a “teaser” memorandum regarding the Company and its clinical programs, in connection with the Potential
Party C Transaction.
On
September 22, 2023, representatives of Company Management provided representatives of Party C Management with a presentation (the “Management
Presentation”) regarding the Company and its clinical programs.
At
the end of the third quarter of 2023, Dr. David Sidransky, Chairman of the Board (“Dr. Sidransky”), held an initial discussion
with Dr. Vered Bisker-Leib, the Chief Operating Officer (COO) of Party D (the “Party D COO”) to discuss a potential strategic
transaction between the Company and Party D (the “Potential Party D Transaction”). The Party D COO is a member of the Company
Board. The Party D COO was thereafter recused from all discussions and deliberations of the Board relating to the Potential Party D Transaction
and any strategic transaction that might be an alternative to the Potential Party D Transaction.
In
late September and early October, 2023, the Company, with assistance from A.G.P./Alliance Global Partners (“A.G.P.”), conducted
an outreach process in connection with the Potential PIPE (the “Potential PIPE Outreach”), and representatives of Company
Management engaged in discussions with approximately 50 potential investors as part of such process, which included certain of the Company’s
existing investors, as well as new investors contacted by A.G.P. on behalf of the Company. In connection with the Potential PIPE Outreach,
the Company received verbal indications of interest from certain potential investors, as well as a non-binding commitment from certain
of its existing investors of an aggregate amount approximately equal to $15 million. During such time, negotiations took place with certain
of these investors regarding the terms and the amount to be raised, and a deal structure of a $50 million initial investment at closing,
plus an additional $50 million upon the achievement of certain milestones, was discussed. As part of the Potential PIPE Outreach, certain
investors conducted due diligence and received access to the Data Room.
On
October 2, 2023, representatives of Company Management held a meeting with representatives of Party B Management to discuss the Potential
Party B Transaction, and at such meeting, representatives of Company Management provided the Management Presentation to representatives
of Party B Management.
On
October 4, 2023, representatives of Company Management had a discussion with representatives of Party A Management to discuss the Potential
Party A Transaction, including any updates with respect thereto.
On
October 6, 2023, Dr. Yuval Cabilly, a member of the Board (“Dr. Cabilly”), had a telephonic discussion with the Party D COO
to discuss the Potential Party D Transaction. The Party D COO, while recused from Board discussions, continued to negotiate with the
Company on behalf of Party D.
On
October 10, 2023, Mr. Kenneth Berlin, Chief Executive Officer of the Company (“Mr. Berlin”), had a telephonic discussion
with the Party D COO to discuss the Potential Party D Transaction.
On
October 11, 2023, the Party D COO sent an email to representatives of Company Management, requesting access to the Data Room to allow
Party D to continue to progress due diligence in connection with the Potential Party D Transaction.
On
October 12, 2023, representatives of Company Management provided representatives of Party D Management (the “Party D Management”)
with access to the Data Room to allow Party D to continue to progress due diligence in connection with the Potential Party D Transaction.
On
October 17, 2023, representatives of Party B Management provided representatives of Company Management with a due diligence request list
in connection with the Potential Party B Transaction (the “Party B Diligence Request List”).
Also
on October 17, 2023, representatives of Company Management and representatives of Party D Management had an introductory call to discuss
the status and process of the Potential Party D Transaction.
On
October 18, 2023, the Company and Biosight consummated the Biosight Merger, pursuant to which Biosight merged into a wholly-owned subsidiary
of the Company and survived the Biosight Merger as a wholly-owned subsidiary of the Company.
Also
on October 18, 2023, representatives of Company Management emailed the Party B CBO, regarding the status of the Potential Party B Transaction.
On
October 24, 2023, the Party B CBO emailed representatives of Company Management to schedule an introductory call, which email was followed
by an introductory call between representatives of Party B Management and representatives of Company Management to discuss the Potential
Party B Transaction.
On
October 31, 2023, representatives of Company Management provided responses to the Party B Diligence Request List to representatives of
Party B Management.
On
November 1, 2023, Mr. Berlin had a telephonic discussion with the Party B CBO to discuss the Potential Party B Transaction.
On
November 6, 2023, representatives of Company Management had a telephonic discussion with representatives of Party B Management to discuss
the Potential Party B Transaction.
On
November 7, 2023, representatives of A.G.P. provided an introduction over email between representatives of Company Management and representatives
of the management of Party E (the “Party E Management”) to discuss a potential strategic transaction between the Company
and Party E (the “Potential Party E Transaction”).
On
November 9, 2023, the Company and Party E entered into a confidentiality agreement (the “Party E NDA”) in furtherance of
Party E’s due diligence of the Company in connection with the Potential Party E Transaction.
Also
on November 9, 2023, Mr. Berlin reached out to the Chief Executive Officer and Chairman of the Board of Party F (the “Party F CEO”)
to discuss a potential strategic transaction between the Company and Party F (the “Potential Party F Transaction”). Mr. Berlin
also provided the Party F CEO with the Management Presentation.
On
November 10, 2023, representatives of Company Management provided representatives of Party E Management with access to the Data Room
to allow Party E to continue to progress due diligence in connection with the Potential Party E Transaction.
Between
November 10, 2023 and December 15, 2023, Mr. Berlin had several separate telephonic discussions with representatives of Party E Management
to discuss the Potential Party E Transaction.
On
November 14, 2023, Mr. Berlin provided representatives of the management of Party F with the Management Presentation in connection with
the Potential Party F Transaction.
Also
on November 14, 2023, the Party B CEO sent an email to representatives of Company Management requesting a discussion regarding next steps
with respect to the Potential Party B Transaction and requesting access to the Data Room.
On
November 15, 2023, representatives of Company Management provided representatives of Party B Management with access to the Data Room.
On
November 16, 2023, the Board held a meeting. Members of Company Management and representatives of Morgan, Lewis & Bockius LLP (“Morgan
Lewis”), the Company’s legal advisor, also participated. At the meeting, the Board discussed, among other things, the Company’s
cash position and potential strategic transactions and financing options, including the Potential PIPE, and a proposed bridge financing,
whereby certain investors would lend up to $4 million to the Company, which amount would be convertible into shares of Company Common
Stock, upon the terms and subject to the conditions of the relevant loan documents (the “Senior Convertible Promissory Notes”).
After discussion, the Board authorized the issuance of the Senior Convertible Promissory Notes.
On
November 17, 2023, in furtherance of its ongoing financing efforts, the Company issued the Senior Convertible Promissory Notes to each
of IBF I, IBF II, Arkin Bio and Biotel, in the aggregate principal amount of $4 million. The Company also issued to (a) the holders of
the Senior Convertible Promissory Notes warrants to purchase up to 15 million shares of Company Common Stock, and (b) the holders of
the August Secured Convertible Notes warrants to purchase up to 7.5 million shares of Company Common Stock. The Company also amended
and restated the August Secured Convertible Notes to conform to the terms of the Senior Convertible Promissory Notes. The Company also
entered into that certain Side Letter Agreement (New Notes), pursuant to which the investor parties thereto were provided with the right
to lend an additional $4 million to the Company on the same terms, including warrant coverage, as the Senior Convertible Promissory Notes,
and entered into that certain Side Letter Agreement For Conversion, pursuant to which the investor parties thereto were provided with
the right to lend an additional $1.48 million to the Company on the same terms, including warrant coverage, as the Senior Convertible
Promissory Notes.
On
November 20, 2023, representatives of Party B Management requested estimates of research and development costs for the clinical trials
of AL102 and BST-236 (in each case for 2023 and forecasted costs to filing).
On
November 22, 2023, representatives of the Party B clinical team provided a series of follow-up questions to the Party B Diligence Request
List (the “Party B Follow-Up Questions”).
Also
on November 22, 2023, Dr. Cabilly and the Party D COO held a telephonic discussion regarding potential terms of the Potential Party D
Transaction, and Dr. Cabilly indicated to the Party D COO that the Company would be interested in consideration consisting of cash, equity
and post-Closing payments determined based on the achievement of certain post-Closing milestones.
On
November 23, 2023, the Company and Party F entered into an amendment to the Party F NDA, extending the term of the Party F NDA.
On
November 28, 2023, representatives of Party B Management sent an email to representatives of Company Management, requesting patient-level
data, and requesting the opportunity to speak with key opinion leaders (KOLs) for the Company’s clinical trial.
On
November 29, 2023, as part of routine industry coverage activities, a representative of A.G.P. had a telephonic conversation with a
representative of the board of directors of Immunome during which, among other things, Immunome’s potential interest in
acquiring a late-stage oncology program was discussed. A potential strategic transaction between the Company and
Immunome was not specifically discussed at the time.
On
December 1, 2023, representatives of Party B Management, including the Chief Financial Officer of Party B, had a telephonic discussion
with representatives of Company Management to discuss the Potential Party B Transaction. Following such call, representatives of Company
Management provided representatives of Party B Management with certain pro forma calculations.
On
December 5, 2023, representatives of Party B Management had a telephonic discussion with representatives of Company Management to discuss
Party B’s request for patient level data.
Also
on December 5, 2023, representatives of Company Management provided additional responses to the Party B Follow-Up Questions.
On December 6, 2023, further to their prior discussion on November 29, 2023, a representative of A.G.P. had a telephonic conversation
on behalf of the Company with a representative of the board of directors of Immunome to discuss a potential strategic transaction between
the Company and Immunome.
On
December 7, 2023, representatives of A.G.P. provided an introduction over email between representatives of Company Management and representatives
of the management (“Immunome Management”) and the board of directors of Immunome to further discuss a potential Immunome
transaction.
Later
that day, Dr. Cabilly provided an introduction over email between representatives of Company Management and representatives of the management
of Party G (“Party G Management”) to discuss a potential strategic transaction between the Company and Party G (the “Potential
Party G Transaction”). Representatives of Company Management provided representatives of Party G Management with the Management
Presentation.
Also
on December 7, 2023, the Company and Party G entered into a confidentiality agreement (the “Party G NDA”).
Also
on December 7, 2023, following the execution of the Party G NDA, representatives of Company Management provided representatives of Party
G Management with access to certain folders in the Data Room, to allow Party G to continue to progress due diligence in connection with
the Potential Party G Transaction. Representatives of Party G Management were only provided access to Data Room folders containing material
related to AL102.
Also
on December 7, 2023, at the direction of the Company, representatives of A.G.P. sent the Management Presentation to representatives of
Immunome Management.
Also
on December 7, 2023, representatives of Company Management provided representatives of Party G Management with access to the Data Room
folders.
On
December 8, 2023, the Party D COO sent Mr. Berlin an initial draft of a term sheet with respect to the Potential Party D Transaction
(the “Initial Party D Proposal”). The Initial Party D Proposal provided for, among other things, a cash purpose price of
$10 million payable at closing, with the Company retaining all current liabilities with respect to vendor obligations of the AL102 program.
On
December 10, 2023, Dr. Cabilly provided an introduction over email between representatives of Company Management and representatives
of the management of Party H (“Party H Management”) to discuss a potential strategic transaction between the Company and
Party H (the “Potential Party H Transaction”).
Also
on December 10, 2023, representatives of the Company provided representatives of Party H with an initial draft confidentiality agreement
(the “Party H NDA”).
Also
on December 10, 2023, Ms. Dana Gelbaum, General Manager and Chief Business Officer of the Company (“Ms. Gelbaum”), had a
telephonic discussion with Party A’s vice president, business and corporate development, who indicated to Ms. Gelbaum that Party
A was not interested in a strategic transaction with the Company that would involve the acquisition or assumption by Party A of the Company’s
assets related to AL102.
On
December 11, 2023, representatives of A.G.P. had a follow-up telephonic discussion with representatives of Immunome regarding
the potential Immunome transaction.
Also
on December 11, 2023, Mr. Berlin and the Party D COO had a telephonic discussion regarding the Potential Party D Transaction.
Also
on December 11, 2023, Mr. Berlin and the Party B CBO had a telephonic discussion regarding the Potential Party B Transaction.
Also
on December 11, 2023, the Company and Immunome entered into a confidentiality agreement (the “Immunome NDA”), which did not
contain a standstill. Representatives of the Company then shared certain information related to the Company’s intellectual property
with representatives of Immunome.
On
December 12, 2023, Representatives of Company Management terminated access to the Data Room for Party D.
On
December 13, 2023, the Board held a meeting. Members of Company Management and representatives of Morgan Lewis also participated. At
the meeting, the Board discussed, among other things, the Company’s cash position and potential strategic transactions and financing
options, including the Potential Party D Transaction, the Potential Party H Transaction and the Potential PIPE.
Also
on December 13, 2023, representatives of Company Management had a telephonic discussion with representatives of Immunome Management to
discuss the potential Immunome transaction and indicated that the Company could be open to different structures.
Also
on December 13, 2024, Mr. Berlin sent an email to the Party D COO outlining a revised structure for the Potential Party D Transaction
and certain proposed revisions to the Initial Party D Proposal. Following such email, Mr. Berlin and the Party D COO had a telephonic
discussion regarding the Potential Party D Transaction.
Between
December 13, 2023 and December 22, 2023, Mr. Berlin had several separate telephonic discussions with representatives of Immunome Management
to discuss the potential Immunome transaction.
On
December 14, 2024, Dr. Bridget Martell, a member of the Board, contacted representatives of management of each of Party I, Party J, Party
K and Party L regarding their participation in the Potential PIPE.
Also
on December 14, 2023, representatives of management of Party M (the “Party M Management”) sent an email to representatives
of Company Management, which included a number of preliminary due diligence questions (the “Initial Party M Questions”) in
connection with Party M’s potential participation in the Potential PIPE.
Also
on December 14, 2023, representatives of Company Management provided representatives of Immunome Management with access to materials
in the Data Room related to the AL101 and AL102 programs to allow Immunome to continue to progress due diligence in connection with the
potential Immunome transaction.
On
December 15, 2024, representatives of Company Management had a telephonic discussion with representatives of Party F Management to discuss
the Potential Party F Transaction.
Also
on December 15, 2023, representatives of Company Management sent an email to representatives of Party M Management, which included responses
to the Initial Party M Questions.
On
December 16, 2023, the Party D COO sent an email to Mr. Berlin, which included a revised term sheet (the “Second Party D Proposal”),
which provided for, among other things, consideration of $10 million in cash and $10 million payable in Party D shares.
At
9:00 AM Eastern Time on December 18, 2023, the Board held a meeting. Members of Company Management and representatives of Morgan Lewis
also participated. At the meeting, the Board discussed, among other things, the terms of the Second Party D Proposal, which had been
received earlier that day and provided, among other things, for total aggregate consideration payments of $10 million in cash and $10
million payable in Party D shares. The Board then discussed ongoing discussions with Immunome, which discussions had been progressing
quickly. The Board discussed that a potential transaction with Immunome would likely take the form of an asset purchase. The Board also
discussed the current status of the Potential PIPE. The Board determined that the Company should continue to try to gather various strategic
and financing alternatives, including those discussed at the Meeting, and then revisit these issues.
Also
on December 18, 2023, representatives of Company Management had a telephonic discussion with the Party D COO regarding revised terms
of the Second Party D Proposal (the “Third Party D Proposal”), which provided for, among other things, for total aggregate
consideration payments of $10 million in cash, $15 million in Party D shares, and a Contingent Value Right of approximately $20-$30 million
in Party D shares, payable upon positive and differentiated Positive Topline Data from RINGSIDE Part B study.
Also
on December 18, 2023,, representatives of Immunome Management sent representatives of Company Management an initial draft of a term
sheet with respect to the potential Immunome transaction (the “Initial Immunome Proposal”). The Initial Immunome
Proposal provided for, among other things, the acquisition of the AL101 and AL102 programs in an asset purchase for aggregate
consideration consisting of $35 million at closing (paid 50% in cash and 50% in unregistered Immunome common stock), a milestone
payment of $35 million in cash payable on receipt of U.S. FDA approval for a first indication for AL102, and the assumption of
certain liabilities related to the AL101 and AL102 programs.
Also
on December 18, 2023, Mr. Berlin had a telephonic discussion with the Party B CBO regarding the Potential Party B Transaction.
Also
on December 18, 2023, Dr. Andres Gutierrez, Executive Vice President and Chief Medical Officer, provided an introduction over email between
representatives of Company Management and representatives of the management of Party N (the “Party N Management”). Also on
December 18, 2023, representatives of Company Management had an initial meeting with representatives of Party N Management to discuss
participation in the Potential PIPE.
Also
on December 18, 2023, Mr. Berlin had a telephonic discussion with Dr. Clay Siegall, President and Chief Executive Officer of Immunome
(“Dr. Siegall”) regarding the terms of the potential Immunome transaction. Mr. Berlin conveyed that the Company was seeking
$40 million in upfront payments, $40 million in a milestone payment payable on public announcement of positive topline data from RINGSIDE
Part B study, the assumption of $4 million in outstanding payables for clinical trial costs and a $5 million breakup fee.
On
December 19, 2023, representatives of Company Management had a telephonic discussion with representatives of Party N Management to discuss
participation in the Potential PIPE.
Also
on December 19, 2023, Mr. Berlin received an email from representatives of management of Party O, informing Mr. Berlin that Party O would
not be able to participate in the Potential PIPE by year-end.
Also
on December 19, 2023, representatives of Company Management sent representatives of Immunome Management proposed revisions to the
Initial Immunome Proposal (the “Proposed Revisions”). Such Proposed Revisions provided, among other things, an asset
purchase transaction for consideration of $40 million at closing (50% in cash and 50% in unregistered Immunome common stock), a
milestone payment of $40 million in cash on public announcement of Positive Topline Data from RINGSIDE Part B study and assumption
of certain liabilities related to the AL101 and AL102 programs, subject to certain caps.
At
9:00 AM Eastern Time on December 20, 2023, the Board held a meeting. Members of Company Management and representatives of Morgan Lewis
also participated. At the meeting, the Board discussed, among other things, the terms of the Initial Immunome Proposal and the Proposed
Revisions. Mr. Berlin noted that he had indicated to Dr. Siegall the Company’s preference for a milestone based on Positive Topline
Data (as opposed to FDA approval), and a portion of the purchase price paid in the form of an advance deposit upon execution of a term
sheet. The Board then discussed the terms of the Third Party D Proposal. Mr. Berlin informed the Board that the Company had received
no further contract from Party H, and that the third parties who had previously expressed interest in the Potential PIPE had similarly
ceased contact. The Board then discussed the status of other potential strategic transactions with various third parties. Following a
discussion, the Board authorized Mr. Berlin to pursue the potential Immunome transaction on terms no less beneficial to the Company as
those expressed in the Initial Immunome Proposal, while trying to improve the terms of the Initial Immunome Proposal if possible.
Following
the Board meeting, the Company continued to engage in discussions with Immunome to improve the terms of its offer.
Later
that day, representatives of Immunome Management submitted a revised term sheet (the “Revised Immunome Proposal”), which
offered $40 million at closing, payable 50% in cash and 50% in unregistered Immunome common stock (to be valued based on the VWAPs
on the 30 trading days ending with the trading day immediately prior to the date on which the definitive agreement for the potential
Immunome transaction was executed), two milestones consisting of a $20 million milestone payable in cash on public announcement of
Positive Topline Data from RINGSIDE Part B study and a $15 million milestone payable in cash on receipt of U.S. FDA approval for a
first indication and the assumption of certain specified liabilities related to the AL101 and AL102 programs, subject to certain
caps. The Revised Immunome Proposal also included an exclusivity agreement with an exclusivity period of 30 days, followed by an
automatic extension of 15 days if the parties were continuing to negotiate in good faith. The Revised Immunome Proposal also
included a $4 million advance deposit (the “Deposit”) payable upon execution of a term sheet and exclusivity agreement,
which would be creditable towards the $20 million in cash due at closing and would be non-refundable except for limited instances to
be described in such exclusivity agreement.
On
December 21, 2023, representatives of Company Management received an email from the Party B CBO informing them that Party B would not
be able to proceed with the Potential Party B Transaction.
Also
on December 21, 2023, Mr. Berlin sent an email to representatives of management of Party P regarding proposed terms for the Potential
PIPE.
Also
on December 21, 2023, the Company sent Immunome a revised term sheet, with minor modifications and a revised exclusivity agreement that,
among other things, removed the 15 day automatic extension to the exclusivity period, and modified the strategic transactions covered
by the exclusivity agreement to permit the additional senior convertible promissory note issuances as permitted by the November financing.
On
December 22, 2023, Mr. Cabilly and the Party D COO had a telephonic discussion regarding the terms of the Third Party D Proposal.
At
3:15 PM Eastern Time on December 22, 2023, the Board held a meeting. Members of Company Management and representatives of Morgan
Lewis also participated. At the meeting, the Board discussed, among other things, the ongoing discussions between representatives of
the Company and representatives of Immunome in an effort to have Immunome improve on the terms of the Initial Immunome Proposal, as
described to the Board at the Board’s December 20, 2023 meeting. The Board discussed the terms of the Initial Immunome
Proposal, which was for $35 million at closing (50% in cash and 50% in unregistered Immunome common stock), a milestone payment of
$35 million in cash on receipt of U.S. FDA approval for a first indication, and the assumption of certain liabilities related to the
AL101 and AL102 programs. The Board then discussed the changes to the Initial Immunome Proposal as contemplated by the Revised
Immunome Proposal, including the $4 million Deposit and that such Deposit was non-refundable except in limited circumstances. Mr.
Berlin informed the Board that both the Initial Immunome Proposal and the Revised Immunome Proposal proposed an exclusivity period
of 30 days, followed by an automatic extension of 15 days if the parties were continuing to negotiate in good faith, and indicated
that the Company would propose an exclusivity period of 35 days, with no automatic extension. Mr. Berlin then described for the
Board the other material terms of the Revised Immunome Proposal, including the structure, proposed timing and economic terms of the
potential Immunome transaction. Dr. Cabilly then informed the Board that the Company had received a non-binding written proposal
from Party D earlier that day (the “Fourth Party D Proposal”) regarding the Potential Party D Transaction, which
contemplated the payment of $20 million in cash at closing, $15 million in shares of Party D common stock (subject to a 12-month
lock up), and six milestones, payable in cash or Party D common stock at the option of Party D, as follows: $10 million on public
announcement of verified Positive Phase 3 Data from RINGSIDE Part B study; $10 million on receipt of U.S. FDA approval for a first
indication; $7.5 million upon the first at MAA (including price and reimbursement approval) approval in three of the following EU
countries: France, Germany, Italy, Spain, and the United Kingdom; $2.5 million upon the at first PMDA approval (including price and
reimbursement approval) in Japan; $15 million upon the first achievement of total net sales of AL102 exceeding $250 million in a
single calendar year; and $25 million upon the first achievement of total net sales of AL102 exceeding $500 million in a single
calendar year. Dr. Cabilly also informed the Board that the Fourth Party D Proposal also contemplated assumption of BMS obligations
related to the AL101 and AL102 programs, subject to certain caps, and included the Deposit payable upon execution of a term sheet
and exclusivity agreement which is creditable towards the $20 million in cash due at closing and would be non-refundable except for
limited instances to be described in the exclusivity agreement. Following a discussion, the Board unanimously agreed to authorize
Mr. Berlin to continue to progress the potential Immunome transaction on the terms of the Revised Immunome Proposal, but also
directed him to submit, prior to agreeing to those terms, a counterproposal to Immunome with terms more favorable to the Company,
including, among other things, an increase in the amount of Immunome shares proposed to be issued to the Company as part of the
closing consideration of the potential Immunome transaction to $25 million, and an increase with respect to the milestone payable in
cash on receipt of U.S. FDA approval for a first indication. The Board also authorized Mr. Berlin to enter into an exclusivity
agreement with Immunome in consideration of the Deposit.
Later
that day, the Company and Immunome entered into a non-binding term sheet outlining the terms and conditions of the potential Immunome
transaction, including $45 million payable at closing, consisting of $20 million in cash (net of the $4 million included in the Deposit)
and $25 million of Immunome shares of common stock (to be valued based on the VWAPs on the 30 trading days ending with the
trading day immediately prior to the date on which the definitive agreement for the potential Immunome transaction was executed), up to $40 million of milestone payments ($5 million of which could be satisfied in cash or shares
of common stock of Immunome, at Immunome’s election), and an exclusivity agreement providing for the $4 million Deposit with an
exclusivity period ending at 11:59 p.m. Pacific Time on the 35th day thereafter (the “Exclusivity Period”).
Later
that day, the Party D COO sent an email to Mr. Berlin proposing revisions to the Fourth Party D Proposal, including, among other things,
consideration payments of $20 million in cash and $24.6 million in Party D shares, and aggregate milestone payments of up to $20 million
payable in cash. Mr. Berlin informed the Party D COO that the Company had entered into an exclusivity agreement with a third party, and
was thus prohibited from engaging in further discussions with Party D.
On December 23, 2023, Representatives of Company
Management terminated access to the Data Room for Party A, Party B and Party E.
From
December 23, 2023 through February 5, 2024, representatives of Immunome Management conducted due diligence, including holding due diligence
calls with representatives of Company Management regarding, among other things, intellectual property, clinical and CMC matters.
On
December 25, 2023, representatives of Party H Management sent an email to representatives of Company Management inquiring about the status
of the Party H NDA. Representatives of Company Management responded to such email informing such representatives of Party H Management
that the Company was under an exclusivity agreement with a third party, and could not continue discussions with Party H.
On
December 27, 2023, Mr. Berlin sent an email to representatives of Party G Management informing such representatives of Party G Management
that the Company was under an exclusivity obligation with a third party and could not continue discussions with Party G. Representatives
of Company Management then terminated Party G’s access to the Data Room.
Also
on December 27, 2023, representatives of Party G Management sent an email to representatives of Company Management inquiring about the
duration of the Company’s exclusivity period. Representatives of Company Management responded to such email informing such representatives
of Party G Management that the Company was under an exclusivity agreement with a third party and could not continue discussions with
Party G.
In
the evening of December 31, 2024, representatives of Cooley LLP (“Cooley”), counsel to Immunome, provided to representatives
of Morgan Lewis an initial draft of the Asset Purchase Agreement.
In
the evening of January 4, 2024, representatives of Morgan Lewis provided to representatives of Cooley a revised draft of the Asset Purchase
Agreement, which, among other things, provided that no representation, warranty or pre-closing covenant would survive the closing and
removed a mutual post-closing indemnification provision, pursuant to which (a) the Company would be required to indemnify Immunome following
the Closing for (i) any breach of representations, warranties and covenants of the Company contained in the Asset Purchase Agreement,
(ii) any excluded liabilities, (iii) any liabilities related to the operation by the Company of its business prior to the Closing, (iv)
any common law fraud or (v) any legal proceeding related to enforcement by Immunome of such indemnification rights, and (b) Immunome
would be required to indemnity the Company following the Closing for (i) any breach of representations, warranties and covenants of Immunome
contained in the Asset Purchase Agreement, (ii) the assumed liabilities, (iii) any common law fraud or (iv) any legal proceeding related
to enforcement by the Company of such indemnification rights. The January 4, 2024 draft of the Asset Purchase Agreement also included
the provision of registration rights with respect to the shares of common stock of Immunome issuable at closing.
On
January 14, 2024, representatives of Party H Management sent an email to representatives of Company Management inquiring about the status
of the Potential Party H Transaction, and whether the Company was still subject to an exclusivity obligation. Representatives of Company
Management responded to such email informing such representatives of Party H Management that the Company was under an exclusivity agreement
with a third party, and could not continue discussions with Party H.
On
January 19, 2024, the Board agreed in writing (a) that the Company would enter into that certain Amendment No. 1 to Exclusivity Agreement
(the “Exclusivity Amendment”), pursuant to which the Exclusivity Period was extended until 11:59 p.m. Pacific Time on February
2, 2024 (the “Exclusivity Extension Expiration Time”), and (b) to authorize Mr. Berlin to extend the Exclusivity Period for
up to an additional seven days following the date of the Exclusivity Extension Expiration Time, in the sole discretion of Mr. Berlin
and in furtherance of the potential Immunome transaction (the “Exclusivity Extension Option”).
Later
that day, the Company and Immunome entered into the Exclusivity Amendment.
On
January 20, 2024, representatives of Party C Management sent an email to representatives of Company Management informing such representatives
of Company Management that Party C would not be able to proceed with the Potential Party C Transaction.
In
the morning of January 24, 2024, representatives of Cooley provided to representatives of Morgan Lewis a revised draft of the Asset Purchase
Agreement, which, among other things, provided that representations, warranties and pre-closing covenants would not survive closing,
but included a mutual post-closing indemnification provision, pursuant to which (a) the Company would be required to indemnify Immunome
following the Closing for (i) any breach of covenants of the Company contained in the Agreement, (ii) any excluded liabilities, (iii)
any liabilities related to the operation by the Company of its business prior to the Closing, (iv) any common law fraud or (v) any legal
proceeding related to enforcement by Immunome of such indemnification rights, and (b) Immunome would be required to indemnity the Company
following the Closing for (i) any breach of covenants of Immunome contained in the Agreement, (ii) the assumed liabilities, (iii) any
common law fraud or (iv) any legal proceeding related to enforcement by the Company of such indemnification rights (such provision as
proposed, the “Revised Indemnification Provision”). Such revised draft of the Asset Purchase Agreement also removed a limited
recourse provision applicable to certain “related parties” of each of the Company and Immunome (the “Limited Recourse
Provision”) and removed the provision of registration rights with respect to the shares of common stock of Immunome issuable at
closing. The January 24 draft of the Asset Purchase Agreement also included an update to the milestone payments in which (x) the milestone
payment associated with the receipt of U.S. FDA approval for a first indication would be $15 million in cash and (y) an additional $10
million cash milestone payment would be due following the first calendar year in which annual net sales of AL102 exceeded $100 million.
In
the afternoon of January 24, 2024, representatives of management of Party Q (the “Party Q Management”) sent an email to representatives
of Company Management inquiring about the status of the Potential PIPE. Representatives of Company Management responded to such email
informing such representatives of Party Q Management that the Company was under an exclusivity agreement with a third party, and could
not continue discussions with Party Q.
In
late January 2024, at the request of the Company, A.G.P. began work to render a financial advisory opinion to the Board in connection
with the Board’s consideration of the potential Immunome transaction.
In
the morning of January 26, 2024, representatives of Cooley provided to representatives of Morgan Lewis an initial draft of the Support
Agreement proposed to be executed by certain director and officer equity holders of the Company and other equity holders of the Company
who, collectively, possess a number of shares (or securities convertible or exercisable into a number of shares) of common stock of the
Company sufficient to approve the potential Immunome transaction, as well as other ancillary agreements to the Asset Purchase Agreement.
The Support Agreement generally required each signing party to (a) vote in favor of the potential Immunome transaction, (b) vote against
any proposal made in opposition to, or in competition with, the potential Immunome transaction and (c) vote against any acquisition proposal
involving a third party.
In
the morning of January 27, 2024, representatives of Morgan Lewis provided to representatives of Cooley a revised draft of the Asset Purchase
Agreement which, among other things, removed the Revised Indemnification Provision and restored the Limited Recourse Provision.
Later
that day, representatives of Cooley had a telephonic discussion with representatives of Morgan Lewis to highlight and discuss what they
considered to be the remaining open points that would be reflected in the revised draft Asset Purchase Agreement to be circulated later
that week. Among other points, these included certain revisions to the representations and warranties, the definition of the term “Fraud”,
the Revised Indemnification Provision, the Limited Recourse Provision, the registration rights and related lock-up period and orderly
market covenants, limitations on dissolution and liquidation of the Company or the payment of dividends or distributions to stockholders
and the purchase by the Company of tail insurance to mitigate against pre-closing liabilities.
In
the evening of January 29, 2024, representatives of Morgan Lewis provided to representatives of Cooley an initial draft of the Disclosure
Schedules.
In
the evening of January 30, 2024, representatives of Cooley provided to representatives of Morgan Lewis a revised draft of the Asset Purchase
Agreement and other ancillary agreements. Among other things, the revised draft of the Asset Purchase Agreement restored the Revised
Indemnification Provision, added registration rights with respect to the shares of common stock of Immunome issuable at closing and removed
the Limited Recourse Provision.
In
the morning of January 31, 2024, representatives of Morgan Lewis provided to representatives of Cooley revised drafts of the Support
Agreement and comments received from representatives of Goldfarb Seligman, counsel to IBF, with respect to the support agreement to be
signed by IBF.
In
the evening of January 31, 2024, representatives of Morgan Lewis provided to representatives of Cooley a revised draft of the Asset Purchase
Agreement and the Disclosure Schedules. Among other things, the revised draft of the Asset Purchase Agreement removed the Revised Indemnification
Provision and restored the Limited Recourse Provision. From January 31 through February 5, 2024, representatives of Morgan Lewis and
Cooley continued negotiating and finalizing the Asset Purchase Agreement, the Disclosure Schedules and the other ancillary agreements.
On
February 2, 2024, the Company and Immunome agreed to extend the Exclusivity Period until 5:00 p.m. Eastern Time on February 5, 2024,
and executed Amendment No. 2 to Exclusivity Agreement to accomplish the same (the “Second Exclusivity Amendment”).
At
8:00 AM Eastern Time on February 5, 2024, the Board held a meeting. Members of Company Management and representatives of A.G.P. and Morgan
Lewis also participated.
The
Board first discussed the Exclusivity Amendment, the Exclusivity Extension Option and the Second Exclusivity Amendment. Upon a
motion duly made, the Board unanimously authorized, adopted and approved and consented to and ratified the form, terms and
provisions of Amendment No. 1 to Exclusivity Agreement, the Exclusivity Extension Option and Amendment No. 2 to Exclusivity Agreement.
The
Board next discussed the potential Immunome transaction. Representatives of Morgan Lewis reviewed the material terms of the Asset Purchase
Agreement, which had been provided to the Board in advance of the meeting, and reported that it was complete and ready for execution,
and also described for the Board the fiduciary duties of directors applicable to the potential Immunome transaction. At the Board’s
request, representatives of A.G.P. reviewed for the Board A.G.P.’s financial analysis of the aggregate consideration provided for
in the potential Immunome transaction, consisting of (a) closing payments of $20 million in cash and 2,175,489 newly-issued unregistered
shares of Immunome common stock, and (b) three contingent milestone payments of $10 million, $17.5 million and $10 million in cash due
upon the achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement. A.G.P. then rendered
an opinion, initially rendered verbally and confirmed in a written opinion dated February 5, 2024, to the Board to the effect that, as
of that date and based on and subject to various assumptions made, procedures followed, matters considered and qualifications and limitations
on the scope of the review undertaken by A.G.P. as set forth in A.G.P.’s written opinion, the aggregate consideration to be paid
to the Company for the assets subject to the potential Immunome transaction was fair, from a financial point of view, to the Company.
The
Board deliberated regarding the potential Immunome transaction. After the Board’s discussions, upon a motion duly made, the Board
(a) determined that the transactions contemplated by the Asset Purchase Agreement were advisable, expedient, fair to and for the best
interests of the Company and its stockholders, and authorized, adopted, approved and consented to and ratified the form, terms and provisions
of the Asset Purchase Agreement and all other agreements, instruments, certificates and documents required to be delivered in connection
therewith (collectively, the “Transaction Documents”), and did thereby consent to, authorize, approve and ratify the execution,
delivery and performance of the Asset Purchase Agreement and the Transaction Documents, and did thereby authorize, adopt and approve
the potential Immunome transaction and all other transactions contemplated by the Asset Purchase Agreement and the Transaction Documents;
(b) directed that, in accordance with Section 271(a) of the Delaware General Corporate Law (the “DGCL”), the Company submit
the Transaction Documents, including the Asset Purchase Agreement and the transactions contemplated thereby, to the Company’s stockholders
for authorization and approval thereof, which authorization and approval the Board expected to be reflected in a written consent of stockholders
of the Company who own a majority of all of the issued and outstanding capital stock of the Company entitled to vote thereon, and recommended
that the stockholders of the Company, in accordance with the DGCL, authorize, adopt and approve the Asset Purchase Agreement, the Transaction
Documents and the transactions contemplated thereby; (c) fixed the close of business on February 8, 2024 as the date of record for the
determination of stockholders of the Company entitled to vote on the authorization, adoption and approval of the Asset Purchase Agreement,
the Transaction Documents and the transactions contemplated thereby; and (d) authorized and directed, in the name and on behalf of the
Company, the appropriate officers of the Company, to prepare, execute, if appropriate, and file or cause to be filed all reports, statements,
documents and information required to be filed by the Company pursuant to the Exchange Act in connection with the Asset Purchase Agreement,
the Transaction Documents and the transactions contemplated thereby, including: (i) an information statement relating to the written
consent of the stockholders reflecting the authorization, adoption and approval of the Asset Purchase Agreement, the Transaction Documents
and the transactions contemplated thereby, including the Transaction in compliance with Rule 14c-2 of the Exchange Act; and (ii) any
necessary Current Reports on Form 8-K. Following that, the meeting adjourned.
During
the afternoon of February 5, 2024, the Asset Purchase Agreement was executed on February 5, 2024, on behalf of the Company by Mr. Berlin
and on behalf of Immunome by Dr. Siegall.
The
Company and Immunome announced the signed agreement by press releases on Tuesday, February 6, 2024, pre-market opening.
Recommendation
of the Board; Reasons for the Asset Sale
Our
Board of Directors believes that effecting the Asset Sale pursuant to the Asset Purchase Agreement is expedient and
in the best interests of Ayala and its stockholders. The decision of our Board of Directors to seek our stockholders’ approval
for the Asset Sale pursuant to the Asset Purchase Agreement followed a lengthy process during which our Board of Directors consulted
with management and legal, financial, accounting and tax advisors and carefully considered the terms of the Asset Purchase Agreement
and the risks, timing, viability and potential impact to the Company and our stockholders of the strategic alternatives potentially available
to us, including, strategic alternatives such as a merger, reverse merger, strategic partnership or other business combination. Based
on such consideration, our Board of Directors determined that the most beneficial strategic proposal available to us was the Asset Sale.
In
reaching its decision to approve the Asset Purchase Agreement and to recommend that our stockholders vote to approve the Asset Sale,
our Board of Directors considered a number of factors, including, but not limited to the following factors:
| |
● |
our
limited capital resources and our recent difficulty in raising capital given the current capital markets for microcap biopharmaceutical
companies, thus limiting our ability to attempt to further develop our business on our own; |
| |
|
|
| |
● |
our
Board of Directors’ understanding of and familiarity with, and discussions with our management regarding, the business, operations,
management, financial condition, operating losses and future business prospects for Ayala; |
| |
|
|
| |
● |
the
Board’s belief that it had obtained Immunome’s best and final offer and that it was unlikely that any other party would
be willing to acquire the Company, or substantially all of its assets, on terms and at a value as attractive to the Company as the
terms of the Immunome offer; |
| |
|
|
| |
● |
the
value of the consideration to be received by Ayala pursuant to the Asset Purchase Agreement, including the fact that approximately
$30,000,000 of the $50,000,000 in upfront consideration is payable in shares of common stock of Immunome that were
priced at $13.79 per share (valued at 30-day volume weighted average price of as of February 1, 2024 prior to the announcement of
the Asset Sale) and the Board’s belief that the stock component will allow Ayala to continue to participate in the appreciation
in the value of the assets it sold and including the fact that Immunome is assuming certain outstanding liabilities of Ayala and
is reimbursing it for certain expenditures between signing and closing as well as assuming the ongoing cost of the Phase 3 trial
of AL102 for the treatment of desmoid tumors, manufacturing expenses and commercialization expenses and Ayala’s obligations
to BMS; |
| |
|
|
| |
● |
the
fact that Immunome would pay a $4,000,000 up-front deposit to Ayala upon execution the non-binding term sheet and exclusivity
agreement for the Asset Sale; |
| |
|
|
| |
● |
the
opportunity to realize additional value of up to an aggregate of $37,500,000 if all three milestones are achieved as described
in the Asset Purchase Agreement, as more fully described in the section captioned “Milestone Consideration”; |
| |
|
|
| |
● |
the
Asset Sale is the result of an active, lengthy and thorough evaluation of strategic alternatives reasonably available to Ayala that
did not result in any actionable alternative proposals that the Board believed were superior to the proposal by Immunome; |
| |
|
|
| |
● |
our
extensive, but unsuccessful, efforts prior to our negotiations with Immunome with respect to the Asset Sale to identify prospective
acquisition or merger candidates to enter into a transaction on terms more favorable than those of the Asset Sale, including with
respect to the terms of a proposal from another third party received shortly before the Board approved the non-binding term sheet
and exclusivity agreement for the Asset Sale; |
| |
● |
the
fact that the programs being purchased by Immunome through the Asset Sale will constitute their most advanced program following the
acquisition, increasing the likelihood in the Board’s view that they will be incentivized to pursue achievement of the milestones; |
| |
|
|
| |
● |
the
prior track record of Immunome’s management for developing and commercializing drug products; |
| |
|
|
| |
● |
the
fact that the likelihood of achieving the milestones contained in the Asset Purchase Agreement was greater in the Board’s view
than the chances of achieving the milestones proposed by the other offer available to the Company when the non-binding term sheet
and exclusivity agreement for the Asset Sale were approved; |
| |
|
|
| |
● |
Immunome’s
obligation to consummate the Asset Sale is not conditioned on Immunome obtaining financing; |
| |
|
|
| |
● |
the
financial presentation, dated February 5, 2024, reviewed by A.G.P. with the Board and the opinion, dated February 5, 2024, of A.G.P.
to the Board as to the fairness, from a financial point of view and as of the date of the opinion, to the Company of the aggregate
consideration to be paid to the Company for the assets being sold in the Asset Sale; |
| |
|
|
| |
● |
the
likelihood that the Asset Sale will be completed, including the reasonableness of the conditions to the Asset Purchase Agreement
and the likelihood that stockholder approval necessary to complete the Asset Sale will be obtained; and |
| |
|
|
| |
● |
that
the Asset Sale is subject to approval of holders of at least a majority of the outstanding shares of common stock entitled to vote,
which ensures that our Board of Directors will not be taking action without the support of a significant portion of our stockholders. |
Our
Board of Directors also considered potential drawbacks or risks relating to the Asset Sale, including the following risks and potentially
negative factors, but determined that these potential risks and factors were outweighed by the expected benefits of the Asset Sale:
| |
● |
the
incurrence of significant costs and expenses in connection with attempting to complete the Asset Sale, including legal, accounting
and other costs; |
| |
|
|
| |
● |
the
fact that the assets being sold to Immunome include substantially all of our non-cash assets; |
| |
|
|
| |
● |
the
fact that the Asset Sale must be completed by the End Date; |
| |
|
|
| |
● |
the
terms of the Asset Purchase Agreement that do not allow us to consider an alternative strategic transaction or to terminate the Asset
Purchase Agreement and accept a superior proposal; |
| |
|
|
| |
● |
the
restrictions on the conduct of our business prior to the completion of the Asset Sale, requiring us to conduct our business only
in the ordinary course, subject to specific limitations and exceptions, which may delay or prevent us from undertaking business opportunities
that may arise pending completion of the Asset Sale; |
| |
|
|
| |
● |
one
or more third parties could assert claims against us, either before or after the Closing, and seek damages or other remedies, and
we might be required to spend substantial time and resources defending any such claims, and any amounts paid to any such third parties
would reduce the net amount received from the Asset Sale; |
| |
|
|
| |
● |
the
fact that gains from the Asset Sale would be taxable to the Company for income tax purposes, and that we may not be able to offset
such taxes with our net operating losses; |
| |
|
|
| |
● |
that
certain of our officers and directors may have interests with respect to the Asset Sale in addition to their interests as stockholders
generally; and |
| |
|
|
| |
● |
following
the Asset Sale, our non-cash assets are expected to consist mainly of assets relating to programs that the Company had worked to
develop in the past, but had more recently substantially deemphasized as it prioritized the development of AL101 and AL102; including
assets acquired from Biosight relating to its programs to develop Aspacytarabine (“BST-236”), which is a novel proprietary
anti-metabolite, prodrug of cytarabine, covalently bound to asparagine, as well as assets relating to former Advaxis’ operations
as a clinical-stage biotechnology company focused on the development and commercialization of proprietary Listeria monocytogenes
(“Lm”)-based antigen delivery products. The Board of Directors may use the proceeds from the Asset Sale at its discretion,
which includes investing in existing programs and for general corporate purposes. |
The
foregoing discussion of the factors considered by the Board of Directors is not intended to be exhaustive, but rather includes the principal
factors considered by the Board. The Board reached the conclusion to approve the Asset Purchase Agreement, the Asset Sale and the other
transactions contemplated by the Asset Purchase Agreement in light of the various factors described above and other factors that the
members of the Board believed were appropriate. The Board did not assign relative weights to the foregoing factors or determine that
any factor was of particular importance. Rather, the Board viewed their positions and recommendations as being based on the totality
of information presented to and considered by it. In considering the factors discussed above, individual directors may have given different
weights to different factors.
At
this time, our Board of Directors has considered all the strategic alternatives available to Ayala and our Board of Directors has determined
that the only strategic proposal available to Ayala at this time is the Asset Sale.
Opinion
of A.G.P./Alliance Global Partners
On
February 5, 2024, at a meeting of the Company’s Board of Directors held to evaluate the Transaction, A.G.P. rendered to
the Company’s Board an opinion, dated February 5, 2024, to the effect that, as of that date and based on and subject to the matters
described in its opinion, the Aggregate Consideration (which was defined in A.G.P.’s opinion as consisting of $20,000,000 in
cash (the “Closing Cash Consideration”), 2,175,489 newly-issued unregistered shares of Immunome common stock (the “Closing
Stock Consideration”) and the contingent payments of $10,000,000, $17,500,000 and $10,000,000 in cash due upon the
achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement (the “Milestone Consideration”))
to be paid to the Company for certain of the Company’s assets and liabilities related to the Company’s AL101 and AL102 programs
(the “Product Assets”) in the Asset Sale was fair, from a financial point of view, to the Company.
The
full text of A.G.P.’s written opinion, dated February 5, 2024, which describes the assumptions made, procedures followed, matters
considered and limitations on the review undertaken, is attached to this information statement as Annex B and is incorporated by reference
in its entirety. A.G.P.’s opinion was provided for the information and assistance of the Company’s Board (in its capacity
as such) in connection with its consideration of the Asset Sale. A.G.P.’s opinion does not constitute a recommendation to the Company’s
Board, the Company, its stockholders or any other person as to how any of them should vote or act with respect to the Asset Sale or any
other matter. A.G.P.’s opinion does not address the underlying business decision of the Company to engage in the Asset Sale, or
the relative merits of the Asset Sale as compared to any strategic alternatives that may be available to the Company. This summary
of A.G.P.’s opinion is qualified in its entirety by reference to the full text of its opinion.
For
purposes of rendering its opinion, A.G.P. reviewed, among other things, the following:
| |
● |
Draft
dated February 4, 2024 of the Asset Purchase Agreement; |
| |
|
|
| |
● |
Historical
financials relating to the Company for the fiscal years ended December 31, 2022 and December 31, 2021 and the interim period ended
September 30, 2023 contained in the Company’s public filings, financial forecasts and projections relating to the Product Assets
for the years 2023 through 2033 prepared by or discussed with the management of the Company and estimates of the probability of achieving
the milestone events associated with the Milestone Consideration provided by the management of the Company; |
| |
|
|
| |
● |
Publicly
available financial and stock market information of certain public companies that were deemed by A.G.P. to be reasonably comparable
to the Product Assets; |
| |
|
|
| |
● |
Financial
terms, to the extent publicly available, of certain acquisition transactions that were deemed by A.G.P. to be reasonably comparable
to the Asset Sale; and |
| |
|
|
| |
● |
Publicly
available stock market information of Immunome, including current and historical market prices and trading volumes of publicly traded
shares of Immunome common stock. |
In
connection with its opinion, A.G.P. performed a selected companies analysis and a selected transactions analysis of the Product Assets
as well as a discounted cash flow analysis of the Product Assets (which discounted cash flow analysis was probability-adjusted for the
milestone events associated with the Milestone Consideration). At the direction of the Company based on the assessments of the management
of the Company as to commercial viability and consistent with the financial forecasts and projections reviewed by A.G.P., no value was
ascribed to AL101 in A.G.P.’s analysis. A.G.P. also participated in certain discussions with representatives of the Company. A.G.P.
was not provided with access to the management of Immunome and did not perform any financial analyses to estimate the value of Immunome
common stock. Rather, A.G.P. assumed that the latest closing price of Immunome common stock on the last trading date immediately prior
to the date of its opinion was a reasonable estimate of value for shares of Immunome common stock to be issued as the Closing Stock Consideration.
Furthermore, A.G.P. did not apply any illiquidity or other discounts, or otherwise give effect to any restrictions or limitations, which
might be attributable to the Closing Stock Consideration.
In
rendering its opinion, A.G.P. assumed and relied upon the accuracy and completeness of all information that was publicly available, provided
by or on behalf of the Company or any other party to the Asset Sale, or otherwise reviewed by or discussed with A.G.P., without (and
without assuming responsibility for) independent verification thereof by A.G.P. Accordingly, A.G.P. did not express an opinion or any
other form of assurance thereon. Moreover, A.G.P. assumed that the financial forecasts and projections and probability estimates referred
to above were prepared reasonably and in good faith and were based upon the best currently available estimates and judgments of the management
of the Company as to the matters covered thereby, and A.G.P. relied upon such forecasts, projections and estimates in its analysis. A.G.P.
was not engaged to assess the reasonableness or achievability of such forecasts, projections or estimates or the assumptions upon which
they were based, and A.G.P. expressed no views as to such forecasts, projections or estimates or the assumptions on which they were based.
In
addition, A.G.P. did not evaluate the solvency of the Company or Immunome or make an independent evaluation or appraisal of any of the
Product Assets or the other assets and liabilities (including any contingent, derivative or off-balance-sheet assets and liabilities)
of the Company, Immunome or any of their respective subsidiaries, and A.G.P. was not furnished with any such evaluation or appraisal.
A.G.P.’s
opinion did not constitute legal, regulatory, accounting, insurance, tax or other similar professional advice. A.G.P.’s opinion
also did not address the underlying business decision of the Company to engage in the Asset Sale, or the relative merits of the Asset
Sale as compared to any strategic alternatives that might be available to the Company. A.G.P.’s opinion addressed only the fairness
from a financial point of view, as of the date hereof, to the Company of the Aggregate Consideration to be paid to the Company for the
Product Assets in the Asset Sale. A.G.P. did not express any view on, and its opinion did not address, any other terms or aspect of the
Asset Sale, including, without limitation, the form or structure of the Asset Sale, the tax treatment thereof, the form or structure
of the Aggregate Consideration (or any component thereof) or the allocation thereof between cash and Immunome common stock or among the
Product Assets, any adjustment to the Closing Cash Consideration, any potential set-off against the Milestone Consideration, the allocation
of expenses between the Company and Immunome in connection with the Asset Sale, any transition services, noncompetition, support, consulting,
lock-up, transfer restriction, registration rights or other agreement or arrangement to be entered into in connection with the Asset
Sale, any potential dissolution of the Company following the consummation of the Asset Sale, any contingent value rights issued in connection
with such dissolution, the fairness of the Asset Sale to, or any consideration received in connection therewith by, the holders of any
class of securities, creditors, or other constituencies of the Company or any other party to the Asset Sale, or the fairness of the amount
or nature of any compensation to be paid or payable to any of the officers, directors or employees of the Company or any other party
to the Asset Sale, or class of such persons in connection with the Asset Sale, whether relative to the Aggregate Consideration to be
paid to the Company in the Asset Sale or otherwise. A.G.P. did not express any opinion as to what the actual value of Immunome common
stock would be when issued in the Asset Sale, the prices at which Immunome common stock or the Company’s common stock would trade
at any time or what the actual amount of the Milestone Consideration would be if and when paid. A.G.P.’s opinion was necessarily
based on economic, monetary, market and other conditions as in effect on, and the information made available to A.G.P. as of, the date
of the opinion, and A.G.P. assumes no responsibility for updating, revising or reaffirming its opinion based on circumstances, developments
or events occurring after the date of A.G.P.’s opinion. A.G.P.’s opinion was provided for the information and assistance
of the Company’s Board (in its capacity as such) in connection with its consideration of the Asset Sale. A.G.P.’s Opinion
did not constitute a recommendation to the Company’s Board, the Company, its stockholders or any other person as to how any of
them should vote or act with respect to the Asset Sale or any other matter.
A.G.P.
assumed that the representations and warranties made in the Asset Purchase Agreement were accurate and assumed that all of the conditions
required to implement the Asset Sale would be satisfied and that the Asset Sale would be completed in accordance with the Asset Purchase
Agreement without any amendments to the Asset Purchase Agreement or any waivers of any terms or conditions of the Asset Purchase Agreement.
A.G.P. also assumed that all governmental, regulatory or other consents and approvals necessary or useful for the consummation of the
Asset Sale would be obtained without any adverse effect on any of the Product Assets, the Company or Immunome or on the Asset Sale in
any way impacting A.G.P.’s analysis. A.G.P. relied upon the fact that the Company was advised by counsel as to all legal matters
with respect to the Asset Sale, including whether all procedures required by law to be taken in connection with the Asset Sale were duly,
validly and timely taken. A.G.P. assumed that the final form of the Asset Purchase Agreement would not differ materially from the draft
reviewed by A.G.P.
Except
as described in this summary, the Company imposed no other instructions or limitations on A.G.P. with respect to the investigations made
or the procedures followed by it in rendering its opinion. This summary is not a complete description of A.G.P.’s opinion or the
financial analyses performed and factors considered by A.G.P. in connection with its opinion. A.G.P. believes that its analysis must
be considered as a whole and that selecting portions of the analysis or the factors considered by it, without considering all factors
and analysis together, could create a misleading view of the process underlying its opinion. A.G.P. did not draw, in isolation, conclusions
from or with regard to any one factor or method of analysis for purposes of its opinion. The preparation of an opinion is a complex process
and is not necessarily susceptible to partial analysis or summary description. Any attempt to do so could lead to undue emphasis on any
one particular factor or analysis and an inaccurate conclusion.
In
performing its analyses, A.G.P. considered industry performance, general business, economic, market and financial conditions and other
matters existing as of the date of its opinion, many of which are beyond the Company’s control. No company, business or transaction
used in the analyses is identical to the Company or the Asset Sale, and an evaluation of the results of those analyses is not entirely
mathematical. Rather, the analyses involve complex considerations and judgments concerning financial and operating characteristics and
other factors that could affect the acquisition, public trading or other values of the companies, business segments or transactions analyzed.
The
assumptions and estimates contained in A.G.P.’s analyses and the ranges of valuations resulting from any particular analysis are
not necessarily indicative of actual values or future results, which may be significantly more or less favorable than those suggested
by its analyses. In addition, analyses relating to the value of businesses or securities do not purport to be appraisals or to reflect
the prices at which businesses or securities actually may be sold or acquired. Accordingly, the assumptions and estimates used in, and
the results derived from, A.G.P.’s analyses are inherently subject to substantial uncertainty.
A.G.P.
was not requested to, and it did not, recommend the specific consideration payable in the Asset Sale. The type and amount of consideration
payable in the Asset Sale was determined through negotiation between the Company and Immunome and was approved by the Company’s
Board. The decision of the Company to enter into the Asset Purchase Agreement was solely that of the Company’s Board. A.G.P.’s
opinion and financial analysis were only one of many factors considered by the Company’s Board in its evaluation of the Asset Sale
and should not be viewed as determinative of the views of the Company’s Board or management with respect to the Asset Sale or the
consideration payable in the Asset Sale.
The
following is a summary of the material financial analyses reviewed with the Company’s Board in connection with A.G.P.’s opinion
dated February 5, 2024. The financial analyses summarized below include information presented in tabular format. In order to fully understand
A.G.P.’s financial analyses, the tables must be read together with the text of each summary. The tables alone do not constitute
a complete description of the financial analyses. Considering the data in the tables below without considering the full narrative description
of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete
view of A.G.P.’s financial analyses.
For
purposes of the “Selected Companies Analysis,” the “Selected Transaction Analysis” and “Discounted
Cash Flow Analysis” summarized below, the “Probability-Adjusted Aggregate Consideration” of approximately $78.8
million refers to the sum of the following:
| |
● |
the
Closing Cash Consideration of $20,000,000; |
| |
● |
the
implied value of the Closing Stock Consideration based on the closing stock price of Immunome on February 2, 2024 of $35,438,724;
and |
| |
● |
the
probability-adjusted Milestone Consideration based on estimates of the probability of achieving the milestone events associated with
the Milestone Consideration provided by the management of the Company of $23,400,000 in the aggregate. |
Selected
Companies Analysis
A.G.P.
reviewed publicly available financial and stock market information of the following 10 selected publicly traded biopharmaceutical and
biotechnology companies which are involved in the development of cancerous and noncancerous tumor treatments and which generate annual
revenue of less than $2 million (shown in descending order of market capitalization):
| |
Springworks
Therapeutics, Inc. |
| |
MedPacto,
Inc. |
| |
GlycoMimetics,
Inc. |
| |
OncolyticsBiotech,
Inc. |
| |
Apollomics,
Inc. |
| |
Intensity
Therapeutics, Inc. |
| |
SELLAS
Life Sciences Group, Inc. |
| |
Aileron
Therapeutics, Inc.
Portage
Biotech, Inc. |
| |
Soligenix,
Inc. |
A.G.P.
reviewed fully-diluted market capitalizations of the selected companies based on closing stock prices on February 2, 2024 and enterprise
values of the selected companies, calculated as fully-diluted market capitalization, plus debt and non-controlling interests, less cash,
of the selected companies. Financial data for the selected companies was based on public filings.
The
low, 25th percentile, median, 75th percentile and high of the market capitalizations and the enterprise values
of the selected companies are indicated in the table below:
| | |
Market
Capitalizations
($ in millions) | | |
Enterprise
Values
($ in millions) | |
| High | |
$ | 3,392 | | |
$ | 2,977 | |
| 75th Percentile | |
$ | 164 | | |
$ | 124 | |
| Median | |
$ | 69 | | |
$ | 52 | |
| First Quartile | |
$ | 22 | | |
$ | 13 | |
| Low | |
$ | 7 | | |
$ | 3 | |
Applying
a selected range of 10% above and below the medians of the market capitalizations and the enterprise values of the selected companies,
this analysis indicated the following approximate implied value reference ranges for the Product Assets, as compared to the Probability-Adjusted
Aggregate Consideration of approximately $78.8 million:
| |
|
Implied
Value Reference Ranges
for
Product Assets |
| Based
on Selected Companies Median Market Capitalization |
|
$61.9
million to $75.6 million |
| Based
on Selected Companies Median Enterprise Value |
|
$47.2
million to $57.7 million |
Selected
Transactions (Premiums Paid) Analysis
Using
publicly available information, A.G.P. reviewed the transaction consideration value paid for the acquired company in the following 14
selected transactions announced since 2018 in which the acquired companies were publicly traded biopharmaceutical and biotechnology companies
involved in the development of cancerous and noncancerous tumor treatments:
| |
Acquiror: |
|
Acquired
Company: |
| |
Pathos
AI, Inc. |
|
Rain
Oncology Inc. |
| |
Eli
Lilly and Company |
|
POINT
Biopharma Global Inc. |
| |
Coherus
BioSciences, Inc. |
|
Surface
Oncology Inc |
| |
Regeneron
Pharmaceuticals, Inc. |
|
Checkmate
Pharmaceuticals, Inc. |
| |
GSK
plc |
|
Sierra
Oncology, Inc. |
| |
Pfizer
Inc. |
|
Trillium
Therapeutics Inc. |
| |
MorphoSys
AG |
|
Constellation
Pharmaceuticals, Inc. |
| |
Amgen |
|
Five
Prime Therapeutics |
| |
Castle
Creek Pharmaceutical Holdings, Inc. |
|
Fibrocell
Science, Inc. |
| |
ESSA
Pharma Inc. |
|
Realm
Therapeutics PLC |
| |
Oneness
Biotech |
|
Fountain
Biopharma |
| |
Merck
& Co. |
|
Immune
Design |
| |
Novartis
AG |
|
Endocyte |
| |
Eli
Lilly and Company |
|
ARMO
BioSciences, Inc. |
A.G.P.
reviewed the premiums paid in the selected transactions relative to the market capitalizations of the acquired companies based on the
closing stock price of the acquired company one day prior to public announcement of the relevant transaction.
The
low, median, average and high premiums to market capitalization in the selected transactions are indicated in the table below:
| Premiums to Market Capitalization |
| High | |
| 869 | % |
| Average | |
| 157 | % |
| Median | |
| 68 | % |
| Low | |
| 17 | % |
A.G.P.
then applied a selected range of 10% above and below the median of the premiums to market capitalization in the selected transactions
to the fully diluted market capitalization of the Company based on the closing stock price of the Company on February 2, 2024. This analysis
indicated the following approximate implied value reference range for the Product Assets, as compared to the Probability-Adjusted Aggregate
Consideration of approximately $78.8 million:
| |
Implied Value Reference
Range for Product Assets |
|
| |
$52.5
million to $64.2 million |
|
Discounted
Cash Flow Analysis
A.G.P.
performed a discounted cash flow analysis to calculate the estimated present value of the implied probability-adjusted standalone
unlevered free cash flows (as reflected by estimated net operating profit after tax) that the Product Assets were forecasted to generate
during the fiscal years ending December 31, 2024 through December 31, 2033. Financial data of the Product Assets, including additional
capital requirements needed to fund commercialization, were based on financial forecasts and projections and probability estimates provided
by the management of the Company (which assumed that AL101 would not be commercialized). A.G.P. calculated terminal values for the Product
Assets by applying a selected range of multiples of 3.3x to 4.3x to the probability-adjusted estimated fiscal year 2033 revenue of the
Product Assets. The unlevered free cash flows and terminal values were then discounted to present value using discount rates ranging
from 18.0% to 22.0%. This analysis indicated the following approximate implied value reference range for the Product Assets, as compared
to the Probability-Adjusted Aggregate Consideration of approximately $78.8 million:
| |
Implied Value Reference
Range for Product Assets |
|
| |
$0
to $77.3 million |
|
Miscellaneous
The
Company selected A.G.P. to provide a financial advisory opinion in connection with the Asset Sale based on A.G.P.’s familiarity
with the Company and A.G.P.’s reputation and experience in healthcare investment banking. A.G.P. is a financial services firm engaged
in the securities, investment management and individual wealth management businesses. Its securities business is engaged in securities
underwriting, trading and brokerage activities, foreign exchange, commodities and derivatives trading, prime brokerage, as well as providing
investment banking, financing and financial advisory services. A.G.P., its affiliates, directors and officers may at any time invest
on a principal basis or manage funds that invest, hold long or short positions, finance positions, and may trade or otherwise structure
and effect transactions, for their own account or for the accounts of their customers, in debt or equity securities or loans of the Company,
or any other company, or any currency or commodity, that may be involved in the Asset Sale, or any related derivative instrument.
A.G.P.
may, in the future, in the ordinary course of its business, perform financial advisory or investment banking services for the Company,
Immunome or any of their respective associates or affiliates.
A.G.P.
has received a fee of $200,000 as compensation for its services in rendering its opinion, no portion of which was contingent upon either
the conclusion in the opinion or the consummation of the Asset Sale. The Company has agreed to indemnify A.G.P. against certain liabilities
arising out of its engagement. In addition to the foregoing engagement, A.G.P. has also been engaged as strategic advisor to the Company
in connection with the Asset Sale and will receive a fee of $500,000 as compensation for such engagement which is contingent upon the
consummation of the Asset Sale.
A.G.P.’s
opinion was approved by A.G.P.’s fairness opinion committee, a committee of A.G.P. investment banking and other professionals,
in accordance with A.G.P.’s customary practice.
Management
Projections Relating to AL102
We
do not, as a matter of course, make public forecasts or projections as to future performance or financial data and are especially wary
of making projections for extended earnings periods given the inherent unpredictability of the underlying assumptions and estimates.
However, in connection with the Asset Sale, we prepared certain unaudited projections (the “Unaudited Projections”) based
on our management’s estimate of the potential revenues and net income relating to AL102 for the treatment of desmoid tumors for
the calendar years 2024 through 2033, which were provided to A.G.P. for purposes of the financial analyses performed by it
in connection with A.G.P.’s opinion to our Board of Directors. We have included below a summary of the Unaudited Projections
to give our stockholders access to certain nonpublic information prepared for purposes of considering and evaluating the Asset Sale.
The inclusion of this information should not be regarded as an indication that we, our Board of Directors, Immunome or any other recipient
considered, or now considers, this information to be necessarily predictive of actual future results, and such data should not be relied
upon as such. Neither we nor any of our affiliates or representatives has made or makes any representations to any person regarding the
ultimate performance of the assets to be acquired in the Asset Sale compared to the information contained in the projections, and none
of them intends to provide any update or revision thereof.
As
prepared by our management, the Unaudited Projections reflected the potential evolving market for AL102, which is being studied in a
Phase 3 clinical trial for the treatment of desmoid tumors. The Unaudited Projections reflect management’s assumptions about the
timing of the receipt of regulatory approval and product launch, prevalence of desmoid tumors, treatment pricing, duration of treatment,
availability of reimbursement, market penetration, market share of AL102, peak sales, the competitive landscape, development and commercialization
expenses, tax rates and other factors that are difficult to predict. The table below sets forth our management’s estimate of the
potential revenues from the U.S. and European Union and net operating profit after tax relating to AL102 for the treatment of desmoid
tumors for the calendar years 2024 through 2033:
Unaudited
Projections
| | |
For the Year Ending December 31, | |
| | |
2024E | | |
2025E | | |
2026E | | |
2027E | | |
2028E | | |
2029E | | |
2030E | | |
2031E | | |
2032E | | |
2033E | |
| | |
(in millions) | |
| Net Revenue | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 8.3 | | |
$ | 33.9 | | |
$ | 76.9 | | |
$ | 132.3 | | |
$ | 217.2 | | |
$ | 286.0 | | |
$ | 336.6 | |
| Net Operating Profit After Tax | |
$ | ( 22.2 | ) | |
$ | ( 23.1 | ) | |
$ | ( 25.6 | ) | |
$ | (20.1 | ) | |
$ | 0.1 | | |
$ | 28.6 | | |
$ | 58.2 | | |
$ | 120.3 | | |
$ | 157.1 | | |
$ | 196.9 | |
Additionally,
in the discounted cash flow analysis performed by A.G.P. in connection with its opinion, the above estimates of net operating profit
after tax were probability-adjusted at the direction of, and as approved by, the Board, to derive the following implied probability-adjusted
unlevered free cash flows:
| | |
Year Ending December 31, | |
| | |
2024E | | |
2025E | | |
2026E | | |
2027E | | |
2028E | | |
2029E | | |
2030E | | |
2031E | | |
2032E | | |
2033E | |
| | |
(in millions) | |
Probability-Adjusted Net
Operating Profit After Tax/Implied Unlevered Free Cash Flows | |
$ | (12.4 | ) | |
$ | (12.9 | ) | |
$ | (14.3 | ) | |
$ | (11.3 | ) | |
$ | 0.0 | | |
$ | 16.0 | | |
$ | 32.6 | | |
$ | 67.4 | | |
$ | 88.0 | | |
$ | 110.3 | |
The implied probability-adjusted
unlevered free cash flows were calculated solely for purposes of the discounted cash flow analysis performed in connection with A.G.P.’s
opinion, and neither the Company nor A.G.P. assumes any responsibility for any use of such estimates, or reliance on such estimates,
for any other purpose.
Cautionary
Statement Regarding Projected Financial Information
The
Unaudited Projections described above were not prepared with a view towards public disclosure or compliance with generally accepted accounting
principles or with published guidelines of the Securities and Exchange Commission or the guidelines established by the American Institute
of Certified Public Accountants regarding forecasts or projections. Our internal forecasts (upon which the Unaudited Projections were
based in part) are, in general, prepared solely for internal use and capital budgeting and other management decisions and are subjective
in many respects and thus susceptible to interpretation and periodic revision based on actual experience and business developments. The
projected financial information described above also reflects numerous assumptions made by our management with respect to industry performance,
general business, economic, market and financial conditions and other matters, all of which are difficult to predict and many of which
are beyond our control. Accordingly, we cannot assure you that the projected results will be realized or that actual results will not
be significantly higher or lower than projected. In addition, the projections do not consider the effect of the Asset Sale or any future
combination of our business with the businesses conducted by Immunome. We do not intend to update or revise the unaudited projected financial
information described above. For a discussion of the risks and uncertainties that may be relevant to our results, see “Cautionary
Statement Regarding Forward-Looking Statements” on page 9.
Interests
of Our Directors and Executive Officers in the Asset Sale
The
Company’s directors and executive officers have interests in the Asset Sale that may be different from, or in addition to, the
interests of the Company stockholders generally. The Board was aware of these interests and considered them, among other matters, in
approving the Asset Sale. These interests are described below.
On
February 5, 2024, the Company’s compensation committee (the “Compensation Committee”) approved the payment of transaction
bonuses, payable in cash and contingent upon Closing of the Asset Sale to each of the following executive officers of the Company in
the applicable respective amounts: Ken Berlin, President and Chief Executive Officer, $1,600,000; Roy Golan, Chief Financial Officer,
$450,000; Dr. Andres Gutierrez, Chief Medical Officer and Executive Vice President, $800,000; David Sidransky, Chairman of the Board,
$160,000; Murray Goldberg, Robert Spiegel, Vered Bisker, and Roni Appel, Directors, $70,000; and Pini Orbach Bridget Martell and Yuval
Cabily, Directors, $50,000.
Quantification
of Outstanding Equity-Based Awards
The
following table summarizes all outstanding equity awards held by our named executive officers on February 8, 2024, all of which have
vested as of the date of this information statement.
| Name | |
Number of Securities Underlying Unexercised Options (#) Exercisable | | |
Number of Securities Underlying Unexercised Options (#) Unexercisable | | |
Option Exercise Price ($) | | |
Option Expiration Date | | |
Number of Shares or Units of Stock That Have Not Vested (#) | | |
Value of Shares or Units of Stock That Have Not Vested ($) | |
| Kenneth Berlin | |
| 625 | | |
| - | (1) | |
| 1,944.00 | | |
| 4/23/2028 | | |
| - | | |
| - | |
| | |
| 267 | | |
| - | (2) | |
| 648.00 | | |
| 11/5/2028 | | |
| - | | |
| - | |
| | |
| 625 | | |
| - | (3) | |
| 24.80 | | |
| 10/24/2029 | | |
| - | | |
| - | |
| | |
| 625 | | |
| - | (4) | |
| 52.80 | | |
| 5/4/2030 | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| - | |
| Roy Golan | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Andres Gutierrez | |
| 208 | | |
| - | (5) | |
| 1,944.00 | | |
| 4/23/2028 | | |
| - | | |
| - | |
| | |
| 104 | | |
| - | (2) | |
| 648.00 | | |
| 11/05/2028 | | |
| - | | |
| - | |
| | |
| 313 | | |
| - | (3) | |
| 24.80 | | |
| 10/24/2029 | | |
| - | | |
| - | |
| | |
| 625 | | |
| - | (4) | |
| 52.80 | | |
| 5/4/2030 | | |
| - | | |
| - | |
| (1) |
Of
these options, one-third vested on December 31, 2018, one-third vested on April 23, 2020, and the award was fully vested on April
23, 2021. |
| |
|
| (2) |
Of
these options, one-third vested on November 5, 2019, one-third vested on November 5, 2020, and the award was fully vested on November
5, 2021. |
| |
|
| (3) |
Of
these options, one-third vested on October 24, 2020, one-third vested on October 24, 2021, and the award was fully vested on October
24, 2022. |
| |
|
| (4) |
Of
these options, one-third vested on May 4, 2021, one-third vested on May 4, 2022, and the award was fully vested on May 4, 2023. |
| |
|
| (5) |
Of
these options, one-third vested on April 23, 2019, one-third vested on April 23, 2020, and the award was fully vested on April 23,
2021. |
Executive
Officer Severance
Kenneth
Berlin has served as our President and Chief Executive Officer and a member of our Board of Directors since April 2018. Mr. Berlin served
as our Interim Chief Financial Officer from September 2020 to May 2022. In the event Mr. Berlin’s employment is terminated without
Just Cause during the period beginning three months prior to a Change in Control (all such capitalized terms as defined in Mr. Berlin’s
employment agreement) and ending 18 months after the Change in Control (such period, the “CIC Protection Period”), or if
Mr. Berlin voluntarily resigns with Good Reason (as defined in Mr. Berlin’s employment agreement), during the CIC Protection Period,
and provided that Mr. Berlin continues to comply with certain covenants set forth in his employment agreement, in addition to the applicable
accrued but unpaid base salary and any earned but unpaid bonus for the prior fiscal year, plus any accrued but unused vacation time and
unpaid expenses that have been earned as of the date of such termination, Mr. Berlin is entitled to the following severance benefits:
(i) an amount equal to two times the sum of the applicable base salary plus an amount equal to Mr. Berlin’s target bonus, payable
in a single lump sum within sixty (60) days of the termination, (ii) a bonus payment for the year in which the employment is terminated
equal to the target bonus percentage, multiplied by the base salary in effect at the time of termination, multiplied by a fraction, the
numerator of which is the number of calendar days Mr. Berlin was employed during such year and the denominator is 365, (iii) continued
health and welfare benefits for 24 months, and (iv) full vesting and exercisability of all stock options and stock awards.
The
Company appointed Dr. Gutierrez as Executive Vice President and Chief Medical Officer, effective April 23, 2018. Prior to the Board’s
approval of the $800,000 transaction bonus payable to Dr. Gutierrez upon consummation of the Asset Sale as set forth in “The Asset
Sale — Interests of Our Directors and Executive Officers in the Asset Sale,” Dr. Gutierrez had been eligible under his employment
agreement to receive severance payments and benefits upon a qualifying termination of employment that occurs during a specified period
before or after a change of control, as defined therein. However, when the Compensation Committee approved the transaction bonus payable
to Dr. Gutierrez, it was in lieu of such severance payments, which will not be paid.
Quantification
of Severance Payments to Executive Officers
Upon
a Change in Control of the Company (such term as defined in the Company’s 2017 Stock Incentive Plan and 2015 Incentive Plan), unvested
equity awards held by Mr. Berlin and Dr. Gutierrez will be accelerated as follows: (i) outstanding stock options and other awards in
the nature of rights that may be exercised shall become fully vested and exercisable, (ii) time-based restrictions on restricted stock,
restricted stock units and other equity awards shall lapse and the awards shall become fully vested, and (iii) performance-based equity
awards, if any, shall become vested and shall be deemed earned based on an assumed achievement of all relevant performance goals at “target”
levels, and shall payout pro rata to reflect the portion of the performance period that had elapsed prior to the Change in Control.
The
table below shows the estimated value of benefits to each of the named executive officers if their employment had been terminated under
various circumstances as of December 31, 2023. The amounts shown in the table exclude accrued but unpaid base salary, unreimbursed employment-related
expenses, accrued but unpaid vacation pay, and the value of equity awards that were vested by their terms as of December 31, 2023. Note
that the amounts set forth in the tables below do not include the transaction bonuses payable to Mr. Berlin, Dr. Gutierrez and Mr. Golan,
as set forth in “The Asset Sale — Interests of Our Directors and Executive Officers in the Asset Sale,” of $1,600,000,
$800,000 and $450,000, respectively.
| | |
Involuntary Termination without a Change in Control ($) | | |
Involuntary Termination in connection with a Change in Control ($) | | |
Death ($) | | |
Disability ($) | | |
Termination for Cause; Voluntary Resignation ($) | |
| | |
| | |
| | |
| | |
| | |
| |
| Kenneth Berlin | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash severance | |
| 870,144 | (1) | |
| 2,157,996 | (5) | |
| - | | |
| 870,144 | (1) | |
| - | |
| Bonus | |
| 382,863 | (7) | |
| 382,863 | (2) | |
| 382,863 | (2) | |
| 382,863 | (7) | |
| - | |
| Health benefits | |
| 26,038 | (3) | |
| 41,660 | (6) | |
| - | | |
| 26,038 | (3) | |
| - | |
| Value of equity acceleration | |
| - | (4) | |
| - | (4) | |
| - | (4) | |
| - | (4) | |
| - | |
| Total | |
| 1,279,045 | | |
| 2,582,519 | | |
| 382,863 | | |
| 1,279,045 | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Andres Gutierrez | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash severance | |
| 535,473 | (1) | |
| 535,473 | (1) | |
| - | | |
| 535,473 | (1) | |
| - | |
| Bonus | |
| 214,189 | (7) | |
| 214,189 | (7) | |
| 214,189 | (7) | |
| 214,189 | (7) | |
| | |
| Health benefits | |
| 36,651 | (3) | |
| 36,651 | (6) | |
| - | | |
| 36,651 | (3) | |
| - | |
| Value of equity acceleration | |
| - | (4) | |
| - | (4) | |
| - | (4) | |
| - | (4) | |
| - | |
| Total | |
| 786,313 | | |
| 786,313 | | |
| 214,189 | | |
| 786,313 | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Roy Golan | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash severance | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Bonus | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Health benefits | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Value of equity acceleration | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| Total | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| (1) |
For
Mr. Berlin, reflects severance payment equal to 1.25 times base salary payable in equal monthly installments for 15 months. For Dr.
Gutierrez, reflects severance payment equal to one times base salary payable in equal monthly installments for 12 months. |
| |
|
| (2) |
Reflects
pro rata bonus determined by multiplying the target bonus amount for the year in which the termination occurs by a fraction, the
numerator of which is the number of calendar days the executive is employed during such year and the denominator of which is 365.
Because the amounts reflected in the table assume the named executive officer’s employment was terminated on December 31, 2023
(the last day of the 2023 fiscal year), the amounts reflected are not pro-rated. |
| |
|
| (3) |
For
Mr. Berlin, reflects the Company’s cost of continued health coverage at active employee rates for 15 months. For Dr. Gutierrez,
reflects the Company’s cost of continued health coverage at active employee rates for 12 months. |
| |
|
| (4) |
Reflects
the value of unvested in-the-money stock options that vest upon the designated event. |
| |
|
| (5) |
For
Mr. Berlin, reflects two times the sum of his base salary and target bonus, payable in equal monthly installments for 24 months.
|
| |
|
| (6) |
Reflects
the full cost of continued health coverage for 24 months for Mr. Berlin. |
| |
|
| (7) |
Represents
a bonus payment equal to the executive’s target bonus. |
Indemnification
of Officers and Directors
In
connection with the Asset Sale, we will continue to indemnify our directors and officers to the maximum extent permitted in accordance
with applicable law, our certificate of incorporation and bylaws. The Board is authorized to obtain and maintain insurance as may be
necessary, appropriate or advisable to cover such indemnification obligations, including seeking an extension in time and coverage of
our insurance policies currently in effect.
Anticipated
Accounting Treatment
Under
generally accepted accounting principles, upon completion of the Asset Sale, we will remove the net assets and assumed liabilities sold
and add the proceeds from the Asset Sale to our consolidated balance sheet, which we anticipate would result in recording a gain from
the Asset Sale.
Use
of Proceeds
Assuming
that the Asset Sale is consummated in accordance with the terms of the Asset Purchase Agreement, Ayala intends to use substantially all
of the after-tax proceeds for general corporate purposes.
U.S.
Federal Income Tax Consequences of the Asset Sale
The
following is a general discussion of the anticipated material U.S. federal income tax consequences of the Asset Sale. This discussion
is a summary for general information only and applies solely to holders of our common stock and to us. The discussion addresses only
the specific U.S. federal income tax consequences set forth below and does not address any other U.S. federal, state, local or foreign
income, estate, gift, transfer, sales, use or other tax consequences that may result from the Asset Sale or any other transaction.
This
discussion does not purport to be a complete analysis of all of the tax considerations applicable to all categories of stockholders,
some of which (such as broker-dealers, stockholders who hold the common stock as part of hedging or conversion transactions, stockholders
whose functional currency is not the U.S. dollar, and tax-exempt organizations) may be subject to special rules.
THIS
DISCUSSION IS NOT INTENDED TO BE USED, AND IT CANNOT BE USED BY ANY TAXPAYER, FOR THE PURPOSE OF AVOIDING PENALTIES THAT MAY BE IMPOSED
ON THE TAXPAYER. EACH TAXPAYER SHOULD SEEK ADVICE BASED ON THE TAXPAYER’S PARTICULAR CIRCUMSTANCES FROM AN INDEPENDENT TAX ADVISOR.
This
discussion is based on the U.S. Internal Revenue Code of 1986, as amended, or the Code, administrative pronouncements, judicial decisions,
final, temporary and proposed Treasury regulations, all as in effect on the date hereof and all of which may be changed, perhaps retroactively,
so as to result in U.S. federal income tax consequences different from those described below. No rulings have been requested or received
from the Internal Revenue Service, or IRS, as to the tax consequences of the Asset Sale and there is no intent to seek any such ruling.
Accordingly, no assurance can be given that the IRS will not challenge the tax treatment of tax consequences of the Asset Sale discussed
below or, if it does challenge the tax treatment, that it will not be successful.
The
Asset Sale will be treated for U.S. federal and state income tax purposes as a taxable transaction upon which we will recognize gain
or loss. The amount of gain or loss we recognize with respect to the sale of a particular asset will be measured by the difference between
the amount realized by us on the sale of that asset and our tax basis in that asset. The amount realized by us on the Asset Sale will
include the amount of cash received, the fair market value of any other property received, and total liabilities assumed or taken by
the Purchaser. For purposes of determining the amount realized by us with respect to specific assets, the total amount realized by us
will generally be allocated among the assets according to the rules set forth in Section 1060(a) of the Code. Our basis in our assets
is generally equal to their cost, as adjusted for certain items, such as depreciation. The determination of whether we will recognize
gain or loss will be made with respect to each of the assets to be sold. Accordingly, we may recognize gain on the sale of certain assets
and loss on the sale of certain others, depending on the amount of consideration allocated to an asset as compared with the basis of
that asset. Further, the sale of certain assets may result in ordinary income or loss, depending on the nature of the asset. To the extent
the Asset Sale results in us recognizing a net gain for U.S. federal income tax purposes, we expect, subject to a final analysis, that
our available net operating loss carryforwards will offset a substantial part of such gain.
Regulatory
Approvals
The
Company is not required to obtain any regulatory approvals in order to consummate the Asset Sale and the Asset Sale is not conditioned
upon any such approvals.
THE ASSET PURCHASE AGREEMENT
This
section describes the material terms of the Asset Purchase Agreement. The description in this section and elsewhere in this information
statement is qualified in its entirety by reference to the complete text of the Asset Purchase Agreement, a copy of which is attached
as Annex A and is incorporated by reference into this information statement. This summary does not purport to be complete and
may not contain all of the information about the Asset Purchase Agreement that is important to you. We encourage you to read the Asset
Purchase Agreement carefully and in its entirety. Such information can be found elsewhere in this information statement and in the public
filings we make with the SEC, as described in the section entitled, “Where You Can Find More Information.”
General
On
February 5, 2024, Ayala entered into the Asset Purchase Agreement with the Purchaser pursuant to which we have agreed, subject to specified
terms and conditions, to sell to the Purchaser substantially all of our assets related to (x) the gamma secretase inhibitors knows as
AL101 and AL202, as set forth on Schedule B to the Asset Purchase Agreement (the “Compounds”), and (y) any product
comprised of or containing either of the Compounds, in any form of formulation (collectively, the “Products”).
Purchase
and Sale of Assets
Acquired
Assets
The
Asset Purchase Agreement contemplates that Ayala will sell to the Purchaser substantially all of the non-cash assets of its business
related to the Compounds and the Products (collectively, the “Acquired Assets”), including, among others:
●
substantially all intellectual property of Ayala related to the Compounds and the Products, together with any and all goodwill symbolized
thereby and associated thereby and associated therewith, any and all rights to royalties, profits, compensation, license payments, and
other payments or remuneration of any kind relating to the Acquired Assets, and any and all rights to obtain renewals, reissues, reexaminations,
supplemental examinations and certificates and extensions of registrations, exclusivities, or other legal protections pertaining to such
Ayala intellectual property, including all documentation related to the patents set forth on Schedule C of the Asset Purchase Agreement;
●
all rights in, to and under the contracts listed on Schedule 2.1(a)(ii) of the Asset Purchase Agreement, as updated by Ayala and delivered
to Purchaser at least five Business Days prior to the anticipated Closing Date (subject to Purchaser’s right to reject the inclusion
of any such contracts on Schedule 2.1(a)(ii) of the Asset Purchase Agreement) (collectively, the “Acquired Business Contracts”);
●
all inventories of active pharmaceutical ingredient for the Compounds and Products, all drug product for the Compounds and Products,
all raw and pack materials, work-in-process, finished goods, Products on stability, reference materials warehouse stock, supplies, consumables
and packaging materials relating to the Products or the manufacture thereof;
●
all regulatory applications, filings, approvals and associated correspondence required or related to the development, manufacture of
commercialization of any Compound or Product in any country or jurisdiction, including all INDs relating to the Compounds and the Products,
and all other regulatory documentation with respect thereto;
●
all permits that constitute Acquired Assets, as set forth on Section 5.1(h) of the disclosure schedules delivered by Ayala on the date
of the Asset Purchase Agreement (the “Disclosure Schedules”);
●
all chemical or biological materials relating to the Compounds or Products, including all patient samples from clinical trials;
●
all rights, claims, credits (including the $2,480,000.00 credit under that certain Master Services Agreement, dated as of July 21, 2020,
by and between Old Ayala, Inc. (formerly Ayala Pharmaceuticals, Inc.) and IQVIA Biotech LLC, as amended, together with that certain Amendment
No. 2 to Initial Work Order Project Code: Al-DES-01/WZ95313, by and between Ayala and IQVIA RDS Inc., to be executed after the date of
the Asset Purchase Agreement), guaranties, warranties, indemnities, causes of action or rights of set-off, and other similar rights against
third parties to the extent relating to or arising from the Compounds, the Products, the Acquired Assets or the Assumed Liabilities (as
defined below); and
●
Ayala’s rights in or to any equipment used in the development or manufacture of the Compounds or Products.
Assumed
Liabilities
The
Purchaser will assume and agree to pay, perform, and discharge only the following liabilities: (i) all liabilities of Ayala and its affiliates
under the Acquired Contracts arising on or after the Closing Date (excluding liabilities related to breach or noncompliance under the
Acquired Contracts by Ayala prior to the Closing Date and any tax liabilities of Ayala) and (ii) any liability or other obligation expressly
set forth in Section 2.2(b) of the Asset Purchase Agreement (the “Specified Liabilities”) in an aggregate amount not to exceed
$4,000,000.00.
Excluded
Assets
The
Purchaser will not acquire any right, title or interest in any assets that are not Acquired Assets (collectively, the “Excluded
Assets”).
Excluded
Liabilities
The
Purchaser will not assume or be liable for or be required to pay, perform, or discharge any liabilities not expressly included in the
definition of Assumed Liabilities (collectively, the “Excluded Liabilities”), including:
●
except as expressly included as an Assumed Liability, all liabilities arising from the Company’s ownership or use of the Acquired
Assets or the conduct of its business, including the Product operations, as of or prior to the Closing;
●
all liabilities related to Excluded Assets;
●
all Ayala tax liabilities;
●
all liabilities of Ayala or any of its affiliates for product liability, infringement, or misappropriation arising from preclinical or
clinical trials of a Product or Product operations conducted by or for Ayala on or prior to Closing;
●
all liabilities, whenever or however arising, including all costs and expenses relating thereto arising under contract, law or permit,
order or any award of any arbitrator of any kind relating to any Ayala employee benefit plan, employment agreement, natural person consulting
or independent contractor agreement, or otherwise relating to an employee or other natural person service provider and his or her potential
or actual service or employment with Ayala or any member of Ayala’s “Controlled Group” under Section 4001(b)(1) of
ERISA;
●
any liabilities that arose or were incurred under any Acquired Business Contract on or prior to the Closing or that relate to any failure
to perform, improper performance, warranty or other breach, default or violation by Ayala on or prior to the Closing;
●
except as expressly including as an Assumed Liability, all liabilities for invoices, bills, accounts payable, or other trade payables
due or owned by the Company or any of its affiliates to any third party relating to or arising from the development, manufacture (or
having manufactured), packaging, importation, marketing, or distribution of a Compound or Product prior to the Closing;
●
any liability arising out of or resulting from non-compliance with any law by Ayala or its affiliates with respect to the Product operations
or the Acquired Assets;
●
any liability of Ayala or its affiliates to pay any fees or commissions to any broker, finder, or agent with respect to the Asset Purchase
Agreement or the Asset Sale or the consummation thereof;
●
any liability arising out of or resulting from any liquidation, dissolution or winding up of the Company, including any legal proceeding,
bankruptcy filing, assignment for the benefit of creditors or similar restructuring or reorganization involving Ayala; and
●
an aggregate amount equal to (a) the amount of any Specified Liabilities in excess of $4,000,000.00, plus (b) the amount of the
expenses set forth on Schedule E of the Asset Purchase Agreement (the “Qualified CMC Expenses”) in excess of $1,000,000.00
(the amounts referred to in clauses (a) and (b), collectively, the “Excess Liabilities”).
The
Purchaser may set off any Excess Liabilities against any unpaid future Milestone Payments due and payable to the Company under the Asset
Purchase Agreement to the extent such Excess Liabilities were not taken into account as part of the calculation of the Closing Cash Payment.
Non-Transferable
Assets
Assets
which are by their terms non-assignable or non-transferable without the consent of a third party are not transferred by the Asset Purchase
Agreement. For a period of twelve (12) months following the Closing Date, Ayala will, at no cost to Purchaser, use commercially reasonable
efforts to obtain the consent of such other third party to the assignment or transfer of any such non-transferrable asset to Purchaser
in all cases in which such consent is required for such assignment or transfer.
Consideration
to be Received in the Asset Sale
Closing
Consideration
At
the Closing, Purchaser shall (i) pay (or cause to be paid) an amount of cash equal to (w) $20,000,000.00, minus (x) the $4,000,000.00
deposit paid by Purchaser to the Company on December 23, 2023 (the “Deposit”) (provided the Deposit has not already been
refunded to Purchaser in accordance with the terms of the Asset Purchase Agreement), plus (y) the aggregate amount of Qualified
CMC Expenses incurred and paid by the Company prior to Closing, minus (z) the aggregate amount of Excess Liabilities (such amount,
the “Closing Cash Payment”); (ii) issue to Ayala 2,175,489 unregistered shares of Purchaser Common Stock (the
“Shares”) and (iii) assume and agree, on and after the Closing Date, to pay, perform
and discharge promptly and fully when due the Assumed Liabilities.
Milestone
Consideration
From
and after the Closing, Purchaser shall pay the following amounts to Ayala: (i) upon the occurrence of a public announcement of positive
topline data from the ongoing RINGSIDE Phase 3/Part B study of AL102 in patients with desmoid tumors (defined as data showing that the
study met its primary endpoint as defined in the study protocol, with a hazard ratio of 0.35 or better): $10,000,000.00; (ii) upon the
grant of NDA regulatory approval of a product containing either AL102 or AL101 in any form or formulation by the FDA in a first indication:
$17,500,000.00 and (iii) within 90 days after the first calendar year in which Annual Net Sales (as defined in the Asset Purchase Agreement)
of AL102 exceed $100,000,000.00: $10,000,000.00. The milestones are payable only once even if a milestone event is achieved for more
than one indication.
Conditions
to Closing
The
closing of the Asset Sale is subject to the satisfaction or, to the extent permissible under applicable law or pursuant to the Asset
Purchase Agreement, waiver of certain conditions on or prior to the closing. Such conditions include:
Conditions
for both Parties:
●
No temporary restraining order, preliminary or permanent injunction, or other order preventing the consummation of the Asset Sale shall
have been issued by any court of competent jurisdiction and remain in effect, and no material law shall have been enacted that makes
consummation of the Asset Sale illegal.
●
Each of Ayala and Purchaser shall have performed and complied with, in all material respects, all of its covenants contained in the Asset
Purchase Agreement at or before the Closing (to the extent that such covenants require performance by such party at or before the Closing).
Conditions
for Purchaser:
●
The fundamental representations and warranties of Ayala set forth in the Asset Purchase Agreement shall be true and correct in all respects
as of the date of the Asset Purchase Agreement and as of the Closing Date (provided that the accuracy of representations and warranties
that by their terms speak of a specified date will be determined as of such date, and all other representations and warranties of Ayala
set forth in the Asset Purchase Agreement (without giving effect to materiality qualifications) shall be true and correct in all material
respects, except where the failure of such representations and warranties to be so true and correct would not reasonably be expected
to, individually or in the aggregate, have a material adverse effect.
●
There shall not have occurred and be continuing a material adverse effect since the date of the Asset Purchase Agreement.
●
Ayala shall have delivered a certificate to the effect that each of the conditions specified in Sections 7.1, 7.2 and 7.4 of the
Asset Purchase Agreement is satisfied in all respects as of the Closing.
●
Ayala shall have delivered to Purchaser each of the documents and materials contemplated to be delivered by Section 4.2(a) of the Asset
Purchase Agreement.
●
At least twenty (20) calendar days shall have elapsed from the date the definitive information statement was mailed to the Company’s
stockholders in accordance with Rule 14c-2 of the Exchange Act.
Conditions
for Ayala:
●
The representations and warranties of Purchaser set forth in the Asset Purchase Agreement (without giving effect to materiality qualifications)
shall be true and correct in all material respects, except where the failure of such representations and warranties to be so true and
correct would not reasonably be expected to, individually or in the aggregate, prevent or materially delay the consummation of the Asset
Sale.
●
Purchaser shall have delivered a certificate to the effect that each of the conditions specified in Sections 8.1 and 8.2 of the Asset
Purchase Agreement is satisfied in all respects as of the Closing.
●
Ayala shall have delivered to Purchaser each of the documents and materials contemplated to be delivered by Section 4.2(b) of the Asset
Purchase Agreement.
Representations
and Warranties
The
representations and warranties of Ayala contained in the Asset Purchase Agreement (i) have been made solely for purposes of the Asset
Purchase Agreement, (ii) have been qualified by matters specifically disclosed in (x) the Disclosure Schedules and (y) any form, report,
schedule, registration statement, definitive proxy statement and other document (together with all amendments thereof and supplements
thereto) filed or furnished by Ayala pursuant to the Securities Act of 1933 or the Exchange Act of 1934, each as amended, with the SEC
since January 1, 2023, (iii) are subject to materiality qualifications contained in the Asset Purchase Agreement, which might differ
from what is viewed as material by investors, (iv) were made only as of the date of the Asset Purchase Agreement or such other date as
is specified in the Asset Purchase Agreement and (v) have been included in the Asset Purchase Agreement for the purpose of allocating
risk between the contracting parties and were not intended to be treated as categorical statements of fact. The Asset Purchase Agreement
should not be read alone, but should instead be read in conjunction with the information provided elsewhere in this document. In addition,
to the extent specific material facts are known by Ayala to exist that it believes contradict the representations and warranties of Ayala
contained in the Asset Purchase Agreement in a material way, we have provided disclosure of those facts.
Ayala
made representations and warranties to Purchaser concerning the following matters:
| |
● |
organization
and existence; |
| |
|
|
| |
● |
authorization;
binding nature of the Asset Purchase Agreement; |
| |
|
|
| |
● |
no
conflict; |
| |
|
|
| |
● |
governmental
approvals and filing; |
| |
|
|
| |
● |
taxes; |
| |
|
|
| |
● |
title
to Acquired Assets; |
| |
|
|
| |
● |
compliance
with laws; |
| |
|
|
| |
● |
permits; |
| |
|
|
| |
● |
material
contracts; |
| |
|
|
| |
● |
inventory; |
| |
|
|
| |
● |
legal
proceedings; |
| |
|
|
| |
● |
regulatory
compliance; |
| |
|
|
| |
● |
intellectual
property; |
| |
|
|
| |
● |
no
brokers; |
| |
|
|
| |
● |
affiliate
transactions; |
| |
|
|
| |
● |
certain
product operations activities; |
| |
|
|
| |
● |
no
government officials; |
| |
|
|
| |
● |
books
and records; |
| |
|
|
| |
● |
solvency; |
| |
|
|
| |
● |
sufficiency
of Acquired Assets; |
| |
|
|
| |
● |
no
transfer of employees; |
| |
● |
absence
of certain changes since December 31, 2023; |
| |
|
|
| |
● |
insurance; |
| |
|
|
| |
● |
SEC
reports; financial statements; |
| |
|
|
| |
● |
receipt
of opinion of financial advisor; |
| |
|
|
| |
● |
Hart-Scott-Rodino
Act; |
| |
|
|
| |
● |
capitalization;
and |
| |
|
|
| |
● |
investment
representations. |
The
Purchaser made representations and warranties to Ayala concerning the following matters:
| |
● |
organization
and existence; |
| |
|
|
| |
● |
authorization;
binding nature of the Asset Purchase Agreement; |
| |
|
|
| |
● |
no
conflict; |
| |
|
|
| |
● |
government
approvals and filing; |
| |
|
|
| |
● |
no
brokers; |
| |
|
|
| |
● |
legal
proceedings; |
| |
|
|
| |
● |
availability
of funds; solvency; |
| |
|
|
| |
● |
SEC
reports; financial statements; |
| |
|
|
| |
● |
no
purchaser vote required; |
| |
|
|
| |
● |
valid
issuance; |
| |
|
|
| |
● |
Hart-Scott-Rodino
Act; and |
| |
|
|
| |
● |
independent
investigation; acknowledgements and confirmations. |
Additional
Agreements
Public
Disclosure
Ayala
and the Purchaser will issue an initial joint press release. Both parties will allow the other party reasonable time to review and comment
on such joint press release. Thereafter, no party will issue any press release related to the transactions unless such press release
(a) has been approved by the other party (which approval shall not be unreasonably withheld, conditioned or delayed) or (b) is required
to be issued by such party under applicable laws based upon advice of counsel.
Confidentiality
Subject
to certain customary exceptions, Ayala agrees that following the closing date it shall hold in strict confidence, unless compelled to
disclose by judicial or administrative process or by other laws, all confidential information of the Purchaser or the Acquired Assets
to which they had access prior to the closing and will not release or disclose such confidential information to any other person, except
to their auditors, attorneys, financial advisors and other consultants, agents and advisors who need to know such information in connection
with Ayala’s business (provided that Ayala takes reasonable steps to ensure that each such person maintain the confidentiality
required hereunder).
Conduct
Pending the Closing
Until
the earlier of the Closing of the Asset Sale or the termination of the Asset Purchase Agreement, Ayala, except (i) as otherwise contemplated,
permitted or required by the Asset Purchase Agreement, (ii) as required by law, (iii) as set forth in the Disclosure Schedules or (iv)
to the extent Purchaser otherwise consents in writing (which consent shall not be unreasonably withheld, conditioned or delayed), (x)
Ayala shall cause the Acquired Assets to be operated in the ordinary course of business in all material respects, and (y) Ayala shall
not:
●
sell, lease, transfer, or otherwise dispose of any of the Acquired Assets;
●
grant or suffer to exist any lien (other than permitted liens) on any of the Acquired Assets;
●
commence any material legal proceeding, or settle, pay, discharge, or satisfy any legal proceeding, where such commencement, settlement,
payment, discharge, or satisfaction would impose any restrictions or limitations upon the Acquired Assets following the closing;
●
terminate, extend, or modify any Acquired Business Contract or material contract, other than to the extent explicitly contemplated or
provided by the Asset Purchase Agreement or any related agreement, or enter into any contract in respect of the Acquired Assets or the
Assumed Liabilities that, if in effect on the Asset Purchase Agreement date, would be a material contract;
●
waive or release any right of material value, in each case related to any Acquired Assets or any Assumed Liabilities;
●
fail to keep in force and effect, or allow to lapse, any insurance policy in respect of the Acquired Assets comparable in amount and
scope of coverage to that maintained as of the date of the Asset Purchase Agreement;
●
correspond, communicate or consult with the FDA or similar governmental authority, in each case with respect to the Compounds or the
Products, other than (x) any such correspondence, communication or consultation in the ordinary course of business, or (y) any such correspondence,
communication or consultation required by applicable law in connection with an adverse event;
●
merge, combine or consolidate itself with any other person or adopt a plan of complete or partial liquidation, dissolution, consolidation,
restructuring, recapitalization or other reorganization, or file a certificate of dissolution in respect of Ayala or any of its subsidiaries
with the secretary of state of the state of Delaware;
●
make any distributions to its stockholders or declare or pay any dividends on shares of Ayala’s capital stock; or
●
agree in writing to do any of the foregoing.
Regulatory
Approvals
Ayala
is not required to obtain any regulatory approvals in order to consummate the Transaction and the Transaction is not conditioned upon
any such approvals.
Further
Assurances and Cooperation; Non-Compete
Further
Assurances
Each
of the parties agrees to use its commercially reasonable efforts to take, or cause to be taken, all actions, and to do, or cause to be
done, and to assist and cooperate with the other parties in doing, all things necessary, proper, or advisable to consummate and make
effective, in the most expeditious manner practicable, the transactions.
Cooperation
Either
Purchaser or Ayala be furnished with additional information, documents, or records relating to the Product operations, the Acquired Assets,
the Excluded Liabilities, or the Assumed Liabilities, and such information, documents, or records are in the possession or control of
the other party, such other party will use its commercially reasonable efforts to furnish or make available such information, documents,
or records (or copies thereof) at the recipient’s reasonable request and at recipient’s cost and expense.
Non-Compete
Ayala
agrees that, from the Asset Purchase Agreement Date until five (5) years following the Closing Date, it shall not, and shall cause its
controlled affiliates, successors, and assigns to not, directly or indirectly, without Purchaser’s prior written consent, (i) develop,
test, manufacture, commercialize, or otherwise exploit any program, pharmaceutical product candidate or product that targets gamma secretase
or that is intended to treat desmoid tumors (a “Competing Product”), anywhere in the world, (ii) own or have the right to
acquire, or manage, operate or control, any person engaged in, or otherwise participate in the research, development, testing, manufacture,
commercialization, or other exploitation of any Competing Product anywhere in the world, or (iii) otherwise knowingly assist or enable
any third party to research, develop, test, manufacture, commercialize, or otherwise exploit any Competing Product anywhere in the world.
Tax
Matters
Following
the Closing Date, Purchaser shall provide a draft allocation schedule to Ayala within 120 days after the Closing Date, and Ayala shall
have 30 days thereafter to review and comment on such allocation schedule. Purchaser shall consider in good faith any comments of Ayala,
and revise the draft allocation schedule accordingly to the extent required to comply with Section 1060 of the Code.
Each
party agrees to prepare and file all applicable tax returns in a manner consistent with the allocation schedule.
Any
taxes attributable to the Acquired Assets covering a period beginning on or before the Closing Date and ending thereafter shall be prorated
between Ayala and the Purchaser.
Ayala
and the Purchaser shall provide the other party with reasonable assistance in the preparation of any tax return, claim for refund, and
the conduct of any audit or other examination by any governmental authority or in connection with judicial or administrative proceedings
relating to any liability for taxes, in each case to the extent related to the Product operations or the Acquired Assets. Each of Ayala
and the Purchaser will provide the other with all records or other information in its possession that is reasonably requested and may
be relevant to the preparation of any tax returns, claim for refund or the conduct of any audit, examination or other proceeding relating
to taxes.
Seller
Stockholder Approval
On
February 8, 2024, Ayala stockholders who collectively held approximately 78.4% of the voting power of the outstanding shares of Common
Stock at the close of business on such date, which was the record date established by the Board with respect to the consent of stockholders,
delivered the Written Consent, in the form attached to the Asset Purchase Agreement as Exhibit F, authorizing, approving and adopting
in all respects the Asset Purchase Agreement and the transactions contemplated thereby, including the Asset Sale and other transactions
contemplated by the Asset Purchase Agreement and the related transaction documents as defined therein. The Written Consent constituted
the necessary action by Ayala’s stockholders to authorize, approve and adopt the Asset Sale under applicable law (including Section
271 of the Delaware General Corporation Law) and Ayala’s organizational documents. As a result, the Company will not be soliciting
your vote to adopt the Asset Purchase Agreement and will not call a stockholders meeting for purposes of voting on the adoption of the
Asset Purchase Agreement.
Non-Solicitation
Until
the earlier of the Closing of the Asset Sale or the termination of the Asset Purchase Agreement, Ayala shall not, and shall cause its
representatives not to, directly or indirectly, (i) solicit, seek, initiate or knowingly encourage, respond to (other than to communicate
that Ayala is subject to an exclusivity obligations and not permitted to respond further as a result) or facilitate the making of any
inquiry, expression of interest, proposal or offer that constitutes, or is reasonably likely to lead to or encourage the initiation or
submission of, any expression of interest, inquiry, proposal or offer from any third party relating to a possible Strategic Transaction
(as such term is defined in the Asset Purchase Agreement), (ii) participate in, maintain or continue any discussions or negotiations
or enter into any agreement with, provide any non-public information to, or provide access to the properties, books or records of Ayala
or any of its direct or indirect subsidiaries to any third party related to or in connection with a possible Strategic Transaction, or
(iii) agree to accept, recommend or endorse, or publicly propose or announce any intention or desire to agree to, accept, recommend or
endorse, or publicly propose or announce any intention or desire to agree to, accept, recommend or endorse, any proposal or offer from
any third party relating to a possible Strategic Transaction; provided, however, that in each case, Ayala shall be permitted to respond
to any unsolicited inquiry from any third party solely for the purposes of informing such person that Ayala is subject to an exclusivity
obligation and not permitted to respond further as a result. During such period, Ayala shall promptly provide Purchaser with an oral
and a written description of any expression of interest, inquiry, proposal or offer relating to a possible Strategic Transaction that
is received by Ayala or any of its representatives. During such period, Ayala shall not release any third party from, or waive any provision
of, any confidentiality agreement to which Ayala is a party.
Deposit
The
Deposit shall be non-refundable to Purchaser, except for the following circumstances: (i) if the Company fails to obtain the Support
Agreements from the Consenting Stockholders, (ii) if the Company fails to deliver the Written Consent three (3) Business Days following
the date of the Asset Purchase Agreement, (iii) if the Asset Purchase Agreement is terminated by Purchaser pursuant to Section 11.1(c)
or Section 11.1(e) of the Asset Purchase Agreement or (iv) if Ayala or any of its representatives breach, in any material
respects the non-solicitation provision of the Asset Purchase Agreement.
Restrictions
on Dissolution
For
a period of six (6) months following the Closing Date, without Purchaser’s prior written consent (which consent shall not be unreasonably
withheld, conditioned or delayed), (a) Ayala shall not make any distributions to its stockholders or declare or pay any dividends on
shares of Ayala’s capital stock, (b) Ayala shall not be wound up, liquidated or dissolved, or initiate any procedures or processes
to be wound up, liquidated or dissolved, (c) Ayala shall not file or cause to be filed on its behalf a petition in bankruptcy or other
similar proceedings and (d) Ayala shall not seek or consent to the appointment of a trustee, receiver, liquidator, assignee for the benefit
of creditors or other person or official with similar duties with respect to Ayala or its assets; provided, however, that Ayala shall
not be restricted from seeking approval from its stockholders to give the board of directors of Ayala discretion to file, a certificate
of dissolution with the Secretary of State of the State of Delaware in accordance with Section 275 of the Delaware General Corporation
Law.
Lock Up; Orderly Market Covenant
For a period of
six (6) months following the Closing Date, Ayala will not sell greater than 50% of the Shares (such 50% portion of the Shares, the “Lock-up
Shares”), or enter into any hedging or similar transaction with the same economic effect as a sale of the Lock-up Shares, subject
to limited exceptions.
For a period of
one (1) year following the Closing Date, Ayala agrees that any transfer of Shares (other than a pro rata distribution to the stockholders
of Seller in connection with any dissolution of Seller, and subject to other limited exceptions) taking place on any single trading day
that exceeds 15% of the average daily trading volume of the Purchaser Common Stock on Nasdaq over the five (5) trading day period ending
on the trading day immediately prior to the trading day on which such transfer is being made, to conduct such Share transfer as a block
trade or other disposition through a market participant designated by Purchaser.
Tail
Insurance
Ayala
agrees that, prior to the Closing, Ayala shall obtain and deliver to Purchaser evidence of a tail policy or similar coverage reasonably
satisfactory to Purchaser that (a) covers claims made in respect of product liability or clinical trial liability for activities conducted
by the Company prior to Closing and such other coverage for incurred but unreported claims arising on or prior to Closing related to
Ayala’s conduct of its business relating to the Acquired Assets prior to the Closing as requested by Purchaser, (b) has an extended
reporting period from the Closing through the fifth (5th) anniversary of the Closing date and (c) is consistent, both in terms
of coverage and limits with Ayala’s insurance policies.
Transition
Services
For
a period of one (1) month following the Closing, Ayala shall provide the transition services set forth on Schedule 6.11 of the Asset
Purchase Agreement.
Employee
Matters
Purchaser
may offer consulting or other arrangements to certain employees, advisors and independent contracts of Ayala who have been involved in
research and development related to the Products and the Compounds and who may have information necessary to enable technology transfer
to Purchaser following the Closing (collectively, the “Key Personnel”), and Ayala will not assert that any such arrangements
violates any employment or other written agreements with such Key Personnel that contain obligations of confidentiality, non-use and
non-disclosure with respect to any information related to the Acquired Assets and/or the Assumed Liabilities, or any non-compete obligations
with respect to the Acquired Assets and/or the Assumed Liabilities.
Registration
Rights
Subject
to certain terms and conditions that are further specified in the Asset Purchase Agreement, and in accordance with the procedures as
provided therein, Purchaser shall use commercially reasonably efforts to file a resale registration statement with respect to the Shares
with the SEC on or before the date that is seven (7) days following the earlier of (i) April 1, 2024 and (ii) the date Purchaser files
its annual report on Form 10-K for the year ended December 31, 2023, to register all of the Shares on Form S-3 under the Securities Act,
and to cause such registration statement to become effective as soon as practicable after the filing thereof. The Asset Purchase Agreement
provides that our method of disposition of the Shares shall be limited to non-underwritten public offerings and pro rata distributions
of the Shares to our stockholders in connection with our dissolution, should that occur.
Termination
Ayala
and Purchaser may terminate the Asset Purchase Agreement by mutual written consent. In addition, there are certain other circumstances
under which the Asset Purchase Agreement may be terminated prior to the Closing, including:
●
by either Ayala or Purchaser if any government authority of competent jurisdiction issues an order permanently restraining, enjoining,
or otherwise prohibiting the consummation of the Asset Sale, and such order becomes final and non-appealable (provided that a party shall
not be entitled to exercise such termination right if, at the time of such termination, such party is in breach of any of its representations,
warranties or covenants under the Asset Purchase Agreement, which breach has caused or resulting in the imposition of such order or the
failure of such order to be resisted, resolved or lifted);
●
by Purchaser if Ayala has breached any of its representations, warranties or covenants under the Asset Purchase Agreement such that an
applicable condition to Closing shall not be satisfied and which breach is not cured by Ayala within the shorter of 30 days after Ayala
receives written notice of such breach from Purchaser or by the date that is six months following the date of the Asset Purchase Agreement
(such date, the “Outside Date”) (provided that Purchaser shall not be entitled to exercise such termination right if, at
the time of such termination, Purchaser is in breach of any of its representations, warranties or covenants under the Asset Purchase
Agreement, such that an applicable condition to Closing shall not be satisfied);
●
by Ayala if Purchaser has breached any of its representations, warranties or covenants under the Asset Purchase Agreement such that an
applicable condition to Closing shall not be satisfied and which breach is not cured by Purchaser within the shorter of 30 days after
Purchaser receives written notice of such breach from Ayala or by the Outside Date (provided that Ayala shall not be entitled to exercise
such termination right if, at the time of such termination, Ayala is in breach of any of its representations, warranties or covenants
under the Asset Purchase Agreement, such that an applicable condition to Closing shall not be satisfied);
●
by either party if the Closing shall not have occurred on or before the Outside Date (provided that a party shall only be entitled to
exercise such termination right if the terminating party is not in breach of any representation, warranty or covenant such that an applicable
condition to Closing shall not be satisfied); or
●
by Purchaser, if Seller fails to deliver, by 11:59 p.m. Eastern Time on the date that is three (3) Business Days after the date of the
Asset Purchase Agreement, the Written Consent.
Amendments
and Waivers
The
Asset Purchase Agreement may not be amended or modified, nor may compliance with any condition or covenant set forth therein be waived,
except in a writing duly and validly executed by Purchaser and Ayala or, in the case of a waiver, the party waiving compliance.
Governing
Law
The
Asset Purchase Agreement is governed by and construed and enforced under the substantive laws of the State of Delaware, excluding any
conflicts or choice of law rule or principle that might otherwise make the Asset Purchase Agreement subject to the substantive law of
another jurisdiction.
MARKET
PRICE OF OUR STOCK
Ayala’s
Common Stock is quoted on the OTCQX under the symbol “ADXS”.
The
closing price of shares of Ayala’s Common Stock on February 5, 2024, the trading day immediately prior to the public announcement
of the Asset Sale on February 6, 2024, as reported on the OTCQX, was $0.5899 per share. Any over-the-counter market quotations reflect
inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
As
of February 8, 2024, there were approximately 122 holders of record of Ayala’s Common Stock and 42,633,400 shares of Ayala’s
Common Stock outstanding. The actual number of holders of Ayala’s Common Stock is greater than this number of record holders, and
includes stockholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees.
Ayala
Dividends
Ayala
has never declared or paid any cash dividends on its capital stock. Ayala currently intends to retain all available funds and future
earnings, if any, for the operation and expansion of its business and does not anticipate declaring or paying any dividends in the foreseeable
future. Pursuant to the Asset Purchase Agreement, Ayala may not make any distributions to its stockholders or declare or pay any dividends
on shares of Ayala’s capital stock for a period of six (6) months following the Closing.
The
payment of dividends, if any, will be at the discretion of the combined company’s board of directors and will depend on its results
of operations, capital requirements, financial condition, prospects, contractual arrangements, any limitations on payment of dividends
present in its future debt agreements, and other factors that the board of directors of the combined company may deem relevant.
ANCILLARY
AGREEMENTS WITH CERTAIN STOCKHOLDERS OF THE COMPANY
The
Support Agreement
On
February 5, 2024, in connection with the execution of the Asset Purchase Agreement, certain of the Company’s officers, directors
and stockholders who collectively are the record or beneficial holders of more than a majority of the issued and outstanding shares of
capital stock of the Company entered into a stockholder support agreement in favor of the Purchaser (the “Support Agreement”)
providing, among other things, that such officers, directors and stockholders will, (i) deliver the Written Consent by 11:59 p.m. Eastern
Time on February 8, 2024; (ii) vote against any proposal made in opposition to, or in competition with, the Asset Purchase Agreement
or the Asset Sale and (iii) vote against any acquisition proposal involving a third party.
The
Support Agreement terminates upon certain events including: (a) the termination of the Asset Purchase Agreement in accordance with its
terms, (b) the Closing and (c) if the Asset Purchase Agreement is amended, without the prior written consent of the stockholders, in
a manner that affects the economic terms of the Asset Purchase Agreement in a manner that is adverse to the Company or its stockholders.
APPRAISAL
RIGHTS
No
appraisal or dissenters’ rights are available to our stockholders under Delaware law or our certificate of incorporation or bylaws
in connection with the Asset Sale.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth information regarding the beneficial ownership of our common stock by (a) each person who is known to us to
be the owner of more than five percent (5%) of our common stock, (b) each of our directors, (c) each of the named executive officers,
and (d) all directors and executive officers and executive employees as a group. For purposes of the table, a person or group of persons
is deemed to have beneficial ownership of any shares that such person has the right to acquire within 60 days of February 14, 2024. The
percentage of ownership is based on 42,633,400 shares outstanding as of February 14, 2024. Unless otherwise indicated by footnote, the
address for each of the beneficial owners set forth in the table below is c/o Ayala Pharmaceuticals, Inc., 9 Deer Park Drive, Suite K-1,
Monmouth Junction, NJ 08852. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment
power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws. The beneficial ownership
of our common stock will remain unchanged following the completion of this offering.
| Name of Beneficial Owner | |
Total # of Shares Beneficially Owned | | |
Percentage of Ownership | |
| Israel Biotech Fund I, L.P. (1) | |
| 12,824,138 | | |
| 30.08 | % |
| Israel Biotech Fund II, L.P. (2) | |
| 11,668,403 | | |
| 27.37 | % |
| Arkin Bio Ventures L.P. (3) | |
| 8,533,051 | | |
| 20.01 | % |
| Kenneth Berlin (4) | |
| 2,413 | | |
| * | % |
| Roy Golan(5) | |
| - | | |
| * | % |
| David Sidransky (6) | |
| 5,145 | | |
| * | % |
| Roni Appel (7) | |
| 484 | | |
| * | % |
| Vered Bisker-Leib (8) | |
| 3,981 | | |
| * | % |
| Murray A. Goldberg (9) | |
| 7,963 | | |
| * | % |
| Robert Spiegel (10) | |
| 1,171 | | |
| * | % |
| Andres Gutierrez (11) | |
| 1,297 | | |
| * | % |
| Yuval Cabilly(12) | |
| - | | |
| * | % |
| Pini Orbach(13) | |
| - | | |
| * | % |
| Bridget Martell(14) | |
| - | | |
| * | % |
| All Current Directors and Officers as a Group (11 People) (15) | |
| 22,454 | | |
| * % | |
*Constitutes
less than 1% of our outstanding Common Stock.
| (1) |
The
address of Israel Biotech Fund I, L.P. (“IBF I”) is c/o IBF Management Ltd., 4 Oppenheimer St. Rehovot Israel. Represents
12,824,138 shares of common stock held of record by IBF I. Yuval Cabilly is the chief executive officer and a director of IBF I and
as such could be deemed to have voting and dispositive power with respect to the shares held by IBF I. David Sidransky is a director
of IBF I and as such could be deemed to have voting and dispositive power with respect to the shares held by IBF I. Mr. Sidransky
and Mr. Cabilly disclaim beneficial ownership of such shares except to the extent of his pecuniary interest therein. |
| |
|
| (2) |
The
address of Israel Biotech Fund II, L.P. (“IBF II”) is c/o IBF Management Ltd., 4 Oppenheimer St. Rehovot Israel. Represents
11,668,403 shares of common stock held of record by IBF II. Yuval Cabilly is the chief executive officer and a director of IBF II
and as such could be deemed to have voting and dispositive power with respect to the shares held by IBF II. David Sidransky is a
director of IBF II and as such could be deemed to have voting and dispositive power with respect to the shares held by IBF II. Mr.
Sidransky and Mr. Cabilly disclaim beneficial ownership of such shares except to the extent of his pecuniary interest therein. |
| |
|
| (3) |
The
address of Arkin Bio Ventures L.P. (“Arkin”) is Ha’Choshlim St., Building C, 6th Floor, Herzliya Pituach 46724,
Israel. Represents 8,533,051 shares of common stock held of record by Arkin. Pini Orbach is Head of Pharma at Arkin and as such could
be deemed to have voting and dispositive power with respect to the shares held by Arkin. Mr. Orbach disclaims beneficial ownership
of such shares except to the extent of his pecuniary interest therein. |
| |
|
| (4) |
Represents
271 issued shares of our Common Stock, and options to purchase 2,142 shares of our Common Stock exercisable within 60 days. |
| |
|
| (5) |
Represents
0 issued shares of our Common Stock, and options to purchase 0 shares of our Common Stock exercisable within 60 days. |
| |
|
| (6) |
Represents
93 issued shares of our Common Stock, and options to purchase 5,052 shares of our Common Stock exercisable within 60 days. |
| |
|
| (7) |
Represents
133 issued shares of our Common Stock, and options to purchase 351 shares of our Common Stock exercisable within 60 days. |
| |
|
| (8) |
Represents
0 issued shares of our Common Stock, and options to purchase 3,981 shares of our Common Stock exercisable within 60 days. |
| |
|
| (9) |
Represents
0 issued shares of our Common Stock, and options to purchase 7,963 shares of our Common Stock exercisable within 60 days. |
| |
|
| (10) |
Represents
0 issued shares of our Common Stock, and options to purchase 1,171 shares of our Common Stock exercisable within 60 days. |
| |
|
| (11) |
Represents
47 issued shares of our Common Stock, and options to purchase 1,250 shares of our Common Stock exercisable within 60 days. |
| |
|
| (12) |
Represents
0 issued shares of our Common Stock, and options to purchase 0 shares of our Common Stock exercisable within 60 days. |
| |
|
| (13) |
Represents
0 issued shares of our Common Stock, and options to purchase 0 shares of our Common Stock exercisable within 60 days. |
| |
|
| (14) |
Represents
0 issued shares of our Common Stock, and options to purchase 0 shares of our Common Stock exercisable within 60 days. |
| |
|
| (15) |
Represents
544 issued shares of our Common Stock, and options to purchase 21,910 shares of our Common Stock exercisable within 60 days. |
WHERE
YOU CAN FIND MORE INFORMATION
The
Company files annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy any document
that the Company files at the Public Reference Room of the SEC at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information
on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. In addition, the SEC maintains a website at http://www.sec.gov,
from which interested persons can electronically access the Company’s SEC filings.
In
addition, all documents the Company files under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this information
statement and before the date of the Annual Meeting are incorporated by reference into and deemed a part of this information statement
from the date of filing of those documents.
Any
person, including any beneficial owner, to whom this information statement is delivered may request copies of reports, proxy statements
or other information concerning the Company (including the documents incorporated by reference herein) without charge, by written or
telephonic request directed to the Corporate Secretary, Ayala Pharmaceuticals, Inc., 9 Deer Park Drive, Suite K-1, Monmouth Junction.
No
provision has been made to grant the Company’s unaffiliated shareholders access to the corporate files of the Company, any other
party to the Asset Purchase Agreement or any of their respective affiliates, or to obtain counsel or appraisal services at the expense
of the Company, any other such party or affiliate.
If
you have any questions concerning the Asset Purchase Agreement, the Asset Sale, or this information statement, or would like additional
copies of this information statement or need help voting your shares of Common Stock, please contact Roy Golan, our Chief Financial Officer,
by mail at Ayala Pharmaceuticals, Inc., 9 Deer Park Drive, Suite K-1, Monmouth Junction, NJ 08852, or by telephone: 908-215-2787.
Stockholders
should not rely on information that purports to be made by or on behalf of the Company other than that contained in or incorporated by
reference in this information statement. The Company has not authorized anyone to provide information on behalf of the Company that is
different from that contained in this information statement. This information statement is dated February , 2024. No assumption should
be made that the information contained in this information statement is accurate as of any date other than that date, and the mailing
of this information statement will not create any implication to the contrary. Notwithstanding the foregoing, in the event of any material
change in any of the information previously disclosed, the Company will, where relevant and if required by applicable law, update such
information through a supplement to this information statement.
ANNEX
A
CONFIDENTIAL
EXECUTION
VERSION
ASSET
PURCHASE AGREEMENT
by
and between
Ayala
Pharmaceuticals, Inc.
and
Immunome,
Inc.
Dated
as of February 5, 2024
TABLE
OF CONTENTS
| |
|
Page |
| |
|
|
| 1. |
Definitions. |
1 |
| |
|
|
| 2. |
Purchase
and Sale of Assets |
20 |
| |
|
|
| |
2.1 |
Acquired
Assets. |
20 |
| |
|
|
|
| |
2.2 |
Assumed
Liabilities |
21 |
| |
|
|
|
| |
2.3 |
Excluded
Liabilities |
21 |
| |
|
|
|
| |
2.4 |
Assets
Incapable of Transfer |
21 |
| |
|
|
|
| 3. |
Consideration |
21 |
| |
|
|
| |
3.1 |
Consideration |
21 |
| |
|
|
|
| |
3.2 |
Closing
Consideration. |
22 |
| |
|
|
|
| |
3.3 |
Milestone
Consideration |
22 |
| |
|
|
|
| |
3.4 |
Withholding |
25 |
| |
|
|
|
| |
3.5 |
Legends |
25 |
| |
|
|
|
| |
3.6 |
Lock
Up |
27 |
| |
|
|
|
| |
3.7 |
Orderly
Market |
27 |
| |
|
|
|
| |
3.8 |
Rule
144 Reporting. |
27 |
| |
|
|
|
| 4. |
Closing |
28 |
| |
|
|
| |
4.1 |
Closing |
28 |
| |
|
|
|
| |
4.2 |
Deliveries
at Closing. |
28 |
| |
|
|
|
| |
4.3 |
Possession |
29 |
| |
|
|
|
| 5. |
Representations
and Warranties |
29 |
| |
|
|
| |
5.1 |
Representations
and Warranties of Seller |
29 |
| |
|
|
|
| |
5.2 |
Representations
and Warranties of Purchaser |
45 |
| |
|
|
|
| 6. |
Additional
Agreements |
49 |
| |
|
|
| |
6.1 |
Public
Disclosures |
49 |
| |
|
|
|
| |
6.2 |
Confidentiality |
49 |
| |
|
|
|
| |
6.3 |
Conduct
Pending the Closing |
49 |
| |
|
|
|
| |
6.4 |
Further
Assurances and Cooperation; Non-Compete. |
50 |
| |
|
|
|
| |
6.5 |
Tax
Matters |
51 |
| |
|
|
|
| |
6.6 |
Seller
Stockholder Approval |
52 |
| |
|
|
|
| |
6.7 |
Non-Solicitation. |
54 |
TABLE
OF CONTENTS
(continued)
| |
|
|
Page |
| |
|
|
|
| |
6.8 |
Deposit |
54 |
| |
|
|
|
| |
6.9 |
Restrictions on Dissolution
and Winding Up |
55 |
| |
|
|
|
| |
6.10 |
Tail Insurance |
55 |
| |
|
|
|
| |
6.11 |
Transition Services |
55 |
| |
|
|
|
| |
6.12 |
Employee Matters |
55 |
| |
|
|
|
| 7. |
Conditions
Precedent to Obligations of Purchaser. |
56 |
| |
|
|
|
| |
7.1 |
Accuracy of Representations
and Warranties |
56 |
| |
|
|
|
| |
7.2 |
Performance of Covenants |
56 |
| |
|
|
|
| |
7.3 |
No Restraints |
56 |
| |
|
|
|
| |
7.4 |
No Material Adverse Effect |
56 |
| |
|
|
|
| |
7.5 |
No Governmental Litigation |
56 |
| |
|
|
|
| |
7.6 |
Closing Certificate |
56 |
| |
|
|
|
| |
7.7 |
Seller Closing Deliverables |
56 |
| |
|
|
|
| |
7.8 |
Seller Information Statement |
57 |
| |
|
|
|
| 8. |
Conditions
Precedent to Obligation of Seller |
57 |
| |
|
|
|
| |
8.1 |
Accuracy of Representations |
57 |
| |
|
|
|
| |
8.2 |
Performance of Covenants |
57 |
| |
|
|
|
| |
8.3 |
No Restraints |
57 |
| |
|
|
|
| |
8.4 |
Closing Certificate |
57 |
| |
|
|
|
| |
8.5 |
Purchaser Closing Deliverables |
57 |
| |
|
|
|
| 9. |
Survival. |
57 |
| |
|
|
|
| |
9.1 |
Survival of Representations;
Warranties and Covenants |
57 |
| |
|
|
|
| 10. |
Registration
Rights. |
57 |
| |
|
|
|
| |
10.1 |
Definitions |
57 |
| |
|
|
|
| |
10.2 |
Registration Procedures. |
58 |
| |
|
|
|
| |
10.3 |
Rule 415; Cutback |
60 |
| |
|
|
|
| |
10.4 |
Registration Right Indemnification. |
61 |
| |
|
|
|
| |
10.5 |
Prospectus Suspension |
62 |
| |
|
|
|
| 11. |
Termination |
62 |
| |
|
|
|
| |
11.1 |
Termination of the Agreement |
62 |
| |
|
|
|
| |
11.2 |
Effect of Termination |
64 |
TABLE
OF CONTENTS
(continued)
| |
|
Page |
| |
|
|
| 12. |
Miscellaneous |
64 |
| |
|
|
|
| |
12.1 |
Governing Law; Jurisdiction |
64 |
| |
|
|
|
| |
12.2 |
Specific Performance |
64 |
| |
|
|
|
| |
12.3 |
WAIVER OF JURY TRIAL |
64 |
| |
|
|
|
| |
12.4 |
Entire Agreement; Severability |
65 |
| |
|
|
|
| |
12.5 |
Incorporation by Reference |
65 |
| |
|
|
|
| |
12.6 |
Amendments and Waivers |
65 |
| |
|
|
|
| |
12.7 |
Notices |
65 |
| |
|
|
|
| |
12.8 |
No Assignment; Binding Effect |
66 |
| |
|
|
|
| |
12.9 |
Third Person Beneficiaries |
67 |
| |
|
|
|
| |
12.10 |
Relationship of the Parties |
67 |
| |
|
|
|
| |
12.11 |
Headings; Interpretation |
67 |
| |
|
|
|
| |
12.12 |
Counterparts; Signatures |
67 |
| |
|
|
|
| |
12.13 |
Expenses |
67 |
| Exhibits |
|
|
| Exhibit
A |
– |
Support
Agreement |
| Exhibit
B |
– |
Assignment
and Assumption Agreement |
| Exhibit
C |
– |
Bill
of Sale |
| Exhibit
D |
– |
CVR
Agreement |
| Exhibit
E |
– |
IP
and Patent Assignment Agreement |
| Exhibit
F |
– |
Stockholder
Written Consent |
| Schedules |
|
|
| Schedule
A |
– |
Requisite
Holders/Consenting Stockholders |
| Schedule
B |
– |
Compounds |
| Schedule
C |
– |
Product
Patents |
| Schedule
C-1 |
– |
Dropped
Patents |
| Schedule
D |
– |
Knowledge
Parties |
| Schedule
E |
– |
Qualified
CMC Expenses |
| Schedule
2.1(a)(ii) |
– |
Acquired
Business Contracts |
| Schedule
2.2(b) |
– |
Certain
Assumed Liabilities |
| Schedule
4.2(a)(v) |
– |
Closing
Third Party Consents/Notices |
| Schedule
6.11 |
– |
Transition
Services |
ASSET
PURCHASE AGREEMENT
This
Asset
Purchase Agreement (this “Agreement”)
is made as of February 5, 2024 (the “Agreement Date”), by and between Ayala Pharmaceuticals, Inc.,
a Delaware corporation (“Seller”), and Immunome, Inc., a Delaware corporation (“Purchaser”).
Seller and Purchaser are sometimes referred to herein individually as a “Party” and collectively as the “Parties”.
Recitals
A.
The Parties intend to provide for the purchase by Purchaser of the Acquired Assets, including the assumption of certain Liabilities relating
to the Acquired Assets, all on the terms and subject to the conditions set forth in this Agreement.
B.
Subject to, and in accordance with, this Agreement, the Parties desire to enter into (i) an Assignment and Assumption Agreement and (ii)
a Bill of Sale.
C.
Concurrently with the execution and delivery of this Agreement and as a condition and inducement to Purchaser’s willingness to
enter into this Agreement, the Persons identified as “Requisite Holders” on Schedule A (such equity holders, the “Requisite
Holders”) have entered into Support Agreements, dated as of the date of this Agreement, in substantially the form attached
hereto as Exhibit A (the “Support Agreement”), pursuant to which the Requisite Holders have, subject to the
terms and conditions set forth therein, agreed to vote all their shares of Seller’s capital stock in favor of the Transactions.
Now,
Therefore, in consideration of the premises and of
the respective representations, warranties, covenants, agreements, and conditions contained herein, and for other good and valuable consideration,
the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound hereby, the Parties agree as follows:
Agreement
1.
Definitions.
In
this Agreement and any Exhibit, Disclosure Schedule, and Schedule attached hereto, the following terms have the meanings specified or
referred to in this Section 1 and shall be equally applicable to both the singular and plural forms.
“Acquired
Assets” has the meaning set forth in Section 2.1(a).
“Acquired
Business Contracts” has the meaning set forth in Section 2.1(a)(ii).
“Affiliate”
means, as to any Person, any other Person that, directly or indirectly through one or more intermediaries, controls, is controlled by,
or is under common control with that Person, but only for so long as such control exists. For purposes of this definition, “control”
(including, with correlative meanings, the terms “controlled by” and “under common control with”), as used with
respect to any Person or group of Persons, means possession, directly or indirectly, of the power to direct or cause the direction of
the management and policies of the Person, whether (a) through direct or indirect beneficial ownership of at least 50% (or such lesser
percentage which is the maximum allowed to be owned by a foreign entity in a particular jurisdiction) of the voting stock or other ownership
interest in such corporation or other entity, or (b) by contract.
“Agreement”
has the meaning set forth in the preamble.
“Allocation
Schedule” has the meaning set forth in Section 6.5(a).
“Annual
Net Sales” means, with respect to a Product, total worldwide Net Sales of such Product during a calendar year.
“Anti-Corruption
Laws” means the Foreign Corrupt Practices Act of 1977, the Anti-Kickback Act of 1986 or any applicable Laws of similar effect,
and the related regulations and published interpretations thereunder.
“Assignment
Agreements” means any agreement with Third Parties (other than an Inventor) assigning right, title, or interest to Seller Patents.
“Assignment
and Assumption Agreement” means the Assignment and Assumption Agreement in substantially the form attached hereto as Exhibit
B.
“Assumed
Liabilities” has the meaning set forth in Section 2.2.
“Bill
of Sale” means the Bill of Sale in substantially the form attached hereto as Exhibit C.
“BMS”
means Bristol-Myers Squibb Company or its successors or assigns to the BMS License.
“BMS
License” means the License Agreement dated November 29, 2017, between Seller and BMS, as amended.
“Business
Day” means any day that is not a Saturday, a Sunday or other day on which banks are required or authorized by Law to be closed
in the Commonwealth of Pennsylvania or the State of New Jersey.
“Closing”
has the meaning set forth in Section 4.1.
“Closing
Consideration” has the meaning set forth in Section 3.2(b).
“Closing
Date” has the meaning set forth in Section 4.1.
“Closing
Cash Payment” has the meaning set forth in Section 3.2(a).
“Code”
means the Internal Revenue Code of 1986.
“Combination
Product” means a product in which one or more active ingredients that are not Products are sold in combination with, in addition
to, or in a bundle with, a Product. Such other active ingredient(s) are referred to as the “Other Product(s)”.
“Commercialization”
means the conduct of all activities undertaken in preparation for and following Regulatory Approval relating to the promotion, sale (including
receiving, accepting, and filling product orders), marketing, and distribution (including importing, exporting, transporting, customs
clearance, warehousing, invoicing, handling, and delivering) of a product, including sales force, detailing, advertising, market research,
market access (including price and reimbursement activities) and sales force training. “Commercialize” and “Commercializing”
have correlative meanings.
“Competing
Product” has the meaning set forth in Section 6.4(c).
“Compounds”
means the gamma secretase inhibitors known as AL101 and AL102, as set forth on Schedule B.
“Consenting
Stockholder” means the Persons identified as “Consenting Stockholders” on Schedule A.
“Conflict”
has the meaning set forth in Section 5.1(c).
“Consideration”
has the meaning set forth in Section 3.1.
“Contract”
means any legally binding written or oral contract, agreement, or instrument, including supply contracts, licenses, understandings or
commitments, customer agreements, subcontracts, leases of personal property, notes, guarantees, pledges, and conditional sales agreements
to which the Person referred to is a party or by which any of its assets are bound.
“Control”
or “Controlled by” means, with respect to any Intellectual Property Rights, the possession by a party of the ability
(whether by ownership, license or other right) to grant access to, or a license or sublicense of, such Intellectual Property Rights without
violating the terms of any agreement or other arrangement with any Third Party.
“Controlled
Group” means Seller and any trade or business, whether or not incorporated, which is treated together with Seller as a single
employer under Section 4001(b)(1) of ERISA or Sections 414(b), (c), (m), or (o) of the Code.
“Cut
Back Shares” has the meaning set forth in Section 10.3.
“CVR”
means a contingent value right issued under the CVR Agreement.
“CVR
Agreement” means the Contingent Value Right Agreement in substantially the form attached hereto as Exhibit D.
“Deposit”
means $4,000,000.00 in immediately available funds, which amount was paid by Purchaser to Seller on December 23, 2023.
“Development”
means all development activities for a pharmaceutical or biological product (whether alone or for use together, or in combination, with
another active agent or ingredient as a combination product or combination therapy) that are directed to obtaining Regulatory Approval(s)
of such product and lifecycle management of such product in any country in the world, including all non-clinical, preclinical, and clinical
studies and trials of such product; toxicology, pharmacokinetic, and pharmacological studies; statistical analyses; assay development;
protocol design and development; the preparation, filing, and prosecution of any Regulatory Approval Application for such product; development
activities directed to label expansion and/or obtaining Regulatory Approval for one or more additional indications following initial
Regulatory Approval; development activities conducted after receipt of Regulatory Approval; and all regulatory affairs related to any
of the foregoing. “Develop”, “Developing” and “Developed” and similar variations
have correlative meanings.
“Development
Milestone Event” has the meaning set forth in Section 3.3(a).
“Diligent
Efforts” means, with respect to the efforts to be expended by Purchaser with respect to any task, objective, activity or decision
to be undertaken with respect to the Development of a Product, the carrying out of such Development activities with a level of effort
and resources consistent with those efforts that Purchaser and its controlled Affiliates devote to the Development of a pharmaceutical
product owned by them or to which they have rights at a similar stage of development, and of similar scientific data and validation,
market potential, profit potential and strategic value and based on conditions then prevailing. Such efforts may take into account, without
limitation, scientific data (including safety and efficacy data), Regulatory Authority-approved labeling, product profile, the competitiveness
of alternative products, pricing and reimbursement for such Product in a country relative to other markets, the likely timing of such
Product’s entry into the market, the proprietary position of such Product, the anticipated profitability of such Product and the
other products in Purchaser’s and its Affiliates’ portfolio (including other products under development), the likelihood
of Regulatory Approval and other relevant scientific, technical and commercial factors. Diligent Efforts shall be determined on a country
by country basis and indication by indication basis and it is anticipated that the level of effort will change over time reflecting the
change in conditions.
“Disclosure
Schedules” has the meaning set forth in Section 5.1.
“Distributor”
means any Third Party wholesaler or distributor engaged for the sale of Product provided that such wholesaler or distributor does not
make any royalty, milestone, profit share or other payment to Purchaser and its Affiliates based on such wholesaler’s or distributor’s
sale of Product.
“DOJ”
means the Antitrust Division of the U.S. Department of Justice.
“Domain
Names” means domain names, uniform resource locators, other names and locators associated with the Internet, and applications
or registrations for the foregoing.
“Dropped
Patents” means each of the following, whether or not pending, issued, expired, withdrawn, rejected, canceled, abandoned, or
closed: (a) the Patents listed on Schedule C-1, and (b) any renewals, divisions, continuations (in whole or in part), or requests
for continued examination of any of such patents, certificates of invention, and patent applications, any and all patents or certificates
of invention issuing thereon, and any and all reissuances, reexaminations, extensions, divisions, renewals, substitutions, confirmations,
registrations, revalidations, revisions, and additions of or to any of the Patents listed on Schedule C-1.
“Effectiveness
Deadline” has the meaning set forth in Section 10.2(b).
“Employee
Benefit Plan” means (i) all “employee benefit plans” (as defined in Section 3(3) of ERISA), (ii) all other employee
benefit plans, policies, agreements or arrangements, (iii) all employment, individual consulting, executive compensation, or other compensation
agreements, or bonus or other incentive compensation, stock purchase, equity or equity-based compensation, deferred compensation, change
in control, retention, severance, sick leave, vacation, recreation, retirement, pension, loans, salary continuation, health, medical,
dental, vision, accident, disability, cafeteria, life insurance and educational assistance plans, policies, agreements or arrangements,
and (iv) any collective bargaining agreement or union contract, in each case, whether written or unwritten and whether or not subject
to ERISA, that are sponsored or maintained by Seller or any member of the Controlled Group for the benefit of current or former employees
or current or former consultants, independent contractors or directors of Seller or any of its Subsidiaries or with respect to which
Seller or any of its Subsidiaries has any Liability.
“Employee
Liabilities” means any and all Liabilities, whenever or however arising, including all costs and expenses relating thereto
arising under Contract, Law or Permit, Order or any award of any arbitrator of any kind relating to any Employee Benefit Plan, employment
agreement, natural person consulting or independent contractor agreement, or otherwise relating to an employee or other natural person
service provider and his or her potential or actual service or employment with Seller or any member of the Controlled Group.
“Enforceability
Exception” has the meaning set forth in Section 5.1(b).
“ERISA”
means the Employee Retirement Income Security Act of 1974.
“Evaluation
Material” has the meaning set forth in Section 5.1(bb).
“Excess
Liabilities” means (a) the excess (if any) of (i) the aggregate amount of Specified Liabilities over (ii) $4,000,000, plus
(b) the excess (if any) of (i) the sum of (A) the Qualified CMC Expenses reimbursed to Seller as of Closing and (B) any Qualified
CMC Expenses assumed and paid by Purchaser after the Closing, over (ii) $1,000,000.
“Exchange
Act” means the Securities Exchange Act of 1934.
“Excluded
Assets” has the meaning set forth in Section 2.1(b).
“Excluded
Liabilities” means all Liabilities of Seller and its Affiliates not expressly included in the definition of Assumed Liabilities,
including:
(a)
except as expressly included as an Assumed Liability, all Liabilities arising from Seller’s ownership or use of the Acquired
Assets or the conduct of its business, including the Product Operations, as of or prior to Closing;
(b)
all Liabilities relating to Excluded Assets;
(c)
all Seller Tax Liabilities;
(d)
all Liabilities of Seller or any of its Affiliates for product liability, infringement, or misappropriation arising from preclinical
or clinical trials of a Product or Product Operations conducted by or for Seller on or prior to Closing;
(e)
all Employee Liabilities;
(f)
any Liabilities that arose or were incurred under any Acquired Business Contract on or prior to Closing or that relate to any failure
to perform, improper performance, warranty or other breach, default, or violation by Seller on or prior to the Closing;
(g)
except as expressly included as an Assumed Liability, all Liabilities for invoices, bills, accounts payable, or other trade payables
due or owned by Seller or any of its Affiliates to any Third Party relating to or arising from the Development, manufacture (or having
manufactured), packaging, importation, marketing, or distribution of a Compound or Product prior to the Closing;
(h)
any Liability arising out of or resulting from non-compliance with any Law by Seller or its Affiliates with respect to the Product
Operations or the Acquired Assets;
(i)
any Liability of Seller or its Affiliates to pay any fees or commissions to any broker, finder, or agent with respect to this Agreement
or any of the Transactions or the consummation thereof;
(j)
any Liability arising out of or resulting from any liquidation, dissolution or winding up of Seller, including any Legal Proceeding,
bankruptcy filing, assignment for the benefit of creditors or similar restructuring or reorganization involving Seller; and
(k)
all Excess Liabilities.
“Exclusivity
Agreement” means the Exclusivity Agreement, dated December 22, 2023, as amended, by and between Purchaser and Seller.
“FDA”
means the U.S. Food and Drug Administration, or any successor agency thereto.
“FDC
Act” means the U.S. Federal Food, Drug, and Cosmetic Act of 1938.
“Filed
SEC Documents” has the meaning set forth in Section 5.1.
“Filing
Date” has the meaning set forth in Section 10.2(a).
“Fraud”
means, with respect to any Party hereto, the making of a representation and warranty set forth in Article 5, with actual knowledge
that such representation and warranty was false when made, with the intent that the Purchaser (in the case of the Seller) or the Seller
(in the case of the Purchaser) rely thereon to its detriment.
“FTC”
means the U.S. Federal Trade Commission or any successor agency thereto.
“Fundamental
Representations” means the representations and warranties contained in Sections 5.1(a) (Organization and Existence),
5.1(b) (Authority and Approval), clause (i) of 5.1(c) (No Conflict), 5.1(f) (Title), 5.1(n)
(No Brokers) and 5.1(t) (Sufficiency of Acquired Assets).
“GAAP”
means U.S. generally accepted accounting principles, consistently applied.
“GCP”
means good clinical practices, standards and procedures promulgated or endorsed by the FDA as set forth in the guidelines entitled “Guidance
for Industry E6 Good Clinical Practice,” including related regulatory requirements imposed by the FDA, in 21 C.F.R parts 11, 16,
50, 54, 56, 58, 312, 324, 320, 511, 514, 601, 812, and 814, and comparable regulatory standards, practices and procedures promulgated
by any other Regulatory Authority, including applicable quality guidelines promulgated under the ICH, in each case, as amended from time
to time and applicable to relevant activities hereunder.
“GLP”
means good laboratory practices promulgated or endorsed by the FDA, as set forth in 21 C.F.R. Part 58, and comparable regulatory standards
promulgated by any other Regulatory Authority, including applicable quality guidelines promulgated under the ICH, in each case, as amended
from time to time and applicable to the relevant activities hereunder.
“GMP”
means good manufacturing practices and standards for the production of drugs promulgated or endorsed by the FDA, as set forth in 21 C.F.R.
Parts 210, 211, 600 through 680, 820, and 1271, as applicable, and comparable regulatory standards promulgated by any other Regulatory
Authority, including applicable guidelines promulgated under the ICH, in each case, as amended from time to time and applicable to the
relevant activities hereunder.
“Governmental
Authority” means any federal, state, local, or any non-U.S. government, governmental, regulatory (including self-regulatory)
or administrative authority, body, agency or commission or any court, tribunal, or judicial or arbitral body.
“HIPAA”
means, collectively: (a) the Health Insurance Portability and Accountability Act of 1996; (b) the Health Information Technology for Economic
and Clinical Health Act (Title XIII of the American Recovery and Reinvestment Act of 2009); and (c) the Omnibus Rule effective March
26, 2013 (78 Fed. Reg. 5566), and other implementing regulations at 45 C.F.R. Parts 160 and 164 and related binding guidance from the
United States Department of Health and Human Services.
“HSR
Act” means the Hart-Scott Rodino Antitrust Improvements Act of 1976, and rules and regulations promulgated thereunder.
“ICH”
means the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
“IND”
means an investigational new drug application (including any amendment or supplement thereto) submitted to the FDA pursuant to U.S. 21
C.F.R. Part 312, or the comparable application submitted to the applicable Regulatory Authority, including any amendments thereto.
“Independent
Expert” has the meaning set forth in Section 3.3(c)(iii).
“Information
Statement” has the meaning set forth in Section 6.6(b).
“Insurance
Policies” has the meaning set forth in Section 5.1(w).
“Intellectual
Property Rights” means any and all of the following and any and all rights associated with the following, in any jurisdiction
throughout the world, by whatever name or term known or designated, whether arising by operation of Law, Contract, or otherwise:
(a)
Patents;
(b)
Trademarks;
(c)
Domain Names;
(d)
all copyrights, mask work rights, moral rights, and common-law rights thereto, and all applications, registrations, and renewals
in connection therewith throughout the world;
(e)
all rights in databases and data collections;
(f)
all Know-How;
(g)
all similar, corresponding, or equivalent rights to any of the foregoing anywhere in the world; and
(h)
all rights to sue for past, present, or future infringement, violations, or misappropriation of any of the foregoing anywhere in
the world.
“Inventor”
means each of the named inventors of each of the Seller Patents, as well as any inventor who should be or should have been named on each
of the Seller Patents.
“Inventor
Assignment Agreement” means an agreement by Seller or its Affiliate with the respective Inventor assigning all right, title,
and interest to the Seller Patents to Seller or its Affiliate. For clarity, any agreement with an employee or contractor of Seller or
its Affiliate that provides for the general assignment of inventions made in the course of employment by or providing services to Seller
or its Affiliate shall not be an “Inventor Assignment Agreement”.
“Inventory”
means all inventories of active pharmaceutical ingredient for the Compounds and Products, all drug product for the Compounds and Products,
all raw and pack materials, work-in-process, finished goods, Products on stability, reference materials warehoused stock, supplies, consumables
and packaging materials relating to the Product or manufacture thereof.
“IP
and Patent Assignment Agreement” means the IP and Patent Assignment and Assumption Agreement in substantially the form attached
hereto as Exhibit E.
“IQVIA
Agreement” means the Master Services Agreement, dated July 21, 2020, by and between Old Ayala, Inc. (formerly Ayala Pharmaceuticals,
Inc.), a Delaware corporation, and IQVIA Biotech LLC, as amended, together with that certain Amendment No. 2 to Initial Work Order Project
Code: Al-DES-01/WZ95313, by and between Seller and IQVIA RDS Inc., to be executed after the date hereof.
“Key
Personnel” has the meaning set forth in Section 6.12.
“Key
Personnel Agreements” has the meaning set forth in Section 6.12.
“Know-How”
means data, knowledge, trade secrets, know-how, discoveries, inventions and technical, business or other proprietary information, including
materials, samples, manufacturing data, chemistry, manufacturing, and controls information, toxicological data, pharmacological data,
data from preclinical, clinical or non-clinical testing or studies, assays, processes, protocols, procedures, methods, devices, designs,
results, technology, platforms, formulations, regulatory filings, applications, approvals and associated correspondence, specifications,
quality control testing data, customer lists, mailing lists and business plans. “Know-How” shall expressly exclude the subject
matter of the Dropped Patents. Notwithstanding the foregoing, solely with respect to any representation by Seller pursuant to this Agreement
(including but not limited to the representations set forth in Section 5.1(m)), “Know-How” shall expressly exclude
subject matter that is solely claimed in the Dropped Patents. For clarity, subject matter that is claimed in both the Product Patents
and the Dropped Patents shall be included within “Know-How.”
“Knowledge”.
An individual shall be deemed to have “Knowledge” of a particular fact or other matter if such individual is actually
aware of such fact or other matter, after reasonable investigation. Seller shall be deemed to have “Knowledge” of
a particular fact or other matter if any individual set forth on Schedule D has Knowledge of such fact or other matter.
“Laws”
means any United States federal, state and local, and any non-U.S., laws, statutes, regulations, rules, codes or ordinances enacted,
adopted, issued or promulgated by any Governmental Authority (including those pertaining to electrical, building, zoning, environmental
and occupational safety and health requirements) or common law, including GCP, GMP and GLP.
“Legal
Proceeding” means any action, suit, charge, complaint, litigation, arbitration, proceeding (including any civil, criminal,
administrative, investigative or appellate proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted
or heard by or before, or otherwise involving, any court or other Governmental Authority or any arbitrator or arbitration panel.
“Liability”
or “Liabilities” means, with respect to any Person, any debt, duty, liability or obligation of any kind (whether known
or unknown, contingent, accrued, due or to become due, secured or unsecured, matured or otherwise), including accounts payable, royalties
payable, and other reserves, accrued bonuses and commissions, accrued vacation and any other form of leave, termination payment obligations,
employee expense obligations, Taxes (including penalties and interest), and all other liabilities and obligations of such Person or any
of its Subsidiaries or Affiliates, regardless of whether such debts, duties, liabilities or obligations are required to be reflected
on a balance sheet in accordance with GAAP.
“License
Grants” means licenses, sublicenses, or other Contracts (whether royalty bearing or non-royalty bearing) under which rights
in any Product IP have been granted to any Person by any of (a) Seller, (b) any Affiliate of Seller and (c) any other Person that, at
the time of the execution of the license, sublicense, or other Contract, as applicable, is or was an Affiliate of Seller.
“Lien”
means any lien, statutory lien, pledge, mortgage, security interest, claim, encumbrance, restriction on use or transfer, easement, right
of way, option, conditional sale, or other title retention agreement of any kind or nature. In the case of an Intellectual Property Right
that is subject to any in-license or out-license, “Lien” includes the terms and conditions of such in-license or out-license,
as applicable.
“Lock-Up
Period” has the meaning set forth in Section 3.6.
“Lock-Up
Shares” has the meaning set forth in Section 3.6.
“Look-Back
Date” means the date that is three (3) years prior to the date of this Agreement.
“made
available” means that Seller has provided to Purchaser that material in question, on before the second (2nd) Business
Day prior to the date hereof, other than material that was delivered to Purchaser at the request of Purchaser made during such period.
“Mandatory
Registration Statement” has the meaning set forth in Section 10.2(a).
“Material
Adverse Effect” means any event, change, circumstance, occurrence, effect, result, or state of facts that, individually or
in the aggregate, (a) is or would reasonably be expected to be materially adverse to the Acquired Assets, taken as a whole or materially
increase the Assumed Liabilities or (b) materially impairs the ability of Seller to consummate, or prevents or materially delays, the
Closing or would reasonably be expected to do so; provided, however, that any events, changes, circumstances, occurrences,
effects, results, or states of facts resulting from the following items shall not be considered when determining whether a Material Adverse
Effect has occurred: (i) changes in economic, political, regulatory, financial or capital market conditions generally or in the industries
in which Seller operates; (ii) any acts of war, sabotage, terrorist activities or Laws or Orders imposed by a Governmental Authority
associated with national security in response thereto; (iii) effects of weather or meteorological events, any epidemic, pandemic or disease
outbreak, any public health emergency (as declared by the World Health Organization or the Health and Human Services Secretary of the
United States) or other similar acts of God; (iv) any change of Law or accounting standards after the Agreement Date; (v) the announcement
or pendency of this Agreement; and (vi) any failure by Seller to meet projections or forecasts or revenue or earnings predictions for
any period (but, for the purposes of clarity, not the underlying cause of such failure); except in the case of each of clauses (i) through
(iv), with respect to Seller, to the extent disproportionately affecting Seller relative to other similarly situated companies in the
industries in which Seller operates.
“Milestone
Payment” means any Development Milestone Payment or Net Sales Milestone Payment.
“Material
Contracts” has the meaning set forth in Section 5.1(i)(i).
“Net
Sales” means, with respect to any Product, the gross amounts invoiced by Purchaser and its Affiliates for sales of such Product
to unaffiliated Third Parties, including to Distributors, less the following deductions provided to unaffiliated entities, each as actually
allowed and taken and not reimbursed by any Third Party:
(a)
trade, quantity and/or cash discounts, charge-back payments, allowances or rebates actually taken and allowed, including promotional
or similar discounts or rebates and discounts or rebates to wholesalers or other distributors, buying groups, healthcare insurance carriers
or other institutions, managed health care organizations, pharmacy benefit managers (or equivalents thereof), national, state/provincial,
local, and other governments, their agencies and purchasers and reimbursors, or to trade customers (managed care and similar types of
rebates and chargebacks);
(b)
any payment actually made in respect to sales to any governmental authority in respect of any government subsidized program, including
Medicare and Medicaid rebates;
(c)
discounts provided in connection with coupon, voucher or similar patient programs;
(d)
credits or allowances actually given or made with respect to Products by reason of rejection, defects, recalls, returns, rebates
and retroactive price reductions;
(e)
a reasonable allowance for bad debt (not to exceed 2% of Net Sales in the aggregate);
(f)
any tax, tariff, duty or government charge (including any sales, value added, excise or similar tax or government charge, but excluding
any income tax) levied on the sale, transportation or delivery of a Product and borne by the seller thereof, as adjusted for rebates
and refunds, without reimbursement from any Third Party, including that portion of the annual fee on prescription drug manufacturers
imposed by the Patient Protection and Affordable Care Act, Pub. L. No. 111-148 (as amended), that Purchaser or its Affiliates, as applicable,
allocate to sales of such Product in accordance with Purchaser’s or its Affiliates’ standard policies and procedures consistently
applied across its products, as applicable, in each case, to the extent non-creditable or refundable;
(g)
any charges for freight, postage, shipping or transportation, or for insurance, in each case to the extent borne by Purchaser and/or
its Affiliates; and
(h)
any administrative fees paid to group purchasing organizations or managed care entities for the sale of Products.
Notwithstanding
the foregoing, amounts received or invoiced by Purchaser or its Affiliates for the sale of Products among Purchaser and its Affiliates
shall not be included in the computation of Net Sales hereunder. Net Sales shall be accounted for in accordance the selling party’s
standard practices in the relevant country of the sale and in accordance with GAAP.
Notwithstanding
the foregoing, “Net Sales” shall not include any amounts invoiced for sales of Products supplied for use in clinical trials
of Products, or under early access, compassionate use, named patient, indigent access, patient assistance or other reduced pricing programs.
Net
Sales for a Combination Product shall be calculated as follows:
(i)
If the Product and the Other Product(s) in such Combination Product each are sold separately in the same country during the same
calendar year, Net Sales will be calculated by multiplying the total Net Sales (as described above) of the Combination Product by the
fraction A/(A+B), where A is the public or list price in such country and calendar year of the Product sold separately in the same formulation
and dosage, and B is the (sum of the) public or list price(s) in such country and calendar year of the Other Product(s) sold separately
in the same formulation and dosage.
(ii)
If the Product is sold independently of the Other Product(s) in such Combination Product in such country and calendar year, but the
public or list price of the Other Product(s) cannot be determined, Net Sales will be calculated by multiplying the total Net Sales (as
described above) of such Combination Product by the fraction A/C, where A is the public or list price in such country and calendar year
of the Product sold independently and C is the public or list price of the Combination Product in such country.
(iii)
If the Other Product(s) in such Combination Product are sold independently of the Product in such country and calendar year, but
the public or list price of the Product cannot be determined, Net Sales will be calculated by multiplying the total Net Sales (as described
above) of such Combination Product by the fraction [1-B/C], where B is the (sum of the) public or list price(s) in such country and calendar
year of the Other Product(s) and C is the public or list price in such country and calendar year of the Combination Product.
(iv)
If neither the Product nor the Other Product(s) in such Combination Product are sold separately in such country and calendar year,
then Purchaser will determine a calculation of Net Sales for the Combination Product in good faith based on the relative values of the
Product and the Other Product(s).
“NDA”
means (a) a New Drug Application or Biologics License Application, as applicable, for any product requesting permission to market a drug
or biologic, and all supplements or amendments thereto, filed pursuant to the requirements of the FDA, including all documents, data
and other information concerning a product which are reasonably necessary for FDA approval to market a product in the United States and
(b) all equivalents of the foregoing in any jurisdiction outside of the United States.
“Net
Sales Information” has the meaning set forth in Section 3.3(c)(ii).
“Net
Sales Report” has the meaning set forth in Section 3.3(c)(i).
“Nondisclosure
Agreements” means any nondisclosure, confidentiality, or similar agreements in effect as of the date of this Agreement to which
Seller is a party that primarily relate to the Acquired Assets or any Product IP, or any other Compound or Product.
“Non-Paying
Party” has the meaning set forth in Section 6.5(b).
“Non-Scheduled
License Grants” has the meaning set forth in Section 5.1(m)(ii).
“Non-Transferable
Assets” has the meaning set forth in Section 2.4.
“Objection
Notice” has the meaning set forth in Section 3.3(c)(iii).
“Opinion”
has the meaning set forth in Section 5.1(y).
“Order”
means and includes any writ, law, rule, regulation, executive order or decree, judgment, injunction, ruling, or other order, whether
temporary, preliminary, or permanent enacted, issued, promulgated, enforced, or entered into by any Governmental Authority.
“Orderly
Trading Period” has the meaning set forth in Section 3.7.
“Ordinary
Course of Business” means the ordinary course of business of Seller consistent with Seller’s past custom and practice.
“Organizational
Document” means (a) the articles or certificate of incorporation, association, or formation and the bylaws of a corporation;
(b) the operating agreement, limited liability company agreement, or similar document governing a limited liability company; (c) any
charter or similar document adopted or filed in connection with the creation, formation, or organization of a Person; and (d) any amendment
to any of the foregoing.
“Outside
Date” has the meaning set forth in Section 11.1(e).
“Party”
or “Parties” has the meaning set forth in the preamble.
“Patent
Documents” means all (a) prosecution files for each of the Seller Patents and any other Product Patents for which Seller or
its Affiliate is responsible for filing or prosecution; (b) Assignment Agreements and all Inventor Assignment Agreements; and (c) documents,
records, and files in the possession and Control of Seller, its counsel, or its agents with respect to (i) the conception and reduction
to practice (and diligence in reduction to practice) of the inventions of any of the Seller Patents, or (ii) the filing, prosecution,
registration, continuation, continuation-in-part, reissuance, correction, enforcement, defense, and maintenance of the Seller Patents
and any other Product Patents for which Seller or its Affiliate is responsible for filing or prosecution.
“Patents”
means (a) all patents, certificates of invention, applications for certificates of invention, priority patent applications, and patent
applications, and (b) any renewals, divisions, continuations (in whole or in part), or requests for continued examination of any of such
patents, certificates of invention, and patent applications, any and all patents or certificates of invention issuing thereon, and any
and all reissuances, reexaminations, extensions, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions,
and additions of or to any of the foregoing.
“Paying
Party” has the meaning set forth in Section 6.5(b).
“Permits”
means any license, permit, registration, listing, approval, qualification, letter, authorization, certificate of authority, qualification,
NDA, IND, or similar document or authority issued or granted by any Regulatory Authority or pursuant to any Law.
“Permitted
Liens” means (a) Liens for Taxes or similar governmental assessments and charges (i) not yet due and payable, or (ii) that
are being contested in good faith through appropriate proceedings and for which adequate reserves have been established, (b) Liens referred
to in the Acquired Business Contracts, (c) in the case of an Intellectual Property Right that is subject to any non-exclusive in-license
or out-license, the terms and conditions of such in-license or out-license as apparent from the face of such license and the license
is included in any Material Contract set forth on Section 5.1(i)(i) of the Disclosure Schedules, in any Contract set forth on Section
5.1(m)(i) or 5.1(m)(ii) of the Disclosure Schedule, or in any Non-Scheduled License Grant and in each case do not materially impair or
limit the use, value, or marketability of the property which they encumber.
“Person”
means any individual, corporation, partnership, joint venture, limited liability company, association, joint-stock company, trust, unincorporated
organization or Governmental Authority.
“Positive
Topline Data” means data showing that the RINGSIDE Part B Study met its primary endpoint of improving progression-free survival
of patients with progressive desmoid tumors, as assessed by blinded independent central review, demonstrating a statistically significant
improvement for AL102 over placebo as defined by the clinical protocol, specifically a hazard ratio of 0.35 or better.
“Pre-Closing
Tax Period” means any taxable period ending on or before the Closing Date and, in the case of any Straddle Period, the portion
of such period beginning before and ending on the Closing Date as determined by Section 6.5(b).
“Product”
means any product comprised of or containing either of the Compounds, in any form or formulation.
“Product
Information” has the meaning set forth in Section 6.12.
“Product
IP” means any and all (a) Product Patents, (b) Product Know-How and (c) other Intellectual Property Rights Controlled by Seller
or any of its Affiliates that is related to any Compounds or Products but excluding (i) Domain Names and (ii) Trademarks.
“Product
Know-How” means all Know-How Controlled by Seller or any of its Affiliates as of the Closing Date that (a) is used or held
for use in or is necessary or useful for the Product Operations or (b) is necessary or useful for the research, Development, manufacture,
or Commercialization of any Compound or Product, including, for the avoidance of doubt, all of Seller’s and its Affiliates’
Know-How made before the Closing Date that (i) constitutes the composition of matter of any Compound or Product, or a method of manufacturing
or using any Compound or Product, or (ii) relates to a companion diagnostic for any Compound or Product.
“Product
Operations” means the research, Development, manufacture, formulation, testing, use, distribution, marketing, sale, promotion,
and other exploitation of any Compound or Product as conducted by Seller and its Affiliates, and any Affiliates of Seller prior to the
Agreement Date, in each case at any time prior to the Closing Date (except as expressly provided elsewhere in this Agreement). “Product
Operations” shall expressly exclude the subject matter of the Dropped Patents. Notwithstanding the foregoing, solely with respect
to any representation by Seller pursuant to this Agreement (including but not limited to the representations set forth in Section 5.1(m)),
“Product Operations” shall expressly exclude subject matter this is solely claimed in the Dropped Patents. For clarity, subject
matter that is claimed in both the Product Patents and the Dropped Patents shall be included within “Product Operations.”
“Product
Patents” means each of the following, whether or not pending, issued, expired, withdrawn, rejected, canceled, abandoned, or
closed: (a) the Patents listed on Schedule C, and (b) any renewals, divisions, continuations (in whole or in part), or requests
for continued examination of any of such patents, certificates of invention, and patent applications, any and all patents or certificates
of invention issuing thereon, and any and all reissuances, reexaminations, extensions, divisions, renewals, substitutions, confirmations,
registrations, revalidations, revisions, and additions of or to any of the Patents listed on Schedule C. The Product Patents shall
expressly exclude the Dropped Patents.
“Purchaser
Common Stock” means shares of Purchaser’s common stock, $0.0001 par value per share.
“Purchaser
Parties” has the meaning set forth in Section 10.4(b).
“Purchaser
Milestone Representative” has the meaning set forth in Section 3.3(c)(iv).
“Purchaser
SEC Reports” has the meaning set forth in Section 5.2(h)(i).
“Registrable
Shares” has the meaning set forth in Section 10.1(b).
“Registration
Liability Cap” has the meaning set forth in Section 10.4(b).
“Qualified
CMC Expenses” shall mean all costs and expenses payable to the CMOs set forth on Schedule E for CMC related work for
the Products arising from purchase orders or Contracts set forth on Schedule E that are approved by Purchaser (with such approval
not to be unreasonably withheld, delayed or conditioned) in an aggregate amount not to exceed $1,000,000 (unless otherwise approved in
writing by Purchaser), which purchase orders or Contracts shall be included in the Acquired Business Contracts.
“Regulatory
Approval” means, with respect to any Product in any regulatory jurisdiction, approval from the applicable Regulatory Authority
sufficient for the manufacture, distribution, use, marketing, and sale of the Product in such jurisdiction in accordance with applicable
Laws.
“Regulatory
Approval Application” means an application submitted to the applicable Regulatory Authority in pursuit of receiving Regulatory
Approval of a Product in a country, regulatory jurisdiction or region, and all amendments and supplements thereto.
“Regulatory
Authority” means any applicable Governmental Authority involved in the granting and maintenance of Regulatory Approvals in
any country worldwide, or the conduct of clinical investigations, including the FDA in the United States.
“Regulatory
Documentation” means all books, records, files, documents, information and correspondence of Seller or its Affiliates to the
extent (a) relating to the Compounds, Products, Acquired Assets, or Assumed Liabilities, (b) used or held for use in the Product Operations,
or (c) generated in the conduct of the Product Operations, including (i) all records with respect to supply sources; (ii) all market
research data, market intelligence reports, statistical programs (if any) used for marketing and sales research with respect to the Products;
(iii) promotional, advertising and marketing materials, sales forecasting models, medical education materials, sales training materials,
web site content, and advertising and display materials relating to the Products; (iv) all records, including vendor and supplier lists,
research, development and manufacturing records, sampling records, standard operating procedures and batch records, related to the manufacturing
process for any Compounds or Products; (v) all data contained in laboratory notebooks to the extent relating to any Compounds or Products
or relating to the biological, physiological, mechanical or formula properties of any of the foregoing, including data related to preclinical
and clinical trials and investigator brochures, and all statistical programs developed (or modified in a manner to the use or function
thereof) to analyze such data; (vi) all adverse experience reports and files related thereto (including source documentation) and all
periodic adverse experience reports and all data contained in electronic data bases relating to periodic adverse experience reports with
respect to any Compounds or Products; (vii) all analytical and quality control data relating to any Compounds or Products; (viii) all
correspondence, minutes, and other communications with the FDA, any other Regulatory Authority and institutional review board or research
ethics committee relating to any Compounds or Product, and (ix) HIPAA authorizations collected in connection with clinical trials, in
each case owned or held by Seller or any of its Affiliates as of or prior to the Closing other than any Excluded Assets.
“Regulatory
Filings” means any and all regulatory applications, filings, approvals and associated correspondence required or related to
the Development, manufacture or Commercialization of any Compound or Product in any country or jurisdiction, including all INDs relating
to the Compounds and the Products.
“Related
Agreements” means the Bill of Sale, Assignment and Assumption Agreement, the Support Agreements, the CVR Agreement and any
agreement, document, or instrument entered into or delivered in connection with this Agreement and the Transactions.
“Representatives”
means officers, directors, employees, stockholders, partners, members, agents, attorneys, accountants, bankers, advisors and representatives.
“Requisite
Holders” has the meaning set forth in the Recitals.
“Resale
Registration Statement” has the meaning set forth in Section 10.1(a).
“Restriction
Termination Date” has the meaning set forth in Section 10.3.
“RINGSIDE
Part B Study” means the Phase 3/Part B double-blind, placebo-controlled part of the Study of AL102 in Patients with Progressing
Desmoid Tumors (RINGSIDE Part B Study)(NCT04871282) being conducted by or on behalf of Seller.
“Rule
144” means Rule 144 as promulgated by the SEC under the Securities Act, or any similar successor rule that may be promulgated
by the SEC.
“Rule
424” means Rule 424 as promulgated by the SEC under the Securities Act, or any similar successor rule that may be promulgated
by the SEC.
“SEC”
means the United States Securities and Exchange Commission.
“SEC
Restrictions” has the meaning set forth in Section 10.3.
“Securities
Act” means the Securities Act of 1933.
“Seller”
has the meaning set forth in the preamble.
“Seller
Common Stock” means shares of Seller’s common stock, par value $0.001 per share.
“Seller
Financial Statements” has the meaning set forth in Section 5.1(x)(ii).
“Seller
IP” means any and all (a) Product Patents owned by Seller or its Affiliate, (b) Product Know-How owned by Seller or its Affiliate
and (c) other Intellectual Property Rights (including any Domain Names or Trademarks) owned by Seller or any of its Affiliates that is
related to any Compounds or Products.
“Seller
Parties” has the meaning set forth in Section 10.4(a).
“Seller
Patents” means any and all Product Patents owned by Seller or its Affiliate.
“Seller
SEC Reports” means each form, report, schedule, registration statement, definitive proxy statement and other document (together
with all amendments thereof and supplements thereto) filed or furnished by Seller pursuant to the Securities Act or the Exchange Act
with the SEC since January 1, 2023, in each case, as such documents have since the time of their filing been amended or supplemented
as of the date that is two (2) Business Days prior to the Agreement Date.
“Seller
Tax Liabilities” means (i) all Taxes of Seller or its Affiliates, or for which Seller or any of its Affiliates is or are liable
(including as a transferee or successor, or by contract or otherwise by operation of Law), for any taxable period (to the extent not
relating to the Acquired Assets, the Product Operations or the Assumed Liabilities) and for any Pre-Closing Tax Period (to the extent
relating to the Acquired Assets, the Product Operations or the Assumed Liabilities) (including any such Tax of Seller or any of its Affiliates
that becomes a Liability of Purchaser under any common law doctrine of de facto merger or transferee or successor liability or otherwise
by operation of contract or Law), (ii) any Taxes of another Person for which Seller is liable (including under Treasury Regulation Section
1.1502-6 or any similar provision of state, local or non-U.S. applicable Law) as a result of being a member of an affiliated, consolidated,
combined or unitary group for Tax purposes on or before the Closing Date or any similar provision of state, local, or foreign applicable
Law, (iii) any Taxes that arise out of the transactions contemplated by this Agreement (including withholding taxes imposed on payments
under this Agreement and fifty-percent of any Transfer Taxes pursuant to Section 6.5(c)), (iv) any Taxes relating to the Excluded
Assets or Excluded Liabilities for any taxable period; and (v) all Taxes relating to the Acquired Assets or the Assumed Liabilities for
any Pre-Closing Tax Period.
“SOX”
has the meaning set forth in Section 5.1(x)(i).
“Specified
Liabilities” has the meaning set forth in Section 2.2(b).
“Stockholder
Written Consent” has the meaning set forth in Section 6.6(a).
“Straddle
Period” has the meaning set forth in Section 6.5(b).
“Straddle
Period Tax” has the meaning set forth in Section 6.5(b).
“Strategic
Transaction” shall mean any transaction involving: (a) the sale, license, disposition or acquisition of the Acquired Assets;
(b) the issuance, grant, disposition or acquisition of (i) any capital stock or other equity security of Seller or any direct or indirect
subsidiary of Seller, (ii) any option, call, warrant or right (whether or not immediately exercisable) to acquire any capital stock or
other equity security of Seller or any direct or indirect subsidiary of Seller, or (iii) any security, instrument or obligation that
is or may become convertible into or exchangeable for any capital stock or other equity security of Seller or any direct or indirect
Subsidiary of Seller; or (c) a merger, amalgamation, consolidation, share exchange, business combination, sale of substantially all the
assets, reorganization, recapitalization, liquidation, dissolution or other similar transaction involving Seller or any of its direct
and indirect subsidiaries; provided, however, that none of the following shall be deemed to constitute a “Strategic
Transaction”: (A) the grant of stock, stock options or other equity or equity-based compensation, or the granting or payment of
cash bonuses, right to proceeds or other payments, by Seller to its employees and contractors if such grant and/or payment is made in
the Ordinary Course of Business or in connection with the Transactions, (B) the issuance of stock by Seller to its employees and contractors
upon the exercise of outstanding stock options, (C) the issuance of stock by Seller in connection with the exercise by the holders thereof
of any of Seller’s currently outstanding convertible debt and equity securities, and (D) the issuance of securities as contemplated
by the right of certain existing investors of Seller to (1) lend an additional $4.0 million to Seller on the same terms as the loan made
to Seller on November 17, 2023, including warrant coverage, and (2) purchase senior convertible promissory notes of Seller up to an aggregate
amount of $1.458 million, all as described in the Part II, Item 5 of Seller’s Quarterly Report on Form 10-Q for the quarter ended
September 30, 2023.
“Subsidiary”
means with respect to any Person, any other Person (a) of which the initial Person directly or indirectly owns or controls more than
50% of the voting equity interests or has the power to elect or direct the election of a majority of the members of the governing body
of such Person or (b) which is required to be consolidated with such Person under GAAP.
“Tail
Policy” has the meaning set forth in Section 6.10.
“Tax”
or “Taxes” means any and all federal, provincial, territorial, state, municipal, local, foreign or other taxes (including
imposts, rates, levies, assessments, and other charges, in each case in the nature of a tax), including all income, excise, franchise,
gains, capital, real property, goods and services, transfer, value added, gross receipts, windfall profits, severance, ad valorem, personal
property, production, sales, use, license, stamp, documentary stamp, mortgage recording, employment, payroll, social security, unemployment,
disability, estimated or withholding taxes, and all customs and import duties, together with all interest, penalties and additions to
tax (and additional amounts imposed with respect to such amounts).
“Tax
Contest” has the meaning set forth in Section 6.5(d).
“Tax
Return” means all U.S. federal, state, local, provincial and non-U.S. returns, declarations, claims for refunds, forms, statements,
reports, schedules, information returns or similar statements or documents and any amendments thereof (including any related or supporting
information or schedule attached thereto) filed or required to be filed with any applicable Governmental Authority in connection with
the determination, assessment or collection of any Tax.
“Third
Party” means any Person other than a Party hereto or an Affiliate of a Party hereto.
“Trademarks”
means trademarks, trade names, corporate names, service marks, brand names, logos, trade dress, slogans, and other indicia of source
or origin together with all translations, adaptations, derivations, and combinations thereof and including all goodwill associated with
the foregoing and all common-law rights thereto, as well as all applications, registrations and renewals in connection therewith.
“Trading
Day” means any day on which the primary market on which shares of Purchaser Common Stock are listed as open for trading.
“Transactions”
means the transactions contemplated pursuant to this Agreement and the other Related Agreements.
“Transfer
Agent” has the meaning set forth in Section 3.5(c).
“Transfer
Taxes” has the meaning set forth in Section 6.5(c).
“Transferred
Permits” has the meaning set forth in Section 5.1(h).
“U.S.”
means the United States of America and its territories and possessions.
“Written
Consent Delivery Time” has the meaning set forth in Section 6.6(a).
2.
Purchase and Sale of Assets
2.1
Acquired Assets.
(a)
Purchase and Sale. Upon the terms and subject to the conditions set forth in this Agreement, Seller shall cause to be sold, transferred
and conveyed to Purchaser, at the Closing, all of Seller’s and its Subsidiaries’ right, title and interest at the Closing
in the (a) Acquired Assets, free and clear of all Liens other than Permitted Liens, and (b) the Dropped Patents. At the Closing, the
sale, transfer, conveyance, assignment, and delivery of the Acquired Assets and the Dropped Patents will be effected pursuant to the
Assignment and Assumption Agreement and the Bill of Sale. Notwithstanding anything to the contrary contained in this Agreement, the transfer
of the Acquired Assets will not include the assumption by Purchaser of any Liability of Seller related to the Acquired Assets, unless
Purchaser expressly assumes that Liability as an Assumed Liability pursuant to Section 2.2. “Acquired Assets”
means the following properties, assets, and rights of Seller:
(i)
all Seller IP together with (A) any and all goodwill symbolized thereby and associated therewith, (B) any and all rights to royalties,
profits, compensation, license payments, and other payments or remuneration of any kind relating to the Acquired Assets, and (C) any
and all rights to obtain renewals, reissues, reexaminations, supplemental examinations and certificates and extensions of registrations,
exclusivities, or other legal protections pertaining to the Seller IP and all Patent Documents;
(ii)
all rights in, to, and under the Contracts listed on Schedule 2.1(a)(ii) (including the BMS License and any additional Contracts
listed on Schedule 2.1(a)(ii) as updated by Seller and delivered to Purchaser at least five Business Days prior to the anticipated
Closing Date; provided that Purchaser shall have the right to reject inclusion of (i) any Contracts proposed to be listed on the
updated Schedule 2.1(a)(ii) or (ii) any Contracts listed on Schedule 2.1(a)(ii) that were materially amended or under which
material rights were waived after the Agreement Date) (collectively, the “Acquired Business Contracts”);
(iii)
all Inventory;
(iv)
all Regulatory Filings and other Regulatory Documentation, including all copies thereof;
(v)
the Transferred Permits;
(vi)
all chemical or biological materials relating to the Compounds or Products, including all patient samples from clinical trials;
(vii)
all rights, claims, credits (including the $2,480,000.00 credit under the IQVIA Agreement), guaranties, warranties, indemnities,
causes of action or rights of set-off, and other similar rights against Third Parties to the extent relating to or arising from the Compounds,
the Products, the Acquired Assets or the Assumed Liabilities; and
(viii)
Seller’s rights in or to any equipment used in the development or manufacturing the Compounds or Products.
(b)
Seller and Purchaser expressly agree and acknowledge that Purchaser is not acquiring any right, title, or interest in any assets
that are not Acquired Assets (collectively, the “Excluded Assets”).
2.2
Assumed Liabilities.
Upon the terms and subject to the conditions of this Agreement, at the Closing, Purchaser will assume and agree to pay, perform, and
discharge only those Liabilities that are Assumed Liabilities. The assumption of the Assumed Liabilities by Purchaser will be effected
pursuant to the Assignment and Assumption Agreement. For purposes of this Agreement, “Assumed Liabilities” means only the
following liabilities of Seller:
(a)
all Liabilities of Seller and its Affiliates under the Acquired Business Contracts arising on or after the Closing Date, including
Liabilities under the BMS License (but excluding (i) Liabilities resulting from any breach or non-compliance with of any such Acquired
Business Contract by Seller or any of its Affiliates or (ii) Seller Tax Liabilities); and
(b)
any Liability or other obligation expressly set forth on Schedule 2.2(b) in an aggregate amount not to exceed $4,000,000 (the
Liabilities identified in this clause (b), the “Specified Liabilities”).
2.3
Excluded Liabilities. The Parties acknowledge and agree that Purchaser will not, and in no event will Purchaser assume or be required
to pay, perform, or discharge any Liabilities other than the Assumed Liabilities, and that, as between the Parties, Seller shall remain
responsible for all Excluded Liabilities.
2.4 Assets Incapable of Transfer. Notwithstanding
anything herein to the contrary, this Agreement will not constitute an assignment or transfer of, an attempted assignment or transfer
of, or an agreement to effect an assignment or transfer of, Acquired Assets that are not assignable or transferable without the consent
of another Person (that has not been obtained at or prior to Closing) (the “Non-Transferable Assets”), if such assignment
or transfer, attempted assignment or transfer, or agreement would constitute a breach of any Acquired Business Contract in the absence
of such consent; provided, however, that, except as provided in Section 4.2(a)(v), Closing shall occur notwithstanding
the foregoing without any adjustment to the Consideration on account thereof. For a period of 12 months following the Closing Date, Seller
will, at no cost to Purchaser, use commercially reasonable efforts to obtain the consent of such other Person to the assignment or transfer
of any such Non- Transferable Asset to Purchaser in all cases in which such consent is required for such assignment or transfer. Purchaser
will reasonably cooperate with Seller in its efforts to obtain such consents. To the extent any such consent cannot be obtained, Seller
will, at no cost to Purchaser, use commercially reasonable efforts to provide an alternate arrangement reasonably satisfactory to Purchaser
designed to provide to Purchaser the economic benefits intended to be assigned or transferred to Purchaser under the relevant Non- Transferable
Asset; provided, however, that Seller shall not be required to undertake any work or take any action that would constitute
a breach of any such Contract included in a Non-Transferable Asset. Without limiting the generality of the foregoing, the beneficial
interest in and to the Acquired Assets, to the fullest extent permitted by the relevant Contract or Permit and applicable Law, will pass
to Purchaser.
3.
Consideration
3.1 Consideration. In consideration for the
purchase and sale of the Acquired Assets, at the Closing, upon the terms and subject to conditions set forth herein, Purchaser shall
pay the Consideration. The “Consideration” means the sum of (a) the Closing Consideration, (b) the Milestone Payments
and (c) the Assumed Liabilities; provided, in the case of clause (b), such payments shall be made only when and to the extent
they are payable pursuant to the terms of this Agreement.
3.2
Closing Consideration.
(a)
At the Closing, Purchaser shall pay (or cause to be paid) to Seller an amount of immediately available funds equal to (i) $20,000,000,
minus (ii) the Deposit (provided the Deposit has not already been refunded to Purchaser in accordance with Section 6.8),
plus (iii) the aggregate amount of documented Qualified CMC Expenses incurred and paid by Seller prior to Closing as documented
to Purchaser at least two (2) Business Days prior to Closing, minus (iv) the aggregate amount of Excess Liabilities (collectively,
the “Closing Cash Payment”), by wire transfer to one or more accounts provided to Purchaser by Seller no later than
two (2) Business Days prior to the Closing.
(b)
At the Closing, Purchaser shall issue to Seller 2,175,489 unregistered shares of Purchaser Common Stock (the “Shares”,
and together with the Closing Cash Payment, the “Closing Consideration”).
(c)
At the Closing, Purchaser shall assume and agree, on and after the Closing Date, to pay, perform and discharge promptly and fully
when due the Assumed Liabilities.
3.3 Milestone Consideration.
(a) Development Milestone Payments. Subject to Closing and the
other terms and conditions of this Agreement, from and after the Closing, when an event set forth in the table below is achieved (each
such event, a “Development Milestone Event”), Purchaser shall pay or issue (or cause to be paid or issued), as applicable,
to Seller, in accordance with and subject to the terms of this Agreement, the one-time, non-refundable, non-creditable payment equal
to the corresponding amount set forth in the table below (each such payment, a “Development Milestone Payment”):
| Development Milestone Event | |
Development Milestone Payment |
| Public announcement of Positive Topline Data | |
$10,000,000 in cash |
| Grant of NDA Regulatory Approval of a Product by the FDA in a first indication | |
$17,500,000 in cash |
Each
of the Development Milestone Payments shall be payable or issuable only one time, for the first achievement of the corresponding Development
Milestone Event, and no Development Milestone Payment would be due for subsequent or repeated achievements of the same Development Milestone
Event, whether by the same Product or a different Product. Each Development Milestone Payment shall be paid or issued within 45 days
after the achievement of the corresponding Development Milestone Event. In no event shall the total Development Milestone Payments exceed
$27,500,000.
(b)
Net Sales Milestone Payment. Subject to Closing and the other terms and conditions of this Agreement, from and after the Closing,
when an event set forth in the table below is achieved (each such event, a “Net Sales Milestone Event”), Purchaser
shall pay (or cause to be paid) to Seller, in accordance with and subject to the terms of this Agreement, the one-time, non-refundable,
non-creditable payment equal to the corresponding amount set forth in the table below (each such payment, a “Net Sales Milestone
Payment”):
| Net Sales Milestone Event | |
Net Sales Milestone Payment |
| First calendar year in which Annual Net Sales of AL102 exceed $100,000,000 | |
$10,000,000 in cash |
The
Net Sales Milestone Payment set forth above shall be payable only one time, for the first achievement of such Net Sales Milestone Event.
In no event shall the total Net Sales Milestone Payment exceed $10,000,000. The Net Sales Milestone Payment shall be paid within 90 days
after the end of the calendar year in which such Net Sales Milestone Event is achieved.
(c)
Net Sales Reporting.
(i)
Purchaser shall, within 90 days following the date of the first calendar year following the date hereof, and within 90 days following
each calendar year thereafter, provide to Seller a report (each, a “Net Sales Report”) setting forth, in reasonable
detail, the Annual Net Sales of AL102 for each such calendar year, calculated in accordance with the definition of “Net Sales”
as set forth herein; provided that Purchaser’s obligation to prepare a Net Sales Report shall terminate automatically upon payment
of the Net Sales Milestone Payment under this Agreement or the CVR Agreement, as applicable.
(ii)
Purchaser shall keep complete and accurate, in all material respects, books and records of Net Sales of AL102 to the extent required
to calculate the Net Sales Milestone Payment payable hereunder (the “Net Sales Information”) and shall maintain the
Net Sales Information until one year after the last day of the calendar year to which such Net Sales Information relates.
(iii)
Notwithstanding anything to the contrary in this Agreement, Seller shall have 30 days after the receipt of a Net Sales Report pursuant
to Section 3.3(c)(i) to dispute any of the calculations therein by providing written notice thereof (including a reasonably detailed
basis thereof) to Purchaser (such notice, an “Objection Notice”). If the Seller does not deliver an Objection Notice
within such 30-day period, it shall be deemed to have irrevocably consented to the applicable Net Sales Report and the calculations therein.
If the Seller delivers an Objection Notice the Parties shall work in good faith to resolve such dispute. If the Parties are unable to
resolve any such dispute within 30 days after Purchaser’s receipt of an Objection Notice, the matters remaining in dispute shall
be submitted for resolution to a nationally recognized independent accounting firm to be mutually agreed upon by Seller and Purchaser
(such agreed firm, the “Independent Expert”). The Independent Expert’s determination of Net Sales shall be within
the range of values proposed by Purchaser and Seller and the Independent Expert shall make such determination in accordance with the
definition of “Net Sales” and the other applicable terms of this Agreement. The Independent Expert’s decision shall
be final, absent manifest error, and the costs of such Independent Expert shall be borne by the Seller if the Annual Net Sales are determined
by the Independent Expert to be $100,000,000 or less and by Purchaser if the Annual Net Sales are determined by the Independent Expert
to be greater than $100,000,000 and the Independent Expert’s final determination. No later than 30 days after such decision and
in accordance with such decision, Purchaser shall pay any amount of any Milestone Payment owing to Seller in accordance with such decision.
(iv)
In the event that, following the Closing Date, Seller is wound up, liquidated or dissolved, Seller shall be entitled to designate,
by written notice to Purchaser, another Person who shall be reasonably acceptable to Purchaser to enforce its rights and assume its obligations
under this Section 3.3 (the “Purchaser Milestone Representative”), and such Purchaser Milestone Representative
shall be a third-party beneficiary of this Agreement for purposes of this Section 3.3. The Purchaser Milestone Representative
shall agree to be bound to a customary confidentiality agreement in form and substance reasonably satisfactory to Purchaser as a condition
to serving in such capacity.
(d)
Purchaser Diligence. Commencing upon the Closing, Purchaser shall use Diligent Efforts to achieve the Development Milestone Events
set forth in Section 3.3(a) for one Product in one indication in the U.S. Neither Purchaser nor any of its Affiliates shall take
any action the primary purpose of which is to avoid the payment of any Milestone Payment. The Parties intend the express provisions of
this Section 3.3(d) to govern their contractual obligations with respect to the rights and obligations under this Section 3.3(d)
and to supersede any standard of efforts or implied covenant of good faith and fair dealing that might otherwise be imposed by applicable
Law with respect thereto.
(e)
Non-Transferable Right. The right of Seller to receive any amounts with respect to Milestone Payments (i) shall not be evidenced
by a certificate or other instrument, (ii) shall not be assignable or otherwise transferable by Seller other than pursuant to a court
Order, by operation of Law (including a consolidation or merger) or without consideration in connection with the dissolution, liquidation
or termination of any corporation, limited liability company, partnership or other entity and (iii) does not represent any right other
than the right to receive the Milestone Payments pursuant to this Agreement. Any attempted transfer of the right to any amounts with
respect to any such payment by any holder thereof (other than as specifically permitted by the immediately preceding sentence) shall
be null and void. Notwithstanding the foregoing, if Seller’s stockholders approve a plan of dissolution in compliance with Section
6.9 and after the date that is six (6) months following the Closing Date Seller decides to make a pro rata distribution of its rights
to receive all of the remaining Milestone Payments under this Section 3.3 to its stockholders, Seller shall notify Purchaser and
the applicable Parties shall enter into the CVR Agreement. Seller shall distribute one CVR for each share of common stock of Seller that
is outstanding as of the record date for such distribution, which will represent an aggregate right to receive, upon achievement of the
applicable Development Milestone Event or Net Sales Milestone Event, the applicable Milestone Payments under this Agreement to the extent
not paid to Seller prior to the date of distribution of the CVRs. If the Parties enter into the CVR Agreement, then the right of Seller
to receive any Milestone Payments under this Agreement shall be extinguished and fully discharged, and the right to receive the Milestone
Payments shall be held solely by the Holders (as defined in the CVR Agreement). In no event shall Purchaser’s total liability to
pay Milestone Payments under this Agreement and under the CVR Agreement (and any CVRs that are issued pursuant thereto) exceed $37,500,000
in the aggregate.
(f)
Right of Set-Off. Purchaser shall have the right, but not the obligation, to withhold from any unpaid future Milestone Payments due
and payable to Seller the amount of any Excess Liabilities to the extent not already taken into account in the calculation of Closing
Cash Payment pursuant to Section 3.2(a) (i.e., Purchaser may withhold an aggregate amount from any Milestone Payments up
to 100% of the amount of its Excess Liabilities). In order to exercise its right of Set-Off, Purchaser must deliver a notice to Seller
which contains (a) a description and calculation of the amount of Excess Liabilities to be set off against the Milestone Payments, (b)
supporting documentation in respect of such Excess Liabilities.
3.4 Withholding. Purchaser and its agents
(as applicable) shall be entitled to deduct and withhold from any amounts payable or otherwise deliverable pursuant to this Agreement
and the CVR Agreement such amounts as such Person is required to deduct or withhold therefrom under any applicable Laws and shall pay
the amounts withheld to the appropriate Governmental Authority, provided, however, that Purchaser use commercially reasonable
efforts, together with Seller, to reduce or eliminate the amount of any such deduction or withholding. Purchaser acknowledges that it
has no knowledge as of the date hereof, pursuant to current Law, of any obligation to deduct and withhold from any amount otherwise payable
pursuant to this Agreement to Seller provided that the IRS Form W-9 described in Section 4.2(a)(iv) is delivered by Seller. To
the extent such amounts are so deducted or withheld and remitted to the appropriate Governmental Authority, Purchaser will send proof
of Tax payment to Seller on a reasonable and timely basis following such Tax payment. Any amounts deducted and withheld by Purchaser
or its agent from payment to Seller shall be treated for all purposes under this Agreement and the CVR Agreement as having been paid
to Seller.
3.5
Legends.
(a)
The Shares shall be placed in a restrictive class bearing a restrictive legend substantially similar to the following:
THE
SHARES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR ANY STATE SECURITIES LAWS. THEY MAY NOT BE SOLD OR OFFERED
FOR SALE IN THE ABSENCE OF AN EFFECTIVE REGISTRATION STATEMENT AS TO THE SECURITIES UNDER THE SECURITIES ACT AND ANY APPLICABLE STATE
SECURITIES LAW OR AN EXEMPTION FROM SUCH REGISTRATION UNDER THE SECURITIES ACT. THE ISSUER OF THESE SHARES MAY REQUIRE AN OPINION OF
COUNSEL REASONABLY SATISFACTORY TO THE ISSUER THAT SUCH OFFER, SALE OR OTHER TRANSFER OTHERWISE COMPLIES WITH THE SECURITIES ACT AND
ANY APPLICABLE STATE SECURITIES LAWS.
Additionally,
during the Lock-up Period (as defined below), the Lock-up Shares (as defined below) will also bear the restrictive legend in substantially
the following form (and a stop-transfer order may be placed against transfer of the Lock-up Shares):
THE
SALE, PLEDGE, HYPOTHECATION OR TRANSFER OF THESE SECURITIES IS SUBJECT TO THE TERMS AND CONDITIONS OF AN ASSET PURCHASE AGREEMENT DATED
FEBRUARY 5, 2024, BETWEEN IMMUNOME, INC. AND AYALA PHARMACEUTICALS, INC.
(b)
Purchaser shall be entitled to place appropriate legends on the book entries or certificates evidencing any shares of Purchaser Common
Stock to be received in the Transactions by Seller to the extent Seller may be considered an “affiliate” of Purchaser for
purposes of Rules 144 and 145 under the Securities Act, reflecting the restrictions set forth in Rules 144 and 145 and to issue appropriate
stop transfer instructions to the transfer agent for Purchaser Common Stock.
(c)
Subject to receipt from Seller by Purchaser and Purchaser’s transfer agent (the “Transfer Agent”) of customary
representations and other documentation reasonably acceptable to Purchaser and the Transfer Agent in connection therewith, Purchaser
shall remove any legend from the book entry position evidencing the Shares issued hereunder and Purchaser will, if required by the Transfer
Agent, use its commercially reasonable efforts to cause an opinion of Purchaser’s counsel be provided, in a form reasonably acceptable
to the Transfer Agent, to the effect that the removal of such restrictive legends in such circumstances may be effected under the Securities
Act, (i) following the time the Resale Registration Statement is declared effective, or (ii) if such Shares have been sold pursuant to
Rule 144 or any other applicable exemption from the registration requirements of the Securities Act. If restrictive legends are no longer
required for such Shares pursuant to the foregoing, Purchaser shall, in accordance with the provisions of this Section 3.5(c)
and within two (2) Trading Days of any request therefor from Seller accompanied by such customary and reasonably acceptable representations
and other documentation referred to above establishing that restrictive legends are no longer required, deliver to the Transfer Agent
irrevocable instructions to make a new, unlegended entry for such book entry Shares. Notwithstanding the foregoing, (A) Purchaser shall
not be obligated to remove or cause to be removed the Lock-up Legend from the Lock-up Shares prior to the expiration of the Lock-up Period,
and (B) promptly following the one (1) year anniversary of the Closing, Purchaser shall remove any legend from the book entry position
evidencing the Shares. Seller agrees with Purchaser that Seller will only sell Shares in accordance with either the registration requirements
of the Securities Act, including any applicable prospectus delivery requirements, or an exemption therefrom, and that if Shares are sold
pursuant to the Resale Registration Statement, they will be sold in compliance with the plan of distribution set forth therein, and acknowledges
that the removal of the restrictive legend from certificates representing Shares as set forth in this Section 3.5 is predicated
upon Purchaser’s reliance upon this understanding.
3.6
Lock Up. Seller agrees that it will hold and will not sell greater than 50% of the Shares (the “Lock-up
Shares”) (or otherwise make any short sale of, grant any option for the purchase of, or enter into any hedging or similar
transaction with the same economic effect as a sale of the Lock-up Shares) until the date that is six (6) months following the
Closing Date (the “Lock-up Period”). Notwithstanding the foregoing, this Section 3.6 will not preclude (a)
distributions of Lock-up Shares to general or limited partners, members, stockholders, Affiliates or wholly-owned subsidiaries of
Seller or any investment fund or other entity controlled or managed by Seller; provided, in each case, that following any
such transfer such Lock-up Shares will remain subject to the provisions of this Section 3.6, or (b) transfers pursuant to a
bona fide third party tender offer for all outstanding shares of Purchaser Common Stock, merger, consolidation or other similar
transaction made to all holders of Purchaser’s securities involving a change of control of Purchaser (including the entering
into any lock-up, voting or similar agreement pursuant to which Seller may agree to transfer, sell, tender or otherwise dispose of
Lock-up Shares or other such securities in connection with such transaction, or vote any Lock-up Shares or other such securities in
favor of any such transaction); provided that in the event that such tender offer, merger, consolidation or other such
transaction is not completed, the Lock-up Shares shall remain subject to the provisions of this Section 3.6. Following the
expiration of the Lock-up Period, Purchaser shall promptly remove, or cause to be removed, the Lock-up Legend from the Lock-up
Shares.
3.7
Orderly Market. Seller agrees that for one (1) year following the Closing Date (the “Orderly Trading Period”),
for any transfer of Shares, other than a pro rata distribution to the stockholders of Seller in connection with a dissolution of Seller
(which for the avoidance of doubt shall not be prohibited or restricted by this Section 3.7), taking place on any single Trading
Day that exceeds 15% of the average daily trading volume of Purchaser Common Stock on Nasdaq over the five (5) Trading Day period ending
on the Trading Day immediately prior to such Trading Day, to conduct such Share transfer as a block trade or other disposition through
a market participant designated by Purchaser. Notwithstanding the foregoing, this Section 3.7 will not preclude sales pursuant
to a bona fide third party tender offer for all outstanding shares of Purchaser Common Stock, merger, consolidation or other similar
transaction made to all holders of Purchaser’s securities involving a change of control of Purchaser (including the entering into
any lock-up, voting or similar agreement pursuant to which Seller may agree to transfer, sell, tender or otherwise dispose of Shares
or other such securities in connection with such transaction, or vote any Shares or other such securities in favor of any such transaction).
3.8
Rule 144 Reporting. With a view to making available the benefits of certain rules and regulations of the SEC that may permit the
sale of the Closing Shares to the public without registration, the Purchaser agrees to use its commercially reasonable efforts
to:
(a)
make and keep adequate current public information with respect to Purchaser available in accordance with Rule 144;
(b)
file with the SEC in a timely manner all reports and other documents required of Purchaser under the Securities Act and the Exchange
Act at any time after it has become subject to such reporting requirements; and
(c)
so long as Seller owns any Closing Shares, furnish to Seller forthwith upon written request a written statement by Purchaser as to its
compliance with the reporting requirements of Rule 144, and of the Securities Act and the Exchange Act, a copy of the most recent annual
or quarterly report of Seller, and such other reports and documents so filed as Seller may reasonably request in availing itself of any
rule or regulation of the SEC allowing Seller to sell any such securities without registration.
4.1
Closing. Subject to the satisfaction or waiver of the conditions set forth in Article 7 and Article 8, and unless this
Agreement shall have been validly terminated in accordance with Article 10, the consummation of the Transactions, including the
closing of the sale of the Acquired Assets and the assumption of the Assumed Liabilities pursuant to this Agreement (the “Closing”)
shall take place remotely via the electronic exchange of documents and signatures, on a date (no later than the third (3rd)
business day after the satisfaction or waiver of the last of the conditions set forth in Article 7 and Article 8 to be
satisfied, other than those conditions that by their nature are to be satisfied at Closing, but subject to the satisfaction or waiver
of such conditions) to be agreed upon by Purchaser and Seller. For purposes of this Agreement, “Closing Date” means
the date on which the Closing actually takes place.
4.2
Deliveries at Closing.
(a)
Deliveries of Seller. On or before the Closing, Seller shall deliver or caused to be delivered to Purchaser the following:
(i)
a duly executed counterpart of the Assignment and Assumption Agreement;
(ii)
a duly executed counterpart of the Bill of Sale;
(iii)
a duly executed counterpart of the IP and Patent Assignment Agreement;
(iv)
a duly executed and valid IRS Form W-9 from Seller;
(v)
duly executed counterparts of all approvals, consents, and waivers that are listed on Schedule 4.2(a)(v), or evidence reasonably
satisfactory to Purchaser that all notices listed on Schedule 4.2(a)(v) have been delivered, except to the extent waived by Purchaser;
(vi)
evidence, reasonably satisfactory to Purchaser, that all Liens (other than Permitted Liens) relating to any of the Acquired Assets
have been removed;
(vii)
evidence, reasonably satisfactory to Purchaser, that the Tail Policy has been obtained;
(viii)
any letter of transfer required to transfer any Regulatory Filings; and
(ix)
evidence, reasonably satisfactory to Purchaser, regarding amounts owed, and credits available, under the IQVIA Agreement.
(b)
Deliveries of Purchaser. On or before Closing, Purchaser shall deliver or caused to be delivered to Seller the following:
(i)
a duly executed counterpart of the Assignment and Assumption Agreement;
(ii)
a duly executed counterpart of the Bill of Sale;
(iii)
a duly executed counterpart of the IP and Patent Assignment Agreement; and
(iv)
evidence that the notice required under Section 15.4.2 of the BMS License has been duly sent to BMS.
4.3
Possession. At the Closing, Seller shall (a) place Purchaser in actual possession and operating control of all Acquired Assets that
are tangible assets and all Patent Documents; and (b) deliver possession of all remaining Acquired Assets to Purchaser at Closing or
as soon as reasonably practicable following the Closing. Without limiting the generality of the foregoing, Seller, at no additional cost,
shall work with Purchaser to transfer all Acquired Assets stored in electronic form wherever stored, in an agreed upon format as soon
as reasonably feasible following the Closing and shall provide to Purchaser, at no additional cost, access to all such Acquired Assets
as requested by Purchaser, prior to the transfer of such electronic data to Purchaser. Further, Seller, at Seller’s expense, shall
make personnel reasonably available and shall support the transfer of all technology included with the Acquired Assets to Purchaser.
Title to Inventory shall be conveyed by delivery of such Inventory into Purchaser’s possession.
| 5. |
Representations and Warranties |
5.1
Representations and Warranties of Seller. For purposes of this Section 5.1, references to “Seller”
shall refer to Seller and each Subsidiary of Seller. Except as set forth in the disclosure schedules delivered by Seller on the
Agreement Date (the “Disclosure Schedules”), or (b) disclosed in Seller SEC Reports other than disclosures
contained in any part of any Seller SEC Reports entitled “Risk Factors,” “Forward-Looking Statements,”
“Cautionary Statement Regarding Forward Looking Statements,” or “Note Regarding Forward Looking Statements,”
or any other disclosures in any Seller SEC Reports are cautionary, predictive or forward looking in nature), Seller makes the
following representations and warranties to Purchaser as of the Agreement Date and as of the Closing Date (except, in each case, to
the extent such representations and warranties speak expressly as of a different date, and then, as of such date) as
follows:
(a)
Organization and Existence. Seller is a corporation duly organized, validly existing and in good standing under the laws of its jurisdiction
of incorporation. Seller has the requisite corporate power and authority to own, use and operate the Acquired Assets as currently conducted.
Seller is duly qualified or licensed to do business and in good standing as a foreign corporation in each jurisdiction in which the ownership
of the Acquired Assets or the conduct of its business requires such qualification or license, except for those jurisdictions where the
failure to be so qualified or licensed and in good standing has not had and would not reasonably be expected to have, individually or
in the aggregate, a material adverse effect on the Acquired Assets or the Assumed Liabilities, taken as a whole. Seller has delivered
to Purchaser accurate and complete copies of the Organizational Documents of Seller.
(b)
Authority; Binding Nature of Agreements. Seller has all requisite power and authority to enter into this Agreement, the Related Agreements
and the other agreements, instruments, and documents to be executed and delivered in connection herewith and therewith to which Seller
is (or becomes) a party, and, subject to the approval of Seller’s stockholders in accordance with Section 6.6, to consummate
the Transactions. Except as contemplated by Section 6.6, the execution and delivery of this Agreement and the Related Agreements
to which Seller is a party, and the consummation of the Transactions by Seller, including the sale of the Acquired Assets, have been
duly authorized by all necessary corporate action, if required, and, except as contemplated by Section 6.6, no further corporate
or stockholder action is required on the part of Seller to authorize this Agreement or any Related Agreements to which Seller is a party
or the Transactions or for Seller to perform its obligations under this Agreement or any other Related Agreements. This Agreement has
been duly executed and delivered by Seller and the other Related Agreements will be duly executed and delivered by Seller, and, assuming
the due execution and delivery of this Agreement by Purchaser and of the Related Agreements by the counterparties thereto, this Agreement
constitutes, and the Related Agreements when so executed and delivered will each constitute, a valid and legally binding obligation of
Seller, enforceable against it in accordance with their respective terms, except as enforceability may be affected by bankruptcy, insolvency,
fraudulent conveyance, reorganization, moratorium and other similar Laws relating to or affecting creditors’ rights generally,
and general equitable principles (whether considered in a Legal Proceeding in equity or at Law) (the “Enforceability Exception”).
The Stockholder Written Consent, when executed and delivered by the Consenting Stockholders in accordance with Section 6.6, shall
be signed by holders of Seller Common Stock having not less than the minimum number of votes necessary to approve the consummation of
the Transactions by Seller’s stockholders by written consent under applicable Law (including Section 271 of the Delaware General
Corporation Law) and Seller’s Organizational Documents.
(c)
No Conflict. The execution and delivery of this Agreement and each of the Related Agreements to which it is a party does not, and
the consummation of the Transactions will not, conflict with or result in any violation of or default under (with or without notice or
lapse of time, or both) or give rise to, any payment obligation, or a right of termination, cancellation, modification or acceleration
of any obligation or loss of any benefit under (any such event, a “Conflict”) (i) any provision of Seller’s
Organizational Documents, (ii) any Acquired Business Contract, or (iii) any material Law or material Order applicable to the Acquired
Assets, or any Product or Compound, except in the cases of clauses (ii) and (iii), where the Conflict, individually or in the aggregate,
has not been and would not reasonably be expected to be material and adverse to the Acquired Assets, taken as a whole, or that would
not reasonably be expected to prevent, materially impede or materially delay the consummation by Seller of the Transactions. Section
5.1(c) of the Disclosure Schedule sets forth all notices, consents, waivers and approvals of parties to any Acquired Business Contract
that are required thereunder in connection with the Transactions or for any Acquired Business Contract to remain in full force and effect
with modification from and after the Closing. The execution and delivery of this Agreement and the Related Agreements does not, and the
consummation of the Transactions will not result in the creation or imposition of any Lien other than Permitted Liens on the Acquired
Assets. Following the Closing, Purchaser will be permitted to exercise all of its rights under the Acquired Business Contracts without
the payment of any additional amounts or consideration other than ongoing fees, royalties, or payments that Seller would otherwise have
been required to pay pursuant to the terms of such Acquired Business Contracts had the Transactions not occurred.
(d)
Governmental Approvals and Filing. No consent, notice, waiver, approval, order, or authorization of, or registration, declaration,
or filing with, any Governmental Authority is required by, or with respect to, Seller in connection with the execution and delivery of
this Agreement, the Related Agreements or any other agreement to which Seller is a party or the consummation of the Transactions, except
for (i) the filings required under Section 6.6, (ii) any approvals in order to close the sale of the Acquired Assets under this
Agreement, and (iii) any consent, notice, waiver, approval, order, or authorization of, or registration, declaration, or filing that
has not been and would not reasonably be expected to be material and adverse to the Acquired Assets, taken as a whole, or that would
not reasonably be expected to prevent, materially impede or materially delay the consummation by Seller of the Transactions.
(e)
Taxes.
(i)
All income and other material Tax Returns required to be filed by Seller or its Affiliates with respect to the Acquired Assets and
Product Operations have been filed and such Tax Returns are true and correct in all material respects. All Taxes due and owing by Seller
(whether or not shown on a Tax Return) attributable to the Acquired Assets or Product Operations have been paid. No power of attorney
that is currently in effect has been granted by Seller with respect to the Acquired Assets or the Product Operations (other than powers
of attorney granted in the ordinary course of business, such as to a payroll provider).
(ii)
The Seller has (i) complied in all respects with all applicable laws relating to the payment, reporting and withholding of Taxes
(including withholding of Taxes pursuant to Sections 1441, 1442, 1445, 1446, 1471, 1472, and 3406 of the Code or similar provisions under
any foreign Law), (ii) withheld (within the time and in the manner prescribed by applicable Law) from employee wages or consulting compensation
and paid over to the proper Governmental Authorities (or is properly holding for such timely payment) all amounts required to be so withheld
and paid over under all applicable Law, including federal and state income Taxes, Federal Insurance Contribution Act, Medicare, Federal
Unemployment Tax Act, relevant state income and employment tax withholding Laws, and (iii) timely filed all withholding Tax Returns,
for all periods through and including the Closing, to the extent any such matter described in sub-clauses (i), (ii) or (iii) of this
clause (e)(ii) relates to the Acquired Assets or the Product Operations.
(iii)
There are no Liens for unpaid Taxes on the Acquired Assets (other than Liens for Taxes not yet due and payable). There is no Tax
Contest pending or, to the Knowledge of Seller, threatened, that relates to the Acquired Assets.
(iv)
Seller is not a “foreign person” within the meaning of Sections 1445 or 1446 of the Code. Seller is and always has been
properly treated as a domestic corporation for U.S. federal and applicable state and local income Tax purposes.
(v)
The Acquired Assets do not include any tax-sharing agreements, United States real property assets, or stock or other ownership interests
in any corporations, partnerships, joint ventures, limited liability companies, business trusts, or other entities.
(vi)
None of the Acquired Assets are (i) property required to be treated as being owned by another Person pursuant to the provisions of
Section 168(f)(8) of the Internal Revenue Code of 1954, as amended and in effect immediately prior to the enactment of the Tax Reform
Act of 1986, (ii) “tax-exempt use property” within the meaning of Section 168(h)(1) of the Code, (iii) “tax-exempt
bond financed property” within the meaning of Section 168(g) of the Code, (iv) subject to Section 168(g)(1)(A) of the Code, or
(v) subject to a “section 467 rental agreement” as defined in Section 467 of the Code.
(vii)
No jurisdiction in which Seller does not file Tax Returns in respect of the Acquired Assets, or the Product Operations, has ever
asserted in writing that Seller may be required to file a Tax Return in such jurisdiction with respect to the Acquired Assets or the
Product Operations. No Taxes have attached to the Acquired Assets and will become a liability of Purchaser as a result of the execution
of this Agreement and purchase of the Acquired Assets.
(viii)
Seller has not received any written notice from any Governmental Authority of any Tax deficiency, Tax examination, or other Tax proceeding
that relates to Taxes attributable to the Product Operations or the Acquired Assets, which deficiency, examination, or proceeding has
not been resolved in full. Seller has not waived any statute of limitations in respect of Taxes attributable to the Product Operations
or the Acquired Assets, which waiver is currently in effect.
(f)
Title. None of the Acquired Assets is subject to any Liens (including Tax-related Liens), other than Liens of the type described
in clauses (a)(i) and (c) of the definition of Permitted Liens. Seller has good and marketable title to all of the Acquired Assets (whether
real or personal, tangible or intangible) and will transfer to Purchaser good and marketable title to all Acquired Assets at the Closing,
free and clear of any Liens other than Permitted Liens. Following the Closing, no Affiliate of Seller will have title to or rights under
any of the Acquired Assets.
(g)
Compliance with Laws.
(i)
Seller is in compliance in all material respects with, and since the Look-Back Date has complied in all material respects with, all
Laws and Orders applicable to the Acquired Assets and the conduct of the Product Operations;
(ii)
Seller, and to the Knowledge of Seller, its Affiliates and each Person acting on their behalf, is and since the Look-Back Date has
been, in compliance, in all material respects, with (A) all applicable Laws relating to the privacy, data protection and security of
any personal information, including HIPAA, (B) all privacy, data protection and security policies of Seller concerning patient medical
records and other personal information, and (C) any contractual requirements to which Seller and its Affiliates are subject that relate
to any of the foregoing. The execution, delivery and performance of this Agreement and the consummation of the Transactions will comply,
in all material respects, with all applicable (1) Laws, (2) policies of Seller concerning patient medical records and other personal
information, and (3) contractual requirements to which Seller or its Affiliates are subject, in each case (1) through (3) relating to
privacy, data protection and security of personal information; and
(iii)
Since the Look-Back Date, Seller has received no written notification or communication from any Governmental Authority (A) asserting
that Seller is not in compliance with any Law or Order with respect to the Acquired Assets or (B) threatening to revoke or suspend any
Transferred Permit owned or held by Seller relating to the Acquired Assets or the Development, manufacturing of Commercialization of
any Products or Compounds.
(h)
Permits. Section 5.1(h) of the Disclosure Schedules contains a complete list of all material Permits that constitute Acquired Assets
(the “Transferred Permits”). Seller possesses all such Transferred Permits, and each such Transferred Permit is validly
and presently in effect (and the continuing validity and effectiveness of such Transferred Permit will not be affected by the consummation
of the Closing), and Seller is not in default (with or without notice or lapse of time, or both) under any Transferred Permit in any
material respect. There are no Legal Proceedings pending, nor to the Knowledge of Seller, threatened, that seek the revocation, cancellation,
suspension, failure to renew or materially adverse modification of any such Transferred Permit. Since the Look-Back Date, all required
filings with respect to each such Transferred Permit have been timely made and all required applications for renewal thereof have been
timely filed.
(i)
Material Contracts.
(i)
Section 5.1(i)(i) of the Disclosure Schedules sets forth a true and accurate list of each Contract (other than purchase orders issued
by Seller to a Third Party that are ancillary to another written Contract with the same Third Party and that do not constitute an Assumed
Liability) in effect as of the date of this Agreement to which Seller is a party or which was entered into by or on behalf of Seller,
or by which any of the Acquired Assets is bound in the following categories (the “Material Contracts”):
(A)
any Contract establishing a joint venture or collaboration, co-promotion or like arrangement, or involving a sharing with another
Person of profits, losses, costs, royalties, milestone payments, or Liabilities of Seller relating to the Acquired Assets or the Development,
manufacture, or Commercialization of any Compound or Product, including the conduct of any clinical trials;
(B)
any Contract containing covenants prohibiting or limiting the right to compete or engage in any aspect of the Product Operations
or prohibiting or restricting Seller’s ability to conduct the Product Operations with any Person or in any geographical area;
(C)
any Contract granting most favored nation or exclusive rights relating to any Compound or Product to any other Person;
(D)
any Contract pursuant to which Seller has obtained or granted any Intellectual Property Rights included in the Acquired Assets (or
that would have been included in the Acquired Assets but for such Contract), including any covenant not to enforce or assert, including
any existing license agreement relating to any Compound or Product or the Product Operations and each other Contract under which Seller
is a licensor or licensee of any Intellectual Property Rights relating to any Compound or Product or the Product Operations other than
any of the following entered into in the Ordinary Course of Business and, in each case, that are not Acquired Business Contracts and
are deemed Excluded Liabilities: (i) Nondisclosure Agreements; (ii) services agreements containing non-exclusive licenses to Intellectual
Property Rights included in the Acquired Assets for the sole purpose of a service provider performing services for or on behalf of Seller;
(iii) agreements with clinical investigators and clinical sites for the conduct of a clinical study, which study is complete or substantially
complete at the relevant clinical sites as of the date of this Agreement; (iv) licenses to commercially available software or cloud or
software as a service agreements; and (v) assignment agreements with employees, including proprietary information and invention assignment
agreements with employees;
(E)
any Contract under which Seller pays or receives milestone or royalty payments relating to any Compound or Product or any Product
IP;
(F)
any Contract relating to the creation of Liens on any Acquired Assets or the guarantee of the payment of Liabilities or performance
of obligations of any other Person by Seller relating to any Compound or Product or any Acquired Assets;
(G)
any Contract entered into by Seller or any of its Affiliates in settlement of any Legal Proceeding or other dispute relating to the
Acquired Assets or the Product Operations, including the conduct of any clinical trials;
(H)
any Contract that limits Seller’s ability to make generally available any versions of any Compound or Product developed by
or for Seller;
(I)
any Contract for the research or Development of any Compound or Product, other than any of the following entered into in the Ordinary
Course of Business and, in each case that are not Acquired Business Contracts and are deemed Excluded Liabilities: (i) standalone indemnity
arrangements with clinical trial sites or clinical trial investigators; (ii) powers of attorney, letters of delegation, declarations
and similar instruments executed by Seller in connection with the regulatory and ethics committee submissions and data processing activities
for clinical studies outside of the U.S. (other than local representative agreements and legal representative agreements or similar arrangements
for local representation entered into with a contract research organization or similar service provider); (iii) Nondisclosure Agreements;
(iv) licenses to commercially available software or cloud or software as a service agreements; and (v) service agreements with quality
assurance auditors, meeting planners, Third Parties providing meeting support services, and non-physician advisory board participants
(i.e. nurse advisors);
(J)
any Contract for the development, manufacture, supply, packaging, labeling, distribution, analytical testing, or storage of the active
pharmaceutical ingredients and other raw materials for any Compound or Product, and related quality agreements;
(K)
any Contract for the ongoing or planned analytical testing or storage of biological specimens collected from subjects participating
in clinical trials of any Compound or Product;
(L)
any Contract for the distribution, promotion, marketing, reselling or other Commercialization of any Compound or Product;
(M)
any Contract for the maintenance of the safety database for any Compound or Product, and any safety data exchange agreements or pharmacovigilance
agreements related to any Compound or Product;
(N)
any Contract with any Governmental Authority relating to any Compound or Product or any of the Acquired Assets, other than clinical
trial agreements and related ancillary agreements with public institutions; and
(O)
any other Contract that is material to the Development, manufacture or sale of any Compound or Product, in each case, as currently
conducted by Seller, other than any Contract relating to (i) real property, (ii) employees, or employee compensation or benefit matters,
including any Employee Benefit Plan, (iii) indebtedness, other than indebtedness associated with any Lien on any Acquired Asset, (iv)
general administration expenses, or (v) insurance.
(ii)
All of the Material Contracts are valid and binding agreements of Seller, enforceable in accordance with their terms, subject to
the Enforceability Exception. Other than Material Contracts entered into on behalf of Seller, Seller has made available or delivered
to Purchaser a correct and complete copy of each written Material Contract. Seller is not in material breach or material default of any
of the Material Contracts or Nondisclosure Agreements, and no event has occurred that with notice or lapse of time, or both, would constitute
a material default by Seller under any Material Contract. To the Knowledge of Seller, no other party to a Material Contract is in material
breach or material default of such Material Contract and no event has occurred that with notice or lapse of time, or both, would constitute
a material default by such other party under any Material Contract or Nondisclosure Agreement. No party has repudiated in writing or,
to the Knowledge of Seller, otherwise provided notice of its intention to repudiate any provision of a Material Contract or Nondisclosure
Agreement. Seller has not given to or received from any other Person any written, or to the Knowledge of Seller other, notice regarding
any material violation or breach of, or default under, any Material Contract or Nondisclosure Agreement.
(iii)
As of the date hereof, (A) the Liabilities of Seller relating to the IQVIA Agreement are $3,800,000, which amount is net of all deposits
(including the deposit referenced in clause (B)), advances and prepayments, and (B) Seller has a credit available under the IQVIA Agreement
in an amount equal to $2,480,000.
(iv)
Section 5.1(i)(iv) of the Disclosure Schedules sets forth an accurate and complete list of all outstanding accrued trade payables
under the Acquired Business Contracts as of the date hereof.
(j)
Inventory. Section 5.1(j) of the Disclosure Schedule lists Seller’s clinical supply Inventory as of January 30, 2024. All of
the Inventory has been manufactured, stored and otherwise maintained in accordance with Seller’s manufacturing practices and with
applicable Laws, in each case, in all material respects, and is in usable condition for the Development of any Product or Compound as
intended, subject to its shelf life. Other than as set forth on Section 5.1(j) of the Disclosure Schedule, the Inventory is in a quantity
with a shelf-life and with available back-up supply that is sufficient for Seller to conduct and complete the RINGSIDE Part B Study in
the Ordinary Course of Business.
(k)
Legal Proceedings.
(i)
As of the Agreement Date, there are no Legal Proceedings pending against or, to the Knowledge of Seller, threatened, pertaining to
the Product Operations, any Compound or Product, or any of the Acquired Assets, and there is no investigation (internal or external),
audit or other Legal Proceeding pending or, to the Knowledge of Seller, threatened, pertaining to the Product Operations, any Compound
or Product, or any of the Acquired Assets or any of the executive officers or directors of Seller, in each case, in relation to any Compound
or Product or any of the Acquired Assets, by or before any Governmental Authority.
(ii)
There is no Order to which Seller is subject or that is pending or, to the Knowledge of Seller, threatened that relates to the Acquired
Assets or the Assumed Liabilities.
(l)
Regulatory Compliance.
(i)
Since the Look-Back Date, Seller has not been in violation of, and is not the subject of any Legal Proceeding with respect to the
violation of, any Law or Order, and has not received any FDA Form 483, “warning letters”, or “untitled letters”,
or other similar Governmental Authority notice of inspectional observations or deficiencies relating to the Product Operations. Since
the Look-Back Date, no Compound or Product has been subject to any import detention or refusal by the FDA or other similar Regulatory
Authority or any safety alert issued by the FDA or other similar Regulatory Authority. No Legal Proceeding is pending, or to the Knowledge
of Seller, threatened, with respect to any violation of any Law or Order by Seller, or to the Knowledge of Seller, by representative
agent, contract manufacturing organization, contract research organization, clinical investigator or clinical trial site acting on behalf
of Seller, and Seller is and since the Look-Back Date, has been in compliance in all material respects with all Laws and Orders, in each
case pertaining to the Product Operations. Since the Look-Back Date, Seller has not received any notice of any such Legal Proceeding
or any Liability on the part of Seller to undertake or to bear all or any portion of the cost of any Product Operations remedial action
of any nature. Seller has filed, maintained or submitted all material reports, documents, forms, notices, applications, records, claims,
submissions and supplements or amendments as required by any Law or Permit for the Product Operations and all such reports, documents,
forms, notices, applications, records, claims, submissions and supplements or amendments were materially complete and accurate on the
date filed (or were corrected or supplemented by a subsequent submission).
(ii)
Except to the extent not required to be conducted in accordance with GMP, all manufacturing operations and the manufacture of any
Products and Compounds by, or on behalf of, Seller are being conducted and since the Look-Back Date, have been conducted, in material
compliance with applicable Laws and Orders and in accordance with GMP. The processes used to produce the Compounds and Products are adequate
to ensure that the Compounds and Products will conform to the specifications established therefor at the time of production. Since the
Look-Back Date, Seller has not received any material written complaints about the Compounds or Products. Since the Look-Back Date, Seller
has not conducted any recoveries or recalls of Products or Compounds.
(iii)
Since the Look-Back Date, all preclinical testing and clinical trials in respect of the Product Operations conducted by or on behalf
of Seller have been conducted in all material respects in accordance with experimental protocols, procedures, and controls, as well as
pursuant to applicable Laws (including GCPs and GLPs) and Orders, as applicable. Since the Look-Back Date, no clinical trial in respect
of any Product or Compound has been placed on clinical hold, terminated, materially delayed, limited or suspended prior to completion
by the FDA or any similar Regulatory Authority, or by any institutional review board that has or has had jurisdiction over such clinical
trial, and neither the FDA nor any other applicable Regulatory Authority, nor any institutional review board that has or has had jurisdiction
over such clinical trial has ordered or commenced, or, to the Knowledge of Seller, threatened to initiate, any such action or alleged
any violation of any Law in connection with any such clinical trial. Seller has provided Purchaser with true and correct copies of final
study reports of all clinical trials in respect of any Products or Compounds that were completed prior to the Agreement Date.
(iv)
All human clinical trials conducted by or on behalf of Seller that are intended to be submitted to Governmental Authorities to support
regulatory approval of the Compounds and Products are being conducted in compliance in all material respects with applicable GCP regulations
and guidance, and all applicable Laws relating to protection of human subjects, including those contained in 21 CFR Parts 50, 54, 56
and 312, and any comparable state and foreign Laws. All required approvals and authorizations for clinical trials to proceed have been
obtained from an appropriate institutional review board, and informed consent, in material compliance with applicable Laws, has been
obtained from all subjects enrolled in the studies.
(v)
Since the Look-Back Date, Seller has complied in all material respects with all adverse event reporting requirements applicable to
the Products and Compounds. Section 5.1(l)(v) of the Disclosure Schedules identifies all serious adverse events and suspected adverse
reactions that occurred during any clinical trial in respect of the Product Operations, as well as any serious and unexpected suspected
adverse reactions that were the subject of an IND safety report.
(vi)
Seller is not the subject of any pending or, to the Knowledge of Seller, threatened investigation by any Governmental Authority in
respect of Seller or the Product Operations, including by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts,
Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto or
any other Governmental Authority that has jurisdiction over the operations of Seller under any similar policy. Neither Seller nor, to
the Knowledge of Seller, any of its officers, employees, or agents acting for Seller, has since the Look-Back Date committed any act,
made any statement or failed to make any statement, relating to the Product Operations that would reasonably be expected to provide a
basis for the FDA to invoke its policy with respect to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities”
set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. Since the Look-Back Date, neither Seller nor any current
or, to the Knowledge of Seller, former officer or employee, or, to the Knowledge of Seller, agent of Seller with respect to the Product
Operations has been convicted of any crime or engaged in any conduct that would reasonably be expected to result in exclusion under 42
U.S.C. Section 1320a-7 or any similar state law or regulation or been debarred by the FDA under Article 306 of the FDC Act, 21 U.S.C.
§335a(a) or (b), or any similar foreign or local law, rule, or regulation.
(vii)
The Compounds and Products are being and have been researched, Developed, manufactured, labeled, stored, and tested in compliance
in all material respects with all applicable requirements under the FDC Act, the Public Health Service Act, and their applicable implementing
regulations, and all comparable state and foreign Laws in those jurisdictions outside the United States in which either (A) human clinical
trials involving the Compounds and Products have been or are being conducted by Seller or (B) the Compounds and Products have been or
are being manufactured by or for Seller.
(viii)
Neither Seller nor, to the Knowledge of Seller, any representative agent, contract manufacturing organization, contract research
organization, clinical investigator or clinical trial site acting on behalf of Seller nor any of its licensors, licensees or assignees
of Product IP has received any written notice that the FDA or any other Regulatory Authority has initiated, or threatened to initiate,
any action to suspend any clinical trial sponsored by Seller with respect to the Compounds and Products, or to recall or suspend the
manufacture of the Compounds and Products.
(ix)
Seller has made available to Purchaser copies of any and all written notices of inspectional observations, establishment inspection
reports, and any other documents received by Seller since the Look-Back Date and prior to the date of this Agreement from the FDA or
comparable foreign Regulatory Authorities that identify lack of compliance with Laws of the FDA or comparable foreign Regulatory Authorities
with respect to Product Operations.
(x)
There are no proceedings pending or, to the Knowledge of Seller, threatened, relating to the Product Operations with respect to a
violation by Seller or by any representative, agent, contract manufacturing organization, contract research organization, clinical investigator
or clinical trial site acting on behalf of Seller, of the FDC Act, FDA regulations adopted thereunder, the Controlled Substances Act,
federal or state whistleblower statutes, or any other Law promulgated by any other Governmental Authorities.
(m)
Intellectual Property.
(i)
Section 5.1(m)(i) of the Disclosure Schedules identifies (A) all registered Product IP, (B) all applications for the registration
of Product IP, and (C) all licenses, sublicenses, and other Contracts (excluding any agreement with an employee or contractor of Seller
or its Affiliate in the Ordinary Course of Business that provides for the general assignment of inventions made in the course of employment
by or providing services to Seller or its Affiliate) (whether royalty bearing or non-royalty bearing) under which Seller is granted rights
by others in any Product IP.
(ii)
Section 5.1(m)(ii) of the Disclosure Schedules identifies all License Grants in the Product IP, other than non-exclusive License
Grants included in services agreements entered into in the Ordinary Course of Business for the sole purpose of a service provider performing
services for or on behalf of Seller (“Non-Scheduled License Grants”) and confidentiality agreements entered into in
the Ordinary Course of Business.
(iii)
Except for the rights identified in Section 5.1(m)(i)(C) of the Disclosure Schedules and subject to the License Grants identified
in Section 5.1(m)(ii) of the Disclosure Schedules, Seller owns and possesses all right, title, and interest in and to, free and clear
of all Liens (other than Permitted Liens), all of the Seller IP. There are no Intellectual Property Rights and there is no Know-How owned
or licensed (including by sublicense, covenant-not-to-assert, or any similar right or authorization) that are necessary to the Product
Operations other than the Product IP. All previously due fees associated with maintaining any Seller IP or other Product IP for which
Seller is responsible for filing, prosecution or maintenance have been paid in full in a timely manner to the proper Governmental Authority,
except where Seller has decided, to the extent described in Section 5.1(m)(iii) of the Disclosure Schedules, abandon or cancel such Product
IP (including any Intellectual Property Rights that would have been Product IP but for such abandonment or cancelation). Upon the Closing,
all of the Product IP shall be available for use by Purchaser on terms and conditions substantially identical to those under which Seller
owned or used the Product IP immediately prior to the Closing. To the Knowledge of Seller, no Product IP is subject to any outstanding
consent or Order restricting the use thereof.
(iv)
Seller has not received any written claim by any Third Party and, to Seller’s Knowledge, there is no claim threatened against
Seller contesting the validity, enforceability, or ownership of any Product IP. The validity or enforceability of registered Product
IP or rights of Seller to use Product IP has not been challenged in any jurisdiction. Each item of registered Product IP is valid, subsisting,
in full force and effect and, to the Knowledge of Seller, enforceable; and has not been cancelled, expired, or abandoned except where
Seller has decided to abandon or cancel such Product IP to the extent described in Section 4.1(m)(iii) of the Disclosure Schedules. No
claim is pending or, to Seller’s Knowledge, threatened challenging Seller’s right to any Product IP or right to use any Product
IP. No claim is pending or, to Seller’s Knowledge, threatened to the effect that any registered Product IP is, or upon consummation
of the Transactions will be, invalid or unenforceable.
(v)
To the Knowledge of Seller, Seller has not infringed, misappropriated, diluted, violated, or otherwise conflicted with any Intellectual
Property Rights of any Third Party in conducting the Product Operations. To the Knowledge of Seller, the Product Operations, including
the use of the Compounds and Products, the manufacture, use, sale, and importation of the Compounds and Products, the possession, use,
disclosure, copying, or distribution of any information, data, or other tangible or intangible assets in the possession of Seller regarding
any Compound or Product included in the Acquired Assets, and the possession or use by Seller of the Product IP, has not and does not;
and, if possessed and used in the same manner as Seller has in conducting the Product Operations, will not infringe, misappropriate,
dilute, violate, or otherwise conflict with any Intellectual Property Rights of any other Person. Seller has not received any written
notice of any claim (including by an offer to license any Intellectual Property Rights) and Seller has not received any written claim
and, to Seller’s Knowledge, there is no threatened claim against Seller asserting that the Product Operations, or the manufacture,
use, sale, and importation of any Compound or Product, may infringe, misappropriate, dilute, violate, or otherwise conflict with the
Intellectual Property Rights of any Person.
(vi)
To Seller’s Knowledge, none of the Product IP is being infringed by any Third Party other than by the License Grants as set
forth on Section 5.1(m)(ii) of the Disclosure Schedules, the Non-Scheduled License Grants or confidentiality agreements entered into
in the Ordinary Course of Business. Seller has not given any notice to any Person asserting infringement, misappropriation, dilution,
violation or conflict by any such Person of any of the Product IP. There are no pending, or, to Seller’s Knowledge, threatened
or potential, claims of infringement, misappropriation, dilution, conflict, or violation of the Product IP by any Person.
(vii)
Seller has obtained from all individuals who participated in any respect in the invention or authorship of any Seller IP effective
written assignments of all ownership rights of such individuals in such Seller IP, and, to Seller’s Knowledge, no Person who as
of the Agreement Date claims to be an inventor of an invention claimed in any registered Seller IP is not identified as an inventor of
such invention in the filed patent documents for such registered Seller IP.
(viii)
Seller and its Affiliates have taken all reasonable measures to protect the secrecy, confidentiality, and value of all Product Know-How
that constitutes trade secrets under applicable Laws (including requiring all employees, consultants, and independent contractors to
execute agreements requiring all such employees, consultants, and independent contractors to maintain the confidentiality of such Product
Know-How) and to Seller’s Knowledge, such Product Know-How has not been used by, or disclosed to, any Third Party except pursuant
to such confidentiality agreements and there has not been a breach by any party to such confidentiality agreements.
(ix)
Neither Seller nor any of its Affiliates has entered into a government funding relationship that would result in any payment obligations
to any Governmental Authority or any rights to any Product or Acquired Asset residing in any Governmental Authority and neither the Product
nor the Product Operations are subject to overriding obligations to the United States federal government as set forth in Public Law 96
517 (35 U.S.C. § 200 -204), or any similar obligations under the applicable Laws of any state or other country.
(x)
To Seller’s Knowledge, the Product Patents constitute all Patents covering any Compounds or Products that are Controlled by
Seller.
(xi)
Seller and its Affiliates have the exclusive right to use all data generated in the course of, or as a result of, any clinical trial
or other testing in humans conducted by or on behalf of Seller in respect of any of the Compounds or Products, except for (A) non-exclusive
research licenses granted in the Ordinary Course of Business to a Third Party university or institution that generated such data for
non-commercial, medical and academic purposes, or to Third Party service providers or vendors solely to enable the performance of services
on Seller’s behalf, or (B) data that constitutes or is included in the medical records of any individual.
(xii)
Seller is not bound by any Contract to indemnify, defend, hold harmless, or reimburse any other Person with respect to, or otherwise
assumed or agreed to discharge or otherwise take responsibility for, any existing intellectual property infringement, misappropriation,
or similar claim.
(n)
No Brokers. No broker, finder, or investment banker is entitled to any brokerage commission, finder’s fee or similar payment
in connection with the Transactions based upon arrangements made by or on behalf of Seller.
(o)
Affiliate Transactions. Neither Seller nor any of its Affiliates, nor, to the Knowledge of Seller, any current director, officer,
employee or any beneficial owner of 5% or more of Seller’s outstanding capital stock of Seller, (i) has any direct or indirect
financial interest (excluding in their capacity as an employee or stockholder of Seller) (A) in, or is a director or officer of, any
Person that is a material client, customer, supplier, lessor, lessee, debtor, creditor or competitor of Seller in respect of the Acquired
Business Contracts or (B) in any material property, asset, or right that is owned or used by or on behalf of Seller exclusively in the
conduct of the Product Operations or (ii) is, or since the Look-Back Date has been, a party to any Acquired Business Contract.
(p)
Certain Product Operations Activities. Since the Look-Back Date, neither Seller nor any of its officers, directors, and to the Knowledge
of Seller, its employees, agents or representatives, or any Affiliate of or any Person associated with or acting for or on behalf of
Seller, has directly or indirectly, acting for or on behalf of Seller, in each case, in connection with the Product Operations:
(i)
used any funds for unlawful contributions, gifts, or entertainment or other unlawful payments, including having made or attempted
to make any improper contribution or gift, bribe, rebate, payoff, influence payment, kickback, or other improper payment to any Person,
private or public, regardless of what form, whether in money, property, or services to (A) obtain favorable treatment for business or
Contracts secured, (B) pay for favorable treatment for business or Contracts secured, or (C) obtain special concessions or for special
concessions already obtained, in each of clauses (A), (B), and (C) in violation of any requirement of applicable Law;
(ii)
made or attempted to make any such contribution or gift, bribe, rebate, payoff, influence payment, kickback, or other improper payment
in violation of any applicable written policy of Seller;
(iii)
established or maintained any fund or asset for the purpose of making any such contribution or gift, bribe, rebate, payoff, influence
payment, kickback, or other improper payment in violation of any applicable Law or applicable written policy of Seller and which Seller
or any of its officers, directors, or employees has willfully failed to record in the Regulatory Documentation. To the extent required
by applicable Law, Seller has established and maintains a compliance program and reasonable internal controls and procedures that, for
all periods prior to the Closing, were appropriate to satisfy the requirements of applicable Anti-Corruption Laws; or
(iv)
consummated any transaction, made any payment, entered into any contract or arrangement or taken any other action in violation of
Section 1128B(b) of the U.S. Social Security Act.
(q)
No Government Officials. To the Knowledge of Seller, no officer or director of Seller is or since the Look-Back Date has been a foreign
or domestic government official or employee or a candidate for any foreign or domestic political office.
(r)
Books and Records. Since the Look-Back Date, Seller has made and kept its books and records, including the Regulatory Documentation
and the Patent Documents, which, in reasonable detail, accurately and fairly reflect the activities of the Product Operations in all
material respects. Since the Look-Back Date, Seller has not engaged in any material transaction, maintained any bank account, or used
any corporate funds in connection with the Product Operations, except as reflected in its normally maintained books and records.
(s)
Solvency. Seller currently owns property that has a fair saleable value greater than the amounts required to pay its debts (including
a reasonable estimate of the amount of all contingent liabilities). No transfer of property is being made and no obligation is being
incurred in connection with the Transactions with the intent to hinder, delay or defraud either present or future creditors of Seller.
(t)
Sufficiency of Acquired Assets. The Acquired Assets are sufficient for the continued conduct of the Product Operations by Purchaser
after the Closing Date in substantially the same manner as currently conducted by Seller and constitute all of the material rights, property,
and assets necessary to conduct the Product Operations as currently conducted by Seller, in each case, other than employees, contractors,
real estate and Employee Benefit Plans necessary to conduct the Product Operations.
(u)
No Transfer of Employees. Unless Purchaser and Seller otherwise agree in writing, no employees of Seller will be transferred to Purchaser
in connection with the Transactions.
(v)
Absence of Changes. Since December 31, 2023, (i) there has not been any condition, change, effect, event occurrence, state of facts
or development that, individually or in the aggregate, has resulted in or would be reasonably expected to result in a Material Adverse
Effect, (ii) there has not been any material loss, damage or destruction to, or any material interruption in the use of, any Acquired
Assets, and (iii) Seller has not taken any action that would have required the consent of Purchaser pursuant to Section 6.3 if
taken after the Agreement Date.
(w)
Insurance. Seller has the insurance of the types and in the amounts set forth in Section 5.1(w) of the Disclosure Schedules (the
“Insurance Policies”) in respect of the Acquired Assets. The Insurance Policies are in full force and effect and all
premiums due and payable under such Insurance Policies have been paid on a timely basis. As of the Agreement Date, there is no material
claim relating to the Acquired Assets pending under the Insurance Policies as to which coverage has been questioned, denied or disputed
by the underwriters of such policies. Seller is in compliance in all material respects with the terms of such policies. To Sellers’
Knowledge as of the Agreement Date, there is no threatened termination of, or material premium increase with respect to, any of such
policies.
(x)
SEC Reports; Financial Statements.
(i)
Since January 1, 2023, Seller has timely filed or furnished each of the Seller SEC Reports. As of their respective dates, after giving
effect to any amendments or supplements thereto, the Seller SEC Reports (A) complied as to form in all material respects with the requirements
of the Securities Act and the Exchange Act, as applicable, and, to the extent applicable, Sarbanes-Oxley Act of 2002 (“SOX”),
and (B) did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary
in order to make the statements therein, in light of the circumstances under which they were made, not misleading, provided, however,
that no representation is made as to the accuracy of any financial projections or forward-looking statements or the completeness of any
information filed or furnished by Seller to the SEC solely for the purposes of complying with Regulation FD promulgated under the Exchange
Act. As of the Agreement Date, there are no material outstanding or unresolved comments in comment letters from the SEC staff with respect
to any of the Seller SEC Reports.
(ii)
The audited financial statements and unaudited interim financial statements (including, in each case, the notes, if any, thereto)
included in the Seller SEC Reports (the “Seller Financial Statements”) complied in all material respects with the
published rules and regulations of the SEC with respect thereto in effect at the time of filing or furnishing the applicable Seller SEC
Report, were prepared in accordance with GAAP, applied on a consistent basis during the periods involved (except as may be indicated
therein or in the notes thereto and except with respect to unaudited statements as permitted by the SEC on Form 8-K, Form 10-Q or any
successor or like form under the Exchange Act) and fairly present in accordance with GAAP (subject, in the case of the unaudited interim
financial statements, to normal, recurring year end audit adjustments that would not be, individually or in the aggregate, materially
adverse to Purchaser) in all material respects the financial position of Seller, as of the respective dates thereof, and the consolidated
results of their operations and cash flows for the respective periods then ended.
(y)
Opinion of Financial Advisor. Prior to the execution of this Agreement, the Board of Directors of Seller has received an opinion
from Alliance Global Partners (the “Opinion”) to the effect that, as of the date of the Opinion and subject to certain
assumptions, qualifications, limitations and other matters set forth therein, the Consideration to be received by Seller in the Transaction
is fair, from a financial point of view, to Seller, and such opinion has not been withdrawn, revoked or modified as of the date of this
Agreement. Promptly following the execution of this Agreement, Seller shall deliver or make available to Purchaser a copy of the Opinion
for informational purposes only.
(z)
HSR Act. Seller (or if different, the Acquired Person as defined in the HSR Act regulations) does not satisfy the higher size of
person jurisdictional threshold under the HSR Act.
(aa)
Investment Representations.
(i)
Seller acknowledges and understands that its investment in the Shares involves substantial risk and, if and when issued by Purchaser
in accordance with this Agreement, (A) will not be registered for sale under the Securities Act or any other applicable securities Laws,
(B) will not be traded on any national exchanges as of the date of this Agreement, and (C) may not be offered, sold or otherwise transferred
except in compliance with the registration requirements of the Securities Act and any other applicable securities Laws or pursuant to
an exemption therefrom, and in each case in compliance with the conditions set forth in this Agreement.
(ii)
Seller acknowledges and understands that it is acquiring the Shares for its own account, for investment purposes only and not with
a view toward, or for sale in connection with, any distribution thereof, or with any present intention of distributing or selling any
Shares, in each case, in violation of the federal securities laws or any other applicable Law.
(iii)
Seller represents that it is an “Accredited Investor” as that term is defined in Rule 501 of Regulation D promulgated
under the Securities Act.
(iv)
Seller understands and agrees that the Shares issuable pursuant to this Agreement may not be transferred, sold, offered for sale,
pledged, hypothecated or otherwise disposed of without registration under the Securities Act and any other provision of applicable United
States federal, United States state, or other Law or pursuant to an applicable exemption therefrom.
(v)
Seller acknowledges and agrees that the book entries or certificates evidencing the Shares shall bear a restrictive legend substantially
similar to the legend set forth in Section 3.5(a).
(vi)
Seller represents that it has received all the information that it considers necessary or appropriate for deciding whether to acquire
Shares and has had the opportunity to ask questions and receive answers from Purchaser regarding the Shares and the business, properties,
prospects, and financial condition of Purchaser and the terms and conditions of the transactions contemplated hereby and to obtain such
additional information (to the extent Purchaser possessed such information or could acquire it without unreasonable effort or expense)
necessary to verify the accuracy of any information furnished to Seller or to which Seller had access. Seller represents that it is a
sophisticated and experienced investor and is capable of evaluating, to its satisfaction, the accounting, tax, financial, legal and other
risks associated with Transactions, and has retained outside legal counsel and has had the opportunity to consult with his, her or its
accounting, tax, financial and legal advisors to be able to evaluate the risks involved in the Transactions and to make an informed investment
decision with respect to the Transactions.
(bb)
Disclaimer of Warranties. Except as set forth in this Section 5.1 (as qualified or modified by the Disclosure Schedules),
(i) the Acquired Assets are being sold on an “as is, where is” basis as of the Closing and (ii) none of Seller, its Affiliates
or any of their respective Representatives have made, or shall be deemed to have made, any other representation or warranty, express
or implied, at law or in equity, in respect of the Acquired Assets or the Assumed Liabilities, including with respect to (A) merchantability
or fitness for any particular purpose, (B) the Product Operations after the Closing, (C) the probable success or profitability of the
Product Operations after the Closing or (D) the accuracy or completeness of any (1) projections, predictions, forecasts, estimates, plans
or budgets of future revenues, expenses or expenditures, future results of operations (or any component thereof), future cash flows (or
any component thereof) or future financial condition (or any component thereof) that may be contained or referred to in the Disclosure
Schedules or elsewhere, or (2) information, documents or materials regarding the business of Seller, the Acquired Assets or the Assumed
Liabilities, including any information furnished or made available to Purchaser, its Affiliates or their respective Representatives in
any confidential information memorandum or presentation, “data room,” “virtual data room,” management presentation
or in any other form in expectation of, or in connection with, the Transactions (the items and information referred to in the immediately
preceding clauses (A) and (B) collectively, the “Evaluation Material”), or the appropriateness or suitability of the
Evaluation Material for the purposes of enabling Purchaser to evaluate the consummation of the Transactions. Any such other representations
or warranties are hereby expressly disclaimed. Except in the case of Fraud, none of Seller, its Affiliates or any of their respective
Representatives will have or be subject to any liability or obligation to Purchaser or any other Person resulting from the distribution
to Purchaser, its Affiliates or their respective Representatives of, or Purchaser’s use of or reliance on, any Evaluation Material
in expectation of the Transactions.
5.2
Representations and Warranties of Purchaser. Purchaser makes the following representations and warranties to Seller as of the
Agreement Date and as of the Closing Date (except, in each case, to the extent such representations and warranties speak expressly
as of a different date, and then, as of such date) as follows:
(a)
Organization and Existence. Purchaser is a corporation, duly organized, validly existing and in good standing under the laws of the
state of its jurisdiction, with full power and authority to own, lease, and operate its business and properties and to carry on its business
as and where such properties and assets are now owned or leased and such business is now conducted.
(b)
Authority and Approval. Purchaser has the power to enter into this Agreement and each of the Related Agreements to which it is to
be a party and to perform its obligations thereunder. The execution, delivery, and performance by Purchaser of this Agreement and the
Related Agreements to which it is to be a party, and the consummation by Purchaser of the Transactions, have been duly authorized by
all required action on the part of Purchaser. This Agreement has been duly executed and delivered by Purchaser and, when executed and
delivered by Purchaser, the Related Agreements to which Purchaser is to be a party will have been duly executed and delivered by Purchaser.
This Agreement has been duly executed and delivered by Purchaser and the other Related Agreements will be duly executed and delivered
by Purchaser, and, assuming the due execution and delivery of this Agreement by Seller and of the Related Agreements by the counterparties
thereto, this Agreement constitutes, and the Related Agreements when so executed and delivered will each constitute, a valid and legally
binding obligation of Purchaser, enforceable against it in accordance with their respective terms, except as enforceability may be affected
by the Enforceability Exception.
(c)
No Conflict. The execution and delivery by Purchaser of this Agreement and each of the Related Agreements to which it is to be a
party, and Purchaser’s compliance with the terms and conditions hereof and thereof, and the consummation by Purchaser of the Transactions,
do not and will not (i) conflict with any of, or require any consent of any Person that has not been obtained under, Purchaser’s
Organizational Documents, (ii) violate any provision of, or require any consent, authorization, or approval under, any Law or any Order
applicable to Purchaser, (iii) conflict with, result in a breach of, constitute a default under (whether with or without notice or the
lapse of time or both), accelerate or permit the acceleration of the performance required by, or require any consent, authorization,
or approval under, any material Contract to which Purchaser is a party or by which it is bound or to which any of its assets or property
is subject, or (iv) result in the creation of any Lien upon the assets or property of Purchaser, except in the case of the immediately
preceding clauses (ii), (iii) and (iv), as would not reasonably be expected to have a material adverse effect on Purchaser or materially
adversely affect the validity or enforceability of this Agreement against Purchaser or materially adversely affect the ability of Purchaser
to consummate the Transactions.
(d)
Governmental Approvals and Filing. No consent, authorization, approval, or action of, filing with, notice to, or exemption from any
Governmental Authority on the part of Purchaser is required in connection with the execution, delivery and performance of this Agreement
or any Related Agreements to which Purchaser is to be a party or the consummation of the Transactions, except for any other consent,
approval or action where the failure to obtain any such consent, approval, or action, to make any such filing, to give any such notice
or obtain any such exemption would not be reasonably expected to (A) have a material adverse effect on Purchaser or (B) materially adversely
affect the validity or enforceability against Purchaser of this Agreement or such Related Agreements or materially adversely affect the
ability of Purchaser to consummate the Transactions.
(e)
No Brokers. No broker, finder, or investment banker is entitled to any brokerage commission, finder’s fee, or similar payment
in connection with the Transactions based upon arrangements made by or on behalf of Purchaser.
(f)
Legal Proceedings. As of the Agreement Date, there are no Legal Proceedings pending, or to the knowledge of Purchaser, threatened,
against Purchaser that would reasonably be expected to prevent or delay the ability of Purchaser to enter into and perform its obligations
under this Agreement, the Related Agreements, or the Transactions. There is no Order to which Purchaser is subject or that is pending
or, to the knowledge of Purchaser, threatened that would reasonably be expected to prevent or delay the ability of Purchaser to enter
into and perform its obligations under this Agreement, the Related Agreements or the Transactions.
(g)
Availability of Funds; Solvency. Purchaser will have available sufficient cash to enable it to pay the cash portions of the Closing
Consideration as required pursuant to Section 3.2 and the cash portions of any Milestone Payments as required pursuant to Section
3.3(a). Purchaser is solvent and currently: (i) is able to pay its debts as they become due; (ii) owns property that has a fair saleable
value greater than the amounts required to pay its debts (including a reasonable estimate of the amount of all contingent liabilities);
and (iii) has adequate capital to carry on its business. No transfer of property is being made and no obligation is being incurred in
connection with the Transactions with the intent to hinder, delay or defraud either present or future creditors of Purchaser.
(h)
SEC Reports, Financial Statements.
(i)
Purchaser has timely filed or furnished each form, report, schedule, registration statement, definitive proxy statement and other
document (together with all amendments thereof and supplements thereto) required to be filed or furnished by Purchaser pursuant to the
Securities Act or the Exchange Act with the SEC since January 1, 2023 (as such documents have since the time of their filing been amended
or supplemented, the “Purchaser SEC Reports”). As of their respective dates, after giving effect to any amendments
or supplements thereto, the Purchaser SEC Reports (A) complied as to form in all material respects with the requirements of the Securities
Act and the Exchange Act, as applicable, and, to the extent applicable, SOX, and (B) did not contain any untrue statement of a material
fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of
the circumstances under which they were made, not misleading, provided, however, that no representation is made as to the
accuracy of any financial projections or forward-looking statements or the completeness of any information filed or furnished by Purchaser
to the SEC solely for the purposes of complying with Regulation FD promulgated under the Exchange Act. As of the Agreement Date, there
are no material outstanding or unresolved comments in comment letters from the SEC staff with respect to any of the Purchaser SEC Reports.
(ii)
The audited financial statements and unaudited interim financial statements (including, in each case, the notes, if any, thereto)
included in the Purchaser SEC Reports (the “Purchaser Financial Statements”) complied in all material respects with
the published rules and regulations of the SEC with respect thereto in effect at the time of filing or furnishing the applicable Purchaser
SEC Report, were prepared in accordance with GAAP, applied on a consistent basis during the periods involved (except as may be indicated
therein or in the notes thereto and except with respect to unaudited statements as permitted by the SEC on Form 8-K, Form 10-Q or any
successor or like form under the Exchange Act) and fairly present (subject, in the case of the unaudited interim financial statements,
to normal, recurring year end audit adjustments that would not be, individually or in the aggregate, materially adverse to Purchaser)
in all material respects the financial position of Purchaser, as of the respective dates thereof, and the consolidated results of their
operations and cash flows for the respective periods then ended.
(iii)
Purchaser maintains a system of internal control over financial reporting (as defined in Rules 13a–15(f) and 15d–15(f)
of the Exchange Act) sufficient to provide reasonable assurances regarding the reliability of financial reporting. Purchaser (A) maintains
disclosure controls and procedures (as defined in Rules 13a–15(e) and 15d–15(e) of the Exchange Act) to provide reasonable
assurance that all information required to be disclosed by Purchaser in the reports that it files or submits under the Exchange Act is
recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and is accumulated
and communicated to Purchaser’s management as appropriate to allow timely decisions regarding required disclosure, and (B) has
disclosed, based on its most recent evaluation of internal control over financial reporting, to Purchaser’s outside auditors and
the audit committee of the board of directors of Purchaser (1) all significant deficiencies and material weaknesses in the design or
operation of internal control over financial reporting that are reasonably likely to adversely affect Purchaser’s ability to record,
process, summarize and report financial information and (2) any fraud, whether or not material, that involves management or other employees
who have a significant role in Purchaser’s internal control over financial reporting, and the information described in the foregoing
clauses (1) and (2) has been disclosed to Seller prior to the Agreement Date. Neither Purchaser nor, to the knowledge of Purchaser, Purchaser’s
independent registered accountant has received or otherwise had or obtained knowledge of any material complaint, allegation, assertion
or claim, whether written or oral, in each case, regarding deficient accounting or auditing practices, procedures, methodologies or methods
of Purchaser or its internal accounting controls or any material inaccuracy in the Purchaser Financial Statements.
(i)
No Purchaser Vote Required. No vote or other action of the stockholders of Purchaser is required by applicable Law, the certificate
of incorporation or bylaws of Purchaser or otherwise in order for Purchaser to consummate the Transactions.
(k)
Valid Issuance. The Shares, when issued as provided in and pursuant to the terms of this Agreement, will be duly authorized and validly
issued, fully paid and nonassessable, and (other than restrictions under applicable securities Laws or as described in Section 3.5)
will be free of restrictions on transfer.
(l)
HSR Act. Purchaser (or if different, the Acquiring Person, as defined in the HSR Act regulations) does not satisfy the higher size
of person jurisdictional threshold under the HSR Act.
(m)
Independent Investigation; Acknowledgements and Confirmations. Purchaser has conducted to its satisfaction its own independent review
and analysis of, and based thereon has formed an independent judgment concerning, the Transactions, the Acquired Assets and the Assumed
Liabilities. In entering into this Agreement, Purchaser has relied solely upon its own review and analysis and the specific representations
and warranties of Seller expressly set forth in Section 5.1 (as qualified or modified by the Disclosure Schedules) and not on
any other representations, warranties, statements or omissions (whether by another Person or Seller). Without prejudicing any claim for
Fraud, Purchaser acknowledges and confirms that, except for the representations and warranties expressly set forth in Section 5.1
(as qualified or modified by the Disclosure Schedules), none of Seller, its Affiliates or any of their respective Representatives
have made, or shall be deemed to have made, and Purchaser has not relied on, is not relying on and hereby disclaims reliance upon, any
other representation or warranty, express or implied, at law or in equity, in respect of the Acquired Assets or the Assumed Liabilities,
including with respect to (a) merchantability or fitness for any particular purpose, (b) the operation of the business to which the Acquired
Assets relate after the Closing, (c) the probable success or profitability of the Product Operations after the Closing or (d) the accuracy
or completeness of any Evaluation Material or the appropriateness or suitability of such information for the purposes of enabling Purchaser
to evaluate the consummation of the Transactions. Except in the case of Fraud, none of Seller, its Affiliates or any of their respective
Representatives will have or be subject to any liability to Purchaser or any other Person resulting from the distribution to Purchaser,
its Affiliates or their respective Representatives of, or Purchaser’s use of or reliance on, any Evaluation Material in expectation
of the Transactions.
(n)
Disclaimer of Warranties. Except as set forth in this Section 5.2 (as qualified or modified by the Disclosure Schedules),
none of Purchaser, its Affiliates or any of their respective Representatives have made, or shall be deemed to have made, any other representation
or warranty, express or implied, at law or in equity, in respect of the Transactions, and any such other representations or warranties
are hereby expressly disclaimed.
6.1
Public Disclosures. The initial press release relating to the Transactions shall be a joint press release, the text of which has
been agreed to by each of Purchaser and Seller. Each of Purchaser and Seller shall allow the other Party reasonable time to review and
comment on such joint press release. Thereafter, no Party shall issue any press release related to the Transactions unless such press
release (a) has been approved by the other Party (which approval shall not be unreasonably withheld, conditioned or delayed) or (b) is
required to be issued by such Party under applicable Laws based upon advice of counsel. Notwithstanding the foregoing, each Party may
make any public statement in response to questions by the press, analysts, investors or those attending industry conferences or financial
analyst calls, or issue press releases, so long as any such public statement or press release is consistent with prior public disclosures
or public statements approved pursuant to this Section 5.1 and which do not reveal nonpublic information about the other Party.
If the filing of this Agreement with the SEC or any stock exchange on which securities issued by a Party or its Affiliate are traded
is required by applicable Laws, the Parties shall coordinate in advance with each other in connection with such filing and each Party
will use reasonable efforts to seek confidential treatment for the terms proposed to be redacted; provided that each Party will
ultimately retain control over what information to disclose to any Governmental Authority or any stock exchange as the case may be.
6.2
Confidentiality. Seller agrees that following the Closing Date it shall hold in strict confidence, unless compelled to disclose
by judicial or administrative process or by other Laws (and then only following reasonable prior written notice to Purchaser, to the
extent practicable, so that Purchaser shall have an opportunity to object (and Seller shall reasonably cooperate with Purchaser in
objecting to any such compulsion)), all confidential information of Purchaser or the Acquired Assets to which they had access prior
to the Closing and will not release or disclose such confidential information to any other Person, except to their auditors,
attorneys, financial advisors and other consultants, agents and advisors who need to know such information in connection with
Seller’s business (provided that Seller takes reasonable steps to ensure that each such Person maintain the confidentiality
required hereunder); provided that the foregoing obligations shall not apply to any such information which comes into the
public domain through no fault of Seller, or of any Person to whom Seller is authorized to release or disclose such information, or
any information that the recipient of such information independently develops or discovers after the Closing without reference to
the disclosed information or breach hereof.
6.3
Conduct Pending the Closing. From the Agreement Date until the earlier of the Closing Date or the termination of this Agreement pursuant
to Section 11.1, except (i) as otherwise contemplated, permitted, or required by this Agreement, (ii) as required by Law, (iii)
as set forth on Section 6.3 of the Disclosure Schedule or (iv) to the extent that Purchaser otherwise consents in writing, which consent
shall not be unreasonably withheld, conditioned or delayed, (x) Seller shall cause the Acquired Assets to be operated in the Ordinary
Course of Business in all material respects and (y) Seller shall not:
(a)
sell, lease, transfer, or otherwise dispose of any of the Acquired Assets;
(b)
grant or suffer to exist any Lien (other than Permitted Liens) on any of the Acquired Assets;
(c)
commence any material Legal Proceeding, or settle, pay, discharge, or satisfy any Legal Proceeding, where such commencement, settlement,
payment, discharge, or satisfaction would impose any restrictions or limitations upon the Acquired Assets following the Closing;
(d)
terminate, extend, or modify any Acquired Business Contract or Material Contract, other than to the extent explicitly contemplated
or provided by this Agreement or any Related Agreement, or enter into any Contract in respect of the Acquired Assets or the Assumed Liabilities
that, if in effect on the Agreement Date, would be a Material Contract;
(e)
waive or release any right of material value, in each case related to any Acquired Assets or any Assumed Liabilities;
(f)
fail to keep in force and effect, or allow to lapse, any insurance policy in respect of the Acquired Assets comparable in amount
and scope of coverage to that maintained as of the Agreement Date;
(g)
correspond, communicate or consult with the FDA or similar Governmental Authority, in each case with respect to the Compounds or
the Products, other than (x) any immaterial communication in the Ordinary Course of Business, or (y) any such correspondence, communication
or consultation required by applicable Law in connection with an adverse event;
(h)
merge, combine or consolidate itself with any other Person or adopt a plan of complete or partial liquidation, dissolution, consolidation,
restructuring, recapitalization or other reorganization, or file a certificate of dissolution in respect of Seller or any of its Subsidiaries
with the Secretary of State of the State of Delaware;
(i)
make any distributions to its stockholders or declare or pay any dividends on shares of Seller’s capital stock; or
(j)
agree in writing to do any of the foregoing.
6.4
Further Assurances and Cooperation; Non-Compete.
(a)
Further Assurances. Upon the terms and subject to the conditions set forth in this Agreement, each of the Parties agrees to use its
commercially reasonable efforts to take, or cause to be taken, all actions, and to do, or cause to be done, and to assist and cooperate
with the other Parties in doing, all things necessary, proper, or advisable to consummate and make effective, in the most expeditious
manner practicable, the Transactions, including using commercially reasonable efforts to accomplish the following: (i) the obtaining
of all necessary actions or non-actions, waivers, consents, and approvals from Governmental Authorities and the making of all necessary
registrations and filings (including filings with Governmental Authorities, if any) and recordings of license grants; (ii) the obtaining
of all necessary consents, approvals, or waivers from Third Parties required in accordance with the transfer of any Acquired Asset from
Seller to Purchaser pursuant to Section 2.1; (iii) the execution and delivery of any additional instruments necessary to consummate
the Transactions, and to fully carry out the purposes of, this Agreement and the Related Agreements; and (iv) the identification and
delivery of all Acquired Assets not previously identified and delivered.
(b)
Cooperation. If, in order to properly prepare any documents or reports required to be filed with any Governmental Authority, it is
necessary that either Purchaser or Seller be furnished with additional information, documents, or records relating to the Product Operations,
the Acquired Assets, the Excluded Liabilities, or the Assumed Liabilities, and such information, documents, or records are in the possession
or control of the other Party, such other Party will use its commercially reasonable efforts to furnish or make available such information,
documents, or records (or copies thereof) at the recipient’s reasonable request and at recipient’s cost and expense; provided,
further, that the Parties agree that any Party receiving such materials shall be subject to the same obligations of confidentiality
described in Section 6.2.
(c)
Non-Compete. Seller agrees that, from the Agreement Date until five (5) years following the Closing Date, it shall not, and shall
cause its controlled Affiliates, successors, and assigns to not, directly or indirectly, without Purchaser’s prior written consent,
(i) Develop, test, manufacture, commercialize, or otherwise exploit any program, pharmaceutical product candidate or product that targets
gamma secretase or that is intended to treat desmoid tumors (a “Competing Product”), anywhere in the world, (ii) own
or have the right to acquire, or manage, operate or control, any Person engaged in, or otherwise participate in the research, Development,
testing, manufacture, Commercialization, or other exploitation of any Competing Product anywhere in the world, or (iii) otherwise knowingly
assist or enable any Third Party to research, Develop, test, manufacture, commercialize, or otherwise exploit any Competing Product anywhere
in the world. Seller acknowledges that the time, geographic, and scope limitations of its obligations under this Section 6.4(c)
are fair and reasonable in all respects. If any term of this provision is invalid or unenforceable in any jurisdiction, it shall not
affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending
term or provision in any other situation or in any other jurisdiction. Further, the Parties agree to replace such invalid or unenforceable
term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and
other purposes of such invalid or unenforceable term. In addition, Seller will not waive or release any employee from any Contract between
Seller and its employees that binds its employees to customary non-compete obligations consistent with this Section 6.4(c) and
applicable Law.
6.5
Tax Matters.
(a)
Allocation. Purchaser and Seller acknowledge and agree that the purchase and sale of the Acquired Assets will be treated for all
Tax purposes as a taxable asset purchase. The Consideration (including any Assumed Liabilities, to the extent properly taken into account
for income Tax purposes) shall be allocated for all Tax purposes consistently with Section 1060 of the Code. Following the Closing Date,
Purchaser shall prepare a draft allocation schedule (the “Allocation Schedule”). Purchaser shall provide the draft
Allocation Schedule to Seller within 120 days after the Closing Date, and Seller shall have 30 days thereafter to review and comment
on such Allocation Schedule. Purchaser shall consider in good faith any such comments of the Seller, and revise the draft Allocation
Schedule accordingly to the extent required to comply with Section 1060 of the Code. Each Party agrees to prepare and file all applicable
Tax Returns in a manner consistent with the Allocation Schedule.
(b)
Straddle Period Taxes. In the case of any real or personal property Taxes (or other Taxes imposed on a periodic basis) (each, a “Straddle
Period Tax”) attributable to the Acquired Assets that are reported on a Tax Return covering a period beginning on or before
the Closing Date and ending thereafter (such period, a “Straddle Period”), any such Straddle Period Taxes shall be
prorated between Purchaser and Seller on a per diem basis. The Party required by applicable Law to pay any such Straddle Period Tax (the
“Paying Party”) shall file the Tax Return related to such Straddle Period Tax within the time period and in the manner
required by applicable Law and shall timely pay such Straddle Period Tax. To the extent any such payment exceeds the obligation of the
Paying Party hereunder, the Paying Party shall provide the other Party (the “Non-Paying Party”) with notice of the
amount of such Straddle Period Taxes, and within 10 days of receipt of such notice of payment, the Non-Paying Party shall reimburse the
Paying Party for the Non-Paying Party’s share of such Straddle Period Taxes.
(c)
Transfer Taxes. All transfer, documentary, sales, use, stamp, value added, goods and services, excise, registration and other similar
Taxes, and all conveyance fees, recording charges and other fees and charges (including any penalties and interest) incurred in connection
with consummation of the Transactions (“Transfer Taxes”) shall be borne 50% by Purchaser and 50% by Seller. The Party
required under applicable Laws to file a Tax Return with respect to Transfer Taxes will, at its own expense, prepare or cause to be prepared,
and file, or cause to be filed, all necessary Tax Returns and other documentation with respect to such Transfer Taxes, and if required
by applicable Law, the Parties will, and will cause their Affiliates to, join in the execution of any such Tax Returns. The Parties agree
to reasonably cooperate to minimize any Transfer Taxes to the extent permitted by applicable Law.
(d)
Cooperation. To the extent relevant to the Product Operations or the Acquired Assets, each Party shall (i) provide the other with
such assistance as may reasonably be requested in connection with the preparation of any Tax Return, claim for refund, and the conduct
of any audit or other examination by any Governmental Authority or in connection with judicial or administrative proceedings relating
to any Liability for Taxes and (ii) provide the other with all records or other information in its possession that is reasonably requested
and may be relevant to the preparation of any Tax Returns, claim for refund, or the conduct of any audit, examination, or other proceeding
(including any judicial or administrative proceeding) relating to Taxes (each, a “Tax Contest”). Such cooperation
shall include obtaining and providing appropriate forms, retaining and providing records and information that are reasonably relevant
to any such Tax Return, claim for refund, or Tax Contest, and making employees available on a mutually convenient basis to provide additional
information and explanation of any materials provided hereunder. Notwithstanding the foregoing, no Party shall be required to provide
or provide access to any income Tax Returns or related workpapers, other than Tax Returns relating to the Acquired Assets or Product
Operations. For the avoidance of any doubt, this Section 6.5(d) shall only apply to cooperation solely related to the Acquired
Assets and the Product Operations, and no party shall be required to provide any information whatsoever otherwise.
6.6
Seller Stockholder Approval.
(a)
Immediately following the execution and delivery of this Agreement (i) Seller shall accept any notices of conversion or notices of
exercise delivered by any Requisite Holders in respect of outstanding convertible notes or warrants, as applicable, and issue any shares
of Seller Common Stock in respect thereof as soon as practicable thereafter, (ii) in lieu of calling a meeting of Seller’s stockholders,
Seller shall submit the Stockholder Written Consent, in the form attached hereto as Exhibit F (the “Stockholder Written
Consent”), to the Requisite Holders. No later than 11:59 pm Eastern Time on the date that is three (3) Business Days following
the Agreement Date (the time that the Stockholder Written Consent is obtained, the “Written Consent Delivery Time”),
Seller shall obtain the Stockholder Written Consent, duly executed and delivered by each Consenting Stockholder, in accordance with the
Delaware General Corporation Law and Seller’s Organizational Documents. The Parties agree and acknowledge that the Stockholder
Written Consent shall be void and of no further effect if this Agreement is terminated in accordance with the terms and conditions hereof.
(b)
Within ten (10) Business Days after the date of this Agreement, Seller shall file with the SEC a preliminary information statement
in accordance with Regulation 14C promulgated under of the Exchange Act (such preliminary information statement and any revised or definitive
information statement, the “Information Statement”) relating to the Stockholder Written Consent. Purchaser shall reasonably
cooperate with Seller in the preparation of the preliminary Information Statement, the definitive Information Statement and any amendments
or supplements thereto and shall promptly furnish to Seller the information relating to Purchaser required by the Exchange Act for inclusion
therein. Prior to filing with the SEC, Seller shall provide Purchaser and its counsel a reasonable opportunity to review and comment
on the Information Statement and shall consider in good faith for inclusion in the Information Statement any comments made by Purchaser
or its counsel. Seller shall use reasonable best efforts to respond as promptly as practicable to any comments of the SEC with respect
to the Information Statement and to cause the Information Statement in definitive form to be mailed to the holders of shares of Seller
Common Stock entitled thereto as promptly as reasonably practicable after (i) the tenth (10th) calendar day after the initial filing
of the preliminary Information Statement with the SEC if by such date the SEC has not informed Purchaser that it intends to review the
Information Statement or (ii) if the SEC has, by the tenth (10th) calendar day after the filing of the initial preliminary Information
Statement with the SEC, informed Seller that it intends to review the Information Statement, the date on which the SEC confirms that
it has no further comments on the Information Statement. Seller shall notify Purchaser promptly of (and in any event no more than one
(1) Business Day after) the receipt of any comments from the SEC or its staff and of any request by the SEC or its staff for any amendments
or supplements to the preliminary Information Statement or the definitive Information Statement, and if required, Seller shall mail to
the holders of shares of Seller Common Stock entitled thereto, as promptly as reasonably practicable, such amendment or supplement. Prior
to filing with the SEC, Seller shall provide Purchaser and its counsel a reasonable opportunity to review and comment on any such amendments
or supplements to the Information Statement and shall reasonably consider in good faith for inclusion in any amendments or supplements
any comments made by Purchaser or its counsel. If at any time prior to the Closing any event shall occur, or fact or information shall
be discovered, that should be set forth in an amendment or supplement to the Information Statement so that such document would not include
any misstatement of a material fact or omit to state any material fact necessary to make the statements therein, in light of the circumstances
under which they are made, not misleading, the Party that discovers such information shall as promptly as practicable notify the other
Party and Seller shall prepare and file with the SEC such amendment or supplement, in consultation with and subject to review by Purchaser
and its counsel as promptly as practicable and, to the extent required by Law, cause such amendment or supplement to be disseminated
to the holders of shares of Seller Common Stock entitled thereto. Notwithstanding the foregoing, in the event that this Agreement is
terminated in accordance with the terms and conditions hereof, Purchaser shall not be required, after the date of termination, to prepare,
file and mail the Information Statement pursuant to this Section 6.6(b).
6.7
Non-Solicitation.
(a)
From the Agreement Date until the earlier of the Closing Date or the termination of this Agreement pursuant to Section 11.1,
Seller shall not, and shall cause its Representatives not to, directly or indirectly: (a) solicit, seek, initiate or knowingly encourage,
respond to (other than to communicate that Seller is subject to an exclusivity obligation and not permitted to respond further as a result)
or facilitate the making of any inquiry, expression of interest, proposal or offer that constitutes, or is reasonably likely to lead
to or encourage the initiation or submission of, any expression of interest, inquiry, proposal or offer from any Person (other than Purchaser)
relating to a possible Strategic Transaction; (b) participate in, maintain or continue any discussions or negotiations or enter into
any agreement with, provide any non-public information to, or provide access to the properties, books or records of Seller or any of
its direct or indirect subsidiaries to any Person (other than Purchaser) relating to or in connection with a possible Strategic Transaction;
or (c) agree to, accept, recommend or endorse, or publicly propose or announce any intention or desire to agree to, accept, recommend
or endorse, any proposal or offer from any Person (other than Purchaser) relating to a possible Strategic Transaction; provided,
however, that in each case, Seller shall be permitted to respond to any unsolicited inquiry from any Person (other than Purchaser),
solely for the purposes of informing such Person that Seller is subject to an exclusivity obligation and not permitted to respond further
as a result.
(b)
From the Agreement Date until the earlier of the Closing Date or the termination of this Agreement pursuant to Section 11.1,
Seller shall promptly provide Purchaser with an oral and a written description of any expression of interest, inquiry, proposal or offer
relating to a possible Strategic Transaction (including the material terms and conditions of any such expression of interest, inquiry,
proposal or offer and the identity of the third party) that is received by Seller or by any of Seller’s Representatives from any
Person (other than Purchaser). From the Agreement Date until the earlier of the Closing Date or the termination of this Agreement pursuant
to Section 11.1, Seller shall not release any third party from, or waive any provision of, any confidentiality agreement to which
Seller is a party.
(c)
It is understood that any violation of the restrictions set forth above by any of the Representatives of Seller shall be deemed to
be a breach of this Section 6.7 by Seller.
6.8
Deposit. Each of Purchaser and Seller acknowledge and agree that the Deposit previously made by Purchaser pursuant to the Exclusivity
Agreement shall be non-refundable, except for the following: (a) if Seller fails to obtain Support Agreements from each of the Requisite
Holders; (b) if Seller fails to obtain the fully executed and delivered Stockholder Written Consents in accordance with Section 6.6(a)
three (3) Business Days following the Agreement Date; (c) (i) if this Agreement is terminated by Purchaser pursuant to Section
11.1(c) in respect of any material breach or failure respect any of Seller’s representations, warranties, covenants, or agreements
contained in this Agreement, or (ii) if this Agreement is terminated by Purchaser pursuant to Section 11.1(e) and such termination
is due to Seller’s failure to obtain an approval, consent or waiver listed on Schedule 4.2(a)(v); or (d) if Seller or any
of its Representatives breach in any material respect the provisions of Section 6.7. Upon the occurrence of any event described
in (a) through (c) above, Seller shall refund the Deposit to Purchaser within three (3) Business Days of the date on which Purchaser
delivers written notice to Seller demanding repayment of the Deposit.
6.9
Restrictions on Dissolution and Winding Up.
(a)
For a period of six (6) months following the Closing Date, without Purchaser’s prior written consent (which consent shall not
be unreasonably withheld, conditioned or delayed), (a) Seller shall not make any distributions to its stockholders or declare or pay
any dividends on shares of Seller’s capital stock, (b) Seller shall not be wound up, liquidated or dissolved, or initiate any procedures
or processes to be wound up, liquidated or dissolved, (c) Seller shall not file or cause to be filed on its behalf a petition in bankruptcy
under any provisions of federal or state bankruptcy or insolvency Law or consent to the entry of an order for relief (or any other order
with similar effect) under any involuntary bankruptcy proceedings (or any other similar proceedings under state law) commenced against
Seller, and (d) Seller shall not seek or cause to be sought on its behalf, nor shall Seller consent to, the appointment of a trustee,
receiver, liquidator, assignee for the benefit of creditors or other Person or official with similar duties with respect to the Seller
or its assets.
(b)
Notwithstanding anything to the contrary in Section 6.9(a), Seller may seek the approval of its stockholders to give the board
of directors of Seller the discretion to file, at a time chosen by the board of directors of Seller but no earlier than the date that
is six (6) months following the Closing Date, a certificate of dissolution with the Secretary of State of the State of Delaware in accordance
with Section 275 of the Delaware General Corporation Law.
6.10
Tail Insurance. Prior to the Closing, Seller shall obtain and deliver to Purchaser evidence of an insurance tail policy or similar
coverage reasonably satisfactory to Purchaser (the “Tail Policy”) that (a) covers claims made in respect of product
liability or clinical trial liability for activities conducted by Seller prior to Closing and such other coverage for incurred but unreported
claims arising on or prior to Closing related to Seller’s conduct of its business relating to the Acquired Assets prior to the
Closing as requested by Purchaser, (b) has an extended reporting period from the Closing through the fifth (5th) anniversary
of the Closing Date and (c) is consistent, both in terms of coverage and limits, with the Insurance Policies.
6.11
Transition Services. For a period of one (1) month following the Closing, Seller shall provide the transition services set forth
on Schedule 6.11.
6.12
Employee Matters. The Parties acknowledge that employees, advisors, and independent contractors of Seller who have been involved
in research and development related to the Products and the Compounds (“Product Information”), may have
information necessary to enable technology transfer to Purchaser following the Closing (the “Key Personnel”).
Purchaser anticipates that it may enter into consulting arrangements with some Key Personnel. The Parties acknowledge that Key
Personnel may have entered into employment agreements or other written agreements with Seller or its Affiliates, that contain (a)
obligations of confidentiality, non-use and non-disclosure with respect to any information related to the Acquired Assets and/or the
Assumed Liabilities, or (b) non-compete obligations with respect to the Acquired Assets and/or the Assumed Liabilities
(“Key Personnel Agreements”). Seller hereby consents and agrees that Purchaser may offer consulting or other
arrangements to one or more Key Personnel and Seller agrees that it will not assert that any such consulting arrangement violates
the Key Personnel Agreements. Seller hereby waives any rights under any Key Personnel Agreements solely with respect to activities
set forth in this Section 6.12.
| 7. |
Conditions Precedent to Obligations of Purchaser. |
The
obligation of Purchaser to effect the Closing and consummate the Transactions is subject to the satisfaction (or waiver by Purchaser),
at or prior to the Closing, of each of the following conditions:
7.1
Accuracy of Representations and Warranties.
The Fundamental Representations shall be true and correct in all respects, in each case as of the Agreement Date and as of the Closing
Date, with the same effect as though made as of the Closing Date (provided that the accuracy of representations and warranties that by
their terms speak as of a specified date will be determined as of such date). All other representations and warranties of Seller set
forth in this Agreement (without giving effect to any limitation as to “materiality” or “Material Adverse Effect”
or any similar limitation contained in this Agreement) shall be true and correct, in each case as of the Agreement Date and as of the
Closing Date, with the same effect as though made as of the Closing Date (provided that the accuracy of representations and warranties
that by their terms speak as of a specified date will be determined as of such date), except where the failure of the representations
and warranties of Seller set forth in this Agreement to be so true and correct would not reasonably be expected to have, individually
or in the aggregate, a Material Adverse Effect.
7.2
Performance of Covenants.
Seller shall have performed and complied with, in all material respects, all of its covenants contained in this Agreement at or before
the Closing (to the extent that such covenants require performance by Seller at or before the Closing).
7.3
No Restraints. No temporary restraining order, preliminary or permanent injunction, or other Order preventing the consummation of
any of the Transactions shall have been issued by any court of competent jurisdiction and remain in effect, and no material Law shall
have been enacted that makes consummation of any of the Transactions illegal.
7.4
No Material Adverse Effect. There shall not have occurred and be continuing a Material Adverse Effect since the Agreement
Date.
7.5
No Governmental Litigation.
There shall not be pending or threatened in writing before any court of competent jurisdiction or other Governmental Authority any Legal
Proceeding (a) to which a Governmental Authority is a party, and (b) that would or would reasonably be expected to (i) restrain, enjoin,
prevent, prohibit or make illegal the consummation of the Transactions, or (ii) prohibit or limit the ownership or operation by Purchaser,
its Affiliates of the Acquired Assets or the Assumed Liabilities, or (iii) compel Purchaser or its Affiliates to dispose of, hold separate
or license any material portion of the business or assets (including the Acquired Assets) of Purchaser or its Subsidiaries, as a result
of the Transactions.
7.6
Closing Certificate.
Seller shall have delivered to Purchaser a certificate to the effect that each of the conditions specified above in Sections 7.1,
7.2 and 7.4 is satisfied in all respects as of the Closing.
7.7
Seller Closing Deliverables. Seller shall have executed and delivered to Purchaser (or caused one or more of its Affiliates to
execute and deliver to Purchaser, as applicable) each of the documents and materials contemplated to be delivered by Section
4.2(a).
7.8
Seller Information Statement. At least twenty (20) calendar days shall have elapsed from the date the definitive Information Statement
was mailed to Seller’s stockholders in accordance with Rule 14c-2 promulgated under the Exchange Act.
| 8. |
Conditions
Precedent to Obligation of Seller |
The
obligation of Seller to effect the Closing and consummate the Transactions is subject to the satisfaction (or waiver by Seller), at or
prior to the Closing, of the following conditions:
8.1
Accuracy of Representations. The representations and warranties of Purchaser set forth in this Agreement (without giving effect to
any limitation as to “materiality” or “Material Adverse Effect” or any similar limitation contained in this Agreement)
shall be true and correct in all material respects, except where the failure of the representations and warranties of Purchaser set forth
in this Agreement to be so true and correct would not reasonably be expected to, individually or in the aggregate, prevent or materially
delay the consummation of any of the Transactions.
8.2
Performance of Covenants. Purchaser shall have performed and complied with, in all material respects, all of its covenants contained
in this Agreement at or before the Closing (to the extent that such covenants require performance by Purchaser at or before the Closing).
8.3
No Restraints. No temporary restraining order, preliminary or permanent injunction, or other Order preventing the consummation of
any of the Transactions by Seller shall have been issued by any court of competent jurisdiction and remain in effect, and no material
Law shall have been enacted that makes consummation of any of the Transactions illegal.
8.4
Closing Certificate. Purchaser shall have delivered to Seller a certificate to the effect that each of the conditions specified above
in Sections 8.1 and 8.2 is satisfied in all respects.
8.5
Purchaser Closing Deliverables. Purchaser shall have executed and delivered to Seller (or caused one or more of its Affiliates to
execute and deliver to Seller, as applicable) each of the documents and materials contemplated to be delivered by Section 4.2(b).
9.1
Survival of Representations; Warranties and Covenants The Parties, intending to modify any applicable statute of limitations, hereby
acknowledge and agree that the representations, warranties, covenants and agreements contained in this Agreement or in any certificate
or other document delivered pursuant to this Agreement shall terminate upon the earlier of (a) the Closing and (b) the termination of
this Agreement pursuant to Article 10; provided, however, that the covenants and agreements that explicitly contemplate
performance after the Closing shall survive the Closing in accordance with their terms until the earlier of (i) the date on which they
have been fully performed or expire in accordance with this Agreement and (ii) 60 days following the expiration of any applicable statute
of limitations.
10.1
Definitions. For the purpose of this Section 10:
(a)
the term “Resale Registration Statement” shall mean any registration statement required to be filed by Section
10.2, and shall include any preliminary prospectus, final prospectus, exhibit or amendment included in or relating to such registration
statements; and
(b)
the term “Registrable Shares” means the Shares; provided, however, that a security shall cease to be a Registrable
Share upon the earliest to occur of the following: (i) a Resale Registration Statement registering such security under the Securities
Act has been declared or becomes effective and such security has been sold or otherwise transferred by the holder thereof pursuant to
and in a manner contemplated by such effective Resale Registration Statement, (ii) such security is sold pursuant to Rule 144 under circumstances
in which any legend borne by such security relating to restrictions on transferability thereof, under the Security Act or otherwise,
is removed by Purchaser, (iii) the first date such security is eligible to be sold pursuant to Rule 144 without any limitation as to
volume of sales, holding period and without the holder complying with any method of sale requirements or notice requirements under Rule
144, or (iv) such security shall cease to be outstanding following its issuance. Notwithstanding the foregoing, no Shares shall be Registrable
Shares following the third (3rd) anniversary of the date on which the Mandatory Registration Statement is declared effective.
10.2
Registration Procedures.
(a)
Purchaser shall use commercially reasonable efforts to file a Resale Registration Statement (the “Mandatory Registration
Statement”) with the SEC on or before the date that is seven (7) days following the earlier of (i) April 1, 2024 and (ii) the
date Purchaser files its annual report on Form 10-K for the year ended December 31, 2023 (such earlier date, the “Filing Date”)
to register all of the Registrable Shares on Form S-3 under the Securities Act (providing for shelf registration of such Registrable
Shares under SEC Rule 415) (or if Purchaser is not then eligible to use Form S-3, Form S-1); provided, that Purchaser’s
obligation to file a Resale Registration Statement (including the Mandatory Registration Statement) is contingent upon Seller furnishing
in writing to Purchaser such information regarding Seller, the securities of Purchaser held by Seller and the intended method of disposition
of such Shares, which shall be limited to non-underwritten public offerings and the pro rata distributions of the Shares to the stockholders
of Seller in connection with the dissolution of Seller, as shall be reasonably requested by Purchaser to effect the registration of such
Shares in compliance with applicable securities laws and which information shall be requested by Purchaser from Seller at least five
(5) Trading Days prior to the anticipated filing date of the Resale Registration Statement.
(b)
Purchaser shall use commercially reasonable efforts to promptly cause such Mandatory Registration Statement to be declared effective
as soon as practicable after the filing thereof, but no later than the earlier of (i) 60 calendar days after the filing thereof in the
event the SEC reviews and has written comments to the Resale Registration Statement and (ii) the fifth (5th) Trading Day after the date
Purchaser is notified (orally or in writing, whichever is earlier) by the SEC that the Resale Registration Statement will not be “reviewed”
or will not be subject to further review (the earlier of (i) and (ii), the “Effectiveness Deadline”); provided,
that if such deadline falls on a Saturday, Sunday or other day that the SEC is closed for business, the Effectiveness Deadline shall
be extended to the next Trading Day.
(c)
Not less than two (2) Trading Days prior to the filing of a Resale Registration Statement or any related prospectus or any amendment
or supplement thereto, Purchaser shall furnish via email to Seller copies of all such documents proposed to be filed (other than any
document that is incorporated or deemed to be incorporated by reference therein) for review by Seller. Purchaser shall reflect in each
such document when so filed with the SEC such comments regarding Seller and the plan of distribution as Seller may reasonably and promptly
propose no later than two (2) Trading Days after Seller has been so furnished with copies of such documents as aforesaid.
(d)
Purchaser shall promptly prepare and file with the SEC such amendments and supplements to such Resale Registration Statements and
the prospectus used in connection therewith as shall be necessary to keep such Resale Registration Statements continuously effective
and free from any material misstatement or omission to state a material fact therein for so long as such Shares remain Registrable Shares,
subject to Purchaser’s right to suspend such registration pursuant to Section 10.5.
(e)
Purchaser shall furnish to Seller such number of copies of prospectuses in conformity with the requirements of the Securities Act
as Seller may reasonably request, in order to facilitate the public sale or other disposition of all or any of the Registrable Shares
by Seller.
(f)
Upon notification by the SEC that that the Resale Registration Statement has been declared effective by the SEC, Purchaser shall,
if required, file the final prospectus under Rule 424 within the applicable time period prescribed by Rule 424.
(g)
For so long as the Shares remain Registrable Shares, Purchaser shall advise Seller:
(i)
within one (1) Trading Day of the effectiveness of the Resale Registration Statement or any post-effective amendments thereto;
(ii)
within five (5) Trading Days of any request by the SEC for amendments to the Resale Registration Statement or amendments to the prospectus
or for additional information relating thereto;
(iii)
within one (1) Trading Day of the issuance by the SEC of any stop order suspending the effectiveness of the Resale Registration Statement
or the initiation of any proceedings for such purpose;
(iv)
within one (1) Trading Day of the suspension of the qualification of the Registrable Shares for sale in any jurisdiction, or the
initiation of any proceeding for any of the preceding purposes; and
(v)
within one (1) Trading Day of the existence of any fact and the happening of any event that makes any statement of a material fact
made in the Resale Registration Statement, the prospectus and amendment or supplement thereto, or any document incorporated by reference
therein, untrue, or that requires the making of any additions to or changes in the Resale Registration Statement or the prospectus in
order to make the statements therein not misleading.
(h)
Purchaser shall cause all Registrable Shares to be listed on each securities exchange, if any, on which equity securities by Purchaser
are then listed.
(i)
Purchaser shall bear all expenses in connection with the procedures in paragraphs (a) through (i) of this Section 10.2 and
the registration of the Registrable Shares on such Resale Registration Statement.
10.3
Rule 415; Cutback. If the SEC prevents Purchaser from including any or all of the Registrable Shares in a Resale Registration Statement
due to limitations on the use of Rule 415 under the Securities Act or requires Seller to be named as an “underwriter,” Purchaser
shall use its commercially reasonable efforts to persuade the SEC that the offering contemplated by the Resale Registration Statement
is a valid secondary offering and not an offering “by or on behalf of the issuer” as defined in Rule 415 and that Seller
is not an “underwriter.” In the event that, despite Purchaser’s commercially reasonable efforts and compliance with
the terms of this Section 10.3, the SEC refuses to alter its position, Purchaser shall (i) remove from the Resale Registration
Statement such portion of the Registrable Shares (the “Cut Back Shares”) and/or (ii) agree to such restrictions and
limitations on the registration and resale of the Registrable Shares as the SEC may require to assure Purchaser’s compliance with
the requirements of Rule 415 (collectively, the “SEC Restrictions”); provided, however, that Purchaser
shall not agree to name Seller as an “underwriter” in such Registration Statement without the prior written consent of Seller.
Seller acknowledges that it shall not have suffered any Losses as to any Cut Back Shares until the date that is five (5) Trading Days
following the date that Purchaser is eligible to bring effective the registration of such Cut Back Shares in accordance with any SEC
Restrictions (such date, the “Restriction Termination Date” of such Cut Back Shares). From and after the Restriction
Termination Date applicable to any Cut Back Shares, all of the provisions of this Section 10 shall again be applicable to such
Cut Back Shares; provided, however, that the Filing Deadline for the Resale Registration Statement including such Cut Back
Shares shall be ten (10) Trading Days after such Restriction Termination Date, and Purchaser shall use commercially reasonable efforts
to cause such Resale Registration Statement to become effective as promptly as practicable.
10.4
Registration Right Indemnification.
(a)
Purchaser agrees to indemnify and hold harmless Seller and its Affiliates and Representatives (each, a “Seller Party”
and collectively the “Seller Parties”) to the fullest extent permitted by applicable Law, from and against any losses,
claims, damages or liabilities (collectively, “Losses”) to which they may become subject (under the Securities Act
or otherwise) insofar as such Losses (or actions or proceedings in respect thereof) arise out of, or are based upon, any material breach
of this Section 10 by Purchaser or any untrue statement or alleged untrue statement of a material fact contained in the Resale
Registration Statement or any omission or alleged omission to state therein a material fact required to be stated therein or necessary
to make the statements therein, in light of the circumstances under which they were made, not misleading or arise out of any failure
by Purchaser to fulfill any undertaking included in the Resale Registration Statement and Purchaser will, as incurred, reimburse the
Seller Parties for any legal or other expenses reasonably incurred in investigating, defending or preparing to defend any such action,
proceeding or claim; provided, however, that Purchaser shall not be liable in any such case to the extent that such Loss arises
out of, or is based upon: (i) an untrue statement or omission or alleged untrue statement or omission made in such Resale Registration
Statement based on written information furnished to Purchaser by or on behalf of Seller specifically for use in preparation of the Resale
Registration Statement; or (ii) any breach of this Section 10 by Seller; provided further, however, that Purchaser shall
not be liable to any Seller Party (or any partner, member, officer, director or controlling Person of Seller) to the extent that any
such Loss is caused by an untrue statement or omission or alleged untrue statement or omission made in any preliminary prospectus if
either (A) (1) Seller failed to send or deliver a copy of the final prospectus with or prior to, or such Seller failed to confirm that
a final prospectus was deemed to be delivered prior to (in accordance with Rule 172 of the Securities Act), the delivery of written confirmation
of the sale by Seller to the Person asserting the claim from which such Loss resulted and (2) the final prospectus corrected such untrue
statement or omission, (B) (1) such untrue statement or omission is corrected in an amendment or supplement to the prospectus and (2)
having previously been furnished by or on behalf of Purchaser with copies of the prospectus as so amended or supplemented or notified
by Purchaser that such amended or supplemented prospectus has been filed with the SEC, in accordance with Rule 172 of the Securities
Act, Seller thereafter fails to deliver such prospectus as so amended or supplemented, with or prior to or Seller fails to confirm that
the prospectus as so amended or supplemented was deemed to be delivered prior to (in accordance with Rule 172 of the Securities Act),
the delivery of written confirmation of the sale by Seller to the Person asserting the claim from which such Loss resulted or (C) Seller
sold Registrable Shares in violation of its covenants contained in Section 3.6.
(b)
Seller agrees to indemnify and hold harmless Purchaser and its Affiliates and Representatives (each, a “Purchaser Party”
and collectively the “Purchaser Parties”) from and against any Losses to which the Purchaser Parties may become subject
(under the Securities Act or otherwise), insofar as such Losses (or actions or proceedings in respect thereof) arise out of, or are based
upon, any material breach of this Agreement by Seller or untrue statement or alleged untrue statement of a material fact contained in
the Resale Registration Statement (or any omission or alleged omission to state therein a material fact required to be stated therein
or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading), but only to the
extent such untrue statement or omission or alleged untrue statement or omission was made based on written information furnished by or
on behalf of Seller specifically for use in preparation of the Resale Registration Statement, and Seller will reimburse each Purchaser
Party for any legal or other expenses reasonably incurred in investigating, defending or preparing to defend any such action, proceeding
or claim; provided, however, that in no event shall any indemnity under this Section 10.4(b) be greater in amount than
the dollar amount of the net proceeds received by Seller upon its sale of the Registrable Shares included in the Resale Registration
Statement giving rise to such indemnification obligation (such amount, the “Registration Liability Cap”).
(c)
Promptly after receipt by any indemnified Person of a notice of a claim or the beginning of any action in respect of which indemnity
is to be sought against an indemnifying Person pursuant to this Section 10.4, such indemnified Person shall notify the indemnifying
Person in writing of such claim or of the commencement of such action, and, subject to the provisions hereinafter stated, in case any
such action shall be brought against an indemnified Person and such indemnifying Person shall have been notified thereof, such indemnifying
Person shall be entitled to participate therein, and, to the extent that it shall wish, to assume the defense thereof, with counsel reasonably
satisfactory to such indemnified Person. After notice from the indemnifying Person to such indemnified Person of its election to assume
the defense thereof, such indemnifying Person shall not be liable to such indemnified Person for any legal expenses subsequently incurred
by such indemnified Person in connection with the defense thereof; provided, however, that if there exists or shall exist a conflict
of interest that would make it inappropriate, based on the opinion of counsel, for the same counsel to represent both the indemnified
Person and such indemnifying Person or any affiliate or associate thereof, the indemnified Person shall be entitled to retain its own
counsel at the expense of such indemnifying Person; provided, further, that no indemnifying Person shall be responsible for the fees
and expense of more than one separate counsel for all indemnified parties. The indemnifying party shall not settle an action without
the consent of the indemnified party, which consent shall not be unreasonably withheld, conditioned or delayed; provided that no consent
shall be required if such settlement contains an unconditional release of the indemnified party from all liability arising out of such
action or claim and does not include a statement as to or an admission of fault, culpability or failure to act by or on behalf of any
indemnified party.
(d)
If the indemnification provided for in this Section 10.4 is held by a court of competent jurisdiction to be unavailable to
an indemnified party with respect to any Losses, the indemnifying party, in lieu of indemnifying such indemnified party thereunder, shall
to the extent permitted by applicable Law contribute to the amount paid or payable by such indemnified party as a result of such loss,
claim, damage or liability in such proportion as is appropriate to reflect the relative fault of the indemnifying party on the one hand
and of the indemnified party on the other, as well as any other relevant equitable considerations; provided, that in no event
shall any contribution by Seller hereunder be greater than the Registration Liability Cap.
(e)
For the avoidance of doubt, nothing contained in this Section 10.4 relate to a claim for Losses by an Indemnified Party pursuant
to Section 9.
10.5
Prospectus Suspension. Seller acknowledges that there may be times when Purchaser must suspend the use of the prospectus forming
a part of the Resale Registration Statement until such time as an amendment to the Resale Registration Statement has been filed by Purchaser
and declared effective by the SEC, or until such time as Purchaser has filed an appropriate report with the SEC pursuant to the Exchange
Act. Seller hereby covenants that it will not sell any Registrable Shares pursuant to said prospectus during the period commencing at
the time at which Purchaser gives Seller notice of the suspension of the use of said prospectus and ending at the time Purchaser gives
Seller notice that Seller may thereafter effect sales pursuant to said prospectus; provided, (a) that such suspension periods
shall in no event exceed (i) on more than three occasions, a period of more than thirty (30) consecutive Trading Days or (ii) more than
an aggregate total of sixty (60) Trading Days, in each case in any 360-day period, and (b) the board of directors of Purchaser has reasonably
determined that, in order for such Resale Registration Statement not to contain a material misstatement or omission, an amendment thereto
would be needed to include information that would at that time not otherwise be required in a current, quarterly or annual report under
the Exchange Act.
11.1
Termination of the Agreement. This Agreement may be terminated, and the Transactions may be abandoned, by written notice delivered
by the terminating Party to the other Party (other than in the case of Section 11.1(a)) at any time prior to the Closing:
(a)
by the mutual written agreement of Purchaser and Seller;
(b)
by either Purchaser or Seller if any Governmental Authority of competent jurisdiction issues an Order permanently restraining, enjoining,
or otherwise prohibiting the consummation of the Transactions, and such Order becomes final and non-appealable; provided, however,
that the right to terminate this Agreement under this Section 11.1(b) shall not be available to a Party whose failure to perform
its covenants or agreements contained in this Agreement has been the cause of or has resulted in the imposition of such Order or the
failure of such Order to be resisted, resolved, or lifted;
(c)
by Purchaser if (i) there exists a breach of any representation or warranty of Seller contained in this Agreement such that the Closing
condition set forth in Section 7.1 would not be satisfied or (ii) Seller shall have breached any of the covenants or agreements
contained in this Agreement to be complied with by Seller such that the Closing condition set forth in Section 7.2 would not be
satisfied; provided, that (A) Purchaser shall not be entitled to terminate this Agreement pursuant to this Section 11.1(c)
unless, in the case of the immediately preceding clauses (i) or (ii), such breach is not cured by Seller within the shorter of 30
days after Seller receives written notice of such breach from Purchaser or by the Outside Date; and (B) Purchaser shall not be entitled
to terminate this Agreement pursuant to this Section 11.1(c) if, at the time of such termination, Purchaser is in breach of any
representation, warranty, covenant or other agreement contained in this Agreement in a manner such that the conditions to Closing set
forth in Section 8.1 or Section 8.2, as applicable, would not have been satisfied;
(d)
by Seller if (i) there exists a breach of any representation or warranty of Purchaser contained in this Agreement such that the Closing
condition set forth in Section 8.1 would not be satisfied or (ii) Purchaser shall have breached any of the covenants or agreements
contained in this Agreement to be complied with by Purchaser such that the Closing condition set forth in Section 8.2 would not
be satisfied; provided, that (A) Seller shall not be entitled to terminate this Agreement pursuant to this Section 11.1(d)
unless, in the case of the immediately preceding clauses (i) or (ii), such breach is not cured by Purchaser within the shorter of
30 days after Purchaser receives written notice of such breach from Seller or by the Outside Date; and (B) Seller shall not be entitled
to terminate this Agreement pursuant to this Section 11.1(d) if, at the time of such termination, Seller is in breach of any representation,
warranty, covenant or other agreement contained in this Agreement in a manner such that the conditions to Closing set forth in Section
7.1 or Section 7.2, as applicable, would not have been satisfied;
(e)
by either Party by written notice to the other if the consummation of the Transactions contemplated hereby shall not have occurred
on or before the date that is six months following the Agreement Date (the “Outside Date”); provided, that
the terminating Party shall only be entitled to exercise such right of termination if the terminating Party is not then in breach
of any representation, warranty, covenant or other agreement contained in this Agreement such that the conditions to Closing set forth
in Article 7 or Article 8, as applicable, would not have been satisfied; or
(f)
by Purchaser if Seller fails to deliver, by 11:59 p.m. Eastern Time on the date that is three (3) Business Days after Agreement Date,
the Stockholder Written Consent executed and delivered by each of the Consenting Stockholders.
11.2
Effect of Termination. In the event of termination by either Party pursuant to Section 11.1, written notice thereof will forthwith
be given to the other Party and the Transactions will be terminated, without further action by either Party. If the Transactions are
terminated as provided herein, this Agreement shall become null and void and have no further force and effect and all obligations of
the Parties under this Agreement shall terminate and there shall be no liability of any Party to any other Party, except that nothing
herein will relieve or release any Party from liability arising from any breach by such Party of this Agreement prior to any such termination.
Notwithstanding the foregoing, the following Sections of this Agreement shall remain in full force and effect following termination of
this Agreement: Section 6.1, Section 6.2, Section 6.8, this Section 11.2, and Article 12.
12.1
Governing Law; Jurisdiction. This Agreement shall be governed by and construed and enforced under the substantive laws of the State
of Delaware, excluding any conflicts or choice of law rule or principle that might otherwise make this Agreement subject to the substantive
law of another jurisdiction. Purchaser and Seller each hereby irrevocably: (i) consents to submit itself in any suit, action or proceeding
arising out of or related to this Agreement or any of the transactions contemplated hereby to the exclusive personal jurisdiction of
the Court of Chancery of the State of Delaware (or, only if such court declines to accept jurisdiction over a particular matter, any
state or federal court within the New Castle County, Delaware); (ii) agrees that it will not attempt to defeat or deny such personal
jurisdiction by motion or other request for leave from such court; and (iii) agrees that it will not bring any action arising out of
or related to this Agreement or any of the transactions contemplated hereby in any court other than any such court.
12.2
Specific Performance. The Parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate
remedy, would occur in the event that the Parties do not perform their obligations under the provisions of this Agreement in accordance
with its specified terms or otherwise breach such provisions. The Parties acknowledge and agree that (i) the Parties shall be entitled
to an injunction or injunctions, specific performance, or other equitable relief, to prevent breaches of this Agreement and to enforce
specifically the terms and provisions hereof in the courts described in Section 12.1 without proof of damages or otherwise, this
being in addition to any other remedy to which they are entitled under this Agreement and (ii) the right of specific performance is an
integral part of the Transactions and without that right, neither Purchaser nor Seller would have entered into this Agreement. Each of
the Parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief on the basis
that the other Parties have an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason
at law or equity. The Parties acknowledge and agree that any Party seeking an injunction or injunctions to prevent breaches of this Agreement
and to enforce specifically the terms and provisions of this Agreement in accordance with this Section 12.2 shall not be required
to provide any bond or other security in connection with any such order or injunction.
12.3
WAIVER OF JURY TRIAL. EACH OF PURCHASER AND SELLER HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY
ACTION, PROCEEDING OR COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE
ACTIONS OF PURCHASER AND SELLER IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT HEREOF.
12.4
Entire Agreement; Severability. This Agreement, together with the Disclosure Schedules, Related Agreements, all Exhibits and Schedules
hereto and thereto constitute the entire agreement, and supersede all prior agreements and understandings, both written and oral, between
the Parties with respect to the subject matter hereof and thereof. If any term, condition or other provision of this Agreement is found
to be invalid, illegal or incapable of being enforced by virtue of any rule of law, public policy or court determination, all other terms,
conditions and provisions of this Agreement shall nevertheless remain in full force and effect. If the final determination of any arbitration
process or final judgment of a court of competent jurisdiction, in each case, to the extent in accordance with the terms of this Agreement,
declares that any term or provision hereof is invalid or unenforceable, the arbitrators or court making the determination of invalidity
or unenforceability shall have the power to limit the term or provision, to delete specific words or phrases, or to replace any invalid
or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention
of the invalid or unenforceable term or provision, and this Agreement shall be enforceable as so modified.
12.5
Incorporation by Reference. The Disclosure Schedules, the Schedules and Exhibits, and the documents referenced herein and therein
constitute integral parts of this Agreement and are hereby incorporated by reference herein.
12.6
Amendments and Waivers. This Agreement may not be amended or modified, nor may compliance with any condition or covenant set forth
herein be waived, except by a writing duly and validly executed by Purchaser and Seller or, in the case of a waiver, the Party waiving
compliance. Except as specifically set forth herein to the contrary, no delay or omission by either Party in exercising any right or
power occurring upon any noncompliance or default by the other Party with respect to any of the terms of this Agreement shall impair
any such right or power or be construed to be a waiver thereof. A waiver by either party of any of the covenants, conditions or agreements
to be performed by the other shall not be construed to be a waiver of any succeeding breach thereof or of any other covenant, condition
or agreement herein contained.
12.7
Notices. Any notice or other communication required or permitted to be delivered to any party under this Agreement shall be in writing
and shall be deemed properly delivered, given and received (a) upon receipt when delivered by hand, (b) upon transmission, if sent by
electronic transmission (in each case with receipt verified by electronic confirmation) or (c) one (1) Business Day after being sent
by courier or express delivery service, provided that in each case the notice or other communication is sent to the address set
forth beneath the name of such party below (or to such other address as such party shall have specified in a written notice given to
the other Parties hereto):
Ayala
Pharmaceuticals, Inc.
9
Deer Park Drive, Suite K-1
Monmouth
Junction, NJ 08852, USA
Attention:
Kenneth A. Berlin
e-mail:
[***]
with
required copies to:
Morgan,
Lewis & Bockius LLP
101
Park Avenue
New
York, NY 10178
Attention:
Robert W. Dickey
Email:
[***]
Immunome,
Inc.
18912
N. Creek Parkway
Bothell,
WA 98011
Attention:
Clay Siegall, Ph.D.
e-mail:
[***]
with
required copies to:
Immunome,
Inc.
665
Stockton Drive, Suite 300
Exton,
PA 19341
Attention:
Sandra Stoneman
e-mail:
[***]
Cooley
LLP
10265
Science Center Drive
San
Diego, California 92121
Attention:
Barbara Borden
e-mail:
[***]
12.8
No Assignment; Binding Effect. This Agreement is not assignable by any Party without the prior written consent of the other Party;
provided, however, for the avoidance of doubt, Purchaser may, with notice to Seller but without Seller’s consent, (a) at
any time, sell, assign, contribute, or otherwise transfer this Agreement and its rights and obligations hereunder in whole or in part
to an Affiliate, (b) assign all or any part of its rights or obligations hereunder to any Person (whether or not an Affiliate of Purchaser)
in connection with a merger or consolidation of Purchaser or the sale of all or substantially all of Purchaser’s operations or
assets or substantially all of Purchaser’s assets that relate to the Acquired Assets and the Assumed Liabilities, and (c) grant
or permit any Lien or assignment to any Person (whether or not an Affiliate of Purchaser) in connection with a financing for Purchaser
(or an Affiliate of Purchaser to which any rights under this Agreement have been assigned or sublicensed) from time to time, in each
case of clauses (a), (b), or (c) above, without Purchaser being relieved of any of its obligations hereunder. This Agreement will be
binding upon and will inure to the benefit of the Parties hereto and their respective successors and permitted assigns.
12.9
Third Person Beneficiaries. This Agreement shall not benefit or create any right or cause of action in or on behalf of any Person
other than the Parties and their respective successors and permitted assigns and nothing herein, whether express or implied, is intended
to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever under or by reason
of this Agreement.
12.10
Relationship of the Parties. Nothing contained in this Agreement shall be deemed or construed to create a partnership, joint venture,
employment, franchise, agency or fiduciary relationship between Purchaser and Seller. Neither party shall have any express or implied
right or authority to assume or create any obligations on behalf of or in the name of the other party or to bind the other party to any
contract, agreement or undertaking with any Third Party.
12.11
Headings; Interpretation. The headings in this Agreement are intended solely for convenience of reference and will be given no effect
in the construction or interpretation of this Agreement. Unless the context otherwise requires, the singular includes the plural, and
the plural includes the singular. Whenever the words “include”, “includes”, or “including” are used
in this Agreement, they are deemed to be followed by the words “without limitation”. Unless otherwise specified, references
in this Agreement to any Article shall include all Sections, subsections, and paragraphs in such Article, references to any Section shall
include all subsections and paragraphs in such Section, and references in this Agreement to any subsection shall include all paragraphs
in such subsection. The word “or” means “and/or” unless the context dictates otherwise because the subjects of
the conjunction are mutually exclusive. The words “herein”, “hereof”, and “hereunder” and other words
of similar import refer to this Agreement as a whole and not to any particular Section or other subdivision. All references to days in
this Agreement mean calendar days, unless otherwise specified. Ambiguities and uncertainties in this Agreement, if any, shall not be
interpreted against either Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to exist. All
references herein to “immediately available funds” or “$” shall be deemed to be references to the lawful money
of the United States. Unless the context otherwise requires, references herein to a statute means such statute as amended from time to
time and includes any successor legislation thereto and any regulations promulgated thereunder.
12.12
Counterparts; Signatures. This Agreement may be executed in one or more counterparts, all of which will be considered one and the
same agreement and will become effective when one or more counterparts have been signed by each of the Parties and delivered to the other
Party. This Agreement may be executed by facsimile signature or by an electronic scan delivered by electronic mail.
12.13
Expenses. Except as otherwise expressly provided in this Agreement, whether or not the Transactions are consummated, each Party hereto
will pay its own costs and expenses incurred incident to its negotiation and preparation of this Agreement and the Related Agreements
and to its performance and compliance with all agreements and conditions contained herein and therein on its part to be performed or
complied with, including the fees, expenses and disbursements of its counsel and accountants.
[Signature
Page to Follow]
In
Witness Whereof, the Parties, intending legally to
be bound, have caused this Asset Purchase Agreement to be duly executed and delivered as of the Agreement Date.
| Immunome,
Inc. |
|
| |
|
|
| By: |
/s/
Clay Siegall |
|
| |
|
|
| Print
Name: |
Clay
Siegal, Ph.D. |
|
| |
|
|
| Title:
|
President
and Chief Executive Officer |
|
In
Witness Whereof, the Parties, intending legally to
be bound, have caused this Asset Purchase Agreement to be duly executed and delivered as of the Agreement Date.
| Ayala
Pharmaceuticals, Inc. |
|
| |
|
|
| By:
|
/s/
Kenneth Berlin |
|
| |
|
|
| Print
Name: |
Kenneth
A. Berlin |
|
| |
|
|
| Title:
|
President
and Chief Executive Officer |
|
Exhibit
A
Support
Agreement
[***]
[Exhibit
A - Form of Support Agreement]
Exhibit
B
Assignment
and Assumption Agreement
[***]
[Exhibit
B – Assignment and Assumption Agreement]
Exhibit
C
Bill
of Sale
[***]
[Exhibit
C – Bill of Sale]
Exhibit
D
CVR
Agreement
[***]
[Exhibit
D – CVR Agreement]
Exhibit
E
IP
and Patent Assignment Agreement
[***]
[Exhibit
E – IP and Patent Assignment Agreement]
Exhibit
F
Stockholder
Written Consent
[***]
[Exhibit
F – Stockholder Written Consent]
Schedule
A
[***]
[Schedule
A – Support Agreement Signatories]
Schedule
B
Compounds
[***]
[Schedule
B – Compounds]
Schedule
C
Product
Patents
[***]
[Schedule
C – Product Patents]
Schedule
C-1
Dropped
Patents
[***]
[Schedule
C-1 – Dropped Patents]
Schedule
D
Knowledge
Parties
[***]
[Schedule
D – Knowledge Parties]
Schedule
E
Qualified
CMC Expenses
[***]
[Schedule
E – Qualified CMC Expenses]
Schedule
2.1(a)(ii)
Acquired
Business Contracts
[***]
[Schedule
2.1(a)(ii) – Acquired Business Contracts]
Schedule
2.2(b)
Specified
Liabilities
[***]
[Schedule
2.2(b) – Assumed Liabilities]
Schedule
4.2(a)(v)
Closing
Third Party Consents/Notices
[***]
[Schedule
4.2(a)(v) – Closing Third Party Consents/Notices]
Schedule
6.11
Transition
Services
[***]
[Schedule
6.11 – Transition Services]
ANNEX
B
[OPINION
OF A.G.P./ALLIANCE GLOBAL PARTNERS]

The
Board of Directors
Ayala
Pharmaceuticals, Inc.
9
Deer Park Drive, Suite K-1
Monmouth
Junction, NJ 08852
Dear
Board of Directors:
Ayala
Pharmaceuticals, Inc., a Delaware company (the “Company”), has engaged A.G.P./Alliance Global Partners (“A.G.P.”)
to provide an opinion (this “Opinion”) to the board of directors of the Company (the “Board”)
as of the date hereof as to the fairness, from a financial point of view, to the Company of the consideration to be paid to the Company
in the contemplated transaction described below (the “Proposed Transaction”).
It
is our understanding that the Proposed Transaction will involve, subject to the terms and conditions of the Asset Purchase Agreement
to be entered into by the Company and Immunome, Inc. (“Immunome”) (such agreement, the “Purchase Agreement”),
the acquisition from the Company by Immunome of all of the Company’s patents, know-how and other intellectual property rights,
as well as certain other properties, assets, and rights of the Company, relating to the gamma secretase inhibitors known as AL101 and
AL102 (or any product comprised of or containing either of the foregoing compounds (a “Product”)) (the foregoing properties,
assets, and rights, collectively, the “Product Assets”) and the assumption by Immunome of specified related liabilities
of the Company. It is also our understanding that, subject to the provisions of the Purchase Agreement, the consideration to be paid
to the Company for the Product Assets in the Proposed Transaction will consist of (a) $20,000,000 in cash (the “Closing Cash
Consideration”), $4,000,000 of which previously paid by Immunome to the Company on December 23, 2023 and the balance of which
to be paid at the closing of the Proposed Transaction, (b) 2,175,489 newly-issued unregistered shares of common stock, par value $0.0001
per share, of Immunome (“Immunome Common Stock”) to be paid at the closing of the Proposed Transaction (the “Closing
Stock Consideration”), and (c) up to three future cash milestone payments as more fully described below (the “Milestone
Consideration” and, together with the Closing Cash Consideration and the Closing Stock Consideration, the “Aggregate
Consideration”).
It
is our understanding that the Purchase Agreement provides that, as part of the Aggregate Consideration, the Milestone Consideration includes
the following contingent future cash payments: (i) $10,000,000 in the event of public announcement of Positive Topline Data (as defined
in the Purchase Agreement), (ii) $17,500,000 in the event of the grant of NDA (as defined in the Purchase Agreement) Regulatory Approval
(as defined in the Purchase Agreement) of a Product by the U.S. Food and Drug Administration in a first indication, and (iii) $10,000,000
in the event of the first calendar year in which Annual Net Sales (as defined in the Purchase Agreement) of AL102 exceed $100,000,000.
For
purposes of rendering this Opinion, A.G.P. has reviewed, among other things, the following:
| 1. | Draft
dated February 4, 2024 of the Purchase Agreement; |
| 2. | Historical
financials relating to the Company for the fiscal years ended December 31, 2022 and December
31, 2021 and the interim period ended September 30, 2023 contained in the Company’s
public filings, financial forecasts and projections relating to the Product Assets for the
years 2023 through 2033 prepared by or discussed with the management of the Company and estimates
of the probability of achieving the milestone events associated with the Milestone Consideration
provided by the management of the Company; |
| 3. | Publicly
available financial and stock market information of certain public companies that were deemed
by us to be reasonably comparable to the Product Assets; |
| 4. | Financial
terms, to the extent publicly available, of certain acquisition transactions that were deemed
by us to be reasonably comparable to the Proposed Transaction; and |
| 5. | Publicly
available stock market information of Immunome, including current and historical market prices
and trading volumes of publicly traded shares of Immunome Common Stock. |
In
connection with this Opinion, A.G.P. has performed a selected companies analysis and a selected transactions analysis of the Product
Assets as well as a discounted cash flow analysis of the Product Assets (which discounted cash flow analysis has been probability-adjusted
for the milestone events associated with the Milestone Consideration). At the direction of the Company based on the assessments of the
management of the Company as to commercial viability and consistent with the financial forecasts and projections reviewed by us, no value
has been ascribed to AL101 in our analysis. A.G.P. also participated in certain discussions with representatives of the Company. A.G.P.
has not been provided with access to the management of Immunome and has not performed any financial analyses to estimate the value of
Immunome Common Stock. Rather, A.G.P. has assumed that the latest closing price of Immunome Common Stock is a reasonable estimate of
value for shares of Immunome Common Stock to be issued as the Closing Stock Consideration. Furthermore, A.G.P. has not applied any illiquidity
or other discounts, or otherwise given effect to any restrictions or limitations, which may be attributable to the Closing Stock Consideration.
In
rendering this Opinion, we have assumed and relied upon the accuracy and completeness of all information that was publicly available,
provided by or on behalf of the Company or any other party to the Proposed Transaction, or otherwise reviewed by or discussed with us,
without (and without assuming responsibility for) independent verification thereof by us. Accordingly, we do not express an opinion or
any other form of assurance thereon. Moreover, we have assumed that the financial forecasts and projections and probability estimates
referred to above have been prepared reasonably and in good faith and are based upon the best currently available estimates and judgments
of the management of the Company as to the matters covered thereby, and we have relied upon such forecasts, projections and estimates
in our analysis. We have not been engaged to assess the reasonableness or achievability of such forecasts, projections or estimates or
the assumptions upon which they are based, and we express no views as to such forecasts, projections or estimates or the assumptions
on which they are based.
In
addition, A.G.P. has not evaluated the solvency of the Company or Immunome or made an independent evaluation or appraisal of any of the
Product Assets or the other assets and liabilities (including any contingent, derivative or off-balance-sheet assets and liabilities)
of the Company, Immunome or any of their respective subsidiaries, and A.G.P. has not been furnished with any such evaluation or appraisal.
This
Opinion does not constitute legal, regulatory, accounting, insurance, tax or other similar professional advice. This Opinion also does
not address the underlying business decision of the Company to engage in the Proposed Transaction, or the relative merits of the Proposed
Transaction as compared to any strategic alternatives that may be available to the Company. This Opinion
addresses only the fairness from a financial point of view, as of the date hereof, to the Company of the Aggregate Consideration
to be paid to the Company for the Product Assets in the Proposed Transaction. A.G.P. does not express any view on, and this Opinion
does not address, any other terms or aspect of the Proposed Transaction, including, without limitation, the form or structure
of the Proposed Transaction, the tax treatment thereof, the form or structure of the Aggregate Consideration (or any component thereof)
or the allocation thereof between cash and Immunome Common Stock or among the Product Assets, any adjustment to the Closing Cash Consideration,
any potential set-off against the Milestone Consideration, the allocation of expenses between the Company and Immunome in connection
with the Proposed Transaction, any transition services, noncompetition, support, consulting, lock-up, transfer restriction, registration
rights or other agreement or arrangement to be entered into in connection with the Proposed Transaction, any potential dissolution of
the Company following the consummation of the Proposed Transaction, any contingent value rights issued in connection with such dissolution,
the fairness of the Proposed Transaction to, or any consideration received in connection therewith by, the holders of any class of securities,
creditors, or other constituencies of the Company or any other party to the Proposed Transaction, or the fairness of the amount or nature
of any compensation to be paid or payable to any of the officers, directors or employees of the Company or any other party to the Proposed
Transaction, or class of such persons in connection with the Proposed Transaction, whether relative to the Aggregate Consideration to
be paid to the Company in the Proposed Transaction or otherwise. A.G.P. does not express any opinion as to what the actual value of Immunome
Common Stock will be when issued in the Proposed Transaction, the prices at which Immunome Common Stock or the Company’s common
stock will trade at any time or what the actual amount of the Milestone Consideration will be if and when paid. This Opinion
is necessarily based on economic, monetary, market and other conditions as in effect on, and the information made available to
A.G.P. as of, the date hereof, and A.G.P. assumes no responsibility for updating, revising or reaffirming this Opinion
based on circumstances, developments or events occurring after the date hereof. The opinion expressed in this Opinion
are provided for the information and assistance of the Board (in its capacity as such) in connection with its consideration of
the Proposed Transaction. This Opinion does not constitute a recommendation to the Board, the Company, its stockholders or any other
person as to how any of them should vote or act with respect to the Proposed Transaction or any other matter.
A.G.P.
has assumed that the representations and warranties made in the Purchase Agreement are accurate and assumed that all of the conditions
required to implement the Proposed Transaction will be satisfied and that the Proposed Transaction will be completed in accordance with
the Purchase Agreement without any amendments thereto or any waivers of any terms or conditions thereof. A.G.P. also has assumed that
all governmental, regulatory or other consents and approvals necessary or useful for the consummation of the Proposed Transaction would
be obtained without any adverse effect on any of the Product Assets, the Company or Immunome or on the Proposed Transaction in any way
impacting A.G.P.’s analysis. A.G.P. has relied upon the fact that the Company has been advised by counsel as to all legal matters
with respect to the Proposed Transaction, including whether all procedures required by law to be taken in connection with the Proposed
Transaction have been duly, validly and timely taken. A.G.P. has assumed that the final form of the Purchase Agreement will not differ
materially from the draft reviewed by A.G.P.
A.G.P.
believes that its analysis must be considered as a whole and that selecting portions of the analysis or the factors considered by it,
without considering all factors and analysis together, could create a misleading view of the process underlying this Opinion. The preparation
of an Opinion is a complex process and is not necessarily susceptible to partial analysis or summary description. Any attempt to do so
could lead to undue emphasis on any particular factor or analysis and an inaccurate conclusion.
A.G.P.
is a financial services firm engaged in the securities, investment management and individual wealth management businesses. Its securities
business is engaged in securities underwriting, trading and brokerage activities, foreign exchange, commodities and derivatives trading,
prime brokerage, as well as providing investment banking, financing and financial advisory services. A.G.P., its affiliates, directors
and officers may at any time invest on a principal basis or manage funds that invest, hold long or short positions, finance positions,
and may trade or otherwise structure and effect transactions, for their own account or for the accounts of their customers, in debt or
equity securities or loans of the Company, or any other company, or any currency or commodity, that may be involved in the Proposed Transaction,
or any related derivative instrument.
A.G.P.
may, in the future, in the ordinary course of its business, perform financial advisory or investment banking services for the Company,
Immunome or any of their respective associates or affiliates.
A.G.P.
will receive a fee as compensation for its services in rendering this Opinion, no portion of which is contingent upon either the conclusion
herein or the consummation of the Proposed Transaction.
The Company has agreed to indemnify A.G.P. against certain liabilities arising out of its engagement. In addition to the foregoing engagement,
A.G.P. has also been engaged as strategic advisor to the Company in connection with the Proposed Transaction and will receive a fee as
compensation for such engagement which is contingent upon the consummation of the Proposed Transaction.
Based
upon and subject to the foregoing, A.G.P. is of the opinion that as of the date hereof the Aggregate Consideration to be paid to the
Company for the Product Assets in the Proposed Transaction is fair from a financial point of view to the Company.
This
Opinion was approved by A.G.P.’s fairness opinion committee,
a committee of A.G.P. investment banking and other professionals, in accordance with A.G.P.’s customary practice.
Yours
truly,
A.G.P./ALLIANCE
GLOBAL PARTNERS
|
|
| |
|
|
| By: |
/s/ Thomas
J. Higgins |
|
| Name: |
Thomas J. Higgins |
|
| Title: |
Managing Director |
|
Exhibit
107
CALCULATION
OF FILING FEE TABLES
Schedule
14C
(Form Type)
Ayala
Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table
1: Transaction Valuation
| | |
Proposed Maximum Aggregate Value of Transaction | | |
Fee Rate | | |
Amount of Filing Fee | |
| Fees to be Paid | |
$ | 104,185,993.94 | (1),(2),(3) | |
| 0.00014760 | | |
$ | 15,377.85 | (4) |
| Fees Previously Paid | |
$ | 0 | | |
| | | |
$ | 0 | |
| Total Transaction Valuation | |
$ | 104,185,993.94 | | |
| | | |
| | |
| Total Fees Due for Filing | |
| | | |
| | | |
$ | 15,377.85 | |
| Total Fees Previously Paid | |
| | | |
| | | |
$ | 0 | |
| Total Fee Offsets | |
| | | |
| | | |
$ | 0 | |
| Net Fee Due | |
| | | |
| | | |
$ | 15,377.85 | |
Table
2: Fee Offset Claims and Sources
Not
Applicable
| (1) |
Title
of each class of securities to which transaction applies: Immunome, Inc. (the “Purchaser”) common stock, par value $0.0001
per share (the “Purchaser Common Stock”). |
| |
|
| (2) |
Aggregate
number of securities to which transaction applies: The number of shares of Purchaser Common Stock to which this transaction applies
is 2,175,489 due at the closing of the asset sale (the “Closing”). |
| |
|
| (3) |
In
accordance with Exchange Act Rule 0-11 and solely for the purpose of calculating the filing fee, the proposed maximum aggregate value
of the transaction was calculated, as of February 20, 2024, based on the sum of (a) the product of 2,175,489 shares of Purchaser
Common Stock due at Closing multiplied by $21.46, representing the average of the high and low prices of the Purchaser Common
Stock reported on the Nasdaq Capital Market on February 14, 2024 and (b) $57,500,000 cash consideration (including $37,500,000
in cash due upon the achievement of certain development and commercial milestone events set forth in the Asset Purchase Agreement). |
| |
|
| (4) |
The
filing fee was calculated in accordance with Rule 0-11 under the Securities and Exchange Act of 1934, as amended, by multiplying
the transaction value by 0.00014760. |
Ayala Pharmaceuticals (CE) (USOTC:ADXS)
Historical Stock Chart
From Feb 2025 to Mar 2025
Ayala Pharmaceuticals (CE) (USOTC:ADXS)
Historical Stock Chart
From Mar 2024 to Mar 2025